
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
PHENOTHRIN
Explanation
This pesticide was evaluated by the 1979 Joint Meeting (FAO, 1980).
Data were not submitted in sufficient time to allow an estimation
of an ADI for man and consideration was deferred. In the absence
of toxicological evaluations, guideline levels for residues found
in crops following good agricultural practice were reported. The
present monograph addendum considers all the available
toxicological information submitted to the Meeting.
Special explanatory note
Phenothrin is a chrysanthemic acid ester of 3-phenoxybenzyl
alcohol. Four stereoisomers of phenothrin can be derived from the
chirality of the cyclopropane ring at the C1 and C3 positions.
The nomenclature does not prescribe the ratio of isomers but rather
the ratio of isomers is a function of synthesis or purification of
the technical product. It is critical to note the isomeric ratio
in the product used in supervised residue trials and in
toxicological studies.
Among the four isomeric esters (d:1, cis: trans), both
1-isomers show little insecticidal activity. In addition, the
d-cis isomer appears to be less effective as an insecticide than
the d-trans. Thus, the d-trans is the isomeric molecule of
choice for insecticidal activity.
Phenothrin is the racemic mixture (+)cis, trans molecules
containing a 20:80 isomeric ratio. An additional compound
(Sumithrin(R)) is the registered trademark for the (d+) cis,
trans (20:80) preparation. Thus Sumithrin(R) is a purified
component of the technical phenothrin.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on the metabolic fate of phenothrin (the d, 1 trans,
cis 80:20 3 phenoxybenzyl chrysanthemate active ingredient) have
not been reported. However, studies on the metabolic fate of one
of the isomeric components of phenothrin, the d-trans
chrysanthemic acid ester of 3-phenoxybenzyl alcohol, have been
performed in rats. 14C-d-trans-phenothrin was orally
administered to male rats at a dosage of 200 mg/kg body weight.
The isomer was rapidly absorbed, translocated in the body, and
excreted predominantly in the urine. In the faeces, up to 9% of the
phenothrin isomer was excreted unchanged.
Within three hours of administration, maximum body burdens were
recorded in all tissues examined. Within 12 hours, these levels
had diminished to values representing about 50% of the maximum
residue found, and within 24 hours the vast majority of
radioactivity had been excreted from the body. Excretion is
extremely rapid. Within three hours, approximately 40% of the
orally administered radioactivity had been excreted and all
radioactivity was recovered within 48 hours (Miyamoto et al,
1974).
Biotransformation
Based upon in vivo studies with the d-trans-phenothrin, the
metabolic fate appears to follow that observed with several other
pyrethroid esters. Phenothrin is hydrolysed to the 3-phenoxybenzyl
alcohol, which in turn is oxidised to 3-phenoxybenzoic acid and
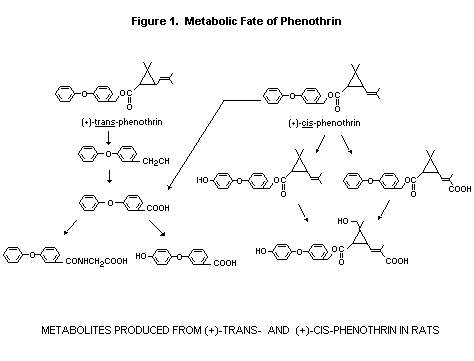
other metabolites. The major degradation pattern of both cis and
trans isomers can be seen in Figure 1. In contrast to the
trans isomer, both in vivo and in vitro studies confirm
that the cis-phenothrin is metabolised at a less rapid rate and
yields a larger proportion of ester metabolites. There exists a
substantial difference between the two isomers in the rate of
hydrolysis as observed by in vitro studies. Based upon both
in vivo and in vitro data, it appears that hydrolytic
cleavage at the ester is probably the major pathway of
biodegradation of phenothrin although oxidative metabolism of the
cis isomer portion would be expected to yield a somewhat
different pattern of metabolites, some of which appear to contain
an intact ester moiety (Miyamoto et al, 1974; Casida et al,
1979; Soderlund and Casida, 1977; Suzuki et al, 1976, Suzuki and
Miyamoto, 1978).
Individual isomers (d-cis and d-trans) of phenothrin were
formulated as a dust or an emulsified concentrate and applied
orally or dermally to rats. The dermal exposure was for 24 hours.
Following oral administration, the isomers of phenothrin were
eliminated from the body primarily in urine and faeces within 6
days. With the cis-isomer the major reaction products were
oxidative metabolites and ester hydrolytic products. With the
trans-isomer the major metabolites were hydrolytic products. The
data confirmed earlier work both in vivo and in vitro,
which had demonstrated a similar reaction with these isomers.
Following dermal administration, the major quantity of the applied
dose was recovered from the intact skin. The emulsifiable
concentrate was found to penetrate the skin to a greater degree
than the dust formulation and a substantial difference was noted
with absorption of individual isomers (the cis-isomer penetrated
more rapidly than the trans). As with oral administration the
absorbed dose was rapidly eliminated from the body.
Once in the body, the isomers were metabolised in a similar manner
irrespective of the initial route of entry. With the cis-isomer,
the major metabolic reactions were oxidative at the cis and
trans-positions of the isobutenyl group; at the trans-position
of the gem-dimethyl group of the cyclopropane ring; at the
4'-position of the alcohol moiety; and slightly at the 2'-position;
additionally, cleavage of the ester bound occurred. In contrast,
hydrolytic cleavage of the ester bond was the most prominent
reaction of the trans-isomer, occurring some 5 times faster
than with the cis-isomer. Hydroxylation at 4'-position occurred
to the same degree as with the cis-isomer. The qualitative
metabolic course followed that identified in Figure 1 (Kaneko, H.
et al, 1980).
Biochemical aspects
A carboxyesterase has been isolated and purified from rat liver
microsomes. This carboxyesterase was found to hydrolyse
trans-phenothrin faster than the cis isomers and may account in
part for the reduced mammalian toxicity of the trans isomers. In
contrast to the increased hydrolytic rate observed with
trans-phenothrin, the cis-phenothrin was found to be oxidised
at a more rapid rate by mouse liver microsomal preparations. These
data may explain the difference in toxicity noted between the cis
and trans isomers of several of the pyrethroid esters (Suzuki and
Miyamoto, 1978; Soderland and Casida, 1977; Casida et al, 1979).
 TOXICOLOGICAL STUDIES
Special Pharmacological studies
A series of pharmacological studies were performed with the
d-isomer of phenothrin (Sumithrin(R)). These studies reported the
pharmacological action of this pyrethroid on respiration, blood
pressure, and a series of tests representative of effects on the
peripheral and central nervous system. Sumithrin(R) was emulsified
(Sorpol(R) 1200 and/or Tween(R) 80) and either diluted with
physiological saline or used directly. The following series of
in vitro and in vivo studies were performed: spasmolytic
activity on isolated ileum from male guinea pigs; spontaneous
contractions of isolated uterus from rats; contractions of a
neuromuscular preparation of phrenic nerve-diaphgram of rats;
respiration, nictitating membrane and blood pressure of male cats
administered Sumithrin(R) intravenously; contractile force, blood
pressure, and heart rate of dogs administered Sumithrin(R)
intravenously; the anticonvulsive activity following induced
convulsion or seizures in mice; the effects on muscular relaxation
activity in mice; coordinative movement in mice;
hexabarbital-induced sleeping time in mice; spontaneous activity in
mice; body temperature of male rats; and electroencephalogram in
male cats.
Sumithrin(R) did not show any pharmacological activity in any of
the in vitro and/or in vivo experiments listed above. A
tentative arousal response in the electroencephalogram of cats
intravenously administered a dose of 4 mg/kg body weight was
recorded. Several other synthetic pyrethroid esters have been
shown to induce this arousal response in the EEG pattern of cats
(Hare et al, 1974).
Special studies on neurotoxicity
Groups of rats exposed by inhalation to the d-isomer of phenothrin
(Sumithrin(R)) at concentration of up to an including 3,760 mg/m3
for four hours showed no adverse effects as a result of exposure.
Examination of the sciatic nerve for possible myelin degeneration
or axon disruption did not reveal any such occurrence (Kohda et
al, 1977).
Groups of rats were administered Sumithrin(R) orally for five
consecutive days at a dosage level of 5,000 mg/kg/day. Mortality
was observed and toxic signs of poisoning were noted in several of
the animals. Toxic signs of poisoning rapidly disappeared at the
conclusion of the treatment. Three days after treatment, all
animals were sacrificed and histopathological examination of
sciatic nerve was carried out to determine whether changes had
occurred. Clinically, there were no toxic signs of poisoning as
evidenced by leg weakness or ataxia. Histological examination
revealed minute changes in axon and myelin, characterised by very
slight axonal swelling, axonal disintegration, and/or demyelination.
There were similar occurrences in the control animals and it was
suggested that the small changes observed were not due to the
administration of Sumithrin(R). It was considered that oral
administration of Sumithrin(R) at excessively high doses does
not induce a neurotoxic effect as has been observed with several
other pyrethroid esters (Okuno, et al, 1978; FAO, 1980).
Special studies on mutagenicity
The host-mediated assay was conducted with phenothrin and
individual isomers of phenothrin in male mice using S.
typhimurium-G46 as an indicator strain. Groups of six male mice
were administered phenothrin orally, twice, at 24-hour intervals at
dosage rates of 0, 500, or 1500 mg/kg/dose. Additionally, the
d-trans and d-cis isomers were administered at dosage levels of
250 and 90 mg/kg respectively. A positive control of
dymethylnitrosamine (50 mg/kg) was used. Immediately after
administration of the chemical, the tester strain (S.
typhimurium) was administered into the abdominal cavity. This
was removed three hours later, cultured and examined for mutation
frequency. There was no increase in reversion frequency observed
with phenothrin or its isomers although the positive control was
found to give an increased reversion rate.
The potential mutagenicity of phenothrin was investigated using
various bacterial test systems including the Reo-Assay (repair
test) with Bacillus subtilis and Salmonella typhimurium
strains.
At dose levels ranging from 0 to 10 mg/disc/plate, phenothrin did
not induce an increase of the growth inhibition zone of the
microorganisms, while mytomycin C showed a substantially larger
inhibition zone. A negative control gave results similar to that
obtained with phenothrin.
Mutation induction studies (the standard "Ames test") with various
strains of E. coli and Salmonella typhimurium (TA1535,
TA1537, TA1538, TA98 and TA100) with and without metabolic
activation enzymes, were performed. Phenothrin and its individual
isomers, at dose levels up to 10 mg per plate, with or without
hepatic drug metabolising enzymes, showed negative results in this
standard mutagenicity assay. Phenothrin and its individual isomers
alone and following metabolic activation, caused no increase in the
mutation rate. Positive controls employed during the course of the
study gave significant numbers of revertants (Shirasu et al,
1977; Suzuki and Miyamoto, 1975). Under the conditions of these
bioassays, phenothrin and its individual d-cis and d-trans
isomers are not mutagenic.
Special studies on teratogenicity
Groups of pregnant New Zealand rabbits (17 rabbits per group) were
administered phenothrin orally at dosage levels of 0, 3, 10 or 30
mg/kg from day 6 to 18 of gestation. All animals were sacrificed
on day 29 of gestation and the young obtained by caesarean section
were examined.
Does treated with phenothrin at the highest dosage level exhibited
a loss in body weight during gestation. There was no mortality
observed in the study. There was a slight decrease in the number
of live young obtained from does treated with 30 mg/kg. Changes in
other reproductive/teratogenic parameters did not show a
dose-response relationship and were not believed to be induced as
a result of phenothrin administration. At 30 mg/kg, there was a
slight reduced foetal weight which was not accompanied by changes
in survival rate or in the occurrence of external or internal
abnormalities.
There was no apparent teratogenic effect of phenothrin as observed
by apparent gross internal or external somatic abnormalities and by
examination for foetal skeletal development following prenatal
exposure. Treatment of pregnant albino rabbits during the period
of foetal organogenesis did not induce abnormal foetal
development. At the high dosage level, possible foetal and/or
maternal toxicity was observed.
Groups of pregnant rabbits (15 dose/group) were administered
Sumithrin(R)) at dosage levels of 0, 10, 100 or 1000 mg/kg/day from
day 6 through 18 of gestation. All does were sacrificed on day 29
or day 30 and caesarean section was performed to remove the foetus.
Following caesarean section, one half of the pups were maintained
for 24 hours to evaluate survival.
