
AMITRAZ
First draft prepared by
J.-J. Larsen
Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
Ministry of Food, Agriculture and fisheries, Sœborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Effects on the thymus and hormone concentrations
Effects on the estrus cycle and hormone
concentrations
Mechanism of action
Studies of metabolites
Acute toxicity
Short-term toxicity
Genotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Amitraz [ N-methylbis(2,4-xylyliminomethyl)amine] was evaluated
by the Joint Meeting in 1980, 1984, and 1990 (Annex 1, references 34,
42, and 59). A toxicological monograph was prepared in 1980 (Annex I,
reference 35) and a monograph addendum was prepared in 1984 (Annex I,
reference 43). A temporary ADI of 0-0.0005 mg/kg bw was allocated in
1980, and an ADI of 0-0.003 mg/kg bw was established in 1984. The 1990
Meeting reviewed the compound at the request of a WHO Member State
which asked for reconsideration of the ADI in view of the acute nature
of the reported toxicological effects and potential dietary exposure.
Since that Meeting, studies have become available on absorption,
distribution, excretion, biotransformation, effects on liver enzymes
and the oestrus cycle, long-term toxicity, dermal and ocular
irritation, and dermal sensitization.
The compound was reviewed by the present Meeting within the CCPR
periodic review programme. This monograph summarizes the new data and
relevant data from the previous monograph and monograph addendum on
this pesticide (Annex 1, references 35 and 43).
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Male and female B6C3F1 mice, either untreated or pre-dosed for
three weeks with 400 ppm amitraz in the diet, were given a single oral
intubation of 0 or 10 mg/kg bw 14C-amitraz (specific activity, 9
mCi/g). Urine and faeces were collected for 96 h after dosing, after
which the mice were killed for tissue analysis. During the first 24 h,
86% of the radiolabelled dose was excreted and 62% was present in the
urine. It was completely excreted by 96 h, with 73% in the urine. The
route and rate of excretion were similar in each sex and in untreated
and pre-dosed mice. The highest concentrations of radiolabel were
found in the liver, adrenals, and eyes and the least in bone and
muscle (Campbell & Needham, 1983).
In rats given oral doses of 14C-labelled amitraz (specific
activity unspecified), 53-85% was recovered in urine within three
days, with 17-47% in faeces and < 0.1% in expired air. Peak plasma
concentrations were found about l h after dosing. The highest
concentrations of residue were found in the liver, kidney, and muscle
within 2 h, diminishing thereafter (Lewis, 1971).
During repeated oral dosing of groups of one male and one female
rat with 4 mg/kg bw per day 14C-labelled amitraz (specific activity
unspecified) for 28 days, the highest concentrations of residue were
found in the thyroid and adrenal glands, liver, kidney, skin, spleen,
and eyes. After dosing had ceased, a considerable decrease in
concentration was observed. The radiolabel in blood was bound mainly
to cells. Seven days after the final dose, small but significant
concentrations of residue were detected in liver, spleen, skin, and
adrenals (Somerville, 1973).
Three male and three female Sprague-Dawley rats were dosed orally
with 10 mg/kg bw 14C-amitraz (specific activity, 7.8 mCi/g) dissolved
in corn oil, and urine and faeces were collected over 96 h, after
which the rats were killed and dissected for tissue analysis. Over the
first 24 h, 82% of the dose was excreted, mainly in the urine; over 96
h, 94% of the dose was recovered, with 82% in urine and 12% in faeces.
The concentrations of residue were highest (0.4-0.5%) in liver
(Campbell & Needham, 1981).
Ten male rats were treated dermally with 1 mg 14C-amitraz
(specific activity, 253 mCi/g), formulated as MitacTM wettable
powder (technical product), diluted with water to a concentration
approximately 20 times the maximum recommended spray dilution. After
10 h, the treated skin was washed with soap and water. Half of the
rats were killed 24 h after treatment, and the remainder were
maintained in metabolism cages for five days. Urine and faeces were
collected at 24-h intervals after the beginning of treatment and were
radioassayed with gastrointestinal tracts, carcasses, treatment sites,
dressings, and washings. Washing of the skin 10 h after treatment
removed 92% of the applied amitraz, while approximately 3% remained on
the skin by 24 h; this percent fell to 1.4% after five days. The small
amount of amitraz absorbed over five days (approximately 3-8% of that
applied) was excreted in the urine and faeces. Excretion by this route
was 90% complete 96 h after treatment. Very low concentrations of
residue were detected in the carcass (0.06%) and gastrointestinal
tract (0.01%) after five days (Challis, 1990).
Male Crl:CD(SD)BR rats (n = 112) were treated dermally with
0.01, 0.1, 1, or 10 mg per animal 14C-amitraz (specific activity, 281
mCi/g), formulated as diluted MitacTM wettable powder (technical
product) for 0.5, 1, 2, 4, or 10 h, and radiolabel was measured in
urine, faeces, residual carcass, and the application site. The results
for animals at 0.01 mg were not reported because of poor, inconsistent
recovery of radiolabel. The mean total recovery of radiolabel was 104%
at 0.1 mg, 100% at 1 mg, and 107% at 10 mg. The distribution of
radiolabel was similar at the three doses. Most of the applied dose
was recovered in application site washings (61-99%) and dressing
washings (10-31%). The concentration of radiolabel retained at the
application site peaked 4 h after application. When the site was
washed, 10 h after application, the concentration of radiolabel
retained at the application site fell to < 3% by 24 h and to 0.06% by
120 h in all three groups. The extent absorbed (percent applied dose)
fell with increasing dose, implying that absorption was nearing
saturation at 0.1 mg/animal. Maximum absorption (12% at 0.1 mg/animal)
was achieved at 120 h after a 10-h exposure. The concentrations of
radiolabelled amitraz and its metabolites in blood were low at all
sacrifices and at all doses. The absorbed radiolabel was eliminated
primarily in urine, with minor quantities in faeces. The
concentrations of radiolabel in residual carcass were low (maximum, 2%
at 24 h in animals receiving 10 mg) at all three doses and all
sacrifices. By 120 h after application, the concentration in residual
carcass was below the limit of detection in all animals (Stewart,
1993).
In separate studies, groups of two male and two female beagle
dogs were dosed orally (4 mg/kg bw by capsule) or dermally (20-21 mg
on an area of 400-500 cm2) with 14C-amitraz (specific activity,
8.6 mCi/g). Peak blood concentrations of radiolabel were observed
during the first 8 h after oral administration. About 80% of the oral
dose was excreted within the first 24 h and 100% within 72 h,
preferentially in the urine. After dermal treatment, peak blood
concentrations occurred within 24-72 h, and only 25-40% was recovered
in urine and faeces over a 10-day collection period, demonstrating the
poor dermal absorption of amitraz (Hornish & Nappier, 1983).
Two male and two female pigs received a single topical dose of
18 mg 14C-amitraz (chemical purity, 98.6%; specific activity,
9 mCi/g) on a shaven dorsal area. The treated area was subjected to a
mild washing procedure 12 h after application, which removed 60-80% of
the applied radiolabel. Over 60 h after dosing, 7% of the applied
radiolabel was detected in excreta. Less than 0.05 ppm residues were
found in most tissues (Campbell & Needham, 1984a).
One male and one female baboon received a single oral dose of 10
mg/kg bw 14C-amitraz (specific activity, 2 mCi/g), and urine and
faeces were collected for 72 h, after which time the animals were
killed. Within the first 24 h, 75-83% of the dose was excreted, with
58-76% in the urine. The concentrations of residues in tissue residues
were similar in animals of each sex: highest in liver and eyes and
lowest in muscle (Campbell & Needham, 1984b).
Two male volunteers received a single oral dose of 0.25 mg/kg bw
14C-amitraz; urine was collected for 72 h after dosing, and the
radiolabel was determined. Urinary excretion was 58-68% of the dose
within the first 24 h and 77-87% within 72 h. Within the first 4 h,
dry mouth, drowsiness, disorientation, slurred speech,
light-headedness, and decrease in pulse rate and blood pressure were
recorded. One person fell asleep 2 h after treatment and subsequently
complained of nausea and vivid dreams. The urinary metabolites were
the same as those found in the other species examined (Campbell &
Needham, 1984c).
(b) Biotransformation
In rats dosed orally with 14C-labelled amitraz (specific
activity unspecified), at least four metabolites were found in urine
and six in faeces, the major component of which was
N-methyl- N'-(2,4-xylyl)formamidine (Lewis, 1971).
After administration of a single oral dose of 14C-amitraz
(specific activity, 9.0 mCi/g; radiochemical purity, > 95%) to groups
of two male and two female Sprague-Dawley CD rats at 1, 10, 50, or
100 mg/kg bw, the compound was rapidly excreted, effectively
metabolized, and completely degraded, with no apparent sex difference.
At all doses, 4-formamido- meta-toluic acid and
4-acetamido- meta-toluic acid were the major metabolites (see Figure
1), which together accounted for up to 32% of the metabolites excreted
in the urine. Excretion of the hydrolysis product,
N-methyl- N'-(2,4-xylyl)formamidine, was dose-dependent: about 4%
of the dose at 1 mg/kg bw and 23-38% at 100 mg/kg bw. Minor
metabolites identified accounted for 2% of the total excretion and
included form-2',4'-xylidide and 4-amino- meta-toluic acid (Campbell
& Needham, 1984d).
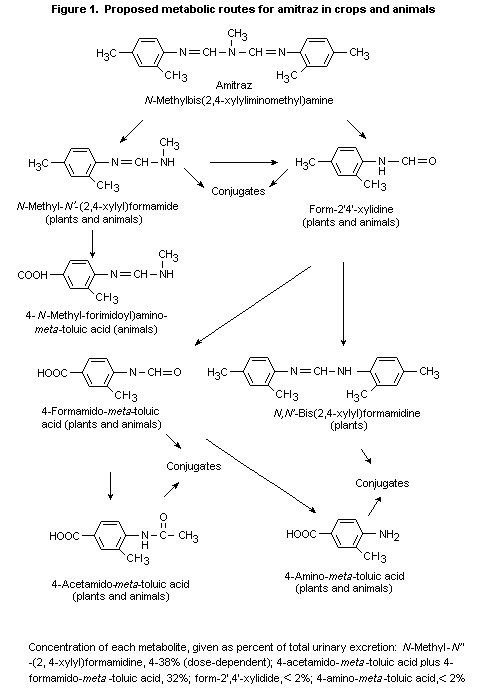 In the study of Challis (1990), described above, the metabolic
profile of rats treated dermally was similar to that of rats treated
orally.
In the study of Hornish & Nappier (1983), described above, the
metabolism of amitraz was essentially the same after oral and dermal
administration. 4-Formamido- meta-toluic acid was the predominant
residue in both blood and urine. The parent compound and the first
hydrolysis products, N-methyl- N'-(2,4-xylyl)formamidine and
form-2',4'-xylidide, were not observed at measurable concentrations in
either blood or urine.
One cow received 0.5 mg/kg bw per day 14C-amitraz (specific
activity, 4.2 mCi/g; radiochemical purity, > 98.8%) by capsule twice
daily for four days. All milk was collected, and samples of urine were
taken. The cow was killed 17 h after the final dose. The urinary
metabolites corresponded to 4-acetamido- meta-toluic acid and
4-formamido- meta-toluic acid, which were converted to
4-amino- meta-toluic acid by acid hydrolysis. The total residue in
milk accounted for only 0.2% of the dose. The greatest concentration
of residue (0.07 ppm) was found after the final dose. The residue was
extracted with methanol (recovery, 90%), and the metabolites present
identified by co-chromatography as 4-acetamido- meta-toluic acid,
4-formamido- meta-toluic acid, and 4-amino- meta-toluic acid (23%);
form-2',4'-xylidide (9%); polar material that was converted to
4-amino- meta-toluic acid by acid hydrolysis (34%); and low-polarity
material (24%). The latter broke down readily to form-2',4'-xylidide
under neutral or basic conditions; it was not present in acetonitrile
extracts of whole milk and was considered to be an artefact produced
by the methanol Soxhlet extraction of the milk. 2,4-Dimethylaniline
was not observed. The edible tissue with the highest concentration of
residue was the liver (3.7 ppm).
Methanol Soxhlet extraction released about 60% of the residue,
and the remainder was solubilized after enzymic digestion and acid
hydrolysis. The residues were identified by co-chromatography as
4-acetamido- meta-toluic acid and 4-formamido- meta-toluic acid
(14%), 4-amino- meta-toluic acid (15%), form-2',4'-xylidide (10%),
and polar material (10%) which on acid hydrolysis was converted to
4-amino- meta-toluic acid and additional low-polarity material. Other
polar material (29% of the residue) was not amenable to
chromatography. 2,4-Dimethylaniline was not observed. The low-polarity
material (14%) broke down on neutral or basic thin-layer
chromatography to a range of compounds, suggesting that it was a
product of condensation between 4-amino- meta-toluic acid and
compounds released from acid-labile conjugates. When the
non-methanol-extractable residue was fed to a dog, analysis of urine
gave similar results to those after enzymic digestion (Phillips et
al., 1987).
Six laying hens (Gallus gallus domesticus) were each dosed
orally with 24.5 mg 14C-amitraz (specific activity, 0.14 mCi/g;
radiochemical purity, > 99%) per day for four days, providing a dose
2200 times greater than the expected daily exposure of birds fed
cotton-meal derived from amitraz-treated plants. The hens were killed
4 or 12 h after the final dose, and the concentrations and nature of
radioactive residues in tissues were determined. The radiolabel was
rapidly excreted, with 68% in 0-24-h excreta. The main route of
metabolism was via 4-amino- meta-toluic acid, since 75% of the
metabolites extracted from the excreta could be accounted for as
either this metabolite or its acid-labile conjugates. The highest
concentrations of tissue residues were found in the liver and kidney
(17 and 25 mg/kg respectively) 4 h after the fourth dose, but these
concentrations had fallen to 12 and 10 mg/kg, respectively, by 12 h
after dosing. The major metabolite detected in the liver was
4-amino- meta-toluic acid, present as both free acid and labile
conjugates, which represented 55% of the total residue.
N-Methyl- N'-(2,4-xylyl)formamidine (4%), form-2',4'-xylidide (4%)
and 2,4-dimethylaniline (2%) were also present. A mixture of at least
seven highly polar compounds, believed to be mono- and di-acids
similar to those previously seen in rats, was a further substantial
residue (27%). Unextractable fibre-bound material represented 2% of
the residue. The concentrations in fat, muscle, and skin represented
0.6-2.7 mg/kg 4 and 12 h after the final dose. In fat, the residues
consisted of form-2',4'-xylidide (42%),
N-methyl- N'-(2,4-xylyl)formamidine (24%), unchanged amitraz (21%),
2,4-dimethylaniline (3%), and 4-amino- meta-toluic acid (3%). In
muscle, 81% of the residues were identified as 4-amino- meta-toluic
acid or its acid-labile conjugates; form-2',4'-xylidide was present as
7% of the residue.
The concentrations of amitraz residues in eggs, collected daily,
were 0.28-0.46 mg/kg throughout the study and did not increase over
the four days of dosing. The residue concentrations in the yolks rose
from 0.1 to 1.4 mg/kg during the study;
N-methyl- N'-(2,4-xylyl)formamidine (54%) and 4-amino- meta-toluic
acid (34%) were the major metabolites. 4-Amino- meta-toluic acid
accounted for 91% of the residue in the egg white as both free acid
and labile conjugates, and form-2',4'-xylidide accounted for 4% of the
residue (Needham & Hemmings, 1988).
Separately reported data on the metabolism of amitraz
in various species were compared. After a single oral dose of
14C-amitraz (specific activity, 9 mCi/g; radiochemical
purity, > 95%) to groups of four male and four female mice,
three male and three female rats, one male and one female
baboon, and two men, the administered radiolabel was rapidly excreted.
In all species examined, urine was the major route of excretion,
accounting for 65-84% of the dose (55-76% within the first 24 h).
Analysis of urine obtained from volunteers dosed with 14C-amitraz
indicated that the metabolism of amitraz was qualitatively similar
to that in the other species. The major metabolites were
4-formamido- meta-toluic acid, 4-acetamido- meta-toluic acid and
N-methyl- N'-(2,4-xylyl)formamidine. In addition, 40-60% of the
metabolites excreted in urine was accounted for by a polar fraction
containing conjugates of 4-formamido- meta-toluic acid and
4-acetamido- meta-toluic acid. The minor metabolites included
4-amino- meta-toluic acid and form-2',4'-xylidide. Excretion of
N-methyl- N'-(2,4-xylyl)formamidine was dose-dependent. The
metabolites identified in the volunteers were
4-formamido- meta-toluic acid plus 4-acetamido- meta-toluic acid
(27% of the total radiolabel in urine),
N-methyl- N'-(2,4-xylyl)formamidine (6%), 4-amino- meta-toluic
acid (4%), form-2',4'-xylidide (4 %), the product of acid hydrolysis
of N-methyl- N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
(1%), and polar material (57%). In both rats and mice, increasing the
dose of amitraz from 1 to 100 mg/kg bw increased the excretion of
N-methyl- N'-(2,4-xylyl)formamidine from approximately 5 to 30% of
the total excretion. Prior dosing of five male and five female mice
with amitraz in the diet at 100 ppm for three weeks, followed by
400 ppm for a further three weeks, had no effect on the metabolism of
a single oral dose of 14C-amitraz (Campbell & Needham, 1984e).
(c) Effects on enzymes and other biochemical parameters
Groups of 18 male and 18 female B6C3F1 mice were dosed orally
with corn oil or amitraz at 100 mg/kg bw per day for two days and,
because of toxic symptoms, at 50 mg/kg bw per day for the following
two days. The animals were then killed, and the livers were assayed
for the activity of microsomal oxidases. A significant increase in
liver weight and in the activity of cytochrome b5 were recorded. The
effect did not appear to be related to increased activity of the
hepatic mixed-function oxidase system, since no significant increase
was seen in the activities of cytochrome P450, aniline hydroxylase, or
para-nitroanisole demethylase or in the concentration of microsomal
protein (Needham, 1984).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of amitraz and its metabolites (purity
unspecified) has been investigated in several species (Table 1). The
toxic signs after oral administration of amitraz to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Rats had intestinal
irritation and bladder distension. The lowest effective doses were 400
mg/kg bw in mice and 200 mg/kg bw in rats. Dermal application of 1600
mg/kg bw to rats had no local or systemic effect. Guinea-pigs were
hyperexcitable after receiving 400 mg/kg bw or more. Rabbits had
central nervous system depression, decreased rectal temperature, pulse
rate, and respiration, nasal discharge, and rales after receiving 100
mg/kg bw, with complete recovery by 48 h. The reactions of dogs to
administration of 100 mg/kg bw and, to a lesser extent, 20 mg/kg bw,
were central nervous system depression, ataxia, muscular weakness,
muscular spasm, uncontrolled vocal spasm and micturition, and
decreased rectal temperature and pulse rate. Haemoconcentration and
increased blood sugar, urea nitrogen, and potassion concentrations
were also seen. No specific pathological lesions were induced. The
lowest dose, 4 mg/kg bw, affected temperature and pulse rate only
slightly . The profile of toxic reactions was consistent with
depression of hypothalamic function. All of the effects were
reversible.
Table 1. Acute toxicity of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
Mouse Oral > 1600 Patton & Sutton (1971)
Rat Oral 600 Patton & Sutton (1971);
Shaw (1973a)
Rat Dermal >1600 Patton & Sutton (1971)
Rat Intraperitoneal 800 Shaw (1971, 1973a)
Rat Inhalation (6 h) 65 Berczy et al. (1972)
Guinea-pig Oral 400-800 Patton & Sutton (1971)
Rabbit Oral > 100 Patton & Sutton (1971)
Rabbit Dermal > 200 Sutton & Williams (1972)
Dog Oral 100 Patton & Sutton (1971)
Baboon Oral 100-250 Patton (1973)
The dermal irritation potential of amitraz (purity, 99%) was
studied in six New Zealand white rabbits weighing 1.9-2.5 kg.
Approximately 24 h after hair had been removed from the dorso-lumbar
region, 0.5 g of technical-grade amitraz was applied under a 2.5-cm
gauze pad moistened with 0.5 ml distilled water, and each treatment
site was occluded with an elastic adhesive dressing for 4 h. At the
end of the exposure period, the dressing was removed and the treatment
site was washed with water. The treated skin was examined on days 1,
2, 3, and 4 after treatment. There was no response to treatment
(Liggett & Smith, 1987a).
