
CARBENDAZIM
First draft prepared by
M. Watson
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and
Food, Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily retake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Hormonal effects and spermatogenesis
Observations in humans
Medical surveillance of workers
Studies of volunteers
Comments
Toxicological evaluation
References
Explanation
Carbendazim was previously evaluated toxicologically by the Joint
Meeting in 1973, 1977, 1983, and 1985 (Annex I, references 20, 28, 40,
and 44). In 1983, an ADI of 0-0.01 mg/kg bw was established on the
basis of a review of data on the toxicity of carbendazim and benomyl
and incorporating a higher-than-normal safety factor in view of the
paucity of data on individual animals in many studies, This ADI was
confirmed in 1985, when additional data were reviewed, but concern
remained due to the absence of individual data.
The compound was reviewed by the present Meeting within the CCPR
periodic review programme, with particular attention to the recent WHO
Environmental Health Criteria monograph on carbendazim (EHC 149). This
monograph summarizes new data on carbendazim and data that were not
previously reviewed and includes relevant data from the previous
monographs.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
14C-Carbendazim administered by gavage to rats at 2 mg/kg bw
per day for 10 consecutive days was cleared from the blood rapidly,
and 59% of the radiolabel was excreted in the urine and 36% in the
faeces. Elimination was biphasic, with a rapid rate during the first
three days and a slower phase thereafter. Residues in the liver
represented 0.3% of the administered dose seven days after the last
administration and 0.08% after 14 days. The levels in blood and organs
other than the liver (kidney, fat, muscle, and gonads) did not exceed
(0.03% of the administered dose after seven days (Christ & Kellner,
1973).
After oral administration of 3 mg/kg bw 14C-carbendazim to
rats, an average Cmax of 1.03 mg/ml was attained in blood within
15-40 min. A dose 100 times higher resulted in a disproportionally
lower Cmax of 16 mg/ml 0.4-4 h after treatment. Excretion occurred
almost exclusively in the urine, irrespective of sex and dose; only
about 1% of the administered dose was found in the faeces. Mice had a
Cmax similar to that seen in rats after an oral dose of 3 mg/kg bw,
but the Cmax at a dose of 300 mg/kg bw was higher than that in rats
(36-53 mg/ml). Faecal excretion was higher in mice than in rats,
representing 10-27% of the administered dose. Pretreatment with
unlabelled carbendazim had no effect on the excretory pattern in
either species. The excretory organs contained the highest tissue
concentrations; those in the gonads were near or below the blood
concentrations (Kellner & Eckert, 1983).
These distribution patterns were confirmed in rats and mice by
whole-body autoradiography after intravenous and oral administration
of 3 mg/kg bw [2-14C]-carbendazim. The radioactivity was almost
completely excreted within 24 h after treatment (Kellner, 1983).
Male albino rats were given a single oral dose of 12 mg/kg bw
14C-carbendazim as a solution in diethyl glycol-ethanol. Urinary
excretion of 14C-carbendazim and two of its metabolites indicated
that about 85% had been absorbed. Rats were also given a single dose
of 12 mg/kg bw 14C-carbendazim as a solution in diethyl glycol-
ethanol by intravenous injection. The highest concentrations of
radiolabel were found in kidney and the lowest in blood; elimination
followed the kinetics of a two-compartment model. By 12 h, only small
quantities of radiolabel were present in blood, liver, and kidney
(Krechniak & Klosowska, 1986).
Three groups of five rats of each sex were given [phenyl(U)-
14C]-carbendazim by gavage: one group received a single dose of
50 mg/kg bw; the second received a single dose of 50 mg/kg bw after 14
days of pretreatment with 50 mg/kg bw per day unlabelled carbendazim;
and the third received a single dose of 1000 mg/kg bw labelled
carbendazim. In all groups, > 98% of the recovered radiolabel had
been excreted in the urine or faeces by the time of sacrifice 72 h
after treatment Urinary excretion accounted for 62-66% of the dose in
males and 54-62% of the dose in females at the low dose with or
without pretreatment. In animals at the high dose, this pathway
accounted for 41% of the dose; elimination in the faeces accounted for
virtually all of the remaining radiolabel. There were no apparent
differences between male and female rats with respect to the extent of
absorption or the extent or rate of elimination of 14C-carbendazim
equivalents within each dose group. The label remaining in tissues
represented < 1% of the administered dose (Monson, 1990).
Percutaneous absorption of carbendazim is negligible. In rats
that were given 0.6 mg over 10% of the body surface, only about 0.2%
of a radiolabelled dose was excreted in urine and faeces within 24 h.
When 60 mg per rat were applied under similar conditions, only 0.03%
was excreted (Dorn & Keller, 1980).
(b) Biotransformation
In a study in which rats were treated by gavage with carbendazim
(Monson, 1990), 5-HBC-S (see Figure 1) was identified as the main
metabolite (21-43% of the dose), except in females at the high dose or
receiving pretreatment (5.5-10%); in all groups of females, 5,6-HOBC-
N-oxide was the predominant metabolite (10-19%). 5,6-DHBC-S and
5,6-DHBC-G were identified as minor metabolites. The total recovery
from faeces represented about 24% for males and 33-38% for females at
the low dose and which had been pretreated and > 60% for males and
females at the high dose. Unchanged carbendazim represented 10-15% of
the administered dose in the faeces of rats at the high dose.
NMRI mice and Wistar rats of each sex were given radiolabelled
carbendazim by gavage as single doses of 3 and 300 mg/kg bw; they were
then given repeated daily doses of unlabelled carbendazim for 28 days,
followed by a single radiolabelled dose. Urine was collected during
the first 6 h, after which time the animals were killed. Almost all
the metabolites in urine were conjugated with sulfuric acid. Cleavage
of these conjugates by ß-glucuronidase-arylsulfatase released 5-HBC as
the only metabolite extractable from water. Mouse urine contained more
compounds that remained polar after enzyme treatment than the urine of
rats. There was no sex difference. The residual content of carbendazim
in the liver was generally lower in rats that were pretreated with
unlabelled carbendazim. The results are summarized in Table 1, which
indicates that the detoxification capacity of mouse liver was
saturated at the higher dose (Dorn et al., 1983).
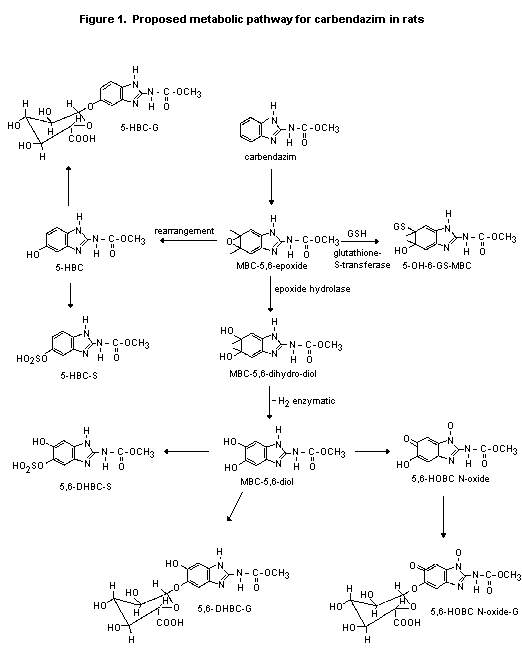 Table 1. Residual content of carbendazim in the livers of rats
Dosage Residual content (%)
Rat Mouse
Single dose
3 mg/kg bw 12 29
300 mg/kg bw 18 26
29-day repeated dose
3 mg/kg bw 2 < 2
300 mg/kg bw 4 28
In the study of Krechniak & Klosowska (1986), 94% of the measured
radiolabel in urine 12 h after treatment was as 5-HBC, 3% as 2-AB, and
3% as carbendazim.
The proposed metabolic pathway for carbendazim in rats is given
in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed for 28 days with diets containing benomyl or carbendazim at a
concentration of 0, 10, 30, 100, 300, 1000, or 3000 ppm. After
sacrifice, liver weights were recorded and microsomal epoxide
hydrolase and cytosolic glutathione- S-transferase were monitored in
subcellular fractions isolated from the liver. The mean absolute liver
weights were elevated in males and females fed 1000 or 3000 ppm
carbendazim and in females fed 300 ppm; however, the only
significantly increase was found in females fed 3000 ppm benomyl. No
apparent liver toxicity or effect on body weight was observed. Both
benomyl and carbendazim induced epoxide hydrolase in male and female
rats and mice fed 1000 or 3000 ppm, and both induced glutathione- S-
transferase at 3000 ppm. The level of induction seemed to be slightly
greater in females than males. There was no substantial difference in
enzyme induction between rats and mice (Guengerich, 1981).
In the same study, but in a separate test, CD-1 male mice were
treated by gavage with carbendazim suspended in 0, 100, or 1000 mg/kg
bw per day corn oil for five days. After sacrifice, liver samples were
homogenized, and subcellular fractions were prepared as described
above. Wet liver weights and the activities of microsomal cytochrome
P450, NADPH-cytochrome- creductase, styrene-7,8-hydrolase,
benzphetamine- N-demethylase, benzo[ a]pyrene hydroxylase,
7-ethoxycoumarin-deethylase, and cytosolic glutathione- S-transferase
were measured. The activities of styrene-7,8-hydrolase and
glutathione- S-transferase were statistically significantly increased
over the control values; that of 7-ethoxycoumarin-deethylase was
significantly decreased. It is noteworthy that the total microsomal
cytochrome P450 level did not increase, indicating that carbendazim
did not induce overall microsomal induction, even at the higher dose.
The increase in styrene-7,8-hydrolase activity shows, however, that
some hepatic microsomal enzymes are induced by carbendazim in vivo.
There appeared to be no substantial difference in enzyme induction
between rats and mice (Guengerich, 1981).
Groups of male Wistar rats and Swiss mice were given carbendazim
in the diet at levels of 0-10 000 ppm for 60 days, and the induction
of liver enzyme activities was examined and compared with that induced
by phenobarbital sodium administered in the drinking-water. Growth and
food consumption were decreased in rats at 10 000 ppm but not in mice
given up to 5000 ppm in the diet. Relative liver weights were
increased in rats fed 2000 or 10 000 ppm and in mice receiving 1000 or
5000 ppm carbendazim. Phenobarbital had similar effects. The protein
concentrations in total homogenates and post-mitochondrial fractions
of liver from rats were not affected by carbendazim, whereas they were
increased in mice at 5000 ppm. Feeding of carbendazim to rats at
2000 ppm or more resulted in slight-to-moderate induction of several
phase-I drug metabolizing enzymes: 7-ethoxycoumarin- O-deethylase,
biphenyl-4-hydroxylase, aniline hydroxylase, 4-methoxybiphenyl- N-
demethylase, and cytochrome- c-reductase. The activities of the
phase-II drug metabolizing enzymes glucuronyl transferase I and II and
the glutathione content were moderately-to-markedly increased at this
dose. Feeding of carbendazim to mice at 1000 ppm or more resulted in
moderate-to-marked increases in the activities of phase-I drug
metabolizing enzymes, including cytochrome P-450 and aminopyrine- N-
demethylase; cytochrome- c-reductase activity was decreased, and
those of glucuronyl transferase and glutathione- S-transferase and
glutathione content were slightly increased. There was no measureable
difference between rats and mice with regard to the metabolism of
carbendazim, although exhaustion of the detoxification mechanism was
more evident in mice at high doses. The detoxification and elimination
of carbendazim and its metabolites proceed more rapidly in rats than
in mice, as reflected in the increased glutathione content of rat
liver and the increased activity of phase-II enzymes (Falke et al.,
1982a,b).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
2. The clinical signs of toxicity after treatment with carbendazim
were generally nonspecific. The acute toxicity of carbendazim in a
number of species is low, LD50 values by various routes of
administration ranging from > 2000 to > 15 000 mg/kg bw. Gross and
histopathological changes were observed in the testes and epididymides
of male rats given carbendazim orally at doses of 1000 mg/kg bw and
more. The testes were small, soft, and discoloured, and more than 70%
of the tubules showed degenerative changes. The sperm count in the
epididymides examined was reduced or nil.
(b) Short-term toxicity
Rats
Groups of six male Sprague Dawley rats were given carbendazim by
gavage at a dose of 0, 200, 3400, or 5000 mg/kg bw per day, five times
per week for two weeks. Two rats at 3400 mg/kg bw per day died. At all
doses, gross and microscopic evidence of adverse effects on testes and
reduction or absence of sperm in the epididymides was seen. The testes
were small and discoloured, with tubular degeneration and evidence of
aspermatogenesis. At 3400 mg/kg bw per day, there were also
morphological changes in the duodenum (oedema and focal necrosis),
bone marrow (reduction in the blood-forming elements), and liver
(decrease in large, globular-shaped vacuoles) (Sherman, 1965; Sherman
& Krauss, 1966).
Groups of 10 male Sprague-Dawley rats were given carbendazim by
gavage at a dose of 0, 10, 20, 30, or 40 mg/kg bw per day for two
weeks. At the high dose, liver weights were increased. There were no
histopathological findings and no effects on spermatogenesis, on
cellularity, or on the incidence of mitosis, as evidenced by tritiated
thymidine incorporation 1 h before sacrifice (Hunter et al., 1973a).
Administration of 1350 ppm carbendazim to Sprague-Dawley rats for
13 weeks resulted in hepatomegaly in animals of each sex, which was
not accompanied by histopathological lesions and was reversible after
six weeks. A dietary level of 450 ppm (equal to 35 mg/kg bw per day)
was the NOAEL (Hunter et al., 1973b).
In a poorly reported study, groups of 10 male and 10 female
litter-mate weanling Wistar rats were treated by gavage with 0, 16,
32, or 64 mg/kg bw per day for 90 days. The erythrocyte counts in
treated rats were lower than those of the controls after 15 days of
exposure; however, no clear dose-response relationship was seen after
30, 60, or 90 days of exposure. Leukocyte counts were decreased after
Table 2. Acute toxicity of carbendazim and carbendazim formulations
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Carbendazim Mouse Oral > 15 000 NR Til et al. (1981)
Carbendazim Rat Oral > 10 000 > 95 Goodman & Sherman (1975)
Carbendazim Rat Oral > 11 000 > 95 Sherman (1965)
Carbendazim Rat Oral > 15 000 > 95 Kramer & Weigand (1971)
Carbendazim Rat Oral > 17 000 NR Sherman & Krauss (1966)
Carbendazim Guinea-pig Oral > 5 000 NR Dashiell (1975)
Carbendazim Rabbit Oral > 8 000 NR Zeller & Kirsch (1971)
Carbendazim Dog Oral > 5 000 NR Scholz & Weigand (1972)
Carbendazim Mouse Intraperitoneal > 15 000 NR Scholz & Weigand (1972)
Carbendazim Rat Intraperitoneal > 2 000 NR Scholz & Weigand (1972)
Carbendazim Rat Inhalation (1-h) > 5.9 NR Sarver (1975)
Carbendazim Rat Dermal > 2 000 NR Kramer & Weigand (1971)
Carbendazim Rabbit Dermal > 10 000 NR Edwards (1974a)
'75% wettable powder' Rat Oral > 5 000 NR Hinckle (1981)
'75% wettable powder' Rat Inhalation (4-h) > 5 > 95 Nash & Ferenz (1982)
'75% wettable powder' Rabbit Dermal > 2 000 > 95 Ford (1982)
'75% wettable powder' Rat Oral > 5 000 NR Grandizio & Saner (1987)
'75% wettable powder' Rabbit dermal > 2 000 > 95 Vick & Brock (1987)
NR, Not reported
15 days, and after 30 and 60 days animals of each sex showed transient
decreases in lymphocyte counts in comparison with controls, although
no clear dose-response relationship was observed among the treated
groups. No change was seen in the activity of whole-blood
cholinesterase. Male rats had significantly increased alkaline
phosphatase activity at a dose of 64 mg/kg bw per day, and blood urea
levels were lowered in males at 32 and 64 mg/kg bw per day after 90
days. Increased serum bilirubin concentrations were seen in males and
females at 32 and 64 mg/kg bw per day; these were attributable to
parenchymal cell damage, as indicated by increased alanine amino-
transferase activity. Dose-related changes in the liver ranged from
sparse infiltration by inflammatory cells to inflammatory and
degenerative changes. Tubular dilatation and hydropic degeneration
were noted in the kidneys of rats at the low dose, and fibrosis and
congestion were seen in rats at the medium and high doses. Increased
lung weights were correlated with bronchopneumonic changes. Slight
changes in weights were reported for several other organs. In view of
the lack of data on individual animals in this experiment and the
apparent variability in the results, no NOAEL could be established
(Janardhan et al., 1987).
Groups of 16 male and 16 female Sprague-Dawley rats were fed
carbendazim (purity, 72%) in the diet for 90 days at 0, 100, 500, or
2500 ppm (as carbendazim). The animals were observed daily for
behavioural changes and body weight, and food consumption was recorded
weekly. Haematological examinations were conducted on 10 male and 10
female rats in each group at 30, 60, and 90 days. Routine urinalyses
were performed on the same animals, and plasma alkaline phosphatase
and alanine aminotransferase levels were determined. After 90-98 days
of continuous feeding, 10 male and 10 female rats in each group were
killed, and selected organs were weighed; these and other organs were
also preserved for microscopic examination. The six male and six
female rats remaining in each group after terminal sacrifice were used
in a study of reproductive toxicity (see below). There were no
clinical signs of toxicity and no compound-related effects on weight
gain, food consumption, or haematological parameters. The relative
liver weights in females fed 2500 ppm were slightly increased in
comparison with control rats. Testicular weight was not affected in
any group. Microscopic examination of selected tissues and organs in
the control and high-dose groups showed no adverse effect attributable
to carbendazim. The NOAEL in this study was 500 ppm, equivalent to
50 mg/kg bw per day (Sherman, 1968).
Rabbits
Groups of six male New Zealand albino rabbits received 0 or
2000 mg/kg bw carbendazim as a 50% aqueous paste on shaved intact
dorsal skin repeatedly, for 6 h/day, for 10 consecutive days. There
were no adverse effects on body weight, clinical symptoms, organ
weights, gross pathology, or histopathology of selected organs;
however, there was focal necrosis of the epidermis and polymorpho-
nuclear cell infiltration of the dermis in five of six exposed
rabbits. No other effects were observed (Dashiell, 1975).
Dogs
Groups of four male and four female one-year-old beagle dogs were
given carbendazim (purity, 53%) in the diet for three, months at 0,
100, 500, or 2500 ppm (as carbendazim); the highest level was
subsequently reduced to 1500 ppm because of reduced food intake and
body weight. Food consumption and body weight were recorded weekly,
and clinical laboratory examinations, including haematological,
biochemical, and urinary measurements, were performed periodically. At
the end of the study, all animals were killed, selected organs were
weighed, and these and other organs were subjected to gross and
microscopic evaluations. No mortality or adverse clinical signs were
observed over the course of the study, and growth and food consumption
were normal, except in animals at 1500-2500 ppm. Urinalysis showed no
change due to treatment, and there were no dose-related effects on
haematological values. Females at the mid- and high doses showed a
trend to increased cholesterol levels over the pre-test and control
levels after one, two, and three months. The weights of the thymus
were increased in males at the low and mid-doses, and those of the
prostate at the mid-dose. Limited histopathological data did not
indicate any compound-related effects. The data for the high-dose
group were compromised by the change in dietary level, which involved
a 'recovery' period during which the animals received control diet
(Sherman, 1970).
Groups of four male and four female beagle dogs were given
carbendazim in the diet at 0, 100, 300, or 1000 ppm for 13 weeks. The
highest level was increased to 2000 ppm after six weeks of treatment.
Body weight, haematological, blood chemistry and urine measurements,
and liver and kidney function tests were performed periodically. The
animals were examined grossly and microscopically at the end of the
study. There were no reported compound-related effects on clinical
behaviour, body weight, food consumption, haematological parameters,
kidney function (phenol red excretion) or liver function (brom-
sulphthalein retention). Blood chemistry was normal, except for a
slight decrease in albumin in males at the mid- and high doses at 12
weeks. Urinalysis showed normal values, except for a high bacterial
count in females at the high dose at week 13. The blood clotting time
was slightly reduced in dogs at the high dose at week 12. There were
slight increases in relative liver and thyroid weights and a decrease
in relative heart weights in the group at the highest dose. No
microscopic changes that could be associated with treatment were
observed in these or other organs. The NOAEL was 300 ppm, equivalent
to 7.5 mg/kg bw per day, on the basis of minor changes in clinical
chemistry and organ weights (Til et al., 1972).
Groups of three male and three female beagle dogs were fed diets
containing 0, 500, 1500, or 4500 ppm carbendazim for 13 weeks. Reduced
body-weight gain, increased liver weight, hepatic periportal
infiltration, and hepatic regeneration were seen at the high dose. No
histopathological changes were seen in any other organs, including the
testes. At 1500 ppm, liver weights were increased, but there were no
histological lesions. The NOAEL was 500 ppm, equal to 12.5 mg/kg bw
per day (Hoffman & Kirsch, 1987).
Groups of five male and five female beagle dogs were fed diets
containing 0, 100, 200, or 500 ppm carbendazim for one year. The dogs
were weighed at regular intervals, and individual food consumption was
monitored throughout the study; clinical pathology was evaluated
periodically. After one year, all of the dogs were killed and selected
tissues were examined microscopically. There were no statistical
differences in mean body weight that could be attributed to treatment,
and the mean daily food consumption of treated dogs was similar to
that of controls. None of the clinical observations was attributable
to carbendazim intake. Dogs fed 500 ppm had elevated levels of serum
cholesterol, which were statistically significant for males at nine
months and for females at one and two months. There were no
microscopic lesions related to treatment. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day (Stadler, 1986).
Groups of four male and four female beagle dogs, one to two years
of age, were given carbendazim (purity, 53%) in the diet at 0, 100,
500, or 2500 ppm (as carbendazim) for two years. Food consumption and
body weights were measured weekly, and animals were examined daily for
clinical signs of toxicity. Haematological, biochemical, and urinary
examinations were performed periodically throughout the study. After
one year, one male and one female in the control and 500-ppm groups
were killed. At the end of the study, the organs were weighed, and
gross and histopathological examinations were performed. No mortality
was reported among the controls or dogs at 100 or 500 ppm; however,
three males at the high dose were sacrificed after 22 and 42 weeks
because of poor nutrition. No females at the high dose died.
Haematological and urinary values were unaffected by treatment. The
dogs at 500 ppm had increased levels of cholesterol, blood urea
nitrogen, total protein, and serum alanine aminotransferase. Swollen,
vacuolated hepatic cells and marginal proliferation of the portal
triads with cellular infiltration were observed in one dog that had
been fed 500 ppm and was sacrificed after one year. The biochemical
evidence of an effect on the liver was corroborated by the finding at
terminal sacrifice of hepatic cirrhosis, swollen, vacuolated hepatic
cells, and mild chronic hepatitis in dogs fed 500 ppm or more of
carbendazim. There were no effects on organ weights. Diffuse
testicular atrophy and aspermatogenesis were observed in two of four
males at 100 ppm but not at higher doses. As similar effects were not
seen at the other doses, these findings are considered not to be
treatment-related. The NOAEL was 100 ppm, on the basis of the effects
on the liver at 500 ppm, equivalent to 2.5 mg/kg bw per day (Sherman,
1972).
Groups of four male and four female beagle dogs, aged 22-27
weeks, were fed carbendazim in the diet at 0, 150, 300, or 2000 ppm
for 104 weeks. After 33 weeks, the dose of 2000 ppm was increased to
5000 ppm. The dogs were examined daily for clinical signs of toxicity
and altered behaviour; body weight and food consumption were recorded
regularly throughout the study, and haematological examinations, blood
chemistry (including liver and kidney function tests), and urinary
measurements were conducted periodically. After 104 weeks, the dogs
were sacrificed, and the tissues were reported to have been examined
grossly and microscopically, although the available data indicate that
the pathological examinations were inadequate. The only death was that
of a female at the high dose which was killed in a moribund state
after week 36. The body weights of males at the mid-dose and of males
and females at the high dose were decreased. Food consumption was
comparable in all groups. Blood clotting times were significantly
reduced in males at the high dose from week 13 to term, and slight
decreases were noted in females at the high dose. Serum alkaline
phosphatase activity was increased at the high dose throughout the
study, but there were no compound-related effects on serum alanine or
aspartate aminotransferase activities. All other haematological
parameters and blood chemistry were comparable with those of
the controls. There were no differences among the groups in
bromsulphthalein retention, phenol red excretion, or urinary values.
Liver and thyroid weights were significantly increased in dogs at the
high dose, but there were no microscopic changes in these organs that
were related to treatment. An increased incidence of prostatitis was
seen in high-dose males in comparison with controls (3/4 versus 1/4).
One male at that dose also had interstitial mononuclear inflammatory
cell infiltrates and atrophic tubules of the testes. Although feeding
of carbendazim in the diet to dogs for two years apparently had no
adverse effect at levels up to and including 300 ppm, the deficiencies
in the reporting of the pathological data preclude determination of an
NOAEL (Reuzel et al., 1976).
(c) Long-term toxicity and carcinogenicity
Mice
Carbendazim was administered in the diet to groups of 100 male
and 100 female specific pathogen-free Swiss mice at 0, 150, 300, or
1000 ppm for 80 weeks. The highest dose was increased to 2000 ppm at
week 4 and to 5000 ppm at week 8 for the remainder of the study.
Animals were examined for behaviour and for clinical signs of
toxicity, and body weights were measured throughout the study. All
animals were examined grossly, liver and kidney weights were recorded,
and tissues were examined microscopically. There were no compound-
related effects on general condition, mortality, or body weight. At
termination of the study, 70% of males and 80% of females were still
alive. The relative liver weights of males and females at the high
dose were significantly higher than those of controls, but kidney
weights were unchanged. After peer review and reclassification of the
data on hepatic tumour incidence, the combined incidence of
hepatocellular adenomas and carcinomas was found to have increased
with increasing doses in both males and females (Table 3). Males
showed more pronounced induction of liver tumours and more frequent
occurrence of hepatocellular carcinomas, which were often found
simultaneously with hepatocellular adenomas, whereas females usually
had only hepatocellular adenomas. It was concluded that carbendazim is
oncogenic in this strain of mouse at a dietary level of 5000 ppm
(Beems et al., 1976; Mohr, 1977).
Table 3. Hepatic rumour incidence in Swiss mice fed carbendazim
Sex Dietary level No.of mice Liver nodular Hepatocellular Hepatocellular
(ppm) examined hyperplasia adenoma carcinoma
Male 0 100 0 9 1
150 94 8 5 3
300 98 11 13 4
100/5000 100 25 14 9
Female 0 94 0 1 1
150 99 5 1 0
300 98 3 3 0
1000/5000 95 11 8 0
Groups of 80 male and 80 female CD-1 mice, aged six to seven
weeks, were given carbendazim (purity, 99%) in the diet at 0, 500,
1500, or 7500 ppm for two years. The highest dose was reduced to
3750 ppm for the males after 66 weeks because of increased mortality
(62 controls, 32 at 7500 ppm); females, however, received 7500 ppm
throughout the study. Treatment affected mortality in male mice, and
those at the high dose were sacrificed at week 73 because only 23 were
still alive. Only nine males at 1500 ppm survived to week 104, whereas
18 male controls were still alive at that time. Females had no similar
increase in mortality. There were no dose-related effects on body
weight or food consumption at any time, although the terminal body
weights of males at the low and mid-doses were lower than those of the
control and high-dose males. Clinical parameters were similar for all
treated and control groups, and haematological measures were
unaffected. Both absolute and relative thymic weights were
significantly decreased in females at 500 and 1500 ppm, but not in the
high-dose group. Absolute liver weights were increased in females at
the high dose and relative liver weights in those at the two highest
doses. The organ weights of male mice were variable, and only those of
the kidney and thymus appeared to be decreased as a result of
treatment. Absolute kidney and thymic weights were depressed in male
mice at all doses, but relative kidney and thymic weights were
significantly decreased only in males at the high dose. Histological
examination revealed dose-related changes in the thymus (lymphoid
depletion) and accumulation of yellow-brown pigment in the renal
tubules of male mice at the mid- and high doses. These mice also had
an increased frequency of sperm stasis in the testes and increased
bilateral germinal cell atrophy; there was no trend for unilateral
germinal cell atrophy, the incidence in controls being greater than or
equal to that of treated males. These effects are therefore considered
not to be compound-related. A significant hepatotoxic effect was seen
in male mice at 1500 and 7500 ppm, as demonstrated by centrilobular
hypertrophy, necrosis, and swelling. The frequency of hepatocellular
adenomas was not increased, as they occurred at equal frequency in
control and treated groups. There was a significant increase in the
incidence of hepatocellular carcinomas, but only at 1500 ppm; however,
too few males at the high dose survived to 17 months (510 days) to
support the conclusion that there is no oncogenic effect at that dose.
The combined incidence of hepatocellular carcinomas, hepatocellular
adenomas, and hepatoblastomas (Table 4) was significantly increased
(P < 0.05) in females at the low, mid-, and high doses and in males
at the mid-dose, but this was not evaluated in high-dose males because
of the high rate of mortality. The study thus shows statistically
significant increases in the incidences of hepatocellular carcinoma
for males at 1500 ppm and for females at all doses, the response being
dose-related. A dose-response relationship could not be determined
because of the high mortality in the high-dose males; the high
mortality rate in male controls also hampered interpretation of
results. No carcinogenic effect was observed in tissues other than the
liver (Wood, 1982).
Carbendazim was administered in the diet of groups of 100-120
male and female NMRKf mice for 96 weeks at a dose of 0, 50, 150, 300,
or 1000 ppm. The highest dose was increased to 2000 ppm at week 4 and
to 5000 ppm at week 8 for the remainder of the study. Animals were
examined for behaviour and general condition and for body weight, food
and water consumption, and mortality. Gross necropsy was performed on
all animals, liver and lung weights were recorded, and all organs and
tissues were examined microscopically. An interim sacrifice was
conducted of 20 males and 20 females in the control group and that at
the highest dose at 18 months. There were no compound-related effects
on behaviour, body-weight gain, food or water consumption, or
mortality. By 22 months, 24-31% of the males and 37-52% of the females
had died. As there was no difference between the treated and control
groups, it was concluded that mortality was not influenced by the
feeding of carbendazim. At 18 and 22 months, the absolute and relative
liver weights of both male and female mice at 5000 ppm were increased.
Macroscopic and microscopic examination of 20 male and 20 female
animals killed after 18 months of receiving 5000 ppm carbendazim
revealed compound-related effects on the liver: all animals had
centrilobular hypertrophy, single-cell necrosis, mitotic cells, and
pigmented Kupffer cells. The tissues of the remaining 100 males and
100 females exposed to 5000 ppm, evaluated at 22 months, showed marked
hypertrophy (greater than in animals treated for 18 months), clear-
cell foci, mitosis, inclusion bodies in enlarged cell nuclei, multiple
cell necrosis, and a greenish-yellow pigment in Kupffer cells.
Neoplastic nodules (adenomas), carcinomas, fibrosarcomas, and other
tumorigenic responses in the liver were equally distributed among the
groups. Although haemangiomas of the liver were found in all treated
groups but not in controls, no dose-related response was evident. Lung
adenomatosis was equally distributed among the groups. The tumour
incidence is summarized in Table 5. There was no effect on the
incidence or time of onset of tumours, and the total number of benign
and malignant tumours was comparable among the different groups of
mice. Thus, there was no evidence that carbendazim administered in the
diet of mice at doses up to and including 5000 ppm for 22 months had a
carcinogenic effect. The NOAEL was thus 300 ppm, equal to 34 mg/kg bw
per day (Donaubauer et al., 1982).
Rats
Groups of 36 male and 36 female weanling Sprague-Dawley rats were
given carbendazim (purity, 50-70%) in the diet for 104 weeks at 0,
100, 500, 2500 (increased to 10 000 ppm after 20 weeks), or 5000 ppm
(as carbendazim). Body weight and food consumption were recorded
weekly for the first year and twice a month thereafter. Behavioural
changes and mortality were observed daily. Haematological, urinary,
and selected clinical chemical examinations were performed
periodically. After one year, each group was reduced to 30 male and 30
female rats by interim sacrifice for gross and microscopic
examinations. At the end of the study, all surviving animals were
sacrificed, and tissues and organs were examined grossly. Microscopic
examinations were conducted on all tissues and organs from the
controls and animals at 2500-10 000 ppm, the livers of animals at 100
and 500 ppm, and the livers, kidneys, testes, and bone marrow of
animals at 5000 ppm. Body-weight gain was depressed in males and
females at 2500-10 000 ppm and in females at 5000 ppm in comparison
with controls. Food consumption was similar in all groups. Reduced
erythrocyte counts and haemoglobin and haematocrit values were seen in
females at 2500-10 000 or 5000 ppm after 9-24 months and in males at
2500-10 000 ppm after 24 months. There were no compound-related
Table 4. Hepatic tumour incidence in CD-1 mice fed carbendazim
Sex Dietary level No. of mice No. of mice alive Median survival Combined incidence
(ppm) examined at termination (weeks) of hepatic tumours
Male 0 80 18 79 13
500 80 14 72 20
1500 80 9 69 23
7500/3500 80 23 64 NA
Female 0 79 22 91 1
500 78 15 91 9
1500 80 13 91 21
7500/3500 78 20 91 15
NA, not analysed
Table 5. Hepatic tumour incidence in NMRK-f mice fed carbendazim
Sex Dietary level No.of mice Clear-cell Basophilic Hepatocellular Liver
(ppm) examined foci loci adenomas haemangiomas
Male 0 97 0 0 3 0
50 99 0 0 2 2
150 99 0 1 0 3
300 95 0 0 0 2
1000/5000 99 3 0 1 0
Female 0 98 0 0 0 0
50 98 0 1 00
150 95 0 0 0 0
300 95 0 0 1 2
1000/5000 95 4 0 0 1
clinical manifestations of toxicity and no effects on urinary
parameters. Alkaline phosphatase and alanine aminotransferase
activities varied throughout the study in animals at 2500-10 000 or
5000 ppm, but there was no consistent dose-response relationship.
Organ weights and organ-to-body weight ratios were unchanged, except
for the livers of females at 2500-10 000 or 5000 ppm, but the increase
in the liver-to-body weight ratio was due to a reduction in body
weight. Histopathological examination of the livers showed no
compound-related effects. Males at 2500-10 000 ppm had a marginal
increase in the frequency of diffuse testicular atrophy and
prostatitis. The NOAEL was 500 ppm, equivalent to 15 mg/kg bw per day
(Sherman, 1972).
Groups of 60 male and 60 female Wistar rats were given
carbendazim (purity, 99%) in the diet at 0, 150, 300, or 2000 ppm for
two years. The dose of 2000 ppm was increased to 5000 ppm after one
week and to 10 000 ppm after two weeks for the remainder of the study.
Animals were examined daily for clinical signs of toxicity. Body
weight and food consumption were measured regularly throughout the
study. Haematological measurements (peripheral blood), blood chemistry
(orbital sinus), and urinalysis were conducted periodically. All
animals were subjected to complete gross necropsy, and selected organs
were weighed. Tissues from 20 male and 20 female rats in the control
and high-dose groups were examined microscopically, and all tumours
and gross abnormalities were examined histologically. No differences
in clinical signs of toxicity or food consumption were seen between
test groups and control animals. Body weights were significantly
reduced in low-dose males from week 88 to term and in high-dose
females from week 12 to term. The results of urinalyses were
comparable among the groups. The haemoglobin level was depressed in
high-dose females a t weeks 26, 52, and 103, and the haematocrit was
depressed in high-dose females at week 103. There were no compound-
related effects in males. Serum aspartate aminotransferase activity
was decreased in high-dose males at termination of the study, but
not in females; high-dose females had increased serum alanine
aminotransferase activity and decreased total serum protein. The only
compound-related effects on organ weights were increased relative
liver weights in high-dose females. There were no compound-related
effects on mortality, and survival at termination of the study was
similar in all groups. There were no histological differences between
control and treated groups, except for an increased incidence of
diffuse proliferation of parafollicular cells of the thyroid in the
high-dose females. The number of tumour-bearing animals and the total
number of tumours were comparable among all groups, and no compound-
related oncogenic effects were reported. The NOAEL was 300 ppm,
equivalent to 15 mg/kg bw per day, on the basis of changes in organ
weights and minor biochemical changes (Til et al., 1976a).
(d) Reproductive toxicity
Groups of six male and six female rats were removed from a 90-day
study in which they were fed dietary levels of 0, 100, 500, or
2500 ppm carbendazim. Each female was exposed to three males from the
same dose group to produce the F1a generation; each litter was
reduced to 10 pups on the fourth day after birth. The F0 animals were
mated again after about one week to produce the F1b litter.
Reproduction indices and status at birth and on days 4, 12, and 21
were recorded, as were body weights at weaning. The data were
extremely limited and were available only for groups. At 100 ppm,
neither the F1a nor the F1b animals became pregnant. There were no
apparent effects on reproduction indices or weanling weights, but the
fertility indexes for all groups, which were only 33-67%, prevented
meaningful interpretation of the data (Sherman, 1968).
Groups of three male and 16 female Sprague-Dawley rats were fed
carbendazim in the diet at 0, 100, 500, 5000, or 10 000 ppm
(20 females at the highest dose) and mated in a standard study of two
litters per generation for three generations. The parental animals
were fed the experimental diet at 21 days of age and mated to produce
the F1 litter at 100 days of age; the numbers of matings,
pregnancies, and young were recorded for each litter at birth. The
litters were culled to 10 pups on day 4. The number of live pups was
again recorded on days 4, 12, and 21, as was pup weight at weaning.
The parents were mated again to produce F1b litters, which were
maintained on the respective diets for 110 days and then mated to
produce the F2a and F2blitters; F3a and F3b litters were
produced similarly. Selected tissues and organs from two males and two
females in each of five F3b litters from the controls and from
the groups fed 5000 and 10 000 ppm were examined grossly and
histopathologically. Reproduction indices, including mating,
fecundity, fertility, gestation, viability, and lactation, were
calculated and compared with control values. Carbendazim had no effect
on fertility, gestation, viability, or lactation, but the average
litter weights at weaning were reduced in all generations fed 5000 and
10 000 ppm. Histopathological examination of F3b weanlings did not
reveal any effects that were considered to be compound-related
(Sherman, 1972).
Carbendazim was administered to groups of 10 male and 20 female
Wistar rats at a dietary level of 0, 150, 300, or 2000 ppm for three
generations, and two successive litters were reared from each female
General condition and behaviour were routinely observed, and
individual body weights were recorded throughout the study. The
numbers of pups in each litter were recorded, and each litter was
culled to eight on day 1. The total weight of each litter was measured
on days 1, 10, and 20. The F1a and F2a litters were discarded at
weaning and the F1b and F2b litters were used to produce
succeeding generations. The F3a offspring were used to study
teratogenicity, while the F3b offspring w ere used in a four-week
evaluation of toxicity. Neither health nor body-weight gain was
affected, but all generations of the treated groups weighed
significantly more than controls. The compound had no effect on
fertility or on survival at birth, at day 10, or at day 20. Litter
size was not affected by treatment, except for a marginal decrease in
F2a litters at all doses. There were no differences in the F2a
litters at 300 and 2000 ppm, but litter size was decreased and
mortality at birth increased at 150 ppm. Birth weight during the
lactation period was comparable in all groups. There were no gross
abnormalities related to treatment. Autopsy of rats in the four-week
study showed increased relative liver weights and decreased relative
spleen weights in females fed 2000 ppm; relative ovarian weights were
also significantly decreased in all dose groups. Histopathological
examination of the livers showed no compound-related change;
histopathological results were not presented for other organs. No
maternal or fetal toxicity was evident, and there were no differences
in visceral anomalies in animals at 0 or 2000 ppm (the only groups
examined). Thoracic vertebral bodies were reduced at 2000 ppm, and
there was a significant reduction in cervical vertebral bodies at
2000 ppm. Controls, however, had more significant changes with regard
to absent or delayed ossification of skeletal structures. There were
no apparent adverse effects on reproduction and no teratogenic effects
at dietary levels of carbendazim up to and including 2000 ppm,
equivalent to about 120 mg/kg bw per day (Koeter et al., 1976).
A serial breeding technique was used to evaluate the fertility of
male Sprague-Dawley rats after exposure by gavage to 10 daily doses of
400 mg/kg bw per day carbendazim. Males, 90 days old and proven to be
fertile, were bred with a new female each week, starting on the third
day of treatment and continuing for 32 weeks after the last day of
treatment. Twelve days after each breeding period, the females were
killed, their uteri were examined for resorptions, and the numbers of
dead and viable fetuses were determined. All males were killed 35
weeks after treatment, and testicular tissue was prepared for
histopathological examination by vascular perfusion. The fertility of
treated males (as indicated by the number of pregnant females) was
depressed during the first week after treatment: 10 of the 24 treated
males failed to induce a pregnancy, as compared with no failure in the
control group. By the fifth week after treatment, 16 of the 24
carbendazim-treated males were infertile. Of these, four recovered
fertility after being infertile for 5-11 consecutive breeding periods,
but the other 12 did not recover during the remainder of the 32-week
period after treatment. Histological examinations of testicular
sections of the latter animals 245 days after treatment revealed
severe seminiferous tubular atrophy (> 85% of tubules were atrophic),
often with epithelium containing only Sertoli cells, surrounded by a
thickened basement membrane. The lumina of < 2% of the tubules
contained spermatozoa. The seminiferous tubules of the treated males
that recovered fertility had various contents of atrophic tubules
(13-85%) 245 days after treatment (Carter et al., 1987).
Groups of 8-12 male and 8-12 female rats were given 0, 50, 100,
200, or 400 mg/kg bw per day carbendazim by gavage from weaning
through puberty, gestation, and lactation and were mated at 84 days of
age. The male rats were killed on days 104-106 and the female rats on
day 27 post partum. In a similar study, Syrian hamsters were given 0
or 400 mg/kg bw per day carbendazim. Various landmarks of puberty were
measured in the parental generation. In females, estrous cyclicity,
litter size, the number of implants, organ weights, and histological
status were assessed. In males, organ weights, testicular and
epididymal sperm counts, sperm motility, sperm morphology, testicular
histological status, and endocrine parameters were assessed. In
addition, the growth, viability and reproductive function of the
offspring (F1) were observed during a four-month period of
continuous breeding. Males were killed after five months for
histopathological investigation. Carbendazim did not alter pubertal
development, growth, or viability in the parental generation of either
species. The reproductive potential of rats treated with 200 or
400 mg/kg bw per day was reduced due to effects on sperm production
and fetal viability. These doses markedly altered sperm morphology,
testicular and epididymal weights, sperm numbers, and testicular
histology; fertility, sperm mobility, and hormonal levels were altered
primarily in males with very low sperm counts. A statistically
significant reduction in the caudal epididymal sperm count was noted
at doses of 50 mg/kg bw per day or more. Testicular and epididymal
sperm counts in male hamsters were significantly lower (about 21%) in
treated than in control males. In F1 male hamsters, testis and
seminal vesicle weights and epididymal sperm counts were significantly
reduced by prenatal exposure to carbendazim at 400 mg/kg bw per day.
Parental female rats exposed at this dose had post-implantation
losses, and a few malformed pups were found in litters of animals at
100 or 200 mg/kg per day. Litter size was significantly reduced at 200
and 400 mg/kg bw per day. Overall, carbendazim was less toxic to
hamsters than to rats (Gray et al., 1988, 1990).
Carbendazim was fed to groups of eight female Holtzmann rats by
gavage at doses of 0, 25, 50, 100, 200, 400, or 1000 mg/kg bw per day
during early pregnancy (days 1-8). A range of parameters, including
the number of implantation sites, body-weight gain, uterine weight,
implantation site size, and serum ovarian and pituitary hormones, was
assessed after sacrifice on day 9. At doses up to 400 mg/kg bw per
day, carbendazim had no significant effect on any of the measured
parameters, but a trend towards increased resorptions was evident. The
highest dose reduced maternal body-weight gain, implantation site
size, and serum luteinizing hormone levels and increased serum
estradiol (Cummings et al., 1990).
(e) Developmental toxicity
Rats
Groups of 27-28 pregnant Sprague-Dawley rats were fed carbendazim
(purity, 53%) in their diet at 0, 200, 500, 2500, 5000, 7500, or
10 000 ppm on days 6-15 of gestation. On day 20 of gestation, all of
the animals were sacrificed and the fetuses were delivered by
caesarean section. The numbers and location of live and dead fetuses
and resorption sites, body weights, crown-rump length, sex, and
visible abnormalities were determined. Two-thirds of the fetuses were
prepared for examination for skeletal abnormalities, and the remainder
were examined for visceral and soft-tissue anomalies. There were no
deaths, no adverse effects on body weight, and no clinical signs of
toxicity. Food intake was reduced at the highest dose during the
period the test diet was administered but returned to control levels
from day 16 to 20. The numbers of implantation sites, resorption
sites, and live and dead fetuses were not adversely affected. Although
data on individual litters were not presented, carbendazim did not
appear to be teratogenic when administered to rats at dietary levels
up to and including 10 000 ppm during the critical period of
organogenesis (Sherman, 1970).
