
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
PIRIMIPHOS-METHYL
RESIDUES
Explanation
Pirimiphos-methyl was evaluated in 1974, 1976, 1977 and 1979.1
An acceptable daily intake (ADI) was established and maximum residue
limits (MRLs) were recommended in a range of commodities.
A number of items of information considered desirable by these
Meetings still appear to be outstanding:
1. Information on residues in fruit and vegetables following
approved uses (1974).
2. Further information on the level and fate of residues in food at
the point of consumption following the use of primiphos-methyl
for the control of various stored product pests (1974 and 1977).
3. Results of studies now in progress on the residues in peanuts and
peanut products (1976).
4. Results from commercial trials in other commodities (1976).
Pirimiphos-methyl has been approved for use in the control of
vectors of human disease and an interim specification has been issued
by the Vector Biology and Control Division of WHO (WHO 1982).
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
A limited amount of new information has been received. From the
results of monitoring of imported fruits and vegetables provided by
Sweden (1983) it is apparent there must be significant uses on citrus,
sweet peppers and tomatoes, at least. Barry et al. (1981) report
measurable residues of pirimiphos-methyl in chickpeas (Australia) -
pigeon peas (Kenya), Moong dall (Tanzania), peanuts (South Africa),
split peas (Kenya) and Sardo cheese (Argentina).
The Spanish Ministry of Agriculture (Spain 1983) advised that
pirimiphos-methyl is registered for use on fruit including citrus and
grapes, olives, beets, potatoes and other vegetables, maize, sorghum,
sugarcane and stored grain.
1 See Annex 2 for FAO and WHO documentation.
The use against mosquito vectors of malaria was described by
Rishikesh et al. (1977) and Shaw et al. (1979) and against the
blackfly vector of onchocerciasis (river blindness) by Le Berre
et al. (1972).
McCallum Deighton (1978) reported that Soderstrom & Armstrong
(1973) showed that raisins treated with pirimiphos-methyl at the rate
of 4 mg/kg gave complete kill of several stored product pests 12
months after treatment. An application of 2 mg/kg killed more than 95
percent under similar conditions.
The same author reports that Spitler & Hartsell (1975) found that
almonds in the shell treated with an initial deposit of 1.6 mg/kg
pirimiphos-methyl showed little damage after 10 months of storage.
When treated at the rate of 3.6 mg/kg, the protection was excellent
and little damage occurred at the end of 12 months.
Similarly, it was reported that trials during 1974-75
demonstrated that packages containing dates treated on the outside,
with pirimiphos-methyl at the rate of 0.5 g/sq m remained free of
insects for at least 6 mo. irrespective of the nature of the packages
(cardboard, wood, palm leaf). The dates remained free of any
detectable residues.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Postharvest Cereal Grains
The extensive world literature on the usefulness, effect and fate
of pirimiphos-methyl when applied to cereal grains for the control of
the whole spectrum of stored-product pests was reviewed. The
application rate necessary to control most species adequately is of
the order of 4 mg/kg but depends on the insect species, the
temperature and humidity of the grain, type of storage structure, and
anticipated period of storage. The application rate ranges from 2-6
mg/kg. Treatment may be confined to pirimiphos-methyl alone but where
tolerant species are a problem it is more practical to combine another
insecticide that is specifically effective against the
pirimiphos-methyl tolerant species than to increase the level of
pirimiphos-methyl sufficient to control the tolerant pests.
In India, Chawla, & Bindra (1971) reported that the rate of
dissipation of pirimiphos-methyl residues from grain was significantly
slower than that of malathion, bromophos or iodofenphos. The Cyprus
Agricultural Research Institute observed (Anonymous 1972), when
testing a range of insecticides as grain treatments against a variety
of stored-grain insects, that by the third mouth most of the chemicals
had lost their effectiveness, whereas pirimiphos-methyl was still
effective four months after application. In the United States, La Hue
(1974) studied the patterns of degradation of a number of potential
grain protectants compared with malathion, the standard treatment.
Pirimiphos-methyl residues were influenced less by high moisture
content of the grain at time of treatment than those of malathion and
fenitrothion.
Bowker (1974) studied the degradation of radio-labelled
pirimiphos-methyl during eight months storage, when applied as a 2
percent dust to wheat at 4 mg/kg. In grain with a moisture content
below 14 percent, only 20 percent degradation was observed during
storage, whereas 70 to 80 percent degradation was recorded, in grain
with about 18 percent moisture. He found no significant levels of
degradation products, other than the hydrolysis product
2-diethylamino-4-hydroxy-6-methyl pyrimidine. In particular, levels of
the very unstable "oxon" and of the N-de-ethylated thionate were all
less than 0.05 mg/kg total residue.
Rowlands (1975) reported results of studies on the breakdown and
recovery of radio-labelled pirimiphos-methyl in wheat treated at 4
mg/kg in hexane solution and stored under laboratory conditions at
21°C. After eight months, 78 percent of the radioactivity was still
recoverable as intact parent compound and 9-10 percent was bound to
lipid and proteinaceous matter in the grain. The latter could only be
recovered by digestion and was thought to be associated chiefly with
the protein. The only metabolite detected was the hydroxy-pyrimidine.
Rowlands & Wilkin (1975) applied solutions of radiolabelled
pirimiphos-methyl and also a 2 percent dust to wheat with a moisture
content of 14 and 18 percent to give 4 mg/kg final treatment. The
samples were then stored in a laboratory at 20°C for six months. They
also found that approximately 10 percent of the radioactivity was
bound to lipoprotein material and was unextractable except by
digesting the aleurone regions of the grain. Such labelled material,
as recovered by this means, appeared to be unchanged
pirimiphos-methyl, but degradation during this extraction and
subsequent purification hampered identification. During the storage
period, the breakdown rates were approximately the same for both
solvent and dust treatments. After six months, 15 and 50 percent
degradation had occurred at the 14 and 18 percent moisture levels,
respectively.
Bengston et al. (1975), in studying the effect of
pirimiphos-methyl against malathion-resistant insects and its fate in
bulk wheat under typical semi-tropical conditions found that the
deposit degraded rather slowly. Deposits of 2 and 4 mg/kg degraded to
1.62 and 2.94 mg/kg after 25 weeks, respectively. The authors
estimated the half-life of such deposits to be of the order of 45
weeks (temperature and moisture conditions not stated).
Weaving (1975) found pirimiphos-methyl, applied to maize and
sorghum so as to give a deposit of 5 and 10 mg/kg, gave complete kill
of Sitophilus zeamais for 12 months under tribal storage conditions
in Rhodesia (now Zimbabwe).
La Hue & Dicke (1976) found that pirimiphos-methyl applied at the
rate of 8.4 mg/kg to high-moisture sorghum grain gave excellent
protection against damage by six different insect pests for a period
of 12 months.
Morallo-Rejesus & Carino (1976) reported pirimiphos-methyl was
more persistent on maize than on sorghum but apparently little
allowance was made in their studies for any difference in temperature
or moisture content.
Desmarchelier (1977) reported that the degradation of
pirimiphos-methyl over a 35-week period was too slow to allow accurate
measurements of temperature effects (experiments conducted over the
range 20 to 30°C and 11.5 to 12.5 percent moisture content).
La Hue (1977) noted that pirimiphos-methyl degraded much more
slowly than did malathion under similar conditions; 83 percent of the
original deposit of pirimiphos-methyl remained on wheat 12 months
after treatment, whereas only 16 percent of the malathion deposit
remained under identical conditions.
Cerna & Benes (1977) provided results of a study carried out in
Czechoslovakia where 400 tonnes of wheat, containing 12.6-14.2 percent
moisture at a temperature of 8-10°C, was treated with
pirimiphos-methyl at a rate to provide 4 mg/kg. The wheat was analysed
at the time of treatment and at intervals thereafter until discharged
for milling at the end of 286 days. Over this period, the residue
levels declined from 3.7 to 1.62 mg/kg.
Bengston et al. (1978) carried out duplicate field experiments
on the control of insect infestation in stored sorghum. Residues of
pirimiphos-methyl declined from 3.9 to 3.0 mg/kg over a period of 24
weeks; the average temperature was 26°C and average moisture content
was 12.4 percent.
Banks & Desmarchelier (1978) discussed the finding that the loss
of insecticide residues from stored grain follows pseudo first-order
reaction kinetics and that this model applies equally well to
pirimiphos-methyl (Desmarchelier 1977; Desmarchelier 1978). They noted
the influence of water vapour and temperature on changes in pesticide
residue levels with time and drew attention to the errors introduced
in calculating the rate of degradation from a "linear" or a
semi-logarithmic model. Desmarchelier & Bengston (1979) further
developed this concept and explained how the mathematical models are
developed and used. They compared the rate of degradation of 12 grain
protectants. The half-life of pirimiphos-methyl at 30°C and 50 percent
relative humidity was given as 70 weeks. This compared with 12 weeks
for malathion under similar conditions. The interesting point is that
the co-efficient of variation with respect to temperature is much
smaller for pirimiphos-methyl than for any of the other compounds
considered.
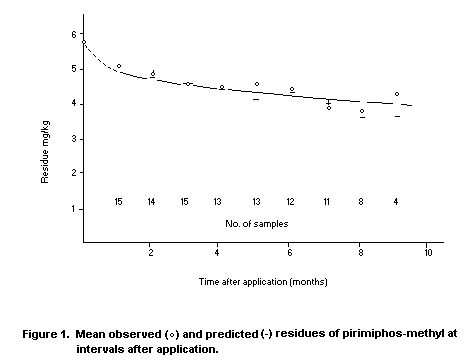
Desmarchelier et al. (in press) provided extensive information
from 21 commercial storages treated with pirimiphos-methyl at 6 mg/kg.
Grain condition and protectant residue levels were regularly
monitored. The residue level declined from 6 to 4 mg/kg over ten
months on grain that remained at 30°C for seven months and then cooled
gradually to 20°C. The moisture content of this grain was between 11
and 12 percent. The mean observed and predicted residue levels of
pirimiphos-methyl were plotted at intervals after application. Figure
1 shows the high level of agreement with the model, based on pseudo
first-order kinetics.
Bengston et al. (1980b) reported the results of duplicate field
trials carried out on bulk wheat in commercial silos, where
pirimiphos-methyl was applied at the rate of 6 mg/kg in conjunction
with phenothrin (2 mg/kg). The effectiveness against a range of
stored-product pests and the residue levels were monitored throughout
a period of nine months, during which the pirimiphos-methyl residue
level did not change appreciably. The moisture content of the grain
ranged from 10.7 to 11.1 percent and the temperature from 26 to 29°C.
Bengston et al. (1980a) reported the results of field trials
with various pesticide combinations carried out on bulk wheat in
commercial silos in Queensland, South Australia and Western Australia.
Once again, the concentration of pirimiphos-methyl declined only
slightly during the eight months storage period.
Desmarchelier et al. (1980b) reported the results of an
extensive collaborative study of residues on wheat of methacrifos,
chlorpyrifos-methyl, fenitrothion, malathion and pirimiphos-methyl.
Pirimiphos-methyl degraded more slowly and to a lesser extent than the
other compounds. The measured values of residues of pirimiphos-methyl
on wheat at the experimental sites agreed with the values predicted
from the calculation using first-order kinetics, grain temperature and
interstitial relative humidity. The rate of degradation was
surprisingly slow.
Desmarchelier et al. (1980a) reported studies on the fate of
pirimiphos-methyl and five other grain protectants or rice and barley
after storage and during processing. The level of residues were
determined on unhusked rice, husked rice, polished rice and barley
over a storage period of six months. The observed levels were close to
those predicted from use of a model relating rate of loss of residue
levels to a rate constant and only two variables, temperature and
equilibrium relative humidity. The difference between predicted and
observed values of residues of pirimiphos-methyl on the four
commodities was 8 percent.
 Residue data were obtained over a year from hard winter wheat,
shelled corn and sorghum grain treated with pirimiphos-methyl stored
in bins in a laboratory (La Hue 1974). Table 1 shows the average
residues of pirimiphos-methyl in the stored grain over the
twelve-month period at 27°C. It is noticeable that the degradation
appears to be independent of the moisture content of the grain under
these conditions. Malathion, included in the same trial, degraded to
about one tenth of the initial dosage within the same period. In the
case of the high moisture sorghum, it was almost entirely destroyed
within one month.
Seth (1974) reported trials to evaluate pirimiphos-methyl for the
control of pests in stored rice in South-east Asia. Rice in husk and
polished rice were treated with emulsifiable concentrate (E.C.) and
dust formulations at rates equivalent to 2, 4 and 8 mg/kg. The rice
was analysed at monthly intervals throughout the four-month trial. In
polished grains, the residue at the end of four months was
approximately 25 percent of the residue found on the first day of the
trial. In rice in husk, it was not possible to deduce the degradation,
since the residue data were expressed on hull and milled grain
separately. However, there appeared to be relatively little loss of
pirimiphos-methyl over five months. Relatively little of the
insecticide (generally less than 0.5 mg/kg) transferred to milled
grain. There was no significant difference between the dust and E.C.
formulations.
FATE OF RESIDUES
In Grain
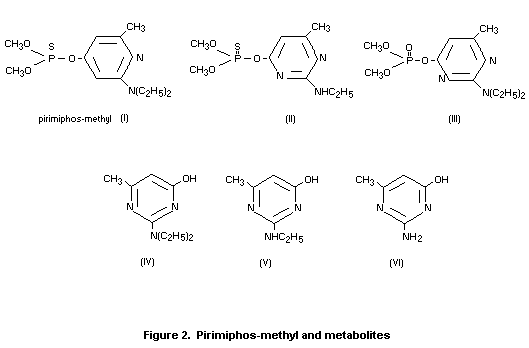
Residues of pirimiphos-methyl on wheat grain are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain to give
principally the parent hydroxypyrimidine (Figure 2, IV), and the
related compounds (V and VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grain.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residue of the chemically-unstable
oxygen analogue (III) was detected. The limit of detection was 0.01
mg/kg (Bowker 1973).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat, so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products. The general pattern of
breakdown on stored rice was similar to that found on wheat grain. The
insecticide and its degradation products were concentrated in the
husk, in which the rate of breakdown appeared to be unaffected by the
moisture content of the rice (Bowker 1973; Bullock 1973).
Table 1. Average Residues of Pirimiphos-methyl in Stored Grain1
Post-treatment residues (mg/kg)
Grain Moisture Intended months
Content dosage
(%) (mg/kg) 24 h 1 3 6 9 12
Wheat 12.5 7.8 6.5 6.1 5.6 6.2 4.9 5.4
Maize 12.5 8.4 7.9 6.3 4.5 4.1 3.4 3.0
Sorghum 17.6 8.4 7.5 6.5 4.3 3.8 3.8 3.7
1 Over 12 months at 27°C.
Residue data were obtained over a year from hard winter wheat,
shelled corn and sorghum grain treated with pirimiphos-methyl stored
in bins in a laboratory (La Hue 1974). Table 1 shows the average
residues of pirimiphos-methyl in the stored grain over the
twelve-month period at 27°C. It is noticeable that the degradation
appears to be independent of the moisture content of the grain under
these conditions. Malathion, included in the same trial, degraded to
about one tenth of the initial dosage within the same period. In the
case of the high moisture sorghum, it was almost entirely destroyed
within one month.
Seth (1974) reported trials to evaluate pirimiphos-methyl for the
control of pests in stored rice in South-east Asia. Rice in husk and
polished rice were treated with emulsifiable concentrate (E.C.) and
dust formulations at rates equivalent to 2, 4 and 8 mg/kg. The rice
was analysed at monthly intervals throughout the four-month trial. In
polished grains, the residue at the end of four months was
approximately 25 percent of the residue found on the first day of the
trial. In rice in husk, it was not possible to deduce the degradation,
since the residue data were expressed on hull and milled grain
separately. However, there appeared to be relatively little loss of
pirimiphos-methyl over five months. Relatively little of the
insecticide (generally less than 0.5 mg/kg) transferred to milled
grain. There was no significant difference between the dust and E.C.
formulations.
FATE OF RESIDUES
In Grain
Residues of pirimiphos-methyl on wheat grain are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain to give
principally the parent hydroxypyrimidine (Figure 2, IV), and the
related compounds (V and VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grain.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residue of the chemically-unstable
oxygen analogue (III) was detected. The limit of detection was 0.01
mg/kg (Bowker 1973).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat, so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products. The general pattern of
breakdown on stored rice was similar to that found on wheat grain. The
insecticide and its degradation products were concentrated in the
husk, in which the rate of breakdown appeared to be unaffected by the
moisture content of the rice (Bowker 1973; Bullock 1973).
Table 1. Average Residues of Pirimiphos-methyl in Stored Grain1
Post-treatment residues (mg/kg)
Grain Moisture Intended months
Content dosage
(%) (mg/kg) 24 h 1 3 6 9 12
Wheat 12.5 7.8 6.5 6.1 5.6 6.2 4.9 5.4
Maize 12.5 8.4 7.9 6.3 4.5 4.1 3.4 3.0
Sorghum 17.6 8.4 7.5 6.5 4.3 3.8 3.8 3.7
1 Over 12 months at 27°C.
 Degradation was marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
was caused by factors within the grain, or by the associated
microflora, was not known. At a higher moisture content (ca. 18
percent) less pirimiphos-methyl was recovered on analysis but
increased levels of the hydrolysis product (IV) were obtained,
suggesting that a more rapid degradation of the insecticide occurred.
Bowker (1973) concluded that grain may lack the enzymatic activity,
believed to be present in plants and soil, to cleave the pyrimidine
N-ethyl bonds. It is likely, therefore, that following the treatment
of wheat and brown rice with pirimiphos-methyl, the major residues
during storage will be the parent compound and the simple hydrolysis
product (IV). Under optimum conditions, the maximum level of compound
(IV) following treatment at 4 mg/kg was found to be 0.17 mg/kg; under
poor storage conditions, with high moisture content grain it was 0.62
mg/kg.
Solutions and also a 2 percent dust of radio-labelled
pirimiphos-methyl were applied in the laboratory to wheat grain of 14
and 18 percent moisture to give levels of 4 mg/kg. Throughout a
storage period of six months, the residues of pirimiphos-methyl were
found almost entirely in the seed coat and aleurone layer, with only
traces present in the germ or endosperm. It was also clear that, as
with malathion but to a greater extent, there was transfer of
insecticide between grains, possibly in the vapour phase. About 10
percent of the total residual radioactivity was bound to lipoprotein
material in the aleurone region of the grain and could be extracted
only by digestion of the aleurone protein. In contrast with other
organophosphates studied, the bound material appeared to be unchanged
pesticide rather than a metabolite. It was undoubtedly a P=S compound,
but degradation during the liberation of the bound material
complicated identification (Rowlands et al. 1974).
In Animals
The metabolism of pirimiphos-methyl was studied in the rat and
dog. Twelve metabolites were detected, none of which showed
anticholinesterase activity. No parent pirimiphos-methyl was detected
in the urine. In both rats and dogs, 2-ethylamino-4-hydroxy-6-methyl
pyrimidine was the major urinary metabolite (Bratt & Jones 1973).
When rats were given a single oral dose of radio-labelled
pirimiphos-methyl at 7.5 mg/kg, the uptake of radioactivity into blood
and its subsequent disappearance from the bloodstream were both very
rapid. More than 50 percent of the radioactivity present in the blood
30 min. after dosing had disappeared at one hour after dosing.
Unchanged pirimiphos-methyl usually represented less than 10 percent
of the total residue in the blood 24 h after dosing. When
radio-labelled pirimiphos-methyl was administered orally to rats at
7.5 mg/kg per day for four days, total radioactive residues in the
liver, kidney and fat did not usually exceed 2 mg/kg pirimiphos-methyl
equivalents. There was no evidence to show that either
pirimiphos-methyl or its metabolites accumulated in the liver, kidney
or fat of rats following daily dosing with the insecticide over four
days (Mills 1976).
The rodioactive residues in the liver and kidney of a goat, dosed
daily for seven consecutive days with 14C-pirimiphos-methyl at 30
mg/kg in the diet, were examined. (Curl and Leahy 1980).
A radioactive residue of 0.25-0.30 mg/kg of
14C-pirimiphos-methyl equivalents was detected in the liver, and 89
percent of this was extracted and analysed by thin layer
chromatography. 5.9 percent of the total radioactive residue in the
liver was unchanged pirimiphos-methyl. The hydroxypyrimidines,
compound (II) (2-diethylamino-6-methyl-pyrimidin-4-ol), compound (III)
(2-ethylamino-6-methyl-pyrimidin-4-ol) and compound (IV)
(2-amino-6-methyl-pyrimidin-4-ol) accounted for 3.7 percent, 21.8
percent and 17.5 percent respectively, of the total radioactive
residue in the liver. Compounds (II), (III) and (IV) were present
mainly as "free" metabolites. However, 13.7 percent of the
hydroxypyrimidines detected were released from the liver by acid
reflux.
The remainder of the radioactivity extracted from the liver
consisted of at least five compounds.
A radioactive residue of 0.59-0.70 mg/kg of
14C-pirimiphos-methyl equivalents was found in the kidney; 91 percent
of this was extracted and analysed by thin-layer chromatography.
Compound (II), compound (III) and compound (IV) accounted for 7.9
percent, 35.3 percent and 17.0 percent respectively, of the total
radioactive residue in the kidney. Compounds (II), (III) and (IV) were
present mainly as "free" metabolites, however, 9.0 percent of the
hydroxypyrimidines detected were released from the kidney by acid
reflux.
The remainder of the radioactivity extracted from the kidney
consisted of at least six compounds.
In Storage and Processing
Bullock (1973, 1974) reported several individual experiments
which demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table 2
summarizes the results of residue trials carried out in the United
Kingdom on wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 3 summarizes results of a residue trial (Bullock 1973)
carried out in the United Kingdom with wheat nominally treated to
contain 8 mg/kg pirimiphos-methyl. These data are substantially in
agreement with those of Bengston et al. (1975) and show that there
is relatively little penetration beyond the seed coat, even throughout
a storage period of nine weeks.
Table 2. Effect of Milling and Baking on Residue in Wheat Admixed
with Pirimiphos-Methyl1
Interval between
Grain treatment and Residues (mg/kg)
fraction sampling (months) Highest Lowest Mean
Whole grain 0 4.2 1.9 2.9 (9)
3.0 -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Whole meal 0 1.3 0.94 1.1 (3)
flour 1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White flour 0 0.88 0.30 0.52 (6)
0.56* 0.53* 0.55*(3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27*(3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread 1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
White bread 0 0.28 0.19 0.23 (6)
0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 Dosage of pesticide was 4 mg/kg. All results are from field
trials except those marked *, which are from a small-scale trial.
Figures in parentheses are the numbers of results on which the
means are based.
Table 3. Residues of Pirimiphos-methyl in Whole Grains and in Milling
and Baking Fractions Treated in a Laboratory Trial1
Interval between Residues (mg/kg)
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52
6.0 0.91 0.56
6.0 1.0 0.57
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 Dosage of pesticide was 8 mg/kg. In all cases, no residues of the
phosphorus-containing compounds (II) or (III) (Figure 1) were
detected. (Limit of detection: 0.01 mg/kg in each case.)
Seth (1974) discussed experiments on the admixture of
pirimiphos-methyl with paddy rice (rice in husk) in Southeast Asia.
Both emulsion and dust formulations were used and were applied at
rates of 2, 4 and 8 mg/kg. A portion of the rice was milled
immediately after treatment and further portions at the end of one,
two, three and five months. It was found that the deposit of
pirimiphos-methyl on both the husk and the milled grain was
proportional to the amount applied, but the concentration on the husk
was always about 25 times that on the milled grain. The residue on the
husk and milled grain declined steadily over the five-month storage
period. Although the concentration on the husk initially was as high
as 12 mg/kg, the residue on the milled rice barely exceeded 0.5 mg/kg
and even at the highest rate of application was always below 1 mg/kg.
