
CYANOFENPHOS JMPR 1975
IDENTITY
Chemical name
O-4-cyanophenyl O-ethyl phenylphosphonothioate
Synonyms
SurecideR
CYP, OMS-870, S-4087, Surazon
Structural formula
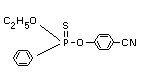 ther information on identity and properties
Physical state: pure compound, white crystalline solid.
Technical product, slightly yellowish solid
with characteristic odour
Melting point: 83°C
Vapour pressure: 10 mm Hg at 225°C
Specific gravity: D20 = 1.29
4
Solubility: practically insoluble in water, soluble in
ketones (acetone, methyl ethyl ketone, methyl
isobutyl ketone, cyclohexanone), hydrocarbons
(benzene, toluene, xylene,
methylnaphthalene), methylene dichloride,
chloroform, acetonitrile, dimethyl formamide,
ethyl cellosolve. Slightly soluble in
methanol, isopropanol, kerosene, soybean oil.
Stability: stable for two years under normal storage
conditions. Half-life values in aqueous
solutions at 60°C and
pH 6.0 - 150 days
pH 8.0 - 227 days
pH 10.0 - 68 days
The composition of the technical product is reported to be as follows.
Active ingredient 92.0 - 95.0%
O,O-diethyl phenylphosphonothioate 3.0 - 5.0%
O,O-di(4-cyanophenyl)phenylphosphonothioate 1.0 - 2.0%
O-ethyl phenylphosphonochloridothioate <0.2%
O,S-diethyl phenylphosphonodithioate <0.2%
S-ethyl O-(4-cyanophenyl)phenylphosphonothioate <0.5%
4-cyanophenol <0.3%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Sumitomo Chemical Company in their submission have abstracted a
study on the absorption and metabolism of C14 labelled cyanofenphos
in the mouse. This was taken from a private communication (S. Kato and
J. Miyamoto, presented at T.R. commission of IUPAC Meeting in
September 1974) which was not available for review. They did present
data on the absorption and biodegradation patterns of O,O dimethyl
O-(4-cyanophenyl) phosphorothiate in the rat (Wakimura and Miyamoto,
1971) and of O-(4 bromo-2,5 dichlorophenyl) O-methyl phenylphosphonate
(Leptophos) in mice and on cotton plants (Holmstead et al., 1973). The
company has implied that cyanofenphos has similar absorption and
biodegradation patterns as the above-mentioned compounds.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Chicken
Cyanofenphos was administered orally as a single dose of 100
mg/kg/ bodyweight to eight white leghorn chickens (1-1.5 years of
age). The birds were observed for three weeks. The same treatment was
repeated and birds observed for a further three weeks. Atropine and
2-PAM were used to protect the birds from acute intoxication. One bird
died during first test period. Birds that survived through the two
treatment periods did not exhibit delayed paralysis of the legs.
Histopathologic examination of sciatic nerve and spinal cord did not
reveal demyelination. A single dose of TOCP (400 mg/kg body weight)
produced paralysis in legs after two weeks with degeneration and
demyelination of the sciatic nerve.
In a further study cyanofenphos was administered at daily doses
of 2 and 4 mg/kg body weight six days a week for four weeks and
observed for a further four weeks. Both groups exhibited anorexia, a
decrease in spontaneous motor activity and in body weight gain. No
paralysis was noted. No anomalies were observed in sciatic nerve and
spinal cord on histopathologic examination (Kadota et al., 1972).
Special studies on reproduction
In a three-generation reproduction study (two litters per
generation) eight male and 16 female rats received dietary
concentrations of 0, 1, 3 and 10 ppm cyanofenphos during the first d
generation. For the second and third generations those selected from
the 1 ppm dietary groups the concentration of cyanofenphos in the diet
was increased to 30 ppm. No treatment related effects were observed on
body weight gain, survival or ophthalmic and pathological examinations
in parental animals. However, reduced mating indices during third
generation and lowered male and female fertility during second and
third generation were consistently observed at 30 ppm. Pup viability
was reduced at 30 ppm. No compound related effects were observed with
respect to weanling weights, gross external observation or
histopathological examination of F3b progeny (Smith et al., 1975).
Special studies on teratogenicity
Groups of pregnant rabbits received 0, 1.0, and 3.0 mg/kg body
weight/day of cyanofenphos via gelatin capsule from day 6 through day
18 of gestation. Animals were sacrificed at day 29 of gestation and
pups removed by Caesarean section. No significant differences were
found in total number of implantation sites, number of resorptions and
number of live young. Body weights of foetuses were similar for
treated groups and were comparable to control. Twenty-four hour
survival was not affected. No visceral or skeletal abnormalities were
noted. The results obtained with the positive control (thalidomide)
demonstrated the susceptibility of this strain to a teratogenic agent
(Ladd and Smith, 1973).
Acute toxicity
Technical compound (purity 92%)
LD50 mg/kg
Species Route Solvent M F Reference
mouse oral Tween 80 36 30 Kadota and Miyamoto, 1973
S.C. Tween 80 68 48 Kadota and Miyamoto, 1973
I.P. Tween 80 27.5 19 Kadota and Miyamoto, 1973
Dermal Tween 80 >1 000 >1 000 Kadota and Miyamoto, 1973
rat oral Tween 80 89 32 Miyamoto and Kadota, 1972
S.C. Tween 80 200 50 Miyamoto and Kadota, 1972
Gum Arabic 1 120 380 Miyamoto and Kadota, 1972
I.P. Tween 80 141 30 Miyamoto and Kadota, 1972
Dermal peanut oil >1 000 640 Miyamoto and Kadota, 1972
hen oral Tween 80 60 Kadota et al., 1972
A single instillation of 0.05 ml suspension of cyanofenphos in
Tween 80 was found to be irritating to the rabbit eye at 0.2% and
above. At 1.0% eye closure, congestion of the conjunctiva and
lacrimation were noted. No changes were observed in cornea,
conjunctival reflex, corneal reflex and pupil diameter.
When cyanofenphos suspension was instilled daily (six days a
week) for four weeks at dosage levels of 0.2 and 1.0%, similar eye
reactions were observed which did not appear to increase in intensity
with time. Myosis was not observed (Kagoshima et al., 1972)
Two groups of guinea pigs were sensitized by injecting
intracutaneously 5% and 1% solutions of cyanofenphos in corn oil every
other day for 20 days. Each animal was challenged with an
intracutaneous injection of cyanofenphos in corn oil and a dermal
application of this compound in acetone 14 days after last sensitizing
dose. Local swelling was observed at injection site both on
sensitizing and challenging the animals. No remarkable changes with
dermal challenge (Koda et al., 1972).
Short-term studies
Dog
Four groups of dogs (four male and four female/group) were fed 0,
10, 30 and 100 ppm cyanofenphos in the diet for 12 months. Two male
dogs at 100 ppm displayed a moderate ataxia of the hindquarters during
the final month of testing. Growth, food consumption, mortality,
haematology, blood chemistry, urinalysis, ophthalmologic examination,
gross and histopathology revealed no compound related effect. Liver to
body and brain weight ratio was increased in four of eight dogs at 100
ppm. Plasma and red blood cell cholinesterase activities at 100 ppm
were decreased when determined at one, three and nine months. Red
blood cell cholinesterase was also reduced at 12 months. No inhibitory
effect on brain cholinesterase was noted at any level (Marias et al.,
1974).
Cow
Groups of four cows were fed diets containing 0, 5, 15 and 50 ppm
cyanofenphos. On day 30 of treatment three cows/group were sacrificed,
the remaining animal of each group was fed the control diet for a
further 30 days. Milk, liver, kidney, muscle and fat were collected
from each animal and analysed for residues.
No adverse reactions due to the feeding of cyanofenphos were
observed. Trace levels of cyanofenphos and its oxon metabolite were
found only in milk and tissues of cows fed 50 ppm. Residue levels were
below the limit of detection (<0.002 ppm) during the withdrawal
period (Ladd and Winegender, 1975).
Long-term studies
Rat
Groups of 60 rats (six weeks of age) were fed cyanofenphos at
dietary levels of 0, 10, 30, 100 and 200 ppm in males and 0, 5, 15, 50
and 100 ppm in females for 24 months. There was no apparent evidence
of significant changes in general behaviour, mortality, body weight
gain, food and water consumption, urinalysis, haematology, clinical
biochemistry, organ to body weight ratio or gross and microscopic
examination of tissues. Plasma, red blood cell and brain
cholinesterase were depressed in males at 100 and 200 ppm and in
females at 50 and 100 ppm (Kadota et al., 1974).
COMMENTS
Although data on the absorption, metabolism and excretion have
been presented on two similar chemicals and reference made to data on
cyanofenphos from a private communication, no metabolic data on
cyanofenphos per se have been received.
There were no teratogenic or neurotoxic effects observed with
cyanofenphos. Reproductive effects were observed in both parental
animal and progeny at 30 ppm. The no-effect level was 10 ppm. In a
short-term (12 month) study with the dog the no-effect level was 30
ppm in the diet based on plasma and erythrocyte cholinesterase
activity. In a long-term study in the rat, the no-effect level for
cholinesterase inhibition was 15 ppm. Based on the available
information and considering the lack of metabolic data a temporary
acceptable daily intake was allocated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg
Dog: 30 ppm in the diet equivalent to 0.75 mg/kg
Estimation of temporary acceptable daily intake
0-0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cyanofenphos is mainly applied against insect pests including
Lepidoptera, Diptera, Orthoptera, Hemiptera and Coleoptera in field
crops, fruits and vegetables. It is also applied for the control of
insects affecting livestock such as blow flies, lice and ticks.
The percentage of cyanofenphos used in different fields of
application varies according to current pest situations. A rough
estimate is 60% for field crops (rice, cotton, legumes, cereals), 20%
for vegetable crops (crucifers, onions, cucurbits), 15% for pome
fruit, stone fruit, citrus and tea, and 5% for veterinary uses.
Approximate dosages and frequencies of application are shown in
Table 1.
TABLE 1. Dosages and frequencies of application of cyanofenphos
Crop Dosage and frequency of application
Rice 0.25-0.75 kg a.i./ha, 2-4 times/season
Pome and stone fruits 0.025-0.75 kg a.i./ha, 2-4 times/season
Vegetables 0.25-0.75 kg a.i./ha, 2-5 times/season
Tea 0.5-1 kg a.i./ha, 1-3 times/season
Cotton 0.5-1 kg a.i./ha, 2-6 times/season
Sheep 0.02-0.05% by plunge dips, once a year
0.04-0.06% by shower dips, once a year
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data on residues resulting from supervised trials were
available. Different countries have different patterns of consumption
and usage of agricultural commodities, e.g. in some countries rice
straw after harvest is used as a feed of farm animals. Table 2
summarizes the residue levels which have been found when the product
is used according to "good agricultural practice" in Japan (Sumitomo,
1975).
FATE OF RESIDUES
In animals
Following oral administration of cyanofenphos to dairy cows at
dietary levels of 5, 15 and 50 ppm for 30 consecutive days, samples of
muscle, fat, liver, kidney and milk were analysed to determine
contents of cyanofenphos and cyanofenphos-oxon. In the tissue samples
residue levels were highest in the fat and decreased in the order fat
>> liver > kidney = muscle. Levels of cyanofenphos ranged from
approximately 1 mg/kg in the fat to 0.012 mg/kg in the kidney and
muscle in the highest dose group. The oxon levels ranged from
approximately 0.08 mg/kg in fat to less than 0.0004 mg/kg in liver,
kidney and muscle from the same group. Residues of cyanofenphos
(<0.002-0.013 mg/kg) were also found in fat samples from the lower
and middle dose groups and in liver samples from the middle group.