Groups of 18-22 pregnant Wistar specific pathogen-free rats were
given carbendazim in the diet at doses of 0, 600, 2000, or 6000 ppm on
days 6-15 of gestation. On day 21 of gestation, all of the rats were
sacrificed and the pups delivered by caesarean section. Dams were
weighed periodically during the test, and food consumption was
measured for specific periods. The number of corpora lutea was
determined, the ovaries were weighed, and the fetuses were weighed and
examined. The numbers of implantation and resorption sites were
recorded, and the empty uterine horns weighed. One-third of the
fetuses were fixed and stained for skeletal examination, and the
remaining two-thirds were examined for soft-tissue anomalies. Although
23 females per group were mated, the pregnancy rate was variable, with
18, 22, 20, and 18 pregnant rats in the groups receiving 0, 600, 2000,
and 6000 ppm, respectively. The mean body-weight gain and food
consumption of the high-dose females were significantly decreased in
comparison with controls. The numbers of live and dead fetuses,
implantation sites, embryonal resorptions, fetal resorptions, and
corpora lutea per dam were comparable in all groups, and ovarian
weights, the weights of the empty uteri, mean fetal weight per litter,
the sex ratio, and pre- and post-implantation losses were not affected
by treatment. No visceral anomalies were reported that were
significantly different from those seen in controls. Misshapen and
fused bones were much more frequent in the high-dose groups then in
any other group, and the incidence of supernumerary ribs was
significantly increased in high-dose females. Ossification was
significantly delayed or absent in pups at the high dose, particularly
in forelimbs, hindlimbs, sternebrae, and skull bones; ossification was
also significantly delayed or absent in cervical vertebral bodies in
all treated groups. It was concluded that carbendazim has no
teratogenic potential when administered in the diet to rats at levels
up to 6000 ppm, although ossification is reduced in a dose-related
manner (Koeter, 1975a).
Groups of 8-10 female Wistar rats were given carbendazim (purity,
98%) by gavage at 0, 20, 40, or 80 mg/kg per day on days 6-15 of
pregnancy. Half of each group of animals was killed on day 21 of
gestation, and half was allowed to deliver normally. All sacrificed
animals were scored for live and dead fetuses and for resorptions;
live fetuses were killed and examined for abnormalities. After normal
deliveries, neonatal deaths and survivors were counted, and survivors
were weighed and examined for gross abnormalities. In rats sacrificed
on day 21, dead and resorbed fetuses accounted for 29% of the
conceptuses among controls, 48% at 20 mg/kg per day, 64%, at 40 mg/kg
per day, and 73% at 80 mg/kg per day. There were no differences among
the various groups with respect to mean weight of live fetuses, and
there were no malformations. The average number of live pups per
litter was close to eight in controls, six at 20 mg/kg per day, and
about five at 40 and 80 mg/kg per day. Mean fetal weight was increased
by about 13% over that of controls at the two highest doses. There
were no stillbirths, neonatal deaths, or gross abnormalities, but
mortality at 21 days post partum was 3.0-3.5 times greater at the
two highest doses than in controls (Janardhan et al., 1984).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethyl-cellulose by gavage to groups of 15-26 Sprague-Dawley
rats on days 6-15 of gestation at daily doses of 10-3000 mg/kg bw per
day. The lowest dose had no effect on dams or their offspring and was
considered to be a no-effect lewd. At 30 mg/kg bw per day,
fetotoxicity was evident: 42% of the fetuses in 19/21 litters had
malformations affecting the head, spine, ribs, and sternum. At
60 mg/kg bw per day, 2/23 animals aborted and 51% of the implantations
in the remaining dams were dead. Malformations were seen in 90% of
fetuses, and all litters were affected. At 100 mg/kg bw per day, 15
pregnant animals produced only four live fetuses in three litters, all
of which were malformed. At 300, 1000, and 3000 mg/kg bw per day,
severe toxicity resulted in early resorptions only. The no-effect
level of 10 mg/kg bw per day was confirmed in a further study
specifically of fetotoxicity and hydrocephaly (Hoffman & Peh,
1987a,b).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethylcellulose by gavage to groups of 25 Sprague-Dawley rats
on days 7-16 of gestation at doses of 0, 5, 10, 20, or 90 mg/kg bw per
day. Maternal toxicity was seen only at the highest dose, in the form
of depressed weight gain during treatment and before sacrifice on day
22. The mean liver weights and liver-to-body weight ratios were
increased. A decreased pregnancy rate was observed at the highest
dose. An increase in the incidence of early resorptions per dam,
decreased litter size, and total resorption of three litters occurred
at the highest dose, but only the reduction in females per litter was
significant. Significant reductions in mean fetal weight were observed
at both 20 and 90 mg/kg bw per day, and a significant increase in the
incidence of fetal malformations was seen at the highest dose. The
malformations consisted primarily of hydrocephaly, microphthalmia,
anophthalmia, malformed scapulae, and axial skeletal malformations
(vertebral, rib, and sternebral fusions, exencephaly, hemivertebrae,
and rib hyperplasia). The NOAEL was 20 mg/kg bw per day for the dams
and 10 mg/kg bw per day for the fetuses (Alverez, 1987).
Carbendazim (purity, 95%) was administered by gavage to female
Holtzman rats at doses of 0, 100, 200, 400, or 600 mg/kg bw per day
during days 1-8 of gestation, and the rats were sacrificed on day 11
or 20. No maternal toxicity was seen at any dose. On day 11, the
crown-rump length, head length, number of somites, and number of
embryos per dam were significantly reduced in groups receiving
200 mg/kg bw per day or more. Open posterior neuropores and limb
anomalies were observed more frequently at doses > 200 mg/kg bw per
day. On day 20, increased resorptions, decreased live litter size and
fetal body weight, and delayed ossification were observed at all
doses. Skeletal malformations seen at the high dose were attributed to
treatment. The authors noted that developmental alterations occurred
after termination of treatment, suggesting either that the anomalies
represent delays in development or that the embryonic cells are
vulnerable at earlier stages than was previously thought (Cummings
et al., 1992).
Rabbits
Groups of 3-11 pregnant New Zealand albino rabbits were given
carbendazim in the diet at 0, 600, 2000, or 6000 ppm on days 6-18 of
gestation. On day 29 of gestation, all of the animals were sacrificed
and the fetuses delivered by caesarean section. Does were weighed
periodically, and food consumption was determined for specific
periods. The number of corpora lutea was determined, the ovaries were
weighed, and the fetuses were weighed and examined; the numbers of
implantation sites and resorption sites were recorded, and the empty
uteri weighed. One-half of fetuses were stained and sectioned for
determination of skeletal anomalies, and the other half was examined
for soft-tissue abnormalities. Only the fetuses in the high-dose and
control groups were examined for visceral anomalies. Although 15
females per group were artificially inseminated, the pregnancy rate
was extremely variable, with nine in controls, eight at 600 ppm, three
at 2000 ppm, and 11 at 6000 ppm. The mean body-weight gain was
significantly decreased in the high-dose group, although food
consumption did not vary among groups. The numbers of live and dead
fetuses, implantation sites, embryonal resorptions, fetal resorptions,
and corpora lutea per doe were comparable in all groups. The ovarian
and uterine weights of animals at the high dose were depressed in
comparison with control females; Pre- and post-implantation losses
were not affected by treatment. The incidence of visceral anomalies
appeared to differ between the high-dose and control groups, but too
few litters and fetuses were examined to allow a conclusion. There
were significantly increased numbers of supernumerary ribs (bilateral)
and skull bones in the high-dose group. Ossification was significantly
delayed or absent in fetuses in the high-dose group, most notably in
the forelimb metacarpals and phalanges. There was also incomplete
ossification of the sternebrae and skull bones, which was significant
at 600 and 6000 ppm, and misshapen sternebrae were present at the
highest dose. Although it can be concluded that carbendazim does not
induce teratogenic effects when administered in the diet to rabbits at
levels up to 6000 ppm, there were too few animals, litters, and
fetuses at 2000 ppm to allow a useful evaluation of compound-related
effects (Koeter, 1975b).
Female albino rabbits were given 0, 40, 80, or 160 mg/kg bw per
day carbendazim by gavage on days 6-18 of pregnancy and were
sacrificed on day 31. All of the animals were scored for live and dead
fetuses and for resorptions; live fetuses were killed and examined for
abnormalities. There were no dead or resorbed fetuses in controls, but
15% of all conceptuses were dead at 40 mg/kg bw per day, 21.7% at 80
mg/kg bw per day, and 33.3% at 160 mg/kg bw per day. There were no
differences among the various groups with respect to mean weight of
live fetuses, and there were no malformations (Janardhan et al.,
1984).
Suspensions of carbendazim in aqueous 0.5% carboxymethylcellulose
were administered by gavage on days 7-19 of presumed gestation to
groups of 20 artificially inseminated New Zealand white rabbits at
doses of 0, 10, 20, or 125 mg/kg bw per day. The highest dose
inhabited average maternal weight up to day 16 of gestation. The
implantation rate was decreased at 20 and 125 mg/kg bw per day, and
the incidence of resorptions was increased at 125 mg/kg bw per day,
resulting in decreased live litter size at these doses. At the highest
dose, decreased fetal body weight was seen, but the effect was not
statistically significant. The average percentage of malformed fetuses
per litter was significantly increased at 125 mg/kg bw per day.
Compound-related malformations at the highest dose consisted of
malformed cervical vertebrae and interrelated malformation of the ribs
and proximate thoracic vertebrae. The NOAEL for maternal toxicity was
20 mg/kg bw per day, and the NOAEL for developmental toxicity was
10 mg/kg bw per day (Christian et al., 1985).
Hamsters
Groups of 7-11 pregnant hamsters were treated orally on day 10 of
gestation with 0, 15, 30, 75, or 150 mg/kg bw carbendazim A
significant increase in malformations, including exencephaly, was
observed at 75 and 150 mg/kg bw. These doses were also embryotoxic,
producing 31 and 45% resorptions, respectively (Minta & Biernacki,
1982).
(f) Genotoxicity
Numerous studies have been conducted to assess the mutagenic
potential of carbendazim. Many of the results are conflicting, and
many of the reports do not provide sufficient detail to evaluate the
reasons for the conflicting data. Since before the mid-1980s
industrially produced carbendazim contained phenazine impurities, the
use of carbendazim with different degrees of purity might account for
some of the discrepancies. Table 6 summarizes those reports that
included sufficient experimental detail and data.
Carbendazim is not a heritable gene mutagen; it does not interact
with cellular DNA, induce point mutations, or result in germ-cell
mutations, as seen in both mammalian and nonmammalian systems in
vitro and in vivo and in somatic and germ cells. Positive results
have occasionally been obtained in tests for gene mutation, but they
may have been associated with the presence of phenazines, which are
mutagenic at very low concentrations in Salmonella typhimurium in
Ames' test and in mouse lymphoma LY5178Y tk+/- cells.
Concentrations of > 4 ppm diaminophenazine and 10 ppm aminohydroxy-
phenazine were mutagenic in Ames' test. Process changes by some of the
major manufacturers of carbendazim have removed the phenazine, and
this contaminant is not present when other benzimidazoles, such as
benomyl and thiophanate-methyl, are metabolized to carbendazim.
Carbendazim does cause numerical chromosomal aberrations (aneuploidy
and/or polyploidy) in experimental systems in vitro and in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A 75% wettable powder formulation induced slight, transient
irritation when applied to the in tact and abraded skin of albino
rabbits. A 55% suspension of the 75% wettable powder formulation in
dimethyl phthalate induced mild irritation when applied to the intact,
shaved skin of albino guinea-pigs, while a 5.5% concentration produced
no irritation (Ford, 1981).
The primary dermal irritation potential of Benlate C (50%
wettable powder) was evaluated by applying a 5-g aliquot to the
intact, clipped skin of six New Zealand white rabbits for 4 h. The
test sites were evaluated for erythema, oedema, and other evidence of
dermal effects and were scored according to the Draize scale 4, 24,
48, and 72 h after application. No dermal irritation was seen at any
time during the study (Vick & Brock, 1987).
Table 6. Results of tests for the genotoxicity of carbendazim
End-point Test system Concentration Results Reference
or dose
Tests for gene mutation
Reverse mutation S. typhimurium TA98, < 2500 µg/plate Negative Gericke (1977)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium TA100, < 200 µg/plate Negative Fiscor et al. (1978)
TA1530, TA1535, TA1950
Reverse mutation S. typhimurium TA1530, < 100 µg/spot Weakly positive Fiscor et al. (1978)
TA1950, G46 his-
Reverse mutation S. typhimurium TA100, < 1000 µg/plate Negative Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP2 hcr
Reverse mutation S. typhimurium TA98, < 300 µg/plate Negative Pandita (1988)
TA100
Reverse mutation S. typhimurium TA97, 5000 µg/plate Positive (only with Albertini (1989)
TA98, TA1537, TA1538 activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Positive, TA1537, Donovan (1982)
TA100, TA1535, TA1537 TA98 (with
activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Negative Russell (1983)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium Positive, only with Arce (19844)
4 ppm DAP or
10 ppm AHP
Reverse mutation S. typhimurium TA98, < 20 000 µg/plate Negative Russell (1977);
TA100, TA1535, TA1537 Donovan (1983)
hprt mutation Chinese hamster ovary cells < 654 µmol/litre Negative Waterer (1980)
tk mutation L5178Y mouse lymphoma < 250 µmol/litre Positive (with Jotz (1980)
cells activation)
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
Tests for chromosomal effects
Sister chromatid exchange Chinese hamster ovary cells < 40 µg/ml Negative Ivett (1984)
Sister chromatid exchange Human lymphocytes < 30 µg/ml Negative Banduhn & Obe (1985)
Sister chromatid exchange Human lymphocytes < 60 µg/ml Marginally positive Pandita (1988)
Chromosomal gain S. cereviriae < 0.1 µg/ml Positive Whittaker et al. (1990)
Aneuploidy S. cereviriae < 5 µg/ml Positive Albertini (1991)
Chromosomal aberrations Human lymphocytes < 10 µmol/litre Not clastogenic but Banduhn & Obe (1985)
induces micronuclei
Chromosomal aberrations Human lymphocytes < 0.5 mg/ml Negative Lamb & Lilly (1980)
DNA damage and repair
Rec assay B. subtilis < 1000 µg/disc Negative Shirasu et al. (1977)
Rec assay S. typhimurium TA1535, < 2000 µg/plate Negative Rashid & Mumma
(1986)
TA1538, E. coli K12,
L. coli WP2
Unscheduled DNA Rat and mouse hepatocytes < 12.5 µg/ml Negative Tong (1981a, b)
synthesis
Unscheduled DNA Rat hepatocytes < 104 µg/ml Negative Litton Bionetics, Inc.
synthesis (1981)
In vivo
Holt-mediated assay, mice S. typhimurium G46 his- Negative Fiscor et al. (1978)
Host-mediated assay, mice S. typhimurium G46 his- 4000 mg/kg bw Negative Shirasu et al. (1977)
Gene mutation Mouse embryos treated in < 300 mg/kg bw Positive at Fahrig & Seller (1979)
utero by dosing mother orally 200 mg/kg bw
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
In vivo (con't)
Chromosomal aberration Rat bone marrow 300 mg/kg bw Negative BASF AG (1975)
orally
Chromosomal aberration Chinese hamster bone 1 000 mg/kg bw Negative Seiler (1976)
marrow orally
Chromosomal aberration ICR mouse nucleated 2 × 1 000 mg/kg bw Spindle effects Seiler (1976)
anaphase cells orally
Micronucleus formation Mouse 500 mg/kg bw orally Negative Seiler (1976)
Micronucleus formation Mouse < 6000 mg/kg bw i.p. Positive Pandita (1988)
Chromosomal aberration Chinese hamster bone < 1000 mg/kg bw i.p. Negative Pandita (1988)
marrow
Dominant lethal mutation NMRI mice 5 × 500 mg/kg bw Negative Hoechst AG (1974)
per day i.p.; 5 × 300
mg/kg bw per day orally
Sex-linked recessive lethal Drosophila melanogaster 0.5 mg/ml in dimethyl Negative Lamb & Lilley (1980)
mutation sulfoxide
Germ-line aneuploidy Drosophila melanogaster < 50 000 ppm Negative Osgood et al. (1991)
Technical-grade carbendazim was not irritating to the eyes of
albino rabbits. A 75% wettable powder formulation induced transient
corneal opacity in six of six unwashed and two of three washed eyes.
Microscopic examination confirmed the corneal opacity as mild to
moderate. Conjunctival irritation (redness, swelling, discharge) was
also transient. All eyes were normal after four days. The irritation
response was probably related to the inert ingredients in the wettable
powder formulation (Edwards, 1974b).
A 50% wettable powder formulation produced slight corneal
opacity, mild or moderate conjunctival redness, and slight or mild
conjunctival oedema in six male New Zealand white rabbits. Three of
the rabbits also had moderate iritis, and one had minimal blood-tinged
discharge. Microscopic examination revealed no corneal injury in any
of the treated eyes. The eyes of the other two rabbits were normal by
72 h. It was concluded that this formulation is a moderate ocular
irritant (Vick & Valentine, 1987).
Ten male albino guinea-pigs exposed to technical-grade
carbendazim or a 75% wettable powder formulation showed no dermal
sensitization after intradermal injections or repeated applications to
intact, shaved skin (Ford, 1981).
A 50% carbendazim formulation was applied to the intact, shaved
skin of 10 male and 10 female Dunkin Hartley albino guinea-pigs; five
male and five female guinea-pigs treated with 80% ethanol in water
served as vehicle controls; two male and two female guinea-pigs
treated with solid test material at the challenge phase only served as
negative controls; and two male and two female guinea-pigs treated
with a 0.3% suspension of 1-chloro-2,4-dinitrobenzene in 80% ethanol
in water served as positive controls. No irritation was observed in
the treated or vehicle or negative controls, but 1-chloro-2,4-
dinitrobenzene produced sensitization in all treated animals (Martin
et al., 1987).
(ii) Neurotoxicity
Groups of 10 white Leghorn hens receved carbendazim at doses of
500, 2500, or 5000 mg/kg bw to test for delayed neurotoxic potential.
Controls received the vehicle, corn oil, and the neurotoxin tri-
ortho-tolyl phosphate. The hens were observed daily for mortality
and clinical neurotoxicity for four weeks. Neurotoxic signs consisting
of leg weakness, ataxia and/or 'goose-stepping' gait were observed in
hens treated with tri- ortho-tolyl phosphate. Less severe, reversible
signs, consisting of slight leg weakness and ataxia, were observed in
hens treated with carbendazim at 5000 mg/kg bw, but no neurotoxic
signs were observed in those treated at lower doses. Microscopic
examination indicated no axonal degeneration or demyelination in
carbendazim-treated birds (Goldenthal, 1978).
(iii) Hormonal effects and spermatogenesis
Since spermatogenesis is an androgen-dependent process, the
effects of carbendazim on the endocrine function of the rat testis
were investigated by feeding 0-400 mg/kg bw per day by gavage for 85
days and measuring the serum levels of pituitary luteinizing hormone,
follicle-stimulating hormone, thyroid-stimulating hormone, and
prolactin, and the levels of androgen-binding protein and testosterone
in serum and in testicular interstitial and seminiferous tubule fluid.
The function al capacity of Leydig cells to secrete testosterone
was assessed in vitro after challenge with human chorionic
gonadotrophin. Doses of 50-100 mg/kg bw per day had no effect on
pituitary or testicular hormone concentrations; 200 mg/kg bw per day
increased the testosterone concentration in the seminiferous tubular
fluid, without affecting serum testosterone or androgen-binding
protein concentrations. The dose of 400 mg/kg bw per day resulted in
increased concentrations of both substances in the interstitial and
seminiferous tubular fluid and of serum androgen-binding protein.
These hormonal changes indicate that carbendazim affects the gonads,
resulting in testicular atrophy. Thus, the elevated seminiferous
tubule fluid testosterone concentrations may be a result of two
factors: (i) greater release of testosterone by the Leydig cells into
the interstitial fluid and/or (ii) decreased testosterone outflow from
the testis into the genera] circulation. The increased level of
androgen-binding protein in the interstitial fluid reflects a change
in its relative secretion into the interstitial fluid and seminiferous
tubules (Rehnberg et al., 1989).
Since extragonadal changes may have contributed to the altered
testicular endocrine profile described above, a further study focused
on the presence of concurrent changes in the hypothalamic and
pituitary control of the testis. Rats were given carbendazim at 50,
100, 200, or 400 mg/kg bw per day by gavage for 85 days. Dose-related
increases in serum follicle-stimulating and luteinizing hormone levels
were noted, but the values for prolactin and thyroid-stimulating
hormone remained unchanged. No significant difference in gonadotropin-
releasing hormone concentration in the mediobasal hypothalamus was
seen, although an increased level was found in the anterior
hypothalamus at 50 mg/kg bw per day, followed by a dose-related
decline (Gray et al., 1988). These findings suggest that
carbendazim-induced testicular damage is accompanied by compensatory
changes in hypothalamic and pituitary regulation of the testis
(Goldman et al., 1989).
These findings imply that carbendazim acts directly on the testis
to induce a number of hormonal and pathological changes. Consistent
with this hypothesis are the results of a study by Nakai et al.
(1992), in which the effects of carbendazim on the testes, efferent
ductules, and spermatozoa were determined after a single oral dose to
male Sprague-Dawley rats. In the first experiment, groups of 86-day-
old rats were treated with 0 or 400 mg/kg bw carbendazim and killed 2,
4, or 8 h later on the same day or 1, 4, 8, 16, or 32 days after
treatment. The first effect of carbendazim was noted after 8 h as an
increase in testicular weight; this continued to increase until day 4,
but on days 16 and 32 the testicular weights were substantially lower
than those of controls in five of 16 animals, indicating individual
variation. A decrease in the percentage of sonication-resistant sperm
heads per testis occurred at 8 h in four of eight rats, but the
decrease was significant only after 24 h, when a mean decrease of 19%
was observed; maximal decreases in total sperm head counts per testis
were seen on day 8, after which some recovery was apparent. Epididymal
weights were increased on day 4, but the percentage of morphologically
normal sperm in the cauda epididymus was decreased at that time. By
day 8, many spermatozoa heads were separated from their flagella, and
10% of the heads were misshapen. Numerous sloughed, round germ cells
and cytoplasmic testicular debris were also evident. No effect on the
percentage of motile sperm was seen at 2, 4, or 8 h or one or four
days after treatment. Sperm motility was significantly decreased on
days 8 and 16, but because of clumping and degeneration of the
spermatozoa, the percentage motility could not be determined.
Similarly damaged sperm were seen in three of eight rats on day 32,
but the percentage of motile sperm that could be measured was similar
to that in controls.
In a second experiment (Nakai et al., 1992), groups of rats
aged 97-105 days were given a single oral dose of 0, 50, 100, 200,
400, or 800 mg/kg bw and were killed 2 or 70 days after treatment. On
day 2, a dose-dependent increase in testicular weight was seen at
doses of 100 mg/kg bw or more. This was accompanied by significant
increases in mean seminiferous tubular diameter at 400 and 800 mg/kg
bw. At 50 mg/kg bw, missing immature germ cells were noted, with round
spermatids from stages I and II and elongated spermatids sloughed from
stage VII epithelium. At 100 mg/kg bw, the disappearance of germ cells
was more severe and sloughing of elongated spermatids extended into
stages XII and XIV. At doses greater than 100 mg/kg bw, germ cells
were missing at all stages except stages IX-XI, and at doses of
400-800 mg/kg bw some seminiferous epithelia were damaged so severely
that it was difficult to identify the stage. In addition, major
pathological changes were seen in the efferent ducts of the testis. In
animals treated with 100 mg/kg bw or more, the rete testis was
swollen, with sloughed germ cells, indicating that ductal blockage had
occurred further down the tract; 50% or more of the efferent ductules
were occluded. The occlusions were characterized as compacted luminal
contents, spermatic granulomas, mineralizations, and obliteration of
the original lumen by fibrotic connective tissue. On day 70, mean
testicular weight and mean seminiferous tubule diameter showed a
dose-dependent decrease. Histologically, these decreases were
associated with a dose-dependent increase in seminiferous tubular
atrophy. The atrophied tubules contained primarily Sertoli cells and
occasional spermatogonia and were surrounded by a thickened basement
membrane. No atrophic tubules were seen in the control rats.
Female hamsters were treated by gavage with a single dose of
carbendazim around the fertilization period, at times selected to
coincide with either of the two microtubule-dependent events initiated
by the ovulatory surge of luteinizing hormone: oocyte maturation
(first meiotic division, occurring late in vaginal proestrous) and
fertilization (second meiotic division, occurring early in vaginal
estrous). In the first experiment, groups of 10 hamsters received 0,
250, 500, 750, or 1000 mg/kg bw carbendazim during melosis I, and
pregnancy outcome was assessed on day 15. The percentage of pregnant
hamsters was significantly reduced at 750 and 1000 mg/kg bw, and, in
those animals that became pregnant, the average number of live pups
was reduced at all doses. In the second experiment, groups of 10
female hamsters were bred overnight and were given a single dose of 0
or 1000 mg/kg bw during meiosis II the next morning. The percentage of
pregnant hamsters was unaffected, but the average number of live pups
(measured at 15 days) was reduced. Thus, administration of carbendazim
at the time of microtubule-dependent meiotic events can result in
early pregnancy loss in hamsters (Perreault et al., 1992).
Rats in pseudopregnancy induced by stimulation of the uterine
cervix with a small brass rod in proestrous and estrous were given 0
or 400 mg/kg bw per day carbendazim for eight days. A uterine decidual
cell response was induced on day 4 of pseudopregnancy, and the animals
were killed on day 9. This response, evaluated as a measure of uterine
competency, was significantly lower in the treated rats than in the
controls (Cummings et al., 1990).
Groups of 12 male C57Bl/6 x C3H/He F1 mice were given
carbendazim at 0, 250, 500, or 1000 mg/kg bw per day by gavage for
five consecutive days, and body weight, testicular weights, and sperm
parameters were measured 7, 24, and 39 days after treatment. Body
weight was unaffected; testicular weight was reduced only in the group
at the highest dose after 7 and 24 days but had recovered by 39 days.
Flow cytometry of testicular cells showed that the relative
percentages of certain testicular populations (round, elongating, and
elongated spermatids) in the group at the highest dose were different
from those in controls 7 and 14 days after treatment (Evenson et al,
1987).
Eight-week-old Wistar-derived male rats were fed 0, 10, 70, or
500 ppm carbendazim in the diet for 182 days. Another group received
500 ppm for 91 days and control diet for a further 91 days. Positive
controls were treated with 0.5 mg/kg bw per day colchicine by gavage
for 12 days. Every 13 days, 10 rats were killed and their testicular
organs were examined by histological, morphometric, enzyme
histochemical, and autoradiographic methods. Males were mated twice
with untreated females on day 182 and were killed on day 208.
Fertility parameters were not affected by treatment. Similarly,
treatment did not affect testicular weight, the area of seminiferous
tubules or interstitial tissue, or epididymal structures and enzyme
activities; however, the incidence of 'degenerating' germ cells
undergoing meiosis and spermatogenesis was increased at 70 and
500 ppm, and a significant increase in the preleptonene spermatocyte
nuclear area was seen in all treated groups. The biological
significance of this finding is difficult to assess. The authors
concluded that the effects seen at 70 ppm (equal to 3 mg/kg bw per
day) indicate that carbendazim affects the physiological 'germinal
elimination process'; however, an independent review of the findings
raised methodological concerns which may preclude definitive analysis
of the data (Hilscher et al., 1992; Russell, 1992).
3. Observations in humans
(a) Medical surveillance of workers
Selected blood profiles from 50 workers involved in the
manufacture of benomyl and carbendazim were compared with those of a
control group of 48 workers who were not exposed to these two
fungicides. White blood cell count, red blood cell count, and
haemoglobin and haematocrit values were comparable in the two groups.
No quantitative estimates of exposure were given for the factory
workers, and no female employees were included in the control group
(Everhart, 1979).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers who were exposed to benomyl between 1970 and
1977. The ages of the workers ranged from 19 to 64 years; 79% of the
workers and 78% of their spouses were aged 20-39. The duration of
exposure ranged from less than one month to 95 months, and more than
51% of the workers had potentially been exposed for one to five
months. The birth rates of the spouses of the exposed workers were
compared with those of four populations in the same county, state,
region, and country (USA). The birth rates of the study population
were generally higher than those of the comparison populations,
indicating no reduction in fertility. Spermatogenesis was not examined
(Gooch, 1978).
(b) Studies of volunteers
Urinary excretion of carbendazim was investigated after oral
administration of 2 mg, application of 17 mg onto a skin area of
100 cm2, or intravenous administration of 1 mg. Total excretion of
5-HBC was proportional to the dose applied, and use of an occlusive
dressing did not significantly increase dermal absorption (Meuling
et al., 1993).
Comments
Carbendazim is readily absorbed by animals after oral exposure
and rapidly metabolized. It is eliminated in the faeces and excreted
in the urine. The tissue distribution showed no bioconcentration. In
rats, 2-[(methoxycarbonyl)amino]-1 H-benzimidazol-5-yl hydrogen
sulfate was identified as the main metabolite. The results of
comparative studies in rats and mice indicate that the detoxification
capacity of mouse liver may become saturated at high doses.
Carbendazim is poorly absorbed via the dermal route in rats. Within 24
h, only about 0.2% of a single dose of 0.6 mg was excreted in the
urine and faeces.
Carbendazim has low acute toxicity, with an oral LD50 in the
rat of > 10 000 mg/kg bw. The clinical signs of toxicity after high
single doses were generally nonspecific. Testicular degeneration has
been observed after single oral doses of > 1000 mg/kg bw in rats.
Wettable powder formulations containing carbendazim have been shown to
be irritating to rabbit skin and eyes but did not induce dermal
sensitization in Buehler-type tests. WHO has classified carbendazim as
unlikely to present an acute hazard in normal use.
In 90-day dietary studies in rats, increased liver weight was
seen at 1350 ppm (the highest dose tested in one study), without any
histological change. The overall NOAEL was 500 ppm, equivalent to
50 mg/kg bw per day. In dogs treated by dietary administration for tip
to two years at levels up to and including 4500 ppm, hepatotoxicity
(increased liver weight, hepatic cirrhosis, swollen vacuolated
hepatocytes, and chronic hepatitis) was seen at 500 ppm and above, and
the NOAEL was 100 ppm, equivalent to 2.5 mg/kg bw per day.
Two two-year studies were conducted in rats given dietary levels
of 0, 100, 500, or 2500-10 000 ppm and 0,150, 300, or 2000-10 000 ppm.
Carbendazim did not have carcinogenic potential, but some effects were
seen at the highest doses tested. In one study there was some evidence
of testicular atrophy, and in the other an increased incidence of
diffuse proliferation of parafollicular cells in the thyroid was seen.
In the latter study, evidence of mild hepatotoxicity was found in the
absence of histopathological change. The NOAEL in both studies was
equivalent to 15 mg/kg bw per day.
Three carcinogenicity studies were performed in mice. In Swiss
mice and CD-1 mice treated for 80 weeks and two years, respectively,
at dietary levels up to 5000 or 7500 ppm, carbendazim increased the
incidence of liver tumours. Because of the minimally increased
incidence of proliferative lesions of the liver at 150 or 500 ppm in
the diet (the lowest doses tested), it was not possible to establish
no-effect levels in these studies. In NMRKf mice, which have a low
spontaneous incidence of liver tumours, no carcinogenic effect was
seen at dietary levels up to and including 5000 ppm (the highest level
tested for 96 weeks), but hepatotoxicity was seen at this dose. The
NOAEL was equal to 34 mg/kg bw per day.
In a three-generation study of reproductive toxicity, rats were
fed dietary levels of 0, 150, 300, or 2000 ppm. Carbendazim had no
effect on reproduction or development at dietary levels up to and
including 2000 ppm (equivalent to 120 mg/kg bw per day). Male
fertility was depressed in rats given carbendazim by gavage at
200 mg/kg bw per day for 85 days. A dose of 50 mg/kg bw per day (the
lowest dose tested) caused a decrease in epididymal sperm counts.
After a single oral dose to rats, disruption of spermatogenesis was
seen at 100 mg/kg bw, but there was no effect at 50 mg/kg bw. The
effect was associated with loss of germ cells resulting from
inhibition of Sertoli-cell microtubules.
Carbendazim was tested for developmental toxicity in rats at
doses of 5-3000 mg/kg bw per day on days 6-15 of gestation. It was
fetotoxic and teratogenic at 20 mg/kg bw per day and above, the main
effects being microphthalmia and hydrocephaly. The NOAEL for
fetotoxicity and teratogenicity was 10 mg/kg bw per day; maternal
toxicity was observed at 90 mg/kg bw per day and above, and the NOAEL
for maternal toxicity was 20 mg/kg bw per day. Rabbits were exposed to
carbendazim by gavage at doses of 0, 10, 20, or 125 mg/kg bw per day
on days 7-19 of gestation. Carbendazim was fetotoxic and teratogenic,
the major effects being malformed vertebrate and ribs. The NOAELs in
this species were 10 mg/kg bw per day for teratogenicity and
fetotoxicity and 20 mg/kg bw per day for maternal toxicity.
Carbendazim has been adequately tested for genotoxicity in a
range of assays. The Meeting concluded that it does not directly
damage genetic material but causes numerical chromosomal aberrations
both in vitro and in vivo as a result of its interference with the
mitotic spindle proteins.
In an epidemiological study of workers exposed to carbendazim,
there was no reduction in fertility, as indicated by the birth rates,
among the study population. Spermatogenesis was not; examined.
An ADI of 0-0.03 mg/kg bw was established on the basis of the
no-effect level of 2.5 mg/kg bw per day in the two-year study in dogs
and a safety factor of 100. The resultant ADI, when compared with the
LOAELs in the studies with Swiss and CD-1 mice, provides an adequate
level of safety.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 34 mg/kg bw per day (96-week study of
toxicity and carcinogenicity in NMRKf mice)
< 150-500 ppm, equivalent to < 22-75 mg/kg bw per day
(studies of toxicity and carcinogenicity in Swiss mice (80
weeks) and CD-1 mice (two years)
Rat: 300-500 ppm, equal to 15 mg/kg bw per day (toxicity in a
two-year studies of toxicity and carcinogenicity)
2000 ppm, equivalent to 120 mg/kg bw per day (study of
reproductive toxicity)
10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
Dog: 100 ppm, equal to 2.5 mg/kg bw per day (two-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to carbendazim
Exposure Relevant route, study type, species Results/remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder non-irritating
Ocular, irritation, rabbit 50% wettable powder irritating
Dermal, sensitization, guinea-pig Non-sensitizing in Buehler test
Inhalation, toxicity, rat LC50 > 5.9 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat and rabbit NOAEL = 10 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary, toxicity, dog NOAEL = 2.5 mg/kg bw per day;
hepatotoxicity
References
Arce, G.T. & Sarrif, A.M. (1984) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report from DuPont Haskell
Laboratory for Toxicology, Newark, Delaware, USA. Submitted to
WHO by AgrEvo, Frankfurt, Germany.
Albertini, S. (1989) Influence of different factors on the induction
of chromosome malsegregation in Saccharomyces cerevisiae D61.M
by Bavistan and assessment of its genotoxic property in the Ames
test and in Saccharomyces cerevisiae DT. Mutat. Res., 216,
327-340.
Albertini, S. (1991) Re-evaluation of file 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae
aneuploidy test systems by the Gene-Tox Program using strain
D61 M of Saccharomyces cerevisiae. Mutat. Res., 260, 165-180.
Alverez, L. (1987) Teratogenicity study of INE-965 (carbendazim) in
rats. Unpublished report from E.I. DuPont de Nemours and Co.,
Haskell Laboratory, Newark, Delaware, USA.
Banduhn, N. & Obe, G. (1985) Mutagenicity of methyl 2-benzimidazole
carbamate, diethylstilbestrol and estradiol: Structural
chromosomal aberrations, sister-chromatid exchanges, C-mitoses,
polyploidies and micronuclei. Mutat. Res., 156, 199-218.
BASF AG (1975) Dominant lethal study in mice. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Beems, R.B., Til, H.P. & van der Heijden, C.A. (1976) Carcinogenicity
study with carbendazim (99% MBC) in mice. Unpublished report from
Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Carter, S.D, Hess, R.A. & Laskey, J.W. (1987) The fungicide methyl
2-benzimidazole carbamate causes infertility in male Sprague-
Dawley rats. Biol. Reprod., 37, 709-718.
Christ, O. & Kellner, H.M. (1973) Animal tests with carbendazim.
Unpublished report from Hoechst AG, Frankfurt, Germany.
Christian, N.S., Hoberman, A.M. & Feussner, E.L. (1985) Developmental
toxicity study of carbendazim administered via gavage to New
Zealand white rabbits. Unpublished report from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by E.I. DuPont de Nemours and Co., Inc.
Cummings, A.M., Harris, S.T. & Rehnberg, G.L. (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat.
Fundam. Appl. Toxicol., 15, 528-535.
Cummings, A.M., Ebron-McCoy, M.T., Rogers, J.M., Barbee, B.D. &
Harris, S.T. (1992) Developmental effects of methyl benzimid-
azolccarbamate following exposure during early pregnancy.
Fundam. Appl. Toxicol., 18, 288-293.
Dashiell, O.L. (1975) Oral LD50 test in guinea pigs (Carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory Newark, Delaware, USA.
Donaubauer, H.H., Schuetz, E., Weigand, W. & Kramer, M. (1982)
Repeated dose (24 month) feeding study, for determination of the
carcinogenic effect of HOE 17411 OFAT204 (carbendazim) in mice.
Unpublished report from Hoechst AG, Pharmaceuticals Research,
Toxicology Section, Frankfurt, Germany.
Donovan, S.D. (1982). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Donovan, S.D. (1983). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Dorn, E. & Keller, H.M. (1980) Carbendazim (60% wettable powder)
absorption via the skin in rats. Unpublished report from Hoechst
AG, Frankfurt, Germany.
Dorn E., Schmidt E., Kellner H.M. & Leist K.H. (1983) HOE 017411-14-C
(carbendazim-14C) metabolic fate in rats and mice, a
comparison. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Edwards, D.F. (1974a) Skin absorption LD50 (carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Edwards, D.F. (1974b) Federal Hazardous Substances Act -- Eye
irritation test in rabbits (carbendazim). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Evenson, D.P., Janca, F.C. & Jost, L.K. (1987) Effects of the
fungicide methyl-benzimidazol-2-yl carbamate (MBC) on mouse germ
cells as determined by flow cytometry. J. Toxicol. Environ.
Health, 20, 387-399.
Everhart, L.P. (1979) Benlate dust exposure survey. Unpublished report
from E.I. DuPont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Fahrig, R & Seiler, J.P. (1979) Dose and effect of methyl-2-
benzimidazolyl-carbamate in the 'mammalian spot test', an
in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-Biol. Interactions, 26, 115-120.
Falke, H.E., Beems, R.B. & Spit, B. (1982a) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 60-day enzyme induction study
in mice. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Falke, H.E., Beems, R.B. & Spit, B. (1982b) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 59-day enzyme induction study
in rats. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Fiscor, G, Bordas, S. & Steward, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Ford, L.S. (1981) Primary skin irritation and sensitization on guinea
pigs. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Ford, L.S. (1982) Acute skin absorption LD50 test on rabbits
(carbendazim 75% wettable powder). Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
from Laboratory for Cancer Research, Hoechst AG, Frankfurt,
Germany.
Goldenthal, E.I. (1978) Neurotoxicity in hens. Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by E.I. DuPont de Nemours and
Co., Inc.
Goldman, J.M., Rehnberg, G.L, Cooper, R.L., Gray, L.E. Jr, Vein, J.F.
& McElroy, W.K. (1989) Effects of the benomyl metabolite,
carbendazim, on the hypothalamic-pituitary reproductive axis in
the male rat. Toxicology, 57, 173-182.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Goodman, N.C. & Sherman, H. (1975) Oral LD50 tests (fasted male and
female rats). Unpublished report from E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Grandizio, A.M. & Sarver, J.W. (1987) Acute oral toxicity study with
INE-965-261 (Benlate C) in male and female rats. Unpublished
report from E.I. DuPont de Nemours and Co., Inc., Haskell
Laboratory, Newark, Delaware, USA.
Gray L.E., Ostby, J., Sigmon, R., Ferrell, J., Rehnberg, G., Linder,
R., Cooper, R., Goldman, J. & Laskey, J. (1988) The development
of a protocol to assess reproductive effects of toxicants in the
rat. Reprod. Toxicol., 2, 281-287.
Gray, L.E., Ostby, J., Linder, R., Goldman, J., Rehnberg, G. & Cooper,
R. (1990) Carbendazim induced alterations of reproductive
development and function in the rat and hamster. Fundam Appl.
Toxicol., 15, 281-297.
Guengerich F.P. (1981) Enzyme induction with Du Pont compounds
H11,201-02 and H10,962-02. Unpublished report from Vanderbilt
University School of Medicine, Nashville, Tennessee, USA.
Submitted to WHO by E.I. Du Pont de Nemours and Co., Inc.,
Newark, Delaware, USA.
Hilscher, W., Ohnesorge, H. & Muller, H. (1992) The effects of
carbendazim on spermatogenesis. Unpublished report from Institut
für Toxicologie, Heinrich-Heine-Universitat, Dusseldorf, Germany.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hinckle, L. (1981) Oral LD50 test in rats -- EPA proposed
guidelines. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Hoechst AG (1974) Mouse dominant lethal study on MBC. Unpublished
report submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hoffman, H.T. & Kirsch, P. (1987) Report on the subchronic toxicity of
methyl benzimidazole-2-carbamate in beagle dogs on oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Hoffman, H.T. & Peh, J. (1987a) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hoffman, H.T. & Peh, J. (1987b) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hunter, B., Batham, P. & Newman, A.J. (1973a) Carbendazim oral
toxicity to rats in oral administration for 2 weeks. Unpublished
report from Huntingdon Research Centre, United Kingdom. Submitted
to WHO by Hoechst AG, Frankfurt, Germany.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. & Newman, A.J.
(1973b) Carbendazim toxicity to rats during dietary
administration for 13 weeks followed by a recovery period of 6
weeks. Unpublished report from Huntingdon Research Centre, UNited
Kingdom. Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Ivett, J.L. (1984) Mutagenicity evaluation of H#15,152 in an
in vitro sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report from Litton Bionetics,
Inc., Kensington, Maryland, USA. Submitted to WHO by E.I. DuPont
de Nemours and Co., Inc., Newark, Delaware, USA.
Janardhan, A., Sattur, P.B. & Sisodia, P. (1984) Teratology of methyl
benzimadazole carbamate in rats and rabbits. Bull. Environ.
Contam. Toxicol 33, 257-263.
Janardhan, A., Rao, A.B. & Sisodia, P. (1987) Sub-chronic toxicity of
methyl benzimidazole carbamate in rats. Bull. Environ. Contam.
Toxicol., 38, 890-898.
Jotz, M.M. (1980) An evaluation of mutagenic potential of benomyl
employing the L5178Y TK+/-mouse lymphoma assay. Unpublished
report from SRI International, Menlo Park, California, USA,
prepared for the US Environmental Protection Agency.
Kellner, H.M. (1983) Carbendazim-(2-14C) whole body autoradiography
studies of the distribution in rats and mice after oral and.
intravenous administration. Unpublished report from Hoechst AG,
Frankfurt, Germany.
Kellner, H.M. & Eckert, H. (1983) Carbendazim-(2-14C) blood levels,
distribution and excretion in rats and mice after oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Koeter, H.B.W.M. (1975a) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the rat Unpublished report from Central Institute
for Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, and BASF AG,
Ludwigshafen, Germany.
Koeter, H.B.W.M. (1975b) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the New Zealand white rabbit. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Kramer, M. & Weigand, W. (1971) (HOE 17411 OF) Toxicological
examination. Unpublished report from Hoechst AG, Pharmaceuticals
Research, Toxicology Section, Frankfurt, Germany.
Krechniak, J. & Klosowska, B. (1986) The fate of 14C-carbendazim in
the rat. Xenobiotica, 16, 809-815.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Litton Bionetics, Inc. (1981) Evaluation of HOE 17411 OF AT204 in the
primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Inc., Kensington,
Maryland, USA. Submitted to WHO by Hoechst AG, Frankfurt,
Germany.
Martin, D.A., Henry, J.E. & Brock, W.J. (1987) Closed-patch repeated
insult dermal sensitization study (Buehler method) with Benlate C
fungicide in guinea-pigs. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Meuling, W.J.A., Opdam, J.J.G. & DeKort, W.L.A.M. (1993) Dose-
excretion study with the fungicide carbendazim in volunteers.
Unpublished report from Central Institute for Nutrition and Food
Research (TNO) The Hague, Netherlands. Submitted to WHO by
Hoechst AG, Frankfurt, Germany.
Minta, M. & Biernacki, B. (1982) Embryotoxicity of carbendazim in
rats, rabbits and hamsters. Bull. Vet. Inst. Pulawy, 2, 42-52.
Mohr, U. (1977) Review of liver sections from mice and rats fed with
carbendazim. Unpublished report from Department of Experimental
Pathology, Medical School, Hanover, Germany. Submitted to WHO by
Hoechst AG, Frankfurt, and BASF AG, Ludwigshafen, Germany.
Monson K.D. (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.
Wilmington, Delaware, USA.
Nakai, M., Hess, R.A., Moore, B.J., Guttroff, R.F., Strader, L.F. &
Linder, R.E. (1992) Acute and long-term effects of a single dose
of the fungicide carbendazim (methyl 2-benzimidazole carbamate)
on the male reproductive system in the rat. J. Androl., 13,
507-518.
Nash, S.D. & Ferenz, R. (1982) Inhalation median lethal concentration
(LC50) in rats -- EPA protocol (carbendazim 75% wettable
powder). Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Osgood, C., Zimmering, S. & Mason, J.M. (1991) Aneuploidy in
Drosophila. II: Further validation of the FIX and ZESTE genetic
test systems employing female Drosophila melanogaster. Mutat.
Res., 259, 147-163.
Pandita, T.K. (1988) Assessment of the mutagenic potential of a
fungicide Bavistin using multiple assays. Mutat. Res., 204,
627-643.
Perreault, S.D., Jeffay, S., Poss, P. & Laskey, J.W. (1992) Use of the
fungicide carbendazim as a model compound to determine the impact
of acute chemical exposure during oocyte maturation and
fertilization on pregnancy outcome in the hamster. Toxicol.
Appl. Pharmacol., 114, 225-231.
