
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
AMITRAZ
IDENTITY
Chemical Name (IUPAC): N-methylbis (2,4-xylyliminomethyl)
methylamine
Synonyms
N,N-di-(2,4-xylyliminomethyl) methylamine
2-methyl-1,3-di-(2,4-xlylimino)-2-azapropane
1,5-di-(2,4-dimethylphenyl)-3-methyl-1,3,5-triazapenta-1,4-diene
Daam(R), Mitac(R), Taktic(R), Triatox(R), BTS 27419, JA 119,
U-36,059.
Previously known as triazid or azaform. In Chemical Abstracts
usage it is:
N'-(2,4-dimethylphenyl)-N-[(2,4-dimethylphenyl) imino]
methyl]-N-methlylmethaniminamide [33089-61-1].
Structural formula
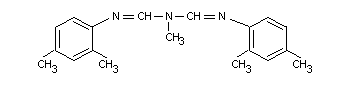 Molecular formula C19H23N3
Other information on identity and properties
Molecular weight 293
State off-white crystalline solid
Melting point 86-87°C
Vapour pressure 3.8 × 10-7 mm Hg at 20°C
Solubility Less than 1 mg/l in water at room temperature;
readily soluble at room temperature in most
organic solvents, 666 g/l in xylene, 500 g/l in
acetone, and 23.8 g/l in methanol.
Stability
Amitraz is a very weak base. Potentiometric titration gives pK
values of 4.0, 1.6 and 1.1, but the significance of the two lower
figures is doubtful because the compound is unstable in acid
solution, undergoing hydrolysis to yield ultimately
2,4-dimethylformanilide (Table 1,III) and methylamine.
Amitraz is relatively stable to heating, either alone or in a dry
inert solvent such as xylene or toluene. A xylene solution can be
boiled for 7 days without detectable change and the pure material
can be heated in air at 190°C with only slight discolouration.
Methanolic solutions are unstable, but solutions in
dimethylformamide or isopropanol are less so.
A stream of oxygen has no effect when passed through a solution of
amitraz in xylene at room temperature for 6 hours, but at reflux
temperature some decomposition occurs. A slow deterioration of the
moist compound occurs on prolonged standing (Martin and Worthing,
1979).
Technical material
The technical material is of a pale straw colour, and typically has
a purity of 97-99% at the time of synthesis. The minimum purity
specified by the manufacturer is 93%.
The principal impurity in the freshly manufactured product is
NN'-bis(2,4-dimethylphenyl) formamidine (Table 1,VI)
Storage stability of technical material
Storage stability tests were carried out in 3 countries on
technical amitraz stabilised with paraformaldehyde sachets inside
sealed drums (Nix, 1979). The results for the longest periods of
storage may be summarised as follows:
Country Period Mean loss of active Content of
of storage ingredient (% of (III) (%)
initial content) Initial Final
Japan 24 months Nil <0.2 0.25
plus travel
to Japan and
back
Nigeria 22 months 4.4 <0.25 1.0
plus travel to
to Nigeria 2.0
and back
Colombia 12 months 0.5 0.25 0.25
plus travel to
to Colombia 0.50
and back
Formulations
Amitraz is available as emulsifiable concentrates (200 g ai per
litre for crop-protection, 125 g ai per litre for animal use) and
wettable powders (500 or 250 g ai per kg).
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
In rats orally dosed with 14C-labelled amitraz recovery within 3
days of the administration dose was 53-85% in urine, 17-47% in
faeces, and <0.1% in expired air. Peak plasma levels occurred
about 1 hour after dosing. Highest residues were found in liver,
kidney and muscle after 0.75-1.5 hours, diminishing thereafter. In
urine at least 4 metabolites were found. In faeces 6 metabolites
were detected of which the major component was identified as BTS
27271 (Lewis, 1971 a).
During repeated oral dosing of 14C-labelled amitraz to rats highest
residues were found in thyroid and adrenal glands, liver, skin,
spleen and eyes. After dosing ceased, a considerable decrease of
residues was observed. Radioactivity in blood was mainly cell
bound. Seven days after the final dose small but significant
residues were detected in liver, spleen, skin and adrenals
(Somerville, 1973 a).
TABLE 1. Impurities Found In Technical Amitraz
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
a. N-2,4-dimethylphenyl-N-
methylformamidine
"formamidine" (II) TLC 0.2% 0-0.25% 2.0%
Molecular formula C19H23N3
Other information on identity and properties
Molecular weight 293
State off-white crystalline solid
Melting point 86-87°C
Vapour pressure 3.8 × 10-7 mm Hg at 20°C
Solubility Less than 1 mg/l in water at room temperature;
readily soluble at room temperature in most
organic solvents, 666 g/l in xylene, 500 g/l in
acetone, and 23.8 g/l in methanol.
Stability
Amitraz is a very weak base. Potentiometric titration gives pK
values of 4.0, 1.6 and 1.1, but the significance of the two lower
figures is doubtful because the compound is unstable in acid
solution, undergoing hydrolysis to yield ultimately
2,4-dimethylformanilide (Table 1,III) and methylamine.
Amitraz is relatively stable to heating, either alone or in a dry
inert solvent such as xylene or toluene. A xylene solution can be
boiled for 7 days without detectable change and the pure material
can be heated in air at 190°C with only slight discolouration.
Methanolic solutions are unstable, but solutions in
dimethylformamide or isopropanol are less so.
A stream of oxygen has no effect when passed through a solution of
amitraz in xylene at room temperature for 6 hours, but at reflux
temperature some decomposition occurs. A slow deterioration of the
moist compound occurs on prolonged standing (Martin and Worthing,
1979).
Technical material
The technical material is of a pale straw colour, and typically has
a purity of 97-99% at the time of synthesis. The minimum purity
specified by the manufacturer is 93%.
The principal impurity in the freshly manufactured product is
NN'-bis(2,4-dimethylphenyl) formamidine (Table 1,VI)
Storage stability of technical material
Storage stability tests were carried out in 3 countries on
technical amitraz stabilised with paraformaldehyde sachets inside
sealed drums (Nix, 1979). The results for the longest periods of
storage may be summarised as follows:
Country Period Mean loss of active Content of
of storage ingredient (% of (III) (%)
initial content) Initial Final
Japan 24 months Nil <0.2 0.25
plus travel
to Japan and
back
Nigeria 22 months 4.4 <0.25 1.0
plus travel to
to Nigeria 2.0
and back
Colombia 12 months 0.5 0.25 0.25
plus travel to
to Colombia 0.50
and back
Formulations
Amitraz is available as emulsifiable concentrates (200 g ai per
litre for crop-protection, 125 g ai per litre for animal use) and
wettable powders (500 or 250 g ai per kg).
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
In rats orally dosed with 14C-labelled amitraz recovery within 3
days of the administration dose was 53-85% in urine, 17-47% in
faeces, and <0.1% in expired air. Peak plasma levels occurred
about 1 hour after dosing. Highest residues were found in liver,
kidney and muscle after 0.75-1.5 hours, diminishing thereafter. In
urine at least 4 metabolites were found. In faeces 6 metabolites
were detected of which the major component was identified as BTS
27271 (Lewis, 1971 a).
During repeated oral dosing of 14C-labelled amitraz to rats highest
residues were found in thyroid and adrenal glands, liver, skin,
spleen and eyes. After dosing ceased, a considerable decrease of
residues was observed. Radioactivity in blood was mainly cell
bound. Seven days after the final dose small but significant
residues were detected in liver, spleen, skin and adrenals
(Somerville, 1973 a).
TABLE 1. Impurities Found In Technical Amitraz
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
a. N-2,4-dimethylphenyl-N-
methylformamidine
"formamidine" (II) TLC 0.2% 0-0.25% 2.0%
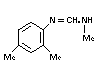 b. 2,4-dimethylformanilide
"formanilide" "DMF" (III) TLC 0.2% 0-0.25% 7.0% by
difference
b. 2,4-dimethylformanilide
"formanilide" "DMF" (III) TLC 0.2% 0-0.25% 7.0% by
difference
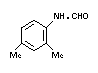 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
c. N,N'-bis-(2,4-dimethyl-phenyl)
formamidine
"diformamidine" (VI) GLC 0.1% 0-3% 6.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
c. N,N'-bis-(2,4-dimethyl-phenyl)
formamidine
"diformamidine" (VI) GLC 0.1% 0-3% 6.0%
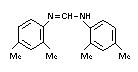 d. 2,4-dimethylaniline
"xylidine" (DMA) (IV) TLC 0.05 0-0.1% 0.3%
d. 2,4-dimethylaniline
"xylidine" (DMA) (IV) TLC 0.05 0-0.1% 0.3%
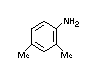 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
e. 1,3,5-tri-(2,4-dimethyl-phenyl)
-1,3,5-triazapenta-1-4-diene (VII) GLC 0.2% 0-0.25% 2.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
e. 1,3,5-tri-(2,4-dimethyl-phenyl)
-1,3,5-triazapenta-1-4-diene (VII) GLC 0.2% 0-0.25% 2.0%
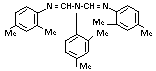 f. N,N'-bis-2,4-dimethyl-phenyl
-N-methyl formamidine (VIII) GLC 0.1% 0-0.25% 2.0%
f. N,N'-bis-2,4-dimethyl-phenyl
-N-methyl formamidine (VIII) GLC 0.1% 0-0.25% 2.0%
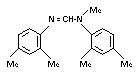 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
g. Ethyl-N-2,4-dimethyl-phenyl
formimidate
"formimidate" (IX) TLC 0.5% 0-0.5% 2.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
g. Ethyl-N-2,4-dimethyl-phenyl
formimidate
"formimidate" (IX) TLC 0.5% 0-0.5% 2.0%
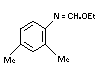 1 Typical Content specifically refers to freshly manufactured material
Twenty-four hours after dermal application of labelled amitraz in
acetone solution to rats approximately 50% of the dose had been
excreted in urine and faeces (Lewis, 1971 b).
Ninety-six hours after a single oral dose of 14C-labelled amitraz to
dogs the recovery in the excreta was 81% (57% in urine and 24% in
faeces). The peak plasma levels varied between 1.5 and 6 hours after
dosing for the male and the female dog respectively (Lewis, 1971 c).
One to four days after dosing significant residues were found in
liver, kidney, skin, bile and the pigmented tissue of the eye (iris,
retina and choroid and sclera) (Lewis, 1971 c; Hamilton and
Somerville, 1974 a).
In calf and mice a similar pattern of distribution and elimination of
radioactivity was observed after oral treatment with 14C-ring
labelled amitraz (Somerville, 1973 b; Hamilton, 1976).
A lactating cow was treated dermally twice with a 7-day interval with
14C-amitraz. In milk the maximal concentration of radioactivity was
0.09 ml/l. Nine days after the second application the concentration
was 0.03 mg/l (Somerville, 1972).
Metabolites of labelled amitraz measured in liver and urine showed
similar patterns for all species examined, i.e. rat, mouse, dog, cat
and calf. At least 6 metabolites were present of which conjugates of
3-methyl-4-amino-benzoic acid (BTS 28369) were predominant. In urine
of dogs and rats a difference in number and type of metabolites
between males and females was found (Jones, 1973 c).
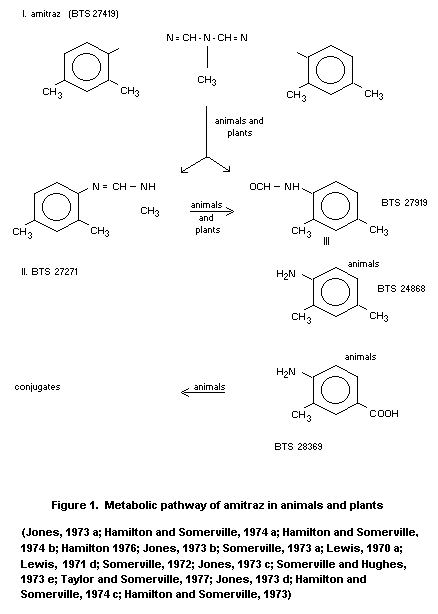
The proposed metabolic pathway is given in figure 1.
In vivo and in vitro degradation studies in dog gastric juice of
amitraz revealed rapid hydrolysis to BTS 27271, BTS 27919 and BTS
24868 (Somerville and Hughes, 1973 e; Taylor and Somerville, 1977).
Administration of BTS 27271 to cats and dogs resulted in a similar
distribution pattern and metabolic degradation as was observed for
amitraz (Jones, 1973 d; Hamilton and Somerville, 1973; Hamilton and
Somerville, 1974 c).
From accumulation-elimination studies of BTS 27271 in dogs the half
lives in retina/choroid and the iris were estimated to be 1.5 and 3
days respectively (Somerville and Hamilton, 1974).
2,4-Xylidine (BTS 24868) administered to rats predominantly oxidized
to 3-methyl-4-amino-benzoic acid (BTS 28369). This metabolite is
excreted as the acetanilide conjugate (Lindstrom, 1961).
3-methyl-4-amino-benzoic acid (BTS 28369) administered in a single
oral dose to dogs gives significant residues in iris, liver and skin,
24 hours after dosing. The excretion of the compound was maximal in
the first 7 hours (Hamilton and Somerville, 1974 b).
1 Typical Content specifically refers to freshly manufactured material
Twenty-four hours after dermal application of labelled amitraz in
acetone solution to rats approximately 50% of the dose had been
excreted in urine and faeces (Lewis, 1971 b).
Ninety-six hours after a single oral dose of 14C-labelled amitraz to
dogs the recovery in the excreta was 81% (57% in urine and 24% in
faeces). The peak plasma levels varied between 1.5 and 6 hours after
dosing for the male and the female dog respectively (Lewis, 1971 c).
One to four days after dosing significant residues were found in
liver, kidney, skin, bile and the pigmented tissue of the eye (iris,
retina and choroid and sclera) (Lewis, 1971 c; Hamilton and
Somerville, 1974 a).
In calf and mice a similar pattern of distribution and elimination of
radioactivity was observed after oral treatment with 14C-ring
labelled amitraz (Somerville, 1973 b; Hamilton, 1976).
A lactating cow was treated dermally twice with a 7-day interval with
14C-amitraz. In milk the maximal concentration of radioactivity was
0.09 ml/l. Nine days after the second application the concentration
was 0.03 mg/l (Somerville, 1972).
Metabolites of labelled amitraz measured in liver and urine showed
similar patterns for all species examined, i.e. rat, mouse, dog, cat
and calf. At least 6 metabolites were present of which conjugates of
3-methyl-4-amino-benzoic acid (BTS 28369) were predominant. In urine
of dogs and rats a difference in number and type of metabolites
between males and females was found (Jones, 1973 c).
The proposed metabolic pathway is given in figure 1.
In vivo and in vitro degradation studies in dog gastric juice of
amitraz revealed rapid hydrolysis to BTS 27271, BTS 27919 and BTS
24868 (Somerville and Hughes, 1973 e; Taylor and Somerville, 1977).
Administration of BTS 27271 to cats and dogs resulted in a similar
distribution pattern and metabolic degradation as was observed for
amitraz (Jones, 1973 d; Hamilton and Somerville, 1973; Hamilton and
Somerville, 1974 c).
From accumulation-elimination studies of BTS 27271 in dogs the half
lives in retina/choroid and the iris were estimated to be 1.5 and 3
days respectively (Somerville and Hamilton, 1974).
2,4-Xylidine (BTS 24868) administered to rats predominantly oxidized
to 3-methyl-4-amino-benzoic acid (BTS 28369). This metabolite is
excreted as the acetanilide conjugate (Lindstrom, 1961).
3-methyl-4-amino-benzoic acid (BTS 28369) administered in a single
oral dose to dogs gives significant residues in iris, liver and skin,
24 hours after dosing. The excretion of the compound was maximal in
the first 7 hours (Hamilton and Somerville, 1974 b).
 TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Effect of amitraz on the oestrous cycle
In female SFP CFLP mice fed a diet containing 400 mg amitraz/kg feed
for up to 33 weeks a reduction in body weight and an increase in food
consumption was observed. Analysis of vaginal smears indicated that
the mean duration of oestrus as well as the incidence of a prolonged
oestrus was significantly increased due to treatment. No effects were
found on the level of ß-oestradiol in plasma (Brown et al, 1978;
Channon and Cryer, 1978).
Female rats maintained on a diet containing 200 mg amitraz/kg for 18
weeks had longer oestrus cycles than control rats of the same age,
resulting from prolonged periods of oestrus or dioestrus (Merryman and
Sutton, 1972).
Effect on pregnancy, parturation and care of the young
Amitraz was administered in doses of 0, 1, 3 or 12 mg/kg bw to
Boots-Wistar rats from day 1 of pregnancy until the young were weaned
at 21 days old. 12 mg/kg bw reduced the weight gain of the dams as
well as the mean number of young born and alive at day 4. No effects
were observed for mothers and offspring for the other dose groups
(Sutton, 1973 c).
Three-generation feeding study
Groups of 10 male and 20 female newly-weaned Boots-Wistar rats were
fed amitraz in dietary concentrations of 0, 15, 50 or 200 mg/kg.
After ten weeks the animals were mated. After the F1 generation was
weaned 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. At 200 mg/kg amitraz causes decrease
of growth and food consumption in the F0 generation, furthermore a
decrease in fertility and viability was found. The 200 mg/kg diet
group was terminated when the F1 generation was weaned, due to the
very low survival. In the 50 mg/kg group no effect was found on the
number of litters and mean litter size, however in all generations a
decrease in number of young alive at 21 days was observed. No further
effects due to treatment were found in this and the other dose group
(Sutton, 1973 d; Lancaster and Williams, 1978).
Special Studies on teratogenicity
Rat
Groups of 11-13 female rats received amitraz in doses of 0, 1, 3 or 12
mg/kg bw daily from day 8 to day 20 of pregnancy. The rate were
killed on day 21 and the uterine content was examined. Average litter
size, foetal viability and implantation index were not affected. In
the highest dose group foetal weight was less than in the controls,
and the calcification of the stermebrae was less advanced (Sutton,
1973 b).
Rabbit
Groups of 8-10 New Zealand rabbits were treated with amitraz in doses
of 0, 1, 5 or 25 mg/kg bw from day 6 to 18 of pregnancy and killed on
day 30. In the highest dose group number of litters and mean litter
size were decreased. Only in this dose group abortions were observed.
No increase in congenital abnormalities was found (Sutton, 1973 a).
Special studies on mutagenicity
Bacterial systems
Amitraz and its major metabolites (BTS 27271, 27919, 28369) were
tested in bacterial in vitro systems, with and without activation,
against Salmonella typhimurium TA 1535, 1437, and 1538 and
Escherichia coli Wp2 and Wp2 uvrA. In these systems no mutagenic
activity was detected (Everest and Wilcox, 1976 a, 1976 b; Tuplin and
Wilcox, 1978).
The impurity BTS 33220 (N,N'-bis-2,4-dimethylphenyl-N-methyl
formamidine) showed weak positive activity against TA 100 in the
presence of microsomes. Amitraz spiked with 1% BTS 33220, however,
did not show any mutagenic action (Wilcox, 1979; Everest and Wilcox,
1979).
In the TA 100 strain BTS 24868 and BTS 27919 exhibited weak but
consistent mutagenic activity (Zimmer at al, 1977).
Amitraz showed no increase in point mutational activity against
Salmonella typhimurium G 46, TA 1532 and TA 1964 when tested in
the mouse perivisceral host-mediated assay at single oral doses up to
400 mg/kg bw (Wilcox, 1976; Everest, 1976).
Dominant Lethal Studies
Female mouse
Groups of 48 female mice were treated orally for 5 consecutive days
with amitraz in doses of 0, 12 or 50 mg/kg bw.
Subgroups of 12 females were mated with untreated males on day 3, 9,
14 or 19 post-dosing. A slight increase in mean post-implantation
loss and decrease in mean viable litter size at 50 mg/kg was found
(Palmer and James, 1977 a).
Male mouse
Male mice of proven fertility were treated with amitraz for 5
consecutive days at doses of 0, 12 or 50 mg/kg. Following treatment
males were mated with untreated females for six consecutive weeks. A
significantly lower implantation rate was found at 50 mg/kg at the
first mating and a higher implantation rate at 12 mg/kg at the 5th
mating. No changes were found in respect to number of embryonic
deaths and post implantation losses (Palmer and James, 1977 b).
DNA damage
Amitraz, BTS 27271, 27919, 24868 and 28369 were tested at several dose
levels in the DNA damage/alkaline elution assay with or without
activation systems, using chinese hamster lung fibroblasts. In this
test system no indication was obtained that amitraz or its metabolites
induce DNA damage (Petzold et al, 1977).
Special studies on carcinogenicity
Mouse
Groups of 50 male and 50 female mice (CFLP strain) were fed a diet
containing 0, 25, 100 or 400 mg/kg for 80 weeks. The animals of the
100 and 400 mg/kg diet groups gained less weight than the control over
the first 40 weeks. The 25 mg/kg diet group gained more weight than
the control group from week 10 onwards and showed a 20% greater body
weight gain at the end of the experiment. Calculated over 80 weeks,
food consumption was increased for male mice at 100 and 400 mg/kg diet
and decreased at 25 and 100 mg/kg diet and increased at 400 mg/kg diet
for female mice. The survival rate was similar in all groups. An
increased incidence of lymphoreticular tumours was observed for female
mice at 400 mg/kg only; 49% compared with 23% in control animals. A
slight, although not statistically significant (p>5%) increase of all
types of liver cell tumours was found in both sexes fed 400 mg/kg. No
other pathological findings related to treatment were reported
(Burnett et al, 1976; Kakuk 1979).
Rat
Amitraz was administered at dietary concentrations of 0, 15, 50 or 200
mg/kg to groups of 40 male and 40 female Ash Wistar rats for 2 years.
Due to treatment in the highest dose group the animals tended to be
nervous, aggressive and excitable. In this group growth rate was
depressed for both sexes and food intake was slightly less in the
first weeks of the experiment. No dose-related anomalies were found
on organ weights and haematological, biochemical and histopathological
examination. Furthermore, no difference in incidence, type and time
of appearance of tumours in control and treated rats was observed
(Sutton and Offer, 1973).
2,4-Xylidine carcinogenicity study
Mouse
2,4-Xylidine (BTS 24868) was fed in the diet of 3 groups of HAM/ICR
mice (25 males and 25 females) in a concentration of 0, 1000 or 2000
mg/kg. Since these doses were not tolerated the experiment was run
again with doses of 0, 125 or 250 mg/kg diet. Dosing was
discontinuated after 18 months. After 24 months the animals were
killed and examined for tumours. In female mice an increased
incidence of lung tumours was observed in the highest dose group (NCI,
1973).
Rat
2,4-Xylidine (BTS 24868) was administered to groups of 25 male Charles
River rats for approximately 3 months at doses of 2000 or 4000 mg/kg
diet; thereafter the doses were changed to 0, 250 or 500 mg/kg diet
respectively, for approximately 2.5 months and subsequently, the doses
were set at 0, 500 or 1000 mg for the rest of the 18 months period.
At that time the feeding of the test compound was discontinued until
sacrifice 24 months after starting the experiment. A slight increase
in lung tumours in the highest dose group was observed. Furthermore
in the treated groups bladder and liver tumours were observed,
which were not found in the controls (NCI, 1973). However, in
contrast to their earlier conclusions, the authors finally stated that
there was no carcinogenic effect in the rat (Weisburger et al,
1978).
Special studies on pharmacological effects
In dogs amitraz and BTS 27271 produce similar effects when given
orally in doses of 4, and 0.5 mg/kg bw respectively either after
single or repeated administration. Signs included sedation, ataxia,
protrusion of the tongue, bradycardia and hypothermia (Morgan et al,
1975 b).
An experiment comparing oral and topical application of amitraz in
pigs revealed a decrease in rectal temperature after oral dosing (25
mg/kg bw and higher), whereas no effects were found after topical
application up to 200 mg/kg bw (Morgan et al, 1975 a).
No flushing could be demonstrated in pigs after topical or
intramuscular administration of amitraz and BTS 27271 although other
pharmacological changes were apparent (Parkinson, 1975).
Amitraz and BTS 27271 did not have any effect on trafuril-induced or
UV-induced erythema in the guinea pig (Patton, 1970; Parkinson and
Yates, 1971).
BTS 27271 was antidiuretic in mice at 90 mg/kg bw whereas amitraz in
the same dose was ineffective (Sim, 1970).
Intravenous administration of amitraz caused vasoconstriction in the
ear vessels of cats (1-10 µg/kg) and fall in blood pressure and
bradycardia (>20 µg/kg) in dogs. BTS 27271 evoked similar effects in
dogs (40 µg/kg). Both compounds had no effect on the response of the
blood vessels of the rabbit ear to antidromic stimulation or to
catecholamines (Anonymous, 1972; Parkinson et al, 1971 a; Parkinson
et al 1971 b; Patton, 1973 b).
Amitraz induced hypothermia in mice under various ambient conditions.
This effect was greater in intensity and duration in females (Sutton,
1972 a; Berry, 1976).
BTS 27419, BTS 27271 and BTS 21103 were tested for their potency of
central monoamine oxidase inhibition in mice and rats, by
investigating the influence on reserpine-induced ptosis and inhibition
of exploratory activity. No effects were observed in mice. At high
doses these compounds antogonise reserpine-induced ptosis in the rat,
suggesting a relative potency in the order BTS 27271 > BTS 27419 >
BTS 21103 (Parkinson, 1974 a).
BTS 27419, BTS 27271 and BTS 21103 potentiated the pressor response to
tyramine in the pithed rat at doses of 40, 80 and 80 mg/kg bw
respectively. Similar effects have been shown with monoamine oxidases
inhibitors (Parkinson, 1974 b).
Special studies on sensitisation and irritation
Neither amitraz nor BTS 27271 showed sensitisation activity in guinea
pigs (Sutton, 1971).
Amitraz was not irritant to the rabbit eye after a single
administration (Sutton and Metcalf, 1972).
Acute toxicity
Acute oral administration of amitraz caused CNS depression in all
species studied. Dogs were more susceptible than the others. Toxic
manifestations: ataxia, subnormal rectal temperature and
cardiovascular disturbance.
TABLE 2. Acute Toxicity of Amitraz
Species Sex Number Route LD50 (mg/kg) Reference
mouse M 5 oral >1600 Patton and Sutton, 1971
rat M 5 oral approx. 800 Patton and Sutton, 1971
Shaw, 1973a
rat M 5 i.p. approx. 800 Shaw, 1971, 1973a
rat M,F 6 inhalation (6 h LC50) Berczy et al, 1972
65 mg/kg
rat M 5 dermal > 1600 Patton and Sutton, 1971
guinea pig F 3 oral 400-800 Patton and Sutton, 1971
Sutton 1970c
rabbit F 2 oral > 100 Patton and Sutton, 1971
rabbit M,F dermal > 200 Sutton and Williams, 1972
dog M,F 2 oral approx. 100 Patton and Sutton, 1971
baboon M,F 2 oral 100-250 Patton, 1973a
BTS 27271 is more toxic than amitraz. Signs of poisoning are similar
to those shown by amitraz. A summary of acute toxicity data of
impurities degradation products and metabolites of amitraz is given in
table 3.
Short-term studies
Mouse
Groups of 10 male and 10 female CFLP-mice (age 4 weeks) were dosed
with 3, 12, 50 or 200 mg/kg/day amitraz by gavage (suspended in 0.4%
cellosize; 0.1 ml/10 g bw) for 90 days. Control groups of 20 males
and females received the same volume of vehicle alone.
Eight mice (5 males and 3 females) given 200 mg/kg/day died within 3
weeks after becoming progressively emaciated and inactive. The
surviving animals of this group were in poor condition. In the other
groups 5 animals died of causes probably not related to treatment.
The 50 and 200 mg/kg groups showed a decreased body weight gain over
the experimental period for both sexes. The body weight gain of male
TABLE 3. Acute Toxicity of Impurities, Degradation Products and Metabolites of Amitraz
compound species number route LD50 (mg/kg) vehicle references
BTS 27271 mouse 5 oral 100-200 HCl salt aq. sol. Sutton, 1970a
rat 5 oral 200 idem Sutton, 1970b
guinea pig 3 oral 200 idem Sutton, 1970c
dog 2 oral >20 gelatin capsules Morgan, 1973a
Morgan and Williams, 1973a
rabbit 2 oral >25 HCl salt aq. sol. Sutton, 1972b
BTS 27919 mouse 5 oral >1600 idem Anonymous, 1971a
rat 4 oral approx. 1600 idem Shaw, 1973b
dog 2 oral >100 gelatin capsules Morgan, 1974a
Morgan and Turnhull, 1973
BTS 28369 dog 2 oral >250 idem Morgan and Sheperd, 1974
Morgan 1974b
BTS 24868 mouse 5 oral 800 - Anonymous, 1971a
BTS 33220 rat 5 oral 800-1600 10% acacia mucilage O'Donovan and Smithson, 1978
BTS 28037 dog 2 oral >250 gelatin capsules Morgan, 1973b
Morgan and Williams, 1973b
and female mice of the 3 and 12 mg/kg groups was slightly decreased.
GPT activity increased in both sexes from 50 mg/kg. Male mice showed
a slight increase in haematocrit at 3 and 12 mg/kg. Reducing
substances in blood were found to be decreased in both sexes at 12 and
50 mg/kg. Organ weight/body weight ratio of the kidney was increased
in males from 12 mg/kg; of the liver increased for both sexes at 50
and 200 mg/kg and of the spleen for males at 50 and 200 mg/kg and for
females at 200 mg/ kg only.
Slight to moderate hepatocyte and/or nuclear enlargement was observed
dose-relatedly at 12, 50 and 200 mg/kg. Furthermore, in the liver of
some females of the highest dose group, intranuclear inclusion, bile
duct proliferation and inflammatory infiltration in the portal tracts
and nodular hyperplasia were observed (Shaw and Williams, 1974).
Four groups of 13 female and 13 male ICR-SLC mice received amitraz in
doses of 0, 3, 12 or 50 mg/kg bw, in the diet (presuming that the
daily consumption was 5 g/animal) for 90 days. Six males died at an
early stage of the experiment. According to the authors death was not
due to treatment. In both sexes growth was depressed in the 12 and 50
mg/kg groups. Food and water intake were slightly decreased at 12 and
50 mg/kg; for males this was more pronounced than for females. In
male mice a slight decrease in food efficiency was observed at 50
mg/kg over the whole experimental period. For male mice relative
brain weight was increased at 50 mg/kg and heart weight at 12 and 50
mg/kg. The relative kidney weight of female mice at 50 mg/kg was
decreased. SGPT and aldaline-phosphatase were decreased whereas the
albumin-globulin ratio was increased in female mice of the two highest
dose groups. Male mice only showed a higher albumin-globulin ratio at
50 mg/kg. At the end of the experiment macroscopically slight black
discolouration of the liver was seen predominantly in the highest dose
group. The only histopathological change probably directly related to
the administration of the compound was a slight centrolobular
degeneration in the liver at 3, 12 and 50 mg/kg (Toyoshima et al,
1972 b).
Rat (inhalation)
Three groups of 6 female and 6 male rats were exposed daily for six
hours to amitraz at concentrations of 0.01, 0.1 or 1.0 mg/l air
respectively for 14 days over a period of three weeks. During
exposure to 0.1 mg/l dyspnoea, eye-irritation and hyposensitivity to
noise were observed. In addition at 1.0 mg/l ataxia, increased nasal
secretion, polyuria, body tremors and slight coma were found. Body
weight gain was markedly reduced in both sexes at 0.1 and 1.0 mg/l
air. Haematological studies revealed a decreased PCV, haemoglobin and
red blood cell count and increased number of neutrophils in both sexes
and a decreased NCHC and number of lymphocytes in males of the highest
dose group (no other groups examined). At 0.1 and 1.0 mg/l
significant alterations of the relative weights of brain, kidney,
thyroid and adrenal glands, and gonads were found for males, and of
brain, kidney and adrenals of female rats. Liver, heart and pituitary
for males and liver for females were increased in the highest dose
group only. No pathological findings related to treatment were found
(Berozy et al 1973).
[text missing] abnormalities, dosing was restarted for 1 week in which
toxic reactions returned. Rats of the 12 mg/kg group appeared
irritable and excitable. In male rats body weight gain was depressed.
A significantly depressed relative liver weight was seen at 50, 12 as
well as 3 mg/kg for males but only at 50 mg/kg for females.
Alkaline-phosphatase activity was decreased at 50 and 12 mg/kg (Sutton
and Williams, 1971).
Rat
Groups of 10 Wistar rats of each sex received amitraz in doses of 0,
3, 12 or 50 mg/kg bw in the diet (presuming the daily food consumption
was 20 g/animal) for 90 days. Body weight gain was decreased in the
50 mg/kg group for both sexes and in the 12 mg/kg group for females
only. Food and water consumption were decreased in all dose groups.
The relative weight of brain, heart, lung, liver, kidney, spleen and
uterus or testes were increased for both sexes in the highest dose
group. In addition the adrenals were decreased and thymus was
increased in weight in male rats of the highest dose group. Male rats
showed a dose-related decrease in number of blood platelets in the 12
and 50 mg/kg group. Female rats showed a significant decrease in
eosinophils in the highest dose group. In serum of male rate of the
highest dose group a decrease was observed in the Alk Pase activity
and blood sugar concentration, and an increased serum potassium
concentration. Urinalysis revealed an increased number of rats with
proteinurea from the 12 mg/kg group onwards. In male rate urine
potassium concentration was significantly decreased at 12 and 50
mg/kg, in females only at 50 mg/kg (Toyoshima et al, 1972 a).
Rabbit (dermal)
Amitraz dissolved in acetone was applied to the intact skin of groups
of New Zealand rabbits (4 males and 4 females) in 15 doses of 0, 50 or
200 mg/kg bw over a 2l-day period. One male and three females at 200
mg/kg and one male at 50 mg/kg died intercurrently. Sedation was
observed in males at 50 mg/kg and in both sexes at 200 mg/kg. In
males of both dose groups slight to moderate erythema, desquamation of
skin and subcutaneous hemorrhage were observed. In both sexes
decreased body weight and food consumption were found in the 50 and
200 mg/kg dose groups. In males at 200 mg/kg underweight testes with
tubular degeneration were found (Sutton, 1973 e).
Dog
Amitraz in gelatine capsules was given to groups of two male and two
female beagle dogs in oral doses of 0, 0.25, 1 or 4 mg/kg once daily
for 90 days. Clinical signs were observed in all dosed groups
consisting of CNS depression, ataxia and vomiting during the first
days within 3-6 hours after dosing. These dogs were subdued
throughout the experimental period. When examined at intervals during
the treatment period the dogs consistently had subnormal rectal
temperatures and pulse rates. These parameters returned to normal
within 6-24 hours after administration. The animals of the 1 and 4
mg/kg dose groups showed a significant increase in blood sugar
concentration which was maximal 6 hours after dosing and returned to
normal within 24 hours. In the same dose groups an increased amount
of glucose and total reducing substances was found in the urine in
weeks 2 and 3. In the highest dose group an increased liver weight
was observed in both sexes. Except in control animals, livers showed
enlargement of the central and midzonal hepatocytes. Hyperplasia of
the small periportal hepatocytes and increase in binucleate cells were
particularly evident in the highest dose group. At all dose levels
thinning of the zonae fasciculata and reticularis, sometimes
associated with slight hyperplasia of the zona glomerulosa in the
adrenales, was observed (Patton and Williams, 1971).
Metabolites
Rat (90-day test with BTS 27271)
Groups of 10 male and 10 female Boots-Wistar rats were dosed orally by
gastric intubation with BTS 27271 for 90 days at doses of 0.25, 1, 3
or 12 mg/kg bw/day. In the 12 mg/kg group the rats were nervous and
difficult to handle. Body weight was slightly lower at 3 and 12 mg/kg
compared to the controls, the males being more affected than the
females. At the end of the experiment the number of erythrocytes was
decreased in the 3 and 12 mg/kg group for females and in the 12 mg/kg
group for males. Haemoglobin and haematocrit values were decreased at
12 mg/kg for males only. Owing to the variation in the number of
lymphocytes the leucocyte counts were decreased for male and increased
for female rats in the highest dose group. Concomitantly a
significant decrease in number of eosinophils was found. Urinalysis
revealed an increased GPT activity in females, and a slight increase
in urine volume and decrease in specific gravity in the males of the
highest dose group. Together with these changes an increase in BUN
was found. In terminal plasma samples a significant increase in
alkaline-phosphatase activity in both sexes given 12mg/kg was found.
The relative weights of adrenals, ovaries and uterus in the females
and the testes in the males given 3 and 12 mg/kg were increased. In
the highest dose group the weight of liver for females and spleen for
males was increased. Microscopic examination of rats given 3 or 12
mg/kg showed a slight increase in lymphoid infiltration with some
leucocytosis and a loss of glycogen in the liver (livers of female
rats were not examined at 3 mg/kg). In the highest dose group the
hearts of the female rats showed slight cellular accumulation (Shaw
and Williams, 1975).
Dog (90-day test with BTS 27271)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg BTS 27271/kg bw in gelatin capsules daily for three months. The
test compound was diluted in lactose to 1% BTS 27271 as free base.
Clinical signs in the 0.25 and 1 mg/kg group were abnormal quietness
and drowsiness. Between 1 and 4 hours after dosing the mean body
temperature and mean heart rates decreased significantly for the 0.25
and 1 mg/kg dose groups. In the highest dose group a significant
increase in liver weight was found. A slight reduction in thymus
weight was observed at 0.25 and 1 mg/kg. In the highest dose group
the urine volume was found to be increased. No histopathological
anomalies were found (Chesterman et al, 1973).
Rat (21-day test with BTS 28369)
BTS 28369 was administered orally to groups of 4-6 male and female
rate (Boots-Wistar) at dose levels of 0, 40, 100 or 250 mg/kg bw/day
for 21 days. In the highest dose group a slightly decreased weight
gain and BUN level was observed for males and an increased relative
weight of the spleen for females. No pathological changes due to
treatment were found (Shaw, 1975).
Dog (90-day test with BTS 28369)
Groups of 4 male and 4 female beagle dogs were dosed 0, 16, 40 or 100
mg BTS 28369/kg bw/day orally in gelatin capsules for 90 days. At 100
mg/kg bw slightly elevated urinary levels of total reducing substances
were found. No dose related effects were observed on behaviour, ECG,
heart rate, rectal temperature, body weight, food consumption,
haematology, blood biochemistry, organ weights and histopathology
(Morgan et al 1974).
Long-term studies
Rat
See under "Special studies on carcinogenicity".
Dog (2 years)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg amitraz/kg bw/day orally in gelatin capsules for 2 years. Three
hours after dosing all dogs given 1 mg/kg bw exhibited CNS depression
on day 1 and 2, blood sugar level was slightly increased at weeks 40
and 53 (the only weeks measured). No effects on haematological,
biochemical and histopathological parameters were reported (Morgan
et al, 1973).
OBSERVATIONS IN HUMANS
After a few complaints from people involved with the development of
amitraz, all workers in this department were interviewed by the
medical staff, which led to the following conclusions:
1) Skin flushing due to capillary dilatation may be caused by amitraz
or its precursor BTS 27271, or both.
2) The response appears to be the result of systemic absorption rather
than topical application.
3) Absorption is more likely to occur when large quantities are
processed. So far skin effects have not followed the use of
formulated materials in the course of animal or crop protection
trials.
4) Apart from the observation that the capillary circulation in the
skin appears to have been sensitised to trauma such as gentle friction
and radiant heat, no other information can be offered to explain the
phenomena described.
5) The effects appear to be temporary and disappear soon after contact
with the materials is discontinued (Moore, 1972).
Amitraz (about 10 mg) was applied to the arms of 9 volunteers as 0.02
ml of a 50% acetone solution. After 6 hours the arms were cleaned.
One volunteer, who had previously reacted to the compound, showed a
slight erythema localized to the patch area.
Three volunteers who had previously reacted to this compound were
tested using a single application of a paraffin-based ointment
containing 50% amitraz. One subject reported a delayed reaction
occurring 14 h after the initial application consisting of a reddening
of his previous reaction to this compound; no throbbing or other
symptomatology was observed and the reaction had faded by the morning
(Anonymous, 1971 b and c).
Six volunteers received a single oral dose of 2 mg BTS 27271 in a
double-blind crossover study. Blood pressure, pulse rate and oral
temperature were different comparing the volunteers receiving the
active treatment to those receiving placebo. This difference existed
just as much initially as during the observation period. Mental
alertness scores show that a number of subjects felt relatively more
alert on placebo (Hall et al, 1975).
In urine of production workers and volunteers the expected terminal
metabolite, BTS 28369 could be detected (Shirley, 1975 b, 1976;
Hughes, 1975).
RESIDUES IN FOOD
USE PATTERN
Amitraz is used for the control of pests on both animals and crops and
is used in countries throughout the world as an insecticide and
acaricide.
Treatment of crops
As an acaricide, amitraz is successfully used against red spider mites
on deciduous fruit crops, citrus, cotton and certain other crops. As
an insecticide it is used to control pear psylla and whitefly, and as
an ovicide against the major cotton lepidopterous pests.
Recommendations for its use on cotton, deciduous top fruit, citrus,
ornamentals and vegetables are shown in Tables 4 and 5.
Treatment of animals
Amitraz is effective against ticks, mange mites, lice and keds on
cattle, sheep, goats and pigs (Tables 6 and 7).
The species of skin parasites controlled by amitraz are shown in Table
8.
TABLE 4. Use Pattern on Crops
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
COTTON Lepidoptera
Cotton Bollworm
Heliothis spp.
Pink Bollworm
Pectinophora gossypiella
Red (Sudan) Bollworm
Diparopsis castanea Spray when eggs first 300,400, 1.5, 2.0,
appear in the crop 600 3.0
Cotton Leafworm and repeat at regular
Spodoptera spp. intervals throughout 500,600, 2.5, 3.0,
Leaf Perforator the season 200 3.5
Bucculatix thurberiella
Spiny (Spotted) Bollworm
Earias spp.
Whitefly Spray when whitefly first appear 500,600, 2.5, 3.0,
Bemisia tabaci in the crop and repeat at regular 700 3.5
intervals throughout the season.
Thrips spp.
Aphida Spray when these pests first 500, 600, 2.5, 3.0,
Aphis spp. appear in the crop and 700 3.5
Jassida repeat at regular intervals
Empoasca spp. throughout the season
Mites
Tetraaychus spp.
DECIDUOUS Panonychus ulmi Apply the first spray at 60-80% 20-60 1-3 400-1200 2-6
TOP FRUIT egg hatch and repeat at 2-3 week
intervals.
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Mites Spray at the first sign of infestation 40-60 2-3 800-1200 4-6
Tetranychus spp. including and repeat at 2-3 week
T. urticae, T. cinnabarinus intervals.
and T. pacificus
Aculus schlectendali Spray at the first sign of 20-30 1.0-1.5 400-600 2.3
Epitrimerus pyri infestation and continue at
regular 2-3 week intervals.
Codling Moth Apply the first spray immediately
Cydia pomonella after adult emergence and repeat
at 2-3 week intervals for at
least 5 applications. 50-70 2.5-3.5 1000-1400 5-7
Aphids
Myzus persicae Apply at the first sign of
Aphys pomi infestation and repeat at regular
Dysaphis pyri 2-3 week intervals. 50-70 2.5-3.5 1000-1400 5-7
Eriosoma lanigerum Will give good suppression
Phorodon humuli of aphids in an intensive
Dysaphis plantaginea spray programme against mites.
Apple Maggot
Rhagoletis pomonella Used in a full season's
White Apple Leaf Hopper programme against mites will
Typhlocyba pomaria give useful suppression of 50-70 2.5-3.5 1000-1400 5-7
San Jose Scale these pests.
Quadraspidictus perniciosus
Plum Curculio
Conotrachelus nenuphar
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Pears only Pear psylla Spray when attack first occurs and
Psylla pyricola repeat at regular intervals. The 30-50 1.5-2.5 600-1000 3-5
higher rate is required to give
consistently good control of
over-wintering adults.
Apples Only Leaf miners
Leucoptera scitella Apply the first spray at petal fall
Phyllonorycter blancardella and repeat at 2-3 week intervals 40-50 2-2.5 800-1000 4-5
with a minimum of 4 applications
CITRUS Mites
Panonychus citri Apply first spray at 60-80% egg 20-50 1-3 400-1200 2-6
hatch and repeat at 2-3 week
intervals.
Tetranychus spp. Spray at first sign of infestation 40-60 2-3 800-1200 4-6
and repeat at 2-3 week intervals.
Phyllocoptruta oleivora Spray at first sign of infestation 20-30 1-1.5 400-600 2-3
and continue at regular 2-3 week
intervals.
Scale Insects
Ceroplastea spp. Apply the first spray during the 50-60 2.5-3 1000-1200 5-6
Saissetia spp. crawler (larval) stage and repeat
at regular intervals.
ORNAMENTALS
Carnations, Mites
Chrysanthemums, Panonychus ulmi Apply the first spray at 60-80% 30-50 1.5-2.5 600-1000 3-5
Roses, Pot plants egg hatch with repeat sprays at
and other annuals 2-3 week intervals.
and perennials
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
VEGETABLES Tetranychus urticae Spray at the first sign of infestation)
SOFT FRUIT Tetranychus pacificus regular intervals )
and other Tetranychus )
species )
Acalitus essigi )
Aphids Under cold conditions ) 46-60 2-3 800-1200 4-6
Aphids gossypii and on some crops under glass, )
Aulocorthum spp. the high rate may be necessary )
Macrosiphum spp. to achieve consistently good )
control
TABLE 5. Use of Amitraz on Crops. Registrations (at August 1980) and Periods
Between Last Application and Harvest
Country Withholding Crop1
Period
Argentina 4 weeks apples
Australia 4 weeks pome fruit and stone fruit
Belgium 4 weeks apples, pears
Bulgaria not available apples
Chile 2 weeks fruit, vegetables
Cyprus 2 weeks apples, peaches, citrus, cucurbits
Czechoslovakia 1 week cucumbers
4 weeks apples, pears green beans, peppers, hops
Denmark 2 weeks apples, pears
East Germany 4 days cucumbers, tomatoes
4 weeks apples, pears
Ecuador 2 weeks cotton, stone fruit, citrus,
strawberries, tomatoes, cucumbers
El Salvador not available cotton
France 30 days fruit trees, pears
Greece 1 week apples, pears, citrus, vegetables
Guatemala not available cotton
Hungary 10 days apples, plums, wild strawberries,
vines, sugarbeet, soybeans
Italy 2 weeks citrus, vegetables, vines,
stone fruit
4 weeks apples, pears
Japan 2 weeks apples, pears, citrus
Jordan not available top fruit
Morocco 4 weeks cotton, top fruit, vegetables
Netherlands 4 weeks apples, pears
New Zealand 2 weeks pome fruit and stone fruit
Nicaragua not available cotton
Pakistan not available cotton
Peru not available cotton
Portugal 4 weeks apples, pears
Romania not available top fruit
South Africa 2 weeks apples
4 weeks cotton
South Korea not available apples, pears, citrus
Spain 1 week fruit, maize
Switzerland 3 weeks apples, pears3
Taiwan not available apples, pears, citrus
Turkey not available apples, pears, citrus, cotton
UK 2 weeks apples, pears
7 weeks2 hops
USA 4 weeks pears
1 Use on ornamentals not included.
2 Shorter withholding period under review - likely to be 4 weeks.
3 Based on informal information, subject to written confirmation
TABLE 6. Use Patterns on Animals
Animal Pests Timing Applications
Cattle ticks A second application 10-14 As a spray containing
mange mites days later may be necessary 0.025% a.i.
lice for full control of mange and lice.
Pigs mange mites Two applications, at low concentration As a spray.
lice 10-14 days after the first High concentration
will provide control. Alternatively 0.1%
a single application at high concentration Low concentration
may be recommended. 0.05% a.i.
Sheep ticks As a dip at 0.05% a.i.
mange mites Replenishments should be
lice made with 0.075% amitraz
keds
Goats ticks A second application 0-14 As a spray containing
mange mites days after the first my 0.05% a.i.
lice be necessary for full control
of mange and lice.
TABLE 7. Use of Amitraz on Animals. Registrations (at August 1980) and Periods Between Last
Application and Slaughter
Country Formulation1 Use/Animals Withholding Period
Argentina 12.5% e.c. Dip for sheep None
Australia 50% d.p. Spray for cattle None
50% d.p. Dip for cattle None
Brazil 12.5% e.c. Spray for cattle and Meat 14 days2
sheep Milk 1 day
Colombia 12.5% e.c. Dip for cattle Meat 14 days2
12.5% e.c. Spray for cattle Milk 1 day
Costa Rica 12.5% e.c. Spray for cattle None
Cyprus 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Denmark 12.5% e.c. Spray for pigs and Meat 6 days
cattle Milk 1 day
Jordan 12.5% e.c. Spray for sheep and goats None
Netherlands 12.5% e.c. Dip for pigs and sheep Pigs None
Sheep 4 weeks
Nicaragua 12.5% e.c. Spray for cattle None
Rhodesia 12.5% e.c. Spray for cattle None
12.5% e.c. Dip for cattle None
South Africa 12.5% e.c. Spray for cattle None
25% d.p. Spray for cattle None
25% d.p. Dip for cattle None
Spain 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Sweden 12.5% e.c. Spray or dip for cattle, Sheep 7 days
sheep and pigs Pigs and cattle 1 day
United Kingdom 12.5% e.c. Spray for pigs and Meat 1 day
cattle Milk None
12.5% e.c. Dip for sheep Meat 7 days
Milk None
Venezuela 12.5% e.c. Spray for cattle Meat 14 days2
Milk 1 day
Yugoslavia 12.5% e.c. Cattle None
1 Emulsifiable concentrate is denoted by "e.c." and dispersible powder by "d.p.".
2 These withholding periods were agreed upon early in the development programme
before most residue work had been carried out.
TABLE 8. Species Of Skin Parasites Controlled By Amitraz
(i) Ticks
Boophilus microplus
B. decoloratus
Rhipicephalus evertsi
Amblyomma hebraeum
A. cayennense
Hyalomma spp.
Ixodes ricinus
I. persulcatus
I. holocyclus
Haemaphysalis bispinosa
H. longicornis
ii) Mites
Psoroptes spp.
Sarcoptes scabiei
Dermanyssus gallinae
Demodex canis
Psorergates spp.
Chorioptes bovis
iii) Lice
Linognathus vituli
L. ovillus
Damalinia bovis
D. ovis
Haematopinus eurvsternus
Solenopotes capillatus
Haematopinus suis
iv) Keds
Melophagus ovinus
RESIDUES RESULTING FROM SUPERVISED TRIALS
The metabolite (II) (see figure 1) closely resembles amitraz in its
toxicological effects and may be solely responsible for the signs seen
when amitraz is ingested (see Toxicity of impurities, degradation
products and metabolites, Table 3). Effort was therefore concentrated
on the measurement of the combined residues of amitraz and (II) in
most countries (see Table 1 for structures).
In the USA however, most of the trials have measured total residues as
'2,4-xylidine moiety (IV). For details of the analysis methods for
both animal and vegetables products see "METHODS OF RESIDUE ANALYSIS".
Residues in crops
a. Apples and pears
Residues have been determined on apples and pears from many countries
representing a wide variety of climatic conditions. Most trials have
demonstrated that the total residue (amitraz plus (II), expressed as
(II)) will not exceed 0.5 mg/kg, given an interval of 14 days between
application and harvest, following use of amitraz at the recommended
level, although the trials on pears in Italy gave somewhat higher
residues. See Table 9. for a summary of the trials and the results.