TABLE 2. Cyanofenphos residues resulting from supervised trials in Japan
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
rough rice straw
Rice EC 0.4, 0.5 2 59 0 0.002 2.26
0.5 4 51 0 0.014 6.14
0.6 2 45 0 0.034 0.546
0.6 4 37 0 0.141 1.26
0.5, 0.75 2 40 0 0.055 0.695
0.75 4 31 0 0.196 4.86
Dust 0.6 1 47 0.011
0.6 2 43-59 <0.002-0.010
0.45, 0.6 2 47 0.005
0.6 3 44-50 <0.002-0.023
0.6 4 32 0.021
42 0.012
0.45, 0.6 2-2 37 0.002
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
green pod
Soybean EC 0.5 2 7 0.142, 0.228 6.00, 6.571
3 7 0.402 8.978
4 7 0.156 8.50
dried bean
2 34-50 0.075, 0.186
3 50 0.108
4 34 0.214
fresh peel
Peach EC 0.75 2 3 (4 samples) 0.004-0.028 6.3-31.3
2 7 " 0.006-0.009 7.8-27.0
2 14 " 0.004-0.012 6.8-13.7
4 3 " 0.007-0.038 7.2-39.4
4 7 " 0.005-0.014 6.4-32.1
4 14 " 0.004-0.014 4.8-18.4
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage EC 0.375 2 28 0.002
2 35 <0.001
2 42 <0.001
3 14 0.123
3 21 0.002
3 28 0.001
0.5 2 7 0.186
2 14 0.092
2 21 0.028
4 7 0.258
4 14 0.108
4 21 0.026
0.75 2 7 1.45
2 14 0.902
2 20 0.721
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage 2 28 0.020
(continued)
2 35 0.015
2 42 0.010
3 14 0.050, 0.056
3 21 0.004, 0.008
3 28 0.001, 0.002
0.5, 0.75 2 + 2 7 2.02
2 + 2 14 2.02
2 + 2 20 1.24
1.5 3 14 0.083
3 21 0.008
3 28 0.002
Onion D 1.35 1 189-234 <0.002
Cucumber D 0.9 1 39-42 <0.001
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Ginger EC 0.7 3 45-52 0.004, 0.008
(root)
6 " 0.006, 0.012
Tea EC 1 1 7 48.6, 49.2
(steamed)
1 14 23.0, 27.1
1 21 4.35, 8.40
1 27-28 1.19, 7.29
Apple EC 1.5 2 45-48 0.412, 0.444
2 60-63 0.292, 0.306
2 77-79 0.288, 0.316
3 30-33 0.590, 0.711
3 45-48 0.332, 0.636
3 61-64 0.522, 0.580
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
root leaves
Radish Dust 1.8 1 65 <0.002 <0.002
1 65 <0.002 <0.002
Granules 0.3 1 24 0.001-0.157
1 65-66 <0.001-0.007
0.6 1 24 0.002-0.080
1 65-66 <0.001-0.001
160 milk samples were analysed for residues of cyanofenphos and
cyanofenphos-oxon during the 30-day feeding period. Residues of
cyanofenphos were detected only in samples from the highest dose
group. The maximum residue found was 0.4 mg/kg on the second and
fourth sampling day. Cyanofenphos-oxon residues reached a peak of
0.061 mg/kg on the fourth sampling day which dropped to about 0.020
mg/kg throughout the remainder of the 30 days (Jenkins et al., 1975).
In plants
The metabolism of cyanofenphos-14C (CN) by bifoliate bean plants
(phaseolus vulgaris, L.) grown in a greenhouse was studied by Chiba et
al. (1973). Little translocation was found when cyanofenphos-14C was
applied to the leaf. It was not taken up from water into the aerial
parts of the plant and most of the radioactivity was in the roots in
the form of intact cyanofenphos. Cyanofenphos seems to be fairly
persistent under these experimental conditions. Approximately 50% of
the compound initially applied remained in the plant samples two weeks
after foliar application.
The persistence of cyanofenphos was also observed in several
other crops including rice grains, vegetables and fruits (Sumitomo,
1975). 4-cyanophenol and desethyl cyanofenphos-oxon were detected as
metabolites by TLC. However, neither cyanofenphos-oxon nor desethyl
cyanofenphos was positively identified.
In soils
Cyanofenphos-14C (CN) was fairly persistent when incorporated
into soil (volcanic ash loam) at 10 mg/kg. 53% of the initial
radioactivity remained after three weeks. In comparison, when
Cyanox-14C (the corresponding O,O-dimethyl phosphorothionate ester of
p-cyanophenol) was incorporated into the soil at the same level, only
6% of the radioactivity remained after two weeks. No degradation
products in soils were identified (Chiba et al., 1973).
Photodecomposition
Aqueous solutions containing 7 mg/l of cyanofenphos-14C (CN)
were exposed to bright sunlight for 20 days, and the resultant
photoproducts were examined. After 20 days exposure, 78.3% of the
recovered radioactivity was intact cyanofenphos, and by extrapolation,
the half-life is presumed to be about 50 days. The predominant
photoproducts were cyanofenphos-oxon and p-cyanophenol, amounting to
7.8% and 4.8% respectively. These two products tend to increase on
longer irradiation. A non-radioactive photoproduct was identified as
ethyl phenylphosphonic acid. The following compounds were not
detected: cyanofenphos-S-isomer, desethyl-cyanofenphos,
desethyl-cyanofenphos-oxon, radioactive carbon dioxide and hydrogen
cyanide.
Unlike aqueous solutions, cyanofenphos deposits on silica gel
chromatoplates underwent rapid photodecomposition with a half-life of
two days, yielding mainly cyanofenphos-oxon and p-cyanophenol. A trace
of 2-hydroxy-5-cyanobenzoic acid was detected. This compound as well
as p-cyanobenzoic acid was also produced, in small quantities, by
irradiation of an acetone solution of cyanofenphos or p-cyanophenol
with UV light. Several pesticides and antioxidants such as rotenone,
phenazine, phenazine-N-oxide, and 2,5-tert-butylhydroquinone
accelerate the photolysis of cyanofenphos, with accompanying increase
of the oxon and p-cyanophenol, while beta-carotene, xanthophyll and
chlorophyll are inactive (Mikami et al., 1975).
METHODS OF RESIDUE ANALYSIS
Residues of cyanofenphos are determined by gas-liquid
chromatography with either flame thermionic or with flame photometric
detection (Sumitomo Chemical Co. Ltd, 1975). The solvents and
techniques used to extract cyanofenphos from samples are similar to
those used for fenitrothion (FAO/WHO, 1975b). Samples with a high
water content such as fruits and vegetables are blended with polar
solvents (acetone, acetonitrile, methanol or
acetonitrile-methanol-water mixtures). Samples with a high content of
fat or oil such as fresh soybeans are extracted with acetone followed
by chloroform. Dry samples such as rice grains are extracted with
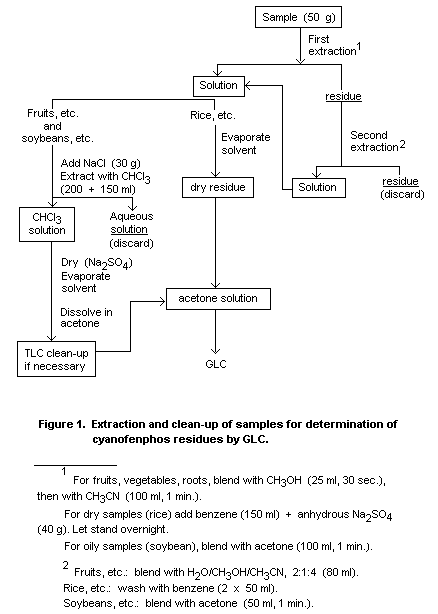
benzene. A suitable extraction scheme is shown in Figure 1.
Sumitomo Chemical Co. Ltd (1975) have reported recoveries of
cyanofenphos added to several crops (Table 3). Gas chromatography was
on a column containing a mixture of 10% DC-200 and 20% OV-1 on
silanized Chromosorb W with the column, injector and detector at
240°C. The carrier gas was helium and EPN was used as an internal
standard. The limit of detection was about 0.001 mg/kg with a
potassium bromide thermionic detector.
TABLE 3. Recoveries of cyanofenphos added to crop samples
Crop Cyanofenphos added, mg/kg Recovery, %
Cabbages 0.2 97.2
Sugar beets 0.02 85.2
Radishes (root) 0.02 92.3
Soybeans 0.02 93.1
Rice grains 0.1 99.3
TLC on silica gel with benzene as the developing solvent is used
to confirm identity and if necessary as a final clean-up step.
Cyanofenphos (Rf about 0.4) is visualized under UV light or by
spraying with palladium chloride solution.
ther information on identity and properties
Physical state: pure compound, white crystalline solid.
Technical product, slightly yellowish solid
with characteristic odour
Melting point: 83°C
Vapour pressure: 10 mm Hg at 225°C
Specific gravity: D20 = 1.29
4
Solubility: practically insoluble in water, soluble in
ketones (acetone, methyl ethyl ketone, methyl
isobutyl ketone, cyclohexanone), hydrocarbons
(benzene, toluene, xylene,
methylnaphthalene), methylene dichloride,
chloroform, acetonitrile, dimethyl formamide,
ethyl cellosolve. Slightly soluble in
methanol, isopropanol, kerosene, soybean oil.
Stability: stable for two years under normal storage
conditions. Half-life values in aqueous
solutions at 60°C and
pH 6.0 - 150 days
pH 8.0 - 227 days
pH 10.0 - 68 days
The composition of the technical product is reported to be as follows.
Active ingredient 92.0 - 95.0%
O,O-diethyl phenylphosphonothioate 3.0 - 5.0%
O,O-di(4-cyanophenyl)phenylphosphonothioate 1.0 - 2.0%
O-ethyl phenylphosphonochloridothioate <0.2%
O,S-diethyl phenylphosphonodithioate <0.2%
S-ethyl O-(4-cyanophenyl)phenylphosphonothioate <0.5%
4-cyanophenol <0.3%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Sumitomo Chemical Company in their submission have abstracted a
study on the absorption and metabolism of C14 labelled cyanofenphos
in the mouse. This was taken from a private communication (S. Kato and
J. Miyamoto, presented at T.R. commission of IUPAC Meeting in
September 1974) which was not available for review. They did present
data on the absorption and biodegradation patterns of O,O dimethyl
O-(4-cyanophenyl) phosphorothiate in the rat (Wakimura and Miyamoto,
1971) and of O-(4 bromo-2,5 dichlorophenyl) O-methyl phenylphosphonate
(Leptophos) in mice and on cotton plants (Holmstead et al., 1973). The
company has implied that cyanofenphos has similar absorption and
biodegradation patterns as the above-mentioned compounds.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Chicken
Cyanofenphos was administered orally as a single dose of 100
mg/kg/ bodyweight to eight white leghorn chickens (1-1.5 years of
age). The birds were observed for three weeks. The same treatment was
repeated and birds observed for a further three weeks. Atropine and
2-PAM were used to protect the birds from acute intoxication. One bird
died during first test period. Birds that survived through the two
treatment periods did not exhibit delayed paralysis of the legs.
Histopathologic examination of sciatic nerve and spinal cord did not
reveal demyelination. A single dose of TOCP (400 mg/kg body weight)
produced paralysis in legs after two weeks with degeneration and
demyelination of the sciatic nerve.
In a further study cyanofenphos was administered at daily doses
of 2 and 4 mg/kg body weight six days a week for four weeks and
observed for a further four weeks. Both groups exhibited anorexia, a
decrease in spontaneous motor activity and in body weight gain. No
paralysis was noted. No anomalies were observed in sciatic nerve and
spinal cord on histopathologic examination (Kadota et al., 1972).
Special studies on reproduction
In a three-generation reproduction study (two litters per
generation) eight male and 16 female rats received dietary
concentrations of 0, 1, 3 and 10 ppm cyanofenphos during the first d
generation. For the second and third generations those selected from
the 1 ppm dietary groups the concentration of cyanofenphos in the diet
was increased to 30 ppm. No treatment related effects were observed on
body weight gain, survival or ophthalmic and pathological examinations
in parental animals. However, reduced mating indices during third
generation and lowered male and female fertility during second and
third generation were consistently observed at 30 ppm. Pup viability
was reduced at 30 ppm. No compound related effects were observed with
respect to weanling weights, gross external observation or
histopathological examination of F3b progeny (Smith et al., 1975).
Special studies on teratogenicity
Groups of pregnant rabbits received 0, 1.0, and 3.0 mg/kg body
weight/day of cyanofenphos via gelatin capsule from day 6 through day
18 of gestation. Animals were sacrificed at day 29 of gestation and
pups removed by Caesarean section. No significant differences were
found in total number of implantation sites, number of resorptions and
number of live young. Body weights of foetuses were similar for
treated groups and were comparable to control. Twenty-four hour
survival was not affected. No visceral or skeletal abnormalities were
noted. The results obtained with the positive control (thalidomide)
demonstrated the susceptibility of this strain to a teratogenic agent
(Ladd and Smith, 1973).
