
DICLORAN JMPR 1998
First draft prepared by
C.E. Moase
Health Evaluation Division, Pest Management Regulatory Agency, Ottawa,
Ontario, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Single-generation reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Cataractogenicity
Haematological effects
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Dicloran was evaluated for toxicological effects by the Joint
Meetings in 1974 and 1977 (Annex 1, references 22 and 28). A temporary
ADI of 0-0.03 mg/kg bw was established in 1974 on the basis of the
results of a two-year study in dogs and short- and long-term studies
in rats. The 1977 Meeting established an ADI of 0-0.03 mg/kg bw on the
basis of these studies, after examination of further data on
oculotoxicity in dogs, metabolism and pharmacokinetics in pigs, and
the effects of dicloran on liver microsomal enzymes, in compliance
with the request of the 1974 Joint Meeting.
Dicloran was reviewed by the present Meeting within the CCPR
periodic review programme. In addition to studies previously reviewed,
newly available studies, including those on metabolism, short-term
toxicity in mice and rats, carcinogenicity in mice, genotoxicity,
reproductive toxicity in rats, and developmental toxicity in rabbits,
were reviewed. This monograph summarizes the new data and includes
relevant data from the previous monograph and monograph addendum
(Annex 1, references 23 and 29).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
The 1974 Joint Meeting concluded that dicloran is rapidly
absorbed, metabolized, and excreted by mammals, including humans, with
the formation of chlorinated phenylenediamine and aminophenol, which
are conjugated and excreted.
When dicloran was administered orally or intraperitoneally at
single doses of 10-40 mg/kg bw to rats, 91% of the oral dose was
excreted in the urine within three days (77% in 24 h) and 1% in the
faeces, and 83% of the intraperitoneal dose was excreted in the urine
within three days (70% in 24 h) and 1.5% in the faeces. A small amount
appeared to be excreted in the bile, amounting to 2% of the dose 6 h
after intraperitoneal injection and 5% within 12 h after oral delivery
(Maté et al., 1967).
14C-Dicloran administered orally to groups of three male
Sprague-Dawley rats at a dose of 1.7 or 8 mg/kg bw was rapidly
excreted. Urinary excretion accounted for approximately 90% of the
administered dose, which was recovered within 48 h; up to 85% was
recovered within 8 h of treatment. The remainder was present in the
faeces. Small quantities of radiolabel were detected in the
gastrointestinal tract, urinary tract, and liver. 14C-Dicloran
administered to three male volunteers at a dose of 50 mg was also
rapidly absorbed and excreted, with 72-79% of the administered dose in
urine and 24-36% in faeces. Although recovery was considered to be
complete, excretion was somewhat slower in humans than in rats, with
49% of the administered dose recovered in urine and faeces within 24 h
and 76% within 48 h; most of the material was excreted within 1.5 days
(Eberts, 1965).
[U-14C]-Dicloran (purity, > 99%) was administered by gavage to
groups of five male and five female Sprague-Dawley rats as a single
dose of 5 or 500 mg/kg bw. Dicloran appeared to be well absorbed from
the intestinal tract and was rapidly excreted in the urine, most of
the radiolabel (90-96%) being excreted within 24 h of administration
of the low dose and 91% within 48 h of the high dose; 93-98% had been
eliminated by day 4 after treatment with both the low and the high
dose. The urine was the principle route of elimination (> 77% at the
low dose, > 70% at the high dose), with an additional 13% eliminated
in the faeces of animals at the low dose and 22% in faeces of those at
the high dose. Radiolabelled residues in the tissues and carcass
accounted for < 1% of the administered dose, the liver and carcass
containing the highest concentrations (O'Boyle & Challis, 1991a,b).
In groups of five hens dosed with 14C-dicloran in capsules (0.37
or 6 mg/hen per day) for five days, dicloran was rapidly absorbed,
metabolized, and eliminated. More than 80% was excreted, and 50-57% of
the administered dose was recovered within 24 h of dosing (Dawson,
1988).
In goats intubated with 14C-dicloran, the administered
radiolabel was completely recovered from urine and faeces by 72 h; 38%
was recovered within the first 24 h from goats treated with 1.5 mg/kg
bw and 96% from those given 8 mg/kg bw. More than 10 times more
residues were found in goats than in Sprague-Dawley rats 72 h after
treatment, with only trace amounts of residues in other tissues. The
liver residues in goats could not be extracted with organic solvents,
whereas most of the radiolabelled residues were extractable from rat
liver. In contrast, muscle residues from goats treated with a single
dose of 8 mg/kg bw were not covalently bound to macromolecules (Jaglan
& Arnold, 1985a,b; Jaglan et al., 1985a). In a lactating goat given
613 mg 14C-dicloran in a capsule daily for five days, 0.5% of the
administered radiolabel was detected in milk. The parent compound and
4-amino-2,6-dichlorophenol were the major residues (Cheng, 1996a).
(b) Biotransformation
Preliminary studies suggested that dicloran metabolites in humans
are similar to those in rats. 2,6-Dichloro-4-hydroxyaniline sulfate
represented about 85% of the total radiolabel excreted in rat urine
(Eberts, 1965).
14C-Dicloran administered to rats intraperitoneally or orally at
a dose of 20 mg/kg bw was metabolized to dichloroamino-phenol and
dichlorophenylenediamine derivatives. The major metabolite in urine
was 2,6-dichloro-4-hydroxyaniline (4-amino-3,5-dichlorophenol), which
represented 50% of the dose and 70% of the urinary activity. This was
excreted as a conjugate, therefore undergoing rapid deactivation
in vivo. The only other metabolite detected in the urine was
2,6-dichlorophenylenediamine (4-amino-2,6-dichloroaniline),
representing 2.4% of urinary activity, although Cheng (1996b; see
below) reported concentrations of < 0.1% of the administered dose.
Studies with mouse liver microsomes in vitro showed limited
conversion of dicloran to the same two metabolites (~20 % each; Maté
et al., 1967). These were also reported to be the principal
metabolites in dogs and monkeys (Bachmann et al., 1971).
Groups of five Sprague-Dawley rats of each sex received repeated
doses of 5 mg/kg bw unlabelled dicloran (purity, > 97%) by gavage for
two weeks or a single oral dose of 500 mg/kg bw before treatment with
[U-14C]-dicloran (purity, > 99%). The compound appeared to be well
absorbed from the intestinal tract and was rapidly excreted in the
urine, most of radiolabel being excreted within 24 h of dosing. There
were no apparent sex-related differences in absorption or elimination,
and urine was the principal route of excretion (~85% of the low dose,
66% of the high dose) in males and females. Within 48 h, 92-93% of the
administered dose had been metabolized and excreted by animals at the
low dose and 82-86% by those at the high dose. The concentrations of
residues in tissues were low, the highest concentrations being found
in liver (0.05-0.06 ppm) and kidney (0.02 ppm) seven days after dosing
with 5 mg/kg bw; the concentrations in other tissues were < 0.01
ppm. The concentrations of radiolabelled residues were comparable in
males and females, and the total residues accounted for 0.2-0.3% of
the administered dose. The major urine metabolites were
2,6-dichloro-4-hydroxyaniline sulfate (22-63% of administered dose)
and 2,6-dichloro-4-hydroxyaniline glucuronide (16-29%). Unchanged
parent compound was detected in faeces of animals at the high dose
only. The major faecal metabolites were derivatives of glutathione
conjugates. The excretion and metabolite profiles were essentially
independent of dose and pretreatment, although there were some
quantitative, sex-dependent differences in the distribution of major
urinary metabolite fractions (Cheng, 1996b).
An integrated pathway for the metabolism of dicloran in rats,
goats, hens, plants, and soil is indicated in Figure 1. The parent
compound and its glutathione conjugates were the major metabolites of
plants, comprising 32-51% of residues in peaches, potatoes, and
lettuces (Hawkins et al., 1988; Smith, 1989; O'Neal, 1997a,b). None of
the metabolites was of toxicological concern.
The highest residues in tissues of hens dosed with capsules
containing 14C-dicloran at 150 g/bird per day for three days were
found in egg yolk, fat, and liver (0.74, 0.21, and 0.10 g/g,
respectively). Only the parent compound was detected in egg yolk and
fat. The residues in liver consisted of 4-amino-3,5-dichloroacetilide
(24%), 2,6-dichloro-4-nitrophenol (21%), and parent (54.8%; Dawson,
1988).
Groups of five hens treated with dicloran at a dose of 0.37 or 6
mg/day for five days excreted more than 80%, 3-11% of which was parent
compound. The percentage of the residues that were extractable (i.e.
unbound) was 87% (in liver) to 100%, with the highest residue
concentrations in liver and egg yolk of hens given either dose and in
abdominal fat of those given the high dose. Parent compound was the
major component of fat (94%) and egg yolk (> 80%), and
2,6-dichloro-4-nitrophenol was the major metabolite in liver (45-58%
of residue). 4-Amino-3,5-dichloroacetilide and
3,5-dichloro-4-hydroxyacetanilide were found at concentrations of
1-12% and 12-33%, respectively, in liver and muscle. The
concentrations of other metabolites represented < 10% of radiolabel
in tissue. The metabolites underwent subsequent sulfate or glutathione
conjugation and excretion (Cheng, 1996c).
The major metabolite in rat urine (2,6-dichloro-4-hydroxyaniline)
was not present in goat urine, and rat urine contained more polar
metabolites than goat urine, whereas goat urine had more polar
conjugates that could not be hydrolysed by glucuronidase or sulfatase.
In goats, dicloran is reduced to 2,6-dichlorophenylenediamine and
acetylated to 3,5-dichloro-4-aminoacetanilide (4-6% in urine), which
is rapidly metabolized and excreted in the urine and faeces. Although
the reactive intermediate is formed in goat liver and is bound
covalently to macromolecules, goat liver-bound residues had little
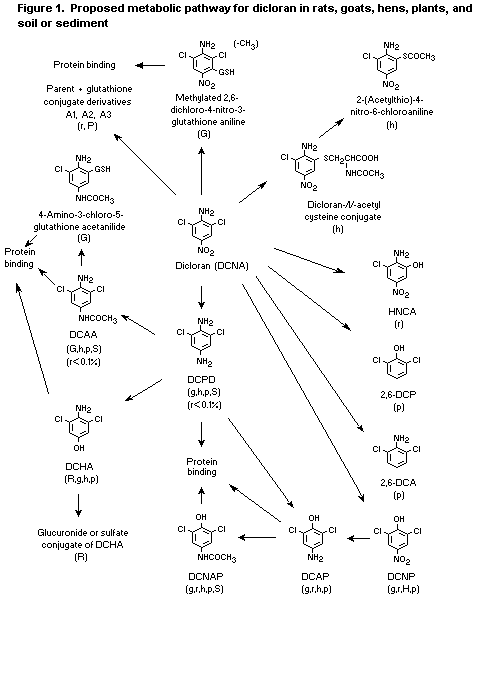 DCNA, 2.6-dichloro-4-nitroaniline; HCNA,
2-hydroxy-4-nitro-6-chloroaniline; DCHA,
2,6-dichloro-4-hydroxyaniline; DCAP, 4-amino-2,6-dichlorophenol;
DCNAP; 3,5-dichloro-4-hydroxyacetanilide; DCDP,
2,6-diclorophenylenediamine; DCAA, 4-amino-3,5-dichloroacetanilide;
DCNP, 2,6-dicholoro-4-nitrophenol; DCP, 2,6-dichlorophenol; DCA,
2,6 dichloroaniline; A1, A2, A3, glutathione conjugate deriviatives.
R, G, H, P, S correspond to metabolites in rats, goats, hens, plants,
and soil or sediment, respectively. Major metabolites are represented
by letters in upper-case, minor metabolites are represented by
lower-case letters.
potential to form reactive intermediates after ingestion by rats
(Jaglan & Arnold, 1985a; Jaglan et al., 1985a,b).
(c) Effects on enzymes and other biochemical parameters
Oral administration of dicloran at a dose of 400 or 1000 mg/kg bw
per day to rats for three months resulted in increased hepatic
demethylase and desulfurase activity, and liver mitochondrial oxygen
consumption was increased (Serrone et al., 1967). Dicloran at oral
doses of > 10 mg/kg bw stimulated rat liver mixed-function
oxidases, and a dose of 500 mg/kg bw decreased mitochondrial oxidation
of succinate without concomitant uncoupling of oxidative
phosphorylation. Oxidative phosphorylation was not affected by single
doses at any concentration. Daily administration of 1000 mg/kg bw to
rats induced uncoupling of oxidative phosphorylation after four days
(Bachmann et al., 1971). These liver enzymes were not stimulated in
rhesus monkeys treated with 160 mg/kg bw per day dicloran for three
months (Serrone et al., 1967), and no effect was seen on brain or
liver mitochondrial function in mice (Bachmann et al., 1971).
The 2,6-dichloro-4-hydroxyaniline metabolite was as active as
2,4-dinitrophenol in vitro in uncoupling oxidative phosphorylation,
whereas dicloran was only one-tenth as effective.
2,6-Dichlorophenylenediamine had no effect on mitochondrial
respiration or oxidative phosphorylation in vitro (Bachmann et al.,
1971).
Dicloran and 2,6-dichloro-4-hydroxyaniline at 10-5 mol/L
inhibited electron transport and uncoupled oxidative phosphorylation
in vitro, whereas 2,6-dichlorophenylenediamine at 10-4 mol/L
induced only slight uncoupling. These inhibitory effects in vitro
cannot be considered serious adverse effects since they were not
confirmed by similar observations in vivo. Mitochondria isolated
from rats treated with dicloran or its metabolites were functionally
normal (Gallo et al., 1976).
In a preliminary study, oral doses of 1000 mg/kg bw dicloran per
day for four days to female Sprague-Dawley rats did not significantly
induce cytochrome P450 activity (Basting et al., 1984). After oral
administration of 100 mg/kg bw twice a day for four days to male
Sprague-Dawley rats, however, significant increases were found in the
activities of cytochrome P450 (55% increase), cytochromeb5 (24%
increase), benzphetamine demethylase, and ethoxycoumarin deethylase
(51-68% increases) in comparison with controls. The pattern of enzyme
induction was reported to indicate that dicloran significantly induces
phenobarbital-type enzymes in rat microsomes (Creedy et al., 1985).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of dicloran are
presented in Table 1. Technical-grade dicloran was of low or slight
acute toxicity by all routes of administration in all species
examined. Oral administration resulted in nasal haemorrhage,
paralysis, depression, and excessive yellow urine and faeces.
Intraperitoneal administration resulted in sleep, depression, and
excessive yellow urine and faeces. The effects of subcutaneous
injection were confined to local damage in which the test substance
became enclosed in a fibrous sac, sometimes followed by abscess
formation and eventual ulceration. Dermal administration resulted in
no systemic clinical effects.
Technical-grade dicloran (purity, 96%) was not irritating to the
intact or abraded skin of male or female New Zealand white rabbits at
a concentration of 500 mg per site (Raczniak & Wood, 1980b) or after
repeated daily applications of 10 mg dry material or 0.1 ml of 10%
aqueous suspension to the abraded or unabraded skin of albino rabbits
over five days (Boots Pure Drug Co., 1962).
Technical-grade dicloran (purity, 96%) caused a minimal
conjunctival response in the eys of New Zealand white rabbits,
consisting of redness and chemosis 24 and 48 h after treatment.
Corneal opacity or injury to superficial layers of the corneal or
conjunctival epithelium was not observed (Raczniak & Wood, 1980c).
Four successive daily applications of 2-3 mg dicloran to the
conjunctival sac of four rabbits resulted in a slight inflammatory
reaction of the cornea, which was of short duration (Boots Pure Drug
Co., 1962).
Dicloran was inactive in two tests for skin sensitization in
guinea-pigs (Boots Pure Drug Co., 1962; Johnston & Schwikert, 1963).
(b) Short-term studies of toxicity
Mice
Groups of Charles River COBS mice were fed diets containing 0,
1250, 2500, 5000, or 10 000 ppm technical-grade dicloran for two
weeks, equivalent to 0, 190, 380, 750, and 1500 mg/kg bw per day. At
10 000 ppm, 20% of animals of each sex died; the only clinical sign
observed was hunched posture. Food consumption and body weight were
significantly reduced. Female mice fed 5000 or 10 000 ppm had
significantly increased absolute liver weights, and animals of each
sex fed doses of 2500 ppm or more had an increased liver:body-weight
ratio. Food consumption was slightly reduced in animals of each sex at
2500 and 5000 ppm, and a slight reduc-tion in food consumption was
noted at 1250 ppm only in females (Mallyon & Rawcliffe, 1985).
Table 1. Acute toxicity of dicloran
Species Strain Sex Route LD50 Reference
(mg/kg bw)
Rat Sherman SPF M/F Oral > 4000 Gaines & Linder (1986)
Rat NR NR Oral 4040 Boots Pure Drug Co. (1962)
Rat NR NR Oral ~ 8000 Serrone et al. (1967)
Rat NR NR Oral > 10 000 Johnston & Ceru (1961)
Mouse NR NR Oral 1500-2500 Boots Pure Drug Co. (1962)
Guinea-pig NR NR Oral 1400 Boots Pure Drug Co. (1962)
Rabbit New Zealand white M/F Dermal (abraded) > 2000 Raczniak & Wood (1980a)
Mouse NR NR Intraperitoneal 5500 Johnston & Ceru (1961)
Mouse NR NR Intraperitoneal 2500 Boots Pure Drug Co. (1962)
Rat NR NR Intraperitoneal 1500 Boots Pure Drug Co. (1962)
Rat NR NR Subcutaneous > 5000 Boots Pure Drug Co. (1962)
Mouse NR NR Subcutaneous > 6000 Boots Pure Drug Co. (1962)
SPF, specific pathogen-free; M/F, male and female; NR, not reported
Groups of 10 male and 10 female CD-1 (ICR) mice received dicloran
(purity, 95.8%) in the diet at 0, 300, 600, or 1200 ppm, equal to 0,
49, 100, and 200 mg/kg bw per day in males and 0, 53, 100, and 220
mg/kg bw per day in females, for 60 days. There were no
treatment-related effects on survival, clinical signs, body weight,
food or water consumption, or food conversion. A significant increase
in methaemoglobin concentrations in the blood of both males and
females was noted at 600 and 1200 ppm (males: 1, 1.3, 1.4, and 1.6%;
females: 0.6, 0.7, 0.9, and 1.2%, control to high dose, respectively).
Cholesterol and bilirubin concentrations were significantly increased
in high-dose females (cholesterol: 2.4 ± 0.58, 2.2 ± 0.50, 2.5 ± 0.2,
3.0 ± 0.65 mmol/L; bilirubin: 4.3 ± 0.6, 5.4 ± 1.4, 5.5 ± 2.4, 5.8 ±
1.7 mol/L, control to high dose, respectively). These changes were
dose-related but were not observed in males, which, unlike females,
had specific liver lesions. Minimal to slight centrilobular
hepatocytic enlargement (2-5/10 versus 0/10 controls) and fatty liver
degeneration (2-4/10 versus 0/10 controls) with an increased severity
of haemosiderosis in the spleen was noted in males at 600 and 1200
ppm, and a slight increase in the degree of haematopoietic activity
was noted in the spleen of males and females combined at the
intermediate and high doses. The NOAEL was 300 ppm, equal to 49 mg/kg
bw per day in males (Mallyon et al., 1986).
Dicloran (purity, 97%) was administered to groups of 15 B6C3HF1
mice of each sex (five of each sex per dose for interim sacrifice) by
gavage at doses of 0, 15, 45, 135, 400, or 600 mg/kg bw per day for 90
days, with an interim sacrifice at 49 days. There were no
treatment-related effects on survival or body weight; data on food
consumption were not provided. Significant polycythaemia was seen in
males, and hypercholesterolaemia at 1.2-2.1 times the control level
and a significant increase in the incidence of splenic extramedullary
haematopoiesis were seen in females at > 45 mg/kg bw per day.
Dose-related increases in cholesterol concentrations (1.3-2.3 times
the control concentration) were also noted in males, which were
statistically significant at > 135 mg/kg bw per day. A deep-yellow
colouration of the urine was noted in all treated groups and was
attributed to the yellow colour of the test material. In the absence
of any associated lesion in the group at the lowest dose, this effect
was considered not to be adverse. Other effects at higher doses
included kidney nephrosis in males at 400 and 600 mg/kg bw per day (5,
4, 6, 5, 15, 14/15, control to high dose, respectively) and females
(3, 5, 2, 4, 8, 12/15), with kidney tubular regeneration in males (0,
0, 3, 1, 8, 11/15), increased liver weights in animals of each sex at
> 135 mg/kg bw per day (7-64% increase), hepatocyte hypertrophy in
males (0, 0, 0, 4, 15, 15/15) and females (0, 0, 0, 0, 5, 12/15) at
> 400 mg/kg bw per day, liver necrosis in males (0, 0, 0, 0, 3,
3/15) and females (0, 0, 0, 0, 1, 1/15) at 400 and 600 mg/kg bw per
day, a significantly higher incidence of splenic extramedullary
haematopoiesis in males at > 135 mg/kg bw per day (0, 1, 2, 13, 15,
15/15) and in females at > 45 mg/kg bw per day (2, 5, 9, 10, 14,
15/15), increased spleen weight at the two higher doses (11-29%
increase), and epithelial hyperplasia of the urinary bladder in males
(0, 1, 2, 10, 12, 15/15) and females (0, 0, 0, 1, 7, 13/15) at doses
> 135 mg/kg bw per day. The NOAEL was 15 mg/kg bw per day (Kakuk,
1986).
Rats
Dicloran (unspecified purity) was administered by gavage to rats
(strain unspecified) at 400 or 1000 mg/kg bw per day for three months.
Deaths occurred among rats at the high dose. Significantly enlarged
livers and unspecified renal changes were observed by light and
electron microscopy, with increases in liver mitochondrial enzyme
activity and mitochondrial oxygen use (Serrone et al., 1967).
Groups of five newly weaned rats (strain unspecified) of each sex
were given dicloran (purity unspecified) by gavage at doses of 0, 140,
or 350 mg/kg bw per day, six days/week for four weeks. The growth of
males at 350 mg/kg bw per day was reduced to 60% of the control value.
At both doses, increased liver weight (120-155% of control) with
hepatic vacuolation were noted. There was no apparent effect on blood
parameters or on the kidneys at the end of the study (Boots Pure Drug
Co., 1962).
Groups of 10 rats (strain unspecified) were given dicloran
(purity unspecified) by gavage at doses of 35, 140, or 350 mg/kg bw
per day, six days/week, for four weeks. Groups of 20 controls of each
sex were available. Growth depression was observed in animals of each
sex at 350 mg/kg bw per day and in males at 140 mg/kg bw per day.
Increased liver weights and microscopic findings were also seen at
these doses. The liver lesions included a dose-related increase in
hepatocytic hypertrophy with increased vacuolization, especially in
the outer lobes. The liver had returned to normal size in half of the
animals which were maintained for two weeks after the end of the
treatment, except for males at the highest dose. The histopathological
changes in the liver caused by repeated short-term dosing was
apparently reversible within two weeks of the end of treatment. The
NOAEL was 35 mg/kg bw per day on the basis of effects on body weight
and liver at 140 mg/kg bw per day (Boots Pure Drug Co., 1962).
Technical-grade dicloran was fed to rats (strain unspecified) for
six months at doses of 0 (25 rats), 30 (15 rats), 300 (15 rats), or
3000 (10 rats) ppm, equal to 0, 2.2, 22, and 230 mg/kg bw per day in
males and 0, 2.5, 25, and 270 mg/kg bw per day in females. Another
10 animals of each sex per group received recrystallized dicloran at
3000 ppm, equivalent to 230 mg/kg bw per day for males and 260 mg/kg
bw per day for females. At 3000 ppm, growth of both males and females
was impaired (70-83% of control), whereas males fed purified material
had only a slight reduction in growth (89% of control). Significantly
increased liver weights (120-144% of control) were noted in both
groups at the high dose, with a significant increase in spleen weight
(129% of control) in females. Haematological parameters were not
affected at 12 weeks or at termination, and no microscopic lesions
were noted. The NOAEL was 300 ppm, equal to 22 mg/kg bw per day, on
the basis of changes in body weight and increased liver and spleen
weights at 3000 ppm (Boots Pure Drug Co., 1962).
Dicloran (purity, 97.4%) was administered to groups of 10 male
and 10 female Sprague-Dawley rats in the diet at doses of 0, 1000,
3000, or 5000 ppm, equal to 0, 75, 230, and 370 mg/kg bw per day in
males and 0, 80, 230, and 420 mg/kg bw per day in females, for 90
days. There were no treatment-related effects on survival or
ophthalmological parameters. Significantly lower body-weight gain was
seen in all treated groups (17-32% less than controls for males and
18-22% less for females). Although palatability may have accounted for
the lower body-weight gains during the first week of the study, food
consumption was comparable in all groups at week 2; however, it was
consistently lower than control values throughout the remainder of the
study (males, 10-14% less than controls; females, 12-21%; weeks 2-13).
Many of the haematological and clinical chemical findings were
considered to be associated with reductions in food consumption. The
treatment-related hepatic effects included increased cholesterol
concentrations, which were dose-related and statistically significant
in males at all doses (1.3-1.5 times the control concentration) and in
females at the intermediate and high doses (1.4-2.0 times control),
minimal to moderate centrilobular hypertrophy in males in all treated
groups (0, 3, 9, 9/10) and in females at the two higher doses (0, 0,
9, 9/10), and significant increases in liver weight in males and
females at the two higher doses (29-66%). Minimal thyroid
follicular-cell hypertrophy was noted in males in all treated groups
(0, 5, 6, 6/10) and in females at the intermediate and high doses (0,
0, 4, 7/10). The dose of 1000 ppm, equal to a daily intake of 75 and
80 mg/kg bw per day for males and females, respectively, was
considered to be at the upper limit of a suitable high-level dose for
a long-term study of toxicity and carcinogenicity in rats. An NOAEL
was not identified because significantly lower body-weight gains were
seen at all doses (Peters et al., 1990).
A second short-term study was conducted to investigate the
effects of dicloran at doses lower than those administered in the
90-day study. Dicloran (purity, 96.4%) was given in the diet to groups
of 10 Sprague-Dawley rats of each sex at doses of 0, 500, or 750 ppm,
equal to 0, 44, and 71 mg/kg bw per day for males, and 0, 48, and 71
mg/kg bw per day for females, for eight weeks. There were no
treatment-related effects on survival. The overall body-weight gain of
females at 750 ppm was significantly less than that of controls (12%
less overall), the greatest difference occurring during the final two
weeks of treatment (41-58% less than control). This decrease
correlated with a slight decrease in food consumption in this group
during treatment, which was 10-11% less than that of controls during
the last two weeks of treatment. Increased liver weight relative to
body weight (13-21%) in animals of each sex at 750 ppm was associated
in 10/10 males with minimal centrilobular hepatocyte enlargement. The
NOAEL was 500 ppm, equal to 44 mg/kg bw per day, on the basis of lower
body-weight gain and decreased food consumption in females at 750 ppm
and increased liver weights in males and females, with associated
centrilobular hepatocyte enlargement in males at 750 ppm; however, a
full histological examination was not carried out (only of the liver
and thyroid), and although alterations were found in the livers of
males at the high dose, the livers of males at the low dose were not
examined histologically (Waterson et al., 1992).
Rabbits
Technical-grade dicloran (purity, 96.2%) was moistened with
distilled water and applied to the intact skin of groups of five male
and female New Zealand white rabbits under an occluded patch for 6 h
per day for 21 consecutive days at doses of 12, 120, or 1200 mg/kg bw
per day. Animals at the two higher doses showed yellow staining of
untreated fur and extremities from day 4 onwards, but there were no
adverse clinical findings. One male and one female rabbit at 12 mg/kg
bw per day had slight, transient erythema lasting for two to four
days, and most rabbits at 120 and 1200 mg/kg bw per day had slight
erythema beginning in the second week of the study, which tended to
persist until study termination. The reaction did not progress beyond
slight erythema, and there did not appear to be a dose-related
difference above 120 mg/kg bw per day. At termination, male rabbits at
1200 mg/kg bw per day had significantly greater (36%) adrenal weights
than controls, but the adrenals were not examined histologically. The
liver weights were 18% greater in females at 1200 mg/kg bw per day
than in controls. No other effects were seen on body weight, food
consumption, or haematological, biochemical, or histological
parameters. The NOAEL was 120 mg/kg bw per day (Elliot et al., 1988).
Rats, rabbits, and dogs
Groups of 10 male and 10 female TUC/SPD rats, one male and one
female New Zealand white rabbit, and two male beagle dogs were exposed
to a dust aerosol of technical-grade dicloran by whole-body exposure
for 6 h per day, five days per week for three weeks. The nominal
concentration of the aerosol was 2 mg/L; the actual concentration and
particle size were not provided. Two rats died on the third day of
exposure, and one rabbit died on day 13. The food consumption and
body-weight gains of the treated animals were depressed. There were no
consistent haematological changes attributable to treatment. The blood
cholesterol concentration was significantly elevated in exposed dogs
and rabbits. The liver weights of exposed animals were increased,
although the histological appearance was unremarkable, with no
evidence of hepatocellular effects in any species (Seaman et al.,
1980).
Monkeys
Oral doses of 160 mg/kg bw per day were lethal to rhesus monkeys
within three months, with a greater effect on females than males. The
colouration of the urine differed from that of rat urine, suggesting a
difference in metabolism in the two species. Centrilobular fatty
infiltration was observed in the liver, and liver and kidney changes
were observed on electron microscopic examination, which included
swelling of mitochondria with distortion of the cristae. Hepatic
microsomal enzyme activity was not enhanced (Serrone et al., 1967).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a screening study, groups of 18 mice of each sex of each of
two susceptible hybrid strains (unspecified) were given dicloran at
215 mg/kg bw per day for three weeks from day 7 after birth.
Thereafter, for 18 months, the mice were fed 600 ppm (equivalent to
100 mg/kg bw per day) in the diet, sacrificed, and examined for
tumours. Dicloran did not cause a significant increase in the
incidence of tumours (Innes et al., 1969).
Dicloran (purity, 96.2-97.2%) was administered to groups of 50
CD-1 mice of each sex at dietary concentrations of 0, 50, 175, or 600
ppm, equal to 0, 7.4, 24, and 86 mg/kg bw per day in males and 0, 10,
36, and 120 mg/kg bw per day in females, for 80 weeks. The doses were
based on a the results of a 60-day dietary study in mice in which a
concentration of 600 ppm was associated with increased methaemoglobin
concentration, splenic pigment change, increased haematopoiesis,
centrilobular hepatocyte enlargement, and fatty degeneration of
hepatocytes. There was no adverse effect on mortality (percent
survival at termination: males, 50, 50, 64, and 56%; females, 84, 76,
76, and 78%, control to high dose, respectively). There were no
treatment-related effects on clinical signs, body weight, palpable
masses, food consumption, food conversion, or differential leukocyte
counts. Clinical chemistry and urinalysis were not performed. There
was no increase in tumour incidence. The liver was the principal
target organ, with increased absolute and relative weights (10-12%) in
males and females at the high dose, which were statistically
significant in females, and histopathological changes. These included
centrilobular hepatocyte enlargement (males: 8, 8, 7, and 26/50;
females: 1, 0, 2, and 5/50), centrilobular haemosiderosis (males: 1,
2, 4, and 12/50; females: 7, 5, 3, and 13/50), focal (4, 2, 5, and
10/50) and single-cell (1, 1, 0, and 6/50) liver necrosis in males,
and vacuolation of centrilobular hepatocytes (4, 3, 3, and 12/50) in
females. Acute inflammatory cell infiltration was seen in males at the
intermediate and high doses (2, 4, 9, and 9/50), but no other hepatic
changes were seen in males at this dose. Other changes at 600 ppm
included a higher incidence of erythropoiesis in the spleens of males
(7, 7, 8, and 15/50), an increased number of females with an
enlarged/distended uterus (5, 9, 9, and 15/50), associated with a
significant increase in the incidence and severity of cystic uterine
endometrial hyperplasia (17, 21, 24, and 31/50), and an increased
number of females at the high dose with distended mammary gland ducts
(9/50 versus 3/50 in control; animals at the low and intermediate
doses were not assessed). An 11% increase in kidney weight in males
was not associated with histopathological lesions. On the basis of the
above findings, the NOAEL was 175 ppm, equal to 24 mg/kg bw per day
(Mallyon & Markham, 1989).
Rats
Groups of 35 rats (strain unspecified) of each sex were fed
dicloran (purity unspecified) in the diet at concentrations of 0, 20,
100, or 3000 ppm for two years, equal to 0, 1.6, 8, and 240 mg/kg bw
per day, with an interim sacrifice of five animals of each sex per
dose at 13 weeks. The dose of 100 ppm had no effect on behaviour,
mortality, or growth, and all values were comparable to those of
controls. At 3000 ppm, growth and food consumption of males and
females were depressed, and haematological parameters (haemoglobin and
packed cell volume) were reduced but only after the first year of
treatment. Gross and microscopic examination at 13 weeks and at the
conclusion of the study showed slightly heavier livers, kidneys, and
testes in males at 3000 ppm. The incidence and location of neoplasms
were similar in treated and control rats. Histological examination
revealed changes in the liver at 3000 ppm, characterized by
hepatic-cell enlargement, increased severity of glycogen depletion,
increased basophilia of the cytoplasm, and the presence of dead cells.
Other observations were increased thyroid hypertrophy and increased
pigmentation of the spleen in males and females at 3000 ppm.
Hepatic-cell changes and slight adrenal cortical atrophy were seen in
several animals at this dose at 13 weeks, but the adrenal changes were
not seen at 104 weeks. The NOAEL was 100 ppm, equal to 8 mg/kg bw per
day (Woodard et al., 1964).
Groups of 25 Boots-Wistar strain rats of each sex were fed
dicloran (purity unspecified) in the diet at a concentration of 0 or
1000 ppm for two years, equal to 59 mg/kg bw per day in males and 71
mg/kg bw per day in females. There was no effect on survival, food
consumption, growth, haematological parameters, or gross or
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular constitution
of the liver, kidney, or spleen. The incidence of tumours in control
and treated groups was similar. The NOAEL was 1000 ppm, equal to 59
mg/kg bw per day, the highest dose tested (Lessel, 1974).
Dogs
Groups of four male and four female beagle dogs were fed dry
diets containing dicloran (purity unspecified) at concentrations of 0,
20, 100, or 3000 ppm for two years, equal to 0, 0.33, 1.7, and 44
mg/kg bw per day for males and 0, 0.36, 1.8, and 62 mg/kg bw per day
for females, with an interim sacrifice of one animal of each sex per
dose at 14 weeks. No compound-related changes in behaviour, food
consumption, or growth were observed. One female at 3000 ppm died at
74 weeks, with treatment-related signs of haemolytic anaemia (reduced
haemoglobin, immature erythrocytes, polychromophilic macrocytes,
increased leukocyte count, and increased myeloid: erythroid ratio in
the bone marrow) before death. One control male lost considerable
weight but survived to the end of the study. All dogs at 3000 ppm had
watery lachrymation, which persisted throughout treatment; the
examination of the eyes was not adequate to determine any ocular
toxicity of dicloran. Yellowing of the sclera, mucous membranes, and
abdominal skin were noted at the high dose. Changes in haematological
and clinical chemical parameters in males and females at the high dose
included significant reductions in haemoglobin from week 26 to
termination and reduced serum protein at termination; increased
activity of alanine and aspartate aminotransferases and increased
prothrombin time, blood urea nitrogen, and bromosulphthalein retention
were observed in one male and the one female that died at the high
dose, both of which had received 20-94% more test material than the
other dogs in this group owing to gradual body-weight loss relative to
the constant concentration in the diet. Gross and microscopic
examination revealed significantly increased liver, spleen, and kidney
weights accompanied by histological changes at 3000 ppm, which
included irregular hepatic-cell size (3/5 versus 0/6 in other groups),
moderate hepatic-cell hypertrophy (3/5 versus 0/6 in controls) and
increased pigmentation of the liver (4/5 versus 0/6 in control), and
spleen (5/5 versus 0/6 in controls). One male and two females at 3000
ppm (including the female that died) and one male at 20 ppm also had
enlarged gall-bladders containing tar-like, viscous bile, although
this was stated to be a spontaneous lesion in dogs (cystic mucinous
hypertrophy). Changes at 100 ppm which included irregular hepatic
cells, slight focal hepatocytic hypertrophy, slight vacuolation,
moderate liver pigmentation, and/or marked spleen pigmentation were
noted in two female dogs. A company re-evaluation attributed the
findings at 100 ppm to enzyme induction, although the
histopathological findings in this group were complicated by the
presence of parasitic hepatitis. No degenerative lesions were noted at
study termination, and there was no progression in the severity of
hepatocellular effects between interim and terminal sacrifice. The
NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day, on the basis of
significant reductions in haemoglobin, reduced serum protein, and
histopathological changes in the liver and spleen at 3000 ppm (Woodard
et al., 1964; Kakuk et al., 1979).
(d) Genotoxicity
The results of studies on the mutagenicity and genotoxicity of
dicloran are presented in Table 2. Dicloran gave occasional positive
responses in the most recent tests for reverse mutation and in a test
for mitotic recombination, with a 2-2.5-fold increase in mitotic
recombination. The results of the test for sex-linked recessive lethal
mutation in Drosophila were equivocal, even after re-analysis in
conjunction with historical control data.
(e) Reproductive toxicity
(i) Single-generation reproductive toxicity
Male rats (strain unspecified) were treated with dicloran (purity
unspecified) in the diet at concentrations of 0, 1000, or 2000 ppm,
equivalent to 0, 50, and 100 mg/kg bw per day, for 90 days. The dose
of 1000 ppm increased liver and kidney weights. When the males were
mated with untreated females, no difference was observed in the number
Table 2. Results of assays for the genotoxicity of dicloran
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, 16, 31, 62, 125, 99.9 Negativeb Everest & Tuplin (1977)
TA100, TA1535, 250, 500, 1000
TA1537, TA1538 µg/plate in DMSO
Reverse mutationa S. typhimurium 100 µg/plate in NR Negative Shirasu et al. (1976)
TA1535, TA1536, DMSO
TA1537, TA1538
Reverse mutationc S. typhimurium TA98, 20-200 µg/plate 99.5 Negatived Jeang & Li (1978)
TA100 in DMSO
Reverse mutatione S. typhimurium TA98, 2, 20, 200 µg/plate 99.5 Negativef Jeang & Li (1980)
TA100 in DMSO
Reverse mutationa S. typhimurium TA98, 6 concentrations NR Negative Waters et al. (1982);
TA100, TA1535, up to 10 000 µg/plate Garrett et al. (1986)
TA1537, TA1538
Reverse mutationa S. typhimurium TA98, 62, 100, 155, 200, NR Positiveg Basting et al. (1983);
TA98 NRF, TA100, 310, 415 µg/plate in Myers-Basting (1986)
TA100 NRF ethyl cellosolve/ethanol
or DMSO
Reverse mutationa S. typhimurium TA98, 50, 150, 500, 1500, 97.5 Positiveh Jones & Fenner (1987)
TA100, TA1535, 5000 µg/plate in
TA1537, TA1538 DMSO
Reverse mutationa S. typhimurium TA98, 10, 33, 100, 333, 98 Positivei Zeiger et al. (1992)
TA100 1000, 2000 µg/plate
in DMSO
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Reverse mutationc E. coli WP2, WP2uvrA- 62, 125, 250, 500, 99.9 Negativej Everest & Tuplin (1977)
1000 µg/plate in
DMSO
Reverse mutationc E. coli B/r try WP2, 20 µg/plate in DMSO NR Negative Shirasu et al. (1976)
WP2 try hcr
Reverse mutationa E. coli WP2uvrA- 6 concentrations NR Negativek Waters et al. (1982);
up to 10 000 µg/plate Garrett et al. (1986)
Mitotic recombationa S. cerevisiae D3 5 concentrations NR Negative Waters et al. (1982)
(unspecified)
Mitotic aneuploidy Neurospora crassa 5 or 10 µg/ml NR Negativel Griffiths et al. (1986;
I-41-5, I-34-8 in DMSO Moustacchi (1986)
DNA repairc B. subtilis H17 rec+, 20 g/disc in DMSO NR Negative Shirasu et al. (1976)
M45 rec-
DNA repaira B. subtilis H17 rec+, 20 g/disc in DMSO 99.5 Negativem Jeang & Li (1978)
M45 rec-
DNA repairc B. subtilis H17 rec+, 2 concentrations NR Negativem Waters et al. (1982);
M45 rec- (not specified) Garrett et al. (1986)
DNA repairc E. coli P3478 (polA-), 2 concentrations NR Negativem Waters et al. (1982);
W3110 (polA+) (not specified) Garrett et al. (1986)
Unscheduled DNA Rat hepatocytes 3, 4, 5, 6, 7, 8, 9, 97.5 Negativen McBride & McGregor
synthesis 10 µg/ml in DMSO (1987)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Mitotic recombination Aspergillus nidulans 2070, 4140, 6210 NR Positiveo Kappas (1978);
and aneuploidy (heterozygous diploid µg/plate in ethanol Moustacchi (1986)
strain)
Cytogenetic Human lymphocytes 2, 10, 20 µg/ml in 97.5 Negativep Allen et al. (1988)
alterationsa DMSO
In vivo
Recessive lethal Drosophila melanogaster Feeding at 1250 and 98 Equivocal Woodruff et al. (1985)
mutation Canton-S 1389 ppm in 5%
ethanol:5% Tween 80
Recessive lethal Drosophila melanogaster Re-analysis of data NR Equivocal Mason et al. (1992)
mutation Canton-S of Woodruff et al.