Total residues determined in the USA, measured as mg/kg of
'2,4-xylidine', indicate a similar decay pattern. For details and
results of the trials in the USA, see Tables 6/10/11.
b. Stone fruits
A summary of residue trials and results on stone fruits is given in
Table 7.
Peaches
Residues of amitraz and its metabolites (II) and (III) have been
measured on peaches grown in France. The results obtained are very
similar to those obtained for apples and pears.
It is concluded that the total residue (amitraz plus (II)) will not
exceed 0.5 mg/kg, given an interval of 14 days between application and
harvest.
Cherries
Combined residues of amitraz and its metabolite (II) were measured on
cherries in Denmark. Residues ranged from 0.09 to 0.33 mg/kg, and
seem unlikely to exceed 0.5 mg/kg given an interval of 28 days from a
single application at recommended rates to harvest of crop.
c. Oranges
Total residues on treated oranges, grown in two areas of the USA, were
measured. Initial deposits on the fruit fell with half lives of about
3 weeks to leave residues of around 0.5 mg/kg (expressed as amitraz)
after 14 days. Fruit harvested after 20 weeks contained a maximum
residue of 0.060 mg/kg equally distributed between peel and fruit (see
Table 13).
TABLE 9. Supervised Residue Trials on Pome Fruits in Various Countries
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
APPLES
United Kingdom 5 1 1.4 1125 0.39- 0.18- 0.18- 0.18- 0.16- 0.07- 0.07- Hughes and Somerville,
0.48 0.44 0.41 0.35 0.41 0.17 0.30 1973a
Hughes, Jones and
Somerville, 1974
Netherlands 2 2 0.54 600 0.06- 0.04- Somerville and
0.07 0.05 Hughes, 1973d
1 1 0.72 1800 0.20 0.17 Goodall and Somerville,
1974 b
France 1 2 0.60 880 0.60 0.47 0.42 0.32 0.17 ) Somerville and
1 1 0.60 1000 0.20 0.32 0.38 0.28 0.03 ) Hughes, 1973f
USA 1 3 1.0 4500 0.37, 0.21 0.14 0.17 0.14 0.19 )
0.38 )
1 3 2.0 4500 0.51, 0.46 0.42 0.35 0.38 0.18 ) Anon., 1973
0.54 )
1 1 1.0 4500 0.07 0.05 0.06 )
1 1 2.0 4500 0.01 0.12 0.11 )
Denmark 1 1 0.9 2250 0.44 0.05 0.04 ) Somerville and
1 1 0.9 2250) 0.85 0.11 0.17 0.03 ) Goodall, 1974
+2 0.67 2250) )
Italy 1 2 0.04 in 100 l
at 15 l per
tree 0.45 0.34 0.23 Goodall and
Somerville, 1974a
TABLE 9. Continued...
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
Switzerland 1 1 0.2% 0.54 0.44 0.28 0.25 Anon., 1977
Fed. Rep. of 5 3 0.75 1500 0.38- 0.33- 0.29- 0.26- 0.16- Richards, 1980b
Germany 1.01 1.03 0.71 0.72 0.781
PEARS
UK 2 1 1.4 1125 0 36, 0.37, 0.12, 0.13, 0.07, 0.09 Hughes, Jones and
0.46 0.38 0.27 0.17 0.15 0.09 Somerville, 1074
USA 1 1 1.32 2950 0.54 0.49 0.10 ) Anon., 1973
1 1 2.64 2950 1.00 0.92 0.84 )
Italy 2 2 0.60 1000 1.80, 1.42 0.02 0.02, 0.01 )
1.90 0.84 0.56 0.18 0.11 ) Goodall and
1 2 (50 g in 100 l. 1.54 0.94 0.53 0.43 0.26 ) Somerville, 1974a
to run off) )
Japan 1 2 1.5 6000 0.30 0.15 0.19 0.06 Summary only
0.21
Switzerland 1 1 0.2% 0.5 0.56 0.39 0.24 Anon., 1977
0.63
Fed. Rep. of
Germany 1 3 0.75 1500 0.10 0.10 0.10 Richards, 1980b
1 The highest figures were all obtained from one anomalous trial. In the others residue levels from 14 days were not above 0.50 mg/kg.
TABLE 10. Supervised Residue Trials on Apples in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Michigan 9 1.3 3590 1.03 1.16
1.79 1.15
Michigan 1 1.2 3590 0.72 0.57 0.28
0.86 0.91 0.38
1.34 1.11 0.74
1.3 3590 0.64 0.66 0.35
Michigan 6 1.2 3590 2.33 1.68 1.79
2.31 1.32
2.33 3.03 2.13
2.89 1.74
2.45 1.66 1.75
2.66 2.19
1.3 3590 2.15 1.12 1.48
0.98 1.87
Michigan 3 1.4 1870 1.49 1.37 1.02
1.66 1.28 0.88
Michigan 1 1.4 1870 0.58 0.36 0.46
0.67 0.25
1.39 374 1.54 0.90 0.53
0.90 0.78
Michigan 1 0.56 140 0.11
0.14
New York 3 1.22 3272 0.43 0.28 0.32
0.40 0.50 0.44
New York 1 1.39 3740 0.32 0.28 0.20
0.52 0.35 0.24
North 3 1.07 2870 0.45 0.76 0.25
Carolina 0.72 0.59 0.42
TABLE 10. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Oregon 1 1.86 467 0.99 1.68 0.87
1.46 0.38 0.81
Washington 1 1.86 3740 0.96 0.89 1.02
0.51 1.00
California 2 0.70 467 1.00 0.63 0.46
0.63 1.15 0.54
0.70 3740 1.71 1.79 0.80
1.24 1.44 1.13
Wisconsin 2 1.39 3740 0.79 0.24 0.24
0.54 0.30 0.27
Anon., 1975
TABLE 11. Supervised Trials on Pears in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
California 3 1.85 1870 0.78 0.66 0.69
0.50 0.69 0.54
California 3 1.86 467 0.52 0.30 0.32
0.45 0.34 0.42
Michigan 3 0.88 2945 0.30
0.47
1.46 2945 1.18 0.62 0.49
0.96 0.42 0.56
Michigan 1 0.56 1122 0.31 0.43 0.32 0.31
0.30 0.41 0.38
Michigan 1 1.32 2945 1.20 0.81 0.66
1.36 0.86 0.25
1.67 1.04 0.27
1.26 0.75 0.31
Michigan 3 1.46 2945 0.89 1.17
1.17 1.36
1.19 0.94
1.22 1.03
1.20 1.03
1.36 1.40
Michigan 2 1.39 1402 0.82 0.65 0.34
1.39 280 1.16 1.09 0.87
0.47
New York 5 1.85 3740 1.69 1.07 0.97
0.87 0.68
New York 6 1.63 3272 1.74 1.37 1.22
2.38 2.38 1.37
Oregon 5 1.87 467 0.92 0.78 0.95
1.28 0.75 0.43
TABLE 11. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
Oregon 6 2.79 5610 1.80 1.48 1.36
2.75 1.22 1.07
Washington 5 1.86 1309 1.19 1.02 0.96 0.51
0.28
0.34 1.10 0.75 0.30
0.40
Washington 5 1.86 3740 1.12 0.55 0.85
0.99 0.65 0.72
Washington 6 8.198 g/l 0.12 0.12
undiluted 0.14 0.12
Anon., 1975
TABLE 12. Supervised Residue Trials on Stone Fruits
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Applications Residues in mg/kg intervals (days)
No. Rate per hectare after application References
Country of No. kg litres 0 7 14 21 28 35 49 63
Crop Trials
Cherries Denmark 2 1 0.675 3,375 0.28 0.09 Humphrey and
Somerville,1976
Sour Cherries Denmark 3 1 0.40 1,000 0.33 0.09 0.17 Hayto, 1978d
Plums UK 2 1 1.26 g/l 0.041 0.241 Hayto, 1977c
Peaches France 4 1 0.60 1,000 0.57- 0.32- 0.24- 0.15- 0.04, )Somerville and
1.03 1.03 0.30 0.24 0.06 )Hughes, 1973b,c
1 1 0.32 1,900 0.72 0.21 0.10 0.21 0.06 Somerville and
Hughes, 1973c
1 These two results were obtained on different varieties of plum at different locations.
d. Cotton
Residues of amitraz and its metabolite (II) have been measured in
cotton from several different territories. Most of the determinations
have been made on cotton seed following a normal spray programme. In
the case of South Africa, the seed was fractionated into cake and oil,
which were analysed separately; much shorter last spray to harvest
intervals were used than in a normal spray programme. The combined
residues (expressed as (II)) never exceeded 0.4 mg/kg. A summary of
these cotton residue trials can be found in Table 14.
Total '2,4-xylidine' residues (expressed as amitraz) measured in the
USA indicated that after 14 days residues were below 0.5 mg/kg except
in one instance. See Table 15.
e. Hops and beer
Treated samples of hops, both green and dried, have been analysed for
residues of amitraz and its metabolite (II). The combined residues, 7
weeks after the final application, were 0.03 and 0.11 mg/kg (expressed
as (II)) for the green and dried hops respectively. No detectable
residues were found in beer made with treated hops.
f. Cucumbers
Residues of amitraz and (II) have been measured in cucumbers treated
in the UK, Netherlands and South Africa. In all cases the combined
residues after three days never exceeded 0.3 mg/kg in the whole fruit
or the skin.
g. Olives
Residues of amitraz and its metabolite (II) have been measured in
olive oil and pressed olives treated in Italy. The results are in
Table 16.
h. Eggplants
Eggplants were treated with amitraz in Italy. Combined residues were
below 0.3 mg/kg after 4 days.
i. Onions
In a similar study, amitraz was applied to onions. Combined residues
after 28 days were an average of 0.06 mg/kg.
Table 16 summarises the residue data on vegetables, hops and beer.
TABLE 14. Supervised Residue Trials with Cotton in Various Countries: Residues in cottonseed, seedcake and oil
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
No.
Rate of Application of Residues in mg/kg at intervals (days) after application References
Country kg/ha Treatments 5 19 21 28 37 41 77 80
Turkey 0.8 3 <0.01 ( Hamilton,
1.0 3 <0.01 ( 1977a
0.8 in 4001 followed 2 <0.01
by 0.6 in 6001 (in seed oil) Hayto, 1978f
Guatemala 0.29 21 <0.01,0.02 )
0.57 21 <0.01,0.01 )
)
Colombia 0.5) ) ) Hayto, 1978a,
0.7)-in 4001 7 )<0.01 )
1.0) ) )
S. Africa 0.4 1 0.05 0.05 0.05 (seedcake) )
0.03 0.18 0.04 (oil) )
)
0.6 1 0.13 0.06 0.11 (seedcake) ) Hayto, 1978c
0.22 0.09 0.04 (oil) )
)
0.8 1 0.28 0.21 0.29 (seedcake) )
0.22 0.34 0.29 (oil) )
Mexico 0.2 kg/l 6 0.02 (seed cotton)
0.05 (seed from whole bolls) Hayto, 1978e
1 The first application was ULV; the second conventional application. Samples tested were cottonseed except where
treated otherwise.
TABLE 15. Residues on Cottonseed in U.S. trials
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
State of No. of Residues in mg/kg at
U.S.A. Rate of Application per hectare Treatments intervals (days) after application
0 6,7 9 14 18 48 85
Texas 0.28 kg in 37.4 l 9 1.04 0.80 0.68
0.14 kg in 37.4 l 9 0.16 0.18 0.21
S. Carolina 0.28 kg in 46.7 l 16 0.16 0.12 0.13
Alabama 0.56 kg in 121.6 l 11 0.41
Mississippi 0.14 kg in 46.7 l 10 0.20
0.14 kg in 28.0 l 4 0.12
0.28 kg in 56.1 l 12 0.27 0.28
Alabama 0.56 kg in 46.7 l 6 0.06
0.28 kg in 46.7 l 6 0.07
Anon., 1978
TABLE 16. Supervised Residue Trials on Vegetables, Hops and Olives (Oil and Cake)
Combined residues of amitraz and N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Crop Country Applications Residues (mg/kg) at intervals (days)
No. Rate per hectare after application References
kg litres 0,1 3 4,5 6,7 14 28
Cucumber UK 1 0.33 g/l 0.09 ) Hamilton 1978
2 0.33 g/l 0.13 0.09 )
Cucumber S. Africa 1 60 g in 100 l drench 0.30 0.30 0.10 ) Hayto, 1979
0.37 )
Cucumber Netherlands 1 2.8 7,000 0.02,<0.01 )
0.03, 0.07 )
)
1 2.4 6,000 0.01 ) Somerville and
0.13 ) Hughes, 1973a,
Eggplant 1 0.51 1,000 0.17 0.27 0.18 0.12 )
) Hayto, 1978b
)
Onion bulbs 1 0.5 0.06 )
TABLE 16. Continued...
Crop Country No. Applications Residues (mg/kg) at intervals (days)
of No. Rate of hectare after application References
Trials kg litres 50 54 90 139
Hops and
beer UK
Green Hops UK 1 1 0.72 0.03 )
)
Dried Hops UK 1 2 0.72 0.11 ) Hamilton, 1977b
)
Beer UK 1 2 0.72 (on hops) 1.68 <0.01 )
Olives Italy 2 1 54 g/hl )
1.68 1.36 (Pressed cake) )
2 1 108 g/hl ) Richards, 1980a
<0.01 <0.01 (Purified oil) )
0.80 1.70 (Pressed cake) )
Residues in animals
Cattle
Trials have been carried out on cattle and calves in order to measure
combined residues of amitraz and (II), total '2,4-xylidine', or (V)
residues, in tissues after single or repeated applications of amitraz
as a spray or dip. Combined residues of amitraz and (II) and total
'2,4-xylidine' residues after hydrolysis were generally comparable to
control values 1 and 7 days after single spray applications. As
expected, some residues of (V) were apparent after hydrolysis of the
tissues. The results, however, were often complicated by
naturally-occurring 4-amino-benzoic acid, which was not resolved from
(V) by the original method of analysis and gave rise to apparent
residues in untreated tissues. The maximum corrected residue of (V)
after a single-spray application was 0.50 mg/kg in the liver.
Long-term trials involving spray or dip programmes indicated no large
accumulation of residues. Maximum residues of amitraz plus (II) were
0.13 mg/kg in the kidney 1 day after a 14-month spray programme.
Liver tissues from the same trial gave rise to corrected maximum
residues of 0.89 mg/kg of (V).
Milk samples and butter fat have been analysed for combined residues
of amitraz plus (II), for residues of (V), and also for total
'2,4-xylidine' residues. No significant residue were found in milk.
Maximum levels of '2,4-xylidine' occurred in butter fat 6 hours after
treatment (0.15 mg/kg amitraz), however milk collected 2 days after
treatment showed no significant residues as did factory butter
produced locally.
Sheep
In sheep, total residues of '2,4-xylidine' one day after a single dip
were below 0.1 mg/kg in muscle, and below 0.5 mg/kg in kidney and fat,
except in the case of back fat in the first trial. Residues of the
terminal metabolite (V) were only significant in liver and kidney
(corrected maximum 0.65 mg/kg). Milk samples from treated sheep gave
no significant residues of amitraz plus (II) after 1 or 2 days.
Pigs
Residues of amitraz plus (II), and total residues of '2,4-xylidine',
were comparable to control values in pigs sprayed once and twice
respectively. Analysis of pigskin indicated combined residues of
amitraz plus (II) of 0.04 mg/kg and 0.01 mg/kg at 1 and 3 days after a
single spray.
Tissues from the double spray trial gave rise to residues of (V).
However, an apparent residue of over 1 mg/kg in the liver was
confirmed at 0.40 mg/kg by combined gas chromatography-mass
spectroscopy. Smaller residues were likewise found in other tissues.
A summary of all residue results from animal trials can be found in
Table 17.
FATE OF RESIDUES
In plants
Experiments using 14C-amitraz demonstrated that, under certain
conditions at least residues of (II) and (III) could occur on and in
fruit, e.g. apples (Somerville and Nicholson, 1972).
The radiotracer experiments also demonstrated that 14C-amitraz,
applied to leaves, was not transported in any measurable quantity to
other parts of the plant, whereas when 14C-amitraz was applied to the
soil, radioactivity could be taken up into the aerial portions of
plants (Somerville and Spiers, 1972; Lewis, 1970b).
The metabolic pathway is summarised in figure 1.
In animals
The metabolism of amitraz in animals is covered fully in Biochemical
Aspects.
Studies with dogs, cats, rats, mice and bovines all yield the same
picture, namely that amitraz does not persist in vivo, but is
rapidly degraded, yielding conjugated 4-amino-3-methylbenzoic acid (V)
which is excreted in the urine. In dogs, evidence has been obtained
indicating that the first step in the degradation is simple hydrolysis
to yield N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethylformanilide (III). This is consistent with the known
properties of amitraz in acid medium. Furthermore, in an experiment
in which (II) was itself fed to dogs, identical excretory products to
those yielded by amitraz were found. In vitro studies have shown
that both (II) and (III) are degraded rapidly in gastric juice to give
2,4-xylidine (IV).
Further evidence that (IV) is an intermediate in amitraz degradation
in animals is provided by Lindstrom (1961) who showed that (IV) was
rapidly converted to (V) in rats.
It is also of interest to note that (V) is found in human urine
following exposure to amitraz indicating that the same pathway of
metabolism exists in humans.
Once ingested, amitraz is rapidly converted to metabolite (II), which
has similar toxicological effects to amitraz itself. Since apple
pomace and citrus pulp may be fed to cattle, analyses were made of
plasma, tissues and milk from calves and cows dosed with (II). No
measurable residues of (II) or (V) were found in the milk of cows
after 21 days continuous dosage (Dickinson and Shirley, 1975). After
a similar period the total residues of (II), (III), and (IV) in
TABLE 17. Supervised Residue Trials on Animals in Various Countries
Country Animal/ Residues at intervals (days) after application.
Commodity Application Amitraz plus N-2,4-dimethyl-N'-methyl-formamidine References
expressed as the latter (mg/kg) unless
stated otherwise.
Type Number Concentration CONTROL 1 4 7/8
UK CATTLE spray once 0.025%
muscle ) <0.01 <0.01 0.01 )
liver ) <0.01 0.05 <0.01 )
kidney ) <0.01 0.03 )
fat <0.01 <0.01 <0.01 )
)
muscle )3 0.051 <0.01 0.02 ) Jones and
liver ) 0.061 0.12 0.02 ) Holland,
kidney ) 0.03 0.01 ) 1974
fat ) 0.091 0.01 0.01 )
UK CATTLE spray once 0.025%
muscle ) 0.02 <0.01 )
liver ) <0.01 <0.01 ) Holland,
kidney ) 0.03 0.01 ) Jones and
fat ) <0.01 0.03 ) Jones, 1974
Australia CATTLE spray repeatedly at 0.025%
7-day intervals
muscle (rump) ) followed by 3 <0.01 <0.01 <0.01 )
(shoulder) ) sprays at 3-day <0.01 <0.01-0.02 <0.01 )
liver ) intervals <0.01 0.01 <0.01 ) Hayto,
kidney ) <0.01 0.03-0.04 0.03 ) 1977b
fat (back) ) <0.01 <0.01 <0.01 )
(omental) ) <0.01 <0.01 <0.01
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
S. Africa CATTLE spray weekly 0.05%
for 14
muscle )3 <0.011 0.05 )
liver ) <0.01 0.06 )
kidney ) 0.13 )
fat ) 0.01 0.06 )
)
muscle )3 0.051 0.02 0.03 ) Holland
liver ) 0.061 0.04 0.03 ) and Jones,
kidney ) 0.11 0.12 ) 1974a
fat ) 0.091 0.03 0.03 )
milk )3 0.02 0.02 0.01 ) Holland and
) Jones, 1974b
S. Africa CATTLE dipped weekly 0.008%
1-3 years
muscle )3 0.0051 0.008 <0.005 )
liver ) 0.0051 0.04 0.01 ) Shirley,
kidney ) 0.0051 0.06 0.01 ) 1977a
fat ) 0.0051 0.02 <0.005 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
Day 0 Day 1 Day 2
am pm am pm am pm
pre-treatment
Australia CATTLE dipped once 0.025% )
)
tissues <0.02 <0.02 )
)
dipped repeatedly 0.025% )
)
milk ) <0.012 <0.01 <0.01 ) McDougall,
butterfat ) <0.012 0.15 0.11 0.02 0.01 <0.01 ) Heath and
factory butter ) all samples 0.012 ) Black, 1979
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 3 5 7
UK SHEEP dipped once 0.05%
muscle ) 0.011 0.08 0.07 )
liver ) 0.011 0.24 0.11 )
kidney ) 0.021 0.40 0.13 ) Shirley,
fat (back) ) <0.0051 0.62 0.34 ) 1977b
(omental) ) <0.0051 0.14 0.08 )
UK SHEEP dipped once 0.05%
muscle (shoulder) 0.012 0.03 0.02 0.01 0.01 )
(hind leg) 0.012 0.03 0.01 0.01 0.01 )
liver 0.012 0.13 0.04 0.03 ) Needham and
kidney 0.012 0.27 0.09 0.07 ) Campbell,
fat (back) 0.012 0.26 0.27 0.25 0.12 ) 1980
(omental) 0.012 0.10 0.08 0.10 0.09 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 2 3 7
UK SHEEP dipped once 0.05%
milk <0.01 0.02 0.01 <0.01 <0.01 Shirley, 1978
UK PIGS sprayed once 0.1% 2 and 7 days
twice at
muscle 7-day <0.01-0.04 <0.01-0.02 )
liver intervals <0.01-0.05 <0.01-0.04 )
kidney <0.01-0.02 <0.01-0.07 ) Shirley,
fat <0.01-0.02 <0.01-0.02 ) 1975a
UK PIGS sprayed once 0.1%
skin <0.01 0.04 0.01 <0.01 Hayto, 1977a
UK PIGS sprayed twice 0.1%
at 8-day
muscle intervals <0.031 <0.03 )
liver <0.031 <0.03 )
kidney <0.031 <0.03 ) Dickinson and
fat <0.031 <0.03 ) Shirley, 1976
1 Total residues measured as '2,4-xylidine' and expressed as N-2,4-dimethyl-N'-methyl-formamidine (mg/kg)
2 Total residues measured as '2,4,xylidine' and expressed as amitraz (mg/kg)
3 No untreated animals in trial, tissues bought locally.
tissues and plasma of calves were found to be < 0.01 mg/kg and < 0.1
mg/l respectively, although the livers and kidneys of calves receiving
the highest dose of 40 mg/kg diet/day contained residues of 0.02 and
0.05 mg/kg respectively (Campbell and Needham, 1979).
METHODS OF RESIDUE ANALYSIS
Methods have been developed to measure combined residues of amitraz,
compound (II), total '2,4-xylidine' (IV) and metabolites (V) and
(III).
Combined residues of amitraz (calculated as (II) and (II) are measured
as mg/kg (II). Fruit for analysis is first subjected to treatment
with acidic methanol to convert amitraz to (II). Solid material is
filtered off, the extract cleaned and analysed by GLC using a
flame-ionisation detector. Sensitivity is 0.01 mg/kg and recoveries
varied between 70 and 100% for different fruits (Hughes, 1974b;
Somerville and Richards, 1980).
Analysis of animal tissues and milk differ from the above only in the
initial preparation of the sample for extraction, and in the cleanup
procedures, which were slightly modified from those used for fruit.
The method is capable of estimating a total residue level of 0.02
mg/kg in lean meat, liver, fat and milk samples; recoveries were over
70% and background levels were less than O.02 mg/kg (II) (Hughes,
1974a).
Methods of assay of total '2,4-xylidine' residues were developed for
analysis of animal tissues. The methods involve hydrolysis of
amitraz, and all its metabolites that contain the moiety, to
'2,4-xylidine' which on conversion to its heptafluorobutyric
derivative can be determined by gas-liquid chromatography with
electron capture detection. The limits of sensitivity are 0.01 mg/kg
(II) equivalents (Nappier, Hornish and Lane, 1976).
Similar methods have been used in the USA for analysis of fruit and
cotton seed, and lower limits of detection have been established at
0.01 mg/kg and 0.05 mg/kg respectively (Nappier and Hornish, 1975;
Nappier and White, 1978).
Compound (V), the main mammalian metabolite of amitraz, exists as a
conjugated derivative in tissues. Analysis of residues of (V)
therefore involves acid hydrolysis followed by high pressure anion
exchange chromatography. The method has a recovery of 70-80% and a
lower level of detection of 0.03 mg/kg. Relatively high background
levels were found in earlier work, probably due to naturally occurring
p-aminobenzoic acid, which eluted with (V). The more recent method
resolves these difficulties (Dickinson and Shirley, 1976).
Compound(III) is formed in the hydrolysis of amitraz and has been
assayed by high pressure liquid chromatography following extraction of
fruit in the usual way. The sensitivity of the method in 0.01 mg/kg
and the recovery is 89%. (Jones, 1973 f).
NATIONAL RESIDUE LIMITS
The following national Maximum Residue Limits (MRLs) were reported to
the Meeting.
Country Withholding Crop MRL1
Period mg/kg
Australia 4 weeks pome fruit and 0.052
stone fruit
Belgium 4 weeks apples, pears 0.4
East Germany 4 days cucumbers, tomatoes 0.2
4 weeks apples, pears 0.2
France 30 days fruit trees, pears 0.15
Hungary 10 days apples, plums, strawberries,
vines, sugarbeet, soybeans 0.052
Italy 4 weeks apples, pears 0.4
2 weeks citrus, vegetables,
vines, stone fruit 0.4
Netherlands 4 weeks apples, pears 0.4
New Zealand 2 weeks pome fruit and stone fruit 0.5
Switzerland4 3 weeks apples, pears 0.3
USA 4 weeks pears 3.03
Fed. Rep. of apples, pome fruit 0.4
Germany
1 Combined residues of amitraz and formamidine (II), expressed as
II, unless otherwise indicated.
2 Amitraz only.
3 Measured as total '2,4-xylidine' (IV), expressed as amitraz.
4 Based on informal information, subject to written confirmation.
EVALUATION
COMMENT AND APPRAISAL
Amitraz is an insecticide and acaricide effective against a wide
variety of phytophagous mites and insects; its use on fruit and
vegetables and on cotton is authorised in a large number of countries.
It also has important veterinary uses, especially against the ticks
and mites of cattle and sheep, including some otherwise resistant
strains. It is formulated as emulsifiable concentrates for both crop
protection and animal use, and as wettable powders for animal use.
Amitraz is well absorbed from the gastro-intestinal tract and
eliminated from most tissues within a few days. The metabolic
breakdown is similar for all species studied, resulting in the
formation of 3-methyl-4-aminobenzoic acid (BTS 28369) and its
conjugates.
With amitraz a number of short and long-term toxicity studies have
been carried out with mice, rats and dogs. From these experiments
dogs appeared to be the most sensitive species. Furthermore,
experiments with amitraz metabolites showed that on the basis of
weight BTS 27271 is more toxic than the parent compound, however this
difference in toxicity may be explained by the difference in molecular
weight between these compounds. Therefore the toxicity of amitraz can
most probably be attributed to the formation of BTS 27271.
For mice and rats a marginal no toxic effect level of 2.5 and 3 mg/kg
bw/day may be estimated. In dogs however the no effect level from the
short and long-term studies was 0.25 mg/kg bw/day. The most
predominant effect particularly in dogs was CNS depression. The
metabolite BTS 27271 has a no effect level of 1 and 0.1 mg/kg bw/day
for rats and dogs respectively.
From reproduction and teratogenicity studies it was shown that amitraz
influences mean litter size and viability of the young, although, in
effective doses, toxicity on the dame was also apparent. Therefore it
can be concluded that amitraz is possibly embryotoxic in doses also
toxic for the dama, but has no clear teratogenic activity. However
reported studies are incomplete with respect to the number of animals
used (teratogenicity test) and the number of litters bred
(multigeneration study). Also no information was obtained about pre-
implantation losses. No information is available about the possible
teratogenic potential of the metabolites.
From mutagenicity tests with amitraz, BTS 27271, 27919, 28369, 33220
and 24868 it was shown that only BTS 24868, 33220 and 27919 exhibit
weak mutagenic activity in in vitro bacterial systems using
Salmonella typhimurium (TA 100 strain). Negative results were
also obtained in the host-mediated and lethal tests with amitraz.
With amitraz and BTS 24868 carcinogenicity experiments were carried
out in mice and rats. Amitraz was negative in the rat study, but from
initial body weights it is concluded that the age of the animals at
the start of the experiment was not within normal standards. In the
female mouse only 400 mg/kg bw caused an increased incidence of
lymphoreticular tumours. In female mice BTS 24868 increased the
number of lung tumours. In male rats (no female tested) also the
number of lung tumours was found to be increased, together with
bladder and liver tumours which were not seen in controls. In these
studies the number of animals used, dosing schedule and experimental
period do not permit an evaluation of a possible carcinogenic action
of BTS 24868.
Human data are inadequate to be included in the evaluation for the
acceptable daily intake.
It should be remarked that a number of experiments and reports
submitted do not meet acceptable standards.
Experiments using 14C-amitraz show that under certain conditions the
metabolites N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethyl-formanilide (III) may occur on or in fruit. If 14C
labelled amitraz is applied to soil some radioactivity may be taken up
into the aerial parts of plants, but when it is applied to leaves
there is no detectable movement to other parts.
Extensive residue trials on apples and pears have taken place in the
United Kingdom, Europe and other parts of the world, especially in the
United States. US registration authorities require that all
components of the residue that give rise to '2,4-xylidine' on
hydrolysis are included in the analyses, whereas elsewhere residue
measurements are based on the determination of the parent compound
plus N-(2,4-dimethylphenyl)-N'-methylformamidine (II), which is the
most toxic metabolite, being more toxic to mammals than amitraz
itself. Further degradation gives compounds of lesser toxicological
significance.
It is suggested that results obtained by the two methods are broadly
comparable when expressed on the same basis, although those based on
hydrolysis to '2,4-xylidine' are sometimes rather higher.
The terminal metabolite in animals, 4-amino-3-methyl benzoic acid (V),
has been detected as a residue in animal tissues (especially in the
liver), but it closely resembles the natural product 4-aminobenzoic
acid (which can interfere with its determination).
The residue analytical methods used in the USA, involving total
'2,4-xylidine' determination, require a specially made
distillation/extraction apparatus, and also involve the formation of a
fluorinated derivative of the 'xylidine' before final determination by
gas chromatography using electron capture detection. These procedures
probably require considerable skill on the part of the analyst.
Convenient analytical methods, using gas chromatography with flame
ionisation detection, are available for the determination of combined
residues of amitraz and (II) in fruits, vegetables, animal tissues and
milk, and these methods could be adapted for regulatory purposes.
The meeting examined residues data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels which were likely to occur when
amitraz was used in practice and when reported intervals between last
application and harvest were observed.
Level of amitraz causing no toxicological effect
Mouse: 2.5 mg/kg bw/day
Rat: 3 mg/kg bw/day
Dog: 0.25 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw/day
RECOMMENDATIONS OF RESIDUE LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. These levels refer
to the sum of amitraz and N-(2,4-dimethylphenyl)-N'-methylformamidine
(II), calculated as II.
Crop FAO MRL Pre-harvest
Commodity (mg/kg) Interval
Group (days)
Pome fruit 10 0.5 14
Peaches 11 0.5 14
Cherries 11 0.5 28
Cucumber 7 0.5 3
Oranges 9 0.5 7
Cottonseed 20 0.5 7
Cottonseed oil class C 0.05
Olive oil class C 0.05 (Prepared from olives
(sprayed 60 days
(before harvest.
Carcass meat
of cattle 25 0.05
of pig 25 0.05
of sheep 25 0.2
Cattle meat by-products 27 0.2
Pig meat by-products 27 0.2
Sheep meat by-products 27 0.2
Milk 28 0.011/
1/ Level at or about the limit of determination
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Long-term carcinogenicity study with
N-2,4-dimethylphenyl-N-methylformamidine.
2. Long-term carcinogenicity study with amitraz in mice.
3. Further information on the mutagenic activity of 2,4-xylidine,
NN-bis(2,4-dimethylphenyl)formamidine and 2,4-dimethylformanilide.
4. Additional residue data from supervised trials on whole olives.
Desirable
1. Information on the teratogenic activity of
N-2,4-dimethylphenyl-N-methylformamidine.
2. Information on species differences in toxicity.
3. Further observations in humans.
4. Additional residue data on citrus fruits.
5. Further trials on hops, onions and eggplants.
REFERENCES
Anonymous. Acute oral studies on triazid impurities. Unpublished
report RD/BS/BL/1268, 1971a; from The Boots Company, submitted to WHO.
Anonymous. Human pharmacology study on BTS 27419. Unpublished report
no. MS 71004; 1971b, from The Boots Company, submitted to WHO.
Anonymous. Supplement to report no. MS 71004; 1971c, Unpublished
report from The Boots Company, submitted to WHO.
Anonymous. Effects of BTS 27149 and BTS 27271 on the responses of
rabbit ear blood vessels to catecholamines and antidromic stimulation.
Unpublished report no. P 72622; 1972, from The Boots Company,
submitted to WHO.
Anonymous. Residue determination for amitraz and its metabolites in
apples and pears. Upjohn Co. reports 315-9760-5,8,11,12; 1973,
(Unpublished).
Anonymous. Residue determination for U-36,059 (amitraz) and
metabolites in apples and pears. Upjohn Co. reports
315-9760-33,35-40,42-49,52,55-65; 1975, (Unpublished).
Anonymous. Residue analysis of amitraz on Swiss apple and pear crops.
Boots Co. Ltd. report no. AR 77016; 1977, (Unpublished).
Anonymous. Residue determination for U-36,059 and metabolites in
cotton seed (Texas, 1977). Upjohn Co. reports 315-9760-131 to 137;
1978, (Unpublished).
Anonymous. Persistence of amitraz on Florida grown Hamlin oranges.
Boots Hercules Agrochemicals Co. report; 1979a, (Unpublished).
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Methods of analysis. Unpublished report dd. 17-9-1979; 1980, from The
Boots Company, submitted to WHO.
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Analytical specifications. Unpublished report, dd. 8-1-1980; 1980,
from The Boots Company, submitted to WHO.
Berczy, Z.S., Binns, R. and Newman, A.J. Acute inhalation toxicity to
the rat of BTS 27419. Unpublished report no. 4971/72/406; 1973, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield,
R.A.S. Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report no. 5454/72/850; 1973, from Huntingdon Research
Centre, submitted to WHO by The Boots Company.
Berry, P.A. A comparison of the hypothermic effects of amitraz (BTS
27419) in male and female CFLP mice. Unpublished report no. P 76058;
1976, from The Boots Company, submitted to WHO.
Brown, D.J., Burnett, R.J., Crowley, J., Cryer, P.C., Lancaster, M.C.
and Turnbull, G.J. Amitraz: Investigations of effects on the thymus
gland and oestrus cycle in mice. Unpublished report no TX 78037; 1978,
from The Boots Company, submitted to WHO.
Burnett, R., Crowley, J., Lessell, B., Patton, D.S.G., Sutton, M.M.,
Turnbull, G.J. and Williams, G.A.H. BTS 27419: 80-Week carcinogenicity
study in mice - final report. Unpublished report no. TX 76039; 1976,
The Boots Company, submitted to WHO.
Campbell, J.K. and Needham, D. Residues of BTS 27271 and its
metabolites in the plasma and tissues of calves after repeated daily
dosing. Boots Co. Ltd. report no. AX 79019; 1979, (Unpublished).
Channon, E.J. and Cryer, P.C. Amitraz: Investigation of effects on the
thymus gland and oestrus cycle in mice - statistical analysis of
vaginal smear data and thymus weights. Unpublished report no. SS
78001; 1978, The Boots Company, submitted to WHO.
Chesterman, H., Skerrett, K., Street, A.E. and Newman, A.J. Boots
27271 oral toxicity study in beagle dogs (Repeated dosage for 13
weeks). Unpublished report no. S 38/73511; 1973, Huntingdon Research
Centre, submitted to WHO by the Boots Company.
Dickinson, W. and Shirley, D.B., Analysis of milk from cows dosed
orally with BTS 27271 at Thurgarton Research Station. Boots Co. Ltd.
report no. F 75018; 1975, (Unpublished).
Dickinson, W. and Shirley, D.B. Residue analyses of tissues from pigs
treated with amitraz at Thurgarton Research Station, UK. Boots Co.
Ltd. report no. F 76015; 1976, (Unpublished).
O'Donovan, M.R. and Smithson, A. Amitraz impurity. BTS 33220: Acute
oral toxicity to male Boots-Wistar rats. Unpublished report no. TX
78079; 1978, from The Boots Company submitted to WHO.
Everest, R.P. BTS 27419: Mutagenicity study in the male mouse
pervisceral cavity hostmediated assay. Unpublished report no. TX
76056; 1976, from The Boots Company submitted to WHO.
Everest, R.P. and Wilcox, P. BTS 27419, BTS 27271, BTS 27919 and BTS
28369: mutagenicity testing in bacterial in vitro systems.
Unpublished report no.76016; 1976a, from The Boots Company submitted
to WHO.
Everest, R.P. and Wilcox, P. BTS 27419: Mutagenicity testing against
Salmonella typhimurium strains TA 1535, TA 1537 and TA 1538 in the
presence and absence of liver microsomes from male and female mice.
Unpublished report no. TX 76057; 1976b, from the Boots Company
submitted to the WHO.
Everest, R.P. and Wilcox, P. In vitro bacterial mutagenicity
testing of an amitraz sample containing 1% BTS 33220. Unpublished
report no. TX 79108; 1979, from The Boots Company submitted to WHO.
Goodall, E. and Somerville, L. Residue analyses of amitraz and its
metabolite BTS 27271 on Italian fruit crops. Boots Co. Ltd. report no.
AX 74026; 1974a, (Unpublished).
Goodall, E. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Dutch apple and tomato crops. Boots Go. Ltd.
report no. AX 74028, 1974b, (Unpublished).
Hall, J.E., Ahlunwalia, H. and Hampson, P. A study of the effects of
oral administration of a metabolite (BTS 27271) of amitraz to
volunteers. Unpublished report no. NS 75002; 1975, from The Boots
Company submitted to WHO.
Hamilton, D.Y. The fate of [14C]-BTS 27419 (amitraz) when
administered to mice at 100 mg/kg as a single oral dose. Unpublished
report no. AX 76013; 1976, from The Boots Company submitted to WHO.
Hamilton, D.Y. Amitraz residues in Turkish cotton seed. Boots Co. Ltd.
report no. AX 77003; 1977a, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
hops and beer from Whitbreads. Boots Co. Ltd. report no. AX 77006;
1977b, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
cucumbers from the UK. Field Trials 1976. Report no. AX 78011; 1978.
Hamilton, D.Y and Somerville, L. Fate of [14C]-BTS 27271 when
administered to dogs as a oral dose. Unpublished report no. AX 73019;
1973, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27419 when
administered at 15 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74006; 1974a, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C] BTS 28369 when
administered at 10 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74012; 1974b, from The Boots Odmpany submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27271 when
administered to cats as a single oral dose. Unpublished report no. AX
74005; 1974c, from The Boots Company submitted to WHO.
Hayto, E.M. Residues of amitraz and its metabolite BTS 27271 in
pigskin from animals treated at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 77007; 1977a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cattle
tissues from Australia. Boots Co. Ltd. report no. AX 77013; 1977b,
(Unpublished)
Hayto, E.M. Combined residues of amitraz and BTS 27271 in plums and
blackcurrants from UK field trials, 1976. Boots Co. Ltd. report no. AX
77014; 1977c, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Guatemala and Colombia, 1977. Boots Co. Ltd. report no. Ax 78002;
1978a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in egg-plants
and onion bulbs from Italy. Boots Co. Ltd. report no. AX 78012; 1978b,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton from
South Africa, 1978. Boots Co. Ltd. report no. AX 78015; 1978c,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in
sour-cherries from Denmark, 1976. Boots Co. Ltd. report no. AX 78018;
1978d, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Mexico, 1977. Boots Co. Ltd. report no. AX 78019; 1978e,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
oil from Turkey, 1977. Boots Co. Ltd. report no. AX 78021; 1978f,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cucumbers
from South Africa, 1978. Boots Co. Ltd. report no. AX 79002; 1979,
(Unpublished)
Holland, G.N., Jones, A.W., and Jones, E.M. Analyses of amitraz and
its metabolite BTS 28369 in control and treated Thurgarton calves.
Boots Co. Ltd. report no. P 74022; 1974, (Unpublished).
Holland, G.N. and Jones, E.M. Analyses of amitraz and its metabolite
BTS 28369 in treated South African cattle. Boots Co. Ltd. report no.
F 74021; 1974a, (Unpublished).
Holland, G.N. and Jones, E.M. Analysis of amitraz in milk from treated
South African cattle. Boots Co. Ltd. report no. F 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of amitraz and BTS 27271 in animal tissues and
milk. Boots Co. Ltd. report no. AX 74018; 1974a, (Unpublished).
Hughes, K.W. Estimation of combined residues of amitraz and BTS 27271
in apples and pears. Boots Co. Ltd. report no. AX 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of BTS 28369 in human urine. Unpublished
report no. F 75009; 1975, from The Boots company submitted to WHO.
Hughes, K.W., Jones, E.M., and Somerville, L. Residue analyses of
amitraz and its metabolites BTS 27271 and BTS 27919 on apple and pear
crops treated with amitraz at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 74002; 1974, (Unpublished).
Hughes, K.W., and Somerville, L. Residue analyses of amitraz and its
metabolie BTS 27271 on apple crops from Thurgarton Research Station,
UK. Boots Co. Ltd. report no. AX 73017; 1973a, (Unpublished).
Humphrey, E.A. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Danish cherries. Boots Co. Ltd. report no. AX
76003; 1976, (Unpublished).
Jones, E.M, Metabolism of [14C]-BTS 27419 in calf and in cow, part I.
Identification of major urinary metabolite. Unpublished report no.
F 73004; 1973a, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in calf. Part 2.
Identification of major liver metabolite. Unpublished report no.
F 73011; 1973b, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in rats. Unpublished report
no. F 73010; 1973c, from the Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BST 27271 in dogs. Unpublished report
no. F 73019; 1973d, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in cats. Unpublished report
no. F 73018; 1973e, from The Boots Company alimitted to WHO.
Jones, E.M. Residue analyses of BTS 27919 on French peach crops
(Report no. 1). Boots Co. Ltd. report no. F 73015; 1973f,
(Unpublished).
Jones. E.M. and Holland, G.N. Analysis of amitraz and its metabolite
BTS 28369 in treated Thurgarton calves. Boots Co. Ltd. report no.
F 74020; 1974 (Unpublished).
Kakuk, T.J. Amitraz. Further evaluation of 80-week mouse study.
Unpublished report no. HGD 79002; 1979, from The Boots Company
submitted to WHO.
Lancaster. M.C. and Williams, G.A.H. Amitraz: Multigeneration feeding
test in rats - Histopathology. Unpublished report no. TX 78064; 1978,
from The Boots Company submitted to WHO.
Lewis, D.K. RD 27.419. Metabolism and residue studies in the calf
using (14C) and (3H)-labelled material. Unpublished report no.
C 70029; 1970a, from The Boots Company submitted to WHO.
Lewis, D.K. Amitraz - a preliminary study of the persistence,
translocation, and metabolism in plants. Boots Co. Ltd. report no.
FM 70158; 1970b, (Unpublished).
Lewis, D.K. Fate of [14C]-triazid applied to rats as a single oral
dose. Unpublished report no. C 71011; 1971a, from The Boots Company
submitted to WHO.
Lewis, D.K. Fate of [14CH]-BTS 27419 applied to rats as a single
dermal dose. Unpublished report no. C 71019; 1971b, from The Boots
Company submitted to WHO.
Lewis. D.K. Fate of [14C]-BTS 27419 applied to dogs as a single oral
dose. Unpublished report no. 71024; 1971c, from The Boots Company
submitted to WHO.
Lewis, L. Fate of [14C]-triazid (BTS 27419) in the calf. Unpublished
report no. C 71026; 1971d, from The Boots Company submitted to WHO.
Lindstrom, H.V. The metabolism of FD and CRED no. I. The fate of
2,4-meta-xylidine in rats. J. Pharm. Exp. Therap. 132, 306-310.
Martin, H., and Worthing, C.R. Pesticide Manual, 6th Edition, p. 15.
Published by the British Crop Protection Council.
McDougall, D.W., Heath, A.B., and Black. R.R. Residues of amitraz in
the tissues, milk, and butter of cattle dipped in Taktic. Aust. J.
Exp. Agric. Anim. Husb., 19: 663-665.
Merryman, D.C. and Sutton, M.M. BTS 27419. Effects on the oestrus
cycle of the rat. Unpublished report no. PN 72003; 1972, from The
Boots Company submitted to WHO.
Moore, W.K.S. Clinical observations during development. 1972,
Unpublished report from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27271. Acute oral toxicity study in dogs.
Unpublished report no. TX 73004; 1973a, from The Boots Company
submitted to WHO.
Morgan, H.E, BTS 28037; Acute oral toxicity study in dogs. Unpublished
report no. TX 73015; 1973b, from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27919: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74004; 1974a, from The
Boots Company submitted to WHO.
Morgan, H.E. BTS 28369: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74006; 1974b, from The Boots
Company submitted to WHO.
Morgan, H.E., Ellicock, L.A., Parkinson, R. and Wood, R. Amitraz:
comparison of oral and topical administration to pigs. Unpublished
report no. TX 75025; 1975a, from The Boots Company submitted to WHO.
Morgan, H.E., Patton, D.S.G. and Turnbull, G.J. BTS 27419: two year
oral toxicity study in dogs. Unpublished report no. TX 73035; 1973,
from The Boots Company submitted to WHO.
Morgan, H.E. and Shepherd, G.M. BTS 28369: Acute oral toxicity study
in dogs. Unpublished report no. TX 74001; 1974, from The Boots Company
submitted to WHO.
Morgan, H.E., Shepherd, G.M., and Turnbull, G.J. BTS 28369: 90-day
oral toxicity study in dogs. Unpublished report no. TX 74037; 1974,
from The Boots Company submitted to WHO.
Morgan, H.E. and Turnbull, G.J. BTS 27919: Acute oral toxicity study
in dogs. Unpublished report no. TX 73034; 1973, from The Boots Company
submitted to WHO.
Morgan, H.E., Turnbull, G.J. and Ellicook, L.A. Amitraz and BTS 27271:
Effects of repeated daily dosing on acute responses in dogs.
Unpublished report no. M 75026; 1975b, from The Boots Company
submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 27271: Acute oral toxicity study
in dogs -Histopathology. Unpublished report no. TX 73030; 1973a, from
The Boots Company submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 28037: Acute oral toxicity study
in dogs. Unpublished report no. TX 73038; 1973b, from The Boots
Company submitted to WHO.
Nappier, J.L. and Hornish, R.E. Total residue method for U-36, 059
(amitraz) in apples, pears, and soils. Upjohn Co. report no.
315-9760-32; 1975, (Unpublished).
Nappier, J.L., Hornish, R.E. and Lane, R.E. Total residue method for
U-36,059 (amitraz) in swine tissue. Upjohn Co. report no. 315-9760-70;
1976, (Unpublished).
Nappier, J.L. and White, M.C. Total residue method for U-36, 059
(amitraz) in cotton seeds. Upjohn Co. report no. 315-78-9760-001;
1978, (Unpublished).
NCI, Final Report. Carcinogenicity of chemicals present in man's
environment. Contract no. NIH-NCI-E-68-311. Reported by Bio-Research
consultants, 1973, submitted to WHO by The Boots Company.
Needham, D. and Campbell, J.K. Amitraz residues in the tissue of sheep
1,3,5 and 7 days after dipping in 0.05% amitraz. Boots Co. Ltd. report
no. AX 80016; 1980, (Unpublished).
Nix, N. Stability data for Amitraz Technical (Paraformaldehyde Sachets
Stabilised). Unpublished report dd. 11-12-1979; 1979, from The Boots
Company submitted to WHO.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
female mouse. Unpublished report no. BTS 81/7758; 1977a, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
male mouse. Unpublished report no. BTS 80/7792; 1977b, from Huntingdon
Research Centre, submitted to WHO by The Boots Company.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
(chlordimeform) on the central actions of reserpine in the mouse and
rat. Unpublished report no. P 74033; 1974a, from The Boots Company
submitted to WHO.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
chlordimeform) on the pressor response to tyramine in the pithed rat.
Unpublished report no. P 74032; 1974b, from The Boots Company
submitted to WHO.
Parkinson, R. BTS 27271 - an attempt to produce and quantify the
flushing response in the pig. Unpublished report no. P 75030; 1975,
from The Boots Company submitted to WHO.
Parkinson, R., Patton, D.S.G. and Yates, D.B.G. BTS 27419. Effects in
conscious dogs of small intravenous doses. Unpublished report no.
P 71535; 1971a, from The Boots Company submitted to WHO.
Parkinson, R., Sim, M.F. and Yates, D. Effects of the acaricide BTS
27419 and the related sulphur analogues BTS 30559 and BTS 30561 on the
cardiovascular system of the cat, particularly the ear vascular bed.
Unpublished report no. P 71576; 1971b, from The Boots Company
submitted to WHO.
Parkinson, R. and Yates, D.B. The effects of BTS 27419 on
Trafuril-induced erythema on the guinea-pig. Unpublished report no.
PM 71022; 1971, from The Boots Company submitted to WHO.
Patton, D.S.G. RD 27271: Effects on u/v erythema in guinea pigs.
Unpublished report no. PM 70100; 1970, from The Boots Company
submitted to WHO.
Patton, D.S.G. BTS 27419: Acute toxicity in baboons. Unpublished
report no. TX 73002; 1973a, from The Boots Company submitted to WHO.
Patton, D.S.G. BTS 27271: Effects in conscious dogs of small
intravenous doses. Unpublished report no. TX 73017; 1973b, from The
Boots Company submitted to WHO.
Patton, D.S.G. and Sutton, M.M. Acute toxicity studies on BTS 27419 an
acaricide. Unpublished report no. P 71544; 1971a, from The Boots
Company submitted to WHO.
Patton, D.S.G. and Williams, G.A.H. BTS 27419: 90-day toxicity study
in dogs. Unpublished report no. P 71547; 1971b, from The Boots Company
submitted to WHO.
Petzold, G.L., Swenberg, J.A. and Bedell, M. Evaluation of amitraz
(U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915 A and
U-54,914) in the DNA/Alkaline Flution assay. Unpublished report no.
7268/77/7268/001; 1977, from The Boots Company submitted to WHO.
Richards, M.E. Combined residues of amitraz and BTS 27271 in olives
from Italy, 1978. Boots Co. Ltd. report nos. AX 79018 and 80029; 1979
and 1980a, (Unpublished).
Richards, M.E. Combined residues of amitraz and BTS 27271 in apples
and pears from West Germany. Boots Co. Ltd. report no. AX 80001;
1980b, (Unpublished).
Shaw, J.W. BTS 27419 - acute intraperitoneal toxicity to rats.
Unpublished report no. PM 71057; 1971, from The Boots Company
submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral and intraperitoneal
toxicities to rats. Unpublished report no. TX 73006; 1973a, from The
Boots Company submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral toxicities to rats
of BTS 27419 and BTS 27919. Unpublished report TX 73010; 1973b, from
The Boots Company submitted to WHO.