Acute toxicity
Technical compound (purity 92%)
LD50 mg/kg
Species Route Solvent M F Reference
mouse oral Tween 80 36 30 Kadota and Miyamoto, 1973
S.C. Tween 80 68 48 Kadota and Miyamoto, 1973
I.P. Tween 80 27.5 19 Kadota and Miyamoto, 1973
Dermal Tween 80 >1 000 >1 000 Kadota and Miyamoto, 1973
rat oral Tween 80 89 32 Miyamoto and Kadota, 1972
S.C. Tween 80 200 50 Miyamoto and Kadota, 1972
Gum Arabic 1 120 380 Miyamoto and Kadota, 1972
I.P. Tween 80 141 30 Miyamoto and Kadota, 1972
Dermal peanut oil >1 000 640 Miyamoto and Kadota, 1972
hen oral Tween 80 60 Kadota et al., 1972
A single instillation of 0.05 ml suspension of cyanofenphos in
Tween 80 was found to be irritating to the rabbit eye at 0.2% and
above. At 1.0% eye closure, congestion of the conjunctiva and
lacrimation were noted. No changes were observed in cornea,
conjunctival reflex, corneal reflex and pupil diameter.
When cyanofenphos suspension was instilled daily (six days a
week) for four weeks at dosage levels of 0.2 and 1.0%, similar eye
reactions were observed which did not appear to increase in intensity
with time. Myosis was not observed (Kagoshima et al., 1972)
Two groups of guinea pigs were sensitized by injecting
intracutaneously 5% and 1% solutions of cyanofenphos in corn oil every
other day for 20 days. Each animal was challenged with an
intracutaneous injection of cyanofenphos in corn oil and a dermal
application of this compound in acetone 14 days after last sensitizing
dose. Local swelling was observed at injection site both on
sensitizing and challenging the animals. No remarkable changes with
dermal challenge (Koda et al., 1972).
Short-term studies
Dog
Four groups of dogs (four male and four female/group) were fed 0,
10, 30 and 100 ppm cyanofenphos in the diet for 12 months. Two male
dogs at 100 ppm displayed a moderate ataxia of the hindquarters during
the final month of testing. Growth, food consumption, mortality,
haematology, blood chemistry, urinalysis, ophthalmologic examination,
gross and histopathology revealed no compound related effect. Liver to
body and brain weight ratio was increased in four of eight dogs at 100
ppm. Plasma and red blood cell cholinesterase activities at 100 ppm
were decreased when determined at one, three and nine months. Red
blood cell cholinesterase was also reduced at 12 months. No inhibitory
effect on brain cholinesterase was noted at any level (Marias et al.,
1974).
Cow
Groups of four cows were fed diets containing 0, 5, 15 and 50 ppm
cyanofenphos. On day 30 of treatment three cows/group were sacrificed,
the remaining animal of each group was fed the control diet for a
further 30 days. Milk, liver, kidney, muscle and fat were collected
from each animal and analysed for residues.
No adverse reactions due to the feeding of cyanofenphos were
observed. Trace levels of cyanofenphos and its oxon metabolite were
found only in milk and tissues of cows fed 50 ppm. Residue levels were
below the limit of detection (<0.002 ppm) during the withdrawal
period (Ladd and Winegender, 1975).
Long-term studies
Rat
Groups of 60 rats (six weeks of age) were fed cyanofenphos at
dietary levels of 0, 10, 30, 100 and 200 ppm in males and 0, 5, 15, 50
and 100 ppm in females for 24 months. There was no apparent evidence
of significant changes in general behaviour, mortality, body weight
gain, food and water consumption, urinalysis, haematology, clinical
biochemistry, organ to body weight ratio or gross and microscopic
examination of tissues. Plasma, red blood cell and brain
cholinesterase were depressed in males at 100 and 200 ppm and in
females at 50 and 100 ppm (Kadota et al., 1974).
COMMENTS
Although data on the absorption, metabolism and excretion have
been presented on two similar chemicals and reference made to data on
cyanofenphos from a private communication, no metabolic data on
cyanofenphos per se have been received.
There were no teratogenic or neurotoxic effects observed with
cyanofenphos. Reproductive effects were observed in both parental
animal and progeny at 30 ppm. The no-effect level was 10 ppm. In a
short-term (12 month) study with the dog the no-effect level was 30
ppm in the diet based on plasma and erythrocyte cholinesterase
activity. In a long-term study in the rat, the no-effect level for
cholinesterase inhibition was 15 ppm. Based on the available
information and considering the lack of metabolic data a temporary
acceptable daily intake was allocated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg
Dog: 30 ppm in the diet equivalent to 0.75 mg/kg
Estimation of temporary acceptable daily intake
0-0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cyanofenphos is mainly applied against insect pests including
Lepidoptera, Diptera, Orthoptera, Hemiptera and Coleoptera in field
crops, fruits and vegetables. It is also applied for the control of
insects affecting livestock such as blow flies, lice and ticks.
The percentage of cyanofenphos used in different fields of
application varies according to current pest situations. A rough
estimate is 60% for field crops (rice, cotton, legumes, cereals), 20%
for vegetable crops (crucifers, onions, cucurbits), 15% for pome
fruit, stone fruit, citrus and tea, and 5% for veterinary uses.
Approximate dosages and frequencies of application are shown in
Table 1.
TABLE 1. Dosages and frequencies of application of cyanofenphos
Crop Dosage and frequency of application
Rice 0.25-0.75 kg a.i./ha, 2-4 times/season
Pome and stone fruits 0.025-0.75 kg a.i./ha, 2-4 times/season
Vegetables 0.25-0.75 kg a.i./ha, 2-5 times/season
Tea 0.5-1 kg a.i./ha, 1-3 times/season
Cotton 0.5-1 kg a.i./ha, 2-6 times/season
Sheep 0.02-0.05% by plunge dips, once a year
0.04-0.06% by shower dips, once a year
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data on residues resulting from supervised trials were
available. Different countries have different patterns of consumption
and usage of agricultural commodities, e.g. in some countries rice
straw after harvest is used as a feed of farm animals. Table 2
summarizes the residue levels which have been found when the product
is used according to "good agricultural practice" in Japan (Sumitomo,
1975).
FATE OF RESIDUES
In animals
Following oral administration of cyanofenphos to dairy cows at
dietary levels of 5, 15 and 50 ppm for 30 consecutive days, samples of
muscle, fat, liver, kidney and milk were analysed to determine
contents of cyanofenphos and cyanofenphos-oxon. In the tissue samples
residue levels were highest in the fat and decreased in the order fat
>> liver > kidney = muscle. Levels of cyanofenphos ranged from
approximately 1 mg/kg in the fat to 0.012 mg/kg in the kidney and
muscle in the highest dose group. The oxon levels ranged from
approximately 0.08 mg/kg in fat to less than 0.0004 mg/kg in liver,
kidney and muscle from the same group. Residues of cyanofenphos
(<0.002-0.013 mg/kg) were also found in fat samples from the lower
and middle dose groups and in liver samples from the middle group.
TABLE 2. Cyanofenphos residues resulting from supervised trials in Japan
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
rough rice straw
Rice EC 0.4, 0.5 2 59 0 0.002 2.26
0.5 4 51 0 0.014 6.14
0.6 2 45 0 0.034 0.546
0.6 4 37 0 0.141 1.26
0.5, 0.75 2 40 0 0.055 0.695
0.75 4 31 0 0.196 4.86
Dust 0.6 1 47 0.011
0.6 2 43-59 <0.002-0.010
0.45, 0.6 2 47 0.005
0.6 3 44-50 <0.002-0.023
0.6 4 32 0.021
42 0.012
0.45, 0.6 2-2 37 0.002
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
green pod
Soybean EC 0.5 2 7 0.142, 0.228 6.00, 6.571
3 7 0.402 8.978
4 7 0.156 8.50
dried bean
2 34-50 0.075, 0.186
3 50 0.108
4 34 0.214
fresh peel
Peach EC 0.75 2 3 (4 samples) 0.004-0.028 6.3-31.3
2 7 " 0.006-0.009 7.8-27.0
2 14 " 0.004-0.012 6.8-13.7
4 3 " 0.007-0.038 7.2-39.4
4 7 " 0.005-0.014 6.4-32.1
4 14 " 0.004-0.014 4.8-18.4
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage EC 0.375 2 28 0.002
2 35 <0.001
2 42 <0.001
3 14 0.123
3 21 0.002
3 28 0.001
0.5 2 7 0.186
2 14 0.092
2 21 0.028
4 7 0.258
4 14 0.108
4 21 0.026
0.75 2 7 1.45
2 14 0.902
2 20 0.721
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage 2 28 0.020
(continued)
2 35 0.015
2 42 0.010
3 14 0.050, 0.056
3 21 0.004, 0.008
3 28 0.001, 0.002
0.5, 0.75 2 + 2 7 2.02
2 + 2 14 2.02
2 + 2 20 1.24
1.5 3 14 0.083
3 21 0.008
3 28 0.002
Onion D 1.35 1 189-234 <0.002
Cucumber D 0.9 1 39-42 <0.001
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Ginger EC 0.7 3 45-52 0.004, 0.008
(root)
6 " 0.006, 0.012
Tea EC 1 1 7 48.6, 49.2
(steamed)
1 14 23.0, 27.1
1 21 4.35, 8.40
1 27-28 1.19, 7.29
Apple EC 1.5 2 45-48 0.412, 0.444
2 60-63 0.292, 0.306
2 77-79 0.288, 0.316
3 30-33 0.590, 0.711
3 45-48 0.332, 0.636
3 61-64 0.522, 0.580
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
root leaves
Radish Dust 1.8 1 65 <0.002 <0.002
1 65 <0.002 <0.002
Granules 0.3 1 24 0.001-0.157
1 65-66 <0.001-0.007
0.6 1 24 0.002-0.080
1 65-66 <0.001-0.001
160 milk samples were analysed for residues of cyanofenphos and
cyanofenphos-oxon during the 30-day feeding period. Residues of
cyanofenphos were detected only in samples from the highest dose
group. The maximum residue found was 0.4 mg/kg on the second and
fourth sampling day. Cyanofenphos-oxon residues reached a peak of
0.061 mg/kg on the fourth sampling day which dropped to about 0.020
mg/kg throughout the remainder of the 30 days (Jenkins et al., 1975).
In plants
The metabolism of cyanofenphos-14C (CN) by bifoliate bean plants
(phaseolus vulgaris, L.) grown in a greenhouse was studied by Chiba et
al. (1973). Little translocation was found when cyanofenphos-14C was
applied to the leaf. It was not taken up from water into the aerial
parts of the plant and most of the radioactivity was in the roots in
the form of intact cyanofenphos. Cyanofenphos seems to be fairly
persistent under these experimental conditions. Approximately 50% of
the compound initially applied remained in the plant samples two weeks
after foliar application.
The persistence of cyanofenphos was also observed in several
other crops including rice grains, vegetables and fruits (Sumitomo,
1975). 4-cyanophenol and desethyl cyanofenphos-oxon were detected as
metabolites by TLC. However, neither cyanofenphos-oxon nor desethyl
cyanofenphos was positively identified.
In soils
Cyanofenphos-14C (CN) was fairly persistent when incorporated
into soil (volcanic ash loam) at 10 mg/kg. 53% of the initial
radioactivity remained after three weeks. In comparison, when
Cyanox-14C (the corresponding O,O-dimethyl phosphorothionate ester of
p-cyanophenol) was incorporated into the soil at the same level, only
6% of the radioactivity remained after two weeks. No degradation
products in soils were identified (Chiba et al., 1973).
Photodecomposition
Aqueous solutions containing 7 mg/l of cyanofenphos-14C (CN)
were exposed to bright sunlight for 20 days, and the resultant
photoproducts were examined. After 20 days exposure, 78.3% of the
recovered radioactivity was intact cyanofenphos, and by extrapolation,
the half-life is presumed to be about 50 days. The predominant
photoproducts were cyanofenphos-oxon and p-cyanophenol, amounting to
7.8% and 4.8% respectively. These two products tend to increase on
longer irradiation. A non-radioactive photoproduct was identified as
ethyl phenylphosphonic acid. The following compounds were not
detected: cyanofenphos-S-isomer, desethyl-cyanofenphos,
desethyl-cyanofenphos-oxon, radioactive carbon dioxide and hydrogen
cyanide.
Unlike aqueous solutions, cyanofenphos deposits on silica gel
chromatoplates underwent rapid photodecomposition with a half-life of
two days, yielding mainly cyanofenphos-oxon and p-cyanophenol. A trace
of 2-hydroxy-5-cyanobenzoic acid was detected. This compound as well
as p-cyanobenzoic acid was also produced, in small quantities, by
irradiation of an acetone solution of cyanofenphos or p-cyanophenol
with UV light. Several pesticides and antioxidants such as rotenone,
phenazine, phenazine-N-oxide, and 2,5-tert-butylhydroquinone
accelerate the photolysis of cyanofenphos, with accompanying increase
of the oxon and p-cyanophenol, while beta-carotene, xanthophyll and
chlorophyll are inactive (Mikami et al., 1975).
