
PIRIMIPHOS-METHYL JMPR 1976
Explanation
Pirimiphos-methyl was first evaluated at the 1974 Joint
FAO/WHO Meeting on Pesticide Residues (FAO/WHO, 1975). A temporary
acceptable daily intake for man was established.
Additional toxicological and residue studies on this compound
have become available and are summarized in this addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Additional data are presented on the concentration of
pirimiphos-methyl and metabolites in rat tissues and on the blood
profile following a single oral dose.
When rats were given a single oral dose of 2-14C-labelled
pirimiphos-methyl at 7.5 mg/kg the uptake of radioactivity into
blood and its subsequent disappearance from the blood stream were
both very rapid. More than 50% of the radioactivity present in the
blood 30 minutes after dosing had disappeared at 1 hour after
dosing. Total radioactive residues in the blood 24 hours after
dosing (generally 0.2-0.3 mg/l pirimiphos-methyl equivalents) did
not increase following daily dosing of 2-14C-labelled
pirimiphos-methyl for a further 3 days. Unchanged pirimiphos-methyl
normally represented less than 10% of the total residue in the
blood 24 hours after dosing.
When 2-14C-labelled pirimiphos-methyl was administered orally
to rats at 7.5 mg/kg/day for four days, total radioactive residues
in the liver, kidney and fat did not normally exceed 2 mg/kg
pirimiphos-methyl equivalents. Levels of unchanged
pirimiphos-methyl where detected were very small and did not exceed
0.15 mg/kg in fat or 0.1 mg/kg in liver and kidneys. There is no
evidence to show that either pirimiphos-methyl or its metabolites
accumulated in the liver, kidney or adipose tissue of rats
following daily dosing with the insecticide over 4 days (Mills,
1976).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Pirimiphos-methyl was tested for dominant lethal mutagenic
activity in mice. Male animals were given orally 15, 80 or 150
mg/kg body weight for 5 days before mating. Groups receiving
ethylmethanesulphonate (EMS) and cyclophosphamide were used as
positive controls. Mating was carried out during 8 consecutive
weeks, each week with new virgin females (2 females per male).
These were killed 15 or 16 days after caging and examined for live
implantations and early and late foetal deaths. The ovaries were
examined for the number of corpora lutea.
Pregnancy frequency was statistically significantly lower in
the 150 mg/kg group. No other effects were noted in the
pirimiphos-methyl groups which give an indication of any mutagenic
activity. Such effects were amply demonstrated in the positive
control groups (McGregor, 1975).
Pirimiphos-methyl has shown evidence of mutagenic activity in
Salmonella typhimurium but not in Escherichia coli. No
metabolic activation was used in this study (Hanna and Dyer, 1975).
Special studies on neurotoxicity
Pirimiphos-methyl was tested for neurotoxicity in the hen. Two
groups of five hens were given single oral doses of 50 and 60 mg/kg
(dose rates for LD50) respectively. They were observed for 21 days.
Four birds survived in the first group and 2 birds in the second
group.
On day 21 of the study one additional bird was added to the
group dosed with 50 mg/kg and three to the group dosed with 60
mg/kg. The birds were protected with intramuscular injections of
atropine and pralidoxime and then re-dosed at 50 or 60 mg/kg. No
neurotoxic symptoms were observed on clinical assessment or during
histological examination after a further 21 days. Tri-o-tolyl
phosphate (500 mg/kg) was used as a positive control compound and
all birds showed signs of ataxia and significant neuropathological
changes. It is concluded that pirimiphosmethyl is not neurotoxic
(Ross et al., 1975)
Special studies on potentiation
A test for potentiation of the acute toxicity of a combination
of pirimiphos-methyl and bioresmethrin was conducted by
administration of a suspension of the two compounds mixed in the
ratio of their LD50 values. The degree of potentiation was assessed
by finding the ratio between the expected toxicity and the observed
toxicity of the mixture. The results obtained suggested that
potentiation between pirimiphos-methyl and bioresmethrin does occur
(ratio of expected to observed) but the extent does not appear
sufficient to be of practical significance (Parkinson, 1975).
Special studies on reproduction
In an assessment of the effect of pirimiphos-methyl on
reproductive functions of the rat, dietary concentration of 5, 10
and 100 ppm were fed continuously to three generations of rats.
There were no consistent dose-related effects on parent animals in
respect of signs of reaction, mortalities, food consumption,
bodyweight change, mating performance, pregnancy rate or duration
of gestation.
There were no consistent dosage related effects on values for
litter size, pup mortality rates, litter weight, mean pup weight or
the incidence of anomalies. Terminal observations of the F3
generation showed no conclusive differences in organ weight. The
only differences to attain statistical significance related to the
higher absolute liver weight of males at 10 ppm and the lower
absolute thyroid weight of males at 100 ppm. However, the
significance of these differences was not maintained following
adjustment for differences in body weight.
For both sexes depression of both plasma and erythrocyte
cholinesterase activity in excess of 20%, considered biologically
significant, was recorded at 100 ppm in each generation, but not at
5 and 10 ppm levels.
Pirimiphos-methyl at 100 ppm has no significant effect on
growth and reproductive functions of the rat (Palmer and Hill,
1976).
Special studies on egg production and hatchability
The study was designed to assess the effect of dietary
inclusion of pirimiphos-methyl on body weight and food consumption
of laying hens and on their egg production and hatchability.
Groups of 35 hens and 4 cockerels were fed doses of 0, 4, 12
and 40 ppm pirimiphos-methyl for 28 days and then allowed a 14 day
recovery experimental period. The effect of pirimiphos-methyl on
number of eggs laid, egg quality, fertility and hatchability, chick
body weight and mortality was examined, together with body weight
and food consumption of parent birds.
At all levels tested pirimiphos-methyl had no significant
effect on egg quality, production, hatchability, or any other
effect that could be observed on any of the parameters measured.
This study was undertaken in view of the large number of
chicks dead in the shell found in a previous experiment, the
validity of which was doubted when a fault was discovered in the
incubator.
In addition, samples of egg, liver and mixed flesh were
analysed for pirimiphos-methyl and two of its metabolites. Values
were below the level of detection in egg yolk following the 7 day
withdrawal period. Except in the mixed flesh from samples taken on
day 21 of the study all samples were below detection (Ross et al.,
1975a,b).
Acute toxicity
2-ethylamino-4-hydroxy-6-methylpyrimidine (I) and
2-amino-4-hydroxy-6-methylpyrimidine (II) have both been identified
as degradation products of pirimiphos-methyl in animals, in plants
and in stored products. Their acute oral mammalian toxicity has
been assessed and found of low order.
The acute oral LD50 of compound (I) in the female rat is 2093
mg/kg. 95% confidence limits: 1841-2380 mg/kg. Toxic symptoms set
in rapidly and were of short duration. They included urinary
incontinence and salivation. Deaths occurred within 24 hours and
surviving animals were fully recovered within four days.
No deaths and no toxic symptoms were observed after the
administration of compound (II) to the tested female rat at 4000
mg/kg (Parkinson, 1974).
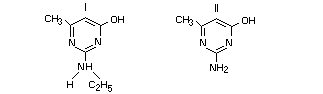 Short-term studies
Rat
Pirimiphos-methyl was administered to young rats at doses of
0, 5, 8, 10 and 50 ppm in the diet for a period of 28 days in order
to determine the no-effect level on plasma cholinesterase activity
and body weight gain. Plasma cholinesterase activity was inhibited
at the 50 ppm level throughout the dosing period by 30% and 50% in
male and female animals respectively. No effect on erythrocyte
cholinesterase activity was detected. There was a tendency towards
poorer food utilization in the group but there was no effect on
body weight gain.
There were sporadic instances of statistically significant
depressions of plasma cholinesterase activity at the 8 and 10 ppm
levels but less than 25% and not dose related. The "no effect"
level for pirimiphos-methyl in the young rat was 10 ppm in the diet
administered for a period of 28 days. This is also the no-effect
level established for pirimiphos-methyl in the rat over two years
(FAO/WHO, 1975; Berry and Gore, 1975).
Dog
In order to examine the potential hepatic effect of
pirimiphos-methyl in the dog, ten more dogs were given 25 mg/kg/day
with six dogs acting as controls. Three of the dogs were killed
after four weeks dosing for histopathological examination. The
remaining animals were maintained at the same dose level for a
total period of 13 weeks except for two dogs whose dose was raised
progressively to 50 mg/kg/day from week eight onwards.
Slight bile duct proliferation was present in two dogs given
25 mg/kg pirimiphos-methyl for 13 weeks. None of the other dosed
animals showed this effect even when the dose was increased to 50
mg pirimiphos-methyl/kg/day in two other dogs.
The effects in the dog liver characterised by bile duct
proliferation in a certain number of dogs given 25 mg/kg/day was
confirmed. However the changes appear to be small and dependent
upon individual animal sensitivity and reversible; the mode of
action could not be ascertained. Focal inflammation was present in
the livers of control and dosed animals. Liver changes were shown
in the rat at levels of 300-600 ppm in the diet and in dogs dosed
at 10 mg/kg/day for two years (Garuti et al., 1976).
Long-term studies
Mouse
Four groups of 52 male and 52 female mice of the CFLP strain
were fed 0, 5, 250 and 500 ppm pirimiphos-methyl in the diet for 80
weeks. Three extra groups of 12 male and 12 female mice were fed 0,
5 and 500 ppm and were used for the investigation of cholinesterase
levels.
There were no significant differences in mortality between
control and treated groups. Macroscopic examination of descendants
gave no indication of treatment-related change. Efficiency of food
utilization and overall body weight gain were not affected. Food
consumption was reduced slightly among females receiving 500 ppm
only. No changes ascribable to the administration of pirimiphos-
methyl were seen during gross and histological examination at
autopsy.
Changes in the kidneys, namely foci of lymphocytic
infiltration in the interstitium, or minimal perivascular or
peribronchiolar lymphoid aggregations in the lung and abscesses and
small haemorrhages in ovaries and other morphological changes in
various tissues were seen in a proportion of animals of all groups
including controls and were considered to be of no toxicological
significance. Similarly the liver tumour incidence was comparable
to that of the controls.
There was no evidence to suggest that treatment with
pirimiphos-methyl altered the tumour profile of the CFLP mouse at
levels up to 500 ppm. However, animals of both sexes showed marked
inhibition of plasma and erythrocyte cholinesterase activities at
the 500 ppm dietary inclusion level. When corrected for differences
prevailing between groups at the start of the study, differences in
erythrocyte cholinesterase activity between the control and 5 ppm
pirimiphos-methyl groups were normally less than 20% and
consistently less than 25%. Plasma cholinesterase activities in
both untreated and 5 ppm dietary inclusion level groups followed
similar, slightly fluctuating patterns during the study, although
levels in both sexes in the treated group appeared inhibited by
comparison with controls. However there was no evidence of
progressive inhibition during treatment and differences between the
control and 5 ppm groups were considered to be of borderline
biological significance (Hunter et al., 1976). A "no-effect" level
at 5 ppm in the diet, equivalent to approximately 0.5 mg/kg/day was
established over an 80 week period.
OBSERVATIONS IN MAN
A group of seven healthy volunteers, consisting of three males
and four females, was given orally a dose of 0.25 mg
pirimiphos-methyl/kg bw, in capsule form for a period of 56 days.
No effect due to the administration of pirimiphos-methyl was seen
on liver function tests, blood count or erythrocyte or plasma
cholinesterase activity (Howard and Gore, 1976).
COMMENTS
Results of studies on the effect of pirimiphos-methyl on rat
reproduction in a three generation test, on avian-egg production
and hatchability and of special tests on mutagenicity were
negative.
Hydronephrosis incidence noted in a previous test has been
shown to be well within the limits of control values pertaining to
the same species.
Two studies were performed to investigate the potential
hepatotoxicity of pirimiphos-methyl. In an 80 week mouse study the
tumour incidence (including that in liver) was comparable in test
and control groups. In a three month dog study slight bile duct
proliferation was observed in animals dosed at 25 mg/kg/day,
however the effect was absent at 50 mg/kg/day. Focal inflammation
occurred equally in the livers of control and dosed animals. In a
56 day human study, 0.25 mg/kg/day did not induce any changes in
liver function tests, blood count or erythrocyte or plasma
cholinesterase activity. Additional biochemical data presented
established that the substance is very rapidly absorbed,
metabolised and excreted.
The Meeting agreed that the previously indicated further
studies have been carried out satisfactorily, and there is no
objection to estimating a permanent acceptable daily intake.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Mouse 5 ppm in the diet equivalent to 0.5 mg/kg bw/day
Rat 10 ppm in the diet equivalent to 0.5 mg/kg bw/day
Man 0.25 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.01 mg/kg bw/day
RESIDUES IN FOOD AND THEIR EVALUATION
The 1974 evaluation of pirimiphos-methyl (FAO/WHO, 1975) was
principally in relation to its use as a treatment of stored grains.
However the insecticide is also used on growing crops, in public
health and in industrial outlets. Therefore, arising out of the
list of requirements in the report of the 1974 Joint Meeting, the
following additional data are now presented.
1. Information on pre-harvest use patterns and residues in fruit
and vegetables.
2. Information on residues in peanuts and other stored products.
3. Further information on the level and fate of residues at the
point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
USE PATTERN
Pre-harvest
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant
actions. It is effective against a wide range of flying and
crawling insects, including aphids, scales, mealy bugs, whiteflies,
bugs, thrips, beetles, caterpillars, moths and ants. It also has
acaricidal activity. It controls both adult and immature stages of
insects such as whiteflies, and is effective against strains of
insects which have developed resistance to malathion.
Because it has only limited biological persistence on leaf
surfaces, pirimiphos-methyl is particularly attractive for uses in
which a broad spectrum of insecticidal activity is required for a
short period. e.g. pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg a.i./ha "low volume" or 0.05-0.1% a.i. "high volume" are
normally recommended. 0.5-0.75 kg a.i./ha (or 20-30 mg a.i. per
m3) is normally effective in controlling greenhouse whiteflies by
fogging. A fogging rate of 40 mg per m3 is used to control
mushroom flies.
The principal formulation is a 50% emulsifiable concentrate.
A 50% oil-based formulation is available for ultra-low volume
application to outdoor crops and a 10% oil-based formulation is
used for whitefly control under glass. An 8% emulsifiable
concentrate is sold to the amateur gardener. Dust and aerosol
formulations are under development for crop uses.
A pre-harvest withholding interval of four days is normally
recommended after spraying. No pre-harvest withholding interval is
normally recommended after the use of a fog to control
1. whiteflies on greenhouse tomatoes, cucumbers and peppers.
2. mushroom flies.
Pirimiphos-methyl is not generally recommended for use on
celery.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Data reviewed by the 1974 Joint Meeting showed that
pirimiphos-methyl is rapidly lost from leaf surfaces during the
first 2-3 days after spraying, mainly by volatilisation. Levels of
the parent insecticide and the major degradation product, compound
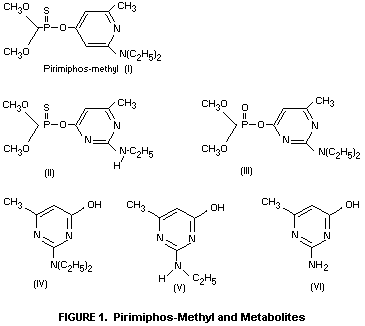
II (Figure 1), represent less than 10% of the applied material
after two to three days. After foliar treatment the hydroxy-
pyrimidine IV, formed by hydrolysis of the insecticide, is unlikely
to be a significant residue - the compound is usually present only
in trace amounts and is degraded photochemically and bound to plant
material.
Photochemical degradation of pirimiphos-methyl on leaf
surfaces leads to the formation of compound II which is not
accumulated.
When applied to water in which rice seedlings were growing,
pirimiphos-methyl was not translocated significantly into the
foliage; in these conditions the hydrolysis product IV is likely to
represent the predominant pyrimidine residue. Compounds V and VI
occur only in trace amounts.
The new data show that pirimiphos-methyl residues on growing
crops decline rapidly during the first few days after spraying.
Bullock (1972) has studied extensively residues of
pirimiphos-methyl in crops grown world-wide. Samples were analysed
for residues of pirimiphos-methyl and of the phosphorus-containing
metabolites II and III. However significant amounts of the
metabolites did not appear and so were not reported.
The highest initial residues are found in leafy vegetables.
However, pirimiphos-methyl residues decline very quickly on these
crops so that after four days levels on vegetables generally are
below 2 mg/kg except on celery and occasionally on lettuce, spinach
or Brussels sprouts where they may be higher (Table 1). Residues on
tree fruits are normally less than 2 mg/kg after four days, while
on soft fruits (currants and berries) they are generally below 1
mg/kg (Table 2). There was good agreement between the results
obtained by Bullock and those obtained by a second laboratory on a
set of apple samples from Japan.
The residues which Bullock found in rice in husk (up to 0.21
mg/kg), in dehusked rice and in maize (<0.01 mg/kg in both cases)
following pre-harvest spray uses are all within the temporary
tolerance levels recommended by the 1974 Joint Meeting for
post-harvest uses on these commodities.
The use of fogs to control whiteflies under glass results in
initial residues of 0.3 mg/kg or less in cucumbers, tomatoes and
peppers (Table 3). Although treatments for whitefly control may
need to be made every 3-5 days, the available data (ICI Plant
Protection Division, 1973) indicate that no accumulation of
pirimiphos-methyl residues occur in these fruits on repeated
application.
There is also no accumulation of residues in mushrooms
following multiple applications of a fog (Table 3). Initial
residues of up to 2.5 mg/kg were found to decline rapidly to below
0.5 mg/kg after two days (Bullock and Stephens, 1974).
The new aerosol and dust formulations yield residues (Table 4)
similar to those observed previously with sprays of the
emulsifiable concentrate formulations on lettuce and blackcurrants
(Boxwell and Bullock, 1976). Residues on tomatoes were marginally
higher than after fogging but were still below 1 mg/kg immediately
after application at normal rates.
Short-term studies
Rat
Pirimiphos-methyl was administered to young rats at doses of
0, 5, 8, 10 and 50 ppm in the diet for a period of 28 days in order
to determine the no-effect level on plasma cholinesterase activity
and body weight gain. Plasma cholinesterase activity was inhibited
at the 50 ppm level throughout the dosing period by 30% and 50% in
male and female animals respectively. No effect on erythrocyte
cholinesterase activity was detected. There was a tendency towards
poorer food utilization in the group but there was no effect on
body weight gain.
There were sporadic instances of statistically significant
depressions of plasma cholinesterase activity at the 8 and 10 ppm
levels but less than 25% and not dose related. The "no effect"
level for pirimiphos-methyl in the young rat was 10 ppm in the diet
administered for a period of 28 days. This is also the no-effect
level established for pirimiphos-methyl in the rat over two years
(FAO/WHO, 1975; Berry and Gore, 1975).
Dog
In order to examine the potential hepatic effect of
pirimiphos-methyl in the dog, ten more dogs were given 25 mg/kg/day
with six dogs acting as controls. Three of the dogs were killed
after four weeks dosing for histopathological examination. The
remaining animals were maintained at the same dose level for a
total period of 13 weeks except for two dogs whose dose was raised
progressively to 50 mg/kg/day from week eight onwards.
Slight bile duct proliferation was present in two dogs given
25 mg/kg pirimiphos-methyl for 13 weeks. None of the other dosed
animals showed this effect even when the dose was increased to 50
mg pirimiphos-methyl/kg/day in two other dogs.
The effects in the dog liver characterised by bile duct
proliferation in a certain number of dogs given 25 mg/kg/day was
confirmed. However the changes appear to be small and dependent
upon individual animal sensitivity and reversible; the mode of
action could not be ascertained. Focal inflammation was present in
the livers of control and dosed animals. Liver changes were shown
in the rat at levels of 300-600 ppm in the diet and in dogs dosed
at 10 mg/kg/day for two years (Garuti et al., 1976).
Long-term studies
Mouse
Four groups of 52 male and 52 female mice of the CFLP strain
were fed 0, 5, 250 and 500 ppm pirimiphos-methyl in the diet for 80
weeks. Three extra groups of 12 male and 12 female mice were fed 0,
5 and 500 ppm and were used for the investigation of cholinesterase
levels.
There were no significant differences in mortality between
control and treated groups. Macroscopic examination of descendants
gave no indication of treatment-related change. Efficiency of food
utilization and overall body weight gain were not affected. Food
consumption was reduced slightly among females receiving 500 ppm
only. No changes ascribable to the administration of pirimiphos-
methyl were seen during gross and histological examination at
autopsy.
Changes in the kidneys, namely foci of lymphocytic
infiltration in the interstitium, or minimal perivascular or
peribronchiolar lymphoid aggregations in the lung and abscesses and
small haemorrhages in ovaries and other morphological changes in
various tissues were seen in a proportion of animals of all groups
including controls and were considered to be of no toxicological
significance. Similarly the liver tumour incidence was comparable
to that of the controls.
There was no evidence to suggest that treatment with
pirimiphos-methyl altered the tumour profile of the CFLP mouse at
levels up to 500 ppm. However, animals of both sexes showed marked
inhibition of plasma and erythrocyte cholinesterase activities at
the 500 ppm dietary inclusion level. When corrected for differences
prevailing between groups at the start of the study, differences in
erythrocyte cholinesterase activity between the control and 5 ppm
pirimiphos-methyl groups were normally less than 20% and
consistently less than 25%. Plasma cholinesterase activities in
both untreated and 5 ppm dietary inclusion level groups followed
similar, slightly fluctuating patterns during the study, although
levels in both sexes in the treated group appeared inhibited by
comparison with controls. However there was no evidence of
progressive inhibition during treatment and differences between the
control and 5 ppm groups were considered to be of borderline
biological significance (Hunter et al., 1976). A "no-effect" level
at 5 ppm in the diet, equivalent to approximately 0.5 mg/kg/day was
established over an 80 week period.
OBSERVATIONS IN MAN
A group of seven healthy volunteers, consisting of three males
and four females, was given orally a dose of 0.25 mg
pirimiphos-methyl/kg bw, in capsule form for a period of 56 days.