(1985)
NR, not reported; DMSO, dimethyl sulfoxide; NRF, nitroreductase-free
a With and without metabolic activation
b The positive controls, cyclophosphamide, 6-aminochrysene, and 2-aminofluorene, gave the expected results.
c Without metabolic activation
d The positive control nitroquinoline N-oxide gave the expected result.
e With metabolic activation
f The positive control sterigmatocystin gave the expected result.
g Positive in TA98 (> 100 µg/plate) only without metabolic activation
h Positive in TA98 and TA1538 (> 500 µg/plate) with and without metabolic activation and in TA100 without metabolic
activation. The positive controls, N-ethyl-N'-nitro-N-nitrosoguanidine, 9-aminoacridine, 2-aminoanthracene, and
2-nitrofluorene, gave the expected results.
i Positive in TA98 (> 100 µg/plate) with and without metabolic activation; positive control unknown
j The positive control ethyl methanesulfonate gave the expected result.
Table 2. (continued)
k The positive controls, 2-aminoanthracene and 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide, gave the expected results.
l This test system has not been fully validated. The positive control para-fluorophenylaniline gave the expected result.
m The positive control methyl methanesulfoxide gave the expected result.
n The positive control, Mischler's ketone [4,4'-bis(dimethylaminobenzophenone) in ethanol], gave the expected result.
o A 2-2.5-fold increase in mitotic recombination. This test system has not been fully validated.
p The positive controls, ethyl methanesulfonate and cyclophosphamide, gave the expected results.
of litters or in the number of animals born or weaned (US
Environmental Protection Agency, 1974).
Dicloran (purity unspecified) was fed to groups of 10 female rats
at concentrations of 0, 500, or 1000 ppm, equivalent to 0, 25, and 50
mg/kg bw per day, for 188 days before mating and then through
gestation and lactation. A reduced number of pups was reported when
females were treated with 1000 ppm. There was no apparent effect on
the survival of pups, although the mean body weight of those at 1000
ppm was slightly reduced (US Environmental Protection Agency, 1974).
(ii) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, with one
litter per generation, dicloran (purity, 99.2%) was administered to
groups of 28 male and 28 (F0) and 24 male and 24 female (F1) female
Sprague-Dawley rats in the diet at doses of 0, 50, 250, or 1250 ppm,
equal to 0, 4, 21, and 110 mg/kg bw per day. The doses were based on
the results of a range-finding study (Wilcox & Barton, 1996) in which
concentrations up to 1000 ppm did not produce any obvious alterations
in body weight, food consumption, duration of gestation, or litter
parameters. The animals were fed the test diet during the 10- (F0) or
11-week (F1) pre-mating period and then randomly allocated to mating
pairs (l:1) for a maximum of seven nights. Treatment was continued for
animals of each sex throughout the mating, gestation, and lactation
periods until termination of the F2 litters.
The treatment-related clinical signs included yellow staining of
the tray paper and, in some cases, yellow staining of the fur of all
animals at the high dose in both generations, due to the yellow colour
of the test material. There were no significant differences in mean
body weights or body-weight gain between control and treated males.
The mean body weights of F0 females at the high dose throughout the
pre-mating period was 2-5% lower than that of controls, resulting in a
12% lower mean overall gain at the end of the pre-mating period. This
was considered to be treatment-related. The mean body weights of F1
females at the high dose were, on average, 6% lower than those of
controls during pre-mating, but the overall mean body-weight gain was
only 5% lower. F0 and F1 females at the high dose had slightly lower
overall body-weight gain during gestation, the greatest difference
occurring during the first week of gestation (64% of control values in
F0 females and 86% in F1). There were no apparent effects on the
food consumption of F0 or F1 males or females during pre-mating and
gestation; however, the food consumption of females of both
generations at the high dose was 11% lower during the first week of
lactation, and these animals weighed more than controls and those
given lower doses at the end of lactation, indicating that pups in the
high-dose group were less demanding with respect to suckling than pups
in the other groups.
At the end of the study, a greater proportion of F0 and F1
females at the high dose were in pro-estrus (4/28 F0 controls versus
19/28 at the high dose; 4/24 F1 controls versus 11/24 at the high
dose). Since there was no indication of altered cycling, this result
was considered to be of no toxicological concern. Sperm-stage analysis
indicated no difference between control F0 and F1 males and those at
the high dose with respect to the number of testicular tubules at
various spermatogenic stages or in tubule diameter. There was a
dose-related increase in the incidence of abnormal sperm, particularly
with respect to tail defects, in F0 males (0.25 ± 0.26 and 0.29 ±
0.31% in the two control groups, 0.59 ± 0.96% at the low dose, 0.6 ±
0.65% at the intermediate dose, and 0.67 ± 0.58% at the high dose);
however, no such increase was observed in F1 males at the high dose
(0.21% versus 0.23% in controls). Furthermore, the mean values for F0
animals were within the historical control range, 0.0-0.8%. Thus,
these findings were considered incidental. The fertility and
gestational indices of control and treated F0 and F1 females were
similar. The male fertility indices of F0 males at the intermediate
and high doses were lower than that of controls (82 and 86%,
respectively), as was that of F1 males at the low dose (75%). Since
the fertility index of F1 males at the intermediate and high doses
approached 100%, the lower fertility indices were considered
incidental.
There were significant increases in absolute and body
weight-adjusted mean liver weights in F1 males and females at the
high dose; no data were collected for F0 animals. The weights of the
kidneys of F1 animals at the high dose were increased, and the
increase was significant in males. Brain weights were significantly
lower in F1 females at the high dose, and thymus weights were
significantly lower in F1 males and females at the high dose relative
to controls. No microscopic examinations were conducted on these
tissues. The mean absolute epididymal weights of F0 males at the high
dose and the absolute and body weight-adjusted epididymal weights of
F1 males at the intermediate and high doses were significantly lower
than those of controls. The authors indicated that the values for the
F1 males were similar to 'recent control values for animals of
similar age'; however, data on these controls were not provided. The
mean absolute and body weight-adjusted testicular weights were
statistically significantly increased in F1 males at the high dose
and non-significantly increased in F0 males at this dose. There was a
dose-related decrease in mean ovarian weights in F1 females, which
was statistically significant in the high-dose group. No
treatment-associated histopathological lesions were seen in the
reproductive tissues that were examined. In the absence of
histological data on non-reproductive tissues, the toxicological
significance of the observed changes in organ weights in these tissues
is equivocal.
No difference was observed in the mean number of implantation
sites per litter, litter size, sex ratio, or litter indices in control
and treated groups. A slight increase in the number of F1 pups that
died during days 4-21 of lactation was not observed in the F2
generation and was considered to be incidental. The group mean weights
of F1 and F2 litters were decreased throughout the lactation period,
as reflected in the lower mean pup body weights at this dose. Although
the magnitude of the difference was not as great in F2 pups as in F1
pups, these observations were consistent and considered to be related
to treatment. The mean litter weights of dams at the low and
intermediate doses were lower than those of controls on day 1 of
gestation, but the mean pup weights were comparable to those of
controls on days 1-21 of lactation, in contrast to the high-dose
group, and were therefore considered not to be related to treatment.
There were no treatment-related clinical observations or
malformations.
The NOAEL for systemic toxicity was 250 ppm, equal to 21 mg/kg bw
per day, on the basis of reduced body-weight gain at 1250 ppm in
females during pre-mating and gestation and increased liver and kidney
weights in males and females at this dose. The NOAEL for reproductive
and developmental toxicity was also 250 ppm, equal to 21 mg/kg bw per
day, on the basis of lower F1 and F2 pup weights at the next highest
dose. There were no histopathological lesions in the reproductive
tissues that would indicate that the changes in organ weights should
be considered adverse (Barton & Wilcox, 1997).
In a three-generation study, with two litters per generation,
dicloran (purity unspecified) was administered to groups of 20 rats
(strain unspecified) of each sex at 0 or 100 ppm, equivalent to 5
mg/kg bw per day, in the diet. On the basis of the reproduction
parameters examined, including the numbers of litters, stillbirths,
live births, birth weight, and lactation indices, dicloran had no
effect on reproduction (Lobdell & Johnston, 1965).
(iii) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley rats (Upj:TUC (SD) SPF) were
dosed by gavage with dicloran (purity, 93.7%) in 0.25% carboxymethyl
cellulose and 0.5% Tween 20 at doses of 0, 100, 200, or 400 mg/kg bw
per day on days 6-15 of gestation, the day of mating being considered
gestation day 0. These doses were derived from the results of a
range-finding study in which 5/5 rats died at doses of 2000 and 4000
mg/kg bw per day, and 3/5 at 1000 mg/kg bw per day. Neither of the two
survivors treated with 1000 mg/kg bw per day had pups, and one had 15
early resorptions. The mean body-weight gain of females treated with
500 mg/kg bw per day was 25% less than that of controls.
The dams were sacrificed on day 20 of gestation, and the uterine
contents were examined; the uteri of non-pregnant dams were stained
with ammonium sulfide, and all fetuses were assessed for viability,
sex, weight, and external malformations and variations. Equal numbers
of fetuses from each group were assigned to either visceral or
skeletal examination. There were no deaths. The treatment-related
clinical signs included yellow staining of the urogenital region in
some animals at each dose due to the colour of the test material, and
slight central nervous system depression throughout the treatment
period in animals at 200 and 400 mg/kg bw per day. The mean
body-weight gain (corrected for gravid uterine weights) of treated
animals was significantly lower (16-33% less) than that of the the
controls. The incidence of dams with totally resorbed litters (early
implants only) was increased in all treated groups (1, 5, 9, 10;
control to high dose, respectively); however, there were no
differences in the mean numbers of implants or resorptions or in the
mean litter sizes of dams with live litters. The fetal weights were
significantly lower at 200 and 400 mg/kg bw per day (7 and 20% less
than control, respectively), and a significant trend for an increased
incidence of delayed or reduced ossification was noted in these two
groups. Increased incidences of other skeletal variations (bipartite
sternebrae) were also seen at the high dose, but there were no
teratogenic effects. (The authors maintained that the low dose was not
maternally or embryotoxic. The conclusion concerning embryotoxicity
was based on statistical significance rather than biological
significance, and an erroneously large control value appears to have
been used for statistical comparison.) On the basis of the
significantly lower mean body-weight gains and the higher incidences
of dams with totally resorbed litters (i.e. early implants only) in
all treated groups, an NOAEL was not identified for maternal or
developmental toxicity. (The authors indicated that
sialodacryoadenitis viral infection was present during much of the
dosing period and indicated that this may have had some effect on
weight gain; however, in previous studies this virus had no
teratogenic effects. If this is a valid conclusion, the increased
incidence of total resorption of litters at all doses should be
considered treatment-related.) (Marks et al., 1982).
Rabbits
Groups of 10, 12, and 14 pregnant New Zealand white rabbits were
fed dicloran (purity unspecified) in the diet at 0, 100, or 1000 ppm,
equivalent to 0, 3, and 30 mg/kg bw per day, on days 8-16 of
gestation, the day of mating being considered gestation day 1.
Pregnant females were allowed to deliver naturally, and the pups and
parental females were sacrificed on post-natal day 21. There were no
effects on maternal body weight during treatment. The mean litter size
was slightly lower in the group at the high dose than in controls,
with values of 7.1, 6.6, and 6.1 for the control, low, and high dose,
respectively. In the absence of data on resorption, however, it could
not be determined whether this effect was treatment-related. The pup
weights at birth were not provided, but the mean pup weights at
weaning (day 21) were comparable in all groups. There were no
malformations and no increase in the incidence of skeletal
malformations in treated pups (Wazeter, 1966).
Groups of 15-16 naturally mated New Zealand white rabbits were
dosed by gavage with dicloran (purity, 98.3%) in 1% carboxymethyl
cellulose at 0, 8, 20, or 50 mg/kg bw per day on days 6-18 of
gestation, the day of mating being considered gestation day 0. These
doses were based on the results of a range-finding study in which a
dose of 100 mg/kg bw per day caused two deaths, marked reduction in
food intake, reduced body-weight gain during treatment, slightly
increased post-implantation loss, and reduced litter and mean fetal
weights, with gross malformations in 4/11 fetuses from one litter. The
dose of 50 mg/kg bw per day resulted in reduced food intake and lower
body-weight gains throughout treatment, and at 20 mg/kg bw per day
there was a slight, transient reduction in food intake, with reduced
body-weight gain and slight body-weight loss on days 9-11 of gestation
(James & Brennan, 1991).
The dams were sacrificed on day 29 of gestation, the uterine
contents were examined, and all fetuses were assessed for viability,
sex, and external, visceral, and skeletal malformations and
variations. There were no treatment-related effects on mortality,
clinical signs, or food consumption. The mean body weights and body-
weight gain were slightly but consistently lower than those of
controls in females at the intermediate and high doses throughout both
the treatment and post-treatment periods. This resulted in an overall
mean body-weight gain in these animals that was 17-32% less than that
of controls during treatment and at termination. The mean gravid
uterine weights were also lower in these animals than in controls,
whereas the carcass weights were similar in all groups, indicating
that the weight changes were attributable primarily to effects on the
litters rather than the dams. The lower body-weight gain of dams at
the intermediate dose in the latter stages of gestation may be due
partially to the smaller litter sizes of this group, which were not
treatment-related; however, the effects seen in females at the
intermediate dose early during treatment (days 6-9) may indicate
maternal toxicity. The differences in body-weight gain in animals at
the high dose were also considered to be toxicologically significant.
Dams at the two higher doses had a slight increase in the frequency of
post-implantation loss (7.4, 9, 4.8, and 10% for control to high dose,
respectively). There were slight increases in the incidence of minor
anomalies of the gall-bladder in three fetuses in three litters and
delays in ossification of all limb epiphyseal sites in four fetuses in
three litters (with none in controls) at 50 mg/kg bw per day. The
NOAEL for maternal toxicity was 8 mg/kg bw per day on the basis of
lower body-weight gain early in the treatment period at 20 and 50
mg/kg bw per day. The NOAEL for developmental toxicity was 20 mg/kg bw
per day (Barton & Wilcox, 1996).
(f) Special studies
(i) Cataractogenicity
Dogs have been shown to develop lesions of the cornea and lens
after prolonged oral administration of dicloran. It has been suggested
that a photochemical product reaction is responsible for the lesion as
it occurs only when dogs are exposed to sunlight. Groups of four to
eight beagle dogs and six Hormel-Hanford miniature swine were fed
dicloran (purity, 95.8%) at concentrations of 0, 0.75, 6, 24, 48, or
192 mg/kg bw per day for periods varying from 50 to 306 days. Corneal
opacity appeared in dogs within 53-104 days after administration of 24
or 48 mg/kg bw per day, when they were exposed to sunlight. Dogs that
were not exposed to sunlight and eyes that had been sutured closed did
not develop lesions. Dogs given 192 mg/kg bw per day refused to eat
after 38 days and were given dicloran by capsule. All of these animals
died 49-53 days after the study began; no eye lesions were detected.
In several dogs with eye damage that were maintained for four months
to one year after dicloran administration had ceased, the pathological
changes seen in the cornea and lens were not reversed. Dicloran did
not appear to affect miniature swine at any concentration, and no
histopathological changes were seen in the eyes. A dose-related
increase in the presence of Heinz bodies was seen in the blood of both
dogs and swine, with at least one dog affected per dose and pigs
affected at doses of 48 mg/kg bw per day or more. No methaemoglobin
formation was found in dogs or pigs. Administration of dicloran as a
dust (0.1 mg) or a 5% solution directly into the eyes of dogs for
three months had no effect on corneal opacity or irritation of the
conjunctivae (Curtis et al., 1968; Bernstein et al, 1970; Earl et al.,
1971).
Ocular toxicity was not observed in rats or rhesus monkeys
(Serrone et al., 1967), and administration of 0.143 mg/kg bw per day
for three months to 20 human volunteers produced no ocular symptoms
(Strough, 1962).
The 1977 Joint Meeting (Annex 1, reference 28) reviewed
additional data requested by the 1974 JMPR and concluded that a
species difference in the kinetics of dicloran may partly explain the
photosensitive ocular toxicity observed in dogs,but not in other
mammals. 14C-Dicloran was administered orally at 100 mg/kg bw per day
for five days to four beagle dogs, four pigs (breed unspecified), and
eight Wistar rats. The concentration of radiolabelled residues was
highest in the dogs and slightly lower in pig tissues, but the tissue
residues in both species were 2.4-11-fold higher than those in liver
or plasma residues of rats. All three species had a high concentration
of residue in the liver, but in dogs and pigs the highest
concentrations were found in the pigmented tissues of the eye, the dog
having two to three times more residue in the iris and pigmented
retina than the pig. Nonpigmented eye tissues (cornea and lens) had
low concentrations. There was no correlation between ocular toxicity
and tissue residues of dicloran and its metabolites. The plasma
concentrations of radiolabel increased more rapidly in the dogs, but
24 h after three days of dosing the plateau plasma concentrations in
the pigs and dogs were similar (10-15 mg/kg bw). High concentrations
of radiolabel were excreted in the bile of dogs and pigs (Hamilton,
1977).
(ii) Haematological effects
Groups of 10 or 15 rats (strain unspecified) of each sex were
treated with dicloran at concentrations of 0, 5, 20, or 100 mg/kg bw
per day by gavage for four months or in the diet at a concentration of
0 or 20 ppm, equivalent to 1 mg/kg bw per day for four months.
Measurements of red blood cells, total and differential leukocyte
counts, platelet count, haematocrit, haemoglobin concentration,
glucose, growth, and food consumption indicated no significant effect
attributable to dicloran at any dose by either route (Evans et al.,
1963).
Because of the known ability of 4-nitroaniline to induce specific
blood dyscrasias, short-term studies were performed to compare
dicloran and 4-nitroaniline. Groups of five weanling rats (strain
unspecified) of each sex were given dicloran by gavage at 0 or 400
mg/kg bw per day, five days/week, for four weeks, and 4-nitroaniline
was administered at 200 or 400 mg/kg bw per day to two comparable
groups over the same interval. In a second experiment, groups of six
male weanling rats were given dicloran by gavage at 0 or 800 mg/kg bw
per day or 4-nitroaniline at 400 or 800 mg/kg bw per day, five days
per week, for two weeks. Haematological parameters were normal in rats
treated at 400 mg/kg bw per day -- no Heinz bodies were detected and
the reticulocyte counts were normal -- although their body-weight gain
was 50-60% of that of controls. At 800 mg/kg bw per day, there was
slight weight loss. Red blood cell count, packed cell volume, and
haemoglobin measurements were normal, while the lymphocyte counts were
reduced to 65% of that of controls. At the end of the study, no
effects were seen on the spleen. In contrast, 4-nitroaniline had
definitive effects on growth at 200 mg/kg bw per day (70% of control),
Heinz bodies were identified in the blood, and the reticulocyte count
was markedly elevated to two to four times the control value. The bone
marrow was not affected. At 400 mg/kg bw per day, growth was reduced
to 60% of the control value, and a reduced red blood cell count (65%
of control with polychromasia and nucleation) was seen, accompanied by
increased spleen weight (200% of control). These effects were more
pronounced at 800 mg/kg bw per day where, in addition, the haemoglobin
concentration was reduced and the lymphocyte count greatly increased.
Although the high doses of dicloran caused lymphopenia, the other
haemotoxic effects seen with 4-nitro-aniline were not observed with
dicloran (Boots Pure Drug Co., 1962).
In studies to substantiate the difference between the effects of
4-nitroaniline and dicloran, a single oral dose of 500 mg/kg bw
dicloran was given to cats. No methaemoglobin was noted at any time
between 1 and 48 h after dosing; however, administration of
4-nitroaniline as a single dose of 100 mg/kg bw resulted in
methaemoglobin over the same time. In addition, the cats given
4-nitroaniline were cyanotic and showed extensive muscle weakness
(Gurd, 1974). Although methaemoglobin was not assessed in all studies,
it is noteworthy that the 60-day study in mice was the only one that
showed an association between dicloran and significantly elevated
methaemoglobin concentrations in the blood at doses of 100 and
200 mg/kg bw per day (Mallyon et al., 1986). No effect on
methaemoglobin was seen in the two-year study in dogs (Woodard et al.,
1964).
3. Observations in humans
In a double-blind clinical study, 20 male volunteers were given
10 mg of dicloran (purity unspecified), equivalent to 0.14 mg/kg bw
per day, and 10 received a placebo, once daily, for 90 days. The dose
and duration were based on the estimated maximum residues obtained
from consumption of fruits and vegetables over one year.
Haematological examinations and tests for liver and kidney function
were performed at various intervals over the course of the study; the
results were found to be normal (Strough, 1962).
Extensive examinations were made on an industrial worker who had
been occupationally exposed to dicloran for three years, with
considerable exposure by inhalation and dermal contact for about 60
days per year. No adverse effects were observed (Brooks & Boyack,
1963).
Comments
Dicloran is rapidly absorbed, metabolized, and eliminated, mainly
in the urine, after oral administration to rats, goats, and humans; it
is also rapidly excreted by hens. Rats excreted most of the radiolabel
(90-96%) within 24 h, with > 70% in the urine and an additional
13-22% in the faeces, depending on the dose. Elimination was
essentially complete (91-97% of the administered dose) by 48 h, with
total tissue residues accounting for 0.2-0.3%. The highest tissue
concentrations were found in the liver (0.05-0.06 mg/kg) and kidneys
(0.02 mg/kg). Unchanged parent compound was detected in the faeces of
animals at the high dose only. The major urinary metabolites were the
sulfate and glucuronide conjugates of 2,6-dichloro-4-hydroxyaniline,
which accounted for 55-79% of the total administered radiolabel, and
the major faecal metabolites were derivatives of glutathione
conjugates. The parent compound and its glutathione conjugates were
the major residues in plants. None of the metabolites was of
toxicological concern. The rapid excretion of dicloran as a conjugate
indicates that it is readily metabolized in vivo.
The major metabolite in rat urine (2,6-dichloro-4-hydroxyaniline)
was not present in goat urine, which contained more polar conjugates
that could not be hydrolysed by glucuronidase or sulfatase. In goats,
dicloran is reduced to 4-amino-2,6-dichloro-aniline and acetylated to
4-amino-3,5-dichloroacetanilide (4-6% in urine), which is rapidly
metabolized and excreted in the urine and faeces, although a reactive
intermediate is formed in goat liver and bound covalently to
macromolecules. Species differences in dicloran metabolism were also
apparent in dogs and mice, which partly explain the photosensitive
oculotoxic effects observed in dogs and the methaemo-globinaemia noted
in mice, which are not seen in other mammals. Preliminary studies
suggested that the metabolites of dicloran in humans are similar to
those in rats. Although recovery was considered complete, excretion
was somewhat slower in humans than in rats, most of the material being
excreted within 1.5 days.
Dicloran has low or slight toxicity when administered by the oral
route, depending on species. It has low dermal toxicity, is not
irritating to the skin, is a mild eye irritant, and is not a skin
sensitizer.
WHO has classified dicloran as unlikely to present an acute
hazard in normal use (WHO, 1996).
The results of short-term studies of toxicity in rabbits treated
dermally and in mice and rats treated in the diet or by gavage and of
long-term studies of toxicity in mice, rats, and dogs treated in the
diet indicate that the liver is the primary target organ. Increased
liver weights and centrilobular hepatic hypertrophy were observed
consistently in all species treated orally, and increased splenic
activity and splenic extramedullary haematopoiesis were noted in
short- and long-term studies in mice.
When dicloran was fed to mice at 0, 300, 600, or 1200 ppm for 60
days, the NOAEL was 300 ppm (equal to 49 mg/kg per day), on the basis
of hepatic lesions, splenic extramedullary haematopoiesis, and
increased methaemoglobin at doses of 600 ppm and above.
In a 90-day study in mice treated by gavage with 0, 15, 45, 135,
400, or 600 mg/kg bw per day, the NOAEL was 15 mg/kg bw per day on the
basis of significant polycythaemia in males and hypercholesterolaemia
and a significant increase in the incidence of splenic extramedullary
haematopoiesis in females at 45 mg/kg bw per day and above.
A NOAEL was not identified in a 90-day study in rats fed diets
containing 0, 1000, 3000, or 5000 ppm dicloran because of
significantly reduced body-weight gain in conjunction with effects on
the liver and thyroid in all treated groups. In an eight-week study in
which rats were given diets containing dicloran at doses of 0, 500, or
750 ppm, the NOAEL was 500 ppm (equal to 44 mg/kg bw per day) on the
basis of reduced body-weight gain, decreased food consumption, and
increased liver weights with associated hepatic histopathological
manifestations at 750 ppm.
In a study in which dicloran was fed to rats for six months at 0,
30, 300, or 3000 ppm, the NOAEL was 300 ppm (equal to 22 mg/kg bw per
day) on the basis of reduced body weights and increased liver and
spleen weights at 3000 ppm.
In a four-week study in rats given a dose of 0, 35, 140, or 350
mg/kg bw per day by gavage, the NOAEL was 35 mg/kg bw per day on the
basis of growth depression and hepatic hypertrophy and vacuolation at
140 mg/kg bw per day.
In an 18-month study of carcinogenicity in mice at dietary
concentrations of 0, 50, 175, or 600 ppm, the NOAEL was 175 ppm (equal
to 25 mg/kg bw per day) on the basis of increased liver weights,
centrilobular hepatocyte enlargement, centrilobular haemosiderosis,
focal and single-cell liver necrosis, and vacuolation of centrilobular
hepatocytes.There was no evidence of carcinogenicity in mice.
Two studies were conducted in rats to assess toxicity over a
two-year period of exposure. Rats were fed diets containing dicloran
at concentrations of 0, 20, 100, or 3000 ppm or 0 or 1000 ppm. The
overall NOAEL was 1000 ppm (equal to 59 mg/kg bw per day), on the
basis of changes in body-weight, food consumption, and haematological
parameters, spleen pigmentation, increased liver weights,
centrilobular hepatocyte enlargement, and thyroid hypertrophy at 3000
ppm. There was no evidence of carcinogenicity in these studies, but
they were considered inadequate for complete evaluation of the
carcinogenetic potential of dicloran.
Dogs fed dicloran at dietary concentrations of 0, 20, 100, or
3000 ppm for two years had treatment-related changes in haematological
and clinical chemical parameters and significant increases in liver,
spleen, and kidney weights, accompanied by histological changes at
3000 ppm that included irregular hepatic-cell size, moderate
hepatic-cell hypertrophy, and increased pigmentation of the liver and
spleen. The NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day.
Dicloran was not mutagenic in most assays, although occasional
positive responses were seen in more recent tests for reverse mutation
and in a test for mitotic recombination. The results of an assay for
sex-linked recessive mutation in Drosophila were equivocal. The
Meeting concluded that dicloran is unlikely to be genotoxic.
Studies of reproductive and developmental toxicity indicated that
dicloran is not a reproductive toxicant and is not teratogenic in rats
or rabbits. Dicloran was embryotoxic at maternally toxic doses in
rabbits, but an NOAEL for maternal or developmental toxicity was not
identified in rats.
In a two-generation study of reproductive toxicity in rats (one
litter per generation) at dietary concentrations of 0, 50, 250, or
1250 ppm, the NOAEL for systemic toxicity was 250 ppm (equal to 21
mg/kg bw per day) on the basis of reduced body-weight gains and
increased liver weights at 1250 ppm. The NOAEL for reproductive and
developmental toxicity was 250 ppm (equal to 21 mg/kg bw per day) on
the basis of reduced weights of F1 and F2 pups at 1250 ppm.
In a study of developmental toxicity, dicloran was administered
by gavage to rats on days 6-15 of gestation at doses of 0, 100, 200,
or 400 mg/kg bw per day. Because of significantly reduced mean
maternal body-weight gains and higher incidences of totally resorbed
litters in all treated groups, NOAEL values for maternal or
developmental toxicity were not identified. Dicloran was not
teratogenic in this study.
In a study of developmental toxicity in rabbits, dicloran was
administered by gavage at doses of 0, 8, 20, or 50 mg/kg bw per day on
days 6-18 of gestation. The NOAEL for maternal toxicity was 8 mg/kg bw
per day, on the basis of reduced maternal body-weight gains early
during treatment with 20 or 50 mg/kg bw per day. The NOAEL for
developmental toxicity was 20 mg/kg bw per day on the basis of a
slight increase in post-implantation losses, a slight increase in the
incidence of minor anomalies of the gall-bladder, and delays in
ossification of all limb epiphyseal sites in fetuses at 50 mg/kg bw
per day.
Dogs have been shown to develop lesions in the cornea and lens
after prolonged oral administration of dicloran. The 1977 Joint
Meeting reviewed additional data requested by the 1974 Meeting and
concluded that the photosensitive oculotoxic effects observed in dogs
but not in other mammals were due partly to a species difference in
the kinetics of dicloran. No oculotoxic effects were seen in any of
the more recent studies, although no further studies have been
conducted in dogs.
In a double-blind clinical study, 20 men were given 10 mg
dicloran (equivalent to 0.14 mg/kg bw per day) and 10 received a
placebo once daily for 90 days. The dose and duration were chosen on
the basis of the maximum residues estimated to be derived from
consumption of fruits and vegetables over one year. There was no
indication that administration of dicloran at this dosage had any
adverse effect.
An ADI of 0-0.01 mg/kg bw per day was established on the basis of
the NOAEL of 1.7 mg/kg bw per day for hepatic and haematological
effects in the two-year study in dogs and a 200-fold safety factor. A
larger than normal safety factor was used because of the inadequacy of
the long-term studies in rats for assessing the carcinogenic potential
of dicloran and because of the lack of a NOAEL for maternal and
developmental toxicity in rats.
An acute RfD was not allocated because dicloran has low or slight
toxicity when administered orally or dermally and because acute
effects occur only at very high doses, resulting in a 10 000-fold
difference between the ADI and the LOAEL for maternal and
developmental toxicity in rats. Therefore, the Meeting concluded that
the acute intake of residues is unlikely to present a risk to
consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 49 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
15 mg/kg bw per day (13-week study of toxicity)
175 ppm, equal to 24 mg/kg bw per day (18-month study
of carcinogenicity)
Rat: 35 mg/kg bw per day (lowest dose tested, four-week
study of toxicity)
500 ppm, equal to 44 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
300 ppm, equal to 22 mg/kg bw per day (six-month study
of toxicity)
1000 ppm, equal to 59 mg/kg bw per day (two two-year
studies of toxicity)
250 ppm, equal to 21 mg/kg bw per day (two-generation
study of reproductive toxicity)
Rabbit: 8 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (developmental toxicity)
Dog: 100 ppm, equal to 1.7 mg/kg bw per day (two-year study
of toxicity)
Human: 0.14 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of an acute reference dose
Not allocated (unnecessary).
Studies that would provide information useful for continued
evaluation of the compound
1. Combined study of toxicity and carcinogenicity in rats.
2. Study of developmental toxicity in rats at less than 100
mg/kg bw per day to establish clear NOAELs for maternal and
developmental toxicity.
3. Assays for genotoxicity in mammals in vivo, such as an
assay for micronucleus formation
4. Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete, > 70% urinary excretion within 24 h
Dermal absorption No data
Distribution Liver, kidney
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 90-96% in urine and faeces within 24 h
Metabolism in animals Metabolites differ in rats and goats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral > 4000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation No data
Skin irritation Not irritating
Eye irritation Minimally irritating
Skin sensitization Not sensitizing (Draize test)
Short term toxicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant oral NOAEL Rat: 44 mg/kg bw/per day (diet); 15 mg/kg bw per day
(gavage)
Lowest relevant dermal NOAEL Rabbit: 120 mg/kg bw per day
Lowest relevant inhalation NOAEL Poor study: data on actual intake, particle size not
provided
Genotoxicity Unlikely to be genotoxic; no study of genotoxicity in
mammals in vivo
Long term toxicity and carcinogenicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant NOAEL Dog: 1.7 mg/kg bw per day (2-year study)
Carcinogenicity No evidence of carcinogenicity in mice. The study in
rats was inadequate.
Reproductive toxicity
Reproduction target/critical effect Increased ovary/testis weights (no histological
findings)
Lowest relevant reproductive NOAEL Rat: 21 mg/kg bw per day (reduced body-weight gain,
increased liver weight)
Developmental target/critical effect Increased resorptions, delayed ossification, not
teratogenic
Lowest relevant developmental NOAEL 20 mg/kg bw per day in rabbits; no NOAEL in rats.
A 10 000-fold difference exists between the ADI and
the LOAEL in rats.
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies
Cataractogenicity Photosensitive oculotoxic effects observed in dogs are
not seen in other mammals
Medical data No indication that administration of dicloran at
10 mg/day to men for 90 days had any adverse effect.
Extensive examinations were made on one industrial
worker occupationally exposed to dicloran over three
years, with considerable inhalation and dermal
exposure for about 60 days/year. No adverse effects
were observed
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 2-year study, dogs 200
Acute reference dose Not allocated (unnecessary)
References
Allen, J.A., Brooker, P.C. & Gray, V.M. (1988) Technical dicloran:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. No. SMS 43/871138 from Huntingdon Research
Centre, United Kingdom. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Bachmann, E., Golberg, L. & Thibodeau, L. (1971) Aspects of the
determination of biphenyl hydroxylase activity in liver homogenate.
Exp. Mol. Pathol., 14, 303-326.
Barton, S.J. & Wilcox, S. (1996) Dicloran: Development toxicity in
rabbits. Unpublished report No. 11379 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Barton, S.J. & Wilcox, S. (1997) Dicloran: Two generation reproduction
study in rats. Unpublished report No. 14271 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Basting, L.A., Cooper, C., Witmer, C.M. & Gallo, M.A. (1983) Toxicity
and mutagenicity of 2,6-dichloro-4-nitroaniline in the Ames test.
Fed. Proc., 42, 623.
Basting, L.A., Witmer, C.M., Iba, M.M. & Gallo, M.A. (1984) The effect
of 2,6-dichloro-4-nitroaniline on hepatic microsomal systems.
Toxicologist, 4, 149.
Bernstein, H., Curtis, J.M., Earl, F.L. & Kuwabara, W. (1970)
Phototoxic corneal and lens opacities. Arch. Ophthalmol., 83,
336-348.
Boots Pure Drug Co. (1962) Dicloran: Experimental data on toxicity.
Unpublished report No. T-17 from Boots Pure Drug Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Boyack, G.A. & Brooks, B.W. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Unpublished report
No. T-21 from Upjohn Co., USA. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Cheng, T. (1996a) Nature of the residue of 14C-dicloran in lactating
goats. Unpublished report No. HWI6564-102 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996b) Metabolism of 14C-dicloran in rats. Unpublished
report No. HWI6564-108 from Hazleton Wisconsin, Inc., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996c) Nature of the residue of 14C-dicloran in laying
hens. Unpublished report No. HWI6564-100 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Creedy, C.L., Hemmings, P.A. & Needham, D. (1985) The effect of
dicloran on the hepatic mixed-function oxidase system of the male rat
following oral administration of 100 mg/kg body weight twice daily for
4 days. Unpublished FBC Ltd report No. METAB/85/24, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Curtis, J.M., Bernstein, H., Earl, F.L. & Smalley, H.E. (1968) Corneal
and lens opacities in dogs treated with 2,6-dichloro-4-nitroaniline.
Toxicol. Appl. Pharmacol., 12, 305.
Dawson, J. R. (1988) Metabolism and residues of dicloran in the laying
hen. Unpublished report No. ENVIR/88/26 from Schering Agrochemicals
Ltd, United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
Earl, F.L., Curtis, J.M., Bernstein, H.N. & Smalley, H.E. (1971)
Ocular effects in dogs and pigs treated with dichloran
(2,6-dichloro-4-nitroaniline). Food Cosmet. Toxicol., 9, 819-828.
Eberts, F.S. (1965) Fate of Botran (2,6,-dichloro-4-nitroaniline) in
rat and man. Unpublished report No. M9 from Upjohn Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Eberts, F.S., Meeks, R.C. & Vliek, R.W. (1963) Excretion of
Botran-14C (2,6,-dichloro-4-nitroaniline) by man. Unpublished Report
No. M3 from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Elliot, P.H., Smith, C., Crook, D., Gibson, W.A., Singh, H. &
Gopinath, C. (1988) Technical dicloran: Twenty-one day dermal toxicity
study in rabbits. Unpublished report No. SMS 60/871292 from Huntingdon
Research Centre Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Evans, J., Mengel, G. & Bostwick, L. (1963) Botran (U-2069) Effect of
oral administration. Final report four-months study. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Everest, R.P. & Tuplin, J.A. (1977) Dicloran, pure reference sample:
Mutagenicity testing in bacterial in vitro systems. Unpublished report
No. TX 77024 from Boots Pure Drug Co., United Kingdom. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Gaines, T.B. & Linder, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. Appl. Toxicol., 7, 299-308.
Gallo, M.A., Bachmann, E. & Golberg, L. (1976) Mitochondrial effects
of 2,6-dichloro-4-nitroaniline and its metabolites. Toxicol. Appl.
Pharmacol., 35, 51-61.
Garrett, N.E., Stack, H.F. & Waters, M.D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res., 168,
301-325.
Griffiths, A.F.J., Brockman, H.E., DeMartini, D.M. & de Serres, F.J.
(1986) The efficacy of Neurospora in detecting agents that cause
aneuploidy. Mutat. Res., 167, 35-45.
Gurd, P.R. (1974) Methemoglobin formation in the cat. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Hamilton, D.Y. (1977) Tissue distribution of [14C]-dichloran in rats,
dogs and pigs following repeated oral dosing at 100 mg/kg/day.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Hawkins, D.R., Kirkpatrick, D. & Shaw, D. (1988) The metabolism of
[14C]-dicloran in peaches: Analysis of samples. Unpublished report
No. HRC/SMS from Huntingdon Research Centre, Ltd United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. & Peters, J. (1967) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Natl Cancer Inst., 42, 1101-1114.
Jaglan, P.S. & Arnold, T.S. (1985a) Comparative metabolism of
[14C]-dichloran in rats and goats: Nature of liver residues.
