
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
CYANOFENPHOS
Explanation
Cyanofenphos was reviewed by the 1975 Joint Meeting (FAO/WHO, 1976)
and a temporary ADI of 0-0.005 mg/kg body weight was allocated.
Data were available from studies on neurotoxicity, reproduction,
and teratogenicity as well as a series of acute studies to develop
basic toxicology data. Short-term studies in the dog and cow and
long-term studies in the rat demonstrated an adverse toxicological
reaction only with respect to cholinesterase inhibition. No-effect
levels in rat and dog were based on this parameter. A lack of
biochemical data with respect to metabolism was the basis for the
temporary nature of the ADI. Studies on absorption, metabolism,
and excretion were required for a further toxicological
consideration.
These studies, as well as additional information on mutagenicity
and delayed neurotoxicity, were made available to the Meeting and
are reviewed in this monograph addendum.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY
INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, and excretion
Cyanofenphos, radiolabelled at the 4-cyano moiety, was administered
orally to both sexes of rats and mice. In studies with mice,
cyanofenphos was administered at dose levels of 1/8 and 1/16 LD50
(LD50=43 mg/kg body weight). Cyanofenphos was rapidly absorbed,
and excreted almost entirely within 24 hours. Maximum distribution
of radioactivity to tissues and organs was observed within 30
minutes of treatment. Excretion was predominantly via urine and
faeces to the extent of 95% and 5%, respectively, within 72 hours.
Within 24 hours, approximately 95% of the administered
radioactivity had been excreted. There was no 14CO2 expired,
reflecting stability of the cyano group (Kato and Yamamoto, 1974).
As noted above with mice, administration of cyanofenphos, as well
as the individual optical isomers of cyanofenphos, to rats (at 4
mg/kg) was followed by rapid elimination of the radiolabelled
chemical in urine and faeces, predominantly within one day. There
were no apparent differences with respect to the elimination
pattern when cyanofenphos (the racemic form) and its isolated
optical isomers were administered to rats as a single oral dose of
4 mg/kg body weight (Ohkawa et al.,1977).
Thus, in both rats and mice, an orally administered dose of
cyanofenphos was rapidly absorbed, distributed and excreted,
predominantly within 24 hours. There was no appreciable
distribution
of the molecule to tissues and organs other than those associated
with metabolism and excretion.
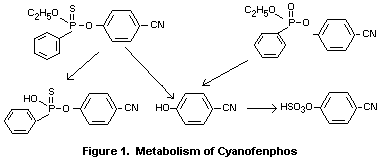
Biotransformation
The nature of the biotransformation pattern of cyanofenphos in both
rats and mice following oral administration appears to be very
similar. In both species, predominant reactions included oxidation
of P=S to P=O and oxidative and/or hydrolytic cleavage of the P-O-
aryl bonds as indicated in Figure 1.
In addition, P-O-dealkylation reactions were observed. In mouse
urine, cyanofenphos (5%), desethyl cyanofenphos (10%), and
4-cyanofenphenol (15%) were found as unconjugated products. The
other urinary metabolites were conjugates (predominantly sulphate
and glucuronide conjugates of 4-cyanophenol) (Kato and Yamamoto,
1974).
A similar (qualitative) pattern of metabolism of cyanofenphos was
observed in rats. When individual isomers and the racemic
cyanofenphos were administered to rats, there was quantitative
differences with respect to the urinary excretion. The excretion
patterns of free and conjugated 4-cyanophenol suggested that a
differential metabolism occurred in the rat when the racemic or
individual optical isomers were administered. Differences in
conjugation rates of the 4-cyanophenol were reflective of the
differing metabolic pathways of the optical and racemic
cyanofenphos.
Further studies with respect to in vitro metabolism using
isolated microsomes from rat liver clarified some of the
differences in the metabolic patterns and suggested that oxidation
of the cyanofenphos was a predominant reaction producing the oxon
(P=C) analogue and hydrolytic cleavage products. It was suggested
that the (+) and the (-) isomers were hydrolysed by different
subcellular mechanisms. The stereo-selectivity in metabolism of
cyanofenphos isomers appears likely to be due to selective
hydrolysis of (-)-cyanofenphos oxon by an arylesterase, which may
account for differences noted in the metabolic breakdown of the
individual optical isomers (Ohkawa et al., 1976).
 TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanofenphos was tested for mutagenicity in the Rec-assay using
B. subtilis (M45 rec- and H 17 wild type) strains to detect
DNA damage. Dosage levels ranged from 0 to 2,000 µg/plate.
Positive and negative controls (Mitomycin C and Kanamycin) were
employed in the study. It was observed that cyanofenphos had no
inhibitory effect on the growth of either microbial strain.
Cyanofenphos was not mutagenic under the conditions of this assay
(Moriya et al., 1976).
