
CARBENDAZIM JMPR 1973
IDENTITY
Chemical names
Methyl-2-benzimidazole carbamate
Methyl benzimidazole-2-ylcarbamate
2-(methoxy-carbamoyl)-benzimidazole
2-(methoxycarbonylamino)benzimidazole
Carbomethoxyaminobenzimidazole
Synonyms
Bavisitin(R), MBC, MCAB, BCM, Hoe 17411, Drosal
Structural formula
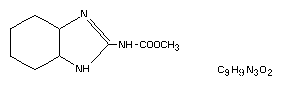 Other information on identity and properties
Molecular weight: 191.2
State: light grey powder
Melting point: 307-312°C (decomposition)
Vapour pressure: 0.1 × 10-6 mmHg (20°C)
Solubility: Soluble in acetic acid and
dimethyl-formamide
Water, pH 4: 28 ppm
Water, pH 7: 8 ppm
Water, pH 8: 7 ppm
Hexane: 0.5 ppm
Benzene: 36 ppm
Methylenechloride: 68 ppm
Ethanol: 300 ppm
Acetone: 300 ppm
Chloroform: 100 ppm
Stability: Stable in acidic and alkaline solutions
at room temperature and when exposed to
light. Stable in storage at 35-50°C
Purity of
technical product: Better than 98%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Methyl-2-benzimidazole carbamate has been proposed as an
intermediate in the degradation of thiophanate and benomyl in plants.
The quantitative aspects of this conversion have not been fully
reported although it appears that, while carbendazim is a major
metabolite, there may only be limited conversion of the two parent
molecules (Douch, 1973). Sims et al. (1969) found only carbendazim but
not benomyl four weeks after treatment of cotton. In aqueous solution
benomyl and thiophanate methyl were reported to convert rapidly to
carbendazim (Clemons and Sisler, 1969; Buchenauer and Edgington,
1973). In pea plant treated with benomyl, only carbendazim was
recovered as a plant residue (Siegel and Zabbia, 1972). In aqueous
solution on glass following irradiation by ultra-violet or sunlight,
conversion of both thiophanate and thiophanate methyl to
ethyl-2-benzimidazole carbamate and carbendazim was effected. There
are no data available on the metabolism of carbendazim in either
mammals or plants.
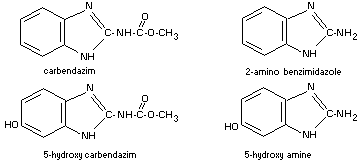
Metabolism of benomyl in mammals also results in the occurrence
of 5-hydroxy carbendazim and other products (Douch, 1973; Gardiner et
al., 1968). This might arise directly from benomyl by oxidation and
hydrolysis or by hydrolysis of benomyl to carbendazim followed by
oxidation. Carbendazim is the major metabolite of benomyl in plants. A
minor metabolite from benomyl is believed to be 2-amino benzimidazole.
The probable metabolic pathway of carbendazim based upon data
derived from thiophanate and benomyl conversion is suggested as
follows:
Other information on identity and properties
Molecular weight: 191.2
State: light grey powder
Melting point: 307-312°C (decomposition)
Vapour pressure: 0.1 × 10-6 mmHg (20°C)
Solubility: Soluble in acetic acid and
dimethyl-formamide
Water, pH 4: 28 ppm
Water, pH 7: 8 ppm
Water, pH 8: 7 ppm
Hexane: 0.5 ppm
Benzene: 36 ppm
Methylenechloride: 68 ppm
Ethanol: 300 ppm
Acetone: 300 ppm
Chloroform: 100 ppm
Stability: Stable in acidic and alkaline solutions
at room temperature and when exposed to
light. Stable in storage at 35-50°C
Purity of
technical product: Better than 98%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Methyl-2-benzimidazole carbamate has been proposed as an
intermediate in the degradation of thiophanate and benomyl in plants.
The quantitative aspects of this conversion have not been fully
reported although it appears that, while carbendazim is a major
metabolite, there may only be limited conversion of the two parent
molecules (Douch, 1973). Sims et al. (1969) found only carbendazim but
not benomyl four weeks after treatment of cotton. In aqueous solution
benomyl and thiophanate methyl were reported to convert rapidly to
carbendazim (Clemons and Sisler, 1969; Buchenauer and Edgington,
1973). In pea plant treated with benomyl, only carbendazim was
recovered as a plant residue (Siegel and Zabbia, 1972). In aqueous
solution on glass following irradiation by ultra-violet or sunlight,
conversion of both thiophanate and thiophanate methyl to
ethyl-2-benzimidazole carbamate and carbendazim was effected. There
are no data available on the metabolism of carbendazim in either
mammals or plants.
Metabolism of benomyl in mammals also results in the occurrence
of 5-hydroxy carbendazim and other products (Douch, 1973; Gardiner et
al., 1968). This might arise directly from benomyl by oxidation and
hydrolysis or by hydrolysis of benomyl to carbendazim followed by
oxidation. Carbendazim is the major metabolite of benomyl in plants. A
minor metabolite from benomyl is believed to be 2-amino benzimidazole.
The probable metabolic pathway of carbendazim based upon data
derived from thiophanate and benomyl conversion is suggested as
follows:
 Clemons and Sisler (1971) have suggested that carbendazim appears
to interfere with DNA synthesis or some closely related process such
as nuclear or cell division in fungi. RNA and protein synthesis were
not affected. Hammerschlag (1973) indicated an effect of carbendazim
on mitosis, DNA synthesis and cytokinesis in two fungi species.
Certain differences in effects of benomyl and carbendazim were
attributed to the occurrence of butylisocyanate, a potential
metabolite of benomyl.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of mice (20 male mice per group) were used in a
dominant lethal study where one group was administered a dose of 1280
mg/kg carbendazim by i.p. injection and three other groups served as
controls (Hofmann and Peh, 1973). One group was a solvent control, one
an unhandled control and one a positive control. Each male mouse was
mated with three females for seven days over a period of eight weeks.
The females were sacrificed at day 18 following introduction to the
males and various reproduction parameters examined, including
implantation, total number of live and dead fetuses, and resorption
sites. There were no apparent effects as a result of MBC
administration to males except a slight growth depression during week
1. During the same week, the mutagenic index was higher in the treated
group than in the untreated controls, although not different from that
in the solvent controls. The effect was considered to be within the
normal variation. A positive control (Trenimon, 2,3,5-triethyleneimino
benzoquinone-1,4) showed definitive effects especially during the
first three weeks of the experiment. Methyl-benzimidazole carbamate
does not appear to be mutagenic to mice as evidenced by this dominant
lethal study (Hofmann and Pen, 1973).
Acute toxicity
Dermal injection of 0.01% (2 µg) to rabbits caused slight
reddening around the injection site (Scholz and Weigand, 1972). There
were no effects noted following five daily dermal applications of 500
mg to unbroken skin of rabbits. Application of 10 mg to the
conjunctival sac of rabbits caused a transient reddening. No such
effects were noted with 5 mg.
Species Sex Route LD50 Reference
(mg/kg)
Rat M & F oral > 15 000 Hofmann, 1971, 1972c;
Scholz and Weigand, 1973a
M oral > 15 000 Scholz and Weigand, 1972b
Species Sex Route LD50 Reference
(mg/kg)
F oral 14 000 Scholz and Weigand, 1972b
M i.p. 7 230 Scholz and Weigand, 1972a
F i.p. 715 000 Scholz and Weigand, 1972a
Mouse M & F i.p. > 5 000 Scholz and Weigand, 1972a;
Hofmann and Peh, 1973
Dog M & F oral > 8 000 Zeller and Kirsch, 1971a;
Scholz and Weigand, 1972a
Rabbit M & F oral > 8 000 Zeller and Kirsch, 1971b
Japanese M oral 10 996 Scholz and Weigand, 1973b
quail (sesamol)
15 595 Scholz and Weigand, 1973b
(starke-schleim)
F 5 826 Scholz and Weigand, 1973b
(sesamol)
9 813 Scholz and Weigand, 1973b
(starke-schleim)
Short-term studies
Rat. Groups of rats (10 males per group) were administered
carbendazim orally for two weeks (by gavage) at dosage levels of 0,
10, 20, 30 and 40 mg/kg per day. The object of the study was to assess
toxicity with special reference to the effect on bone marrow and the
male reproduction system. One hour before sacrifice, each animal was
injected with tritiated thymidine. At the conclusion of the study, the
following tissues were removed, organ weights were recorded and organ
to body weight ratios were calculated: adrenal glands, brain, heart,
kidney, liver, pituitary gland, spleen, testes and thyroid gland.
Microscopic examination was perfumed on the bone marrow and testes of
all rats from the control and high-dose group. Microscopic
radio-autography was used to determine bone marrow mitosis. Bone
marrow was dissected, fixed and processed in paraffin wax. Sections
were mounted on microscope slides and exposed to Kodak stripping film.
After 20 days, the slides were developed, fixed and counted as well as
examined following staining with haematoxylin.
There were no effects noted in behaviour, mortality, food
consumption and body weight gain. On gross examination of tissues, it
was observed that the absolute and relative liver weight of rats at
the 40 mg/kg dosage was higher than the controls. A decrease in
relative testes weight, although significant, did not appear to be
dose related and did not appear to be significant in this study,
especially as there were no histological abnormalities observed in
testes. Bone marrow examinations in both control and treated animals
were within normal limits and showed no significant abnormalities
either histologically or on radio-autography of the bone marrow cells.
This study indicates that a dosage level of 30 mg/kg per day
administered orally for two weeks would be a no-effect level with an
effect noted at 40 mg/kg per day with regard to liver to body weight
ratio (Hunter et al., 1973a).
Groups of Sprague-Dawley strain rats (10 male and 10 female per
group) were fed carbendazim in the diet at dosage levels of 0, 2000,
4000 and 8000 ppm for 28 days. Haematological examination, clinical
chemistry and urinalyses were performed at three weeks of testing.
Gross and microscopic examination of tissues and organs was Performed
at the end of the feeding study. Food consumption and growth were
depressed at 4000 and 8000 ppm. Urinalysis and blood chemistry were
not affected by the treatment, while a tendency was noted at 8000 ppm
of an increase in haemoglobin concentration and white and red blood
cell counts. Gross Pathology appeared normal and at histopathological
examination degeneration of testicular tissue was found at 4000 and
8000 ppm. Also spermatogenesis and oogenesis were affected at these
feeding levels. Some changes were found in the weights of different
organs relative to body or heart weight. of most significance was the
increase in absolute and relative liver weight, which was also found
in the lowest dosage group, 2000 ppm in the diet. No changes relative
to the controls were found at histological examination of livers from
the dosed groups (Hofmann and Kitsch, 1972).
Groups of Sprague-Dawley albino rats (30 male and 30 female rats
per group) were fed carbendazim at dose levels of 0, 50, 150, 450 and
1350 ppm in the diet for 13 weeks. At the conclusion of this study, 10
males and 10 females from each group were maintained on a control diet
for a recovery period of six weeks. Food consumption and behavioural
changes were examined daily and body weights weekly. Urine analysis,
haematological and blood chemistry investigations were carried out
periodically. Gross and microscopic examinations of tissues and organs
were performed at the end of the study. Food intake in females
receiving 1350 ppm was slightly depressed during recovery phase. Body
weight gains in all groups at any dose level remained closely
comparable to controls. Growth, urine analysis, blood chemistry and
haematological findings were within the normal range except females
receiving 1350 ppm who exhibited depression of total serum proteins.
Relative liver weight in males and females was significantly
increased at 1350 ppm. Absolute liver weight was elevated
significantly at 1300 ppm in females. In rats allowed a recovery
period of six weeks, no treatment-related differences in relative or
absolute liver weight was observed. Gross and microscopic examinations
of tissues and organs did not show any consistent finding at any dose
level (Hunter et al., 1973b).
Groups of rats (20 male and 20 female rats per group) were fed
carbendazim at dose levels of 0, 80, 400, 2000 and 10 000 ppm in the
diet for 93 days. Food consumption and behaviour were examined daily
and body weight gain was measured weekly. Haematological examinations,
blood chemistry and urinalyses were perfumed at 0, 45 and 92 days. At
the conclusion of the study, half of the animals were sacrificed and
the remaining animals were maintained on normal feed for two weeks
after which they were sacrificed. Gross and microscopic examinations
were made on tissues and organs. A minor reduction in growth occurred
at 10 000 ppm and mortality was noted at this level. There was no
effect on food consumption at any dose level. There were no effects on
behaviour, and haematological and urinalyses values were normal. Blood
chemistry was normal except that, at the conclusion of the study,
there appeared to be a slight increase of uric acid in the blood at 10
000 ppm in both males and females. Gross examination of the tissues
and organs of females sacrificed immediately at the conclusion of the
feeding study indicated an increase in the absolute liver weight. The
absolute liver weight of males was increased at the two highest dose
levels only. There was a slight increase in liver to body weight ratio
in females sacrificed 12 days after the feedings study ended. This was
not reflected in males.
The increase in absolute liver weight observed in females at 80
ppm which did not begin to occur until 2000 ppm in males appears to be
the most sensitive parameter noted in the study. As this effect was
not observed to influence the liver to body weight ratio, except at
dietary concentrations of 400 ppm and above, the no-effect level in
rats was 80 ppm. Gross pathology was not consistently different from
the controls at any dose level. At histological examination no lesions
were demonstrated to be a result of feeding carbendazim at levels
including 10 000 ppm. No histological changes of the liver tissue were
found (Scholz et al., 1973).
Dog. Groups of dogs (three male and three female beagle dogs per
group) were fed carbendazim in the diet at dose levels of 0, 500, 1500
and 4500 ppm for three months. Haematological examinations, blood
chemistry and urinalyses were performed at 0, 6 and 12 weeks. There
were no signs of poisoning observed in the study and animals showed no
differences in behavioural response. A reduction in body weight was
observed at 4500 ppm. Clinical chemistry and haematological values
were normal. An increase in absolute and relative liver weight in
males was noted at 4500 ppm and in females at 1500 ppm. The relative
weight of adrenals was slightly increased in males at 1500 ppm. The
heart weight was significantly reduced in females at 4500 ppm.
Histological examination of the liver in the 4500 ppm dose group
revealed perivenous infiltrates and zones of asymmetric proliferation
in the periportal areas or in the liver parenchyma with liver cell
regeneration and local hyperaemia. These changes together with signs
of broncho pneumonia were considered to be of infectious origin and
not related to the carbendazim treatment. No other tissues showed any
adverse histological effects. A slight and not significant effect on
the liver weight of female dogs of the level 500 ppm of carbendazim in
the diet is considered marginal, but cannot be completely dismissed on
the basis of examination of three animals (Kirsch et al., 1973).
Long-term studies
None available.
Comments
Carbendazim, methyl 2-benzimidazole carbamate, is a fungicide of
low acute toxicity. Its fate in animals is not known and needs to be
clarified.
Limited studies on mice and rats have indicated no mutagenic
potential or adverse effect on the male sex organs although high
levels resulted in testicular damage. Data is not available on the
effect of carbendazim on reproduction or on its teratogenic potential.
In short-term studies in the rat and the dog an increase in the liver
weight was the most significant Pinding. This effect was marginal at
80 ppm in the rat and at 500 ppm in the dog and was substantial at 400
ppm and at 1500 ppm in the rat and dog respectively. No histological
changes were seen in the liver. Because of the observed effect on the
liver and as no long-term or reproduction studies were available, an
acceptable daily intake for man was not established.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Carbendazim, or MBC, was introduced as a commercial fungicide in
1972 and has through 1972-1973 obtained registration and approval for
specified pre-harvest treatments in a number of countries in
temperate, subtropical and tropical regions (see Table 1). It is
available as wettable powder formulations or as dispersions containing
60 and 20% active ingredient, respectively.