These experiments clearly showed that there is minimum transfer of
pirimiphos-methyl from the husk to the kernel of rice, even during
prolonged storage. The degree of penetration is comparable to that
reported by Kadoum & La Hue (1974) for malathion on wheat, maize and
sorghum.
Residues of pirimiphos-methyl in flour are relatively stable
during baking to bread and biscuits. However, because of the dilution
which the flour undergoes during these processes, flour initially
containing 1 mg/kg pirimiphos-methyl is likely to yield
bread/ biscuits containing residues of ca. 0.5 mg/kg.
Bullock et al. (1976) studied the fate of pirimiphos-methyl
during processing of flour into bread and biscuits. In studies with
the radio-labelled compound, flour was dosed with 2-14C-labelled
pirimiphos-methyl and baked to produce white bread, wholemeal bread
and biscuits. Although pirimiphos-methyl is known to be a relatively
volative compound, there was no significant loss of radioactivity by
volatilization. Distribution of radioactivity throughout the bread was
fairly uniform. Unchanged pirimiphos-methyl accounted for 75-90
percent of the radioactivity in the baked product. The major
degradation product formed during the baking was hydroxypyrimidine,
which accounted for 3-10 percent of the radioactivity in the final
product.
Similar results were obtained by residue analysis in a second set
of studies. After correcting for the weight increase when flour is
converted to bread, residues of pirimiphos-methyl fell by 11-18
percent. Likewise, residue analysis of biscuits showed average losses
of 8 percent. However, owing to the dilution of the flour that occurs
during baking, the residue of pirimiphos-methyl in bread will be lower
than that in the corresponding flour. Bullock et al. (1976) found
levels of pirimiphos-methyl in bread and biscuits to be about 50
percent of those in the flour from which they were derived. These
findings agreed well with the earlier work of Bullock (1973, 1974). No
residues of the phosphorus-containing compounds (II) and (III) were
detected in bread baked from flour treated with pirimiphos-methyl at
1 mg/kg or in biscuits baked from flour containing up to 5 mg/kg
(Bullock et al. 1976)
The hydroxypyrimidine (IV) also undergoes only slight degradation
during the baking process. This compound constitutes only a minor part
of the residue in stored grains (FAO/,WHO 1975). Bullock et al
(1976) used radio-labelled compound (IV) and found that it degraded by
less than 10 percent during the baking of bread. It can, therefore, be
concluded that residues of compound (IV) in baked products will never
exceed 0.2 mg/kg and will normally be considerably lower.
Bullock & May (1976) studied the fate of residues in wheat during
processing to semolina and pasta. White semolina, prepared from durum
wheat treated at 10 mg/kg, contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90 percent of those in the corresponding semolina; 70
percent of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However, the weight of pasta increased by
100 percent on cooking, so that the concentration of pirimiphos-methyl
in cooked pasta is likely to be approximately 35 percent of that in
the corresponding semolina.
Results of experiments in Czechoslovakia (Cerna & Benes 1977)
indicated that residues of pirimiphos-methyl in grain, which had been
in store for nine months, were substantially removed by the milling
process. The bulk of the residue was removed in the bran; there was no
substantial difference in the concentration in the different bran
fractions. When white flour was made into white bread, there was a
further loss of approximately 50 percent so that the concentration of
the residue in the bread was only 10-15 percent of its concentration
in raw grain. Some residue was destroyed during milling.
Tempone (1979) reported a series of studies on the effects of
insecticides on barley malting and the resulting residues. In the
first of these trials, barley treated with pirimiphos-methyl at the
rate of 6 mg/kg or 18 mg/kg was converted into malt but no residues
were detected in the wort (unfermented extracts from the malted
barley), the limit of determination being 0.004 mg/kg. Wort, prepared
from barley malted after being in store for three, six and nine
months, likewise showed no residues. In the second series of similar
trials, the residue was determined in the malt (germinated grain that
had been calcined). Residues could be found in the malt, but at a
level of from 10 to 20 percent of that in the barley before malting.
Barley kept in storage for three months after being treated with
pirimiphos-methyl transferred significantly less residue to the malt
than did the barley malted immediately after treatment, no doubt
because there was significantly less residue on the outside of the
kernel, where it would be protected from the enzymatic activity within
the barley grain during germination.
Bengston et al. (1980 a, b) arranged for wheat from bulk grain
treated with pirimiphos-methyl and held in commercial silos to be
processed through to wholemeal bread and white bread. The results
obtained in these trials are reported in Tables 4 and 5. During
processing from wheat to white bread, residues were reduced by 85-91
percent.
Table 4. Mean Residues of Pirimiphos-methyl Following Milling and Baking of Stored Wheat
Site Storage Residues (mg/kg)
(weeks)
Wheat Bran Pollard Wholemeal White Wholemeal White
flour flour bread bread
Site B 13 5-7 12-16 2-4 2.6 0.1-0.2 1.0-2.0 0.05-0.1
Site D 19 2.5-3.0 6-8 1-2 1.2 0.05-0.1 0.5-1.0 0.05
Table 5. Reduction in Pirimiphos-methyl Residues Following Milling and Baking of Stored Wheat
Site Reduction in residues(%)
Wheat to Wheat to Wholemeal flour White Flour Wheat to Wheat to
Wholemeal White to Wholemeal to White Wholemeal White
Flour Flour Bread Bread Bread Bread
Site B 5 68 47 54 50 85
Site D 0 78 55 58 56 91
Desmarchelier et al. (1980b), after treating bulk wheat in
commercial silos with pirimiphos-methyl, arranged for a portion to be
milled and for the white flour to be converted into white bread. The
results are given in Table 6. It is of interest to note that the
residues in flour and bread were higher in the hard wheat which was
held for 22 weeks before milling, than in the soft wheat which was
held of 11 weeks before milling.
Desmarchelier et al. (1980a) studied the fate of
primiphos-methyl, and a number of other grain protectant insecticides,
applied to unhusked rice, husked rice, polished rice and barley over a
storage period of six months and subsequently during the processing
and cooking of these grains. The results of these trials are given in
Table 7, and show that only about 10-15 percent of the residue present
on husked rice or polished rice was destroyed in the cooking process.
However, if the treatment was applied to unhusked rice, approximately
70 percent of the residue was removed with the husk and a great deal
more when the husked rice was milled for the removal of the bran.
Subsequent cooking of the husked rice or polished rice brought about a
further substantial reduction in the residue level.
These same workers found that pirimiphos-methyl, applied to
barley destined for malting, was substantially lost during the malting
process. Barley treated at the rate of 6 mg/kg and held in storage for
six months was found to contain 4.9 mg/kg pirimiphos-methyl. When this
grain was malted, the pirimiphos-methyl residue in the prepared malt
was only 0.9 mg/kg. The work of Tempone (1979) showed that little, if
any, of the residue in the malt is extracted into the wort.
There have been no reports of pirimiphos-methyl being metabolized
by attack at either of the O,O-dimethyl groups (to give a desmethyl
derivative) as is common with certain other organophosphorus
triesters. Morallo-Rejesus & Carino (1976) noted, but did not
identify, breakdown products in shelled maize stored in
pirimiphos-methyl treated bags.
Rowlands (1981), reporting work carried out some years previously
using radio-labelled compound, treated small quantities (10 g) of
wheat that were held in store for up to 6 months. Aliquots were
removed at intervals and, after crushing, were extracted first with
hexane, followed in turn by chloroform, methanol and acetonitrile and
then, after sequential digestion by three enzymes, further extraction
by chloroform. The results are recorded in Table 8.
It is clear from Table 8 that very soon after treatment, a
portion of the aged pirimiphos-methyl residue is not extractable from
wheat unless it is digested out enzymatically. It is thought that this
is due to a complex formed by intact pirimiphos-methyl within the
aleurone layer (Rowlands 1975).
Table 6. Residues of Primiphos-methyl in Wheat, Its Milling Products and Bread
Residues (mg/kg)
Type of Storage
Wheat (weeks) Initial deposit Whole grain bran pollard flour bread
Soft 11 5.2 4.2 9.1 8.3 0.2 0.1
Hard 22 6.1 4.5 12.4 10.4 1.3 0.35
Table 7. Residues of Pirimiphos-methyl on Husked, Polished and Unhusked Rice After Storage and Processing
Grain Application Residues (mg/kg)
level
(mg/kg)
After After After After
3 6 Cooking at Cooking at
Months Months 3 Months 6 Months
Husked
rice 6 6.5 4.9 4.9 4.0
Polished
rice 6 6.0 6.0 4.9 4.9
Residues (mg/kg) after
6 months storage and
Milling Milling &
Cooking
Husked Polished Husked Polished
rice rice rice rice
Unhusked
rice 6.0 6.0 4.6 1.4 0.3 0.6 0.1
Table 8. Recovery of Pirimiphos-methyl From Crushed Wheat (18% mo) By Sequential extraction
Extraction by 14C activity recovered %1 at time of extraction after
application (0 h)
0 h 1 h 7 days 14 days 1 month 6 months
Hexane 97 89 84 78 72 67
Chloroform nil 1 4 7 8 8
Methanol trace 1 2 2 6 7
Acetonitrile trace 2 1 4 2 2
Digest Sequence2
(1) nil 2 1 4 2 2
(2) 4 5 7 7 9 12
(3) nil 2 2 1 2 1
Total extracted 101 102 101 103 101 99
1 Average of three results ± 3 percent.
2 Digest sequence: (1) = lipase pH 7.4; (2) = papain pH 4,5; (3) - cellulase pH 4.5,
All at 37°C for 24 h
Studies reported by Rowlands (1981) on the uptake from dust or
solvent application as determined by the separated tissue of the wheat
grains after storage, showed that intact pirimiphos-methyl and free
pyrimidinols occur chiefly in the seedcoat. These findings received
some confirmation in the work of Mensah et al. (1979), who found
that pirimiphos-methyl emulsion, applied to wheat that was then milled
six months later, had accumulated in the bran and middlings fractions,
irrespective of the moisture content of the stored wheat (12 percent
and 16 percent). They found little or no residue in white flour.
Rowlands (1981) found only pirimiphos-methyl (I) and the
corresponding pirimidinol (IV) as residues in pirimiphos-methyl
treated wheat with 12 percent moisture that was kept in sealed jars
for up to eight months. However, in wheat with 18 percent moisture, a
small (less than 10 percent of the total residue) quantity of the
N-des-ethyl pirimidinol (V) was found after six and eight months. (See
Figure 2 for conformations of these compounds).
Further to the work of Thomas & Rowlands (1975) on the uptake and
degradation of pirimiphos-methyl by Cheshire cheese, similar studies
were carried out using Stilton cheeses, which differ in physical
characteristics and are stored differently.
In these studies a wooden plank was treated with
14C-ring-labelled pirimiphos-methyl and allowed to dry for 24 h
before two young Stilton cheeses were placed on the treated surface.
One of the cheeses was first covered by a layer of cheesecloth. The
cheeses were turned every two days. Replicate core samples were taken
with a cork borer from each face of both cheeses. The core was
analysed for pirimiphos-methyl. Some of the cores were sectioned with
a microtome and the concentration of pirimiphos-methyl in the cheese
at various depths was determined by means of a scintillation counter.
The findings are reported in Table 9.
This experiment has shown that only a small amount of
pirimiphos-methyl penetrated into the cheeses under the various
storage techniques used with Stilton cheese. No breakdown of the
pirimiphos-methyl could be detected in the cheese or the cheesecloth
throughout the seven weeks of storage.
These results indicate that the MRL of 0.5 mg/kg for
pirimiphos-methyl in cheese would not be exceeded when the insecticide
was used for the control of cheese mites during the making and storage
of Stilton cheese.
Mensah et al. (1979) reported results of small-scale trials, in
which water-based emulsions of malathion, bromophos, iodofenphos and
pirimiphos-methyl were applied at two dosage rates to spring wheat of
12 percent and 15 percent moisture content, to compare the fate of the
residues. Pirimiphos-methyl degraded at a comparatively slower rate
than the other three compounds, but the rate of degradation was
significantly higher on the high moisture grain. The results are given
in Table 10. The residue levels were concentrated in the seed coat,
resulting in high levels in bran and middlings. When applied at the
rate of 6 mg/kg, the residues in these fractions exceeded the MRL
recommended by the Meeting.
Results were reported of small-scale trials in which the efficacy
and fate of pirimiphos-methyl residue on wheat and milling fractions
were assessed. Two rates (7.3 mg/kg and 14.6 mg/kg) were applied and
the wheat was stored for up to 12 months. The results are reported in
Table 11. These show that the residues in bran and middlings (shorts)
exceeded the MRL following application at the lower rate and storage
for less than six months. At the higher rate (which is well in excess
of the recommended rate) the residues greatly exceeded the MRL.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of pirimiphos-methyl were detected in the United States
in several samples of imported foods (Barry et al. 1981) and the
identification of these residues by GC-MS and GLC was reported. The
level and source of residues found are reported in Table 12.
The Swedish National Food Institute (Sweden 1983) indicated that
during the period 1-1-80 to 30-4-83, 8 654 samples of fruit and
vegetables were analysed for pesticide residues. From among these,
eight samples were found to contain pirimiphos-methyl at levels up to
1.4 mg/kg, as indicated in Table 13.
METHODS OF RESIDUE ANALYSIS
Barry et al. (1981) have provided details of GC-MS
identification and analytical behaviour of pirimiphos-methyl in a
variety of foods as part of an investigation of unknown residues
detected in imported foods.
Zakitis & McCray (1982), having reviewed the analytical
methodology for residues of pirimiphos-methyl in a variety of
substrates, concluded that none of the eight known methods met all the
desired criteria for analysis of residues in water, fish and snails,
viz. specificity, simplicity of sample preparation and recovery after
clean-up. Water was simply extracted with hexane prior to
determination by GC. Fish and snails were extracted with acetone in
the presence of sufficient anhydrous sodium sulphate to form a dry
powder. The acetone was filtered and the solids were extracted three
more times with acetone. The combined, filtered extracts were
evaporated to low volume before being transferred to de-ionized water
for extraction with hexane as for analysis of water. Recoveries ranged
from 93 to 101 percent. The method is being used for environmental
studies involving the use of pirimiphos-methyl for disease vector
control. The limit of determination in snails is 0.5 mg/kg. Below this
concentration, some naturally occurring substance interferes with the
determination of pirimiphos-methyl. Good recoveries were obtained from
dechlorinated water at concentrations as low as 0.005 mg/l and in fish
down to 0.05 mg/kg.
Table 9. Pirimiphos-Methyl Residues From Microtomed Sections of Two Cheeses1
Residues (mg/kg)
Storage
period End Depth (mm) into cheese
(days) sampled 0-1 1-2 2-3 3-4 4-5 5-6 6-7 7-8 8-9 9-10
Cheese in direct contact with treated surface
31 1 41.15 7.48 4.03 1.73 1.25 0.90 0.65 0.55 0.40 0.10
2 27.50 7.70 2.32 1.09 0.71 0.50 0.28 0.20 0.16 -
49 1 29.57 9.26 2.87 2.04 1.52 1.13 0.82 0.71 0.51 0.27
2 23.73 6.54 2.82 2.71 1.52 1.07 0.74 0.55 0.51 0.37
Cheese on cloth barrier
31 1 18.04 3.40 0.65 0.30 0.18 0.08 - - - -
2 35.28 11.48 4.19 0.62 0.36 0.27 0.22 0.16 - -
49 1 19.58 5.69 1.72 0.72 0.45 0.28 - - - -
2 12.26 7.13 1.84 1.11 0.84 0.66 0.46 0.31 - -
1 Dashes indicate that no pirimiphos-methyl was detected.
Table 10. Mean1 Pirimiphos-methyl Residues (mg/kg) on Dry and Tough Wheat and Milled Fractions After Storage
of Treated Wheat
12% moisture content (dry) 16% moisture content (tough)
Storage
Dosage period Whole Whole
(mg/kg) (months) wheat2 Bran Middling3 Flour wheat2 Bran Middling3 Flour
4 1 3.29±0.07 15.66±0.79 21.44±1.96 1.94±0.25 3.24±0.03 19.81±1.63 15.35±0.58 1.57±0.03
3 2.98±0.10 13.93±0.26 15.10±1.00 1.93±0.12 3.04±0.01 13.45±0.80 13.51±0.36 1.16±0.06
6 2.54±0.07 13.16±0.51 13.80±1.36 1.56±0.06 2.26±0.18 11.98±0.67 10.68±0.60 0.81±0.23
6 1 4.93±0.13 20.87±0.82 23.15±1.47 2.86±0.14 4.92±0.04 27.33±1.41 21.83±1.73 1.91±0.07
3 4.54±0.10 20.54±1.38 19.34±0.74 2.42±0.22 4.44±0.04 20.90±2.92 17.89±0.59 1.50±0.08
6 3.66±0.23 19.32±0.73 16.05±1.35 2.23±0.13 3.86±0.26 18.60±0.73 16.89±0.56 1.33±0.14
1 Mean of 3 replicates ± SE.
2 Ground wheat.
3 Shorts, wheat germ and coarse particles of flour.
Table 11. Average Pirimiphos-methyl Residue (mg/kg) on Hard Winter Wheat
and Wheat Fractions Stored up to 12 Months
Rate of application
7.3 mg/kg 14.6 mg/kg
Storage Whole Whole
Period wheat1 Bran Shorts Flour wheat1 Bran Shorts Flour
24 h 7.15 25.50 18.29 0.83 14.75 50.41 36.01 2.01
Months
1 6.88 21.45 17.89 1.38 13.13 41.43 31.01 2.94
3 6.60 20.11 20.82 1.47 12.98 39.97 42.97 3.15
6 5.78 18.06 20.46 1.47 11.17 30.52 46.58 2.91
9 5.43 17.34 17.90 1.17 10.39 34.52 31.47 2.44
12 5.27 13.47 15.35 1.05 9.72 32.29 26.98 2.14
1 Before tempering to 15% for milling.
Table 12. Pirimiphos-methyl residues in imported foods
Food Country of Origin Residue (mg/kg)
Chickpeas Australia 0.07
Dried green peas Australia 3.0
Pigeon peas Kenya 0.19
Moong dall Tanzania 0.03
Peanut butter South Africa 0.23
Split peas Kenya 0.1-0.171
Sardo cheese Argentina 0.21-1.41,2
1 Represents range found in multiple samples of the same
commodity.
2 Determined on fat basis.
Table 13. Primiphos-methyl Residues Detected in Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.21 0.21-0.53 0.54-1.05 >1.05 (mg/kg)
Mandarin Import 292 291 1 0.21
Orange Import 622 621 1 0.41
Pepper Import 206 201 3 1 1 1.4
(sweet)
Tomato Sweden 205 205
Import 500 499 1 0.30
1 Total number of samples analysed:8 654
Period: 81-01-01 -- 83-04-30
A number of the methods reviewed by Zakitis & McCray (1982) have
not been considered previously by the Meeting. They are briefly
mentioned for information.
A general review of analytical methods was published by Bullock
(1976) including procedures suitable for animal tissues. There is also
a TLC-UV method for residues in water, soil and plants (Krasnykh,
1978), an HPLC method for determining pirimiphos-methyl and five
metabolites in samples of plasma and urine (Beasley & Lawrence, 1979),
and gas chromatographic methods for residues in stored grain (Varca
et al. 1975), peanuts (Redlinger & Simonaitis 1977) and milled
fractions of wheat (Mensah et al. 1979). In addition, a
gas-chromatographic method has been proposed for the simultaneous
determination of residues of pirimiphos-methyl and malathion in
peanuts (Simonaitis et al. 1981), and GC-MS was used to identify
residues of pirimiphos-methyl in imported foods (Barry et al. 1981).
The residue in stored grains (barley, maize, oats, rice in husk
and wheat) consisted mainly of the parent compound. Neither the oxygen
analogue (O,O-dimethyl-O(2-diethylamino-6-methyl-pirimidine-4yl)
phosphate), (metabolite III) nor the desethyl analogue
(O, O-dimethyl-O(2-ethylamino-6-methyl-pyrimidin-4-yl)
phosphorothionate), (metabolite II) was detectable. No metabolite was
detectable in milled products or in white and wholemeal breads. A
large number of plant samples (leafy vegetables, carrots, celery,
spring onions, potatoes, sugarbeets, cucumber, tomatoes, peppers,
mushrooms, lettuce, blackcurrants, apples, pears, plums, lemons,
oranges, olives) were analysed for residues of pirimiphos-methyl and
the phosphorous-containing metabolites. However, significant amounts
of the metabolites did not appear and so were not reported. The
residues listed in various tables of the 1976 Evaluations are of the
parent compound only.
Analyses of wheat grains treated with labelled compounds
confirmed the low level of both metabolites. No oxygen analogue was
detected. The level of desethyl analogue was below 0.05 mg/kg over a
period of 32 weeks, while the parent compound was present at 4 mg/kg.
The oxygen analogue was not detectable in whole rice seedlings. The
levels of parent compound in mg/kg and desethyl analogue (expressed as
parent equivalent) were as follows:
Compound Residue (mg/kg at intervals (days) after treatment
1 3 6 8 10 13
pirimiphos-methyl 0.5 0.51 12 0.6 0.51 0.32
metabolite (II) and
unknown compound 0.17 0.11 0.14 0.12 0.15 0.14
The oxygen analogue reached its maximum concentration on plant
leaves approximately 1 to 3 days after treatment. The ratio of maximum
levels of oxygen analogue and the parent compound were about 0.3 and 3
on cotton and citrus leaves, respectively, while the two compounds
were present in nearly equal concentration on bean leaves. The
disappearance of pirimiphos-methyl residues was rapid on the leaf
surfaces of all plants due to evaporation. The total 14C represented
10-12 percent of the applied material after three days and the
phosphorous containing metabolites were below 10 percent (Bowker
1973).
The Meeting concluded that the metabolites can be excluded from
the definition of the residue. The MRLs need not be changed because
the metabolites represent such a minor proportion of the total
residue. The MRLs refer to the present compound alone.
APPRAISAL
Pirimiphos-methyl was evaluated in 1974, 1976, 1977 and 1979 and
a number of items of information previously requested appear to be
still outstanding.
It would appear that there are a number of uses that give rise to
residues in food moving in international trade, which have not yet
been considered by the Meeting and about which relevant information
will be available in 1984.
An extensive amount of information about the use and fate of
pirimiphos-methyl when applied after harvest to cereal grains has
appeared in open scientific literature and this was reviewed by the
Meeting. The review only serves to confirm the evaluations made
previously. All authors have drawn attention to the stability of
pirimiphos-methyl deposits on treated grain and the retention of the
bulk of the deposit in the seed coat. This is reflected in the low
transfer of residue into white flour and milled rice and the
relatively higher residues in wheat bran.
This review has confirmed that the degradation and metabolism of
pirimiphos-methyl on grain leads only to the production of
2-diethylamino-4-hydroxy-6-methyl pyrimidine. No
cholinesterase-inhibiting metabolites occur. However, between 4
percent and 15 percent of the residue of the parent compound forms a
complex with the aleurone layer of the grain and resists extraction by
a variety of solvents. It may be dislodged by digesting the substrate
with proteolytic enzymes.
The Meeting, having reviewed all available data, concluded that
the metabolites can be excluded from the definition of the residue.
The MRLs, which remain the same, refer to the parent compound alone.
A further study of the use of pirimiphos-methyl for the control
of cheese mites in cheese stores confirmed that the MRL previously
recommended adequately covers the resulting residues.
Several reviews have been made of analytical methods suitable for
dealing with various substrates.
The Meeting considered that the information evaluated in 1983 did
not indicate any need to amend or modify recommendations previously
made for MRLs in a range of commodities.
The Meeting reviewed the requests made by earlier Meetings for
additional information in the light of knowledge about the current use
of this insecticide and considered that they had been satisfied.