No effect due to the administration of pirimiphos-methyl was seen
on liver function tests, blood count or erythrocyte or plasma
cholinesterase activity (Howard and Gore, 1976).
COMMENTS
Results of studies on the effect of pirimiphos-methyl on rat
reproduction in a three generation test, on avian-egg production
and hatchability and of special tests on mutagenicity were
negative.
Hydronephrosis incidence noted in a previous test has been
shown to be well within the limits of control values pertaining to
the same species.
Two studies were performed to investigate the potential
hepatotoxicity of pirimiphos-methyl. In an 80 week mouse study the
tumour incidence (including that in liver) was comparable in test
and control groups. In a three month dog study slight bile duct
proliferation was observed in animals dosed at 25 mg/kg/day,
however the effect was absent at 50 mg/kg/day. Focal inflammation
occurred equally in the livers of control and dosed animals. In a
56 day human study, 0.25 mg/kg/day did not induce any changes in
liver function tests, blood count or erythrocyte or plasma
cholinesterase activity. Additional biochemical data presented
established that the substance is very rapidly absorbed,
metabolised and excreted.
The Meeting agreed that the previously indicated further
studies have been carried out satisfactorily, and there is no
objection to estimating a permanent acceptable daily intake.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Mouse 5 ppm in the diet equivalent to 0.5 mg/kg bw/day
Rat 10 ppm in the diet equivalent to 0.5 mg/kg bw/day
Man 0.25 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.01 mg/kg bw/day
RESIDUES IN FOOD AND THEIR EVALUATION
The 1974 evaluation of pirimiphos-methyl (FAO/WHO, 1975) was
principally in relation to its use as a treatment of stored grains.
However the insecticide is also used on growing crops, in public
health and in industrial outlets. Therefore, arising out of the
list of requirements in the report of the 1974 Joint Meeting, the
following additional data are now presented.
1. Information on pre-harvest use patterns and residues in fruit
and vegetables.
2. Information on residues in peanuts and other stored products.
3. Further information on the level and fate of residues at the
point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
USE PATTERN
Pre-harvest
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant
actions. It is effective against a wide range of flying and
crawling insects, including aphids, scales, mealy bugs, whiteflies,
bugs, thrips, beetles, caterpillars, moths and ants. It also has
acaricidal activity. It controls both adult and immature stages of
insects such as whiteflies, and is effective against strains of
insects which have developed resistance to malathion.
Because it has only limited biological persistence on leaf
surfaces, pirimiphos-methyl is particularly attractive for uses in
which a broad spectrum of insecticidal activity is required for a
short period. e.g. pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg a.i./ha "low volume" or 0.05-0.1% a.i. "high volume" are
normally recommended. 0.5-0.75 kg a.i./ha (or 20-30 mg a.i. per
m3) is normally effective in controlling greenhouse whiteflies by
fogging. A fogging rate of 40 mg per m3 is used to control
mushroom flies.
The principal formulation is a 50% emulsifiable concentrate.
A 50% oil-based formulation is available for ultra-low volume
application to outdoor crops and a 10% oil-based formulation is
used for whitefly control under glass. An 8% emulsifiable
concentrate is sold to the amateur gardener. Dust and aerosol
formulations are under development for crop uses.
A pre-harvest withholding interval of four days is normally
recommended after spraying. No pre-harvest withholding interval is
normally recommended after the use of a fog to control
1. whiteflies on greenhouse tomatoes, cucumbers and peppers.
2. mushroom flies.
Pirimiphos-methyl is not generally recommended for use on
celery.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Data reviewed by the 1974 Joint Meeting showed that
pirimiphos-methyl is rapidly lost from leaf surfaces during the
first 2-3 days after spraying, mainly by volatilisation. Levels of
the parent insecticide and the major degradation product, compound
II (Figure 1), represent less than 10% of the applied material
after two to three days. After foliar treatment the hydroxy-
pyrimidine IV, formed by hydrolysis of the insecticide, is unlikely
to be a significant residue - the compound is usually present only
in trace amounts and is degraded photochemically and bound to plant
material.
Photochemical degradation of pirimiphos-methyl on leaf
surfaces leads to the formation of compound II which is not
accumulated.
When applied to water in which rice seedlings were growing,
pirimiphos-methyl was not translocated significantly into the
foliage; in these conditions the hydrolysis product IV is likely to
represent the predominant pyrimidine residue. Compounds V and VI
occur only in trace amounts.
The new data show that pirimiphos-methyl residues on growing
crops decline rapidly during the first few days after spraying.
Bullock (1972) has studied extensively residues of
pirimiphos-methyl in crops grown world-wide. Samples were analysed
for residues of pirimiphos-methyl and of the phosphorus-containing
metabolites II and III. However significant amounts of the
metabolites did not appear and so were not reported.
The highest initial residues are found in leafy vegetables.
However, pirimiphos-methyl residues decline very quickly on these
crops so that after four days levels on vegetables generally are
below 2 mg/kg except on celery and occasionally on lettuce, spinach
or Brussels sprouts where they may be higher (Table 1). Residues on
tree fruits are normally less than 2 mg/kg after four days, while
on soft fruits (currants and berries) they are generally below 1
mg/kg (Table 2). There was good agreement between the results
obtained by Bullock and those obtained by a second laboratory on a
set of apple samples from Japan.
The residues which Bullock found in rice in husk (up to 0.21
mg/kg), in dehusked rice and in maize (<0.01 mg/kg in both cases)
following pre-harvest spray uses are all within the temporary
tolerance levels recommended by the 1974 Joint Meeting for
post-harvest uses on these commodities.
The use of fogs to control whiteflies under glass results in
initial residues of 0.3 mg/kg or less in cucumbers, tomatoes and
peppers (Table 3). Although treatments for whitefly control may
need to be made every 3-5 days, the available data (ICI Plant
Protection Division, 1973) indicate that no accumulation of
pirimiphos-methyl residues occur in these fruits on repeated
application.
There is also no accumulation of residues in mushrooms
following multiple applications of a fog (Table 3). Initial
residues of up to 2.5 mg/kg were found to decline rapidly to below
0.5 mg/kg after two days (Bullock and Stephens, 1974).
The new aerosol and dust formulations yield residues (Table 4)
similar to those observed previously with sprays of the
emulsifiable concentrate formulations on lettuce and blackcurrants
(Boxwell and Bullock, 1976). Residues on tomatoes were marginally
higher than after fogging but were still below 1 mg/kg immediately
after application at normal rates.
 Stored product uses
In contrast to its short persistence on growing crops,
pirimiphos-methyl possesses exceptional stability on stored
products such as grains. Although high temperatures and high
moisture levels in grain reduce the life of the deposit, the effect
of these influences is much less than with other grain protectants
approved or evaluated to date (FAO/WHO, 1975).
During degradation, residues of pirimiphos-methyl on wheat
grains are degraded by hydrolysis of the phosphorus-ester side
chain, to give principally the parent hydroxy-pyrimidine IV (Figure
1) and also the related compounds V and VI. Levels of compound II
are always extremely low (approximately 0.05 mg/kg over a period of
32 weeks in wheat grain treated at 4 mg/kg). No residues of the
chemically unstable oxygen analogue III have been detected. The
limit of detection for compound III is 0.01 mg/kg. Residues of the
hydroxypyrimidine IV in stored grains are normally below 0.5 mg/kg.
Peanuts
Pirimiphos-methyl residues in undecorticated peanuts treated at
the intended rate of 20 mg/kg did not exceed 5.0 mg/kg. Two trials
were conducted in the U.S.A. in 1971-2 in which pirimiphos-methyl was
applied at 20 and 50 mg/kg to undecorticated peanuts. Analytical
results indicated that the target rates of treatment were achieved.
Very little change occurred in the level of residues during the first
month following application. Even at the end of three months
substantially all of the deposit remained. The data suggest that the
half-life is of the order of 5 months from the time of application.
Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied. These data have recently been published (Redlinger, 1976).
A larger scale trial was conducted in the U.S.A. during 1972-73
(Ussary, 1975). Pirimiphos-methyl was applied at 10, 20 and 30 mg/kg
as an admixture treatment to undecorticated peanuts. Analytical
results again indicated that the target rate of treatment was
achieved. 20-50% of the initial residue was present on the
undecorticated peanuts after twelve months storage, compared with 10%
in the case of malathion. During four commercial scale trials in the
U.S.A. in 1973-74, in which pirimiphos-methyl was applied at the
proposed commercial application rate of 20 mg/kg, 30-60% of the
initial residue was present at bin emptying, that is after 4 ´-6
months storage (Ussary, 1975).
TABLE 1. Residues of pirimiphos-methyl resulting from supervised spray trials on leafy vegetables and root crops (EC formulations)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Beans West Germany 50% EC 0.15 kg/ha 1 0.15-0.25 <0.01-0.07 <0.01-0.01
(Bush) 0.3 kg/ha 1 0.05-0.47 <0.01-0.08 <0.01-0.04
(French) U.K. 50% EC or 0.05% 1 0.06-0.07 0.01-0.03 <0.01-0.01
8% EC 0.1% 1 0.08-0.14 0.10-0.14 <0.01-0.06
0.2% 1 - 0.26 0.14
Peas U.K. 50% EC 0.1 % 1 - - <0.01
0.2 % 1-2 - <0.01 <0.01
Lettuce U.K. 50% EC or 0.025% 3 0.19-0.73 0.07-0.13 0.30-0.12
25% EC 0.05% 1-3 11-18 0.60-1.8 0.37-0.88
0.1% 1-3 19-39 1.8-2.9 0.63-2.1
West Germany 50% EC 0.025% 1 2.6-2.7 0.23 0.07-0.13
0.05% 1 3.4-7.3 0.52 0.14-0.71
0.1% 4.7 - 0.52
West Germany 25% EC (0.15 kg/ha 1 4.6-8.1 0.02-0.24 0.02-0.19
0.3 kg/ha 1 6-13 0.05-0.50 0.01-0.11
Spinach West Germany 25% EC 0.025% 1 3.2-6.7 0.35-1.0 0.22
0.05% 1 12-14 1.5-1.6 0.19-0.43
0.1% 1 21 3.4 0.75
Cabbage U.K. 25% EC 1.0 kg/ha 1 - 0.30-0.50** <0.02-0.28
2.0 kg/ha 1 - 0.31-1.8 0.09-0.40
West Germany 25% EC 0.15 kg/ha 1 0.13-1.4 0.05-0.18 <0.01-0.09
0.3 kg/ha 1 0.57-4.6 <0.01-0.69 <0.01-0.40
0.6 kg/ha 1 4.1 1.5 0.18
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Cabbage Malaysia 25% EC 1.0 kg/ha 18 - - 0.01***
2.0 kg/ha 18 - - 0.01-0.02***
Cauliflower U.K. 50% EC or 0.1% 1 0.20-2.0 0.01-0.11 <0.01
8% EC
(Hearts) 0.2% 1 0.15-1.4 0.04-0.24 <0.01-0.04
(leaves) 50% EC or 0.1% 1 0.66-12 0.50-1.3 0.02-0.35
8% EC 0.2% 1 1.5-13 0.65-1.1 0.04-2.2
Brussels U.K. 50% EC 1.4 kg/ha 3 1.7-3.3 0.30-1.7 0.28-0.66
sprouts (0.1%)
(buttons) 2.28 kg/ha 3 2.9-6.8 0.96-4.5 0.67-2.3
(0.2%)
Brussels Netherlands 50% EC 0.5 kg/ha 1 0.26* 0.14** 0.07
sprouts (0.05 %)
(buttons) 0.75 kg/ha 1 0.49* 0.39** 0.21
(0.075%)
Spring U.K. 50% EC or 0.05% 1 0.28-1.6 <0.01-0.07 <0.01-0.08
onions 8% EC 0.1% 1 2.3-4.2 0.04-0.49 <0.01-0.46
Celery U.K. 8% EC 0.05% 1 18-34 3.1-8.8 1.2-2.7
0.1% 1 26-69 5.1-14 3.70-8.0
Carrots U.K. 50% EC 1.14 kg/ha 1 0.32-0.95 0.09-0.16 0.10-0.22
(0.1%)
2.28 kg/ha 1 0.57-2.2 0.17-0.48 0.12-0.50
(0.2%)
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Potatoes U.K. 50% EC 0.1% 2 <0.01 <0.01
0.2% 2 <0.01 <0.01 <0.01-0.02
Sugar West Germany 25% EC 0.05% 1 18 - 1.7
beets (0.3 kg/ha)
0.1% 1 38 - 3.1
(0.6 kg/ha)
* Samples taken after 1 day
** Samples taken after 3 days
*** Samples taken after 9-15 days
TABLE 2 Residues of pirimiphos-methyl resulting from supervised spray trials on tree and sort fruits (EC and ULV formulations
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Apples U.K. 50% EC or 0.1 % 3 0.51-1.3 0.25-0.67 0.10-0.80
8 % 0.05% 1
0.2 % 3 0.76-2.8 0.58-1.9 0.16-1.5
0.1% 1
France 50% EC 0.075% 6 - - - 2 weeks: 0.32
Japan 50% EC 0.1 % 1-3 - - - 10 days: 0.15-1.4
Pears Spain 50% EC 0.1 % 1 - - - 12 weeks: <0.01
Plums U.K. 50% EC 0.1 % 2 0.47-0.96 0.24-0.55 0.02-0.25
0.2 % 2 1.6-2.2 1.3-1.5 0.06-0.55
Lemons Italy 25% EC 0.05 % 1 - - - 9 weeks: 0.25 in peel
0.03 in flesh
0.075% 1 - - - 0.75 in peel
0.03 in flesh
0.01 % - - 0.27-0.35 - -
in peel
0.02-0.03
in flesh
Oranges Australia 25% EC 0.075% 2 - - - 4´ months: 0.69 in peel
<0.01 in flesh
0.1 2 - - - 0.92 in peel
<0.01 in flesh
TABLE 2 (Cont'd.)
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Oranges South 25% EC 0.075% 1 - - - 2-8 months: <0.01-0.01 in peel
Africa <0.01 in flesh
0.01-0.125% 1 - - - <0.01-0.1 in peel
<0.01-0.1 in flesh
Olives Spain 50%ULV 0.25 kg/ha 2 - - 0.46 9-20 days: 0.07-0.36
0.5 kg/ha 2 - - - 0.16-0.45
1.0 kg/ha 2 - - - 0.18-1.4
Blackcurrants U.K. 8% EC 0.05 % 2 0.25-0.75 0.06-0.20 0.01-0.18
0.1 % 2 0.86-2.3 0.25-0.62 0.08-0.34
Raspberries U.K. 50% EC or 0.05% 1-2 0.81 0.23 0.13 2-5 weeks: <0.01-0.03
8% EC 0.01% 1-2 2.5 0.53 0.31 <0.01-0.09
Strawberries U.K. 50% EC or 0.05% 1 0.14-0.79 0.17-0.39 0.04-0.16 4´-7 weeks: <0.01-0.05
8% 0.1% 1 0.40-2.9 0.31-0.71 0.13-0.25 <0.01-0.05
TABLE 3. Levels of pirimiphos-methyl resulting from supervised residue trials on greenhouse crops and mushrooms using a 10% fogging
formulation
Number of Residues (mg/kg) at intervals after last application
crop Country Rate (a.i.) Applications 0 days 1 day 2 days 3 days 4 days
Cucumbers U.K. 0.75 kg/ha 1-4 0.06-0.30 0.03-0.11 0.02-0.11 0.01-0.04 <0.01
(=20 mg/m3)
Netherlands 20 mg/m3 1-5 0.08-0.19 0.03-0.08 0.02 <0.01-0.02 0.02
20 mg/m3 5
+30mg/m3 1-5 - 0.04-0.20 - 0.01-0.08 0.02-0.03
Tomatoes U.K. 0.5 kg/ha 2 - 0.15 - - -
(= 15 mg/m3)
1 kg/ha or 1-4 - 0.16-0.62 0.16 0.12 -
30 mg/m3
Netherlands 0.75 kg/ha 1-3 - <0.01-0.12 <0.01-0.18 0.08 -
(= 20 mg/m3)
30 mg/m3 1-5 - 0.06-0.17 - 0.08-0.14 0.08-0.11
(= 1 kg/ha)
Peppers U.K. 1 kg/ha 1 - 0.26 - - -
Mushrooms U.K. 40 mg/m3 1 and 6 0.99 0.19 0.12 - 0.14-0.16
(two trials) -2.5 -1.4 -0.44 - (4-5 days)
TABLE 4. Residues of pirimiphos-methyl resulting from supervised trials in the U.K. using dust and aerosol
formulations (single application date per trial)
Residues (mg/kg) at intervals after
Crop Formulation Rate 0 days 2 days 4 days 7-8 days
Lettuce Dust Normal 2.1-10 0.53-7.9 0.33-2.1 0.06-1.9
Overdosed 8.3-47 2.2-19 1.1-5.6 0.32-5.2
Aerosol Normal 1.6-21 0.53-11 0.34-2.8 0.16-1.8
Overdosed 13-40 6.2-25 2.0-7.6 0.75-3.8
Tomatoes Dust Normal 0.01-0.93 <0.01-0.15 0.07-0.09 0.04-0.07
Overdosed 0.18-1.4 0.08-0.89 0.05-0.13 0.02-0.03
Aerosol Normal 0.09-0.67 0.04-0.43 0.02-0.38 0.03-0.05
Overdosed 0.03-4.1 0.01-1.3 0.02-0.46 0.01-0.03
Blackcurrants Dust Normal 0.10-0.59 0.03-0.05 - -
Overdosed 0.44-1.4 0.08-0.22 - -
Aerosol Normal <0.01-0.27 <0.01-0.04 - -
Overdosed 0.04-0.59 <0.01-0.29 - -
During these studies there were wide variations between different
samples analysed from the same bin. These were probably caused by
non-representative sampling. Dust, estimated to constitute up to 10%
of the contents of the bins, settles into layers in the bins. It
contains several hundred mg/kg pirimiphos-methyl and in large bins it
was impossible to sample the dust in a representative manner. However,
residues in individual samples of undecorticated peanuts in the U.S.A.
trials were generally below 5.0 mg/kg during the period after
treatment at the proposed recommended rate of 20 mg/kg.
The walls of the four warehouses used in the commercial scale
trials were sprayed with a 0.5% aqueous suspension of
pirimiphos-methyl and probe samples were taken adjacent to the walls
during the storage period. Residues in the undecorticated peanuts were
similar to those from the bulk of the warehouse and it was concluded
that there was no major transfer of pirimiphos-methyl from the walls
to the peanuts (Ussary, 1975).
Rice
The 1974 Joint Meeting recommended temporary tolerances of 10
mg/kg in rice (in husk), 20 mg/kg in rice bran, 2 mg/kg in dehusked
rice and 1 mg/kg in polished rice.
Cogburn (1976) in the U.S.A. found that an admixture treatment of
pirimiphos-methyl at 10 mg/kg protected rice from all insect
infestation for twelve months. At this rate residues in the rice in
husk, bran, dehusked rice and polished rice were all within the
temporary tolerance levels proposed by the 1974 Joint Meeting.
Dates
In supervised trials in Iraq, MacCallum, Deighton and Pascoe
(1976) found that 500 mg pirimiphos-methyl per square metre sprayed
onto packaging materials (cardboard or wooden boxes) was effective in
protecting export dates from insect attack. A deposit of 125 mg per
square metre was effective for the treatment of waxed paper liners. At
these rates pirimiphos-methyl residues in the dates during the
following six months were invariably small - i.e. less than 0.5 mg/kg.
Cheese
There is very little movement of pirimiphos-methyl into cheese
stored on surfaces treated with the insecticide for mite control.
Thomas and Rowlands (1975) treated a wooden plank at 440 mg per
square metre with 2-14C-labelled pirimiphos-methyl and then stored a
22 kg Cheshire cheese, wrapped in a cloth, on it for fourteen weeks.
The cheese was turned to stand on alternate ends weekly, as in normal
storage practice. The radioactive residue was confined to the outer 4
mm of the cheese in which there was no detectable breakdown of
pirimiphos-methyl.
Levels of pirimiphos-methyl were approximately 40 mg/kg in the
outer 1 mm and 5-10 mg/kg in the outer 6 mm of the cheese. On a "whole
cheese" basis, this represents a residue of approximately 0.4 mg/kg.
FATE OF RESIDUES
In animals
Extensive data were evaluated by the 1974 Joint Meeting on the
fate of pirimiphos-methyl in goats, cows, hens, their edible tissues
and in foods of animal origin.
When fed to these animals, pirimiphos-methyl is rapidly
metabolised and the metabolites are excreted, principally in the
urine. Only very small quantities are excreted into milk or eggs.
Cattle receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites II and
III could be detected in meat, milk, butter or eggs (limit of
detection: 0.01 mg/kg or below).
Since the 1974 Joint Meeting, data on the fate in pigs and
additional data on the fate in hens have become available.
Pigs
Davis et al. (1976) maintained groups of pigs for 21 and 29 days
on diets containing 3, 10 and 34 mg/kg pirimiphos-methyl. No residues
of pirimiphos-methyl or of its phosphorus containing metabolites II or
III were detected in the kidney, liver, lung, heart or muscle (limit
of detection: 0.005 mg/kg in each case). Small residues of
pirimiphos-methyl and compound II were found in the fat of pigs fed
pirimiphos-methyl at 10 and 34 ppm in the diet (Table 15). No residues
were detected in the fat of pigs returned for eight days to untreated
diet prior to slaughter. No residues of the P=0 analogue (III) were
detected in any fat tissues (limit of detection 0.005 mg/kg).
No residues of the hydroxypyrimidines IV and V were found in any
of the above-mentioned tissues (limits of detection 0.01 mg/kg for
compound IV and 0.04 mg/kg for compound V).
Hens
Ross et al. (1975b) confirmed the low residue levels in eggs and
meat previously reported by other authors (FAO/WHO, 1975).