Rashid, M.A. & Mumma, R.O. (1986) Screening pesticides for their
availability to damage bacterial DNA. J. Environ. Health Sci.,
B21, 319-334
Rehnberg, G.L., Cooper, R.L., Goldman, J.M., Gray, L.E, Hein, J.F. &
McElroy, W.K. (1989) Serum and testicular testosterone and
androgen binding protein profiles following subchronic treatment
with carbendazim. Toxicol. Appl. Pharmacol., 101, 55-61.
Reuzel, P.G.J., Hendriksen, C.F.M & Til, H.P (1976) Long-term
(two-year) toxicity study with carbendazim in beagle dogs.
Unpublished report from the Central Institute for Nutrition and
Food Research for BASF. Submitted to WHO by E.I. DuPont de
Nemours and Co., Wilmington, Delaware, USA.
Russell, J.F. (1977) Mutagenicity evaluation of 2-benzimidazole
carbamic acid, 5-hydroxymethyl in Salmonella typhimurium.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1983) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from E.I. DuPont de Nemours and
Co., Inc.. Haskell Laboratory, Newark, Delaware, USA.
Russell, L.D.. (1992). Review of Prof. Hilscher's report «Effects of
carbendazim on spermatogenesis». Unpublished report from
University of Illinois, USA. Submitted to WHO by Hoechst AG,
Germany.
Sarver, J.W. (1975) Acute inhalation toxicity -- one hour head only,
(carbendazim). Unpublished report from E.I. DuPont de Nemours,
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Scholz, J. & Weigand, W. (1972) Carbendazim (HOE 17411 OF, batch
496/I). Toxicological examination. Unpublished report from
Hoechst AG, Pharmaceuticals Research, Toxicology Section,
Frankfurt, Germany.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1965) Acute oral test. Unpublished report from E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. (1968) Ninety-day feeding study in rats using a wettable
powder formulation (70% MBC). Unpublished report from E.I. DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1970) Three-month feeding study in dogs using a wettable
powder formulation (50% MBC). Unpublished report from E.I DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1972) Long-term feeding studies in rats and dogs with
2-benzimadazole carbamic acid, methyl ester (INE-965) (50% and
70% MBC wettable powder formulations). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. and Krauss, W.C. (1966). Acute oral test (carbendazim).
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware.
Shirasu, Y., Morlya, M. & Kato, K. (1977) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Stadler, J.C. (1986) One-year feeding study in dogs with carbendazim.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Til, H.P., van den Muelen, H.C, Feron, V.J., Seinen, W. & de Groot,
A.P. (1972) Sub-chronic (90 day) toxicity study with W17411 in
beagle dogs. Unpublished report from Central Institute for
Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Til, H.P., Koellen, C. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with carbendazim in
rats. Unpublished report from Central Institute for Nutrition and
Food Research (TNO), The Hague, Netherlands. Submitted to WHO by
BASF AG, Ludwigshafen, and Hoechst AG, Frankfurt, Germany.
Til, H.P., Koeter, H.B.W.M. & van der Heijden, C.A. (1976b)
Multigeneration study with carbendazim in rats. Unpublished
report from Central Institute for Nutrition and Food Research
(TNO), The Hague, Netherlands. Submitted to WHO by Hoechst AG,
Frankfurt, Germany.
Til, H.P., Beems, R.B. & de Groot A.P. (1981) Determination of the
acute oral toxicity of carbendazim in mice. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Tong, C. (1981a) Hepatocyte primary culture/DNA repair assay on
compound 11, 201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Tong, C. (1981b) Hepatocyte primary culture/DNA repair assay on
compound 11,201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Vick, D.A. & Brock, W.J. (1987) Acute dermal toxicity study of Benlate
C fungicide in rabbits. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Vick, D.A. & Valentine, R. (1987) Primary eye irritation study with
Benlate C fungicide in rabbits. Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Waterer, J.C. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory Newark, Delaware, USA.
Whittaker, S.G., Moser, S.F., Maloney, D.H., Plegorsch, W.W., Resnick,
M.A. & Fogel, S. (1990) The detection of mitotic and meiotic
chromosome gain in the yeast Saccharomyces cerevisiae: Effects
of methyl benzimidazol-2-yl carbamate, methyl methanesulfonate,
ethyl methanesulfonate, dimethyl sulfoxide, propionitrile and
cyclophosphamide monohydrate. Mutat. Res., 242, 231-258.
Wood, C.K. (1982) Long-term feeding study with 2-benzimidazole-
carbamate, methyl ester (> 99% MBC, INE-965) in mice.
Unpublished report from E.I. DuPont de Nemours and Co, Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Zeller, H. & Kirsch, P. (1971) Acute oral toxicity of methyl-2-
benzimidazole carbamate to the rabbit. Unpublished report from
Hoechst AG, Frankfurt, Germany.
Table 1. Residual content of carbendazim in the livers of rats
Dosage Residual content (%)
Rat Mouse
Single dose
3 mg/kg bw 12 29
300 mg/kg bw 18 26
29-day repeated dose
3 mg/kg bw 2 < 2
300 mg/kg bw 4 28
In the study of Krechniak & Klosowska (1986), 94% of the measured
radiolabel in urine 12 h after treatment was as 5-HBC, 3% as 2-AB, and
3% as carbendazim.
The proposed metabolic pathway for carbendazim in rats is given
in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed for 28 days with diets containing benomyl or carbendazim at a
concentration of 0, 10, 30, 100, 300, 1000, or 3000 ppm. After
sacrifice, liver weights were recorded and microsomal epoxide
hydrolase and cytosolic glutathione- S-transferase were monitored in
subcellular fractions isolated from the liver. The mean absolute liver
weights were elevated in males and females fed 1000 or 3000 ppm
carbendazim and in females fed 300 ppm; however, the only
significantly increase was found in females fed 3000 ppm benomyl. No
apparent liver toxicity or effect on body weight was observed. Both
benomyl and carbendazim induced epoxide hydrolase in male and female
rats and mice fed 1000 or 3000 ppm, and both induced glutathione- S-
transferase at 3000 ppm. The level of induction seemed to be slightly
greater in females than males. There was no substantial difference in
enzyme induction between rats and mice (Guengerich, 1981).
In the same study, but in a separate test, CD-1 male mice were
treated by gavage with carbendazim suspended in 0, 100, or 1000 mg/kg
bw per day corn oil for five days. After sacrifice, liver samples were
homogenized, and subcellular fractions were prepared as described
above. Wet liver weights and the activities of microsomal cytochrome
P450, NADPH-cytochrome- creductase, styrene-7,8-hydrolase,
benzphetamine- N-demethylase, benzo[ a]pyrene hydroxylase,
7-ethoxycoumarin-deethylase, and cytosolic glutathione- S-transferase
were measured. The activities of styrene-7,8-hydrolase and
glutathione- S-transferase were statistically significantly increased
over the control values; that of 7-ethoxycoumarin-deethylase was
significantly decreased. It is noteworthy that the total microsomal
cytochrome P450 level did not increase, indicating that carbendazim
did not induce overall microsomal induction, even at the higher dose.
The increase in styrene-7,8-hydrolase activity shows, however, that
some hepatic microsomal enzymes are induced by carbendazim in vivo.
There appeared to be no substantial difference in enzyme induction
between rats and mice (Guengerich, 1981).
Groups of male Wistar rats and Swiss mice were given carbendazim
in the diet at levels of 0-10 000 ppm for 60 days, and the induction
of liver enzyme activities was examined and compared with that induced
by phenobarbital sodium administered in the drinking-water. Growth and
food consumption were decreased in rats at 10 000 ppm but not in mice
given up to 5000 ppm in the diet. Relative liver weights were
increased in rats fed 2000 or 10 000 ppm and in mice receiving 1000 or
5000 ppm carbendazim. Phenobarbital had similar effects. The protein
concentrations in total homogenates and post-mitochondrial fractions
of liver from rats were not affected by carbendazim, whereas they were
increased in mice at 5000 ppm. Feeding of carbendazim to rats at
2000 ppm or more resulted in slight-to-moderate induction of several
phase-I drug metabolizing enzymes: 7-ethoxycoumarin- O-deethylase,
biphenyl-4-hydroxylase, aniline hydroxylase, 4-methoxybiphenyl- N-
demethylase, and cytochrome- c-reductase. The activities of the
phase-II drug metabolizing enzymes glucuronyl transferase I and II and
the glutathione content were moderately-to-markedly increased at this
dose. Feeding of carbendazim to mice at 1000 ppm or more resulted in
moderate-to-marked increases in the activities of phase-I drug
metabolizing enzymes, including cytochrome P-450 and aminopyrine- N-
demethylase; cytochrome- c-reductase activity was decreased, and
those of glucuronyl transferase and glutathione- S-transferase and
glutathione content were slightly increased. There was no measureable
difference between rats and mice with regard to the metabolism of
carbendazim, although exhaustion of the detoxification mechanism was
more evident in mice at high doses. The detoxification and elimination
of carbendazim and its metabolites proceed more rapidly in rats than
in mice, as reflected in the increased glutathione content of rat
liver and the increased activity of phase-II enzymes (Falke et al.,
1982a,b).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
2. The clinical signs of toxicity after treatment with carbendazim
were generally nonspecific. The acute toxicity of carbendazim in a
number of species is low, LD50 values by various routes of
administration ranging from > 2000 to > 15 000 mg/kg bw. Gross and
histopathological changes were observed in the testes and epididymides
of male rats given carbendazim orally at doses of 1000 mg/kg bw and
more. The testes were small, soft, and discoloured, and more than 70%
of the tubules showed degenerative changes. The sperm count in the
epididymides examined was reduced or nil.
(b) Short-term toxicity
Rats
Groups of six male Sprague Dawley rats were given carbendazim by
gavage at a dose of 0, 200, 3400, or 5000 mg/kg bw per day, five times
per week for two weeks. Two rats at 3400 mg/kg bw per day died. At all
doses, gross and microscopic evidence of adverse effects on testes and
reduction or absence of sperm in the epididymides was seen. The testes
were small and discoloured, with tubular degeneration and evidence of
aspermatogenesis. At 3400 mg/kg bw per day, there were also
morphological changes in the duodenum (oedema and focal necrosis),
bone marrow (reduction in the blood-forming elements), and liver
(decrease in large, globular-shaped vacuoles) (Sherman, 1965; Sherman
& Krauss, 1966).
Groups of 10 male Sprague-Dawley rats were given carbendazim by
gavage at a dose of 0, 10, 20, 30, or 40 mg/kg bw per day for two
weeks. At the high dose, liver weights were increased. There were no
histopathological findings and no effects on spermatogenesis, on
cellularity, or on the incidence of mitosis, as evidenced by tritiated
thymidine incorporation 1 h before sacrifice (Hunter et al., 1973a).
Administration of 1350 ppm carbendazim to Sprague-Dawley rats for
13 weeks resulted in hepatomegaly in animals of each sex, which was
not accompanied by histopathological lesions and was reversible after
six weeks. A dietary level of 450 ppm (equal to 35 mg/kg bw per day)
was the NOAEL (Hunter et al., 1973b).
In a poorly reported study, groups of 10 male and 10 female
litter-mate weanling Wistar rats were treated by gavage with 0, 16,
32, or 64 mg/kg bw per day for 90 days. The erythrocyte counts in
treated rats were lower than those of the controls after 15 days of
exposure; however, no clear dose-response relationship was seen after
30, 60, or 90 days of exposure. Leukocyte counts were decreased after
Table 2. Acute toxicity of carbendazim and carbendazim formulations
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Carbendazim Mouse Oral > 15 000 NR Til et al. (1981)
Carbendazim Rat Oral > 10 000 > 95 Goodman & Sherman (1975)
Carbendazim Rat Oral > 11 000 > 95 Sherman (1965)
Carbendazim Rat Oral > 15 000 > 95 Kramer & Weigand (1971)
Carbendazim Rat Oral > 17 000 NR Sherman & Krauss (1966)
Carbendazim Guinea-pig Oral > 5 000 NR Dashiell (1975)
Carbendazim Rabbit Oral > 8 000 NR Zeller & Kirsch (1971)
Carbendazim Dog Oral > 5 000 NR Scholz & Weigand (1972)
Carbendazim Mouse Intraperitoneal > 15 000 NR Scholz & Weigand (1972)
Carbendazim Rat Intraperitoneal > 2 000 NR Scholz & Weigand (1972)
Carbendazim Rat Inhalation (1-h) > 5.9 NR Sarver (1975)
Carbendazim Rat Dermal > 2 000 NR Kramer & Weigand (1971)
Carbendazim Rabbit Dermal > 10 000 NR Edwards (1974a)
'75% wettable powder' Rat Oral > 5 000 NR Hinckle (1981)
'75% wettable powder' Rat Inhalation (4-h) > 5 > 95 Nash & Ferenz (1982)
'75% wettable powder' Rabbit Dermal > 2 000 > 95 Ford (1982)
'75% wettable powder' Rat Oral > 5 000 NR Grandizio & Saner (1987)
'75% wettable powder' Rabbit dermal > 2 000 > 95 Vick & Brock (1987)
NR, Not reported
15 days, and after 30 and 60 days animals of each sex showed transient
decreases in lymphocyte counts in comparison with controls, although
no clear dose-response relationship was observed among the treated
groups. No change was seen in the activity of whole-blood
cholinesterase. Male rats had significantly increased alkaline
phosphatase activity at a dose of 64 mg/kg bw per day, and blood urea
levels were lowered in males at 32 and 64 mg/kg bw per day after 90
days. Increased serum bilirubin concentrations were seen in males and
females at 32 and 64 mg/kg bw per day; these were attributable to
parenchymal cell damage, as indicated by increased alanine amino-
transferase activity. Dose-related changes in the liver ranged from
sparse infiltration by inflammatory cells to inflammatory and
degenerative changes. Tubular dilatation and hydropic degeneration
were noted in the kidneys of rats at the low dose, and fibrosis and
congestion were seen in rats at the medium and high doses. Increased
lung weights were correlated with bronchopneumonic changes. Slight
changes in weights were reported for several other organs. In view of
the lack of data on individual animals in this experiment and the
apparent variability in the results, no NOAEL could be established
(Janardhan et al., 1987).
Groups of 16 male and 16 female Sprague-Dawley rats were fed
carbendazim (purity, 72%) in the diet for 90 days at 0, 100, 500, or
2500 ppm (as carbendazim). The animals were observed daily for
behavioural changes and body weight, and food consumption was recorded
weekly. Haematological examinations were conducted on 10 male and 10
female rats in each group at 30, 60, and 90 days. Routine urinalyses
were performed on the same animals, and plasma alkaline phosphatase
and alanine aminotransferase levels were determined. After 90-98 days
of continuous feeding, 10 male and 10 female rats in each group were
killed, and selected organs were weighed; these and other organs were
also preserved for microscopic examination. The six male and six
female rats remaining in each group after terminal sacrifice were used
in a study of reproductive toxicity (see below). There were no
clinical signs of toxicity and no compound-related effects on weight
gain, food consumption, or haematological parameters. The relative
liver weights in females fed 2500 ppm were slightly increased in
comparison with control rats. Testicular weight was not affected in
any group. Microscopic examination of selected tissues and organs in
the control and high-dose groups showed no adverse effect attributable
to carbendazim. The NOAEL in this study was 500 ppm, equivalent to
50 mg/kg bw per day (Sherman, 1968).
Rabbits
Groups of six male New Zealand albino rabbits received 0 or
2000 mg/kg bw carbendazim as a 50% aqueous paste on shaved intact
dorsal skin repeatedly, for 6 h/day, for 10 consecutive days. There
were no adverse effects on body weight, clinical symptoms, organ
weights, gross pathology, or histopathology of selected organs;
however, there was focal necrosis of the epidermis and polymorpho-
nuclear cell infiltration of the dermis in five of six exposed
rabbits. No other effects were observed (Dashiell, 1975).
Dogs
Groups of four male and four female one-year-old beagle dogs were
given carbendazim (purity, 53%) in the diet for three, months at 0,
100, 500, or 2500 ppm (as carbendazim); the highest level was
subsequently reduced to 1500 ppm because of reduced food intake and
body weight. Food consumption and body weight were recorded weekly,
and clinical laboratory examinations, including haematological,
biochemical, and urinary measurements, were performed periodically. At
the end of the study, all animals were killed, selected organs were
weighed, and these and other organs were subjected to gross and
microscopic evaluations. No mortality or adverse clinical signs were
observed over the course of the study, and growth and food consumption
were normal, except in animals at 1500-2500 ppm. Urinalysis showed no
change due to treatment, and there were no dose-related effects on
haematological values. Females at the mid- and high doses showed a
trend to increased cholesterol levels over the pre-test and control
levels after one, two, and three months. The weights of the thymus
were increased in males at the low and mid-doses, and those of the
prostate at the mid-dose. Limited histopathological data did not
indicate any compound-related effects. The data for the high-dose
group were compromised by the change in dietary level, which involved
a 'recovery' period during which the animals received control diet
(Sherman, 1970).
Groups of four male and four female beagle dogs were given
carbendazim in the diet at 0, 100, 300, or 1000 ppm for 13 weeks. The
highest level was increased to 2000 ppm after six weeks of treatment.
Body weight, haematological, blood chemistry and urine measurements,
and liver and kidney function tests were performed periodically. The
animals were examined grossly and microscopically at the end of the
study. There were no reported compound-related effects on clinical
behaviour, body weight, food consumption, haematological parameters,
kidney function (phenol red excretion) or liver function (brom-
sulphthalein retention). Blood chemistry was normal, except for a
slight decrease in albumin in males at the mid- and high doses at 12
weeks. Urinalysis showed normal values, except for a high bacterial
count in females at the high dose at week 13. The blood clotting time
was slightly reduced in dogs at the high dose at week 12. There were
slight increases in relative liver and thyroid weights and a decrease
in relative heart weights in the group at the highest dose. No
microscopic changes that could be associated with treatment were
observed in these or other organs. The NOAEL was 300 ppm, equivalent
to 7.5 mg/kg bw per day, on the basis of minor changes in clinical
chemistry and organ weights (Til et al., 1972).
Groups of three male and three female beagle dogs were fed diets
containing 0, 500, 1500, or 4500 ppm carbendazim for 13 weeks. Reduced
body-weight gain, increased liver weight, hepatic periportal
infiltration, and hepatic regeneration were seen at the high dose. No
histopathological changes were seen in any other organs, including the
testes. At 1500 ppm, liver weights were increased, but there were no
histological lesions. The NOAEL was 500 ppm, equal to 12.5 mg/kg bw
per day (Hoffman & Kirsch, 1987).
Groups of five male and five female beagle dogs were fed diets
containing 0, 100, 200, or 500 ppm carbendazim for one year. The dogs
were weighed at regular intervals, and individual food consumption was
monitored throughout the study; clinical pathology was evaluated
periodically. After one year, all of the dogs were killed and selected
tissues were examined microscopically. There were no statistical
differences in mean body weight that could be attributed to treatment,
and the mean daily food consumption of treated dogs was similar to
that of controls. None of the clinical observations was attributable
to carbendazim intake. Dogs fed 500 ppm had elevated levels of serum
cholesterol, which were statistically significant for males at nine
months and for females at one and two months. There were no
microscopic lesions related to treatment. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day (Stadler, 1986).
Groups of four male and four female beagle dogs, one to two years
of age, were given carbendazim (purity, 53%) in the diet at 0, 100,
500, or 2500 ppm (as carbendazim) for two years. Food consumption and
body weights were measured weekly, and animals were examined daily for
clinical signs of toxicity. Haematological, biochemical, and urinary
examinations were performed periodically throughout the study. After
one year, one male and one female in the control and 500-ppm groups
were killed. At the end of the study, the organs were weighed, and
gross and histopathological examinations were performed. No mortality
was reported among the controls or dogs at 100 or 500 ppm; however,
three males at the high dose were sacrificed after 22 and 42 weeks
because of poor nutrition. No females at the high dose died.
Haematological and urinary values were unaffected by treatment. The
dogs at 500 ppm had increased levels of cholesterol, blood urea
nitrogen, total protein, and serum alanine aminotransferase. Swollen,
vacuolated hepatic cells and marginal proliferation of the portal
triads with cellular infiltration were observed in one dog that had
been fed 500 ppm and was sacrificed after one year. The biochemical
evidence of an effect on the liver was corroborated by the finding at
terminal sacrifice of hepatic cirrhosis, swollen, vacuolated hepatic
cells, and mild chronic hepatitis in dogs fed 500 ppm or more of
carbendazim. There were no effects on organ weights. Diffuse
testicular atrophy and aspermatogenesis were observed in two of four
males at 100 ppm but not at higher doses. As similar effects were not
seen at the other doses, these findings are considered not to be
treatment-related. The NOAEL was 100 ppm, on the basis of the effects
on the liver at 500 ppm, equivalent to 2.5 mg/kg bw per day (Sherman,
1972).
Groups of four male and four female beagle dogs, aged 22-27
weeks, were fed carbendazim in the diet at 0, 150, 300, or 2000 ppm
for 104 weeks. After 33 weeks, the dose of 2000 ppm was increased to
5000 ppm. The dogs were examined daily for clinical signs of toxicity
and altered behaviour; body weight and food consumption were recorded
regularly throughout the study, and haematological examinations, blood
chemistry (including liver and kidney function tests), and urinary
measurements were conducted periodically. After 104 weeks, the dogs
were sacrificed, and the tissues were reported to have been examined
grossly and microscopically, although the available data indicate that
the pathological examinations were inadequate. The only death was that
of a female at the high dose which was killed in a moribund state
after week 36. The body weights of males at the mid-dose and of males
and females at the high dose were decreased. Food consumption was
comparable in all groups. Blood clotting times were significantly
reduced in males at the high dose from week 13 to term, and slight
decreases were noted in females at the high dose. Serum alkaline
phosphatase activity was increased at the high dose throughout the
study, but there were no compound-related effects on serum alanine or
aspartate aminotransferase activities. All other haematological
parameters and blood chemistry were comparable with those of
the controls. There were no differences among the groups in
bromsulphthalein retention, phenol red excretion, or urinary values.
Liver and thyroid weights were significantly increased in dogs at the
high dose, but there were no microscopic changes in these organs that
were related to treatment. An increased incidence of prostatitis was
seen in high-dose males in comparison with controls (3/4 versus 1/4).
One male at that dose also had interstitial mononuclear inflammatory
cell infiltrates and atrophic tubules of the testes. Although feeding
of carbendazim in the diet to dogs for two years apparently had no
adverse effect at levels up to and including 300 ppm, the deficiencies
in the reporting of the pathological data preclude determination of an
NOAEL (Reuzel et al., 1976).
(c) Long-term toxicity and carcinogenicity
Mice
Carbendazim was administered in the diet to groups of 100 male
and 100 female specific pathogen-free Swiss mice at 0, 150, 300, or
1000 ppm for 80 weeks. The highest dose was increased to 2000 ppm at
week 4 and to 5000 ppm at week 8 for the remainder of the study.
Animals were examined for behaviour and for clinical signs of
toxicity, and body weights were measured throughout the study. All
animals were examined grossly, liver and kidney weights were recorded,
and tissues were examined microscopically. There were no compound-
related effects on general condition, mortality, or body weight. At
termination of the study, 70% of males and 80% of females were still
alive. The relative liver weights of males and females at the high
dose were significantly higher than those of controls, but kidney
weights were unchanged. After peer review and reclassification of the
data on hepatic tumour incidence, the combined incidence of
hepatocellular adenomas and carcinomas was found to have increased
with increasing doses in both males and females (Table 3). Males
showed more pronounced induction of liver tumours and more frequent
occurrence of hepatocellular carcinomas, which were often found
simultaneously with hepatocellular adenomas, whereas females usually
had only hepatocellular adenomas. It was concluded that carbendazim is
oncogenic in this strain of mouse at a dietary level of 5000 ppm
(Beems et al., 1976; Mohr, 1977).
Table 3. Hepatic rumour incidence in Swiss mice fed carbendazim
Sex Dietary level No.of mice Liver nodular Hepatocellular Hepatocellular
(ppm) examined hyperplasia adenoma carcinoma
Male 0 100 0 9 1
150 94 8 5 3
300 98 11 13 4
100/5000 100 25 14 9
Female 0 94 0 1 1
150 99 5 1 0
300 98 3 3 0
1000/5000 95 11 8 0
Groups of 80 male and 80 female CD-1 mice, aged six to seven
weeks, were given carbendazim (purity, 99%) in the diet at 0, 500,
1500, or 7500 ppm for two years. The highest dose was reduced to
3750 ppm for the males after 66 weeks because of increased mortality
(62 controls, 32 at 7500 ppm); females, however, received 7500 ppm
throughout the study. Treatment affected mortality in male mice, and
those at the high dose were sacrificed at week 73 because only 23 were
still alive. Only nine males at 1500 ppm survived to week 104, whereas
18 male controls were still alive at that time. Females had no similar
increase in mortality. There were no dose-related effects on body
weight or food consumption at any time, although the terminal body
weights of males at the low and mid-doses were lower than those of the
control and high-dose males. Clinical parameters were similar for all
treated and control groups, and haematological measures were
unaffected. Both absolute and relative thymic weights were
significantly decreased in females at 500 and 1500 ppm, but not in the
high-dose group. Absolute liver weights were increased in females at
the high dose and relative liver weights in those at the two highest
doses. The organ weights of male mice were variable, and only those of
the kidney and thymus appeared to be decreased as a result of
treatment. Absolute kidney and thymic weights were depressed in male
mice at all doses, but relative kidney and thymic weights were
significantly decreased only in males at the high dose. Histological
examination revealed dose-related changes in the thymus (lymphoid
depletion) and accumulation of yellow-brown pigment in the renal
tubules of male mice at the mid- and high doses. These mice also had
an increased frequency of sperm stasis in the testes and increased
bilateral germinal cell atrophy; there was no trend for unilateral
germinal cell atrophy, the incidence in controls being greater than or
equal to that of treated males. These effects are therefore considered
not to be compound-related. A significant hepatotoxic effect was seen
in male mice at 1500 and 7500 ppm, as demonstrated by centrilobular
hypertrophy, necrosis, and swelling. The frequency of hepatocellular
adenomas was not increased, as they occurred at equal frequency in
control and treated groups. There was a significant increase in the
incidence of hepatocellular carcinomas, but only at 1500 ppm; however,
too few males at the high dose survived to 17 months (510 days) to
support the conclusion that there is no oncogenic effect at that dose.
The combined incidence of hepatocellular carcinomas, hepatocellular
adenomas, and hepatoblastomas (Table 4) was significantly increased
(P < 0.05) in females at the low, mid-, and high doses and in males
at the mid-dose, but this was not evaluated in high-dose males because
of the high rate of mortality. The study thus shows statistically
significant increases in the incidences of hepatocellular carcinoma
for males at 1500 ppm and for females at all doses, the response being
dose-related. A dose-response relationship could not be determined
because of the high mortality in the high-dose males; the high
mortality rate in male controls also hampered interpretation of
results. No carcinogenic effect was observed in tissues other than the
liver (Wood, 1982).
Carbendazim was administered in the diet of groups of 100-120
male and female NMRKf mice for 96 weeks at a dose of 0, 50, 150, 300,
or 1000 ppm. The highest dose was increased to 2000 ppm at week 4 and
to 5000 ppm at week 8 for the remainder of the study. Animals were
examined for behaviour and general condition and for body weight, food
and water consumption, and mortality. Gross necropsy was performed on
all animals, liver and lung weights were recorded, and all organs and
tissues were examined microscopically. An interim sacrifice was
conducted of 20 males and 20 females in the control group and that at
the highest dose at 18 months. There were no compound-related effects
on behaviour, body-weight gain, food or water consumption, or
mortality. By 22 months, 24-31% of the males and 37-52% of the females
had died. As there was no difference between the treated and control
groups, it was concluded that mortality was not influenced by the
feeding of carbendazim. At 18 and 22 months, the absolute and relative
liver weights of both male and female mice at 5000 ppm were increased.
Macroscopic and microscopic examination of 20 male and 20 female
animals killed after 18 months of receiving 5000 ppm carbendazim
revealed compound-related effects on the liver: all animals had
centrilobular hypertrophy, single-cell necrosis, mitotic cells, and
pigmented Kupffer cells. The tissues of the remaining 100 males and
100 females exposed to 5000 ppm, evaluated at 22 months, showed marked
hypertrophy (greater than in animals treated for 18 months), clear-
cell foci, mitosis, inclusion bodies in enlarged cell nuclei, multiple
cell necrosis, and a greenish-yellow pigment in Kupffer cells.
Neoplastic nodules (adenomas), carcinomas, fibrosarcomas, and other
tumorigenic responses in the liver were equally distributed among the
groups. Although haemangiomas of the liver were found in all treated
groups but not in controls, no dose-related response was evident. Lung
adenomatosis was equally distributed among the groups. The tumour
incidence is summarized in Table 5. There was no effect on the
incidence or time of onset of tumours, and the total number of benign
and malignant tumours was comparable among the different groups of
mice. Thus, there was no evidence that carbendazim administered in the
diet of mice at doses up to and including 5000 ppm for 22 months had a
carcinogenic effect. The NOAEL was thus 300 ppm, equal to 34 mg/kg bw
per day (Donaubauer et al., 1982).
Rats
Groups of 36 male and 36 female weanling Sprague-Dawley rats were
given carbendazim (purity, 50-70%) in the diet for 104 weeks at 0,
100, 500, 2500 (increased to 10 000 ppm after 20 weeks), or 5000 ppm
(as carbendazim). Body weight and food consumption were recorded
weekly for the first year and twice a month thereafter. Behavioural
changes and mortality were observed daily. Haematological, urinary,
and selected clinical chemical examinations were performed
periodically. After one year, each group was reduced to 30 male and 30
female rats by interim sacrifice for gross and microscopic
examinations. At the end of the study, all surviving animals were
sacrificed, and tissues and organs were examined grossly. Microscopic
examinations were conducted on all tissues and organs from the
controls and animals at 2500-10 000 ppm, the livers of animals at 100
and 500 ppm, and the livers, kidneys, testes, and bone marrow of
animals at 5000 ppm. Body-weight gain was depressed in males and
females at 2500-10 000 ppm and in females at 5000 ppm in comparison
with controls. Food consumption was similar in all groups. Reduced
erythrocyte counts and haemoglobin and haematocrit values were seen in
females at 2500-10 000 or 5000 ppm after 9-24 months and in males at
2500-10 000 ppm after 24 months. There were no compound-related
Table 4. Hepatic tumour incidence in CD-1 mice fed carbendazim
Sex Dietary level No. of mice No. of mice alive Median survival Combined incidence
(ppm) examined at termination (weeks) of hepatic tumours
Male 0 80 18 79 13
500 80 14 72 20
1500 80 9 69 23
7500/3500 80 23 64 NA
Female 0 79 22 91 1
500 78 15 91 9
1500 80 13 91 21
7500/3500 78 20 91 15
NA, not analysed
Table 5. Hepatic tumour incidence in NMRK-f mice fed carbendazim
Sex Dietary level No.of mice Clear-cell Basophilic Hepatocellular Liver
(ppm) examined foci loci adenomas haemangiomas
Male 0 97 0 0 3 0
50 99 0 0 2 2
150 99 0 1 0 3
300 95 0 0 0 2
1000/5000 99 3 0 1 0
Female 0 98 0 0 0 0
50 98 0 1 00
150 95 0 0 0 0
300 95 0 0 1 2
1000/5000 95 4 0 0 1
clinical manifestations of toxicity and no effects on urinary
parameters. Alkaline phosphatase and alanine aminotransferase
activities varied throughout the study in animals at 2500-10 000 or
5000 ppm, but there was no consistent dose-response relationship.
Organ weights and organ-to-body weight ratios were unchanged, except
for the livers of females at 2500-10 000 or 5000 ppm, but the increase
in the liver-to-body weight ratio was due to a reduction in body
weight. Histopathological examination of the livers showed no
compound-related effects. Males at 2500-10 000 ppm had a marginal
increase in the frequency of diffuse testicular atrophy and
prostatitis. The NOAEL was 500 ppm, equivalent to 15 mg/kg bw per day
(Sherman, 1972).
Groups of 60 male and 60 female Wistar rats were given
carbendazim (purity, 99%) in the diet at 0, 150, 300, or 2000 ppm for
two years. The dose of 2000 ppm was increased to 5000 ppm after one
week and to 10 000 ppm after two weeks for the remainder of the study.
Animals were examined daily for clinical signs of toxicity. Body
weight and food consumption were measured regularly throughout the
study. Haematological measurements (peripheral blood), blood chemistry
(orbital sinus), and urinalysis were conducted periodically. All
animals were subjected to complete gross necropsy, and selected organs
were weighed. Tissues from 20 male and 20 female rats in the control
and high-dose groups were examined microscopically, and all tumours
and gross abnormalities were examined histologically. No differences
in clinical signs of toxicity or food consumption were seen between
test groups and control animals. Body weights were significantly
reduced in low-dose males from week 88 to term and in high-dose
females from week 12 to term. The results of urinalyses were
comparable among the groups. The haemoglobin level was depressed in
high-dose females a t weeks 26, 52, and 103, and the haematocrit was
depressed in high-dose females at week 103. There were no compound-
related effects in males. Serum aspartate aminotransferase activity
was decreased in high-dose males at termination of the study, but
not in females; high-dose females had increased serum alanine
aminotransferase activity and decreased total serum protein. The only
compound-related effects on organ weights were increased relative
liver weights in high-dose females. There were no compound-related
effects on mortality, and survival at termination of the study was
similar in all groups. There were no histological differences between
control and treated groups, except for an increased incidence of
diffuse proliferation of parafollicular cells of the thyroid in the
high-dose females. The number of tumour-bearing animals and the total
number of tumours were comparable among all groups, and no compound-
related oncogenic effects were reported. The NOAEL was 300 ppm,
equivalent to 15 mg/kg bw per day, on the basis of changes in organ
weights and minor biochemical changes (Til et al., 1976a).
(d) Reproductive toxicity
Groups of six male and six female rats were removed from a 90-day
study in which they were fed dietary levels of 0, 100, 500, or
2500 ppm carbendazim. Each female was exposed to three males from the
same dose group to produce the F1a generation; each litter was
reduced to 10 pups on the fourth day after birth. The F0 animals were
mated again after about one week to produce the F1b litter.
Reproduction indices and status at birth and on days 4, 12, and 21
were recorded, as were body weights at weaning. The data were
extremely limited and were available only for groups. At 100 ppm,
neither the F1a nor the F1b animals became pregnant. There were no
apparent effects on reproduction indices or weanling weights, but the
fertility indexes for all groups, which were only 33-67%, prevented
meaningful interpretation of the data (Sherman, 1968).
Groups of three male and 16 female Sprague-Dawley rats were fed
carbendazim in the diet at 0, 100, 500, 5000, or 10 000 ppm
(20 females at the highest dose) and mated in a standard study of two
litters per generation for three generations. The parental animals
were fed the experimental diet at 21 days of age and mated to produce
the F1 litter at 100 days of age; the numbers of matings,
pregnancies, and young were recorded for each litter at birth. The
litters were culled to 10 pups on day 4. The number of live pups was
again recorded on days 4, 12, and 21, as was pup weight at weaning.
The parents were mated again to produce F1b litters, which were
maintained on the respective diets for 110 days and then mated to
produce the F2a and F2blitters; F3a and F3b litters were
produced similarly. Selected tissues and organs from two males and two
females in each of five F3b litters from the controls and from
the groups fed 5000 and 10 000 ppm were examined grossly and
histopathologically. Reproduction indices, including mating,
fecundity, fertility, gestation, viability, and lactation, were
calculated and compared with control values. Carbendazim had no effect
on fertility, gestation, viability, or lactation, but the average
litter weights at weaning were reduced in all generations fed 5000 and
10 000 ppm. Histopathological examination of F3b weanlings did not
reveal any effects that were considered to be compound-related
(Sherman, 1972).
Carbendazim was administered to groups of 10 male and 20 female
Wistar rats at a dietary level of 0, 150, 300, or 2000 ppm for three
generations, and two successive litters were reared from each female
General condition and behaviour were routinely observed, and
individual body weights were recorded throughout the study. The
numbers of pups in each litter were recorded, and each litter was
culled to eight on day 1. The total weight of each litter was measured
on days 1, 10, and 20. The F1a and F2a litters were discarded at
weaning and the F1b and F2b litters were used to produce
succeeding generations. The F3a offspring were used to study
teratogenicity, while the F3b offspring w ere used in a four-week
evaluation of toxicity. Neither health nor body-weight gain was
affected, but all generations of the treated groups weighed
significantly more than controls. The compound had no effect on
fertility or on survival at birth, at day 10, or at day 20. Litter
size was not affected by treatment, except for a marginal decrease in
F2a litters at all doses. There were no differences in the F2a
litters at 300 and 2000 ppm, but litter size was decreased and
mortality at birth increased at 150 ppm. Birth weight during the
lactation period was comparable in all groups. There were no gross
abnormalities related to treatment. Autopsy of rats in the four-week
study showed increased relative liver weights and decreased relative
spleen weights in females fed 2000 ppm; relative ovarian weights were
also significantly decreased in all dose groups. Histopathological
examination of the livers showed no compound-related change;
histopathological results were not presented for other organs. No
maternal or fetal toxicity was evident, and there were no differences
in visceral anomalies in animals at 0 or 2000 ppm (the only groups
examined). Thoracic vertebral bodies were reduced at 2000 ppm, and
there was a significant reduction in cervical vertebral bodies at
2000 ppm. Controls, however, had more significant changes with regard
to absent or delayed ossification of skeletal structures. There were
no apparent adverse effects on reproduction and no teratogenic effects
at dietary levels of carbendazim up to and including 2000 ppm,
equivalent to about 120 mg/kg bw per day (Koeter et al., 1976).
A serial breeding technique was used to evaluate the fertility of
male Sprague-Dawley rats after exposure by gavage to 10 daily doses of
400 mg/kg bw per day carbendazim. Males, 90 days old and proven to be
fertile, were bred with a new female each week, starting on the third
day of treatment and continuing for 32 weeks after the last day of
treatment. Twelve days after each breeding period, the females were
killed, their uteri were examined for resorptions, and the numbers of
dead and viable fetuses were determined. All males were killed 35
weeks after treatment, and testicular tissue was prepared for
histopathological examination by vascular perfusion. The fertility of
treated males (as indicated by the number of pregnant females) was
depressed during the first week after treatment: 10 of the 24 treated
males failed to induce a pregnancy, as compared with no failure in the
control group. By the fifth week after treatment, 16 of the 24
carbendazim-treated males were infertile. Of these, four recovered
fertility after being infertile for 5-11 consecutive breeding periods,
but the other 12 did not recover during the remainder of the 32-week
period after treatment. Histological examinations of testicular
sections of the latter animals 245 days after treatment revealed
severe seminiferous tubular atrophy (> 85% of tubules were atrophic),
often with epithelium containing only Sertoli cells, surrounded by a
thickened basement membrane. The lumina of < 2% of the tubules
contained spermatozoa. The seminiferous tubules of the treated males
that recovered fertility had various contents of atrophic tubules
(13-85%) 245 days after treatment (Carter et al., 1987).
Groups of 8-12 male and 8-12 female rats were given 0, 50, 100,
200, or 400 mg/kg bw per day carbendazim by gavage from weaning
through puberty, gestation, and lactation and were mated at 84 days of
age. The male rats were killed on days 104-106 and the female rats on
day 27 post partum. In a similar study, Syrian hamsters were given 0
or 400 mg/kg bw per day carbendazim. Various landmarks of puberty were
measured in the parental generation. In females, estrous cyclicity,
litter size, the number of implants, organ weights, and histological
status were assessed. In males, organ weights, testicular and
epididymal sperm counts, sperm motility, sperm morphology, testicular
histological status, and endocrine parameters were assessed. In
addition, the growth, viability and reproductive function of the
offspring (F1) were observed during a four-month period of
continuous breeding. Males were killed after five months for
histopathological investigation. Carbendazim did not alter pubertal
development, growth, or viability in the parental generation of either
species. The reproductive potential of rats treated with 200 or
400 mg/kg bw per day was reduced due to effects on sperm production
and fetal viability. These doses markedly altered sperm morphology,
testicular and epididymal weights, sperm numbers, and testicular
histology; fertility, sperm mobility, and hormonal levels were altered
primarily in males with very low sperm counts. A statistically
significant reduction in the caudal epididymal sperm count was noted
at doses of 50 mg/kg bw per day or more. Testicular and epididymal
sperm counts in male hamsters were significantly lower (about 21%) in
treated than in control males. In F1 male hamsters, testis and
seminal vesicle weights and epididymal sperm counts were significantly
reduced by prenatal exposure to carbendazim at 400 mg/kg bw per day.
Parental female rats exposed at this dose had post-implantation
losses, and a few malformed pups were found in litters of animals at
100 or 200 mg/kg per day. Litter size was significantly reduced at 200
and 400 mg/kg bw per day. Overall, carbendazim was less toxic to
hamsters than to rats (Gray et al., 1988, 1990).
Carbendazim was fed to groups of eight female Holtzmann rats by
gavage at doses of 0, 25, 50, 100, 200, 400, or 1000 mg/kg bw per day
during early pregnancy (days 1-8). A range of parameters, including
the number of implantation sites, body-weight gain, uterine weight,
implantation site size, and serum ovarian and pituitary hormones, was
assessed after sacrifice on day 9. At doses up to 400 mg/kg bw per
day, carbendazim had no significant effect on any of the measured
parameters, but a trend towards increased resorptions was evident. The
highest dose reduced maternal body-weight gain, implantation site
size, and serum luteinizing hormone levels and increased serum
estradiol (Cummings et al., 1990).
(e) Developmental toxicity
Rats
Groups of 27-28 pregnant Sprague-Dawley rats were fed carbendazim
(purity, 53%) in their diet at 0, 200, 500, 2500, 5000, 7500, or
10 000 ppm on days 6-15 of gestation. On day 20 of gestation, all of
the animals were sacrificed and the fetuses were delivered by
caesarean section. The numbers and location of live and dead fetuses
and resorption sites, body weights, crown-rump length, sex, and
visible abnormalities were determined. Two-thirds of the fetuses were
prepared for examination for skeletal abnormalities, and the remainder
were examined for visceral and soft-tissue anomalies. There were no
deaths, no adverse effects on body weight, and no clinical signs of
toxicity. Food intake was reduced at the highest dose during the
period the test diet was administered but returned to control levels
from day 16 to 20. The numbers of implantation sites, resorption
sites, and live and dead fetuses were not adversely affected. Although
data on individual litters were not presented, carbendazim did not
appear to be teratogenic when administered to rats at dietary levels
up to and including 10 000 ppm during the critical period of
organogenesis (Sherman, 1970).
Groups of 18-22 pregnant Wistar specific pathogen-free rats were
given carbendazim in the diet at doses of 0, 600, 2000, or 6000 ppm on
days 6-15 of gestation. On day 21 of gestation, all of the rats were
sacrificed and the pups delivered by caesarean section. Dams were
weighed periodically during the test, and food consumption was
measured for specific periods. The number of corpora lutea was
determined, the ovaries were weighed, and the fetuses were weighed and
examined. The numbers of implantation and resorption sites were
recorded, and the empty uterine horns weighed. One-third of the
fetuses were fixed and stained for skeletal examination, and the
remaining two-thirds were examined for soft-tissue anomalies. Although
23 females per group were mated, the pregnancy rate was variable, with
18, 22, 20, and 18 pregnant rats in the groups receiving 0, 600, 2000,
and 6000 ppm, respectively. The mean body-weight gain and food
consumption of the high-dose females were significantly decreased in
comparison with controls. The numbers of live and dead fetuses,
implantation sites, embryonal resorptions, fetal resorptions, and
corpora lutea per dam were comparable in all groups, and ovarian
weights, the weights of the empty uteri, mean fetal weight per litter,
the sex ratio, and pre- and post-implantation losses were not affected
by treatment. No visceral anomalies were reported that were
significantly different from those seen in controls. Misshapen and
fused bones were much more frequent in the high-dose groups then in
any other group, and the incidence of supernumerary ribs was
significantly increased in high-dose females. Ossification was
significantly delayed or absent in pups at the high dose, particularly
in forelimbs, hindlimbs, sternebrae, and skull bones; ossification was
also significantly delayed or absent in cervical vertebral bodies in
all treated groups. It was concluded that carbendazim has no
teratogenic potential when administered in the diet to rats at levels
up to 6000 ppm, although ossification is reduced in a dose-related
manner (Koeter, 1975a).