Unpublished report No. 218-9760-85-002A from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jaglan, P.S. & Arnold, T.S. (1985b) Nature of muscle residue from the
treatment of a goat with [14C]-dichloran
(2,6-dichloro-4-nitroaniline). Unpublished report No. 218-9760-85-003
from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jaglan, P.S., Gosline, R.E. & Arnold, T.S. (1985a) Comparative
metabolism of [14C]-dichloran in rats and goats. Unpublished report
No. 218-9760-85-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Jaglan, P.S., Arnold, T.S., Gosline, R.E. & Subacz, C.J. (1985b)
Bioavailability of [14C]-dichloran (U-2069;
2,6-dichloro-4-nitroaniline) derived goat liver residues by the rats.
Unpublished report No. 218-9760-85-004 from Upjohn Co. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
James, P. & Brennan, C. (1991) Technical dichloran: Rabbit oral
developmental toxicity (teratogenicity) range finding study.
Unpublished report No. TOX/91/199-98 (T128) from Schering AG, Germany.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1978) Screening of pesticides for
mutagenicity in microbial systems. Taiwan Plant Protection Center
Research Report No. 12. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1980) Screening of pesticides for
mutagenicity in microbial systems II: with mammalian microsomal
activation. Natl Sci. Council Monthly, 8, 551-559.
Johnston, R.L. & Ceru, J.G. (1961) Acute oral LD50 -- rat and acute
intraperitoneal LD50 -- mouse using DCNA with 0.09% p-nitroaniline.
Unpublished report No. T-16 from Upjohn Co., USA. Submitted to WHO by
Gowan Company, Yuma, Arizona, USA.
Johnston, R.L. & Schwikert, R.S. (1963) Botran: Skin sensitization in
guinea pigs. Unpublished report No. 5567-64-RLJ-196B from Upjohn Co.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jones, E. & Fenner, L.A. (1987) Technical dicloran: Ames bacterial
mutagenicity test. Unpublished report No. TOX/87/199-85 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Kakuk, T. (1986) 90-Day oral toxicity study in B6C3F1 mice with DCNA.
Unpublished report No. 7263/86/003 from Upjohn Co., USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Kakuk, T.J., Weddon, T.W. & Thomas, R.W. (1979) Reevaluation of
potential hepatic effects of Botran in beagle dogs: Supplemental
report. Unpublished report No. 001-9610-79-005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Kappas, A. (1978) On the mechanisms of induced somatic recombination
by certain fungicides in Aspergillus nidulans. Mutat. Res., 51,
189-197.
Lessel, B. (1974) Two-year feeding trial in rats on dicloran.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Lobdell, B. & Johnson, C. (1965) U-2069: Effect on reproduction
capacity through three generations in the rat. Unpublished report from
Woodard Research Corporation. Cited from the 1974 JMPR Monograph
(Annex 1, reference 22).
Mallyon, B.A. & Markham, L.P. (1989) Technical dicloran: Oncogenicity
study in the mouse. Unpublished report No. TOX/86006 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Mallyon, B.A. & Rawcliffe, L.M. (1985) Technical dicloran:
Palatability in the diet to the mouse. Unpublished report No.
TOX/85/199-74 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Mallyon, B.A., Rawliffe, L.M. & Major, I.R. (1986) Technical dicloran:
60-day dietary study in the mouse. Unpublished report No.
TOX/86/199-75 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Marks, T.A., Poppe, S.M., Moerdyk, D.L., Stuckhardt, J.L., Morris,
D.F. & Greenberg, J.H. (1982) U-2069: A segment II teratology study in
the rat. Unpublished report No. 9610/82/7263/005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Mason, J.M., Valencia, R. & Zimmering, S. (1992) Chemical mutagenesis
testing in Drosophila: VIII: Reexamination of equivocal results.
Environ. Mol. Mutag., 19, 227-234.
Maté, C., Ryan, A.J. & Wright, S.E. (1967) Metabolism of some 4-
nitroaniline derivatives in the rat. Food Cosmet. Toxicol., 5,
657-663.
McBride, D. & McGregor, D.B. (1987) Technical dicloran: Assessment of
unscheduled DNA synthesis using rat hepatocyte cultures. Unpublished
report No. 4414 from Inveresk Research International, Musselborgh,
United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Moustacchi, E., Carere, A., Morpurgo, G. Ramel, C. & Würgler, F.E.
(1986) Assays for genetic changes in fungi. In: Montesano, R.,
Bartsch, H., Vainio, H., Wilbourn, J. & Yamasaki, H., eds,
Long-term and Short-term Assays for Carcinogens: A Critical
Appraisal (IARC Scientific Publications No. 83), Lyon, International
Agency for Research on Cancer, pp. 203-249.
Myers-Basting, L.A. (1986) Mutagenicity and characterization of the in
vitro metabolism of 2,6-dichloro-4-nitroaniline. Unpublished report
No. (T101) from Rutgers University, New Jersey, USA. Submitted to WHO
by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I.R. (1991a) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 5 mg/kg body-weight. Unpublished report No.
TOX/90/199-95 (M48) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I. R. (1991b) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 500 mg/kg body-weight. Unpublished report No.
TOX/90/199-96 (M49) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997a) Metabolic fate and distribution of 14C-dicloran in
potatoes. Unpublished report No. 1947 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997b) Metabolic fate and distribution of 14C-dicloran in
lettuce. Unpublished report No. 1949 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Peters, D.H., Stuart, V., Smith, H.L., Crook, D., Gibson, W.A.,
Majeed, S.K. & Gopinath, C. (1991) Technical dicloran: Rat 13-week
dietary toxicity study. Unpublished report No. TOX 89324 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980a) Primary eye irritation evaluation
in New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-001 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980b) Primary dermal irritation study in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T. J. & Wood, D. R. (1980c) Acute dermal toxicity screen in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-003 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Seaman, W.J., Weddon, T.E. & Kakuk, T.J. (1980) Three-week inhalation
study in rats, rabbits and dogs with Botran. Unpublished report No.
218-9610-80-004 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Serrone, D.M., Pakdaman, P., Stein, A.A. & Coulston, F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
rhesus monkeys. Toxicol. Appl. Pharmacol., 10, 404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Smith, S (1989) Metabolism of [14C]-dicloran in peaches under field
and glasshouse conditions. Unpublished report No. ENVIR/89/12 from
Schering Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Strough, A.R. (1962) Botran clinical study. Unpublished report from
Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
US Environmental Protection Agency (1974) Unpublished data from EPA
laboratories. Cited from the 1974 JMPR Monograph, (Annex 1, reference
22).
Waters, M.D., Sandhu, S.S., Simmon, V.F, Mortelmans, K.E., Mitchell,
A.D., Jorgenson, T.A., Jones, D.C.L., Valencia, R. & Garrett, N. E.
(1982) Study of pesticide genotoxicity. Basic Life Sci., 21,
275-326.
Waterson, L.A., Gibson, W.A., Morrow, J. & Gopinath, C. (1992)
Technical dicloran rat 8-week repeat dose study. Unpublished report
No. 91/199-97 from Huntingdon Research Centre, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wazeter, F.X. (1966) Somers test in the albino rabbit. Unpublished
report No. 100-037 from International Research and Development Corp.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wilcox, S., & Barton, S.J. (1996) Dicloran: Range-finding study for a
two generation reproduction study in rats. Unpublished report No.
14271 from Inveresk Research International, United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Woodard, M. W., Cockrell, K.O. & Woodard, G. (1964) Safety evaluation
by oral administration to rats and dogs for 104 weeks. Unpublished
report No. U2069 from Woodard Research Corporation, USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila: V: Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V: results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
DCNA, 2.6-dichloro-4-nitroaniline; HCNA,
2-hydroxy-4-nitro-6-chloroaniline; DCHA,
2,6-dichloro-4-hydroxyaniline; DCAP, 4-amino-2,6-dichlorophenol;
DCNAP; 3,5-dichloro-4-hydroxyacetanilide; DCDP,
2,6-diclorophenylenediamine; DCAA, 4-amino-3,5-dichloroacetanilide;
DCNP, 2,6-dicholoro-4-nitrophenol; DCP, 2,6-dichlorophenol; DCA,
2,6 dichloroaniline; A1, A2, A3, glutathione conjugate deriviatives.
R, G, H, P, S correspond to metabolites in rats, goats, hens, plants,
and soil or sediment, respectively. Major metabolites are represented
by letters in upper-case, minor metabolites are represented by
lower-case letters.
potential to form reactive intermediates after ingestion by rats
(Jaglan & Arnold, 1985a; Jaglan et al., 1985a,b).
(c) Effects on enzymes and other biochemical parameters
Oral administration of dicloran at a dose of 400 or 1000 mg/kg bw
per day to rats for three months resulted in increased hepatic
demethylase and desulfurase activity, and liver mitochondrial oxygen
consumption was increased (Serrone et al., 1967). Dicloran at oral
doses of > 10 mg/kg bw stimulated rat liver mixed-function
oxidases, and a dose of 500 mg/kg bw decreased mitochondrial oxidation
of succinate without concomitant uncoupling of oxidative
phosphorylation. Oxidative phosphorylation was not affected by single
doses at any concentration. Daily administration of 1000 mg/kg bw to
rats induced uncoupling of oxidative phosphorylation after four days
(Bachmann et al., 1971). These liver enzymes were not stimulated in
rhesus monkeys treated with 160 mg/kg bw per day dicloran for three
months (Serrone et al., 1967), and no effect was seen on brain or
liver mitochondrial function in mice (Bachmann et al., 1971).
The 2,6-dichloro-4-hydroxyaniline metabolite was as active as
2,4-dinitrophenol in vitro in uncoupling oxidative phosphorylation,
whereas dicloran was only one-tenth as effective.
2,6-Dichlorophenylenediamine had no effect on mitochondrial
respiration or oxidative phosphorylation in vitro (Bachmann et al.,
1971).
Dicloran and 2,6-dichloro-4-hydroxyaniline at 10-5 mol/L
inhibited electron transport and uncoupled oxidative phosphorylation
in vitro, whereas 2,6-dichlorophenylenediamine at 10-4 mol/L
induced only slight uncoupling. These inhibitory effects in vitro
cannot be considered serious adverse effects since they were not
confirmed by similar observations in vivo. Mitochondria isolated
from rats treated with dicloran or its metabolites were functionally
normal (Gallo et al., 1976).
In a preliminary study, oral doses of 1000 mg/kg bw dicloran per
day for four days to female Sprague-Dawley rats did not significantly
induce cytochrome P450 activity (Basting et al., 1984). After oral
administration of 100 mg/kg bw twice a day for four days to male
Sprague-Dawley rats, however, significant increases were found in the
activities of cytochrome P450 (55% increase), cytochromeb5 (24%
increase), benzphetamine demethylase, and ethoxycoumarin deethylase
(51-68% increases) in comparison with controls. The pattern of enzyme
induction was reported to indicate that dicloran significantly induces
phenobarbital-type enzymes in rat microsomes (Creedy et al., 1985).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of dicloran are
presented in Table 1. Technical-grade dicloran was of low or slight
acute toxicity by all routes of administration in all species
examined. Oral administration resulted in nasal haemorrhage,
paralysis, depression, and excessive yellow urine and faeces.
Intraperitoneal administration resulted in sleep, depression, and
excessive yellow urine and faeces. The effects of subcutaneous
injection were confined to local damage in which the test substance
became enclosed in a fibrous sac, sometimes followed by abscess
formation and eventual ulceration. Dermal administration resulted in
no systemic clinical effects.
Technical-grade dicloran (purity, 96%) was not irritating to the
intact or abraded skin of male or female New Zealand white rabbits at
a concentration of 500 mg per site (Raczniak & Wood, 1980b) or after
repeated daily applications of 10 mg dry material or 0.1 ml of 10%
aqueous suspension to the abraded or unabraded skin of albino rabbits
over five days (Boots Pure Drug Co., 1962).
Technical-grade dicloran (purity, 96%) caused a minimal
conjunctival response in the eys of New Zealand white rabbits,
consisting of redness and chemosis 24 and 48 h after treatment.
Corneal opacity or injury to superficial layers of the corneal or
conjunctival epithelium was not observed (Raczniak & Wood, 1980c).
Four successive daily applications of 2-3 mg dicloran to the
conjunctival sac of four rabbits resulted in a slight inflammatory
reaction of the cornea, which was of short duration (Boots Pure Drug
Co., 1962).
Dicloran was inactive in two tests for skin sensitization in
guinea-pigs (Boots Pure Drug Co., 1962; Johnston & Schwikert, 1963).
(b) Short-term studies of toxicity
Mice
Groups of Charles River COBS mice were fed diets containing 0,
1250, 2500, 5000, or 10 000 ppm technical-grade dicloran for two
weeks, equivalent to 0, 190, 380, 750, and 1500 mg/kg bw per day. At
10 000 ppm, 20% of animals of each sex died; the only clinical sign
observed was hunched posture. Food consumption and body weight were
significantly reduced. Female mice fed 5000 or 10 000 ppm had
significantly increased absolute liver weights, and animals of each
sex fed doses of 2500 ppm or more had an increased liver:body-weight
ratio. Food consumption was slightly reduced in animals of each sex at
2500 and 5000 ppm, and a slight reduc-tion in food consumption was
noted at 1250 ppm only in females (Mallyon & Rawcliffe, 1985).
Table 1. Acute toxicity of dicloran
Species Strain Sex Route LD50 Reference
(mg/kg bw)
Rat Sherman SPF M/F Oral > 4000 Gaines & Linder (1986)
Rat NR NR Oral 4040 Boots Pure Drug Co. (1962)
Rat NR NR Oral ~ 8000 Serrone et al. (1967)
Rat NR NR Oral > 10 000 Johnston & Ceru (1961)
Mouse NR NR Oral 1500-2500 Boots Pure Drug Co. (1962)
Guinea-pig NR NR Oral 1400 Boots Pure Drug Co. (1962)
Rabbit New Zealand white M/F Dermal (abraded) > 2000 Raczniak & Wood (1980a)
Mouse NR NR Intraperitoneal 5500 Johnston & Ceru (1961)
Mouse NR NR Intraperitoneal 2500 Boots Pure Drug Co. (1962)
Rat NR NR Intraperitoneal 1500 Boots Pure Drug Co. (1962)
Rat NR NR Subcutaneous > 5000 Boots Pure Drug Co. (1962)
Mouse NR NR Subcutaneous > 6000 Boots Pure Drug Co. (1962)
SPF, specific pathogen-free; M/F, male and female; NR, not reported
Groups of 10 male and 10 female CD-1 (ICR) mice received dicloran
(purity, 95.8%) in the diet at 0, 300, 600, or 1200 ppm, equal to 0,
49, 100, and 200 mg/kg bw per day in males and 0, 53, 100, and 220
mg/kg bw per day in females, for 60 days. There were no
treatment-related effects on survival, clinical signs, body weight,
food or water consumption, or food conversion. A significant increase
in methaemoglobin concentrations in the blood of both males and
females was noted at 600 and 1200 ppm (males: 1, 1.3, 1.4, and 1.6%;
females: 0.6, 0.7, 0.9, and 1.2%, control to high dose, respectively).
Cholesterol and bilirubin concentrations were significantly increased
in high-dose females (cholesterol: 2.4 ± 0.58, 2.2 ± 0.50, 2.5 ± 0.2,
3.0 ± 0.65 mmol/L; bilirubin: 4.3 ± 0.6, 5.4 ± 1.4, 5.5 ± 2.4, 5.8 ±
1.7 mol/L, control to high dose, respectively). These changes were
dose-related but were not observed in males, which, unlike females,
had specific liver lesions. Minimal to slight centrilobular
hepatocytic enlargement (2-5/10 versus 0/10 controls) and fatty liver
degeneration (2-4/10 versus 0/10 controls) with an increased severity
of haemosiderosis in the spleen was noted in males at 600 and 1200
ppm, and a slight increase in the degree of haematopoietic activity
was noted in the spleen of males and females combined at the
intermediate and high doses. The NOAEL was 300 ppm, equal to 49 mg/kg
bw per day in males (Mallyon et al., 1986).
Dicloran (purity, 97%) was administered to groups of 15 B6C3HF1
mice of each sex (five of each sex per dose for interim sacrifice) by
gavage at doses of 0, 15, 45, 135, 400, or 600 mg/kg bw per day for 90
days, with an interim sacrifice at 49 days. There were no
treatment-related effects on survival or body weight; data on food
consumption were not provided. Significant polycythaemia was seen in
males, and hypercholesterolaemia at 1.2-2.1 times the control level
and a significant increase in the incidence of splenic extramedullary
haematopoiesis were seen in females at > 45 mg/kg bw per day.
Dose-related increases in cholesterol concentrations (1.3-2.3 times
the control concentration) were also noted in males, which were
statistically significant at > 135 mg/kg bw per day. A deep-yellow
colouration of the urine was noted in all treated groups and was
attributed to the yellow colour of the test material. In the absence
of any associated lesion in the group at the lowest dose, this effect
was considered not to be adverse. Other effects at higher doses
included kidney nephrosis in males at 400 and 600 mg/kg bw per day (5,
4, 6, 5, 15, 14/15, control to high dose, respectively) and females
(3, 5, 2, 4, 8, 12/15), with kidney tubular regeneration in males (0,
0, 3, 1, 8, 11/15), increased liver weights in animals of each sex at
> 135 mg/kg bw per day (7-64% increase), hepatocyte hypertrophy in
males (0, 0, 0, 4, 15, 15/15) and females (0, 0, 0, 0, 5, 12/15) at
> 400 mg/kg bw per day, liver necrosis in males (0, 0, 0, 0, 3,
3/15) and females (0, 0, 0, 0, 1, 1/15) at 400 and 600 mg/kg bw per
day, a significantly higher incidence of splenic extramedullary
haematopoiesis in males at > 135 mg/kg bw per day (0, 1, 2, 13, 15,
15/15) and in females at > 45 mg/kg bw per day (2, 5, 9, 10, 14,
15/15), increased spleen weight at the two higher doses (11-29%
increase), and epithelial hyperplasia of the urinary bladder in males
(0, 1, 2, 10, 12, 15/15) and females (0, 0, 0, 1, 7, 13/15) at doses
> 135 mg/kg bw per day. The NOAEL was 15 mg/kg bw per day (Kakuk,
1986).
Rats
Dicloran (unspecified purity) was administered by gavage to rats
(strain unspecified) at 400 or 1000 mg/kg bw per day for three months.
Deaths occurred among rats at the high dose. Significantly enlarged
livers and unspecified renal changes were observed by light and
electron microscopy, with increases in liver mitochondrial enzyme
activity and mitochondrial oxygen use (Serrone et al., 1967).
Groups of five newly weaned rats (strain unspecified) of each sex
were given dicloran (purity unspecified) by gavage at doses of 0, 140,
or 350 mg/kg bw per day, six days/week for four weeks. The growth of
males at 350 mg/kg bw per day was reduced to 60% of the control value.
At both doses, increased liver weight (120-155% of control) with
hepatic vacuolation were noted. There was no apparent effect on blood
parameters or on the kidneys at the end of the study (Boots Pure Drug
Co., 1962).
Groups of 10 rats (strain unspecified) were given dicloran
(purity unspecified) by gavage at doses of 35, 140, or 350 mg/kg bw
per day, six days/week, for four weeks. Groups of 20 controls of each
sex were available. Growth depression was observed in animals of each
sex at 350 mg/kg bw per day and in males at 140 mg/kg bw per day.
Increased liver weights and microscopic findings were also seen at
these doses. The liver lesions included a dose-related increase in
hepatocytic hypertrophy with increased vacuolization, especially in
the outer lobes. The liver had returned to normal size in half of the
animals which were maintained for two weeks after the end of the
treatment, except for males at the highest dose. The histopathological
changes in the liver caused by repeated short-term dosing was
apparently reversible within two weeks of the end of treatment. The
NOAEL was 35 mg/kg bw per day on the basis of effects on body weight
and liver at 140 mg/kg bw per day (Boots Pure Drug Co., 1962).
Technical-grade dicloran was fed to rats (strain unspecified) for
six months at doses of 0 (25 rats), 30 (15 rats), 300 (15 rats), or
3000 (10 rats) ppm, equal to 0, 2.2, 22, and 230 mg/kg bw per day in
males and 0, 2.5, 25, and 270 mg/kg bw per day in females. Another
10 animals of each sex per group received recrystallized dicloran at
3000 ppm, equivalent to 230 mg/kg bw per day for males and 260 mg/kg
bw per day for females. At 3000 ppm, growth of both males and females
was impaired (70-83% of control), whereas males fed purified material
had only a slight reduction in growth (89% of control). Significantly
increased liver weights (120-144% of control) were noted in both
groups at the high dose, with a significant increase in spleen weight
(129% of control) in females. Haematological parameters were not
affected at 12 weeks or at termination, and no microscopic lesions
were noted. The NOAEL was 300 ppm, equal to 22 mg/kg bw per day, on
the basis of changes in body weight and increased liver and spleen
weights at 3000 ppm (Boots Pure Drug Co., 1962).
Dicloran (purity, 97.4%) was administered to groups of 10 male
and 10 female Sprague-Dawley rats in the diet at doses of 0, 1000,
3000, or 5000 ppm, equal to 0, 75, 230, and 370 mg/kg bw per day in
males and 0, 80, 230, and 420 mg/kg bw per day in females, for 90
days. There were no treatment-related effects on survival or
ophthalmological parameters. Significantly lower body-weight gain was
seen in all treated groups (17-32% less than controls for males and
18-22% less for females). Although palatability may have accounted for
the lower body-weight gains during the first week of the study, food
consumption was comparable in all groups at week 2; however, it was
consistently lower than control values throughout the remainder of the
study (males, 10-14% less than controls; females, 12-21%; weeks 2-13).
Many of the haematological and clinical chemical findings were
considered to be associated with reductions in food consumption. The
treatment-related hepatic effects included increased cholesterol
concentrations, which were dose-related and statistically significant
in males at all doses (1.3-1.5 times the control concentration) and in
females at the intermediate and high doses (1.4-2.0 times control),
minimal to moderate centrilobular hypertrophy in males in all treated
groups (0, 3, 9, 9/10) and in females at the two higher doses (0, 0,
9, 9/10), and significant increases in liver weight in males and
females at the two higher doses (29-66%). Minimal thyroid
follicular-cell hypertrophy was noted in males in all treated groups
(0, 5, 6, 6/10) and in females at the intermediate and high doses (0,
0, 4, 7/10). The dose of 1000 ppm, equal to a daily intake of 75 and
80 mg/kg bw per day for males and females, respectively, was
considered to be at the upper limit of a suitable high-level dose for
a long-term study of toxicity and carcinogenicity in rats. An NOAEL
was not identified because significantly lower body-weight gains were
seen at all doses (Peters et al., 1990).
A second short-term study was conducted to investigate the
effects of dicloran at doses lower than those administered in the
90-day study. Dicloran (purity, 96.4%) was given in the diet to groups
of 10 Sprague-Dawley rats of each sex at doses of 0, 500, or 750 ppm,
equal to 0, 44, and 71 mg/kg bw per day for males, and 0, 48, and 71
mg/kg bw per day for females, for eight weeks. There were no
treatment-related effects on survival. The overall body-weight gain of
females at 750 ppm was significantly less than that of controls (12%
less overall), the greatest difference occurring during the final two
weeks of treatment (41-58% less than control). This decrease
correlated with a slight decrease in food consumption in this group
during treatment, which was 10-11% less than that of controls during
the last two weeks of treatment. Increased liver weight relative to
body weight (13-21%) in animals of each sex at 750 ppm was associated
in 10/10 males with minimal centrilobular hepatocyte enlargement. The
NOAEL was 500 ppm, equal to 44 mg/kg bw per day, on the basis of lower
body-weight gain and decreased food consumption in females at 750 ppm
and increased liver weights in males and females, with associated
centrilobular hepatocyte enlargement in males at 750 ppm; however, a
full histological examination was not carried out (only of the liver
and thyroid), and although alterations were found in the livers of
males at the high dose, the livers of males at the low dose were not
examined histologically (Waterson et al., 1992).
Rabbits
Technical-grade dicloran (purity, 96.2%) was moistened with
distilled water and applied to the intact skin of groups of five male
and female New Zealand white rabbits under an occluded patch for 6 h
per day for 21 consecutive days at doses of 12, 120, or 1200 mg/kg bw
per day. Animals at the two higher doses showed yellow staining of
untreated fur and extremities from day 4 onwards, but there were no
adverse clinical findings. One male and one female rabbit at 12 mg/kg
bw per day had slight, transient erythema lasting for two to four
days, and most rabbits at 120 and 1200 mg/kg bw per day had slight
erythema beginning in the second week of the study, which tended to
persist until study termination. The reaction did not progress beyond
slight erythema, and there did not appear to be a dose-related
difference above 120 mg/kg bw per day. At termination, male rabbits at
1200 mg/kg bw per day had significantly greater (36%) adrenal weights
than controls, but the adrenals were not examined histologically. The
liver weights were 18% greater in females at 1200 mg/kg bw per day
than in controls. No other effects were seen on body weight, food
consumption, or haematological, biochemical, or histological
parameters. The NOAEL was 120 mg/kg bw per day (Elliot et al., 1988).
Rats, rabbits, and dogs
Groups of 10 male and 10 female TUC/SPD rats, one male and one
female New Zealand white rabbit, and two male beagle dogs were exposed
to a dust aerosol of technical-grade dicloran by whole-body exposure
for 6 h per day, five days per week for three weeks. The nominal
concentration of the aerosol was 2 mg/L; the actual concentration and
particle size were not provided. Two rats died on the third day of
exposure, and one rabbit died on day 13. The food consumption and
body-weight gains of the treated animals were depressed. There were no
consistent haematological changes attributable to treatment. The blood
cholesterol concentration was significantly elevated in exposed dogs
and rabbits. The liver weights of exposed animals were increased,
although the histological appearance was unremarkable, with no
evidence of hepatocellular effects in any species (Seaman et al.,
1980).
Monkeys
Oral doses of 160 mg/kg bw per day were lethal to rhesus monkeys
within three months, with a greater effect on females than males. The
colouration of the urine differed from that of rat urine, suggesting a
difference in metabolism in the two species. Centrilobular fatty
infiltration was observed in the liver, and liver and kidney changes
were observed on electron microscopic examination, which included
swelling of mitochondria with distortion of the cristae. Hepatic
microsomal enzyme activity was not enhanced (Serrone et al., 1967).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a screening study, groups of 18 mice of each sex of each of
two susceptible hybrid strains (unspecified) were given dicloran at
215 mg/kg bw per day for three weeks from day 7 after birth.
Thereafter, for 18 months, the mice were fed 600 ppm (equivalent to
100 mg/kg bw per day) in the diet, sacrificed, and examined for
tumours. Dicloran did not cause a significant increase in the
incidence of tumours (Innes et al., 1969).
Dicloran (purity, 96.2-97.2%) was administered to groups of 50
CD-1 mice of each sex at dietary concentrations of 0, 50, 175, or 600
ppm, equal to 0, 7.4, 24, and 86 mg/kg bw per day in males and 0, 10,
36, and 120 mg/kg bw per day in females, for 80 weeks. The doses were
based on a the results of a 60-day dietary study in mice in which a
concentration of 600 ppm was associated with increased methaemoglobin
concentration, splenic pigment change, increased haematopoiesis,
centrilobular hepatocyte enlargement, and fatty degeneration of
hepatocytes. There was no adverse effect on mortality (percent
survival at termination: males, 50, 50, 64, and 56%; females, 84, 76,
76, and 78%, control to high dose, respectively). There were no
treatment-related effects on clinical signs, body weight, palpable
masses, food consumption, food conversion, or differential leukocyte
counts. Clinical chemistry and urinalysis were not performed. There
was no increase in tumour incidence. The liver was the principal
target organ, with increased absolute and relative weights (10-12%) in
males and females at the high dose, which were statistically
significant in females, and histopathological changes. These included
centrilobular hepatocyte enlargement (males: 8, 8, 7, and 26/50;
females: 1, 0, 2, and 5/50), centrilobular haemosiderosis (males: 1,
2, 4, and 12/50; females: 7, 5, 3, and 13/50), focal (4, 2, 5, and
10/50) and single-cell (1, 1, 0, and 6/50) liver necrosis in males,
and vacuolation of centrilobular hepatocytes (4, 3, 3, and 12/50) in
females. Acute inflammatory cell infiltration was seen in males at the
intermediate and high doses (2, 4, 9, and 9/50), but no other hepatic
changes were seen in males at this dose. Other changes at 600 ppm
included a higher incidence of erythropoiesis in the spleens of males
(7, 7, 8, and 15/50), an increased number of females with an
enlarged/distended uterus (5, 9, 9, and 15/50), associated with a
significant increase in the incidence and severity of cystic uterine
endometrial hyperplasia (17, 21, 24, and 31/50), and an increased
number of females at the high dose with distended mammary gland ducts
(9/50 versus 3/50 in control; animals at the low and intermediate
doses were not assessed). An 11% increase in kidney weight in males
was not associated with histopathological lesions. On the basis of the
above findings, the NOAEL was 175 ppm, equal to 24 mg/kg bw per day
(Mallyon & Markham, 1989).
Rats
Groups of 35 rats (strain unspecified) of each sex were fed
dicloran (purity unspecified) in the diet at concentrations of 0, 20,
100, or 3000 ppm for two years, equal to 0, 1.6, 8, and 240 mg/kg bw
per day, with an interim sacrifice of five animals of each sex per
dose at 13 weeks. The dose of 100 ppm had no effect on behaviour,
mortality, or growth, and all values were comparable to those of
controls. At 3000 ppm, growth and food consumption of males and
females were depressed, and haematological parameters (haemoglobin and
packed cell volume) were reduced but only after the first year of
treatment. Gross and microscopic examination at 13 weeks and at the
conclusion of the study showed slightly heavier livers, kidneys, and
testes in males at 3000 ppm. The incidence and location of neoplasms
were similar in treated and control rats. Histological examination
revealed changes in the liver at 3000 ppm, characterized by
hepatic-cell enlargement, increased severity of glycogen depletion,
increased basophilia of the cytoplasm, and the presence of dead cells.
Other observations were increased thyroid hypertrophy and increased
pigmentation of the spleen in males and females at 3000 ppm.
Hepatic-cell changes and slight adrenal cortical atrophy were seen in
several animals at this dose at 13 weeks, but the adrenal changes were
not seen at 104 weeks. The NOAEL was 100 ppm, equal to 8 mg/kg bw per
day (Woodard et al., 1964).
Groups of 25 Boots-Wistar strain rats of each sex were fed
dicloran (purity unspecified) in the diet at a concentration of 0 or
1000 ppm for two years, equal to 59 mg/kg bw per day in males and 71
mg/kg bw per day in females. There was no effect on survival, food
consumption, growth, haematological parameters, or gross or
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular constitution
of the liver, kidney, or spleen. The incidence of tumours in control
and treated groups was similar. The NOAEL was 1000 ppm, equal to 59
mg/kg bw per day, the highest dose tested (Lessel, 1974).
Dogs
Groups of four male and four female beagle dogs were fed dry
diets containing dicloran (purity unspecified) at concentrations of 0,
20, 100, or 3000 ppm for two years, equal to 0, 0.33, 1.7, and 44
mg/kg bw per day for males and 0, 0.36, 1.8, and 62 mg/kg bw per day
for females, with an interim sacrifice of one animal of each sex per
dose at 14 weeks. No compound-related changes in behaviour, food
consumption, or growth were observed. One female at 3000 ppm died at
74 weeks, with treatment-related signs of haemolytic anaemia (reduced
haemoglobin, immature erythrocytes, polychromophilic macrocytes,
increased leukocyte count, and increased myeloid: erythroid ratio in
the bone marrow) before death. One control male lost considerable
weight but survived to the end of the study. All dogs at 3000 ppm had
watery lachrymation, which persisted throughout treatment; the
examination of the eyes was not adequate to determine any ocular
toxicity of dicloran. Yellowing of the sclera, mucous membranes, and
abdominal skin were noted at the high dose. Changes in haematological
and clinical chemical parameters in males and females at the high dose
included significant reductions in haemoglobin from week 26 to
termination and reduced serum protein at termination; increased
activity of alanine and aspartate aminotransferases and increased
prothrombin time, blood urea nitrogen, and bromosulphthalein retention
were observed in one male and the one female that died at the high
dose, both of which had received 20-94% more test material than the
other dogs in this group owing to gradual body-weight loss relative to
the constant concentration in the diet. Gross and microscopic
examination revealed significantly increased liver, spleen, and kidney
weights accompanied by histological changes at 3000 ppm, which
included irregular hepatic-cell size (3/5 versus 0/6 in other groups),
moderate hepatic-cell hypertrophy (3/5 versus 0/6 in controls) and
increased pigmentation of the liver (4/5 versus 0/6 in control), and
spleen (5/5 versus 0/6 in controls). One male and two females at 3000
ppm (including the female that died) and one male at 20 ppm also had
enlarged gall-bladders containing tar-like, viscous bile, although
this was stated to be a spontaneous lesion in dogs (cystic mucinous
hypertrophy). Changes at 100 ppm which included irregular hepatic
cells, slight focal hepatocytic hypertrophy, slight vacuolation,
moderate liver pigmentation, and/or marked spleen pigmentation were
noted in two female dogs. A company re-evaluation attributed the
findings at 100 ppm to enzyme induction, although the
histopathological findings in this group were complicated by the
presence of parasitic hepatitis. No degenerative lesions were noted at
study termination, and there was no progression in the severity of
hepatocellular effects between interim and terminal sacrifice. The
NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day, on the basis of
significant reductions in haemoglobin, reduced serum protein, and
histopathological changes in the liver and spleen at 3000 ppm (Woodard
et al., 1964; Kakuk et al., 1979).
(d) Genotoxicity
The results of studies on the mutagenicity and genotoxicity of
dicloran are presented in Table 2. Dicloran gave occasional positive
responses in the most recent tests for reverse mutation and in a test
for mitotic recombination, with a 2-2.5-fold increase in mitotic
recombination. The results of the test for sex-linked recessive lethal
mutation in Drosophila were equivocal, even after re-analysis in
conjunction with historical control data.
(e) Reproductive toxicity
(i) Single-generation reproductive toxicity
Male rats (strain unspecified) were treated with dicloran (purity
unspecified) in the diet at concentrations of 0, 1000, or 2000 ppm,
equivalent to 0, 50, and 100 mg/kg bw per day, for 90 days. The dose
of 1000 ppm increased liver and kidney weights. When the males were
mated with untreated females, no difference was observed in the number
Table 2. Results of assays for the genotoxicity of dicloran
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, 16, 31, 62, 125, 99.9 Negativeb Everest & Tuplin (1977)
TA100, TA1535, 250, 500, 1000
TA1537, TA1538 µg/plate in DMSO
Reverse mutationa S. typhimurium 100 µg/plate in NR Negative Shirasu et al. (1976)
TA1535, TA1536, DMSO
TA1537, TA1538
Reverse mutationc S. typhimurium TA98, 20-200 µg/plate 99.5 Negatived Jeang & Li (1978)
TA100 in DMSO
Reverse mutatione S. typhimurium TA98, 2, 20, 200 µg/plate 99.5 Negativef Jeang & Li (1980)
TA100 in DMSO
Reverse mutationa S. typhimurium TA98, 6 concentrations NR Negative Waters et al. (1982);
TA100, TA1535, up to 10 000 µg/plate Garrett et al. (1986)
TA1537, TA1538
Reverse mutationa S. typhimurium TA98, 62, 100, 155, 200, NR Positiveg Basting et al. (1983);
TA98 NRF, TA100, 310, 415 µg/plate in Myers-Basting (1986)
TA100 NRF ethyl cellosolve/ethanol
or DMSO
Reverse mutationa S. typhimurium TA98, 50, 150, 500, 1500, 97.5 Positiveh Jones & Fenner (1987)
TA100, TA1535, 5000 µg/plate in
TA1537, TA1538 DMSO
Reverse mutationa S. typhimurium TA98, 10, 33, 100, 333, 98 Positivei Zeiger et al. (1992)
TA100 1000, 2000 µg/plate
in DMSO
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Reverse mutationc E. coli WP2, WP2uvrA- 62, 125, 250, 500, 99.9 Negativej Everest & Tuplin (1977)
1000 µg/plate in
DMSO
Reverse mutationc E. coli B/r try WP2, 20 µg/plate in DMSO NR Negative Shirasu et al. (1976)
WP2 try hcr
Reverse mutationa E. coli WP2uvrA- 6 concentrations NR Negativek Waters et al. (1982);
up to 10 000 µg/plate Garrett et al. (1986)
Mitotic recombationa S. cerevisiae D3 5 concentrations NR Negative Waters et al. (1982)
(unspecified)
Mitotic aneuploidy Neurospora crassa 5 or 10 µg/ml NR Negativel Griffiths et al. (1986;
I-41-5, I-34-8 in DMSO Moustacchi (1986)
DNA repairc B. subtilis H17 rec+, 20 g/disc in DMSO NR Negative Shirasu et al. (1976)
M45 rec-
DNA repaira B. subtilis H17 rec+, 20 g/disc in DMSO 99.5 Negativem Jeang & Li (1978)
M45 rec-
DNA repairc B. subtilis H17 rec+, 2 concentrations NR Negativem Waters et al. (1982);
M45 rec- (not specified) Garrett et al. (1986)
DNA repairc E. coli P3478 (polA-), 2 concentrations NR Negativem Waters et al. (1982);
W3110 (polA+) (not specified) Garrett et al. (1986)
Unscheduled DNA Rat hepatocytes 3, 4, 5, 6, 7, 8, 9, 97.5 Negativen McBride & McGregor
synthesis 10 µg/ml in DMSO (1987)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Mitotic recombination Aspergillus nidulans 2070, 4140, 6210 NR Positiveo Kappas (1978);
and aneuploidy (heterozygous diploid µg/plate in ethanol Moustacchi (1986)
strain)
Cytogenetic Human lymphocytes 2, 10, 20 µg/ml in 97.5 Negativep Allen et al. (1988)
alterationsa DMSO
In vivo
Recessive lethal Drosophila melanogaster Feeding at 1250 and 98 Equivocal Woodruff et al. (1985)
mutation Canton-S 1389 ppm in 5%
ethanol:5% Tween 80
Recessive lethal Drosophila melanogaster Re-analysis of data NR Equivocal Mason et al. (1992)
mutation Canton-S of Woodruff et al.
(1985)
NR, not reported; DMSO, dimethyl sulfoxide; NRF, nitroreductase-free
a With and without metabolic activation
b The positive controls, cyclophosphamide, 6-aminochrysene, and 2-aminofluorene, gave the expected results.
c Without metabolic activation
d The positive control nitroquinoline N-oxide gave the expected result.
e With metabolic activation
f The positive control sterigmatocystin gave the expected result.
g Positive in TA98 (> 100 µg/plate) only without metabolic activation
h Positive in TA98 and TA1538 (> 500 µg/plate) with and without metabolic activation and in TA100 without metabolic
activation. The positive controls, N-ethyl-N'-nitro-N-nitrosoguanidine, 9-aminoacridine, 2-aminoanthracene, and
2-nitrofluorene, gave the expected results.
i Positive in TA98 (> 100 µg/plate) with and without metabolic activation; positive control unknown
j The positive control ethyl methanesulfonate gave the expected result.
Table 2. (continued)
k The positive controls, 2-aminoanthracene and 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide, gave the expected results.
l This test system has not been fully validated. The positive control para-fluorophenylaniline gave the expected result.
m The positive control methyl methanesulfoxide gave the expected result.
n The positive control, Mischler's ketone [4,4'-bis(dimethylaminobenzophenone) in ethanol], gave the expected result.
o A 2-2.5-fold increase in mitotic recombination. This test system has not been fully validated.
p The positive controls, ethyl methanesulfonate and cyclophosphamide, gave the expected results.
of litters or in the number of animals born or weaned (US
Environmental Protection Agency, 1974).
