
ETHEPHON JMPR 1977
IDENTITY
Ethephon has been adopted as a common name by the American National
Standards Institute (ANSI), but not by the International Organization
for Standardization (ISO).
Chemical Name
2-chloroethylphosphonic acid.
Synonym
Ethrel (R)
Structural Formula
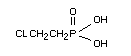 Chemical properties
a) State: White waxy solid
b) Melting point: 144.5°C
c) Solubility: very soluble in water, alcohol, acetone, propylene
glycol and other polar solvents
d) Flammability: non-flammable
Purity
Company request disclosure to be withheld.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
No toxicological information on ethephon was made available to the
1977 Joint Meeting. Therefore no toxicological evaluation could be
accomplished and no acceptable daily intake for humans was
established.
RESIDUES IN FOOD AND THEIR EVALUATION
TABLE 1. United States Registered Uses for Ethephon
Application Timing or
Crop Rate, kg,ha Purpose Pre-harvest interval
Apples 0.70-1.4 fruit loosening and 7-21 days
uniform ripening
Blackberries 2.8 " 3 days
Blueberries 2.24 uniform ripening -
Cantaloupes 0.84 fruit loosening -
Cherries 1.4 fruit loosening and 7-14
uniform ripening
Cranberries 1.68 uniform coloring -
Figs 1.12 uniform ripening, 14-21 days
Filberts 1.96 " -
Lemons 0.1 kg/hl spray degreening of fruit
(Postharvest)
Peppers 1.12 uniform ripening and coloring -
Tangerines 1.39 uniform coloring -
Tomatoes 1.83 uniform ripening -
Pineapples 6.72 flower bud development 6-8 months
1.12 uniform ripening and fruit 7 days
loosening
Walnuts 1.4 uniform ripening 5-10 days
USE PATTERN
The Meeting was informed that ethephon is used in the United States,
Canada, New Zealand, the United Kingdom, France, Australia and the
Netherlands as a growth regulator. United States Registered Uses are
shown in Table 1. Use patterns in other countries are generally
comparable.
In addition to the uses described in Table 1, the Meeting was informed
that the product is used on blackcurrants in the United Kingdom and on
glasshouse tomatoes in the Netherlands. Australia has under
consideration proposed uses for peaches, blackcurrants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were available from the United States (in summary form),
Canada, the Netherlands, the United Kingdom and France. Tables 2 and 3
summarize the residues found on a variety of commodities and their
by-products in the U.S.A. Pre-harvest intervals, concentration factors
and application rates are also given.
Table 4 contains a summary of residue values on blackcurrants from
residue trials conducted in the U.K. and reported to the Meeting. The
trials were carried out in the years 1969-1971.
In other trials from the U.K. in 1971 on apples, all residues were
reported as <0.5 mg/kg from spray concentrations of 750 mg/kg.
Samples were taken at intervals after treatment ranging from 3 to 16
days. At dosages of 1000 mg/kg and above, residues of about 1-3 mg/kg
were found on pears. No residues were detected on gooseberries or
tomatoes even on the day of treatment with spray concentration of 480
and 250 mg/kg respectively.
Supervised residue trials in France were reported to the Meeting. At
spray concentrations of 480 mg/kg, initial deposits on apples were
0.80 - 1.09 mg/kg and declined to 0.30 - 0.99 mg/kg on day 7. At 960
mg/kg spray concentration initial deposits were 0.60 - 1.34 mg/kg,
declining to 0.59 - 1.24 mg/kg on day 7.
French residue trials on tomatoes were carried out at several spray
concentrations (480, 720, and 960 mg/kg) and samples taken at
intervals from 0 to 28 days. The residues showed no tendency to
decline gradually in that period. There is apparently continuous
absorption and translocation into the fruit until foliar deposits are
exhausted or weathered, with peak residues occurring about the 10-15th
day. Overall, residues were in the range 0.07 - 0.65 mg/kg.
TABLE 2. Residues of ethephon in various commodities, resulting from supervised trials in USA
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Apples 0.37 1 3 - 13 0.16 to 2.3
0.74 1 3 - 13 0.48 to 4.5
0.56 to 2.8 1 3 - 19 0.12 to 4.5
Blackberries 1.12 to 2.8 (1x) 1 0 - 4 0.4 - 23.4
Blueberries 1.12 to 2.24 (1X) 1 7 - 14 0.9 - 12.9
Canteloupes 0.89 to 1.79 1 3 - 6 0.08 - 0.46
(1X = 0.84) 0.89 to 1.79 1 3 - 10 0.11 - 1.2
1.79 1 1 1.8
1.79 1 8 whole melon 0.21
rind 0.18
seeds 0.06
flesh 0.03
Cherries 0.70 -2.8 (2X) 1 6 - 14 0.69 - 12.2
0.58 -2.8 (2X) 1 6 - 10 <0.01 - 8.1
0.58 1 1 1.50 - 3.4
Cranberries 1.12 - 2.8 1 0 - 3 1.79 - 10.1
(1X = 1.68) 1.12 - 2.8 1 6 - 11 0.09 - 5.6
1.12 - 2.8 1 14 - 21 <0.01 -0.78
Figs 0.70 - 2.8 1 14 0.84 - 4.4
0.70 - 2.8 1 26 0.47 - 2.6
1.05 - 5.25(b) 1 15 - 41 0.22 - 0.47
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Filberts 0.69 - 1.87 1 20 - 39 <0.01 - 0.16
(1X 1.96) 2.8 -5.6 1 19 - 20 0.04 - 0.31
2.8 1 7 <0.01 - 0.01
1.87 - 2.06(c) 1 28 - 39 <0.01 - 0.14
2.8 - 4.2 (c) 1 7 0.04 - 0.07
5.6 1 7 0.13 - 0.20
Lemons (1x) 0.1 - 0.2 1 post-harvest 0.6 - 1.9
kg/hl spray
Peppers 0.84 - 1.4 1 5 - 15 0.20 - 13.5
0.84 -1.12 (1x) (d) 1 5 - 8 9.13 - 28.7
0.84 -1.12 (d) 1 15 <0.01 - 3.
Tangerines 0.90 - 1.80 (1.33X) 1 3 - 9 Peel 0.33 -0.72
flesh
<0.01 - 0.10e)
Tomatoes 0.478 - 2.24 1 3 - 10 0.08 - 1.7
(1X = 1.83 0.478 - 2.24 1 12 - 16 0.03 - 1.9
0.93 - 2.4 1 18 - 21 0.03 - 0.41
0.56 - 0.90 1 1 - 3 <0.01 - 0.47
0.56 - 0.90 1 4 - 6 0.02 - 1.2
0.56 - 0.90 1 12 - 16 0.11 - 0.29
1.23 - 1.88 1 8 - 10 0.18 - 1.2
1.23 - 1.88 1 14 0.29 - 1.1
Coffee Beans 120 - 240 mg/plant 1 13 - 30 <0.01 - 0.05
360 - 480 mg/plant 1 13 - 30 <0.01 - 0.07
600 - 960 (3x)mg/plant 1 13 - 30 <0.01 - 0.15
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Pineapples 2.24 -18 (2.5x) 1f) 6 months <0.05
0.56 1g) 7 fruit 0.05 - 0.3
shell & core 0.1 - 0.3
whole fruit 0.03 - 0.3h)
1.12(1X) 1g) 14 fruit 0.05 - 0.7
shell & core 0.2 - 1.8
whole fruit 0.1 - 1.2
1.56j) 2i) 7 flesh 0.03 - 0.16
1.12j) 2i) 7 flesh 0.04 - 0.43
2.24j) 2i) 7 flesh 0.08 - 0.59
1.12j) 2i) 7 shell 0.11 - 0.54
2.24j) 2i) 7 shell 0.31 - 0.44
Pineapple forage 1.12 3k) 7 0.80 - 2.19
and fodder 2.24 3k) 7 1.75 - 5.35
Walnuts 1.4 1 7 - 36 0.04 - 0.08
2.1 1 14 - 20 0.02 - 0.08
2.8 1 5 - 7 <0.01 - 0.57
0.84 1 7 <0.01 - 0.04
1.4 1 7 0.01 - 0.06
0.84 1) 1 7 <0.01 - 0.1
1.4 1) 1 7 <0.01 - 0.04
Apples
(Canada) 300 mg/kg spray 1 0 1.59
300 mg/kg spray 1 10 0.65
TABLE 2. (Continued)
a) Numbers in parenthesis refer to the maximum
registered application rate in the United States,
e.g. 2X = 2 times the maxim= registered rate
b) Dried figs
c) Dried filberts
d) Chili peppers
e) Assuming that 25% of the weight of the whole tangerine
is peel, the calculated level on the whole fruit from a
value of 0.72 mg/kg on the peel is 0.18 mg/kg
f) Bud flowering use
g) Pre-harvest use
h) Calculated or actual values
i) One bud-flowering and one pre-harvest treatment
j) Treated with 1.12, 2.24, or 4.48 kg/ha to promote bud
flowering 7 to 7 1/2 months prior to harvest then treated
at pre-harvest rates given
k) One 1.12, 2.24, or 4.48 kg/ha treatment 7 to 7 1/2 months
prior to harvest then one 2.24 kg/ha treatment 2 1/2 to 3 1/2
months prior to harvest then one treatment at pre-harvest
rates given
l) Dried walnuts
TABLE 3. Residues in feed by-products (supervised trials in USA)
Concentration factor
Crop By-product in by-product
Lemons Dried lemon pulp 1.2
molasses 1.5
Lemon juice & oil no concentration
Apples Dried Apple pomace no concentration
Tangerines Dried tangerine peel 1.6
Tangerine molasses 4.3
Pineapples Dried Pineapple bran no concentration
TABLE 4. Average residues, corrected for recovery, mg/kg, in blackcurrants
Time interval SPRAY CONCENTRATION, mg/kg