FURTHER WORK OR INFORMATION
Desirable
Further information about residues in peanuts, oil seeds, lentils
and citrus.
REFERENCES - RESIDUES
Anonymous. Chemical control of stored grain insects 1972. Cyprus
1972 Agric. Res. Inst. Ann. Rep., p. 69.
Banks, H.J. & Desmarchelier, J.M. New chemical approaches to pest
1978 control in stored grain. Chem. Aust. 45(6): 276-281.
Barry, T.L., Petzinger, G. & Gretch, F.M. GC-MS identification and
1981 analytical behaviour of pirimiphos-methyl in imported foods.
Bull. Environ. Contam. Toxicol., 27: 524-528.
Beasley, C.J. & Lawrence, D.K.: J. Chromatog., 168: 461.
1979
Bengston, M., Cooper, L.M. & Grant-Taylor, F.J. A comparison of
1975 bioresmethrin, chloropyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in
wheat. J. Agric. Anim. Sci., 32: 51-78.
Bengston, M., Cooper, L.M., Davies, R., Desmarchelier, J., Hart R. &
1978 Phillips, M. Grain protectants for the control of
malathion-resistant insects in stored sorghum. (To be
submitted for publication).
Bengston, M., Connell, M. Davies, R., Desmarchelier, J., Elder, B.,
1980a Hart, R., Phillips, M., Ridley, E., Ripp, E., Snelson, J. &
Sticka, R. Chloropyrifos-methyl plus bioremethrin,
methacrifos, pirimiphos-methyl plus bioresmethrin and
synergised bioresmethrin as grain protectants for wheat.
Pestic. Sci., 11: 61-76.
Bengston, M., Connell, M., Davies, R., Desmarchelier, J., Phillips,
1980b M., Snelson, J. & Sticks, R. Fenitrothion plus phenothrin
and pirimiphos-methyl plus carbaryl, as grain protectant
combinations for wheat. Pestic. Sci., 11: 471-482.
Bowker, D.M. Pirimiphos-methyl: fate of stored wheat and rice grain in
1973 the laboratory. Report No. AR2457 AR,submitted to FAO by ICI
Plant Protection Ltd, (Unpublished).
Bratt, H. & Jones, L.A. Pirimiphos-methyl: Metabolism in rats and
1973 dogs. Report submitted to FAO by ICI Industrial Hygiene
Research Laboratories. (Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain. Report
1973 No. AR2472 AR submitted to FAO by ICI Plant Protection Ltd.
(Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain, bread,
1974 flour and milled products. Report submitted to FAO by ICI
Plant Protection Ltd. (Unpublished).
Bullock, D.J.W. In Analytical Methods for Pesticides and Plant
Growth
1976 Regulators, VIII, 185, G. Zweig Ed., Academic Press, New
York.
Bullock, D.J.W. & May, M.S. Pirimiphos-methyl: Residue transfer from
1976 durum wheat to semolina and pasta. Report No. TMJI345A
submitted to FAO by ICI Plant Protection Ltd. (Unpublished).
Bullock, D.J.W., Harrison, P.J. & Day, S.R. Degradation of residues in
1976 flour during baking. Report No. AR2666A submitted to FAO by
ICI Plant Protection Ltd. (Unpublished).
Cerna, V. & Benes, V. Residues of pirimiphos-methyl in wheat, mill
1977 products and white bread. Report by Czechoslovakian
Institute of Hygiene in Epidemiology, Prague.
Chawla, R.P. & Bindra, O.S. (1971). Residues of some promising grain
1971 protectants on stored wheat. Proc. Symp. Progress and
Problems in Pesticide Residue Analysis (Bindra & Kalra,
Eds). Punjab Agric. Univ. Ludhiana & Indian Counc. Agric.
Res., New Delhi. p. 68-74.
Curl, E.A. & Leary, J.P. The radioactive residues found in the liver
1980 and kidney of a goat after administration of a multiple dose
of pirimiphos-methyl. Report RJO144B submitted to FAO by ICI
Plant Protection Ltd.
Desmarchelier, J.M. Selective treatments including combinations of
1977 pyrethroid and organophosphorus insecticides for control of
stored product Coleoptera at two temperatures. J. Stored
Prod. Res., 13: 129-137.
Desmarchelier, J.M. Loss of fenitrothion on grains in storage. Pestic.
1978 Sci. 9: 33-38.
Desmarchelier, J.M. & Bengston, M. The residual behaviour of chemicals
1979 in stored grain. Proceedings of the Second International
Working Conference in Stored-Product Entomology, Ibadan,
Nigeria, 10-16 September 1979.
Desmarchelier, J.M., Bengston, M., Connell, M., Henning, R., Ridley,
in E., Ripp, E., Sierakowski, C., Sticka, R., Snelson, J.
press & Wilson, A. Extensive pilot usage of the grain protectant
combinations, fenitrothion plus bioresmethrin and
pirimiphos-methyl plus bioresmethrin. J. Pestic. Sci.
Desmarchelier, J.M., Goldring, M. & Horan, R. Predicted and observed
1980a residues of bio-resmethrin, carbaryl, fenitrothion,
phenothrin, methacrifos and pirimiphos-methyl on rice and
barley after storage, and losses of these insecticides
during processing. J. Pestic. Sci., 5: 539-545.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W., Moore, B.,
1980b Phillips, M., Snelson, J., Sticka, R. & Tucker, K. A
collaborative study of residues on wheat of methacrifos,
chloropyrifos-methyl, fenitrothion, malathion and
pirimiphos-methyl. II. Rates of decay. CSIRO, Division of
Entomology Report No. 20. 21 p.
Kadoum, A.M. & La Hue, D.W. Penetration of malathion in stored corn,
1974 wheat and sorghum grain. J. Econ. Entomol., 67(4): 477-478.
Krasnykh, A.A. Khim. Sel'sk. Khoz. 16, (11) 36 (Russ).
1978
La Hue, D.W. Pesticide residues of potential protectants of grain.
1974 Proc. 1st Int. Working Conf. on Stored Product Entomology,
Savannah, Georgia, USA. p. 635-638.
La Hue, D.W. Pirimiphos-methyl: Effect on populations of Tribolium
1977 confusum and T. castaneum in wheat. J. Econ. Entomol.,
70(1): 135-137.
La Hue, D.W. & Dicke, E.B. Evaluation of selected insecticides applied
1976 to high moisture sorghum grain to prevent stored grain
insect attack. USDA Agricultural Research Service, Marketing
Research Report No. 1063.
Le Berre, R., Escaffre, H., Pendriez, B., Grebaut, S. & Pengalet, P.
1972 Control of Simulium damnosum, the vector of human
onchocerciasus in West Africa II. Test by classic
application of new insecticides and new formulations, 70
Oncho du 8 Mai ORSTOMOCCGE, BP 171, Bobo-Dioulasso, Upper
Volta.
McCallum Deighton, J. Pirimiphos-methyl and other insecticides in the
1978 control of stored-product insects. Outlook Agric., 9(5)
240-248.
Mensah, G.W.K., Watters, F.L. & Webster, G.R.B. Insecticide residues
1979 in milled fractions of dry or tough wheat treated with
malathion, bromophos, iodofenphos and pirimiphos-methyl. J.
Econ. Entomol., 72: 728-731.
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. Report No. CTL/P/247 submitted to FAO
by ICI Central Toxicology. (Unpublished).
Morallo-Rejesus, B. & Carino F.O. The residual toxicity of five
1976 insecticides on three varieties of corn and sorghum.
Philipp. Agric., 60(3&4): 96-104.
Redlinger, L.M. & Simonaitis, R.A. Field tests with pirimiphos-methyl
1977 as a protectant for farmers stock peanuts. Peanut Sci.
4: 27.
Rishikesh, N., Mathis, H.L., King, J.S. & Nambiar, R.V. A field trial
1977 of pirimiphos-methyl for the control of Anopheles gambiae
and Anopheles funestus in Nigeria. WHO Geneva, VBC/77/671.
(Unpublished).
Rowlands, D.G. The metabolism of contact insecticides in stored grains
1975 1970-1974. Residue Rev., 58: 113.
Rowlands, D.G. The metabolism of pirimiphos-methyl by stored grains
1981 under laboratory conditions. Report No. 54, Pest Control
Chemistry Department, Pest Infestation Laboratory, Slough,
U.K. Ministry of Agriculture, Fisheries & Food.
Rowlands, D.G. et al. Preliminary report of studies with pirimiphos-
1974 methyl as a grain protectant. Submission by United Kingdom
to FAO.
Rowlands, D.G. & Wilkin, D.R. Metabolism of pesticides in stored
1975 cereals. Report of Director, Pest Infestation Control
Laboratory, Slough, U.K. 1971-1973.
Seth, A.K. Use of Actellic for stored rice insect problems in S.E.
1974 Asia. Symp. 1st Int. Working Conf. Stored Prod. Entomol.,
7-11 October 1974, Savannah, Georgia, USA.
Shaw, R.F., Fanara, D.M., Pradhan, G.D., Supratman, Supalin, Bang,
1979 Y.H., & Fleming, G.A. A village-scale trial of
pirimiphos-methyl (OMS-1424) for control of the malaria
vector (Anopheles aconitus) in Central Java, Indonesia.
WHO, Geneva, VBC/79/722. (Unpublished)
Simonaitis, R.A., Zehner, J.M. & Redlinger, L.M. J. HRC & CC 4: 169.
1981
Soderstrom, E.L. & Armstrong, J.W. pp-511: (Pirimiphos-methyl-ISO)
1973 evaluated as a protectant for raisins and walnuts. J. Econ.
Entomol., 66(6): 1305-1306.
Spain. Registered uses of pirimiphos-methyl. Data submitted to FAO by
1983 the Ministry of Agriculture, Madrid.
Spitler, G.H. & Hartsell, P.L. Pirimiphos-methyl as a protectant for
1975 stored inshell almonds. J. Econ. Entomol., 68(6): 777-780.
Sweden. Monitoring of imported fruits and vegetables for residues of
1983 pirimiphos-methyl. Data submitted to FAO by the Swedish
National Food Institute.
Tempone, M.J. Studies on the effects of insecticides on barley
1979 malting. Research Project Series No. 50, Victorian
Department of Agriculture, Melbourne, Australia.
Thomas, K.P. & Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by Cheshire cheese. J. Stored Prod. Res.,
11:53.
Varca, L.M., Magallona, E.D. & Pasco, J.A. Philipp. Entomol., 3: 55.
1975
Weaving, A.J.S. Grain protectants for use under tribal storage
1975 conditions in Rhodesia - Comparative toxicities of some
insecticides on maize and sorghum. J. Stored Prod. Res.,
11: 65-71.
WHO. Interim specifications for pirimiphos-methyl, technical, water
1982 dispersible powder and emulsion concentrate VBC/15/82.04.
WHO Vector Biology Control Division, Geneva.
Zakitis, L.H. & McCray, E.M. Gas chromatographic analysis of residues
1982 of pirimiphos-methyl in water, fish and snails. Bull.
Environ. Contam. Toxicol., 28: 334-340.
Degradation was marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
was caused by factors within the grain, or by the associated
microflora, was not known. At a higher moisture content (ca. 18
percent) less pirimiphos-methyl was recovered on analysis but
increased levels of the hydrolysis product (IV) were obtained,
suggesting that a more rapid degradation of the insecticide occurred.
Bowker (1973) concluded that grain may lack the enzymatic activity,
believed to be present in plants and soil, to cleave the pyrimidine
N-ethyl bonds. It is likely, therefore, that following the treatment
of wheat and brown rice with pirimiphos-methyl, the major residues
during storage will be the parent compound and the simple hydrolysis
product (IV). Under optimum conditions, the maximum level of compound
(IV) following treatment at 4 mg/kg was found to be 0.17 mg/kg; under
poor storage conditions, with high moisture content grain it was 0.62
mg/kg.
Solutions and also a 2 percent dust of radio-labelled
pirimiphos-methyl were applied in the laboratory to wheat grain of 14
and 18 percent moisture to give levels of 4 mg/kg. Throughout a
storage period of six months, the residues of pirimiphos-methyl were
found almost entirely in the seed coat and aleurone layer, with only
traces present in the germ or endosperm. It was also clear that, as
with malathion but to a greater extent, there was transfer of
insecticide between grains, possibly in the vapour phase. About 10
percent of the total residual radioactivity was bound to lipoprotein
material in the aleurone region of the grain and could be extracted
only by digestion of the aleurone protein. In contrast with other
organophosphates studied, the bound material appeared to be unchanged
pesticide rather than a metabolite. It was undoubtedly a P=S compound,
but degradation during the liberation of the bound material
complicated identification (Rowlands et al. 1974).
In Animals
The metabolism of pirimiphos-methyl was studied in the rat and
dog. Twelve metabolites were detected, none of which showed
anticholinesterase activity. No parent pirimiphos-methyl was detected
in the urine. In both rats and dogs, 2-ethylamino-4-hydroxy-6-methyl
pyrimidine was the major urinary metabolite (Bratt & Jones 1973).
When rats were given a single oral dose of radio-labelled
pirimiphos-methyl at 7.5 mg/kg, the uptake of radioactivity into blood
and its subsequent disappearance from the bloodstream were both very
rapid. More than 50 percent of the radioactivity present in the blood
30 min. after dosing had disappeared at one hour after dosing.
Unchanged pirimiphos-methyl usually represented less than 10 percent
of the total residue in the blood 24 h after dosing. When
radio-labelled pirimiphos-methyl was administered orally to rats at
7.5 mg/kg per day for four days, total radioactive residues in the
liver, kidney and fat did not usually exceed 2 mg/kg pirimiphos-methyl
equivalents. There was no evidence to show that either
pirimiphos-methyl or its metabolites accumulated in the liver, kidney
or fat of rats following daily dosing with the insecticide over four
days (Mills 1976).
The rodioactive residues in the liver and kidney of a goat, dosed
daily for seven consecutive days with 14C-pirimiphos-methyl at 30
mg/kg in the diet, were examined. (Curl and Leahy 1980).
A radioactive residue of 0.25-0.30 mg/kg of
14C-pirimiphos-methyl equivalents was detected in the liver, and 89
percent of this was extracted and analysed by thin layer
chromatography. 5.9 percent of the total radioactive residue in the
liver was unchanged pirimiphos-methyl. The hydroxypyrimidines,
compound (II) (2-diethylamino-6-methyl-pyrimidin-4-ol), compound (III)
(2-ethylamino-6-methyl-pyrimidin-4-ol) and compound (IV)
(2-amino-6-methyl-pyrimidin-4-ol) accounted for 3.7 percent, 21.8
percent and 17.5 percent respectively, of the total radioactive
residue in the liver. Compounds (II), (III) and (IV) were present
mainly as "free" metabolites. However, 13.7 percent of the
hydroxypyrimidines detected were released from the liver by acid
reflux.
The remainder of the radioactivity extracted from the liver
consisted of at least five compounds.
A radioactive residue of 0.59-0.70 mg/kg of
14C-pirimiphos-methyl equivalents was found in the kidney; 91 percent
of this was extracted and analysed by thin-layer chromatography.
Compound (II), compound (III) and compound (IV) accounted for 7.9
percent, 35.3 percent and 17.0 percent respectively, of the total
radioactive residue in the kidney. Compounds (II), (III) and (IV) were
present mainly as "free" metabolites, however, 9.0 percent of the
hydroxypyrimidines detected were released from the kidney by acid
reflux.
The remainder of the radioactivity extracted from the kidney
consisted of at least six compounds.
In Storage and Processing
Bullock (1973, 1974) reported several individual experiments
which demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table 2
summarizes the results of residue trials carried out in the United
Kingdom on wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 3 summarizes results of a residue trial (Bullock 1973)
carried out in the United Kingdom with wheat nominally treated to
contain 8 mg/kg pirimiphos-methyl. These data are substantially in
agreement with those of Bengston et al. (1975) and show that there
is relatively little penetration beyond the seed coat, even throughout
a storage period of nine weeks.
Table 2. Effect of Milling and Baking on Residue in Wheat Admixed
with Pirimiphos-Methyl1
Interval between
Grain treatment and Residues (mg/kg)
fraction sampling (months) Highest Lowest Mean
Whole grain 0 4.2 1.9 2.9 (9)
3.0 -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Whole meal 0 1.3 0.94 1.1 (3)
flour 1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White flour 0 0.88 0.30 0.52 (6)
0.56* 0.53* 0.55*(3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27*(3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread 1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
White bread 0 0.28 0.19 0.23 (6)
0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 Dosage of pesticide was 4 mg/kg. All results are from field
trials except those marked *, which are from a small-scale trial.
Figures in parentheses are the numbers of results on which the
means are based.
Table 3. Residues of Pirimiphos-methyl in Whole Grains and in Milling
and Baking Fractions Treated in a Laboratory Trial1
Interval between Residues (mg/kg)
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52
6.0 0.91 0.56
6.0 1.0 0.57
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 Dosage of pesticide was 8 mg/kg. In all cases, no residues of the
phosphorus-containing compounds (II) or (III) (Figure 1) were
detected. (Limit of detection: 0.01 mg/kg in each case.)
Seth (1974) discussed experiments on the admixture of
pirimiphos-methyl with paddy rice (rice in husk) in Southeast Asia.
Both emulsion and dust formulations were used and were applied at
rates of 2, 4 and 8 mg/kg. A portion of the rice was milled
immediately after treatment and further portions at the end of one,
two, three and five months. It was found that the deposit of
pirimiphos-methyl on both the husk and the milled grain was
proportional to the amount applied, but the concentration on the husk
was always about 25 times that on the milled grain. The residue on the
husk and milled grain declined steadily over the five-month storage
period. Although the concentration on the husk initially was as high
as 12 mg/kg, the residue on the milled rice barely exceeded 0.5 mg/kg
and even at the highest rate of application was always below 1 mg/kg.
These experiments clearly showed that there is minimum transfer of
pirimiphos-methyl from the husk to the kernel of rice, even during
prolonged storage. The degree of penetration is comparable to that
reported by Kadoum & La Hue (1974) for malathion on wheat, maize and
sorghum.
Residues of pirimiphos-methyl in flour are relatively stable
during baking to bread and biscuits. However, because of the dilution
which the flour undergoes during these processes, flour initially
containing 1 mg/kg pirimiphos-methyl is likely to yield
bread/ biscuits containing residues of ca. 0.5 mg/kg.
Bullock et al. (1976) studied the fate of pirimiphos-methyl
during processing of flour into bread and biscuits. In studies with
the radio-labelled compound, flour was dosed with 2-14C-labelled
pirimiphos-methyl and baked to produce white bread, wholemeal bread
and biscuits. Although pirimiphos-methyl is known to be a relatively
volative compound, there was no significant loss of radioactivity by
volatilization. Distribution of radioactivity throughout the bread was
fairly uniform. Unchanged pirimiphos-methyl accounted for 75-90
percent of the radioactivity in the baked product. The major
degradation product formed during the baking was hydroxypyrimidine,
which accounted for 3-10 percent of the radioactivity in the final
product.
Similar results were obtained by residue analysis in a second set
of studies. After correcting for the weight increase when flour is
converted to bread, residues of pirimiphos-methyl fell by 11-18
percent. Likewise, residue analysis of biscuits showed average losses
of 8 percent. However, owing to the dilution of the flour that occurs
during baking, the residue of pirimiphos-methyl in bread will be lower
than that in the corresponding flour. Bullock et al. (1976) found
levels of pirimiphos-methyl in bread and biscuits to be about 50
percent of those in the flour from which they were derived. These
findings agreed well with the earlier work of Bullock (1973, 1974). No
residues of the phosphorus-containing compounds (II) and (III) were
detected in bread baked from flour treated with pirimiphos-methyl at
1 mg/kg or in biscuits baked from flour containing up to 5 mg/kg
(Bullock et al. 1976)
The hydroxypyrimidine (IV) also undergoes only slight degradation
during the baking process. This compound constitutes only a minor part
of the residue in stored grains (FAO/,WHO 1975). Bullock et al
(1976) used radio-labelled compound (IV) and found that it degraded by
less than 10 percent during the baking of bread. It can, therefore, be
concluded that residues of compound (IV) in baked products will never
exceed 0.2 mg/kg and will normally be considerably lower.
Bullock & May (1976) studied the fate of residues in wheat during
processing to semolina and pasta. White semolina, prepared from durum
wheat treated at 10 mg/kg, contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90 percent of those in the corresponding semolina; 70
percent of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However, the weight of pasta increased by
100 percent on cooking, so that the concentration of pirimiphos-methyl
in cooked pasta is likely to be approximately 35 percent of that in
the corresponding semolina.
Results of experiments in Czechoslovakia (Cerna & Benes 1977)
indicated that residues of pirimiphos-methyl in grain, which had been
in store for nine months, were substantially removed by the milling
process. The bulk of the residue was removed in the bran; there was no
substantial difference in the concentration in the different bran
fractions. When white flour was made into white bread, there was a
further loss of approximately 50 percent so that the concentration of
the residue in the bread was only 10-15 percent of its concentration
in raw grain. Some residue was destroyed during milling.
Tempone (1979) reported a series of studies on the effects of
insecticides on barley malting and the resulting residues. In the
first of these trials, barley treated with pirimiphos-methyl at the
rate of 6 mg/kg or 18 mg/kg was converted into malt but no residues
were detected in the wort (unfermented extracts from the malted
barley), the limit of determination being 0.004 mg/kg. Wort, prepared
from barley malted after being in store for three, six and nine
months, likewise showed no residues. In the second series of similar
trials, the residue was determined in the malt (germinated grain that
had been calcined). Residues could be found in the malt, but at a
level of from 10 to 20 percent of that in the barley before malting.
Barley kept in storage for three months after being treated with
pirimiphos-methyl transferred significantly less residue to the malt
than did the barley malted immediately after treatment, no doubt
because there was significantly less residue on the outside of the
kernel, where it would be protected from the enzymatic activity within
the barley grain during germination.
Bengston et al. (1980 a, b) arranged for wheat from bulk grain
treated with pirimiphos-methyl and held in commercial silos to be
processed through to wholemeal bread and white bread. The results
obtained in these trials are reported in Tables 4 and 5. During
processing from wheat to white bread, residues were reduced by 85-91
percent.
Table 4. Mean Residues of Pirimiphos-methyl Following Milling and Baking of Stored Wheat
Site Storage Residues (mg/kg)
(weeks)
Wheat Bran Pollard Wholemeal White Wholemeal White
flour flour bread bread
Site B 13 5-7 12-16 2-4 2.6 0.1-0.2 1.0-2.0 0.05-0.1
Site D 19 2.5-3.0 6-8 1-2 1.2 0.05-0.1 0.5-1.0 0.05
Table 5. Reduction in Pirimiphos-methyl Residues Following Milling and Baking of Stored Wheat
Site Reduction in residues(%)
Wheat to Wheat to Wholemeal flour White Flour Wheat to Wheat to
Wholemeal White to Wholemeal to White Wholemeal White
Flour Flour Bread Bread Bread Bread
Site B 5 68 47 54 50 85
Site D 0 78 55 58 56 91
Desmarchelier et al. (1980b), after treating bulk wheat in
commercial silos with pirimiphos-methyl, arranged for a portion to be
milled and for the white flour to be converted into white bread. The
results are given in Table 6. It is of interest to note that the
residues in flour and bread were higher in the hard wheat which was
held for 22 weeks before milling, than in the soft wheat which was
held of 11 weeks before milling.
Desmarchelier et al. (1980a) studied the fate of
primiphos-methyl, and a number of other grain protectant insecticides,
applied to unhusked rice, husked rice, polished rice and barley over a
storage period of six months and subsequently during the processing
and cooking of these grains. The results of these trials are given in
Table 7, and show that only about 10-15 percent of the residue present
on husked rice or polished rice was destroyed in the cooking process.
