
ACEPHATE JMPR 1976
IDENTITY
Chemical name
OS-dimethyl acetylphosphoramidothioate
Synonyms
OrtheneR, Ortho 12420, RE 12420, Ortran, Ent. 27822
Structural formula
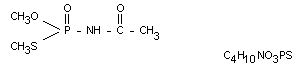 Other information on identity and properties
Molecular weight 183.16
State White solid
Melting point 72-80°C (80-90% purity)
Specific gravity 1.35 (80-90% purity)
Volatility Low. Some minor products
of degradation are volatile
Stability Relatively stable. Samples should be
stored cool
Solubility Water, ca. 65% (w/v)
Aromatic solvents, < 5%(w/v)
Acetone )
Ethanol ) >10%(w/v)
Impurities in the technical material
The technical product contains 80-90% acephate.
Among the impurities are:
OO-dimethyl acetylphosphoramidothioate max. 5%
OS-dimethyl phosphoramidothioate max. 1%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Male and female rats were pretreated with single daily oral
doses of analytical grade acephate (25 mg/kg/day) for 7 days
followed by a dose of S-methyl 14C acephate. There was no
difference in the quantitative or qualitative excretion pattern in
males or females. The excretion was rapid. The majority of the
radioactive material recovered was found within 12 hours. Urine
contained 82-95% of the administered dose, 1-4% was exhaled, 1% was
found in feces. Less than 1% was seen as a residue in tissues and
organs 72 hours after the last application. The liver was the
tissue with the highest 14C residue reflecting the normal portal
detoxication mechanism. Urinary metabolites were identified as
unchanged acephate (73-77%), O,S-dimethyl phosphorothioate (3-6%)
and S-methyl acetylphosphoramidothioate (3-4%). No methamidophos
was observed (Lee, 1972).
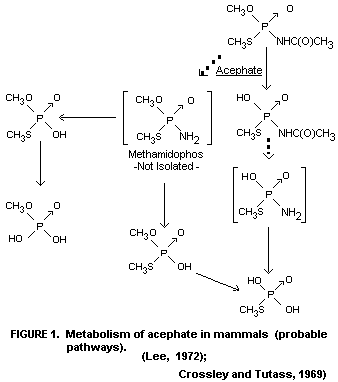
Since only the mentioned metabolites could be detected, the
metabolic pathway of acephate is not fully elucidated. A probable
scheme may be as shown in Figure 1.
The metabolic fate of two intermediates used in the
manufacture of acephate was examined in rats. These products were:
Other information on identity and properties
Molecular weight 183.16
State White solid
Melting point 72-80°C (80-90% purity)
Specific gravity 1.35 (80-90% purity)
Volatility Low. Some minor products
of degradation are volatile
Stability Relatively stable. Samples should be
stored cool
Solubility Water, ca. 65% (w/v)
Aromatic solvents, < 5%(w/v)
Acetone )
Ethanol ) >10%(w/v)
Impurities in the technical material
The technical product contains 80-90% acephate.
Among the impurities are:
OO-dimethyl acetylphosphoramidothioate max. 5%
OS-dimethyl phosphoramidothioate max. 1%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Male and female rats were pretreated with single daily oral
doses of analytical grade acephate (25 mg/kg/day) for 7 days
followed by a dose of S-methyl 14C acephate. There was no
difference in the quantitative or qualitative excretion pattern in
males or females. The excretion was rapid. The majority of the
radioactive material recovered was found within 12 hours. Urine
contained 82-95% of the administered dose, 1-4% was exhaled, 1% was
found in feces. Less than 1% was seen as a residue in tissues and
organs 72 hours after the last application. The liver was the
tissue with the highest 14C residue reflecting the normal portal
detoxication mechanism. Urinary metabolites were identified as
unchanged acephate (73-77%), O,S-dimethyl phosphorothioate (3-6%)
and S-methyl acetylphosphoramidothioate (3-4%). No methamidophos
was observed (Lee, 1972).
Since only the mentioned metabolites could be detected, the
metabolic pathway of acephate is not fully elucidated. A probable
scheme may be as shown in Figure 1.
The metabolic fate of two intermediates used in the
manufacture of acephate was examined in rats. These products were:
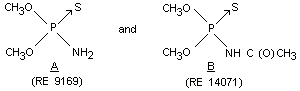
 Immature female rats were administered the above products
individually in corn oil by oral intubation at a dose of 50 mg/kg.
Urinary metabolites were characterized as similar to those found in
human urine from workers exposed in manufacturing procedures.
Qualitative determination of metabolites suggested the following
metabolic sequence.
Immature female rats were administered the above products
individually in corn oil by oral intubation at a dose of 50 mg/kg.
Urinary metabolites were characterized as similar to those found in
human urine from workers exposed in manufacturing procedures.
Qualitative determination of metabolites suggested the following
metabolic sequence.
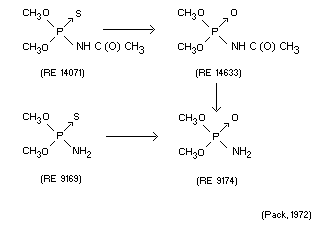 (Pack, 1972)
Effects on enzymes and other biochemical parameters
Acephate and its major plant metabolite methamidophos, as are
other organophosphate esters, are cholinesterase inhibitors. The
I50 values (molar concentrations for 50% inhibition) for plasma and
bovine RBC cholinesterase (acetylcholine hydrolysis) are given
below:
Human Plasma Bovine RBC
µg/ml I50, Molar µg/ml I50, Molar
Acephate >500 >2.7 X 10-3 >500 >2.7 X 10-3
Methamidophos 23 1.6 X 10-4 4.3 3.1 X 10-5
(Tucker, 1972)
Depression of cholinesterase activity was measured following
oral administration of acephate to male rats. Following a dose of
900 mg/kg RBC cholinesterase was substantially depressed while
plasma was unaffected. At the time of sacrifice, all animals
displayed some typical signs of organophosphate poisoning.
Administration of a dose of parathion (15 mg/kg) resulted in a
slightly higher reduction of RBC cholinesterase activity (50-30%)
with no effect on plasma enzymes (Cavalli and Spence, 1970c).
Groups of rats (15 females/group) were orally administered
acephate at levels corresponding to 1200, 100, 30, and 0 ppm as
daily equivalents in the diet for up to 3 weeks. Another group was
administered methamidophos at a dose corresponding to 10 ppm. A
slight depression of RBC-cholinesterase was noted at 100 and 30 ppm
acephate. The 1200 ppm level caused a significant depression of
activity. The plasma cholinesterase was only slightly depressed by
the highest dose level. There was no indication of a build-up of
cholinesterase inhibition over the 3 week test interval.
Methamidophos was inhibitory at 10 ppm. Methamidophos was shown to
be substantially more active an inhibitor than acephate (Cavalli
and Spence, 1970f).
TOXICOLOGICAL STUDIES
Special studies on potentiation
A study was performed on the acute toxicity of acephate alone
and in combination with 5 cholinesterase inhibiting pesticides
(azinphos-methyl, carbaryl, malathion, parathionmethyl and
oxydemeton-methyl) on male rats. The LD50 values for equitoxic
mixtures of the paired pesticides differed from single LD50 values
especially with carbaryl and malathion suggesting that potentiation
may be a factor in the interaction (Rittenhouse et al., 1972a).
A similar study in rats with methamidophos and acephate
indicated that there was no significant potentiation (Kretchmar et
al., 1972).
Special studies on neurotoxicity
Hen
Groups of adult hens (6 hens/group) were administered acephate
orally at dosage levels of 0 and 568 mg/kg (an LD50 level). A
second dose was administered to survivors at 50 day 21 and these
were observed for an additional 21 days; tri-o-cresyl phosphate
(TOCP) was administered at 500 mg/kg as a positive control. Samples
of spinal cord, peripheral nerve and brain were examined
histologically for signs of myelin disruption.
Of the hens treated with TOCP, 3 of 6 died and the remaining
hens displayed signs of clinical ataxia commonly noted with this
toxicant. One of the 6 hens treated with acephate died after the
first dosing and 3 more after the second. The remaining hens
displayed none of the signs of ataxia and on histological examination
there was no evidence of myelin degeneration. Acephate does not induce
ataxia as evidenced by TOCP in hens (Fletcher, et al., 1972b).
Special studies on reproduction
Rat
Groups of rats (8 males and 16 females/group) were fed
acephate in the diet at dosage levels of 0, 30, 100 and 300 ppm and
subjected to a standard 3-generation, 2-litter per generation
reproduction study. Gross and histopathologic examinations were
performed on the parental animals of all 3 generations, upon
weanlings of the second litter and upon weanlings of the F3b litter
for the control and high dose level. No pathologic or microscopic
changes were noted in the tissues and organs examined.
Adverse reproductive effects were noted at 100 and 300 ppm.
Reduced mating and fertility indices (reflected by copulation and
pregnancies) were noted. There were no effects noted on the ability
of mothers to maintain pregnancy although survival of pups after
birth was affected at various times during the 3 generation trial.
Weanling body weight of pups fed acephate was similar to control
values. There were no apparent malformations noted in the pups born
during this study. The results indicate a no-effect level in this
study based on all observable parameters to be 30 ppm (Haley et
al., 1973).
Special studies on teratogenicity
Rabbits
Groups of pregnant rabbits (17 does/group) were administered
acephate by capsule daily from day 6 to day 18 of gestation at
doses of 0, 1 and 3 mg/kg body weight in a standard teratology
study. Thalidomide (37.5 mg/kg/day) was used as a positive control.
There were no deaths or abnormal behavior attributed to acephate.
There were no effects on the reproduction or teratogenicity
parameters examined. Total implantation sites, resorptions,
abortions, number of young and examinations of skeletal tissue were
normal. Acephate did not elicit a teratogenic response in rabbits
while the positive control induced resorption, reduced the
incidence of pups and resulted in an increase in the number of
terata formed (Ladd et al., 1972).
Rats
Groups of pregnant rats (17-20 females/group) were
administered acephate by gavage daily from day 6 to day 15 of
gestation (10 doses) at dosage levels of 0, 25, 50, 100 and 200
mg/kg. Upon sacrifice at day 20 examinations made of the maternal
and fetal parameters did not indicate a teratological potential for
acephate in rats. Maternal effects were observed as reduced weight
gain at 100 and 200 mg/kg and as an increase in resorption sites at
200 mg/kg. All treated groups reflected acute parasympathomimetic
signs of poisoning (lacrimation) initially after treatment. No
effects were noted with respect to abortion or on the fetal
development, both internal and external tissue development including
skeletal abnormalities. This study again demonstrated a negative
teratogenic potential for acephate (Haley et al., 1971).
Special studies on mutagenicity
Mice (dominant lethal)
Groups of mice (12 males/group) were administered acephate by
intra peritoneal injection at dose levels of 0, 25, and 50 mg/kg.
The mice were then mated individually with 3 virgin females weekly
for 6 consecutive weeks in a dominant lethal test to investigate
the effects on maturation of male mouse germ cells. A positive
control (MMS, methyl methanesulfonate) was also used. The females
were sacrificed 1 week after removal from the breeding cage.
Analyses were made of implantation sites, resorption sites and
embryo development to evaluate the in vivo mutagenic potential of
acephate. In the first week after mating, an increase of
pre-implantation loss was noted at 50 mg/kg. There were no
significant differences between groups with respect to
implantation, resorption or embryo development. The in vivo
mutagenic data do not indicate acephate to have a potential for
mutagenic development (Arnold et al., 1971).
In contrast to the previously referenced in vivo study, a
series of in vitro microbiological tests has shown acephate to
elicit a positive response in tested strains of bacteria. In two
species of bacteria, Salmonella typhimurium and Escherichia
coli, acephate was positive only in some of the latter strains
(Hanna and Dyer, 1975).
Special studies on carcinogenicity
Mice
Groups of mice (Swiss White Mice - 50 males and 50
females/group) were fed acephate in the diet at levels of 0, 300
and 600 ppm for 18 months. A positive control
(N-nitrosodimethylamine) was fed at 10 ppm over this interval. Gross
pathologic examination for tumor formation and histopathologic
examination of tumors from animals sacrificed in extremis or
dying during the study showed no evidence of carcinogenicity due to
acephate. A similar negative finding was observed in the mice
sacrificed at the conclusion of the study. There was no unusual
behavior pattern exhibited by the acephate-fed mice and the
incidence and pattern of mortality were not influenced by acephate
in the diet. This study suggests that acephate is not a carcinogen
in mice (Reyna et al., 1973).
Special studies on skin sensitization
In two identical studies, groups of rabbits (8 rabbits/group)
were administered acephate dermally in acetone at a dose level of
0.1 ml of a 1% w/v solution or a control acetone treatment daily
for 16 days. Ten days after the last application, a challenge dose
at a remote site was applied and dermal irritation graded for a
sensitization response. None of the animals showed an adverse
reaction suggesting that by the dermal route acephate is not a
sensitizing agent (Cavalli and Spence, 1970g and 1970h).
Groups of guinea pigs (10 albino guinea pigs/group) were
dermally administered acephate (0.1 ml of a 1% w/v aqueous
solution) every other day (4-6 hours/day) for 10 treatments. Two
weeks after the last exposure, the animals were challenged and
examined for sensitization. There were no indications that acephate
induced a sensitization reaction under these conditions. A positive
reaction was obtained with chlorodinitrobenzene, a known sensitizer
(Ebbens et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of acephate
LD50
Species Route Sex (mg/kg) Reference
Rat Oral M&F 1100 Kretchmar et al, 1971
Oral M&F 1494 Mastri et al, 1970b
Oral M&F 2025 Kretchmar et al, 1972
Oral M 945 Cavalli & Spence, 1970b
Oral F 866 Cavalli & Spence, 1970b
Oral M 1230 Rittenhouse et al, 1972
IP M 1106 Coquet, 1972
Inhalation M&F >1.81 mg/L(4 hr.) Elliott et al, 1972
Inhalation M&F >12.1 mg/L(1 hr.) Kruke, 1972a
Mouse Oral M&F 362 Mastri et al, 1970a
Rabbit Oral M 707 Narcisse et al, 1972a
Dermal M&F 10,250 Mastri et al, 1970b
(24 hour 75% spray suspension)
Dermal M&F 500 Narcisse et al, 1972b
(no irritation)
(24 hr - 75-S formulation)
Dermal M >2000 Cavalli & Spence, 1970a
TABLE 1. (continued)
LD50
Species Route Sex (mg/kg) Reference
Dog Oral M&F <681 Mastalski et al, 1970
Oral M&F 215 Mastalski et al, 1970
(emetic dose)
Chicken Oral F >100 Edds & Bradley, 1971
Oral F 568 Fletcher et al, 1972a
Eye irritation
Acephate (100 mg) was instilled into the conjunctival sac of
rabbits (six animals/group) in an examination for eye irritancy
properties. Conjunctival irritation was evident with all forms of
acephate. Where acephate was washed out after a 5 minute exposure
period, conjunctival irritation and corneal damage were observed.
The irritation was observed after one week while all eyes were
normal at 14 days (Narcisse et al., 1971a).
Vapor inhalation
Groups of rats (5 male and 5 female/group) were exposed to an
atmosphere saturated with acephate vapor (derived from passing air
through crystalline or melted acephate at 90°C). Exposure for 1
hour to vapor derived from either method did not result in any
mortality. Animals were examined over a 14 day observation period
(Cavalli and Spence, 1970d). In an additional study, 5 female rats
were exposed for four hours to vapors of acephate derived only from
passing air through crystalline material. There was no mortality or
morbidity and cholinesterase (undefined) activity was normal
(Cavalli and Spence, 1970e).
Aerosol inhalation
Groups of rats (5 male and 5 female rats/group) were exposed
to an aerosol concentration of 12.1 mg/1 (90% of particles below 10
µ) for one hour. There were no signs of poisoning and no mortality;
gross effects on tissues and organs were not noted (Kruke et al.,
1972a).
Groups of rats (10 male and 10 female rats/group) were exposed
in a 380 l dynamic chamber to aerosols of acephate 1 hour per day,
5 days per week for two weeks. The aerosol was generated from
aqueous solutions of 0, 0.25, 1.0 and 4% acephate and had a
particle size generally under 10 microns. There was no mortality
and weight gain and general behavior were normal. At the highest
dose, female uric acid content was increased. There were no other
effects noted an clinical chemistry, urinalysis or hematology
parameters including cholinesterase activity. Gross and microscopic
examination of tissues and organs showed no effect of acephate
exposure (Kruke et al., 1972b).
Short-term studies
Rabbit - (Dermal)
Groups of rabbits (8 male and 8 female albino rabbits/group)
were administered acephate to either intact or abraded skin. An
aqueous solution was applied daily, 6 hours/day, 5 days/week, for
3 weeks with the daily doses being 0, 0.5, 1.0 and 2.0 g/kg. At the
conclusion of the study, the animals were sacrificed. There was
mortality (2/16) observed at the highest dose and
parasympathomimetic signs of poisoning were observed at 1.0 and 2.0
g/kg. These two groups also lost weight while no differences in growth
or body weight were observed at 0.5 g/kg. The two highest doses showed
substantial changes in hematology and blood chemistry parameters:
reduced RBC count, reduced hemoglobin and hematocrit values and
reduced BUN values. In male rabbits only at the low dose group, LDH
and SGOT values were reduced. RBC cholinesterase was depressed at all
dose levels to about 50% in both sexes whereas plasma cholinesterase
was not affected. On gross examination increased liver weights were
observed at the two highest dose groups. There were no effects noted
on microscopic examination including bone marrow (M/E ratio) studies
(Rittenhouse et al., 1972b).
Hen
Groups of chickens (25 females and 4-6 males/group) were fed
acephate in the diet at dosage levels of 0, 3, 10 and 30 ppm for up to
13 weeks (followed by a 28 day recovery period with control diets).
Growth, egg production and egg quality was normal. The hatchability of
eggs at the highest dose was slightly reduced, and reduction was noted
in growth and development of hatched chicks in the 10 and 30 ppm
groups. The viability of the hatched chicks was diminished in the
highest dose group. There were no gross pathological changes in any of
the birds (Fletcher, 1972a).
Rat
Groups of rats (15 males and 15 females/group) were fed acephate
in the diet for 90 days at levels of 0, 30, 100 and 300 ppm. There was
no mortality attributed to acephate in the diet. Growth, food
consumption and observable behavior were normal. Urinalyses, blood
chemistry and hematology values were normal. Gross and microscopic
analysis of tissues and organs were performed at the conclusion of the
study. In the males a decreased liver weight was noted at 300 ppm.
There were no histological lesions associated with this finding.
Cholinesterase activity data were not reported (Plank et al., 1971).
TABLE 2. Acute toxicity of metabolites or possible impurities
LD50
Compound Structure Species Route Sex (mg/kg) Reference
OO-dimethyl (CH3O)2P(S)NH2 Rat Oral M 633 Cavalli et al, 1968a
phosphoramidothioate (RE 9169) Oral F 549
Signs of poisoning include CNS depression (paresis, hyporeflexia and anesthesia)
Rabbit Dermal M 2500 Cavalli et al, 1969
OS-dimethyl (CH3O)(CH3S)P Rat Oral M 21 Cavalli et al, 1968b
Phosphoramidothioate (O)NH2 Oral F 19
(RE 9006)
Signs of poisoning are standard for parasympathomimetic agents.
Rabbit Dermal M 118 Cavalli et al, 1968c
OO-dimethyl acetyl (CH3O)2P(S)NHC Rat Oral M 1468 Cavalli et al, 1970
phosphoramidothioate (O)CH3 Oral F 1250
(RE 14071)
Signs of poisoning are standard for CNS depression - hyporeflexia, hypothermia, pyloerection, constipation, and possibly
tachycardia.
Rabbit Dermal M >2000 Rittenhouse et al,1972C
OO-dimethyl (CH3O)2(CH3S) Rat Oral M 83 Narcisse et al, 1971b
S-methyl phosphorothioate P(0) Oral F 63
(RE 15283)
The signs of poisoning include salivation, tremors, weakness, and prostration - death occurred 4-10 days after dosing.
TABLE 2. (Cont'd.)
LD50
Compound Structure Species Route Sex (mg/kg) Reference
Rabbit Dermal M 109 Narcisse et al, 1971c
OS-dimethyl (CH3O)(CH3S)P Rat Oral >2000 McCaskey,1972a
phosphorothioate (O) (OH)
(RE 18421) Rabbit Dermal M >2000 Rittenhouse et al, 1972d
S-methyl (CH3S)P(O)OH Rat Oral >2000 McCaskey, 1972b
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al, 1972e
RE 17245)
O-methyl (CH3O)P(O)SH Rat Oral >2000 McCaskey, 1972c
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al.,
(RE 17246) 1972f
Groups of rats (from 15 to 35 males and females per group) were
fed acephate in the diet at levels of 0, 1, 5, 10, 30, 100 and 300 ppm
for 90 days and a control diet for an additional 4 weeks. Animals were
sacrificed at various intervals and cholinesterase activity was
measured using erythrocytes, plasma and brain as enzyme sources.
Contrary to acute or short term oral administration studies this
dietary study showed little effect on erythrocyte cholinesterase
activity at levels below 100 ppm. In contrast, 30 ppm affected plasma
cholinesterase in weeks 6 and 13 of the test period. Brain
cholinesterase was depressed consistently at 30 ppm. In general,
females were more susceptible than males. Recovery of all blood
enzymes was rapid (within 1 week) while brain cholinesterase was
slower. All enzyme levels were normal within 4 weeks of feeding
control diets (Plank et al., 1972).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet at dosage levels of 0, 10, 30 and 100 ppm for 90
days. At the conclusion of the feeding period half of the groups were
sacrificed and the remainder maintained for a 40 day recovery period.
RBC cholinesterase activity was depressed in both sexes at 100 and 30
ppm while plasma was unaffected. Brain cholinesterase was depressed at
30 ppm. Recovery of enzyme activity was noted at 28 days after feeding
stopped and animals were fed control diets.
Growth, food consumption and behavior were unaffected over the
course of the study. There were no deaths, and animals appeared
normal. Analysis of blood and urine and hematologic parameters showed
no effects of acephate. Gross and microscopic analysis of tissues and
organs also showed no effects (Lindberg et al., 1971). [A no-effect
level in the study is 10 ppm (equivalent to 0.32 mg/kg bw) while 30
ppm produced a minimal depression of cholinesterase.]
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet for two years at levels of 0, 10, 30 and 100 ppm.
There was no effect on food consumption, growth or behavior. Two dogs
which did not survive the study died as a result of factors believed
to be unrelated to the presence of acephate in the diet. No effects
were noted on blood chemistry, hematology or urine analysis
parameters. Gross and microscopic examination of a variety of tissues
and organs showed no effects attributed to acephate. Depression of
cholinesterase activity was noted in this study in brain and red blood
cells. Plasma cholinesterase was unaffected at 100 ppm while RBC
showed reduced activity. Brain cholinesterase was only slightly (N10%)
depressed at the highest dose level. A no-effect level based on RBC
cholinesterase depression is 30 ppm over the two year study (Hartke et
al., 1972).
Long-term studies
Rat
Groups of rats (35 males and 35 females/group) were fed acephate
in the diet for two years at levels of 0, 30, 100 and 300 ppm. A
depression of growth was observed in both sexes at 12 months at 100
and 300 ppm. At 24 months the body weight of both sexes was depressed
only at 300 ppm. Food consumption was normal. There were no effects on
mortality, behavior or on hematology, blood chemistry or urine test
parameters. On gross examination male and female liver weight at 300
ppm was depressed, but nothing abnormal was noted on histopathologic
examination of the liver or other tissues and organs. There was no
suggestion of an increased tumor incidence. Blood and brain
cholinesterase depression examined during the course of this study
provided the only effects noted. At 24 months (the only interval
reported) RBC and plasma cholinesterase values were normal. Brain
cholinesterase was minimally depressed (26-27% of normal) at 30 ppm.
The depression was dose dependent suggesting a no-effect level to be
somewhat below 30 ppm. Cholinesterase was not reported for time
intervals other than 24 months. A no-effect level based on
cholinesterase depression cannot be determined in this study. A
systemic no-effect level of 30 ppm may be estimated based on growth
reduction over the first year test interval (Plank and Keplinger,
1973).
OBSERVATIONS IN MAN
In a series of reports from areas in the U.S.A. where acephate
has been used in field applications, 95 individuals exposed from 0.5
to > 1000 hours under a variety of conditions expressed no symptoms
or adverse effects of exposure. The survey was primarily subjective as
clinical measurements were not made (Anonymous, 1976a).
In a similar survey individuals exposed to acephate in pilot
plant and formulation processes did not show adverse effects. Several
of these workers were exposed for up to 15 months (Anonymous, 1976b).
Analysis of urine from these individuals confirmed exposure to
acephate. Urinary levels up to 5 ppm acephate were observed (Pack,
1972b). As expected, other metabolites or technical products of
production were observed in the urine of individuals exposed in the
pilot plant. In addition to acephate, these products included the
oxygen analog of acephate, methamidophos, the oxygen analog of
methamidophos and trimethyl phosphate. The individuals exposed to
technical acephate in formulating processes showed only methamidophos
and trimethyl phosphate as urinary metabolites. Blood and urinary
samples from 5 individuals were examined over a 10 week period. All of
the clinical parameters were normal as were standard tests including
EKG, X-ray and cholinesterase values (Swencicki and Hartz, 1972).
In a follow-up of agricultural field workers exposed to acephate,
levels of 1/50 those found in the pilot plant survey were observed in
the urine. There was no evidence of methamidophos in the urine. As
expected there were no adverse effects noted (Pack, 1972c).
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. They received either a
combination (methamidophos/acephate) of 1:9, 1:4 or corn oil as a
control daily (divided into 3 equal doses) for 21 days. The 1:9 group
was administered the combination for consecutive periods of 21 days at
doses of 0.1, 0.2 and 0.3 mg/kg/day followed by a 7 day recovery
period after which the dose was increased to 0.4 mg/kg/day for 10
days. The 1:4 group received the combination at doses of 0.1 and 0.2
mg/kg/day for 21 consecutive days before the dosing was halted. The
protocol called for a total of 4 dose levels (up to 0.4 mg/kg/day)
each to be administered for 21 days. Blood cholinesterase (plasma and
RBC) was examined periodically.
Results are summarized in Table 3. No effects were noted on RBC
cholinesterase at any treatment level. There was marginal depression
of the plasma cholinesterase noted In the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The plasma
cholinesterase, of the males was substantially affected at the 0.3
mg/kg dose and was not retested at the higher dose level. The group
(both males and females) receiving the higher methamidophos:acephate
ratio (1:4) showed reduction of plasma cholinesterase after two weeks
of receiving a dose of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not
used in the 1:4 dose group. This study indicated the minimal dose of
acephate necessary to decrease plasma cholinesterase for the
combination of acephate and methamidophos was 0.2 mg/kg/day of a 1:4
combination of the two toxicants. No effects with this combination
were noted at 0.1 mg/kg (Garofalo et al., 1973). Thus, a dose level of
0.03 mg methamidophos/kg bw combined with a dose of 0.2 mg acephate/kg
bw (ranging from 0.08 to 0.18 mg acephate/kg bw) would be expected to
reduce cholinesterase activity in man. A no-effect level evaluated
from this study might be 0.2 mg acephate/kg bw in combination with
0.02 mg methamidophos/kg bw.
COMMENTS
Acephate, a relatively non-toxic organophosphorus pesticide, is
rapidly absorbed, metabolised and excreted from the body. A toxic
metabolite, methamidophos, found to occur in plants and soil has not
been identified in mammals. Methamidophos, a potent
parasympathomimetic agent acting by inhibition of
acetylcholinesterase, is also rapidly degraded and excreted in
mammals. Neither compound persists or accumulates in the body nor
appears to affect biochemical systems other than cholinesterase.
Neurotoxicity studies in hens and potentiation studies in rats with
other antiesterase compounds were negative. Special studies on
reproduction showed effects at dietary levels of 100 ppm and above as
evidenced by reduced survival of offspring. In vivo mutagenesis
and teratogenesis testing was negative as was a special study with
mice for carcinogenicity. A positive mutagenesis response in a single
bacterial strain was observed in vitro. In a 2 year study with
dogs and an 18 month study with mice no observable effects other than
cholinesterase depression were noted. In a 2 year rat study, a slight
depression of growth and of brain cholinesterase activity at the
lowest level tested precluded a definition of a no-effect level.
However, a no-effect level in both rats and dogs was suggested based
on 90 day dietary studies which exposed animals at lower levels. In
random observations with humans occupationally exposed, no adverse
effects were noted. In a study to define a no-effect level in humans
based on cholinesterase depression, administration of a combination of
methamidophos and acephate (1:4 ratio) at a total dose of 0.2
mg/kg/day reduced plasma cholinesterase. A calculation of the
individual doses received suggested in one case that 0.16 mg
acephate/kg body weight in combination with 0.04 mg methamidophos/kg
(bw) would cause depression of cholinesterase while a dose of 0.08 mg
or 0.18 mg acephate/kg (bw) both in combination with 0.02 mg
methamidophos did not reduce plasma cholinesterase activity. Based on
the studies with both rat and dog, with further assurance afforded by
the observations in man, an ADI was recommended. This also took into
account the slight effect noted in the rat 2 year study where 30 ppm
(equivalent to 1.5 mg/kg) induced a marginal effect on brain
cholinesterase.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg body weight
Dog: 10 ppm in the diet equivalent to 0.32 mg/kg body weight
Man: 0.18 mg/kg body weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of acephate as a contact and systemic insecticide is
reported from many countries of the American continents, Australia,
Asia and Europe. It is mostly marketed as soluble powders (s.p.), 75%.
Occasionally, however, it is also registered as a 50% s.p., as low
concentration dusts and as 1-5% granules.
TABLE 3. Effect on plasma cholinesterase of combined doses of methamidophos and acephate
Methamidophos: Acephate Ratio
1:4 1:9
Total
dose methamidophos:acephate Plasma Inhibition methamidophos:acephate Plasma inhibition
(mg/kg) (mg/kg) Males Females (mg/kg) Males Females
0.1 0.02 : 0.08 - - 0.01 : 0.09 - -
0.2 0.04 : 0.16 + + 0.02 : 0.18 - -
0.3 0.06 : 0.24 * * 0.03 : 0.27 + -
0.4 0.08 : 0.32 * * 0.04 : 0.36 * -
* Discontinued treatment
Acephate is claimed to be especially active against insects of
the families Lepidoptera, Homoptera and Hemiptera. A list of suggested
uses which is typical in many countries is given in Table 4. These
uses cover suggested protection of both horticultural and agricultural
crops (fruits, vegetables, root-crops and several industrial crops)
against larvae, aphids, caterpillars, berry moths, leaf miners,
beetles etc. Usual application rates are also indicated in Table 4.
They range from 1-2 kg a.i. per ha on field crops and from 0.05-0.1%
on fruit trees, bushes etc.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable amount of residue data on acephate and its
behavior in supervised trials and controlled experiments has been
presented by the originating manufacturer (Chevron Chemical Company).
Most of the data were generated under American climatic and
agricultural conditions, although in some cases supplementary data
from other sources are available.
TABLE 4. Suggested uses of acephate
Concentration or
Crop Pest dosage of a. i.
Fruit crops Aphids 0.04-0.075%
(pome, stone, Codling moths
citrus) Chewing and sucking insects
Tortrix
Citrus, olive scale
Grape Grape berry moths 0.075%
Vegetables Aphids 0.05%
Chewing and sucking insects
Caterpillars
Moths
Beets Leaf miners 0.8-1.0 kg/ha
Ornamentals Aphids and Tortrix 0.05-0.75%
Hops Aphids 0.05-0.25%
Tobacco Aphids 1 kg/ha
Budworms
Flea beetle
Corn Corn borer 1.5-2 kg/ha
Generally the residue data from field trials indicate that
acephate is a relatively non-persistent, systemic insecticide. When
applied at the recommended rate of about 1 kg a.i./ha, several
treatments are often applied, commonly at weekly intervals for as many
weeks as necessary for insect control. A first metabolite, OS-dimethyl
phosphoramidothioate which is itself the insecticide methamidophos, is
regularly found in plant material together with acephate, although
only in the ratio of 1:10 - 1:5.
Alfalfa, cotton and soybean
Extensive trials have been made on these three important crops
(Table 5). Repeated treatments of alfalfa with 1 kg of acephate per ha
leave total residues on fresh cut green hay of less than 6 mg/kg after
21 days and a maximum of about 2 mg/kg after 28 days. Curing of the
hay in the open after harvest did not significantly change the residue
level. The apparent half-life of acephate on green hay is
approximately 7 days.
In cottonseed, the total residues ranged from zero to a maximum
of About 1.5 mg/kg 21 days after the last treatment with 1 kg/ha. The
maximum residue levels from a series of experiments are shown in Table
5. The halflife of such residues is of the order of 14 days. When
processed, essentially all the residue remains in the cottonseed meal
while crude oil and refined oil contain practically no acephate or
methamidophos metabolite (less than 0.03 mg/kg). Immediately after
treatment at the 1 kg/ha level, green leaves of cotton plants carried
residues ranging from 60-200 mg/kg. These residues declined, but they
were still significant, from 7 to 50 mg/kg, after 21 days.
In soybeans, mature and with shell removed, the total residue
varied from zero to 0.2 mg/kg 14 days after the last application of
acephate at the 1 kg/ha rate. Some positive findings are shown in
Table 5, which illustrates that when processed, most of the residue
remained in the mill feed, while trace residues only (less than
0.03 mg/kg) were found in the crude oil and the soya protein flour.
Vegetables
Residue data are available from several countries (e.g.
Australia, the Netherlands, New Zealand, Philippines, U.S.A.) on
vegetables, mostly leafy vegetables such as broccoli, Brussels
sprouts, cabbages, cauliflower and lettuce. Repeated applications of
1 kg acephate per ha are usually recommended and the residue data
indicate that there is a direct relationship between dosage and
residue level.
Residues decline at rates corresponding to the rate of growth
dilution or somewhat faster and as usual, the outer leaves of the
vegetables carry higher residues than the inner parts. This is
exemplified in Table 6 which illustrates typical residue levels of
both acephate and its metabolite methamidophos found in broccoli.
Outer leaves of the vegetables may or may not be discarded as
so-called trimmings before marketing and the final residue on the
marketed product is therefore dependent on the degree of trimming. To
take account of this situation a maximum possible residue on a given
crop may be calculated on the basis of actual analyses of the crop and
its trimmings, assuming maximum trimming. Such predictions of residues
are shown in Table 7 for 5 vegetable crops and compared with residues
actually found. The Table shows that actual residues of acephate + its
metabolite methamidophos are unlikely to exceed 5-10 mg/kg at
appropriate preharvest intervals from 3 to 14 days.