There were no abnormalities observed in the maternal parameters
(including: body weight, food consumption, clinical observations,
and necropsy) or on foetal data (including: implantation sites,
corpora lutea, resorptions sites and live or dead foetuses). Data
on foetal weight and condition were normal. Data on foetal
survival and from internal and external examinations for
abnormalities showed no significant effects of administration of
Sumithrin(R) during gestation (Rutter, 1974).
The teratogenic potential of Sumithrin(R) was examined in mice.
Groups of pregnant mice (17-18 mice/group) were orally administered
Sumithrin(R) emulsified in 0.1% Tween(R) 80 at dosage levels of 0,
30, 300 or 3000 mg/kg from day 7 to 12 of gestation. A separate
group of mice (7 mice per group) receiving dosage levels of 0, 300
or 3000 mg/kg were utilized in the study to evaluate postnatal
effects. On day 18 of gestation, mice were sacrificed and pups
delivered by caesarean section. The second group of mice were
allowed to deliver naturally and the young were maintained for 29
days.
There were no adverse effects of the administration of Sumithrin(R)
with respect to maternal and embryo well-being as evidenced by
maternal growth, foetal mortality and external and internal
examination of foetuses for teratogenic or embryotoxic effects.
Sumithrin(R) was neither teratogenic nor embryotoxic in mice at
dosage levels up to and including 3000 mg/kg (Nakamoto et al,
1973).
Special studies on reproduction
Groups of rats (8 male and 16 female Charles River rats/group) were
fed phenothrin in the diet at dosage levels of 0, 200, 600, or 2000
mg/kg and subjected to a standard three-generation, two-litter per
generation reproduction study. Data were reported on the
significant reproductive indices (mating index, fecundity index,
male fertility index, female fertility index and the incidence of
parturition). The first litters obtained were examined for
physical abnormalities at birth and for the numbers of viable and
stillborn pups of each litter. Records of survival were made at 1,
4, 12, and 21 days after birth and a final examination was made at
weaning. Litters were reduced to 10 pups on the fifth day of
lactation. Following weaning, the parental females were mated and
the procedure was repeated to obtain a second generation. Eight
males and 16 females of the second generation were selected at
weaning as parental animals for a succeeding generation.
Gross examination of tissues and organs was performed on the second
litters of all generations. A complete microscopic examination was
conducted on five male and five female animals from the control and
high dose group of the second litter from each generation. In
addition, gross and histopathologic examination was conducted on 10
animals of each sex of the final litter of the third generation.
There was no significant mortality or complications with respect to
parental animals over the course of the study. Although there were
sporadic changes in some of the reproductive data during the three
parental generations, the reproductive parameters showed no
significant, dose-related adverse effects attributable to
phenothrin. Gross and microscopic findings suggested no adverse
effect as a result of dietary phenothrin and it was concluded that
phenothrin had no effect on reproduction in a standard,
three-generation reproduction study in rats (Takatsuka et al,
1980).
Acute toxicity
The acute toxicity in male and female rats and mice is extremely
low. The LD50 was greater than 5,000 mg/kg body weight when
phenothrin was administered orally, subcutaneously, or by
intraperitoneal injection. An intravenous LD50 in rats was reported
to range from 452 to 492 mg/kg and in mice ranged from 354 to 405
mg/kg. There were no sex differences noted in acute toxicity
studies.
Signs of poisoning were noted rapidly following the intravenous
administration of phenothrin. The signs of poisoning include:
fibrillation, tremor, slow respiration, salivation, lacrimation,
ataxia, and paralysis. The signs of poisoning, evident at one-half
to one hour following administration, were rapidly diminished
spontaneously to the point where, at 24 hours, there were no signs
of toxicity (Segawa, 1976).
The LC50 following a four-hour inhalation exposure was determined
to be greater than 1,210 mg/kg3 for phenothrin with both rats and
mice (Kohda et al, 1979). With Sumithrin an LC50 exceeds 3,760
mg/m3 for a 4-hour acute exposure (Kohda, et al, 1977).
Acute oral toxicity of metabolites of phenothrin
Chemical Species LD50 (mg/kg)
3-phenoxybenzyl alcohol rat 1330
3-phenoxybenazldehyde rat 600
Acute intraperitoneal toxicity of several phenothrin metabolites in
mice
Chemical Male Female
3-phenoxybenzyl alcohol 371 424
3-(4'-hydroxyphenoxy)benzyl alcohol 750-1000 750-1000
3-(2'-hydroxyphenoxy)benzyl alcohol 876 778
3-phenoxybenzoic acid 154 169
3-(4'-hydroxyphenoxy)benzoic acid 783 745
3-(2'-hydroxyphenoxy)benzoic acid 859 912
3-phenoxybenzaldehyde 415 416
(These data on phenothrin metabolites were originally reported in
the toxicological review of permethrin, see FAO, 1980).
Short-term studies
Rats
Groups of rats (15 male and 15 female rats per group) were exposed
to phenothrin by inhalation at concentrations of 0, 43 or 220
mg/m3 for four weeks. The animals were exposed for five
consecutive days per week with an exposure period of four hours per
days. Phenothrin, dissolved in deodorised kerosene, was
aerosolised and admitted to the exposure chamber after larger sized
particles were removed. The range of particle size to which the
animals were exposed was not reported. However, the concentration
of the phenothrin in the chamber was measured.
At the conclusion of the four-week treatment interval, 10 animals
of each sex of each group were sacrificed. Haematological
examinations, clinical chemistry examinations, and gross and
microscopic examinations of selected tissues and organs were
performed on all animals sacrificed at the conclusion of the study.
In addition, groups of five animals of each sex were maintained
and examined for gross pathological changes three weeks after
completion of the exposure.
There was no mortality or evidence of acute poisoning observed in
any of the animals. Data on body weight, haematology and clinical
chemistry parameters, and gross and microscopic examinations of
tissues and organs showed that subchronic exposure to concentration
of phenothrin up to 220 mg/m3 for four weeks did not result in any
adverse toxicological effect (Kohda et al, 1979).
Long-term studies
Mice
Groups of mice (50 male and 50 female Swiss white mice/group) were
fed phenothrin in the diet at dosage levels of 0, 300, 1,000 or
3,000 mg/kg for 18 months in a standard carcinogenicity study. The
mice were examined daily for clinical signs of toxicity and
mortality. The animals were weighed once at the initiation of the
study and monthly thereafter. At the conclusion of the study,
haematologic and clinical-blood chemistry studies were performed
using 10 mice of each sex from each dosage group. At the
conclusion of the 18-month feeding trial, all mice were sacrificed
for gross and microscopic examination of tissues and organs.
Statistical evaluation of the data was performed to identify group
differences.
There was no mortality, and clinical signs of poisoning were not
significant in the study. Growth, as evidence by body weight of
males fed 3,000 mg/kg, was slightly depressed throughout the major
portion of the study. Haematologic values were normal. Clinical
chemistry parameters were normal (with the exception of a
statistically significant elevation in SGPT activity in females;
the increase was not dose-dependant and was not present in males
and additionally, as the data from the female control group
appeared to be significantly lower than that observed in the male
control group, it is believed that the statistically significant
difference in the SGPT activity is not attributable to phenothrin
in the diet). Gross examination of tissues and organs at the
conclusion of the study showed an increased liver weight at the
high-dose level in both males and females. There were no other
gross pathological findings.
Microscopic examination of liver revealed no unusual findings
associated with the enlargement. In lungs of mice, congestion was
observed. In addition, amyloidosis in alveoli was found in all
groups in a dose-dependant manner. The two highest dose groups
showed a statistically significant difference from control values
with respect to this occurrence. There was no significant increase
in neoplasm associated with the presence of phenothrin in the diet
(Murakami et al, 1980). Based on this bioassay phenothrin is not
carcinogenic to the mouse.
Rats
Groups of rats (50 male and 50 female rats per group) were fed
phenothrin in the diet at dosage levels of 0, 200, 600, 2,000 or
6,000 mg/kg for two years. Animals were examined weekly for the
first 13 weeks and at monthly intervals thereafter. Food
consumption data were reported for these same intervals.
Ophthalmological examinations, haematological studies, clinical
chemistry studies, and urinalyses were performed at periodic
intervals over the course of the study. At the conclusion of the
study, all animals were sacrificed and examined for gross
abnormalities. Extensive microscopic examinations were conducted
on a variety of tissues and organs and where gross lesions were
observed. All data were statistically analyzed to evaluate
significant intergroup differences.
There were no abnormal clinical behaviourial problems associated
with this study. The survival rate of all animals of all groups
was similar to that of controls and there were no effects of
phenothrin on mortality. Growth, as evidenced by body-weight
reduction, was significantly affected at 6,000 mg/kg in both males
and females. The growth of males, while significantly reduced
during the early parts of the experiment, was not substantially
different from that of the controls after the first four months.
Body weights of females fed 6,000 mg/kg were slightly lower than
the controls throughout the study. Food consumption was observed
to be slightly less in the high-dose male and female animals but
was not consistently different from control animals in any of the
other dose groups. Ophthalmological examinations revealed some
abnormalities, all of which appeared to be age-related changes.
There were no significant haematological differences. An increase
in SGPT activity in the 6,000 mg/kg male group was significantly
different than controls. There were no differences observed with
females. Urinalysis values were normal throughout the study.
OBSERVATIONS IN MAN
A group of eight male human volunteers were administered
Sumithrin(R) (d-phenothrin) dermally at a dose of 32 mg/kg/day for
3 consecutive days. There were no signs of toxicity or dermal
irritation. Blood biochemistry and haematology parameters were
normal and dermal absorption was slight as blood levels of
d-phenothrin isomers were below the level of analytical detection
0.006 mg/kg). The daily dosages used in this dermal study ranged
from 0.67 mg/kg body weight to 0.44 mg/kg body weight with no
adverse effects noted (Hashimoto et al, 198O).
EVALUATION
COMMENTS
Phenothrin, the chrysanthemic acid ester of 3-phenoxybenzyl
alcohol, a synthetic pyrethroid used as an insecticide, is a
mixture of cis and trans racemates, usually with a
cis:trans ratio of 20:80.
Phenothrin is rapidly absorbed, distributed, metabolized and
excreted from the body and the metabolic fate of the molecule has
been well defined. Phenothrin has a very low order of acute
toxicity. At very high doses, phenothrin exhibited central nervous
system effects as noted with several other pyrethroids. Axon and
myelin degeneration were not demonstrated following acute treatment
with phenothrin.
Phenothrin is not mutagenic in microbial or mammalian tests, is not
teratogenic and was found to have no effect on rat reproduction in
a standard three-generation bioassay.
Long-term studies in both the rat and the mouse showed phenothrin
to have a low order of toxicity. There was no carcinogenic
potential in either rat or mouse.
In a human volunteer study no adverse effects were reported when
people were exposed dermally to Sumithrin(R) (also known as
phenothrin) a mixture enriched in the (1R)-cis and (1R)-trans
isomers but still with a cis:trans ratio of 20:80.
Adequate data were presented to allocate a temporary ADI for man on
the basis of no-effect levels observed in long-term studies in the
rat and mouse. A temporary ADI was suggested, since all long-term
studies were in rodent species and the dog has been noted to be a
species that has shown greater sensitivity to other pyrethroids.
Further studies on the dog were therefore required. Additionally,
further studies on the metabolic fate of phenothrin are desirable
in order to confirm the rapid nature of its degradation and
elimination from the body. Observations in man are valuable in
assessing the overall hazards associated with this new class of
pesticides.
Level causing no toxicological effect
Mouse: 300 mg/kg in the diet equivalent to 45 mg/kg bw/day
Rat: 2,000 mg/kg in the diet equivalent to 100 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.2 mg/kg bw/day
RESIDUES AND ANALYTICAL ASPECTS
Since a temporary ADI has been allocated the existing guideline
levels should be replaced by temporary MRLs.
FURTHER DATA AND INFORMATION
Required (by 1984)
1. A two-year dog study.
Desirable
1. Additional metabolism data with phenothrin technical product.
2. Further observations in man with phenothrin.
REFERENCES
Casida, J.E., Gaughan, L.C. and Ruzo, L.O. Comparative Metabolism
of Pyrethroids derived from 3-Phenoxybenzyl and
alpha-Cyano-3-phenoxybenzyl Alcohols. Advances in Pesticide
Science, Ed. by Geisebühler, H., Brooke, G.T., and Kearney, P.C.