Cyanofenphos was examined in the standard "Ames" assay using 5
strains of S. typhimurium (TA100, TA98, TA1537, and TA1538)
and E. coli WP-2 (hor and uvr A). The results of studies,
with and without metabolic activation, at dosage levels ranging
from 0 to 5,000 µg/plate, were negative. A positive control using
either MMS, ENNG, 2-nitrofluorene, or 9-aminoacridine was employed
and assured the quality of the assay (Kishida et al.) 1980;
Moriya et al., 1976).
In a host-mediated assay, administration of cyanofenphos to groups
of mice (6 male mice/group) at dosage levels of O, 5 or 20
mg/kg/day for 2 days did not result in an increased mutation rate
of the Salmonella indicator strain. The positive control
(dimethylnitrosamine) gave a significant increase in the number of
mutants (Moriya et al., 1976).
Based upon these three bioassays in microbial test systems,
cyanofenphos is not mutagenic.
Special studies on delayed neurotoxicity
Chickens
Cyanofenphos was orally administered to adult hens in dosage levels
ranging from 0 to 500 mg/kg body weight. Hens given dosage levels
of 100 mg/kg and above received multiple doses of atropine sulphate
to protect them from cholinergic signs of poisoning. Depending
upon the acute oral dose of cyanofenphos, clinical signs of delayed
neurotoxicity were noted, accompanied by histological evidence for
myelin and/or axon degeneration (axonpathy). At dosage levels of
100 mg/kg, animals exhibited paralytic signs accompanied by
histological evidence of degeneration in the spinal cord. In those
animals surviving higher doses, more prominent signs of ataxia,
paralysis and histologically-noted disruption in both the spinal
cord and sciatic nerve were evident. Ataxia was reported at 10
mg/kg, but the clinical signs were not accompanied by histologic
changes in the spinal cord or sciatic nerve. The ataxic condition
did not degenerate to a state of paralysis. At 50 mg/kg, ataxia
and histopathologic changes were noted; again, paralysis was not
seen clinically (Abou-Donia and Graham, 1979).
Published and unpublished studies examining this phenomena have
confirmed the observation of delayed neuropathy (or axonopathy)
with cyanofenphos (El-Sebae et al., 1980; Soliman and Curley,
1980; Soliman et al., 1980), although these studies have
suggested that the neurotoxic dose inducing neuropathy in hens is
considerably higher 240mg/kg) than reported above. Transient
ataxia was noted at doses of 160 mg/kg and below, but no paralysis.
Sheep
Further studies on the delayed neurotoxic potential of cyanofenphos
were carried out with sheep, a species susceptible to this effect
(El-Sebae et al., 1979; Soliman, 1980). As with other
organophosphates capable of inducing delayed neurotoxicity in hens,
cyanofenphos was found to induce this effect in adult sheep
following continuous oral administration (1 mg/kg administered for
60 days; 2 mg/kg for 45 days; and 4 mg/kg for 30 days).
Thus, under conditions of these in vivo assays, cyanofenphos
induced a delayed neurotoxic reaction (axonopathy) in two
susceptible species following either a single acute or multiple
oral dose.
EVALUATION
COMMENTS
Studies on pharmacokinetics and metabolism with cyanofenphos in
rats and mice show that cyanofenphos is rapidly absorbed, degraded,
and excreted in both species. There is no bioaccumulation and the
metabolism in animals follows a pattern of degradation noted with
other organophosphate esters.
In vivo and in vitro studies to evaluate mutagenicity with
cyanofenphos are negative. Additional studies on the
delayed-neurotoxic potential of cyanofenphos in hens and sheep, two
species susceptible to the syndrome, have shown that cyanofenphos
induces a delayed neurotoxic effect similar to that noted with
certain other organophosphorous esters. These findings contrast
with previous studies which reported a negative delayed neurotoxic
reaction in chickens. Questions were raised on the possible
impurities in the technical product which might have contributed to
this event. This could not be evaluated with the available data.
Studies have shown sheep to be extremely susceptible to
cyanofenphos-induced delayed neurotoxicity. Longer-term,
subchronic studies in this species have failed to show a definitive
no-effect level.
The Meeting considered that the delayed neurotoxicity is another
toxicological property and based its considerations of a no-effect
level on cholinesterase level depression in rat and dog.
As a result of these considerations, a temporary ADI was
reaffirmed. Further work to define fully a no-effect level in
sheep, or another appropriate species, for delayed neurotoxicity is
required to evaluate fully the toxicological profile for this
chemical.
Level causing no toxicological effect
Rat: 10 mg/kg in the diet equivalent to 0.5 mg/kg bw/day.
Dog: 30 mg/kg in the diet equivalent to 0.75 mg/kg bw/day.