METHODS OF RESIDUE ANALYSIS
Residues of cyanofenphos are determined by gas-liquid
chromatography with either flame thermionic or with flame photometric
detection (Sumitomo Chemical Co. Ltd, 1975). The solvents and
techniques used to extract cyanofenphos from samples are similar to
those used for fenitrothion (FAO/WHO, 1975b). Samples with a high
water content such as fruits and vegetables are blended with polar
solvents (acetone, acetonitrile, methanol or
acetonitrile-methanol-water mixtures). Samples with a high content of
fat or oil such as fresh soybeans are extracted with acetone followed
by chloroform. Dry samples such as rice grains are extracted with
benzene. A suitable extraction scheme is shown in Figure 1.
Sumitomo Chemical Co. Ltd (1975) have reported recoveries of
cyanofenphos added to several crops (Table 3). Gas chromatography was
on a column containing a mixture of 10% DC-200 and 20% OV-1 on
silanized Chromosorb W with the column, injector and detector at
240°C. The carrier gas was helium and EPN was used as an internal
standard. The limit of detection was about 0.001 mg/kg with a
potassium bromide thermionic detector.
TABLE 3. Recoveries of cyanofenphos added to crop samples
Crop Cyanofenphos added, mg/kg Recovery, %
Cabbages 0.2 97.2
Sugar beets 0.02 85.2
Radishes (root) 0.02 92.3
Soybeans 0.02 93.1
Rice grains 0.1 99.3
TLC on silica gel with benzene as the developing solvent is used
to confirm identity and if necessary as a final clean-up step.
Cyanofenphos (Rf about 0.4) is visualized under UV light or by
spraying with palladium chloride solution.
 APPRAISAL
Cyanofenphos has a wide insecticidal activity, and since it is
moderately persistent it offers a possible alternative to the
persistent organochlorine compounds. Considerations of cost limit its
use for this purpose at present, however.
Although the persistence of cyanofenphos can lead to residues in
and on crops at harvest, the potentially toxic metabolite
cyanofenphos-oxon has not been found in plants.
The translocation of cyanofenphos in plants is very limited.
Although radio tracer studies have been undertaken, the identities of
the metabolites in plants have not been fully elucidated. Studies with
the cyanofenphos molecule labelled in other positions might provide a
more detailed understanding of the metabolic pathways. In particular
the fate of the P-C ring bond in plants and animals remains to be
fully determined for cyanofenphos and some other phosphonates.
No information was available on the tendency to partition into
vegetable oils and animal fats. Information is needed on the
distribution of residues in rice and their fate in processing and
cooking.
Residue data from many supervised trials conducted in Japan were
made available and were considered sufficient to make recommendations
for temporary maximum residue limits on several commodities.
RECOMMENDATIONS
The following temporary maximum residue limits are based on the
determination of the parent compound only.
TEMPORARY MAXIMUM RESIDUE LIMITS
Pre-harvest intervals
Limit, on which recommendations
Commodity mg/kg are based, days
Cabbage 2 7
Peaches 1 14
Soybean (fresh, without pods) 0.5 7
Soybean, dry 0.5 30
Radishes (roots) 0.2 21
Rice (hulled) 0.2 30
Cucumber 0.05* 40
Ginger 0.05* 40
Onions 0.05* 150
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1978)
1. Data on absorption, metabolism and excretion in at least one
mammalian species.
2. Studies to identify and investigate toxicity of plant
metabolites.
3. Distribution of residues in rice and their fate during processing
and cooking.
4. Information on residues in dry green and dry manufactured
(fermented) tea.
5. Studies on residues in sheep following dipping and spraying.
6. Results of ongoing studies on the feeding of poultry to determine
the fate of residues in tissues and eggs.
7. Further information on the fate in cabbage during processing and
cooking.
DESIRABLE
1. Observations in man including cholinesterase studies.
2. Appropriate mutagenic study.
3. A further long-term study.
4. Information from trials in countries other than Japan.
5. Studies to determine whether cyanofenphos residues can be
determined by current multiresidue methods.
REFERENCES
Chiba, M., Kato, S. and Yamamoto, I. (1973) Metabolism of Cyanox and
Surecide in plant and degradation in soil. Unpublished report
submitted from Tokyo University of Agriculture, Tokyo, Japan. Private
Communication to Sumitomo Chemical Co. Presented by J. Miyamoto at
Commission on Terminal Residues, IUPAC Meeting, 1974.
Holmstead, R. L., Fukuto, T. R. and March, R. B. (1973) The Metabolism
of O-(4-Bromo-2,5-Dichlorophenyl) O-Methyl Phenylphosphonothioate
(Leptophos) in White Mice and on Cotton Plants. Archives of
Environmental Contamination and Toxicology, 1:133-147.
Jenkins, D. H., Kinoshita, F. K. and Keplinger, M. L. (1975) Meat and
milk residue study with Surecide in dairy cattle. Unpublished report
from Industrial BIOTEST Laboratories, U.S.A., submitted by Sumitomo
Chemical Co. Ltd., Osaka.
Kadota, T., Kagoshima, M., Miyamota, J. and Nobuyuki, I. (1972) Acute
Toxicity and Delayed Neurotoxicity of B-4087 (Surecide) in Hens.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health organization by Sumitomo Chemical Co., Japan.
Kadota, T. and Miyamota, J. (1973) Acute Toxicity of Surecide in Mice.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health Organization by Sumitomo Chemical Co., Japan.
Kadota, T., Hosokawa, T., Kohda, H., Miyamoto, J. and Itoh, N. (1974)
Two year Chronic Toxicity Study with Surecide in Rats. Unpublished
report from the Research Department, Sumitomo, submitted to the World
Health Organization by Sumitomo Chemical Co., Japan.
Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Acute and Subacute
Eye Irritation Tests of Surecide in Rabbits. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Koda, H., Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Skin
Sensitization Test of Surecide in Guinea Pigs. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
organization by Sumitomo Chemical Co., Japan.
Ladd, R. and Smith, P. S. (1973) Teratogenic Study with Surecide in
Albino Rabbits. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Ladd, R. and Winegender, R. J. (1975) Meat and Milk Residue Study with
Surecide in Dairy Cattle. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Marias, A., Burtner, B. R., Kennedy, G. L. and Keplinger, M. L. (1974)
12 Month Chronic Oral Toxicity Study with Surecide Technical in Beagle
Dogs. Unpublished report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Mikami, N., Ohkawa, H. and Miyamoto, J. (1975) Photo-decomposition of
Surecide (O-ethyl O-(4-cyanophenyl) phenylphosphonothioate).
Unpublished report submitted by Sumitomo Chemical Co. Ltd, Osaka.
Presented by Miyamoto, J. at TR Commission of IUPAC Meeting in 1975.
Miyamota, J. and Kadota, T. (1972) Comparative Acute Toxicological
Studies with Surecide and EPN in Rats. Unpublished report from the
Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Smith, S., Kennedy, G. L., Kinoshita, F. K. and Keplinger, M. L.
(1975) Three Generation Reproduction Study with Surecide in Albino
Rats. Unpublished report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Sumitomo Chemical Co. Ltd (1975) Unpublished report.
APPRAISAL
Cyanofenphos has a wide insecticidal activity, and since it is
moderately persistent it offers a possible alternative to the
persistent organochlorine compounds. Considerations of cost limit its
use for this purpose at present, however.
Although the persistence of cyanofenphos can lead to residues in
and on crops at harvest, the potentially toxic metabolite
cyanofenphos-oxon has not been found in plants.
The translocation of cyanofenphos in plants is very limited.
Although radio tracer studies have been undertaken, the identities of
the metabolites in plants have not been fully elucidated. Studies with
the cyanofenphos molecule labelled in other positions might provide a
more detailed understanding of the metabolic pathways. In particular
the fate of the P-C ring bond in plants and animals remains to be
fully determined for cyanofenphos and some other phosphonates.
No information was available on the tendency to partition into
vegetable oils and animal fats. Information is needed on the
distribution of residues in rice and their fate in processing and
cooking.
Residue data from many supervised trials conducted in Japan were
made available and were considered sufficient to make recommendations
for temporary maximum residue limits on several commodities.
RECOMMENDATIONS
The following temporary maximum residue limits are based on the
determination of the parent compound only.
TEMPORARY MAXIMUM RESIDUE LIMITS
Pre-harvest intervals
Limit, on which recommendations
Commodity mg/kg are based, days
Cabbage 2 7
Peaches 1 14
Soybean (fresh, without pods) 0.5 7
Soybean, dry 0.5 30
Radishes (roots) 0.2 21
Rice (hulled) 0.2 30
Cucumber 0.05* 40
Ginger 0.05* 40
Onions 0.05* 150
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1978)
1. Data on absorption, metabolism and excretion in at least one
mammalian species.
2. Studies to identify and investigate toxicity of plant
metabolites.
3. Distribution of residues in rice and their fate during processing
and cooking.
4. Information on residues in dry green and dry manufactured
(fermented) tea.
5. Studies on residues in sheep following dipping and spraying.
6. Results of ongoing studies on the feeding of poultry to determine
the fate of residues in tissues and eggs.
7. Further information on the fate in cabbage during processing and
cooking.
DESIRABLE
1. Observations in man including cholinesterase studies.
2. Appropriate mutagenic study.
3. A further long-term study.
4. Information from trials in countries other than Japan.
5. Studies to determine whether cyanofenphos residues can be
determined by current multiresidue methods.
REFERENCES
Chiba, M., Kato, S. and Yamamoto, I. (1973) Metabolism of Cyanox and
Surecide in plant and degradation in soil. Unpublished report
submitted from Tokyo University of Agriculture, Tokyo, Japan. Private
Communication to Sumitomo Chemical Co. Presented by J. Miyamoto at
Commission on Terminal Residues, IUPAC Meeting, 1974.
Holmstead, R. L., Fukuto, T. R. and March, R. B. (1973) The Metabolism
of O-(4-Bromo-2,5-Dichlorophenyl) O-Methyl Phenylphosphonothioate
(Leptophos) in White Mice and on Cotton Plants. Archives of
Environmental Contamination and Toxicology, 1:133-147.
Jenkins, D. H., Kinoshita, F. K. and Keplinger, M. L. (1975) Meat and
milk residue study with Surecide in dairy cattle. Unpublished report
from Industrial BIOTEST Laboratories, U.S.A., submitted by Sumitomo
Chemical Co. Ltd., Osaka.
Kadota, T., Kagoshima, M., Miyamota, J. and Nobuyuki, I. (1972) Acute
Toxicity and Delayed Neurotoxicity of B-4087 (Surecide) in Hens.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health organization by Sumitomo Chemical Co., Japan.
Kadota, T. and Miyamota, J. (1973) Acute Toxicity of Surecide in Mice.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health Organization by Sumitomo Chemical Co., Japan.
Kadota, T., Hosokawa, T., Kohda, H., Miyamoto, J. and Itoh, N. (1974)
Two year Chronic Toxicity Study with Surecide in Rats. Unpublished
report from the Research Department, Sumitomo, submitted to the World
Health Organization by Sumitomo Chemical Co., Japan.
Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Acute and Subacute
Eye Irritation Tests of Surecide in Rabbits. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Koda, H., Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Skin
Sensitization Test of Surecide in Guinea Pigs. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
organization by Sumitomo Chemical Co., Japan.
Ladd, R. and Smith, P. S. (1973) Teratogenic Study with Surecide in
Albino Rabbits. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Ladd, R. and Winegender, R. J. (1975) Meat and Milk Residue Study with
Surecide in Dairy Cattle. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Marias, A., Burtner, B. R., Kennedy, G. L. and Keplinger, M. L. (1974)
12 Month Chronic Oral Toxicity Study with Surecide Technical in Beagle
Dogs. Unpublished report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Mikami, N., Ohkawa, H. and Miyamoto, J. (1975) Photo-decomposition of
Surecide (O-ethyl O-(4-cyanophenyl) phenylphosphonothioate).
Unpublished report submitted by Sumitomo Chemical Co. Ltd, Osaka.
Presented by Miyamoto, J. at TR Commission of IUPAC Meeting in 1975.
Miyamota, J. and Kadota, T. (1972) Comparative Acute Toxicological
Studies with Surecide and EPN in Rats. Unpublished report from the
Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Smith, S., Kennedy, G. L., Kinoshita, F. K. and Keplinger, M. L.
