
DICLORAN JMPR 1974
IDENTITY
Chemical name
2,6-dichloro-4-nitroaniline
Synonyms
Dicloran (B.S.I.), DCNA, Botran(R), Allisan(R), Ditranil(R)
Structural formula
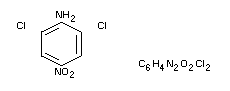 Other information on identity and properties
Molecular weight: 207
State: Yellow, crystalline solid with practically
no odour
Melting point: 192-194°C
Vapour pressure: 1.2 x 10-6 mm Hg (20°C)
Solubility: Water7 mg/l
Cyclohexane 0.006 g/100 ml
Petroleum ether 0.02 g/100 ml
Carbon tetrachloride 0.06g/100 ml
Ethanol 0.29 g/100 ml
Benzene 0.46 g/100 ml
Glacial acetic acid 8.80 g/100 ml
Chloroform 1.2 g/100 ml
Acetone 3.4 g/100 ml
Stability: Stable to hydrolysis and relatively stable
to oxidation. Readily reduced to
phenylenediamine by zinc and acid. Stable
to light and heat.
Composition and
purity: The technical product contains
2,6-dichloro-4-nitroaniline not less than
96% (on dry weight basis);
2,4-dichloro-6-nitroaniline not more than
2%; 2-chloro-4-nitroaniline not more than
l%; Chloranil not more than 1%;
P-nitroaniline not more than 0.1%; Loss on
drying not more than 1% (at 60°C, <5 mm
Hg); Sulphated ash not more than 0.25%;
Sodium chlorate not more than 100 ppm.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
2,6-Dichloro-4-nitroaniline-14C administered to three male human
subjects at a level of 50 mg was found to be rapidly absorbed and
excreted. Excretion was somewhat slower than measured in the rat with
the major quantity of material being excreted within 1.5 days.
Preliminary studies suggest that 2,6-dichloro-4-nitroaniline
metabolites are similar to those obtained from the rat. Approximately
85% of the total urinary excretion from the rat was found to be of
2,6-dichloro-4 hydroxy aniline sulfate.
2,6-Dichloro-4-nitroaniline-14C administered to male rats at a
dosage of either 1.7 mg/kg or 8 mg/kg orally was rapidly excreted
from the body. Urinary excretion accounted for approximately 90% of
the recovered material with the remainder being located in the
faeces. The majority of material (about 90%) was recovered within 48
hours and over half was recovered within 8 hours after treatment.
2,6-Dichloro-4-nitroaniline was not observed in any body tissues with
the exception of small quantities detected in the G.I. tract, urinary
tract, and liver (Eberts, 1965).
2,6-Dichloro-4-nitroaniline administered to rats (ip or orally,
20 mg/kg) was metabolized to the dichloroamimophenol and the
dichlorophenylenediamine derivatives. Following both routes of
administration the majority of material was recovered from the urine
within 24 hours and total recovery was noted within 72 hours. Very
small quantities were obtained in the faeces. In vitro studies using
mouse liver microsomes showed limited conversion of
2,6-dichloro-4-nitroaniline to the same two metabolites (Máté et al.,
1967).
Biotransformations in plants and micro-organisms are discussed
under "Fate of residues".
Effects on enzymes and other biochemical parameters
Following high level subacute oral administration of
2,6-dichloro-4-nitroaniline to rats, an increase of hepatic
demethylase and desulfurase activity was noted. Liver mitochondrial
O2 consumption was also increased in rats. The liver enzymes were
not stimulated in monkey following similar treatment (Serrone, 1967).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A carcinogenic screening using high levels of
2,6-dichloro-4-nitroaniline administered to susceptible mice was
negative. Groups of mice (18 males and 18 females of each of two
hybrid strains) were administered 2,6-dichloro-4-nitroaniline at 215
mg/kg/day for three weeks from day seven after birth. Thereafter for
18 months the mice were fed 603 ppm in the diet, sacrificed and
examined for tumours. 2,6-Dichloro-4-nitroaniline did not cause a
significant increase in tumours (Innes et al., 1969).
Effects on blood and blood forming tissues
Cat
In studies to substantiate the difference between effects of
4-nitroanaline and 2,6-dichloro-4-nitroaniline, a study on the effect
of these materials on methaemoglobinemia in the cat was performed
(Gurd, 1974). After a single oral dose of 2,6-dichloro-4-nitroaniline
(500 mg/kg), no methaemoglobin was noted at any time between 1 and 48
hours after dosing. Administration of 4-nitroaniline at a single dose
of 100 mg/kg resulted in methaemoglobin, observed over the same time
course. In addition, the cats subjected to this experiment were noted
to be cyanotic and have extensive muscle weakness following
4-nitroaniline.
Ocular toxicity
Dog and miniature swine
Dogs have been shown to develop lesions in the cornea and lens
following prolonged oral administration of
2,6-dichloro-4-nitroaniline. It has been suggested that a
photochemical product reaction may be responsible for the lesion as
it occurred only when dogs were exposed to sun light.
Dogs and miniature swine were fed 2,6-dichloro-4-nitroaniline in
the diet at levels of 0, 0.75, 6, 24, 48 and 192 mg/kg/day for periods
of time varying from 50 to 306 days. Corneal opacity appeared in dogs
within 53 to 104 days after administration of 24 or 48 mg/kg, and when
exposed to sunlight. Dogs unexposed to sunlight and those with one eye
sutured closed failed to develop lesions in the unexposed eyes. Dogs
administered 192 mg/kg refused to eat after 38 days and were
administered 2,6-dichloro-4-nitroaniline by capsule. All of these
animals died 49 - 53 days after the study began. Eye lesions were not
detected in this high level group. Several dogs showing eye damage
were maintained for four months after 2,6-dichloro-4-nitroaniline
administration had ceased. Pathological changes seen in the cornea and
lens were not reversible. Administration of
2,6-dichloro-4-nitroaniline at all levels did not appear to affect
miniature swine. Sporadic instances of the presence of Heinz bodies
in blood were observed in both swine and dogs. Administration of
2,6-dichloro-4-nitroaniline as dust or 5% solution directly to the
eyes for three months had no effect on corneal opacity or irritation
of the conjunctiva (Earl et al., 1971; Bernstein et al. 1970; Curtis
et al. 1968).
Special studies on reproduction
Rat
In a standard three generation, two litters per generation,
reproduction study, 2,6-dichloro-4-nitroaniline was administered to
rats (20 males and 20 females per group) at levels of 0 and 100 ppm in
the diet. On the basis of the reproduction parameters examined,
including number of litters, stillbirths, live births, birthweight,
lactation indices, etc. no evidence of an effect of dicloran on
reproduction was indicated (Lobdell and Johnston, 1965).
Male rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 1000, and 2000 ppm for 90 days. The males were mated with
untreated females. There were no differences observed in the number of
litters or in the number of animals born or weaned. Feeding
2,6-dichloro-4-nitroaniline in the diet to male rats resulted in an
increased liver weight at 1000 ppm. An increased kidney weight was
seen at 1000 ppm (EPA, 1974).
Female rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 500, and 1000 ppm for 188 days prior to mating. The rats
were continued on the diet through gestation and lactation. From the
small number of animals in this experiment (10 females/group), it is
difficult to make definitive conclusions concerning the effect on
reproduction of 2,6-dichloro-4-nitroaniline administered to females.
The data suggested a reduced number of pups when the females were fed
a level of 1000 ppm in the diet. There was no apparent effect on
survival of pups, although the mean body weight of pups at 1000 ppm
was slightly reduced. It might be considered that 1000 ppm in the diet
of females for six months might have a slight effect on reproduction.
No effects were seen at 500 ppm (EPA, 1974).
Rabbit
Groups of pregnant New Zealand white rabbits (10, 12, and 14 does
respectively) were fed 2,6-dichloro-4-nitroaniline in the diet at 0,
100, and 1000 ppm from day 8 until day 16 of gestation. In no case was
there evidence of an adverse effect of 2,6-dichloro-4-nitroaniline on
reproduction, affecting either the parents or the offspring (Anonymous
1974).
Sensitization
Rat, guinea pig, rabbit
An examination was made of the potential skin sensitization
properties of 2,6-dichloro-4-nitroaniline in guinea pigs. Ten
subcutaneous injections were administered to male guinea pigs (total
dose 0.95 mg). Two weeks after the last injection a re-injection of
0.05 mg was made. Twenty-four hour readings showed no apparent
sensitization (Johnston and Sweickert, 1963)
Rats, guinea pigs and rabbits were administered
2,6-dichloro-4-nitroaniline via inhalation exposure to an 8% dust
for seven hours. It was estimated that the exposure averaged 0.4
mg/l. No deaths were observed although reddening of the lungs and
pale kidneys were seen in rats and guinea pigs (Horn, 1961).
TABLE 1 Acute toxicity of 2,6-dichloro-4-nitroaniline
LD50
Species Route (mg/kg) References
Rat oral 4000-10 000 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
Ip 1460-5471 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
SC >5000 Lessel, 1974a
Guinea Pig oral 1450 Lessel, 1974a
Mouse oral 1500-2500 Lessel, 1974a
Feenstra, 1961
Ip 2500-8000 Lessel, 1974a
Feenstra, 1961
SC >6000 Lessel, 1974a
dermal >5000 Lessel, 1974a
Cat oral >500 Lessel, 1974a
oral 200 x 7 daily doses Lessel, 1974a
Signs of poisoning in the mouse included defaecation and
urination, depression and lethargy leading to sleep. In rats the same
signs were noted including nasal haemorrhage and paralysis. Death
occurred up to four days after administration of 2,6-dichloro-4-
nitroaniline (Feenstra, 1961). Cyanosis, muscle weakness, and other
typical signs of aniline poisoning were not observed.
Two formulations (8% dust and 50% W.P.) were applied to intact
and abraded skin of rabbits daily for five days. No irritation of skin
was observed (Johnston and Schwikert 1961a). When these materials were
instilled into the conjunctival sac of rabbits, no ocular irritation
was noted (Johnston and Schwikert, 1961b).
Short Term Studies
Rat
Groups of rats (either 10 males and females or 15 males and
females per group) were treated with 2,6-dichloro-4-nitroaniline to
examine potential haematopoietic effects. One series of animals was
administered 2,6-dichloro-4-nitroaniline at levels of 0, 5, 20 and 100
mg/kg/day by gavage for four months. Another group was administered
2,6-dichloro-4-nitroaniline in the diet at levels of 0 and 20 ppm for
four months. Haematological examinations (RBC, total and differential
leucocyte, platelet, haematocrit and haemoglobin concentration), blood
sugar, as well as growth and food consumption data indicated no
significant effects attributable to 2,6-dichloro-4-nitroaniline at any
of the dose levels or treatments (Evans et al., 1963).
Groups of rats were fed 2,6-dichloro-4-nitroaniline (either
technical or recrystallized material) in the diet for six months.
Groups of 15 males and 15 females were fed 30 and 300 ppm, groups of
10 males and 10 females were fed 3000 ppm of either the technical or
pure material; and groups of 25 males and 25 females were fed a
control diet. At 3000 ppm in the diet, growth of both males and
females fed technical 2,6-dichloro-4-nitroaniline was impaired with
only the males fed the purified material showing a slight reduction in
growth. In both high level groups livers were enlarged at 3000 ppm.
There was no effect on haematology or on tissues and organs. There was
no sign of damage when the tissues were examined microscopically. No
effects were noted at 300 ppm over the six month period (Lessel,
1974d).
Groups of rats (5 males and 5 females per group) were
administered 2,6-dichloro-4-nitroaniline orally at doses of 0, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth of males was
reduced at 350 mg/kg. At both doses, liver enlargement and
histological changes were noted. There was no apparent effect on blood
parameters or on the kidneys when examined at the termination of the
experiment (Lessel, 1974c).
Groups of rats (10 males and 10 females per group, 20 of each sex
were used in the controls) were administered
2,6-dichloro-4-nitroaniline orally at dose levels of 0, 35, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth depression was
observed in both males and females at the highest dose level. Growth
depression was also noted at the intermediate level in males only.
Liver enlargement was again observed at 140 mg/kg. No effects were
noted at 35 mg/kg. Microscopic examination revealed the presence of
enlarged liver cells with increased vacuolization especially at the
outer lobes. Some animals were maintained for two weeks after the
conclusion of the treatment. After this two week period, liver size
was normal in all but the highest male dosage level. Liver hypertrophy
caused by repeated short term dosing is apparently reversible within a
two week period on cessation of treatment. In this study daily acute
administration 35 mg/kg was observed to have no effect on the rat
(Lessel, 1974c).
Because of the known effect of 4-nitroaniline in inducing
specific blood dyscrasias, subacute feeding experiments were performed
to compare 2,6-dichloro-4-nitroaniline with this material. Groups of
weanling rats (5 males and 5 females/group) were administered
2,6-dichloro-4-nitroaniline by gavage at 0 and 400 mg/kg, 5 days/week
for four weeks. 4-Nitro-aniline was administered at 200 and 400 mg/kg
to two other comparable groups over the same interval. In a second
experiment, groups of male weanling rats (6 rats per group) were
administered 2,6-dichloro-4-nitroaniline by oral gavage at 0 and 400
mg/kg and 4-nitroaniline at 200 and 400 mg/kg, twice daily, 5
days/week, for two weeks. With 2,6-dichloro-4-nitroaniline at 400
mg/kg haematology was normal - no Heinz bodies were detected and the
reticulocyte count was normal. At the 800 mg/kg dose there was a
slight weight loss. The red blood cell and haemoglobin counts were
normal while lymphocyte counts were slightly reduced. It was noted at
the conclusion of the study that there was no effect of this compound
on the spleen. In contrast, 4-nitroaniline had definitive effects on
growth at 200 mg/kg per day. Heinz bodies were identified in the blood
and the reticulocyte count was greatly elevated (marked
reticulocytosis). Bone marrow was not affected. At 200 mg/kg (2x/day)
there was a reduction of growth, reduced RBC count (with polychromasia
and nucleation) accompanied by an enlarged spleen. These effects were
more pronounced at the higher dose where, in addition, a haemoglobin
was reduced and the lymphocyte count greatly increased. At high
levels, although 2,6-dichloro-4-nitroaniline caused lymphopenia, the
hemotoxic effects normally associated with 4-nitroaniline were not
observed (Lessel, 1974b).
Mortality was observed when rats were administered
2,6-dichloro-4-nitroaniline at 1000 mg/kg. No mortality was noted when
400 mg/kg was administered for three months. Liver and kidney changes
were observed with light and electron microscopic examinations
(Serrone, 1967).
Dog
Groups of dogs (8 males and 8 females per group) were fed
2,6-dichloro-4-nitroaniline in the dry diet at levels of 0, 20, 100
and 3000 ppm for two years. One female dog at 3000 ppm died at 74
weeks. This death war attributable to the presence of
2,6-dichloro-4-nitroaniline in the diet. One male control dog lost
considerable weight but survived to the end of the experiment. No
compound-related changes in behaviour, food consumption, or growth
were observed. A watery lacrimation was noted for all dogs at 3000 ppm
which persisted during the entire testing interval. A yellowing of the
sclera, mucous membranes, and abdominal skin was noted at the high
level of feeding. The dog that died showed a picture of haemolytic
anemia prior to death (reduced haemoglobin, immature erythrocytes,
polychromophylic macrocytes, increased leucocyte count and increased
M:E ratio in the bone marrow). At 3000 ppm, clinical chemistry was
altered in both males and females with an elevation observed in the
activities of the SGOT and SGPT enzymes, a reduced blood protein,
increased prothrombin time, BUN, BSP and urinary albumin content. At
the conclusion of the study, gross and microscopic examination
revealed an increase in liver weight accompanied by histological
changes at 3000 ppm in the diet. Histological changes in the animals
fed 3000 ppm in the diet included irregular hepatic cell size, hepatic
cell hypertrophy and increased pigmentation of hepatic cells and of
liver macrophages. Slight changes at 100 ppm were noted in two dogs. A
no-effect level in this study is estimated to be between 100 and 3000
ppm in the dry diet (Woodard et al., 1964).
Monkey
Daily oral administration to Rhesus monkey at 160 mg/kg was
lethal within three months with a greater effect noted on females than
males. Coloration of monkey urine differed from rat urine suggesting a
difference in metabolism in the two species. Liver and kidney changes
were observed after light and electron microscopic examination.
Centrolobular fatty degeneration was observed. Swelling of
mitochondria with distortion of the cristal was also observed. There
were differences in the comparative effects of
2,6-dichloro-4-nitroaniline on liver metabolizing enzymes of monkey
and rat (Serrone, 1967).
Long-Term Studies
Rat
Groups of rats (35 males and 35 females/group) were fed
2,6-dichloro-4-nitroaniline in the diet at levels of 0, 20, 100 and
3000 ppm for two years. At 100 ppm there was no effect on behaviour,
mortality or growth. At this level all values from treated animals
were comparable to control values. Growth and food consumption of both
males and females was depressed at 3000 ppm. Haematological parameters
(haemoglobin and packed cell volume) were reduced at 3000 ppm. These
haematological changes were noted only after the first year of
treatment. Gross and microscopic examination performed at 13 weeks and
at the conclusion of study showed slightly higher liver weights,
kidney weights, testicular weights and (possibly) thyroid weights at
3000 ppm. The incidence and location of neoplasms in all treatments
did not differ from those in the controls. Histological examination
revealed liver changes at 3000 ppm, characterized by hepatic cell
enlargement, glycogen depletion, increased basophilia of the
cytoplasm, and the presence of necrobiotic hepatic cells. Histological
examination performed at 13 weeks also indicated hepatic cell changes
and slight adrenal cortical atrophy in several animals at 3000 ppm.
The adrenal changes were not noted at 104 weeks. An estimated
no-effect level in this study is between 100 and 3000 ppm in the diet
(Woodard et al., 1964).
Groups of rats (25 males and 25 females/group, Boots-Wistar
strain) were fed 2,6-dichloro-4-nitro-aniline in the diet at
concentrations of 0 and 1000 ppm for two years. There was no effect on
survival, food consumption, growth, haematology, or upon gross and
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular makeup of
liver, kidney, or spleen. The incidence of tumors in the control and
treatment group was similar. From the results of this experiment a
suggested no-effect level is greater than 1000 ppm (Lessel, 1974e).
OBSERVATIONS IN MAN
In a clinical double blind study, two groups of adult males were
administered 2,6-dichloro-4-nitroaniline (20 individuals) or a placebo
(10 individuals) once a day for ninety days.
2,6-Dichloro-4-nitroaniline was administered at a level of 10 mg per
day. Hematological, liver function, and kidney function tests were
performed at various intervals over the course of the study and were
found to be normal. There were no indications that administration of
2,6-dichloro-4-nitroaniline at 10 mg per day to adult males had any
adverse effect (Stough, 1962).
Extensive examinations were made on one industrial worker
occupationally exposed to 2,6-dichloro-4-nitroaniline over a period of
three years. It was reported that for about 60 days per year
considerable inhalation and dermal exposure had occurred. No adverse
effects were observed with the individual (Brooks and Boyack, 1963).
Another investigation in man on the potential ocular problem
associated with 2,6-dichloro-4-nitroaniline was again negative
(Manger, 1972).
COMMENTS
2,6-Dichloro-4-nitroaniline has a low order of toxicity to
mammals, including man. 2,6-Dichloro-4-nitroaniline is rapidly
metabolized in plants, and fragments of the molecule are
reincorporated as natural plant constituents. In mammals,
2,6-dichloro-4-nitroaniline is rapidly absorbed, metabolized and
excreted. Metabolism in mammals results in formation of the
chlorinated phenylenediamine and aminophenol which are conjugated and
excreted. 2,6-Dichloro-4-nitroaniline does not induce
methemoglobinemia as evidenced with 4-nitroaniline.
2,6-Dichloro-4-nitroaniline does not affect reproduction in rodents
and has shown no evidence of being a teratogen under the experimental
protocol used. Following subacute feeding, dogs exposed to sunlight
developed cataracts. Specific experiments with rabbits, rats and
swine did not duplicate these results. Long term feeding studies in
rat and two year feeding studies in dog resulted in growth
retardation accompanied by an increased liver and kidney size at
high levels. No-effect levels based on a two year dog study and short
and long term rat studies formed the basis for allocating a temporary
ADI for man. A short term study in man is reassuring in estimation of
the temporary ADI although no conclusions could be drawn on the
possibility of ocular damage to man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of 2,6-dichloro-4-nitroaniline as a commercial
fungicide is recorded in Canada, France, Holland, Italy, Japan, New
Zealand, South Africa and USA.
It is claimed to be effective against several Basidiomycetes
and Deuteromycetes species of fungi, being fungistatic to the
mycelium and spores of Botrytis cenera (Clark et al., 1960; Clark
and Hams, 1961; Sharples, 1962) and to several Rhizopus fruit rot
fungi (Ogawa et al., 1961, 1962; Cappelini and Stretch, 1962).
It is mostly marketed as 4% or 8% (w/w) dust formulations or as
50% (w/w) wettable powders, alone or mixed with thiram (TMTD) or
pentachloronitrobenzene. Smoke formulations containing 40% dicloran
are also available.
Pre-harvest treatments recommended by the manufacturers include
soil treatments for lettuce under glass, dusting or spraying of soft
fruits, cotton, leafy vegetables, strawberries, onions, garlic,
tomatoes and ornamentals. Post-harvest dips of peaches, nectarines and
carrots are practised at rates of 750-1000 mg/kg a.i.
Phytotoxicity
Under extreme experimental conditions a "bronzing" discolouration
of lettuce leaves and marginal "off-taste" taints in fruits have
occasionally been found, but none of these effects have ever been
reported in practical use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data on 2,6-dichloro-4-nitroaniline in several fruit and
vegetable crops have been presented by the originating company (The
Boots Company Ltd., 1972). The data derive from field trials and
post-harvest experiments carried out mainly in the USA and UK.
Summaries of residue ranges and experimental conditions extracted from
these data are presented in Tables 2, 3 and 4.
Peaches and apricots
Extensive trials with single or repeated spraying of peaches and
apricots at recommended dosage rates (usually 0.12-0.18% a.i.) and
higher rates have been carried out during the period from 1960 to 1965
(Tables 2 and 4). The initial deposits of 2,6-dichloro-4-nitroaniline
on peaches are generally between 5 and 15 mg/kg with occasional values
up to 25-30 mg/kg. A typical residue was 5 mg/kg after 6 days, 3 mg/kg
after 9 days and 1.5 mg/kg after 14 days. The highest recorded residue
14 days after treatment at a dosage rate of 0.18% was 4.9 mg/kg.
The average half-life of 2,6-dichloro-4-nitroaniline on peaches
has been calculated to be 5 days.
Spraying apricots gave residues very similar to those found in
sprayed peaches (Table 4).
Dusting peaches using 8% a.i. dicloran powders at rates of 3-5 kg
per ha gave maximum initial deposits of 3.7 mg/kg.
A suggested practice of wrapping apricots and peaches in tissues
impregnated with 2,6-dichloro-4-nitroaniline (from 500-3000 mg/kg, in
the wrap) showed a definite transfer of the chemical to the fruits.
Residues from such post harvest application were from 1.6 - 3.5 mg/kg
in peaches and 1.9 - 3.3 mg/kg in apricots (Table 3).
TABLE 2 Residues of dicloran in peaches
Application Number Number Number Range of
(% a.i. w/v or PHI of of of results
kg a.i./ha) (days) applications trials results mg/kg
0.06% 3 3 1 4 0.1-0.3
0.09% 0 1-3 3 9 6.1-16.5
1 1-3 5 9 1.5-11.6
2-3 1-3 2 7 0.2-4.7
7 1 1 1 2.3
0.12% 0 1-3 1 6 10.8-14.0
1 1-2 1 2 3.4-5.5
3 1-3 2 10 0.2-6.3
7 1 1 1 3.2
0.18% 0 2 1 6 6.0-11.4
1 2 1 6 9.2-13.7
2 2 1 6 5.7-13.8
4 2 1 6 4.2-9.4
6 2 1 6 4.5-7.8
9 2 1 6 1.9-6.0
14 2 1 6 0.6-4.9
0.18% 0 3 1 4 18.9-29.5
3 3 1 4 22.4-29.6
0.24% 1 3 1 4 3.0-8.3
3 3 1 4 2.7-11.1
3.1 kg/ha
(8% dust) 0 1 1 2 0.14-0.35
4.5-4.9 kg/ha
(8% dust) 0 1 3 21 0.22-3.7
TABLE 3 Residues in fruits wrapped in tissues impregnated with
dicloran
Fruit Dicloran in wrap mg/kg Dicloran in fruits
Apricot 500 1.9
1000 2.6
2000 3.3
Peach 500 1.6
1000 2.3
2000 3.8
3000 3.5
TABLE 4 Residues of 2.6-dichloro-4-nitroaniline in fruit and vegetables
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Apricots 0.09% 0 1 1 12 1.9-7.9
2 3 1 2 0.9-1.4
4 1-2 1 4 1.4-3.7
11 1 1 2 0.1-0.7
Apricots 0.12% 0 1 1 1 10.1
1 1 1 1 8.0
7 1 1 1 3.8
Cherry 0.12% 0 1 1 1 10.3
1 1 2 5 6.4-14.8
7 3 1 1 4.9
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
0.24% 1 1 1 2 0.7-11.2
7 1 1 1 2.9
0.36% 1 5 1 2 2.7-3.1
1200 ppm post-harvest 6 21 1.5-6.4
1200 ppm post-harvest
and washing 1 2 0.3-0.3
1350 ppm post-harvest 1 2 10.2-13.7
1800 ppm post-harvest 1 2 7.7-8.4
Grapes 1.7 kg/ha
(dust) 0 2-4 2 6 0.2-3.9
7 2 1 2 0.3-1.1
2.3-3.3
kg/ha (dust) 1 4 1 4 1.1-1.3
7 1-2 2 4 0.2-1.1
1000 ppm post-harvest dip 1 4 0.6-5.1
Plums
(dried) 1000 ppm post-harvest dip 1 3 1.0-4.7*
post-harvest dust 1 3 2.0-8.1
0.12% 1 1 1 1 2.5
Strawberry 0.16-0.18% 1 4 1 1 0.32
3 1 1 2 4.0-5.7
9-11 3-4 2 4 0.2-1.6
0.24% 1 3 1 2 0.9-1.7
Blackberry 1.1 kg/ha
(spray) 4-11 1 1 2 1.2-2.3
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
1.8 kg/ha
(dust) 4-11 1 1 2 <0.05-0.3
Currants 0.11-0.15% 33 4 1 3 1.7-3.3
Raspberry 0.15-0.18% 5 4 2 2 17.0-20.2
9-10 4-5 2 2 2.2-11.8
13-14 3-4 2 2 2.6-7.5
0.36% 5 4 1 1 39.0
10 4 1 1 28.4
14 4 1 1 16.3
Carrots 900- post-harvest dip
1000 ppm (stored 0-3 days) 2 12 3.6-9.4
peeled 1 1 2.1
canned 1 7 <0.05-0.2
Lettuce 2.2 kg/ha 0 2 1 4 50.1 (average)
7 2 1 9 18.0 "
RL50 approx 14 2 1 9 3.0 "
3-4 days
21 2 1 9 2.4 "
28 2 1 8 0.8 "
35 2 1 8 0.04 "
1.3 g/m2
(dust) 120 1 1 1 0.1
1.3 g/m2 (soil
treatment;
pre-planting) 114-140 1 3 5 0.2-2.1
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Gherkins
(indoor) 0.05% 0 1 1 1 5.5
2 1 1 1 1.1
4 1 1 1 0.6
5 1 1 1 0.35
Tomato
(indoor) 0.1% 2 1 1 1 0.25
4 1 1 1 0.2
6 1 1 1 0.04
Beans,
French 3 kg/ha 12 4 1 2 1.3-1.9
20 2 1 2 0.8-1.5
* Loss by drying.
