
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
PHENOTHRIN
IDENTITY
Chemical Name: 3-phenoxybenzyl
(±)-cis-trans-chrystanthemate
Synonyms: 3-2539, Wellcide (R)
Structural Formula: C23H26O3
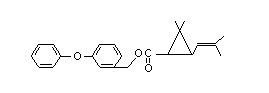 Other information on identity and properties
Molecular weight: 350.5
State: colorless liquid
Specific gravity: d25 1.058
25
Viscosity: 195.1 cp at 20°C
87.4 cp at 30°C
Vapour pressure: 1.2 × 10-6 mmHg at 20°c
Refractive index: n25 1.5483
D
Solubility:
(g/l at room Highly soluble in the following solvents
temperature) solvents (>500 g/l methanol, isopropanol,
ethylcellosolve, diethyl ether, xylene,
n-hexane alpha-methyl, naphthalene,
cyclohexane, chloroform, acetonitrile,
dimethyl formamide, kerosene.
Solubility in water: 1.4 ± 0.1 ppm at 30°C
Stability: No breakdown after one year at room
temperature out of the light. Stable in
neutral and weak acidic conditions. Stable
in most solvents except methanol, ethyl
cellosolve, o-cresol, dimethylsulfoxide.
Typical percent purity of the technical material
% by weight
3-phenoxy (±)-cis, trans-chrysanthemate 92.5 - 94.5
The meeting was also provided by the manufacturer with complete
information on the chemical nature and quantity of the residual
manufacturing impurities in the technical material. This information
is on file with the Secretariat of the meeting. The data in this
monograph are from phenothrin meeting these specifications as are the
recommendations for residue limits.
RESIDUES IN FOOD AND THEIR EVALUATIONS
Use Pattern
Phenothrin is a new pyrethroid being currently developed. It is more
stable than natural pyrethrins and other novel pyrethroids developed
earlier, but it is relatively degradable by sunlight. In the light of
its insecticidal properties and its relatively low toxicity to
mammals, phenothrin and its optically active isomers show much promise
as a safe and useful insecticide in various fields including
households, public health, food storage, ornamentals and livestock
production.
Principal uses currently are:
1. in household aerosols and sprays formulated in combination with
tetramethrin, piperonyl butoxide, carbamates and/or fenitrothion
for control of various species of plant hoppers and for control of
house-flies, mosquitoes, cockroaches and other household or public
health insect pests.
2. in grain protection and disinfestation.
Treatment of plants
Repeated and multiple applications of organophosphorus compounds (e.g.
malathion) and carbamates (e.g. carbaryl) for control of green rice
leaf hoppers in paddy fields have resulted in resistant strains in
some parts of Japan. Several efforts have been made to solve the
problem.
Yoshioka and others reported laboratory and field trials in which not
only susceptible but also resistant leaf hoppers and other hoppers
were controlled by the treatment of 30 to 40 kg/ha of combined dust
formulations containing various compounds including small amounts of
phenothrin (e.g. 0.8% by wt) and carbamates such as BPMC,
O-sec-buthylphenyl-N-methyl carbamate, MTMC, m-tolyl-N-methyl
carbamate and MPMC, 3,4-xylyl-N-methyl carbamate (e.g. 2.0% by wt).
It is expected these formulations will be used for control of hoppers
within the next one or two years.
Phenothrin has advantages of a short residual life on food plants,
quick insecticidal action and efficient control of pest strains
resistant to organochlorine and organophosphorus insecticides. It is
currently being developed for controlling various pests on a range of
fruit and vegetables.
Post-harvest use
Phenothrin offers advantages of improved cost/efficiency (of
pyrethrins and bioresmethrin), reliable availability and a high degree
of mammalian safety as a potential grain protectant. Phenothrin
applied at 2 mg/kg has a similar level of efficiency as commercial
applications of bioresmethrin and pyrethrins in the control of insects
resistant to chlorinated hydrocarbon and/or organophosphorus grain
protectants.
The pesticide is stable in grain masses, but is subject to degradation
on exposure to ultraviolet light (Nambu et al., 1979b).
In a series of practical commercial trials undertaken in Australia and
involving exposures to various insects known to be resistant to other
commonly-used pesticides, the potentialities of phenothrin for the
protection of grains in store were well demonstrated (Desmarchelier
et al., 1979; Bengston, et al., 1978; Working Party of Grain
Protectants, 1979 a and b).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatment
Supervised trials on rice, green peppers and cabbage have been carried
out in Japan. Replicated results are available from each crop
evaluated (Takimoto et al., 1977). The results are given in Table 1.
Post-harvest treatment
All trials on wheat, sorghum and barley grains were carried out in
Australia except one in Japan under laboratory conditions with
C-14-labelled (+)-trans and (+)-cis phenothrin. Most of the
Australian trials involved samples from commercial storages of up to
7,435 tonnes treated with phenothrin on the proviso that the grain was
ultimately used only as animal feed-stuff. The temperature ranged
from 18°C to 36°C and the moisture from 9.0% to 13.0%. Applications
were made with phenothrin or d-phenothrin mostly in combination with
piperonyl butoxide and/or fenitrothion. The application rates were
mostly up to 3.0 mg/kg, but on wheat grain phenothrin was applied at
up to 8 mg/kg. Wheat grains were stored for up to 9 months, barley
for up to 5 months and sorghum up to 6 months, and samples withdrawn
for residue analysis at each indicated times. The residues of
phenothrin and d-phenothrin are shown in Tables 2 and 3 respectively.
Relatively high amounts of phenothrin and d-phenothrin were detected
after 5-9 months in those grains. For example, in some cases more
than half of the applied phenothrin and d-phenothrin remained in grain
even after 9 months. In the experiment with C-14-labelled
(+)-trans- and (+)-cis phenothrin, only slight decomposition of
both isomers was found months after treatment.
Distribution of C-14-labelled (+)-trans and (+)-cis phenothrin in
germ, endosperm and seed coat was examined after 0, 1, 3 and 6 months
of storage at 15°C and 30°C (Nambu et al. 1979b). The results are
presented in Table 4. Most of the applied isomers were localized at
the seed coat and the distribution pattern did not change materially
during 6 month storage. Those results were evidenced by
radioautograms.
Table 1. Residues of phenothrin in rice, green peppers and cabbage
(Tests undertaken in Japan using a 25% EC formulation (Takimoto et al, 1977)
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Rice Straw Hulled rice
(0.375) 6 3-10 7 0.62 0.016
14 0.45 0.009
Rice Straw Hulled rice
(0.375) 6 6-9 7 1.25 0.078
14 1.58 0.040
Green pepper
(0.50) 3 7 1 0.324
3 0.660
7 0.334
6 7 1 1.038
3 0.625
7 0.428
(0.50) 3 7 1 0.333
3 0.268
7 0.469
6 7 1 0.363
3 0.445
7 0.244
Cabbage
(0.375) 4 7 3 <0.005
7 <0.005
14 <0.005
21 <0.005
Table 1. Continued...
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Cabbage 8 7 3 0.005
(cont'd) 7 0.017
14 <0.005
21 <0.005
Cabbage
(0.375) 5 7-8 3 <0.005
7 <0.005
14 <0.005
21 <0.005
9 6-8 3 0.011
7 <0.005
14 <0.005
21 <0.005
Table 2. Residues of phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1977) 6* 3 4.4
6 4.4
9 3.2,3.2** Welcome
8* 3 6.7 Australasia,
6 6.9 1977
9 5.8, 5.2**
4* 3 3.0
6 2.7
9 1.6,2.8**
(1977/78) 1.9+ 0.25 1.8
1.5 1.42 Working Party
3 1.45 on Grain
4.5 1.71 Protectants,
6 1.38 1978
9 1.1
(1978) 1.85+ 3 1.29
4.5 1.3
6 1.1
7 1.21
(1978) 2.08+ 1 1.21
2.00+ 1 1.56
2.00+ 1 1.23 Working Party
1.89+ 1 1.23 on Grain
2.1+ 1 1.50 Protectants,
2.02+ 1 1.30 1979a
2.29+ 1 1.30
Wheat
(1979) 1.49+ 1 1.85,1.28**
2 1.35
3 1.75
4 1.75
2.01+ 1 2.25,1.62**
2 1.8
3 2.05, 1.45**
4 1.6
Table 2. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
(1979) 2.01+ 20 days 2.3
48 days 1.9
75 days 2.3 Working Party
81 days 1.6 on Grain
1.49+ 24 days 1.9 Protectants,
55 days 1.5 1979b
79 days 1.9
83 days 1.8
Sorghum
(1978) 2.0+ 1 week 1.86
12 weeks 2.72 Bengston
17 weeks 1.59 1978
26 weeks 1.56
* Nominal application
** Residues were determined in two different laboratories from the
same sample
+ Rate of application calculated from total grams phenothrin used
divided by tons of grain treated.
Table 3. Residues of d-phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.7
2 0.9
3 1.1
5 1.0
6 0.5
8 1.1
1.7+ 1 2.4
2 2.1
3 1.4
4 1.0
5 1.1
6 0.9 Desmarchelier
7 0.9 et al., 1979c
8 1.0
9 1.0
1.9+ 1 1.6
2 0.8
4 0.8
5 1.0
6 0.5
8 0.6
9 0.6
2.0+ 1 1.5
2 1.7
5 1.6
2.0+ 1 0.7
3 1.0
1.8+ 1 1.5
2 1.3
4 0.7
6 0.7
1.4+ 1 1.5
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.3
2 1.3
4 1.1 Desmarchelier
5 1.1 et al., 1979c
6 0.9
7 0.8
9 0.8
1.9+ 1 0.9
2 0.7
3 0.8
6 0.8
2.1+ 1 1.0
3 0.7
4 1.1
5 0.9
2.0+ 1 1.0
3 1.0
4 0.9
5 1.5 Desmarchelier
7 1.5 et al., 1979c
2.6+ 2 1.3
3 0.6
6 1.1
2.2+ 2 1.0
3 0.8
5 0.8
6 1.2
4 1/2 3.87*,
3.93** Nambu et al,
1 3.80, 1979b
3.90
(+)-trans-
phenothrin 3 3.78, 3.80
6 3.72, 3.80
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 4 1/2 3.77*, 3.80**
1 3.84, 3.94
(+)-trans
phenothrin 3 3.87, 3.80
6 3.69, 3.66
4 1/2 3.68*, 3.67**
1 3.82, 3.78
(+)-trans
phenothrin 3 3.88,
3.80 Nambu et al.
6 3.69, 1979b
3.66
4 1/2 3.85*, 3.88**
1 3.80, 4.00
(+)-cis-
phenothrin 3 3.80, 3.93
6 3.73, 3.80
4 1/2 3.82*, 3.99**
1 4.03, 3.95
(+)-cis-
phenothrin 3 3.88, 4.02
6 3.84, 3.55
4 1/2 3.90*, 4.10**
1 4.03, 3.83
(+)-cis-
phenothrin 3 3.91, 3.89
6 3.76, 3.72
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Barley
(1979/79) 2.6+ 1 2.0
3 1.1
5 1.5
Desmarchelier
et al, 1979c
2.9+ 2 1.5
3 1.4
4 1.1
* Grain was stored at the temperature of 15°C
** Grain was stored at the temperature of 30°C
+ Rate of application calculated from total grams
phenothrin used divided by tons of grain treated.
Table 4. Distribution of (+)-trans and (+)-cis phenothrin after storage of treated grain
(Nambu et al., 1979b)
Compound Temperature Months Residue (mg/kg)
Whole grain Germ Endosperm Seed coat
(+)-trans 15°C 0 3.81 0.08 0.62 2.82
-phenothrin
(4 mg/kg) 1 3.80 0.1 0.64 2.86
3 3.78 0.09 0.63 3.09
6 3.72 0.08 0.51 2.97
30°C 1 3.90 0.12 0.77 2.95
3 3.80 0.11 0.69 2.97
6 3.80 0.12 0.58 3.06
(+)-trans
-phenothrin 15°C 6 3.69 0.10 0.62 2.94
+ PBO 30°C 6 3.66 0.10 0.60 2.65
-Fenitrothion
(4+20+4,
mg/kg)
(+)-cis-
phenothrin 15°C 0 3.85 0.09 0.67 3.14
(4 mg/kg) 1 3.80 0.08 0.70 3.08
3 3.80 0.08 0.64 3.11
6 3.73 0.11 0.62 2.63
30°C 1 4.00 0.08 0.80 3.02
3 3.93 0.12 0.68 3.28
6 3.80 0.14 0.58 2.75
(+)-cis
phenothrin 15°C 6 3.76 0.12 0.58 2.92
+ PBO 30°C 6 3.72 0.10 0.59 2.79
+ Fenitrothion
(4+20+4
mg/kg)
PBO: Technical piperonyl butoxide
FATE OF RESIDUES
In animals
(+)-trans-phenothrin labelled with C-14 in the methylene group of the
alcohol moiety was administered orally to male Sprague-Dawley rats at
the rate of 200 mg/kg. The administered C-14 was almost completely
eliminated via the urine (ca. 60%) and faeces (ca. 40%) during three
days. No detectable C-14-C02 was expired. The levels of total C-14
and phenothrin isomers in the blood, liver, kidney and brain reached
maxima at three hours after administration and then gradually
decreased to one-tenth to one-twentieth of the maxima in 24 hours.
The major metabolites identified were 3-phenoxybenzoic acid (free and
glycine conjugate), 3-(4-hydroxybenzoic) acid and 3-phenoxybenzyl
alcohol. A small amount of unmetabolized (+)-trans-phenothrin was
excreted into the faeces (Miyamoto et al., 1974).
Metabolism of (+)-cis-phenothrin in male rats was also studied.
About 65% of the administered C-14 was recovered in faeces for three
days post-treatment. The faeces contained three ester metabolites
which resulted from hydroxylation at the 4'-phenoxy position of the
alcohol moiety, oxidation of trans-isobutenyl methyl group and
hydroxylation of cis-geminal-dimethyl group of the acid moiety. A
small amount of 3-phenoxybenzoic acid was also found (Suzuki et al.,
1976).
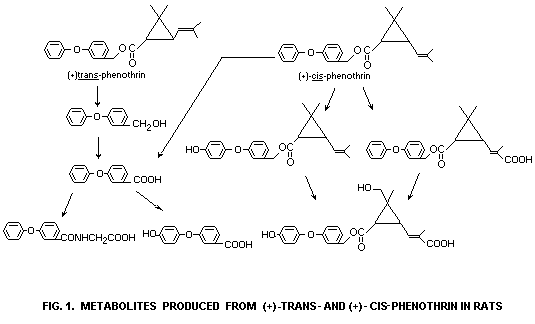
Thus, metabolism of phenothrin isomers proceeded rapidly in the rat
mainly via hydrolysis of the ester linkage and oxidation at several
positions of both alcohol and acid moieties (Fig. 1). The metabolites
derived from the alcohol moiety were almost completely excreted into
the urine and faeces.
Metabolism studies in vitro revealed that liver microsomal enzymes
from rats, mice, guinea pigs, rabbits and dogs hydrolyse
(+)-trans-phenothrin much faster than the cis-isomers, but the
oxidation rate by mouse liver microsomes was somewhat faster with the
(+)-cis isomer than with the (+)-trans isomer (Suzuki and
Miyamoto, 1978; Soderland and Casida, 1977; Casida et al., 1979).
Other information on identity and properties
Molecular weight: 350.5
State: colorless liquid
Specific gravity: d25 1.058
25
Viscosity: 195.1 cp at 20°C
87.4 cp at 30°C
Vapour pressure: 1.2 × 10-6 mmHg at 20°c
Refractive index: n25 1.5483
D
Solubility:
(g/l at room Highly soluble in the following solvents
temperature) solvents (>500 g/l methanol, isopropanol,
ethylcellosolve, diethyl ether, xylene,
n-hexane alpha-methyl, naphthalene,
cyclohexane, chloroform, acetonitrile,
dimethyl formamide, kerosene.
Solubility in water: 1.4 ± 0.1 ppm at 30°C
Stability: No breakdown after one year at room
temperature out of the light. Stable in
neutral and weak acidic conditions. Stable
in most solvents except methanol, ethyl
cellosolve, o-cresol, dimethylsulfoxide.
Typical percent purity of the technical material
% by weight
3-phenoxy (±)-cis, trans-chrysanthemate 92.5 - 94.5
The meeting was also provided by the manufacturer with complete
information on the chemical nature and quantity of the residual
manufacturing impurities in the technical material. This information
is on file with the Secretariat of the meeting. The data in this
monograph are from phenothrin meeting these specifications as are the
recommendations for residue limits.
RESIDUES IN FOOD AND THEIR EVALUATIONS
Use Pattern
Phenothrin is a new pyrethroid being currently developed. It is more
stable than natural pyrethrins and other novel pyrethroids developed
earlier, but it is relatively degradable by sunlight. In the light of
its insecticidal properties and its relatively low toxicity to
mammals, phenothrin and its optically active isomers show much promise
as a safe and useful insecticide in various fields including
households, public health, food storage, ornamentals and livestock
production.
Principal uses currently are:
1. in household aerosols and sprays formulated in combination with
tetramethrin, piperonyl butoxide, carbamates and/or fenitrothion
for control of various species of plant hoppers and for control of
house-flies, mosquitoes, cockroaches and other household or public
health insect pests.
2. in grain protection and disinfestation.
Treatment of plants
Repeated and multiple applications of organophosphorus compounds (e.g.
malathion) and carbamates (e.g. carbaryl) for control of green rice
leaf hoppers in paddy fields have resulted in resistant strains in
some parts of Japan. Several efforts have been made to solve the
problem.
Yoshioka and others reported laboratory and field trials in which not
only susceptible but also resistant leaf hoppers and other hoppers
were controlled by the treatment of 30 to 40 kg/ha of combined dust
formulations containing various compounds including small amounts of
phenothrin (e.g. 0.8% by wt) and carbamates such as BPMC,
O-sec-buthylphenyl-N-methyl carbamate, MTMC, m-tolyl-N-methyl
carbamate and MPMC, 3,4-xylyl-N-methyl carbamate (e.g. 2.0% by wt).
It is expected these formulations will be used for control of hoppers
within the next one or two years.
Phenothrin has advantages of a short residual life on food plants,
quick insecticidal action and efficient control of pest strains
resistant to organochlorine and organophosphorus insecticides. It is
currently being developed for controlling various pests on a range of
fruit and vegetables.
Post-harvest use
Phenothrin offers advantages of improved cost/efficiency (of
pyrethrins and bioresmethrin), reliable availability and a high degree
of mammalian safety as a potential grain protectant. Phenothrin
applied at 2 mg/kg has a similar level of efficiency as commercial
applications of bioresmethrin and pyrethrins in the control of insects
resistant to chlorinated hydrocarbon and/or organophosphorus grain
protectants.
The pesticide is stable in grain masses, but is subject to degradation
on exposure to ultraviolet light (Nambu et al., 1979b).
In a series of practical commercial trials undertaken in Australia and
involving exposures to various insects known to be resistant to other
commonly-used pesticides, the potentialities of phenothrin for the
protection of grains in store were well demonstrated (Desmarchelier
et al., 1979; Bengston, et al., 1978; Working Party of Grain
Protectants, 1979 a and b).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatment
Supervised trials on rice, green peppers and cabbage have been carried
out in Japan. Replicated results are available from each crop
evaluated (Takimoto et al., 1977). The results are given in Table 1.