Carbendazim is generally recognized as the chemical entity, which
is mainly responsible for the fungitoxic activity of some other
systemic benzimidazole fungicides, including benomyl and
thiophanate-methyl. From these, MBC is formed as the major metabolite
in and on plant material. Accordingly, the antifungal effects of
carbendazim are described as practically similar to the two mentioned
chemicals, i.e. it is a broad-spectrum, systemic fungicide which is
active against moulds, rots and blight. It is claimed effective
against apple scab, powdery mildew, botrytis and Penicillim induced
decay of citrus fruits.
TABLE 1. INFORMATION ON REGISTERED AND APPROVED USES OF
CARBENDAZIM (SEPTEMBER 1973)
Countries in which Registered for following crops
registered
Australia stone fruit, strawberries, apples,
pears, peanuts, cucumbers
Austria apples, cucumbers, grapes, lettuce
Colombia bananas, coffee, rice
Costa Rica tropical crops, grapes, vegetables,
ornamentals, hops
El Salvador coffee, vegetables, cotton
England apples, blackcurrants, beans,
gooseberries, tomatoes, cucumbers,
ornamentals, bulbs, grapes
Ecuador bananas, rice
France grapes, cereals, fruit
Guatemala coffee, vegetables, cotton
Holland bulbs
Morocco bananas, pineapples
New Zealand apples, citrus, roses, stone fruit
Spain stone fruit, ornamentals, vegetables,
pome fruit
South Africa cotton, tobacco, vegetables
Switzerland strawberries
Uruguay vegetables, grapes, sugar beet, fruit
Residues resulting from supervised trials
Carbendazim residue data from pre-and post-harvest uses in
different countries covering a range of single or repeated
applications on fruits and vegetables has been presented by the
manufacturers (Hoechst, 1973). A summarized list of residue ranges
extracted from these data are presented in Table 2.
Generally, most sprayings and dipping procedures tend to leave
residues, the level of which is primarily governed by the applied
dosages (concentration of active ingredient and number of treatments).
The decrease in carbendazim residue levels with time seems consistent
with a normal growth dilution effect following application, while
chemical and/or physical losses seem to be of lesser importance,
justifying the classification of carbendazim as a relatively
persistent fungicide.
Relatively stable deposits of carbendazim remain after the
dipping of apples, citrus and bananas. In these fruits the residues
are mostly concentrated on the peels. Citrus fruits, however,
regularly show MBC residues penetrating into the edible inner parts of
fruit in concentration ranges up to about one tenth of the
corresponding peel concentrations.
Fate of residues
Plant material
Gorbach et al. (1973) showed that MBC after foliar application to
cucumber plants under greenhouse conditions mostly remained on the
leaf surfaces. Only about 10% penetrated into the plant tissue. A
translocation to other parts of the plant thereafter was hardly
detectable (about 0.1% of the applied amount), but the dominant route
of transportation was with the transpiration stream, i.e. towards the
leaf edges.
Solel and Edgington (1973) and others showed that plant
penetration after foliar applications and movement through the cuticle
is less for MBC than for bonomyl. They found, however, a definite
translocation (1.11-1.68% of the applied amount) of MBC to roots,
stems and shoots, when the application rates were 5-10 times higher
than those used in normal practice (Solel et al., 1973). They suggest
that a downstream movement in treated plants is insignificant at lower
concentrations.
MBC is more readily absorbed into plants through the roots
(Gorbach et al., 1973). Seven days after adding 14C-carbendazim to a
hydroponic-culture of cucumber plants up to 16 ppm was found in the
plant material. Of these, 95% were present as MBC. The remaining 5% of
radio-active material behaved like a single substance on TLC,
concluded to be 2-aminobenzimidazole (2-AB).
TABLE 2. RESIDUES OF CARBENDAZIM IN VARIOUS CROPS FROM PRE- AND POST-HARVEST TREATMENTS
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Apples 50-60 g/100 l Dipping 1.1-2.4 (27) Germany
100-500 g/ha 4-11 × 0.3-1.9 (53) Germany
600-900 g/ha 7-11 × 0.2-2.1 (27) Germany
1200-2400 g/ha 9-15 × 1.6-8.4 (36) Germany
490 g/ha 3 2.2-3.6 (12) Netherlands
825 /h. 3 2.1-4.9 (12) Netherlands
Bananas 5-20 g/100 l Dipping Int.: n.d.-0.2 (8) Ivory Coast
Peel: 0.5-2.0 (8) Ivory Coast
15-50 g/100 1 Storage Int.: n.d.-0.2 (35) Costa Rica
Spray Peel: 0.1-0.9 (35) Costa Rica
Barley 300-500 g/100 kg Seed n.d.-n.d. (10) Germany
treatment
Celery 450-1200 g/ha 7 × n.d-2.0 (12) England
Cherries 3-4 g/tree 2 × n.d.-0.14 (6) Germany
Coffee 660-1380 g/h. 1-7 n.d.-n.d. (5) Kenya
(raw beans)
Cucumber 250-500 g/ha. 2 × n.d.-0.35 (28) Gernany
Gherkins 1000 g/ha 1 × 0.3-1.5 (8) Netherlands
(under glass)
Dwarf beans 450-1200 g/ha. 1 n.d-0.1 (6) England
Gooseberries 720-900 g/ha. 4 1.6-2.2 (5) England
Grapes 100-1120 g/ha. 4-10 0.3-9.3 (57) Germany
Lemon 250 g/1100 l Dipping Int: 0.1-0.2 (13) Israel
TABLE 2. (Cont'd.)
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Peel: 4.3-12.7 (13) Israel
Mandarins 25-75 g/100 l Dipping Int: 0.2-1.2 (16) Spain
Peel: 2.1-10.5 (16) Spain
Oranges 23-75 g/100 l Dipping Int: n.d-1.4 (27) Spain
Peel. 0.2-3.7 (27) Spain
250 g/100 l Dipping Int.: 0.1-0.7 (24) Israel
Peel: 4.0-16.0 (24) Israel
600 g/ha 1 × Int: n.d. (2) South Africa
Peel: n.d. (2) South Africa
Peaches 400 g/h. 4 0.2-1.1 (12) Germany
Strawberries 375 g/ha. 4-7 × n.d.-18a (32) Netherlands
600-2160 g/h. 2-3 × n.d.-1.2b (30) Germany
Sugar beets 90-180 g/ha 2-3 Leaves: 0.1-4.8 (12) Germany
Beets: n.d. (12) Germany
Tomatoes 0.026-0.053 g/plant 4-8 × 0.5-1.4c (22) Netherlands
600-660 g/ha
Winter wheat 125-480 g/ha 1 × Straw (0-14 days): 3.4 (16) Germany
Straw (21-136 days): <0.1 (16) Germany
Grains: n.d. (4) Germary
Melons 0.01 g/plant 3 × Int.: n.d. Netherlands
Peel: 0.6-0.8 (3) Netherlands
Mushroom 6 kg/ha 1 × 0.5 (1) Netherlands
a Sampled at 0-14 days after last application.
b Sampled at 9-39 days after last application.
c Surface residues only. Interior: not detectable.
n.d. - not detected = < 40.05 ppm.
Carbendazim treatment of sugar beets by spraying gives
significant deposits of MBC on the leaves, but uptake of active
material by the beet roots is not detectable (Anon., 1973).
Animal tissues
Studies of the metabolic degradation of MBC after direct feeding
to animals has not been Presented, but by analogy to the available
evidence on metabolism of benomyl it is anticipated that hydroxylation
may take place to produce 5-hydroxymethyl-2-benzimidazolecarbamate,
followed by urinary excretion as glucuronide or sulfate conjugates
(Gorbach, 1973).
Soil
Carbendazim is a relatively stable compound and it is known to
show some persistence in soil (Gorbach, 1973). Only data on low dosage
treatments (90-180 g/ha) of sugar beets in the soil are presently
available and these indicate no detectable residues (i.e. <0.01 ppm).
Methods of residue analysis
So far the most reliable analytical methods for the determination
of benomyl are based on the conversion to MBC and eventual
saponification to 2-aminobenzimidazole (Pease and Gardiner, 1969;
Pease and Holt, 1971). These methods can easily be adapted for the
determination of MBC.
Details of U.V.-spectrophotometric methods used by the
manufacturers of MBC have been presented (BASF, 1972; Gorbach, 1972).
MBC is extracted from the slightly alkaline sample material with ethyl
acetate. The ethyl acetate solution is extracted into 1 N sulfuric or
hydrochloric acid and again transferred into ethyl acetate after pH
adjustment. This procedure is repeated and MBC is finally partitioned
into 1 N acid solution for spectrophotometric measurement at 281 nm.
The detection limit of the method is given as 0.05 ppm and recoveries
are 70-80% for various crops containing 0.1-0.5 ppm MBC.
National tolerances
According to the information available to the meeting, the
following tolerances for carbendazim have been established:
Australia Apples and pears 2 ppm
Cucumbers 0.5 ppm
Peanut kernels 0.1 ppm
Federal Republic Citrus fruit 10 ppm
of Germany Grapes 3.0 ppm
(MBC residues Berries 2.0 ppm
calculated as Vegetables (except cucumbers),
benomyl) bananas, citrus fruits
(without peel), stone fruit 1.0 ppm
Cucumbers, cereals 0.5 ppm
Bananas (without peel) 0.2 ppm
Other plant produce 0.1 ppm
Netherlands Fruit and vegetables 2 ppm
Raw cereals (provisional) 0.5 ppm
Appraisal
Carbendazim is recognized as one of the major fungitoxic
principles of the systemic benzimidazole fungicides, benmyi and
thiophanate-methyl. Marketed as a fungicide on its own merits, it has
obtained official registration in various countries and it is claimed
to be effective in combating several fungal diseases, including apple
scab, mildew and botrytis, and is applied both pre-harvest and
post-harvest.
A fair amount of data on residue in crops following approved
foliar applications and dipping procedures has been made available to
the Joint Meeting. Data were derived from supervised trials. Some
studies involved excessive rates resulting in residue levels higher
than would be likely following good agricultural practice.
The residue levels reported generally reflect the dosages applied
and the decline of residue levels mostly follows the pattern which
characterizes growth dilution.
Carbendazim is a chemically stable and relatively persistent
fungicide which only metabolizes to a limited extent in plants and in
soil. The only detected metabolite is 2-aminobenzimidazole, which
constitutes less than 5% of the total residues in leaves.
Although direct experimental data is lacking, carbendazim is
expected to show relatively high persistence in soils. It is readily
absorbed through the root systems of plants and, accordingly, there
may be a possibility of unintentional uptake into subsequent crops.
Data, on the other hand, that indicates that carbendazim may not reach
through the soils into groundwater has been made available to the
meeting.
By analogy to other benzimidazole compounds, carbendazim may be
anticipated to metabolize in the animal into hydroxylated analogues
which may appear in meat and milk products.
Analytical methods which may be adapted for regulatory purposes
are available.
RECOMMENDATIONS
As no ADI could be established, no tolerances are recommended.
Following officially acceptable use in various countries, residues of
carbendazim can occur in the following commodities up to the levels
indicated. The guideline levels indicated below are not likely to be
exceeded when following good agricultural practice, including
pre-harvest and postharvest treatments where applicable.
For the underlined commodities, individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides of
which MBC is recognized and identified as the major metabolic and/or
chemical entity (i.e. benomyl and thiophanatemethyl).
Guideline levels ppm
Citrus fruit (whole), peaches, cherries, grapes 10
Apples, pears, strawberries, tomatoes, lettuce,
gooseberries 5
Gherkins, beans (dwarf), celery, plums 2
Mushrooms, bananas (whole) 1
Cucumbers, melons, bananas (pulp) 0.5
Raw cereals, sugar beet, coffee beans (raw) 0.11
Sugar beet tops 5
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Reproduction and teratogenicity studies.
3. Metabolism and distribution studies in several animal species.
4. Elucidation of the effect on the liver in female rats and dogs.
5. Information on the nature and level of residues in meat, milk,
and eggs, after feeding animals on crops or feedstuffs treated
with carbendazim.
1 At or about the limit of determination
Desirable
1. Further studies to define the apparent "high-level" effects on
male reproductive organs.
2. Information on the possible uptake from soils into subsequent
crops.
3. Information on residues in food in commerce.
REFERENCES
Anonymous (1973) Evaluation of carbomethoxyaminobenzimidazole for the
1973 meeting of the Working Party of Experts on Pesticide Residues, 21
November - 5 December 1973, Geneva. Documents submitted by Farbewerke
Hoechst AG and BASF Aktiengesellschaft (Unpublished)
BASF Aktiengesellschaft (1972) Landwirtschaftliche Versuchsstation
1972 Limbürgerhof. Spektralphotometrische Bestimmung von BCM. Dated
August 1972 (Unpublished)
Buchenauerp H, Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci. 4: 343-348
Clemons, G.P. and Sisler, H.D. (1969) Formation of a fungitoxic
derivative from Benlate. Phytopath. 59: 705
Clemons, G.P. and Sisler, H.D. (1971) Localization of the site of
action of a fungitoxic benomyl derivative. Pest Biochem. and Physiol.
1: 32-43
Douch, P.G.C. (1973) The metabolism of benomyl fungicide in mammals.
Xenobiotica, 3(6): 367-380
Gardiner, J.A, Brantley, R.K. and Sherman, H. (1968) Isolation and
identification of a metabolite of methyl
1-(butylcarboyl)-2-benzimidazole carbamate in rat urine. J. Agr. Food
Chem. 16: 1050-1052
Gorbach, S. (1972) Analysenmethode zur Beatimmung von 2-Carbomethoxy
aminobenzimidazole in Pflanzlichen Material. Paper presented by
Farbewerke Hoechst AG (Unpublished)
Gorbach, S. (1973) Ubersicht das Abbauverhaltens von Carbendazim (BCM)
in Tier und Pflanze (Stand August 1973). Summary report presented by
Farbowerke Hoechst AG (Unpublished)
Gorbach, S, Thier, W. and SchuIze, E,F. (1973) Untersuchungen zum
Rüchstandsverhalten und Metabolismus von Hoe 17411 OF-2-14C an
Gurkenpflanzen unter Gewächshaus bedingungen. Forschungsbericht No.
221/73 submitted by Farbewerke Hoechst AG (Unpublished)
Hammerschlag, R.S. (1973) Benomyl and methyl-2-benzimidazole carbamate
(MBO: Biochemical, cytological and chemical aspects of toxicity to
Ustilago maydis and Saccharomyces cerevesiae. Diss. Abstracts Int.
33(11): 5092B (Reviewed in Abstract in Health Aspect of Pesticides
No. 73-2456)
Hofmann, H. Th. (1971) Acute oral toxicity of methyl-2-benzimidazole
carbamate to the rat. Unpublished report submitted by BASF AG
Hofmann, H. (1972) Bericht uber die Prufung der akuten oralen oxizitdt
von 2-(methoxy-carbamoyl)-benzimidazole an der Ratte. Unpublished
report submitted by BASF AG
Hofmann, H. Th. and Kirsch, P. (1972) Bericht uber die Prufung von
2-(methoxy-carbamoyl)-benzimidazole im 28-tage-futterungsversuch an
der Ratte. Unpublished report submitted by BASF AG
Hofmann, H. Th. and Peh, J. (1973) Bericht uber die Prufung von MCB
(2-(methoxy-carbamoyl)-benzimidazole) auf mutagene Wirkung nach
intraperitonealer applikation an der mannlichen Mans. Unpublished
report submitted by BASF AG
Hunter, B., Batham, F. and Newman, A.J. (1973a) BCM oral toxicity to
rats. Daily administration for 2 weeks. Unpublished report from
Huntingdon Research Centre submitted by BASF AG
Hunter, B. Benson, H.G., Street, A.E., Heywood, R. and Newman, A.J.