The ocular irritation potential of amitraz (purity unspecified)
was studied in six New Zealand white rabbits weighing 2.1-2.8 kg. The
eyes of each animal were examined before instillation of 45 mg (0.1 ml
crystalline powder) of technical-grade amitraz (purity, 98.4%) into
one eye. Both eyes were examined 1 h before and 1, 2, 3, 4, and 7 days
after instillation. Only minimal or slight conjunctival irritation was
observed (Liggett & Smith, 1987b).
Groups of 12 guinea-pigs weighing 300-350 g were treated daily
with 0.1 ml of acetone or 10% amitraz (purity unspecified) or with 1%
dinitrochlorobenzene on the outer surface of each ear for three days.
One week after the start of treatment, the backs and flanks were
clipped and treated with challenge doses of 0.2 ml acetone, amitraz
(10%), or dinitrochlorobenzene (0.25%). The degree of erythema was
assessed after 24 h. Sensitizing activity was observed after treatment
with the positive control but not with amitraz (Sutton, 1971).
The dermal sensitizing effect of amitraz was studied in the
Buehler test. During induction, five male and five female guinea-pigs
received 500 mg technical-grade amitraz (purity, 99%) on the anterior
right flank for 6 h under a dry compress on days 1, 8, and 15. A dry
compress alone was applied on the anterior left flank. After a
two-week rest period, each animal received a challenge of 500 mg
technical-grade amitraz to the right flank and a dry compress alone to
the left flank. The cutaneous reactions were evaluated 24 and 48 h
after challenge. The animals were then sacrificed, and cutaneous
samples were taken from the challenge site in all animals. No clinical
signs or deaths related to treatment were observed, and no cutaneous
reactions were found 24 and 48 h after the challenge with amitraz
(Clouzeau, 1992).
Delayed contact hypersensivity in response to amitraz (purity,
98.4%) was tested in the maximization test described by Magnusson and
Kligman. After random allocation of 20 female guinea-pigs to a control
group and 20 to a test group, the animals were induced by intradermal
injection of 5% w/w amitraz in Alembicol D (a coconut oil) into a
4 × 6-cm clipped area of the dorsal skin on the scapular region. One
week after the injections, the same area was clipped again and given a
topical application of 15 or 30% w/w amitraz in Alembicol D on a
2 × 4-cm patch saturated with the test solution, which was placed on
the skin, covered with impermeable plastic adhesive tape, secured by
an elastic adhesive bandage, and left in place for 48 h. The test and
control animals were challenged topically as described above, two
weeks after the induction, with 15 or 30% w/w technical-grade amitraz
in Alembicol D. The challenge sites were evaluated 24, 48, and 72 h
after removal of the patches, and an arbitrary scale of 0-4 was used
to score the reactions. The dermal reactions observed in the test
animals were more marked and persistent than those seen in the
controls (Kynoch & Parcell, 1988).
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female B6C3F1 mice were fed diets
containing 0, 100, 200, 400, 600, or 800 ppm amitraz (purity, 98.3%),
equal to 0, 13, 26, 53, 96, and 110 mg/kg bw per day for males and 0,
17, 35, 68, 110, and 150 mg/kg bw per day for females, for 13 weeks.
Clinical signs, body weight, and food consumption were recorded
throughout the study. All mice were killed at the end of treatment and
examined grossly but not histopathologically. The only overt sign of
reaction to treatment was an increase in aggressive behaviour, as
evidenced by fighting and resultant cutaneous lesions, among males
treated at doses > 400 ppm. The overall body-weight gain was
statistically significant lower in the males (40%) treated at > 400
ppm and in females (34%) at > 200 ppm. Food consumption did not
differ statistically significantly between the control and treated
groups. The NOAEL was 100 ppm, equal to 17 mg/kg bw per day, on the
basis of the overall reduced body-weight gain of females (Colley et
al., 1981).
Rats
Groups of 21 male and 21 female Ash-Wistar rats were given
amitraz (purity unspecified) suspended in 0.4% Cellosize solution by
oral intubation at a dose of 0, 3, or 12 mg/kg bw per day for 90 days.
They were then killed either immediately or after a three-week
recovery. Treatment of rats with 50 mg/kg bw per day was discontinued
after seven days because of depressed growth and behavioural
disturbances, and treatment with 200 mg/kg bw per day was discontinued
after seven days because of irritability and debilitation. Body
weights were recorded three times per week. Haematological
examinations were made on some control rats and on some rats receiving
12 mg/kg bw per day at 4, 8, and 12 weeks. At autopsy, blood was
withdrawn from the heart to estimate plasma alkaline phosphatase
activity, bilirubin, aspartate and alanine aminotransferase activity,
and sodium and potassium concentrations. Organs were weighed and
prepared for microscopic examination. Significant reductions were seen
in the overall body-weight gain (8%) and absolute (8%) and relative
weights (6%) of the liver of male rats receiving 12 mg/kg bw per day.
The NOAEL was 3 mg/kg bw per day on the basis of these last effects
(Sutton & Williams, 1971).
Groups of 12 rats (strain not given) were exposed daily for 6 h
to dusts of amitraz (purity unspecified) at a concentration of 0,
0.01, 0.1, or 1 mg/L air, on 14 days over three weeks. During exposure
to 0.1 mg/L, signs of mild dyspnoea, slight eye irritation, and
hyposensitivity to noise were recorded. After termination of exposure,
the rats were hypersensitive to touch and became aggressive. Exposure
to 1 mg/L elicited signs similar to but more severe than those seen in
the previous group. In addition, ataxia, increased nasal secretion,
polyuria, body tremors, and coma were observed in the exposed rats.
Consumption of food and water was reduced, and there was body-weight
loss. Reductions in packed cell volume, haemoglobin, red cells, and
plasma protein concentrations in blood may have been related to
treatment. The body tremors, aggressive behaviour, and coma indicate
that the central nervous system was affected (Berczy et al., 1973).
Rabbits
Groups of four to eight male and female New Zealand white rabbits
weighing 2.5-4.5 kg (the health status of which must be regarded as
suspect due to general infection) were treated on the intact skin of
the back (without occlusion) with amitraz (purity unspecified) in
acetone at a dose of 0, 50, or 200 mg/kg bw per day for a total of 15
doses over 21 days. Body weight, food consumption, heart rate, and
rectal temperature were determined. Haematological and blood
biochemical parameters and organ weights were measured, and
histopathological examinations were carried out. A transient sedative
effect and local skin reactions were seen in animals of each sex at 50
and 200 mg/kg bw per day. Body weights and food consumption were
adversely affected, and some deaths occurred that were possibly
related to treatment. Variable tubular degeneration was seen in the
testes, which were underweight. At 50 mg/kg bw per day, body weights
and food consumption were less adversely affected than at the high
dose, and the death of only one male was considered related to
treatment (Sutton, 1973a).
Dogs
Groups of two male and two female beagles received amitraz
(purity unspecified) in capsules at a dose of 0, 0.25, 1, or 4 mg/kg
bw per day for 90 days. The animals were examined clinically before
dosing began and during weeks 2, 4, 8, and 13 of dosing. Food
consumption was recorded daily and body weights weekly.
Haematological, blood biochemical, and urinary parameters were
measured before and at intervals during treatment. During week 13 of
dosing, control animals and those at 4 mg/kg bw per day were placed in
metabolism cages, and their water intake and urine excretion over 24 h
were recorded. The faeces were tested daily for occult blood during
the first week of dosing. At the end of treatment, the animals were
sacrificed, and several organs were weighed and prepared for
histological examination.
On the three first days of dosing, all four animals at 4 mg/kg bw
per day showed central nervous system depression, vomiting, ataxia,
and reduced rectal temperature and pulse rate, which recurred
consistently within 6 h. Hyperglycaemia and occasional glycosuria were
also induced. The livers were slightly enlarged and showed low-grade
microscopic changes. The dose of 1 mg/kg bw per day had similar but
less severe effects. At 0.25 mg/kg bw per day, only minor changes were
seen, although central nervous system depression was seen in one dog
on one occasion 3 h after dosing during week 8 of treatment. The NOAEL
was 0.25 mg/kg bw per day on the basis of central nervous system
depression and reduced rectal temperature and pulse rate (Patton &
Williams, 1971).
Groups of four male and four female beagles were given 0, 0.1,
0.25, or 1 mg/kg bw per day amitraz (purity unspecified) in gelatine
capsules for two years. They were examined clinically before and
immediately after dosing on the first two days and again during weeks
4, 13, 26, 39, 52, 78, and 102. Heart rate and rectal temperature were
monitored serially for up to 24 h after dosing on day l and during
weeks 39, 52, 79, and 103. Signs of toxicity and faecal appearance
were recorded daily and body weights weekly. Haematological, blood
biochemical, and urinary parameters were measured before dosing and
during weeks 4, 13, 26, 52, 78, and 102. In addition, serial blood
samples were obtained during weeks 40 and 53 for estimating blood
sugar and during weeks 91 to 96 for estimating methaemoglobin. The
animals were sacrificed and examined macroscopically on the day after
the final dose, and their organs were weighed and prepared for
histopathological examination.
All eight animals given 1 mg/kg bw per day showed signs of slight
central nervous system depression 3 h after dosing on days 1 and 2,
but all appeared normal by the following morning. On both days, one
dog had a slightly subnormal temperature (38.3°C) at 3 h, which had
returned to normal (38.7°C) by 24 h. Subsequently, all of the animals
in this group appeared clinically normal, apart from one bitch that
was slightly hypothermic 3 h after dosing during weeks 52 and 79. The
NOAEL was 0.25 mg/kg bw per day on the basis of central nervous system
depression (Morgan et al., 1973).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CFLP mice were fed diets
containing 0, 25, 100, or 400 ppm amitraz (purity unspecified),
equivalent to 3, 8, 15, and 60 mg/kg bw per day (wrongly given as 25,
100, and 400 mg/kg bw per day in reference 34, Annex 1), for 80 weeks.
Animals at 100 or 400 ppm gained less weight than the controls over
the first 40 weeks, whereas those at 25 ppm gained more weight than
the controls from week 10 onwards and showed a 20% greater body-weight
gain at the end of the experiment. Calculated over 80 weeks, food
consumption was increased for male mice at 100 and 400 ppm and female
mice at 400 ppm, and decreased for males at 25 and 100 ppm. The
survival rate was similar in all groups. An increased incidence of
lymphoreticular tumours was observed in female mice at 400 ppm (49%
compared with 23% in controls). A slight, not statistically
significant increase in the incidence of all types of liver-cell
tumour was found in animals of each sex fed 400 ppm. No other
pathological finding related to treatment was reported. The NOAEL for
carcinogenicity was 100 ppm, equivalent to 15 mg/kg bw per day, on the
basis of an increased incidence of lymphoreticular tumours in females
at 400 ppm (Burnett et al, 1976; Kakuk, 1979).
Groups of 75 male and 75 female B6C3F1 hybrid mice, 33 days old,
were given diets containing 25, 100, or 400 ppm amitraz (purity,
97.9%), equal to 2.3, 9.6, and 45 mg/kg bw per day for males and 2.6,
11, and 50 mg/kg bw per day for females, for 104 weeks. The untreated
controls consisted of 100 male and 100 female mice. Clinical signs,
food consumption, and body weight were recorded throughout the study.
Survivors were killed at the end of the dosing period and all animals
were subjected to a complete gross autopsy. All tissues were collected
from all animals and preserved for future examination.
Histopathological examination was performed on all tissues from the
high-dose group and controls, and on the liver, pancreas, spleen,
lung, stomach, pituitary, thyroid, adrenals, ovaries, uterus, sternum,
and all abnormal tissues found grossly from animals at the low and
intermediate doses.
Males at 400 ppm were hyperactive and behaved aggressively. The
incidence of cutaneous ulceration and inflammation of the perigenital
and perianal areas was greater in mice at 100 and 400 ppm than in
those at 25 ppm or in controls, and the incidence of urogenital
swelling was greater in all treated mice than in controls. The
incidences of gross adverse effects in treated females were comparable
to the control incidences. There was clearly increased mortality of
males at 400 ppm (20/75) and of males and females combined (37/150).
Mean body-weight gain was reduced in mice at 400 ppm (by 30-50%)
throughout the study, with a significant decrease in females at 100
ppm over the last 74 weeks of the study. Food consumption was
marginally reduced initially in animals at 100 and 400 ppm but was
comparable to that of controls from week 25 to termination. Increased
liver and lymph node involvement was seen in males and females at 400
ppm, and the incidence of preputial gland enlargement in males at 400
ppm was greater than that in controls (20/75 vs 12/100). The
prominence of the limiting ridge of the stomach was greater than in
controls for females at all doses and for males at 100 and 400 ppm.
Decreased production of myeloid elements accompanied by an increase in
erythroid elements resulted in a significant decrease in the
myeloid:erythroid ratio for males at 400 ppm and females at 100 or
400 ppm. The incidences of spleen haematopoiesis in males and of
stomach focal hyperkeratosis in males and females at all three doses
were greater than that in controls. There was apparent liver
involvement in females, with an increased incidence of hepatocellular
carcinoma at 400 ppm (15/75 vs 2/100 hepatocarcinoma; 16/75 vs 4/100
hepatocellular adenoma). This was accompanied by a dose-related
increase in the incidence of hyperplastic nodules and telangiectatic
and basophilic foci of the liver in animals at 100 and 400 ppm. Males
at 400 ppm also had an apparent increase in the incidence of
hyperplastic nodules and telangiectatic and basophilic foci of the
liver. The NOAEL for carcinogenicity was 100 ppm, equal to 11 mg/kg bw
per day, on the basis of hepatocellular carcinoma in females at
400 ppm. This dose was considered to be greater than the conventional
maximum tolerated dose. The NOAEL for long-term toxicity was 25 ppm,
equal to 2.3 mg/kg bw per day, on the basis of general toxicity
(Colley et al., 1983).
Rats
Groups of 40 male and 40 female Ash-Wistar rats received amitraz
(purity, 97.8%) at a dietary concentration of 0, 15, 50, or 200 ppm
(equal to 0, 0.77, 2.5, or 10 mg/kg bw per day in males and 0, 0.97,
3.1, or 13 mg/kg bw per day in females) for two years. Body weights
were measured twice per week during the period of maximal growth and
later weekly. Food consumption was determined weekly during this
growth period and later monthly. Haemato-logical, biochemical, and
urinary analysis were performed during and at the end of the study,
and organs were weighed and examined grossly and histologically at the
end of study.
Rats at 200 ppm were occasionally nervous, excitable, and
aggressive, and their food consumption was temporarily reduced. The
overall body-weight gain of males was significantly reduced (10%).
Rats at 50 and 15 ppm showed no adverse reactions to treatment. The
incidence, type, and time to appearance of tumours were not
significantly different in treated and control groups. The NOAEL for
toxicity was 50 ppm, equal to 2.5 mg/kg bw per day, on the basis of
effects on the central nervous system and reduced overall body-weight
gain in males (Sutton & Offer, 1973).
(d) Genotoxicity
The results of tests for the genotoxicity of amitraz are
summarized in Table 2. The results of the tests in vitro and
in vivo were negative.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a three-generation study of reproductive toxicity, groups of
10 male and 20 female newly weaned Boots-Wistar rats were fed amitraz
(purity, 99.8%) at a dietary concentration of 0, 15, 50, or 200 ppm,
equal to 0, 1.3, 4.4, and 16 mg/kg bw per day in males and 0, 1.5,
5.1, and 20 mg/kg bw per day in females. After the F1 generation had
been weaned, 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. Amitraz at 200 ppm decreased the
growth, food consumption, fertility, and viability of offspring of the
F0 generation, and this dose was eliminated when the F1 generation
had been weaned, because of the very low survival. No effect was found
on the number of litters or mean litter size at 50 ppm; however, a
decreased number of young alive at 21 days was observed in all
generations. No further effects due to treatment were found in this or
the other group. The NOAEL was 50 ppm, equal to 4.4 mg/kg bw per day,
for maternal toxicity and 15 ppm, equal to 1.3 mg/kg bw per day, for
developmental toxicity (Sutton, 1973b).
(ii) Developmental toxicity
In a study of developmental toxicity, groups of 11-13 female
Boots-Wistar rats received amitraz (purity, 99.8%) at a dose of 0, 1,
3, or 12 mg/kg bw per day on days 8-20 of gestation. The rats were
killed on day 21, and the uterine contents were examined. There were
no deaths or clinical signs of toxicity. Food consumption, body-weight
gain, average litter size, fetal viability, and the implantation index
were not affected. At 12 mg/kg bw, fetal weight was lower than in the
controls, and the calcification of the sternebrae was less advanced.
The NOAEL for maternal toxicity was 12 mg/kg bw per day, and that for
developmental toxicity was 3 mg/kg bw per day, on the basis of reduced
fetal weight and reduced calcification of the sternebrae (Sutton,
1973c).
Table 2. Results of tests for the genotoxicity of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium 31-500 µg/plate 99.9 Negative Everest &
TA1535, TA1537, Wilcox (1976)
TA1558
Reverse mutation S. typhimurium 0, 33, 100, 333, 1000, 98.4 Negative McGregor &
TA98, TA100, TA1535, 3300, 10 000 µg/plate Printice (1983)
TA1537, TA1538
Chromosomal Human lymphocytes 0, 5, 10, 20 µg/ml -S9; 99.5 Negative Brooker et al.
aberration 0, 3, 5, 30 µg/ml +S9 (1988)
Cell mutation Mouse L5178Y tk+/- 0.06-33 µg/ml +S9; 98.4 Negative McGregor &
cells 0.06-20 µg/ml -S9 Riach (1983a)
Unscheduled Human embryonic 20, 60, 100, 140, 180, 100 Negative McGregor &
DNA synthesis fibroblasts 220, 260, 300 µg/ml ±S9 Riach (1983b)
DNA damage Chinese hamster 0.01-0.3 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Reverse mutation S. typhimurium 0, 100, 200, 400 mg/kg NR Negative Everest (1976);
(host-mediated, G46, TA1532 bw, single dose Wilcox (1976)
mouse) TA1964
Dominant lethal Female CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977a)
for 5 days
Table 2. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
Dominant lethal Male CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977b)
for 5 days
NR, not reported; S9, 9000 × g microsomal fraction from rodent liver
In another study of developmental toxicity, groups of 24 mated
Sprague-Dawley rats weighing 215-280 g were given 0, 7.5, 15, or 30
mg/kg bw per day amitraz (purity, 99.7%) by gavage on days 6-15 of
gestation. Immediately after mating, the females were assigned to
treatment groups by a randomization process based on stratified body
weight. Each female was then individually identified by ear notching.
All females were examined once or twice daily for clinical signs of
ill health, toxicity, or behavioural changes, and body weights and
food intake were recorded on days 0, 6, 10, 15, and 20 of gestation.
On day 20 of gestation, the females were killed and their uterine
contents examined.
One control female was found dead on day 10 of gestation; there
were no other deaths. The principal clinical sign was fur staining,
which was slightly more frequent at 30 mg/kg bw per day. At this dose,
a slight loss of body weight up to day 10 of gestation was followed by
reduced body-weight gain at termination. The body-weight gain of rats
at 15 mg/kg bw per day was slightly reduced (10%), but that of animals
at 7.5 mg/kg bw per day was not adversely affected by treatment. Food
intake of rats at 30 mg/kg bw per day, and to a lesser extent those at
15 mg/kg bw per day, was initially lower than in the control group;
there was no effect on food intake at 7.5 mg/kg bw per day. The
pregnancy rate was high in all groups, and no adverse findings were
made at necropsy. The treatment did not adversely affect the number of
implantations, the incidence of post-implantation loss, or the number
or sex ratio of fetuses. Animals at 30 mg/kg bw per day had a
statistically significantly increased incidence of dilated ureters and
increased bilateral renal pelvic cavitation. Although the incidence of
the latter lesion at 15 mg/kg bw per day was statistically
significantly higher than that of the concurrent controls
(p < 0.05), the group percentage increase (5.7%) was comparable to
the upper range of the relevant historical control values (5.4%). The
NOAEL was 7.5 mg/kg bw per day for both maternal toxicity, on the
basis of reduced body-weight gain, and for developmental toxicity, on
the basis of increased incidences of dilated ureters and renal pelvic
cavitation (McIntyre, 1987a).