Groups of laying hens were maintained for 28 days on diets
containing 0, 4, 12 and 40 ppm pirimiphos-methyl. At all but the
highest dose level, pirimiphos-methyl levels in the eggs were below
0.01 mg/kg. At the 40 ppm dietary inclusion level, pirimiphos-methyl
levels in the egg yolk reached a plateau of 0.03-0.04 mg/kg after
seven days. After the hens had been returned to untreated diet for
seven days, pirimiphos-methyl residues in the yolks were below the
limit of detection (i.e. less than 0.008 mg/kg). Residues in the
albumen were negligible (less than 0.01 mg/kg).
Residues of pirimiphos-methyl in meat tissues were below 0.01
mg/kg at all dose levels.
No residues of the phosphorus-containing metabolites II and III
were detected in any samples of eggs or meat (limit of detection:
0.006-0.02 mg/kg).
In processing
Initial data on the fate of pirimiphos-methyl residues or
processing of stored products were reviewed by the 1974 Joint Meeting
(FAO/WHO, 1975). Subsequent studies are described below.
TABLE 5. Residues of pirimiphos-methyl and its desethyl metabolite
in the fat of pigs fed pirimiphos-methyl in the diet
Desethyl
Days Level in diet Pirimiphos-methyl Metabolite
on diet (ppm) (mg/kg) (mg/kg)
21 control ND ND
3 ND ND
10 0.008 0.016
34 0.034 0.034
29 3 ND ND
10 0.006 0.009
34 0.05 0.036
29 control ND ND
+ 8 days 3 ND ND
recovery
3 ND ND
10 ND ND
10 ND ND
34 ND ND
34 ND ND
ND: Not detected, less than 0.005 mg/kg
Wheat
Residues of pirimiphos-methyl in flour are relatively stable to
the conditions found during baking to bread and biscuits. However,
because of the dilution which the flour undergoes during these
processes flour initially containing 1 mg/kg pirimiphos-methyl is
likely to yield bread/biscuits containing residues of the order of
0.5 mg/kg.
Bullock and co-workers (1976) have studied extensively the fate
of pirimiphos-methyl during processing to bread and biscuits. In
studies with the radiolabelled compound, flour was dosed with
2-14C-labelled pirimiphos-methyl and baked to produce white bread,
wholemeal bread and biscuits. Although pirimiphos-methyl is known to
be a relatively volatile compound, there was no significant loss of
radioactivity by volatilisation. Distribution of radioactivity
throughout the bread was fairly uniform. Unchanged pirimiphos-methyl
accounted for 75-90% of the radioactivity in the baked product. The
major degradation products formed during baking were the
hydroxypyrimidines IV and VI, which accounted for 3-10% of the
radioactivity in the final product.
Similar results were obtained by residue analysis in a second set
of studies. After correction for the weight increase when flour is
baked to bread, residues of pirimiphos-methyl fell by 11-18%.
Likewise, residue analysis of biscuits showed average losses of 8%.
However, owing to the dilution of the flour which occurs during
baking, the residue level of pirimiphos-methyl in bread will be lower
than that in the corresponding flour.
During extensive residue studies Bullock et al.(1976) found that
levels of pirimiphos-methyl in bread and biscuits are likely to be
about 50% of those in the flour from which they were derived. These
findings agree well with the earlier work of Bullock (1973, 1974)
which was reviewed by the 1974 Joint Meeting. No residues of the
phosphorus-containing compounds II and III were detected in bread
baked from flour treated with pirimiphos-methyl at 1 mg/kg or in
biscuits baked from flour treated at up to 5 mg/kg (limit of
detection: 0.03 mg/kg)(Bullock et al., 1976).
The hydroxypyrimidine IV also undergoes little degradation during
the baking process. This compound has previously been shown to
constitute a minor part (generally less than 0.5 mg/kg) of the residue
in stored grains (FAO/WHO 1975). When bread was baked from flour
containing 2-14C-labelled compound IV, the unchanged
hydroxypyrimidine constituted more than 90% of the radioactive residue
in the bread (Bullock et al., 1976). By analogy with pirimiphos-methyl
residues, it can be concluded that residues of compound IV in baked
products will not exceed 0.2 mg/kg and will normally be considerably
lower.
The pattern and levels of residues found in semolina and cooked
pasta prepared from pirimiphos-methyl treated wheat are similar to
those found in flour and bread/biscuits respectively. Unchanged
pirimiphos-methyl constitutes the major proportion of the residue.
Bullock and May (1976) studied the fate of residues in wheat on
processing to semolina and pasta. White semolina prepared from Durum
wheat treated at 10 mg/kg contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90% of those in the corresponding semolina.
70% of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However the weight of pasta increases by
100% on cooking so that the concentration of pirimiphos-methyl in
cooked pasta is likely to be approximately 35% of that in the
corresponding semolina.
Peanuts
Pirimiphos-methyl residues in peanuts are found mainly on the
hulls. There is little transfer of residue to the kernels.
Pirimiphos-methyl residues in processed fractions obtained from the
kernel are normally very much smaller than in the whole raw
agricultural commodity.
Ussary (1975) has reviewed the available unpublished data for
pirimiphos-methyl residues in peanut fractions. He concluded that
residues in the kernels depend upon the degree of contamination by the
hulls which occurs in the differing shelling processes. Nevertheless
in the commercial scale trials in the U.S.A. during 1973-74, where
peanuts were treated at 20 mg/kg by admixture, pirimiphos-methyl
levels in the sound mature kernels were normally less than 5 mg/kg.
Undecorticated peanuts containing a mean residue of 10 mg/kg
pirimiphos-methyl yielded oil containing 9-14 mg/kg pirimiphos-methyl.
In the 1972-3 trials in the U.S.A. undecorticated peanuts treated
at 20 mg/kg yielded peanut butter containing mean residues of 3 mg/kg
(Ussary, 1975).
In the series of admixture trials in the U.S.A, pirimiphos-methyl
residues in the peanut hulls were normally below 50 mg/kg (Ussary,
1975).
Residues in food in commerce
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis, by gas chromatography
with a phosphorus detector, was reviewed by the 1974 Joint Meeting.
The method was recently published (Zweig, 1976). It is suitable for
the determination of residues of pirimiphos-methyl and the phosphorus-
containing metabolites II and III in crops. The limit of determination
is normally 0.01 mg/kg in each case.
NATIONAL TOLERANCES
The following national tolerances have been reported to the
Meeting.
Country Commodity Tolerance, mg/kg
France Cereals 4
Fruit and vegetables 2
Netherlands Cucumbers, courgettes,
peppers and tomatoes 0.2
West Germany Cereals 4*
Grain parts 2*
In the UK, where tolerances are not normally specified,
pirimiphos-methyl is registered for uniform admixture with small grain
cereals at 4 mg/kg.
APPRAISAL
Pirimiphos-methyl was evaluated by the 1974 Joint Meeting when,
in addition to the required toxicological information, further data
were considered desirable on its use on stored products other than
grain, on residues on fruit and vegetables following approved uses,
and on terminal levels in various foods. These data have been
provided.
In use on outdoor and indoor crops, since pirimiphos-methyl has
only a limited biological persistence on leaf surfaces it can be used
where a broad spectrum of insecticidal activity is required for a
short period, e.g., pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg. a.i./ha "low volume" or 0.05 to 0.1% a.i. "high volume"
are normally recommended. A fogging rate of 20-30 mg a.i./m3 is
normally effective in controlling greenhouse whiteflies, and a fogging
rate of 40 mg/m3 is used to control mushroom flies.
*Temporary tolerance.
A pre-harvest withholding interval of four days is normally
recommended after spraying. but 0 to 3 days after the use of a fog to
control whiteflies on greenhouse tomatoes, cucumbers and peppers or
mushroom flies.
Pirimiphos-methyl residues on growing crops decline rapidly
during the first few days after spraying. On the leaf surfaces and
other aerial parts of the plant the compound is lost principally by
volatilization; photochemical or other degradation also occurs.
However, there are no significant amounts of the degradation compounds
containing the intact phosphorus ester grouping and the
phosphorus-containing residue can be expressed as the parent compound.
Following treatment at recommended rates, levels on vegetables
are normally less than 2 mg/kg after four days but higher applications
can occasionally lead to residues on some green leafy vegetables
(lettuce, spinach, Brussels sprouts) not exceeding 5 mg/kg. On celery,
on which it is not generally recommended, residues my be higher than
this.
Residues on tree fruit are below 2 mg/kg after 4 days whilst on
soft fruits they are below 1 mg/kg. When used for fogging under glass,
initial levels are 0.3 mg/kg or less on cucumbers, tomatoes and
peppers.
Residues of pirimiphos-methyl are relatively stable in the
conditions found during baking to bread and biscuits. Flour containing
1 mg/kg of pirimiphos-methyl is likely to yield bread or biscuits
containing about 0.5 mg/kg of the unchanged compound; the main
degradation products do not contain phosphorus. Approximately similar
changes occur when durum wheat is milled to white or whole meal
semolina and this is subsequently made into pasta and cooked.
Preparation of white semolina reduces the residue by about 80% (most
of the pericarp is removed) but what is left is largely retained in
pasta, even on cooking, although the proportion is halved in cooked
pasta by uptake of water.
These results, and the additional results obtained in stored
rice, do not suggest a need for any alteration in the maximum residue
levels proposed temporarily for wheat and wheat products and rice at
the 1974 Joint Meeting and these are confirmed as maximum residue
limits.
Data evaluated by the 1974 Joint Meeting on the fate of
pirimiphos-methyl in goats, cows, hens, their edible tissues and foods
from them has now been supplemented by work with pigs which has shown
no accumulation in the fat or meat of pigs maintained on diets
containing pirimiphos-methyl at levels up to 34 mg/kg. Any residues of
the parent compound or its phosphorus-containing metabolites were
below the limit of detection (0.005 mg/kg) in muscle, kidney, liver,
lung and heart, and very small in fat. When hens were fed for 28 days
on diets containing up to 40 mg/kg of pirimiphos-methyl small residues
(0.03 - 0.04 mg/kg) were found in eggs at the highest level of
treatment. Residues of pirimiphos-methyl in meat tissues were below
0.01 mg/kg at all doses, and neither the desethyl compound nor the
oxon was detectable in any samples of eggs or meat.
RECOMMENDATIONS
As an ADI has now been established, the temporary tolerances
recommended by the 1974 Meeting are converted to maximum residue
limits. The following additional limits are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl expressed as pirimiphos-methyl.
Commodity Limit, mg/kg
Peanut hulls, peanuts (whole) 50
Peanut oil 10
Lettuce, mushroom, olives,
peanuts (kernels), spinach 5
Apples, Brussels sprouts, cabbage,
cauliflower, cherries, pears, plums 2
Blackcurrants, carrots, cucumber,
gooseberries, peppers, raspberries,
spring onions, strawberries, tomato 1
Beans with pod, cheese, citrus, dates 0.5
Peas, potatoes 0.05*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Results of studies now in progress on the residues in peanuts and
peanut products.
2. Results from commercial trials on other commodities.
REFERENCES
Berry, D., and Gore, C.W. Pirimiphos-methyl (PP511): Determination
1975 of no effect level during a 28 day rat feeding
study. Unpublished report received from ICI
Central Toxicology Laboratory. Submitted to the
World Health Organization by ICI.
Boxwell, J.W., and Bullock, D.J.W. Pirimiphos-methyl: Residues on
1976 crops following application of aerosol and dust
formulations (UK). ICI Plant Protection Division
Report No. TMJ1305A. (Unpublished)
Bullock, D.J.W. Residue summary: Pirimiphos-methyl in crops. ICI
1972 Plant Protection Ltd. Report No. TMJ595/1.
(Unpublished)
Bullock, D.J.W., Harrison, P.J., and Day, S.R. Pirimiphos-methyl:
1976 Degradation of residues in flour during baking.
ICI Plant Protection Division Report No. AR2666A.
(Unpublished)
Bullock, D.J.W., and May, M.S. Pirimiphos-methyl: Residue transfer
1976 from durum wheat to semolina and pasta. ICI Plant
Protection Division Report No. TMJ1345A.
(Unpublished).
Bullock, D.J.W., and Stephens, P.P. Pirimiphos-methyl: Residues in
1974 mushrooms following application of
pirimiphos-methyl by `Fumovap' gun and Swingfog
machine. ICI Plant Protection Ltd. Report No.
AR2543A. (Unpublished).
Cogburn, R.G. Pirimiphos-methyl and a protectant for stored rough
1976 rice: Small bin tests. J. Econ. Ent., 69(3): 369.
Davis, J.A., Day, S.R., Hemingway, R.J., Jegatheeswaren, T.,
1976 and Bullock, D.J.W. Pirimiphos-methyl: residue
transfer study with pigs. ICI Plant Protection
Division Report No. AR2665A. (Unpublished)
FAO/WHO 1974 Evaluations of some pesticide residues in food. AGP:
1975 1974/M/11; WHO Pesticide Residues Series No. 4.
Garuti, A., Gore, C.W., Ishmael, J., and Kalinowski, A.E.
1976 Pirimiphos-methyl (PP511): A study of hepatic
changes in the dog. Unpublished report from ICI.
Hanna, P.J., and Dyer, K.F. Mutagenicity of organophosphorus
1975 compounds. Mutat. Res., 28: 405.
Hodge, M.C.E., and Moore, S. Pirimiphos-methyl (PP511) teratological
1972 studies in the rat. Unpublished report from ICI
Industrial Hygiene Research Laboratories.
Howard, S.K., and Gore, C.W. The human response to long-term
1976 oral administration of low doses of
pirimiphos-methyl. Unpublished report from ICI.
Hunter, B., Graham, C., Street, Ae., Offer, J.M., and Printice,
1976 D.E. Long-term feeding of pirimiphos-methyl
(PP511) in mice. (Final report 0-80 weeks).
Unpublished report from Huntingdon Research Centre
No. ICI/34/7650, submitted by ICI.
ICI Plant Protection Ltd. Pirimiphos-methyl: Residues data for fogging
1973 use on cucumbers, tomatoes and peppers.
(Unpublished).
MacCullum Deighton, J., and Pascoe, R. Pirimiphos-methyl: Protection
1976 of stored dates: Trials in Iraq 1974/5. ICI
Plan-Protection Ltd. Report No. AR2642A.
(Unpublished)
McGregor, D.B. Dominant lethal study in mice of pirimiphos methyl.
1975 Unpublished report from Inveresk Research
International (No. 289).
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. ICI Central Toxicology
Report No. CTL/P/247.
Palmer, A.K., and Hill, P.A. Effect of pirimiphos-methyl (PP511)
1976 on reproductive functions of multiple generations
in the rat. Huntingdon Research Centre Report,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl metabolites (R4039 and R35510):
1974 Acute oral toxicity. Unpublished report from ICI
Central Toxicology Laboratory No. CTL/P/137,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl potentiation studies with
1975 bioresmethrin. Unpublished report from ICI Central
Toxicology Laboratory (No. CTL/P/166B). Submitted
by ICI.
Redlinger, L.M. Pirimiphos-methyl as a protectant for farmers
1976 stock peanuts. J.Econ. Ent., 69(3): 377.
Ross, D.B., Burroughs, S.J., and Roberts, N.L. Examination of
1975a pirimiphos-methyl for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon
Research Centre (No. ICI/49/75220) submitted by
ICI.
Ross, D.B., Christopher, D.H., Cameron, D.M., Dollery, R.,
1976 Almond, R.H., and Roberts, N.L. Egg production and
hatchability following inclusion of
pirimiphos-methyl at various levels in the diet of
the laying hen. Huntingdon Research Centre Report
No. ICI/31A/75924.
Thomas, K.P., and Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by cheshire cheese. J. Stored
Prod. Res., 11: 53.
Ussary, J.P. ActellicR residues in peanuts. ICI United States
1975 Inc. Interim Report. (Unpublished)
Zweig, G. Analytical methods for pesticides and plant growth
1976 regulators. Academic Press, 8: 125.
Stored product uses
In contrast to its short persistence on growing crops,
pirimiphos-methyl possesses exceptional stability on stored
products such as grains. Although high temperatures and high
moisture levels in grain reduce the life of the deposit, the effect
of these influences is much less than with other grain protectants
approved or evaluated to date (FAO/WHO, 1975).
During degradation, residues of pirimiphos-methyl on wheat
grains are degraded by hydrolysis of the phosphorus-ester side
chain, to give principally the parent hydroxy-pyrimidine IV (Figure
1) and also the related compounds V and VI. Levels of compound II
are always extremely low (approximately 0.05 mg/kg over a period of
32 weeks in wheat grain treated at 4 mg/kg). No residues of the
chemically unstable oxygen analogue III have been detected. The
limit of detection for compound III is 0.01 mg/kg. Residues of the
hydroxypyrimidine IV in stored grains are normally below 0.5 mg/kg.
Peanuts
Pirimiphos-methyl residues in undecorticated peanuts treated at
the intended rate of 20 mg/kg did not exceed 5.0 mg/kg. Two trials
were conducted in the U.S.A. in 1971-2 in which pirimiphos-methyl was
applied at 20 and 50 mg/kg to undecorticated peanuts. Analytical
results indicated that the target rates of treatment were achieved.
Very little change occurred in the level of residues during the first
month following application. Even at the end of three months
substantially all of the deposit remained. The data suggest that the
half-life is of the order of 5 months from the time of application.
Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied. These data have recently been published (Redlinger, 1976).
A larger scale trial was conducted in the U.S.A. during 1972-73
(Ussary, 1975). Pirimiphos-methyl was applied at 10, 20 and 30 mg/kg
as an admixture treatment to undecorticated peanuts. Analytical
results again indicated that the target rate of treatment was
achieved. 20-50% of the initial residue was present on the
undecorticated peanuts after twelve months storage, compared with 10%
in the case of malathion. During four commercial scale trials in the
U.S.A. in 1973-74, in which pirimiphos-methyl was applied at the
proposed commercial application rate of 20 mg/kg, 30-60% of the
initial residue was present at bin emptying, that is after 4 ´-6
months storage (Ussary, 1975).
TABLE 1. Residues of pirimiphos-methyl resulting from supervised spray trials on leafy vegetables and root crops (EC formulations)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Beans West Germany 50% EC 0.15 kg/ha 1 0.15-0.25 <0.01-0.07 <0.01-0.01
(Bush) 0.3 kg/ha 1 0.05-0.47 <0.01-0.08 <0.01-0.04
(French) U.K. 50% EC or 0.05% 1 0.06-0.07 0.01-0.03 <0.01-0.01
8% EC 0.1% 1 0.08-0.14 0.10-0.14 <0.01-0.06
0.2% 1 - 0.26 0.14
Peas U.K. 50% EC 0.1 % 1 - - <0.01
0.2 % 1-2 - <0.01 <0.01
Lettuce U.K. 50% EC or 0.025% 3 0.19-0.73 0.07-0.13 0.30-0.12
25% EC 0.05% 1-3 11-18 0.60-1.8 0.37-0.88
0.1% 1-3 19-39 1.8-2.9 0.63-2.1
West Germany 50% EC 0.025% 1 2.6-2.7 0.23 0.07-0.13
0.05% 1 3.4-7.3 0.52 0.14-0.71
0.1% 4.7 - 0.52
West Germany 25% EC (0.15 kg/ha 1 4.6-8.1 0.02-0.24 0.02-0.19
0.3 kg/ha 1 6-13 0.05-0.50 0.01-0.11
Spinach West Germany 25% EC 0.025% 1 3.2-6.7 0.35-1.0 0.22
0.05% 1 12-14 1.5-1.6 0.19-0.43
0.1% 1 21 3.4 0.75
Cabbage U.K. 25% EC 1.0 kg/ha 1 - 0.30-0.50** <0.02-0.28
2.0 kg/ha 1 - 0.31-1.8 0.09-0.40
West Germany 25% EC 0.15 kg/ha 1 0.13-1.4 0.05-0.18 <0.01-0.09
0.3 kg/ha 1 0.57-4.6 <0.01-0.69 <0.01-0.40
0.6 kg/ha 1 4.1 1.5 0.18
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Cabbage Malaysia 25% EC 1.0 kg/ha 18 - - 0.01***
2.0 kg/ha 18 - - 0.01-0.02***
Cauliflower U.K. 50% EC or 0.1% 1 0.20-2.0 0.01-0.11 <0.01
8% EC
(Hearts) 0.2% 1 0.15-1.4 0.04-0.24 <0.01-0.04
(leaves) 50% EC or 0.1% 1 0.66-12 0.50-1.3 0.02-0.35
8% EC 0.2% 1 1.5-13 0.65-1.1 0.04-2.2
Brussels U.K. 50% EC 1.4 kg/ha 3 1.7-3.3 0.30-1.7 0.28-0.66
sprouts (0.1%)
(buttons) 2.28 kg/ha 3 2.9-6.8 0.96-4.5 0.67-2.3
(0.2%)
Brussels Netherlands 50% EC 0.5 kg/ha 1 0.26* 0.14** 0.07
sprouts (0.05 %)
(buttons) 0.75 kg/ha 1 0.49* 0.39** 0.21
(0.075%)
Spring U.K. 50% EC or 0.05% 1 0.28-1.6 <0.01-0.07 <0.01-0.08
onions 8% EC 0.1% 1 2.3-4.2 0.04-0.49 <0.01-0.46
Celery U.K. 8% EC 0.05% 1 18-34 3.1-8.8 1.2-2.7
0.1% 1 26-69 5.1-14 3.70-8.0
Carrots U.K. 50% EC 1.14 kg/ha 1 0.32-0.95 0.09-0.16 0.10-0.22
(0.1%)
2.28 kg/ha 1 0.57-2.2 0.17-0.48 0.12-0.50
(0.2%)
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Potatoes U.K. 50% EC 0.1% 2 <0.01 <0.01
0.2% 2 <0.01 <0.01 <0.01-0.02
Sugar West Germany 25% EC 0.05% 1 18 - 1.7
beets (0.3 kg/ha)
0.1% 1 38 - 3.1
(0.6 kg/ha)
* Samples taken after 1 day
** Samples taken after 3 days
*** Samples taken after 9-15 days
TABLE 2 Residues of pirimiphos-methyl resulting from supervised spray trials on tree and sort fruits (EC and ULV formulations
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Apples U.K. 50% EC or 0.1 % 3 0.51-1.3 0.25-0.67 0.10-0.80
8 % 0.05% 1
0.2 % 3 0.76-2.8 0.58-1.9 0.16-1.5
0.1% 1
France 50% EC 0.075% 6 - - - 2 weeks: 0.32
Japan 50% EC 0.1 % 1-3 - - - 10 days: 0.15-1.4
Pears Spain 50% EC 0.1 % 1 - - - 12 weeks: <0.01
Plums U.K. 50% EC 0.1 % 2 0.47-0.96 0.24-0.55 0.02-0.25
0.2 % 2 1.6-2.2 1.3-1.5 0.06-0.55
Lemons Italy 25% EC 0.05 % 1 - - - 9 weeks: 0.25 in peel
0.03 in flesh
0.075% 1 - - - 0.75 in peel
0.03 in flesh
0.01 % - - 0.27-0.35 - -
in peel
0.02-0.03
in flesh
Oranges Australia 25% EC 0.075% 2 - - - 4´ months: 0.69 in peel
<0.01 in flesh
0.1 2 - - - 0.92 in peel
<0.01 in flesh
TABLE 2 (Cont'd.)