Groups of 8-10 female Wistar rats were given carbendazim (purity,
98%) by gavage at 0, 20, 40, or 80 mg/kg per day on days 6-15 of
pregnancy. Half of each group of animals was killed on day 21 of
gestation, and half was allowed to deliver normally. All sacrificed
animals were scored for live and dead fetuses and for resorptions;
live fetuses were killed and examined for abnormalities. After normal
deliveries, neonatal deaths and survivors were counted, and survivors
were weighed and examined for gross abnormalities. In rats sacrificed
on day 21, dead and resorbed fetuses accounted for 29% of the
conceptuses among controls, 48% at 20 mg/kg per day, 64%, at 40 mg/kg
per day, and 73% at 80 mg/kg per day. There were no differences among
the various groups with respect to mean weight of live fetuses, and
there were no malformations. The average number of live pups per
litter was close to eight in controls, six at 20 mg/kg per day, and
about five at 40 and 80 mg/kg per day. Mean fetal weight was increased
by about 13% over that of controls at the two highest doses. There
were no stillbirths, neonatal deaths, or gross abnormalities, but
mortality at 21 days post partum was 3.0-3.5 times greater at the
two highest doses than in controls (Janardhan et al., 1984).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethyl-cellulose by gavage to groups of 15-26 Sprague-Dawley
rats on days 6-15 of gestation at daily doses of 10-3000 mg/kg bw per
day. The lowest dose had no effect on dams or their offspring and was
considered to be a no-effect lewd. At 30 mg/kg bw per day,
fetotoxicity was evident: 42% of the fetuses in 19/21 litters had
malformations affecting the head, spine, ribs, and sternum. At
60 mg/kg bw per day, 2/23 animals aborted and 51% of the implantations
in the remaining dams were dead. Malformations were seen in 90% of
fetuses, and all litters were affected. At 100 mg/kg bw per day, 15
pregnant animals produced only four live fetuses in three litters, all
of which were malformed. At 300, 1000, and 3000 mg/kg bw per day,
severe toxicity resulted in early resorptions only. The no-effect
level of 10 mg/kg bw per day was confirmed in a further study
specifically of fetotoxicity and hydrocephaly (Hoffman & Peh,
1987a,b).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethylcellulose by gavage to groups of 25 Sprague-Dawley rats
on days 7-16 of gestation at doses of 0, 5, 10, 20, or 90 mg/kg bw per
day. Maternal toxicity was seen only at the highest dose, in the form
of depressed weight gain during treatment and before sacrifice on day
22. The mean liver weights and liver-to-body weight ratios were
increased. A decreased pregnancy rate was observed at the highest
dose. An increase in the incidence of early resorptions per dam,
decreased litter size, and total resorption of three litters occurred
at the highest dose, but only the reduction in females per litter was
significant. Significant reductions in mean fetal weight were observed
at both 20 and 90 mg/kg bw per day, and a significant increase in the
incidence of fetal malformations was seen at the highest dose. The
malformations consisted primarily of hydrocephaly, microphthalmia,
anophthalmia, malformed scapulae, and axial skeletal malformations
(vertebral, rib, and sternebral fusions, exencephaly, hemivertebrae,
and rib hyperplasia). The NOAEL was 20 mg/kg bw per day for the dams
and 10 mg/kg bw per day for the fetuses (Alverez, 1987).
Carbendazim (purity, 95%) was administered by gavage to female
Holtzman rats at doses of 0, 100, 200, 400, or 600 mg/kg bw per day
during days 1-8 of gestation, and the rats were sacrificed on day 11
or 20. No maternal toxicity was seen at any dose. On day 11, the
crown-rump length, head length, number of somites, and number of
embryos per dam were significantly reduced in groups receiving
200 mg/kg bw per day or more. Open posterior neuropores and limb
anomalies were observed more frequently at doses > 200 mg/kg bw per
day. On day 20, increased resorptions, decreased live litter size and
fetal body weight, and delayed ossification were observed at all
doses. Skeletal malformations seen at the high dose were attributed to
treatment. The authors noted that developmental alterations occurred
after termination of treatment, suggesting either that the anomalies
represent delays in development or that the embryonic cells are
vulnerable at earlier stages than was previously thought (Cummings
et al., 1992).
Rabbits
Groups of 3-11 pregnant New Zealand albino rabbits were given
carbendazim in the diet at 0, 600, 2000, or 6000 ppm on days 6-18 of
gestation. On day 29 of gestation, all of the animals were sacrificed
and the fetuses delivered by caesarean section. Does were weighed
periodically, and food consumption was determined for specific
periods. The number of corpora lutea was determined, the ovaries were
weighed, and the fetuses were weighed and examined; the numbers of
implantation sites and resorption sites were recorded, and the empty
uteri weighed. One-half of fetuses were stained and sectioned for
determination of skeletal anomalies, and the other half was examined
for soft-tissue abnormalities. Only the fetuses in the high-dose and
control groups were examined for visceral anomalies. Although 15
females per group were artificially inseminated, the pregnancy rate
was extremely variable, with nine in controls, eight at 600 ppm, three
at 2000 ppm, and 11 at 6000 ppm. The mean body-weight gain was
significantly decreased in the high-dose group, although food
consumption did not vary among groups. The numbers of live and dead
fetuses, implantation sites, embryonal resorptions, fetal resorptions,
and corpora lutea per doe were comparable in all groups. The ovarian
and uterine weights of animals at the high dose were depressed in
comparison with control females; Pre- and post-implantation losses
were not affected by treatment. The incidence of visceral anomalies
appeared to differ between the high-dose and control groups, but too
few litters and fetuses were examined to allow a conclusion. There
were significantly increased numbers of supernumerary ribs (bilateral)
and skull bones in the high-dose group. Ossification was significantly
delayed or absent in fetuses in the high-dose group, most notably in
the forelimb metacarpals and phalanges. There was also incomplete
ossification of the sternebrae and skull bones, which was significant
at 600 and 6000 ppm, and misshapen sternebrae were present at the
highest dose. Although it can be concluded that carbendazim does not
induce teratogenic effects when administered in the diet to rabbits at
levels up to 6000 ppm, there were too few animals, litters, and
fetuses at 2000 ppm to allow a useful evaluation of compound-related
effects (Koeter, 1975b).
Female albino rabbits were given 0, 40, 80, or 160 mg/kg bw per
day carbendazim by gavage on days 6-18 of pregnancy and were
sacrificed on day 31. All of the animals were scored for live and dead
fetuses and for resorptions; live fetuses were killed and examined for
abnormalities. There were no dead or resorbed fetuses in controls, but
15% of all conceptuses were dead at 40 mg/kg bw per day, 21.7% at 80
mg/kg bw per day, and 33.3% at 160 mg/kg bw per day. There were no
differences among the various groups with respect to mean weight of
live fetuses, and there were no malformations (Janardhan et al.,
1984).
Suspensions of carbendazim in aqueous 0.5% carboxymethylcellulose
were administered by gavage on days 7-19 of presumed gestation to
groups of 20 artificially inseminated New Zealand white rabbits at
doses of 0, 10, 20, or 125 mg/kg bw per day. The highest dose
inhabited average maternal weight up to day 16 of gestation. The
implantation rate was decreased at 20 and 125 mg/kg bw per day, and
the incidence of resorptions was increased at 125 mg/kg bw per day,
resulting in decreased live litter size at these doses. At the highest
dose, decreased fetal body weight was seen, but the effect was not
statistically significant. The average percentage of malformed fetuses
per litter was significantly increased at 125 mg/kg bw per day.
Compound-related malformations at the highest dose consisted of
malformed cervical vertebrae and interrelated malformation of the ribs
and proximate thoracic vertebrae. The NOAEL for maternal toxicity was
20 mg/kg bw per day, and the NOAEL for developmental toxicity was
10 mg/kg bw per day (Christian et al., 1985).
Hamsters
Groups of 7-11 pregnant hamsters were treated orally on day 10 of
gestation with 0, 15, 30, 75, or 150 mg/kg bw carbendazim A
significant increase in malformations, including exencephaly, was
observed at 75 and 150 mg/kg bw. These doses were also embryotoxic,
producing 31 and 45% resorptions, respectively (Minta & Biernacki,
1982).
(f) Genotoxicity
Numerous studies have been conducted to assess the mutagenic
potential of carbendazim. Many of the results are conflicting, and
many of the reports do not provide sufficient detail to evaluate the
reasons for the conflicting data. Since before the mid-1980s
industrially produced carbendazim contained phenazine impurities, the
use of carbendazim with different degrees of purity might account for
some of the discrepancies. Table 6 summarizes those reports that
included sufficient experimental detail and data.
Carbendazim is not a heritable gene mutagen; it does not interact
with cellular DNA, induce point mutations, or result in germ-cell
mutations, as seen in both mammalian and nonmammalian systems in
vitro and in vivo and in somatic and germ cells. Positive results
have occasionally been obtained in tests for gene mutation, but they
may have been associated with the presence of phenazines, which are
mutagenic at very low concentrations in Salmonella typhimurium in
Ames' test and in mouse lymphoma LY5178Y tk+/- cells.
Concentrations of > 4 ppm diaminophenazine and 10 ppm aminohydroxy-
phenazine were mutagenic in Ames' test. Process changes by some of the
major manufacturers of carbendazim have removed the phenazine, and
this contaminant is not present when other benzimidazoles, such as
benomyl and thiophanate-methyl, are metabolized to carbendazim.
Carbendazim does cause numerical chromosomal aberrations (aneuploidy
and/or polyploidy) in experimental systems in vitro and in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A 75% wettable powder formulation induced slight, transient
irritation when applied to the in tact and abraded skin of albino
rabbits. A 55% suspension of the 75% wettable powder formulation in
dimethyl phthalate induced mild irritation when applied to the intact,
shaved skin of albino guinea-pigs, while a 5.5% concentration produced
no irritation (Ford, 1981).
The primary dermal irritation potential of Benlate C (50%
wettable powder) was evaluated by applying a 5-g aliquot to the
intact, clipped skin of six New Zealand white rabbits for 4 h. The
test sites were evaluated for erythema, oedema, and other evidence of
dermal effects and were scored according to the Draize scale 4, 24,
48, and 72 h after application. No dermal irritation was seen at any
time during the study (Vick & Brock, 1987).
Table 6. Results of tests for the genotoxicity of carbendazim
End-point Test system Concentration Results Reference
or dose
Tests for gene mutation
Reverse mutation S. typhimurium TA98, < 2500 µg/plate Negative Gericke (1977)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium TA100, < 200 µg/plate Negative Fiscor et al. (1978)
TA1530, TA1535, TA1950
Reverse mutation S. typhimurium TA1530, < 100 µg/spot Weakly positive Fiscor et al. (1978)
TA1950, G46 his-
Reverse mutation S. typhimurium TA100, < 1000 µg/plate Negative Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP2 hcr
Reverse mutation S. typhimurium TA98, < 300 µg/plate Negative Pandita (1988)
TA100
Reverse mutation S. typhimurium TA97, 5000 µg/plate Positive (only with Albertini (1989)
TA98, TA1537, TA1538 activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Positive, TA1537, Donovan (1982)
TA100, TA1535, TA1537 TA98 (with
activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Negative Russell (1983)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium Positive, only with Arce (19844)
4 ppm DAP or
10 ppm AHP
Reverse mutation S. typhimurium TA98, < 20 000 µg/plate Negative Russell (1977);
TA100, TA1535, TA1537 Donovan (1983)
hprt mutation Chinese hamster ovary cells < 654 µmol/litre Negative Waterer (1980)
tk mutation L5178Y mouse lymphoma < 250 µmol/litre Positive (with Jotz (1980)
cells activation)
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
Tests for chromosomal effects
Sister chromatid exchange Chinese hamster ovary cells < 40 µg/ml Negative Ivett (1984)
Sister chromatid exchange Human lymphocytes < 30 µg/ml Negative Banduhn & Obe (1985)
Sister chromatid exchange Human lymphocytes < 60 µg/ml Marginally positive Pandita (1988)
Chromosomal gain S. cereviriae < 0.1 µg/ml Positive Whittaker et al. (1990)
Aneuploidy S. cereviriae < 5 µg/ml Positive Albertini (1991)
Chromosomal aberrations Human lymphocytes < 10 µmol/litre Not clastogenic but Banduhn & Obe (1985)
induces micronuclei
Chromosomal aberrations Human lymphocytes < 0.5 mg/ml Negative Lamb & Lilly (1980)
DNA damage and repair
Rec assay B. subtilis < 1000 µg/disc Negative Shirasu et al. (1977)
Rec assay S. typhimurium TA1535, < 2000 µg/plate Negative Rashid & Mumma
(1986)
TA1538, E. coli K12,
L. coli WP2
Unscheduled DNA Rat and mouse hepatocytes < 12.5 µg/ml Negative Tong (1981a, b)
synthesis
Unscheduled DNA Rat hepatocytes < 104 µg/ml Negative Litton Bionetics, Inc.
synthesis (1981)
In vivo
Holt-mediated assay, mice S. typhimurium G46 his- Negative Fiscor et al. (1978)
Host-mediated assay, mice S. typhimurium G46 his- 4000 mg/kg bw Negative Shirasu et al. (1977)
Gene mutation Mouse embryos treated in < 300 mg/kg bw Positive at Fahrig & Seller (1979)
utero by dosing mother orally 200 mg/kg bw
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
In vivo (con't)
Chromosomal aberration Rat bone marrow 300 mg/kg bw Negative BASF AG (1975)
orally
Chromosomal aberration Chinese hamster bone 1 000 mg/kg bw Negative Seiler (1976)
marrow orally
Chromosomal aberration ICR mouse nucleated 2 × 1 000 mg/kg bw Spindle effects Seiler (1976)
anaphase cells orally
Micronucleus formation Mouse 500 mg/kg bw orally Negative Seiler (1976)
Micronucleus formation Mouse < 6000 mg/kg bw i.p. Positive Pandita (1988)
Chromosomal aberration Chinese hamster bone < 1000 mg/kg bw i.p. Negative Pandita (1988)
marrow
Dominant lethal mutation NMRI mice 5 × 500 mg/kg bw Negative Hoechst AG (1974)
per day i.p.; 5 × 300
mg/kg bw per day orally
Sex-linked recessive lethal Drosophila melanogaster 0.5 mg/ml in dimethyl Negative Lamb & Lilley (1980)
mutation sulfoxide
Germ-line aneuploidy Drosophila melanogaster < 50 000 ppm Negative Osgood et al. (1991)
Technical-grade carbendazim was not irritating to the eyes of
albino rabbits. A 75% wettable powder formulation induced transient
corneal opacity in six of six unwashed and two of three washed eyes.
Microscopic examination confirmed the corneal opacity as mild to
moderate. Conjunctival irritation (redness, swelling, discharge) was
also transient. All eyes were normal after four days. The irritation
response was probably related to the inert ingredients in the wettable
powder formulation (Edwards, 1974b).
A 50% wettable powder formulation produced slight corneal
opacity, mild or moderate conjunctival redness, and slight or mild
conjunctival oedema in six male New Zealand white rabbits. Three of
the rabbits also had moderate iritis, and one had minimal blood-tinged
discharge. Microscopic examination revealed no corneal injury in any
of the treated eyes. The eyes of the other two rabbits were normal by
72 h. It was concluded that this formulation is a moderate ocular
irritant (Vick & Valentine, 1987).
Ten male albino guinea-pigs exposed to technical-grade
carbendazim or a 75% wettable powder formulation showed no dermal
sensitization after intradermal injections or repeated applications to
intact, shaved skin (Ford, 1981).
A 50% carbendazim formulation was applied to the intact, shaved
skin of 10 male and 10 female Dunkin Hartley albino guinea-pigs; five
male and five female guinea-pigs treated with 80% ethanol in water
served as vehicle controls; two male and two female guinea-pigs
treated with solid test material at the challenge phase only served as
negative controls; and two male and two female guinea-pigs treated
with a 0.3% suspension of 1-chloro-2,4-dinitrobenzene in 80% ethanol
in water served as positive controls. No irritation was observed in
the treated or vehicle or negative controls, but 1-chloro-2,4-
dinitrobenzene produced sensitization in all treated animals (Martin
et al., 1987).
(ii) Neurotoxicity
Groups of 10 white Leghorn hens receved carbendazim at doses of
500, 2500, or 5000 mg/kg bw to test for delayed neurotoxic potential.
Controls received the vehicle, corn oil, and the neurotoxin tri-
ortho-tolyl phosphate. The hens were observed daily for mortality
and clinical neurotoxicity for four weeks. Neurotoxic signs consisting
of leg weakness, ataxia and/or 'goose-stepping' gait were observed in
hens treated with tri- ortho-tolyl phosphate. Less severe, reversible
signs, consisting of slight leg weakness and ataxia, were observed in
hens treated with carbendazim at 5000 mg/kg bw, but no neurotoxic
signs were observed in those treated at lower doses. Microscopic
examination indicated no axonal degeneration or demyelination in
carbendazim-treated birds (Goldenthal, 1978).
(iii) Hormonal effects and spermatogenesis
Since spermatogenesis is an androgen-dependent process, the
effects of carbendazim on the endocrine function of the rat testis
were investigated by feeding 0-400 mg/kg bw per day by gavage for 85
days and measuring the serum levels of pituitary luteinizing hormone,
follicle-stimulating hormone, thyroid-stimulating hormone, and
prolactin, and the levels of androgen-binding protein and testosterone
in serum and in testicular interstitial and seminiferous tubule fluid.
The function al capacity of Leydig cells to secrete testosterone
was assessed in vitro after challenge with human chorionic
gonadotrophin. Doses of 50-100 mg/kg bw per day had no effect on
pituitary or testicular hormone concentrations; 200 mg/kg bw per day
increased the testosterone concentration in the seminiferous tubular
fluid, without affecting serum testosterone or androgen-binding
protein concentrations. The dose of 400 mg/kg bw per day resulted in
increased concentrations of both substances in the interstitial and
seminiferous tubular fluid and of serum androgen-binding protein.
These hormonal changes indicate that carbendazim affects the gonads,
resulting in testicular atrophy. Thus, the elevated seminiferous
tubule fluid testosterone concentrations may be a result of two
factors: (i) greater release of testosterone by the Leydig cells into
the interstitial fluid and/or (ii) decreased testosterone outflow from
the testis into the genera] circulation. The increased level of
androgen-binding protein in the interstitial fluid reflects a change
in its relative secretion into the interstitial fluid and seminiferous
tubules (Rehnberg et al., 1989).
Since extragonadal changes may have contributed to the altered
testicular endocrine profile described above, a further study focused
on the presence of concurrent changes in the hypothalamic and
pituitary control of the testis. Rats were given carbendazim at 50,
100, 200, or 400 mg/kg bw per day by gavage for 85 days. Dose-related
increases in serum follicle-stimulating and luteinizing hormone levels
were noted, but the values for prolactin and thyroid-stimulating
hormone remained unchanged. No significant difference in gonadotropin-
releasing hormone concentration in the mediobasal hypothalamus was
seen, although an increased level was found in the anterior
hypothalamus at 50 mg/kg bw per day, followed by a dose-related
decline (Gray et al., 1988). These findings suggest that
carbendazim-induced testicular damage is accompanied by compensatory
changes in hypothalamic and pituitary regulation of the testis
(Goldman et al., 1989).
These findings imply that carbendazim acts directly on the testis
to induce a number of hormonal and pathological changes. Consistent
with this hypothesis are the results of a study by Nakai et al.
(1992), in which the effects of carbendazim on the testes, efferent
ductules, and spermatozoa were determined after a single oral dose to
male Sprague-Dawley rats. In the first experiment, groups of 86-day-
old rats were treated with 0 or 400 mg/kg bw carbendazim and killed 2,
4, or 8 h later on the same day or 1, 4, 8, 16, or 32 days after
treatment. The first effect of carbendazim was noted after 8 h as an
increase in testicular weight; this continued to increase until day 4,
but on days 16 and 32 the testicular weights were substantially lower
than those of controls in five of 16 animals, indicating individual
variation. A decrease in the percentage of sonication-resistant sperm
heads per testis occurred at 8 h in four of eight rats, but the
decrease was significant only after 24 h, when a mean decrease of 19%
was observed; maximal decreases in total sperm head counts per testis
were seen on day 8, after which some recovery was apparent. Epididymal
weights were increased on day 4, but the percentage of morphologically
normal sperm in the cauda epididymus was decreased at that time. By
day 8, many spermatozoa heads were separated from their flagella, and
10% of the heads were misshapen. Numerous sloughed, round germ cells
and cytoplasmic testicular debris were also evident. No effect on the
percentage of motile sperm was seen at 2, 4, or 8 h or one or four
days after treatment. Sperm motility was significantly decreased on
days 8 and 16, but because of clumping and degeneration of the
spermatozoa, the percentage motility could not be determined.
Similarly damaged sperm were seen in three of eight rats on day 32,
but the percentage of motile sperm that could be measured was similar
to that in controls.
In a second experiment (Nakai et al., 1992), groups of rats
aged 97-105 days were given a single oral dose of 0, 50, 100, 200,
400, or 800 mg/kg bw and were killed 2 or 70 days after treatment. On
day 2, a dose-dependent increase in testicular weight was seen at
doses of 100 mg/kg bw or more. This was accompanied by significant
increases in mean seminiferous tubular diameter at 400 and 800 mg/kg
bw. At 50 mg/kg bw, missing immature germ cells were noted, with round
spermatids from stages I and II and elongated spermatids sloughed from
stage VII epithelium. At 100 mg/kg bw, the disappearance of germ cells
was more severe and sloughing of elongated spermatids extended into
stages XII and XIV. At doses greater than 100 mg/kg bw, germ cells
were missing at all stages except stages IX-XI, and at doses of
400-800 mg/kg bw some seminiferous epithelia were damaged so severely
that it was difficult to identify the stage. In addition, major
pathological changes were seen in the efferent ducts of the testis. In
animals treated with 100 mg/kg bw or more, the rete testis was
swollen, with sloughed germ cells, indicating that ductal blockage had
occurred further down the tract; 50% or more of the efferent ductules
were occluded. The occlusions were characterized as compacted luminal
contents, spermatic granulomas, mineralizations, and obliteration of
the original lumen by fibrotic connective tissue. On day 70, mean
testicular weight and mean seminiferous tubule diameter showed a
dose-dependent decrease. Histologically, these decreases were
associated with a dose-dependent increase in seminiferous tubular
atrophy. The atrophied tubules contained primarily Sertoli cells and
occasional spermatogonia and were surrounded by a thickened basement
membrane. No atrophic tubules were seen in the control rats.
Female hamsters were treated by gavage with a single dose of
carbendazim around the fertilization period, at times selected to
coincide with either of the two microtubule-dependent events initiated
by the ovulatory surge of luteinizing hormone: oocyte maturation
(first meiotic division, occurring late in vaginal proestrous) and
fertilization (second meiotic division, occurring early in vaginal
estrous). In the first experiment, groups of 10 hamsters received 0,
250, 500, 750, or 1000 mg/kg bw carbendazim during melosis I, and
pregnancy outcome was assessed on day 15. The percentage of pregnant
hamsters was significantly reduced at 750 and 1000 mg/kg bw, and, in
those animals that became pregnant, the average number of live pups
was reduced at all doses. In the second experiment, groups of 10
female hamsters were bred overnight and were given a single dose of 0
or 1000 mg/kg bw during meiosis II the next morning. The percentage of
pregnant hamsters was unaffected, but the average number of live pups
(measured at 15 days) was reduced. Thus, administration of carbendazim
at the time of microtubule-dependent meiotic events can result in
early pregnancy loss in hamsters (Perreault et al., 1992).
Rats in pseudopregnancy induced by stimulation of the uterine
cervix with a small brass rod in proestrous and estrous were given 0
or 400 mg/kg bw per day carbendazim for eight days. A uterine decidual
cell response was induced on day 4 of pseudopregnancy, and the animals
were killed on day 9. This response, evaluated as a measure of uterine
competency, was significantly lower in the treated rats than in the
controls (Cummings et al., 1990).
Groups of 12 male C57Bl/6 x C3H/He F1 mice were given
carbendazim at 0, 250, 500, or 1000 mg/kg bw per day by gavage for
five consecutive days, and body weight, testicular weights, and sperm
parameters were measured 7, 24, and 39 days after treatment. Body
weight was unaffected; testicular weight was reduced only in the group
at the highest dose after 7 and 24 days but had recovered by 39 days.
Flow cytometry of testicular cells showed that the relative
percentages of certain testicular populations (round, elongating, and
elongated spermatids) in the group at the highest dose were different
from those in controls 7 and 14 days after treatment (Evenson et al,
1987).
Eight-week-old Wistar-derived male rats were fed 0, 10, 70, or
500 ppm carbendazim in the diet for 182 days. Another group received
500 ppm for 91 days and control diet for a further 91 days. Positive
controls were treated with 0.5 mg/kg bw per day colchicine by gavage
for 12 days. Every 13 days, 10 rats were killed and their testicular
organs were examined by histological, morphometric, enzyme
histochemical, and autoradiographic methods. Males were mated twice
with untreated females on day 182 and were killed on day 208.
Fertility parameters were not affected by treatment. Similarly,
treatment did not affect testicular weight, the area of seminiferous
tubules or interstitial tissue, or epididymal structures and enzyme
activities; however, the incidence of 'degenerating' germ cells
undergoing meiosis and spermatogenesis was increased at 70 and
500 ppm, and a significant increase in the preleptonene spermatocyte
nuclear area was seen in all treated groups. The biological
significance of this finding is difficult to assess. The authors
concluded that the effects seen at 70 ppm (equal to 3 mg/kg bw per
day) indicate that carbendazim affects the physiological 'germinal
elimination process'; however, an independent review of the findings
raised methodological concerns which may preclude definitive analysis
of the data (Hilscher et al., 1992; Russell, 1992).
3. Observations in humans
(a) Medical surveillance of workers
Selected blood profiles from 50 workers involved in the
manufacture of benomyl and carbendazim were compared with those of a
control group of 48 workers who were not exposed to these two
fungicides. White blood cell count, red blood cell count, and
haemoglobin and haematocrit values were comparable in the two groups.
No quantitative estimates of exposure were given for the factory
workers, and no female employees were included in the control group
(Everhart, 1979).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers who were exposed to benomyl between 1970 and
1977. The ages of the workers ranged from 19 to 64 years; 79% of the
workers and 78% of their spouses were aged 20-39. The duration of
exposure ranged from less than one month to 95 months, and more than
51% of the workers had potentially been exposed for one to five
months. The birth rates of the spouses of the exposed workers were
compared with those of four populations in the same county, state,
region, and country (USA). The birth rates of the study population
were generally higher than those of the comparison populations,
indicating no reduction in fertility. Spermatogenesis was not examined
(Gooch, 1978).
(b) Studies of volunteers
Urinary excretion of carbendazim was investigated after oral
administration of 2 mg, application of 17 mg onto a skin area of
100 cm2, or intravenous administration of 1 mg. Total excretion of
5-HBC was proportional to the dose applied, and use of an occlusive
dressing did not significantly increase dermal absorption (Meuling
et al., 1993).
Comments
Carbendazim is readily absorbed by animals after oral exposure
and rapidly metabolized. It is eliminated in the faeces and excreted
in the urine. The tissue distribution showed no bioconcentration. In
rats, 2-[(methoxycarbonyl)amino]-1 H-benzimidazol-5-yl hydrogen
sulfate was identified as the main metabolite. The results of
comparative studies in rats and mice indicate that the detoxification
capacity of mouse liver may become saturated at high doses.
Carbendazim is poorly absorbed via the dermal route in rats. Within 24
h, only about 0.2% of a single dose of 0.6 mg was excreted in the
urine and faeces.
Carbendazim has low acute toxicity, with an oral LD50 in the
rat of > 10 000 mg/kg bw. The clinical signs of toxicity after high
single doses were generally nonspecific. Testicular degeneration has
been observed after single oral doses of > 1000 mg/kg bw in rats.
Wettable powder formulations containing carbendazim have been shown to
be irritating to rabbit skin and eyes but did not induce dermal
sensitization in Buehler-type tests. WHO has classified carbendazim as
unlikely to present an acute hazard in normal use.
In 90-day dietary studies in rats, increased liver weight was
seen at 1350 ppm (the highest dose tested in one study), without any
histological change. The overall NOAEL was 500 ppm, equivalent to
50 mg/kg bw per day. In dogs treated by dietary administration for tip
to two years at levels up to and including 4500 ppm, hepatotoxicity
(increased liver weight, hepatic cirrhosis, swollen vacuolated
hepatocytes, and chronic hepatitis) was seen at 500 ppm and above, and
the NOAEL was 100 ppm, equivalent to 2.5 mg/kg bw per day.
Two two-year studies were conducted in rats given dietary levels
of 0, 100, 500, or 2500-10 000 ppm and 0,150, 300, or 2000-10 000 ppm.
Carbendazim did not have carcinogenic potential, but some effects were
seen at the highest doses tested. In one study there was some evidence
of testicular atrophy, and in the other an increased incidence of
diffuse proliferation of parafollicular cells in the thyroid was seen.
In the latter study, evidence of mild hepatotoxicity was found in the
absence of histopathological change. The NOAEL in both studies was
equivalent to 15 mg/kg bw per day.
Three carcinogenicity studies were performed in mice. In Swiss
mice and CD-1 mice treated for 80 weeks and two years, respectively,
at dietary levels up to 5000 or 7500 ppm, carbendazim increased the
incidence of liver tumours. Because of the minimally increased
incidence of proliferative lesions of the liver at 150 or 500 ppm in
the diet (the lowest doses tested), it was not possible to establish
no-effect levels in these studies. In NMRKf mice, which have a low
spontaneous incidence of liver tumours, no carcinogenic effect was
seen at dietary levels up to and including 5000 ppm (the highest level
tested for 96 weeks), but hepatotoxicity was seen at this dose. The
NOAEL was equal to 34 mg/kg bw per day.
In a three-generation study of reproductive toxicity, rats were
fed dietary levels of 0, 150, 300, or 2000 ppm. Carbendazim had no
effect on reproduction or development at dietary levels up to and
including 2000 ppm (equivalent to 120 mg/kg bw per day). Male
fertility was depressed in rats given carbendazim by gavage at
200 mg/kg bw per day for 85 days. A dose of 50 mg/kg bw per day (the
lowest dose tested) caused a decrease in epididymal sperm counts.
After a single oral dose to rats, disruption of spermatogenesis was
seen at 100 mg/kg bw, but there was no effect at 50 mg/kg bw. The
effect was associated with loss of germ cells resulting from
inhibition of Sertoli-cell microtubules.
Carbendazim was tested for developmental toxicity in rats at
doses of 5-3000 mg/kg bw per day on days 6-15 of gestation. It was
fetotoxic and teratogenic at 20 mg/kg bw per day and above, the main
effects being microphthalmia and hydrocephaly. The NOAEL for
fetotoxicity and teratogenicity was 10 mg/kg bw per day; maternal
toxicity was observed at 90 mg/kg bw per day and above, and the NOAEL
for maternal toxicity was 20 mg/kg bw per day. Rabbits were exposed to
carbendazim by gavage at doses of 0, 10, 20, or 125 mg/kg bw per day
on days 7-19 of gestation. Carbendazim was fetotoxic and teratogenic,
the major effects being malformed vertebrate and ribs. The NOAELs in
this species were 10 mg/kg bw per day for teratogenicity and
fetotoxicity and 20 mg/kg bw per day for maternal toxicity.
Carbendazim has been adequately tested for genotoxicity in a
range of assays. The Meeting concluded that it does not directly
damage genetic material but causes numerical chromosomal aberrations
both in vitro and in vivo as a result of its interference with the
mitotic spindle proteins.
In an epidemiological study of workers exposed to carbendazim,
there was no reduction in fertility, as indicated by the birth rates,
among the study population. Spermatogenesis was not; examined.
An ADI of 0-0.03 mg/kg bw was established on the basis of the
no-effect level of 2.5 mg/kg bw per day in the two-year study in dogs
and a safety factor of 100. The resultant ADI, when compared with the
LOAELs in the studies with Swiss and CD-1 mice, provides an adequate
level of safety.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 34 mg/kg bw per day (96-week study of
toxicity and carcinogenicity in NMRKf mice)
< 150-500 ppm, equivalent to < 22-75 mg/kg bw per day
(studies of toxicity and carcinogenicity in Swiss mice (80
weeks) and CD-1 mice (two years)
Rat: 300-500 ppm, equal to 15 mg/kg bw per day (toxicity in a
two-year studies of toxicity and carcinogenicity)
2000 ppm, equivalent to 120 mg/kg bw per day (study of
reproductive toxicity)
10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
Dog: 100 ppm, equal to 2.5 mg/kg bw per day (two-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to carbendazim
Exposure Relevant route, study type, species Results/remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder non-irritating
Ocular, irritation, rabbit 50% wettable powder irritating
Dermal, sensitization, guinea-pig Non-sensitizing in Buehler test
Inhalation, toxicity, rat LC50 > 5.9 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat and rabbit NOAEL = 10 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary, toxicity, dog NOAEL = 2.5 mg/kg bw per day;
hepatotoxicity
References
Arce, G.T. & Sarrif, A.M. (1984) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report from DuPont Haskell
Laboratory for Toxicology, Newark, Delaware, USA. Submitted to
WHO by AgrEvo, Frankfurt, Germany.
Albertini, S. (1989) Influence of different factors on the induction
of chromosome malsegregation in Saccharomyces cerevisiae D61.M
by Bavistan and assessment of its genotoxic property in the Ames
test and in Saccharomyces cerevisiae DT. Mutat. Res., 216,
327-340.
Albertini, S. (1991) Re-evaluation of file 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae
aneuploidy test systems by the Gene-Tox Program using strain
D61 M of Saccharomyces cerevisiae. Mutat. Res., 260, 165-180.
Alverez, L. (1987) Teratogenicity study of INE-965 (carbendazim) in
rats. Unpublished report from E.I. DuPont de Nemours and Co.,
Haskell Laboratory, Newark, Delaware, USA.
Banduhn, N. & Obe, G. (1985) Mutagenicity of methyl 2-benzimidazole
carbamate, diethylstilbestrol and estradiol: Structural
chromosomal aberrations, sister-chromatid exchanges, C-mitoses,
polyploidies and micronuclei. Mutat. Res., 156, 199-218.
BASF AG (1975) Dominant lethal study in mice. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Beems, R.B., Til, H.P. & van der Heijden, C.A. (1976) Carcinogenicity
study with carbendazim (99% MBC) in mice. Unpublished report from
Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Carter, S.D, Hess, R.A. & Laskey, J.W. (1987) The fungicide methyl
2-benzimidazole carbamate causes infertility in male Sprague-
Dawley rats. Biol. Reprod., 37, 709-718.
Christ, O. & Kellner, H.M. (1973) Animal tests with carbendazim.
Unpublished report from Hoechst AG, Frankfurt, Germany.
Christian, N.S., Hoberman, A.M. & Feussner, E.L. (1985) Developmental
toxicity study of carbendazim administered via gavage to New
Zealand white rabbits. Unpublished report from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by E.I. DuPont de Nemours and Co., Inc.
Cummings, A.M., Harris, S.T. & Rehnberg, G.L. (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat.
Fundam. Appl. Toxicol., 15, 528-535.
Cummings, A.M., Ebron-McCoy, M.T., Rogers, J.M., Barbee, B.D. &
Harris, S.T. (1992) Developmental effects of methyl benzimid-
azolccarbamate following exposure during early pregnancy.
Fundam. Appl. Toxicol., 18, 288-293.
Dashiell, O.L. (1975) Oral LD50 test in guinea pigs (Carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory Newark, Delaware, USA.
Donaubauer, H.H., Schuetz, E., Weigand, W. & Kramer, M. (1982)
Repeated dose (24 month) feeding study, for determination of the
carcinogenic effect of HOE 17411 OFAT204 (carbendazim) in mice.
Unpublished report from Hoechst AG, Pharmaceuticals Research,
Toxicology Section, Frankfurt, Germany.
Donovan, S.D. (1982). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Donovan, S.D. (1983). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Dorn, E. & Keller, H.M. (1980) Carbendazim (60% wettable powder)
absorption via the skin in rats. Unpublished report from Hoechst
AG, Frankfurt, Germany.
Dorn E., Schmidt E., Kellner H.M. & Leist K.H. (1983) HOE 017411-14-C
(carbendazim-14C) metabolic fate in rats and mice, a
comparison. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Edwards, D.F. (1974a) Skin absorption LD50 (carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Edwards, D.F. (1974b) Federal Hazardous Substances Act -- Eye
irritation test in rabbits (carbendazim). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Evenson, D.P., Janca, F.C. & Jost, L.K. (1987) Effects of the
fungicide methyl-benzimidazol-2-yl carbamate (MBC) on mouse germ
cells as determined by flow cytometry. J. Toxicol. Environ.
Health, 20, 387-399.
Everhart, L.P. (1979) Benlate dust exposure survey. Unpublished report
from E.I. DuPont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Fahrig, R & Seiler, J.P. (1979) Dose and effect of methyl-2-
benzimidazolyl-carbamate in the 'mammalian spot test', an
in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-Biol. Interactions, 26, 115-120.
Falke, H.E., Beems, R.B. & Spit, B. (1982a) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 60-day enzyme induction study
in mice. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Falke, H.E., Beems, R.B. & Spit, B. (1982b) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 59-day enzyme induction study
in rats. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Fiscor, G, Bordas, S. & Steward, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Ford, L.S. (1981) Primary skin irritation and sensitization on guinea
pigs. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Ford, L.S. (1982) Acute skin absorption LD50 test on rabbits
(carbendazim 75% wettable powder). Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
from Laboratory for Cancer Research, Hoechst AG, Frankfurt,
Germany.
Goldenthal, E.I. (1978) Neurotoxicity in hens. Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by E.I. DuPont de Nemours and
Co., Inc.
Goldman, J.M., Rehnberg, G.L, Cooper, R.L., Gray, L.E. Jr, Vein, J.F.
& McElroy, W.K. (1989) Effects of the benomyl metabolite,
carbendazim, on the hypothalamic-pituitary reproductive axis in
the male rat. Toxicology, 57, 173-182.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Goodman, N.C. & Sherman, H. (1975) Oral LD50 tests (fasted male and
female rats). Unpublished report from E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Grandizio, A.M. & Sarver, J.W. (1987) Acute oral toxicity study with
INE-965-261 (Benlate C) in male and female rats. Unpublished
report from E.I. DuPont de Nemours and Co., Inc., Haskell
Laboratory, Newark, Delaware, USA.
Gray L.E., Ostby, J., Sigmon, R., Ferrell, J., Rehnberg, G., Linder,
R., Cooper, R., Goldman, J. & Laskey, J. (1988) The development
of a protocol to assess reproductive effects of toxicants in the
rat. Reprod. Toxicol., 2, 281-287.
Gray, L.E., Ostby, J., Linder, R., Goldman, J., Rehnberg, G. & Cooper,
R. (1990) Carbendazim induced alterations of reproductive
development and function in the rat and hamster. Fundam Appl.
Toxicol., 15, 281-297.
Guengerich F.P. (1981) Enzyme induction with Du Pont compounds
H11,201-02 and H10,962-02. Unpublished report from Vanderbilt
University School of Medicine, Nashville, Tennessee, USA.
Submitted to WHO by E.I. Du Pont de Nemours and Co., Inc.,
Newark, Delaware, USA.
Hilscher, W., Ohnesorge, H. & Muller, H. (1992) The effects of
carbendazim on spermatogenesis. Unpublished report from Institut
für Toxicologie, Heinrich-Heine-Universitat, Dusseldorf, Germany.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hinckle, L. (1981) Oral LD50 test in rats -- EPA proposed
guidelines. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Hoechst AG (1974) Mouse dominant lethal study on MBC. Unpublished
report submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hoffman, H.T. & Kirsch, P. (1987) Report on the subchronic toxicity of
methyl benzimidazole-2-carbamate in beagle dogs on oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Hoffman, H.T. & Peh, J. (1987a) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hoffman, H.T. & Peh, J. (1987b) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hunter, B., Batham, P. & Newman, A.J. (1973a) Carbendazim oral
toxicity to rats in oral administration for 2 weeks. Unpublished
report from Huntingdon Research Centre, United Kingdom. Submitted
to WHO by Hoechst AG, Frankfurt, Germany.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. & Newman, A.J.
(1973b) Carbendazim toxicity to rats during dietary
administration for 13 weeks followed by a recovery period of 6
weeks. Unpublished report from Huntingdon Research Centre, UNited
Kingdom. Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Ivett, J.L. (1984) Mutagenicity evaluation of H#15,152 in an
in vitro sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report from Litton Bionetics,
Inc., Kensington, Maryland, USA. Submitted to WHO by E.I. DuPont
de Nemours and Co., Inc., Newark, Delaware, USA.
Janardhan, A., Sattur, P.B. & Sisodia, P. (1984) Teratology of methyl
benzimadazole carbamate in rats and rabbits. Bull. Environ.
Contam. Toxicol 33, 257-263.
Janardhan, A., Rao, A.B. & Sisodia, P. (1987) Sub-chronic toxicity of
methyl benzimidazole carbamate in rats. Bull. Environ. Contam.
Toxicol., 38, 890-898.
Jotz, M.M. (1980) An evaluation of mutagenic potential of benomyl
employing the L5178Y TK+/-mouse lymphoma assay. Unpublished
report from SRI International, Menlo Park, California, USA,
prepared for the US Environmental Protection Agency.
Kellner, H.M. (1983) Carbendazim-(2-14C) whole body autoradiography
studies of the distribution in rats and mice after oral and.
intravenous administration. Unpublished report from Hoechst AG,
Frankfurt, Germany.
Kellner, H.M. & Eckert, H. (1983) Carbendazim-(2-14C) blood levels,
distribution and excretion in rats and mice after oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Koeter, H.B.W.M. (1975a) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the rat Unpublished report from Central Institute
for Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, and BASF AG,
Ludwigshafen, Germany.
Koeter, H.B.W.M. (1975b) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the New Zealand white rabbit. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Kramer, M. & Weigand, W. (1971) (HOE 17411 OF) Toxicological
examination. Unpublished report from Hoechst AG, Pharmaceuticals
Research, Toxicology Section, Frankfurt, Germany.
Krechniak, J. & Klosowska, B. (1986) The fate of 14C-carbendazim in
the rat. Xenobiotica, 16, 809-815.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Litton Bionetics, Inc. (1981) Evaluation of HOE 17411 OF AT204 in the
primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Inc., Kensington,
Maryland, USA. Submitted to WHO by Hoechst AG, Frankfurt,
Germany.
Martin, D.A., Henry, J.E. & Brock, W.J. (1987) Closed-patch repeated
insult dermal sensitization study (Buehler method) with Benlate C
fungicide in guinea-pigs. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Meuling, W.J.A., Opdam, J.J.G. & DeKort, W.L.A.M. (1993) Dose-
excretion study with the fungicide carbendazim in volunteers.
Unpublished report from Central Institute for Nutrition and Food
Research (TNO) The Hague, Netherlands. Submitted to WHO by
Hoechst AG, Frankfurt, Germany.
Minta, M. & Biernacki, B. (1982) Embryotoxicity of carbendazim in
rats, rabbits and hamsters. Bull. Vet. Inst. Pulawy, 2, 42-52.
Mohr, U. (1977) Review of liver sections from mice and rats fed with
carbendazim. Unpublished report from Department of Experimental
Pathology, Medical School, Hanover, Germany. Submitted to WHO by
Hoechst AG, Frankfurt, and BASF AG, Ludwigshafen, Germany.
Monson K.D. (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.
Wilmington, Delaware, USA.
Nakai, M., Hess, R.A., Moore, B.J., Guttroff, R.F., Strader, L.F. &
Linder, R.E. (1992) Acute and long-term effects of a single dose
of the fungicide carbendazim (methyl 2-benzimidazole carbamate)
on the male reproductive system in the rat. J. Androl., 13,
507-518.
Nash, S.D. & Ferenz, R. (1982) Inhalation median lethal concentration
(LC50) in rats -- EPA protocol (carbendazim 75% wettable
powder). Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Osgood, C., Zimmering, S. & Mason, J.M. (1991) Aneuploidy in
Drosophila. II: Further validation of the FIX and ZESTE genetic
test systems employing female Drosophila melanogaster. Mutat.
Res., 259, 147-163.
Pandita, T.K. (1988) Assessment of the mutagenic potential of a
fungicide Bavistin using multiple assays. Mutat. Res., 204,
627-643.
Perreault, S.D., Jeffay, S., Poss, P. & Laskey, J.W. (1992) Use of the
fungicide carbendazim as a model compound to determine the impact
of acute chemical exposure during oocyte maturation and
fertilization on pregnancy outcome in the hamster. Toxicol.
Appl. Pharmacol., 114, 225-231.
Rashid, M.A. & Mumma, R.O. (1986) Screening pesticides for their
availability to damage bacterial DNA. J. Environ. Health Sci.,
B21, 319-334
Rehnberg, G.L., Cooper, R.L., Goldman, J.M., Gray, L.E, Hein, J.F. &
McElroy, W.K. (1989) Serum and testicular testosterone and
androgen binding protein profiles following subchronic treatment
with carbendazim. Toxicol. Appl. Pharmacol., 101, 55-61.
Reuzel, P.G.J., Hendriksen, C.F.M & Til, H.P (1976) Long-term
(two-year) toxicity study with carbendazim in beagle dogs.
Unpublished report from the Central Institute for Nutrition and
Food Research for BASF. Submitted to WHO by E.I. DuPont de
Nemours and Co., Wilmington, Delaware, USA.
Russell, J.F. (1977) Mutagenicity evaluation of 2-benzimidazole
carbamic acid, 5-hydroxymethyl in Salmonella typhimurium.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1983) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from E.I. DuPont de Nemours and
Co., Inc.. Haskell Laboratory, Newark, Delaware, USA.
Russell, L.D.. (1992). Review of Prof. Hilscher's report «Effects of
carbendazim on spermatogenesis». Unpublished report from
University of Illinois, USA. Submitted to WHO by Hoechst AG,
Germany.
Sarver, J.W. (1975) Acute inhalation toxicity -- one hour head only,
(carbendazim). Unpublished report from E.I. DuPont de Nemours,
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Scholz, J. & Weigand, W. (1972) Carbendazim (HOE 17411 OF, batch
496/I). Toxicological examination. Unpublished report from
Hoechst AG, Pharmaceuticals Research, Toxicology Section,
Frankfurt, Germany.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1965) Acute oral test. Unpublished report from E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. (1968) Ninety-day feeding study in rats using a wettable
powder formulation (70% MBC). Unpublished report from E.I. DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1970) Three-month feeding study in dogs using a wettable
powder formulation (50% MBC). Unpublished report from E.I DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1972) Long-term feeding studies in rats and dogs with
2-benzimadazole carbamic acid, methyl ester (INE-965) (50% and
70% MBC wettable powder formulations). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. and Krauss, W.C. (1966). Acute oral test (carbendazim).
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware.