Pergamon Press, London, Part 2, p. 182-89.
Hara, Y., Miyagishi, A., Ohtuska, M., Asami, Y., Kurokawa, H. and
Miyamoto, J. Pharmacological Study of Sumithrin(R): Effects of
Respiration, Blood Pressure, and Peripheral Central Nervous
Systems. (1974) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Hashimoto, S., Okano, S., Yamada, H. and Miyamoto, J. Human
volunteer study with d-phenothrin powder. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Hiromori, T., Koyama, Y., Okuno, Y., Arai, M., Ito, N. and
Kiyamoto, J. Two-year Chronic Toxicity Study of S-2539 in Rats.
(1980) Unpublished report from Sumitomo Chemical Co. of a study
performed by Industrial Bio-Test Laboratories, Inc. validated by
Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
Kaneko, H., Ohkawa, H. and Miyamoto, J. Absorption and Metabolism
of Dermally Applied d-phenothrin in Rat. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Kohda H., Nishimoto K., Kadota, K. and Miyamoto, J. Acute and
Subacute Inhalation Toxicity Studies of S-2539 Forte in Rats and
Mice. (1979) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Kohda H., Okuno Y., Kadota, K. and Miyamoto, J. Acute Inhalation
Toxicity of d-phenothrin (S-2559 Forte) in Rats. (1977)
Unpublished report from Sumitomo Chemical Co., submitted to the
World Health Organization by Sumitomo Chemical Co.
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., jr.,
Kinoshita, F.K. and Keplinger, M.L. Teratogenic Study with S-2539
in Albino Rabbits. (1976) Unpublished report from Industrial
Bio-Test Laboratories, submitted to the World Health Organization
by Sumitomo Chemical Co.
Miyamoto, J., Suzuki, T. and Nakae, C. Metabolism of Phenothrin of
3-Phenoxybenzyl d-trans Chrysanthemumate in Mammals. Pestic.
Biochem. Physiol. 4: 438-450.
Murikami, J., Ito, S., Okuno, Y., Arai, M., Ito, N. and Miyamoto,
J. Eighteen-month Chronic Oral Toxicity and Tumorigenicity Study of
S-2539 in Mice. (1980) Unpublished report from Sumitomo Chemical
Co. of a study performed by Industrial Bio-Test Laboratories, Inc.
validated by Sumitomo Chemical Co. and submitted to the World
Health Organization by Sumitomo Chemical Co.
Nakamoto, N., Kato, T. and Miyamoto, J. Teratogenicity Study of
S-2539 Forte in Mice. (1973) Unpublished report from Sumitomo
Chemical Co. submitted to the World Health Organization by Sumitomo
Chemical Co.
Okuno Y., Kadota and Miyamoto, J. Neurotoxicity Study of
d-Phenothrin (S-2539 Forte(R)) in Rats by Repeated Oral
Administration. (1978) Unpublished report from Sumitomo Chemical
Co., submitted to the World Health Organization by Sumitomo
Chemical Co.
Rutter, H.A. Teratogenicity Study in Rabbits: S-2539 Forte. (1974)
Unpublished report from Hazleton Laboratories Inc., submitted to
the World Health Organization by Sumitomo Chemical Co.
Segawa, T. Acute Toxicity Study of S-2539 in Rats and Mice. (1976)
Unpublished report from Hiroshima University School of Medicine,
submitted to the World Health Organization by Sumitomo Chemical Co.
Shirasu, Y., Morishita, M. and Kato, K. Mutation Test of S-2539 on
Bacteria. (1974) Unpublished report from The Institute of
Environmental Toxicology, submitted to the World Health
Organization by Sumitomo Chemical Co.
Soderland, D.M. and Casida, J.E. Effects of Pyrethroid Structure on
Rates of Hydrolysis and Oxidation by Mouse Liver Microsomal
Enzymes. Pestic. Biochem. Physio. 7: 391-401.
Suzuki, H. and Miyamoto, J. Mutagenicity of Some Synthetic
Pryethroids in Bacterial Test Systems. (1975) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Suzuki, T. and Miyamoto, J. Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome. Pestic. Biochem.
Physio. 8: 186-98.
Suzuki, T., Ohno, N. and Miyamoto, J. New Metabolites of (+)-cis
Fenothrin, 3-Phenoxybenzyl, (+)-cis Chrysanthemumate, in Rats. J.
Pesticide Sci. 1: 151-52.
Tekatsuka, M., Okuno, Y., Suzuki, T., Kadota, T., Yasuda, M. and
Miyamoto, J. Three-generation reproduction study of S-2539 in rats.
Unpublished report from Industrial Bio-Test Laboratories, validated
by Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
TOXICOLOGICAL STUDIES
Special Pharmacological studies
A series of pharmacological studies were performed with the
d-isomer of phenothrin (Sumithrin(R)). These studies reported the
pharmacological action of this pyrethroid on respiration, blood
pressure, and a series of tests representative of effects on the
peripheral and central nervous system. Sumithrin(R) was emulsified
(Sorpol(R) 1200 and/or Tween(R) 80) and either diluted with
physiological saline or used directly. The following series of
in vitro and in vivo studies were performed: spasmolytic
activity on isolated ileum from male guinea pigs; spontaneous
contractions of isolated uterus from rats; contractions of a
neuromuscular preparation of phrenic nerve-diaphgram of rats;
respiration, nictitating membrane and blood pressure of male cats
administered Sumithrin(R) intravenously; contractile force, blood
pressure, and heart rate of dogs administered Sumithrin(R)
intravenously; the anticonvulsive activity following induced
convulsion or seizures in mice; the effects on muscular relaxation
activity in mice; coordinative movement in mice;
hexabarbital-induced sleeping time in mice; spontaneous activity in
mice; body temperature of male rats; and electroencephalogram in
male cats.
Sumithrin(R) did not show any pharmacological activity in any of
the in vitro and/or in vivo experiments listed above. A
tentative arousal response in the electroencephalogram of cats
intravenously administered a dose of 4 mg/kg body weight was
recorded. Several other synthetic pyrethroid esters have been
shown to induce this arousal response in the EEG pattern of cats
(Hare et al, 1974).
Special studies on neurotoxicity
Groups of rats exposed by inhalation to the d-isomer of phenothrin
(Sumithrin(R)) at concentration of up to an including 3,760 mg/m3
for four hours showed no adverse effects as a result of exposure.
Examination of the sciatic nerve for possible myelin degeneration
or axon disruption did not reveal any such occurrence (Kohda et
al, 1977).
Groups of rats were administered Sumithrin(R) orally for five
consecutive days at a dosage level of 5,000 mg/kg/day. Mortality
was observed and toxic signs of poisoning were noted in several of
the animals. Toxic signs of poisoning rapidly disappeared at the
conclusion of the treatment. Three days after treatment, all
animals were sacrificed and histopathological examination of
sciatic nerve was carried out to determine whether changes had
occurred. Clinically, there were no toxic signs of poisoning as
evidenced by leg weakness or ataxia. Histological examination
revealed minute changes in axon and myelin, characterised by very
slight axonal swelling, axonal disintegration, and/or demyelination.
There were similar occurrences in the control animals and it was
suggested that the small changes observed were not due to the
administration of Sumithrin(R). It was considered that oral
administration of Sumithrin(R) at excessively high doses does
not induce a neurotoxic effect as has been observed with several
other pyrethroid esters (Okuno, et al, 1978; FAO, 1980).
Special studies on mutagenicity
The host-mediated assay was conducted with phenothrin and
individual isomers of phenothrin in male mice using S.
typhimurium-G46 as an indicator strain. Groups of six male mice
were administered phenothrin orally, twice, at 24-hour intervals at
dosage rates of 0, 500, or 1500 mg/kg/dose. Additionally, the
d-trans and d-cis isomers were administered at dosage levels of
250 and 90 mg/kg respectively. A positive control of
dymethylnitrosamine (50 mg/kg) was used. Immediately after
administration of the chemical, the tester strain (S.
typhimurium) was administered into the abdominal cavity. This
was removed three hours later, cultured and examined for mutation
frequency. There was no increase in reversion frequency observed
with phenothrin or its isomers although the positive control was
found to give an increased reversion rate.
The potential mutagenicity of phenothrin was investigated using
various bacterial test systems including the Reo-Assay (repair
test) with Bacillus subtilis and Salmonella typhimurium
strains.
At dose levels ranging from 0 to 10 mg/disc/plate, phenothrin did
not induce an increase of the growth inhibition zone of the
microorganisms, while mytomycin C showed a substantially larger
inhibition zone. A negative control gave results similar to that
obtained with phenothrin.
Mutation induction studies (the standard "Ames test") with various
strains of E. coli and Salmonella typhimurium (TA1535,
TA1537, TA1538, TA98 and TA100) with and without metabolic
activation enzymes, were performed. Phenothrin and its individual
isomers, at dose levels up to 10 mg per plate, with or without
hepatic drug metabolising enzymes, showed negative results in this
standard mutagenicity assay. Phenothrin and its individual isomers
alone and following metabolic activation, caused no increase in the
mutation rate. Positive controls employed during the course of the
study gave significant numbers of revertants (Shirasu et al,
1977; Suzuki and Miyamoto, 1975). Under the conditions of these
bioassays, phenothrin and its individual d-cis and d-trans
isomers are not mutagenic.
Special studies on teratogenicity
Groups of pregnant New Zealand rabbits (17 rabbits per group) were
administered phenothrin orally at dosage levels of 0, 3, 10 or 30
mg/kg from day 6 to 18 of gestation. All animals were sacrificed
on day 29 of gestation and the young obtained by caesarean section
were examined.
Does treated with phenothrin at the highest dosage level exhibited
a loss in body weight during gestation. There was no mortality
observed in the study. There was a slight decrease in the number
of live young obtained from does treated with 30 mg/kg. Changes in
other reproductive/teratogenic parameters did not show a
dose-response relationship and were not believed to be induced as
a result of phenothrin administration. At 30 mg/kg, there was a
slight reduced foetal weight which was not accompanied by changes
in survival rate or in the occurrence of external or internal
abnormalities.
There was no apparent teratogenic effect of phenothrin as observed
by apparent gross internal or external somatic abnormalities and by
examination for foetal skeletal development following prenatal
exposure. Treatment of pregnant albino rabbits during the period
of foetal organogenesis did not induce abnormal foetal
development. At the high dosage level, possible foetal and/or
maternal toxicity was observed.
Groups of pregnant rabbits (15 dose/group) were administered
Sumithrin(R)) at dosage levels of 0, 10, 100 or 1000 mg/kg/day from
day 6 through 18 of gestation. All does were sacrificed on day 29
or day 30 and caesarean section was performed to remove the foetus.
Following caesarean section, one half of the pups were maintained
for 24 hours to evaluate survival.
There were no abnormalities observed in the maternal parameters
(including: body weight, food consumption, clinical observations,
and necropsy) or on foetal data (including: implantation sites,
corpora lutea, resorptions sites and live or dead foetuses). Data
on foetal weight and condition were normal. Data on foetal
survival and from internal and external examinations for
abnormalities showed no significant effects of administration of
Sumithrin(R) during gestation (Rutter, 1974).
The teratogenic potential of Sumithrin(R) was examined in mice.
Groups of pregnant mice (17-18 mice/group) were orally administered
Sumithrin(R) emulsified in 0.1% Tween(R) 80 at dosage levels of 0,
30, 300 or 3000 mg/kg from day 7 to 12 of gestation. A separate
group of mice (7 mice per group) receiving dosage levels of 0, 300
or 3000 mg/kg were utilized in the study to evaluate postnatal
effects. On day 18 of gestation, mice were sacrificed and pups
delivered by caesarean section. The second group of mice were
allowed to deliver naturally and the young were maintained for 29
days.
There were no adverse effects of the administration of Sumithrin(R)
with respect to maternal and embryo well-being as evidenced by
maternal growth, foetal mortality and external and internal
examination of foetuses for teratogenic or embryotoxic effects.
Sumithrin(R) was neither teratogenic nor embryotoxic in mice at
dosage levels up to and including 3000 mg/kg (Nakamoto et al,
1973).