Rabbits
In a study of developmental toxicity, groups of 8-10 New Zealand
rabbits were treated with amitraz (purity unspecified) at a dose of 0,
1, 5, or 25 mg/kg bw per day on days 6-18 of pregnancy and were killed
on day 30. At 25 mg/kg bw per day, the number of litters and mean
litter size were decreased and abortions were observed. No increase in
the incidence of congenital abnormalities was found. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, the highest dose tested,
and the NOAEL for developmental toxicity was 5 mg/kg bw per day on the
basis of a reduced number of litters and litter size (Sutton, 1973d).
In another study of developmental toxicity, groups of 16 mated
female New Zealand white rabbits weighing 3-4 kg were given amitraz
(purity, 99.7%) by gavage at a dose of 0, 3, 6, or 12 mg/kg bw per day
on days 7-19 of gestation. All females were examined once or twice
daily for clinical signs of ill health, toxicity, or behavioural
changes, and body weight and food intake were recorded on days 0, 7,
13, 19, 24, and 28 of gestation. On day 28 of gestation, the surviving
females were killed and their uterine contents examined.
One female at 12 mg/kg bw per day died, and three females at this
dose were killed because of poor clinical condition or abortion. Two
of the deaths were considered to be directly related to treatment. Two
females at 6 mg/kg bw per day died; at 3 mg/kg bw per day, one female
was killed and a further two died. Two control animals also died.
Langour, polypnoea, and squinting were observed in all treated groups,
and the incidence, severity, and duration of these signs appeared to
be dose-related. At 12 mg/kg bw per day, body-weight loss followed by
a reduction in body-weight gain were observed during dosing, but the
weight gain was similar to that of controls on cessation of dosing.
Treatment at 3 or 6 mg/kg bw per day had no effect on body weight.
Food intake was reduced during dosing and up to day 24 at 12 mg/kg bw
per day, but no adverse effect was seen at 6 or 3 mg/kg bw per day. No
adverse effects were seen at necropsy. Three of the surviving females
at 12 mg/kg bw per day lost their litters on day 28 of gestation.
Litter paraments were not affected at 6 or 3 mg/kg bw per day, as
assessed by the numbers of corpora lutea, implantation sites, and
viable fetuses. The mean litter and fetal weights and sex ratio of
fetuses were not adversely affected by treatment. No major fetal
defects were recorded that were considered to be related to treatment,
and there were no treatment-related effects on the incidence of minor
or variant anomalies. There was no NOAEL for maternal toxicity because
of deaths of animals at all doses and in the control group. The NOAEL
for developmental toxicity was 6 mg/kg bw per day, on the basis of
litter loss (McIntyre, 1987b).
(f) Special studies
(i) Effects on the thymus and hormone concentrations
Diets containing 400 ppm amitraz (purity, 97.1%), equal to 110
mg/kg bw per day for males and 150 mg/kg bw per day for females, were
given to 24 male CFLP mice for up to 18 weeks and to 52 female CFLP
mice for up to 33 weeks. A group of 36 males and 64 females given
plain diet served as controls. Body weight and food consumption were
measured regularly throughout the study, and the animals were examined
for overt signs of toxicity at least once a week. Vaginal smears were
monitored every morning during weeks 6-9, 15-18, 23-26, and 30-33.
Twelve male and 12 female controls were killed during the first week,
and 12 males and 12 females from both treated and control groups were
killed after 9 and 18 weeks. The remaining animals were killed after
33 weeks. The ß-estradiol concentration of the blood of female mice
was estimated at weeks 9 and 18, and the thymuses from all animals
were weighed at autopsy. Detailed histological examinations were
conducted on a selected range of tissues.
Body-weight gain was reduced in amitraz-treated mice, to a
slightly greater degre in the males, and food consumption was markedly
increased, particularly in the females. Examination of vaginal smears
indicated prolongation of estrus in treated mice, but there was no
measurable effect on the circulating concentrations of ß-estradiol.
The thymus weights and histological appearance were not affected by
treatment. Histopathological examination revealed two lymphoreticular
tumours in the treated females and two in controls. In comparison with
the controls, females given amitraz for 33 weeks had a higher
incidence of foci of inflammatory cells in the liver (Brown et al.
1978).
(ii) Effects on the estrus cycle and hormone concentrations
Mice
The effects of amitraz on the estrus cycle and hormone
concentrations were evaluated in groups of 70 female B6C3F1 mice fed
diets containing amitraz (purity, 98-100%) at 0, 25, 100, or 400 ppm,
equivalent to 0, 3.8, 15, and 60 mg/kg bw per day, for up to 28 weeks.
Permanently stained and mounted vaginal smears were prepared from each
animal daily for 30 days after 13 weeks of treatment, and the stage of
the oestrus cycle on each day was determined by microscopic
examination of the cell population on each slide. Blood samples were
taken at necropsy after overnight starvation and assayed for
dehydroepiandrosterone sulfate, estradiol, progesterone, testosterone,
lutropin, follitropin, prolactin, thyroxine, triiodothyronine, and
thyroid hormone uptake, as indicators of hormonal status and routine
clinical chemical parameters. The fresh liver weight was recorded at
necropsy.
Pro-estrus was prolonged, and a trend towards reduced duration of
diestrus was evident in animals at 400 ppm. The blood concentrations
of progesterone were lower and the concentrations of
dehydroepiandrosterone sulfate were higher in animals at 400 ppm and
to a lesser degree in those at 100 ppm when compared with controls.
The relative liver weights were increased at 400 (by 4%) and 100 ppm
(by 5%). There were no treatment-related effects at 25 ppm. The NOAEL
was 25 ppm, equivalent to 3.8 mg/kg bw per day, on the basis of lower
blood concentrations of progesterone, higher concentrations of
dehydroepiandrosterone sulfate, and increased relative liver weight
(Hounsell & Rush, 1984).
Rats
Groups of 20 female 22-week-old Boots-Wistar rats were fed diets
containing 0 or 200 ppm (equivalent to 0 and 12 mg/kg bw per day)
amitraz (purity unspecified) for 18 weeks. Vaginal smears were taken
routinely over 32 days. After fixation in methanol, the smears were
stained with 1% aqueous methylene blue and examined for keratinized
cells under 60 × magnification. Estrus was characterized by the
presence of keratinized cells only, and the cycle length was taken as
the interval between the first day of estrus in successive cycles.
The average cycle length of the controls was 4.3 days; two of the
controls had prolonged periods in estrus (four and six days), and a
third had a period of prolonged diestrus with a cycle lasting 18 days.
In the remaining animals, the shortest cycles were two days and the
longest six days. The low incidence of prolonged estrus among these
animals confirms that the technique of smearing did not affect vaginal
cytology. In the treated rats, the average cycle length was 6.1 days,
and the range was 2-16 days. Six rats had one or more periods of
prolonged diestrus; four of these had been acyclic in a preliminary
test. Estrus lasted for three to eight consecutive days in seven rats,
two of which had shown a similar tendency in a preliminary test. The
second test showed that the cycle length was significantly altered by
treatment, either estrus or diestrus being prolonged. Treated rats
thus had longer oestrus cycles than controls, resulting from prolonged
periods of estrus or diestrus (Merryman & Sutton, 1972).
(iii) Mechanism of action
The effects of amitraz and its major metabolite,
N-methyl- N'-(2,4-xylyl)formamidine, given orally or intravenously
on the cardiovascular system, pupil diameter, and the respiratory
system were studied in conscious and anaesthetized rats, cats, and
dogs. Both compounds caused a fall in blood pressure, sometimes
preceded by hypertension and bradycardia. The threshold for the effect
in conscious rats dosed orally with amitraz was 1 mg/kg bw. Amitraz
also caused mydriasis, sedation, and a reduced respiratory rate. The
bradycardia, sedation, and mydriatic effect was antagonized by the
alpha2-blocking agent yohimbine but not by the alpha1-blocking agent
prazosin, indicating that the effects were caused by stimulation of
presynaptic alpha2-adrenoceptors. The lethal effect of amitraz in mice
and dogs has also been shown to be inhibited by yohimbine (Parkinson &
Sim, 1970; Cullen & Reynoldson, 1983; Hsu & Kakuk, 1984; Moser &
MacPhail, 1985; Hsu et al., 1986; Hovda & McManus, 1993).
Amitraz and N-methyl- N'-(2,4-xylyl)formamidine were tested
for their ability to potentiate the pressor responses to tyramine in a
pithed rat preparation, since a structurally related compound,
chlordimeform, inhibited monoamine oxidase in vitro in rat liver
homogenates and centrally in vivo through an effect on brain
serotonin concentrations. These effects were obvious only with nearly
toxic oral doses of 80 mg/kg bw amitraz and 40 mg/kg bw
N-methyl- N'-(2,4-xylyl)formamidine (Parkinson, 1974).
Amitraz and chlordimeform were tested for their ability to
inhibit prostaglandin synthesis after intraperitoneal administration
in rats. Both compounds had antipyretic and antiinflam-matory effects
at doses of 5-80 mg/kg bw. They reduced yeast-induced fever, with
potencies intermediate between those of indomethacin and aspirin, and
antagonized carrageenan-induced swelling of the hind paw. They also
inhibited the synthesis of prostaglandin E2 from arachidonic acid by
bovine seminal vesicle microsomes. The potency of amitraz in this
assay was the same as that of aspirin (Yim et al., 1978).
(g) Studies on metabolites
(i) Acute toxicity
The acute toxicity of metabolites of amitraz (purity unspecified)
has been investigated in several species (Table 3).
Table 3. Acute toxicity of metabolites of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
N-Methyl-N'-(2,4-xylyl)formamidine
Mouse Oral 150 Sutton (1970a)
Rat Oral 200 Sutton (1970b)
Dog Oral >20 Morgan (1973);
Morgan & Williams (1974)
4-Amino-meta-toluic acid
Mouse Oral >1600 Shaw & Williams (1973a)
Rat Oral >1600 Shaw & Williams (1973b)
Form-2',4'-xylidide
Rat Oral 1600 Shaw (1973b)
(ii) Short-term toxicity
Rats
Groups of 10 male and 10 female newly-weaned Boots-Wistar rats
were dosed by gastric intubation with
N-methyl- N'-(2,4-xylyl)formamidine (purity unspecified) for 90
days at 0, 0.25, 1, 3, or 12 mg/kg bw per day. Body weights were
recorded three times per week, and the rats were observed each day for
signs of toxicity. At 3 mg/kg bw per day, an initial reduction in
body-weight gain was seen in males. At 12 mg/kg bw per day, the rats
became nervous and difficult to handle, and two deaths occurred. The
growth rate was reduced in animals of each sex but to a greater extent
in the males. At the end of experiment, haemoglobin and haematocrit
values were decreased in males, and the number of erythrocytes was
decreased in females. Slight biochemical changes were seen. At
autopsy, the weights of the adrenals, ovaries, uterus, and liver in
females and spleen and testes in males were increased, but the only
histopathological changes were a slight increase in lymphoid
infiltration, some sinusoidal leukocytosis, and loss of glycogen in
the livers of most males and slight cellular accumulations in the
hearts of some females. The NOAEL was 1 mg/kg bw per day on the basis
of the reduced body-weight gain and increased organ weights (Shaw &
Williams, 1975).
Groups of five male and five female Wistar rats were dosed by
gastric intubation with 4-amino- meta-toluic acid (purity
unspecified) at a dose of 0, 40, 100, or 250 mg/kg bw per day for 21
days. At 250 mg/kg bw per day, slight decreases in weight gain and in
blood urea nitrogen concentration were observed in males and an
increased relative weight of the spleen in females. No gross
pathological changes due to treatment were found. The NOAEL was
250 mg/kg bw per day, the highest dose tested (Shaw, 1975).
Dogs
Groups of four male and four female beagles were given gelatin
capsules containing N-methyl- N'-(2,4-xylyl)formamidine (purity
unspecified) as free base diluted in lactose to 1% at a dose of 0,
0.1, 0.25, or 1 mg/kg bw per day for 90 days. Clinical signs were
recorded daily, food consumption twice daily, and body weight once a
week. Ophthalmoscopic examination and recordings of temperature and
heart rate were carried out before dosing and after 6 and 12 weeks.
Haematological, clinical chemical, and urinary analyses were perfomed,
and gross and histopathological examinations were carried out.
At 0.25 and 1 mg/kg bw per day, abnormal quietness and drowsiness
and significantly lower body temperature (by up to 16.2°C) were
observed 0.5-4 h after dosing. At 1 mg/kg bw per day, the heart rate
was significantly reduced (by up to 40 beats/min) 1-2 h after dosing,
and the liver weight and urine volume were increased. A slight
reduction in thymus weight was observed at 0.25 and 1 mg/kg bw per
day. No histopathological anomalies were found. The NOAEL was 0.1
mg/kg bw per day on the basis of central nervous system depression and
lowered body temperature (Chesterman et al., 1973).
Groups of four male and four female beagles were given gelatin
capsules containing 4-amino- meta-toluic acid (purity unspecified) at
a dose of 0, 16, 40, or 100 mg/kg bw per day for 90 days. Slightly
increased urinary concentrations of total reducing substances other
than glucose were found at the highest dose. No dose-related effects
were observed on behaviour, electrocardiogram, heart rate, rectal
temperature, body weight, food consumption, haematological or blood
chemical parameters, organ weights, or histopathological appearance
(Morgan et al., 1974).
(iii) Genotoxicity
The results of tests for the genotoxicity of potential
metabolites of amitraz are summarized in Table 4. Except for a single
positive response to 2.4-dimethylaniline in the assay for forward
mutation in mouse lymphoma cells, in the presence of metabolic
activation, the results of the tests in vitro and in vivo were
negative.
3. Observations in humans
In a double-blind, randomized cross-over study, six healthy male
volunteers aged 18-45 years and weighing 60-70 kg received sequential
single oral doses of 0, 0.063, and 0.13 mg/kg bw amitraz, two to three
weeks apart. Each dose was given with 150 ml water, 30 min after
breakfast. Pulse rate, respiration rate, blood pressure, and
temperature were measured at - 1, - 0.5, 1, 3, 6, 12, 24, and 36 h,
and electrocardiograms were performed at -1, 1, 3, 6, 12, 24, and 36 h
with respect to dosing. Pupil responsiveness and psychomotor
performance were evaluated before treatment and at 2.5 and 8 h. Urine
was collected at 0-36 h and 36-60 h. There were no clinically
significant changes in vital signs or electrocardiographic parameters.
Moreover, haematological, blood chemical, and urinary parameters,
pupil responsiveness, and psychomotor performance were unaffected by
treatment. The NOAEL was 0.13 mg/kg bw, the highest dose tested (Cass,
1992).
Two human volunteers who received a single oral dose of 0.25
mg/kg bw 14C-amitraz showed drowsiness, disorientation, slurred
speech, decreased pulse rate and blood pressure, and other effects
(Campbell & Needham, 1984c).
In a double-blind cross-over study, four male and two female
volunteers, aged 21-42 years and in normal health, received two doses
of 2 mg (about 0.03 mg/kg bw) of the amitraz metabolite,
N-(2,4-dimethylphenyl)- N'-methylformamidine, one week apart in a
capsule with 100 ml of water, or placebo. Blood pressure, pulse rate,
and temperature were measured at half-hourly intervals over 7 h, and
mental alertness was assessed at 0, 3, and 7 h. An electrocardiograph
was performed, and urine was collected before dosing and at 0-7 h and
7-24 h. The urine samples were analysed to estimate the amount of the
metabolite, 3-methyl-4-aminobenzoic acid, that had been excreted.
Mental alertness was estimated at 0, 3, and 7 h. No difference was
seen from those receiving the placebo. The NOAEL was 0.03 mg/kg bw,
the only dose tested (Hall et al., 1975).
Symptoms of central nervous system depression ranging from
sedation to coma lasting more than 24 h, depressed respiration,
hypotension, and bradycardia have been described in 11 reports after
accidental or intentional ingestion of uncertain amounts of amitraz.
In nine cases, recovery was complete after symptomatic and supportive
treatment (Groppi, 1977; Ros & Aken, 1994; Pronczuk et al., 1995).
Table 4. Results of tests for the genotoxicity of metabolites of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
N-Methyl-N'-(2,4-xylyl)formamidine
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983a)
TA1337, TA1538
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Form-2',4'-xylidide
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983b)
TA1337, TA1538
DNA damage Chinese hamster 0.01-1.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Forward mutation Mouse L5178Y tk+/- 1, 3.3, 10, 33, 100, 200, NR Positive + S9 McGregor &
lymphoma cells 300, 333, 400, 500, Negative -S9 Riach (1984)
600 µg/ml
Cell transformation C3H/10 T1/2 clone 8 5, 10, 20 µg/ml +S9 NR Negative McGregor et al.
mouse embryo fibroblasts 100, 200, 400 µg/ml -S9 (1984)
DNA damage Chinese hamster 0.03-2.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
4-Amino-meta-toluic acid
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Micronucleus Mouse bone 56, 113, 225 mg/kg bw NR Negative Hounsell &
formation marrow twice, 24 h apart Walker (1983)
Comments
Amitraz was well absorbed, extensively metabolized, and rapidly
excreted, mainly in the urine, after oral administration to mice,
rats, dogs, pigs, hens, cows, baboons, and humans. After oral
treatment of mice with 14C-amitraz, 86% of the radiolabelled dose was
excreted, 62% in the urine, within the first 24 h. All of it had been
excreted by 96 h, with 73% in the urine of animals of each sex. The
concentrations of residues were highest in liver, adrenal glands, and
eyes. After oral administration of 14C-amitraz to rats, 94% of the
dose was recovered within three days, with 82% in urine and 12% in
faeces. After oral administration of 14C-amitraz to two humans,
77-87% was recovered within three days. Amitraz is hydrolysed to two
components, N-methyl- N-(2,4-xylyl)formamidine and
form-2',4'-xylidide. The former is the pharmacologically active
compound and accounted for 5-30% of the total urinary excretion in
mice and rats; it was further metabolized to 4-amino- meta-toluic
acid and the acetyl and formyl conjugates, 4-acetamido- and 4-
formamido- meta-toluic acids. These five metabolites were also found
in plants.
Amitraz has low acute oral toxicity in rats but is more toxic in
dogs. The LD50 values ranged from 100 mg/kg bw in dogs to > 1600
mg/kg bw in mice, indicating that dogs are more sensitive. The toxic
signs after oral administration to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Amitraz had no
sensitizing potential in guinea-pigs, and no local irritation was
found in rabbits after a single application to skin or eyes. There was
evidence of delayed contact hypersensitivity after application of
amitraz either topically or intradermally.
WHO (1996) has classified amitraz as slightly hazardous.
In a 13-week study in which mice were fed diets providing 0, 100,
200, 400, 600, or 800 ppm, the NOAEL was 100 ppm, equal to 17 mg/kg bw
per day, on the basis of reduced overall body-weight gain (by 34%).
In a 90-day study, rats were given amitraz at doses of 0, 3, or
12 mg/kg bw per day by gavage. The NOAEL was 3 mg/kg bw per day, on
the basis of reduced terminal body-weight gain, absolute liver weight,
and relative liver weight.
In a 90-day study in dogs, amitraz was administered at doses of
0, 0.25, 1, or 4 mg/kg per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression
and reductions in rectal temperature and pulse rate.
In a two-year study, dogs were given amitraz at doses of 0, 0.1,
0.25, or 1 mg/kg bw per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression.
In two 90-day studies, the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine was administered to rats at
doses of 0, 0.25, 1, 3, or 12 mg/kg bw per day by gastric intubation
or to dogs at 0, 0.1, 0.25, or 1 mg/kg bw per day by gelatine
capsules. The NOAEL was 1 mg/kg bw per day in rats, on the basis of
reduced body-weight gain and increased organ weights, and 0.1 mg/kg bw
per day in dogs, on the basis of central nervous system depression and
lowered body temperature.
In a 21-day study in rats and a 90-day study in dogs, the amitraz
metabolite 4-amino- meta-toluic acid was given at doses of 0, 40,
100, or 250 mg/kg bw per day by gastric intubation to rats or 0, 16,
40, or 100 mg/kg bw per day by gelatin capsules to dogs. The NOAEL was
250 mg/kg bw per day in rats and 100 mg/kg bw per day in dogs, (the
highest doses tested).
In an 80-week study, mice were fed diets containing 0, 25, 100,
or 400 ppm, equivalent to 0, 3.8, 15, and 60 mg/kg bw per day (wrongly
given as 25, 100, or 400 mg/kg bw per day in the 1980 JMPR report).
The NOAEL for carcinogenicity was 100 ppm, equivalent to
15 mg/kg bw per day, on the basis of an increased incidence of
lymphoreticular tumours in females at 400 ppm.