Estimation of a temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
FURTHER WORK OR INFORMATION
Required (by 1983)
Further subchronic studies with sheep (or another appropriate,
susceptible mammalian species) to determine no-effect level with
respect to delayed neurotoxicity.
Desirable
1. Observations in man including cholinesterase studies.
2. A further long-term study.
3. Observations in man primarily exposed through high-level
occupational conditions to monitor for clinical signs of delayed
neurotoxicity (axonopathy).
REFERENCES
Abou-Donia, M.B. and Graham, D.G. Delayed neurotoxicity of a single
oral dose of O-ethyl 0-4-cyanophenyl phosphonothioate in the hen.
Neurotoxicol. 1: 449-466.
El-Sebae, A.H., Othman, M.A.S., Hammam, S.M., Tantawy, G. and
Soliman, S.A. Delayed neurotoxicity of cyanofenphos in chickens. J.
Environ. Sci. Health B15(3): 267-285.
El-Sebae, A.H., Soliman, S.A. and Ahmed, N.S. Delayed neuropathy in
sheep by the phosphonothioate insecticide cyanofenphos. J. Environ.
Sci. Health, B14(3): 247-263.
Kato, S. and Yamamoto, I. Metabolism of Surecide,
O-(4-cyanophenyl)phenylphosphonothioate in mice. (1974) Unpublished
report from Sumitomo Chemical Co., Ltd., submitted to the World
Health Organization by Sumitomo Chemical Co., Ltd.
Kishida, F., Suzuki, H. and Miyamoto, J. Studies on mutagenicity of
Surecide in bacterial system. (1980) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted to the World Health
Organization by Sumitomo Chemical Co., Ltd.
Moriya, M., Watanabe, Y. and Shirasu, Y. Mutagenicity of Surecide
in bacterial test systems. (1976) Unpublished report from the
Institute of Environmental Toxicology (Japan), submitted to the
World Health Organization by Sumitomo Chemical Co. Ltd.
Ohkawa, H., Mikami, N. and Miyamato, J. Stereoselectivity in
metabolism of the optical isomers of cyanofenphos (O-p-cyanophenyl
O-ethyl phenylphosphonothioate) in rats and liver microsomes.
Agric. Biol. Chem. 41: 369-376.
Soliman, A. (1980) Unpublished studies from the Health Effects
Research Laboratory, U.S. Environmental Protection Agency,
submitted as a personal communication to the World Health
Organization by the U.S. Environmental Protection Agency.
Soliman, S.A. and Curley, A. In vivo inhibition of chicken
brain neurotoxic esterase by leptophos and cyanofenphos as
determined by a new direct method. (1980) Unpublished studies from
the Health Effects Research Laboratory, U.S. Environmental
Protection Agency submitted to the World Health Organization by the
U.S. Environmental Protection Agency.
Soliman, S.A., Curley, A., Farmer, J. and Novak, R. In vivo
inhibition of chicken brain acetylcholinesterase and neurotoxic
esterase in relation to the delayed neurotoxicity of leptophos and
cyanofenphos. (1980) Unpublished studies for the Health Effects
Research Laboratory, U.S. Environmental Protection Agency submitted
to the World Health Organization by the U.S. Environmental
Protection Agency.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanofenphos was tested for mutagenicity in the Rec-assay using
B. subtilis (M45 rec- and H 17 wild type) strains to detect
DNA damage. Dosage levels ranged from 0 to 2,000 µg/plate.
Positive and negative controls (Mitomycin C and Kanamycin) were
employed in the study. It was observed that cyanofenphos had no
inhibitory effect on the growth of either microbial strain.
Cyanofenphos was not mutagenic under the conditions of this assay
(Moriya et al., 1976).
Cyanofenphos was examined in the standard "Ames" assay using 5
strains of S. typhimurium (TA100, TA98, TA1537, and TA1538)
and E. coli WP-2 (hor and uvr A). The results of studies,
with and without metabolic activation, at dosage levels ranging
from 0 to 5,000 µg/plate, were negative. A positive control using
either MMS, ENNG, 2-nitrofluorene, or 9-aminoacridine was employed
and assured the quality of the assay (Kishida et al.) 1980;
Moriya et al., 1976).
In a host-mediated assay, administration of cyanofenphos to groups
of mice (6 male mice/group) at dosage levels of O, 5 or 20
mg/kg/day for 2 days did not result in an increased mutation rate
of the Salmonella indicator strain. The positive control
(dimethylnitrosamine) gave a significant increase in the number of
mutants (Moriya et al., 1976).
Based upon these three bioassays in microbial test systems,
cyanofenphos is not mutagenic.