Sugar beets, potatoes and tomatoes
After repeated foliar treatments of sugar beets with acephate, at
a weekly rate of 1 kg/ha, high residues were found on the green leaves
(up to 25-30 mg/kg), while little or no residue (at or below 0.1
mg/kg) was found in the roots. 21 days after the last application the
residues in leaves had declined to about 5-6 mg/kg and in the roots to
zero-0.05 mg/kg. Conversion of the roots to wet pulp completely
removed any residues.
In potatoes after similar treatment the total residues in tubers
are reported to range from 0.04-0.27 mg/kg (Chevron, 1976). This level
did not change during 3 days, but there was some loss of total residue
by the 14th day after the last application. This loss was calculated
to proceed with a half-life of about 21 days.
Total residues of acephate in tomatoes (mature green or ripe
fruits) are reported to range from 0.3-2.4 mg/kg after repeated foliar
treatments at 1 kg/ha (Chevron, 1976). The half-life was 7-14 days on
these fruits.
Residues in the pulp and peel of potatoes and tomatoes are
discussed further below ("Fate of residues in processing and
cooking"). Some residue data are given in Tables 14 and 15.
Citrus fruits
Supervised trials on acephate treatments of oranges, grapefruits,
lemons and tangeloes have been reported from New Zealand (1976) and
the U.S.A. From the results, Table 8, it is clear that a significant
systemic penetration takes place from the peel into the fruit flesh.
In the grapefruit, the acephate concentration seems to be equilibrated
throughout the whole fruit.
TABLE 5. Residues of acephate and its metabolite in alfalfa, cottonseed* and soybeans
Application Days after Residues, mg/kg
Rate, last
Crop kg a.i./ha No. treatment Acephate Methamidophos Total
Green hay (Air spray) 2 21 1.70 - 1.75 0.25 - 0.26 1.95 - 2.01
1 28 0.46 - 0.55 0.07 - 0.10 0.56 - 0.62
Cured hay 1 2 21 2.78 - 5.00 0.36 - 0.57 3.14 - 5.57
(4 days) (Air spray) 28 0.89 - 0.96 0.09 - 0.11 1.00 - 1.05
Green hay 1 2 21 - - -
(Ground spray) 28 0.76 - 1.56 0.24 - 0.52 1.00 - 2.08
Cured hay 1 2 21 - - -
(4 days) (Ground spray) 28 0.33 - 0.52 0.12 - 0.15 0.45 - 0.67
Green hay 1 3 0 27.0 - 32.0 1.29 - 1.65 33.3
(Ground spray) 14 8.9 - 11.9 1.40 - 1.41 10.3
21 3.28 - 4.40 0.59 - 0.74 3.87 - 5.14
28 - 0.21 - 0.29 -
Green hay 1 3 21 5.01 - 5.12 0.54 - 0.59 5.55 - 5.71
(Ground spray) 28 - - -
Cottonseed, 1 8 21 0.48 - 1.15 0.13 - 0.26 0.61 - 1.41
fuzzy 2 8 21 0.47 - 0.83 0.14 - 0.23 0.61 - 1.06
Cottonseed 1 8 21 1.00 - 1.19 0.23 - 0.31 1.23 - 1.50
meal 2 8 21 1.27 - 1.48 0.32 - 0.34 1.59 - 1.82
Crude cottonseed 1 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
oil 2 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
Soybeans 1 3 14 0.08 - 0.20 0.00 - 0.01 0.08 - 0.21
Soybeans, protein 1 3 14 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
flour
Soybeans, 1 3 14 0.02 - 0.03 0.00 - 0.00 0.02 - 0.03
crude oil
Soybeans, 1 3 14 0.34 - 0.23 0.04 - 0.07 0.27 - 0.41
mill feed
* Residues tabulated for cottonseed and soybeans are maximum levels found in a series of experiments.
TABLE 6. Residues of acephate and its metabolite methamidophos in broccoli
Application Days after Residues, mg/kg
Rate last
Crop part kg a.i./ha No. treatment Acephate Methamidophos Total
Heads, 1 3 0 - - -
trimmed 3 - - -
7 4.48 - 5.25 0.36 - 0.45 4.84 - 5.70
14 3.42 - 3.44 0.32 - 0.36 3.74 - 3.80
1 6 0 6.63 - 6.82 0.65 - 0.69 7.32 - 7.47
3 6.63 - 6.63 0.58 - 0.68 7.21 - 7.31
7 5.17 - 6.08 0.75 - 0.75 5.92 - 6.83
14 1.98 - 2.27 0.39 - 0.40 2.66 - 2.38
2 6 0 20.0 - 21.6 1.56 - 1.74 21.7 - 23.2
3 20.4 - 23.0 1.56 - 1.78 22.2 - 24.6
7 14.0 - 16.7 1.70 - 1.70 15.7 - 18.4
14 4.92 - 5.14 0.80 - 0.96 5.7 - 6.1
1 9 0 14.5 - 16.0 1.78 - 1.82 16.3 - 17.8
3 16.0 - 17.4 1.40 - 1.60 17.4 - 19.0
7 10.1 - 13.2 1.24 - 1.27 11.3 - 14.5
14 2.48 - 2.56 0.75 - 0.76 3.24 - 3.31
2 9 0 28.3 - 30.3 3.46 - 3.59 32.1 - 33.9
3 23.5 - 25.3 2.33 - 2.49 23.8 - 27.8
7 21.6 - 24.9 2.47 - 2.59 24.1 - 27.5
14 5.46 - 6.57 1.11 - 1.45 6.57 - 8.02
Trimmings 2 6 0 45.4 - 47.9 2.17 - 2.25 47.7 - 50.1
3 45.4 - 52.9 2.05 - 2.23 47.5 - 55.1
7 20.8 - 40.4 1.38 - 1.65 22.2 - 42.1
14 14.6 - 16.1 1.05 - 1.21 15.7 - 17.3
TABLE 7. Predicted maximum+ and actual residues of acephate
+ methamidophos metabolite in some vegetables
Crop Total Residue,
Application Days after mg/kg
Rate, last Calculated+
Crop kg a.i./ha No. treatment maximum+ Found1
Broccoli 1 3-9 14 5.47 3.80
(max.25%
trimming) 2 6-9 14 9.89 8.02
Brussels 1 8 3 4.79 3.92
sprouts
(max. 15% 2 8 3 12.93 10.18
trimming)
Cabbage 1 9 3 7.29 2.51
(max. 20%
trimming) 2 9 3 12.82 6.38
Cauliflower 1 7-9 3 6.85 2.30
(max.50%
trimming) 2 7-9 3 7.90 4.36
Lettuce 1 6-8 3 7.35 3.29
(max. 50%
trimming) 2 6-8 3 16.17 5.54
+ Based on results from trimmed heads and trimmings, assuming maximum
possible trimming.
1 Reported results on heads trimmed according to commercial practice.
FATE OF RESIDUES
In animals
The metabolic fate of acephate in rats has been described above
("Biotransformation"). Proposed metabolic pathways in mammals are
shown in the same section (Figure 1).
TABLE 8. Residues of acephate and its metabolite methamidophos in citrus fruits
Application Days after Residues, mg/kg
Concentration last Acephate Methamidophos
Country Crop a.i. No. treatment Peel Flesh Peel Flesh
New Zealand Oranges 0.075% 6 1 9.0 0.6 0.98 0.01
(25% peel) 8 9.0 0.7 0.8 0.3
15 7.6 0.7 0.9 0.09
24 5.3 0.6 0.6 n.d.
Grapefruit 0.075% 6 1 2.9 3.0 n.d. 0.2
(30% peel) 8 3.1 1.9 0.2 0.3
15 2.6 3.6 0.2 0.2
24 1.4 5.4 n.d. 0.2
Whole fruit Whole fruit
U.S.A. Oranges 0.06% 2-3 20-25 0.33 - 1.1 0.06 - 0.10
40 0.19 - 1.5 0.02 - 0.08
100 0.00 - 0.00 0.00 - 0.00
Lemons 0.06% 3 20-25 0.08 - 0.18 0.02 - 0.03
0.12% 3 20-25 0.42 - 0.44 0.05 - 0.06
Tangeloes 0.06% 3 20 1.4 - 1.6 0.16 - 0.17
Dairy cows and pigs
9 dairy cows were fed at levels of 3, 10 and 30 ppm of acephate
+0.6, 2 and 6 ppm of its metabolite methamidophos in the diet for 30
days. The ratio of acephate to methamidophos was 5:1 because this is
typical of the ratio in most field crops after treatment with
acephate.
The lowest dosage did not give detectable residues in the cows'
milk. Milk from the higher dosed animals, however, contained between
0.1 and 0.2% of the daily doses and at levels which rapidly reached a
plateau where they remained during the 30 days treatment period.
Average residues found in the milk are shown in Table 9. At withdrawal
of the daily intake, residues in the milk decreased rapidly. By the
morning of the second day after withdrawal neither acephate nor its
metabolite could be observed in the milk.
Some individual cows were sacrificed after 3-4 weeks of feeding
with acephate and its metabolite. They showed significant but moderate
levels of residues in various tissues (Table 10). The highest residue
of acephate was 0.57 mg/kg in the kidney of an animal at the highest
dose level, and no tendency to accumulation in tissues was noted. 6
days after withdrawal, no residues could be detected in liver, kidney,
heart, muscle or fat.
TABLE 9. Average1 residues of acephate and its metabolite
methamidophos in cows' milk
Dose level Residue in milk
(ppm in dry feed) mg/kg ± S.D.1
Acephate Methamidophos Acephate Methamidophos
3 0.6 0.00 0.00
10 2 0.09 ± 0.005 0.00
30 6 0.27 ± 0.06 0.022 ± 0.006
1 Average for 30 days of three cows per dose level.
Essentially the same results were obtained by feeding acephate
and its metabolite to pigs. A linear relationship could be established
between dose levels and tissue residues. The residues ranged from 0.15
to 0.58 mg/kg of total residue at the high dose level (i.e. 30 ppm of
acephate + 6 ppm of metabolite) in tissues which were analysed after 4
weeks of feeding, namely: muscle, liver, kidney, subcutaneous and
peritoneal fat, heart and brain. In pigs as in cows, the residues were
undetectable in all tissues in less than 6 days after withdrawal of
the diet.
Chicken and quail
Tissue samples and eggs were taken from chickens and quail which
had been subjected to acephate feeding in subacute and chronic
toxicological studies. Three dose levels were followed: 3, 10 and 30
ppm of acephate in the diet for 92-140 days. No residues were detected
in any of the experiments in the fat, kidney or liver of the birds. In
eggs and muscle tissue, low levels of acephate were observed at the 10
and 30 ppm feeding levels, namely 0.07 and 0.19 mg/kg respectively in
chicken eggs, 0.16 and 0.31 mg/kg in quail eggs, 0.01 and 0.02 mg/kg
in chicken muscle and 0.01 and 0.04 mg/kg in quail muscle (Table 11).
At the high feeding levels in these studies, trace amounts of
methamidophos (OS-dimethyl phosphoramidothioate), which is otherwise
recognised as a plant metabolite only, were observed in muscles as
well as in eggs.
In plants
Studies using 14C-labelled acephate show that the compound is
readily degraded by plants such as bean seedlings, tomato and cabbage.
The half-life of acephate in such greenhouse-grown plants has been
shown to be of the order of 5-10 days. Among several theoretically
possible metabolites only one, methamidophos or OS-dimethyl
phosphoramidothioate, is found to be of practical significance.
Another of the possible metabolites is believed to be present although
it could not be analytically observed. This is OS-dimethyl
phosphorothioate, which is thought to be transitional step in the
complete breakdown to phosphate. The suggested metabolic pathways of
acephate in plants are shown in Figure 2.
(Pack, 1972)
Effects on enzymes and other biochemical parameters
Acephate and its major plant metabolite methamidophos, as are
other organophosphate esters, are cholinesterase inhibitors. The
I50 values (molar concentrations for 50% inhibition) for plasma and
bovine RBC cholinesterase (acetylcholine hydrolysis) are given
below:
Human Plasma Bovine RBC
µg/ml I50, Molar µg/ml I50, Molar
Acephate >500 >2.7 X 10-3 >500 >2.7 X 10-3
Methamidophos 23 1.6 X 10-4 4.3 3.1 X 10-5
(Tucker, 1972)
Depression of cholinesterase activity was measured following
oral administration of acephate to male rats. Following a dose of
900 mg/kg RBC cholinesterase was substantially depressed while
plasma was unaffected. At the time of sacrifice, all animals
displayed some typical signs of organophosphate poisoning.
Administration of a dose of parathion (15 mg/kg) resulted in a
slightly higher reduction of RBC cholinesterase activity (50-30%)
with no effect on plasma enzymes (Cavalli and Spence, 1970c).
Groups of rats (15 females/group) were orally administered
acephate at levels corresponding to 1200, 100, 30, and 0 ppm as
daily equivalents in the diet for up to 3 weeks. Another group was
administered methamidophos at a dose corresponding to 10 ppm. A
slight depression of RBC-cholinesterase was noted at 100 and 30 ppm
acephate. The 1200 ppm level caused a significant depression of
activity. The plasma cholinesterase was only slightly depressed by
the highest dose level. There was no indication of a build-up of
cholinesterase inhibition over the 3 week test interval.
Methamidophos was inhibitory at 10 ppm. Methamidophos was shown to
be substantially more active an inhibitor than acephate (Cavalli
and Spence, 1970f).
TOXICOLOGICAL STUDIES
Special studies on potentiation
A study was performed on the acute toxicity of acephate alone
and in combination with 5 cholinesterase inhibiting pesticides
(azinphos-methyl, carbaryl, malathion, parathionmethyl and
oxydemeton-methyl) on male rats. The LD50 values for equitoxic
mixtures of the paired pesticides differed from single LD50 values
especially with carbaryl and malathion suggesting that potentiation
may be a factor in the interaction (Rittenhouse et al., 1972a).
A similar study in rats with methamidophos and acephate
indicated that there was no significant potentiation (Kretchmar et
al., 1972).
Special studies on neurotoxicity
Hen
Groups of adult hens (6 hens/group) were administered acephate
orally at dosage levels of 0 and 568 mg/kg (an LD50 level). A
second dose was administered to survivors at 50 day 21 and these
were observed for an additional 21 days; tri-o-cresyl phosphate
(TOCP) was administered at 500 mg/kg as a positive control. Samples
of spinal cord, peripheral nerve and brain were examined
histologically for signs of myelin disruption.
Of the hens treated with TOCP, 3 of 6 died and the remaining
hens displayed signs of clinical ataxia commonly noted with this
toxicant. One of the 6 hens treated with acephate died after the
first dosing and 3 more after the second. The remaining hens
displayed none of the signs of ataxia and on histological examination
there was no evidence of myelin degeneration. Acephate does not induce
ataxia as evidenced by TOCP in hens (Fletcher, et al., 1972b).
Special studies on reproduction
Rat
Groups of rats (8 males and 16 females/group) were fed
acephate in the diet at dosage levels of 0, 30, 100 and 300 ppm and
subjected to a standard 3-generation, 2-litter per generation
reproduction study. Gross and histopathologic examinations were
performed on the parental animals of all 3 generations, upon
weanlings of the second litter and upon weanlings of the F3b litter
for the control and high dose level. No pathologic or microscopic
changes were noted in the tissues and organs examined.
Adverse reproductive effects were noted at 100 and 300 ppm.
Reduced mating and fertility indices (reflected by copulation and
pregnancies) were noted. There were no effects noted on the ability
of mothers to maintain pregnancy although survival of pups after
birth was affected at various times during the 3 generation trial.
Weanling body weight of pups fed acephate was similar to control
values. There were no apparent malformations noted in the pups born
during this study. The results indicate a no-effect level in this
study based on all observable parameters to be 30 ppm (Haley et
al., 1973).
Special studies on teratogenicity
Rabbits
Groups of pregnant rabbits (17 does/group) were administered
acephate by capsule daily from day 6 to day 18 of gestation at
doses of 0, 1 and 3 mg/kg body weight in a standard teratology
study. Thalidomide (37.5 mg/kg/day) was used as a positive control.
There were no deaths or abnormal behavior attributed to acephate.
There were no effects on the reproduction or teratogenicity
parameters examined. Total implantation sites, resorptions,
abortions, number of young and examinations of skeletal tissue were
normal. Acephate did not elicit a teratogenic response in rabbits
while the positive control induced resorption, reduced the
incidence of pups and resulted in an increase in the number of
terata formed (Ladd et al., 1972).
Rats
Groups of pregnant rats (17-20 females/group) were
administered acephate by gavage daily from day 6 to day 15 of
gestation (10 doses) at dosage levels of 0, 25, 50, 100 and 200
mg/kg. Upon sacrifice at day 20 examinations made of the maternal
and fetal parameters did not indicate a teratological potential for
acephate in rats. Maternal effects were observed as reduced weight
gain at 100 and 200 mg/kg and as an increase in resorption sites at
200 mg/kg. All treated groups reflected acute parasympathomimetic
signs of poisoning (lacrimation) initially after treatment. No
effects were noted with respect to abortion or on the fetal
development, both internal and external tissue development including
skeletal abnormalities. This study again demonstrated a negative
teratogenic potential for acephate (Haley et al., 1971).
Special studies on mutagenicity
Mice (dominant lethal)
Groups of mice (12 males/group) were administered acephate by
intra peritoneal injection at dose levels of 0, 25, and 50 mg/kg.
The mice were then mated individually with 3 virgin females weekly
for 6 consecutive weeks in a dominant lethal test to investigate
the effects on maturation of male mouse germ cells. A positive
control (MMS, methyl methanesulfonate) was also used. The females
were sacrificed 1 week after removal from the breeding cage.
Analyses were made of implantation sites, resorption sites and
embryo development to evaluate the in vivo mutagenic potential of
acephate. In the first week after mating, an increase of
pre-implantation loss was noted at 50 mg/kg. There were no
significant differences between groups with respect to
implantation, resorption or embryo development. The in vivo
mutagenic data do not indicate acephate to have a potential for
mutagenic development (Arnold et al., 1971).
In contrast to the previously referenced in vivo study, a
series of in vitro microbiological tests has shown acephate to
elicit a positive response in tested strains of bacteria. In two
species of bacteria, Salmonella typhimurium and Escherichia
coli, acephate was positive only in some of the latter strains
(Hanna and Dyer, 1975).
Special studies on carcinogenicity
Mice
Groups of mice (Swiss White Mice - 50 males and 50
females/group) were fed acephate in the diet at levels of 0, 300
and 600 ppm for 18 months. A positive control
(N-nitrosodimethylamine) was fed at 10 ppm over this interval. Gross
pathologic examination for tumor formation and histopathologic
examination of tumors from animals sacrificed in extremis or
dying during the study showed no evidence of carcinogenicity due to
acephate. A similar negative finding was observed in the mice
sacrificed at the conclusion of the study. There was no unusual
behavior pattern exhibited by the acephate-fed mice and the
incidence and pattern of mortality were not influenced by acephate
in the diet. This study suggests that acephate is not a carcinogen
in mice (Reyna et al., 1973).
Special studies on skin sensitization
In two identical studies, groups of rabbits (8 rabbits/group)
were administered acephate dermally in acetone at a dose level of
0.1 ml of a 1% w/v solution or a control acetone treatment daily
for 16 days. Ten days after the last application, a challenge dose
at a remote site was applied and dermal irritation graded for a
sensitization response. None of the animals showed an adverse
reaction suggesting that by the dermal route acephate is not a
sensitizing agent (Cavalli and Spence, 1970g and 1970h).
Groups of guinea pigs (10 albino guinea pigs/group) were
dermally administered acephate (0.1 ml of a 1% w/v aqueous
solution) every other day (4-6 hours/day) for 10 treatments. Two
weeks after the last exposure, the animals were challenged and
examined for sensitization. There were no indications that acephate
induced a sensitization reaction under these conditions. A positive
reaction was obtained with chlorodinitrobenzene, a known sensitizer
(Ebbens et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of acephate
LD50
Species Route Sex (mg/kg) Reference
Rat Oral M&F 1100 Kretchmar et al, 1971
Oral M&F 1494 Mastri et al, 1970b
Oral M&F 2025 Kretchmar et al, 1972
Oral M 945 Cavalli & Spence, 1970b
Oral F 866 Cavalli & Spence, 1970b
Oral M 1230 Rittenhouse et al, 1972
IP M 1106 Coquet, 1972
Inhalation M&F >1.81 mg/L(4 hr.) Elliott et al, 1972
Inhalation M&F >12.1 mg/L(1 hr.) Kruke, 1972a
Mouse Oral M&F 362 Mastri et al, 1970a
Rabbit Oral M 707 Narcisse et al, 1972a
Dermal M&F 10,250 Mastri et al, 1970b
(24 hour 75% spray suspension)
Dermal M&F 500 Narcisse et al, 1972b
(no irritation)
(24 hr - 75-S formulation)
Dermal M >2000 Cavalli & Spence, 1970a
TABLE 1. (continued)
LD50
Species Route Sex (mg/kg) Reference
Dog Oral M&F <681 Mastalski et al, 1970
Oral M&F 215 Mastalski et al, 1970
(emetic dose)
Chicken Oral F >100 Edds & Bradley, 1971
Oral F 568 Fletcher et al, 1972a
Eye irritation
Acephate (100 mg) was instilled into the conjunctival sac of
rabbits (six animals/group) in an examination for eye irritancy
properties. Conjunctival irritation was evident with all forms of
acephate. Where acephate was washed out after a 5 minute exposure
period, conjunctival irritation and corneal damage were observed.
The irritation was observed after one week while all eyes were
normal at 14 days (Narcisse et al., 1971a).
Vapor inhalation
Groups of rats (5 male and 5 female/group) were exposed to an
atmosphere saturated with acephate vapor (derived from passing air
through crystalline or melted acephate at 90°C). Exposure for 1
hour to vapor derived from either method did not result in any
mortality. Animals were examined over a 14 day observation period
(Cavalli and Spence, 1970d). In an additional study, 5 female rats
were exposed for four hours to vapors of acephate derived only from
passing air through crystalline material. There was no mortality or
morbidity and cholinesterase (undefined) activity was normal
(Cavalli and Spence, 1970e).
Aerosol inhalation
Groups of rats (5 male and 5 female rats/group) were exposed
to an aerosol concentration of 12.1 mg/1 (90% of particles below 10
µ) for one hour. There were no signs of poisoning and no mortality;
gross effects on tissues and organs were not noted (Kruke et al.,
1972a).
Groups of rats (10 male and 10 female rats/group) were exposed
in a 380 l dynamic chamber to aerosols of acephate 1 hour per day,
5 days per week for two weeks. The aerosol was generated from
aqueous solutions of 0, 0.25, 1.0 and 4% acephate and had a
particle size generally under 10 microns. There was no mortality
and weight gain and general behavior were normal. At the highest
dose, female uric acid content was increased. There were no other
effects noted an clinical chemistry, urinalysis or hematology
parameters including cholinesterase activity. Gross and microscopic
examination of tissues and organs showed no effect of acephate
exposure (Kruke et al., 1972b).
Short-term studies
Rabbit - (Dermal)
Groups of rabbits (8 male and 8 female albino rabbits/group)
were administered acephate to either intact or abraded skin. An
aqueous solution was applied daily, 6 hours/day, 5 days/week, for
3 weeks with the daily doses being 0, 0.5, 1.0 and 2.0 g/kg. At the
conclusion of the study, the animals were sacrificed. There was
mortality (2/16) observed at the highest dose and
parasympathomimetic signs of poisoning were observed at 1.0 and 2.0
g/kg. These two groups also lost weight while no differences in growth
or body weight were observed at 0.5 g/kg. The two highest doses showed
substantial changes in hematology and blood chemistry parameters:
reduced RBC count, reduced hemoglobin and hematocrit values and
reduced BUN values. In male rabbits only at the low dose group, LDH
and SGOT values were reduced. RBC cholinesterase was depressed at all
dose levels to about 50% in both sexes whereas plasma cholinesterase
was not affected. On gross examination increased liver weights were
observed at the two highest dose groups. There were no effects noted
on microscopic examination including bone marrow (M/E ratio) studies
(Rittenhouse et al., 1972b).
Hen
Groups of chickens (25 females and 4-6 males/group) were fed
acephate in the diet at dosage levels of 0, 3, 10 and 30 ppm for up to
13 weeks (followed by a 28 day recovery period with control diets).
Growth, egg production and egg quality was normal. The hatchability of
eggs at the highest dose was slightly reduced, and reduction was noted
in growth and development of hatched chicks in the 10 and 30 ppm
groups. The viability of the hatched chicks was diminished in the
highest dose group. There were no gross pathological changes in any of
the birds (Fletcher, 1972a).
Rat
Groups of rats (15 males and 15 females/group) were fed acephate
in the diet for 90 days at levels of 0, 30, 100 and 300 ppm. There was
no mortality attributed to acephate in the diet. Growth, food
consumption and observable behavior were normal. Urinalyses, blood
chemistry and hematology values were normal. Gross and microscopic
analysis of tissues and organs were performed at the conclusion of the
study. In the males a decreased liver weight was noted at 300 ppm.
There were no histological lesions associated with this finding.
Cholinesterase activity data were not reported (Plank et al., 1971).
TABLE 2. Acute toxicity of metabolites or possible impurities
LD50
Compound Structure Species Route Sex (mg/kg) Reference
OO-dimethyl (CH3O)2P(S)NH2 Rat Oral M 633 Cavalli et al, 1968a
phosphoramidothioate (RE 9169) Oral F 549
Signs of poisoning include CNS depression (paresis, hyporeflexia and anesthesia)
Rabbit Dermal M 2500 Cavalli et al, 1969
OS-dimethyl (CH3O)(CH3S)P Rat Oral M 21 Cavalli et al, 1968b
Phosphoramidothioate (O)NH2 Oral F 19
(RE 9006)
Signs of poisoning are standard for parasympathomimetic agents.
Rabbit Dermal M 118 Cavalli et al, 1968c
OO-dimethyl acetyl (CH3O)2P(S)NHC Rat Oral M 1468 Cavalli et al, 1970
phosphoramidothioate (O)CH3 Oral F 1250
(RE 14071)
Signs of poisoning are standard for CNS depression - hyporeflexia, hypothermia, pyloerection, constipation, and possibly
tachycardia.
Rabbit Dermal M >2000 Rittenhouse et al,1972C
OO-dimethyl (CH3O)2(CH3S) Rat Oral M 83 Narcisse et al, 1971b
S-methyl phosphorothioate P(0) Oral F 63
(RE 15283)
The signs of poisoning include salivation, tremors, weakness, and prostration - death occurred 4-10 days after dosing.
TABLE 2. (Cont'd.)
LD50
Compound Structure Species Route Sex (mg/kg) Reference
Rabbit Dermal M 109 Narcisse et al, 1971c
OS-dimethyl (CH3O)(CH3S)P Rat Oral >2000 McCaskey,1972a
phosphorothioate (O) (OH)
(RE 18421) Rabbit Dermal M >2000 Rittenhouse et al, 1972d
S-methyl (CH3S)P(O)OH Rat Oral >2000 McCaskey, 1972b
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al, 1972e
RE 17245)
O-methyl (CH3O)P(O)SH Rat Oral >2000 McCaskey, 1972c
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al.,
(RE 17246) 1972f
Groups of rats (from 15 to 35 males and females per group) were
fed acephate in the diet at levels of 0, 1, 5, 10, 30, 100 and 300 ppm
for 90 days and a control diet for an additional 4 weeks. Animals were
sacrificed at various intervals and cholinesterase activity was
measured using erythrocytes, plasma and brain as enzyme sources.
Contrary to acute or short term oral administration studies this
dietary study showed little effect on erythrocyte cholinesterase
activity at levels below 100 ppm. In contrast, 30 ppm affected plasma
cholinesterase in weeks 6 and 13 of the test period. Brain
cholinesterase was depressed consistently at 30 ppm. In general,
females were more susceptible than males. Recovery of all blood
enzymes was rapid (within 1 week) while brain cholinesterase was
slower. All enzyme levels were normal within 4 weeks of feeding
control diets (Plank et al., 1972).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet at dosage levels of 0, 10, 30 and 100 ppm for 90
days. At the conclusion of the feeding period half of the groups were
sacrificed and the remainder maintained for a 40 day recovery period.
RBC cholinesterase activity was depressed in both sexes at 100 and 30
ppm while plasma was unaffected. Brain cholinesterase was depressed at
30 ppm. Recovery of enzyme activity was noted at 28 days after feeding
stopped and animals were fed control diets.
Growth, food consumption and behavior were unaffected over the
course of the study. There were no deaths, and animals appeared
normal. Analysis of blood and urine and hematologic parameters showed
no effects of acephate. Gross and microscopic analysis of tissues and
organs also showed no effects (Lindberg et al., 1971). [A no-effect
level in the study is 10 ppm (equivalent to 0.32 mg/kg bw) while 30
ppm produced a minimal depression of cholinesterase.]
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet for two years at levels of 0, 10, 30 and 100 ppm.
There was no effect on food consumption, growth or behavior. Two dogs
which did not survive the study died as a result of factors believed
to be unrelated to the presence of acephate in the diet. No effects
were noted on blood chemistry, hematology or urine analysis
parameters. Gross and microscopic examination of a variety of tissues
and organs showed no effects attributed to acephate. Depression of
cholinesterase activity was noted in this study in brain and red blood
cells. Plasma cholinesterase was unaffected at 100 ppm while RBC
showed reduced activity. Brain cholinesterase was only slightly (N10%)
depressed at the highest dose level. A no-effect level based on RBC
cholinesterase depression is 30 ppm over the two year study (Hartke et
al., 1972).
Long-term studies
Rat
Groups of rats (35 males and 35 females/group) were fed acephate
in the diet for two years at levels of 0, 30, 100 and 300 ppm. A
depression of growth was observed in both sexes at 12 months at 100
and 300 ppm. At 24 months the body weight of both sexes was depressed
only at 300 ppm. Food consumption was normal. There were no effects on
mortality, behavior or on hematology, blood chemistry or urine test
parameters. On gross examination male and female liver weight at 300
ppm was depressed, but nothing abnormal was noted on histopathologic
examination of the liver or other tissues and organs. There was no
suggestion of an increased tumor incidence. Blood and brain
cholinesterase depression examined during the course of this study
provided the only effects noted. At 24 months (the only interval
reported) RBC and plasma cholinesterase values were normal. Brain
cholinesterase was minimally depressed (26-27% of normal) at 30 ppm.
The depression was dose dependent suggesting a no-effect level to be
somewhat below 30 ppm. Cholinesterase was not reported for time
intervals other than 24 months. A no-effect level based on
cholinesterase depression cannot be determined in this study. A
systemic no-effect level of 30 ppm may be estimated based on growth
reduction over the first year test interval (Plank and Keplinger,
1973).
OBSERVATIONS IN MAN
In a series of reports from areas in the U.S.A. where acephate
has been used in field applications, 95 individuals exposed from 0.5
to > 1000 hours under a variety of conditions expressed no symptoms
or adverse effects of exposure. The survey was primarily subjective as
clinical measurements were not made (Anonymous, 1976a).
In a similar survey individuals exposed to acephate in pilot
plant and formulation processes did not show adverse effects. Several
of these workers were exposed for up to 15 months (Anonymous, 1976b).
Analysis of urine from these individuals confirmed exposure to
acephate. Urinary levels up to 5 ppm acephate were observed (Pack,
1972b). As expected, other metabolites or technical products of
production were observed in the urine of individuals exposed in the
pilot plant. In addition to acephate, these products included the
oxygen analog of acephate, methamidophos, the oxygen analog of
methamidophos and trimethyl phosphate. The individuals exposed to
technical acephate in formulating processes showed only methamidophos
and trimethyl phosphate as urinary metabolites. Blood and urinary
samples from 5 individuals were examined over a 10 week period. All of
the clinical parameters were normal as were standard tests including
EKG, X-ray and cholinesterase values (Swencicki and Hartz, 1972).
In a follow-up of agricultural field workers exposed to acephate,
levels of 1/50 those found in the pilot plant survey were observed in
the urine. There was no evidence of methamidophos in the urine. As
expected there were no adverse effects noted (Pack, 1972c).
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. They received either a
combination (methamidophos/acephate) of 1:9, 1:4 or corn oil as a
control daily (divided into 3 equal doses) for 21 days. The 1:9 group
was administered the combination for consecutive periods of 21 days at
doses of 0.1, 0.2 and 0.3 mg/kg/day followed by a 7 day recovery
period after which the dose was increased to 0.4 mg/kg/day for 10
days. The 1:4 group received the combination at doses of 0.1 and 0.2
mg/kg/day for 21 consecutive days before the dosing was halted. The
protocol called for a total of 4 dose levels (up to 0.4 mg/kg/day)
each to be administered for 21 days. Blood cholinesterase (plasma and
RBC) was examined periodically.
Results are summarized in Table 3. No effects were noted on RBC
cholinesterase at any treatment level. There was marginal depression
of the plasma cholinesterase noted In the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The plasma
cholinesterase, of the males was substantially affected at the 0.3
mg/kg dose and was not retested at the higher dose level. The group
(both males and females) receiving the higher methamidophos:acephate
ratio (1:4) showed reduction of plasma cholinesterase after two weeks
of receiving a dose of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not
used in the 1:4 dose group. This study indicated the minimal dose of
acephate necessary to decrease plasma cholinesterase for the
combination of acephate and methamidophos was 0.2 mg/kg/day of a 1:4
combination of the two toxicants. No effects with this combination
were noted at 0.1 mg/kg (Garofalo et al., 1973). Thus, a dose level of
0.03 mg methamidophos/kg bw combined with a dose of 0.2 mg acephate/kg
bw (ranging from 0.08 to 0.18 mg acephate/kg bw) would be expected to
reduce cholinesterase activity in man. A no-effect level evaluated
from this study might be 0.2 mg acephate/kg bw in combination with
0.02 mg methamidophos/kg bw.
COMMENTS
Acephate, a relatively non-toxic organophosphorus pesticide, is
rapidly absorbed, metabolised and excreted from the body. A toxic
metabolite, methamidophos, found to occur in plants and soil has not
been identified in mammals. Methamidophos, a potent
parasympathomimetic agent acting by inhibition of
acetylcholinesterase, is also rapidly degraded and excreted in
mammals. Neither compound persists or accumulates in the body nor
appears to affect biochemical systems other than cholinesterase.
Neurotoxicity studies in hens and potentiation studies in rats with
other antiesterase compounds were negative. Special studies on
reproduction showed effects at dietary levels of 100 ppm and above as
evidenced by reduced survival of offspring. In vivo mutagenesis
and teratogenesis testing was negative as was a special study with
mice for carcinogenicity. A positive mutagenesis response in a single
bacterial strain was observed in vitro. In a 2 year study with
dogs and an 18 month study with mice no observable effects other than
cholinesterase depression were noted. In a 2 year rat study, a slight
depression of growth and of brain cholinesterase activity at the
lowest level tested precluded a definition of a no-effect level.