Dicloran (purity unspecified) was fed to groups of 10 female rats
at concentrations of 0, 500, or 1000 ppm, equivalent to 0, 25, and 50
mg/kg bw per day, for 188 days before mating and then through
gestation and lactation. A reduced number of pups was reported when
females were treated with 1000 ppm. There was no apparent effect on
the survival of pups, although the mean body weight of those at 1000
ppm was slightly reduced (US Environmental Protection Agency, 1974).
(ii) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, with one
litter per generation, dicloran (purity, 99.2%) was administered to
groups of 28 male and 28 (F0) and 24 male and 24 female (F1) female
Sprague-Dawley rats in the diet at doses of 0, 50, 250, or 1250 ppm,
equal to 0, 4, 21, and 110 mg/kg bw per day. The doses were based on
the results of a range-finding study (Wilcox & Barton, 1996) in which
concentrations up to 1000 ppm did not produce any obvious alterations
in body weight, food consumption, duration of gestation, or litter
parameters. The animals were fed the test diet during the 10- (F0) or
11-week (F1) pre-mating period and then randomly allocated to mating
pairs (l:1) for a maximum of seven nights. Treatment was continued for
animals of each sex throughout the mating, gestation, and lactation
periods until termination of the F2 litters.
The treatment-related clinical signs included yellow staining of
the tray paper and, in some cases, yellow staining of the fur of all
animals at the high dose in both generations, due to the yellow colour
of the test material. There were no significant differences in mean
body weights or body-weight gain between control and treated males.
The mean body weights of F0 females at the high dose throughout the
pre-mating period was 2-5% lower than that of controls, resulting in a
12% lower mean overall gain at the end of the pre-mating period. This
was considered to be treatment-related. The mean body weights of F1
females at the high dose were, on average, 6% lower than those of
controls during pre-mating, but the overall mean body-weight gain was
only 5% lower. F0 and F1 females at the high dose had slightly lower
overall body-weight gain during gestation, the greatest difference
occurring during the first week of gestation (64% of control values in
F0 females and 86% in F1). There were no apparent effects on the
food consumption of F0 or F1 males or females during pre-mating and
gestation; however, the food consumption of females of both
generations at the high dose was 11% lower during the first week of
lactation, and these animals weighed more than controls and those
given lower doses at the end of lactation, indicating that pups in the
high-dose group were less demanding with respect to suckling than pups
in the other groups.
At the end of the study, a greater proportion of F0 and F1
females at the high dose were in pro-estrus (4/28 F0 controls versus
19/28 at the high dose; 4/24 F1 controls versus 11/24 at the high
dose). Since there was no indication of altered cycling, this result
was considered to be of no toxicological concern. Sperm-stage analysis
indicated no difference between control F0 and F1 males and those at
the high dose with respect to the number of testicular tubules at
various spermatogenic stages or in tubule diameter. There was a
dose-related increase in the incidence of abnormal sperm, particularly
with respect to tail defects, in F0 males (0.25 ± 0.26 and 0.29 ±
0.31% in the two control groups, 0.59 ± 0.96% at the low dose, 0.6 ±
0.65% at the intermediate dose, and 0.67 ± 0.58% at the high dose);
however, no such increase was observed in F1 males at the high dose
(0.21% versus 0.23% in controls). Furthermore, the mean values for F0
animals were within the historical control range, 0.0-0.8%. Thus,
these findings were considered incidental. The fertility and
gestational indices of control and treated F0 and F1 females were
similar. The male fertility indices of F0 males at the intermediate
and high doses were lower than that of controls (82 and 86%,
respectively), as was that of F1 males at the low dose (75%). Since
the fertility index of F1 males at the intermediate and high doses
approached 100%, the lower fertility indices were considered
incidental.
There were significant increases in absolute and body
weight-adjusted mean liver weights in F1 males and females at the
high dose; no data were collected for F0 animals. The weights of the
kidneys of F1 animals at the high dose were increased, and the
increase was significant in males. Brain weights were significantly
lower in F1 females at the high dose, and thymus weights were
significantly lower in F1 males and females at the high dose relative
to controls. No microscopic examinations were conducted on these
tissues. The mean absolute epididymal weights of F0 males at the high
dose and the absolute and body weight-adjusted epididymal weights of
F1 males at the intermediate and high doses were significantly lower
than those of controls. The authors indicated that the values for the
F1 males were similar to 'recent control values for animals of
similar age'; however, data on these controls were not provided. The
mean absolute and body weight-adjusted testicular weights were
statistically significantly increased in F1 males at the high dose
and non-significantly increased in F0 males at this dose. There was a
dose-related decrease in mean ovarian weights in F1 females, which
was statistically significant in the high-dose group. No
treatment-associated histopathological lesions were seen in the
reproductive tissues that were examined. In the absence of
histological data on non-reproductive tissues, the toxicological
significance of the observed changes in organ weights in these tissues
is equivocal.
No difference was observed in the mean number of implantation
sites per litter, litter size, sex ratio, or litter indices in control
and treated groups. A slight increase in the number of F1 pups that
died during days 4-21 of lactation was not observed in the F2
generation and was considered to be incidental. The group mean weights
of F1 and F2 litters were decreased throughout the lactation period,
as reflected in the lower mean pup body weights at this dose. Although
the magnitude of the difference was not as great in F2 pups as in F1
pups, these observations were consistent and considered to be related
to treatment. The mean litter weights of dams at the low and
intermediate doses were lower than those of controls on day 1 of
gestation, but the mean pup weights were comparable to those of
controls on days 1-21 of lactation, in contrast to the high-dose
group, and were therefore considered not to be related to treatment.
There were no treatment-related clinical observations or
malformations.
The NOAEL for systemic toxicity was 250 ppm, equal to 21 mg/kg bw
per day, on the basis of reduced body-weight gain at 1250 ppm in
females during pre-mating and gestation and increased liver and kidney
weights in males and females at this dose. The NOAEL for reproductive
and developmental toxicity was also 250 ppm, equal to 21 mg/kg bw per
day, on the basis of lower F1 and F2 pup weights at the next highest
dose. There were no histopathological lesions in the reproductive
tissues that would indicate that the changes in organ weights should
be considered adverse (Barton & Wilcox, 1997).
In a three-generation study, with two litters per generation,
dicloran (purity unspecified) was administered to groups of 20 rats
(strain unspecified) of each sex at 0 or 100 ppm, equivalent to 5
mg/kg bw per day, in the diet. On the basis of the reproduction
parameters examined, including the numbers of litters, stillbirths,
live births, birth weight, and lactation indices, dicloran had no
effect on reproduction (Lobdell & Johnston, 1965).
(iii) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley rats (Upj:TUC (SD) SPF) were
dosed by gavage with dicloran (purity, 93.7%) in 0.25% carboxymethyl
cellulose and 0.5% Tween 20 at doses of 0, 100, 200, or 400 mg/kg bw
per day on days 6-15 of gestation, the day of mating being considered
gestation day 0. These doses were derived from the results of a
range-finding study in which 5/5 rats died at doses of 2000 and 4000
mg/kg bw per day, and 3/5 at 1000 mg/kg bw per day. Neither of the two
survivors treated with 1000 mg/kg bw per day had pups, and one had 15
early resorptions. The mean body-weight gain of females treated with
500 mg/kg bw per day was 25% less than that of controls.
The dams were sacrificed on day 20 of gestation, and the uterine
contents were examined; the uteri of non-pregnant dams were stained
with ammonium sulfide, and all fetuses were assessed for viability,
sex, weight, and external malformations and variations. Equal numbers
of fetuses from each group were assigned to either visceral or
skeletal examination. There were no deaths. The treatment-related
clinical signs included yellow staining of the urogenital region in
some animals at each dose due to the colour of the test material, and
slight central nervous system depression throughout the treatment
period in animals at 200 and 400 mg/kg bw per day. The mean
body-weight gain (corrected for gravid uterine weights) of treated
animals was significantly lower (16-33% less) than that of the the
controls. The incidence of dams with totally resorbed litters (early
implants only) was increased in all treated groups (1, 5, 9, 10;
control to high dose, respectively); however, there were no
differences in the mean numbers of implants or resorptions or in the
mean litter sizes of dams with live litters. The fetal weights were
significantly lower at 200 and 400 mg/kg bw per day (7 and 20% less
than control, respectively), and a significant trend for an increased
incidence of delayed or reduced ossification was noted in these two
groups. Increased incidences of other skeletal variations (bipartite
sternebrae) were also seen at the high dose, but there were no
teratogenic effects. (The authors maintained that the low dose was not
maternally or embryotoxic. The conclusion concerning embryotoxicity
was based on statistical significance rather than biological
significance, and an erroneously large control value appears to have
been used for statistical comparison.) On the basis of the
significantly lower mean body-weight gains and the higher incidences
of dams with totally resorbed litters (i.e. early implants only) in
all treated groups, an NOAEL was not identified for maternal or
developmental toxicity. (The authors indicated that
sialodacryoadenitis viral infection was present during much of the
dosing period and indicated that this may have had some effect on
weight gain; however, in previous studies this virus had no
teratogenic effects. If this is a valid conclusion, the increased
incidence of total resorption of litters at all doses should be
considered treatment-related.) (Marks et al., 1982).
Rabbits
Groups of 10, 12, and 14 pregnant New Zealand white rabbits were
fed dicloran (purity unspecified) in the diet at 0, 100, or 1000 ppm,
equivalent to 0, 3, and 30 mg/kg bw per day, on days 8-16 of
gestation, the day of mating being considered gestation day 1.
Pregnant females were allowed to deliver naturally, and the pups and
parental females were sacrificed on post-natal day 21. There were no
effects on maternal body weight during treatment. The mean litter size
was slightly lower in the group at the high dose than in controls,
with values of 7.1, 6.6, and 6.1 for the control, low, and high dose,
respectively. In the absence of data on resorption, however, it could
not be determined whether this effect was treatment-related. The pup
weights at birth were not provided, but the mean pup weights at
weaning (day 21) were comparable in all groups. There were no
malformations and no increase in the incidence of skeletal
malformations in treated pups (Wazeter, 1966).
Groups of 15-16 naturally mated New Zealand white rabbits were
dosed by gavage with dicloran (purity, 98.3%) in 1% carboxymethyl
cellulose at 0, 8, 20, or 50 mg/kg bw per day on days 6-18 of
gestation, the day of mating being considered gestation day 0. These
doses were based on the results of a range-finding study in which a
dose of 100 mg/kg bw per day caused two deaths, marked reduction in
food intake, reduced body-weight gain during treatment, slightly
increased post-implantation loss, and reduced litter and mean fetal
weights, with gross malformations in 4/11 fetuses from one litter. The
dose of 50 mg/kg bw per day resulted in reduced food intake and lower
body-weight gains throughout treatment, and at 20 mg/kg bw per day
there was a slight, transient reduction in food intake, with reduced
body-weight gain and slight body-weight loss on days 9-11 of gestation
(James & Brennan, 1991).
The dams were sacrificed on day 29 of gestation, the uterine
contents were examined, and all fetuses were assessed for viability,
sex, and external, visceral, and skeletal malformations and
variations. There were no treatment-related effects on mortality,
clinical signs, or food consumption. The mean body weights and body-
weight gain were slightly but consistently lower than those of
controls in females at the intermediate and high doses throughout both
the treatment and post-treatment periods. This resulted in an overall
mean body-weight gain in these animals that was 17-32% less than that
of controls during treatment and at termination. The mean gravid
uterine weights were also lower in these animals than in controls,
whereas the carcass weights were similar in all groups, indicating
that the weight changes were attributable primarily to effects on the
litters rather than the dams. The lower body-weight gain of dams at
the intermediate dose in the latter stages of gestation may be due
partially to the smaller litter sizes of this group, which were not
treatment-related; however, the effects seen in females at the
intermediate dose early during treatment (days 6-9) may indicate
maternal toxicity. The differences in body-weight gain in animals at
the high dose were also considered to be toxicologically significant.
Dams at the two higher doses had a slight increase in the frequency of
post-implantation loss (7.4, 9, 4.8, and 10% for control to high dose,
respectively). There were slight increases in the incidence of minor
anomalies of the gall-bladder in three fetuses in three litters and
delays in ossification of all limb epiphyseal sites in four fetuses in
three litters (with none in controls) at 50 mg/kg bw per day. The
NOAEL for maternal toxicity was 8 mg/kg bw per day on the basis of
lower body-weight gain early in the treatment period at 20 and 50
mg/kg bw per day. The NOAEL for developmental toxicity was 20 mg/kg bw
per day (Barton & Wilcox, 1996).
(f) Special studies
(i) Cataractogenicity
Dogs have been shown to develop lesions of the cornea and lens
after prolonged oral administration of dicloran. It has been suggested
that a photochemical product reaction is responsible for the lesion as
it occurs only when dogs are exposed to sunlight. Groups of four to
eight beagle dogs and six Hormel-Hanford miniature swine were fed
dicloran (purity, 95.8%) at concentrations of 0, 0.75, 6, 24, 48, or
192 mg/kg bw per day for periods varying from 50 to 306 days. Corneal
opacity appeared in dogs within 53-104 days after administration of 24
or 48 mg/kg bw per day, when they were exposed to sunlight. Dogs that
were not exposed to sunlight and eyes that had been sutured closed did
not develop lesions. Dogs given 192 mg/kg bw per day refused to eat
after 38 days and were given dicloran by capsule. All of these animals
died 49-53 days after the study began; no eye lesions were detected.
In several dogs with eye damage that were maintained for four months
to one year after dicloran administration had ceased, the pathological
changes seen in the cornea and lens were not reversed. Dicloran did
not appear to affect miniature swine at any concentration, and no
histopathological changes were seen in the eyes. A dose-related
increase in the presence of Heinz bodies was seen in the blood of both
dogs and swine, with at least one dog affected per dose and pigs
affected at doses of 48 mg/kg bw per day or more. No methaemoglobin
formation was found in dogs or pigs. Administration of dicloran as a
dust (0.1 mg) or a 5% solution directly into the eyes of dogs for
three months had no effect on corneal opacity or irritation of the
conjunctivae (Curtis et al., 1968; Bernstein et al, 1970; Earl et al.,
1971).
Ocular toxicity was not observed in rats or rhesus monkeys
(Serrone et al., 1967), and administration of 0.143 mg/kg bw per day
for three months to 20 human volunteers produced no ocular symptoms
(Strough, 1962).
The 1977 Joint Meeting (Annex 1, reference 28) reviewed
additional data requested by the 1974 JMPR and concluded that a
species difference in the kinetics of dicloran may partly explain the
photosensitive ocular toxicity observed in dogs,but not in other
mammals. 14C-Dicloran was administered orally at 100 mg/kg bw per day
for five days to four beagle dogs, four pigs (breed unspecified), and
eight Wistar rats. The concentration of radiolabelled residues was
highest in the dogs and slightly lower in pig tissues, but the tissue
residues in both species were 2.4-11-fold higher than those in liver
or plasma residues of rats. All three species had a high concentration
of residue in the liver, but in dogs and pigs the highest
concentrations were found in the pigmented tissues of the eye, the dog
having two to three times more residue in the iris and pigmented
retina than the pig. Nonpigmented eye tissues (cornea and lens) had
low concentrations. There was no correlation between ocular toxicity
and tissue residues of dicloran and its metabolites. The plasma
concentrations of radiolabel increased more rapidly in the dogs, but
24 h after three days of dosing the plateau plasma concentrations in
the pigs and dogs were similar (10-15 mg/kg bw). High concentrations
of radiolabel were excreted in the bile of dogs and pigs (Hamilton,
1977).
(ii) Haematological effects
Groups of 10 or 15 rats (strain unspecified) of each sex were
treated with dicloran at concentrations of 0, 5, 20, or 100 mg/kg bw
per day by gavage for four months or in the diet at a concentration of
0 or 20 ppm, equivalent to 1 mg/kg bw per day for four months.
Measurements of red blood cells, total and differential leukocyte
counts, platelet count, haematocrit, haemoglobin concentration,
glucose, growth, and food consumption indicated no significant effect
attributable to dicloran at any dose by either route (Evans et al.,
1963).
Because of the known ability of 4-nitroaniline to induce specific
blood dyscrasias, short-term studies were performed to compare
dicloran and 4-nitroaniline. Groups of five weanling rats (strain
unspecified) of each sex were given dicloran by gavage at 0 or 400
mg/kg bw per day, five days/week, for four weeks, and 4-nitroaniline
was administered at 200 or 400 mg/kg bw per day to two comparable
groups over the same interval. In a second experiment, groups of six
male weanling rats were given dicloran by gavage at 0 or 800 mg/kg bw
per day or 4-nitroaniline at 400 or 800 mg/kg bw per day, five days
per week, for two weeks. Haematological parameters were normal in rats
treated at 400 mg/kg bw per day -- no Heinz bodies were detected and
the reticulocyte counts were normal -- although their body-weight gain
was 50-60% of that of controls. At 800 mg/kg bw per day, there was
slight weight loss. Red blood cell count, packed cell volume, and
haemoglobin measurements were normal, while the lymphocyte counts were
reduced to 65% of that of controls. At the end of the study, no
effects were seen on the spleen. In contrast, 4-nitroaniline had
definitive effects on growth at 200 mg/kg bw per day (70% of control),
Heinz bodies were identified in the blood, and the reticulocyte count
was markedly elevated to two to four times the control value. The bone
marrow was not affected. At 400 mg/kg bw per day, growth was reduced
to 60% of the control value, and a reduced red blood cell count (65%
of control with polychromasia and nucleation) was seen, accompanied by
increased spleen weight (200% of control). These effects were more
pronounced at 800 mg/kg bw per day where, in addition, the haemoglobin
concentration was reduced and the lymphocyte count greatly increased.
Although the high doses of dicloran caused lymphopenia, the other
haemotoxic effects seen with 4-nitro-aniline were not observed with
dicloran (Boots Pure Drug Co., 1962).
In studies to substantiate the difference between the effects of
4-nitroaniline and dicloran, a single oral dose of 500 mg/kg bw
dicloran was given to cats. No methaemoglobin was noted at any time
between 1 and 48 h after dosing; however, administration of
4-nitroaniline as a single dose of 100 mg/kg bw resulted in
methaemoglobin over the same time. In addition, the cats given
4-nitroaniline were cyanotic and showed extensive muscle weakness
(Gurd, 1974). Although methaemoglobin was not assessed in all studies,
it is noteworthy that the 60-day study in mice was the only one that
showed an association between dicloran and significantly elevated
methaemoglobin concentrations in the blood at doses of 100 and
200 mg/kg bw per day (Mallyon et al., 1986). No effect on
methaemoglobin was seen in the two-year study in dogs (Woodard et al.,
1964).
3. Observations in humans
In a double-blind clinical study, 20 male volunteers were given
10 mg of dicloran (purity unspecified), equivalent to 0.14 mg/kg bw
per day, and 10 received a placebo, once daily, for 90 days. The dose
and duration were based on the estimated maximum residues obtained
from consumption of fruits and vegetables over one year.
Haematological examinations and tests for liver and kidney function
were performed at various intervals over the course of the study; the
results were found to be normal (Strough, 1962).
Extensive examinations were made on an industrial worker who had
been occupationally exposed to dicloran for three years, with
considerable exposure by inhalation and dermal contact for about 60
days per year. No adverse effects were observed (Brooks & Boyack,
1963).
Comments
Dicloran is rapidly absorbed, metabolized, and eliminated, mainly
in the urine, after oral administration to rats, goats, and humans; it
is also rapidly excreted by hens. Rats excreted most of the radiolabel
(90-96%) within 24 h, with > 70% in the urine and an additional
13-22% in the faeces, depending on the dose. Elimination was
essentially complete (91-97% of the administered dose) by 48 h, with
total tissue residues accounting for 0.2-0.3%. The highest tissue
concentrations were found in the liver (0.05-0.06 mg/kg) and kidneys
(0.02 mg/kg). Unchanged parent compound was detected in the faeces of
animals at the high dose only. The major urinary metabolites were the
sulfate and glucuronide conjugates of 2,6-dichloro-4-hydroxyaniline,
which accounted for 55-79% of the total administered radiolabel, and
the major faecal metabolites were derivatives of glutathione
conjugates. The parent compound and its glutathione conjugates were
the major residues in plants. None of the metabolites was of
toxicological concern. The rapid excretion of dicloran as a conjugate
indicates that it is readily metabolized in vivo.
The major metabolite in rat urine (2,6-dichloro-4-hydroxyaniline)
was not present in goat urine, which contained more polar conjugates
that could not be hydrolysed by glucuronidase or sulfatase. In goats,
dicloran is reduced to 4-amino-2,6-dichloro-aniline and acetylated to
4-amino-3,5-dichloroacetanilide (4-6% in urine), which is rapidly
metabolized and excreted in the urine and faeces, although a reactive
intermediate is formed in goat liver and bound covalently to
macromolecules. Species differences in dicloran metabolism were also
apparent in dogs and mice, which partly explain the photosensitive
oculotoxic effects observed in dogs and the methaemo-globinaemia noted
in mice, which are not seen in other mammals. Preliminary studies
suggested that the metabolites of dicloran in humans are similar to
those in rats. Although recovery was considered complete, excretion
was somewhat slower in humans than in rats, most of the material being
excreted within 1.5 days.
Dicloran has low or slight toxicity when administered by the oral
route, depending on species. It has low dermal toxicity, is not
irritating to the skin, is a mild eye irritant, and is not a skin
sensitizer.
WHO has classified dicloran as unlikely to present an acute
hazard in normal use (WHO, 1996).
The results of short-term studies of toxicity in rabbits treated
dermally and in mice and rats treated in the diet or by gavage and of
long-term studies of toxicity in mice, rats, and dogs treated in the
diet indicate that the liver is the primary target organ. Increased
liver weights and centrilobular hepatic hypertrophy were observed
consistently in all species treated orally, and increased splenic
activity and splenic extramedullary haematopoiesis were noted in
short- and long-term studies in mice.
When dicloran was fed to mice at 0, 300, 600, or 1200 ppm for 60
days, the NOAEL was 300 ppm (equal to 49 mg/kg per day), on the basis
of hepatic lesions, splenic extramedullary haematopoiesis, and
increased methaemoglobin at doses of 600 ppm and above.
In a 90-day study in mice treated by gavage with 0, 15, 45, 135,
400, or 600 mg/kg bw per day, the NOAEL was 15 mg/kg bw per day on the
basis of significant polycythaemia in males and hypercholesterolaemia
and a significant increase in the incidence of splenic extramedullary
haematopoiesis in females at 45 mg/kg bw per day and above.
A NOAEL was not identified in a 90-day study in rats fed diets
containing 0, 1000, 3000, or 5000 ppm dicloran because of
significantly reduced body-weight gain in conjunction with effects on
the liver and thyroid in all treated groups. In an eight-week study in
which rats were given diets containing dicloran at doses of 0, 500, or
750 ppm, the NOAEL was 500 ppm (equal to 44 mg/kg bw per day) on the
basis of reduced body-weight gain, decreased food consumption, and
increased liver weights with associated hepatic histopathological
manifestations at 750 ppm.
In a study in which dicloran was fed to rats for six months at 0,
30, 300, or 3000 ppm, the NOAEL was 300 ppm (equal to 22 mg/kg bw per
day) on the basis of reduced body weights and increased liver and
spleen weights at 3000 ppm.
In a four-week study in rats given a dose of 0, 35, 140, or 350
mg/kg bw per day by gavage, the NOAEL was 35 mg/kg bw per day on the
basis of growth depression and hepatic hypertrophy and vacuolation at
140 mg/kg bw per day.
In an 18-month study of carcinogenicity in mice at dietary
concentrations of 0, 50, 175, or 600 ppm, the NOAEL was 175 ppm (equal
to 25 mg/kg bw per day) on the basis of increased liver weights,
centrilobular hepatocyte enlargement, centrilobular haemosiderosis,
focal and single-cell liver necrosis, and vacuolation of centrilobular
hepatocytes.There was no evidence of carcinogenicity in mice.
Two studies were conducted in rats to assess toxicity over a
two-year period of exposure. Rats were fed diets containing dicloran
at concentrations of 0, 20, 100, or 3000 ppm or 0 or 1000 ppm. The
overall NOAEL was 1000 ppm (equal to 59 mg/kg bw per day), on the
basis of changes in body-weight, food consumption, and haematological
parameters, spleen pigmentation, increased liver weights,
centrilobular hepatocyte enlargement, and thyroid hypertrophy at 3000
ppm. There was no evidence of carcinogenicity in these studies, but
they were considered inadequate for complete evaluation of the
carcinogenetic potential of dicloran.
Dogs fed dicloran at dietary concentrations of 0, 20, 100, or
3000 ppm for two years had treatment-related changes in haematological
and clinical chemical parameters and significant increases in liver,
spleen, and kidney weights, accompanied by histological changes at
3000 ppm that included irregular hepatic-cell size, moderate
hepatic-cell hypertrophy, and increased pigmentation of the liver and
spleen. The NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day.
Dicloran was not mutagenic in most assays, although occasional
positive responses were seen in more recent tests for reverse mutation
and in a test for mitotic recombination. The results of an assay for
sex-linked recessive mutation in Drosophila were equivocal. The
Meeting concluded that dicloran is unlikely to be genotoxic.
Studies of reproductive and developmental toxicity indicated that
dicloran is not a reproductive toxicant and is not teratogenic in rats
or rabbits. Dicloran was embryotoxic at maternally toxic doses in
rabbits, but an NOAEL for maternal or developmental toxicity was not
identified in rats.
In a two-generation study of reproductive toxicity in rats (one
litter per generation) at dietary concentrations of 0, 50, 250, or
1250 ppm, the NOAEL for systemic toxicity was 250 ppm (equal to 21
mg/kg bw per day) on the basis of reduced body-weight gains and
increased liver weights at 1250 ppm. The NOAEL for reproductive and
developmental toxicity was 250 ppm (equal to 21 mg/kg bw per day) on
the basis of reduced weights of F1 and F2 pups at 1250 ppm.
In a study of developmental toxicity, dicloran was administered
by gavage to rats on days 6-15 of gestation at doses of 0, 100, 200,
or 400 mg/kg bw per day. Because of significantly reduced mean
maternal body-weight gains and higher incidences of totally resorbed
litters in all treated groups, NOAEL values for maternal or
developmental toxicity were not identified. Dicloran was not
teratogenic in this study.
In a study of developmental toxicity in rabbits, dicloran was
administered by gavage at doses of 0, 8, 20, or 50 mg/kg bw per day on
days 6-18 of gestation. The NOAEL for maternal toxicity was 8 mg/kg bw
per day, on the basis of reduced maternal body-weight gains early
during treatment with 20 or 50 mg/kg bw per day. The NOAEL for
developmental toxicity was 20 mg/kg bw per day on the basis of a
slight increase in post-implantation losses, a slight increase in the
incidence of minor anomalies of the gall-bladder, and delays in
ossification of all limb epiphyseal sites in fetuses at 50 mg/kg bw
per day.
Dogs have been shown to develop lesions in the cornea and lens
after prolonged oral administration of dicloran. The 1977 Joint
Meeting reviewed additional data requested by the 1974 Meeting and
concluded that the photosensitive oculotoxic effects observed in dogs
but not in other mammals were due partly to a species difference in
the kinetics of dicloran. No oculotoxic effects were seen in any of
the more recent studies, although no further studies have been
conducted in dogs.
In a double-blind clinical study, 20 men were given 10 mg
dicloran (equivalent to 0.14 mg/kg bw per day) and 10 received a
placebo once daily for 90 days. The dose and duration were chosen on
the basis of the maximum residues estimated to be derived from
consumption of fruits and vegetables over one year. There was no
indication that administration of dicloran at this dosage had any
adverse effect.
An ADI of 0-0.01 mg/kg bw per day was established on the basis of
the NOAEL of 1.7 mg/kg bw per day for hepatic and haematological
effects in the two-year study in dogs and a 200-fold safety factor. A
larger than normal safety factor was used because of the inadequacy of
the long-term studies in rats for assessing the carcinogenic potential
of dicloran and because of the lack of a NOAEL for maternal and
developmental toxicity in rats.
An acute RfD was not allocated because dicloran has low or slight
toxicity when administered orally or dermally and because acute
effects occur only at very high doses, resulting in a 10 000-fold
difference between the ADI and the LOAEL for maternal and
developmental toxicity in rats. Therefore, the Meeting concluded that
the acute intake of residues is unlikely to present a risk to
consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 49 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
15 mg/kg bw per day (13-week study of toxicity)
175 ppm, equal to 24 mg/kg bw per day (18-month study
of carcinogenicity)
Rat: 35 mg/kg bw per day (lowest dose tested, four-week
study of toxicity)
500 ppm, equal to 44 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
300 ppm, equal to 22 mg/kg bw per day (six-month study
of toxicity)
1000 ppm, equal to 59 mg/kg bw per day (two two-year
studies of toxicity)
250 ppm, equal to 21 mg/kg bw per day (two-generation
study of reproductive toxicity)
Rabbit: 8 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (developmental toxicity)
Dog: 100 ppm, equal to 1.7 mg/kg bw per day (two-year study
of toxicity)
Human: 0.14 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of an acute reference dose
Not allocated (unnecessary).
Studies that would provide information useful for continued
evaluation of the compound
1. Combined study of toxicity and carcinogenicity in rats.
2. Study of developmental toxicity in rats at less than 100
mg/kg bw per day to establish clear NOAELs for maternal and
developmental toxicity.
3. Assays for genotoxicity in mammals in vivo, such as an
assay for micronucleus formation
4. Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete, > 70% urinary excretion within 24 h
Dermal absorption No data
Distribution Liver, kidney
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 90-96% in urine and faeces within 24 h
Metabolism in animals Metabolites differ in rats and goats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral > 4000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation No data
Skin irritation Not irritating
Eye irritation Minimally irritating
Skin sensitization Not sensitizing (Draize test)
Short term toxicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant oral NOAEL Rat: 44 mg/kg bw/per day (diet); 15 mg/kg bw per day
(gavage)
Lowest relevant dermal NOAEL Rabbit: 120 mg/kg bw per day
Lowest relevant inhalation NOAEL Poor study: data on actual intake, particle size not
provided
Genotoxicity Unlikely to be genotoxic; no study of genotoxicity in
mammals in vivo
Long term toxicity and carcinogenicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant NOAEL Dog: 1.7 mg/kg bw per day (2-year study)
Carcinogenicity No evidence of carcinogenicity in mice. The study in
rats was inadequate.
Reproductive toxicity
Reproduction target/critical effect Increased ovary/testis weights (no histological
findings)
Lowest relevant reproductive NOAEL Rat: 21 mg/kg bw per day (reduced body-weight gain,
increased liver weight)
Developmental target/critical effect Increased resorptions, delayed ossification, not
teratogenic
Lowest relevant developmental NOAEL 20 mg/kg bw per day in rabbits; no NOAEL in rats.
A 10 000-fold difference exists between the ADI and
the LOAEL in rats.
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies
Cataractogenicity Photosensitive oculotoxic effects observed in dogs are
not seen in other mammals
Medical data No indication that administration of dicloran at
10 mg/day to men for 90 days had any adverse effect.
Extensive examinations were made on one industrial
worker occupationally exposed to dicloran over three
years, with considerable inhalation and dermal
exposure for about 60 days/year. No adverse effects
were observed
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 2-year study, dogs 200
Acute reference dose Not allocated (unnecessary)
References
Allen, J.A., Brooker, P.C. & Gray, V.M. (1988) Technical dicloran:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. No. SMS 43/871138 from Huntingdon Research
Centre, United Kingdom. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Bachmann, E., Golberg, L. & Thibodeau, L. (1971) Aspects of the
determination of biphenyl hydroxylase activity in liver homogenate.
Exp. Mol. Pathol., 14, 303-326.
Barton, S.J. & Wilcox, S. (1996) Dicloran: Development toxicity in
rabbits. Unpublished report No. 11379 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Barton, S.J. & Wilcox, S. (1997) Dicloran: Two generation reproduction
study in rats. Unpublished report No. 14271 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Basting, L.A., Cooper, C., Witmer, C.M. & Gallo, M.A. (1983) Toxicity
and mutagenicity of 2,6-dichloro-4-nitroaniline in the Ames test.
Fed. Proc., 42, 623.
Basting, L.A., Witmer, C.M., Iba, M.M. & Gallo, M.A. (1984) The effect
of 2,6-dichloro-4-nitroaniline on hepatic microsomal systems.
Toxicologist, 4, 149.
Bernstein, H., Curtis, J.M., Earl, F.L. & Kuwabara, W. (1970)
Phototoxic corneal and lens opacities. Arch. Ophthalmol., 83,
336-348.
Boots Pure Drug Co. (1962) Dicloran: Experimental data on toxicity.
Unpublished report No. T-17 from Boots Pure Drug Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Boyack, G.A. & Brooks, B.W. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Unpublished report
No. T-21 from Upjohn Co., USA. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Cheng, T. (1996a) Nature of the residue of 14C-dicloran in lactating
goats. Unpublished report No. HWI6564-102 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996b) Metabolism of 14C-dicloran in rats. Unpublished
report No. HWI6564-108 from Hazleton Wisconsin, Inc., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996c) Nature of the residue of 14C-dicloran in laying
hens. Unpublished report No. HWI6564-100 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Creedy, C.L., Hemmings, P.A. & Needham, D. (1985) The effect of
dicloran on the hepatic mixed-function oxidase system of the male rat
following oral administration of 100 mg/kg body weight twice daily for
4 days. Unpublished FBC Ltd report No. METAB/85/24, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Curtis, J.M., Bernstein, H., Earl, F.L. & Smalley, H.E. (1968) Corneal
and lens opacities in dogs treated with 2,6-dichloro-4-nitroaniline.
Toxicol. Appl. Pharmacol., 12, 305.
Dawson, J. R. (1988) Metabolism and residues of dicloran in the laying
hen. Unpublished report No. ENVIR/88/26 from Schering Agrochemicals
Ltd, United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
Earl, F.L., Curtis, J.M., Bernstein, H.N. & Smalley, H.E. (1971)
Ocular effects in dogs and pigs treated with dichloran
(2,6-dichloro-4-nitroaniline). Food Cosmet. Toxicol., 9, 819-828.
Eberts, F.S. (1965) Fate of Botran (2,6,-dichloro-4-nitroaniline) in
rat and man. Unpublished report No. M9 from Upjohn Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Eberts, F.S., Meeks, R.C. & Vliek, R.W. (1963) Excretion of
Botran-14C (2,6,-dichloro-4-nitroaniline) by man. Unpublished Report
No. M3 from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Elliot, P.H., Smith, C., Crook, D., Gibson, W.A., Singh, H. &
Gopinath, C. (1988) Technical dicloran: Twenty-one day dermal toxicity
study in rabbits. Unpublished report No. SMS 60/871292 from Huntingdon
Research Centre Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Evans, J., Mengel, G. & Bostwick, L. (1963) Botran (U-2069) Effect of
oral administration. Final report four-months study. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Everest, R.P. & Tuplin, J.A. (1977) Dicloran, pure reference sample:
Mutagenicity testing in bacterial in vitro systems. Unpublished report
No. TX 77024 from Boots Pure Drug Co., United Kingdom. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Gaines, T.B. & Linder, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. Appl. Toxicol., 7, 299-308.
Gallo, M.A., Bachmann, E. & Golberg, L. (1976) Mitochondrial effects
of 2,6-dichloro-4-nitroaniline and its metabolites. Toxicol. Appl.
Pharmacol., 35, 51-61.
Garrett, N.E., Stack, H.F. & Waters, M.D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res., 168,
301-325.
Griffiths, A.F.J., Brockman, H.E., DeMartini, D.M. & de Serres, F.J.
(1986) The efficacy of Neurospora in detecting agents that cause
aneuploidy. Mutat. Res., 167, 35-45.
Gurd, P.R. (1974) Methemoglobin formation in the cat. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Hamilton, D.Y. (1977) Tissue distribution of [14C]-dichloran in rats,
dogs and pigs following repeated oral dosing at 100 mg/kg/day.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Hawkins, D.R., Kirkpatrick, D. & Shaw, D. (1988) The metabolism of
[14C]-dicloran in peaches: Analysis of samples. Unpublished report
No. HRC/SMS from Huntingdon Research Centre, Ltd United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. & Peters, J. (1967) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Natl Cancer Inst., 42, 1101-1114.
Jaglan, P.S. & Arnold, T.S. (1985a) Comparative metabolism of
[14C]-dichloran in rats and goats: Nature of liver residues.
Unpublished report No. 218-9760-85-002A from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jaglan, P.S. & Arnold, T.S. (1985b) Nature of muscle residue from the
treatment of a goat with [14C]-dichloran
(2,6-dichloro-4-nitroaniline). Unpublished report No. 218-9760-85-003
from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jaglan, P.S., Gosline, R.E. & Arnold, T.S. (1985a) Comparative
metabolism of [14C]-dichloran in rats and goats. Unpublished report
No. 218-9760-85-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Jaglan, P.S., Arnold, T.S., Gosline, R.E. & Subacz, C.J. (1985b)
Bioavailability of [14C]-dichloran (U-2069;
2,6-dichloro-4-nitroaniline) derived goat liver residues by the rats.
Unpublished report No. 218-9760-85-004 from Upjohn Co. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
James, P. & Brennan, C. (1991) Technical dichloran: Rabbit oral
developmental toxicity (teratogenicity) range finding study.
Unpublished report No. TOX/91/199-98 (T128) from Schering AG, Germany.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1978) Screening of pesticides for
mutagenicity in microbial systems. Taiwan Plant Protection Center
Research Report No. 12. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1980) Screening of pesticides for
mutagenicity in microbial systems II: with mammalian microsomal
activation. Natl Sci. Council Monthly, 8, 551-559.
Johnston, R.L. & Ceru, J.G. (1961) Acute oral LD50 -- rat and acute
intraperitoneal LD50 -- mouse using DCNA with 0.09% p-nitroaniline.
Unpublished report No. T-16 from Upjohn Co., USA. Submitted to WHO by
Gowan Company, Yuma, Arizona, USA.
Johnston, R.L. & Schwikert, R.S. (1963) Botran: Skin sensitization in
guinea pigs. Unpublished report No. 5567-64-RLJ-196B from Upjohn Co.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jones, E. & Fenner, L.A. (1987) Technical dicloran: Ames bacterial
mutagenicity test. Unpublished report No. TOX/87/199-85 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Kakuk, T. (1986) 90-Day oral toxicity study in B6C3F1 mice with DCNA.
Unpublished report No. 7263/86/003 from Upjohn Co., USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Kakuk, T.J., Weddon, T.W. & Thomas, R.W. (1979) Reevaluation of
potential hepatic effects of Botran in beagle dogs: Supplemental
report. Unpublished report No. 001-9610-79-005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Kappas, A. (1978) On the mechanisms of induced somatic recombination
by certain fungicides in Aspergillus nidulans. Mutat. Res., 51,
189-197.
Lessel, B. (1974) Two-year feeding trial in rats on dicloran.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Lobdell, B. & Johnson, C. (1965) U-2069: Effect on reproduction
capacity through three generations in the rat. Unpublished report from
Woodard Research Corporation. Cited from the 1974 JMPR Monograph
(Annex 1, reference 22).
Mallyon, B.A. & Markham, L.P. (1989) Technical dicloran: Oncogenicity
study in the mouse. Unpublished report No. TOX/86006 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Mallyon, B.A. & Rawcliffe, L.M. (1985) Technical dicloran:
Palatability in the diet to the mouse. Unpublished report No.
TOX/85/199-74 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Mallyon, B.A., Rawliffe, L.M. & Major, I.R. (1986) Technical dicloran:
60-day dietary study in the mouse. Unpublished report No.