Special studies on reproduction
Groups of rats (8 male and 16 female Charles River rats/group) were
fed phenothrin in the diet at dosage levels of 0, 200, 600, or 2000
mg/kg and subjected to a standard three-generation, two-litter per
generation reproduction study. Data were reported on the
significant reproductive indices (mating index, fecundity index,
male fertility index, female fertility index and the incidence of
parturition). The first litters obtained were examined for
physical abnormalities at birth and for the numbers of viable and
stillborn pups of each litter. Records of survival were made at 1,
4, 12, and 21 days after birth and a final examination was made at
weaning. Litters were reduced to 10 pups on the fifth day of
lactation. Following weaning, the parental females were mated and
the procedure was repeated to obtain a second generation. Eight
males and 16 females of the second generation were selected at
weaning as parental animals for a succeeding generation.
Gross examination of tissues and organs was performed on the second
litters of all generations. A complete microscopic examination was
conducted on five male and five female animals from the control and
high dose group of the second litter from each generation. In
addition, gross and histopathologic examination was conducted on 10
animals of each sex of the final litter of the third generation.
There was no significant mortality or complications with respect to
parental animals over the course of the study. Although there were
sporadic changes in some of the reproductive data during the three
parental generations, the reproductive parameters showed no
significant, dose-related adverse effects attributable to
phenothrin. Gross and microscopic findings suggested no adverse
effect as a result of dietary phenothrin and it was concluded that
phenothrin had no effect on reproduction in a standard,
three-generation reproduction study in rats (Takatsuka et al,
1980).
Acute toxicity
The acute toxicity in male and female rats and mice is extremely
low. The LD50 was greater than 5,000 mg/kg body weight when
phenothrin was administered orally, subcutaneously, or by
intraperitoneal injection. An intravenous LD50 in rats was reported
to range from 452 to 492 mg/kg and in mice ranged from 354 to 405
mg/kg. There were no sex differences noted in acute toxicity
studies.
Signs of poisoning were noted rapidly following the intravenous
administration of phenothrin. The signs of poisoning include:
fibrillation, tremor, slow respiration, salivation, lacrimation,
ataxia, and paralysis. The signs of poisoning, evident at one-half
to one hour following administration, were rapidly diminished
spontaneously to the point where, at 24 hours, there were no signs
of toxicity (Segawa, 1976).
The LC50 following a four-hour inhalation exposure was determined
to be greater than 1,210 mg/kg3 for phenothrin with both rats and
mice (Kohda et al, 1979). With Sumithrin an LC50 exceeds 3,760
mg/m3 for a 4-hour acute exposure (Kohda, et al, 1977).
Acute oral toxicity of metabolites of phenothrin
Chemical Species LD50 (mg/kg)
3-phenoxybenzyl alcohol rat 1330
3-phenoxybenazldehyde rat 600
Acute intraperitoneal toxicity of several phenothrin metabolites in
mice
Chemical Male Female
3-phenoxybenzyl alcohol 371 424
3-(4'-hydroxyphenoxy)benzyl alcohol 750-1000 750-1000
3-(2'-hydroxyphenoxy)benzyl alcohol 876 778
3-phenoxybenzoic acid 154 169
3-(4'-hydroxyphenoxy)benzoic acid 783 745
3-(2'-hydroxyphenoxy)benzoic acid 859 912
3-phenoxybenzaldehyde 415 416
(These data on phenothrin metabolites were originally reported in
the toxicological review of permethrin, see FAO, 1980).
Short-term studies
Rats
Groups of rats (15 male and 15 female rats per group) were exposed
to phenothrin by inhalation at concentrations of 0, 43 or 220
mg/m3 for four weeks. The animals were exposed for five
consecutive days per week with an exposure period of four hours per
days. Phenothrin, dissolved in deodorised kerosene, was
aerosolised and admitted to the exposure chamber after larger sized
particles were removed. The range of particle size to which the
animals were exposed was not reported. However, the concentration
of the phenothrin in the chamber was measured.
At the conclusion of the four-week treatment interval, 10 animals
of each sex of each group were sacrificed. Haematological
examinations, clinical chemistry examinations, and gross and
microscopic examinations of selected tissues and organs were
performed on all animals sacrificed at the conclusion of the study.
In addition, groups of five animals of each sex were maintained
and examined for gross pathological changes three weeks after
completion of the exposure.
There was no mortality or evidence of acute poisoning observed in
any of the animals. Data on body weight, haematology and clinical
chemistry parameters, and gross and microscopic examinations of
tissues and organs showed that subchronic exposure to concentration
of phenothrin up to 220 mg/m3 for four weeks did not result in any
adverse toxicological effect (Kohda et al, 1979).
Long-term studies
Mice
Groups of mice (50 male and 50 female Swiss white mice/group) were
fed phenothrin in the diet at dosage levels of 0, 300, 1,000 or
3,000 mg/kg for 18 months in a standard carcinogenicity study. The
mice were examined daily for clinical signs of toxicity and
mortality. The animals were weighed once at the initiation of the
study and monthly thereafter. At the conclusion of the study,
haematologic and clinical-blood chemistry studies were performed
using 10 mice of each sex from each dosage group. At the
conclusion of the 18-month feeding trial, all mice were sacrificed
for gross and microscopic examination of tissues and organs.
Statistical evaluation of the data was performed to identify group
differences.
There was no mortality, and clinical signs of poisoning were not
significant in the study. Growth, as evidence by body weight of
males fed 3,000 mg/kg, was slightly depressed throughout the major
portion of the study. Haematologic values were normal. Clinical
chemistry parameters were normal (with the exception of a
statistically significant elevation in SGPT activity in females;
the increase was not dose-dependant and was not present in males
and additionally, as the data from the female control group
appeared to be significantly lower than that observed in the male
control group, it is believed that the statistically significant
difference in the SGPT activity is not attributable to phenothrin
in the diet). Gross examination of tissues and organs at the
conclusion of the study showed an increased liver weight at the
high-dose level in both males and females. There were no other
gross pathological findings.
Microscopic examination of liver revealed no unusual findings
associated with the enlargement. In lungs of mice, congestion was
observed. In addition, amyloidosis in alveoli was found in all
groups in a dose-dependant manner. The two highest dose groups
showed a statistically significant difference from control values
with respect to this occurrence. There was no significant increase
in neoplasm associated with the presence of phenothrin in the diet
(Murakami et al, 1980). Based on this bioassay phenothrin is not
carcinogenic to the mouse.
Rats
Groups of rats (50 male and 50 female rats per group) were fed
phenothrin in the diet at dosage levels of 0, 200, 600, 2,000 or
6,000 mg/kg for two years. Animals were examined weekly for the
first 13 weeks and at monthly intervals thereafter. Food
consumption data were reported for these same intervals.
Ophthalmological examinations, haematological studies, clinical
chemistry studies, and urinalyses were performed at periodic
intervals over the course of the study. At the conclusion of the
study, all animals were sacrificed and examined for gross
abnormalities. Extensive microscopic examinations were conducted
on a variety of tissues and organs and where gross lesions were
observed. All data were statistically analyzed to evaluate
significant intergroup differences.
There were no abnormal clinical behaviourial problems associated
with this study. The survival rate of all animals of all groups
was similar to that of controls and there were no effects of
phenothrin on mortality. Growth, as evidenced by body-weight
reduction, was significantly affected at 6,000 mg/kg in both males
and females. The growth of males, while significantly reduced
during the early parts of the experiment, was not substantially
different from that of the controls after the first four months.
Body weights of females fed 6,000 mg/kg were slightly lower than
the controls throughout the study. Food consumption was observed
to be slightly less in the high-dose male and female animals but
was not consistently different from control animals in any of the
other dose groups. Ophthalmological examinations revealed some
abnormalities, all of which appeared to be age-related changes.
There were no significant haematological differences. An increase
in SGPT activity in the 6,000 mg/kg male group was significantly
different than controls. There were no differences observed with
females. Urinalysis values were normal throughout the study.
OBSERVATIONS IN MAN
A group of eight male human volunteers were administered
Sumithrin(R) (d-phenothrin) dermally at a dose of 32 mg/kg/day for
3 consecutive days. There were no signs of toxicity or dermal
irritation. Blood biochemistry and haematology parameters were
normal and dermal absorption was slight as blood levels of
d-phenothrin isomers were below the level of analytical detection
0.006 mg/kg). The daily dosages used in this dermal study ranged
from 0.67 mg/kg body weight to 0.44 mg/kg body weight with no
adverse effects noted (Hashimoto et al, 198O).
EVALUATION
COMMENTS
Phenothrin, the chrysanthemic acid ester of 3-phenoxybenzyl
alcohol, a synthetic pyrethroid used as an insecticide, is a
mixture of cis and trans racemates, usually with a
cis:trans ratio of 20:80.
Phenothrin is rapidly absorbed, distributed, metabolized and
excreted from the body and the metabolic fate of the molecule has
been well defined. Phenothrin has a very low order of acute
toxicity. At very high doses, phenothrin exhibited central nervous
system effects as noted with several other pyrethroids. Axon and
myelin degeneration were not demonstrated following acute treatment
with phenothrin.
Phenothrin is not mutagenic in microbial or mammalian tests, is not
teratogenic and was found to have no effect on rat reproduction in
a standard three-generation bioassay.
Long-term studies in both the rat and the mouse showed phenothrin
to have a low order of toxicity. There was no carcinogenic
potential in either rat or mouse.
In a human volunteer study no adverse effects were reported when
people were exposed dermally to Sumithrin(R) (also known as
phenothrin) a mixture enriched in the (1R)-cis and (1R)-trans
isomers but still with a cis:trans ratio of 20:80.
Adequate data were presented to allocate a temporary ADI for man on
the basis of no-effect levels observed in long-term studies in the
rat and mouse. A temporary ADI was suggested, since all long-term
studies were in rodent species and the dog has been noted to be a
species that has shown greater sensitivity to other pyrethroids.
Further studies on the dog were therefore required. Additionally,
further studies on the metabolic fate of phenothrin are desirable
in order to confirm the rapid nature of its degradation and
elimination from the body. Observations in man are valuable in
assessing the overall hazards associated with this new class of
pesticides.
Level causing no toxicological effect
Mouse: 300 mg/kg in the diet equivalent to 45 mg/kg bw/day
Rat: 2,000 mg/kg in the diet equivalent to 100 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.2 mg/kg bw/day
RESIDUES AND ANALYTICAL ASPECTS
Since a temporary ADI has been allocated the existing guideline
levels should be replaced by temporary MRLs.
FURTHER DATA AND INFORMATION
Required (by 1984)
1. A two-year dog study.
Desirable
1. Additional metabolism data with phenothrin technical product.
2. Further observations in man with phenothrin.
REFERENCES
Casida, J.E., Gaughan, L.C. and Ruzo, L.O. Comparative Metabolism
of Pyrethroids derived from 3-Phenoxybenzyl and
alpha-Cyano-3-phenoxybenzyl Alcohols. Advances in Pesticide
Science, Ed. by Geisebühler, H., Brooke, G.T., and Kearney, P.C.
Pergamon Press, London, Part 2, p. 182-89.
Hara, Y., Miyagishi, A., Ohtuska, M., Asami, Y., Kurokawa, H. and
Miyamoto, J. Pharmacological Study of Sumithrin(R): Effects of
Respiration, Blood Pressure, and Peripheral Central Nervous
Systems. (1974) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Hashimoto, S., Okano, S., Yamada, H. and Miyamoto, J. Human
volunteer study with d-phenothrin powder. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Hiromori, T., Koyama, Y., Okuno, Y., Arai, M., Ito, N. and
Kiyamoto, J. Two-year Chronic Toxicity Study of S-2539 in Rats.
(1980) Unpublished report from Sumitomo Chemical Co. of a study
performed by Industrial Bio-Test Laboratories, Inc. validated by
Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
Kaneko, H., Ohkawa, H. and Miyamoto, J. Absorption and Metabolism
of Dermally Applied d-phenothrin in Rat. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Kohda H., Nishimoto K., Kadota, K. and Miyamoto, J. Acute and
Subacute Inhalation Toxicity Studies of S-2539 Forte in Rats and
Mice. (1979) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Kohda H., Okuno Y., Kadota, K. and Miyamoto, J. Acute Inhalation
Toxicity of d-phenothrin (S-2559 Forte) in Rats. (1977)
Unpublished report from Sumitomo Chemical Co., submitted to the
World Health Organization by Sumitomo Chemical Co.
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., jr.,
Kinoshita, F.K. and Keplinger, M.L. Teratogenic Study with S-2539
in Albino Rabbits. (1976) Unpublished report from Industrial
Bio-Test Laboratories, submitted to the World Health Organization
by Sumitomo Chemical Co.