Post-harvest treatment
All trials on wheat, sorghum and barley grains were carried out in
Australia except one in Japan under laboratory conditions with
C-14-labelled (+)-trans and (+)-cis phenothrin. Most of the
Australian trials involved samples from commercial storages of up to
7,435 tonnes treated with phenothrin on the proviso that the grain was
ultimately used only as animal feed-stuff. The temperature ranged
from 18°C to 36°C and the moisture from 9.0% to 13.0%. Applications
were made with phenothrin or d-phenothrin mostly in combination with
piperonyl butoxide and/or fenitrothion. The application rates were
mostly up to 3.0 mg/kg, but on wheat grain phenothrin was applied at
up to 8 mg/kg. Wheat grains were stored for up to 9 months, barley
for up to 5 months and sorghum up to 6 months, and samples withdrawn
for residue analysis at each indicated times. The residues of
phenothrin and d-phenothrin are shown in Tables 2 and 3 respectively.
Relatively high amounts of phenothrin and d-phenothrin were detected
after 5-9 months in those grains. For example, in some cases more
than half of the applied phenothrin and d-phenothrin remained in grain
even after 9 months. In the experiment with C-14-labelled
(+)-trans- and (+)-cis phenothrin, only slight decomposition of
both isomers was found months after treatment.
Distribution of C-14-labelled (+)-trans and (+)-cis phenothrin in
germ, endosperm and seed coat was examined after 0, 1, 3 and 6 months
of storage at 15°C and 30°C (Nambu et al. 1979b). The results are
presented in Table 4. Most of the applied isomers were localized at
the seed coat and the distribution pattern did not change materially
during 6 month storage. Those results were evidenced by
radioautograms.
Table 1. Residues of phenothrin in rice, green peppers and cabbage
(Tests undertaken in Japan using a 25% EC formulation (Takimoto et al, 1977)
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Rice Straw Hulled rice
(0.375) 6 3-10 7 0.62 0.016
14 0.45 0.009
Rice Straw Hulled rice
(0.375) 6 6-9 7 1.25 0.078
14 1.58 0.040
Green pepper
(0.50) 3 7 1 0.324
3 0.660
7 0.334
6 7 1 1.038
3 0.625
7 0.428
(0.50) 3 7 1 0.333
3 0.268
7 0.469
6 7 1 0.363
3 0.445
7 0.244
Cabbage
(0.375) 4 7 3 <0.005
7 <0.005
14 <0.005
21 <0.005
Table 1. Continued...
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Cabbage 8 7 3 0.005
(cont'd) 7 0.017
14 <0.005
21 <0.005
Cabbage
(0.375) 5 7-8 3 <0.005
7 <0.005
14 <0.005
21 <0.005
9 6-8 3 0.011
7 <0.005
14 <0.005
21 <0.005
Table 2. Residues of phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1977) 6* 3 4.4
6 4.4
9 3.2,3.2** Welcome
8* 3 6.7 Australasia,
6 6.9 1977
9 5.8, 5.2**
4* 3 3.0
6 2.7
9 1.6,2.8**
(1977/78) 1.9+ 0.25 1.8
1.5 1.42 Working Party
3 1.45 on Grain
4.5 1.71 Protectants,
6 1.38 1978
9 1.1
(1978) 1.85+ 3 1.29
4.5 1.3
6 1.1
7 1.21
(1978) 2.08+ 1 1.21
2.00+ 1 1.56
2.00+ 1 1.23 Working Party
1.89+ 1 1.23 on Grain
2.1+ 1 1.50 Protectants,
2.02+ 1 1.30 1979a
2.29+ 1 1.30
Wheat
(1979) 1.49+ 1 1.85,1.28**
2 1.35
3 1.75
4 1.75
2.01+ 1 2.25,1.62**
2 1.8
3 2.05, 1.45**
4 1.6
Table 2. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
(1979) 2.01+ 20 days 2.3
48 days 1.9
75 days 2.3 Working Party
81 days 1.6 on Grain
1.49+ 24 days 1.9 Protectants,
55 days 1.5 1979b
79 days 1.9
83 days 1.8
Sorghum
(1978) 2.0+ 1 week 1.86
12 weeks 2.72 Bengston
17 weeks 1.59 1978
26 weeks 1.56
* Nominal application
** Residues were determined in two different laboratories from the
same sample
+ Rate of application calculated from total grams phenothrin used
divided by tons of grain treated.
Table 3. Residues of d-phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.7
2 0.9
3 1.1
5 1.0
6 0.5
8 1.1
1.7+ 1 2.4
2 2.1
3 1.4
4 1.0
5 1.1
6 0.9 Desmarchelier
7 0.9 et al., 1979c
8 1.0
9 1.0
1.9+ 1 1.6
2 0.8
4 0.8
5 1.0
6 0.5
8 0.6
9 0.6
2.0+ 1 1.5
2 1.7
5 1.6
2.0+ 1 0.7
3 1.0
1.8+ 1 1.5
2 1.3
4 0.7
6 0.7
1.4+ 1 1.5
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.3
2 1.3
4 1.1 Desmarchelier
5 1.1 et al., 1979c
6 0.9
7 0.8
9 0.8
1.9+ 1 0.9
2 0.7
3 0.8
6 0.8
2.1+ 1 1.0
3 0.7
4 1.1
5 0.9
2.0+ 1 1.0
3 1.0
4 0.9
5 1.5 Desmarchelier
7 1.5 et al., 1979c
2.6+ 2 1.3
3 0.6
6 1.1
2.2+ 2 1.0
3 0.8
5 0.8
6 1.2
4 1/2 3.87*,
3.93** Nambu et al,
1 3.80, 1979b
3.90
(+)-trans-
phenothrin 3 3.78, 3.80
6 3.72, 3.80
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 4 1/2 3.77*, 3.80**
1 3.84, 3.94
(+)-trans
phenothrin 3 3.87, 3.80
6 3.69, 3.66
4 1/2 3.68*, 3.67**
1 3.82, 3.78
(+)-trans
phenothrin 3 3.88,
3.80 Nambu et al.
6 3.69, 1979b
3.66
4 1/2 3.85*, 3.88**
1 3.80, 4.00
(+)-cis-
phenothrin 3 3.80, 3.93
6 3.73, 3.80
4 1/2 3.82*, 3.99**
1 4.03, 3.95
(+)-cis-
phenothrin 3 3.88, 4.02
6 3.84, 3.55
4 1/2 3.90*, 4.10**
1 4.03, 3.83
(+)-cis-
phenothrin 3 3.91, 3.89
6 3.76, 3.72
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Barley
(1979/79) 2.6+ 1 2.0
3 1.1
5 1.5
Desmarchelier
et al, 1979c
2.9+ 2 1.5
3 1.4
4 1.1
* Grain was stored at the temperature of 15°C
** Grain was stored at the temperature of 30°C
+ Rate of application calculated from total grams
phenothrin used divided by tons of grain treated.
Table 4. Distribution of (+)-trans and (+)-cis phenothrin after storage of treated grain
(Nambu et al., 1979b)
Compound Temperature Months Residue (mg/kg)
Whole grain Germ Endosperm Seed coat
(+)-trans 15°C 0 3.81 0.08 0.62 2.82
-phenothrin
(4 mg/kg) 1 3.80 0.1 0.64 2.86
3 3.78 0.09 0.63 3.09
6 3.72 0.08 0.51 2.97
30°C 1 3.90 0.12 0.77 2.95
3 3.80 0.11 0.69 2.97
6 3.80 0.12 0.58 3.06
(+)-trans
-phenothrin 15°C 6 3.69 0.10 0.62 2.94
+ PBO 30°C 6 3.66 0.10 0.60 2.65
-Fenitrothion
(4+20+4,
mg/kg)
(+)-cis-
phenothrin 15°C 0 3.85 0.09 0.67 3.14
(4 mg/kg) 1 3.80 0.08 0.70 3.08
3 3.80 0.08 0.64 3.11
6 3.73 0.11 0.62 2.63
30°C 1 4.00 0.08 0.80 3.02
3 3.93 0.12 0.68 3.28
6 3.80 0.14 0.58 2.75
(+)-cis
phenothrin 15°C 6 3.76 0.12 0.58 2.92
+ PBO 30°C 6 3.72 0.10 0.59 2.79
+ Fenitrothion
(4+20+4
mg/kg)
PBO: Technical piperonyl butoxide
FATE OF RESIDUES
In animals
(+)-trans-phenothrin labelled with C-14 in the methylene group of the
alcohol moiety was administered orally to male Sprague-Dawley rats at
the rate of 200 mg/kg. The administered C-14 was almost completely
eliminated via the urine (ca. 60%) and faeces (ca. 40%) during three
days. No detectable C-14-C02 was expired. The levels of total C-14
and phenothrin isomers in the blood, liver, kidney and brain reached
maxima at three hours after administration and then gradually
decreased to one-tenth to one-twentieth of the maxima in 24 hours.
The major metabolites identified were 3-phenoxybenzoic acid (free and
glycine conjugate), 3-(4-hydroxybenzoic) acid and 3-phenoxybenzyl
alcohol. A small amount of unmetabolized (+)-trans-phenothrin was
excreted into the faeces (Miyamoto et al., 1974).
Metabolism of (+)-cis-phenothrin in male rats was also studied.
About 65% of the administered C-14 was recovered in faeces for three
days post-treatment. The faeces contained three ester metabolites
which resulted from hydroxylation at the 4'-phenoxy position of the
alcohol moiety, oxidation of trans-isobutenyl methyl group and
hydroxylation of cis-geminal-dimethyl group of the acid moiety. A
small amount of 3-phenoxybenzoic acid was also found (Suzuki et al.,
1976).
Thus, metabolism of phenothrin isomers proceeded rapidly in the rat
mainly via hydrolysis of the ester linkage and oxidation at several
positions of both alcohol and acid moieties (Fig. 1). The metabolites
derived from the alcohol moiety were almost completely excreted into
the urine and faeces.
Metabolism studies in vitro revealed that liver microsomal enzymes
from rats, mice, guinea pigs, rabbits and dogs hydrolyse
(+)-trans-phenothrin much faster than the cis-isomers, but the
oxidation rate by mouse liver microsomes was somewhat faster with the
(+)-cis isomer than with the (+)-trans isomer (Suzuki and
Miyamoto, 1978; Soderland and Casida, 1977; Casida et al., 1979).
 In Plants
When (+)-trans and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were each applied to the leaf
surface of bean and rice plants at rate of 48-50 g/20 cm2, both
isomers rapidly disappeared from the treated leaves of both plants
with half-lives of less than one day. On and/or in these plants, both
isomers underwent ozonolysis at the isobutenyl double bond to yield
the corresponding ozonides which were rapidly decomposed to the
corresponding aldehydes and carboxylic acids. These ester products
were subsequently metabolized via cleavage of the ester linkage,
hydroxylation at 2'- and 4'- positions of the alcohol moiety, and
oxidation of the benzyl alcohols to the benzoic acids. The resultant
phenols and carboxylic acids were further conjugated with sugars. The
parent compounds and their degradation products were hardly
transferred from the application site to other parts of plants (Nambu
et al., 1979a).
Bean plant seedlings were planted in Kodaira light clay and Katano
sandy loam soils treated with 1.0 mg/kg of C-14, phenothrin isomers.
After 30 days, 0.01 - 0.03 mg/kg and 0.04 - 0.09 mg/kg equivalents of
C-14 were found in pods and seeds, and shoots respectively, although
0.21 - 3.48 mg/kg of C-14 was retained in roots. No parent compound
was detected in shoots (Nambu et al., 1979a).
In soil
(+)-trans and (+)-cis-phenothrin were applied to Kodaira light
clay and Katano sandy loam soils at the rate of 1.0 mg/kg, and the
treated soils were held at 25 ± 2°C in the dark. Under upland
conditions, both isomers were rapidly decomposed in these soils with
half-lives of 1-2 days. On the other hand, the rate of degradation of
both isomers was much slower under flooded conditions, and half-lives
were 1-2 weeks and 1-2 months for the (+)-trans and (+)-cis
isomers, respectively. Phenothrin isomers were decomposed in the
soils via cleavage of the ester linkage, hydroxylation at 4'-position
of the alcohol moiety, cleavage of the diphenyl ether linkage and
oxidation of benzyl alcohols to benzoic acids. These products were
not persistent in the soils under upland conditions and the labelled
carbon was finally decomposed to C-14-CO2 (Nambu et al., 1979b).
Phenothrin was impregnated at a concentration of 1 g/kg on to a
laterite from Uganda. The treated soil was stored at 25°C and 20 or
80% relative humidity (R.H.). The half-life of the insecticide in
days was 40 with 20% RH and 150 with 80% RH (Barlow et al., 1977).
(+)-trans and (+)-cis-phenothrin and their degradation products
were hardly eluted with water from Kodaira light clay and Katano sandy
loam soils, whereas polar products including 3-phenoxybenzoic acid
were slightly eluted from Muko sand (Nambu et al., 1979b).
In storage and processing
(+)-trans- and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were applied to wheat grains at
a concentration of 4 mg/kg alone or together with 20 mg/kg piperonyl
butoxide and 4 mg/kg fenitrothion. The treated grains with 11-12%
moisture content were stored at 15 and 30°C in the dark. During 6
months storage period, both isomers were slightly decomposed
regardless of the presence and absence of piperonyl butoxide and
fenitrothion. The residue levels of phenothrin isomers were 3.64-3.78
mg/kg in whole wheat grain, 0.79-0.83 mg/kg in flour and 9.24-11.4
mg/kg in bran after 6 months. The C-14 residues were mainly located
in the seed coat as evidenced by radio-autography and radioanalysis.
Small amounts (less than 2% of the applied C-14) of 3-phenoxybenzyl
alcohol and 3-phenoxybenzoic acid were found. The phenothrin residues
in flour hardly decreased to a small extent through baking processes
leaving 0.66-0.69 mg/kg of phenothrin isomers in bread (Nambu et al,
1979b).
All studies were carried out in Australia to determine the residues of
phenothrin or d-phenothrin in the products after processing and
cooking of wheat, oats, barley and rice grain, except one experiment
with wheat grain in Japan using labelled compounds.
Wheat grain containing 0.5-3.9 mg/kg of phenothrin was used for the
studies, and the residue results after processing and cooking are
summarised in Table 5. Although higher residues were found in bran
and pollard after processing, the residues in flour were reduced and
reached 10.6-25.0% of those found in whole grain. After cooking the
residues in bread did not exceed 0.2 mg/kg in Australian trials, but
in the Japanese trial those residues of C-14-labelled (+)-trans and
(+)-cis phenothrin were relatively high (0.69 mg/kg and 0.66 mg/kg)
corresponding to the higher residues in flour (0.79 mg/kg and 0.91
mg/kg). This is supposed to be caused by contamination of a large
portion of testa in flour from the crease area of grain, while in
normal commercial flour milling situations only a very small portion
of testa would go into flour. In gluten, the residue was higher (1.9
mg/kg) than that in flour (0.3 mg/kg). However, the pesticide
residues in gluten does not cause concern, because normally there
would be a 2% maximum addition of gluten in bread.
Oats containing 1.2 mg/kg of phenothrin were processed and cooked
(Desmarchelier, 1979a). Most of the residues were carried in oat
hulls, and low residue levels were found in both groats (0.3 mg/kg)
and rolled oats (0.1 mg/kg). In porridge the residue was below the
detection limit of 0.05 mg/kg.
Barley containing 3.2 mg/kg and 0.92 mg/kg phenothrin was processed
into malt, wort and cattle feed. Of these products, the highest
residues were found in the malt, but these levels, 0.63 mg/kg and 0.15
mg/kg were less than one-fifth of that in barley. In wort, the
residues were less than the detection limit of 0.02 mg/kg.
Table 5. Residues of phenothrin and d-phenothrin prior to and after processing and cooking of wheat
Residue (mg/kg)
Compound Reference
Wholemeal White
Wheat Bran Pollard Flour Bread Bread Gluten Starch
Phenothrin 1.2 5.6 3.6 0.21 0.74 0.16 - - Bengston 1979
3.0 10.0 4.1 0.82 - 0.18 - - Desmarchelier, 1979a
1.5 6.0 3.0 0.4 - 0.1 - - "
0.9 3.0 2.0 0.15 - 0.05 - - "
0.5 1.6 0.9 0.09 - <0.05 - - "
3.9 8.1 1.6 0.65 - - 1.9 <0.05 "
1.2 4.0 1.8 0.3 0.4-0.6 0.1-0.2 - - Ardley, 1979
2.0 - - - - <0.1 - - Mollard, 1979
4.3 - - - - <0.1 - - "
(+)-trans-phenothrin 3.78 11.4 - 0.79 - 0.69 - - Nambu et al
1979b
(+)-cis-phenothrin 3.64 9.24 - 0.91 - 0.66 - -
A nominal application of 8 mg/kg phenothrin was applied to husked
(brown) rice and polished (white) rice. Six months after application,
husked (6.2 mg/kg of phenothrin) and polished (5.9 mg/kg) rice were
cooked in a minimum amount of boiling water for 25 minutes. Unhusked
rice was also treated with d-phenothrin at a rate of 8 mg/kg, and
after 6 months of storage when 4.7 mg/kg of phenothrin was detected,
rice was milled into husked and polished rice, and cooked as described
above. As shown in Table 6, the cooking of rice had the effect of
reducing residues by from 34% to 60%. It also has indicated that the
milling of rice from husked (paddy) to unhusked (brown) or polished
(white) is a major means of residue reduction, that is, 90% and 97%
respectively.
Table 6. Residues of d-phenothrin prior to and following processing
and cooking of rice
Residue (mg/kg)
Unhusked Husked Polished Cooked
- 6.2 - 4.1
- - 5.9 3.1
4.7 0.50 - 0.2
4.7 - 0.15 <0.1
(Desmarchelier et al., 1979d)
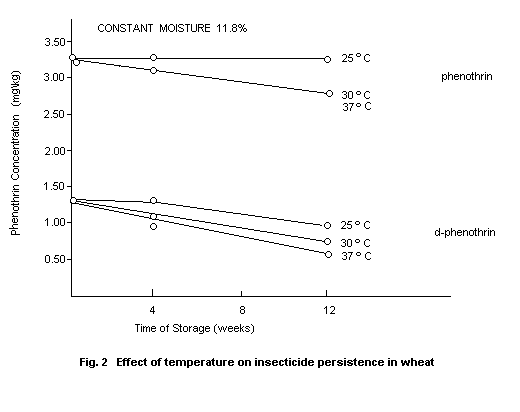
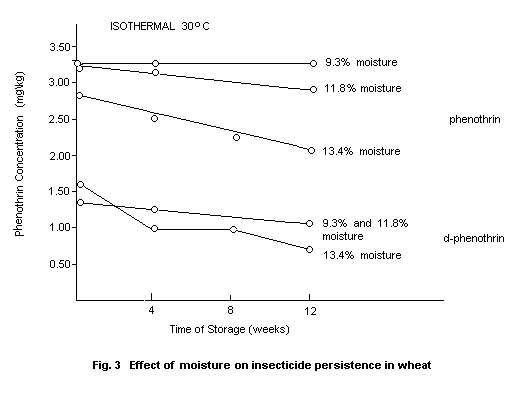
Phenothrin and d-phenothrin were applied to grain at the level of 4
mg/kg and 2 mg/kg, respectively, and have encountered levels of
moisture and three temperature levels. As shown in Fig. 2 and 3, both
increasing temperature (25, 30 and 37°C) at a constant moisture of
11.8% and increasing moisture (9.3, 11.8 and 13.4%) at a constant
temperature of 30°C led to increased breakdown of phenothrin and
d-phenothrin (Maguire, 1977).