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Oranges South 25% EC 0.075% 1 - - - 2-8 months: <0.01-0.01 in peel
Africa <0.01 in flesh
0.01-0.125% 1 - - - <0.01-0.1 in peel
<0.01-0.1 in flesh
Olives Spain 50%ULV 0.25 kg/ha 2 - - 0.46 9-20 days: 0.07-0.36
0.5 kg/ha 2 - - - 0.16-0.45
1.0 kg/ha 2 - - - 0.18-1.4
Blackcurrants U.K. 8% EC 0.05 % 2 0.25-0.75 0.06-0.20 0.01-0.18
0.1 % 2 0.86-2.3 0.25-0.62 0.08-0.34
Raspberries U.K. 50% EC or 0.05% 1-2 0.81 0.23 0.13 2-5 weeks: <0.01-0.03
8% EC 0.01% 1-2 2.5 0.53 0.31 <0.01-0.09
Strawberries U.K. 50% EC or 0.05% 1 0.14-0.79 0.17-0.39 0.04-0.16 4´-7 weeks: <0.01-0.05
8% 0.1% 1 0.40-2.9 0.31-0.71 0.13-0.25 <0.01-0.05
TABLE 3. Levels of pirimiphos-methyl resulting from supervised residue trials on greenhouse crops and mushrooms using a 10% fogging
formulation
Number of Residues (mg/kg) at intervals after last application
crop Country Rate (a.i.) Applications 0 days 1 day 2 days 3 days 4 days
Cucumbers U.K. 0.75 kg/ha 1-4 0.06-0.30 0.03-0.11 0.02-0.11 0.01-0.04 <0.01
(=20 mg/m3)
Netherlands 20 mg/m3 1-5 0.08-0.19 0.03-0.08 0.02 <0.01-0.02 0.02
20 mg/m3 5
+30mg/m3 1-5 - 0.04-0.20 - 0.01-0.08 0.02-0.03
Tomatoes U.K. 0.5 kg/ha 2 - 0.15 - - -
(= 15 mg/m3)
1 kg/ha or 1-4 - 0.16-0.62 0.16 0.12 -
30 mg/m3
Netherlands 0.75 kg/ha 1-3 - <0.01-0.12 <0.01-0.18 0.08 -
(= 20 mg/m3)
30 mg/m3 1-5 - 0.06-0.17 - 0.08-0.14 0.08-0.11
(= 1 kg/ha)
Peppers U.K. 1 kg/ha 1 - 0.26 - - -
Mushrooms U.K. 40 mg/m3 1 and 6 0.99 0.19 0.12 - 0.14-0.16
(two trials) -2.5 -1.4 -0.44 - (4-5 days)
TABLE 4. Residues of pirimiphos-methyl resulting from supervised trials in the U.K. using dust and aerosol
formulations (single application date per trial)
Residues (mg/kg) at intervals after
Crop Formulation Rate 0 days 2 days 4 days 7-8 days
Lettuce Dust Normal 2.1-10 0.53-7.9 0.33-2.1 0.06-1.9
Overdosed 8.3-47 2.2-19 1.1-5.6 0.32-5.2
Aerosol Normal 1.6-21 0.53-11 0.34-2.8 0.16-1.8
Overdosed 13-40 6.2-25 2.0-7.6 0.75-3.8
Tomatoes Dust Normal 0.01-0.93 <0.01-0.15 0.07-0.09 0.04-0.07
Overdosed 0.18-1.4 0.08-0.89 0.05-0.13 0.02-0.03
Aerosol Normal 0.09-0.67 0.04-0.43 0.02-0.38 0.03-0.05
Overdosed 0.03-4.1 0.01-1.3 0.02-0.46 0.01-0.03
Blackcurrants Dust Normal 0.10-0.59 0.03-0.05 - -
Overdosed 0.44-1.4 0.08-0.22 - -
Aerosol Normal <0.01-0.27 <0.01-0.04 - -
Overdosed 0.04-0.59 <0.01-0.29 - -
During these studies there were wide variations between different
samples analysed from the same bin. These were probably caused by
non-representative sampling. Dust, estimated to constitute up to 10%
of the contents of the bins, settles into layers in the bins. It
contains several hundred mg/kg pirimiphos-methyl and in large bins it
was impossible to sample the dust in a representative manner. However,
residues in individual samples of undecorticated peanuts in the U.S.A.
trials were generally below 5.0 mg/kg during the period after
treatment at the proposed recommended rate of 20 mg/kg.
The walls of the four warehouses used in the commercial scale
trials were sprayed with a 0.5% aqueous suspension of
pirimiphos-methyl and probe samples were taken adjacent to the walls
during the storage period. Residues in the undecorticated peanuts were
similar to those from the bulk of the warehouse and it was concluded
that there was no major transfer of pirimiphos-methyl from the walls
to the peanuts (Ussary, 1975).
Rice
The 1974 Joint Meeting recommended temporary tolerances of 10
mg/kg in rice (in husk), 20 mg/kg in rice bran, 2 mg/kg in dehusked
rice and 1 mg/kg in polished rice.
Cogburn (1976) in the U.S.A. found that an admixture treatment of
pirimiphos-methyl at 10 mg/kg protected rice from all insect
infestation for twelve months. At this rate residues in the rice in
husk, bran, dehusked rice and polished rice were all within the
temporary tolerance levels proposed by the 1974 Joint Meeting.
Dates
In supervised trials in Iraq, MacCallum, Deighton and Pascoe
(1976) found that 500 mg pirimiphos-methyl per square metre sprayed
onto packaging materials (cardboard or wooden boxes) was effective in
protecting export dates from insect attack. A deposit of 125 mg per
square metre was effective for the treatment of waxed paper liners. At
these rates pirimiphos-methyl residues in the dates during the
following six months were invariably small - i.e. less than 0.5 mg/kg.
Cheese
There is very little movement of pirimiphos-methyl into cheese
stored on surfaces treated with the insecticide for mite control.
Thomas and Rowlands (1975) treated a wooden plank at 440 mg per
square metre with 2-14C-labelled pirimiphos-methyl and then stored a
22 kg Cheshire cheese, wrapped in a cloth, on it for fourteen weeks.
The cheese was turned to stand on alternate ends weekly, as in normal
storage practice. The radioactive residue was confined to the outer 4
mm of the cheese in which there was no detectable breakdown of
pirimiphos-methyl.
Levels of pirimiphos-methyl were approximately 40 mg/kg in the
outer 1 mm and 5-10 mg/kg in the outer 6 mm of the cheese. On a "whole
cheese" basis, this represents a residue of approximately 0.4 mg/kg.
FATE OF RESIDUES
In animals
Extensive data were evaluated by the 1974 Joint Meeting on the
fate of pirimiphos-methyl in goats, cows, hens, their edible tissues
and in foods of animal origin.
When fed to these animals, pirimiphos-methyl is rapidly
metabolised and the metabolites are excreted, principally in the
urine. Only very small quantities are excreted into milk or eggs.
Cattle receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites II and
III could be detected in meat, milk, butter or eggs (limit of
detection: 0.01 mg/kg or below).
Since the 1974 Joint Meeting, data on the fate in pigs and
additional data on the fate in hens have become available.
Pigs
Davis et al. (1976) maintained groups of pigs for 21 and 29 days
on diets containing 3, 10 and 34 mg/kg pirimiphos-methyl. No residues
of pirimiphos-methyl or of its phosphorus containing metabolites II or
III were detected in the kidney, liver, lung, heart or muscle (limit
of detection: 0.005 mg/kg in each case). Small residues of
pirimiphos-methyl and compound II were found in the fat of pigs fed
pirimiphos-methyl at 10 and 34 ppm in the diet (Table 15). No residues
were detected in the fat of pigs returned for eight days to untreated
diet prior to slaughter. No residues of the P=0 analogue (III) were
detected in any fat tissues (limit of detection 0.005 mg/kg).
No residues of the hydroxypyrimidines IV and V were found in any
of the above-mentioned tissues (limits of detection 0.01 mg/kg for
compound IV and 0.04 mg/kg for compound V).
Hens
Ross et al. (1975b) confirmed the low residue levels in eggs and
meat previously reported by other authors (FAO/WHO, 1975).
Groups of laying hens were maintained for 28 days on diets
containing 0, 4, 12 and 40 ppm pirimiphos-methyl. At all but the
highest dose level, pirimiphos-methyl levels in the eggs were below
0.01 mg/kg. At the 40 ppm dietary inclusion level, pirimiphos-methyl
levels in the egg yolk reached a plateau of 0.03-0.04 mg/kg after
seven days. After the hens had been returned to untreated diet for
seven days, pirimiphos-methyl residues in the yolks were below the
limit of detection (i.e. less than 0.008 mg/kg). Residues in the
albumen were negligible (less than 0.01 mg/kg).
Residues of pirimiphos-methyl in meat tissues were below 0.01
mg/kg at all dose levels.
No residues of the phosphorus-containing metabolites II and III
were detected in any samples of eggs or meat (limit of detection:
0.006-0.02 mg/kg).
In processing
Initial data on the fate of pirimiphos-methyl residues or
processing of stored products were reviewed by the 1974 Joint Meeting
(FAO/WHO, 1975). Subsequent studies are described below.
TABLE 5. Residues of pirimiphos-methyl and its desethyl metabolite
in the fat of pigs fed pirimiphos-methyl in the diet
Desethyl
Days Level in diet Pirimiphos-methyl Metabolite
on diet (ppm) (mg/kg) (mg/kg)
21 control ND ND
3 ND ND
10 0.008 0.016
34 0.034 0.034
29 3 ND ND
10 0.006 0.009
34 0.05 0.036
29 control ND ND
+ 8 days 3 ND ND
recovery
3 ND ND
10 ND ND
10 ND ND
34 ND ND
34 ND ND
ND: Not detected, less than 0.005 mg/kg
Wheat
Residues of pirimiphos-methyl in flour are relatively stable to
the conditions found during baking to bread and biscuits. However,
because of the dilution which the flour undergoes during these
processes flour initially containing 1 mg/kg pirimiphos-methyl is
likely to yield bread/biscuits containing residues of the order of
0.5 mg/kg.
Bullock and co-workers (1976) have studied extensively the fate
of pirimiphos-methyl during processing to bread and biscuits. In
studies with the radiolabelled compound, flour was dosed with
2-14C-labelled pirimiphos-methyl and baked to produce white bread,
wholemeal bread and biscuits. Although pirimiphos-methyl is known to
be a relatively volatile compound, there was no significant loss of
radioactivity by volatilisation. Distribution of radioactivity
throughout the bread was fairly uniform. Unchanged pirimiphos-methyl
accounted for 75-90% of the radioactivity in the baked product. The
major degradation products formed during baking were the
hydroxypyrimidines IV and VI, which accounted for 3-10% of the
radioactivity in the final product.
Similar results were obtained by residue analysis in a second set
of studies. After correction for the weight increase when flour is
baked to bread, residues of pirimiphos-methyl fell by 11-18%.
Likewise, residue analysis of biscuits showed average losses of 8%.
However, owing to the dilution of the flour which occurs during
baking, the residue level of pirimiphos-methyl in bread will be lower
than that in the corresponding flour.
During extensive residue studies Bullock et al.(1976) found that
levels of pirimiphos-methyl in bread and biscuits are likely to be
about 50% of those in the flour from which they were derived. These
findings agree well with the earlier work of Bullock (1973, 1974)
which was reviewed by the 1974 Joint Meeting. No residues of the
phosphorus-containing compounds II and III were detected in bread
baked from flour treated with pirimiphos-methyl at 1 mg/kg or in
biscuits baked from flour treated at up to 5 mg/kg (limit of
detection: 0.03 mg/kg)(Bullock et al., 1976).
The hydroxypyrimidine IV also undergoes little degradation during
the baking process. This compound has previously been shown to
constitute a minor part (generally less than 0.5 mg/kg) of the residue
in stored grains (FAO/WHO 1975). When bread was baked from flour
containing 2-14C-labelled compound IV, the unchanged
hydroxypyrimidine constituted more than 90% of the radioactive residue
in the bread (Bullock et al., 1976). By analogy with pirimiphos-methyl
residues, it can be concluded that residues of compound IV in baked
products will not exceed 0.2 mg/kg and will normally be considerably
lower.
The pattern and levels of residues found in semolina and cooked
pasta prepared from pirimiphos-methyl treated wheat are similar to
those found in flour and bread/biscuits respectively. Unchanged
pirimiphos-methyl constitutes the major proportion of the residue.
Bullock and May (1976) studied the fate of residues in wheat on
processing to semolina and pasta. White semolina prepared from Durum
wheat treated at 10 mg/kg contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90% of those in the corresponding semolina.
70% of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However the weight of pasta increases by
100% on cooking so that the concentration of pirimiphos-methyl in
cooked pasta is likely to be approximately 35% of that in the
corresponding semolina.
Peanuts
Pirimiphos-methyl residues in peanuts are found mainly on the
hulls. There is little transfer of residue to the kernels.
Pirimiphos-methyl residues in processed fractions obtained from the
kernel are normally very much smaller than in the whole raw
agricultural commodity.
Ussary (1975) has reviewed the available unpublished data for
pirimiphos-methyl residues in peanut fractions. He concluded that
residues in the kernels depend upon the degree of contamination by the
hulls which occurs in the differing shelling processes. Nevertheless
in the commercial scale trials in the U.S.A. during 1973-74, where
peanuts were treated at 20 mg/kg by admixture, pirimiphos-methyl
levels in the sound mature kernels were normally less than 5 mg/kg.
Undecorticated peanuts containing a mean residue of 10 mg/kg
pirimiphos-methyl yielded oil containing 9-14 mg/kg pirimiphos-methyl.
In the 1972-3 trials in the U.S.A. undecorticated peanuts treated
at 20 mg/kg yielded peanut butter containing mean residues of 3 mg/kg
(Ussary, 1975).
In the series of admixture trials in the U.S.A, pirimiphos-methyl
residues in the peanut hulls were normally below 50 mg/kg (Ussary,
1975).
Residues in food in commerce
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis, by gas chromatography
with a phosphorus detector, was reviewed by the 1974 Joint Meeting.
The method was recently published (Zweig, 1976). It is suitable for
the determination of residues of pirimiphos-methyl and the phosphorus-
containing metabolites II and III in crops. The limit of determination
is normally 0.01 mg/kg in each case.
NATIONAL TOLERANCES
The following national tolerances have been reported to the
Meeting.
Country Commodity Tolerance, mg/kg
France Cereals 4
Fruit and vegetables 2
Netherlands Cucumbers, courgettes,
peppers and tomatoes 0.2
West Germany Cereals 4*
Grain parts 2*
In the UK, where tolerances are not normally specified,
pirimiphos-methyl is registered for uniform admixture with small grain
cereals at 4 mg/kg.
APPRAISAL
Pirimiphos-methyl was evaluated by the 1974 Joint Meeting when,
in addition to the required toxicological information, further data
were considered desirable on its use on stored products other than
grain, on residues on fruit and vegetables following approved uses,
and on terminal levels in various foods. These data have been
provided.
In use on outdoor and indoor crops, since pirimiphos-methyl has
only a limited biological persistence on leaf surfaces it can be used
where a broad spectrum of insecticidal activity is required for a
short period, e.g., pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg. a.i./ha "low volume" or 0.05 to 0.1% a.i. "high volume"
are normally recommended. A fogging rate of 20-30 mg a.i./m3 is
normally effective in controlling greenhouse whiteflies, and a fogging
rate of 40 mg/m3 is used to control mushroom flies.
*Temporary tolerance.
A pre-harvest withholding interval of four days is normally
recommended after spraying. but 0 to 3 days after the use of a fog to
control whiteflies on greenhouse tomatoes, cucumbers and peppers or
mushroom flies.
Pirimiphos-methyl residues on growing crops decline rapidly
during the first few days after spraying. On the leaf surfaces and
other aerial parts of the plant the compound is lost principally by
volatilization; photochemical or other degradation also occurs.
However, there are no significant amounts of the degradation compounds
containing the intact phosphorus ester grouping and the
phosphorus-containing residue can be expressed as the parent compound.
Following treatment at recommended rates, levels on vegetables
are normally less than 2 mg/kg after four days but higher applications
can occasionally lead to residues on some green leafy vegetables
(lettuce, spinach, Brussels sprouts) not exceeding 5 mg/kg. On celery,
on which it is not generally recommended, residues my be higher than
this.
Residues on tree fruit are below 2 mg/kg after 4 days whilst on
soft fruits they are below 1 mg/kg. When used for fogging under glass,
initial levels are 0.3 mg/kg or less on cucumbers, tomatoes and
peppers.
Residues of pirimiphos-methyl are relatively stable in the
conditions found during baking to bread and biscuits. Flour containing
1 mg/kg of pirimiphos-methyl is likely to yield bread or biscuits
containing about 0.5 mg/kg of the unchanged compound; the main
degradation products do not contain phosphorus. Approximately similar
changes occur when durum wheat is milled to white or whole meal
semolina and this is subsequently made into pasta and cooked.
Preparation of white semolina reduces the residue by about 80% (most
of the pericarp is removed) but what is left is largely retained in
pasta, even on cooking, although the proportion is halved in cooked
pasta by uptake of water.
These results, and the additional results obtained in stored
rice, do not suggest a need for any alteration in the maximum residue
levels proposed temporarily for wheat and wheat products and rice at
the 1974 Joint Meeting and these are confirmed as maximum residue
limits.
Data evaluated by the 1974 Joint Meeting on the fate of
pirimiphos-methyl in goats, cows, hens, their edible tissues and foods
from them has now been supplemented by work with pigs which has shown
no accumulation in the fat or meat of pigs maintained on diets
containing pirimiphos-methyl at levels up to 34 mg/kg. Any residues of
the parent compound or its phosphorus-containing metabolites were
below the limit of detection (0.005 mg/kg) in muscle, kidney, liver,
lung and heart, and very small in fat. When hens were fed for 28 days
on diets containing up to 40 mg/kg of pirimiphos-methyl small residues
(0.03 - 0.04 mg/kg) were found in eggs at the highest level of
treatment. Residues of pirimiphos-methyl in meat tissues were below
0.01 mg/kg at all doses, and neither the desethyl compound nor the
oxon was detectable in any samples of eggs or meat.
RECOMMENDATIONS
As an ADI has now been established, the temporary tolerances
recommended by the 1974 Meeting are converted to maximum residue
limits. The following additional limits are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl expressed as pirimiphos-methyl.
Commodity Limit, mg/kg
Peanut hulls, peanuts (whole) 50
Peanut oil 10
Lettuce, mushroom, olives,
peanuts (kernels), spinach 5
Apples, Brussels sprouts, cabbage,
cauliflower, cherries, pears, plums 2
Blackcurrants, carrots, cucumber,
gooseberries, peppers, raspberries,
spring onions, strawberries, tomato 1
Beans with pod, cheese, citrus, dates 0.5
Peas, potatoes 0.05*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Results of studies now in progress on the residues in peanuts and
peanut products.
2. Results from commercial trials on other commodities.
REFERENCES
Berry, D., and Gore, C.W. Pirimiphos-methyl (PP511): Determination
1975 of no effect level during a 28 day rat feeding
study. Unpublished report received from ICI
Central Toxicology Laboratory. Submitted to the
World Health Organization by ICI.
Boxwell, J.W., and Bullock, D.J.W. Pirimiphos-methyl: Residues on
1976 crops following application of aerosol and dust
formulations (UK). ICI Plant Protection Division
Report No. TMJ1305A. (Unpublished)
Bullock, D.J.W. Residue summary: Pirimiphos-methyl in crops. ICI
1972 Plant Protection Ltd. Report No. TMJ595/1.
(Unpublished)
Bullock, D.J.W., Harrison, P.J., and Day, S.R. Pirimiphos-methyl:
1976 Degradation of residues in flour during baking.
ICI Plant Protection Division Report No. AR2666A.
(Unpublished)
Bullock, D.J.W., and May, M.S. Pirimiphos-methyl: Residue transfer
1976 from durum wheat to semolina and pasta. ICI Plant
Protection Division Report No. TMJ1345A.
(Unpublished).
Bullock, D.J.W., and Stephens, P.P. Pirimiphos-methyl: Residues in
1974 mushrooms following application of
pirimiphos-methyl by `Fumovap' gun and Swingfog
machine. ICI Plant Protection Ltd. Report No.
AR2543A. (Unpublished).
Cogburn, R.G. Pirimiphos-methyl and a protectant for stored rough
1976 rice: Small bin tests. J. Econ. Ent., 69(3): 369.
Davis, J.A., Day, S.R., Hemingway, R.J., Jegatheeswaren, T.,
1976 and Bullock, D.J.W. Pirimiphos-methyl: residue
transfer study with pigs. ICI Plant Protection
Division Report No. AR2665A. (Unpublished)
FAO/WHO 1974 Evaluations of some pesticide residues in food. AGP:
1975 1974/M/11; WHO Pesticide Residues Series No. 4.