In a two-year study in which mice were fed diets providing 0, 25,
100, or 400 ppm, the NOAEL for carcinogenicity was 100 ppm, equal to
11 mg/kg bw per day, on the basis of the occurrence of hepatocellular
carcinoma in females at 400 ppm. This dose was considered to be
greater than the conventional maximum tolerated dose. The NOAEL for
toxicity was 25 ppm, equal to 2.3 mg/kg bw per day, on the basis of
generalized toxic effects.
In a two-year study, rats were fed diets containing 0, 15, 50, or
200 ppm. The NOAEL for toxicity was 50 ppm, equal to 2.5 mg/kg bw per
day, on the basis of effects on the central nervous system and reduced
overall body-weight gain in males. There was no evidence of
carcinogenicity.
The genotoxic potential of amitraz has been adequately evaluated
in a range of assays in vitro and in vivo. The Meeting concluded
that amitraz is not genotoxic.
In view of the lack of genotoxicity and the finding of tumours
only in mice and only at concentrations at which severe toxicity was
observed, the Meeting concluded that amitraz is not likely to pose a
carcinogenic risk to humans.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 15, 50, or 200 ppm, the NOAEL was 50 ppm,
equal to 4.4 mg/kg bw per day, for maternal toxicity and 15 ppm, equal
to 1.3 mg/kg bw per day for developmental toxicity. No teratogenic
effect was observed.
In two studies of developmental toxicity, pregnant rats were
given amitraz at 0, 1, 3, or 12 mg/kg bw per day by gavage on days
8-20 of gestation or 0, 7.5, 15 or 30 mg/kg bw per day by gavage on
days 6-15. The NOAEL for maternal toxicity was 7.5-12 mg/kg bw per
day, and that for developmental toxicity was 3-7.5 mg/kg bw per day.
In two studies of developmental toxicity in rabbits, amitraz was
given at doses of 0, 1, 5, or 25 mg/kg bw per day by gavage on days
6-18 of gestation or 0, 3, 6, or 12 mg/kg bw per day by gavage on days
7-19. The NOAEL for maternal toxicity was 25 mg/kg bw per day in one
study, but a NOAEL was not identified in the other because all of the
treated animals died. The NOAEL for developmental toxicity was 3-6
mg/kg bw per day.
The effect of amitraz on the estrus cycle and hormone levels was
evaluated in mice fed diets containing 0, 25, 100, or 400 ppm for 28
weeks and in rats fed diets containing 0 or 200 ppm for 18 weeks. In
mice, pro-estrus was prolonged at 400 ppm; blood levels of
progesterone were reduced and those of dehydroepiandrosterone were
increased at 100 and 400 ppm. The NOAEL was 25 ppm, equivalent to
3.8 mg/kg bw per day, on the basis of the changed hormone levels. The
estrus cycles were longer in treated than in control rats, with no
NOAEL.
In a double-blind, randomized, cross-over study of tolerance, six
healthy adult male volunteers received sequential single oral doses of
0, 0.063, and 0.13 mg/kg bw amitraz, two to three weeks apart. The
NOAEL was 0.13 mg/kg bw, the highest dose tested.
Two human volunteers who received single oral doses of 0.25 mg/kg
bw 14C-amitraz showed effects including drowsiness, disorientation,
slurred speech, and decreased pulse rate and blood pressure.
In a double-blind cross-over study of tolerance, six adult
volunteers received two single doses of placebo or 2 mg (about 0.03
mg/kg bw) of the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine one week apart. The NOAEL was
0.03 mg/kg bw, the only dose tested.
The Meeting established an ADI of 0-0.01 mg/kg bw on the basis of
the NOAEL of 1.3 mg/kg bw per day in the study of reproductive
toxicity in rats and a safety factor of 100. The pharmacological
effects on the central nervous system seen in dogs, with a NOAEL of
0.25 mg/kg bw per day, were considered not to be relevant for setting
the ADI because they were reversible and the dogs became tolerant.
Moreover, a NOAEL of 0.13 mg/kg bw per day was seen for such effects
in humans.
The Meeting established an acute RfD of 0.01 mg/kg bw, on the
basis of the NOAEL of 0.13 mg/kg bw per day in the study in humans and
a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 2.3 mg/kg bw per day (toxicity in a
two-year study of carcinogenicity)
Rat: 3 mg/kg bw per day (toxicity in a 90-day study of
toxicity)
50 ppm, equal to 2.5 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
50 ppm, equal to 4.4 mg/kg bw per day (maternal
toxicity in a three-generation study of reproductive
toxicity)
15 ppm, equal to 1.3 mg/kg bw per day (developmental
toxicity in a three-generation study of reproductive
toxicity)
12 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
5 mg/kg bw per day (developmental toxicity)
Dog: 0.25 mg/kg bw per day (toxicity in a two-year study of
toxicity)
Human: 0.13 mg/kg bw (toxicity after single oral doses)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies to further characterize the effects on the
reproductive system of female rodents
2. Further observations in humans
List of end-points relevant for setting guidance values for dietary
and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete
Distribution Liver, adrenals, eyes
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 80-100% in 96 h
Metabolism in animals Metabolites same in rodents, dogs,
humans
Toxicologically significant
compounds (animals, plants, N-Methyl-N'-(2,4-xylyl)formamidine
and environment)
Acute toxicity
Rat: LD50 oral 600 mg/kg bw
Rabbit: LD50 dermal > 200 mg/kg bw
Rat: LC50 inhalation 65 mg/L
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing (Buehler test)
Short-term toxicity
Target/critical effect Central nervous system depression, dog
Lowest relevant oral NOAEL 0.25 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 50 mg/kg bw per day
Lowest relevant inhalation Rat: 0.01 mg/L air
NOAEL
Genotoxicity Unlikely to be genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Lymphoreticular tumours, hepatocellular
carcinomas
Lowest relevant NOAEL Mouse: 11 mg/kg bw per day (80-week and
2-year studies)
Carcinogenicity Unlikely to be carcinogenic
Reproductive toxicity
Reproduction target/critical Decrease in number of young alive
effect at 21 days
Lowest relevant reproductive Rat: 1.3 mg/kg bw per day
NOAEL (developmental toxicity)
Developmental target/critical Reduced fetal weight
effect
Lowest relevant developmental Rat: 3 mg/kg bw per day
NOAEL (developmental toxicity)
Neurotoxicity/Delayed Acute central nervous system
neurotoxicity depression
Other toxicological studies Prolongation of estrus cycles and
reduction of blood concentrationof
progesterone (mouse, rat)
Medical data Central nervous system depression in
two volunteers after a single oral dose
of 0.25 mg/kg bw
Summary Value Study Safety
factor
ADI 0-0.01 mg/kg bw Reproductive toxicity, 100
rat
Acute reference 0.01 mg/kg bw Single oral dose in six
dose volunteers 10
References
Berczy, Z.S., Binns, R. & Newman, A.J. (1972) Acute inhalation
toxicity to the rat of BTS 27419. Unpublished report No. 4971/72/406
from Huntingdon Research Centre Ltd, Huntingdon, Cambridge-shire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. & Whitfield,
R.A.S. (1973) Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report No. 5454/72/850 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brown, D.J., Burnett, R., Crowley, J., Cryer, P.C., Lancaster, M.C. &
Turnbull, G.J. (1978) Amitraz: Investigation of effects on the thymus
gland and oestrous cycle in mice. Unpublished report No. TX 78037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brooker, P.C., Akhurst, L.C. & Gray, V.M. (1988) Technical amitraz,
metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. SMS 127/881149 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Burnett, R., Crowley, J., Lessel, B., Patton, D.S.G., Sutton, M.M. &
Turnbull, G.J. (1976) BTS 27419: 80-week carcinogenicity study in
mice--final report. Unpublished report No. TX 76039 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1981) Excretion and residues of
14C-amitraz in male and female rats given a single oral dose of 10
mg/kg. Unpublished report No. METAB/81/20 from FBC Ltd, Chesterford
Park Research Station, Saffron Walden, Essex, United Kingdom.
Submitted to WHO by Schering Agro-chemicals Ltd.
Campbell, J.K. & Needham, D. (1983) Excretion and tissue residues of
14C amitraz in male and female mice given a single oral dose of 10 mg
14C-amitraz/kg bodyweight. Unpublished report No. METAB/83/34 from
FBC Ltd, Chesterford Park Research Station, Saffron Walden, Essex,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984a) Dermal absorption of 14C-amitraz
in EC formulation by male and female pigs given a single topical
application of 18 mg A.I. Unpublished report No. METAB/84/9 from FBC
Ltd, Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. Needham, D. (1984b) Excretion and tissue residues of
14C-amitraz in a male and a female baboon given a single oral dose of
10 mg 14C-amitraz per kg bodyweight. Unpublished report No.
METAB/83/44 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984c) Urinary excretion of 14C-amitraz
by two male humans following a single oral dose of 0.25 mg/kg
bodyweight. Unpublished report No. METAB/84/10 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984d) The metabolism of 14C-amitraz by
male and female rats. Unpublished report No. METAB/84/2 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984e) A comparison of the metabolism of
14C-amitraz in rat, mouse, baboon and human. Unpublished report No.
METAB/84/1 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Cass, L.M.R. (1992) Report of a double blind tolerance study of
amitraz in six adult healthy volunteers. Unpublished report No.
TOX/92/179-209 from Simbec Research Ltd, Merthyr Tydfil,
Mid-Glamorgan, Wales. Submitted to WHO by Schering Agrochemicals Ltd.
Challis I.R. (1990) Dermal absorption of amitraz in the rat.
Unpublished report No. TOX/90/179-178 from Schering Agrochemicals Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Chesterman, H., Skerrett, K., Street, A.E. & Cherry, C.P. (1973) Boots
27 271, oral toxicity study in beagle dogs (repeated dosage for 13
weeks). Unpublished report No. BTS 38/73511 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO
by Schering Agrochemicals Ltd.
Clouzeau, J. (1992) Technical amitraz: Guinea pig sensitization study
(Buehler test). Unpublished report No. TOX 91/179-203 from Centre
International de Toxicologie, Miserey, Evreux, France. Submitted to
WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Gibson, W.A. & Prentice, D.E.
(1981) Amitraz toxicity to B6C3F1 mice by dietary administration for
13 weeks. Unpublished report No. BTS 158/80759 from Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Street, A.E., Gibson W.A. &
Gopinath, C. (1983) Amitraz, 104 week tumorigenicity study in mice.
Unpublished report No. BTS 153/8262/A from Huntingdon Research Centre
plc, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agro-chemicals Ltd.
Cullen, L.K. & Reynoldson, J.K. (1983) Effects of amitraz on the
cardiovascular and respiratory systems in the dog. Unpublished report
No. T242 from School of Veterinary Studies, Murdoch University,
Murdoch, Western Australia. Submitted to WHO by Schering Agrochemicals
Ltd.
Everest, R.P. (1976) BTS 27 419: Mutagenicity study in the male mouse
perivisceral cavity host-mediated assay. Unpublished report No. TX
76056 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Everest, R.P. & Wilcox, P. (1976) BTS 27 419: Mutagenicity testing
against Salmonella typhimurium strains TA1535, TA1537 and TA1538 in
the presence and absence of liver microsomes from male and female
mice. Unpublished report No. TX 76057 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Groppi, L. (1977) Medical report on a patient who ingested Mitac
(Edrizar) in Italy in 1977. Report No. T84 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Hall, J.E., Ahluwalia, H. & Hampson, P. (1975) A study of the effects
of oral administration of a metabolite (BTS 27 271) of amitraz to
volunteers. Unpublished report No. MS 75002 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hornish R.E. & Nappier, J.M. (1983) The absorption, metabolism, and
excretion of Mitaban(R) (U-36,059) in the dog from oral and dermal
exposure. Unpublished report No. 527-9760-83-001 from Agricultural
Research and Development Laboratories, The Upjohn Company, Kalamazoo,
Michigan, USA. Submitted to WHO by Schering Agrochemicals Ltd.
Hounsell, I.A.G & Walker, A.K. (1983) A micronucleus study in mice
using BTS 24868 (2,4-dimethylaniline). Unpublished report No.
TOX/84/179-97 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hounsell, I.A. & Rush, K.C. (1984) Technical amitraz: The effect of
dietary administration on the oestrus cycle and hormones in the mouse.
Unpublished report No. TOX/84/179-97 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Hovda, L.R. & McManus, A.C. (1993) Yohimbine for treatment of amitraz
poisoning in dogs. Vet. Hum. Toxicol., 35, 329.
Hsu, W.H. & Kakuk, T.J. (1984) Effect of amitraz on heart rate and
pupil diameter in rats: Mediated by alpha2-adrenoceptors. Toxicol.
Appl. Pharmacol., 73, 411-415.
Hsu, W.H., Lu, Z.-X. & Hembrough, F.B. (1986) Effect of amitraz on
heart rate and aortic blood pressure in conscious dogs: Influence of
atropine, prazosin, tolazoline, and yohimbine. Toxicol. Appl.
Pharmacol., 84, 418-422.
Kakuk, T.J. (1979) Pathological findings on the matched control and
amitraz 400 ppm females from the Boots 80 week mouse study by various
pathologists with commentary. Unpublished report No. 315-78-9610-005
from Agricultural Research and Development Laboratories, The Upjohn
Company, Kalamazoo, Michigan, USA. Submitted to WHO by Schering
Agrochemicals Ltd.
Kynoch, S.R. & Parcell, B.I. (1988) Technical amitraz: Assessment of
delayed contact hypersensitivity in the guinea-pig. Unpublished report
No. TOX 88/179-145 from Huntingdon Research Centre Ltd, Huntingdon,
Cambridgeshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Lancaster, M.C. & Williams, G.A.H. (1978) Amitraz: Multigeneration
feeding test in rats -- Histopathology. Unpublished report No. TX
78064 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Lewis, D.K. (1971) Fate of [14C]-BTS 27419 applied to rats as a
single oral dose. Unpublished report No. C 71011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987a) Technical amitraz: Irritant
effects on rabbit skin. Unpublished report No. TOX/87/179-137 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987b) Technical amitraz: Irritant
effects on the rabbit eye. Unpublished report No. TOX/87/179-142 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Printice, R.D. (1983) Technical amitraz: Ames
bacterial mutagenicity test. Unpublished report No. 2590 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983a) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. 2669 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by Schering
Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983b) Technical amitraz: Unscheduled
DNA synthesis in human embryonic cells. Unpublished report No. 2634
from Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1984) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. TOX/84/179-97 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B., Brown, A.G. & Riach, G.G. (1984) Technical BTS 24868:
Induction of cell transformation in C3H10T1/2 cells. Unpublished
report No. TOX/84/179-97 from FBC Ltd, Chesterford Park Research
Station, Saffron Walden, Essex, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
McIntyre, M. (1987a) Technical amitraz: Teratogenicity study in the
rat. Unpublished report No. 5562-194/11 from Hazleton UK, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
McIntyre, M. (1987b) Technical amitraz: Teratogenicity study in the
rabbit. Unpublished report No. 5536-194/13 from Hazleton UK,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Merryman, D.C. & Sutton, M.M. (1972) Effects on the oestrus cycle of
the rat. Unpublished report No. PM 72003 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. (1973) BTS 27 271: Acute oral toxicity study in dogs.
Unpublished report No. TX 73004 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. & Williams, G.A.H. (1974) BTS 27 271: Acute oral toxicity
study in dogs--Histopathology. Unpublished report No. TX 73 030 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Morgan, H.E., Patton, D.S.G. & Turnbull, G.J. (1973) BTS 27 419:
Two-year oral toxicity study in dogs. Unpublished report No. TX 73035
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Morgan, H.E., Shepherd, G.M. & Turnbull, G.J. (1974) BTS 28 369:
90-day oral toxicity study in dogs. Unpublished report No. TX 74037
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Moser, V.C. & MacPhail, R.C. (1985) Yohimbine attenuates the delayed
lethality induced in mice by amitraz, a formamidine pesticide.
Toxicol. Lett., 28, 99-104.
Needham, D. (1984) The effect of Amitraz on the hepatic mixed-function
oxidase system of male and female mice following oral administration.
Unpublished report No. METAB/84/8 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Needham, D. & Hemmings, P.A. (1988) The metabolism and distribution of
amitraz residues in the laying hen following the daily oral
administration of 24.5 mg 14C-amitraz/per bird. Unpublished report
No. Envir/88/6 from Schering Agrochemicals Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977a) Dominant lethal assay of amitraz in
the female mouse. Unpublished report No. BTS 81/7758 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977b) Dominant lethal assay of amitraz in
the male mouse. Unpublished report No. BTS 80/7792 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Parkinson, R. (1974) The effects of BTS 27 271, BTS 27 419 and BTS 21
103 (chlordimeform) on the pressor response to tyramine in the pithed
rat. Unpublished report No. P74 032 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Parkinson, R. & Sim, M.F. (1970) Some pharmacological effects of the
acaricide RD 27,271 and related compounds. Unpublished report No.
P70508 from The Boots Pure Drug Company, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Patton, D.S.G. (1973) BTS 27419: Acute toxicity in baboons.
Unpublished report No. TX 73002 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Patton, D.S.G & Sutton, M.M. (1971) Acute toxicity studies on BTS 27
419, an acaricide. Unpublished report No. P71544 from The Boots Pure
Drug Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Patton, D.S.G. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in dogs. Unpublished report No. P71547 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Petzold, G.L., Swenberg, J.A. & Bedell, M. (1977) Evaluation of
amitraz (U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915A
and U-54,914) in the DNA damage/alkaline elution assay. Unpublished
report No. 7268/77/7268/001 from The Boots Company Ltd, Nottingham,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Phillips, M.W.A., Swalwell, L.M. & Needham, D. (1987) Identification
of metabolites of amitraz in the milk and meat of a cow dosed for 4
days with amitraz. Unpublished report No. Envir/87/46 from Schering
Agrochemicals Ltd, Chesterford Park Research Station, Saffron Walden,
Essex, United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Pronczuk, J., Heuhs, L., Scaiola, G., Bogdan, M. & Fogel de Korc, E.
(1995) Clinical cholinergic presentation of acute amitraz poisoning.
Report No. T368 from Department of Toxicology, Hospital de Clinicas,
Montevideo, Uruguay. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983a) Technical BTS 27271,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61A/83580
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983b) Technical BTS 27919,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61B/83581
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Ros, J.J.W. & van Aken, J. (1994) A case of poising with amitraz, an
agricultural pesticide. Ned. Tijdschr. Geneeskd., 138, 776-778.
Shaw, J.W. (1971) BTS 27 419 -- Acute intraperitoneal toxicity to
rats. Unpublished report No. PM 71057 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. (1973a) BTS 27 419: Comparison of the acute oral and
intraperitoneal toxicities to rats. Unpublished report No. TXM 73006
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1973b) BTS 27 419: Comparison of the acute oral toxicities
to rats of BTS 27 419 and BTS 27 919. Unpublished report No. TXM 73010
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1975) BTS 27 419 metabolite: 21 Day chronic oral toxicity
in rats of BTS 28 369. Unpublished report No. TX 75058 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973a) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to mice. Unpublished report No. TXM 73 036 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973b) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to rats. Unpublished report No. TXM 73 037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, G.A.H. (1975) BTS 27 419 metabolite: 90 day
chronic oral toxicity in rats of BTS 27 271. Unpublished report No. TX
75059 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Somerville, L. (1973) Fate of 14C-BTS 27419 administered to rats in
repeated oral doses. Unpublished report No. AX 73011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Stewart, F.P. (1993) (14C)-Amitraz: Dermal absorption in the rat.
Unpublished report No. 194/69-1011 from Hazleton Europe, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. (1970a) RD 27,271. Acute oral toxicity to mice.
Unpublished report No. PM 70043 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agro-
chemicals Ltd.
Sutton, M.M. (1970b) RD 27,271 Acute toxicity to rats. Unpublished
report No. PM 70042 from The Boots Pure Drug Company, Nottingham,
United Kingdom. Submitted to WHO by Schering Agro-chemicals Ltd.
Sutton, M.M. (1971) BTS 27 419. Contact sensitisation in the guinea
pig. Unpublished report No. PM 71919 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973a) BTS 27 419: Three week dermal toxicity to
rabbits. Unpublished report No. TX 73 026 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973b) BTS 27 419: Multigeneration feeding test in rats.
Unpublished report No. TX 73 036 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973c) BTS 27 419: Teratogenicity in the rat.