(1973b) BCM toxicity to rats during dietary administration for 13
weeks followed by a recovery period of 6 weeks. Unpublished report by
Huntingdon Research Centre submitted by BASF AG
Kirsch, P., Koob, K. Freisberg, K.O. and Birnsteil, H. (1973) Bericht
über die Prufung der subchronischen - Toxizität von
2-(methoxy-carbamoyl)-benzimidazole (MCB) fur beagle-Hune bei oraler
verabreichung. Unpublished report submitted by BASF AG
Pease, H.L. and Gardiner, J. A. (1969) Fluorimetric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.F. (1971) Improved method for determining
benomyl residues. J. Assoc. Off. Agric. Chem. 54: 1399
Scholz, Schultes, D. and Bahr, Bericht (1973) über einen
subchromischen Futterungs-versuch (93 tags) von HOE 17411 OF Techn.
Wirkstoff. Unpublished report submitted by Farbewerke Hoechst AG
Scholz, and Weigand, (1972a) W 17411 = 2-carbomethoxyamino
benzimidazole toxikologischePrufung. Unpublished report submitted by
Farbewerke Hoechst AG
Scholz, and Weigand, (1972b) HOE 17411 = 2 carbomethoxyamino
benzimidazole Toxikologische Prufung, Unpublished report submitted
byFarbewerke Hoechst AG
Scholz, and Weigand, (1972) 17411 2-carbomethoxyamino benzimidazole
20% ige Oldispersion. Unpublished report submitted by Farbewerke
Hoechst AG
Scholz, and Weigand, (1973) HOE 174110 F 60 Spritzpulver
toxikologische Prufung. Unpublished report submitted by Farbewerke
Hoechst AG
Siegel, M.R. and Zabbia, A.J., jr. (1972) Distribution and metabolic
fate of the fungicide benomyl in dwarf pea. Phytopathology, 62(6):
630-633
Sims, J.J., Mee, H. and Erwin, D.C. (1969) Methyl-2-benzimidazole
carbamate, a fungitoxic compound isolated from cotton plants treated
with methyl 1-(butylearbamoyl)2-benzimidazole carbamate (benomyl).
Phytopath. 59: 1775
Solel, Z., Schooley, J.M. and Edgington, L.V. (1973) Uptake and
translocation of benomyl and carbendazim (methyl benzimidazole-2-yl
carbamate) in the symplast. Pest. Sci. 4: 713
Solel, Z. and Edgington, L.V. (1973) Transcuticular movement of 1973
fungicides. Phytopathology, 63: 505
Zeller, H.,and Kirsch, P. (1971a) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the dog. Unpublished report
submitted by BASF AG
Zeller, H. and Kirsch, P. (1971b) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the rabbit. Unpublished report
submitted by BASF AG
Clemons and Sisler (1971) have suggested that carbendazim appears
to interfere with DNA synthesis or some closely related process such
as nuclear or cell division in fungi. RNA and protein synthesis were
not affected. Hammerschlag (1973) indicated an effect of carbendazim
on mitosis, DNA synthesis and cytokinesis in two fungi species.
Certain differences in effects of benomyl and carbendazim were
attributed to the occurrence of butylisocyanate, a potential
metabolite of benomyl.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of mice (20 male mice per group) were used in a
dominant lethal study where one group was administered a dose of 1280
mg/kg carbendazim by i.p. injection and three other groups served as
controls (Hofmann and Peh, 1973). One group was a solvent control, one
an unhandled control and one a positive control. Each male mouse was
mated with three females for seven days over a period of eight weeks.
The females were sacrificed at day 18 following introduction to the
males and various reproduction parameters examined, including
implantation, total number of live and dead fetuses, and resorption
sites. There were no apparent effects as a result of MBC
administration to males except a slight growth depression during week
1. During the same week, the mutagenic index was higher in the treated
group than in the untreated controls, although not different from that
in the solvent controls. The effect was considered to be within the
normal variation. A positive control (Trenimon, 2,3,5-triethyleneimino
benzoquinone-1,4) showed definitive effects especially during the
first three weeks of the experiment. Methyl-benzimidazole carbamate
does not appear to be mutagenic to mice as evidenced by this dominant
lethal study (Hofmann and Pen, 1973).
Acute toxicity
Dermal injection of 0.01% (2 µg) to rabbits caused slight
reddening around the injection site (Scholz and Weigand, 1972). There
were no effects noted following five daily dermal applications of 500
mg to unbroken skin of rabbits. Application of 10 mg to the
conjunctival sac of rabbits caused a transient reddening. No such
effects were noted with 5 mg.
Species Sex Route LD50 Reference
(mg/kg)
Rat M & F oral > 15 000 Hofmann, 1971, 1972c;
Scholz and Weigand, 1973a
M oral > 15 000 Scholz and Weigand, 1972b
Species Sex Route LD50 Reference
(mg/kg)
F oral 14 000 Scholz and Weigand, 1972b
M i.p. 7 230 Scholz and Weigand, 1972a
F i.p. 715 000 Scholz and Weigand, 1972a
Mouse M & F i.p. > 5 000 Scholz and Weigand, 1972a;
Hofmann and Peh, 1973
Dog M & F oral > 8 000 Zeller and Kirsch, 1971a;
Scholz and Weigand, 1972a
Rabbit M & F oral > 8 000 Zeller and Kirsch, 1971b
Japanese M oral 10 996 Scholz and Weigand, 1973b
quail (sesamol)
15 595 Scholz and Weigand, 1973b
(starke-schleim)
F 5 826 Scholz and Weigand, 1973b
(sesamol)
9 813 Scholz and Weigand, 1973b
(starke-schleim)
Short-term studies
Rat. Groups of rats (10 males per group) were administered
carbendazim orally for two weeks (by gavage) at dosage levels of 0,
10, 20, 30 and 40 mg/kg per day. The object of the study was to assess
toxicity with special reference to the effect on bone marrow and the
male reproduction system. One hour before sacrifice, each animal was
injected with tritiated thymidine. At the conclusion of the study, the
following tissues were removed, organ weights were recorded and organ
to body weight ratios were calculated: adrenal glands, brain, heart,
kidney, liver, pituitary gland, spleen, testes and thyroid gland.
Microscopic examination was perfumed on the bone marrow and testes of
all rats from the control and high-dose group. Microscopic
radio-autography was used to determine bone marrow mitosis. Bone
marrow was dissected, fixed and processed in paraffin wax. Sections
were mounted on microscope slides and exposed to Kodak stripping film.
After 20 days, the slides were developed, fixed and counted as well as
examined following staining with haematoxylin.
There were no effects noted in behaviour, mortality, food
consumption and body weight gain. On gross examination of tissues, it
was observed that the absolute and relative liver weight of rats at
the 40 mg/kg dosage was higher than the controls. A decrease in
relative testes weight, although significant, did not appear to be
dose related and did not appear to be significant in this study,
especially as there were no histological abnormalities observed in
testes. Bone marrow examinations in both control and treated animals
were within normal limits and showed no significant abnormalities
either histologically or on radio-autography of the bone marrow cells.
This study indicates that a dosage level of 30 mg/kg per day
administered orally for two weeks would be a no-effect level with an
effect noted at 40 mg/kg per day with regard to liver to body weight
ratio (Hunter et al., 1973a).
Groups of Sprague-Dawley strain rats (10 male and 10 female per
group) were fed carbendazim in the diet at dosage levels of 0, 2000,
4000 and 8000 ppm for 28 days. Haematological examination, clinical
chemistry and urinalyses were performed at three weeks of testing.
Gross and microscopic examination of tissues and organs was Performed
at the end of the feeding study. Food consumption and growth were
depressed at 4000 and 8000 ppm. Urinalysis and blood chemistry were
not affected by the treatment, while a tendency was noted at 8000 ppm
of an increase in haemoglobin concentration and white and red blood
cell counts. Gross Pathology appeared normal and at histopathological
examination degeneration of testicular tissue was found at 4000 and
8000 ppm. Also spermatogenesis and oogenesis were affected at these
feeding levels. Some changes were found in the weights of different
organs relative to body or heart weight. of most significance was the
increase in absolute and relative liver weight, which was also found
in the lowest dosage group, 2000 ppm in the diet. No changes relative
to the controls were found at histological examination of livers from
the dosed groups (Hofmann and Kitsch, 1972).
Groups of Sprague-Dawley albino rats (30 male and 30 female rats
per group) were fed carbendazim at dose levels of 0, 50, 150, 450 and
1350 ppm in the diet for 13 weeks. At the conclusion of this study, 10
males and 10 females from each group were maintained on a control diet
for a recovery period of six weeks. Food consumption and behavioural
changes were examined daily and body weights weekly. Urine analysis,
haematological and blood chemistry investigations were carried out
periodically. Gross and microscopic examinations of tissues and organs
were performed at the end of the study. Food intake in females
receiving 1350 ppm was slightly depressed during recovery phase. Body
weight gains in all groups at any dose level remained closely
comparable to controls. Growth, urine analysis, blood chemistry and
haematological findings were within the normal range except females
receiving 1350 ppm who exhibited depression of total serum proteins.
Relative liver weight in males and females was significantly
increased at 1350 ppm. Absolute liver weight was elevated
significantly at 1300 ppm in females. In rats allowed a recovery
period of six weeks, no treatment-related differences in relative or
absolute liver weight was observed. Gross and microscopic examinations
of tissues and organs did not show any consistent finding at any dose
level (Hunter et al., 1973b).
Groups of rats (20 male and 20 female rats per group) were fed
carbendazim at dose levels of 0, 80, 400, 2000 and 10 000 ppm in the
diet for 93 days. Food consumption and behaviour were examined daily
and body weight gain was measured weekly. Haematological examinations,
blood chemistry and urinalyses were perfumed at 0, 45 and 92 days. At
the conclusion of the study, half of the animals were sacrificed and
the remaining animals were maintained on normal feed for two weeks
after which they were sacrificed. Gross and microscopic examinations
were made on tissues and organs. A minor reduction in growth occurred
at 10 000 ppm and mortality was noted at this level. There was no
effect on food consumption at any dose level. There were no effects on
behaviour, and haematological and urinalyses values were normal. Blood
chemistry was normal except that, at the conclusion of the study,
there appeared to be a slight increase of uric acid in the blood at 10
000 ppm in both males and females. Gross examination of the tissues
and organs of females sacrificed immediately at the conclusion of the
feeding study indicated an increase in the absolute liver weight. The
absolute liver weight of males was increased at the two highest dose
levels only. There was a slight increase in liver to body weight ratio
in females sacrificed 12 days after the feedings study ended. This was
not reflected in males.
The increase in absolute liver weight observed in females at 80
ppm which did not begin to occur until 2000 ppm in males appears to be
the most sensitive parameter noted in the study. As this effect was
not observed to influence the liver to body weight ratio, except at
dietary concentrations of 400 ppm and above, the no-effect level in
rats was 80 ppm. Gross pathology was not consistently different from
the controls at any dose level. At histological examination no lesions
were demonstrated to be a result of feeding carbendazim at levels
including 10 000 ppm. No histological changes of the liver tissue were
found (Scholz et al., 1973).
Dog. Groups of dogs (three male and three female beagle dogs per
group) were fed carbendazim in the diet at dose levels of 0, 500, 1500
and 4500 ppm for three months. Haematological examinations, blood
chemistry and urinalyses were performed at 0, 6 and 12 weeks. There
were no signs of poisoning observed in the study and animals showed no
differences in behavioural response. A reduction in body weight was
observed at 4500 ppm. Clinical chemistry and haematological values
were normal. An increase in absolute and relative liver weight in
males was noted at 4500 ppm and in females at 1500 ppm. The relative
weight of adrenals was slightly increased in males at 1500 ppm. The
heart weight was significantly reduced in females at 4500 ppm.
Histological examination of the liver in the 4500 ppm dose group
revealed perivenous infiltrates and zones of asymmetric proliferation
in the periportal areas or in the liver parenchyma with liver cell
regeneration and local hyperaemia. These changes together with signs
of broncho pneumonia were considered to be of infectious origin and
not related to the carbendazim treatment. No other tissues showed any
adverse histological effects. A slight and not significant effect on
the liver weight of female dogs of the level 500 ppm of carbendazim in
the diet is considered marginal, but cannot be completely dismissed on
the basis of examination of three animals (Kirsch et al., 1973).
Long-term studies
None available.
Comments
Carbendazim, methyl 2-benzimidazole carbamate, is a fungicide of
low acute toxicity. Its fate in animals is not known and needs to be
clarified.
Limited studies on mice and rats have indicated no mutagenic
potential or adverse effect on the male sex organs although high
levels resulted in testicular damage. Data is not available on the
effect of carbendazim on reproduction or on its teratogenic potential.
In short-term studies in the rat and the dog an increase in the liver
weight was the most significant Pinding. This effect was marginal at
80 ppm in the rat and at 500 ppm in the dog and was substantial at 400
ppm and at 1500 ppm in the rat and dog respectively. No histological
changes were seen in the liver. Because of the observed effect on the
liver and as no long-term or reproduction studies were available, an
acceptable daily intake for man was not established.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Carbendazim, or MBC, was introduced as a commercial fungicide in
1972 and has through 1972-1973 obtained registration and approval for
specified pre-harvest treatments in a number of countries in
temperate, subtropical and tropical regions (see Table 1). It is
available as wettable powder formulations or as dispersions containing
60 and 20% active ingredient, respectively.
Carbendazim is generally recognized as the chemical entity, which
is mainly responsible for the fungitoxic activity of some other
systemic benzimidazole fungicides, including benomyl and
thiophanate-methyl. From these, MBC is formed as the major metabolite
in and on plant material. Accordingly, the antifungal effects of
carbendazim are described as practically similar to the two mentioned
chemicals, i.e. it is a broad-spectrum, systemic fungicide which is
active against moulds, rots and blight. It is claimed effective
against apple scab, powdery mildew, botrytis and Penicillim induced
decay of citrus fruits.
TABLE 1. INFORMATION ON REGISTERED AND APPROVED USES OF
CARBENDAZIM (SEPTEMBER 1973)
Countries in which Registered for following crops
registered
Australia stone fruit, strawberries, apples,
pears, peanuts, cucumbers
Austria apples, cucumbers, grapes, lettuce
Colombia bananas, coffee, rice
Costa Rica tropical crops, grapes, vegetables,
ornamentals, hops
El Salvador coffee, vegetables, cotton
England apples, blackcurrants, beans,
gooseberries, tomatoes, cucumbers,
ornamentals, bulbs, grapes
Ecuador bananas, rice
France grapes, cereals, fruit
Guatemala coffee, vegetables, cotton
Holland bulbs
Morocco bananas, pineapples
New Zealand apples, citrus, roses, stone fruit
Spain stone fruit, ornamentals, vegetables,
pome fruit
South Africa cotton, tobacco, vegetables
Switzerland strawberries
Uruguay vegetables, grapes, sugar beet, fruit
Residues resulting from supervised trials
Carbendazim residue data from pre-and post-harvest uses in
different countries covering a range of single or repeated
applications on fruits and vegetables has been presented by the
manufacturers (Hoechst, 1973). A summarized list of residue ranges
extracted from these data are presented in Table 2.
Generally, most sprayings and dipping procedures tend to leave
residues, the level of which is primarily governed by the applied
dosages (concentration of active ingredient and number of treatments).
The decrease in carbendazim residue levels with time seems consistent
with a normal growth dilution effect following application, while
chemical and/or physical losses seem to be of lesser importance,
justifying the classification of carbendazim as a relatively
persistent fungicide.
Relatively stable deposits of carbendazim remain after the
dipping of apples, citrus and bananas. In these fruits the residues
are mostly concentrated on the peels. Citrus fruits, however,
regularly show MBC residues penetrating into the edible inner parts of
fruit in concentration ranges up to about one tenth of the
corresponding peel concentrations.