Garuti, A., Gore, C.W., Ishmael, J., and Kalinowski, A.E.
1976 Pirimiphos-methyl (PP511): A study of hepatic
changes in the dog. Unpublished report from ICI.
Hanna, P.J., and Dyer, K.F. Mutagenicity of organophosphorus
1975 compounds. Mutat. Res., 28: 405.
Hodge, M.C.E., and Moore, S. Pirimiphos-methyl (PP511) teratological
1972 studies in the rat. Unpublished report from ICI
Industrial Hygiene Research Laboratories.
Howard, S.K., and Gore, C.W. The human response to long-term
1976 oral administration of low doses of
pirimiphos-methyl. Unpublished report from ICI.
Hunter, B., Graham, C., Street, Ae., Offer, J.M., and Printice,
1976 D.E. Long-term feeding of pirimiphos-methyl
(PP511) in mice. (Final report 0-80 weeks).
Unpublished report from Huntingdon Research Centre
No. ICI/34/7650, submitted by ICI.
ICI Plant Protection Ltd. Pirimiphos-methyl: Residues data for fogging
1973 use on cucumbers, tomatoes and peppers.
(Unpublished).
MacCullum Deighton, J., and Pascoe, R. Pirimiphos-methyl: Protection
1976 of stored dates: Trials in Iraq 1974/5. ICI
Plan-Protection Ltd. Report No. AR2642A.
(Unpublished)
McGregor, D.B. Dominant lethal study in mice of pirimiphos methyl.
1975 Unpublished report from Inveresk Research
International (No. 289).
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. ICI Central Toxicology
Report No. CTL/P/247.
Palmer, A.K., and Hill, P.A. Effect of pirimiphos-methyl (PP511)
1976 on reproductive functions of multiple generations
in the rat. Huntingdon Research Centre Report,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl metabolites (R4039 and R35510):
1974 Acute oral toxicity. Unpublished report from ICI
Central Toxicology Laboratory No. CTL/P/137,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl potentiation studies with
1975 bioresmethrin. Unpublished report from ICI Central
Toxicology Laboratory (No. CTL/P/166B). Submitted
by ICI.
Redlinger, L.M. Pirimiphos-methyl as a protectant for farmers
1976 stock peanuts. J.Econ. Ent., 69(3): 377.
Ross, D.B., Burroughs, S.J., and Roberts, N.L. Examination of
1975a pirimiphos-methyl for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon
Research Centre (No. ICI/49/75220) submitted by
ICI.
Ross, D.B., Christopher, D.H., Cameron, D.M., Dollery, R.,
1976 Almond, R.H., and Roberts, N.L. Egg production and
hatchability following inclusion of
pirimiphos-methyl at various levels in the diet of
the laying hen. Huntingdon Research Centre Report
No. ICI/31A/75924.
Thomas, K.P., and Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by cheshire cheese. J. Stored
Prod. Res., 11: 53.
Ussary, J.P. ActellicR residues in peanuts. ICI United States
1975 Inc. Interim Report. (Unpublished)
Zweig, G. Analytical methods for pesticides and plant growth
1976 regulators. Academic Press, 8: 125.
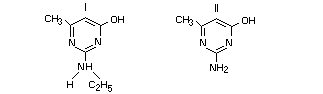 Short-term studies
Rat
Pirimiphos-methyl was administered to young rats at doses of
0, 5, 8, 10 and 50 ppm in the diet for a period of 28 days in order
to determine the no-effect level on plasma cholinesterase activity
and body weight gain. Plasma cholinesterase activity was inhibited
at the 50 ppm level throughout the dosing period by 30% and 50% in
male and female animals respectively. No effect on erythrocyte
cholinesterase activity was detected. There was a tendency towards
poorer food utilization in the group but there was no effect on
body weight gain.
There were sporadic instances of statistically significant
depressions of plasma cholinesterase activity at the 8 and 10 ppm
levels but less than 25% and not dose related. The "no effect"
level for pirimiphos-methyl in the young rat was 10 ppm in the diet
administered for a period of 28 days. This is also the no-effect
level established for pirimiphos-methyl in the rat over two years
(FAO/WHO, 1975; Berry and Gore, 1975).
Dog
In order to examine the potential hepatic effect of
pirimiphos-methyl in the dog, ten more dogs were given 25 mg/kg/day
with six dogs acting as controls. Three of the dogs were killed
after four weeks dosing for histopathological examination. The
remaining animals were maintained at the same dose level for a
total period of 13 weeks except for two dogs whose dose was raised
progressively to 50 mg/kg/day from week eight onwards.
Slight bile duct proliferation was present in two dogs given
25 mg/kg pirimiphos-methyl for 13 weeks. None of the other dosed
animals showed this effect even when the dose was increased to 50
mg pirimiphos-methyl/kg/day in two other dogs.
The effects in the dog liver characterised by bile duct
proliferation in a certain number of dogs given 25 mg/kg/day was
confirmed. However the changes appear to be small and dependent
upon individual animal sensitivity and reversible; the mode of
action could not be ascertained. Focal inflammation was present in
the livers of control and dosed animals. Liver changes were shown
in the rat at levels of 300-600 ppm in the diet and in dogs dosed
at 10 mg/kg/day for two years (Garuti et al., 1976).
Long-term studies
Mouse
Four groups of 52 male and 52 female mice of the CFLP strain
were fed 0, 5, 250 and 500 ppm pirimiphos-methyl in the diet for 80
weeks. Three extra groups of 12 male and 12 female mice were fed 0,
5 and 500 ppm and were used for the investigation of cholinesterase
levels.
There were no significant differences in mortality between
control and treated groups. Macroscopic examination of descendants
gave no indication of treatment-related change. Efficiency of food
utilization and overall body weight gain were not affected. Food
consumption was reduced slightly among females receiving 500 ppm
only. No changes ascribable to the administration of pirimiphos-
methyl were seen during gross and histological examination at
autopsy.
Changes in the kidneys, namely foci of lymphocytic
infiltration in the interstitium, or minimal perivascular or
peribronchiolar lymphoid aggregations in the lung and abscesses and
small haemorrhages in ovaries and other morphological changes in
various tissues were seen in a proportion of animals of all groups
including controls and were considered to be of no toxicological
significance. Similarly the liver tumour incidence was comparable
to that of the controls.
There was no evidence to suggest that treatment with
pirimiphos-methyl altered the tumour profile of the CFLP mouse at
levels up to 500 ppm. However, animals of both sexes showed marked
inhibition of plasma and erythrocyte cholinesterase activities at
the 500 ppm dietary inclusion level. When corrected for differences
prevailing between groups at the start of the study, differences in
erythrocyte cholinesterase activity between the control and 5 ppm
pirimiphos-methyl groups were normally less than 20% and
consistently less than 25%. Plasma cholinesterase activities in
both untreated and 5 ppm dietary inclusion level groups followed
similar, slightly fluctuating patterns during the study, although
levels in both sexes in the treated group appeared inhibited by
comparison with controls. However there was no evidence of
progressive inhibition during treatment and differences between the
control and 5 ppm groups were considered to be of borderline
biological significance (Hunter et al., 1976). A "no-effect" level
at 5 ppm in the diet, equivalent to approximately 0.5 mg/kg/day was
established over an 80 week period.
OBSERVATIONS IN MAN
A group of seven healthy volunteers, consisting of three males
and four females, was given orally a dose of 0.25 mg
pirimiphos-methyl/kg bw, in capsule form for a period of 56 days.
No effect due to the administration of pirimiphos-methyl was seen
on liver function tests, blood count or erythrocyte or plasma
cholinesterase activity (Howard and Gore, 1976).
COMMENTS
Results of studies on the effect of pirimiphos-methyl on rat
reproduction in a three generation test, on avian-egg production
and hatchability and of special tests on mutagenicity were
negative.
Hydronephrosis incidence noted in a previous test has been
shown to be well within the limits of control values pertaining to
the same species.
Two studies were performed to investigate the potential
hepatotoxicity of pirimiphos-methyl. In an 80 week mouse study the
tumour incidence (including that in liver) was comparable in test
and control groups. In a three month dog study slight bile duct
proliferation was observed in animals dosed at 25 mg/kg/day,
however the effect was absent at 50 mg/kg/day. Focal inflammation
occurred equally in the livers of control and dosed animals. In a
56 day human study, 0.25 mg/kg/day did not induce any changes in
liver function tests, blood count or erythrocyte or plasma
cholinesterase activity. Additional biochemical data presented
established that the substance is very rapidly absorbed,
metabolised and excreted.
The Meeting agreed that the previously indicated further
studies have been carried out satisfactorily, and there is no
objection to estimating a permanent acceptable daily intake.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Mouse 5 ppm in the diet equivalent to 0.5 mg/kg bw/day
Rat 10 ppm in the diet equivalent to 0.5 mg/kg bw/day
Man 0.25 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.01 mg/kg bw/day
RESIDUES IN FOOD AND THEIR EVALUATION
The 1974 evaluation of pirimiphos-methyl (FAO/WHO, 1975) was
principally in relation to its use as a treatment of stored grains.
However the insecticide is also used on growing crops, in public
health and in industrial outlets. Therefore, arising out of the
list of requirements in the report of the 1974 Joint Meeting, the
following additional data are now presented.
1. Information on pre-harvest use patterns and residues in fruit
and vegetables.
2. Information on residues in peanuts and other stored products.
3. Further information on the level and fate of residues at the
point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
USE PATTERN
Pre-harvest
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant
actions. It is effective against a wide range of flying and
crawling insects, including aphids, scales, mealy bugs, whiteflies,
bugs, thrips, beetles, caterpillars, moths and ants. It also has
acaricidal activity. It controls both adult and immature stages of
insects such as whiteflies, and is effective against strains of
insects which have developed resistance to malathion.
Because it has only limited biological persistence on leaf
surfaces, pirimiphos-methyl is particularly attractive for uses in
which a broad spectrum of insecticidal activity is required for a
short period. e.g. pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg a.i./ha "low volume" or 0.05-0.1% a.i. "high volume" are
normally recommended. 0.5-0.75 kg a.i./ha (or 20-30 mg a.i. per
m3) is normally effective in controlling greenhouse whiteflies by
fogging. A fogging rate of 40 mg per m3 is used to control
mushroom flies.
The principal formulation is a 50% emulsifiable concentrate.
A 50% oil-based formulation is available for ultra-low volume
application to outdoor crops and a 10% oil-based formulation is
used for whitefly control under glass. An 8% emulsifiable
concentrate is sold to the amateur gardener. Dust and aerosol
formulations are under development for crop uses.
A pre-harvest withholding interval of four days is normally
recommended after spraying. No pre-harvest withholding interval is
normally recommended after the use of a fog to control
1. whiteflies on greenhouse tomatoes, cucumbers and peppers.
2. mushroom flies.
Pirimiphos-methyl is not generally recommended for use on
celery.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Data reviewed by the 1974 Joint Meeting showed that
pirimiphos-methyl is rapidly lost from leaf surfaces during the
first 2-3 days after spraying, mainly by volatilisation. Levels of
the parent insecticide and the major degradation product, compound
II (Figure 1), represent less than 10% of the applied material
after two to three days. After foliar treatment the hydroxy-
pyrimidine IV, formed by hydrolysis of the insecticide, is unlikely
to be a significant residue - the compound is usually present only
in trace amounts and is degraded photochemically and bound to plant
material.
Photochemical degradation of pirimiphos-methyl on leaf
surfaces leads to the formation of compound II which is not
accumulated.
When applied to water in which rice seedlings were growing,
pirimiphos-methyl was not translocated significantly into the
foliage; in these conditions the hydrolysis product IV is likely to
represent the predominant pyrimidine residue. Compounds V and VI
occur only in trace amounts.
The new data show that pirimiphos-methyl residues on growing
crops decline rapidly during the first few days after spraying.
Bullock (1972) has studied extensively residues of
pirimiphos-methyl in crops grown world-wide. Samples were analysed
for residues of pirimiphos-methyl and of the phosphorus-containing
metabolites II and III. However significant amounts of the
metabolites did not appear and so were not reported.
The highest initial residues are found in leafy vegetables.
However, pirimiphos-methyl residues decline very quickly on these
crops so that after four days levels on vegetables generally are
below 2 mg/kg except on celery and occasionally on lettuce, spinach
or Brussels sprouts where they may be higher (Table 1). Residues on
tree fruits are normally less than 2 mg/kg after four days, while
on soft fruits (currants and berries) they are generally below 1
mg/kg (Table 2). There was good agreement between the results
obtained by Bullock and those obtained by a second laboratory on a
set of apple samples from Japan.
The residues which Bullock found in rice in husk (up to 0.21
mg/kg), in dehusked rice and in maize (<0.01 mg/kg in both cases)
following pre-harvest spray uses are all within the temporary
tolerance levels recommended by the 1974 Joint Meeting for
post-harvest uses on these commodities.
The use of fogs to control whiteflies under glass results in
initial residues of 0.3 mg/kg or less in cucumbers, tomatoes and
peppers (Table 3). Although treatments for whitefly control may
need to be made every 3-5 days, the available data (ICI Plant
Protection Division, 1973) indicate that no accumulation of
pirimiphos-methyl residues occur in these fruits on repeated
application.
There is also no accumulation of residues in mushrooms
following multiple applications of a fog (Table 3). Initial
residues of up to 2.5 mg/kg were found to decline rapidly to below
0.5 mg/kg after two days (Bullock and Stephens, 1974).
The new aerosol and dust formulations yield residues (Table 4)
similar to those observed previously with sprays of the
emulsifiable concentrate formulations on lettuce and blackcurrants
(Boxwell and Bullock, 1976). Residues on tomatoes were marginally
higher than after fogging but were still below 1 mg/kg immediately
after application at normal rates.
Short-term studies
Rat
Pirimiphos-methyl was administered to young rats at doses of
0, 5, 8, 10 and 50 ppm in the diet for a period of 28 days in order
to determine the no-effect level on plasma cholinesterase activity
and body weight gain. Plasma cholinesterase activity was inhibited
at the 50 ppm level throughout the dosing period by 30% and 50% in
male and female animals respectively. No effect on erythrocyte
cholinesterase activity was detected. There was a tendency towards
poorer food utilization in the group but there was no effect on
body weight gain.
There were sporadic instances of statistically significant
depressions of plasma cholinesterase activity at the 8 and 10 ppm
levels but less than 25% and not dose related. The "no effect"
level for pirimiphos-methyl in the young rat was 10 ppm in the diet
administered for a period of 28 days. This is also the no-effect
level established for pirimiphos-methyl in the rat over two years
(FAO/WHO, 1975; Berry and Gore, 1975).
Dog
In order to examine the potential hepatic effect of
pirimiphos-methyl in the dog, ten more dogs were given 25 mg/kg/day
with six dogs acting as controls. Three of the dogs were killed
after four weeks dosing for histopathological examination. The
remaining animals were maintained at the same dose level for a
total period of 13 weeks except for two dogs whose dose was raised
progressively to 50 mg/kg/day from week eight onwards.
Slight bile duct proliferation was present in two dogs given
25 mg/kg pirimiphos-methyl for 13 weeks. None of the other dosed
animals showed this effect even when the dose was increased to 50
mg pirimiphos-methyl/kg/day in two other dogs.
The effects in the dog liver characterised by bile duct
proliferation in a certain number of dogs given 25 mg/kg/day was
confirmed. However the changes appear to be small and dependent
upon individual animal sensitivity and reversible; the mode of
action could not be ascertained. Focal inflammation was present in
the livers of control and dosed animals. Liver changes were shown
in the rat at levels of 300-600 ppm in the diet and in dogs dosed
at 10 mg/kg/day for two years (Garuti et al., 1976).
Long-term studies
Mouse
Four groups of 52 male and 52 female mice of the CFLP strain
were fed 0, 5, 250 and 500 ppm pirimiphos-methyl in the diet for 80
weeks. Three extra groups of 12 male and 12 female mice were fed 0,
5 and 500 ppm and were used for the investigation of cholinesterase
levels.
There were no significant differences in mortality between
control and treated groups. Macroscopic examination of descendants
gave no indication of treatment-related change. Efficiency of food
utilization and overall body weight gain were not affected. Food
consumption was reduced slightly among females receiving 500 ppm
only. No changes ascribable to the administration of pirimiphos-
methyl were seen during gross and histological examination at
autopsy.
Changes in the kidneys, namely foci of lymphocytic
infiltration in the interstitium, or minimal perivascular or
peribronchiolar lymphoid aggregations in the lung and abscesses and
small haemorrhages in ovaries and other morphological changes in
various tissues were seen in a proportion of animals of all groups
including controls and were considered to be of no toxicological
significance. Similarly the liver tumour incidence was comparable
to that of the controls.
There was no evidence to suggest that treatment with
pirimiphos-methyl altered the tumour profile of the CFLP mouse at
levels up to 500 ppm. However, animals of both sexes showed marked
inhibition of plasma and erythrocyte cholinesterase activities at
the 500 ppm dietary inclusion level. When corrected for differences
prevailing between groups at the start of the study, differences in
erythrocyte cholinesterase activity between the control and 5 ppm
pirimiphos-methyl groups were normally less than 20% and
consistently less than 25%. Plasma cholinesterase activities in
both untreated and 5 ppm dietary inclusion level groups followed
similar, slightly fluctuating patterns during the study, although
levels in both sexes in the treated group appeared inhibited by
comparison with controls. However there was no evidence of
progressive inhibition during treatment and differences between the
control and 5 ppm groups were considered to be of borderline
biological significance (Hunter et al., 1976). A "no-effect" level
at 5 ppm in the diet, equivalent to approximately 0.5 mg/kg/day was
established over an 80 week period.
OBSERVATIONS IN MAN
A group of seven healthy volunteers, consisting of three males
and four females, was given orally a dose of 0.25 mg
pirimiphos-methyl/kg bw, in capsule form for a period of 56 days.
No effect due to the administration of pirimiphos-methyl was seen
on liver function tests, blood count or erythrocyte or plasma
cholinesterase activity (Howard and Gore, 1976).
COMMENTS
Results of studies on the effect of pirimiphos-methyl on rat
reproduction in a three generation test, on avian-egg production
and hatchability and of special tests on mutagenicity were
negative.
Hydronephrosis incidence noted in a previous test has been
shown to be well within the limits of control values pertaining to
the same species.
Two studies were performed to investigate the potential
hepatotoxicity of pirimiphos-methyl. In an 80 week mouse study the
tumour incidence (including that in liver) was comparable in test
and control groups. In a three month dog study slight bile duct
proliferation was observed in animals dosed at 25 mg/kg/day,
however the effect was absent at 50 mg/kg/day. Focal inflammation
occurred equally in the livers of control and dosed animals. In a
56 day human study, 0.25 mg/kg/day did not induce any changes in
liver function tests, blood count or erythrocyte or plasma
cholinesterase activity. Additional biochemical data presented
established that the substance is very rapidly absorbed,
metabolised and excreted.
The Meeting agreed that the previously indicated further
studies have been carried out satisfactorily, and there is no
objection to estimating a permanent acceptable daily intake.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effects
Mouse 5 ppm in the diet equivalent to 0.5 mg/kg bw/day
Rat 10 ppm in the diet equivalent to 0.5 mg/kg bw/day
Man 0.25 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.01 mg/kg bw/day
RESIDUES IN FOOD AND THEIR EVALUATION
The 1974 evaluation of pirimiphos-methyl (FAO/WHO, 1975) was
principally in relation to its use as a treatment of stored grains.
However the insecticide is also used on growing crops, in public
health and in industrial outlets. Therefore, arising out of the
list of requirements in the report of the 1974 Joint Meeting, the
following additional data are now presented.
1. Information on pre-harvest use patterns and residues in fruit
and vegetables.
2. Information on residues in peanuts and other stored products.
3. Further information on the level and fate of residues at the
point of consumption following the use of pirimiphos-methyl
for the control of various stored product pests.
USE PATTERN
Pre-harvest
Pirimiphos-methyl is a fast-acting broad-spectrum
organophosphorus insecticide with both contact and fumigant
actions. It is effective against a wide range of flying and
crawling insects, including aphids, scales, mealy bugs, whiteflies,
bugs, thrips, beetles, caterpillars, moths and ants. It also has
acaricidal activity. It controls both adult and immature stages of
insects such as whiteflies, and is effective against strains of
insects which have developed resistance to malathion.
Because it has only limited biological persistence on leaf
surfaces, pirimiphos-methyl is particularly attractive for uses in
which a broad spectrum of insecticidal activity is required for a
short period. e.g. pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg a.i./ha "low volume" or 0.05-0.1% a.i. "high volume" are
normally recommended. 0.5-0.75 kg a.i./ha (or 20-30 mg a.i. per
m3) is normally effective in controlling greenhouse whiteflies by
fogging. A fogging rate of 40 mg per m3 is used to control
mushroom flies.
The principal formulation is a 50% emulsifiable concentrate.
A 50% oil-based formulation is available for ultra-low volume
application to outdoor crops and a 10% oil-based formulation is
used for whitefly control under glass. An 8% emulsifiable
concentrate is sold to the amateur gardener. Dust and aerosol
formulations are under development for crop uses.
A pre-harvest withholding interval of four days is normally
recommended after spraying. No pre-harvest withholding interval is
normally recommended after the use of a fog to control
1. whiteflies on greenhouse tomatoes, cucumbers and peppers.
2. mushroom flies.
Pirimiphos-methyl is not generally recommended for use on
celery.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Data reviewed by the 1974 Joint Meeting showed that
pirimiphos-methyl is rapidly lost from leaf surfaces during the
first 2-3 days after spraying, mainly by volatilisation. Levels of
the parent insecticide and the major degradation product, compound
II (Figure 1), represent less than 10% of the applied material
after two to three days. After foliar treatment the hydroxy-
pyrimidine IV, formed by hydrolysis of the insecticide, is unlikely
to be a significant residue - the compound is usually present only
in trace amounts and is degraded photochemically and bound to plant
material.
Photochemical degradation of pirimiphos-methyl on leaf
surfaces leads to the formation of compound II which is not
accumulated.