Special studies on delayed neurotoxicity
Chickens
Cyanofenphos was orally administered to adult hens in dosage levels
ranging from 0 to 500 mg/kg body weight. Hens given dosage levels
of 100 mg/kg and above received multiple doses of atropine sulphate
to protect them from cholinergic signs of poisoning. Depending
upon the acute oral dose of cyanofenphos, clinical signs of delayed
neurotoxicity were noted, accompanied by histological evidence for
myelin and/or axon degeneration (axonpathy). At dosage levels of
100 mg/kg, animals exhibited paralytic signs accompanied by
histological evidence of degeneration in the spinal cord. In those
animals surviving higher doses, more prominent signs of ataxia,
paralysis and histologically-noted disruption in both the spinal
cord and sciatic nerve were evident. Ataxia was reported at 10
mg/kg, but the clinical signs were not accompanied by histologic
changes in the spinal cord or sciatic nerve. The ataxic condition
did not degenerate to a state of paralysis. At 50 mg/kg, ataxia
and histopathologic changes were noted; again, paralysis was not
seen clinically (Abou-Donia and Graham, 1979).
Published and unpublished studies examining this phenomena have
confirmed the observation of delayed neuropathy (or axonopathy)
with cyanofenphos (El-Sebae et al., 1980; Soliman and Curley,
1980; Soliman et al., 1980), although these studies have
suggested that the neurotoxic dose inducing neuropathy in hens is
considerably higher 240mg/kg) than reported above. Transient
ataxia was noted at doses of 160 mg/kg and below, but no paralysis.
Sheep
Further studies on the delayed neurotoxic potential of cyanofenphos
were carried out with sheep, a species susceptible to this effect
(El-Sebae et al., 1979; Soliman, 1980). As with other
organophosphates capable of inducing delayed neurotoxicity in hens,
cyanofenphos was found to induce this effect in adult sheep
following continuous oral administration (1 mg/kg administered for
60 days; 2 mg/kg for 45 days; and 4 mg/kg for 30 days).
Thus, under conditions of these in vivo assays, cyanofenphos
induced a delayed neurotoxic reaction (axonopathy) in two
susceptible species following either a single acute or multiple
oral dose.
EVALUATION
COMMENTS
Studies on pharmacokinetics and metabolism with cyanofenphos in
rats and mice show that cyanofenphos is rapidly absorbed, degraded,
and excreted in both species. There is no bioaccumulation and the
metabolism in animals follows a pattern of degradation noted with
other organophosphate esters.
In vivo and in vitro studies to evaluate mutagenicity with
cyanofenphos are negative. Additional studies on the
delayed-neurotoxic potential of cyanofenphos in hens and sheep, two
species susceptible to the syndrome, have shown that cyanofenphos
induces a delayed neurotoxic effect similar to that noted with
certain other organophosphorous esters. These findings contrast
with previous studies which reported a negative delayed neurotoxic
reaction in chickens. Questions were raised on the possible
impurities in the technical product which might have contributed to
this event. This could not be evaluated with the available data.
Studies have shown sheep to be extremely susceptible to
cyanofenphos-induced delayed neurotoxicity. Longer-term,
subchronic studies in this species have failed to show a definitive
no-effect level.
The Meeting considered that the delayed neurotoxicity is another
toxicological property and based its considerations of a no-effect
level on cholinesterase level depression in rat and dog.
As a result of these considerations, a temporary ADI was
reaffirmed. Further work to define fully a no-effect level in
sheep, or another appropriate species, for delayed neurotoxicity is
required to evaluate fully the toxicological profile for this
chemical.
Level causing no toxicological effect
Rat: 10 mg/kg in the diet equivalent to 0.5 mg/kg bw/day.
Dog: 30 mg/kg in the diet equivalent to 0.75 mg/kg bw/day.
Estimation of a temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
FURTHER WORK OR INFORMATION
Required (by 1983)
Further subchronic studies with sheep (or another appropriate,
susceptible mammalian species) to determine no-effect level with
respect to delayed neurotoxicity.
Desirable
1. Observations in man including cholinesterase studies.
2. A further long-term study.
3. Observations in man primarily exposed through high-level
occupational conditions to monitor for clinical signs of delayed
neurotoxicity (axonopathy).
REFERENCES
Abou-Donia, M.B. and Graham, D.G. Delayed neurotoxicity of a single
oral dose of O-ethyl 0-4-cyanophenyl phosphonothioate in the hen.
Neurotoxicol. 1: 449-466.
El-Sebae, A.H., Othman, M.A.S., Hammam, S.M., Tantawy, G. and
Soliman, S.A. Delayed neurotoxicity of cyanofenphos in chickens. J.
Environ. Sci. Health B15(3): 267-285.
El-Sebae, A.H., Soliman, S.A. and Ahmed, N.S. Delayed neuropathy in
sheep by the phosphonothioate insecticide cyanofenphos. J. Environ.