Miyamoto, J., Suzuki, T. and Nakae, C. Metabolism of Phenothrin of
3-Phenoxybenzyl d-trans Chrysanthemumate in Mammals. Pestic.
Biochem. Physiol. 4: 438-450.
Murikami, J., Ito, S., Okuno, Y., Arai, M., Ito, N. and Miyamoto,
J. Eighteen-month Chronic Oral Toxicity and Tumorigenicity Study of
S-2539 in Mice. (1980) Unpublished report from Sumitomo Chemical
Co. of a study performed by Industrial Bio-Test Laboratories, Inc.
validated by Sumitomo Chemical Co. and submitted to the World
Health Organization by Sumitomo Chemical Co.
Nakamoto, N., Kato, T. and Miyamoto, J. Teratogenicity Study of
S-2539 Forte in Mice. (1973) Unpublished report from Sumitomo
Chemical Co. submitted to the World Health Organization by Sumitomo
Chemical Co.
Okuno Y., Kadota and Miyamoto, J. Neurotoxicity Study of
d-Phenothrin (S-2539 Forte(R)) in Rats by Repeated Oral
Administration. (1978) Unpublished report from Sumitomo Chemical
Co., submitted to the World Health Organization by Sumitomo
Chemical Co.
Rutter, H.A. Teratogenicity Study in Rabbits: S-2539 Forte. (1974)
Unpublished report from Hazleton Laboratories Inc., submitted to
the World Health Organization by Sumitomo Chemical Co.
Segawa, T. Acute Toxicity Study of S-2539 in Rats and Mice. (1976)
Unpublished report from Hiroshima University School of Medicine,
submitted to the World Health Organization by Sumitomo Chemical Co.
Shirasu, Y., Morishita, M. and Kato, K. Mutation Test of S-2539 on
Bacteria. (1974) Unpublished report from The Institute of
Environmental Toxicology, submitted to the World Health
Organization by Sumitomo Chemical Co.
Soderland, D.M. and Casida, J.E. Effects of Pyrethroid Structure on
Rates of Hydrolysis and Oxidation by Mouse Liver Microsomal
Enzymes. Pestic. Biochem. Physio. 7: 391-401.
Suzuki, H. and Miyamoto, J. Mutagenicity of Some Synthetic
Pryethroids in Bacterial Test Systems. (1975) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Suzuki, T. and Miyamoto, J. Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome. Pestic. Biochem.
Physio. 8: 186-98.
Suzuki, T., Ohno, N. and Miyamoto, J. New Metabolites of (+)-cis
Fenothrin, 3-Phenoxybenzyl, (+)-cis Chrysanthemumate, in Rats. J.
Pesticide Sci. 1: 151-52.
Tekatsuka, M., Okuno, Y., Suzuki, T., Kadota, T., Yasuda, M. and
Miyamoto, J. Three-generation reproduction study of S-2539 in rats.
Unpublished report from Industrial Bio-Test Laboratories, validated
by Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
 TOXICOLOGICAL STUDIES
Special Pharmacological studies
A series of pharmacological studies were performed with the
d-isomer of phenothrin (Sumithrin(R)). These studies reported the
pharmacological action of this pyrethroid on respiration, blood
pressure, and a series of tests representative of effects on the
peripheral and central nervous system. Sumithrin(R) was emulsified
(Sorpol(R) 1200 and/or Tween(R) 80) and either diluted with
physiological saline or used directly. The following series of
in vitro and in vivo studies were performed: spasmolytic
activity on isolated ileum from male guinea pigs; spontaneous
contractions of isolated uterus from rats; contractions of a
neuromuscular preparation of phrenic nerve-diaphgram of rats;
respiration, nictitating membrane and blood pressure of male cats
administered Sumithrin(R) intravenously; contractile force, blood
pressure, and heart rate of dogs administered Sumithrin(R)
intravenously; the anticonvulsive activity following induced
convulsion or seizures in mice; the effects on muscular relaxation
activity in mice; coordinative movement in mice;
hexabarbital-induced sleeping time in mice; spontaneous activity in
mice; body temperature of male rats; and electroencephalogram in
male cats.
Sumithrin(R) did not show any pharmacological activity in any of
the in vitro and/or in vivo experiments listed above. A
tentative arousal response in the electroencephalogram of cats
intravenously administered a dose of 4 mg/kg body weight was
recorded. Several other synthetic pyrethroid esters have been
shown to induce this arousal response in the EEG pattern of cats
(Hare et al, 1974).
Special studies on neurotoxicity
Groups of rats exposed by inhalation to the d-isomer of phenothrin
(Sumithrin(R)) at concentration of up to an including 3,760 mg/m3
for four hours showed no adverse effects as a result of exposure.
Examination of the sciatic nerve for possible myelin degeneration
or axon disruption did not reveal any such occurrence (Kohda et
al, 1977).
Groups of rats were administered Sumithrin(R) orally for five
consecutive days at a dosage level of 5,000 mg/kg/day. Mortality
was observed and toxic signs of poisoning were noted in several of
the animals. Toxic signs of poisoning rapidly disappeared at the
conclusion of the treatment. Three days after treatment, all
animals were sacrificed and histopathological examination of
sciatic nerve was carried out to determine whether changes had
occurred. Clinically, there were no toxic signs of poisoning as
evidenced by leg weakness or ataxia. Histological examination
revealed minute changes in axon and myelin, characterised by very
slight axonal swelling, axonal disintegration, and/or demyelination.
There were similar occurrences in the control animals and it was
suggested that the small changes observed were not due to the
administration of Sumithrin(R). It was considered that oral
administration of Sumithrin(R) at excessively high doses does
not induce a neurotoxic effect as has been observed with several
other pyrethroid esters (Okuno, et al, 1978; FAO, 1980).
Special studies on mutagenicity
The host-mediated assay was conducted with phenothrin and
individual isomers of phenothrin in male mice using S.
typhimurium-G46 as an indicator strain. Groups of six male mice
were administered phenothrin orally, twice, at 24-hour intervals at
dosage rates of 0, 500, or 1500 mg/kg/dose. Additionally, the
d-trans and d-cis isomers were administered at dosage levels of
250 and 90 mg/kg respectively. A positive control of
dymethylnitrosamine (50 mg/kg) was used. Immediately after
administration of the chemical, the tester strain (S.
typhimurium) was administered into the abdominal cavity. This
was removed three hours later, cultured and examined for mutation
frequency. There was no increase in reversion frequency observed
with phenothrin or its isomers although the positive control was
found to give an increased reversion rate.
The potential mutagenicity of phenothrin was investigated using
various bacterial test systems including the Reo-Assay (repair
test) with Bacillus subtilis and Salmonella typhimurium
strains.
At dose levels ranging from 0 to 10 mg/disc/plate, phenothrin did
not induce an increase of the growth inhibition zone of the
microorganisms, while mytomycin C showed a substantially larger
inhibition zone. A negative control gave results similar to that
obtained with phenothrin.
Mutation induction studies (the standard "Ames test") with various
strains of E. coli and Salmonella typhimurium (TA1535,
TA1537, TA1538, TA98 and TA100) with and without metabolic
activation enzymes, were performed. Phenothrin and its individual
isomers, at dose levels up to 10 mg per plate, with or without
hepatic drug metabolising enzymes, showed negative results in this
standard mutagenicity assay. Phenothrin and its individual isomers
alone and following metabolic activation, caused no increase in the
mutation rate. Positive controls employed during the course of the
study gave significant numbers of revertants (Shirasu et al,
1977; Suzuki and Miyamoto, 1975). Under the conditions of these
bioassays, phenothrin and its individual d-cis and d-trans
isomers are not mutagenic.
Special studies on teratogenicity
Groups of pregnant New Zealand rabbits (17 rabbits per group) were
administered phenothrin orally at dosage levels of 0, 3, 10 or 30
mg/kg from day 6 to 18 of gestation. All animals were sacrificed
on day 29 of gestation and the young obtained by caesarean section
were examined.
Does treated with phenothrin at the highest dosage level exhibited
a loss in body weight during gestation. There was no mortality
observed in the study. There was a slight decrease in the number
of live young obtained from does treated with 30 mg/kg. Changes in
other reproductive/teratogenic parameters did not show a
dose-response relationship and were not believed to be induced as
a result of phenothrin administration. At 30 mg/kg, there was a
slight reduced foetal weight which was not accompanied by changes
in survival rate or in the occurrence of external or internal
abnormalities.
There was no apparent teratogenic effect of phenothrin as observed
by apparent gross internal or external somatic abnormalities and by
examination for foetal skeletal development following prenatal
exposure. Treatment of pregnant albino rabbits during the period
of foetal organogenesis did not induce abnormal foetal
development. At the high dosage level, possible foetal and/or
maternal toxicity was observed.
Groups of pregnant rabbits (15 dose/group) were administered
Sumithrin(R)) at dosage levels of 0, 10, 100 or 1000 mg/kg/day from
day 6 through 18 of gestation. All does were sacrificed on day 29
or day 30 and caesarean section was performed to remove the foetus.
Following caesarean section, one half of the pups were maintained
for 24 hours to evaluate survival.
There were no abnormalities observed in the maternal parameters
(including: body weight, food consumption, clinical observations,
and necropsy) or on foetal data (including: implantation sites,
corpora lutea, resorptions sites and live or dead foetuses). Data
on foetal weight and condition were normal. Data on foetal
survival and from internal and external examinations for
abnormalities showed no significant effects of administration of
Sumithrin(R) during gestation (Rutter, 1974).
The teratogenic potential of Sumithrin(R) was examined in mice.
Groups of pregnant mice (17-18 mice/group) were orally administered
Sumithrin(R) emulsified in 0.1% Tween(R) 80 at dosage levels of 0,
30, 300 or 3000 mg/kg from day 7 to 12 of gestation. A separate
group of mice (7 mice per group) receiving dosage levels of 0, 300
or 3000 mg/kg were utilized in the study to evaluate postnatal
effects. On day 18 of gestation, mice were sacrificed and pups
delivered by caesarean section. The second group of mice were
allowed to deliver naturally and the young were maintained for 29
days.
There were no adverse effects of the administration of Sumithrin(R)
with respect to maternal and embryo well-being as evidenced by
maternal growth, foetal mortality and external and internal
examination of foetuses for teratogenic or embryotoxic effects.
Sumithrin(R) was neither teratogenic nor embryotoxic in mice at
dosage levels up to and including 3000 mg/kg (Nakamoto et al,
1973).
Special studies on reproduction
Groups of rats (8 male and 16 female Charles River rats/group) were
fed phenothrin in the diet at dosage levels of 0, 200, 600, or 2000
mg/kg and subjected to a standard three-generation, two-litter per
generation reproduction study. Data were reported on the
significant reproductive indices (mating index, fecundity index,
male fertility index, female fertility index and the incidence of
parturition). The first litters obtained were examined for
physical abnormalities at birth and for the numbers of viable and
stillborn pups of each litter. Records of survival were made at 1,
4, 12, and 21 days after birth and a final examination was made at
weaning. Litters were reduced to 10 pups on the fifth day of
lactation. Following weaning, the parental females were mated and
the procedure was repeated to obtain a second generation. Eight
males and 16 females of the second generation were selected at
weaning as parental animals for a succeeding generation.
Gross examination of tissues and organs was performed on the second
litters of all generations. A complete microscopic examination was
conducted on five male and five female animals from the control and
high dose group of the second litter from each generation. In
addition, gross and histopathologic examination was conducted on 10
animals of each sex of the final litter of the third generation.
There was no significant mortality or complications with respect to
parental animals over the course of the study. Although there were
sporadic changes in some of the reproductive data during the three
parental generations, the reproductive parameters showed no
significant, dose-related adverse effects attributable to
phenothrin. Gross and microscopic findings suggested no adverse
effect as a result of dietary phenothrin and it was concluded that
phenothrin had no effect on reproduction in a standard,
three-generation reproduction study in rats (Takatsuka et al,
1980).
Acute toxicity
The acute toxicity in male and female rats and mice is extremely
low. The LD50 was greater than 5,000 mg/kg body weight when
phenothrin was administered orally, subcutaneously, or by
intraperitoneal injection. An intravenous LD50 in rats was reported
to range from 452 to 492 mg/kg and in mice ranged from 354 to 405
mg/kg. There were no sex differences noted in acute toxicity
studies.