Cherries, grapes and plums
The residue levels of 2,6-dichloro-4-nitroaniline from
recommended uses on these fruits are similar to those in peaches and
apricots (Table 4).
After dipping procedures or post-harvest dusting of plums,
dicloran was retained on the fruits at levels of 4.7 and 8.1 mg/kg
respectively. These residues were reduced to 1.0 and 2.0 mg/kg
respectively, when the fruits were subjected to an 8 day air-drying
period at ambient temperature.
A post-harvest treatment of cherries by spraying on the packing
line with 1200-1800 ppm suspensions gave residues of
2,6-dichloro-4-nitroaniline from 1.5 - 13.7 mg/kg. In storage
experiments these residues were reduced at a rate corresponding to a
half-life of 11 days at 20°C.
Small fruits and berries
Repeated spraying of strawberries at different locations (0.06
- 0.24% a.i.) have been reported to give maximum initial deposits of
dicloran of 9 mg/kg. However, residues at harvest after a 9 - 11 day
interval from the last application were generally well below 1 mg/kg,
with an occasional individual sample at 1.6 mg/kg. Among the berries,
raspberries in some cases showed higher residues. After application of
0.36% a.i. the initial residue was 39 mg/kg which was reduced to 16.3
mg/kg at 14 days. At lower application rates, residues were
proportionately lower.
Experiments with other berries (blackberries, currants and
boysenberries) generally gave residues below 5 mg/kg.
Lettuce
Roburn (1960) followed the disappearance of
2,6-dichloro-4-nitroaniline after post-planting treatments of lettuce
plants with 1/4 oz of 4% dust per sq. yd. and found a reduction in
deposits greater than that due to growth dilution. Volatilization and
chemical degradation were suggested as contributing factors.
The half-life values decreased progressively in these trials,
being about 3-5 days during the last weeks of the growing season. A
three weeks pre-harvest interval for dicloran treatments of lettuce is
recommended on this basis in the United Kingdom.
Later experiments by Boyack and Boot (1962 a, b, c) confirmed
Roburn's findings, indicating half-life values of 3-4 days on
greenhouse lettuce with average residues of 2.4 mg/kg after an
interval of 21 days (Table 4).
Uptake of 2,6-dichloro-4-nitroaniline through the roots of
lettuce plants after pre-planting soil treatments has been
demonstrated by Boyack and Boot (1962a), Lemin and co-workers (Lemin,
1963, 1965; Moe and Lemin, 1963a, 1964) and Groves and Chough (1970).
Residues from such practices at the time of harvest will be near or
below the detection limit, i.e. less than 0.05-0.1 mg/kg.
In recent experiments in the Netherlands lettuces have been
treated both by a pre-planting soil application and by dusting the
young plants immediately after planting. (Pieters, 1974). Residues in
these experiments ranged from 0.1 - 2.1 mg/kg (Table 4).
Carrots
Post-harvest dipping in 900-1000 ppm suspensions caused residues
of 3.6-9.4 mg/kg at 0-3 days. After storage for 5 months the remaining
residues were 2.1-3.7 mg/kg.
Other vegetables
Residue data on a few other vegetable crops, e.g. tomatoes,
gherkins and French beans, mostly grown under glasshouse conditions,
are available (Pieters, 1974). The data are insufficient to evaluate
quantitatively the rates of dissipation, but residues are generally
low at the time of harvest, i.e. from a few days to 1-2 weeks after
treatment (Table 4).
FATE OF RESIDUES
In animals
The metabolism of 2,6-dichloro-4-nitroaniline by the rat, mouse
and to a limited extent man, has been described above under
"Biotransformation". No such experimental evidence is available on
livestock animals, nor on the excretion of dicloran or its metabolites
in milk.
In plants
Studies by Lemin (1965), Lemin et al. (1963) and Moe and Lemin
(1963a, 1964) have shown that 2,6-dichloro-4-nitroaniline is absorbed
by plant roots and translocated to the plant material in tomato and
lettuce. These experiments include uptake from both nutrient solutions
and treated soils.
Application of 14C-labelled 2,6-dichloro-4-nitroaniline gave
evidence of rapid degradation into polar metabolites and 14C was
found in carbohydrate constituents of the plant tissue, presumably
owing to the incorporation of degradation fragments. Transitional
metabolites such as dechlorinated components, reduction products or
2,6-dichloro-p-phenylene-diamine were not detected.
Amino acids, chlorophyll or uronic acid did not contain
radioactivity. Neither could 2,6-dichloro-4-aminophenol, known to be
formed by animal metabolism (see previous section), be found in plant
tissues.
Exhaustive extractions of peaches 13 days after treatment with
14C-labelled 2,6-dichloro-4-nitroaniline revealed (in addition to
unchanged parent compound) 14C-labelled phenylalanine and labelled
flavonoid glycosides (Moe and Lemin, 1963b). As in the above
experiments, no transitional metabolites could be traced.
Groves and Chough (1970) report rapid absorption of dicloran and
incorporation of fragments into tissue constituents by a number of
plants.
In processing and storage
Washing or canning processes have been shown to reduce residues
of 2,6-dichloro-4-nitroaniline considerably (Boots Company Ltd.,
1972). On lettuce, for example, residues of 18-25 mg/kg still
remaining two weeks after the last application were reduced to 5.6-6.0
by washing with water (Roburn, 1958). In peaches, freshly treated with
2,6-dichloro-4-nitroaniline, the residues which still remained after
an industrial canning procedure were below 0.1 mg/kg.
Carrots treated by post-harvest dipping showed only moderate
reduction of the residues by peeling, from 3.7 to 2.1 mg/kg,
indicating a significant penetration into the inner parts. A
subsequent canning procedure, however, reduced the levels to 0.1-0.2
mg/kg. Storage of dipped carrots for 5 months at 40°F gave a decrease
in the residue of about 25%.
In soils
2,6-Dichloro-4-nitroaniline is generally regarded as being
relatively stable in soils at field moisture capacity. Its
persistence, however, is greatly affected by conditioning factors such
as soil composition, water content, microculture etc. (Wang and
Broadbent, 1973). The absorption of the chemical from soils by oats
was found by Groves and Chough (1970) and Wang (1972) to be inversely
related to the clay and organic matter content of the soils,
suggesting a binding of the chemical to these soil constituents. Later
exhaustive extraction experiments showed that a reversible binding
probably occurs (Groves and Chough, 1971).
The same authors (Groves and Chough, 1970, 1971) showed that
14C-labelled dicloran can be metabolized by soil organisms which
degrade the compound to 14C-carbon dioxide and other volatile
compounds. The rate of microbial breakdown could be increased by
repeated applications of dicloran to the same soil or by incubation of
the soil with dicloran. A culture of rod-shaped bacteria was isolated
and found active in the decomposition of the fungicide. Microbial
breakdown is also held responsible for the rapid degradation of DCNA
which is seen in soil under flooded conditions (Wang and Broadbent,
1973).
Van Alfen and Kosuge (1974) have identified both
2,6-dichloro-p-phenylenediamine and its acetylated derivative,
4-amino-3,5-dichloroacetanilide, in cultures of Pseudomonas cepacia
and Escherichia coli B. In soil and through the action of
horseradish peroxidase, the phenylenediamine was converted to one of
three isomers of the azine resulting from oxidative dimerization (Van
Alfen, 1973).
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Market sample surveys carried out in the Netherlands in 1973 on
domestically grown fruit and vegetables showed that residues of
2,6-dichloro-4-nitroaniline were frequently present in some crops
(Table 5). In vegetables such as endive, lettuce and chicory sprouts
about 50-80% of samples were positive with individual residues up to 8
mg/kg. Crops such as cucumbers, tomatoes and paprika were less
frequently positive (3-25%). At the time of this survey, the tolerance
for vegetables in the Netherlands was 10 mg/kg.
TABLE 5 Dicloran residues in marketed vegetables (Netherlands, 1973)
Dicloran
residues,
mg/kg number of samples
(range)
Cucumber Endive Lettuce Paprika Tomato Chicory
not detectable 31 34 136 12 30 68
0.01 - 0.1 1 16 139 2 4 50
0.1 - 0.3 - 7 126 2 - 21
0.3 - 1.0 - 8 180 - - 3
1.0 - 3.0 - 4 123 - - 1
3.0 - 10.0 - 1 35 - - -
Total number 32 70 739 16 34 143
[text missing]
pre-harvest treatments on fruits, vegetables and ornamentals and
post-harvest treatments on peaches, nectarines, cherries and carrots.
It is registered in several countries, usually as 4 or 8% (w/w) dusts
or as 50% (w/w) wettable powders.
The technical grade typically contains about 96%
2,6-dichloro-4-nitroaniline. The remaining parts consist of chemically
related compounds and impurities which have been identified and
quantified.
Concentrations and rates of application vary, depending on the
crop and method of application. Normal spraying concentrations are
0.05% to 0.18% applied at rates of 2-5 kg a.i. per ha. Post-harvest
dips are practised at rates of 1000 - 2000 mg per litre.
The residue data available are mainly obtained from the USA and
UK, in a few cases supplemented from other countries. Most of the data
derive from field trials or experiments under practical conditions
likely to represent the results of good agricultural practice.
2,6-dichloro-4-nitroaniline is a non-systemic compound of
relatively low persistence. The dissipation of the compound on fruits
and vegetables is often faster than can be explained by normal
weathering or growth dilution. Half-life periods of 11 days have been
found for residues during storage of post-harvest treated cherries.
2,6-dichloro-4-nitroaniline is absorbed by plant roots from the
soil. The fate of residues in plant material has been followed with
the radio-labelled compound, which indicated a rapid degradation into
polar metabolites and 14CO2 followed by incorporation of 14C into
normal metabolic plant constituents. No indications of transitional
metabolites from such degradation have been found.
Similar degradation of 2,6-dichloro-4-nitroaniline occurs in
soils, although in this case microbial breakdown may also produce
smaller amounts of reduction products and acetylated derivatives of
the parent compound.
Studies were available on the metabolic fate of residues in rats
indicating a fairly rapid process of absorption, metabolism and
excretion, especially as aminophenol and phenylenediamine derivatives,
in the urine. There was no evidence of tissue storage in these
experiments. Preliminary studies in man suggest a metabolic pattern
similar to that of rats. No information, however, has been recorded on
metabolism in livestock animals or on transfer of residues into meat
or milk.
METHODS OF RESIDUE ANALYSIS
A colorimetric method of residue analysis, based on the
development of a yellow colour characteristic of some mononitro
aromatic compounds in the presence of strong alkali and acetone, is
described by Kilgore et al. (1962). Extraction is with benzene,
followed by clean-up on Florisil alone or in combination with an
acetonitrile-petroleum ether partitioning procedure. Blank values for
fruits were about 0.01-0.02 mg/kg and recoveries in the range 75 -
100% (average 86%). The colorimetric method described by Groves and
Chough (1966) and Heagy (1969) has a similar sensitivity, based on
diazotization and coupling to N-(1-naphthyl)ethylenediamine has a
similar sensitivity.
An alternative method developed by Roburn (1961) utilizes the
reduction of 2,6-dichloro-4-nitroaniline by zinc and hydrochloric acid
to the corresponding 2,6-dichloro-p-phenylenediamine which, in the
presence of aniline, can be oxidized to produce an intense blue
colour. Less than 2 µg can be detected by this method, corresponding
to 0.05 mg/kg in fruit and 0.2 mg/kg in lettuce. Recovery of surface
deposits was approximately 100%, but for macerated samples it was only
71%. Chloronitroanilines in general give similar reactions but a
series of other pesticide chemicals did not interfere, indicating a
fair degree of specificity of the method.
A thin-layer chromatographic spot test sensitive to 0.5 µg of
2,6-dichloro-4-nitroaniline is given by Groves et al. (1966) based on
their colour reaction mentioned above.
Gas-chromatographic methods for the determination of
2,6-dichloro-4-nitroaniline have been developed by Beckmann and
Bevenue (1962) and Kilgore (1964) using electron capture and
microcoulometric detectors, in combination with the Kilgore extraction
procedure already mentioned. Recoveries of 85-105% were established by
Brewerton et al. (1967) for the analysis of dicloran in soils, fruit
and vegetables down to the 0.1 mg/kg level by the GLC/EC technique.
These methods could be adapted for regulatory purposes as part of a
multi-residue procedure.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance
Country Crop mg/kg
Australia Beans, lettuce, stone fruits, tomatoes 20
Belgium Fruit and vegetables (exc. potatoes) 10
Canada Apricots, nectarines, peaches
(pre-harvest and post-harvest),
sweet cherries pre-harvest and
post-harvest), snap beans 20
Strawberries, raspberries 15
Celery, grapes, lettuce, rhubarb,
sweet potatoes 10
Carrots, cucumbers, garlic, onions,
plums, tomatoes 5
Germany All fruits and vegetables 0.1
Tolerance
Country Crop mg/kg
Italy General 10
Switzerland Lettuce (tentatively) 0.5
Netherlands Lettuce, endive 3
Chicory (sprouts) 1
Cucumbers, gherkins, melons, bell
peppers, tomatoes 0.3
Other vegetables 0.1
USA Apricots, nectarines (pre-harvest and
post-harvest), peaches (pre-harvest
and post-harvest), sweet cherries
(pre-harvest and post-harvest), snap
beans 20
Blackberries, boysenberries, celery,
plums (fresh prunes) (pre-harvest and
post-harvest), raspberries 15
Carrots (pre-harvest and post-harvest),
grapes lettuce, rhubarb, sweet
potatoes (pre-harvest and post-harvest) 10
Cucumbers, garlic, onions, tomatoes 5
Potatoes 0.25
Cotton seed 0.1
APPRAISAL
2,6-dichloro-4-nitroaniline is a fungicide active against the
mycelium and spores of Botrytis cinerea, B. sclerotinia and other
fungi, including several Rhizopus fruit rots.
Practical use patterns comprise foliar applications and soil
treatments under indoor and outdoor growing conditions.
The residue data from supervised trials on several crops were
considered sufficiently extensive to enable recommendations to be
made, including some for post-harvest treatments.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for specific determination.
These methods are suitable for regulatory purposes.
RECOMMENDATIONS
Temporary tolerances are recommended for
2,6-dichloro-4-nitroaniline in the following commodities at the
levels indicated. The levels are not likely to be exceeded when
2,6-dichloro-4-nitroaniline is applied in accordance with good
agricultural practice including both pre-harvest and post-harvest
applications and taking into account that preharvest intervals may
apply for certain treatments.
TEMPORARY TOLERANCES
Limit Basis on which recommendations
Crop (mg/kg) are made
Cherries 15 pre and post-harvest
Peaches 15 pre and post-harvest
Apricots 10 pre and post-harvest
Carrot 10 post-harvest only
Grapes 10 pre and post-harvest
Lettuce 10 pre-harvest only
Plums 10 pre and post-harvest
Raspberry 10 pre-harvest only
Strawberry 10 pre-harvest only
Blackberry 5 pre-harvest only
Currant (red, black
and white) 5 pre-harvest only
Beans (French) 2 pre-harvest only
Gherkins 0.5 pre-harvest (indoor) only
Tomato 0.5 pre-harvest only
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further studies on the ocular disturbance observed in dogs to
confirm and clarify this effect.
2. Information on fate in livestock animals in so far as plant
material containing residues may be fed to animals.
DESIRABLE
1. Effect on hepatic microsome systems in several species.
2. Further observations in man.
3. Further information on fate of residues during storage, transport
and processing of fruit and vegetables.
4. Information on transfer of residues from grapes to wine and on
possible influence on wine processing.
5. Further information on soil residues and their possible uptake
into subsequent crops.
6. Further data to clarify inconsistencies in the residue levels
found in different berries.
REFERENCES
Anonymous. (1974) "Somers test" in rabbits. Technical summary reports
submitted to WHO by UpJohn Co. (Unpublished)
Beckmann, H. and Bevenue, A. (1962) Pesticide residue analysis Gas
chromatographic analysis of 2,6-dichloro-4-nitroaniline. J. Food Sci.,
27:602-604.
Bernstein, H.N., Curtis, J., Earl, F.L. and Kuwabara, T. (1970)
Phototoxic corneal and lens opacities in dogs receiving a fungicide,
2,6-dichloro-4-nitroaniline. Archs Ophthal. (Chicago), 83:336-348.
Boots Co. Ltd. (1972) Dicloran. Registration Document Vol. I, II, III
and VI. Reports submitted by The Boots Company Ltd. Agricultural
Research, Nottingham. (Unpublished)
Boyack, G. and Boot, D. (1962a) Assay for 2,6-dichloro-4-nitroaniline
in lettuce grown in treated soil under glass. Dicloran, Registration
document from The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G, and Boot, D. (1962b) Assay for 2,6-dichloro-4-nitroaniline
on sprayed greenhouse lettuce. Dicloran, Registration document from
The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G. and Boot, D. (1962c) Residue of 2,6-dichloro-4-nitroaniline
(DCNA) on lettuce dusted with Botran. Dicloran, Registration document
from The Boots Co. Ltd. Vol. II, Appendix 26.
Brewerton, H.V., Clark, P.J. and McGrath, H.J.W. (1967) Dicloran
analysis by gas chromatography. New Zealand J. Sci., 10:124-127.
Brooks, B. and Boyack, G. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Report submitted by
The Upjohn Co. (Unpublished)
Cappellini, R.A. and Stretch, A.W. (1962) Control of post-harvest
decays of peaches. Pl. Dis. Reptr., 46:31-33.
Clark, N.G., Hams, A.F., Higgons, D.J. and Stevenson, H.A. (1960) A
new fungicide active against Botrytis spp. Chemy Ind., 1960:572-573.
Clark, N.G. and Hams, A.F. (1961) Antifungal activity of substituted
nitroanilines and related compounds. J. Sci. Food Agric., 12:751-757.
Curtis, J., Bernstein, H.N., Earl, F.L. and Smalley, H., Jr. (1968)
Corneal and lens opacities in dogs treated with
2,6-dichloro-4-nitroaniline. Toxic. appl. Pharmac., 12:305.
Earl, F.L., Curtis, J., Bernstein, H.N. and Smalley, H., Jr. (1971)
Ocular effects in dogs and pigs treated with dichloran. Food Cosmet.
Toxic., 9:819-828.
Eberts, F.S. (1965) Fate of Botran (2,6-dichloro-4-nitroaniline) in
rat and man. Dicloran, Registration Document, Vol. IV, Appendix 54.
Boots Co. Ltd. (Unpublished). Botran Symposium, Augusta, Mich. 1965.
Cited in Chemical Abstracts 1968, 68:abstract 11908g
EPA. (1974) Data from EPA Laboratories. (Unpublished)
Evans, J., Mengel, G. and Bostwick, L. (1963) Botran(R) (U-2069)
Effect of oral administration. Final report, four months study. Report
submitted by the Upjohn Co. (Unpublished)
Feenstra, E. (1961) Report submitted by The Upjohn Co. (Unpublished)
Groves, K. and Chough, K.S. (1966) Determination of small quantities
of 2,6-dichloro-4-nitroaniline (dicloran). J. agr. Food Chem.,
14:668-669.
Groves, K. and Chough, K.S. (1970) Fate of the fungicide
2,6-dichloro-4-nitroaniline (DCNA) in plants and soils. J. agr. Food
Chem., 18:1127-1128.
Groves, K. and Chough, K.S. (1971) Extraction of
3-amino-1,2,4-triazole (Amitrole) and 2,6-dichloro-4-nitroaniline
(DCNA) from soils. J. agr. Food Chem., 19:840-841.
Gurd, P.R. (1974) Methaemoglobin formation in the cat. Report from
Boots Pure Drug Company Limited. Submitted to WHO by the Boots Co.
Ltd. (Unpublished)
Heagy, J.A. (1969) Colorimetric determination of
2,6-dichloro-4-nitroaniline (dicloran) residues in foods. J. Ass. off.
analyt. Chem., 52:797-799.
Horn, J. (1961) U-2069 - Acute inhalation toxicity. Study from Woodard
Research Corporation submitted by The Boots Co., Ltd. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: A preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Johnston, R. and Schwikert, R. (1961a) Skin irritation in rabbits.
Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1961b) Dichloran - eye irritation in
rabbits. Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1963) Skin sensitization in guinea
pigs. Report from The Upjohn Co. (Unpublished)
Kilgore, W.W. (1964) 2,6-dichloro-4-nitroaniline. Analytical methods
for pesticides, plant growth regulators and food additives. Gunter
Zweig (Editor). Vol. III. p. 61-68.
Kilgore, W.W., Cheng, K.W. and Ogawa, J.M. (1962) Extraction and
determination of 2,6-dichloro-4-nitroaniline in processed fruits. J.
agr. Food Chem., 10:399-401.
Lemin, A.J. (1965) Translocation and metabolism of
2,6-dichloro-4-nitroaniline by lettuce and tomato. J. agr. Food Chem.,
13:557-560.
Lemin, A.J. Moe, L.D. and Smith, A.H. (1963) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce. Dicloran, Registration
document from the Boots Co. Ltd. Vol. II, Appendix 34 A.
Lessel, B. (1974a) Experimental data on toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974b) Effect on blood and blood forming tissues. Report
from The Boots Co. Ltd. (Unpublished)
Lessel, B. (1974c) Short term (4-week) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974d) Chronic (6 month) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974e) Two year feeding trial in rats on dicloran
(2,6-dichloro-4-nitroaniline). Report submitted by the Boots Co. Ltd.
(Unpublished)
Lobdell, B. and Johnson, C. (1965) U-2069 - Effect on reproduction
capacity through three generations in the rat. Report from Woodard
Research Corporation submitted by The Boots Co. Ltd. (Unpublished)
Manger, W.H. (1972) Use and handling of dicloran formulations and
influence on human eyesight. Report from Biological Laboratory,
Aseptafabrick B.V. Delft, Netherlands. (Unpublished)
Maté, C., Ryan, A.J. and Wright, S.E. (1967) Metabolism of some
4-nitroaniline derivatives in the rat. Food Cosmet. Toxic., 5:657-663.
Moe, L.D. and Lemin, A.J. (1963a) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part II, dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 B.
Moe, L.D. and Lemin, A.J. (1963b) The metabolism of
2,6-dichloro-4-nitroaniline-C14 in peaches. Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 35.
Moe, L.D. and Lemin, A.J. (1964) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part III. Dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 C.
Ogawa, J.M., Lyda, S.D. and Weber, D.J. (1961)
2,6-dichloro-4-nitroaniline effective against Rhizopus fruit rot
of sweet cherries. Pl. Dis. Reptr., 45:636-638.
Ogawa, J.M., Mathre, J.M., Weber, D.J. and Lyda, S.D. (1963) Effects
of 2,6-dichloro-4-nitroaniline on Rhizopus species and its
comparison with other fungicides on control of Rhizopus rot of
peaches. Phytopathology, 53:950-955.
Pieters, A.J. (1974) Information on dicloran residues in The
Netherlands submitted to the Joint Meeting 8 November 1974.
(Unpublished)
Roburn, J. (1958) Experiments to demonstrate the effect of washing
lettuce treated with dicloran ("Alisan"). Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 33.
Roburn, J. (1960) Allisan residue trials on lettuce, February to April
1960. Lenton memo nr. 5. Lenton Experimental Station, Nottingham.
(Unpublished)
Roburn, J. (1961) Colorimetric determination of
2,6-dichloro-4-nitroaniline in plants and soil. J. Sci. Fd. Agric.,
12:766-772.
Serrone, D., Pakdaman, P., Stern, A. and Coulston F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
Rhesus monkey. Tox. appl. Pharmacol., 10:404.
Sharples, R.O. (1962) The fungitoxic effects of dicloran on Botrytis
cinerea. Lenton Experimental Station, Nottingham. (Unpublished)
Stough, A.R. (1962) Results of Botran(R) clinical study. Results from
The Upjohn Co. submitted by The Boots Co. Ltd. (Unpublished)
Van Alfen, N.K. (1972) Microbial metabolism of the fungicide
2,6-dichloro-4-nitroaniline in soil by micro-organisms. Dissertation,
Dept. of Plant Pathology, Univ. of California, Davis.
Van Alfen, N.K. and Kosuge, T. (1974) Microbial metabolism of the
fungicide 2,6-dichloro-4-nitroaniline. J. agr. Food Chem., 22:221-224.
Wang, C.H. (1972) The effect of soil properties on losses of two
chloronitrobenzene fungicides from soils. Dissertation, Dept. of Soil
Sciences, Univ. of California, Davis.
Wang, C.H. and Broadbent, F.E. (1973) Effect of soil treatments on
losses of two chloronitrobenzene fungicides. J. environ. Quality, 2
(4):511-515.
Woodard, M., Cockrell, K. and Woodard, G. (1964) Safety evaluation by
oral administration to rats and dogs for 104 weeks. Report from
Woodard Research Corporation submitted by The Boots Co. Ltd.