Shaw, J.W. BTS 27419 metabolite: 21-day chronic oral toxicity in rats
of BTS 28369. Unpublished report no. TX 75058; 1975, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTS 27419. 90-day chronic toxicity
study in mice. Unpublished report no. TX 74016; 1974, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTU 27419 metabolite: 90-day chronic
oral toxicity in rats of BTS 27271. Unpublished report no. TX 75059;
1975, from The Boots Company submitted to WHO.
Shirley, D.B. Analysis of tissue from pigs treated with amitraz at
Thurgarton Research Station. Boots Co. Ltd. report no. F 75015; 1975a,
(Unpublished).
Shirley, D.B. The analysis of BTS 28369 in the urine of amitraz
production workers. Unpublished report no. F 75019; 1975b, from The
Boots Company submitted to WHO.
Shirley, D.B. The analysis of BTS 28369 in the urine of three amitraz
production workers over a period of three weeks. Unpublished report
no. F 75024; 1976.
Shirley, D.B. Amitraz residues in cattle from Wiltonside, South
Africa. Boots Co. Ltd. report no. F 77002; 1977a, (Unpublished).
Shirley, D.B. Residue analyses of tissues from sheep treated with
amitraz at Thurgarton Research Station, UK. Boots Co. Ltd. report no.
F 77004, 1977b, (Unpublished).
Shirley, D.B. Analysis of amitraz residues in sheep's milk. Boots Co.
Ltd. report no. F 78013; 1978, (Unpublished).
Sim, M.F. The diuretic action of the acaricides RD 27271 and RD 27419
in mice. Unpublished report no. PM 70111; 1970, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 in the lactating cow.
Unpublished report no. C 72007; 1972, from The Boots Company submitted
to WHO.
Somerville, L. Fate of 14C-BTS 27419 administered to rats in repeated
oral doses. Unpublished report no. AX 73011; 1973a, from The Boots
Company submitted to WHO.
Somerville, L. Fate of 14C-ring-labelled BTS 27419 in the calf.
Unpublished report no. AX 73006; 1973b, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 when administered to cats as a
single oral dose. Unpublished report no. AX 73018; 1973c, from The
Boots Company submitted to WHO.
Somerville, L. and Goodall, E. Residue analyses of amitraz and its
metabolite BTS 27271 on Danish apple crops. Boots Co. Ltd. report no.
AX 74023; 1974, (Unpublished).
Somerville, L. and Hamilton, D.Y. Studies on the accumulation and
elimination of radio-labelled residues from dogs' eyes following oral
administration of (14C)-BTS 27271. Unpublished report no. AX 74013;
1974, from The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch cucumber crops. Boots Co. Ltd. report
no. AX 73008; 1973a, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 1). Boots Co.
Ltd. report no. AX 73010; 1973b, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 2). Boots Co.
Ltd. report no. AX 73013; 1973c, (Unpublished)
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch apple crops. Boots Co. Ltd. report no.
AX 73014; 1973d, (Unpublished).
Somerville, L. and Hughes, K.W. The conversion of BTS 27419 to BTS
27271 in the dog stomach. Unpublished report no. AX 73021; 1973e, from
The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French apple crops. Boots Co. Ltd. report no.
AX 73016; 1973f, (Unpublished).
Somerville, L. and Nicholson, J.E. Amitraz. Metabolism in apples,
variety Cox's Orange Pippin. Boots Co. Ltd. report no. AX 72003; 1972,
(Unpublished).
Somerville, L. and Richards, M.E. The analysis of fruit for combined
residues of amitraz and BTS 27271. Boots Co. Ltd. report no. AX 80002;
1980, (Unpublished).
Somerville, L. and Spiers, M.J. Amitraz. Metabolism in apple leaves.
Boots Co. Ltd. report no. AX 72002; 1972, (Unpublished).
Sutton, M.M., RD 27271. Acute oral toxicity to mice. Unpublished
report no. PM 70043; 1970a, from The Boots Company submitted to WHO.
Sutton, M.M. RD 27271. Acute toxicity to rats. Unpublished report no.
PM 70042; 1970b, from The Boots Company submitted to WHO.
Sutton, M.M. Acaricides: RD 27419 and RD 27271 acute toxicity to
guinea pigs. Unpublished report no. PM 70108; 1970c, from The Boots
Company submitted to WHO.
Sutton, M.M. BTS 27419. Contact sensitization in the guinea pig.
Unpublished report no. PM 71010; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Effect on thermo-regulation in mice.
Unpublished report no. P 72609; 1972a, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27271: acute oral toxicity to rabbits. Unpublished
report no. TXM 72044; 1972b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rabbit. Unpublished
report no. TX 73029; 1973a from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rat. Unpublished report
no. TX 73028; 1973b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Effects on pregnancy, parturition and care of
the young in rate. Unpublished report no. TX 73031; 1973c, from The
Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Multigeneration feeding test in rats.
Unpublished report no. TX 73036; 1973d, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Three-week dermal toxicity to rabbits.
Unpublished report no. TX 73026; 1973e, from The Boots Company
submitted to WHO.
Sutton, M.M. and Metcalf, W. BTS 27419. Eye-irritancy in the rabbit.
Unpublished report no. TXM 72037; 1972, from The Boots Company
submitted to WHO.
Sutton, M.M. and Offer, J. BTS 27419: carcinogenicity and long-term
toxicity study in rats. Unpublished report no. TX 73043; 1972, from
The Boots Company submitted to WHO.
Sutton, M.M. and Williams, G.A.H. BTS 27419: 90-day toxicity study in
rate. Unpublished report no. P 71548; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. and Williams, P.A. BTS 27419: acute dermal toxicity to
rabbits. Unpublished 1972 report no. YM 72011; 1972 from The Boots
Company submitted to WHO.
Taylor, J. and Somerville, L. The conversion of amitraz to BTS 24068
in dog gastric juice. Unpublished report no. AX 77010; 1977, from The
Boots Company submitted to WHO.
Toyoshima, S., Fujita. H., Sato, H., Sato. R., Sato, S. and Kashima,
M. JA-119 (BTS 27419). Three-month feeding study on rats. Unpublished
report E 76014; 1972a, from The Boots Company submitted to WHO.
Toyoshima, S., Fujita H., Sato, H., Sato, R., Sato, S. and Kashima, M.
JA-119 (BTS 27419). Three month feeding study on mice. Unpublished
report no. K 76015; 1972b, from The Boots Company submitted to WHO.
Tuplin, J.A. and Wilcox, A. Amitraz. Ames-tests on a sample produced
by Nissan Chemical Industries. Unpublished report no. TX 78068; 1978,
from The Boots Company submitted to WHO.
Weisburger, E.K. et al. Testing of twenty-one environmental aromatic
amines or derivatives for long-term toxicity or carcinogenicity. J.
Environ. Path. Toxicol. 2, 325-356.
Wilcox, P. BTS 27419: Mutagenicity study in the intraperitoneal
host-mediated assay. Unpublished report no. TX 76028; 1976, from The
Boots Company submitted to WHO.
Wilcox, P. Amitraz impurity BTS 33220: in vitro bacterial
mutagenicity testing. Unpublished report no. TXA 79025; 1979, from The
Boots Company submitted to WHO.
Zimmer, D.M., Mazurek, J.H. and Bhuyan, B.K. Evaluation of amitraz
(U-36.059) and its metabolites in the Salmonella microsome test.
Unpublished report no. 72068/77/7269/002; 1977, from The Boots Company
submitted to WHO.
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Effect of amitraz on the oestrous cycle
In female SFP CFLP mice fed a diet containing 400 mg amitraz/kg feed
for up to 33 weeks a reduction in body weight and an increase in food
consumption was observed. Analysis of vaginal smears indicated that
the mean duration of oestrus as well as the incidence of a prolonged
oestrus was significantly increased due to treatment. No effects were
found on the level of ß-oestradiol in plasma (Brown et al, 1978;
Channon and Cryer, 1978).
Female rats maintained on a diet containing 200 mg amitraz/kg for 18
weeks had longer oestrus cycles than control rats of the same age,
resulting from prolonged periods of oestrus or dioestrus (Merryman and
Sutton, 1972).
Effect on pregnancy, parturation and care of the young
Amitraz was administered in doses of 0, 1, 3 or 12 mg/kg bw to
Boots-Wistar rats from day 1 of pregnancy until the young were weaned
at 21 days old. 12 mg/kg bw reduced the weight gain of the dams as
well as the mean number of young born and alive at day 4. No effects
were observed for mothers and offspring for the other dose groups
(Sutton, 1973 c).
Three-generation feeding study
Groups of 10 male and 20 female newly-weaned Boots-Wistar rats were
fed amitraz in dietary concentrations of 0, 15, 50 or 200 mg/kg.
After ten weeks the animals were mated. After the F1 generation was
weaned 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. At 200 mg/kg amitraz causes decrease
of growth and food consumption in the F0 generation, furthermore a
decrease in fertility and viability was found. The 200 mg/kg diet
group was terminated when the F1 generation was weaned, due to the
very low survival. In the 50 mg/kg group no effect was found on the
number of litters and mean litter size, however in all generations a
decrease in number of young alive at 21 days was observed. No further
effects due to treatment were found in this and the other dose group
(Sutton, 1973 d; Lancaster and Williams, 1978).
Special Studies on teratogenicity
Rat
Groups of 11-13 female rats received amitraz in doses of 0, 1, 3 or 12
mg/kg bw daily from day 8 to day 20 of pregnancy. The rate were
killed on day 21 and the uterine content was examined. Average litter
size, foetal viability and implantation index were not affected. In
the highest dose group foetal weight was less than in the controls,
and the calcification of the stermebrae was less advanced (Sutton,
1973 b).
Rabbit
Groups of 8-10 New Zealand rabbits were treated with amitraz in doses
of 0, 1, 5 or 25 mg/kg bw from day 6 to 18 of pregnancy and killed on
day 30. In the highest dose group number of litters and mean litter
size were decreased. Only in this dose group abortions were observed.
No increase in congenital abnormalities was found (Sutton, 1973 a).
Special studies on mutagenicity
Bacterial systems
Amitraz and its major metabolites (BTS 27271, 27919, 28369) were
tested in bacterial in vitro systems, with and without activation,
against Salmonella typhimurium TA 1535, 1437, and 1538 and
Escherichia coli Wp2 and Wp2 uvrA. In these systems no mutagenic
activity was detected (Everest and Wilcox, 1976 a, 1976 b; Tuplin and
Wilcox, 1978).
The impurity BTS 33220 (N,N'-bis-2,4-dimethylphenyl-N-methyl
formamidine) showed weak positive activity against TA 100 in the
presence of microsomes. Amitraz spiked with 1% BTS 33220, however,
did not show any mutagenic action (Wilcox, 1979; Everest and Wilcox,
1979).
In the TA 100 strain BTS 24868 and BTS 27919 exhibited weak but
consistent mutagenic activity (Zimmer at al, 1977).
Amitraz showed no increase in point mutational activity against
Salmonella typhimurium G 46, TA 1532 and TA 1964 when tested in
the mouse perivisceral host-mediated assay at single oral doses up to
400 mg/kg bw (Wilcox, 1976; Everest, 1976).
Dominant Lethal Studies
Female mouse
Groups of 48 female mice were treated orally for 5 consecutive days
with amitraz in doses of 0, 12 or 50 mg/kg bw.
Subgroups of 12 females were mated with untreated males on day 3, 9,
14 or 19 post-dosing. A slight increase in mean post-implantation
loss and decrease in mean viable litter size at 50 mg/kg was found
(Palmer and James, 1977 a).
Male mouse
Male mice of proven fertility were treated with amitraz for 5
consecutive days at doses of 0, 12 or 50 mg/kg. Following treatment
males were mated with untreated females for six consecutive weeks. A
significantly lower implantation rate was found at 50 mg/kg at the
first mating and a higher implantation rate at 12 mg/kg at the 5th
mating. No changes were found in respect to number of embryonic
deaths and post implantation losses (Palmer and James, 1977 b).
DNA damage
Amitraz, BTS 27271, 27919, 24868 and 28369 were tested at several dose
levels in the DNA damage/alkaline elution assay with or without
activation systems, using chinese hamster lung fibroblasts. In this
test system no indication was obtained that amitraz or its metabolites
induce DNA damage (Petzold et al, 1977).
Special studies on carcinogenicity
Mouse
Groups of 50 male and 50 female mice (CFLP strain) were fed a diet
containing 0, 25, 100 or 400 mg/kg for 80 weeks. The animals of the
100 and 400 mg/kg diet groups gained less weight than the control over
the first 40 weeks. The 25 mg/kg diet group gained more weight than
the control group from week 10 onwards and showed a 20% greater body
weight gain at the end of the experiment. Calculated over 80 weeks,
food consumption was increased for male mice at 100 and 400 mg/kg diet
and decreased at 25 and 100 mg/kg diet and increased at 400 mg/kg diet
for female mice. The survival rate was similar in all groups. An
increased incidence of lymphoreticular tumours was observed for female
mice at 400 mg/kg only; 49% compared with 23% in control animals. A
slight, although not statistically significant (p>5%) increase of all
types of liver cell tumours was found in both sexes fed 400 mg/kg. No
other pathological findings related to treatment were reported
(Burnett et al, 1976; Kakuk 1979).
Rat
Amitraz was administered at dietary concentrations of 0, 15, 50 or 200
mg/kg to groups of 40 male and 40 female Ash Wistar rats for 2 years.
Due to treatment in the highest dose group the animals tended to be
nervous, aggressive and excitable. In this group growth rate was
depressed for both sexes and food intake was slightly less in the
first weeks of the experiment. No dose-related anomalies were found
on organ weights and haematological, biochemical and histopathological
examination. Furthermore, no difference in incidence, type and time
of appearance of tumours in control and treated rats was observed
(Sutton and Offer, 1973).
2,4-Xylidine carcinogenicity study
Mouse
2,4-Xylidine (BTS 24868) was fed in the diet of 3 groups of HAM/ICR
mice (25 males and 25 females) in a concentration of 0, 1000 or 2000
mg/kg. Since these doses were not tolerated the experiment was run
again with doses of 0, 125 or 250 mg/kg diet. Dosing was
discontinuated after 18 months. After 24 months the animals were
killed and examined for tumours. In female mice an increased
incidence of lung tumours was observed in the highest dose group (NCI,
1973).
Rat
2,4-Xylidine (BTS 24868) was administered to groups of 25 male Charles
River rats for approximately 3 months at doses of 2000 or 4000 mg/kg
diet; thereafter the doses were changed to 0, 250 or 500 mg/kg diet
respectively, for approximately 2.5 months and subsequently, the doses
were set at 0, 500 or 1000 mg for the rest of the 18 months period.
At that time the feeding of the test compound was discontinued until
sacrifice 24 months after starting the experiment. A slight increase
in lung tumours in the highest dose group was observed. Furthermore
in the treated groups bladder and liver tumours were observed,
which were not found in the controls (NCI, 1973). However, in
contrast to their earlier conclusions, the authors finally stated that
there was no carcinogenic effect in the rat (Weisburger et al,
1978).
Special studies on pharmacological effects
In dogs amitraz and BTS 27271 produce similar effects when given
orally in doses of 4, and 0.5 mg/kg bw respectively either after
single or repeated administration. Signs included sedation, ataxia,
protrusion of the tongue, bradycardia and hypothermia (Morgan et al,
1975 b).
An experiment comparing oral and topical application of amitraz in
pigs revealed a decrease in rectal temperature after oral dosing (25
mg/kg bw and higher), whereas no effects were found after topical
application up to 200 mg/kg bw (Morgan et al, 1975 a).
No flushing could be demonstrated in pigs after topical or
intramuscular administration of amitraz and BTS 27271 although other
pharmacological changes were apparent (Parkinson, 1975).
Amitraz and BTS 27271 did not have any effect on trafuril-induced or
UV-induced erythema in the guinea pig (Patton, 1970; Parkinson and
Yates, 1971).
BTS 27271 was antidiuretic in mice at 90 mg/kg bw whereas amitraz in
the same dose was ineffective (Sim, 1970).
Intravenous administration of amitraz caused vasoconstriction in the
ear vessels of cats (1-10 µg/kg) and fall in blood pressure and
bradycardia (>20 µg/kg) in dogs. BTS 27271 evoked similar effects in
dogs (40 µg/kg). Both compounds had no effect on the response of the
blood vessels of the rabbit ear to antidromic stimulation or to
catecholamines (Anonymous, 1972; Parkinson et al, 1971 a; Parkinson
et al 1971 b; Patton, 1973 b).
Amitraz induced hypothermia in mice under various ambient conditions.
This effect was greater in intensity and duration in females (Sutton,
1972 a; Berry, 1976).
BTS 27419, BTS 27271 and BTS 21103 were tested for their potency of
central monoamine oxidase inhibition in mice and rats, by
investigating the influence on reserpine-induced ptosis and inhibition
of exploratory activity. No effects were observed in mice. At high
doses these compounds antogonise reserpine-induced ptosis in the rat,
suggesting a relative potency in the order BTS 27271 > BTS 27419 >
BTS 21103 (Parkinson, 1974 a).
BTS 27419, BTS 27271 and BTS 21103 potentiated the pressor response to
tyramine in the pithed rat at doses of 40, 80 and 80 mg/kg bw
respectively. Similar effects have been shown with monoamine oxidases
inhibitors (Parkinson, 1974 b).
Special studies on sensitisation and irritation
Neither amitraz nor BTS 27271 showed sensitisation activity in guinea
pigs (Sutton, 1971).
Amitraz was not irritant to the rabbit eye after a single
administration (Sutton and Metcalf, 1972).
Acute toxicity
Acute oral administration of amitraz caused CNS depression in all
species studied. Dogs were more susceptible than the others. Toxic
manifestations: ataxia, subnormal rectal temperature and
cardiovascular disturbance.
TABLE 2. Acute Toxicity of Amitraz
Species Sex Number Route LD50 (mg/kg) Reference
mouse M 5 oral >1600 Patton and Sutton, 1971
rat M 5 oral approx. 800 Patton and Sutton, 1971
Shaw, 1973a
rat M 5 i.p. approx. 800 Shaw, 1971, 1973a
rat M,F 6 inhalation (6 h LC50) Berczy et al, 1972
65 mg/kg
rat M 5 dermal > 1600 Patton and Sutton, 1971
guinea pig F 3 oral 400-800 Patton and Sutton, 1971
Sutton 1970c
rabbit F 2 oral > 100 Patton and Sutton, 1971
rabbit M,F dermal > 200 Sutton and Williams, 1972
dog M,F 2 oral approx. 100 Patton and Sutton, 1971
baboon M,F 2 oral 100-250 Patton, 1973a
BTS 27271 is more toxic than amitraz. Signs of poisoning are similar
to those shown by amitraz. A summary of acute toxicity data of
impurities degradation products and metabolites of amitraz is given in
table 3.
Short-term studies
Mouse
Groups of 10 male and 10 female CFLP-mice (age 4 weeks) were dosed
with 3, 12, 50 or 200 mg/kg/day amitraz by gavage (suspended in 0.4%
cellosize; 0.1 ml/10 g bw) for 90 days. Control groups of 20 males
and females received the same volume of vehicle alone.
Eight mice (5 males and 3 females) given 200 mg/kg/day died within 3
weeks after becoming progressively emaciated and inactive. The
surviving animals of this group were in poor condition. In the other
groups 5 animals died of causes probably not related to treatment.
The 50 and 200 mg/kg groups showed a decreased body weight gain over
the experimental period for both sexes. The body weight gain of male
TABLE 3. Acute Toxicity of Impurities, Degradation Products and Metabolites of Amitraz
compound species number route LD50 (mg/kg) vehicle references
BTS 27271 mouse 5 oral 100-200 HCl salt aq. sol. Sutton, 1970a
rat 5 oral 200 idem Sutton, 1970b
guinea pig 3 oral 200 idem Sutton, 1970c
dog 2 oral >20 gelatin capsules Morgan, 1973a
Morgan and Williams, 1973a
rabbit 2 oral >25 HCl salt aq. sol. Sutton, 1972b
BTS 27919 mouse 5 oral >1600 idem Anonymous, 1971a
rat 4 oral approx. 1600 idem Shaw, 1973b
dog 2 oral >100 gelatin capsules Morgan, 1974a
Morgan and Turnhull, 1973
BTS 28369 dog 2 oral >250 idem Morgan and Sheperd, 1974
Morgan 1974b
BTS 24868 mouse 5 oral 800 - Anonymous, 1971a
BTS 33220 rat 5 oral 800-1600 10% acacia mucilage O'Donovan and Smithson, 1978
BTS 28037 dog 2 oral >250 gelatin capsules Morgan, 1973b
Morgan and Williams, 1973b
and female mice of the 3 and 12 mg/kg groups was slightly decreased.
GPT activity increased in both sexes from 50 mg/kg. Male mice showed
a slight increase in haematocrit at 3 and 12 mg/kg. Reducing
substances in blood were found to be decreased in both sexes at 12 and
50 mg/kg. Organ weight/body weight ratio of the kidney was increased
in males from 12 mg/kg; of the liver increased for both sexes at 50
and 200 mg/kg and of the spleen for males at 50 and 200 mg/kg and for
females at 200 mg/ kg only.
Slight to moderate hepatocyte and/or nuclear enlargement was observed
dose-relatedly at 12, 50 and 200 mg/kg. Furthermore, in the liver of
some females of the highest dose group, intranuclear inclusion, bile
duct proliferation and inflammatory infiltration in the portal tracts
and nodular hyperplasia were observed (Shaw and Williams, 1974).
Four groups of 13 female and 13 male ICR-SLC mice received amitraz in
doses of 0, 3, 12 or 50 mg/kg bw, in the diet (presuming that the
daily consumption was 5 g/animal) for 90 days. Six males died at an
early stage of the experiment. According to the authors death was not
due to treatment. In both sexes growth was depressed in the 12 and 50
mg/kg groups. Food and water intake were slightly decreased at 12 and
50 mg/kg; for males this was more pronounced than for females. In
male mice a slight decrease in food efficiency was observed at 50
mg/kg over the whole experimental period. For male mice relative
brain weight was increased at 50 mg/kg and heart weight at 12 and 50
mg/kg. The relative kidney weight of female mice at 50 mg/kg was
decreased. SGPT and aldaline-phosphatase were decreased whereas the
albumin-globulin ratio was increased in female mice of the two highest
dose groups. Male mice only showed a higher albumin-globulin ratio at
50 mg/kg. At the end of the experiment macroscopically slight black
discolouration of the liver was seen predominantly in the highest dose
group. The only histopathological change probably directly related to
the administration of the compound was a slight centrolobular
degeneration in the liver at 3, 12 and 50 mg/kg (Toyoshima et al,
1972 b).
Rat (inhalation)
Three groups of 6 female and 6 male rats were exposed daily for six
hours to amitraz at concentrations of 0.01, 0.1 or 1.0 mg/l air
respectively for 14 days over a period of three weeks. During
exposure to 0.1 mg/l dyspnoea, eye-irritation and hyposensitivity to
noise were observed. In addition at 1.0 mg/l ataxia, increased nasal
secretion, polyuria, body tremors and slight coma were found. Body
weight gain was markedly reduced in both sexes at 0.1 and 1.0 mg/l
air. Haematological studies revealed a decreased PCV, haemoglobin and
red blood cell count and increased number of neutrophils in both sexes
and a decreased NCHC and number of lymphocytes in males of the highest
dose group (no other groups examined). At 0.1 and 1.0 mg/l
significant alterations of the relative weights of brain, kidney,
thyroid and adrenal glands, and gonads were found for males, and of
brain, kidney and adrenals of female rats. Liver, heart and pituitary
for males and liver for females were increased in the highest dose
group only. No pathological findings related to treatment were found
(Berozy et al 1973).
[text missing] abnormalities, dosing was restarted for 1 week in which
toxic reactions returned. Rats of the 12 mg/kg group appeared
irritable and excitable. In male rats body weight gain was depressed.
A significantly depressed relative liver weight was seen at 50, 12 as
well as 3 mg/kg for males but only at 50 mg/kg for females.
Alkaline-phosphatase activity was decreased at 50 and 12 mg/kg (Sutton
and Williams, 1971).
Rat
Groups of 10 Wistar rats of each sex received amitraz in doses of 0,
3, 12 or 50 mg/kg bw in the diet (presuming the daily food consumption
was 20 g/animal) for 90 days. Body weight gain was decreased in the
50 mg/kg group for both sexes and in the 12 mg/kg group for females
only. Food and water consumption were decreased in all dose groups.
The relative weight of brain, heart, lung, liver, kidney, spleen and
uterus or testes were increased for both sexes in the highest dose
group. In addition the adrenals were decreased and thymus was
increased in weight in male rats of the highest dose group. Male rats
showed a dose-related decrease in number of blood platelets in the 12
and 50 mg/kg group. Female rats showed a significant decrease in
eosinophils in the highest dose group. In serum of male rate of the
highest dose group a decrease was observed in the Alk Pase activity
and blood sugar concentration, and an increased serum potassium
concentration. Urinalysis revealed an increased number of rats with
proteinurea from the 12 mg/kg group onwards. In male rate urine
potassium concentration was significantly decreased at 12 and 50
mg/kg, in females only at 50 mg/kg (Toyoshima et al, 1972 a).
Rabbit (dermal)
Amitraz dissolved in acetone was applied to the intact skin of groups
of New Zealand rabbits (4 males and 4 females) in 15 doses of 0, 50 or
200 mg/kg bw over a 2l-day period. One male and three females at 200
mg/kg and one male at 50 mg/kg died intercurrently. Sedation was
observed in males at 50 mg/kg and in both sexes at 200 mg/kg. In
males of both dose groups slight to moderate erythema, desquamation of
skin and subcutaneous hemorrhage were observed. In both sexes
decreased body weight and food consumption were found in the 50 and
200 mg/kg dose groups. In males at 200 mg/kg underweight testes with
tubular degeneration were found (Sutton, 1973 e).
Dog
Amitraz in gelatine capsules was given to groups of two male and two
female beagle dogs in oral doses of 0, 0.25, 1 or 4 mg/kg once daily
for 90 days. Clinical signs were observed in all dosed groups
consisting of CNS depression, ataxia and vomiting during the first
days within 3-6 hours after dosing. These dogs were subdued
throughout the experimental period. When examined at intervals during
the treatment period the dogs consistently had subnormal rectal
temperatures and pulse rates. These parameters returned to normal
within 6-24 hours after administration. The animals of the 1 and 4
mg/kg dose groups showed a significant increase in blood sugar
concentration which was maximal 6 hours after dosing and returned to
normal within 24 hours. In the same dose groups an increased amount
of glucose and total reducing substances was found in the urine in
weeks 2 and 3. In the highest dose group an increased liver weight
was observed in both sexes. Except in control animals, livers showed
enlargement of the central and midzonal hepatocytes. Hyperplasia of
the small periportal hepatocytes and increase in binucleate cells were
particularly evident in the highest dose group. At all dose levels
thinning of the zonae fasciculata and reticularis, sometimes
associated with slight hyperplasia of the zona glomerulosa in the
adrenales, was observed (Patton and Williams, 1971).
Metabolites
Rat (90-day test with BTS 27271)
Groups of 10 male and 10 female Boots-Wistar rats were dosed orally by
gastric intubation with BTS 27271 for 90 days at doses of 0.25, 1, 3
or 12 mg/kg bw/day. In the 12 mg/kg group the rats were nervous and
difficult to handle. Body weight was slightly lower at 3 and 12 mg/kg
compared to the controls, the males being more affected than the
females. At the end of the experiment the number of erythrocytes was
decreased in the 3 and 12 mg/kg group for females and in the 12 mg/kg
group for males. Haemoglobin and haematocrit values were decreased at
12 mg/kg for males only. Owing to the variation in the number of
lymphocytes the leucocyte counts were decreased for male and increased
for female rats in the highest dose group. Concomitantly a
significant decrease in number of eosinophils was found. Urinalysis
revealed an increased GPT activity in females, and a slight increase
in urine volume and decrease in specific gravity in the males of the
highest dose group. Together with these changes an increase in BUN
was found. In terminal plasma samples a significant increase in
alkaline-phosphatase activity in both sexes given 12mg/kg was found.
The relative weights of adrenals, ovaries and uterus in the females
and the testes in the males given 3 and 12 mg/kg were increased. In
the highest dose group the weight of liver for females and spleen for
males was increased. Microscopic examination of rats given 3 or 12
mg/kg showed a slight increase in lymphoid infiltration with some
leucocytosis and a loss of glycogen in the liver (livers of female
rats were not examined at 3 mg/kg). In the highest dose group the
hearts of the female rats showed slight cellular accumulation (Shaw
and Williams, 1975).
Dog (90-day test with BTS 27271)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg BTS 27271/kg bw in gelatin capsules daily for three months. The
test compound was diluted in lactose to 1% BTS 27271 as free base.
Clinical signs in the 0.25 and 1 mg/kg group were abnormal quietness
and drowsiness. Between 1 and 4 hours after dosing the mean body
temperature and mean heart rates decreased significantly for the 0.25
and 1 mg/kg dose groups. In the highest dose group a significant
increase in liver weight was found. A slight reduction in thymus
weight was observed at 0.25 and 1 mg/kg. In the highest dose group
the urine volume was found to be increased. No histopathological
anomalies were found (Chesterman et al, 1973).
Rat (21-day test with BTS 28369)
BTS 28369 was administered orally to groups of 4-6 male and female
rate (Boots-Wistar) at dose levels of 0, 40, 100 or 250 mg/kg bw/day
for 21 days. In the highest dose group a slightly decreased weight
gain and BUN level was observed for males and an increased relative
weight of the spleen for females. No pathological changes due to
treatment were found (Shaw, 1975).
Dog (90-day test with BTS 28369)
Groups of 4 male and 4 female beagle dogs were dosed 0, 16, 40 or 100
mg BTS 28369/kg bw/day orally in gelatin capsules for 90 days. At 100
mg/kg bw slightly elevated urinary levels of total reducing substances
were found. No dose related effects were observed on behaviour, ECG,
heart rate, rectal temperature, body weight, food consumption,
haematology, blood biochemistry, organ weights and histopathology
(Morgan et al 1974).
Long-term studies
Rat
See under "Special studies on carcinogenicity".
Dog (2 years)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg amitraz/kg bw/day orally in gelatin capsules for 2 years. Three
hours after dosing all dogs given 1 mg/kg bw exhibited CNS depression
on day 1 and 2, blood sugar level was slightly increased at weeks 40
and 53 (the only weeks measured). No effects on haematological,
biochemical and histopathological parameters were reported (Morgan
et al, 1973).
OBSERVATIONS IN HUMANS
After a few complaints from people involved with the development of
amitraz, all workers in this department were interviewed by the
medical staff, which led to the following conclusions:
1) Skin flushing due to capillary dilatation may be caused by amitraz
or its precursor BTS 27271, or both.
2) The response appears to be the result of systemic absorption rather
than topical application.
3) Absorption is more likely to occur when large quantities are
processed. So far skin effects have not followed the use of
formulated materials in the course of animal or crop protection
trials.
4) Apart from the observation that the capillary circulation in the
skin appears to have been sensitised to trauma such as gentle friction
and radiant heat, no other information can be offered to explain the
phenomena described.
5) The effects appear to be temporary and disappear soon after contact
with the materials is discontinued (Moore, 1972).
Amitraz (about 10 mg) was applied to the arms of 9 volunteers as 0.02
ml of a 50% acetone solution. After 6 hours the arms were cleaned.
One volunteer, who had previously reacted to the compound, showed a
slight erythema localized to the patch area.
Three volunteers who had previously reacted to this compound were
tested using a single application of a paraffin-based ointment
containing 50% amitraz. One subject reported a delayed reaction
occurring 14 h after the initial application consisting of a reddening
of his previous reaction to this compound; no throbbing or other
symptomatology was observed and the reaction had faded by the morning
(Anonymous, 1971 b and c).
Six volunteers received a single oral dose of 2 mg BTS 27271 in a
double-blind crossover study. Blood pressure, pulse rate and oral
temperature were different comparing the volunteers receiving the
active treatment to those receiving placebo. This difference existed
just as much initially as during the observation period. Mental
alertness scores show that a number of subjects felt relatively more
alert on placebo (Hall et al, 1975).
In urine of production workers and volunteers the expected terminal
metabolite, BTS 28369 could be detected (Shirley, 1975 b, 1976;
Hughes, 1975).
RESIDUES IN FOOD
USE PATTERN
Amitraz is used for the control of pests on both animals and crops and
is used in countries throughout the world as an insecticide and
acaricide.
Treatment of crops
As an acaricide, amitraz is successfully used against red spider mites
on deciduous fruit crops, citrus, cotton and certain other crops. As
an insecticide it is used to control pear psylla and whitefly, and as
an ovicide against the major cotton lepidopterous pests.
Recommendations for its use on cotton, deciduous top fruit, citrus,
ornamentals and vegetables are shown in Tables 4 and 5.
Treatment of animals
Amitraz is effective against ticks, mange mites, lice and keds on
cattle, sheep, goats and pigs (Tables 6 and 7).
The species of skin parasites controlled by amitraz are shown in Table
8.
TABLE 4. Use Pattern on Crops
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
COTTON Lepidoptera
Cotton Bollworm
Heliothis spp.
Pink Bollworm
Pectinophora gossypiella
Red (Sudan) Bollworm
Diparopsis castanea Spray when eggs first 300,400, 1.5, 2.0,
appear in the crop 600 3.0
Cotton Leafworm and repeat at regular
Spodoptera spp. intervals throughout 500,600, 2.5, 3.0,
Leaf Perforator the season 200 3.5
Bucculatix thurberiella
Spiny (Spotted) Bollworm
Earias spp.
Whitefly Spray when whitefly first appear 500,600, 2.5, 3.0,
Bemisia tabaci in the crop and repeat at regular 700 3.5
intervals throughout the season.
Thrips spp.
Aphida Spray when these pests first 500, 600, 2.5, 3.0,
Aphis spp. appear in the crop and 700 3.5
Jassida repeat at regular intervals
Empoasca spp. throughout the season
Mites
Tetraaychus spp.
DECIDUOUS Panonychus ulmi Apply the first spray at 60-80% 20-60 1-3 400-1200 2-6
TOP FRUIT egg hatch and repeat at 2-3 week
intervals.
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Mites Spray at the first sign of infestation 40-60 2-3 800-1200 4-6
Tetranychus spp. including and repeat at 2-3 week
T. urticae, T. cinnabarinus intervals.
and T. pacificus
Aculus schlectendali Spray at the first sign of 20-30 1.0-1.5 400-600 2.3
Epitrimerus pyri infestation and continue at
regular 2-3 week intervals.
Codling Moth Apply the first spray immediately
Cydia pomonella after adult emergence and repeat
at 2-3 week intervals for at
least 5 applications. 50-70 2.5-3.5 1000-1400 5-7
Aphids
Myzus persicae Apply at the first sign of
Aphys pomi infestation and repeat at regular
Dysaphis pyri 2-3 week intervals. 50-70 2.5-3.5 1000-1400 5-7
Eriosoma lanigerum Will give good suppression
Phorodon humuli of aphids in an intensive
Dysaphis plantaginea spray programme against mites.
Apple Maggot
Rhagoletis pomonella Used in a full season's
White Apple Leaf Hopper programme against mites will
Typhlocyba pomaria give useful suppression of 50-70 2.5-3.5 1000-1400 5-7
San Jose Scale these pests.
Quadraspidictus perniciosus
Plum Curculio
Conotrachelus nenuphar
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Pears only Pear psylla Spray when attack first occurs and
Psylla pyricola repeat at regular intervals. The 30-50 1.5-2.5 600-1000 3-5
higher rate is required to give
consistently good control of
over-wintering adults.
Apples Only Leaf miners
Leucoptera scitella Apply the first spray at petal fall
Phyllonorycter blancardella and repeat at 2-3 week intervals 40-50 2-2.5 800-1000 4-5
with a minimum of 4 applications
CITRUS Mites
Panonychus citri Apply first spray at 60-80% egg 20-50 1-3 400-1200 2-6
hatch and repeat at 2-3 week
intervals.
Tetranychus spp. Spray at first sign of infestation 40-60 2-3 800-1200 4-6
and repeat at 2-3 week intervals.
Phyllocoptruta oleivora Spray at first sign of infestation 20-30 1-1.5 400-600 2-3
and continue at regular 2-3 week
intervals.
Scale Insects
Ceroplastea spp. Apply the first spray during the 50-60 2.5-3 1000-1200 5-6
Saissetia spp. crawler (larval) stage and repeat
at regular intervals.
ORNAMENTALS
Carnations, Mites
Chrysanthemums, Panonychus ulmi Apply the first spray at 60-80% 30-50 1.5-2.5 600-1000 3-5
Roses, Pot plants egg hatch with repeat sprays at
and other annuals 2-3 week intervals.
and perennials
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
VEGETABLES Tetranychus urticae Spray at the first sign of infestation)
SOFT FRUIT Tetranychus pacificus regular intervals )
and other Tetranychus )
species )
Acalitus essigi )
Aphids Under cold conditions ) 46-60 2-3 800-1200 4-6
Aphids gossypii and on some crops under glass, )
Aulocorthum spp. the high rate may be necessary )
Macrosiphum spp. to achieve consistently good )
control
TABLE 5. Use of Amitraz on Crops. Registrations (at August 1980) and Periods
Between Last Application and Harvest
Country Withholding Crop1
Period
Argentina 4 weeks apples
Australia 4 weeks pome fruit and stone fruit
Belgium 4 weeks apples, pears
Bulgaria not available apples
Chile 2 weeks fruit, vegetables
Cyprus 2 weeks apples, peaches, citrus, cucurbits
Czechoslovakia 1 week cucumbers
4 weeks apples, pears green beans, peppers, hops
Denmark 2 weeks apples, pears
East Germany 4 days cucumbers, tomatoes
4 weeks apples, pears
Ecuador 2 weeks cotton, stone fruit, citrus,
strawberries, tomatoes, cucumbers
El Salvador not available cotton
France 30 days fruit trees, pears
Greece 1 week apples, pears, citrus, vegetables
Guatemala not available cotton
Hungary 10 days apples, plums, wild strawberries,
vines, sugarbeet, soybeans
Italy 2 weeks citrus, vegetables, vines,
stone fruit
4 weeks apples, pears
Japan 2 weeks apples, pears, citrus
Jordan not available top fruit
Morocco 4 weeks cotton, top fruit, vegetables
Netherlands 4 weeks apples, pears
New Zealand 2 weeks pome fruit and stone fruit
Nicaragua not available cotton
Pakistan not available cotton
Peru not available cotton
Portugal 4 weeks apples, pears
Romania not available top fruit
South Africa 2 weeks apples
4 weeks cotton
South Korea not available apples, pears, citrus
Spain 1 week fruit, maize
Switzerland 3 weeks apples, pears3
Taiwan not available apples, pears, citrus
Turkey not available apples, pears, citrus, cotton
UK 2 weeks apples, pears
7 weeks2 hops
USA 4 weeks pears
1 Use on ornamentals not included.
2 Shorter withholding period under review - likely to be 4 weeks.
3 Based on informal information, subject to written confirmation
TABLE 6. Use Patterns on Animals
Animal Pests Timing Applications
Cattle ticks A second application 10-14 As a spray containing
mange mites days later may be necessary 0.025% a.i.
lice for full control of mange and lice.
Pigs mange mites Two applications, at low concentration As a spray.
lice 10-14 days after the first High concentration
will provide control. Alternatively 0.1%
a single application at high concentration Low concentration
may be recommended. 0.05% a.i.
Sheep ticks As a dip at 0.05% a.i.
mange mites Replenishments should be
lice made with 0.075% amitraz
keds
Goats ticks A second application 0-14 As a spray containing
mange mites days after the first my 0.05% a.i.
lice be necessary for full control
of mange and lice.
TABLE 7. Use of Amitraz on Animals. Registrations (at August 1980) and Periods Between Last
Application and Slaughter
Country Formulation1 Use/Animals Withholding Period
Argentina 12.5% e.c. Dip for sheep None
Australia 50% d.p. Spray for cattle None
50% d.p. Dip for cattle None
Brazil 12.5% e.c. Spray for cattle and Meat 14 days2
sheep Milk 1 day
Colombia 12.5% e.c. Dip for cattle Meat 14 days2
12.5% e.c. Spray for cattle Milk 1 day
Costa Rica 12.5% e.c. Spray for cattle None
Cyprus 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Denmark 12.5% e.c. Spray for pigs and Meat 6 days
cattle Milk 1 day
Jordan 12.5% e.c. Spray for sheep and goats None
Netherlands 12.5% e.c. Dip for pigs and sheep Pigs None
Sheep 4 weeks
Nicaragua 12.5% e.c. Spray for cattle None
Rhodesia 12.5% e.c. Spray for cattle None
12.5% e.c. Dip for cattle None
South Africa 12.5% e.c. Spray for cattle None
25% d.p. Spray for cattle None
25% d.p. Dip for cattle None
Spain 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Sweden 12.5% e.c. Spray or dip for cattle, Sheep 7 days
sheep and pigs Pigs and cattle 1 day
United Kingdom 12.5% e.c. Spray for pigs and Meat 1 day
cattle Milk None
12.5% e.c. Dip for sheep Meat 7 days
Milk None
Venezuela 12.5% e.c. Spray for cattle Meat 14 days2
Milk 1 day
Yugoslavia 12.5% e.c. Cattle None
1 Emulsifiable concentrate is denoted by "e.c." and dispersible powder by "d.p.".
2 These withholding periods were agreed upon early in the development programme
before most residue work had been carried out.
TABLE 8. Species Of Skin Parasites Controlled By Amitraz
(i) Ticks
Boophilus microplus
B. decoloratus
Rhipicephalus evertsi
Amblyomma hebraeum
A. cayennense
Hyalomma spp.
Ixodes ricinus
I. persulcatus
I. holocyclus
Haemaphysalis bispinosa
H. longicornis
ii) Mites
Psoroptes spp.
Sarcoptes scabiei
Dermanyssus gallinae
Demodex canis
Psorergates spp.
Chorioptes bovis
iii) Lice
Linognathus vituli
L. ovillus
Damalinia bovis
D. ovis
Haematopinus eurvsternus
Solenopotes capillatus
Haematopinus suis
iv) Keds
Melophagus ovinus
RESIDUES RESULTING FROM SUPERVISED TRIALS
The metabolite (II) (see figure 1) closely resembles amitraz in its
toxicological effects and may be solely responsible for the signs seen
when amitraz is ingested (see Toxicity of impurities, degradation
products and metabolites, Table 3). Effort was therefore concentrated
on the measurement of the combined residues of amitraz and (II) in
most countries (see Table 1 for structures).
In the USA however, most of the trials have measured total residues as
'2,4-xylidine moiety (IV). For details of the analysis methods for
both animal and vegetables products see "METHODS OF RESIDUE ANALYSIS".
Residues in crops
a. Apples and pears
Residues have been determined on apples and pears from many countries
representing a wide variety of climatic conditions. Most trials have
demonstrated that the total residue (amitraz plus (II), expressed as
(II)) will not exceed 0.5 mg/kg, given an interval of 14 days between
application and harvest, following use of amitraz at the recommended
level, although the trials on pears in Italy gave somewhat higher
residues. See Table 9. for a summary of the trials and the results.
Total residues determined in the USA, measured as mg/kg of
'2,4-xylidine', indicate a similar decay pattern. For details and
results of the trials in the USA, see Tables 6/10/11.
b. Stone fruits
A summary of residue trials and results on stone fruits is given in
Table 7.
Peaches
Residues of amitraz and its metabolites (II) and (III) have been
measured on peaches grown in France. The results obtained are very
similar to those obtained for apples and pears.
It is concluded that the total residue (amitraz plus (II)) will not
exceed 0.5 mg/kg, given an interval of 14 days between application and
harvest.
Cherries
Combined residues of amitraz and its metabolite (II) were measured on
cherries in Denmark. Residues ranged from 0.09 to 0.33 mg/kg, and
seem unlikely to exceed 0.5 mg/kg given an interval of 28 days from a
single application at recommended rates to harvest of crop.
c. Oranges
Total residues on treated oranges, grown in two areas of the USA, were
measured. Initial deposits on the fruit fell with half lives of about
3 weeks to leave residues of around 0.5 mg/kg (expressed as amitraz)
after 14 days. Fruit harvested after 20 weeks contained a maximum
residue of 0.060 mg/kg equally distributed between peel and fruit (see
Table 13).
TABLE 9. Supervised Residue Trials on Pome Fruits in Various Countries
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
APPLES
United Kingdom 5 1 1.4 1125 0.39- 0.18- 0.18- 0.18- 0.16- 0.07- 0.07- Hughes and Somerville,
0.48 0.44 0.41 0.35 0.41 0.17 0.30 1973a
Hughes, Jones and
Somerville, 1974
Netherlands 2 2 0.54 600 0.06- 0.04- Somerville and
0.07 0.05 Hughes, 1973d
1 1 0.72 1800 0.20 0.17 Goodall and Somerville,
1974 b
France 1 2 0.60 880 0.60 0.47 0.42 0.32 0.17 ) Somerville and
1 1 0.60 1000 0.20 0.32 0.38 0.28 0.03 ) Hughes, 1973f
USA 1 3 1.0 4500 0.37, 0.21 0.14 0.17 0.14 0.19 )
0.38 )
1 3 2.0 4500 0.51, 0.46 0.42 0.35 0.38 0.18 ) Anon., 1973
0.54 )
1 1 1.0 4500 0.07 0.05 0.06 )
1 1 2.0 4500 0.01 0.12 0.11 )
Denmark 1 1 0.9 2250 0.44 0.05 0.04 ) Somerville and
1 1 0.9 2250) 0.85 0.11 0.17 0.03 ) Goodall, 1974
+2 0.67 2250) )
Italy 1 2 0.04 in 100 l
at 15 l per
tree 0.45 0.34 0.23 Goodall and
Somerville, 1974a
TABLE 9. Continued...
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
Switzerland 1 1 0.2% 0.54 0.44 0.28 0.25 Anon., 1977
Fed. Rep. of 5 3 0.75 1500 0.38- 0.33- 0.29- 0.26- 0.16- Richards, 1980b
Germany 1.01 1.03 0.71 0.72 0.781
PEARS
UK 2 1 1.4 1125 0 36, 0.37, 0.12, 0.13, 0.07, 0.09 Hughes, Jones and
0.46 0.38 0.27 0.17 0.15 0.09 Somerville, 1074
USA 1 1 1.32 2950 0.54 0.49 0.10 ) Anon., 1973
1 1 2.64 2950 1.00 0.92 0.84 )
Italy 2 2 0.60 1000 1.80, 1.42 0.02 0.02, 0.01 )
1.90 0.84 0.56 0.18 0.11 ) Goodall and
1 2 (50 g in 100 l. 1.54 0.94 0.53 0.43 0.26 ) Somerville, 1974a
to run off) )
Japan 1 2 1.5 6000 0.30 0.15 0.19 0.06 Summary only
0.21
Switzerland 1 1 0.2% 0.5 0.56 0.39 0.24 Anon., 1977
0.63
Fed. Rep. of
Germany 1 3 0.75 1500 0.10 0.10 0.10 Richards, 1980b
1 The highest figures were all obtained from one anomalous trial. In the others residue levels from 14 days were not above 0.50 mg/kg.
TABLE 10. Supervised Residue Trials on Apples in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Michigan 9 1.3 3590 1.03 1.16
1.79 1.15
Michigan 1 1.2 3590 0.72 0.57 0.28
0.86 0.91 0.38
1.34 1.11 0.74
1.3 3590 0.64 0.66 0.35
Michigan 6 1.2 3590 2.33 1.68 1.79
2.31 1.32
2.33 3.03 2.13
2.89 1.74
2.45 1.66 1.75
2.66 2.19
1.3 3590 2.15 1.12 1.48
0.98 1.87
Michigan 3 1.4 1870 1.49 1.37 1.02
1.66 1.28 0.88
Michigan 1 1.4 1870 0.58 0.36 0.46
0.67 0.25
1.39 374 1.54 0.90 0.53
0.90 0.78
Michigan 1 0.56 140 0.11
0.14
New York 3 1.22 3272 0.43 0.28 0.32
0.40 0.50 0.44
New York 1 1.39 3740 0.32 0.28 0.20
0.52 0.35 0.24
North 3 1.07 2870 0.45 0.76 0.25
Carolina 0.72 0.59 0.42
TABLE 10. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Oregon 1 1.86 467 0.99 1.68 0.87
1.46 0.38 0.81
Washington 1 1.86 3740 0.96 0.89 1.02
0.51 1.00
California 2 0.70 467 1.00 0.63 0.46
0.63 1.15 0.54
0.70 3740 1.71 1.79 0.80
1.24 1.44 1.13
Wisconsin 2 1.39 3740 0.79 0.24 0.24
0.54 0.30 0.27
Anon., 1975
TABLE 11. Supervised Trials on Pears in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
California 3 1.85 1870 0.78 0.66 0.69
0.50 0.69 0.54
California 3 1.86 467 0.52 0.30 0.32
0.45 0.34 0.42
Michigan 3 0.88 2945 0.30
0.47
1.46 2945 1.18 0.62 0.49
0.96 0.42 0.56
Michigan 1 0.56 1122 0.31 0.43 0.32 0.31
0.30 0.41 0.38
Michigan 1 1.32 2945 1.20 0.81 0.66
1.36 0.86 0.25
1.67 1.04 0.27
1.26 0.75 0.31
Michigan 3 1.46 2945 0.89 1.17
1.17 1.36
1.19 0.94
1.22 1.03
1.20 1.03
1.36 1.40
Michigan 2 1.39 1402 0.82 0.65 0.34
1.39 280 1.16 1.09 0.87
0.47
New York 5 1.85 3740 1.69 1.07 0.97
0.87 0.68
New York 6 1.63 3272 1.74 1.37 1.22
2.38 2.38 1.37
Oregon 5 1.87 467 0.92 0.78 0.95
1.28 0.75 0.43
TABLE 11. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
Oregon 6 2.79 5610 1.80 1.48 1.36
2.75 1.22 1.07
Washington 5 1.86 1309 1.19 1.02 0.96 0.51
0.28
0.34 1.10 0.75 0.30
0.40
Washington 5 1.86 3740 1.12 0.55 0.85
0.99 0.65 0.72
Washington 6 8.198 g/l 0.12 0.12
undiluted 0.14 0.12
Anon., 1975
TABLE 12. Supervised Residue Trials on Stone Fruits
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Applications Residues in mg/kg intervals (days)
No. Rate per hectare after application References
Country of No. kg litres 0 7 14 21 28 35 49 63
Crop Trials
Cherries Denmark 2 1 0.675 3,375 0.28 0.09 Humphrey and
Somerville,1976
Sour Cherries Denmark 3 1 0.40 1,000 0.33 0.09 0.17 Hayto, 1978d
Plums UK 2 1 1.26 g/l 0.041 0.241 Hayto, 1977c
Peaches France 4 1 0.60 1,000 0.57- 0.32- 0.24- 0.15- 0.04, )Somerville and
1.03 1.03 0.30 0.24 0.06 )Hughes, 1973b,c
1 1 0.32 1,900 0.72 0.21 0.10 0.21 0.06 Somerville and
Hughes, 1973c
1 These two results were obtained on different varieties of plum at different locations.
d. Cotton
Residues of amitraz and its metabolite (II) have been measured in
cotton from several different territories. Most of the determinations
have been made on cotton seed following a normal spray programme. In
the case of South Africa, the seed was fractionated into cake and oil,
which were analysed separately; much shorter last spray to harvest
intervals were used than in a normal spray programme. The combined
residues (expressed as (II)) never exceeded 0.4 mg/kg. A summary of
these cotton residue trials can be found in Table 14.