Sci. Health, B14(3): 247-263.
Kato, S. and Yamamoto, I. Metabolism of Surecide,
O-(4-cyanophenyl)phenylphosphonothioate in mice. (1974) Unpublished
report from Sumitomo Chemical Co., Ltd., submitted to the World
Health Organization by Sumitomo Chemical Co., Ltd.
Kishida, F., Suzuki, H. and Miyamoto, J. Studies on mutagenicity of
Surecide in bacterial system. (1980) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted to the World Health
Organization by Sumitomo Chemical Co., Ltd.
Moriya, M., Watanabe, Y. and Shirasu, Y. Mutagenicity of Surecide
in bacterial test systems. (1976) Unpublished report from the
Institute of Environmental Toxicology (Japan), submitted to the
World Health Organization by Sumitomo Chemical Co. Ltd.
Ohkawa, H., Mikami, N. and Miyamato, J. Stereoselectivity in
metabolism of the optical isomers of cyanofenphos (O-p-cyanophenyl
O-ethyl phenylphosphonothioate) in rats and liver microsomes.
Agric. Biol. Chem. 41: 369-376.
Soliman, A. (1980) Unpublished studies from the Health Effects
Research Laboratory, U.S. Environmental Protection Agency,
submitted as a personal communication to the World Health
Organization by the U.S. Environmental Protection Agency.
Soliman, S.A. and Curley, A. In vivo inhibition of chicken
brain neurotoxic esterase by leptophos and cyanofenphos as
determined by a new direct method. (1980) Unpublished studies from
the Health Effects Research Laboratory, U.S. Environmental
Protection Agency submitted to the World Health Organization by the
U.S. Environmental Protection Agency.
Soliman, S.A., Curley, A., Farmer, J. and Novak, R. In vivo
inhibition of chicken brain acetylcholinesterase and neurotoxic
esterase in relation to the delayed neurotoxicity of leptophos and
cyanofenphos. (1980) Unpublished studies for the Health Effects
Research Laboratory, U.S. Environmental Protection Agency submitted
to the World Health Organization by the U.S. Environmental
Protection Agency.
 TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanofenphos was tested for mutagenicity in the Rec-assay using
B. subtilis (M45 rec- and H 17 wild type) strains to detect
DNA damage. Dosage levels ranged from 0 to 2,000 µg/plate.
Positive and negative controls (Mitomycin C and Kanamycin) were
employed in the study. It was observed that cyanofenphos had no
inhibitory effect on the growth of either microbial strain.
Cyanofenphos was not mutagenic under the conditions of this assay
(Moriya et al., 1976).
Cyanofenphos was examined in the standard "Ames" assay using 5
strains of S. typhimurium (TA100, TA98, TA1537, and TA1538)
and E. coli WP-2 (hor and uvr A). The results of studies,
with and without metabolic activation, at dosage levels ranging
from 0 to 5,000 µg/plate, were negative. A positive control using
either MMS, ENNG, 2-nitrofluorene, or 9-aminoacridine was employed
and assured the quality of the assay (Kishida et al.) 1980;
Moriya et al., 1976).
In a host-mediated assay, administration of cyanofenphos to groups
of mice (6 male mice/group) at dosage levels of O, 5 or 20
mg/kg/day for 2 days did not result in an increased mutation rate
of the Salmonella indicator strain. The positive control
(dimethylnitrosamine) gave a significant increase in the number of
mutants (Moriya et al., 1976).
Based upon these three bioassays in microbial test systems,
cyanofenphos is not mutagenic.
Special studies on delayed neurotoxicity
Chickens
Cyanofenphos was orally administered to adult hens in dosage levels
ranging from 0 to 500 mg/kg body weight. Hens given dosage levels
of 100 mg/kg and above received multiple doses of atropine sulphate
to protect them from cholinergic signs of poisoning. Depending
upon the acute oral dose of cyanofenphos, clinical signs of delayed
neurotoxicity were noted, accompanied by histological evidence for
myelin and/or axon degeneration (axonpathy). At dosage levels of
100 mg/kg, animals exhibited paralytic signs accompanied by
histological evidence of degeneration in the spinal cord. In those
animals surviving higher doses, more prominent signs of ataxia,
paralysis and histologically-noted disruption in both the spinal
cord and sciatic nerve were evident. Ataxia was reported at 10
mg/kg, but the clinical signs were not accompanied by histologic
changes in the spinal cord or sciatic nerve. The ataxic condition
did not degenerate to a state of paralysis. At 50 mg/kg, ataxia
and histopathologic changes were noted; again, paralysis was not
seen clinically (Abou-Donia and Graham, 1979).