(Unpublished)
Other information on identity and properties
Molecular weight: 207
State: Yellow, crystalline solid with practically
no odour
Melting point: 192-194°C
Vapour pressure: 1.2 x 10-6 mm Hg (20°C)
Solubility: Water7 mg/l
Cyclohexane 0.006 g/100 ml
Petroleum ether 0.02 g/100 ml
Carbon tetrachloride 0.06g/100 ml
Ethanol 0.29 g/100 ml
Benzene 0.46 g/100 ml
Glacial acetic acid 8.80 g/100 ml
Chloroform 1.2 g/100 ml
Acetone 3.4 g/100 ml
Stability: Stable to hydrolysis and relatively stable
to oxidation. Readily reduced to
phenylenediamine by zinc and acid. Stable
to light and heat.
Composition and
purity: The technical product contains
2,6-dichloro-4-nitroaniline not less than
96% (on dry weight basis);
2,4-dichloro-6-nitroaniline not more than
2%; 2-chloro-4-nitroaniline not more than
l%; Chloranil not more than 1%;
P-nitroaniline not more than 0.1%; Loss on
drying not more than 1% (at 60°C, <5 mm
Hg); Sulphated ash not more than 0.25%;
Sodium chlorate not more than 100 ppm.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
2,6-Dichloro-4-nitroaniline-14C administered to three male human
subjects at a level of 50 mg was found to be rapidly absorbed and
excreted. Excretion was somewhat slower than measured in the rat with
the major quantity of material being excreted within 1.5 days.
Preliminary studies suggest that 2,6-dichloro-4-nitroaniline
metabolites are similar to those obtained from the rat. Approximately
85% of the total urinary excretion from the rat was found to be of
2,6-dichloro-4 hydroxy aniline sulfate.
2,6-Dichloro-4-nitroaniline-14C administered to male rats at a
dosage of either 1.7 mg/kg or 8 mg/kg orally was rapidly excreted
from the body. Urinary excretion accounted for approximately 90% of
the recovered material with the remainder being located in the
faeces. The majority of material (about 90%) was recovered within 48
hours and over half was recovered within 8 hours after treatment.
2,6-Dichloro-4-nitroaniline was not observed in any body tissues with
the exception of small quantities detected in the G.I. tract, urinary
tract, and liver (Eberts, 1965).
2,6-Dichloro-4-nitroaniline administered to rats (ip or orally,
20 mg/kg) was metabolized to the dichloroamimophenol and the
dichlorophenylenediamine derivatives. Following both routes of
administration the majority of material was recovered from the urine
within 24 hours and total recovery was noted within 72 hours. Very
small quantities were obtained in the faeces. In vitro studies using
mouse liver microsomes showed limited conversion of
2,6-dichloro-4-nitroaniline to the same two metabolites (Máté et al.,
1967).
Biotransformations in plants and micro-organisms are discussed
under "Fate of residues".
Effects on enzymes and other biochemical parameters
Following high level subacute oral administration of
2,6-dichloro-4-nitroaniline to rats, an increase of hepatic
demethylase and desulfurase activity was noted. Liver mitochondrial
O2 consumption was also increased in rats. The liver enzymes were
not stimulated in monkey following similar treatment (Serrone, 1967).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A carcinogenic screening using high levels of
2,6-dichloro-4-nitroaniline administered to susceptible mice was
negative. Groups of mice (18 males and 18 females of each of two
hybrid strains) were administered 2,6-dichloro-4-nitroaniline at 215
mg/kg/day for three weeks from day seven after birth. Thereafter for
18 months the mice were fed 603 ppm in the diet, sacrificed and
examined for tumours. 2,6-Dichloro-4-nitroaniline did not cause a
significant increase in tumours (Innes et al., 1969).
Effects on blood and blood forming tissues
Cat
In studies to substantiate the difference between effects of
4-nitroanaline and 2,6-dichloro-4-nitroaniline, a study on the effect
of these materials on methaemoglobinemia in the cat was performed
(Gurd, 1974). After a single oral dose of 2,6-dichloro-4-nitroaniline
(500 mg/kg), no methaemoglobin was noted at any time between 1 and 48
hours after dosing. Administration of 4-nitroaniline at a single dose
of 100 mg/kg resulted in methaemoglobin, observed over the same time
course. In addition, the cats subjected to this experiment were noted
to be cyanotic and have extensive muscle weakness following
4-nitroaniline.
Ocular toxicity
Dog and miniature swine
Dogs have been shown to develop lesions in the cornea and lens
following prolonged oral administration of
2,6-dichloro-4-nitroaniline. It has been suggested that a
photochemical product reaction may be responsible for the lesion as
it occurred only when dogs were exposed to sun light.
Dogs and miniature swine were fed 2,6-dichloro-4-nitroaniline in
the diet at levels of 0, 0.75, 6, 24, 48 and 192 mg/kg/day for periods
of time varying from 50 to 306 days. Corneal opacity appeared in dogs
within 53 to 104 days after administration of 24 or 48 mg/kg, and when
exposed to sunlight. Dogs unexposed to sunlight and those with one eye
sutured closed failed to develop lesions in the unexposed eyes. Dogs
administered 192 mg/kg refused to eat after 38 days and were
administered 2,6-dichloro-4-nitroaniline by capsule. All of these
animals died 49 - 53 days after the study began. Eye lesions were not
detected in this high level group. Several dogs showing eye damage
were maintained for four months after 2,6-dichloro-4-nitroaniline
administration had ceased. Pathological changes seen in the cornea and
lens were not reversible. Administration of
2,6-dichloro-4-nitroaniline at all levels did not appear to affect
miniature swine. Sporadic instances of the presence of Heinz bodies
in blood were observed in both swine and dogs. Administration of
2,6-dichloro-4-nitroaniline as dust or 5% solution directly to the
eyes for three months had no effect on corneal opacity or irritation
of the conjunctiva (Earl et al., 1971; Bernstein et al. 1970; Curtis
et al. 1968).
Special studies on reproduction
Rat
In a standard three generation, two litters per generation,
reproduction study, 2,6-dichloro-4-nitroaniline was administered to
rats (20 males and 20 females per group) at levels of 0 and 100 ppm in
the diet. On the basis of the reproduction parameters examined,
including number of litters, stillbirths, live births, birthweight,
lactation indices, etc. no evidence of an effect of dicloran on
reproduction was indicated (Lobdell and Johnston, 1965).
Male rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 1000, and 2000 ppm for 90 days. The males were mated with
untreated females. There were no differences observed in the number of
litters or in the number of animals born or weaned. Feeding
2,6-dichloro-4-nitroaniline in the diet to male rats resulted in an
increased liver weight at 1000 ppm. An increased kidney weight was
seen at 1000 ppm (EPA, 1974).
Female rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 500, and 1000 ppm for 188 days prior to mating. The rats
were continued on the diet through gestation and lactation. From the
small number of animals in this experiment (10 females/group), it is
difficult to make definitive conclusions concerning the effect on
reproduction of 2,6-dichloro-4-nitroaniline administered to females.
The data suggested a reduced number of pups when the females were fed
a level of 1000 ppm in the diet. There was no apparent effect on
survival of pups, although the mean body weight of pups at 1000 ppm
was slightly reduced. It might be considered that 1000 ppm in the diet
of females for six months might have a slight effect on reproduction.
No effects were seen at 500 ppm (EPA, 1974).
Rabbit
Groups of pregnant New Zealand white rabbits (10, 12, and 14 does
respectively) were fed 2,6-dichloro-4-nitroaniline in the diet at 0,
100, and 1000 ppm from day 8 until day 16 of gestation. In no case was
there evidence of an adverse effect of 2,6-dichloro-4-nitroaniline on
reproduction, affecting either the parents or the offspring (Anonymous
1974).
Sensitization
Rat, guinea pig, rabbit
An examination was made of the potential skin sensitization
properties of 2,6-dichloro-4-nitroaniline in guinea pigs. Ten
subcutaneous injections were administered to male guinea pigs (total
dose 0.95 mg). Two weeks after the last injection a re-injection of
0.05 mg was made. Twenty-four hour readings showed no apparent
sensitization (Johnston and Sweickert, 1963)
Rats, guinea pigs and rabbits were administered
2,6-dichloro-4-nitroaniline via inhalation exposure to an 8% dust
for seven hours. It was estimated that the exposure averaged 0.4
mg/l. No deaths were observed although reddening of the lungs and
pale kidneys were seen in rats and guinea pigs (Horn, 1961).
TABLE 1 Acute toxicity of 2,6-dichloro-4-nitroaniline
LD50
Species Route (mg/kg) References
Rat oral 4000-10 000 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
Ip 1460-5471 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
SC >5000 Lessel, 1974a
Guinea Pig oral 1450 Lessel, 1974a
Mouse oral 1500-2500 Lessel, 1974a
Feenstra, 1961
Ip 2500-8000 Lessel, 1974a
Feenstra, 1961
SC >6000 Lessel, 1974a
dermal >5000 Lessel, 1974a
Cat oral >500 Lessel, 1974a
oral 200 x 7 daily doses Lessel, 1974a
Signs of poisoning in the mouse included defaecation and
urination, depression and lethargy leading to sleep. In rats the same
signs were noted including nasal haemorrhage and paralysis. Death
occurred up to four days after administration of 2,6-dichloro-4-
nitroaniline (Feenstra, 1961). Cyanosis, muscle weakness, and other
typical signs of aniline poisoning were not observed.
Two formulations (8% dust and 50% W.P.) were applied to intact
and abraded skin of rabbits daily for five days. No irritation of skin
was observed (Johnston and Schwikert 1961a). When these materials were
instilled into the conjunctival sac of rabbits, no ocular irritation
was noted (Johnston and Schwikert, 1961b).
Short Term Studies
Rat
Groups of rats (either 10 males and females or 15 males and
females per group) were treated with 2,6-dichloro-4-nitroaniline to
examine potential haematopoietic effects. One series of animals was
administered 2,6-dichloro-4-nitroaniline at levels of 0, 5, 20 and 100
mg/kg/day by gavage for four months. Another group was administered
2,6-dichloro-4-nitroaniline in the diet at levels of 0 and 20 ppm for
four months. Haematological examinations (RBC, total and differential
leucocyte, platelet, haematocrit and haemoglobin concentration), blood
sugar, as well as growth and food consumption data indicated no
significant effects attributable to 2,6-dichloro-4-nitroaniline at any
of the dose levels or treatments (Evans et al., 1963).
Groups of rats were fed 2,6-dichloro-4-nitroaniline (either
technical or recrystallized material) in the diet for six months.
Groups of 15 males and 15 females were fed 30 and 300 ppm, groups of
10 males and 10 females were fed 3000 ppm of either the technical or
pure material; and groups of 25 males and 25 females were fed a
control diet. At 3000 ppm in the diet, growth of both males and
females fed technical 2,6-dichloro-4-nitroaniline was impaired with
only the males fed the purified material showing a slight reduction in
growth. In both high level groups livers were enlarged at 3000 ppm.
There was no effect on haematology or on tissues and organs. There was
no sign of damage when the tissues were examined microscopically. No
effects were noted at 300 ppm over the six month period (Lessel,
1974d).
Groups of rats (5 males and 5 females per group) were
administered 2,6-dichloro-4-nitroaniline orally at doses of 0, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth of males was
reduced at 350 mg/kg. At both doses, liver enlargement and
histological changes were noted. There was no apparent effect on blood
parameters or on the kidneys when examined at the termination of the
experiment (Lessel, 1974c).
Groups of rats (10 males and 10 females per group, 20 of each sex
were used in the controls) were administered
2,6-dichloro-4-nitroaniline orally at dose levels of 0, 35, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth depression was
observed in both males and females at the highest dose level. Growth
depression was also noted at the intermediate level in males only.
Liver enlargement was again observed at 140 mg/kg. No effects were
noted at 35 mg/kg. Microscopic examination revealed the presence of
enlarged liver cells with increased vacuolization especially at the
outer lobes. Some animals were maintained for two weeks after the
conclusion of the treatment. After this two week period, liver size
was normal in all but the highest male dosage level. Liver hypertrophy
caused by repeated short term dosing is apparently reversible within a
two week period on cessation of treatment. In this study daily acute
administration 35 mg/kg was observed to have no effect on the rat
(Lessel, 1974c).
Because of the known effect of 4-nitroaniline in inducing
specific blood dyscrasias, subacute feeding experiments were performed
to compare 2,6-dichloro-4-nitroaniline with this material. Groups of
weanling rats (5 males and 5 females/group) were administered
2,6-dichloro-4-nitroaniline by gavage at 0 and 400 mg/kg, 5 days/week
for four weeks. 4-Nitro-aniline was administered at 200 and 400 mg/kg
to two other comparable groups over the same interval. In a second
experiment, groups of male weanling rats (6 rats per group) were
administered 2,6-dichloro-4-nitroaniline by oral gavage at 0 and 400
mg/kg and 4-nitroaniline at 200 and 400 mg/kg, twice daily, 5
days/week, for two weeks. With 2,6-dichloro-4-nitroaniline at 400
mg/kg haematology was normal - no Heinz bodies were detected and the
reticulocyte count was normal. At the 800 mg/kg dose there was a
slight weight loss. The red blood cell and haemoglobin counts were
normal while lymphocyte counts were slightly reduced. It was noted at
the conclusion of the study that there was no effect of this compound
on the spleen. In contrast, 4-nitroaniline had definitive effects on
growth at 200 mg/kg per day. Heinz bodies were identified in the blood
and the reticulocyte count was greatly elevated (marked
reticulocytosis). Bone marrow was not affected. At 200 mg/kg (2x/day)
there was a reduction of growth, reduced RBC count (with polychromasia
and nucleation) accompanied by an enlarged spleen. These effects were
more pronounced at the higher dose where, in addition, a haemoglobin
was reduced and the lymphocyte count greatly increased. At high
levels, although 2,6-dichloro-4-nitroaniline caused lymphopenia, the
hemotoxic effects normally associated with 4-nitroaniline were not
observed (Lessel, 1974b).
Mortality was observed when rats were administered
2,6-dichloro-4-nitroaniline at 1000 mg/kg. No mortality was noted when
400 mg/kg was administered for three months. Liver and kidney changes
were observed with light and electron microscopic examinations
(Serrone, 1967).
Dog
Groups of dogs (8 males and 8 females per group) were fed
2,6-dichloro-4-nitroaniline in the dry diet at levels of 0, 20, 100
and 3000 ppm for two years. One female dog at 3000 ppm died at 74
weeks. This death war attributable to the presence of
2,6-dichloro-4-nitroaniline in the diet. One male control dog lost
considerable weight but survived to the end of the experiment. No
compound-related changes in behaviour, food consumption, or growth
were observed. A watery lacrimation was noted for all dogs at 3000 ppm
which persisted during the entire testing interval. A yellowing of the
sclera, mucous membranes, and abdominal skin was noted at the high
level of feeding. The dog that died showed a picture of haemolytic
anemia prior to death (reduced haemoglobin, immature erythrocytes,
polychromophylic macrocytes, increased leucocyte count and increased
M:E ratio in the bone marrow). At 3000 ppm, clinical chemistry was
altered in both males and females with an elevation observed in the
activities of the SGOT and SGPT enzymes, a reduced blood protein,
increased prothrombin time, BUN, BSP and urinary albumin content. At
the conclusion of the study, gross and microscopic examination
revealed an increase in liver weight accompanied by histological
changes at 3000 ppm in the diet. Histological changes in the animals
fed 3000 ppm in the diet included irregular hepatic cell size, hepatic
cell hypertrophy and increased pigmentation of hepatic cells and of
liver macrophages. Slight changes at 100 ppm were noted in two dogs. A
no-effect level in this study is estimated to be between 100 and 3000
ppm in the dry diet (Woodard et al., 1964).
Monkey
Daily oral administration to Rhesus monkey at 160 mg/kg was
lethal within three months with a greater effect noted on females than
males. Coloration of monkey urine differed from rat urine suggesting a
difference in metabolism in the two species. Liver and kidney changes
were observed after light and electron microscopic examination.
Centrolobular fatty degeneration was observed. Swelling of
mitochondria with distortion of the cristal was also observed. There
were differences in the comparative effects of
2,6-dichloro-4-nitroaniline on liver metabolizing enzymes of monkey
and rat (Serrone, 1967).
Long-Term Studies
Rat
Groups of rats (35 males and 35 females/group) were fed
2,6-dichloro-4-nitroaniline in the diet at levels of 0, 20, 100 and
3000 ppm for two years. At 100 ppm there was no effect on behaviour,
mortality or growth. At this level all values from treated animals
were comparable to control values. Growth and food consumption of both
males and females was depressed at 3000 ppm. Haematological parameters
(haemoglobin and packed cell volume) were reduced at 3000 ppm. These
haematological changes were noted only after the first year of
treatment. Gross and microscopic examination performed at 13 weeks and
at the conclusion of study showed slightly higher liver weights,
kidney weights, testicular weights and (possibly) thyroid weights at
3000 ppm. The incidence and location of neoplasms in all treatments
did not differ from those in the controls. Histological examination
revealed liver changes at 3000 ppm, characterized by hepatic cell
enlargement, glycogen depletion, increased basophilia of the
cytoplasm, and the presence of necrobiotic hepatic cells. Histological
examination performed at 13 weeks also indicated hepatic cell changes
and slight adrenal cortical atrophy in several animals at 3000 ppm.
The adrenal changes were not noted at 104 weeks. An estimated
no-effect level in this study is between 100 and 3000 ppm in the diet
(Woodard et al., 1964).
Groups of rats (25 males and 25 females/group, Boots-Wistar
strain) were fed 2,6-dichloro-4-nitro-aniline in the diet at
concentrations of 0 and 1000 ppm for two years. There was no effect on
survival, food consumption, growth, haematology, or upon gross and
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular makeup of
liver, kidney, or spleen. The incidence of tumors in the control and
treatment group was similar. From the results of this experiment a
suggested no-effect level is greater than 1000 ppm (Lessel, 1974e).
OBSERVATIONS IN MAN
In a clinical double blind study, two groups of adult males were
administered 2,6-dichloro-4-nitroaniline (20 individuals) or a placebo
(10 individuals) once a day for ninety days.
2,6-Dichloro-4-nitroaniline was administered at a level of 10 mg per
day. Hematological, liver function, and kidney function tests were
performed at various intervals over the course of the study and were
found to be normal. There were no indications that administration of
2,6-dichloro-4-nitroaniline at 10 mg per day to adult males had any
adverse effect (Stough, 1962).
Extensive examinations were made on one industrial worker
occupationally exposed to 2,6-dichloro-4-nitroaniline over a period of
three years. It was reported that for about 60 days per year
considerable inhalation and dermal exposure had occurred. No adverse
effects were observed with the individual (Brooks and Boyack, 1963).
Another investigation in man on the potential ocular problem
associated with 2,6-dichloro-4-nitroaniline was again negative
(Manger, 1972).
COMMENTS
2,6-Dichloro-4-nitroaniline has a low order of toxicity to
mammals, including man. 2,6-Dichloro-4-nitroaniline is rapidly
metabolized in plants, and fragments of the molecule are
reincorporated as natural plant constituents. In mammals,
2,6-dichloro-4-nitroaniline is rapidly absorbed, metabolized and
excreted. Metabolism in mammals results in formation of the
chlorinated phenylenediamine and aminophenol which are conjugated and
excreted. 2,6-Dichloro-4-nitroaniline does not induce
methemoglobinemia as evidenced with 4-nitroaniline.
2,6-Dichloro-4-nitroaniline does not affect reproduction in rodents
and has shown no evidence of being a teratogen under the experimental
protocol used. Following subacute feeding, dogs exposed to sunlight
developed cataracts. Specific experiments with rabbits, rats and
swine did not duplicate these results. Long term feeding studies in
rat and two year feeding studies in dog resulted in growth
retardation accompanied by an increased liver and kidney size at
high levels. No-effect levels based on a two year dog study and short
and long term rat studies formed the basis for allocating a temporary
ADI for man. A short term study in man is reassuring in estimation of
the temporary ADI although no conclusions could be drawn on the
possibility of ocular damage to man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of 2,6-dichloro-4-nitroaniline as a commercial
fungicide is recorded in Canada, France, Holland, Italy, Japan, New
Zealand, South Africa and USA.
It is claimed to be effective against several Basidiomycetes
and Deuteromycetes species of fungi, being fungistatic to the
mycelium and spores of Botrytis cenera (Clark et al., 1960; Clark
and Hams, 1961; Sharples, 1962) and to several Rhizopus fruit rot
fungi (Ogawa et al., 1961, 1962; Cappelini and Stretch, 1962).
It is mostly marketed as 4% or 8% (w/w) dust formulations or as
50% (w/w) wettable powders, alone or mixed with thiram (TMTD) or
pentachloronitrobenzene. Smoke formulations containing 40% dicloran
are also available.
Pre-harvest treatments recommended by the manufacturers include
soil treatments for lettuce under glass, dusting or spraying of soft
fruits, cotton, leafy vegetables, strawberries, onions, garlic,
tomatoes and ornamentals. Post-harvest dips of peaches, nectarines and
carrots are practised at rates of 750-1000 mg/kg a.i.
Phytotoxicity
Under extreme experimental conditions a "bronzing" discolouration
of lettuce leaves and marginal "off-taste" taints in fruits have
occasionally been found, but none of these effects have ever been
reported in practical use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data on 2,6-dichloro-4-nitroaniline in several fruit and
vegetable crops have been presented by the originating company (The
Boots Company Ltd., 1972). The data derive from field trials and
post-harvest experiments carried out mainly in the USA and UK.
Summaries of residue ranges and experimental conditions extracted from
these data are presented in Tables 2, 3 and 4.
Peaches and apricots
Extensive trials with single or repeated spraying of peaches and
apricots at recommended dosage rates (usually 0.12-0.18% a.i.) and
higher rates have been carried out during the period from 1960 to 1965
(Tables 2 and 4). The initial deposits of 2,6-dichloro-4-nitroaniline
on peaches are generally between 5 and 15 mg/kg with occasional values
up to 25-30 mg/kg. A typical residue was 5 mg/kg after 6 days, 3 mg/kg
after 9 days and 1.5 mg/kg after 14 days. The highest recorded residue
14 days after treatment at a dosage rate of 0.18% was 4.9 mg/kg.
The average half-life of 2,6-dichloro-4-nitroaniline on peaches
has been calculated to be 5 days.
Spraying apricots gave residues very similar to those found in
sprayed peaches (Table 4).
Dusting peaches using 8% a.i. dicloran powders at rates of 3-5 kg
per ha gave maximum initial deposits of 3.7 mg/kg.
A suggested practice of wrapping apricots and peaches in tissues
impregnated with 2,6-dichloro-4-nitroaniline (from 500-3000 mg/kg, in
the wrap) showed a definite transfer of the chemical to the fruits.
Residues from such post harvest application were from 1.6 - 3.5 mg/kg
in peaches and 1.9 - 3.3 mg/kg in apricots (Table 3).
TABLE 2 Residues of dicloran in peaches
Application Number Number Number Range of
(% a.i. w/v or PHI of of of results
kg a.i./ha) (days) applications trials results mg/kg
0.06% 3 3 1 4 0.1-0.3
0.09% 0 1-3 3 9 6.1-16.5
1 1-3 5 9 1.5-11.6
2-3 1-3 2 7 0.2-4.7
7 1 1 1 2.3
0.12% 0 1-3 1 6 10.8-14.0
1 1-2 1 2 3.4-5.5
3 1-3 2 10 0.2-6.3
7 1 1 1 3.2
0.18% 0 2 1 6 6.0-11.4
1 2 1 6 9.2-13.7
2 2 1 6 5.7-13.8
4 2 1 6 4.2-9.4
6 2 1 6 4.5-7.8
9 2 1 6 1.9-6.0
14 2 1 6 0.6-4.9
0.18% 0 3 1 4 18.9-29.5
3 3 1 4 22.4-29.6
0.24% 1 3 1 4 3.0-8.3
3 3 1 4 2.7-11.1
3.1 kg/ha
(8% dust) 0 1 1 2 0.14-0.35
4.5-4.9 kg/ha
(8% dust) 0 1 3 21 0.22-3.7
TABLE 3 Residues in fruits wrapped in tissues impregnated with
dicloran
Fruit Dicloran in wrap mg/kg Dicloran in fruits
Apricot 500 1.9
1000 2.6
2000 3.3
Peach 500 1.6
1000 2.3
2000 3.8
3000 3.5
TABLE 4 Residues of 2.6-dichloro-4-nitroaniline in fruit and vegetables
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Apricots 0.09% 0 1 1 12 1.9-7.9
2 3 1 2 0.9-1.4
4 1-2 1 4 1.4-3.7
11 1 1 2 0.1-0.7
Apricots 0.12% 0 1 1 1 10.1
1 1 1 1 8.0
7 1 1 1 3.8
Cherry 0.12% 0 1 1 1 10.3
1 1 2 5 6.4-14.8
7 3 1 1 4.9
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
0.24% 1 1 1 2 0.7-11.2
7 1 1 1 2.9
0.36% 1 5 1 2 2.7-3.1
1200 ppm post-harvest 6 21 1.5-6.4
1200 ppm post-harvest
and washing 1 2 0.3-0.3
1350 ppm post-harvest 1 2 10.2-13.7
1800 ppm post-harvest 1 2 7.7-8.4
Grapes 1.7 kg/ha
(dust) 0 2-4 2 6 0.2-3.9
7 2 1 2 0.3-1.1
2.3-3.3
kg/ha (dust) 1 4 1 4 1.1-1.3
7 1-2 2 4 0.2-1.1
1000 ppm post-harvest dip 1 4 0.6-5.1
Plums
(dried) 1000 ppm post-harvest dip 1 3 1.0-4.7*
post-harvest dust 1 3 2.0-8.1
0.12% 1 1 1 1 2.5
Strawberry 0.16-0.18% 1 4 1 1 0.32
3 1 1 2 4.0-5.7
9-11 3-4 2 4 0.2-1.6
0.24% 1 3 1 2 0.9-1.7
Blackberry 1.1 kg/ha
(spray) 4-11 1 1 2 1.2-2.3
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
1.8 kg/ha
(dust) 4-11 1 1 2 <0.05-0.3
Currants 0.11-0.15% 33 4 1 3 1.7-3.3
Raspberry 0.15-0.18% 5 4 2 2 17.0-20.2
9-10 4-5 2 2 2.2-11.8
13-14 3-4 2 2 2.6-7.5
0.36% 5 4 1 1 39.0
10 4 1 1 28.4
14 4 1 1 16.3
Carrots 900- post-harvest dip
1000 ppm (stored 0-3 days) 2 12 3.6-9.4
peeled 1 1 2.1
canned 1 7 <0.05-0.2
Lettuce 2.2 kg/ha 0 2 1 4 50.1 (average)
7 2 1 9 18.0 "
RL50 approx 14 2 1 9 3.0 "
3-4 days
21 2 1 9 2.4 "
28 2 1 8 0.8 "
35 2 1 8 0.04 "
1.3 g/m2
(dust) 120 1 1 1 0.1
1.3 g/m2 (soil
treatment;
pre-planting) 114-140 1 3 5 0.2-2.1
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Gherkins
(indoor) 0.05% 0 1 1 1 5.5
2 1 1 1 1.1
4 1 1 1 0.6
5 1 1 1 0.35
Tomato
(indoor) 0.1% 2 1 1 1 0.25
4 1 1 1 0.2
6 1 1 1 0.04
Beans,
French 3 kg/ha 12 4 1 2 1.3-1.9
20 2 1 2 0.8-1.5
* Loss by drying.