Shirasu, Y., Morlya, M. & Kato, K. (1977) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Stadler, J.C. (1986) One-year feeding study in dogs with carbendazim.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Til, H.P., van den Muelen, H.C, Feron, V.J., Seinen, W. & de Groot,
A.P. (1972) Sub-chronic (90 day) toxicity study with W17411 in
beagle dogs. Unpublished report from Central Institute for
Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Til, H.P., Koellen, C. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with carbendazim in
rats. Unpublished report from Central Institute for Nutrition and
Food Research (TNO), The Hague, Netherlands. Submitted to WHO by
BASF AG, Ludwigshafen, and Hoechst AG, Frankfurt, Germany.
Til, H.P., Koeter, H.B.W.M. & van der Heijden, C.A. (1976b)
Multigeneration study with carbendazim in rats. Unpublished
report from Central Institute for Nutrition and Food Research
(TNO), The Hague, Netherlands. Submitted to WHO by Hoechst AG,
Frankfurt, Germany.
Til, H.P., Beems, R.B. & de Groot A.P. (1981) Determination of the
acute oral toxicity of carbendazim in mice. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Tong, C. (1981a) Hepatocyte primary culture/DNA repair assay on
compound 11, 201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Tong, C. (1981b) Hepatocyte primary culture/DNA repair assay on
compound 11,201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Vick, D.A. & Brock, W.J. (1987) Acute dermal toxicity study of Benlate
C fungicide in rabbits. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Vick, D.A. & Valentine, R. (1987) Primary eye irritation study with
Benlate C fungicide in rabbits. Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Waterer, J.C. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory Newark, Delaware, USA.
Whittaker, S.G., Moser, S.F., Maloney, D.H., Plegorsch, W.W., Resnick,
M.A. & Fogel, S. (1990) The detection of mitotic and meiotic
chromosome gain in the yeast Saccharomyces cerevisiae: Effects
of methyl benzimidazol-2-yl carbamate, methyl methanesulfonate,
ethyl methanesulfonate, dimethyl sulfoxide, propionitrile and
cyclophosphamide monohydrate. Mutat. Res., 242, 231-258.
Wood, C.K. (1982) Long-term feeding study with 2-benzimidazole-
carbamate, methyl ester (> 99% MBC, INE-965) in mice.
Unpublished report from E.I. DuPont de Nemours and Co, Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Zeller, H. & Kirsch, P. (1971) Acute oral toxicity of methyl-2-
benzimidazole carbamate to the rabbit. Unpublished report from
Hoechst AG, Frankfurt, Germany.
 Table 1. Residual content of carbendazim in the livers of rats
Dosage Residual content (%)
Rat Mouse
Single dose
3 mg/kg bw 12 29
300 mg/kg bw 18 26
29-day repeated dose
3 mg/kg bw 2 < 2
300 mg/kg bw 4 28
In the study of Krechniak & Klosowska (1986), 94% of the measured
radiolabel in urine 12 h after treatment was as 5-HBC, 3% as 2-AB, and
3% as carbendazim.
The proposed metabolic pathway for carbendazim in rats is given
in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed for 28 days with diets containing benomyl or carbendazim at a
concentration of 0, 10, 30, 100, 300, 1000, or 3000 ppm. After
sacrifice, liver weights were recorded and microsomal epoxide
hydrolase and cytosolic glutathione- S-transferase were monitored in
subcellular fractions isolated from the liver. The mean absolute liver
weights were elevated in males and females fed 1000 or 3000 ppm
carbendazim and in females fed 300 ppm; however, the only
significantly increase was found in females fed 3000 ppm benomyl. No
apparent liver toxicity or effect on body weight was observed. Both
benomyl and carbendazim induced epoxide hydrolase in male and female
rats and mice fed 1000 or 3000 ppm, and both induced glutathione- S-
transferase at 3000 ppm. The level of induction seemed to be slightly
greater in females than males. There was no substantial difference in
enzyme induction between rats and mice (Guengerich, 1981).
In the same study, but in a separate test, CD-1 male mice were
treated by gavage with carbendazim suspended in 0, 100, or 1000 mg/kg
bw per day corn oil for five days. After sacrifice, liver samples were
homogenized, and subcellular fractions were prepared as described
above. Wet liver weights and the activities of microsomal cytochrome
P450, NADPH-cytochrome- creductase, styrene-7,8-hydrolase,
benzphetamine- N-demethylase, benzo[ a]pyrene hydroxylase,
7-ethoxycoumarin-deethylase, and cytosolic glutathione- S-transferase
were measured. The activities of styrene-7,8-hydrolase and
glutathione- S-transferase were statistically significantly increased
over the control values; that of 7-ethoxycoumarin-deethylase was
significantly decreased. It is noteworthy that the total microsomal
cytochrome P450 level did not increase, indicating that carbendazim
did not induce overall microsomal induction, even at the higher dose.
The increase in styrene-7,8-hydrolase activity shows, however, that
some hepatic microsomal enzymes are induced by carbendazim in vivo.
There appeared to be no substantial difference in enzyme induction
between rats and mice (Guengerich, 1981).
Groups of male Wistar rats and Swiss mice were given carbendazim
in the diet at levels of 0-10 000 ppm for 60 days, and the induction
of liver enzyme activities was examined and compared with that induced
by phenobarbital sodium administered in the drinking-water. Growth and
food consumption were decreased in rats at 10 000 ppm but not in mice
given up to 5000 ppm in the diet. Relative liver weights were
increased in rats fed 2000 or 10 000 ppm and in mice receiving 1000 or
5000 ppm carbendazim. Phenobarbital had similar effects. The protein
concentrations in total homogenates and post-mitochondrial fractions
of liver from rats were not affected by carbendazim, whereas they were
increased in mice at 5000 ppm. Feeding of carbendazim to rats at
2000 ppm or more resulted in slight-to-moderate induction of several
phase-I drug metabolizing enzymes: 7-ethoxycoumarin- O-deethylase,
biphenyl-4-hydroxylase, aniline hydroxylase, 4-methoxybiphenyl- N-
demethylase, and cytochrome- c-reductase. The activities of the
phase-II drug metabolizing enzymes glucuronyl transferase I and II and
the glutathione content were moderately-to-markedly increased at this
dose. Feeding of carbendazim to mice at 1000 ppm or more resulted in
moderate-to-marked increases in the activities of phase-I drug
metabolizing enzymes, including cytochrome P-450 and aminopyrine- N-
demethylase; cytochrome- c-reductase activity was decreased, and
those of glucuronyl transferase and glutathione- S-transferase and
glutathione content were slightly increased. There was no measureable
difference between rats and mice with regard to the metabolism of
carbendazim, although exhaustion of the detoxification mechanism was
more evident in mice at high doses. The detoxification and elimination
of carbendazim and its metabolites proceed more rapidly in rats than
in mice, as reflected in the increased glutathione content of rat
liver and the increased activity of phase-II enzymes (Falke et al.,
1982a,b).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
2. The clinical signs of toxicity after treatment with carbendazim
were generally nonspecific. The acute toxicity of carbendazim in a
number of species is low, LD50 values by various routes of
administration ranging from > 2000 to > 15 000 mg/kg bw. Gross and
histopathological changes were observed in the testes and epididymides
of male rats given carbendazim orally at doses of 1000 mg/kg bw and
more. The testes were small, soft, and discoloured, and more than 70%
of the tubules showed degenerative changes. The sperm count in the
epididymides examined was reduced or nil.
(b) Short-term toxicity
Rats
Groups of six male Sprague Dawley rats were given carbendazim by
gavage at a dose of 0, 200, 3400, or 5000 mg/kg bw per day, five times
per week for two weeks. Two rats at 3400 mg/kg bw per day died. At all
doses, gross and microscopic evidence of adverse effects on testes and
reduction or absence of sperm in the epididymides was seen. The testes
were small and discoloured, with tubular degeneration and evidence of
aspermatogenesis. At 3400 mg/kg bw per day, there were also
morphological changes in the duodenum (oedema and focal necrosis),
bone marrow (reduction in the blood-forming elements), and liver
(decrease in large, globular-shaped vacuoles) (Sherman, 1965; Sherman
& Krauss, 1966).
Groups of 10 male Sprague-Dawley rats were given carbendazim by
gavage at a dose of 0, 10, 20, 30, or 40 mg/kg bw per day for two
weeks. At the high dose, liver weights were increased. There were no
histopathological findings and no effects on spermatogenesis, on
cellularity, or on the incidence of mitosis, as evidenced by tritiated
thymidine incorporation 1 h before sacrifice (Hunter et al., 1973a).
Administration of 1350 ppm carbendazim to Sprague-Dawley rats for
13 weeks resulted in hepatomegaly in animals of each sex, which was
not accompanied by histopathological lesions and was reversible after
six weeks. A dietary level of 450 ppm (equal to 35 mg/kg bw per day)
was the NOAEL (Hunter et al., 1973b).
In a poorly reported study, groups of 10 male and 10 female
litter-mate weanling Wistar rats were treated by gavage with 0, 16,
32, or 64 mg/kg bw per day for 90 days. The erythrocyte counts in
treated rats were lower than those of the controls after 15 days of
exposure; however, no clear dose-response relationship was seen after
30, 60, or 90 days of exposure. Leukocyte counts were decreased after
Table 2. Acute toxicity of carbendazim and carbendazim formulations
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Carbendazim Mouse Oral > 15 000 NR Til et al. (1981)
Carbendazim Rat Oral > 10 000 > 95 Goodman & Sherman (1975)
Carbendazim Rat Oral > 11 000 > 95 Sherman (1965)
Carbendazim Rat Oral > 15 000 > 95 Kramer & Weigand (1971)
Carbendazim Rat Oral > 17 000 NR Sherman & Krauss (1966)
Carbendazim Guinea-pig Oral > 5 000 NR Dashiell (1975)
Carbendazim Rabbit Oral > 8 000 NR Zeller & Kirsch (1971)
Carbendazim Dog Oral > 5 000 NR Scholz & Weigand (1972)
Carbendazim Mouse Intraperitoneal > 15 000 NR Scholz & Weigand (1972)
Carbendazim Rat Intraperitoneal > 2 000 NR Scholz & Weigand (1972)
Carbendazim Rat Inhalation (1-h) > 5.9 NR Sarver (1975)
Carbendazim Rat Dermal > 2 000 NR Kramer & Weigand (1971)
Carbendazim Rabbit Dermal > 10 000 NR Edwards (1974a)
'75% wettable powder' Rat Oral > 5 000 NR Hinckle (1981)
'75% wettable powder' Rat Inhalation (4-h) > 5 > 95 Nash & Ferenz (1982)
'75% wettable powder' Rabbit Dermal > 2 000 > 95 Ford (1982)
'75% wettable powder' Rat Oral > 5 000 NR Grandizio & Saner (1987)
'75% wettable powder' Rabbit dermal > 2 000 > 95 Vick & Brock (1987)
NR, Not reported
15 days, and after 30 and 60 days animals of each sex showed transient
decreases in lymphocyte counts in comparison with controls, although
no clear dose-response relationship was observed among the treated
groups. No change was seen in the activity of whole-blood
cholinesterase. Male rats had significantly increased alkaline
phosphatase activity at a dose of 64 mg/kg bw per day, and blood urea
levels were lowered in males at 32 and 64 mg/kg bw per day after 90
days. Increased serum bilirubin concentrations were seen in males and
females at 32 and 64 mg/kg bw per day; these were attributable to
parenchymal cell damage, as indicated by increased alanine amino-
transferase activity. Dose-related changes in the liver ranged from
sparse infiltration by inflammatory cells to inflammatory and
degenerative changes. Tubular dilatation and hydropic degeneration
were noted in the kidneys of rats at the low dose, and fibrosis and
congestion were seen in rats at the medium and high doses. Increased
lung weights were correlated with bronchopneumonic changes. Slight
changes in weights were reported for several other organs. In view of
the lack of data on individual animals in this experiment and the
apparent variability in the results, no NOAEL could be established
(Janardhan et al., 1987).
Groups of 16 male and 16 female Sprague-Dawley rats were fed
carbendazim (purity, 72%) in the diet for 90 days at 0, 100, 500, or
2500 ppm (as carbendazim). The animals were observed daily for
behavioural changes and body weight, and food consumption was recorded
weekly. Haematological examinations were conducted on 10 male and 10
female rats in each group at 30, 60, and 90 days. Routine urinalyses
were performed on the same animals, and plasma alkaline phosphatase
and alanine aminotransferase levels were determined. After 90-98 days
of continuous feeding, 10 male and 10 female rats in each group were
killed, and selected organs were weighed; these and other organs were
also preserved for microscopic examination. The six male and six
female rats remaining in each group after terminal sacrifice were used
in a study of reproductive toxicity (see below). There were no
clinical signs of toxicity and no compound-related effects on weight
gain, food consumption, or haematological parameters. The relative
liver weights in females fed 2500 ppm were slightly increased in
comparison with control rats. Testicular weight was not affected in
any group. Microscopic examination of selected tissues and organs in
the control and high-dose groups showed no adverse effect attributable
to carbendazim. The NOAEL in this study was 500 ppm, equivalent to
50 mg/kg bw per day (Sherman, 1968).
Rabbits
Groups of six male New Zealand albino rabbits received 0 or
2000 mg/kg bw carbendazim as a 50% aqueous paste on shaved intact
dorsal skin repeatedly, for 6 h/day, for 10 consecutive days. There
were no adverse effects on body weight, clinical symptoms, organ
weights, gross pathology, or histopathology of selected organs;
however, there was focal necrosis of the epidermis and polymorpho-
nuclear cell infiltration of the dermis in five of six exposed
rabbits. No other effects were observed (Dashiell, 1975).
Dogs
Groups of four male and four female one-year-old beagle dogs were
given carbendazim (purity, 53%) in the diet for three, months at 0,
100, 500, or 2500 ppm (as carbendazim); the highest level was
subsequently reduced to 1500 ppm because of reduced food intake and
body weight. Food consumption and body weight were recorded weekly,
and clinical laboratory examinations, including haematological,
biochemical, and urinary measurements, were performed periodically. At
the end of the study, all animals were killed, selected organs were
weighed, and these and other organs were subjected to gross and
microscopic evaluations. No mortality or adverse clinical signs were
observed over the course of the study, and growth and food consumption
were normal, except in animals at 1500-2500 ppm. Urinalysis showed no
change due to treatment, and there were no dose-related effects on
haematological values. Females at the mid- and high doses showed a
trend to increased cholesterol levels over the pre-test and control
levels after one, two, and three months. The weights of the thymus
were increased in males at the low and mid-doses, and those of the
prostate at the mid-dose. Limited histopathological data did not
indicate any compound-related effects. The data for the high-dose
group were compromised by the change in dietary level, which involved
a 'recovery' period during which the animals received control diet
(Sherman, 1970).
Groups of four male and four female beagle dogs were given
carbendazim in the diet at 0, 100, 300, or 1000 ppm for 13 weeks. The
highest level was increased to 2000 ppm after six weeks of treatment.
Body weight, haematological, blood chemistry and urine measurements,
and liver and kidney function tests were performed periodically. The
animals were examined grossly and microscopically at the end of the
study. There were no reported compound-related effects on clinical
behaviour, body weight, food consumption, haematological parameters,
kidney function (phenol red excretion) or liver function (brom-
sulphthalein retention). Blood chemistry was normal, except for a
slight decrease in albumin in males at the mid- and high doses at 12
weeks. Urinalysis showed normal values, except for a high bacterial
count in females at the high dose at week 13. The blood clotting time
was slightly reduced in dogs at the high dose at week 12. There were
slight increases in relative liver and thyroid weights and a decrease
in relative heart weights in the group at the highest dose. No
microscopic changes that could be associated with treatment were
observed in these or other organs. The NOAEL was 300 ppm, equivalent
to 7.5 mg/kg bw per day, on the basis of minor changes in clinical
chemistry and organ weights (Til et al., 1972).
Groups of three male and three female beagle dogs were fed diets
containing 0, 500, 1500, or 4500 ppm carbendazim for 13 weeks. Reduced
body-weight gain, increased liver weight, hepatic periportal
infiltration, and hepatic regeneration were seen at the high dose. No
histopathological changes were seen in any other organs, including the
testes. At 1500 ppm, liver weights were increased, but there were no
histological lesions. The NOAEL was 500 ppm, equal to 12.5 mg/kg bw
per day (Hoffman & Kirsch, 1987).
Groups of five male and five female beagle dogs were fed diets
containing 0, 100, 200, or 500 ppm carbendazim for one year. The dogs
were weighed at regular intervals, and individual food consumption was
monitored throughout the study; clinical pathology was evaluated
periodically. After one year, all of the dogs were killed and selected
tissues were examined microscopically. There were no statistical
differences in mean body weight that could be attributed to treatment,
and the mean daily food consumption of treated dogs was similar to
that of controls. None of the clinical observations was attributable
to carbendazim intake. Dogs fed 500 ppm had elevated levels of serum
cholesterol, which were statistically significant for males at nine
months and for females at one and two months. There were no
microscopic lesions related to treatment. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day (Stadler, 1986).
Groups of four male and four female beagle dogs, one to two years
of age, were given carbendazim (purity, 53%) in the diet at 0, 100,
500, or 2500 ppm (as carbendazim) for two years. Food consumption and
body weights were measured weekly, and animals were examined daily for
clinical signs of toxicity. Haematological, biochemical, and urinary
examinations were performed periodically throughout the study. After
one year, one male and one female in the control and 500-ppm groups
were killed. At the end of the study, the organs were weighed, and
gross and histopathological examinations were performed. No mortality
was reported among the controls or dogs at 100 or 500 ppm; however,
three males at the high dose were sacrificed after 22 and 42 weeks
because of poor nutrition. No females at the high dose died.
Haematological and urinary values were unaffected by treatment. The
dogs at 500 ppm had increased levels of cholesterol, blood urea
nitrogen, total protein, and serum alanine aminotransferase. Swollen,
vacuolated hepatic cells and marginal proliferation of the portal
triads with cellular infiltration were observed in one dog that had
been fed 500 ppm and was sacrificed after one year. The biochemical
evidence of an effect on the liver was corroborated by the finding at
terminal sacrifice of hepatic cirrhosis, swollen, vacuolated hepatic
cells, and mild chronic hepatitis in dogs fed 500 ppm or more of
carbendazim. There were no effects on organ weights. Diffuse
testicular atrophy and aspermatogenesis were observed in two of four
males at 100 ppm but not at higher doses. As similar effects were not
seen at the other doses, these findings are considered not to be
treatment-related. The NOAEL was 100 ppm, on the basis of the effects
on the liver at 500 ppm, equivalent to 2.5 mg/kg bw per day (Sherman,
1972).
Groups of four male and four female beagle dogs, aged 22-27
weeks, were fed carbendazim in the diet at 0, 150, 300, or 2000 ppm
for 104 weeks. After 33 weeks, the dose of 2000 ppm was increased to
5000 ppm. The dogs were examined daily for clinical signs of toxicity
and altered behaviour; body weight and food consumption were recorded
regularly throughout the study, and haematological examinations, blood
chemistry (including liver and kidney function tests), and urinary
measurements were conducted periodically. After 104 weeks, the dogs
were sacrificed, and the tissues were reported to have been examined
grossly and microscopically, although the available data indicate that
the pathological examinations were inadequate. The only death was that
of a female at the high dose which was killed in a moribund state
after week 36. The body weights of males at the mid-dose and of males
and females at the high dose were decreased. Food consumption was
comparable in all groups. Blood clotting times were significantly
reduced in males at the high dose from week 13 to term, and slight
decreases were noted in females at the high dose. Serum alkaline
phosphatase activity was increased at the high dose throughout the
study, but there were no compound-related effects on serum alanine or
aspartate aminotransferase activities. All other haematological
parameters and blood chemistry were comparable with those of
the controls. There were no differences among the groups in
bromsulphthalein retention, phenol red excretion, or urinary values.
Liver and thyroid weights were significantly increased in dogs at the
high dose, but there were no microscopic changes in these organs that
were related to treatment. An increased incidence of prostatitis was
seen in high-dose males in comparison with controls (3/4 versus 1/4).
One male at that dose also had interstitial mononuclear inflammatory
cell infiltrates and atrophic tubules of the testes. Although feeding
of carbendazim in the diet to dogs for two years apparently had no
adverse effect at levels up to and including 300 ppm, the deficiencies
in the reporting of the pathological data preclude determination of an
NOAEL (Reuzel et al., 1976).
(c) Long-term toxicity and carcinogenicity
Mice
Carbendazim was administered in the diet to groups of 100 male
and 100 female specific pathogen-free Swiss mice at 0, 150, 300, or
1000 ppm for 80 weeks. The highest dose was increased to 2000 ppm at
week 4 and to 5000 ppm at week 8 for the remainder of the study.
Animals were examined for behaviour and for clinical signs of
toxicity, and body weights were measured throughout the study. All
animals were examined grossly, liver and kidney weights were recorded,
and tissues were examined microscopically. There were no compound-
related effects on general condition, mortality, or body weight. At
termination of the study, 70% of males and 80% of females were still
alive. The relative liver weights of males and females at the high
dose were significantly higher than those of controls, but kidney
weights were unchanged. After peer review and reclassification of the
data on hepatic tumour incidence, the combined incidence of
hepatocellular adenomas and carcinomas was found to have increased
with increasing doses in both males and females (Table 3). Males
showed more pronounced induction of liver tumours and more frequent
occurrence of hepatocellular carcinomas, which were often found
simultaneously with hepatocellular adenomas, whereas females usually
had only hepatocellular adenomas. It was concluded that carbendazim is
oncogenic in this strain of mouse at a dietary level of 5000 ppm
(Beems et al., 1976; Mohr, 1977).
Table 3. Hepatic rumour incidence in Swiss mice fed carbendazim
Sex Dietary level No.of mice Liver nodular Hepatocellular Hepatocellular
(ppm) examined hyperplasia adenoma carcinoma
Male 0 100 0 9 1
150 94 8 5 3
300 98 11 13 4
100/5000 100 25 14 9
Female 0 94 0 1 1
150 99 5 1 0
300 98 3 3 0
1000/5000 95 11 8 0
Groups of 80 male and 80 female CD-1 mice, aged six to seven
weeks, were given carbendazim (purity, 99%) in the diet at 0, 500,
1500, or 7500 ppm for two years. The highest dose was reduced to
3750 ppm for the males after 66 weeks because of increased mortality
(62 controls, 32 at 7500 ppm); females, however, received 7500 ppm
throughout the study. Treatment affected mortality in male mice, and
those at the high dose were sacrificed at week 73 because only 23 were
still alive. Only nine males at 1500 ppm survived to week 104, whereas
18 male controls were still alive at that time. Females had no similar
increase in mortality. There were no dose-related effects on body
weight or food consumption at any time, although the terminal body
weights of males at the low and mid-doses were lower than those of the
control and high-dose males. Clinical parameters were similar for all
treated and control groups, and haematological measures were
unaffected. Both absolute and relative thymic weights were
significantly decreased in females at 500 and 1500 ppm, but not in the
high-dose group. Absolute liver weights were increased in females at
the high dose and relative liver weights in those at the two highest
doses. The organ weights of male mice were variable, and only those of
the kidney and thymus appeared to be decreased as a result of
treatment. Absolute kidney and thymic weights were depressed in male
mice at all doses, but relative kidney and thymic weights were
significantly decreased only in males at the high dose. Histological
examination revealed dose-related changes in the thymus (lymphoid
depletion) and accumulation of yellow-brown pigment in the renal
tubules of male mice at the mid- and high doses. These mice also had
an increased frequency of sperm stasis in the testes and increased
bilateral germinal cell atrophy; there was no trend for unilateral
germinal cell atrophy, the incidence in controls being greater than or
equal to that of treated males. These effects are therefore considered
not to be compound-related. A significant hepatotoxic effect was seen
in male mice at 1500 and 7500 ppm, as demonstrated by centrilobular
hypertrophy, necrosis, and swelling. The frequency of hepatocellular
adenomas was not increased, as they occurred at equal frequency in
control and treated groups. There was a significant increase in the
incidence of hepatocellular carcinomas, but only at 1500 ppm; however,
too few males at the high dose survived to 17 months (510 days) to
support the conclusion that there is no oncogenic effect at that dose.
The combined incidence of hepatocellular carcinomas, hepatocellular
adenomas, and hepatoblastomas (Table 4) was significantly increased
(P < 0.05) in females at the low, mid-, and high doses and in males
at the mid-dose, but this was not evaluated in high-dose males because
of the high rate of mortality. The study thus shows statistically
significant increases in the incidences of hepatocellular carcinoma
for males at 1500 ppm and for females at all doses, the response being
dose-related. A dose-response relationship could not be determined
because of the high mortality in the high-dose males; the high
mortality rate in male controls also hampered interpretation of
results. No carcinogenic effect was observed in tissues other than the
liver (Wood, 1982).
Carbendazim was administered in the diet of groups of 100-120
male and female NMRKf mice for 96 weeks at a dose of 0, 50, 150, 300,
or 1000 ppm. The highest dose was increased to 2000 ppm at week 4 and
to 5000 ppm at week 8 for the remainder of the study. Animals were
examined for behaviour and general condition and for body weight, food
and water consumption, and mortality. Gross necropsy was performed on
all animals, liver and lung weights were recorded, and all organs and
tissues were examined microscopically. An interim sacrifice was
conducted of 20 males and 20 females in the control group and that at
the highest dose at 18 months. There were no compound-related effects
on behaviour, body-weight gain, food or water consumption, or
mortality. By 22 months, 24-31% of the males and 37-52% of the females
had died. As there was no difference between the treated and control
groups, it was concluded that mortality was not influenced by the
feeding of carbendazim. At 18 and 22 months, the absolute and relative
liver weights of both male and female mice at 5000 ppm were increased.
Macroscopic and microscopic examination of 20 male and 20 female
animals killed after 18 months of receiving 5000 ppm carbendazim
revealed compound-related effects on the liver: all animals had
centrilobular hypertrophy, single-cell necrosis, mitotic cells, and
pigmented Kupffer cells. The tissues of the remaining 100 males and
100 females exposed to 5000 ppm, evaluated at 22 months, showed marked
hypertrophy (greater than in animals treated for 18 months), clear-
cell foci, mitosis, inclusion bodies in enlarged cell nuclei, multiple
cell necrosis, and a greenish-yellow pigment in Kupffer cells.
Neoplastic nodules (adenomas), carcinomas, fibrosarcomas, and other
tumorigenic responses in the liver were equally distributed among the
groups. Although haemangiomas of the liver were found in all treated
groups but not in controls, no dose-related response was evident. Lung
adenomatosis was equally distributed among the groups. The tumour
incidence is summarized in Table 5. There was no effect on the
incidence or time of onset of tumours, and the total number of benign
and malignant tumours was comparable among the different groups of
mice. Thus, there was no evidence that carbendazim administered in the
diet of mice at doses up to and including 5000 ppm for 22 months had a
carcinogenic effect. The NOAEL was thus 300 ppm, equal to 34 mg/kg bw
per day (Donaubauer et al., 1982).
Rats
Groups of 36 male and 36 female weanling Sprague-Dawley rats were
given carbendazim (purity, 50-70%) in the diet for 104 weeks at 0,
100, 500, 2500 (increased to 10 000 ppm after 20 weeks), or 5000 ppm
(as carbendazim). Body weight and food consumption were recorded
weekly for the first year and twice a month thereafter. Behavioural
changes and mortality were observed daily. Haematological, urinary,
and selected clinical chemical examinations were performed
periodically. After one year, each group was reduced to 30 male and 30
female rats by interim sacrifice for gross and microscopic
examinations. At the end of the study, all surviving animals were
sacrificed, and tissues and organs were examined grossly. Microscopic
examinations were conducted on all tissues and organs from the
controls and animals at 2500-10 000 ppm, the livers of animals at 100
and 500 ppm, and the livers, kidneys, testes, and bone marrow of
animals at 5000 ppm. Body-weight gain was depressed in males and
females at 2500-10 000 ppm and in females at 5000 ppm in comparison
with controls. Food consumption was similar in all groups. Reduced
erythrocyte counts and haemoglobin and haematocrit values were seen in
females at 2500-10 000 or 5000 ppm after 9-24 months and in males at
2500-10 000 ppm after 24 months. There were no compound-related
Table 4. Hepatic tumour incidence in CD-1 mice fed carbendazim
Sex Dietary level No. of mice No. of mice alive Median survival Combined incidence
(ppm) examined at termination (weeks) of hepatic tumours
Male 0 80 18 79 13
500 80 14 72 20
1500 80 9 69 23
7500/3500 80 23 64 NA
Female 0 79 22 91 1
500 78 15 91 9
1500 80 13 91 21
7500/3500 78 20 91 15
NA, not analysed
Table 5. Hepatic tumour incidence in NMRK-f mice fed carbendazim
Sex Dietary level No.of mice Clear-cell Basophilic Hepatocellular Liver
(ppm) examined foci loci adenomas haemangiomas
Male 0 97 0 0 3 0
50 99 0 0 2 2
150 99 0 1 0 3
300 95 0 0 0 2
1000/5000 99 3 0 1 0
Female 0 98 0 0 0 0
50 98 0 1 00
150 95 0 0 0 0
300 95 0 0 1 2
1000/5000 95 4 0 0 1
clinical manifestations of toxicity and no effects on urinary
parameters. Alkaline phosphatase and alanine aminotransferase
activities varied throughout the study in animals at 2500-10 000 or
5000 ppm, but there was no consistent dose-response relationship.
Organ weights and organ-to-body weight ratios were unchanged, except
for the livers of females at 2500-10 000 or 5000 ppm, but the increase
in the liver-to-body weight ratio was due to a reduction in body
weight. Histopathological examination of the livers showed no
compound-related effects. Males at 2500-10 000 ppm had a marginal
increase in the frequency of diffuse testicular atrophy and
prostatitis. The NOAEL was 500 ppm, equivalent to 15 mg/kg bw per day
(Sherman, 1972).
Groups of 60 male and 60 female Wistar rats were given
carbendazim (purity, 99%) in the diet at 0, 150, 300, or 2000 ppm for
two years. The dose of 2000 ppm was increased to 5000 ppm after one
week and to 10 000 ppm after two weeks for the remainder of the study.
Animals were examined daily for clinical signs of toxicity. Body
weight and food consumption were measured regularly throughout the
study. Haematological measurements (peripheral blood), blood chemistry
(orbital sinus), and urinalysis were conducted periodically. All
animals were subjected to complete gross necropsy, and selected organs
were weighed. Tissues from 20 male and 20 female rats in the control
and high-dose groups were examined microscopically, and all tumours
and gross abnormalities were examined histologically. No differences
in clinical signs of toxicity or food consumption were seen between
test groups and control animals. Body weights were significantly
reduced in low-dose males from week 88 to term and in high-dose
females from week 12 to term. The results of urinalyses were
comparable among the groups. The haemoglobin level was depressed in
high-dose females a t weeks 26, 52, and 103, and the haematocrit was
depressed in high-dose females at week 103. There were no compound-
related effects in males. Serum aspartate aminotransferase activity
was decreased in high-dose males at termination of the study, but
not in females; high-dose females had increased serum alanine
aminotransferase activity and decreased total serum protein. The only
compound-related effects on organ weights were increased relative
liver weights in high-dose females. There were no compound-related
effects on mortality, and survival at termination of the study was
similar in all groups. There were no histological differences between
control and treated groups, except for an increased incidence of
diffuse proliferation of parafollicular cells of the thyroid in the
high-dose females. The number of tumour-bearing animals and the total
number of tumours were comparable among all groups, and no compound-
related oncogenic effects were reported. The NOAEL was 300 ppm,
equivalent to 15 mg/kg bw per day, on the basis of changes in organ
weights and minor biochemical changes (Til et al., 1976a).
(d) Reproductive toxicity
Groups of six male and six female rats were removed from a 90-day
study in which they were fed dietary levels of 0, 100, 500, or
2500 ppm carbendazim. Each female was exposed to three males from the
same dose group to produce the F1a generation; each litter was
reduced to 10 pups on the fourth day after birth. The F0 animals were
mated again after about one week to produce the F1b litter.
Reproduction indices and status at birth and on days 4, 12, and 21
were recorded, as were body weights at weaning. The data were
extremely limited and were available only for groups. At 100 ppm,
neither the F1a nor the F1b animals became pregnant. There were no
apparent effects on reproduction indices or weanling weights, but the
fertility indexes for all groups, which were only 33-67%, prevented
meaningful interpretation of the data (Sherman, 1968).
Groups of three male and 16 female Sprague-Dawley rats were fed
carbendazim in the diet at 0, 100, 500, 5000, or 10 000 ppm
(20 females at the highest dose) and mated in a standard study of two
litters per generation for three generations. The parental animals
were fed the experimental diet at 21 days of age and mated to produce
the F1 litter at 100 days of age; the numbers of matings,
pregnancies, and young were recorded for each litter at birth. The
litters were culled to 10 pups on day 4. The number of live pups was
again recorded on days 4, 12, and 21, as was pup weight at weaning.
The parents were mated again to produce F1b litters, which were
maintained on the respective diets for 110 days and then mated to
produce the F2a and F2blitters; F3a and F3b litters were
produced similarly. Selected tissues and organs from two males and two
females in each of five F3b litters from the controls and from
the groups fed 5000 and 10 000 ppm were examined grossly and
histopathologically. Reproduction indices, including mating,
fecundity, fertility, gestation, viability, and lactation, were
calculated and compared with control values. Carbendazim had no effect
on fertility, gestation, viability, or lactation, but the average
litter weights at weaning were reduced in all generations fed 5000 and
10 000 ppm. Histopathological examination of F3b weanlings did not
reveal any effects that were considered to be compound-related
(Sherman, 1972).
Carbendazim was administered to groups of 10 male and 20 female
Wistar rats at a dietary level of 0, 150, 300, or 2000 ppm for three
generations, and two successive litters were reared from each female
General condition and behaviour were routinely observed, and
individual body weights were recorded throughout the study. The
numbers of pups in each litter were recorded, and each litter was
culled to eight on day 1. The total weight of each litter was measured
on days 1, 10, and 20. The F1a and F2a litters were discarded at
weaning and the F1b and F2b litters were used to produce
succeeding generations. The F3a offspring were used to study
teratogenicity, while the F3b offspring w ere used in a four-week
evaluation of toxicity. Neither health nor body-weight gain was
affected, but all generations of the treated groups weighed
significantly more than controls. The compound had no effect on
fertility or on survival at birth, at day 10, or at day 20. Litter
size was not affected by treatment, except for a marginal decrease in
F2a litters at all doses. There were no differences in the F2a
litters at 300 and 2000 ppm, but litter size was decreased and
mortality at birth increased at 150 ppm. Birth weight during the
lactation period was comparable in all groups. There were no gross
abnormalities related to treatment. Autopsy of rats in the four-week
study showed increased relative liver weights and decreased relative
spleen weights in females fed 2000 ppm; relative ovarian weights were
also significantly decreased in all dose groups. Histopathological
examination of the livers showed no compound-related change;
histopathological results were not presented for other organs. No
maternal or fetal toxicity was evident, and there were no differences
in visceral anomalies in animals at 0 or 2000 ppm (the only groups
examined). Thoracic vertebral bodies were reduced at 2000 ppm, and
there was a significant reduction in cervical vertebral bodies at
2000 ppm. Controls, however, had more significant changes with regard
to absent or delayed ossification of skeletal structures. There were
no apparent adverse effects on reproduction and no teratogenic effects
at dietary levels of carbendazim up to and including 2000 ppm,
equivalent to about 120 mg/kg bw per day (Koeter et al., 1976).
A serial breeding technique was used to evaluate the fertility of
male Sprague-Dawley rats after exposure by gavage to 10 daily doses of
400 mg/kg bw per day carbendazim. Males, 90 days old and proven to be
fertile, were bred with a new female each week, starting on the third
day of treatment and continuing for 32 weeks after the last day of
treatment. Twelve days after each breeding period, the females were
killed, their uteri were examined for resorptions, and the numbers of
dead and viable fetuses were determined. All males were killed 35
weeks after treatment, and testicular tissue was prepared for
histopathological examination by vascular perfusion. The fertility of
treated males (as indicated by the number of pregnant females) was
depressed during the first week after treatment: 10 of the 24 treated
males failed to induce a pregnancy, as compared with no failure in the
control group. By the fifth week after treatment, 16 of the 24
carbendazim-treated males were infertile. Of these, four recovered
fertility after being infertile for 5-11 consecutive breeding periods,
but the other 12 did not recover during the remainder of the 32-week
period after treatment. Histological examinations of testicular
sections of the latter animals 245 days after treatment revealed
severe seminiferous tubular atrophy (> 85% of tubules were atrophic),
often with epithelium containing only Sertoli cells, surrounded by a
thickened basement membrane. The lumina of < 2% of the tubules
contained spermatozoa. The seminiferous tubules of the treated males
that recovered fertility had various contents of atrophic tubules
(13-85%) 245 days after treatment (Carter et al., 1987).
Groups of 8-12 male and 8-12 female rats were given 0, 50, 100,
200, or 400 mg/kg bw per day carbendazim by gavage from weaning
through puberty, gestation, and lactation and were mated at 84 days of
age. The male rats were killed on days 104-106 and the female rats on
day 27 post partum. In a similar study, Syrian hamsters were given 0
or 400 mg/kg bw per day carbendazim. Various landmarks of puberty were
measured in the parental generation. In females, estrous cyclicity,
litter size, the number of implants, organ weights, and histological
status were assessed. In males, organ weights, testicular and
epididymal sperm counts, sperm motility, sperm morphology, testicular
histological status, and endocrine parameters were assessed. In
addition, the growth, viability and reproductive function of the
offspring (F1) were observed during a four-month period of
continuous breeding. Males were killed after five months for
histopathological investigation. Carbendazim did not alter pubertal
development, growth, or viability in the parental generation of either
species. The reproductive potential of rats treated with 200 or
400 mg/kg bw per day was reduced due to effects on sperm production
and fetal viability. These doses markedly altered sperm morphology,
testicular and epididymal weights, sperm numbers, and testicular
histology; fertility, sperm mobility, and hormonal levels were altered
primarily in males with very low sperm counts. A statistically
significant reduction in the caudal epididymal sperm count was noted
at doses of 50 mg/kg bw per day or more. Testicular and epididymal
sperm counts in male hamsters were significantly lower (about 21%) in
treated than in control males. In F1 male hamsters, testis and
seminal vesicle weights and epididymal sperm counts were significantly
reduced by prenatal exposure to carbendazim at 400 mg/kg bw per day.
Parental female rats exposed at this dose had post-implantation
losses, and a few malformed pups were found in litters of animals at
100 or 200 mg/kg per day. Litter size was significantly reduced at 200
and 400 mg/kg bw per day. Overall, carbendazim was less toxic to
hamsters than to rats (Gray et al., 1988, 1990).
Carbendazim was fed to groups of eight female Holtzmann rats by
gavage at doses of 0, 25, 50, 100, 200, 400, or 1000 mg/kg bw per day
during early pregnancy (days 1-8). A range of parameters, including
the number of implantation sites, body-weight gain, uterine weight,
implantation site size, and serum ovarian and pituitary hormones, was
assessed after sacrifice on day 9. At doses up to 400 mg/kg bw per
day, carbendazim had no significant effect on any of the measured
parameters, but a trend towards increased resorptions was evident. The
highest dose reduced maternal body-weight gain, implantation site
size, and serum luteinizing hormone levels and increased serum
estradiol (Cummings et al., 1990).
(e) Developmental toxicity
Rats
Groups of 27-28 pregnant Sprague-Dawley rats were fed carbendazim
(purity, 53%) in their diet at 0, 200, 500, 2500, 5000, 7500, or
10 000 ppm on days 6-15 of gestation. On day 20 of gestation, all of
the animals were sacrificed and the fetuses were delivered by
caesarean section. The numbers and location of live and dead fetuses
and resorption sites, body weights, crown-rump length, sex, and
visible abnormalities were determined. Two-thirds of the fetuses were
prepared for examination for skeletal abnormalities, and the remainder
were examined for visceral and soft-tissue anomalies. There were no
deaths, no adverse effects on body weight, and no clinical signs of
toxicity. Food intake was reduced at the highest dose during the
period the test diet was administered but returned to control levels
from day 16 to 20. The numbers of implantation sites, resorption
sites, and live and dead fetuses were not adversely affected. Although
data on individual litters were not presented, carbendazim did not
appear to be teratogenic when administered to rats at dietary levels
up to and including 10 000 ppm during the critical period of
organogenesis (Sherman, 1970).
Groups of 18-22 pregnant Wistar specific pathogen-free rats were
given carbendazim in the diet at doses of 0, 600, 2000, or 6000 ppm on
days 6-15 of gestation. On day 21 of gestation, all of the rats were
sacrificed and the pups delivered by caesarean section. Dams were
weighed periodically during the test, and food consumption was
measured for specific periods. The number of corpora lutea was
determined, the ovaries were weighed, and the fetuses were weighed and
examined. The numbers of implantation and resorption sites were
recorded, and the empty uterine horns weighed. One-third of the
fetuses were fixed and stained for skeletal examination, and the
remaining two-thirds were examined for soft-tissue anomalies. Although
23 females per group were mated, the pregnancy rate was variable, with
18, 22, 20, and 18 pregnant rats in the groups receiving 0, 600, 2000,
and 6000 ppm, respectively. The mean body-weight gain and food
consumption of the high-dose females were significantly decreased in
comparison with controls. The numbers of live and dead fetuses,
implantation sites, embryonal resorptions, fetal resorptions, and
corpora lutea per dam were comparable in all groups, and ovarian
weights, the weights of the empty uteri, mean fetal weight per litter,
the sex ratio, and pre- and post-implantation losses were not affected
by treatment. No visceral anomalies were reported that were
significantly different from those seen in controls. Misshapen and
fused bones were much more frequent in the high-dose groups then in
any other group, and the incidence of supernumerary ribs was
significantly increased in high-dose females. Ossification was
significantly delayed or absent in pups at the high dose, particularly
in forelimbs, hindlimbs, sternebrae, and skull bones; ossification was
also significantly delayed or absent in cervical vertebral bodies in
all treated groups. It was concluded that carbendazim has no
teratogenic potential when administered in the diet to rats at levels
up to 6000 ppm, although ossification is reduced in a dose-related
manner (Koeter, 1975a).
Groups of 8-10 female Wistar rats were given carbendazim (purity,
98%) by gavage at 0, 20, 40, or 80 mg/kg per day on days 6-15 of
pregnancy. Half of each group of animals was killed on day 21 of
gestation, and half was allowed to deliver normally. All sacrificed
animals were scored for live and dead fetuses and for resorptions;
live fetuses were killed and examined for abnormalities. After normal
deliveries, neonatal deaths and survivors were counted, and survivors
were weighed and examined for gross abnormalities. In rats sacrificed
on day 21, dead and resorbed fetuses accounted for 29% of the
conceptuses among controls, 48% at 20 mg/kg per day, 64%, at 40 mg/kg
per day, and 73% at 80 mg/kg per day. There were no differences among
the various groups with respect to mean weight of live fetuses, and
there were no malformations. The average number of live pups per
litter was close to eight in controls, six at 20 mg/kg per day, and
about five at 40 and 80 mg/kg per day. Mean fetal weight was increased
by about 13% over that of controls at the two highest doses. There
were no stillbirths, neonatal deaths, or gross abnormalities, but
mortality at 21 days post partum was 3.0-3.5 times greater at the
two highest doses than in controls (Janardhan et al., 1984).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethyl-cellulose by gavage to groups of 15-26 Sprague-Dawley
rats on days 6-15 of gestation at daily doses of 10-3000 mg/kg bw per
day. The lowest dose had no effect on dams or their offspring and was
considered to be a no-effect lewd. At 30 mg/kg bw per day,
fetotoxicity was evident: 42% of the fetuses in 19/21 litters had
malformations affecting the head, spine, ribs, and sternum. At
60 mg/kg bw per day, 2/23 animals aborted and 51% of the implantations
in the remaining dams were dead. Malformations were seen in 90% of
fetuses, and all litters were affected. At 100 mg/kg bw per day, 15
pregnant animals produced only four live fetuses in three litters, all
of which were malformed. At 300, 1000, and 3000 mg/kg bw per day,
severe toxicity resulted in early resorptions only. The no-effect
level of 10 mg/kg bw per day was confirmed in a further study
specifically of fetotoxicity and hydrocephaly (Hoffman & Peh,
1987a,b).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethylcellulose by gavage to groups of 25 Sprague-Dawley rats
on days 7-16 of gestation at doses of 0, 5, 10, 20, or 90 mg/kg bw per
day. Maternal toxicity was seen only at the highest dose, in the form
of depressed weight gain during treatment and before sacrifice on day
22. The mean liver weights and liver-to-body weight ratios were
increased. A decreased pregnancy rate was observed at the highest
dose. An increase in the incidence of early resorptions per dam,
decreased litter size, and total resorption of three litters occurred
at the highest dose, but only the reduction in females per litter was
significant. Significant reductions in mean fetal weight were observed
at both 20 and 90 mg/kg bw per day, and a significant increase in the
incidence of fetal malformations was seen at the highest dose. The
malformations consisted primarily of hydrocephaly, microphthalmia,
anophthalmia, malformed scapulae, and axial skeletal malformations
(vertebral, rib, and sternebral fusions, exencephaly, hemivertebrae,
and rib hyperplasia). The NOAEL was 20 mg/kg bw per day for the dams
and 10 mg/kg bw per day for the fetuses (Alverez, 1987).
Carbendazim (purity, 95%) was administered by gavage to female
Holtzman rats at doses of 0, 100, 200, 400, or 600 mg/kg bw per day
during days 1-8 of gestation, and the rats were sacrificed on day 11
or 20. No maternal toxicity was seen at any dose. On day 11, the
crown-rump length, head length, number of somites, and number of
embryos per dam were significantly reduced in groups receiving
200 mg/kg bw per day or more. Open posterior neuropores and limb
anomalies were observed more frequently at doses > 200 mg/kg bw per
day. On day 20, increased resorptions, decreased live litter size and
fetal body weight, and delayed ossification were observed at all
doses. Skeletal malformations seen at the high dose were attributed to
treatment. The authors noted that developmental alterations occurred
after termination of treatment, suggesting either that the anomalies
represent delays in development or that the embryonic cells are
vulnerable at earlier stages than was previously thought (Cummings
et al., 1992).