However, a no-effect level in both rats and dogs was suggested based
on 90 day dietary studies which exposed animals at lower levels. In
random observations with humans occupationally exposed, no adverse
effects were noted. In a study to define a no-effect level in humans
based on cholinesterase depression, administration of a combination of
methamidophos and acephate (1:4 ratio) at a total dose of 0.2
mg/kg/day reduced plasma cholinesterase. A calculation of the
individual doses received suggested in one case that 0.16 mg
acephate/kg body weight in combination with 0.04 mg methamidophos/kg
(bw) would cause depression of cholinesterase while a dose of 0.08 mg
or 0.18 mg acephate/kg (bw) both in combination with 0.02 mg
methamidophos did not reduce plasma cholinesterase activity. Based on
the studies with both rat and dog, with further assurance afforded by
the observations in man, an ADI was recommended. This also took into
account the slight effect noted in the rat 2 year study where 30 ppm
(equivalent to 1.5 mg/kg) induced a marginal effect on brain
cholinesterase.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg body weight
Dog: 10 ppm in the diet equivalent to 0.32 mg/kg body weight
Man: 0.18 mg/kg body weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of acephate as a contact and systemic insecticide is
reported from many countries of the American continents, Australia,
Asia and Europe. It is mostly marketed as soluble powders (s.p.), 75%.
Occasionally, however, it is also registered as a 50% s.p., as low
concentration dusts and as 1-5% granules.
TABLE 3. Effect on plasma cholinesterase of combined doses of methamidophos and acephate
Methamidophos: Acephate Ratio
1:4 1:9
Total
dose methamidophos:acephate Plasma Inhibition methamidophos:acephate Plasma inhibition
(mg/kg) (mg/kg) Males Females (mg/kg) Males Females
0.1 0.02 : 0.08 - - 0.01 : 0.09 - -
0.2 0.04 : 0.16 + + 0.02 : 0.18 - -
0.3 0.06 : 0.24 * * 0.03 : 0.27 + -
0.4 0.08 : 0.32 * * 0.04 : 0.36 * -
* Discontinued treatment
Acephate is claimed to be especially active against insects of
the families Lepidoptera, Homoptera and Hemiptera. A list of suggested
uses which is typical in many countries is given in Table 4. These
uses cover suggested protection of both horticultural and agricultural
crops (fruits, vegetables, root-crops and several industrial crops)
against larvae, aphids, caterpillars, berry moths, leaf miners,
beetles etc. Usual application rates are also indicated in Table 4.
They range from 1-2 kg a.i. per ha on field crops and from 0.05-0.1%
on fruit trees, bushes etc.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable amount of residue data on acephate and its
behavior in supervised trials and controlled experiments has been
presented by the originating manufacturer (Chevron Chemical Company).
Most of the data were generated under American climatic and
agricultural conditions, although in some cases supplementary data
from other sources are available.
TABLE 4. Suggested uses of acephate
Concentration or
Crop Pest dosage of a. i.
Fruit crops Aphids 0.04-0.075%
(pome, stone, Codling moths
citrus) Chewing and sucking insects
Tortrix
Citrus, olive scale
Grape Grape berry moths 0.075%
Vegetables Aphids 0.05%
Chewing and sucking insects
Caterpillars
Moths
Beets Leaf miners 0.8-1.0 kg/ha
Ornamentals Aphids and Tortrix 0.05-0.75%
Hops Aphids 0.05-0.25%
Tobacco Aphids 1 kg/ha
Budworms
Flea beetle
Corn Corn borer 1.5-2 kg/ha
Generally the residue data from field trials indicate that
acephate is a relatively non-persistent, systemic insecticide. When
applied at the recommended rate of about 1 kg a.i./ha, several
treatments are often applied, commonly at weekly intervals for as many
weeks as necessary for insect control. A first metabolite, OS-dimethyl
phosphoramidothioate which is itself the insecticide methamidophos, is
regularly found in plant material together with acephate, although
only in the ratio of 1:10 - 1:5.
Alfalfa, cotton and soybean
Extensive trials have been made on these three important crops
(Table 5). Repeated treatments of alfalfa with 1 kg of acephate per ha
leave total residues on fresh cut green hay of less than 6 mg/kg after
21 days and a maximum of about 2 mg/kg after 28 days. Curing of the
hay in the open after harvest did not significantly change the residue
level. The apparent half-life of acephate on green hay is
approximately 7 days.
In cottonseed, the total residues ranged from zero to a maximum
of About 1.5 mg/kg 21 days after the last treatment with 1 kg/ha. The
maximum residue levels from a series of experiments are shown in Table
5. The halflife of such residues is of the order of 14 days. When
processed, essentially all the residue remains in the cottonseed meal
while crude oil and refined oil contain practically no acephate or
methamidophos metabolite (less than 0.03 mg/kg). Immediately after
treatment at the 1 kg/ha level, green leaves of cotton plants carried
residues ranging from 60-200 mg/kg. These residues declined, but they
were still significant, from 7 to 50 mg/kg, after 21 days.
In soybeans, mature and with shell removed, the total residue
varied from zero to 0.2 mg/kg 14 days after the last application of
acephate at the 1 kg/ha rate. Some positive findings are shown in
Table 5, which illustrates that when processed, most of the residue
remained in the mill feed, while trace residues only (less than
0.03 mg/kg) were found in the crude oil and the soya protein flour.
Vegetables
Residue data are available from several countries (e.g.
Australia, the Netherlands, New Zealand, Philippines, U.S.A.) on
vegetables, mostly leafy vegetables such as broccoli, Brussels
sprouts, cabbages, cauliflower and lettuce. Repeated applications of
1 kg acephate per ha are usually recommended and the residue data
indicate that there is a direct relationship between dosage and
residue level.
Residues decline at rates corresponding to the rate of growth
dilution or somewhat faster and as usual, the outer leaves of the
vegetables carry higher residues than the inner parts. This is
exemplified in Table 6 which illustrates typical residue levels of
both acephate and its metabolite methamidophos found in broccoli.
Outer leaves of the vegetables may or may not be discarded as
so-called trimmings before marketing and the final residue on the
marketed product is therefore dependent on the degree of trimming. To
take account of this situation a maximum possible residue on a given
crop may be calculated on the basis of actual analyses of the crop and
its trimmings, assuming maximum trimming. Such predictions of residues
are shown in Table 7 for 5 vegetable crops and compared with residues
actually found. The Table shows that actual residues of acephate + its
metabolite methamidophos are unlikely to exceed 5-10 mg/kg at
appropriate preharvest intervals from 3 to 14 days.
Sugar beets, potatoes and tomatoes
After repeated foliar treatments of sugar beets with acephate, at
a weekly rate of 1 kg/ha, high residues were found on the green leaves
(up to 25-30 mg/kg), while little or no residue (at or below 0.1
mg/kg) was found in the roots. 21 days after the last application the
residues in leaves had declined to about 5-6 mg/kg and in the roots to
zero-0.05 mg/kg. Conversion of the roots to wet pulp completely
removed any residues.
In potatoes after similar treatment the total residues in tubers
are reported to range from 0.04-0.27 mg/kg (Chevron, 1976). This level
did not change during 3 days, but there was some loss of total residue
by the 14th day after the last application. This loss was calculated
to proceed with a half-life of about 21 days.
Total residues of acephate in tomatoes (mature green or ripe
fruits) are reported to range from 0.3-2.4 mg/kg after repeated foliar
treatments at 1 kg/ha (Chevron, 1976). The half-life was 7-14 days on
these fruits.
Residues in the pulp and peel of potatoes and tomatoes are
discussed further below ("Fate of residues in processing and
cooking"). Some residue data are given in Tables 14 and 15.
Citrus fruits
Supervised trials on acephate treatments of oranges, grapefruits,
lemons and tangeloes have been reported from New Zealand (1976) and
the U.S.A. From the results, Table 8, it is clear that a significant
systemic penetration takes place from the peel into the fruit flesh.
In the grapefruit, the acephate concentration seems to be equilibrated
throughout the whole fruit.
TABLE 5. Residues of acephate and its metabolite in alfalfa, cottonseed* and soybeans
Application Days after Residues, mg/kg
Rate, last
Crop kg a.i./ha No. treatment Acephate Methamidophos Total
Green hay (Air spray) 2 21 1.70 - 1.75 0.25 - 0.26 1.95 - 2.01
1 28 0.46 - 0.55 0.07 - 0.10 0.56 - 0.62
Cured hay 1 2 21 2.78 - 5.00 0.36 - 0.57 3.14 - 5.57
(4 days) (Air spray) 28 0.89 - 0.96 0.09 - 0.11 1.00 - 1.05
Green hay 1 2 21 - - -
(Ground spray) 28 0.76 - 1.56 0.24 - 0.52 1.00 - 2.08
Cured hay 1 2 21 - - -
(4 days) (Ground spray) 28 0.33 - 0.52 0.12 - 0.15 0.45 - 0.67
Green hay 1 3 0 27.0 - 32.0 1.29 - 1.65 33.3
(Ground spray) 14 8.9 - 11.9 1.40 - 1.41 10.3
21 3.28 - 4.40 0.59 - 0.74 3.87 - 5.14
28 - 0.21 - 0.29 -
Green hay 1 3 21 5.01 - 5.12 0.54 - 0.59 5.55 - 5.71
(Ground spray) 28 - - -
Cottonseed, 1 8 21 0.48 - 1.15 0.13 - 0.26 0.61 - 1.41
fuzzy 2 8 21 0.47 - 0.83 0.14 - 0.23 0.61 - 1.06
Cottonseed 1 8 21 1.00 - 1.19 0.23 - 0.31 1.23 - 1.50
meal 2 8 21 1.27 - 1.48 0.32 - 0.34 1.59 - 1.82
Crude cottonseed 1 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
oil 2 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
Soybeans 1 3 14 0.08 - 0.20 0.00 - 0.01 0.08 - 0.21
Soybeans, protein 1 3 14 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
flour
Soybeans, 1 3 14 0.02 - 0.03 0.00 - 0.00 0.02 - 0.03
crude oil
Soybeans, 1 3 14 0.34 - 0.23 0.04 - 0.07 0.27 - 0.41
mill feed
* Residues tabulated for cottonseed and soybeans are maximum levels found in a series of experiments.
TABLE 6. Residues of acephate and its metabolite methamidophos in broccoli
Application Days after Residues, mg/kg
Rate last
Crop part kg a.i./ha No. treatment Acephate Methamidophos Total
Heads, 1 3 0 - - -
trimmed 3 - - -
7 4.48 - 5.25 0.36 - 0.45 4.84 - 5.70
14 3.42 - 3.44 0.32 - 0.36 3.74 - 3.80
1 6 0 6.63 - 6.82 0.65 - 0.69 7.32 - 7.47
3 6.63 - 6.63 0.58 - 0.68 7.21 - 7.31
7 5.17 - 6.08 0.75 - 0.75 5.92 - 6.83
14 1.98 - 2.27 0.39 - 0.40 2.66 - 2.38
2 6 0 20.0 - 21.6 1.56 - 1.74 21.7 - 23.2
3 20.4 - 23.0 1.56 - 1.78 22.2 - 24.6
7 14.0 - 16.7 1.70 - 1.70 15.7 - 18.4
14 4.92 - 5.14 0.80 - 0.96 5.7 - 6.1
1 9 0 14.5 - 16.0 1.78 - 1.82 16.3 - 17.8
3 16.0 - 17.4 1.40 - 1.60 17.4 - 19.0
7 10.1 - 13.2 1.24 - 1.27 11.3 - 14.5
14 2.48 - 2.56 0.75 - 0.76 3.24 - 3.31
2 9 0 28.3 - 30.3 3.46 - 3.59 32.1 - 33.9
3 23.5 - 25.3 2.33 - 2.49 23.8 - 27.8
7 21.6 - 24.9 2.47 - 2.59 24.1 - 27.5
14 5.46 - 6.57 1.11 - 1.45 6.57 - 8.02
Trimmings 2 6 0 45.4 - 47.9 2.17 - 2.25 47.7 - 50.1
3 45.4 - 52.9 2.05 - 2.23 47.5 - 55.1
7 20.8 - 40.4 1.38 - 1.65 22.2 - 42.1
14 14.6 - 16.1 1.05 - 1.21 15.7 - 17.3
TABLE 7. Predicted maximum+ and actual residues of acephate
+ methamidophos metabolite in some vegetables
Crop Total Residue,
Application Days after mg/kg
Rate, last Calculated+
Crop kg a.i./ha No. treatment maximum+ Found1
Broccoli 1 3-9 14 5.47 3.80
(max.25%
trimming) 2 6-9 14 9.89 8.02
Brussels 1 8 3 4.79 3.92
sprouts
(max. 15% 2 8 3 12.93 10.18
trimming)
Cabbage 1 9 3 7.29 2.51
(max. 20%
trimming) 2 9 3 12.82 6.38
Cauliflower 1 7-9 3 6.85 2.30
(max.50%
trimming) 2 7-9 3 7.90 4.36
Lettuce 1 6-8 3 7.35 3.29
(max. 50%
trimming) 2 6-8 3 16.17 5.54
+ Based on results from trimmed heads and trimmings, assuming maximum
possible trimming.
1 Reported results on heads trimmed according to commercial practice.
FATE OF RESIDUES
In animals
The metabolic fate of acephate in rats has been described above
("Biotransformation"). Proposed metabolic pathways in mammals are
shown in the same section (Figure 1).
TABLE 8. Residues of acephate and its metabolite methamidophos in citrus fruits
Application Days after Residues, mg/kg
Concentration last Acephate Methamidophos
Country Crop a.i. No. treatment Peel Flesh Peel Flesh
New Zealand Oranges 0.075% 6 1 9.0 0.6 0.98 0.01
(25% peel) 8 9.0 0.7 0.8 0.3
15 7.6 0.7 0.9 0.09
24 5.3 0.6 0.6 n.d.
Grapefruit 0.075% 6 1 2.9 3.0 n.d. 0.2
(30% peel) 8 3.1 1.9 0.2 0.3
15 2.6 3.6 0.2 0.2
24 1.4 5.4 n.d. 0.2
Whole fruit Whole fruit
U.S.A. Oranges 0.06% 2-3 20-25 0.33 - 1.1 0.06 - 0.10
40 0.19 - 1.5 0.02 - 0.08
100 0.00 - 0.00 0.00 - 0.00
Lemons 0.06% 3 20-25 0.08 - 0.18 0.02 - 0.03
0.12% 3 20-25 0.42 - 0.44 0.05 - 0.06
Tangeloes 0.06% 3 20 1.4 - 1.6 0.16 - 0.17
Dairy cows and pigs
9 dairy cows were fed at levels of 3, 10 and 30 ppm of acephate
+0.6, 2 and 6 ppm of its metabolite methamidophos in the diet for 30
days. The ratio of acephate to methamidophos was 5:1 because this is
typical of the ratio in most field crops after treatment with
acephate.
The lowest dosage did not give detectable residues in the cows'
milk. Milk from the higher dosed animals, however, contained between
0.1 and 0.2% of the daily doses and at levels which rapidly reached a
plateau where they remained during the 30 days treatment period.
Average residues found in the milk are shown in Table 9. At withdrawal
of the daily intake, residues in the milk decreased rapidly. By the
morning of the second day after withdrawal neither acephate nor its
metabolite could be observed in the milk.
Some individual cows were sacrificed after 3-4 weeks of feeding
with acephate and its metabolite. They showed significant but moderate
levels of residues in various tissues (Table 10). The highest residue
of acephate was 0.57 mg/kg in the kidney of an animal at the highest
dose level, and no tendency to accumulation in tissues was noted. 6
days after withdrawal, no residues could be detected in liver, kidney,
heart, muscle or fat.
TABLE 9. Average1 residues of acephate and its metabolite
methamidophos in cows' milk
Dose level Residue in milk
(ppm in dry feed) mg/kg ± S.D.1
Acephate Methamidophos Acephate Methamidophos
3 0.6 0.00 0.00
10 2 0.09 ± 0.005 0.00
30 6 0.27 ± 0.06 0.022 ± 0.006
1 Average for 30 days of three cows per dose level.
Essentially the same results were obtained by feeding acephate
and its metabolite to pigs. A linear relationship could be established
between dose levels and tissue residues. The residues ranged from 0.15
to 0.58 mg/kg of total residue at the high dose level (i.e. 30 ppm of
acephate + 6 ppm of metabolite) in tissues which were analysed after 4
weeks of feeding, namely: muscle, liver, kidney, subcutaneous and
peritoneal fat, heart and brain. In pigs as in cows, the residues were
undetectable in all tissues in less than 6 days after withdrawal of
the diet.
Chicken and quail
Tissue samples and eggs were taken from chickens and quail which
had been subjected to acephate feeding in subacute and chronic
toxicological studies. Three dose levels were followed: 3, 10 and 30
ppm of acephate in the diet for 92-140 days. No residues were detected
in any of the experiments in the fat, kidney or liver of the birds. In
eggs and muscle tissue, low levels of acephate were observed at the 10
and 30 ppm feeding levels, namely 0.07 and 0.19 mg/kg respectively in
chicken eggs, 0.16 and 0.31 mg/kg in quail eggs, 0.01 and 0.02 mg/kg
in chicken muscle and 0.01 and 0.04 mg/kg in quail muscle (Table 11).
At the high feeding levels in these studies, trace amounts of
methamidophos (OS-dimethyl phosphoramidothioate), which is otherwise
recognised as a plant metabolite only, were observed in muscles as
well as in eggs.
In plants
Studies using 14C-labelled acephate show that the compound is
readily degraded by plants such as bean seedlings, tomato and cabbage.
The half-life of acephate in such greenhouse-grown plants has been
shown to be of the order of 5-10 days. Among several theoretically
possible metabolites only one, methamidophos or OS-dimethyl
phosphoramidothioate, is found to be of practical significance.
Another of the possible metabolites is believed to be present although
it could not be analytically observed. This is OS-dimethyl
phosphorothioate, which is thought to be transitional step in the
complete breakdown to phosphate. The suggested metabolic pathways of
acephate in plants are shown in Figure 2.
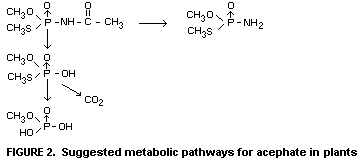 TABLE 10. Residues of acephate and its metabolite methamidophos in cows' tissues
Dose level, acephate + methamidophos (ppm in dry feed)
3 + 0.6 10 + 2 30 + 6
Tissue Acephate Methamidophos Acephate Methamidophos Acephate Methamidophos
Liver 0.00 0.00 0.00 0.00 0.08 0.00
Heart 0.03 0.00 0.10 0.01 0.32 0.06
Kidney 0.03 0.00 0.21 0.01 0.57 0.05
Muscle 0.03 0.00 0.08 0.00 0.28 0.04
Subcutaneous fat 0.00 0.00 0.03 0.00 0.13 0.02
Peritoneal fat 0.00 0.00 0.02 0.00 0.04 0.00
Limits of detection: acephate 0.01 mg/kg
methamidophos 0.005 mg/kg
TABLE 11. Acephate residues in muscle tissue and eggs from chickens
and quail
Chickens Quail
Feeding Days Residues
level(ppm of mg/kg Days of Residues, mg/kg
in diet) feeding Muscle Eggs feeding Muscle Eggs
3 7 - 0.00 148 - -
10 7 0.01 0.07 148 0.01 0.14-0.19
30 7 0.12 0.19 148 0.04 0.28-0.34
Acephate is readily translocated from soil to plants. Tracer
studies show that it is taken up and nearly quantitatively moved
through the roots and throughout the entire plant into the foliage.
For example, 93-98% of the total residue in radish plants was found to
be located in the foliage about 28 days after a soil treatment, with
acephate. Translocation in the other direction, i.e. from the leaves
to the roots or other parts does not take place.
In soil
The fate of acephate in soil has been extensively studied in both
the laboratory and field.
Using S-methyl-14C-acephate for soil treatment, it has been
shown that 14CO2 is released as the acephate decreases. Further, the
metabolite methamidophos is regularly observed. This is the same
metabolite as seen in plants, and it has been noted that it does not
concentrate in soils as it is rapidly degraded. At higher levels of
fortification a further soil metabolite, methyl acetylphosphoramidate
has been identified. Independent studies of this compound indicate
that it has a half-life in soil of about 11 days.
Quantitatively, the decomposition of acephate in soils is
relatively rapid. When 9 soils were studied under laboratory
conditions it was extremely rapid for 8 of them (clay, loams, and
sandy soils) in which half-lives were from 0.5 to 4 days. In the
ninth, a muck soil, decomposition was slower with a half-life of 6-13
days, generally with the higher values associated with dry soils.
Usually half-lives under field conditions are slightly longer
than those obtained in laboratory studies. Data have been presented
from 18 soil tests in 6 major crop producing areas in the United
States indicating relatively rapid declines with half-lives varying
TABLE 12. Carry-over of acephate residues to second crop in crop rotation
Application Residue, mg/kg
First Rate A*, Second B,** Methamidophos
crop kg a.i./ha No. days crop days Acephate metabolite
Cabbage 2 5 14 Radish 42 0.00-0.02 0.00-0.00
Mustard 42 0.00-0.00 0.00-0.00
tops
Lettuce 1 8 20 Lettuce 76 0.00-0.00 0.00-0.00
Beans 85 0.00-0.00 0.00-0.00
Carrots, 99 0.00-0.00 0.00-0.00
roots
Carrots, 99 0.00-0.00 0.00-0.00
tops
Cabbage 1 6 11 Mustard 68 0.03-0.07 0.00-0.00
greens
*A: interval from last treatment to planting of following crop.
**B: interval from last treatment to harvest of following crop.
from 2 to 15 days. Maximum applications in these tests were 9
treatments at 1 and 2 kg/ha and no residues of acephate or
methamidophos could be detected 60 days after the last application.
The lowest detectable levels were 0.01 mg/kg of acephate and 0.005
mg/kg of methamidophos.
The decomposition of acephate in soil has been shown to be mainly
biological in nature. For instance, by sterilization of the soil the
otherwise rapid decline in residues is practically halted. On the
other hand, no adverse effects from soil treatments up to 3 x 20 mg/kg
could be detected on fungal, bacterial or actinomycetes populations,
nor were respiration, ammonification, nitrification or sulphur
oxidation rates affected in any of 3 tested soils.
Earthworms have been studied and found not to concentrate or
store acephate or any of its metabolites in their bodies, and it is
concluded from the study that acephate in soils is not metabolized by
earthworms.
As might be expected, the rapid degradation of acephate in soil
will generally prevent any carry-over from one series of applications
to a following crop. This has been confirmed both in fast crop
rotations and with aged treated soils using lettuce, carrots and other
vegetables as the second crop (Table 12).
Leaching in soils
In laboratory studies using column leaching or soil thin-layer
techniques, it has been clearly shown that acephate and its metabolite
methamidophos are readily moved by water through soil with little, if
any, retention by the soil particles. This is in contrast to the
behaviour of weathered or aged residues. 20 days after soil treatment
at 2 mg/kg no leaching could be detected, while 15% of the applied
14C remained in the soil incorporated in natural soil constituents
after metabolism. This means that leaching into ground water is a
possibility to be considered, at least in extreme cases of high
dosages and when measured immediately after treatment. In one such
case, 0.06 mg/kg of acephate and 0.02 mg/kg of methamidophos were
observed in water sampled in a depth of 15-30 cm.
Likewise, acephate and methamidophos could be moved by run-off
water and detected in both water and soil particles which were
collected at the edge of a vegetable field and analysed separately.
After spraying with 1 kg/ha followed by overhead irrigation
corresponding to 5 cm of rain, residues in the run-off water varied
from 0.04-0.09 mg/kg in one experiment and up to 0.32 mg/kg in
another.
In water
Acephate is found to be relatively stable in water. Hydrolysis in
neutral and near-neutral solutions takes place with a half-life of
about 50 days at 21°C with the formation of
OS-dimethylphosphorothioate. This product is stable at acid but not
alkaline pH. Under alkaline conditions it forms S-methyl
phosphorothioate. Demethylacephate (S-methyl
acetylphosphoramidothioate) is also formed in neutral water. The major
plant and soil metabolite, methamidophos, is not formed in water by
direct hydrolysis. The presence of this metabolite in plants etc. is
therefore due to biochemical mechanisms.
Acephate in water is stable to sunlight which, therefore, cannot
be a major factor in the loss of acephate in the field.
In two field experiments, acephate was studied in natural ponds.
Acephate was added to the water at about 0.1 mg/kg and its fate was
followed by analysis of water, bottom mud and submerged vegetation for
44 days. Some uptake of acephate in bottom mud and vegetation
occurred. A rapid degradation, however was noted shortly afterwards
with half-lives varying from 3 to 10 days in the mud and in
vegetation. The results from one of these studies are summarised in
Table 13.
In the environment
The water solubility of acephate and its possible movement in
leaching and run-off waters etc. have attracted special attention and
the effects of acephate on microorganisms and fish have been followed
in several special studies.
TABLE 13. Residues in a natural pond (Iowa) after treatment
of the water with 0.1 mg acephate per litre
Residues in sample material, mg/kg1
Days after Submerged
treatment Pond water Bottom mud vegetation
0 0.11-0.14 0.04-0.03 0.18-0.52
3 0.09-0.10 0.04-0.06 0.17-0.23
9/10 0.08-0.08 0.00-0.00 0.09-0.09
23/24 0.04-0.04 0.00-0.00 0.04-0.04
44/45 0.00-0.03 0.00-0.00 0.00-0.00
1 No methamidophos metabolite was detected in this study.
Cylindrotheca fusiformis is a marine diatom which is known as
part of basic food chains. Tests show that this organism does not
concentrate acephate or its metabolite methamidophos from culture
solutions containing 1-100 mg/kg of acephate and 1-10 mg/kg of the
metabolite.
The fresh water species, Daphnia magna, is also part of food
chains. In studies with water containing 1 mg/l of acephate and 0.1
mg/1 of methamidophos, adult Daphnia behaved similarly to the marine
diatom, i.e. the concentrations of the compounds in the organisms
remained practically equal to their concentrations in the water
without any indication of further build-up. In parallel experiments
for comparison, DDT was found to concentrate 1000-7000 times in
diatoms and 550 times in Daphnia.
Bluegill sunfish were exposed to 1.0 or 0.01 mg/l of acephate for
35 days followed by a 14 days period in an uncontaminated water
system. 7-10 days after the exposure started, relatively constant
levels of acephate of the order of 5-10 times the concentration in the
water were reached. Thereafter, no further accumulation was observed
and after removal of the fish a rapid elimination of acephate from
their tissues occurred. Within 3 days more than 50% had disappeared.
In none of these experiments were any toxic effects from acephate
on the organisms under study observed.
These observations have been further substantiated in a special
model ecosystem including both a terrestrial and a fresh water aquatic
phase. The following kinds of organisms were involved in the study:
algae, fresh water plants, water flea, mosquito, snail, clam, crab,
mosquito fish, sorghum seedlings and a species of caterpillar. After
treatment with acephate in the model system none of these organisms
accumulated acephate or its metabolites or concentrated them in any of
the tissues.
It was concluded from the studies that acephate would not persist
in the environment and could safely be used in and near aquatic
systems.
In processing and cooking
Commercial processing of ripe tomatoes into canned fruits, puree
and juice reduced the total residue of acephate and its plant
metabolite methamidophos. The decrease was found to be about 3-7 fold
from fresh to processed products in a series of experiments, with no
significant difference between canned tomato, tomato puree and tomato
juice, as shown in Table 14.
These results indicate that acephate residues in tomatoes are not
confined to the outer fruit-layers and the peel. Owing to the systemic
nature of the insecticide a certain amount penetrates into the fruit
flesh. This is further confirmed by direct separate analyses of the
peel and flesh of both tomatoes and potatoes. Table 15 shows that in
potatoes both acephate and its metabolite methamidophos are evenly
distributed throughout the potato. In the tomato penetration into the
interior parts also takes place, although to a smaller extent as there
is a lower concentration in the pulp than in the peel.
Washing with water was not effective in removing residues of
acephate from vegetables (Table 16), which is to be expected since
residues are to a great extent internal. Boiling for 30 minutes
removed up to 60% of the acephate and methamidophos from Brussels
sprouts, however, owing to the water-solubility of the compounds. The
extracted residue was fully recovered in the boiling water.
Storage for up to 3 months at -20°C of various processed products
containing acephate, e.g. canned tomatoes, blanched and frozen
Brussels sprouts and potato tubers, has been shown not to change the
residue level.
Further examples of acephate behavior during the processing of
cotton seed, soybeans and sugar beets have been mentioned above.
METHODS OF RESIDUE ANALYSIS
Analytical methods based on thin-layer chromatography and on
gas-liquid chromatography for the final determination of acephate have
been reported (Chevron, 1972). The principal plant and soil metabolite
methamidophos (0S-dimethyl phosphoramidothioate) is determined
simultaneously in these methods.
The methods are described for animal and vegetable products,
including oily and dry crop samples, and for soil. They involve an
extraction with ethyl acetate, with acetonitrile/hexane partition for
samples containing fat and oil, and then clean-up by silicic acid
column chromatography with 10% methanol in ether as eluant. In its
most recent development the GLC determination is with thermionic
detection (Chevron, 1974).
The limits of determination reported for the GLC methods are
about 0.01 mg/kg for acephate in various substrates and from
0.005-0.01 for methamidophos.
Acephate has been reported to be recovered by the recently
published multi-residue method of Luke et al. (1975) in which acetone
extraction is followed by partition between methylene chloride and
petroleum ether to remove water. The recoveries of acephate in this
procedure are better than 80%.
TABLE 14. Acephate and its metabolite methamidophos in fresh and processed tomatoes
Application Days after Residue, mg/kg
Rate, last
Product kg a.i./ha No. treatment Acephate Methamidophos Total
Fresh ripe 1 6 7 1.7 0.21-0.30 1.91
tomato 7 7 1.5-2.0 0.35-0.41 1.85-2.41
2 6 7 3.2-3.7 0.58-0.69 3.89-4.28
7 7 2.6-3.5 0.55-0.77 3.15-4.27
Canned 1 6 7 0.46-0.57 0.18-0.21 0.64-0.78
tomato 7 7 0.44-0.47 0.25-0.26 0.69-0.73
2 6 7 1.1-1.3 0.39-0.42 1.49-1.72
7 7 0.82-0.98 0.45-0.55 1.27-1.53
Tomato 1 6 7 0.51-0.57 0.21-0.24 0.72-0.81
puree 7 7 0.71-0.71 0.25-0.26 0.96-0.97
2 6 7 1.3-1.3 0.40-0.42 1.70-1.72
7 7 1.1-1.2 0.44-0.46 1.54-1.66
Tomato 1 6 7 0.63-0.71 0.23-0.23 0.86-0.94
juice 7 7 0.79-0.81 0.31-0.33 1.12-1.12
2 6 7 1.2-1.4 0.43-0.43 1.63-1.83
7 7 1.2-1.3 0.53-0.55 1.73-1.85
TABLE 15. Acephate residues in peel and pulp of potatoes and tomatoes
Days after
last Residue, mg/kg
Crop part Treatment treatment Acephate Methamidophos
Potato
peel 1-2 kg/ha 3 0.24-0.86 0.03-0.06
pulp - 3 0.34-0.87 0.02-0.04
peel - 14 0.24-0.25 0.03-0.04
pulp - 14 0.24-0.28 0.03-0.03
Tomato
peel 1-2 kg/ha 3 6.8-9.7 0.57-0.78
pulp - 3 1.5-2.2 0.38-0.50
peel - 14 2.4-2.5 0.56-0.60
pulp - 14 1.1-1.2 0.38-0.40
Further validation of the GLC methods for acephate is reported to
be in progress.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the meeting are shown in Table
17.
APPRAISAL
Acephate is a relatively new organophosphorus insecticide with
both contact and systemic action on sucking and biting insects. Since
its first marketing in 1970/71 it has been registered in many
countries for both horticultural and agricultural crop protection.
Technical acephate is described as a chemical of 80-90% purity of
which the main impurities are known. It is usually formulated as 50%
or 75% soluble powders, low concentration dusts and 1.5% granules and
it is often applied in repeated pre-harvest treatments at the rate of
1-2 kg/ha or 0.05-0.1% spray dilutions.
The residue data available are mainly obtained from experiments
and supervised trials made under North American climatic conditions,
in a few cases supplemented from other countries. Most of the data are
from trials under practical conditions which are likely to represent
the results of good agricultural practice.
The systemic nature of acephate is demonstrated by a significant
penetration of residues into the flesh of fruit and the internal parts
of plants such as tomatoes, potatoes and citrus. It shows relatively
low persistence.
Uptake from soils and ready translocation through plants to the
foliage occurs. The metabolism of acephate has been studied and it is
suggested that a complete degradation into phosphates and CO2 may
take place leading to the incorporation of these compounds into plant
constituents. A major metabolite, 0S-dimethyl phosphoramidothioate is
considered to represent a transitional step. This metabolite is itself
the insecticide methamidophos, and it is regularly found as a part
(10-15%) of acephate residues in plant material.
The fate of acephate residues when fed to livestock has been
investigated. Continued feeding gave rise to residues which settled at
low levels in meat, milk and eggs as well as in most tissues. In all
cases examined, viz cow, pig, chicken and quail, residues
disappeared rapidly becoming undetectable within 1 to 6 days of the
rapid withdrawal of acephate feeding.
Owing to the water-soluble character of acephate its fate in
different environmental systems, particularly in soil and water, has
been thoroughly investigated. In soils, degradation is relatively
rapid and follows the pathways demonstrated in metabolism. Some
leaching through the upper soil layers can be traced in extreme cases.
Degradation of acephate in the various components of different
ecosystems is relatively fast and no signs of accumulation or
concentration in any tissues of living organisms have been found.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for the specific determination
of acephate, although full validation of the methods has not yet been
published.
TABLE 17. National tolerances reported to the Meeting
Tolerance, Pre-harvest
Country Crop mg/kg interval, days
Australia Cauliflower, cabbage,
lettuce 10
Brussels sprouts, broccoli,
tomatoes 6
Cottonseed 2.5
Soybeans 1
Potatoes 0.5
Fat of meat, meat and milk 0.1
Netherlands Brassicas 1 (+ 0.1 mg/kg methamidophos)
Potato 0.02 (+ 0.01 mg/kg methamidophos)1
New Zealand Brassicas 2.0
Switzerland Pome fruit, stone fruit 2.5
Vegetables 1.5
U.S.A. Celery,* 10 21
Lettuce (head)* 10
Cottonseed meal 8
Cottonseed hulls, bell peppers*
soybean meal 4
Beans(succulent and dry)* 3 14
Cottonseed 2 21
Almonds, soybeans 1 35,14
Meat, fat, meat by products of cattle,
goats, hogs, horses, poultry & sheep 0.1
Milk, eggs 0.1
1 At or about the limit of determination.
* Not more than 1 mg/kg of OS-dimethyl phosphoramidothioate.