Photochemical Degradation
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 and 7 days. Under these conditions phenothrin was more unstable
than permethrin and more stable than bioresmethrin (Elliot et al.,
1973). In a separate study, decomposition of phenothrin on glass by
irradiation of 270-370 mm light was determined. The half-life for
phenothrin was one day (Barlow et al., 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Due to the newness of phenothrin and its largely experimental status,
no information was available on residues in food in commerce or at
consumption.
In Plants
When (+)-trans and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were each applied to the leaf
surface of bean and rice plants at rate of 48-50 g/20 cm2, both
isomers rapidly disappeared from the treated leaves of both plants
with half-lives of less than one day. On and/or in these plants, both
isomers underwent ozonolysis at the isobutenyl double bond to yield
the corresponding ozonides which were rapidly decomposed to the
corresponding aldehydes and carboxylic acids. These ester products
were subsequently metabolized via cleavage of the ester linkage,
hydroxylation at 2'- and 4'- positions of the alcohol moiety, and
oxidation of the benzyl alcohols to the benzoic acids. The resultant
phenols and carboxylic acids were further conjugated with sugars. The
parent compounds and their degradation products were hardly
transferred from the application site to other parts of plants (Nambu
et al., 1979a).
Bean plant seedlings were planted in Kodaira light clay and Katano
sandy loam soils treated with 1.0 mg/kg of C-14, phenothrin isomers.
After 30 days, 0.01 - 0.03 mg/kg and 0.04 - 0.09 mg/kg equivalents of
C-14 were found in pods and seeds, and shoots respectively, although
0.21 - 3.48 mg/kg of C-14 was retained in roots. No parent compound
was detected in shoots (Nambu et al., 1979a).
In soil
(+)-trans and (+)-cis-phenothrin were applied to Kodaira light
clay and Katano sandy loam soils at the rate of 1.0 mg/kg, and the
treated soils were held at 25 ± 2°C in the dark. Under upland
conditions, both isomers were rapidly decomposed in these soils with
half-lives of 1-2 days. On the other hand, the rate of degradation of
both isomers was much slower under flooded conditions, and half-lives
were 1-2 weeks and 1-2 months for the (+)-trans and (+)-cis
isomers, respectively. Phenothrin isomers were decomposed in the
soils via cleavage of the ester linkage, hydroxylation at 4'-position
of the alcohol moiety, cleavage of the diphenyl ether linkage and
oxidation of benzyl alcohols to benzoic acids. These products were
not persistent in the soils under upland conditions and the labelled
carbon was finally decomposed to C-14-CO2 (Nambu et al., 1979b).
Phenothrin was impregnated at a concentration of 1 g/kg on to a
laterite from Uganda. The treated soil was stored at 25°C and 20 or
80% relative humidity (R.H.). The half-life of the insecticide in
days was 40 with 20% RH and 150 with 80% RH (Barlow et al., 1977).
(+)-trans and (+)-cis-phenothrin and their degradation products
were hardly eluted with water from Kodaira light clay and Katano sandy
loam soils, whereas polar products including 3-phenoxybenzoic acid
were slightly eluted from Muko sand (Nambu et al., 1979b).
In storage and processing
(+)-trans- and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were applied to wheat grains at
a concentration of 4 mg/kg alone or together with 20 mg/kg piperonyl
butoxide and 4 mg/kg fenitrothion. The treated grains with 11-12%
moisture content were stored at 15 and 30°C in the dark. During 6
months storage period, both isomers were slightly decomposed
regardless of the presence and absence of piperonyl butoxide and
fenitrothion. The residue levels of phenothrin isomers were 3.64-3.78
mg/kg in whole wheat grain, 0.79-0.83 mg/kg in flour and 9.24-11.4
mg/kg in bran after 6 months. The C-14 residues were mainly located
in the seed coat as evidenced by radio-autography and radioanalysis.
Small amounts (less than 2% of the applied C-14) of 3-phenoxybenzyl
alcohol and 3-phenoxybenzoic acid were found. The phenothrin residues
in flour hardly decreased to a small extent through baking processes
leaving 0.66-0.69 mg/kg of phenothrin isomers in bread (Nambu et al,
1979b).
All studies were carried out in Australia to determine the residues of
phenothrin or d-phenothrin in the products after processing and
cooking of wheat, oats, barley and rice grain, except one experiment
with wheat grain in Japan using labelled compounds.
Wheat grain containing 0.5-3.9 mg/kg of phenothrin was used for the
studies, and the residue results after processing and cooking are
summarised in Table 5. Although higher residues were found in bran
and pollard after processing, the residues in flour were reduced and
reached 10.6-25.0% of those found in whole grain. After cooking the
residues in bread did not exceed 0.2 mg/kg in Australian trials, but
in the Japanese trial those residues of C-14-labelled (+)-trans and
(+)-cis phenothrin were relatively high (0.69 mg/kg and 0.66 mg/kg)
corresponding to the higher residues in flour (0.79 mg/kg and 0.91
mg/kg). This is supposed to be caused by contamination of a large
portion of testa in flour from the crease area of grain, while in
normal commercial flour milling situations only a very small portion
of testa would go into flour. In gluten, the residue was higher (1.9
mg/kg) than that in flour (0.3 mg/kg). However, the pesticide
residues in gluten does not cause concern, because normally there
would be a 2% maximum addition of gluten in bread.
Oats containing 1.2 mg/kg of phenothrin were processed and cooked
(Desmarchelier, 1979a). Most of the residues were carried in oat
hulls, and low residue levels were found in both groats (0.3 mg/kg)
and rolled oats (0.1 mg/kg). In porridge the residue was below the
detection limit of 0.05 mg/kg.
Barley containing 3.2 mg/kg and 0.92 mg/kg phenothrin was processed
into malt, wort and cattle feed. Of these products, the highest
residues were found in the malt, but these levels, 0.63 mg/kg and 0.15
mg/kg were less than one-fifth of that in barley. In wort, the
residues were less than the detection limit of 0.02 mg/kg.
Table 5. Residues of phenothrin and d-phenothrin prior to and after processing and cooking of wheat
Residue (mg/kg)
Compound Reference
Wholemeal White
Wheat Bran Pollard Flour Bread Bread Gluten Starch
Phenothrin 1.2 5.6 3.6 0.21 0.74 0.16 - - Bengston 1979
3.0 10.0 4.1 0.82 - 0.18 - - Desmarchelier, 1979a
1.5 6.0 3.0 0.4 - 0.1 - - "
0.9 3.0 2.0 0.15 - 0.05 - - "
0.5 1.6 0.9 0.09 - <0.05 - - "
3.9 8.1 1.6 0.65 - - 1.9 <0.05 "
1.2 4.0 1.8 0.3 0.4-0.6 0.1-0.2 - - Ardley, 1979
2.0 - - - - <0.1 - - Mollard, 1979
4.3 - - - - <0.1 - - "
(+)-trans-phenothrin 3.78 11.4 - 0.79 - 0.69 - - Nambu et al
1979b
(+)-cis-phenothrin 3.64 9.24 - 0.91 - 0.66 - -
A nominal application of 8 mg/kg phenothrin was applied to husked
(brown) rice and polished (white) rice. Six months after application,
husked (6.2 mg/kg of phenothrin) and polished (5.9 mg/kg) rice were
cooked in a minimum amount of boiling water for 25 minutes. Unhusked
rice was also treated with d-phenothrin at a rate of 8 mg/kg, and
after 6 months of storage when 4.7 mg/kg of phenothrin was detected,
rice was milled into husked and polished rice, and cooked as described
above. As shown in Table 6, the cooking of rice had the effect of
reducing residues by from 34% to 60%. It also has indicated that the
milling of rice from husked (paddy) to unhusked (brown) or polished
(white) is a major means of residue reduction, that is, 90% and 97%
respectively.
Table 6. Residues of d-phenothrin prior to and following processing
and cooking of rice
Residue (mg/kg)
Unhusked Husked Polished Cooked
- 6.2 - 4.1
- - 5.9 3.1
4.7 0.50 - 0.2
4.7 - 0.15 <0.1
(Desmarchelier et al., 1979d)
Phenothrin and d-phenothrin were applied to grain at the level of 4
mg/kg and 2 mg/kg, respectively, and have encountered levels of
moisture and three temperature levels. As shown in Fig. 2 and 3, both
increasing temperature (25, 30 and 37°C) at a constant moisture of
11.8% and increasing moisture (9.3, 11.8 and 13.4%) at a constant
temperature of 30°C led to increased breakdown of phenothrin and
d-phenothrin (Maguire, 1977).
Photochemical Degradation
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 and 7 days. Under these conditions phenothrin was more unstable
than permethrin and more stable than bioresmethrin (Elliot et al.,
1973). In a separate study, decomposition of phenothrin on glass by
irradiation of 270-370 mm light was determined. The half-life for
phenothrin was one day (Barlow et al., 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Due to the newness of phenothrin and its largely experimental status,
no information was available on residues in food in commerce or at
consumption.
 METHODS OF RESIDUE ANALYSIS
Residue determination of intact phenothrin by gas-liquid
chromatography (GLC) is difficult due to poor sensitivity to the GLC
detectors commonly used in residue analysis, though some residue data
of d-phenothrin on stored sorghum (0.4 - 0.95 mg/kg) were obtained by
GLC equipped with flame ionization detector (Bengston et al., 1977).
Kato et al. (1979) successfully applied combined gas-liquid
chromatograph/Mass spectrometry (GLC/MS) to determine intact
d-phenothrin residues in blood. Mass fragmentography of d-phenothrin
gave as minimum detectable amounts 0.1 ng and 0.04 ng at mass ions,
m/e 350 (parent) and 183, respectively, thereby allowing confirmation
as well as quantitation of 0.005 mg/kg or even less. Desmarchelier
(1979a) also briefly reported utility of GLC/MS for analysis of
phenothrin in stored grains at residue levels below 0.01 mg/kg without
presenting detailed data.
As a determinative procedure Simpson (1979) adopted high pressure
liquid chromatography in combination with cleanup by partitioning
between n-hexane and acetonitrile, and alumina column chromatography.
By this method phenothrin residues of 1.56-2.72 mg/kg were quantitated
in stored sorghum, though data on detection limits and recoveries are
not presented (Bengston et al., 1978; Simpson 1979).
Derivatization methods have been more commonly adopted for residue
analysis. Takimoto et al., (1977) analyzed phenothrin residues by
the procedures which included potassium hydroxide-catalyzed hydrolysis
of phenothrin in aqueous methanol and subsequent pyridine-catalyzed
esterification of the liberated alcohol moiety with
2,4-dichlorobenzoyl chloride. When analyzed by GLC fitted with 0.8
m-long glass column packed either with 4% FFAP on Gas-chrom Q or with
3% OV-101/3% Apiezon Grease L on Gas-chrom Q, 3-phenoxy-benzyl
2,4-dichlorobenzoate showed excellent sensitivity, i.e. 0.04-0.1 ng as
minimum detectability, to electron-capture detector, which permitted
detection of phenothrin at 0.005-0.02 mg/kg.
Desmarchelier (1976; 1979a) extended the colorimetric procedures which
had originally been devised by McClellan (1964) for residue analysis
of pyrethrum. The procedures were based upon a color reaction of a
modified Deniges reagent with chrysanthemic acid formed by hydrolysis
of phenothrin under catalysis of sodium hydroxide in aqueous alcoholic
solution. The method appears less laborious but less selective and
less sensitive than that reported by Takimoto et al., (1977). In
addition, a significant difference in extinction coefficient observed
at 584 nm between (+)-cis and (+)-trans chrysanthemic acid
necessitates prior separation of cis and trans isomers for
accurate determination of phenothrin.
Thin-layer chromatography (TLC) based on the above colour reaction has
also been applied as a semiquantitative procedure (Desmarchelier 1976,
1979a).
The solvent or solvent combinations selected for extraction of
phenothrin depend upon the nature of substrates. From the substrated
with high-moisture contents like cabbage and green pepper, phenothrin
was effectively extracted by chopping and blending either with polar
solvents, e.g., methanolacetonitrile, or with nonpolar-polar solvent
mixtures, e.g., benzene-ethanol (Takimoto et al., 1977). For aged
phenothrin residues on cooked rice 24-hour extraction with polar
solvents, e.g. methanol and ethanol, gave complete recoveries whereas
the extraction with non-polar solvents, e.g. light petroleum and
hexane, resulted in poor recoveries (Desmarchelier 1979b). From dry
and low-moisture substrates including rice, barley, wheat, oats and
sorghum phenothrin is efficiently extracted with either polar,
non-polar or dual solvent system. Hulled rice grain was ground and
macerated overnight in methanol (Takimoto et al., 1977). Wheat and
barley were soaked overnight as whole grain either in light petroleum
in ethanol (Desmarchelier 1979b). Desmarchelier (1976) reported that
use of light petroleum simplified cleanup in the analysis of grains.
As cleanup procedures both liquid chromatography (LC; Florisil and
alumina) and TLC (silica-gel) are used for intact phenothrin residues
(Takimoto et al., 1977; Simpson 1979). The Florisil column
chromatography coupled with benzene-ethyl acetate extraction resulted
in 93% recovery, as determined by GLC/MS, from the blood fortified at
0.05 mg/kg (Kato et al., 1979). Cleanup procedures employed for the
derivatization methods obviously reflect the nature of the derivatives
as well as substrates. The method of converting phenothrin to
3-phenoxybenzyl 2,4,dichlorobenzoate which provided 81-88% recoveries
for cabbage, green pepper and hulled rice fortified at 0.1-1 mg/kg
included cleanup by either LC or TLC before both hydrolysis and GLC
quantitation (Takimoto et al., 1977). The colorimetric method
employed partition cleanup between aqueous phases and nonpolar
solvents, e.g. light petroleum or chloroform, and satisfactory
recoveries of 87-91% were achieved from cooked rice (Desmarchelier,
1979b).
NATIONAL LIMITS REPORTED TO THE MEETING
No information was available.
APPRAISAL
Phenothrin is more stable than natural pyrethrins and synthetic
pyrethroids developed earlier, but it is relatively degradable by
sunlight. The principal current uses are (1) in household aerosols
and sprays formulated in combination with tetramethrin, piperonyl
butoxide, carbamates and/or fenitrothion for control of various
species of plant hoppers and for control of houseflies, mosquitoes,
cockroaches and other household or public health pests, and (2) in
grain protection. Supervised trials have been undertaken on rice,
green peppers and cabbage.
The most important food use at present is for the protection of stored
grains. Extensive trials on wheat, sorghum and barley grains were
carried out in Australia and Japan. Wheat was stored up to 9 months,
barley up to 5 months, and sorghum up to 6 months and samples were
withdrawn for residue analysis at a variety of intervals. Treatment
levels of approximately 2 mg a.i./kg usually gave residues of about 2
mg/kg at 1 month after treatment, falling to about 1 mg/kg after 9
months. Similar results were obtained for sorghum and barley.
Increasing temperature and moisture resulted in increased breakdown of
phenothrin, but the effect was not large. Wheat residues were found
mostly in the seed coat.
Processing of wheat, oats, barley and rice resulted in somewhat higher
residues in wheat bran, wheat pollard, and oat hulls and lower
residues in wheat flour, groats, rolled oats, barley malt, wort,
barley cattle feed and polished rice. Cooking resulted in reduced
residues in bread, porridge and boiled rice.
In studies with 14C-labelled (+)-trans and (+)-cis- phenothrin
applied to bean and rice plant leaf surfaces, both isomers disappeared
rapidly with half-lives of less than one day. Both isomers underwent
ozonolysis and sequential metabolism to give final products consisting
of sugar conjugates of phenols and carboxylic acids. No translocation
of parent compounds and their degradation products from the
application site occurred. Bean seedlings planted in 14C-phenothrin
treated soil did not show translocation of parent compound into the
shoots.
Stored wheat (11-12% moisture) treated with 14C-phenothrin isomers at
4 mg/kg either alone or in combination with piperonyl butoxide and
fenitrothion was held at either 15 or 30°C in the dark for six months.
During this interval only slight degradation occurred with 3.6-3.8
mg/kg remaining in whole wheat grain, mainly in the seed coat. Small
amounts ('2% of applied 14C) of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were found.
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 to 7 days. Photostability of phenothrin was greater than
bioresmethrin and less than permethrin. Phenothrin films on glass
irradiated by 270-370 nm light had a half-life of one day.
Residue determination of intact phenothrin by GLC is complicated by
its molecular structure which results in poor sensitivity in selective
detectors such as the electron capture, necessitating the use of the
non-selective flame ionization detector with its attendant rigorous
cleanup requirements. Combined GC-Mass spectrometry has been
successfully used to determine d-phenothrin in blood at levels as low
as 0.04 ng, allowing quantitation at 0.005 mg/kg. GC-MS has also been
used for residues in stored grains, but such techniques will probably
not find wide acceptance for some time. The colorimetric procedure
for pyrethrum has been extended to phenothrin, but since the method is
based on a colour reaction involving chrysanthemic acid, it would not
distinguish between most synthetic and natural pyrethroids. High
performance liquid chromatography with UV absorption detection has
also been used to determine residues in stored sorghum. Rapid
improvements in LC column technology combined with variable
wavelength UV detectors should result in methods of greater
selectivity and sensitivity and merits further development. Although
tedious, the derivatization method developed by Takimoto, et al.
appears to be the most suitable for regulatory analysis. The method
gives overall recoveries of 81-88%.
RECOMMENDATIONS
Because phenothrin was not evaluated for an ADI in 1979, only
guideline levels can be presented at this time.
The following residue levels for phenothrin as combined (+)-trans
and (+) cis-isomers are those which need not be exceeded if the
compound is used as recommended for the protection of stored grains.
Commodity Guideline level
mg/kg
Wheat bran 15
Cereal grains 5
FURTHER WORK OR INFORMATION
Desirable
1. Further residue data from supervised trials on commodities for
which phenothrin uses are developed, especially stored products,
vegetables, rice and cereal brans and straws used as animal feed.
2. Continued development of improved methods of residue analysis for
the intact isomer molecules.
REFERENCES
Ardley, J.H. "Phenothrin-bread residues AWS-CSIRO pilot trials"
Reference PH-11/52 Welcome Australasia Ltd. (1979).
Ardley, J.H. and Halls, G.R.H. "Phenothrin (S-2539) A potential grain
protectant". Chemical Marketing and Economics Division of the
American Chemical Society, Preprints pp 38-54, Honolulu, Hawaii. 1979.
Barlow, F., Hadaway, A.B., Flower, L.S., Grose, J.E. and Turner, C.R.