Rabbits
Groups of 3-11 pregnant New Zealand albino rabbits were given
carbendazim in the diet at 0, 600, 2000, or 6000 ppm on days 6-18 of
gestation. On day 29 of gestation, all of the animals were sacrificed
and the fetuses delivered by caesarean section. Does were weighed
periodically, and food consumption was determined for specific
periods. The number of corpora lutea was determined, the ovaries were
weighed, and the fetuses were weighed and examined; the numbers of
implantation sites and resorption sites were recorded, and the empty
uteri weighed. One-half of fetuses were stained and sectioned for
determination of skeletal anomalies, and the other half was examined
for soft-tissue abnormalities. Only the fetuses in the high-dose and
control groups were examined for visceral anomalies. Although 15
females per group were artificially inseminated, the pregnancy rate
was extremely variable, with nine in controls, eight at 600 ppm, three
at 2000 ppm, and 11 at 6000 ppm. The mean body-weight gain was
significantly decreased in the high-dose group, although food
consumption did not vary among groups. The numbers of live and dead
fetuses, implantation sites, embryonal resorptions, fetal resorptions,
and corpora lutea per doe were comparable in all groups. The ovarian
and uterine weights of animals at the high dose were depressed in
comparison with control females; Pre- and post-implantation losses
were not affected by treatment. The incidence of visceral anomalies
appeared to differ between the high-dose and control groups, but too
few litters and fetuses were examined to allow a conclusion. There
were significantly increased numbers of supernumerary ribs (bilateral)
and skull bones in the high-dose group. Ossification was significantly
delayed or absent in fetuses in the high-dose group, most notably in
the forelimb metacarpals and phalanges. There was also incomplete
ossification of the sternebrae and skull bones, which was significant
at 600 and 6000 ppm, and misshapen sternebrae were present at the
highest dose. Although it can be concluded that carbendazim does not
induce teratogenic effects when administered in the diet to rabbits at
levels up to 6000 ppm, there were too few animals, litters, and
fetuses at 2000 ppm to allow a useful evaluation of compound-related
effects (Koeter, 1975b).
Female albino rabbits were given 0, 40, 80, or 160 mg/kg bw per
day carbendazim by gavage on days 6-18 of pregnancy and were
sacrificed on day 31. All of the animals were scored for live and dead
fetuses and for resorptions; live fetuses were killed and examined for
abnormalities. There were no dead or resorbed fetuses in controls, but
15% of all conceptuses were dead at 40 mg/kg bw per day, 21.7% at 80
mg/kg bw per day, and 33.3% at 160 mg/kg bw per day. There were no
differences among the various groups with respect to mean weight of
live fetuses, and there were no malformations (Janardhan et al.,
1984).
Suspensions of carbendazim in aqueous 0.5% carboxymethylcellulose
were administered by gavage on days 7-19 of presumed gestation to
groups of 20 artificially inseminated New Zealand white rabbits at
doses of 0, 10, 20, or 125 mg/kg bw per day. The highest dose
inhabited average maternal weight up to day 16 of gestation. The
implantation rate was decreased at 20 and 125 mg/kg bw per day, and
the incidence of resorptions was increased at 125 mg/kg bw per day,
resulting in decreased live litter size at these doses. At the highest
dose, decreased fetal body weight was seen, but the effect was not
statistically significant. The average percentage of malformed fetuses
per litter was significantly increased at 125 mg/kg bw per day.
Compound-related malformations at the highest dose consisted of
malformed cervical vertebrae and interrelated malformation of the ribs
and proximate thoracic vertebrae. The NOAEL for maternal toxicity was
20 mg/kg bw per day, and the NOAEL for developmental toxicity was
10 mg/kg bw per day (Christian et al., 1985).
Hamsters
Groups of 7-11 pregnant hamsters were treated orally on day 10 of
gestation with 0, 15, 30, 75, or 150 mg/kg bw carbendazim A
significant increase in malformations, including exencephaly, was
observed at 75 and 150 mg/kg bw. These doses were also embryotoxic,
producing 31 and 45% resorptions, respectively (Minta & Biernacki,
1982).
(f) Genotoxicity
Numerous studies have been conducted to assess the mutagenic
potential of carbendazim. Many of the results are conflicting, and
many of the reports do not provide sufficient detail to evaluate the
reasons for the conflicting data. Since before the mid-1980s
industrially produced carbendazim contained phenazine impurities, the
use of carbendazim with different degrees of purity might account for
some of the discrepancies. Table 6 summarizes those reports that
included sufficient experimental detail and data.
Carbendazim is not a heritable gene mutagen; it does not interact
with cellular DNA, induce point mutations, or result in germ-cell
mutations, as seen in both mammalian and nonmammalian systems in
vitro and in vivo and in somatic and germ cells. Positive results
have occasionally been obtained in tests for gene mutation, but they
may have been associated with the presence of phenazines, which are
mutagenic at very low concentrations in Salmonella typhimurium in
Ames' test and in mouse lymphoma LY5178Y tk+/- cells.
Concentrations of > 4 ppm diaminophenazine and 10 ppm aminohydroxy-
phenazine were mutagenic in Ames' test. Process changes by some of the
major manufacturers of carbendazim have removed the phenazine, and
this contaminant is not present when other benzimidazoles, such as
benomyl and thiophanate-methyl, are metabolized to carbendazim.
Carbendazim does cause numerical chromosomal aberrations (aneuploidy
and/or polyploidy) in experimental systems in vitro and in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A 75% wettable powder formulation induced slight, transient
irritation when applied to the in tact and abraded skin of albino
rabbits. A 55% suspension of the 75% wettable powder formulation in
dimethyl phthalate induced mild irritation when applied to the intact,
shaved skin of albino guinea-pigs, while a 5.5% concentration produced
no irritation (Ford, 1981).
The primary dermal irritation potential of Benlate C (50%
wettable powder) was evaluated by applying a 5-g aliquot to the
intact, clipped skin of six New Zealand white rabbits for 4 h. The
test sites were evaluated for erythema, oedema, and other evidence of
dermal effects and were scored according to the Draize scale 4, 24,
48, and 72 h after application. No dermal irritation was seen at any
time during the study (Vick & Brock, 1987).
Table 6. Results of tests for the genotoxicity of carbendazim
End-point Test system Concentration Results Reference
or dose
Tests for gene mutation
Reverse mutation S. typhimurium TA98, < 2500 µg/plate Negative Gericke (1977)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium TA100, < 200 µg/plate Negative Fiscor et al. (1978)
TA1530, TA1535, TA1950
Reverse mutation S. typhimurium TA1530, < 100 µg/spot Weakly positive Fiscor et al. (1978)
TA1950, G46 his-
Reverse mutation S. typhimurium TA100, < 1000 µg/plate Negative Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP2 hcr
Reverse mutation S. typhimurium TA98, < 300 µg/plate Negative Pandita (1988)
TA100
Reverse mutation S. typhimurium TA97, 5000 µg/plate Positive (only with Albertini (1989)
TA98, TA1537, TA1538 activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Positive, TA1537, Donovan (1982)
TA100, TA1535, TA1537 TA98 (with
activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Negative Russell (1983)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium Positive, only with Arce (19844)
4 ppm DAP or
10 ppm AHP
Reverse mutation S. typhimurium TA98, < 20 000 µg/plate Negative Russell (1977);
TA100, TA1535, TA1537 Donovan (1983)
hprt mutation Chinese hamster ovary cells < 654 µmol/litre Negative Waterer (1980)
tk mutation L5178Y mouse lymphoma < 250 µmol/litre Positive (with Jotz (1980)
cells activation)
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
Tests for chromosomal effects
Sister chromatid exchange Chinese hamster ovary cells < 40 µg/ml Negative Ivett (1984)
Sister chromatid exchange Human lymphocytes < 30 µg/ml Negative Banduhn & Obe (1985)
Sister chromatid exchange Human lymphocytes < 60 µg/ml Marginally positive Pandita (1988)
Chromosomal gain S. cereviriae < 0.1 µg/ml Positive Whittaker et al. (1990)
Aneuploidy S. cereviriae < 5 µg/ml Positive Albertini (1991)
Chromosomal aberrations Human lymphocytes < 10 µmol/litre Not clastogenic but Banduhn & Obe (1985)
induces micronuclei
Chromosomal aberrations Human lymphocytes < 0.5 mg/ml Negative Lamb & Lilly (1980)
DNA damage and repair
Rec assay B. subtilis < 1000 µg/disc Negative Shirasu et al. (1977)
Rec assay S. typhimurium TA1535, < 2000 µg/plate Negative Rashid & Mumma
(1986)
TA1538, E. coli K12,
L. coli WP2
Unscheduled DNA Rat and mouse hepatocytes < 12.5 µg/ml Negative Tong (1981a, b)
synthesis
Unscheduled DNA Rat hepatocytes < 104 µg/ml Negative Litton Bionetics, Inc.
synthesis (1981)
In vivo
Holt-mediated assay, mice S. typhimurium G46 his- Negative Fiscor et al. (1978)
Host-mediated assay, mice S. typhimurium G46 his- 4000 mg/kg bw Negative Shirasu et al. (1977)
Gene mutation Mouse embryos treated in < 300 mg/kg bw Positive at Fahrig & Seller (1979)
utero by dosing mother orally 200 mg/kg bw
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
In vivo (con't)
Chromosomal aberration Rat bone marrow 300 mg/kg bw Negative BASF AG (1975)
orally
Chromosomal aberration Chinese hamster bone 1 000 mg/kg bw Negative Seiler (1976)
marrow orally
Chromosomal aberration ICR mouse nucleated 2 × 1 000 mg/kg bw Spindle effects Seiler (1976)
anaphase cells orally
Micronucleus formation Mouse 500 mg/kg bw orally Negative Seiler (1976)
Micronucleus formation Mouse < 6000 mg/kg bw i.p. Positive Pandita (1988)
Chromosomal aberration Chinese hamster bone < 1000 mg/kg bw i.p. Negative Pandita (1988)
marrow
Dominant lethal mutation NMRI mice 5 × 500 mg/kg bw Negative Hoechst AG (1974)
per day i.p.; 5 × 300
mg/kg bw per day orally
Sex-linked recessive lethal Drosophila melanogaster 0.5 mg/ml in dimethyl Negative Lamb & Lilley (1980)
mutation sulfoxide
Germ-line aneuploidy Drosophila melanogaster < 50 000 ppm Negative Osgood et al. (1991)
Technical-grade carbendazim was not irritating to the eyes of
albino rabbits. A 75% wettable powder formulation induced transient
corneal opacity in six of six unwashed and two of three washed eyes.
Microscopic examination confirmed the corneal opacity as mild to
moderate. Conjunctival irritation (redness, swelling, discharge) was
also transient. All eyes were normal after four days. The irritation
response was probably related to the inert ingredients in the wettable
powder formulation (Edwards, 1974b).
A 50% wettable powder formulation produced slight corneal
opacity, mild or moderate conjunctival redness, and slight or mild
conjunctival oedema in six male New Zealand white rabbits. Three of
the rabbits also had moderate iritis, and one had minimal blood-tinged
discharge. Microscopic examination revealed no corneal injury in any
of the treated eyes. The eyes of the other two rabbits were normal by
72 h. It was concluded that this formulation is a moderate ocular
irritant (Vick & Valentine, 1987).
Ten male albino guinea-pigs exposed to technical-grade
carbendazim or a 75% wettable powder formulation showed no dermal
sensitization after intradermal injections or repeated applications to
intact, shaved skin (Ford, 1981).
A 50% carbendazim formulation was applied to the intact, shaved
skin of 10 male and 10 female Dunkin Hartley albino guinea-pigs; five
male and five female guinea-pigs treated with 80% ethanol in water
served as vehicle controls; two male and two female guinea-pigs
treated with solid test material at the challenge phase only served as
negative controls; and two male and two female guinea-pigs treated
with a 0.3% suspension of 1-chloro-2,4-dinitrobenzene in 80% ethanol
in water served as positive controls. No irritation was observed in
the treated or vehicle or negative controls, but 1-chloro-2,4-
dinitrobenzene produced sensitization in all treated animals (Martin
et al., 1987).
(ii) Neurotoxicity
Groups of 10 white Leghorn hens receved carbendazim at doses of
500, 2500, or 5000 mg/kg bw to test for delayed neurotoxic potential.
Controls received the vehicle, corn oil, and the neurotoxin tri-
ortho-tolyl phosphate. The hens were observed daily for mortality
and clinical neurotoxicity for four weeks. Neurotoxic signs consisting
of leg weakness, ataxia and/or 'goose-stepping' gait were observed in
hens treated with tri- ortho-tolyl phosphate. Less severe, reversible
signs, consisting of slight leg weakness and ataxia, were observed in
hens treated with carbendazim at 5000 mg/kg bw, but no neurotoxic
signs were observed in those treated at lower doses. Microscopic
examination indicated no axonal degeneration or demyelination in
carbendazim-treated birds (Goldenthal, 1978).
(iii) Hormonal effects and spermatogenesis
Since spermatogenesis is an androgen-dependent process, the
effects of carbendazim on the endocrine function of the rat testis
were investigated by feeding 0-400 mg/kg bw per day by gavage for 85
days and measuring the serum levels of pituitary luteinizing hormone,
follicle-stimulating hormone, thyroid-stimulating hormone, and
prolactin, and the levels of androgen-binding protein and testosterone
in serum and in testicular interstitial and seminiferous tubule fluid.
The function al capacity of Leydig cells to secrete testosterone
was assessed in vitro after challenge with human chorionic
gonadotrophin. Doses of 50-100 mg/kg bw per day had no effect on
pituitary or testicular hormone concentrations; 200 mg/kg bw per day
increased the testosterone concentration in the seminiferous tubular
fluid, without affecting serum testosterone or androgen-binding
protein concentrations. The dose of 400 mg/kg bw per day resulted in
increased concentrations of both substances in the interstitial and
seminiferous tubular fluid and of serum androgen-binding protein.
These hormonal changes indicate that carbendazim affects the gonads,
resulting in testicular atrophy. Thus, the elevated seminiferous
tubule fluid testosterone concentrations may be a result of two
factors: (i) greater release of testosterone by the Leydig cells into
the interstitial fluid and/or (ii) decreased testosterone outflow from
the testis into the genera] circulation. The increased level of
androgen-binding protein in the interstitial fluid reflects a change
in its relative secretion into the interstitial fluid and seminiferous
tubules (Rehnberg et al., 1989).
Since extragonadal changes may have contributed to the altered
testicular endocrine profile described above, a further study focused
on the presence of concurrent changes in the hypothalamic and
pituitary control of the testis. Rats were given carbendazim at 50,
100, 200, or 400 mg/kg bw per day by gavage for 85 days. Dose-related
increases in serum follicle-stimulating and luteinizing hormone levels
were noted, but the values for prolactin and thyroid-stimulating
hormone remained unchanged. No significant difference in gonadotropin-
releasing hormone concentration in the mediobasal hypothalamus was
seen, although an increased level was found in the anterior
hypothalamus at 50 mg/kg bw per day, followed by a dose-related
decline (Gray et al., 1988). These findings suggest that
carbendazim-induced testicular damage is accompanied by compensatory
changes in hypothalamic and pituitary regulation of the testis
(Goldman et al., 1989).
These findings imply that carbendazim acts directly on the testis
to induce a number of hormonal and pathological changes. Consistent
with this hypothesis are the results of a study by Nakai et al.
(1992), in which the effects of carbendazim on the testes, efferent
ductules, and spermatozoa were determined after a single oral dose to
male Sprague-Dawley rats. In the first experiment, groups of 86-day-
old rats were treated with 0 or 400 mg/kg bw carbendazim and killed 2,
4, or 8 h later on the same day or 1, 4, 8, 16, or 32 days after
treatment. The first effect of carbendazim was noted after 8 h as an
increase in testicular weight; this continued to increase until day 4,
but on days 16 and 32 the testicular weights were substantially lower
than those of controls in five of 16 animals, indicating individual
variation. A decrease in the percentage of sonication-resistant sperm
heads per testis occurred at 8 h in four of eight rats, but the
decrease was significant only after 24 h, when a mean decrease of 19%
was observed; maximal decreases in total sperm head counts per testis
were seen on day 8, after which some recovery was apparent. Epididymal
weights were increased on day 4, but the percentage of morphologically
normal sperm in the cauda epididymus was decreased at that time. By
day 8, many spermatozoa heads were separated from their flagella, and
10% of the heads were misshapen. Numerous sloughed, round germ cells
and cytoplasmic testicular debris were also evident. No effect on the
percentage of motile sperm was seen at 2, 4, or 8 h or one or four
days after treatment. Sperm motility was significantly decreased on
days 8 and 16, but because of clumping and degeneration of the
spermatozoa, the percentage motility could not be determined.
Similarly damaged sperm were seen in three of eight rats on day 32,
but the percentage of motile sperm that could be measured was similar
to that in controls.
In a second experiment (Nakai et al., 1992), groups of rats
aged 97-105 days were given a single oral dose of 0, 50, 100, 200,
400, or 800 mg/kg bw and were killed 2 or 70 days after treatment. On
day 2, a dose-dependent increase in testicular weight was seen at
doses of 100 mg/kg bw or more. This was accompanied by significant
increases in mean seminiferous tubular diameter at 400 and 800 mg/kg
bw. At 50 mg/kg bw, missing immature germ cells were noted, with round
spermatids from stages I and II and elongated spermatids sloughed from
stage VII epithelium. At 100 mg/kg bw, the disappearance of germ cells
was more severe and sloughing of elongated spermatids extended into
stages XII and XIV. At doses greater than 100 mg/kg bw, germ cells
were missing at all stages except stages IX-XI, and at doses of
400-800 mg/kg bw some seminiferous epithelia were damaged so severely
that it was difficult to identify the stage. In addition, major
pathological changes were seen in the efferent ducts of the testis. In
animals treated with 100 mg/kg bw or more, the rete testis was
swollen, with sloughed germ cells, indicating that ductal blockage had
occurred further down the tract; 50% or more of the efferent ductules
were occluded. The occlusions were characterized as compacted luminal
contents, spermatic granulomas, mineralizations, and obliteration of
the original lumen by fibrotic connective tissue. On day 70, mean
testicular weight and mean seminiferous tubule diameter showed a
dose-dependent decrease. Histologically, these decreases were
associated with a dose-dependent increase in seminiferous tubular
atrophy. The atrophied tubules contained primarily Sertoli cells and
occasional spermatogonia and were surrounded by a thickened basement
membrane. No atrophic tubules were seen in the control rats.
Female hamsters were treated by gavage with a single dose of
carbendazim around the fertilization period, at times selected to
coincide with either of the two microtubule-dependent events initiated
by the ovulatory surge of luteinizing hormone: oocyte maturation
(first meiotic division, occurring late in vaginal proestrous) and
fertilization (second meiotic division, occurring early in vaginal
estrous). In the first experiment, groups of 10 hamsters received 0,
250, 500, 750, or 1000 mg/kg bw carbendazim during melosis I, and
pregnancy outcome was assessed on day 15. The percentage of pregnant
hamsters was significantly reduced at 750 and 1000 mg/kg bw, and, in
those animals that became pregnant, the average number of live pups
was reduced at all doses. In the second experiment, groups of 10
female hamsters were bred overnight and were given a single dose of 0
or 1000 mg/kg bw during meiosis II the next morning. The percentage of
pregnant hamsters was unaffected, but the average number of live pups
(measured at 15 days) was reduced. Thus, administration of carbendazim
at the time of microtubule-dependent meiotic events can result in
early pregnancy loss in hamsters (Perreault et al., 1992).
Rats in pseudopregnancy induced by stimulation of the uterine
cervix with a small brass rod in proestrous and estrous were given 0
or 400 mg/kg bw per day carbendazim for eight days. A uterine decidual
cell response was induced on day 4 of pseudopregnancy, and the animals
were killed on day 9. This response, evaluated as a measure of uterine
competency, was significantly lower in the treated rats than in the
controls (Cummings et al., 1990).
Groups of 12 male C57Bl/6 x C3H/He F1 mice were given
carbendazim at 0, 250, 500, or 1000 mg/kg bw per day by gavage for
five consecutive days, and body weight, testicular weights, and sperm
parameters were measured 7, 24, and 39 days after treatment. Body
weight was unaffected; testicular weight was reduced only in the group
at the highest dose after 7 and 24 days but had recovered by 39 days.
Flow cytometry of testicular cells showed that the relative
percentages of certain testicular populations (round, elongating, and
elongated spermatids) in the group at the highest dose were different
from those in controls 7 and 14 days after treatment (Evenson et al,
1987).
Eight-week-old Wistar-derived male rats were fed 0, 10, 70, or
500 ppm carbendazim in the diet for 182 days. Another group received
500 ppm for 91 days and control diet for a further 91 days. Positive
controls were treated with 0.5 mg/kg bw per day colchicine by gavage
for 12 days. Every 13 days, 10 rats were killed and their testicular
organs were examined by histological, morphometric, enzyme
histochemical, and autoradiographic methods. Males were mated twice
with untreated females on day 182 and were killed on day 208.
Fertility parameters were not affected by treatment. Similarly,
treatment did not affect testicular weight, the area of seminiferous
tubules or interstitial tissue, or epididymal structures and enzyme
activities; however, the incidence of 'degenerating' germ cells
undergoing meiosis and spermatogenesis was increased at 70 and
500 ppm, and a significant increase in the preleptonene spermatocyte
nuclear area was seen in all treated groups. The biological
significance of this finding is difficult to assess. The authors
concluded that the effects seen at 70 ppm (equal to 3 mg/kg bw per
day) indicate that carbendazim affects the physiological 'germinal
elimination process'; however, an independent review of the findings
raised methodological concerns which may preclude definitive analysis
of the data (Hilscher et al., 1992; Russell, 1992).
3. Observations in humans
(a) Medical surveillance of workers
Selected blood profiles from 50 workers involved in the
manufacture of benomyl and carbendazim were compared with those of a
control group of 48 workers who were not exposed to these two
fungicides. White blood cell count, red blood cell count, and
haemoglobin and haematocrit values were comparable in the two groups.
No quantitative estimates of exposure were given for the factory
workers, and no female employees were included in the control group
(Everhart, 1979).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers who were exposed to benomyl between 1970 and
1977. The ages of the workers ranged from 19 to 64 years; 79% of the
workers and 78% of their spouses were aged 20-39. The duration of
exposure ranged from less than one month to 95 months, and more than
51% of the workers had potentially been exposed for one to five
months. The birth rates of the spouses of the exposed workers were
compared with those of four populations in the same county, state,
region, and country (USA). The birth rates of the study population
were generally higher than those of the comparison populations,
indicating no reduction in fertility. Spermatogenesis was not examined
(Gooch, 1978).
(b) Studies of volunteers
Urinary excretion of carbendazim was investigated after oral
administration of 2 mg, application of 17 mg onto a skin area of
100 cm2, or intravenous administration of 1 mg. Total excretion of
5-HBC was proportional to the dose applied, and use of an occlusive
dressing did not significantly increase dermal absorption (Meuling
et al., 1993).
Comments
Carbendazim is readily absorbed by animals after oral exposure
and rapidly metabolized. It is eliminated in the faeces and excreted
in the urine. The tissue distribution showed no bioconcentration. In
rats, 2-[(methoxycarbonyl)amino]-1 H-benzimidazol-5-yl hydrogen
sulfate was identified as the main metabolite. The results of
comparative studies in rats and mice indicate that the detoxification
capacity of mouse liver may become saturated at high doses.
Carbendazim is poorly absorbed via the dermal route in rats. Within 24
h, only about 0.2% of a single dose of 0.6 mg was excreted in the
urine and faeces.
Carbendazim has low acute toxicity, with an oral LD50 in the
rat of > 10 000 mg/kg bw. The clinical signs of toxicity after high
single doses were generally nonspecific. Testicular degeneration has
been observed after single oral doses of > 1000 mg/kg bw in rats.
Wettable powder formulations containing carbendazim have been shown to
be irritating to rabbit skin and eyes but did not induce dermal
sensitization in Buehler-type tests. WHO has classified carbendazim as
unlikely to present an acute hazard in normal use.
In 90-day dietary studies in rats, increased liver weight was
seen at 1350 ppm (the highest dose tested in one study), without any
histological change. The overall NOAEL was 500 ppm, equivalent to
50 mg/kg bw per day. In dogs treated by dietary administration for tip
to two years at levels up to and including 4500 ppm, hepatotoxicity
(increased liver weight, hepatic cirrhosis, swollen vacuolated
hepatocytes, and chronic hepatitis) was seen at 500 ppm and above, and
the NOAEL was 100 ppm, equivalent to 2.5 mg/kg bw per day.
Two two-year studies were conducted in rats given dietary levels
of 0, 100, 500, or 2500-10 000 ppm and 0,150, 300, or 2000-10 000 ppm.
Carbendazim did not have carcinogenic potential, but some effects were
seen at the highest doses tested. In one study there was some evidence
of testicular atrophy, and in the other an increased incidence of
diffuse proliferation of parafollicular cells in the thyroid was seen.
In the latter study, evidence of mild hepatotoxicity was found in the
absence of histopathological change. The NOAEL in both studies was
equivalent to 15 mg/kg bw per day.
Three carcinogenicity studies were performed in mice. In Swiss
mice and CD-1 mice treated for 80 weeks and two years, respectively,
at dietary levels up to 5000 or 7500 ppm, carbendazim increased the
incidence of liver tumours. Because of the minimally increased
incidence of proliferative lesions of the liver at 150 or 500 ppm in
the diet (the lowest doses tested), it was not possible to establish
no-effect levels in these studies. In NMRKf mice, which have a low
spontaneous incidence of liver tumours, no carcinogenic effect was
seen at dietary levels up to and including 5000 ppm (the highest level
tested for 96 weeks), but hepatotoxicity was seen at this dose. The
NOAEL was equal to 34 mg/kg bw per day.
In a three-generation study of reproductive toxicity, rats were
fed dietary levels of 0, 150, 300, or 2000 ppm. Carbendazim had no
effect on reproduction or development at dietary levels up to and
including 2000 ppm (equivalent to 120 mg/kg bw per day). Male
fertility was depressed in rats given carbendazim by gavage at
200 mg/kg bw per day for 85 days. A dose of 50 mg/kg bw per day (the
lowest dose tested) caused a decrease in epididymal sperm counts.
After a single oral dose to rats, disruption of spermatogenesis was
seen at 100 mg/kg bw, but there was no effect at 50 mg/kg bw. The
effect was associated with loss of germ cells resulting from
inhibition of Sertoli-cell microtubules.
Carbendazim was tested for developmental toxicity in rats at
doses of 5-3000 mg/kg bw per day on days 6-15 of gestation. It was
fetotoxic and teratogenic at 20 mg/kg bw per day and above, the main
effects being microphthalmia and hydrocephaly. The NOAEL for
fetotoxicity and teratogenicity was 10 mg/kg bw per day; maternal
toxicity was observed at 90 mg/kg bw per day and above, and the NOAEL
for maternal toxicity was 20 mg/kg bw per day. Rabbits were exposed to
carbendazim by gavage at doses of 0, 10, 20, or 125 mg/kg bw per day
on days 7-19 of gestation. Carbendazim was fetotoxic and teratogenic,
the major effects being malformed vertebrate and ribs. The NOAELs in
this species were 10 mg/kg bw per day for teratogenicity and
fetotoxicity and 20 mg/kg bw per day for maternal toxicity.
Carbendazim has been adequately tested for genotoxicity in a
range of assays. The Meeting concluded that it does not directly
damage genetic material but causes numerical chromosomal aberrations
both in vitro and in vivo as a result of its interference with the
mitotic spindle proteins.
In an epidemiological study of workers exposed to carbendazim,
there was no reduction in fertility, as indicated by the birth rates,
among the study population. Spermatogenesis was not; examined.
An ADI of 0-0.03 mg/kg bw was established on the basis of the
no-effect level of 2.5 mg/kg bw per day in the two-year study in dogs
and a safety factor of 100. The resultant ADI, when compared with the
LOAELs in the studies with Swiss and CD-1 mice, provides an adequate
level of safety.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 34 mg/kg bw per day (96-week study of
toxicity and carcinogenicity in NMRKf mice)
< 150-500 ppm, equivalent to < 22-75 mg/kg bw per day
(studies of toxicity and carcinogenicity in Swiss mice (80
weeks) and CD-1 mice (two years)
Rat: 300-500 ppm, equal to 15 mg/kg bw per day (toxicity in a
two-year studies of toxicity and carcinogenicity)
2000 ppm, equivalent to 120 mg/kg bw per day (study of
reproductive toxicity)
10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
Dog: 100 ppm, equal to 2.5 mg/kg bw per day (two-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to carbendazim
Exposure Relevant route, study type, species Results/remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder non-irritating
Ocular, irritation, rabbit 50% wettable powder irritating
Dermal, sensitization, guinea-pig Non-sensitizing in Buehler test
Inhalation, toxicity, rat LC50 > 5.9 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat and rabbit NOAEL = 10 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary, toxicity, dog NOAEL = 2.5 mg/kg bw per day;
hepatotoxicity
References
Arce, G.T. & Sarrif, A.M. (1984) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report from DuPont Haskell
Laboratory for Toxicology, Newark, Delaware, USA. Submitted to
WHO by AgrEvo, Frankfurt, Germany.
Albertini, S. (1989) Influence of different factors on the induction
of chromosome malsegregation in Saccharomyces cerevisiae D61.M
by Bavistan and assessment of its genotoxic property in the Ames
test and in Saccharomyces cerevisiae DT. Mutat. Res., 216,
327-340.
Albertini, S. (1991) Re-evaluation of file 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae
aneuploidy test systems by the Gene-Tox Program using strain
D61 M of Saccharomyces cerevisiae. Mutat. Res., 260, 165-180.
Alverez, L. (1987) Teratogenicity study of INE-965 (carbendazim) in
rats. Unpublished report from E.I. DuPont de Nemours and Co.,
Haskell Laboratory, Newark, Delaware, USA.
Banduhn, N. & Obe, G. (1985) Mutagenicity of methyl 2-benzimidazole
carbamate, diethylstilbestrol and estradiol: Structural
chromosomal aberrations, sister-chromatid exchanges, C-mitoses,
polyploidies and micronuclei. Mutat. Res., 156, 199-218.
BASF AG (1975) Dominant lethal study in mice. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Beems, R.B., Til, H.P. & van der Heijden, C.A. (1976) Carcinogenicity
study with carbendazim (99% MBC) in mice. Unpublished report from
Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Carter, S.D, Hess, R.A. & Laskey, J.W. (1987) The fungicide methyl
2-benzimidazole carbamate causes infertility in male Sprague-
Dawley rats. Biol. Reprod., 37, 709-718.
Christ, O. & Kellner, H.M. (1973) Animal tests with carbendazim.
Unpublished report from Hoechst AG, Frankfurt, Germany.
Christian, N.S., Hoberman, A.M. & Feussner, E.L. (1985) Developmental
toxicity study of carbendazim administered via gavage to New
Zealand white rabbits. Unpublished report from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by E.I. DuPont de Nemours and Co., Inc.
Cummings, A.M., Harris, S.T. & Rehnberg, G.L. (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat.
Fundam. Appl. Toxicol., 15, 528-535.
Cummings, A.M., Ebron-McCoy, M.T., Rogers, J.M., Barbee, B.D. &
Harris, S.T. (1992) Developmental effects of methyl benzimid-
azolccarbamate following exposure during early pregnancy.
Fundam. Appl. Toxicol., 18, 288-293.
Dashiell, O.L. (1975) Oral LD50 test in guinea pigs (Carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory Newark, Delaware, USA.
Donaubauer, H.H., Schuetz, E., Weigand, W. & Kramer, M. (1982)
Repeated dose (24 month) feeding study, for determination of the
carcinogenic effect of HOE 17411 OFAT204 (carbendazim) in mice.
Unpublished report from Hoechst AG, Pharmaceuticals Research,
Toxicology Section, Frankfurt, Germany.
Donovan, S.D. (1982). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Donovan, S.D. (1983). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Dorn, E. & Keller, H.M. (1980) Carbendazim (60% wettable powder)
absorption via the skin in rats. Unpublished report from Hoechst
AG, Frankfurt, Germany.
Dorn E., Schmidt E., Kellner H.M. & Leist K.H. (1983) HOE 017411-14-C
(carbendazim-14C) metabolic fate in rats and mice, a
comparison. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Edwards, D.F. (1974a) Skin absorption LD50 (carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Edwards, D.F. (1974b) Federal Hazardous Substances Act -- Eye
irritation test in rabbits (carbendazim). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Evenson, D.P., Janca, F.C. & Jost, L.K. (1987) Effects of the
fungicide methyl-benzimidazol-2-yl carbamate (MBC) on mouse germ
cells as determined by flow cytometry. J. Toxicol. Environ.
Health, 20, 387-399.
Everhart, L.P. (1979) Benlate dust exposure survey. Unpublished report
from E.I. DuPont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Fahrig, R & Seiler, J.P. (1979) Dose and effect of methyl-2-
benzimidazolyl-carbamate in the 'mammalian spot test', an
in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-Biol. Interactions, 26, 115-120.
Falke, H.E., Beems, R.B. & Spit, B. (1982a) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 60-day enzyme induction study
in mice. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Falke, H.E., Beems, R.B. & Spit, B. (1982b) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 59-day enzyme induction study
in rats. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Fiscor, G, Bordas, S. & Steward, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Ford, L.S. (1981) Primary skin irritation and sensitization on guinea
pigs. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Ford, L.S. (1982) Acute skin absorption LD50 test on rabbits
(carbendazim 75% wettable powder). Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
from Laboratory for Cancer Research, Hoechst AG, Frankfurt,
Germany.
Goldenthal, E.I. (1978) Neurotoxicity in hens. Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by E.I. DuPont de Nemours and
Co., Inc.
Goldman, J.M., Rehnberg, G.L, Cooper, R.L., Gray, L.E. Jr, Vein, J.F.
& McElroy, W.K. (1989) Effects of the benomyl metabolite,
carbendazim, on the hypothalamic-pituitary reproductive axis in
the male rat. Toxicology, 57, 173-182.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Goodman, N.C. & Sherman, H. (1975) Oral LD50 tests (fasted male and
female rats). Unpublished report from E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Grandizio, A.M. & Sarver, J.W. (1987) Acute oral toxicity study with
INE-965-261 (Benlate C) in male and female rats. Unpublished
report from E.I. DuPont de Nemours and Co., Inc., Haskell
Laboratory, Newark, Delaware, USA.
Gray L.E., Ostby, J., Sigmon, R., Ferrell, J., Rehnberg, G., Linder,
R., Cooper, R., Goldman, J. & Laskey, J. (1988) The development
of a protocol to assess reproductive effects of toxicants in the
rat. Reprod. Toxicol., 2, 281-287.
Gray, L.E., Ostby, J., Linder, R., Goldman, J., Rehnberg, G. & Cooper,
R. (1990) Carbendazim induced alterations of reproductive
development and function in the rat and hamster. Fundam Appl.
Toxicol., 15, 281-297.
Guengerich F.P. (1981) Enzyme induction with Du Pont compounds
H11,201-02 and H10,962-02. Unpublished report from Vanderbilt
University School of Medicine, Nashville, Tennessee, USA.
Submitted to WHO by E.I. Du Pont de Nemours and Co., Inc.,
Newark, Delaware, USA.
Hilscher, W., Ohnesorge, H. & Muller, H. (1992) The effects of
carbendazim on spermatogenesis. Unpublished report from Institut
für Toxicologie, Heinrich-Heine-Universitat, Dusseldorf, Germany.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hinckle, L. (1981) Oral LD50 test in rats -- EPA proposed
guidelines. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Hoechst AG (1974) Mouse dominant lethal study on MBC. Unpublished
report submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hoffman, H.T. & Kirsch, P. (1987) Report on the subchronic toxicity of
methyl benzimidazole-2-carbamate in beagle dogs on oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Hoffman, H.T. & Peh, J. (1987a) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hoffman, H.T. & Peh, J. (1987b) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hunter, B., Batham, P. & Newman, A.J. (1973a) Carbendazim oral
toxicity to rats in oral administration for 2 weeks. Unpublished
report from Huntingdon Research Centre, United Kingdom. Submitted
to WHO by Hoechst AG, Frankfurt, Germany.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. & Newman, A.J.
(1973b) Carbendazim toxicity to rats during dietary
administration for 13 weeks followed by a recovery period of 6
weeks. Unpublished report from Huntingdon Research Centre, UNited
Kingdom. Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Ivett, J.L. (1984) Mutagenicity evaluation of H#15,152 in an
in vitro sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report from Litton Bionetics,
Inc., Kensington, Maryland, USA. Submitted to WHO by E.I. DuPont
de Nemours and Co., Inc., Newark, Delaware, USA.
Janardhan, A., Sattur, P.B. & Sisodia, P. (1984) Teratology of methyl
benzimadazole carbamate in rats and rabbits. Bull. Environ.
Contam. Toxicol 33, 257-263.
Janardhan, A., Rao, A.B. & Sisodia, P. (1987) Sub-chronic toxicity of
methyl benzimidazole carbamate in rats. Bull. Environ. Contam.
Toxicol., 38, 890-898.
Jotz, M.M. (1980) An evaluation of mutagenic potential of benomyl
employing the L5178Y TK+/-mouse lymphoma assay. Unpublished
report from SRI International, Menlo Park, California, USA,
prepared for the US Environmental Protection Agency.
Kellner, H.M. (1983) Carbendazim-(2-14C) whole body autoradiography
studies of the distribution in rats and mice after oral and.
intravenous administration. Unpublished report from Hoechst AG,
Frankfurt, Germany.
Kellner, H.M. & Eckert, H. (1983) Carbendazim-(2-14C) blood levels,
distribution and excretion in rats and mice after oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Koeter, H.B.W.M. (1975a) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the rat Unpublished report from Central Institute
for Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, and BASF AG,
Ludwigshafen, Germany.
Koeter, H.B.W.M. (1975b) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the New Zealand white rabbit. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Kramer, M. & Weigand, W. (1971) (HOE 17411 OF) Toxicological
examination. Unpublished report from Hoechst AG, Pharmaceuticals
Research, Toxicology Section, Frankfurt, Germany.
Krechniak, J. & Klosowska, B. (1986) The fate of 14C-carbendazim in
the rat. Xenobiotica, 16, 809-815.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Litton Bionetics, Inc. (1981) Evaluation of HOE 17411 OF AT204 in the
primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Inc., Kensington,
Maryland, USA. Submitted to WHO by Hoechst AG, Frankfurt,
Germany.
Martin, D.A., Henry, J.E. & Brock, W.J. (1987) Closed-patch repeated
insult dermal sensitization study (Buehler method) with Benlate C
fungicide in guinea-pigs. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Meuling, W.J.A., Opdam, J.J.G. & DeKort, W.L.A.M. (1993) Dose-
excretion study with the fungicide carbendazim in volunteers.
Unpublished report from Central Institute for Nutrition and Food
Research (TNO) The Hague, Netherlands. Submitted to WHO by
Hoechst AG, Frankfurt, Germany.
Minta, M. & Biernacki, B. (1982) Embryotoxicity of carbendazim in
rats, rabbits and hamsters. Bull. Vet. Inst. Pulawy, 2, 42-52.
Mohr, U. (1977) Review of liver sections from mice and rats fed with
carbendazim. Unpublished report from Department of Experimental
Pathology, Medical School, Hanover, Germany. Submitted to WHO by
Hoechst AG, Frankfurt, and BASF AG, Ludwigshafen, Germany.
Monson K.D. (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.
Wilmington, Delaware, USA.
Nakai, M., Hess, R.A., Moore, B.J., Guttroff, R.F., Strader, L.F. &
Linder, R.E. (1992) Acute and long-term effects of a single dose
of the fungicide carbendazim (methyl 2-benzimidazole carbamate)
on the male reproductive system in the rat. J. Androl., 13,
507-518.
Nash, S.D. & Ferenz, R. (1982) Inhalation median lethal concentration
(LC50) in rats -- EPA protocol (carbendazim 75% wettable
powder). Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Osgood, C., Zimmering, S. & Mason, J.M. (1991) Aneuploidy in
Drosophila. II: Further validation of the FIX and ZESTE genetic
test systems employing female Drosophila melanogaster. Mutat.
Res., 259, 147-163.
Pandita, T.K. (1988) Assessment of the mutagenic potential of a
fungicide Bavistin using multiple assays. Mutat. Res., 204,
627-643.
Perreault, S.D., Jeffay, S., Poss, P. & Laskey, J.W. (1992) Use of the
fungicide carbendazim as a model compound to determine the impact
of acute chemical exposure during oocyte maturation and
fertilization on pregnancy outcome in the hamster. Toxicol.
Appl. Pharmacol., 114, 225-231.
Rashid, M.A. & Mumma, R.O. (1986) Screening pesticides for their
availability to damage bacterial DNA. J. Environ. Health Sci.,
B21, 319-334
Rehnberg, G.L., Cooper, R.L., Goldman, J.M., Gray, L.E, Hein, J.F. &
McElroy, W.K. (1989) Serum and testicular testosterone and
androgen binding protein profiles following subchronic treatment
with carbendazim. Toxicol. Appl. Pharmacol., 101, 55-61.
Reuzel, P.G.J., Hendriksen, C.F.M & Til, H.P (1976) Long-term
(two-year) toxicity study with carbendazim in beagle dogs.
Unpublished report from the Central Institute for Nutrition and
Food Research for BASF. Submitted to WHO by E.I. DuPont de
Nemours and Co., Wilmington, Delaware, USA.
Russell, J.F. (1977) Mutagenicity evaluation of 2-benzimidazole
carbamic acid, 5-hydroxymethyl in Salmonella typhimurium.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1983) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from E.I. DuPont de Nemours and
Co., Inc.. Haskell Laboratory, Newark, Delaware, USA.
Russell, L.D.. (1992). Review of Prof. Hilscher's report «Effects of
carbendazim on spermatogenesis». Unpublished report from
University of Illinois, USA. Submitted to WHO by Hoechst AG,
Germany.
Sarver, J.W. (1975) Acute inhalation toxicity -- one hour head only,
(carbendazim). Unpublished report from E.I. DuPont de Nemours,
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Scholz, J. & Weigand, W. (1972) Carbendazim (HOE 17411 OF, batch
496/I). Toxicological examination. Unpublished report from
Hoechst AG, Pharmaceuticals Research, Toxicology Section,
Frankfurt, Germany.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1965) Acute oral test. Unpublished report from E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. (1968) Ninety-day feeding study in rats using a wettable
powder formulation (70% MBC). Unpublished report from E.I. DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1970) Three-month feeding study in dogs using a wettable
powder formulation (50% MBC). Unpublished report from E.I DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1972) Long-term feeding studies in rats and dogs with
2-benzimadazole carbamic acid, methyl ester (INE-965) (50% and
70% MBC wettable powder formulations). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. and Krauss, W.C. (1966). Acute oral test (carbendazim).
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware.
Shirasu, Y., Morlya, M. & Kato, K. (1977) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Stadler, J.C. (1986) One-year feeding study in dogs with carbendazim.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Til, H.P., van den Muelen, H.C, Feron, V.J., Seinen, W. & de Groot,
A.P. (1972) Sub-chronic (90 day) toxicity study with W17411 in
beagle dogs. Unpublished report from Central Institute for
Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Til, H.P., Koellen, C. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with carbendazim in
rats. Unpublished report from Central Institute for Nutrition and
Food Research (TNO), The Hague, Netherlands. Submitted to WHO by
BASF AG, Ludwigshafen, and Hoechst AG, Frankfurt, Germany.
Til, H.P., Koeter, H.B.W.M. & van der Heijden, C.A. (1976b)
Multigeneration study with carbendazim in rats. Unpublished
report from Central Institute for Nutrition and Food Research
(TNO), The Hague, Netherlands. Submitted to WHO by Hoechst AG,
Frankfurt, Germany.
Til, H.P., Beems, R.B. & de Groot A.P. (1981) Determination of the
acute oral toxicity of carbendazim in mice. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Tong, C. (1981a) Hepatocyte primary culture/DNA repair assay on
compound 11, 201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Tong, C. (1981b) Hepatocyte primary culture/DNA repair assay on
compound 11,201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Vick, D.A. & Brock, W.J. (1987) Acute dermal toxicity study of Benlate
C fungicide in rabbits. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Vick, D.A. & Valentine, R. (1987) Primary eye irritation study with
Benlate C fungicide in rabbits. Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Waterer, J.C. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory Newark, Delaware, USA.
Whittaker, S.G., Moser, S.F., Maloney, D.H., Plegorsch, W.W., Resnick,
M.A. & Fogel, S. (1990) The detection of mitotic and meiotic
chromosome gain in the yeast Saccharomyces cerevisiae: Effects
of methyl benzimidazol-2-yl carbamate, methyl methanesulfonate,
ethyl methanesulfonate, dimethyl sulfoxide, propionitrile and
cyclophosphamide monohydrate. Mutat. Res., 242, 231-258.
Wood, C.K. (1982) Long-term feeding study with 2-benzimidazole-
carbamate, methyl ester (> 99% MBC, INE-965) in mice.
Unpublished report from E.I. DuPont de Nemours and Co, Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Zeller, H. & Kirsch, P. (1971) Acute oral toxicity of methyl-2-
benzimidazole carbamate to the rabbit. Unpublished report from
Hoechst AG, Frankfurt, Germany.
Table 1. Residual content of carbendazim in the livers of rats
Dosage Residual content (%)
Rat Mouse
Single dose
3 mg/kg bw 12 29
300 mg/kg bw 18 26
29-day repeated dose
3 mg/kg bw 2 < 2
300 mg/kg bw 4 28
In the study of Krechniak & Klosowska (1986), 94% of the measured
radiolabel in urine 12 h after treatment was as 5-HBC, 3% as 2-AB, and
3% as carbendazim.