RECOMMENDATIONS
The following maximum residue limits are recommended for
acephate. The plant metabolite 0S-dimethyl phosphoramidothioate is the
insecticide methamidophos for which separate recommendations are made
(see "Methamidophos").
Pre-harvest intervals on which
Commodity Limit recommendations are based
mg/kg days
Alfalfa 10 21
Lettuce 10 14
Sugar beet leaves 10 21
Broccoli 5 14
Brussels sprouts 5 14
Cabbage 5 14
Cauliflower 5 14
Citrus 5 7
Tomatoes 5
Cottonseed 2 21
Potatoes 1
Soybeans 1 14
Sugar beets 1
Meat and fat of cattle,
pigs and poultry 0.1
Milk 0.1
Eggs 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the metabolic fate of acephate,
preferably in non-rodent species.
2. Further studies to elucidate the contribution of acephate and
methamidophos alone or in combination in depressing
cholinesterase activity.
3. Validation of methods of residue analysis for regulatory
purposes, which is reported to be in progress.
REFERENCES
Anonymous. Human exposure reports from field application
1976a of orthene by research personnel. Unpublished reports
compiled by Chevron Chemical Company,. submitted to the
World Health Organization by Chevron Chemical Company.
Anonymous. Human exposure reports from operation of orthene
1976b pilot plant and formulation of orthene 75S. Unpublished
reports compiled by Chevron Chemical Company, submitted
to the World Health Organization by Chevron Chemical
Company.
Arnold, D., Kennedy, G., and Keplinger, M. Mutagenic study with
1971 orthene in albino mice. Unpublished report from
Industrial Bio-Test Laboratories, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S125 acute oral toxicity of
1968a RE9169 (SX 196) in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-86 acute oral toxicity
1968b of monitor technical in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, Jr. S-98 acute dermal toxicity
1968c of monitor technical. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-126 acute dermal
1969 toxicity of RE9169 (SX 198) in rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Cavalli, R., Christensen, H., and Spence, J. S-183 the acute oral
1970 toxicity of RE14.071. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-160 acute dermal toxicity of
1970a RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-181 acute oral toxicity of ortho
1970b RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-174 effect of RE12,420 on the
1970c acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. The acute inhalation toxicity of
1970d RE12,420. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of RE12,420 on the
1970e acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of repeated doses of RE12,420
1970f on cholinesterase activity in rats. Unpublished report
from Standard Oil Company of California, submitted to
the World Health Organization by Chevron Chemical
Company.
Cavalli, R., and Spence, J. Skin sensitization potential of RE12,420
1970g (75% SS). Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Skin sensitization potential of
1970h RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Chevron Chemical Company. Comparison of methods RM-12B with RM-12
1972 and RM-12A. File 740.01, January 21.
Chevron Chemical Company. Orthene and themmetabolite ortho 9006.
1974 Residue analysis by thermionic gas chromatography. File
740.01, April 25.
Chevron Chemical Company. Acephate (OrtheneR). Identity and
1976 residues in food. Vol. 1, 2 and 3. Information, data
and laboratory reports covering the research period
1971-1973 and submitted to the Joint Meeting 1976. On
file in FAO, Plant Protection Division.
Coquet, B. Determination of the lethal dose 50% by intra-peritoneal
1972 route in the male rat of ortho 12,420 technical (85%
acephate). Unpublished report from Centre de Recherche
et d'Elevage des Oncins, submitted to the World Health
Organization by Chevron Chemical Company.
Crossley, J., and Tutass, H. Metabolism of monitor insecticide
1969 by rats. Unpublished report from Ortho, Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Ebbens, K., Mastri, C., Keplinger, M., and Fancher, O. Skin
1971 sensitization test with orthene technical-SX-284 in
albino guinea pigs. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Edds, G., and Bradley, R. Acute toxicity study - white Leghorn
1971 chicks. Unpublished report from the University of
Florida, Gainesville, submitted to the World Health
Organization by Chevron Chemical Company.
Elliott, C., Shadberg, K., Goode, J., and Keplinger, M. Acute dust
1972 inhalation toxicity study with orthene-85% technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Toxicity reproduction
1972a and residue study with orthen technical SX-331 in white
Leghorn chickens. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Neurotoxicity study
1972b with orthene technical, SX.284 in adult hens.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Garofalo, M., Palazzolo, R., and Sanders, R. A study of the effects
1973 of orthene and monitor on plasma and erythrocyte
cholinesterase activity in human subjects during
subacute oral administration. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to
the World Health Organization by Chevron Chemical
Company.
Haley, S., Plank, J., Wright, P., and Keplinger, M. Teratogenic
1971 study with orthene technical in albino rats.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Haley, S., Kennedy, G., and Keplinger, M. Three generation
1973 reproduction study with RE 12,420 technical in albino
rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Hartke, K., Burtner, G., Kennedy, G., and Keplinger, M. Two-year
1972 chronic oral toxicity study with RE 12,420 in beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
ICI, Australia. Data submitted through the Codex Contact Point,
1976 Canberra, Australia.
Kretchmar, B., Mastri, C., Keplinger, M., and Fancher, O. Acute
1971 oral toxicity study to determine the efficacy of
atropine sulfate and 2-PAM as antidotes for RE 12,420
technical intoxication in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Kretchmar, B., Mastri, C., and Keplinger, M. Acute oral potentiation
1972 study with monitor technical and orthene technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc. submitted to the World
Health Organization by Chevron Chemical Company.
Kruke, V., Narcisse, J., Cavalli, R., and Spence, J. Acute inhalation
1972a toxicity of orthene 75S. Unpublished report from
Standard Oil Company of California. submitted to the
World Health Organization by Chevron Chemical Company.
Kruke, V., Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 14-Day aerosol inhalation study in adult rats.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Ladd, R., Jenkins, D., Wright, P., and Keplinger, M. Teratogenic
1972 study with orthene technical in albino rabbits.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Lee, H. Metabolism of orthene in rats. Unpublished report from Ortho
1972 Division of Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Lindberg, D., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 study with orthene in Beagle dogs. Unpublished study
from Industrial Bio-Test Laboratories, Inc., submitted
to the World Health Organization by Chevron Chemical
Company.
Luke, M.A., Froberg. J.E., and Masumoto. H.T. Extraction and
1975 cleanup of organochlorine, organophosphate,
organonitrogen, and hydrocarbon pesticides in produce
for determination by gas-liquid chromatography. J. Ass.
off. analyt. Chem., 58:1020-1026.
Magellona, E.D. Report from the Codex Contact Point, Philippines
1976 on acephate uses on rice and cabbage.
Mastalski, K., Jenkins, D., Keplinger, M., and Fancher, O. Acute
1970 oral toxicity study on RE 12,420 technical in Beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Mastri, C., Keplinger, M., and Fancher, O. Acute oral toxicity
1970a study on RE 12,420 technical in white mice. Unpublished
report from Industrial BioTest Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Mastri, C., Keplinger, M., and Francher, O. Acute oral and dermal
1970b toxicity studies on RE 12,420 75% spray suspension
formulation. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE18,421. Unpublished report from
1972a Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,245. Unpublished report from
1972b Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,246. Unpublished report from
1972c Chevron Chemical Company submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. Eye irritation potential
1971a of orthene technical, orthene 75S (CC-2153) and orthene
75S (CC-2152). Unpublished report from Standard Oil
Company of California, submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-269 the acute oral
1971b toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-270 the acute dermal
1971c toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The acute oral toxicity
1972a of orthene 75-S to adult male rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The skin irritation
1972b potential of orthene 75-S. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
New Zealand. Submission on acephate from the National Codex
1976 Committee of New Zealand.
Pack, D. The metabolism of RE9169 and RE14,071 in rats. Unpublished
1972a report from Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Pack, D. Orthene occupational exposure-pilot plant and formulations.
1972b Unpublished report from Ortho Division of Chevron
Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Pack, D. Orthene insecticide occupational exposure - field station
1972c personnel. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Plank, J., and Keplinger, M. Two-year chronic oral toxicity study
1973 with RE12,420 technical in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Plank, J., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 subacute oral toxicity study with RE12,420 technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Plank, J., Wright, P., and Keplinger, M. 90-Day cholinesterase
1972 study with ortho 12,420 technical in albino rats.
Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. The
1972a acute oral toxicity to rats of orthene in combination
with five other cholinesterase inhibition of materials.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Reyna, M., Kennedy, G., and Keplinger, M. 18-Month carcinogenic
1973 study with RE12,420 technical in Swiss white mice.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 21-Day subacute dermal study in rabbits with
orthene-75S. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972c S-184 the acute dermal toxicity of RE14,071.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972d S-533. The acute dermal toxicity of RE18,421.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. S-514
1972e The acute dermal toxicity of RE17,245. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972P S-515 the acute dermal toxicity of RE17,246.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Swencicki, R., and Hartz, W. Orthene human exposure study
1972 clinical evaluation. Unpublished study from Standard
Oil Company of California, Submitted to the World
Health Organization by Chevron Chemical Company.
Tucker, B. Acetylcholinesterase inhibition of orthene and
1972 ortho 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
TABLE 10. Residues of acephate and its metabolite methamidophos in cows' tissues
Dose level, acephate + methamidophos (ppm in dry feed)
3 + 0.6 10 + 2 30 + 6
Tissue Acephate Methamidophos Acephate Methamidophos Acephate Methamidophos
Liver 0.00 0.00 0.00 0.00 0.08 0.00
Heart 0.03 0.00 0.10 0.01 0.32 0.06
Kidney 0.03 0.00 0.21 0.01 0.57 0.05
Muscle 0.03 0.00 0.08 0.00 0.28 0.04
Subcutaneous fat 0.00 0.00 0.03 0.00 0.13 0.02
Peritoneal fat 0.00 0.00 0.02 0.00 0.04 0.00
Limits of detection: acephate 0.01 mg/kg
methamidophos 0.005 mg/kg
TABLE 11. Acephate residues in muscle tissue and eggs from chickens
and quail
Chickens Quail
Feeding Days Residues
level(ppm of mg/kg Days of Residues, mg/kg
in diet) feeding Muscle Eggs feeding Muscle Eggs
3 7 - 0.00 148 - -
10 7 0.01 0.07 148 0.01 0.14-0.19
30 7 0.12 0.19 148 0.04 0.28-0.34
Acephate is readily translocated from soil to plants. Tracer
studies show that it is taken up and nearly quantitatively moved
through the roots and throughout the entire plant into the foliage.
For example, 93-98% of the total residue in radish plants was found to
be located in the foliage about 28 days after a soil treatment, with
acephate. Translocation in the other direction, i.e. from the leaves
to the roots or other parts does not take place.
In soil
The fate of acephate in soil has been extensively studied in both
the laboratory and field.
Using S-methyl-14C-acephate for soil treatment, it has been
shown that 14CO2 is released as the acephate decreases. Further, the
metabolite methamidophos is regularly observed. This is the same
metabolite as seen in plants, and it has been noted that it does not
concentrate in soils as it is rapidly degraded. At higher levels of
fortification a further soil metabolite, methyl acetylphosphoramidate
has been identified. Independent studies of this compound indicate
that it has a half-life in soil of about 11 days.
Quantitatively, the decomposition of acephate in soils is
relatively rapid. When 9 soils were studied under laboratory
conditions it was extremely rapid for 8 of them (clay, loams, and
sandy soils) in which half-lives were from 0.5 to 4 days. In the
ninth, a muck soil, decomposition was slower with a half-life of 6-13
days, generally with the higher values associated with dry soils.
Usually half-lives under field conditions are slightly longer
than those obtained in laboratory studies. Data have been presented
from 18 soil tests in 6 major crop producing areas in the United
States indicating relatively rapid declines with half-lives varying
TABLE 12. Carry-over of acephate residues to second crop in crop rotation
Application Residue, mg/kg
First Rate A*, Second B,** Methamidophos
crop kg a.i./ha No. days crop days Acephate metabolite
Cabbage 2 5 14 Radish 42 0.00-0.02 0.00-0.00
Mustard 42 0.00-0.00 0.00-0.00
tops
Lettuce 1 8 20 Lettuce 76 0.00-0.00 0.00-0.00
Beans 85 0.00-0.00 0.00-0.00
Carrots, 99 0.00-0.00 0.00-0.00
roots
Carrots, 99 0.00-0.00 0.00-0.00
tops
Cabbage 1 6 11 Mustard 68 0.03-0.07 0.00-0.00
greens
*A: interval from last treatment to planting of following crop.
**B: interval from last treatment to harvest of following crop.
from 2 to 15 days. Maximum applications in these tests were 9
treatments at 1 and 2 kg/ha and no residues of acephate or
methamidophos could be detected 60 days after the last application.
The lowest detectable levels were 0.01 mg/kg of acephate and 0.005
mg/kg of methamidophos.
The decomposition of acephate in soil has been shown to be mainly
biological in nature. For instance, by sterilization of the soil the
otherwise rapid decline in residues is practically halted. On the
other hand, no adverse effects from soil treatments up to 3 x 20 mg/kg
could be detected on fungal, bacterial or actinomycetes populations,
nor were respiration, ammonification, nitrification or sulphur
oxidation rates affected in any of 3 tested soils.
Earthworms have been studied and found not to concentrate or
store acephate or any of its metabolites in their bodies, and it is
concluded from the study that acephate in soils is not metabolized by
earthworms.
As might be expected, the rapid degradation of acephate in soil
will generally prevent any carry-over from one series of applications
to a following crop. This has been confirmed both in fast crop
rotations and with aged treated soils using lettuce, carrots and other
vegetables as the second crop (Table 12).
Leaching in soils
In laboratory studies using column leaching or soil thin-layer
techniques, it has been clearly shown that acephate and its metabolite
methamidophos are readily moved by water through soil with little, if
any, retention by the soil particles. This is in contrast to the
behaviour of weathered or aged residues. 20 days after soil treatment
at 2 mg/kg no leaching could be detected, while 15% of the applied
14C remained in the soil incorporated in natural soil constituents
after metabolism. This means that leaching into ground water is a
possibility to be considered, at least in extreme cases of high
dosages and when measured immediately after treatment. In one such
case, 0.06 mg/kg of acephate and 0.02 mg/kg of methamidophos were
observed in water sampled in a depth of 15-30 cm.
Likewise, acephate and methamidophos could be moved by run-off
water and detected in both water and soil particles which were
collected at the edge of a vegetable field and analysed separately.
After spraying with 1 kg/ha followed by overhead irrigation
corresponding to 5 cm of rain, residues in the run-off water varied
from 0.04-0.09 mg/kg in one experiment and up to 0.32 mg/kg in
another.
In water
Acephate is found to be relatively stable in water. Hydrolysis in
neutral and near-neutral solutions takes place with a half-life of
about 50 days at 21°C with the formation of
OS-dimethylphosphorothioate. This product is stable at acid but not
alkaline pH. Under alkaline conditions it forms S-methyl
phosphorothioate. Demethylacephate (S-methyl
acetylphosphoramidothioate) is also formed in neutral water. The major
plant and soil metabolite, methamidophos, is not formed in water by
direct hydrolysis. The presence of this metabolite in plants etc. is
therefore due to biochemical mechanisms.
Acephate in water is stable to sunlight which, therefore, cannot
be a major factor in the loss of acephate in the field.
In two field experiments, acephate was studied in natural ponds.
Acephate was added to the water at about 0.1 mg/kg and its fate was
followed by analysis of water, bottom mud and submerged vegetation for
44 days. Some uptake of acephate in bottom mud and vegetation
occurred. A rapid degradation, however was noted shortly afterwards
with half-lives varying from 3 to 10 days in the mud and in
vegetation. The results from one of these studies are summarised in
Table 13.
In the environment
The water solubility of acephate and its possible movement in
leaching and run-off waters etc. have attracted special attention and
the effects of acephate on microorganisms and fish have been followed
in several special studies.
TABLE 13. Residues in a natural pond (Iowa) after treatment
of the water with 0.1 mg acephate per litre
Residues in sample material, mg/kg1
Days after Submerged
treatment Pond water Bottom mud vegetation
0 0.11-0.14 0.04-0.03 0.18-0.52
3 0.09-0.10 0.04-0.06 0.17-0.23
9/10 0.08-0.08 0.00-0.00 0.09-0.09
23/24 0.04-0.04 0.00-0.00 0.04-0.04
44/45 0.00-0.03 0.00-0.00 0.00-0.00
1 No methamidophos metabolite was detected in this study.
Cylindrotheca fusiformis is a marine diatom which is known as
part of basic food chains. Tests show that this organism does not
concentrate acephate or its metabolite methamidophos from culture
solutions containing 1-100 mg/kg of acephate and 1-10 mg/kg of the
metabolite.
The fresh water species, Daphnia magna, is also part of food
chains. In studies with water containing 1 mg/l of acephate and 0.1
mg/1 of methamidophos, adult Daphnia behaved similarly to the marine
diatom, i.e. the concentrations of the compounds in the organisms
remained practically equal to their concentrations in the water
without any indication of further build-up. In parallel experiments
for comparison, DDT was found to concentrate 1000-7000 times in
diatoms and 550 times in Daphnia.
Bluegill sunfish were exposed to 1.0 or 0.01 mg/l of acephate for
35 days followed by a 14 days period in an uncontaminated water
system. 7-10 days after the exposure started, relatively constant
levels of acephate of the order of 5-10 times the concentration in the
water were reached. Thereafter, no further accumulation was observed
and after removal of the fish a rapid elimination of acephate from
their tissues occurred. Within 3 days more than 50% had disappeared.
In none of these experiments were any toxic effects from acephate
on the organisms under study observed.
These observations have been further substantiated in a special
model ecosystem including both a terrestrial and a fresh water aquatic
phase. The following kinds of organisms were involved in the study:
algae, fresh water plants, water flea, mosquito, snail, clam, crab,
mosquito fish, sorghum seedlings and a species of caterpillar. After
treatment with acephate in the model system none of these organisms
accumulated acephate or its metabolites or concentrated them in any of
the tissues.
It was concluded from the studies that acephate would not persist
in the environment and could safely be used in and near aquatic
systems.
In processing and cooking
Commercial processing of ripe tomatoes into canned fruits, puree
and juice reduced the total residue of acephate and its plant
metabolite methamidophos. The decrease was found to be about 3-7 fold
from fresh to processed products in a series of experiments, with no
significant difference between canned tomato, tomato puree and tomato
juice, as shown in Table 14.
These results indicate that acephate residues in tomatoes are not
confined to the outer fruit-layers and the peel. Owing to the systemic
nature of the insecticide a certain amount penetrates into the fruit
flesh. This is further confirmed by direct separate analyses of the
peel and flesh of both tomatoes and potatoes. Table 15 shows that in
potatoes both acephate and its metabolite methamidophos are evenly
distributed throughout the potato. In the tomato penetration into the
interior parts also takes place, although to a smaller extent as there
is a lower concentration in the pulp than in the peel.
Washing with water was not effective in removing residues of
acephate from vegetables (Table 16), which is to be expected since
residues are to a great extent internal. Boiling for 30 minutes
removed up to 60% of the acephate and methamidophos from Brussels
sprouts, however, owing to the water-solubility of the compounds. The
extracted residue was fully recovered in the boiling water.
Storage for up to 3 months at -20°C of various processed products
containing acephate, e.g. canned tomatoes, blanched and frozen
Brussels sprouts and potato tubers, has been shown not to change the
residue level.
Further examples of acephate behavior during the processing of
cotton seed, soybeans and sugar beets have been mentioned above.
METHODS OF RESIDUE ANALYSIS
Analytical methods based on thin-layer chromatography and on
gas-liquid chromatography for the final determination of acephate have
been reported (Chevron, 1972). The principal plant and soil metabolite
methamidophos (0S-dimethyl phosphoramidothioate) is determined
simultaneously in these methods.
The methods are described for animal and vegetable products,
including oily and dry crop samples, and for soil. They involve an
extraction with ethyl acetate, with acetonitrile/hexane partition for
samples containing fat and oil, and then clean-up by silicic acid
column chromatography with 10% methanol in ether as eluant. In its
most recent development the GLC determination is with thermionic
detection (Chevron, 1974).
The limits of determination reported for the GLC methods are
about 0.01 mg/kg for acephate in various substrates and from
0.005-0.01 for methamidophos.
Acephate has been reported to be recovered by the recently
published multi-residue method of Luke et al. (1975) in which acetone
extraction is followed by partition between methylene chloride and
petroleum ether to remove water. The recoveries of acephate in this
procedure are better than 80%.
TABLE 14. Acephate and its metabolite methamidophos in fresh and processed tomatoes
Application Days after Residue, mg/kg
Rate, last
Product kg a.i./ha No. treatment Acephate Methamidophos Total
Fresh ripe 1 6 7 1.7 0.21-0.30 1.91
tomato 7 7 1.5-2.0 0.35-0.41 1.85-2.41
2 6 7 3.2-3.7 0.58-0.69 3.89-4.28
7 7 2.6-3.5 0.55-0.77 3.15-4.27
Canned 1 6 7 0.46-0.57 0.18-0.21 0.64-0.78
tomato 7 7 0.44-0.47 0.25-0.26 0.69-0.73
2 6 7 1.1-1.3 0.39-0.42 1.49-1.72
7 7 0.82-0.98 0.45-0.55 1.27-1.53
Tomato 1 6 7 0.51-0.57 0.21-0.24 0.72-0.81
puree 7 7 0.71-0.71 0.25-0.26 0.96-0.97
2 6 7 1.3-1.3 0.40-0.42 1.70-1.72
7 7 1.1-1.2 0.44-0.46 1.54-1.66
Tomato 1 6 7 0.63-0.71 0.23-0.23 0.86-0.94
juice 7 7 0.79-0.81 0.31-0.33 1.12-1.12
2 6 7 1.2-1.4 0.43-0.43 1.63-1.83
7 7 1.2-1.3 0.53-0.55 1.73-1.85
TABLE 15. Acephate residues in peel and pulp of potatoes and tomatoes
Days after
last Residue, mg/kg
Crop part Treatment treatment Acephate Methamidophos
Potato
peel 1-2 kg/ha 3 0.24-0.86 0.03-0.06
pulp - 3 0.34-0.87 0.02-0.04
peel - 14 0.24-0.25 0.03-0.04
pulp - 14 0.24-0.28 0.03-0.03
Tomato
peel 1-2 kg/ha 3 6.8-9.7 0.57-0.78
pulp - 3 1.5-2.2 0.38-0.50
peel - 14 2.4-2.5 0.56-0.60
pulp - 14 1.1-1.2 0.38-0.40
Further validation of the GLC methods for acephate is reported to
be in progress.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the meeting are shown in Table
17.
APPRAISAL
Acephate is a relatively new organophosphorus insecticide with
both contact and systemic action on sucking and biting insects. Since
its first marketing in 1970/71 it has been registered in many
countries for both horticultural and agricultural crop protection.
Technical acephate is described as a chemical of 80-90% purity of
which the main impurities are known. It is usually formulated as 50%
or 75% soluble powders, low concentration dusts and 1.5% granules and
it is often applied in repeated pre-harvest treatments at the rate of
1-2 kg/ha or 0.05-0.1% spray dilutions.
The residue data available are mainly obtained from experiments
and supervised trials made under North American climatic conditions,
in a few cases supplemented from other countries. Most of the data are
from trials under practical conditions which are likely to represent
the results of good agricultural practice.
The systemic nature of acephate is demonstrated by a significant
penetration of residues into the flesh of fruit and the internal parts
of plants such as tomatoes, potatoes and citrus. It shows relatively
low persistence.
Uptake from soils and ready translocation through plants to the
foliage occurs. The metabolism of acephate has been studied and it is
suggested that a complete degradation into phosphates and CO2 may
take place leading to the incorporation of these compounds into plant
constituents. A major metabolite, 0S-dimethyl phosphoramidothioate is
considered to represent a transitional step. This metabolite is itself
the insecticide methamidophos, and it is regularly found as a part
(10-15%) of acephate residues in plant material.
The fate of acephate residues when fed to livestock has been
investigated. Continued feeding gave rise to residues which settled at
low levels in meat, milk and eggs as well as in most tissues. In all
cases examined, viz cow, pig, chicken and quail, residues
disappeared rapidly becoming undetectable within 1 to 6 days of the
rapid withdrawal of acephate feeding.
Owing to the water-soluble character of acephate its fate in
different environmental systems, particularly in soil and water, has
been thoroughly investigated. In soils, degradation is relatively
rapid and follows the pathways demonstrated in metabolism. Some
leaching through the upper soil layers can be traced in extreme cases.
Degradation of acephate in the various components of different
ecosystems is relatively fast and no signs of accumulation or
concentration in any tissues of living organisms have been found.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for the specific determination
of acephate, although full validation of the methods has not yet been
published.
TABLE 17. National tolerances reported to the Meeting
Tolerance, Pre-harvest
Country Crop mg/kg interval, days
Australia Cauliflower, cabbage,
lettuce 10
Brussels sprouts, broccoli,
tomatoes 6
Cottonseed 2.5
Soybeans 1
Potatoes 0.5
Fat of meat, meat and milk 0.1
Netherlands Brassicas 1 (+ 0.1 mg/kg methamidophos)
Potato 0.02 (+ 0.01 mg/kg methamidophos)1
New Zealand Brassicas 2.0
Switzerland Pome fruit, stone fruit 2.5
Vegetables 1.5
U.S.A. Celery,* 10 21
Lettuce (head)* 10
Cottonseed meal 8
Cottonseed hulls, bell peppers*
soybean meal 4
Beans(succulent and dry)* 3 14
Cottonseed 2 21
Almonds, soybeans 1 35,14
Meat, fat, meat by products of cattle,
goats, hogs, horses, poultry & sheep 0.1
Milk, eggs 0.1
1 At or about the limit of determination.
* Not more than 1 mg/kg of OS-dimethyl phosphoramidothioate.
RECOMMENDATIONS
The following maximum residue limits are recommended for
acephate. The plant metabolite 0S-dimethyl phosphoramidothioate is the
insecticide methamidophos for which separate recommendations are made
(see "Methamidophos").
Pre-harvest intervals on which
Commodity Limit recommendations are based
mg/kg days
Alfalfa 10 21
Lettuce 10 14
Sugar beet leaves 10 21
Broccoli 5 14
Brussels sprouts 5 14
Cabbage 5 14
Cauliflower 5 14
Citrus 5 7
Tomatoes 5
Cottonseed 2 21
Potatoes 1
Soybeans 1 14
Sugar beets 1
Meat and fat of cattle,
pigs and poultry 0.1
Milk 0.1
Eggs 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the metabolic fate of acephate,
preferably in non-rodent species.
2. Further studies to elucidate the contribution of acephate and
methamidophos alone or in combination in depressing
cholinesterase activity.
3. Validation of methods of residue analysis for regulatory
purposes, which is reported to be in progress.
REFERENCES
Anonymous. Human exposure reports from field application
1976a of orthene by research personnel. Unpublished reports
compiled by Chevron Chemical Company,. submitted to the
World Health Organization by Chevron Chemical Company.
Anonymous. Human exposure reports from operation of orthene
1976b pilot plant and formulation of orthene 75S. Unpublished
reports compiled by Chevron Chemical Company, submitted
to the World Health Organization by Chevron Chemical
Company.
Arnold, D., Kennedy, G., and Keplinger, M. Mutagenic study with
1971 orthene in albino mice. Unpublished report from
Industrial Bio-Test Laboratories, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S125 acute oral toxicity of
1968a RE9169 (SX 196) in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-86 acute oral toxicity
1968b of monitor technical in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, Jr. S-98 acute dermal toxicity
1968c of monitor technical. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-126 acute dermal
1969 toxicity of RE9169 (SX 198) in rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Cavalli, R., Christensen, H., and Spence, J. S-183 the acute oral
1970 toxicity of RE14.071. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-160 acute dermal toxicity of
1970a RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-181 acute oral toxicity of ortho
1970b RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-174 effect of RE12,420 on the
1970c acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. The acute inhalation toxicity of
1970d RE12,420. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of RE12,420 on the
1970e acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of repeated doses of RE12,420
1970f on cholinesterase activity in rats. Unpublished report
from Standard Oil Company of California, submitted to
the World Health Organization by Chevron Chemical
Company.
Cavalli, R., and Spence, J. Skin sensitization potential of RE12,420
1970g (75% SS). Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Skin sensitization potential of
1970h RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Chevron Chemical Company. Comparison of methods RM-12B with RM-12
1972 and RM-12A. File 740.01, January 21.
Chevron Chemical Company. Orthene and themmetabolite ortho 9006.
1974 Residue analysis by thermionic gas chromatography. File
740.01, April 25.
Chevron Chemical Company. Acephate (OrtheneR). Identity and
1976 residues in food. Vol. 1, 2 and 3. Information, data
and laboratory reports covering the research period
1971-1973 and submitted to the Joint Meeting 1976. On
file in FAO, Plant Protection Division.
Coquet, B. Determination of the lethal dose 50% by intra-peritoneal
1972 route in the male rat of ortho 12,420 technical (85%
acephate). Unpublished report from Centre de Recherche
et d'Elevage des Oncins, submitted to the World Health
Organization by Chevron Chemical Company.
Crossley, J., and Tutass, H. Metabolism of monitor insecticide
1969 by rats. Unpublished report from Ortho, Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Ebbens, K., Mastri, C., Keplinger, M., and Fancher, O. Skin
1971 sensitization test with orthene technical-SX-284 in
albino guinea pigs. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Edds, G., and Bradley, R. Acute toxicity study - white Leghorn
1971 chicks. Unpublished report from the University of
Florida, Gainesville, submitted to the World Health
Organization by Chevron Chemical Company.
Elliott, C., Shadberg, K., Goode, J., and Keplinger, M. Acute dust
1972 inhalation toxicity study with orthene-85% technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Toxicity reproduction
1972a and residue study with orthen technical SX-331 in white
Leghorn chickens. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Neurotoxicity study
1972b with orthene technical, SX.284 in adult hens.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Garofalo, M., Palazzolo, R., and Sanders, R. A study of the effects
1973 of orthene and monitor on plasma and erythrocyte
cholinesterase activity in human subjects during
subacute oral administration. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to
the World Health Organization by Chevron Chemical
Company.
Haley, S., Plank, J., Wright, P., and Keplinger, M. Teratogenic
1971 study with orthene technical in albino rats.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Haley, S., Kennedy, G., and Keplinger, M. Three generation
1973 reproduction study with RE 12,420 technical in albino
rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Hartke, K., Burtner, G., Kennedy, G., and Keplinger, M. Two-year
1972 chronic oral toxicity study with RE 12,420 in beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
ICI, Australia. Data submitted through the Codex Contact Point,
1976 Canberra, Australia.
Kretchmar, B., Mastri, C., Keplinger, M., and Fancher, O. Acute
1971 oral toxicity study to determine the efficacy of
atropine sulfate and 2-PAM as antidotes for RE 12,420
technical intoxication in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Kretchmar, B., Mastri, C., and Keplinger, M. Acute oral potentiation
1972 study with monitor technical and orthene technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc. submitted to the World
Health Organization by Chevron Chemical Company.
Kruke, V., Narcisse, J., Cavalli, R., and Spence, J. Acute inhalation
1972a toxicity of orthene 75S. Unpublished report from
Standard Oil Company of California. submitted to the
World Health Organization by Chevron Chemical Company.
Kruke, V., Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 14-Day aerosol inhalation study in adult rats.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Ladd, R., Jenkins, D., Wright, P., and Keplinger, M. Teratogenic
1972 study with orthene technical in albino rabbits.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Lee, H. Metabolism of orthene in rats. Unpublished report from Ortho
1972 Division of Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Lindberg, D., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 study with orthene in Beagle dogs. Unpublished study
from Industrial Bio-Test Laboratories, Inc., submitted
to the World Health Organization by Chevron Chemical
Company.
Luke, M.A., Froberg. J.E., and Masumoto. H.T. Extraction and
1975 cleanup of organochlorine, organophosphate,
organonitrogen, and hydrocarbon pesticides in produce
for determination by gas-liquid chromatography. J. Ass.
off. analyt. Chem., 58:1020-1026.
Magellona, E.D. Report from the Codex Contact Point, Philippines
1976 on acephate uses on rice and cabbage.
Mastalski, K., Jenkins, D., Keplinger, M., and Fancher, O. Acute
1970 oral toxicity study on RE 12,420 technical in Beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Mastri, C., Keplinger, M., and Fancher, O. Acute oral toxicity
1970a study on RE 12,420 technical in white mice. Unpublished
report from Industrial BioTest Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Mastri, C., Keplinger, M., and Francher, O. Acute oral and dermal
1970b toxicity studies on RE 12,420 75% spray suspension
formulation. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE18,421. Unpublished report from
1972a Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,245. Unpublished report from
1972b Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,246. Unpublished report from
1972c Chevron Chemical Company submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. Eye irritation potential
1971a of orthene technical, orthene 75S (CC-2153) and orthene
75S (CC-2152). Unpublished report from Standard Oil
Company of California, submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-269 the acute oral
1971b toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-270 the acute dermal
1971c toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The acute oral toxicity
1972a of orthene 75-S to adult male rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The skin irritation
1972b potential of orthene 75-S. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
New Zealand. Submission on acephate from the National Codex
1976 Committee of New Zealand.
Pack, D. The metabolism of RE9169 and RE14,071 in rats. Unpublished
1972a report from Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Pack, D. Orthene occupational exposure-pilot plant and formulations.
1972b Unpublished report from Ortho Division of Chevron
Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Pack, D. Orthene insecticide occupational exposure - field station
1972c personnel. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Plank, J., and Keplinger, M. Two-year chronic oral toxicity study
1973 with RE12,420 technical in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Plank, J., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 subacute oral toxicity study with RE12,420 technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Plank, J., Wright, P., and Keplinger, M. 90-Day cholinesterase
1972 study with ortho 12,420 technical in albino rats.
Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. The
1972a acute oral toxicity to rats of orthene in combination
with five other cholinesterase inhibition of materials.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Reyna, M., Kennedy, G., and Keplinger, M. 18-Month carcinogenic
1973 study with RE12,420 technical in Swiss white mice.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 21-Day subacute dermal study in rabbits with
orthene-75S. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972c S-184 the acute dermal toxicity of RE14,071.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972d S-533. The acute dermal toxicity of RE18,421.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. S-514
1972e The acute dermal toxicity of RE17,245. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972P S-515 the acute dermal toxicity of RE17,246.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Swencicki, R., and Hartz, W. Orthene human exposure study
1972 clinical evaluation. Unpublished study from Standard
Oil Company of California, Submitted to the World
Health Organization by Chevron Chemical Company.
Tucker, B. Acetylcholinesterase inhibition of orthene and
1972 ortho 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
 Other information on identity and properties
Molecular weight 183.16
State White solid
Melting point 72-80°C (80-90% purity)
Specific gravity 1.35 (80-90% purity)
Volatility Low. Some minor products
of degradation are volatile
Stability Relatively stable. Samples should be
stored cool
Solubility Water, ca. 65% (w/v)
Aromatic solvents, < 5%(w/v)
Acetone )
Ethanol ) >10%(w/v)
Impurities in the technical material
The technical product contains 80-90% acephate.