Signs of poisoning were noted rapidly following the intravenous
administration of phenothrin. The signs of poisoning include:
fibrillation, tremor, slow respiration, salivation, lacrimation,
ataxia, and paralysis. The signs of poisoning, evident at one-half
to one hour following administration, were rapidly diminished
spontaneously to the point where, at 24 hours, there were no signs
of toxicity (Segawa, 1976).
The LC50 following a four-hour inhalation exposure was determined
to be greater than 1,210 mg/kg3 for phenothrin with both rats and
mice (Kohda et al, 1979). With Sumithrin an LC50 exceeds 3,760
mg/m3 for a 4-hour acute exposure (Kohda, et al, 1977).
Acute oral toxicity of metabolites of phenothrin
Chemical Species LD50 (mg/kg)
3-phenoxybenzyl alcohol rat 1330
3-phenoxybenazldehyde rat 600
Acute intraperitoneal toxicity of several phenothrin metabolites in
mice
Chemical Male Female
3-phenoxybenzyl alcohol 371 424
3-(4'-hydroxyphenoxy)benzyl alcohol 750-1000 750-1000
3-(2'-hydroxyphenoxy)benzyl alcohol 876 778
3-phenoxybenzoic acid 154 169
3-(4'-hydroxyphenoxy)benzoic acid 783 745
3-(2'-hydroxyphenoxy)benzoic acid 859 912
3-phenoxybenzaldehyde 415 416
(These data on phenothrin metabolites were originally reported in
the toxicological review of permethrin, see FAO, 1980).
Short-term studies
Rats
Groups of rats (15 male and 15 female rats per group) were exposed
to phenothrin by inhalation at concentrations of 0, 43 or 220
mg/m3 for four weeks. The animals were exposed for five
consecutive days per week with an exposure period of four hours per
days. Phenothrin, dissolved in deodorised kerosene, was
aerosolised and admitted to the exposure chamber after larger sized
particles were removed. The range of particle size to which the
animals were exposed was not reported. However, the concentration
of the phenothrin in the chamber was measured.
At the conclusion of the four-week treatment interval, 10 animals
of each sex of each group were sacrificed. Haematological
examinations, clinical chemistry examinations, and gross and
microscopic examinations of selected tissues and organs were
performed on all animals sacrificed at the conclusion of the study.
In addition, groups of five animals of each sex were maintained
and examined for gross pathological changes three weeks after
completion of the exposure.
There was no mortality or evidence of acute poisoning observed in
any of the animals. Data on body weight, haematology and clinical
chemistry parameters, and gross and microscopic examinations of
tissues and organs showed that subchronic exposure to concentration
of phenothrin up to 220 mg/m3 for four weeks did not result in any
adverse toxicological effect (Kohda et al, 1979).
Long-term studies
Mice
Groups of mice (50 male and 50 female Swiss white mice/group) were
fed phenothrin in the diet at dosage levels of 0, 300, 1,000 or
3,000 mg/kg for 18 months in a standard carcinogenicity study. The
mice were examined daily for clinical signs of toxicity and
mortality. The animals were weighed once at the initiation of the
study and monthly thereafter. At the conclusion of the study,
haematologic and clinical-blood chemistry studies were performed
using 10 mice of each sex from each dosage group. At the
conclusion of the 18-month feeding trial, all mice were sacrificed
for gross and microscopic examination of tissues and organs.
Statistical evaluation of the data was performed to identify group
differences.
There was no mortality, and clinical signs of poisoning were not
significant in the study. Growth, as evidence by body weight of
males fed 3,000 mg/kg, was slightly depressed throughout the major
portion of the study. Haematologic values were normal. Clinical
chemistry parameters were normal (with the exception of a
statistically significant elevation in SGPT activity in females;
the increase was not dose-dependant and was not present in males
and additionally, as the data from the female control group
appeared to be significantly lower than that observed in the male
control group, it is believed that the statistically significant
difference in the SGPT activity is not attributable to phenothrin
in the diet). Gross examination of tissues and organs at the
conclusion of the study showed an increased liver weight at the
high-dose level in both males and females. There were no other
gross pathological findings.
Microscopic examination of liver revealed no unusual findings
associated with the enlargement. In lungs of mice, congestion was
observed. In addition, amyloidosis in alveoli was found in all
groups in a dose-dependant manner. The two highest dose groups
showed a statistically significant difference from control values
with respect to this occurrence. There was no significant increase
in neoplasm associated with the presence of phenothrin in the diet
(Murakami et al, 1980). Based on this bioassay phenothrin is not
carcinogenic to the mouse.
Rats
Groups of rats (50 male and 50 female rats per group) were fed
phenothrin in the diet at dosage levels of 0, 200, 600, 2,000 or
6,000 mg/kg for two years. Animals were examined weekly for the
first 13 weeks and at monthly intervals thereafter. Food
consumption data were reported for these same intervals.
Ophthalmological examinations, haematological studies, clinical
chemistry studies, and urinalyses were performed at periodic
intervals over the course of the study. At the conclusion of the
study, all animals were sacrificed and examined for gross
abnormalities. Extensive microscopic examinations were conducted
on a variety of tissues and organs and where gross lesions were
observed. All data were statistically analyzed to evaluate
significant intergroup differences.
There were no abnormal clinical behaviourial problems associated
with this study. The survival rate of all animals of all groups
was similar to that of controls and there were no effects of
phenothrin on mortality. Growth, as evidenced by body-weight
reduction, was significantly affected at 6,000 mg/kg in both males
and females. The growth of males, while significantly reduced
during the early parts of the experiment, was not substantially
different from that of the controls after the first four months.
Body weights of females fed 6,000 mg/kg were slightly lower than
the controls throughout the study. Food consumption was observed
to be slightly less in the high-dose male and female animals but
was not consistently different from control animals in any of the
other dose groups. Ophthalmological examinations revealed some
abnormalities, all of which appeared to be age-related changes.
There were no significant haematological differences. An increase
in SGPT activity in the 6,000 mg/kg male group was significantly
different than controls. There were no differences observed with
females. Urinalysis values were normal throughout the study.
OBSERVATIONS IN MAN
A group of eight male human volunteers were administered
Sumithrin(R) (d-phenothrin) dermally at a dose of 32 mg/kg/day for
3 consecutive days. There were no signs of toxicity or dermal
irritation. Blood biochemistry and haematology parameters were
normal and dermal absorption was slight as blood levels of
d-phenothrin isomers were below the level of analytical detection
0.006 mg/kg). The daily dosages used in this dermal study ranged
from 0.67 mg/kg body weight to 0.44 mg/kg body weight with no
adverse effects noted (Hashimoto et al, 198O).
EVALUATION
COMMENTS
Phenothrin, the chrysanthemic acid ester of 3-phenoxybenzyl
alcohol, a synthetic pyrethroid used as an insecticide, is a
mixture of cis and trans racemates, usually with a
cis:trans ratio of 20:80.
Phenothrin is rapidly absorbed, distributed, metabolized and
excreted from the body and the metabolic fate of the molecule has
been well defined. Phenothrin has a very low order of acute
toxicity. At very high doses, phenothrin exhibited central nervous
system effects as noted with several other pyrethroids. Axon and
myelin degeneration were not demonstrated following acute treatment
with phenothrin.
Phenothrin is not mutagenic in microbial or mammalian tests, is not
teratogenic and was found to have no effect on rat reproduction in
a standard three-generation bioassay.
Long-term studies in both the rat and the mouse showed phenothrin
to have a low order of toxicity. There was no carcinogenic
potential in either rat or mouse.
In a human volunteer study no adverse effects were reported when
people were exposed dermally to Sumithrin(R) (also known as
phenothrin) a mixture enriched in the (1R)-cis and (1R)-trans
isomers but still with a cis:trans ratio of 20:80.
Adequate data were presented to allocate a temporary ADI for man on
the basis of no-effect levels observed in long-term studies in the
rat and mouse. A temporary ADI was suggested, since all long-term
studies were in rodent species and the dog has been noted to be a
species that has shown greater sensitivity to other pyrethroids.
Further studies on the dog were therefore required. Additionally,
further studies on the metabolic fate of phenothrin are desirable
in order to confirm the rapid nature of its degradation and
elimination from the body. Observations in man are valuable in
assessing the overall hazards associated with this new class of
pesticides.
Level causing no toxicological effect
Mouse: 300 mg/kg in the diet equivalent to 45 mg/kg bw/day
Rat: 2,000 mg/kg in the diet equivalent to 100 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.2 mg/kg bw/day
RESIDUES AND ANALYTICAL ASPECTS
Since a temporary ADI has been allocated the existing guideline
levels should be replaced by temporary MRLs.
FURTHER DATA AND INFORMATION
Required (by 1984)
1. A two-year dog study.
Desirable
1. Additional metabolism data with phenothrin technical product.
2. Further observations in man with phenothrin.
REFERENCES
Casida, J.E., Gaughan, L.C. and Ruzo, L.O. Comparative Metabolism
of Pyrethroids derived from 3-Phenoxybenzyl and
alpha-Cyano-3-phenoxybenzyl Alcohols. Advances in Pesticide
Science, Ed. by Geisebühler, H., Brooke, G.T., and Kearney, P.C.
Pergamon Press, London, Part 2, p. 182-89.
Hara, Y., Miyagishi, A., Ohtuska, M., Asami, Y., Kurokawa, H. and
Miyamoto, J. Pharmacological Study of Sumithrin(R): Effects of
Respiration, Blood Pressure, and Peripheral Central Nervous
Systems. (1974) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Hashimoto, S., Okano, S., Yamada, H. and Miyamoto, J. Human
volunteer study with d-phenothrin powder. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Hiromori, T., Koyama, Y., Okuno, Y., Arai, M., Ito, N. and
Kiyamoto, J. Two-year Chronic Toxicity Study of S-2539 in Rats.
(1980) Unpublished report from Sumitomo Chemical Co. of a study
performed by Industrial Bio-Test Laboratories, Inc. validated by
Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
Kaneko, H., Ohkawa, H. and Miyamoto, J. Absorption and Metabolism
of Dermally Applied d-phenothrin in Rat. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Kohda H., Nishimoto K., Kadota, K. and Miyamoto, J. Acute and
Subacute Inhalation Toxicity Studies of S-2539 Forte in Rats and
Mice. (1979) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Kohda H., Okuno Y., Kadota, K. and Miyamoto, J. Acute Inhalation
Toxicity of d-phenothrin (S-2559 Forte) in Rats. (1977)
Unpublished report from Sumitomo Chemical Co., submitted to the
World Health Organization by Sumitomo Chemical Co.
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., jr.,
Kinoshita, F.K. and Keplinger, M.L. Teratogenic Study with S-2539
in Albino Rabbits. (1976) Unpublished report from Industrial
Bio-Test Laboratories, submitted to the World Health Organization
by Sumitomo Chemical Co.
Miyamoto, J., Suzuki, T. and Nakae, C. Metabolism of Phenothrin of
3-Phenoxybenzyl d-trans Chrysanthemumate in Mammals. Pestic.
Biochem. Physiol. 4: 438-450.
Murikami, J., Ito, S., Okuno, Y., Arai, M., Ito, N. and Miyamoto,
J. Eighteen-month Chronic Oral Toxicity and Tumorigenicity Study of
S-2539 in Mice. (1980) Unpublished report from Sumitomo Chemical
Co. of a study performed by Industrial Bio-Test Laboratories, Inc.
validated by Sumitomo Chemical Co. and submitted to the World
Health Organization by Sumitomo Chemical Co.
Nakamoto, N., Kato, T. and Miyamoto, J. Teratogenicity Study of
S-2539 Forte in Mice. (1973) Unpublished report from Sumitomo
Chemical Co. submitted to the World Health Organization by Sumitomo
Chemical Co.
Okuno Y., Kadota and Miyamoto, J. Neurotoxicity Study of
d-Phenothrin (S-2539 Forte(R)) in Rats by Repeated Oral
Administration. (1978) Unpublished report from Sumitomo Chemical
Co., submitted to the World Health Organization by Sumitomo
Chemical Co.
Rutter, H.A. Teratogenicity Study in Rabbits: S-2539 Forte. (1974)
Unpublished report from Hazleton Laboratories Inc., submitted to
the World Health Organization by Sumitomo Chemical Co.
Segawa, T. Acute Toxicity Study of S-2539 in Rats and Mice. (1976)
Unpublished report from Hiroshima University School of Medicine,
submitted to the World Health Organization by Sumitomo Chemical Co.