When applied to water in which rice seedlings were growing,
pirimiphos-methyl was not translocated significantly into the
foliage; in these conditions the hydrolysis product IV is likely to
represent the predominant pyrimidine residue. Compounds V and VI
occur only in trace amounts.
The new data show that pirimiphos-methyl residues on growing
crops decline rapidly during the first few days after spraying.
Bullock (1972) has studied extensively residues of
pirimiphos-methyl in crops grown world-wide. Samples were analysed
for residues of pirimiphos-methyl and of the phosphorus-containing
metabolites II and III. However significant amounts of the
metabolites did not appear and so were not reported.
The highest initial residues are found in leafy vegetables.
However, pirimiphos-methyl residues decline very quickly on these
crops so that after four days levels on vegetables generally are
below 2 mg/kg except on celery and occasionally on lettuce, spinach
or Brussels sprouts where they may be higher (Table 1). Residues on
tree fruits are normally less than 2 mg/kg after four days, while
on soft fruits (currants and berries) they are generally below 1
mg/kg (Table 2). There was good agreement between the results
obtained by Bullock and those obtained by a second laboratory on a
set of apple samples from Japan.
The residues which Bullock found in rice in husk (up to 0.21
mg/kg), in dehusked rice and in maize (<0.01 mg/kg in both cases)
following pre-harvest spray uses are all within the temporary
tolerance levels recommended by the 1974 Joint Meeting for
post-harvest uses on these commodities.
The use of fogs to control whiteflies under glass results in
initial residues of 0.3 mg/kg or less in cucumbers, tomatoes and
peppers (Table 3). Although treatments for whitefly control may
need to be made every 3-5 days, the available data (ICI Plant
Protection Division, 1973) indicate that no accumulation of
pirimiphos-methyl residues occur in these fruits on repeated
application.
There is also no accumulation of residues in mushrooms
following multiple applications of a fog (Table 3). Initial
residues of up to 2.5 mg/kg were found to decline rapidly to below
0.5 mg/kg after two days (Bullock and Stephens, 1974).
The new aerosol and dust formulations yield residues (Table 4)
similar to those observed previously with sprays of the
emulsifiable concentrate formulations on lettuce and blackcurrants
(Boxwell and Bullock, 1976). Residues on tomatoes were marginally
higher than after fogging but were still below 1 mg/kg immediately
after application at normal rates.
 Stored product uses
In contrast to its short persistence on growing crops,
pirimiphos-methyl possesses exceptional stability on stored
products such as grains. Although high temperatures and high
moisture levels in grain reduce the life of the deposit, the effect
of these influences is much less than with other grain protectants
approved or evaluated to date (FAO/WHO, 1975).
During degradation, residues of pirimiphos-methyl on wheat
grains are degraded by hydrolysis of the phosphorus-ester side
chain, to give principally the parent hydroxy-pyrimidine IV (Figure
1) and also the related compounds V and VI. Levels of compound II
are always extremely low (approximately 0.05 mg/kg over a period of
32 weeks in wheat grain treated at 4 mg/kg). No residues of the
chemically unstable oxygen analogue III have been detected. The
limit of detection for compound III is 0.01 mg/kg. Residues of the
hydroxypyrimidine IV in stored grains are normally below 0.5 mg/kg.
Peanuts
Pirimiphos-methyl residues in undecorticated peanuts treated at
the intended rate of 20 mg/kg did not exceed 5.0 mg/kg. Two trials
were conducted in the U.S.A. in 1971-2 in which pirimiphos-methyl was
applied at 20 and 50 mg/kg to undecorticated peanuts. Analytical
results indicated that the target rates of treatment were achieved.
Very little change occurred in the level of residues during the first
month following application. Even at the end of three months
substantially all of the deposit remained. The data suggest that the
half-life is of the order of 5 months from the time of application.
Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied. These data have recently been published (Redlinger, 1976).
A larger scale trial was conducted in the U.S.A. during 1972-73
(Ussary, 1975). Pirimiphos-methyl was applied at 10, 20 and 30 mg/kg
as an admixture treatment to undecorticated peanuts. Analytical
results again indicated that the target rate of treatment was
achieved. 20-50% of the initial residue was present on the
undecorticated peanuts after twelve months storage, compared with 10%
in the case of malathion. During four commercial scale trials in the
U.S.A. in 1973-74, in which pirimiphos-methyl was applied at the
proposed commercial application rate of 20 mg/kg, 30-60% of the
initial residue was present at bin emptying, that is after 4 ´-6
months storage (Ussary, 1975).
TABLE 1. Residues of pirimiphos-methyl resulting from supervised spray trials on leafy vegetables and root crops (EC formulations)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Beans West Germany 50% EC 0.15 kg/ha 1 0.15-0.25 <0.01-0.07 <0.01-0.01
(Bush) 0.3 kg/ha 1 0.05-0.47 <0.01-0.08 <0.01-0.04
(French) U.K. 50% EC or 0.05% 1 0.06-0.07 0.01-0.03 <0.01-0.01
8% EC 0.1% 1 0.08-0.14 0.10-0.14 <0.01-0.06
0.2% 1 - 0.26 0.14
Peas U.K. 50% EC 0.1 % 1 - - <0.01
0.2 % 1-2 - <0.01 <0.01
Lettuce U.K. 50% EC or 0.025% 3 0.19-0.73 0.07-0.13 0.30-0.12
25% EC 0.05% 1-3 11-18 0.60-1.8 0.37-0.88
0.1% 1-3 19-39 1.8-2.9 0.63-2.1
West Germany 50% EC 0.025% 1 2.6-2.7 0.23 0.07-0.13
0.05% 1 3.4-7.3 0.52 0.14-0.71
0.1% 4.7 - 0.52
West Germany 25% EC (0.15 kg/ha 1 4.6-8.1 0.02-0.24 0.02-0.19
0.3 kg/ha 1 6-13 0.05-0.50 0.01-0.11
Spinach West Germany 25% EC 0.025% 1 3.2-6.7 0.35-1.0 0.22
0.05% 1 12-14 1.5-1.6 0.19-0.43
0.1% 1 21 3.4 0.75
Cabbage U.K. 25% EC 1.0 kg/ha 1 - 0.30-0.50** <0.02-0.28
2.0 kg/ha 1 - 0.31-1.8 0.09-0.40
West Germany 25% EC 0.15 kg/ha 1 0.13-1.4 0.05-0.18 <0.01-0.09
0.3 kg/ha 1 0.57-4.6 <0.01-0.69 <0.01-0.40
0.6 kg/ha 1 4.1 1.5 0.18
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Cabbage Malaysia 25% EC 1.0 kg/ha 18 - - 0.01***
2.0 kg/ha 18 - - 0.01-0.02***
Cauliflower U.K. 50% EC or 0.1% 1 0.20-2.0 0.01-0.11 <0.01
8% EC
(Hearts) 0.2% 1 0.15-1.4 0.04-0.24 <0.01-0.04
(leaves) 50% EC or 0.1% 1 0.66-12 0.50-1.3 0.02-0.35
8% EC 0.2% 1 1.5-13 0.65-1.1 0.04-2.2
Brussels U.K. 50% EC 1.4 kg/ha 3 1.7-3.3 0.30-1.7 0.28-0.66
sprouts (0.1%)
(buttons) 2.28 kg/ha 3 2.9-6.8 0.96-4.5 0.67-2.3
(0.2%)
Brussels Netherlands 50% EC 0.5 kg/ha 1 0.26* 0.14** 0.07
sprouts (0.05 %)
(buttons) 0.75 kg/ha 1 0.49* 0.39** 0.21
(0.075%)
Spring U.K. 50% EC or 0.05% 1 0.28-1.6 <0.01-0.07 <0.01-0.08
onions 8% EC 0.1% 1 2.3-4.2 0.04-0.49 <0.01-0.46
Celery U.K. 8% EC 0.05% 1 18-34 3.1-8.8 1.2-2.7
0.1% 1 26-69 5.1-14 3.70-8.0
Carrots U.K. 50% EC 1.14 kg/ha 1 0.32-0.95 0.09-0.16 0.10-0.22
(0.1%)
2.28 kg/ha 1 0.57-2.2 0.17-0.48 0.12-0.50
(0.2%)
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Potatoes U.K. 50% EC 0.1% 2 <0.01 <0.01
0.2% 2 <0.01 <0.01 <0.01-0.02
Sugar West Germany 25% EC 0.05% 1 18 - 1.7
beets (0.3 kg/ha)
0.1% 1 38 - 3.1
(0.6 kg/ha)
* Samples taken after 1 day
** Samples taken after 3 days
*** Samples taken after 9-15 days
TABLE 2 Residues of pirimiphos-methyl resulting from supervised spray trials on tree and sort fruits (EC and ULV formulations
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Apples U.K. 50% EC or 0.1 % 3 0.51-1.3 0.25-0.67 0.10-0.80
8 % 0.05% 1
0.2 % 3 0.76-2.8 0.58-1.9 0.16-1.5
0.1% 1
France 50% EC 0.075% 6 - - - 2 weeks: 0.32
Japan 50% EC 0.1 % 1-3 - - - 10 days: 0.15-1.4
Pears Spain 50% EC 0.1 % 1 - - - 12 weeks: <0.01
Plums U.K. 50% EC 0.1 % 2 0.47-0.96 0.24-0.55 0.02-0.25
0.2 % 2 1.6-2.2 1.3-1.5 0.06-0.55
Lemons Italy 25% EC 0.05 % 1 - - - 9 weeks: 0.25 in peel
0.03 in flesh
0.075% 1 - - - 0.75 in peel
0.03 in flesh
0.01 % - - 0.27-0.35 - -
in peel
0.02-0.03
in flesh
Oranges Australia 25% EC 0.075% 2 - - - 4´ months: 0.69 in peel
<0.01 in flesh
0.1 2 - - - 0.92 in peel
<0.01 in flesh
TABLE 2 (Cont'd.)
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Oranges South 25% EC 0.075% 1 - - - 2-8 months: <0.01-0.01 in peel
Africa <0.01 in flesh
0.01-0.125% 1 - - - <0.01-0.1 in peel
<0.01-0.1 in flesh
Olives Spain 50%ULV 0.25 kg/ha 2 - - 0.46 9-20 days: 0.07-0.36
0.5 kg/ha 2 - - - 0.16-0.45
1.0 kg/ha 2 - - - 0.18-1.4
Blackcurrants U.K. 8% EC 0.05 % 2 0.25-0.75 0.06-0.20 0.01-0.18
0.1 % 2 0.86-2.3 0.25-0.62 0.08-0.34
Raspberries U.K. 50% EC or 0.05% 1-2 0.81 0.23 0.13 2-5 weeks: <0.01-0.03
8% EC 0.01% 1-2 2.5 0.53 0.31 <0.01-0.09
Strawberries U.K. 50% EC or 0.05% 1 0.14-0.79 0.17-0.39 0.04-0.16 4´-7 weeks: <0.01-0.05
8% 0.1% 1 0.40-2.9 0.31-0.71 0.13-0.25 <0.01-0.05
TABLE 3. Levels of pirimiphos-methyl resulting from supervised residue trials on greenhouse crops and mushrooms using a 10% fogging
formulation
Number of Residues (mg/kg) at intervals after last application
crop Country Rate (a.i.) Applications 0 days 1 day 2 days 3 days 4 days
Cucumbers U.K. 0.75 kg/ha 1-4 0.06-0.30 0.03-0.11 0.02-0.11 0.01-0.04 <0.01
(=20 mg/m3)
Netherlands 20 mg/m3 1-5 0.08-0.19 0.03-0.08 0.02 <0.01-0.02 0.02
20 mg/m3 5
+30mg/m3 1-5 - 0.04-0.20 - 0.01-0.08 0.02-0.03
Tomatoes U.K. 0.5 kg/ha 2 - 0.15 - - -
(= 15 mg/m3)
1 kg/ha or 1-4 - 0.16-0.62 0.16 0.12 -
30 mg/m3
Netherlands 0.75 kg/ha 1-3 - <0.01-0.12 <0.01-0.18 0.08 -
(= 20 mg/m3)
30 mg/m3 1-5 - 0.06-0.17 - 0.08-0.14 0.08-0.11
(= 1 kg/ha)
Peppers U.K. 1 kg/ha 1 - 0.26 - - -
Mushrooms U.K. 40 mg/m3 1 and 6 0.99 0.19 0.12 - 0.14-0.16
(two trials) -2.5 -1.4 -0.44 - (4-5 days)
TABLE 4. Residues of pirimiphos-methyl resulting from supervised trials in the U.K. using dust and aerosol
formulations (single application date per trial)
Residues (mg/kg) at intervals after
Crop Formulation Rate 0 days 2 days 4 days 7-8 days
Lettuce Dust Normal 2.1-10 0.53-7.9 0.33-2.1 0.06-1.9
Overdosed 8.3-47 2.2-19 1.1-5.6 0.32-5.2
Aerosol Normal 1.6-21 0.53-11 0.34-2.8 0.16-1.8
Overdosed 13-40 6.2-25 2.0-7.6 0.75-3.8
Tomatoes Dust Normal 0.01-0.93 <0.01-0.15 0.07-0.09 0.04-0.07
Overdosed 0.18-1.4 0.08-0.89 0.05-0.13 0.02-0.03
Aerosol Normal 0.09-0.67 0.04-0.43 0.02-0.38 0.03-0.05
Overdosed 0.03-4.1 0.01-1.3 0.02-0.46 0.01-0.03
Blackcurrants Dust Normal 0.10-0.59 0.03-0.05 - -
Overdosed 0.44-1.4 0.08-0.22 - -
Aerosol Normal <0.01-0.27 <0.01-0.04 - -
Overdosed 0.04-0.59 <0.01-0.29 - -
During these studies there were wide variations between different
samples analysed from the same bin. These were probably caused by
non-representative sampling. Dust, estimated to constitute up to 10%
of the contents of the bins, settles into layers in the bins. It
contains several hundred mg/kg pirimiphos-methyl and in large bins it
was impossible to sample the dust in a representative manner. However,
residues in individual samples of undecorticated peanuts in the U.S.A.
trials were generally below 5.0 mg/kg during the period after
treatment at the proposed recommended rate of 20 mg/kg.
The walls of the four warehouses used in the commercial scale
trials were sprayed with a 0.5% aqueous suspension of
pirimiphos-methyl and probe samples were taken adjacent to the walls
during the storage period. Residues in the undecorticated peanuts were
similar to those from the bulk of the warehouse and it was concluded
that there was no major transfer of pirimiphos-methyl from the walls
to the peanuts (Ussary, 1975).
Rice
The 1974 Joint Meeting recommended temporary tolerances of 10
mg/kg in rice (in husk), 20 mg/kg in rice bran, 2 mg/kg in dehusked
rice and 1 mg/kg in polished rice.
Cogburn (1976) in the U.S.A. found that an admixture treatment of
pirimiphos-methyl at 10 mg/kg protected rice from all insect
infestation for twelve months. At this rate residues in the rice in
husk, bran, dehusked rice and polished rice were all within the
temporary tolerance levels proposed by the 1974 Joint Meeting.
Dates
In supervised trials in Iraq, MacCallum, Deighton and Pascoe
(1976) found that 500 mg pirimiphos-methyl per square metre sprayed
onto packaging materials (cardboard or wooden boxes) was effective in
protecting export dates from insect attack. A deposit of 125 mg per
square metre was effective for the treatment of waxed paper liners. At
these rates pirimiphos-methyl residues in the dates during the
following six months were invariably small - i.e. less than 0.5 mg/kg.
Cheese
There is very little movement of pirimiphos-methyl into cheese
stored on surfaces treated with the insecticide for mite control.
Thomas and Rowlands (1975) treated a wooden plank at 440 mg per
square metre with 2-14C-labelled pirimiphos-methyl and then stored a
22 kg Cheshire cheese, wrapped in a cloth, on it for fourteen weeks.
The cheese was turned to stand on alternate ends weekly, as in normal
storage practice. The radioactive residue was confined to the outer 4
mm of the cheese in which there was no detectable breakdown of
pirimiphos-methyl.
Levels of pirimiphos-methyl were approximately 40 mg/kg in the
outer 1 mm and 5-10 mg/kg in the outer 6 mm of the cheese. On a "whole
cheese" basis, this represents a residue of approximately 0.4 mg/kg.
FATE OF RESIDUES
In animals
Extensive data were evaluated by the 1974 Joint Meeting on the
fate of pirimiphos-methyl in goats, cows, hens, their edible tissues
and in foods of animal origin.
When fed to these animals, pirimiphos-methyl is rapidly
metabolised and the metabolites are excreted, principally in the
urine. Only very small quantities are excreted into milk or eggs.
Cattle receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites II and
III could be detected in meat, milk, butter or eggs (limit of
detection: 0.01 mg/kg or below).
Since the 1974 Joint Meeting, data on the fate in pigs and
additional data on the fate in hens have become available.
Pigs
Davis et al. (1976) maintained groups of pigs for 21 and 29 days
on diets containing 3, 10 and 34 mg/kg pirimiphos-methyl. No residues
of pirimiphos-methyl or of its phosphorus containing metabolites II or
III were detected in the kidney, liver, lung, heart or muscle (limit
of detection: 0.005 mg/kg in each case). Small residues of
pirimiphos-methyl and compound II were found in the fat of pigs fed
pirimiphos-methyl at 10 and 34 ppm in the diet (Table 15). No residues
were detected in the fat of pigs returned for eight days to untreated
diet prior to slaughter. No residues of the P=0 analogue (III) were
detected in any fat tissues (limit of detection 0.005 mg/kg).
No residues of the hydroxypyrimidines IV and V were found in any
of the above-mentioned tissues (limits of detection 0.01 mg/kg for
compound IV and 0.04 mg/kg for compound V).
Hens
Ross et al. (1975b) confirmed the low residue levels in eggs and
meat previously reported by other authors (FAO/WHO, 1975).
Groups of laying hens were maintained for 28 days on diets
containing 0, 4, 12 and 40 ppm pirimiphos-methyl. At all but the
highest dose level, pirimiphos-methyl levels in the eggs were below
0.01 mg/kg. At the 40 ppm dietary inclusion level, pirimiphos-methyl
levels in the egg yolk reached a plateau of 0.03-0.04 mg/kg after
seven days. After the hens had been returned to untreated diet for
seven days, pirimiphos-methyl residues in the yolks were below the
limit of detection (i.e. less than 0.008 mg/kg). Residues in the
albumen were negligible (less than 0.01 mg/kg).
Residues of pirimiphos-methyl in meat tissues were below 0.01
mg/kg at all dose levels.
No residues of the phosphorus-containing metabolites II and III
were detected in any samples of eggs or meat (limit of detection:
0.006-0.02 mg/kg).
In processing
Initial data on the fate of pirimiphos-methyl residues or
processing of stored products were reviewed by the 1974 Joint Meeting
(FAO/WHO, 1975). Subsequent studies are described below.
TABLE 5. Residues of pirimiphos-methyl and its desethyl metabolite
in the fat of pigs fed pirimiphos-methyl in the diet
Desethyl
Days Level in diet Pirimiphos-methyl Metabolite
on diet (ppm) (mg/kg) (mg/kg)
21 control ND ND
3 ND ND
10 0.008 0.016
34 0.034 0.034
29 3 ND ND
10 0.006 0.009
34 0.05 0.036
29 control ND ND
+ 8 days 3 ND ND
recovery
3 ND ND
10 ND ND
10 ND ND
34 ND ND
34 ND ND
ND: Not detected, less than 0.005 mg/kg
Wheat
Residues of pirimiphos-methyl in flour are relatively stable to
the conditions found during baking to bread and biscuits. However,
because of the dilution which the flour undergoes during these
processes flour initially containing 1 mg/kg pirimiphos-methyl is
likely to yield bread/biscuits containing residues of the order of
0.5 mg/kg.
Bullock and co-workers (1976) have studied extensively the fate
of pirimiphos-methyl during processing to bread and biscuits. In
studies with the radiolabelled compound, flour was dosed with
2-14C-labelled pirimiphos-methyl and baked to produce white bread,
wholemeal bread and biscuits. Although pirimiphos-methyl is known to
be a relatively volatile compound, there was no significant loss of
radioactivity by volatilisation. Distribution of radioactivity
throughout the bread was fairly uniform. Unchanged pirimiphos-methyl
accounted for 75-90% of the radioactivity in the baked product. The
major degradation products formed during baking were the
hydroxypyrimidines IV and VI, which accounted for 3-10% of the
radioactivity in the final product.
Similar results were obtained by residue analysis in a second set
of studies. After correction for the weight increase when flour is
baked to bread, residues of pirimiphos-methyl fell by 11-18%.
Likewise, residue analysis of biscuits showed average losses of 8%.
However, owing to the dilution of the flour which occurs during
baking, the residue level of pirimiphos-methyl in bread will be lower
than that in the corresponding flour.
During extensive residue studies Bullock et al.(1976) found that
levels of pirimiphos-methyl in bread and biscuits are likely to be
about 50% of those in the flour from which they were derived. These
findings agree well with the earlier work of Bullock (1973, 1974)
which was reviewed by the 1974 Joint Meeting. No residues of the
phosphorus-containing compounds II and III were detected in bread
baked from flour treated with pirimiphos-methyl at 1 mg/kg or in
biscuits baked from flour treated at up to 5 mg/kg (limit of
detection: 0.03 mg/kg)(Bullock et al., 1976).
The hydroxypyrimidine IV also undergoes little degradation during
the baking process. This compound has previously been shown to
constitute a minor part (generally less than 0.5 mg/kg) of the residue
in stored grains (FAO/WHO 1975). When bread was baked from flour
containing 2-14C-labelled compound IV, the unchanged
hydroxypyrimidine constituted more than 90% of the radioactive residue
in the bread (Bullock et al., 1976). By analogy with pirimiphos-methyl
residues, it can be concluded that residues of compound IV in baked
products will not exceed 0.2 mg/kg and will normally be considerably
lower.