(days)
250 500 375 750
0 1.6,1.8,2.6 2.9,3.9,3.5
1.8 4.6
1 4.8,4.7,5.5 7.4,8.8,6.4
2 3.9,6.6 6.5,9.5
4.1 5.9
3 0.30 5.2,2.5 10.0,5.9
4 3.6,5.5,4.5 6.7,7.3,6.6
5 0.28,0.22 0.22,0.28
6 0.18 0.54
7 0.56,0.22 0.48,0.20 3.2,3.3 8.2,4.8
TABLE 5. Residues from supervised trials in the Netherlands
Crop Application No. Year Interval Residue
rate (days) (mg/kg)
Apples 0.67 1 1975 7 0.71-1.14
kg a.i./ha
0.94 1 1975 7 0.43-1.49
kg a.i./ha
Tomatoes 0.012 1 1977 3 1.1 -1.7
kg a.i./ha
" 1 " 7 1.0 -1.5
Blackcurrants 250 mg/kg 1 1969, 1970 7 0.20-0.56
500 mg/kg 1 1969, 1970 5-7 0.20-0.54
375 mg/kg 1 1971 0 1.6, 1.8
" " 1 " 1 4.8, 4.7
" " 1 " 2 3.9, 6.6
" " 1 " 4 3.6, 5.5
" " 1 " 7 3.2, 3.3
750 mg/kg 1 " 0 2.9, 3.9
" " 1 " 1 7.4, 8.8
" " 1 " 2 6.5, 9.5
" " 1 " 4 6.7, 7.3
" " 1 " 7 8.2, 4.8
Tomatoes 480 mg/kg 1 1974 0 0.07
" " 1 " 3 0.12
" " 1 " 7 0.44
" " 1 " 10 0.24
" " 1 " 15 0.32
" " 1 " 21 0.18
720 mg/kg 1 " 28 0.19
" " 1 " 0 0.08
" " 1 " 3 0.19
" " 1 " 7 0.27
" " 1 " 10 0.52
" " 1 " 15 0.55
" " 1 " 21 0.25
" " 1 " 28 0.23
A country statement from the Netherlands contained reports on residue
trials on currants, apples, tomatoes, pears, cherries and
gooseberries. The analytical results did not differ significantly from
those in the trials previously discussed. A summary of the typical
residues found is given in Table 5.
FATE OF RESIDUES
In animals
Metabolism studies using 14C-labelled ethephon were carried out on
the rat and dog. The data demonstrated that ethephon is rapidly
eliminated by both species. After 72 hours the rats and dogs retained
< 1% of the ingested radioactivity. In both species 40-68% of the
radioactivity was excreted as ethephon, 29-35% as ethylene, and less
than 0.1 to 0.5% as carbon dioxide. This metabolism pattern is
considered similar to that found in plants.
In plants
Radiotracer studies using 15C-labelled ethephon have been carried
out on grapes, walnuts, pineapples, cherries, tangerines, oranges,
tomatoes, filberts, figs, cantaloupes and apples. All of these studies
consistently demonstrate that ethephon translocates in plants and
exists in or on plants as the parent compound until degraded into
ethylene, phosphate, and chloride ion. Thus the terminal residue of
concern is the parent.
Other studies have indicated the presence of a very labile conjugate
of ethephon which produces the parent molecule under chromatographic
conditions.
In soils
Studies conducted on soils indicate the same pathway for degradation
found in animals and plants. When applied to the soil ethephon
released ethylene gas. The presence of soil organisms does not appear
to alter the pathway for degradation.
In processing and cooking
See "Evidence of residues in food in commerce or at consumption which
follows.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Ethephon is not detected by the multi-residue screening procedures
used in government market basket surveys and monitoring programmes and
therefore no data for residues of ethephon in commerce in the United
States are available.
A survey carried out in the Netherlands in 1975 showed that of some 68
samples of tomatoes analyzed (mainly greenhouse tomatoes), 34 samples
had non-detectable residues, 16 samples showed residues ranging from
0.05 to 1.0 mg/kg, and 18 samples had ethephon residues ranging from
1.1 to 8.1 mg/kg. No indication of whether the tomato samples were
washed was given. Similar results were obtained in a 1976 survey.
Degradation studies on pineapples, tomatoes, grapes etc., indicate
that washing, cooking or other preparation decreases the level of
ethephon found on a commodity and therefore there would be little
likelihood that ethephon residues would occur on a commodity at the
time of consumption. This information is also supported by the fact
that ethephon is not extremely stable.
METHODS OF RESIDUE ANALYSIS
Ethephon residues in crops are determined by a GLG method published in
PAM II (1974). The method involves freeze-drying the samples, soxhlet
extraction with methanol, concentrations, pH adjustment, precipitation
of interfering materials, esterification with diazomethane and GLC
analysis using a phosphrous-specific alkali thermionic detector. The
method determines the parent only and can be used on a number of
different crops.
This method had been tested in government laboratories on pineapple.
Depending on crop blanks the lower limit of detection is about
0.05 mg/kg.
A confirmatory procedure utilizing an alternative GLC column of
different polarity and a microcoulometric detector is also published
in PAM II (1974).
APPRAISAL
Ethephon is a growth regulator used to produce various beneficial
effects on a variety of fruits, nuts, and vegetables. Information
available to the Meeting indicated that national tolerances for
ethephon have been established. by the governments of the USA, New
Zealand, Netherlands, Canada and Australia.
Ethephon must be absorbed and translocated in plants or fruit in order
to produce the desired effects. Radio-tracer studies show that the
compound is metabolized in plants to ethylene and phosphate and
chloride ions. However, the parent compound itself is sufficiently
persistent to require guideline levels (until maximum residue limits
can be established) for fruits and vegetables in commerce. Animal
studies show that it is rapidly eliminated from the body.
Summaries of extensive residue trials in the USA submitted by the
basic manufacturer to the US Government were made available to the
Meeting. Country statements from Canada, the Netherlands, France and
the United Kingdom contained results of supervised trials which
generally agreed with the US findings. Additionally, the Netherlands
and the UK reported on residue trials on blackcurrants which were
adequate to support a recommendation for a guideline level for that
commodity.
National Tolerances Reported to the Meeting
Commodity United States New Zealand Netherlands Canada Australia
Apples 5 2 2 3
Blackberries 30
Blueberries 20
Cantaloupes 2
Cherries 10 1 15
Cherries, sour 1 2
Cherries, sweet 8
Coffee beans 0.1
Cranberries 5
Figs 5
Filberts 0.5
Lemons 2
Peppers 30
Pineapples 2
Pineapples 3 2
Fooder and
forage
Tangerines 0.5
Tangerine hybrids 0.5
Tomatoes 2 1 1 3 2
Walnuts 0.5
Ethephon residues are determined by a "specific" GLC method which has
been validated in government laboratories. The lower limit of
determination is about 0.05 mg/kg. it is not detected by any of the
multi-residue procedures used in the USA or Canadian Government
surveillance programmes. Surveillance programmes in the Netherlands
indicate that tomatoes in commerce may contain residues approaching 3
mg/kg from glasshouse use.
The necessary toxicological data required to allocate an ADI were not
available to the Meeting. However, information relating to national
use patterns, analytical methods and residue trials was sufficient to
set guideline levels.
EVALUATION
The following guideline levels for ethephon are recorded.
Commodity Guideline level, mg/kg Intervals on
which levels are
based (days)
peppers 30 0
blackberries 30 3
blueberries 20 7-14
cherries 10 7-14
apples 5 7-21
blackcurrants 5 0
cranberries 5 0
figs 5 14-21
tomatoes 3 0
pineapples 2 7
cantaloupes 2 0
lemons 2 (post-harvest)
filberts 0.5 0
tangerines 0.5 5-10
walnuts 0.5 5-10
coffee (beans) 0.1 -
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI) can be
established and maximum residue limits (MRL) can be recommended)
1. Submission of full toxicological data.
DESIRABLE
1. Country statements on national use patterns and supervised residue
trials, and information on occurrence of residues on commodities in
commerce.