Cherries, grapes and plums
The residue levels of 2,6-dichloro-4-nitroaniline from
recommended uses on these fruits are similar to those in peaches and
apricots (Table 4).
After dipping procedures or post-harvest dusting of plums,
dicloran was retained on the fruits at levels of 4.7 and 8.1 mg/kg
respectively. These residues were reduced to 1.0 and 2.0 mg/kg
respectively, when the fruits were subjected to an 8 day air-drying
period at ambient temperature.
A post-harvest treatment of cherries by spraying on the packing
line with 1200-1800 ppm suspensions gave residues of
2,6-dichloro-4-nitroaniline from 1.5 - 13.7 mg/kg. In storage
experiments these residues were reduced at a rate corresponding to a
half-life of 11 days at 20°C.
Small fruits and berries
Repeated spraying of strawberries at different locations (0.06
- 0.24% a.i.) have been reported to give maximum initial deposits of
dicloran of 9 mg/kg. However, residues at harvest after a 9 - 11 day
interval from the last application were generally well below 1 mg/kg,
with an occasional individual sample at 1.6 mg/kg. Among the berries,
raspberries in some cases showed higher residues. After application of
0.36% a.i. the initial residue was 39 mg/kg which was reduced to 16.3
mg/kg at 14 days. At lower application rates, residues were
proportionately lower.
Experiments with other berries (blackberries, currants and
boysenberries) generally gave residues below 5 mg/kg.
Lettuce
Roburn (1960) followed the disappearance of
2,6-dichloro-4-nitroaniline after post-planting treatments of lettuce
plants with 1/4 oz of 4% dust per sq. yd. and found a reduction in
deposits greater than that due to growth dilution. Volatilization and
chemical degradation were suggested as contributing factors.
The half-life values decreased progressively in these trials,
being about 3-5 days during the last weeks of the growing season. A
three weeks pre-harvest interval for dicloran treatments of lettuce is
recommended on this basis in the United Kingdom.
Later experiments by Boyack and Boot (1962 a, b, c) confirmed
Roburn's findings, indicating half-life values of 3-4 days on
greenhouse lettuce with average residues of 2.4 mg/kg after an
interval of 21 days (Table 4).
Uptake of 2,6-dichloro-4-nitroaniline through the roots of
lettuce plants after pre-planting soil treatments has been
demonstrated by Boyack and Boot (1962a), Lemin and co-workers (Lemin,
1963, 1965; Moe and Lemin, 1963a, 1964) and Groves and Chough (1970).
Residues from such practices at the time of harvest will be near or
below the detection limit, i.e. less than 0.05-0.1 mg/kg.
In recent experiments in the Netherlands lettuces have been
treated both by a pre-planting soil application and by dusting the
young plants immediately after planting. (Pieters, 1974). Residues in
these experiments ranged from 0.1 - 2.1 mg/kg (Table 4).
Carrots
Post-harvest dipping in 900-1000 ppm suspensions caused residues
of 3.6-9.4 mg/kg at 0-3 days. After storage for 5 months the remaining
residues were 2.1-3.7 mg/kg.
Other vegetables
Residue data on a few other vegetable crops, e.g. tomatoes,
gherkins and French beans, mostly grown under glasshouse conditions,
are available (Pieters, 1974). The data are insufficient to evaluate
quantitatively the rates of dissipation, but residues are generally
low at the time of harvest, i.e. from a few days to 1-2 weeks after
treatment (Table 4).
FATE OF RESIDUES
In animals
The metabolism of 2,6-dichloro-4-nitroaniline by the rat, mouse
and to a limited extent man, has been described above under
"Biotransformation". No such experimental evidence is available on
livestock animals, nor on the excretion of dicloran or its metabolites
in milk.
In plants
Studies by Lemin (1965), Lemin et al. (1963) and Moe and Lemin
(1963a, 1964) have shown that 2,6-dichloro-4-nitroaniline is absorbed
by plant roots and translocated to the plant material in tomato and
lettuce. These experiments include uptake from both nutrient solutions
and treated soils.
Application of 14C-labelled 2,6-dichloro-4-nitroaniline gave
evidence of rapid degradation into polar metabolites and 14C was
found in carbohydrate constituents of the plant tissue, presumably
owing to the incorporation of degradation fragments. Transitional
metabolites such as dechlorinated components, reduction products or
2,6-dichloro-p-phenylene-diamine were not detected.
Amino acids, chlorophyll or uronic acid did not contain
radioactivity. Neither could 2,6-dichloro-4-aminophenol, known to be
formed by animal metabolism (see previous section), be found in plant
tissues.
Exhaustive extractions of peaches 13 days after treatment with
14C-labelled 2,6-dichloro-4-nitroaniline revealed (in addition to
unchanged parent compound) 14C-labelled phenylalanine and labelled
flavonoid glycosides (Moe and Lemin, 1963b). As in the above
experiments, no transitional metabolites could be traced.
Groves and Chough (1970) report rapid absorption of dicloran and
incorporation of fragments into tissue constituents by a number of
plants.
In processing and storage
Washing or canning processes have been shown to reduce residues
of 2,6-dichloro-4-nitroaniline considerably (Boots Company Ltd.,
1972). On lettuce, for example, residues of 18-25 mg/kg still
remaining two weeks after the last application were reduced to 5.6-6.0
by washing with water (Roburn, 1958). In peaches, freshly treated with
2,6-dichloro-4-nitroaniline, the residues which still remained after
an industrial canning procedure were below 0.1 mg/kg.
Carrots treated by post-harvest dipping showed only moderate
reduction of the residues by peeling, from 3.7 to 2.1 mg/kg,
indicating a significant penetration into the inner parts. A
subsequent canning procedure, however, reduced the levels to 0.1-0.2
mg/kg. Storage of dipped carrots for 5 months at 40°F gave a decrease
in the residue of about 25%.
In soils
2,6-Dichloro-4-nitroaniline is generally regarded as being
relatively stable in soils at field moisture capacity. Its
persistence, however, is greatly affected by conditioning factors such
as soil composition, water content, microculture etc. (Wang and
Broadbent, 1973). The absorption of the chemical from soils by oats
was found by Groves and Chough (1970) and Wang (1972) to be inversely
related to the clay and organic matter content of the soils,
suggesting a binding of the chemical to these soil constituents. Later
exhaustive extraction experiments showed that a reversible binding
probably occurs (Groves and Chough, 1971).
The same authors (Groves and Chough, 1970, 1971) showed that
14C-labelled dicloran can be metabolized by soil organisms which
degrade the compound to 14C-carbon dioxide and other volatile
compounds. The rate of microbial breakdown could be increased by
repeated applications of dicloran to the same soil or by incubation of
the soil with dicloran. A culture of rod-shaped bacteria was isolated
and found active in the decomposition of the fungicide. Microbial
breakdown is also held responsible for the rapid degradation of DCNA
which is seen in soil under flooded conditions (Wang and Broadbent,
1973).
Van Alfen and Kosuge (1974) have identified both
2,6-dichloro-p-phenylenediamine and its acetylated derivative,
4-amino-3,5-dichloroacetanilide, in cultures of Pseudomonas cepacia
and Escherichia coli B. In soil and through the action of
horseradish peroxidase, the phenylenediamine was converted to one of
three isomers of the azine resulting from oxidative dimerization (Van
Alfen, 1973).
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Market sample surveys carried out in the Netherlands in 1973 on
domestically grown fruit and vegetables showed that residues of
2,6-dichloro-4-nitroaniline were frequently present in some crops
(Table 5). In vegetables such as endive, lettuce and chicory sprouts
about 50-80% of samples were positive with individual residues up to 8
mg/kg. Crops such as cucumbers, tomatoes and paprika were less
frequently positive (3-25%). At the time of this survey, the tolerance
for vegetables in the Netherlands was 10 mg/kg.
TABLE 5 Dicloran residues in marketed vegetables (Netherlands, 1973)
Dicloran
residues,
mg/kg number of samples
(range)
Cucumber Endive Lettuce Paprika Tomato Chicory
not detectable 31 34 136 12 30 68
0.01 - 0.1 1 16 139 2 4 50
0.1 - 0.3 - 7 126 2 - 21
0.3 - 1.0 - 8 180 - - 3
1.0 - 3.0 - 4 123 - - 1
3.0 - 10.0 - 1 35 - - -
Total number 32 70 739 16 34 143
[text missing]
pre-harvest treatments on fruits, vegetables and ornamentals and
post-harvest treatments on peaches, nectarines, cherries and carrots.
It is registered in several countries, usually as 4 or 8% (w/w) dusts
or as 50% (w/w) wettable powders.
The technical grade typically contains about 96%
2,6-dichloro-4-nitroaniline. The remaining parts consist of chemically
related compounds and impurities which have been identified and
quantified.
Concentrations and rates of application vary, depending on the
crop and method of application. Normal spraying concentrations are
0.05% to 0.18% applied at rates of 2-5 kg a.i. per ha. Post-harvest
dips are practised at rates of 1000 - 2000 mg per litre.
The residue data available are mainly obtained from the USA and
UK, in a few cases supplemented from other countries. Most of the data
derive from field trials or experiments under practical conditions
likely to represent the results of good agricultural practice.
2,6-dichloro-4-nitroaniline is a non-systemic compound of
relatively low persistence. The dissipation of the compound on fruits
and vegetables is often faster than can be explained by normal
weathering or growth dilution. Half-life periods of 11 days have been
found for residues during storage of post-harvest treated cherries.
2,6-dichloro-4-nitroaniline is absorbed by plant roots from the
soil. The fate of residues in plant material has been followed with
the radio-labelled compound, which indicated a rapid degradation into
polar metabolites and 14CO2 followed by incorporation of 14C into
normal metabolic plant constituents. No indications of transitional
metabolites from such degradation have been found.
Similar degradation of 2,6-dichloro-4-nitroaniline occurs in
soils, although in this case microbial breakdown may also produce
smaller amounts of reduction products and acetylated derivatives of
the parent compound.
Studies were available on the metabolic fate of residues in rats
indicating a fairly rapid process of absorption, metabolism and
excretion, especially as aminophenol and phenylenediamine derivatives,
in the urine. There was no evidence of tissue storage in these
experiments. Preliminary studies in man suggest a metabolic pattern
similar to that of rats. No information, however, has been recorded on
metabolism in livestock animals or on transfer of residues into meat
or milk.
METHODS OF RESIDUE ANALYSIS
A colorimetric method of residue analysis, based on the
development of a yellow colour characteristic of some mononitro
aromatic compounds in the presence of strong alkali and acetone, is
described by Kilgore et al. (1962). Extraction is with benzene,
followed by clean-up on Florisil alone or in combination with an
acetonitrile-petroleum ether partitioning procedure. Blank values for
fruits were about 0.01-0.02 mg/kg and recoveries in the range 75 -
100% (average 86%). The colorimetric method described by Groves and
Chough (1966) and Heagy (1969) has a similar sensitivity, based on
diazotization and coupling to N-(1-naphthyl)ethylenediamine has a
similar sensitivity.
An alternative method developed by Roburn (1961) utilizes the
reduction of 2,6-dichloro-4-nitroaniline by zinc and hydrochloric acid
to the corresponding 2,6-dichloro-p-phenylenediamine which, in the
presence of aniline, can be oxidized to produce an intense blue
colour. Less than 2 µg can be detected by this method, corresponding
to 0.05 mg/kg in fruit and 0.2 mg/kg in lettuce. Recovery of surface
deposits was approximately 100%, but for macerated samples it was only
71%. Chloronitroanilines in general give similar reactions but a
series of other pesticide chemicals did not interfere, indicating a
fair degree of specificity of the method.
A thin-layer chromatographic spot test sensitive to 0.5 µg of
2,6-dichloro-4-nitroaniline is given by Groves et al. (1966) based on
their colour reaction mentioned above.
Gas-chromatographic methods for the determination of
2,6-dichloro-4-nitroaniline have been developed by Beckmann and
Bevenue (1962) and Kilgore (1964) using electron capture and
microcoulometric detectors, in combination with the Kilgore extraction
procedure already mentioned. Recoveries of 85-105% were established by
Brewerton et al. (1967) for the analysis of dicloran in soils, fruit
and vegetables down to the 0.1 mg/kg level by the GLC/EC technique.
These methods could be adapted for regulatory purposes as part of a
multi-residue procedure.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance
Country Crop mg/kg
Australia Beans, lettuce, stone fruits, tomatoes 20
Belgium Fruit and vegetables (exc. potatoes) 10
Canada Apricots, nectarines, peaches
(pre-harvest and post-harvest),
sweet cherries pre-harvest and
post-harvest), snap beans 20
Strawberries, raspberries 15
Celery, grapes, lettuce, rhubarb,
sweet potatoes 10
Carrots, cucumbers, garlic, onions,
plums, tomatoes 5
Germany All fruits and vegetables 0.1
Tolerance
Country Crop mg/kg
Italy General 10
Switzerland Lettuce (tentatively) 0.5
Netherlands Lettuce, endive 3
Chicory (sprouts) 1
Cucumbers, gherkins, melons, bell
peppers, tomatoes 0.3
Other vegetables 0.1
USA Apricots, nectarines (pre-harvest and
post-harvest), peaches (pre-harvest
and post-harvest), sweet cherries
(pre-harvest and post-harvest), snap
beans 20
Blackberries, boysenberries, celery,
plums (fresh prunes) (pre-harvest and
post-harvest), raspberries 15
Carrots (pre-harvest and post-harvest),
grapes lettuce, rhubarb, sweet
potatoes (pre-harvest and post-harvest) 10
Cucumbers, garlic, onions, tomatoes 5
Potatoes 0.25
Cotton seed 0.1
APPRAISAL
2,6-dichloro-4-nitroaniline is a fungicide active against the
mycelium and spores of Botrytis cinerea, B. sclerotinia and other
fungi, including several Rhizopus fruit rots.
Practical use patterns comprise foliar applications and soil
treatments under indoor and outdoor growing conditions.
The residue data from supervised trials on several crops were
considered sufficiently extensive to enable recommendations to be
made, including some for post-harvest treatments.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for specific determination.
These methods are suitable for regulatory purposes.
RECOMMENDATIONS
Temporary tolerances are recommended for
2,6-dichloro-4-nitroaniline in the following commodities at the
levels indicated. The levels are not likely to be exceeded when
2,6-dichloro-4-nitroaniline is applied in accordance with good
agricultural practice including both pre-harvest and post-harvest
applications and taking into account that preharvest intervals may
apply for certain treatments.
TEMPORARY TOLERANCES
Limit Basis on which recommendations
Crop (mg/kg) are made
Cherries 15 pre and post-harvest
Peaches 15 pre and post-harvest
Apricots 10 pre and post-harvest
Carrot 10 post-harvest only
Grapes 10 pre and post-harvest
Lettuce 10 pre-harvest only
Plums 10 pre and post-harvest
Raspberry 10 pre-harvest only
Strawberry 10 pre-harvest only
Blackberry 5 pre-harvest only
Currant (red, black
and white) 5 pre-harvest only
Beans (French) 2 pre-harvest only
Gherkins 0.5 pre-harvest (indoor) only
Tomato 0.5 pre-harvest only
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further studies on the ocular disturbance observed in dogs to
confirm and clarify this effect.
2. Information on fate in livestock animals in so far as plant
material containing residues may be fed to animals.
DESIRABLE
1. Effect on hepatic microsome systems in several species.
2. Further observations in man.
3. Further information on fate of residues during storage, transport
and processing of fruit and vegetables.
4. Information on transfer of residues from grapes to wine and on
possible influence on wine processing.
5. Further information on soil residues and their possible uptake
into subsequent crops.
6. Further data to clarify inconsistencies in the residue levels
found in different berries.
REFERENCES
Anonymous. (1974) "Somers test" in rabbits. Technical summary reports
submitted to WHO by UpJohn Co. (Unpublished)
Beckmann, H. and Bevenue, A. (1962) Pesticide residue analysis Gas
chromatographic analysis of 2,6-dichloro-4-nitroaniline. J. Food Sci.,
27:602-604.
Bernstein, H.N., Curtis, J., Earl, F.L. and Kuwabara, T. (1970)
Phototoxic corneal and lens opacities in dogs receiving a fungicide,
2,6-dichloro-4-nitroaniline. Archs Ophthal. (Chicago), 83:336-348.
Boots Co. Ltd. (1972) Dicloran. Registration Document Vol. I, II, III
and VI. Reports submitted by The Boots Company Ltd. Agricultural
Research, Nottingham. (Unpublished)
Boyack, G. and Boot, D. (1962a) Assay for 2,6-dichloro-4-nitroaniline
in lettuce grown in treated soil under glass. Dicloran, Registration
document from The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G, and Boot, D. (1962b) Assay for 2,6-dichloro-4-nitroaniline
on sprayed greenhouse lettuce. Dicloran, Registration document from
The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G. and Boot, D. (1962c) Residue of 2,6-dichloro-4-nitroaniline
(DCNA) on lettuce dusted with Botran. Dicloran, Registration document
from The Boots Co. Ltd. Vol. II, Appendix 26.
Brewerton, H.V., Clark, P.J. and McGrath, H.J.W. (1967) Dicloran
analysis by gas chromatography. New Zealand J. Sci., 10:124-127.
Brooks, B. and Boyack, G. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Report submitted by
The Upjohn Co. (Unpublished)
Cappellini, R.A. and Stretch, A.W. (1962) Control of post-harvest
decays of peaches. Pl. Dis. Reptr., 46:31-33.
Clark, N.G., Hams, A.F., Higgons, D.J. and Stevenson, H.A. (1960) A
new fungicide active against Botrytis spp. Chemy Ind., 1960:572-573.
Clark, N.G. and Hams, A.F. (1961) Antifungal activity of substituted
nitroanilines and related compounds. J. Sci. Food Agric., 12:751-757.
Curtis, J., Bernstein, H.N., Earl, F.L. and Smalley, H., Jr. (1968)
Corneal and lens opacities in dogs treated with
2,6-dichloro-4-nitroaniline. Toxic. appl. Pharmac., 12:305.
Earl, F.L., Curtis, J., Bernstein, H.N. and Smalley, H., Jr. (1971)
Ocular effects in dogs and pigs treated with dichloran. Food Cosmet.
Toxic., 9:819-828.
Eberts, F.S. (1965) Fate of Botran (2,6-dichloro-4-nitroaniline) in
rat and man. Dicloran, Registration Document, Vol. IV, Appendix 54.
Boots Co. Ltd. (Unpublished). Botran Symposium, Augusta, Mich. 1965.
Cited in Chemical Abstracts 1968, 68:abstract 11908g
EPA. (1974) Data from EPA Laboratories. (Unpublished)
Evans, J., Mengel, G. and Bostwick, L. (1963) Botran(R) (U-2069)
Effect of oral administration. Final report, four months study. Report
submitted by the Upjohn Co. (Unpublished)
Feenstra, E. (1961) Report submitted by The Upjohn Co. (Unpublished)
Groves, K. and Chough, K.S. (1966) Determination of small quantities
of 2,6-dichloro-4-nitroaniline (dicloran). J. agr. Food Chem.,
14:668-669.
Groves, K. and Chough, K.S. (1970) Fate of the fungicide
2,6-dichloro-4-nitroaniline (DCNA) in plants and soils. J. agr. Food
Chem., 18:1127-1128.
Groves, K. and Chough, K.S. (1971) Extraction of
3-amino-1,2,4-triazole (Amitrole) and 2,6-dichloro-4-nitroaniline
(DCNA) from soils. J. agr. Food Chem., 19:840-841.
Gurd, P.R. (1974) Methaemoglobin formation in the cat. Report from
Boots Pure Drug Company Limited. Submitted to WHO by the Boots Co.
Ltd. (Unpublished)
Heagy, J.A. (1969) Colorimetric determination of
2,6-dichloro-4-nitroaniline (dicloran) residues in foods. J. Ass. off.
analyt. Chem., 52:797-799.
Horn, J. (1961) U-2069 - Acute inhalation toxicity. Study from Woodard
Research Corporation submitted by The Boots Co., Ltd. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: A preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Johnston, R. and Schwikert, R. (1961a) Skin irritation in rabbits.
Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1961b) Dichloran - eye irritation in
rabbits. Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1963) Skin sensitization in guinea
pigs. Report from The Upjohn Co. (Unpublished)
Kilgore, W.W. (1964) 2,6-dichloro-4-nitroaniline. Analytical methods
for pesticides, plant growth regulators and food additives. Gunter
Zweig (Editor). Vol. III. p. 61-68.
Kilgore, W.W., Cheng, K.W. and Ogawa, J.M. (1962) Extraction and
determination of 2,6-dichloro-4-nitroaniline in processed fruits. J.
agr. Food Chem., 10:399-401.
Lemin, A.J. (1965) Translocation and metabolism of
2,6-dichloro-4-nitroaniline by lettuce and tomato. J. agr. Food Chem.,
13:557-560.
Lemin, A.J. Moe, L.D. and Smith, A.H. (1963) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce. Dicloran, Registration
document from the Boots Co. Ltd. Vol. II, Appendix 34 A.
Lessel, B. (1974a) Experimental data on toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974b) Effect on blood and blood forming tissues. Report
from The Boots Co. Ltd. (Unpublished)
Lessel, B. (1974c) Short term (4-week) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974d) Chronic (6 month) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974e) Two year feeding trial in rats on dicloran
(2,6-dichloro-4-nitroaniline). Report submitted by the Boots Co. Ltd.
(Unpublished)
Lobdell, B. and Johnson, C. (1965) U-2069 - Effect on reproduction
capacity through three generations in the rat. Report from Woodard
Research Corporation submitted by The Boots Co. Ltd. (Unpublished)
Manger, W.H. (1972) Use and handling of dicloran formulations and
influence on human eyesight. Report from Biological Laboratory,
Aseptafabrick B.V. Delft, Netherlands. (Unpublished)
Maté, C., Ryan, A.J. and Wright, S.E. (1967) Metabolism of some
4-nitroaniline derivatives in the rat. Food Cosmet. Toxic., 5:657-663.
Moe, L.D. and Lemin, A.J. (1963a) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part II, dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 B.
Moe, L.D. and Lemin, A.J. (1963b) The metabolism of
2,6-dichloro-4-nitroaniline-C14 in peaches. Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 35.
Moe, L.D. and Lemin, A.J. (1964) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part III. Dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 C.
Ogawa, J.M., Lyda, S.D. and Weber, D.J. (1961)
2,6-dichloro-4-nitroaniline effective against Rhizopus fruit rot
of sweet cherries. Pl. Dis. Reptr., 45:636-638.
Ogawa, J.M., Mathre, J.M., Weber, D.J. and Lyda, S.D. (1963) Effects
of 2,6-dichloro-4-nitroaniline on Rhizopus species and its
comparison with other fungicides on control of Rhizopus rot of
peaches. Phytopathology, 53:950-955.
Pieters, A.J. (1974) Information on dicloran residues in The
Netherlands submitted to the Joint Meeting 8 November 1974.
(Unpublished)
Roburn, J. (1958) Experiments to demonstrate the effect of washing
lettuce treated with dicloran ("Alisan"). Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 33.
Roburn, J. (1960) Allisan residue trials on lettuce, February to April
1960. Lenton memo nr. 5. Lenton Experimental Station, Nottingham.
(Unpublished)
Roburn, J. (1961) Colorimetric determination of
2,6-dichloro-4-nitroaniline in plants and soil. J. Sci. Fd. Agric.,
12:766-772.
Serrone, D., Pakdaman, P., Stern, A. and Coulston F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
Rhesus monkey. Tox. appl. Pharmacol., 10:404.
Sharples, R.O. (1962) The fungitoxic effects of dicloran on Botrytis
cinerea. Lenton Experimental Station, Nottingham. (Unpublished)
Stough, A.R. (1962) Results of Botran(R) clinical study. Results from
The Upjohn Co. submitted by The Boots Co. Ltd. (Unpublished)
Van Alfen, N.K. (1972) Microbial metabolism of the fungicide
2,6-dichloro-4-nitroaniline in soil by micro-organisms. Dissertation,
Dept. of Plant Pathology, Univ. of California, Davis.
Van Alfen, N.K. and Kosuge, T. (1974) Microbial metabolism of the
fungicide 2,6-dichloro-4-nitroaniline. J. agr. Food Chem., 22:221-224.
Wang, C.H. (1972) The effect of soil properties on losses of two
chloronitrobenzene fungicides from soils. Dissertation, Dept. of Soil
Sciences, Univ. of California, Davis.
Wang, C.H. and Broadbent, F.E. (1973) Effect of soil treatments on
losses of two chloronitrobenzene fungicides. J. environ. Quality, 2
(4):511-515.
Woodard, M., Cockrell, K. and Woodard, G. (1964) Safety evaluation by
oral administration to rats and dogs for 104 weeks. Report from
Woodard Research Corporation submitted by The Boots Co. Ltd.