Total '2,4-xylidine' residues (expressed as amitraz) measured in the
USA indicated that after 14 days residues were below 0.5 mg/kg except
in one instance. See Table 15.
e. Hops and beer
Treated samples of hops, both green and dried, have been analysed for
residues of amitraz and its metabolite (II). The combined residues, 7
weeks after the final application, were 0.03 and 0.11 mg/kg (expressed
as (II)) for the green and dried hops respectively. No detectable
residues were found in beer made with treated hops.
f. Cucumbers
Residues of amitraz and (II) have been measured in cucumbers treated
in the UK, Netherlands and South Africa. In all cases the combined
residues after three days never exceeded 0.3 mg/kg in the whole fruit
or the skin.
g. Olives
Residues of amitraz and its metabolite (II) have been measured in
olive oil and pressed olives treated in Italy. The results are in
Table 16.
h. Eggplants
Eggplants were treated with amitraz in Italy. Combined residues were
below 0.3 mg/kg after 4 days.
i. Onions
In a similar study, amitraz was applied to onions. Combined residues
after 28 days were an average of 0.06 mg/kg.
Table 16 summarises the residue data on vegetables, hops and beer.
TABLE 14. Supervised Residue Trials with Cotton in Various Countries: Residues in cottonseed, seedcake and oil
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
No.
Rate of Application of Residues in mg/kg at intervals (days) after application References
Country kg/ha Treatments 5 19 21 28 37 41 77 80
Turkey 0.8 3 <0.01 ( Hamilton,
1.0 3 <0.01 ( 1977a
0.8 in 4001 followed 2 <0.01
by 0.6 in 6001 (in seed oil) Hayto, 1978f
Guatemala 0.29 21 <0.01,0.02 )
0.57 21 <0.01,0.01 )
)
Colombia 0.5) ) ) Hayto, 1978a,
0.7)-in 4001 7 )<0.01 )
1.0) ) )
S. Africa 0.4 1 0.05 0.05 0.05 (seedcake) )
0.03 0.18 0.04 (oil) )
)
0.6 1 0.13 0.06 0.11 (seedcake) ) Hayto, 1978c
0.22 0.09 0.04 (oil) )
)
0.8 1 0.28 0.21 0.29 (seedcake) )
0.22 0.34 0.29 (oil) )
Mexico 0.2 kg/l 6 0.02 (seed cotton)
0.05 (seed from whole bolls) Hayto, 1978e
1 The first application was ULV; the second conventional application. Samples tested were cottonseed except where
treated otherwise.
TABLE 15. Residues on Cottonseed in U.S. trials
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
State of No. of Residues in mg/kg at
U.S.A. Rate of Application per hectare Treatments intervals (days) after application
0 6,7 9 14 18 48 85
Texas 0.28 kg in 37.4 l 9 1.04 0.80 0.68
0.14 kg in 37.4 l 9 0.16 0.18 0.21
S. Carolina 0.28 kg in 46.7 l 16 0.16 0.12 0.13
Alabama 0.56 kg in 121.6 l 11 0.41
Mississippi 0.14 kg in 46.7 l 10 0.20
0.14 kg in 28.0 l 4 0.12
0.28 kg in 56.1 l 12 0.27 0.28
Alabama 0.56 kg in 46.7 l 6 0.06
0.28 kg in 46.7 l 6 0.07
Anon., 1978
TABLE 16. Supervised Residue Trials on Vegetables, Hops and Olives (Oil and Cake)
Combined residues of amitraz and N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Crop Country Applications Residues (mg/kg) at intervals (days)
No. Rate per hectare after application References
kg litres 0,1 3 4,5 6,7 14 28
Cucumber UK 1 0.33 g/l 0.09 ) Hamilton 1978
2 0.33 g/l 0.13 0.09 )
Cucumber S. Africa 1 60 g in 100 l drench 0.30 0.30 0.10 ) Hayto, 1979
0.37 )
Cucumber Netherlands 1 2.8 7,000 0.02,<0.01 )
0.03, 0.07 )
)
1 2.4 6,000 0.01 ) Somerville and
0.13 ) Hughes, 1973a,
Eggplant 1 0.51 1,000 0.17 0.27 0.18 0.12 )
) Hayto, 1978b
)
Onion bulbs 1 0.5 0.06 )
TABLE 16. Continued...
Crop Country No. Applications Residues (mg/kg) at intervals (days)
of No. Rate of hectare after application References
Trials kg litres 50 54 90 139
Hops and
beer UK
Green Hops UK 1 1 0.72 0.03 )
)
Dried Hops UK 1 2 0.72 0.11 ) Hamilton, 1977b
)
Beer UK 1 2 0.72 (on hops) 1.68 <0.01 )
Olives Italy 2 1 54 g/hl )
1.68 1.36 (Pressed cake) )
2 1 108 g/hl ) Richards, 1980a
<0.01 <0.01 (Purified oil) )
0.80 1.70 (Pressed cake) )
Residues in animals
Cattle
Trials have been carried out on cattle and calves in order to measure
combined residues of amitraz and (II), total '2,4-xylidine', or (V)
residues, in tissues after single or repeated applications of amitraz
as a spray or dip. Combined residues of amitraz and (II) and total
'2,4-xylidine' residues after hydrolysis were generally comparable to
control values 1 and 7 days after single spray applications. As
expected, some residues of (V) were apparent after hydrolysis of the
tissues. The results, however, were often complicated by
naturally-occurring 4-amino-benzoic acid, which was not resolved from
(V) by the original method of analysis and gave rise to apparent
residues in untreated tissues. The maximum corrected residue of (V)
after a single-spray application was 0.50 mg/kg in the liver.
Long-term trials involving spray or dip programmes indicated no large
accumulation of residues. Maximum residues of amitraz plus (II) were
0.13 mg/kg in the kidney 1 day after a 14-month spray programme.
Liver tissues from the same trial gave rise to corrected maximum
residues of 0.89 mg/kg of (V).
Milk samples and butter fat have been analysed for combined residues
of amitraz plus (II), for residues of (V), and also for total
'2,4-xylidine' residues. No significant residue were found in milk.
Maximum levels of '2,4-xylidine' occurred in butter fat 6 hours after
treatment (0.15 mg/kg amitraz), however milk collected 2 days after
treatment showed no significant residues as did factory butter
produced locally.
Sheep
In sheep, total residues of '2,4-xylidine' one day after a single dip
were below 0.1 mg/kg in muscle, and below 0.5 mg/kg in kidney and fat,
except in the case of back fat in the first trial. Residues of the
terminal metabolite (V) were only significant in liver and kidney
(corrected maximum 0.65 mg/kg). Milk samples from treated sheep gave
no significant residues of amitraz plus (II) after 1 or 2 days.
Pigs
Residues of amitraz plus (II), and total residues of '2,4-xylidine',
were comparable to control values in pigs sprayed once and twice
respectively. Analysis of pigskin indicated combined residues of
amitraz plus (II) of 0.04 mg/kg and 0.01 mg/kg at 1 and 3 days after a
single spray.
Tissues from the double spray trial gave rise to residues of (V).
However, an apparent residue of over 1 mg/kg in the liver was
confirmed at 0.40 mg/kg by combined gas chromatography-mass
spectroscopy. Smaller residues were likewise found in other tissues.
A summary of all residue results from animal trials can be found in
Table 17.
FATE OF RESIDUES
In plants
Experiments using 14C-amitraz demonstrated that, under certain
conditions at least residues of (II) and (III) could occur on and in
fruit, e.g. apples (Somerville and Nicholson, 1972).
The radiotracer experiments also demonstrated that 14C-amitraz,
applied to leaves, was not transported in any measurable quantity to
other parts of the plant, whereas when 14C-amitraz was applied to the
soil, radioactivity could be taken up into the aerial portions of
plants (Somerville and Spiers, 1972; Lewis, 1970b).
The metabolic pathway is summarised in figure 1.
In animals
The metabolism of amitraz in animals is covered fully in Biochemical
Aspects.
Studies with dogs, cats, rats, mice and bovines all yield the same
picture, namely that amitraz does not persist in vivo, but is
rapidly degraded, yielding conjugated 4-amino-3-methylbenzoic acid (V)
which is excreted in the urine. In dogs, evidence has been obtained
indicating that the first step in the degradation is simple hydrolysis
to yield N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethylformanilide (III). This is consistent with the known
properties of amitraz in acid medium. Furthermore, in an experiment
in which (II) was itself fed to dogs, identical excretory products to
those yielded by amitraz were found. In vitro studies have shown
that both (II) and (III) are degraded rapidly in gastric juice to give
2,4-xylidine (IV).
Further evidence that (IV) is an intermediate in amitraz degradation
in animals is provided by Lindstrom (1961) who showed that (IV) was
rapidly converted to (V) in rats.
It is also of interest to note that (V) is found in human urine
following exposure to amitraz indicating that the same pathway of
metabolism exists in humans.
Once ingested, amitraz is rapidly converted to metabolite (II), which
has similar toxicological effects to amitraz itself. Since apple
pomace and citrus pulp may be fed to cattle, analyses were made of
plasma, tissues and milk from calves and cows dosed with (II). No
measurable residues of (II) or (V) were found in the milk of cows
after 21 days continuous dosage (Dickinson and Shirley, 1975). After
a similar period the total residues of (II), (III), and (IV) in
TABLE 17. Supervised Residue Trials on Animals in Various Countries
Country Animal/ Residues at intervals (days) after application.
Commodity Application Amitraz plus N-2,4-dimethyl-N'-methyl-formamidine References
expressed as the latter (mg/kg) unless
stated otherwise.
Type Number Concentration CONTROL 1 4 7/8
UK CATTLE spray once 0.025%
muscle ) <0.01 <0.01 0.01 )
liver ) <0.01 0.05 <0.01 )
kidney ) <0.01 0.03 )
fat <0.01 <0.01 <0.01 )
)
muscle )3 0.051 <0.01 0.02 ) Jones and
liver ) 0.061 0.12 0.02 ) Holland,
kidney ) 0.03 0.01 ) 1974
fat ) 0.091 0.01 0.01 )
UK CATTLE spray once 0.025%
muscle ) 0.02 <0.01 )
liver ) <0.01 <0.01 ) Holland,
kidney ) 0.03 0.01 ) Jones and
fat ) <0.01 0.03 ) Jones, 1974
Australia CATTLE spray repeatedly at 0.025%
7-day intervals
muscle (rump) ) followed by 3 <0.01 <0.01 <0.01 )
(shoulder) ) sprays at 3-day <0.01 <0.01-0.02 <0.01 )
liver ) intervals <0.01 0.01 <0.01 ) Hayto,
kidney ) <0.01 0.03-0.04 0.03 ) 1977b
fat (back) ) <0.01 <0.01 <0.01 )
(omental) ) <0.01 <0.01 <0.01
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
S. Africa CATTLE spray weekly 0.05%
for 14
muscle )3 <0.011 0.05 )
liver ) <0.01 0.06 )
kidney ) 0.13 )
fat ) 0.01 0.06 )
)
muscle )3 0.051 0.02 0.03 ) Holland
liver ) 0.061 0.04 0.03 ) and Jones,
kidney ) 0.11 0.12 ) 1974a
fat ) 0.091 0.03 0.03 )
milk )3 0.02 0.02 0.01 ) Holland and
) Jones, 1974b
S. Africa CATTLE dipped weekly 0.008%
1-3 years
muscle )3 0.0051 0.008 <0.005 )
liver ) 0.0051 0.04 0.01 ) Shirley,
kidney ) 0.0051 0.06 0.01 ) 1977a
fat ) 0.0051 0.02 <0.005 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
Day 0 Day 1 Day 2
am pm am pm am pm
pre-treatment
Australia CATTLE dipped once 0.025% )
)
tissues <0.02 <0.02 )
)
dipped repeatedly 0.025% )
)
milk ) <0.012 <0.01 <0.01 ) McDougall,
butterfat ) <0.012 0.15 0.11 0.02 0.01 <0.01 ) Heath and
factory butter ) all samples 0.012 ) Black, 1979
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 3 5 7
UK SHEEP dipped once 0.05%
muscle ) 0.011 0.08 0.07 )
liver ) 0.011 0.24 0.11 )
kidney ) 0.021 0.40 0.13 ) Shirley,
fat (back) ) <0.0051 0.62 0.34 ) 1977b
(omental) ) <0.0051 0.14 0.08 )
UK SHEEP dipped once 0.05%
muscle (shoulder) 0.012 0.03 0.02 0.01 0.01 )
(hind leg) 0.012 0.03 0.01 0.01 0.01 )
liver 0.012 0.13 0.04 0.03 ) Needham and
kidney 0.012 0.27 0.09 0.07 ) Campbell,
fat (back) 0.012 0.26 0.27 0.25 0.12 ) 1980
(omental) 0.012 0.10 0.08 0.10 0.09 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 2 3 7
UK SHEEP dipped once 0.05%
milk <0.01 0.02 0.01 <0.01 <0.01 Shirley, 1978
UK PIGS sprayed once 0.1% 2 and 7 days
twice at
muscle 7-day <0.01-0.04 <0.01-0.02 )
liver intervals <0.01-0.05 <0.01-0.04 )
kidney <0.01-0.02 <0.01-0.07 ) Shirley,
fat <0.01-0.02 <0.01-0.02 ) 1975a
UK PIGS sprayed once 0.1%
skin <0.01 0.04 0.01 <0.01 Hayto, 1977a
UK PIGS sprayed twice 0.1%
at 8-day
muscle intervals <0.031 <0.03 )
liver <0.031 <0.03 )
kidney <0.031 <0.03 ) Dickinson and
fat <0.031 <0.03 ) Shirley, 1976
1 Total residues measured as '2,4-xylidine' and expressed as N-2,4-dimethyl-N'-methyl-formamidine (mg/kg)
2 Total residues measured as '2,4,xylidine' and expressed as amitraz (mg/kg)
3 No untreated animals in trial, tissues bought locally.
tissues and plasma of calves were found to be < 0.01 mg/kg and < 0.1
mg/l respectively, although the livers and kidneys of calves receiving
the highest dose of 40 mg/kg diet/day contained residues of 0.02 and
0.05 mg/kg respectively (Campbell and Needham, 1979).
METHODS OF RESIDUE ANALYSIS
Methods have been developed to measure combined residues of amitraz,
compound (II), total '2,4-xylidine' (IV) and metabolites (V) and
(III).
Combined residues of amitraz (calculated as (II) and (II) are measured
as mg/kg (II). Fruit for analysis is first subjected to treatment
with acidic methanol to convert amitraz to (II). Solid material is
filtered off, the extract cleaned and analysed by GLC using a
flame-ionisation detector. Sensitivity is 0.01 mg/kg and recoveries
varied between 70 and 100% for different fruits (Hughes, 1974b;
Somerville and Richards, 1980).
Analysis of animal tissues and milk differ from the above only in the
initial preparation of the sample for extraction, and in the cleanup
procedures, which were slightly modified from those used for fruit.
The method is capable of estimating a total residue level of 0.02
mg/kg in lean meat, liver, fat and milk samples; recoveries were over
70% and background levels were less than O.02 mg/kg (II) (Hughes,
1974a).
Methods of assay of total '2,4-xylidine' residues were developed for
analysis of animal tissues. The methods involve hydrolysis of
amitraz, and all its metabolites that contain the moiety, to
'2,4-xylidine' which on conversion to its heptafluorobutyric
derivative can be determined by gas-liquid chromatography with
electron capture detection. The limits of sensitivity are 0.01 mg/kg
(II) equivalents (Nappier, Hornish and Lane, 1976).
Similar methods have been used in the USA for analysis of fruit and
cotton seed, and lower limits of detection have been established at
0.01 mg/kg and 0.05 mg/kg respectively (Nappier and Hornish, 1975;
Nappier and White, 1978).
Compound (V), the main mammalian metabolite of amitraz, exists as a
conjugated derivative in tissues. Analysis of residues of (V)
therefore involves acid hydrolysis followed by high pressure anion
exchange chromatography. The method has a recovery of 70-80% and a
lower level of detection of 0.03 mg/kg. Relatively high background
levels were found in earlier work, probably due to naturally occurring
p-aminobenzoic acid, which eluted with (V). The more recent method
resolves these difficulties (Dickinson and Shirley, 1976).
Compound(III) is formed in the hydrolysis of amitraz and has been
assayed by high pressure liquid chromatography following extraction of
fruit in the usual way. The sensitivity of the method in 0.01 mg/kg
and the recovery is 89%. (Jones, 1973 f).
NATIONAL RESIDUE LIMITS
The following national Maximum Residue Limits (MRLs) were reported to
the Meeting.
Country Withholding Crop MRL1
Period mg/kg
Australia 4 weeks pome fruit and 0.052
stone fruit
Belgium 4 weeks apples, pears 0.4
East Germany 4 days cucumbers, tomatoes 0.2
4 weeks apples, pears 0.2
France 30 days fruit trees, pears 0.15
Hungary 10 days apples, plums, strawberries,
vines, sugarbeet, soybeans 0.052
Italy 4 weeks apples, pears 0.4
2 weeks citrus, vegetables,
vines, stone fruit 0.4
Netherlands 4 weeks apples, pears 0.4
New Zealand 2 weeks pome fruit and stone fruit 0.5
Switzerland4 3 weeks apples, pears 0.3
USA 4 weeks pears 3.03
Fed. Rep. of apples, pome fruit 0.4
Germany
1 Combined residues of amitraz and formamidine (II), expressed as
II, unless otherwise indicated.
2 Amitraz only.
3 Measured as total '2,4-xylidine' (IV), expressed as amitraz.
4 Based on informal information, subject to written confirmation.
EVALUATION
COMMENT AND APPRAISAL
Amitraz is an insecticide and acaricide effective against a wide
variety of phytophagous mites and insects; its use on fruit and
vegetables and on cotton is authorised in a large number of countries.
It also has important veterinary uses, especially against the ticks
and mites of cattle and sheep, including some otherwise resistant
strains. It is formulated as emulsifiable concentrates for both crop
protection and animal use, and as wettable powders for animal use.
Amitraz is well absorbed from the gastro-intestinal tract and
eliminated from most tissues within a few days. The metabolic
breakdown is similar for all species studied, resulting in the
formation of 3-methyl-4-aminobenzoic acid (BTS 28369) and its
conjugates.
With amitraz a number of short and long-term toxicity studies have
been carried out with mice, rats and dogs. From these experiments
dogs appeared to be the most sensitive species. Furthermore,
experiments with amitraz metabolites showed that on the basis of
weight BTS 27271 is more toxic than the parent compound, however this
difference in toxicity may be explained by the difference in molecular
weight between these compounds. Therefore the toxicity of amitraz can
most probably be attributed to the formation of BTS 27271.
For mice and rats a marginal no toxic effect level of 2.5 and 3 mg/kg
bw/day may be estimated. In dogs however the no effect level from the
short and long-term studies was 0.25 mg/kg bw/day. The most
predominant effect particularly in dogs was CNS depression. The
metabolite BTS 27271 has a no effect level of 1 and 0.1 mg/kg bw/day
for rats and dogs respectively.
From reproduction and teratogenicity studies it was shown that amitraz
influences mean litter size and viability of the young, although, in
effective doses, toxicity on the dame was also apparent. Therefore it
can be concluded that amitraz is possibly embryotoxic in doses also
toxic for the dama, but has no clear teratogenic activity. However
reported studies are incomplete with respect to the number of animals
used (teratogenicity test) and the number of litters bred
(multigeneration study). Also no information was obtained about pre-
implantation losses. No information is available about the possible
teratogenic potential of the metabolites.
From mutagenicity tests with amitraz, BTS 27271, 27919, 28369, 33220
and 24868 it was shown that only BTS 24868, 33220 and 27919 exhibit
weak mutagenic activity in in vitro bacterial systems using
Salmonella typhimurium (TA 100 strain). Negative results were
also obtained in the host-mediated and lethal tests with amitraz.
With amitraz and BTS 24868 carcinogenicity experiments were carried
out in mice and rats. Amitraz was negative in the rat study, but from
initial body weights it is concluded that the age of the animals at
the start of the experiment was not within normal standards. In the
female mouse only 400 mg/kg bw caused an increased incidence of
lymphoreticular tumours. In female mice BTS 24868 increased the
number of lung tumours. In male rats (no female tested) also the
number of lung tumours was found to be increased, together with
bladder and liver tumours which were not seen in controls. In these
studies the number of animals used, dosing schedule and experimental
period do not permit an evaluation of a possible carcinogenic action
of BTS 24868.
Human data are inadequate to be included in the evaluation for the
acceptable daily intake.
It should be remarked that a number of experiments and reports
submitted do not meet acceptable standards.
Experiments using 14C-amitraz show that under certain conditions the
metabolites N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethyl-formanilide (III) may occur on or in fruit. If 14C
labelled amitraz is applied to soil some radioactivity may be taken up
into the aerial parts of plants, but when it is applied to leaves
there is no detectable movement to other parts.
Extensive residue trials on apples and pears have taken place in the
United Kingdom, Europe and other parts of the world, especially in the
United States. US registration authorities require that all
components of the residue that give rise to '2,4-xylidine' on
hydrolysis are included in the analyses, whereas elsewhere residue
measurements are based on the determination of the parent compound
plus N-(2,4-dimethylphenyl)-N'-methylformamidine (II), which is the
most toxic metabolite, being more toxic to mammals than amitraz
itself. Further degradation gives compounds of lesser toxicological
significance.
It is suggested that results obtained by the two methods are broadly
comparable when expressed on the same basis, although those based on
hydrolysis to '2,4-xylidine' are sometimes rather higher.
The terminal metabolite in animals, 4-amino-3-methyl benzoic acid (V),
has been detected as a residue in animal tissues (especially in the
liver), but it closely resembles the natural product 4-aminobenzoic
acid (which can interfere with its determination).
The residue analytical methods used in the USA, involving total
'2,4-xylidine' determination, require a specially made
distillation/extraction apparatus, and also involve the formation of a
fluorinated derivative of the 'xylidine' before final determination by
gas chromatography using electron capture detection. These procedures
probably require considerable skill on the part of the analyst.
Convenient analytical methods, using gas chromatography with flame
ionisation detection, are available for the determination of combined
residues of amitraz and (II) in fruits, vegetables, animal tissues and
milk, and these methods could be adapted for regulatory purposes.
The meeting examined residues data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels which were likely to occur when
amitraz was used in practice and when reported intervals between last
application and harvest were observed.
Level of amitraz causing no toxicological effect
Mouse: 2.5 mg/kg bw/day
Rat: 3 mg/kg bw/day
Dog: 0.25 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw/day
RECOMMENDATIONS OF RESIDUE LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. These levels refer
to the sum of amitraz and N-(2,4-dimethylphenyl)-N'-methylformamidine
(II), calculated as II.
Crop FAO MRL Pre-harvest
Commodity (mg/kg) Interval
Group (days)
Pome fruit 10 0.5 14
Peaches 11 0.5 14
Cherries 11 0.5 28
Cucumber 7 0.5 3
Oranges 9 0.5 7
Cottonseed 20 0.5 7
Cottonseed oil class C 0.05
Olive oil class C 0.05 (Prepared from olives
(sprayed 60 days
(before harvest.
Carcass meat
of cattle 25 0.05
of pig 25 0.05
of sheep 25 0.2
Cattle meat by-products 27 0.2
Pig meat by-products 27 0.2
Sheep meat by-products 27 0.2
Milk 28 0.011/
1/ Level at or about the limit of determination
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Long-term carcinogenicity study with
N-2,4-dimethylphenyl-N-methylformamidine.
2. Long-term carcinogenicity study with amitraz in mice.
3. Further information on the mutagenic activity of 2,4-xylidine,
NN-bis(2,4-dimethylphenyl)formamidine and 2,4-dimethylformanilide.
4. Additional residue data from supervised trials on whole olives.
Desirable
1. Information on the teratogenic activity of
N-2,4-dimethylphenyl-N-methylformamidine.
2. Information on species differences in toxicity.
3. Further observations in humans.
4. Additional residue data on citrus fruits.
5. Further trials on hops, onions and eggplants.
REFERENCES
Anonymous. Acute oral studies on triazid impurities. Unpublished
report RD/BS/BL/1268, 1971a; from The Boots Company, submitted to WHO.
Anonymous. Human pharmacology study on BTS 27419. Unpublished report
no. MS 71004; 1971b, from The Boots Company, submitted to WHO.
Anonymous. Supplement to report no. MS 71004; 1971c, Unpublished
report from The Boots Company, submitted to WHO.
Anonymous. Effects of BTS 27149 and BTS 27271 on the responses of
rabbit ear blood vessels to catecholamines and antidromic stimulation.
Unpublished report no. P 72622; 1972, from The Boots Company,
submitted to WHO.
Anonymous. Residue determination for amitraz and its metabolites in
apples and pears. Upjohn Co. reports 315-9760-5,8,11,12; 1973,
(Unpublished).
Anonymous. Residue determination for U-36,059 (amitraz) and
metabolites in apples and pears. Upjohn Co. reports
315-9760-33,35-40,42-49,52,55-65; 1975, (Unpublished).
Anonymous. Residue analysis of amitraz on Swiss apple and pear crops.
Boots Co. Ltd. report no. AR 77016; 1977, (Unpublished).
Anonymous. Residue determination for U-36,059 and metabolites in
cotton seed (Texas, 1977). Upjohn Co. reports 315-9760-131 to 137;
1978, (Unpublished).
Anonymous. Persistence of amitraz on Florida grown Hamlin oranges.
Boots Hercules Agrochemicals Co. report; 1979a, (Unpublished).
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Methods of analysis. Unpublished report dd. 17-9-1979; 1980, from The
Boots Company, submitted to WHO.
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Analytical specifications. Unpublished report, dd. 8-1-1980; 1980,
from The Boots Company, submitted to WHO.
Berczy, Z.S., Binns, R. and Newman, A.J. Acute inhalation toxicity to
the rat of BTS 27419. Unpublished report no. 4971/72/406; 1973, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield,
R.A.S. Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report no. 5454/72/850; 1973, from Huntingdon Research
Centre, submitted to WHO by The Boots Company.
Berry, P.A. A comparison of the hypothermic effects of amitraz (BTS
27419) in male and female CFLP mice. Unpublished report no. P 76058;
1976, from The Boots Company, submitted to WHO.
Brown, D.J., Burnett, R.J., Crowley, J., Cryer, P.C., Lancaster, M.C.
and Turnbull, G.J. Amitraz: Investigations of effects on the thymus
gland and oestrus cycle in mice. Unpublished report no TX 78037; 1978,
from The Boots Company, submitted to WHO.
Burnett, R., Crowley, J., Lessell, B., Patton, D.S.G., Sutton, M.M.,
Turnbull, G.J. and Williams, G.A.H. BTS 27419: 80-Week carcinogenicity
study in mice - final report. Unpublished report no. TX 76039; 1976,
The Boots Company, submitted to WHO.
Campbell, J.K. and Needham, D. Residues of BTS 27271 and its
metabolites in the plasma and tissues of calves after repeated daily
dosing. Boots Co. Ltd. report no. AX 79019; 1979, (Unpublished).
Channon, E.J. and Cryer, P.C. Amitraz: Investigation of effects on the
thymus gland and oestrus cycle in mice - statistical analysis of
vaginal smear data and thymus weights. Unpublished report no. SS
78001; 1978, The Boots Company, submitted to WHO.
Chesterman, H., Skerrett, K., Street, A.E. and Newman, A.J. Boots
27271 oral toxicity study in beagle dogs (Repeated dosage for 13
weeks). Unpublished report no. S 38/73511; 1973, Huntingdon Research
Centre, submitted to WHO by the Boots Company.
Dickinson, W. and Shirley, D.B., Analysis of milk from cows dosed
orally with BTS 27271 at Thurgarton Research Station. Boots Co. Ltd.
report no. F 75018; 1975, (Unpublished).
Dickinson, W. and Shirley, D.B. Residue analyses of tissues from pigs
treated with amitraz at Thurgarton Research Station, UK. Boots Co.
Ltd. report no. F 76015; 1976, (Unpublished).
O'Donovan, M.R. and Smithson, A. Amitraz impurity. BTS 33220: Acute
oral toxicity to male Boots-Wistar rats. Unpublished report no. TX
78079; 1978, from The Boots Company submitted to WHO.
Everest, R.P. BTS 27419: Mutagenicity study in the male mouse
pervisceral cavity hostmediated assay. Unpublished report no. TX
76056; 1976, from The Boots Company submitted to WHO.
Everest, R.P. and Wilcox, P. BTS 27419, BTS 27271, BTS 27919 and BTS
28369: mutagenicity testing in bacterial in vitro systems.
Unpublished report no.76016; 1976a, from The Boots Company submitted
to WHO.
Everest, R.P. and Wilcox, P. BTS 27419: Mutagenicity testing against
Salmonella typhimurium strains TA 1535, TA 1537 and TA 1538 in the
presence and absence of liver microsomes from male and female mice.
Unpublished report no. TX 76057; 1976b, from the Boots Company
submitted to the WHO.
Everest, R.P. and Wilcox, P. In vitro bacterial mutagenicity
testing of an amitraz sample containing 1% BTS 33220. Unpublished
report no. TX 79108; 1979, from The Boots Company submitted to WHO.
Goodall, E. and Somerville, L. Residue analyses of amitraz and its
metabolite BTS 27271 on Italian fruit crops. Boots Co. Ltd. report no.
AX 74026; 1974a, (Unpublished).
Goodall, E. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Dutch apple and tomato crops. Boots Go. Ltd.
report no. AX 74028, 1974b, (Unpublished).
Hall, J.E., Ahlunwalia, H. and Hampson, P. A study of the effects of
oral administration of a metabolite (BTS 27271) of amitraz to
volunteers. Unpublished report no. NS 75002; 1975, from The Boots
Company submitted to WHO.
Hamilton, D.Y. The fate of [14C]-BTS 27419 (amitraz) when
administered to mice at 100 mg/kg as a single oral dose. Unpublished
report no. AX 76013; 1976, from The Boots Company submitted to WHO.
Hamilton, D.Y. Amitraz residues in Turkish cotton seed. Boots Co. Ltd.
report no. AX 77003; 1977a, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
hops and beer from Whitbreads. Boots Co. Ltd. report no. AX 77006;
1977b, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
cucumbers from the UK. Field Trials 1976. Report no. AX 78011; 1978.
Hamilton, D.Y and Somerville, L. Fate of [14C]-BTS 27271 when
administered to dogs as a oral dose. Unpublished report no. AX 73019;
1973, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27419 when
administered at 15 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74006; 1974a, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C] BTS 28369 when
administered at 10 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74012; 1974b, from The Boots Odmpany submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27271 when
administered to cats as a single oral dose. Unpublished report no. AX
74005; 1974c, from The Boots Company submitted to WHO.
Hayto, E.M. Residues of amitraz and its metabolite BTS 27271 in
pigskin from animals treated at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 77007; 1977a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cattle
tissues from Australia. Boots Co. Ltd. report no. AX 77013; 1977b,
(Unpublished)
Hayto, E.M. Combined residues of amitraz and BTS 27271 in plums and
blackcurrants from UK field trials, 1976. Boots Co. Ltd. report no. AX
77014; 1977c, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Guatemala and Colombia, 1977. Boots Co. Ltd. report no. Ax 78002;
1978a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in egg-plants
and onion bulbs from Italy. Boots Co. Ltd. report no. AX 78012; 1978b,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton from
South Africa, 1978. Boots Co. Ltd. report no. AX 78015; 1978c,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in
sour-cherries from Denmark, 1976. Boots Co. Ltd. report no. AX 78018;
1978d, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Mexico, 1977. Boots Co. Ltd. report no. AX 78019; 1978e,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
oil from Turkey, 1977. Boots Co. Ltd. report no. AX 78021; 1978f,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cucumbers
from South Africa, 1978. Boots Co. Ltd. report no. AX 79002; 1979,
(Unpublished)
Holland, G.N., Jones, A.W., and Jones, E.M. Analyses of amitraz and
its metabolite BTS 28369 in control and treated Thurgarton calves.
Boots Co. Ltd. report no. P 74022; 1974, (Unpublished).
Holland, G.N. and Jones, E.M. Analyses of amitraz and its metabolite
BTS 28369 in treated South African cattle. Boots Co. Ltd. report no.
F 74021; 1974a, (Unpublished).
Holland, G.N. and Jones, E.M. Analysis of amitraz in milk from treated
South African cattle. Boots Co. Ltd. report no. F 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of amitraz and BTS 27271 in animal tissues and
milk. Boots Co. Ltd. report no. AX 74018; 1974a, (Unpublished).
Hughes, K.W. Estimation of combined residues of amitraz and BTS 27271
in apples and pears. Boots Co. Ltd. report no. AX 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of BTS 28369 in human urine. Unpublished
report no. F 75009; 1975, from The Boots company submitted to WHO.
Hughes, K.W., Jones, E.M., and Somerville, L. Residue analyses of
amitraz and its metabolites BTS 27271 and BTS 27919 on apple and pear
crops treated with amitraz at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 74002; 1974, (Unpublished).
Hughes, K.W., and Somerville, L. Residue analyses of amitraz and its
metabolie BTS 27271 on apple crops from Thurgarton Research Station,
UK. Boots Co. Ltd. report no. AX 73017; 1973a, (Unpublished).
Humphrey, E.A. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Danish cherries. Boots Co. Ltd. report no. AX
76003; 1976, (Unpublished).
Jones, E.M, Metabolism of [14C]-BTS 27419 in calf and in cow, part I.
Identification of major urinary metabolite. Unpublished report no.
F 73004; 1973a, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in calf. Part 2.
Identification of major liver metabolite. Unpublished report no.
F 73011; 1973b, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in rats. Unpublished report
no. F 73010; 1973c, from the Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BST 27271 in dogs. Unpublished report
no. F 73019; 1973d, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in cats. Unpublished report
no. F 73018; 1973e, from The Boots Company alimitted to WHO.
Jones, E.M. Residue analyses of BTS 27919 on French peach crops
(Report no. 1). Boots Co. Ltd. report no. F 73015; 1973f,
(Unpublished).
Jones. E.M. and Holland, G.N. Analysis of amitraz and its metabolite
BTS 28369 in treated Thurgarton calves. Boots Co. Ltd. report no.
F 74020; 1974 (Unpublished).
Kakuk, T.J. Amitraz. Further evaluation of 80-week mouse study.
Unpublished report no. HGD 79002; 1979, from The Boots Company
submitted to WHO.
Lancaster. M.C. and Williams, G.A.H. Amitraz: Multigeneration feeding
test in rats - Histopathology. Unpublished report no. TX 78064; 1978,
from The Boots Company submitted to WHO.
Lewis, D.K. RD 27.419. Metabolism and residue studies in the calf
using (14C) and (3H)-labelled material. Unpublished report no.
C 70029; 1970a, from The Boots Company submitted to WHO.
Lewis, D.K. Amitraz - a preliminary study of the persistence,
translocation, and metabolism in plants. Boots Co. Ltd. report no.
FM 70158; 1970b, (Unpublished).
Lewis, D.K. Fate of [14C]-triazid applied to rats as a single oral
dose. Unpublished report no. C 71011; 1971a, from The Boots Company
submitted to WHO.
Lewis, D.K. Fate of [14CH]-BTS 27419 applied to rats as a single
dermal dose. Unpublished report no. C 71019; 1971b, from The Boots
Company submitted to WHO.
Lewis. D.K. Fate of [14C]-BTS 27419 applied to dogs as a single oral
dose. Unpublished report no. 71024; 1971c, from The Boots Company
submitted to WHO.
Lewis, L. Fate of [14C]-triazid (BTS 27419) in the calf. Unpublished
report no. C 71026; 1971d, from The Boots Company submitted to WHO.
Lindstrom, H.V. The metabolism of FD and CRED no. I. The fate of
2,4-meta-xylidine in rats. J. Pharm. Exp. Therap. 132, 306-310.
Martin, H., and Worthing, C.R. Pesticide Manual, 6th Edition, p. 15.
Published by the British Crop Protection Council.
McDougall, D.W., Heath, A.B., and Black. R.R. Residues of amitraz in
the tissues, milk, and butter of cattle dipped in Taktic. Aust. J.
Exp. Agric. Anim. Husb., 19: 663-665.
Merryman, D.C. and Sutton, M.M. BTS 27419. Effects on the oestrus
cycle of the rat. Unpublished report no. PN 72003; 1972, from The
Boots Company submitted to WHO.
Moore, W.K.S. Clinical observations during development. 1972,
Unpublished report from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27271. Acute oral toxicity study in dogs.
Unpublished report no. TX 73004; 1973a, from The Boots Company
submitted to WHO.
Morgan, H.E, BTS 28037; Acute oral toxicity study in dogs. Unpublished
report no. TX 73015; 1973b, from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27919: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74004; 1974a, from The
Boots Company submitted to WHO.
Morgan, H.E. BTS 28369: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74006; 1974b, from The Boots
Company submitted to WHO.
Morgan, H.E., Ellicock, L.A., Parkinson, R. and Wood, R. Amitraz:
comparison of oral and topical administration to pigs. Unpublished
report no. TX 75025; 1975a, from The Boots Company submitted to WHO.
Morgan, H.E., Patton, D.S.G. and Turnbull, G.J. BTS 27419: two year
oral toxicity study in dogs. Unpublished report no. TX 73035; 1973,
from The Boots Company submitted to WHO.
Morgan, H.E. and Shepherd, G.M. BTS 28369: Acute oral toxicity study
in dogs. Unpublished report no. TX 74001; 1974, from The Boots Company
submitted to WHO.
Morgan, H.E., Shepherd, G.M., and Turnbull, G.J. BTS 28369: 90-day
oral toxicity study in dogs. Unpublished report no. TX 74037; 1974,
from The Boots Company submitted to WHO.
Morgan, H.E. and Turnbull, G.J. BTS 27919: Acute oral toxicity study
in dogs. Unpublished report no. TX 73034; 1973, from The Boots Company
submitted to WHO.
Morgan, H.E., Turnbull, G.J. and Ellicook, L.A. Amitraz and BTS 27271:
Effects of repeated daily dosing on acute responses in dogs.
Unpublished report no. M 75026; 1975b, from The Boots Company
submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 27271: Acute oral toxicity study
in dogs -Histopathology. Unpublished report no. TX 73030; 1973a, from
The Boots Company submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 28037: Acute oral toxicity study
in dogs. Unpublished report no. TX 73038; 1973b, from The Boots
Company submitted to WHO.
Nappier, J.L. and Hornish, R.E. Total residue method for U-36, 059
(amitraz) in apples, pears, and soils. Upjohn Co. report no.
315-9760-32; 1975, (Unpublished).
Nappier, J.L., Hornish, R.E. and Lane, R.E. Total residue method for
U-36,059 (amitraz) in swine tissue. Upjohn Co. report no. 315-9760-70;
1976, (Unpublished).
Nappier, J.L. and White, M.C. Total residue method for U-36, 059
(amitraz) in cotton seeds. Upjohn Co. report no. 315-78-9760-001;
1978, (Unpublished).
NCI, Final Report. Carcinogenicity of chemicals present in man's
environment. Contract no. NIH-NCI-E-68-311. Reported by Bio-Research
consultants, 1973, submitted to WHO by The Boots Company.
Needham, D. and Campbell, J.K. Amitraz residues in the tissue of sheep
1,3,5 and 7 days after dipping in 0.05% amitraz. Boots Co. Ltd. report
no. AX 80016; 1980, (Unpublished).
Nix, N. Stability data for Amitraz Technical (Paraformaldehyde Sachets
Stabilised). Unpublished report dd. 11-12-1979; 1979, from The Boots
Company submitted to WHO.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
female mouse. Unpublished report no. BTS 81/7758; 1977a, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
male mouse. Unpublished report no. BTS 80/7792; 1977b, from Huntingdon
Research Centre, submitted to WHO by The Boots Company.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
(chlordimeform) on the central actions of reserpine in the mouse and
rat. Unpublished report no. P 74033; 1974a, from The Boots Company
submitted to WHO.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
chlordimeform) on the pressor response to tyramine in the pithed rat.
Unpublished report no. P 74032; 1974b, from The Boots Company
submitted to WHO.
Parkinson, R. BTS 27271 - an attempt to produce and quantify the
flushing response in the pig. Unpublished report no. P 75030; 1975,
from The Boots Company submitted to WHO.
Parkinson, R., Patton, D.S.G. and Yates, D.B.G. BTS 27419. Effects in
conscious dogs of small intravenous doses. Unpublished report no.
P 71535; 1971a, from The Boots Company submitted to WHO.
Parkinson, R., Sim, M.F. and Yates, D. Effects of the acaricide BTS
27419 and the related sulphur analogues BTS 30559 and BTS 30561 on the
cardiovascular system of the cat, particularly the ear vascular bed.
Unpublished report no. P 71576; 1971b, from The Boots Company
submitted to WHO.
Parkinson, R. and Yates, D.B. The effects of BTS 27419 on
Trafuril-induced erythema on the guinea-pig. Unpublished report no.
PM 71022; 1971, from The Boots Company submitted to WHO.
Patton, D.S.G. RD 27271: Effects on u/v erythema in guinea pigs.
Unpublished report no. PM 70100; 1970, from The Boots Company
submitted to WHO.
Patton, D.S.G. BTS 27419: Acute toxicity in baboons. Unpublished
report no. TX 73002; 1973a, from The Boots Company submitted to WHO.
Patton, D.S.G. BTS 27271: Effects in conscious dogs of small
intravenous doses. Unpublished report no. TX 73017; 1973b, from The
Boots Company submitted to WHO.
Patton, D.S.G. and Sutton, M.M. Acute toxicity studies on BTS 27419 an
acaricide. Unpublished report no. P 71544; 1971a, from The Boots
Company submitted to WHO.
Patton, D.S.G. and Williams, G.A.H. BTS 27419: 90-day toxicity study
in dogs. Unpublished report no. P 71547; 1971b, from The Boots Company
submitted to WHO.
Petzold, G.L., Swenberg, J.A. and Bedell, M. Evaluation of amitraz
(U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915 A and
U-54,914) in the DNA/Alkaline Flution assay. Unpublished report no.
7268/77/7268/001; 1977, from The Boots Company submitted to WHO.
Richards, M.E. Combined residues of amitraz and BTS 27271 in olives
from Italy, 1978. Boots Co. Ltd. report nos. AX 79018 and 80029; 1979
and 1980a, (Unpublished).
Richards, M.E. Combined residues of amitraz and BTS 27271 in apples
and pears from West Germany. Boots Co. Ltd. report no. AX 80001;
1980b, (Unpublished).
Shaw, J.W. BTS 27419 - acute intraperitoneal toxicity to rats.
Unpublished report no. PM 71057; 1971, from The Boots Company
submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral and intraperitoneal
toxicities to rats. Unpublished report no. TX 73006; 1973a, from The
Boots Company submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral toxicities to rats
of BTS 27419 and BTS 27919. Unpublished report TX 73010; 1973b, from
The Boots Company submitted to WHO.
Shaw, J.W. BTS 27419 metabolite: 21-day chronic oral toxicity in rats
of BTS 28369. Unpublished report no. TX 75058; 1975, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTS 27419. 90-day chronic toxicity
study in mice. Unpublished report no. TX 74016; 1974, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTU 27419 metabolite: 90-day chronic
oral toxicity in rats of BTS 27271. Unpublished report no. TX 75059;
1975, from The Boots Company submitted to WHO.
Shirley, D.B. Analysis of tissue from pigs treated with amitraz at
Thurgarton Research Station. Boots Co. Ltd. report no. F 75015; 1975a,
(Unpublished).
Shirley, D.B. The analysis of BTS 28369 in the urine of amitraz
production workers. Unpublished report no. F 75019; 1975b, from The
Boots Company submitted to WHO.
Shirley, D.B. The analysis of BTS 28369 in the urine of three amitraz
production workers over a period of three weeks. Unpublished report
no. F 75024; 1976.
Shirley, D.B. Amitraz residues in cattle from Wiltonside, South
Africa. Boots Co. Ltd. report no. F 77002; 1977a, (Unpublished).
Shirley, D.B. Residue analyses of tissues from sheep treated with
amitraz at Thurgarton Research Station, UK. Boots Co. Ltd. report no.
F 77004, 1977b, (Unpublished).
Shirley, D.B. Analysis of amitraz residues in sheep's milk. Boots Co.
Ltd. report no. F 78013; 1978, (Unpublished).
Sim, M.F. The diuretic action of the acaricides RD 27271 and RD 27419
in mice. Unpublished report no. PM 70111; 1970, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 in the lactating cow.
Unpublished report no. C 72007; 1972, from The Boots Company submitted
to WHO.
Somerville, L. Fate of 14C-BTS 27419 administered to rats in repeated
oral doses. Unpublished report no. AX 73011; 1973a, from The Boots
Company submitted to WHO.
Somerville, L. Fate of 14C-ring-labelled BTS 27419 in the calf.
Unpublished report no. AX 73006; 1973b, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 when administered to cats as a
single oral dose. Unpublished report no. AX 73018; 1973c, from The
Boots Company submitted to WHO.
Somerville, L. and Goodall, E. Residue analyses of amitraz and its
metabolite BTS 27271 on Danish apple crops. Boots Co. Ltd. report no.
AX 74023; 1974, (Unpublished).
Somerville, L. and Hamilton, D.Y. Studies on the accumulation and
elimination of radio-labelled residues from dogs' eyes following oral
administration of (14C)-BTS 27271. Unpublished report no. AX 74013;
1974, from The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch cucumber crops. Boots Co. Ltd. report
no. AX 73008; 1973a, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 1). Boots Co.
Ltd. report no. AX 73010; 1973b, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 2). Boots Co.
Ltd. report no. AX 73013; 1973c, (Unpublished)
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch apple crops. Boots Co. Ltd. report no.
AX 73014; 1973d, (Unpublished).
Somerville, L. and Hughes, K.W. The conversion of BTS 27419 to BTS
27271 in the dog stomach. Unpublished report no. AX 73021; 1973e, from
The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French apple crops. Boots Co. Ltd. report no.
AX 73016; 1973f, (Unpublished).
Somerville, L. and Nicholson, J.E. Amitraz. Metabolism in apples,
variety Cox's Orange Pippin. Boots Co. Ltd. report no. AX 72003; 1972,
(Unpublished).
Somerville, L. and Richards, M.E. The analysis of fruit for combined
residues of amitraz and BTS 27271. Boots Co. Ltd. report no. AX 80002;
1980, (Unpublished).
Somerville, L. and Spiers, M.J. Amitraz. Metabolism in apple leaves.
Boots Co. Ltd. report no. AX 72002; 1972, (Unpublished).
Sutton, M.M., RD 27271. Acute oral toxicity to mice. Unpublished
report no. PM 70043; 1970a, from The Boots Company submitted to WHO.
Sutton, M.M. RD 27271. Acute toxicity to rats. Unpublished report no.
PM 70042; 1970b, from The Boots Company submitted to WHO.
Sutton, M.M. Acaricides: RD 27419 and RD 27271 acute toxicity to
guinea pigs. Unpublished report no. PM 70108; 1970c, from The Boots
Company submitted to WHO.
Sutton, M.M. BTS 27419. Contact sensitization in the guinea pig.
Unpublished report no. PM 71010; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Effect on thermo-regulation in mice.
Unpublished report no. P 72609; 1972a, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27271: acute oral toxicity to rabbits. Unpublished
report no. TXM 72044; 1972b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rabbit. Unpublished
report no. TX 73029; 1973a from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rat. Unpublished report
no. TX 73028; 1973b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Effects on pregnancy, parturition and care of
the young in rate. Unpublished report no. TX 73031; 1973c, from The
Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Multigeneration feeding test in rats.
Unpublished report no. TX 73036; 1973d, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Three-week dermal toxicity to rabbits.
Unpublished report no. TX 73026; 1973e, from The Boots Company
submitted to WHO.
Sutton, M.M. and Metcalf, W. BTS 27419. Eye-irritancy in the rabbit.
Unpublished report no. TXM 72037; 1972, from The Boots Company
submitted to WHO.
Sutton, M.M. and Offer, J. BTS 27419: carcinogenicity and long-term
toxicity study in rats. Unpublished report no. TX 73043; 1972, from
The Boots Company submitted to WHO.
Sutton, M.M. and Williams, G.A.H. BTS 27419: 90-day toxicity study in
rate. Unpublished report no. P 71548; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. and Williams, P.A. BTS 27419: acute dermal toxicity to
rabbits. Unpublished 1972 report no. YM 72011; 1972 from The Boots
Company submitted to WHO.
Taylor, J. and Somerville, L. The conversion of amitraz to BTS 24068
in dog gastric juice. Unpublished report no. AX 77010; 1977, from The
Boots Company submitted to WHO.
Toyoshima, S., Fujita. H., Sato, H., Sato. R., Sato, S. and Kashima,
M. JA-119 (BTS 27419). Three-month feeding study on rats. Unpublished
report E 76014; 1972a, from The Boots Company submitted to WHO.
Toyoshima, S., Fujita H., Sato, H., Sato, R., Sato, S. and Kashima, M.
JA-119 (BTS 27419). Three month feeding study on mice. Unpublished
report no. K 76015; 1972b, from The Boots Company submitted to WHO.
Tuplin, J.A. and Wilcox, A. Amitraz. Ames-tests on a sample produced
by Nissan Chemical Industries. Unpublished report no. TX 78068; 1978,
from The Boots Company submitted to WHO.
Weisburger, E.K. et al. Testing of twenty-one environmental aromatic
amines or derivatives for long-term toxicity or carcinogenicity. J.
Environ. Path. Toxicol. 2, 325-356.
Wilcox, P. BTS 27419: Mutagenicity study in the intraperitoneal
host-mediated assay. Unpublished report no. TX 76028; 1976, from The
Boots Company submitted to WHO.
Wilcox, P. Amitraz impurity BTS 33220: in vitro bacterial
mutagenicity testing. Unpublished report no. TXA 79025; 1979, from The
Boots Company submitted to WHO.
Zimmer, D.M., Mazurek, J.H. and Bhuyan, B.K. Evaluation of amitraz
(U-36.059) and its metabolites in the Salmonella microsome test.
Unpublished report no. 72068/77/7269/002; 1977, from The Boots Company
submitted to WHO.
 Molecular formula C19H23N3
Other information on identity and properties
Molecular weight 293
State off-white crystalline solid
Melting point 86-87°C
Vapour pressure 3.8 × 10-7 mm Hg at 20°C
Solubility Less than 1 mg/l in water at room temperature;
readily soluble at room temperature in most
organic solvents, 666 g/l in xylene, 500 g/l in
acetone, and 23.8 g/l in methanol.
Stability
Amitraz is a very weak base. Potentiometric titration gives pK
values of 4.0, 1.6 and 1.1, but the significance of the two lower
figures is doubtful because the compound is unstable in acid
solution, undergoing hydrolysis to yield ultimately
2,4-dimethylformanilide (Table 1,III) and methylamine.
Amitraz is relatively stable to heating, either alone or in a dry
inert solvent such as xylene or toluene. A xylene solution can be
boiled for 7 days without detectable change and the pure material
can be heated in air at 190°C with only slight discolouration.