REFERENCES
PAM II, (1974) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education, and Welfare, Food and Drug
Administration.
Chemical properties
a) State: White waxy solid
b) Melting point: 144.5°C
c) Solubility: very soluble in water, alcohol, acetone, propylene
glycol and other polar solvents
d) Flammability: non-flammable
Purity
Company request disclosure to be withheld.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
No toxicological information on ethephon was made available to the
1977 Joint Meeting. Therefore no toxicological evaluation could be
accomplished and no acceptable daily intake for humans was
established.
RESIDUES IN FOOD AND THEIR EVALUATION
TABLE 1. United States Registered Uses for Ethephon
Application Timing or
Crop Rate, kg,ha Purpose Pre-harvest interval
Apples 0.70-1.4 fruit loosening and 7-21 days
uniform ripening
Blackberries 2.8 " 3 days
Blueberries 2.24 uniform ripening -
Cantaloupes 0.84 fruit loosening -
Cherries 1.4 fruit loosening and 7-14
uniform ripening
Cranberries 1.68 uniform coloring -
Figs 1.12 uniform ripening, 14-21 days
Filberts 1.96 " -
Lemons 0.1 kg/hl spray degreening of fruit
(Postharvest)
Peppers 1.12 uniform ripening and coloring -
Tangerines 1.39 uniform coloring -
Tomatoes 1.83 uniform ripening -
Pineapples 6.72 flower bud development 6-8 months
1.12 uniform ripening and fruit 7 days
loosening
Walnuts 1.4 uniform ripening 5-10 days
USE PATTERN
The Meeting was informed that ethephon is used in the United States,
Canada, New Zealand, the United Kingdom, France, Australia and the
Netherlands as a growth regulator. United States Registered Uses are
shown in Table 1. Use patterns in other countries are generally
comparable.
In addition to the uses described in Table 1, the Meeting was informed
that the product is used on blackcurrants in the United Kingdom and on
glasshouse tomatoes in the Netherlands. Australia has under
consideration proposed uses for peaches, blackcurrants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were available from the United States (in summary form),
Canada, the Netherlands, the United Kingdom and France. Tables 2 and 3
summarize the residues found on a variety of commodities and their
by-products in the U.S.A. Pre-harvest intervals, concentration factors
and application rates are also given.
Table 4 contains a summary of residue values on blackcurrants from
residue trials conducted in the U.K. and reported to the Meeting. The
trials were carried out in the years 1969-1971.
In other trials from the U.K. in 1971 on apples, all residues were
reported as <0.5 mg/kg from spray concentrations of 750 mg/kg.
Samples were taken at intervals after treatment ranging from 3 to 16
days. At dosages of 1000 mg/kg and above, residues of about 1-3 mg/kg
were found on pears. No residues were detected on gooseberries or
tomatoes even on the day of treatment with spray concentration of 480
and 250 mg/kg respectively.
Supervised residue trials in France were reported to the Meeting. At
spray concentrations of 480 mg/kg, initial deposits on apples were
0.80 - 1.09 mg/kg and declined to 0.30 - 0.99 mg/kg on day 7. At 960
mg/kg spray concentration initial deposits were 0.60 - 1.34 mg/kg,
declining to 0.59 - 1.24 mg/kg on day 7.
French residue trials on tomatoes were carried out at several spray
concentrations (480, 720, and 960 mg/kg) and samples taken at
intervals from 0 to 28 days. The residues showed no tendency to
decline gradually in that period. There is apparently continuous
absorption and translocation into the fruit until foliar deposits are
exhausted or weathered, with peak residues occurring about the 10-15th
day. Overall, residues were in the range 0.07 - 0.65 mg/kg.
TABLE 2. Residues of ethephon in various commodities, resulting from supervised trials in USA
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Apples 0.37 1 3 - 13 0.16 to 2.3
0.74 1 3 - 13 0.48 to 4.5
0.56 to 2.8 1 3 - 19 0.12 to 4.5
Blackberries 1.12 to 2.8 (1x) 1 0 - 4 0.4 - 23.4
Blueberries 1.12 to 2.24 (1X) 1 7 - 14 0.9 - 12.9
Canteloupes 0.89 to 1.79 1 3 - 6 0.08 - 0.46
(1X = 0.84) 0.89 to 1.79 1 3 - 10 0.11 - 1.2
1.79 1 1 1.8
1.79 1 8 whole melon 0.21
rind 0.18
seeds 0.06
flesh 0.03
Cherries 0.70 -2.8 (2X) 1 6 - 14 0.69 - 12.2
0.58 -2.8 (2X) 1 6 - 10 <0.01 - 8.1
0.58 1 1 1.50 - 3.4
Cranberries 1.12 - 2.8 1 0 - 3 1.79 - 10.1
(1X = 1.68) 1.12 - 2.8 1 6 - 11 0.09 - 5.6
1.12 - 2.8 1 14 - 21 <0.01 -0.78
Figs 0.70 - 2.8 1 14 0.84 - 4.4
0.70 - 2.8 1 26 0.47 - 2.6
1.05 - 5.25(b) 1 15 - 41 0.22 - 0.47
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Filberts 0.69 - 1.87 1 20 - 39 <0.01 - 0.16
(1X 1.96) 2.8 -5.6 1 19 - 20 0.04 - 0.31
2.8 1 7 <0.01 - 0.01
1.87 - 2.06(c) 1 28 - 39 <0.01 - 0.14
2.8 - 4.2 (c) 1 7 0.04 - 0.07
5.6 1 7 0.13 - 0.20
Lemons (1x) 0.1 - 0.2 1 post-harvest 0.6 - 1.9
kg/hl spray
Peppers 0.84 - 1.4 1 5 - 15 0.20 - 13.5
0.84 -1.12 (1x) (d) 1 5 - 8 9.13 - 28.7
0.84 -1.12 (d) 1 15 <0.01 - 3.
Tangerines 0.90 - 1.80 (1.33X) 1 3 - 9 Peel 0.33 -0.72
flesh
<0.01 - 0.10e)
Tomatoes 0.478 - 2.24 1 3 - 10 0.08 - 1.7
(1X = 1.83 0.478 - 2.24 1 12 - 16 0.03 - 1.9
0.93 - 2.4 1 18 - 21 0.03 - 0.41
0.56 - 0.90 1 1 - 3 <0.01 - 0.47
0.56 - 0.90 1 4 - 6 0.02 - 1.2
0.56 - 0.90 1 12 - 16 0.11 - 0.29
1.23 - 1.88 1 8 - 10 0.18 - 1.2
1.23 - 1.88 1 14 0.29 - 1.1
Coffee Beans 120 - 240 mg/plant 1 13 - 30 <0.01 - 0.05
360 - 480 mg/plant 1 13 - 30 <0.01 - 0.07
600 - 960 (3x)mg/plant 1 13 - 30 <0.01 - 0.15
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Pineapples 2.24 -18 (2.5x) 1f) 6 months <0.05
0.56 1g) 7 fruit 0.05 - 0.3
shell & core 0.1 - 0.3
whole fruit 0.03 - 0.3h)
1.12(1X) 1g) 14 fruit 0.05 - 0.7
shell & core 0.2 - 1.8
whole fruit 0.1 - 1.2
1.56j) 2i) 7 flesh 0.03 - 0.16
1.12j) 2i) 7 flesh 0.04 - 0.43
2.24j) 2i) 7 flesh 0.08 - 0.59
1.12j) 2i) 7 shell 0.11 - 0.54
2.24j) 2i) 7 shell 0.31 - 0.44
Pineapple forage 1.12 3k) 7 0.80 - 2.19
and fodder 2.24 3k) 7 1.75 - 5.35
Walnuts 1.4 1 7 - 36 0.04 - 0.08
2.1 1 14 - 20 0.02 - 0.08
2.8 1 5 - 7 <0.01 - 0.57
0.84 1 7 <0.01 - 0.04
1.4 1 7 0.01 - 0.06
0.84 1) 1 7 <0.01 - 0.1
1.4 1) 1 7 <0.01 - 0.04
Apples
(Canada) 300 mg/kg spray 1 0 1.59
300 mg/kg spray 1 10 0.65
TABLE 2. (Continued)
a) Numbers in parenthesis refer to the maximum
registered application rate in the United States,
e.g. 2X = 2 times the maxim= registered rate
b) Dried figs
c) Dried filberts
d) Chili peppers
e) Assuming that 25% of the weight of the whole tangerine
is peel, the calculated level on the whole fruit from a
value of 0.72 mg/kg on the peel is 0.18 mg/kg
f) Bud flowering use
g) Pre-harvest use
h) Calculated or actual values
i) One bud-flowering and one pre-harvest treatment
j) Treated with 1.12, 2.24, or 4.48 kg/ha to promote bud
flowering 7 to 7 1/2 months prior to harvest then treated
at pre-harvest rates given
k) One 1.12, 2.24, or 4.48 kg/ha treatment 7 to 7 1/2 months
prior to harvest then one 2.24 kg/ha treatment 2 1/2 to 3 1/2
months prior to harvest then one treatment at pre-harvest
rates given
l) Dried walnuts
TABLE 3. Residues in feed by-products (supervised trials in USA)
Concentration factor
Crop By-product in by-product
Lemons Dried lemon pulp 1.2
molasses 1.5
Lemon juice & oil no concentration
Apples Dried Apple pomace no concentration
Tangerines Dried tangerine peel 1.6
Tangerine molasses 4.3
Pineapples Dried Pineapple bran no concentration
TABLE 4. Average residues, corrected for recovery, mg/kg, in blackcurrants
Time interval SPRAY CONCENTRATION, mg/kg
(days)
250 500 375 750
0 1.6,1.8,2.6 2.9,3.9,3.5
1.8 4.6
1 4.8,4.7,5.5 7.4,8.8,6.4
2 3.9,6.6 6.5,9.5
4.1 5.9
3 0.30 5.2,2.5 10.0,5.9