(Unpublished)
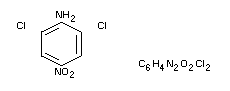 Other information on identity and properties
Molecular weight: 207
State: Yellow, crystalline solid with practically
no odour
Melting point: 192-194°C
Vapour pressure: 1.2 x 10-6 mm Hg (20°C)
Solubility: Water7 mg/l
Cyclohexane 0.006 g/100 ml
Petroleum ether 0.02 g/100 ml
Carbon tetrachloride 0.06g/100 ml
Ethanol 0.29 g/100 ml
Benzene 0.46 g/100 ml
Glacial acetic acid 8.80 g/100 ml
Chloroform 1.2 g/100 ml
Acetone 3.4 g/100 ml
Stability: Stable to hydrolysis and relatively stable
to oxidation. Readily reduced to
phenylenediamine by zinc and acid. Stable
to light and heat.
Composition and
purity: The technical product contains
2,6-dichloro-4-nitroaniline not less than
96% (on dry weight basis);
2,4-dichloro-6-nitroaniline not more than
2%; 2-chloro-4-nitroaniline not more than
l%; Chloranil not more than 1%;
P-nitroaniline not more than 0.1%; Loss on
drying not more than 1% (at 60°C, <5 mm
Hg); Sulphated ash not more than 0.25%;
Sodium chlorate not more than 100 ppm.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
2,6-Dichloro-4-nitroaniline-14C administered to three male human
subjects at a level of 50 mg was found to be rapidly absorbed and
excreted. Excretion was somewhat slower than measured in the rat with
the major quantity of material being excreted within 1.5 days.
Preliminary studies suggest that 2,6-dichloro-4-nitroaniline
metabolites are similar to those obtained from the rat. Approximately
85% of the total urinary excretion from the rat was found to be of
2,6-dichloro-4 hydroxy aniline sulfate.
2,6-Dichloro-4-nitroaniline-14C administered to male rats at a
dosage of either 1.7 mg/kg or 8 mg/kg orally was rapidly excreted
from the body. Urinary excretion accounted for approximately 90% of
the recovered material with the remainder being located in the
faeces. The majority of material (about 90%) was recovered within 48
hours and over half was recovered within 8 hours after treatment.
2,6-Dichloro-4-nitroaniline was not observed in any body tissues with
the exception of small quantities detected in the G.I. tract, urinary
tract, and liver (Eberts, 1965).
2,6-Dichloro-4-nitroaniline administered to rats (ip or orally,
20 mg/kg) was metabolized to the dichloroamimophenol and the
dichlorophenylenediamine derivatives. Following both routes of
administration the majority of material was recovered from the urine
within 24 hours and total recovery was noted within 72 hours. Very
small quantities were obtained in the faeces. In vitro studies using
mouse liver microsomes showed limited conversion of
2,6-dichloro-4-nitroaniline to the same two metabolites (Máté et al.,
1967).
Biotransformations in plants and micro-organisms are discussed
under "Fate of residues".
Effects on enzymes and other biochemical parameters
Following high level subacute oral administration of
2,6-dichloro-4-nitroaniline to rats, an increase of hepatic
demethylase and desulfurase activity was noted. Liver mitochondrial
O2 consumption was also increased in rats. The liver enzymes were
not stimulated in monkey following similar treatment (Serrone, 1967).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A carcinogenic screening using high levels of
2,6-dichloro-4-nitroaniline administered to susceptible mice was
negative. Groups of mice (18 males and 18 females of each of two
hybrid strains) were administered 2,6-dichloro-4-nitroaniline at 215
mg/kg/day for three weeks from day seven after birth. Thereafter for
18 months the mice were fed 603 ppm in the diet, sacrificed and
examined for tumours. 2,6-Dichloro-4-nitroaniline did not cause a
significant increase in tumours (Innes et al., 1969).
Effects on blood and blood forming tissues
Cat
In studies to substantiate the difference between effects of
4-nitroanaline and 2,6-dichloro-4-nitroaniline, a study on the effect
of these materials on methaemoglobinemia in the cat was performed
(Gurd, 1974). After a single oral dose of 2,6-dichloro-4-nitroaniline
(500 mg/kg), no methaemoglobin was noted at any time between 1 and 48
hours after dosing. Administration of 4-nitroaniline at a single dose
of 100 mg/kg resulted in methaemoglobin, observed over the same time
course. In addition, the cats subjected to this experiment were noted
to be cyanotic and have extensive muscle weakness following
4-nitroaniline.
Ocular toxicity
Dog and miniature swine
Dogs have been shown to develop lesions in the cornea and lens
following prolonged oral administration of
2,6-dichloro-4-nitroaniline. It has been suggested that a
photochemical product reaction may be responsible for the lesion as
it occurred only when dogs were exposed to sun light.
Dogs and miniature swine were fed 2,6-dichloro-4-nitroaniline in
the diet at levels of 0, 0.75, 6, 24, 48 and 192 mg/kg/day for periods
of time varying from 50 to 306 days. Corneal opacity appeared in dogs
within 53 to 104 days after administration of 24 or 48 mg/kg, and when
exposed to sunlight. Dogs unexposed to sunlight and those with one eye
sutured closed failed to develop lesions in the unexposed eyes. Dogs
administered 192 mg/kg refused to eat after 38 days and were
administered 2,6-dichloro-4-nitroaniline by capsule. All of these
animals died 49 - 53 days after the study began. Eye lesions were not
detected in this high level group. Several dogs showing eye damage
were maintained for four months after 2,6-dichloro-4-nitroaniline
administration had ceased. Pathological changes seen in the cornea and
lens were not reversible. Administration of
2,6-dichloro-4-nitroaniline at all levels did not appear to affect
miniature swine. Sporadic instances of the presence of Heinz bodies
in blood were observed in both swine and dogs. Administration of
2,6-dichloro-4-nitroaniline as dust or 5% solution directly to the
eyes for three months had no effect on corneal opacity or irritation
of the conjunctiva (Earl et al., 1971; Bernstein et al. 1970; Curtis
et al. 1968).
Special studies on reproduction
Rat
In a standard three generation, two litters per generation,
reproduction study, 2,6-dichloro-4-nitroaniline was administered to
rats (20 males and 20 females per group) at levels of 0 and 100 ppm in
the diet. On the basis of the reproduction parameters examined,
including number of litters, stillbirths, live births, birthweight,
lactation indices, etc. no evidence of an effect of dicloran on
reproduction was indicated (Lobdell and Johnston, 1965).
Male rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 1000, and 2000 ppm for 90 days. The males were mated with
untreated females. There were no differences observed in the number of
litters or in the number of animals born or weaned. Feeding
2,6-dichloro-4-nitroaniline in the diet to male rats resulted in an
increased liver weight at 1000 ppm. An increased kidney weight was
seen at 1000 ppm (EPA, 1974).
Female rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 500, and 1000 ppm for 188 days prior to mating. The rats
were continued on the diet through gestation and lactation. From the
small number of animals in this experiment (10 females/group), it is
difficult to make definitive conclusions concerning the effect on
reproduction of 2,6-dichloro-4-nitroaniline administered to females.
The data suggested a reduced number of pups when the females were fed
a level of 1000 ppm in the diet. There was no apparent effect on
survival of pups, although the mean body weight of pups at 1000 ppm
was slightly reduced. It might be considered that 1000 ppm in the diet
of females for six months might have a slight effect on reproduction.
No effects were seen at 500 ppm (EPA, 1974).
Rabbit
Groups of pregnant New Zealand white rabbits (10, 12, and 14 does
respectively) were fed 2,6-dichloro-4-nitroaniline in the diet at 0,
100, and 1000 ppm from day 8 until day 16 of gestation. In no case was
there evidence of an adverse effect of 2,6-dichloro-4-nitroaniline on
reproduction, affecting either the parents or the offspring (Anonymous
1974).
Sensitization
Rat, guinea pig, rabbit
An examination was made of the potential skin sensitization
properties of 2,6-dichloro-4-nitroaniline in guinea pigs. Ten
subcutaneous injections were administered to male guinea pigs (total
dose 0.95 mg). Two weeks after the last injection a re-injection of
0.05 mg was made. Twenty-four hour readings showed no apparent
sensitization (Johnston and Sweickert, 1963)
Rats, guinea pigs and rabbits were administered
2,6-dichloro-4-nitroaniline via inhalation exposure to an 8% dust
for seven hours. It was estimated that the exposure averaged 0.4
mg/l. No deaths were observed although reddening of the lungs and
pale kidneys were seen in rats and guinea pigs (Horn, 1961).
TABLE 1 Acute toxicity of 2,6-dichloro-4-nitroaniline
LD50
Species Route (mg/kg) References
Rat oral 4000-10 000 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
Ip 1460-5471 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
SC >5000 Lessel, 1974a
Guinea Pig oral 1450 Lessel, 1974a
Mouse oral 1500-2500 Lessel, 1974a
Feenstra, 1961
Ip 2500-8000 Lessel, 1974a
Feenstra, 1961
SC >6000 Lessel, 1974a
dermal >5000 Lessel, 1974a
Cat oral >500 Lessel, 1974a
oral 200 x 7 daily doses Lessel, 1974a
Signs of poisoning in the mouse included defaecation and
urination, depression and lethargy leading to sleep. In rats the same
signs were noted including nasal haemorrhage and paralysis. Death
occurred up to four days after administration of 2,6-dichloro-4-
nitroaniline (Feenstra, 1961). Cyanosis, muscle weakness, and other
typical signs of aniline poisoning were not observed.
Two formulations (8% dust and 50% W.P.) were applied to intact
and abraded skin of rabbits daily for five days. No irritation of skin
was observed (Johnston and Schwikert 1961a). When these materials were
instilled into the conjunctival sac of rabbits, no ocular irritation
was noted (Johnston and Schwikert, 1961b).
Short Term Studies
Rat
Groups of rats (either 10 males and females or 15 males and
females per group) were treated with 2,6-dichloro-4-nitroaniline to
examine potential haematopoietic effects. One series of animals was
administered 2,6-dichloro-4-nitroaniline at levels of 0, 5, 20 and 100
mg/kg/day by gavage for four months. Another group was administered
2,6-dichloro-4-nitroaniline in the diet at levels of 0 and 20 ppm for
four months. Haematological examinations (RBC, total and differential
leucocyte, platelet, haematocrit and haemoglobin concentration), blood
sugar, as well as growth and food consumption data indicated no
significant effects attributable to 2,6-dichloro-4-nitroaniline at any
of the dose levels or treatments (Evans et al., 1963).
Groups of rats were fed 2,6-dichloro-4-nitroaniline (either
technical or recrystallized material) in the diet for six months.
Groups of 15 males and 15 females were fed 30 and 300 ppm, groups of
10 males and 10 females were fed 3000 ppm of either the technical or
pure material; and groups of 25 males and 25 females were fed a
control diet. At 3000 ppm in the diet, growth of both males and
females fed technical 2,6-dichloro-4-nitroaniline was impaired with
only the males fed the purified material showing a slight reduction in
growth. In both high level groups livers were enlarged at 3000 ppm.
There was no effect on haematology or on tissues and organs. There was
no sign of damage when the tissues were examined microscopically. No
effects were noted at 300 ppm over the six month period (Lessel,
1974d).
Groups of rats (5 males and 5 females per group) were
administered 2,6-dichloro-4-nitroaniline orally at doses of 0, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth of males was
reduced at 350 mg/kg. At both doses, liver enlargement and
histological changes were noted. There was no apparent effect on blood
parameters or on the kidneys when examined at the termination of the
experiment (Lessel, 1974c).
Groups of rats (10 males and 10 females per group, 20 of each sex
were used in the controls) were administered
2,6-dichloro-4-nitroaniline orally at dose levels of 0, 35, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth depression was
observed in both males and females at the highest dose level. Growth
depression was also noted at the intermediate level in males only.
Liver enlargement was again observed at 140 mg/kg. No effects were
noted at 35 mg/kg. Microscopic examination revealed the presence of
enlarged liver cells with increased vacuolization especially at the
outer lobes. Some animals were maintained for two weeks after the
conclusion of the treatment. After this two week period, liver size
was normal in all but the highest male dosage level. Liver hypertrophy
caused by repeated short term dosing is apparently reversible within a
two week period on cessation of treatment. In this study daily acute
administration 35 mg/kg was observed to have no effect on the rat
(Lessel, 1974c).
Because of the known effect of 4-nitroaniline in inducing
specific blood dyscrasias, subacute feeding experiments were performed
to compare 2,6-dichloro-4-nitroaniline with this material. Groups of
weanling rats (5 males and 5 females/group) were administered
2,6-dichloro-4-nitroaniline by gavage at 0 and 400 mg/kg, 5 days/week
for four weeks. 4-Nitro-aniline was administered at 200 and 400 mg/kg
to two other comparable groups over the same interval. In a second
experiment, groups of male weanling rats (6 rats per group) were
administered 2,6-dichloro-4-nitroaniline by oral gavage at 0 and 400
mg/kg and 4-nitroaniline at 200 and 400 mg/kg, twice daily, 5
days/week, for two weeks. With 2,6-dichloro-4-nitroaniline at 400
mg/kg haematology was normal - no Heinz bodies were detected and the
reticulocyte count was normal. At the 800 mg/kg dose there was a
slight weight loss. The red blood cell and haemoglobin counts were
normal while lymphocyte counts were slightly reduced. It was noted at
the conclusion of the study that there was no effect of this compound
on the spleen. In contrast, 4-nitroaniline had definitive effects on
growth at 200 mg/kg per day. Heinz bodies were identified in the blood
and the reticulocyte count was greatly elevated (marked
reticulocytosis). Bone marrow was not affected. At 200 mg/kg (2x/day)
there was a reduction of growth, reduced RBC count (with polychromasia
and nucleation) accompanied by an enlarged spleen. These effects were
more pronounced at the higher dose where, in addition, a haemoglobin
was reduced and the lymphocyte count greatly increased. At high
levels, although 2,6-dichloro-4-nitroaniline caused lymphopenia, the
hemotoxic effects normally associated with 4-nitroaniline were not
observed (Lessel, 1974b).
Mortality was observed when rats were administered
2,6-dichloro-4-nitroaniline at 1000 mg/kg. No mortality was noted when
400 mg/kg was administered for three months. Liver and kidney changes
were observed with light and electron microscopic examinations
(Serrone, 1967).
Dog
Groups of dogs (8 males and 8 females per group) were fed
2,6-dichloro-4-nitroaniline in the dry diet at levels of 0, 20, 100
and 3000 ppm for two years. One female dog at 3000 ppm died at 74
weeks. This death war attributable to the presence of
2,6-dichloro-4-nitroaniline in the diet. One male control dog lost
considerable weight but survived to the end of the experiment. No
compound-related changes in behaviour, food consumption, or growth
were observed. A watery lacrimation was noted for all dogs at 3000 ppm
which persisted during the entire testing interval. A yellowing of the
sclera, mucous membranes, and abdominal skin was noted at the high
level of feeding. The dog that died showed a picture of haemolytic
anemia prior to death (reduced haemoglobin, immature erythrocytes,
polychromophylic macrocytes, increased leucocyte count and increased
M:E ratio in the bone marrow). At 3000 ppm, clinical chemistry was
altered in both males and females with an elevation observed in the
activities of the SGOT and SGPT enzymes, a reduced blood protein,
increased prothrombin time, BUN, BSP and urinary albumin content. At
the conclusion of the study, gross and microscopic examination
revealed an increase in liver weight accompanied by histological
changes at 3000 ppm in the diet. Histological changes in the animals
fed 3000 ppm in the diet included irregular hepatic cell size, hepatic
cell hypertrophy and increased pigmentation of hepatic cells and of
liver macrophages. Slight changes at 100 ppm were noted in two dogs. A
no-effect level in this study is estimated to be between 100 and 3000
ppm in the dry diet (Woodard et al., 1964).
Monkey
Daily oral administration to Rhesus monkey at 160 mg/kg was
lethal within three months with a greater effect noted on females than
males. Coloration of monkey urine differed from rat urine suggesting a
difference in metabolism in the two species. Liver and kidney changes
were observed after light and electron microscopic examination.
Centrolobular fatty degeneration was observed. Swelling of
mitochondria with distortion of the cristal was also observed. There
were differences in the comparative effects of
2,6-dichloro-4-nitroaniline on liver metabolizing enzymes of monkey
and rat (Serrone, 1967).
Long-Term Studies
Rat
Groups of rats (35 males and 35 females/group) were fed
2,6-dichloro-4-nitroaniline in the diet at levels of 0, 20, 100 and
3000 ppm for two years. At 100 ppm there was no effect on behaviour,
mortality or growth. At this level all values from treated animals
were comparable to control values. Growth and food consumption of both
males and females was depressed at 3000 ppm. Haematological parameters
(haemoglobin and packed cell volume) were reduced at 3000 ppm. These
haematological changes were noted only after the first year of
treatment. Gross and microscopic examination performed at 13 weeks and
at the conclusion of study showed slightly higher liver weights,
kidney weights, testicular weights and (possibly) thyroid weights at
3000 ppm. The incidence and location of neoplasms in all treatments
did not differ from those in the controls. Histological examination
revealed liver changes at 3000 ppm, characterized by hepatic cell
enlargement, glycogen depletion, increased basophilia of the
cytoplasm, and the presence of necrobiotic hepatic cells. Histological
examination performed at 13 weeks also indicated hepatic cell changes
and slight adrenal cortical atrophy in several animals at 3000 ppm.
The adrenal changes were not noted at 104 weeks. An estimated
no-effect level in this study is between 100 and 3000 ppm in the diet
(Woodard et al., 1964).
Groups of rats (25 males and 25 females/group, Boots-Wistar
strain) were fed 2,6-dichloro-4-nitro-aniline in the diet at
concentrations of 0 and 1000 ppm for two years. There was no effect on
survival, food consumption, growth, haematology, or upon gross and
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular makeup of
liver, kidney, or spleen. The incidence of tumors in the control and
treatment group was similar. From the results of this experiment a
suggested no-effect level is greater than 1000 ppm (Lessel, 1974e).
OBSERVATIONS IN MAN
In a clinical double blind study, two groups of adult males were
administered 2,6-dichloro-4-nitroaniline (20 individuals) or a placebo
(10 individuals) once a day for ninety days.
2,6-Dichloro-4-nitroaniline was administered at a level of 10 mg per
day. Hematological, liver function, and kidney function tests were
performed at various intervals over the course of the study and were
found to be normal. There were no indications that administration of
2,6-dichloro-4-nitroaniline at 10 mg per day to adult males had any
adverse effect (Stough, 1962).
Extensive examinations were made on one industrial worker
occupationally exposed to 2,6-dichloro-4-nitroaniline over a period of
three years. It was reported that for about 60 days per year
considerable inhalation and dermal exposure had occurred. No adverse
effects were observed with the individual (Brooks and Boyack, 1963).
Another investigation in man on the potential ocular problem
associated with 2,6-dichloro-4-nitroaniline was again negative
(Manger, 1972).
COMMENTS
2,6-Dichloro-4-nitroaniline has a low order of toxicity to
mammals, including man. 2,6-Dichloro-4-nitroaniline is rapidly
metabolized in plants, and fragments of the molecule are
reincorporated as natural plant constituents. In mammals,
2,6-dichloro-4-nitroaniline is rapidly absorbed, metabolized and
excreted. Metabolism in mammals results in formation of the
chlorinated phenylenediamine and aminophenol which are conjugated and
excreted. 2,6-Dichloro-4-nitroaniline does not induce
methemoglobinemia as evidenced with 4-nitroaniline.
2,6-Dichloro-4-nitroaniline does not affect reproduction in rodents
and has shown no evidence of being a teratogen under the experimental
protocol used. Following subacute feeding, dogs exposed to sunlight
developed cataracts. Specific experiments with rabbits, rats and
swine did not duplicate these results. Long term feeding studies in
rat and two year feeding studies in dog resulted in growth
retardation accompanied by an increased liver and kidney size at
high levels. No-effect levels based on a two year dog study and short
and long term rat studies formed the basis for allocating a temporary
ADI for man. A short term study in man is reassuring in estimation of
the temporary ADI although no conclusions could be drawn on the
possibility of ocular damage to man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of 2,6-dichloro-4-nitroaniline as a commercial
fungicide is recorded in Canada, France, Holland, Italy, Japan, New
Zealand, South Africa and USA.
It is claimed to be effective against several Basidiomycetes
and Deuteromycetes species of fungi, being fungistatic to the
mycelium and spores of Botrytis cenera (Clark et al., 1960; Clark
and Hams, 1961; Sharples, 1962) and to several Rhizopus fruit rot
fungi (Ogawa et al., 1961, 1962; Cappelini and Stretch, 1962).
It is mostly marketed as 4% or 8% (w/w) dust formulations or as
50% (w/w) wettable powders, alone or mixed with thiram (TMTD) or
pentachloronitrobenzene. Smoke formulations containing 40% dicloran
are also available.
Pre-harvest treatments recommended by the manufacturers include
soil treatments for lettuce under glass, dusting or spraying of soft
fruits, cotton, leafy vegetables, strawberries, onions, garlic,
tomatoes and ornamentals. Post-harvest dips of peaches, nectarines and
carrots are practised at rates of 750-1000 mg/kg a.i.
Phytotoxicity
Under extreme experimental conditions a "bronzing" discolouration
of lettuce leaves and marginal "off-taste" taints in fruits have
occasionally been found, but none of these effects have ever been
reported in practical use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data on 2,6-dichloro-4-nitroaniline in several fruit and
vegetable crops have been presented by the originating company (The
Boots Company Ltd., 1972). The data derive from field trials and
post-harvest experiments carried out mainly in the USA and UK.
Summaries of residue ranges and experimental conditions extracted from
these data are presented in Tables 2, 3 and 4.
Peaches and apricots
Extensive trials with single or repeated spraying of peaches and
apricots at recommended dosage rates (usually 0.12-0.18% a.i.) and
higher rates have been carried out during the period from 1960 to 1965
(Tables 2 and 4). The initial deposits of 2,6-dichloro-4-nitroaniline
on peaches are generally between 5 and 15 mg/kg with occasional values
up to 25-30 mg/kg. A typical residue was 5 mg/kg after 6 days, 3 mg/kg
after 9 days and 1.5 mg/kg after 14 days. The highest recorded residue
14 days after treatment at a dosage rate of 0.18% was 4.9 mg/kg.
The average half-life of 2,6-dichloro-4-nitroaniline on peaches
has been calculated to be 5 days.
Spraying apricots gave residues very similar to those found in
sprayed peaches (Table 4).
Dusting peaches using 8% a.i. dicloran powders at rates of 3-5 kg
per ha gave maximum initial deposits of 3.7 mg/kg.
A suggested practice of wrapping apricots and peaches in tissues
impregnated with 2,6-dichloro-4-nitroaniline (from 500-3000 mg/kg, in
the wrap) showed a definite transfer of the chemical to the fruits.
Residues from such post harvest application were from 1.6 - 3.5 mg/kg
in peaches and 1.9 - 3.3 mg/kg in apricots (Table 3).
TABLE 2 Residues of dicloran in peaches
Application Number Number Number Range of
(% a.i. w/v or PHI of of of results
kg a.i./ha) (days) applications trials results mg/kg
0.06% 3 3 1 4 0.1-0.3
0.09% 0 1-3 3 9 6.1-16.5
1 1-3 5 9 1.5-11.6
2-3 1-3 2 7 0.2-4.7
7 1 1 1 2.3
0.12% 0 1-3 1 6 10.8-14.0
1 1-2 1 2 3.4-5.5
3 1-3 2 10 0.2-6.3
7 1 1 1 3.2
0.18% 0 2 1 6 6.0-11.4
1 2 1 6 9.2-13.7
2 2 1 6 5.7-13.8
4 2 1 6 4.2-9.4
6 2 1 6 4.5-7.8
9 2 1 6 1.9-6.0
14 2 1 6 0.6-4.9
0.18% 0 3 1 4 18.9-29.5
3 3 1 4 22.4-29.6
0.24% 1 3 1 4 3.0-8.3
3 3 1 4 2.7-11.1
3.1 kg/ha
(8% dust) 0 1 1 2 0.14-0.35
4.5-4.9 kg/ha
(8% dust) 0 1 3 21 0.22-3.7
TABLE 3 Residues in fruits wrapped in tissues impregnated with
dicloran
Fruit Dicloran in wrap mg/kg Dicloran in fruits
Apricot 500 1.9
1000 2.6
2000 3.3
Peach 500 1.6
1000 2.3
2000 3.8
3000 3.5
TABLE 4 Residues of 2.6-dichloro-4-nitroaniline in fruit and vegetables
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Apricots 0.09% 0 1 1 12 1.9-7.9
2 3 1 2 0.9-1.4
4 1-2 1 4 1.4-3.7
11 1 1 2 0.1-0.7
Apricots 0.12% 0 1 1 1 10.1
1 1 1 1 8.0
7 1 1 1 3.8
Cherry 0.12% 0 1 1 1 10.3
1 1 2 5 6.4-14.8
7 3 1 1 4.9
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
0.24% 1 1 1 2 0.7-11.2
7 1 1 1 2.9
0.36% 1 5 1 2 2.7-3.1
1200 ppm post-harvest 6 21 1.5-6.4
1200 ppm post-harvest
and washing 1 2 0.3-0.3
1350 ppm post-harvest 1 2 10.2-13.7
1800 ppm post-harvest 1 2 7.7-8.4
Grapes 1.7 kg/ha
(dust) 0 2-4 2 6 0.2-3.9
7 2 1 2 0.3-1.1
2.3-3.3
kg/ha (dust) 1 4 1 4 1.1-1.3
7 1-2 2 4 0.2-1.1
1000 ppm post-harvest dip 1 4 0.6-5.1
Plums
(dried) 1000 ppm post-harvest dip 1 3 1.0-4.7*
post-harvest dust 1 3 2.0-8.1
0.12% 1 1 1 1 2.5
Strawberry 0.16-0.18% 1 4 1 1 0.32
3 1 1 2 4.0-5.7
9-11 3-4 2 4 0.2-1.6
0.24% 1 3 1 2 0.9-1.7
Blackberry 1.1 kg/ha
(spray) 4-11 1 1 2 1.2-2.3
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
1.8 kg/ha
(dust) 4-11 1 1 2 <0.05-0.3
Currants 0.11-0.15% 33 4 1 3 1.7-3.3
Raspberry 0.15-0.18% 5 4 2 2 17.0-20.2
9-10 4-5 2 2 2.2-11.8
13-14 3-4 2 2 2.6-7.5
0.36% 5 4 1 1 39.0
10 4 1 1 28.4
14 4 1 1 16.3
Carrots 900- post-harvest dip
1000 ppm (stored 0-3 days) 2 12 3.6-9.4
peeled 1 1 2.1
canned 1 7 <0.05-0.2
Lettuce 2.2 kg/ha 0 2 1 4 50.1 (average)
7 2 1 9 18.0 "
RL50 approx 14 2 1 9 3.0 "
3-4 days
21 2 1 9 2.4 "
28 2 1 8 0.8 "
35 2 1 8 0.04 "
1.3 g/m2
(dust) 120 1 1 1 0.1
1.3 g/m2 (soil
treatment;
pre-planting) 114-140 1 3 5 0.2-2.1
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Gherkins
(indoor) 0.05% 0 1 1 1 5.5
2 1 1 1 1.1
4 1 1 1 0.6
5 1 1 1 0.35
Tomato
(indoor) 0.1% 2 1 1 1 0.25
4 1 1 1 0.2
6 1 1 1 0.04
Beans,
French 3 kg/ha 12 4 1 2 1.3-1.9
20 2 1 2 0.8-1.5
* Loss by drying.