The proposed metabolic pathway for carbendazim in rats is given
in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed for 28 days with diets containing benomyl or carbendazim at a
concentration of 0, 10, 30, 100, 300, 1000, or 3000 ppm. After
sacrifice, liver weights were recorded and microsomal epoxide
hydrolase and cytosolic glutathione- S-transferase were monitored in
subcellular fractions isolated from the liver. The mean absolute liver
weights were elevated in males and females fed 1000 or 3000 ppm
carbendazim and in females fed 300 ppm; however, the only
significantly increase was found in females fed 3000 ppm benomyl. No
apparent liver toxicity or effect on body weight was observed. Both
benomyl and carbendazim induced epoxide hydrolase in male and female
rats and mice fed 1000 or 3000 ppm, and both induced glutathione- S-
transferase at 3000 ppm. The level of induction seemed to be slightly
greater in females than males. There was no substantial difference in
enzyme induction between rats and mice (Guengerich, 1981).
In the same study, but in a separate test, CD-1 male mice were
treated by gavage with carbendazim suspended in 0, 100, or 1000 mg/kg
bw per day corn oil for five days. After sacrifice, liver samples were
homogenized, and subcellular fractions were prepared as described
above. Wet liver weights and the activities of microsomal cytochrome
P450, NADPH-cytochrome- creductase, styrene-7,8-hydrolase,
benzphetamine- N-demethylase, benzo[ a]pyrene hydroxylase,
7-ethoxycoumarin-deethylase, and cytosolic glutathione- S-transferase
were measured. The activities of styrene-7,8-hydrolase and
glutathione- S-transferase were statistically significantly increased
over the control values; that of 7-ethoxycoumarin-deethylase was
significantly decreased. It is noteworthy that the total microsomal
cytochrome P450 level did not increase, indicating that carbendazim
did not induce overall microsomal induction, even at the higher dose.
The increase in styrene-7,8-hydrolase activity shows, however, that
some hepatic microsomal enzymes are induced by carbendazim in vivo.
There appeared to be no substantial difference in enzyme induction
between rats and mice (Guengerich, 1981).
Groups of male Wistar rats and Swiss mice were given carbendazim
in the diet at levels of 0-10 000 ppm for 60 days, and the induction
of liver enzyme activities was examined and compared with that induced
by phenobarbital sodium administered in the drinking-water. Growth and
food consumption were decreased in rats at 10 000 ppm but not in mice
given up to 5000 ppm in the diet. Relative liver weights were
increased in rats fed 2000 or 10 000 ppm and in mice receiving 1000 or
5000 ppm carbendazim. Phenobarbital had similar effects. The protein
concentrations in total homogenates and post-mitochondrial fractions
of liver from rats were not affected by carbendazim, whereas they were
increased in mice at 5000 ppm. Feeding of carbendazim to rats at
2000 ppm or more resulted in slight-to-moderate induction of several
phase-I drug metabolizing enzymes: 7-ethoxycoumarin- O-deethylase,
biphenyl-4-hydroxylase, aniline hydroxylase, 4-methoxybiphenyl- N-
demethylase, and cytochrome- c-reductase. The activities of the
phase-II drug metabolizing enzymes glucuronyl transferase I and II and
the glutathione content were moderately-to-markedly increased at this
dose. Feeding of carbendazim to mice at 1000 ppm or more resulted in
moderate-to-marked increases in the activities of phase-I drug
metabolizing enzymes, including cytochrome P-450 and aminopyrine- N-
demethylase; cytochrome- c-reductase activity was decreased, and
those of glucuronyl transferase and glutathione- S-transferase and
glutathione content were slightly increased. There was no measureable
difference between rats and mice with regard to the metabolism of
carbendazim, although exhaustion of the detoxification mechanism was
more evident in mice at high doses. The detoxification and elimination
of carbendazim and its metabolites proceed more rapidly in rats than
in mice, as reflected in the increased glutathione content of rat
liver and the increased activity of phase-II enzymes (Falke et al.,
1982a,b).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
2. The clinical signs of toxicity after treatment with carbendazim
were generally nonspecific. The acute toxicity of carbendazim in a
number of species is low, LD50 values by various routes of
administration ranging from > 2000 to > 15 000 mg/kg bw. Gross and
histopathological changes were observed in the testes and epididymides
of male rats given carbendazim orally at doses of 1000 mg/kg bw and
more. The testes were small, soft, and discoloured, and more than 70%
of the tubules showed degenerative changes. The sperm count in the
epididymides examined was reduced or nil.
(b) Short-term toxicity
Rats
Groups of six male Sprague Dawley rats were given carbendazim by
gavage at a dose of 0, 200, 3400, or 5000 mg/kg bw per day, five times
per week for two weeks. Two rats at 3400 mg/kg bw per day died. At all
doses, gross and microscopic evidence of adverse effects on testes and
reduction or absence of sperm in the epididymides was seen. The testes
were small and discoloured, with tubular degeneration and evidence of
aspermatogenesis. At 3400 mg/kg bw per day, there were also
morphological changes in the duodenum (oedema and focal necrosis),
bone marrow (reduction in the blood-forming elements), and liver
(decrease in large, globular-shaped vacuoles) (Sherman, 1965; Sherman
& Krauss, 1966).
Groups of 10 male Sprague-Dawley rats were given carbendazim by
gavage at a dose of 0, 10, 20, 30, or 40 mg/kg bw per day for two
weeks. At the high dose, liver weights were increased. There were no
histopathological findings and no effects on spermatogenesis, on
cellularity, or on the incidence of mitosis, as evidenced by tritiated
thymidine incorporation 1 h before sacrifice (Hunter et al., 1973a).
Administration of 1350 ppm carbendazim to Sprague-Dawley rats for
13 weeks resulted in hepatomegaly in animals of each sex, which was
not accompanied by histopathological lesions and was reversible after
six weeks. A dietary level of 450 ppm (equal to 35 mg/kg bw per day)
was the NOAEL (Hunter et al., 1973b).
In a poorly reported study, groups of 10 male and 10 female
litter-mate weanling Wistar rats were treated by gavage with 0, 16,
32, or 64 mg/kg bw per day for 90 days. The erythrocyte counts in
treated rats were lower than those of the controls after 15 days of
exposure; however, no clear dose-response relationship was seen after
30, 60, or 90 days of exposure. Leukocyte counts were decreased after
Table 2. Acute toxicity of carbendazim and carbendazim formulations
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Carbendazim Mouse Oral > 15 000 NR Til et al. (1981)
Carbendazim Rat Oral > 10 000 > 95 Goodman & Sherman (1975)
Carbendazim Rat Oral > 11 000 > 95 Sherman (1965)
Carbendazim Rat Oral > 15 000 > 95 Kramer & Weigand (1971)
Carbendazim Rat Oral > 17 000 NR Sherman & Krauss (1966)
Carbendazim Guinea-pig Oral > 5 000 NR Dashiell (1975)
Carbendazim Rabbit Oral > 8 000 NR Zeller & Kirsch (1971)
Carbendazim Dog Oral > 5 000 NR Scholz & Weigand (1972)
Carbendazim Mouse Intraperitoneal > 15 000 NR Scholz & Weigand (1972)
Carbendazim Rat Intraperitoneal > 2 000 NR Scholz & Weigand (1972)
Carbendazim Rat Inhalation (1-h) > 5.9 NR Sarver (1975)
Carbendazim Rat Dermal > 2 000 NR Kramer & Weigand (1971)
Carbendazim Rabbit Dermal > 10 000 NR Edwards (1974a)
'75% wettable powder' Rat Oral > 5 000 NR Hinckle (1981)
'75% wettable powder' Rat Inhalation (4-h) > 5 > 95 Nash & Ferenz (1982)
'75% wettable powder' Rabbit Dermal > 2 000 > 95 Ford (1982)
'75% wettable powder' Rat Oral > 5 000 NR Grandizio & Saner (1987)
'75% wettable powder' Rabbit dermal > 2 000 > 95 Vick & Brock (1987)
NR, Not reported
15 days, and after 30 and 60 days animals of each sex showed transient
decreases in lymphocyte counts in comparison with controls, although
no clear dose-response relationship was observed among the treated
groups. No change was seen in the activity of whole-blood
cholinesterase. Male rats had significantly increased alkaline
phosphatase activity at a dose of 64 mg/kg bw per day, and blood urea
levels were lowered in males at 32 and 64 mg/kg bw per day after 90
days. Increased serum bilirubin concentrations were seen in males and
females at 32 and 64 mg/kg bw per day; these were attributable to
parenchymal cell damage, as indicated by increased alanine amino-
transferase activity. Dose-related changes in the liver ranged from
sparse infiltration by inflammatory cells to inflammatory and
degenerative changes. Tubular dilatation and hydropic degeneration
were noted in the kidneys of rats at the low dose, and fibrosis and
congestion were seen in rats at the medium and high doses. Increased
lung weights were correlated with bronchopneumonic changes. Slight
changes in weights were reported for several other organs. In view of
the lack of data on individual animals in this experiment and the
apparent variability in the results, no NOAEL could be established
(Janardhan et al., 1987).
Groups of 16 male and 16 female Sprague-Dawley rats were fed
carbendazim (purity, 72%) in the diet for 90 days at 0, 100, 500, or
2500 ppm (as carbendazim). The animals were observed daily for
behavioural changes and body weight, and food consumption was recorded
weekly. Haematological examinations were conducted on 10 male and 10
female rats in each group at 30, 60, and 90 days. Routine urinalyses
were performed on the same animals, and plasma alkaline phosphatase
and alanine aminotransferase levels were determined. After 90-98 days
of continuous feeding, 10 male and 10 female rats in each group were
killed, and selected organs were weighed; these and other organs were
also preserved for microscopic examination. The six male and six
female rats remaining in each group after terminal sacrifice were used
in a study of reproductive toxicity (see below). There were no
clinical signs of toxicity and no compound-related effects on weight
gain, food consumption, or haematological parameters. The relative
liver weights in females fed 2500 ppm were slightly increased in
comparison with control rats. Testicular weight was not affected in
any group. Microscopic examination of selected tissues and organs in
the control and high-dose groups showed no adverse effect attributable
to carbendazim. The NOAEL in this study was 500 ppm, equivalent to
50 mg/kg bw per day (Sherman, 1968).
Rabbits
Groups of six male New Zealand albino rabbits received 0 or
2000 mg/kg bw carbendazim as a 50% aqueous paste on shaved intact
dorsal skin repeatedly, for 6 h/day, for 10 consecutive days. There
were no adverse effects on body weight, clinical symptoms, organ
weights, gross pathology, or histopathology of selected organs;
however, there was focal necrosis of the epidermis and polymorpho-
nuclear cell infiltration of the dermis in five of six exposed
rabbits. No other effects were observed (Dashiell, 1975).
Dogs
Groups of four male and four female one-year-old beagle dogs were
given carbendazim (purity, 53%) in the diet for three, months at 0,
100, 500, or 2500 ppm (as carbendazim); the highest level was
subsequently reduced to 1500 ppm because of reduced food intake and
body weight. Food consumption and body weight were recorded weekly,
and clinical laboratory examinations, including haematological,
biochemical, and urinary measurements, were performed periodically. At
the end of the study, all animals were killed, selected organs were
weighed, and these and other organs were subjected to gross and
microscopic evaluations. No mortality or adverse clinical signs were
observed over the course of the study, and growth and food consumption
were normal, except in animals at 1500-2500 ppm. Urinalysis showed no
change due to treatment, and there were no dose-related effects on
haematological values. Females at the mid- and high doses showed a
trend to increased cholesterol levels over the pre-test and control
levels after one, two, and three months. The weights of the thymus
were increased in males at the low and mid-doses, and those of the
prostate at the mid-dose. Limited histopathological data did not
indicate any compound-related effects. The data for the high-dose
group were compromised by the change in dietary level, which involved
a 'recovery' period during which the animals received control diet
(Sherman, 1970).
Groups of four male and four female beagle dogs were given
carbendazim in the diet at 0, 100, 300, or 1000 ppm for 13 weeks. The
highest level was increased to 2000 ppm after six weeks of treatment.
Body weight, haematological, blood chemistry and urine measurements,
and liver and kidney function tests were performed periodically. The
animals were examined grossly and microscopically at the end of the
study. There were no reported compound-related effects on clinical
behaviour, body weight, food consumption, haematological parameters,
kidney function (phenol red excretion) or liver function (brom-
sulphthalein retention). Blood chemistry was normal, except for a
slight decrease in albumin in males at the mid- and high doses at 12
weeks. Urinalysis showed normal values, except for a high bacterial
count in females at the high dose at week 13. The blood clotting time
was slightly reduced in dogs at the high dose at week 12. There were
slight increases in relative liver and thyroid weights and a decrease
in relative heart weights in the group at the highest dose. No
microscopic changes that could be associated with treatment were
observed in these or other organs. The NOAEL was 300 ppm, equivalent
to 7.5 mg/kg bw per day, on the basis of minor changes in clinical
chemistry and organ weights (Til et al., 1972).
Groups of three male and three female beagle dogs were fed diets
containing 0, 500, 1500, or 4500 ppm carbendazim for 13 weeks. Reduced
body-weight gain, increased liver weight, hepatic periportal
infiltration, and hepatic regeneration were seen at the high dose. No
histopathological changes were seen in any other organs, including the
testes. At 1500 ppm, liver weights were increased, but there were no
histological lesions. The NOAEL was 500 ppm, equal to 12.5 mg/kg bw
per day (Hoffman & Kirsch, 1987).
Groups of five male and five female beagle dogs were fed diets
containing 0, 100, 200, or 500 ppm carbendazim for one year. The dogs
were weighed at regular intervals, and individual food consumption was
monitored throughout the study; clinical pathology was evaluated
periodically. After one year, all of the dogs were killed and selected
tissues were examined microscopically. There were no statistical
differences in mean body weight that could be attributed to treatment,
and the mean daily food consumption of treated dogs was similar to
that of controls. None of the clinical observations was attributable
to carbendazim intake. Dogs fed 500 ppm had elevated levels of serum
cholesterol, which were statistically significant for males at nine
months and for females at one and two months. There were no
microscopic lesions related to treatment. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day (Stadler, 1986).
Groups of four male and four female beagle dogs, one to two years
of age, were given carbendazim (purity, 53%) in the diet at 0, 100,
500, or 2500 ppm (as carbendazim) for two years. Food consumption and
body weights were measured weekly, and animals were examined daily for
clinical signs of toxicity. Haematological, biochemical, and urinary
examinations were performed periodically throughout the study. After
one year, one male and one female in the control and 500-ppm groups
were killed. At the end of the study, the organs were weighed, and
gross and histopathological examinations were performed. No mortality
was reported among the controls or dogs at 100 or 500 ppm; however,
three males at the high dose were sacrificed after 22 and 42 weeks
because of poor nutrition. No females at the high dose died.
Haematological and urinary values were unaffected by treatment. The
dogs at 500 ppm had increased levels of cholesterol, blood urea
nitrogen, total protein, and serum alanine aminotransferase. Swollen,
vacuolated hepatic cells and marginal proliferation of the portal
triads with cellular infiltration were observed in one dog that had
been fed 500 ppm and was sacrificed after one year. The biochemical
evidence of an effect on the liver was corroborated by the finding at
terminal sacrifice of hepatic cirrhosis, swollen, vacuolated hepatic
cells, and mild chronic hepatitis in dogs fed 500 ppm or more of
carbendazim. There were no effects on organ weights. Diffuse
testicular atrophy and aspermatogenesis were observed in two of four
males at 100 ppm but not at higher doses. As similar effects were not
seen at the other doses, these findings are considered not to be
treatment-related. The NOAEL was 100 ppm, on the basis of the effects
on the liver at 500 ppm, equivalent to 2.5 mg/kg bw per day (Sherman,
1972).
Groups of four male and four female beagle dogs, aged 22-27
weeks, were fed carbendazim in the diet at 0, 150, 300, or 2000 ppm
for 104 weeks. After 33 weeks, the dose of 2000 ppm was increased to
5000 ppm. The dogs were examined daily for clinical signs of toxicity
and altered behaviour; body weight and food consumption were recorded
regularly throughout the study, and haematological examinations, blood
chemistry (including liver and kidney function tests), and urinary
measurements were conducted periodically. After 104 weeks, the dogs
were sacrificed, and the tissues were reported to have been examined
grossly and microscopically, although the available data indicate that
the pathological examinations were inadequate. The only death was that
of a female at the high dose which was killed in a moribund state
after week 36. The body weights of males at the mid-dose and of males
and females at the high dose were decreased. Food consumption was
comparable in all groups. Blood clotting times were significantly
reduced in males at the high dose from week 13 to term, and slight
decreases were noted in females at the high dose. Serum alkaline
phosphatase activity was increased at the high dose throughout the
study, but there were no compound-related effects on serum alanine or
aspartate aminotransferase activities. All other haematological
parameters and blood chemistry were comparable with those of
the controls. There were no differences among the groups in
bromsulphthalein retention, phenol red excretion, or urinary values.
Liver and thyroid weights were significantly increased in dogs at the
high dose, but there were no microscopic changes in these organs that
were related to treatment. An increased incidence of prostatitis was
seen in high-dose males in comparison with controls (3/4 versus 1/4).
One male at that dose also had interstitial mononuclear inflammatory
cell infiltrates and atrophic tubules of the testes. Although feeding
of carbendazim in the diet to dogs for two years apparently had no
adverse effect at levels up to and including 300 ppm, the deficiencies
in the reporting of the pathological data preclude determination of an
NOAEL (Reuzel et al., 1976).
(c) Long-term toxicity and carcinogenicity
Mice
Carbendazim was administered in the diet to groups of 100 male
and 100 female specific pathogen-free Swiss mice at 0, 150, 300, or
1000 ppm for 80 weeks. The highest dose was increased to 2000 ppm at
week 4 and to 5000 ppm at week 8 for the remainder of the study.
Animals were examined for behaviour and for clinical signs of
toxicity, and body weights were measured throughout the study. All
animals were examined grossly, liver and kidney weights were recorded,
and tissues were examined microscopically. There were no compound-
related effects on general condition, mortality, or body weight. At
termination of the study, 70% of males and 80% of females were still
alive. The relative liver weights of males and females at the high
dose were significantly higher than those of controls, but kidney
weights were unchanged. After peer review and reclassification of the
data on hepatic tumour incidence, the combined incidence of
hepatocellular adenomas and carcinomas was found to have increased
with increasing doses in both males and females (Table 3). Males
showed more pronounced induction of liver tumours and more frequent
occurrence of hepatocellular carcinomas, which were often found
simultaneously with hepatocellular adenomas, whereas females usually
had only hepatocellular adenomas. It was concluded that carbendazim is
oncogenic in this strain of mouse at a dietary level of 5000 ppm
(Beems et al., 1976; Mohr, 1977).
Table 3. Hepatic rumour incidence in Swiss mice fed carbendazim
Sex Dietary level No.of mice Liver nodular Hepatocellular Hepatocellular
(ppm) examined hyperplasia adenoma carcinoma
Male 0 100 0 9 1
150 94 8 5 3
300 98 11 13 4
100/5000 100 25 14 9
Female 0 94 0 1 1
150 99 5 1 0
300 98 3 3 0
1000/5000 95 11 8 0
Groups of 80 male and 80 female CD-1 mice, aged six to seven
weeks, were given carbendazim (purity, 99%) in the diet at 0, 500,
1500, or 7500 ppm for two years. The highest dose was reduced to
3750 ppm for the males after 66 weeks because of increased mortality
(62 controls, 32 at 7500 ppm); females, however, received 7500 ppm
throughout the study. Treatment affected mortality in male mice, and
those at the high dose were sacrificed at week 73 because only 23 were
still alive. Only nine males at 1500 ppm survived to week 104, whereas
18 male controls were still alive at that time. Females had no similar
increase in mortality. There were no dose-related effects on body
weight or food consumption at any time, although the terminal body
weights of males at the low and mid-doses were lower than those of the
control and high-dose males. Clinical parameters were similar for all
treated and control groups, and haematological measures were
unaffected. Both absolute and relative thymic weights were
significantly decreased in females at 500 and 1500 ppm, but not in the
high-dose group. Absolute liver weights were increased in females at
the high dose and relative liver weights in those at the two highest
doses. The organ weights of male mice were variable, and only those of
the kidney and thymus appeared to be decreased as a result of
treatment. Absolute kidney and thymic weights were depressed in male
mice at all doses, but relative kidney and thymic weights were
significantly decreased only in males at the high dose. Histological
examination revealed dose-related changes in the thymus (lymphoid
depletion) and accumulation of yellow-brown pigment in the renal
tubules of male mice at the mid- and high doses. These mice also had
an increased frequency of sperm stasis in the testes and increased
bilateral germinal cell atrophy; there was no trend for unilateral
germinal cell atrophy, the incidence in controls being greater than or
equal to that of treated males. These effects are therefore considered
not to be compound-related. A significant hepatotoxic effect was seen
in male mice at 1500 and 7500 ppm, as demonstrated by centrilobular
hypertrophy, necrosis, and swelling. The frequency of hepatocellular
adenomas was not increased, as they occurred at equal frequency in
control and treated groups. There was a significant increase in the
incidence of hepatocellular carcinomas, but only at 1500 ppm; however,
too few males at the high dose survived to 17 months (510 days) to
support the conclusion that there is no oncogenic effect at that dose.
The combined incidence of hepatocellular carcinomas, hepatocellular
adenomas, and hepatoblastomas (Table 4) was significantly increased
(P < 0.05) in females at the low, mid-, and high doses and in males
at the mid-dose, but this was not evaluated in high-dose males because
of the high rate of mortality. The study thus shows statistically
significant increases in the incidences of hepatocellular carcinoma
for males at 1500 ppm and for females at all doses, the response being
dose-related. A dose-response relationship could not be determined
because of the high mortality in the high-dose males; the high
mortality rate in male controls also hampered interpretation of
results. No carcinogenic effect was observed in tissues other than the
liver (Wood, 1982).
Carbendazim was administered in the diet of groups of 100-120
male and female NMRKf mice for 96 weeks at a dose of 0, 50, 150, 300,
or 1000 ppm. The highest dose was increased to 2000 ppm at week 4 and
to 5000 ppm at week 8 for the remainder of the study. Animals were
examined for behaviour and general condition and for body weight, food
and water consumption, and mortality. Gross necropsy was performed on
all animals, liver and lung weights were recorded, and all organs and
tissues were examined microscopically. An interim sacrifice was
conducted of 20 males and 20 females in the control group and that at
the highest dose at 18 months. There were no compound-related effects
on behaviour, body-weight gain, food or water consumption, or
mortality. By 22 months, 24-31% of the males and 37-52% of the females
had died. As there was no difference between the treated and control
groups, it was concluded that mortality was not influenced by the
feeding of carbendazim. At 18 and 22 months, the absolute and relative
liver weights of both male and female mice at 5000 ppm were increased.
Macroscopic and microscopic examination of 20 male and 20 female
animals killed after 18 months of receiving 5000 ppm carbendazim
revealed compound-related effects on the liver: all animals had
centrilobular hypertrophy, single-cell necrosis, mitotic cells, and
pigmented Kupffer cells. The tissues of the remaining 100 males and
100 females exposed to 5000 ppm, evaluated at 22 months, showed marked
hypertrophy (greater than in animals treated for 18 months), clear-
cell foci, mitosis, inclusion bodies in enlarged cell nuclei, multiple
cell necrosis, and a greenish-yellow pigment in Kupffer cells.
Neoplastic nodules (adenomas), carcinomas, fibrosarcomas, and other
tumorigenic responses in the liver were equally distributed among the
groups. Although haemangiomas of the liver were found in all treated
groups but not in controls, no dose-related response was evident. Lung
adenomatosis was equally distributed among the groups. The tumour
incidence is summarized in Table 5. There was no effect on the
incidence or time of onset of tumours, and the total number of benign
and malignant tumours was comparable among the different groups of
mice. Thus, there was no evidence that carbendazim administered in the
diet of mice at doses up to and including 5000 ppm for 22 months had a
carcinogenic effect. The NOAEL was thus 300 ppm, equal to 34 mg/kg bw
per day (Donaubauer et al., 1982).
Rats
Groups of 36 male and 36 female weanling Sprague-Dawley rats were
given carbendazim (purity, 50-70%) in the diet for 104 weeks at 0,
100, 500, 2500 (increased to 10 000 ppm after 20 weeks), or 5000 ppm
(as carbendazim). Body weight and food consumption were recorded
weekly for the first year and twice a month thereafter. Behavioural
changes and mortality were observed daily. Haematological, urinary,
and selected clinical chemical examinations were performed
periodically. After one year, each group was reduced to 30 male and 30
female rats by interim sacrifice for gross and microscopic
examinations. At the end of the study, all surviving animals were
sacrificed, and tissues and organs were examined grossly. Microscopic
examinations were conducted on all tissues and organs from the
controls and animals at 2500-10 000 ppm, the livers of animals at 100
and 500 ppm, and the livers, kidneys, testes, and bone marrow of
animals at 5000 ppm. Body-weight gain was depressed in males and
females at 2500-10 000 ppm and in females at 5000 ppm in comparison
with controls. Food consumption was similar in all groups. Reduced
erythrocyte counts and haemoglobin and haematocrit values were seen in
females at 2500-10 000 or 5000 ppm after 9-24 months and in males at
2500-10 000 ppm after 24 months. There were no compound-related
Table 4. Hepatic tumour incidence in CD-1 mice fed carbendazim
Sex Dietary level No. of mice No. of mice alive Median survival Combined incidence
(ppm) examined at termination (weeks) of hepatic tumours
Male 0 80 18 79 13
500 80 14 72 20
1500 80 9 69 23
7500/3500 80 23 64 NA
Female 0 79 22 91 1
500 78 15 91 9
1500 80 13 91 21
7500/3500 78 20 91 15
NA, not analysed
Table 5. Hepatic tumour incidence in NMRK-f mice fed carbendazim
Sex Dietary level No.of mice Clear-cell Basophilic Hepatocellular Liver
(ppm) examined foci loci adenomas haemangiomas
Male 0 97 0 0 3 0
50 99 0 0 2 2
150 99 0 1 0 3
300 95 0 0 0 2
1000/5000 99 3 0 1 0
Female 0 98 0 0 0 0
50 98 0 1 00
150 95 0 0 0 0
300 95 0 0 1 2
1000/5000 95 4 0 0 1
clinical manifestations of toxicity and no effects on urinary
parameters. Alkaline phosphatase and alanine aminotransferase
activities varied throughout the study in animals at 2500-10 000 or
5000 ppm, but there was no consistent dose-response relationship.
Organ weights and organ-to-body weight ratios were unchanged, except
for the livers of females at 2500-10 000 or 5000 ppm, but the increase
in the liver-to-body weight ratio was due to a reduction in body
weight. Histopathological examination of the livers showed no
compound-related effects. Males at 2500-10 000 ppm had a marginal
increase in the frequency of diffuse testicular atrophy and
prostatitis. The NOAEL was 500 ppm, equivalent to 15 mg/kg bw per day
(Sherman, 1972).
Groups of 60 male and 60 female Wistar rats were given
carbendazim (purity, 99%) in the diet at 0, 150, 300, or 2000 ppm for
two years. The dose of 2000 ppm was increased to 5000 ppm after one
week and to 10 000 ppm after two weeks for the remainder of the study.
Animals were examined daily for clinical signs of toxicity. Body
weight and food consumption were measured regularly throughout the
study. Haematological measurements (peripheral blood), blood chemistry
(orbital sinus), and urinalysis were conducted periodically. All
animals were subjected to complete gross necropsy, and selected organs
were weighed. Tissues from 20 male and 20 female rats in the control
and high-dose groups were examined microscopically, and all tumours
and gross abnormalities were examined histologically. No differences
in clinical signs of toxicity or food consumption were seen between
test groups and control animals. Body weights were significantly
reduced in low-dose males from week 88 to term and in high-dose
females from week 12 to term. The results of urinalyses were
comparable among the groups. The haemoglobin level was depressed in
high-dose females a t weeks 26, 52, and 103, and the haematocrit was
depressed in high-dose females at week 103. There were no compound-
related effects in males. Serum aspartate aminotransferase activity
was decreased in high-dose males at termination of the study, but
not in females; high-dose females had increased serum alanine
aminotransferase activity and decreased total serum protein. The only
compound-related effects on organ weights were increased relative
liver weights in high-dose females. There were no compound-related
effects on mortality, and survival at termination of the study was
similar in all groups. There were no histological differences between
control and treated groups, except for an increased incidence of
diffuse proliferation of parafollicular cells of the thyroid in the
high-dose females. The number of tumour-bearing animals and the total
number of tumours were comparable among all groups, and no compound-
related oncogenic effects were reported. The NOAEL was 300 ppm,
equivalent to 15 mg/kg bw per day, on the basis of changes in organ
weights and minor biochemical changes (Til et al., 1976a).
(d) Reproductive toxicity
Groups of six male and six female rats were removed from a 90-day
study in which they were fed dietary levels of 0, 100, 500, or
2500 ppm carbendazim. Each female was exposed to three males from the
same dose group to produce the F1a generation; each litter was
reduced to 10 pups on the fourth day after birth. The F0 animals were
mated again after about one week to produce the F1b litter.
Reproduction indices and status at birth and on days 4, 12, and 21
were recorded, as were body weights at weaning. The data were
extremely limited and were available only for groups. At 100 ppm,
neither the F1a nor the F1b animals became pregnant. There were no
apparent effects on reproduction indices or weanling weights, but the
fertility indexes for all groups, which were only 33-67%, prevented
meaningful interpretation of the data (Sherman, 1968).
Groups of three male and 16 female Sprague-Dawley rats were fed
carbendazim in the diet at 0, 100, 500, 5000, or 10 000 ppm
(20 females at the highest dose) and mated in a standard study of two
litters per generation for three generations. The parental animals
were fed the experimental diet at 21 days of age and mated to produce
the F1 litter at 100 days of age; the numbers of matings,
pregnancies, and young were recorded for each litter at birth. The
litters were culled to 10 pups on day 4. The number of live pups was
again recorded on days 4, 12, and 21, as was pup weight at weaning.
The parents were mated again to produce F1b litters, which were
maintained on the respective diets for 110 days and then mated to
produce the F2a and F2blitters; F3a and F3b litters were
produced similarly. Selected tissues and organs from two males and two
females in each of five F3b litters from the controls and from
the groups fed 5000 and 10 000 ppm were examined grossly and
histopathologically. Reproduction indices, including mating,
fecundity, fertility, gestation, viability, and lactation, were
calculated and compared with control values. Carbendazim had no effect
on fertility, gestation, viability, or lactation, but the average
litter weights at weaning were reduced in all generations fed 5000 and
10 000 ppm. Histopathological examination of F3b weanlings did not
reveal any effects that were considered to be compound-related
(Sherman, 1972).
Carbendazim was administered to groups of 10 male and 20 female
Wistar rats at a dietary level of 0, 150, 300, or 2000 ppm for three
generations, and two successive litters were reared from each female
General condition and behaviour were routinely observed, and
individual body weights were recorded throughout the study. The
numbers of pups in each litter were recorded, and each litter was
culled to eight on day 1. The total weight of each litter was measured
on days 1, 10, and 20. The F1a and F2a litters were discarded at
weaning and the F1b and F2b litters were used to produce
succeeding generations. The F3a offspring were used to study
teratogenicity, while the F3b offspring w ere used in a four-week
evaluation of toxicity. Neither health nor body-weight gain was
affected, but all generations of the treated groups weighed
significantly more than controls. The compound had no effect on
fertility or on survival at birth, at day 10, or at day 20. Litter
size was not affected by treatment, except for a marginal decrease in
F2a litters at all doses. There were no differences in the F2a
litters at 300 and 2000 ppm, but litter size was decreased and
mortality at birth increased at 150 ppm. Birth weight during the
lactation period was comparable in all groups. There were no gross
abnormalities related to treatment. Autopsy of rats in the four-week
study showed increased relative liver weights and decreased relative
spleen weights in females fed 2000 ppm; relative ovarian weights were
also significantly decreased in all dose groups. Histopathological
examination of the livers showed no compound-related change;
histopathological results were not presented for other organs. No
maternal or fetal toxicity was evident, and there were no differences
in visceral anomalies in animals at 0 or 2000 ppm (the only groups
examined). Thoracic vertebral bodies were reduced at 2000 ppm, and
there was a significant reduction in cervical vertebral bodies at
2000 ppm. Controls, however, had more significant changes with regard
to absent or delayed ossification of skeletal structures. There were
no apparent adverse effects on reproduction and no teratogenic effects
at dietary levels of carbendazim up to and including 2000 ppm,
equivalent to about 120 mg/kg bw per day (Koeter et al., 1976).
A serial breeding technique was used to evaluate the fertility of
male Sprague-Dawley rats after exposure by gavage to 10 daily doses of
400 mg/kg bw per day carbendazim. Males, 90 days old and proven to be
fertile, were bred with a new female each week, starting on the third
day of treatment and continuing for 32 weeks after the last day of
treatment. Twelve days after each breeding period, the females were
killed, their uteri were examined for resorptions, and the numbers of
dead and viable fetuses were determined. All males were killed 35
weeks after treatment, and testicular tissue was prepared for
histopathological examination by vascular perfusion. The fertility of
treated males (as indicated by the number of pregnant females) was
depressed during the first week after treatment: 10 of the 24 treated
males failed to induce a pregnancy, as compared with no failure in the
control group. By the fifth week after treatment, 16 of the 24
carbendazim-treated males were infertile. Of these, four recovered
fertility after being infertile for 5-11 consecutive breeding periods,
but the other 12 did not recover during the remainder of the 32-week
period after treatment. Histological examinations of testicular
sections of the latter animals 245 days after treatment revealed
severe seminiferous tubular atrophy (> 85% of tubules were atrophic),
often with epithelium containing only Sertoli cells, surrounded by a
thickened basement membrane. The lumina of < 2% of the tubules
contained spermatozoa. The seminiferous tubules of the treated males
that recovered fertility had various contents of atrophic tubules
(13-85%) 245 days after treatment (Carter et al., 1987).
Groups of 8-12 male and 8-12 female rats were given 0, 50, 100,
200, or 400 mg/kg bw per day carbendazim by gavage from weaning
through puberty, gestation, and lactation and were mated at 84 days of
age. The male rats were killed on days 104-106 and the female rats on
day 27 post partum. In a similar study, Syrian hamsters were given 0
or 400 mg/kg bw per day carbendazim. Various landmarks of puberty were
measured in the parental generation. In females, estrous cyclicity,
litter size, the number of implants, organ weights, and histological
status were assessed. In males, organ weights, testicular and
epididymal sperm counts, sperm motility, sperm morphology, testicular
histological status, and endocrine parameters were assessed. In
addition, the growth, viability and reproductive function of the
offspring (F1) were observed during a four-month period of
continuous breeding. Males were killed after five months for
histopathological investigation. Carbendazim did not alter pubertal
development, growth, or viability in the parental generation of either
species. The reproductive potential of rats treated with 200 or
400 mg/kg bw per day was reduced due to effects on sperm production
and fetal viability. These doses markedly altered sperm morphology,
testicular and epididymal weights, sperm numbers, and testicular
histology; fertility, sperm mobility, and hormonal levels were altered
primarily in males with very low sperm counts. A statistically
significant reduction in the caudal epididymal sperm count was noted
at doses of 50 mg/kg bw per day or more. Testicular and epididymal
sperm counts in male hamsters were significantly lower (about 21%) in
treated than in control males. In F1 male hamsters, testis and
seminal vesicle weights and epididymal sperm counts were significantly
reduced by prenatal exposure to carbendazim at 400 mg/kg bw per day.
Parental female rats exposed at this dose had post-implantation
losses, and a few malformed pups were found in litters of animals at
100 or 200 mg/kg per day. Litter size was significantly reduced at 200
and 400 mg/kg bw per day. Overall, carbendazim was less toxic to
hamsters than to rats (Gray et al., 1988, 1990).
Carbendazim was fed to groups of eight female Holtzmann rats by
gavage at doses of 0, 25, 50, 100, 200, 400, or 1000 mg/kg bw per day
during early pregnancy (days 1-8). A range of parameters, including
the number of implantation sites, body-weight gain, uterine weight,
implantation site size, and serum ovarian and pituitary hormones, was
assessed after sacrifice on day 9. At doses up to 400 mg/kg bw per
day, carbendazim had no significant effect on any of the measured
parameters, but a trend towards increased resorptions was evident. The
highest dose reduced maternal body-weight gain, implantation site
size, and serum luteinizing hormone levels and increased serum
estradiol (Cummings et al., 1990).
(e) Developmental toxicity
Rats
Groups of 27-28 pregnant Sprague-Dawley rats were fed carbendazim
(purity, 53%) in their diet at 0, 200, 500, 2500, 5000, 7500, or
10 000 ppm on days 6-15 of gestation. On day 20 of gestation, all of
the animals were sacrificed and the fetuses were delivered by
caesarean section. The numbers and location of live and dead fetuses
and resorption sites, body weights, crown-rump length, sex, and
visible abnormalities were determined. Two-thirds of the fetuses were
prepared for examination for skeletal abnormalities, and the remainder
were examined for visceral and soft-tissue anomalies. There were no
deaths, no adverse effects on body weight, and no clinical signs of
toxicity. Food intake was reduced at the highest dose during the
period the test diet was administered but returned to control levels
from day 16 to 20. The numbers of implantation sites, resorption
sites, and live and dead fetuses were not adversely affected. Although
data on individual litters were not presented, carbendazim did not
appear to be teratogenic when administered to rats at dietary levels
up to and including 10 000 ppm during the critical period of
organogenesis (Sherman, 1970).
Groups of 18-22 pregnant Wistar specific pathogen-free rats were
given carbendazim in the diet at doses of 0, 600, 2000, or 6000 ppm on
days 6-15 of gestation. On day 21 of gestation, all of the rats were
sacrificed and the pups delivered by caesarean section. Dams were
weighed periodically during the test, and food consumption was
measured for specific periods. The number of corpora lutea was
determined, the ovaries were weighed, and the fetuses were weighed and
examined. The numbers of implantation and resorption sites were
recorded, and the empty uterine horns weighed. One-third of the
fetuses were fixed and stained for skeletal examination, and the
remaining two-thirds were examined for soft-tissue anomalies. Although
23 females per group were mated, the pregnancy rate was variable, with
18, 22, 20, and 18 pregnant rats in the groups receiving 0, 600, 2000,
and 6000 ppm, respectively. The mean body-weight gain and food
consumption of the high-dose females were significantly decreased in
comparison with controls. The numbers of live and dead fetuses,
implantation sites, embryonal resorptions, fetal resorptions, and
corpora lutea per dam were comparable in all groups, and ovarian
weights, the weights of the empty uteri, mean fetal weight per litter,
the sex ratio, and pre- and post-implantation losses were not affected
by treatment. No visceral anomalies were reported that were
significantly different from those seen in controls. Misshapen and
fused bones were much more frequent in the high-dose groups then in
any other group, and the incidence of supernumerary ribs was
significantly increased in high-dose females. Ossification was
significantly delayed or absent in pups at the high dose, particularly
in forelimbs, hindlimbs, sternebrae, and skull bones; ossification was
also significantly delayed or absent in cervical vertebral bodies in
all treated groups. It was concluded that carbendazim has no
teratogenic potential when administered in the diet to rats at levels
up to 6000 ppm, although ossification is reduced in a dose-related
manner (Koeter, 1975a).
Groups of 8-10 female Wistar rats were given carbendazim (purity,
98%) by gavage at 0, 20, 40, or 80 mg/kg per day on days 6-15 of
pregnancy. Half of each group of animals was killed on day 21 of
gestation, and half was allowed to deliver normally. All sacrificed
animals were scored for live and dead fetuses and for resorptions;
live fetuses were killed and examined for abnormalities. After normal
deliveries, neonatal deaths and survivors were counted, and survivors
were weighed and examined for gross abnormalities. In rats sacrificed
on day 21, dead and resorbed fetuses accounted for 29% of the
conceptuses among controls, 48% at 20 mg/kg per day, 64%, at 40 mg/kg
per day, and 73% at 80 mg/kg per day. There were no differences among
the various groups with respect to mean weight of live fetuses, and
there were no malformations. The average number of live pups per
litter was close to eight in controls, six at 20 mg/kg per day, and
about five at 40 and 80 mg/kg per day. Mean fetal weight was increased
by about 13% over that of controls at the two highest doses. There
were no stillbirths, neonatal deaths, or gross abnormalities, but
mortality at 21 days post partum was 3.0-3.5 times greater at the
two highest doses than in controls (Janardhan et al., 1984).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethyl-cellulose by gavage to groups of 15-26 Sprague-Dawley
rats on days 6-15 of gestation at daily doses of 10-3000 mg/kg bw per
day. The lowest dose had no effect on dams or their offspring and was
considered to be a no-effect lewd. At 30 mg/kg bw per day,
fetotoxicity was evident: 42% of the fetuses in 19/21 litters had
malformations affecting the head, spine, ribs, and sternum. At
60 mg/kg bw per day, 2/23 animals aborted and 51% of the implantations
in the remaining dams were dead. Malformations were seen in 90% of
fetuses, and all litters were affected. At 100 mg/kg bw per day, 15
pregnant animals produced only four live fetuses in three litters, all
of which were malformed. At 300, 1000, and 3000 mg/kg bw per day,
severe toxicity resulted in early resorptions only. The no-effect
level of 10 mg/kg bw per day was confirmed in a further study
specifically of fetotoxicity and hydrocephaly (Hoffman & Peh,
1987a,b).
Carbendazim was administered in a 0.5% aqueous suspension of
carboxymethylcellulose by gavage to groups of 25 Sprague-Dawley rats
on days 7-16 of gestation at doses of 0, 5, 10, 20, or 90 mg/kg bw per
day. Maternal toxicity was seen only at the highest dose, in the form
of depressed weight gain during treatment and before sacrifice on day
22. The mean liver weights and liver-to-body weight ratios were
increased. A decreased pregnancy rate was observed at the highest
dose. An increase in the incidence of early resorptions per dam,
decreased litter size, and total resorption of three litters occurred
at the highest dose, but only the reduction in females per litter was
significant. Significant reductions in mean fetal weight were observed
at both 20 and 90 mg/kg bw per day, and a significant increase in the
incidence of fetal malformations was seen at the highest dose. The
malformations consisted primarily of hydrocephaly, microphthalmia,
anophthalmia, malformed scapulae, and axial skeletal malformations
(vertebral, rib, and sternebral fusions, exencephaly, hemivertebrae,
and rib hyperplasia). The NOAEL was 20 mg/kg bw per day for the dams
and 10 mg/kg bw per day for the fetuses (Alverez, 1987).
Carbendazim (purity, 95%) was administered by gavage to female
Holtzman rats at doses of 0, 100, 200, 400, or 600 mg/kg bw per day
during days 1-8 of gestation, and the rats were sacrificed on day 11
or 20. No maternal toxicity was seen at any dose. On day 11, the
crown-rump length, head length, number of somites, and number of
embryos per dam were significantly reduced in groups receiving
200 mg/kg bw per day or more. Open posterior neuropores and limb
anomalies were observed more frequently at doses > 200 mg/kg bw per
day. On day 20, increased resorptions, decreased live litter size and
fetal body weight, and delayed ossification were observed at all
doses. Skeletal malformations seen at the high dose were attributed to
treatment. The authors noted that developmental alterations occurred
after termination of treatment, suggesting either that the anomalies
represent delays in development or that the embryonic cells are
vulnerable at earlier stages than was previously thought (Cummings
et al., 1992).
Rabbits
Groups of 3-11 pregnant New Zealand albino rabbits were given
carbendazim in the diet at 0, 600, 2000, or 6000 ppm on days 6-18 of
gestation. On day 29 of gestation, all of the animals were sacrificed
and the fetuses delivered by caesarean section. Does were weighed
periodically, and food consumption was determined for specific
periods. The number of corpora lutea was determined, the ovaries were
weighed, and the fetuses were weighed and examined; the numbers of
implantation sites and resorption sites were recorded, and the empty
uteri weighed. One-half of fetuses were stained and sectioned for
determination of skeletal anomalies, and the other half was examined
for soft-tissue abnormalities. Only the fetuses in the high-dose and
control groups were examined for visceral anomalies. Although 15
females per group were artificially inseminated, the pregnancy rate
was extremely variable, with nine in controls, eight at 600 ppm, three
at 2000 ppm, and 11 at 6000 ppm. The mean body-weight gain was
significantly decreased in the high-dose group, although food
consumption did not vary among groups. The numbers of live and dead
fetuses, implantation sites, embryonal resorptions, fetal resorptions,
and corpora lutea per doe were comparable in all groups. The ovarian
and uterine weights of animals at the high dose were depressed in
comparison with control females; Pre- and post-implantation losses
were not affected by treatment. The incidence of visceral anomalies
appeared to differ between the high-dose and control groups, but too
few litters and fetuses were examined to allow a conclusion. There
were significantly increased numbers of supernumerary ribs (bilateral)
and skull bones in the high-dose group. Ossification was significantly
delayed or absent in fetuses in the high-dose group, most notably in
the forelimb metacarpals and phalanges. There was also incomplete
ossification of the sternebrae and skull bones, which was significant
at 600 and 6000 ppm, and misshapen sternebrae were present at the
highest dose. Although it can be concluded that carbendazim does not
induce teratogenic effects when administered in the diet to rabbits at
levels up to 6000 ppm, there were too few animals, litters, and
fetuses at 2000 ppm to allow a useful evaluation of compound-related
effects (Koeter, 1975b).