(1975) Three Generation Reproduction Study with Surecide in Albino
Rats. Unpublished report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Sumitomo Chemical Co. Ltd (1975) Unpublished report.
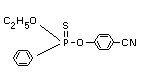 ther information on identity and properties
Physical state: pure compound, white crystalline solid.
Technical product, slightly yellowish solid
with characteristic odour
Melting point: 83°C
Vapour pressure: 10 mm Hg at 225°C
Specific gravity: D20 = 1.29
4
Solubility: practically insoluble in water, soluble in
ketones (acetone, methyl ethyl ketone, methyl
isobutyl ketone, cyclohexanone), hydrocarbons
(benzene, toluene, xylene,
methylnaphthalene), methylene dichloride,
chloroform, acetonitrile, dimethyl formamide,
ethyl cellosolve. Slightly soluble in
methanol, isopropanol, kerosene, soybean oil.
Stability: stable for two years under normal storage
conditions. Half-life values in aqueous
solutions at 60°C and
pH 6.0 - 150 days
pH 8.0 - 227 days
pH 10.0 - 68 days
The composition of the technical product is reported to be as follows.
Active ingredient 92.0 - 95.0%
O,O-diethyl phenylphosphonothioate 3.0 - 5.0%
O,O-di(4-cyanophenyl)phenylphosphonothioate 1.0 - 2.0%
O-ethyl phenylphosphonochloridothioate <0.2%
O,S-diethyl phenylphosphonodithioate <0.2%
S-ethyl O-(4-cyanophenyl)phenylphosphonothioate <0.5%
4-cyanophenol <0.3%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Sumitomo Chemical Company in their submission have abstracted a
study on the absorption and metabolism of C14 labelled cyanofenphos
in the mouse. This was taken from a private communication (S. Kato and
J. Miyamoto, presented at T.R. commission of IUPAC Meeting in
September 1974) which was not available for review. They did present
data on the absorption and biodegradation patterns of O,O dimethyl
O-(4-cyanophenyl) phosphorothiate in the rat (Wakimura and Miyamoto,
1971) and of O-(4 bromo-2,5 dichlorophenyl) O-methyl phenylphosphonate
(Leptophos) in mice and on cotton plants (Holmstead et al., 1973). The
company has implied that cyanofenphos has similar absorption and
biodegradation patterns as the above-mentioned compounds.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Chicken
Cyanofenphos was administered orally as a single dose of 100
mg/kg/ bodyweight to eight white leghorn chickens (1-1.5 years of
age). The birds were observed for three weeks. The same treatment was
repeated and birds observed for a further three weeks. Atropine and
2-PAM were used to protect the birds from acute intoxication. One bird
died during first test period. Birds that survived through the two
treatment periods did not exhibit delayed paralysis of the legs.
Histopathologic examination of sciatic nerve and spinal cord did not
reveal demyelination. A single dose of TOCP (400 mg/kg body weight)
produced paralysis in legs after two weeks with degeneration and
demyelination of the sciatic nerve.
In a further study cyanofenphos was administered at daily doses
of 2 and 4 mg/kg body weight six days a week for four weeks and
observed for a further four weeks. Both groups exhibited anorexia, a
decrease in spontaneous motor activity and in body weight gain. No
paralysis was noted. No anomalies were observed in sciatic nerve and
spinal cord on histopathologic examination (Kadota et al., 1972).
Special studies on reproduction
In a three-generation reproduction study (two litters per
generation) eight male and 16 female rats received dietary
concentrations of 0, 1, 3 and 10 ppm cyanofenphos during the first d
generation. For the second and third generations those selected from
the 1 ppm dietary groups the concentration of cyanofenphos in the diet
was increased to 30 ppm. No treatment related effects were observed on
body weight gain, survival or ophthalmic and pathological examinations
in parental animals. However, reduced mating indices during third
generation and lowered male and female fertility during second and
third generation were consistently observed at 30 ppm. Pup viability
was reduced at 30 ppm. No compound related effects were observed with
respect to weanling weights, gross external observation or
histopathological examination of F3b progeny (Smith et al., 1975).
Special studies on teratogenicity
Groups of pregnant rabbits received 0, 1.0, and 3.0 mg/kg body
weight/day of cyanofenphos via gelatin capsule from day 6 through day
18 of gestation. Animals were sacrificed at day 29 of gestation and
pups removed by Caesarean section. No significant differences were
found in total number of implantation sites, number of resorptions and
number of live young. Body weights of foetuses were similar for
treated groups and were comparable to control. Twenty-four hour
survival was not affected. No visceral or skeletal abnormalities were
noted. The results obtained with the positive control (thalidomide)
demonstrated the susceptibility of this strain to a teratogenic agent
(Ladd and Smith, 1973).
Acute toxicity
Technical compound (purity 92%)
LD50 mg/kg
Species Route Solvent M F Reference
mouse oral Tween 80 36 30 Kadota and Miyamoto, 1973
S.C. Tween 80 68 48 Kadota and Miyamoto, 1973
I.P. Tween 80 27.5 19 Kadota and Miyamoto, 1973
Dermal Tween 80 >1 000 >1 000 Kadota and Miyamoto, 1973
rat oral Tween 80 89 32 Miyamoto and Kadota, 1972
S.C. Tween 80 200 50 Miyamoto and Kadota, 1972
Gum Arabic 1 120 380 Miyamoto and Kadota, 1972
I.P. Tween 80 141 30 Miyamoto and Kadota, 1972
Dermal peanut oil >1 000 640 Miyamoto and Kadota, 1972
hen oral Tween 80 60 Kadota et al., 1972
A single instillation of 0.05 ml suspension of cyanofenphos in
Tween 80 was found to be irritating to the rabbit eye at 0.2% and
above. At 1.0% eye closure, congestion of the conjunctiva and
lacrimation were noted. No changes were observed in cornea,
conjunctival reflex, corneal reflex and pupil diameter.
When cyanofenphos suspension was instilled daily (six days a
week) for four weeks at dosage levels of 0.2 and 1.0%, similar eye
reactions were observed which did not appear to increase in intensity
with time. Myosis was not observed (Kagoshima et al., 1972)
Two groups of guinea pigs were sensitized by injecting
intracutaneously 5% and 1% solutions of cyanofenphos in corn oil every
other day for 20 days. Each animal was challenged with an
intracutaneous injection of cyanofenphos in corn oil and a dermal
application of this compound in acetone 14 days after last sensitizing
dose. Local swelling was observed at injection site both on
sensitizing and challenging the animals. No remarkable changes with
dermal challenge (Koda et al., 1972).
Short-term studies
Dog
Four groups of dogs (four male and four female/group) were fed 0,
10, 30 and 100 ppm cyanofenphos in the diet for 12 months. Two male
dogs at 100 ppm displayed a moderate ataxia of the hindquarters during
the final month of testing. Growth, food consumption, mortality,
haematology, blood chemistry, urinalysis, ophthalmologic examination,
gross and histopathology revealed no compound related effect. Liver to
body and brain weight ratio was increased in four of eight dogs at 100
ppm. Plasma and red blood cell cholinesterase activities at 100 ppm
were decreased when determined at one, three and nine months. Red
blood cell cholinesterase was also reduced at 12 months. No inhibitory
effect on brain cholinesterase was noted at any level (Marias et al.,
1974).
Cow
Groups of four cows were fed diets containing 0, 5, 15 and 50 ppm
cyanofenphos. On day 30 of treatment three cows/group were sacrificed,
the remaining animal of each group was fed the control diet for a
further 30 days. Milk, liver, kidney, muscle and fat were collected
from each animal and analysed for residues.
No adverse reactions due to the feeding of cyanofenphos were
observed. Trace levels of cyanofenphos and its oxon metabolite were
found only in milk and tissues of cows fed 50 ppm. Residue levels were
below the limit of detection (<0.002 ppm) during the withdrawal
period (Ladd and Winegender, 1975).
Long-term studies
Rat
Groups of 60 rats (six weeks of age) were fed cyanofenphos at
dietary levels of 0, 10, 30, 100 and 200 ppm in males and 0, 5, 15, 50
and 100 ppm in females for 24 months. There was no apparent evidence
of significant changes in general behaviour, mortality, body weight
gain, food and water consumption, urinalysis, haematology, clinical
biochemistry, organ to body weight ratio or gross and microscopic
examination of tissues. Plasma, red blood cell and brain
cholinesterase were depressed in males at 100 and 200 ppm and in
females at 50 and 100 ppm (Kadota et al., 1974).
COMMENTS
Although data on the absorption, metabolism and excretion have
been presented on two similar chemicals and reference made to data on
cyanofenphos from a private communication, no metabolic data on
cyanofenphos per se have been received.
There were no teratogenic or neurotoxic effects observed with
cyanofenphos. Reproductive effects were observed in both parental
animal and progeny at 30 ppm. The no-effect level was 10 ppm. In a
short-term (12 month) study with the dog the no-effect level was 30
ppm in the diet based on plasma and erythrocyte cholinesterase
activity. In a long-term study in the rat, the no-effect level for
cholinesterase inhibition was 15 ppm. Based on the available
information and considering the lack of metabolic data a temporary
acceptable daily intake was allocated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg
Dog: 30 ppm in the diet equivalent to 0.75 mg/kg
Estimation of temporary acceptable daily intake
0-0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cyanofenphos is mainly applied against insect pests including
Lepidoptera, Diptera, Orthoptera, Hemiptera and Coleoptera in field
crops, fruits and vegetables. It is also applied for the control of
insects affecting livestock such as blow flies, lice and ticks.
The percentage of cyanofenphos used in different fields of
application varies according to current pest situations. A rough
estimate is 60% for field crops (rice, cotton, legumes, cereals), 20%
for vegetable crops (crucifers, onions, cucurbits), 15% for pome
fruit, stone fruit, citrus and tea, and 5% for veterinary uses.
Approximate dosages and frequencies of application are shown in
Table 1.
TABLE 1. Dosages and frequencies of application of cyanofenphos
Crop Dosage and frequency of application
Rice 0.25-0.75 kg a.i./ha, 2-4 times/season
Pome and stone fruits 0.025-0.75 kg a.i./ha, 2-4 times/season
Vegetables 0.25-0.75 kg a.i./ha, 2-5 times/season
Tea 0.5-1 kg a.i./ha, 1-3 times/season
Cotton 0.5-1 kg a.i./ha, 2-6 times/season
Sheep 0.02-0.05% by plunge dips, once a year
0.04-0.06% by shower dips, once a year
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data on residues resulting from supervised trials were
available. Different countries have different patterns of consumption
and usage of agricultural commodities, e.g. in some countries rice
straw after harvest is used as a feed of farm animals. Table 2
summarizes the residue levels which have been found when the product
is used according to "good agricultural practice" in Japan (Sumitomo,
1975).
FATE OF RESIDUES
In animals
Following oral administration of cyanofenphos to dairy cows at
dietary levels of 5, 15 and 50 ppm for 30 consecutive days, samples of
muscle, fat, liver, kidney and milk were analysed to determine
contents of cyanofenphos and cyanofenphos-oxon. In the tissue samples
residue levels were highest in the fat and decreased in the order fat
>> liver > kidney = muscle. Levels of cyanofenphos ranged from
approximately 1 mg/kg in the fat to 0.012 mg/kg in the kidney and
muscle in the highest dose group. The oxon levels ranged from
approximately 0.08 mg/kg in fat to less than 0.0004 mg/kg in liver,
kidney and muscle from the same group. Residues of cyanofenphos
(<0.002-0.013 mg/kg) were also found in fat samples from the lower
and middle dose groups and in liver samples from the middle group.