"Some laboratory investigations relevant to the possible use of new
pyrethroids in control of mosquitoes and tse-tse flies". Pestic. Sci.,
8, 291-300.
Bengston, M. "The fate of residues of phenothrin in bran, pollard,
flow and bread", 1979.
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. "Grain protectants for the control of
malathion- resistant insects in stored sorghum".
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional grain protectants for the control of
malathion-resistant insects in stored sorghum".
Casida, J.E., Gaughan, L.C. and Ruzo, L.C. "Comparative Metabolism of
Pyrethroids Derived from 3-phenoxybenzyl and a-Cyano-3-Phenoxybenzyl
Alcohols". Advances in Pesticide Science Ed. by Geissbühler, Brooks,
G.T., and Kearney, P.C. Pergamon Press, London, Part 2, P. 182.
Desmarchelier, J.M. "Analysis of pyrethroids on grains" J. Stored
Prod. Res. 12, 245-252.
"Selective treatments, including combinations of pyrethroid and
organophosphorus insecticides, for control of stored product
Coleoptera at two temperatures". J. Stored Prod. Res. 13, 129-137.
"The fate of phenothrin residues during processing of wheat, barley
and oats". Dept. Primary Industry, Canberra, Australia. (1979a).
"Analysis of bioresmethrin, phenothrin, d-phenothrin, pyrethrum I
and carbaryl on grains, extraction studies on these insecticides plus
fenitrothion, methacrifos, pirimiphos-methyl and dichlorovos on 7
grains and cooked rice and an improved procedure for esterification on
phenols". Submitted to J. Pesticide Sci. (Japan). (1979b).
Desmarchelier, J.M., Bengston, M., Davies, R., Elder, B., Hart, R.,
Henning, R., Murry, W., Ridley, E., Ripp, E., Sierakawski,C., Sticka,
R., Snelson, J., Wallbank, B. and Wilson, A. "Pilot usage of the
grain protectants chloropyrifosmethyl plus bioresmethrin, fenitrothion
plus d-phenothrin, methacrifos and pirimiphos.methyl plus carbaryl".
(1979c).
Desmarchelier, J.M., Goldring, M. and Horgan, R. "Predicted and
observed residues of bioresmethrin, carbaryl, dichlorovos
fenitrothion, d-phenothrin, methacrifos and pirimiphos methyl on rice
and barley after storage and losses of these insecticides during
processing" Dept. Primary Industry, Canberra, Australia. (1979d).
Elliott, M., Farnham, A.W., James, N.F., Needham, P.H., Pulman, D.A.
and Stevenson, J.H. "NRDC 143, A More stable pyrethroid". Proceeding
7th British Insecticide and Fungicide Conference, p. 721. (1973).
Kato, T., Fujita,, K. and Miyamoto, J. "Analytical method of
d-phenothrin (SumithrinR) in blood". Sumitomo Chemical Co., Ltd.
(1979).
Maguire, M.J. "Persistence of insecticides on stored grain at three
temperature levels and three moisture levels". (1978).
McClellan, D.B. "Pyrethrum-Pyrethrum I and Pyrethrum-II" pp. 399-413,
in Analytical Methods for Pesticides, Plant Growth Regulators and Food
Additives, Vol. II (Zweig, G., ed.) Academic Press, New York).
Miyamoto, J., Suzuki, T., and Nakae, C. "Metabolism of Phenothrin or
3-phenoxybenzyl d-trans-Chrysanthemumate in Mammals". Pestic. Biochem.
Physiol., 4, 438.
Mollard, V. "Samples from Bread Research Institute". Reference PH
10/141 Wellcome Australasia Ltd.
Nambu, K., Ohkawa, H. and Miyamoto, J. "Metabolic fate of phenothrin
in plants and soils" Sumitomo Chemical Co., Ltd. (1979a).
Nambu, K., Takimoto, Y. and Miyamoto, J. "Degradation and fate of
phenothrin in stored wheat grains". Sumitomo Chemical Co., Ltd.
(1979b).
Simpson, B.W. "Method for pyrethroid residues on wheat, sorghum
pollard, flour and bread". (1979).
Soderland, D.M. and Casida, J.E. "Effects of Pyrethroid Structure on
Rats of Hydrolysis and Oxidation by Mouse Liver Microsomal Enzymes"
Pestic. Biochem. Physiol. 7, 391-401.
Suzuki, T., Ohno, N. and Miyamoto, J. "New Metabolites of
(+)-cis-Fenothrin, 3 Phenoxybenzyl (+)-cis-Chrysanthemumate, in
Rats" J. Pesticide Sci. 1, 151.
Suzuki, T. and Miyamoto, J. "Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome". Pestic. Biochem.
Physiol., 8, 186.
Takimoto, Y., Kato, T. and Miyamoto, J. "Residue analyses of
phenothrin (S-2539) in crops". Sumitomo Chemical Co., Ltd. (1977).
Wellcome Australasia. "Evaluation of phenothrin as a potential grain
protectant". (1977).
Working Party on Grain Protectants. "Final report on silo scale
experimental application of grain protectant chemicals, 1977-1978".
Working Party of Grain Protectants "Interim report on pilot usage
trials 1978-1979". (1979a).
Working Party on Grain Protectants "Summary of pilot usage trials,
1978-1979". (1979b).
METHODS OF RESIDUE ANALYSIS
Residue determination of intact phenothrin by gas-liquid
chromatography (GLC) is difficult due to poor sensitivity to the GLC
detectors commonly used in residue analysis, though some residue data
of d-phenothrin on stored sorghum (0.4 - 0.95 mg/kg) were obtained by
GLC equipped with flame ionization detector (Bengston et al., 1977).
Kato et al. (1979) successfully applied combined gas-liquid
chromatograph/Mass spectrometry (GLC/MS) to determine intact
d-phenothrin residues in blood. Mass fragmentography of d-phenothrin
gave as minimum detectable amounts 0.1 ng and 0.04 ng at mass ions,
m/e 350 (parent) and 183, respectively, thereby allowing confirmation
as well as quantitation of 0.005 mg/kg or even less. Desmarchelier
(1979a) also briefly reported utility of GLC/MS for analysis of
phenothrin in stored grains at residue levels below 0.01 mg/kg without
presenting detailed data.
As a determinative procedure Simpson (1979) adopted high pressure
liquid chromatography in combination with cleanup by partitioning
between n-hexane and acetonitrile, and alumina column chromatography.
By this method phenothrin residues of 1.56-2.72 mg/kg were quantitated
in stored sorghum, though data on detection limits and recoveries are
not presented (Bengston et al., 1978; Simpson 1979).
Derivatization methods have been more commonly adopted for residue
analysis. Takimoto et al., (1977) analyzed phenothrin residues by
the procedures which included potassium hydroxide-catalyzed hydrolysis
of phenothrin in aqueous methanol and subsequent pyridine-catalyzed
esterification of the liberated alcohol moiety with
2,4-dichlorobenzoyl chloride. When analyzed by GLC fitted with 0.8
m-long glass column packed either with 4% FFAP on Gas-chrom Q or with
3% OV-101/3% Apiezon Grease L on Gas-chrom Q, 3-phenoxy-benzyl
2,4-dichlorobenzoate showed excellent sensitivity, i.e. 0.04-0.1 ng as
minimum detectability, to electron-capture detector, which permitted
detection of phenothrin at 0.005-0.02 mg/kg.
Desmarchelier (1976; 1979a) extended the colorimetric procedures which
had originally been devised by McClellan (1964) for residue analysis
of pyrethrum. The procedures were based upon a color reaction of a
modified Deniges reagent with chrysanthemic acid formed by hydrolysis
of phenothrin under catalysis of sodium hydroxide in aqueous alcoholic
solution. The method appears less laborious but less selective and
less sensitive than that reported by Takimoto et al., (1977). In
addition, a significant difference in extinction coefficient observed
at 584 nm between (+)-cis and (+)-trans chrysanthemic acid
necessitates prior separation of cis and trans isomers for
accurate determination of phenothrin.
Thin-layer chromatography (TLC) based on the above colour reaction has
also been applied as a semiquantitative procedure (Desmarchelier 1976,
1979a).
The solvent or solvent combinations selected for extraction of
phenothrin depend upon the nature of substrates. From the substrated
with high-moisture contents like cabbage and green pepper, phenothrin
was effectively extracted by chopping and blending either with polar
solvents, e.g., methanolacetonitrile, or with nonpolar-polar solvent
mixtures, e.g., benzene-ethanol (Takimoto et al., 1977). For aged
phenothrin residues on cooked rice 24-hour extraction with polar
solvents, e.g. methanol and ethanol, gave complete recoveries whereas
the extraction with non-polar solvents, e.g. light petroleum and
hexane, resulted in poor recoveries (Desmarchelier 1979b). From dry
and low-moisture substrates including rice, barley, wheat, oats and
sorghum phenothrin is efficiently extracted with either polar,
non-polar or dual solvent system. Hulled rice grain was ground and
macerated overnight in methanol (Takimoto et al., 1977). Wheat and
barley were soaked overnight as whole grain either in light petroleum
in ethanol (Desmarchelier 1979b). Desmarchelier (1976) reported that
use of light petroleum simplified cleanup in the analysis of grains.
As cleanup procedures both liquid chromatography (LC; Florisil and
alumina) and TLC (silica-gel) are used for intact phenothrin residues
(Takimoto et al., 1977; Simpson 1979). The Florisil column
chromatography coupled with benzene-ethyl acetate extraction resulted
in 93% recovery, as determined by GLC/MS, from the blood fortified at
0.05 mg/kg (Kato et al., 1979). Cleanup procedures employed for the
derivatization methods obviously reflect the nature of the derivatives
as well as substrates. The method of converting phenothrin to
3-phenoxybenzyl 2,4,dichlorobenzoate which provided 81-88% recoveries
for cabbage, green pepper and hulled rice fortified at 0.1-1 mg/kg
included cleanup by either LC or TLC before both hydrolysis and GLC
quantitation (Takimoto et al., 1977). The colorimetric method
employed partition cleanup between aqueous phases and nonpolar
solvents, e.g. light petroleum or chloroform, and satisfactory
recoveries of 87-91% were achieved from cooked rice (Desmarchelier,
1979b).
NATIONAL LIMITS REPORTED TO THE MEETING
No information was available.
APPRAISAL
Phenothrin is more stable than natural pyrethrins and synthetic
pyrethroids developed earlier, but it is relatively degradable by
sunlight. The principal current uses are (1) in household aerosols
and sprays formulated in combination with tetramethrin, piperonyl
butoxide, carbamates and/or fenitrothion for control of various
species of plant hoppers and for control of houseflies, mosquitoes,
cockroaches and other household or public health pests, and (2) in
grain protection. Supervised trials have been undertaken on rice,
green peppers and cabbage.
The most important food use at present is for the protection of stored
grains. Extensive trials on wheat, sorghum and barley grains were
carried out in Australia and Japan. Wheat was stored up to 9 months,
barley up to 5 months, and sorghum up to 6 months and samples were
withdrawn for residue analysis at a variety of intervals. Treatment
levels of approximately 2 mg a.i./kg usually gave residues of about 2
mg/kg at 1 month after treatment, falling to about 1 mg/kg after 9
months. Similar results were obtained for sorghum and barley.
Increasing temperature and moisture resulted in increased breakdown of
phenothrin, but the effect was not large. Wheat residues were found
mostly in the seed coat.
Processing of wheat, oats, barley and rice resulted in somewhat higher
residues in wheat bran, wheat pollard, and oat hulls and lower
residues in wheat flour, groats, rolled oats, barley malt, wort,
barley cattle feed and polished rice. Cooking resulted in reduced
residues in bread, porridge and boiled rice.
In studies with 14C-labelled (+)-trans and (+)-cis- phenothrin
applied to bean and rice plant leaf surfaces, both isomers disappeared
rapidly with half-lives of less than one day. Both isomers underwent
ozonolysis and sequential metabolism to give final products consisting
of sugar conjugates of phenols and carboxylic acids. No translocation
of parent compounds and their degradation products from the
application site occurred. Bean seedlings planted in 14C-phenothrin
treated soil did not show translocation of parent compound into the
shoots.
Stored wheat (11-12% moisture) treated with 14C-phenothrin isomers at
4 mg/kg either alone or in combination with piperonyl butoxide and
fenitrothion was held at either 15 or 30°C in the dark for six months.
During this interval only slight degradation occurred with 3.6-3.8
mg/kg remaining in whole wheat grain, mainly in the seed coat. Small
amounts ('2% of applied 14C) of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were found.
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 to 7 days. Photostability of phenothrin was greater than
bioresmethrin and less than permethrin. Phenothrin films on glass
irradiated by 270-370 nm light had a half-life of one day.
Residue determination of intact phenothrin by GLC is complicated by
its molecular structure which results in poor sensitivity in selective
detectors such as the electron capture, necessitating the use of the
non-selective flame ionization detector with its attendant rigorous
cleanup requirements. Combined GC-Mass spectrometry has been
successfully used to determine d-phenothrin in blood at levels as low
as 0.04 ng, allowing quantitation at 0.005 mg/kg. GC-MS has also been
used for residues in stored grains, but such techniques will probably
not find wide acceptance for some time. The colorimetric procedure
for pyrethrum has been extended to phenothrin, but since the method is
based on a colour reaction involving chrysanthemic acid, it would not
distinguish between most synthetic and natural pyrethroids. High
performance liquid chromatography with UV absorption detection has
also been used to determine residues in stored sorghum. Rapid
improvements in LC column technology combined with variable
wavelength UV detectors should result in methods of greater
selectivity and sensitivity and merits further development. Although
tedious, the derivatization method developed by Takimoto, et al.
appears to be the most suitable for regulatory analysis. The method
gives overall recoveries of 81-88%.
RECOMMENDATIONS
Because phenothrin was not evaluated for an ADI in 1979, only
guideline levels can be presented at this time.
The following residue levels for phenothrin as combined (+)-trans
and (+) cis-isomers are those which need not be exceeded if the
compound is used as recommended for the protection of stored grains.
Commodity Guideline level
mg/kg
Wheat bran 15
Cereal grains 5
FURTHER WORK OR INFORMATION
Desirable
1. Further residue data from supervised trials on commodities for
which phenothrin uses are developed, especially stored products,
vegetables, rice and cereal brans and straws used as animal feed.
2. Continued development of improved methods of residue analysis for
the intact isomer molecules.
REFERENCES
Ardley, J.H. "Phenothrin-bread residues AWS-CSIRO pilot trials"
Reference PH-11/52 Welcome Australasia Ltd. (1979).
Ardley, J.H. and Halls, G.R.H. "Phenothrin (S-2539) A potential grain
protectant". Chemical Marketing and Economics Division of the
American Chemical Society, Preprints pp 38-54, Honolulu, Hawaii. 1979.
Barlow, F., Hadaway, A.B., Flower, L.S., Grose, J.E. and Turner, C.R.
"Some laboratory investigations relevant to the possible use of new
pyrethroids in control of mosquitoes and tse-tse flies". Pestic. Sci.,
8, 291-300.
Bengston, M. "The fate of residues of phenothrin in bran, pollard,
flow and bread", 1979.
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. "Grain protectants for the control of
malathion- resistant insects in stored sorghum".
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional grain protectants for the control of
malathion-resistant insects in stored sorghum".
Casida, J.E., Gaughan, L.C. and Ruzo, L.C. "Comparative Metabolism of
Pyrethroids Derived from 3-phenoxybenzyl and a-Cyano-3-Phenoxybenzyl
Alcohols". Advances in Pesticide Science Ed. by Geissbühler, Brooks,
G.T., and Kearney, P.C. Pergamon Press, London, Part 2, P. 182.
Desmarchelier, J.M. "Analysis of pyrethroids on grains" J. Stored
Prod. Res. 12, 245-252.
"Selective treatments, including combinations of pyrethroid and
organophosphorus insecticides, for control of stored product
Coleoptera at two temperatures". J. Stored Prod. Res. 13, 129-137.
"The fate of phenothrin residues during processing of wheat, barley
and oats". Dept. Primary Industry, Canberra, Australia. (1979a).
"Analysis of bioresmethrin, phenothrin, d-phenothrin, pyrethrum I
and carbaryl on grains, extraction studies on these insecticides plus
fenitrothion, methacrifos, pirimiphos-methyl and dichlorovos on 7
grains and cooked rice and an improved procedure for esterification on
phenols". Submitted to J. Pesticide Sci. (Japan). (1979b).
Desmarchelier, J.M., Bengston, M., Davies, R., Elder, B., Hart, R.,
Henning, R., Murry, W., Ridley, E., Ripp, E., Sierakawski,C., Sticka,
R., Snelson, J., Wallbank, B. and Wilson, A. "Pilot usage of the
grain protectants chloropyrifosmethyl plus bioresmethrin, fenitrothion
plus d-phenothrin, methacrifos and pirimiphos.methyl plus carbaryl".
(1979c).
Desmarchelier, J.M., Goldring, M. and Horgan, R. "Predicted and
observed residues of bioresmethrin, carbaryl, dichlorovos
fenitrothion, d-phenothrin, methacrifos and pirimiphos methyl on rice
and barley after storage and losses of these insecticides during
processing" Dept. Primary Industry, Canberra, Australia. (1979d).
Elliott, M., Farnham, A.W., James, N.F., Needham, P.H., Pulman, D.A.
and Stevenson, J.H. "NRDC 143, A More stable pyrethroid". Proceeding
7th British Insecticide and Fungicide Conference, p. 721. (1973).
Kato, T., Fujita,, K. and Miyamoto, J. "Analytical method of
d-phenothrin (SumithrinR) in blood". Sumitomo Chemical Co., Ltd.
(1979).
Maguire, M.J. "Persistence of insecticides on stored grain at three
temperature levels and three moisture levels". (1978).
McClellan, D.B. "Pyrethrum-Pyrethrum I and Pyrethrum-II" pp. 399-413,
in Analytical Methods for Pesticides, Plant Growth Regulators and Food
Additives, Vol. II (Zweig, G., ed.) Academic Press, New York).
Miyamoto, J., Suzuki, T., and Nakae, C. "Metabolism of Phenothrin or
3-phenoxybenzyl d-trans-Chrysanthemumate in Mammals". Pestic. Biochem.
Physiol., 4, 438.
Mollard, V. "Samples from Bread Research Institute". Reference PH
10/141 Wellcome Australasia Ltd.
Nambu, K., Ohkawa, H. and Miyamoto, J. "Metabolic fate of phenothrin
in plants and soils" Sumitomo Chemical Co., Ltd. (1979a).
Nambu, K., Takimoto, Y. and Miyamoto, J. "Degradation and fate of
phenothrin in stored wheat grains". Sumitomo Chemical Co., Ltd.
(1979b).
Simpson, B.W. "Method for pyrethroid residues on wheat, sorghum
pollard, flour and bread". (1979).
Soderland, D.M. and Casida, J.E. "Effects of Pyrethroid Structure on
Rats of Hydrolysis and Oxidation by Mouse Liver Microsomal Enzymes"
Pestic. Biochem. Physiol. 7, 391-401.