Shirasu, Y., Morishita, M. and Kato, K. Mutation Test of S-2539 on
Bacteria. (1974) Unpublished report from The Institute of
Environmental Toxicology, submitted to the World Health
Organization by Sumitomo Chemical Co.
Soderland, D.M. and Casida, J.E. Effects of Pyrethroid Structure on
Rates of Hydrolysis and Oxidation by Mouse Liver Microsomal
Enzymes. Pestic. Biochem. Physio. 7: 391-401.
Suzuki, H. and Miyamoto, J. Mutagenicity of Some Synthetic
Pryethroids in Bacterial Test Systems. (1975) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Suzuki, T. and Miyamoto, J. Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome. Pestic. Biochem.
Physio. 8: 186-98.
Suzuki, T., Ohno, N. and Miyamoto, J. New Metabolites of (+)-cis
Fenothrin, 3-Phenoxybenzyl, (+)-cis Chrysanthemumate, in Rats. J.
Pesticide Sci. 1: 151-52.
Tekatsuka, M., Okuno, Y., Suzuki, T., Kadota, T., Yasuda, M. and
Miyamoto, J. Three-generation reproduction study of S-2539 in rats.
Unpublished report from Industrial Bio-Test Laboratories, validated
by Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
TOXICOLOGICAL STUDIES
Special Pharmacological studies
A series of pharmacological studies were performed with the
d-isomer of phenothrin (Sumithrin(R)). These studies reported the
pharmacological action of this pyrethroid on respiration, blood
pressure, and a series of tests representative of effects on the
peripheral and central nervous system. Sumithrin(R) was emulsified
(Sorpol(R) 1200 and/or Tween(R) 80) and either diluted with
physiological saline or used directly. The following series of
in vitro and in vivo studies were performed: spasmolytic
activity on isolated ileum from male guinea pigs; spontaneous
contractions of isolated uterus from rats; contractions of a
neuromuscular preparation of phrenic nerve-diaphgram of rats;
respiration, nictitating membrane and blood pressure of male cats
administered Sumithrin(R) intravenously; contractile force, blood
pressure, and heart rate of dogs administered Sumithrin(R)
intravenously; the anticonvulsive activity following induced
convulsion or seizures in mice; the effects on muscular relaxation
activity in mice; coordinative movement in mice;
hexabarbital-induced sleeping time in mice; spontaneous activity in
mice; body temperature of male rats; and electroencephalogram in
male cats.
Sumithrin(R) did not show any pharmacological activity in any of
the in vitro and/or in vivo experiments listed above. A
tentative arousal response in the electroencephalogram of cats
intravenously administered a dose of 4 mg/kg body weight was
recorded. Several other synthetic pyrethroid esters have been
shown to induce this arousal response in the EEG pattern of cats
(Hare et al, 1974).
Special studies on neurotoxicity
Groups of rats exposed by inhalation to the d-isomer of phenothrin
(Sumithrin(R)) at concentration of up to an including 3,760 mg/m3
for four hours showed no adverse effects as a result of exposure.
Examination of the sciatic nerve for possible myelin degeneration
or axon disruption did not reveal any such occurrence (Kohda et
al, 1977).
Groups of rats were administered Sumithrin(R) orally for five
consecutive days at a dosage level of 5,000 mg/kg/day. Mortality
was observed and toxic signs of poisoning were noted in several of
the animals. Toxic signs of poisoning rapidly disappeared at the
conclusion of the treatment. Three days after treatment, all
animals were sacrificed and histopathological examination of
sciatic nerve was carried out to determine whether changes had
occurred. Clinically, there were no toxic signs of poisoning as
evidenced by leg weakness or ataxia. Histological examination
revealed minute changes in axon and myelin, characterised by very
slight axonal swelling, axonal disintegration, and/or demyelination.
There were similar occurrences in the control animals and it was
suggested that the small changes observed were not due to the
administration of Sumithrin(R). It was considered that oral
administration of Sumithrin(R) at excessively high doses does
not induce a neurotoxic effect as has been observed with several
other pyrethroid esters (Okuno, et al, 1978; FAO, 1980).
Special studies on mutagenicity
The host-mediated assay was conducted with phenothrin and
individual isomers of phenothrin in male mice using S.
typhimurium-G46 as an indicator strain. Groups of six male mice
were administered phenothrin orally, twice, at 24-hour intervals at
dosage rates of 0, 500, or 1500 mg/kg/dose. Additionally, the
d-trans and d-cis isomers were administered at dosage levels of
250 and 90 mg/kg respectively. A positive control of
dymethylnitrosamine (50 mg/kg) was used. Immediately after
administration of the chemical, the tester strain (S.
typhimurium) was administered into the abdominal cavity. This
was removed three hours later, cultured and examined for mutation
frequency. There was no increase in reversion frequency observed
with phenothrin or its isomers although the positive control was
found to give an increased reversion rate.
The potential mutagenicity of phenothrin was investigated using
various bacterial test systems including the Reo-Assay (repair
test) with Bacillus subtilis and Salmonella typhimurium
strains.
At dose levels ranging from 0 to 10 mg/disc/plate, phenothrin did
not induce an increase of the growth inhibition zone of the
microorganisms, while mytomycin C showed a substantially larger
inhibition zone. A negative control gave results similar to that
obtained with phenothrin.
Mutation induction studies (the standard "Ames test") with various
strains of E. coli and Salmonella typhimurium (TA1535,
TA1537, TA1538, TA98 and TA100) with and without metabolic
activation enzymes, were performed. Phenothrin and its individual
isomers, at dose levels up to 10 mg per plate, with or without
hepatic drug metabolising enzymes, showed negative results in this
standard mutagenicity assay. Phenothrin and its individual isomers
alone and following metabolic activation, caused no increase in the
mutation rate. Positive controls employed during the course of the
study gave significant numbers of revertants (Shirasu et al,
1977; Suzuki and Miyamoto, 1975). Under the conditions of these
bioassays, phenothrin and its individual d-cis and d-trans
isomers are not mutagenic.
Special studies on teratogenicity
Groups of pregnant New Zealand rabbits (17 rabbits per group) were
administered phenothrin orally at dosage levels of 0, 3, 10 or 30
mg/kg from day 6 to 18 of gestation. All animals were sacrificed
on day 29 of gestation and the young obtained by caesarean section
were examined.
Does treated with phenothrin at the highest dosage level exhibited
a loss in body weight during gestation. There was no mortality
observed in the study. There was a slight decrease in the number
of live young obtained from does treated with 30 mg/kg. Changes in
other reproductive/teratogenic parameters did not show a
dose-response relationship and were not believed to be induced as
a result of phenothrin administration. At 30 mg/kg, there was a
slight reduced foetal weight which was not accompanied by changes
in survival rate or in the occurrence of external or internal
abnormalities.
There was no apparent teratogenic effect of phenothrin as observed
by apparent gross internal or external somatic abnormalities and by
examination for foetal skeletal development following prenatal
exposure. Treatment of pregnant albino rabbits during the period
of foetal organogenesis did not induce abnormal foetal
development. At the high dosage level, possible foetal and/or
maternal toxicity was observed.
Groups of pregnant rabbits (15 dose/group) were administered
Sumithrin(R)) at dosage levels of 0, 10, 100 or 1000 mg/kg/day from
day 6 through 18 of gestation. All does were sacrificed on day 29
or day 30 and caesarean section was performed to remove the foetus.
Following caesarean section, one half of the pups were maintained
for 24 hours to evaluate survival.
There were no abnormalities observed in the maternal parameters
(including: body weight, food consumption, clinical observations,
and necropsy) or on foetal data (including: implantation sites,
corpora lutea, resorptions sites and live or dead foetuses). Data
on foetal weight and condition were normal. Data on foetal
survival and from internal and external examinations for
abnormalities showed no significant effects of administration of
Sumithrin(R) during gestation (Rutter, 1974).
The teratogenic potential of Sumithrin(R) was examined in mice.
Groups of pregnant mice (17-18 mice/group) were orally administered
Sumithrin(R) emulsified in 0.1% Tween(R) 80 at dosage levels of 0,
30, 300 or 3000 mg/kg from day 7 to 12 of gestation. A separate
group of mice (7 mice per group) receiving dosage levels of 0, 300
or 3000 mg/kg were utilized in the study to evaluate postnatal
effects. On day 18 of gestation, mice were sacrificed and pups
delivered by caesarean section. The second group of mice were
allowed to deliver naturally and the young were maintained for 29
days.
There were no adverse effects of the administration of Sumithrin(R)
with respect to maternal and embryo well-being as evidenced by
maternal growth, foetal mortality and external and internal
examination of foetuses for teratogenic or embryotoxic effects.
Sumithrin(R) was neither teratogenic nor embryotoxic in mice at
dosage levels up to and including 3000 mg/kg (Nakamoto et al,
1973).
Special studies on reproduction
Groups of rats (8 male and 16 female Charles River rats/group) were
fed phenothrin in the diet at dosage levels of 0, 200, 600, or 2000
mg/kg and subjected to a standard three-generation, two-litter per
generation reproduction study. Data were reported on the
significant reproductive indices (mating index, fecundity index,
male fertility index, female fertility index and the incidence of
parturition). The first litters obtained were examined for
physical abnormalities at birth and for the numbers of viable and
stillborn pups of each litter. Records of survival were made at 1,
4, 12, and 21 days after birth and a final examination was made at
weaning. Litters were reduced to 10 pups on the fifth day of
lactation. Following weaning, the parental females were mated and
the procedure was repeated to obtain a second generation. Eight
males and 16 females of the second generation were selected at
weaning as parental animals for a succeeding generation.
Gross examination of tissues and organs was performed on the second
litters of all generations. A complete microscopic examination was
conducted on five male and five female animals from the control and
high dose group of the second litter from each generation. In
addition, gross and histopathologic examination was conducted on 10
animals of each sex of the final litter of the third generation.
There was no significant mortality or complications with respect to
parental animals over the course of the study. Although there were
sporadic changes in some of the reproductive data during the three
parental generations, the reproductive parameters showed no
significant, dose-related adverse effects attributable to
phenothrin. Gross and microscopic findings suggested no adverse
effect as a result of dietary phenothrin and it was concluded that
phenothrin had no effect on reproduction in a standard,
three-generation reproduction study in rats (Takatsuka et al,
1980).
Acute toxicity
The acute toxicity in male and female rats and mice is extremely
low. The LD50 was greater than 5,000 mg/kg body weight when
phenothrin was administered orally, subcutaneously, or by
intraperitoneal injection. An intravenous LD50 in rats was reported
to range from 452 to 492 mg/kg and in mice ranged from 354 to 405
mg/kg. There were no sex differences noted in acute toxicity
studies.
Signs of poisoning were noted rapidly following the intravenous
administration of phenothrin. The signs of poisoning include:
fibrillation, tremor, slow respiration, salivation, lacrimation,
ataxia, and paralysis. The signs of poisoning, evident at one-half
to one hour following administration, were rapidly diminished
spontaneously to the point where, at 24 hours, there were no signs
of toxicity (Segawa, 1976).
The LC50 following a four-hour inhalation exposure was determined
to be greater than 1,210 mg/kg3 for phenothrin with both rats and
mice (Kohda et al, 1979). With Sumithrin an LC50 exceeds 3,760
mg/m3 for a 4-hour acute exposure (Kohda, et al, 1977).
Acute oral toxicity of metabolites of phenothrin
Chemical Species LD50 (mg/kg)
3-phenoxybenzyl alcohol rat 1330
3-phenoxybenazldehyde rat 600
Acute intraperitoneal toxicity of several phenothrin metabolites in
mice
Chemical Male Female
3-phenoxybenzyl alcohol 371 424
3-(4'-hydroxyphenoxy)benzyl alcohol 750-1000 750-1000
3-(2'-hydroxyphenoxy)benzyl alcohol 876 778
3-phenoxybenzoic acid 154 169
3-(4'-hydroxyphenoxy)benzoic acid 783 745
3-(2'-hydroxyphenoxy)benzoic acid 859 912
3-phenoxybenzaldehyde 415 416
(These data on phenothrin metabolites were originally reported in
the toxicological review of permethrin, see FAO, 1980).
Short-term studies
Rats
Groups of rats (15 male and 15 female rats per group) were exposed
to phenothrin by inhalation at concentrations of 0, 43 or 220
mg/m3 for four weeks. The animals were exposed for five
consecutive days per week with an exposure period of four hours per
days. Phenothrin, dissolved in deodorised kerosene, was
aerosolised and admitted to the exposure chamber after larger sized
particles were removed. The range of particle size to which the
animals were exposed was not reported. However, the concentration
of the phenothrin in the chamber was measured.