TOX/86/199-75 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Marks, T.A., Poppe, S.M., Moerdyk, D.L., Stuckhardt, J.L., Morris,
D.F. & Greenberg, J.H. (1982) U-2069: A segment II teratology study in
the rat. Unpublished report No. 9610/82/7263/005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Mason, J.M., Valencia, R. & Zimmering, S. (1992) Chemical mutagenesis
testing in Drosophila: VIII: Reexamination of equivocal results.
Environ. Mol. Mutag., 19, 227-234.
Maté, C., Ryan, A.J. & Wright, S.E. (1967) Metabolism of some 4-
nitroaniline derivatives in the rat. Food Cosmet. Toxicol., 5,
657-663.
McBride, D. & McGregor, D.B. (1987) Technical dicloran: Assessment of
unscheduled DNA synthesis using rat hepatocyte cultures. Unpublished
report No. 4414 from Inveresk Research International, Musselborgh,
United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Moustacchi, E., Carere, A., Morpurgo, G. Ramel, C. & Würgler, F.E.
(1986) Assays for genetic changes in fungi. In: Montesano, R.,
Bartsch, H., Vainio, H., Wilbourn, J. & Yamasaki, H., eds,
Long-term and Short-term Assays for Carcinogens: A Critical
Appraisal (IARC Scientific Publications No. 83), Lyon, International
Agency for Research on Cancer, pp. 203-249.
Myers-Basting, L.A. (1986) Mutagenicity and characterization of the in
vitro metabolism of 2,6-dichloro-4-nitroaniline. Unpublished report
No. (T101) from Rutgers University, New Jersey, USA. Submitted to WHO
by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I.R. (1991a) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 5 mg/kg body-weight. Unpublished report No.
TOX/90/199-95 (M48) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I. R. (1991b) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 500 mg/kg body-weight. Unpublished report No.
TOX/90/199-96 (M49) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997a) Metabolic fate and distribution of 14C-dicloran in
potatoes. Unpublished report No. 1947 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997b) Metabolic fate and distribution of 14C-dicloran in
lettuce. Unpublished report No. 1949 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Peters, D.H., Stuart, V., Smith, H.L., Crook, D., Gibson, W.A.,
Majeed, S.K. & Gopinath, C. (1991) Technical dicloran: Rat 13-week
dietary toxicity study. Unpublished report No. TOX 89324 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980a) Primary eye irritation evaluation
in New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-001 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980b) Primary dermal irritation study in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T. J. & Wood, D. R. (1980c) Acute dermal toxicity screen in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-003 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Seaman, W.J., Weddon, T.E. & Kakuk, T.J. (1980) Three-week inhalation
study in rats, rabbits and dogs with Botran. Unpublished report No.
218-9610-80-004 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Serrone, D.M., Pakdaman, P., Stein, A.A. & Coulston, F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
rhesus monkeys. Toxicol. Appl. Pharmacol., 10, 404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Smith, S (1989) Metabolism of [14C]-dicloran in peaches under field
and glasshouse conditions. Unpublished report No. ENVIR/89/12 from
Schering Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Strough, A.R. (1962) Botran clinical study. Unpublished report from
Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
US Environmental Protection Agency (1974) Unpublished data from EPA
laboratories. Cited from the 1974 JMPR Monograph, (Annex 1, reference
22).
Waters, M.D., Sandhu, S.S., Simmon, V.F, Mortelmans, K.E., Mitchell,
A.D., Jorgenson, T.A., Jones, D.C.L., Valencia, R. & Garrett, N. E.
(1982) Study of pesticide genotoxicity. Basic Life Sci., 21,
275-326.
Waterson, L.A., Gibson, W.A., Morrow, J. & Gopinath, C. (1992)
Technical dicloran rat 8-week repeat dose study. Unpublished report
No. 91/199-97 from Huntingdon Research Centre, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wazeter, F.X. (1966) Somers test in the albino rabbit. Unpublished
report No. 100-037 from International Research and Development Corp.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wilcox, S., & Barton, S.J. (1996) Dicloran: Range-finding study for a
two generation reproduction study in rats. Unpublished report No.
14271 from Inveresk Research International, United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Woodard, M. W., Cockrell, K.O. & Woodard, G. (1964) Safety evaluation
by oral administration to rats and dogs for 104 weeks. Unpublished
report No. U2069 from Woodard Research Corporation, USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila: V: Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V: results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
 DCNA, 2.6-dichloro-4-nitroaniline; HCNA,
2-hydroxy-4-nitro-6-chloroaniline; DCHA,
2,6-dichloro-4-hydroxyaniline; DCAP, 4-amino-2,6-dichlorophenol;
DCNAP; 3,5-dichloro-4-hydroxyacetanilide; DCDP,
2,6-diclorophenylenediamine; DCAA, 4-amino-3,5-dichloroacetanilide;
DCNP, 2,6-dicholoro-4-nitrophenol; DCP, 2,6-dichlorophenol; DCA,
2,6 dichloroaniline; A1, A2, A3, glutathione conjugate deriviatives.
R, G, H, P, S correspond to metabolites in rats, goats, hens, plants,
and soil or sediment, respectively. Major metabolites are represented
by letters in upper-case, minor metabolites are represented by
lower-case letters.
potential to form reactive intermediates after ingestion by rats
(Jaglan & Arnold, 1985a; Jaglan et al., 1985a,b).
(c) Effects on enzymes and other biochemical parameters
Oral administration of dicloran at a dose of 400 or 1000 mg/kg bw
per day to rats for three months resulted in increased hepatic
demethylase and desulfurase activity, and liver mitochondrial oxygen
consumption was increased (Serrone et al., 1967). Dicloran at oral
doses of > 10 mg/kg bw stimulated rat liver mixed-function
oxidases, and a dose of 500 mg/kg bw decreased mitochondrial oxidation
of succinate without concomitant uncoupling of oxidative
phosphorylation. Oxidative phosphorylation was not affected by single
doses at any concentration. Daily administration of 1000 mg/kg bw to
rats induced uncoupling of oxidative phosphorylation after four days
(Bachmann et al., 1971). These liver enzymes were not stimulated in
rhesus monkeys treated with 160 mg/kg bw per day dicloran for three
months (Serrone et al., 1967), and no effect was seen on brain or
liver mitochondrial function in mice (Bachmann et al., 1971).
The 2,6-dichloro-4-hydroxyaniline metabolite was as active as
2,4-dinitrophenol in vitro in uncoupling oxidative phosphorylation,
whereas dicloran was only one-tenth as effective.
2,6-Dichlorophenylenediamine had no effect on mitochondrial
respiration or oxidative phosphorylation in vitro (Bachmann et al.,
1971).
Dicloran and 2,6-dichloro-4-hydroxyaniline at 10-5 mol/L
inhibited electron transport and uncoupled oxidative phosphorylation
in vitro, whereas 2,6-dichlorophenylenediamine at 10-4 mol/L
induced only slight uncoupling. These inhibitory effects in vitro
cannot be considered serious adverse effects since they were not
confirmed by similar observations in vivo. Mitochondria isolated
from rats treated with dicloran or its metabolites were functionally
normal (Gallo et al., 1976).
In a preliminary study, oral doses of 1000 mg/kg bw dicloran per
day for four days to female Sprague-Dawley rats did not significantly
induce cytochrome P450 activity (Basting et al., 1984). After oral
administration of 100 mg/kg bw twice a day for four days to male
Sprague-Dawley rats, however, significant increases were found in the
activities of cytochrome P450 (55% increase), cytochromeb5 (24%
increase), benzphetamine demethylase, and ethoxycoumarin deethylase
(51-68% increases) in comparison with controls. The pattern of enzyme
induction was reported to indicate that dicloran significantly induces
phenobarbital-type enzymes in rat microsomes (Creedy et al., 1985).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of dicloran are
presented in Table 1. Technical-grade dicloran was of low or slight
acute toxicity by all routes of administration in all species
examined. Oral administration resulted in nasal haemorrhage,
paralysis, depression, and excessive yellow urine and faeces.
Intraperitoneal administration resulted in sleep, depression, and
excessive yellow urine and faeces. The effects of subcutaneous
injection were confined to local damage in which the test substance
became enclosed in a fibrous sac, sometimes followed by abscess
formation and eventual ulceration. Dermal administration resulted in
no systemic clinical effects.
Technical-grade dicloran (purity, 96%) was not irritating to the
intact or abraded skin of male or female New Zealand white rabbits at
a concentration of 500 mg per site (Raczniak & Wood, 1980b) or after
repeated daily applications of 10 mg dry material or 0.1 ml of 10%
aqueous suspension to the abraded or unabraded skin of albino rabbits
over five days (Boots Pure Drug Co., 1962).
Technical-grade dicloran (purity, 96%) caused a minimal
conjunctival response in the eys of New Zealand white rabbits,
consisting of redness and chemosis 24 and 48 h after treatment.
Corneal opacity or injury to superficial layers of the corneal or
conjunctival epithelium was not observed (Raczniak & Wood, 1980c).
Four successive daily applications of 2-3 mg dicloran to the
conjunctival sac of four rabbits resulted in a slight inflammatory
reaction of the cornea, which was of short duration (Boots Pure Drug
Co., 1962).
Dicloran was inactive in two tests for skin sensitization in
guinea-pigs (Boots Pure Drug Co., 1962; Johnston & Schwikert, 1963).
(b) Short-term studies of toxicity
Mice
Groups of Charles River COBS mice were fed diets containing 0,
1250, 2500, 5000, or 10 000 ppm technical-grade dicloran for two
weeks, equivalent to 0, 190, 380, 750, and 1500 mg/kg bw per day. At
10 000 ppm, 20% of animals of each sex died; the only clinical sign
observed was hunched posture. Food consumption and body weight were
significantly reduced. Female mice fed 5000 or 10 000 ppm had
significantly increased absolute liver weights, and animals of each
sex fed doses of 2500 ppm or more had an increased liver:body-weight
ratio. Food consumption was slightly reduced in animals of each sex at
2500 and 5000 ppm, and a slight reduc-tion in food consumption was
noted at 1250 ppm only in females (Mallyon & Rawcliffe, 1985).
Table 1. Acute toxicity of dicloran
Species Strain Sex Route LD50 Reference
(mg/kg bw)
Rat Sherman SPF M/F Oral > 4000 Gaines & Linder (1986)
Rat NR NR Oral 4040 Boots Pure Drug Co. (1962)
Rat NR NR Oral ~ 8000 Serrone et al. (1967)
Rat NR NR Oral > 10 000 Johnston & Ceru (1961)
Mouse NR NR Oral 1500-2500 Boots Pure Drug Co. (1962)
Guinea-pig NR NR Oral 1400 Boots Pure Drug Co. (1962)
Rabbit New Zealand white M/F Dermal (abraded) > 2000 Raczniak & Wood (1980a)
Mouse NR NR Intraperitoneal 5500 Johnston & Ceru (1961)
Mouse NR NR Intraperitoneal 2500 Boots Pure Drug Co. (1962)
Rat NR NR Intraperitoneal 1500 Boots Pure Drug Co. (1962)
Rat NR NR Subcutaneous > 5000 Boots Pure Drug Co. (1962)
Mouse NR NR Subcutaneous > 6000 Boots Pure Drug Co. (1962)
SPF, specific pathogen-free; M/F, male and female; NR, not reported
Groups of 10 male and 10 female CD-1 (ICR) mice received dicloran
(purity, 95.8%) in the diet at 0, 300, 600, or 1200 ppm, equal to 0,
49, 100, and 200 mg/kg bw per day in males and 0, 53, 100, and 220
mg/kg bw per day in females, for 60 days. There were no
treatment-related effects on survival, clinical signs, body weight,
food or water consumption, or food conversion. A significant increase
in methaemoglobin concentrations in the blood of both males and
females was noted at 600 and 1200 ppm (males: 1, 1.3, 1.4, and 1.6%;
females: 0.6, 0.7, 0.9, and 1.2%, control to high dose, respectively).
Cholesterol and bilirubin concentrations were significantly increased
in high-dose females (cholesterol: 2.4 ± 0.58, 2.2 ± 0.50, 2.5 ± 0.2,
3.0 ± 0.65 mmol/L; bilirubin: 4.3 ± 0.6, 5.4 ± 1.4, 5.5 ± 2.4, 5.8 ±
1.7 mol/L, control to high dose, respectively). These changes were
dose-related but were not observed in males, which, unlike females,
had specific liver lesions. Minimal to slight centrilobular
hepatocytic enlargement (2-5/10 versus 0/10 controls) and fatty liver
degeneration (2-4/10 versus 0/10 controls) with an increased severity
of haemosiderosis in the spleen was noted in males at 600 and 1200
ppm, and a slight increase in the degree of haematopoietic activity
was noted in the spleen of males and females combined at the
intermediate and high doses. The NOAEL was 300 ppm, equal to 49 mg/kg
bw per day in males (Mallyon et al., 1986).
Dicloran (purity, 97%) was administered to groups of 15 B6C3HF1
mice of each sex (five of each sex per dose for interim sacrifice) by
gavage at doses of 0, 15, 45, 135, 400, or 600 mg/kg bw per day for 90
days, with an interim sacrifice at 49 days. There were no
treatment-related effects on survival or body weight; data on food
consumption were not provided. Significant polycythaemia was seen in
males, and hypercholesterolaemia at 1.2-2.1 times the control level
and a significant increase in the incidence of splenic extramedullary
haematopoiesis were seen in females at > 45 mg/kg bw per day.
Dose-related increases in cholesterol concentrations (1.3-2.3 times
the control concentration) were also noted in males, which were
statistically significant at > 135 mg/kg bw per day. A deep-yellow
colouration of the urine was noted in all treated groups and was
attributed to the yellow colour of the test material. In the absence
of any associated lesion in the group at the lowest dose, this effect
was considered not to be adverse. Other effects at higher doses
included kidney nephrosis in males at 400 and 600 mg/kg bw per day (5,
4, 6, 5, 15, 14/15, control to high dose, respectively) and females
(3, 5, 2, 4, 8, 12/15), with kidney tubular regeneration in males (0,
0, 3, 1, 8, 11/15), increased liver weights in animals of each sex at
> 135 mg/kg bw per day (7-64% increase), hepatocyte hypertrophy in
males (0, 0, 0, 4, 15, 15/15) and females (0, 0, 0, 0, 5, 12/15) at
> 400 mg/kg bw per day, liver necrosis in males (0, 0, 0, 0, 3,
3/15) and females (0, 0, 0, 0, 1, 1/15) at 400 and 600 mg/kg bw per
day, a significantly higher incidence of splenic extramedullary
haematopoiesis in males at > 135 mg/kg bw per day (0, 1, 2, 13, 15,
15/15) and in females at > 45 mg/kg bw per day (2, 5, 9, 10, 14,
15/15), increased spleen weight at the two higher doses (11-29%
increase), and epithelial hyperplasia of the urinary bladder in males
(0, 1, 2, 10, 12, 15/15) and females (0, 0, 0, 1, 7, 13/15) at doses
> 135 mg/kg bw per day. The NOAEL was 15 mg/kg bw per day (Kakuk,
1986).
Rats
Dicloran (unspecified purity) was administered by gavage to rats
(strain unspecified) at 400 or 1000 mg/kg bw per day for three months.
Deaths occurred among rats at the high dose. Significantly enlarged
livers and unspecified renal changes were observed by light and
electron microscopy, with increases in liver mitochondrial enzyme
activity and mitochondrial oxygen use (Serrone et al., 1967).
Groups of five newly weaned rats (strain unspecified) of each sex
were given dicloran (purity unspecified) by gavage at doses of 0, 140,
or 350 mg/kg bw per day, six days/week for four weeks. The growth of
males at 350 mg/kg bw per day was reduced to 60% of the control value.
At both doses, increased liver weight (120-155% of control) with
hepatic vacuolation were noted. There was no apparent effect on blood
parameters or on the kidneys at the end of the study (Boots Pure Drug
Co., 1962).
Groups of 10 rats (strain unspecified) were given dicloran
(purity unspecified) by gavage at doses of 35, 140, or 350 mg/kg bw
per day, six days/week, for four weeks. Groups of 20 controls of each
sex were available. Growth depression was observed in animals of each
sex at 350 mg/kg bw per day and in males at 140 mg/kg bw per day.
Increased liver weights and microscopic findings were also seen at
these doses. The liver lesions included a dose-related increase in
hepatocytic hypertrophy with increased vacuolization, especially in
the outer lobes. The liver had returned to normal size in half of the
animals which were maintained for two weeks after the end of the
treatment, except for males at the highest dose. The histopathological
changes in the liver caused by repeated short-term dosing was
apparently reversible within two weeks of the end of treatment. The
NOAEL was 35 mg/kg bw per day on the basis of effects on body weight
and liver at 140 mg/kg bw per day (Boots Pure Drug Co., 1962).
Technical-grade dicloran was fed to rats (strain unspecified) for
six months at doses of 0 (25 rats), 30 (15 rats), 300 (15 rats), or
3000 (10 rats) ppm, equal to 0, 2.2, 22, and 230 mg/kg bw per day in
males and 0, 2.5, 25, and 270 mg/kg bw per day in females. Another
10 animals of each sex per group received recrystallized dicloran at
3000 ppm, equivalent to 230 mg/kg bw per day for males and 260 mg/kg
bw per day for females. At 3000 ppm, growth of both males and females
was impaired (70-83% of control), whereas males fed purified material
had only a slight reduction in growth (89% of control). Significantly
increased liver weights (120-144% of control) were noted in both
groups at the high dose, with a significant increase in spleen weight
(129% of control) in females. Haematological parameters were not
affected at 12 weeks or at termination, and no microscopic lesions
were noted. The NOAEL was 300 ppm, equal to 22 mg/kg bw per day, on
the basis of changes in body weight and increased liver and spleen
weights at 3000 ppm (Boots Pure Drug Co., 1962).
Dicloran (purity, 97.4%) was administered to groups of 10 male
and 10 female Sprague-Dawley rats in the diet at doses of 0, 1000,
3000, or 5000 ppm, equal to 0, 75, 230, and 370 mg/kg bw per day in
males and 0, 80, 230, and 420 mg/kg bw per day in females, for 90
days. There were no treatment-related effects on survival or
ophthalmological parameters. Significantly lower body-weight gain was
seen in all treated groups (17-32% less than controls for males and
18-22% less for females). Although palatability may have accounted for
the lower body-weight gains during the first week of the study, food
consumption was comparable in all groups at week 2; however, it was
consistently lower than control values throughout the remainder of the
study (males, 10-14% less than controls; females, 12-21%; weeks 2-13).
Many of the haematological and clinical chemical findings were
considered to be associated with reductions in food consumption. The
treatment-related hepatic effects included increased cholesterol
concentrations, which were dose-related and statistically significant
in males at all doses (1.3-1.5 times the control concentration) and in
females at the intermediate and high doses (1.4-2.0 times control),
minimal to moderate centrilobular hypertrophy in males in all treated
groups (0, 3, 9, 9/10) and in females at the two higher doses (0, 0,
9, 9/10), and significant increases in liver weight in males and
females at the two higher doses (29-66%). Minimal thyroid
follicular-cell hypertrophy was noted in males in all treated groups
(0, 5, 6, 6/10) and in females at the intermediate and high doses (0,
0, 4, 7/10). The dose of 1000 ppm, equal to a daily intake of 75 and
80 mg/kg bw per day for males and females, respectively, was
considered to be at the upper limit of a suitable high-level dose for
a long-term study of toxicity and carcinogenicity in rats. An NOAEL
was not identified because significantly lower body-weight gains were
seen at all doses (Peters et al., 1990).
A second short-term study was conducted to investigate the
effects of dicloran at doses lower than those administered in the
90-day study. Dicloran (purity, 96.4%) was given in the diet to groups
of 10 Sprague-Dawley rats of each sex at doses of 0, 500, or 750 ppm,
equal to 0, 44, and 71 mg/kg bw per day for males, and 0, 48, and 71
mg/kg bw per day for females, for eight weeks. There were no
treatment-related effects on survival. The overall body-weight gain of
females at 750 ppm was significantly less than that of controls (12%
less overall), the greatest difference occurring during the final two
weeks of treatment (41-58% less than control). This decrease
correlated with a slight decrease in food consumption in this group
during treatment, which was 10-11% less than that of controls during
the last two weeks of treatment. Increased liver weight relative to
body weight (13-21%) in animals of each sex at 750 ppm was associated
in 10/10 males with minimal centrilobular hepatocyte enlargement. The
NOAEL was 500 ppm, equal to 44 mg/kg bw per day, on the basis of lower
body-weight gain and decreased food consumption in females at 750 ppm
and increased liver weights in males and females, with associated
centrilobular hepatocyte enlargement in males at 750 ppm; however, a
full histological examination was not carried out (only of the liver
and thyroid), and although alterations were found in the livers of
males at the high dose, the livers of males at the low dose were not
examined histologically (Waterson et al., 1992).
Rabbits
Technical-grade dicloran (purity, 96.2%) was moistened with
distilled water and applied to the intact skin of groups of five male
and female New Zealand white rabbits under an occluded patch for 6 h
per day for 21 consecutive days at doses of 12, 120, or 1200 mg/kg bw
per day. Animals at the two higher doses showed yellow staining of
untreated fur and extremities from day 4 onwards, but there were no
adverse clinical findings. One male and one female rabbit at 12 mg/kg
bw per day had slight, transient erythema lasting for two to four
days, and most rabbits at 120 and 1200 mg/kg bw per day had slight
erythema beginning in the second week of the study, which tended to
persist until study termination. The reaction did not progress beyond
slight erythema, and there did not appear to be a dose-related
difference above 120 mg/kg bw per day. At termination, male rabbits at
1200 mg/kg bw per day had significantly greater (36%) adrenal weights
than controls, but the adrenals were not examined histologically. The
liver weights were 18% greater in females at 1200 mg/kg bw per day
than in controls. No other effects were seen on body weight, food
consumption, or haematological, biochemical, or histological
parameters. The NOAEL was 120 mg/kg bw per day (Elliot et al., 1988).
Rats, rabbits, and dogs
Groups of 10 male and 10 female TUC/SPD rats, one male and one
female New Zealand white rabbit, and two male beagle dogs were exposed
to a dust aerosol of technical-grade dicloran by whole-body exposure
for 6 h per day, five days per week for three weeks. The nominal
concentration of the aerosol was 2 mg/L; the actual concentration and
particle size were not provided. Two rats died on the third day of
exposure, and one rabbit died on day 13. The food consumption and
body-weight gains of the treated animals were depressed. There were no
consistent haematological changes attributable to treatment. The blood
cholesterol concentration was significantly elevated in exposed dogs
and rabbits. The liver weights of exposed animals were increased,
although the histological appearance was unremarkable, with no
evidence of hepatocellular effects in any species (Seaman et al.,
1980).
Monkeys
Oral doses of 160 mg/kg bw per day were lethal to rhesus monkeys
within three months, with a greater effect on females than males. The
colouration of the urine differed from that of rat urine, suggesting a
difference in metabolism in the two species. Centrilobular fatty
infiltration was observed in the liver, and liver and kidney changes
were observed on electron microscopic examination, which included
swelling of mitochondria with distortion of the cristae. Hepatic
microsomal enzyme activity was not enhanced (Serrone et al., 1967).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a screening study, groups of 18 mice of each sex of each of
two susceptible hybrid strains (unspecified) were given dicloran at
215 mg/kg bw per day for three weeks from day 7 after birth.
Thereafter, for 18 months, the mice were fed 600 ppm (equivalent to
100 mg/kg bw per day) in the diet, sacrificed, and examined for
tumours. Dicloran did not cause a significant increase in the
incidence of tumours (Innes et al., 1969).
Dicloran (purity, 96.2-97.2%) was administered to groups of 50
CD-1 mice of each sex at dietary concentrations of 0, 50, 175, or 600
ppm, equal to 0, 7.4, 24, and 86 mg/kg bw per day in males and 0, 10,
36, and 120 mg/kg bw per day in females, for 80 weeks. The doses were
based on a the results of a 60-day dietary study in mice in which a
concentration of 600 ppm was associated with increased methaemoglobin
concentration, splenic pigment change, increased haematopoiesis,
centrilobular hepatocyte enlargement, and fatty degeneration of
hepatocytes. There was no adverse effect on mortality (percent
survival at termination: males, 50, 50, 64, and 56%; females, 84, 76,
76, and 78%, control to high dose, respectively). There were no
treatment-related effects on clinical signs, body weight, palpable
masses, food consumption, food conversion, or differential leukocyte
counts. Clinical chemistry and urinalysis were not performed. There
was no increase in tumour incidence. The liver was the principal
target organ, with increased absolute and relative weights (10-12%) in
males and females at the high dose, which were statistically
significant in females, and histopathological changes. These included
centrilobular hepatocyte enlargement (males: 8, 8, 7, and 26/50;
females: 1, 0, 2, and 5/50), centrilobular haemosiderosis (males: 1,
2, 4, and 12/50; females: 7, 5, 3, and 13/50), focal (4, 2, 5, and
10/50) and single-cell (1, 1, 0, and 6/50) liver necrosis in males,
and vacuolation of centrilobular hepatocytes (4, 3, 3, and 12/50) in
females. Acute inflammatory cell infiltration was seen in males at the
intermediate and high doses (2, 4, 9, and 9/50), but no other hepatic
changes were seen in males at this dose. Other changes at 600 ppm
included a higher incidence of erythropoiesis in the spleens of males
(7, 7, 8, and 15/50), an increased number of females with an
enlarged/distended uterus (5, 9, 9, and 15/50), associated with a
significant increase in the incidence and severity of cystic uterine
endometrial hyperplasia (17, 21, 24, and 31/50), and an increased
number of females at the high dose with distended mammary gland ducts
(9/50 versus 3/50 in control; animals at the low and intermediate
doses were not assessed). An 11% increase in kidney weight in males
was not associated with histopathological lesions. On the basis of the
above findings, the NOAEL was 175 ppm, equal to 24 mg/kg bw per day
(Mallyon & Markham, 1989).
Rats
Groups of 35 rats (strain unspecified) of each sex were fed
dicloran (purity unspecified) in the diet at concentrations of 0, 20,
100, or 3000 ppm for two years, equal to 0, 1.6, 8, and 240 mg/kg bw
per day, with an interim sacrifice of five animals of each sex per
dose at 13 weeks. The dose of 100 ppm had no effect on behaviour,
mortality, or growth, and all values were comparable to those of
controls. At 3000 ppm, growth and food consumption of males and
females were depressed, and haematological parameters (haemoglobin and
packed cell volume) were reduced but only after the first year of
treatment. Gross and microscopic examination at 13 weeks and at the
conclusion of the study showed slightly heavier livers, kidneys, and
testes in males at 3000 ppm. The incidence and location of neoplasms
were similar in treated and control rats. Histological examination
revealed changes in the liver at 3000 ppm, characterized by
hepatic-cell enlargement, increased severity of glycogen depletion,
increased basophilia of the cytoplasm, and the presence of dead cells.
Other observations were increased thyroid hypertrophy and increased
pigmentation of the spleen in males and females at 3000 ppm.
Hepatic-cell changes and slight adrenal cortical atrophy were seen in
several animals at this dose at 13 weeks, but the adrenal changes were
not seen at 104 weeks. The NOAEL was 100 ppm, equal to 8 mg/kg bw per
day (Woodard et al., 1964).
Groups of 25 Boots-Wistar strain rats of each sex were fed
dicloran (purity unspecified) in the diet at a concentration of 0 or
1000 ppm for two years, equal to 59 mg/kg bw per day in males and 71
mg/kg bw per day in females. There was no effect on survival, food
consumption, growth, haematological parameters, or gross or
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular constitution
of the liver, kidney, or spleen. The incidence of tumours in control
and treated groups was similar. The NOAEL was 1000 ppm, equal to 59
mg/kg bw per day, the highest dose tested (Lessel, 1974).
Dogs
Groups of four male and four female beagle dogs were fed dry
diets containing dicloran (purity unspecified) at concentrations of 0,
20, 100, or 3000 ppm for two years, equal to 0, 0.33, 1.7, and 44
mg/kg bw per day for males and 0, 0.36, 1.8, and 62 mg/kg bw per day
for females, with an interim sacrifice of one animal of each sex per
dose at 14 weeks. No compound-related changes in behaviour, food
consumption, or growth were observed. One female at 3000 ppm died at
74 weeks, with treatment-related signs of haemolytic anaemia (reduced
haemoglobin, immature erythrocytes, polychromophilic macrocytes,
increased leukocyte count, and increased myeloid: erythroid ratio in
the bone marrow) before death. One control male lost considerable
weight but survived to the end of the study. All dogs at 3000 ppm had
watery lachrymation, which persisted throughout treatment; the
examination of the eyes was not adequate to determine any ocular
toxicity of dicloran. Yellowing of the sclera, mucous membranes, and
abdominal skin were noted at the high dose. Changes in haematological
and clinical chemical parameters in males and females at the high dose
included significant reductions in haemoglobin from week 26 to
termination and reduced serum protein at termination; increased
activity of alanine and aspartate aminotransferases and increased
prothrombin time, blood urea nitrogen, and bromosulphthalein retention
were observed in one male and the one female that died at the high
dose, both of which had received 20-94% more test material than the
other dogs in this group owing to gradual body-weight loss relative to
the constant concentration in the diet. Gross and microscopic
examination revealed significantly increased liver, spleen, and kidney
weights accompanied by histological changes at 3000 ppm, which
included irregular hepatic-cell size (3/5 versus 0/6 in other groups),
moderate hepatic-cell hypertrophy (3/5 versus 0/6 in controls) and
increased pigmentation of the liver (4/5 versus 0/6 in control), and
spleen (5/5 versus 0/6 in controls). One male and two females at 3000
ppm (including the female that died) and one male at 20 ppm also had
enlarged gall-bladders containing tar-like, viscous bile, although
this was stated to be a spontaneous lesion in dogs (cystic mucinous
hypertrophy). Changes at 100 ppm which included irregular hepatic
cells, slight focal hepatocytic hypertrophy, slight vacuolation,
moderate liver pigmentation, and/or marked spleen pigmentation were
noted in two female dogs. A company re-evaluation attributed the
findings at 100 ppm to enzyme induction, although the
histopathological findings in this group were complicated by the
presence of parasitic hepatitis. No degenerative lesions were noted at
study termination, and there was no progression in the severity of
hepatocellular effects between interim and terminal sacrifice. The
NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day, on the basis of
significant reductions in haemoglobin, reduced serum protein, and
histopathological changes in the liver and spleen at 3000 ppm (Woodard
et al., 1964; Kakuk et al., 1979).
(d) Genotoxicity
The results of studies on the mutagenicity and genotoxicity of
dicloran are presented in Table 2. Dicloran gave occasional positive
responses in the most recent tests for reverse mutation and in a test
for mitotic recombination, with a 2-2.5-fold increase in mitotic
recombination. The results of the test for sex-linked recessive lethal
mutation in Drosophila were equivocal, even after re-analysis in
conjunction with historical control data.
(e) Reproductive toxicity
(i) Single-generation reproductive toxicity
Male rats (strain unspecified) were treated with dicloran (purity
unspecified) in the diet at concentrations of 0, 1000, or 2000 ppm,
equivalent to 0, 50, and 100 mg/kg bw per day, for 90 days. The dose
of 1000 ppm increased liver and kidney weights. When the males were
mated with untreated females, no difference was observed in the number
Table 2. Results of assays for the genotoxicity of dicloran
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, 16, 31, 62, 125, 99.9 Negativeb Everest & Tuplin (1977)
TA100, TA1535, 250, 500, 1000
TA1537, TA1538 µg/plate in DMSO
Reverse mutationa S. typhimurium 100 µg/plate in NR Negative Shirasu et al. (1976)
TA1535, TA1536, DMSO
TA1537, TA1538
Reverse mutationc S. typhimurium TA98, 20-200 µg/plate 99.5 Negatived Jeang & Li (1978)
TA100 in DMSO
Reverse mutatione S. typhimurium TA98, 2, 20, 200 µg/plate 99.5 Negativef Jeang & Li (1980)
TA100 in DMSO
Reverse mutationa S. typhimurium TA98, 6 concentrations NR Negative Waters et al. (1982);
TA100, TA1535, up to 10 000 µg/plate Garrett et al. (1986)
TA1537, TA1538
Reverse mutationa S. typhimurium TA98, 62, 100, 155, 200, NR Positiveg Basting et al. (1983);
TA98 NRF, TA100, 310, 415 µg/plate in Myers-Basting (1986)
TA100 NRF ethyl cellosolve/ethanol
or DMSO
Reverse mutationa S. typhimurium TA98, 50, 150, 500, 1500, 97.5 Positiveh Jones & Fenner (1987)
TA100, TA1535, 5000 µg/plate in
TA1537, TA1538 DMSO
Reverse mutationa S. typhimurium TA98, 10, 33, 100, 333, 98 Positivei Zeiger et al. (1992)
TA100 1000, 2000 µg/plate
in DMSO
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Reverse mutationc E. coli WP2, WP2uvrA- 62, 125, 250, 500, 99.9 Negativej Everest & Tuplin (1977)
1000 µg/plate in
DMSO
Reverse mutationc E. coli B/r try WP2, 20 µg/plate in DMSO NR Negative Shirasu et al. (1976)
WP2 try hcr
Reverse mutationa E. coli WP2uvrA- 6 concentrations NR Negativek Waters et al. (1982);
up to 10 000 µg/plate Garrett et al. (1986)
Mitotic recombationa S. cerevisiae D3 5 concentrations NR Negative Waters et al. (1982)
(unspecified)
Mitotic aneuploidy Neurospora crassa 5 or 10 µg/ml NR Negativel Griffiths et al. (1986;
I-41-5, I-34-8 in DMSO Moustacchi (1986)
DNA repairc B. subtilis H17 rec+, 20 g/disc in DMSO NR Negative Shirasu et al. (1976)
M45 rec-
DNA repaira B. subtilis H17 rec+, 20 g/disc in DMSO 99.5 Negativem Jeang & Li (1978)
M45 rec-
DNA repairc B. subtilis H17 rec+, 2 concentrations NR Negativem Waters et al. (1982);
M45 rec- (not specified) Garrett et al. (1986)
DNA repairc E. coli P3478 (polA-), 2 concentrations NR Negativem Waters et al. (1982);
W3110 (polA+) (not specified) Garrett et al. (1986)
Unscheduled DNA Rat hepatocytes 3, 4, 5, 6, 7, 8, 9, 97.5 Negativen McBride & McGregor
synthesis 10 µg/ml in DMSO (1987)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Mitotic recombination Aspergillus nidulans 2070, 4140, 6210 NR Positiveo Kappas (1978);
and aneuploidy (heterozygous diploid µg/plate in ethanol Moustacchi (1986)
strain)
Cytogenetic Human lymphocytes 2, 10, 20 µg/ml in 97.5 Negativep Allen et al. (1988)
alterationsa DMSO
In vivo
Recessive lethal Drosophila melanogaster Feeding at 1250 and 98 Equivocal Woodruff et al. (1985)
mutation Canton-S 1389 ppm in 5%
ethanol:5% Tween 80
Recessive lethal Drosophila melanogaster Re-analysis of data NR Equivocal Mason et al. (1992)
mutation Canton-S of Woodruff et al.
(1985)
NR, not reported; DMSO, dimethyl sulfoxide; NRF, nitroreductase-free
a With and without metabolic activation
b The positive controls, cyclophosphamide, 6-aminochrysene, and 2-aminofluorene, gave the expected results.
c Without metabolic activation
d The positive control nitroquinoline N-oxide gave the expected result.
e With metabolic activation
f The positive control sterigmatocystin gave the expected result.
g Positive in TA98 (> 100 µg/plate) only without metabolic activation
h Positive in TA98 and TA1538 (> 500 µg/plate) with and without metabolic activation and in TA100 without metabolic
activation. The positive controls, N-ethyl-N'-nitro-N-nitrosoguanidine, 9-aminoacridine, 2-aminoanthracene, and
2-nitrofluorene, gave the expected results.
i Positive in TA98 (> 100 µg/plate) with and without metabolic activation; positive control unknown
j The positive control ethyl methanesulfonate gave the expected result.
Table 2. (continued)
k The positive controls, 2-aminoanthracene and 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide, gave the expected results.
l This test system has not been fully validated. The positive control para-fluorophenylaniline gave the expected result.
m The positive control methyl methanesulfoxide gave the expected result.
n The positive control, Mischler's ketone [4,4'-bis(dimethylaminobenzophenone) in ethanol], gave the expected result.
o A 2-2.5-fold increase in mitotic recombination. This test system has not been fully validated.
p The positive controls, ethyl methanesulfonate and cyclophosphamide, gave the expected results.
of litters or in the number of animals born or weaned (US
Environmental Protection Agency, 1974).
Dicloran (purity unspecified) was fed to groups of 10 female rats
at concentrations of 0, 500, or 1000 ppm, equivalent to 0, 25, and 50
mg/kg bw per day, for 188 days before mating and then through
gestation and lactation. A reduced number of pups was reported when
females were treated with 1000 ppm. There was no apparent effect on
the survival of pups, although the mean body weight of those at 1000
ppm was slightly reduced (US Environmental Protection Agency, 1974).
(ii) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, with one
litter per generation, dicloran (purity, 99.2%) was administered to
groups of 28 male and 28 (F0) and 24 male and 24 female (F1) female
Sprague-Dawley rats in the diet at doses of 0, 50, 250, or 1250 ppm,
equal to 0, 4, 21, and 110 mg/kg bw per day. The doses were based on
the results of a range-finding study (Wilcox & Barton, 1996) in which
concentrations up to 1000 ppm did not produce any obvious alterations
in body weight, food consumption, duration of gestation, or litter
parameters. The animals were fed the test diet during the 10- (F0) or
11-week (F1) pre-mating period and then randomly allocated to mating
pairs (l:1) for a maximum of seven nights. Treatment was continued for
animals of each sex throughout the mating, gestation, and lactation
periods until termination of the F2 litters.
The treatment-related clinical signs included yellow staining of
the tray paper and, in some cases, yellow staining of the fur of all
animals at the high dose in both generations, due to the yellow colour
of the test material. There were no significant differences in mean
body weights or body-weight gain between control and treated males.
The mean body weights of F0 females at the high dose throughout the
pre-mating period was 2-5% lower than that of controls, resulting in a
12% lower mean overall gain at the end of the pre-mating period. This
was considered to be treatment-related. The mean body weights of F1
females at the high dose were, on average, 6% lower than those of
controls during pre-mating, but the overall mean body-weight gain was
only 5% lower. F0 and F1 females at the high dose had slightly lower
overall body-weight gain during gestation, the greatest difference
occurring during the first week of gestation (64% of control values in
F0 females and 86% in F1). There were no apparent effects on the
food consumption of F0 or F1 males or females during pre-mating and
gestation; however, the food consumption of females of both
generations at the high dose was 11% lower during the first week of
lactation, and these animals weighed more than controls and those
given lower doses at the end of lactation, indicating that pups in the
high-dose group were less demanding with respect to suckling than pups
in the other groups.
At the end of the study, a greater proportion of F0 and F1
females at the high dose were in pro-estrus (4/28 F0 controls versus
19/28 at the high dose; 4/24 F1 controls versus 11/24 at the high
dose). Since there was no indication of altered cycling, this result
was considered to be of no toxicological concern. Sperm-stage analysis
indicated no difference between control F0 and F1 males and those at
the high dose with respect to the number of testicular tubules at
various spermatogenic stages or in tubule diameter. There was a
dose-related increase in the incidence of abnormal sperm, particularly
with respect to tail defects, in F0 males (0.25 ± 0.26 and 0.29 ±
0.31% in the two control groups, 0.59 ± 0.96% at the low dose, 0.6 ±
0.65% at the intermediate dose, and 0.67 ± 0.58% at the high dose);
however, no such increase was observed in F1 males at the high dose
(0.21% versus 0.23% in controls). Furthermore, the mean values for F0
animals were within the historical control range, 0.0-0.8%. Thus,
these findings were considered incidental. The fertility and
gestational indices of control and treated F0 and F1 females were
similar. The male fertility indices of F0 males at the intermediate
and high doses were lower than that of controls (82 and 86%,
respectively), as was that of F1 males at the low dose (75%). Since
the fertility index of F1 males at the intermediate and high doses
approached 100%, the lower fertility indices were considered
incidental.