Suzuki, T., Ohno, N. and Miyamoto, J. "New Metabolites of
(+)-cis-Fenothrin, 3 Phenoxybenzyl (+)-cis-Chrysanthemumate, in
Rats" J. Pesticide Sci. 1, 151.
Suzuki, T. and Miyamoto, J. "Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome". Pestic. Biochem.
Physiol., 8, 186.
Takimoto, Y., Kato, T. and Miyamoto, J. "Residue analyses of
phenothrin (S-2539) in crops". Sumitomo Chemical Co., Ltd. (1977).
Wellcome Australasia. "Evaluation of phenothrin as a potential grain
protectant". (1977).
Working Party on Grain Protectants. "Final report on silo scale
experimental application of grain protectant chemicals, 1977-1978".
Working Party of Grain Protectants "Interim report on pilot usage
trials 1978-1979". (1979a).
Working Party on Grain Protectants "Summary of pilot usage trials,
1978-1979". (1979b).
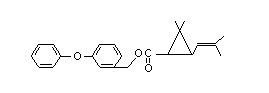 Other information on identity and properties
Molecular weight: 350.5
State: colorless liquid
Specific gravity: d25 1.058
25
Viscosity: 195.1 cp at 20°C
87.4 cp at 30°C
Vapour pressure: 1.2 × 10-6 mmHg at 20°c
Refractive index: n25 1.5483
D
Solubility:
(g/l at room Highly soluble in the following solvents
temperature) solvents (>500 g/l methanol, isopropanol,
ethylcellosolve, diethyl ether, xylene,
n-hexane alpha-methyl, naphthalene,
cyclohexane, chloroform, acetonitrile,
dimethyl formamide, kerosene.
Solubility in water: 1.4 ± 0.1 ppm at 30°C
Stability: No breakdown after one year at room
temperature out of the light. Stable in
neutral and weak acidic conditions. Stable
in most solvents except methanol, ethyl
cellosolve, o-cresol, dimethylsulfoxide.
Typical percent purity of the technical material
% by weight
3-phenoxy (±)-cis, trans-chrysanthemate 92.5 - 94.5
The meeting was also provided by the manufacturer with complete
information on the chemical nature and quantity of the residual
manufacturing impurities in the technical material. This information
is on file with the Secretariat of the meeting. The data in this
monograph are from phenothrin meeting these specifications as are the
recommendations for residue limits.
RESIDUES IN FOOD AND THEIR EVALUATIONS
Use Pattern
Phenothrin is a new pyrethroid being currently developed. It is more
stable than natural pyrethrins and other novel pyrethroids developed
earlier, but it is relatively degradable by sunlight. In the light of
its insecticidal properties and its relatively low toxicity to
mammals, phenothrin and its optically active isomers show much promise
as a safe and useful insecticide in various fields including
households, public health, food storage, ornamentals and livestock
production.
Principal uses currently are:
1. in household aerosols and sprays formulated in combination with
tetramethrin, piperonyl butoxide, carbamates and/or fenitrothion
for control of various species of plant hoppers and for control of
house-flies, mosquitoes, cockroaches and other household or public
health insect pests.
2. in grain protection and disinfestation.
Treatment of plants
Repeated and multiple applications of organophosphorus compounds (e.g.
malathion) and carbamates (e.g. carbaryl) for control of green rice
leaf hoppers in paddy fields have resulted in resistant strains in
some parts of Japan. Several efforts have been made to solve the
problem.
Yoshioka and others reported laboratory and field trials in which not
only susceptible but also resistant leaf hoppers and other hoppers
were controlled by the treatment of 30 to 40 kg/ha of combined dust
formulations containing various compounds including small amounts of
phenothrin (e.g. 0.8% by wt) and carbamates such as BPMC,
O-sec-buthylphenyl-N-methyl carbamate, MTMC, m-tolyl-N-methyl
carbamate and MPMC, 3,4-xylyl-N-methyl carbamate (e.g. 2.0% by wt).
It is expected these formulations will be used for control of hoppers
within the next one or two years.
Phenothrin has advantages of a short residual life on food plants,
quick insecticidal action and efficient control of pest strains
resistant to organochlorine and organophosphorus insecticides. It is
currently being developed for controlling various pests on a range of
fruit and vegetables.
Post-harvest use
Phenothrin offers advantages of improved cost/efficiency (of
pyrethrins and bioresmethrin), reliable availability and a high degree
of mammalian safety as a potential grain protectant. Phenothrin
applied at 2 mg/kg has a similar level of efficiency as commercial
applications of bioresmethrin and pyrethrins in the control of insects
resistant to chlorinated hydrocarbon and/or organophosphorus grain
protectants.
The pesticide is stable in grain masses, but is subject to degradation
on exposure to ultraviolet light (Nambu et al., 1979b).
In a series of practical commercial trials undertaken in Australia and
involving exposures to various insects known to be resistant to other
commonly-used pesticides, the potentialities of phenothrin for the
protection of grains in store were well demonstrated (Desmarchelier
et al., 1979; Bengston, et al., 1978; Working Party of Grain
Protectants, 1979 a and b).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatment
Supervised trials on rice, green peppers and cabbage have been carried
out in Japan. Replicated results are available from each crop
evaluated (Takimoto et al., 1977). The results are given in Table 1.
Post-harvest treatment
All trials on wheat, sorghum and barley grains were carried out in
Australia except one in Japan under laboratory conditions with
C-14-labelled (+)-trans and (+)-cis phenothrin. Most of the
Australian trials involved samples from commercial storages of up to
7,435 tonnes treated with phenothrin on the proviso that the grain was
ultimately used only as animal feed-stuff. The temperature ranged
from 18°C to 36°C and the moisture from 9.0% to 13.0%. Applications
were made with phenothrin or d-phenothrin mostly in combination with
piperonyl butoxide and/or fenitrothion. The application rates were
mostly up to 3.0 mg/kg, but on wheat grain phenothrin was applied at
up to 8 mg/kg. Wheat grains were stored for up to 9 months, barley
for up to 5 months and sorghum up to 6 months, and samples withdrawn
for residue analysis at each indicated times. The residues of
phenothrin and d-phenothrin are shown in Tables 2 and 3 respectively.
Relatively high amounts of phenothrin and d-phenothrin were detected
after 5-9 months in those grains. For example, in some cases more
than half of the applied phenothrin and d-phenothrin remained in grain
even after 9 months. In the experiment with C-14-labelled
(+)-trans- and (+)-cis phenothrin, only slight decomposition of
both isomers was found months after treatment.
Distribution of C-14-labelled (+)-trans and (+)-cis phenothrin in
germ, endosperm and seed coat was examined after 0, 1, 3 and 6 months
of storage at 15°C and 30°C (Nambu et al. 1979b). The results are
presented in Table 4. Most of the applied isomers were localized at
the seed coat and the distribution pattern did not change materially
during 6 month storage. Those results were evidenced by
radioautograms.
Table 1. Residues of phenothrin in rice, green peppers and cabbage
(Tests undertaken in Japan using a 25% EC formulation (Takimoto et al, 1977)
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Rice Straw Hulled rice
(0.375) 6 3-10 7 0.62 0.016
14 0.45 0.009
Rice Straw Hulled rice
(0.375) 6 6-9 7 1.25 0.078
14 1.58 0.040
Green pepper
(0.50) 3 7 1 0.324
3 0.660
7 0.334
6 7 1 1.038
3 0.625
7 0.428
(0.50) 3 7 1 0.333
3 0.268
7 0.469
6 7 1 0.363
3 0.445
7 0.244
Cabbage
(0.375) 4 7 3 <0.005
7 <0.005
14 <0.005
21 <0.005
Table 1. Continued...
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Cabbage 8 7 3 0.005
(cont'd) 7 0.017
14 <0.005
21 <0.005
Cabbage
(0.375) 5 7-8 3 <0.005
7 <0.005
14 <0.005
21 <0.005
9 6-8 3 0.011
7 <0.005
14 <0.005
21 <0.005
Table 2. Residues of phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1977) 6* 3 4.4
6 4.4
9 3.2,3.2** Welcome
8* 3 6.7 Australasia,
6 6.9 1977
9 5.8, 5.2**
4* 3 3.0
6 2.7
9 1.6,2.8**
(1977/78) 1.9+ 0.25 1.8
1.5 1.42 Working Party
3 1.45 on Grain
4.5 1.71 Protectants,
6 1.38 1978
9 1.1
(1978) 1.85+ 3 1.29
4.5 1.3
6 1.1
7 1.21
(1978) 2.08+ 1 1.21
2.00+ 1 1.56
2.00+ 1 1.23 Working Party
1.89+ 1 1.23 on Grain
2.1+ 1 1.50 Protectants,
2.02+ 1 1.30 1979a
2.29+ 1 1.30
Wheat
(1979) 1.49+ 1 1.85,1.28**
2 1.35
3 1.75
4 1.75
2.01+ 1 2.25,1.62**
2 1.8
3 2.05, 1.45**
4 1.6
Table 2. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
(1979) 2.01+ 20 days 2.3
48 days 1.9
75 days 2.3 Working Party
81 days 1.6 on Grain
1.49+ 24 days 1.9 Protectants,
55 days 1.5 1979b
79 days 1.9
83 days 1.8
Sorghum
(1978) 2.0+ 1 week 1.86
12 weeks 2.72 Bengston
17 weeks 1.59 1978
26 weeks 1.56
* Nominal application
** Residues were determined in two different laboratories from the
same sample
+ Rate of application calculated from total grams phenothrin used
divided by tons of grain treated.
Table 3. Residues of d-phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.7
2 0.9
3 1.1
5 1.0
6 0.5
8 1.1
1.7+ 1 2.4
2 2.1
3 1.4
4 1.0
5 1.1
6 0.9 Desmarchelier
7 0.9 et al., 1979c
8 1.0
9 1.0
1.9+ 1 1.6
2 0.8
4 0.8
5 1.0
6 0.5
8 0.6
9 0.6
2.0+ 1 1.5
2 1.7
5 1.6
2.0+ 1 0.7
3 1.0
1.8+ 1 1.5
2 1.3
4 0.7
6 0.7
1.4+ 1 1.5
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.3
2 1.3
4 1.1 Desmarchelier
5 1.1 et al., 1979c
6 0.9
7 0.8
9 0.8
1.9+ 1 0.9
2 0.7
3 0.8
6 0.8
2.1+ 1 1.0
3 0.7
4 1.1
5 0.9
2.0+ 1 1.0
3 1.0
4 0.9
5 1.5 Desmarchelier
7 1.5 et al., 1979c
2.6+ 2 1.3
3 0.6
6 1.1
2.2+ 2 1.0
3 0.8
5 0.8
6 1.2
4 1/2 3.87*,
3.93** Nambu et al,
1 3.80, 1979b
3.90
(+)-trans-
phenothrin 3 3.78, 3.80
6 3.72, 3.80
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 4 1/2 3.77*, 3.80**
1 3.84, 3.94
(+)-trans
phenothrin 3 3.87, 3.80
6 3.69, 3.66
4 1/2 3.68*, 3.67**
1 3.82, 3.78
(+)-trans
phenothrin 3 3.88,
3.80 Nambu et al.
6 3.69, 1979b
3.66
4 1/2 3.85*, 3.88**
1 3.80, 4.00
(+)-cis-
phenothrin 3 3.80, 3.93
6 3.73, 3.80
4 1/2 3.82*, 3.99**
1 4.03, 3.95
(+)-cis-
phenothrin 3 3.88, 4.02
6 3.84, 3.55
4 1/2 3.90*, 4.10**
1 4.03, 3.83
(+)-cis-
phenothrin 3 3.91, 3.89
6 3.76, 3.72
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Barley
(1979/79) 2.6+ 1 2.0
3 1.1
5 1.5
Desmarchelier
et al, 1979c
2.9+ 2 1.5
3 1.4
4 1.1
* Grain was stored at the temperature of 15°C
** Grain was stored at the temperature of 30°C
+ Rate of application calculated from total grams
phenothrin used divided by tons of grain treated.
Table 4. Distribution of (+)-trans and (+)-cis phenothrin after storage of treated grain
(Nambu et al., 1979b)
Compound Temperature Months Residue (mg/kg)
Whole grain Germ Endosperm Seed coat
(+)-trans 15°C 0 3.81 0.08 0.62 2.82
-phenothrin
(4 mg/kg) 1 3.80 0.1 0.64 2.86
3 3.78 0.09 0.63 3.09
6 3.72 0.08 0.51 2.97
30°C 1 3.90 0.12 0.77 2.95
3 3.80 0.11 0.69 2.97
6 3.80 0.12 0.58 3.06
(+)-trans
-phenothrin 15°C 6 3.69 0.10 0.62 2.94
+ PBO 30°C 6 3.66 0.10 0.60 2.65
-Fenitrothion
(4+20+4,
mg/kg)
(+)-cis-
phenothrin 15°C 0 3.85 0.09 0.67 3.14
(4 mg/kg) 1 3.80 0.08 0.70 3.08
3 3.80 0.08 0.64 3.11
6 3.73 0.11 0.62 2.63
30°C 1 4.00 0.08 0.80 3.02
3 3.93 0.12 0.68 3.28
6 3.80 0.14 0.58 2.75
(+)-cis
phenothrin 15°C 6 3.76 0.12 0.58 2.92
+ PBO 30°C 6 3.72 0.10 0.59 2.79
+ Fenitrothion
(4+20+4
mg/kg)
PBO: Technical piperonyl butoxide
FATE OF RESIDUES
In animals
(+)-trans-phenothrin labelled with C-14 in the methylene group of the
alcohol moiety was administered orally to male Sprague-Dawley rats at
the rate of 200 mg/kg. The administered C-14 was almost completely
eliminated via the urine (ca. 60%) and faeces (ca. 40%) during three
days. No detectable C-14-C02 was expired. The levels of total C-14
and phenothrin isomers in the blood, liver, kidney and brain reached
maxima at three hours after administration and then gradually
decreased to one-tenth to one-twentieth of the maxima in 24 hours.
The major metabolites identified were 3-phenoxybenzoic acid (free and
glycine conjugate), 3-(4-hydroxybenzoic) acid and 3-phenoxybenzyl
alcohol. A small amount of unmetabolized (+)-trans-phenothrin was
excreted into the faeces (Miyamoto et al., 1974).
Metabolism of (+)-cis-phenothrin in male rats was also studied.
About 65% of the administered C-14 was recovered in faeces for three
days post-treatment. The faeces contained three ester metabolites
which resulted from hydroxylation at the 4'-phenoxy position of the
alcohol moiety, oxidation of trans-isobutenyl methyl group and
hydroxylation of cis-geminal-dimethyl group of the acid moiety. A
small amount of 3-phenoxybenzoic acid was also found (Suzuki et al.,
1976).
Thus, metabolism of phenothrin isomers proceeded rapidly in the rat
mainly via hydrolysis of the ester linkage and oxidation at several
positions of both alcohol and acid moieties (Fig. 1). The metabolites
derived from the alcohol moiety were almost completely excreted into
the urine and faeces.
Metabolism studies in vitro revealed that liver microsomal enzymes
from rats, mice, guinea pigs, rabbits and dogs hydrolyse
(+)-trans-phenothrin much faster than the cis-isomers, but the
oxidation rate by mouse liver microsomes was somewhat faster with the
(+)-cis isomer than with the (+)-trans isomer (Suzuki and
Miyamoto, 1978; Soderland and Casida, 1977; Casida et al., 1979).
Other information on identity and properties
Molecular weight: 350.5
State: colorless liquid
Specific gravity: d25 1.058
25
Viscosity: 195.1 cp at 20°C
87.4 cp at 30°C
Vapour pressure: 1.2 × 10-6 mmHg at 20°c
Refractive index: n25 1.5483
D
Solubility:
(g/l at room Highly soluble in the following solvents
temperature) solvents (>500 g/l methanol, isopropanol,
ethylcellosolve, diethyl ether, xylene,
n-hexane alpha-methyl, naphthalene,
cyclohexane, chloroform, acetonitrile,
dimethyl formamide, kerosene.
Solubility in water: 1.4 ± 0.1 ppm at 30°C
Stability: No breakdown after one year at room
temperature out of the light. Stable in
neutral and weak acidic conditions. Stable
in most solvents except methanol, ethyl
cellosolve, o-cresol, dimethylsulfoxide.
Typical percent purity of the technical material
% by weight
3-phenoxy (±)-cis, trans-chrysanthemate 92.5 - 94.5
The meeting was also provided by the manufacturer with complete
information on the chemical nature and quantity of the residual
manufacturing impurities in the technical material. This information
is on file with the Secretariat of the meeting. The data in this
monograph are from phenothrin meeting these specifications as are the
recommendations for residue limits.
RESIDUES IN FOOD AND THEIR EVALUATIONS
Use Pattern
Phenothrin is a new pyrethroid being currently developed. It is more
stable than natural pyrethrins and other novel pyrethroids developed
earlier, but it is relatively degradable by sunlight. In the light of
its insecticidal properties and its relatively low toxicity to
mammals, phenothrin and its optically active isomers show much promise
as a safe and useful insecticide in various fields including
households, public health, food storage, ornamentals and livestock
production.
Principal uses currently are:
1. in household aerosols and sprays formulated in combination with
tetramethrin, piperonyl butoxide, carbamates and/or fenitrothion
for control of various species of plant hoppers and for control of
house-flies, mosquitoes, cockroaches and other household or public
health insect pests.
2. in grain protection and disinfestation.
Treatment of plants
Repeated and multiple applications of organophosphorus compounds (e.g.
malathion) and carbamates (e.g. carbaryl) for control of green rice
leaf hoppers in paddy fields have resulted in resistant strains in
some parts of Japan. Several efforts have been made to solve the
problem.
Yoshioka and others reported laboratory and field trials in which not
only susceptible but also resistant leaf hoppers and other hoppers
were controlled by the treatment of 30 to 40 kg/ha of combined dust
formulations containing various compounds including small amounts of
phenothrin (e.g. 0.8% by wt) and carbamates such as BPMC,
O-sec-buthylphenyl-N-methyl carbamate, MTMC, m-tolyl-N-methyl
carbamate and MPMC, 3,4-xylyl-N-methyl carbamate (e.g. 2.0% by wt).
It is expected these formulations will be used for control of hoppers
within the next one or two years.
Phenothrin has advantages of a short residual life on food plants,
quick insecticidal action and efficient control of pest strains
resistant to organochlorine and organophosphorus insecticides. It is
currently being developed for controlling various pests on a range of
fruit and vegetables.
Post-harvest use
Phenothrin offers advantages of improved cost/efficiency (of
pyrethrins and bioresmethrin), reliable availability and a high degree
of mammalian safety as a potential grain protectant. Phenothrin
applied at 2 mg/kg has a similar level of efficiency as commercial
applications of bioresmethrin and pyrethrins in the control of insects
resistant to chlorinated hydrocarbon and/or organophosphorus grain
protectants.
The pesticide is stable in grain masses, but is subject to degradation
on exposure to ultraviolet light (Nambu et al., 1979b).
In a series of practical commercial trials undertaken in Australia and
involving exposures to various insects known to be resistant to other
commonly-used pesticides, the potentialities of phenothrin for the
protection of grains in store were well demonstrated (Desmarchelier
et al., 1979; Bengston, et al., 1978; Working Party of Grain
Protectants, 1979 a and b).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest treatment
Supervised trials on rice, green peppers and cabbage have been carried
out in Japan. Replicated results are available from each crop
evaluated (Takimoto et al., 1977). The results are given in Table 1.