Methanolic solutions are unstable, but solutions in
dimethylformamide or isopropanol are less so.
A stream of oxygen has no effect when passed through a solution of
amitraz in xylene at room temperature for 6 hours, but at reflux
temperature some decomposition occurs. A slow deterioration of the
moist compound occurs on prolonged standing (Martin and Worthing,
1979).
Technical material
The technical material is of a pale straw colour, and typically has
a purity of 97-99% at the time of synthesis. The minimum purity
specified by the manufacturer is 93%.
The principal impurity in the freshly manufactured product is
NN'-bis(2,4-dimethylphenyl) formamidine (Table 1,VI)
Storage stability of technical material
Storage stability tests were carried out in 3 countries on
technical amitraz stabilised with paraformaldehyde sachets inside
sealed drums (Nix, 1979). The results for the longest periods of
storage may be summarised as follows:
Country Period Mean loss of active Content of
of storage ingredient (% of (III) (%)
initial content) Initial Final
Japan 24 months Nil <0.2 0.25
plus travel
to Japan and
back
Nigeria 22 months 4.4 <0.25 1.0
plus travel to
to Nigeria 2.0
and back
Colombia 12 months 0.5 0.25 0.25
plus travel to
to Colombia 0.50
and back
Formulations
Amitraz is available as emulsifiable concentrates (200 g ai per
litre for crop-protection, 125 g ai per litre for animal use) and
wettable powders (500 or 250 g ai per kg).
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
In rats orally dosed with 14C-labelled amitraz recovery within 3
days of the administration dose was 53-85% in urine, 17-47% in
faeces, and <0.1% in expired air. Peak plasma levels occurred
about 1 hour after dosing. Highest residues were found in liver,
kidney and muscle after 0.75-1.5 hours, diminishing thereafter. In
urine at least 4 metabolites were found. In faeces 6 metabolites
were detected of which the major component was identified as BTS
27271 (Lewis, 1971 a).
During repeated oral dosing of 14C-labelled amitraz to rats highest
residues were found in thyroid and adrenal glands, liver, skin,
spleen and eyes. After dosing ceased, a considerable decrease of
residues was observed. Radioactivity in blood was mainly cell
bound. Seven days after the final dose small but significant
residues were detected in liver, spleen, skin and adrenals
(Somerville, 1973 a).
TABLE 1. Impurities Found In Technical Amitraz
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
a. N-2,4-dimethylphenyl-N-
methylformamidine
"formamidine" (II) TLC 0.2% 0-0.25% 2.0%
Molecular formula C19H23N3
Other information on identity and properties
Molecular weight 293
State off-white crystalline solid
Melting point 86-87°C
Vapour pressure 3.8 × 10-7 mm Hg at 20°C
Solubility Less than 1 mg/l in water at room temperature;
readily soluble at room temperature in most
organic solvents, 666 g/l in xylene, 500 g/l in
acetone, and 23.8 g/l in methanol.
Stability
Amitraz is a very weak base. Potentiometric titration gives pK
values of 4.0, 1.6 and 1.1, but the significance of the two lower
figures is doubtful because the compound is unstable in acid
solution, undergoing hydrolysis to yield ultimately
2,4-dimethylformanilide (Table 1,III) and methylamine.
Amitraz is relatively stable to heating, either alone or in a dry
inert solvent such as xylene or toluene. A xylene solution can be
boiled for 7 days without detectable change and the pure material
can be heated in air at 190°C with only slight discolouration.
Methanolic solutions are unstable, but solutions in
dimethylformamide or isopropanol are less so.
A stream of oxygen has no effect when passed through a solution of
amitraz in xylene at room temperature for 6 hours, but at reflux
temperature some decomposition occurs. A slow deterioration of the
moist compound occurs on prolonged standing (Martin and Worthing,
1979).
Technical material
The technical material is of a pale straw colour, and typically has
a purity of 97-99% at the time of synthesis. The minimum purity
specified by the manufacturer is 93%.
The principal impurity in the freshly manufactured product is
NN'-bis(2,4-dimethylphenyl) formamidine (Table 1,VI)
Storage stability of technical material
Storage stability tests were carried out in 3 countries on
technical amitraz stabilised with paraformaldehyde sachets inside
sealed drums (Nix, 1979). The results for the longest periods of
storage may be summarised as follows:
Country Period Mean loss of active Content of
of storage ingredient (% of (III) (%)
initial content) Initial Final
Japan 24 months Nil <0.2 0.25
plus travel
to Japan and
back
Nigeria 22 months 4.4 <0.25 1.0
plus travel to
to Nigeria 2.0
and back
Colombia 12 months 0.5 0.25 0.25
plus travel to
to Colombia 0.50
and back
Formulations
Amitraz is available as emulsifiable concentrates (200 g ai per
litre for crop-protection, 125 g ai per litre for animal use) and
wettable powders (500 or 250 g ai per kg).
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
In rats orally dosed with 14C-labelled amitraz recovery within 3
days of the administration dose was 53-85% in urine, 17-47% in
faeces, and <0.1% in expired air. Peak plasma levels occurred
about 1 hour after dosing. Highest residues were found in liver,
kidney and muscle after 0.75-1.5 hours, diminishing thereafter. In
urine at least 4 metabolites were found. In faeces 6 metabolites
were detected of which the major component was identified as BTS
27271 (Lewis, 1971 a).
During repeated oral dosing of 14C-labelled amitraz to rats highest
residues were found in thyroid and adrenal glands, liver, skin,
spleen and eyes. After dosing ceased, a considerable decrease of
residues was observed. Radioactivity in blood was mainly cell
bound. Seven days after the final dose small but significant
residues were detected in liver, spleen, skin and adrenals
(Somerville, 1973 a).
TABLE 1. Impurities Found In Technical Amitraz
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
a. N-2,4-dimethylphenyl-N-
methylformamidine
"formamidine" (II) TLC 0.2% 0-0.25% 2.0%
 b. 2,4-dimethylformanilide
"formanilide" "DMF" (III) TLC 0.2% 0-0.25% 7.0% by
difference
b. 2,4-dimethylformanilide
"formanilide" "DMF" (III) TLC 0.2% 0-0.25% 7.0% by
difference
 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
c. N,N'-bis-(2,4-dimethyl-phenyl)
formamidine
"diformamidine" (VI) GLC 0.1% 0-3% 6.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
c. N,N'-bis-(2,4-dimethyl-phenyl)
formamidine
"diformamidine" (VI) GLC 0.1% 0-3% 6.0%
 d. 2,4-dimethylaniline
"xylidine" (DMA) (IV) TLC 0.05 0-0.1% 0.3%
d. 2,4-dimethylaniline
"xylidine" (DMA) (IV) TLC 0.05 0-0.1% 0.3%
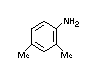 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
e. 1,3,5-tri-(2,4-dimethyl-phenyl)
-1,3,5-triazapenta-1-4-diene (VII) GLC 0.2% 0-0.25% 2.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
e. 1,3,5-tri-(2,4-dimethyl-phenyl)
-1,3,5-triazapenta-1-4-diene (VII) GLC 0.2% 0-0.25% 2.0%
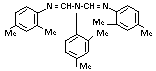 f. N,N'-bis-2,4-dimethyl-phenyl
-N-methyl formamidine (VIII) GLC 0.1% 0-0.25% 2.0%
f. N,N'-bis-2,4-dimethyl-phenyl
-N-methyl formamidine (VIII) GLC 0.1% 0-0.25% 2.0%
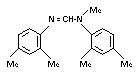 TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
g. Ethyl-N-2,4-dimethyl-phenyl
formimidate
"formimidate" (IX) TLC 0.5% 0-0.5% 2.0%
TABLE 1. Continued...
Chemical Name Referred to Method of Limit of Typical Specified
in text as Analysis Detection Content1 Limit
g. Ethyl-N-2,4-dimethyl-phenyl
formimidate
"formimidate" (IX) TLC 0.5% 0-0.5% 2.0%
 1 Typical Content specifically refers to freshly manufactured material
Twenty-four hours after dermal application of labelled amitraz in
acetone solution to rats approximately 50% of the dose had been
excreted in urine and faeces (Lewis, 1971 b).
Ninety-six hours after a single oral dose of 14C-labelled amitraz to
dogs the recovery in the excreta was 81% (57% in urine and 24% in
faeces). The peak plasma levels varied between 1.5 and 6 hours after
dosing for the male and the female dog respectively (Lewis, 1971 c).
One to four days after dosing significant residues were found in
liver, kidney, skin, bile and the pigmented tissue of the eye (iris,
retina and choroid and sclera) (Lewis, 1971 c; Hamilton and
Somerville, 1974 a).
In calf and mice a similar pattern of distribution and elimination of
radioactivity was observed after oral treatment with 14C-ring
labelled amitraz (Somerville, 1973 b; Hamilton, 1976).
A lactating cow was treated dermally twice with a 7-day interval with
14C-amitraz. In milk the maximal concentration of radioactivity was
0.09 ml/l. Nine days after the second application the concentration
was 0.03 mg/l (Somerville, 1972).
Metabolites of labelled amitraz measured in liver and urine showed
similar patterns for all species examined, i.e. rat, mouse, dog, cat
and calf. At least 6 metabolites were present of which conjugates of
3-methyl-4-amino-benzoic acid (BTS 28369) were predominant. In urine
of dogs and rats a difference in number and type of metabolites
between males and females was found (Jones, 1973 c).
The proposed metabolic pathway is given in figure 1.
In vivo and in vitro degradation studies in dog gastric juice of
amitraz revealed rapid hydrolysis to BTS 27271, BTS 27919 and BTS
24868 (Somerville and Hughes, 1973 e; Taylor and Somerville, 1977).
Administration of BTS 27271 to cats and dogs resulted in a similar
distribution pattern and metabolic degradation as was observed for
amitraz (Jones, 1973 d; Hamilton and Somerville, 1973; Hamilton and
Somerville, 1974 c).
From accumulation-elimination studies of BTS 27271 in dogs the half
lives in retina/choroid and the iris were estimated to be 1.5 and 3
days respectively (Somerville and Hamilton, 1974).
2,4-Xylidine (BTS 24868) administered to rats predominantly oxidized
to 3-methyl-4-amino-benzoic acid (BTS 28369). This metabolite is
excreted as the acetanilide conjugate (Lindstrom, 1961).
3-methyl-4-amino-benzoic acid (BTS 28369) administered in a single
oral dose to dogs gives significant residues in iris, liver and skin,
24 hours after dosing. The excretion of the compound was maximal in
the first 7 hours (Hamilton and Somerville, 1974 b).
1 Typical Content specifically refers to freshly manufactured material
Twenty-four hours after dermal application of labelled amitraz in
acetone solution to rats approximately 50% of the dose had been
excreted in urine and faeces (Lewis, 1971 b).
Ninety-six hours after a single oral dose of 14C-labelled amitraz to
dogs the recovery in the excreta was 81% (57% in urine and 24% in
faeces). The peak plasma levels varied between 1.5 and 6 hours after
dosing for the male and the female dog respectively (Lewis, 1971 c).
One to four days after dosing significant residues were found in
liver, kidney, skin, bile and the pigmented tissue of the eye (iris,
retina and choroid and sclera) (Lewis, 1971 c; Hamilton and
Somerville, 1974 a).
In calf and mice a similar pattern of distribution and elimination of
radioactivity was observed after oral treatment with 14C-ring
labelled amitraz (Somerville, 1973 b; Hamilton, 1976).
A lactating cow was treated dermally twice with a 7-day interval with
14C-amitraz. In milk the maximal concentration of radioactivity was
0.09 ml/l. Nine days after the second application the concentration
was 0.03 mg/l (Somerville, 1972).
Metabolites of labelled amitraz measured in liver and urine showed
similar patterns for all species examined, i.e. rat, mouse, dog, cat
and calf. At least 6 metabolites were present of which conjugates of
3-methyl-4-amino-benzoic acid (BTS 28369) were predominant. In urine
of dogs and rats a difference in number and type of metabolites
between males and females was found (Jones, 1973 c).
The proposed metabolic pathway is given in figure 1.
In vivo and in vitro degradation studies in dog gastric juice of
amitraz revealed rapid hydrolysis to BTS 27271, BTS 27919 and BTS
24868 (Somerville and Hughes, 1973 e; Taylor and Somerville, 1977).
Administration of BTS 27271 to cats and dogs resulted in a similar
distribution pattern and metabolic degradation as was observed for
amitraz (Jones, 1973 d; Hamilton and Somerville, 1973; Hamilton and
Somerville, 1974 c).
From accumulation-elimination studies of BTS 27271 in dogs the half
lives in retina/choroid and the iris were estimated to be 1.5 and 3
days respectively (Somerville and Hamilton, 1974).
2,4-Xylidine (BTS 24868) administered to rats predominantly oxidized
to 3-methyl-4-amino-benzoic acid (BTS 28369). This metabolite is
excreted as the acetanilide conjugate (Lindstrom, 1961).
3-methyl-4-amino-benzoic acid (BTS 28369) administered in a single
oral dose to dogs gives significant residues in iris, liver and skin,
24 hours after dosing. The excretion of the compound was maximal in
the first 7 hours (Hamilton and Somerville, 1974 b).
 TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Effect of amitraz on the oestrous cycle
In female SFP CFLP mice fed a diet containing 400 mg amitraz/kg feed
for up to 33 weeks a reduction in body weight and an increase in food
consumption was observed. Analysis of vaginal smears indicated that
the mean duration of oestrus as well as the incidence of a prolonged
oestrus was significantly increased due to treatment. No effects were
found on the level of ß-oestradiol in plasma (Brown et al, 1978;
Channon and Cryer, 1978).
Female rats maintained on a diet containing 200 mg amitraz/kg for 18
weeks had longer oestrus cycles than control rats of the same age,
resulting from prolonged periods of oestrus or dioestrus (Merryman and
Sutton, 1972).
Effect on pregnancy, parturation and care of the young
Amitraz was administered in doses of 0, 1, 3 or 12 mg/kg bw to
Boots-Wistar rats from day 1 of pregnancy until the young were weaned
at 21 days old. 12 mg/kg bw reduced the weight gain of the dams as
well as the mean number of young born and alive at day 4. No effects
were observed for mothers and offspring for the other dose groups
(Sutton, 1973 c).
Three-generation feeding study
Groups of 10 male and 20 female newly-weaned Boots-Wistar rats were
fed amitraz in dietary concentrations of 0, 15, 50 or 200 mg/kg.
After ten weeks the animals were mated. After the F1 generation was
weaned 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. At 200 mg/kg amitraz causes decrease
of growth and food consumption in the F0 generation, furthermore a
decrease in fertility and viability was found. The 200 mg/kg diet
group was terminated when the F1 generation was weaned, due to the
very low survival. In the 50 mg/kg group no effect was found on the
number of litters and mean litter size, however in all generations a
decrease in number of young alive at 21 days was observed. No further
effects due to treatment were found in this and the other dose group
(Sutton, 1973 d; Lancaster and Williams, 1978).
Special Studies on teratogenicity
Rat
Groups of 11-13 female rats received amitraz in doses of 0, 1, 3 or 12
mg/kg bw daily from day 8 to day 20 of pregnancy. The rate were
killed on day 21 and the uterine content was examined. Average litter
size, foetal viability and implantation index were not affected. In
the highest dose group foetal weight was less than in the controls,
and the calcification of the stermebrae was less advanced (Sutton,
1973 b).
Rabbit
Groups of 8-10 New Zealand rabbits were treated with amitraz in doses
of 0, 1, 5 or 25 mg/kg bw from day 6 to 18 of pregnancy and killed on
day 30. In the highest dose group number of litters and mean litter
size were decreased. Only in this dose group abortions were observed.
No increase in congenital abnormalities was found (Sutton, 1973 a).
Special studies on mutagenicity
Bacterial systems
Amitraz and its major metabolites (BTS 27271, 27919, 28369) were
tested in bacterial in vitro systems, with and without activation,
against Salmonella typhimurium TA 1535, 1437, and 1538 and
Escherichia coli Wp2 and Wp2 uvrA. In these systems no mutagenic
activity was detected (Everest and Wilcox, 1976 a, 1976 b; Tuplin and
Wilcox, 1978).
The impurity BTS 33220 (N,N'-bis-2,4-dimethylphenyl-N-methyl
formamidine) showed weak positive activity against TA 100 in the
presence of microsomes. Amitraz spiked with 1% BTS 33220, however,
did not show any mutagenic action (Wilcox, 1979; Everest and Wilcox,
1979).
In the TA 100 strain BTS 24868 and BTS 27919 exhibited weak but
consistent mutagenic activity (Zimmer at al, 1977).
Amitraz showed no increase in point mutational activity against
Salmonella typhimurium G 46, TA 1532 and TA 1964 when tested in
the mouse perivisceral host-mediated assay at single oral doses up to
400 mg/kg bw (Wilcox, 1976; Everest, 1976).
Dominant Lethal Studies
Female mouse
Groups of 48 female mice were treated orally for 5 consecutive days
with amitraz in doses of 0, 12 or 50 mg/kg bw.
Subgroups of 12 females were mated with untreated males on day 3, 9,
14 or 19 post-dosing. A slight increase in mean post-implantation
loss and decrease in mean viable litter size at 50 mg/kg was found
(Palmer and James, 1977 a).
Male mouse
Male mice of proven fertility were treated with amitraz for 5
consecutive days at doses of 0, 12 or 50 mg/kg. Following treatment
males were mated with untreated females for six consecutive weeks. A
significantly lower implantation rate was found at 50 mg/kg at the
first mating and a higher implantation rate at 12 mg/kg at the 5th
mating. No changes were found in respect to number of embryonic
deaths and post implantation losses (Palmer and James, 1977 b).
DNA damage
Amitraz, BTS 27271, 27919, 24868 and 28369 were tested at several dose
levels in the DNA damage/alkaline elution assay with or without
activation systems, using chinese hamster lung fibroblasts. In this
test system no indication was obtained that amitraz or its metabolites
induce DNA damage (Petzold et al, 1977).
Special studies on carcinogenicity
Mouse
Groups of 50 male and 50 female mice (CFLP strain) were fed a diet
containing 0, 25, 100 or 400 mg/kg for 80 weeks. The animals of the
100 and 400 mg/kg diet groups gained less weight than the control over
the first 40 weeks. The 25 mg/kg diet group gained more weight than
the control group from week 10 onwards and showed a 20% greater body
weight gain at the end of the experiment. Calculated over 80 weeks,
food consumption was increased for male mice at 100 and 400 mg/kg diet
and decreased at 25 and 100 mg/kg diet and increased at 400 mg/kg diet
for female mice. The survival rate was similar in all groups. An
increased incidence of lymphoreticular tumours was observed for female
mice at 400 mg/kg only; 49% compared with 23% in control animals. A
slight, although not statistically significant (p>5%) increase of all
types of liver cell tumours was found in both sexes fed 400 mg/kg. No
other pathological findings related to treatment were reported
(Burnett et al, 1976; Kakuk 1979).
Rat
Amitraz was administered at dietary concentrations of 0, 15, 50 or 200
mg/kg to groups of 40 male and 40 female Ash Wistar rats for 2 years.
Due to treatment in the highest dose group the animals tended to be
nervous, aggressive and excitable. In this group growth rate was
depressed for both sexes and food intake was slightly less in the
first weeks of the experiment. No dose-related anomalies were found
on organ weights and haematological, biochemical and histopathological
examination. Furthermore, no difference in incidence, type and time
of appearance of tumours in control and treated rats was observed
(Sutton and Offer, 1973).
2,4-Xylidine carcinogenicity study
Mouse
2,4-Xylidine (BTS 24868) was fed in the diet of 3 groups of HAM/ICR
mice (25 males and 25 females) in a concentration of 0, 1000 or 2000
mg/kg. Since these doses were not tolerated the experiment was run
again with doses of 0, 125 or 250 mg/kg diet. Dosing was
discontinuated after 18 months. After 24 months the animals were
killed and examined for tumours. In female mice an increased
incidence of lung tumours was observed in the highest dose group (NCI,
1973).
Rat
2,4-Xylidine (BTS 24868) was administered to groups of 25 male Charles
River rats for approximately 3 months at doses of 2000 or 4000 mg/kg
diet; thereafter the doses were changed to 0, 250 or 500 mg/kg diet
respectively, for approximately 2.5 months and subsequently, the doses
were set at 0, 500 or 1000 mg for the rest of the 18 months period.
At that time the feeding of the test compound was discontinued until
sacrifice 24 months after starting the experiment. A slight increase
in lung tumours in the highest dose group was observed. Furthermore
in the treated groups bladder and liver tumours were observed,
which were not found in the controls (NCI, 1973). However, in
contrast to their earlier conclusions, the authors finally stated that
there was no carcinogenic effect in the rat (Weisburger et al,
1978).
Special studies on pharmacological effects
In dogs amitraz and BTS 27271 produce similar effects when given
orally in doses of 4, and 0.5 mg/kg bw respectively either after
single or repeated administration. Signs included sedation, ataxia,
protrusion of the tongue, bradycardia and hypothermia (Morgan et al,
1975 b).
An experiment comparing oral and topical application of amitraz in
pigs revealed a decrease in rectal temperature after oral dosing (25
mg/kg bw and higher), whereas no effects were found after topical
application up to 200 mg/kg bw (Morgan et al, 1975 a).
No flushing could be demonstrated in pigs after topical or
intramuscular administration of amitraz and BTS 27271 although other
pharmacological changes were apparent (Parkinson, 1975).
Amitraz and BTS 27271 did not have any effect on trafuril-induced or
UV-induced erythema in the guinea pig (Patton, 1970; Parkinson and
Yates, 1971).
BTS 27271 was antidiuretic in mice at 90 mg/kg bw whereas amitraz in
the same dose was ineffective (Sim, 1970).
Intravenous administration of amitraz caused vasoconstriction in the
ear vessels of cats (1-10 µg/kg) and fall in blood pressure and
bradycardia (>20 µg/kg) in dogs. BTS 27271 evoked similar effects in
dogs (40 µg/kg). Both compounds had no effect on the response of the
blood vessels of the rabbit ear to antidromic stimulation or to
catecholamines (Anonymous, 1972; Parkinson et al, 1971 a; Parkinson
et al 1971 b; Patton, 1973 b).
Amitraz induced hypothermia in mice under various ambient conditions.
This effect was greater in intensity and duration in females (Sutton,
1972 a; Berry, 1976).
BTS 27419, BTS 27271 and BTS 21103 were tested for their potency of
central monoamine oxidase inhibition in mice and rats, by
investigating the influence on reserpine-induced ptosis and inhibition
of exploratory activity. No effects were observed in mice. At high
doses these compounds antogonise reserpine-induced ptosis in the rat,
suggesting a relative potency in the order BTS 27271 > BTS 27419 >
BTS 21103 (Parkinson, 1974 a).
BTS 27419, BTS 27271 and BTS 21103 potentiated the pressor response to
tyramine in the pithed rat at doses of 40, 80 and 80 mg/kg bw
respectively. Similar effects have been shown with monoamine oxidases
inhibitors (Parkinson, 1974 b).
Special studies on sensitisation and irritation
Neither amitraz nor BTS 27271 showed sensitisation activity in guinea
pigs (Sutton, 1971).
Amitraz was not irritant to the rabbit eye after a single
administration (Sutton and Metcalf, 1972).
Acute toxicity
Acute oral administration of amitraz caused CNS depression in all
species studied. Dogs were more susceptible than the others. Toxic
manifestations: ataxia, subnormal rectal temperature and
cardiovascular disturbance.
TABLE 2. Acute Toxicity of Amitraz
Species Sex Number Route LD50 (mg/kg) Reference
mouse M 5 oral >1600 Patton and Sutton, 1971
rat M 5 oral approx. 800 Patton and Sutton, 1971
Shaw, 1973a
rat M 5 i.p. approx. 800 Shaw, 1971, 1973a
rat M,F 6 inhalation (6 h LC50) Berczy et al, 1972
65 mg/kg
rat M 5 dermal > 1600 Patton and Sutton, 1971
guinea pig F 3 oral 400-800 Patton and Sutton, 1971
Sutton 1970c
rabbit F 2 oral > 100 Patton and Sutton, 1971
rabbit M,F dermal > 200 Sutton and Williams, 1972
dog M,F 2 oral approx. 100 Patton and Sutton, 1971
baboon M,F 2 oral 100-250 Patton, 1973a
BTS 27271 is more toxic than amitraz. Signs of poisoning are similar
to those shown by amitraz. A summary of acute toxicity data of
impurities degradation products and metabolites of amitraz is given in
table 3.
Short-term studies
Mouse
Groups of 10 male and 10 female CFLP-mice (age 4 weeks) were dosed
with 3, 12, 50 or 200 mg/kg/day amitraz by gavage (suspended in 0.4%
cellosize; 0.1 ml/10 g bw) for 90 days. Control groups of 20 males
and females received the same volume of vehicle alone.
Eight mice (5 males and 3 females) given 200 mg/kg/day died within 3
weeks after becoming progressively emaciated and inactive. The
surviving animals of this group were in poor condition. In the other
groups 5 animals died of causes probably not related to treatment.
The 50 and 200 mg/kg groups showed a decreased body weight gain over
the experimental period for both sexes. The body weight gain of male
TABLE 3. Acute Toxicity of Impurities, Degradation Products and Metabolites of Amitraz
compound species number route LD50 (mg/kg) vehicle references
BTS 27271 mouse 5 oral 100-200 HCl salt aq. sol. Sutton, 1970a
rat 5 oral 200 idem Sutton, 1970b
guinea pig 3 oral 200 idem Sutton, 1970c
dog 2 oral >20 gelatin capsules Morgan, 1973a
Morgan and Williams, 1973a
rabbit 2 oral >25 HCl salt aq. sol. Sutton, 1972b
BTS 27919 mouse 5 oral >1600 idem Anonymous, 1971a
rat 4 oral approx. 1600 idem Shaw, 1973b
dog 2 oral >100 gelatin capsules Morgan, 1974a
Morgan and Turnhull, 1973
BTS 28369 dog 2 oral >250 idem Morgan and Sheperd, 1974
Morgan 1974b
BTS 24868 mouse 5 oral 800 - Anonymous, 1971a
BTS 33220 rat 5 oral 800-1600 10% acacia mucilage O'Donovan and Smithson, 1978
BTS 28037 dog 2 oral >250 gelatin capsules Morgan, 1973b
Morgan and Williams, 1973b
and female mice of the 3 and 12 mg/kg groups was slightly decreased.
GPT activity increased in both sexes from 50 mg/kg. Male mice showed
a slight increase in haematocrit at 3 and 12 mg/kg. Reducing
substances in blood were found to be decreased in both sexes at 12 and
50 mg/kg. Organ weight/body weight ratio of the kidney was increased
in males from 12 mg/kg; of the liver increased for both sexes at 50
and 200 mg/kg and of the spleen for males at 50 and 200 mg/kg and for
females at 200 mg/ kg only.
Slight to moderate hepatocyte and/or nuclear enlargement was observed
dose-relatedly at 12, 50 and 200 mg/kg. Furthermore, in the liver of
some females of the highest dose group, intranuclear inclusion, bile
duct proliferation and inflammatory infiltration in the portal tracts
and nodular hyperplasia were observed (Shaw and Williams, 1974).
Four groups of 13 female and 13 male ICR-SLC mice received amitraz in
doses of 0, 3, 12 or 50 mg/kg bw, in the diet (presuming that the
daily consumption was 5 g/animal) for 90 days. Six males died at an
early stage of the experiment. According to the authors death was not
due to treatment. In both sexes growth was depressed in the 12 and 50
mg/kg groups. Food and water intake were slightly decreased at 12 and
50 mg/kg; for males this was more pronounced than for females. In
male mice a slight decrease in food efficiency was observed at 50
mg/kg over the whole experimental period. For male mice relative
brain weight was increased at 50 mg/kg and heart weight at 12 and 50
mg/kg. The relative kidney weight of female mice at 50 mg/kg was
decreased. SGPT and aldaline-phosphatase were decreased whereas the
albumin-globulin ratio was increased in female mice of the two highest
dose groups. Male mice only showed a higher albumin-globulin ratio at
50 mg/kg. At the end of the experiment macroscopically slight black
discolouration of the liver was seen predominantly in the highest dose
group. The only histopathological change probably directly related to
the administration of the compound was a slight centrolobular
degeneration in the liver at 3, 12 and 50 mg/kg (Toyoshima et al,
1972 b).
Rat (inhalation)
Three groups of 6 female and 6 male rats were exposed daily for six
hours to amitraz at concentrations of 0.01, 0.1 or 1.0 mg/l air
respectively for 14 days over a period of three weeks. During
exposure to 0.1 mg/l dyspnoea, eye-irritation and hyposensitivity to
noise were observed. In addition at 1.0 mg/l ataxia, increased nasal
secretion, polyuria, body tremors and slight coma were found. Body
weight gain was markedly reduced in both sexes at 0.1 and 1.0 mg/l
air. Haematological studies revealed a decreased PCV, haemoglobin and
red blood cell count and increased number of neutrophils in both sexes
and a decreased NCHC and number of lymphocytes in males of the highest
dose group (no other groups examined). At 0.1 and 1.0 mg/l
significant alterations of the relative weights of brain, kidney,
thyroid and adrenal glands, and gonads were found for males, and of
brain, kidney and adrenals of female rats. Liver, heart and pituitary
for males and liver for females were increased in the highest dose
group only. No pathological findings related to treatment were found
(Berozy et al 1973).
[text missing] abnormalities, dosing was restarted for 1 week in which
toxic reactions returned. Rats of the 12 mg/kg group appeared
irritable and excitable. In male rats body weight gain was depressed.
A significantly depressed relative liver weight was seen at 50, 12 as
well as 3 mg/kg for males but only at 50 mg/kg for females.
Alkaline-phosphatase activity was decreased at 50 and 12 mg/kg (Sutton
and Williams, 1971).
Rat
Groups of 10 Wistar rats of each sex received amitraz in doses of 0,
3, 12 or 50 mg/kg bw in the diet (presuming the daily food consumption
was 20 g/animal) for 90 days. Body weight gain was decreased in the
50 mg/kg group for both sexes and in the 12 mg/kg group for females
only. Food and water consumption were decreased in all dose groups.
The relative weight of brain, heart, lung, liver, kidney, spleen and
uterus or testes were increased for both sexes in the highest dose
group. In addition the adrenals were decreased and thymus was
increased in weight in male rats of the highest dose group. Male rats
showed a dose-related decrease in number of blood platelets in the 12
and 50 mg/kg group. Female rats showed a significant decrease in
eosinophils in the highest dose group. In serum of male rate of the
highest dose group a decrease was observed in the Alk Pase activity
and blood sugar concentration, and an increased serum potassium
concentration. Urinalysis revealed an increased number of rats with
proteinurea from the 12 mg/kg group onwards. In male rate urine
potassium concentration was significantly decreased at 12 and 50
mg/kg, in females only at 50 mg/kg (Toyoshima et al, 1972 a).
Rabbit (dermal)
Amitraz dissolved in acetone was applied to the intact skin of groups
of New Zealand rabbits (4 males and 4 females) in 15 doses of 0, 50 or
200 mg/kg bw over a 2l-day period. One male and three females at 200
mg/kg and one male at 50 mg/kg died intercurrently. Sedation was
observed in males at 50 mg/kg and in both sexes at 200 mg/kg. In
males of both dose groups slight to moderate erythema, desquamation of
skin and subcutaneous hemorrhage were observed. In both sexes
decreased body weight and food consumption were found in the 50 and
200 mg/kg dose groups. In males at 200 mg/kg underweight testes with
tubular degeneration were found (Sutton, 1973 e).
Dog
Amitraz in gelatine capsules was given to groups of two male and two
female beagle dogs in oral doses of 0, 0.25, 1 or 4 mg/kg once daily
for 90 days. Clinical signs were observed in all dosed groups
consisting of CNS depression, ataxia and vomiting during the first
days within 3-6 hours after dosing. These dogs were subdued
throughout the experimental period. When examined at intervals during
the treatment period the dogs consistently had subnormal rectal
temperatures and pulse rates. These parameters returned to normal
within 6-24 hours after administration. The animals of the 1 and 4
mg/kg dose groups showed a significant increase in blood sugar
concentration which was maximal 6 hours after dosing and returned to
normal within 24 hours. In the same dose groups an increased amount
of glucose and total reducing substances was found in the urine in
weeks 2 and 3. In the highest dose group an increased liver weight
was observed in both sexes. Except in control animals, livers showed
enlargement of the central and midzonal hepatocytes. Hyperplasia of
the small periportal hepatocytes and increase in binucleate cells were
particularly evident in the highest dose group. At all dose levels
thinning of the zonae fasciculata and reticularis, sometimes
associated with slight hyperplasia of the zona glomerulosa in the
adrenales, was observed (Patton and Williams, 1971).
Metabolites
Rat (90-day test with BTS 27271)
Groups of 10 male and 10 female Boots-Wistar rats were dosed orally by
gastric intubation with BTS 27271 for 90 days at doses of 0.25, 1, 3
or 12 mg/kg bw/day. In the 12 mg/kg group the rats were nervous and
difficult to handle. Body weight was slightly lower at 3 and 12 mg/kg
compared to the controls, the males being more affected than the
females. At the end of the experiment the number of erythrocytes was
decreased in the 3 and 12 mg/kg group for females and in the 12 mg/kg
group for males. Haemoglobin and haematocrit values were decreased at
12 mg/kg for males only. Owing to the variation in the number of
lymphocytes the leucocyte counts were decreased for male and increased
for female rats in the highest dose group. Concomitantly a
significant decrease in number of eosinophils was found. Urinalysis
revealed an increased GPT activity in females, and a slight increase
in urine volume and decrease in specific gravity in the males of the
highest dose group. Together with these changes an increase in BUN
was found. In terminal plasma samples a significant increase in
alkaline-phosphatase activity in both sexes given 12mg/kg was found.
The relative weights of adrenals, ovaries and uterus in the females
and the testes in the males given 3 and 12 mg/kg were increased. In
the highest dose group the weight of liver for females and spleen for
males was increased. Microscopic examination of rats given 3 or 12
mg/kg showed a slight increase in lymphoid infiltration with some
leucocytosis and a loss of glycogen in the liver (livers of female
rats were not examined at 3 mg/kg). In the highest dose group the
hearts of the female rats showed slight cellular accumulation (Shaw
and Williams, 1975).
Dog (90-day test with BTS 27271)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg BTS 27271/kg bw in gelatin capsules daily for three months. The
test compound was diluted in lactose to 1% BTS 27271 as free base.
Clinical signs in the 0.25 and 1 mg/kg group were abnormal quietness
and drowsiness. Between 1 and 4 hours after dosing the mean body
temperature and mean heart rates decreased significantly for the 0.25
and 1 mg/kg dose groups. In the highest dose group a significant
increase in liver weight was found. A slight reduction in thymus
weight was observed at 0.25 and 1 mg/kg. In the highest dose group
the urine volume was found to be increased. No histopathological
anomalies were found (Chesterman et al, 1973).
Rat (21-day test with BTS 28369)
BTS 28369 was administered orally to groups of 4-6 male and female
rate (Boots-Wistar) at dose levels of 0, 40, 100 or 250 mg/kg bw/day
for 21 days. In the highest dose group a slightly decreased weight
gain and BUN level was observed for males and an increased relative
weight of the spleen for females. No pathological changes due to
treatment were found (Shaw, 1975).
Dog (90-day test with BTS 28369)
Groups of 4 male and 4 female beagle dogs were dosed 0, 16, 40 or 100
mg BTS 28369/kg bw/day orally in gelatin capsules for 90 days. At 100
mg/kg bw slightly elevated urinary levels of total reducing substances
were found. No dose related effects were observed on behaviour, ECG,
heart rate, rectal temperature, body weight, food consumption,
haematology, blood biochemistry, organ weights and histopathology
(Morgan et al 1974).
Long-term studies
Rat
See under "Special studies on carcinogenicity".
Dog (2 years)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg amitraz/kg bw/day orally in gelatin capsules for 2 years. Three
hours after dosing all dogs given 1 mg/kg bw exhibited CNS depression
on day 1 and 2, blood sugar level was slightly increased at weeks 40
and 53 (the only weeks measured). No effects on haematological,
biochemical and histopathological parameters were reported (Morgan
et al, 1973).
OBSERVATIONS IN HUMANS
After a few complaints from people involved with the development of
amitraz, all workers in this department were interviewed by the
medical staff, which led to the following conclusions:
1) Skin flushing due to capillary dilatation may be caused by amitraz
or its precursor BTS 27271, or both.
2) The response appears to be the result of systemic absorption rather
than topical application.
3) Absorption is more likely to occur when large quantities are
processed. So far skin effects have not followed the use of
formulated materials in the course of animal or crop protection
trials.
4) Apart from the observation that the capillary circulation in the
skin appears to have been sensitised to trauma such as gentle friction
and radiant heat, no other information can be offered to explain the
phenomena described.
5) The effects appear to be temporary and disappear soon after contact
with the materials is discontinued (Moore, 1972).
Amitraz (about 10 mg) was applied to the arms of 9 volunteers as 0.02
ml of a 50% acetone solution. After 6 hours the arms were cleaned.
One volunteer, who had previously reacted to the compound, showed a
slight erythema localized to the patch area.
Three volunteers who had previously reacted to this compound were
tested using a single application of a paraffin-based ointment
containing 50% amitraz. One subject reported a delayed reaction
occurring 14 h after the initial application consisting of a reddening
of his previous reaction to this compound; no throbbing or other
symptomatology was observed and the reaction had faded by the morning
(Anonymous, 1971 b and c).
Six volunteers received a single oral dose of 2 mg BTS 27271 in a
double-blind crossover study. Blood pressure, pulse rate and oral
temperature were different comparing the volunteers receiving the
active treatment to those receiving placebo. This difference existed
just as much initially as during the observation period. Mental
alertness scores show that a number of subjects felt relatively more
alert on placebo (Hall et al, 1975).
In urine of production workers and volunteers the expected terminal
metabolite, BTS 28369 could be detected (Shirley, 1975 b, 1976;
Hughes, 1975).
RESIDUES IN FOOD
USE PATTERN
Amitraz is used for the control of pests on both animals and crops and
is used in countries throughout the world as an insecticide and
acaricide.
Treatment of crops
As an acaricide, amitraz is successfully used against red spider mites
on deciduous fruit crops, citrus, cotton and certain other crops. As
an insecticide it is used to control pear psylla and whitefly, and as
an ovicide against the major cotton lepidopterous pests.
Recommendations for its use on cotton, deciduous top fruit, citrus,
ornamentals and vegetables are shown in Tables 4 and 5.
Treatment of animals
Amitraz is effective against ticks, mange mites, lice and keds on
cattle, sheep, goats and pigs (Tables 6 and 7).
The species of skin parasites controlled by amitraz are shown in Table
8.
TABLE 4. Use Pattern on Crops
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
COTTON Lepidoptera
Cotton Bollworm
Heliothis spp.
Pink Bollworm
Pectinophora gossypiella
Red (Sudan) Bollworm
Diparopsis castanea Spray when eggs first 300,400, 1.5, 2.0,
appear in the crop 600 3.0
Cotton Leafworm and repeat at regular
Spodoptera spp. intervals throughout 500,600, 2.5, 3.0,
Leaf Perforator the season 200 3.5
Bucculatix thurberiella
Spiny (Spotted) Bollworm
Earias spp.
Whitefly Spray when whitefly first appear 500,600, 2.5, 3.0,
Bemisia tabaci in the crop and repeat at regular 700 3.5
intervals throughout the season.
Thrips spp.
Aphida Spray when these pests first 500, 600, 2.5, 3.0,
Aphis spp. appear in the crop and 700 3.5
Jassida repeat at regular intervals
Empoasca spp. throughout the season
Mites
Tetraaychus spp.
DECIDUOUS Panonychus ulmi Apply the first spray at 60-80% 20-60 1-3 400-1200 2-6
TOP FRUIT egg hatch and repeat at 2-3 week
intervals.
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Mites Spray at the first sign of infestation 40-60 2-3 800-1200 4-6
Tetranychus spp. including and repeat at 2-3 week
T. urticae, T. cinnabarinus intervals.
and T. pacificus
Aculus schlectendali Spray at the first sign of 20-30 1.0-1.5 400-600 2.3
Epitrimerus pyri infestation and continue at
regular 2-3 week intervals.
Codling Moth Apply the first spray immediately
Cydia pomonella after adult emergence and repeat
at 2-3 week intervals for at
least 5 applications. 50-70 2.5-3.5 1000-1400 5-7
Aphids
Myzus persicae Apply at the first sign of
Aphys pomi infestation and repeat at regular
Dysaphis pyri 2-3 week intervals. 50-70 2.5-3.5 1000-1400 5-7
Eriosoma lanigerum Will give good suppression
Phorodon humuli of aphids in an intensive
Dysaphis plantaginea spray programme against mites.
Apple Maggot
Rhagoletis pomonella Used in a full season's
White Apple Leaf Hopper programme against mites will
Typhlocyba pomaria give useful suppression of 50-70 2.5-3.5 1000-1400 5-7
San Jose Scale these pests.
Quadraspidictus perniciosus
Plum Curculio
Conotrachelus nenuphar
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Pears only Pear psylla Spray when attack first occurs and
Psylla pyricola repeat at regular intervals. The 30-50 1.5-2.5 600-1000 3-5
higher rate is required to give
consistently good control of
over-wintering adults.
Apples Only Leaf miners
Leucoptera scitella Apply the first spray at petal fall
Phyllonorycter blancardella and repeat at 2-3 week intervals 40-50 2-2.5 800-1000 4-5
with a minimum of 4 applications
CITRUS Mites
Panonychus citri Apply first spray at 60-80% egg 20-50 1-3 400-1200 2-6
hatch and repeat at 2-3 week
intervals.
Tetranychus spp. Spray at first sign of infestation 40-60 2-3 800-1200 4-6
and repeat at 2-3 week intervals.
Phyllocoptruta oleivora Spray at first sign of infestation 20-30 1-1.5 400-600 2-3
and continue at regular 2-3 week
intervals.
Scale Insects
Ceroplastea spp. Apply the first spray during the 50-60 2.5-3 1000-1200 5-6
Saissetia spp. crawler (larval) stage and repeat
at regular intervals.
ORNAMENTALS
Carnations, Mites
Chrysanthemums, Panonychus ulmi Apply the first spray at 60-80% 30-50 1.5-2.5 600-1000 3-5
Roses, Pot plants egg hatch with repeat sprays at
and other annuals 2-3 week intervals.
and perennials
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
VEGETABLES Tetranychus urticae Spray at the first sign of infestation)
SOFT FRUIT Tetranychus pacificus regular intervals )
and other Tetranychus )
species )
Acalitus essigi )
Aphids Under cold conditions ) 46-60 2-3 800-1200 4-6
Aphids gossypii and on some crops under glass, )
Aulocorthum spp. the high rate may be necessary )
Macrosiphum spp. to achieve consistently good )
control
TABLE 5. Use of Amitraz on Crops. Registrations (at August 1980) and Periods
Between Last Application and Harvest
Country Withholding Crop1
Period
Argentina 4 weeks apples
Australia 4 weeks pome fruit and stone fruit
Belgium 4 weeks apples, pears
Bulgaria not available apples
Chile 2 weeks fruit, vegetables
Cyprus 2 weeks apples, peaches, citrus, cucurbits
Czechoslovakia 1 week cucumbers
4 weeks apples, pears green beans, peppers, hops
Denmark 2 weeks apples, pears
East Germany 4 days cucumbers, tomatoes
4 weeks apples, pears
Ecuador 2 weeks cotton, stone fruit, citrus,
strawberries, tomatoes, cucumbers
El Salvador not available cotton
France 30 days fruit trees, pears
Greece 1 week apples, pears, citrus, vegetables
Guatemala not available cotton
Hungary 10 days apples, plums, wild strawberries,
vines, sugarbeet, soybeans
Italy 2 weeks citrus, vegetables, vines,
stone fruit
4 weeks apples, pears
Japan 2 weeks apples, pears, citrus
Jordan not available top fruit
Morocco 4 weeks cotton, top fruit, vegetables
Netherlands 4 weeks apples, pears
New Zealand 2 weeks pome fruit and stone fruit
Nicaragua not available cotton
Pakistan not available cotton
Peru not available cotton
Portugal 4 weeks apples, pears
Romania not available top fruit
South Africa 2 weeks apples
4 weeks cotton
South Korea not available apples, pears, citrus
Spain 1 week fruit, maize
Switzerland 3 weeks apples, pears3
Taiwan not available apples, pears, citrus
Turkey not available apples, pears, citrus, cotton
UK 2 weeks apples, pears
7 weeks2 hops
USA 4 weeks pears
1 Use on ornamentals not included.
2 Shorter withholding period under review - likely to be 4 weeks.
3 Based on informal information, subject to written confirmation
TABLE 6. Use Patterns on Animals
Animal Pests Timing Applications
Cattle ticks A second application 10-14 As a spray containing
mange mites days later may be necessary 0.025% a.i.
lice for full control of mange and lice.
Pigs mange mites Two applications, at low concentration As a spray.
lice 10-14 days after the first High concentration
will provide control. Alternatively 0.1%
a single application at high concentration Low concentration
may be recommended. 0.05% a.i.
Sheep ticks As a dip at 0.05% a.i.
mange mites Replenishments should be
lice made with 0.075% amitraz
keds
Goats ticks A second application 0-14 As a spray containing
mange mites days after the first my 0.05% a.i.
lice be necessary for full control
of mange and lice.
TABLE 7. Use of Amitraz on Animals. Registrations (at August 1980) and Periods Between Last
Application and Slaughter
Country Formulation1 Use/Animals Withholding Period
Argentina 12.5% e.c. Dip for sheep None
Australia 50% d.p. Spray for cattle None
50% d.p. Dip for cattle None
Brazil 12.5% e.c. Spray for cattle and Meat 14 days2
sheep Milk 1 day
Colombia 12.5% e.c. Dip for cattle Meat 14 days2
12.5% e.c. Spray for cattle Milk 1 day
Costa Rica 12.5% e.c. Spray for cattle None
Cyprus 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Denmark 12.5% e.c. Spray for pigs and Meat 6 days
cattle Milk 1 day
Jordan 12.5% e.c. Spray for sheep and goats None
Netherlands 12.5% e.c. Dip for pigs and sheep Pigs None
Sheep 4 weeks
Nicaragua 12.5% e.c. Spray for cattle None
Rhodesia 12.5% e.c. Spray for cattle None
12.5% e.c. Dip for cattle None
South Africa 12.5% e.c. Spray for cattle None
25% d.p. Spray for cattle None
25% d.p. Dip for cattle None
Spain 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Sweden 12.5% e.c. Spray or dip for cattle, Sheep 7 days
sheep and pigs Pigs and cattle 1 day
United Kingdom 12.5% e.c. Spray for pigs and Meat 1 day
cattle Milk None
12.5% e.c. Dip for sheep Meat 7 days
Milk None
Venezuela 12.5% e.c. Spray for cattle Meat 14 days2
Milk 1 day
Yugoslavia 12.5% e.c. Cattle None
1 Emulsifiable concentrate is denoted by "e.c." and dispersible powder by "d.p.".
2 These withholding periods were agreed upon early in the development programme
before most residue work had been carried out.
TABLE 8. Species Of Skin Parasites Controlled By Amitraz
(i) Ticks
Boophilus microplus
B. decoloratus
Rhipicephalus evertsi
Amblyomma hebraeum
A. cayennense
Hyalomma spp.
Ixodes ricinus
I. persulcatus
I. holocyclus
Haemaphysalis bispinosa
H. longicornis
ii) Mites
Psoroptes spp.
Sarcoptes scabiei
Dermanyssus gallinae
Demodex canis
Psorergates spp.
Chorioptes bovis
iii) Lice
Linognathus vituli
L. ovillus
Damalinia bovis
D. ovis
Haematopinus eurvsternus
Solenopotes capillatus
Haematopinus suis
iv) Keds
Melophagus ovinus
RESIDUES RESULTING FROM SUPERVISED TRIALS
The metabolite (II) (see figure 1) closely resembles amitraz in its
toxicological effects and may be solely responsible for the signs seen
when amitraz is ingested (see Toxicity of impurities, degradation
products and metabolites, Table 3). Effort was therefore concentrated
on the measurement of the combined residues of amitraz and (II) in
most countries (see Table 1 for structures).
In the USA however, most of the trials have measured total residues as
'2,4-xylidine moiety (IV). For details of the analysis methods for
both animal and vegetables products see "METHODS OF RESIDUE ANALYSIS".
Residues in crops
a. Apples and pears
Residues have been determined on apples and pears from many countries
representing a wide variety of climatic conditions. Most trials have
demonstrated that the total residue (amitraz plus (II), expressed as
(II)) will not exceed 0.5 mg/kg, given an interval of 14 days between
application and harvest, following use of amitraz at the recommended
level, although the trials on pears in Italy gave somewhat higher
residues. See Table 9. for a summary of the trials and the results.
Total residues determined in the USA, measured as mg/kg of
'2,4-xylidine', indicate a similar decay pattern. For details and
results of the trials in the USA, see Tables 6/10/11.
b. Stone fruits
A summary of residue trials and results on stone fruits is given in
Table 7.
Peaches
Residues of amitraz and its metabolites (II) and (III) have been
measured on peaches grown in France. The results obtained are very
similar to those obtained for apples and pears.
It is concluded that the total residue (amitraz plus (II)) will not
exceed 0.5 mg/kg, given an interval of 14 days between application and
harvest.
Cherries
Combined residues of amitraz and its metabolite (II) were measured on
cherries in Denmark. Residues ranged from 0.09 to 0.33 mg/kg, and
seem unlikely to exceed 0.5 mg/kg given an interval of 28 days from a
single application at recommended rates to harvest of crop.
c. Oranges
Total residues on treated oranges, grown in two areas of the USA, were
measured. Initial deposits on the fruit fell with half lives of about
3 weeks to leave residues of around 0.5 mg/kg (expressed as amitraz)
after 14 days. Fruit harvested after 20 weeks contained a maximum
residue of 0.060 mg/kg equally distributed between peel and fruit (see
Table 13).
TABLE 9. Supervised Residue Trials on Pome Fruits in Various Countries
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
APPLES
United Kingdom 5 1 1.4 1125 0.39- 0.18- 0.18- 0.18- 0.16- 0.07- 0.07- Hughes and Somerville,
0.48 0.44 0.41 0.35 0.41 0.17 0.30 1973a
Hughes, Jones and
Somerville, 1974
Netherlands 2 2 0.54 600 0.06- 0.04- Somerville and
0.07 0.05 Hughes, 1973d
1 1 0.72 1800 0.20 0.17 Goodall and Somerville,
1974 b
France 1 2 0.60 880 0.60 0.47 0.42 0.32 0.17 ) Somerville and
1 1 0.60 1000 0.20 0.32 0.38 0.28 0.03 ) Hughes, 1973f
USA 1 3 1.0 4500 0.37, 0.21 0.14 0.17 0.14 0.19 )
0.38 )
1 3 2.0 4500 0.51, 0.46 0.42 0.35 0.38 0.18 ) Anon., 1973
0.54 )
1 1 1.0 4500 0.07 0.05 0.06 )
1 1 2.0 4500 0.01 0.12 0.11 )
Denmark 1 1 0.9 2250 0.44 0.05 0.04 ) Somerville and
1 1 0.9 2250) 0.85 0.11 0.17 0.03 ) Goodall, 1974
+2 0.67 2250) )
Italy 1 2 0.04 in 100 l
at 15 l per
tree 0.45 0.34 0.23 Goodall and
Somerville, 1974a
TABLE 9. Continued...