Fate of residues
Plant material
Gorbach et al. (1973) showed that MBC after foliar application to
cucumber plants under greenhouse conditions mostly remained on the
leaf surfaces. Only about 10% penetrated into the plant tissue. A
translocation to other parts of the plant thereafter was hardly
detectable (about 0.1% of the applied amount), but the dominant route
of transportation was with the transpiration stream, i.e. towards the
leaf edges.
Solel and Edgington (1973) and others showed that plant
penetration after foliar applications and movement through the cuticle
is less for MBC than for bonomyl. They found, however, a definite
translocation (1.11-1.68% of the applied amount) of MBC to roots,
stems and shoots, when the application rates were 5-10 times higher
than those used in normal practice (Solel et al., 1973). They suggest
that a downstream movement in treated plants is insignificant at lower
concentrations.
MBC is more readily absorbed into plants through the roots
(Gorbach et al., 1973). Seven days after adding 14C-carbendazim to a
hydroponic-culture of cucumber plants up to 16 ppm was found in the
plant material. Of these, 95% were present as MBC. The remaining 5% of
radio-active material behaved like a single substance on TLC,
concluded to be 2-aminobenzimidazole (2-AB).
TABLE 2. RESIDUES OF CARBENDAZIM IN VARIOUS CROPS FROM PRE- AND POST-HARVEST TREATMENTS
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Apples 50-60 g/100 l Dipping 1.1-2.4 (27) Germany
100-500 g/ha 4-11 × 0.3-1.9 (53) Germany
600-900 g/ha 7-11 × 0.2-2.1 (27) Germany
1200-2400 g/ha 9-15 × 1.6-8.4 (36) Germany
490 g/ha 3 2.2-3.6 (12) Netherlands
825 /h. 3 2.1-4.9 (12) Netherlands
Bananas 5-20 g/100 l Dipping Int.: n.d.-0.2 (8) Ivory Coast
Peel: 0.5-2.0 (8) Ivory Coast
15-50 g/100 1 Storage Int.: n.d.-0.2 (35) Costa Rica
Spray Peel: 0.1-0.9 (35) Costa Rica
Barley 300-500 g/100 kg Seed n.d.-n.d. (10) Germany
treatment
Celery 450-1200 g/ha 7 × n.d-2.0 (12) England
Cherries 3-4 g/tree 2 × n.d.-0.14 (6) Germany
Coffee 660-1380 g/h. 1-7 n.d.-n.d. (5) Kenya
(raw beans)
Cucumber 250-500 g/ha. 2 × n.d.-0.35 (28) Gernany
Gherkins 1000 g/ha 1 × 0.3-1.5 (8) Netherlands
(under glass)
Dwarf beans 450-1200 g/ha. 1 n.d-0.1 (6) England
Gooseberries 720-900 g/ha. 4 1.6-2.2 (5) England
Grapes 100-1120 g/ha. 4-10 0.3-9.3 (57) Germany
Lemon 250 g/1100 l Dipping Int: 0.1-0.2 (13) Israel
TABLE 2. (Cont'd.)
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Peel: 4.3-12.7 (13) Israel
Mandarins 25-75 g/100 l Dipping Int: 0.2-1.2 (16) Spain
Peel: 2.1-10.5 (16) Spain
Oranges 23-75 g/100 l Dipping Int: n.d-1.4 (27) Spain
Peel. 0.2-3.7 (27) Spain
250 g/100 l Dipping Int.: 0.1-0.7 (24) Israel
Peel: 4.0-16.0 (24) Israel
600 g/ha 1 × Int: n.d. (2) South Africa
Peel: n.d. (2) South Africa
Peaches 400 g/h. 4 0.2-1.1 (12) Germany
Strawberries 375 g/ha. 4-7 × n.d.-18a (32) Netherlands
600-2160 g/h. 2-3 × n.d.-1.2b (30) Germany
Sugar beets 90-180 g/ha 2-3 Leaves: 0.1-4.8 (12) Germany
Beets: n.d. (12) Germany
Tomatoes 0.026-0.053 g/plant 4-8 × 0.5-1.4c (22) Netherlands
600-660 g/ha
Winter wheat 125-480 g/ha 1 × Straw (0-14 days): 3.4 (16) Germany
Straw (21-136 days): <0.1 (16) Germany
Grains: n.d. (4) Germary
Melons 0.01 g/plant 3 × Int.: n.d. Netherlands
Peel: 0.6-0.8 (3) Netherlands
Mushroom 6 kg/ha 1 × 0.5 (1) Netherlands
a Sampled at 0-14 days after last application.
b Sampled at 9-39 days after last application.
c Surface residues only. Interior: not detectable.
n.d. - not detected = < 40.05 ppm.
Carbendazim treatment of sugar beets by spraying gives
significant deposits of MBC on the leaves, but uptake of active
material by the beet roots is not detectable (Anon., 1973).
Animal tissues
Studies of the metabolic degradation of MBC after direct feeding
to animals has not been Presented, but by analogy to the available
evidence on metabolism of benomyl it is anticipated that hydroxylation
may take place to produce 5-hydroxymethyl-2-benzimidazolecarbamate,
followed by urinary excretion as glucuronide or sulfate conjugates
(Gorbach, 1973).
Soil
Carbendazim is a relatively stable compound and it is known to
show some persistence in soil (Gorbach, 1973). Only data on low dosage
treatments (90-180 g/ha) of sugar beets in the soil are presently
available and these indicate no detectable residues (i.e. <0.01 ppm).
Methods of residue analysis
So far the most reliable analytical methods for the determination
of benomyl are based on the conversion to MBC and eventual
saponification to 2-aminobenzimidazole (Pease and Gardiner, 1969;
Pease and Holt, 1971). These methods can easily be adapted for the
determination of MBC.
Details of U.V.-spectrophotometric methods used by the
manufacturers of MBC have been presented (BASF, 1972; Gorbach, 1972).
MBC is extracted from the slightly alkaline sample material with ethyl
acetate. The ethyl acetate solution is extracted into 1 N sulfuric or
hydrochloric acid and again transferred into ethyl acetate after pH
adjustment. This procedure is repeated and MBC is finally partitioned
into 1 N acid solution for spectrophotometric measurement at 281 nm.
The detection limit of the method is given as 0.05 ppm and recoveries
are 70-80% for various crops containing 0.1-0.5 ppm MBC.
National tolerances
According to the information available to the meeting, the
following tolerances for carbendazim have been established:
Australia Apples and pears 2 ppm
Cucumbers 0.5 ppm
Peanut kernels 0.1 ppm
Federal Republic Citrus fruit 10 ppm
of Germany Grapes 3.0 ppm
(MBC residues Berries 2.0 ppm
calculated as Vegetables (except cucumbers),
benomyl) bananas, citrus fruits
(without peel), stone fruit 1.0 ppm
Cucumbers, cereals 0.5 ppm
Bananas (without peel) 0.2 ppm
Other plant produce 0.1 ppm
Netherlands Fruit and vegetables 2 ppm
Raw cereals (provisional) 0.5 ppm
Appraisal
Carbendazim is recognized as one of the major fungitoxic
principles of the systemic benzimidazole fungicides, benmyi and
thiophanate-methyl. Marketed as a fungicide on its own merits, it has
obtained official registration in various countries and it is claimed
to be effective in combating several fungal diseases, including apple
scab, mildew and botrytis, and is applied both pre-harvest and
post-harvest.
A fair amount of data on residue in crops following approved
foliar applications and dipping procedures has been made available to
the Joint Meeting. Data were derived from supervised trials. Some
studies involved excessive rates resulting in residue levels higher
than would be likely following good agricultural practice.
The residue levels reported generally reflect the dosages applied
and the decline of residue levels mostly follows the pattern which
characterizes growth dilution.
Carbendazim is a chemically stable and relatively persistent
fungicide which only metabolizes to a limited extent in plants and in
soil. The only detected metabolite is 2-aminobenzimidazole, which
constitutes less than 5% of the total residues in leaves.
Although direct experimental data is lacking, carbendazim is
expected to show relatively high persistence in soils. It is readily
absorbed through the root systems of plants and, accordingly, there
may be a possibility of unintentional uptake into subsequent crops.
Data, on the other hand, that indicates that carbendazim may not reach
through the soils into groundwater has been made available to the
meeting.
By analogy to other benzimidazole compounds, carbendazim may be
anticipated to metabolize in the animal into hydroxylated analogues
which may appear in meat and milk products.
Analytical methods which may be adapted for regulatory purposes
are available.
RECOMMENDATIONS
As no ADI could be established, no tolerances are recommended.
Following officially acceptable use in various countries, residues of
carbendazim can occur in the following commodities up to the levels
indicated. The guideline levels indicated below are not likely to be
exceeded when following good agricultural practice, including
pre-harvest and postharvest treatments where applicable.
For the underlined commodities, individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides of
which MBC is recognized and identified as the major metabolic and/or
chemical entity (i.e. benomyl and thiophanatemethyl).
Guideline levels ppm
Citrus fruit (whole), peaches, cherries, grapes 10
Apples, pears, strawberries, tomatoes, lettuce,
gooseberries 5
Gherkins, beans (dwarf), celery, plums 2
Mushrooms, bananas (whole) 1
Cucumbers, melons, bananas (pulp) 0.5
Raw cereals, sugar beet, coffee beans (raw) 0.11
Sugar beet tops 5
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Reproduction and teratogenicity studies.
3. Metabolism and distribution studies in several animal species.
4. Elucidation of the effect on the liver in female rats and dogs.
5. Information on the nature and level of residues in meat, milk,
and eggs, after feeding animals on crops or feedstuffs treated
with carbendazim.
1 At or about the limit of determination
Desirable
1. Further studies to define the apparent "high-level" effects on
male reproductive organs.
2. Information on the possible uptake from soils into subsequent
crops.
3. Information on residues in food in commerce.
REFERENCES
Anonymous (1973) Evaluation of carbomethoxyaminobenzimidazole for the
1973 meeting of the Working Party of Experts on Pesticide Residues, 21
November - 5 December 1973, Geneva. Documents submitted by Farbewerke
Hoechst AG and BASF Aktiengesellschaft (Unpublished)
BASF Aktiengesellschaft (1972) Landwirtschaftliche Versuchsstation
1972 Limbürgerhof. Spektralphotometrische Bestimmung von BCM. Dated
August 1972 (Unpublished)
Buchenauerp H, Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci. 4: 343-348
Clemons, G.P. and Sisler, H.D. (1969) Formation of a fungitoxic
derivative from Benlate. Phytopath. 59: 705
Clemons, G.P. and Sisler, H.D. (1971) Localization of the site of
action of a fungitoxic benomyl derivative. Pest Biochem. and Physiol.
1: 32-43
Douch, P.G.C. (1973) The metabolism of benomyl fungicide in mammals.
Xenobiotica, 3(6): 367-380
Gardiner, J.A, Brantley, R.K. and Sherman, H. (1968) Isolation and
identification of a metabolite of methyl
1-(butylcarboyl)-2-benzimidazole carbamate in rat urine. J. Agr. Food
Chem. 16: 1050-1052
Gorbach, S. (1972) Analysenmethode zur Beatimmung von 2-Carbomethoxy
aminobenzimidazole in Pflanzlichen Material. Paper presented by
Farbewerke Hoechst AG (Unpublished)
Gorbach, S. (1973) Ubersicht das Abbauverhaltens von Carbendazim (BCM)
in Tier und Pflanze (Stand August 1973). Summary report presented by
Farbowerke Hoechst AG (Unpublished)
Gorbach, S, Thier, W. and SchuIze, E,F. (1973) Untersuchungen zum
Rüchstandsverhalten und Metabolismus von Hoe 17411 OF-2-14C an
Gurkenpflanzen unter Gewächshaus bedingungen. Forschungsbericht No.
221/73 submitted by Farbewerke Hoechst AG (Unpublished)
Hammerschlag, R.S. (1973) Benomyl and methyl-2-benzimidazole carbamate
(MBO: Biochemical, cytological and chemical aspects of toxicity to
Ustilago maydis and Saccharomyces cerevesiae. Diss. Abstracts Int.
33(11): 5092B (Reviewed in Abstract in Health Aspect of Pesticides
No. 73-2456)
Hofmann, H. Th. (1971) Acute oral toxicity of methyl-2-benzimidazole
carbamate to the rat. Unpublished report submitted by BASF AG
Hofmann, H. (1972) Bericht uber die Prufung der akuten oralen oxizitdt
von 2-(methoxy-carbamoyl)-benzimidazole an der Ratte. Unpublished
report submitted by BASF AG
Hofmann, H. Th. and Kirsch, P. (1972) Bericht uber die Prufung von
2-(methoxy-carbamoyl)-benzimidazole im 28-tage-futterungsversuch an
der Ratte. Unpublished report submitted by BASF AG
Hofmann, H. Th. and Peh, J. (1973) Bericht uber die Prufung von MCB
(2-(methoxy-carbamoyl)-benzimidazole) auf mutagene Wirkung nach
intraperitonealer applikation an der mannlichen Mans. Unpublished
report submitted by BASF AG
Hunter, B., Batham, F. and Newman, A.J. (1973a) BCM oral toxicity to
rats. Daily administration for 2 weeks. Unpublished report from
Huntingdon Research Centre submitted by BASF AG
Hunter, B. Benson, H.G., Street, A.E., Heywood, R. and Newman, A.J.
(1973b) BCM toxicity to rats during dietary administration for 13
weeks followed by a recovery period of 6 weeks. Unpublished report by
Huntingdon Research Centre submitted by BASF AG
Kirsch, P., Koob, K. Freisberg, K.O. and Birnsteil, H. (1973) Bericht
über die Prufung der subchronischen - Toxizität von
2-(methoxy-carbamoyl)-benzimidazole (MCB) fur beagle-Hune bei oraler
verabreichung. Unpublished report submitted by BASF AG
Pease, H.L. and Gardiner, J. A. (1969) Fluorimetric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.F. (1971) Improved method for determining
benomyl residues. J. Assoc. Off. Agric. Chem. 54: 1399
Scholz, Schultes, D. and Bahr, Bericht (1973) über einen
subchromischen Futterungs-versuch (93 tags) von HOE 17411 OF Techn.
Wirkstoff. Unpublished report submitted by Farbewerke Hoechst AG
Scholz, and Weigand, (1972a) W 17411 = 2-carbomethoxyamino
benzimidazole toxikologischePrufung. Unpublished report submitted by
Farbewerke Hoechst AG
Scholz, and Weigand, (1972b) HOE 17411 = 2 carbomethoxyamino
benzimidazole Toxikologische Prufung, Unpublished report submitted
byFarbewerke Hoechst AG
Scholz, and Weigand, (1972) 17411 2-carbomethoxyamino benzimidazole
20% ige Oldispersion. Unpublished report submitted by Farbewerke
Hoechst AG
Scholz, and Weigand, (1973) HOE 174110 F 60 Spritzpulver
toxikologische Prufung. Unpublished report submitted by Farbewerke
Hoechst AG
Siegel, M.R. and Zabbia, A.J., jr. (1972) Distribution and metabolic
fate of the fungicide benomyl in dwarf pea. Phytopathology, 62(6):
630-633
Sims, J.J., Mee, H. and Erwin, D.C. (1969) Methyl-2-benzimidazole
carbamate, a fungitoxic compound isolated from cotton plants treated
with methyl 1-(butylearbamoyl)2-benzimidazole carbamate (benomyl).