Unpublished report No. TX 73028 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973d) BTS 27 419: Teratogenicity in the rabbit.
Unpublished report No. TX 73029 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. & Offer, J. (1973) BTS 27 419: Carcinogenicity and
long-term toxicity study in rats. Unpublished report No. TX 73043 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Sutton, M.M. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in rats. Unpublished report No. P71548 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. & Williams, P.A. (1972) BTS 27 419: Acute dermal toxicity
to rabbits. Unpublished report No. YM 72 011 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Wilcox, P. (1976) BTS 27 419: Mutagenicity study in the
intraperitoneal host-mediated assay. Unpublished report No. TX 76028
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yim, G.K.W., Holsapple, M.P., Pfister, W.R. & Hollingworth, R.M.
(1978) Prostaglandin synthesis inhibited by formamidine pesticides.
Life Sci., 23, 2509-2516.
In the study of Challis (1990), described above, the metabolic
profile of rats treated dermally was similar to that of rats treated
orally.
In the study of Hornish & Nappier (1983), described above, the
metabolism of amitraz was essentially the same after oral and dermal
administration. 4-Formamido- meta-toluic acid was the predominant
residue in both blood and urine. The parent compound and the first
hydrolysis products, N-methyl- N'-(2,4-xylyl)formamidine and
form-2',4'-xylidide, were not observed at measurable concentrations in
either blood or urine.
One cow received 0.5 mg/kg bw per day 14C-amitraz (specific
activity, 4.2 mCi/g; radiochemical purity, > 98.8%) by capsule twice
daily for four days. All milk was collected, and samples of urine were
taken. The cow was killed 17 h after the final dose. The urinary
metabolites corresponded to 4-acetamido- meta-toluic acid and
4-formamido- meta-toluic acid, which were converted to
4-amino- meta-toluic acid by acid hydrolysis. The total residue in
milk accounted for only 0.2% of the dose. The greatest concentration
of residue (0.07 ppm) was found after the final dose. The residue was
extracted with methanol (recovery, 90%), and the metabolites present
identified by co-chromatography as 4-acetamido- meta-toluic acid,
4-formamido- meta-toluic acid, and 4-amino- meta-toluic acid (23%);
form-2',4'-xylidide (9%); polar material that was converted to
4-amino- meta-toluic acid by acid hydrolysis (34%); and low-polarity
material (24%). The latter broke down readily to form-2',4'-xylidide
under neutral or basic conditions; it was not present in acetonitrile
extracts of whole milk and was considered to be an artefact produced
by the methanol Soxhlet extraction of the milk. 2,4-Dimethylaniline
was not observed. The edible tissue with the highest concentration of
residue was the liver (3.7 ppm).
Methanol Soxhlet extraction released about 60% of the residue,
and the remainder was solubilized after enzymic digestion and acid
hydrolysis. The residues were identified by co-chromatography as
4-acetamido- meta-toluic acid and 4-formamido- meta-toluic acid
(14%), 4-amino- meta-toluic acid (15%), form-2',4'-xylidide (10%),
and polar material (10%) which on acid hydrolysis was converted to
4-amino- meta-toluic acid and additional low-polarity material. Other
polar material (29% of the residue) was not amenable to
chromatography. 2,4-Dimethylaniline was not observed. The low-polarity
material (14%) broke down on neutral or basic thin-layer
chromatography to a range of compounds, suggesting that it was a
product of condensation between 4-amino- meta-toluic acid and
compounds released from acid-labile conjugates. When the
non-methanol-extractable residue was fed to a dog, analysis of urine
gave similar results to those after enzymic digestion (Phillips et
al., 1987).
Six laying hens (Gallus gallus domesticus) were each dosed
orally with 24.5 mg 14C-amitraz (specific activity, 0.14 mCi/g;
radiochemical purity, > 99%) per day for four days, providing a dose
2200 times greater than the expected daily exposure of birds fed
cotton-meal derived from amitraz-treated plants. The hens were killed
4 or 12 h after the final dose, and the concentrations and nature of
radioactive residues in tissues were determined. The radiolabel was
rapidly excreted, with 68% in 0-24-h excreta. The main route of
metabolism was via 4-amino- meta-toluic acid, since 75% of the
metabolites extracted from the excreta could be accounted for as
either this metabolite or its acid-labile conjugates. The highest
concentrations of tissue residues were found in the liver and kidney
(17 and 25 mg/kg respectively) 4 h after the fourth dose, but these
concentrations had fallen to 12 and 10 mg/kg, respectively, by 12 h
after dosing. The major metabolite detected in the liver was
4-amino- meta-toluic acid, present as both free acid and labile
conjugates, which represented 55% of the total residue.
N-Methyl- N'-(2,4-xylyl)formamidine (4%), form-2',4'-xylidide (4%)
and 2,4-dimethylaniline (2%) were also present. A mixture of at least
seven highly polar compounds, believed to be mono- and di-acids
similar to those previously seen in rats, was a further substantial
residue (27%). Unextractable fibre-bound material represented 2% of
the residue. The concentrations in fat, muscle, and skin represented
0.6-2.7 mg/kg 4 and 12 h after the final dose. In fat, the residues
consisted of form-2',4'-xylidide (42%),
N-methyl- N'-(2,4-xylyl)formamidine (24%), unchanged amitraz (21%),
2,4-dimethylaniline (3%), and 4-amino- meta-toluic acid (3%). In
muscle, 81% of the residues were identified as 4-amino- meta-toluic
acid or its acid-labile conjugates; form-2',4'-xylidide was present as
7% of the residue.
The concentrations of amitraz residues in eggs, collected daily,
were 0.28-0.46 mg/kg throughout the study and did not increase over
the four days of dosing. The residue concentrations in the yolks rose
from 0.1 to 1.4 mg/kg during the study;
N-methyl- N'-(2,4-xylyl)formamidine (54%) and 4-amino- meta-toluic
acid (34%) were the major metabolites. 4-Amino- meta-toluic acid
accounted for 91% of the residue in the egg white as both free acid
and labile conjugates, and form-2',4'-xylidide accounted for 4% of the
residue (Needham & Hemmings, 1988).
Separately reported data on the metabolism of amitraz
in various species were compared. After a single oral dose of
14C-amitraz (specific activity, 9 mCi/g; radiochemical
purity, > 95%) to groups of four male and four female mice,
three male and three female rats, one male and one female
baboon, and two men, the administered radiolabel was rapidly excreted.
In all species examined, urine was the major route of excretion,
accounting for 65-84% of the dose (55-76% within the first 24 h).
Analysis of urine obtained from volunteers dosed with 14C-amitraz
indicated that the metabolism of amitraz was qualitatively similar
to that in the other species. The major metabolites were
4-formamido- meta-toluic acid, 4-acetamido- meta-toluic acid and
N-methyl- N'-(2,4-xylyl)formamidine. In addition, 40-60% of the
metabolites excreted in urine was accounted for by a polar fraction
containing conjugates of 4-formamido- meta-toluic acid and
4-acetamido- meta-toluic acid. The minor metabolites included
4-amino- meta-toluic acid and form-2',4'-xylidide. Excretion of
N-methyl- N'-(2,4-xylyl)formamidine was dose-dependent. The
metabolites identified in the volunteers were
4-formamido- meta-toluic acid plus 4-acetamido- meta-toluic acid
(27% of the total radiolabel in urine),
N-methyl- N'-(2,4-xylyl)formamidine (6%), 4-amino- meta-toluic
acid (4%), form-2',4'-xylidide (4 %), the product of acid hydrolysis
of N-methyl- N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
(1%), and polar material (57%). In both rats and mice, increasing the
dose of amitraz from 1 to 100 mg/kg bw increased the excretion of
N-methyl- N'-(2,4-xylyl)formamidine from approximately 5 to 30% of
the total excretion. Prior dosing of five male and five female mice
with amitraz in the diet at 100 ppm for three weeks, followed by
400 ppm for a further three weeks, had no effect on the metabolism of
a single oral dose of 14C-amitraz (Campbell & Needham, 1984e).
(c) Effects on enzymes and other biochemical parameters
Groups of 18 male and 18 female B6C3F1 mice were dosed orally
with corn oil or amitraz at 100 mg/kg bw per day for two days and,
because of toxic symptoms, at 50 mg/kg bw per day for the following
two days. The animals were then killed, and the livers were assayed
for the activity of microsomal oxidases. A significant increase in
liver weight and in the activity of cytochrome b5 were recorded. The
effect did not appear to be related to increased activity of the
hepatic mixed-function oxidase system, since no significant increase
was seen in the activities of cytochrome P450, aniline hydroxylase, or
para-nitroanisole demethylase or in the concentration of microsomal
protein (Needham, 1984).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of amitraz and its metabolites (purity
unspecified) has been investigated in several species (Table 1). The
toxic signs after oral administration of amitraz to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Rats had intestinal
irritation and bladder distension. The lowest effective doses were 400
mg/kg bw in mice and 200 mg/kg bw in rats. Dermal application of 1600
mg/kg bw to rats had no local or systemic effect. Guinea-pigs were
hyperexcitable after receiving 400 mg/kg bw or more. Rabbits had
central nervous system depression, decreased rectal temperature, pulse
rate, and respiration, nasal discharge, and rales after receiving 100
mg/kg bw, with complete recovery by 48 h. The reactions of dogs to
administration of 100 mg/kg bw and, to a lesser extent, 20 mg/kg bw,
were central nervous system depression, ataxia, muscular weakness,
muscular spasm, uncontrolled vocal spasm and micturition, and
decreased rectal temperature and pulse rate. Haemoconcentration and
increased blood sugar, urea nitrogen, and potassion concentrations
were also seen. No specific pathological lesions were induced. The
lowest dose, 4 mg/kg bw, affected temperature and pulse rate only
slightly . The profile of toxic reactions was consistent with
depression of hypothalamic function. All of the effects were
reversible.
Table 1. Acute toxicity of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
Mouse Oral > 1600 Patton & Sutton (1971)
Rat Oral 600 Patton & Sutton (1971);
Shaw (1973a)
Rat Dermal >1600 Patton & Sutton (1971)
Rat Intraperitoneal 800 Shaw (1971, 1973a)
Rat Inhalation (6 h) 65 Berczy et al. (1972)
Guinea-pig Oral 400-800 Patton & Sutton (1971)
Rabbit Oral > 100 Patton & Sutton (1971)
Rabbit Dermal > 200 Sutton & Williams (1972)
Dog Oral 100 Patton & Sutton (1971)
Baboon Oral 100-250 Patton (1973)
The dermal irritation potential of amitraz (purity, 99%) was
studied in six New Zealand white rabbits weighing 1.9-2.5 kg.
Approximately 24 h after hair had been removed from the dorso-lumbar
region, 0.5 g of technical-grade amitraz was applied under a 2.5-cm
gauze pad moistened with 0.5 ml distilled water, and each treatment
site was occluded with an elastic adhesive dressing for 4 h. At the
end of the exposure period, the dressing was removed and the treatment
site was washed with water. The treated skin was examined on days 1,
2, 3, and 4 after treatment. There was no response to treatment
(Liggett & Smith, 1987a).
The ocular irritation potential of amitraz (purity unspecified)
was studied in six New Zealand white rabbits weighing 2.1-2.8 kg. The
eyes of each animal were examined before instillation of 45 mg (0.1 ml
crystalline powder) of technical-grade amitraz (purity, 98.4%) into
one eye. Both eyes were examined 1 h before and 1, 2, 3, 4, and 7 days
after instillation. Only minimal or slight conjunctival irritation was
observed (Liggett & Smith, 1987b).
Groups of 12 guinea-pigs weighing 300-350 g were treated daily
with 0.1 ml of acetone or 10% amitraz (purity unspecified) or with 1%
dinitrochlorobenzene on the outer surface of each ear for three days.
One week after the start of treatment, the backs and flanks were
clipped and treated with challenge doses of 0.2 ml acetone, amitraz
(10%), or dinitrochlorobenzene (0.25%). The degree of erythema was
assessed after 24 h. Sensitizing activity was observed after treatment
with the positive control but not with amitraz (Sutton, 1971).
The dermal sensitizing effect of amitraz was studied in the
Buehler test. During induction, five male and five female guinea-pigs
received 500 mg technical-grade amitraz (purity, 99%) on the anterior
right flank for 6 h under a dry compress on days 1, 8, and 15. A dry
compress alone was applied on the anterior left flank. After a
two-week rest period, each animal received a challenge of 500 mg
technical-grade amitraz to the right flank and a dry compress alone to
the left flank. The cutaneous reactions were evaluated 24 and 48 h
after challenge. The animals were then sacrificed, and cutaneous
samples were taken from the challenge site in all animals. No clinical
signs or deaths related to treatment were observed, and no cutaneous
reactions were found 24 and 48 h after the challenge with amitraz
(Clouzeau, 1992).
Delayed contact hypersensivity in response to amitraz (purity,
98.4%) was tested in the maximization test described by Magnusson and
Kligman. After random allocation of 20 female guinea-pigs to a control
group and 20 to a test group, the animals were induced by intradermal
injection of 5% w/w amitraz in Alembicol D (a coconut oil) into a
4 × 6-cm clipped area of the dorsal skin on the scapular region. One
week after the injections, the same area was clipped again and given a
topical application of 15 or 30% w/w amitraz in Alembicol D on a
2 × 4-cm patch saturated with the test solution, which was placed on
the skin, covered with impermeable plastic adhesive tape, secured by
an elastic adhesive bandage, and left in place for 48 h. The test and
control animals were challenged topically as described above, two
weeks after the induction, with 15 or 30% w/w technical-grade amitraz
in Alembicol D. The challenge sites were evaluated 24, 48, and 72 h
after removal of the patches, and an arbitrary scale of 0-4 was used
to score the reactions. The dermal reactions observed in the test
animals were more marked and persistent than those seen in the
controls (Kynoch & Parcell, 1988).
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female B6C3F1 mice were fed diets
containing 0, 100, 200, 400, 600, or 800 ppm amitraz (purity, 98.3%),
equal to 0, 13, 26, 53, 96, and 110 mg/kg bw per day for males and 0,
17, 35, 68, 110, and 150 mg/kg bw per day for females, for 13 weeks.
Clinical signs, body weight, and food consumption were recorded
throughout the study. All mice were killed at the end of treatment and
examined grossly but not histopathologically. The only overt sign of
reaction to treatment was an increase in aggressive behaviour, as
evidenced by fighting and resultant cutaneous lesions, among males
treated at doses > 400 ppm. The overall body-weight gain was
statistically significant lower in the males (40%) treated at > 400
ppm and in females (34%) at > 200 ppm. Food consumption did not
differ statistically significantly between the control and treated
groups. The NOAEL was 100 ppm, equal to 17 mg/kg bw per day, on the
basis of the overall reduced body-weight gain of females (Colley et
al., 1981).
Rats
Groups of 21 male and 21 female Ash-Wistar rats were given
amitraz (purity unspecified) suspended in 0.4% Cellosize solution by
oral intubation at a dose of 0, 3, or 12 mg/kg bw per day for 90 days.
They were then killed either immediately or after a three-week
recovery. Treatment of rats with 50 mg/kg bw per day was discontinued
after seven days because of depressed growth and behavioural
disturbances, and treatment with 200 mg/kg bw per day was discontinued
after seven days because of irritability and debilitation. Body
weights were recorded three times per week. Haematological
examinations were made on some control rats and on some rats receiving
12 mg/kg bw per day at 4, 8, and 12 weeks. At autopsy, blood was
withdrawn from the heart to estimate plasma alkaline phosphatase
activity, bilirubin, aspartate and alanine aminotransferase activity,
and sodium and potassium concentrations. Organs were weighed and
prepared for microscopic examination. Significant reductions were seen
in the overall body-weight gain (8%) and absolute (8%) and relative
weights (6%) of the liver of male rats receiving 12 mg/kg bw per day.
The NOAEL was 3 mg/kg bw per day on the basis of these last effects
(Sutton & Williams, 1971).
Groups of 12 rats (strain not given) were exposed daily for 6 h
to dusts of amitraz (purity unspecified) at a concentration of 0,
0.01, 0.1, or 1 mg/L air, on 14 days over three weeks. During exposure
to 0.1 mg/L, signs of mild dyspnoea, slight eye irritation, and
hyposensitivity to noise were recorded. After termination of exposure,
the rats were hypersensitive to touch and became aggressive. Exposure
to 1 mg/L elicited signs similar to but more severe than those seen in
the previous group. In addition, ataxia, increased nasal secretion,
polyuria, body tremors, and coma were observed in the exposed rats.
Consumption of food and water was reduced, and there was body-weight
loss. Reductions in packed cell volume, haemoglobin, red cells, and
plasma protein concentrations in blood may have been related to
treatment. The body tremors, aggressive behaviour, and coma indicate
that the central nervous system was affected (Berczy et al., 1973).
Rabbits
Groups of four to eight male and female New Zealand white rabbits
weighing 2.5-4.5 kg (the health status of which must be regarded as
suspect due to general infection) were treated on the intact skin of
the back (without occlusion) with amitraz (purity unspecified) in
acetone at a dose of 0, 50, or 200 mg/kg bw per day for a total of 15
doses over 21 days. Body weight, food consumption, heart rate, and
rectal temperature were determined. Haematological and blood
biochemical parameters and organ weights were measured, and
histopathological examinations were carried out. A transient sedative
effect and local skin reactions were seen in animals of each sex at 50
and 200 mg/kg bw per day. Body weights and food consumption were
adversely affected, and some deaths occurred that were possibly
related to treatment. Variable tubular degeneration was seen in the
testes, which were underweight. At 50 mg/kg bw per day, body weights
and food consumption were less adversely affected than at the high
dose, and the death of only one male was considered related to
treatment (Sutton, 1973a).
Dogs
Groups of two male and two female beagles received amitraz
(purity unspecified) in capsules at a dose of 0, 0.25, 1, or 4 mg/kg
bw per day for 90 days. The animals were examined clinically before
dosing began and during weeks 2, 4, 8, and 13 of dosing. Food
consumption was recorded daily and body weights weekly.
Haematological, blood biochemical, and urinary parameters were
measured before and at intervals during treatment. During week 13 of
dosing, control animals and those at 4 mg/kg bw per day were placed in
metabolism cages, and their water intake and urine excretion over 24 h
were recorded. The faeces were tested daily for occult blood during
the first week of dosing. At the end of treatment, the animals were
sacrificed, and several organs were weighed and prepared for
histological examination.
On the three first days of dosing, all four animals at 4 mg/kg bw
per day showed central nervous system depression, vomiting, ataxia,
and reduced rectal temperature and pulse rate, which recurred
consistently within 6 h. Hyperglycaemia and occasional glycosuria were
also induced. The livers were slightly enlarged and showed low-grade
microscopic changes. The dose of 1 mg/kg bw per day had similar but
less severe effects. At 0.25 mg/kg bw per day, only minor changes were
seen, although central nervous system depression was seen in one dog
on one occasion 3 h after dosing during week 8 of treatment. The NOAEL
was 0.25 mg/kg bw per day on the basis of central nervous system
depression and reduced rectal temperature and pulse rate (Patton &
Williams, 1971).
Groups of four male and four female beagles were given 0, 0.1,
0.25, or 1 mg/kg bw per day amitraz (purity unspecified) in gelatine
capsules for two years. They were examined clinically before and
immediately after dosing on the first two days and again during weeks
4, 13, 26, 39, 52, 78, and 102. Heart rate and rectal temperature were
monitored serially for up to 24 h after dosing on day l and during
weeks 39, 52, 79, and 103. Signs of toxicity and faecal appearance
were recorded daily and body weights weekly. Haematological, blood
biochemical, and urinary parameters were measured before dosing and
during weeks 4, 13, 26, 52, 78, and 102. In addition, serial blood
samples were obtained during weeks 40 and 53 for estimating blood
sugar and during weeks 91 to 96 for estimating methaemoglobin. The
animals were sacrificed and examined macroscopically on the day after
the final dose, and their organs were weighed and prepared for
histopathological examination.
All eight animals given 1 mg/kg bw per day showed signs of slight
central nervous system depression 3 h after dosing on days 1 and 2,
but all appeared normal by the following morning. On both days, one
dog had a slightly subnormal temperature (38.3°C) at 3 h, which had
returned to normal (38.7°C) by 24 h. Subsequently, all of the animals
in this group appeared clinically normal, apart from one bitch that
was slightly hypothermic 3 h after dosing during weeks 52 and 79. The
NOAEL was 0.25 mg/kg bw per day on the basis of central nervous system
depression (Morgan et al., 1973).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CFLP mice were fed diets
containing 0, 25, 100, or 400 ppm amitraz (purity unspecified),
equivalent to 3, 8, 15, and 60 mg/kg bw per day (wrongly given as 25,
100, and 400 mg/kg bw per day in reference 34, Annex 1), for 80 weeks.