Post-harvest treatment
All trials on wheat, sorghum and barley grains were carried out in
Australia except one in Japan under laboratory conditions with
C-14-labelled (+)-trans and (+)-cis phenothrin. Most of the
Australian trials involved samples from commercial storages of up to
7,435 tonnes treated with phenothrin on the proviso that the grain was
ultimately used only as animal feed-stuff. The temperature ranged
from 18°C to 36°C and the moisture from 9.0% to 13.0%. Applications
were made with phenothrin or d-phenothrin mostly in combination with
piperonyl butoxide and/or fenitrothion. The application rates were
mostly up to 3.0 mg/kg, but on wheat grain phenothrin was applied at
up to 8 mg/kg. Wheat grains were stored for up to 9 months, barley
for up to 5 months and sorghum up to 6 months, and samples withdrawn
for residue analysis at each indicated times. The residues of
phenothrin and d-phenothrin are shown in Tables 2 and 3 respectively.
Relatively high amounts of phenothrin and d-phenothrin were detected
after 5-9 months in those grains. For example, in some cases more
than half of the applied phenothrin and d-phenothrin remained in grain
even after 9 months. In the experiment with C-14-labelled
(+)-trans- and (+)-cis phenothrin, only slight decomposition of
both isomers was found months after treatment.
Distribution of C-14-labelled (+)-trans and (+)-cis phenothrin in
germ, endosperm and seed coat was examined after 0, 1, 3 and 6 months
of storage at 15°C and 30°C (Nambu et al. 1979b). The results are
presented in Table 4. Most of the applied isomers were localized at
the seed coat and the distribution pattern did not change materially
during 6 month storage. Those results were evidenced by
radioautograms.
Table 1. Residues of phenothrin in rice, green peppers and cabbage
(Tests undertaken in Japan using a 25% EC formulation (Takimoto et al, 1977)
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Rice Straw Hulled rice
(0.375) 6 3-10 7 0.62 0.016
14 0.45 0.009
Rice Straw Hulled rice
(0.375) 6 6-9 7 1.25 0.078
14 1.58 0.040
Green pepper
(0.50) 3 7 1 0.324
3 0.660
7 0.334
6 7 1 1.038
3 0.625
7 0.428
(0.50) 3 7 1 0.333
3 0.268
7 0.469
6 7 1 0.363
3 0.445
7 0.244
Cabbage
(0.375) 4 7 3 <0.005
7 <0.005
14 <0.005
21 <0.005
Table 1. Continued...
Crop Interval between Treatment
(Rate of Number of applications to sampling Residue
application applications (days) interval (mg/kg)
kg ai/ha) (days)
Cabbage 8 7 3 0.005
(cont'd) 7 0.017
14 <0.005
21 <0.005
Cabbage
(0.375) 5 7-8 3 <0.005
7 <0.005
14 <0.005
21 <0.005
9 6-8 3 0.011
7 <0.005
14 <0.005
21 <0.005
Table 2. Residues of phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1977) 6* 3 4.4
6 4.4
9 3.2,3.2** Welcome
8* 3 6.7 Australasia,
6 6.9 1977
9 5.8, 5.2**
4* 3 3.0
6 2.7
9 1.6,2.8**
(1977/78) 1.9+ 0.25 1.8
1.5 1.42 Working Party
3 1.45 on Grain
4.5 1.71 Protectants,
6 1.38 1978
9 1.1
(1978) 1.85+ 3 1.29
4.5 1.3
6 1.1
7 1.21
(1978) 2.08+ 1 1.21
2.00+ 1 1.56
2.00+ 1 1.23 Working Party
1.89+ 1 1.23 on Grain
2.1+ 1 1.50 Protectants,
2.02+ 1 1.30 1979a
2.29+ 1 1.30
Wheat
(1979) 1.49+ 1 1.85,1.28**
2 1.35
3 1.75
4 1.75
2.01+ 1 2.25,1.62**
2 1.8
3 2.05, 1.45**
4 1.6
Table 2. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
(1979) 2.01+ 20 days 2.3
48 days 1.9
75 days 2.3 Working Party
81 days 1.6 on Grain
1.49+ 24 days 1.9 Protectants,
55 days 1.5 1979b
79 days 1.9
83 days 1.8
Sorghum
(1978) 2.0+ 1 week 1.86
12 weeks 2.72 Bengston
17 weeks 1.59 1978
26 weeks 1.56
* Nominal application
** Residues were determined in two different laboratories from the
same sample
+ Rate of application calculated from total grams phenothrin used
divided by tons of grain treated.
Table 3. Residues of d-phenothrin in grain after storage
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.7
2 0.9
3 1.1
5 1.0
6 0.5
8 1.1
1.7+ 1 2.4
2 2.1
3 1.4
4 1.0
5 1.1
6 0.9 Desmarchelier
7 0.9 et al., 1979c
8 1.0
9 1.0
1.9+ 1 1.6
2 0.8
4 0.8
5 1.0
6 0.5
8 0.6
9 0.6
2.0+ 1 1.5
2 1.7
5 1.6
2.0+ 1 0.7
3 1.0
1.8+ 1 1.5
2 1.3
4 0.7
6 0.7
1.4+ 1 1.5
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 2.0+ 1 1.3
2 1.3
4 1.1 Desmarchelier
5 1.1 et al., 1979c
6 0.9
7 0.8
9 0.8
1.9+ 1 0.9
2 0.7
3 0.8
6 0.8
2.1+ 1 1.0
3 0.7
4 1.1
5 0.9
2.0+ 1 1.0
3 1.0
4 0.9
5 1.5 Desmarchelier
7 1.5 et al., 1979c
2.6+ 2 1.3
3 0.6
6 1.1
2.2+ 2 1.0
3 0.8
5 0.8
6 1.2
4 1/2 3.87*,
3.93** Nambu et al,
1 3.80, 1979b
3.90
(+)-trans-
phenothrin 3 3.78, 3.80
6 3.72, 3.80
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Wheat
(1978/79) 4 1/2 3.77*, 3.80**
1 3.84, 3.94
(+)-trans
phenothrin 3 3.87, 3.80
6 3.69, 3.66
4 1/2 3.68*, 3.67**
1 3.82, 3.78
(+)-trans
phenothrin 3 3.88,
3.80 Nambu et al.
6 3.69, 1979b
3.66
4 1/2 3.85*, 3.88**
1 3.80, 4.00
(+)-cis-
phenothrin 3 3.80, 3.93
6 3.73, 3.80
4 1/2 3.82*, 3.99**
1 4.03, 3.95
(+)-cis-
phenothrin 3 3.88, 4.02
6 3.84, 3.55
4 1/2 3.90*, 4.10**
1 4.03, 3.83
(+)-cis-
phenothrin 3 3.91, 3.89
6 3.76, 3.72
Table 3. Continued...
Rate of Treatment
Grain application to sampling Residue
(Year) (mg ai/kg) interval (mg/kg) References
(months)
Barley
(1979/79) 2.6+ 1 2.0
3 1.1
5 1.5
Desmarchelier
et al, 1979c
2.9+ 2 1.5
3 1.4
4 1.1
* Grain was stored at the temperature of 15°C
** Grain was stored at the temperature of 30°C
+ Rate of application calculated from total grams
phenothrin used divided by tons of grain treated.
Table 4. Distribution of (+)-trans and (+)-cis phenothrin after storage of treated grain
(Nambu et al., 1979b)
Compound Temperature Months Residue (mg/kg)
Whole grain Germ Endosperm Seed coat
(+)-trans 15°C 0 3.81 0.08 0.62 2.82
-phenothrin
(4 mg/kg) 1 3.80 0.1 0.64 2.86
3 3.78 0.09 0.63 3.09
6 3.72 0.08 0.51 2.97
30°C 1 3.90 0.12 0.77 2.95
3 3.80 0.11 0.69 2.97
6 3.80 0.12 0.58 3.06
(+)-trans
-phenothrin 15°C 6 3.69 0.10 0.62 2.94
+ PBO 30°C 6 3.66 0.10 0.60 2.65
-Fenitrothion
(4+20+4,
mg/kg)
(+)-cis-
phenothrin 15°C 0 3.85 0.09 0.67 3.14
(4 mg/kg) 1 3.80 0.08 0.70 3.08
3 3.80 0.08 0.64 3.11
6 3.73 0.11 0.62 2.63
30°C 1 4.00 0.08 0.80 3.02
3 3.93 0.12 0.68 3.28
6 3.80 0.14 0.58 2.75
(+)-cis
phenothrin 15°C 6 3.76 0.12 0.58 2.92
+ PBO 30°C 6 3.72 0.10 0.59 2.79
+ Fenitrothion
(4+20+4
mg/kg)
PBO: Technical piperonyl butoxide
FATE OF RESIDUES
In animals
(+)-trans-phenothrin labelled with C-14 in the methylene group of the
alcohol moiety was administered orally to male Sprague-Dawley rats at
the rate of 200 mg/kg. The administered C-14 was almost completely
eliminated via the urine (ca. 60%) and faeces (ca. 40%) during three
days. No detectable C-14-C02 was expired. The levels of total C-14
and phenothrin isomers in the blood, liver, kidney and brain reached
maxima at three hours after administration and then gradually
decreased to one-tenth to one-twentieth of the maxima in 24 hours.
The major metabolites identified were 3-phenoxybenzoic acid (free and
glycine conjugate), 3-(4-hydroxybenzoic) acid and 3-phenoxybenzyl
alcohol. A small amount of unmetabolized (+)-trans-phenothrin was
excreted into the faeces (Miyamoto et al., 1974).
Metabolism of (+)-cis-phenothrin in male rats was also studied.
About 65% of the administered C-14 was recovered in faeces for three
days post-treatment. The faeces contained three ester metabolites
which resulted from hydroxylation at the 4'-phenoxy position of the
alcohol moiety, oxidation of trans-isobutenyl methyl group and
hydroxylation of cis-geminal-dimethyl group of the acid moiety. A
small amount of 3-phenoxybenzoic acid was also found (Suzuki et al.,
1976).
Thus, metabolism of phenothrin isomers proceeded rapidly in the rat
mainly via hydrolysis of the ester linkage and oxidation at several
positions of both alcohol and acid moieties (Fig. 1). The metabolites
derived from the alcohol moiety were almost completely excreted into
the urine and faeces.
Metabolism studies in vitro revealed that liver microsomal enzymes
from rats, mice, guinea pigs, rabbits and dogs hydrolyse
(+)-trans-phenothrin much faster than the cis-isomers, but the
oxidation rate by mouse liver microsomes was somewhat faster with the
(+)-cis isomer than with the (+)-trans isomer (Suzuki and
Miyamoto, 1978; Soderland and Casida, 1977; Casida et al., 1979).
 In Plants
When (+)-trans and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were each applied to the leaf
surface of bean and rice plants at rate of 48-50 g/20 cm2, both
isomers rapidly disappeared from the treated leaves of both plants
with half-lives of less than one day. On and/or in these plants, both
isomers underwent ozonolysis at the isobutenyl double bond to yield
the corresponding ozonides which were rapidly decomposed to the
corresponding aldehydes and carboxylic acids. These ester products
were subsequently metabolized via cleavage of the ester linkage,
hydroxylation at 2'- and 4'- positions of the alcohol moiety, and
oxidation of the benzyl alcohols to the benzoic acids. The resultant
phenols and carboxylic acids were further conjugated with sugars. The
parent compounds and their degradation products were hardly
transferred from the application site to other parts of plants (Nambu
et al., 1979a).
Bean plant seedlings were planted in Kodaira light clay and Katano
sandy loam soils treated with 1.0 mg/kg of C-14, phenothrin isomers.
After 30 days, 0.01 - 0.03 mg/kg and 0.04 - 0.09 mg/kg equivalents of
C-14 were found in pods and seeds, and shoots respectively, although
0.21 - 3.48 mg/kg of C-14 was retained in roots. No parent compound
was detected in shoots (Nambu et al., 1979a).
In soil
(+)-trans and (+)-cis-phenothrin were applied to Kodaira light
clay and Katano sandy loam soils at the rate of 1.0 mg/kg, and the
treated soils were held at 25 ± 2°C in the dark. Under upland
conditions, both isomers were rapidly decomposed in these soils with
half-lives of 1-2 days. On the other hand, the rate of degradation of
both isomers was much slower under flooded conditions, and half-lives
were 1-2 weeks and 1-2 months for the (+)-trans and (+)-cis
isomers, respectively. Phenothrin isomers were decomposed in the
soils via cleavage of the ester linkage, hydroxylation at 4'-position
of the alcohol moiety, cleavage of the diphenyl ether linkage and
oxidation of benzyl alcohols to benzoic acids. These products were
not persistent in the soils under upland conditions and the labelled
carbon was finally decomposed to C-14-CO2 (Nambu et al., 1979b).
Phenothrin was impregnated at a concentration of 1 g/kg on to a
laterite from Uganda. The treated soil was stored at 25°C and 20 or
80% relative humidity (R.H.). The half-life of the insecticide in
days was 40 with 20% RH and 150 with 80% RH (Barlow et al., 1977).
(+)-trans and (+)-cis-phenothrin and their degradation products
were hardly eluted with water from Kodaira light clay and Katano sandy
loam soils, whereas polar products including 3-phenoxybenzoic acid
were slightly eluted from Muko sand (Nambu et al., 1979b).
In storage and processing
(+)-trans- and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were applied to wheat grains at
a concentration of 4 mg/kg alone or together with 20 mg/kg piperonyl
butoxide and 4 mg/kg fenitrothion. The treated grains with 11-12%
moisture content were stored at 15 and 30°C in the dark. During 6
months storage period, both isomers were slightly decomposed
regardless of the presence and absence of piperonyl butoxide and
fenitrothion. The residue levels of phenothrin isomers were 3.64-3.78
mg/kg in whole wheat grain, 0.79-0.83 mg/kg in flour and 9.24-11.4
mg/kg in bran after 6 months. The C-14 residues were mainly located
in the seed coat as evidenced by radio-autography and radioanalysis.
Small amounts (less than 2% of the applied C-14) of 3-phenoxybenzyl
alcohol and 3-phenoxybenzoic acid were found. The phenothrin residues
in flour hardly decreased to a small extent through baking processes
leaving 0.66-0.69 mg/kg of phenothrin isomers in bread (Nambu et al,
1979b).
All studies were carried out in Australia to determine the residues of
phenothrin or d-phenothrin in the products after processing and
cooking of wheat, oats, barley and rice grain, except one experiment
with wheat grain in Japan using labelled compounds.
Wheat grain containing 0.5-3.9 mg/kg of phenothrin was used for the
studies, and the residue results after processing and cooking are
summarised in Table 5. Although higher residues were found in bran
and pollard after processing, the residues in flour were reduced and
reached 10.6-25.0% of those found in whole grain. After cooking the
residues in bread did not exceed 0.2 mg/kg in Australian trials, but
in the Japanese trial those residues of C-14-labelled (+)-trans and
(+)-cis phenothrin were relatively high (0.69 mg/kg and 0.66 mg/kg)
corresponding to the higher residues in flour (0.79 mg/kg and 0.91
mg/kg). This is supposed to be caused by contamination of a large
portion of testa in flour from the crease area of grain, while in
normal commercial flour milling situations only a very small portion
of testa would go into flour. In gluten, the residue was higher (1.9
mg/kg) than that in flour (0.3 mg/kg). However, the pesticide
residues in gluten does not cause concern, because normally there
would be a 2% maximum addition of gluten in bread.
Oats containing 1.2 mg/kg of phenothrin were processed and cooked
(Desmarchelier, 1979a). Most of the residues were carried in oat
hulls, and low residue levels were found in both groats (0.3 mg/kg)
and rolled oats (0.1 mg/kg). In porridge the residue was below the
detection limit of 0.05 mg/kg.
Barley containing 3.2 mg/kg and 0.92 mg/kg phenothrin was processed
into malt, wort and cattle feed. Of these products, the highest
residues were found in the malt, but these levels, 0.63 mg/kg and 0.15
mg/kg were less than one-fifth of that in barley. In wort, the
residues were less than the detection limit of 0.02 mg/kg.
Table 5. Residues of phenothrin and d-phenothrin prior to and after processing and cooking of wheat
Residue (mg/kg)
Compound Reference
Wholemeal White
Wheat Bran Pollard Flour Bread Bread Gluten Starch
Phenothrin 1.2 5.6 3.6 0.21 0.74 0.16 - - Bengston 1979
3.0 10.0 4.1 0.82 - 0.18 - - Desmarchelier, 1979a
1.5 6.0 3.0 0.4 - 0.1 - - "
0.9 3.0 2.0 0.15 - 0.05 - - "
0.5 1.6 0.9 0.09 - <0.05 - - "
3.9 8.1 1.6 0.65 - - 1.9 <0.05 "
1.2 4.0 1.8 0.3 0.4-0.6 0.1-0.2 - - Ardley, 1979
2.0 - - - - <0.1 - - Mollard, 1979
4.3 - - - - <0.1 - - "
(+)-trans-phenothrin 3.78 11.4 - 0.79 - 0.69 - - Nambu et al
1979b
(+)-cis-phenothrin 3.64 9.24 - 0.91 - 0.66 - -
A nominal application of 8 mg/kg phenothrin was applied to husked
(brown) rice and polished (white) rice. Six months after application,
husked (6.2 mg/kg of phenothrin) and polished (5.9 mg/kg) rice were
cooked in a minimum amount of boiling water for 25 minutes. Unhusked
rice was also treated with d-phenothrin at a rate of 8 mg/kg, and
after 6 months of storage when 4.7 mg/kg of phenothrin was detected,
rice was milled into husked and polished rice, and cooked as described
above. As shown in Table 6, the cooking of rice had the effect of
reducing residues by from 34% to 60%. It also has indicated that the
milling of rice from husked (paddy) to unhusked (brown) or polished
(white) is a major means of residue reduction, that is, 90% and 97%
respectively.
Table 6. Residues of d-phenothrin prior to and following processing
and cooking of rice
Residue (mg/kg)
Unhusked Husked Polished Cooked
- 6.2 - 4.1
- - 5.9 3.1
4.7 0.50 - 0.2
4.7 - 0.15 <0.1
(Desmarchelier et al., 1979d)
Phenothrin and d-phenothrin were applied to grain at the level of 4
mg/kg and 2 mg/kg, respectively, and have encountered levels of
moisture and three temperature levels. As shown in Fig. 2 and 3, both
increasing temperature (25, 30 and 37°C) at a constant moisture of
11.8% and increasing moisture (9.3, 11.8 and 13.4%) at a constant
temperature of 30°C led to increased breakdown of phenothrin and
d-phenothrin (Maguire, 1977).
Photochemical Degradation
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 and 7 days. Under these conditions phenothrin was more unstable
than permethrin and more stable than bioresmethrin (Elliot et al.,
1973). In a separate study, decomposition of phenothrin on glass by
irradiation of 270-370 mm light was determined. The half-life for
phenothrin was one day (Barlow et al., 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Due to the newness of phenothrin and its largely experimental status,
no information was available on residues in food in commerce or at
consumption.