4 3.6,5.5,4.5 6.7,7.3,6.6
5 0.28,0.22 0.22,0.28
6 0.18 0.54
7 0.56,0.22 0.48,0.20 3.2,3.3 8.2,4.8
TABLE 5. Residues from supervised trials in the Netherlands
Crop Application No. Year Interval Residue
rate (days) (mg/kg)
Apples 0.67 1 1975 7 0.71-1.14
kg a.i./ha
0.94 1 1975 7 0.43-1.49
kg a.i./ha
Tomatoes 0.012 1 1977 3 1.1 -1.7
kg a.i./ha
" 1 " 7 1.0 -1.5
Blackcurrants 250 mg/kg 1 1969, 1970 7 0.20-0.56
500 mg/kg 1 1969, 1970 5-7 0.20-0.54
375 mg/kg 1 1971 0 1.6, 1.8
" " 1 " 1 4.8, 4.7
" " 1 " 2 3.9, 6.6
" " 1 " 4 3.6, 5.5
" " 1 " 7 3.2, 3.3
750 mg/kg 1 " 0 2.9, 3.9
" " 1 " 1 7.4, 8.8
" " 1 " 2 6.5, 9.5
" " 1 " 4 6.7, 7.3
" " 1 " 7 8.2, 4.8
Tomatoes 480 mg/kg 1 1974 0 0.07
" " 1 " 3 0.12
" " 1 " 7 0.44
" " 1 " 10 0.24
" " 1 " 15 0.32
" " 1 " 21 0.18
720 mg/kg 1 " 28 0.19
" " 1 " 0 0.08
" " 1 " 3 0.19
" " 1 " 7 0.27
" " 1 " 10 0.52
" " 1 " 15 0.55
" " 1 " 21 0.25
" " 1 " 28 0.23
A country statement from the Netherlands contained reports on residue
trials on currants, apples, tomatoes, pears, cherries and
gooseberries. The analytical results did not differ significantly from
those in the trials previously discussed. A summary of the typical
residues found is given in Table 5.
FATE OF RESIDUES
In animals
Metabolism studies using 14C-labelled ethephon were carried out on
the rat and dog. The data demonstrated that ethephon is rapidly
eliminated by both species. After 72 hours the rats and dogs retained
< 1% of the ingested radioactivity. In both species 40-68% of the
radioactivity was excreted as ethephon, 29-35% as ethylene, and less
than 0.1 to 0.5% as carbon dioxide. This metabolism pattern is
considered similar to that found in plants.
In plants
Radiotracer studies using 15C-labelled ethephon have been carried
out on grapes, walnuts, pineapples, cherries, tangerines, oranges,
tomatoes, filberts, figs, cantaloupes and apples. All of these studies
consistently demonstrate that ethephon translocates in plants and
exists in or on plants as the parent compound until degraded into
ethylene, phosphate, and chloride ion. Thus the terminal residue of
concern is the parent.
Other studies have indicated the presence of a very labile conjugate
of ethephon which produces the parent molecule under chromatographic
conditions.
In soils
Studies conducted on soils indicate the same pathway for degradation
found in animals and plants. When applied to the soil ethephon
released ethylene gas. The presence of soil organisms does not appear
to alter the pathway for degradation.
In processing and cooking
See "Evidence of residues in food in commerce or at consumption which
follows.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Ethephon is not detected by the multi-residue screening procedures
used in government market basket surveys and monitoring programmes and
therefore no data for residues of ethephon in commerce in the United
States are available.
A survey carried out in the Netherlands in 1975 showed that of some 68
samples of tomatoes analyzed (mainly greenhouse tomatoes), 34 samples
had non-detectable residues, 16 samples showed residues ranging from
0.05 to 1.0 mg/kg, and 18 samples had ethephon residues ranging from
1.1 to 8.1 mg/kg. No indication of whether the tomato samples were
washed was given. Similar results were obtained in a 1976 survey.
Degradation studies on pineapples, tomatoes, grapes etc., indicate
that washing, cooking or other preparation decreases the level of
ethephon found on a commodity and therefore there would be little
likelihood that ethephon residues would occur on a commodity at the
time of consumption. This information is also supported by the fact
that ethephon is not extremely stable.
METHODS OF RESIDUE ANALYSIS
Ethephon residues in crops are determined by a GLG method published in
PAM II (1974). The method involves freeze-drying the samples, soxhlet
extraction with methanol, concentrations, pH adjustment, precipitation
of interfering materials, esterification with diazomethane and GLC
analysis using a phosphrous-specific alkali thermionic detector. The
method determines the parent only and can be used on a number of
different crops.
This method had been tested in government laboratories on pineapple.
Depending on crop blanks the lower limit of detection is about
0.05 mg/kg.
A confirmatory procedure utilizing an alternative GLC column of
different polarity and a microcoulometric detector is also published
in PAM II (1974).
APPRAISAL
Ethephon is a growth regulator used to produce various beneficial
effects on a variety of fruits, nuts, and vegetables. Information
available to the Meeting indicated that national tolerances for
ethephon have been established. by the governments of the USA, New
Zealand, Netherlands, Canada and Australia.
Ethephon must be absorbed and translocated in plants or fruit in order
to produce the desired effects. Radio-tracer studies show that the
compound is metabolized in plants to ethylene and phosphate and
chloride ions. However, the parent compound itself is sufficiently
persistent to require guideline levels (until maximum residue limits
can be established) for fruits and vegetables in commerce. Animal
studies show that it is rapidly eliminated from the body.
Summaries of extensive residue trials in the USA submitted by the
basic manufacturer to the US Government were made available to the
Meeting. Country statements from Canada, the Netherlands, France and
the United Kingdom contained results of supervised trials which
generally agreed with the US findings. Additionally, the Netherlands
and the UK reported on residue trials on blackcurrants which were
adequate to support a recommendation for a guideline level for that
commodity.
National Tolerances Reported to the Meeting
Commodity United States New Zealand Netherlands Canada Australia
Apples 5 2 2 3
Blackberries 30
Blueberries 20
Cantaloupes 2
Cherries 10 1 15
Cherries, sour 1 2
Cherries, sweet 8
Coffee beans 0.1
Cranberries 5
Figs 5
Filberts 0.5
Lemons 2
Peppers 30
Pineapples 2
Pineapples 3 2
Fooder and
forage
Tangerines 0.5
Tangerine hybrids 0.5
Tomatoes 2 1 1 3 2
Walnuts 0.5
Ethephon residues are determined by a "specific" GLC method which has
been validated in government laboratories. The lower limit of
determination is about 0.05 mg/kg. it is not detected by any of the
multi-residue procedures used in the USA or Canadian Government
surveillance programmes. Surveillance programmes in the Netherlands
indicate that tomatoes in commerce may contain residues approaching 3
mg/kg from glasshouse use.
The necessary toxicological data required to allocate an ADI were not
available to the Meeting. However, information relating to national
use patterns, analytical methods and residue trials was sufficient to
set guideline levels.
EVALUATION
The following guideline levels for ethephon are recorded.
Commodity Guideline level, mg/kg Intervals on
which levels are
based (days)
peppers 30 0
blackberries 30 3
blueberries 20 7-14
cherries 10 7-14
apples 5 7-21
blackcurrants 5 0
cranberries 5 0
figs 5 14-21
tomatoes 3 0
pineapples 2 7
cantaloupes 2 0
lemons 2 (post-harvest)
filberts 0.5 0
tangerines 0.5 5-10
walnuts 0.5 5-10
coffee (beans) 0.1 -
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI) can be
established and maximum residue limits (MRL) can be recommended)
1. Submission of full toxicological data.
DESIRABLE
1. Country statements on national use patterns and supervised residue
trials, and information on occurrence of residues on commodities in
commerce.
REFERENCES
PAM II, (1974) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education, and Welfare, Food and Drug
Administration.