Animals at 100 or 400 ppm gained less weight than the controls over
the first 40 weeks, whereas those at 25 ppm gained more weight than
the controls from week 10 onwards and showed a 20% greater body-weight
gain at the end of the experiment. Calculated over 80 weeks, food
consumption was increased for male mice at 100 and 400 ppm and female
mice at 400 ppm, and decreased for males at 25 and 100 ppm. The
survival rate was similar in all groups. An increased incidence of
lymphoreticular tumours was observed in female mice at 400 ppm (49%
compared with 23% in controls). A slight, not statistically
significant increase in the incidence of all types of liver-cell
tumour was found in animals of each sex fed 400 ppm. No other
pathological finding related to treatment was reported. The NOAEL for
carcinogenicity was 100 ppm, equivalent to 15 mg/kg bw per day, on the
basis of an increased incidence of lymphoreticular tumours in females
at 400 ppm (Burnett et al, 1976; Kakuk, 1979).
Groups of 75 male and 75 female B6C3F1 hybrid mice, 33 days old,
were given diets containing 25, 100, or 400 ppm amitraz (purity,
97.9%), equal to 2.3, 9.6, and 45 mg/kg bw per day for males and 2.6,
11, and 50 mg/kg bw per day for females, for 104 weeks. The untreated
controls consisted of 100 male and 100 female mice. Clinical signs,
food consumption, and body weight were recorded throughout the study.
Survivors were killed at the end of the dosing period and all animals
were subjected to a complete gross autopsy. All tissues were collected
from all animals and preserved for future examination.
Histopathological examination was performed on all tissues from the
high-dose group and controls, and on the liver, pancreas, spleen,
lung, stomach, pituitary, thyroid, adrenals, ovaries, uterus, sternum,
and all abnormal tissues found grossly from animals at the low and
intermediate doses.
Males at 400 ppm were hyperactive and behaved aggressively. The
incidence of cutaneous ulceration and inflammation of the perigenital
and perianal areas was greater in mice at 100 and 400 ppm than in
those at 25 ppm or in controls, and the incidence of urogenital
swelling was greater in all treated mice than in controls. The
incidences of gross adverse effects in treated females were comparable
to the control incidences. There was clearly increased mortality of
males at 400 ppm (20/75) and of males and females combined (37/150).
Mean body-weight gain was reduced in mice at 400 ppm (by 30-50%)
throughout the study, with a significant decrease in females at 100
ppm over the last 74 weeks of the study. Food consumption was
marginally reduced initially in animals at 100 and 400 ppm but was
comparable to that of controls from week 25 to termination. Increased
liver and lymph node involvement was seen in males and females at 400
ppm, and the incidence of preputial gland enlargement in males at 400
ppm was greater than that in controls (20/75 vs 12/100). The
prominence of the limiting ridge of the stomach was greater than in
controls for females at all doses and for males at 100 and 400 ppm.
Decreased production of myeloid elements accompanied by an increase in
erythroid elements resulted in a significant decrease in the
myeloid:erythroid ratio for males at 400 ppm and females at 100 or
400 ppm. The incidences of spleen haematopoiesis in males and of
stomach focal hyperkeratosis in males and females at all three doses
were greater than that in controls. There was apparent liver
involvement in females, with an increased incidence of hepatocellular
carcinoma at 400 ppm (15/75 vs 2/100 hepatocarcinoma; 16/75 vs 4/100
hepatocellular adenoma). This was accompanied by a dose-related
increase in the incidence of hyperplastic nodules and telangiectatic
and basophilic foci of the liver in animals at 100 and 400 ppm. Males
at 400 ppm also had an apparent increase in the incidence of
hyperplastic nodules and telangiectatic and basophilic foci of the
liver. The NOAEL for carcinogenicity was 100 ppm, equal to 11 mg/kg bw
per day, on the basis of hepatocellular carcinoma in females at
400 ppm. This dose was considered to be greater than the conventional
maximum tolerated dose. The NOAEL for long-term toxicity was 25 ppm,
equal to 2.3 mg/kg bw per day, on the basis of general toxicity
(Colley et al., 1983).
Rats
Groups of 40 male and 40 female Ash-Wistar rats received amitraz
(purity, 97.8%) at a dietary concentration of 0, 15, 50, or 200 ppm
(equal to 0, 0.77, 2.5, or 10 mg/kg bw per day in males and 0, 0.97,
3.1, or 13 mg/kg bw per day in females) for two years. Body weights
were measured twice per week during the period of maximal growth and
later weekly. Food consumption was determined weekly during this
growth period and later monthly. Haemato-logical, biochemical, and
urinary analysis were performed during and at the end of the study,
and organs were weighed and examined grossly and histologically at the
end of study.
Rats at 200 ppm were occasionally nervous, excitable, and
aggressive, and their food consumption was temporarily reduced. The
overall body-weight gain of males was significantly reduced (10%).
Rats at 50 and 15 ppm showed no adverse reactions to treatment. The
incidence, type, and time to appearance of tumours were not
significantly different in treated and control groups. The NOAEL for
toxicity was 50 ppm, equal to 2.5 mg/kg bw per day, on the basis of
effects on the central nervous system and reduced overall body-weight
gain in males (Sutton & Offer, 1973).
(d) Genotoxicity
The results of tests for the genotoxicity of amitraz are
summarized in Table 2. The results of the tests in vitro and
in vivo were negative.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a three-generation study of reproductive toxicity, groups of
10 male and 20 female newly weaned Boots-Wistar rats were fed amitraz
(purity, 99.8%) at a dietary concentration of 0, 15, 50, or 200 ppm,
equal to 0, 1.3, 4.4, and 16 mg/kg bw per day in males and 0, 1.5,
5.1, and 20 mg/kg bw per day in females. After the F1 generation had
been weaned, 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. Amitraz at 200 ppm decreased the
growth, food consumption, fertility, and viability of offspring of the
F0 generation, and this dose was eliminated when the F1 generation
had been weaned, because of the very low survival. No effect was found
on the number of litters or mean litter size at 50 ppm; however, a
decreased number of young alive at 21 days was observed in all
generations. No further effects due to treatment were found in this or
the other group. The NOAEL was 50 ppm, equal to 4.4 mg/kg bw per day,
for maternal toxicity and 15 ppm, equal to 1.3 mg/kg bw per day, for
developmental toxicity (Sutton, 1973b).
(ii) Developmental toxicity
In a study of developmental toxicity, groups of 11-13 female
Boots-Wistar rats received amitraz (purity, 99.8%) at a dose of 0, 1,
3, or 12 mg/kg bw per day on days 8-20 of gestation. The rats were
killed on day 21, and the uterine contents were examined. There were
no deaths or clinical signs of toxicity. Food consumption, body-weight
gain, average litter size, fetal viability, and the implantation index
were not affected. At 12 mg/kg bw, fetal weight was lower than in the
controls, and the calcification of the sternebrae was less advanced.
The NOAEL for maternal toxicity was 12 mg/kg bw per day, and that for
developmental toxicity was 3 mg/kg bw per day, on the basis of reduced
fetal weight and reduced calcification of the sternebrae (Sutton,
1973c).
Table 2. Results of tests for the genotoxicity of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium 31-500 µg/plate 99.9 Negative Everest &
TA1535, TA1537, Wilcox (1976)
TA1558
Reverse mutation S. typhimurium 0, 33, 100, 333, 1000, 98.4 Negative McGregor &
TA98, TA100, TA1535, 3300, 10 000 µg/plate Printice (1983)
TA1537, TA1538
Chromosomal Human lymphocytes 0, 5, 10, 20 µg/ml -S9; 99.5 Negative Brooker et al.
aberration 0, 3, 5, 30 µg/ml +S9 (1988)
Cell mutation Mouse L5178Y tk+/- 0.06-33 µg/ml +S9; 98.4 Negative McGregor &
cells 0.06-20 µg/ml -S9 Riach (1983a)
Unscheduled Human embryonic 20, 60, 100, 140, 180, 100 Negative McGregor &
DNA synthesis fibroblasts 220, 260, 300 µg/ml ±S9 Riach (1983b)
DNA damage Chinese hamster 0.01-0.3 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Reverse mutation S. typhimurium 0, 100, 200, 400 mg/kg NR Negative Everest (1976);
(host-mediated, G46, TA1532 bw, single dose Wilcox (1976)
mouse) TA1964
Dominant lethal Female CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977a)
for 5 days
Table 2. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
Dominant lethal Male CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977b)
for 5 days
NR, not reported; S9, 9000 × g microsomal fraction from rodent liver
In another study of developmental toxicity, groups of 24 mated
Sprague-Dawley rats weighing 215-280 g were given 0, 7.5, 15, or 30
mg/kg bw per day amitraz (purity, 99.7%) by gavage on days 6-15 of
gestation. Immediately after mating, the females were assigned to
treatment groups by a randomization process based on stratified body
weight. Each female was then individually identified by ear notching.
All females were examined once or twice daily for clinical signs of
ill health, toxicity, or behavioural changes, and body weights and
food intake were recorded on days 0, 6, 10, 15, and 20 of gestation.
On day 20 of gestation, the females were killed and their uterine
contents examined.
One control female was found dead on day 10 of gestation; there
were no other deaths. The principal clinical sign was fur staining,
which was slightly more frequent at 30 mg/kg bw per day. At this dose,
a slight loss of body weight up to day 10 of gestation was followed by
reduced body-weight gain at termination. The body-weight gain of rats
at 15 mg/kg bw per day was slightly reduced (10%), but that of animals
at 7.5 mg/kg bw per day was not adversely affected by treatment. Food
intake of rats at 30 mg/kg bw per day, and to a lesser extent those at
15 mg/kg bw per day, was initially lower than in the control group;
there was no effect on food intake at 7.5 mg/kg bw per day. The
pregnancy rate was high in all groups, and no adverse findings were
made at necropsy. The treatment did not adversely affect the number of
implantations, the incidence of post-implantation loss, or the number
or sex ratio of fetuses. Animals at 30 mg/kg bw per day had a
statistically significantly increased incidence of dilated ureters and
increased bilateral renal pelvic cavitation. Although the incidence of
the latter lesion at 15 mg/kg bw per day was statistically
significantly higher than that of the concurrent controls
(p < 0.05), the group percentage increase (5.7%) was comparable to
the upper range of the relevant historical control values (5.4%). The
NOAEL was 7.5 mg/kg bw per day for both maternal toxicity, on the
basis of reduced body-weight gain, and for developmental toxicity, on
the basis of increased incidences of dilated ureters and renal pelvic
cavitation (McIntyre, 1987a).
Rabbits
In a study of developmental toxicity, groups of 8-10 New Zealand
rabbits were treated with amitraz (purity unspecified) at a dose of 0,
1, 5, or 25 mg/kg bw per day on days 6-18 of pregnancy and were killed
on day 30. At 25 mg/kg bw per day, the number of litters and mean
litter size were decreased and abortions were observed. No increase in
the incidence of congenital abnormalities was found. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, the highest dose tested,
and the NOAEL for developmental toxicity was 5 mg/kg bw per day on the
basis of a reduced number of litters and litter size (Sutton, 1973d).
In another study of developmental toxicity, groups of 16 mated
female New Zealand white rabbits weighing 3-4 kg were given amitraz
(purity, 99.7%) by gavage at a dose of 0, 3, 6, or 12 mg/kg bw per day
on days 7-19 of gestation. All females were examined once or twice
daily for clinical signs of ill health, toxicity, or behavioural
changes, and body weight and food intake were recorded on days 0, 7,
13, 19, 24, and 28 of gestation. On day 28 of gestation, the surviving
females were killed and their uterine contents examined.
One female at 12 mg/kg bw per day died, and three females at this
dose were killed because of poor clinical condition or abortion. Two
of the deaths were considered to be directly related to treatment. Two
females at 6 mg/kg bw per day died; at 3 mg/kg bw per day, one female
was killed and a further two died. Two control animals also died.
Langour, polypnoea, and squinting were observed in all treated groups,
and the incidence, severity, and duration of these signs appeared to
be dose-related. At 12 mg/kg bw per day, body-weight loss followed by
a reduction in body-weight gain were observed during dosing, but the
weight gain was similar to that of controls on cessation of dosing.
Treatment at 3 or 6 mg/kg bw per day had no effect on body weight.
Food intake was reduced during dosing and up to day 24 at 12 mg/kg bw
per day, but no adverse effect was seen at 6 or 3 mg/kg bw per day. No
adverse effects were seen at necropsy. Three of the surviving females
at 12 mg/kg bw per day lost their litters on day 28 of gestation.
Litter paraments were not affected at 6 or 3 mg/kg bw per day, as
assessed by the numbers of corpora lutea, implantation sites, and
viable fetuses. The mean litter and fetal weights and sex ratio of
fetuses were not adversely affected by treatment. No major fetal
defects were recorded that were considered to be related to treatment,
and there were no treatment-related effects on the incidence of minor
or variant anomalies. There was no NOAEL for maternal toxicity because
of deaths of animals at all doses and in the control group. The NOAEL
for developmental toxicity was 6 mg/kg bw per day, on the basis of
litter loss (McIntyre, 1987b).
(f) Special studies
(i) Effects on the thymus and hormone concentrations
Diets containing 400 ppm amitraz (purity, 97.1%), equal to 110
mg/kg bw per day for males and 150 mg/kg bw per day for females, were
given to 24 male CFLP mice for up to 18 weeks and to 52 female CFLP
mice for up to 33 weeks. A group of 36 males and 64 females given
plain diet served as controls. Body weight and food consumption were
measured regularly throughout the study, and the animals were examined
for overt signs of toxicity at least once a week. Vaginal smears were
monitored every morning during weeks 6-9, 15-18, 23-26, and 30-33.
Twelve male and 12 female controls were killed during the first week,
and 12 males and 12 females from both treated and control groups were
killed after 9 and 18 weeks. The remaining animals were killed after
33 weeks. The ß-estradiol concentration of the blood of female mice
was estimated at weeks 9 and 18, and the thymuses from all animals
were weighed at autopsy. Detailed histological examinations were
conducted on a selected range of tissues.
Body-weight gain was reduced in amitraz-treated mice, to a
slightly greater degre in the males, and food consumption was markedly
increased, particularly in the females. Examination of vaginal smears
indicated prolongation of estrus in treated mice, but there was no
measurable effect on the circulating concentrations of ß-estradiol.
The thymus weights and histological appearance were not affected by
treatment. Histopathological examination revealed two lymphoreticular
tumours in the treated females and two in controls. In comparison with
the controls, females given amitraz for 33 weeks had a higher
incidence of foci of inflammatory cells in the liver (Brown et al.
1978).
(ii) Effects on the estrus cycle and hormone concentrations
Mice
The effects of amitraz on the estrus cycle and hormone
concentrations were evaluated in groups of 70 female B6C3F1 mice fed
diets containing amitraz (purity, 98-100%) at 0, 25, 100, or 400 ppm,
equivalent to 0, 3.8, 15, and 60 mg/kg bw per day, for up to 28 weeks.
Permanently stained and mounted vaginal smears were prepared from each
animal daily for 30 days after 13 weeks of treatment, and the stage of
the oestrus cycle on each day was determined by microscopic
examination of the cell population on each slide. Blood samples were
taken at necropsy after overnight starvation and assayed for
dehydroepiandrosterone sulfate, estradiol, progesterone, testosterone,
lutropin, follitropin, prolactin, thyroxine, triiodothyronine, and
thyroid hormone uptake, as indicators of hormonal status and routine
clinical chemical parameters. The fresh liver weight was recorded at
necropsy.
Pro-estrus was prolonged, and a trend towards reduced duration of
diestrus was evident in animals at 400 ppm. The blood concentrations
of progesterone were lower and the concentrations of
dehydroepiandrosterone sulfate were higher in animals at 400 ppm and
to a lesser degree in those at 100 ppm when compared with controls.
The relative liver weights were increased at 400 (by 4%) and 100 ppm
(by 5%). There were no treatment-related effects at 25 ppm. The NOAEL
was 25 ppm, equivalent to 3.8 mg/kg bw per day, on the basis of lower
blood concentrations of progesterone, higher concentrations of
dehydroepiandrosterone sulfate, and increased relative liver weight
(Hounsell & Rush, 1984).
Rats
Groups of 20 female 22-week-old Boots-Wistar rats were fed diets
containing 0 or 200 ppm (equivalent to 0 and 12 mg/kg bw per day)
amitraz (purity unspecified) for 18 weeks. Vaginal smears were taken
routinely over 32 days. After fixation in methanol, the smears were
stained with 1% aqueous methylene blue and examined for keratinized
cells under 60 × magnification. Estrus was characterized by the
presence of keratinized cells only, and the cycle length was taken as
the interval between the first day of estrus in successive cycles.
The average cycle length of the controls was 4.3 days; two of the
controls had prolonged periods in estrus (four and six days), and a
third had a period of prolonged diestrus with a cycle lasting 18 days.
In the remaining animals, the shortest cycles were two days and the
longest six days. The low incidence of prolonged estrus among these
animals confirms that the technique of smearing did not affect vaginal
cytology. In the treated rats, the average cycle length was 6.1 days,
and the range was 2-16 days. Six rats had one or more periods of
prolonged diestrus; four of these had been acyclic in a preliminary
test. Estrus lasted for three to eight consecutive days in seven rats,
two of which had shown a similar tendency in a preliminary test. The
second test showed that the cycle length was significantly altered by
treatment, either estrus or diestrus being prolonged. Treated rats
thus had longer oestrus cycles than controls, resulting from prolonged
periods of estrus or diestrus (Merryman & Sutton, 1972).
(iii) Mechanism of action
The effects of amitraz and its major metabolite,
N-methyl- N'-(2,4-xylyl)formamidine, given orally or intravenously
on the cardiovascular system, pupil diameter, and the respiratory
system were studied in conscious and anaesthetized rats, cats, and
dogs. Both compounds caused a fall in blood pressure, sometimes
preceded by hypertension and bradycardia. The threshold for the effect
in conscious rats dosed orally with amitraz was 1 mg/kg bw. Amitraz
also caused mydriasis, sedation, and a reduced respiratory rate. The
bradycardia, sedation, and mydriatic effect was antagonized by the
alpha2-blocking agent yohimbine but not by the alpha1-blocking agent
prazosin, indicating that the effects were caused by stimulation of
presynaptic alpha2-adrenoceptors. The lethal effect of amitraz in mice
and dogs has also been shown to be inhibited by yohimbine (Parkinson &
Sim, 1970; Cullen & Reynoldson, 1983; Hsu & Kakuk, 1984; Moser &
MacPhail, 1985; Hsu et al., 1986; Hovda & McManus, 1993).
Amitraz and N-methyl- N'-(2,4-xylyl)formamidine were tested
for their ability to potentiate the pressor responses to tyramine in a
pithed rat preparation, since a structurally related compound,
chlordimeform, inhibited monoamine oxidase in vitro in rat liver
homogenates and centrally in vivo through an effect on brain
serotonin concentrations. These effects were obvious only with nearly
toxic oral doses of 80 mg/kg bw amitraz and 40 mg/kg bw
N-methyl- N'-(2,4-xylyl)formamidine (Parkinson, 1974).
Amitraz and chlordimeform were tested for their ability to
inhibit prostaglandin synthesis after intraperitoneal administration
in rats. Both compounds had antipyretic and antiinflam-matory effects
at doses of 5-80 mg/kg bw. They reduced yeast-induced fever, with
potencies intermediate between those of indomethacin and aspirin, and
antagonized carrageenan-induced swelling of the hind paw. They also
inhibited the synthesis of prostaglandin E2 from arachidonic acid by
bovine seminal vesicle microsomes. The potency of amitraz in this
assay was the same as that of aspirin (Yim et al., 1978).
(g) Studies on metabolites
(i) Acute toxicity
The acute toxicity of metabolites of amitraz (purity unspecified)
has been investigated in several species (Table 3).