However, if the treatment was applied to unhusked rice, approximately
70 percent of the residue was removed with the husk and a great deal
more when the husked rice was milled for the removal of the bran.
Subsequent cooking of the husked rice or polished rice brought about a
further substantial reduction in the residue level.
These same workers found that pirimiphos-methyl, applied to
barley destined for malting, was substantially lost during the malting
process. Barley treated at the rate of 6 mg/kg and held in storage for
six months was found to contain 4.9 mg/kg pirimiphos-methyl. When this
grain was malted, the pirimiphos-methyl residue in the prepared malt
was only 0.9 mg/kg. The work of Tempone (1979) showed that little, if
any, of the residue in the malt is extracted into the wort.
There have been no reports of pirimiphos-methyl being metabolized
by attack at either of the O,O-dimethyl groups (to give a desmethyl
derivative) as is common with certain other organophosphorus
triesters. Morallo-Rejesus & Carino (1976) noted, but did not
identify, breakdown products in shelled maize stored in
pirimiphos-methyl treated bags.
Rowlands (1981), reporting work carried out some years previously
using radio-labelled compound, treated small quantities (10 g) of
wheat that were held in store for up to 6 months. Aliquots were
removed at intervals and, after crushing, were extracted first with
hexane, followed in turn by chloroform, methanol and acetonitrile and
then, after sequential digestion by three enzymes, further extraction
by chloroform. The results are recorded in Table 8.
It is clear from Table 8 that very soon after treatment, a
portion of the aged pirimiphos-methyl residue is not extractable from
wheat unless it is digested out enzymatically. It is thought that this
is due to a complex formed by intact pirimiphos-methyl within the
aleurone layer (Rowlands 1975).
Table 6. Residues of Primiphos-methyl in Wheat, Its Milling Products and Bread
Residues (mg/kg)
Type of Storage
Wheat (weeks) Initial deposit Whole grain bran pollard flour bread
Soft 11 5.2 4.2 9.1 8.3 0.2 0.1
Hard 22 6.1 4.5 12.4 10.4 1.3 0.35
Table 7. Residues of Pirimiphos-methyl on Husked, Polished and Unhusked Rice After Storage and Processing
Grain Application Residues (mg/kg)
level
(mg/kg)
After After After After
3 6 Cooking at Cooking at
Months Months 3 Months 6 Months
Husked
rice 6 6.5 4.9 4.9 4.0
Polished
rice 6 6.0 6.0 4.9 4.9
Residues (mg/kg) after
6 months storage and
Milling Milling &
Cooking
Husked Polished Husked Polished
rice rice rice rice
Unhusked
rice 6.0 6.0 4.6 1.4 0.3 0.6 0.1
Table 8. Recovery of Pirimiphos-methyl From Crushed Wheat (18% mo) By Sequential extraction
Extraction by 14C activity recovered %1 at time of extraction after
application (0 h)
0 h 1 h 7 days 14 days 1 month 6 months
Hexane 97 89 84 78 72 67
Chloroform nil 1 4 7 8 8
Methanol trace 1 2 2 6 7
Acetonitrile trace 2 1 4 2 2
Digest Sequence2
(1) nil 2 1 4 2 2
(2) 4 5 7 7 9 12
(3) nil 2 2 1 2 1
Total extracted 101 102 101 103 101 99
1 Average of three results ± 3 percent.
2 Digest sequence: (1) = lipase pH 7.4; (2) = papain pH 4,5; (3) - cellulase pH 4.5,
All at 37°C for 24 h
Studies reported by Rowlands (1981) on the uptake from dust or
solvent application as determined by the separated tissue of the wheat
grains after storage, showed that intact pirimiphos-methyl and free
pyrimidinols occur chiefly in the seedcoat. These findings received
some confirmation in the work of Mensah et al. (1979), who found
that pirimiphos-methyl emulsion, applied to wheat that was then milled
six months later, had accumulated in the bran and middlings fractions,
irrespective of the moisture content of the stored wheat (12 percent
and 16 percent). They found little or no residue in white flour.
Rowlands (1981) found only pirimiphos-methyl (I) and the
corresponding pirimidinol (IV) as residues in pirimiphos-methyl
treated wheat with 12 percent moisture that was kept in sealed jars
for up to eight months. However, in wheat with 18 percent moisture, a
small (less than 10 percent of the total residue) quantity of the
N-des-ethyl pirimidinol (V) was found after six and eight months. (See
Figure 2 for conformations of these compounds).
Further to the work of Thomas & Rowlands (1975) on the uptake and
degradation of pirimiphos-methyl by Cheshire cheese, similar studies
were carried out using Stilton cheeses, which differ in physical
characteristics and are stored differently.
In these studies a wooden plank was treated with
14C-ring-labelled pirimiphos-methyl and allowed to dry for 24 h
before two young Stilton cheeses were placed on the treated surface.
One of the cheeses was first covered by a layer of cheesecloth. The
cheeses were turned every two days. Replicate core samples were taken
with a cork borer from each face of both cheeses. The core was
analysed for pirimiphos-methyl. Some of the cores were sectioned with
a microtome and the concentration of pirimiphos-methyl in the cheese
at various depths was determined by means of a scintillation counter.
The findings are reported in Table 9.
This experiment has shown that only a small amount of
pirimiphos-methyl penetrated into the cheeses under the various
storage techniques used with Stilton cheese. No breakdown of the
pirimiphos-methyl could be detected in the cheese or the cheesecloth
throughout the seven weeks of storage.
These results indicate that the MRL of 0.5 mg/kg for
pirimiphos-methyl in cheese would not be exceeded when the insecticide
was used for the control of cheese mites during the making and storage
of Stilton cheese.
Mensah et al. (1979) reported results of small-scale trials, in
which water-based emulsions of malathion, bromophos, iodofenphos and
pirimiphos-methyl were applied at two dosage rates to spring wheat of
12 percent and 15 percent moisture content, to compare the fate of the
residues. Pirimiphos-methyl degraded at a comparatively slower rate
than the other three compounds, but the rate of degradation was
significantly higher on the high moisture grain. The results are given
in Table 10. The residue levels were concentrated in the seed coat,
resulting in high levels in bran and middlings. When applied at the
rate of 6 mg/kg, the residues in these fractions exceeded the MRL
recommended by the Meeting.
Results were reported of small-scale trials in which the efficacy
and fate of pirimiphos-methyl residue on wheat and milling fractions
were assessed. Two rates (7.3 mg/kg and 14.6 mg/kg) were applied and
the wheat was stored for up to 12 months. The results are reported in
Table 11. These show that the residues in bran and middlings (shorts)
exceeded the MRL following application at the lower rate and storage
for less than six months. At the higher rate (which is well in excess
of the recommended rate) the residues greatly exceeded the MRL.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of pirimiphos-methyl were detected in the United States
in several samples of imported foods (Barry et al. 1981) and the
identification of these residues by GC-MS and GLC was reported. The
level and source of residues found are reported in Table 12.
The Swedish National Food Institute (Sweden 1983) indicated that
during the period 1-1-80 to 30-4-83, 8 654 samples of fruit and
vegetables were analysed for pesticide residues. From among these,
eight samples were found to contain pirimiphos-methyl at levels up to
1.4 mg/kg, as indicated in Table 13.
METHODS OF RESIDUE ANALYSIS
Barry et al. (1981) have provided details of GC-MS
identification and analytical behaviour of pirimiphos-methyl in a
variety of foods as part of an investigation of unknown residues
detected in imported foods.
Zakitis & McCray (1982), having reviewed the analytical
methodology for residues of pirimiphos-methyl in a variety of
substrates, concluded that none of the eight known methods met all the
desired criteria for analysis of residues in water, fish and snails,
viz. specificity, simplicity of sample preparation and recovery after
clean-up. Water was simply extracted with hexane prior to
determination by GC. Fish and snails were extracted with acetone in
the presence of sufficient anhydrous sodium sulphate to form a dry
powder. The acetone was filtered and the solids were extracted three
more times with acetone. The combined, filtered extracts were
evaporated to low volume before being transferred to de-ionized water
for extraction with hexane as for analysis of water. Recoveries ranged
from 93 to 101 percent. The method is being used for environmental
studies involving the use of pirimiphos-methyl for disease vector
control. The limit of determination in snails is 0.5 mg/kg. Below this
concentration, some naturally occurring substance interferes with the
determination of pirimiphos-methyl. Good recoveries were obtained from
dechlorinated water at concentrations as low as 0.005 mg/l and in fish
down to 0.05 mg/kg.
Table 9. Pirimiphos-Methyl Residues From Microtomed Sections of Two Cheeses1
Residues (mg/kg)
Storage
period End Depth (mm) into cheese
(days) sampled 0-1 1-2 2-3 3-4 4-5 5-6 6-7 7-8 8-9 9-10
Cheese in direct contact with treated surface
31 1 41.15 7.48 4.03 1.73 1.25 0.90 0.65 0.55 0.40 0.10
2 27.50 7.70 2.32 1.09 0.71 0.50 0.28 0.20 0.16 -
49 1 29.57 9.26 2.87 2.04 1.52 1.13 0.82 0.71 0.51 0.27
2 23.73 6.54 2.82 2.71 1.52 1.07 0.74 0.55 0.51 0.37
Cheese on cloth barrier
31 1 18.04 3.40 0.65 0.30 0.18 0.08 - - - -
2 35.28 11.48 4.19 0.62 0.36 0.27 0.22 0.16 - -
49 1 19.58 5.69 1.72 0.72 0.45 0.28 - - - -
2 12.26 7.13 1.84 1.11 0.84 0.66 0.46 0.31 - -
1 Dashes indicate that no pirimiphos-methyl was detected.
Table 10. Mean1 Pirimiphos-methyl Residues (mg/kg) on Dry and Tough Wheat and Milled Fractions After Storage
of Treated Wheat
12% moisture content (dry) 16% moisture content (tough)
Storage
Dosage period Whole Whole
(mg/kg) (months) wheat2 Bran Middling3 Flour wheat2 Bran Middling3 Flour
4 1 3.29±0.07 15.66±0.79 21.44±1.96 1.94±0.25 3.24±0.03 19.81±1.63 15.35±0.58 1.57±0.03
3 2.98±0.10 13.93±0.26 15.10±1.00 1.93±0.12 3.04±0.01 13.45±0.80 13.51±0.36 1.16±0.06
6 2.54±0.07 13.16±0.51 13.80±1.36 1.56±0.06 2.26±0.18 11.98±0.67 10.68±0.60 0.81±0.23
6 1 4.93±0.13 20.87±0.82 23.15±1.47 2.86±0.14 4.92±0.04 27.33±1.41 21.83±1.73 1.91±0.07
3 4.54±0.10 20.54±1.38 19.34±0.74 2.42±0.22 4.44±0.04 20.90±2.92 17.89±0.59 1.50±0.08
6 3.66±0.23 19.32±0.73 16.05±1.35 2.23±0.13 3.86±0.26 18.60±0.73 16.89±0.56 1.33±0.14
1 Mean of 3 replicates ± SE.
2 Ground wheat.
3 Shorts, wheat germ and coarse particles of flour.
Table 11. Average Pirimiphos-methyl Residue (mg/kg) on Hard Winter Wheat
and Wheat Fractions Stored up to 12 Months
Rate of application
7.3 mg/kg 14.6 mg/kg
Storage Whole Whole
Period wheat1 Bran Shorts Flour wheat1 Bran Shorts Flour
24 h 7.15 25.50 18.29 0.83 14.75 50.41 36.01 2.01
Months
1 6.88 21.45 17.89 1.38 13.13 41.43 31.01 2.94
3 6.60 20.11 20.82 1.47 12.98 39.97 42.97 3.15
6 5.78 18.06 20.46 1.47 11.17 30.52 46.58 2.91
9 5.43 17.34 17.90 1.17 10.39 34.52 31.47 2.44
12 5.27 13.47 15.35 1.05 9.72 32.29 26.98 2.14
1 Before tempering to 15% for milling.
Table 12. Pirimiphos-methyl residues in imported foods
Food Country of Origin Residue (mg/kg)
Chickpeas Australia 0.07
Dried green peas Australia 3.0
Pigeon peas Kenya 0.19
Moong dall Tanzania 0.03
Peanut butter South Africa 0.23
Split peas Kenya 0.1-0.171
Sardo cheese Argentina 0.21-1.41,2
1 Represents range found in multiple samples of the same
commodity.
2 Determined on fat basis.
Table 13. Primiphos-methyl Residues Detected in Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.21 0.21-0.53 0.54-1.05 >1.05 (mg/kg)
Mandarin Import 292 291 1 0.21
Orange Import 622 621 1 0.41
Pepper Import 206 201 3 1 1 1.4
(sweet)
Tomato Sweden 205 205
Import 500 499 1 0.30
1 Total number of samples analysed:8 654
Period: 81-01-01 -- 83-04-30
A number of the methods reviewed by Zakitis & McCray (1982) have
not been considered previously by the Meeting. They are briefly
mentioned for information.
A general review of analytical methods was published by Bullock
(1976) including procedures suitable for animal tissues. There is also
a TLC-UV method for residues in water, soil and plants (Krasnykh,
1978), an HPLC method for determining pirimiphos-methyl and five
metabolites in samples of plasma and urine (Beasley & Lawrence, 1979),
and gas chromatographic methods for residues in stored grain (Varca
et al. 1975), peanuts (Redlinger & Simonaitis 1977) and milled
fractions of wheat (Mensah et al. 1979). In addition, a
gas-chromatographic method has been proposed for the simultaneous
determination of residues of pirimiphos-methyl and malathion in
peanuts (Simonaitis et al. 1981), and GC-MS was used to identify
residues of pirimiphos-methyl in imported foods (Barry et al. 1981).
The residue in stored grains (barley, maize, oats, rice in husk
and wheat) consisted mainly of the parent compound. Neither the oxygen
analogue (O,O-dimethyl-O(2-diethylamino-6-methyl-pirimidine-4yl)
phosphate), (metabolite III) nor the desethyl analogue
(O, O-dimethyl-O(2-ethylamino-6-methyl-pyrimidin-4-yl)
phosphorothionate), (metabolite II) was detectable. No metabolite was
detectable in milled products or in white and wholemeal breads. A
large number of plant samples (leafy vegetables, carrots, celery,
spring onions, potatoes, sugarbeets, cucumber, tomatoes, peppers,
mushrooms, lettuce, blackcurrants, apples, pears, plums, lemons,
oranges, olives) were analysed for residues of pirimiphos-methyl and
the phosphorous-containing metabolites. However, significant amounts
of the metabolites did not appear and so were not reported. The
residues listed in various tables of the 1976 Evaluations are of the
parent compound only.
Analyses of wheat grains treated with labelled compounds
confirmed the low level of both metabolites. No oxygen analogue was
detected. The level of desethyl analogue was below 0.05 mg/kg over a
period of 32 weeks, while the parent compound was present at 4 mg/kg.
The oxygen analogue was not detectable in whole rice seedlings. The
levels of parent compound in mg/kg and desethyl analogue (expressed as
parent equivalent) were as follows:
Compound Residue (mg/kg at intervals (days) after treatment
1 3 6 8 10 13
pirimiphos-methyl 0.5 0.51 12 0.6 0.51 0.32
metabolite (II) and
unknown compound 0.17 0.11 0.14 0.12 0.15 0.14
The oxygen analogue reached its maximum concentration on plant
leaves approximately 1 to 3 days after treatment. The ratio of maximum
levels of oxygen analogue and the parent compound were about 0.3 and 3
on cotton and citrus leaves, respectively, while the two compounds
were present in nearly equal concentration on bean leaves. The
disappearance of pirimiphos-methyl residues was rapid on the leaf
surfaces of all plants due to evaporation. The total 14C represented
10-12 percent of the applied material after three days and the
phosphorous containing metabolites were below 10 percent (Bowker
1973).
The Meeting concluded that the metabolites can be excluded from
the definition of the residue. The MRLs need not be changed because
the metabolites represent such a minor proportion of the total
residue. The MRLs refer to the present compound alone.
APPRAISAL
Pirimiphos-methyl was evaluated in 1974, 1976, 1977 and 1979 and
a number of items of information previously requested appear to be
still outstanding.
It would appear that there are a number of uses that give rise to
residues in food moving in international trade, which have not yet
been considered by the Meeting and about which relevant information
will be available in 1984.
An extensive amount of information about the use and fate of
pirimiphos-methyl when applied after harvest to cereal grains has
appeared in open scientific literature and this was reviewed by the
Meeting. The review only serves to confirm the evaluations made
previously. All authors have drawn attention to the stability of
pirimiphos-methyl deposits on treated grain and the retention of the
bulk of the deposit in the seed coat. This is reflected in the low
transfer of residue into white flour and milled rice and the
relatively higher residues in wheat bran.
This review has confirmed that the degradation and metabolism of
pirimiphos-methyl on grain leads only to the production of
2-diethylamino-4-hydroxy-6-methyl pyrimidine. No
cholinesterase-inhibiting metabolites occur. However, between 4
percent and 15 percent of the residue of the parent compound forms a
complex with the aleurone layer of the grain and resists extraction by
a variety of solvents. It may be dislodged by digesting the substrate
with proteolytic enzymes.
The Meeting, having reviewed all available data, concluded that
the metabolites can be excluded from the definition of the residue.
The MRLs, which remain the same, refer to the parent compound alone.
A further study of the use of pirimiphos-methyl for the control
of cheese mites in cheese stores confirmed that the MRL previously
recommended adequately covers the resulting residues.
Several reviews have been made of analytical methods suitable for
dealing with various substrates.
The Meeting considered that the information evaluated in 1983 did
not indicate any need to amend or modify recommendations previously
made for MRLs in a range of commodities.
The Meeting reviewed the requests made by earlier Meetings for
additional information in the light of knowledge about the current use
of this insecticide and considered that they had been satisfied.
FURTHER WORK OR INFORMATION
Desirable
Further information about residues in peanuts, oil seeds, lentils
and citrus.
REFERENCES - RESIDUES
Anonymous. Chemical control of stored grain insects 1972. Cyprus
1972 Agric. Res. Inst. Ann. Rep., p. 69.
Banks, H.J. & Desmarchelier, J.M. New chemical approaches to pest
1978 control in stored grain. Chem. Aust. 45(6): 276-281.
Barry, T.L., Petzinger, G. & Gretch, F.M. GC-MS identification and
1981 analytical behaviour of pirimiphos-methyl in imported foods.
Bull. Environ. Contam. Toxicol., 27: 524-528.
Beasley, C.J. & Lawrence, D.K.: J. Chromatog., 168: 461.
1979
Bengston, M., Cooper, L.M. & Grant-Taylor, F.J. A comparison of
1975 bioresmethrin, chloropyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in
wheat. J. Agric. Anim. Sci., 32: 51-78.
Bengston, M., Cooper, L.M., Davies, R., Desmarchelier, J., Hart R. &
1978 Phillips, M. Grain protectants for the control of
malathion-resistant insects in stored sorghum. (To be
submitted for publication).
Bengston, M., Connell, M. Davies, R., Desmarchelier, J., Elder, B.,
1980a Hart, R., Phillips, M., Ridley, E., Ripp, E., Snelson, J. &
Sticka, R. Chloropyrifos-methyl plus bioremethrin,
methacrifos, pirimiphos-methyl plus bioresmethrin and
synergised bioresmethrin as grain protectants for wheat.
Pestic. Sci., 11: 61-76.
Bengston, M., Connell, M., Davies, R., Desmarchelier, J., Phillips,
1980b M., Snelson, J. & Sticks, R. Fenitrothion plus phenothrin
and pirimiphos-methyl plus carbaryl, as grain protectant
combinations for wheat. Pestic. Sci., 11: 471-482.
Bowker, D.M. Pirimiphos-methyl: fate of stored wheat and rice grain in
1973 the laboratory. Report No. AR2457 AR,submitted to FAO by ICI
Plant Protection Ltd, (Unpublished).
Bratt, H. & Jones, L.A. Pirimiphos-methyl: Metabolism in rats and
1973 dogs. Report submitted to FAO by ICI Industrial Hygiene
Research Laboratories. (Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain. Report
1973 No. AR2472 AR submitted to FAO by ICI Plant Protection Ltd.
(Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain, bread,
1974 flour and milled products. Report submitted to FAO by ICI
Plant Protection Ltd. (Unpublished).
Bullock, D.J.W. In Analytical Methods for Pesticides and Plant
Growth
1976 Regulators, VIII, 185, G. Zweig Ed., Academic Press, New
York.
Bullock, D.J.W. & May, M.S. Pirimiphos-methyl: Residue transfer from
1976 durum wheat to semolina and pasta. Report No. TMJI345A
submitted to FAO by ICI Plant Protection Ltd. (Unpublished).
Bullock, D.J.W., Harrison, P.J. & Day, S.R. Degradation of residues in
1976 flour during baking. Report No. AR2666A submitted to FAO by
ICI Plant Protection Ltd. (Unpublished).
Cerna, V. & Benes, V. Residues of pirimiphos-methyl in wheat, mill
1977 products and white bread. Report by Czechoslovakian
Institute of Hygiene in Epidemiology, Prague.
Chawla, R.P. & Bindra, O.S. (1971). Residues of some promising grain
1971 protectants on stored wheat. Proc. Symp. Progress and
Problems in Pesticide Residue Analysis (Bindra & Kalra,
Eds). Punjab Agric. Univ. Ludhiana & Indian Counc. Agric.
Res., New Delhi. p. 68-74.
Curl, E.A. & Leary, J.P. The radioactive residues found in the liver
1980 and kidney of a goat after administration of a multiple dose
of pirimiphos-methyl. Report RJO144B submitted to FAO by ICI
Plant Protection Ltd.
Desmarchelier, J.M. Selective treatments including combinations of
1977 pyrethroid and organophosphorus insecticides for control of
stored product Coleoptera at two temperatures. J. Stored
Prod. Res., 13: 129-137.
Desmarchelier, J.M. Loss of fenitrothion on grains in storage. Pestic.
1978 Sci. 9: 33-38.
Desmarchelier, J.M. & Bengston, M. The residual behaviour of chemicals
1979 in stored grain. Proceedings of the Second International
Working Conference in Stored-Product Entomology, Ibadan,
Nigeria, 10-16 September 1979.
Desmarchelier, J.M., Bengston, M., Connell, M., Henning, R., Ridley,
in E., Ripp, E., Sierakowski, C., Sticka, R., Snelson, J.
press & Wilson, A. Extensive pilot usage of the grain protectant
combinations, fenitrothion plus bioresmethrin and
pirimiphos-methyl plus bioresmethrin. J. Pestic. Sci.
Desmarchelier, J.M., Goldring, M. & Horan, R. Predicted and observed
1980a residues of bio-resmethrin, carbaryl, fenitrothion,
phenothrin, methacrifos and pirimiphos-methyl on rice and
barley after storage, and losses of these insecticides
during processing. J. Pestic. Sci., 5: 539-545.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W., Moore, B.,
1980b Phillips, M., Snelson, J., Sticka, R. & Tucker, K. A
collaborative study of residues on wheat of methacrifos,
chloropyrifos-methyl, fenitrothion, malathion and
pirimiphos-methyl. II. Rates of decay. CSIRO, Division of
Entomology Report No. 20. 21 p.
Kadoum, A.M. & La Hue, D.W. Penetration of malathion in stored corn,
1974 wheat and sorghum grain. J. Econ. Entomol., 67(4): 477-478.
Krasnykh, A.A. Khim. Sel'sk. Khoz. 16, (11) 36 (Russ).
1978
La Hue, D.W. Pesticide residues of potential protectants of grain.
1974 Proc. 1st Int. Working Conf. on Stored Product Entomology,
Savannah, Georgia, USA. p. 635-638.
La Hue, D.W. Pirimiphos-methyl: Effect on populations of Tribolium
1977 confusum and T. castaneum in wheat. J. Econ. Entomol.,
70(1): 135-137.