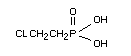 Chemical properties
a) State: White waxy solid
b) Melting point: 144.5°C
c) Solubility: very soluble in water, alcohol, acetone, propylene
glycol and other polar solvents
d) Flammability: non-flammable
Purity
Company request disclosure to be withheld.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
No toxicological information on ethephon was made available to the
1977 Joint Meeting. Therefore no toxicological evaluation could be
accomplished and no acceptable daily intake for humans was
established.
RESIDUES IN FOOD AND THEIR EVALUATION
TABLE 1. United States Registered Uses for Ethephon
Application Timing or
Crop Rate, kg,ha Purpose Pre-harvest interval
Apples 0.70-1.4 fruit loosening and 7-21 days
uniform ripening
Blackberries 2.8 " 3 days
Blueberries 2.24 uniform ripening -
Cantaloupes 0.84 fruit loosening -
Cherries 1.4 fruit loosening and 7-14
uniform ripening
Cranberries 1.68 uniform coloring -
Figs 1.12 uniform ripening, 14-21 days
Filberts 1.96 " -
Lemons 0.1 kg/hl spray degreening of fruit
(Postharvest)
Peppers 1.12 uniform ripening and coloring -
Tangerines 1.39 uniform coloring -
Tomatoes 1.83 uniform ripening -
Pineapples 6.72 flower bud development 6-8 months
1.12 uniform ripening and fruit 7 days
loosening
Walnuts 1.4 uniform ripening 5-10 days
USE PATTERN
The Meeting was informed that ethephon is used in the United States,
Canada, New Zealand, the United Kingdom, France, Australia and the
Netherlands as a growth regulator. United States Registered Uses are
shown in Table 1. Use patterns in other countries are generally
comparable.
In addition to the uses described in Table 1, the Meeting was informed
that the product is used on blackcurrants in the United Kingdom and on
glasshouse tomatoes in the Netherlands. Australia has under
consideration proposed uses for peaches, blackcurrants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were available from the United States (in summary form),
Canada, the Netherlands, the United Kingdom and France. Tables 2 and 3
summarize the residues found on a variety of commodities and their
by-products in the U.S.A. Pre-harvest intervals, concentration factors
and application rates are also given.
Table 4 contains a summary of residue values on blackcurrants from
residue trials conducted in the U.K. and reported to the Meeting. The
trials were carried out in the years 1969-1971.
In other trials from the U.K. in 1971 on apples, all residues were
reported as <0.5 mg/kg from spray concentrations of 750 mg/kg.
Samples were taken at intervals after treatment ranging from 3 to 16
days. At dosages of 1000 mg/kg and above, residues of about 1-3 mg/kg
were found on pears. No residues were detected on gooseberries or
tomatoes even on the day of treatment with spray concentration of 480
and 250 mg/kg respectively.
Supervised residue trials in France were reported to the Meeting. At
spray concentrations of 480 mg/kg, initial deposits on apples were
0.80 - 1.09 mg/kg and declined to 0.30 - 0.99 mg/kg on day 7. At 960
mg/kg spray concentration initial deposits were 0.60 - 1.34 mg/kg,
declining to 0.59 - 1.24 mg/kg on day 7.
French residue trials on tomatoes were carried out at several spray
concentrations (480, 720, and 960 mg/kg) and samples taken at
intervals from 0 to 28 days. The residues showed no tendency to
decline gradually in that period. There is apparently continuous
absorption and translocation into the fruit until foliar deposits are
exhausted or weathered, with peak residues occurring about the 10-15th
day. Overall, residues were in the range 0.07 - 0.65 mg/kg.
TABLE 2. Residues of ethephon in various commodities, resulting from supervised trials in USA
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Apples 0.37 1 3 - 13 0.16 to 2.3
0.74 1 3 - 13 0.48 to 4.5
0.56 to 2.8 1 3 - 19 0.12 to 4.5
Blackberries 1.12 to 2.8 (1x) 1 0 - 4 0.4 - 23.4
Blueberries 1.12 to 2.24 (1X) 1 7 - 14 0.9 - 12.9
Canteloupes 0.89 to 1.79 1 3 - 6 0.08 - 0.46
(1X = 0.84) 0.89 to 1.79 1 3 - 10 0.11 - 1.2
1.79 1 1 1.8
1.79 1 8 whole melon 0.21
rind 0.18
seeds 0.06
flesh 0.03
Cherries 0.70 -2.8 (2X) 1 6 - 14 0.69 - 12.2
0.58 -2.8 (2X) 1 6 - 10 <0.01 - 8.1
0.58 1 1 1.50 - 3.4
Cranberries 1.12 - 2.8 1 0 - 3 1.79 - 10.1
(1X = 1.68) 1.12 - 2.8 1 6 - 11 0.09 - 5.6
1.12 - 2.8 1 14 - 21 <0.01 -0.78
Figs 0.70 - 2.8 1 14 0.84 - 4.4
0.70 - 2.8 1 26 0.47 - 2.6
1.05 - 5.25(b) 1 15 - 41 0.22 - 0.47
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Filberts 0.69 - 1.87 1 20 - 39 <0.01 - 0.16
(1X 1.96) 2.8 -5.6 1 19 - 20 0.04 - 0.31
2.8 1 7 <0.01 - 0.01
1.87 - 2.06(c) 1 28 - 39 <0.01 - 0.14
2.8 - 4.2 (c) 1 7 0.04 - 0.07
5.6 1 7 0.13 - 0.20
Lemons (1x) 0.1 - 0.2 1 post-harvest 0.6 - 1.9
kg/hl spray
Peppers 0.84 - 1.4 1 5 - 15 0.20 - 13.5
0.84 -1.12 (1x) (d) 1 5 - 8 9.13 - 28.7
0.84 -1.12 (d) 1 15 <0.01 - 3.
Tangerines 0.90 - 1.80 (1.33X) 1 3 - 9 Peel 0.33 -0.72
flesh
<0.01 - 0.10e)
Tomatoes 0.478 - 2.24 1 3 - 10 0.08 - 1.7
(1X = 1.83 0.478 - 2.24 1 12 - 16 0.03 - 1.9
0.93 - 2.4 1 18 - 21 0.03 - 0.41
0.56 - 0.90 1 1 - 3 <0.01 - 0.47
0.56 - 0.90 1 4 - 6 0.02 - 1.2
0.56 - 0.90 1 12 - 16 0.11 - 0.29
1.23 - 1.88 1 8 - 10 0.18 - 1.2
1.23 - 1.88 1 14 0.29 - 1.1
Coffee Beans 120 - 240 mg/plant 1 13 - 30 <0.01 - 0.05
360 - 480 mg/plant 1 13 - 30 <0.01 - 0.07
600 - 960 (3x)mg/plant 1 13 - 30 <0.01 - 0.15
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Pineapples 2.24 -18 (2.5x) 1f) 6 months <0.05
0.56 1g) 7 fruit 0.05 - 0.3
shell & core 0.1 - 0.3
whole fruit 0.03 - 0.3h)
1.12(1X) 1g) 14 fruit 0.05 - 0.7
shell & core 0.2 - 1.8
whole fruit 0.1 - 1.2
1.56j) 2i) 7 flesh 0.03 - 0.16
1.12j) 2i) 7 flesh 0.04 - 0.43
2.24j) 2i) 7 flesh 0.08 - 0.59
1.12j) 2i) 7 shell 0.11 - 0.54
2.24j) 2i) 7 shell 0.31 - 0.44
Pineapple forage 1.12 3k) 7 0.80 - 2.19
and fodder 2.24 3k) 7 1.75 - 5.35
Walnuts 1.4 1 7 - 36 0.04 - 0.08
2.1 1 14 - 20 0.02 - 0.08
2.8 1 5 - 7 <0.01 - 0.57
0.84 1 7 <0.01 - 0.04
1.4 1 7 0.01 - 0.06
0.84 1) 1 7 <0.01 - 0.1
1.4 1) 1 7 <0.01 - 0.04
Apples
(Canada) 300 mg/kg spray 1 0 1.59
300 mg/kg spray 1 10 0.65
TABLE 2. (Continued)
a) Numbers in parenthesis refer to the maximum
registered application rate in the United States,
e.g. 2X = 2 times the maxim= registered rate
b) Dried figs
c) Dried filberts
d) Chili peppers
e) Assuming that 25% of the weight of the whole tangerine
is peel, the calculated level on the whole fruit from a
value of 0.72 mg/kg on the peel is 0.18 mg/kg
f) Bud flowering use
g) Pre-harvest use
h) Calculated or actual values
i) One bud-flowering and one pre-harvest treatment
j) Treated with 1.12, 2.24, or 4.48 kg/ha to promote bud
flowering 7 to 7 1/2 months prior to harvest then treated
at pre-harvest rates given
k) One 1.12, 2.24, or 4.48 kg/ha treatment 7 to 7 1/2 months
prior to harvest then one 2.24 kg/ha treatment 2 1/2 to 3 1/2
months prior to harvest then one treatment at pre-harvest
rates given
l) Dried walnuts
TABLE 3. Residues in feed by-products (supervised trials in USA)
Concentration factor
Crop By-product in by-product
Lemons Dried lemon pulp 1.2
molasses 1.5
Lemon juice & oil no concentration
Apples Dried Apple pomace no concentration
Tangerines Dried tangerine peel 1.6
Tangerine molasses 4.3
Pineapples Dried Pineapple bran no concentration
TABLE 4. Average residues, corrected for recovery, mg/kg, in blackcurrants
Time interval SPRAY CONCENTRATION, mg/kg
(days)
250 500 375 750
0 1.6,1.8,2.6 2.9,3.9,3.5
1.8 4.6
1 4.8,4.7,5.5 7.4,8.8,6.4
2 3.9,6.6 6.5,9.5
4.1 5.9
3 0.30 5.2,2.5 10.0,5.9
4 3.6,5.5,4.5 6.7,7.3,6.6
5 0.28,0.22 0.22,0.28
6 0.18 0.54