There were significant increases in absolute and body
weight-adjusted mean liver weights in F1 males and females at the
high dose; no data were collected for F0 animals. The weights of the
kidneys of F1 animals at the high dose were increased, and the
increase was significant in males. Brain weights were significantly
lower in F1 females at the high dose, and thymus weights were
significantly lower in F1 males and females at the high dose relative
to controls. No microscopic examinations were conducted on these
tissues. The mean absolute epididymal weights of F0 males at the high
dose and the absolute and body weight-adjusted epididymal weights of
F1 males at the intermediate and high doses were significantly lower
than those of controls. The authors indicated that the values for the
F1 males were similar to 'recent control values for animals of
similar age'; however, data on these controls were not provided. The
mean absolute and body weight-adjusted testicular weights were
statistically significantly increased in F1 males at the high dose
and non-significantly increased in F0 males at this dose. There was a
dose-related decrease in mean ovarian weights in F1 females, which
was statistically significant in the high-dose group. No
treatment-associated histopathological lesions were seen in the
reproductive tissues that were examined. In the absence of
histological data on non-reproductive tissues, the toxicological
significance of the observed changes in organ weights in these tissues
is equivocal.
No difference was observed in the mean number of implantation
sites per litter, litter size, sex ratio, or litter indices in control
and treated groups. A slight increase in the number of F1 pups that
died during days 4-21 of lactation was not observed in the F2
generation and was considered to be incidental. The group mean weights
of F1 and F2 litters were decreased throughout the lactation period,
as reflected in the lower mean pup body weights at this dose. Although
the magnitude of the difference was not as great in F2 pups as in F1
pups, these observations were consistent and considered to be related
to treatment. The mean litter weights of dams at the low and
intermediate doses were lower than those of controls on day 1 of
gestation, but the mean pup weights were comparable to those of
controls on days 1-21 of lactation, in contrast to the high-dose
group, and were therefore considered not to be related to treatment.
There were no treatment-related clinical observations or
malformations.
The NOAEL for systemic toxicity was 250 ppm, equal to 21 mg/kg bw
per day, on the basis of reduced body-weight gain at 1250 ppm in
females during pre-mating and gestation and increased liver and kidney
weights in males and females at this dose. The NOAEL for reproductive
and developmental toxicity was also 250 ppm, equal to 21 mg/kg bw per
day, on the basis of lower F1 and F2 pup weights at the next highest
dose. There were no histopathological lesions in the reproductive
tissues that would indicate that the changes in organ weights should
be considered adverse (Barton & Wilcox, 1997).
In a three-generation study, with two litters per generation,
dicloran (purity unspecified) was administered to groups of 20 rats
(strain unspecified) of each sex at 0 or 100 ppm, equivalent to 5
mg/kg bw per day, in the diet. On the basis of the reproduction
parameters examined, including the numbers of litters, stillbirths,
live births, birth weight, and lactation indices, dicloran had no
effect on reproduction (Lobdell & Johnston, 1965).
(iii) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley rats (Upj:TUC (SD) SPF) were
dosed by gavage with dicloran (purity, 93.7%) in 0.25% carboxymethyl
cellulose and 0.5% Tween 20 at doses of 0, 100, 200, or 400 mg/kg bw
per day on days 6-15 of gestation, the day of mating being considered
gestation day 0. These doses were derived from the results of a
range-finding study in which 5/5 rats died at doses of 2000 and 4000
mg/kg bw per day, and 3/5 at 1000 mg/kg bw per day. Neither of the two
survivors treated with 1000 mg/kg bw per day had pups, and one had 15
early resorptions. The mean body-weight gain of females treated with
500 mg/kg bw per day was 25% less than that of controls.
The dams were sacrificed on day 20 of gestation, and the uterine
contents were examined; the uteri of non-pregnant dams were stained
with ammonium sulfide, and all fetuses were assessed for viability,
sex, weight, and external malformations and variations. Equal numbers
of fetuses from each group were assigned to either visceral or
skeletal examination. There were no deaths. The treatment-related
clinical signs included yellow staining of the urogenital region in
some animals at each dose due to the colour of the test material, and
slight central nervous system depression throughout the treatment
period in animals at 200 and 400 mg/kg bw per day. The mean
body-weight gain (corrected for gravid uterine weights) of treated
animals was significantly lower (16-33% less) than that of the the
controls. The incidence of dams with totally resorbed litters (early
implants only) was increased in all treated groups (1, 5, 9, 10;
control to high dose, respectively); however, there were no
differences in the mean numbers of implants or resorptions or in the
mean litter sizes of dams with live litters. The fetal weights were
significantly lower at 200 and 400 mg/kg bw per day (7 and 20% less
than control, respectively), and a significant trend for an increased
incidence of delayed or reduced ossification was noted in these two
groups. Increased incidences of other skeletal variations (bipartite
sternebrae) were also seen at the high dose, but there were no
teratogenic effects. (The authors maintained that the low dose was not
maternally or embryotoxic. The conclusion concerning embryotoxicity
was based on statistical significance rather than biological
significance, and an erroneously large control value appears to have
been used for statistical comparison.) On the basis of the
significantly lower mean body-weight gains and the higher incidences
of dams with totally resorbed litters (i.e. early implants only) in
all treated groups, an NOAEL was not identified for maternal or
developmental toxicity. (The authors indicated that
sialodacryoadenitis viral infection was present during much of the
dosing period and indicated that this may have had some effect on
weight gain; however, in previous studies this virus had no
teratogenic effects. If this is a valid conclusion, the increased
incidence of total resorption of litters at all doses should be
considered treatment-related.) (Marks et al., 1982).
Rabbits
Groups of 10, 12, and 14 pregnant New Zealand white rabbits were
fed dicloran (purity unspecified) in the diet at 0, 100, or 1000 ppm,
equivalent to 0, 3, and 30 mg/kg bw per day, on days 8-16 of
gestation, the day of mating being considered gestation day 1.
Pregnant females were allowed to deliver naturally, and the pups and
parental females were sacrificed on post-natal day 21. There were no
effects on maternal body weight during treatment. The mean litter size
was slightly lower in the group at the high dose than in controls,
with values of 7.1, 6.6, and 6.1 for the control, low, and high dose,
respectively. In the absence of data on resorption, however, it could
not be determined whether this effect was treatment-related. The pup
weights at birth were not provided, but the mean pup weights at
weaning (day 21) were comparable in all groups. There were no
malformations and no increase in the incidence of skeletal
malformations in treated pups (Wazeter, 1966).
Groups of 15-16 naturally mated New Zealand white rabbits were
dosed by gavage with dicloran (purity, 98.3%) in 1% carboxymethyl
cellulose at 0, 8, 20, or 50 mg/kg bw per day on days 6-18 of
gestation, the day of mating being considered gestation day 0. These
doses were based on the results of a range-finding study in which a
dose of 100 mg/kg bw per day caused two deaths, marked reduction in
food intake, reduced body-weight gain during treatment, slightly
increased post-implantation loss, and reduced litter and mean fetal
weights, with gross malformations in 4/11 fetuses from one litter. The
dose of 50 mg/kg bw per day resulted in reduced food intake and lower
body-weight gains throughout treatment, and at 20 mg/kg bw per day
there was a slight, transient reduction in food intake, with reduced
body-weight gain and slight body-weight loss on days 9-11 of gestation
(James & Brennan, 1991).
The dams were sacrificed on day 29 of gestation, the uterine
contents were examined, and all fetuses were assessed for viability,
sex, and external, visceral, and skeletal malformations and
variations. There were no treatment-related effects on mortality,
clinical signs, or food consumption. The mean body weights and body-
weight gain were slightly but consistently lower than those of
controls in females at the intermediate and high doses throughout both
the treatment and post-treatment periods. This resulted in an overall
mean body-weight gain in these animals that was 17-32% less than that
of controls during treatment and at termination. The mean gravid
uterine weights were also lower in these animals than in controls,
whereas the carcass weights were similar in all groups, indicating
that the weight changes were attributable primarily to effects on the
litters rather than the dams. The lower body-weight gain of dams at
the intermediate dose in the latter stages of gestation may be due
partially to the smaller litter sizes of this group, which were not
treatment-related; however, the effects seen in females at the
intermediate dose early during treatment (days 6-9) may indicate
maternal toxicity. The differences in body-weight gain in animals at
the high dose were also considered to be toxicologically significant.
Dams at the two higher doses had a slight increase in the frequency of
post-implantation loss (7.4, 9, 4.8, and 10% for control to high dose,
respectively). There were slight increases in the incidence of minor
anomalies of the gall-bladder in three fetuses in three litters and
delays in ossification of all limb epiphyseal sites in four fetuses in
three litters (with none in controls) at 50 mg/kg bw per day. The
NOAEL for maternal toxicity was 8 mg/kg bw per day on the basis of
lower body-weight gain early in the treatment period at 20 and 50
mg/kg bw per day. The NOAEL for developmental toxicity was 20 mg/kg bw
per day (Barton & Wilcox, 1996).
(f) Special studies
(i) Cataractogenicity
Dogs have been shown to develop lesions of the cornea and lens
after prolonged oral administration of dicloran. It has been suggested
that a photochemical product reaction is responsible for the lesion as
it occurs only when dogs are exposed to sunlight. Groups of four to
eight beagle dogs and six Hormel-Hanford miniature swine were fed
dicloran (purity, 95.8%) at concentrations of 0, 0.75, 6, 24, 48, or
192 mg/kg bw per day for periods varying from 50 to 306 days. Corneal
opacity appeared in dogs within 53-104 days after administration of 24
or 48 mg/kg bw per day, when they were exposed to sunlight. Dogs that
were not exposed to sunlight and eyes that had been sutured closed did
not develop lesions. Dogs given 192 mg/kg bw per day refused to eat
after 38 days and were given dicloran by capsule. All of these animals
died 49-53 days after the study began; no eye lesions were detected.
In several dogs with eye damage that were maintained for four months
to one year after dicloran administration had ceased, the pathological
changes seen in the cornea and lens were not reversed. Dicloran did
not appear to affect miniature swine at any concentration, and no
histopathological changes were seen in the eyes. A dose-related
increase in the presence of Heinz bodies was seen in the blood of both
dogs and swine, with at least one dog affected per dose and pigs
affected at doses of 48 mg/kg bw per day or more. No methaemoglobin
formation was found in dogs or pigs. Administration of dicloran as a
dust (0.1 mg) or a 5% solution directly into the eyes of dogs for
three months had no effect on corneal opacity or irritation of the
conjunctivae (Curtis et al., 1968; Bernstein et al, 1970; Earl et al.,
1971).
Ocular toxicity was not observed in rats or rhesus monkeys
(Serrone et al., 1967), and administration of 0.143 mg/kg bw per day
for three months to 20 human volunteers produced no ocular symptoms
(Strough, 1962).
The 1977 Joint Meeting (Annex 1, reference 28) reviewed
additional data requested by the 1974 JMPR and concluded that a
species difference in the kinetics of dicloran may partly explain the
photosensitive ocular toxicity observed in dogs,but not in other
mammals. 14C-Dicloran was administered orally at 100 mg/kg bw per day
for five days to four beagle dogs, four pigs (breed unspecified), and
eight Wistar rats. The concentration of radiolabelled residues was
highest in the dogs and slightly lower in pig tissues, but the tissue
residues in both species were 2.4-11-fold higher than those in liver
or plasma residues of rats. All three species had a high concentration
of residue in the liver, but in dogs and pigs the highest
concentrations were found in the pigmented tissues of the eye, the dog
having two to three times more residue in the iris and pigmented
retina than the pig. Nonpigmented eye tissues (cornea and lens) had
low concentrations. There was no correlation between ocular toxicity
and tissue residues of dicloran and its metabolites. The plasma
concentrations of radiolabel increased more rapidly in the dogs, but
24 h after three days of dosing the plateau plasma concentrations in
the pigs and dogs were similar (10-15 mg/kg bw). High concentrations
of radiolabel were excreted in the bile of dogs and pigs (Hamilton,
1977).
(ii) Haematological effects
Groups of 10 or 15 rats (strain unspecified) of each sex were
treated with dicloran at concentrations of 0, 5, 20, or 100 mg/kg bw
per day by gavage for four months or in the diet at a concentration of
0 or 20 ppm, equivalent to 1 mg/kg bw per day for four months.
Measurements of red blood cells, total and differential leukocyte
counts, platelet count, haematocrit, haemoglobin concentration,
glucose, growth, and food consumption indicated no significant effect
attributable to dicloran at any dose by either route (Evans et al.,
1963).
Because of the known ability of 4-nitroaniline to induce specific
blood dyscrasias, short-term studies were performed to compare
dicloran and 4-nitroaniline. Groups of five weanling rats (strain
unspecified) of each sex were given dicloran by gavage at 0 or 400
mg/kg bw per day, five days/week, for four weeks, and 4-nitroaniline
was administered at 200 or 400 mg/kg bw per day to two comparable
groups over the same interval. In a second experiment, groups of six
male weanling rats were given dicloran by gavage at 0 or 800 mg/kg bw
per day or 4-nitroaniline at 400 or 800 mg/kg bw per day, five days
per week, for two weeks. Haematological parameters were normal in rats
treated at 400 mg/kg bw per day -- no Heinz bodies were detected and
the reticulocyte counts were normal -- although their body-weight gain
was 50-60% of that of controls. At 800 mg/kg bw per day, there was
slight weight loss. Red blood cell count, packed cell volume, and
haemoglobin measurements were normal, while the lymphocyte counts were
reduced to 65% of that of controls. At the end of the study, no
effects were seen on the spleen. In contrast, 4-nitroaniline had
definitive effects on growth at 200 mg/kg bw per day (70% of control),
Heinz bodies were identified in the blood, and the reticulocyte count
was markedly elevated to two to four times the control value. The bone
marrow was not affected. At 400 mg/kg bw per day, growth was reduced
to 60% of the control value, and a reduced red blood cell count (65%
of control with polychromasia and nucleation) was seen, accompanied by
increased spleen weight (200% of control). These effects were more
pronounced at 800 mg/kg bw per day where, in addition, the haemoglobin
concentration was reduced and the lymphocyte count greatly increased.
Although the high doses of dicloran caused lymphopenia, the other
haemotoxic effects seen with 4-nitro-aniline were not observed with
dicloran (Boots Pure Drug Co., 1962).
In studies to substantiate the difference between the effects of
4-nitroaniline and dicloran, a single oral dose of 500 mg/kg bw
dicloran was given to cats. No methaemoglobin was noted at any time
between 1 and 48 h after dosing; however, administration of
4-nitroaniline as a single dose of 100 mg/kg bw resulted in
methaemoglobin over the same time. In addition, the cats given
4-nitroaniline were cyanotic and showed extensive muscle weakness
(Gurd, 1974). Although methaemoglobin was not assessed in all studies,
it is noteworthy that the 60-day study in mice was the only one that
showed an association between dicloran and significantly elevated
methaemoglobin concentrations in the blood at doses of 100 and
200 mg/kg bw per day (Mallyon et al., 1986). No effect on
methaemoglobin was seen in the two-year study in dogs (Woodard et al.,
1964).
3. Observations in humans
In a double-blind clinical study, 20 male volunteers were given
10 mg of dicloran (purity unspecified), equivalent to 0.14 mg/kg bw
per day, and 10 received a placebo, once daily, for 90 days. The dose
and duration were based on the estimated maximum residues obtained
from consumption of fruits and vegetables over one year.
Haematological examinations and tests for liver and kidney function
were performed at various intervals over the course of the study; the
results were found to be normal (Strough, 1962).
Extensive examinations were made on an industrial worker who had
been occupationally exposed to dicloran for three years, with
considerable exposure by inhalation and dermal contact for about 60
days per year. No adverse effects were observed (Brooks & Boyack,
1963).
Comments
Dicloran is rapidly absorbed, metabolized, and eliminated, mainly
in the urine, after oral administration to rats, goats, and humans; it
is also rapidly excreted by hens. Rats excreted most of the radiolabel
(90-96%) within 24 h, with > 70% in the urine and an additional
13-22% in the faeces, depending on the dose. Elimination was
essentially complete (91-97% of the administered dose) by 48 h, with
total tissue residues accounting for 0.2-0.3%. The highest tissue
concentrations were found in the liver (0.05-0.06 mg/kg) and kidneys
(0.02 mg/kg). Unchanged parent compound was detected in the faeces of
animals at the high dose only. The major urinary metabolites were the
sulfate and glucuronide conjugates of 2,6-dichloro-4-hydroxyaniline,
which accounted for 55-79% of the total administered radiolabel, and
the major faecal metabolites were derivatives of glutathione
conjugates. The parent compound and its glutathione conjugates were
the major residues in plants. None of the metabolites was of
toxicological concern. The rapid excretion of dicloran as a conjugate
indicates that it is readily metabolized in vivo.
The major metabolite in rat urine (2,6-dichloro-4-hydroxyaniline)
was not present in goat urine, which contained more polar conjugates
that could not be hydrolysed by glucuronidase or sulfatase. In goats,
dicloran is reduced to 4-amino-2,6-dichloro-aniline and acetylated to
4-amino-3,5-dichloroacetanilide (4-6% in urine), which is rapidly
metabolized and excreted in the urine and faeces, although a reactive
intermediate is formed in goat liver and bound covalently to
macromolecules. Species differences in dicloran metabolism were also
apparent in dogs and mice, which partly explain the photosensitive
oculotoxic effects observed in dogs and the methaemo-globinaemia noted
in mice, which are not seen in other mammals. Preliminary studies
suggested that the metabolites of dicloran in humans are similar to
those in rats. Although recovery was considered complete, excretion
was somewhat slower in humans than in rats, most of the material being
excreted within 1.5 days.
Dicloran has low or slight toxicity when administered by the oral
route, depending on species. It has low dermal toxicity, is not
irritating to the skin, is a mild eye irritant, and is not a skin
sensitizer.
WHO has classified dicloran as unlikely to present an acute
hazard in normal use (WHO, 1996).
The results of short-term studies of toxicity in rabbits treated
dermally and in mice and rats treated in the diet or by gavage and of
long-term studies of toxicity in mice, rats, and dogs treated in the
diet indicate that the liver is the primary target organ. Increased
liver weights and centrilobular hepatic hypertrophy were observed
consistently in all species treated orally, and increased splenic
activity and splenic extramedullary haematopoiesis were noted in
short- and long-term studies in mice.
When dicloran was fed to mice at 0, 300, 600, or 1200 ppm for 60
days, the NOAEL was 300 ppm (equal to 49 mg/kg per day), on the basis
of hepatic lesions, splenic extramedullary haematopoiesis, and
increased methaemoglobin at doses of 600 ppm and above.
In a 90-day study in mice treated by gavage with 0, 15, 45, 135,
400, or 600 mg/kg bw per day, the NOAEL was 15 mg/kg bw per day on the
basis of significant polycythaemia in males and hypercholesterolaemia
and a significant increase in the incidence of splenic extramedullary
haematopoiesis in females at 45 mg/kg bw per day and above.
A NOAEL was not identified in a 90-day study in rats fed diets
containing 0, 1000, 3000, or 5000 ppm dicloran because of
significantly reduced body-weight gain in conjunction with effects on
the liver and thyroid in all treated groups. In an eight-week study in
which rats were given diets containing dicloran at doses of 0, 500, or
750 ppm, the NOAEL was 500 ppm (equal to 44 mg/kg bw per day) on the
basis of reduced body-weight gain, decreased food consumption, and
increased liver weights with associated hepatic histopathological
manifestations at 750 ppm.
In a study in which dicloran was fed to rats for six months at 0,
30, 300, or 3000 ppm, the NOAEL was 300 ppm (equal to 22 mg/kg bw per
day) on the basis of reduced body weights and increased liver and
spleen weights at 3000 ppm.
In a four-week study in rats given a dose of 0, 35, 140, or 350
mg/kg bw per day by gavage, the NOAEL was 35 mg/kg bw per day on the
basis of growth depression and hepatic hypertrophy and vacuolation at
140 mg/kg bw per day.
In an 18-month study of carcinogenicity in mice at dietary
concentrations of 0, 50, 175, or 600 ppm, the NOAEL was 175 ppm (equal
to 25 mg/kg bw per day) on the basis of increased liver weights,
centrilobular hepatocyte enlargement, centrilobular haemosiderosis,
focal and single-cell liver necrosis, and vacuolation of centrilobular
hepatocytes.There was no evidence of carcinogenicity in mice.
Two studies were conducted in rats to assess toxicity over a
two-year period of exposure. Rats were fed diets containing dicloran
at concentrations of 0, 20, 100, or 3000 ppm or 0 or 1000 ppm. The
overall NOAEL was 1000 ppm (equal to 59 mg/kg bw per day), on the
basis of changes in body-weight, food consumption, and haematological
parameters, spleen pigmentation, increased liver weights,
centrilobular hepatocyte enlargement, and thyroid hypertrophy at 3000
ppm. There was no evidence of carcinogenicity in these studies, but
they were considered inadequate for complete evaluation of the
carcinogenetic potential of dicloran.
Dogs fed dicloran at dietary concentrations of 0, 20, 100, or
3000 ppm for two years had treatment-related changes in haematological
and clinical chemical parameters and significant increases in liver,
spleen, and kidney weights, accompanied by histological changes at
3000 ppm that included irregular hepatic-cell size, moderate
hepatic-cell hypertrophy, and increased pigmentation of the liver and
spleen. The NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day.
Dicloran was not mutagenic in most assays, although occasional
positive responses were seen in more recent tests for reverse mutation
and in a test for mitotic recombination. The results of an assay for
sex-linked recessive mutation in Drosophila were equivocal. The
Meeting concluded that dicloran is unlikely to be genotoxic.
Studies of reproductive and developmental toxicity indicated that
dicloran is not a reproductive toxicant and is not teratogenic in rats
or rabbits. Dicloran was embryotoxic at maternally toxic doses in
rabbits, but an NOAEL for maternal or developmental toxicity was not
identified in rats.
In a two-generation study of reproductive toxicity in rats (one
litter per generation) at dietary concentrations of 0, 50, 250, or
1250 ppm, the NOAEL for systemic toxicity was 250 ppm (equal to 21
mg/kg bw per day) on the basis of reduced body-weight gains and
increased liver weights at 1250 ppm. The NOAEL for reproductive and
developmental toxicity was 250 ppm (equal to 21 mg/kg bw per day) on
the basis of reduced weights of F1 and F2 pups at 1250 ppm.
In a study of developmental toxicity, dicloran was administered
by gavage to rats on days 6-15 of gestation at doses of 0, 100, 200,
or 400 mg/kg bw per day. Because of significantly reduced mean
maternal body-weight gains and higher incidences of totally resorbed
litters in all treated groups, NOAEL values for maternal or
developmental toxicity were not identified. Dicloran was not
teratogenic in this study.
In a study of developmental toxicity in rabbits, dicloran was
administered by gavage at doses of 0, 8, 20, or 50 mg/kg bw per day on
days 6-18 of gestation. The NOAEL for maternal toxicity was 8 mg/kg bw
per day, on the basis of reduced maternal body-weight gains early
during treatment with 20 or 50 mg/kg bw per day. The NOAEL for
developmental toxicity was 20 mg/kg bw per day on the basis of a
slight increase in post-implantation losses, a slight increase in the
incidence of minor anomalies of the gall-bladder, and delays in
ossification of all limb epiphyseal sites in fetuses at 50 mg/kg bw
per day.
Dogs have been shown to develop lesions in the cornea and lens
after prolonged oral administration of dicloran. The 1977 Joint
Meeting reviewed additional data requested by the 1974 Meeting and
concluded that the photosensitive oculotoxic effects observed in dogs
but not in other mammals were due partly to a species difference in
the kinetics of dicloran. No oculotoxic effects were seen in any of
the more recent studies, although no further studies have been
conducted in dogs.
In a double-blind clinical study, 20 men were given 10 mg
dicloran (equivalent to 0.14 mg/kg bw per day) and 10 received a
placebo once daily for 90 days. The dose and duration were chosen on
the basis of the maximum residues estimated to be derived from
consumption of fruits and vegetables over one year. There was no
indication that administration of dicloran at this dosage had any
adverse effect.
An ADI of 0-0.01 mg/kg bw per day was established on the basis of
the NOAEL of 1.7 mg/kg bw per day for hepatic and haematological
effects in the two-year study in dogs and a 200-fold safety factor. A
larger than normal safety factor was used because of the inadequacy of
the long-term studies in rats for assessing the carcinogenic potential
of dicloran and because of the lack of a NOAEL for maternal and
developmental toxicity in rats.
An acute RfD was not allocated because dicloran has low or slight
toxicity when administered orally or dermally and because acute
effects occur only at very high doses, resulting in a 10 000-fold
difference between the ADI and the LOAEL for maternal and
developmental toxicity in rats. Therefore, the Meeting concluded that
the acute intake of residues is unlikely to present a risk to
consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 49 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
15 mg/kg bw per day (13-week study of toxicity)
175 ppm, equal to 24 mg/kg bw per day (18-month study
of carcinogenicity)
Rat: 35 mg/kg bw per day (lowest dose tested, four-week
study of toxicity)
500 ppm, equal to 44 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
300 ppm, equal to 22 mg/kg bw per day (six-month study
of toxicity)
1000 ppm, equal to 59 mg/kg bw per day (two two-year
studies of toxicity)
250 ppm, equal to 21 mg/kg bw per day (two-generation
study of reproductive toxicity)
Rabbit: 8 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (developmental toxicity)
Dog: 100 ppm, equal to 1.7 mg/kg bw per day (two-year study
of toxicity)
Human: 0.14 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of an acute reference dose
Not allocated (unnecessary).
Studies that would provide information useful for continued
evaluation of the compound
1. Combined study of toxicity and carcinogenicity in rats.
2. Study of developmental toxicity in rats at less than 100
mg/kg bw per day to establish clear NOAELs for maternal and
developmental toxicity.
3. Assays for genotoxicity in mammals in vivo, such as an
assay for micronucleus formation
4. Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete, > 70% urinary excretion within 24 h
Dermal absorption No data
Distribution Liver, kidney
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 90-96% in urine and faeces within 24 h
Metabolism in animals Metabolites differ in rats and goats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral > 4000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation No data
Skin irritation Not irritating
Eye irritation Minimally irritating
Skin sensitization Not sensitizing (Draize test)
Short term toxicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant oral NOAEL Rat: 44 mg/kg bw/per day (diet); 15 mg/kg bw per day
(gavage)
Lowest relevant dermal NOAEL Rabbit: 120 mg/kg bw per day
Lowest relevant inhalation NOAEL Poor study: data on actual intake, particle size not
provided
Genotoxicity Unlikely to be genotoxic; no study of genotoxicity in
mammals in vivo
Long term toxicity and carcinogenicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant NOAEL Dog: 1.7 mg/kg bw per day (2-year study)
Carcinogenicity No evidence of carcinogenicity in mice. The study in
rats was inadequate.
Reproductive toxicity
Reproduction target/critical effect Increased ovary/testis weights (no histological
findings)
Lowest relevant reproductive NOAEL Rat: 21 mg/kg bw per day (reduced body-weight gain,
increased liver weight)
Developmental target/critical effect Increased resorptions, delayed ossification, not
teratogenic
Lowest relevant developmental NOAEL 20 mg/kg bw per day in rabbits; no NOAEL in rats.
A 10 000-fold difference exists between the ADI and
the LOAEL in rats.
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies
Cataractogenicity Photosensitive oculotoxic effects observed in dogs are
not seen in other mammals
Medical data No indication that administration of dicloran at
10 mg/day to men for 90 days had any adverse effect.
Extensive examinations were made on one industrial
worker occupationally exposed to dicloran over three
years, with considerable inhalation and dermal
exposure for about 60 days/year. No adverse effects
were observed
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 2-year study, dogs 200
Acute reference dose Not allocated (unnecessary)
References
Allen, J.A., Brooker, P.C. & Gray, V.M. (1988) Technical dicloran:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. No. SMS 43/871138 from Huntingdon Research
Centre, United Kingdom. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Bachmann, E., Golberg, L. & Thibodeau, L. (1971) Aspects of the
determination of biphenyl hydroxylase activity in liver homogenate.
Exp. Mol. Pathol., 14, 303-326.
Barton, S.J. & Wilcox, S. (1996) Dicloran: Development toxicity in
rabbits. Unpublished report No. 11379 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Barton, S.J. & Wilcox, S. (1997) Dicloran: Two generation reproduction
study in rats. Unpublished report No. 14271 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Basting, L.A., Cooper, C., Witmer, C.M. & Gallo, M.A. (1983) Toxicity
and mutagenicity of 2,6-dichloro-4-nitroaniline in the Ames test.
Fed. Proc., 42, 623.
Basting, L.A., Witmer, C.M., Iba, M.M. & Gallo, M.A. (1984) The effect
of 2,6-dichloro-4-nitroaniline on hepatic microsomal systems.
Toxicologist, 4, 149.
Bernstein, H., Curtis, J.M., Earl, F.L. & Kuwabara, W. (1970)
Phototoxic corneal and lens opacities. Arch. Ophthalmol., 83,
336-348.
Boots Pure Drug Co. (1962) Dicloran: Experimental data on toxicity.
Unpublished report No. T-17 from Boots Pure Drug Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Boyack, G.A. & Brooks, B.W. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Unpublished report
No. T-21 from Upjohn Co., USA. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Cheng, T. (1996a) Nature of the residue of 14C-dicloran in lactating
goats. Unpublished report No. HWI6564-102 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996b) Metabolism of 14C-dicloran in rats. Unpublished
report No. HWI6564-108 from Hazleton Wisconsin, Inc., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996c) Nature of the residue of 14C-dicloran in laying
hens. Unpublished report No. HWI6564-100 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Creedy, C.L., Hemmings, P.A. & Needham, D. (1985) The effect of
dicloran on the hepatic mixed-function oxidase system of the male rat
following oral administration of 100 mg/kg body weight twice daily for
4 days. Unpublished FBC Ltd report No. METAB/85/24, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Curtis, J.M., Bernstein, H., Earl, F.L. & Smalley, H.E. (1968) Corneal
and lens opacities in dogs treated with 2,6-dichloro-4-nitroaniline.
Toxicol. Appl. Pharmacol., 12, 305.
Dawson, J. R. (1988) Metabolism and residues of dicloran in the laying
hen. Unpublished report No. ENVIR/88/26 from Schering Agrochemicals
Ltd, United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
Earl, F.L., Curtis, J.M., Bernstein, H.N. & Smalley, H.E. (1971)
Ocular effects in dogs and pigs treated with dichloran
(2,6-dichloro-4-nitroaniline). Food Cosmet. Toxicol., 9, 819-828.
Eberts, F.S. (1965) Fate of Botran (2,6,-dichloro-4-nitroaniline) in
rat and man. Unpublished report No. M9 from Upjohn Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Eberts, F.S., Meeks, R.C. & Vliek, R.W. (1963) Excretion of
Botran-14C (2,6,-dichloro-4-nitroaniline) by man. Unpublished Report
No. M3 from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Elliot, P.H., Smith, C., Crook, D., Gibson, W.A., Singh, H. &
Gopinath, C. (1988) Technical dicloran: Twenty-one day dermal toxicity
study in rabbits. Unpublished report No. SMS 60/871292 from Huntingdon
Research Centre Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Evans, J., Mengel, G. & Bostwick, L. (1963) Botran (U-2069) Effect of
oral administration. Final report four-months study. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Everest, R.P. & Tuplin, J.A. (1977) Dicloran, pure reference sample:
Mutagenicity testing in bacterial in vitro systems. Unpublished report
No. TX 77024 from Boots Pure Drug Co., United Kingdom. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Gaines, T.B. & Linder, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. Appl. Toxicol., 7, 299-308.
Gallo, M.A., Bachmann, E. & Golberg, L. (1976) Mitochondrial effects
of 2,6-dichloro-4-nitroaniline and its metabolites. Toxicol. Appl.
Pharmacol., 35, 51-61.
Garrett, N.E., Stack, H.F. & Waters, M.D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res., 168,
301-325.
Griffiths, A.F.J., Brockman, H.E., DeMartini, D.M. & de Serres, F.J.
(1986) The efficacy of Neurospora in detecting agents that cause
aneuploidy. Mutat. Res., 167, 35-45.
Gurd, P.R. (1974) Methemoglobin formation in the cat. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Hamilton, D.Y. (1977) Tissue distribution of [14C]-dichloran in rats,
dogs and pigs following repeated oral dosing at 100 mg/kg/day.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Hawkins, D.R., Kirkpatrick, D. & Shaw, D. (1988) The metabolism of
[14C]-dicloran in peaches: Analysis of samples. Unpublished report
No. HRC/SMS from Huntingdon Research Centre, Ltd United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. & Peters, J. (1967) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Natl Cancer Inst., 42, 1101-1114.
Jaglan, P.S. & Arnold, T.S. (1985a) Comparative metabolism of
[14C]-dichloran in rats and goats: Nature of liver residues.
Unpublished report No. 218-9760-85-002A from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jaglan, P.S. & Arnold, T.S. (1985b) Nature of muscle residue from the
treatment of a goat with [14C]-dichloran
(2,6-dichloro-4-nitroaniline). Unpublished report No. 218-9760-85-003
from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jaglan, P.S., Gosline, R.E. & Arnold, T.S. (1985a) Comparative
metabolism of [14C]-dichloran in rats and goats. Unpublished report
No. 218-9760-85-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Jaglan, P.S., Arnold, T.S., Gosline, R.E. & Subacz, C.J. (1985b)
Bioavailability of [14C]-dichloran (U-2069;
2,6-dichloro-4-nitroaniline) derived goat liver residues by the rats.
Unpublished report No. 218-9760-85-004 from Upjohn Co. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
James, P. & Brennan, C. (1991) Technical dichloran: Rabbit oral
developmental toxicity (teratogenicity) range finding study.
Unpublished report No. TOX/91/199-98 (T128) from Schering AG, Germany.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1978) Screening of pesticides for
mutagenicity in microbial systems. Taiwan Plant Protection Center
Research Report No. 12. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1980) Screening of pesticides for
mutagenicity in microbial systems II: with mammalian microsomal
activation. Natl Sci. Council Monthly, 8, 551-559.
Johnston, R.L. & Ceru, J.G. (1961) Acute oral LD50 -- rat and acute
intraperitoneal LD50 -- mouse using DCNA with 0.09% p-nitroaniline.
Unpublished report No. T-16 from Upjohn Co., USA. Submitted to WHO by
Gowan Company, Yuma, Arizona, USA.
Johnston, R.L. & Schwikert, R.S. (1963) Botran: Skin sensitization in
guinea pigs. Unpublished report No. 5567-64-RLJ-196B from Upjohn Co.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jones, E. & Fenner, L.A. (1987) Technical dicloran: Ames bacterial
mutagenicity test. Unpublished report No. TOX/87/199-85 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Kakuk, T. (1986) 90-Day oral toxicity study in B6C3F1 mice with DCNA.
Unpublished report No. 7263/86/003 from Upjohn Co., USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Kakuk, T.J., Weddon, T.W. & Thomas, R.W. (1979) Reevaluation of
potential hepatic effects of Botran in beagle dogs: Supplemental
report. Unpublished report No. 001-9610-79-005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Kappas, A. (1978) On the mechanisms of induced somatic recombination
by certain fungicides in Aspergillus nidulans. Mutat. Res., 51,
189-197.
Lessel, B. (1974) Two-year feeding trial in rats on dicloran.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Lobdell, B. & Johnson, C. (1965) U-2069: Effect on reproduction
capacity through three generations in the rat. Unpublished report from
Woodard Research Corporation. Cited from the 1974 JMPR Monograph
(Annex 1, reference 22).
Mallyon, B.A. & Markham, L.P. (1989) Technical dicloran: Oncogenicity
study in the mouse. Unpublished report No. TOX/86006 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Mallyon, B.A. & Rawcliffe, L.M. (1985) Technical dicloran:
Palatability in the diet to the mouse. Unpublished report No.
TOX/85/199-74 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Mallyon, B.A., Rawliffe, L.M. & Major, I.R. (1986) Technical dicloran:
60-day dietary study in the mouse. Unpublished report No.
TOX/86/199-75 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Marks, T.A., Poppe, S.M., Moerdyk, D.L., Stuckhardt, J.L., Morris,
D.F. & Greenberg, J.H. (1982) U-2069: A segment II teratology study in
the rat. Unpublished report No. 9610/82/7263/005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Mason, J.M., Valencia, R. & Zimmering, S. (1992) Chemical mutagenesis
testing in Drosophila: VIII: Reexamination of equivocal results.
Environ. Mol. Mutag., 19, 227-234.
Maté, C., Ryan, A.J. & Wright, S.E. (1967) Metabolism of some 4-
nitroaniline derivatives in the rat. Food Cosmet. Toxicol., 5,
657-663.
McBride, D. & McGregor, D.B. (1987) Technical dicloran: Assessment of
unscheduled DNA synthesis using rat hepatocyte cultures. Unpublished
report No. 4414 from Inveresk Research International, Musselborgh,
United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Moustacchi, E., Carere, A., Morpurgo, G. Ramel, C. & Würgler, F.E.
(1986) Assays for genetic changes in fungi. In: Montesano, R.,
Bartsch, H., Vainio, H., Wilbourn, J. & Yamasaki, H., eds,
Long-term and Short-term Assays for Carcinogens: A Critical
Appraisal (IARC Scientific Publications No. 83), Lyon, International
Agency for Research on Cancer, pp. 203-249.
Myers-Basting, L.A. (1986) Mutagenicity and characterization of the in
vitro metabolism of 2,6-dichloro-4-nitroaniline. Unpublished report
No. (T101) from Rutgers University, New Jersey, USA. Submitted to WHO
by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I.R. (1991a) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 5 mg/kg body-weight. Unpublished report No.
TOX/90/199-95 (M48) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I. R. (1991b) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 500 mg/kg body-weight. Unpublished report No.
TOX/90/199-96 (M49) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997a) Metabolic fate and distribution of 14C-dicloran in
potatoes. Unpublished report No. 1947 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997b) Metabolic fate and distribution of 14C-dicloran in
lettuce. Unpublished report No. 1949 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Peters, D.H., Stuart, V., Smith, H.L., Crook, D., Gibson, W.A.,
Majeed, S.K. & Gopinath, C. (1991) Technical dicloran: Rat 13-week
dietary toxicity study. Unpublished report No. TOX 89324 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980a) Primary eye irritation evaluation
in New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-001 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980b) Primary dermal irritation study in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T. J. & Wood, D. R. (1980c) Acute dermal toxicity screen in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-003 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Seaman, W.J., Weddon, T.E. & Kakuk, T.J. (1980) Three-week inhalation
study in rats, rabbits and dogs with Botran. Unpublished report No.
218-9610-80-004 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Serrone, D.M., Pakdaman, P., Stein, A.A. & Coulston, F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
rhesus monkeys. Toxicol. Appl. Pharmacol., 10, 404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Smith, S (1989) Metabolism of [14C]-dicloran in peaches under field
and glasshouse conditions. Unpublished report No. ENVIR/89/12 from
Schering Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Strough, A.R. (1962) Botran clinical study. Unpublished report from
Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
US Environmental Protection Agency (1974) Unpublished data from EPA
laboratories. Cited from the 1974 JMPR Monograph, (Annex 1, reference
22).
Waters, M.D., Sandhu, S.S., Simmon, V.F, Mortelmans, K.E., Mitchell,
A.D., Jorgenson, T.A., Jones, D.C.L., Valencia, R. & Garrett, N. E.
(1982) Study of pesticide genotoxicity. Basic Life Sci., 21,
275-326.