Published and unpublished studies examining this phenomena have
confirmed the observation of delayed neuropathy (or axonopathy)
with cyanofenphos (El-Sebae et al., 1980; Soliman and Curley,
1980; Soliman et al., 1980), although these studies have
suggested that the neurotoxic dose inducing neuropathy in hens is
considerably higher 240mg/kg) than reported above. Transient
ataxia was noted at doses of 160 mg/kg and below, but no paralysis.
Sheep
Further studies on the delayed neurotoxic potential of cyanofenphos
were carried out with sheep, a species susceptible to this effect
(El-Sebae et al., 1979; Soliman, 1980). As with other
organophosphates capable of inducing delayed neurotoxicity in hens,
cyanofenphos was found to induce this effect in adult sheep
following continuous oral administration (1 mg/kg administered for
60 days; 2 mg/kg for 45 days; and 4 mg/kg for 30 days).
Thus, under conditions of these in vivo assays, cyanofenphos
induced a delayed neurotoxic reaction (axonopathy) in two
susceptible species following either a single acute or multiple
oral dose.
EVALUATION
COMMENTS
Studies on pharmacokinetics and metabolism with cyanofenphos in
rats and mice show that cyanofenphos is rapidly absorbed, degraded,
and excreted in both species. There is no bioaccumulation and the
metabolism in animals follows a pattern of degradation noted with
other organophosphate esters.
In vivo and in vitro studies to evaluate mutagenicity with
cyanofenphos are negative. Additional studies on the
delayed-neurotoxic potential of cyanofenphos in hens and sheep, two
species susceptible to the syndrome, have shown that cyanofenphos
induces a delayed neurotoxic effect similar to that noted with
certain other organophosphorous esters. These findings contrast
with previous studies which reported a negative delayed neurotoxic
reaction in chickens. Questions were raised on the possible
impurities in the technical product which might have contributed to
this event. This could not be evaluated with the available data.
Studies have shown sheep to be extremely susceptible to
cyanofenphos-induced delayed neurotoxicity. Longer-term,
subchronic studies in this species have failed to show a definitive
no-effect level.
The Meeting considered that the delayed neurotoxicity is another
toxicological property and based its considerations of a no-effect
level on cholinesterase level depression in rat and dog.
As a result of these considerations, a temporary ADI was
reaffirmed. Further work to define fully a no-effect level in
sheep, or another appropriate species, for delayed neurotoxicity is
required to evaluate fully the toxicological profile for this
chemical.
Level causing no toxicological effect
Rat: 10 mg/kg in the diet equivalent to 0.5 mg/kg bw/day.
Dog: 30 mg/kg in the diet equivalent to 0.75 mg/kg bw/day.
Estimation of a temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
FURTHER WORK OR INFORMATION
Required (by 1983)
Further subchronic studies with sheep (or another appropriate,
susceptible mammalian species) to determine no-effect level with
respect to delayed neurotoxicity.
Desirable
1. Observations in man including cholinesterase studies.
2. A further long-term study.
3. Observations in man primarily exposed through high-level
occupational conditions to monitor for clinical signs of delayed
neurotoxicity (axonopathy).
REFERENCES
Abou-Donia, M.B. and Graham, D.G. Delayed neurotoxicity of a single
oral dose of O-ethyl 0-4-cyanophenyl phosphonothioate in the hen.
Neurotoxicol. 1: 449-466.
El-Sebae, A.H., Othman, M.A.S., Hammam, S.M., Tantawy, G. and
Soliman, S.A. Delayed neurotoxicity of cyanofenphos in chickens. J.
Environ. Sci. Health B15(3): 267-285.
El-Sebae, A.H., Soliman, S.A. and Ahmed, N.S. Delayed neuropathy in
sheep by the phosphonothioate insecticide cyanofenphos. J. Environ.
Sci. Health, B14(3): 247-263.
Kato, S. and Yamamoto, I. Metabolism of Surecide,
O-(4-cyanophenyl)phenylphosphonothioate in mice. (1974) Unpublished
report from Sumitomo Chemical Co., Ltd., submitted to the World
Health Organization by Sumitomo Chemical Co., Ltd.
Kishida, F., Suzuki, H. and Miyamoto, J. Studies on mutagenicity of
Surecide in bacterial system. (1980) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted to the World Health
Organization by Sumitomo Chemical Co., Ltd.
Moriya, M., Watanabe, Y. and Shirasu, Y. Mutagenicity of Surecide
in bacterial test systems. (1976) Unpublished report from the
Institute of Environmental Toxicology (Japan), submitted to the
World Health Organization by Sumitomo Chemical Co. Ltd.
Ohkawa, H., Mikami, N. and Miyamato, J. Stereoselectivity in
metabolism of the optical isomers of cyanofenphos (O-p-cyanophenyl
O-ethyl phenylphosphonothioate) in rats and liver microsomes.