7 0.56,0.22 0.48,0.20 3.2,3.3 8.2,4.8
TABLE 5. Residues from supervised trials in the Netherlands
Crop Application No. Year Interval Residue
rate (days) (mg/kg)
Apples 0.67 1 1975 7 0.71-1.14
kg a.i./ha
0.94 1 1975 7 0.43-1.49
kg a.i./ha
Tomatoes 0.012 1 1977 3 1.1 -1.7
kg a.i./ha
" 1 " 7 1.0 -1.5
Blackcurrants 250 mg/kg 1 1969, 1970 7 0.20-0.56
500 mg/kg 1 1969, 1970 5-7 0.20-0.54
375 mg/kg 1 1971 0 1.6, 1.8
" " 1 " 1 4.8, 4.7
" " 1 " 2 3.9, 6.6
" " 1 " 4 3.6, 5.5
" " 1 " 7 3.2, 3.3
750 mg/kg 1 " 0 2.9, 3.9
" " 1 " 1 7.4, 8.8
" " 1 " 2 6.5, 9.5
" " 1 " 4 6.7, 7.3
" " 1 " 7 8.2, 4.8
Tomatoes 480 mg/kg 1 1974 0 0.07
" " 1 " 3 0.12
" " 1 " 7 0.44
" " 1 " 10 0.24
" " 1 " 15 0.32
" " 1 " 21 0.18
720 mg/kg 1 " 28 0.19
" " 1 " 0 0.08
" " 1 " 3 0.19
" " 1 " 7 0.27
" " 1 " 10 0.52
" " 1 " 15 0.55
" " 1 " 21 0.25
" " 1 " 28 0.23
A country statement from the Netherlands contained reports on residue
trials on currants, apples, tomatoes, pears, cherries and
gooseberries. The analytical results did not differ significantly from
those in the trials previously discussed. A summary of the typical
residues found is given in Table 5.
FATE OF RESIDUES
In animals
Metabolism studies using 14C-labelled ethephon were carried out on
the rat and dog. The data demonstrated that ethephon is rapidly
eliminated by both species. After 72 hours the rats and dogs retained
< 1% of the ingested radioactivity. In both species 40-68% of the
radioactivity was excreted as ethephon, 29-35% as ethylene, and less
than 0.1 to 0.5% as carbon dioxide. This metabolism pattern is
considered similar to that found in plants.
In plants
Radiotracer studies using 15C-labelled ethephon have been carried
out on grapes, walnuts, pineapples, cherries, tangerines, oranges,
tomatoes, filberts, figs, cantaloupes and apples. All of these studies
consistently demonstrate that ethephon translocates in plants and
exists in or on plants as the parent compound until degraded into
ethylene, phosphate, and chloride ion. Thus the terminal residue of
concern is the parent.
Other studies have indicated the presence of a very labile conjugate
of ethephon which produces the parent molecule under chromatographic
conditions.
In soils
Studies conducted on soils indicate the same pathway for degradation
found in animals and plants. When applied to the soil ethephon
released ethylene gas. The presence of soil organisms does not appear
to alter the pathway for degradation.
In processing and cooking
See "Evidence of residues in food in commerce or at consumption which
follows.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Ethephon is not detected by the multi-residue screening procedures
used in government market basket surveys and monitoring programmes and
therefore no data for residues of ethephon in commerce in the United
States are available.
A survey carried out in the Netherlands in 1975 showed that of some 68
samples of tomatoes analyzed (mainly greenhouse tomatoes), 34 samples
had non-detectable residues, 16 samples showed residues ranging from
0.05 to 1.0 mg/kg, and 18 samples had ethephon residues ranging from
1.1 to 8.1 mg/kg. No indication of whether the tomato samples were
washed was given. Similar results were obtained in a 1976 survey.
Degradation studies on pineapples, tomatoes, grapes etc., indicate
that washing, cooking or other preparation decreases the level of
ethephon found on a commodity and therefore there would be little
likelihood that ethephon residues would occur on a commodity at the
time of consumption. This information is also supported by the fact
that ethephon is not extremely stable.
METHODS OF RESIDUE ANALYSIS
Ethephon residues in crops are determined by a GLG method published in
PAM II (1974). The method involves freeze-drying the samples, soxhlet
extraction with methanol, concentrations, pH adjustment, precipitation
of interfering materials, esterification with diazomethane and GLC
analysis using a phosphrous-specific alkali thermionic detector. The
method determines the parent only and can be used on a number of
different crops.
This method had been tested in government laboratories on pineapple.
Depending on crop blanks the lower limit of detection is about
0.05 mg/kg.
A confirmatory procedure utilizing an alternative GLC column of
different polarity and a microcoulometric detector is also published
in PAM II (1974).
APPRAISAL
Ethephon is a growth regulator used to produce various beneficial
effects on a variety of fruits, nuts, and vegetables. Information
available to the Meeting indicated that national tolerances for
ethephon have been established. by the governments of the USA, New
Zealand, Netherlands, Canada and Australia.
Ethephon must be absorbed and translocated in plants or fruit in order
to produce the desired effects. Radio-tracer studies show that the
compound is metabolized in plants to ethylene and phosphate and
chloride ions. However, the parent compound itself is sufficiently
persistent to require guideline levels (until maximum residue limits
can be established) for fruits and vegetables in commerce. Animal
studies show that it is rapidly eliminated from the body.
Summaries of extensive residue trials in the USA submitted by the
basic manufacturer to the US Government were made available to the
Meeting. Country statements from Canada, the Netherlands, France and
the United Kingdom contained results of supervised trials which
generally agreed with the US findings. Additionally, the Netherlands
and the UK reported on residue trials on blackcurrants which were
adequate to support a recommendation for a guideline level for that
commodity.
National Tolerances Reported to the Meeting
Commodity United States New Zealand Netherlands Canada Australia
Apples 5 2 2 3
Blackberries 30
Blueberries 20
Cantaloupes 2
Cherries 10 1 15
Cherries, sour 1 2
Cherries, sweet 8
Coffee beans 0.1
Cranberries 5
Figs 5
Filberts 0.5
Lemons 2
Peppers 30
Pineapples 2
Pineapples 3 2
Fooder and
forage
Tangerines 0.5
Tangerine hybrids 0.5
Tomatoes 2 1 1 3 2
Walnuts 0.5
Ethephon residues are determined by a "specific" GLC method which has
been validated in government laboratories. The lower limit of
determination is about 0.05 mg/kg. it is not detected by any of the
multi-residue procedures used in the USA or Canadian Government
surveillance programmes. Surveillance programmes in the Netherlands
indicate that tomatoes in commerce may contain residues approaching 3
mg/kg from glasshouse use.
The necessary toxicological data required to allocate an ADI were not
available to the Meeting. However, information relating to national
use patterns, analytical methods and residue trials was sufficient to
set guideline levels.
EVALUATION
The following guideline levels for ethephon are recorded.
Commodity Guideline level, mg/kg Intervals on
which levels are
based (days)
peppers 30 0
blackberries 30 3
blueberries 20 7-14
cherries 10 7-14
apples 5 7-21
blackcurrants 5 0
cranberries 5 0
figs 5 14-21
tomatoes 3 0
pineapples 2 7
cantaloupes 2 0
lemons 2 (post-harvest)
filberts 0.5 0
tangerines 0.5 5-10
walnuts 0.5 5-10
coffee (beans) 0.1 -
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI) can be
established and maximum residue limits (MRL) can be recommended)
1. Submission of full toxicological data.
DESIRABLE
1. Country statements on national use patterns and supervised residue
trials, and information on occurrence of residues on commodities in
commerce.
REFERENCES
PAM II, (1974) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education, and Welfare, Food and Drug
Administration.