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
Switzerland 1 1 0.2% 0.54 0.44 0.28 0.25 Anon., 1977
Fed. Rep. of 5 3 0.75 1500 0.38- 0.33- 0.29- 0.26- 0.16- Richards, 1980b
Germany 1.01 1.03 0.71 0.72 0.781
PEARS
UK 2 1 1.4 1125 0 36, 0.37, 0.12, 0.13, 0.07, 0.09 Hughes, Jones and
0.46 0.38 0.27 0.17 0.15 0.09 Somerville, 1074
USA 1 1 1.32 2950 0.54 0.49 0.10 ) Anon., 1973
1 1 2.64 2950 1.00 0.92 0.84 )
Italy 2 2 0.60 1000 1.80, 1.42 0.02 0.02, 0.01 )
1.90 0.84 0.56 0.18 0.11 ) Goodall and
1 2 (50 g in 100 l. 1.54 0.94 0.53 0.43 0.26 ) Somerville, 1974a
to run off) )
Japan 1 2 1.5 6000 0.30 0.15 0.19 0.06 Summary only
0.21
Switzerland 1 1 0.2% 0.5 0.56 0.39 0.24 Anon., 1977
0.63
Fed. Rep. of
Germany 1 3 0.75 1500 0.10 0.10 0.10 Richards, 1980b
1 The highest figures were all obtained from one anomalous trial. In the others residue levels from 14 days were not above 0.50 mg/kg.
TABLE 10. Supervised Residue Trials on Apples in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Michigan 9 1.3 3590 1.03 1.16
1.79 1.15
Michigan 1 1.2 3590 0.72 0.57 0.28
0.86 0.91 0.38
1.34 1.11 0.74
1.3 3590 0.64 0.66 0.35
Michigan 6 1.2 3590 2.33 1.68 1.79
2.31 1.32
2.33 3.03 2.13
2.89 1.74
2.45 1.66 1.75
2.66 2.19
1.3 3590 2.15 1.12 1.48
0.98 1.87
Michigan 3 1.4 1870 1.49 1.37 1.02
1.66 1.28 0.88
Michigan 1 1.4 1870 0.58 0.36 0.46
0.67 0.25
1.39 374 1.54 0.90 0.53
0.90 0.78
Michigan 1 0.56 140 0.11
0.14
New York 3 1.22 3272 0.43 0.28 0.32
0.40 0.50 0.44
New York 1 1.39 3740 0.32 0.28 0.20
0.52 0.35 0.24
North 3 1.07 2870 0.45 0.76 0.25
Carolina 0.72 0.59 0.42
TABLE 10. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Oregon 1 1.86 467 0.99 1.68 0.87
1.46 0.38 0.81
Washington 1 1.86 3740 0.96 0.89 1.02
0.51 1.00
California 2 0.70 467 1.00 0.63 0.46
0.63 1.15 0.54
0.70 3740 1.71 1.79 0.80
1.24 1.44 1.13
Wisconsin 2 1.39 3740 0.79 0.24 0.24
0.54 0.30 0.27
Anon., 1975
TABLE 11. Supervised Trials on Pears in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
California 3 1.85 1870 0.78 0.66 0.69
0.50 0.69 0.54
California 3 1.86 467 0.52 0.30 0.32
0.45 0.34 0.42
Michigan 3 0.88 2945 0.30
0.47
1.46 2945 1.18 0.62 0.49
0.96 0.42 0.56
Michigan 1 0.56 1122 0.31 0.43 0.32 0.31
0.30 0.41 0.38
Michigan 1 1.32 2945 1.20 0.81 0.66
1.36 0.86 0.25
1.67 1.04 0.27
1.26 0.75 0.31
Michigan 3 1.46 2945 0.89 1.17
1.17 1.36
1.19 0.94
1.22 1.03
1.20 1.03
1.36 1.40
Michigan 2 1.39 1402 0.82 0.65 0.34
1.39 280 1.16 1.09 0.87
0.47
New York 5 1.85 3740 1.69 1.07 0.97
0.87 0.68
New York 6 1.63 3272 1.74 1.37 1.22
2.38 2.38 1.37
Oregon 5 1.87 467 0.92 0.78 0.95
1.28 0.75 0.43
TABLE 11. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
Oregon 6 2.79 5610 1.80 1.48 1.36
2.75 1.22 1.07
Washington 5 1.86 1309 1.19 1.02 0.96 0.51
0.28
0.34 1.10 0.75 0.30
0.40
Washington 5 1.86 3740 1.12 0.55 0.85
0.99 0.65 0.72
Washington 6 8.198 g/l 0.12 0.12
undiluted 0.14 0.12
Anon., 1975
TABLE 12. Supervised Residue Trials on Stone Fruits
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Applications Residues in mg/kg intervals (days)
No. Rate per hectare after application References
Country of No. kg litres 0 7 14 21 28 35 49 63
Crop Trials
Cherries Denmark 2 1 0.675 3,375 0.28 0.09 Humphrey and
Somerville,1976
Sour Cherries Denmark 3 1 0.40 1,000 0.33 0.09 0.17 Hayto, 1978d
Plums UK 2 1 1.26 g/l 0.041 0.241 Hayto, 1977c
Peaches France 4 1 0.60 1,000 0.57- 0.32- 0.24- 0.15- 0.04, )Somerville and
1.03 1.03 0.30 0.24 0.06 )Hughes, 1973b,c
1 1 0.32 1,900 0.72 0.21 0.10 0.21 0.06 Somerville and
Hughes, 1973c
1 These two results were obtained on different varieties of plum at different locations.
d. Cotton
Residues of amitraz and its metabolite (II) have been measured in
cotton from several different territories. Most of the determinations
have been made on cotton seed following a normal spray programme. In
the case of South Africa, the seed was fractionated into cake and oil,
which were analysed separately; much shorter last spray to harvest
intervals were used than in a normal spray programme. The combined
residues (expressed as (II)) never exceeded 0.4 mg/kg. A summary of
these cotton residue trials can be found in Table 14.
Total '2,4-xylidine' residues (expressed as amitraz) measured in the
USA indicated that after 14 days residues were below 0.5 mg/kg except
in one instance. See Table 15.
e. Hops and beer
Treated samples of hops, both green and dried, have been analysed for
residues of amitraz and its metabolite (II). The combined residues, 7
weeks after the final application, were 0.03 and 0.11 mg/kg (expressed
as (II)) for the green and dried hops respectively. No detectable
residues were found in beer made with treated hops.
f. Cucumbers
Residues of amitraz and (II) have been measured in cucumbers treated
in the UK, Netherlands and South Africa. In all cases the combined
residues after three days never exceeded 0.3 mg/kg in the whole fruit
or the skin.
g. Olives
Residues of amitraz and its metabolite (II) have been measured in
olive oil and pressed olives treated in Italy. The results are in
Table 16.
h. Eggplants
Eggplants were treated with amitraz in Italy. Combined residues were
below 0.3 mg/kg after 4 days.
i. Onions
In a similar study, amitraz was applied to onions. Combined residues
after 28 days were an average of 0.06 mg/kg.
Table 16 summarises the residue data on vegetables, hops and beer.
TABLE 14. Supervised Residue Trials with Cotton in Various Countries: Residues in cottonseed, seedcake and oil
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
No.
Rate of Application of Residues in mg/kg at intervals (days) after application References
Country kg/ha Treatments 5 19 21 28 37 41 77 80
Turkey 0.8 3 <0.01 ( Hamilton,
1.0 3 <0.01 ( 1977a
0.8 in 4001 followed 2 <0.01
by 0.6 in 6001 (in seed oil) Hayto, 1978f
Guatemala 0.29 21 <0.01,0.02 )
0.57 21 <0.01,0.01 )
)
Colombia 0.5) ) ) Hayto, 1978a,
0.7)-in 4001 7 )<0.01 )
1.0) ) )
S. Africa 0.4 1 0.05 0.05 0.05 (seedcake) )
0.03 0.18 0.04 (oil) )
)
0.6 1 0.13 0.06 0.11 (seedcake) ) Hayto, 1978c
0.22 0.09 0.04 (oil) )
)
0.8 1 0.28 0.21 0.29 (seedcake) )
0.22 0.34 0.29 (oil) )
Mexico 0.2 kg/l 6 0.02 (seed cotton)
0.05 (seed from whole bolls) Hayto, 1978e
1 The first application was ULV; the second conventional application. Samples tested were cottonseed except where
treated otherwise.
TABLE 15. Residues on Cottonseed in U.S. trials
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
State of No. of Residues in mg/kg at
U.S.A. Rate of Application per hectare Treatments intervals (days) after application
0 6,7 9 14 18 48 85
Texas 0.28 kg in 37.4 l 9 1.04 0.80 0.68
0.14 kg in 37.4 l 9 0.16 0.18 0.21
S. Carolina 0.28 kg in 46.7 l 16 0.16 0.12 0.13
Alabama 0.56 kg in 121.6 l 11 0.41
Mississippi 0.14 kg in 46.7 l 10 0.20
0.14 kg in 28.0 l 4 0.12
0.28 kg in 56.1 l 12 0.27 0.28
Alabama 0.56 kg in 46.7 l 6 0.06
0.28 kg in 46.7 l 6 0.07
Anon., 1978
TABLE 16. Supervised Residue Trials on Vegetables, Hops and Olives (Oil and Cake)
Combined residues of amitraz and N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Crop Country Applications Residues (mg/kg) at intervals (days)
No. Rate per hectare after application References
kg litres 0,1 3 4,5 6,7 14 28
Cucumber UK 1 0.33 g/l 0.09 ) Hamilton 1978
2 0.33 g/l 0.13 0.09 )
Cucumber S. Africa 1 60 g in 100 l drench 0.30 0.30 0.10 ) Hayto, 1979
0.37 )
Cucumber Netherlands 1 2.8 7,000 0.02,<0.01 )
0.03, 0.07 )
)
1 2.4 6,000 0.01 ) Somerville and
0.13 ) Hughes, 1973a,
Eggplant 1 0.51 1,000 0.17 0.27 0.18 0.12 )
) Hayto, 1978b
)
Onion bulbs 1 0.5 0.06 )
TABLE 16. Continued...
Crop Country No. Applications Residues (mg/kg) at intervals (days)
of No. Rate of hectare after application References
Trials kg litres 50 54 90 139
Hops and
beer UK
Green Hops UK 1 1 0.72 0.03 )
)
Dried Hops UK 1 2 0.72 0.11 ) Hamilton, 1977b
)
Beer UK 1 2 0.72 (on hops) 1.68 <0.01 )
Olives Italy 2 1 54 g/hl )
1.68 1.36 (Pressed cake) )
2 1 108 g/hl ) Richards, 1980a
<0.01 <0.01 (Purified oil) )
0.80 1.70 (Pressed cake) )
Residues in animals
Cattle
Trials have been carried out on cattle and calves in order to measure
combined residues of amitraz and (II), total '2,4-xylidine', or (V)
residues, in tissues after single or repeated applications of amitraz
as a spray or dip. Combined residues of amitraz and (II) and total
'2,4-xylidine' residues after hydrolysis were generally comparable to
control values 1 and 7 days after single spray applications. As
expected, some residues of (V) were apparent after hydrolysis of the
tissues. The results, however, were often complicated by
naturally-occurring 4-amino-benzoic acid, which was not resolved from
(V) by the original method of analysis and gave rise to apparent
residues in untreated tissues. The maximum corrected residue of (V)
after a single-spray application was 0.50 mg/kg in the liver.
Long-term trials involving spray or dip programmes indicated no large
accumulation of residues. Maximum residues of amitraz plus (II) were
0.13 mg/kg in the kidney 1 day after a 14-month spray programme.
Liver tissues from the same trial gave rise to corrected maximum
residues of 0.89 mg/kg of (V).
Milk samples and butter fat have been analysed for combined residues
of amitraz plus (II), for residues of (V), and also for total
'2,4-xylidine' residues. No significant residue were found in milk.
Maximum levels of '2,4-xylidine' occurred in butter fat 6 hours after
treatment (0.15 mg/kg amitraz), however milk collected 2 days after
treatment showed no significant residues as did factory butter
produced locally.
Sheep
In sheep, total residues of '2,4-xylidine' one day after a single dip
were below 0.1 mg/kg in muscle, and below 0.5 mg/kg in kidney and fat,
except in the case of back fat in the first trial. Residues of the
terminal metabolite (V) were only significant in liver and kidney
(corrected maximum 0.65 mg/kg). Milk samples from treated sheep gave
no significant residues of amitraz plus (II) after 1 or 2 days.
Pigs
Residues of amitraz plus (II), and total residues of '2,4-xylidine',
were comparable to control values in pigs sprayed once and twice
respectively. Analysis of pigskin indicated combined residues of
amitraz plus (II) of 0.04 mg/kg and 0.01 mg/kg at 1 and 3 days after a
single spray.
Tissues from the double spray trial gave rise to residues of (V).
However, an apparent residue of over 1 mg/kg in the liver was
confirmed at 0.40 mg/kg by combined gas chromatography-mass
spectroscopy. Smaller residues were likewise found in other tissues.
A summary of all residue results from animal trials can be found in
Table 17.
FATE OF RESIDUES
In plants
Experiments using 14C-amitraz demonstrated that, under certain
conditions at least residues of (II) and (III) could occur on and in
fruit, e.g. apples (Somerville and Nicholson, 1972).
The radiotracer experiments also demonstrated that 14C-amitraz,
applied to leaves, was not transported in any measurable quantity to
other parts of the plant, whereas when 14C-amitraz was applied to the
soil, radioactivity could be taken up into the aerial portions of
plants (Somerville and Spiers, 1972; Lewis, 1970b).
The metabolic pathway is summarised in figure 1.
In animals
The metabolism of amitraz in animals is covered fully in Biochemical
Aspects.
Studies with dogs, cats, rats, mice and bovines all yield the same
picture, namely that amitraz does not persist in vivo, but is
rapidly degraded, yielding conjugated 4-amino-3-methylbenzoic acid (V)
which is excreted in the urine. In dogs, evidence has been obtained
indicating that the first step in the degradation is simple hydrolysis
to yield N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethylformanilide (III). This is consistent with the known
properties of amitraz in acid medium. Furthermore, in an experiment
in which (II) was itself fed to dogs, identical excretory products to
those yielded by amitraz were found. In vitro studies have shown
that both (II) and (III) are degraded rapidly in gastric juice to give
2,4-xylidine (IV).
Further evidence that (IV) is an intermediate in amitraz degradation
in animals is provided by Lindstrom (1961) who showed that (IV) was
rapidly converted to (V) in rats.
It is also of interest to note that (V) is found in human urine
following exposure to amitraz indicating that the same pathway of
metabolism exists in humans.
Once ingested, amitraz is rapidly converted to metabolite (II), which
has similar toxicological effects to amitraz itself. Since apple
pomace and citrus pulp may be fed to cattle, analyses were made of
plasma, tissues and milk from calves and cows dosed with (II). No
measurable residues of (II) or (V) were found in the milk of cows
after 21 days continuous dosage (Dickinson and Shirley, 1975). After
a similar period the total residues of (II), (III), and (IV) in
TABLE 17. Supervised Residue Trials on Animals in Various Countries
Country Animal/ Residues at intervals (days) after application.
Commodity Application Amitraz plus N-2,4-dimethyl-N'-methyl-formamidine References
expressed as the latter (mg/kg) unless
stated otherwise.
Type Number Concentration CONTROL 1 4 7/8
UK CATTLE spray once 0.025%
muscle ) <0.01 <0.01 0.01 )
liver ) <0.01 0.05 <0.01 )
kidney ) <0.01 0.03 )
fat <0.01 <0.01 <0.01 )
)
muscle )3 0.051 <0.01 0.02 ) Jones and
liver ) 0.061 0.12 0.02 ) Holland,
kidney ) 0.03 0.01 ) 1974
fat ) 0.091 0.01 0.01 )
UK CATTLE spray once 0.025%
muscle ) 0.02 <0.01 )
liver ) <0.01 <0.01 ) Holland,
kidney ) 0.03 0.01 ) Jones and
fat ) <0.01 0.03 ) Jones, 1974
Australia CATTLE spray repeatedly at 0.025%
7-day intervals
muscle (rump) ) followed by 3 <0.01 <0.01 <0.01 )
(shoulder) ) sprays at 3-day <0.01 <0.01-0.02 <0.01 )
liver ) intervals <0.01 0.01 <0.01 ) Hayto,
kidney ) <0.01 0.03-0.04 0.03 ) 1977b
fat (back) ) <0.01 <0.01 <0.01 )
(omental) ) <0.01 <0.01 <0.01
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
S. Africa CATTLE spray weekly 0.05%
for 14
muscle )3 <0.011 0.05 )
liver ) <0.01 0.06 )
kidney ) 0.13 )
fat ) 0.01 0.06 )
)
muscle )3 0.051 0.02 0.03 ) Holland
liver ) 0.061 0.04 0.03 ) and Jones,
kidney ) 0.11 0.12 ) 1974a
fat ) 0.091 0.03 0.03 )
milk )3 0.02 0.02 0.01 ) Holland and
) Jones, 1974b
S. Africa CATTLE dipped weekly 0.008%
1-3 years
muscle )3 0.0051 0.008 <0.005 )
liver ) 0.0051 0.04 0.01 ) Shirley,
kidney ) 0.0051 0.06 0.01 ) 1977a
fat ) 0.0051 0.02 <0.005 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
Day 0 Day 1 Day 2
am pm am pm am pm
pre-treatment
Australia CATTLE dipped once 0.025% )
)
tissues <0.02 <0.02 )
)
dipped repeatedly 0.025% )
)
milk ) <0.012 <0.01 <0.01 ) McDougall,
butterfat ) <0.012 0.15 0.11 0.02 0.01 <0.01 ) Heath and
factory butter ) all samples 0.012 ) Black, 1979
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 3 5 7
UK SHEEP dipped once 0.05%
muscle ) 0.011 0.08 0.07 )
liver ) 0.011 0.24 0.11 )
kidney ) 0.021 0.40 0.13 ) Shirley,
fat (back) ) <0.0051 0.62 0.34 ) 1977b
(omental) ) <0.0051 0.14 0.08 )
UK SHEEP dipped once 0.05%
muscle (shoulder) 0.012 0.03 0.02 0.01 0.01 )
(hind leg) 0.012 0.03 0.01 0.01 0.01 )
liver 0.012 0.13 0.04 0.03 ) Needham and
kidney 0.012 0.27 0.09 0.07 ) Campbell,
fat (back) 0.012 0.26 0.27 0.25 0.12 ) 1980
(omental) 0.012 0.10 0.08 0.10 0.09 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 2 3 7
UK SHEEP dipped once 0.05%
milk <0.01 0.02 0.01 <0.01 <0.01 Shirley, 1978
UK PIGS sprayed once 0.1% 2 and 7 days
twice at
muscle 7-day <0.01-0.04 <0.01-0.02 )
liver intervals <0.01-0.05 <0.01-0.04 )
kidney <0.01-0.02 <0.01-0.07 ) Shirley,
fat <0.01-0.02 <0.01-0.02 ) 1975a
UK PIGS sprayed once 0.1%
skin <0.01 0.04 0.01 <0.01 Hayto, 1977a
UK PIGS sprayed twice 0.1%
at 8-day
muscle intervals <0.031 <0.03 )
liver <0.031 <0.03 )
kidney <0.031 <0.03 ) Dickinson and
fat <0.031 <0.03 ) Shirley, 1976
1 Total residues measured as '2,4-xylidine' and expressed as N-2,4-dimethyl-N'-methyl-formamidine (mg/kg)
2 Total residues measured as '2,4,xylidine' and expressed as amitraz (mg/kg)
3 No untreated animals in trial, tissues bought locally.
tissues and plasma of calves were found to be < 0.01 mg/kg and < 0.1
mg/l respectively, although the livers and kidneys of calves receiving
the highest dose of 40 mg/kg diet/day contained residues of 0.02 and
0.05 mg/kg respectively (Campbell and Needham, 1979).
METHODS OF RESIDUE ANALYSIS
Methods have been developed to measure combined residues of amitraz,
compound (II), total '2,4-xylidine' (IV) and metabolites (V) and
(III).
Combined residues of amitraz (calculated as (II) and (II) are measured
as mg/kg (II). Fruit for analysis is first subjected to treatment
with acidic methanol to convert amitraz to (II). Solid material is
filtered off, the extract cleaned and analysed by GLC using a
flame-ionisation detector. Sensitivity is 0.01 mg/kg and recoveries
varied between 70 and 100% for different fruits (Hughes, 1974b;
Somerville and Richards, 1980).
Analysis of animal tissues and milk differ from the above only in the
initial preparation of the sample for extraction, and in the cleanup
procedures, which were slightly modified from those used for fruit.
The method is capable of estimating a total residue level of 0.02
mg/kg in lean meat, liver, fat and milk samples; recoveries were over
70% and background levels were less than O.02 mg/kg (II) (Hughes,
1974a).
Methods of assay of total '2,4-xylidine' residues were developed for
analysis of animal tissues. The methods involve hydrolysis of
amitraz, and all its metabolites that contain the moiety, to
'2,4-xylidine' which on conversion to its heptafluorobutyric
derivative can be determined by gas-liquid chromatography with
electron capture detection. The limits of sensitivity are 0.01 mg/kg
(II) equivalents (Nappier, Hornish and Lane, 1976).
Similar methods have been used in the USA for analysis of fruit and
cotton seed, and lower limits of detection have been established at
0.01 mg/kg and 0.05 mg/kg respectively (Nappier and Hornish, 1975;
Nappier and White, 1978).
Compound (V), the main mammalian metabolite of amitraz, exists as a
conjugated derivative in tissues. Analysis of residues of (V)
therefore involves acid hydrolysis followed by high pressure anion
exchange chromatography. The method has a recovery of 70-80% and a
lower level of detection of 0.03 mg/kg. Relatively high background
levels were found in earlier work, probably due to naturally occurring
p-aminobenzoic acid, which eluted with (V). The more recent method
resolves these difficulties (Dickinson and Shirley, 1976).
Compound(III) is formed in the hydrolysis of amitraz and has been
assayed by high pressure liquid chromatography following extraction of
fruit in the usual way. The sensitivity of the method in 0.01 mg/kg
and the recovery is 89%. (Jones, 1973 f).
NATIONAL RESIDUE LIMITS
The following national Maximum Residue Limits (MRLs) were reported to
the Meeting.
Country Withholding Crop MRL1
Period mg/kg
Australia 4 weeks pome fruit and 0.052
stone fruit
Belgium 4 weeks apples, pears 0.4
East Germany 4 days cucumbers, tomatoes 0.2
4 weeks apples, pears 0.2
France 30 days fruit trees, pears 0.15
Hungary 10 days apples, plums, strawberries,
vines, sugarbeet, soybeans 0.052
Italy 4 weeks apples, pears 0.4
2 weeks citrus, vegetables,
vines, stone fruit 0.4
Netherlands 4 weeks apples, pears 0.4
New Zealand 2 weeks pome fruit and stone fruit 0.5
Switzerland4 3 weeks apples, pears 0.3
USA 4 weeks pears 3.03
Fed. Rep. of apples, pome fruit 0.4
Germany
1 Combined residues of amitraz and formamidine (II), expressed as
II, unless otherwise indicated.
2 Amitraz only.
3 Measured as total '2,4-xylidine' (IV), expressed as amitraz.
4 Based on informal information, subject to written confirmation.
EVALUATION
COMMENT AND APPRAISAL
Amitraz is an insecticide and acaricide effective against a wide
variety of phytophagous mites and insects; its use on fruit and
vegetables and on cotton is authorised in a large number of countries.
It also has important veterinary uses, especially against the ticks
and mites of cattle and sheep, including some otherwise resistant
strains. It is formulated as emulsifiable concentrates for both crop
protection and animal use, and as wettable powders for animal use.
Amitraz is well absorbed from the gastro-intestinal tract and
eliminated from most tissues within a few days. The metabolic
breakdown is similar for all species studied, resulting in the
formation of 3-methyl-4-aminobenzoic acid (BTS 28369) and its
conjugates.
With amitraz a number of short and long-term toxicity studies have
been carried out with mice, rats and dogs. From these experiments
dogs appeared to be the most sensitive species. Furthermore,
experiments with amitraz metabolites showed that on the basis of
weight BTS 27271 is more toxic than the parent compound, however this
difference in toxicity may be explained by the difference in molecular
weight between these compounds. Therefore the toxicity of amitraz can
most probably be attributed to the formation of BTS 27271.
For mice and rats a marginal no toxic effect level of 2.5 and 3 mg/kg
bw/day may be estimated. In dogs however the no effect level from the
short and long-term studies was 0.25 mg/kg bw/day. The most
predominant effect particularly in dogs was CNS depression. The
metabolite BTS 27271 has a no effect level of 1 and 0.1 mg/kg bw/day
for rats and dogs respectively.
From reproduction and teratogenicity studies it was shown that amitraz
influences mean litter size and viability of the young, although, in
effective doses, toxicity on the dame was also apparent. Therefore it
can be concluded that amitraz is possibly embryotoxic in doses also
toxic for the dama, but has no clear teratogenic activity. However
reported studies are incomplete with respect to the number of animals
used (teratogenicity test) and the number of litters bred
(multigeneration study). Also no information was obtained about pre-
implantation losses. No information is available about the possible
teratogenic potential of the metabolites.
From mutagenicity tests with amitraz, BTS 27271, 27919, 28369, 33220
and 24868 it was shown that only BTS 24868, 33220 and 27919 exhibit
weak mutagenic activity in in vitro bacterial systems using
Salmonella typhimurium (TA 100 strain). Negative results were
also obtained in the host-mediated and lethal tests with amitraz.
With amitraz and BTS 24868 carcinogenicity experiments were carried
out in mice and rats. Amitraz was negative in the rat study, but from
initial body weights it is concluded that the age of the animals at
the start of the experiment was not within normal standards. In the
female mouse only 400 mg/kg bw caused an increased incidence of
lymphoreticular tumours. In female mice BTS 24868 increased the
number of lung tumours. In male rats (no female tested) also the
number of lung tumours was found to be increased, together with
bladder and liver tumours which were not seen in controls. In these
studies the number of animals used, dosing schedule and experimental
period do not permit an evaluation of a possible carcinogenic action
of BTS 24868.
Human data are inadequate to be included in the evaluation for the
acceptable daily intake.
It should be remarked that a number of experiments and reports
submitted do not meet acceptable standards.
Experiments using 14C-amitraz show that under certain conditions the
metabolites N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethyl-formanilide (III) may occur on or in fruit. If 14C
labelled amitraz is applied to soil some radioactivity may be taken up
into the aerial parts of plants, but when it is applied to leaves
there is no detectable movement to other parts.
Extensive residue trials on apples and pears have taken place in the
United Kingdom, Europe and other parts of the world, especially in the
United States. US registration authorities require that all
components of the residue that give rise to '2,4-xylidine' on
hydrolysis are included in the analyses, whereas elsewhere residue
measurements are based on the determination of the parent compound
plus N-(2,4-dimethylphenyl)-N'-methylformamidine (II), which is the
most toxic metabolite, being more toxic to mammals than amitraz
itself. Further degradation gives compounds of lesser toxicological
significance.
It is suggested that results obtained by the two methods are broadly
comparable when expressed on the same basis, although those based on
hydrolysis to '2,4-xylidine' are sometimes rather higher.
The terminal metabolite in animals, 4-amino-3-methyl benzoic acid (V),
has been detected as a residue in animal tissues (especially in the
liver), but it closely resembles the natural product 4-aminobenzoic
acid (which can interfere with its determination).
The residue analytical methods used in the USA, involving total
'2,4-xylidine' determination, require a specially made
distillation/extraction apparatus, and also involve the formation of a
fluorinated derivative of the 'xylidine' before final determination by
gas chromatography using electron capture detection. These procedures
probably require considerable skill on the part of the analyst.
Convenient analytical methods, using gas chromatography with flame
ionisation detection, are available for the determination of combined
residues of amitraz and (II) in fruits, vegetables, animal tissues and
milk, and these methods could be adapted for regulatory purposes.
The meeting examined residues data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels which were likely to occur when
amitraz was used in practice and when reported intervals between last
application and harvest were observed.
Level of amitraz causing no toxicological effect
Mouse: 2.5 mg/kg bw/day
Rat: 3 mg/kg bw/day
Dog: 0.25 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw/day
RECOMMENDATIONS OF RESIDUE LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. These levels refer
to the sum of amitraz and N-(2,4-dimethylphenyl)-N'-methylformamidine
(II), calculated as II.
Crop FAO MRL Pre-harvest
Commodity (mg/kg) Interval
Group (days)
Pome fruit 10 0.5 14
Peaches 11 0.5 14
Cherries 11 0.5 28
Cucumber 7 0.5 3
Oranges 9 0.5 7
Cottonseed 20 0.5 7
Cottonseed oil class C 0.05
Olive oil class C 0.05 (Prepared from olives
(sprayed 60 days
(before harvest.
Carcass meat
of cattle 25 0.05
of pig 25 0.05
of sheep 25 0.2
Cattle meat by-products 27 0.2
Pig meat by-products 27 0.2
Sheep meat by-products 27 0.2
Milk 28 0.011/
1/ Level at or about the limit of determination
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Long-term carcinogenicity study with
N-2,4-dimethylphenyl-N-methylformamidine.
2. Long-term carcinogenicity study with amitraz in mice.
3. Further information on the mutagenic activity of 2,4-xylidine,
NN-bis(2,4-dimethylphenyl)formamidine and 2,4-dimethylformanilide.
4. Additional residue data from supervised trials on whole olives.
Desirable
1. Information on the teratogenic activity of
N-2,4-dimethylphenyl-N-methylformamidine.
2. Information on species differences in toxicity.
3. Further observations in humans.
4. Additional residue data on citrus fruits.
5. Further trials on hops, onions and eggplants.
REFERENCES
Anonymous. Acute oral studies on triazid impurities. Unpublished
report RD/BS/BL/1268, 1971a; from The Boots Company, submitted to WHO.
Anonymous. Human pharmacology study on BTS 27419. Unpublished report
no. MS 71004; 1971b, from The Boots Company, submitted to WHO.
Anonymous. Supplement to report no. MS 71004; 1971c, Unpublished
report from The Boots Company, submitted to WHO.
Anonymous. Effects of BTS 27149 and BTS 27271 on the responses of
rabbit ear blood vessels to catecholamines and antidromic stimulation.
Unpublished report no. P 72622; 1972, from The Boots Company,
submitted to WHO.
Anonymous. Residue determination for amitraz and its metabolites in
apples and pears. Upjohn Co. reports 315-9760-5,8,11,12; 1973,
(Unpublished).
Anonymous. Residue determination for U-36,059 (amitraz) and
metabolites in apples and pears. Upjohn Co. reports
315-9760-33,35-40,42-49,52,55-65; 1975, (Unpublished).
Anonymous. Residue analysis of amitraz on Swiss apple and pear crops.
Boots Co. Ltd. report no. AR 77016; 1977, (Unpublished).
Anonymous. Residue determination for U-36,059 and metabolites in
cotton seed (Texas, 1977). Upjohn Co. reports 315-9760-131 to 137;
1978, (Unpublished).
Anonymous. Persistence of amitraz on Florida grown Hamlin oranges.
Boots Hercules Agrochemicals Co. report; 1979a, (Unpublished).
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Methods of analysis. Unpublished report dd. 17-9-1979; 1980, from The
Boots Company, submitted to WHO.
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Analytical specifications. Unpublished report, dd. 8-1-1980; 1980,
from The Boots Company, submitted to WHO.
Berczy, Z.S., Binns, R. and Newman, A.J. Acute inhalation toxicity to
the rat of BTS 27419. Unpublished report no. 4971/72/406; 1973, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield,
R.A.S. Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report no. 5454/72/850; 1973, from Huntingdon Research
Centre, submitted to WHO by The Boots Company.
Berry, P.A. A comparison of the hypothermic effects of amitraz (BTS
27419) in male and female CFLP mice. Unpublished report no. P 76058;
1976, from The Boots Company, submitted to WHO.
Brown, D.J., Burnett, R.J., Crowley, J., Cryer, P.C., Lancaster, M.C.
and Turnbull, G.J. Amitraz: Investigations of effects on the thymus
gland and oestrus cycle in mice. Unpublished report no TX 78037; 1978,
from The Boots Company, submitted to WHO.
Burnett, R., Crowley, J., Lessell, B., Patton, D.S.G., Sutton, M.M.,
Turnbull, G.J. and Williams, G.A.H. BTS 27419: 80-Week carcinogenicity
study in mice - final report. Unpublished report no. TX 76039; 1976,
The Boots Company, submitted to WHO.
Campbell, J.K. and Needham, D. Residues of BTS 27271 and its
metabolites in the plasma and tissues of calves after repeated daily
dosing. Boots Co. Ltd. report no. AX 79019; 1979, (Unpublished).
Channon, E.J. and Cryer, P.C. Amitraz: Investigation of effects on the
thymus gland and oestrus cycle in mice - statistical analysis of
vaginal smear data and thymus weights. Unpublished report no. SS
78001; 1978, The Boots Company, submitted to WHO.
Chesterman, H., Skerrett, K., Street, A.E. and Newman, A.J. Boots
27271 oral toxicity study in beagle dogs (Repeated dosage for 13
weeks). Unpublished report no. S 38/73511; 1973, Huntingdon Research
Centre, submitted to WHO by the Boots Company.
Dickinson, W. and Shirley, D.B., Analysis of milk from cows dosed
orally with BTS 27271 at Thurgarton Research Station. Boots Co. Ltd.
report no. F 75018; 1975, (Unpublished).
Dickinson, W. and Shirley, D.B. Residue analyses of tissues from pigs
treated with amitraz at Thurgarton Research Station, UK. Boots Co.
Ltd. report no. F 76015; 1976, (Unpublished).
O'Donovan, M.R. and Smithson, A. Amitraz impurity. BTS 33220: Acute
oral toxicity to male Boots-Wistar rats. Unpublished report no. TX
78079; 1978, from The Boots Company submitted to WHO.
Everest, R.P. BTS 27419: Mutagenicity study in the male mouse
pervisceral cavity hostmediated assay. Unpublished report no. TX
76056; 1976, from The Boots Company submitted to WHO.
Everest, R.P. and Wilcox, P. BTS 27419, BTS 27271, BTS 27919 and BTS
28369: mutagenicity testing in bacterial in vitro systems.
Unpublished report no.76016; 1976a, from The Boots Company submitted
to WHO.
Everest, R.P. and Wilcox, P. BTS 27419: Mutagenicity testing against
Salmonella typhimurium strains TA 1535, TA 1537 and TA 1538 in the
presence and absence of liver microsomes from male and female mice.
Unpublished report no. TX 76057; 1976b, from the Boots Company
submitted to the WHO.
Everest, R.P. and Wilcox, P. In vitro bacterial mutagenicity
testing of an amitraz sample containing 1% BTS 33220. Unpublished
report no. TX 79108; 1979, from The Boots Company submitted to WHO.
Goodall, E. and Somerville, L. Residue analyses of amitraz and its
metabolite BTS 27271 on Italian fruit crops. Boots Co. Ltd. report no.
AX 74026; 1974a, (Unpublished).
Goodall, E. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Dutch apple and tomato crops. Boots Go. Ltd.
report no. AX 74028, 1974b, (Unpublished).
Hall, J.E., Ahlunwalia, H. and Hampson, P. A study of the effects of
oral administration of a metabolite (BTS 27271) of amitraz to
volunteers. Unpublished report no. NS 75002; 1975, from The Boots
Company submitted to WHO.
Hamilton, D.Y. The fate of [14C]-BTS 27419 (amitraz) when
administered to mice at 100 mg/kg as a single oral dose. Unpublished
report no. AX 76013; 1976, from The Boots Company submitted to WHO.
Hamilton, D.Y. Amitraz residues in Turkish cotton seed. Boots Co. Ltd.
report no. AX 77003; 1977a, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
hops and beer from Whitbreads. Boots Co. Ltd. report no. AX 77006;
1977b, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
cucumbers from the UK. Field Trials 1976. Report no. AX 78011; 1978.
Hamilton, D.Y and Somerville, L. Fate of [14C]-BTS 27271 when
administered to dogs as a oral dose. Unpublished report no. AX 73019;
1973, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27419 when
administered at 15 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74006; 1974a, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C] BTS 28369 when
administered at 10 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74012; 1974b, from The Boots Odmpany submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27271 when
administered to cats as a single oral dose. Unpublished report no. AX
74005; 1974c, from The Boots Company submitted to WHO.
Hayto, E.M. Residues of amitraz and its metabolite BTS 27271 in
pigskin from animals treated at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 77007; 1977a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cattle
tissues from Australia. Boots Co. Ltd. report no. AX 77013; 1977b,
(Unpublished)
Hayto, E.M. Combined residues of amitraz and BTS 27271 in plums and
blackcurrants from UK field trials, 1976. Boots Co. Ltd. report no. AX
77014; 1977c, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Guatemala and Colombia, 1977. Boots Co. Ltd. report no. Ax 78002;
1978a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in egg-plants
and onion bulbs from Italy. Boots Co. Ltd. report no. AX 78012; 1978b,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton from
South Africa, 1978. Boots Co. Ltd. report no. AX 78015; 1978c,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in
sour-cherries from Denmark, 1976. Boots Co. Ltd. report no. AX 78018;
1978d, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Mexico, 1977. Boots Co. Ltd. report no. AX 78019; 1978e,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
oil from Turkey, 1977. Boots Co. Ltd. report no. AX 78021; 1978f,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cucumbers
from South Africa, 1978. Boots Co. Ltd. report no. AX 79002; 1979,
(Unpublished)
Holland, G.N., Jones, A.W., and Jones, E.M. Analyses of amitraz and
its metabolite BTS 28369 in control and treated Thurgarton calves.
Boots Co. Ltd. report no. P 74022; 1974, (Unpublished).
Holland, G.N. and Jones, E.M. Analyses of amitraz and its metabolite
BTS 28369 in treated South African cattle. Boots Co. Ltd. report no.
F 74021; 1974a, (Unpublished).
Holland, G.N. and Jones, E.M. Analysis of amitraz in milk from treated
South African cattle. Boots Co. Ltd. report no. F 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of amitraz and BTS 27271 in animal tissues and
milk. Boots Co. Ltd. report no. AX 74018; 1974a, (Unpublished).
Hughes, K.W. Estimation of combined residues of amitraz and BTS 27271
in apples and pears. Boots Co. Ltd. report no. AX 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of BTS 28369 in human urine. Unpublished
report no. F 75009; 1975, from The Boots company submitted to WHO.
Hughes, K.W., Jones, E.M., and Somerville, L. Residue analyses of
amitraz and its metabolites BTS 27271 and BTS 27919 on apple and pear
crops treated with amitraz at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 74002; 1974, (Unpublished).
Hughes, K.W., and Somerville, L. Residue analyses of amitraz and its
metabolie BTS 27271 on apple crops from Thurgarton Research Station,
UK. Boots Co. Ltd. report no. AX 73017; 1973a, (Unpublished).
Humphrey, E.A. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Danish cherries. Boots Co. Ltd. report no. AX
76003; 1976, (Unpublished).
Jones, E.M, Metabolism of [14C]-BTS 27419 in calf and in cow, part I.
Identification of major urinary metabolite. Unpublished report no.
F 73004; 1973a, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in calf. Part 2.
Identification of major liver metabolite. Unpublished report no.
F 73011; 1973b, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in rats. Unpublished report
no. F 73010; 1973c, from the Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BST 27271 in dogs. Unpublished report
no. F 73019; 1973d, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in cats. Unpublished report
no. F 73018; 1973e, from The Boots Company alimitted to WHO.
Jones, E.M. Residue analyses of BTS 27919 on French peach crops
(Report no. 1). Boots Co. Ltd. report no. F 73015; 1973f,
(Unpublished).
Jones. E.M. and Holland, G.N. Analysis of amitraz and its metabolite
BTS 28369 in treated Thurgarton calves. Boots Co. Ltd. report no.
F 74020; 1974 (Unpublished).
Kakuk, T.J. Amitraz. Further evaluation of 80-week mouse study.
Unpublished report no. HGD 79002; 1979, from The Boots Company
submitted to WHO.
Lancaster. M.C. and Williams, G.A.H. Amitraz: Multigeneration feeding
test in rats - Histopathology. Unpublished report no. TX 78064; 1978,
from The Boots Company submitted to WHO.
Lewis, D.K. RD 27.419. Metabolism and residue studies in the calf
using (14C) and (3H)-labelled material. Unpublished report no.
C 70029; 1970a, from The Boots Company submitted to WHO.
Lewis, D.K. Amitraz - a preliminary study of the persistence,
translocation, and metabolism in plants. Boots Co. Ltd. report no.
FM 70158; 1970b, (Unpublished).
Lewis, D.K. Fate of [14C]-triazid applied to rats as a single oral
dose. Unpublished report no. C 71011; 1971a, from The Boots Company
submitted to WHO.
Lewis, D.K. Fate of [14CH]-BTS 27419 applied to rats as a single
dermal dose. Unpublished report no. C 71019; 1971b, from The Boots
Company submitted to WHO.
Lewis. D.K. Fate of [14C]-BTS 27419 applied to dogs as a single oral
dose. Unpublished report no. 71024; 1971c, from The Boots Company
submitted to WHO.
Lewis, L. Fate of [14C]-triazid (BTS 27419) in the calf. Unpublished
report no. C 71026; 1971d, from The Boots Company submitted to WHO.
Lindstrom, H.V. The metabolism of FD and CRED no. I. The fate of
2,4-meta-xylidine in rats. J. Pharm. Exp. Therap. 132, 306-310.
Martin, H., and Worthing, C.R. Pesticide Manual, 6th Edition, p. 15.
Published by the British Crop Protection Council.
McDougall, D.W., Heath, A.B., and Black. R.R. Residues of amitraz in
the tissues, milk, and butter of cattle dipped in Taktic. Aust. J.
Exp. Agric. Anim. Husb., 19: 663-665.
Merryman, D.C. and Sutton, M.M. BTS 27419. Effects on the oestrus
cycle of the rat. Unpublished report no. PN 72003; 1972, from The
Boots Company submitted to WHO.
Moore, W.K.S. Clinical observations during development. 1972,
Unpublished report from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27271. Acute oral toxicity study in dogs.
Unpublished report no. TX 73004; 1973a, from The Boots Company
submitted to WHO.
Morgan, H.E, BTS 28037; Acute oral toxicity study in dogs. Unpublished
report no. TX 73015; 1973b, from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27919: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74004; 1974a, from The
Boots Company submitted to WHO.
Morgan, H.E. BTS 28369: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74006; 1974b, from The Boots
Company submitted to WHO.
Morgan, H.E., Ellicock, L.A., Parkinson, R. and Wood, R. Amitraz:
comparison of oral and topical administration to pigs. Unpublished
report no. TX 75025; 1975a, from The Boots Company submitted to WHO.
Morgan, H.E., Patton, D.S.G. and Turnbull, G.J. BTS 27419: two year
oral toxicity study in dogs. Unpublished report no. TX 73035; 1973,
from The Boots Company submitted to WHO.
Morgan, H.E. and Shepherd, G.M. BTS 28369: Acute oral toxicity study
in dogs. Unpublished report no. TX 74001; 1974, from The Boots Company
submitted to WHO.
Morgan, H.E., Shepherd, G.M., and Turnbull, G.J. BTS 28369: 90-day
oral toxicity study in dogs. Unpublished report no. TX 74037; 1974,
from The Boots Company submitted to WHO.
Morgan, H.E. and Turnbull, G.J. BTS 27919: Acute oral toxicity study
in dogs. Unpublished report no. TX 73034; 1973, from The Boots Company
submitted to WHO.
Morgan, H.E., Turnbull, G.J. and Ellicook, L.A. Amitraz and BTS 27271:
Effects of repeated daily dosing on acute responses in dogs.
Unpublished report no. M 75026; 1975b, from The Boots Company
submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 27271: Acute oral toxicity study
in dogs -Histopathology. Unpublished report no. TX 73030; 1973a, from
The Boots Company submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 28037: Acute oral toxicity study
in dogs. Unpublished report no. TX 73038; 1973b, from The Boots
Company submitted to WHO.
Nappier, J.L. and Hornish, R.E. Total residue method for U-36, 059
(amitraz) in apples, pears, and soils. Upjohn Co. report no.
315-9760-32; 1975, (Unpublished).
Nappier, J.L., Hornish, R.E. and Lane, R.E. Total residue method for
U-36,059 (amitraz) in swine tissue. Upjohn Co. report no. 315-9760-70;
1976, (Unpublished).
Nappier, J.L. and White, M.C. Total residue method for U-36, 059
(amitraz) in cotton seeds. Upjohn Co. report no. 315-78-9760-001;
1978, (Unpublished).
NCI, Final Report. Carcinogenicity of chemicals present in man's
environment. Contract no. NIH-NCI-E-68-311. Reported by Bio-Research
consultants, 1973, submitted to WHO by The Boots Company.
Needham, D. and Campbell, J.K. Amitraz residues in the tissue of sheep
1,3,5 and 7 days after dipping in 0.05% amitraz. Boots Co. Ltd. report
no. AX 80016; 1980, (Unpublished).
Nix, N. Stability data for Amitraz Technical (Paraformaldehyde Sachets
Stabilised). Unpublished report dd. 11-12-1979; 1979, from The Boots
Company submitted to WHO.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
female mouse. Unpublished report no. BTS 81/7758; 1977a, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
male mouse. Unpublished report no. BTS 80/7792; 1977b, from Huntingdon
Research Centre, submitted to WHO by The Boots Company.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
(chlordimeform) on the central actions of reserpine in the mouse and
rat. Unpublished report no. P 74033; 1974a, from The Boots Company
submitted to WHO.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
chlordimeform) on the pressor response to tyramine in the pithed rat.
Unpublished report no. P 74032; 1974b, from The Boots Company
submitted to WHO.
Parkinson, R. BTS 27271 - an attempt to produce and quantify the
flushing response in the pig. Unpublished report no. P 75030; 1975,
from The Boots Company submitted to WHO.
Parkinson, R., Patton, D.S.G. and Yates, D.B.G. BTS 27419. Effects in
conscious dogs of small intravenous doses. Unpublished report no.
P 71535; 1971a, from The Boots Company submitted to WHO.
Parkinson, R., Sim, M.F. and Yates, D. Effects of the acaricide BTS
27419 and the related sulphur analogues BTS 30559 and BTS 30561 on the
cardiovascular system of the cat, particularly the ear vascular bed.
Unpublished report no. P 71576; 1971b, from The Boots Company
submitted to WHO.
Parkinson, R. and Yates, D.B. The effects of BTS 27419 on
Trafuril-induced erythema on the guinea-pig. Unpublished report no.
PM 71022; 1971, from The Boots Company submitted to WHO.
Patton, D.S.G. RD 27271: Effects on u/v erythema in guinea pigs.
Unpublished report no. PM 70100; 1970, from The Boots Company
submitted to WHO.
Patton, D.S.G. BTS 27419: Acute toxicity in baboons. Unpublished
report no. TX 73002; 1973a, from The Boots Company submitted to WHO.
Patton, D.S.G. BTS 27271: Effects in conscious dogs of small
intravenous doses. Unpublished report no. TX 73017; 1973b, from The
Boots Company submitted to WHO.
Patton, D.S.G. and Sutton, M.M. Acute toxicity studies on BTS 27419 an
acaricide. Unpublished report no. P 71544; 1971a, from The Boots
Company submitted to WHO.
Patton, D.S.G. and Williams, G.A.H. BTS 27419: 90-day toxicity study
in dogs. Unpublished report no. P 71547; 1971b, from The Boots Company
submitted to WHO.
Petzold, G.L., Swenberg, J.A. and Bedell, M. Evaluation of amitraz
(U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915 A and
U-54,914) in the DNA/Alkaline Flution assay. Unpublished report no.
7268/77/7268/001; 1977, from The Boots Company submitted to WHO.
Richards, M.E. Combined residues of amitraz and BTS 27271 in olives
from Italy, 1978. Boots Co. Ltd. report nos. AX 79018 and 80029; 1979
and 1980a, (Unpublished).
Richards, M.E. Combined residues of amitraz and BTS 27271 in apples
and pears from West Germany. Boots Co. Ltd. report no. AX 80001;
1980b, (Unpublished).
Shaw, J.W. BTS 27419 - acute intraperitoneal toxicity to rats.
Unpublished report no. PM 71057; 1971, from The Boots Company
submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral and intraperitoneal
toxicities to rats. Unpublished report no. TX 73006; 1973a, from The
Boots Company submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral toxicities to rats
of BTS 27419 and BTS 27919. Unpublished report TX 73010; 1973b, from
The Boots Company submitted to WHO.
Shaw, J.W. BTS 27419 metabolite: 21-day chronic oral toxicity in rats
of BTS 28369. Unpublished report no. TX 75058; 1975, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTS 27419. 90-day chronic toxicity
study in mice. Unpublished report no. TX 74016; 1974, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTU 27419 metabolite: 90-day chronic
oral toxicity in rats of BTS 27271. Unpublished report no. TX 75059;
1975, from The Boots Company submitted to WHO.
Shirley, D.B. Analysis of tissue from pigs treated with amitraz at
Thurgarton Research Station. Boots Co. Ltd. report no. F 75015; 1975a,
(Unpublished).
Shirley, D.B. The analysis of BTS 28369 in the urine of amitraz
production workers. Unpublished report no. F 75019; 1975b, from The
Boots Company submitted to WHO.
Shirley, D.B. The analysis of BTS 28369 in the urine of three amitraz
production workers over a period of three weeks. Unpublished report
no. F 75024; 1976.
Shirley, D.B. Amitraz residues in cattle from Wiltonside, South
Africa. Boots Co. Ltd. report no. F 77002; 1977a, (Unpublished).
Shirley, D.B. Residue analyses of tissues from sheep treated with
amitraz at Thurgarton Research Station, UK. Boots Co. Ltd. report no.
F 77004, 1977b, (Unpublished).
Shirley, D.B. Analysis of amitraz residues in sheep's milk. Boots Co.
Ltd. report no. F 78013; 1978, (Unpublished).
Sim, M.F. The diuretic action of the acaricides RD 27271 and RD 27419
in mice. Unpublished report no. PM 70111; 1970, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 in the lactating cow.
Unpublished report no. C 72007; 1972, from The Boots Company submitted
to WHO.
Somerville, L. Fate of 14C-BTS 27419 administered to rats in repeated
oral doses. Unpublished report no. AX 73011; 1973a, from The Boots
Company submitted to WHO.
Somerville, L. Fate of 14C-ring-labelled BTS 27419 in the calf.
Unpublished report no. AX 73006; 1973b, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 when administered to cats as a
single oral dose. Unpublished report no. AX 73018; 1973c, from The
Boots Company submitted to WHO.
Somerville, L. and Goodall, E. Residue analyses of amitraz and its
metabolite BTS 27271 on Danish apple crops. Boots Co. Ltd. report no.
AX 74023; 1974, (Unpublished).
Somerville, L. and Hamilton, D.Y. Studies on the accumulation and
elimination of radio-labelled residues from dogs' eyes following oral
administration of (14C)-BTS 27271. Unpublished report no. AX 74013;
1974, from The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch cucumber crops. Boots Co. Ltd. report
no. AX 73008; 1973a, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 1). Boots Co.