La Hue, D.W. & Dicke, E.B. Evaluation of selected insecticides applied
1976 to high moisture sorghum grain to prevent stored grain
insect attack. USDA Agricultural Research Service, Marketing
Research Report No. 1063.
Le Berre, R., Escaffre, H., Pendriez, B., Grebaut, S. & Pengalet, P.
1972 Control of Simulium damnosum, the vector of human
onchocerciasus in West Africa II. Test by classic
application of new insecticides and new formulations, 70
Oncho du 8 Mai ORSTOMOCCGE, BP 171, Bobo-Dioulasso, Upper
Volta.
McCallum Deighton, J. Pirimiphos-methyl and other insecticides in the
1978 control of stored-product insects. Outlook Agric., 9(5)
240-248.
Mensah, G.W.K., Watters, F.L. & Webster, G.R.B. Insecticide residues
1979 in milled fractions of dry or tough wheat treated with
malathion, bromophos, iodofenphos and pirimiphos-methyl. J.
Econ. Entomol., 72: 728-731.
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. Report No. CTL/P/247 submitted to FAO
by ICI Central Toxicology. (Unpublished).
Morallo-Rejesus, B. & Carino F.O. The residual toxicity of five
1976 insecticides on three varieties of corn and sorghum.
Philipp. Agric., 60(3&4): 96-104.
Redlinger, L.M. & Simonaitis, R.A. Field tests with pirimiphos-methyl
1977 as a protectant for farmers stock peanuts. Peanut Sci.
4: 27.
Rishikesh, N., Mathis, H.L., King, J.S. & Nambiar, R.V. A field trial
1977 of pirimiphos-methyl for the control of Anopheles gambiae
and Anopheles funestus in Nigeria. WHO Geneva, VBC/77/671.
(Unpublished).
Rowlands, D.G. The metabolism of contact insecticides in stored grains
1975 1970-1974. Residue Rev., 58: 113.
Rowlands, D.G. The metabolism of pirimiphos-methyl by stored grains
1981 under laboratory conditions. Report No. 54, Pest Control
Chemistry Department, Pest Infestation Laboratory, Slough,
U.K. Ministry of Agriculture, Fisheries & Food.
Rowlands, D.G. et al. Preliminary report of studies with pirimiphos-
1974 methyl as a grain protectant. Submission by United Kingdom
to FAO.
Rowlands, D.G. & Wilkin, D.R. Metabolism of pesticides in stored
1975 cereals. Report of Director, Pest Infestation Control
Laboratory, Slough, U.K. 1971-1973.
Seth, A.K. Use of Actellic for stored rice insect problems in S.E.
1974 Asia. Symp. 1st Int. Working Conf. Stored Prod. Entomol.,
7-11 October 1974, Savannah, Georgia, USA.
Shaw, R.F., Fanara, D.M., Pradhan, G.D., Supratman, Supalin, Bang,
1979 Y.H., & Fleming, G.A. A village-scale trial of
pirimiphos-methyl (OMS-1424) for control of the malaria
vector (Anopheles aconitus) in Central Java, Indonesia.
WHO, Geneva, VBC/79/722. (Unpublished)
Simonaitis, R.A., Zehner, J.M. & Redlinger, L.M. J. HRC & CC 4: 169.
1981
Soderstrom, E.L. & Armstrong, J.W. pp-511: (Pirimiphos-methyl-ISO)
1973 evaluated as a protectant for raisins and walnuts. J. Econ.
Entomol., 66(6): 1305-1306.
Spain. Registered uses of pirimiphos-methyl. Data submitted to FAO by
1983 the Ministry of Agriculture, Madrid.
Spitler, G.H. & Hartsell, P.L. Pirimiphos-methyl as a protectant for
1975 stored inshell almonds. J. Econ. Entomol., 68(6): 777-780.
Sweden. Monitoring of imported fruits and vegetables for residues of
1983 pirimiphos-methyl. Data submitted to FAO by the Swedish
National Food Institute.
Tempone, M.J. Studies on the effects of insecticides on barley
1979 malting. Research Project Series No. 50, Victorian
Department of Agriculture, Melbourne, Australia.
Thomas, K.P. & Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by Cheshire cheese. J. Stored Prod. Res.,
11:53.
Varca, L.M., Magallona, E.D. & Pasco, J.A. Philipp. Entomol., 3: 55.
1975
Weaving, A.J.S. Grain protectants for use under tribal storage
1975 conditions in Rhodesia - Comparative toxicities of some
insecticides on maize and sorghum. J. Stored Prod. Res.,
11: 65-71.
WHO. Interim specifications for pirimiphos-methyl, technical, water
1982 dispersible powder and emulsion concentrate VBC/15/82.04.
WHO Vector Biology Control Division, Geneva.
Zakitis, L.H. & McCray, E.M. Gas chromatographic analysis of residues
1982 of pirimiphos-methyl in water, fish and snails. Bull.
Environ. Contam. Toxicol., 28: 334-340.
 Residue data were obtained over a year from hard winter wheat,
shelled corn and sorghum grain treated with pirimiphos-methyl stored
in bins in a laboratory (La Hue 1974). Table 1 shows the average
residues of pirimiphos-methyl in the stored grain over the
twelve-month period at 27°C. It is noticeable that the degradation
appears to be independent of the moisture content of the grain under
these conditions. Malathion, included in the same trial, degraded to
about one tenth of the initial dosage within the same period. In the
case of the high moisture sorghum, it was almost entirely destroyed
within one month.
Seth (1974) reported trials to evaluate pirimiphos-methyl for the
control of pests in stored rice in South-east Asia. Rice in husk and
polished rice were treated with emulsifiable concentrate (E.C.) and
dust formulations at rates equivalent to 2, 4 and 8 mg/kg. The rice
was analysed at monthly intervals throughout the four-month trial. In
polished grains, the residue at the end of four months was
approximately 25 percent of the residue found on the first day of the
trial. In rice in husk, it was not possible to deduce the degradation,
since the residue data were expressed on hull and milled grain
separately. However, there appeared to be relatively little loss of
pirimiphos-methyl over five months. Relatively little of the
insecticide (generally less than 0.5 mg/kg) transferred to milled
grain. There was no significant difference between the dust and E.C.
formulations.
FATE OF RESIDUES
In Grain
Residues of pirimiphos-methyl on wheat grain are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain to give
principally the parent hydroxypyrimidine (Figure 2, IV), and the
related compounds (V and VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grain.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residue of the chemically-unstable
oxygen analogue (III) was detected. The limit of detection was 0.01
mg/kg (Bowker 1973).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat, so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products. The general pattern of
breakdown on stored rice was similar to that found on wheat grain. The
insecticide and its degradation products were concentrated in the
husk, in which the rate of breakdown appeared to be unaffected by the
moisture content of the rice (Bowker 1973; Bullock 1973).
Table 1. Average Residues of Pirimiphos-methyl in Stored Grain1
Post-treatment residues (mg/kg)
Grain Moisture Intended months
Content dosage
(%) (mg/kg) 24 h 1 3 6 9 12
Wheat 12.5 7.8 6.5 6.1 5.6 6.2 4.9 5.4
Maize 12.5 8.4 7.9 6.3 4.5 4.1 3.4 3.0
Sorghum 17.6 8.4 7.5 6.5 4.3 3.8 3.8 3.7
1 Over 12 months at 27°C.
Residue data were obtained over a year from hard winter wheat,
shelled corn and sorghum grain treated with pirimiphos-methyl stored
in bins in a laboratory (La Hue 1974). Table 1 shows the average
residues of pirimiphos-methyl in the stored grain over the
twelve-month period at 27°C. It is noticeable that the degradation
appears to be independent of the moisture content of the grain under
these conditions. Malathion, included in the same trial, degraded to
about one tenth of the initial dosage within the same period. In the
case of the high moisture sorghum, it was almost entirely destroyed
within one month.
Seth (1974) reported trials to evaluate pirimiphos-methyl for the
control of pests in stored rice in South-east Asia. Rice in husk and
polished rice were treated with emulsifiable concentrate (E.C.) and
dust formulations at rates equivalent to 2, 4 and 8 mg/kg. The rice
was analysed at monthly intervals throughout the four-month trial. In
polished grains, the residue at the end of four months was
approximately 25 percent of the residue found on the first day of the
trial. In rice in husk, it was not possible to deduce the degradation,
since the residue data were expressed on hull and milled grain
separately. However, there appeared to be relatively little loss of
pirimiphos-methyl over five months. Relatively little of the
insecticide (generally less than 0.5 mg/kg) transferred to milled
grain. There was no significant difference between the dust and E.C.
formulations.
FATE OF RESIDUES
In Grain
Residues of pirimiphos-methyl on wheat grain are degraded and
detoxified by hydrolysis of the phosphorus-ester side chain to give
principally the parent hydroxypyrimidine (Figure 2, IV), and the
related compounds (V and VI). At a given temperature, the rate of
breakdown increases with increasing moisture content of the grain.
Levels of the N-desethyl phosphorus compound (II) were always
extremely low (approximately 0.05 mg/kg over a period of 32 weeks in
wheat grain treated at 4 mg/kg). No residue of the chemically-unstable
oxygen analogue (III) was detected. The limit of detection was 0.01
mg/kg (Bowker 1973).
Radio-autograms of grain sectioned after four months showed that
the insecticide and its degradation products were concentrated in the
seed coat, so that residues in white flour and bread are likely to be
lower than in bran and wholemeal products. The general pattern of
breakdown on stored rice was similar to that found on wheat grain. The
insecticide and its degradation products were concentrated in the
husk, in which the rate of breakdown appeared to be unaffected by the
moisture content of the rice (Bowker 1973; Bullock 1973).
Table 1. Average Residues of Pirimiphos-methyl in Stored Grain1
Post-treatment residues (mg/kg)
Grain Moisture Intended months
Content dosage
(%) (mg/kg) 24 h 1 3 6 9 12
Wheat 12.5 7.8 6.5 6.1 5.6 6.2 4.9 5.4
Maize 12.5 8.4 7.9 6.3 4.5 4.1 3.4 3.0
Sorghum 17.6 8.4 7.5 6.5 4.3 3.8 3.8 3.7
1 Over 12 months at 27°C.
 Degradation was marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
was caused by factors within the grain, or by the associated
microflora, was not known. At a higher moisture content (ca. 18
percent) less pirimiphos-methyl was recovered on analysis but
increased levels of the hydrolysis product (IV) were obtained,
suggesting that a more rapid degradation of the insecticide occurred.
Bowker (1973) concluded that grain may lack the enzymatic activity,
believed to be present in plants and soil, to cleave the pyrimidine
N-ethyl bonds. It is likely, therefore, that following the treatment
of wheat and brown rice with pirimiphos-methyl, the major residues
during storage will be the parent compound and the simple hydrolysis
product (IV). Under optimum conditions, the maximum level of compound
(IV) following treatment at 4 mg/kg was found to be 0.17 mg/kg; under
poor storage conditions, with high moisture content grain it was 0.62
mg/kg.
Solutions and also a 2 percent dust of radio-labelled
pirimiphos-methyl were applied in the laboratory to wheat grain of 14
and 18 percent moisture to give levels of 4 mg/kg. Throughout a
storage period of six months, the residues of pirimiphos-methyl were
found almost entirely in the seed coat and aleurone layer, with only
traces present in the germ or endosperm. It was also clear that, as
with malathion but to a greater extent, there was transfer of
insecticide between grains, possibly in the vapour phase. About 10
percent of the total residual radioactivity was bound to lipoprotein
material in the aleurone region of the grain and could be extracted
only by digestion of the aleurone protein. In contrast with other
organophosphates studied, the bound material appeared to be unchanged
pesticide rather than a metabolite. It was undoubtedly a P=S compound,
but degradation during the liberation of the bound material
complicated identification (Rowlands et al. 1974).
In Animals
The metabolism of pirimiphos-methyl was studied in the rat and
dog. Twelve metabolites were detected, none of which showed
anticholinesterase activity. No parent pirimiphos-methyl was detected
in the urine. In both rats and dogs, 2-ethylamino-4-hydroxy-6-methyl
pyrimidine was the major urinary metabolite (Bratt & Jones 1973).
When rats were given a single oral dose of radio-labelled
pirimiphos-methyl at 7.5 mg/kg, the uptake of radioactivity into blood
and its subsequent disappearance from the bloodstream were both very
rapid. More than 50 percent of the radioactivity present in the blood
30 min. after dosing had disappeared at one hour after dosing.
Unchanged pirimiphos-methyl usually represented less than 10 percent
of the total residue in the blood 24 h after dosing. When
radio-labelled pirimiphos-methyl was administered orally to rats at
7.5 mg/kg per day for four days, total radioactive residues in the
liver, kidney and fat did not usually exceed 2 mg/kg pirimiphos-methyl
equivalents. There was no evidence to show that either
pirimiphos-methyl or its metabolites accumulated in the liver, kidney
or fat of rats following daily dosing with the insecticide over four
days (Mills 1976).
The rodioactive residues in the liver and kidney of a goat, dosed
daily for seven consecutive days with 14C-pirimiphos-methyl at 30
mg/kg in the diet, were examined. (Curl and Leahy 1980).
A radioactive residue of 0.25-0.30 mg/kg of
14C-pirimiphos-methyl equivalents was detected in the liver, and 89
percent of this was extracted and analysed by thin layer
chromatography. 5.9 percent of the total radioactive residue in the
liver was unchanged pirimiphos-methyl. The hydroxypyrimidines,
compound (II) (2-diethylamino-6-methyl-pyrimidin-4-ol), compound (III)
(2-ethylamino-6-methyl-pyrimidin-4-ol) and compound (IV)
(2-amino-6-methyl-pyrimidin-4-ol) accounted for 3.7 percent, 21.8
percent and 17.5 percent respectively, of the total radioactive
residue in the liver. Compounds (II), (III) and (IV) were present
mainly as "free" metabolites. However, 13.7 percent of the
hydroxypyrimidines detected were released from the liver by acid
reflux.
The remainder of the radioactivity extracted from the liver
consisted of at least five compounds.
A radioactive residue of 0.59-0.70 mg/kg of
14C-pirimiphos-methyl equivalents was found in the kidney; 91 percent
of this was extracted and analysed by thin-layer chromatography.
Compound (II), compound (III) and compound (IV) accounted for 7.9
percent, 35.3 percent and 17.0 percent respectively, of the total
radioactive residue in the kidney. Compounds (II), (III) and (IV) were
present mainly as "free" metabolites, however, 9.0 percent of the
hydroxypyrimidines detected were released from the kidney by acid
reflux.
The remainder of the radioactivity extracted from the kidney
consisted of at least six compounds.
In Storage and Processing
Bullock (1973, 1974) reported several individual experiments
which demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table 2
summarizes the results of residue trials carried out in the United
Kingdom on wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 3 summarizes results of a residue trial (Bullock 1973)
carried out in the United Kingdom with wheat nominally treated to
contain 8 mg/kg pirimiphos-methyl. These data are substantially in
agreement with those of Bengston et al. (1975) and show that there
is relatively little penetration beyond the seed coat, even throughout
a storage period of nine weeks.
Table 2. Effect of Milling and Baking on Residue in Wheat Admixed
with Pirimiphos-Methyl1
Interval between
Grain treatment and Residues (mg/kg)
fraction sampling (months) Highest Lowest Mean
Whole grain 0 4.2 1.9 2.9 (9)
3.0 -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Whole meal 0 1.3 0.94 1.1 (3)
flour 1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White flour 0 0.88 0.30 0.52 (6)
0.56* 0.53* 0.55*(3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27*(3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread 1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
White bread 0 0.28 0.19 0.23 (6)
0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 Dosage of pesticide was 4 mg/kg. All results are from field
trials except those marked *, which are from a small-scale trial.
Figures in parentheses are the numbers of results on which the
means are based.
Table 3. Residues of Pirimiphos-methyl in Whole Grains and in Milling
and Baking Fractions Treated in a Laboratory Trial1
Interval between Residues (mg/kg)
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52
6.0 0.91 0.56
6.0 1.0 0.57
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 Dosage of pesticide was 8 mg/kg. In all cases, no residues of the
phosphorus-containing compounds (II) or (III) (Figure 1) were
detected. (Limit of detection: 0.01 mg/kg in each case.)
Seth (1974) discussed experiments on the admixture of
pirimiphos-methyl with paddy rice (rice in husk) in Southeast Asia.
Both emulsion and dust formulations were used and were applied at
rates of 2, 4 and 8 mg/kg. A portion of the rice was milled
immediately after treatment and further portions at the end of one,
two, three and five months. It was found that the deposit of
pirimiphos-methyl on both the husk and the milled grain was
proportional to the amount applied, but the concentration on the husk
was always about 25 times that on the milled grain. The residue on the
husk and milled grain declined steadily over the five-month storage
period. Although the concentration on the husk initially was as high
as 12 mg/kg, the residue on the milled rice barely exceeded 0.5 mg/kg
and even at the highest rate of application was always below 1 mg/kg.
These experiments clearly showed that there is minimum transfer of
pirimiphos-methyl from the husk to the kernel of rice, even during
prolonged storage. The degree of penetration is comparable to that
reported by Kadoum & La Hue (1974) for malathion on wheat, maize and
sorghum.
Residues of pirimiphos-methyl in flour are relatively stable
during baking to bread and biscuits. However, because of the dilution
which the flour undergoes during these processes, flour initially
containing 1 mg/kg pirimiphos-methyl is likely to yield
bread/ biscuits containing residues of ca. 0.5 mg/kg.
Bullock et al. (1976) studied the fate of pirimiphos-methyl
during processing of flour into bread and biscuits. In studies with
the radio-labelled compound, flour was dosed with 2-14C-labelled
pirimiphos-methyl and baked to produce white bread, wholemeal bread
and biscuits. Although pirimiphos-methyl is known to be a relatively
volative compound, there was no significant loss of radioactivity by
volatilization. Distribution of radioactivity throughout the bread was
fairly uniform. Unchanged pirimiphos-methyl accounted for 75-90
percent of the radioactivity in the baked product. The major
degradation product formed during the baking was hydroxypyrimidine,
which accounted for 3-10 percent of the radioactivity in the final
product.
Similar results were obtained by residue analysis in a second set
of studies. After correcting for the weight increase when flour is
converted to bread, residues of pirimiphos-methyl fell by 11-18
percent. Likewise, residue analysis of biscuits showed average losses
of 8 percent. However, owing to the dilution of the flour that occurs
during baking, the residue of pirimiphos-methyl in bread will be lower
than that in the corresponding flour. Bullock et al. (1976) found
levels of pirimiphos-methyl in bread and biscuits to be about 50
percent of those in the flour from which they were derived. These
findings agreed well with the earlier work of Bullock (1973, 1974). No
residues of the phosphorus-containing compounds (II) and (III) were
detected in bread baked from flour treated with pirimiphos-methyl at
1 mg/kg or in biscuits baked from flour containing up to 5 mg/kg
(Bullock et al. 1976)
The hydroxypyrimidine (IV) also undergoes only slight degradation
during the baking process. This compound constitutes only a minor part
of the residue in stored grains (FAO/,WHO 1975). Bullock et al
(1976) used radio-labelled compound (IV) and found that it degraded by
less than 10 percent during the baking of bread. It can, therefore, be
concluded that residues of compound (IV) in baked products will never
exceed 0.2 mg/kg and will normally be considerably lower.
Bullock & May (1976) studied the fate of residues in wheat during
processing to semolina and pasta. White semolina, prepared from durum
wheat treated at 10 mg/kg, contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90 percent of those in the corresponding semolina; 70
percent of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However, the weight of pasta increased by
100 percent on cooking, so that the concentration of pirimiphos-methyl
in cooked pasta is likely to be approximately 35 percent of that in
the corresponding semolina.
Results of experiments in Czechoslovakia (Cerna & Benes 1977)
indicated that residues of pirimiphos-methyl in grain, which had been
in store for nine months, were substantially removed by the milling
process. The bulk of the residue was removed in the bran; there was no
substantial difference in the concentration in the different bran
fractions. When white flour was made into white bread, there was a
further loss of approximately 50 percent so that the concentration of
the residue in the bread was only 10-15 percent of its concentration
in raw grain. Some residue was destroyed during milling.
Tempone (1979) reported a series of studies on the effects of
insecticides on barley malting and the resulting residues. In the
first of these trials, barley treated with pirimiphos-methyl at the
rate of 6 mg/kg or 18 mg/kg was converted into malt but no residues
were detected in the wort (unfermented extracts from the malted
barley), the limit of determination being 0.004 mg/kg. Wort, prepared
from barley malted after being in store for three, six and nine
months, likewise showed no residues. In the second series of similar
trials, the residue was determined in the malt (germinated grain that
had been calcined). Residues could be found in the malt, but at a
level of from 10 to 20 percent of that in the barley before malting.
Barley kept in storage for three months after being treated with
pirimiphos-methyl transferred significantly less residue to the malt
than did the barley malted immediately after treatment, no doubt
because there was significantly less residue on the outside of the
kernel, where it would be protected from the enzymatic activity within
the barley grain during germination.
Bengston et al. (1980 a, b) arranged for wheat from bulk grain
treated with pirimiphos-methyl and held in commercial silos to be
processed through to wholemeal bread and white bread. The results
obtained in these trials are reported in Tables 4 and 5. During
processing from wheat to white bread, residues were reduced by 85-91
percent.
Table 4. Mean Residues of Pirimiphos-methyl Following Milling and Baking of Stored Wheat
Site Storage Residues (mg/kg)
(weeks)
Wheat Bran Pollard Wholemeal White Wholemeal White
flour flour bread bread
Site B 13 5-7 12-16 2-4 2.6 0.1-0.2 1.0-2.0 0.05-0.1
Site D 19 2.5-3.0 6-8 1-2 1.2 0.05-0.1 0.5-1.0 0.05
Table 5. Reduction in Pirimiphos-methyl Residues Following Milling and Baking of Stored Wheat
Site Reduction in residues(%)
Wheat to Wheat to Wholemeal flour White Flour Wheat to Wheat to
Wholemeal White to Wholemeal to White Wholemeal White
Flour Flour Bread Bread Bread Bread
Site B 5 68 47 54 50 85
Site D 0 78 55 58 56 91
Desmarchelier et al. (1980b), after treating bulk wheat in
commercial silos with pirimiphos-methyl, arranged for a portion to be
milled and for the white flour to be converted into white bread. The
results are given in Table 6. It is of interest to note that the
residues in flour and bread were higher in the hard wheat which was
held for 22 weeks before milling, than in the soft wheat which was
held of 11 weeks before milling.
Desmarchelier et al. (1980a) studied the fate of
primiphos-methyl, and a number of other grain protectant insecticides,
applied to unhusked rice, husked rice, polished rice and barley over a
storage period of six months and subsequently during the processing
and cooking of these grains. The results of these trials are given in
Table 7, and show that only about 10-15 percent of the residue present
on husked rice or polished rice was destroyed in the cooking process.
However, if the treatment was applied to unhusked rice, approximately
70 percent of the residue was removed with the husk and a great deal
more when the husked rice was milled for the removal of the bran.
Subsequent cooking of the husked rice or polished rice brought about a
further substantial reduction in the residue level.
These same workers found that pirimiphos-methyl, applied to
barley destined for malting, was substantially lost during the malting
process. Barley treated at the rate of 6 mg/kg and held in storage for
six months was found to contain 4.9 mg/kg pirimiphos-methyl. When this
grain was malted, the pirimiphos-methyl residue in the prepared malt
was only 0.9 mg/kg. The work of Tempone (1979) showed that little, if
any, of the residue in the malt is extracted into the wort.
There have been no reports of pirimiphos-methyl being metabolized
by attack at either of the O,O-dimethyl groups (to give a desmethyl
derivative) as is common with certain other organophosphorus
triesters. Morallo-Rejesus & Carino (1976) noted, but did not
identify, breakdown products in shelled maize stored in
pirimiphos-methyl treated bags.