At the conclusion of the four-week treatment interval, 10 animals
of each sex of each group were sacrificed. Haematological
examinations, clinical chemistry examinations, and gross and
microscopic examinations of selected tissues and organs were
performed on all animals sacrificed at the conclusion of the study.
In addition, groups of five animals of each sex were maintained
and examined for gross pathological changes three weeks after
completion of the exposure.
There was no mortality or evidence of acute poisoning observed in
any of the animals. Data on body weight, haematology and clinical
chemistry parameters, and gross and microscopic examinations of
tissues and organs showed that subchronic exposure to concentration
of phenothrin up to 220 mg/m3 for four weeks did not result in any
adverse toxicological effect (Kohda et al, 1979).
Long-term studies
Mice
Groups of mice (50 male and 50 female Swiss white mice/group) were
fed phenothrin in the diet at dosage levels of 0, 300, 1,000 or
3,000 mg/kg for 18 months in a standard carcinogenicity study. The
mice were examined daily for clinical signs of toxicity and
mortality. The animals were weighed once at the initiation of the
study and monthly thereafter. At the conclusion of the study,
haematologic and clinical-blood chemistry studies were performed
using 10 mice of each sex from each dosage group. At the
conclusion of the 18-month feeding trial, all mice were sacrificed
for gross and microscopic examination of tissues and organs.
Statistical evaluation of the data was performed to identify group
differences.
There was no mortality, and clinical signs of poisoning were not
significant in the study. Growth, as evidence by body weight of
males fed 3,000 mg/kg, was slightly depressed throughout the major
portion of the study. Haematologic values were normal. Clinical
chemistry parameters were normal (with the exception of a
statistically significant elevation in SGPT activity in females;
the increase was not dose-dependant and was not present in males
and additionally, as the data from the female control group
appeared to be significantly lower than that observed in the male
control group, it is believed that the statistically significant
difference in the SGPT activity is not attributable to phenothrin
in the diet). Gross examination of tissues and organs at the
conclusion of the study showed an increased liver weight at the
high-dose level in both males and females. There were no other
gross pathological findings.
Microscopic examination of liver revealed no unusual findings
associated with the enlargement. In lungs of mice, congestion was
observed. In addition, amyloidosis in alveoli was found in all
groups in a dose-dependant manner. The two highest dose groups
showed a statistically significant difference from control values
with respect to this occurrence. There was no significant increase
in neoplasm associated with the presence of phenothrin in the diet
(Murakami et al, 1980). Based on this bioassay phenothrin is not
carcinogenic to the mouse.
Rats
Groups of rats (50 male and 50 female rats per group) were fed
phenothrin in the diet at dosage levels of 0, 200, 600, 2,000 or
6,000 mg/kg for two years. Animals were examined weekly for the
first 13 weeks and at monthly intervals thereafter. Food
consumption data were reported for these same intervals.
Ophthalmological examinations, haematological studies, clinical
chemistry studies, and urinalyses were performed at periodic
intervals over the course of the study. At the conclusion of the
study, all animals were sacrificed and examined for gross
abnormalities. Extensive microscopic examinations were conducted
on a variety of tissues and organs and where gross lesions were
observed. All data were statistically analyzed to evaluate
significant intergroup differences.
There were no abnormal clinical behaviourial problems associated
with this study. The survival rate of all animals of all groups
was similar to that of controls and there were no effects of
phenothrin on mortality. Growth, as evidenced by body-weight
reduction, was significantly affected at 6,000 mg/kg in both males
and females. The growth of males, while significantly reduced
during the early parts of the experiment, was not substantially
different from that of the controls after the first four months.
Body weights of females fed 6,000 mg/kg were slightly lower than
the controls throughout the study. Food consumption was observed
to be slightly less in the high-dose male and female animals but
was not consistently different from control animals in any of the
other dose groups. Ophthalmological examinations revealed some
abnormalities, all of which appeared to be age-related changes.
There were no significant haematological differences. An increase
in SGPT activity in the 6,000 mg/kg male group was significantly
different than controls. There were no differences observed with
females. Urinalysis values were normal throughout the study.
OBSERVATIONS IN MAN
A group of eight male human volunteers were administered
Sumithrin(R) (d-phenothrin) dermally at a dose of 32 mg/kg/day for
3 consecutive days. There were no signs of toxicity or dermal
irritation. Blood biochemistry and haematology parameters were
normal and dermal absorption was slight as blood levels of
d-phenothrin isomers were below the level of analytical detection
0.006 mg/kg). The daily dosages used in this dermal study ranged
from 0.67 mg/kg body weight to 0.44 mg/kg body weight with no
adverse effects noted (Hashimoto et al, 198O).
EVALUATION
COMMENTS
Phenothrin, the chrysanthemic acid ester of 3-phenoxybenzyl
alcohol, a synthetic pyrethroid used as an insecticide, is a
mixture of cis and trans racemates, usually with a
cis:trans ratio of 20:80.
Phenothrin is rapidly absorbed, distributed, metabolized and
excreted from the body and the metabolic fate of the molecule has
been well defined. Phenothrin has a very low order of acute
toxicity. At very high doses, phenothrin exhibited central nervous
system effects as noted with several other pyrethroids. Axon and
myelin degeneration were not demonstrated following acute treatment
with phenothrin.
Phenothrin is not mutagenic in microbial or mammalian tests, is not
teratogenic and was found to have no effect on rat reproduction in
a standard three-generation bioassay.
Long-term studies in both the rat and the mouse showed phenothrin
to have a low order of toxicity. There was no carcinogenic
potential in either rat or mouse.
In a human volunteer study no adverse effects were reported when
people were exposed dermally to Sumithrin(R) (also known as
phenothrin) a mixture enriched in the (1R)-cis and (1R)-trans
isomers but still with a cis:trans ratio of 20:80.
Adequate data were presented to allocate a temporary ADI for man on
the basis of no-effect levels observed in long-term studies in the
rat and mouse. A temporary ADI was suggested, since all long-term
studies were in rodent species and the dog has been noted to be a
species that has shown greater sensitivity to other pyrethroids.
Further studies on the dog were therefore required. Additionally,
further studies on the metabolic fate of phenothrin are desirable
in order to confirm the rapid nature of its degradation and
elimination from the body. Observations in man are valuable in
assessing the overall hazards associated with this new class of
pesticides.
Level causing no toxicological effect
Mouse: 300 mg/kg in the diet equivalent to 45 mg/kg bw/day
Rat: 2,000 mg/kg in the diet equivalent to 100 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0-0.2 mg/kg bw/day
RESIDUES AND ANALYTICAL ASPECTS
Since a temporary ADI has been allocated the existing guideline
levels should be replaced by temporary MRLs.
FURTHER DATA AND INFORMATION
Required (by 1984)
1. A two-year dog study.
Desirable
1. Additional metabolism data with phenothrin technical product.
2. Further observations in man with phenothrin.
REFERENCES
Casida, J.E., Gaughan, L.C. and Ruzo, L.O. Comparative Metabolism
of Pyrethroids derived from 3-Phenoxybenzyl and
alpha-Cyano-3-phenoxybenzyl Alcohols. Advances in Pesticide
Science, Ed. by Geisebühler, H., Brooke, G.T., and Kearney, P.C.
Pergamon Press, London, Part 2, p. 182-89.
Hara, Y., Miyagishi, A., Ohtuska, M., Asami, Y., Kurokawa, H. and
Miyamoto, J. Pharmacological Study of Sumithrin(R): Effects of
Respiration, Blood Pressure, and Peripheral Central Nervous
Systems. (1974) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Hashimoto, S., Okano, S., Yamada, H. and Miyamoto, J. Human
volunteer study with d-phenothrin powder. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Hiromori, T., Koyama, Y., Okuno, Y., Arai, M., Ito, N. and
Kiyamoto, J. Two-year Chronic Toxicity Study of S-2539 in Rats.
(1980) Unpublished report from Sumitomo Chemical Co. of a study
performed by Industrial Bio-Test Laboratories, Inc. validated by
Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
Kaneko, H., Ohkawa, H. and Miyamoto, J. Absorption and Metabolism
of Dermally Applied d-phenothrin in Rat. (1980) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Kohda H., Nishimoto K., Kadota, K. and Miyamoto, J. Acute and
Subacute Inhalation Toxicity Studies of S-2539 Forte in Rats and
Mice. (1979) Unpublished report from Sumitomo Chemical Co.,
submitted to the World Health Organization by Sumitomo Chemical Co.
Kohda H., Okuno Y., Kadota, K. and Miyamoto, J. Acute Inhalation
Toxicity of d-phenothrin (S-2559 Forte) in Rats. (1977)
Unpublished report from Sumitomo Chemical Co., submitted to the
World Health Organization by Sumitomo Chemical Co.
Ladd, R., Smith, P.S., Jenkins, D.H., Kennedy, G.L., jr.,
Kinoshita, F.K. and Keplinger, M.L. Teratogenic Study with S-2539
in Albino Rabbits. (1976) Unpublished report from Industrial
Bio-Test Laboratories, submitted to the World Health Organization
by Sumitomo Chemical Co.
Miyamoto, J., Suzuki, T. and Nakae, C. Metabolism of Phenothrin of
3-Phenoxybenzyl d-trans Chrysanthemumate in Mammals. Pestic.
Biochem. Physiol. 4: 438-450.
Murikami, J., Ito, S., Okuno, Y., Arai, M., Ito, N. and Miyamoto,
J. Eighteen-month Chronic Oral Toxicity and Tumorigenicity Study of
S-2539 in Mice. (1980) Unpublished report from Sumitomo Chemical
Co. of a study performed by Industrial Bio-Test Laboratories, Inc.
validated by Sumitomo Chemical Co. and submitted to the World
Health Organization by Sumitomo Chemical Co.
Nakamoto, N., Kato, T. and Miyamoto, J. Teratogenicity Study of
S-2539 Forte in Mice. (1973) Unpublished report from Sumitomo
Chemical Co. submitted to the World Health Organization by Sumitomo
Chemical Co.
Okuno Y., Kadota and Miyamoto, J. Neurotoxicity Study of
d-Phenothrin (S-2539 Forte(R)) in Rats by Repeated Oral
Administration. (1978) Unpublished report from Sumitomo Chemical
Co., submitted to the World Health Organization by Sumitomo
Chemical Co.
Rutter, H.A. Teratogenicity Study in Rabbits: S-2539 Forte. (1974)
Unpublished report from Hazleton Laboratories Inc., submitted to
the World Health Organization by Sumitomo Chemical Co.
Segawa, T. Acute Toxicity Study of S-2539 in Rats and Mice. (1976)
Unpublished report from Hiroshima University School of Medicine,
submitted to the World Health Organization by Sumitomo Chemical Co.
Shirasu, Y., Morishita, M. and Kato, K. Mutation Test of S-2539 on
Bacteria. (1974) Unpublished report from The Institute of
Environmental Toxicology, submitted to the World Health
Organization by Sumitomo Chemical Co.
Soderland, D.M. and Casida, J.E. Effects of Pyrethroid Structure on
Rates of Hydrolysis and Oxidation by Mouse Liver Microsomal
Enzymes. Pestic. Biochem. Physio. 7: 391-401.
Suzuki, H. and Miyamoto, J. Mutagenicity of Some Synthetic
Pryethroids in Bacterial Test Systems. (1975) Unpublished report
from Sumitomo Chemical Co., submitted to the World Health
Organization by Sumitomo Chemical Co.
Suzuki, T. and Miyamoto, J. Purification and Properties of
Pyrethroid Carboxyesterase in Rat Liver Microsome. Pestic. Biochem.
Physio. 8: 186-98.
Suzuki, T., Ohno, N. and Miyamoto, J. New Metabolites of (+)-cis
Fenothrin, 3-Phenoxybenzyl, (+)-cis Chrysanthemumate, in Rats. J.
Pesticide Sci. 1: 151-52.
Tekatsuka, M., Okuno, Y., Suzuki, T., Kadota, T., Yasuda, M. and
Miyamoto, J. Three-generation reproduction study of S-2539 in rats.
Unpublished report from Industrial Bio-Test Laboratories, validated
by Sumitomo Chemical Co. and submitted to the World Health
Organization by Sumitomo Chemical Co.