Ltd. report no. AX 73010; 1973b, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 2). Boots Co.
Ltd. report no. AX 73013; 1973c, (Unpublished)
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch apple crops. Boots Co. Ltd. report no.
AX 73014; 1973d, (Unpublished).
Somerville, L. and Hughes, K.W. The conversion of BTS 27419 to BTS
27271 in the dog stomach. Unpublished report no. AX 73021; 1973e, from
The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French apple crops. Boots Co. Ltd. report no.
AX 73016; 1973f, (Unpublished).
Somerville, L. and Nicholson, J.E. Amitraz. Metabolism in apples,
variety Cox's Orange Pippin. Boots Co. Ltd. report no. AX 72003; 1972,
(Unpublished).
Somerville, L. and Richards, M.E. The analysis of fruit for combined
residues of amitraz and BTS 27271. Boots Co. Ltd. report no. AX 80002;
1980, (Unpublished).
Somerville, L. and Spiers, M.J. Amitraz. Metabolism in apple leaves.
Boots Co. Ltd. report no. AX 72002; 1972, (Unpublished).
Sutton, M.M., RD 27271. Acute oral toxicity to mice. Unpublished
report no. PM 70043; 1970a, from The Boots Company submitted to WHO.
Sutton, M.M. RD 27271. Acute toxicity to rats. Unpublished report no.
PM 70042; 1970b, from The Boots Company submitted to WHO.
Sutton, M.M. Acaricides: RD 27419 and RD 27271 acute toxicity to
guinea pigs. Unpublished report no. PM 70108; 1970c, from The Boots
Company submitted to WHO.
Sutton, M.M. BTS 27419. Contact sensitization in the guinea pig.
Unpublished report no. PM 71010; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Effect on thermo-regulation in mice.
Unpublished report no. P 72609; 1972a, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27271: acute oral toxicity to rabbits. Unpublished
report no. TXM 72044; 1972b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rabbit. Unpublished
report no. TX 73029; 1973a from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rat. Unpublished report
no. TX 73028; 1973b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Effects on pregnancy, parturition and care of
the young in rate. Unpublished report no. TX 73031; 1973c, from The
Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Multigeneration feeding test in rats.
Unpublished report no. TX 73036; 1973d, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Three-week dermal toxicity to rabbits.
Unpublished report no. TX 73026; 1973e, from The Boots Company
submitted to WHO.
Sutton, M.M. and Metcalf, W. BTS 27419. Eye-irritancy in the rabbit.
Unpublished report no. TXM 72037; 1972, from The Boots Company
submitted to WHO.
Sutton, M.M. and Offer, J. BTS 27419: carcinogenicity and long-term
toxicity study in rats. Unpublished report no. TX 73043; 1972, from
The Boots Company submitted to WHO.
Sutton, M.M. and Williams, G.A.H. BTS 27419: 90-day toxicity study in
rate. Unpublished report no. P 71548; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. and Williams, P.A. BTS 27419: acute dermal toxicity to
rabbits. Unpublished 1972 report no. YM 72011; 1972 from The Boots
Company submitted to WHO.
Taylor, J. and Somerville, L. The conversion of amitraz to BTS 24068
in dog gastric juice. Unpublished report no. AX 77010; 1977, from The
Boots Company submitted to WHO.
Toyoshima, S., Fujita. H., Sato, H., Sato. R., Sato, S. and Kashima,
M. JA-119 (BTS 27419). Three-month feeding study on rats. Unpublished
report E 76014; 1972a, from The Boots Company submitted to WHO.
Toyoshima, S., Fujita H., Sato, H., Sato, R., Sato, S. and Kashima, M.
JA-119 (BTS 27419). Three month feeding study on mice. Unpublished
report no. K 76015; 1972b, from The Boots Company submitted to WHO.
Tuplin, J.A. and Wilcox, A. Amitraz. Ames-tests on a sample produced
by Nissan Chemical Industries. Unpublished report no. TX 78068; 1978,
from The Boots Company submitted to WHO.
Weisburger, E.K. et al. Testing of twenty-one environmental aromatic
amines or derivatives for long-term toxicity or carcinogenicity. J.
Environ. Path. Toxicol. 2, 325-356.
Wilcox, P. BTS 27419: Mutagenicity study in the intraperitoneal
host-mediated assay. Unpublished report no. TX 76028; 1976, from The
Boots Company submitted to WHO.
Wilcox, P. Amitraz impurity BTS 33220: in vitro bacterial
mutagenicity testing. Unpublished report no. TXA 79025; 1979, from The
Boots Company submitted to WHO.
Zimmer, D.M., Mazurek, J.H. and Bhuyan, B.K. Evaluation of amitraz
(U-36.059) and its metabolites in the Salmonella microsome test.
Unpublished report no. 72068/77/7269/002; 1977, from The Boots Company
submitted to WHO.
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Effect of amitraz on the oestrous cycle
In female SFP CFLP mice fed a diet containing 400 mg amitraz/kg feed
for up to 33 weeks a reduction in body weight and an increase in food
consumption was observed. Analysis of vaginal smears indicated that
the mean duration of oestrus as well as the incidence of a prolonged
oestrus was significantly increased due to treatment. No effects were
found on the level of ß-oestradiol in plasma (Brown et al, 1978;
Channon and Cryer, 1978).
Female rats maintained on a diet containing 200 mg amitraz/kg for 18
weeks had longer oestrus cycles than control rats of the same age,
resulting from prolonged periods of oestrus or dioestrus (Merryman and
Sutton, 1972).
Effect on pregnancy, parturation and care of the young
Amitraz was administered in doses of 0, 1, 3 or 12 mg/kg bw to
Boots-Wistar rats from day 1 of pregnancy until the young were weaned
at 21 days old. 12 mg/kg bw reduced the weight gain of the dams as
well as the mean number of young born and alive at day 4. No effects
were observed for mothers and offspring for the other dose groups
(Sutton, 1973 c).
Three-generation feeding study
Groups of 10 male and 20 female newly-weaned Boots-Wistar rats were
fed amitraz in dietary concentrations of 0, 15, 50 or 200 mg/kg.
After ten weeks the animals were mated. After the F1 generation was
weaned 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. At 200 mg/kg amitraz causes decrease
of growth and food consumption in the F0 generation, furthermore a
decrease in fertility and viability was found. The 200 mg/kg diet
group was terminated when the F1 generation was weaned, due to the
very low survival. In the 50 mg/kg group no effect was found on the
number of litters and mean litter size, however in all generations a
decrease in number of young alive at 21 days was observed. No further
effects due to treatment were found in this and the other dose group
(Sutton, 1973 d; Lancaster and Williams, 1978).
Special Studies on teratogenicity
Rat
Groups of 11-13 female rats received amitraz in doses of 0, 1, 3 or 12
mg/kg bw daily from day 8 to day 20 of pregnancy. The rate were
killed on day 21 and the uterine content was examined. Average litter
size, foetal viability and implantation index were not affected. In
the highest dose group foetal weight was less than in the controls,
and the calcification of the stermebrae was less advanced (Sutton,
1973 b).
Rabbit
Groups of 8-10 New Zealand rabbits were treated with amitraz in doses
of 0, 1, 5 or 25 mg/kg bw from day 6 to 18 of pregnancy and killed on
day 30. In the highest dose group number of litters and mean litter
size were decreased. Only in this dose group abortions were observed.
No increase in congenital abnormalities was found (Sutton, 1973 a).
Special studies on mutagenicity
Bacterial systems
Amitraz and its major metabolites (BTS 27271, 27919, 28369) were
tested in bacterial in vitro systems, with and without activation,
against Salmonella typhimurium TA 1535, 1437, and 1538 and
Escherichia coli Wp2 and Wp2 uvrA. In these systems no mutagenic
activity was detected (Everest and Wilcox, 1976 a, 1976 b; Tuplin and
Wilcox, 1978).
The impurity BTS 33220 (N,N'-bis-2,4-dimethylphenyl-N-methyl
formamidine) showed weak positive activity against TA 100 in the
presence of microsomes. Amitraz spiked with 1% BTS 33220, however,
did not show any mutagenic action (Wilcox, 1979; Everest and Wilcox,
1979).
In the TA 100 strain BTS 24868 and BTS 27919 exhibited weak but
consistent mutagenic activity (Zimmer at al, 1977).
Amitraz showed no increase in point mutational activity against
Salmonella typhimurium G 46, TA 1532 and TA 1964 when tested in
the mouse perivisceral host-mediated assay at single oral doses up to
400 mg/kg bw (Wilcox, 1976; Everest, 1976).
Dominant Lethal Studies
Female mouse
Groups of 48 female mice were treated orally for 5 consecutive days
with amitraz in doses of 0, 12 or 50 mg/kg bw.
Subgroups of 12 females were mated with untreated males on day 3, 9,
14 or 19 post-dosing. A slight increase in mean post-implantation
loss and decrease in mean viable litter size at 50 mg/kg was found
(Palmer and James, 1977 a).
Male mouse
Male mice of proven fertility were treated with amitraz for 5
consecutive days at doses of 0, 12 or 50 mg/kg. Following treatment
males were mated with untreated females for six consecutive weeks. A
significantly lower implantation rate was found at 50 mg/kg at the
first mating and a higher implantation rate at 12 mg/kg at the 5th
mating. No changes were found in respect to number of embryonic
deaths and post implantation losses (Palmer and James, 1977 b).
DNA damage
Amitraz, BTS 27271, 27919, 24868 and 28369 were tested at several dose
levels in the DNA damage/alkaline elution assay with or without
activation systems, using chinese hamster lung fibroblasts. In this
test system no indication was obtained that amitraz or its metabolites
induce DNA damage (Petzold et al, 1977).
Special studies on carcinogenicity
Mouse
Groups of 50 male and 50 female mice (CFLP strain) were fed a diet
containing 0, 25, 100 or 400 mg/kg for 80 weeks. The animals of the
100 and 400 mg/kg diet groups gained less weight than the control over
the first 40 weeks. The 25 mg/kg diet group gained more weight than
the control group from week 10 onwards and showed a 20% greater body
weight gain at the end of the experiment. Calculated over 80 weeks,
food consumption was increased for male mice at 100 and 400 mg/kg diet
and decreased at 25 and 100 mg/kg diet and increased at 400 mg/kg diet
for female mice. The survival rate was similar in all groups. An
increased incidence of lymphoreticular tumours was observed for female
mice at 400 mg/kg only; 49% compared with 23% in control animals. A
slight, although not statistically significant (p>5%) increase of all
types of liver cell tumours was found in both sexes fed 400 mg/kg. No
other pathological findings related to treatment were reported
(Burnett et al, 1976; Kakuk 1979).
Rat
Amitraz was administered at dietary concentrations of 0, 15, 50 or 200
mg/kg to groups of 40 male and 40 female Ash Wistar rats for 2 years.
Due to treatment in the highest dose group the animals tended to be
nervous, aggressive and excitable. In this group growth rate was
depressed for both sexes and food intake was slightly less in the
first weeks of the experiment. No dose-related anomalies were found
on organ weights and haematological, biochemical and histopathological
examination. Furthermore, no difference in incidence, type and time
of appearance of tumours in control and treated rats was observed
(Sutton and Offer, 1973).
2,4-Xylidine carcinogenicity study
Mouse
2,4-Xylidine (BTS 24868) was fed in the diet of 3 groups of HAM/ICR
mice (25 males and 25 females) in a concentration of 0, 1000 or 2000
mg/kg. Since these doses were not tolerated the experiment was run
again with doses of 0, 125 or 250 mg/kg diet. Dosing was
discontinuated after 18 months. After 24 months the animals were
killed and examined for tumours. In female mice an increased
incidence of lung tumours was observed in the highest dose group (NCI,
1973).
Rat
2,4-Xylidine (BTS 24868) was administered to groups of 25 male Charles
River rats for approximately 3 months at doses of 2000 or 4000 mg/kg
diet; thereafter the doses were changed to 0, 250 or 500 mg/kg diet
respectively, for approximately 2.5 months and subsequently, the doses
were set at 0, 500 or 1000 mg for the rest of the 18 months period.
At that time the feeding of the test compound was discontinued until
sacrifice 24 months after starting the experiment. A slight increase
in lung tumours in the highest dose group was observed. Furthermore
in the treated groups bladder and liver tumours were observed,
which were not found in the controls (NCI, 1973). However, in
contrast to their earlier conclusions, the authors finally stated that
there was no carcinogenic effect in the rat (Weisburger et al,
1978).
Special studies on pharmacological effects
In dogs amitraz and BTS 27271 produce similar effects when given
orally in doses of 4, and 0.5 mg/kg bw respectively either after
single or repeated administration. Signs included sedation, ataxia,
protrusion of the tongue, bradycardia and hypothermia (Morgan et al,
1975 b).
An experiment comparing oral and topical application of amitraz in
pigs revealed a decrease in rectal temperature after oral dosing (25
mg/kg bw and higher), whereas no effects were found after topical
application up to 200 mg/kg bw (Morgan et al, 1975 a).
No flushing could be demonstrated in pigs after topical or
intramuscular administration of amitraz and BTS 27271 although other
pharmacological changes were apparent (Parkinson, 1975).
Amitraz and BTS 27271 did not have any effect on trafuril-induced or
UV-induced erythema in the guinea pig (Patton, 1970; Parkinson and
Yates, 1971).
BTS 27271 was antidiuretic in mice at 90 mg/kg bw whereas amitraz in
the same dose was ineffective (Sim, 1970).
Intravenous administration of amitraz caused vasoconstriction in the
ear vessels of cats (1-10 µg/kg) and fall in blood pressure and
bradycardia (>20 µg/kg) in dogs. BTS 27271 evoked similar effects in
dogs (40 µg/kg). Both compounds had no effect on the response of the
blood vessels of the rabbit ear to antidromic stimulation or to
catecholamines (Anonymous, 1972; Parkinson et al, 1971 a; Parkinson
et al 1971 b; Patton, 1973 b).
Amitraz induced hypothermia in mice under various ambient conditions.
This effect was greater in intensity and duration in females (Sutton,
1972 a; Berry, 1976).
BTS 27419, BTS 27271 and BTS 21103 were tested for their potency of
central monoamine oxidase inhibition in mice and rats, by
investigating the influence on reserpine-induced ptosis and inhibition
of exploratory activity. No effects were observed in mice. At high
doses these compounds antogonise reserpine-induced ptosis in the rat,
suggesting a relative potency in the order BTS 27271 > BTS 27419 >
BTS 21103 (Parkinson, 1974 a).
BTS 27419, BTS 27271 and BTS 21103 potentiated the pressor response to
tyramine in the pithed rat at doses of 40, 80 and 80 mg/kg bw
respectively. Similar effects have been shown with monoamine oxidases
inhibitors (Parkinson, 1974 b).
Special studies on sensitisation and irritation
Neither amitraz nor BTS 27271 showed sensitisation activity in guinea
pigs (Sutton, 1971).
Amitraz was not irritant to the rabbit eye after a single
administration (Sutton and Metcalf, 1972).
Acute toxicity
Acute oral administration of amitraz caused CNS depression in all
species studied. Dogs were more susceptible than the others. Toxic
manifestations: ataxia, subnormal rectal temperature and
cardiovascular disturbance.
TABLE 2. Acute Toxicity of Amitraz
Species Sex Number Route LD50 (mg/kg) Reference
mouse M 5 oral >1600 Patton and Sutton, 1971
rat M 5 oral approx. 800 Patton and Sutton, 1971
Shaw, 1973a
rat M 5 i.p. approx. 800 Shaw, 1971, 1973a
rat M,F 6 inhalation (6 h LC50) Berczy et al, 1972
65 mg/kg
rat M 5 dermal > 1600 Patton and Sutton, 1971
guinea pig F 3 oral 400-800 Patton and Sutton, 1971
Sutton 1970c
rabbit F 2 oral > 100 Patton and Sutton, 1971
rabbit M,F dermal > 200 Sutton and Williams, 1972
dog M,F 2 oral approx. 100 Patton and Sutton, 1971
baboon M,F 2 oral 100-250 Patton, 1973a
BTS 27271 is more toxic than amitraz. Signs of poisoning are similar
to those shown by amitraz. A summary of acute toxicity data of
impurities degradation products and metabolites of amitraz is given in
table 3.
Short-term studies
Mouse
Groups of 10 male and 10 female CFLP-mice (age 4 weeks) were dosed
with 3, 12, 50 or 200 mg/kg/day amitraz by gavage (suspended in 0.4%
cellosize; 0.1 ml/10 g bw) for 90 days. Control groups of 20 males
and females received the same volume of vehicle alone.
Eight mice (5 males and 3 females) given 200 mg/kg/day died within 3
weeks after becoming progressively emaciated and inactive. The
surviving animals of this group were in poor condition. In the other
groups 5 animals died of causes probably not related to treatment.
The 50 and 200 mg/kg groups showed a decreased body weight gain over
the experimental period for both sexes. The body weight gain of male
TABLE 3. Acute Toxicity of Impurities, Degradation Products and Metabolites of Amitraz
compound species number route LD50 (mg/kg) vehicle references
BTS 27271 mouse 5 oral 100-200 HCl salt aq. sol. Sutton, 1970a
rat 5 oral 200 idem Sutton, 1970b
guinea pig 3 oral 200 idem Sutton, 1970c
dog 2 oral >20 gelatin capsules Morgan, 1973a
Morgan and Williams, 1973a
rabbit 2 oral >25 HCl salt aq. sol. Sutton, 1972b
BTS 27919 mouse 5 oral >1600 idem Anonymous, 1971a
rat 4 oral approx. 1600 idem Shaw, 1973b
dog 2 oral >100 gelatin capsules Morgan, 1974a
Morgan and Turnhull, 1973
BTS 28369 dog 2 oral >250 idem Morgan and Sheperd, 1974
Morgan 1974b
BTS 24868 mouse 5 oral 800 - Anonymous, 1971a
BTS 33220 rat 5 oral 800-1600 10% acacia mucilage O'Donovan and Smithson, 1978
BTS 28037 dog 2 oral >250 gelatin capsules Morgan, 1973b
Morgan and Williams, 1973b
and female mice of the 3 and 12 mg/kg groups was slightly decreased.
GPT activity increased in both sexes from 50 mg/kg. Male mice showed
a slight increase in haematocrit at 3 and 12 mg/kg. Reducing
substances in blood were found to be decreased in both sexes at 12 and
50 mg/kg. Organ weight/body weight ratio of the kidney was increased
in males from 12 mg/kg; of the liver increased for both sexes at 50
and 200 mg/kg and of the spleen for males at 50 and 200 mg/kg and for
females at 200 mg/ kg only.
Slight to moderate hepatocyte and/or nuclear enlargement was observed
dose-relatedly at 12, 50 and 200 mg/kg. Furthermore, in the liver of
some females of the highest dose group, intranuclear inclusion, bile
duct proliferation and inflammatory infiltration in the portal tracts
and nodular hyperplasia were observed (Shaw and Williams, 1974).
Four groups of 13 female and 13 male ICR-SLC mice received amitraz in
doses of 0, 3, 12 or 50 mg/kg bw, in the diet (presuming that the
daily consumption was 5 g/animal) for 90 days. Six males died at an
early stage of the experiment. According to the authors death was not
due to treatment. In both sexes growth was depressed in the 12 and 50
mg/kg groups. Food and water intake were slightly decreased at 12 and
50 mg/kg; for males this was more pronounced than for females. In
male mice a slight decrease in food efficiency was observed at 50
mg/kg over the whole experimental period. For male mice relative
brain weight was increased at 50 mg/kg and heart weight at 12 and 50
mg/kg. The relative kidney weight of female mice at 50 mg/kg was
decreased. SGPT and aldaline-phosphatase were decreased whereas the
albumin-globulin ratio was increased in female mice of the two highest
dose groups. Male mice only showed a higher albumin-globulin ratio at
50 mg/kg. At the end of the experiment macroscopically slight black
discolouration of the liver was seen predominantly in the highest dose
group. The only histopathological change probably directly related to
the administration of the compound was a slight centrolobular
degeneration in the liver at 3, 12 and 50 mg/kg (Toyoshima et al,
1972 b).
Rat (inhalation)
Three groups of 6 female and 6 male rats were exposed daily for six
hours to amitraz at concentrations of 0.01, 0.1 or 1.0 mg/l air
respectively for 14 days over a period of three weeks. During
exposure to 0.1 mg/l dyspnoea, eye-irritation and hyposensitivity to
noise were observed. In addition at 1.0 mg/l ataxia, increased nasal
secretion, polyuria, body tremors and slight coma were found. Body
weight gain was markedly reduced in both sexes at 0.1 and 1.0 mg/l
air. Haematological studies revealed a decreased PCV, haemoglobin and
red blood cell count and increased number of neutrophils in both sexes
and a decreased NCHC and number of lymphocytes in males of the highest
dose group (no other groups examined). At 0.1 and 1.0 mg/l
significant alterations of the relative weights of brain, kidney,
thyroid and adrenal glands, and gonads were found for males, and of
brain, kidney and adrenals of female rats. Liver, heart and pituitary
for males and liver for females were increased in the highest dose
group only. No pathological findings related to treatment were found
(Berozy et al 1973).
[text missing] abnormalities, dosing was restarted for 1 week in which
toxic reactions returned. Rats of the 12 mg/kg group appeared
irritable and excitable. In male rats body weight gain was depressed.
A significantly depressed relative liver weight was seen at 50, 12 as
well as 3 mg/kg for males but only at 50 mg/kg for females.
Alkaline-phosphatase activity was decreased at 50 and 12 mg/kg (Sutton
and Williams, 1971).
Rat
Groups of 10 Wistar rats of each sex received amitraz in doses of 0,
3, 12 or 50 mg/kg bw in the diet (presuming the daily food consumption
was 20 g/animal) for 90 days. Body weight gain was decreased in the
50 mg/kg group for both sexes and in the 12 mg/kg group for females
only. Food and water consumption were decreased in all dose groups.
The relative weight of brain, heart, lung, liver, kidney, spleen and
uterus or testes were increased for both sexes in the highest dose
group. In addition the adrenals were decreased and thymus was
increased in weight in male rats of the highest dose group. Male rats
showed a dose-related decrease in number of blood platelets in the 12
and 50 mg/kg group. Female rats showed a significant decrease in
eosinophils in the highest dose group. In serum of male rate of the
highest dose group a decrease was observed in the Alk Pase activity
and blood sugar concentration, and an increased serum potassium
concentration. Urinalysis revealed an increased number of rats with
proteinurea from the 12 mg/kg group onwards. In male rate urine
potassium concentration was significantly decreased at 12 and 50
mg/kg, in females only at 50 mg/kg (Toyoshima et al, 1972 a).
Rabbit (dermal)
Amitraz dissolved in acetone was applied to the intact skin of groups
of New Zealand rabbits (4 males and 4 females) in 15 doses of 0, 50 or
200 mg/kg bw over a 2l-day period. One male and three females at 200
mg/kg and one male at 50 mg/kg died intercurrently. Sedation was
observed in males at 50 mg/kg and in both sexes at 200 mg/kg. In
males of both dose groups slight to moderate erythema, desquamation of
skin and subcutaneous hemorrhage were observed. In both sexes
decreased body weight and food consumption were found in the 50 and
200 mg/kg dose groups. In males at 200 mg/kg underweight testes with
tubular degeneration were found (Sutton, 1973 e).
Dog
Amitraz in gelatine capsules was given to groups of two male and two
female beagle dogs in oral doses of 0, 0.25, 1 or 4 mg/kg once daily
for 90 days. Clinical signs were observed in all dosed groups
consisting of CNS depression, ataxia and vomiting during the first
days within 3-6 hours after dosing. These dogs were subdued
throughout the experimental period. When examined at intervals during
the treatment period the dogs consistently had subnormal rectal
temperatures and pulse rates. These parameters returned to normal
within 6-24 hours after administration. The animals of the 1 and 4
mg/kg dose groups showed a significant increase in blood sugar
concentration which was maximal 6 hours after dosing and returned to
normal within 24 hours. In the same dose groups an increased amount
of glucose and total reducing substances was found in the urine in
weeks 2 and 3. In the highest dose group an increased liver weight
was observed in both sexes. Except in control animals, livers showed
enlargement of the central and midzonal hepatocytes. Hyperplasia of
the small periportal hepatocytes and increase in binucleate cells were
particularly evident in the highest dose group. At all dose levels
thinning of the zonae fasciculata and reticularis, sometimes
associated with slight hyperplasia of the zona glomerulosa in the
adrenales, was observed (Patton and Williams, 1971).
Metabolites
Rat (90-day test with BTS 27271)
Groups of 10 male and 10 female Boots-Wistar rats were dosed orally by
gastric intubation with BTS 27271 for 90 days at doses of 0.25, 1, 3
or 12 mg/kg bw/day. In the 12 mg/kg group the rats were nervous and
difficult to handle. Body weight was slightly lower at 3 and 12 mg/kg
compared to the controls, the males being more affected than the
females. At the end of the experiment the number of erythrocytes was
decreased in the 3 and 12 mg/kg group for females and in the 12 mg/kg
group for males. Haemoglobin and haematocrit values were decreased at
12 mg/kg for males only. Owing to the variation in the number of
lymphocytes the leucocyte counts were decreased for male and increased
for female rats in the highest dose group. Concomitantly a
significant decrease in number of eosinophils was found. Urinalysis
revealed an increased GPT activity in females, and a slight increase
in urine volume and decrease in specific gravity in the males of the
highest dose group. Together with these changes an increase in BUN
was found. In terminal plasma samples a significant increase in
alkaline-phosphatase activity in both sexes given 12mg/kg was found.
The relative weights of adrenals, ovaries and uterus in the females
and the testes in the males given 3 and 12 mg/kg were increased. In
the highest dose group the weight of liver for females and spleen for
males was increased. Microscopic examination of rats given 3 or 12
mg/kg showed a slight increase in lymphoid infiltration with some
leucocytosis and a loss of glycogen in the liver (livers of female
rats were not examined at 3 mg/kg). In the highest dose group the
hearts of the female rats showed slight cellular accumulation (Shaw
and Williams, 1975).
Dog (90-day test with BTS 27271)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg BTS 27271/kg bw in gelatin capsules daily for three months. The
test compound was diluted in lactose to 1% BTS 27271 as free base.
Clinical signs in the 0.25 and 1 mg/kg group were abnormal quietness
and drowsiness. Between 1 and 4 hours after dosing the mean body
temperature and mean heart rates decreased significantly for the 0.25
and 1 mg/kg dose groups. In the highest dose group a significant
increase in liver weight was found. A slight reduction in thymus
weight was observed at 0.25 and 1 mg/kg. In the highest dose group
the urine volume was found to be increased. No histopathological
anomalies were found (Chesterman et al, 1973).
Rat (21-day test with BTS 28369)
BTS 28369 was administered orally to groups of 4-6 male and female
rate (Boots-Wistar) at dose levels of 0, 40, 100 or 250 mg/kg bw/day
for 21 days. In the highest dose group a slightly decreased weight
gain and BUN level was observed for males and an increased relative
weight of the spleen for females. No pathological changes due to
treatment were found (Shaw, 1975).
Dog (90-day test with BTS 28369)
Groups of 4 male and 4 female beagle dogs were dosed 0, 16, 40 or 100
mg BTS 28369/kg bw/day orally in gelatin capsules for 90 days. At 100
mg/kg bw slightly elevated urinary levels of total reducing substances
were found. No dose related effects were observed on behaviour, ECG,
heart rate, rectal temperature, body weight, food consumption,
haematology, blood biochemistry, organ weights and histopathology
(Morgan et al 1974).
Long-term studies
Rat
See under "Special studies on carcinogenicity".
Dog (2 years)
Groups of 4 male and 4 female beagle dogs were given 0, 0.1, 0.25 or 1
mg amitraz/kg bw/day orally in gelatin capsules for 2 years. Three
hours after dosing all dogs given 1 mg/kg bw exhibited CNS depression
on day 1 and 2, blood sugar level was slightly increased at weeks 40
and 53 (the only weeks measured). No effects on haematological,
biochemical and histopathological parameters were reported (Morgan
et al, 1973).
OBSERVATIONS IN HUMANS
After a few complaints from people involved with the development of
amitraz, all workers in this department were interviewed by the
medical staff, which led to the following conclusions:
1) Skin flushing due to capillary dilatation may be caused by amitraz
or its precursor BTS 27271, or both.
2) The response appears to be the result of systemic absorption rather
than topical application.
3) Absorption is more likely to occur when large quantities are
processed. So far skin effects have not followed the use of
formulated materials in the course of animal or crop protection
trials.
4) Apart from the observation that the capillary circulation in the
skin appears to have been sensitised to trauma such as gentle friction
and radiant heat, no other information can be offered to explain the
phenomena described.
5) The effects appear to be temporary and disappear soon after contact
with the materials is discontinued (Moore, 1972).
Amitraz (about 10 mg) was applied to the arms of 9 volunteers as 0.02
ml of a 50% acetone solution. After 6 hours the arms were cleaned.
One volunteer, who had previously reacted to the compound, showed a
slight erythema localized to the patch area.
Three volunteers who had previously reacted to this compound were
tested using a single application of a paraffin-based ointment
containing 50% amitraz. One subject reported a delayed reaction
occurring 14 h after the initial application consisting of a reddening
of his previous reaction to this compound; no throbbing or other
symptomatology was observed and the reaction had faded by the morning
(Anonymous, 1971 b and c).
Six volunteers received a single oral dose of 2 mg BTS 27271 in a
double-blind crossover study. Blood pressure, pulse rate and oral
temperature were different comparing the volunteers receiving the
active treatment to those receiving placebo. This difference existed
just as much initially as during the observation period. Mental
alertness scores show that a number of subjects felt relatively more
alert on placebo (Hall et al, 1975).
In urine of production workers and volunteers the expected terminal
metabolite, BTS 28369 could be detected (Shirley, 1975 b, 1976;
Hughes, 1975).
RESIDUES IN FOOD
USE PATTERN
Amitraz is used for the control of pests on both animals and crops and
is used in countries throughout the world as an insecticide and
acaricide.
Treatment of crops
As an acaricide, amitraz is successfully used against red spider mites
on deciduous fruit crops, citrus, cotton and certain other crops. As
an insecticide it is used to control pear psylla and whitefly, and as
an ovicide against the major cotton lepidopterous pests.
Recommendations for its use on cotton, deciduous top fruit, citrus,
ornamentals and vegetables are shown in Tables 4 and 5.
Treatment of animals
Amitraz is effective against ticks, mange mites, lice and keds on
cattle, sheep, goats and pigs (Tables 6 and 7).
The species of skin parasites controlled by amitraz are shown in Table
8.
TABLE 4. Use Pattern on Crops
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
COTTON Lepidoptera
Cotton Bollworm
Heliothis spp.
Pink Bollworm
Pectinophora gossypiella
Red (Sudan) Bollworm
Diparopsis castanea Spray when eggs first 300,400, 1.5, 2.0,
appear in the crop 600 3.0
Cotton Leafworm and repeat at regular
Spodoptera spp. intervals throughout 500,600, 2.5, 3.0,
Leaf Perforator the season 200 3.5
Bucculatix thurberiella
Spiny (Spotted) Bollworm
Earias spp.
Whitefly Spray when whitefly first appear 500,600, 2.5, 3.0,
Bemisia tabaci in the crop and repeat at regular 700 3.5
intervals throughout the season.
Thrips spp.
Aphida Spray when these pests first 500, 600, 2.5, 3.0,
Aphis spp. appear in the crop and 700 3.5
Jassida repeat at regular intervals
Empoasca spp. throughout the season
Mites
Tetraaychus spp.
DECIDUOUS Panonychus ulmi Apply the first spray at 60-80% 20-60 1-3 400-1200 2-6
TOP FRUIT egg hatch and repeat at 2-3 week
intervals.
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Mites Spray at the first sign of infestation 40-60 2-3 800-1200 4-6
Tetranychus spp. including and repeat at 2-3 week
T. urticae, T. cinnabarinus intervals.
and T. pacificus
Aculus schlectendali Spray at the first sign of 20-30 1.0-1.5 400-600 2.3
Epitrimerus pyri infestation and continue at
regular 2-3 week intervals.
Codling Moth Apply the first spray immediately
Cydia pomonella after adult emergence and repeat
at 2-3 week intervals for at
least 5 applications. 50-70 2.5-3.5 1000-1400 5-7
Aphids
Myzus persicae Apply at the first sign of
Aphys pomi infestation and repeat at regular
Dysaphis pyri 2-3 week intervals. 50-70 2.5-3.5 1000-1400 5-7
Eriosoma lanigerum Will give good suppression
Phorodon humuli of aphids in an intensive
Dysaphis plantaginea spray programme against mites.
Apple Maggot
Rhagoletis pomonella Used in a full season's
White Apple Leaf Hopper programme against mites will
Typhlocyba pomaria give useful suppression of 50-70 2.5-3.5 1000-1400 5-7
San Jose Scale these pests.
Quadraspidictus perniciosus
Plum Curculio
Conotrachelus nenuphar
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
Pears only Pear psylla Spray when attack first occurs and
Psylla pyricola repeat at regular intervals. The 30-50 1.5-2.5 600-1000 3-5
higher rate is required to give
consistently good control of
over-wintering adults.
Apples Only Leaf miners
Leucoptera scitella Apply the first spray at petal fall
Phyllonorycter blancardella and repeat at 2-3 week intervals 40-50 2-2.5 800-1000 4-5
with a minimum of 4 applications
CITRUS Mites
Panonychus citri Apply first spray at 60-80% egg 20-50 1-3 400-1200 2-6
hatch and repeat at 2-3 week
intervals.
Tetranychus spp. Spray at first sign of infestation 40-60 2-3 800-1200 4-6
and repeat at 2-3 week intervals.
Phyllocoptruta oleivora Spray at first sign of infestation 20-30 1-1.5 400-600 2-3
and continue at regular 2-3 week
intervals.
Scale Insects
Ceroplastea spp. Apply the first spray during the 50-60 2.5-3 1000-1200 5-6
Saissetia spp. crawler (larval) stage and repeat
at regular intervals.
ORNAMENTALS
Carnations, Mites
Chrysanthemums, Panonychus ulmi Apply the first spray at 60-80% 30-50 1.5-2.5 600-1000 3-5
Roses, Pot plants egg hatch with repeat sprays at
and other annuals 2-3 week intervals.
and perennials
TABLE 4. Continued...
APPLICATION RATES
CROP PEST TIMING High Volume Low Volume
g a.i./hl litres g a.i./ha litres
AMITRAZ 20% EC AMITRAZ
1000 l water 20% EC/ha
VEGETABLES Tetranychus urticae Spray at the first sign of infestation)
SOFT FRUIT Tetranychus pacificus regular intervals )
and other Tetranychus )
species )
Acalitus essigi )
Aphids Under cold conditions ) 46-60 2-3 800-1200 4-6
Aphids gossypii and on some crops under glass, )
Aulocorthum spp. the high rate may be necessary )
Macrosiphum spp. to achieve consistently good )
control
TABLE 5. Use of Amitraz on Crops. Registrations (at August 1980) and Periods
Between Last Application and Harvest
Country Withholding Crop1
Period
Argentina 4 weeks apples
Australia 4 weeks pome fruit and stone fruit
Belgium 4 weeks apples, pears
Bulgaria not available apples
Chile 2 weeks fruit, vegetables
Cyprus 2 weeks apples, peaches, citrus, cucurbits
Czechoslovakia 1 week cucumbers
4 weeks apples, pears green beans, peppers, hops
Denmark 2 weeks apples, pears
East Germany 4 days cucumbers, tomatoes
4 weeks apples, pears
Ecuador 2 weeks cotton, stone fruit, citrus,
strawberries, tomatoes, cucumbers
El Salvador not available cotton
France 30 days fruit trees, pears
Greece 1 week apples, pears, citrus, vegetables
Guatemala not available cotton
Hungary 10 days apples, plums, wild strawberries,
vines, sugarbeet, soybeans
Italy 2 weeks citrus, vegetables, vines,
stone fruit
4 weeks apples, pears
Japan 2 weeks apples, pears, citrus
Jordan not available top fruit
Morocco 4 weeks cotton, top fruit, vegetables
Netherlands 4 weeks apples, pears
New Zealand 2 weeks pome fruit and stone fruit
Nicaragua not available cotton
Pakistan not available cotton
Peru not available cotton
Portugal 4 weeks apples, pears
Romania not available top fruit
South Africa 2 weeks apples
4 weeks cotton
South Korea not available apples, pears, citrus
Spain 1 week fruit, maize
Switzerland 3 weeks apples, pears3
Taiwan not available apples, pears, citrus
Turkey not available apples, pears, citrus, cotton
UK 2 weeks apples, pears
7 weeks2 hops
USA 4 weeks pears
1 Use on ornamentals not included.
2 Shorter withholding period under review - likely to be 4 weeks.
3 Based on informal information, subject to written confirmation
TABLE 6. Use Patterns on Animals
Animal Pests Timing Applications
Cattle ticks A second application 10-14 As a spray containing
mange mites days later may be necessary 0.025% a.i.
lice for full control of mange and lice.
Pigs mange mites Two applications, at low concentration As a spray.
lice 10-14 days after the first High concentration
will provide control. Alternatively 0.1%
a single application at high concentration Low concentration
may be recommended. 0.05% a.i.
Sheep ticks As a dip at 0.05% a.i.
mange mites Replenishments should be
lice made with 0.075% amitraz
keds
Goats ticks A second application 0-14 As a spray containing
mange mites days after the first my 0.05% a.i.
lice be necessary for full control
of mange and lice.
TABLE 7. Use of Amitraz on Animals. Registrations (at August 1980) and Periods Between Last
Application and Slaughter
Country Formulation1 Use/Animals Withholding Period
Argentina 12.5% e.c. Dip for sheep None
Australia 50% d.p. Spray for cattle None
50% d.p. Dip for cattle None
Brazil 12.5% e.c. Spray for cattle and Meat 14 days2
sheep Milk 1 day
Colombia 12.5% e.c. Dip for cattle Meat 14 days2
12.5% e.c. Spray for cattle Milk 1 day
Costa Rica 12.5% e.c. Spray for cattle None
Cyprus 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Denmark 12.5% e.c. Spray for pigs and Meat 6 days
cattle Milk 1 day
Jordan 12.5% e.c. Spray for sheep and goats None
Netherlands 12.5% e.c. Dip for pigs and sheep Pigs None
Sheep 4 weeks
Nicaragua 12.5% e.c. Spray for cattle None
Rhodesia 12.5% e.c. Spray for cattle None
12.5% e.c. Dip for cattle None
South Africa 12.5% e.c. Spray for cattle None
25% d.p. Spray for cattle None
25% d.p. Dip for cattle None
Spain 12.5% e.c. Spray for cattle, sheep,
goats and pigs None
Sweden 12.5% e.c. Spray or dip for cattle, Sheep 7 days
sheep and pigs Pigs and cattle 1 day
United Kingdom 12.5% e.c. Spray for pigs and Meat 1 day
cattle Milk None
12.5% e.c. Dip for sheep Meat 7 days
Milk None
Venezuela 12.5% e.c. Spray for cattle Meat 14 days2
Milk 1 day
Yugoslavia 12.5% e.c. Cattle None
1 Emulsifiable concentrate is denoted by "e.c." and dispersible powder by "d.p.".
2 These withholding periods were agreed upon early in the development programme
before most residue work had been carried out.
TABLE 8. Species Of Skin Parasites Controlled By Amitraz
(i) Ticks
Boophilus microplus
B. decoloratus
Rhipicephalus evertsi
Amblyomma hebraeum
A. cayennense
Hyalomma spp.
Ixodes ricinus
I. persulcatus
I. holocyclus
Haemaphysalis bispinosa
H. longicornis
ii) Mites
Psoroptes spp.
Sarcoptes scabiei
Dermanyssus gallinae
Demodex canis
Psorergates spp.
Chorioptes bovis
iii) Lice
Linognathus vituli
L. ovillus
Damalinia bovis
D. ovis
Haematopinus eurvsternus
Solenopotes capillatus
Haematopinus suis
iv) Keds
Melophagus ovinus
RESIDUES RESULTING FROM SUPERVISED TRIALS
The metabolite (II) (see figure 1) closely resembles amitraz in its
toxicological effects and may be solely responsible for the signs seen
when amitraz is ingested (see Toxicity of impurities, degradation
products and metabolites, Table 3). Effort was therefore concentrated
on the measurement of the combined residues of amitraz and (II) in
most countries (see Table 1 for structures).
In the USA however, most of the trials have measured total residues as
'2,4-xylidine moiety (IV). For details of the analysis methods for
both animal and vegetables products see "METHODS OF RESIDUE ANALYSIS".
Residues in crops
a. Apples and pears
Residues have been determined on apples and pears from many countries
representing a wide variety of climatic conditions. Most trials have
demonstrated that the total residue (amitraz plus (II), expressed as
(II)) will not exceed 0.5 mg/kg, given an interval of 14 days between
application and harvest, following use of amitraz at the recommended
level, although the trials on pears in Italy gave somewhat higher
residues. See Table 9. for a summary of the trials and the results.
Total residues determined in the USA, measured as mg/kg of
'2,4-xylidine', indicate a similar decay pattern. For details and
results of the trials in the USA, see Tables 6/10/11.
b. Stone fruits
A summary of residue trials and results on stone fruits is given in
Table 7.
Peaches
Residues of amitraz and its metabolites (II) and (III) have been
measured on peaches grown in France. The results obtained are very
similar to those obtained for apples and pears.
It is concluded that the total residue (amitraz plus (II)) will not
exceed 0.5 mg/kg, given an interval of 14 days between application and
harvest.
Cherries
Combined residues of amitraz and its metabolite (II) were measured on
cherries in Denmark. Residues ranged from 0.09 to 0.33 mg/kg, and
seem unlikely to exceed 0.5 mg/kg given an interval of 28 days from a
single application at recommended rates to harvest of crop.
c. Oranges
Total residues on treated oranges, grown in two areas of the USA, were
measured. Initial deposits on the fruit fell with half lives of about
3 weeks to leave residues of around 0.5 mg/kg (expressed as amitraz)
after 14 days. Fruit harvested after 20 weeks contained a maximum
residue of 0.060 mg/kg equally distributed between peel and fruit (see
Table 13).
TABLE 9. Supervised Residue Trials on Pome Fruits in Various Countries
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
APPLES
United Kingdom 5 1 1.4 1125 0.39- 0.18- 0.18- 0.18- 0.16- 0.07- 0.07- Hughes and Somerville,
0.48 0.44 0.41 0.35 0.41 0.17 0.30 1973a
Hughes, Jones and
Somerville, 1974
Netherlands 2 2 0.54 600 0.06- 0.04- Somerville and
0.07 0.05 Hughes, 1973d
1 1 0.72 1800 0.20 0.17 Goodall and Somerville,
1974 b
France 1 2 0.60 880 0.60 0.47 0.42 0.32 0.17 ) Somerville and
1 1 0.60 1000 0.20 0.32 0.38 0.28 0.03 ) Hughes, 1973f
USA 1 3 1.0 4500 0.37, 0.21 0.14 0.17 0.14 0.19 )
0.38 )
1 3 2.0 4500 0.51, 0.46 0.42 0.35 0.38 0.18 ) Anon., 1973
0.54 )
1 1 1.0 4500 0.07 0.05 0.06 )
1 1 2.0 4500 0.01 0.12 0.11 )
Denmark 1 1 0.9 2250 0.44 0.05 0.04 ) Somerville and
1 1 0.9 2250) 0.85 0.11 0.17 0.03 ) Goodall, 1974
+2 0.67 2250) )
Italy 1 2 0.04 in 100 l
at 15 l per
tree 0.45 0.34 0.23 Goodall and
Somerville, 1974a
TABLE 9. Continued...
Crop/Country No. of
trials Applications Residues amitraz plus N-2,4-dimethylphenyl-N'-ethyl
formamidine expressed as the latter (mg/kg) at intervals References
(days) after application
No. Rate per hectare
kg litres 0 7 14 21 28 35 42 49 56 63
Switzerland 1 1 0.2% 0.54 0.44 0.28 0.25 Anon., 1977
Fed. Rep. of 5 3 0.75 1500 0.38- 0.33- 0.29- 0.26- 0.16- Richards, 1980b
Germany 1.01 1.03 0.71 0.72 0.781
PEARS
UK 2 1 1.4 1125 0 36, 0.37, 0.12, 0.13, 0.07, 0.09 Hughes, Jones and
0.46 0.38 0.27 0.17 0.15 0.09 Somerville, 1074
USA 1 1 1.32 2950 0.54 0.49 0.10 ) Anon., 1973
1 1 2.64 2950 1.00 0.92 0.84 )
Italy 2 2 0.60 1000 1.80, 1.42 0.02 0.02, 0.01 )
1.90 0.84 0.56 0.18 0.11 ) Goodall and
1 2 (50 g in 100 l. 1.54 0.94 0.53 0.43 0.26 ) Somerville, 1974a
to run off) )
Japan 1 2 1.5 6000 0.30 0.15 0.19 0.06 Summary only
0.21
Switzerland 1 1 0.2% 0.5 0.56 0.39 0.24 Anon., 1977
0.63
Fed. Rep. of
Germany 1 3 0.75 1500 0.10 0.10 0.10 Richards, 1980b
1 The highest figures were all obtained from one anomalous trial. In the others residue levels from 14 days were not above 0.50 mg/kg.
TABLE 10. Supervised Residue Trials on Apples in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Michigan 9 1.3 3590 1.03 1.16
1.79 1.15
Michigan 1 1.2 3590 0.72 0.57 0.28
0.86 0.91 0.38
1.34 1.11 0.74
1.3 3590 0.64 0.66 0.35
Michigan 6 1.2 3590 2.33 1.68 1.79
2.31 1.32
2.33 3.03 2.13
2.89 1.74
2.45 1.66 1.75
2.66 2.19
1.3 3590 2.15 1.12 1.48
0.98 1.87
Michigan 3 1.4 1870 1.49 1.37 1.02
1.66 1.28 0.88
Michigan 1 1.4 1870 0.58 0.36 0.46
0.67 0.25
1.39 374 1.54 0.90 0.53
0.90 0.78
Michigan 1 0.56 140 0.11
0.14
New York 3 1.22 3272 0.43 0.28 0.32
0.40 0.50 0.44
New York 1 1.39 3740 0.32 0.28 0.20
0.52 0.35 0.24
North 3 1.07 2870 0.45 0.76 0.25
Carolina 0.72 0.59 0.42
TABLE 10. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Application Residues in mg/kg at
intervals (days) after application
State Rate per hectare 0,1,2 7,8 14,15 21,23 28
in USA No. kg litres
Oregon 1 1.86 467 0.99 1.68 0.87
1.46 0.38 0.81
Washington 1 1.86 3740 0.96 0.89 1.02
0.51 1.00
California 2 0.70 467 1.00 0.63 0.46
0.63 1.15 0.54
0.70 3740 1.71 1.79 0.80
1.24 1.44 1.13
Wisconsin 2 1.39 3740 0.79 0.24 0.24
0.54 0.30 0.27
Anon., 1975
TABLE 11. Supervised Trials on Pears in USA
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
California 3 1.85 1870 0.78 0.66 0.69
0.50 0.69 0.54
California 3 1.86 467 0.52 0.30 0.32
0.45 0.34 0.42
Michigan 3 0.88 2945 0.30
0.47
1.46 2945 1.18 0.62 0.49
0.96 0.42 0.56
Michigan 1 0.56 1122 0.31 0.43 0.32 0.31
0.30 0.41 0.38
Michigan 1 1.32 2945 1.20 0.81 0.66
1.36 0.86 0.25
1.67 1.04 0.27
1.26 0.75 0.31
Michigan 3 1.46 2945 0.89 1.17
1.17 1.36
1.19 0.94
1.22 1.03
1.20 1.03
1.36 1.40
Michigan 2 1.39 1402 0.82 0.65 0.34
1.39 280 1.16 1.09 0.87
0.47
New York 5 1.85 3740 1.69 1.07 0.97
0.87 0.68
New York 6 1.63 3272 1.74 1.37 1.22
2.38 2.38 1.37
Oregon 5 1.87 467 0.92 0.78 0.95
1.28 0.75 0.43
TABLE 11. Continued...
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
Location Applications Residues in mg/kg at
intervals (days) after application
(State
in USA) No. Rate per hectare
kg litres 0,1 3 7,8 14,15 16,17
Oregon 6 2.79 5610 1.80 1.48 1.36
2.75 1.22 1.07
Washington 5 1.86 1309 1.19 1.02 0.96 0.51
0.28
0.34 1.10 0.75 0.30
0.40
Washington 5 1.86 3740 1.12 0.55 0.85
0.99 0.65 0.72
Washington 6 8.198 g/l 0.12 0.12
undiluted 0.14 0.12
Anon., 1975
TABLE 12. Supervised Residue Trials on Stone Fruits
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Applications Residues in mg/kg intervals (days)
No. Rate per hectare after application References
Country of No. kg litres 0 7 14 21 28 35 49 63
Crop Trials
Cherries Denmark 2 1 0.675 3,375 0.28 0.09 Humphrey and
Somerville,1976
Sour Cherries Denmark 3 1 0.40 1,000 0.33 0.09 0.17 Hayto, 1978d
Plums UK 2 1 1.26 g/l 0.041 0.241 Hayto, 1977c
Peaches France 4 1 0.60 1,000 0.57- 0.32- 0.24- 0.15- 0.04, )Somerville and
1.03 1.03 0.30 0.24 0.06 )Hughes, 1973b,c
1 1 0.32 1,900 0.72 0.21 0.10 0.21 0.06 Somerville and
Hughes, 1973c
1 These two results were obtained on different varieties of plum at different locations.
d. Cotton
Residues of amitraz and its metabolite (II) have been measured in
cotton from several different territories. Most of the determinations
have been made on cotton seed following a normal spray programme. In
the case of South Africa, the seed was fractionated into cake and oil,
which were analysed separately; much shorter last spray to harvest
intervals were used than in a normal spray programme. The combined
residues (expressed as (II)) never exceeded 0.4 mg/kg. A summary of
these cotton residue trials can be found in Table 14.
Total '2,4-xylidine' residues (expressed as amitraz) measured in the
USA indicated that after 14 days residues were below 0.5 mg/kg except
in one instance. See Table 15.
e. Hops and beer
Treated samples of hops, both green and dried, have been analysed for
residues of amitraz and its metabolite (II). The combined residues, 7
weeks after the final application, were 0.03 and 0.11 mg/kg (expressed
as (II)) for the green and dried hops respectively. No detectable
residues were found in beer made with treated hops.
f. Cucumbers
Residues of amitraz and (II) have been measured in cucumbers treated
in the UK, Netherlands and South Africa. In all cases the combined
residues after three days never exceeded 0.3 mg/kg in the whole fruit
or the skin.
g. Olives
Residues of amitraz and its metabolite (II) have been measured in
olive oil and pressed olives treated in Italy. The results are in
Table 16.
h. Eggplants
Eggplants were treated with amitraz in Italy. Combined residues were
below 0.3 mg/kg after 4 days.
i. Onions
In a similar study, amitraz was applied to onions. Combined residues
after 28 days were an average of 0.06 mg/kg.
Table 16 summarises the residue data on vegetables, hops and beer.
TABLE 14. Supervised Residue Trials with Cotton in Various Countries: Residues in cottonseed, seedcake and oil
Residues of amitraz plus N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
No.