Chemical properties
a) State: White waxy solid
b) Melting point: 144.5°C
c) Solubility: very soluble in water, alcohol, acetone, propylene
glycol and other polar solvents
d) Flammability: non-flammable
Purity
Company request disclosure to be withheld.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
No toxicological information on ethephon was made available to the
1977 Joint Meeting. Therefore no toxicological evaluation could be
accomplished and no acceptable daily intake for humans was
established.
RESIDUES IN FOOD AND THEIR EVALUATION
TABLE 1. United States Registered Uses for Ethephon
Application Timing or
Crop Rate, kg,ha Purpose Pre-harvest interval
Apples 0.70-1.4 fruit loosening and 7-21 days
uniform ripening
Blackberries 2.8 " 3 days
Blueberries 2.24 uniform ripening -
Cantaloupes 0.84 fruit loosening -
Cherries 1.4 fruit loosening and 7-14
uniform ripening
Cranberries 1.68 uniform coloring -
Figs 1.12 uniform ripening, 14-21 days
Filberts 1.96 " -
Lemons 0.1 kg/hl spray degreening of fruit
(Postharvest)
Peppers 1.12 uniform ripening and coloring -
Tangerines 1.39 uniform coloring -
Tomatoes 1.83 uniform ripening -
Pineapples 6.72 flower bud development 6-8 months
1.12 uniform ripening and fruit 7 days
loosening
Walnuts 1.4 uniform ripening 5-10 days
USE PATTERN
The Meeting was informed that ethephon is used in the United States,
Canada, New Zealand, the United Kingdom, France, Australia and the
Netherlands as a growth regulator. United States Registered Uses are
shown in Table 1. Use patterns in other countries are generally
comparable.
In addition to the uses described in Table 1, the Meeting was informed
that the product is used on blackcurrants in the United Kingdom and on
glasshouse tomatoes in the Netherlands. Australia has under
consideration proposed uses for peaches, blackcurrants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were available from the United States (in summary form),
Canada, the Netherlands, the United Kingdom and France. Tables 2 and 3
summarize the residues found on a variety of commodities and their
by-products in the U.S.A. Pre-harvest intervals, concentration factors
and application rates are also given.
Table 4 contains a summary of residue values on blackcurrants from
residue trials conducted in the U.K. and reported to the Meeting. The
trials were carried out in the years 1969-1971.
In other trials from the U.K. in 1971 on apples, all residues were
reported as <0.5 mg/kg from spray concentrations of 750 mg/kg.
Samples were taken at intervals after treatment ranging from 3 to 16
days. At dosages of 1000 mg/kg and above, residues of about 1-3 mg/kg
were found on pears. No residues were detected on gooseberries or
tomatoes even on the day of treatment with spray concentration of 480
and 250 mg/kg respectively.
Supervised residue trials in France were reported to the Meeting. At
spray concentrations of 480 mg/kg, initial deposits on apples were
0.80 - 1.09 mg/kg and declined to 0.30 - 0.99 mg/kg on day 7. At 960
mg/kg spray concentration initial deposits were 0.60 - 1.34 mg/kg,
declining to 0.59 - 1.24 mg/kg on day 7.
French residue trials on tomatoes were carried out at several spray
concentrations (480, 720, and 960 mg/kg) and samples taken at
intervals from 0 to 28 days. The residues showed no tendency to
decline gradually in that period. There is apparently continuous
absorption and translocation into the fruit until foliar deposits are
exhausted or weathered, with peak residues occurring about the 10-15th
day. Overall, residues were in the range 0.07 - 0.65 mg/kg.
TABLE 2. Residues of ethephon in various commodities, resulting from supervised trials in USA
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Apples 0.37 1 3 - 13 0.16 to 2.3
0.74 1 3 - 13 0.48 to 4.5
0.56 to 2.8 1 3 - 19 0.12 to 4.5
Blackberries 1.12 to 2.8 (1x) 1 0 - 4 0.4 - 23.4
Blueberries 1.12 to 2.24 (1X) 1 7 - 14 0.9 - 12.9
Canteloupes 0.89 to 1.79 1 3 - 6 0.08 - 0.46
(1X = 0.84) 0.89 to 1.79 1 3 - 10 0.11 - 1.2
1.79 1 1 1.8
1.79 1 8 whole melon 0.21
rind 0.18
seeds 0.06
flesh 0.03
Cherries 0.70 -2.8 (2X) 1 6 - 14 0.69 - 12.2
0.58 -2.8 (2X) 1 6 - 10 <0.01 - 8.1
0.58 1 1 1.50 - 3.4
Cranberries 1.12 - 2.8 1 0 - 3 1.79 - 10.1
(1X = 1.68) 1.12 - 2.8 1 6 - 11 0.09 - 5.6
1.12 - 2.8 1 14 - 21 <0.01 -0.78
Figs 0.70 - 2.8 1 14 0.84 - 4.4
0.70 - 2.8 1 26 0.47 - 2.6
1.05 - 5.25(b) 1 15 - 41 0.22 - 0.47
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Filberts 0.69 - 1.87 1 20 - 39 <0.01 - 0.16
(1X 1.96) 2.8 -5.6 1 19 - 20 0.04 - 0.31
2.8 1 7 <0.01 - 0.01
1.87 - 2.06(c) 1 28 - 39 <0.01 - 0.14
2.8 - 4.2 (c) 1 7 0.04 - 0.07
5.6 1 7 0.13 - 0.20
Lemons (1x) 0.1 - 0.2 1 post-harvest 0.6 - 1.9
kg/hl spray
Peppers 0.84 - 1.4 1 5 - 15 0.20 - 13.5
0.84 -1.12 (1x) (d) 1 5 - 8 9.13 - 28.7
0.84 -1.12 (d) 1 15 <0.01 - 3.
Tangerines 0.90 - 1.80 (1.33X) 1 3 - 9 Peel 0.33 -0.72
flesh
<0.01 - 0.10e)
Tomatoes 0.478 - 2.24 1 3 - 10 0.08 - 1.7
(1X = 1.83 0.478 - 2.24 1 12 - 16 0.03 - 1.9
0.93 - 2.4 1 18 - 21 0.03 - 0.41
0.56 - 0.90 1 1 - 3 <0.01 - 0.47
0.56 - 0.90 1 4 - 6 0.02 - 1.2
0.56 - 0.90 1 12 - 16 0.11 - 0.29
1.23 - 1.88 1 8 - 10 0.18 - 1.2
1.23 - 1.88 1 14 0.29 - 1.1
Coffee Beans 120 - 240 mg/plant 1 13 - 30 <0.01 - 0.05
360 - 480 mg/plant 1 13 - 30 <0.01 - 0.07
600 - 960 (3x)mg/plant 1 13 - 30 <0.01 - 0.15
TABLE 2. (Continued)
Crop Application rate, No. of Pre-harvest Residue range
a.i. kg/haa) treatments interval (days) (mg/kg)
Pineapples 2.24 -18 (2.5x) 1f) 6 months <0.05
0.56 1g) 7 fruit 0.05 - 0.3
shell & core 0.1 - 0.3
whole fruit 0.03 - 0.3h)
1.12(1X) 1g) 14 fruit 0.05 - 0.7
shell & core 0.2 - 1.8
whole fruit 0.1 - 1.2
1.56j) 2i) 7 flesh 0.03 - 0.16
1.12j) 2i) 7 flesh 0.04 - 0.43
2.24j) 2i) 7 flesh 0.08 - 0.59
1.12j) 2i) 7 shell 0.11 - 0.54
2.24j) 2i) 7 shell 0.31 - 0.44
Pineapple forage 1.12 3k) 7 0.80 - 2.19
and fodder 2.24 3k) 7 1.75 - 5.35
Walnuts 1.4 1 7 - 36 0.04 - 0.08
2.1 1 14 - 20 0.02 - 0.08
2.8 1 5 - 7 <0.01 - 0.57
0.84 1 7 <0.01 - 0.04
1.4 1 7 0.01 - 0.06
0.84 1) 1 7 <0.01 - 0.1
1.4 1) 1 7 <0.01 - 0.04
Apples
(Canada) 300 mg/kg spray 1 0 1.59
300 mg/kg spray 1 10 0.65
TABLE 2. (Continued)
a) Numbers in parenthesis refer to the maximum
registered application rate in the United States,
e.g. 2X = 2 times the maxim= registered rate
b) Dried figs
c) Dried filberts
d) Chili peppers
e) Assuming that 25% of the weight of the whole tangerine
is peel, the calculated level on the whole fruit from a
value of 0.72 mg/kg on the peel is 0.18 mg/kg
f) Bud flowering use
g) Pre-harvest use
h) Calculated or actual values
i) One bud-flowering and one pre-harvest treatment
j) Treated with 1.12, 2.24, or 4.48 kg/ha to promote bud
flowering 7 to 7 1/2 months prior to harvest then treated
at pre-harvest rates given
k) One 1.12, 2.24, or 4.48 kg/ha treatment 7 to 7 1/2 months
prior to harvest then one 2.24 kg/ha treatment 2 1/2 to 3 1/2
months prior to harvest then one treatment at pre-harvest
rates given
l) Dried walnuts
TABLE 3. Residues in feed by-products (supervised trials in USA)
Concentration factor
Crop By-product in by-product
Lemons Dried lemon pulp 1.2
molasses 1.5
Lemon juice & oil no concentration
Apples Dried Apple pomace no concentration
Tangerines Dried tangerine peel 1.6
Tangerine molasses 4.3
Pineapples Dried Pineapple bran no concentration
TABLE 4. Average residues, corrected for recovery, mg/kg, in blackcurrants
Time interval SPRAY CONCENTRATION, mg/kg
(days)
250 500 375 750
0 1.6,1.8,2.6 2.9,3.9,3.5
1.8 4.6
1 4.8,4.7,5.5 7.4,8.8,6.4
2 3.9,6.6 6.5,9.5
4.1 5.9
3 0.30 5.2,2.5 10.0,5.9
4 3.6,5.5,4.5 6.7,7.3,6.6
5 0.28,0.22 0.22,0.28