The pattern and levels of residues found in semolina and cooked
pasta prepared from pirimiphos-methyl treated wheat are similar to
those found in flour and bread/biscuits respectively. Unchanged
pirimiphos-methyl constitutes the major proportion of the residue.
Bullock and May (1976) studied the fate of residues in wheat on
processing to semolina and pasta. White semolina prepared from Durum
wheat treated at 10 mg/kg contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90% of those in the corresponding semolina.
70% of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However the weight of pasta increases by
100% on cooking so that the concentration of pirimiphos-methyl in
cooked pasta is likely to be approximately 35% of that in the
corresponding semolina.
Peanuts
Pirimiphos-methyl residues in peanuts are found mainly on the
hulls. There is little transfer of residue to the kernels.
Pirimiphos-methyl residues in processed fractions obtained from the
kernel are normally very much smaller than in the whole raw
agricultural commodity.
Ussary (1975) has reviewed the available unpublished data for
pirimiphos-methyl residues in peanut fractions. He concluded that
residues in the kernels depend upon the degree of contamination by the
hulls which occurs in the differing shelling processes. Nevertheless
in the commercial scale trials in the U.S.A. during 1973-74, where
peanuts were treated at 20 mg/kg by admixture, pirimiphos-methyl
levels in the sound mature kernels were normally less than 5 mg/kg.
Undecorticated peanuts containing a mean residue of 10 mg/kg
pirimiphos-methyl yielded oil containing 9-14 mg/kg pirimiphos-methyl.
In the 1972-3 trials in the U.S.A. undecorticated peanuts treated
at 20 mg/kg yielded peanut butter containing mean residues of 3 mg/kg
(Ussary, 1975).
In the series of admixture trials in the U.S.A, pirimiphos-methyl
residues in the peanut hulls were normally below 50 mg/kg (Ussary,
1975).
Residues in food in commerce
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis, by gas chromatography
with a phosphorus detector, was reviewed by the 1974 Joint Meeting.
The method was recently published (Zweig, 1976). It is suitable for
the determination of residues of pirimiphos-methyl and the phosphorus-
containing metabolites II and III in crops. The limit of determination
is normally 0.01 mg/kg in each case.
NATIONAL TOLERANCES
The following national tolerances have been reported to the
Meeting.
Country Commodity Tolerance, mg/kg
France Cereals 4
Fruit and vegetables 2
Netherlands Cucumbers, courgettes,
peppers and tomatoes 0.2
West Germany Cereals 4*
Grain parts 2*
In the UK, where tolerances are not normally specified,
pirimiphos-methyl is registered for uniform admixture with small grain
cereals at 4 mg/kg.
APPRAISAL
Pirimiphos-methyl was evaluated by the 1974 Joint Meeting when,
in addition to the required toxicological information, further data
were considered desirable on its use on stored products other than
grain, on residues on fruit and vegetables following approved uses,
and on terminal levels in various foods. These data have been
provided.
In use on outdoor and indoor crops, since pirimiphos-methyl has
only a limited biological persistence on leaf surfaces it can be used
where a broad spectrum of insecticidal activity is required for a
short period, e.g., pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg. a.i./ha "low volume" or 0.05 to 0.1% a.i. "high volume"
are normally recommended. A fogging rate of 20-30 mg a.i./m3 is
normally effective in controlling greenhouse whiteflies, and a fogging
rate of 40 mg/m3 is used to control mushroom flies.
*Temporary tolerance.
A pre-harvest withholding interval of four days is normally
recommended after spraying. but 0 to 3 days after the use of a fog to
control whiteflies on greenhouse tomatoes, cucumbers and peppers or
mushroom flies.
Pirimiphos-methyl residues on growing crops decline rapidly
during the first few days after spraying. On the leaf surfaces and
other aerial parts of the plant the compound is lost principally by
volatilization; photochemical or other degradation also occurs.
However, there are no significant amounts of the degradation compounds
containing the intact phosphorus ester grouping and the
phosphorus-containing residue can be expressed as the parent compound.
Following treatment at recommended rates, levels on vegetables
are normally less than 2 mg/kg after four days but higher applications
can occasionally lead to residues on some green leafy vegetables
(lettuce, spinach, Brussels sprouts) not exceeding 5 mg/kg. On celery,
on which it is not generally recommended, residues my be higher than
this.
Residues on tree fruit are below 2 mg/kg after 4 days whilst on
soft fruits they are below 1 mg/kg. When used for fogging under glass,
initial levels are 0.3 mg/kg or less on cucumbers, tomatoes and
peppers.
Residues of pirimiphos-methyl are relatively stable in the
conditions found during baking to bread and biscuits. Flour containing
1 mg/kg of pirimiphos-methyl is likely to yield bread or biscuits
containing about 0.5 mg/kg of the unchanged compound; the main
degradation products do not contain phosphorus. Approximately similar
changes occur when durum wheat is milled to white or whole meal
semolina and this is subsequently made into pasta and cooked.
Preparation of white semolina reduces the residue by about 80% (most
of the pericarp is removed) but what is left is largely retained in
pasta, even on cooking, although the proportion is halved in cooked
pasta by uptake of water.
These results, and the additional results obtained in stored
rice, do not suggest a need for any alteration in the maximum residue
levels proposed temporarily for wheat and wheat products and rice at
the 1974 Joint Meeting and these are confirmed as maximum residue
limits.
Data evaluated by the 1974 Joint Meeting on the fate of
pirimiphos-methyl in goats, cows, hens, their edible tissues and foods
from them has now been supplemented by work with pigs which has shown
no accumulation in the fat or meat of pigs maintained on diets
containing pirimiphos-methyl at levels up to 34 mg/kg. Any residues of
the parent compound or its phosphorus-containing metabolites were
below the limit of detection (0.005 mg/kg) in muscle, kidney, liver,
lung and heart, and very small in fat. When hens were fed for 28 days
on diets containing up to 40 mg/kg of pirimiphos-methyl small residues
(0.03 - 0.04 mg/kg) were found in eggs at the highest level of
treatment. Residues of pirimiphos-methyl in meat tissues were below
0.01 mg/kg at all doses, and neither the desethyl compound nor the
oxon was detectable in any samples of eggs or meat.
RECOMMENDATIONS
As an ADI has now been established, the temporary tolerances
recommended by the 1974 Meeting are converted to maximum residue
limits. The following additional limits are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl expressed as pirimiphos-methyl.
Commodity Limit, mg/kg
Peanut hulls, peanuts (whole) 50
Peanut oil 10
Lettuce, mushroom, olives,
peanuts (kernels), spinach 5
Apples, Brussels sprouts, cabbage,
cauliflower, cherries, pears, plums 2
Blackcurrants, carrots, cucumber,
gooseberries, peppers, raspberries,
spring onions, strawberries, tomato 1
Beans with pod, cheese, citrus, dates 0.5
Peas, potatoes 0.05*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Results of studies now in progress on the residues in peanuts and
peanut products.
2. Results from commercial trials on other commodities.
REFERENCES
Berry, D., and Gore, C.W. Pirimiphos-methyl (PP511): Determination
1975 of no effect level during a 28 day rat feeding
study. Unpublished report received from ICI
Central Toxicology Laboratory. Submitted to the
World Health Organization by ICI.
Boxwell, J.W., and Bullock, D.J.W. Pirimiphos-methyl: Residues on
1976 crops following application of aerosol and dust
formulations (UK). ICI Plant Protection Division
Report No. TMJ1305A. (Unpublished)
Bullock, D.J.W. Residue summary: Pirimiphos-methyl in crops. ICI
1972 Plant Protection Ltd. Report No. TMJ595/1.
(Unpublished)
Bullock, D.J.W., Harrison, P.J., and Day, S.R. Pirimiphos-methyl:
1976 Degradation of residues in flour during baking.
ICI Plant Protection Division Report No. AR2666A.
(Unpublished)
Bullock, D.J.W., and May, M.S. Pirimiphos-methyl: Residue transfer
1976 from durum wheat to semolina and pasta. ICI Plant
Protection Division Report No. TMJ1345A.
(Unpublished).
Bullock, D.J.W., and Stephens, P.P. Pirimiphos-methyl: Residues in
1974 mushrooms following application of
pirimiphos-methyl by `Fumovap' gun and Swingfog
machine. ICI Plant Protection Ltd. Report No.
AR2543A. (Unpublished).
Cogburn, R.G. Pirimiphos-methyl and a protectant for stored rough
1976 rice: Small bin tests. J. Econ. Ent., 69(3): 369.
Davis, J.A., Day, S.R., Hemingway, R.J., Jegatheeswaren, T.,
1976 and Bullock, D.J.W. Pirimiphos-methyl: residue
transfer study with pigs. ICI Plant Protection
Division Report No. AR2665A. (Unpublished)
FAO/WHO 1974 Evaluations of some pesticide residues in food. AGP:
1975 1974/M/11; WHO Pesticide Residues Series No. 4.
Garuti, A., Gore, C.W., Ishmael, J., and Kalinowski, A.E.
1976 Pirimiphos-methyl (PP511): A study of hepatic
changes in the dog. Unpublished report from ICI.
Hanna, P.J., and Dyer, K.F. Mutagenicity of organophosphorus
1975 compounds. Mutat. Res., 28: 405.
Hodge, M.C.E., and Moore, S. Pirimiphos-methyl (PP511) teratological
1972 studies in the rat. Unpublished report from ICI
Industrial Hygiene Research Laboratories.
Howard, S.K., and Gore, C.W. The human response to long-term
1976 oral administration of low doses of
pirimiphos-methyl. Unpublished report from ICI.
Hunter, B., Graham, C., Street, Ae., Offer, J.M., and Printice,
1976 D.E. Long-term feeding of pirimiphos-methyl
(PP511) in mice. (Final report 0-80 weeks).
Unpublished report from Huntingdon Research Centre
No. ICI/34/7650, submitted by ICI.
ICI Plant Protection Ltd. Pirimiphos-methyl: Residues data for fogging
1973 use on cucumbers, tomatoes and peppers.
(Unpublished).
MacCullum Deighton, J., and Pascoe, R. Pirimiphos-methyl: Protection
1976 of stored dates: Trials in Iraq 1974/5. ICI
Plan-Protection Ltd. Report No. AR2642A.
(Unpublished)
McGregor, D.B. Dominant lethal study in mice of pirimiphos methyl.
1975 Unpublished report from Inveresk Research
International (No. 289).
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. ICI Central Toxicology
Report No. CTL/P/247.
Palmer, A.K., and Hill, P.A. Effect of pirimiphos-methyl (PP511)
1976 on reproductive functions of multiple generations
in the rat. Huntingdon Research Centre Report,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl metabolites (R4039 and R35510):
1974 Acute oral toxicity. Unpublished report from ICI
Central Toxicology Laboratory No. CTL/P/137,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl potentiation studies with
1975 bioresmethrin. Unpublished report from ICI Central
Toxicology Laboratory (No. CTL/P/166B). Submitted
by ICI.
Redlinger, L.M. Pirimiphos-methyl as a protectant for farmers
1976 stock peanuts. J.Econ. Ent., 69(3): 377.
Ross, D.B., Burroughs, S.J., and Roberts, N.L. Examination of
1975a pirimiphos-methyl for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon
Research Centre (No. ICI/49/75220) submitted by
ICI.
Ross, D.B., Christopher, D.H., Cameron, D.M., Dollery, R.,
1976 Almond, R.H., and Roberts, N.L. Egg production and
hatchability following inclusion of
pirimiphos-methyl at various levels in the diet of
the laying hen. Huntingdon Research Centre Report
No. ICI/31A/75924.
Thomas, K.P., and Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by cheshire cheese. J. Stored
Prod. Res., 11: 53.
Ussary, J.P. ActellicR residues in peanuts. ICI United States
1975 Inc. Interim Report. (Unpublished)
Zweig, G. Analytical methods for pesticides and plant growth
1976 regulators. Academic Press, 8: 125.
Stored product uses
In contrast to its short persistence on growing crops,
pirimiphos-methyl possesses exceptional stability on stored
products such as grains. Although high temperatures and high
moisture levels in grain reduce the life of the deposit, the effect
of these influences is much less than with other grain protectants
approved or evaluated to date (FAO/WHO, 1975).
During degradation, residues of pirimiphos-methyl on wheat
grains are degraded by hydrolysis of the phosphorus-ester side
chain, to give principally the parent hydroxy-pyrimidine IV (Figure
1) and also the related compounds V and VI. Levels of compound II
are always extremely low (approximately 0.05 mg/kg over a period of
32 weeks in wheat grain treated at 4 mg/kg). No residues of the
chemically unstable oxygen analogue III have been detected. The
limit of detection for compound III is 0.01 mg/kg. Residues of the
hydroxypyrimidine IV in stored grains are normally below 0.5 mg/kg.
Peanuts
Pirimiphos-methyl residues in undecorticated peanuts treated at
the intended rate of 20 mg/kg did not exceed 5.0 mg/kg. Two trials
were conducted in the U.S.A. in 1971-2 in which pirimiphos-methyl was
applied at 20 and 50 mg/kg to undecorticated peanuts. Analytical
results indicated that the target rates of treatment were achieved.
Very little change occurred in the level of residues during the first
month following application. Even at the end of three months
substantially all of the deposit remained. The data suggest that the
half-life is of the order of 5 months from the time of application.
Twelve months after application the residue in the whole
undecorticated nuts was approximately 20-25% of the original amount
applied. These data have recently been published (Redlinger, 1976).
A larger scale trial was conducted in the U.S.A. during 1972-73
(Ussary, 1975). Pirimiphos-methyl was applied at 10, 20 and 30 mg/kg
as an admixture treatment to undecorticated peanuts. Analytical
results again indicated that the target rate of treatment was
achieved. 20-50% of the initial residue was present on the
undecorticated peanuts after twelve months storage, compared with 10%
in the case of malathion. During four commercial scale trials in the
U.S.A. in 1973-74, in which pirimiphos-methyl was applied at the
proposed commercial application rate of 20 mg/kg, 30-60% of the
initial residue was present at bin emptying, that is after 4 ´-6
months storage (Ussary, 1975).
TABLE 1. Residues of pirimiphos-methyl resulting from supervised spray trials on leafy vegetables and root crops (EC formulations)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Beans West Germany 50% EC 0.15 kg/ha 1 0.15-0.25 <0.01-0.07 <0.01-0.01
(Bush) 0.3 kg/ha 1 0.05-0.47 <0.01-0.08 <0.01-0.04
(French) U.K. 50% EC or 0.05% 1 0.06-0.07 0.01-0.03 <0.01-0.01
8% EC 0.1% 1 0.08-0.14 0.10-0.14 <0.01-0.06
0.2% 1 - 0.26 0.14
Peas U.K. 50% EC 0.1 % 1 - - <0.01
0.2 % 1-2 - <0.01 <0.01
Lettuce U.K. 50% EC or 0.025% 3 0.19-0.73 0.07-0.13 0.30-0.12
25% EC 0.05% 1-3 11-18 0.60-1.8 0.37-0.88
0.1% 1-3 19-39 1.8-2.9 0.63-2.1
West Germany 50% EC 0.025% 1 2.6-2.7 0.23 0.07-0.13
0.05% 1 3.4-7.3 0.52 0.14-0.71
0.1% 4.7 - 0.52
West Germany 25% EC (0.15 kg/ha 1 4.6-8.1 0.02-0.24 0.02-0.19
0.3 kg/ha 1 6-13 0.05-0.50 0.01-0.11
Spinach West Germany 25% EC 0.025% 1 3.2-6.7 0.35-1.0 0.22
0.05% 1 12-14 1.5-1.6 0.19-0.43
0.1% 1 21 3.4 0.75
Cabbage U.K. 25% EC 1.0 kg/ha 1 - 0.30-0.50** <0.02-0.28
2.0 kg/ha 1 - 0.31-1.8 0.09-0.40
West Germany 25% EC 0.15 kg/ha 1 0.13-1.4 0.05-0.18 <0.01-0.09
0.3 kg/ha 1 0.57-4.6 <0.01-0.69 <0.01-0.40
0.6 kg/ha 1 4.1 1.5 0.18
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Cabbage Malaysia 25% EC 1.0 kg/ha 18 - - 0.01***
2.0 kg/ha 18 - - 0.01-0.02***
Cauliflower U.K. 50% EC or 0.1% 1 0.20-2.0 0.01-0.11 <0.01
8% EC
(Hearts) 0.2% 1 0.15-1.4 0.04-0.24 <0.01-0.04
(leaves) 50% EC or 0.1% 1 0.66-12 0.50-1.3 0.02-0.35
8% EC 0.2% 1 1.5-13 0.65-1.1 0.04-2.2
Brussels U.K. 50% EC 1.4 kg/ha 3 1.7-3.3 0.30-1.7 0.28-0.66
sprouts (0.1%)
(buttons) 2.28 kg/ha 3 2.9-6.8 0.96-4.5 0.67-2.3
(0.2%)
Brussels Netherlands 50% EC 0.5 kg/ha 1 0.26* 0.14** 0.07
sprouts (0.05 %)
(buttons) 0.75 kg/ha 1 0.49* 0.39** 0.21
(0.075%)
Spring U.K. 50% EC or 0.05% 1 0.28-1.6 <0.01-0.07 <0.01-0.08
onions 8% EC 0.1% 1 2.3-4.2 0.04-0.49 <0.01-0.46
Celery U.K. 8% EC 0.05% 1 18-34 3.1-8.8 1.2-2.7
0.1% 1 26-69 5.1-14 3.70-8.0
Carrots U.K. 50% EC 1.14 kg/ha 1 0.32-0.95 0.09-0.16 0.10-0.22
(0.1%)
2.28 kg/ha 1 0.57-2.2 0.17-0.48 0.12-0.50
(0.2%)
TABLE 1. (Cont'd.)
No. of Residues (mg/kg) at intervals (days)
Rate a.i. applications after last application
Crop Country Formulation % or kg/ha 0 4 7
Potatoes U.K. 50% EC 0.1% 2 <0.01 <0.01
0.2% 2 <0.01 <0.01 <0.01-0.02
Sugar West Germany 25% EC 0.05% 1 18 - 1.7
beets (0.3 kg/ha)
0.1% 1 38 - 3.1
(0.6 kg/ha)
* Samples taken after 1 day
** Samples taken after 3 days
*** Samples taken after 9-15 days
TABLE 2 Residues of pirimiphos-methyl resulting from supervised spray trials on tree and sort fruits (EC and ULV formulations
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Apples U.K. 50% EC or 0.1 % 3 0.51-1.3 0.25-0.67 0.10-0.80
8 % 0.05% 1
0.2 % 3 0.76-2.8 0.58-1.9 0.16-1.5
0.1% 1
France 50% EC 0.075% 6 - - - 2 weeks: 0.32
Japan 50% EC 0.1 % 1-3 - - - 10 days: 0.15-1.4
Pears Spain 50% EC 0.1 % 1 - - - 12 weeks: <0.01
Plums U.K. 50% EC 0.1 % 2 0.47-0.96 0.24-0.55 0.02-0.25
0.2 % 2 1.6-2.2 1.3-1.5 0.06-0.55
Lemons Italy 25% EC 0.05 % 1 - - - 9 weeks: 0.25 in peel
0.03 in flesh
0.075% 1 - - - 0.75 in peel
0.03 in flesh
0.01 % - - 0.27-0.35 - -
in peel
0.02-0.03
in flesh
Oranges Australia 25% EC 0.075% 2 - - - 4´ months: 0.69 in peel
<0.01 in flesh
0.1 2 - - - 0.92 in peel
<0.01 in flesh
TABLE 2 (Cont'd.)
No. of Residues (mg/kg) at intervals after last application
Formulation Rate a.i., Application
Crop Country % or kg/ha 0 days 4 days 7 days Other
Oranges South 25% EC 0.075% 1 - - - 2-8 months: <0.01-0.01 in peel
Africa <0.01 in flesh
0.01-0.125% 1 - - - <0.01-0.1 in peel
<0.01-0.1 in flesh
Olives Spain 50%ULV 0.25 kg/ha 2 - - 0.46 9-20 days: 0.07-0.36
0.5 kg/ha 2 - - - 0.16-0.45
1.0 kg/ha 2 - - - 0.18-1.4
Blackcurrants U.K. 8% EC 0.05 % 2 0.25-0.75 0.06-0.20 0.01-0.18
0.1 % 2 0.86-2.3 0.25-0.62 0.08-0.34
Raspberries U.K. 50% EC or 0.05% 1-2 0.81 0.23 0.13 2-5 weeks: <0.01-0.03
8% EC 0.01% 1-2 2.5 0.53 0.31 <0.01-0.09
Strawberries U.K. 50% EC or 0.05% 1 0.14-0.79 0.17-0.39 0.04-0.16 4´-7 weeks: <0.01-0.05
8% 0.1% 1 0.40-2.9 0.31-0.71 0.13-0.25 <0.01-0.05
TABLE 3. Levels of pirimiphos-methyl resulting from supervised residue trials on greenhouse crops and mushrooms using a 10% fogging
formulation
Number of Residues (mg/kg) at intervals after last application
crop Country Rate (a.i.) Applications 0 days 1 day 2 days 3 days 4 days
Cucumbers U.K. 0.75 kg/ha 1-4 0.06-0.30 0.03-0.11 0.02-0.11 0.01-0.04 <0.01
(=20 mg/m3)
Netherlands 20 mg/m3 1-5 0.08-0.19 0.03-0.08 0.02 <0.01-0.02 0.02
20 mg/m3 5
+30mg/m3 1-5 - 0.04-0.20 - 0.01-0.08 0.02-0.03
Tomatoes U.K. 0.5 kg/ha 2 - 0.15 - - -
(= 15 mg/m3)
1 kg/ha or 1-4 - 0.16-0.62 0.16 0.12 -
30 mg/m3
Netherlands 0.75 kg/ha 1-3 - <0.01-0.12 <0.01-0.18 0.08 -
(= 20 mg/m3)
30 mg/m3 1-5 - 0.06-0.17 - 0.08-0.14 0.08-0.11
(= 1 kg/ha)
Peppers U.K. 1 kg/ha 1 - 0.26 - - -
Mushrooms U.K. 40 mg/m3 1 and 6 0.99 0.19 0.12 - 0.14-0.16
(two trials) -2.5 -1.4 -0.44 - (4-5 days)
TABLE 4. Residues of pirimiphos-methyl resulting from supervised trials in the U.K. using dust and aerosol
formulations (single application date per trial)
Residues (mg/kg) at intervals after
Crop Formulation Rate 0 days 2 days 4 days 7-8 days
Lettuce Dust Normal 2.1-10 0.53-7.9 0.33-2.1 0.06-1.9
Overdosed 8.3-47 2.2-19 1.1-5.6 0.32-5.2
Aerosol Normal 1.6-21 0.53-11 0.34-2.8 0.16-1.8
Overdosed 13-40 6.2-25 2.0-7.6 0.75-3.8
Tomatoes Dust Normal 0.01-0.93 <0.01-0.15 0.07-0.09 0.04-0.07
Overdosed 0.18-1.4 0.08-0.89 0.05-0.13 0.02-0.03
Aerosol Normal 0.09-0.67 0.04-0.43 0.02-0.38 0.03-0.05
Overdosed 0.03-4.1 0.01-1.3 0.02-0.46 0.01-0.03
Blackcurrants Dust Normal 0.10-0.59 0.03-0.05 - -
Overdosed 0.44-1.4 0.08-0.22 - -
Aerosol Normal <0.01-0.27 <0.01-0.04 - -
Overdosed 0.04-0.59 <0.01-0.29 - -
During these studies there were wide variations between different
samples analysed from the same bin. These were probably caused by
non-representative sampling. Dust, estimated to constitute up to 10%
of the contents of the bins, settles into layers in the bins. It
contains several hundred mg/kg pirimiphos-methyl and in large bins it
was impossible to sample the dust in a representative manner. However,
residues in individual samples of undecorticated peanuts in the U.S.A.
trials were generally below 5.0 mg/kg during the period after
treatment at the proposed recommended rate of 20 mg/kg.