Rowlands (1981), reporting work carried out some years previously
using radio-labelled compound, treated small quantities (10 g) of
wheat that were held in store for up to 6 months. Aliquots were
removed at intervals and, after crushing, were extracted first with
hexane, followed in turn by chloroform, methanol and acetonitrile and
then, after sequential digestion by three enzymes, further extraction
by chloroform. The results are recorded in Table 8.
It is clear from Table 8 that very soon after treatment, a
portion of the aged pirimiphos-methyl residue is not extractable from
wheat unless it is digested out enzymatically. It is thought that this
is due to a complex formed by intact pirimiphos-methyl within the
aleurone layer (Rowlands 1975).
Table 6. Residues of Primiphos-methyl in Wheat, Its Milling Products and Bread
Residues (mg/kg)
Type of Storage
Wheat (weeks) Initial deposit Whole grain bran pollard flour bread
Soft 11 5.2 4.2 9.1 8.3 0.2 0.1
Hard 22 6.1 4.5 12.4 10.4 1.3 0.35
Table 7. Residues of Pirimiphos-methyl on Husked, Polished and Unhusked Rice After Storage and Processing
Grain Application Residues (mg/kg)
level
(mg/kg)
After After After After
3 6 Cooking at Cooking at
Months Months 3 Months 6 Months
Husked
rice 6 6.5 4.9 4.9 4.0
Polished
rice 6 6.0 6.0 4.9 4.9
Residues (mg/kg) after
6 months storage and
Milling Milling &
Cooking
Husked Polished Husked Polished
rice rice rice rice
Unhusked
rice 6.0 6.0 4.6 1.4 0.3 0.6 0.1
Table 8. Recovery of Pirimiphos-methyl From Crushed Wheat (18% mo) By Sequential extraction
Extraction by 14C activity recovered %1 at time of extraction after
application (0 h)
0 h 1 h 7 days 14 days 1 month 6 months
Hexane 97 89 84 78 72 67
Chloroform nil 1 4 7 8 8
Methanol trace 1 2 2 6 7
Acetonitrile trace 2 1 4 2 2
Digest Sequence2
(1) nil 2 1 4 2 2
(2) 4 5 7 7 9 12
(3) nil 2 2 1 2 1
Total extracted 101 102 101 103 101 99
1 Average of three results ± 3 percent.
2 Digest sequence: (1) = lipase pH 7.4; (2) = papain pH 4,5; (3) - cellulase pH 4.5,
All at 37°C for 24 h
Studies reported by Rowlands (1981) on the uptake from dust or
solvent application as determined by the separated tissue of the wheat
grains after storage, showed that intact pirimiphos-methyl and free
pyrimidinols occur chiefly in the seedcoat. These findings received
some confirmation in the work of Mensah et al. (1979), who found
that pirimiphos-methyl emulsion, applied to wheat that was then milled
six months later, had accumulated in the bran and middlings fractions,
irrespective of the moisture content of the stored wheat (12 percent
and 16 percent). They found little or no residue in white flour.
Rowlands (1981) found only pirimiphos-methyl (I) and the
corresponding pirimidinol (IV) as residues in pirimiphos-methyl
treated wheat with 12 percent moisture that was kept in sealed jars
for up to eight months. However, in wheat with 18 percent moisture, a
small (less than 10 percent of the total residue) quantity of the
N-des-ethyl pirimidinol (V) was found after six and eight months. (See
Figure 2 for conformations of these compounds).
Further to the work of Thomas & Rowlands (1975) on the uptake and
degradation of pirimiphos-methyl by Cheshire cheese, similar studies
were carried out using Stilton cheeses, which differ in physical
characteristics and are stored differently.
In these studies a wooden plank was treated with
14C-ring-labelled pirimiphos-methyl and allowed to dry for 24 h
before two young Stilton cheeses were placed on the treated surface.
One of the cheeses was first covered by a layer of cheesecloth. The
cheeses were turned every two days. Replicate core samples were taken
with a cork borer from each face of both cheeses. The core was
analysed for pirimiphos-methyl. Some of the cores were sectioned with
a microtome and the concentration of pirimiphos-methyl in the cheese
at various depths was determined by means of a scintillation counter.
The findings are reported in Table 9.
This experiment has shown that only a small amount of
pirimiphos-methyl penetrated into the cheeses under the various
storage techniques used with Stilton cheese. No breakdown of the
pirimiphos-methyl could be detected in the cheese or the cheesecloth
throughout the seven weeks of storage.
These results indicate that the MRL of 0.5 mg/kg for
pirimiphos-methyl in cheese would not be exceeded when the insecticide
was used for the control of cheese mites during the making and storage
of Stilton cheese.
Mensah et al. (1979) reported results of small-scale trials, in
which water-based emulsions of malathion, bromophos, iodofenphos and
pirimiphos-methyl were applied at two dosage rates to spring wheat of
12 percent and 15 percent moisture content, to compare the fate of the
residues. Pirimiphos-methyl degraded at a comparatively slower rate
than the other three compounds, but the rate of degradation was
significantly higher on the high moisture grain. The results are given
in Table 10. The residue levels were concentrated in the seed coat,
resulting in high levels in bran and middlings. When applied at the
rate of 6 mg/kg, the residues in these fractions exceeded the MRL
recommended by the Meeting.
Results were reported of small-scale trials in which the efficacy
and fate of pirimiphos-methyl residue on wheat and milling fractions
were assessed. Two rates (7.3 mg/kg and 14.6 mg/kg) were applied and
the wheat was stored for up to 12 months. The results are reported in
Table 11. These show that the residues in bran and middlings (shorts)
exceeded the MRL following application at the lower rate and storage
for less than six months. At the higher rate (which is well in excess
of the recommended rate) the residues greatly exceeded the MRL.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of pirimiphos-methyl were detected in the United States
in several samples of imported foods (Barry et al. 1981) and the
identification of these residues by GC-MS and GLC was reported. The
level and source of residues found are reported in Table 12.
The Swedish National Food Institute (Sweden 1983) indicated that
during the period 1-1-80 to 30-4-83, 8 654 samples of fruit and
vegetables were analysed for pesticide residues. From among these,
eight samples were found to contain pirimiphos-methyl at levels up to
1.4 mg/kg, as indicated in Table 13.
METHODS OF RESIDUE ANALYSIS
Barry et al. (1981) have provided details of GC-MS
identification and analytical behaviour of pirimiphos-methyl in a
variety of foods as part of an investigation of unknown residues
detected in imported foods.
Zakitis & McCray (1982), having reviewed the analytical
methodology for residues of pirimiphos-methyl in a variety of
substrates, concluded that none of the eight known methods met all the
desired criteria for analysis of residues in water, fish and snails,
viz. specificity, simplicity of sample preparation and recovery after
clean-up. Water was simply extracted with hexane prior to
determination by GC. Fish and snails were extracted with acetone in
the presence of sufficient anhydrous sodium sulphate to form a dry
powder. The acetone was filtered and the solids were extracted three
more times with acetone. The combined, filtered extracts were
evaporated to low volume before being transferred to de-ionized water
for extraction with hexane as for analysis of water. Recoveries ranged
from 93 to 101 percent. The method is being used for environmental
studies involving the use of pirimiphos-methyl for disease vector
control. The limit of determination in snails is 0.5 mg/kg. Below this
concentration, some naturally occurring substance interferes with the
determination of pirimiphos-methyl. Good recoveries were obtained from
dechlorinated water at concentrations as low as 0.005 mg/l and in fish
down to 0.05 mg/kg.
Table 9. Pirimiphos-Methyl Residues From Microtomed Sections of Two Cheeses1
Residues (mg/kg)
Storage
period End Depth (mm) into cheese
(days) sampled 0-1 1-2 2-3 3-4 4-5 5-6 6-7 7-8 8-9 9-10
Cheese in direct contact with treated surface
31 1 41.15 7.48 4.03 1.73 1.25 0.90 0.65 0.55 0.40 0.10
2 27.50 7.70 2.32 1.09 0.71 0.50 0.28 0.20 0.16 -
49 1 29.57 9.26 2.87 2.04 1.52 1.13 0.82 0.71 0.51 0.27
2 23.73 6.54 2.82 2.71 1.52 1.07 0.74 0.55 0.51 0.37
Cheese on cloth barrier
31 1 18.04 3.40 0.65 0.30 0.18 0.08 - - - -
2 35.28 11.48 4.19 0.62 0.36 0.27 0.22 0.16 - -
49 1 19.58 5.69 1.72 0.72 0.45 0.28 - - - -
2 12.26 7.13 1.84 1.11 0.84 0.66 0.46 0.31 - -
1 Dashes indicate that no pirimiphos-methyl was detected.
Table 10. Mean1 Pirimiphos-methyl Residues (mg/kg) on Dry and Tough Wheat and Milled Fractions After Storage
of Treated Wheat
12% moisture content (dry) 16% moisture content (tough)
Storage
Dosage period Whole Whole
(mg/kg) (months) wheat2 Bran Middling3 Flour wheat2 Bran Middling3 Flour
4 1 3.29±0.07 15.66±0.79 21.44±1.96 1.94±0.25 3.24±0.03 19.81±1.63 15.35±0.58 1.57±0.03
3 2.98±0.10 13.93±0.26 15.10±1.00 1.93±0.12 3.04±0.01 13.45±0.80 13.51±0.36 1.16±0.06
6 2.54±0.07 13.16±0.51 13.80±1.36 1.56±0.06 2.26±0.18 11.98±0.67 10.68±0.60 0.81±0.23
6 1 4.93±0.13 20.87±0.82 23.15±1.47 2.86±0.14 4.92±0.04 27.33±1.41 21.83±1.73 1.91±0.07
3 4.54±0.10 20.54±1.38 19.34±0.74 2.42±0.22 4.44±0.04 20.90±2.92 17.89±0.59 1.50±0.08
6 3.66±0.23 19.32±0.73 16.05±1.35 2.23±0.13 3.86±0.26 18.60±0.73 16.89±0.56 1.33±0.14
1 Mean of 3 replicates ± SE.
2 Ground wheat.
3 Shorts, wheat germ and coarse particles of flour.
Table 11. Average Pirimiphos-methyl Residue (mg/kg) on Hard Winter Wheat
and Wheat Fractions Stored up to 12 Months
Rate of application
7.3 mg/kg 14.6 mg/kg
Storage Whole Whole
Period wheat1 Bran Shorts Flour wheat1 Bran Shorts Flour
24 h 7.15 25.50 18.29 0.83 14.75 50.41 36.01 2.01
Months
1 6.88 21.45 17.89 1.38 13.13 41.43 31.01 2.94
3 6.60 20.11 20.82 1.47 12.98 39.97 42.97 3.15
6 5.78 18.06 20.46 1.47 11.17 30.52 46.58 2.91
9 5.43 17.34 17.90 1.17 10.39 34.52 31.47 2.44
12 5.27 13.47 15.35 1.05 9.72 32.29 26.98 2.14
1 Before tempering to 15% for milling.
Table 12. Pirimiphos-methyl residues in imported foods
Food Country of Origin Residue (mg/kg)
Chickpeas Australia 0.07
Dried green peas Australia 3.0
Pigeon peas Kenya 0.19
Moong dall Tanzania 0.03
Peanut butter South Africa 0.23
Split peas Kenya 0.1-0.171
Sardo cheese Argentina 0.21-1.41,2
1 Represents range found in multiple samples of the same
commodity.
2 Determined on fat basis.
Table 13. Primiphos-methyl Residues Detected in Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.21 0.21-0.53 0.54-1.05 >1.05 (mg/kg)
Mandarin Import 292 291 1 0.21
Orange Import 622 621 1 0.41
Pepper Import 206 201 3 1 1 1.4
(sweet)
Tomato Sweden 205 205
Import 500 499 1 0.30
1 Total number of samples analysed:8 654
Period: 81-01-01 -- 83-04-30
A number of the methods reviewed by Zakitis & McCray (1982) have
not been considered previously by the Meeting. They are briefly
mentioned for information.
A general review of analytical methods was published by Bullock
(1976) including procedures suitable for animal tissues. There is also
a TLC-UV method for residues in water, soil and plants (Krasnykh,
1978), an HPLC method for determining pirimiphos-methyl and five
metabolites in samples of plasma and urine (Beasley & Lawrence, 1979),
and gas chromatographic methods for residues in stored grain (Varca
et al. 1975), peanuts (Redlinger & Simonaitis 1977) and milled
fractions of wheat (Mensah et al. 1979). In addition, a
gas-chromatographic method has been proposed for the simultaneous
determination of residues of pirimiphos-methyl and malathion in
peanuts (Simonaitis et al. 1981), and GC-MS was used to identify
residues of pirimiphos-methyl in imported foods (Barry et al. 1981).
The residue in stored grains (barley, maize, oats, rice in husk
and wheat) consisted mainly of the parent compound. Neither the oxygen
analogue (O,O-dimethyl-O(2-diethylamino-6-methyl-pirimidine-4yl)
phosphate), (metabolite III) nor the desethyl analogue
(O, O-dimethyl-O(2-ethylamino-6-methyl-pyrimidin-4-yl)
phosphorothionate), (metabolite II) was detectable. No metabolite was
detectable in milled products or in white and wholemeal breads. A
large number of plant samples (leafy vegetables, carrots, celery,
spring onions, potatoes, sugarbeets, cucumber, tomatoes, peppers,
mushrooms, lettuce, blackcurrants, apples, pears, plums, lemons,
oranges, olives) were analysed for residues of pirimiphos-methyl and
the phosphorous-containing metabolites. However, significant amounts
of the metabolites did not appear and so were not reported. The
residues listed in various tables of the 1976 Evaluations are of the
parent compound only.
Analyses of wheat grains treated with labelled compounds
confirmed the low level of both metabolites. No oxygen analogue was
detected. The level of desethyl analogue was below 0.05 mg/kg over a
period of 32 weeks, while the parent compound was present at 4 mg/kg.
The oxygen analogue was not detectable in whole rice seedlings. The
levels of parent compound in mg/kg and desethyl analogue (expressed as
parent equivalent) were as follows:
Compound Residue (mg/kg at intervals (days) after treatment
1 3 6 8 10 13
pirimiphos-methyl 0.5 0.51 12 0.6 0.51 0.32
metabolite (II) and
unknown compound 0.17 0.11 0.14 0.12 0.15 0.14
The oxygen analogue reached its maximum concentration on plant
leaves approximately 1 to 3 days after treatment. The ratio of maximum
levels of oxygen analogue and the parent compound were about 0.3 and 3
on cotton and citrus leaves, respectively, while the two compounds
were present in nearly equal concentration on bean leaves. The
disappearance of pirimiphos-methyl residues was rapid on the leaf
surfaces of all plants due to evaporation. The total 14C represented
10-12 percent of the applied material after three days and the
phosphorous containing metabolites were below 10 percent (Bowker
1973).
The Meeting concluded that the metabolites can be excluded from
the definition of the residue. The MRLs need not be changed because
the metabolites represent such a minor proportion of the total
residue. The MRLs refer to the present compound alone.
APPRAISAL
Pirimiphos-methyl was evaluated in 1974, 1976, 1977 and 1979 and
a number of items of information previously requested appear to be
still outstanding.
It would appear that there are a number of uses that give rise to
residues in food moving in international trade, which have not yet
been considered by the Meeting and about which relevant information
will be available in 1984.
An extensive amount of information about the use and fate of
pirimiphos-methyl when applied after harvest to cereal grains has
appeared in open scientific literature and this was reviewed by the
Meeting. The review only serves to confirm the evaluations made
previously. All authors have drawn attention to the stability of
pirimiphos-methyl deposits on treated grain and the retention of the
bulk of the deposit in the seed coat. This is reflected in the low
transfer of residue into white flour and milled rice and the
relatively higher residues in wheat bran.
This review has confirmed that the degradation and metabolism of
pirimiphos-methyl on grain leads only to the production of
2-diethylamino-4-hydroxy-6-methyl pyrimidine. No
cholinesterase-inhibiting metabolites occur. However, between 4
percent and 15 percent of the residue of the parent compound forms a
complex with the aleurone layer of the grain and resists extraction by
a variety of solvents. It may be dislodged by digesting the substrate
with proteolytic enzymes.
The Meeting, having reviewed all available data, concluded that
the metabolites can be excluded from the definition of the residue.
The MRLs, which remain the same, refer to the parent compound alone.
A further study of the use of pirimiphos-methyl for the control
of cheese mites in cheese stores confirmed that the MRL previously
recommended adequately covers the resulting residues.
Several reviews have been made of analytical methods suitable for
dealing with various substrates.
The Meeting considered that the information evaluated in 1983 did
not indicate any need to amend or modify recommendations previously
made for MRLs in a range of commodities.
The Meeting reviewed the requests made by earlier Meetings for
additional information in the light of knowledge about the current use
of this insecticide and considered that they had been satisfied.
FURTHER WORK OR INFORMATION
Desirable
Further information about residues in peanuts, oil seeds, lentils
and citrus.
REFERENCES - RESIDUES
Anonymous. Chemical control of stored grain insects 1972. Cyprus
1972 Agric. Res. Inst. Ann. Rep., p. 69.
Banks, H.J. & Desmarchelier, J.M. New chemical approaches to pest
1978 control in stored grain. Chem. Aust. 45(6): 276-281.
Barry, T.L., Petzinger, G. & Gretch, F.M. GC-MS identification and
1981 analytical behaviour of pirimiphos-methyl in imported foods.
Bull. Environ. Contam. Toxicol., 27: 524-528.
Beasley, C.J. & Lawrence, D.K.: J. Chromatog., 168: 461.
1979
Bengston, M., Cooper, L.M. & Grant-Taylor, F.J. A comparison of
1975 bioresmethrin, chloropyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in
wheat. J. Agric. Anim. Sci., 32: 51-78.
Bengston, M., Cooper, L.M., Davies, R., Desmarchelier, J., Hart R. &
1978 Phillips, M. Grain protectants for the control of
malathion-resistant insects in stored sorghum. (To be
submitted for publication).
Bengston, M., Connell, M. Davies, R., Desmarchelier, J., Elder, B.,
1980a Hart, R., Phillips, M., Ridley, E., Ripp, E., Snelson, J. &
Sticka, R. Chloropyrifos-methyl plus bioremethrin,
methacrifos, pirimiphos-methyl plus bioresmethrin and
synergised bioresmethrin as grain protectants for wheat.
Pestic. Sci., 11: 61-76.
Bengston, M., Connell, M., Davies, R., Desmarchelier, J., Phillips,
1980b M., Snelson, J. & Sticks, R. Fenitrothion plus phenothrin
and pirimiphos-methyl plus carbaryl, as grain protectant
combinations for wheat. Pestic. Sci., 11: 471-482.
Bowker, D.M. Pirimiphos-methyl: fate of stored wheat and rice grain in
1973 the laboratory. Report No. AR2457 AR,submitted to FAO by ICI
Plant Protection Ltd, (Unpublished).
Bratt, H. & Jones, L.A. Pirimiphos-methyl: Metabolism in rats and
1973 dogs. Report submitted to FAO by ICI Industrial Hygiene
Research Laboratories. (Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain. Report
1973 No. AR2472 AR submitted to FAO by ICI Plant Protection Ltd.
(Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain, bread,
1974 flour and milled products. Report submitted to FAO by ICI
Plant Protection Ltd. (Unpublished).
Bullock, D.J.W. In Analytical Methods for Pesticides and Plant
Growth
1976 Regulators, VIII, 185, G. Zweig Ed., Academic Press, New
York.
Bullock, D.J.W. & May, M.S. Pirimiphos-methyl: Residue transfer from
1976 durum wheat to semolina and pasta. Report No. TMJI345A
submitted to FAO by ICI Plant Protection Ltd. (Unpublished).
Bullock, D.J.W., Harrison, P.J. & Day, S.R. Degradation of residues in
1976 flour during baking. Report No. AR2666A submitted to FAO by
ICI Plant Protection Ltd. (Unpublished).
Cerna, V. & Benes, V. Residues of pirimiphos-methyl in wheat, mill
1977 products and white bread. Report by Czechoslovakian
Institute of Hygiene in Epidemiology, Prague.
Chawla, R.P. & Bindra, O.S. (1971). Residues of some promising grain
1971 protectants on stored wheat. Proc. Symp. Progress and
Problems in Pesticide Residue Analysis (Bindra & Kalra,
Eds). Punjab Agric. Univ. Ludhiana & Indian Counc. Agric.
Res., New Delhi. p. 68-74.
Curl, E.A. & Leary, J.P. The radioactive residues found in the liver
1980 and kidney of a goat after administration of a multiple dose
of pirimiphos-methyl. Report RJO144B submitted to FAO by ICI
Plant Protection Ltd.
Desmarchelier, J.M. Selective treatments including combinations of
1977 pyrethroid and organophosphorus insecticides for control of
stored product Coleoptera at two temperatures. J. Stored
Prod. Res., 13: 129-137.
Desmarchelier, J.M. Loss of fenitrothion on grains in storage. Pestic.
1978 Sci. 9: 33-38.
Desmarchelier, J.M. & Bengston, M. The residual behaviour of chemicals
1979 in stored grain. Proceedings of the Second International
Working Conference in Stored-Product Entomology, Ibadan,
Nigeria, 10-16 September 1979.
Desmarchelier, J.M., Bengston, M., Connell, M., Henning, R., Ridley,
in E., Ripp, E., Sierakowski, C., Sticka, R., Snelson, J.
press & Wilson, A. Extensive pilot usage of the grain protectant
combinations, fenitrothion plus bioresmethrin and
pirimiphos-methyl plus bioresmethrin. J. Pestic. Sci.
Desmarchelier, J.M., Goldring, M. & Horan, R. Predicted and observed
1980a residues of bio-resmethrin, carbaryl, fenitrothion,
phenothrin, methacrifos and pirimiphos-methyl on rice and
barley after storage, and losses of these insecticides
during processing. J. Pestic. Sci., 5: 539-545.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W., Moore, B.,
1980b Phillips, M., Snelson, J., Sticka, R. & Tucker, K. A
collaborative study of residues on wheat of methacrifos,
chloropyrifos-methyl, fenitrothion, malathion and
pirimiphos-methyl. II. Rates of decay. CSIRO, Division of
Entomology Report No. 20. 21 p.
Kadoum, A.M. & La Hue, D.W. Penetration of malathion in stored corn,
1974 wheat and sorghum grain. J. Econ. Entomol., 67(4): 477-478.
Krasnykh, A.A. Khim. Sel'sk. Khoz. 16, (11) 36 (Russ).
1978
La Hue, D.W. Pesticide residues of potential protectants of grain.
1974 Proc. 1st Int. Working Conf. on Stored Product Entomology,
Savannah, Georgia, USA. p. 635-638.
La Hue, D.W. Pirimiphos-methyl: Effect on populations of Tribolium
1977 confusum and T. castaneum in wheat. J. Econ. Entomol.,
70(1): 135-137.
La Hue, D.W. & Dicke, E.B. Evaluation of selected insecticides applied
1976 to high moisture sorghum grain to prevent stored grain
insect attack. USDA Agricultural Research Service, Marketing
Research Report No. 1063.
Le Berre, R., Escaffre, H., Pendriez, B., Grebaut, S. & Pengalet, P.
1972 Control of Simulium damnosum, the vector of human
onchocerciasus in West Africa II. Test by classic
application of new insecticides and new formulations, 70
Oncho du 8 Mai ORSTOMOCCGE, BP 171, Bobo-Dioulasso, Upper
Volta.
McCallum Deighton, J. Pirimiphos-methyl and other insecticides in the
1978 control of stored-product insects. Outlook Agric., 9(5)
240-248.
Mensah, G.W.K., Watters, F.L. & Webster, G.R.B. Insecticide residues
1979 in milled fractions of dry or tough wheat treated with
malathion, bromophos, iodofenphos and pirimiphos-methyl. J.