Cherries, grapes and plums
The residue levels of 2,6-dichloro-4-nitroaniline from
recommended uses on these fruits are similar to those in peaches and
apricots (Table 4).
After dipping procedures or post-harvest dusting of plums,
dicloran was retained on the fruits at levels of 4.7 and 8.1 mg/kg
respectively. These residues were reduced to 1.0 and 2.0 mg/kg
respectively, when the fruits were subjected to an 8 day air-drying
period at ambient temperature.
A post-harvest treatment of cherries by spraying on the packing
line with 1200-1800 ppm suspensions gave residues of
2,6-dichloro-4-nitroaniline from 1.5 - 13.7 mg/kg. In storage
experiments these residues were reduced at a rate corresponding to a
half-life of 11 days at 20°C.
Small fruits and berries
Repeated spraying of strawberries at different locations (0.06
- 0.24% a.i.) have been reported to give maximum initial deposits of
dicloran of 9 mg/kg. However, residues at harvest after a 9 - 11 day
interval from the last application were generally well below 1 mg/kg,
with an occasional individual sample at 1.6 mg/kg. Among the berries,
raspberries in some cases showed higher residues. After application of
0.36% a.i. the initial residue was 39 mg/kg which was reduced to 16.3
mg/kg at 14 days. At lower application rates, residues were
proportionately lower.
Experiments with other berries (blackberries, currants and
boysenberries) generally gave residues below 5 mg/kg.
Lettuce
Roburn (1960) followed the disappearance of
2,6-dichloro-4-nitroaniline after post-planting treatments of lettuce
plants with 1/4 oz of 4% dust per sq. yd. and found a reduction in
deposits greater than that due to growth dilution. Volatilization and
chemical degradation were suggested as contributing factors.
The half-life values decreased progressively in these trials,
being about 3-5 days during the last weeks of the growing season. A
three weeks pre-harvest interval for dicloran treatments of lettuce is
recommended on this basis in the United Kingdom.
Later experiments by Boyack and Boot (1962 a, b, c) confirmed
Roburn's findings, indicating half-life values of 3-4 days on
greenhouse lettuce with average residues of 2.4 mg/kg after an
interval of 21 days (Table 4).
Uptake of 2,6-dichloro-4-nitroaniline through the roots of
lettuce plants after pre-planting soil treatments has been
demonstrated by Boyack and Boot (1962a), Lemin and co-workers (Lemin,
1963, 1965; Moe and Lemin, 1963a, 1964) and Groves and Chough (1970).
Residues from such practices at the time of harvest will be near or
below the detection limit, i.e. less than 0.05-0.1 mg/kg.
In recent experiments in the Netherlands lettuces have been
treated both by a pre-planting soil application and by dusting the
young plants immediately after planting. (Pieters, 1974). Residues in
these experiments ranged from 0.1 - 2.1 mg/kg (Table 4).
Carrots
Post-harvest dipping in 900-1000 ppm suspensions caused residues
of 3.6-9.4 mg/kg at 0-3 days. After storage for 5 months the remaining
residues were 2.1-3.7 mg/kg.
Other vegetables
Residue data on a few other vegetable crops, e.g. tomatoes,
gherkins and French beans, mostly grown under glasshouse conditions,
are available (Pieters, 1974). The data are insufficient to evaluate
quantitatively the rates of dissipation, but residues are generally
low at the time of harvest, i.e. from a few days to 1-2 weeks after
treatment (Table 4).
FATE OF RESIDUES
In animals
The metabolism of 2,6-dichloro-4-nitroaniline by the rat, mouse
and to a limited extent man, has been described above under
"Biotransformation". No such experimental evidence is available on
livestock animals, nor on the excretion of dicloran or its metabolites
in milk.
In plants
Studies by Lemin (1965), Lemin et al. (1963) and Moe and Lemin
(1963a, 1964) have shown that 2,6-dichloro-4-nitroaniline is absorbed
by plant roots and translocated to the plant material in tomato and
lettuce. These experiments include uptake from both nutrient solutions
and treated soils.
Application of 14C-labelled 2,6-dichloro-4-nitroaniline gave
evidence of rapid degradation into polar metabolites and 14C was
found in carbohydrate constituents of the plant tissue, presumably
owing to the incorporation of degradation fragments. Transitional
metabolites such as dechlorinated components, reduction products or
2,6-dichloro-p-phenylene-diamine were not detected.
Amino acids, chlorophyll or uronic acid did not contain
radioactivity. Neither could 2,6-dichloro-4-aminophenol, known to be
formed by animal metabolism (see previous section), be found in plant
tissues.
Exhaustive extractions of peaches 13 days after treatment with
14C-labelled 2,6-dichloro-4-nitroaniline revealed (in addition to
unchanged parent compound) 14C-labelled phenylalanine and labelled
flavonoid glycosides (Moe and Lemin, 1963b). As in the above
experiments, no transitional metabolites could be traced.
Groves and Chough (1970) report rapid absorption of dicloran and
incorporation of fragments into tissue constituents by a number of
plants.
In processing and storage
Washing or canning processes have been shown to reduce residues
of 2,6-dichloro-4-nitroaniline considerably (Boots Company Ltd.,
1972). On lettuce, for example, residues of 18-25 mg/kg still
remaining two weeks after the last application were reduced to 5.6-6.0
by washing with water (Roburn, 1958). In peaches, freshly treated with
2,6-dichloro-4-nitroaniline, the residues which still remained after
an industrial canning procedure were below 0.1 mg/kg.
Carrots treated by post-harvest dipping showed only moderate
reduction of the residues by peeling, from 3.7 to 2.1 mg/kg,
indicating a significant penetration into the inner parts. A
subsequent canning procedure, however, reduced the levels to 0.1-0.2
mg/kg. Storage of dipped carrots for 5 months at 40°F gave a decrease
in the residue of about 25%.
In soils
2,6-Dichloro-4-nitroaniline is generally regarded as being
relatively stable in soils at field moisture capacity. Its
persistence, however, is greatly affected by conditioning factors such
as soil composition, water content, microculture etc. (Wang and
Broadbent, 1973). The absorption of the chemical from soils by oats
was found by Groves and Chough (1970) and Wang (1972) to be inversely
related to the clay and organic matter content of the soils,
suggesting a binding of the chemical to these soil constituents. Later
exhaustive extraction experiments showed that a reversible binding
probably occurs (Groves and Chough, 1971).
The same authors (Groves and Chough, 1970, 1971) showed that
14C-labelled dicloran can be metabolized by soil organisms which
degrade the compound to 14C-carbon dioxide and other volatile
compounds. The rate of microbial breakdown could be increased by
repeated applications of dicloran to the same soil or by incubation of
the soil with dicloran. A culture of rod-shaped bacteria was isolated
and found active in the decomposition of the fungicide. Microbial
breakdown is also held responsible for the rapid degradation of DCNA
which is seen in soil under flooded conditions (Wang and Broadbent,
1973).
Van Alfen and Kosuge (1974) have identified both
2,6-dichloro-p-phenylenediamine and its acetylated derivative,
4-amino-3,5-dichloroacetanilide, in cultures of Pseudomonas cepacia
and Escherichia coli B. In soil and through the action of
horseradish peroxidase, the phenylenediamine was converted to one of
three isomers of the azine resulting from oxidative dimerization (Van
Alfen, 1973).
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Market sample surveys carried out in the Netherlands in 1973 on
domestically grown fruit and vegetables showed that residues of
2,6-dichloro-4-nitroaniline were frequently present in some crops
(Table 5). In vegetables such as endive, lettuce and chicory sprouts
about 50-80% of samples were positive with individual residues up to 8
mg/kg. Crops such as cucumbers, tomatoes and paprika were less
frequently positive (3-25%). At the time of this survey, the tolerance
for vegetables in the Netherlands was 10 mg/kg.
TABLE 5 Dicloran residues in marketed vegetables (Netherlands, 1973)
Dicloran
residues,
mg/kg number of samples
(range)
Cucumber Endive Lettuce Paprika Tomato Chicory
not detectable 31 34 136 12 30 68
0.01 - 0.1 1 16 139 2 4 50
0.1 - 0.3 - 7 126 2 - 21
0.3 - 1.0 - 8 180 - - 3
1.0 - 3.0 - 4 123 - - 1
3.0 - 10.0 - 1 35 - - -
Total number 32 70 739 16 34 143
[text missing]
pre-harvest treatments on fruits, vegetables and ornamentals and
post-harvest treatments on peaches, nectarines, cherries and carrots.
It is registered in several countries, usually as 4 or 8% (w/w) dusts
or as 50% (w/w) wettable powders.
The technical grade typically contains about 96%
2,6-dichloro-4-nitroaniline. The remaining parts consist of chemically
related compounds and impurities which have been identified and
quantified.
Concentrations and rates of application vary, depending on the
crop and method of application. Normal spraying concentrations are
0.05% to 0.18% applied at rates of 2-5 kg a.i. per ha. Post-harvest
dips are practised at rates of 1000 - 2000 mg per litre.
The residue data available are mainly obtained from the USA and
UK, in a few cases supplemented from other countries. Most of the data
derive from field trials or experiments under practical conditions
likely to represent the results of good agricultural practice.
2,6-dichloro-4-nitroaniline is a non-systemic compound of
relatively low persistence. The dissipation of the compound on fruits
and vegetables is often faster than can be explained by normal
weathering or growth dilution. Half-life periods of 11 days have been
found for residues during storage of post-harvest treated cherries.
2,6-dichloro-4-nitroaniline is absorbed by plant roots from the
soil. The fate of residues in plant material has been followed with
the radio-labelled compound, which indicated a rapid degradation into
polar metabolites and 14CO2 followed by incorporation of 14C into
normal metabolic plant constituents. No indications of transitional
metabolites from such degradation have been found.
Similar degradation of 2,6-dichloro-4-nitroaniline occurs in
soils, although in this case microbial breakdown may also produce
smaller amounts of reduction products and acetylated derivatives of
the parent compound.
Studies were available on the metabolic fate of residues in rats
indicating a fairly rapid process of absorption, metabolism and
excretion, especially as aminophenol and phenylenediamine derivatives,
in the urine. There was no evidence of tissue storage in these
experiments. Preliminary studies in man suggest a metabolic pattern
similar to that of rats. No information, however, has been recorded on
metabolism in livestock animals or on transfer of residues into meat
or milk.
METHODS OF RESIDUE ANALYSIS
A colorimetric method of residue analysis, based on the
development of a yellow colour characteristic of some mononitro
aromatic compounds in the presence of strong alkali and acetone, is
described by Kilgore et al. (1962). Extraction is with benzene,
followed by clean-up on Florisil alone or in combination with an
acetonitrile-petroleum ether partitioning procedure. Blank values for
fruits were about 0.01-0.02 mg/kg and recoveries in the range 75 -
100% (average 86%). The colorimetric method described by Groves and
Chough (1966) and Heagy (1969) has a similar sensitivity, based on
diazotization and coupling to N-(1-naphthyl)ethylenediamine has a
similar sensitivity.
An alternative method developed by Roburn (1961) utilizes the
reduction of 2,6-dichloro-4-nitroaniline by zinc and hydrochloric acid
to the corresponding 2,6-dichloro-p-phenylenediamine which, in the
presence of aniline, can be oxidized to produce an intense blue
colour. Less than 2 µg can be detected by this method, corresponding
to 0.05 mg/kg in fruit and 0.2 mg/kg in lettuce. Recovery of surface
deposits was approximately 100%, but for macerated samples it was only
71%. Chloronitroanilines in general give similar reactions but a
series of other pesticide chemicals did not interfere, indicating a
fair degree of specificity of the method.
A thin-layer chromatographic spot test sensitive to 0.5 µg of
2,6-dichloro-4-nitroaniline is given by Groves et al. (1966) based on
their colour reaction mentioned above.
Gas-chromatographic methods for the determination of
2,6-dichloro-4-nitroaniline have been developed by Beckmann and
Bevenue (1962) and Kilgore (1964) using electron capture and
microcoulometric detectors, in combination with the Kilgore extraction
procedure already mentioned. Recoveries of 85-105% were established by
Brewerton et al. (1967) for the analysis of dicloran in soils, fruit
and vegetables down to the 0.1 mg/kg level by the GLC/EC technique.
These methods could be adapted for regulatory purposes as part of a
multi-residue procedure.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance
Country Crop mg/kg
Australia Beans, lettuce, stone fruits, tomatoes 20
Belgium Fruit and vegetables (exc. potatoes) 10
Canada Apricots, nectarines, peaches
(pre-harvest and post-harvest),
sweet cherries pre-harvest and
post-harvest), snap beans 20
Strawberries, raspberries 15
Celery, grapes, lettuce, rhubarb,
sweet potatoes 10
Carrots, cucumbers, garlic, onions,
plums, tomatoes 5
Germany All fruits and vegetables 0.1
Tolerance
Country Crop mg/kg
Italy General 10
Switzerland Lettuce (tentatively) 0.5
Netherlands Lettuce, endive 3
Chicory (sprouts) 1
Cucumbers, gherkins, melons, bell
peppers, tomatoes 0.3
Other vegetables 0.1
USA Apricots, nectarines (pre-harvest and
post-harvest), peaches (pre-harvest
and post-harvest), sweet cherries
(pre-harvest and post-harvest), snap
beans 20
Blackberries, boysenberries, celery,
plums (fresh prunes) (pre-harvest and
post-harvest), raspberries 15
Carrots (pre-harvest and post-harvest),
grapes lettuce, rhubarb, sweet
potatoes (pre-harvest and post-harvest) 10
Cucumbers, garlic, onions, tomatoes 5
Potatoes 0.25
Cotton seed 0.1
APPRAISAL
2,6-dichloro-4-nitroaniline is a fungicide active against the
mycelium and spores of Botrytis cinerea, B. sclerotinia and other
fungi, including several Rhizopus fruit rots.
Practical use patterns comprise foliar applications and soil
treatments under indoor and outdoor growing conditions.
The residue data from supervised trials on several crops were
considered sufficiently extensive to enable recommendations to be
made, including some for post-harvest treatments.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for specific determination.
These methods are suitable for regulatory purposes.
RECOMMENDATIONS
Temporary tolerances are recommended for
2,6-dichloro-4-nitroaniline in the following commodities at the
levels indicated. The levels are not likely to be exceeded when
2,6-dichloro-4-nitroaniline is applied in accordance with good
agricultural practice including both pre-harvest and post-harvest
applications and taking into account that preharvest intervals may
apply for certain treatments.
TEMPORARY TOLERANCES
Limit Basis on which recommendations
Crop (mg/kg) are made
Cherries 15 pre and post-harvest
Peaches 15 pre and post-harvest
Apricots 10 pre and post-harvest
Carrot 10 post-harvest only
Grapes 10 pre and post-harvest
Lettuce 10 pre-harvest only
Plums 10 pre and post-harvest
Raspberry 10 pre-harvest only
Strawberry 10 pre-harvest only
Blackberry 5 pre-harvest only
Currant (red, black
and white) 5 pre-harvest only
Beans (French) 2 pre-harvest only
Gherkins 0.5 pre-harvest (indoor) only
Tomato 0.5 pre-harvest only
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further studies on the ocular disturbance observed in dogs to
confirm and clarify this effect.
2. Information on fate in livestock animals in so far as plant
material containing residues may be fed to animals.
DESIRABLE
1. Effect on hepatic microsome systems in several species.
2. Further observations in man.
3. Further information on fate of residues during storage, transport
and processing of fruit and vegetables.
4. Information on transfer of residues from grapes to wine and on
possible influence on wine processing.
5. Further information on soil residues and their possible uptake
into subsequent crops.
6. Further data to clarify inconsistencies in the residue levels
found in different berries.
REFERENCES
Anonymous. (1974) "Somers test" in rabbits. Technical summary reports
submitted to WHO by UpJohn Co. (Unpublished)
Beckmann, H. and Bevenue, A. (1962) Pesticide residue analysis Gas
chromatographic analysis of 2,6-dichloro-4-nitroaniline. J. Food Sci.,
27:602-604.
Bernstein, H.N., Curtis, J., Earl, F.L. and Kuwabara, T. (1970)
Phototoxic corneal and lens opacities in dogs receiving a fungicide,
2,6-dichloro-4-nitroaniline. Archs Ophthal. (Chicago), 83:336-348.
Boots Co. Ltd. (1972) Dicloran. Registration Document Vol. I, II, III
and VI. Reports submitted by The Boots Company Ltd. Agricultural
Research, Nottingham. (Unpublished)
Boyack, G. and Boot, D. (1962a) Assay for 2,6-dichloro-4-nitroaniline
in lettuce grown in treated soil under glass. Dicloran, Registration
document from The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G, and Boot, D. (1962b) Assay for 2,6-dichloro-4-nitroaniline
on sprayed greenhouse lettuce. Dicloran, Registration document from
The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G. and Boot, D. (1962c) Residue of 2,6-dichloro-4-nitroaniline
(DCNA) on lettuce dusted with Botran. Dicloran, Registration document
from The Boots Co. Ltd. Vol. II, Appendix 26.
Brewerton, H.V., Clark, P.J. and McGrath, H.J.W. (1967) Dicloran
analysis by gas chromatography. New Zealand J. Sci., 10:124-127.
Brooks, B. and Boyack, G. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Report submitted by
The Upjohn Co. (Unpublished)
Cappellini, R.A. and Stretch, A.W. (1962) Control of post-harvest
decays of peaches. Pl. Dis. Reptr., 46:31-33.
Clark, N.G., Hams, A.F., Higgons, D.J. and Stevenson, H.A. (1960) A
new fungicide active against Botrytis spp. Chemy Ind., 1960:572-573.
Clark, N.G. and Hams, A.F. (1961) Antifungal activity of substituted
nitroanilines and related compounds. J. Sci. Food Agric., 12:751-757.
Curtis, J., Bernstein, H.N., Earl, F.L. and Smalley, H., Jr. (1968)
Corneal and lens opacities in dogs treated with
2,6-dichloro-4-nitroaniline. Toxic. appl. Pharmac., 12:305.
Earl, F.L., Curtis, J., Bernstein, H.N. and Smalley, H., Jr. (1971)
Ocular effects in dogs and pigs treated with dichloran. Food Cosmet.
Toxic., 9:819-828.
Eberts, F.S. (1965) Fate of Botran (2,6-dichloro-4-nitroaniline) in
rat and man. Dicloran, Registration Document, Vol. IV, Appendix 54.
Boots Co. Ltd. (Unpublished). Botran Symposium, Augusta, Mich. 1965.
Cited in Chemical Abstracts 1968, 68:abstract 11908g
EPA. (1974) Data from EPA Laboratories. (Unpublished)
Evans, J., Mengel, G. and Bostwick, L. (1963) Botran(R) (U-2069)
Effect of oral administration. Final report, four months study. Report
submitted by the Upjohn Co. (Unpublished)
Feenstra, E. (1961) Report submitted by The Upjohn Co. (Unpublished)
Groves, K. and Chough, K.S. (1966) Determination of small quantities
of 2,6-dichloro-4-nitroaniline (dicloran). J. agr. Food Chem.,
14:668-669.
Groves, K. and Chough, K.S. (1970) Fate of the fungicide
2,6-dichloro-4-nitroaniline (DCNA) in plants and soils. J. agr. Food
Chem., 18:1127-1128.
Groves, K. and Chough, K.S. (1971) Extraction of
3-amino-1,2,4-triazole (Amitrole) and 2,6-dichloro-4-nitroaniline
(DCNA) from soils. J. agr. Food Chem., 19:840-841.
Gurd, P.R. (1974) Methaemoglobin formation in the cat. Report from
Boots Pure Drug Company Limited. Submitted to WHO by the Boots Co.
Ltd. (Unpublished)
Heagy, J.A. (1969) Colorimetric determination of
2,6-dichloro-4-nitroaniline (dicloran) residues in foods. J. Ass. off.
analyt. Chem., 52:797-799.
Horn, J. (1961) U-2069 - Acute inhalation toxicity. Study from Woodard
Research Corporation submitted by The Boots Co., Ltd. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: A preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Johnston, R. and Schwikert, R. (1961a) Skin irritation in rabbits.
Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1961b) Dichloran - eye irritation in
rabbits. Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1963) Skin sensitization in guinea
pigs. Report from The Upjohn Co. (Unpublished)
Kilgore, W.W. (1964) 2,6-dichloro-4-nitroaniline. Analytical methods
for pesticides, plant growth regulators and food additives. Gunter
Zweig (Editor). Vol. III. p. 61-68.
Kilgore, W.W., Cheng, K.W. and Ogawa, J.M. (1962) Extraction and
determination of 2,6-dichloro-4-nitroaniline in processed fruits. J.
agr. Food Chem., 10:399-401.
Lemin, A.J. (1965) Translocation and metabolism of
2,6-dichloro-4-nitroaniline by lettuce and tomato. J. agr. Food Chem.,
13:557-560.
Lemin, A.J. Moe, L.D. and Smith, A.H. (1963) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce. Dicloran, Registration
document from the Boots Co. Ltd. Vol. II, Appendix 34 A.
Lessel, B. (1974a) Experimental data on toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974b) Effect on blood and blood forming tissues. Report
from The Boots Co. Ltd. (Unpublished)
Lessel, B. (1974c) Short term (4-week) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974d) Chronic (6 month) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974e) Two year feeding trial in rats on dicloran
(2,6-dichloro-4-nitroaniline). Report submitted by the Boots Co. Ltd.
(Unpublished)
Lobdell, B. and Johnson, C. (1965) U-2069 - Effect on reproduction
capacity through three generations in the rat. Report from Woodard
Research Corporation submitted by The Boots Co. Ltd. (Unpublished)
Manger, W.H. (1972) Use and handling of dicloran formulations and
influence on human eyesight. Report from Biological Laboratory,
Aseptafabrick B.V. Delft, Netherlands. (Unpublished)
Maté, C., Ryan, A.J. and Wright, S.E. (1967) Metabolism of some
4-nitroaniline derivatives in the rat. Food Cosmet. Toxic., 5:657-663.
Moe, L.D. and Lemin, A.J. (1963a) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part II, dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 B.
Moe, L.D. and Lemin, A.J. (1963b) The metabolism of
2,6-dichloro-4-nitroaniline-C14 in peaches. Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 35.
Moe, L.D. and Lemin, A.J. (1964) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part III. Dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 C.
Ogawa, J.M., Lyda, S.D. and Weber, D.J. (1961)
2,6-dichloro-4-nitroaniline effective against Rhizopus fruit rot
of sweet cherries. Pl. Dis. Reptr., 45:636-638.
Ogawa, J.M., Mathre, J.M., Weber, D.J. and Lyda, S.D. (1963) Effects
of 2,6-dichloro-4-nitroaniline on Rhizopus species and its
comparison with other fungicides on control of Rhizopus rot of
peaches. Phytopathology, 53:950-955.
Pieters, A.J. (1974) Information on dicloran residues in The
Netherlands submitted to the Joint Meeting 8 November 1974.
(Unpublished)
Roburn, J. (1958) Experiments to demonstrate the effect of washing
lettuce treated with dicloran ("Alisan"). Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 33.
Roburn, J. (1960) Allisan residue trials on lettuce, February to April
1960. Lenton memo nr. 5. Lenton Experimental Station, Nottingham.
(Unpublished)
Roburn, J. (1961) Colorimetric determination of
2,6-dichloro-4-nitroaniline in plants and soil. J. Sci. Fd. Agric.,
12:766-772.
Serrone, D., Pakdaman, P., Stern, A. and Coulston F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
Rhesus monkey. Tox. appl. Pharmacol., 10:404.
Sharples, R.O. (1962) The fungitoxic effects of dicloran on Botrytis
cinerea. Lenton Experimental Station, Nottingham. (Unpublished)
Stough, A.R. (1962) Results of Botran(R) clinical study. Results from
The Upjohn Co. submitted by The Boots Co. Ltd. (Unpublished)
Van Alfen, N.K. (1972) Microbial metabolism of the fungicide
2,6-dichloro-4-nitroaniline in soil by micro-organisms. Dissertation,
Dept. of Plant Pathology, Univ. of California, Davis.
Van Alfen, N.K. and Kosuge, T. (1974) Microbial metabolism of the
fungicide 2,6-dichloro-4-nitroaniline. J. agr. Food Chem., 22:221-224.
Wang, C.H. (1972) The effect of soil properties on losses of two
chloronitrobenzene fungicides from soils. Dissertation, Dept. of Soil
Sciences, Univ. of California, Davis.
Wang, C.H. and Broadbent, F.E. (1973) Effect of soil treatments on
losses of two chloronitrobenzene fungicides. J. environ. Quality, 2
(4):511-515.
Woodard, M., Cockrell, K. and Woodard, G. (1964) Safety evaluation by
oral administration to rats and dogs for 104 weeks. Report from
Woodard Research Corporation submitted by The Boots Co. Ltd.
(Unpublished)
Other information on identity and properties
Molecular weight: 207
State: Yellow, crystalline solid with practically
no odour
Melting point: 192-194°C
Vapour pressure: 1.2 x 10-6 mm Hg (20°C)
Solubility: Water7 mg/l
Cyclohexane 0.006 g/100 ml
Petroleum ether 0.02 g/100 ml
Carbon tetrachloride 0.06g/100 ml
Ethanol 0.29 g/100 ml
Benzene 0.46 g/100 ml
Glacial acetic acid 8.80 g/100 ml
Chloroform 1.2 g/100 ml
Acetone 3.4 g/100 ml
Stability: Stable to hydrolysis and relatively stable
to oxidation. Readily reduced to
phenylenediamine by zinc and acid. Stable
to light and heat.