Phytopath. 59: 1775
Solel, Z., Schooley, J.M. and Edgington, L.V. (1973) Uptake and
translocation of benomyl and carbendazim (methyl benzimidazole-2-yl
carbamate) in the symplast. Pest. Sci. 4: 713
Solel, Z. and Edgington, L.V. (1973) Transcuticular movement of 1973
fungicides. Phytopathology, 63: 505
Zeller, H.,and Kirsch, P. (1971a) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the dog. Unpublished report
submitted by BASF AG
Zeller, H. and Kirsch, P. (1971b) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the rabbit. Unpublished report
submitted by BASF AG
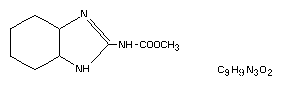 Other information on identity and properties
Molecular weight: 191.2
State: light grey powder
Melting point: 307-312°C (decomposition)
Vapour pressure: 0.1 × 10-6 mmHg (20°C)
Solubility: Soluble in acetic acid and
dimethyl-formamide
Water, pH 4: 28 ppm
Water, pH 7: 8 ppm
Water, pH 8: 7 ppm
Hexane: 0.5 ppm
Benzene: 36 ppm
Methylenechloride: 68 ppm
Ethanol: 300 ppm
Acetone: 300 ppm
Chloroform: 100 ppm
Stability: Stable in acidic and alkaline solutions
at room temperature and when exposed to
light. Stable in storage at 35-50°C
Purity of
technical product: Better than 98%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Methyl-2-benzimidazole carbamate has been proposed as an
intermediate in the degradation of thiophanate and benomyl in plants.
The quantitative aspects of this conversion have not been fully
reported although it appears that, while carbendazim is a major
metabolite, there may only be limited conversion of the two parent
molecules (Douch, 1973). Sims et al. (1969) found only carbendazim but
not benomyl four weeks after treatment of cotton. In aqueous solution
benomyl and thiophanate methyl were reported to convert rapidly to
carbendazim (Clemons and Sisler, 1969; Buchenauer and Edgington,
1973). In pea plant treated with benomyl, only carbendazim was
recovered as a plant residue (Siegel and Zabbia, 1972). In aqueous
solution on glass following irradiation by ultra-violet or sunlight,
conversion of both thiophanate and thiophanate methyl to
ethyl-2-benzimidazole carbamate and carbendazim was effected. There
are no data available on the metabolism of carbendazim in either
mammals or plants.
Metabolism of benomyl in mammals also results in the occurrence
of 5-hydroxy carbendazim and other products (Douch, 1973; Gardiner et
al., 1968). This might arise directly from benomyl by oxidation and
hydrolysis or by hydrolysis of benomyl to carbendazim followed by
oxidation. Carbendazim is the major metabolite of benomyl in plants. A
minor metabolite from benomyl is believed to be 2-amino benzimidazole.
The probable metabolic pathway of carbendazim based upon data
derived from thiophanate and benomyl conversion is suggested as
follows:
Other information on identity and properties
Molecular weight: 191.2
State: light grey powder
Melting point: 307-312°C (decomposition)
Vapour pressure: 0.1 × 10-6 mmHg (20°C)
Solubility: Soluble in acetic acid and
dimethyl-formamide
Water, pH 4: 28 ppm
Water, pH 7: 8 ppm
Water, pH 8: 7 ppm
Hexane: 0.5 ppm
Benzene: 36 ppm
Methylenechloride: 68 ppm
Ethanol: 300 ppm
Acetone: 300 ppm
Chloroform: 100 ppm
Stability: Stable in acidic and alkaline solutions
at room temperature and when exposed to
light. Stable in storage at 35-50°C
Purity of
technical product: Better than 98%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Methyl-2-benzimidazole carbamate has been proposed as an
intermediate in the degradation of thiophanate and benomyl in plants.
The quantitative aspects of this conversion have not been fully
reported although it appears that, while carbendazim is a major
metabolite, there may only be limited conversion of the two parent
molecules (Douch, 1973). Sims et al. (1969) found only carbendazim but
not benomyl four weeks after treatment of cotton. In aqueous solution
benomyl and thiophanate methyl were reported to convert rapidly to
carbendazim (Clemons and Sisler, 1969; Buchenauer and Edgington,
1973). In pea plant treated with benomyl, only carbendazim was
recovered as a plant residue (Siegel and Zabbia, 1972). In aqueous
solution on glass following irradiation by ultra-violet or sunlight,
conversion of both thiophanate and thiophanate methyl to
ethyl-2-benzimidazole carbamate and carbendazim was effected. There
are no data available on the metabolism of carbendazim in either
mammals or plants.
Metabolism of benomyl in mammals also results in the occurrence
of 5-hydroxy carbendazim and other products (Douch, 1973; Gardiner et
al., 1968). This might arise directly from benomyl by oxidation and
hydrolysis or by hydrolysis of benomyl to carbendazim followed by
oxidation. Carbendazim is the major metabolite of benomyl in plants. A
minor metabolite from benomyl is believed to be 2-amino benzimidazole.
The probable metabolic pathway of carbendazim based upon data
derived from thiophanate and benomyl conversion is suggested as
follows:
 Clemons and Sisler (1971) have suggested that carbendazim appears
to interfere with DNA synthesis or some closely related process such
as nuclear or cell division in fungi. RNA and protein synthesis were
not affected. Hammerschlag (1973) indicated an effect of carbendazim
on mitosis, DNA synthesis and cytokinesis in two fungi species.
Certain differences in effects of benomyl and carbendazim were
attributed to the occurrence of butylisocyanate, a potential
metabolite of benomyl.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of mice (20 male mice per group) were used in a
dominant lethal study where one group was administered a dose of 1280
mg/kg carbendazim by i.p. injection and three other groups served as
controls (Hofmann and Peh, 1973). One group was a solvent control, one
an unhandled control and one a positive control. Each male mouse was
mated with three females for seven days over a period of eight weeks.
The females were sacrificed at day 18 following introduction to the
males and various reproduction parameters examined, including
implantation, total number of live and dead fetuses, and resorption
sites. There were no apparent effects as a result of MBC
administration to males except a slight growth depression during week
1. During the same week, the mutagenic index was higher in the treated
group than in the untreated controls, although not different from that
in the solvent controls. The effect was considered to be within the
normal variation. A positive control (Trenimon, 2,3,5-triethyleneimino
benzoquinone-1,4) showed definitive effects especially during the
first three weeks of the experiment. Methyl-benzimidazole carbamate
does not appear to be mutagenic to mice as evidenced by this dominant
lethal study (Hofmann and Pen, 1973).
Acute toxicity
Dermal injection of 0.01% (2 µg) to rabbits caused slight
reddening around the injection site (Scholz and Weigand, 1972). There
were no effects noted following five daily dermal applications of 500
mg to unbroken skin of rabbits. Application of 10 mg to the
conjunctival sac of rabbits caused a transient reddening. No such
effects were noted with 5 mg.
Species Sex Route LD50 Reference
(mg/kg)
Rat M & F oral > 15 000 Hofmann, 1971, 1972c;
Scholz and Weigand, 1973a
M oral > 15 000 Scholz and Weigand, 1972b
Species Sex Route LD50 Reference
(mg/kg)
F oral 14 000 Scholz and Weigand, 1972b
M i.p. 7 230 Scholz and Weigand, 1972a
F i.p. 715 000 Scholz and Weigand, 1972a
Mouse M & F i.p. > 5 000 Scholz and Weigand, 1972a;
Hofmann and Peh, 1973
Dog M & F oral > 8 000 Zeller and Kirsch, 1971a;
Scholz and Weigand, 1972a
Rabbit M & F oral > 8 000 Zeller and Kirsch, 1971b
Japanese M oral 10 996 Scholz and Weigand, 1973b
quail (sesamol)
15 595 Scholz and Weigand, 1973b
(starke-schleim)
F 5 826 Scholz and Weigand, 1973b
(sesamol)
9 813 Scholz and Weigand, 1973b
(starke-schleim)
Short-term studies
Rat. Groups of rats (10 males per group) were administered
carbendazim orally for two weeks (by gavage) at dosage levels of 0,
10, 20, 30 and 40 mg/kg per day. The object of the study was to assess
toxicity with special reference to the effect on bone marrow and the
male reproduction system. One hour before sacrifice, each animal was
injected with tritiated thymidine. At the conclusion of the study, the
following tissues were removed, organ weights were recorded and organ
to body weight ratios were calculated: adrenal glands, brain, heart,
kidney, liver, pituitary gland, spleen, testes and thyroid gland.
Microscopic examination was perfumed on the bone marrow and testes of
all rats from the control and high-dose group. Microscopic
radio-autography was used to determine bone marrow mitosis. Bone
marrow was dissected, fixed and processed in paraffin wax. Sections
were mounted on microscope slides and exposed to Kodak stripping film.
After 20 days, the slides were developed, fixed and counted as well as
examined following staining with haematoxylin.
There were no effects noted in behaviour, mortality, food
consumption and body weight gain. On gross examination of tissues, it
was observed that the absolute and relative liver weight of rats at
the 40 mg/kg dosage was higher than the controls. A decrease in
relative testes weight, although significant, did not appear to be
dose related and did not appear to be significant in this study,
especially as there were no histological abnormalities observed in
testes. Bone marrow examinations in both control and treated animals
were within normal limits and showed no significant abnormalities
either histologically or on radio-autography of the bone marrow cells.
This study indicates that a dosage level of 30 mg/kg per day
administered orally for two weeks would be a no-effect level with an
effect noted at 40 mg/kg per day with regard to liver to body weight
ratio (Hunter et al., 1973a).
Groups of Sprague-Dawley strain rats (10 male and 10 female per
group) were fed carbendazim in the diet at dosage levels of 0, 2000,
4000 and 8000 ppm for 28 days. Haematological examination, clinical
chemistry and urinalyses were performed at three weeks of testing.
Gross and microscopic examination of tissues and organs was Performed
at the end of the feeding study. Food consumption and growth were
depressed at 4000 and 8000 ppm. Urinalysis and blood chemistry were
not affected by the treatment, while a tendency was noted at 8000 ppm
of an increase in haemoglobin concentration and white and red blood
cell counts. Gross Pathology appeared normal and at histopathological
examination degeneration of testicular tissue was found at 4000 and
8000 ppm. Also spermatogenesis and oogenesis were affected at these
feeding levels. Some changes were found in the weights of different
organs relative to body or heart weight. of most significance was the
increase in absolute and relative liver weight, which was also found
in the lowest dosage group, 2000 ppm in the diet. No changes relative
to the controls were found at histological examination of livers from
the dosed groups (Hofmann and Kitsch, 1972).
Groups of Sprague-Dawley albino rats (30 male and 30 female rats
per group) were fed carbendazim at dose levels of 0, 50, 150, 450 and
1350 ppm in the diet for 13 weeks. At the conclusion of this study, 10
males and 10 females from each group were maintained on a control diet
for a recovery period of six weeks. Food consumption and behavioural
changes were examined daily and body weights weekly. Urine analysis,
haematological and blood chemistry investigations were carried out
periodically. Gross and microscopic examinations of tissues and organs
were performed at the end of the study. Food intake in females
receiving 1350 ppm was slightly depressed during recovery phase. Body
weight gains in all groups at any dose level remained closely
comparable to controls. Growth, urine analysis, blood chemistry and
haematological findings were within the normal range except females
receiving 1350 ppm who exhibited depression of total serum proteins.
Relative liver weight in males and females was significantly
increased at 1350 ppm. Absolute liver weight was elevated
significantly at 1300 ppm in females. In rats allowed a recovery
period of six weeks, no treatment-related differences in relative or
absolute liver weight was observed. Gross and microscopic examinations
of tissues and organs did not show any consistent finding at any dose
level (Hunter et al., 1973b).
Groups of rats (20 male and 20 female rats per group) were fed
carbendazim at dose levels of 0, 80, 400, 2000 and 10 000 ppm in the
diet for 93 days. Food consumption and behaviour were examined daily
and body weight gain was measured weekly. Haematological examinations,
blood chemistry and urinalyses were perfumed at 0, 45 and 92 days. At
the conclusion of the study, half of the animals were sacrificed and
the remaining animals were maintained on normal feed for two weeks
after which they were sacrificed. Gross and microscopic examinations
were made on tissues and organs. A minor reduction in growth occurred
at 10 000 ppm and mortality was noted at this level. There was no
effect on food consumption at any dose level. There were no effects on
behaviour, and haematological and urinalyses values were normal. Blood
chemistry was normal except that, at the conclusion of the study,
there appeared to be a slight increase of uric acid in the blood at 10
000 ppm in both males and females. Gross examination of the tissues
and organs of females sacrificed immediately at the conclusion of the
feeding study indicated an increase in the absolute liver weight. The
absolute liver weight of males was increased at the two highest dose
levels only. There was a slight increase in liver to body weight ratio
in females sacrificed 12 days after the feedings study ended. This was
not reflected in males.
The increase in absolute liver weight observed in females at 80
ppm which did not begin to occur until 2000 ppm in males appears to be
the most sensitive parameter noted in the study. As this effect was
not observed to influence the liver to body weight ratio, except at
dietary concentrations of 400 ppm and above, the no-effect level in
rats was 80 ppm. Gross pathology was not consistently different from
the controls at any dose level. At histological examination no lesions
were demonstrated to be a result of feeding carbendazim at levels
including 10 000 ppm. No histological changes of the liver tissue were
found (Scholz et al., 1973).
Dog. Groups of dogs (three male and three female beagle dogs per
group) were fed carbendazim in the diet at dose levels of 0, 500, 1500
and 4500 ppm for three months. Haematological examinations, blood
chemistry and urinalyses were performed at 0, 6 and 12 weeks. There
were no signs of poisoning observed in the study and animals showed no
differences in behavioural response. A reduction in body weight was
observed at 4500 ppm. Clinical chemistry and haematological values
were normal. An increase in absolute and relative liver weight in
males was noted at 4500 ppm and in females at 1500 ppm. The relative
weight of adrenals was slightly increased in males at 1500 ppm. The
heart weight was significantly reduced in females at 4500 ppm.
Histological examination of the liver in the 4500 ppm dose group
revealed perivenous infiltrates and zones of asymmetric proliferation
in the periportal areas or in the liver parenchyma with liver cell
regeneration and local hyperaemia. These changes together with signs
of broncho pneumonia were considered to be of infectious origin and
not related to the carbendazim treatment. No other tissues showed any
adverse histological effects. A slight and not significant effect on
the liver weight of female dogs of the level 500 ppm of carbendazim in
the diet is considered marginal, but cannot be completely dismissed on
the basis of examination of three animals (Kirsch et al., 1973).
Long-term studies
None available.
Comments
Carbendazim, methyl 2-benzimidazole carbamate, is a fungicide of
low acute toxicity. Its fate in animals is not known and needs to be
clarified.
Limited studies on mice and rats have indicated no mutagenic
potential or adverse effect on the male sex organs although high
levels resulted in testicular damage. Data is not available on the
effect of carbendazim on reproduction or on its teratogenic potential.
In short-term studies in the rat and the dog an increase in the liver
weight was the most significant Pinding. This effect was marginal at
80 ppm in the rat and at 500 ppm in the dog and was substantial at 400
ppm and at 1500 ppm in the rat and dog respectively. No histological
changes were seen in the liver. Because of the observed effect on the
liver and as no long-term or reproduction studies were available, an
acceptable daily intake for man was not established.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Carbendazim, or MBC, was introduced as a commercial fungicide in
1972 and has through 1972-1973 obtained registration and approval for
specified pre-harvest treatments in a number of countries in
temperate, subtropical and tropical regions (see Table 1). It is
available as wettable powder formulations or as dispersions containing
60 and 20% active ingredient, respectively.
Carbendazim is generally recognized as the chemical entity, which
is mainly responsible for the fungitoxic activity of some other
systemic benzimidazole fungicides, including benomyl and
thiophanate-methyl. From these, MBC is formed as the major metabolite
in and on plant material. Accordingly, the antifungal effects of
carbendazim are described as practically similar to the two mentioned
chemicals, i.e. it is a broad-spectrum, systemic fungicide which is
active against moulds, rots and blight. It is claimed effective
against apple scab, powdery mildew, botrytis and Penicillim induced
decay of citrus fruits.