In Plants
When (+)-trans and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were each applied to the leaf
surface of bean and rice plants at rate of 48-50 g/20 cm2, both
isomers rapidly disappeared from the treated leaves of both plants
with half-lives of less than one day. On and/or in these plants, both
isomers underwent ozonolysis at the isobutenyl double bond to yield
the corresponding ozonides which were rapidly decomposed to the
corresponding aldehydes and carboxylic acids. These ester products
were subsequently metabolized via cleavage of the ester linkage,
hydroxylation at 2'- and 4'- positions of the alcohol moiety, and
oxidation of the benzyl alcohols to the benzoic acids. The resultant
phenols and carboxylic acids were further conjugated with sugars. The
parent compounds and their degradation products were hardly
transferred from the application site to other parts of plants (Nambu
et al., 1979a).
Bean plant seedlings were planted in Kodaira light clay and Katano
sandy loam soils treated with 1.0 mg/kg of C-14, phenothrin isomers.
After 30 days, 0.01 - 0.03 mg/kg and 0.04 - 0.09 mg/kg equivalents of
C-14 were found in pods and seeds, and shoots respectively, although
0.21 - 3.48 mg/kg of C-14 was retained in roots. No parent compound
was detected in shoots (Nambu et al., 1979a).
In soil
(+)-trans and (+)-cis-phenothrin were applied to Kodaira light
clay and Katano sandy loam soils at the rate of 1.0 mg/kg, and the
treated soils were held at 25 ± 2°C in the dark. Under upland
conditions, both isomers were rapidly decomposed in these soils with
half-lives of 1-2 days. On the other hand, the rate of degradation of
both isomers was much slower under flooded conditions, and half-lives
were 1-2 weeks and 1-2 months for the (+)-trans and (+)-cis
isomers, respectively. Phenothrin isomers were decomposed in the
soils via cleavage of the ester linkage, hydroxylation at 4'-position
of the alcohol moiety, cleavage of the diphenyl ether linkage and
oxidation of benzyl alcohols to benzoic acids. These products were
not persistent in the soils under upland conditions and the labelled
carbon was finally decomposed to C-14-CO2 (Nambu et al., 1979b).
Phenothrin was impregnated at a concentration of 1 g/kg on to a
laterite from Uganda. The treated soil was stored at 25°C and 20 or
80% relative humidity (R.H.). The half-life of the insecticide in
days was 40 with 20% RH and 150 with 80% RH (Barlow et al., 1977).
(+)-trans and (+)-cis-phenothrin and their degradation products
were hardly eluted with water from Kodaira light clay and Katano sandy
loam soils, whereas polar products including 3-phenoxybenzoic acid
were slightly eluted from Muko sand (Nambu et al., 1979b).
In storage and processing
(+)-trans- and (+)-cis-phenothrin labelled with C-14 at the
methylene group of the alcohol moiety were applied to wheat grains at
a concentration of 4 mg/kg alone or together with 20 mg/kg piperonyl
butoxide and 4 mg/kg fenitrothion. The treated grains with 11-12%
moisture content were stored at 15 and 30°C in the dark. During 6
months storage period, both isomers were slightly decomposed
regardless of the presence and absence of piperonyl butoxide and
fenitrothion. The residue levels of phenothrin isomers were 3.64-3.78
mg/kg in whole wheat grain, 0.79-0.83 mg/kg in flour and 9.24-11.4
mg/kg in bran after 6 months. The C-14 residues were mainly located
in the seed coat as evidenced by radio-autography and radioanalysis.
Small amounts (less than 2% of the applied C-14) of 3-phenoxybenzyl
alcohol and 3-phenoxybenzoic acid were found. The phenothrin residues
in flour hardly decreased to a small extent through baking processes
leaving 0.66-0.69 mg/kg of phenothrin isomers in bread (Nambu et al,
1979b).
All studies were carried out in Australia to determine the residues of
phenothrin or d-phenothrin in the products after processing and
cooking of wheat, oats, barley and rice grain, except one experiment
with wheat grain in Japan using labelled compounds.
Wheat grain containing 0.5-3.9 mg/kg of phenothrin was used for the
studies, and the residue results after processing and cooking are
summarised in Table 5. Although higher residues were found in bran
and pollard after processing, the residues in flour were reduced and
reached 10.6-25.0% of those found in whole grain. After cooking the
residues in bread did not exceed 0.2 mg/kg in Australian trials, but
in the Japanese trial those residues of C-14-labelled (+)-trans and
(+)-cis phenothrin were relatively high (0.69 mg/kg and 0.66 mg/kg)
corresponding to the higher residues in flour (0.79 mg/kg and 0.91
mg/kg). This is supposed to be caused by contamination of a large
portion of testa in flour from the crease area of grain, while in
normal commercial flour milling situations only a very small portion
of testa would go into flour. In gluten, the residue was higher (1.9
mg/kg) than that in flour (0.3 mg/kg). However, the pesticide
residues in gluten does not cause concern, because normally there
would be a 2% maximum addition of gluten in bread.
Oats containing 1.2 mg/kg of phenothrin were processed and cooked
(Desmarchelier, 1979a). Most of the residues were carried in oat
hulls, and low residue levels were found in both groats (0.3 mg/kg)
and rolled oats (0.1 mg/kg). In porridge the residue was below the
detection limit of 0.05 mg/kg.
Barley containing 3.2 mg/kg and 0.92 mg/kg phenothrin was processed
into malt, wort and cattle feed. Of these products, the highest
residues were found in the malt, but these levels, 0.63 mg/kg and 0.15
mg/kg were less than one-fifth of that in barley. In wort, the
residues were less than the detection limit of 0.02 mg/kg.
Table 5. Residues of phenothrin and d-phenothrin prior to and after processing and cooking of wheat
Residue (mg/kg)
Compound Reference
Wholemeal White
Wheat Bran Pollard Flour Bread Bread Gluten Starch
Phenothrin 1.2 5.6 3.6 0.21 0.74 0.16 - - Bengston 1979
3.0 10.0 4.1 0.82 - 0.18 - - Desmarchelier, 1979a
1.5 6.0 3.0 0.4 - 0.1 - - "
0.9 3.0 2.0 0.15 - 0.05 - - "
0.5 1.6 0.9 0.09 - <0.05 - - "
3.9 8.1 1.6 0.65 - - 1.9 <0.05 "
1.2 4.0 1.8 0.3 0.4-0.6 0.1-0.2 - - Ardley, 1979
2.0 - - - - <0.1 - - Mollard, 1979
4.3 - - - - <0.1 - - "
(+)-trans-phenothrin 3.78 11.4 - 0.79 - 0.69 - - Nambu et al
1979b
(+)-cis-phenothrin 3.64 9.24 - 0.91 - 0.66 - -
A nominal application of 8 mg/kg phenothrin was applied to husked
(brown) rice and polished (white) rice. Six months after application,
husked (6.2 mg/kg of phenothrin) and polished (5.9 mg/kg) rice were
cooked in a minimum amount of boiling water for 25 minutes. Unhusked
rice was also treated with d-phenothrin at a rate of 8 mg/kg, and
after 6 months of storage when 4.7 mg/kg of phenothrin was detected,
rice was milled into husked and polished rice, and cooked as described
above. As shown in Table 6, the cooking of rice had the effect of
reducing residues by from 34% to 60%. It also has indicated that the
milling of rice from husked (paddy) to unhusked (brown) or polished
(white) is a major means of residue reduction, that is, 90% and 97%
respectively.
Table 6. Residues of d-phenothrin prior to and following processing
and cooking of rice
Residue (mg/kg)
Unhusked Husked Polished Cooked
- 6.2 - 4.1
- - 5.9 3.1
4.7 0.50 - 0.2
4.7 - 0.15 <0.1
(Desmarchelier et al., 1979d)
Phenothrin and d-phenothrin were applied to grain at the level of 4
mg/kg and 2 mg/kg, respectively, and have encountered levels of
moisture and three temperature levels. As shown in Fig. 2 and 3, both
increasing temperature (25, 30 and 37°C) at a constant moisture of
11.8% and increasing moisture (9.3, 11.8 and 13.4%) at a constant
temperature of 30°C led to increased breakdown of phenothrin and
d-phenothrin (Maguire, 1977).
Photochemical Degradation
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 and 7 days. Under these conditions phenothrin was more unstable
than permethrin and more stable than bioresmethrin (Elliot et al.,
1973). In a separate study, decomposition of phenothrin on glass by
irradiation of 270-370 mm light was determined. The half-life for
phenothrin was one day (Barlow et al., 1977).
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Due to the newness of phenothrin and its largely experimental status,
no information was available on residues in food in commerce or at
consumption.

 METHODS OF RESIDUE ANALYSIS
Residue determination of intact phenothrin by gas-liquid
chromatography (GLC) is difficult due to poor sensitivity to the GLC
detectors commonly used in residue analysis, though some residue data
of d-phenothrin on stored sorghum (0.4 - 0.95 mg/kg) were obtained by
GLC equipped with flame ionization detector (Bengston et al., 1977).
Kato et al. (1979) successfully applied combined gas-liquid
chromatograph/Mass spectrometry (GLC/MS) to determine intact
d-phenothrin residues in blood. Mass fragmentography of d-phenothrin
gave as minimum detectable amounts 0.1 ng and 0.04 ng at mass ions,
m/e 350 (parent) and 183, respectively, thereby allowing confirmation
as well as quantitation of 0.005 mg/kg or even less. Desmarchelier
(1979a) also briefly reported utility of GLC/MS for analysis of
phenothrin in stored grains at residue levels below 0.01 mg/kg without
presenting detailed data.
As a determinative procedure Simpson (1979) adopted high pressure
liquid chromatography in combination with cleanup by partitioning
between n-hexane and acetonitrile, and alumina column chromatography.
By this method phenothrin residues of 1.56-2.72 mg/kg were quantitated
in stored sorghum, though data on detection limits and recoveries are
not presented (Bengston et al., 1978; Simpson 1979).
Derivatization methods have been more commonly adopted for residue
analysis. Takimoto et al., (1977) analyzed phenothrin residues by
the procedures which included potassium hydroxide-catalyzed hydrolysis
of phenothrin in aqueous methanol and subsequent pyridine-catalyzed
esterification of the liberated alcohol moiety with
2,4-dichlorobenzoyl chloride. When analyzed by GLC fitted with 0.8
m-long glass column packed either with 4% FFAP on Gas-chrom Q or with
3% OV-101/3% Apiezon Grease L on Gas-chrom Q, 3-phenoxy-benzyl
2,4-dichlorobenzoate showed excellent sensitivity, i.e. 0.04-0.1 ng as
minimum detectability, to electron-capture detector, which permitted
detection of phenothrin at 0.005-0.02 mg/kg.
Desmarchelier (1976; 1979a) extended the colorimetric procedures which
had originally been devised by McClellan (1964) for residue analysis
of pyrethrum. The procedures were based upon a color reaction of a
modified Deniges reagent with chrysanthemic acid formed by hydrolysis
of phenothrin under catalysis of sodium hydroxide in aqueous alcoholic
solution. The method appears less laborious but less selective and
less sensitive than that reported by Takimoto et al., (1977). In
addition, a significant difference in extinction coefficient observed
at 584 nm between (+)-cis and (+)-trans chrysanthemic acid
necessitates prior separation of cis and trans isomers for
accurate determination of phenothrin.
Thin-layer chromatography (TLC) based on the above colour reaction has
also been applied as a semiquantitative procedure (Desmarchelier 1976,
1979a).
The solvent or solvent combinations selected for extraction of
phenothrin depend upon the nature of substrates. From the substrated
with high-moisture contents like cabbage and green pepper, phenothrin
was effectively extracted by chopping and blending either with polar
solvents, e.g., methanolacetonitrile, or with nonpolar-polar solvent
mixtures, e.g., benzene-ethanol (Takimoto et al., 1977). For aged
phenothrin residues on cooked rice 24-hour extraction with polar
solvents, e.g. methanol and ethanol, gave complete recoveries whereas
the extraction with non-polar solvents, e.g. light petroleum and
hexane, resulted in poor recoveries (Desmarchelier 1979b). From dry
and low-moisture substrates including rice, barley, wheat, oats and
sorghum phenothrin is efficiently extracted with either polar,
non-polar or dual solvent system. Hulled rice grain was ground and
macerated overnight in methanol (Takimoto et al., 1977). Wheat and
barley were soaked overnight as whole grain either in light petroleum
in ethanol (Desmarchelier 1979b). Desmarchelier (1976) reported that
use of light petroleum simplified cleanup in the analysis of grains.
As cleanup procedures both liquid chromatography (LC; Florisil and
alumina) and TLC (silica-gel) are used for intact phenothrin residues
(Takimoto et al., 1977; Simpson 1979). The Florisil column
chromatography coupled with benzene-ethyl acetate extraction resulted
in 93% recovery, as determined by GLC/MS, from the blood fortified at
0.05 mg/kg (Kato et al., 1979). Cleanup procedures employed for the
derivatization methods obviously reflect the nature of the derivatives
as well as substrates. The method of converting phenothrin to
3-phenoxybenzyl 2,4,dichlorobenzoate which provided 81-88% recoveries
for cabbage, green pepper and hulled rice fortified at 0.1-1 mg/kg
included cleanup by either LC or TLC before both hydrolysis and GLC
quantitation (Takimoto et al., 1977). The colorimetric method
employed partition cleanup between aqueous phases and nonpolar
solvents, e.g. light petroleum or chloroform, and satisfactory
recoveries of 87-91% were achieved from cooked rice (Desmarchelier,
1979b).
NATIONAL LIMITS REPORTED TO THE MEETING
No information was available.
APPRAISAL
Phenothrin is more stable than natural pyrethrins and synthetic
pyrethroids developed earlier, but it is relatively degradable by
sunlight. The principal current uses are (1) in household aerosols
and sprays formulated in combination with tetramethrin, piperonyl
butoxide, carbamates and/or fenitrothion for control of various
species of plant hoppers and for control of houseflies, mosquitoes,
cockroaches and other household or public health pests, and (2) in
grain protection. Supervised trials have been undertaken on rice,
green peppers and cabbage.
The most important food use at present is for the protection of stored
grains. Extensive trials on wheat, sorghum and barley grains were
carried out in Australia and Japan. Wheat was stored up to 9 months,
barley up to 5 months, and sorghum up to 6 months and samples were
withdrawn for residue analysis at a variety of intervals. Treatment
levels of approximately 2 mg a.i./kg usually gave residues of about 2
mg/kg at 1 month after treatment, falling to about 1 mg/kg after 9
months. Similar results were obtained for sorghum and barley.
Increasing temperature and moisture resulted in increased breakdown of
phenothrin, but the effect was not large. Wheat residues were found
mostly in the seed coat.
Processing of wheat, oats, barley and rice resulted in somewhat higher
residues in wheat bran, wheat pollard, and oat hulls and lower
residues in wheat flour, groats, rolled oats, barley malt, wort,
barley cattle feed and polished rice. Cooking resulted in reduced
residues in bread, porridge and boiled rice.
In studies with 14C-labelled (+)-trans and (+)-cis- phenothrin
applied to bean and rice plant leaf surfaces, both isomers disappeared
rapidly with half-lives of less than one day. Both isomers underwent
ozonolysis and sequential metabolism to give final products consisting
of sugar conjugates of phenols and carboxylic acids. No translocation
of parent compounds and their degradation products from the
application site occurred. Bean seedlings planted in 14C-phenothrin
treated soil did not show translocation of parent compound into the
shoots.
Stored wheat (11-12% moisture) treated with 14C-phenothrin isomers at
4 mg/kg either alone or in combination with piperonyl butoxide and
fenitrothion was held at either 15 or 30°C in the dark for six months.
During this interval only slight degradation occurred with 3.6-3.8
mg/kg remaining in whole wheat grain, mainly in the seed coat. Small
amounts ('2% of applied 14C) of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were found.
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 to 7 days. Photostability of phenothrin was greater than
bioresmethrin and less than permethrin. Phenothrin films on glass
irradiated by 270-370 nm light had a half-life of one day.
Residue determination of intact phenothrin by GLC is complicated by
its molecular structure which results in poor sensitivity in selective
detectors such as the electron capture, necessitating the use of the
non-selective flame ionization detector with its attendant rigorous
cleanup requirements. Combined GC-Mass spectrometry has been
successfully used to determine d-phenothrin in blood at levels as low
as 0.04 ng, allowing quantitation at 0.005 mg/kg. GC-MS has also been
used for residues in stored grains, but such techniques will probably
not find wide acceptance for some time. The colorimetric procedure
for pyrethrum has been extended to phenothrin, but since the method is
based on a colour reaction involving chrysanthemic acid, it would not
distinguish between most synthetic and natural pyrethroids. High
performance liquid chromatography with UV absorption detection has
also been used to determine residues in stored sorghum. Rapid
improvements in LC column technology combined with variable
wavelength UV detectors should result in methods of greater
selectivity and sensitivity and merits further development. Although
tedious, the derivatization method developed by Takimoto, et al.
appears to be the most suitable for regulatory analysis. The method
gives overall recoveries of 81-88%.
RECOMMENDATIONS
Because phenothrin was not evaluated for an ADI in 1979, only
guideline levels can be presented at this time.
The following residue levels for phenothrin as combined (+)-trans
and (+) cis-isomers are those which need not be exceeded if the
compound is used as recommended for the protection of stored grains.
Commodity Guideline level
mg/kg
Wheat bran 15
Cereal grains 5
FURTHER WORK OR INFORMATION
Desirable
1. Further residue data from supervised trials on commodities for
which phenothrin uses are developed, especially stored products,
vegetables, rice and cereal brans and straws used as animal feed.
2. Continued development of improved methods of residue analysis for
the intact isomer molecules.
REFERENCES
Ardley, J.H. "Phenothrin-bread residues AWS-CSIRO pilot trials"
Reference PH-11/52 Welcome Australasia Ltd. (1979).
Ardley, J.H. and Halls, G.R.H. "Phenothrin (S-2539) A potential grain
protectant". Chemical Marketing and Economics Division of the
American Chemical Society, Preprints pp 38-54, Honolulu, Hawaii. 1979.
Barlow, F., Hadaway, A.B., Flower, L.S., Grose, J.E. and Turner, C.R.
"Some laboratory investigations relevant to the possible use of new
pyrethroids in control of mosquitoes and tse-tse flies". Pestic. Sci.,
8, 291-300.
Bengston, M. "The fate of residues of phenothrin in bran, pollard,
flow and bread", 1979.
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. "Grain protectants for the control of
malathion- resistant insects in stored sorghum".
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional grain protectants for the control of
malathion-resistant insects in stored sorghum".
Casida, J.E., Gaughan, L.C. and Ruzo, L.C. "Comparative Metabolism of
Pyrethroids Derived from 3-phenoxybenzyl and a-Cyano-3-Phenoxybenzyl
Alcohols". Advances in Pesticide Science Ed. by Geissbühler, Brooks,
G.T., and Kearney, P.C. Pergamon Press, London, Part 2, P. 182.
Desmarchelier, J.M. "Analysis of pyrethroids on grains" J. Stored
Prod. Res. 12, 245-252.