Female albino rabbits were given 0, 40, 80, or 160 mg/kg bw per
day carbendazim by gavage on days 6-18 of pregnancy and were
sacrificed on day 31. All of the animals were scored for live and dead
fetuses and for resorptions; live fetuses were killed and examined for
abnormalities. There were no dead or resorbed fetuses in controls, but
15% of all conceptuses were dead at 40 mg/kg bw per day, 21.7% at 80
mg/kg bw per day, and 33.3% at 160 mg/kg bw per day. There were no
differences among the various groups with respect to mean weight of
live fetuses, and there were no malformations (Janardhan et al.,
1984).
Suspensions of carbendazim in aqueous 0.5% carboxymethylcellulose
were administered by gavage on days 7-19 of presumed gestation to
groups of 20 artificially inseminated New Zealand white rabbits at
doses of 0, 10, 20, or 125 mg/kg bw per day. The highest dose
inhabited average maternal weight up to day 16 of gestation. The
implantation rate was decreased at 20 and 125 mg/kg bw per day, and
the incidence of resorptions was increased at 125 mg/kg bw per day,
resulting in decreased live litter size at these doses. At the highest
dose, decreased fetal body weight was seen, but the effect was not
statistically significant. The average percentage of malformed fetuses
per litter was significantly increased at 125 mg/kg bw per day.
Compound-related malformations at the highest dose consisted of
malformed cervical vertebrae and interrelated malformation of the ribs
and proximate thoracic vertebrae. The NOAEL for maternal toxicity was
20 mg/kg bw per day, and the NOAEL for developmental toxicity was
10 mg/kg bw per day (Christian et al., 1985).
Hamsters
Groups of 7-11 pregnant hamsters were treated orally on day 10 of
gestation with 0, 15, 30, 75, or 150 mg/kg bw carbendazim A
significant increase in malformations, including exencephaly, was
observed at 75 and 150 mg/kg bw. These doses were also embryotoxic,
producing 31 and 45% resorptions, respectively (Minta & Biernacki,
1982).
(f) Genotoxicity
Numerous studies have been conducted to assess the mutagenic
potential of carbendazim. Many of the results are conflicting, and
many of the reports do not provide sufficient detail to evaluate the
reasons for the conflicting data. Since before the mid-1980s
industrially produced carbendazim contained phenazine impurities, the
use of carbendazim with different degrees of purity might account for
some of the discrepancies. Table 6 summarizes those reports that
included sufficient experimental detail and data.
Carbendazim is not a heritable gene mutagen; it does not interact
with cellular DNA, induce point mutations, or result in germ-cell
mutations, as seen in both mammalian and nonmammalian systems in
vitro and in vivo and in somatic and germ cells. Positive results
have occasionally been obtained in tests for gene mutation, but they
may have been associated with the presence of phenazines, which are
mutagenic at very low concentrations in Salmonella typhimurium in
Ames' test and in mouse lymphoma LY5178Y tk+/- cells.
Concentrations of > 4 ppm diaminophenazine and 10 ppm aminohydroxy-
phenazine were mutagenic in Ames' test. Process changes by some of the
major manufacturers of carbendazim have removed the phenazine, and
this contaminant is not present when other benzimidazoles, such as
benomyl and thiophanate-methyl, are metabolized to carbendazim.
Carbendazim does cause numerical chromosomal aberrations (aneuploidy
and/or polyploidy) in experimental systems in vitro and in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A 75% wettable powder formulation induced slight, transient
irritation when applied to the in tact and abraded skin of albino
rabbits. A 55% suspension of the 75% wettable powder formulation in
dimethyl phthalate induced mild irritation when applied to the intact,
shaved skin of albino guinea-pigs, while a 5.5% concentration produced
no irritation (Ford, 1981).
The primary dermal irritation potential of Benlate C (50%
wettable powder) was evaluated by applying a 5-g aliquot to the
intact, clipped skin of six New Zealand white rabbits for 4 h. The
test sites were evaluated for erythema, oedema, and other evidence of
dermal effects and were scored according to the Draize scale 4, 24,
48, and 72 h after application. No dermal irritation was seen at any
time during the study (Vick & Brock, 1987).
Table 6. Results of tests for the genotoxicity of carbendazim
End-point Test system Concentration Results Reference
or dose
Tests for gene mutation
Reverse mutation S. typhimurium TA98, < 2500 µg/plate Negative Gericke (1977)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium TA100, < 200 µg/plate Negative Fiscor et al. (1978)
TA1530, TA1535, TA1950
Reverse mutation S. typhimurium TA1530, < 100 µg/spot Weakly positive Fiscor et al. (1978)
TA1950, G46 his-
Reverse mutation S. typhimurium TA100, < 1000 µg/plate Negative Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP2 hcr
Reverse mutation S. typhimurium TA98, < 300 µg/plate Negative Pandita (1988)
TA100
Reverse mutation S. typhimurium TA97, 5000 µg/plate Positive (only with Albertini (1989)
TA98, TA1537, TA1538 activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Positive, TA1537, Donovan (1982)
TA100, TA1535, TA1537 TA98 (with
activation)
Reverse mutation S. typhimurium TA98, < 10 000 µg/plate Negative Russell (1983)
TA100, TA1535, TA1537
Reverse mutation S. typhimurium Positive, only with Arce (19844)
4 ppm DAP or
10 ppm AHP
Reverse mutation S. typhimurium TA98, < 20 000 µg/plate Negative Russell (1977);
TA100, TA1535, TA1537 Donovan (1983)
hprt mutation Chinese hamster ovary cells < 654 µmol/litre Negative Waterer (1980)
tk mutation L5178Y mouse lymphoma < 250 µmol/litre Positive (with Jotz (1980)
cells activation)
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
Tests for chromosomal effects
Sister chromatid exchange Chinese hamster ovary cells < 40 µg/ml Negative Ivett (1984)
Sister chromatid exchange Human lymphocytes < 30 µg/ml Negative Banduhn & Obe (1985)
Sister chromatid exchange Human lymphocytes < 60 µg/ml Marginally positive Pandita (1988)
Chromosomal gain S. cereviriae < 0.1 µg/ml Positive Whittaker et al. (1990)
Aneuploidy S. cereviriae < 5 µg/ml Positive Albertini (1991)
Chromosomal aberrations Human lymphocytes < 10 µmol/litre Not clastogenic but Banduhn & Obe (1985)
induces micronuclei
Chromosomal aberrations Human lymphocytes < 0.5 mg/ml Negative Lamb & Lilly (1980)
DNA damage and repair
Rec assay B. subtilis < 1000 µg/disc Negative Shirasu et al. (1977)
Rec assay S. typhimurium TA1535, < 2000 µg/plate Negative Rashid & Mumma
(1986)
TA1538, E. coli K12,
L. coli WP2
Unscheduled DNA Rat and mouse hepatocytes < 12.5 µg/ml Negative Tong (1981a, b)
synthesis
Unscheduled DNA Rat hepatocytes < 104 µg/ml Negative Litton Bionetics, Inc.
synthesis (1981)
In vivo
Holt-mediated assay, mice S. typhimurium G46 his- Negative Fiscor et al. (1978)
Host-mediated assay, mice S. typhimurium G46 his- 4000 mg/kg bw Negative Shirasu et al. (1977)
Gene mutation Mouse embryos treated in < 300 mg/kg bw Positive at Fahrig & Seller (1979)
utero by dosing mother orally 200 mg/kg bw
Table 6. (Con't)
End-point Test system Concentration Results Reference
or dose
In vivo (con't)
Chromosomal aberration Rat bone marrow 300 mg/kg bw Negative BASF AG (1975)
orally
Chromosomal aberration Chinese hamster bone 1 000 mg/kg bw Negative Seiler (1976)
marrow orally
Chromosomal aberration ICR mouse nucleated 2 × 1 000 mg/kg bw Spindle effects Seiler (1976)
anaphase cells orally
Micronucleus formation Mouse 500 mg/kg bw orally Negative Seiler (1976)
Micronucleus formation Mouse < 6000 mg/kg bw i.p. Positive Pandita (1988)
Chromosomal aberration Chinese hamster bone < 1000 mg/kg bw i.p. Negative Pandita (1988)
marrow
Dominant lethal mutation NMRI mice 5 × 500 mg/kg bw Negative Hoechst AG (1974)
per day i.p.; 5 × 300
mg/kg bw per day orally
Sex-linked recessive lethal Drosophila melanogaster 0.5 mg/ml in dimethyl Negative Lamb & Lilley (1980)
mutation sulfoxide
Germ-line aneuploidy Drosophila melanogaster < 50 000 ppm Negative Osgood et al. (1991)
Technical-grade carbendazim was not irritating to the eyes of
albino rabbits. A 75% wettable powder formulation induced transient
corneal opacity in six of six unwashed and two of three washed eyes.
Microscopic examination confirmed the corneal opacity as mild to
moderate. Conjunctival irritation (redness, swelling, discharge) was
also transient. All eyes were normal after four days. The irritation
response was probably related to the inert ingredients in the wettable
powder formulation (Edwards, 1974b).
A 50% wettable powder formulation produced slight corneal
opacity, mild or moderate conjunctival redness, and slight or mild
conjunctival oedema in six male New Zealand white rabbits. Three of
the rabbits also had moderate iritis, and one had minimal blood-tinged
discharge. Microscopic examination revealed no corneal injury in any
of the treated eyes. The eyes of the other two rabbits were normal by
72 h. It was concluded that this formulation is a moderate ocular
irritant (Vick & Valentine, 1987).
Ten male albino guinea-pigs exposed to technical-grade
carbendazim or a 75% wettable powder formulation showed no dermal
sensitization after intradermal injections or repeated applications to
intact, shaved skin (Ford, 1981).
A 50% carbendazim formulation was applied to the intact, shaved
skin of 10 male and 10 female Dunkin Hartley albino guinea-pigs; five
male and five female guinea-pigs treated with 80% ethanol in water
served as vehicle controls; two male and two female guinea-pigs
treated with solid test material at the challenge phase only served as
negative controls; and two male and two female guinea-pigs treated
with a 0.3% suspension of 1-chloro-2,4-dinitrobenzene in 80% ethanol
in water served as positive controls. No irritation was observed in
the treated or vehicle or negative controls, but 1-chloro-2,4-
dinitrobenzene produced sensitization in all treated animals (Martin
et al., 1987).
(ii) Neurotoxicity
Groups of 10 white Leghorn hens receved carbendazim at doses of
500, 2500, or 5000 mg/kg bw to test for delayed neurotoxic potential.
Controls received the vehicle, corn oil, and the neurotoxin tri-
ortho-tolyl phosphate. The hens were observed daily for mortality
and clinical neurotoxicity for four weeks. Neurotoxic signs consisting
of leg weakness, ataxia and/or 'goose-stepping' gait were observed in
hens treated with tri- ortho-tolyl phosphate. Less severe, reversible
signs, consisting of slight leg weakness and ataxia, were observed in
hens treated with carbendazim at 5000 mg/kg bw, but no neurotoxic
signs were observed in those treated at lower doses. Microscopic
examination indicated no axonal degeneration or demyelination in
carbendazim-treated birds (Goldenthal, 1978).
(iii) Hormonal effects and spermatogenesis
Since spermatogenesis is an androgen-dependent process, the
effects of carbendazim on the endocrine function of the rat testis
were investigated by feeding 0-400 mg/kg bw per day by gavage for 85
days and measuring the serum levels of pituitary luteinizing hormone,
follicle-stimulating hormone, thyroid-stimulating hormone, and
prolactin, and the levels of androgen-binding protein and testosterone
in serum and in testicular interstitial and seminiferous tubule fluid.
The function al capacity of Leydig cells to secrete testosterone
was assessed in vitro after challenge with human chorionic
gonadotrophin. Doses of 50-100 mg/kg bw per day had no effect on
pituitary or testicular hormone concentrations; 200 mg/kg bw per day
increased the testosterone concentration in the seminiferous tubular
fluid, without affecting serum testosterone or androgen-binding
protein concentrations. The dose of 400 mg/kg bw per day resulted in
increased concentrations of both substances in the interstitial and
seminiferous tubular fluid and of serum androgen-binding protein.
These hormonal changes indicate that carbendazim affects the gonads,
resulting in testicular atrophy. Thus, the elevated seminiferous
tubule fluid testosterone concentrations may be a result of two
factors: (i) greater release of testosterone by the Leydig cells into
the interstitial fluid and/or (ii) decreased testosterone outflow from
the testis into the genera] circulation. The increased level of
androgen-binding protein in the interstitial fluid reflects a change
in its relative secretion into the interstitial fluid and seminiferous
tubules (Rehnberg et al., 1989).
Since extragonadal changes may have contributed to the altered
testicular endocrine profile described above, a further study focused
on the presence of concurrent changes in the hypothalamic and
pituitary control of the testis. Rats were given carbendazim at 50,
100, 200, or 400 mg/kg bw per day by gavage for 85 days. Dose-related
increases in serum follicle-stimulating and luteinizing hormone levels
were noted, but the values for prolactin and thyroid-stimulating
hormone remained unchanged. No significant difference in gonadotropin-
releasing hormone concentration in the mediobasal hypothalamus was
seen, although an increased level was found in the anterior
hypothalamus at 50 mg/kg bw per day, followed by a dose-related
decline (Gray et al., 1988). These findings suggest that
carbendazim-induced testicular damage is accompanied by compensatory
changes in hypothalamic and pituitary regulation of the testis
(Goldman et al., 1989).
These findings imply that carbendazim acts directly on the testis
to induce a number of hormonal and pathological changes. Consistent
with this hypothesis are the results of a study by Nakai et al.
(1992), in which the effects of carbendazim on the testes, efferent
ductules, and spermatozoa were determined after a single oral dose to
male Sprague-Dawley rats. In the first experiment, groups of 86-day-
old rats were treated with 0 or 400 mg/kg bw carbendazim and killed 2,
4, or 8 h later on the same day or 1, 4, 8, 16, or 32 days after
treatment. The first effect of carbendazim was noted after 8 h as an
increase in testicular weight; this continued to increase until day 4,
but on days 16 and 32 the testicular weights were substantially lower
than those of controls in five of 16 animals, indicating individual
variation. A decrease in the percentage of sonication-resistant sperm
heads per testis occurred at 8 h in four of eight rats, but the
decrease was significant only after 24 h, when a mean decrease of 19%
was observed; maximal decreases in total sperm head counts per testis
were seen on day 8, after which some recovery was apparent. Epididymal
weights were increased on day 4, but the percentage of morphologically
normal sperm in the cauda epididymus was decreased at that time. By
day 8, many spermatozoa heads were separated from their flagella, and
10% of the heads were misshapen. Numerous sloughed, round germ cells
and cytoplasmic testicular debris were also evident. No effect on the
percentage of motile sperm was seen at 2, 4, or 8 h or one or four
days after treatment. Sperm motility was significantly decreased on
days 8 and 16, but because of clumping and degeneration of the
spermatozoa, the percentage motility could not be determined.
Similarly damaged sperm were seen in three of eight rats on day 32,
but the percentage of motile sperm that could be measured was similar
to that in controls.
In a second experiment (Nakai et al., 1992), groups of rats
aged 97-105 days were given a single oral dose of 0, 50, 100, 200,
400, or 800 mg/kg bw and were killed 2 or 70 days after treatment. On
day 2, a dose-dependent increase in testicular weight was seen at
doses of 100 mg/kg bw or more. This was accompanied by significant
increases in mean seminiferous tubular diameter at 400 and 800 mg/kg
bw. At 50 mg/kg bw, missing immature germ cells were noted, with round
spermatids from stages I and II and elongated spermatids sloughed from
stage VII epithelium. At 100 mg/kg bw, the disappearance of germ cells
was more severe and sloughing of elongated spermatids extended into
stages XII and XIV. At doses greater than 100 mg/kg bw, germ cells
were missing at all stages except stages IX-XI, and at doses of
400-800 mg/kg bw some seminiferous epithelia were damaged so severely
that it was difficult to identify the stage. In addition, major
pathological changes were seen in the efferent ducts of the testis. In
animals treated with 100 mg/kg bw or more, the rete testis was
swollen, with sloughed germ cells, indicating that ductal blockage had
occurred further down the tract; 50% or more of the efferent ductules
were occluded. The occlusions were characterized as compacted luminal
contents, spermatic granulomas, mineralizations, and obliteration of
the original lumen by fibrotic connective tissue. On day 70, mean
testicular weight and mean seminiferous tubule diameter showed a
dose-dependent decrease. Histologically, these decreases were
associated with a dose-dependent increase in seminiferous tubular
atrophy. The atrophied tubules contained primarily Sertoli cells and
occasional spermatogonia and were surrounded by a thickened basement
membrane. No atrophic tubules were seen in the control rats.
Female hamsters were treated by gavage with a single dose of
carbendazim around the fertilization period, at times selected to
coincide with either of the two microtubule-dependent events initiated
by the ovulatory surge of luteinizing hormone: oocyte maturation
(first meiotic division, occurring late in vaginal proestrous) and
fertilization (second meiotic division, occurring early in vaginal
estrous). In the first experiment, groups of 10 hamsters received 0,
250, 500, 750, or 1000 mg/kg bw carbendazim during melosis I, and
pregnancy outcome was assessed on day 15. The percentage of pregnant
hamsters was significantly reduced at 750 and 1000 mg/kg bw, and, in
those animals that became pregnant, the average number of live pups
was reduced at all doses. In the second experiment, groups of 10
female hamsters were bred overnight and were given a single dose of 0
or 1000 mg/kg bw during meiosis II the next morning. The percentage of
pregnant hamsters was unaffected, but the average number of live pups
(measured at 15 days) was reduced. Thus, administration of carbendazim
at the time of microtubule-dependent meiotic events can result in
early pregnancy loss in hamsters (Perreault et al., 1992).
Rats in pseudopregnancy induced by stimulation of the uterine
cervix with a small brass rod in proestrous and estrous were given 0
or 400 mg/kg bw per day carbendazim for eight days. A uterine decidual
cell response was induced on day 4 of pseudopregnancy, and the animals
were killed on day 9. This response, evaluated as a measure of uterine
competency, was significantly lower in the treated rats than in the
controls (Cummings et al., 1990).
Groups of 12 male C57Bl/6 x C3H/He F1 mice were given
carbendazim at 0, 250, 500, or 1000 mg/kg bw per day by gavage for
five consecutive days, and body weight, testicular weights, and sperm
parameters were measured 7, 24, and 39 days after treatment. Body
weight was unaffected; testicular weight was reduced only in the group
at the highest dose after 7 and 24 days but had recovered by 39 days.
Flow cytometry of testicular cells showed that the relative
percentages of certain testicular populations (round, elongating, and
elongated spermatids) in the group at the highest dose were different
from those in controls 7 and 14 days after treatment (Evenson et al,
1987).
Eight-week-old Wistar-derived male rats were fed 0, 10, 70, or
500 ppm carbendazim in the diet for 182 days. Another group received
500 ppm for 91 days and control diet for a further 91 days. Positive
controls were treated with 0.5 mg/kg bw per day colchicine by gavage
for 12 days. Every 13 days, 10 rats were killed and their testicular
organs were examined by histological, morphometric, enzyme
histochemical, and autoradiographic methods. Males were mated twice
with untreated females on day 182 and were killed on day 208.
Fertility parameters were not affected by treatment. Similarly,
treatment did not affect testicular weight, the area of seminiferous
tubules or interstitial tissue, or epididymal structures and enzyme
activities; however, the incidence of 'degenerating' germ cells
undergoing meiosis and spermatogenesis was increased at 70 and
500 ppm, and a significant increase in the preleptonene spermatocyte
nuclear area was seen in all treated groups. The biological
significance of this finding is difficult to assess. The authors
concluded that the effects seen at 70 ppm (equal to 3 mg/kg bw per
day) indicate that carbendazim affects the physiological 'germinal
elimination process'; however, an independent review of the findings
raised methodological concerns which may preclude definitive analysis
of the data (Hilscher et al., 1992; Russell, 1992).
3. Observations in humans
(a) Medical surveillance of workers
Selected blood profiles from 50 workers involved in the
manufacture of benomyl and carbendazim were compared with those of a
control group of 48 workers who were not exposed to these two
fungicides. White blood cell count, red blood cell count, and
haemoglobin and haematocrit values were comparable in the two groups.
No quantitative estimates of exposure were given for the factory
workers, and no female employees were included in the control group
(Everhart, 1979).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers who were exposed to benomyl between 1970 and
1977. The ages of the workers ranged from 19 to 64 years; 79% of the
workers and 78% of their spouses were aged 20-39. The duration of
exposure ranged from less than one month to 95 months, and more than
51% of the workers had potentially been exposed for one to five
months. The birth rates of the spouses of the exposed workers were
compared with those of four populations in the same county, state,
region, and country (USA). The birth rates of the study population
were generally higher than those of the comparison populations,
indicating no reduction in fertility. Spermatogenesis was not examined
(Gooch, 1978).
(b) Studies of volunteers
Urinary excretion of carbendazim was investigated after oral
administration of 2 mg, application of 17 mg onto a skin area of
100 cm2, or intravenous administration of 1 mg. Total excretion of
5-HBC was proportional to the dose applied, and use of an occlusive
dressing did not significantly increase dermal absorption (Meuling
et al., 1993).
Comments
Carbendazim is readily absorbed by animals after oral exposure
and rapidly metabolized. It is eliminated in the faeces and excreted
in the urine. The tissue distribution showed no bioconcentration. In
rats, 2-[(methoxycarbonyl)amino]-1 H-benzimidazol-5-yl hydrogen
sulfate was identified as the main metabolite. The results of
comparative studies in rats and mice indicate that the detoxification
capacity of mouse liver may become saturated at high doses.
Carbendazim is poorly absorbed via the dermal route in rats. Within 24
h, only about 0.2% of a single dose of 0.6 mg was excreted in the
urine and faeces.
Carbendazim has low acute toxicity, with an oral LD50 in the
rat of > 10 000 mg/kg bw. The clinical signs of toxicity after high
single doses were generally nonspecific. Testicular degeneration has
been observed after single oral doses of > 1000 mg/kg bw in rats.
Wettable powder formulations containing carbendazim have been shown to
be irritating to rabbit skin and eyes but did not induce dermal
sensitization in Buehler-type tests. WHO has classified carbendazim as
unlikely to present an acute hazard in normal use.
In 90-day dietary studies in rats, increased liver weight was
seen at 1350 ppm (the highest dose tested in one study), without any
histological change. The overall NOAEL was 500 ppm, equivalent to
50 mg/kg bw per day. In dogs treated by dietary administration for tip
to two years at levels up to and including 4500 ppm, hepatotoxicity
(increased liver weight, hepatic cirrhosis, swollen vacuolated
hepatocytes, and chronic hepatitis) was seen at 500 ppm and above, and
the NOAEL was 100 ppm, equivalent to 2.5 mg/kg bw per day.
Two two-year studies were conducted in rats given dietary levels
of 0, 100, 500, or 2500-10 000 ppm and 0,150, 300, or 2000-10 000 ppm.
Carbendazim did not have carcinogenic potential, but some effects were
seen at the highest doses tested. In one study there was some evidence
of testicular atrophy, and in the other an increased incidence of
diffuse proliferation of parafollicular cells in the thyroid was seen.
In the latter study, evidence of mild hepatotoxicity was found in the
absence of histopathological change. The NOAEL in both studies was
equivalent to 15 mg/kg bw per day.
Three carcinogenicity studies were performed in mice. In Swiss
mice and CD-1 mice treated for 80 weeks and two years, respectively,
at dietary levels up to 5000 or 7500 ppm, carbendazim increased the
incidence of liver tumours. Because of the minimally increased
incidence of proliferative lesions of the liver at 150 or 500 ppm in
the diet (the lowest doses tested), it was not possible to establish
no-effect levels in these studies. In NMRKf mice, which have a low
spontaneous incidence of liver tumours, no carcinogenic effect was
seen at dietary levels up to and including 5000 ppm (the highest level
tested for 96 weeks), but hepatotoxicity was seen at this dose. The
NOAEL was equal to 34 mg/kg bw per day.
In a three-generation study of reproductive toxicity, rats were
fed dietary levels of 0, 150, 300, or 2000 ppm. Carbendazim had no
effect on reproduction or development at dietary levels up to and
including 2000 ppm (equivalent to 120 mg/kg bw per day). Male
fertility was depressed in rats given carbendazim by gavage at
200 mg/kg bw per day for 85 days. A dose of 50 mg/kg bw per day (the
lowest dose tested) caused a decrease in epididymal sperm counts.
After a single oral dose to rats, disruption of spermatogenesis was
seen at 100 mg/kg bw, but there was no effect at 50 mg/kg bw. The
effect was associated with loss of germ cells resulting from
inhibition of Sertoli-cell microtubules.
Carbendazim was tested for developmental toxicity in rats at
doses of 5-3000 mg/kg bw per day on days 6-15 of gestation. It was
fetotoxic and teratogenic at 20 mg/kg bw per day and above, the main
effects being microphthalmia and hydrocephaly. The NOAEL for
fetotoxicity and teratogenicity was 10 mg/kg bw per day; maternal
toxicity was observed at 90 mg/kg bw per day and above, and the NOAEL
for maternal toxicity was 20 mg/kg bw per day. Rabbits were exposed to
carbendazim by gavage at doses of 0, 10, 20, or 125 mg/kg bw per day
on days 7-19 of gestation. Carbendazim was fetotoxic and teratogenic,
the major effects being malformed vertebrate and ribs. The NOAELs in
this species were 10 mg/kg bw per day for teratogenicity and
fetotoxicity and 20 mg/kg bw per day for maternal toxicity.
Carbendazim has been adequately tested for genotoxicity in a
range of assays. The Meeting concluded that it does not directly
damage genetic material but causes numerical chromosomal aberrations
both in vitro and in vivo as a result of its interference with the
mitotic spindle proteins.
In an epidemiological study of workers exposed to carbendazim,
there was no reduction in fertility, as indicated by the birth rates,
among the study population. Spermatogenesis was not; examined.
An ADI of 0-0.03 mg/kg bw was established on the basis of the
no-effect level of 2.5 mg/kg bw per day in the two-year study in dogs
and a safety factor of 100. The resultant ADI, when compared with the
LOAELs in the studies with Swiss and CD-1 mice, provides an adequate
level of safety.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 34 mg/kg bw per day (96-week study of
toxicity and carcinogenicity in NMRKf mice)
< 150-500 ppm, equivalent to < 22-75 mg/kg bw per day
(studies of toxicity and carcinogenicity in Swiss mice (80
weeks) and CD-1 mice (two years)
Rat: 300-500 ppm, equal to 15 mg/kg bw per day (toxicity in a
two-year studies of toxicity and carcinogenicity)
2000 ppm, equivalent to 120 mg/kg bw per day (study of
reproductive toxicity)
10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 10 mg/kg bw per day (study of developmental toxicity)
10 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
20 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
Dog: 100 ppm, equal to 2.5 mg/kg bw per day (two-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to carbendazim
Exposure Relevant route, study type, species Results/remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, toxicity, rat LD50 > 2000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder non-irritating
Ocular, irritation, rabbit 50% wettable powder irritating
Dermal, sensitization, guinea-pig Non-sensitizing in Buehler test
Inhalation, toxicity, rat LC50 > 5.9 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat and rabbit NOAEL = 10 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary, toxicity, dog NOAEL = 2.5 mg/kg bw per day;
hepatotoxicity
References
Arce, G.T. & Sarrif, A.M. (1984) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report from DuPont Haskell
Laboratory for Toxicology, Newark, Delaware, USA. Submitted to
WHO by AgrEvo, Frankfurt, Germany.
Albertini, S. (1989) Influence of different factors on the induction
of chromosome malsegregation in Saccharomyces cerevisiae D61.M
by Bavistan and assessment of its genotoxic property in the Ames
test and in Saccharomyces cerevisiae DT. Mutat. Res., 216,
327-340.
Albertini, S. (1991) Re-evaluation of file 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae
aneuploidy test systems by the Gene-Tox Program using strain
D61 M of Saccharomyces cerevisiae. Mutat. Res., 260, 165-180.
Alverez, L. (1987) Teratogenicity study of INE-965 (carbendazim) in
rats. Unpublished report from E.I. DuPont de Nemours and Co.,
Haskell Laboratory, Newark, Delaware, USA.
Banduhn, N. & Obe, G. (1985) Mutagenicity of methyl 2-benzimidazole
carbamate, diethylstilbestrol and estradiol: Structural
chromosomal aberrations, sister-chromatid exchanges, C-mitoses,
polyploidies and micronuclei. Mutat. Res., 156, 199-218.
BASF AG (1975) Dominant lethal study in mice. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Beems, R.B., Til, H.P. & van der Heijden, C.A. (1976) Carcinogenicity
study with carbendazim (99% MBC) in mice. Unpublished report from
Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Carter, S.D, Hess, R.A. & Laskey, J.W. (1987) The fungicide methyl
2-benzimidazole carbamate causes infertility in male Sprague-
Dawley rats. Biol. Reprod., 37, 709-718.
Christ, O. & Kellner, H.M. (1973) Animal tests with carbendazim.
Unpublished report from Hoechst AG, Frankfurt, Germany.
Christian, N.S., Hoberman, A.M. & Feussner, E.L. (1985) Developmental
toxicity study of carbendazim administered via gavage to New
Zealand white rabbits. Unpublished report from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by E.I. DuPont de Nemours and Co., Inc.
Cummings, A.M., Harris, S.T. & Rehnberg, G.L. (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat.
Fundam. Appl. Toxicol., 15, 528-535.
Cummings, A.M., Ebron-McCoy, M.T., Rogers, J.M., Barbee, B.D. &
Harris, S.T. (1992) Developmental effects of methyl benzimid-
azolccarbamate following exposure during early pregnancy.
Fundam. Appl. Toxicol., 18, 288-293.
Dashiell, O.L. (1975) Oral LD50 test in guinea pigs (Carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory Newark, Delaware, USA.
Donaubauer, H.H., Schuetz, E., Weigand, W. & Kramer, M. (1982)
Repeated dose (24 month) feeding study, for determination of the
carcinogenic effect of HOE 17411 OFAT204 (carbendazim) in mice.
Unpublished report from Hoechst AG, Pharmaceuticals Research,
Toxicology Section, Frankfurt, Germany.
Donovan, S.D. (1982). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Donovan, S.D. (1983). Mutagenicity evaluation in Salmonella
typhimiurium. Unpublished report from E.I. DuPont de Nemours
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Dorn, E. & Keller, H.M. (1980) Carbendazim (60% wettable powder)
absorption via the skin in rats. Unpublished report from Hoechst
AG, Frankfurt, Germany.
Dorn E., Schmidt E., Kellner H.M. & Leist K.H. (1983) HOE 017411-14-C
(carbendazim-14C) metabolic fate in rats and mice, a
comparison. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Edwards, D.F. (1974a) Skin absorption LD50 (carbendazim).
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Edwards, D.F. (1974b) Federal Hazardous Substances Act -- Eye
irritation test in rabbits (carbendazim). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Evenson, D.P., Janca, F.C. & Jost, L.K. (1987) Effects of the
fungicide methyl-benzimidazol-2-yl carbamate (MBC) on mouse germ
cells as determined by flow cytometry. J. Toxicol. Environ.
Health, 20, 387-399.
Everhart, L.P. (1979) Benlate dust exposure survey. Unpublished report
from E.I. DuPont de Nemours and Co., Inc., Wilmington, Delaware,
USA.
Fahrig, R & Seiler, J.P. (1979) Dose and effect of methyl-2-
benzimidazolyl-carbamate in the 'mammalian spot test', an
in vivo method for the detection of genetic alterations in
somatic cells of mice. Chem.-Biol. Interactions, 26, 115-120.
Falke, H.E., Beems, R.B. & Spit, B. (1982a) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 60-day enzyme induction study
in mice. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Falke, H.E., Beems, R.B. & Spit, B. (1982b) Carbendazim -- technical
grade (Code: HOE 17411 OF AT 204) 59-day enzyme induction study
in rats. Unpublished report from TNO, Bilthoven, Netherlands.
Submitted to WHO by AgrEvo, Frankfurt, Germany.
Fiscor, G, Bordas, S. & Steward, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Ford, L.S. (1981) Primary skin irritation and sensitization on guinea
pigs. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Ford, L.S. (1982) Acute skin absorption LD50 test on rabbits
(carbendazim 75% wettable powder). Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Gericke, D. (1977) Test for mutagenicity in bacteria strains in the
absence and presence of a liver preparation. Unpublished report
from Laboratory for Cancer Research, Hoechst AG, Frankfurt,
Germany.
Goldenthal, E.I. (1978) Neurotoxicity in hens. Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by E.I. DuPont de Nemours and
Co., Inc.
Goldman, J.M., Rehnberg, G.L, Cooper, R.L., Gray, L.E. Jr, Vein, J.F.
& McElroy, W.K. (1989) Effects of the benomyl metabolite,
carbendazim, on the hypothalamic-pituitary reproductive axis in
the male rat. Toxicology, 57, 173-182.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Goodman, N.C. & Sherman, H. (1975) Oral LD50 tests (fasted male and
female rats). Unpublished report from E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Grandizio, A.M. & Sarver, J.W. (1987) Acute oral toxicity study with
INE-965-261 (Benlate C) in male and female rats. Unpublished
report from E.I. DuPont de Nemours and Co., Inc., Haskell
Laboratory, Newark, Delaware, USA.
Gray L.E., Ostby, J., Sigmon, R., Ferrell, J., Rehnberg, G., Linder,
R., Cooper, R., Goldman, J. & Laskey, J. (1988) The development
of a protocol to assess reproductive effects of toxicants in the
rat. Reprod. Toxicol., 2, 281-287.
Gray, L.E., Ostby, J., Linder, R., Goldman, J., Rehnberg, G. & Cooper,
R. (1990) Carbendazim induced alterations of reproductive
development and function in the rat and hamster. Fundam Appl.
Toxicol., 15, 281-297.
Guengerich F.P. (1981) Enzyme induction with Du Pont compounds
H11,201-02 and H10,962-02. Unpublished report from Vanderbilt
University School of Medicine, Nashville, Tennessee, USA.
Submitted to WHO by E.I. Du Pont de Nemours and Co., Inc.,
Newark, Delaware, USA.
Hilscher, W., Ohnesorge, H. & Muller, H. (1992) The effects of
carbendazim on spermatogenesis. Unpublished report from Institut
für Toxicologie, Heinrich-Heine-Universitat, Dusseldorf, Germany.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hinckle, L. (1981) Oral LD50 test in rats -- EPA proposed
guidelines. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Hoechst AG (1974) Mouse dominant lethal study on MBC. Unpublished
report submitted to WHO by Hoechst AG, Frankfurt, Germany.
Hoffman, H.T. & Kirsch, P. (1987) Report on the subchronic toxicity of
methyl benzimidazole-2-carbamate in beagle dogs on oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Hoffman, H.T. & Peh, J. (1987a) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hoffman, H.T. & Peh, J. (1987b) Report on the study to determine the
prenatal toxicity of methyl benzimidazole-2-carbamate (MBC) in
rats. Unpublished report from BASF AG, Ludwigshafen, Germany.
Hunter, B., Batham, P. & Newman, A.J. (1973a) Carbendazim oral
toxicity to rats in oral administration for 2 weeks. Unpublished
report from Huntingdon Research Centre, United Kingdom. Submitted
to WHO by Hoechst AG, Frankfurt, Germany.
Hunter, B., Benson, H.G., Street, A.E., Heywood, R. & Newman, A.J.
(1973b) Carbendazim toxicity to rats during dietary
administration for 13 weeks followed by a recovery period of 6
weeks. Unpublished report from Huntingdon Research Centre, UNited
Kingdom. Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Ivett, J.L. (1984) Mutagenicity evaluation of H#15,152 in an
in vitro sister chromatid exchange assay in Chinese hamster
ovary (CHO) cells. Unpublished report from Litton Bionetics,
Inc., Kensington, Maryland, USA. Submitted to WHO by E.I. DuPont
de Nemours and Co., Inc., Newark, Delaware, USA.
Janardhan, A., Sattur, P.B. & Sisodia, P. (1984) Teratology of methyl
benzimadazole carbamate in rats and rabbits. Bull. Environ.
Contam. Toxicol 33, 257-263.
Janardhan, A., Rao, A.B. & Sisodia, P. (1987) Sub-chronic toxicity of
methyl benzimidazole carbamate in rats. Bull. Environ. Contam.
Toxicol., 38, 890-898.
Jotz, M.M. (1980) An evaluation of mutagenic potential of benomyl
employing the L5178Y TK+/-mouse lymphoma assay. Unpublished
report from SRI International, Menlo Park, California, USA,
prepared for the US Environmental Protection Agency.
Kellner, H.M. (1983) Carbendazim-(2-14C) whole body autoradiography
studies of the distribution in rats and mice after oral and.
intravenous administration. Unpublished report from Hoechst AG,
Frankfurt, Germany.
Kellner, H.M. & Eckert, H. (1983) Carbendazim-(2-14C) blood levels,
distribution and excretion in rats and mice after oral
administration. Unpublished report from Hoechst AG, Frankfurt,
Germany.
Koeter, H.B.W.M. (1975a) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the rat Unpublished report from Central Institute
for Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, and BASF AG,
Ludwigshafen, Germany.
Koeter, H.B.W.M. (1975b) Effect of HOE 17411F (=BAS 3460F) on
pregnancy of the New Zealand white rabbit. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by Hoechst AG, Frankfurt,
and BASF AG, Ludwigshafen, Germany.
Kramer, M. & Weigand, W. (1971) (HOE 17411 OF) Toxicological
examination. Unpublished report from Hoechst AG, Pharmaceuticals
Research, Toxicology Section, Frankfurt, Germany.
Krechniak, J. & Klosowska, B. (1986) The fate of 14C-carbendazim in
the rat. Xenobiotica, 16, 809-815.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Litton Bionetics, Inc. (1981) Evaluation of HOE 17411 OF AT204 in the
primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Inc., Kensington,
Maryland, USA. Submitted to WHO by Hoechst AG, Frankfurt,
Germany.
Martin, D.A., Henry, J.E. & Brock, W.J. (1987) Closed-patch repeated
insult dermal sensitization study (Buehler method) with Benlate C
fungicide in guinea-pigs. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Meuling, W.J.A., Opdam, J.J.G. & DeKort, W.L.A.M. (1993) Dose-
excretion study with the fungicide carbendazim in volunteers.
Unpublished report from Central Institute for Nutrition and Food
Research (TNO) The Hague, Netherlands. Submitted to WHO by
Hoechst AG, Frankfurt, Germany.
Minta, M. & Biernacki, B. (1982) Embryotoxicity of carbendazim in
rats, rabbits and hamsters. Bull. Vet. Inst. Pulawy, 2, 42-52.
Mohr, U. (1977) Review of liver sections from mice and rats fed with
carbendazim. Unpublished report from Department of Experimental
Pathology, Medical School, Hanover, Germany. Submitted to WHO by
Hoechst AG, Frankfurt, and BASF AG, Ludwigshafen, Germany.
Monson K.D. (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.
Wilmington, Delaware, USA.
Nakai, M., Hess, R.A., Moore, B.J., Guttroff, R.F., Strader, L.F. &
Linder, R.E. (1992) Acute and long-term effects of a single dose
of the fungicide carbendazim (methyl 2-benzimidazole carbamate)
on the male reproductive system in the rat. J. Androl., 13,
507-518.
Nash, S.D. & Ferenz, R. (1982) Inhalation median lethal concentration
(LC50) in rats -- EPA protocol (carbendazim 75% wettable
powder). Unpublished report from E.I. DuPont de Nemours and Co.,
Inc., Haskell Laboratory, Newark, Delaware, USA.
Osgood, C., Zimmering, S. & Mason, J.M. (1991) Aneuploidy in
Drosophila. II: Further validation of the FIX and ZESTE genetic
test systems employing female Drosophila melanogaster. Mutat.
Res., 259, 147-163.
Pandita, T.K. (1988) Assessment of the mutagenic potential of a
fungicide Bavistin using multiple assays. Mutat. Res., 204,
627-643.
Perreault, S.D., Jeffay, S., Poss, P. & Laskey, J.W. (1992) Use of the
fungicide carbendazim as a model compound to determine the impact
of acute chemical exposure during oocyte maturation and
fertilization on pregnancy outcome in the hamster. Toxicol.
Appl. Pharmacol., 114, 225-231.
Rashid, M.A. & Mumma, R.O. (1986) Screening pesticides for their
availability to damage bacterial DNA. J. Environ. Health Sci.,
B21, 319-334
Rehnberg, G.L., Cooper, R.L., Goldman, J.M., Gray, L.E, Hein, J.F. &
McElroy, W.K. (1989) Serum and testicular testosterone and
androgen binding protein profiles following subchronic treatment
with carbendazim. Toxicol. Appl. Pharmacol., 101, 55-61.
Reuzel, P.G.J., Hendriksen, C.F.M & Til, H.P (1976) Long-term
(two-year) toxicity study with carbendazim in beagle dogs.
Unpublished report from the Central Institute for Nutrition and
Food Research for BASF. Submitted to WHO by E.I. DuPont de
Nemours and Co., Wilmington, Delaware, USA.
Russell, J.F. (1977) Mutagenicity evaluation of 2-benzimidazole
carbamic acid, 5-hydroxymethyl in Salmonella typhimurium.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1983) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from E.I. DuPont de Nemours and
Co., Inc.. Haskell Laboratory, Newark, Delaware, USA.
Russell, L.D.. (1992). Review of Prof. Hilscher's report «Effects of
carbendazim on spermatogenesis». Unpublished report from
University of Illinois, USA. Submitted to WHO by Hoechst AG,
Germany.
Sarver, J.W. (1975) Acute inhalation toxicity -- one hour head only,
(carbendazim). Unpublished report from E.I. DuPont de Nemours,
and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Scholz, J. & Weigand, W. (1972) Carbendazim (HOE 17411 OF, batch
496/I). Toxicological examination. Unpublished report from
Hoechst AG, Pharmaceuticals Research, Toxicology Section,
Frankfurt, Germany.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives. VI. Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and
the Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1965) Acute oral test. Unpublished report from E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. (1968) Ninety-day feeding study in rats using a wettable
powder formulation (70% MBC). Unpublished report from E.I. DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1970) Three-month feeding study in dogs using a wettable
powder formulation (50% MBC). Unpublished report from E.I DuPont
de Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware,
USA.
Sherman, H. (1972) Long-term feeding studies in rats and dogs with
2-benzimadazole carbamic acid, methyl ester (INE-965) (50% and
70% MBC wettable powder formulations). Unpublished report from
E.I. DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Sherman, H. and Krauss, W.C. (1966). Acute oral test (carbendazim).
Unpublished report from E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware.
Shirasu, Y., Morlya, M. & Kato, K. (1977) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Stadler, J.C. (1986) One-year feeding study in dogs with carbendazim.
Unpublished report from E.I. DuPont de Nemours and Co., Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Til, H.P., van den Muelen, H.C, Feron, V.J., Seinen, W. & de Groot,
A.P. (1972) Sub-chronic (90 day) toxicity study with W17411 in
beagle dogs. Unpublished report from Central Institute for
Nutrition and Food Research (TNO), The Hague, Netherlands.
Submitted to WHO by Hoechst AG, Frankfurt, Germany.
Til, H.P., Koellen, C. & van der Heijden, C.A. (1976a) Combined
chronic toxicity and carcinogenicity study with carbendazim in
rats. Unpublished report from Central Institute for Nutrition and
Food Research (TNO), The Hague, Netherlands. Submitted to WHO by
BASF AG, Ludwigshafen, and Hoechst AG, Frankfurt, Germany.
Til, H.P., Koeter, H.B.W.M. & van der Heijden, C.A. (1976b)
Multigeneration study with carbendazim in rats. Unpublished
report from Central Institute for Nutrition and Food Research
(TNO), The Hague, Netherlands. Submitted to WHO by Hoechst AG,
Frankfurt, Germany.
Til, H.P., Beems, R.B. & de Groot A.P. (1981) Determination of the
acute oral toxicity of carbendazim in mice. Unpublished report
from Central Institute for Nutrition and Food Research (TNO), The
Hague, Netherlands. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Tong, C. (1981a) Hepatocyte primary culture/DNA repair assay on
compound 11, 201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Tong, C. (1981b) Hepatocyte primary culture/DNA repair assay on
compound 11,201-01 (MBC) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA. Submitted to WHO by E.I. DuPont de Nemours and Co.,
Inc.
Vick, D.A. & Brock, W.J. (1987) Acute dermal toxicity study of Benlate
C fungicide in rabbits. Unpublished report from E.I. DuPont de
Nemours and Co., Inc., Haskell Laboratory, Newark, Delaware, USA.
Vick, D.A. & Valentine, R. (1987) Primary eye irritation study with
Benlate C fungicide in rabbits. Unpublished report from E.I.
DuPont de Nemours and Co., Inc., Haskell Laboratory, Newark,
Delaware, USA.
Waterer, J.C. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from E.I. DuPont de Nemours and
Co., Inc., Haskell Laboratory Newark, Delaware, USA.
Whittaker, S.G., Moser, S.F., Maloney, D.H., Plegorsch, W.W., Resnick,
M.A. & Fogel, S. (1990) The detection of mitotic and meiotic
chromosome gain in the yeast Saccharomyces cerevisiae: Effects
of methyl benzimidazol-2-yl carbamate, methyl methanesulfonate,
ethyl methanesulfonate, dimethyl sulfoxide, propionitrile and
cyclophosphamide monohydrate. Mutat. Res., 242, 231-258.
Wood, C.K. (1982) Long-term feeding study with 2-benzimidazole-
carbamate, methyl ester (> 99% MBC, INE-965) in mice.
Unpublished report from E.I. DuPont de Nemours and Co, Inc.,
Haskell Laboratory, Newark, Delaware, USA.
Zeller, H. & Kirsch, P. (1971) Acute oral toxicity of methyl-2-
benzimidazole carbamate to the rabbit. Unpublished report from
Hoechst AG, Frankfurt, Germany.