Agric. Biol. Chem. 41: 369-376.
Soliman, A. (1980) Unpublished studies from the Health Effects
Research Laboratory, U.S. Environmental Protection Agency,
submitted as a personal communication to the World Health
Organization by the U.S. Environmental Protection Agency.
Soliman, S.A. and Curley, A. In vivo inhibition of chicken
brain neurotoxic esterase by leptophos and cyanofenphos as
determined by a new direct method. (1980) Unpublished studies from
the Health Effects Research Laboratory, U.S. Environmental
Protection Agency submitted to the World Health Organization by the
U.S. Environmental Protection Agency.
Soliman, S.A., Curley, A., Farmer, J. and Novak, R. In vivo
inhibition of chicken brain acetylcholinesterase and neurotoxic
esterase in relation to the delayed neurotoxicity of leptophos and
cyanofenphos. (1980) Unpublished studies for the Health Effects
Research Laboratory, U.S. Environmental Protection Agency submitted
to the World Health Organization by the U.S. Environmental
Protection Agency.
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanofenphos was tested for mutagenicity in the Rec-assay using
B. subtilis (M45 rec- and H 17 wild type) strains to detect
DNA damage. Dosage levels ranged from 0 to 2,000 µg/plate.
Positive and negative controls (Mitomycin C and Kanamycin) were
employed in the study. It was observed that cyanofenphos had no
inhibitory effect on the growth of either microbial strain.
Cyanofenphos was not mutagenic under the conditions of this assay
(Moriya et al., 1976).
Cyanofenphos was examined in the standard "Ames" assay using 5
strains of S. typhimurium (TA100, TA98, TA1537, and TA1538)
and E. coli WP-2 (hor and uvr A). The results of studies,
with and without metabolic activation, at dosage levels ranging
from 0 to 5,000 µg/plate, were negative. A positive control using
either MMS, ENNG, 2-nitrofluorene, or 9-aminoacridine was employed
and assured the quality of the assay (Kishida et al.) 1980;
Moriya et al., 1976).
In a host-mediated assay, administration of cyanofenphos to groups
of mice (6 male mice/group) at dosage levels of O, 5 or 20
mg/kg/day for 2 days did not result in an increased mutation rate
of the Salmonella indicator strain. The positive control
(dimethylnitrosamine) gave a significant increase in the number of
mutants (Moriya et al., 1976).
Based upon these three bioassays in microbial test systems,
cyanofenphos is not mutagenic.
Special studies on delayed neurotoxicity
Chickens
Cyanofenphos was orally administered to adult hens in dosage levels
ranging from 0 to 500 mg/kg body weight. Hens given dosage levels
of 100 mg/kg and above received multiple doses of atropine sulphate
to protect them from cholinergic signs of poisoning. Depending
upon the acute oral dose of cyanofenphos, clinical signs of delayed
neurotoxicity were noted, accompanied by histological evidence for
myelin and/or axon degeneration (axonpathy). At dosage levels of
100 mg/kg, animals exhibited paralytic signs accompanied by
histological evidence of degeneration in the spinal cord. In those
animals surviving higher doses, more prominent signs of ataxia,
paralysis and histologically-noted disruption in both the spinal
cord and sciatic nerve were evident. Ataxia was reported at 10
mg/kg, but the clinical signs were not accompanied by histologic
changes in the spinal cord or sciatic nerve. The ataxic condition
did not degenerate to a state of paralysis. At 50 mg/kg, ataxia
and histopathologic changes were noted; again, paralysis was not
seen clinically (Abou-Donia and Graham, 1979).
Published and unpublished studies examining this phenomena have
confirmed the observation of delayed neuropathy (or axonopathy)
with cyanofenphos (El-Sebae et al., 1980; Soliman and Curley,
1980; Soliman et al., 1980), although these studies have
suggested that the neurotoxic dose inducing neuropathy in hens is
considerably higher 240mg/kg) than reported above. Transient
ataxia was noted at doses of 160 mg/kg and below, but no paralysis.
Sheep
Further studies on the delayed neurotoxic potential of cyanofenphos
were carried out with sheep, a species susceptible to this effect
(El-Sebae et al., 1979; Soliman, 1980). As with other
organophosphates capable of inducing delayed neurotoxicity in hens,
cyanofenphos was found to induce this effect in adult sheep
following continuous oral administration (1 mg/kg administered for
60 days; 2 mg/kg for 45 days; and 4 mg/kg for 30 days).
Thus, under conditions of these in vivo assays, cyanofenphos
induced a delayed neurotoxic reaction (axonopathy) in two
susceptible species following either a single acute or multiple
oral dose.