Rate of Application of Residues in mg/kg at intervals (days) after application References
Country kg/ha Treatments 5 19 21 28 37 41 77 80
Turkey 0.8 3 <0.01 ( Hamilton,
1.0 3 <0.01 ( 1977a
0.8 in 4001 followed 2 <0.01
by 0.6 in 6001 (in seed oil) Hayto, 1978f
Guatemala 0.29 21 <0.01,0.02 )
0.57 21 <0.01,0.01 )
)
Colombia 0.5) ) ) Hayto, 1978a,
0.7)-in 4001 7 )<0.01 )
1.0) ) )
S. Africa 0.4 1 0.05 0.05 0.05 (seedcake) )
0.03 0.18 0.04 (oil) )
)
0.6 1 0.13 0.06 0.11 (seedcake) ) Hayto, 1978c
0.22 0.09 0.04 (oil) )
)
0.8 1 0.28 0.21 0.29 (seedcake) )
0.22 0.34 0.29 (oil) )
Mexico 0.2 kg/l 6 0.02 (seed cotton)
0.05 (seed from whole bolls) Hayto, 1978e
1 The first application was ULV; the second conventional application. Samples tested were cottonseed except where
treated otherwise.
TABLE 15. Residues on Cottonseed in U.S. trials
Residues expressed as amitraz calculated from total '2,4-xylidine' moiety
State of No. of Residues in mg/kg at
U.S.A. Rate of Application per hectare Treatments intervals (days) after application
0 6,7 9 14 18 48 85
Texas 0.28 kg in 37.4 l 9 1.04 0.80 0.68
0.14 kg in 37.4 l 9 0.16 0.18 0.21
S. Carolina 0.28 kg in 46.7 l 16 0.16 0.12 0.13
Alabama 0.56 kg in 121.6 l 11 0.41
Mississippi 0.14 kg in 46.7 l 10 0.20
0.14 kg in 28.0 l 4 0.12
0.28 kg in 56.1 l 12 0.27 0.28
Alabama 0.56 kg in 46.7 l 6 0.06
0.28 kg in 46.7 l 6 0.07
Anon., 1978
TABLE 16. Supervised Residue Trials on Vegetables, Hops and Olives (Oil and Cake)
Combined residues of amitraz and N-2,4-dimethylphenyl-N'-methylformamidine expressed as the latter
Crop Country Applications Residues (mg/kg) at intervals (days)
No. Rate per hectare after application References
kg litres 0,1 3 4,5 6,7 14 28
Cucumber UK 1 0.33 g/l 0.09 ) Hamilton 1978
2 0.33 g/l 0.13 0.09 )
Cucumber S. Africa 1 60 g in 100 l drench 0.30 0.30 0.10 ) Hayto, 1979
0.37 )
Cucumber Netherlands 1 2.8 7,000 0.02,<0.01 )
0.03, 0.07 )
)
1 2.4 6,000 0.01 ) Somerville and
0.13 ) Hughes, 1973a,
Eggplant 1 0.51 1,000 0.17 0.27 0.18 0.12 )
) Hayto, 1978b
)
Onion bulbs 1 0.5 0.06 )
TABLE 16. Continued...
Crop Country No. Applications Residues (mg/kg) at intervals (days)
of No. Rate of hectare after application References
Trials kg litres 50 54 90 139
Hops and
beer UK
Green Hops UK 1 1 0.72 0.03 )
)
Dried Hops UK 1 2 0.72 0.11 ) Hamilton, 1977b
)
Beer UK 1 2 0.72 (on hops) 1.68 <0.01 )
Olives Italy 2 1 54 g/hl )
1.68 1.36 (Pressed cake) )
2 1 108 g/hl ) Richards, 1980a
<0.01 <0.01 (Purified oil) )
0.80 1.70 (Pressed cake) )
Residues in animals
Cattle
Trials have been carried out on cattle and calves in order to measure
combined residues of amitraz and (II), total '2,4-xylidine', or (V)
residues, in tissues after single or repeated applications of amitraz
as a spray or dip. Combined residues of amitraz and (II) and total
'2,4-xylidine' residues after hydrolysis were generally comparable to
control values 1 and 7 days after single spray applications. As
expected, some residues of (V) were apparent after hydrolysis of the
tissues. The results, however, were often complicated by
naturally-occurring 4-amino-benzoic acid, which was not resolved from
(V) by the original method of analysis and gave rise to apparent
residues in untreated tissues. The maximum corrected residue of (V)
after a single-spray application was 0.50 mg/kg in the liver.
Long-term trials involving spray or dip programmes indicated no large
accumulation of residues. Maximum residues of amitraz plus (II) were
0.13 mg/kg in the kidney 1 day after a 14-month spray programme.
Liver tissues from the same trial gave rise to corrected maximum
residues of 0.89 mg/kg of (V).
Milk samples and butter fat have been analysed for combined residues
of amitraz plus (II), for residues of (V), and also for total
'2,4-xylidine' residues. No significant residue were found in milk.
Maximum levels of '2,4-xylidine' occurred in butter fat 6 hours after
treatment (0.15 mg/kg amitraz), however milk collected 2 days after
treatment showed no significant residues as did factory butter
produced locally.
Sheep
In sheep, total residues of '2,4-xylidine' one day after a single dip
were below 0.1 mg/kg in muscle, and below 0.5 mg/kg in kidney and fat,
except in the case of back fat in the first trial. Residues of the
terminal metabolite (V) were only significant in liver and kidney
(corrected maximum 0.65 mg/kg). Milk samples from treated sheep gave
no significant residues of amitraz plus (II) after 1 or 2 days.
Pigs
Residues of amitraz plus (II), and total residues of '2,4-xylidine',
were comparable to control values in pigs sprayed once and twice
respectively. Analysis of pigskin indicated combined residues of
amitraz plus (II) of 0.04 mg/kg and 0.01 mg/kg at 1 and 3 days after a
single spray.
Tissues from the double spray trial gave rise to residues of (V).
However, an apparent residue of over 1 mg/kg in the liver was
confirmed at 0.40 mg/kg by combined gas chromatography-mass
spectroscopy. Smaller residues were likewise found in other tissues.
A summary of all residue results from animal trials can be found in
Table 17.
FATE OF RESIDUES
In plants
Experiments using 14C-amitraz demonstrated that, under certain
conditions at least residues of (II) and (III) could occur on and in
fruit, e.g. apples (Somerville and Nicholson, 1972).
The radiotracer experiments also demonstrated that 14C-amitraz,
applied to leaves, was not transported in any measurable quantity to
other parts of the plant, whereas when 14C-amitraz was applied to the
soil, radioactivity could be taken up into the aerial portions of
plants (Somerville and Spiers, 1972; Lewis, 1970b).
The metabolic pathway is summarised in figure 1.
In animals
The metabolism of amitraz in animals is covered fully in Biochemical
Aspects.
Studies with dogs, cats, rats, mice and bovines all yield the same
picture, namely that amitraz does not persist in vivo, but is
rapidly degraded, yielding conjugated 4-amino-3-methylbenzoic acid (V)
which is excreted in the urine. In dogs, evidence has been obtained
indicating that the first step in the degradation is simple hydrolysis
to yield N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethylformanilide (III). This is consistent with the known
properties of amitraz in acid medium. Furthermore, in an experiment
in which (II) was itself fed to dogs, identical excretory products to
those yielded by amitraz were found. In vitro studies have shown
that both (II) and (III) are degraded rapidly in gastric juice to give
2,4-xylidine (IV).
Further evidence that (IV) is an intermediate in amitraz degradation
in animals is provided by Lindstrom (1961) who showed that (IV) was
rapidly converted to (V) in rats.
It is also of interest to note that (V) is found in human urine
following exposure to amitraz indicating that the same pathway of
metabolism exists in humans.
Once ingested, amitraz is rapidly converted to metabolite (II), which
has similar toxicological effects to amitraz itself. Since apple
pomace and citrus pulp may be fed to cattle, analyses were made of
plasma, tissues and milk from calves and cows dosed with (II). No
measurable residues of (II) or (V) were found in the milk of cows
after 21 days continuous dosage (Dickinson and Shirley, 1975). After
a similar period the total residues of (II), (III), and (IV) in
TABLE 17. Supervised Residue Trials on Animals in Various Countries
Country Animal/ Residues at intervals (days) after application.
Commodity Application Amitraz plus N-2,4-dimethyl-N'-methyl-formamidine References
expressed as the latter (mg/kg) unless
stated otherwise.
Type Number Concentration CONTROL 1 4 7/8
UK CATTLE spray once 0.025%
muscle ) <0.01 <0.01 0.01 )
liver ) <0.01 0.05 <0.01 )
kidney ) <0.01 0.03 )
fat <0.01 <0.01 <0.01 )
)
muscle )3 0.051 <0.01 0.02 ) Jones and
liver ) 0.061 0.12 0.02 ) Holland,
kidney ) 0.03 0.01 ) 1974
fat ) 0.091 0.01 0.01 )
UK CATTLE spray once 0.025%
muscle ) 0.02 <0.01 )
liver ) <0.01 <0.01 ) Holland,
kidney ) 0.03 0.01 ) Jones and
fat ) <0.01 0.03 ) Jones, 1974
Australia CATTLE spray repeatedly at 0.025%
7-day intervals
muscle (rump) ) followed by 3 <0.01 <0.01 <0.01 )
(shoulder) ) sprays at 3-day <0.01 <0.01-0.02 <0.01 )
liver ) intervals <0.01 0.01 <0.01 ) Hayto,
kidney ) <0.01 0.03-0.04 0.03 ) 1977b
fat (back) ) <0.01 <0.01 <0.01 )
(omental) ) <0.01 <0.01 <0.01
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
S. Africa CATTLE spray weekly 0.05%
for 14
muscle )3 <0.011 0.05 )
liver ) <0.01 0.06 )
kidney ) 0.13 )
fat ) 0.01 0.06 )
)
muscle )3 0.051 0.02 0.03 ) Holland
liver ) 0.061 0.04 0.03 ) and Jones,
kidney ) 0.11 0.12 ) 1974a
fat ) 0.091 0.03 0.03 )
milk )3 0.02 0.02 0.01 ) Holland and
) Jones, 1974b
S. Africa CATTLE dipped weekly 0.008%
1-3 years
muscle )3 0.0051 0.008 <0.005 )
liver ) 0.0051 0.04 0.01 ) Shirley,
kidney ) 0.0051 0.06 0.01 ) 1977a
fat ) 0.0051 0.02 <0.005 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 4 7/8
Day 0 Day 1 Day 2
am pm am pm am pm
pre-treatment
Australia CATTLE dipped once 0.025% )
)
tissues <0.02 <0.02 )
)
dipped repeatedly 0.025% )
)
milk ) <0.012 <0.01 <0.01 ) McDougall,
butterfat ) <0.012 0.15 0.11 0.02 0.01 <0.01 ) Heath and
factory butter ) all samples 0.012 ) Black, 1979
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 3 5 7
UK SHEEP dipped once 0.05%
muscle ) 0.011 0.08 0.07 )
liver ) 0.011 0.24 0.11 )
kidney ) 0.021 0.40 0.13 ) Shirley,
fat (back) ) <0.0051 0.62 0.34 ) 1977b
(omental) ) <0.0051 0.14 0.08 )
UK SHEEP dipped once 0.05%
muscle (shoulder) 0.012 0.03 0.02 0.01 0.01 )
(hind leg) 0.012 0.03 0.01 0.01 0.01 )
liver 0.012 0.13 0.04 0.03 ) Needham and
kidney 0.012 0.27 0.09 0.07 ) Campbell,
fat (back) 0.012 0.26 0.27 0.25 0.12 ) 1980
(omental) 0.012 0.10 0.08 0.10 0.09 )
TABLE 17. Continued...
Country Animal/ Residues at intervals (days) after application.
Commodity Application References
Type Number Concentration CONTROL 1 2 3 7
UK SHEEP dipped once 0.05%
milk <0.01 0.02 0.01 <0.01 <0.01 Shirley, 1978
UK PIGS sprayed once 0.1% 2 and 7 days
twice at
muscle 7-day <0.01-0.04 <0.01-0.02 )
liver intervals <0.01-0.05 <0.01-0.04 )
kidney <0.01-0.02 <0.01-0.07 ) Shirley,
fat <0.01-0.02 <0.01-0.02 ) 1975a
UK PIGS sprayed once 0.1%
skin <0.01 0.04 0.01 <0.01 Hayto, 1977a
UK PIGS sprayed twice 0.1%
at 8-day
muscle intervals <0.031 <0.03 )
liver <0.031 <0.03 )
kidney <0.031 <0.03 ) Dickinson and
fat <0.031 <0.03 ) Shirley, 1976
1 Total residues measured as '2,4-xylidine' and expressed as N-2,4-dimethyl-N'-methyl-formamidine (mg/kg)
2 Total residues measured as '2,4,xylidine' and expressed as amitraz (mg/kg)
3 No untreated animals in trial, tissues bought locally.
tissues and plasma of calves were found to be < 0.01 mg/kg and < 0.1
mg/l respectively, although the livers and kidneys of calves receiving
the highest dose of 40 mg/kg diet/day contained residues of 0.02 and
0.05 mg/kg respectively (Campbell and Needham, 1979).
METHODS OF RESIDUE ANALYSIS
Methods have been developed to measure combined residues of amitraz,
compound (II), total '2,4-xylidine' (IV) and metabolites (V) and
(III).
Combined residues of amitraz (calculated as (II) and (II) are measured
as mg/kg (II). Fruit for analysis is first subjected to treatment
with acidic methanol to convert amitraz to (II). Solid material is
filtered off, the extract cleaned and analysed by GLC using a
flame-ionisation detector. Sensitivity is 0.01 mg/kg and recoveries
varied between 70 and 100% for different fruits (Hughes, 1974b;
Somerville and Richards, 1980).
Analysis of animal tissues and milk differ from the above only in the
initial preparation of the sample for extraction, and in the cleanup
procedures, which were slightly modified from those used for fruit.
The method is capable of estimating a total residue level of 0.02
mg/kg in lean meat, liver, fat and milk samples; recoveries were over
70% and background levels were less than O.02 mg/kg (II) (Hughes,
1974a).
Methods of assay of total '2,4-xylidine' residues were developed for
analysis of animal tissues. The methods involve hydrolysis of
amitraz, and all its metabolites that contain the moiety, to
'2,4-xylidine' which on conversion to its heptafluorobutyric
derivative can be determined by gas-liquid chromatography with
electron capture detection. The limits of sensitivity are 0.01 mg/kg
(II) equivalents (Nappier, Hornish and Lane, 1976).
Similar methods have been used in the USA for analysis of fruit and
cotton seed, and lower limits of detection have been established at
0.01 mg/kg and 0.05 mg/kg respectively (Nappier and Hornish, 1975;
Nappier and White, 1978).
Compound (V), the main mammalian metabolite of amitraz, exists as a
conjugated derivative in tissues. Analysis of residues of (V)
therefore involves acid hydrolysis followed by high pressure anion
exchange chromatography. The method has a recovery of 70-80% and a
lower level of detection of 0.03 mg/kg. Relatively high background
levels were found in earlier work, probably due to naturally occurring
p-aminobenzoic acid, which eluted with (V). The more recent method
resolves these difficulties (Dickinson and Shirley, 1976).
Compound(III) is formed in the hydrolysis of amitraz and has been
assayed by high pressure liquid chromatography following extraction of
fruit in the usual way. The sensitivity of the method in 0.01 mg/kg
and the recovery is 89%. (Jones, 1973 f).
NATIONAL RESIDUE LIMITS
The following national Maximum Residue Limits (MRLs) were reported to
the Meeting.
Country Withholding Crop MRL1
Period mg/kg
Australia 4 weeks pome fruit and 0.052
stone fruit
Belgium 4 weeks apples, pears 0.4
East Germany 4 days cucumbers, tomatoes 0.2
4 weeks apples, pears 0.2
France 30 days fruit trees, pears 0.15
Hungary 10 days apples, plums, strawberries,
vines, sugarbeet, soybeans 0.052
Italy 4 weeks apples, pears 0.4
2 weeks citrus, vegetables,
vines, stone fruit 0.4
Netherlands 4 weeks apples, pears 0.4
New Zealand 2 weeks pome fruit and stone fruit 0.5
Switzerland4 3 weeks apples, pears 0.3
USA 4 weeks pears 3.03
Fed. Rep. of apples, pome fruit 0.4
Germany
1 Combined residues of amitraz and formamidine (II), expressed as
II, unless otherwise indicated.
2 Amitraz only.
3 Measured as total '2,4-xylidine' (IV), expressed as amitraz.
4 Based on informal information, subject to written confirmation.
EVALUATION
COMMENT AND APPRAISAL
Amitraz is an insecticide and acaricide effective against a wide
variety of phytophagous mites and insects; its use on fruit and
vegetables and on cotton is authorised in a large number of countries.
It also has important veterinary uses, especially against the ticks
and mites of cattle and sheep, including some otherwise resistant
strains. It is formulated as emulsifiable concentrates for both crop
protection and animal use, and as wettable powders for animal use.
Amitraz is well absorbed from the gastro-intestinal tract and
eliminated from most tissues within a few days. The metabolic
breakdown is similar for all species studied, resulting in the
formation of 3-methyl-4-aminobenzoic acid (BTS 28369) and its
conjugates.
With amitraz a number of short and long-term toxicity studies have
been carried out with mice, rats and dogs. From these experiments
dogs appeared to be the most sensitive species. Furthermore,
experiments with amitraz metabolites showed that on the basis of
weight BTS 27271 is more toxic than the parent compound, however this
difference in toxicity may be explained by the difference in molecular
weight between these compounds. Therefore the toxicity of amitraz can
most probably be attributed to the formation of BTS 27271.
For mice and rats a marginal no toxic effect level of 2.5 and 3 mg/kg
bw/day may be estimated. In dogs however the no effect level from the
short and long-term studies was 0.25 mg/kg bw/day. The most
predominant effect particularly in dogs was CNS depression. The
metabolite BTS 27271 has a no effect level of 1 and 0.1 mg/kg bw/day
for rats and dogs respectively.
From reproduction and teratogenicity studies it was shown that amitraz
influences mean litter size and viability of the young, although, in
effective doses, toxicity on the dame was also apparent. Therefore it
can be concluded that amitraz is possibly embryotoxic in doses also
toxic for the dama, but has no clear teratogenic activity. However
reported studies are incomplete with respect to the number of animals
used (teratogenicity test) and the number of litters bred
(multigeneration study). Also no information was obtained about pre-
implantation losses. No information is available about the possible
teratogenic potential of the metabolites.
From mutagenicity tests with amitraz, BTS 27271, 27919, 28369, 33220
and 24868 it was shown that only BTS 24868, 33220 and 27919 exhibit
weak mutagenic activity in in vitro bacterial systems using
Salmonella typhimurium (TA 100 strain). Negative results were
also obtained in the host-mediated and lethal tests with amitraz.
With amitraz and BTS 24868 carcinogenicity experiments were carried
out in mice and rats. Amitraz was negative in the rat study, but from
initial body weights it is concluded that the age of the animals at
the start of the experiment was not within normal standards. In the
female mouse only 400 mg/kg bw caused an increased incidence of
lymphoreticular tumours. In female mice BTS 24868 increased the
number of lung tumours. In male rats (no female tested) also the
number of lung tumours was found to be increased, together with
bladder and liver tumours which were not seen in controls. In these
studies the number of animals used, dosing schedule and experimental
period do not permit an evaluation of a possible carcinogenic action
of BTS 24868.
Human data are inadequate to be included in the evaluation for the
acceptable daily intake.
It should be remarked that a number of experiments and reports
submitted do not meet acceptable standards.
Experiments using 14C-amitraz show that under certain conditions the
metabolites N-(2,4-dimethylphenyl)-N'-methylformamidine (II) and
2,4-dimethyl-formanilide (III) may occur on or in fruit. If 14C
labelled amitraz is applied to soil some radioactivity may be taken up
into the aerial parts of plants, but when it is applied to leaves
there is no detectable movement to other parts.
Extensive residue trials on apples and pears have taken place in the
United Kingdom, Europe and other parts of the world, especially in the
United States. US registration authorities require that all
components of the residue that give rise to '2,4-xylidine' on
hydrolysis are included in the analyses, whereas elsewhere residue
measurements are based on the determination of the parent compound
plus N-(2,4-dimethylphenyl)-N'-methylformamidine (II), which is the
most toxic metabolite, being more toxic to mammals than amitraz
itself. Further degradation gives compounds of lesser toxicological
significance.
It is suggested that results obtained by the two methods are broadly
comparable when expressed on the same basis, although those based on
hydrolysis to '2,4-xylidine' are sometimes rather higher.
The terminal metabolite in animals, 4-amino-3-methyl benzoic acid (V),
has been detected as a residue in animal tissues (especially in the
liver), but it closely resembles the natural product 4-aminobenzoic
acid (which can interfere with its determination).
The residue analytical methods used in the USA, involving total
'2,4-xylidine' determination, require a specially made
distillation/extraction apparatus, and also involve the formation of a
fluorinated derivative of the 'xylidine' before final determination by
gas chromatography using electron capture detection. These procedures
probably require considerable skill on the part of the analyst.
Convenient analytical methods, using gas chromatography with flame
ionisation detection, are available for the determination of combined
residues of amitraz and (II) in fruits, vegetables, animal tissues and
milk, and these methods could be adapted for regulatory purposes.
The meeting examined residues data from supervised trials reflecting
established or proposed good agricultural practice on a number of
crops and commodities. From these data the meeting was able to
estimate the maximum residue levels which were likely to occur when
amitraz was used in practice and when reported intervals between last
application and harvest were observed.
Level of amitraz causing no toxicological effect
Mouse: 2.5 mg/kg bw/day
Rat: 3 mg/kg bw/day
Dog: 0.25 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw/day
RECOMMENDATIONS OF RESIDUE LIMITS
The meeting concludes that the maximum residue levels listed below are
suitable for establishing maximum residue limits. These levels refer
to the sum of amitraz and N-(2,4-dimethylphenyl)-N'-methylformamidine
(II), calculated as II.
Crop FAO MRL Pre-harvest
Commodity (mg/kg) Interval
Group (days)
Pome fruit 10 0.5 14
Peaches 11 0.5 14
Cherries 11 0.5 28
Cucumber 7 0.5 3
Oranges 9 0.5 7
Cottonseed 20 0.5 7
Cottonseed oil class C 0.05
Olive oil class C 0.05 (Prepared from olives
(sprayed 60 days
(before harvest.
Carcass meat
of cattle 25 0.05
of pig 25 0.05
of sheep 25 0.2
Cattle meat by-products 27 0.2
Pig meat by-products 27 0.2
Sheep meat by-products 27 0.2
Milk 28 0.011/
1/ Level at or about the limit of determination
FURTHER WORK OR INFORMATION
Required (by 1984)
1. Long-term carcinogenicity study with
N-2,4-dimethylphenyl-N-methylformamidine.
2. Long-term carcinogenicity study with amitraz in mice.
3. Further information on the mutagenic activity of 2,4-xylidine,
NN-bis(2,4-dimethylphenyl)formamidine and 2,4-dimethylformanilide.
4. Additional residue data from supervised trials on whole olives.
Desirable
1. Information on the teratogenic activity of
N-2,4-dimethylphenyl-N-methylformamidine.
2. Information on species differences in toxicity.
3. Further observations in humans.
4. Additional residue data on citrus fruits.
5. Further trials on hops, onions and eggplants.
REFERENCES
Anonymous. Acute oral studies on triazid impurities. Unpublished
report RD/BS/BL/1268, 1971a; from The Boots Company, submitted to WHO.
Anonymous. Human pharmacology study on BTS 27419. Unpublished report
no. MS 71004; 1971b, from The Boots Company, submitted to WHO.
Anonymous. Supplement to report no. MS 71004; 1971c, Unpublished
report from The Boots Company, submitted to WHO.
Anonymous. Effects of BTS 27149 and BTS 27271 on the responses of
rabbit ear blood vessels to catecholamines and antidromic stimulation.
Unpublished report no. P 72622; 1972, from The Boots Company,
submitted to WHO.
Anonymous. Residue determination for amitraz and its metabolites in
apples and pears. Upjohn Co. reports 315-9760-5,8,11,12; 1973,
(Unpublished).
Anonymous. Residue determination for U-36,059 (amitraz) and
metabolites in apples and pears. Upjohn Co. reports
315-9760-33,35-40,42-49,52,55-65; 1975, (Unpublished).
Anonymous. Residue analysis of amitraz on Swiss apple and pear crops.
Boots Co. Ltd. report no. AR 77016; 1977, (Unpublished).
Anonymous. Residue determination for U-36,059 and metabolites in
cotton seed (Texas, 1977). Upjohn Co. reports 315-9760-131 to 137;
1978, (Unpublished).
Anonymous. Persistence of amitraz on Florida grown Hamlin oranges.
Boots Hercules Agrochemicals Co. report; 1979a, (Unpublished).
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Methods of analysis. Unpublished report dd. 17-9-1979; 1980, from The
Boots Company, submitted to WHO.
Anonymous. Amitraz technical paraform sachets stabilised (8143).
Analytical specifications. Unpublished report, dd. 8-1-1980; 1980,
from The Boots Company, submitted to WHO.
Berczy, Z.S., Binns, R. and Newman, A.J. Acute inhalation toxicity to
the rat of BTS 27419. Unpublished report no. 4971/72/406; 1973, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. and Whitfield,
R.A.S. Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report no. 5454/72/850; 1973, from Huntingdon Research
Centre, submitted to WHO by The Boots Company.
Berry, P.A. A comparison of the hypothermic effects of amitraz (BTS
27419) in male and female CFLP mice. Unpublished report no. P 76058;
1976, from The Boots Company, submitted to WHO.
Brown, D.J., Burnett, R.J., Crowley, J., Cryer, P.C., Lancaster, M.C.
and Turnbull, G.J. Amitraz: Investigations of effects on the thymus
gland and oestrus cycle in mice. Unpublished report no TX 78037; 1978,
from The Boots Company, submitted to WHO.
Burnett, R., Crowley, J., Lessell, B., Patton, D.S.G., Sutton, M.M.,
Turnbull, G.J. and Williams, G.A.H. BTS 27419: 80-Week carcinogenicity
study in mice - final report. Unpublished report no. TX 76039; 1976,
The Boots Company, submitted to WHO.
Campbell, J.K. and Needham, D. Residues of BTS 27271 and its
metabolites in the plasma and tissues of calves after repeated daily
dosing. Boots Co. Ltd. report no. AX 79019; 1979, (Unpublished).
Channon, E.J. and Cryer, P.C. Amitraz: Investigation of effects on the
thymus gland and oestrus cycle in mice - statistical analysis of
vaginal smear data and thymus weights. Unpublished report no. SS
78001; 1978, The Boots Company, submitted to WHO.
Chesterman, H., Skerrett, K., Street, A.E. and Newman, A.J. Boots
27271 oral toxicity study in beagle dogs (Repeated dosage for 13
weeks). Unpublished report no. S 38/73511; 1973, Huntingdon Research
Centre, submitted to WHO by the Boots Company.
Dickinson, W. and Shirley, D.B., Analysis of milk from cows dosed
orally with BTS 27271 at Thurgarton Research Station. Boots Co. Ltd.
report no. F 75018; 1975, (Unpublished).
Dickinson, W. and Shirley, D.B. Residue analyses of tissues from pigs
treated with amitraz at Thurgarton Research Station, UK. Boots Co.
Ltd. report no. F 76015; 1976, (Unpublished).
O'Donovan, M.R. and Smithson, A. Amitraz impurity. BTS 33220: Acute
oral toxicity to male Boots-Wistar rats. Unpublished report no. TX
78079; 1978, from The Boots Company submitted to WHO.
Everest, R.P. BTS 27419: Mutagenicity study in the male mouse
pervisceral cavity hostmediated assay. Unpublished report no. TX
76056; 1976, from The Boots Company submitted to WHO.
Everest, R.P. and Wilcox, P. BTS 27419, BTS 27271, BTS 27919 and BTS
28369: mutagenicity testing in bacterial in vitro systems.
Unpublished report no.76016; 1976a, from The Boots Company submitted
to WHO.
Everest, R.P. and Wilcox, P. BTS 27419: Mutagenicity testing against
Salmonella typhimurium strains TA 1535, TA 1537 and TA 1538 in the
presence and absence of liver microsomes from male and female mice.
Unpublished report no. TX 76057; 1976b, from the Boots Company
submitted to the WHO.
Everest, R.P. and Wilcox, P. In vitro bacterial mutagenicity
testing of an amitraz sample containing 1% BTS 33220. Unpublished
report no. TX 79108; 1979, from The Boots Company submitted to WHO.
Goodall, E. and Somerville, L. Residue analyses of amitraz and its
metabolite BTS 27271 on Italian fruit crops. Boots Co. Ltd. report no.
AX 74026; 1974a, (Unpublished).
Goodall, E. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Dutch apple and tomato crops. Boots Go. Ltd.
report no. AX 74028, 1974b, (Unpublished).
Hall, J.E., Ahlunwalia, H. and Hampson, P. A study of the effects of
oral administration of a metabolite (BTS 27271) of amitraz to
volunteers. Unpublished report no. NS 75002; 1975, from The Boots
Company submitted to WHO.
Hamilton, D.Y. The fate of [14C]-BTS 27419 (amitraz) when
administered to mice at 100 mg/kg as a single oral dose. Unpublished
report no. AX 76013; 1976, from The Boots Company submitted to WHO.
Hamilton, D.Y. Amitraz residues in Turkish cotton seed. Boots Co. Ltd.
report no. AX 77003; 1977a, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
hops and beer from Whitbreads. Boots Co. Ltd. report no. AX 77006;
1977b, (Unpublished).
Hamilton, D.Y. Residues of amitraz and its metabolite BTS 27271 in
cucumbers from the UK. Field Trials 1976. Report no. AX 78011; 1978.
Hamilton, D.Y and Somerville, L. Fate of [14C]-BTS 27271 when
administered to dogs as a oral dose. Unpublished report no. AX 73019;
1973, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27419 when
administered at 15 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74006; 1974a, from The Boots Company submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C] BTS 28369 when
administered at 10 mg/kg to dogs as a single oral dose. Unpublished
report no. AX 74012; 1974b, from The Boots Odmpany submitted to WHO.
Hamilton, D.Y. and Somerville, L. Fate of [14C]-BTS 27271 when
administered to cats as a single oral dose. Unpublished report no. AX
74005; 1974c, from The Boots Company submitted to WHO.
Hayto, E.M. Residues of amitraz and its metabolite BTS 27271 in
pigskin from animals treated at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 77007; 1977a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cattle
tissues from Australia. Boots Co. Ltd. report no. AX 77013; 1977b,
(Unpublished)
Hayto, E.M. Combined residues of amitraz and BTS 27271 in plums and
blackcurrants from UK field trials, 1976. Boots Co. Ltd. report no. AX
77014; 1977c, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Guatemala and Colombia, 1977. Boots Co. Ltd. report no. Ax 78002;
1978a, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in egg-plants
and onion bulbs from Italy. Boots Co. Ltd. report no. AX 78012; 1978b,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton from
South Africa, 1978. Boots Co. Ltd. report no. AX 78015; 1978c,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in
sour-cherries from Denmark, 1976. Boots Co. Ltd. report no. AX 78018;
1978d, (Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
from Mexico, 1977. Boots Co. Ltd. report no. AX 78019; 1978e,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cotton seed
oil from Turkey, 1977. Boots Co. Ltd. report no. AX 78021; 1978f,
(Unpublished).
Hayto, E.M. Combined residues of amitraz and BTS 27271 in cucumbers
from South Africa, 1978. Boots Co. Ltd. report no. AX 79002; 1979,
(Unpublished)
Holland, G.N., Jones, A.W., and Jones, E.M. Analyses of amitraz and
its metabolite BTS 28369 in control and treated Thurgarton calves.
Boots Co. Ltd. report no. P 74022; 1974, (Unpublished).
Holland, G.N. and Jones, E.M. Analyses of amitraz and its metabolite
BTS 28369 in treated South African cattle. Boots Co. Ltd. report no.
F 74021; 1974a, (Unpublished).
Holland, G.N. and Jones, E.M. Analysis of amitraz in milk from treated
South African cattle. Boots Co. Ltd. report no. F 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of amitraz and BTS 27271 in animal tissues and
milk. Boots Co. Ltd. report no. AX 74018; 1974a, (Unpublished).
Hughes, K.W. Estimation of combined residues of amitraz and BTS 27271
in apples and pears. Boots Co. Ltd. report no. AX 74024; 1974b,
(Unpublished).
Hughes, K.W. Estimation of BTS 28369 in human urine. Unpublished
report no. F 75009; 1975, from The Boots company submitted to WHO.
Hughes, K.W., Jones, E.M., and Somerville, L. Residue analyses of
amitraz and its metabolites BTS 27271 and BTS 27919 on apple and pear
crops treated with amitraz at Thurgarton Research Station, UK. Boots
Co. Ltd. report no. AX 74002; 1974, (Unpublished).
Hughes, K.W., and Somerville, L. Residue analyses of amitraz and its
metabolie BTS 27271 on apple crops from Thurgarton Research Station,
UK. Boots Co. Ltd. report no. AX 73017; 1973a, (Unpublished).
Humphrey, E.A. and Somerville, L. Estimation of combined residues of
amitraz and BTS 27271 on Danish cherries. Boots Co. Ltd. report no. AX
76003; 1976, (Unpublished).
Jones, E.M, Metabolism of [14C]-BTS 27419 in calf and in cow, part I.
Identification of major urinary metabolite. Unpublished report no.
F 73004; 1973a, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in calf. Part 2.
Identification of major liver metabolite. Unpublished report no.
F 73011; 1973b, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in rats. Unpublished report
no. F 73010; 1973c, from the Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BST 27271 in dogs. Unpublished report
no. F 73019; 1973d, from The Boots Company submitted to WHO.
Jones, E.M. Metabolism of [14C]-BTS 27419 in cats. Unpublished report
no. F 73018; 1973e, from The Boots Company alimitted to WHO.
Jones, E.M. Residue analyses of BTS 27919 on French peach crops
(Report no. 1). Boots Co. Ltd. report no. F 73015; 1973f,
(Unpublished).
Jones. E.M. and Holland, G.N. Analysis of amitraz and its metabolite
BTS 28369 in treated Thurgarton calves. Boots Co. Ltd. report no.
F 74020; 1974 (Unpublished).
Kakuk, T.J. Amitraz. Further evaluation of 80-week mouse study.
Unpublished report no. HGD 79002; 1979, from The Boots Company
submitted to WHO.
Lancaster. M.C. and Williams, G.A.H. Amitraz: Multigeneration feeding
test in rats - Histopathology. Unpublished report no. TX 78064; 1978,
from The Boots Company submitted to WHO.
Lewis, D.K. RD 27.419. Metabolism and residue studies in the calf
using (14C) and (3H)-labelled material. Unpublished report no.
C 70029; 1970a, from The Boots Company submitted to WHO.
Lewis, D.K. Amitraz - a preliminary study of the persistence,
translocation, and metabolism in plants. Boots Co. Ltd. report no.
FM 70158; 1970b, (Unpublished).
Lewis, D.K. Fate of [14C]-triazid applied to rats as a single oral
dose. Unpublished report no. C 71011; 1971a, from The Boots Company
submitted to WHO.
Lewis, D.K. Fate of [14CH]-BTS 27419 applied to rats as a single
dermal dose. Unpublished report no. C 71019; 1971b, from The Boots
Company submitted to WHO.
Lewis. D.K. Fate of [14C]-BTS 27419 applied to dogs as a single oral
dose. Unpublished report no. 71024; 1971c, from The Boots Company
submitted to WHO.
Lewis, L. Fate of [14C]-triazid (BTS 27419) in the calf. Unpublished
report no. C 71026; 1971d, from The Boots Company submitted to WHO.
Lindstrom, H.V. The metabolism of FD and CRED no. I. The fate of
2,4-meta-xylidine in rats. J. Pharm. Exp. Therap. 132, 306-310.
Martin, H., and Worthing, C.R. Pesticide Manual, 6th Edition, p. 15.
Published by the British Crop Protection Council.
McDougall, D.W., Heath, A.B., and Black. R.R. Residues of amitraz in
the tissues, milk, and butter of cattle dipped in Taktic. Aust. J.
Exp. Agric. Anim. Husb., 19: 663-665.
Merryman, D.C. and Sutton, M.M. BTS 27419. Effects on the oestrus
cycle of the rat. Unpublished report no. PN 72003; 1972, from The
Boots Company submitted to WHO.
Moore, W.K.S. Clinical observations during development. 1972,
Unpublished report from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27271. Acute oral toxicity study in dogs.
Unpublished report no. TX 73004; 1973a, from The Boots Company
submitted to WHO.
Morgan, H.E, BTS 28037; Acute oral toxicity study in dogs. Unpublished
report no. TX 73015; 1973b, from The Boots Company submitted to WHO.
Morgan, H.E. BTS 27919: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74004; 1974a, from The
Boots Company submitted to WHO.
Morgan, H.E. BTS 28369: Acute oral toxicity study in dogs:
Histopathology. Unpublished report no. TX 74006; 1974b, from The Boots
Company submitted to WHO.
Morgan, H.E., Ellicock, L.A., Parkinson, R. and Wood, R. Amitraz:
comparison of oral and topical administration to pigs. Unpublished
report no. TX 75025; 1975a, from The Boots Company submitted to WHO.
Morgan, H.E., Patton, D.S.G. and Turnbull, G.J. BTS 27419: two year
oral toxicity study in dogs. Unpublished report no. TX 73035; 1973,
from The Boots Company submitted to WHO.
Morgan, H.E. and Shepherd, G.M. BTS 28369: Acute oral toxicity study
in dogs. Unpublished report no. TX 74001; 1974, from The Boots Company
submitted to WHO.
Morgan, H.E., Shepherd, G.M., and Turnbull, G.J. BTS 28369: 90-day
oral toxicity study in dogs. Unpublished report no. TX 74037; 1974,
from The Boots Company submitted to WHO.
Morgan, H.E. and Turnbull, G.J. BTS 27919: Acute oral toxicity study
in dogs. Unpublished report no. TX 73034; 1973, from The Boots Company
submitted to WHO.
Morgan, H.E., Turnbull, G.J. and Ellicook, L.A. Amitraz and BTS 27271:
Effects of repeated daily dosing on acute responses in dogs.
Unpublished report no. M 75026; 1975b, from The Boots Company
submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 27271: Acute oral toxicity study
in dogs -Histopathology. Unpublished report no. TX 73030; 1973a, from
The Boots Company submitted to WHO.
Morgan, H.E. and Williams, G.A.H. BTS 28037: Acute oral toxicity study
in dogs. Unpublished report no. TX 73038; 1973b, from The Boots
Company submitted to WHO.
Nappier, J.L. and Hornish, R.E. Total residue method for U-36, 059
(amitraz) in apples, pears, and soils. Upjohn Co. report no.
315-9760-32; 1975, (Unpublished).
Nappier, J.L., Hornish, R.E. and Lane, R.E. Total residue method for
U-36,059 (amitraz) in swine tissue. Upjohn Co. report no. 315-9760-70;
1976, (Unpublished).
Nappier, J.L. and White, M.C. Total residue method for U-36, 059
(amitraz) in cotton seeds. Upjohn Co. report no. 315-78-9760-001;
1978, (Unpublished).
NCI, Final Report. Carcinogenicity of chemicals present in man's
environment. Contract no. NIH-NCI-E-68-311. Reported by Bio-Research
consultants, 1973, submitted to WHO by The Boots Company.
Needham, D. and Campbell, J.K. Amitraz residues in the tissue of sheep
1,3,5 and 7 days after dipping in 0.05% amitraz. Boots Co. Ltd. report
no. AX 80016; 1980, (Unpublished).
Nix, N. Stability data for Amitraz Technical (Paraformaldehyde Sachets
Stabilised). Unpublished report dd. 11-12-1979; 1979, from The Boots
Company submitted to WHO.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
female mouse. Unpublished report no. BTS 81/7758; 1977a, from
Huntingdon Research Centre, submitted to WHO by The Boots Company.
Palmer, A.K. and James, P.A. Dominant lethal assay of amitraz in the
male mouse. Unpublished report no. BTS 80/7792; 1977b, from Huntingdon
Research Centre, submitted to WHO by The Boots Company.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
(chlordimeform) on the central actions of reserpine in the mouse and
rat. Unpublished report no. P 74033; 1974a, from The Boots Company
submitted to WHO.
Parkinson, R. The effects of BTS 27271, BTS 27419 and BTS 21103
chlordimeform) on the pressor response to tyramine in the pithed rat.
Unpublished report no. P 74032; 1974b, from The Boots Company
submitted to WHO.
Parkinson, R. BTS 27271 - an attempt to produce and quantify the
flushing response in the pig. Unpublished report no. P 75030; 1975,
from The Boots Company submitted to WHO.
Parkinson, R., Patton, D.S.G. and Yates, D.B.G. BTS 27419. Effects in
conscious dogs of small intravenous doses. Unpublished report no.
P 71535; 1971a, from The Boots Company submitted to WHO.
Parkinson, R., Sim, M.F. and Yates, D. Effects of the acaricide BTS
27419 and the related sulphur analogues BTS 30559 and BTS 30561 on the
cardiovascular system of the cat, particularly the ear vascular bed.
Unpublished report no. P 71576; 1971b, from The Boots Company
submitted to WHO.
Parkinson, R. and Yates, D.B. The effects of BTS 27419 on
Trafuril-induced erythema on the guinea-pig. Unpublished report no.
PM 71022; 1971, from The Boots Company submitted to WHO.
Patton, D.S.G. RD 27271: Effects on u/v erythema in guinea pigs.
Unpublished report no. PM 70100; 1970, from The Boots Company
submitted to WHO.
Patton, D.S.G. BTS 27419: Acute toxicity in baboons. Unpublished
report no. TX 73002; 1973a, from The Boots Company submitted to WHO.
Patton, D.S.G. BTS 27271: Effects in conscious dogs of small
intravenous doses. Unpublished report no. TX 73017; 1973b, from The
Boots Company submitted to WHO.
Patton, D.S.G. and Sutton, M.M. Acute toxicity studies on BTS 27419 an
acaricide. Unpublished report no. P 71544; 1971a, from The Boots
Company submitted to WHO.
Patton, D.S.G. and Williams, G.A.H. BTS 27419: 90-day toxicity study
in dogs. Unpublished report no. P 71547; 1971b, from The Boots Company
submitted to WHO.
Petzold, G.L., Swenberg, J.A. and Bedell, M. Evaluation of amitraz
(U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915 A and
U-54,914) in the DNA/Alkaline Flution assay. Unpublished report no.
7268/77/7268/001; 1977, from The Boots Company submitted to WHO.
Richards, M.E. Combined residues of amitraz and BTS 27271 in olives
from Italy, 1978. Boots Co. Ltd. report nos. AX 79018 and 80029; 1979
and 1980a, (Unpublished).
Richards, M.E. Combined residues of amitraz and BTS 27271 in apples
and pears from West Germany. Boots Co. Ltd. report no. AX 80001;
1980b, (Unpublished).
Shaw, J.W. BTS 27419 - acute intraperitoneal toxicity to rats.
Unpublished report no. PM 71057; 1971, from The Boots Company
submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral and intraperitoneal
toxicities to rats. Unpublished report no. TX 73006; 1973a, from The
Boots Company submitted to WHO.
Shaw, J.W. BTS 27419. Comparison of the acute oral toxicities to rats
of BTS 27419 and BTS 27919. Unpublished report TX 73010; 1973b, from
The Boots Company submitted to WHO.
Shaw, J.W. BTS 27419 metabolite: 21-day chronic oral toxicity in rats
of BTS 28369. Unpublished report no. TX 75058; 1975, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTS 27419. 90-day chronic toxicity
study in mice. Unpublished report no. TX 74016; 1974, from The Boots
Company submitted to WHO.
Shaw, J.W. and Williams, G.A.H. BTU 27419 metabolite: 90-day chronic
oral toxicity in rats of BTS 27271. Unpublished report no. TX 75059;
1975, from The Boots Company submitted to WHO.
Shirley, D.B. Analysis of tissue from pigs treated with amitraz at
Thurgarton Research Station. Boots Co. Ltd. report no. F 75015; 1975a,
(Unpublished).
Shirley, D.B. The analysis of BTS 28369 in the urine of amitraz
production workers. Unpublished report no. F 75019; 1975b, from The
Boots Company submitted to WHO.
Shirley, D.B. The analysis of BTS 28369 in the urine of three amitraz
production workers over a period of three weeks. Unpublished report
no. F 75024; 1976.
Shirley, D.B. Amitraz residues in cattle from Wiltonside, South
Africa. Boots Co. Ltd. report no. F 77002; 1977a, (Unpublished).
Shirley, D.B. Residue analyses of tissues from sheep treated with
amitraz at Thurgarton Research Station, UK. Boots Co. Ltd. report no.
F 77004, 1977b, (Unpublished).
Shirley, D.B. Analysis of amitraz residues in sheep's milk. Boots Co.
Ltd. report no. F 78013; 1978, (Unpublished).
Sim, M.F. The diuretic action of the acaricides RD 27271 and RD 27419
in mice. Unpublished report no. PM 70111; 1970, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 in the lactating cow.
Unpublished report no. C 72007; 1972, from The Boots Company submitted
to WHO.
Somerville, L. Fate of 14C-BTS 27419 administered to rats in repeated
oral doses. Unpublished report no. AX 73011; 1973a, from The Boots
Company submitted to WHO.
Somerville, L. Fate of 14C-ring-labelled BTS 27419 in the calf.
Unpublished report no. AX 73006; 1973b, from The Boots Company
submitted to WHO.
Somerville, L. Fate of 14C-BTS 27419 when administered to cats as a
single oral dose. Unpublished report no. AX 73018; 1973c, from The
Boots Company submitted to WHO.
Somerville, L. and Goodall, E. Residue analyses of amitraz and its
metabolite BTS 27271 on Danish apple crops. Boots Co. Ltd. report no.
AX 74023; 1974, (Unpublished).
Somerville, L. and Hamilton, D.Y. Studies on the accumulation and
elimination of radio-labelled residues from dogs' eyes following oral
administration of (14C)-BTS 27271. Unpublished report no. AX 74013;
1974, from The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch cucumber crops. Boots Co. Ltd. report
no. AX 73008; 1973a, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 1). Boots Co.
Ltd. report no. AX 73010; 1973b, (Unpublished).
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French peach crops (Report no. 2). Boots Co.
Ltd. report no. AX 73013; 1973c, (Unpublished)
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on Dutch apple crops. Boots Co. Ltd. report no.
AX 73014; 1973d, (Unpublished).
Somerville, L. and Hughes, K.W. The conversion of BTS 27419 to BTS
27271 in the dog stomach. Unpublished report no. AX 73021; 1973e, from
The Boots Company submitted to WHO.
Somerville, L. and Hughes, K.W. Residue analyses of amitraz and its
metabolite BTS 27271 on French apple crops. Boots Co. Ltd. report no.
AX 73016; 1973f, (Unpublished).
Somerville, L. and Nicholson, J.E. Amitraz. Metabolism in apples,
variety Cox's Orange Pippin. Boots Co. Ltd. report no. AX 72003; 1972,
(Unpublished).
Somerville, L. and Richards, M.E. The analysis of fruit for combined
residues of amitraz and BTS 27271. Boots Co. Ltd. report no. AX 80002;
1980, (Unpublished).
Somerville, L. and Spiers, M.J. Amitraz. Metabolism in apple leaves.
Boots Co. Ltd. report no. AX 72002; 1972, (Unpublished).
Sutton, M.M., RD 27271. Acute oral toxicity to mice. Unpublished
report no. PM 70043; 1970a, from The Boots Company submitted to WHO.
Sutton, M.M. RD 27271. Acute toxicity to rats. Unpublished report no.
PM 70042; 1970b, from The Boots Company submitted to WHO.
Sutton, M.M. Acaricides: RD 27419 and RD 27271 acute toxicity to
guinea pigs. Unpublished report no. PM 70108; 1970c, from The Boots
Company submitted to WHO.
Sutton, M.M. BTS 27419. Contact sensitization in the guinea pig.
Unpublished report no. PM 71010; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Effect on thermo-regulation in mice.
Unpublished report no. P 72609; 1972a, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27271: acute oral toxicity to rabbits. Unpublished
report no. TXM 72044; 1972b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rabbit. Unpublished
report no. TX 73029; 1973a from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Teratogenicity in the rat. Unpublished report
no. TX 73028; 1973b, from The Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Effects on pregnancy, parturition and care of
the young in rate. Unpublished report no. TX 73031; 1973c, from The
Boots Company submitted to WHO.
Sutton, M.M. BTS 27419: Multigeneration feeding test in rats.
Unpublished report no. TX 73036; 1973d, from The Boots Company
submitted to WHO.
Sutton, M.M. BTS 27419: Three-week dermal toxicity to rabbits.
Unpublished report no. TX 73026; 1973e, from The Boots Company
submitted to WHO.
Sutton, M.M. and Metcalf, W. BTS 27419. Eye-irritancy in the rabbit.
Unpublished report no. TXM 72037; 1972, from The Boots Company
submitted to WHO.
Sutton, M.M. and Offer, J. BTS 27419: carcinogenicity and long-term
toxicity study in rats. Unpublished report no. TX 73043; 1972, from
The Boots Company submitted to WHO.
Sutton, M.M. and Williams, G.A.H. BTS 27419: 90-day toxicity study in
rate. Unpublished report no. P 71548; 1971, from The Boots Company
submitted to WHO.
Sutton, M.M. and Williams, P.A. BTS 27419: acute dermal toxicity to
rabbits. Unpublished 1972 report no. YM 72011; 1972 from The Boots
Company submitted to WHO.
Taylor, J. and Somerville, L. The conversion of amitraz to BTS 24068
in dog gastric juice. Unpublished report no. AX 77010; 1977, from The
Boots Company submitted to WHO.
Toyoshima, S., Fujita. H., Sato, H., Sato. R., Sato, S. and Kashima,
M. JA-119 (BTS 27419). Three-month feeding study on rats. Unpublished
report E 76014; 1972a, from The Boots Company submitted to WHO.
Toyoshima, S., Fujita H., Sato, H., Sato, R., Sato, S. and Kashima, M.
JA-119 (BTS 27419). Three month feeding study on mice. Unpublished
report no. K 76015; 1972b, from The Boots Company submitted to WHO.
Tuplin, J.A. and Wilcox, A. Amitraz. Ames-tests on a sample produced
by Nissan Chemical Industries. Unpublished report no. TX 78068; 1978,
from The Boots Company submitted to WHO.
Weisburger, E.K. et al. Testing of twenty-one environmental aromatic
amines or derivatives for long-term toxicity or carcinogenicity. J.
Environ. Path. Toxicol. 2, 325-356.
Wilcox, P. BTS 27419: Mutagenicity study in the intraperitoneal
host-mediated assay. Unpublished report no. TX 76028; 1976, from The
Boots Company submitted to WHO.
Wilcox, P. Amitraz impurity BTS 33220: in vitro bacterial
mutagenicity testing. Unpublished report no. TXA 79025; 1979, from The
Boots Company submitted to WHO.
Zimmer, D.M., Mazurek, J.H. and Bhuyan, B.K. Evaluation of amitraz
(U-36.059) and its metabolites in the Salmonella microsome test.
Unpublished report no. 72068/77/7269/002; 1977, from The Boots Company
submitted to WHO.