TABLE 1. INFORMATION ON REGISTERED AND APPROVED USES OF
CARBENDAZIM (SEPTEMBER 1973)
Countries in which Registered for following crops
registered
Australia stone fruit, strawberries, apples,
pears, peanuts, cucumbers
Austria apples, cucumbers, grapes, lettuce
Colombia bananas, coffee, rice
Costa Rica tropical crops, grapes, vegetables,
ornamentals, hops
El Salvador coffee, vegetables, cotton
England apples, blackcurrants, beans,
gooseberries, tomatoes, cucumbers,
ornamentals, bulbs, grapes
Ecuador bananas, rice
France grapes, cereals, fruit
Guatemala coffee, vegetables, cotton
Holland bulbs
Morocco bananas, pineapples
New Zealand apples, citrus, roses, stone fruit
Spain stone fruit, ornamentals, vegetables,
pome fruit
South Africa cotton, tobacco, vegetables
Switzerland strawberries
Uruguay vegetables, grapes, sugar beet, fruit
Residues resulting from supervised trials
Carbendazim residue data from pre-and post-harvest uses in
different countries covering a range of single or repeated
applications on fruits and vegetables has been presented by the
manufacturers (Hoechst, 1973). A summarized list of residue ranges
extracted from these data are presented in Table 2.
Generally, most sprayings and dipping procedures tend to leave
residues, the level of which is primarily governed by the applied
dosages (concentration of active ingredient and number of treatments).
The decrease in carbendazim residue levels with time seems consistent
with a normal growth dilution effect following application, while
chemical and/or physical losses seem to be of lesser importance,
justifying the classification of carbendazim as a relatively
persistent fungicide.
Relatively stable deposits of carbendazim remain after the
dipping of apples, citrus and bananas. In these fruits the residues
are mostly concentrated on the peels. Citrus fruits, however,
regularly show MBC residues penetrating into the edible inner parts of
fruit in concentration ranges up to about one tenth of the
corresponding peel concentrations.
Fate of residues
Plant material
Gorbach et al. (1973) showed that MBC after foliar application to
cucumber plants under greenhouse conditions mostly remained on the
leaf surfaces. Only about 10% penetrated into the plant tissue. A
translocation to other parts of the plant thereafter was hardly
detectable (about 0.1% of the applied amount), but the dominant route
of transportation was with the transpiration stream, i.e. towards the
leaf edges.
Solel and Edgington (1973) and others showed that plant
penetration after foliar applications and movement through the cuticle
is less for MBC than for bonomyl. They found, however, a definite
translocation (1.11-1.68% of the applied amount) of MBC to roots,
stems and shoots, when the application rates were 5-10 times higher
than those used in normal practice (Solel et al., 1973). They suggest
that a downstream movement in treated plants is insignificant at lower
concentrations.
MBC is more readily absorbed into plants through the roots
(Gorbach et al., 1973). Seven days after adding 14C-carbendazim to a
hydroponic-culture of cucumber plants up to 16 ppm was found in the
plant material. Of these, 95% were present as MBC. The remaining 5% of
radio-active material behaved like a single substance on TLC,
concluded to be 2-aminobenzimidazole (2-AB).
TABLE 2. RESIDUES OF CARBENDAZIM IN VARIOUS CROPS FROM PRE- AND POST-HARVEST TREATMENTS
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Apples 50-60 g/100 l Dipping 1.1-2.4 (27) Germany
100-500 g/ha 4-11 × 0.3-1.9 (53) Germany
600-900 g/ha 7-11 × 0.2-2.1 (27) Germany
1200-2400 g/ha 9-15 × 1.6-8.4 (36) Germany
490 g/ha 3 2.2-3.6 (12) Netherlands
825 /h. 3 2.1-4.9 (12) Netherlands
Bananas 5-20 g/100 l Dipping Int.: n.d.-0.2 (8) Ivory Coast
Peel: 0.5-2.0 (8) Ivory Coast
15-50 g/100 1 Storage Int.: n.d.-0.2 (35) Costa Rica
Spray Peel: 0.1-0.9 (35) Costa Rica
Barley 300-500 g/100 kg Seed n.d.-n.d. (10) Germany
treatment
Celery 450-1200 g/ha 7 × n.d-2.0 (12) England
Cherries 3-4 g/tree 2 × n.d.-0.14 (6) Germany
Coffee 660-1380 g/h. 1-7 n.d.-n.d. (5) Kenya
(raw beans)
Cucumber 250-500 g/ha. 2 × n.d.-0.35 (28) Gernany
Gherkins 1000 g/ha 1 × 0.3-1.5 (8) Netherlands
(under glass)
Dwarf beans 450-1200 g/ha. 1 n.d-0.1 (6) England
Gooseberries 720-900 g/ha. 4 1.6-2.2 (5) England
Grapes 100-1120 g/ha. 4-10 0.3-9.3 (57) Germany
Lemon 250 g/1100 l Dipping Int: 0.1-0.2 (13) Israel
TABLE 2. (Cont'd.)
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Peel: 4.3-12.7 (13) Israel
Mandarins 25-75 g/100 l Dipping Int: 0.2-1.2 (16) Spain
Peel: 2.1-10.5 (16) Spain
Oranges 23-75 g/100 l Dipping Int: n.d-1.4 (27) Spain
Peel. 0.2-3.7 (27) Spain
250 g/100 l Dipping Int.: 0.1-0.7 (24) Israel
Peel: 4.0-16.0 (24) Israel
600 g/ha 1 × Int: n.d. (2) South Africa
Peel: n.d. (2) South Africa
Peaches 400 g/h. 4 0.2-1.1 (12) Germany
Strawberries 375 g/ha. 4-7 × n.d.-18a (32) Netherlands
600-2160 g/h. 2-3 × n.d.-1.2b (30) Germany
Sugar beets 90-180 g/ha 2-3 Leaves: 0.1-4.8 (12) Germany
Beets: n.d. (12) Germany
Tomatoes 0.026-0.053 g/plant 4-8 × 0.5-1.4c (22) Netherlands
600-660 g/ha
Winter wheat 125-480 g/ha 1 × Straw (0-14 days): 3.4 (16) Germany
Straw (21-136 days): <0.1 (16) Germany
Grains: n.d. (4) Germary
Melons 0.01 g/plant 3 × Int.: n.d. Netherlands
Peel: 0.6-0.8 (3) Netherlands
Mushroom 6 kg/ha 1 × 0.5 (1) Netherlands
a Sampled at 0-14 days after last application.
b Sampled at 9-39 days after last application.
c Surface residues only. Interior: not detectable.
n.d. - not detected = < 40.05 ppm.
Carbendazim treatment of sugar beets by spraying gives
significant deposits of MBC on the leaves, but uptake of active
material by the beet roots is not detectable (Anon., 1973).
Animal tissues
Studies of the metabolic degradation of MBC after direct feeding
to animals has not been Presented, but by analogy to the available
evidence on metabolism of benomyl it is anticipated that hydroxylation
may take place to produce 5-hydroxymethyl-2-benzimidazolecarbamate,
followed by urinary excretion as glucuronide or sulfate conjugates
(Gorbach, 1973).
Soil
Carbendazim is a relatively stable compound and it is known to
show some persistence in soil (Gorbach, 1973). Only data on low dosage
treatments (90-180 g/ha) of sugar beets in the soil are presently
available and these indicate no detectable residues (i.e. <0.01 ppm).
Methods of residue analysis
So far the most reliable analytical methods for the determination
of benomyl are based on the conversion to MBC and eventual
saponification to 2-aminobenzimidazole (Pease and Gardiner, 1969;
Pease and Holt, 1971). These methods can easily be adapted for the
determination of MBC.
Details of U.V.-spectrophotometric methods used by the
manufacturers of MBC have been presented (BASF, 1972; Gorbach, 1972).
MBC is extracted from the slightly alkaline sample material with ethyl
acetate. The ethyl acetate solution is extracted into 1 N sulfuric or
hydrochloric acid and again transferred into ethyl acetate after pH
adjustment. This procedure is repeated and MBC is finally partitioned
into 1 N acid solution for spectrophotometric measurement at 281 nm.
The detection limit of the method is given as 0.05 ppm and recoveries
are 70-80% for various crops containing 0.1-0.5 ppm MBC.
National tolerances
According to the information available to the meeting, the
following tolerances for carbendazim have been established:
Australia Apples and pears 2 ppm
Cucumbers 0.5 ppm
Peanut kernels 0.1 ppm
Federal Republic Citrus fruit 10 ppm
of Germany Grapes 3.0 ppm
(MBC residues Berries 2.0 ppm
calculated as Vegetables (except cucumbers),
benomyl) bananas, citrus fruits
(without peel), stone fruit 1.0 ppm
Cucumbers, cereals 0.5 ppm
Bananas (without peel) 0.2 ppm
Other plant produce 0.1 ppm
Netherlands Fruit and vegetables 2 ppm
Raw cereals (provisional) 0.5 ppm
Appraisal
Carbendazim is recognized as one of the major fungitoxic
principles of the systemic benzimidazole fungicides, benmyi and
thiophanate-methyl. Marketed as a fungicide on its own merits, it has
obtained official registration in various countries and it is claimed
to be effective in combating several fungal diseases, including apple
scab, mildew and botrytis, and is applied both pre-harvest and
post-harvest.
A fair amount of data on residue in crops following approved
foliar applications and dipping procedures has been made available to
the Joint Meeting. Data were derived from supervised trials. Some
studies involved excessive rates resulting in residue levels higher
than would be likely following good agricultural practice.
The residue levels reported generally reflect the dosages applied
and the decline of residue levels mostly follows the pattern which
characterizes growth dilution.
Carbendazim is a chemically stable and relatively persistent
fungicide which only metabolizes to a limited extent in plants and in
soil. The only detected metabolite is 2-aminobenzimidazole, which
constitutes less than 5% of the total residues in leaves.
Although direct experimental data is lacking, carbendazim is
expected to show relatively high persistence in soils. It is readily
absorbed through the root systems of plants and, accordingly, there
may be a possibility of unintentional uptake into subsequent crops.
Data, on the other hand, that indicates that carbendazim may not reach
through the soils into groundwater has been made available to the
meeting.
By analogy to other benzimidazole compounds, carbendazim may be
anticipated to metabolize in the animal into hydroxylated analogues
which may appear in meat and milk products.
Analytical methods which may be adapted for regulatory purposes
are available.
RECOMMENDATIONS
As no ADI could be established, no tolerances are recommended.
Following officially acceptable use in various countries, residues of
carbendazim can occur in the following commodities up to the levels
indicated. The guideline levels indicated below are not likely to be
exceeded when following good agricultural practice, including
pre-harvest and postharvest treatments where applicable.
For the underlined commodities, individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides of
which MBC is recognized and identified as the major metabolic and/or
chemical entity (i.e. benomyl and thiophanatemethyl).
Guideline levels ppm
Citrus fruit (whole), peaches, cherries, grapes 10
Apples, pears, strawberries, tomatoes, lettuce,
gooseberries 5
Gherkins, beans (dwarf), celery, plums 2
Mushrooms, bananas (whole) 1
Cucumbers, melons, bananas (pulp) 0.5
Raw cereals, sugar beet, coffee beans (raw) 0.11
Sugar beet tops 5
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Reproduction and teratogenicity studies.
3. Metabolism and distribution studies in several animal species.
4. Elucidation of the effect on the liver in female rats and dogs.
5. Information on the nature and level of residues in meat, milk,
and eggs, after feeding animals on crops or feedstuffs treated
with carbendazim.
1 At or about the limit of determination
Desirable
1. Further studies to define the apparent "high-level" effects on
male reproductive organs.
2. Information on the possible uptake from soils into subsequent
crops.
3. Information on residues in food in commerce.
REFERENCES
Anonymous (1973) Evaluation of carbomethoxyaminobenzimidazole for the
1973 meeting of the Working Party of Experts on Pesticide Residues, 21
November - 5 December 1973, Geneva. Documents submitted by Farbewerke
Hoechst AG and BASF Aktiengesellschaft (Unpublished)
BASF Aktiengesellschaft (1972) Landwirtschaftliche Versuchsstation
1972 Limbürgerhof. Spektralphotometrische Bestimmung von BCM. Dated
August 1972 (Unpublished)
Buchenauerp H, Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci. 4: 343-348
Clemons, G.P. and Sisler, H.D. (1969) Formation of a fungitoxic
derivative from Benlate. Phytopath. 59: 705
Clemons, G.P. and Sisler, H.D. (1971) Localization of the site of
action of a fungitoxic benomyl derivative. Pest Biochem. and Physiol.
1: 32-43
Douch, P.G.C. (1973) The metabolism of benomyl fungicide in mammals.
Xenobiotica, 3(6): 367-380
Gardiner, J.A, Brantley, R.K. and Sherman, H. (1968) Isolation and
identification of a metabolite of methyl
1-(butylcarboyl)-2-benzimidazole carbamate in rat urine. J. Agr. Food
Chem. 16: 1050-1052
Gorbach, S. (1972) Analysenmethode zur Beatimmung von 2-Carbomethoxy
aminobenzimidazole in Pflanzlichen Material. Paper presented by
Farbewerke Hoechst AG (Unpublished)
Gorbach, S. (1973) Ubersicht das Abbauverhaltens von Carbendazim (BCM)
in Tier und Pflanze (Stand August 1973). Summary report presented by
Farbowerke Hoechst AG (Unpublished)
Gorbach, S, Thier, W. and SchuIze, E,F. (1973) Untersuchungen zum
Rüchstandsverhalten und Metabolismus von Hoe 17411 OF-2-14C an
Gurkenpflanzen unter Gewächshaus bedingungen. Forschungsbericht No.
221/73 submitted by Farbewerke Hoechst AG (Unpublished)
Hammerschlag, R.S. (1973) Benomyl and methyl-2-benzimidazole carbamate
(MBO: Biochemical, cytological and chemical aspects of toxicity to
Ustilago maydis and Saccharomyces cerevesiae. Diss. Abstracts Int.
33(11): 5092B (Reviewed in Abstract in Health Aspect of Pesticides
No. 73-2456)
Hofmann, H. Th. (1971) Acute oral toxicity of methyl-2-benzimidazole
carbamate to the rat. Unpublished report submitted by BASF AG
Hofmann, H. (1972) Bericht uber die Prufung der akuten oralen oxizitdt
von 2-(methoxy-carbamoyl)-benzimidazole an der Ratte. Unpublished
report submitted by BASF AG
Hofmann, H. Th. and Kirsch, P. (1972) Bericht uber die Prufung von
2-(methoxy-carbamoyl)-benzimidazole im 28-tage-futterungsversuch an
der Ratte. Unpublished report submitted by BASF AG
Hofmann, H. Th. and Peh, J. (1973) Bericht uber die Prufung von MCB
(2-(methoxy-carbamoyl)-benzimidazole) auf mutagene Wirkung nach
intraperitonealer applikation an der mannlichen Mans. Unpublished
report submitted by BASF AG
Hunter, B., Batham, F. and Newman, A.J. (1973a) BCM oral toxicity to
rats. Daily administration for 2 weeks. Unpublished report from
Huntingdon Research Centre submitted by BASF AG
Hunter, B. Benson, H.G., Street, A.E., Heywood, R. and Newman, A.J.
(1973b) BCM toxicity to rats during dietary administration for 13
weeks followed by a recovery period of 6 weeks. Unpublished report by
Huntingdon Research Centre submitted by BASF AG
Kirsch, P., Koob, K. Freisberg, K.O. and Birnsteil, H. (1973) Bericht
über die Prufung der subchronischen - Toxizität von
2-(methoxy-carbamoyl)-benzimidazole (MCB) fur beagle-Hune bei oraler
verabreichung. Unpublished report submitted by BASF AG
Pease, H.L. and Gardiner, J. A. (1969) Fluorimetric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.F. (1971) Improved method for determining
benomyl residues. J. Assoc. Off. Agric. Chem. 54: 1399
Scholz, Schultes, D. and Bahr, Bericht (1973) über einen
subchromischen Futterungs-versuch (93 tags) von HOE 17411 OF Techn.