Waterson, L.A., Gibson, W.A., Morrow, J. & Gopinath, C. (1992)
Technical dicloran rat 8-week repeat dose study. Unpublished report
No. 91/199-97 from Huntingdon Research Centre, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wazeter, F.X. (1966) Somers test in the albino rabbit. Unpublished
report No. 100-037 from International Research and Development Corp.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wilcox, S., & Barton, S.J. (1996) Dicloran: Range-finding study for a
two generation reproduction study in rats. Unpublished report No.
14271 from Inveresk Research International, United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Woodard, M. W., Cockrell, K.O. & Woodard, G. (1964) Safety evaluation
by oral administration to rats and dogs for 104 weeks. Unpublished
report No. U2069 from Woodard Research Corporation, USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila: V: Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V: results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
DCNA, 2.6-dichloro-4-nitroaniline; HCNA,
2-hydroxy-4-nitro-6-chloroaniline; DCHA,
2,6-dichloro-4-hydroxyaniline; DCAP, 4-amino-2,6-dichlorophenol;
DCNAP; 3,5-dichloro-4-hydroxyacetanilide; DCDP,
2,6-diclorophenylenediamine; DCAA, 4-amino-3,5-dichloroacetanilide;
DCNP, 2,6-dicholoro-4-nitrophenol; DCP, 2,6-dichlorophenol; DCA,
2,6 dichloroaniline; A1, A2, A3, glutathione conjugate deriviatives.
R, G, H, P, S correspond to metabolites in rats, goats, hens, plants,
and soil or sediment, respectively. Major metabolites are represented
by letters in upper-case, minor metabolites are represented by
lower-case letters.
potential to form reactive intermediates after ingestion by rats
(Jaglan & Arnold, 1985a; Jaglan et al., 1985a,b).
(c) Effects on enzymes and other biochemical parameters
Oral administration of dicloran at a dose of 400 or 1000 mg/kg bw
per day to rats for three months resulted in increased hepatic
demethylase and desulfurase activity, and liver mitochondrial oxygen
consumption was increased (Serrone et al., 1967). Dicloran at oral
doses of > 10 mg/kg bw stimulated rat liver mixed-function
oxidases, and a dose of 500 mg/kg bw decreased mitochondrial oxidation
of succinate without concomitant uncoupling of oxidative
phosphorylation. Oxidative phosphorylation was not affected by single
doses at any concentration. Daily administration of 1000 mg/kg bw to
rats induced uncoupling of oxidative phosphorylation after four days
(Bachmann et al., 1971). These liver enzymes were not stimulated in
rhesus monkeys treated with 160 mg/kg bw per day dicloran for three
months (Serrone et al., 1967), and no effect was seen on brain or
liver mitochondrial function in mice (Bachmann et al., 1971).
The 2,6-dichloro-4-hydroxyaniline metabolite was as active as
2,4-dinitrophenol in vitro in uncoupling oxidative phosphorylation,
whereas dicloran was only one-tenth as effective.
2,6-Dichlorophenylenediamine had no effect on mitochondrial
respiration or oxidative phosphorylation in vitro (Bachmann et al.,
1971).
Dicloran and 2,6-dichloro-4-hydroxyaniline at 10-5 mol/L
inhibited electron transport and uncoupled oxidative phosphorylation
in vitro, whereas 2,6-dichlorophenylenediamine at 10-4 mol/L
induced only slight uncoupling. These inhibitory effects in vitro
cannot be considered serious adverse effects since they were not
confirmed by similar observations in vivo. Mitochondria isolated
from rats treated with dicloran or its metabolites were functionally
normal (Gallo et al., 1976).
In a preliminary study, oral doses of 1000 mg/kg bw dicloran per
day for four days to female Sprague-Dawley rats did not significantly
induce cytochrome P450 activity (Basting et al., 1984). After oral
administration of 100 mg/kg bw twice a day for four days to male
Sprague-Dawley rats, however, significant increases were found in the
activities of cytochrome P450 (55% increase), cytochromeb5 (24%
increase), benzphetamine demethylase, and ethoxycoumarin deethylase
(51-68% increases) in comparison with controls. The pattern of enzyme
induction was reported to indicate that dicloran significantly induces
phenobarbital-type enzymes in rat microsomes (Creedy et al., 1985).
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of dicloran are
presented in Table 1. Technical-grade dicloran was of low or slight
acute toxicity by all routes of administration in all species
examined. Oral administration resulted in nasal haemorrhage,
paralysis, depression, and excessive yellow urine and faeces.
Intraperitoneal administration resulted in sleep, depression, and
excessive yellow urine and faeces. The effects of subcutaneous
injection were confined to local damage in which the test substance
became enclosed in a fibrous sac, sometimes followed by abscess
formation and eventual ulceration. Dermal administration resulted in
no systemic clinical effects.
Technical-grade dicloran (purity, 96%) was not irritating to the
intact or abraded skin of male or female New Zealand white rabbits at
a concentration of 500 mg per site (Raczniak & Wood, 1980b) or after
repeated daily applications of 10 mg dry material or 0.1 ml of 10%
aqueous suspension to the abraded or unabraded skin of albino rabbits
over five days (Boots Pure Drug Co., 1962).
Technical-grade dicloran (purity, 96%) caused a minimal
conjunctival response in the eys of New Zealand white rabbits,
consisting of redness and chemosis 24 and 48 h after treatment.
Corneal opacity or injury to superficial layers of the corneal or
conjunctival epithelium was not observed (Raczniak & Wood, 1980c).
Four successive daily applications of 2-3 mg dicloran to the
conjunctival sac of four rabbits resulted in a slight inflammatory
reaction of the cornea, which was of short duration (Boots Pure Drug
Co., 1962).
Dicloran was inactive in two tests for skin sensitization in
guinea-pigs (Boots Pure Drug Co., 1962; Johnston & Schwikert, 1963).
(b) Short-term studies of toxicity
Mice
Groups of Charles River COBS mice were fed diets containing 0,
1250, 2500, 5000, or 10 000 ppm technical-grade dicloran for two
weeks, equivalent to 0, 190, 380, 750, and 1500 mg/kg bw per day. At
10 000 ppm, 20% of animals of each sex died; the only clinical sign
observed was hunched posture. Food consumption and body weight were
significantly reduced. Female mice fed 5000 or 10 000 ppm had
significantly increased absolute liver weights, and animals of each
sex fed doses of 2500 ppm or more had an increased liver:body-weight
ratio. Food consumption was slightly reduced in animals of each sex at
2500 and 5000 ppm, and a slight reduc-tion in food consumption was
noted at 1250 ppm only in females (Mallyon & Rawcliffe, 1985).
Table 1. Acute toxicity of dicloran
Species Strain Sex Route LD50 Reference
(mg/kg bw)
Rat Sherman SPF M/F Oral > 4000 Gaines & Linder (1986)
Rat NR NR Oral 4040 Boots Pure Drug Co. (1962)
Rat NR NR Oral ~ 8000 Serrone et al. (1967)
Rat NR NR Oral > 10 000 Johnston & Ceru (1961)
Mouse NR NR Oral 1500-2500 Boots Pure Drug Co. (1962)
Guinea-pig NR NR Oral 1400 Boots Pure Drug Co. (1962)
Rabbit New Zealand white M/F Dermal (abraded) > 2000 Raczniak & Wood (1980a)
Mouse NR NR Intraperitoneal 5500 Johnston & Ceru (1961)
Mouse NR NR Intraperitoneal 2500 Boots Pure Drug Co. (1962)
Rat NR NR Intraperitoneal 1500 Boots Pure Drug Co. (1962)
Rat NR NR Subcutaneous > 5000 Boots Pure Drug Co. (1962)
Mouse NR NR Subcutaneous > 6000 Boots Pure Drug Co. (1962)
SPF, specific pathogen-free; M/F, male and female; NR, not reported
Groups of 10 male and 10 female CD-1 (ICR) mice received dicloran
(purity, 95.8%) in the diet at 0, 300, 600, or 1200 ppm, equal to 0,
49, 100, and 200 mg/kg bw per day in males and 0, 53, 100, and 220
mg/kg bw per day in females, for 60 days. There were no
treatment-related effects on survival, clinical signs, body weight,
food or water consumption, or food conversion. A significant increase
in methaemoglobin concentrations in the blood of both males and
females was noted at 600 and 1200 ppm (males: 1, 1.3, 1.4, and 1.6%;
females: 0.6, 0.7, 0.9, and 1.2%, control to high dose, respectively).
Cholesterol and bilirubin concentrations were significantly increased
in high-dose females (cholesterol: 2.4 ± 0.58, 2.2 ± 0.50, 2.5 ± 0.2,
3.0 ± 0.65 mmol/L; bilirubin: 4.3 ± 0.6, 5.4 ± 1.4, 5.5 ± 2.4, 5.8 ±
1.7 mol/L, control to high dose, respectively). These changes were
dose-related but were not observed in males, which, unlike females,
had specific liver lesions. Minimal to slight centrilobular
hepatocytic enlargement (2-5/10 versus 0/10 controls) and fatty liver
degeneration (2-4/10 versus 0/10 controls) with an increased severity
of haemosiderosis in the spleen was noted in males at 600 and 1200
ppm, and a slight increase in the degree of haematopoietic activity
was noted in the spleen of males and females combined at the
intermediate and high doses. The NOAEL was 300 ppm, equal to 49 mg/kg
bw per day in males (Mallyon et al., 1986).
Dicloran (purity, 97%) was administered to groups of 15 B6C3HF1
mice of each sex (five of each sex per dose for interim sacrifice) by
gavage at doses of 0, 15, 45, 135, 400, or 600 mg/kg bw per day for 90
days, with an interim sacrifice at 49 days. There were no
treatment-related effects on survival or body weight; data on food
consumption were not provided. Significant polycythaemia was seen in
males, and hypercholesterolaemia at 1.2-2.1 times the control level
and a significant increase in the incidence of splenic extramedullary
haematopoiesis were seen in females at > 45 mg/kg bw per day.
Dose-related increases in cholesterol concentrations (1.3-2.3 times
the control concentration) were also noted in males, which were
statistically significant at > 135 mg/kg bw per day. A deep-yellow
colouration of the urine was noted in all treated groups and was
attributed to the yellow colour of the test material. In the absence
of any associated lesion in the group at the lowest dose, this effect
was considered not to be adverse. Other effects at higher doses
included kidney nephrosis in males at 400 and 600 mg/kg bw per day (5,
4, 6, 5, 15, 14/15, control to high dose, respectively) and females
(3, 5, 2, 4, 8, 12/15), with kidney tubular regeneration in males (0,
0, 3, 1, 8, 11/15), increased liver weights in animals of each sex at
> 135 mg/kg bw per day (7-64% increase), hepatocyte hypertrophy in
males (0, 0, 0, 4, 15, 15/15) and females (0, 0, 0, 0, 5, 12/15) at
> 400 mg/kg bw per day, liver necrosis in males (0, 0, 0, 0, 3,
3/15) and females (0, 0, 0, 0, 1, 1/15) at 400 and 600 mg/kg bw per
day, a significantly higher incidence of splenic extramedullary
haematopoiesis in males at > 135 mg/kg bw per day (0, 1, 2, 13, 15,
15/15) and in females at > 45 mg/kg bw per day (2, 5, 9, 10, 14,
15/15), increased spleen weight at the two higher doses (11-29%
increase), and epithelial hyperplasia of the urinary bladder in males
(0, 1, 2, 10, 12, 15/15) and females (0, 0, 0, 1, 7, 13/15) at doses
> 135 mg/kg bw per day. The NOAEL was 15 mg/kg bw per day (Kakuk,
1986).
Rats
Dicloran (unspecified purity) was administered by gavage to rats
(strain unspecified) at 400 or 1000 mg/kg bw per day for three months.
Deaths occurred among rats at the high dose. Significantly enlarged
livers and unspecified renal changes were observed by light and
electron microscopy, with increases in liver mitochondrial enzyme
activity and mitochondrial oxygen use (Serrone et al., 1967).
Groups of five newly weaned rats (strain unspecified) of each sex
were given dicloran (purity unspecified) by gavage at doses of 0, 140,
or 350 mg/kg bw per day, six days/week for four weeks. The growth of
males at 350 mg/kg bw per day was reduced to 60% of the control value.
At both doses, increased liver weight (120-155% of control) with
hepatic vacuolation were noted. There was no apparent effect on blood
parameters or on the kidneys at the end of the study (Boots Pure Drug
Co., 1962).
Groups of 10 rats (strain unspecified) were given dicloran
(purity unspecified) by gavage at doses of 35, 140, or 350 mg/kg bw
per day, six days/week, for four weeks. Groups of 20 controls of each
sex were available. Growth depression was observed in animals of each
sex at 350 mg/kg bw per day and in males at 140 mg/kg bw per day.
Increased liver weights and microscopic findings were also seen at
these doses. The liver lesions included a dose-related increase in
hepatocytic hypertrophy with increased vacuolization, especially in
the outer lobes. The liver had returned to normal size in half of the
animals which were maintained for two weeks after the end of the
treatment, except for males at the highest dose. The histopathological
changes in the liver caused by repeated short-term dosing was
apparently reversible within two weeks of the end of treatment. The
NOAEL was 35 mg/kg bw per day on the basis of effects on body weight
and liver at 140 mg/kg bw per day (Boots Pure Drug Co., 1962).
Technical-grade dicloran was fed to rats (strain unspecified) for
six months at doses of 0 (25 rats), 30 (15 rats), 300 (15 rats), or
3000 (10 rats) ppm, equal to 0, 2.2, 22, and 230 mg/kg bw per day in
males and 0, 2.5, 25, and 270 mg/kg bw per day in females. Another
10 animals of each sex per group received recrystallized dicloran at
3000 ppm, equivalent to 230 mg/kg bw per day for males and 260 mg/kg
bw per day for females. At 3000 ppm, growth of both males and females
was impaired (70-83% of control), whereas males fed purified material
had only a slight reduction in growth (89% of control). Significantly
increased liver weights (120-144% of control) were noted in both
groups at the high dose, with a significant increase in spleen weight
(129% of control) in females. Haematological parameters were not
affected at 12 weeks or at termination, and no microscopic lesions
were noted. The NOAEL was 300 ppm, equal to 22 mg/kg bw per day, on
the basis of changes in body weight and increased liver and spleen
weights at 3000 ppm (Boots Pure Drug Co., 1962).
Dicloran (purity, 97.4%) was administered to groups of 10 male
and 10 female Sprague-Dawley rats in the diet at doses of 0, 1000,
3000, or 5000 ppm, equal to 0, 75, 230, and 370 mg/kg bw per day in
males and 0, 80, 230, and 420 mg/kg bw per day in females, for 90
days. There were no treatment-related effects on survival or
ophthalmological parameters. Significantly lower body-weight gain was
seen in all treated groups (17-32% less than controls for males and
18-22% less for females). Although palatability may have accounted for
the lower body-weight gains during the first week of the study, food
consumption was comparable in all groups at week 2; however, it was
consistently lower than control values throughout the remainder of the
study (males, 10-14% less than controls; females, 12-21%; weeks 2-13).
Many of the haematological and clinical chemical findings were
considered to be associated with reductions in food consumption. The
treatment-related hepatic effects included increased cholesterol
concentrations, which were dose-related and statistically significant
in males at all doses (1.3-1.5 times the control concentration) and in
females at the intermediate and high doses (1.4-2.0 times control),
minimal to moderate centrilobular hypertrophy in males in all treated
groups (0, 3, 9, 9/10) and in females at the two higher doses (0, 0,
9, 9/10), and significant increases in liver weight in males and
females at the two higher doses (29-66%). Minimal thyroid
follicular-cell hypertrophy was noted in males in all treated groups
(0, 5, 6, 6/10) and in females at the intermediate and high doses (0,
0, 4, 7/10). The dose of 1000 ppm, equal to a daily intake of 75 and
80 mg/kg bw per day for males and females, respectively, was
considered to be at the upper limit of a suitable high-level dose for
a long-term study of toxicity and carcinogenicity in rats. An NOAEL
was not identified because significantly lower body-weight gains were
seen at all doses (Peters et al., 1990).
A second short-term study was conducted to investigate the
effects of dicloran at doses lower than those administered in the
90-day study. Dicloran (purity, 96.4%) was given in the diet to groups
of 10 Sprague-Dawley rats of each sex at doses of 0, 500, or 750 ppm,
equal to 0, 44, and 71 mg/kg bw per day for males, and 0, 48, and 71
mg/kg bw per day for females, for eight weeks. There were no
treatment-related effects on survival. The overall body-weight gain of
females at 750 ppm was significantly less than that of controls (12%
less overall), the greatest difference occurring during the final two
weeks of treatment (41-58% less than control). This decrease
correlated with a slight decrease in food consumption in this group
during treatment, which was 10-11% less than that of controls during
the last two weeks of treatment. Increased liver weight relative to
body weight (13-21%) in animals of each sex at 750 ppm was associated
in 10/10 males with minimal centrilobular hepatocyte enlargement. The
NOAEL was 500 ppm, equal to 44 mg/kg bw per day, on the basis of lower
body-weight gain and decreased food consumption in females at 750 ppm
and increased liver weights in males and females, with associated
centrilobular hepatocyte enlargement in males at 750 ppm; however, a
full histological examination was not carried out (only of the liver
and thyroid), and although alterations were found in the livers of
males at the high dose, the livers of males at the low dose were not
examined histologically (Waterson et al., 1992).
Rabbits
Technical-grade dicloran (purity, 96.2%) was moistened with
distilled water and applied to the intact skin of groups of five male
and female New Zealand white rabbits under an occluded patch for 6 h
per day for 21 consecutive days at doses of 12, 120, or 1200 mg/kg bw
per day. Animals at the two higher doses showed yellow staining of
untreated fur and extremities from day 4 onwards, but there were no
adverse clinical findings. One male and one female rabbit at 12 mg/kg
bw per day had slight, transient erythema lasting for two to four
days, and most rabbits at 120 and 1200 mg/kg bw per day had slight
erythema beginning in the second week of the study, which tended to
persist until study termination. The reaction did not progress beyond
slight erythema, and there did not appear to be a dose-related
difference above 120 mg/kg bw per day. At termination, male rabbits at
1200 mg/kg bw per day had significantly greater (36%) adrenal weights
than controls, but the adrenals were not examined histologically. The
liver weights were 18% greater in females at 1200 mg/kg bw per day
than in controls. No other effects were seen on body weight, food
consumption, or haematological, biochemical, or histological
parameters. The NOAEL was 120 mg/kg bw per day (Elliot et al., 1988).
Rats, rabbits, and dogs
Groups of 10 male and 10 female TUC/SPD rats, one male and one
female New Zealand white rabbit, and two male beagle dogs were exposed
to a dust aerosol of technical-grade dicloran by whole-body exposure
for 6 h per day, five days per week for three weeks. The nominal
concentration of the aerosol was 2 mg/L; the actual concentration and
particle size were not provided. Two rats died on the third day of
exposure, and one rabbit died on day 13. The food consumption and
body-weight gains of the treated animals were depressed. There were no
consistent haematological changes attributable to treatment. The blood
cholesterol concentration was significantly elevated in exposed dogs
and rabbits. The liver weights of exposed animals were increased,
although the histological appearance was unremarkable, with no
evidence of hepatocellular effects in any species (Seaman et al.,
1980).
Monkeys
Oral doses of 160 mg/kg bw per day were lethal to rhesus monkeys
within three months, with a greater effect on females than males. The
colouration of the urine differed from that of rat urine, suggesting a
difference in metabolism in the two species. Centrilobular fatty
infiltration was observed in the liver, and liver and kidney changes
were observed on electron microscopic examination, which included
swelling of mitochondria with distortion of the cristae. Hepatic
microsomal enzyme activity was not enhanced (Serrone et al., 1967).
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a screening study, groups of 18 mice of each sex of each of
two susceptible hybrid strains (unspecified) were given dicloran at
215 mg/kg bw per day for three weeks from day 7 after birth.
Thereafter, for 18 months, the mice were fed 600 ppm (equivalent to
100 mg/kg bw per day) in the diet, sacrificed, and examined for
tumours. Dicloran did not cause a significant increase in the
incidence of tumours (Innes et al., 1969).
Dicloran (purity, 96.2-97.2%) was administered to groups of 50
CD-1 mice of each sex at dietary concentrations of 0, 50, 175, or 600
ppm, equal to 0, 7.4, 24, and 86 mg/kg bw per day in males and 0, 10,
36, and 120 mg/kg bw per day in females, for 80 weeks. The doses were
based on a the results of a 60-day dietary study in mice in which a
concentration of 600 ppm was associated with increased methaemoglobin
concentration, splenic pigment change, increased haematopoiesis,
centrilobular hepatocyte enlargement, and fatty degeneration of
hepatocytes. There was no adverse effect on mortality (percent
survival at termination: males, 50, 50, 64, and 56%; females, 84, 76,
76, and 78%, control to high dose, respectively). There were no
treatment-related effects on clinical signs, body weight, palpable
masses, food consumption, food conversion, or differential leukocyte
counts. Clinical chemistry and urinalysis were not performed. There
was no increase in tumour incidence. The liver was the principal
target organ, with increased absolute and relative weights (10-12%) in
males and females at the high dose, which were statistically
significant in females, and histopathological changes. These included
centrilobular hepatocyte enlargement (males: 8, 8, 7, and 26/50;
females: 1, 0, 2, and 5/50), centrilobular haemosiderosis (males: 1,
2, 4, and 12/50; females: 7, 5, 3, and 13/50), focal (4, 2, 5, and
10/50) and single-cell (1, 1, 0, and 6/50) liver necrosis in males,
and vacuolation of centrilobular hepatocytes (4, 3, 3, and 12/50) in
females. Acute inflammatory cell infiltration was seen in males at the
intermediate and high doses (2, 4, 9, and 9/50), but no other hepatic
changes were seen in males at this dose. Other changes at 600 ppm
included a higher incidence of erythropoiesis in the spleens of males
(7, 7, 8, and 15/50), an increased number of females with an
enlarged/distended uterus (5, 9, 9, and 15/50), associated with a
significant increase in the incidence and severity of cystic uterine
endometrial hyperplasia (17, 21, 24, and 31/50), and an increased
number of females at the high dose with distended mammary gland ducts
(9/50 versus 3/50 in control; animals at the low and intermediate
doses were not assessed). An 11% increase in kidney weight in males
was not associated with histopathological lesions. On the basis of the
above findings, the NOAEL was 175 ppm, equal to 24 mg/kg bw per day
(Mallyon & Markham, 1989).
Rats
Groups of 35 rats (strain unspecified) of each sex were fed
dicloran (purity unspecified) in the diet at concentrations of 0, 20,
100, or 3000 ppm for two years, equal to 0, 1.6, 8, and 240 mg/kg bw
per day, with an interim sacrifice of five animals of each sex per
dose at 13 weeks. The dose of 100 ppm had no effect on behaviour,
mortality, or growth, and all values were comparable to those of
controls. At 3000 ppm, growth and food consumption of males and
females were depressed, and haematological parameters (haemoglobin and
packed cell volume) were reduced but only after the first year of
treatment. Gross and microscopic examination at 13 weeks and at the
conclusion of the study showed slightly heavier livers, kidneys, and
testes in males at 3000 ppm. The incidence and location of neoplasms
were similar in treated and control rats. Histological examination
revealed changes in the liver at 3000 ppm, characterized by
hepatic-cell enlargement, increased severity of glycogen depletion,
increased basophilia of the cytoplasm, and the presence of dead cells.
Other observations were increased thyroid hypertrophy and increased
pigmentation of the spleen in males and females at 3000 ppm.
Hepatic-cell changes and slight adrenal cortical atrophy were seen in
several animals at this dose at 13 weeks, but the adrenal changes were
not seen at 104 weeks. The NOAEL was 100 ppm, equal to 8 mg/kg bw per
day (Woodard et al., 1964).
Groups of 25 Boots-Wistar strain rats of each sex were fed
dicloran (purity unspecified) in the diet at a concentration of 0 or
1000 ppm for two years, equal to 59 mg/kg bw per day in males and 71
mg/kg bw per day in females. There was no effect on survival, food
consumption, growth, haematological parameters, or gross or
histological appearance of tissues and organs at the conclusion of the
study. There were no differences in the size or cellular constitution
of the liver, kidney, or spleen. The incidence of tumours in control
and treated groups was similar. The NOAEL was 1000 ppm, equal to 59
mg/kg bw per day, the highest dose tested (Lessel, 1974).
Dogs
Groups of four male and four female beagle dogs were fed dry
diets containing dicloran (purity unspecified) at concentrations of 0,
20, 100, or 3000 ppm for two years, equal to 0, 0.33, 1.7, and 44
mg/kg bw per day for males and 0, 0.36, 1.8, and 62 mg/kg bw per day
for females, with an interim sacrifice of one animal of each sex per
dose at 14 weeks. No compound-related changes in behaviour, food
consumption, or growth were observed. One female at 3000 ppm died at
74 weeks, with treatment-related signs of haemolytic anaemia (reduced
haemoglobin, immature erythrocytes, polychromophilic macrocytes,
increased leukocyte count, and increased myeloid: erythroid ratio in
the bone marrow) before death. One control male lost considerable
weight but survived to the end of the study. All dogs at 3000 ppm had
watery lachrymation, which persisted throughout treatment; the
examination of the eyes was not adequate to determine any ocular
toxicity of dicloran. Yellowing of the sclera, mucous membranes, and
abdominal skin were noted at the high dose. Changes in haematological
and clinical chemical parameters in males and females at the high dose
included significant reductions in haemoglobin from week 26 to
termination and reduced serum protein at termination; increased
activity of alanine and aspartate aminotransferases and increased
prothrombin time, blood urea nitrogen, and bromosulphthalein retention
were observed in one male and the one female that died at the high
dose, both of which had received 20-94% more test material than the
other dogs in this group owing to gradual body-weight loss relative to
the constant concentration in the diet. Gross and microscopic
examination revealed significantly increased liver, spleen, and kidney
weights accompanied by histological changes at 3000 ppm, which
included irregular hepatic-cell size (3/5 versus 0/6 in other groups),
moderate hepatic-cell hypertrophy (3/5 versus 0/6 in controls) and
increased pigmentation of the liver (4/5 versus 0/6 in control), and
spleen (5/5 versus 0/6 in controls). One male and two females at 3000
ppm (including the female that died) and one male at 20 ppm also had
enlarged gall-bladders containing tar-like, viscous bile, although
this was stated to be a spontaneous lesion in dogs (cystic mucinous
hypertrophy). Changes at 100 ppm which included irregular hepatic
cells, slight focal hepatocytic hypertrophy, slight vacuolation,
moderate liver pigmentation, and/or marked spleen pigmentation were
noted in two female dogs. A company re-evaluation attributed the
findings at 100 ppm to enzyme induction, although the
histopathological findings in this group were complicated by the
presence of parasitic hepatitis. No degenerative lesions were noted at
study termination, and there was no progression in the severity of
hepatocellular effects between interim and terminal sacrifice. The
NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day, on the basis of
significant reductions in haemoglobin, reduced serum protein, and
histopathological changes in the liver and spleen at 3000 ppm (Woodard
et al., 1964; Kakuk et al., 1979).
(d) Genotoxicity
The results of studies on the mutagenicity and genotoxicity of
dicloran are presented in Table 2. Dicloran gave occasional positive
responses in the most recent tests for reverse mutation and in a test
for mitotic recombination, with a 2-2.5-fold increase in mitotic
recombination. The results of the test for sex-linked recessive lethal
mutation in Drosophila were equivocal, even after re-analysis in
conjunction with historical control data.
(e) Reproductive toxicity
(i) Single-generation reproductive toxicity
Male rats (strain unspecified) were treated with dicloran (purity
unspecified) in the diet at concentrations of 0, 1000, or 2000 ppm,
equivalent to 0, 50, and 100 mg/kg bw per day, for 90 days. The dose
of 1000 ppm increased liver and kidney weights. When the males were
mated with untreated females, no difference was observed in the number
Table 2. Results of assays for the genotoxicity of dicloran
End-point Test object Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, 16, 31, 62, 125, 99.9 Negativeb Everest & Tuplin (1977)
TA100, TA1535, 250, 500, 1000
TA1537, TA1538 µg/plate in DMSO
Reverse mutationa S. typhimurium 100 µg/plate in NR Negative Shirasu et al. (1976)
TA1535, TA1536, DMSO
TA1537, TA1538
Reverse mutationc S. typhimurium TA98, 20-200 µg/plate 99.5 Negatived Jeang & Li (1978)
TA100 in DMSO
Reverse mutatione S. typhimurium TA98, 2, 20, 200 µg/plate 99.5 Negativef Jeang & Li (1980)
TA100 in DMSO
Reverse mutationa S. typhimurium TA98, 6 concentrations NR Negative Waters et al. (1982);
TA100, TA1535, up to 10 000 µg/plate Garrett et al. (1986)
TA1537, TA1538
Reverse mutationa S. typhimurium TA98, 62, 100, 155, 200, NR Positiveg Basting et al. (1983);
TA98 NRF, TA100, 310, 415 µg/plate in Myers-Basting (1986)
TA100 NRF ethyl cellosolve/ethanol
or DMSO
Reverse mutationa S. typhimurium TA98, 50, 150, 500, 1500, 97.5 Positiveh Jones & Fenner (1987)
TA100, TA1535, 5000 µg/plate in
TA1537, TA1538 DMSO
Reverse mutationa S. typhimurium TA98, 10, 33, 100, 333, 98 Positivei Zeiger et al. (1992)
TA100 1000, 2000 µg/plate
in DMSO
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Reverse mutationc E. coli WP2, WP2uvrA- 62, 125, 250, 500, 99.9 Negativej Everest & Tuplin (1977)
1000 µg/plate in
DMSO
Reverse mutationc E. coli B/r try WP2, 20 µg/plate in DMSO NR Negative Shirasu et al. (1976)
WP2 try hcr
Reverse mutationa E. coli WP2uvrA- 6 concentrations NR Negativek Waters et al. (1982);
up to 10 000 µg/plate Garrett et al. (1986)
Mitotic recombationa S. cerevisiae D3 5 concentrations NR Negative Waters et al. (1982)
(unspecified)
Mitotic aneuploidy Neurospora crassa 5 or 10 µg/ml NR Negativel Griffiths et al. (1986;
I-41-5, I-34-8 in DMSO Moustacchi (1986)
DNA repairc B. subtilis H17 rec+, 20 g/disc in DMSO NR Negative Shirasu et al. (1976)
M45 rec-
DNA repaira B. subtilis H17 rec+, 20 g/disc in DMSO 99.5 Negativem Jeang & Li (1978)
M45 rec-
DNA repairc B. subtilis H17 rec+, 2 concentrations NR Negativem Waters et al. (1982);
M45 rec- (not specified) Garrett et al. (1986)
DNA repairc E. coli P3478 (polA-), 2 concentrations NR Negativem Waters et al. (1982);
W3110 (polA+) (not specified) Garrett et al. (1986)
Unscheduled DNA Rat hepatocytes 3, 4, 5, 6, 7, 8, 9, 97.5 Negativen McBride & McGregor
synthesis 10 µg/ml in DMSO (1987)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Mitotic recombination Aspergillus nidulans 2070, 4140, 6210 NR Positiveo Kappas (1978);
and aneuploidy (heterozygous diploid µg/plate in ethanol Moustacchi (1986)
strain)
Cytogenetic Human lymphocytes 2, 10, 20 µg/ml in 97.5 Negativep Allen et al. (1988)
alterationsa DMSO
In vivo
Recessive lethal Drosophila melanogaster Feeding at 1250 and 98 Equivocal Woodruff et al. (1985)
mutation Canton-S 1389 ppm in 5%
ethanol:5% Tween 80
Recessive lethal Drosophila melanogaster Re-analysis of data NR Equivocal Mason et al. (1992)
mutation Canton-S of Woodruff et al.
(1985)
NR, not reported; DMSO, dimethyl sulfoxide; NRF, nitroreductase-free
a With and without metabolic activation
b The positive controls, cyclophosphamide, 6-aminochrysene, and 2-aminofluorene, gave the expected results.
c Without metabolic activation
d The positive control nitroquinoline N-oxide gave the expected result.
e With metabolic activation
f The positive control sterigmatocystin gave the expected result.
g Positive in TA98 (> 100 µg/plate) only without metabolic activation
h Positive in TA98 and TA1538 (> 500 µg/plate) with and without metabolic activation and in TA100 without metabolic
activation. The positive controls, N-ethyl-N'-nitro-N-nitrosoguanidine, 9-aminoacridine, 2-aminoanthracene, and
2-nitrofluorene, gave the expected results.
i Positive in TA98 (> 100 µg/plate) with and without metabolic activation; positive control unknown
j The positive control ethyl methanesulfonate gave the expected result.
Table 2. (continued)
k The positive controls, 2-aminoanthracene and 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide, gave the expected results.
l This test system has not been fully validated. The positive control para-fluorophenylaniline gave the expected result.
m The positive control methyl methanesulfoxide gave the expected result.
n The positive control, Mischler's ketone [4,4'-bis(dimethylaminobenzophenone) in ethanol], gave the expected result.
o A 2-2.5-fold increase in mitotic recombination. This test system has not been fully validated.
p The positive controls, ethyl methanesulfonate and cyclophosphamide, gave the expected results.
of litters or in the number of animals born or weaned (US
Environmental Protection Agency, 1974).
Dicloran (purity unspecified) was fed to groups of 10 female rats
at concentrations of 0, 500, or 1000 ppm, equivalent to 0, 25, and 50
mg/kg bw per day, for 188 days before mating and then through
gestation and lactation. A reduced number of pups was reported when
females were treated with 1000 ppm. There was no apparent effect on
the survival of pups, although the mean body weight of those at 1000
ppm was slightly reduced (US Environmental Protection Agency, 1974).
(ii) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity, with one
litter per generation, dicloran (purity, 99.2%) was administered to
groups of 28 male and 28 (F0) and 24 male and 24 female (F1) female
Sprague-Dawley rats in the diet at doses of 0, 50, 250, or 1250 ppm,
equal to 0, 4, 21, and 110 mg/kg bw per day. The doses were based on
the results of a range-finding study (Wilcox & Barton, 1996) in which
concentrations up to 1000 ppm did not produce any obvious alterations
in body weight, food consumption, duration of gestation, or litter
parameters. The animals were fed the test diet during the 10- (F0) or
11-week (F1) pre-mating period and then randomly allocated to mating
pairs (l:1) for a maximum of seven nights. Treatment was continued for
animals of each sex throughout the mating, gestation, and lactation
periods until termination of the F2 litters.
The treatment-related clinical signs included yellow staining of
the tray paper and, in some cases, yellow staining of the fur of all
animals at the high dose in both generations, due to the yellow colour
of the test material. There were no significant differences in mean
body weights or body-weight gain between control and treated males.
The mean body weights of F0 females at the high dose throughout the
pre-mating period was 2-5% lower than that of controls, resulting in a
12% lower mean overall gain at the end of the pre-mating period. This
was considered to be treatment-related. The mean body weights of F1
females at the high dose were, on average, 6% lower than those of
controls during pre-mating, but the overall mean body-weight gain was
only 5% lower. F0 and F1 females at the high dose had slightly lower
overall body-weight gain during gestation, the greatest difference
occurring during the first week of gestation (64% of control values in
F0 females and 86% in F1). There were no apparent effects on the
food consumption of F0 or F1 males or females during pre-mating and
gestation; however, the food consumption of females of both
generations at the high dose was 11% lower during the first week of
lactation, and these animals weighed more than controls and those
given lower doses at the end of lactation, indicating that pups in the
high-dose group were less demanding with respect to suckling than pups
in the other groups.
At the end of the study, a greater proportion of F0 and F1
females at the high dose were in pro-estrus (4/28 F0 controls versus
19/28 at the high dose; 4/24 F1 controls versus 11/24 at the high
dose). Since there was no indication of altered cycling, this result
was considered to be of no toxicological concern. Sperm-stage analysis
indicated no difference between control F0 and F1 males and those at
the high dose with respect to the number of testicular tubules at
various spermatogenic stages or in tubule diameter. There was a
dose-related increase in the incidence of abnormal sperm, particularly
with respect to tail defects, in F0 males (0.25 ± 0.26 and 0.29 ±
0.31% in the two control groups, 0.59 ± 0.96% at the low dose, 0.6 ±
0.65% at the intermediate dose, and 0.67 ± 0.58% at the high dose);
however, no such increase was observed in F1 males at the high dose
(0.21% versus 0.23% in controls). Furthermore, the mean values for F0
animals were within the historical control range, 0.0-0.8%. Thus,
these findings were considered incidental. The fertility and
gestational indices of control and treated F0 and F1 females were
similar. The male fertility indices of F0 males at the intermediate
and high doses were lower than that of controls (82 and 86%,
respectively), as was that of F1 males at the low dose (75%). Since
the fertility index of F1 males at the intermediate and high doses
approached 100%, the lower fertility indices were considered
incidental.
There were significant increases in absolute and body
weight-adjusted mean liver weights in F1 males and females at the
high dose; no data were collected for F0 animals. The weights of the
kidneys of F1 animals at the high dose were increased, and the
increase was significant in males. Brain weights were significantly
lower in F1 females at the high dose, and thymus weights were
significantly lower in F1 males and females at the high dose relative
to controls. No microscopic examinations were conducted on these
tissues. The mean absolute epididymal weights of F0 males at the high
dose and the absolute and body weight-adjusted epididymal weights of
F1 males at the intermediate and high doses were significantly lower
than those of controls. The authors indicated that the values for the
F1 males were similar to 'recent control values for animals of
similar age'; however, data on these controls were not provided. The
mean absolute and body weight-adjusted testicular weights were
statistically significantly increased in F1 males at the high dose
and non-significantly increased in F0 males at this dose. There was a
dose-related decrease in mean ovarian weights in F1 females, which
was statistically significant in the high-dose group. No
treatment-associated histopathological lesions were seen in the
reproductive tissues that were examined. In the absence of
histological data on non-reproductive tissues, the toxicological
significance of the observed changes in organ weights in these tissues
is equivocal.
No difference was observed in the mean number of implantation
sites per litter, litter size, sex ratio, or litter indices in control
and treated groups. A slight increase in the number of F1 pups that
died during days 4-21 of lactation was not observed in the F2
generation and was considered to be incidental. The group mean weights
of F1 and F2 litters were decreased throughout the lactation period,
as reflected in the lower mean pup body weights at this dose. Although
the magnitude of the difference was not as great in F2 pups as in F1
pups, these observations were consistent and considered to be related
to treatment. The mean litter weights of dams at the low and
intermediate doses were lower than those of controls on day 1 of
gestation, but the mean pup weights were comparable to those of
controls on days 1-21 of lactation, in contrast to the high-dose
group, and were therefore considered not to be related to treatment.
There were no treatment-related clinical observations or
malformations.
The NOAEL for systemic toxicity was 250 ppm, equal to 21 mg/kg bw
per day, on the basis of reduced body-weight gain at 1250 ppm in
females during pre-mating and gestation and increased liver and kidney
weights in males and females at this dose. The NOAEL for reproductive
and developmental toxicity was also 250 ppm, equal to 21 mg/kg bw per
day, on the basis of lower F1 and F2 pup weights at the next highest
dose. There were no histopathological lesions in the reproductive
tissues that would indicate that the changes in organ weights should
be considered adverse (Barton & Wilcox, 1997).
In a three-generation study, with two litters per generation,
dicloran (purity unspecified) was administered to groups of 20 rats
(strain unspecified) of each sex at 0 or 100 ppm, equivalent to 5
mg/kg bw per day, in the diet. On the basis of the reproduction
parameters examined, including the numbers of litters, stillbirths,
live births, birth weight, and lactation indices, dicloran had no
effect on reproduction (Lobdell & Johnston, 1965).