EVALUATION
COMMENTS
Studies on pharmacokinetics and metabolism with cyanofenphos in
rats and mice show that cyanofenphos is rapidly absorbed, degraded,
and excreted in both species. There is no bioaccumulation and the
metabolism in animals follows a pattern of degradation noted with
other organophosphate esters.
In vivo and in vitro studies to evaluate mutagenicity with
cyanofenphos are negative. Additional studies on the
delayed-neurotoxic potential of cyanofenphos in hens and sheep, two
species susceptible to the syndrome, have shown that cyanofenphos
induces a delayed neurotoxic effect similar to that noted with
certain other organophosphorous esters. These findings contrast
with previous studies which reported a negative delayed neurotoxic
reaction in chickens. Questions were raised on the possible
impurities in the technical product which might have contributed to
this event. This could not be evaluated with the available data.
Studies have shown sheep to be extremely susceptible to
cyanofenphos-induced delayed neurotoxicity. Longer-term,
subchronic studies in this species have failed to show a definitive
no-effect level.
The Meeting considered that the delayed neurotoxicity is another
toxicological property and based its considerations of a no-effect
level on cholinesterase level depression in rat and dog.
As a result of these considerations, a temporary ADI was
reaffirmed. Further work to define fully a no-effect level in
sheep, or another appropriate species, for delayed neurotoxicity is
required to evaluate fully the toxicological profile for this
chemical.
Level causing no toxicological effect
Rat: 10 mg/kg in the diet equivalent to 0.5 mg/kg bw/day.
Dog: 30 mg/kg in the diet equivalent to 0.75 mg/kg bw/day.
Estimation of a temporary acceptable daily intake for man
0-0.001 mg/kg bw/day.
FURTHER WORK OR INFORMATION
Required (by 1983)
Further subchronic studies with sheep (or another appropriate,
susceptible mammalian species) to determine no-effect level with
respect to delayed neurotoxicity.
Desirable
1. Observations in man including cholinesterase studies.
2. A further long-term study.
3. Observations in man primarily exposed through high-level
occupational conditions to monitor for clinical signs of delayed
neurotoxicity (axonopathy).
REFERENCES
Abou-Donia, M.B. and Graham, D.G. Delayed neurotoxicity of a single
oral dose of O-ethyl 0-4-cyanophenyl phosphonothioate in the hen.
Neurotoxicol. 1: 449-466.
El-Sebae, A.H., Othman, M.A.S., Hammam, S.M., Tantawy, G. and
Soliman, S.A. Delayed neurotoxicity of cyanofenphos in chickens. J.
Environ. Sci. Health B15(3): 267-285.
El-Sebae, A.H., Soliman, S.A. and Ahmed, N.S. Delayed neuropathy in
sheep by the phosphonothioate insecticide cyanofenphos. J. Environ.
Sci. Health, B14(3): 247-263.
Kato, S. and Yamamoto, I. Metabolism of Surecide,
O-(4-cyanophenyl)phenylphosphonothioate in mice. (1974) Unpublished
report from Sumitomo Chemical Co., Ltd., submitted to the World
Health Organization by Sumitomo Chemical Co., Ltd.
Kishida, F., Suzuki, H. and Miyamoto, J. Studies on mutagenicity of
Surecide in bacterial system. (1980) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted to the World Health
Organization by Sumitomo Chemical Co., Ltd.
Moriya, M., Watanabe, Y. and Shirasu, Y. Mutagenicity of Surecide
in bacterial test systems. (1976) Unpublished report from the
Institute of Environmental Toxicology (Japan), submitted to the
World Health Organization by Sumitomo Chemical Co. Ltd.
Ohkawa, H., Mikami, N. and Miyamato, J. Stereoselectivity in
metabolism of the optical isomers of cyanofenphos (O-p-cyanophenyl
O-ethyl phenylphosphonothioate) in rats and liver microsomes.
Agric. Biol. Chem. 41: 369-376.
Soliman, A. (1980) Unpublished studies from the Health Effects
Research Laboratory, U.S. Environmental Protection Agency,
submitted as a personal communication to the World Health
Organization by the U.S. Environmental Protection Agency.
Soliman, S.A. and Curley, A. In vivo inhibition of chicken
brain neurotoxic esterase by leptophos and cyanofenphos as
determined by a new direct method. (1980) Unpublished studies from
the Health Effects Research Laboratory, U.S. Environmental
Protection Agency submitted to the World Health Organization by the
U.S. Environmental Protection Agency.
Soliman, S.A., Curley, A., Farmer, J. and Novak, R. In vivo
inhibition of chicken brain acetylcholinesterase and neurotoxic
esterase in relation to the delayed neurotoxicity of leptophos and
cyanofenphos. (1980) Unpublished studies for the Health Effects
Research Laboratory, U.S. Environmental Protection Agency submitted
to the World Health Organization by the U.S. Environmental
Protection Agency.