Wirkstoff. Unpublished report submitted by Farbewerke Hoechst AG
Scholz, and Weigand, (1972a) W 17411 = 2-carbomethoxyamino
benzimidazole toxikologischePrufung. Unpublished report submitted by
Farbewerke Hoechst AG
Scholz, and Weigand, (1972b) HOE 17411 = 2 carbomethoxyamino
benzimidazole Toxikologische Prufung, Unpublished report submitted
byFarbewerke Hoechst AG
Scholz, and Weigand, (1972) 17411 2-carbomethoxyamino benzimidazole
20% ige Oldispersion. Unpublished report submitted by Farbewerke
Hoechst AG
Scholz, and Weigand, (1973) HOE 174110 F 60 Spritzpulver
toxikologische Prufung. Unpublished report submitted by Farbewerke
Hoechst AG
Siegel, M.R. and Zabbia, A.J., jr. (1972) Distribution and metabolic
fate of the fungicide benomyl in dwarf pea. Phytopathology, 62(6):
630-633
Sims, J.J., Mee, H. and Erwin, D.C. (1969) Methyl-2-benzimidazole
carbamate, a fungitoxic compound isolated from cotton plants treated
with methyl 1-(butylearbamoyl)2-benzimidazole carbamate (benomyl).
Phytopath. 59: 1775
Solel, Z., Schooley, J.M. and Edgington, L.V. (1973) Uptake and
translocation of benomyl and carbendazim (methyl benzimidazole-2-yl
carbamate) in the symplast. Pest. Sci. 4: 713
Solel, Z. and Edgington, L.V. (1973) Transcuticular movement of 1973
fungicides. Phytopathology, 63: 505
Zeller, H.,and Kirsch, P. (1971a) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the dog. Unpublished report
submitted by BASF AG
Zeller, H. and Kirsch, P. (1971b) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the rabbit. Unpublished report
submitted by BASF AG
Clemons and Sisler (1971) have suggested that carbendazim appears
to interfere with DNA synthesis or some closely related process such
as nuclear or cell division in fungi. RNA and protein synthesis were
not affected. Hammerschlag (1973) indicated an effect of carbendazim
on mitosis, DNA synthesis and cytokinesis in two fungi species.
Certain differences in effects of benomyl and carbendazim were
attributed to the occurrence of butylisocyanate, a potential
metabolite of benomyl.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Mouse. Groups of mice (20 male mice per group) were used in a
dominant lethal study where one group was administered a dose of 1280
mg/kg carbendazim by i.p. injection and three other groups served as
controls (Hofmann and Peh, 1973). One group was a solvent control, one
an unhandled control and one a positive control. Each male mouse was
mated with three females for seven days over a period of eight weeks.
The females were sacrificed at day 18 following introduction to the
males and various reproduction parameters examined, including
implantation, total number of live and dead fetuses, and resorption
sites. There were no apparent effects as a result of MBC
administration to males except a slight growth depression during week
1. During the same week, the mutagenic index was higher in the treated
group than in the untreated controls, although not different from that
in the solvent controls. The effect was considered to be within the
normal variation. A positive control (Trenimon, 2,3,5-triethyleneimino
benzoquinone-1,4) showed definitive effects especially during the
first three weeks of the experiment. Methyl-benzimidazole carbamate
does not appear to be mutagenic to mice as evidenced by this dominant
lethal study (Hofmann and Pen, 1973).
Acute toxicity
Dermal injection of 0.01% (2 µg) to rabbits caused slight
reddening around the injection site (Scholz and Weigand, 1972). There
were no effects noted following five daily dermal applications of 500
mg to unbroken skin of rabbits. Application of 10 mg to the
conjunctival sac of rabbits caused a transient reddening. No such
effects were noted with 5 mg.
Species Sex Route LD50 Reference
(mg/kg)
Rat M & F oral > 15 000 Hofmann, 1971, 1972c;
Scholz and Weigand, 1973a
M oral > 15 000 Scholz and Weigand, 1972b
Species Sex Route LD50 Reference
(mg/kg)
F oral 14 000 Scholz and Weigand, 1972b
M i.p. 7 230 Scholz and Weigand, 1972a
F i.p. 715 000 Scholz and Weigand, 1972a
Mouse M & F i.p. > 5 000 Scholz and Weigand, 1972a;
Hofmann and Peh, 1973
Dog M & F oral > 8 000 Zeller and Kirsch, 1971a;
Scholz and Weigand, 1972a
Rabbit M & F oral > 8 000 Zeller and Kirsch, 1971b
Japanese M oral 10 996 Scholz and Weigand, 1973b
quail (sesamol)
15 595 Scholz and Weigand, 1973b
(starke-schleim)
F 5 826 Scholz and Weigand, 1973b
(sesamol)
9 813 Scholz and Weigand, 1973b
(starke-schleim)
Short-term studies
Rat. Groups of rats (10 males per group) were administered
carbendazim orally for two weeks (by gavage) at dosage levels of 0,
10, 20, 30 and 40 mg/kg per day. The object of the study was to assess
toxicity with special reference to the effect on bone marrow and the
male reproduction system. One hour before sacrifice, each animal was
injected with tritiated thymidine. At the conclusion of the study, the
following tissues were removed, organ weights were recorded and organ
to body weight ratios were calculated: adrenal glands, brain, heart,
kidney, liver, pituitary gland, spleen, testes and thyroid gland.
Microscopic examination was perfumed on the bone marrow and testes of
all rats from the control and high-dose group. Microscopic
radio-autography was used to determine bone marrow mitosis. Bone
marrow was dissected, fixed and processed in paraffin wax. Sections
were mounted on microscope slides and exposed to Kodak stripping film.
After 20 days, the slides were developed, fixed and counted as well as
examined following staining with haematoxylin.
There were no effects noted in behaviour, mortality, food
consumption and body weight gain. On gross examination of tissues, it
was observed that the absolute and relative liver weight of rats at
the 40 mg/kg dosage was higher than the controls. A decrease in
relative testes weight, although significant, did not appear to be
dose related and did not appear to be significant in this study,
especially as there were no histological abnormalities observed in
testes. Bone marrow examinations in both control and treated animals
were within normal limits and showed no significant abnormalities
either histologically or on radio-autography of the bone marrow cells.
This study indicates that a dosage level of 30 mg/kg per day
administered orally for two weeks would be a no-effect level with an
effect noted at 40 mg/kg per day with regard to liver to body weight
ratio (Hunter et al., 1973a).
Groups of Sprague-Dawley strain rats (10 male and 10 female per
group) were fed carbendazim in the diet at dosage levels of 0, 2000,
4000 and 8000 ppm for 28 days. Haematological examination, clinical
chemistry and urinalyses were performed at three weeks of testing.
Gross and microscopic examination of tissues and organs was Performed
at the end of the feeding study. Food consumption and growth were
depressed at 4000 and 8000 ppm. Urinalysis and blood chemistry were
not affected by the treatment, while a tendency was noted at 8000 ppm
of an increase in haemoglobin concentration and white and red blood
cell counts. Gross Pathology appeared normal and at histopathological
examination degeneration of testicular tissue was found at 4000 and
8000 ppm. Also spermatogenesis and oogenesis were affected at these
feeding levels. Some changes were found in the weights of different
organs relative to body or heart weight. of most significance was the
increase in absolute and relative liver weight, which was also found
in the lowest dosage group, 2000 ppm in the diet. No changes relative
to the controls were found at histological examination of livers from
the dosed groups (Hofmann and Kitsch, 1972).
Groups of Sprague-Dawley albino rats (30 male and 30 female rats
per group) were fed carbendazim at dose levels of 0, 50, 150, 450 and
1350 ppm in the diet for 13 weeks. At the conclusion of this study, 10
males and 10 females from each group were maintained on a control diet
for a recovery period of six weeks. Food consumption and behavioural
changes were examined daily and body weights weekly. Urine analysis,
haematological and blood chemistry investigations were carried out
periodically. Gross and microscopic examinations of tissues and organs
were performed at the end of the study. Food intake in females
receiving 1350 ppm was slightly depressed during recovery phase. Body
weight gains in all groups at any dose level remained closely
comparable to controls. Growth, urine analysis, blood chemistry and
haematological findings were within the normal range except females
receiving 1350 ppm who exhibited depression of total serum proteins.
Relative liver weight in males and females was significantly
increased at 1350 ppm. Absolute liver weight was elevated
significantly at 1300 ppm in females. In rats allowed a recovery
period of six weeks, no treatment-related differences in relative or
absolute liver weight was observed. Gross and microscopic examinations
of tissues and organs did not show any consistent finding at any dose
level (Hunter et al., 1973b).
Groups of rats (20 male and 20 female rats per group) were fed
carbendazim at dose levels of 0, 80, 400, 2000 and 10 000 ppm in the
diet for 93 days. Food consumption and behaviour were examined daily
and body weight gain was measured weekly. Haematological examinations,
blood chemistry and urinalyses were perfumed at 0, 45 and 92 days. At
the conclusion of the study, half of the animals were sacrificed and
the remaining animals were maintained on normal feed for two weeks
after which they were sacrificed. Gross and microscopic examinations
were made on tissues and organs. A minor reduction in growth occurred
at 10 000 ppm and mortality was noted at this level. There was no
effect on food consumption at any dose level. There were no effects on
behaviour, and haematological and urinalyses values were normal. Blood
chemistry was normal except that, at the conclusion of the study,
there appeared to be a slight increase of uric acid in the blood at 10
000 ppm in both males and females. Gross examination of the tissues
and organs of females sacrificed immediately at the conclusion of the
feeding study indicated an increase in the absolute liver weight. The
absolute liver weight of males was increased at the two highest dose
levels only. There was a slight increase in liver to body weight ratio
in females sacrificed 12 days after the feedings study ended. This was
not reflected in males.
The increase in absolute liver weight observed in females at 80
ppm which did not begin to occur until 2000 ppm in males appears to be
the most sensitive parameter noted in the study. As this effect was
not observed to influence the liver to body weight ratio, except at
dietary concentrations of 400 ppm and above, the no-effect level in
rats was 80 ppm. Gross pathology was not consistently different from
the controls at any dose level. At histological examination no lesions
were demonstrated to be a result of feeding carbendazim at levels
including 10 000 ppm. No histological changes of the liver tissue were
found (Scholz et al., 1973).
Dog. Groups of dogs (three male and three female beagle dogs per
group) were fed carbendazim in the diet at dose levels of 0, 500, 1500
and 4500 ppm for three months. Haematological examinations, blood
chemistry and urinalyses were performed at 0, 6 and 12 weeks. There
were no signs of poisoning observed in the study and animals showed no
differences in behavioural response. A reduction in body weight was
observed at 4500 ppm. Clinical chemistry and haematological values
were normal. An increase in absolute and relative liver weight in
males was noted at 4500 ppm and in females at 1500 ppm. The relative
weight of adrenals was slightly increased in males at 1500 ppm. The
heart weight was significantly reduced in females at 4500 ppm.
Histological examination of the liver in the 4500 ppm dose group
revealed perivenous infiltrates and zones of asymmetric proliferation
in the periportal areas or in the liver parenchyma with liver cell
regeneration and local hyperaemia. These changes together with signs
of broncho pneumonia were considered to be of infectious origin and
not related to the carbendazim treatment. No other tissues showed any
adverse histological effects. A slight and not significant effect on
the liver weight of female dogs of the level 500 ppm of carbendazim in
the diet is considered marginal, but cannot be completely dismissed on
the basis of examination of three animals (Kirsch et al., 1973).
Long-term studies
None available.
Comments
Carbendazim, methyl 2-benzimidazole carbamate, is a fungicide of
low acute toxicity. Its fate in animals is not known and needs to be
clarified.
Limited studies on mice and rats have indicated no mutagenic
potential or adverse effect on the male sex organs although high
levels resulted in testicular damage. Data is not available on the
effect of carbendazim on reproduction or on its teratogenic potential.
In short-term studies in the rat and the dog an increase in the liver
weight was the most significant Pinding. This effect was marginal at
80 ppm in the rat and at 500 ppm in the dog and was substantial at 400
ppm and at 1500 ppm in the rat and dog respectively. No histological
changes were seen in the liver. Because of the observed effect on the
liver and as no long-term or reproduction studies were available, an
acceptable daily intake for man was not established.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Carbendazim, or MBC, was introduced as a commercial fungicide in
1972 and has through 1972-1973 obtained registration and approval for
specified pre-harvest treatments in a number of countries in
temperate, subtropical and tropical regions (see Table 1). It is
available as wettable powder formulations or as dispersions containing
60 and 20% active ingredient, respectively.
Carbendazim is generally recognized as the chemical entity, which
is mainly responsible for the fungitoxic activity of some other
systemic benzimidazole fungicides, including benomyl and
thiophanate-methyl. From these, MBC is formed as the major metabolite
in and on plant material. Accordingly, the antifungal effects of
carbendazim are described as practically similar to the two mentioned
chemicals, i.e. it is a broad-spectrum, systemic fungicide which is
active against moulds, rots and blight. It is claimed effective
against apple scab, powdery mildew, botrytis and Penicillim induced
decay of citrus fruits.
TABLE 1. INFORMATION ON REGISTERED AND APPROVED USES OF
CARBENDAZIM (SEPTEMBER 1973)
Countries in which Registered for following crops
registered
Australia stone fruit, strawberries, apples,
pears, peanuts, cucumbers
Austria apples, cucumbers, grapes, lettuce
Colombia bananas, coffee, rice
Costa Rica tropical crops, grapes, vegetables,
ornamentals, hops
El Salvador coffee, vegetables, cotton
England apples, blackcurrants, beans,
gooseberries, tomatoes, cucumbers,
ornamentals, bulbs, grapes
Ecuador bananas, rice
France grapes, cereals, fruit
Guatemala coffee, vegetables, cotton
Holland bulbs
Morocco bananas, pineapples
New Zealand apples, citrus, roses, stone fruit
Spain stone fruit, ornamentals, vegetables,
pome fruit
South Africa cotton, tobacco, vegetables
Switzerland strawberries
Uruguay vegetables, grapes, sugar beet, fruit
Residues resulting from supervised trials
Carbendazim residue data from pre-and post-harvest uses in
different countries covering a range of single or repeated
applications on fruits and vegetables has been presented by the
manufacturers (Hoechst, 1973). A summarized list of residue ranges
extracted from these data are presented in Table 2.
Generally, most sprayings and dipping procedures tend to leave
residues, the level of which is primarily governed by the applied
dosages (concentration of active ingredient and number of treatments).
The decrease in carbendazim residue levels with time seems consistent
with a normal growth dilution effect following application, while
chemical and/or physical losses seem to be of lesser importance,
justifying the classification of carbendazim as a relatively
persistent fungicide.
Relatively stable deposits of carbendazim remain after the
dipping of apples, citrus and bananas. In these fruits the residues
are mostly concentrated on the peels. Citrus fruits, however,
regularly show MBC residues penetrating into the edible inner parts of
fruit in concentration ranges up to about one tenth of the
corresponding peel concentrations.
Fate of residues
Plant material
Gorbach et al. (1973) showed that MBC after foliar application to
cucumber plants under greenhouse conditions mostly remained on the
leaf surfaces. Only about 10% penetrated into the plant tissue. A
translocation to other parts of the plant thereafter was hardly
detectable (about 0.1% of the applied amount), but the dominant route
of transportation was with the transpiration stream, i.e. towards the
leaf edges.