TABLE 2. Cyanofenphos residues resulting from supervised trials in Japan
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
rough rice straw
Rice EC 0.4, 0.5 2 59 0 0.002 2.26
0.5 4 51 0 0.014 6.14
0.6 2 45 0 0.034 0.546
0.6 4 37 0 0.141 1.26
0.5, 0.75 2 40 0 0.055 0.695
0.75 4 31 0 0.196 4.86
Dust 0.6 1 47 0.011
0.6 2 43-59 <0.002-0.010
0.45, 0.6 2 47 0.005
0.6 3 44-50 <0.002-0.023
0.6 4 32 0.021
42 0.012
0.45, 0.6 2-2 37 0.002
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
green pod
Soybean EC 0.5 2 7 0.142, 0.228 6.00, 6.571
3 7 0.402 8.978
4 7 0.156 8.50
dried bean
2 34-50 0.075, 0.186
3 50 0.108
4 34 0.214
fresh peel
Peach EC 0.75 2 3 (4 samples) 0.004-0.028 6.3-31.3
2 7 " 0.006-0.009 7.8-27.0
2 14 " 0.004-0.012 6.8-13.7
4 3 " 0.007-0.038 7.2-39.4
4 7 " 0.005-0.014 6.4-32.1
4 14 " 0.004-0.014 4.8-18.4
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage EC 0.375 2 28 0.002
2 35 <0.001
2 42 <0.001
3 14 0.123
3 21 0.002
3 28 0.001
0.5 2 7 0.186
2 14 0.092
2 21 0.028
4 7 0.258
4 14 0.108
4 21 0.026
0.75 2 7 1.45
2 14 0.902
2 20 0.721
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage 2 28 0.020
(continued)
2 35 0.015
2 42 0.010
3 14 0.050, 0.056
3 21 0.004, 0.008
3 28 0.001, 0.002
0.5, 0.75 2 + 2 7 2.02
2 + 2 14 2.02
2 + 2 20 1.24
1.5 3 14 0.083
3 21 0.008
3 28 0.002
Onion D 1.35 1 189-234 <0.002
Cucumber D 0.9 1 39-42 <0.001
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Ginger EC 0.7 3 45-52 0.004, 0.008
(root)
6 " 0.006, 0.012
Tea EC 1 1 7 48.6, 49.2
(steamed)
1 14 23.0, 27.1
1 21 4.35, 8.40
1 27-28 1.19, 7.29
Apple EC 1.5 2 45-48 0.412, 0.444
2 60-63 0.292, 0.306
2 77-79 0.288, 0.316
3 30-33 0.590, 0.711
3 45-48 0.332, 0.636
3 61-64 0.522, 0.580
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
root leaves
Radish Dust 1.8 1 65 <0.002 <0.002
1 65 <0.002 <0.002
Granules 0.3 1 24 0.001-0.157
1 65-66 <0.001-0.007
0.6 1 24 0.002-0.080
1 65-66 <0.001-0.001
160 milk samples were analysed for residues of cyanofenphos and
cyanofenphos-oxon during the 30-day feeding period. Residues of
cyanofenphos were detected only in samples from the highest dose
group. The maximum residue found was 0.4 mg/kg on the second and
fourth sampling day. Cyanofenphos-oxon residues reached a peak of
0.061 mg/kg on the fourth sampling day which dropped to about 0.020
mg/kg throughout the remainder of the 30 days (Jenkins et al., 1975).
In plants
The metabolism of cyanofenphos-14C (CN) by bifoliate bean plants
(phaseolus vulgaris, L.) grown in a greenhouse was studied by Chiba et
al. (1973). Little translocation was found when cyanofenphos-14C was
applied to the leaf. It was not taken up from water into the aerial
parts of the plant and most of the radioactivity was in the roots in
the form of intact cyanofenphos. Cyanofenphos seems to be fairly
persistent under these experimental conditions. Approximately 50% of
the compound initially applied remained in the plant samples two weeks
after foliar application.
The persistence of cyanofenphos was also observed in several
other crops including rice grains, vegetables and fruits (Sumitomo,
1975). 4-cyanophenol and desethyl cyanofenphos-oxon were detected as
metabolites by TLC. However, neither cyanofenphos-oxon nor desethyl
cyanofenphos was positively identified.
In soils
Cyanofenphos-14C (CN) was fairly persistent when incorporated
into soil (volcanic ash loam) at 10 mg/kg. 53% of the initial
radioactivity remained after three weeks. In comparison, when
Cyanox-14C (the corresponding O,O-dimethyl phosphorothionate ester of
p-cyanophenol) was incorporated into the soil at the same level, only
6% of the radioactivity remained after two weeks. No degradation
products in soils were identified (Chiba et al., 1973).
Photodecomposition
Aqueous solutions containing 7 mg/l of cyanofenphos-14C (CN)
were exposed to bright sunlight for 20 days, and the resultant
photoproducts were examined. After 20 days exposure, 78.3% of the
recovered radioactivity was intact cyanofenphos, and by extrapolation,
the half-life is presumed to be about 50 days. The predominant
photoproducts were cyanofenphos-oxon and p-cyanophenol, amounting to
7.8% and 4.8% respectively. These two products tend to increase on
longer irradiation. A non-radioactive photoproduct was identified as
ethyl phenylphosphonic acid. The following compounds were not
detected: cyanofenphos-S-isomer, desethyl-cyanofenphos,
desethyl-cyanofenphos-oxon, radioactive carbon dioxide and hydrogen
cyanide.
Unlike aqueous solutions, cyanofenphos deposits on silica gel
chromatoplates underwent rapid photodecomposition with a half-life of
two days, yielding mainly cyanofenphos-oxon and p-cyanophenol. A trace
of 2-hydroxy-5-cyanobenzoic acid was detected. This compound as well
as p-cyanobenzoic acid was also produced, in small quantities, by
irradiation of an acetone solution of cyanofenphos or p-cyanophenol
with UV light. Several pesticides and antioxidants such as rotenone,
phenazine, phenazine-N-oxide, and 2,5-tert-butylhydroquinone
accelerate the photolysis of cyanofenphos, with accompanying increase
of the oxon and p-cyanophenol, while beta-carotene, xanthophyll and
chlorophyll are inactive (Mikami et al., 1975).
METHODS OF RESIDUE ANALYSIS
Residues of cyanofenphos are determined by gas-liquid
chromatography with either flame thermionic or with flame photometric
detection (Sumitomo Chemical Co. Ltd, 1975). The solvents and
techniques used to extract cyanofenphos from samples are similar to
those used for fenitrothion (FAO/WHO, 1975b). Samples with a high
water content such as fruits and vegetables are blended with polar
solvents (acetone, acetonitrile, methanol or
acetonitrile-methanol-water mixtures). Samples with a high content of
fat or oil such as fresh soybeans are extracted with acetone followed
by chloroform. Dry samples such as rice grains are extracted with
benzene. A suitable extraction scheme is shown in Figure 1.
Sumitomo Chemical Co. Ltd (1975) have reported recoveries of
cyanofenphos added to several crops (Table 3). Gas chromatography was
on a column containing a mixture of 10% DC-200 and 20% OV-1 on
silanized Chromosorb W with the column, injector and detector at
240°C. The carrier gas was helium and EPN was used as an internal
standard. The limit of detection was about 0.001 mg/kg with a
potassium bromide thermionic detector.
TABLE 3. Recoveries of cyanofenphos added to crop samples
Crop Cyanofenphos added, mg/kg Recovery, %
Cabbages 0.2 97.2
Sugar beets 0.02 85.2
Radishes (root) 0.02 92.3
Soybeans 0.02 93.1
Rice grains 0.1 99.3
TLC on silica gel with benzene as the developing solvent is used
to confirm identity and if necessary as a final clean-up step.
Cyanofenphos (Rf about 0.4) is visualized under UV light or by
spraying with palladium chloride solution.
ther information on identity and properties
Physical state: pure compound, white crystalline solid.
Technical product, slightly yellowish solid
with characteristic odour
Melting point: 83°C
Vapour pressure: 10 mm Hg at 225°C
Specific gravity: D20 = 1.29
4
Solubility: practically insoluble in water, soluble in
ketones (acetone, methyl ethyl ketone, methyl
isobutyl ketone, cyclohexanone), hydrocarbons
(benzene, toluene, xylene,
methylnaphthalene), methylene dichloride,
chloroform, acetonitrile, dimethyl formamide,
ethyl cellosolve. Slightly soluble in
methanol, isopropanol, kerosene, soybean oil.
Stability: stable for two years under normal storage
conditions. Half-life values in aqueous
solutions at 60°C and
pH 6.0 - 150 days
pH 8.0 - 227 days
pH 10.0 - 68 days
The composition of the technical product is reported to be as follows.
Active ingredient 92.0 - 95.0%
O,O-diethyl phenylphosphonothioate 3.0 - 5.0%
O,O-di(4-cyanophenyl)phenylphosphonothioate 1.0 - 2.0%
O-ethyl phenylphosphonochloridothioate <0.2%
O,S-diethyl phenylphosphonodithioate <0.2%
S-ethyl O-(4-cyanophenyl)phenylphosphonothioate <0.5%
4-cyanophenol <0.3%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Sumitomo Chemical Company in their submission have abstracted a
study on the absorption and metabolism of C14 labelled cyanofenphos
in the mouse. This was taken from a private communication (S. Kato and
J. Miyamoto, presented at T.R. commission of IUPAC Meeting in
September 1974) which was not available for review. They did present
data on the absorption and biodegradation patterns of O,O dimethyl
O-(4-cyanophenyl) phosphorothiate in the rat (Wakimura and Miyamoto,
1971) and of O-(4 bromo-2,5 dichlorophenyl) O-methyl phenylphosphonate
(Leptophos) in mice and on cotton plants (Holmstead et al., 1973). The
company has implied that cyanofenphos has similar absorption and
biodegradation patterns as the above-mentioned compounds.
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Chicken
Cyanofenphos was administered orally as a single dose of 100
mg/kg/ bodyweight to eight white leghorn chickens (1-1.5 years of
age). The birds were observed for three weeks. The same treatment was
repeated and birds observed for a further three weeks. Atropine and
2-PAM were used to protect the birds from acute intoxication. One bird
died during first test period. Birds that survived through the two
treatment periods did not exhibit delayed paralysis of the legs.
Histopathologic examination of sciatic nerve and spinal cord did not
reveal demyelination. A single dose of TOCP (400 mg/kg body weight)
produced paralysis in legs after two weeks with degeneration and
demyelination of the sciatic nerve.
In a further study cyanofenphos was administered at daily doses
of 2 and 4 mg/kg body weight six days a week for four weeks and
observed for a further four weeks. Both groups exhibited anorexia, a
decrease in spontaneous motor activity and in body weight gain. No
paralysis was noted. No anomalies were observed in sciatic nerve and
spinal cord on histopathologic examination (Kadota et al., 1972).
Special studies on reproduction
In a three-generation reproduction study (two litters per
generation) eight male and 16 female rats received dietary
concentrations of 0, 1, 3 and 10 ppm cyanofenphos during the first d
generation. For the second and third generations those selected from
the 1 ppm dietary groups the concentration of cyanofenphos in the diet
was increased to 30 ppm. No treatment related effects were observed on
body weight gain, survival or ophthalmic and pathological examinations
in parental animals. However, reduced mating indices during third
generation and lowered male and female fertility during second and
third generation were consistently observed at 30 ppm. Pup viability
was reduced at 30 ppm. No compound related effects were observed with
respect to weanling weights, gross external observation or
histopathological examination of F3b progeny (Smith et al., 1975).
Special studies on teratogenicity
Groups of pregnant rabbits received 0, 1.0, and 3.0 mg/kg body
weight/day of cyanofenphos via gelatin capsule from day 6 through day
18 of gestation. Animals were sacrificed at day 29 of gestation and
pups removed by Caesarean section. No significant differences were
found in total number of implantation sites, number of resorptions and
number of live young. Body weights of foetuses were similar for
treated groups and were comparable to control. Twenty-four hour
survival was not affected. No visceral or skeletal abnormalities were
noted. The results obtained with the positive control (thalidomide)
demonstrated the susceptibility of this strain to a teratogenic agent
(Ladd and Smith, 1973).
Acute toxicity
Technical compound (purity 92%)
LD50 mg/kg
Species Route Solvent M F Reference
mouse oral Tween 80 36 30 Kadota and Miyamoto, 1973
S.C. Tween 80 68 48 Kadota and Miyamoto, 1973
I.P. Tween 80 27.5 19 Kadota and Miyamoto, 1973
Dermal Tween 80 >1 000 >1 000 Kadota and Miyamoto, 1973
rat oral Tween 80 89 32 Miyamoto and Kadota, 1972
S.C. Tween 80 200 50 Miyamoto and Kadota, 1972
Gum Arabic 1 120 380 Miyamoto and Kadota, 1972
I.P. Tween 80 141 30 Miyamoto and Kadota, 1972
Dermal peanut oil >1 000 640 Miyamoto and Kadota, 1972
hen oral Tween 80 60 Kadota et al., 1972
A single instillation of 0.05 ml suspension of cyanofenphos in
Tween 80 was found to be irritating to the rabbit eye at 0.2% and
above. At 1.0% eye closure, congestion of the conjunctiva and
lacrimation were noted. No changes were observed in cornea,
conjunctival reflex, corneal reflex and pupil diameter.