Composition and
purity: The technical product contains
2,6-dichloro-4-nitroaniline not less than
96% (on dry weight basis);
2,4-dichloro-6-nitroaniline not more than
2%; 2-chloro-4-nitroaniline not more than
l%; Chloranil not more than 1%;
P-nitroaniline not more than 0.1%; Loss on
drying not more than 1% (at 60°C, <5 mm
Hg); Sulphated ash not more than 0.25%;
Sodium chlorate not more than 100 ppm.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
2,6-Dichloro-4-nitroaniline-14C administered to three male human
subjects at a level of 50 mg was found to be rapidly absorbed and
excreted. Excretion was somewhat slower than measured in the rat with
the major quantity of material being excreted within 1.5 days.
Preliminary studies suggest that 2,6-dichloro-4-nitroaniline
metabolites are similar to those obtained from the rat. Approximately
85% of the total urinary excretion from the rat was found to be of
2,6-dichloro-4 hydroxy aniline sulfate.
2,6-Dichloro-4-nitroaniline-14C administered to male rats at a
dosage of either 1.7 mg/kg or 8 mg/kg orally was rapidly excreted
from the body. Urinary excretion accounted for approximately 90% of
the recovered material with the remainder being located in the
faeces. The majority of material (about 90%) was recovered within 48
hours and over half was recovered within 8 hours after treatment.
2,6-Dichloro-4-nitroaniline was not observed in any body tissues with
the exception of small quantities detected in the G.I. tract, urinary
tract, and liver (Eberts, 1965).
2,6-Dichloro-4-nitroaniline administered to rats (ip or orally,
20 mg/kg) was metabolized to the dichloroamimophenol and the
dichlorophenylenediamine derivatives. Following both routes of
administration the majority of material was recovered from the urine
within 24 hours and total recovery was noted within 72 hours. Very
small quantities were obtained in the faeces. In vitro studies using
mouse liver microsomes showed limited conversion of
2,6-dichloro-4-nitroaniline to the same two metabolites (Máté et al.,
1967).
Biotransformations in plants and micro-organisms are discussed
under "Fate of residues".
Effects on enzymes and other biochemical parameters
Following high level subacute oral administration of
2,6-dichloro-4-nitroaniline to rats, an increase of hepatic
demethylase and desulfurase activity was noted. Liver mitochondrial
O2 consumption was also increased in rats. The liver enzymes were
not stimulated in monkey following similar treatment (Serrone, 1967).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A carcinogenic screening using high levels of
2,6-dichloro-4-nitroaniline administered to susceptible mice was
negative. Groups of mice (18 males and 18 females of each of two
hybrid strains) were administered 2,6-dichloro-4-nitroaniline at 215
mg/kg/day for three weeks from day seven after birth. Thereafter for
18 months the mice were fed 603 ppm in the diet, sacrificed and
examined for tumours. 2,6-Dichloro-4-nitroaniline did not cause a
significant increase in tumours (Innes et al., 1969).
Effects on blood and blood forming tissues
Cat
In studies to substantiate the difference between effects of
4-nitroanaline and 2,6-dichloro-4-nitroaniline, a study on the effect
of these materials on methaemoglobinemia in the cat was performed
(Gurd, 1974). After a single oral dose of 2,6-dichloro-4-nitroaniline
(500 mg/kg), no methaemoglobin was noted at any time between 1 and 48
hours after dosing. Administration of 4-nitroaniline at a single dose
of 100 mg/kg resulted in methaemoglobin, observed over the same time
course. In addition, the cats subjected to this experiment were noted
to be cyanotic and have extensive muscle weakness following
4-nitroaniline.
Ocular toxicity
Dog and miniature swine
Dogs have been shown to develop lesions in the cornea and lens
following prolonged oral administration of
2,6-dichloro-4-nitroaniline. It has been suggested that a
photochemical product reaction may be responsible for the lesion as
it occurred only when dogs were exposed to sun light.
Dogs and miniature swine were fed 2,6-dichloro-4-nitroaniline in
the diet at levels of 0, 0.75, 6, 24, 48 and 192 mg/kg/day for periods
of time varying from 50 to 306 days. Corneal opacity appeared in dogs
within 53 to 104 days after administration of 24 or 48 mg/kg, and when
exposed to sunlight. Dogs unexposed to sunlight and those with one eye
sutured closed failed to develop lesions in the unexposed eyes. Dogs
administered 192 mg/kg refused to eat after 38 days and were
administered 2,6-dichloro-4-nitroaniline by capsule. All of these
animals died 49 - 53 days after the study began. Eye lesions were not
detected in this high level group. Several dogs showing eye damage
were maintained for four months after 2,6-dichloro-4-nitroaniline
administration had ceased. Pathological changes seen in the cornea and
lens were not reversible. Administration of
2,6-dichloro-4-nitroaniline at all levels did not appear to affect
miniature swine. Sporadic instances of the presence of Heinz bodies
in blood were observed in both swine and dogs. Administration of
2,6-dichloro-4-nitroaniline as dust or 5% solution directly to the
eyes for three months had no effect on corneal opacity or irritation
of the conjunctiva (Earl et al., 1971; Bernstein et al. 1970; Curtis
et al. 1968).
Special studies on reproduction
Rat
In a standard three generation, two litters per generation,
reproduction study, 2,6-dichloro-4-nitroaniline was administered to
rats (20 males and 20 females per group) at levels of 0 and 100 ppm in
the diet. On the basis of the reproduction parameters examined,
including number of litters, stillbirths, live births, birthweight,
lactation indices, etc. no evidence of an effect of dicloran on
reproduction was indicated (Lobdell and Johnston, 1965).
Male rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 1000, and 2000 ppm for 90 days. The males were mated with
untreated females. There were no differences observed in the number of
litters or in the number of animals born or weaned. Feeding
2,6-dichloro-4-nitroaniline in the diet to male rats resulted in an
increased liver weight at 1000 ppm. An increased kidney weight was
seen at 1000 ppm (EPA, 1974).
Female rats were fed 2,6-dichloro-4-nitroaniline in the diet at
levels of 0, 500, and 1000 ppm for 188 days prior to mating. The rats
were continued on the diet through gestation and lactation. From the
small number of animals in this experiment (10 females/group), it is
difficult to make definitive conclusions concerning the effect on
reproduction of 2,6-dichloro-4-nitroaniline administered to females.
The data suggested a reduced number of pups when the females were fed
a level of 1000 ppm in the diet. There was no apparent effect on
survival of pups, although the mean body weight of pups at 1000 ppm
was slightly reduced. It might be considered that 1000 ppm in the diet
of females for six months might have a slight effect on reproduction.
No effects were seen at 500 ppm (EPA, 1974).
Rabbit
Groups of pregnant New Zealand white rabbits (10, 12, and 14 does
respectively) were fed 2,6-dichloro-4-nitroaniline in the diet at 0,
100, and 1000 ppm from day 8 until day 16 of gestation. In no case was
there evidence of an adverse effect of 2,6-dichloro-4-nitroaniline on
reproduction, affecting either the parents or the offspring (Anonymous
1974).
Sensitization
Rat, guinea pig, rabbit
An examination was made of the potential skin sensitization
properties of 2,6-dichloro-4-nitroaniline in guinea pigs. Ten
subcutaneous injections were administered to male guinea pigs (total
dose 0.95 mg). Two weeks after the last injection a re-injection of
0.05 mg was made. Twenty-four hour readings showed no apparent
sensitization (Johnston and Sweickert, 1963)
Rats, guinea pigs and rabbits were administered
2,6-dichloro-4-nitroaniline via inhalation exposure to an 8% dust
for seven hours. It was estimated that the exposure averaged 0.4
mg/l. No deaths were observed although reddening of the lungs and
pale kidneys were seen in rats and guinea pigs (Horn, 1961).
TABLE 1 Acute toxicity of 2,6-dichloro-4-nitroaniline
LD50
Species Route (mg/kg) References
Rat oral 4000-10 000 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
Ip 1460-5471 Serrone, 1967
Lessel, 1974a
Feenstra, 1961
SC >5000 Lessel, 1974a
Guinea Pig oral 1450 Lessel, 1974a
Mouse oral 1500-2500 Lessel, 1974a
Feenstra, 1961
Ip 2500-8000 Lessel, 1974a
Feenstra, 1961
SC >6000 Lessel, 1974a
dermal >5000 Lessel, 1974a
Cat oral >500 Lessel, 1974a
oral 200 x 7 daily doses Lessel, 1974a
Signs of poisoning in the mouse included defaecation and
urination, depression and lethargy leading to sleep. In rats the same
signs were noted including nasal haemorrhage and paralysis. Death
occurred up to four days after administration of 2,6-dichloro-4-
nitroaniline (Feenstra, 1961). Cyanosis, muscle weakness, and other
typical signs of aniline poisoning were not observed.
Two formulations (8% dust and 50% W.P.) were applied to intact
and abraded skin of rabbits daily for five days. No irritation of skin
was observed (Johnston and Schwikert 1961a). When these materials were
instilled into the conjunctival sac of rabbits, no ocular irritation
was noted (Johnston and Schwikert, 1961b).
Short Term Studies
Rat
Groups of rats (either 10 males and females or 15 males and
females per group) were treated with 2,6-dichloro-4-nitroaniline to
examine potential haematopoietic effects. One series of animals was
administered 2,6-dichloro-4-nitroaniline at levels of 0, 5, 20 and 100
mg/kg/day by gavage for four months. Another group was administered
2,6-dichloro-4-nitroaniline in the diet at levels of 0 and 20 ppm for
four months. Haematological examinations (RBC, total and differential
leucocyte, platelet, haematocrit and haemoglobin concentration), blood
sugar, as well as growth and food consumption data indicated no
significant effects attributable to 2,6-dichloro-4-nitroaniline at any
of the dose levels or treatments (Evans et al., 1963).
Groups of rats were fed 2,6-dichloro-4-nitroaniline (either
technical or recrystallized material) in the diet for six months.
Groups of 15 males and 15 females were fed 30 and 300 ppm, groups of
10 males and 10 females were fed 3000 ppm of either the technical or
pure material; and groups of 25 males and 25 females were fed a
control diet. At 3000 ppm in the diet, growth of both males and
females fed technical 2,6-dichloro-4-nitroaniline was impaired with
only the males fed the purified material showing a slight reduction in
growth. In both high level groups livers were enlarged at 3000 ppm.
There was no effect on haematology or on tissues and organs. There was
no sign of damage when the tissues were examined microscopically. No
effects were noted at 300 ppm over the six month period (Lessel,
1974d).
Groups of rats (5 males and 5 females per group) were
administered 2,6-dichloro-4-nitroaniline orally at doses of 0, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth of males was
reduced at 350 mg/kg. At both doses, liver enlargement and
histological changes were noted. There was no apparent effect on blood
parameters or on the kidneys when examined at the termination of the
experiment (Lessel, 1974c).
Groups of rats (10 males and 10 females per group, 20 of each sex
were used in the controls) were administered
2,6-dichloro-4-nitroaniline orally at dose levels of 0, 35, 140 and
350 mg/kg/day, 6 days/week, for four weeks. Growth depression was
observed in both males and females at the highest dose level. Growth
depression was also noted at the intermediate level in males only.
Liver enlargement was again observed at 140 mg/kg. No effects were
noted at 35 mg/kg. Microscopic examination revealed the presence of
enlarged liver cells with increased vacuolization especially at the
outer lobes. Some animals were maintained for two weeks after the
conclusion of the treatment. After this two week period, liver size
was normal in all but the highest male dosage level. Liver hypertrophy
caused by repeated short term dosing is apparently reversible within a
two week period on cessation of treatment. In this study daily acute
administration 35 mg/kg was observed to have no effect on the rat
(Lessel, 1974c).
Because of the known effect of 4-nitroaniline in inducing
specific blood dyscrasias, subacute feeding experiments were performed
to compare 2,6-dichloro-4-nitroaniline with this material. Groups of
weanling rats (5 males and 5 females/group) were administered
2,6-dichloro-4-nitroaniline by gavage at 0 and 400 mg/kg, 5 days/week
for four weeks. 4-Nitro-aniline was administered at 200 and 400 mg/kg
to two other comparable groups over the same interval. In a second
experiment, groups of male weanling rats (6 rats per group) were
administered 2,6-dichloro-4-nitroaniline by oral gavage at 0 and 400
mg/kg and 4-nitroaniline at 200 and 400 mg/kg, twice daily, 5
days/week, for two weeks. With 2,6-dichloro-4-nitroaniline at 400
mg/kg haematology was normal - no Heinz bodies were detected and the
reticulocyte count was normal. At the 800 mg/kg dose there was a
slight weight loss. The red blood cell and haemoglobin counts were
normal while lymphocyte counts were slightly reduced. It was noted at
the conclusion of the study that there was no effect of this compound
on the spleen. In contrast, 4-nitroaniline had definitive effects on
growth at 200 mg/kg per day. Heinz bodies were identified in the blood
and the reticulocyte count was greatly elevated (marked
reticulocytosis). Bone marrow was not affected. At 200 mg/kg (2x/day)
there was a reduction of growth, reduced RBC count (with polychromasia
and nucleation) accompanied by an enlarged spleen. These effects were
more pronounced at the higher dose where, in addition, a haemoglobin
was reduced and the lymphocyte count greatly increased. At high
levels, although 2,6-dichloro-4-nitroaniline caused lymphopenia, the
hemotoxic effects normally associated with 4-nitroaniline were not
observed (Lessel, 1974b).
Mortality was observed when rats were administered
2,6-dichloro-4-nitroaniline at 1000 mg/kg. No mortality was noted when
400 mg/kg was administered for three months. Liver and kidney changes
were observed with light and electron microscopic examinations
(Serrone, 1967).
Dog
Groups of dogs (8 males and 8 females per group) were fed
2,6-dichloro-4-nitroaniline in the dry diet at levels of 0, 20, 100
and 3000 ppm for two years. One female dog at 3000 ppm died at 74
weeks. This death war attributable to the presence of
2,6-dichloro-4-nitroaniline in the diet. One male control dog lost
considerable weight but survived to the end of the experiment. No
compound-related changes in behaviour, food consumption, or growth
were observed. A watery lacrimation was noted for all dogs at 3000 ppm
which persisted during the entire testing interval. A yellowing of the
sclera, mucous membranes, and abdominal skin was noted at the high
level of feeding. The dog that died showed a picture of haemolytic
anemia prior to death (reduced haemoglobin, immature erythrocytes,
polychromophylic macrocytes, increased leucocyte count and increased
M:E ratio in the bone marrow). At 3000 ppm, clinical chemistry was
altered in both males and females with an elevation observed in the
activities of the SGOT and SGPT enzymes, a reduced blood protein,
increased prothrombin time, BUN, BSP and urinary albumin content. At
the conclusion of the study, gross and microscopic examination
revealed an increase in liver weight accompanied by histological
changes at 3000 ppm in the diet. Histological changes in the animals
fed 3000 ppm in the diet included irregular hepatic cell size, hepatic
cell hypertrophy and increased pigmentation of hepatic cells and of
liver macrophages. Slight changes at 100 ppm were noted in two dogs. A
no-effect level in this study is estimated to be between 100 and 3000
ppm in the dry diet (Woodard et al., 1964).
Monkey
Daily oral administration to Rhesus monkey at 160 mg/kg was
lethal within three months with a greater effect noted on females than
males. Coloration of monkey urine differed from rat urine suggesting a
difference in metabolism in the two species. Liver and kidney changes
were observed after light and electron microscopic examination.
Centrolobular fatty degeneration was observed. Swelling of
mitochondria with distortion of the cristal was also observed. There
were differences in the comparative effects of
2,6-dichloro-4-nitroaniline on liver metabolizing enzymes of monkey
and rat (Serrone, 1967).
Long-Term Studies
Rat
Groups of rats (35 males and 35 females/group) were fed
2,6-dichloro-4-nitroaniline in the diet at levels of 0, 20, 100 and
3000 ppm for two years. At 100 ppm there was no effect on behaviour,
mortality or growth. At this level all values from treated animals
were comparable to control values. Growth and food consumption of both
males and females was depressed at 3000 ppm. Haematological parameters
(haemoglobin and packed cell volume) were reduced at 3000 ppm. These
haematological changes were noted only after the first year of
treatment. Gross and microscopic examination performed at 13 weeks and
at the conclusion of study showed slightly higher liver weights,
kidney weights, testicular weights and (possibly) thyroid weights at
3000 ppm. The incidence and location of neoplasms in all treatments
did not differ from those in the controls. Histological examination
revealed liver changes at 3000 ppm, characterized by hepatic cell
enlargement, glycogen depletion, increased basophilia of the
cytoplasm, and the presence of necrobiotic hepatic cells. Histological
examination performed at 13 weeks also indicated hepatic cell changes
and slight adrenal cortical atrophy in several animals at 3000 ppm.
The adrenal changes were not noted at 104 weeks. An estimated
no-effect level in this study is between 100 and 3000 ppm in the diet
(Woodard et al., 1964).
Groups of rats (25 males and 25 females/group, Boots-Wistar
strain) were fed 2,6-dichloro-4-nitro-aniline in the diet at
concentrations of 0 and 1000 ppm for two years. There was no effect on
survival, food consumption, growth, haematology, or upon gross and
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular makeup of
liver, kidney, or spleen. The incidence of tumors in the control and
treatment group was similar. From the results of this experiment a
suggested no-effect level is greater than 1000 ppm (Lessel, 1974e).
OBSERVATIONS IN MAN
In a clinical double blind study, two groups of adult males were
administered 2,6-dichloro-4-nitroaniline (20 individuals) or a placebo
(10 individuals) once a day for ninety days.
2,6-Dichloro-4-nitroaniline was administered at a level of 10 mg per
day. Hematological, liver function, and kidney function tests were
performed at various intervals over the course of the study and were
found to be normal. There were no indications that administration of
2,6-dichloro-4-nitroaniline at 10 mg per day to adult males had any
adverse effect (Stough, 1962).
Extensive examinations were made on one industrial worker
occupationally exposed to 2,6-dichloro-4-nitroaniline over a period of
three years. It was reported that for about 60 days per year
considerable inhalation and dermal exposure had occurred. No adverse
effects were observed with the individual (Brooks and Boyack, 1963).
Another investigation in man on the potential ocular problem
associated with 2,6-dichloro-4-nitroaniline was again negative
(Manger, 1972).
COMMENTS
2,6-Dichloro-4-nitroaniline has a low order of toxicity to
mammals, including man. 2,6-Dichloro-4-nitroaniline is rapidly
metabolized in plants, and fragments of the molecule are
reincorporated as natural plant constituents. In mammals,
2,6-dichloro-4-nitroaniline is rapidly absorbed, metabolized and
excreted. Metabolism in mammals results in formation of the
chlorinated phenylenediamine and aminophenol which are conjugated and
excreted. 2,6-Dichloro-4-nitroaniline does not induce
methemoglobinemia as evidenced with 4-nitroaniline.
2,6-Dichloro-4-nitroaniline does not affect reproduction in rodents
and has shown no evidence of being a teratogen under the experimental
protocol used. Following subacute feeding, dogs exposed to sunlight
developed cataracts. Specific experiments with rabbits, rats and
swine did not duplicate these results. Long term feeding studies in
rat and two year feeding studies in dog resulted in growth
retardation accompanied by an increased liver and kidney size at
high levels. No-effect levels based on a two year dog study and short
and long term rat studies formed the basis for allocating a temporary
ADI for man. A short term study in man is reassuring in estimation of
the temporary ADI although no conclusions could be drawn on the
possibility of ocular damage to man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR
MAN
0 - 0.03 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of 2,6-dichloro-4-nitroaniline as a commercial
fungicide is recorded in Canada, France, Holland, Italy, Japan, New
Zealand, South Africa and USA.
It is claimed to be effective against several Basidiomycetes
and Deuteromycetes species of fungi, being fungistatic to the
mycelium and spores of Botrytis cenera (Clark et al., 1960; Clark
and Hams, 1961; Sharples, 1962) and to several Rhizopus fruit rot
fungi (Ogawa et al., 1961, 1962; Cappelini and Stretch, 1962).
It is mostly marketed as 4% or 8% (w/w) dust formulations or as
50% (w/w) wettable powders, alone or mixed with thiram (TMTD) or
pentachloronitrobenzene. Smoke formulations containing 40% dicloran
are also available.
Pre-harvest treatments recommended by the manufacturers include
soil treatments for lettuce under glass, dusting or spraying of soft
fruits, cotton, leafy vegetables, strawberries, onions, garlic,
tomatoes and ornamentals. Post-harvest dips of peaches, nectarines and
carrots are practised at rates of 750-1000 mg/kg a.i.
Phytotoxicity
Under extreme experimental conditions a "bronzing" discolouration
of lettuce leaves and marginal "off-taste" taints in fruits have
occasionally been found, but none of these effects have ever been
reported in practical use.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data on 2,6-dichloro-4-nitroaniline in several fruit and
vegetable crops have been presented by the originating company (The
Boots Company Ltd., 1972). The data derive from field trials and
post-harvest experiments carried out mainly in the USA and UK.
Summaries of residue ranges and experimental conditions extracted from
these data are presented in Tables 2, 3 and 4.
Peaches and apricots
Extensive trials with single or repeated spraying of peaches and
apricots at recommended dosage rates (usually 0.12-0.18% a.i.) and
higher rates have been carried out during the period from 1960 to 1965
(Tables 2 and 4). The initial deposits of 2,6-dichloro-4-nitroaniline
on peaches are generally between 5 and 15 mg/kg with occasional values
up to 25-30 mg/kg. A typical residue was 5 mg/kg after 6 days, 3 mg/kg
after 9 days and 1.5 mg/kg after 14 days. The highest recorded residue
14 days after treatment at a dosage rate of 0.18% was 4.9 mg/kg.
The average half-life of 2,6-dichloro-4-nitroaniline on peaches
has been calculated to be 5 days.
Spraying apricots gave residues very similar to those found in
sprayed peaches (Table 4).
Dusting peaches using 8% a.i. dicloran powders at rates of 3-5 kg
per ha gave maximum initial deposits of 3.7 mg/kg.
A suggested practice of wrapping apricots and peaches in tissues
impregnated with 2,6-dichloro-4-nitroaniline (from 500-3000 mg/kg, in
the wrap) showed a definite transfer of the chemical to the fruits.
Residues from such post harvest application were from 1.6 - 3.5 mg/kg
in peaches and 1.9 - 3.3 mg/kg in apricots (Table 3).
TABLE 2 Residues of dicloran in peaches
Application Number Number Number Range of
(% a.i. w/v or PHI of of of results
kg a.i./ha) (days) applications trials results mg/kg
0.06% 3 3 1 4 0.1-0.3
0.09% 0 1-3 3 9 6.1-16.5
1 1-3 5 9 1.5-11.6
2-3 1-3 2 7 0.2-4.7
7 1 1 1 2.3
0.12% 0 1-3 1 6 10.8-14.0
1 1-2 1 2 3.4-5.5
3 1-3 2 10 0.2-6.3
7 1 1 1 3.2
0.18% 0 2 1 6 6.0-11.4
1 2 1 6 9.2-13.7
2 2 1 6 5.7-13.8
4 2 1 6 4.2-9.4
6 2 1 6 4.5-7.8
9 2 1 6 1.9-6.0
14 2 1 6 0.6-4.9
0.18% 0 3 1 4 18.9-29.5
3 3 1 4 22.4-29.6
0.24% 1 3 1 4 3.0-8.3
3 3 1 4 2.7-11.1
3.1 kg/ha
(8% dust) 0 1 1 2 0.14-0.35
4.5-4.9 kg/ha
(8% dust) 0 1 3 21 0.22-3.7
TABLE 3 Residues in fruits wrapped in tissues impregnated with
dicloran
Fruit Dicloran in wrap mg/kg Dicloran in fruits
Apricot 500 1.9
1000 2.6
2000 3.3
Peach 500 1.6
1000 2.3
2000 3.8
3000 3.5
TABLE 4 Residues of 2.6-dichloro-4-nitroaniline in fruit and vegetables
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Apricots 0.09% 0 1 1 12 1.9-7.9
2 3 1 2 0.9-1.4
4 1-2 1 4 1.4-3.7
11 1 1 2 0.1-0.7
Apricots 0.12% 0 1 1 1 10.1
1 1 1 1 8.0
7 1 1 1 3.8
Cherry 0.12% 0 1 1 1 10.3
1 1 2 5 6.4-14.8
7 3 1 1 4.9
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
0.24% 1 1 1 2 0.7-11.2
7 1 1 1 2.9
0.36% 1 5 1 2 2.7-3.1
1200 ppm post-harvest 6 21 1.5-6.4
1200 ppm post-harvest
and washing 1 2 0.3-0.3
1350 ppm post-harvest 1 2 10.2-13.7
1800 ppm post-harvest 1 2 7.7-8.4
Grapes 1.7 kg/ha
(dust) 0 2-4 2 6 0.2-3.9
7 2 1 2 0.3-1.1
2.3-3.3
kg/ha (dust) 1 4 1 4 1.1-1.3
7 1-2 2 4 0.2-1.1
1000 ppm post-harvest dip 1 4 0.6-5.1
Plums
(dried) 1000 ppm post-harvest dip 1 3 1.0-4.7*
post-harvest dust 1 3 2.0-8.1
0.12% 1 1 1 1 2.5
Strawberry 0.16-0.18% 1 4 1 1 0.32
3 1 1 2 4.0-5.7
9-11 3-4 2 4 0.2-1.6
0.24% 1 3 1 2 0.9-1.7
Blackberry 1.1 kg/ha
(spray) 4-11 1 1 2 1.2-2.3
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
1.8 kg/ha
(dust) 4-11 1 1 2 <0.05-0.3
Currants 0.11-0.15% 33 4 1 3 1.7-3.3
Raspberry 0.15-0.18% 5 4 2 2 17.0-20.2
9-10 4-5 2 2 2.2-11.8
13-14 3-4 2 2 2.6-7.5
0.36% 5 4 1 1 39.0
10 4 1 1 28.4
14 4 1 1 16.3
Carrots 900- post-harvest dip
1000 ppm (stored 0-3 days) 2 12 3.6-9.4
peeled 1 1 2.1
canned 1 7 <0.05-0.2
Lettuce 2.2 kg/ha 0 2 1 4 50.1 (average)
7 2 1 9 18.0 "
RL50 approx 14 2 1 9 3.0 "
3-4 days
21 2 1 9 2.4 "
28 2 1 8 0.8 "
35 2 1 8 0.04 "
1.3 g/m2
(dust) 120 1 1 1 0.1
1.3 g/m2 (soil
treatment;
pre-planting) 114-140 1 3 5 0.2-2.1
TABLE 4 (Cont'd.)