(iii) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley rats (Upj:TUC (SD) SPF) were
dosed by gavage with dicloran (purity, 93.7%) in 0.25% carboxymethyl
cellulose and 0.5% Tween 20 at doses of 0, 100, 200, or 400 mg/kg bw
per day on days 6-15 of gestation, the day of mating being considered
gestation day 0. These doses were derived from the results of a
range-finding study in which 5/5 rats died at doses of 2000 and 4000
mg/kg bw per day, and 3/5 at 1000 mg/kg bw per day. Neither of the two
survivors treated with 1000 mg/kg bw per day had pups, and one had 15
early resorptions. The mean body-weight gain of females treated with
500 mg/kg bw per day was 25% less than that of controls.
The dams were sacrificed on day 20 of gestation, and the uterine
contents were examined; the uteri of non-pregnant dams were stained
with ammonium sulfide, and all fetuses were assessed for viability,
sex, weight, and external malformations and variations. Equal numbers
of fetuses from each group were assigned to either visceral or
skeletal examination. There were no deaths. The treatment-related
clinical signs included yellow staining of the urogenital region in
some animals at each dose due to the colour of the test material, and
slight central nervous system depression throughout the treatment
period in animals at 200 and 400 mg/kg bw per day. The mean
body-weight gain (corrected for gravid uterine weights) of treated
animals was significantly lower (16-33% less) than that of the the
controls. The incidence of dams with totally resorbed litters (early
implants only) was increased in all treated groups (1, 5, 9, 10;
control to high dose, respectively); however, there were no
differences in the mean numbers of implants or resorptions or in the
mean litter sizes of dams with live litters. The fetal weights were
significantly lower at 200 and 400 mg/kg bw per day (7 and 20% less
than control, respectively), and a significant trend for an increased
incidence of delayed or reduced ossification was noted in these two
groups. Increased incidences of other skeletal variations (bipartite
sternebrae) were also seen at the high dose, but there were no
teratogenic effects. (The authors maintained that the low dose was not
maternally or embryotoxic. The conclusion concerning embryotoxicity
was based on statistical significance rather than biological
significance, and an erroneously large control value appears to have
been used for statistical comparison.) On the basis of the
significantly lower mean body-weight gains and the higher incidences
of dams with totally resorbed litters (i.e. early implants only) in
all treated groups, an NOAEL was not identified for maternal or
developmental toxicity. (The authors indicated that
sialodacryoadenitis viral infection was present during much of the
dosing period and indicated that this may have had some effect on
weight gain; however, in previous studies this virus had no
teratogenic effects. If this is a valid conclusion, the increased
incidence of total resorption of litters at all doses should be
considered treatment-related.) (Marks et al., 1982).
Rabbits
Groups of 10, 12, and 14 pregnant New Zealand white rabbits were
fed dicloran (purity unspecified) in the diet at 0, 100, or 1000 ppm,
equivalent to 0, 3, and 30 mg/kg bw per day, on days 8-16 of
gestation, the day of mating being considered gestation day 1.
Pregnant females were allowed to deliver naturally, and the pups and
parental females were sacrificed on post-natal day 21. There were no
effects on maternal body weight during treatment. The mean litter size
was slightly lower in the group at the high dose than in controls,
with values of 7.1, 6.6, and 6.1 for the control, low, and high dose,
respectively. In the absence of data on resorption, however, it could
not be determined whether this effect was treatment-related. The pup
weights at birth were not provided, but the mean pup weights at
weaning (day 21) were comparable in all groups. There were no
malformations and no increase in the incidence of skeletal
malformations in treated pups (Wazeter, 1966).
Groups of 15-16 naturally mated New Zealand white rabbits were
dosed by gavage with dicloran (purity, 98.3%) in 1% carboxymethyl
cellulose at 0, 8, 20, or 50 mg/kg bw per day on days 6-18 of
gestation, the day of mating being considered gestation day 0. These
doses were based on the results of a range-finding study in which a
dose of 100 mg/kg bw per day caused two deaths, marked reduction in
food intake, reduced body-weight gain during treatment, slightly
increased post-implantation loss, and reduced litter and mean fetal
weights, with gross malformations in 4/11 fetuses from one litter. The
dose of 50 mg/kg bw per day resulted in reduced food intake and lower
body-weight gains throughout treatment, and at 20 mg/kg bw per day
there was a slight, transient reduction in food intake, with reduced
body-weight gain and slight body-weight loss on days 9-11 of gestation
(James & Brennan, 1991).
The dams were sacrificed on day 29 of gestation, the uterine
contents were examined, and all fetuses were assessed for viability,
sex, and external, visceral, and skeletal malformations and
variations. There were no treatment-related effects on mortality,
clinical signs, or food consumption. The mean body weights and body-
weight gain were slightly but consistently lower than those of
controls in females at the intermediate and high doses throughout both
the treatment and post-treatment periods. This resulted in an overall
mean body-weight gain in these animals that was 17-32% less than that
of controls during treatment and at termination. The mean gravid
uterine weights were also lower in these animals than in controls,
whereas the carcass weights were similar in all groups, indicating
that the weight changes were attributable primarily to effects on the
litters rather than the dams. The lower body-weight gain of dams at
the intermediate dose in the latter stages of gestation may be due
partially to the smaller litter sizes of this group, which were not
treatment-related; however, the effects seen in females at the
intermediate dose early during treatment (days 6-9) may indicate
maternal toxicity. The differences in body-weight gain in animals at
the high dose were also considered to be toxicologically significant.
Dams at the two higher doses had a slight increase in the frequency of
post-implantation loss (7.4, 9, 4.8, and 10% for control to high dose,
respectively). There were slight increases in the incidence of minor
anomalies of the gall-bladder in three fetuses in three litters and
delays in ossification of all limb epiphyseal sites in four fetuses in
three litters (with none in controls) at 50 mg/kg bw per day. The
NOAEL for maternal toxicity was 8 mg/kg bw per day on the basis of
lower body-weight gain early in the treatment period at 20 and 50
mg/kg bw per day. The NOAEL for developmental toxicity was 20 mg/kg bw
per day (Barton & Wilcox, 1996).
(f) Special studies
(i) Cataractogenicity
Dogs have been shown to develop lesions of the cornea and lens
after prolonged oral administration of dicloran. It has been suggested
that a photochemical product reaction is responsible for the lesion as
it occurs only when dogs are exposed to sunlight. Groups of four to
eight beagle dogs and six Hormel-Hanford miniature swine were fed
dicloran (purity, 95.8%) at concentrations of 0, 0.75, 6, 24, 48, or
192 mg/kg bw per day for periods varying from 50 to 306 days. Corneal
opacity appeared in dogs within 53-104 days after administration of 24
or 48 mg/kg bw per day, when they were exposed to sunlight. Dogs that
were not exposed to sunlight and eyes that had been sutured closed did
not develop lesions. Dogs given 192 mg/kg bw per day refused to eat
after 38 days and were given dicloran by capsule. All of these animals
died 49-53 days after the study began; no eye lesions were detected.
In several dogs with eye damage that were maintained for four months
to one year after dicloran administration had ceased, the pathological
changes seen in the cornea and lens were not reversed. Dicloran did
not appear to affect miniature swine at any concentration, and no
histopathological changes were seen in the eyes. A dose-related
increase in the presence of Heinz bodies was seen in the blood of both
dogs and swine, with at least one dog affected per dose and pigs
affected at doses of 48 mg/kg bw per day or more. No methaemoglobin
formation was found in dogs or pigs. Administration of dicloran as a
dust (0.1 mg) or a 5% solution directly into the eyes of dogs for
three months had no effect on corneal opacity or irritation of the
conjunctivae (Curtis et al., 1968; Bernstein et al, 1970; Earl et al.,
1971).
Ocular toxicity was not observed in rats or rhesus monkeys
(Serrone et al., 1967), and administration of 0.143 mg/kg bw per day
for three months to 20 human volunteers produced no ocular symptoms
(Strough, 1962).
The 1977 Joint Meeting (Annex 1, reference 28) reviewed
additional data requested by the 1974 JMPR and concluded that a
species difference in the kinetics of dicloran may partly explain the
photosensitive ocular toxicity observed in dogs,but not in other
mammals. 14C-Dicloran was administered orally at 100 mg/kg bw per day
for five days to four beagle dogs, four pigs (breed unspecified), and
eight Wistar rats. The concentration of radiolabelled residues was
highest in the dogs and slightly lower in pig tissues, but the tissue
residues in both species were 2.4-11-fold higher than those in liver
or plasma residues of rats. All three species had a high concentration
of residue in the liver, but in dogs and pigs the highest
concentrations were found in the pigmented tissues of the eye, the dog
having two to three times more residue in the iris and pigmented
retina than the pig. Nonpigmented eye tissues (cornea and lens) had
low concentrations. There was no correlation between ocular toxicity
and tissue residues of dicloran and its metabolites. The plasma
concentrations of radiolabel increased more rapidly in the dogs, but
24 h after three days of dosing the plateau plasma concentrations in
the pigs and dogs were similar (10-15 mg/kg bw). High concentrations
of radiolabel were excreted in the bile of dogs and pigs (Hamilton,
1977).
(ii) Haematological effects
Groups of 10 or 15 rats (strain unspecified) of each sex were
treated with dicloran at concentrations of 0, 5, 20, or 100 mg/kg bw
per day by gavage for four months or in the diet at a concentration of
0 or 20 ppm, equivalent to 1 mg/kg bw per day for four months.
Measurements of red blood cells, total and differential leukocyte
counts, platelet count, haematocrit, haemoglobin concentration,
glucose, growth, and food consumption indicated no significant effect
attributable to dicloran at any dose by either route (Evans et al.,
1963).
Because of the known ability of 4-nitroaniline to induce specific
blood dyscrasias, short-term studies were performed to compare
dicloran and 4-nitroaniline. Groups of five weanling rats (strain
unspecified) of each sex were given dicloran by gavage at 0 or 400
mg/kg bw per day, five days/week, for four weeks, and 4-nitroaniline
was administered at 200 or 400 mg/kg bw per day to two comparable
groups over the same interval. In a second experiment, groups of six
male weanling rats were given dicloran by gavage at 0 or 800 mg/kg bw
per day or 4-nitroaniline at 400 or 800 mg/kg bw per day, five days
per week, for two weeks. Haematological parameters were normal in rats
treated at 400 mg/kg bw per day -- no Heinz bodies were detected and
the reticulocyte counts were normal -- although their body-weight gain
was 50-60% of that of controls. At 800 mg/kg bw per day, there was
slight weight loss. Red blood cell count, packed cell volume, and
haemoglobin measurements were normal, while the lymphocyte counts were
reduced to 65% of that of controls. At the end of the study, no
effects were seen on the spleen. In contrast, 4-nitroaniline had
definitive effects on growth at 200 mg/kg bw per day (70% of control),
Heinz bodies were identified in the blood, and the reticulocyte count
was markedly elevated to two to four times the control value. The bone
marrow was not affected. At 400 mg/kg bw per day, growth was reduced
to 60% of the control value, and a reduced red blood cell count (65%
of control with polychromasia and nucleation) was seen, accompanied by
increased spleen weight (200% of control). These effects were more
pronounced at 800 mg/kg bw per day where, in addition, the haemoglobin
concentration was reduced and the lymphocyte count greatly increased.
Although the high doses of dicloran caused lymphopenia, the other
haemotoxic effects seen with 4-nitro-aniline were not observed with
dicloran (Boots Pure Drug Co., 1962).
In studies to substantiate the difference between the effects of
4-nitroaniline and dicloran, a single oral dose of 500 mg/kg bw
dicloran was given to cats. No methaemoglobin was noted at any time
between 1 and 48 h after dosing; however, administration of
4-nitroaniline as a single dose of 100 mg/kg bw resulted in
methaemoglobin over the same time. In addition, the cats given
4-nitroaniline were cyanotic and showed extensive muscle weakness
(Gurd, 1974). Although methaemoglobin was not assessed in all studies,
it is noteworthy that the 60-day study in mice was the only one that
showed an association between dicloran and significantly elevated
methaemoglobin concentrations in the blood at doses of 100 and
200 mg/kg bw per day (Mallyon et al., 1986). No effect on
methaemoglobin was seen in the two-year study in dogs (Woodard et al.,
1964).
3. Observations in humans
In a double-blind clinical study, 20 male volunteers were given
10 mg of dicloran (purity unspecified), equivalent to 0.14 mg/kg bw
per day, and 10 received a placebo, once daily, for 90 days. The dose
and duration were based on the estimated maximum residues obtained
from consumption of fruits and vegetables over one year.
Haematological examinations and tests for liver and kidney function
were performed at various intervals over the course of the study; the
results were found to be normal (Strough, 1962).
Extensive examinations were made on an industrial worker who had
been occupationally exposed to dicloran for three years, with
considerable exposure by inhalation and dermal contact for about 60
days per year. No adverse effects were observed (Brooks & Boyack,
1963).
Comments
Dicloran is rapidly absorbed, metabolized, and eliminated, mainly
in the urine, after oral administration to rats, goats, and humans; it
is also rapidly excreted by hens. Rats excreted most of the radiolabel
(90-96%) within 24 h, with > 70% in the urine and an additional
13-22% in the faeces, depending on the dose. Elimination was
essentially complete (91-97% of the administered dose) by 48 h, with
total tissue residues accounting for 0.2-0.3%. The highest tissue
concentrations were found in the liver (0.05-0.06 mg/kg) and kidneys
(0.02 mg/kg). Unchanged parent compound was detected in the faeces of
animals at the high dose only. The major urinary metabolites were the
sulfate and glucuronide conjugates of 2,6-dichloro-4-hydroxyaniline,
which accounted for 55-79% of the total administered radiolabel, and
the major faecal metabolites were derivatives of glutathione
conjugates. The parent compound and its glutathione conjugates were
the major residues in plants. None of the metabolites was of
toxicological concern. The rapid excretion of dicloran as a conjugate
indicates that it is readily metabolized in vivo.
The major metabolite in rat urine (2,6-dichloro-4-hydroxyaniline)
was not present in goat urine, which contained more polar conjugates
that could not be hydrolysed by glucuronidase or sulfatase. In goats,
dicloran is reduced to 4-amino-2,6-dichloro-aniline and acetylated to
4-amino-3,5-dichloroacetanilide (4-6% in urine), which is rapidly
metabolized and excreted in the urine and faeces, although a reactive
intermediate is formed in goat liver and bound covalently to
macromolecules. Species differences in dicloran metabolism were also
apparent in dogs and mice, which partly explain the photosensitive
oculotoxic effects observed in dogs and the methaemo-globinaemia noted
in mice, which are not seen in other mammals. Preliminary studies
suggested that the metabolites of dicloran in humans are similar to
those in rats. Although recovery was considered complete, excretion
was somewhat slower in humans than in rats, most of the material being
excreted within 1.5 days.
Dicloran has low or slight toxicity when administered by the oral
route, depending on species. It has low dermal toxicity, is not
irritating to the skin, is a mild eye irritant, and is not a skin
sensitizer.
WHO has classified dicloran as unlikely to present an acute
hazard in normal use (WHO, 1996).
The results of short-term studies of toxicity in rabbits treated
dermally and in mice and rats treated in the diet or by gavage and of
long-term studies of toxicity in mice, rats, and dogs treated in the
diet indicate that the liver is the primary target organ. Increased
liver weights and centrilobular hepatic hypertrophy were observed
consistently in all species treated orally, and increased splenic
activity and splenic extramedullary haematopoiesis were noted in
short- and long-term studies in mice.
When dicloran was fed to mice at 0, 300, 600, or 1200 ppm for 60
days, the NOAEL was 300 ppm (equal to 49 mg/kg per day), on the basis
of hepatic lesions, splenic extramedullary haematopoiesis, and
increased methaemoglobin at doses of 600 ppm and above.
In a 90-day study in mice treated by gavage with 0, 15, 45, 135,
400, or 600 mg/kg bw per day, the NOAEL was 15 mg/kg bw per day on the
basis of significant polycythaemia in males and hypercholesterolaemia
and a significant increase in the incidence of splenic extramedullary
haematopoiesis in females at 45 mg/kg bw per day and above.
A NOAEL was not identified in a 90-day study in rats fed diets
containing 0, 1000, 3000, or 5000 ppm dicloran because of
significantly reduced body-weight gain in conjunction with effects on
the liver and thyroid in all treated groups. In an eight-week study in
which rats were given diets containing dicloran at doses of 0, 500, or
750 ppm, the NOAEL was 500 ppm (equal to 44 mg/kg bw per day) on the
basis of reduced body-weight gain, decreased food consumption, and
increased liver weights with associated hepatic histopathological
manifestations at 750 ppm.
In a study in which dicloran was fed to rats for six months at 0,
30, 300, or 3000 ppm, the NOAEL was 300 ppm (equal to 22 mg/kg bw per
day) on the basis of reduced body weights and increased liver and
spleen weights at 3000 ppm.
In a four-week study in rats given a dose of 0, 35, 140, or 350
mg/kg bw per day by gavage, the NOAEL was 35 mg/kg bw per day on the
basis of growth depression and hepatic hypertrophy and vacuolation at
140 mg/kg bw per day.
In an 18-month study of carcinogenicity in mice at dietary
concentrations of 0, 50, 175, or 600 ppm, the NOAEL was 175 ppm (equal
to 25 mg/kg bw per day) on the basis of increased liver weights,
centrilobular hepatocyte enlargement, centrilobular haemosiderosis,
focal and single-cell liver necrosis, and vacuolation of centrilobular
hepatocytes.There was no evidence of carcinogenicity in mice.
Two studies were conducted in rats to assess toxicity over a
two-year period of exposure. Rats were fed diets containing dicloran
at concentrations of 0, 20, 100, or 3000 ppm or 0 or 1000 ppm. The
overall NOAEL was 1000 ppm (equal to 59 mg/kg bw per day), on the
basis of changes in body-weight, food consumption, and haematological
parameters, spleen pigmentation, increased liver weights,
centrilobular hepatocyte enlargement, and thyroid hypertrophy at 3000
ppm. There was no evidence of carcinogenicity in these studies, but
they were considered inadequate for complete evaluation of the
carcinogenetic potential of dicloran.
Dogs fed dicloran at dietary concentrations of 0, 20, 100, or
3000 ppm for two years had treatment-related changes in haematological
and clinical chemical parameters and significant increases in liver,
spleen, and kidney weights, accompanied by histological changes at
3000 ppm that included irregular hepatic-cell size, moderate
hepatic-cell hypertrophy, and increased pigmentation of the liver and
spleen. The NOAEL was 100 ppm, equal to 1.7 mg/kg bw per day.
Dicloran was not mutagenic in most assays, although occasional
positive responses were seen in more recent tests for reverse mutation
and in a test for mitotic recombination. The results of an assay for
sex-linked recessive mutation in Drosophila were equivocal. The
Meeting concluded that dicloran is unlikely to be genotoxic.
Studies of reproductive and developmental toxicity indicated that
dicloran is not a reproductive toxicant and is not teratogenic in rats
or rabbits. Dicloran was embryotoxic at maternally toxic doses in
rabbits, but an NOAEL for maternal or developmental toxicity was not
identified in rats.
In a two-generation study of reproductive toxicity in rats (one
litter per generation) at dietary concentrations of 0, 50, 250, or
1250 ppm, the NOAEL for systemic toxicity was 250 ppm (equal to 21
mg/kg bw per day) on the basis of reduced body-weight gains and
increased liver weights at 1250 ppm. The NOAEL for reproductive and
developmental toxicity was 250 ppm (equal to 21 mg/kg bw per day) on
the basis of reduced weights of F1 and F2 pups at 1250 ppm.
In a study of developmental toxicity, dicloran was administered
by gavage to rats on days 6-15 of gestation at doses of 0, 100, 200,
or 400 mg/kg bw per day. Because of significantly reduced mean
maternal body-weight gains and higher incidences of totally resorbed
litters in all treated groups, NOAEL values for maternal or
developmental toxicity were not identified. Dicloran was not
teratogenic in this study.
In a study of developmental toxicity in rabbits, dicloran was
administered by gavage at doses of 0, 8, 20, or 50 mg/kg bw per day on
days 6-18 of gestation. The NOAEL for maternal toxicity was 8 mg/kg bw
per day, on the basis of reduced maternal body-weight gains early
during treatment with 20 or 50 mg/kg bw per day. The NOAEL for
developmental toxicity was 20 mg/kg bw per day on the basis of a
slight increase in post-implantation losses, a slight increase in the
incidence of minor anomalies of the gall-bladder, and delays in
ossification of all limb epiphyseal sites in fetuses at 50 mg/kg bw
per day.
Dogs have been shown to develop lesions in the cornea and lens
after prolonged oral administration of dicloran. The 1977 Joint
Meeting reviewed additional data requested by the 1974 Meeting and
concluded that the photosensitive oculotoxic effects observed in dogs
but not in other mammals were due partly to a species difference in
the kinetics of dicloran. No oculotoxic effects were seen in any of
the more recent studies, although no further studies have been
conducted in dogs.
In a double-blind clinical study, 20 men were given 10 mg
dicloran (equivalent to 0.14 mg/kg bw per day) and 10 received a
placebo once daily for 90 days. The dose and duration were chosen on
the basis of the maximum residues estimated to be derived from
consumption of fruits and vegetables over one year. There was no
indication that administration of dicloran at this dosage had any
adverse effect.
An ADI of 0-0.01 mg/kg bw per day was established on the basis of
the NOAEL of 1.7 mg/kg bw per day for hepatic and haematological
effects in the two-year study in dogs and a 200-fold safety factor. A
larger than normal safety factor was used because of the inadequacy of
the long-term studies in rats for assessing the carcinogenic potential
of dicloran and because of the lack of a NOAEL for maternal and
developmental toxicity in rats.
An acute RfD was not allocated because dicloran has low or slight
toxicity when administered orally or dermally and because acute
effects occur only at very high doses, resulting in a 10 000-fold
difference between the ADI and the LOAEL for maternal and
developmental toxicity in rats. Therefore, the Meeting concluded that
the acute intake of residues is unlikely to present a risk to
consumers.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 300 ppm, equal to 49 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
15 mg/kg bw per day (13-week study of toxicity)
175 ppm, equal to 24 mg/kg bw per day (18-month study
of carcinogenicity)
Rat: 35 mg/kg bw per day (lowest dose tested, four-week
study of toxicity)
500 ppm, equal to 44 mg/kg bw per day (lowest dose
tested, eight-week study of toxicity)
300 ppm, equal to 22 mg/kg bw per day (six-month study
of toxicity)
1000 ppm, equal to 59 mg/kg bw per day (two two-year
studies of toxicity)
250 ppm, equal to 21 mg/kg bw per day (two-generation
study of reproductive toxicity)
Rabbit: 8 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (developmental toxicity)
Dog: 100 ppm, equal to 1.7 mg/kg bw per day (two-year study
of toxicity)
Human: 0.14 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of an acute reference dose
Not allocated (unnecessary).
Studies that would provide information useful for continued
evaluation of the compound
1. Combined study of toxicity and carcinogenicity in rats.
2. Study of developmental toxicity in rats at less than 100
mg/kg bw per day to establish clear NOAELs for maternal and
developmental toxicity.
3. Assays for genotoxicity in mammals in vivo, such as an
assay for micronucleus formation
4. Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete, > 70% urinary excretion within 24 h
Dermal absorption No data
Distribution Liver, kidney
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 90-96% in urine and faeces within 24 h
Metabolism in animals Metabolites differ in rats and goats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral > 4000 mg/kg bw
Rabbit: LD50 dermal > 2000 mg/kg bw
Rat: LC50 inhalation No data
Skin irritation Not irritating
Eye irritation Minimally irritating
Skin sensitization Not sensitizing (Draize test)
Short term toxicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant oral NOAEL Rat: 44 mg/kg bw/per day (diet); 15 mg/kg bw per day
(gavage)
Lowest relevant dermal NOAEL Rabbit: 120 mg/kg bw per day
Lowest relevant inhalation NOAEL Poor study: data on actual intake, particle size not
provided
Genotoxicity Unlikely to be genotoxic; no study of genotoxicity in
mammals in vivo
Long term toxicity and carcinogenicity
Target/critical effect Liver/centrilobular hepatotoxicity (mice, rats, dogs)
Spleen/extramedullary haematopoiesis (mice)
Lowest relevant NOAEL Dog: 1.7 mg/kg bw per day (2-year study)
Carcinogenicity No evidence of carcinogenicity in mice. The study in
rats was inadequate.
Reproductive toxicity
Reproduction target/critical effect Increased ovary/testis weights (no histological
findings)
Lowest relevant reproductive NOAEL Rat: 21 mg/kg bw per day (reduced body-weight gain,
increased liver weight)
Developmental target/critical effect Increased resorptions, delayed ossification, not
teratogenic
Lowest relevant developmental NOAEL 20 mg/kg bw per day in rabbits; no NOAEL in rats.
A 10 000-fold difference exists between the ADI and
the LOAEL in rats.
Neurotoxicity/Delayed neurotoxicity No data
Other toxicological studies
Cataractogenicity Photosensitive oculotoxic effects observed in dogs are
not seen in other mammals
Medical data No indication that administration of dicloran at
10 mg/day to men for 90 days had any adverse effect.
Extensive examinations were made on one industrial
worker occupationally exposed to dicloran over three
years, with considerable inhalation and dermal
exposure for about 60 days/year. No adverse effects
were observed
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 2-year study, dogs 200
Acute reference dose Not allocated (unnecessary)
References
Allen, J.A., Brooker, P.C. & Gray, V.M. (1988) Technical dicloran:
Metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. No. SMS 43/871138 from Huntingdon Research
Centre, United Kingdom. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Bachmann, E., Golberg, L. & Thibodeau, L. (1971) Aspects of the
determination of biphenyl hydroxylase activity in liver homogenate.
Exp. Mol. Pathol., 14, 303-326.
Barton, S.J. & Wilcox, S. (1996) Dicloran: Development toxicity in
rabbits. Unpublished report No. 11379 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Barton, S.J. & Wilcox, S. (1997) Dicloran: Two generation reproduction
study in rats. Unpublished report No. 14271 from Inveresk Research
International, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Basting, L.A., Cooper, C., Witmer, C.M. & Gallo, M.A. (1983) Toxicity
and mutagenicity of 2,6-dichloro-4-nitroaniline in the Ames test.
Fed. Proc., 42, 623.
Basting, L.A., Witmer, C.M., Iba, M.M. & Gallo, M.A. (1984) The effect
of 2,6-dichloro-4-nitroaniline on hepatic microsomal systems.
Toxicologist, 4, 149.
Bernstein, H., Curtis, J.M., Earl, F.L. & Kuwabara, W. (1970)
Phototoxic corneal and lens opacities. Arch. Ophthalmol., 83,
336-348.
Boots Pure Drug Co. (1962) Dicloran: Experimental data on toxicity.
Unpublished report No. T-17 from Boots Pure Drug Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Boyack, G.A. & Brooks, B.W. (1963) Medical examination of a man after
prolonged exposure to 2,6-dichloro-4-nitroaniline. Unpublished report
No. T-21 from Upjohn Co., USA. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Cheng, T. (1996a) Nature of the residue of 14C-dicloran in lactating
goats. Unpublished report No. HWI6564-102 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996b) Metabolism of 14C-dicloran in rats. Unpublished
report No. HWI6564-108 from Hazleton Wisconsin, Inc., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Cheng, T. (1996c) Nature of the residue of 14C-dicloran in laying
hens. Unpublished report No. HWI6564-100 from Hazleton Wisconsin,
Inc., USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Creedy, C.L., Hemmings, P.A. & Needham, D. (1985) The effect of
dicloran on the hepatic mixed-function oxidase system of the male rat
following oral administration of 100 mg/kg body weight twice daily for
4 days. Unpublished FBC Ltd report No. METAB/85/24, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Curtis, J.M., Bernstein, H., Earl, F.L. & Smalley, H.E. (1968) Corneal
and lens opacities in dogs treated with 2,6-dichloro-4-nitroaniline.
Toxicol. Appl. Pharmacol., 12, 305.
Dawson, J. R. (1988) Metabolism and residues of dicloran in the laying
hen. Unpublished report No. ENVIR/88/26 from Schering Agrochemicals
Ltd, United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
Earl, F.L., Curtis, J.M., Bernstein, H.N. & Smalley, H.E. (1971)
Ocular effects in dogs and pigs treated with dichloran
(2,6-dichloro-4-nitroaniline). Food Cosmet. Toxicol., 9, 819-828.
Eberts, F.S. (1965) Fate of Botran (2,6,-dichloro-4-nitroaniline) in
rat and man. Unpublished report No. M9 from Upjohn Co., USA. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Eberts, F.S., Meeks, R.C. & Vliek, R.W. (1963) Excretion of
Botran-14C (2,6,-dichloro-4-nitroaniline) by man. Unpublished Report
No. M3 from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Elliot, P.H., Smith, C., Crook, D., Gibson, W.A., Singh, H. &
Gopinath, C. (1988) Technical dicloran: Twenty-one day dermal toxicity
study in rabbits. Unpublished report No. SMS 60/871292 from Huntingdon
Research Centre Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Evans, J., Mengel, G. & Bostwick, L. (1963) Botran (U-2069) Effect of
oral administration. Final report four-months study. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Everest, R.P. & Tuplin, J.A. (1977) Dicloran, pure reference sample:
Mutagenicity testing in bacterial in vitro systems. Unpublished report
No. TX 77024 from Boots Pure Drug Co., United Kingdom. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Gaines, T.B. & Linder, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. Appl. Toxicol., 7, 299-308.
Gallo, M.A., Bachmann, E. & Golberg, L. (1976) Mitochondrial effects
of 2,6-dichloro-4-nitroaniline and its metabolites. Toxicol. Appl.
Pharmacol., 35, 51-61.
Garrett, N.E., Stack, H.F. & Waters, M.D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res., 168,
301-325.
Griffiths, A.F.J., Brockman, H.E., DeMartini, D.M. & de Serres, F.J.
(1986) The efficacy of Neurospora in detecting agents that cause
aneuploidy. Mutat. Res., 167, 35-45.
Gurd, P.R. (1974) Methemoglobin formation in the cat. Unpublished.
Cited from the 1974 JMPR Monograph, (Annex 1, reference 22).
Hamilton, D.Y. (1977) Tissue distribution of [14C]-dichloran in rats,
dogs and pigs following repeated oral dosing at 100 mg/kg/day.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Hawkins, D.R., Kirkpatrick, D. & Shaw, D. (1988) The metabolism of
[14C]-dicloran in peaches: Analysis of samples. Unpublished report
No. HRC/SMS from Huntingdon Research Centre, Ltd United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, L. & Peters, J. (1967) Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Natl Cancer Inst., 42, 1101-1114.
Jaglan, P.S. & Arnold, T.S. (1985a) Comparative metabolism of
[14C]-dichloran in rats and goats: Nature of liver residues.
Unpublished report No. 218-9760-85-002A from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jaglan, P.S. & Arnold, T.S. (1985b) Nature of muscle residue from the
treatment of a goat with [14C]-dichloran
(2,6-dichloro-4-nitroaniline). Unpublished report No. 218-9760-85-003
from Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jaglan, P.S., Gosline, R.E. & Arnold, T.S. (1985a) Comparative
metabolism of [14C]-dichloran in rats and goats. Unpublished report
No. 218-9760-85-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Jaglan, P.S., Arnold, T.S., Gosline, R.E. & Subacz, C.J. (1985b)
Bioavailability of [14C]-dichloran (U-2069;
2,6-dichloro-4-nitroaniline) derived goat liver residues by the rats.
Unpublished report No. 218-9760-85-004 from Upjohn Co. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
James, P. & Brennan, C. (1991) Technical dichloran: Rabbit oral
developmental toxicity (teratogenicity) range finding study.
Unpublished report No. TOX/91/199-98 (T128) from Schering AG, Germany.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1978) Screening of pesticides for
mutagenicity in microbial systems. Taiwan Plant Protection Center
Research Report No. 12. Submitted to WHO by Gowan Company, Yuma,
Arizona, USA.
Jeang, C.-L. & Li, G.-C. (1980) Screening of pesticides for
mutagenicity in microbial systems II: with mammalian microsomal
activation. Natl Sci. Council Monthly, 8, 551-559.
Johnston, R.L. & Ceru, J.G. (1961) Acute oral LD50 -- rat and acute
intraperitoneal LD50 -- mouse using DCNA with 0.09% p-nitroaniline.
Unpublished report No. T-16 from Upjohn Co., USA. Submitted to WHO by
Gowan Company, Yuma, Arizona, USA.
Johnston, R.L. & Schwikert, R.S. (1963) Botran: Skin sensitization in
guinea pigs. Unpublished report No. 5567-64-RLJ-196B from Upjohn Co.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Jones, E. & Fenner, L.A. (1987) Technical dicloran: Ames bacterial
mutagenicity test. Unpublished report No. TOX/87/199-85 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Kakuk, T. (1986) 90-Day oral toxicity study in B6C3F1 mice with DCNA.
Unpublished report No. 7263/86/003 from Upjohn Co., USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Kakuk, T.J., Weddon, T.W. & Thomas, R.W. (1979) Reevaluation of
potential hepatic effects of Botran in beagle dogs: Supplemental
report. Unpublished report No. 001-9610-79-005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Kappas, A. (1978) On the mechanisms of induced somatic recombination
by certain fungicides in Aspergillus nidulans. Mutat. Res., 51,
189-197.
Lessel, B. (1974) Two-year feeding trial in rats on dicloran.
Unpublished report from Boots Pure Drug Co., United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Lobdell, B. & Johnson, C. (1965) U-2069: Effect on reproduction
capacity through three generations in the rat. Unpublished report from
Woodard Research Corporation. Cited from the 1974 JMPR Monograph
(Annex 1, reference 22).
Mallyon, B.A. & Markham, L.P. (1989) Technical dicloran: Oncogenicity
study in the mouse. Unpublished report No. TOX/86006 from Schering
Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan Company,
Yuma, Arizona, USA.
Mallyon, B.A. & Rawcliffe, L.M. (1985) Technical dicloran:
Palatability in the diet to the mouse. Unpublished report No.
TOX/85/199-74 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Mallyon, B.A., Rawliffe, L.M. & Major, I.R. (1986) Technical dicloran:
60-day dietary study in the mouse. Unpublished report No.
TOX/86/199-75 from FBC Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Marks, T.A., Poppe, S.M., Moerdyk, D.L., Stuckhardt, J.L., Morris,
D.F. & Greenberg, J.H. (1982) U-2069: A segment II teratology study in
the rat. Unpublished report No. 9610/82/7263/005 from Upjohn Co., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Mason, J.M., Valencia, R. & Zimmering, S. (1992) Chemical mutagenesis
testing in Drosophila: VIII: Reexamination of equivocal results.
Environ. Mol. Mutag., 19, 227-234.
Maté, C., Ryan, A.J. & Wright, S.E. (1967) Metabolism of some 4-
nitroaniline derivatives in the rat. Food Cosmet. Toxicol., 5,
657-663.
McBride, D. & McGregor, D.B. (1987) Technical dicloran: Assessment of
unscheduled DNA synthesis using rat hepatocyte cultures. Unpublished
report No. 4414 from Inveresk Research International, Musselborgh,
United Kingdom. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Moustacchi, E., Carere, A., Morpurgo, G. Ramel, C. & Würgler, F.E.
(1986) Assays for genetic changes in fungi. In: Montesano, R.,
Bartsch, H., Vainio, H., Wilbourn, J. & Yamasaki, H., eds,
Long-term and Short-term Assays for Carcinogens: A Critical
Appraisal (IARC Scientific Publications No. 83), Lyon, International
Agency for Research on Cancer, pp. 203-249.
Myers-Basting, L.A. (1986) Mutagenicity and characterization of the in
vitro metabolism of 2,6-dichloro-4-nitroaniline. Unpublished report
No. (T101) from Rutgers University, New Jersey, USA. Submitted to WHO
by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I.R. (1991a) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 5 mg/kg body-weight. Unpublished report No.
TOX/90/199-95 (M48) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Boyle, F. & Challis, I. R. (1991b) The excretion and distribution of
radiolabelled residues in the rat following oral dosing with
[14C]-dicloran at 500 mg/kg body-weight. Unpublished report No.
TOX/90/199-96 (M49) from Schering Agrochemicals Ltd, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997a) Metabolic fate and distribution of 14C-dicloran in
potatoes. Unpublished report No. 1947 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
O'Neal, S. (1997b) Metabolic fate and distribution of 14C-dicloran in
lettuce. Unpublished report No. 1949 from PTRL East Inc., USA.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Peters, D.H., Stuart, V., Smith, H.L., Crook, D., Gibson, W.A.,
Majeed, S.K. & Gopinath, C. (1991) Technical dicloran: Rat 13-week
dietary toxicity study. Unpublished report No. TOX 89324 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980a) Primary eye irritation evaluation
in New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-001 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T.J. & Wood, D.R. (1980b) Primary dermal irritation study in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-002 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Raczniak, T. J. & Wood, D. R. (1980c) Acute dermal toxicity screen in
New Zealand white rabbits with Botran technical. Unpublished report
No. 218-9610-80-003 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Seaman, W.J., Weddon, T.E. & Kakuk, T.J. (1980) Three-week inhalation
study in rats, rabbits and dogs with Botran. Unpublished report No.
218-9610-80-004 from Upjohn Co., USA. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Serrone, D.M., Pakdaman, P., Stein, A.A. & Coulston, F. (1967)
Comparative toxicology of 2,6-dichloro-4-nitroaniline in rats and
rhesus monkeys. Toxicol. Appl. Pharmacol., 10, 404.
Shirasu, Y., Moriya, M., Kato, K. Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Smith, S (1989) Metabolism of [14C]-dicloran in peaches under field
and glasshouse conditions. Unpublished report No. ENVIR/89/12 from
Schering Agrochemicals Ltd, United Kingdom. Submitted to WHO by Gowan
Company, Yuma, Arizona, USA.
Strough, A.R. (1962) Botran clinical study. Unpublished report from
Upjohn Co., USA. Submitted to WHO by Gowan Company, Yuma, Arizona,
USA.
US Environmental Protection Agency (1974) Unpublished data from EPA
laboratories. Cited from the 1974 JMPR Monograph, (Annex 1, reference
22).
Waters, M.D., Sandhu, S.S., Simmon, V.F, Mortelmans, K.E., Mitchell,
A.D., Jorgenson, T.A., Jones, D.C.L., Valencia, R. & Garrett, N. E.
(1982) Study of pesticide genotoxicity. Basic Life Sci., 21,
275-326.
Waterson, L.A., Gibson, W.A., Morrow, J. & Gopinath, C. (1992)
Technical dicloran rat 8-week repeat dose study. Unpublished report
No. 91/199-97 from Huntingdon Research Centre, United Kingdom.
Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wazeter, F.X. (1966) Somers test in the albino rabbit. Unpublished
report No. 100-037 from International Research and Development Corp.,
USA. Submitted to WHO by Gowan Company, Yuma, Arizona, USA.
Wilcox, S., & Barton, S.J. (1996) Dicloran: Range-finding study for a
two generation reproduction study in rats. Unpublished report No.
14271 from Inveresk Research International, United Kingdom. Submitted
to WHO by Gowan Company, Yuma, Arizona, USA.
Woodard, M. W., Cockrell, K.O. & Woodard, G. (1964) Safety evaluation
by oral administration to rats and dogs for 104 weeks. Unpublished
report No. U2069 from Woodard Research Corporation, USA. Submitted to
WHO by Gowan Company, Yuma, Arizona, USA.
Woodruff, R.C., Mason, J.M., Valencia, R. & Zimmering, S. (1985)
Chemical mutagenesis testing in Drosophila: V: Results of 53 coded
compounds tested for the National Toxicology Program. Environ.
Mutag., 7, 677-702.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T. & Mortelmans, K.
(1992) Salmonella mutagenicity tests: V: results from the testing of
311 chemicals. Environ. Mol. Mutag., 19, 2-141.