When cyanofenphos suspension was instilled daily (six days a
week) for four weeks at dosage levels of 0.2 and 1.0%, similar eye
reactions were observed which did not appear to increase in intensity
with time. Myosis was not observed (Kagoshima et al., 1972)
Two groups of guinea pigs were sensitized by injecting
intracutaneously 5% and 1% solutions of cyanofenphos in corn oil every
other day for 20 days. Each animal was challenged with an
intracutaneous injection of cyanofenphos in corn oil and a dermal
application of this compound in acetone 14 days after last sensitizing
dose. Local swelling was observed at injection site both on
sensitizing and challenging the animals. No remarkable changes with
dermal challenge (Koda et al., 1972).
Short-term studies
Dog
Four groups of dogs (four male and four female/group) were fed 0,
10, 30 and 100 ppm cyanofenphos in the diet for 12 months. Two male
dogs at 100 ppm displayed a moderate ataxia of the hindquarters during
the final month of testing. Growth, food consumption, mortality,
haematology, blood chemistry, urinalysis, ophthalmologic examination,
gross and histopathology revealed no compound related effect. Liver to
body and brain weight ratio was increased in four of eight dogs at 100
ppm. Plasma and red blood cell cholinesterase activities at 100 ppm
were decreased when determined at one, three and nine months. Red
blood cell cholinesterase was also reduced at 12 months. No inhibitory
effect on brain cholinesterase was noted at any level (Marias et al.,
1974).
Cow
Groups of four cows were fed diets containing 0, 5, 15 and 50 ppm
cyanofenphos. On day 30 of treatment three cows/group were sacrificed,
the remaining animal of each group was fed the control diet for a
further 30 days. Milk, liver, kidney, muscle and fat were collected
from each animal and analysed for residues.
No adverse reactions due to the feeding of cyanofenphos were
observed. Trace levels of cyanofenphos and its oxon metabolite were
found only in milk and tissues of cows fed 50 ppm. Residue levels were
below the limit of detection (<0.002 ppm) during the withdrawal
period (Ladd and Winegender, 1975).
Long-term studies
Rat
Groups of 60 rats (six weeks of age) were fed cyanofenphos at
dietary levels of 0, 10, 30, 100 and 200 ppm in males and 0, 5, 15, 50
and 100 ppm in females for 24 months. There was no apparent evidence
of significant changes in general behaviour, mortality, body weight
gain, food and water consumption, urinalysis, haematology, clinical
biochemistry, organ to body weight ratio or gross and microscopic
examination of tissues. Plasma, red blood cell and brain
cholinesterase were depressed in males at 100 and 200 ppm and in
females at 50 and 100 ppm (Kadota et al., 1974).
COMMENTS
Although data on the absorption, metabolism and excretion have
been presented on two similar chemicals and reference made to data on
cyanofenphos from a private communication, no metabolic data on
cyanofenphos per se have been received.
There were no teratogenic or neurotoxic effects observed with
cyanofenphos. Reproductive effects were observed in both parental
animal and progeny at 30 ppm. The no-effect level was 10 ppm. In a
short-term (12 month) study with the dog the no-effect level was 30
ppm in the diet based on plasma and erythrocyte cholinesterase
activity. In a long-term study in the rat, the no-effect level for
cholinesterase inhibition was 15 ppm. Based on the available
information and considering the lack of metabolic data a temporary
acceptable daily intake was allocated.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg
Dog: 30 ppm in the diet equivalent to 0.75 mg/kg
Estimation of temporary acceptable daily intake
0-0.005 mg/kg
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Cyanofenphos is mainly applied against insect pests including
Lepidoptera, Diptera, Orthoptera, Hemiptera and Coleoptera in field
crops, fruits and vegetables. It is also applied for the control of
insects affecting livestock such as blow flies, lice and ticks.
The percentage of cyanofenphos used in different fields of
application varies according to current pest situations. A rough
estimate is 60% for field crops (rice, cotton, legumes, cereals), 20%
for vegetable crops (crucifers, onions, cucurbits), 15% for pome
fruit, stone fruit, citrus and tea, and 5% for veterinary uses.
Approximate dosages and frequencies of application are shown in
Table 1.
TABLE 1. Dosages and frequencies of application of cyanofenphos
Crop Dosage and frequency of application
Rice 0.25-0.75 kg a.i./ha, 2-4 times/season
Pome and stone fruits 0.025-0.75 kg a.i./ha, 2-4 times/season
Vegetables 0.25-0.75 kg a.i./ha, 2-5 times/season
Tea 0.5-1 kg a.i./ha, 1-3 times/season
Cotton 0.5-1 kg a.i./ha, 2-6 times/season
Sheep 0.02-0.05% by plunge dips, once a year
0.04-0.06% by shower dips, once a year
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data on residues resulting from supervised trials were
available. Different countries have different patterns of consumption
and usage of agricultural commodities, e.g. in some countries rice
straw after harvest is used as a feed of farm animals. Table 2
summarizes the residue levels which have been found when the product
is used according to "good agricultural practice" in Japan (Sumitomo,
1975).
FATE OF RESIDUES
In animals
Following oral administration of cyanofenphos to dairy cows at
dietary levels of 5, 15 and 50 ppm for 30 consecutive days, samples of
muscle, fat, liver, kidney and milk were analysed to determine
contents of cyanofenphos and cyanofenphos-oxon. In the tissue samples
residue levels were highest in the fat and decreased in the order fat
>> liver > kidney = muscle. Levels of cyanofenphos ranged from
approximately 1 mg/kg in the fat to 0.012 mg/kg in the kidney and
muscle in the highest dose group. The oxon levels ranged from
approximately 0.08 mg/kg in fat to less than 0.0004 mg/kg in liver,
kidney and muscle from the same group. Residues of cyanofenphos
(<0.002-0.013 mg/kg) were also found in fat samples from the lower
and middle dose groups and in liver samples from the middle group.
TABLE 2. Cyanofenphos residues resulting from supervised trials in Japan
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
rough rice straw
Rice EC 0.4, 0.5 2 59 0 0.002 2.26
0.5 4 51 0 0.014 6.14
0.6 2 45 0 0.034 0.546
0.6 4 37 0 0.141 1.26
0.5, 0.75 2 40 0 0.055 0.695
0.75 4 31 0 0.196 4.86
Dust 0.6 1 47 0.011
0.6 2 43-59 <0.002-0.010
0.45, 0.6 2 47 0.005
0.6 3 44-50 <0.002-0.023
0.6 4 32 0.021
42 0.012
0.45, 0.6 2-2 37 0.002
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
green pod
Soybean EC 0.5 2 7 0.142, 0.228 6.00, 6.571
3 7 0.402 8.978
4 7 0.156 8.50
dried bean
2 34-50 0.075, 0.186
3 50 0.108
4 34 0.214
fresh peel
Peach EC 0.75 2 3 (4 samples) 0.004-0.028 6.3-31.3
2 7 " 0.006-0.009 7.8-27.0
2 14 " 0.004-0.012 6.8-13.7
4 3 " 0.007-0.038 7.2-39.4
4 7 " 0.005-0.014 6.4-32.1
4 14 " 0.004-0.014 4.8-18.4
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage EC 0.375 2 28 0.002
2 35 <0.001
2 42 <0.001
3 14 0.123
3 21 0.002
3 28 0.001
0.5 2 7 0.186
2 14 0.092
2 21 0.028
4 7 0.258
4 14 0.108
4 21 0.026
0.75 2 7 1.45
2 14 0.902
2 20 0.721
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Cabbage 2 28 0.020
(continued)
2 35 0.015
2 42 0.010
3 14 0.050, 0.056
3 21 0.004, 0.008
3 28 0.001, 0.002
0.5, 0.75 2 + 2 7 2.02
2 + 2 14 2.02
2 + 2 20 1.24
1.5 3 14 0.083
3 21 0.008
3 28 0.002
Onion D 1.35 1 189-234 <0.002
Cucumber D 0.9 1 39-42 <0.001
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
Ginger EC 0.7 3 45-52 0.004, 0.008
(root)
6 " 0.006, 0.012
Tea EC 1 1 7 48.6, 49.2
(steamed)
1 14 23.0, 27.1
1 21 4.35, 8.40
1 27-28 1.19, 7.29
Apple EC 1.5 2 45-48 0.412, 0.444
2 60-63 0.292, 0.306
2 77-79 0.288, 0.316
3 30-33 0.590, 0.711
3 45-48 0.332, 0.636
3 61-64 0.522, 0.580
TABLE 2. (Cont'd.)
Rate of Number of Pre-harvest
application applications interval Residues
Crop Formulation (kg a.i./ha) per season (days) (mg/kg)
root leaves
Radish Dust 1.8 1 65 <0.002 <0.002
1 65 <0.002 <0.002
Granules 0.3 1 24 0.001-0.157
1 65-66 <0.001-0.007
0.6 1 24 0.002-0.080
1 65-66 <0.001-0.001
160 milk samples were analysed for residues of cyanofenphos and
cyanofenphos-oxon during the 30-day feeding period. Residues of
cyanofenphos were detected only in samples from the highest dose
group. The maximum residue found was 0.4 mg/kg on the second and
fourth sampling day. Cyanofenphos-oxon residues reached a peak of
0.061 mg/kg on the fourth sampling day which dropped to about 0.020
mg/kg throughout the remainder of the 30 days (Jenkins et al., 1975).
In plants
The metabolism of cyanofenphos-14C (CN) by bifoliate bean plants
(phaseolus vulgaris, L.) grown in a greenhouse was studied by Chiba et
al. (1973). Little translocation was found when cyanofenphos-14C was
applied to the leaf. It was not taken up from water into the aerial
parts of the plant and most of the radioactivity was in the roots in
the form of intact cyanofenphos. Cyanofenphos seems to be fairly
persistent under these experimental conditions. Approximately 50% of
the compound initially applied remained in the plant samples two weeks
after foliar application.
The persistence of cyanofenphos was also observed in several
other crops including rice grains, vegetables and fruits (Sumitomo,
1975). 4-cyanophenol and desethyl cyanofenphos-oxon were detected as
metabolites by TLC. However, neither cyanofenphos-oxon nor desethyl
cyanofenphos was positively identified.
In soils
Cyanofenphos-14C (CN) was fairly persistent when incorporated
into soil (volcanic ash loam) at 10 mg/kg. 53% of the initial
radioactivity remained after three weeks. In comparison, when
Cyanox-14C (the corresponding O,O-dimethyl phosphorothionate ester of
p-cyanophenol) was incorporated into the soil at the same level, only
6% of the radioactivity remained after two weeks. No degradation
products in soils were identified (Chiba et al., 1973).
Photodecomposition
Aqueous solutions containing 7 mg/l of cyanofenphos-14C (CN)
were exposed to bright sunlight for 20 days, and the resultant
photoproducts were examined. After 20 days exposure, 78.3% of the
recovered radioactivity was intact cyanofenphos, and by extrapolation,
the half-life is presumed to be about 50 days. The predominant
photoproducts were cyanofenphos-oxon and p-cyanophenol, amounting to
7.8% and 4.8% respectively. These two products tend to increase on
longer irradiation. A non-radioactive photoproduct was identified as
ethyl phenylphosphonic acid. The following compounds were not
detected: cyanofenphos-S-isomer, desethyl-cyanofenphos,
desethyl-cyanofenphos-oxon, radioactive carbon dioxide and hydrogen
cyanide.
Unlike aqueous solutions, cyanofenphos deposits on silica gel
chromatoplates underwent rapid photodecomposition with a half-life of
two days, yielding mainly cyanofenphos-oxon and p-cyanophenol. A trace
of 2-hydroxy-5-cyanobenzoic acid was detected. This compound as well
as p-cyanobenzoic acid was also produced, in small quantities, by
irradiation of an acetone solution of cyanofenphos or p-cyanophenol
with UV light. Several pesticides and antioxidants such as rotenone,
phenazine, phenazine-N-oxide, and 2,5-tert-butylhydroquinone
accelerate the photolysis of cyanofenphos, with accompanying increase
of the oxon and p-cyanophenol, while beta-carotene, xanthophyll and
chlorophyll are inactive (Mikami et al., 1975).
METHODS OF RESIDUE ANALYSIS
Residues of cyanofenphos are determined by gas-liquid
chromatography with either flame thermionic or with flame photometric
detection (Sumitomo Chemical Co. Ltd, 1975). The solvents and
techniques used to extract cyanofenphos from samples are similar to
those used for fenitrothion (FAO/WHO, 1975b). Samples with a high
water content such as fruits and vegetables are blended with polar
solvents (acetone, acetonitrile, methanol or
acetonitrile-methanol-water mixtures). Samples with a high content of
fat or oil such as fresh soybeans are extracted with acetone followed
by chloroform. Dry samples such as rice grains are extracted with
benzene. A suitable extraction scheme is shown in Figure 1.