Number Number Number Range of
PHI of of of results
Crop Application (days) application trials results (ppm)
Gherkins
(indoor) 0.05% 0 1 1 1 5.5
2 1 1 1 1.1
4 1 1 1 0.6
5 1 1 1 0.35
Tomato
(indoor) 0.1% 2 1 1 1 0.25
4 1 1 1 0.2
6 1 1 1 0.04
Beans,
French 3 kg/ha 12 4 1 2 1.3-1.9
20 2 1 2 0.8-1.5
* Loss by drying.
Cherries, grapes and plums
The residue levels of 2,6-dichloro-4-nitroaniline from
recommended uses on these fruits are similar to those in peaches and
apricots (Table 4).
After dipping procedures or post-harvest dusting of plums,
dicloran was retained on the fruits at levels of 4.7 and 8.1 mg/kg
respectively. These residues were reduced to 1.0 and 2.0 mg/kg
respectively, when the fruits were subjected to an 8 day air-drying
period at ambient temperature.
A post-harvest treatment of cherries by spraying on the packing
line with 1200-1800 ppm suspensions gave residues of
2,6-dichloro-4-nitroaniline from 1.5 - 13.7 mg/kg. In storage
experiments these residues were reduced at a rate corresponding to a
half-life of 11 days at 20°C.
Small fruits and berries
Repeated spraying of strawberries at different locations (0.06
- 0.24% a.i.) have been reported to give maximum initial deposits of
dicloran of 9 mg/kg. However, residues at harvest after a 9 - 11 day
interval from the last application were generally well below 1 mg/kg,
with an occasional individual sample at 1.6 mg/kg. Among the berries,
raspberries in some cases showed higher residues. After application of
0.36% a.i. the initial residue was 39 mg/kg which was reduced to 16.3
mg/kg at 14 days. At lower application rates, residues were
proportionately lower.
Experiments with other berries (blackberries, currants and
boysenberries) generally gave residues below 5 mg/kg.
Lettuce
Roburn (1960) followed the disappearance of
2,6-dichloro-4-nitroaniline after post-planting treatments of lettuce
plants with 1/4 oz of 4% dust per sq. yd. and found a reduction in
deposits greater than that due to growth dilution. Volatilization and
chemical degradation were suggested as contributing factors.
The half-life values decreased progressively in these trials,
being about 3-5 days during the last weeks of the growing season. A
three weeks pre-harvest interval for dicloran treatments of lettuce is
recommended on this basis in the United Kingdom.
Later experiments by Boyack and Boot (1962 a, b, c) confirmed
Roburn's findings, indicating half-life values of 3-4 days on
greenhouse lettuce with average residues of 2.4 mg/kg after an
interval of 21 days (Table 4).
Uptake of 2,6-dichloro-4-nitroaniline through the roots of
lettuce plants after pre-planting soil treatments has been
demonstrated by Boyack and Boot (1962a), Lemin and co-workers (Lemin,
1963, 1965; Moe and Lemin, 1963a, 1964) and Groves and Chough (1970).
Residues from such practices at the time of harvest will be near or
below the detection limit, i.e. less than 0.05-0.1 mg/kg.
In recent experiments in the Netherlands lettuces have been
treated both by a pre-planting soil application and by dusting the
young plants immediately after planting. (Pieters, 1974). Residues in
these experiments ranged from 0.1 - 2.1 mg/kg (Table 4).
Carrots
Post-harvest dipping in 900-1000 ppm suspensions caused residues
of 3.6-9.4 mg/kg at 0-3 days. After storage for 5 months the remaining
residues were 2.1-3.7 mg/kg.
Other vegetables
Residue data on a few other vegetable crops, e.g. tomatoes,
gherkins and French beans, mostly grown under glasshouse conditions,
are available (Pieters, 1974). The data are insufficient to evaluate
quantitatively the rates of dissipation, but residues are generally
low at the time of harvest, i.e. from a few days to 1-2 weeks after
treatment (Table 4).
FATE OF RESIDUES
In animals
The metabolism of 2,6-dichloro-4-nitroaniline by the rat, mouse
and to a limited extent man, has been described above under
"Biotransformation". No such experimental evidence is available on
livestock animals, nor on the excretion of dicloran or its metabolites
in milk.
In plants
Studies by Lemin (1965), Lemin et al. (1963) and Moe and Lemin
(1963a, 1964) have shown that 2,6-dichloro-4-nitroaniline is absorbed
by plant roots and translocated to the plant material in tomato and
lettuce. These experiments include uptake from both nutrient solutions
and treated soils.
Application of 14C-labelled 2,6-dichloro-4-nitroaniline gave
evidence of rapid degradation into polar metabolites and 14C was
found in carbohydrate constituents of the plant tissue, presumably
owing to the incorporation of degradation fragments. Transitional
metabolites such as dechlorinated components, reduction products or
2,6-dichloro-p-phenylene-diamine were not detected.
Amino acids, chlorophyll or uronic acid did not contain
radioactivity. Neither could 2,6-dichloro-4-aminophenol, known to be
formed by animal metabolism (see previous section), be found in plant
tissues.
Exhaustive extractions of peaches 13 days after treatment with
14C-labelled 2,6-dichloro-4-nitroaniline revealed (in addition to
unchanged parent compound) 14C-labelled phenylalanine and labelled
flavonoid glycosides (Moe and Lemin, 1963b). As in the above
experiments, no transitional metabolites could be traced.
Groves and Chough (1970) report rapid absorption of dicloran and
incorporation of fragments into tissue constituents by a number of
plants.
In processing and storage
Washing or canning processes have been shown to reduce residues
of 2,6-dichloro-4-nitroaniline considerably (Boots Company Ltd.,
1972). On lettuce, for example, residues of 18-25 mg/kg still
remaining two weeks after the last application were reduced to 5.6-6.0
by washing with water (Roburn, 1958). In peaches, freshly treated with
2,6-dichloro-4-nitroaniline, the residues which still remained after
an industrial canning procedure were below 0.1 mg/kg.
Carrots treated by post-harvest dipping showed only moderate
reduction of the residues by peeling, from 3.7 to 2.1 mg/kg,
indicating a significant penetration into the inner parts. A
subsequent canning procedure, however, reduced the levels to 0.1-0.2
mg/kg. Storage of dipped carrots for 5 months at 40°F gave a decrease
in the residue of about 25%.
In soils
2,6-Dichloro-4-nitroaniline is generally regarded as being
relatively stable in soils at field moisture capacity. Its
persistence, however, is greatly affected by conditioning factors such
as soil composition, water content, microculture etc. (Wang and
Broadbent, 1973). The absorption of the chemical from soils by oats
was found by Groves and Chough (1970) and Wang (1972) to be inversely
related to the clay and organic matter content of the soils,
suggesting a binding of the chemical to these soil constituents. Later
exhaustive extraction experiments showed that a reversible binding
probably occurs (Groves and Chough, 1971).
The same authors (Groves and Chough, 1970, 1971) showed that
14C-labelled dicloran can be metabolized by soil organisms which
degrade the compound to 14C-carbon dioxide and other volatile
compounds. The rate of microbial breakdown could be increased by
repeated applications of dicloran to the same soil or by incubation of
the soil with dicloran. A culture of rod-shaped bacteria was isolated
and found active in the decomposition of the fungicide. Microbial
breakdown is also held responsible for the rapid degradation of DCNA
which is seen in soil under flooded conditions (Wang and Broadbent,
1973).
Van Alfen and Kosuge (1974) have identified both
2,6-dichloro-p-phenylenediamine and its acetylated derivative,
4-amino-3,5-dichloroacetanilide, in cultures of Pseudomonas cepacia
and Escherichia coli B. In soil and through the action of
horseradish peroxidase, the phenylenediamine was converted to one of
three isomers of the azine resulting from oxidative dimerization (Van
Alfen, 1973).
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Market sample surveys carried out in the Netherlands in 1973 on
domestically grown fruit and vegetables showed that residues of
2,6-dichloro-4-nitroaniline were frequently present in some crops
(Table 5). In vegetables such as endive, lettuce and chicory sprouts
about 50-80% of samples were positive with individual residues up to 8
mg/kg. Crops such as cucumbers, tomatoes and paprika were less
frequently positive (3-25%). At the time of this survey, the tolerance
for vegetables in the Netherlands was 10 mg/kg.
TABLE 5 Dicloran residues in marketed vegetables (Netherlands, 1973)
Dicloran
residues,
mg/kg number of samples
(range)
Cucumber Endive Lettuce Paprika Tomato Chicory
not detectable 31 34 136 12 30 68
0.01 - 0.1 1 16 139 2 4 50
0.1 - 0.3 - 7 126 2 - 21
0.3 - 1.0 - 8 180 - - 3
1.0 - 3.0 - 4 123 - - 1
3.0 - 10.0 - 1 35 - - -
Total number 32 70 739 16 34 143
[text missing]
pre-harvest treatments on fruits, vegetables and ornamentals and
post-harvest treatments on peaches, nectarines, cherries and carrots.
It is registered in several countries, usually as 4 or 8% (w/w) dusts
or as 50% (w/w) wettable powders.
The technical grade typically contains about 96%
2,6-dichloro-4-nitroaniline. The remaining parts consist of chemically
related compounds and impurities which have been identified and
quantified.
Concentrations and rates of application vary, depending on the
crop and method of application. Normal spraying concentrations are
0.05% to 0.18% applied at rates of 2-5 kg a.i. per ha. Post-harvest
dips are practised at rates of 1000 - 2000 mg per litre.
The residue data available are mainly obtained from the USA and
UK, in a few cases supplemented from other countries. Most of the data
derive from field trials or experiments under practical conditions
likely to represent the results of good agricultural practice.
2,6-dichloro-4-nitroaniline is a non-systemic compound of
relatively low persistence. The dissipation of the compound on fruits
and vegetables is often faster than can be explained by normal
weathering or growth dilution. Half-life periods of 11 days have been
found for residues during storage of post-harvest treated cherries.
2,6-dichloro-4-nitroaniline is absorbed by plant roots from the
soil. The fate of residues in plant material has been followed with
the radio-labelled compound, which indicated a rapid degradation into
polar metabolites and 14CO2 followed by incorporation of 14C into
normal metabolic plant constituents. No indications of transitional
metabolites from such degradation have been found.
Similar degradation of 2,6-dichloro-4-nitroaniline occurs in
soils, although in this case microbial breakdown may also produce
smaller amounts of reduction products and acetylated derivatives of
the parent compound.
Studies were available on the metabolic fate of residues in rats
indicating a fairly rapid process of absorption, metabolism and
excretion, especially as aminophenol and phenylenediamine derivatives,
in the urine. There was no evidence of tissue storage in these
experiments. Preliminary studies in man suggest a metabolic pattern
similar to that of rats. No information, however, has been recorded on
metabolism in livestock animals or on transfer of residues into meat
or milk.
METHODS OF RESIDUE ANALYSIS
A colorimetric method of residue analysis, based on the
development of a yellow colour characteristic of some mononitro
aromatic compounds in the presence of strong alkali and acetone, is
described by Kilgore et al. (1962). Extraction is with benzene,
followed by clean-up on Florisil alone or in combination with an
acetonitrile-petroleum ether partitioning procedure. Blank values for
fruits were about 0.01-0.02 mg/kg and recoveries in the range 75 -
100% (average 86%). The colorimetric method described by Groves and
Chough (1966) and Heagy (1969) has a similar sensitivity, based on
diazotization and coupling to N-(1-naphthyl)ethylenediamine has a
similar sensitivity.
An alternative method developed by Roburn (1961) utilizes the
reduction of 2,6-dichloro-4-nitroaniline by zinc and hydrochloric acid
to the corresponding 2,6-dichloro-p-phenylenediamine which, in the
presence of aniline, can be oxidized to produce an intense blue
colour. Less than 2 µg can be detected by this method, corresponding
to 0.05 mg/kg in fruit and 0.2 mg/kg in lettuce. Recovery of surface
deposits was approximately 100%, but for macerated samples it was only
71%. Chloronitroanilines in general give similar reactions but a
series of other pesticide chemicals did not interfere, indicating a
fair degree of specificity of the method.
A thin-layer chromatographic spot test sensitive to 0.5 µg of
2,6-dichloro-4-nitroaniline is given by Groves et al. (1966) based on
their colour reaction mentioned above.
Gas-chromatographic methods for the determination of
2,6-dichloro-4-nitroaniline have been developed by Beckmann and
Bevenue (1962) and Kilgore (1964) using electron capture and
microcoulometric detectors, in combination with the Kilgore extraction
procedure already mentioned. Recoveries of 85-105% were established by
Brewerton et al. (1967) for the analysis of dicloran in soils, fruit
and vegetables down to the 0.1 mg/kg level by the GLC/EC technique.
These methods could be adapted for regulatory purposes as part of a
multi-residue procedure.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Tolerance
Country Crop mg/kg
Australia Beans, lettuce, stone fruits, tomatoes 20
Belgium Fruit and vegetables (exc. potatoes) 10
Canada Apricots, nectarines, peaches
(pre-harvest and post-harvest),
sweet cherries pre-harvest and
post-harvest), snap beans 20
Strawberries, raspberries 15
Celery, grapes, lettuce, rhubarb,
sweet potatoes 10
Carrots, cucumbers, garlic, onions,
plums, tomatoes 5
Germany All fruits and vegetables 0.1
Tolerance
Country Crop mg/kg
Italy General 10
Switzerland Lettuce (tentatively) 0.5
Netherlands Lettuce, endive 3
Chicory (sprouts) 1
Cucumbers, gherkins, melons, bell
peppers, tomatoes 0.3
Other vegetables 0.1
USA Apricots, nectarines (pre-harvest and
post-harvest), peaches (pre-harvest
and post-harvest), sweet cherries
(pre-harvest and post-harvest), snap
beans 20
Blackberries, boysenberries, celery,
plums (fresh prunes) (pre-harvest and
post-harvest), raspberries 15
Carrots (pre-harvest and post-harvest),
grapes lettuce, rhubarb, sweet
potatoes (pre-harvest and post-harvest) 10
Cucumbers, garlic, onions, tomatoes 5
Potatoes 0.25
Cotton seed 0.1
APPRAISAL
2,6-dichloro-4-nitroaniline is a fungicide active against the
mycelium and spores of Botrytis cinerea, B. sclerotinia and other
fungi, including several Rhizopus fruit rots.
Practical use patterns comprise foliar applications and soil
treatments under indoor and outdoor growing conditions.
The residue data from supervised trials on several crops were
considered sufficiently extensive to enable recommendations to be
made, including some for post-harvest treatments.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for specific determination.
These methods are suitable for regulatory purposes.
RECOMMENDATIONS
Temporary tolerances are recommended for
2,6-dichloro-4-nitroaniline in the following commodities at the
levels indicated. The levels are not likely to be exceeded when
2,6-dichloro-4-nitroaniline is applied in accordance with good
agricultural practice including both pre-harvest and post-harvest
applications and taking into account that preharvest intervals may
apply for certain treatments.
TEMPORARY TOLERANCES
Limit Basis on which recommendations
Crop (mg/kg) are made
Cherries 15 pre and post-harvest
Peaches 15 pre and post-harvest
Apricots 10 pre and post-harvest
Carrot 10 post-harvest only
Grapes 10 pre and post-harvest
Lettuce 10 pre-harvest only
Plums 10 pre and post-harvest
Raspberry 10 pre-harvest only
Strawberry 10 pre-harvest only
Blackberry 5 pre-harvest only
Currant (red, black
and white) 5 pre-harvest only
Beans (French) 2 pre-harvest only
Gherkins 0.5 pre-harvest (indoor) only
Tomato 0.5 pre-harvest only
FURTHER WORK OR INFORMATION
REQUIRED (by 1977)
1. Further studies on the ocular disturbance observed in dogs to
confirm and clarify this effect.
2. Information on fate in livestock animals in so far as plant
material containing residues may be fed to animals.
DESIRABLE
1. Effect on hepatic microsome systems in several species.
2. Further observations in man.
3. Further information on fate of residues during storage, transport
and processing of fruit and vegetables.
4. Information on transfer of residues from grapes to wine and on
possible influence on wine processing.
5. Further information on soil residues and their possible uptake
into subsequent crops.
6. Further data to clarify inconsistencies in the residue levels
found in different berries.
REFERENCES
Anonymous. (1974) "Somers test" in rabbits. Technical summary reports
submitted to WHO by UpJohn Co. (Unpublished)
Beckmann, H. and Bevenue, A. (1962) Pesticide residue analysis Gas
chromatographic analysis of 2,6-dichloro-4-nitroaniline. J. Food Sci.,
27:602-604.
Bernstein, H.N., Curtis, J., Earl, F.L. and Kuwabara, T. (1970)
Phototoxic corneal and lens opacities in dogs receiving a fungicide,
2,6-dichloro-4-nitroaniline. Archs Ophthal. (Chicago), 83:336-348.
Boots Co. Ltd. (1972) Dicloran. Registration Document Vol. I, II, III
and VI. Reports submitted by The Boots Company Ltd. Agricultural
Research, Nottingham. (Unpublished)
Boyack, G. and Boot, D. (1962a) Assay for 2,6-dichloro-4-nitroaniline
in lettuce grown in treated soil under glass. Dicloran, Registration
document from The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G, and Boot, D. (1962b) Assay for 2,6-dichloro-4-nitroaniline
on sprayed greenhouse lettuce. Dicloran, Registration document from
The Boots Co. Ltd. Vol. II, Appendix 26.
Boyack, G. and Boot, D. (1962c) Residue of 2,6-dichloro-4-nitroaniline
(DCNA) on lettuce dusted with Botran. Dicloran, Registration document
from The Boots Co. Ltd. Vol. II, Appendix 26.
Brewerton, H.V., Clark, P.J. and McGrath, H.J.W. (1967) Dicloran
analysis by gas chromatography. New Zealand J. Sci., 10:124-127.
Brooks, B. and Boyack, G. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Report submitted by
The Upjohn Co. (Unpublished)
Cappellini, R.A. and Stretch, A.W. (1962) Control of post-harvest
decays of peaches. Pl. Dis. Reptr., 46:31-33.
Clark, N.G., Hams, A.F., Higgons, D.J. and Stevenson, H.A. (1960) A
new fungicide active against Botrytis spp. Chemy Ind., 1960:572-573.
Clark, N.G. and Hams, A.F. (1961) Antifungal activity of substituted
nitroanilines and related compounds. J. Sci. Food Agric., 12:751-757.
Curtis, J., Bernstein, H.N., Earl, F.L. and Smalley, H., Jr. (1968)
Corneal and lens opacities in dogs treated with
2,6-dichloro-4-nitroaniline. Toxic. appl. Pharmac., 12:305.
Earl, F.L., Curtis, J., Bernstein, H.N. and Smalley, H., Jr. (1971)
Ocular effects in dogs and pigs treated with dichloran. Food Cosmet.
Toxic., 9:819-828.
Eberts, F.S. (1965) Fate of Botran (2,6-dichloro-4-nitroaniline) in
rat and man. Dicloran, Registration Document, Vol. IV, Appendix 54.
Boots Co. Ltd. (Unpublished). Botran Symposium, Augusta, Mich. 1965.
Cited in Chemical Abstracts 1968, 68:abstract 11908g
EPA. (1974) Data from EPA Laboratories. (Unpublished)
Evans, J., Mengel, G. and Bostwick, L. (1963) Botran(R) (U-2069)
Effect of oral administration. Final report, four months study. Report
submitted by the Upjohn Co. (Unpublished)
Feenstra, E. (1961) Report submitted by The Upjohn Co. (Unpublished)
Groves, K. and Chough, K.S. (1966) Determination of small quantities
of 2,6-dichloro-4-nitroaniline (dicloran). J. agr. Food Chem.,
14:668-669.
Groves, K. and Chough, K.S. (1970) Fate of the fungicide
2,6-dichloro-4-nitroaniline (DCNA) in plants and soils. J. agr. Food
Chem., 18:1127-1128.
Groves, K. and Chough, K.S. (1971) Extraction of
3-amino-1,2,4-triazole (Amitrole) and 2,6-dichloro-4-nitroaniline
(DCNA) from soils. J. agr. Food Chem., 19:840-841.
Gurd, P.R. (1974) Methaemoglobin formation in the cat. Report from
Boots Pure Drug Company Limited. Submitted to WHO by the Boots Co.
Ltd. (Unpublished)
Heagy, J.A. (1969) Colorimetric determination of
2,6-dichloro-4-nitroaniline (dicloran) residues in foods. J. Ass. off.
analyt. Chem., 52:797-799.
Horn, J. (1961) U-2069 - Acute inhalation toxicity. Study from Woodard
Research Corporation submitted by The Boots Co., Ltd. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. and Peters, J. (1969) Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: A preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Johnston, R. and Schwikert, R. (1961a) Skin irritation in rabbits.
Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1961b) Dichloran - eye irritation in
rabbits. Report from The Upjohn Co. submitted by The Boots Co., Ltd.
(Unpublished)
Johnston, R. and Schwikert, R. (1963) Skin sensitization in guinea
pigs. Report from The Upjohn Co. (Unpublished)
Kilgore, W.W. (1964) 2,6-dichloro-4-nitroaniline. Analytical methods
for pesticides, plant growth regulators and food additives. Gunter
Zweig (Editor). Vol. III. p. 61-68.
Kilgore, W.W., Cheng, K.W. and Ogawa, J.M. (1962) Extraction and
determination of 2,6-dichloro-4-nitroaniline in processed fruits. J.
agr. Food Chem., 10:399-401.
Lemin, A.J. (1965) Translocation and metabolism of
2,6-dichloro-4-nitroaniline by lettuce and tomato. J. agr. Food Chem.,
13:557-560.
Lemin, A.J. Moe, L.D. and Smith, A.H. (1963) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce. Dicloran, Registration
document from the Boots Co. Ltd. Vol. II, Appendix 34 A.
Lessel, B. (1974a) Experimental data on toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974b) Effect on blood and blood forming tissues. Report
from The Boots Co. Ltd. (Unpublished)
Lessel, B. (1974c) Short term (4-week) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974d) Chronic (6 month) oral toxicity. Report from The
Boots Co. Ltd. (Unpublished)
Lessel, B. (1974e) Two year feeding trial in rats on dicloran
(2,6-dichloro-4-nitroaniline). Report submitted by the Boots Co. Ltd.
(Unpublished)
Lobdell, B. and Johnson, C. (1965) U-2069 - Effect on reproduction
capacity through three generations in the rat. Report from Woodard
Research Corporation submitted by The Boots Co. Ltd. (Unpublished)
Manger, W.H. (1972) Use and handling of dicloran formulations and
influence on human eyesight. Report from Biological Laboratory,
Aseptafabrick B.V. Delft, Netherlands. (Unpublished)
Maté, C., Ryan, A.J. and Wright, S.E. (1967) Metabolism of some
4-nitroaniline derivatives in the rat. Food Cosmet. Toxic., 5:657-663.
Moe, L.D. and Lemin, A.J. (1963a) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part II, dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 B.
Moe, L.D. and Lemin, A.J. (1963b) The metabolism of
2,6-dichloro-4-nitroaniline-C14 in peaches. Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 35.
Moe, L.D. and Lemin, A.J. (1964) The metabolism of
2,6-dichloro-4-nitroaniline by bibb lettuce, Part III. Dicloran,
Registration document from The Boots Co., Ltd. Vol. II, Appendix 34 C.
Ogawa, J.M., Lyda, S.D. and Weber, D.J. (1961)
2,6-dichloro-4-nitroaniline effective against Rhizopus fruit rot
of sweet cherries. Pl. Dis. Reptr., 45:636-638.
Ogawa, J.M., Mathre, J.M., Weber, D.J. and Lyda, S.D. (1963) Effects
of 2,6-dichloro-4-nitroaniline on Rhizopus species and its
comparison with other fungicides on control of Rhizopus rot of
peaches. Phytopathology, 53:950-955.
Pieters, A.J. (1974) Information on dicloran residues in The
Netherlands submitted to the Joint Meeting 8 November 1974.
(Unpublished)
Roburn, J. (1958) Experiments to demonstrate the effect of washing
lettuce treated with dicloran ("Alisan"). Dicloran, Registration
document from the Boots Co., Ltd. Vol. II, Appendix 33.
Roburn, J. (1960) Allisan residue trials on lettuce, February to April
1960. Lenton memo nr. 5. Lenton Experimental Station, Nottingham.
(Unpublished)
Roburn, J. (1961) Colorimetric determination of
2,6-dichloro-4-nitroaniline in plants and soil. J. Sci. Fd. Agric.,
12:766-772.
Serrone, D., Pakdaman, P., Stern, A. and Coulston F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
Rhesus monkey. Tox. appl. Pharmacol., 10:404.
Sharples, R.O. (1962) The fungitoxic effects of dicloran on Botrytis
cinerea. Lenton Experimental Station, Nottingham. (Unpublished)
Stough, A.R. (1962) Results of Botran(R) clinical study. Results from
The Upjohn Co. submitted by The Boots Co. Ltd. (Unpublished)
Van Alfen, N.K. (1972) Microbial metabolism of the fungicide
2,6-dichloro-4-nitroaniline in soil by micro-organisms. Dissertation,
Dept. of Plant Pathology, Univ. of California, Davis.
Van Alfen, N.K. and Kosuge, T. (1974) Microbial metabolism of the
fungicide 2,6-dichloro-4-nitroaniline. J. agr. Food Chem., 22:221-224.
Wang, C.H. (1972) The effect of soil properties on losses of two
chloronitrobenzene fungicides from soils. Dissertation, Dept. of Soil
Sciences, Univ. of California, Davis.
Wang, C.H. and Broadbent, F.E. (1973) Effect of soil treatments on
losses of two chloronitrobenzene fungicides. J. environ. Quality, 2
(4):511-515.
Woodard, M., Cockrell, K. and Woodard, G. (1964) Safety evaluation by
oral administration to rats and dogs for 104 weeks. Report from
Woodard Research Corporation submitted by The Boots Co. Ltd.
(Unpublished)