Solel and Edgington (1973) and others showed that plant
penetration after foliar applications and movement through the cuticle
is less for MBC than for bonomyl. They found, however, a definite
translocation (1.11-1.68% of the applied amount) of MBC to roots,
stems and shoots, when the application rates were 5-10 times higher
than those used in normal practice (Solel et al., 1973). They suggest
that a downstream movement in treated plants is insignificant at lower
concentrations.
MBC is more readily absorbed into plants through the roots
(Gorbach et al., 1973). Seven days after adding 14C-carbendazim to a
hydroponic-culture of cucumber plants up to 16 ppm was found in the
plant material. Of these, 95% were present as MBC. The remaining 5% of
radio-active material behaved like a single substance on TLC,
concluded to be 2-aminobenzimidazole (2-AB).
TABLE 2. RESIDUES OF CARBENDAZIM IN VARIOUS CROPS FROM PRE- AND POST-HARVEST TREATMENTS
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Apples 50-60 g/100 l Dipping 1.1-2.4 (27) Germany
100-500 g/ha 4-11 × 0.3-1.9 (53) Germany
600-900 g/ha 7-11 × 0.2-2.1 (27) Germany
1200-2400 g/ha 9-15 × 1.6-8.4 (36) Germany
490 g/ha 3 2.2-3.6 (12) Netherlands
825 /h. 3 2.1-4.9 (12) Netherlands
Bananas 5-20 g/100 l Dipping Int.: n.d.-0.2 (8) Ivory Coast
Peel: 0.5-2.0 (8) Ivory Coast
15-50 g/100 1 Storage Int.: n.d.-0.2 (35) Costa Rica
Spray Peel: 0.1-0.9 (35) Costa Rica
Barley 300-500 g/100 kg Seed n.d.-n.d. (10) Germany
treatment
Celery 450-1200 g/ha 7 × n.d-2.0 (12) England
Cherries 3-4 g/tree 2 × n.d.-0.14 (6) Germany
Coffee 660-1380 g/h. 1-7 n.d.-n.d. (5) Kenya
(raw beans)
Cucumber 250-500 g/ha. 2 × n.d.-0.35 (28) Gernany
Gherkins 1000 g/ha 1 × 0.3-1.5 (8) Netherlands
(under glass)
Dwarf beans 450-1200 g/ha. 1 n.d-0.1 (6) England
Gooseberries 720-900 g/ha. 4 1.6-2.2 (5) England
Grapes 100-1120 g/ha. 4-10 0.3-9.3 (57) Germany
Lemon 250 g/1100 l Dipping Int: 0.1-0.2 (13) Israel
TABLE 2. (Cont'd.)
Application MBC-residues (ppm)
Crop Country
Rate Frequency Range (number)
Peel: 4.3-12.7 (13) Israel
Mandarins 25-75 g/100 l Dipping Int: 0.2-1.2 (16) Spain
Peel: 2.1-10.5 (16) Spain
Oranges 23-75 g/100 l Dipping Int: n.d-1.4 (27) Spain
Peel. 0.2-3.7 (27) Spain
250 g/100 l Dipping Int.: 0.1-0.7 (24) Israel
Peel: 4.0-16.0 (24) Israel
600 g/ha 1 × Int: n.d. (2) South Africa
Peel: n.d. (2) South Africa
Peaches 400 g/h. 4 0.2-1.1 (12) Germany
Strawberries 375 g/ha. 4-7 × n.d.-18a (32) Netherlands
600-2160 g/h. 2-3 × n.d.-1.2b (30) Germany
Sugar beets 90-180 g/ha 2-3 Leaves: 0.1-4.8 (12) Germany
Beets: n.d. (12) Germany
Tomatoes 0.026-0.053 g/plant 4-8 × 0.5-1.4c (22) Netherlands
600-660 g/ha
Winter wheat 125-480 g/ha 1 × Straw (0-14 days): 3.4 (16) Germany
Straw (21-136 days): <0.1 (16) Germany
Grains: n.d. (4) Germary
Melons 0.01 g/plant 3 × Int.: n.d. Netherlands
Peel: 0.6-0.8 (3) Netherlands
Mushroom 6 kg/ha 1 × 0.5 (1) Netherlands
a Sampled at 0-14 days after last application.
b Sampled at 9-39 days after last application.
c Surface residues only. Interior: not detectable.
n.d. - not detected = < 40.05 ppm.
Carbendazim treatment of sugar beets by spraying gives
significant deposits of MBC on the leaves, but uptake of active
material by the beet roots is not detectable (Anon., 1973).
Animal tissues
Studies of the metabolic degradation of MBC after direct feeding
to animals has not been Presented, but by analogy to the available
evidence on metabolism of benomyl it is anticipated that hydroxylation
may take place to produce 5-hydroxymethyl-2-benzimidazolecarbamate,
followed by urinary excretion as glucuronide or sulfate conjugates
(Gorbach, 1973).
Soil
Carbendazim is a relatively stable compound and it is known to
show some persistence in soil (Gorbach, 1973). Only data on low dosage
treatments (90-180 g/ha) of sugar beets in the soil are presently
available and these indicate no detectable residues (i.e. <0.01 ppm).
Methods of residue analysis
So far the most reliable analytical methods for the determination
of benomyl are based on the conversion to MBC and eventual
saponification to 2-aminobenzimidazole (Pease and Gardiner, 1969;
Pease and Holt, 1971). These methods can easily be adapted for the
determination of MBC.
Details of U.V.-spectrophotometric methods used by the
manufacturers of MBC have been presented (BASF, 1972; Gorbach, 1972).
MBC is extracted from the slightly alkaline sample material with ethyl
acetate. The ethyl acetate solution is extracted into 1 N sulfuric or
hydrochloric acid and again transferred into ethyl acetate after pH
adjustment. This procedure is repeated and MBC is finally partitioned
into 1 N acid solution for spectrophotometric measurement at 281 nm.
The detection limit of the method is given as 0.05 ppm and recoveries
are 70-80% for various crops containing 0.1-0.5 ppm MBC.
National tolerances
According to the information available to the meeting, the
following tolerances for carbendazim have been established:
Australia Apples and pears 2 ppm
Cucumbers 0.5 ppm
Peanut kernels 0.1 ppm
Federal Republic Citrus fruit 10 ppm
of Germany Grapes 3.0 ppm
(MBC residues Berries 2.0 ppm
calculated as Vegetables (except cucumbers),
benomyl) bananas, citrus fruits
(without peel), stone fruit 1.0 ppm
Cucumbers, cereals 0.5 ppm
Bananas (without peel) 0.2 ppm
Other plant produce 0.1 ppm
Netherlands Fruit and vegetables 2 ppm
Raw cereals (provisional) 0.5 ppm
Appraisal
Carbendazim is recognized as one of the major fungitoxic
principles of the systemic benzimidazole fungicides, benmyi and
thiophanate-methyl. Marketed as a fungicide on its own merits, it has
obtained official registration in various countries and it is claimed
to be effective in combating several fungal diseases, including apple
scab, mildew and botrytis, and is applied both pre-harvest and
post-harvest.
A fair amount of data on residue in crops following approved
foliar applications and dipping procedures has been made available to
the Joint Meeting. Data were derived from supervised trials. Some
studies involved excessive rates resulting in residue levels higher
than would be likely following good agricultural practice.
The residue levels reported generally reflect the dosages applied
and the decline of residue levels mostly follows the pattern which
characterizes growth dilution.
Carbendazim is a chemically stable and relatively persistent
fungicide which only metabolizes to a limited extent in plants and in
soil. The only detected metabolite is 2-aminobenzimidazole, which
constitutes less than 5% of the total residues in leaves.
Although direct experimental data is lacking, carbendazim is
expected to show relatively high persistence in soils. It is readily
absorbed through the root systems of plants and, accordingly, there
may be a possibility of unintentional uptake into subsequent crops.
Data, on the other hand, that indicates that carbendazim may not reach
through the soils into groundwater has been made available to the
meeting.
By analogy to other benzimidazole compounds, carbendazim may be
anticipated to metabolize in the animal into hydroxylated analogues
which may appear in meat and milk products.
Analytical methods which may be adapted for regulatory purposes
are available.
RECOMMENDATIONS
As no ADI could be established, no tolerances are recommended.
Following officially acceptable use in various countries, residues of
carbendazim can occur in the following commodities up to the levels
indicated. The guideline levels indicated below are not likely to be
exceeded when following good agricultural practice, including
pre-harvest and postharvest treatments where applicable.
For the underlined commodities, individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides of
which MBC is recognized and identified as the major metabolic and/or
chemical entity (i.e. benomyl and thiophanatemethyl).
Guideline levels ppm
Citrus fruit (whole), peaches, cherries, grapes 10
Apples, pears, strawberries, tomatoes, lettuce,
gooseberries 5
Gherkins, beans (dwarf), celery, plums 2
Mushrooms, bananas (whole) 1
Cucumbers, melons, bananas (pulp) 0.5
Raw cereals, sugar beet, coffee beans (raw) 0.11
Sugar beet tops 5
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Long-term studies to investigate chronic toxicity and
carcinogenicity.
2. Reproduction and teratogenicity studies.
3. Metabolism and distribution studies in several animal species.
4. Elucidation of the effect on the liver in female rats and dogs.
5. Information on the nature and level of residues in meat, milk,
and eggs, after feeding animals on crops or feedstuffs treated
with carbendazim.
1 At or about the limit of determination
Desirable
1. Further studies to define the apparent "high-level" effects on
male reproductive organs.
2. Information on the possible uptake from soils into subsequent
crops.
3. Information on residues in food in commerce.
REFERENCES
Anonymous (1973) Evaluation of carbomethoxyaminobenzimidazole for the
1973 meeting of the Working Party of Experts on Pesticide Residues, 21
November - 5 December 1973, Geneva. Documents submitted by Farbewerke
Hoechst AG and BASF Aktiengesellschaft (Unpublished)
BASF Aktiengesellschaft (1972) Landwirtschaftliche Versuchsstation
1972 Limbürgerhof. Spektralphotometrische Bestimmung von BCM. Dated
August 1972 (Unpublished)
Buchenauerp H, Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci. 4: 343-348
Clemons, G.P. and Sisler, H.D. (1969) Formation of a fungitoxic
derivative from Benlate. Phytopath. 59: 705
Clemons, G.P. and Sisler, H.D. (1971) Localization of the site of
action of a fungitoxic benomyl derivative. Pest Biochem. and Physiol.
1: 32-43
Douch, P.G.C. (1973) The metabolism of benomyl fungicide in mammals.
Xenobiotica, 3(6): 367-380
Gardiner, J.A, Brantley, R.K. and Sherman, H. (1968) Isolation and
identification of a metabolite of methyl
1-(butylcarboyl)-2-benzimidazole carbamate in rat urine. J. Agr. Food
Chem. 16: 1050-1052
Gorbach, S. (1972) Analysenmethode zur Beatimmung von 2-Carbomethoxy
aminobenzimidazole in Pflanzlichen Material. Paper presented by
Farbewerke Hoechst AG (Unpublished)
Gorbach, S. (1973) Ubersicht das Abbauverhaltens von Carbendazim (BCM)
in Tier und Pflanze (Stand August 1973). Summary report presented by
Farbowerke Hoechst AG (Unpublished)
Gorbach, S, Thier, W. and SchuIze, E,F. (1973) Untersuchungen zum
Rüchstandsverhalten und Metabolismus von Hoe 17411 OF-2-14C an
Gurkenpflanzen unter Gewächshaus bedingungen. Forschungsbericht No.
221/73 submitted by Farbewerke Hoechst AG (Unpublished)
Hammerschlag, R.S. (1973) Benomyl and methyl-2-benzimidazole carbamate
(MBO: Biochemical, cytological and chemical aspects of toxicity to
Ustilago maydis and Saccharomyces cerevesiae. Diss. Abstracts Int.
33(11): 5092B (Reviewed in Abstract in Health Aspect of Pesticides
No. 73-2456)
Hofmann, H. Th. (1971) Acute oral toxicity of methyl-2-benzimidazole
carbamate to the rat. Unpublished report submitted by BASF AG
Hofmann, H. (1972) Bericht uber die Prufung der akuten oralen oxizitdt
von 2-(methoxy-carbamoyl)-benzimidazole an der Ratte. Unpublished
report submitted by BASF AG
Hofmann, H. Th. and Kirsch, P. (1972) Bericht uber die Prufung von
2-(methoxy-carbamoyl)-benzimidazole im 28-tage-futterungsversuch an
der Ratte. Unpublished report submitted by BASF AG
Hofmann, H. Th. and Peh, J. (1973) Bericht uber die Prufung von MCB
(2-(methoxy-carbamoyl)-benzimidazole) auf mutagene Wirkung nach
intraperitonealer applikation an der mannlichen Mans. Unpublished
report submitted by BASF AG
Hunter, B., Batham, F. and Newman, A.J. (1973a) BCM oral toxicity to
rats. Daily administration for 2 weeks. Unpublished report from
Huntingdon Research Centre submitted by BASF AG
Hunter, B. Benson, H.G., Street, A.E., Heywood, R. and Newman, A.J.
(1973b) BCM toxicity to rats during dietary administration for 13
weeks followed by a recovery period of 6 weeks. Unpublished report by
Huntingdon Research Centre submitted by BASF AG
Kirsch, P., Koob, K. Freisberg, K.O. and Birnsteil, H. (1973) Bericht
über die Prufung der subchronischen - Toxizität von
2-(methoxy-carbamoyl)-benzimidazole (MCB) fur beagle-Hune bei oraler
verabreichung. Unpublished report submitted by BASF AG
Pease, H.L. and Gardiner, J. A. (1969) Fluorimetric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.F. (1971) Improved method for determining
benomyl residues. J. Assoc. Off. Agric. Chem. 54: 1399
Scholz, Schultes, D. and Bahr, Bericht (1973) über einen
subchromischen Futterungs-versuch (93 tags) von HOE 17411 OF Techn.
Wirkstoff. Unpublished report submitted by Farbewerke Hoechst AG
Scholz, and Weigand, (1972a) W 17411 = 2-carbomethoxyamino
benzimidazole toxikologischePrufung. Unpublished report submitted by
Farbewerke Hoechst AG
Scholz, and Weigand, (1972b) HOE 17411 = 2 carbomethoxyamino
benzimidazole Toxikologische Prufung, Unpublished report submitted
byFarbewerke Hoechst AG
Scholz, and Weigand, (1972) 17411 2-carbomethoxyamino benzimidazole
20% ige Oldispersion. Unpublished report submitted by Farbewerke
Hoechst AG
Scholz, and Weigand, (1973) HOE 174110 F 60 Spritzpulver
toxikologische Prufung. Unpublished report submitted by Farbewerke
Hoechst AG
Siegel, M.R. and Zabbia, A.J., jr. (1972) Distribution and metabolic
fate of the fungicide benomyl in dwarf pea. Phytopathology, 62(6):
630-633
Sims, J.J., Mee, H. and Erwin, D.C. (1969) Methyl-2-benzimidazole
carbamate, a fungitoxic compound isolated from cotton plants treated
with methyl 1-(butylearbamoyl)2-benzimidazole carbamate (benomyl).
Phytopath. 59: 1775
Solel, Z., Schooley, J.M. and Edgington, L.V. (1973) Uptake and
translocation of benomyl and carbendazim (methyl benzimidazole-2-yl
carbamate) in the symplast. Pest. Sci. 4: 713
Solel, Z. and Edgington, L.V. (1973) Transcuticular movement of 1973
fungicides. Phytopathology, 63: 505
Zeller, H.,and Kirsch, P. (1971a) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the dog. Unpublished report
submitted by BASF AG
Zeller, H. and Kirsch, P. (1971b) Acute oral toxicity of
methyl-2-benzimidazole carbamate to the rabbit. Unpublished report
submitted by BASF AG