Sumitomo Chemical Co. Ltd (1975) have reported recoveries of
cyanofenphos added to several crops (Table 3). Gas chromatography was
on a column containing a mixture of 10% DC-200 and 20% OV-1 on
silanized Chromosorb W with the column, injector and detector at
240°C. The carrier gas was helium and EPN was used as an internal
standard. The limit of detection was about 0.001 mg/kg with a
potassium bromide thermionic detector.
TABLE 3. Recoveries of cyanofenphos added to crop samples
Crop Cyanofenphos added, mg/kg Recovery, %
Cabbages 0.2 97.2
Sugar beets 0.02 85.2
Radishes (root) 0.02 92.3
Soybeans 0.02 93.1
Rice grains 0.1 99.3
TLC on silica gel with benzene as the developing solvent is used
to confirm identity and if necessary as a final clean-up step.
Cyanofenphos (Rf about 0.4) is visualized under UV light or by
spraying with palladium chloride solution.
 APPRAISAL
Cyanofenphos has a wide insecticidal activity, and since it is
moderately persistent it offers a possible alternative to the
persistent organochlorine compounds. Considerations of cost limit its
use for this purpose at present, however.
Although the persistence of cyanofenphos can lead to residues in
and on crops at harvest, the potentially toxic metabolite
cyanofenphos-oxon has not been found in plants.
The translocation of cyanofenphos in plants is very limited.
Although radio tracer studies have been undertaken, the identities of
the metabolites in plants have not been fully elucidated. Studies with
the cyanofenphos molecule labelled in other positions might provide a
more detailed understanding of the metabolic pathways. In particular
the fate of the P-C ring bond in plants and animals remains to be
fully determined for cyanofenphos and some other phosphonates.
No information was available on the tendency to partition into
vegetable oils and animal fats. Information is needed on the
distribution of residues in rice and their fate in processing and
cooking.
Residue data from many supervised trials conducted in Japan were
made available and were considered sufficient to make recommendations
for temporary maximum residue limits on several commodities.
RECOMMENDATIONS
The following temporary maximum residue limits are based on the
determination of the parent compound only.
TEMPORARY MAXIMUM RESIDUE LIMITS
Pre-harvest intervals
Limit, on which recommendations
Commodity mg/kg are based, days
Cabbage 2 7
Peaches 1 14
Soybean (fresh, without pods) 0.5 7
Soybean, dry 0.5 30
Radishes (roots) 0.2 21
Rice (hulled) 0.2 30
Cucumber 0.05* 40
Ginger 0.05* 40
Onions 0.05* 150
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1978)
1. Data on absorption, metabolism and excretion in at least one
mammalian species.
2. Studies to identify and investigate toxicity of plant
metabolites.
3. Distribution of residues in rice and their fate during processing
and cooking.
4. Information on residues in dry green and dry manufactured
(fermented) tea.
5. Studies on residues in sheep following dipping and spraying.
6. Results of ongoing studies on the feeding of poultry to determine
the fate of residues in tissues and eggs.
7. Further information on the fate in cabbage during processing and
cooking.
DESIRABLE
1. Observations in man including cholinesterase studies.
2. Appropriate mutagenic study.
3. A further long-term study.
4. Information from trials in countries other than Japan.
5. Studies to determine whether cyanofenphos residues can be
determined by current multiresidue methods.
REFERENCES
Chiba, M., Kato, S. and Yamamoto, I. (1973) Metabolism of Cyanox and
Surecide in plant and degradation in soil. Unpublished report
submitted from Tokyo University of Agriculture, Tokyo, Japan. Private
Communication to Sumitomo Chemical Co. Presented by J. Miyamoto at
Commission on Terminal Residues, IUPAC Meeting, 1974.
Holmstead, R. L., Fukuto, T. R. and March, R. B. (1973) The Metabolism
of O-(4-Bromo-2,5-Dichlorophenyl) O-Methyl Phenylphosphonothioate
(Leptophos) in White Mice and on Cotton Plants. Archives of
Environmental Contamination and Toxicology, 1:133-147.
Jenkins, D. H., Kinoshita, F. K. and Keplinger, M. L. (1975) Meat and
milk residue study with Surecide in dairy cattle. Unpublished report
from Industrial BIOTEST Laboratories, U.S.A., submitted by Sumitomo
Chemical Co. Ltd., Osaka.
Kadota, T., Kagoshima, M., Miyamota, J. and Nobuyuki, I. (1972) Acute
Toxicity and Delayed Neurotoxicity of B-4087 (Surecide) in Hens.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health organization by Sumitomo Chemical Co., Japan.
Kadota, T. and Miyamota, J. (1973) Acute Toxicity of Surecide in Mice.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health Organization by Sumitomo Chemical Co., Japan.
Kadota, T., Hosokawa, T., Kohda, H., Miyamoto, J. and Itoh, N. (1974)
Two year Chronic Toxicity Study with Surecide in Rats. Unpublished
report from the Research Department, Sumitomo, submitted to the World
Health Organization by Sumitomo Chemical Co., Japan.
Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Acute and Subacute
Eye Irritation Tests of Surecide in Rabbits. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Koda, H., Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Skin
Sensitization Test of Surecide in Guinea Pigs. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
organization by Sumitomo Chemical Co., Japan.
Ladd, R. and Smith, P. S. (1973) Teratogenic Study with Surecide in
Albino Rabbits. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Ladd, R. and Winegender, R. J. (1975) Meat and Milk Residue Study with
Surecide in Dairy Cattle. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Marias, A., Burtner, B. R., Kennedy, G. L. and Keplinger, M. L. (1974)
12 Month Chronic Oral Toxicity Study with Surecide Technical in Beagle
Dogs. Unpublished report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Mikami, N., Ohkawa, H. and Miyamoto, J. (1975) Photo-decomposition of
Surecide (O-ethyl O-(4-cyanophenyl) phenylphosphonothioate).
Unpublished report submitted by Sumitomo Chemical Co. Ltd, Osaka.
Presented by Miyamoto, J. at TR Commission of IUPAC Meeting in 1975.
Miyamota, J. and Kadota, T. (1972) Comparative Acute Toxicological
Studies with Surecide and EPN in Rats. Unpublished report from the
Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Smith, S., Kennedy, G. L., Kinoshita, F. K. and Keplinger, M. L.
(1975) Three Generation Reproduction Study with Surecide in Albino
Rats. Unpublished report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Sumitomo Chemical Co. Ltd (1975) Unpublished report.
APPRAISAL
Cyanofenphos has a wide insecticidal activity, and since it is
moderately persistent it offers a possible alternative to the
persistent organochlorine compounds. Considerations of cost limit its
use for this purpose at present, however.
Although the persistence of cyanofenphos can lead to residues in
and on crops at harvest, the potentially toxic metabolite
cyanofenphos-oxon has not been found in plants.
The translocation of cyanofenphos in plants is very limited.
Although radio tracer studies have been undertaken, the identities of
the metabolites in plants have not been fully elucidated. Studies with
the cyanofenphos molecule labelled in other positions might provide a
more detailed understanding of the metabolic pathways. In particular
the fate of the P-C ring bond in plants and animals remains to be
fully determined for cyanofenphos and some other phosphonates.
No information was available on the tendency to partition into
vegetable oils and animal fats. Information is needed on the
distribution of residues in rice and their fate in processing and
cooking.
Residue data from many supervised trials conducted in Japan were
made available and were considered sufficient to make recommendations
for temporary maximum residue limits on several commodities.
RECOMMENDATIONS
The following temporary maximum residue limits are based on the
determination of the parent compound only.
TEMPORARY MAXIMUM RESIDUE LIMITS
Pre-harvest intervals
Limit, on which recommendations
Commodity mg/kg are based, days
Cabbage 2 7
Peaches 1 14
Soybean (fresh, without pods) 0.5 7
Soybean, dry 0.5 30
Radishes (roots) 0.2 21
Rice (hulled) 0.2 30
Cucumber 0.05* 40
Ginger 0.05* 40
Onions 0.05* 150
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1978)
1. Data on absorption, metabolism and excretion in at least one
mammalian species.
2. Studies to identify and investigate toxicity of plant
metabolites.
3. Distribution of residues in rice and their fate during processing
and cooking.
4. Information on residues in dry green and dry manufactured
(fermented) tea.
5. Studies on residues in sheep following dipping and spraying.
6. Results of ongoing studies on the feeding of poultry to determine
the fate of residues in tissues and eggs.
7. Further information on the fate in cabbage during processing and
cooking.
DESIRABLE
1. Observations in man including cholinesterase studies.
2. Appropriate mutagenic study.
3. A further long-term study.
4. Information from trials in countries other than Japan.
5. Studies to determine whether cyanofenphos residues can be
determined by current multiresidue methods.
REFERENCES
Chiba, M., Kato, S. and Yamamoto, I. (1973) Metabolism of Cyanox and
Surecide in plant and degradation in soil. Unpublished report
submitted from Tokyo University of Agriculture, Tokyo, Japan. Private
Communication to Sumitomo Chemical Co. Presented by J. Miyamoto at
Commission on Terminal Residues, IUPAC Meeting, 1974.
Holmstead, R. L., Fukuto, T. R. and March, R. B. (1973) The Metabolism
of O-(4-Bromo-2,5-Dichlorophenyl) O-Methyl Phenylphosphonothioate
(Leptophos) in White Mice and on Cotton Plants. Archives of
Environmental Contamination and Toxicology, 1:133-147.
Jenkins, D. H., Kinoshita, F. K. and Keplinger, M. L. (1975) Meat and
milk residue study with Surecide in dairy cattle. Unpublished report
from Industrial BIOTEST Laboratories, U.S.A., submitted by Sumitomo
Chemical Co. Ltd., Osaka.
Kadota, T., Kagoshima, M., Miyamota, J. and Nobuyuki, I. (1972) Acute
Toxicity and Delayed Neurotoxicity of B-4087 (Surecide) in Hens.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health organization by Sumitomo Chemical Co., Japan.
Kadota, T. and Miyamota, J. (1973) Acute Toxicity of Surecide in Mice.
Unpublished report from the Research Department, Sumitomo, submitted
to the World Health Organization by Sumitomo Chemical Co., Japan.
Kadota, T., Hosokawa, T., Kohda, H., Miyamoto, J. and Itoh, N. (1974)
Two year Chronic Toxicity Study with Surecide in Rats. Unpublished
report from the Research Department, Sumitomo, submitted to the World
Health Organization by Sumitomo Chemical Co., Japan.
Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Acute and Subacute
Eye Irritation Tests of Surecide in Rabbits. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Koda, H., Kagoshima, M., Kadota, T. and Miyamota, J. (1972) Skin
Sensitization Test of Surecide in Guinea Pigs. Unpublished report from
the Research Department, Sumitomo, submitted to the World Health
organization by Sumitomo Chemical Co., Japan.
Ladd, R. and Smith, P. S. (1973) Teratogenic Study with Surecide in
Albino Rabbits. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Ladd, R. and Winegender, R. J. (1975) Meat and Milk Residue Study with
Surecide in Dairy Cattle. Unpublished report from Industrial Bio-Test
Laboratories submitted to the World Health Organization by Sumitomo
Chemical Co., Japan.
Marias, A., Burtner, B. R., Kennedy, G. L. and Keplinger, M. L. (1974)
12 Month Chronic Oral Toxicity Study with Surecide Technical in Beagle
Dogs. Unpublished report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Mikami, N., Ohkawa, H. and Miyamoto, J. (1975) Photo-decomposition of
Surecide (O-ethyl O-(4-cyanophenyl) phenylphosphonothioate).
Unpublished report submitted by Sumitomo Chemical Co. Ltd, Osaka.
Presented by Miyamoto, J. at TR Commission of IUPAC Meeting in 1975.
Miyamota, J. and Kadota, T. (1972) Comparative Acute Toxicological
Studies with Surecide and EPN in Rats. Unpublished report from the
Research Department, Sumitomo, submitted to the World Health
Organization by Sumitomo Chemical Co., Japan.
Smith, S., Kennedy, G. L., Kinoshita, F. K. and Keplinger, M. L.
(1975) Three Generation Reproduction Study with Surecide in Albino
Rats. Unpublished report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo Chemical Co.,
Japan.
Sumitomo Chemical Co. Ltd (1975) Unpublished report.