6 0.18 0.54
7 0.56,0.22 0.48,0.20 3.2,3.3 8.2,4.8
TABLE 5. Residues from supervised trials in the Netherlands
Crop Application No. Year Interval Residue
rate (days) (mg/kg)
Apples 0.67 1 1975 7 0.71-1.14
kg a.i./ha
0.94 1 1975 7 0.43-1.49
kg a.i./ha
Tomatoes 0.012 1 1977 3 1.1 -1.7
kg a.i./ha
" 1 " 7 1.0 -1.5
Blackcurrants 250 mg/kg 1 1969, 1970 7 0.20-0.56
500 mg/kg 1 1969, 1970 5-7 0.20-0.54
375 mg/kg 1 1971 0 1.6, 1.8
" " 1 " 1 4.8, 4.7
" " 1 " 2 3.9, 6.6
" " 1 " 4 3.6, 5.5
" " 1 " 7 3.2, 3.3
750 mg/kg 1 " 0 2.9, 3.9
" " 1 " 1 7.4, 8.8
" " 1 " 2 6.5, 9.5
" " 1 " 4 6.7, 7.3
" " 1 " 7 8.2, 4.8
Tomatoes 480 mg/kg 1 1974 0 0.07
" " 1 " 3 0.12
" " 1 " 7 0.44
" " 1 " 10 0.24
" " 1 " 15 0.32
" " 1 " 21 0.18
720 mg/kg 1 " 28 0.19
" " 1 " 0 0.08
" " 1 " 3 0.19
" " 1 " 7 0.27
" " 1 " 10 0.52
" " 1 " 15 0.55
" " 1 " 21 0.25
" " 1 " 28 0.23
A country statement from the Netherlands contained reports on residue
trials on currants, apples, tomatoes, pears, cherries and
gooseberries. The analytical results did not differ significantly from
those in the trials previously discussed. A summary of the typical
residues found is given in Table 5.
FATE OF RESIDUES
In animals
Metabolism studies using 14C-labelled ethephon were carried out on
the rat and dog. The data demonstrated that ethephon is rapidly
eliminated by both species. After 72 hours the rats and dogs retained
< 1% of the ingested radioactivity. In both species 40-68% of the
radioactivity was excreted as ethephon, 29-35% as ethylene, and less
than 0.1 to 0.5% as carbon dioxide. This metabolism pattern is
considered similar to that found in plants.
In plants
Radiotracer studies using 15C-labelled ethephon have been carried
out on grapes, walnuts, pineapples, cherries, tangerines, oranges,
tomatoes, filberts, figs, cantaloupes and apples. All of these studies
consistently demonstrate that ethephon translocates in plants and
exists in or on plants as the parent compound until degraded into
ethylene, phosphate, and chloride ion. Thus the terminal residue of
concern is the parent.
Other studies have indicated the presence of a very labile conjugate
of ethephon which produces the parent molecule under chromatographic
conditions.
In soils
Studies conducted on soils indicate the same pathway for degradation
found in animals and plants. When applied to the soil ethephon
released ethylene gas. The presence of soil organisms does not appear
to alter the pathway for degradation.
In processing and cooking
See "Evidence of residues in food in commerce or at consumption which
follows.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Ethephon is not detected by the multi-residue screening procedures
used in government market basket surveys and monitoring programmes and
therefore no data for residues of ethephon in commerce in the United
States are available.
A survey carried out in the Netherlands in 1975 showed that of some 68
samples of tomatoes analyzed (mainly greenhouse tomatoes), 34 samples
had non-detectable residues, 16 samples showed residues ranging from
0.05 to 1.0 mg/kg, and 18 samples had ethephon residues ranging from
1.1 to 8.1 mg/kg. No indication of whether the tomato samples were
washed was given. Similar results were obtained in a 1976 survey.
Degradation studies on pineapples, tomatoes, grapes etc., indicate
that washing, cooking or other preparation decreases the level of
ethephon found on a commodity and therefore there would be little
likelihood that ethephon residues would occur on a commodity at the
time of consumption. This information is also supported by the fact
that ethephon is not extremely stable.
METHODS OF RESIDUE ANALYSIS
Ethephon residues in crops are determined by a GLG method published in
PAM II (1974). The method involves freeze-drying the samples, soxhlet
extraction with methanol, concentrations, pH adjustment, precipitation
of interfering materials, esterification with diazomethane and GLC
analysis using a phosphrous-specific alkali thermionic detector. The
method determines the parent only and can be used on a number of
different crops.
This method had been tested in government laboratories on pineapple.
Depending on crop blanks the lower limit of detection is about
0.05 mg/kg.
A confirmatory procedure utilizing an alternative GLC column of
different polarity and a microcoulometric detector is also published
in PAM II (1974).
APPRAISAL
Ethephon is a growth regulator used to produce various beneficial
effects on a variety of fruits, nuts, and vegetables. Information
available to the Meeting indicated that national tolerances for
ethephon have been established. by the governments of the USA, New
Zealand, Netherlands, Canada and Australia.
Ethephon must be absorbed and translocated in plants or fruit in order
to produce the desired effects. Radio-tracer studies show that the
compound is metabolized in plants to ethylene and phosphate and
chloride ions. However, the parent compound itself is sufficiently
persistent to require guideline levels (until maximum residue limits
can be established) for fruits and vegetables in commerce. Animal
studies show that it is rapidly eliminated from the body.
Summaries of extensive residue trials in the USA submitted by the
basic manufacturer to the US Government were made available to the
Meeting. Country statements from Canada, the Netherlands, France and
the United Kingdom contained results of supervised trials which
generally agreed with the US findings. Additionally, the Netherlands
and the UK reported on residue trials on blackcurrants which were
adequate to support a recommendation for a guideline level for that
commodity.
National Tolerances Reported to the Meeting
Commodity United States New Zealand Netherlands Canada Australia
Apples 5 2 2 3
Blackberries 30
Blueberries 20
Cantaloupes 2
Cherries 10 1 15
Cherries, sour 1 2
Cherries, sweet 8
Coffee beans 0.1
Cranberries 5
Figs 5
Filberts 0.5
Lemons 2
Peppers 30
Pineapples 2
Pineapples 3 2
Fooder and
forage
Tangerines 0.5
Tangerine hybrids 0.5
Tomatoes 2 1 1 3 2
Walnuts 0.5
Ethephon residues are determined by a "specific" GLC method which has
been validated in government laboratories. The lower limit of
determination is about 0.05 mg/kg. it is not detected by any of the
multi-residue procedures used in the USA or Canadian Government
surveillance programmes. Surveillance programmes in the Netherlands
indicate that tomatoes in commerce may contain residues approaching 3
mg/kg from glasshouse use.
The necessary toxicological data required to allocate an ADI were not
available to the Meeting. However, information relating to national
use patterns, analytical methods and residue trials was sufficient to
set guideline levels.
EVALUATION
The following guideline levels for ethephon are recorded.
Commodity Guideline level, mg/kg Intervals on
which levels are
based (days)
peppers 30 0
blackberries 30 3
blueberries 20 7-14
cherries 10 7-14
apples 5 7-21
blackcurrants 5 0
cranberries 5 0
figs 5 14-21
tomatoes 3 0
pineapples 2 7
cantaloupes 2 0
lemons 2 (post-harvest)
filberts 0.5 0
tangerines 0.5 5-10
walnuts 0.5 5-10
coffee (beans) 0.1 -
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI) can be
established and maximum residue limits (MRL) can be recommended)
1. Submission of full toxicological data.
DESIRABLE
1. Country statements on national use patterns and supervised residue
trials, and information on occurrence of residues on commodities in
commerce.
REFERENCES
PAM II, (1974) Pesticide Analytical Manual, Vol. II, revised; U.S.
Department of Health, Education, and Welfare, Food and Drug
Administration.