Table 3. Acute toxicity of metabolites of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
N-Methyl-N'-(2,4-xylyl)formamidine
Mouse Oral 150 Sutton (1970a)
Rat Oral 200 Sutton (1970b)
Dog Oral >20 Morgan (1973);
Morgan & Williams (1974)
4-Amino-meta-toluic acid
Mouse Oral >1600 Shaw & Williams (1973a)
Rat Oral >1600 Shaw & Williams (1973b)
Form-2',4'-xylidide
Rat Oral 1600 Shaw (1973b)
(ii) Short-term toxicity
Rats
Groups of 10 male and 10 female newly-weaned Boots-Wistar rats
were dosed by gastric intubation with
N-methyl- N'-(2,4-xylyl)formamidine (purity unspecified) for 90
days at 0, 0.25, 1, 3, or 12 mg/kg bw per day. Body weights were
recorded three times per week, and the rats were observed each day for
signs of toxicity. At 3 mg/kg bw per day, an initial reduction in
body-weight gain was seen in males. At 12 mg/kg bw per day, the rats
became nervous and difficult to handle, and two deaths occurred. The
growth rate was reduced in animals of each sex but to a greater extent
in the males. At the end of experiment, haemoglobin and haematocrit
values were decreased in males, and the number of erythrocytes was
decreased in females. Slight biochemical changes were seen. At
autopsy, the weights of the adrenals, ovaries, uterus, and liver in
females and spleen and testes in males were increased, but the only
histopathological changes were a slight increase in lymphoid
infiltration, some sinusoidal leukocytosis, and loss of glycogen in
the livers of most males and slight cellular accumulations in the
hearts of some females. The NOAEL was 1 mg/kg bw per day on the basis
of the reduced body-weight gain and increased organ weights (Shaw &
Williams, 1975).
Groups of five male and five female Wistar rats were dosed by
gastric intubation with 4-amino- meta-toluic acid (purity
unspecified) at a dose of 0, 40, 100, or 250 mg/kg bw per day for 21
days. At 250 mg/kg bw per day, slight decreases in weight gain and in
blood urea nitrogen concentration were observed in males and an
increased relative weight of the spleen in females. No gross
pathological changes due to treatment were found. The NOAEL was
250 mg/kg bw per day, the highest dose tested (Shaw, 1975).
Dogs
Groups of four male and four female beagles were given gelatin
capsules containing N-methyl- N'-(2,4-xylyl)formamidine (purity
unspecified) as free base diluted in lactose to 1% at a dose of 0,
0.1, 0.25, or 1 mg/kg bw per day for 90 days. Clinical signs were
recorded daily, food consumption twice daily, and body weight once a
week. Ophthalmoscopic examination and recordings of temperature and
heart rate were carried out before dosing and after 6 and 12 weeks.
Haematological, clinical chemical, and urinary analyses were perfomed,
and gross and histopathological examinations were carried out.
At 0.25 and 1 mg/kg bw per day, abnormal quietness and drowsiness
and significantly lower body temperature (by up to 16.2°C) were
observed 0.5-4 h after dosing. At 1 mg/kg bw per day, the heart rate
was significantly reduced (by up to 40 beats/min) 1-2 h after dosing,
and the liver weight and urine volume were increased. A slight
reduction in thymus weight was observed at 0.25 and 1 mg/kg bw per
day. No histopathological anomalies were found. The NOAEL was 0.1
mg/kg bw per day on the basis of central nervous system depression and
lowered body temperature (Chesterman et al., 1973).
Groups of four male and four female beagles were given gelatin
capsules containing 4-amino- meta-toluic acid (purity unspecified) at
a dose of 0, 16, 40, or 100 mg/kg bw per day for 90 days. Slightly
increased urinary concentrations of total reducing substances other
than glucose were found at the highest dose. No dose-related effects
were observed on behaviour, electrocardiogram, heart rate, rectal
temperature, body weight, food consumption, haematological or blood
chemical parameters, organ weights, or histopathological appearance
(Morgan et al., 1974).
(iii) Genotoxicity
The results of tests for the genotoxicity of potential
metabolites of amitraz are summarized in Table 4. Except for a single
positive response to 2.4-dimethylaniline in the assay for forward
mutation in mouse lymphoma cells, in the presence of metabolic
activation, the results of the tests in vitro and in vivo were
negative.
3. Observations in humans
In a double-blind, randomized cross-over study, six healthy male
volunteers aged 18-45 years and weighing 60-70 kg received sequential
single oral doses of 0, 0.063, and 0.13 mg/kg bw amitraz, two to three
weeks apart. Each dose was given with 150 ml water, 30 min after
breakfast. Pulse rate, respiration rate, blood pressure, and
temperature were measured at - 1, - 0.5, 1, 3, 6, 12, 24, and 36 h,
and electrocardiograms were performed at -1, 1, 3, 6, 12, 24, and 36 h
with respect to dosing. Pupil responsiveness and psychomotor
performance were evaluated before treatment and at 2.5 and 8 h. Urine
was collected at 0-36 h and 36-60 h. There were no clinically
significant changes in vital signs or electrocardiographic parameters.
Moreover, haematological, blood chemical, and urinary parameters,
pupil responsiveness, and psychomotor performance were unaffected by
treatment. The NOAEL was 0.13 mg/kg bw, the highest dose tested (Cass,
1992).
Two human volunteers who received a single oral dose of 0.25
mg/kg bw 14C-amitraz showed drowsiness, disorientation, slurred
speech, decreased pulse rate and blood pressure, and other effects
(Campbell & Needham, 1984c).
In a double-blind cross-over study, four male and two female
volunteers, aged 21-42 years and in normal health, received two doses
of 2 mg (about 0.03 mg/kg bw) of the amitraz metabolite,
N-(2,4-dimethylphenyl)- N'-methylformamidine, one week apart in a
capsule with 100 ml of water, or placebo. Blood pressure, pulse rate,
and temperature were measured at half-hourly intervals over 7 h, and
mental alertness was assessed at 0, 3, and 7 h. An electrocardiograph
was performed, and urine was collected before dosing and at 0-7 h and
7-24 h. The urine samples were analysed to estimate the amount of the
metabolite, 3-methyl-4-aminobenzoic acid, that had been excreted.
Mental alertness was estimated at 0, 3, and 7 h. No difference was
seen from those receiving the placebo. The NOAEL was 0.03 mg/kg bw,
the only dose tested (Hall et al., 1975).
Symptoms of central nervous system depression ranging from
sedation to coma lasting more than 24 h, depressed respiration,
hypotension, and bradycardia have been described in 11 reports after
accidental or intentional ingestion of uncertain amounts of amitraz.
In nine cases, recovery was complete after symptomatic and supportive
treatment (Groppi, 1977; Ros & Aken, 1994; Pronczuk et al., 1995).
Table 4. Results of tests for the genotoxicity of metabolites of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
N-Methyl-N'-(2,4-xylyl)formamidine
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983a)
TA1337, TA1538
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Form-2',4'-xylidide
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983b)
TA1337, TA1538
DNA damage Chinese hamster 0.01-1.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Forward mutation Mouse L5178Y tk+/- 1, 3.3, 10, 33, 100, 200, NR Positive + S9 McGregor &
lymphoma cells 300, 333, 400, 500, Negative -S9 Riach (1984)
600 µg/ml
Cell transformation C3H/10 T1/2 clone 8 5, 10, 20 µg/ml +S9 NR Negative McGregor et al.
mouse embryo fibroblasts 100, 200, 400 µg/ml -S9 (1984)
DNA damage Chinese hamster 0.03-2.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
4-Amino-meta-toluic acid
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Micronucleus Mouse bone 56, 113, 225 mg/kg bw NR Negative Hounsell &
formation marrow twice, 24 h apart Walker (1983)
Comments
Amitraz was well absorbed, extensively metabolized, and rapidly
excreted, mainly in the urine, after oral administration to mice,
rats, dogs, pigs, hens, cows, baboons, and humans. After oral
treatment of mice with 14C-amitraz, 86% of the radiolabelled dose was
excreted, 62% in the urine, within the first 24 h. All of it had been
excreted by 96 h, with 73% in the urine of animals of each sex. The
concentrations of residues were highest in liver, adrenal glands, and
eyes. After oral administration of 14C-amitraz to rats, 94% of the
dose was recovered within three days, with 82% in urine and 12% in
faeces. After oral administration of 14C-amitraz to two humans,
77-87% was recovered within three days. Amitraz is hydrolysed to two
components, N-methyl- N-(2,4-xylyl)formamidine and
form-2',4'-xylidide. The former is the pharmacologically active
compound and accounted for 5-30% of the total urinary excretion in
mice and rats; it was further metabolized to 4-amino- meta-toluic
acid and the acetyl and formyl conjugates, 4-acetamido- and 4-
formamido- meta-toluic acids. These five metabolites were also found
in plants.
Amitraz has low acute oral toxicity in rats but is more toxic in
dogs. The LD50 values ranged from 100 mg/kg bw in dogs to > 1600
mg/kg bw in mice, indicating that dogs are more sensitive. The toxic
signs after oral administration to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Amitraz had no
sensitizing potential in guinea-pigs, and no local irritation was
found in rabbits after a single application to skin or eyes. There was
evidence of delayed contact hypersensitivity after application of
amitraz either topically or intradermally.
WHO (1996) has classified amitraz as slightly hazardous.
In a 13-week study in which mice were fed diets providing 0, 100,
200, 400, 600, or 800 ppm, the NOAEL was 100 ppm, equal to 17 mg/kg bw
per day, on the basis of reduced overall body-weight gain (by 34%).
In a 90-day study, rats were given amitraz at doses of 0, 3, or
12 mg/kg bw per day by gavage. The NOAEL was 3 mg/kg bw per day, on
the basis of reduced terminal body-weight gain, absolute liver weight,
and relative liver weight.
In a 90-day study in dogs, amitraz was administered at doses of
0, 0.25, 1, or 4 mg/kg per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression
and reductions in rectal temperature and pulse rate.
In a two-year study, dogs were given amitraz at doses of 0, 0.1,
0.25, or 1 mg/kg bw per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression.
In two 90-day studies, the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine was administered to rats at
doses of 0, 0.25, 1, 3, or 12 mg/kg bw per day by gastric intubation
or to dogs at 0, 0.1, 0.25, or 1 mg/kg bw per day by gelatine
capsules. The NOAEL was 1 mg/kg bw per day in rats, on the basis of
reduced body-weight gain and increased organ weights, and 0.1 mg/kg bw
per day in dogs, on the basis of central nervous system depression and
lowered body temperature.
In a 21-day study in rats and a 90-day study in dogs, the amitraz
metabolite 4-amino- meta-toluic acid was given at doses of 0, 40,
100, or 250 mg/kg bw per day by gastric intubation to rats or 0, 16,
40, or 100 mg/kg bw per day by gelatin capsules to dogs. The NOAEL was
250 mg/kg bw per day in rats and 100 mg/kg bw per day in dogs, (the
highest doses tested).
In an 80-week study, mice were fed diets containing 0, 25, 100,
or 400 ppm, equivalent to 0, 3.8, 15, and 60 mg/kg bw per day (wrongly
given as 25, 100, or 400 mg/kg bw per day in the 1980 JMPR report).
The NOAEL for carcinogenicity was 100 ppm, equivalent to
15 mg/kg bw per day, on the basis of an increased incidence of
lymphoreticular tumours in females at 400 ppm.
In a two-year study in which mice were fed diets providing 0, 25,
100, or 400 ppm, the NOAEL for carcinogenicity was 100 ppm, equal to
11 mg/kg bw per day, on the basis of the occurrence of hepatocellular
carcinoma in females at 400 ppm. This dose was considered to be
greater than the conventional maximum tolerated dose. The NOAEL for
toxicity was 25 ppm, equal to 2.3 mg/kg bw per day, on the basis of
generalized toxic effects.
In a two-year study, rats were fed diets containing 0, 15, 50, or
200 ppm. The NOAEL for toxicity was 50 ppm, equal to 2.5 mg/kg bw per
day, on the basis of effects on the central nervous system and reduced
overall body-weight gain in males. There was no evidence of
carcinogenicity.
The genotoxic potential of amitraz has been adequately evaluated
in a range of assays in vitro and in vivo. The Meeting concluded
that amitraz is not genotoxic.
In view of the lack of genotoxicity and the finding of tumours
only in mice and only at concentrations at which severe toxicity was
observed, the Meeting concluded that amitraz is not likely to pose a
carcinogenic risk to humans.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 15, 50, or 200 ppm, the NOAEL was 50 ppm,
equal to 4.4 mg/kg bw per day, for maternal toxicity and 15 ppm, equal
to 1.3 mg/kg bw per day for developmental toxicity. No teratogenic
effect was observed.
In two studies of developmental toxicity, pregnant rats were
given amitraz at 0, 1, 3, or 12 mg/kg bw per day by gavage on days
8-20 of gestation or 0, 7.5, 15 or 30 mg/kg bw per day by gavage on
days 6-15. The NOAEL for maternal toxicity was 7.5-12 mg/kg bw per
day, and that for developmental toxicity was 3-7.5 mg/kg bw per day.
In two studies of developmental toxicity in rabbits, amitraz was
given at doses of 0, 1, 5, or 25 mg/kg bw per day by gavage on days
6-18 of gestation or 0, 3, 6, or 12 mg/kg bw per day by gavage on days
7-19. The NOAEL for maternal toxicity was 25 mg/kg bw per day in one
study, but a NOAEL was not identified in the other because all of the
treated animals died. The NOAEL for developmental toxicity was 3-6
mg/kg bw per day.
The effect of amitraz on the estrus cycle and hormone levels was
evaluated in mice fed diets containing 0, 25, 100, or 400 ppm for 28
weeks and in rats fed diets containing 0 or 200 ppm for 18 weeks. In
mice, pro-estrus was prolonged at 400 ppm; blood levels of
progesterone were reduced and those of dehydroepiandrosterone were
increased at 100 and 400 ppm. The NOAEL was 25 ppm, equivalent to
3.8 mg/kg bw per day, on the basis of the changed hormone levels. The
estrus cycles were longer in treated than in control rats, with no
NOAEL.
In a double-blind, randomized, cross-over study of tolerance, six
healthy adult male volunteers received sequential single oral doses of
0, 0.063, and 0.13 mg/kg bw amitraz, two to three weeks apart. The
NOAEL was 0.13 mg/kg bw, the highest dose tested.
Two human volunteers who received single oral doses of 0.25 mg/kg
bw 14C-amitraz showed effects including drowsiness, disorientation,
slurred speech, and decreased pulse rate and blood pressure.
In a double-blind cross-over study of tolerance, six adult
volunteers received two single doses of placebo or 2 mg (about 0.03
mg/kg bw) of the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine one week apart. The NOAEL was
0.03 mg/kg bw, the only dose tested.
The Meeting established an ADI of 0-0.01 mg/kg bw on the basis of
the NOAEL of 1.3 mg/kg bw per day in the study of reproductive
toxicity in rats and a safety factor of 100. The pharmacological
effects on the central nervous system seen in dogs, with a NOAEL of
0.25 mg/kg bw per day, were considered not to be relevant for setting
the ADI because they were reversible and the dogs became tolerant.
Moreover, a NOAEL of 0.13 mg/kg bw per day was seen for such effects
in humans.
The Meeting established an acute RfD of 0.01 mg/kg bw, on the
basis of the NOAEL of 0.13 mg/kg bw per day in the study in humans and
a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 2.3 mg/kg bw per day (toxicity in a
two-year study of carcinogenicity)
Rat: 3 mg/kg bw per day (toxicity in a 90-day study of
toxicity)
50 ppm, equal to 2.5 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
50 ppm, equal to 4.4 mg/kg bw per day (maternal
toxicity in a three-generation study of reproductive
toxicity)
15 ppm, equal to 1.3 mg/kg bw per day (developmental
toxicity in a three-generation study of reproductive
toxicity)
12 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
5 mg/kg bw per day (developmental toxicity)
Dog: 0.25 mg/kg bw per day (toxicity in a two-year study of
toxicity)
Human: 0.13 mg/kg bw (toxicity after single oral doses)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies to further characterize the effects on the
reproductive system of female rodents
2. Further observations in humans
List of end-points relevant for setting guidance values for dietary
and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete
Distribution Liver, adrenals, eyes
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 80-100% in 96 h
Metabolism in animals Metabolites same in rodents, dogs,
humans
Toxicologically significant
compounds (animals, plants, N-Methyl-N'-(2,4-xylyl)formamidine
and environment)
Acute toxicity
Rat: LD50 oral 600 mg/kg bw
Rabbit: LD50 dermal > 200 mg/kg bw
Rat: LC50 inhalation 65 mg/L
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing (Buehler test)
Short-term toxicity
Target/critical effect Central nervous system depression, dog
Lowest relevant oral NOAEL 0.25 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 50 mg/kg bw per day
Lowest relevant inhalation Rat: 0.01 mg/L air
NOAEL
Genotoxicity Unlikely to be genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Lymphoreticular tumours, hepatocellular
carcinomas
Lowest relevant NOAEL Mouse: 11 mg/kg bw per day (80-week and
2-year studies)
Carcinogenicity Unlikely to be carcinogenic
Reproductive toxicity
Reproduction target/critical Decrease in number of young alive
effect at 21 days
Lowest relevant reproductive Rat: 1.3 mg/kg bw per day
NOAEL (developmental toxicity)
Developmental target/critical Reduced fetal weight
effect
Lowest relevant developmental Rat: 3 mg/kg bw per day
NOAEL (developmental toxicity)
Neurotoxicity/Delayed Acute central nervous system
neurotoxicity depression
Other toxicological studies Prolongation of estrus cycles and
reduction of blood concentrationof
progesterone (mouse, rat)
Medical data Central nervous system depression in
two volunteers after a single oral dose
of 0.25 mg/kg bw
Summary Value Study Safety
factor
ADI 0-0.01 mg/kg bw Reproductive toxicity, 100
rat
Acute reference 0.01 mg/kg bw Single oral dose in six
dose volunteers 10
References
Berczy, Z.S., Binns, R. & Newman, A.J. (1972) Acute inhalation
toxicity to the rat of BTS 27419. Unpublished report No. 4971/72/406
from Huntingdon Research Centre Ltd, Huntingdon, Cambridge-shire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. & Whitfield,
R.A.S. (1973) Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report No. 5454/72/850 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brown, D.J., Burnett, R., Crowley, J., Cryer, P.C., Lancaster, M.C. &
Turnbull, G.J. (1978) Amitraz: Investigation of effects on the thymus
gland and oestrous cycle in mice. Unpublished report No. TX 78037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brooker, P.C., Akhurst, L.C. & Gray, V.M. (1988) Technical amitraz,
metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. SMS 127/881149 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Burnett, R., Crowley, J., Lessel, B., Patton, D.S.G., Sutton, M.M. &
Turnbull, G.J. (1976) BTS 27419: 80-week carcinogenicity study in
mice--final report. Unpublished report No. TX 76039 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1981) Excretion and residues of
14C-amitraz in male and female rats given a single oral dose of 10
mg/kg. Unpublished report No. METAB/81/20 from FBC Ltd, Chesterford
Park Research Station, Saffron Walden, Essex, United Kingdom.
Submitted to WHO by Schering Agro-chemicals Ltd.
Campbell, J.K. & Needham, D. (1983) Excretion and tissue residues of
14C amitraz in male and female mice given a single oral dose of 10 mg
14C-amitraz/kg bodyweight. Unpublished report No. METAB/83/34 from
FBC Ltd, Chesterford Park Research Station, Saffron Walden, Essex,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984a) Dermal absorption of 14C-amitraz
in EC formulation by male and female pigs given a single topical
application of 18 mg A.I. Unpublished report No. METAB/84/9 from FBC
Ltd, Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. Needham, D. (1984b) Excretion and tissue residues of
14C-amitraz in a male and a female baboon given a single oral dose of
10 mg 14C-amitraz per kg bodyweight. Unpublished report No.
METAB/83/44 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984c) Urinary excretion of 14C-amitraz
by two male humans following a single oral dose of 0.25 mg/kg
bodyweight. Unpublished report No. METAB/84/10 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984d) The metabolism of 14C-amitraz by
male and female rats. Unpublished report No. METAB/84/2 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984e) A comparison of the metabolism of
14C-amitraz in rat, mouse, baboon and human. Unpublished report No.
METAB/84/1 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Cass, L.M.R. (1992) Report of a double blind tolerance study of
amitraz in six adult healthy volunteers. Unpublished report No.
TOX/92/179-209 from Simbec Research Ltd, Merthyr Tydfil,
Mid-Glamorgan, Wales. Submitted to WHO by Schering Agrochemicals Ltd.
Challis I.R. (1990) Dermal absorption of amitraz in the rat.