Econ. Entomol., 72: 728-731.
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. Report No. CTL/P/247 submitted to FAO
by ICI Central Toxicology. (Unpublished).
Morallo-Rejesus, B. & Carino F.O. The residual toxicity of five
1976 insecticides on three varieties of corn and sorghum.
Philipp. Agric., 60(3&4): 96-104.
Redlinger, L.M. & Simonaitis, R.A. Field tests with pirimiphos-methyl
1977 as a protectant for farmers stock peanuts. Peanut Sci.
4: 27.
Rishikesh, N., Mathis, H.L., King, J.S. & Nambiar, R.V. A field trial
1977 of pirimiphos-methyl for the control of Anopheles gambiae
and Anopheles funestus in Nigeria. WHO Geneva, VBC/77/671.
(Unpublished).
Rowlands, D.G. The metabolism of contact insecticides in stored grains
1975 1970-1974. Residue Rev., 58: 113.
Rowlands, D.G. The metabolism of pirimiphos-methyl by stored grains
1981 under laboratory conditions. Report No. 54, Pest Control
Chemistry Department, Pest Infestation Laboratory, Slough,
U.K. Ministry of Agriculture, Fisheries & Food.
Rowlands, D.G. et al. Preliminary report of studies with pirimiphos-
1974 methyl as a grain protectant. Submission by United Kingdom
to FAO.
Rowlands, D.G. & Wilkin, D.R. Metabolism of pesticides in stored
1975 cereals. Report of Director, Pest Infestation Control
Laboratory, Slough, U.K. 1971-1973.
Seth, A.K. Use of Actellic for stored rice insect problems in S.E.
1974 Asia. Symp. 1st Int. Working Conf. Stored Prod. Entomol.,
7-11 October 1974, Savannah, Georgia, USA.
Shaw, R.F., Fanara, D.M., Pradhan, G.D., Supratman, Supalin, Bang,
1979 Y.H., & Fleming, G.A. A village-scale trial of
pirimiphos-methyl (OMS-1424) for control of the malaria
vector (Anopheles aconitus) in Central Java, Indonesia.
WHO, Geneva, VBC/79/722. (Unpublished)
Simonaitis, R.A., Zehner, J.M. & Redlinger, L.M. J. HRC & CC 4: 169.
1981
Soderstrom, E.L. & Armstrong, J.W. pp-511: (Pirimiphos-methyl-ISO)
1973 evaluated as a protectant for raisins and walnuts. J. Econ.
Entomol., 66(6): 1305-1306.
Spain. Registered uses of pirimiphos-methyl. Data submitted to FAO by
1983 the Ministry of Agriculture, Madrid.
Spitler, G.H. & Hartsell, P.L. Pirimiphos-methyl as a protectant for
1975 stored inshell almonds. J. Econ. Entomol., 68(6): 777-780.
Sweden. Monitoring of imported fruits and vegetables for residues of
1983 pirimiphos-methyl. Data submitted to FAO by the Swedish
National Food Institute.
Tempone, M.J. Studies on the effects of insecticides on barley
1979 malting. Research Project Series No. 50, Victorian
Department of Agriculture, Melbourne, Australia.
Thomas, K.P. & Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by Cheshire cheese. J. Stored Prod. Res.,
11:53.
Varca, L.M., Magallona, E.D. & Pasco, J.A. Philipp. Entomol., 3: 55.
1975
Weaving, A.J.S. Grain protectants for use under tribal storage
1975 conditions in Rhodesia - Comparative toxicities of some
insecticides on maize and sorghum. J. Stored Prod. Res.,
11: 65-71.
WHO. Interim specifications for pirimiphos-methyl, technical, water
1982 dispersible powder and emulsion concentrate VBC/15/82.04.
WHO Vector Biology Control Division, Geneva.
Zakitis, L.H. & McCray, E.M. Gas chromatographic analysis of residues
1982 of pirimiphos-methyl in water, fish and snails. Bull.
Environ. Contam. Toxicol., 28: 334-340.
Degradation was marginally more rapid in contact with the grain
than in the isolated formulation but whether this additional breakdown
was caused by factors within the grain, or by the associated
microflora, was not known. At a higher moisture content (ca. 18
percent) less pirimiphos-methyl was recovered on analysis but
increased levels of the hydrolysis product (IV) were obtained,
suggesting that a more rapid degradation of the insecticide occurred.
Bowker (1973) concluded that grain may lack the enzymatic activity,
believed to be present in plants and soil, to cleave the pyrimidine
N-ethyl bonds. It is likely, therefore, that following the treatment
of wheat and brown rice with pirimiphos-methyl, the major residues
during storage will be the parent compound and the simple hydrolysis
product (IV). Under optimum conditions, the maximum level of compound
(IV) following treatment at 4 mg/kg was found to be 0.17 mg/kg; under
poor storage conditions, with high moisture content grain it was 0.62
mg/kg.
Solutions and also a 2 percent dust of radio-labelled
pirimiphos-methyl were applied in the laboratory to wheat grain of 14
and 18 percent moisture to give levels of 4 mg/kg. Throughout a
storage period of six months, the residues of pirimiphos-methyl were
found almost entirely in the seed coat and aleurone layer, with only
traces present in the germ or endosperm. It was also clear that, as
with malathion but to a greater extent, there was transfer of
insecticide between grains, possibly in the vapour phase. About 10
percent of the total residual radioactivity was bound to lipoprotein
material in the aleurone region of the grain and could be extracted
only by digestion of the aleurone protein. In contrast with other
organophosphates studied, the bound material appeared to be unchanged
pesticide rather than a metabolite. It was undoubtedly a P=S compound,
but degradation during the liberation of the bound material
complicated identification (Rowlands et al. 1974).
In Animals
The metabolism of pirimiphos-methyl was studied in the rat and
dog. Twelve metabolites were detected, none of which showed
anticholinesterase activity. No parent pirimiphos-methyl was detected
in the urine. In both rats and dogs, 2-ethylamino-4-hydroxy-6-methyl
pyrimidine was the major urinary metabolite (Bratt & Jones 1973).
When rats were given a single oral dose of radio-labelled
pirimiphos-methyl at 7.5 mg/kg, the uptake of radioactivity into blood
and its subsequent disappearance from the bloodstream were both very
rapid. More than 50 percent of the radioactivity present in the blood
30 min. after dosing had disappeared at one hour after dosing.
Unchanged pirimiphos-methyl usually represented less than 10 percent
of the total residue in the blood 24 h after dosing. When
radio-labelled pirimiphos-methyl was administered orally to rats at
7.5 mg/kg per day for four days, total radioactive residues in the
liver, kidney and fat did not usually exceed 2 mg/kg pirimiphos-methyl
equivalents. There was no evidence to show that either
pirimiphos-methyl or its metabolites accumulated in the liver, kidney
or fat of rats following daily dosing with the insecticide over four
days (Mills 1976).
The rodioactive residues in the liver and kidney of a goat, dosed
daily for seven consecutive days with 14C-pirimiphos-methyl at 30
mg/kg in the diet, were examined. (Curl and Leahy 1980).
A radioactive residue of 0.25-0.30 mg/kg of
14C-pirimiphos-methyl equivalents was detected in the liver, and 89
percent of this was extracted and analysed by thin layer
chromatography. 5.9 percent of the total radioactive residue in the
liver was unchanged pirimiphos-methyl. The hydroxypyrimidines,
compound (II) (2-diethylamino-6-methyl-pyrimidin-4-ol), compound (III)
(2-ethylamino-6-methyl-pyrimidin-4-ol) and compound (IV)
(2-amino-6-methyl-pyrimidin-4-ol) accounted for 3.7 percent, 21.8
percent and 17.5 percent respectively, of the total radioactive
residue in the liver. Compounds (II), (III) and (IV) were present
mainly as "free" metabolites. However, 13.7 percent of the
hydroxypyrimidines detected were released from the liver by acid
reflux.
The remainder of the radioactivity extracted from the liver
consisted of at least five compounds.
A radioactive residue of 0.59-0.70 mg/kg of
14C-pirimiphos-methyl equivalents was found in the kidney; 91 percent
of this was extracted and analysed by thin-layer chromatography.
Compound (II), compound (III) and compound (IV) accounted for 7.9
percent, 35.3 percent and 17.0 percent respectively, of the total
radioactive residue in the kidney. Compounds (II), (III) and (IV) were
present mainly as "free" metabolites, however, 9.0 percent of the
hydroxypyrimidines detected were released from the kidney by acid
reflux.
The remainder of the radioactivity extracted from the kidney
consisted of at least six compounds.
In Storage and Processing
Bullock (1973, 1974) reported several individual experiments
which demonstrated that residue levels of pirimiphos-methyl are
significantly reduced during the milling and baking processes. Table 2
summarizes the results of residue trials carried out in the United
Kingdom on wheat that had been treated to contain nominally 4 mg/kg
pirimiphos-methyl.
Table 3 summarizes results of a residue trial (Bullock 1973)
carried out in the United Kingdom with wheat nominally treated to
contain 8 mg/kg pirimiphos-methyl. These data are substantially in
agreement with those of Bengston et al. (1975) and show that there
is relatively little penetration beyond the seed coat, even throughout
a storage period of nine weeks.
Table 2. Effect of Milling and Baking on Residue in Wheat Admixed
with Pirimiphos-Methyl1
Interval between
Grain treatment and Residues (mg/kg)
fraction sampling (months) Highest Lowest Mean
Whole grain 0 4.2 1.9 2.9 (9)
3.0 -* 3.0*
1 4.1 1.5 2.8 (9)
2 4.1 1.6 2.6 (8)
2.5* 2.4* 2.5* (3)
3 3.6 1.3 2.3 (7)
Whole meal 0 1.3 0.94 1.1 (3)
flour 1 2.1 1.1 1.7 (4)
2 2.2 1.2 1.7 (3)
1.5* 1.4* 1.5* (3)
3 2.1 1.0 1.5 (4)
White flour 0 0.88 0.30 0.52 (6)
0.56* 0.53* 0.55*(3)
1 0.77 0.44 0.59 (5)
2 0.64 0.24 0.56 (6)
0.29* 0.24* 0.27*(3)
3 0.67 0.38 0.56 (3)
Wholemeal 0 0.72 0.53 0.64 (4)
bread 1 0.91 0.55 0.79 (4)
2 1.1 0.65 0.93 (4)
0.97* 0.82* 0.88* (3)
3 0.54 0.21 0.49 (3)
White bread 0 0.28 0.19 0.23 (6)
0.26* 0.24* 0.25* (3)
1 0.36 0.22 0.30 (5)
2 0.45 0.31 0.36 (8)
0.15* 0.13* 0.14* (3)
3 0.54 0.21 0.43 (3)
1 Dosage of pesticide was 4 mg/kg. All results are from field
trials except those marked *, which are from a small-scale trial.
Figures in parentheses are the numbers of results on which the
means are based.
Table 3. Residues of Pirimiphos-methyl in Whole Grains and in Milling
and Baking Fractions Treated in a Laboratory Trial1
Interval between Residues (mg/kg)
treatment and Whole White White Wholemeal Wholemeal
sampling grain flour bread flour bread
0 days 6.0 0.86 0.52
6.0 0.91 0.56
6.0 1.0 0.57
9 weeks 4.8 0.47 0.28 3.2 1.7
5.2 0.59 0.33 3.0 1.6
5.2 0.60 0.30 3.2 1.5
1 Dosage of pesticide was 8 mg/kg. In all cases, no residues of the
phosphorus-containing compounds (II) or (III) (Figure 1) were
detected. (Limit of detection: 0.01 mg/kg in each case.)
Seth (1974) discussed experiments on the admixture of
pirimiphos-methyl with paddy rice (rice in husk) in Southeast Asia.
Both emulsion and dust formulations were used and were applied at
rates of 2, 4 and 8 mg/kg. A portion of the rice was milled
immediately after treatment and further portions at the end of one,
two, three and five months. It was found that the deposit of
pirimiphos-methyl on both the husk and the milled grain was
proportional to the amount applied, but the concentration on the husk
was always about 25 times that on the milled grain. The residue on the
husk and milled grain declined steadily over the five-month storage
period. Although the concentration on the husk initially was as high
as 12 mg/kg, the residue on the milled rice barely exceeded 0.5 mg/kg
and even at the highest rate of application was always below 1 mg/kg.
These experiments clearly showed that there is minimum transfer of
pirimiphos-methyl from the husk to the kernel of rice, even during
prolonged storage. The degree of penetration is comparable to that
reported by Kadoum & La Hue (1974) for malathion on wheat, maize and
sorghum.
Residues of pirimiphos-methyl in flour are relatively stable
during baking to bread and biscuits. However, because of the dilution
which the flour undergoes during these processes, flour initially
containing 1 mg/kg pirimiphos-methyl is likely to yield
bread/ biscuits containing residues of ca. 0.5 mg/kg.
Bullock et al. (1976) studied the fate of pirimiphos-methyl
during processing of flour into bread and biscuits. In studies with
the radio-labelled compound, flour was dosed with 2-14C-labelled
pirimiphos-methyl and baked to produce white bread, wholemeal bread
and biscuits. Although pirimiphos-methyl is known to be a relatively
volative compound, there was no significant loss of radioactivity by
volatilization. Distribution of radioactivity throughout the bread was
fairly uniform. Unchanged pirimiphos-methyl accounted for 75-90
percent of the radioactivity in the baked product. The major
degradation product formed during the baking was hydroxypyrimidine,
which accounted for 3-10 percent of the radioactivity in the final
product.
Similar results were obtained by residue analysis in a second set
of studies. After correcting for the weight increase when flour is
converted to bread, residues of pirimiphos-methyl fell by 11-18
percent. Likewise, residue analysis of biscuits showed average losses
of 8 percent. However, owing to the dilution of the flour that occurs
during baking, the residue of pirimiphos-methyl in bread will be lower
than that in the corresponding flour. Bullock et al. (1976) found
levels of pirimiphos-methyl in bread and biscuits to be about 50
percent of those in the flour from which they were derived. These
findings agreed well with the earlier work of Bullock (1973, 1974). No
residues of the phosphorus-containing compounds (II) and (III) were
detected in bread baked from flour treated with pirimiphos-methyl at
1 mg/kg or in biscuits baked from flour containing up to 5 mg/kg
(Bullock et al. 1976)
The hydroxypyrimidine (IV) also undergoes only slight degradation
during the baking process. This compound constitutes only a minor part
of the residue in stored grains (FAO/,WHO 1975). Bullock et al
(1976) used radio-labelled compound (IV) and found that it degraded by
less than 10 percent during the baking of bread. It can, therefore, be
concluded that residues of compound (IV) in baked products will never
exceed 0.2 mg/kg and will normally be considerably lower.
Bullock & May (1976) studied the fate of residues in wheat during
processing to semolina and pasta. White semolina, prepared from durum
wheat treated at 10 mg/kg, contained only 1.6 mg/kg pirimiphos-methyl.
Pirimiphos-methyl levels in both white and wholemeal pasta were
approximately 85-90 percent of those in the corresponding semolina; 70
percent of the pirimiphos-methyl residue in semolina was transferred
unchanged to cooked pasta. However, the weight of pasta increased by
100 percent on cooking, so that the concentration of pirimiphos-methyl
in cooked pasta is likely to be approximately 35 percent of that in
the corresponding semolina.
Results of experiments in Czechoslovakia (Cerna & Benes 1977)
indicated that residues of pirimiphos-methyl in grain, which had been
in store for nine months, were substantially removed by the milling
process. The bulk of the residue was removed in the bran; there was no
substantial difference in the concentration in the different bran
fractions. When white flour was made into white bread, there was a
further loss of approximately 50 percent so that the concentration of
the residue in the bread was only 10-15 percent of its concentration
in raw grain. Some residue was destroyed during milling.
Tempone (1979) reported a series of studies on the effects of
insecticides on barley malting and the resulting residues. In the
first of these trials, barley treated with pirimiphos-methyl at the
rate of 6 mg/kg or 18 mg/kg was converted into malt but no residues
were detected in the wort (unfermented extracts from the malted
barley), the limit of determination being 0.004 mg/kg. Wort, prepared
from barley malted after being in store for three, six and nine
months, likewise showed no residues. In the second series of similar
trials, the residue was determined in the malt (germinated grain that
had been calcined). Residues could be found in the malt, but at a
level of from 10 to 20 percent of that in the barley before malting.
Barley kept in storage for three months after being treated with
pirimiphos-methyl transferred significantly less residue to the malt
than did the barley malted immediately after treatment, no doubt
because there was significantly less residue on the outside of the
kernel, where it would be protected from the enzymatic activity within
the barley grain during germination.
Bengston et al. (1980 a, b) arranged for wheat from bulk grain
treated with pirimiphos-methyl and held in commercial silos to be
processed through to wholemeal bread and white bread. The results
obtained in these trials are reported in Tables 4 and 5. During
processing from wheat to white bread, residues were reduced by 85-91
percent.
Table 4. Mean Residues of Pirimiphos-methyl Following Milling and Baking of Stored Wheat
Site Storage Residues (mg/kg)
(weeks)
Wheat Bran Pollard Wholemeal White Wholemeal White
flour flour bread bread
Site B 13 5-7 12-16 2-4 2.6 0.1-0.2 1.0-2.0 0.05-0.1
Site D 19 2.5-3.0 6-8 1-2 1.2 0.05-0.1 0.5-1.0 0.05
Table 5. Reduction in Pirimiphos-methyl Residues Following Milling and Baking of Stored Wheat
Site Reduction in residues(%)
Wheat to Wheat to Wholemeal flour White Flour Wheat to Wheat to
Wholemeal White to Wholemeal to White Wholemeal White
Flour Flour Bread Bread Bread Bread
Site B 5 68 47 54 50 85
Site D 0 78 55 58 56 91
Desmarchelier et al. (1980b), after treating bulk wheat in
commercial silos with pirimiphos-methyl, arranged for a portion to be
milled and for the white flour to be converted into white bread. The
results are given in Table 6. It is of interest to note that the
residues in flour and bread were higher in the hard wheat which was
held for 22 weeks before milling, than in the soft wheat which was
held of 11 weeks before milling.
Desmarchelier et al. (1980a) studied the fate of
primiphos-methyl, and a number of other grain protectant insecticides,
applied to unhusked rice, husked rice, polished rice and barley over a
storage period of six months and subsequently during the processing
and cooking of these grains. The results of these trials are given in
Table 7, and show that only about 10-15 percent of the residue present
on husked rice or polished rice was destroyed in the cooking process.
However, if the treatment was applied to unhusked rice, approximately
70 percent of the residue was removed with the husk and a great deal
more when the husked rice was milled for the removal of the bran.
Subsequent cooking of the husked rice or polished rice brought about a
further substantial reduction in the residue level.
These same workers found that pirimiphos-methyl, applied to
barley destined for malting, was substantially lost during the malting
process. Barley treated at the rate of 6 mg/kg and held in storage for
six months was found to contain 4.9 mg/kg pirimiphos-methyl. When this
grain was malted, the pirimiphos-methyl residue in the prepared malt
was only 0.9 mg/kg. The work of Tempone (1979) showed that little, if
any, of the residue in the malt is extracted into the wort.
There have been no reports of pirimiphos-methyl being metabolized
by attack at either of the O,O-dimethyl groups (to give a desmethyl
derivative) as is common with certain other organophosphorus
triesters. Morallo-Rejesus & Carino (1976) noted, but did not
identify, breakdown products in shelled maize stored in
pirimiphos-methyl treated bags.
Rowlands (1981), reporting work carried out some years previously
using radio-labelled compound, treated small quantities (10 g) of
wheat that were held in store for up to 6 months. Aliquots were
removed at intervals and, after crushing, were extracted first with
hexane, followed in turn by chloroform, methanol and acetonitrile and
then, after sequential digestion by three enzymes, further extraction
by chloroform. The results are recorded in Table 8.
It is clear from Table 8 that very soon after treatment, a
portion of the aged pirimiphos-methyl residue is not extractable from
wheat unless it is digested out enzymatically. It is thought that this
is due to a complex formed by intact pirimiphos-methyl within the
aleurone layer (Rowlands 1975).
Table 6. Residues of Primiphos-methyl in Wheat, Its Milling Products and Bread
Residues (mg/kg)
Type of Storage
Wheat (weeks) Initial deposit Whole grain bran pollard flour bread
Soft 11 5.2 4.2 9.1 8.3 0.2 0.1
Hard 22 6.1 4.5 12.4 10.4 1.3 0.35
Table 7. Residues of Pirimiphos-methyl on Husked, Polished and Unhusked Rice After Storage and Processing
Grain Application Residues (mg/kg)
level
(mg/kg)
After After After After
3 6 Cooking at Cooking at
Months Months 3 Months 6 Months
Husked
rice 6 6.5 4.9 4.9 4.0
Polished
rice 6 6.0 6.0 4.9 4.9
Residues (mg/kg) after
6 months storage and
Milling Milling &
Cooking
Husked Polished Husked Polished
rice rice rice rice
Unhusked
rice 6.0 6.0 4.6 1.4 0.3 0.6 0.1
Table 8. Recovery of Pirimiphos-methyl From Crushed Wheat (18% mo) By Sequential extraction
Extraction by 14C activity recovered %1 at time of extraction after
application (0 h)
0 h 1 h 7 days 14 days 1 month 6 months
Hexane 97 89 84 78 72 67
Chloroform nil 1 4 7 8 8
Methanol trace 1 2 2 6 7
Acetonitrile trace 2 1 4 2 2
Digest Sequence2
(1) nil 2 1 4 2 2
(2) 4 5 7 7 9 12
(3) nil 2 2 1 2 1
Total extracted 101 102 101 103 101 99
1 Average of three results ± 3 percent.
2 Digest sequence: (1) = lipase pH 7.4; (2) = papain pH 4,5; (3) - cellulase pH 4.5,
All at 37°C for 24 h
Studies reported by Rowlands (1981) on the uptake from dust or
solvent application as determined by the separated tissue of the wheat
grains after storage, showed that intact pirimiphos-methyl and free
pyrimidinols occur chiefly in the seedcoat. These findings received
some confirmation in the work of Mensah et al. (1979), who found
that pirimiphos-methyl emulsion, applied to wheat that was then milled
six months later, had accumulated in the bran and middlings fractions,
irrespective of the moisture content of the stored wheat (12 percent
and 16 percent). They found little or no residue in white flour.
Rowlands (1981) found only pirimiphos-methyl (I) and the
corresponding pirimidinol (IV) as residues in pirimiphos-methyl
treated wheat with 12 percent moisture that was kept in sealed jars
for up to eight months. However, in wheat with 18 percent moisture, a
small (less than 10 percent of the total residue) quantity of the
N-des-ethyl pirimidinol (V) was found after six and eight months. (See
Figure 2 for conformations of these compounds).
Further to the work of Thomas & Rowlands (1975) on the uptake and
degradation of pirimiphos-methyl by Cheshire cheese, similar studies
were carried out using Stilton cheeses, which differ in physical
characteristics and are stored differently.
In these studies a wooden plank was treated with
14C-ring-labelled pirimiphos-methyl and allowed to dry for 24 h
before two young Stilton cheeses were placed on the treated surface.
One of the cheeses was first covered by a layer of cheesecloth. The
cheeses were turned every two days. Replicate core samples were taken
with a cork borer from each face of both cheeses. The core was
analysed for pirimiphos-methyl. Some of the cores were sectioned with
a microtome and the concentration of pirimiphos-methyl in the cheese
at various depths was determined by means of a scintillation counter.
The findings are reported in Table 9.
This experiment has shown that only a small amount of
pirimiphos-methyl penetrated into the cheeses under the various
storage techniques used with Stilton cheese. No breakdown of the
pirimiphos-methyl could be detected in the cheese or the cheesecloth
throughout the seven weeks of storage.