Among the impurities are:
OO-dimethyl acetylphosphoramidothioate max. 5%
OS-dimethyl phosphoramidothioate max. 1%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Male and female rats were pretreated with single daily oral
doses of analytical grade acephate (25 mg/kg/day) for 7 days
followed by a dose of S-methyl 14C acephate. There was no
difference in the quantitative or qualitative excretion pattern in
males or females. The excretion was rapid. The majority of the
radioactive material recovered was found within 12 hours. Urine
contained 82-95% of the administered dose, 1-4% was exhaled, 1% was
found in feces. Less than 1% was seen as a residue in tissues and
organs 72 hours after the last application. The liver was the
tissue with the highest 14C residue reflecting the normal portal
detoxication mechanism. Urinary metabolites were identified as
unchanged acephate (73-77%), O,S-dimethyl phosphorothioate (3-6%)
and S-methyl acetylphosphoramidothioate (3-4%). No methamidophos
was observed (Lee, 1972).
Since only the mentioned metabolites could be detected, the
metabolic pathway of acephate is not fully elucidated. A probable
scheme may be as shown in Figure 1.
The metabolic fate of two intermediates used in the
manufacture of acephate was examined in rats. These products were:
Other information on identity and properties
Molecular weight 183.16
State White solid
Melting point 72-80°C (80-90% purity)
Specific gravity 1.35 (80-90% purity)
Volatility Low. Some minor products
of degradation are volatile
Stability Relatively stable. Samples should be
stored cool
Solubility Water, ca. 65% (w/v)
Aromatic solvents, < 5%(w/v)
Acetone )
Ethanol ) >10%(w/v)
Impurities in the technical material
The technical product contains 80-90% acephate.
Among the impurities are:
OO-dimethyl acetylphosphoramidothioate max. 5%
OS-dimethyl phosphoramidothioate max. 1%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Male and female rats were pretreated with single daily oral
doses of analytical grade acephate (25 mg/kg/day) for 7 days
followed by a dose of S-methyl 14C acephate. There was no
difference in the quantitative or qualitative excretion pattern in
males or females. The excretion was rapid. The majority of the
radioactive material recovered was found within 12 hours. Urine
contained 82-95% of the administered dose, 1-4% was exhaled, 1% was
found in feces. Less than 1% was seen as a residue in tissues and
organs 72 hours after the last application. The liver was the
tissue with the highest 14C residue reflecting the normal portal
detoxication mechanism. Urinary metabolites were identified as
unchanged acephate (73-77%), O,S-dimethyl phosphorothioate (3-6%)
and S-methyl acetylphosphoramidothioate (3-4%). No methamidophos
was observed (Lee, 1972).
Since only the mentioned metabolites could be detected, the
metabolic pathway of acephate is not fully elucidated. A probable
scheme may be as shown in Figure 1.
The metabolic fate of two intermediates used in the
manufacture of acephate was examined in rats. These products were:
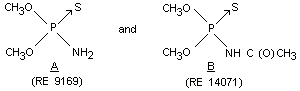
 Immature female rats were administered the above products
individually in corn oil by oral intubation at a dose of 50 mg/kg.
Urinary metabolites were characterized as similar to those found in
human urine from workers exposed in manufacturing procedures.
Qualitative determination of metabolites suggested the following
metabolic sequence.
Immature female rats were administered the above products
individually in corn oil by oral intubation at a dose of 50 mg/kg.
Urinary metabolites were characterized as similar to those found in
human urine from workers exposed in manufacturing procedures.
Qualitative determination of metabolites suggested the following
metabolic sequence.
 (Pack, 1972)
Effects on enzymes and other biochemical parameters
Acephate and its major plant metabolite methamidophos, as are
other organophosphate esters, are cholinesterase inhibitors. The
I50 values (molar concentrations for 50% inhibition) for plasma and
bovine RBC cholinesterase (acetylcholine hydrolysis) are given
below:
Human Plasma Bovine RBC
µg/ml I50, Molar µg/ml I50, Molar
Acephate >500 >2.7 X 10-3 >500 >2.7 X 10-3
Methamidophos 23 1.6 X 10-4 4.3 3.1 X 10-5
(Tucker, 1972)
Depression of cholinesterase activity was measured following
oral administration of acephate to male rats. Following a dose of
900 mg/kg RBC cholinesterase was substantially depressed while
plasma was unaffected. At the time of sacrifice, all animals
displayed some typical signs of organophosphate poisoning.
Administration of a dose of parathion (15 mg/kg) resulted in a
slightly higher reduction of RBC cholinesterase activity (50-30%)
with no effect on plasma enzymes (Cavalli and Spence, 1970c).
Groups of rats (15 females/group) were orally administered
acephate at levels corresponding to 1200, 100, 30, and 0 ppm as
daily equivalents in the diet for up to 3 weeks. Another group was
administered methamidophos at a dose corresponding to 10 ppm. A
slight depression of RBC-cholinesterase was noted at 100 and 30 ppm
acephate. The 1200 ppm level caused a significant depression of
activity. The plasma cholinesterase was only slightly depressed by
the highest dose level. There was no indication of a build-up of
cholinesterase inhibition over the 3 week test interval.
Methamidophos was inhibitory at 10 ppm. Methamidophos was shown to
be substantially more active an inhibitor than acephate (Cavalli
and Spence, 1970f).
TOXICOLOGICAL STUDIES
Special studies on potentiation
A study was performed on the acute toxicity of acephate alone
and in combination with 5 cholinesterase inhibiting pesticides
(azinphos-methyl, carbaryl, malathion, parathionmethyl and
oxydemeton-methyl) on male rats. The LD50 values for equitoxic
mixtures of the paired pesticides differed from single LD50 values
especially with carbaryl and malathion suggesting that potentiation
may be a factor in the interaction (Rittenhouse et al., 1972a).
A similar study in rats with methamidophos and acephate
indicated that there was no significant potentiation (Kretchmar et
al., 1972).
Special studies on neurotoxicity
Hen
Groups of adult hens (6 hens/group) were administered acephate
orally at dosage levels of 0 and 568 mg/kg (an LD50 level). A
second dose was administered to survivors at 50 day 21 and these
were observed for an additional 21 days; tri-o-cresyl phosphate
(TOCP) was administered at 500 mg/kg as a positive control. Samples
of spinal cord, peripheral nerve and brain were examined
histologically for signs of myelin disruption.
Of the hens treated with TOCP, 3 of 6 died and the remaining
hens displayed signs of clinical ataxia commonly noted with this
toxicant. One of the 6 hens treated with acephate died after the
first dosing and 3 more after the second. The remaining hens
displayed none of the signs of ataxia and on histological examination
there was no evidence of myelin degeneration. Acephate does not induce
ataxia as evidenced by TOCP in hens (Fletcher, et al., 1972b).
Special studies on reproduction
Rat
Groups of rats (8 males and 16 females/group) were fed
acephate in the diet at dosage levels of 0, 30, 100 and 300 ppm and
subjected to a standard 3-generation, 2-litter per generation
reproduction study. Gross and histopathologic examinations were
performed on the parental animals of all 3 generations, upon
weanlings of the second litter and upon weanlings of the F3b litter
for the control and high dose level. No pathologic or microscopic
changes were noted in the tissues and organs examined.
Adverse reproductive effects were noted at 100 and 300 ppm.
Reduced mating and fertility indices (reflected by copulation and
pregnancies) were noted. There were no effects noted on the ability
of mothers to maintain pregnancy although survival of pups after
birth was affected at various times during the 3 generation trial.
Weanling body weight of pups fed acephate was similar to control
values. There were no apparent malformations noted in the pups born
during this study. The results indicate a no-effect level in this
study based on all observable parameters to be 30 ppm (Haley et
al., 1973).
Special studies on teratogenicity
Rabbits
Groups of pregnant rabbits (17 does/group) were administered
acephate by capsule daily from day 6 to day 18 of gestation at
doses of 0, 1 and 3 mg/kg body weight in a standard teratology
study. Thalidomide (37.5 mg/kg/day) was used as a positive control.
There were no deaths or abnormal behavior attributed to acephate.
There were no effects on the reproduction or teratogenicity
parameters examined. Total implantation sites, resorptions,
abortions, number of young and examinations of skeletal tissue were
normal. Acephate did not elicit a teratogenic response in rabbits
while the positive control induced resorption, reduced the
incidence of pups and resulted in an increase in the number of
terata formed (Ladd et al., 1972).
Rats
Groups of pregnant rats (17-20 females/group) were
administered acephate by gavage daily from day 6 to day 15 of
gestation (10 doses) at dosage levels of 0, 25, 50, 100 and 200
mg/kg. Upon sacrifice at day 20 examinations made of the maternal
and fetal parameters did not indicate a teratological potential for
acephate in rats. Maternal effects were observed as reduced weight
gain at 100 and 200 mg/kg and as an increase in resorption sites at
200 mg/kg. All treated groups reflected acute parasympathomimetic
signs of poisoning (lacrimation) initially after treatment. No
effects were noted with respect to abortion or on the fetal
development, both internal and external tissue development including
skeletal abnormalities. This study again demonstrated a negative
teratogenic potential for acephate (Haley et al., 1971).
Special studies on mutagenicity
Mice (dominant lethal)
Groups of mice (12 males/group) were administered acephate by
intra peritoneal injection at dose levels of 0, 25, and 50 mg/kg.
The mice were then mated individually with 3 virgin females weekly
for 6 consecutive weeks in a dominant lethal test to investigate
the effects on maturation of male mouse germ cells. A positive
control (MMS, methyl methanesulfonate) was also used. The females
were sacrificed 1 week after removal from the breeding cage.
Analyses were made of implantation sites, resorption sites and
embryo development to evaluate the in vivo mutagenic potential of
acephate. In the first week after mating, an increase of
pre-implantation loss was noted at 50 mg/kg. There were no
significant differences between groups with respect to
implantation, resorption or embryo development. The in vivo
mutagenic data do not indicate acephate to have a potential for
mutagenic development (Arnold et al., 1971).
In contrast to the previously referenced in vivo study, a
series of in vitro microbiological tests has shown acephate to
elicit a positive response in tested strains of bacteria. In two
species of bacteria, Salmonella typhimurium and Escherichia
coli, acephate was positive only in some of the latter strains
(Hanna and Dyer, 1975).
Special studies on carcinogenicity
Mice
Groups of mice (Swiss White Mice - 50 males and 50
females/group) were fed acephate in the diet at levels of 0, 300
and 600 ppm for 18 months. A positive control
(N-nitrosodimethylamine) was fed at 10 ppm over this interval. Gross
pathologic examination for tumor formation and histopathologic
examination of tumors from animals sacrificed in extremis or
dying during the study showed no evidence of carcinogenicity due to
acephate. A similar negative finding was observed in the mice
sacrificed at the conclusion of the study. There was no unusual
behavior pattern exhibited by the acephate-fed mice and the
incidence and pattern of mortality were not influenced by acephate
in the diet. This study suggests that acephate is not a carcinogen
in mice (Reyna et al., 1973).
Special studies on skin sensitization
In two identical studies, groups of rabbits (8 rabbits/group)
were administered acephate dermally in acetone at a dose level of
0.1 ml of a 1% w/v solution or a control acetone treatment daily
for 16 days. Ten days after the last application, a challenge dose
at a remote site was applied and dermal irritation graded for a
sensitization response. None of the animals showed an adverse
reaction suggesting that by the dermal route acephate is not a
sensitizing agent (Cavalli and Spence, 1970g and 1970h).
Groups of guinea pigs (10 albino guinea pigs/group) were
dermally administered acephate (0.1 ml of a 1% w/v aqueous
solution) every other day (4-6 hours/day) for 10 treatments. Two
weeks after the last exposure, the animals were challenged and
examined for sensitization. There were no indications that acephate
induced a sensitization reaction under these conditions. A positive
reaction was obtained with chlorodinitrobenzene, a known sensitizer
(Ebbens et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of acephate
LD50
Species Route Sex (mg/kg) Reference
Rat Oral M&F 1100 Kretchmar et al, 1971
Oral M&F 1494 Mastri et al, 1970b
Oral M&F 2025 Kretchmar et al, 1972
Oral M 945 Cavalli & Spence, 1970b
Oral F 866 Cavalli & Spence, 1970b
Oral M 1230 Rittenhouse et al, 1972
IP M 1106 Coquet, 1972
Inhalation M&F >1.81 mg/L(4 hr.) Elliott et al, 1972
Inhalation M&F >12.1 mg/L(1 hr.) Kruke, 1972a
Mouse Oral M&F 362 Mastri et al, 1970a
Rabbit Oral M 707 Narcisse et al, 1972a
Dermal M&F 10,250 Mastri et al, 1970b
(24 hour 75% spray suspension)
Dermal M&F 500 Narcisse et al, 1972b
(no irritation)
(24 hr - 75-S formulation)
Dermal M >2000 Cavalli & Spence, 1970a
TABLE 1. (continued)
LD50
Species Route Sex (mg/kg) Reference
Dog Oral M&F <681 Mastalski et al, 1970
Oral M&F 215 Mastalski et al, 1970
(emetic dose)
Chicken Oral F >100 Edds & Bradley, 1971
Oral F 568 Fletcher et al, 1972a
Eye irritation
Acephate (100 mg) was instilled into the conjunctival sac of
rabbits (six animals/group) in an examination for eye irritancy
properties. Conjunctival irritation was evident with all forms of
acephate. Where acephate was washed out after a 5 minute exposure
period, conjunctival irritation and corneal damage were observed.
The irritation was observed after one week while all eyes were
normal at 14 days (Narcisse et al., 1971a).
Vapor inhalation
Groups of rats (5 male and 5 female/group) were exposed to an
atmosphere saturated with acephate vapor (derived from passing air
through crystalline or melted acephate at 90°C). Exposure for 1
hour to vapor derived from either method did not result in any
mortality. Animals were examined over a 14 day observation period
(Cavalli and Spence, 1970d). In an additional study, 5 female rats
were exposed for four hours to vapors of acephate derived only from
passing air through crystalline material. There was no mortality or
morbidity and cholinesterase (undefined) activity was normal
(Cavalli and Spence, 1970e).
Aerosol inhalation
Groups of rats (5 male and 5 female rats/group) were exposed
to an aerosol concentration of 12.1 mg/1 (90% of particles below 10
µ) for one hour. There were no signs of poisoning and no mortality;
gross effects on tissues and organs were not noted (Kruke et al.,
1972a).
Groups of rats (10 male and 10 female rats/group) were exposed
in a 380 l dynamic chamber to aerosols of acephate 1 hour per day,
5 days per week for two weeks. The aerosol was generated from
aqueous solutions of 0, 0.25, 1.0 and 4% acephate and had a
particle size generally under 10 microns. There was no mortality
and weight gain and general behavior were normal. At the highest
dose, female uric acid content was increased. There were no other
effects noted an clinical chemistry, urinalysis or hematology
parameters including cholinesterase activity. Gross and microscopic
examination of tissues and organs showed no effect of acephate
exposure (Kruke et al., 1972b).
Short-term studies
Rabbit - (Dermal)
Groups of rabbits (8 male and 8 female albino rabbits/group)
were administered acephate to either intact or abraded skin. An
aqueous solution was applied daily, 6 hours/day, 5 days/week, for
3 weeks with the daily doses being 0, 0.5, 1.0 and 2.0 g/kg. At the
conclusion of the study, the animals were sacrificed. There was
mortality (2/16) observed at the highest dose and
parasympathomimetic signs of poisoning were observed at 1.0 and 2.0
g/kg. These two groups also lost weight while no differences in growth
or body weight were observed at 0.5 g/kg. The two highest doses showed
substantial changes in hematology and blood chemistry parameters:
reduced RBC count, reduced hemoglobin and hematocrit values and
reduced BUN values. In male rabbits only at the low dose group, LDH
and SGOT values were reduced. RBC cholinesterase was depressed at all
dose levels to about 50% in both sexes whereas plasma cholinesterase
was not affected. On gross examination increased liver weights were
observed at the two highest dose groups. There were no effects noted
on microscopic examination including bone marrow (M/E ratio) studies
(Rittenhouse et al., 1972b).
Hen
Groups of chickens (25 females and 4-6 males/group) were fed
acephate in the diet at dosage levels of 0, 3, 10 and 30 ppm for up to
13 weeks (followed by a 28 day recovery period with control diets).
Growth, egg production and egg quality was normal. The hatchability of
eggs at the highest dose was slightly reduced, and reduction was noted
in growth and development of hatched chicks in the 10 and 30 ppm
groups. The viability of the hatched chicks was diminished in the
highest dose group. There were no gross pathological changes in any of
the birds (Fletcher, 1972a).
Rat
Groups of rats (15 males and 15 females/group) were fed acephate
in the diet for 90 days at levels of 0, 30, 100 and 300 ppm. There was
no mortality attributed to acephate in the diet. Growth, food
consumption and observable behavior were normal. Urinalyses, blood
chemistry and hematology values were normal. Gross and microscopic
analysis of tissues and organs were performed at the conclusion of the
study. In the males a decreased liver weight was noted at 300 ppm.
There were no histological lesions associated with this finding.
Cholinesterase activity data were not reported (Plank et al., 1971).
TABLE 2. Acute toxicity of metabolites or possible impurities
LD50
Compound Structure Species Route Sex (mg/kg) Reference
OO-dimethyl (CH3O)2P(S)NH2 Rat Oral M 633 Cavalli et al, 1968a
phosphoramidothioate (RE 9169) Oral F 549
Signs of poisoning include CNS depression (paresis, hyporeflexia and anesthesia)
Rabbit Dermal M 2500 Cavalli et al, 1969
OS-dimethyl (CH3O)(CH3S)P Rat Oral M 21 Cavalli et al, 1968b
Phosphoramidothioate (O)NH2 Oral F 19
(RE 9006)
Signs of poisoning are standard for parasympathomimetic agents.
Rabbit Dermal M 118 Cavalli et al, 1968c
OO-dimethyl acetyl (CH3O)2P(S)NHC Rat Oral M 1468 Cavalli et al, 1970
phosphoramidothioate (O)CH3 Oral F 1250
(RE 14071)
Signs of poisoning are standard for CNS depression - hyporeflexia, hypothermia, pyloerection, constipation, and possibly
tachycardia.
Rabbit Dermal M >2000 Rittenhouse et al,1972C
OO-dimethyl (CH3O)2(CH3S) Rat Oral M 83 Narcisse et al, 1971b
S-methyl phosphorothioate P(0) Oral F 63
(RE 15283)
The signs of poisoning include salivation, tremors, weakness, and prostration - death occurred 4-10 days after dosing.
TABLE 2. (Cont'd.)
LD50
Compound Structure Species Route Sex (mg/kg) Reference
Rabbit Dermal M 109 Narcisse et al, 1971c
OS-dimethyl (CH3O)(CH3S)P Rat Oral >2000 McCaskey,1972a
phosphorothioate (O) (OH)
(RE 18421) Rabbit Dermal M >2000 Rittenhouse et al, 1972d
S-methyl (CH3S)P(O)OH Rat Oral >2000 McCaskey, 1972b
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al, 1972e
RE 17245)
O-methyl (CH3O)P(O)SH Rat Oral >2000 McCaskey, 1972c
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al.,
(RE 17246) 1972f
Groups of rats (from 15 to 35 males and females per group) were
fed acephate in the diet at levels of 0, 1, 5, 10, 30, 100 and 300 ppm
for 90 days and a control diet for an additional 4 weeks. Animals were
sacrificed at various intervals and cholinesterase activity was
measured using erythrocytes, plasma and brain as enzyme sources.
Contrary to acute or short term oral administration studies this
dietary study showed little effect on erythrocyte cholinesterase
activity at levels below 100 ppm. In contrast, 30 ppm affected plasma
cholinesterase in weeks 6 and 13 of the test period. Brain
cholinesterase was depressed consistently at 30 ppm. In general,
females were more susceptible than males. Recovery of all blood
enzymes was rapid (within 1 week) while brain cholinesterase was
slower. All enzyme levels were normal within 4 weeks of feeding
control diets (Plank et al., 1972).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet at dosage levels of 0, 10, 30 and 100 ppm for 90
days. At the conclusion of the feeding period half of the groups were
sacrificed and the remainder maintained for a 40 day recovery period.
RBC cholinesterase activity was depressed in both sexes at 100 and 30
ppm while plasma was unaffected. Brain cholinesterase was depressed at
30 ppm. Recovery of enzyme activity was noted at 28 days after feeding
stopped and animals were fed control diets.
Growth, food consumption and behavior were unaffected over the
course of the study. There were no deaths, and animals appeared
normal. Analysis of blood and urine and hematologic parameters showed
no effects of acephate. Gross and microscopic analysis of tissues and
organs also showed no effects (Lindberg et al., 1971). [A no-effect
level in the study is 10 ppm (equivalent to 0.32 mg/kg bw) while 30
ppm produced a minimal depression of cholinesterase.]
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet for two years at levels of 0, 10, 30 and 100 ppm.
There was no effect on food consumption, growth or behavior. Two dogs
which did not survive the study died as a result of factors believed
to be unrelated to the presence of acephate in the diet. No effects
were noted on blood chemistry, hematology or urine analysis
parameters. Gross and microscopic examination of a variety of tissues
and organs showed no effects attributed to acephate. Depression of
cholinesterase activity was noted in this study in brain and red blood
cells. Plasma cholinesterase was unaffected at 100 ppm while RBC
showed reduced activity. Brain cholinesterase was only slightly (N10%)
depressed at the highest dose level. A no-effect level based on RBC
cholinesterase depression is 30 ppm over the two year study (Hartke et
al., 1972).
Long-term studies
Rat
Groups of rats (35 males and 35 females/group) were fed acephate
in the diet for two years at levels of 0, 30, 100 and 300 ppm. A
depression of growth was observed in both sexes at 12 months at 100
and 300 ppm. At 24 months the body weight of both sexes was depressed
only at 300 ppm. Food consumption was normal. There were no effects on
mortality, behavior or on hematology, blood chemistry or urine test
parameters. On gross examination male and female liver weight at 300
ppm was depressed, but nothing abnormal was noted on histopathologic
examination of the liver or other tissues and organs. There was no
suggestion of an increased tumor incidence. Blood and brain
cholinesterase depression examined during the course of this study
provided the only effects noted. At 24 months (the only interval
reported) RBC and plasma cholinesterase values were normal. Brain
cholinesterase was minimally depressed (26-27% of normal) at 30 ppm.
The depression was dose dependent suggesting a no-effect level to be
somewhat below 30 ppm. Cholinesterase was not reported for time
intervals other than 24 months. A no-effect level based on
cholinesterase depression cannot be determined in this study. A
systemic no-effect level of 30 ppm may be estimated based on growth
reduction over the first year test interval (Plank and Keplinger,
1973).
OBSERVATIONS IN MAN
In a series of reports from areas in the U.S.A. where acephate
has been used in field applications, 95 individuals exposed from 0.5
to > 1000 hours under a variety of conditions expressed no symptoms
or adverse effects of exposure. The survey was primarily subjective as
clinical measurements were not made (Anonymous, 1976a).
In a similar survey individuals exposed to acephate in pilot
plant and formulation processes did not show adverse effects. Several
of these workers were exposed for up to 15 months (Anonymous, 1976b).
Analysis of urine from these individuals confirmed exposure to
acephate. Urinary levels up to 5 ppm acephate were observed (Pack,
1972b). As expected, other metabolites or technical products of
production were observed in the urine of individuals exposed in the
pilot plant. In addition to acephate, these products included the
oxygen analog of acephate, methamidophos, the oxygen analog of
methamidophos and trimethyl phosphate. The individuals exposed to
technical acephate in formulating processes showed only methamidophos
and trimethyl phosphate as urinary metabolites. Blood and urinary
samples from 5 individuals were examined over a 10 week period. All of
the clinical parameters were normal as were standard tests including
EKG, X-ray and cholinesterase values (Swencicki and Hartz, 1972).
In a follow-up of agricultural field workers exposed to acephate,
levels of 1/50 those found in the pilot plant survey were observed in
the urine. There was no evidence of methamidophos in the urine. As
expected there were no adverse effects noted (Pack, 1972c).
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. They received either a
combination (methamidophos/acephate) of 1:9, 1:4 or corn oil as a
control daily (divided into 3 equal doses) for 21 days. The 1:9 group
was administered the combination for consecutive periods of 21 days at
doses of 0.1, 0.2 and 0.3 mg/kg/day followed by a 7 day recovery
period after which the dose was increased to 0.4 mg/kg/day for 10
days. The 1:4 group received the combination at doses of 0.1 and 0.2
mg/kg/day for 21 consecutive days before the dosing was halted. The
protocol called for a total of 4 dose levels (up to 0.4 mg/kg/day)
each to be administered for 21 days. Blood cholinesterase (plasma and
RBC) was examined periodically.
Results are summarized in Table 3. No effects were noted on RBC
cholinesterase at any treatment level. There was marginal depression
of the plasma cholinesterase noted In the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The plasma
cholinesterase, of the males was substantially affected at the 0.3
mg/kg dose and was not retested at the higher dose level. The group
(both males and females) receiving the higher methamidophos:acephate
ratio (1:4) showed reduction of plasma cholinesterase after two weeks
of receiving a dose of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not
used in the 1:4 dose group. This study indicated the minimal dose of
acephate necessary to decrease plasma cholinesterase for the
combination of acephate and methamidophos was 0.2 mg/kg/day of a 1:4
combination of the two toxicants. No effects with this combination
were noted at 0.1 mg/kg (Garofalo et al., 1973). Thus, a dose level of
0.03 mg methamidophos/kg bw combined with a dose of 0.2 mg acephate/kg
bw (ranging from 0.08 to 0.18 mg acephate/kg bw) would be expected to
reduce cholinesterase activity in man. A no-effect level evaluated
from this study might be 0.2 mg acephate/kg bw in combination with
0.02 mg methamidophos/kg bw.
COMMENTS
Acephate, a relatively non-toxic organophosphorus pesticide, is
rapidly absorbed, metabolised and excreted from the body. A toxic
metabolite, methamidophos, found to occur in plants and soil has not
been identified in mammals. Methamidophos, a potent
parasympathomimetic agent acting by inhibition of
acetylcholinesterase, is also rapidly degraded and excreted in
mammals. Neither compound persists or accumulates in the body nor
appears to affect biochemical systems other than cholinesterase.
Neurotoxicity studies in hens and potentiation studies in rats with
other antiesterase compounds were negative. Special studies on
reproduction showed effects at dietary levels of 100 ppm and above as
evidenced by reduced survival of offspring. In vivo mutagenesis
and teratogenesis testing was negative as was a special study with
mice for carcinogenicity. A positive mutagenesis response in a single
bacterial strain was observed in vitro. In a 2 year study with
dogs and an 18 month study with mice no observable effects other than
cholinesterase depression were noted. In a 2 year rat study, a slight
depression of growth and of brain cholinesterase activity at the
lowest level tested precluded a definition of a no-effect level.
However, a no-effect level in both rats and dogs was suggested based
on 90 day dietary studies which exposed animals at lower levels. In
random observations with humans occupationally exposed, no adverse
effects were noted. In a study to define a no-effect level in humans
based on cholinesterase depression, administration of a combination of
methamidophos and acephate (1:4 ratio) at a total dose of 0.2
mg/kg/day reduced plasma cholinesterase. A calculation of the
individual doses received suggested in one case that 0.16 mg
acephate/kg body weight in combination with 0.04 mg methamidophos/kg
(bw) would cause depression of cholinesterase while a dose of 0.08 mg
or 0.18 mg acephate/kg (bw) both in combination with 0.02 mg
methamidophos did not reduce plasma cholinesterase activity. Based on
the studies with both rat and dog, with further assurance afforded by
the observations in man, an ADI was recommended. This also took into
account the slight effect noted in the rat 2 year study where 30 ppm
(equivalent to 1.5 mg/kg) induced a marginal effect on brain
cholinesterase.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg body weight
Dog: 10 ppm in the diet equivalent to 0.32 mg/kg body weight
Man: 0.18 mg/kg body weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of acephate as a contact and systemic insecticide is
reported from many countries of the American continents, Australia,
Asia and Europe. It is mostly marketed as soluble powders (s.p.), 75%.
Occasionally, however, it is also registered as a 50% s.p., as low
concentration dusts and as 1-5% granules.
TABLE 3. Effect on plasma cholinesterase of combined doses of methamidophos and acephate
Methamidophos: Acephate Ratio
1:4 1:9
Total
dose methamidophos:acephate Plasma Inhibition methamidophos:acephate Plasma inhibition
(mg/kg) (mg/kg) Males Females (mg/kg) Males Females
0.1 0.02 : 0.08 - - 0.01 : 0.09 - -
0.2 0.04 : 0.16 + + 0.02 : 0.18 - -
0.3 0.06 : 0.24 * * 0.03 : 0.27 + -
0.4 0.08 : 0.32 * * 0.04 : 0.36 * -
* Discontinued treatment
Acephate is claimed to be especially active against insects of
the families Lepidoptera, Homoptera and Hemiptera. A list of suggested
uses which is typical in many countries is given in Table 4. These
uses cover suggested protection of both horticultural and agricultural
crops (fruits, vegetables, root-crops and several industrial crops)
against larvae, aphids, caterpillars, berry moths, leaf miners,
beetles etc. Usual application rates are also indicated in Table 4.
They range from 1-2 kg a.i. per ha on field crops and from 0.05-0.1%
on fruit trees, bushes etc.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable amount of residue data on acephate and its
behavior in supervised trials and controlled experiments has been
presented by the originating manufacturer (Chevron Chemical Company).
Most of the data were generated under American climatic and
agricultural conditions, although in some cases supplementary data
from other sources are available.
TABLE 4. Suggested uses of acephate
Concentration or
Crop Pest dosage of a. i.
Fruit crops Aphids 0.04-0.075%
(pome, stone, Codling moths
citrus) Chewing and sucking insects
Tortrix
Citrus, olive scale
Grape Grape berry moths 0.075%
Vegetables Aphids 0.05%
Chewing and sucking insects
Caterpillars
Moths
Beets Leaf miners 0.8-1.0 kg/ha
Ornamentals Aphids and Tortrix 0.05-0.75%
Hops Aphids 0.05-0.25%
Tobacco Aphids 1 kg/ha
Budworms
Flea beetle
Corn Corn borer 1.5-2 kg/ha
Generally the residue data from field trials indicate that
acephate is a relatively non-persistent, systemic insecticide. When
applied at the recommended rate of about 1 kg a.i./ha, several
treatments are often applied, commonly at weekly intervals for as many
weeks as necessary for insect control. A first metabolite, OS-dimethyl
phosphoramidothioate which is itself the insecticide methamidophos, is
regularly found in plant material together with acephate, although
only in the ratio of 1:10 - 1:5.
Alfalfa, cotton and soybean
Extensive trials have been made on these three important crops
(Table 5). Repeated treatments of alfalfa with 1 kg of acephate per ha
leave total residues on fresh cut green hay of less than 6 mg/kg after
21 days and a maximum of about 2 mg/kg after 28 days. Curing of the
hay in the open after harvest did not significantly change the residue
level. The apparent half-life of acephate on green hay is
approximately 7 days.
In cottonseed, the total residues ranged from zero to a maximum
of About 1.5 mg/kg 21 days after the last treatment with 1 kg/ha. The
maximum residue levels from a series of experiments are shown in Table
5. The halflife of such residues is of the order of 14 days. When
processed, essentially all the residue remains in the cottonseed meal
while crude oil and refined oil contain practically no acephate or
methamidophos metabolite (less than 0.03 mg/kg). Immediately after
treatment at the 1 kg/ha level, green leaves of cotton plants carried
residues ranging from 60-200 mg/kg. These residues declined, but they
were still significant, from 7 to 50 mg/kg, after 21 days.
In soybeans, mature and with shell removed, the total residue
varied from zero to 0.2 mg/kg 14 days after the last application of
acephate at the 1 kg/ha rate. Some positive findings are shown in
Table 5, which illustrates that when processed, most of the residue
remained in the mill feed, while trace residues only (less than
0.03 mg/kg) were found in the crude oil and the soya protein flour.
Vegetables
Residue data are available from several countries (e.g.
Australia, the Netherlands, New Zealand, Philippines, U.S.A.) on
vegetables, mostly leafy vegetables such as broccoli, Brussels
sprouts, cabbages, cauliflower and lettuce. Repeated applications of
1 kg acephate per ha are usually recommended and the residue data
indicate that there is a direct relationship between dosage and
residue level.
Residues decline at rates corresponding to the rate of growth
dilution or somewhat faster and as usual, the outer leaves of the
vegetables carry higher residues than the inner parts. This is
exemplified in Table 6 which illustrates typical residue levels of
both acephate and its metabolite methamidophos found in broccoli.
Outer leaves of the vegetables may or may not be discarded as
so-called trimmings before marketing and the final residue on the
marketed product is therefore dependent on the degree of trimming. To
take account of this situation a maximum possible residue on a given
crop may be calculated on the basis of actual analyses of the crop and
its trimmings, assuming maximum trimming. Such predictions of residues
are shown in Table 7 for 5 vegetable crops and compared with residues
actually found. The Table shows that actual residues of acephate + its
metabolite methamidophos are unlikely to exceed 5-10 mg/kg at
appropriate preharvest intervals from 3 to 14 days.
Sugar beets, potatoes and tomatoes
After repeated foliar treatments of sugar beets with acephate, at
a weekly rate of 1 kg/ha, high residues were found on the green leaves
(up to 25-30 mg/kg), while little or no residue (at or below 0.1
mg/kg) was found in the roots. 21 days after the last application the
residues in leaves had declined to about 5-6 mg/kg and in the roots to
zero-0.05 mg/kg. Conversion of the roots to wet pulp completely
removed any residues.
In potatoes after similar treatment the total residues in tubers
are reported to range from 0.04-0.27 mg/kg (Chevron, 1976). This level
did not change during 3 days, but there was some loss of total residue
by the 14th day after the last application. This loss was calculated
to proceed with a half-life of about 21 days.
Total residues of acephate in tomatoes (mature green or ripe
fruits) are reported to range from 0.3-2.4 mg/kg after repeated foliar
treatments at 1 kg/ha (Chevron, 1976). The half-life was 7-14 days on
these fruits.
Residues in the pulp and peel of potatoes and tomatoes are
discussed further below ("Fate of residues in processing and
cooking"). Some residue data are given in Tables 14 and 15.
Citrus fruits
Supervised trials on acephate treatments of oranges, grapefruits,
lemons and tangeloes have been reported from New Zealand (1976) and
the U.S.A. From the results, Table 8, it is clear that a significant
systemic penetration takes place from the peel into the fruit flesh.
In the grapefruit, the acephate concentration seems to be equilibrated
throughout the whole fruit.