The walls of the four warehouses used in the commercial scale
trials were sprayed with a 0.5% aqueous suspension of
pirimiphos-methyl and probe samples were taken adjacent to the walls
during the storage period. Residues in the undecorticated peanuts were
similar to those from the bulk of the warehouse and it was concluded
that there was no major transfer of pirimiphos-methyl from the walls
to the peanuts (Ussary, 1975).
Rice
The 1974 Joint Meeting recommended temporary tolerances of 10
mg/kg in rice (in husk), 20 mg/kg in rice bran, 2 mg/kg in dehusked
rice and 1 mg/kg in polished rice.
Cogburn (1976) in the U.S.A. found that an admixture treatment of
pirimiphos-methyl at 10 mg/kg protected rice from all insect
infestation for twelve months. At this rate residues in the rice in
husk, bran, dehusked rice and polished rice were all within the
temporary tolerance levels proposed by the 1974 Joint Meeting.
Dates
In supervised trials in Iraq, MacCallum, Deighton and Pascoe
(1976) found that 500 mg pirimiphos-methyl per square metre sprayed
onto packaging materials (cardboard or wooden boxes) was effective in
protecting export dates from insect attack. A deposit of 125 mg per
square metre was effective for the treatment of waxed paper liners. At
these rates pirimiphos-methyl residues in the dates during the
following six months were invariably small - i.e. less than 0.5 mg/kg.
Cheese
There is very little movement of pirimiphos-methyl into cheese
stored on surfaces treated with the insecticide for mite control.
Thomas and Rowlands (1975) treated a wooden plank at 440 mg per
square metre with 2-14C-labelled pirimiphos-methyl and then stored a
22 kg Cheshire cheese, wrapped in a cloth, on it for fourteen weeks.
The cheese was turned to stand on alternate ends weekly, as in normal
storage practice. The radioactive residue was confined to the outer 4
mm of the cheese in which there was no detectable breakdown of
pirimiphos-methyl.
Levels of pirimiphos-methyl were approximately 40 mg/kg in the
outer 1 mm and 5-10 mg/kg in the outer 6 mm of the cheese. On a "whole
cheese" basis, this represents a residue of approximately 0.4 mg/kg.
FATE OF RESIDUES
In animals
Extensive data were evaluated by the 1974 Joint Meeting on the
fate of pirimiphos-methyl in goats, cows, hens, their edible tissues
and in foods of animal origin.
When fed to these animals, pirimiphos-methyl is rapidly
metabolised and the metabolites are excreted, principally in the
urine. Only very small quantities are excreted into milk or eggs.
Cattle receiving pirimiphos-methyl in their ration do not accumulate
significant quantities in edible tissues, including fat. Such
organophosphorus residues as are detectable are of the parent
compound. No residues of the phosphorus-containing metabolites II and
III could be detected in meat, milk, butter or eggs (limit of
detection: 0.01 mg/kg or below).
Since the 1974 Joint Meeting, data on the fate in pigs and
additional data on the fate in hens have become available.
Pigs
Davis et al. (1976) maintained groups of pigs for 21 and 29 days
on diets containing 3, 10 and 34 mg/kg pirimiphos-methyl. No residues
of pirimiphos-methyl or of its phosphorus containing metabolites II or
III were detected in the kidney, liver, lung, heart or muscle (limit
of detection: 0.005 mg/kg in each case). Small residues of
pirimiphos-methyl and compound II were found in the fat of pigs fed
pirimiphos-methyl at 10 and 34 ppm in the diet (Table 15). No residues
were detected in the fat of pigs returned for eight days to untreated
diet prior to slaughter. No residues of the P=0 analogue (III) were
detected in any fat tissues (limit of detection 0.005 mg/kg).
No residues of the hydroxypyrimidines IV and V were found in any
of the above-mentioned tissues (limits of detection 0.01 mg/kg for
compound IV and 0.04 mg/kg for compound V).
Hens
Ross et al. (1975b) confirmed the low residue levels in eggs and
meat previously reported by other authors (FAO/WHO, 1975).
Groups of laying hens were maintained for 28 days on diets
containing 0, 4, 12 and 40 ppm pirimiphos-methyl. At all but the
highest dose level, pirimiphos-methyl levels in the eggs were below
0.01 mg/kg. At the 40 ppm dietary inclusion level, pirimiphos-methyl
levels in the egg yolk reached a plateau of 0.03-0.04 mg/kg after
seven days. After the hens had been returned to untreated diet for
seven days, pirimiphos-methyl residues in the yolks were below the
limit of detection (i.e. less than 0.008 mg/kg). Residues in the
albumen were negligible (less than 0.01 mg/kg).
Residues of pirimiphos-methyl in meat tissues were below 0.01
mg/kg at all dose levels.
No residues of the phosphorus-containing metabolites II and III
were detected in any samples of eggs or meat (limit of detection:
0.006-0.02 mg/kg).
In processing
Initial data on the fate of pirimiphos-methyl residues or
processing of stored products were reviewed by the 1974 Joint Meeting
(FAO/WHO, 1975). Subsequent studies are described below.
TABLE 5. Residues of pirimiphos-methyl and its desethyl metabolite
in the fat of pigs fed pirimiphos-methyl in the diet
Desethyl
Days Level in diet Pirimiphos-methyl Metabolite
on diet (ppm) (mg/kg) (mg/kg)
21 control ND ND
3 ND ND
10 0.008 0.016
34 0.034 0.034
29 3 ND ND
10 0.006 0.009
34 0.05 0.036
29 control ND ND
+ 8 days 3 ND ND
recovery
3 ND ND
10 ND ND
10 ND ND
34 ND ND
34 ND ND
ND: Not detected, less than 0.005 mg/kg
Wheat
Residues of pirimiphos-methyl in flour are relatively stable to
the conditions found during baking to bread and biscuits. However,
because of the dilution which the flour undergoes during these
processes flour initially containing 1 mg/kg pirimiphos-methyl is
likely to yield bread/biscuits containing residues of the order of
0.5 mg/kg.
Bullock and co-workers (1976) have studied extensively the fate
of pirimiphos-methyl during processing to bread and biscuits. In
studies with the radiolabelled compound, flour was dosed with
2-14C-labelled pirimiphos-methyl and baked to produce white bread,
wholemeal bread and biscuits. Although pirimiphos-methyl is known to
be a relatively volatile compound, there was no significant loss of
radioactivity by volatilisation. Distribution of radioactivity
throughout the bread was fairly uniform. Unchanged pirimiphos-methyl
accounted for 75-90% of the radioactivity in the baked product. The
major degradation products formed during baking were the
hydroxypyrimidines IV and VI, which accounted for 3-10% of the
radioactivity in the final product.
Similar results were obtained by residue analysis in a second set
of studies. After correction for the weight increase when flour is
baked to bread, residues of pirimiphos-methyl fell by 11-18%.
Likewise, residue analysis of biscuits showed average losses of 8%.
However, owing to the dilution of the flour which occurs during
baking, the residue level of pirimiphos-methyl in bread will be lower
than that in the corresponding flour.
During extensive residue studies Bullock et al.(1976) found that
levels of pirimiphos-methyl in bread and biscuits are likely to be
about 50% of those in the flour from which they were derived. These
findings agree well with the earlier work of Bullock (1973, 1974)
which was reviewed by the 1974 Joint Meeting. No residues of the
phosphorus-containing compounds II and III were detected in bread
baked from flour treated with pirimiphos-methyl at 1 mg/kg or in
biscuits baked from flour treated at up to 5 mg/kg (limit of
detection: 0.03 mg/kg)(Bullock et al., 1976).
The hydroxypyrimidine IV also undergoes little degradation during
the baking process. This compound has previously been shown to
constitute a minor part (generally less than 0.5 mg/kg) of the residue
in stored grains (FAO/WHO 1975). When bread was baked from flour
containing 2-14C-labelled compound IV, the unchanged
hydroxypyrimidine constituted more than 90% of the radioactive residue
in the bread (Bullock et al., 1976). By analogy with pirimiphos-methyl
residues, it can be concluded that residues of compound IV in baked
products will not exceed 0.2 mg/kg and will normally be considerably
lower.
The pattern and levels of residues found in semolina and cooked
pasta prepared from pirimiphos-methyl treated wheat are similar to
those found in flour and bread/biscuits respectively. Unchanged
pirimiphos-methyl constitutes the major proportion of the residue.
Bullock and May (1976) studied the fate of residues in wheat on
processing to semolina and pasta. White semolina prepared from Durum
wheat treated at 10 mg/kg contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90% of those in the corresponding semolina.
70% of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However the weight of pasta increases by
100% on cooking so that the concentration of pirimiphos-methyl in
cooked pasta is likely to be approximately 35% of that in the
corresponding semolina.
Peanuts
Pirimiphos-methyl residues in peanuts are found mainly on the
hulls. There is little transfer of residue to the kernels.
Pirimiphos-methyl residues in processed fractions obtained from the
kernel are normally very much smaller than in the whole raw
agricultural commodity.
Ussary (1975) has reviewed the available unpublished data for
pirimiphos-methyl residues in peanut fractions. He concluded that
residues in the kernels depend upon the degree of contamination by the
hulls which occurs in the differing shelling processes. Nevertheless
in the commercial scale trials in the U.S.A. during 1973-74, where
peanuts were treated at 20 mg/kg by admixture, pirimiphos-methyl
levels in the sound mature kernels were normally less than 5 mg/kg.
Undecorticated peanuts containing a mean residue of 10 mg/kg
pirimiphos-methyl yielded oil containing 9-14 mg/kg pirimiphos-methyl.
In the 1972-3 trials in the U.S.A. undecorticated peanuts treated
at 20 mg/kg yielded peanut butter containing mean residues of 3 mg/kg
(Ussary, 1975).
In the series of admixture trials in the U.S.A, pirimiphos-methyl
residues in the peanut hulls were normally below 50 mg/kg (Ussary,
1975).
Residues in food in commerce
Pirimiphos-methyl has not yet been widely used and therefore no
information is available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis, by gas chromatography
with a phosphorus detector, was reviewed by the 1974 Joint Meeting.
The method was recently published (Zweig, 1976). It is suitable for
the determination of residues of pirimiphos-methyl and the phosphorus-
containing metabolites II and III in crops. The limit of determination
is normally 0.01 mg/kg in each case.
NATIONAL TOLERANCES
The following national tolerances have been reported to the
Meeting.
Country Commodity Tolerance, mg/kg
France Cereals 4
Fruit and vegetables 2
Netherlands Cucumbers, courgettes,
peppers and tomatoes 0.2
West Germany Cereals 4*
Grain parts 2*
In the UK, where tolerances are not normally specified,
pirimiphos-methyl is registered for uniform admixture with small grain
cereals at 4 mg/kg.
APPRAISAL
Pirimiphos-methyl was evaluated by the 1974 Joint Meeting when,
in addition to the required toxicological information, further data
were considered desirable on its use on stored products other than
grain, on residues on fruit and vegetables following approved uses,
and on terminal levels in various foods. These data have been
provided.
In use on outdoor and indoor crops, since pirimiphos-methyl has
only a limited biological persistence on leaf surfaces it can be used
where a broad spectrum of insecticidal activity is required for a
short period, e.g., pre-harvest clean-up of vegetables. Rates of
0.5-1.0 kg. a.i./ha "low volume" or 0.05 to 0.1% a.i. "high volume"
are normally recommended. A fogging rate of 20-30 mg a.i./m3 is
normally effective in controlling greenhouse whiteflies, and a fogging
rate of 40 mg/m3 is used to control mushroom flies.
*Temporary tolerance.
A pre-harvest withholding interval of four days is normally
recommended after spraying. but 0 to 3 days after the use of a fog to
control whiteflies on greenhouse tomatoes, cucumbers and peppers or
mushroom flies.
Pirimiphos-methyl residues on growing crops decline rapidly
during the first few days after spraying. On the leaf surfaces and
other aerial parts of the plant the compound is lost principally by
volatilization; photochemical or other degradation also occurs.
However, there are no significant amounts of the degradation compounds
containing the intact phosphorus ester grouping and the
phosphorus-containing residue can be expressed as the parent compound.
Following treatment at recommended rates, levels on vegetables
are normally less than 2 mg/kg after four days but higher applications
can occasionally lead to residues on some green leafy vegetables
(lettuce, spinach, Brussels sprouts) not exceeding 5 mg/kg. On celery,
on which it is not generally recommended, residues my be higher than
this.
Residues on tree fruit are below 2 mg/kg after 4 days whilst on
soft fruits they are below 1 mg/kg. When used for fogging under glass,
initial levels are 0.3 mg/kg or less on cucumbers, tomatoes and
peppers.
Residues of pirimiphos-methyl are relatively stable in the
conditions found during baking to bread and biscuits. Flour containing
1 mg/kg of pirimiphos-methyl is likely to yield bread or biscuits
containing about 0.5 mg/kg of the unchanged compound; the main
degradation products do not contain phosphorus. Approximately similar
changes occur when durum wheat is milled to white or whole meal
semolina and this is subsequently made into pasta and cooked.
Preparation of white semolina reduces the residue by about 80% (most
of the pericarp is removed) but what is left is largely retained in
pasta, even on cooking, although the proportion is halved in cooked
pasta by uptake of water.
These results, and the additional results obtained in stored
rice, do not suggest a need for any alteration in the maximum residue
levels proposed temporarily for wheat and wheat products and rice at
the 1974 Joint Meeting and these are confirmed as maximum residue
limits.
Data evaluated by the 1974 Joint Meeting on the fate of
pirimiphos-methyl in goats, cows, hens, their edible tissues and foods
from them has now been supplemented by work with pigs which has shown
no accumulation in the fat or meat of pigs maintained on diets
containing pirimiphos-methyl at levels up to 34 mg/kg. Any residues of
the parent compound or its phosphorus-containing metabolites were
below the limit of detection (0.005 mg/kg) in muscle, kidney, liver,
lung and heart, and very small in fat. When hens were fed for 28 days
on diets containing up to 40 mg/kg of pirimiphos-methyl small residues
(0.03 - 0.04 mg/kg) were found in eggs at the highest level of
treatment. Residues of pirimiphos-methyl in meat tissues were below
0.01 mg/kg at all doses, and neither the desethyl compound nor the
oxon was detectable in any samples of eggs or meat.
RECOMMENDATIONS
As an ADI has now been established, the temporary tolerances
recommended by the 1974 Meeting are converted to maximum residue
limits. The following additional limits are recommended. They refer to
the sum of pirimiphos-methyl, its oxygen analogue and N-desethyl-
pirimiphos-methyl expressed as pirimiphos-methyl.
Commodity Limit, mg/kg
Peanut hulls, peanuts (whole) 50
Peanut oil 10
Lettuce, mushroom, olives,
peanuts (kernels), spinach 5
Apples, Brussels sprouts, cabbage,
cauliflower, cherries, pears, plums 2
Blackcurrants, carrots, cucumber,
gooseberries, peppers, raspberries,
spring onions, strawberries, tomato 1
Beans with pod, cheese, citrus, dates 0.5
Peas, potatoes 0.05*
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Results of studies now in progress on the residues in peanuts and
peanut products.
2. Results from commercial trials on other commodities.
REFERENCES
Berry, D., and Gore, C.W. Pirimiphos-methyl (PP511): Determination
1975 of no effect level during a 28 day rat feeding
study. Unpublished report received from ICI
Central Toxicology Laboratory. Submitted to the
World Health Organization by ICI.
Boxwell, J.W., and Bullock, D.J.W. Pirimiphos-methyl: Residues on
1976 crops following application of aerosol and dust
formulations (UK). ICI Plant Protection Division
Report No. TMJ1305A. (Unpublished)
Bullock, D.J.W. Residue summary: Pirimiphos-methyl in crops. ICI
1972 Plant Protection Ltd. Report No. TMJ595/1.
(Unpublished)
Bullock, D.J.W., Harrison, P.J., and Day, S.R. Pirimiphos-methyl:
1976 Degradation of residues in flour during baking.
ICI Plant Protection Division Report No. AR2666A.
(Unpublished)
Bullock, D.J.W., and May, M.S. Pirimiphos-methyl: Residue transfer
1976 from durum wheat to semolina and pasta. ICI Plant
Protection Division Report No. TMJ1345A.
(Unpublished).
Bullock, D.J.W., and Stephens, P.P. Pirimiphos-methyl: Residues in
1974 mushrooms following application of
pirimiphos-methyl by `Fumovap' gun and Swingfog
machine. ICI Plant Protection Ltd. Report No.
AR2543A. (Unpublished).
Cogburn, R.G. Pirimiphos-methyl and a protectant for stored rough
1976 rice: Small bin tests. J. Econ. Ent., 69(3): 369.
Davis, J.A., Day, S.R., Hemingway, R.J., Jegatheeswaren, T.,
1976 and Bullock, D.J.W. Pirimiphos-methyl: residue
transfer study with pigs. ICI Plant Protection
Division Report No. AR2665A. (Unpublished)
FAO/WHO 1974 Evaluations of some pesticide residues in food. AGP:
1975 1974/M/11; WHO Pesticide Residues Series No. 4.
Garuti, A., Gore, C.W., Ishmael, J., and Kalinowski, A.E.
1976 Pirimiphos-methyl (PP511): A study of hepatic
changes in the dog. Unpublished report from ICI.
Hanna, P.J., and Dyer, K.F. Mutagenicity of organophosphorus
1975 compounds. Mutat. Res., 28: 405.
Hodge, M.C.E., and Moore, S. Pirimiphos-methyl (PP511) teratological
1972 studies in the rat. Unpublished report from ICI
Industrial Hygiene Research Laboratories.
Howard, S.K., and Gore, C.W. The human response to long-term
1976 oral administration of low doses of
pirimiphos-methyl. Unpublished report from ICI.
Hunter, B., Graham, C., Street, Ae., Offer, J.M., and Printice,
1976 D.E. Long-term feeding of pirimiphos-methyl
(PP511) in mice. (Final report 0-80 weeks).
Unpublished report from Huntingdon Research Centre
No. ICI/34/7650, submitted by ICI.
ICI Plant Protection Ltd. Pirimiphos-methyl: Residues data for fogging
1973 use on cucumbers, tomatoes and peppers.
(Unpublished).
MacCullum Deighton, J., and Pascoe, R. Pirimiphos-methyl: Protection
1976 of stored dates: Trials in Iraq 1974/5. ICI
Plan-Protection Ltd. Report No. AR2642A.
(Unpublished)
McGregor, D.B. Dominant lethal study in mice of pirimiphos methyl.
1975 Unpublished report from Inveresk Research
International (No. 289).
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. ICI Central Toxicology
Report No. CTL/P/247.
Palmer, A.K., and Hill, P.A. Effect of pirimiphos-methyl (PP511)
1976 on reproductive functions of multiple generations
in the rat. Huntingdon Research Centre Report,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl metabolites (R4039 and R35510):
1974 Acute oral toxicity. Unpublished report from ICI
Central Toxicology Laboratory No. CTL/P/137,
submitted by ICI.
Parkinson, G.R. Pirimiphos-methyl potentiation studies with
1975 bioresmethrin. Unpublished report from ICI Central
Toxicology Laboratory (No. CTL/P/166B). Submitted
by ICI.
Redlinger, L.M. Pirimiphos-methyl as a protectant for farmers
1976 stock peanuts. J.Econ. Ent., 69(3): 377.
Ross, D.B., Burroughs, S.J., and Roberts, N.L. Examination of
1975a pirimiphos-methyl for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon
Research Centre (No. ICI/49/75220) submitted by
ICI.
Ross, D.B., Christopher, D.H., Cameron, D.M., Dollery, R.,
1976 Almond, R.H., and Roberts, N.L. Egg production and
hatchability following inclusion of
pirimiphos-methyl at various levels in the diet of
the laying hen. Huntingdon Research Centre Report
No. ICI/31A/75924.
Thomas, K.P., and Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by cheshire cheese. J. Stored
Prod. Res., 11: 53.
Ussary, J.P. ActellicR residues in peanuts. ICI United States
1975 Inc. Interim Report. (Unpublished)
Zweig, G. Analytical methods for pesticides and plant growth
1976 regulators. Academic Press, 8: 125.