These results indicate that the MRL of 0.5 mg/kg for
pirimiphos-methyl in cheese would not be exceeded when the insecticide
was used for the control of cheese mites during the making and storage
of Stilton cheese.
Mensah et al. (1979) reported results of small-scale trials, in
which water-based emulsions of malathion, bromophos, iodofenphos and
pirimiphos-methyl were applied at two dosage rates to spring wheat of
12 percent and 15 percent moisture content, to compare the fate of the
residues. Pirimiphos-methyl degraded at a comparatively slower rate
than the other three compounds, but the rate of degradation was
significantly higher on the high moisture grain. The results are given
in Table 10. The residue levels were concentrated in the seed coat,
resulting in high levels in bran and middlings. When applied at the
rate of 6 mg/kg, the residues in these fractions exceeded the MRL
recommended by the Meeting.
Results were reported of small-scale trials in which the efficacy
and fate of pirimiphos-methyl residue on wheat and milling fractions
were assessed. Two rates (7.3 mg/kg and 14.6 mg/kg) were applied and
the wheat was stored for up to 12 months. The results are reported in
Table 11. These show that the residues in bran and middlings (shorts)
exceeded the MRL following application at the lower rate and storage
for less than six months. At the higher rate (which is well in excess
of the recommended rate) the residues greatly exceeded the MRL.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of pirimiphos-methyl were detected in the United States
in several samples of imported foods (Barry et al. 1981) and the
identification of these residues by GC-MS and GLC was reported. The
level and source of residues found are reported in Table 12.
The Swedish National Food Institute (Sweden 1983) indicated that
during the period 1-1-80 to 30-4-83, 8 654 samples of fruit and
vegetables were analysed for pesticide residues. From among these,
eight samples were found to contain pirimiphos-methyl at levels up to
1.4 mg/kg, as indicated in Table 13.
METHODS OF RESIDUE ANALYSIS
Barry et al. (1981) have provided details of GC-MS
identification and analytical behaviour of pirimiphos-methyl in a
variety of foods as part of an investigation of unknown residues
detected in imported foods.
Zakitis & McCray (1982), having reviewed the analytical
methodology for residues of pirimiphos-methyl in a variety of
substrates, concluded that none of the eight known methods met all the
desired criteria for analysis of residues in water, fish and snails,
viz. specificity, simplicity of sample preparation and recovery after
clean-up. Water was simply extracted with hexane prior to
determination by GC. Fish and snails were extracted with acetone in
the presence of sufficient anhydrous sodium sulphate to form a dry
powder. The acetone was filtered and the solids were extracted three
more times with acetone. The combined, filtered extracts were
evaporated to low volume before being transferred to de-ionized water
for extraction with hexane as for analysis of water. Recoveries ranged
from 93 to 101 percent. The method is being used for environmental
studies involving the use of pirimiphos-methyl for disease vector
control. The limit of determination in snails is 0.5 mg/kg. Below this
concentration, some naturally occurring substance interferes with the
determination of pirimiphos-methyl. Good recoveries were obtained from
dechlorinated water at concentrations as low as 0.005 mg/l and in fish
down to 0.05 mg/kg.
Table 9. Pirimiphos-Methyl Residues From Microtomed Sections of Two Cheeses1
Residues (mg/kg)
Storage
period End Depth (mm) into cheese
(days) sampled 0-1 1-2 2-3 3-4 4-5 5-6 6-7 7-8 8-9 9-10
Cheese in direct contact with treated surface
31 1 41.15 7.48 4.03 1.73 1.25 0.90 0.65 0.55 0.40 0.10
2 27.50 7.70 2.32 1.09 0.71 0.50 0.28 0.20 0.16 -
49 1 29.57 9.26 2.87 2.04 1.52 1.13 0.82 0.71 0.51 0.27
2 23.73 6.54 2.82 2.71 1.52 1.07 0.74 0.55 0.51 0.37
Cheese on cloth barrier
31 1 18.04 3.40 0.65 0.30 0.18 0.08 - - - -
2 35.28 11.48 4.19 0.62 0.36 0.27 0.22 0.16 - -
49 1 19.58 5.69 1.72 0.72 0.45 0.28 - - - -
2 12.26 7.13 1.84 1.11 0.84 0.66 0.46 0.31 - -
1 Dashes indicate that no pirimiphos-methyl was detected.
Table 10. Mean1 Pirimiphos-methyl Residues (mg/kg) on Dry and Tough Wheat and Milled Fractions After Storage
of Treated Wheat
12% moisture content (dry) 16% moisture content (tough)
Storage
Dosage period Whole Whole
(mg/kg) (months) wheat2 Bran Middling3 Flour wheat2 Bran Middling3 Flour
4 1 3.29±0.07 15.66±0.79 21.44±1.96 1.94±0.25 3.24±0.03 19.81±1.63 15.35±0.58 1.57±0.03
3 2.98±0.10 13.93±0.26 15.10±1.00 1.93±0.12 3.04±0.01 13.45±0.80 13.51±0.36 1.16±0.06
6 2.54±0.07 13.16±0.51 13.80±1.36 1.56±0.06 2.26±0.18 11.98±0.67 10.68±0.60 0.81±0.23
6 1 4.93±0.13 20.87±0.82 23.15±1.47 2.86±0.14 4.92±0.04 27.33±1.41 21.83±1.73 1.91±0.07
3 4.54±0.10 20.54±1.38 19.34±0.74 2.42±0.22 4.44±0.04 20.90±2.92 17.89±0.59 1.50±0.08
6 3.66±0.23 19.32±0.73 16.05±1.35 2.23±0.13 3.86±0.26 18.60±0.73 16.89±0.56 1.33±0.14
1 Mean of 3 replicates ± SE.
2 Ground wheat.
3 Shorts, wheat germ and coarse particles of flour.
Table 11. Average Pirimiphos-methyl Residue (mg/kg) on Hard Winter Wheat
and Wheat Fractions Stored up to 12 Months
Rate of application
7.3 mg/kg 14.6 mg/kg
Storage Whole Whole
Period wheat1 Bran Shorts Flour wheat1 Bran Shorts Flour
24 h 7.15 25.50 18.29 0.83 14.75 50.41 36.01 2.01
Months
1 6.88 21.45 17.89 1.38 13.13 41.43 31.01 2.94
3 6.60 20.11 20.82 1.47 12.98 39.97 42.97 3.15
6 5.78 18.06 20.46 1.47 11.17 30.52 46.58 2.91
9 5.43 17.34 17.90 1.17 10.39 34.52 31.47 2.44
12 5.27 13.47 15.35 1.05 9.72 32.29 26.98 2.14
1 Before tempering to 15% for milling.
Table 12. Pirimiphos-methyl residues in imported foods
Food Country of Origin Residue (mg/kg)
Chickpeas Australia 0.07
Dried green peas Australia 3.0
Pigeon peas Kenya 0.19
Moong dall Tanzania 0.03
Peanut butter South Africa 0.23
Split peas Kenya 0.1-0.171
Sardo cheese Argentina 0.21-1.41,2
1 Represents range found in multiple samples of the same
commodity.
2 Determined on fat basis.
Table 13. Primiphos-methyl Residues Detected in Sweden1
Food Origin No. of No. of samples with residues within Maximum
samples given ranges (mg/kg) residue
analysed <0.21 0.21-0.53 0.54-1.05 >1.05 (mg/kg)
Mandarin Import 292 291 1 0.21
Orange Import 622 621 1 0.41
Pepper Import 206 201 3 1 1 1.4
(sweet)
Tomato Sweden 205 205
Import 500 499 1 0.30
1 Total number of samples analysed:8 654
Period: 81-01-01 -- 83-04-30
A number of the methods reviewed by Zakitis & McCray (1982) have
not been considered previously by the Meeting. They are briefly
mentioned for information.
A general review of analytical methods was published by Bullock
(1976) including procedures suitable for animal tissues. There is also
a TLC-UV method for residues in water, soil and plants (Krasnykh,
1978), an HPLC method for determining pirimiphos-methyl and five
metabolites in samples of plasma and urine (Beasley & Lawrence, 1979),
and gas chromatographic methods for residues in stored grain (Varca
et al. 1975), peanuts (Redlinger & Simonaitis 1977) and milled
fractions of wheat (Mensah et al. 1979). In addition, a
gas-chromatographic method has been proposed for the simultaneous
determination of residues of pirimiphos-methyl and malathion in
peanuts (Simonaitis et al. 1981), and GC-MS was used to identify
residues of pirimiphos-methyl in imported foods (Barry et al. 1981).
The residue in stored grains (barley, maize, oats, rice in husk
and wheat) consisted mainly of the parent compound. Neither the oxygen
analogue (O,O-dimethyl-O(2-diethylamino-6-methyl-pirimidine-4yl)
phosphate), (metabolite III) nor the desethyl analogue
(O, O-dimethyl-O(2-ethylamino-6-methyl-pyrimidin-4-yl)
phosphorothionate), (metabolite II) was detectable. No metabolite was
detectable in milled products or in white and wholemeal breads. A
large number of plant samples (leafy vegetables, carrots, celery,
spring onions, potatoes, sugarbeets, cucumber, tomatoes, peppers,
mushrooms, lettuce, blackcurrants, apples, pears, plums, lemons,
oranges, olives) were analysed for residues of pirimiphos-methyl and
the phosphorous-containing metabolites. However, significant amounts
of the metabolites did not appear and so were not reported. The
residues listed in various tables of the 1976 Evaluations are of the
parent compound only.
Analyses of wheat grains treated with labelled compounds
confirmed the low level of both metabolites. No oxygen analogue was
detected. The level of desethyl analogue was below 0.05 mg/kg over a
period of 32 weeks, while the parent compound was present at 4 mg/kg.
The oxygen analogue was not detectable in whole rice seedlings. The
levels of parent compound in mg/kg and desethyl analogue (expressed as
parent equivalent) were as follows:
Compound Residue (mg/kg at intervals (days) after treatment
1 3 6 8 10 13
pirimiphos-methyl 0.5 0.51 12 0.6 0.51 0.32
metabolite (II) and
unknown compound 0.17 0.11 0.14 0.12 0.15 0.14
The oxygen analogue reached its maximum concentration on plant
leaves approximately 1 to 3 days after treatment. The ratio of maximum
levels of oxygen analogue and the parent compound were about 0.3 and 3
on cotton and citrus leaves, respectively, while the two compounds
were present in nearly equal concentration on bean leaves. The
disappearance of pirimiphos-methyl residues was rapid on the leaf
surfaces of all plants due to evaporation. The total 14C represented
10-12 percent of the applied material after three days and the
phosphorous containing metabolites were below 10 percent (Bowker
1973).
The Meeting concluded that the metabolites can be excluded from
the definition of the residue. The MRLs need not be changed because
the metabolites represent such a minor proportion of the total
residue. The MRLs refer to the present compound alone.
APPRAISAL
Pirimiphos-methyl was evaluated in 1974, 1976, 1977 and 1979 and
a number of items of information previously requested appear to be
still outstanding.
It would appear that there are a number of uses that give rise to
residues in food moving in international trade, which have not yet
been considered by the Meeting and about which relevant information
will be available in 1984.
An extensive amount of information about the use and fate of
pirimiphos-methyl when applied after harvest to cereal grains has
appeared in open scientific literature and this was reviewed by the
Meeting. The review only serves to confirm the evaluations made
previously. All authors have drawn attention to the stability of
pirimiphos-methyl deposits on treated grain and the retention of the
bulk of the deposit in the seed coat. This is reflected in the low
transfer of residue into white flour and milled rice and the
relatively higher residues in wheat bran.
This review has confirmed that the degradation and metabolism of
pirimiphos-methyl on grain leads only to the production of
2-diethylamino-4-hydroxy-6-methyl pyrimidine. No
cholinesterase-inhibiting metabolites occur. However, between 4
percent and 15 percent of the residue of the parent compound forms a
complex with the aleurone layer of the grain and resists extraction by
a variety of solvents. It may be dislodged by digesting the substrate
with proteolytic enzymes.
The Meeting, having reviewed all available data, concluded that
the metabolites can be excluded from the definition of the residue.
The MRLs, which remain the same, refer to the parent compound alone.
A further study of the use of pirimiphos-methyl for the control
of cheese mites in cheese stores confirmed that the MRL previously
recommended adequately covers the resulting residues.
Several reviews have been made of analytical methods suitable for
dealing with various substrates.
The Meeting considered that the information evaluated in 1983 did
not indicate any need to amend or modify recommendations previously
made for MRLs in a range of commodities.
The Meeting reviewed the requests made by earlier Meetings for
additional information in the light of knowledge about the current use
of this insecticide and considered that they had been satisfied.
FURTHER WORK OR INFORMATION
Desirable
Further information about residues in peanuts, oil seeds, lentils
and citrus.
REFERENCES - RESIDUES
Anonymous. Chemical control of stored grain insects 1972. Cyprus
1972 Agric. Res. Inst. Ann. Rep., p. 69.
Banks, H.J. & Desmarchelier, J.M. New chemical approaches to pest
1978 control in stored grain. Chem. Aust. 45(6): 276-281.
Barry, T.L., Petzinger, G. & Gretch, F.M. GC-MS identification and
1981 analytical behaviour of pirimiphos-methyl in imported foods.
Bull. Environ. Contam. Toxicol., 27: 524-528.
Beasley, C.J. & Lawrence, D.K.: J. Chromatog., 168: 461.
1979
Bengston, M., Cooper, L.M. & Grant-Taylor, F.J. A comparison of
1975 bioresmethrin, chloropyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in
wheat. J. Agric. Anim. Sci., 32: 51-78.
Bengston, M., Cooper, L.M., Davies, R., Desmarchelier, J., Hart R. &
1978 Phillips, M. Grain protectants for the control of
malathion-resistant insects in stored sorghum. (To be
submitted for publication).
Bengston, M., Connell, M. Davies, R., Desmarchelier, J., Elder, B.,
1980a Hart, R., Phillips, M., Ridley, E., Ripp, E., Snelson, J. &
Sticka, R. Chloropyrifos-methyl plus bioremethrin,
methacrifos, pirimiphos-methyl plus bioresmethrin and
synergised bioresmethrin as grain protectants for wheat.
Pestic. Sci., 11: 61-76.
Bengston, M., Connell, M., Davies, R., Desmarchelier, J., Phillips,
1980b M., Snelson, J. & Sticks, R. Fenitrothion plus phenothrin
and pirimiphos-methyl plus carbaryl, as grain protectant
combinations for wheat. Pestic. Sci., 11: 471-482.
Bowker, D.M. Pirimiphos-methyl: fate of stored wheat and rice grain in
1973 the laboratory. Report No. AR2457 AR,submitted to FAO by ICI
Plant Protection Ltd, (Unpublished).
Bratt, H. & Jones, L.A. Pirimiphos-methyl: Metabolism in rats and
1973 dogs. Report submitted to FAO by ICI Industrial Hygiene
Research Laboratories. (Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain. Report
1973 No. AR2472 AR submitted to FAO by ICI Plant Protection Ltd.
(Unpublished).
Bullock, D.J.W. Pirimiphos-methyl: Residues in stored grain, bread,
1974 flour and milled products. Report submitted to FAO by ICI
Plant Protection Ltd. (Unpublished).
Bullock, D.J.W. In Analytical Methods for Pesticides and Plant
Growth
1976 Regulators, VIII, 185, G. Zweig Ed., Academic Press, New
York.
Bullock, D.J.W. & May, M.S. Pirimiphos-methyl: Residue transfer from
1976 durum wheat to semolina and pasta. Report No. TMJI345A
submitted to FAO by ICI Plant Protection Ltd. (Unpublished).
Bullock, D.J.W., Harrison, P.J. & Day, S.R. Degradation of residues in
1976 flour during baking. Report No. AR2666A submitted to FAO by
ICI Plant Protection Ltd. (Unpublished).
Cerna, V. & Benes, V. Residues of pirimiphos-methyl in wheat, mill
1977 products and white bread. Report by Czechoslovakian
Institute of Hygiene in Epidemiology, Prague.
Chawla, R.P. & Bindra, O.S. (1971). Residues of some promising grain
1971 protectants on stored wheat. Proc. Symp. Progress and
Problems in Pesticide Residue Analysis (Bindra & Kalra,
Eds). Punjab Agric. Univ. Ludhiana & Indian Counc. Agric.
Res., New Delhi. p. 68-74.
Curl, E.A. & Leary, J.P. The radioactive residues found in the liver
1980 and kidney of a goat after administration of a multiple dose
of pirimiphos-methyl. Report RJO144B submitted to FAO by ICI
Plant Protection Ltd.
Desmarchelier, J.M. Selective treatments including combinations of
1977 pyrethroid and organophosphorus insecticides for control of
stored product Coleoptera at two temperatures. J. Stored
Prod. Res., 13: 129-137.
Desmarchelier, J.M. Loss of fenitrothion on grains in storage. Pestic.
1978 Sci. 9: 33-38.
Desmarchelier, J.M. & Bengston, M. The residual behaviour of chemicals
1979 in stored grain. Proceedings of the Second International
Working Conference in Stored-Product Entomology, Ibadan,
Nigeria, 10-16 September 1979.
Desmarchelier, J.M., Bengston, M., Connell, M., Henning, R., Ridley,
in E., Ripp, E., Sierakowski, C., Sticka, R., Snelson, J.
press & Wilson, A. Extensive pilot usage of the grain protectant
combinations, fenitrothion plus bioresmethrin and
pirimiphos-methyl plus bioresmethrin. J. Pestic. Sci.
Desmarchelier, J.M., Goldring, M. & Horan, R. Predicted and observed
1980a residues of bio-resmethrin, carbaryl, fenitrothion,
phenothrin, methacrifos and pirimiphos-methyl on rice and
barley after storage, and losses of these insecticides
during processing. J. Pestic. Sci., 5: 539-545.
Desmarchelier, J.M., Bengston, M., Connell, M., Minett, W., Moore, B.,
1980b Phillips, M., Snelson, J., Sticka, R. & Tucker, K. A
collaborative study of residues on wheat of methacrifos,
chloropyrifos-methyl, fenitrothion, malathion and
pirimiphos-methyl. II. Rates of decay. CSIRO, Division of
Entomology Report No. 20. 21 p.
Kadoum, A.M. & La Hue, D.W. Penetration of malathion in stored corn,
1974 wheat and sorghum grain. J. Econ. Entomol., 67(4): 477-478.
Krasnykh, A.A. Khim. Sel'sk. Khoz. 16, (11) 36 (Russ).
1978
La Hue, D.W. Pesticide residues of potential protectants of grain.
1974 Proc. 1st Int. Working Conf. on Stored Product Entomology,
Savannah, Georgia, USA. p. 635-638.
La Hue, D.W. Pirimiphos-methyl: Effect on populations of Tribolium
1977 confusum and T. castaneum in wheat. J. Econ. Entomol.,
70(1): 135-137.
La Hue, D.W. & Dicke, E.B. Evaluation of selected insecticides applied
1976 to high moisture sorghum grain to prevent stored grain
insect attack. USDA Agricultural Research Service, Marketing
Research Report No. 1063.
Le Berre, R., Escaffre, H., Pendriez, B., Grebaut, S. & Pengalet, P.
1972 Control of Simulium damnosum, the vector of human
onchocerciasus in West Africa II. Test by classic
application of new insecticides and new formulations, 70
Oncho du 8 Mai ORSTOMOCCGE, BP 171, Bobo-Dioulasso, Upper
Volta.
McCallum Deighton, J. Pirimiphos-methyl and other insecticides in the
1978 control of stored-product insects. Outlook Agric., 9(5)
240-248.
Mensah, G.W.K., Watters, F.L. & Webster, G.R.B. Insecticide residues
1979 in milled fractions of dry or tough wheat treated with
malathion, bromophos, iodofenphos and pirimiphos-methyl. J.
Econ. Entomol., 72: 728-731.
Mills, I.H. Pirimiphos-methyl: Blood concentrations and tissue
1976 retention in the rat. Report No. CTL/P/247 submitted to FAO
by ICI Central Toxicology. (Unpublished).
Morallo-Rejesus, B. & Carino F.O. The residual toxicity of five
1976 insecticides on three varieties of corn and sorghum.
Philipp. Agric., 60(3&4): 96-104.
Redlinger, L.M. & Simonaitis, R.A. Field tests with pirimiphos-methyl
1977 as a protectant for farmers stock peanuts. Peanut Sci.
4: 27.
Rishikesh, N., Mathis, H.L., King, J.S. & Nambiar, R.V. A field trial
1977 of pirimiphos-methyl for the control of Anopheles gambiae
and Anopheles funestus in Nigeria. WHO Geneva, VBC/77/671.
(Unpublished).
Rowlands, D.G. The metabolism of contact insecticides in stored grains
1975 1970-1974. Residue Rev., 58: 113.
Rowlands, D.G. The metabolism of pirimiphos-methyl by stored grains
1981 under laboratory conditions. Report No. 54, Pest Control
Chemistry Department, Pest Infestation Laboratory, Slough,
U.K. Ministry of Agriculture, Fisheries & Food.
Rowlands, D.G. et al. Preliminary report of studies with pirimiphos-
1974 methyl as a grain protectant. Submission by United Kingdom
to FAO.
Rowlands, D.G. & Wilkin, D.R. Metabolism of pesticides in stored
1975 cereals. Report of Director, Pest Infestation Control
Laboratory, Slough, U.K. 1971-1973.
Seth, A.K. Use of Actellic for stored rice insect problems in S.E.
1974 Asia. Symp. 1st Int. Working Conf. Stored Prod. Entomol.,
7-11 October 1974, Savannah, Georgia, USA.
Shaw, R.F., Fanara, D.M., Pradhan, G.D., Supratman, Supalin, Bang,
1979 Y.H., & Fleming, G.A. A village-scale trial of
pirimiphos-methyl (OMS-1424) for control of the malaria
vector (Anopheles aconitus) in Central Java, Indonesia.
WHO, Geneva, VBC/79/722. (Unpublished)
Simonaitis, R.A., Zehner, J.M. & Redlinger, L.M. J. HRC & CC 4: 169.
1981
Soderstrom, E.L. & Armstrong, J.W. pp-511: (Pirimiphos-methyl-ISO)
1973 evaluated as a protectant for raisins and walnuts. J. Econ.
Entomol., 66(6): 1305-1306.
Spain. Registered uses of pirimiphos-methyl. Data submitted to FAO by
1983 the Ministry of Agriculture, Madrid.
Spitler, G.H. & Hartsell, P.L. Pirimiphos-methyl as a protectant for
1975 stored inshell almonds. J. Econ. Entomol., 68(6): 777-780.
Sweden. Monitoring of imported fruits and vegetables for residues of
1983 pirimiphos-methyl. Data submitted to FAO by the Swedish
National Food Institute.
Tempone, M.J. Studies on the effects of insecticides on barley
1979 malting. Research Project Series No. 50, Victorian
Department of Agriculture, Melbourne, Australia.
Thomas, K.P. & Rowlands, D.G. The uptake and degradation of
1975 pirimiphos-methyl by Cheshire cheese. J. Stored Prod. Res.,
11:53.
Varca, L.M., Magallona, E.D. & Pasco, J.A. Philipp. Entomol., 3: 55.
1975
Weaving, A.J.S. Grain protectants for use under tribal storage
1975 conditions in Rhodesia - Comparative toxicities of some
insecticides on maize and sorghum. J. Stored Prod. Res.,
11: 65-71.
WHO. Interim specifications for pirimiphos-methyl, technical, water
1982 dispersible powder and emulsion concentrate VBC/15/82.04.
WHO Vector Biology Control Division, Geneva.
Zakitis, L.H. & McCray, E.M. Gas chromatographic analysis of residues
1982 of pirimiphos-methyl in water, fish and snails. Bull.
Environ. Contam. Toxicol., 28: 334-340.