TABLE 5. Residues of acephate and its metabolite in alfalfa, cottonseed* and soybeans
Application Days after Residues, mg/kg
Rate, last
Crop kg a.i./ha No. treatment Acephate Methamidophos Total
Green hay (Air spray) 2 21 1.70 - 1.75 0.25 - 0.26 1.95 - 2.01
1 28 0.46 - 0.55 0.07 - 0.10 0.56 - 0.62
Cured hay 1 2 21 2.78 - 5.00 0.36 - 0.57 3.14 - 5.57
(4 days) (Air spray) 28 0.89 - 0.96 0.09 - 0.11 1.00 - 1.05
Green hay 1 2 21 - - -
(Ground spray) 28 0.76 - 1.56 0.24 - 0.52 1.00 - 2.08
Cured hay 1 2 21 - - -
(4 days) (Ground spray) 28 0.33 - 0.52 0.12 - 0.15 0.45 - 0.67
Green hay 1 3 0 27.0 - 32.0 1.29 - 1.65 33.3
(Ground spray) 14 8.9 - 11.9 1.40 - 1.41 10.3
21 3.28 - 4.40 0.59 - 0.74 3.87 - 5.14
28 - 0.21 - 0.29 -
Green hay 1 3 21 5.01 - 5.12 0.54 - 0.59 5.55 - 5.71
(Ground spray) 28 - - -
Cottonseed, 1 8 21 0.48 - 1.15 0.13 - 0.26 0.61 - 1.41
fuzzy 2 8 21 0.47 - 0.83 0.14 - 0.23 0.61 - 1.06
Cottonseed 1 8 21 1.00 - 1.19 0.23 - 0.31 1.23 - 1.50
meal 2 8 21 1.27 - 1.48 0.32 - 0.34 1.59 - 1.82
Crude cottonseed 1 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
oil 2 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
Soybeans 1 3 14 0.08 - 0.20 0.00 - 0.01 0.08 - 0.21
Soybeans, protein 1 3 14 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
flour
Soybeans, 1 3 14 0.02 - 0.03 0.00 - 0.00 0.02 - 0.03
crude oil
Soybeans, 1 3 14 0.34 - 0.23 0.04 - 0.07 0.27 - 0.41
mill feed
* Residues tabulated for cottonseed and soybeans are maximum levels found in a series of experiments.
TABLE 6. Residues of acephate and its metabolite methamidophos in broccoli
Application Days after Residues, mg/kg
Rate last
Crop part kg a.i./ha No. treatment Acephate Methamidophos Total
Heads, 1 3 0 - - -
trimmed 3 - - -
7 4.48 - 5.25 0.36 - 0.45 4.84 - 5.70
14 3.42 - 3.44 0.32 - 0.36 3.74 - 3.80
1 6 0 6.63 - 6.82 0.65 - 0.69 7.32 - 7.47
3 6.63 - 6.63 0.58 - 0.68 7.21 - 7.31
7 5.17 - 6.08 0.75 - 0.75 5.92 - 6.83
14 1.98 - 2.27 0.39 - 0.40 2.66 - 2.38
2 6 0 20.0 - 21.6 1.56 - 1.74 21.7 - 23.2
3 20.4 - 23.0 1.56 - 1.78 22.2 - 24.6
7 14.0 - 16.7 1.70 - 1.70 15.7 - 18.4
14 4.92 - 5.14 0.80 - 0.96 5.7 - 6.1
1 9 0 14.5 - 16.0 1.78 - 1.82 16.3 - 17.8
3 16.0 - 17.4 1.40 - 1.60 17.4 - 19.0
7 10.1 - 13.2 1.24 - 1.27 11.3 - 14.5
14 2.48 - 2.56 0.75 - 0.76 3.24 - 3.31
2 9 0 28.3 - 30.3 3.46 - 3.59 32.1 - 33.9
3 23.5 - 25.3 2.33 - 2.49 23.8 - 27.8
7 21.6 - 24.9 2.47 - 2.59 24.1 - 27.5
14 5.46 - 6.57 1.11 - 1.45 6.57 - 8.02
Trimmings 2 6 0 45.4 - 47.9 2.17 - 2.25 47.7 - 50.1
3 45.4 - 52.9 2.05 - 2.23 47.5 - 55.1
7 20.8 - 40.4 1.38 - 1.65 22.2 - 42.1
14 14.6 - 16.1 1.05 - 1.21 15.7 - 17.3
TABLE 7. Predicted maximum+ and actual residues of acephate
+ methamidophos metabolite in some vegetables
Crop Total Residue,
Application Days after mg/kg
Rate, last Calculated+
Crop kg a.i./ha No. treatment maximum+ Found1
Broccoli 1 3-9 14 5.47 3.80
(max.25%
trimming) 2 6-9 14 9.89 8.02
Brussels 1 8 3 4.79 3.92
sprouts
(max. 15% 2 8 3 12.93 10.18
trimming)
Cabbage 1 9 3 7.29 2.51
(max. 20%
trimming) 2 9 3 12.82 6.38
Cauliflower 1 7-9 3 6.85 2.30
(max.50%
trimming) 2 7-9 3 7.90 4.36
Lettuce 1 6-8 3 7.35 3.29
(max. 50%
trimming) 2 6-8 3 16.17 5.54
+ Based on results from trimmed heads and trimmings, assuming maximum
possible trimming.
1 Reported results on heads trimmed according to commercial practice.
FATE OF RESIDUES
In animals
The metabolic fate of acephate in rats has been described above
("Biotransformation"). Proposed metabolic pathways in mammals are
shown in the same section (Figure 1).
TABLE 8. Residues of acephate and its metabolite methamidophos in citrus fruits
Application Days after Residues, mg/kg
Concentration last Acephate Methamidophos
Country Crop a.i. No. treatment Peel Flesh Peel Flesh
New Zealand Oranges 0.075% 6 1 9.0 0.6 0.98 0.01
(25% peel) 8 9.0 0.7 0.8 0.3
15 7.6 0.7 0.9 0.09
24 5.3 0.6 0.6 n.d.
Grapefruit 0.075% 6 1 2.9 3.0 n.d. 0.2
(30% peel) 8 3.1 1.9 0.2 0.3
15 2.6 3.6 0.2 0.2
24 1.4 5.4 n.d. 0.2
Whole fruit Whole fruit
U.S.A. Oranges 0.06% 2-3 20-25 0.33 - 1.1 0.06 - 0.10
40 0.19 - 1.5 0.02 - 0.08
100 0.00 - 0.00 0.00 - 0.00
Lemons 0.06% 3 20-25 0.08 - 0.18 0.02 - 0.03
0.12% 3 20-25 0.42 - 0.44 0.05 - 0.06
Tangeloes 0.06% 3 20 1.4 - 1.6 0.16 - 0.17
Dairy cows and pigs
9 dairy cows were fed at levels of 3, 10 and 30 ppm of acephate
+0.6, 2 and 6 ppm of its metabolite methamidophos in the diet for 30
days. The ratio of acephate to methamidophos was 5:1 because this is
typical of the ratio in most field crops after treatment with
acephate.
The lowest dosage did not give detectable residues in the cows'
milk. Milk from the higher dosed animals, however, contained between
0.1 and 0.2% of the daily doses and at levels which rapidly reached a
plateau where they remained during the 30 days treatment period.
Average residues found in the milk are shown in Table 9. At withdrawal
of the daily intake, residues in the milk decreased rapidly. By the
morning of the second day after withdrawal neither acephate nor its
metabolite could be observed in the milk.
Some individual cows were sacrificed after 3-4 weeks of feeding
with acephate and its metabolite. They showed significant but moderate
levels of residues in various tissues (Table 10). The highest residue
of acephate was 0.57 mg/kg in the kidney of an animal at the highest
dose level, and no tendency to accumulation in tissues was noted. 6
days after withdrawal, no residues could be detected in liver, kidney,
heart, muscle or fat.
TABLE 9. Average1 residues of acephate and its metabolite
methamidophos in cows' milk
Dose level Residue in milk
(ppm in dry feed) mg/kg ± S.D.1
Acephate Methamidophos Acephate Methamidophos
3 0.6 0.00 0.00
10 2 0.09 ± 0.005 0.00
30 6 0.27 ± 0.06 0.022 ± 0.006
1 Average for 30 days of three cows per dose level.
Essentially the same results were obtained by feeding acephate
and its metabolite to pigs. A linear relationship could be established
between dose levels and tissue residues. The residues ranged from 0.15
to 0.58 mg/kg of total residue at the high dose level (i.e. 30 ppm of
acephate + 6 ppm of metabolite) in tissues which were analysed after 4
weeks of feeding, namely: muscle, liver, kidney, subcutaneous and
peritoneal fat, heart and brain. In pigs as in cows, the residues were
undetectable in all tissues in less than 6 days after withdrawal of
the diet.
Chicken and quail
Tissue samples and eggs were taken from chickens and quail which
had been subjected to acephate feeding in subacute and chronic
toxicological studies. Three dose levels were followed: 3, 10 and 30
ppm of acephate in the diet for 92-140 days. No residues were detected
in any of the experiments in the fat, kidney or liver of the birds. In
eggs and muscle tissue, low levels of acephate were observed at the 10
and 30 ppm feeding levels, namely 0.07 and 0.19 mg/kg respectively in
chicken eggs, 0.16 and 0.31 mg/kg in quail eggs, 0.01 and 0.02 mg/kg
in chicken muscle and 0.01 and 0.04 mg/kg in quail muscle (Table 11).
At the high feeding levels in these studies, trace amounts of
methamidophos (OS-dimethyl phosphoramidothioate), which is otherwise
recognised as a plant metabolite only, were observed in muscles as
well as in eggs.
In plants
Studies using 14C-labelled acephate show that the compound is
readily degraded by plants such as bean seedlings, tomato and cabbage.
The half-life of acephate in such greenhouse-grown plants has been
shown to be of the order of 5-10 days. Among several theoretically
possible metabolites only one, methamidophos or OS-dimethyl
phosphoramidothioate, is found to be of practical significance.
Another of the possible metabolites is believed to be present although
it could not be analytically observed. This is OS-dimethyl
phosphorothioate, which is thought to be transitional step in the
complete breakdown to phosphate. The suggested metabolic pathways of
acephate in plants are shown in Figure 2.
(Pack, 1972)
Effects on enzymes and other biochemical parameters
Acephate and its major plant metabolite methamidophos, as are
other organophosphate esters, are cholinesterase inhibitors. The
I50 values (molar concentrations for 50% inhibition) for plasma and
bovine RBC cholinesterase (acetylcholine hydrolysis) are given
below:
Human Plasma Bovine RBC
µg/ml I50, Molar µg/ml I50, Molar
Acephate >500 >2.7 X 10-3 >500 >2.7 X 10-3
Methamidophos 23 1.6 X 10-4 4.3 3.1 X 10-5
(Tucker, 1972)
Depression of cholinesterase activity was measured following
oral administration of acephate to male rats. Following a dose of
900 mg/kg RBC cholinesterase was substantially depressed while
plasma was unaffected. At the time of sacrifice, all animals
displayed some typical signs of organophosphate poisoning.
Administration of a dose of parathion (15 mg/kg) resulted in a
slightly higher reduction of RBC cholinesterase activity (50-30%)
with no effect on plasma enzymes (Cavalli and Spence, 1970c).
Groups of rats (15 females/group) were orally administered
acephate at levels corresponding to 1200, 100, 30, and 0 ppm as
daily equivalents in the diet for up to 3 weeks. Another group was
administered methamidophos at a dose corresponding to 10 ppm. A
slight depression of RBC-cholinesterase was noted at 100 and 30 ppm
acephate. The 1200 ppm level caused a significant depression of
activity. The plasma cholinesterase was only slightly depressed by
the highest dose level. There was no indication of a build-up of
cholinesterase inhibition over the 3 week test interval.
Methamidophos was inhibitory at 10 ppm. Methamidophos was shown to
be substantially more active an inhibitor than acephate (Cavalli
and Spence, 1970f).
TOXICOLOGICAL STUDIES
Special studies on potentiation
A study was performed on the acute toxicity of acephate alone
and in combination with 5 cholinesterase inhibiting pesticides
(azinphos-methyl, carbaryl, malathion, parathionmethyl and
oxydemeton-methyl) on male rats. The LD50 values for equitoxic
mixtures of the paired pesticides differed from single LD50 values
especially with carbaryl and malathion suggesting that potentiation
may be a factor in the interaction (Rittenhouse et al., 1972a).
A similar study in rats with methamidophos and acephate
indicated that there was no significant potentiation (Kretchmar et
al., 1972).
Special studies on neurotoxicity
Hen
Groups of adult hens (6 hens/group) were administered acephate
orally at dosage levels of 0 and 568 mg/kg (an LD50 level). A
second dose was administered to survivors at 50 day 21 and these
were observed for an additional 21 days; tri-o-cresyl phosphate
(TOCP) was administered at 500 mg/kg as a positive control. Samples
of spinal cord, peripheral nerve and brain were examined
histologically for signs of myelin disruption.
Of the hens treated with TOCP, 3 of 6 died and the remaining
hens displayed signs of clinical ataxia commonly noted with this
toxicant. One of the 6 hens treated with acephate died after the
first dosing and 3 more after the second. The remaining hens
displayed none of the signs of ataxia and on histological examination
there was no evidence of myelin degeneration. Acephate does not induce
ataxia as evidenced by TOCP in hens (Fletcher, et al., 1972b).
Special studies on reproduction
Rat
Groups of rats (8 males and 16 females/group) were fed
acephate in the diet at dosage levels of 0, 30, 100 and 300 ppm and
subjected to a standard 3-generation, 2-litter per generation
reproduction study. Gross and histopathologic examinations were
performed on the parental animals of all 3 generations, upon
weanlings of the second litter and upon weanlings of the F3b litter
for the control and high dose level. No pathologic or microscopic
changes were noted in the tissues and organs examined.
Adverse reproductive effects were noted at 100 and 300 ppm.
Reduced mating and fertility indices (reflected by copulation and
pregnancies) were noted. There were no effects noted on the ability
of mothers to maintain pregnancy although survival of pups after
birth was affected at various times during the 3 generation trial.
Weanling body weight of pups fed acephate was similar to control
values. There were no apparent malformations noted in the pups born
during this study. The results indicate a no-effect level in this
study based on all observable parameters to be 30 ppm (Haley et
al., 1973).
Special studies on teratogenicity
Rabbits
Groups of pregnant rabbits (17 does/group) were administered
acephate by capsule daily from day 6 to day 18 of gestation at
doses of 0, 1 and 3 mg/kg body weight in a standard teratology
study. Thalidomide (37.5 mg/kg/day) was used as a positive control.
There were no deaths or abnormal behavior attributed to acephate.
There were no effects on the reproduction or teratogenicity
parameters examined. Total implantation sites, resorptions,
abortions, number of young and examinations of skeletal tissue were
normal. Acephate did not elicit a teratogenic response in rabbits
while the positive control induced resorption, reduced the
incidence of pups and resulted in an increase in the number of
terata formed (Ladd et al., 1972).
Rats
Groups of pregnant rats (17-20 females/group) were
administered acephate by gavage daily from day 6 to day 15 of
gestation (10 doses) at dosage levels of 0, 25, 50, 100 and 200
mg/kg. Upon sacrifice at day 20 examinations made of the maternal
and fetal parameters did not indicate a teratological potential for
acephate in rats. Maternal effects were observed as reduced weight
gain at 100 and 200 mg/kg and as an increase in resorption sites at
200 mg/kg. All treated groups reflected acute parasympathomimetic
signs of poisoning (lacrimation) initially after treatment. No
effects were noted with respect to abortion or on the fetal
development, both internal and external tissue development including
skeletal abnormalities. This study again demonstrated a negative
teratogenic potential for acephate (Haley et al., 1971).
Special studies on mutagenicity
Mice (dominant lethal)
Groups of mice (12 males/group) were administered acephate by
intra peritoneal injection at dose levels of 0, 25, and 50 mg/kg.
The mice were then mated individually with 3 virgin females weekly
for 6 consecutive weeks in a dominant lethal test to investigate
the effects on maturation of male mouse germ cells. A positive
control (MMS, methyl methanesulfonate) was also used. The females
were sacrificed 1 week after removal from the breeding cage.
Analyses were made of implantation sites, resorption sites and
embryo development to evaluate the in vivo mutagenic potential of
acephate. In the first week after mating, an increase of
pre-implantation loss was noted at 50 mg/kg. There were no
significant differences between groups with respect to
implantation, resorption or embryo development. The in vivo
mutagenic data do not indicate acephate to have a potential for
mutagenic development (Arnold et al., 1971).
In contrast to the previously referenced in vivo study, a
series of in vitro microbiological tests has shown acephate to
elicit a positive response in tested strains of bacteria. In two
species of bacteria, Salmonella typhimurium and Escherichia
coli, acephate was positive only in some of the latter strains
(Hanna and Dyer, 1975).
Special studies on carcinogenicity
Mice
Groups of mice (Swiss White Mice - 50 males and 50
females/group) were fed acephate in the diet at levels of 0, 300
and 600 ppm for 18 months. A positive control
(N-nitrosodimethylamine) was fed at 10 ppm over this interval. Gross
pathologic examination for tumor formation and histopathologic
examination of tumors from animals sacrificed in extremis or
dying during the study showed no evidence of carcinogenicity due to
acephate. A similar negative finding was observed in the mice
sacrificed at the conclusion of the study. There was no unusual
behavior pattern exhibited by the acephate-fed mice and the
incidence and pattern of mortality were not influenced by acephate
in the diet. This study suggests that acephate is not a carcinogen
in mice (Reyna et al., 1973).
Special studies on skin sensitization
In two identical studies, groups of rabbits (8 rabbits/group)
were administered acephate dermally in acetone at a dose level of
0.1 ml of a 1% w/v solution or a control acetone treatment daily
for 16 days. Ten days after the last application, a challenge dose
at a remote site was applied and dermal irritation graded for a
sensitization response. None of the animals showed an adverse
reaction suggesting that by the dermal route acephate is not a
sensitizing agent (Cavalli and Spence, 1970g and 1970h).
Groups of guinea pigs (10 albino guinea pigs/group) were
dermally administered acephate (0.1 ml of a 1% w/v aqueous
solution) every other day (4-6 hours/day) for 10 treatments. Two
weeks after the last exposure, the animals were challenged and
examined for sensitization. There were no indications that acephate
induced a sensitization reaction under these conditions. A positive
reaction was obtained with chlorodinitrobenzene, a known sensitizer
(Ebbens et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of acephate
LD50
Species Route Sex (mg/kg) Reference
Rat Oral M&F 1100 Kretchmar et al, 1971
Oral M&F 1494 Mastri et al, 1970b
Oral M&F 2025 Kretchmar et al, 1972
Oral M 945 Cavalli & Spence, 1970b
Oral F 866 Cavalli & Spence, 1970b
Oral M 1230 Rittenhouse et al, 1972
IP M 1106 Coquet, 1972
Inhalation M&F >1.81 mg/L(4 hr.) Elliott et al, 1972
Inhalation M&F >12.1 mg/L(1 hr.) Kruke, 1972a
Mouse Oral M&F 362 Mastri et al, 1970a
Rabbit Oral M 707 Narcisse et al, 1972a
Dermal M&F 10,250 Mastri et al, 1970b
(24 hour 75% spray suspension)
Dermal M&F 500 Narcisse et al, 1972b
(no irritation)
(24 hr - 75-S formulation)
Dermal M >2000 Cavalli & Spence, 1970a
TABLE 1. (continued)
LD50
Species Route Sex (mg/kg) Reference
Dog Oral M&F <681 Mastalski et al, 1970
Oral M&F 215 Mastalski et al, 1970
(emetic dose)
Chicken Oral F >100 Edds & Bradley, 1971
Oral F 568 Fletcher et al, 1972a
Eye irritation
Acephate (100 mg) was instilled into the conjunctival sac of
rabbits (six animals/group) in an examination for eye irritancy
properties. Conjunctival irritation was evident with all forms of
acephate. Where acephate was washed out after a 5 minute exposure
period, conjunctival irritation and corneal damage were observed.
The irritation was observed after one week while all eyes were
normal at 14 days (Narcisse et al., 1971a).
Vapor inhalation
Groups of rats (5 male and 5 female/group) were exposed to an
atmosphere saturated with acephate vapor (derived from passing air
through crystalline or melted acephate at 90°C). Exposure for 1
hour to vapor derived from either method did not result in any
mortality. Animals were examined over a 14 day observation period
(Cavalli and Spence, 1970d). In an additional study, 5 female rats
were exposed for four hours to vapors of acephate derived only from
passing air through crystalline material. There was no mortality or
morbidity and cholinesterase (undefined) activity was normal
(Cavalli and Spence, 1970e).
Aerosol inhalation
Groups of rats (5 male and 5 female rats/group) were exposed
to an aerosol concentration of 12.1 mg/1 (90% of particles below 10
µ) for one hour. There were no signs of poisoning and no mortality;
gross effects on tissues and organs were not noted (Kruke et al.,
1972a).
Groups of rats (10 male and 10 female rats/group) were exposed
in a 380 l dynamic chamber to aerosols of acephate 1 hour per day,
5 days per week for two weeks. The aerosol was generated from
aqueous solutions of 0, 0.25, 1.0 and 4% acephate and had a
particle size generally under 10 microns. There was no mortality
and weight gain and general behavior were normal. At the highest
dose, female uric acid content was increased. There were no other
effects noted an clinical chemistry, urinalysis or hematology
parameters including cholinesterase activity. Gross and microscopic
examination of tissues and organs showed no effect of acephate
exposure (Kruke et al., 1972b).
Short-term studies
Rabbit - (Dermal)
Groups of rabbits (8 male and 8 female albino rabbits/group)
were administered acephate to either intact or abraded skin. An
aqueous solution was applied daily, 6 hours/day, 5 days/week, for
3 weeks with the daily doses being 0, 0.5, 1.0 and 2.0 g/kg. At the
conclusion of the study, the animals were sacrificed. There was
mortality (2/16) observed at the highest dose and
parasympathomimetic signs of poisoning were observed at 1.0 and 2.0
g/kg. These two groups also lost weight while no differences in growth
or body weight were observed at 0.5 g/kg. The two highest doses showed
substantial changes in hematology and blood chemistry parameters:
reduced RBC count, reduced hemoglobin and hematocrit values and
reduced BUN values. In male rabbits only at the low dose group, LDH
and SGOT values were reduced. RBC cholinesterase was depressed at all
dose levels to about 50% in both sexes whereas plasma cholinesterase
was not affected. On gross examination increased liver weights were
observed at the two highest dose groups. There were no effects noted
on microscopic examination including bone marrow (M/E ratio) studies
(Rittenhouse et al., 1972b).
Hen
Groups of chickens (25 females and 4-6 males/group) were fed
acephate in the diet at dosage levels of 0, 3, 10 and 30 ppm for up to
13 weeks (followed by a 28 day recovery period with control diets).
Growth, egg production and egg quality was normal. The hatchability of
eggs at the highest dose was slightly reduced, and reduction was noted
in growth and development of hatched chicks in the 10 and 30 ppm
groups. The viability of the hatched chicks was diminished in the
highest dose group. There were no gross pathological changes in any of
the birds (Fletcher, 1972a).
Rat
Groups of rats (15 males and 15 females/group) were fed acephate
in the diet for 90 days at levels of 0, 30, 100 and 300 ppm. There was
no mortality attributed to acephate in the diet. Growth, food
consumption and observable behavior were normal. Urinalyses, blood
chemistry and hematology values were normal. Gross and microscopic
analysis of tissues and organs were performed at the conclusion of the
study. In the males a decreased liver weight was noted at 300 ppm.
There were no histological lesions associated with this finding.
Cholinesterase activity data were not reported (Plank et al., 1971).
TABLE 2. Acute toxicity of metabolites or possible impurities
LD50
Compound Structure Species Route Sex (mg/kg) Reference
OO-dimethyl (CH3O)2P(S)NH2 Rat Oral M 633 Cavalli et al, 1968a
phosphoramidothioate (RE 9169) Oral F 549
Signs of poisoning include CNS depression (paresis, hyporeflexia and anesthesia)
Rabbit Dermal M 2500 Cavalli et al, 1969
OS-dimethyl (CH3O)(CH3S)P Rat Oral M 21 Cavalli et al, 1968b
Phosphoramidothioate (O)NH2 Oral F 19
(RE 9006)
Signs of poisoning are standard for parasympathomimetic agents.
Rabbit Dermal M 118 Cavalli et al, 1968c
OO-dimethyl acetyl (CH3O)2P(S)NHC Rat Oral M 1468 Cavalli et al, 1970
phosphoramidothioate (O)CH3 Oral F 1250
(RE 14071)
Signs of poisoning are standard for CNS depression - hyporeflexia, hypothermia, pyloerection, constipation, and possibly
tachycardia.
Rabbit Dermal M >2000 Rittenhouse et al,1972C
OO-dimethyl (CH3O)2(CH3S) Rat Oral M 83 Narcisse et al, 1971b
S-methyl phosphorothioate P(0) Oral F 63
(RE 15283)
The signs of poisoning include salivation, tremors, weakness, and prostration - death occurred 4-10 days after dosing.
TABLE 2. (Cont'd.)
LD50
Compound Structure Species Route Sex (mg/kg) Reference
Rabbit Dermal M 109 Narcisse et al, 1971c
OS-dimethyl (CH3O)(CH3S)P Rat Oral >2000 McCaskey,1972a
phosphorothioate (O) (OH)
(RE 18421) Rabbit Dermal M >2000 Rittenhouse et al, 1972d
S-methyl (CH3S)P(O)OH Rat Oral >2000 McCaskey, 1972b
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al, 1972e
RE 17245)
O-methyl (CH3O)P(O)SH Rat Oral >2000 McCaskey, 1972c
acetylphosphoramidothioate NHC(O)CH3 Rabbit Dermal M >2000 Rittenhouse et al.,
(RE 17246) 1972f
Groups of rats (from 15 to 35 males and females per group) were
fed acephate in the diet at levels of 0, 1, 5, 10, 30, 100 and 300 ppm
for 90 days and a control diet for an additional 4 weeks. Animals were
sacrificed at various intervals and cholinesterase activity was
measured using erythrocytes, plasma and brain as enzyme sources.
Contrary to acute or short term oral administration studies this
dietary study showed little effect on erythrocyte cholinesterase
activity at levels below 100 ppm. In contrast, 30 ppm affected plasma
cholinesterase in weeks 6 and 13 of the test period. Brain
cholinesterase was depressed consistently at 30 ppm. In general,
females were more susceptible than males. Recovery of all blood
enzymes was rapid (within 1 week) while brain cholinesterase was
slower. All enzyme levels were normal within 4 weeks of feeding
control diets (Plank et al., 1972).
Dog
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet at dosage levels of 0, 10, 30 and 100 ppm for 90
days. At the conclusion of the feeding period half of the groups were
sacrificed and the remainder maintained for a 40 day recovery period.
RBC cholinesterase activity was depressed in both sexes at 100 and 30
ppm while plasma was unaffected. Brain cholinesterase was depressed at
30 ppm. Recovery of enzyme activity was noted at 28 days after feeding
stopped and animals were fed control diets.
Growth, food consumption and behavior were unaffected over the
course of the study. There were no deaths, and animals appeared
normal. Analysis of blood and urine and hematologic parameters showed
no effects of acephate. Gross and microscopic analysis of tissues and
organs also showed no effects (Lindberg et al., 1971). [A no-effect
level in the study is 10 ppm (equivalent to 0.32 mg/kg bw) while 30
ppm produced a minimal depression of cholinesterase.]
Groups of dogs (4 male and 4 female beagle dogs/group) were fed
acephate in the diet for two years at levels of 0, 10, 30 and 100 ppm.
There was no effect on food consumption, growth or behavior. Two dogs
which did not survive the study died as a result of factors believed
to be unrelated to the presence of acephate in the diet. No effects
were noted on blood chemistry, hematology or urine analysis
parameters. Gross and microscopic examination of a variety of tissues
and organs showed no effects attributed to acephate. Depression of
cholinesterase activity was noted in this study in brain and red blood
cells. Plasma cholinesterase was unaffected at 100 ppm while RBC
showed reduced activity. Brain cholinesterase was only slightly (N10%)
depressed at the highest dose level. A no-effect level based on RBC
cholinesterase depression is 30 ppm over the two year study (Hartke et
al., 1972).
Long-term studies
Rat
Groups of rats (35 males and 35 females/group) were fed acephate
in the diet for two years at levels of 0, 30, 100 and 300 ppm. A
depression of growth was observed in both sexes at 12 months at 100
and 300 ppm. At 24 months the body weight of both sexes was depressed
only at 300 ppm. Food consumption was normal. There were no effects on
mortality, behavior or on hematology, blood chemistry or urine test
parameters. On gross examination male and female liver weight at 300
ppm was depressed, but nothing abnormal was noted on histopathologic
examination of the liver or other tissues and organs. There was no
suggestion of an increased tumor incidence. Blood and brain
cholinesterase depression examined during the course of this study
provided the only effects noted. At 24 months (the only interval
reported) RBC and plasma cholinesterase values were normal. Brain
cholinesterase was minimally depressed (26-27% of normal) at 30 ppm.
The depression was dose dependent suggesting a no-effect level to be
somewhat below 30 ppm. Cholinesterase was not reported for time
intervals other than 24 months. A no-effect level based on
cholinesterase depression cannot be determined in this study. A
systemic no-effect level of 30 ppm may be estimated based on growth
reduction over the first year test interval (Plank and Keplinger,
1973).
OBSERVATIONS IN MAN
In a series of reports from areas in the U.S.A. where acephate
has been used in field applications, 95 individuals exposed from 0.5
to > 1000 hours under a variety of conditions expressed no symptoms
or adverse effects of exposure. The survey was primarily subjective as
clinical measurements were not made (Anonymous, 1976a).
In a similar survey individuals exposed to acephate in pilot
plant and formulation processes did not show adverse effects. Several
of these workers were exposed for up to 15 months (Anonymous, 1976b).
Analysis of urine from these individuals confirmed exposure to
acephate. Urinary levels up to 5 ppm acephate were observed (Pack,
1972b). As expected, other metabolites or technical products of
production were observed in the urine of individuals exposed in the
pilot plant. In addition to acephate, these products included the
oxygen analog of acephate, methamidophos, the oxygen analog of
methamidophos and trimethyl phosphate. The individuals exposed to
technical acephate in formulating processes showed only methamidophos
and trimethyl phosphate as urinary metabolites. Blood and urinary
samples from 5 individuals were examined over a 10 week period. All of
the clinical parameters were normal as were standard tests including
EKG, X-ray and cholinesterase values (Swencicki and Hartz, 1972).
In a follow-up of agricultural field workers exposed to acephate,
levels of 1/50 those found in the pilot plant survey were observed in
the urine. There was no evidence of methamidophos in the urine. As
expected there were no adverse effects noted (Pack, 1972c).
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. They received either a
combination (methamidophos/acephate) of 1:9, 1:4 or corn oil as a
control daily (divided into 3 equal doses) for 21 days. The 1:9 group
was administered the combination for consecutive periods of 21 days at
doses of 0.1, 0.2 and 0.3 mg/kg/day followed by a 7 day recovery
period after which the dose was increased to 0.4 mg/kg/day for 10
days. The 1:4 group received the combination at doses of 0.1 and 0.2
mg/kg/day for 21 consecutive days before the dosing was halted. The
protocol called for a total of 4 dose levels (up to 0.4 mg/kg/day)
each to be administered for 21 days. Blood cholinesterase (plasma and
RBC) was examined periodically.
Results are summarized in Table 3. No effects were noted on RBC
cholinesterase at any treatment level. There was marginal depression
of the plasma cholinesterase noted In the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The plasma
cholinesterase, of the males was substantially affected at the 0.3
mg/kg dose and was not retested at the higher dose level. The group
(both males and females) receiving the higher methamidophos:acephate
ratio (1:4) showed reduction of plasma cholinesterase after two weeks
of receiving a dose of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not
used in the 1:4 dose group. This study indicated the minimal dose of
acephate necessary to decrease plasma cholinesterase for the
combination of acephate and methamidophos was 0.2 mg/kg/day of a 1:4
combination of the two toxicants. No effects with this combination
were noted at 0.1 mg/kg (Garofalo et al., 1973). Thus, a dose level of
0.03 mg methamidophos/kg bw combined with a dose of 0.2 mg acephate/kg
bw (ranging from 0.08 to 0.18 mg acephate/kg bw) would be expected to
reduce cholinesterase activity in man. A no-effect level evaluated
from this study might be 0.2 mg acephate/kg bw in combination with
0.02 mg methamidophos/kg bw.
COMMENTS
Acephate, a relatively non-toxic organophosphorus pesticide, is
rapidly absorbed, metabolised and excreted from the body. A toxic
metabolite, methamidophos, found to occur in plants and soil has not
been identified in mammals. Methamidophos, a potent
parasympathomimetic agent acting by inhibition of
acetylcholinesterase, is also rapidly degraded and excreted in
mammals. Neither compound persists or accumulates in the body nor
appears to affect biochemical systems other than cholinesterase.
Neurotoxicity studies in hens and potentiation studies in rats with
other antiesterase compounds were negative. Special studies on
reproduction showed effects at dietary levels of 100 ppm and above as
evidenced by reduced survival of offspring. In vivo mutagenesis
and teratogenesis testing was negative as was a special study with
mice for carcinogenicity. A positive mutagenesis response in a single
bacterial strain was observed in vitro. In a 2 year study with
dogs and an 18 month study with mice no observable effects other than
cholinesterase depression were noted. In a 2 year rat study, a slight
depression of growth and of brain cholinesterase activity at the
lowest level tested precluded a definition of a no-effect level.
However, a no-effect level in both rats and dogs was suggested based
on 90 day dietary studies which exposed animals at lower levels. In
random observations with humans occupationally exposed, no adverse
effects were noted. In a study to define a no-effect level in humans
based on cholinesterase depression, administration of a combination of
methamidophos and acephate (1:4 ratio) at a total dose of 0.2
mg/kg/day reduced plasma cholinesterase. A calculation of the
individual doses received suggested in one case that 0.16 mg
acephate/kg body weight in combination with 0.04 mg methamidophos/kg
(bw) would cause depression of cholinesterase while a dose of 0.08 mg
or 0.18 mg acephate/kg (bw) both in combination with 0.02 mg
methamidophos did not reduce plasma cholinesterase activity. Based on
the studies with both rat and dog, with further assurance afforded by
the observations in man, an ADI was recommended. This also took into
account the slight effect noted in the rat 2 year study where 30 ppm
(equivalent to 1.5 mg/kg) induced a marginal effect on brain
cholinesterase.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 10 ppm in the diet equivalent to 0.5 mg/kg body weight
Dog: 10 ppm in the diet equivalent to 0.32 mg/kg body weight
Man: 0.18 mg/kg body weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.02 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Registration of acephate as a contact and systemic insecticide is
reported from many countries of the American continents, Australia,
Asia and Europe. It is mostly marketed as soluble powders (s.p.), 75%.