"Selective treatments, including combinations of pyrethroid and
organophosphorus insecticides, for control of stored product
Coleoptera at two temperatures". J. Stored Prod. Res. 13, 129-137.
"The fate of phenothrin residues during processing of wheat, barley
and oats". Dept. Primary Industry, Canberra, Australia. (1979a).
"Analysis of bioresmethrin, phenothrin, d-phenothrin, pyrethrum I
and carbaryl on grains, extraction studies on these insecticides plus
fenitrothion, methacrifos, pirimiphos-methyl and dichlorovos on 7
grains and cooked rice and an improved procedure for esterification on
phenols". Submitted to J. Pesticide Sci. (Japan). (1979b).
Desmarchelier, J.M., Bengston, M., Davies, R., Elder, B., Hart, R.,
Henning, R., Murry, W., Ridley, E., Ripp, E., Sierakawski,C., Sticka,
R., Snelson, J., Wallbank, B. and Wilson, A. "Pilot usage of the
grain protectants chloropyrifosmethyl plus bioresmethrin, fenitrothion
plus d-phenothrin, methacrifos and pirimiphos.methyl plus carbaryl".
(1979c).
Desmarchelier, J.M., Goldring, M. and Horgan, R. "Predicted and
observed residues of bioresmethrin, carbaryl, dichlorovos
fenitrothion, d-phenothrin, methacrifos and pirimiphos methyl on rice
and barley after storage and losses of these insecticides during
processing" Dept. Primary Industry, Canberra, Australia. (1979d).
Elliott, M., Farnham, A.W., James, N.F., Needham, P.H., Pulman, D.A.
and Stevenson, J.H. "NRDC 143, A More stable pyrethroid". Proceeding
7th British Insecticide and Fungicide Conference, p. 721. (1973).
Kato, T., Fujita,, K. and Miyamoto, J. "Analytical method of
d-phenothrin (SumithrinR) in blood". Sumitomo Chemical Co., Ltd.
(1979).
Maguire, M.J. "Persistence of insecticides on stored grain at three
temperature levels and three moisture levels". (1978).
McClellan, D.B. "Pyrethrum-Pyrethrum I and Pyrethrum-II" pp. 399-413,
in Analytical Methods for Pesticides, Plant Growth Regulators and Food
Additives, Vol. II (Zweig, G., ed.) Academic Press, New York).
Miyamoto, J., Suzuki, T., and Nakae, C. "Metabolism of Phenothrin or
3-phenoxybenzyl d-trans-Chrysanthemumate in Mammals". Pestic. Biochem.
Physiol., 4, 438.
Mollard, V. "Samples from Bread Research Institute". Reference PH
10/141 Wellcome Australasia Ltd.
Nambu, K., Ohkawa, H. and Miyamoto, J. "Metabolic fate of phenothrin
in plants and soils" Sumitomo Chemical Co., Ltd. (1979a).
Nambu, K., Takimoto, Y. and Miyamoto, J. "Degradation and fate of
phenothrin in stored wheat grains". Sumitomo Chemical Co., Ltd.
(1979b).
Simpson, B.W. "Method for pyrethroid residues on wheat, sorghum
pollard, flour and bread". (1979).
Soderland, D.M. and Casida, J.E. "Effects of Pyrethroid Structure on
Rats of Hydrolysis and Oxidation by Mouse Liver Microsomal Enzymes"
Pestic. Biochem. Physiol. 7, 391-401.
Suzuki, T., Ohno, N. and Miyamoto, J. "New Metabolites of
(+)-cis-Fenothrin, 3 Phenoxybenzyl (+)-cis-Chrysanthemumate, in
Rats" J. Pesticide Sci. 1, 151.
Suzuki, T. and Miyamoto, J. "Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome". Pestic. Biochem.
Physiol., 8, 186.
Takimoto, Y., Kato, T. and Miyamoto, J. "Residue analyses of
phenothrin (S-2539) in crops". Sumitomo Chemical Co., Ltd. (1977).
Wellcome Australasia. "Evaluation of phenothrin as a potential grain
protectant". (1977).
Working Party on Grain Protectants. "Final report on silo scale
experimental application of grain protectant chemicals, 1977-1978".
Working Party of Grain Protectants "Interim report on pilot usage
trials 1978-1979". (1979a).
Working Party on Grain Protectants "Summary of pilot usage trials,
1978-1979". (1979b).
METHODS OF RESIDUE ANALYSIS
Residue determination of intact phenothrin by gas-liquid
chromatography (GLC) is difficult due to poor sensitivity to the GLC
detectors commonly used in residue analysis, though some residue data
of d-phenothrin on stored sorghum (0.4 - 0.95 mg/kg) were obtained by
GLC equipped with flame ionization detector (Bengston et al., 1977).
Kato et al. (1979) successfully applied combined gas-liquid
chromatograph/Mass spectrometry (GLC/MS) to determine intact
d-phenothrin residues in blood. Mass fragmentography of d-phenothrin
gave as minimum detectable amounts 0.1 ng and 0.04 ng at mass ions,
m/e 350 (parent) and 183, respectively, thereby allowing confirmation
as well as quantitation of 0.005 mg/kg or even less. Desmarchelier
(1979a) also briefly reported utility of GLC/MS for analysis of
phenothrin in stored grains at residue levels below 0.01 mg/kg without
presenting detailed data.
As a determinative procedure Simpson (1979) adopted high pressure
liquid chromatography in combination with cleanup by partitioning
between n-hexane and acetonitrile, and alumina column chromatography.
By this method phenothrin residues of 1.56-2.72 mg/kg were quantitated
in stored sorghum, though data on detection limits and recoveries are
not presented (Bengston et al., 1978; Simpson 1979).
Derivatization methods have been more commonly adopted for residue
analysis. Takimoto et al., (1977) analyzed phenothrin residues by
the procedures which included potassium hydroxide-catalyzed hydrolysis
of phenothrin in aqueous methanol and subsequent pyridine-catalyzed
esterification of the liberated alcohol moiety with
2,4-dichlorobenzoyl chloride. When analyzed by GLC fitted with 0.8
m-long glass column packed either with 4% FFAP on Gas-chrom Q or with
3% OV-101/3% Apiezon Grease L on Gas-chrom Q, 3-phenoxy-benzyl
2,4-dichlorobenzoate showed excellent sensitivity, i.e. 0.04-0.1 ng as
minimum detectability, to electron-capture detector, which permitted
detection of phenothrin at 0.005-0.02 mg/kg.
Desmarchelier (1976; 1979a) extended the colorimetric procedures which
had originally been devised by McClellan (1964) for residue analysis
of pyrethrum. The procedures were based upon a color reaction of a
modified Deniges reagent with chrysanthemic acid formed by hydrolysis
of phenothrin under catalysis of sodium hydroxide in aqueous alcoholic
solution. The method appears less laborious but less selective and
less sensitive than that reported by Takimoto et al., (1977). In
addition, a significant difference in extinction coefficient observed
at 584 nm between (+)-cis and (+)-trans chrysanthemic acid
necessitates prior separation of cis and trans isomers for
accurate determination of phenothrin.
Thin-layer chromatography (TLC) based on the above colour reaction has
also been applied as a semiquantitative procedure (Desmarchelier 1976,
1979a).
The solvent or solvent combinations selected for extraction of
phenothrin depend upon the nature of substrates. From the substrated
with high-moisture contents like cabbage and green pepper, phenothrin
was effectively extracted by chopping and blending either with polar
solvents, e.g., methanolacetonitrile, or with nonpolar-polar solvent
mixtures, e.g., benzene-ethanol (Takimoto et al., 1977). For aged
phenothrin residues on cooked rice 24-hour extraction with polar
solvents, e.g. methanol and ethanol, gave complete recoveries whereas
the extraction with non-polar solvents, e.g. light petroleum and
hexane, resulted in poor recoveries (Desmarchelier 1979b). From dry
and low-moisture substrates including rice, barley, wheat, oats and
sorghum phenothrin is efficiently extracted with either polar,
non-polar or dual solvent system. Hulled rice grain was ground and
macerated overnight in methanol (Takimoto et al., 1977). Wheat and
barley were soaked overnight as whole grain either in light petroleum
in ethanol (Desmarchelier 1979b). Desmarchelier (1976) reported that
use of light petroleum simplified cleanup in the analysis of grains.
As cleanup procedures both liquid chromatography (LC; Florisil and
alumina) and TLC (silica-gel) are used for intact phenothrin residues
(Takimoto et al., 1977; Simpson 1979). The Florisil column
chromatography coupled with benzene-ethyl acetate extraction resulted
in 93% recovery, as determined by GLC/MS, from the blood fortified at
0.05 mg/kg (Kato et al., 1979). Cleanup procedures employed for the
derivatization methods obviously reflect the nature of the derivatives
as well as substrates. The method of converting phenothrin to
3-phenoxybenzyl 2,4,dichlorobenzoate which provided 81-88% recoveries
for cabbage, green pepper and hulled rice fortified at 0.1-1 mg/kg
included cleanup by either LC or TLC before both hydrolysis and GLC
quantitation (Takimoto et al., 1977). The colorimetric method
employed partition cleanup between aqueous phases and nonpolar
solvents, e.g. light petroleum or chloroform, and satisfactory
recoveries of 87-91% were achieved from cooked rice (Desmarchelier,
1979b).
NATIONAL LIMITS REPORTED TO THE MEETING
No information was available.
APPRAISAL
Phenothrin is more stable than natural pyrethrins and synthetic
pyrethroids developed earlier, but it is relatively degradable by
sunlight. The principal current uses are (1) in household aerosols
and sprays formulated in combination with tetramethrin, piperonyl
butoxide, carbamates and/or fenitrothion for control of various
species of plant hoppers and for control of houseflies, mosquitoes,
cockroaches and other household or public health pests, and (2) in
grain protection. Supervised trials have been undertaken on rice,
green peppers and cabbage.
The most important food use at present is for the protection of stored
grains. Extensive trials on wheat, sorghum and barley grains were
carried out in Australia and Japan. Wheat was stored up to 9 months,
barley up to 5 months, and sorghum up to 6 months and samples were
withdrawn for residue analysis at a variety of intervals. Treatment
levels of approximately 2 mg a.i./kg usually gave residues of about 2
mg/kg at 1 month after treatment, falling to about 1 mg/kg after 9
months. Similar results were obtained for sorghum and barley.
Increasing temperature and moisture resulted in increased breakdown of
phenothrin, but the effect was not large. Wheat residues were found
mostly in the seed coat.
Processing of wheat, oats, barley and rice resulted in somewhat higher
residues in wheat bran, wheat pollard, and oat hulls and lower
residues in wheat flour, groats, rolled oats, barley malt, wort,
barley cattle feed and polished rice. Cooking resulted in reduced
residues in bread, porridge and boiled rice.
In studies with 14C-labelled (+)-trans and (+)-cis- phenothrin
applied to bean and rice plant leaf surfaces, both isomers disappeared
rapidly with half-lives of less than one day. Both isomers underwent
ozonolysis and sequential metabolism to give final products consisting
of sugar conjugates of phenols and carboxylic acids. No translocation
of parent compounds and their degradation products from the
application site occurred. Bean seedlings planted in 14C-phenothrin
treated soil did not show translocation of parent compound into the
shoots.
Stored wheat (11-12% moisture) treated with 14C-phenothrin isomers at
4 mg/kg either alone or in combination with piperonyl butoxide and
fenitrothion was held at either 15 or 30°C in the dark for six months.
During this interval only slight degradation occurred with 3.6-3.8
mg/kg remaining in whole wheat grain, mainly in the seed coat. Small
amounts ('2% of applied 14C) of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were found.
When exposed to daylight in England near a window and out of doors
under quartz plates, phenothrin decomposed rapidly with half-lives of
4 to 7 days. Photostability of phenothrin was greater than
bioresmethrin and less than permethrin. Phenothrin films on glass
irradiated by 270-370 nm light had a half-life of one day.
Residue determination of intact phenothrin by GLC is complicated by
its molecular structure which results in poor sensitivity in selective
detectors such as the electron capture, necessitating the use of the
non-selective flame ionization detector with its attendant rigorous
cleanup requirements. Combined GC-Mass spectrometry has been
successfully used to determine d-phenothrin in blood at levels as low
as 0.04 ng, allowing quantitation at 0.005 mg/kg. GC-MS has also been
used for residues in stored grains, but such techniques will probably
not find wide acceptance for some time. The colorimetric procedure
for pyrethrum has been extended to phenothrin, but since the method is
based on a colour reaction involving chrysanthemic acid, it would not
distinguish between most synthetic and natural pyrethroids. High
performance liquid chromatography with UV absorption detection has
also been used to determine residues in stored sorghum. Rapid
improvements in LC column technology combined with variable
wavelength UV detectors should result in methods of greater
selectivity and sensitivity and merits further development. Although
tedious, the derivatization method developed by Takimoto, et al.
appears to be the most suitable for regulatory analysis. The method
gives overall recoveries of 81-88%.
RECOMMENDATIONS
Because phenothrin was not evaluated for an ADI in 1979, only
guideline levels can be presented at this time.
The following residue levels for phenothrin as combined (+)-trans
and (+) cis-isomers are those which need not be exceeded if the
compound is used as recommended for the protection of stored grains.
Commodity Guideline level
mg/kg
Wheat bran 15
Cereal grains 5
FURTHER WORK OR INFORMATION
Desirable
1. Further residue data from supervised trials on commodities for
which phenothrin uses are developed, especially stored products,
vegetables, rice and cereal brans and straws used as animal feed.
2. Continued development of improved methods of residue analysis for
the intact isomer molecules.
REFERENCES
Ardley, J.H. "Phenothrin-bread residues AWS-CSIRO pilot trials"
Reference PH-11/52 Welcome Australasia Ltd. (1979).
Ardley, J.H. and Halls, G.R.H. "Phenothrin (S-2539) A potential grain
protectant". Chemical Marketing and Economics Division of the
American Chemical Society, Preprints pp 38-54, Honolulu, Hawaii. 1979.
Barlow, F., Hadaway, A.B., Flower, L.S., Grose, J.E. and Turner, C.R.
"Some laboratory investigations relevant to the possible use of new
pyrethroids in control of mosquitoes and tse-tse flies". Pestic. Sci.,
8, 291-300.
Bengston, M. "The fate of residues of phenothrin in bran, pollard,
flow and bread", 1979.
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. "Grain protectants for the control of
malathion- resistant insects in stored sorghum".
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional grain protectants for the control of
malathion-resistant insects in stored sorghum".
Casida, J.E., Gaughan, L.C. and Ruzo, L.C. "Comparative Metabolism of
Pyrethroids Derived from 3-phenoxybenzyl and a-Cyano-3-Phenoxybenzyl
Alcohols". Advances in Pesticide Science Ed. by Geissbühler, Brooks,
G.T., and Kearney, P.C. Pergamon Press, London, Part 2, P. 182.
Desmarchelier, J.M. "Analysis of pyrethroids on grains" J. Stored
Prod. Res. 12, 245-252.
"Selective treatments, including combinations of pyrethroid and
organophosphorus insecticides, for control of stored product
Coleoptera at two temperatures". J. Stored Prod. Res. 13, 129-137.
"The fate of phenothrin residues during processing of wheat, barley
and oats". Dept. Primary Industry, Canberra, Australia. (1979a).
"Analysis of bioresmethrin, phenothrin, d-phenothrin, pyrethrum I
and carbaryl on grains, extraction studies on these insecticides plus
fenitrothion, methacrifos, pirimiphos-methyl and dichlorovos on 7
grains and cooked rice and an improved procedure for esterification on
phenols". Submitted to J. Pesticide Sci. (Japan). (1979b).
Desmarchelier, J.M., Bengston, M., Davies, R., Elder, B., Hart, R.,
Henning, R., Murry, W., Ridley, E., Ripp, E., Sierakawski,C., Sticka,
R., Snelson, J., Wallbank, B. and Wilson, A. "Pilot usage of the
grain protectants chloropyrifosmethyl plus bioresmethrin, fenitrothion
plus d-phenothrin, methacrifos and pirimiphos.methyl plus carbaryl".
(1979c).
Desmarchelier, J.M., Goldring, M. and Horgan, R. "Predicted and
observed residues of bioresmethrin, carbaryl, dichlorovos
fenitrothion, d-phenothrin, methacrifos and pirimiphos methyl on rice
and barley after storage and losses of these insecticides during
processing" Dept. Primary Industry, Canberra, Australia. (1979d).
Elliott, M., Farnham, A.W., James, N.F., Needham, P.H., Pulman, D.A.
and Stevenson, J.H. "NRDC 143, A More stable pyrethroid". Proceeding
7th British Insecticide and Fungicide Conference, p. 721. (1973).
Kato, T., Fujita,, K. and Miyamoto, J. "Analytical method of
d-phenothrin (SumithrinR) in blood". Sumitomo Chemical Co., Ltd.
(1979).
Maguire, M.J. "Persistence of insecticides on stored grain at three
temperature levels and three moisture levels". (1978).
McClellan, D.B. "Pyrethrum-Pyrethrum I and Pyrethrum-II" pp. 399-413,
in Analytical Methods for Pesticides, Plant Growth Regulators and Food
Additives, Vol. II (Zweig, G., ed.) Academic Press, New York).
Miyamoto, J., Suzuki, T., and Nakae, C. "Metabolism of Phenothrin or
3-phenoxybenzyl d-trans-Chrysanthemumate in Mammals". Pestic. Biochem.
Physiol., 4, 438.
Mollard, V. "Samples from Bread Research Institute". Reference PH
10/141 Wellcome Australasia Ltd.
Nambu, K., Ohkawa, H. and Miyamoto, J. "Metabolic fate of phenothrin
in plants and soils" Sumitomo Chemical Co., Ltd. (1979a).
Nambu, K., Takimoto, Y. and Miyamoto, J. "Degradation and fate of
phenothrin in stored wheat grains". Sumitomo Chemical Co., Ltd.
(1979b).
Simpson, B.W. "Method for pyrethroid residues on wheat, sorghum
pollard, flour and bread". (1979).
Soderland, D.M. and Casida, J.E. "Effects of Pyrethroid Structure on
Rats of Hydrolysis and Oxidation by Mouse Liver Microsomal Enzymes"
Pestic. Biochem. Physiol. 7, 391-401.
Suzuki, T., Ohno, N. and Miyamoto, J. "New Metabolites of
(+)-cis-Fenothrin, 3 Phenoxybenzyl (+)-cis-Chrysanthemumate, in
Rats" J. Pesticide Sci. 1, 151.
Suzuki, T. and Miyamoto, J. "Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome". Pestic. Biochem.
Physiol., 8, 186.
Takimoto, Y., Kato, T. and Miyamoto, J. "Residue analyses of
phenothrin (S-2539) in crops". Sumitomo Chemical Co., Ltd. (1977).
Wellcome Australasia. "Evaluation of phenothrin as a potential grain
protectant". (1977).
Working Party on Grain Protectants. "Final report on silo scale
experimental application of grain protectant chemicals, 1977-1978".
Working Party of Grain Protectants "Interim report on pilot usage
trials 1978-1979". (1979a).
Working Party on Grain Protectants "Summary of pilot usage trials,
1978-1979". (1979b).