Occasionally, however, it is also registered as a 50% s.p., as low
concentration dusts and as 1-5% granules.
TABLE 3. Effect on plasma cholinesterase of combined doses of methamidophos and acephate
Methamidophos: Acephate Ratio
1:4 1:9
Total
dose methamidophos:acephate Plasma Inhibition methamidophos:acephate Plasma inhibition
(mg/kg) (mg/kg) Males Females (mg/kg) Males Females
0.1 0.02 : 0.08 - - 0.01 : 0.09 - -
0.2 0.04 : 0.16 + + 0.02 : 0.18 - -
0.3 0.06 : 0.24 * * 0.03 : 0.27 + -
0.4 0.08 : 0.32 * * 0.04 : 0.36 * -
* Discontinued treatment
Acephate is claimed to be especially active against insects of
the families Lepidoptera, Homoptera and Hemiptera. A list of suggested
uses which is typical in many countries is given in Table 4. These
uses cover suggested protection of both horticultural and agricultural
crops (fruits, vegetables, root-crops and several industrial crops)
against larvae, aphids, caterpillars, berry moths, leaf miners,
beetles etc. Usual application rates are also indicated in Table 4.
They range from 1-2 kg a.i. per ha on field crops and from 0.05-0.1%
on fruit trees, bushes etc.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable amount of residue data on acephate and its
behavior in supervised trials and controlled experiments has been
presented by the originating manufacturer (Chevron Chemical Company).
Most of the data were generated under American climatic and
agricultural conditions, although in some cases supplementary data
from other sources are available.
TABLE 4. Suggested uses of acephate
Concentration or
Crop Pest dosage of a. i.
Fruit crops Aphids 0.04-0.075%
(pome, stone, Codling moths
citrus) Chewing and sucking insects
Tortrix
Citrus, olive scale
Grape Grape berry moths 0.075%
Vegetables Aphids 0.05%
Chewing and sucking insects
Caterpillars
Moths
Beets Leaf miners 0.8-1.0 kg/ha
Ornamentals Aphids and Tortrix 0.05-0.75%
Hops Aphids 0.05-0.25%
Tobacco Aphids 1 kg/ha
Budworms
Flea beetle
Corn Corn borer 1.5-2 kg/ha
Generally the residue data from field trials indicate that
acephate is a relatively non-persistent, systemic insecticide. When
applied at the recommended rate of about 1 kg a.i./ha, several
treatments are often applied, commonly at weekly intervals for as many
weeks as necessary for insect control. A first metabolite, OS-dimethyl
phosphoramidothioate which is itself the insecticide methamidophos, is
regularly found in plant material together with acephate, although
only in the ratio of 1:10 - 1:5.
Alfalfa, cotton and soybean
Extensive trials have been made on these three important crops
(Table 5). Repeated treatments of alfalfa with 1 kg of acephate per ha
leave total residues on fresh cut green hay of less than 6 mg/kg after
21 days and a maximum of about 2 mg/kg after 28 days. Curing of the
hay in the open after harvest did not significantly change the residue
level. The apparent half-life of acephate on green hay is
approximately 7 days.
In cottonseed, the total residues ranged from zero to a maximum
of About 1.5 mg/kg 21 days after the last treatment with 1 kg/ha. The
maximum residue levels from a series of experiments are shown in Table
5. The halflife of such residues is of the order of 14 days. When
processed, essentially all the residue remains in the cottonseed meal
while crude oil and refined oil contain practically no acephate or
methamidophos metabolite (less than 0.03 mg/kg). Immediately after
treatment at the 1 kg/ha level, green leaves of cotton plants carried
residues ranging from 60-200 mg/kg. These residues declined, but they
were still significant, from 7 to 50 mg/kg, after 21 days.
In soybeans, mature and with shell removed, the total residue
varied from zero to 0.2 mg/kg 14 days after the last application of
acephate at the 1 kg/ha rate. Some positive findings are shown in
Table 5, which illustrates that when processed, most of the residue
remained in the mill feed, while trace residues only (less than
0.03 mg/kg) were found in the crude oil and the soya protein flour.
Vegetables
Residue data are available from several countries (e.g.
Australia, the Netherlands, New Zealand, Philippines, U.S.A.) on
vegetables, mostly leafy vegetables such as broccoli, Brussels
sprouts, cabbages, cauliflower and lettuce. Repeated applications of
1 kg acephate per ha are usually recommended and the residue data
indicate that there is a direct relationship between dosage and
residue level.
Residues decline at rates corresponding to the rate of growth
dilution or somewhat faster and as usual, the outer leaves of the
vegetables carry higher residues than the inner parts. This is
exemplified in Table 6 which illustrates typical residue levels of
both acephate and its metabolite methamidophos found in broccoli.
Outer leaves of the vegetables may or may not be discarded as
so-called trimmings before marketing and the final residue on the
marketed product is therefore dependent on the degree of trimming. To
take account of this situation a maximum possible residue on a given
crop may be calculated on the basis of actual analyses of the crop and
its trimmings, assuming maximum trimming. Such predictions of residues
are shown in Table 7 for 5 vegetable crops and compared with residues
actually found. The Table shows that actual residues of acephate + its
metabolite methamidophos are unlikely to exceed 5-10 mg/kg at
appropriate preharvest intervals from 3 to 14 days.
Sugar beets, potatoes and tomatoes
After repeated foliar treatments of sugar beets with acephate, at
a weekly rate of 1 kg/ha, high residues were found on the green leaves
(up to 25-30 mg/kg), while little or no residue (at or below 0.1
mg/kg) was found in the roots. 21 days after the last application the
residues in leaves had declined to about 5-6 mg/kg and in the roots to
zero-0.05 mg/kg. Conversion of the roots to wet pulp completely
removed any residues.
In potatoes after similar treatment the total residues in tubers
are reported to range from 0.04-0.27 mg/kg (Chevron, 1976). This level
did not change during 3 days, but there was some loss of total residue
by the 14th day after the last application. This loss was calculated
to proceed with a half-life of about 21 days.
Total residues of acephate in tomatoes (mature green or ripe
fruits) are reported to range from 0.3-2.4 mg/kg after repeated foliar
treatments at 1 kg/ha (Chevron, 1976). The half-life was 7-14 days on
these fruits.
Residues in the pulp and peel of potatoes and tomatoes are
discussed further below ("Fate of residues in processing and
cooking"). Some residue data are given in Tables 14 and 15.
Citrus fruits
Supervised trials on acephate treatments of oranges, grapefruits,
lemons and tangeloes have been reported from New Zealand (1976) and
the U.S.A. From the results, Table 8, it is clear that a significant
systemic penetration takes place from the peel into the fruit flesh.
In the grapefruit, the acephate concentration seems to be equilibrated
throughout the whole fruit.
TABLE 5. Residues of acephate and its metabolite in alfalfa, cottonseed* and soybeans
Application Days after Residues, mg/kg
Rate, last
Crop kg a.i./ha No. treatment Acephate Methamidophos Total
Green hay (Air spray) 2 21 1.70 - 1.75 0.25 - 0.26 1.95 - 2.01
1 28 0.46 - 0.55 0.07 - 0.10 0.56 - 0.62
Cured hay 1 2 21 2.78 - 5.00 0.36 - 0.57 3.14 - 5.57
(4 days) (Air spray) 28 0.89 - 0.96 0.09 - 0.11 1.00 - 1.05
Green hay 1 2 21 - - -
(Ground spray) 28 0.76 - 1.56 0.24 - 0.52 1.00 - 2.08
Cured hay 1 2 21 - - -
(4 days) (Ground spray) 28 0.33 - 0.52 0.12 - 0.15 0.45 - 0.67
Green hay 1 3 0 27.0 - 32.0 1.29 - 1.65 33.3
(Ground spray) 14 8.9 - 11.9 1.40 - 1.41 10.3
21 3.28 - 4.40 0.59 - 0.74 3.87 - 5.14
28 - 0.21 - 0.29 -
Green hay 1 3 21 5.01 - 5.12 0.54 - 0.59 5.55 - 5.71
(Ground spray) 28 - - -
Cottonseed, 1 8 21 0.48 - 1.15 0.13 - 0.26 0.61 - 1.41
fuzzy 2 8 21 0.47 - 0.83 0.14 - 0.23 0.61 - 1.06
Cottonseed 1 8 21 1.00 - 1.19 0.23 - 0.31 1.23 - 1.50
meal 2 8 21 1.27 - 1.48 0.32 - 0.34 1.59 - 1.82
Crude cottonseed 1 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
oil 2 8 21 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
Soybeans 1 3 14 0.08 - 0.20 0.00 - 0.01 0.08 - 0.21
Soybeans, protein 1 3 14 0.00 - 0.00 0.00 - 0.00 0.00 - 0.00
flour
Soybeans, 1 3 14 0.02 - 0.03 0.00 - 0.00 0.02 - 0.03
crude oil
Soybeans, 1 3 14 0.34 - 0.23 0.04 - 0.07 0.27 - 0.41
mill feed
* Residues tabulated for cottonseed and soybeans are maximum levels found in a series of experiments.
TABLE 6. Residues of acephate and its metabolite methamidophos in broccoli
Application Days after Residues, mg/kg
Rate last
Crop part kg a.i./ha No. treatment Acephate Methamidophos Total
Heads, 1 3 0 - - -
trimmed 3 - - -
7 4.48 - 5.25 0.36 - 0.45 4.84 - 5.70
14 3.42 - 3.44 0.32 - 0.36 3.74 - 3.80
1 6 0 6.63 - 6.82 0.65 - 0.69 7.32 - 7.47
3 6.63 - 6.63 0.58 - 0.68 7.21 - 7.31
7 5.17 - 6.08 0.75 - 0.75 5.92 - 6.83
14 1.98 - 2.27 0.39 - 0.40 2.66 - 2.38
2 6 0 20.0 - 21.6 1.56 - 1.74 21.7 - 23.2
3 20.4 - 23.0 1.56 - 1.78 22.2 - 24.6
7 14.0 - 16.7 1.70 - 1.70 15.7 - 18.4
14 4.92 - 5.14 0.80 - 0.96 5.7 - 6.1
1 9 0 14.5 - 16.0 1.78 - 1.82 16.3 - 17.8
3 16.0 - 17.4 1.40 - 1.60 17.4 - 19.0
7 10.1 - 13.2 1.24 - 1.27 11.3 - 14.5
14 2.48 - 2.56 0.75 - 0.76 3.24 - 3.31
2 9 0 28.3 - 30.3 3.46 - 3.59 32.1 - 33.9
3 23.5 - 25.3 2.33 - 2.49 23.8 - 27.8
7 21.6 - 24.9 2.47 - 2.59 24.1 - 27.5
14 5.46 - 6.57 1.11 - 1.45 6.57 - 8.02
Trimmings 2 6 0 45.4 - 47.9 2.17 - 2.25 47.7 - 50.1
3 45.4 - 52.9 2.05 - 2.23 47.5 - 55.1
7 20.8 - 40.4 1.38 - 1.65 22.2 - 42.1
14 14.6 - 16.1 1.05 - 1.21 15.7 - 17.3
TABLE 7. Predicted maximum+ and actual residues of acephate
+ methamidophos metabolite in some vegetables
Crop Total Residue,
Application Days after mg/kg
Rate, last Calculated+
Crop kg a.i./ha No. treatment maximum+ Found1
Broccoli 1 3-9 14 5.47 3.80
(max.25%
trimming) 2 6-9 14 9.89 8.02
Brussels 1 8 3 4.79 3.92
sprouts
(max. 15% 2 8 3 12.93 10.18
trimming)
Cabbage 1 9 3 7.29 2.51
(max. 20%
trimming) 2 9 3 12.82 6.38
Cauliflower 1 7-9 3 6.85 2.30
(max.50%
trimming) 2 7-9 3 7.90 4.36
Lettuce 1 6-8 3 7.35 3.29
(max. 50%
trimming) 2 6-8 3 16.17 5.54
+ Based on results from trimmed heads and trimmings, assuming maximum
possible trimming.
1 Reported results on heads trimmed according to commercial practice.
FATE OF RESIDUES
In animals
The metabolic fate of acephate in rats has been described above
("Biotransformation"). Proposed metabolic pathways in mammals are
shown in the same section (Figure 1).
TABLE 8. Residues of acephate and its metabolite methamidophos in citrus fruits
Application Days after Residues, mg/kg
Concentration last Acephate Methamidophos
Country Crop a.i. No. treatment Peel Flesh Peel Flesh
New Zealand Oranges 0.075% 6 1 9.0 0.6 0.98 0.01
(25% peel) 8 9.0 0.7 0.8 0.3
15 7.6 0.7 0.9 0.09
24 5.3 0.6 0.6 n.d.
Grapefruit 0.075% 6 1 2.9 3.0 n.d. 0.2
(30% peel) 8 3.1 1.9 0.2 0.3
15 2.6 3.6 0.2 0.2
24 1.4 5.4 n.d. 0.2
Whole fruit Whole fruit
U.S.A. Oranges 0.06% 2-3 20-25 0.33 - 1.1 0.06 - 0.10
40 0.19 - 1.5 0.02 - 0.08
100 0.00 - 0.00 0.00 - 0.00
Lemons 0.06% 3 20-25 0.08 - 0.18 0.02 - 0.03
0.12% 3 20-25 0.42 - 0.44 0.05 - 0.06
Tangeloes 0.06% 3 20 1.4 - 1.6 0.16 - 0.17
Dairy cows and pigs
9 dairy cows were fed at levels of 3, 10 and 30 ppm of acephate
+0.6, 2 and 6 ppm of its metabolite methamidophos in the diet for 30
days. The ratio of acephate to methamidophos was 5:1 because this is
typical of the ratio in most field crops after treatment with
acephate.
The lowest dosage did not give detectable residues in the cows'
milk. Milk from the higher dosed animals, however, contained between
0.1 and 0.2% of the daily doses and at levels which rapidly reached a
plateau where they remained during the 30 days treatment period.
Average residues found in the milk are shown in Table 9. At withdrawal
of the daily intake, residues in the milk decreased rapidly. By the
morning of the second day after withdrawal neither acephate nor its
metabolite could be observed in the milk.
Some individual cows were sacrificed after 3-4 weeks of feeding
with acephate and its metabolite. They showed significant but moderate
levels of residues in various tissues (Table 10). The highest residue
of acephate was 0.57 mg/kg in the kidney of an animal at the highest
dose level, and no tendency to accumulation in tissues was noted. 6
days after withdrawal, no residues could be detected in liver, kidney,
heart, muscle or fat.
TABLE 9. Average1 residues of acephate and its metabolite
methamidophos in cows' milk
Dose level Residue in milk
(ppm in dry feed) mg/kg ± S.D.1
Acephate Methamidophos Acephate Methamidophos
3 0.6 0.00 0.00
10 2 0.09 ± 0.005 0.00
30 6 0.27 ± 0.06 0.022 ± 0.006
1 Average for 30 days of three cows per dose level.
Essentially the same results were obtained by feeding acephate
and its metabolite to pigs. A linear relationship could be established
between dose levels and tissue residues. The residues ranged from 0.15
to 0.58 mg/kg of total residue at the high dose level (i.e. 30 ppm of
acephate + 6 ppm of metabolite) in tissues which were analysed after 4
weeks of feeding, namely: muscle, liver, kidney, subcutaneous and
peritoneal fat, heart and brain. In pigs as in cows, the residues were
undetectable in all tissues in less than 6 days after withdrawal of
the diet.
Chicken and quail
Tissue samples and eggs were taken from chickens and quail which
had been subjected to acephate feeding in subacute and chronic
toxicological studies. Three dose levels were followed: 3, 10 and 30
ppm of acephate in the diet for 92-140 days. No residues were detected
in any of the experiments in the fat, kidney or liver of the birds. In
eggs and muscle tissue, low levels of acephate were observed at the 10
and 30 ppm feeding levels, namely 0.07 and 0.19 mg/kg respectively in
chicken eggs, 0.16 and 0.31 mg/kg in quail eggs, 0.01 and 0.02 mg/kg
in chicken muscle and 0.01 and 0.04 mg/kg in quail muscle (Table 11).
At the high feeding levels in these studies, trace amounts of
methamidophos (OS-dimethyl phosphoramidothioate), which is otherwise
recognised as a plant metabolite only, were observed in muscles as
well as in eggs.
In plants
Studies using 14C-labelled acephate show that the compound is
readily degraded by plants such as bean seedlings, tomato and cabbage.
The half-life of acephate in such greenhouse-grown plants has been
shown to be of the order of 5-10 days. Among several theoretically
possible metabolites only one, methamidophos or OS-dimethyl
phosphoramidothioate, is found to be of practical significance.
Another of the possible metabolites is believed to be present although
it could not be analytically observed. This is OS-dimethyl
phosphorothioate, which is thought to be transitional step in the
complete breakdown to phosphate. The suggested metabolic pathways of
acephate in plants are shown in Figure 2.
 TABLE 10. Residues of acephate and its metabolite methamidophos in cows' tissues
Dose level, acephate + methamidophos (ppm in dry feed)
3 + 0.6 10 + 2 30 + 6
Tissue Acephate Methamidophos Acephate Methamidophos Acephate Methamidophos
Liver 0.00 0.00 0.00 0.00 0.08 0.00
Heart 0.03 0.00 0.10 0.01 0.32 0.06
Kidney 0.03 0.00 0.21 0.01 0.57 0.05
Muscle 0.03 0.00 0.08 0.00 0.28 0.04
Subcutaneous fat 0.00 0.00 0.03 0.00 0.13 0.02
Peritoneal fat 0.00 0.00 0.02 0.00 0.04 0.00
Limits of detection: acephate 0.01 mg/kg
methamidophos 0.005 mg/kg
TABLE 11. Acephate residues in muscle tissue and eggs from chickens
and quail
Chickens Quail
Feeding Days Residues
level(ppm of mg/kg Days of Residues, mg/kg
in diet) feeding Muscle Eggs feeding Muscle Eggs
3 7 - 0.00 148 - -
10 7 0.01 0.07 148 0.01 0.14-0.19
30 7 0.12 0.19 148 0.04 0.28-0.34
Acephate is readily translocated from soil to plants. Tracer
studies show that it is taken up and nearly quantitatively moved
through the roots and throughout the entire plant into the foliage.
For example, 93-98% of the total residue in radish plants was found to
be located in the foliage about 28 days after a soil treatment, with
acephate. Translocation in the other direction, i.e. from the leaves
to the roots or other parts does not take place.
In soil
The fate of acephate in soil has been extensively studied in both
the laboratory and field.
Using S-methyl-14C-acephate for soil treatment, it has been
shown that 14CO2 is released as the acephate decreases. Further, the
metabolite methamidophos is regularly observed. This is the same
metabolite as seen in plants, and it has been noted that it does not
concentrate in soils as it is rapidly degraded. At higher levels of
fortification a further soil metabolite, methyl acetylphosphoramidate
has been identified. Independent studies of this compound indicate
that it has a half-life in soil of about 11 days.
Quantitatively, the decomposition of acephate in soils is
relatively rapid. When 9 soils were studied under laboratory
conditions it was extremely rapid for 8 of them (clay, loams, and
sandy soils) in which half-lives were from 0.5 to 4 days. In the
ninth, a muck soil, decomposition was slower with a half-life of 6-13
days, generally with the higher values associated with dry soils.
Usually half-lives under field conditions are slightly longer
than those obtained in laboratory studies. Data have been presented
from 18 soil tests in 6 major crop producing areas in the United
States indicating relatively rapid declines with half-lives varying
TABLE 12. Carry-over of acephate residues to second crop in crop rotation
Application Residue, mg/kg
First Rate A*, Second B,** Methamidophos
crop kg a.i./ha No. days crop days Acephate metabolite
Cabbage 2 5 14 Radish 42 0.00-0.02 0.00-0.00
Mustard 42 0.00-0.00 0.00-0.00
tops
Lettuce 1 8 20 Lettuce 76 0.00-0.00 0.00-0.00
Beans 85 0.00-0.00 0.00-0.00
Carrots, 99 0.00-0.00 0.00-0.00
roots
Carrots, 99 0.00-0.00 0.00-0.00
tops
Cabbage 1 6 11 Mustard 68 0.03-0.07 0.00-0.00
greens
*A: interval from last treatment to planting of following crop.
**B: interval from last treatment to harvest of following crop.
from 2 to 15 days. Maximum applications in these tests were 9
treatments at 1 and 2 kg/ha and no residues of acephate or
methamidophos could be detected 60 days after the last application.
The lowest detectable levels were 0.01 mg/kg of acephate and 0.005
mg/kg of methamidophos.
The decomposition of acephate in soil has been shown to be mainly
biological in nature. For instance, by sterilization of the soil the
otherwise rapid decline in residues is practically halted. On the
other hand, no adverse effects from soil treatments up to 3 x 20 mg/kg
could be detected on fungal, bacterial or actinomycetes populations,
nor were respiration, ammonification, nitrification or sulphur
oxidation rates affected in any of 3 tested soils.
Earthworms have been studied and found not to concentrate or
store acephate or any of its metabolites in their bodies, and it is
concluded from the study that acephate in soils is not metabolized by
earthworms.
As might be expected, the rapid degradation of acephate in soil
will generally prevent any carry-over from one series of applications
to a following crop. This has been confirmed both in fast crop
rotations and with aged treated soils using lettuce, carrots and other
vegetables as the second crop (Table 12).
Leaching in soils
In laboratory studies using column leaching or soil thin-layer
techniques, it has been clearly shown that acephate and its metabolite
methamidophos are readily moved by water through soil with little, if
any, retention by the soil particles. This is in contrast to the
behaviour of weathered or aged residues. 20 days after soil treatment
at 2 mg/kg no leaching could be detected, while 15% of the applied
14C remained in the soil incorporated in natural soil constituents
after metabolism. This means that leaching into ground water is a
possibility to be considered, at least in extreme cases of high
dosages and when measured immediately after treatment. In one such
case, 0.06 mg/kg of acephate and 0.02 mg/kg of methamidophos were
observed in water sampled in a depth of 15-30 cm.
Likewise, acephate and methamidophos could be moved by run-off
water and detected in both water and soil particles which were
collected at the edge of a vegetable field and analysed separately.
After spraying with 1 kg/ha followed by overhead irrigation
corresponding to 5 cm of rain, residues in the run-off water varied
from 0.04-0.09 mg/kg in one experiment and up to 0.32 mg/kg in
another.
In water
Acephate is found to be relatively stable in water. Hydrolysis in
neutral and near-neutral solutions takes place with a half-life of
about 50 days at 21°C with the formation of
OS-dimethylphosphorothioate. This product is stable at acid but not
alkaline pH. Under alkaline conditions it forms S-methyl
phosphorothioate. Demethylacephate (S-methyl
acetylphosphoramidothioate) is also formed in neutral water. The major
plant and soil metabolite, methamidophos, is not formed in water by
direct hydrolysis. The presence of this metabolite in plants etc. is
therefore due to biochemical mechanisms.
Acephate in water is stable to sunlight which, therefore, cannot
be a major factor in the loss of acephate in the field.
In two field experiments, acephate was studied in natural ponds.
Acephate was added to the water at about 0.1 mg/kg and its fate was
followed by analysis of water, bottom mud and submerged vegetation for
44 days. Some uptake of acephate in bottom mud and vegetation
occurred. A rapid degradation, however was noted shortly afterwards
with half-lives varying from 3 to 10 days in the mud and in
vegetation. The results from one of these studies are summarised in
Table 13.
In the environment
The water solubility of acephate and its possible movement in
leaching and run-off waters etc. have attracted special attention and
the effects of acephate on microorganisms and fish have been followed
in several special studies.
TABLE 13. Residues in a natural pond (Iowa) after treatment
of the water with 0.1 mg acephate per litre
Residues in sample material, mg/kg1
Days after Submerged
treatment Pond water Bottom mud vegetation
0 0.11-0.14 0.04-0.03 0.18-0.52
3 0.09-0.10 0.04-0.06 0.17-0.23
9/10 0.08-0.08 0.00-0.00 0.09-0.09
23/24 0.04-0.04 0.00-0.00 0.04-0.04
44/45 0.00-0.03 0.00-0.00 0.00-0.00
1 No methamidophos metabolite was detected in this study.
Cylindrotheca fusiformis is a marine diatom which is known as
part of basic food chains. Tests show that this organism does not
concentrate acephate or its metabolite methamidophos from culture
solutions containing 1-100 mg/kg of acephate and 1-10 mg/kg of the
metabolite.
The fresh water species, Daphnia magna, is also part of food
chains. In studies with water containing 1 mg/l of acephate and 0.1
mg/1 of methamidophos, adult Daphnia behaved similarly to the marine
diatom, i.e. the concentrations of the compounds in the organisms
remained practically equal to their concentrations in the water
without any indication of further build-up. In parallel experiments
for comparison, DDT was found to concentrate 1000-7000 times in
diatoms and 550 times in Daphnia.
Bluegill sunfish were exposed to 1.0 or 0.01 mg/l of acephate for
35 days followed by a 14 days period in an uncontaminated water
system. 7-10 days after the exposure started, relatively constant
levels of acephate of the order of 5-10 times the concentration in the
water were reached. Thereafter, no further accumulation was observed
and after removal of the fish a rapid elimination of acephate from
their tissues occurred. Within 3 days more than 50% had disappeared.
In none of these experiments were any toxic effects from acephate
on the organisms under study observed.
These observations have been further substantiated in a special
model ecosystem including both a terrestrial and a fresh water aquatic
phase. The following kinds of organisms were involved in the study:
algae, fresh water plants, water flea, mosquito, snail, clam, crab,
mosquito fish, sorghum seedlings and a species of caterpillar. After
treatment with acephate in the model system none of these organisms
accumulated acephate or its metabolites or concentrated them in any of
the tissues.
It was concluded from the studies that acephate would not persist
in the environment and could safely be used in and near aquatic
systems.
In processing and cooking
Commercial processing of ripe tomatoes into canned fruits, puree
and juice reduced the total residue of acephate and its plant
metabolite methamidophos. The decrease was found to be about 3-7 fold
from fresh to processed products in a series of experiments, with no
significant difference between canned tomato, tomato puree and tomato
juice, as shown in Table 14.
These results indicate that acephate residues in tomatoes are not
confined to the outer fruit-layers and the peel. Owing to the systemic
nature of the insecticide a certain amount penetrates into the fruit
flesh. This is further confirmed by direct separate analyses of the
peel and flesh of both tomatoes and potatoes. Table 15 shows that in
potatoes both acephate and its metabolite methamidophos are evenly
distributed throughout the potato. In the tomato penetration into the
interior parts also takes place, although to a smaller extent as there
is a lower concentration in the pulp than in the peel.
Washing with water was not effective in removing residues of
acephate from vegetables (Table 16), which is to be expected since
residues are to a great extent internal. Boiling for 30 minutes
removed up to 60% of the acephate and methamidophos from Brussels
sprouts, however, owing to the water-solubility of the compounds. The
extracted residue was fully recovered in the boiling water.
Storage for up to 3 months at -20°C of various processed products
containing acephate, e.g. canned tomatoes, blanched and frozen
Brussels sprouts and potato tubers, has been shown not to change the
residue level.
Further examples of acephate behavior during the processing of
cotton seed, soybeans and sugar beets have been mentioned above.
METHODS OF RESIDUE ANALYSIS
Analytical methods based on thin-layer chromatography and on
gas-liquid chromatography for the final determination of acephate have
been reported (Chevron, 1972). The principal plant and soil metabolite
methamidophos (0S-dimethyl phosphoramidothioate) is determined
simultaneously in these methods.
The methods are described for animal and vegetable products,
including oily and dry crop samples, and for soil. They involve an
extraction with ethyl acetate, with acetonitrile/hexane partition for
samples containing fat and oil, and then clean-up by silicic acid
column chromatography with 10% methanol in ether as eluant. In its
most recent development the GLC determination is with thermionic
detection (Chevron, 1974).
The limits of determination reported for the GLC methods are
about 0.01 mg/kg for acephate in various substrates and from
0.005-0.01 for methamidophos.
Acephate has been reported to be recovered by the recently
published multi-residue method of Luke et al. (1975) in which acetone
extraction is followed by partition between methylene chloride and
petroleum ether to remove water. The recoveries of acephate in this
procedure are better than 80%.
TABLE 14. Acephate and its metabolite methamidophos in fresh and processed tomatoes
Application Days after Residue, mg/kg
Rate, last
Product kg a.i./ha No. treatment Acephate Methamidophos Total
Fresh ripe 1 6 7 1.7 0.21-0.30 1.91
tomato 7 7 1.5-2.0 0.35-0.41 1.85-2.41
2 6 7 3.2-3.7 0.58-0.69 3.89-4.28
7 7 2.6-3.5 0.55-0.77 3.15-4.27
Canned 1 6 7 0.46-0.57 0.18-0.21 0.64-0.78
tomato 7 7 0.44-0.47 0.25-0.26 0.69-0.73
2 6 7 1.1-1.3 0.39-0.42 1.49-1.72
7 7 0.82-0.98 0.45-0.55 1.27-1.53
Tomato 1 6 7 0.51-0.57 0.21-0.24 0.72-0.81
puree 7 7 0.71-0.71 0.25-0.26 0.96-0.97
2 6 7 1.3-1.3 0.40-0.42 1.70-1.72
7 7 1.1-1.2 0.44-0.46 1.54-1.66
Tomato 1 6 7 0.63-0.71 0.23-0.23 0.86-0.94
juice 7 7 0.79-0.81 0.31-0.33 1.12-1.12
2 6 7 1.2-1.4 0.43-0.43 1.63-1.83
7 7 1.2-1.3 0.53-0.55 1.73-1.85
TABLE 15. Acephate residues in peel and pulp of potatoes and tomatoes
Days after
last Residue, mg/kg
Crop part Treatment treatment Acephate Methamidophos
Potato
peel 1-2 kg/ha 3 0.24-0.86 0.03-0.06
pulp - 3 0.34-0.87 0.02-0.04
peel - 14 0.24-0.25 0.03-0.04
pulp - 14 0.24-0.28 0.03-0.03
Tomato
peel 1-2 kg/ha 3 6.8-9.7 0.57-0.78
pulp - 3 1.5-2.2 0.38-0.50
peel - 14 2.4-2.5 0.56-0.60
pulp - 14 1.1-1.2 0.38-0.40
Further validation of the GLC methods for acephate is reported to
be in progress.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the meeting are shown in Table
17.
APPRAISAL
Acephate is a relatively new organophosphorus insecticide with
both contact and systemic action on sucking and biting insects. Since
its first marketing in 1970/71 it has been registered in many
countries for both horticultural and agricultural crop protection.
Technical acephate is described as a chemical of 80-90% purity of
which the main impurities are known. It is usually formulated as 50%
or 75% soluble powders, low concentration dusts and 1.5% granules and
it is often applied in repeated pre-harvest treatments at the rate of
1-2 kg/ha or 0.05-0.1% spray dilutions.
The residue data available are mainly obtained from experiments
and supervised trials made under North American climatic conditions,
in a few cases supplemented from other countries. Most of the data are
from trials under practical conditions which are likely to represent
the results of good agricultural practice.
The systemic nature of acephate is demonstrated by a significant
penetration of residues into the flesh of fruit and the internal parts
of plants such as tomatoes, potatoes and citrus. It shows relatively
low persistence.
Uptake from soils and ready translocation through plants to the
foliage occurs. The metabolism of acephate has been studied and it is
suggested that a complete degradation into phosphates and CO2 may
take place leading to the incorporation of these compounds into plant
constituents. A major metabolite, 0S-dimethyl phosphoramidothioate is
considered to represent a transitional step. This metabolite is itself
the insecticide methamidophos, and it is regularly found as a part
(10-15%) of acephate residues in plant material.
The fate of acephate residues when fed to livestock has been
investigated. Continued feeding gave rise to residues which settled at
low levels in meat, milk and eggs as well as in most tissues. In all
cases examined, viz cow, pig, chicken and quail, residues
disappeared rapidly becoming undetectable within 1 to 6 days of the
rapid withdrawal of acephate feeding.
Owing to the water-soluble character of acephate its fate in
different environmental systems, particularly in soil and water, has
been thoroughly investigated. In soils, degradation is relatively
rapid and follows the pathways demonstrated in metabolism. Some
leaching through the upper soil layers can be traced in extreme cases.
Degradation of acephate in the various components of different
ecosystems is relatively fast and no signs of accumulation or
concentration in any tissues of living organisms have been found.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for the specific determination
of acephate, although full validation of the methods has not yet been
published.
TABLE 17. National tolerances reported to the Meeting
Tolerance, Pre-harvest
Country Crop mg/kg interval, days
Australia Cauliflower, cabbage,
lettuce 10
Brussels sprouts, broccoli,
tomatoes 6
Cottonseed 2.5
Soybeans 1
Potatoes 0.5
Fat of meat, meat and milk 0.1
Netherlands Brassicas 1 (+ 0.1 mg/kg methamidophos)
Potato 0.02 (+ 0.01 mg/kg methamidophos)1
New Zealand Brassicas 2.0
Switzerland Pome fruit, stone fruit 2.5
Vegetables 1.5
U.S.A. Celery,* 10 21
Lettuce (head)* 10
Cottonseed meal 8
Cottonseed hulls, bell peppers*
soybean meal 4
Beans(succulent and dry)* 3 14
Cottonseed 2 21
Almonds, soybeans 1 35,14
Meat, fat, meat by products of cattle,
goats, hogs, horses, poultry & sheep 0.1
Milk, eggs 0.1
1 At or about the limit of determination.
* Not more than 1 mg/kg of OS-dimethyl phosphoramidothioate.
RECOMMENDATIONS
The following maximum residue limits are recommended for
acephate. The plant metabolite 0S-dimethyl phosphoramidothioate is the
insecticide methamidophos for which separate recommendations are made
(see "Methamidophos").
Pre-harvest intervals on which
Commodity Limit recommendations are based
mg/kg days
Alfalfa 10 21
Lettuce 10 14
Sugar beet leaves 10 21
Broccoli 5 14
Brussels sprouts 5 14
Cabbage 5 14
Cauliflower 5 14
Citrus 5 7
Tomatoes 5
Cottonseed 2 21
Potatoes 1
Soybeans 1 14
Sugar beets 1
Meat and fat of cattle,
pigs and poultry 0.1
Milk 0.1
Eggs 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the metabolic fate of acephate,
preferably in non-rodent species.
2. Further studies to elucidate the contribution of acephate and
methamidophos alone or in combination in depressing
cholinesterase activity.
3. Validation of methods of residue analysis for regulatory
purposes, which is reported to be in progress.
REFERENCES
Anonymous. Human exposure reports from field application
1976a of orthene by research personnel. Unpublished reports
compiled by Chevron Chemical Company,. submitted to the
World Health Organization by Chevron Chemical Company.
Anonymous. Human exposure reports from operation of orthene
1976b pilot plant and formulation of orthene 75S. Unpublished
reports compiled by Chevron Chemical Company, submitted
to the World Health Organization by Chevron Chemical
Company.
Arnold, D., Kennedy, G., and Keplinger, M. Mutagenic study with
1971 orthene in albino mice. Unpublished report from
Industrial Bio-Test Laboratories, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S125 acute oral toxicity of
1968a RE9169 (SX 196) in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-86 acute oral toxicity
1968b of monitor technical in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, Jr. S-98 acute dermal toxicity
1968c of monitor technical. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-126 acute dermal
1969 toxicity of RE9169 (SX 198) in rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Cavalli, R., Christensen, H., and Spence, J. S-183 the acute oral
1970 toxicity of RE14.071. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-160 acute dermal toxicity of
1970a RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-181 acute oral toxicity of ortho
1970b RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-174 effect of RE12,420 on the
1970c acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. The acute inhalation toxicity of
1970d RE12,420. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of RE12,420 on the
1970e acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of repeated doses of RE12,420
1970f on cholinesterase activity in rats. Unpublished report
from Standard Oil Company of California, submitted to
the World Health Organization by Chevron Chemical
Company.
Cavalli, R., and Spence, J. Skin sensitization potential of RE12,420
1970g (75% SS). Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Skin sensitization potential of
1970h RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Chevron Chemical Company. Comparison of methods RM-12B with RM-12
1972 and RM-12A. File 740.01, January 21.
Chevron Chemical Company. Orthene and themmetabolite ortho 9006.
1974 Residue analysis by thermionic gas chromatography. File
740.01, April 25.
Chevron Chemical Company. Acephate (OrtheneR). Identity and
1976 residues in food. Vol. 1, 2 and 3. Information, data
and laboratory reports covering the research period
1971-1973 and submitted to the Joint Meeting 1976. On
file in FAO, Plant Protection Division.
Coquet, B. Determination of the lethal dose 50% by intra-peritoneal
1972 route in the male rat of ortho 12,420 technical (85%
acephate). Unpublished report from Centre de Recherche
et d'Elevage des Oncins, submitted to the World Health
Organization by Chevron Chemical Company.
Crossley, J., and Tutass, H. Metabolism of monitor insecticide
1969 by rats. Unpublished report from Ortho, Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Ebbens, K., Mastri, C., Keplinger, M., and Fancher, O. Skin
1971 sensitization test with orthene technical-SX-284 in
albino guinea pigs. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Edds, G., and Bradley, R. Acute toxicity study - white Leghorn
1971 chicks. Unpublished report from the University of
Florida, Gainesville, submitted to the World Health
Organization by Chevron Chemical Company.
Elliott, C., Shadberg, K., Goode, J., and Keplinger, M. Acute dust
1972 inhalation toxicity study with orthene-85% technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Toxicity reproduction
1972a and residue study with orthen technical SX-331 in white
Leghorn chickens. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Neurotoxicity study
1972b with orthene technical, SX.284 in adult hens.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Garofalo, M., Palazzolo, R., and Sanders, R. A study of the effects
1973 of orthene and monitor on plasma and erythrocyte
cholinesterase activity in human subjects during
subacute oral administration. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to
the World Health Organization by Chevron Chemical
Company.
Haley, S., Plank, J., Wright, P., and Keplinger, M. Teratogenic
1971 study with orthene technical in albino rats.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Haley, S., Kennedy, G., and Keplinger, M. Three generation
1973 reproduction study with RE 12,420 technical in albino
rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Hartke, K., Burtner, G., Kennedy, G., and Keplinger, M. Two-year
1972 chronic oral toxicity study with RE 12,420 in beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
ICI, Australia. Data submitted through the Codex Contact Point,
1976 Canberra, Australia.
Kretchmar, B., Mastri, C., Keplinger, M., and Fancher, O. Acute
1971 oral toxicity study to determine the efficacy of
atropine sulfate and 2-PAM as antidotes for RE 12,420
technical intoxication in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Kretchmar, B., Mastri, C., and Keplinger, M. Acute oral potentiation
1972 study with monitor technical and orthene technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc. submitted to the World
Health Organization by Chevron Chemical Company.
Kruke, V., Narcisse, J., Cavalli, R., and Spence, J. Acute inhalation
1972a toxicity of orthene 75S. Unpublished report from
Standard Oil Company of California. submitted to the
World Health Organization by Chevron Chemical Company.
Kruke, V., Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 14-Day aerosol inhalation study in adult rats.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Ladd, R., Jenkins, D., Wright, P., and Keplinger, M. Teratogenic
1972 study with orthene technical in albino rabbits.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Lee, H. Metabolism of orthene in rats. Unpublished report from Ortho
1972 Division of Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Lindberg, D., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 study with orthene in Beagle dogs. Unpublished study
from Industrial Bio-Test Laboratories, Inc., submitted
to the World Health Organization by Chevron Chemical
Company.
Luke, M.A., Froberg. J.E., and Masumoto. H.T. Extraction and
1975 cleanup of organochlorine, organophosphate,
organonitrogen, and hydrocarbon pesticides in produce
for determination by gas-liquid chromatography. J. Ass.
off. analyt. Chem., 58:1020-1026.
Magellona, E.D. Report from the Codex Contact Point, Philippines
1976 on acephate uses on rice and cabbage.
Mastalski, K., Jenkins, D., Keplinger, M., and Fancher, O. Acute
1970 oral toxicity study on RE 12,420 technical in Beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Mastri, C., Keplinger, M., and Fancher, O. Acute oral toxicity
1970a study on RE 12,420 technical in white mice. Unpublished
report from Industrial BioTest Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Mastri, C., Keplinger, M., and Francher, O. Acute oral and dermal
1970b toxicity studies on RE 12,420 75% spray suspension
formulation. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE18,421. Unpublished report from
1972a Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,245. Unpublished report from
1972b Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,246. Unpublished report from
1972c Chevron Chemical Company submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. Eye irritation potential
1971a of orthene technical, orthene 75S (CC-2153) and orthene
75S (CC-2152). Unpublished report from Standard Oil
Company of California, submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-269 the acute oral
1971b toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-270 the acute dermal
1971c toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The acute oral toxicity
1972a of orthene 75-S to adult male rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The skin irritation
1972b potential of orthene 75-S. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
New Zealand. Submission on acephate from the National Codex
1976 Committee of New Zealand.
Pack, D. The metabolism of RE9169 and RE14,071 in rats. Unpublished
1972a report from Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Pack, D. Orthene occupational exposure-pilot plant and formulations.
1972b Unpublished report from Ortho Division of Chevron
Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Pack, D. Orthene insecticide occupational exposure - field station
1972c personnel. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Plank, J., and Keplinger, M. Two-year chronic oral toxicity study
1973 with RE12,420 technical in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Plank, J., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 subacute oral toxicity study with RE12,420 technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Plank, J., Wright, P., and Keplinger, M. 90-Day cholinesterase
1972 study with ortho 12,420 technical in albino rats.
Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. The
1972a acute oral toxicity to rats of orthene in combination
with five other cholinesterase inhibition of materials.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Reyna, M., Kennedy, G., and Keplinger, M. 18-Month carcinogenic
1973 study with RE12,420 technical in Swiss white mice.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 21-Day subacute dermal study in rabbits with
orthene-75S. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972c S-184 the acute dermal toxicity of RE14,071.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972d S-533. The acute dermal toxicity of RE18,421.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. S-514
1972e The acute dermal toxicity of RE17,245. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972P S-515 the acute dermal toxicity of RE17,246.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Swencicki, R., and Hartz, W. Orthene human exposure study
1972 clinical evaluation. Unpublished study from Standard
Oil Company of California, Submitted to the World
Health Organization by Chevron Chemical Company.
Tucker, B. Acetylcholinesterase inhibition of orthene and
1972 ortho 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
TABLE 10. Residues of acephate and its metabolite methamidophos in cows' tissues
Dose level, acephate + methamidophos (ppm in dry feed)
3 + 0.6 10 + 2 30 + 6
Tissue Acephate Methamidophos Acephate Methamidophos Acephate Methamidophos
Liver 0.00 0.00 0.00 0.00 0.08 0.00
Heart 0.03 0.00 0.10 0.01 0.32 0.06
Kidney 0.03 0.00 0.21 0.01 0.57 0.05
Muscle 0.03 0.00 0.08 0.00 0.28 0.04
Subcutaneous fat 0.00 0.00 0.03 0.00 0.13 0.02
Peritoneal fat 0.00 0.00 0.02 0.00 0.04 0.00
Limits of detection: acephate 0.01 mg/kg
methamidophos 0.005 mg/kg
TABLE 11. Acephate residues in muscle tissue and eggs from chickens
and quail
Chickens Quail
Feeding Days Residues
level(ppm of mg/kg Days of Residues, mg/kg
in diet) feeding Muscle Eggs feeding Muscle Eggs
3 7 - 0.00 148 - -
10 7 0.01 0.07 148 0.01 0.14-0.19
30 7 0.12 0.19 148 0.04 0.28-0.34
Acephate is readily translocated from soil to plants. Tracer
studies show that it is taken up and nearly quantitatively moved
through the roots and throughout the entire plant into the foliage.
For example, 93-98% of the total residue in radish plants was found to
be located in the foliage about 28 days after a soil treatment, with
acephate. Translocation in the other direction, i.e. from the leaves
to the roots or other parts does not take place.
In soil
The fate of acephate in soil has been extensively studied in both
the laboratory and field.
Using S-methyl-14C-acephate for soil treatment, it has been
shown that 14CO2 is released as the acephate decreases. Further, the
metabolite methamidophos is regularly observed. This is the same
metabolite as seen in plants, and it has been noted that it does not
concentrate in soils as it is rapidly degraded. At higher levels of
fortification a further soil metabolite, methyl acetylphosphoramidate
has been identified. Independent studies of this compound indicate
that it has a half-life in soil of about 11 days.
Quantitatively, the decomposition of acephate in soils is
relatively rapid. When 9 soils were studied under laboratory
conditions it was extremely rapid for 8 of them (clay, loams, and
sandy soils) in which half-lives were from 0.5 to 4 days. In the
ninth, a muck soil, decomposition was slower with a half-life of 6-13
days, generally with the higher values associated with dry soils.
Usually half-lives under field conditions are slightly longer
than those obtained in laboratory studies. Data have been presented
from 18 soil tests in 6 major crop producing areas in the United
States indicating relatively rapid declines with half-lives varying
TABLE 12. Carry-over of acephate residues to second crop in crop rotation
Application Residue, mg/kg
First Rate A*, Second B,** Methamidophos
crop kg a.i./ha No. days crop days Acephate metabolite
Cabbage 2 5 14 Radish 42 0.00-0.02 0.00-0.00
Mustard 42 0.00-0.00 0.00-0.00
tops
Lettuce 1 8 20 Lettuce 76 0.00-0.00 0.00-0.00
Beans 85 0.00-0.00 0.00-0.00
Carrots, 99 0.00-0.00 0.00-0.00
roots
Carrots, 99 0.00-0.00 0.00-0.00
tops
Cabbage 1 6 11 Mustard 68 0.03-0.07 0.00-0.00
greens
*A: interval from last treatment to planting of following crop.
**B: interval from last treatment to harvest of following crop.
from 2 to 15 days. Maximum applications in these tests were 9
treatments at 1 and 2 kg/ha and no residues of acephate or
methamidophos could be detected 60 days after the last application.
The lowest detectable levels were 0.01 mg/kg of acephate and 0.005
mg/kg of methamidophos.
The decomposition of acephate in soil has been shown to be mainly
biological in nature. For instance, by sterilization of the soil the
otherwise rapid decline in residues is practically halted. On the
other hand, no adverse effects from soil treatments up to 3 x 20 mg/kg
could be detected on fungal, bacterial or actinomycetes populations,
nor were respiration, ammonification, nitrification or sulphur
oxidation rates affected in any of 3 tested soils.
Earthworms have been studied and found not to concentrate or
store acephate or any of its metabolites in their bodies, and it is
concluded from the study that acephate in soils is not metabolized by
earthworms.
As might be expected, the rapid degradation of acephate in soil
will generally prevent any carry-over from one series of applications
to a following crop. This has been confirmed both in fast crop
rotations and with aged treated soils using lettuce, carrots and other
vegetables as the second crop (Table 12).
Leaching in soils
In laboratory studies using column leaching or soil thin-layer
techniques, it has been clearly shown that acephate and its metabolite
methamidophos are readily moved by water through soil with little, if
any, retention by the soil particles. This is in contrast to the
behaviour of weathered or aged residues. 20 days after soil treatment
at 2 mg/kg no leaching could be detected, while 15% of the applied
14C remained in the soil incorporated in natural soil constituents
after metabolism. This means that leaching into ground water is a
possibility to be considered, at least in extreme cases of high
dosages and when measured immediately after treatment. In one such
case, 0.06 mg/kg of acephate and 0.02 mg/kg of methamidophos were
observed in water sampled in a depth of 15-30 cm.
Likewise, acephate and methamidophos could be moved by run-off
water and detected in both water and soil particles which were
collected at the edge of a vegetable field and analysed separately.
After spraying with 1 kg/ha followed by overhead irrigation
corresponding to 5 cm of rain, residues in the run-off water varied
from 0.04-0.09 mg/kg in one experiment and up to 0.32 mg/kg in
another.
In water
Acephate is found to be relatively stable in water. Hydrolysis in
neutral and near-neutral solutions takes place with a half-life of
about 50 days at 21°C with the formation of
OS-dimethylphosphorothioate. This product is stable at acid but not
alkaline pH. Under alkaline conditions it forms S-methyl
phosphorothioate. Demethylacephate (S-methyl
acetylphosphoramidothioate) is also formed in neutral water. The major
plant and soil metabolite, methamidophos, is not formed in water by
direct hydrolysis. The presence of this metabolite in plants etc. is
therefore due to biochemical mechanisms.
Acephate in water is stable to sunlight which, therefore, cannot
be a major factor in the loss of acephate in the field.
In two field experiments, acephate was studied in natural ponds.
Acephate was added to the water at about 0.1 mg/kg and its fate was
followed by analysis of water, bottom mud and submerged vegetation for
44 days. Some uptake of acephate in bottom mud and vegetation
occurred. A rapid degradation, however was noted shortly afterwards
with half-lives varying from 3 to 10 days in the mud and in
vegetation. The results from one of these studies are summarised in
Table 13.
In the environment
The water solubility of acephate and its possible movement in
leaching and run-off waters etc. have attracted special attention and
the effects of acephate on microorganisms and fish have been followed
in several special studies.
TABLE 13. Residues in a natural pond (Iowa) after treatment
of the water with 0.1 mg acephate per litre
Residues in sample material, mg/kg1
Days after Submerged
treatment Pond water Bottom mud vegetation
0 0.11-0.14 0.04-0.03 0.18-0.52
3 0.09-0.10 0.04-0.06 0.17-0.23
9/10 0.08-0.08 0.00-0.00 0.09-0.09
23/24 0.04-0.04 0.00-0.00 0.04-0.04
44/45 0.00-0.03 0.00-0.00 0.00-0.00
1 No methamidophos metabolite was detected in this study.
Cylindrotheca fusiformis is a marine diatom which is known as
part of basic food chains. Tests show that this organism does not
concentrate acephate or its metabolite methamidophos from culture
solutions containing 1-100 mg/kg of acephate and 1-10 mg/kg of the
metabolite.
The fresh water species, Daphnia magna, is also part of food
chains. In studies with water containing 1 mg/l of acephate and 0.1
mg/1 of methamidophos, adult Daphnia behaved similarly to the marine
diatom, i.e. the concentrations of the compounds in the organisms
remained practically equal to their concentrations in the water
without any indication of further build-up. In parallel experiments
for comparison, DDT was found to concentrate 1000-7000 times in
diatoms and 550 times in Daphnia.
Bluegill sunfish were exposed to 1.0 or 0.01 mg/l of acephate for
35 days followed by a 14 days period in an uncontaminated water
system. 7-10 days after the exposure started, relatively constant
levels of acephate of the order of 5-10 times the concentration in the
water were reached. Thereafter, no further accumulation was observed
and after removal of the fish a rapid elimination of acephate from
their tissues occurred. Within 3 days more than 50% had disappeared.
In none of these experiments were any toxic effects from acephate
on the organisms under study observed.
These observations have been further substantiated in a special
model ecosystem including both a terrestrial and a fresh water aquatic
phase. The following kinds of organisms were involved in the study:
algae, fresh water plants, water flea, mosquito, snail, clam, crab,
mosquito fish, sorghum seedlings and a species of caterpillar. After
treatment with acephate in the model system none of these organisms
accumulated acephate or its metabolites or concentrated them in any of
the tissues.
It was concluded from the studies that acephate would not persist
in the environment and could safely be used in and near aquatic
systems.
In processing and cooking
Commercial processing of ripe tomatoes into canned fruits, puree
and juice reduced the total residue of acephate and its plant
metabolite methamidophos. The decrease was found to be about 3-7 fold
from fresh to processed products in a series of experiments, with no
significant difference between canned tomato, tomato puree and tomato
juice, as shown in Table 14.
These results indicate that acephate residues in tomatoes are not
confined to the outer fruit-layers and the peel. Owing to the systemic
nature of the insecticide a certain amount penetrates into the fruit
flesh. This is further confirmed by direct separate analyses of the
peel and flesh of both tomatoes and potatoes. Table 15 shows that in
potatoes both acephate and its metabolite methamidophos are evenly
distributed throughout the potato. In the tomato penetration into the
interior parts also takes place, although to a smaller extent as there
is a lower concentration in the pulp than in the peel.
Washing with water was not effective in removing residues of
acephate from vegetables (Table 16), which is to be expected since
residues are to a great extent internal. Boiling for 30 minutes
removed up to 60% of the acephate and methamidophos from Brussels
sprouts, however, owing to the water-solubility of the compounds. The
extracted residue was fully recovered in the boiling water.
Storage for up to 3 months at -20°C of various processed products
containing acephate, e.g. canned tomatoes, blanched and frozen
Brussels sprouts and potato tubers, has been shown not to change the
residue level.
Further examples of acephate behavior during the processing of
cotton seed, soybeans and sugar beets have been mentioned above.
METHODS OF RESIDUE ANALYSIS
Analytical methods based on thin-layer chromatography and on
gas-liquid chromatography for the final determination of acephate have
been reported (Chevron, 1972). The principal plant and soil metabolite
methamidophos (0S-dimethyl phosphoramidothioate) is determined
simultaneously in these methods.
The methods are described for animal and vegetable products,
including oily and dry crop samples, and for soil. They involve an
extraction with ethyl acetate, with acetonitrile/hexane partition for
samples containing fat and oil, and then clean-up by silicic acid
column chromatography with 10% methanol in ether as eluant. In its
most recent development the GLC determination is with thermionic
detection (Chevron, 1974).
The limits of determination reported for the GLC methods are
about 0.01 mg/kg for acephate in various substrates and from
0.005-0.01 for methamidophos.
Acephate has been reported to be recovered by the recently
published multi-residue method of Luke et al. (1975) in which acetone
extraction is followed by partition between methylene chloride and
petroleum ether to remove water. The recoveries of acephate in this
procedure are better than 80%.
TABLE 14. Acephate and its metabolite methamidophos in fresh and processed tomatoes
Application Days after Residue, mg/kg
Rate, last
Product kg a.i./ha No. treatment Acephate Methamidophos Total
Fresh ripe 1 6 7 1.7 0.21-0.30 1.91
tomato 7 7 1.5-2.0 0.35-0.41 1.85-2.41
2 6 7 3.2-3.7 0.58-0.69 3.89-4.28
7 7 2.6-3.5 0.55-0.77 3.15-4.27
Canned 1 6 7 0.46-0.57 0.18-0.21 0.64-0.78
tomato 7 7 0.44-0.47 0.25-0.26 0.69-0.73
2 6 7 1.1-1.3 0.39-0.42 1.49-1.72
7 7 0.82-0.98 0.45-0.55 1.27-1.53
Tomato 1 6 7 0.51-0.57 0.21-0.24 0.72-0.81
puree 7 7 0.71-0.71 0.25-0.26 0.96-0.97
2 6 7 1.3-1.3 0.40-0.42 1.70-1.72
7 7 1.1-1.2 0.44-0.46 1.54-1.66
Tomato 1 6 7 0.63-0.71 0.23-0.23 0.86-0.94
juice 7 7 0.79-0.81 0.31-0.33 1.12-1.12
2 6 7 1.2-1.4 0.43-0.43 1.63-1.83
7 7 1.2-1.3 0.53-0.55 1.73-1.85
TABLE 15. Acephate residues in peel and pulp of potatoes and tomatoes
Days after
last Residue, mg/kg
Crop part Treatment treatment Acephate Methamidophos
Potato
peel 1-2 kg/ha 3 0.24-0.86 0.03-0.06
pulp - 3 0.34-0.87 0.02-0.04
peel - 14 0.24-0.25 0.03-0.04
pulp - 14 0.24-0.28 0.03-0.03
Tomato
peel 1-2 kg/ha 3 6.8-9.7 0.57-0.78
pulp - 3 1.5-2.2 0.38-0.50
peel - 14 2.4-2.5 0.56-0.60
pulp - 14 1.1-1.2 0.38-0.40
Further validation of the GLC methods for acephate is reported to
be in progress.
NATIONAL TOLERANCES REPORTED TO THE MEETING
National tolerances reported to the meeting are shown in Table
17.
APPRAISAL
Acephate is a relatively new organophosphorus insecticide with
both contact and systemic action on sucking and biting insects. Since
its first marketing in 1970/71 it has been registered in many
countries for both horticultural and agricultural crop protection.
Technical acephate is described as a chemical of 80-90% purity of
which the main impurities are known. It is usually formulated as 50%
or 75% soluble powders, low concentration dusts and 1.5% granules and
it is often applied in repeated pre-harvest treatments at the rate of
1-2 kg/ha or 0.05-0.1% spray dilutions.
The residue data available are mainly obtained from experiments
and supervised trials made under North American climatic conditions,
in a few cases supplemented from other countries. Most of the data are
from trials under practical conditions which are likely to represent
the results of good agricultural practice.
The systemic nature of acephate is demonstrated by a significant
penetration of residues into the flesh of fruit and the internal parts
of plants such as tomatoes, potatoes and citrus. It shows relatively
low persistence.
Uptake from soils and ready translocation through plants to the
foliage occurs. The metabolism of acephate has been studied and it is
suggested that a complete degradation into phosphates and CO2 may
take place leading to the incorporation of these compounds into plant
constituents. A major metabolite, 0S-dimethyl phosphoramidothioate is
considered to represent a transitional step. This metabolite is itself
the insecticide methamidophos, and it is regularly found as a part
(10-15%) of acephate residues in plant material.
The fate of acephate residues when fed to livestock has been
investigated. Continued feeding gave rise to residues which settled at
low levels in meat, milk and eggs as well as in most tissues. In all
cases examined, viz cow, pig, chicken and quail, residues
disappeared rapidly becoming undetectable within 1 to 6 days of the
rapid withdrawal of acephate feeding.
Owing to the water-soluble character of acephate its fate in
different environmental systems, particularly in soil and water, has
been thoroughly investigated. In soils, degradation is relatively
rapid and follows the pathways demonstrated in metabolism. Some
leaching through the upper soil layers can be traced in extreme cases.
Degradation of acephate in the various components of different
ecosystems is relatively fast and no signs of accumulation or
concentration in any tissues of living organisms have been found.
Gas-chromatographic methods combined with appropriate extraction
and clean-up procedures are available for the specific determination
of acephate, although full validation of the methods has not yet been
published.
TABLE 17. National tolerances reported to the Meeting
Tolerance, Pre-harvest
Country Crop mg/kg interval, days
Australia Cauliflower, cabbage,
lettuce 10
Brussels sprouts, broccoli,
tomatoes 6
Cottonseed 2.5
Soybeans 1
Potatoes 0.5
Fat of meat, meat and milk 0.1
Netherlands Brassicas 1 (+ 0.1 mg/kg methamidophos)
Potato 0.02 (+ 0.01 mg/kg methamidophos)1
New Zealand Brassicas 2.0
Switzerland Pome fruit, stone fruit 2.5
Vegetables 1.5
U.S.A. Celery,* 10 21
Lettuce (head)* 10
Cottonseed meal 8
Cottonseed hulls, bell peppers*
soybean meal 4
Beans(succulent and dry)* 3 14
Cottonseed 2 21
Almonds, soybeans 1 35,14
Meat, fat, meat by products of cattle,
goats, hogs, horses, poultry & sheep 0.1
Milk, eggs 0.1
1 At or about the limit of determination.
* Not more than 1 mg/kg of OS-dimethyl phosphoramidothioate.
RECOMMENDATIONS
The following maximum residue limits are recommended for
acephate. The plant metabolite 0S-dimethyl phosphoramidothioate is the
insecticide methamidophos for which separate recommendations are made
(see "Methamidophos").
Pre-harvest intervals on which
Commodity Limit recommendations are based
mg/kg days
Alfalfa 10 21
Lettuce 10 14
Sugar beet leaves 10 21
Broccoli 5 14
Brussels sprouts 5 14
Cabbage 5 14
Cauliflower 5 14
Citrus 5 7
Tomatoes 5
Cottonseed 2 21
Potatoes 1
Soybeans 1 14
Sugar beets 1
Meat and fat of cattle,
pigs and poultry 0.1
Milk 0.1
Eggs 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies to elucidate the metabolic fate of acephate,
preferably in non-rodent species.
2. Further studies to elucidate the contribution of acephate and
methamidophos alone or in combination in depressing
cholinesterase activity.
3. Validation of methods of residue analysis for regulatory
purposes, which is reported to be in progress.
REFERENCES
Anonymous. Human exposure reports from field application
1976a of orthene by research personnel. Unpublished reports
compiled by Chevron Chemical Company,. submitted to the
World Health Organization by Chevron Chemical Company.
Anonymous. Human exposure reports from operation of orthene
1976b pilot plant and formulation of orthene 75S. Unpublished
reports compiled by Chevron Chemical Company, submitted
to the World Health Organization by Chevron Chemical
Company.
Arnold, D., Kennedy, G., and Keplinger, M. Mutagenic study with
1971 orthene in albino mice. Unpublished report from
Industrial Bio-Test Laboratories, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S125 acute oral toxicity of
1968a RE9169 (SX 196) in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-86 acute oral toxicity
1968b of monitor technical in rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, Jr. S-98 acute dermal toxicity
1968c of monitor technical. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., Hallesy, D., and Spence, J. S-126 acute dermal
1969 toxicity of RE9169 (SX 198) in rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Cavalli, R., Christensen, H., and Spence, J. S-183 the acute oral
1970 toxicity of RE14.071. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-160 acute dermal toxicity of
1970a RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-181 acute oral toxicity of ortho
1970b RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. S-174 effect of RE12,420 on the
1970c acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. The acute inhalation toxicity of
1970d RE12,420. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of RE12,420 on the
1970e acetylcholinesterase of rats. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Effect of repeated doses of RE12,420
1970f on cholinesterase activity in rats. Unpublished report
from Standard Oil Company of California, submitted to
the World Health Organization by Chevron Chemical
Company.
Cavalli, R., and Spence, J. Skin sensitization potential of RE12,420
1970g (75% SS). Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Cavalli, R., and Spence, J. Skin sensitization potential of
1970h RE12,420. Unpublished report from Standard Oil Company
of California, submitted to the World Health
Organization by Chevron Chemical Company.
Chevron Chemical Company. Comparison of methods RM-12B with RM-12
1972 and RM-12A. File 740.01, January 21.
Chevron Chemical Company. Orthene and themmetabolite ortho 9006.
1974 Residue analysis by thermionic gas chromatography. File
740.01, April 25.
Chevron Chemical Company. Acephate (OrtheneR). Identity and
1976 residues in food. Vol. 1, 2 and 3. Information, data
and laboratory reports covering the research period
1971-1973 and submitted to the Joint Meeting 1976. On
file in FAO, Plant Protection Division.
Coquet, B. Determination of the lethal dose 50% by intra-peritoneal
1972 route in the male rat of ortho 12,420 technical (85%
acephate). Unpublished report from Centre de Recherche
et d'Elevage des Oncins, submitted to the World Health
Organization by Chevron Chemical Company.
Crossley, J., and Tutass, H. Metabolism of monitor insecticide
1969 by rats. Unpublished report from Ortho, Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Ebbens, K., Mastri, C., Keplinger, M., and Fancher, O. Skin
1971 sensitization test with orthene technical-SX-284 in
albino guinea pigs. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Edds, G., and Bradley, R. Acute toxicity study - white Leghorn
1971 chicks. Unpublished report from the University of
Florida, Gainesville, submitted to the World Health
Organization by Chevron Chemical Company.
Elliott, C., Shadberg, K., Goode, J., and Keplinger, M. Acute dust
1972 inhalation toxicity study with orthene-85% technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Toxicity reproduction
1972a and residue study with orthen technical SX-331 in white
Leghorn chickens. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Fletcher, D., Jenkins, D., and Keplinger, M. Neurotoxicity study
1972b with orthene technical, SX.284 in adult hens.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Garofalo, M., Palazzolo, R., and Sanders, R. A study of the effects
1973 of orthene and monitor on plasma and erythrocyte
cholinesterase activity in human subjects during
subacute oral administration. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted to
the World Health Organization by Chevron Chemical
Company.
Haley, S., Plank, J., Wright, P., and Keplinger, M. Teratogenic
1971 study with orthene technical in albino rats.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Haley, S., Kennedy, G., and Keplinger, M. Three generation
1973 reproduction study with RE 12,420 technical in albino
rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Hartke, K., Burtner, G., Kennedy, G., and Keplinger, M. Two-year
1972 chronic oral toxicity study with RE 12,420 in beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
ICI, Australia. Data submitted through the Codex Contact Point,
1976 Canberra, Australia.
Kretchmar, B., Mastri, C., Keplinger, M., and Fancher, O. Acute
1971 oral toxicity study to determine the efficacy of
atropine sulfate and 2-PAM as antidotes for RE 12,420
technical intoxication in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Kretchmar, B., Mastri, C., and Keplinger, M. Acute oral potentiation
1972 study with monitor technical and orthene technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc. submitted to the World
Health Organization by Chevron Chemical Company.
Kruke, V., Narcisse, J., Cavalli, R., and Spence, J. Acute inhalation
1972a toxicity of orthene 75S. Unpublished report from
Standard Oil Company of California. submitted to the
World Health Organization by Chevron Chemical Company.
Kruke, V., Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 14-Day aerosol inhalation study in adult rats.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Ladd, R., Jenkins, D., Wright, P., and Keplinger, M. Teratogenic
1972 study with orthene technical in albino rabbits.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Lee, H. Metabolism of orthene in rats. Unpublished report from Ortho
1972 Division of Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Lindberg, D., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 study with orthene in Beagle dogs. Unpublished study
from Industrial Bio-Test Laboratories, Inc., submitted
to the World Health Organization by Chevron Chemical
Company.
Luke, M.A., Froberg. J.E., and Masumoto. H.T. Extraction and
1975 cleanup of organochlorine, organophosphate,
organonitrogen, and hydrocarbon pesticides in produce
for determination by gas-liquid chromatography. J. Ass.
off. analyt. Chem., 58:1020-1026.
Magellona, E.D. Report from the Codex Contact Point, Philippines
1976 on acephate uses on rice and cabbage.
Mastalski, K., Jenkins, D., Keplinger, M., and Fancher, O. Acute
1970 oral toxicity study on RE 12,420 technical in Beagle
dogs. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Mastri, C., Keplinger, M., and Fancher, O. Acute oral toxicity
1970a study on RE 12,420 technical in white mice. Unpublished
report from Industrial BioTest Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Mastri, C., Keplinger, M., and Francher, O. Acute oral and dermal
1970b toxicity studies on RE 12,420 75% spray suspension
formulation. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE18,421. Unpublished report from
1972a Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,245. Unpublished report from
1972b Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
McCasky, B. Acute toxicity of RE17,246. Unpublished report from
1972c Chevron Chemical Company submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. Eye irritation potential
1971a of orthene technical, orthene 75S (CC-2153) and orthene
75S (CC-2152). Unpublished report from Standard Oil
Company of California, submitted to the World Health
Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-269 the acute oral
1971b toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. S-270 the acute dermal
1971c toxicity of RE15,283. Unpublished report from Standard
Oil Company of California, submitted to the World
Health Organization by Chevron Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The acute oral toxicity
1972a of orthene 75-S to adult male rabbits. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Narcisse, J., Cavalli, R., and Spence, J. The skin irritation
1972b potential of orthene 75-S. Unpublished report from
Standard Oil Company of California, submitted to the
World Health Organization by Chevron Chemical Company.
New Zealand. Submission on acephate from the National Codex
1976 Committee of New Zealand.
Pack, D. The metabolism of RE9169 and RE14,071 in rats. Unpublished
1972a report from Chevron Chemical Company, submitted to the
World Health Organization by Chevron Chemical Company.
Pack, D. Orthene occupational exposure-pilot plant and formulations.
1972b Unpublished report from Ortho Division of Chevron
Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Pack, D. Orthene insecticide occupational exposure - field station
1972c personnel. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
Plank, J., and Keplinger, M. Two-year chronic oral toxicity study
1973 with RE12,420 technical in albino rats. Unpublished
report from Industrial Bio-Test Laboratories, Inc.,
submitted to the World Health Organization by Chevron
Chemical Company.
Plank, J., Wright, P., Keplinger, M., and Fancher, O. 90-Day
1971 subacute oral toxicity study with RE12,420 technical in
albino rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the World
Health Organization by Chevron Chemical Company.
Plank, J., Wright, P., and Keplinger, M. 90-Day cholinesterase
1972 study with ortho 12,420 technical in albino rats.
Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. The
1972a acute oral toxicity to rats of orthene in combination
with five other cholinesterase inhibition of materials.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Reyna, M., Kennedy, G., and Keplinger, M. 18-Month carcinogenic
1973 study with RE12,420 technical in Swiss white mice.
Unpublished report from Industrial Bio Test
Laboratories, Inc., submitted to the World Health
Organization by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972b 21-Day subacute dermal study in rabbits with
orthene-75S. Unpublished report from Standard Oil of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972c S-184 the acute dermal toxicity of RE14,071.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972d S-533. The acute dermal toxicity of RE18,421.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J. S-514
1972e The acute dermal toxicity of RE17,245. Unpublished
report from Standard Oil Company of California,
submitted to the World Health Organization by Chevron
Chemical Company.
Rittenhouse, J., Narcisse, J., Cavalli, R., and Spence, J.
1972P S-515 the acute dermal toxicity of RE17,246.
Unpublished report from Standard Oil Company of
California, submitted to the World Health Organization
by Chevron Chemical Company.
Swencicki, R., and Hartz, W. Orthene human exposure study
1972 clinical evaluation. Unpublished study from Standard
Oil Company of California, Submitted to the World
Health Organization by Chevron Chemical Company.
Tucker, B. Acetylcholinesterase inhibition of orthene and
1972 ortho 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the World Health
Organization by Chevron Chemical Company.