Unpublished report No. TOX/90/179-178 from Schering Agrochemicals Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Chesterman, H., Skerrett, K., Street, A.E. & Cherry, C.P. (1973) Boots
27 271, oral toxicity study in beagle dogs (repeated dosage for 13
weeks). Unpublished report No. BTS 38/73511 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO
by Schering Agrochemicals Ltd.
Clouzeau, J. (1992) Technical amitraz: Guinea pig sensitization study
(Buehler test). Unpublished report No. TOX 91/179-203 from Centre
International de Toxicologie, Miserey, Evreux, France. Submitted to
WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Gibson, W.A. & Prentice, D.E.
(1981) Amitraz toxicity to B6C3F1 mice by dietary administration for
13 weeks. Unpublished report No. BTS 158/80759 from Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Street, A.E., Gibson W.A. &
Gopinath, C. (1983) Amitraz, 104 week tumorigenicity study in mice.
Unpublished report No. BTS 153/8262/A from Huntingdon Research Centre
plc, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agro-chemicals Ltd.
Cullen, L.K. & Reynoldson, J.K. (1983) Effects of amitraz on the
cardiovascular and respiratory systems in the dog. Unpublished report
No. T242 from School of Veterinary Studies, Murdoch University,
Murdoch, Western Australia. Submitted to WHO by Schering Agrochemicals
Ltd.
Everest, R.P. (1976) BTS 27 419: Mutagenicity study in the male mouse
perivisceral cavity host-mediated assay. Unpublished report No. TX
76056 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Everest, R.P. & Wilcox, P. (1976) BTS 27 419: Mutagenicity testing
against Salmonella typhimurium strains TA1535, TA1537 and TA1538 in
the presence and absence of liver microsomes from male and female
mice. Unpublished report No. TX 76057 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Groppi, L. (1977) Medical report on a patient who ingested Mitac
(Edrizar) in Italy in 1977. Report No. T84 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Hall, J.E., Ahluwalia, H. & Hampson, P. (1975) A study of the effects
of oral administration of a metabolite (BTS 27 271) of amitraz to
volunteers. Unpublished report No. MS 75002 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hornish R.E. & Nappier, J.M. (1983) The absorption, metabolism, and
excretion of Mitaban(R) (U-36,059) in the dog from oral and dermal
exposure. Unpublished report No. 527-9760-83-001 from Agricultural
Research and Development Laboratories, The Upjohn Company, Kalamazoo,
Michigan, USA. Submitted to WHO by Schering Agrochemicals Ltd.
Hounsell, I.A.G & Walker, A.K. (1983) A micronucleus study in mice
using BTS 24868 (2,4-dimethylaniline). Unpublished report No.
TOX/84/179-97 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hounsell, I.A. & Rush, K.C. (1984) Technical amitraz: The effect of
dietary administration on the oestrus cycle and hormones in the mouse.
Unpublished report No. TOX/84/179-97 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Hovda, L.R. & McManus, A.C. (1993) Yohimbine for treatment of amitraz
poisoning in dogs. Vet. Hum. Toxicol., 35, 329.
Hsu, W.H. & Kakuk, T.J. (1984) Effect of amitraz on heart rate and
pupil diameter in rats: Mediated by alpha2-adrenoceptors. Toxicol.
Appl. Pharmacol., 73, 411-415.
Hsu, W.H., Lu, Z.-X. & Hembrough, F.B. (1986) Effect of amitraz on
heart rate and aortic blood pressure in conscious dogs: Influence of
atropine, prazosin, tolazoline, and yohimbine. Toxicol. Appl.
Pharmacol., 84, 418-422.
Kakuk, T.J. (1979) Pathological findings on the matched control and
amitraz 400 ppm females from the Boots 80 week mouse study by various
pathologists with commentary. Unpublished report No. 315-78-9610-005
from Agricultural Research and Development Laboratories, The Upjohn
Company, Kalamazoo, Michigan, USA. Submitted to WHO by Schering
Agrochemicals Ltd.
Kynoch, S.R. & Parcell, B.I. (1988) Technical amitraz: Assessment of
delayed contact hypersensitivity in the guinea-pig. Unpublished report
No. TOX 88/179-145 from Huntingdon Research Centre Ltd, Huntingdon,
Cambridgeshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Lancaster, M.C. & Williams, G.A.H. (1978) Amitraz: Multigeneration
feeding test in rats -- Histopathology. Unpublished report No. TX
78064 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Lewis, D.K. (1971) Fate of [14C]-BTS 27419 applied to rats as a
single oral dose. Unpublished report No. C 71011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987a) Technical amitraz: Irritant
effects on rabbit skin. Unpublished report No. TOX/87/179-137 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987b) Technical amitraz: Irritant
effects on the rabbit eye. Unpublished report No. TOX/87/179-142 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Printice, R.D. (1983) Technical amitraz: Ames
bacterial mutagenicity test. Unpublished report No. 2590 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983a) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. 2669 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by Schering
Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983b) Technical amitraz: Unscheduled
DNA synthesis in human embryonic cells. Unpublished report No. 2634
from Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1984) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. TOX/84/179-97 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B., Brown, A.G. & Riach, G.G. (1984) Technical BTS 24868:
Induction of cell transformation in C3H10T1/2 cells. Unpublished
report No. TOX/84/179-97 from FBC Ltd, Chesterford Park Research
Station, Saffron Walden, Essex, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
McIntyre, M. (1987a) Technical amitraz: Teratogenicity study in the
rat. Unpublished report No. 5562-194/11 from Hazleton UK, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
McIntyre, M. (1987b) Technical amitraz: Teratogenicity study in the
rabbit. Unpublished report No. 5536-194/13 from Hazleton UK,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Merryman, D.C. & Sutton, M.M. (1972) Effects on the oestrus cycle of
the rat. Unpublished report No. PM 72003 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. (1973) BTS 27 271: Acute oral toxicity study in dogs.
Unpublished report No. TX 73004 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. & Williams, G.A.H. (1974) BTS 27 271: Acute oral toxicity
study in dogs--Histopathology. Unpublished report No. TX 73 030 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Morgan, H.E., Patton, D.S.G. & Turnbull, G.J. (1973) BTS 27 419:
Two-year oral toxicity study in dogs. Unpublished report No. TX 73035
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Morgan, H.E., Shepherd, G.M. & Turnbull, G.J. (1974) BTS 28 369:
90-day oral toxicity study in dogs. Unpublished report No. TX 74037
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Moser, V.C. & MacPhail, R.C. (1985) Yohimbine attenuates the delayed
lethality induced in mice by amitraz, a formamidine pesticide.
Toxicol. Lett., 28, 99-104.
Needham, D. (1984) The effect of Amitraz on the hepatic mixed-function
oxidase system of male and female mice following oral administration.
Unpublished report No. METAB/84/8 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Needham, D. & Hemmings, P.A. (1988) The metabolism and distribution of
amitraz residues in the laying hen following the daily oral
administration of 24.5 mg 14C-amitraz/per bird. Unpublished report
No. Envir/88/6 from Schering Agrochemicals Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977a) Dominant lethal assay of amitraz in
the female mouse. Unpublished report No. BTS 81/7758 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977b) Dominant lethal assay of amitraz in
the male mouse. Unpublished report No. BTS 80/7792 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Parkinson, R. (1974) The effects of BTS 27 271, BTS 27 419 and BTS 21
103 (chlordimeform) on the pressor response to tyramine in the pithed
rat. Unpublished report No. P74 032 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Parkinson, R. & Sim, M.F. (1970) Some pharmacological effects of the
acaricide RD 27,271 and related compounds. Unpublished report No.
P70508 from The Boots Pure Drug Company, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Patton, D.S.G. (1973) BTS 27419: Acute toxicity in baboons.
Unpublished report No. TX 73002 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Patton, D.S.G & Sutton, M.M. (1971) Acute toxicity studies on BTS 27
419, an acaricide. Unpublished report No. P71544 from The Boots Pure
Drug Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Patton, D.S.G. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in dogs. Unpublished report No. P71547 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Petzold, G.L., Swenberg, J.A. & Bedell, M. (1977) Evaluation of
amitraz (U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915A
and U-54,914) in the DNA damage/alkaline elution assay. Unpublished
report No. 7268/77/7268/001 from The Boots Company Ltd, Nottingham,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Phillips, M.W.A., Swalwell, L.M. & Needham, D. (1987) Identification
of metabolites of amitraz in the milk and meat of a cow dosed for 4
days with amitraz. Unpublished report No. Envir/87/46 from Schering
Agrochemicals Ltd, Chesterford Park Research Station, Saffron Walden,
Essex, United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Pronczuk, J., Heuhs, L., Scaiola, G., Bogdan, M. & Fogel de Korc, E.
(1995) Clinical cholinergic presentation of acute amitraz poisoning.
Report No. T368 from Department of Toxicology, Hospital de Clinicas,
Montevideo, Uruguay. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983a) Technical BTS 27271,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61A/83580
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983b) Technical BTS 27919,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61B/83581
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Ros, J.J.W. & van Aken, J. (1994) A case of poising with amitraz, an
agricultural pesticide. Ned. Tijdschr. Geneeskd., 138, 776-778.
Shaw, J.W. (1971) BTS 27 419 -- Acute intraperitoneal toxicity to
rats. Unpublished report No. PM 71057 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. (1973a) BTS 27 419: Comparison of the acute oral and
intraperitoneal toxicities to rats. Unpublished report No. TXM 73006
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1973b) BTS 27 419: Comparison of the acute oral toxicities
to rats of BTS 27 419 and BTS 27 919. Unpublished report No. TXM 73010
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1975) BTS 27 419 metabolite: 21 Day chronic oral toxicity
in rats of BTS 28 369. Unpublished report No. TX 75058 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973a) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to mice. Unpublished report No. TXM 73 036 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973b) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to rats. Unpublished report No. TXM 73 037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, G.A.H. (1975) BTS 27 419 metabolite: 90 day
chronic oral toxicity in rats of BTS 27 271. Unpublished report No. TX
75059 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Somerville, L. (1973) Fate of 14C-BTS 27419 administered to rats in
repeated oral doses. Unpublished report No. AX 73011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Stewart, F.P. (1993) (14C)-Amitraz: Dermal absorption in the rat.
Unpublished report No. 194/69-1011 from Hazleton Europe, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. (1970a) RD 27,271. Acute oral toxicity to mice.
Unpublished report No. PM 70043 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agro-
chemicals Ltd.
Sutton, M.M. (1970b) RD 27,271 Acute toxicity to rats. Unpublished
report No. PM 70042 from The Boots Pure Drug Company, Nottingham,
United Kingdom. Submitted to WHO by Schering Agro-chemicals Ltd.
Sutton, M.M. (1971) BTS 27 419. Contact sensitisation in the guinea
pig. Unpublished report No. PM 71919 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973a) BTS 27 419: Three week dermal toxicity to
rabbits. Unpublished report No. TX 73 026 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973b) BTS 27 419: Multigeneration feeding test in rats.
Unpublished report No. TX 73 036 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973c) BTS 27 419: Teratogenicity in the rat.
Unpublished report No. TX 73028 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973d) BTS 27 419: Teratogenicity in the rabbit.
Unpublished report No. TX 73029 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. & Offer, J. (1973) BTS 27 419: Carcinogenicity and
long-term toxicity study in rats. Unpublished report No. TX 73043 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Sutton, M.M. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in rats. Unpublished report No. P71548 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. & Williams, P.A. (1972) BTS 27 419: Acute dermal toxicity
to rabbits. Unpublished report No. YM 72 011 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Wilcox, P. (1976) BTS 27 419: Mutagenicity study in the
intraperitoneal host-mediated assay. Unpublished report No. TX 76028
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yim, G.K.W., Holsapple, M.P., Pfister, W.R. & Hollingworth, R.M.
(1978) Prostaglandin synthesis inhibited by formamidine pesticides.
Life Sci., 23, 2509-2516.
 In the study of Challis (1990), described above, the metabolic
profile of rats treated dermally was similar to that of rats treated
orally.
In the study of Hornish & Nappier (1983), described above, the
metabolism of amitraz was essentially the same after oral and dermal
administration. 4-Formamido- meta-toluic acid was the predominant
residue in both blood and urine. The parent compound and the first
hydrolysis products, N-methyl- N'-(2,4-xylyl)formamidine and
form-2',4'-xylidide, were not observed at measurable concentrations in
either blood or urine.
One cow received 0.5 mg/kg bw per day 14C-amitraz (specific
activity, 4.2 mCi/g; radiochemical purity, > 98.8%) by capsule twice
daily for four days. All milk was collected, and samples of urine were
taken. The cow was killed 17 h after the final dose. The urinary
metabolites corresponded to 4-acetamido- meta-toluic acid and
4-formamido- meta-toluic acid, which were converted to
4-amino- meta-toluic acid by acid hydrolysis. The total residue in
milk accounted for only 0.2% of the dose. The greatest concentration
of residue (0.07 ppm) was found after the final dose. The residue was
extracted with methanol (recovery, 90%), and the metabolites present
identified by co-chromatography as 4-acetamido- meta-toluic acid,
4-formamido- meta-toluic acid, and 4-amino- meta-toluic acid (23%);
form-2',4'-xylidide (9%); polar material that was converted to
4-amino- meta-toluic acid by acid hydrolysis (34%); and low-polarity
material (24%). The latter broke down readily to form-2',4'-xylidide
under neutral or basic conditions; it was not present in acetonitrile
extracts of whole milk and was considered to be an artefact produced
by the methanol Soxhlet extraction of the milk. 2,4-Dimethylaniline
was not observed. The edible tissue with the highest concentration of
residue was the liver (3.7 ppm).
Methanol Soxhlet extraction released about 60% of the residue,
and the remainder was solubilized after enzymic digestion and acid
hydrolysis. The residues were identified by co-chromatography as
4-acetamido- meta-toluic acid and 4-formamido- meta-toluic acid
(14%), 4-amino- meta-toluic acid (15%), form-2',4'-xylidide (10%),
and polar material (10%) which on acid hydrolysis was converted to
4-amino- meta-toluic acid and additional low-polarity material. Other
polar material (29% of the residue) was not amenable to
chromatography. 2,4-Dimethylaniline was not observed. The low-polarity
material (14%) broke down on neutral or basic thin-layer
chromatography to a range of compounds, suggesting that it was a
product of condensation between 4-amino- meta-toluic acid and
compounds released from acid-labile conjugates. When the
non-methanol-extractable residue was fed to a dog, analysis of urine
gave similar results to those after enzymic digestion (Phillips et
al., 1987).
Six laying hens (Gallus gallus domesticus) were each dosed
orally with 24.5 mg 14C-amitraz (specific activity, 0.14 mCi/g;
radiochemical purity, > 99%) per day for four days, providing a dose
2200 times greater than the expected daily exposure of birds fed
cotton-meal derived from amitraz-treated plants. The hens were killed
4 or 12 h after the final dose, and the concentrations and nature of
radioactive residues in tissues were determined. The radiolabel was
rapidly excreted, with 68% in 0-24-h excreta. The main route of
metabolism was via 4-amino- meta-toluic acid, since 75% of the
metabolites extracted from the excreta could be accounted for as
either this metabolite or its acid-labile conjugates. The highest
concentrations of tissue residues were found in the liver and kidney
(17 and 25 mg/kg respectively) 4 h after the fourth dose, but these
concentrations had fallen to 12 and 10 mg/kg, respectively, by 12 h
after dosing. The major metabolite detected in the liver was
4-amino- meta-toluic acid, present as both free acid and labile
conjugates, which represented 55% of the total residue.
N-Methyl- N'-(2,4-xylyl)formamidine (4%), form-2',4'-xylidide (4%)
and 2,4-dimethylaniline (2%) were also present. A mixture of at least
seven highly polar compounds, believed to be mono- and di-acids
similar to those previously seen in rats, was a further substantial
residue (27%). Unextractable fibre-bound material represented 2% of
the residue. The concentrations in fat, muscle, and skin represented
0.6-2.7 mg/kg 4 and 12 h after the final dose. In fat, the residues
consisted of form-2',4'-xylidide (42%),
N-methyl- N'-(2,4-xylyl)formamidine (24%), unchanged amitraz (21%),
2,4-dimethylaniline (3%), and 4-amino- meta-toluic acid (3%). In
muscle, 81% of the residues were identified as 4-amino- meta-toluic
acid or its acid-labile conjugates; form-2',4'-xylidide was present as
7% of the residue.
The concentrations of amitraz residues in eggs, collected daily,
were 0.28-0.46 mg/kg throughout the study and did not increase over
the four days of dosing. The residue concentrations in the yolks rose
from 0.1 to 1.4 mg/kg during the study;
N-methyl- N'-(2,4-xylyl)formamidine (54%) and 4-amino- meta-toluic
acid (34%) were the major metabolites. 4-Amino- meta-toluic acid
accounted for 91% of the residue in the egg white as both free acid
and labile conjugates, and form-2',4'-xylidide accounted for 4% of the
residue (Needham & Hemmings, 1988).
Separately reported data on the metabolism of amitraz
in various species were compared. After a single oral dose of
14C-amitraz (specific activity, 9 mCi/g; radiochemical
purity, > 95%) to groups of four male and four female mice,
three male and three female rats, one male and one female
baboon, and two men, the administered radiolabel was rapidly excreted.
In all species examined, urine was the major route of excretion,
accounting for 65-84% of the dose (55-76% within the first 24 h).
Analysis of urine obtained from volunteers dosed with 14C-amitraz
indicated that the metabolism of amitraz was qualitatively similar
to that in the other species. The major metabolites were
4-formamido- meta-toluic acid, 4-acetamido- meta-toluic acid and
N-methyl- N'-(2,4-xylyl)formamidine. In addition, 40-60% of the
metabolites excreted in urine was accounted for by a polar fraction
containing conjugates of 4-formamido- meta-toluic acid and
4-acetamido- meta-toluic acid. The minor metabolites included
4-amino- meta-toluic acid and form-2',4'-xylidide. Excretion of
N-methyl- N'-(2,4-xylyl)formamidine was dose-dependent. The
metabolites identified in the volunteers were
4-formamido- meta-toluic acid plus 4-acetamido- meta-toluic acid
(27% of the total radiolabel in urine),
N-methyl- N'-(2,4-xylyl)formamidine (6%), 4-amino- meta-toluic
acid (4%), form-2',4'-xylidide (4 %), the product of acid hydrolysis
of N-methyl- N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
(1%), and polar material (57%). In both rats and mice, increasing the
dose of amitraz from 1 to 100 mg/kg bw increased the excretion of
N-methyl- N'-(2,4-xylyl)formamidine from approximately 5 to 30% of
the total excretion. Prior dosing of five male and five female mice
with amitraz in the diet at 100 ppm for three weeks, followed by
400 ppm for a further three weeks, had no effect on the metabolism of
a single oral dose of 14C-amitraz (Campbell & Needham, 1984e).
(c) Effects on enzymes and other biochemical parameters
Groups of 18 male and 18 female B6C3F1 mice were dosed orally
with corn oil or amitraz at 100 mg/kg bw per day for two days and,
because of toxic symptoms, at 50 mg/kg bw per day for the following
two days. The animals were then killed, and the livers were assayed
for the activity of microsomal oxidases. A significant increase in
liver weight and in the activity of cytochrome b5 were recorded. The
effect did not appear to be related to increased activity of the
hepatic mixed-function oxidase system, since no significant increase
was seen in the activities of cytochrome P450, aniline hydroxylase, or
para-nitroanisole demethylase or in the concentration of microsomal
protein (Needham, 1984).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of amitraz and its metabolites (purity
unspecified) has been investigated in several species (Table 1). The
toxic signs after oral administration of amitraz to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Rats had intestinal
irritation and bladder distension. The lowest effective doses were 400
mg/kg bw in mice and 200 mg/kg bw in rats. Dermal application of 1600
mg/kg bw to rats had no local or systemic effect. Guinea-pigs were
hyperexcitable after receiving 400 mg/kg bw or more. Rabbits had
central nervous system depression, decreased rectal temperature, pulse
rate, and respiration, nasal discharge, and rales after receiving 100
mg/kg bw, with complete recovery by 48 h. The reactions of dogs to
administration of 100 mg/kg bw and, to a lesser extent, 20 mg/kg bw,
were central nervous system depression, ataxia, muscular weakness,
muscular spasm, uncontrolled vocal spasm and micturition, and
decreased rectal temperature and pulse rate. Haemoconcentration and
increased blood sugar, urea nitrogen, and potassion concentrations
were also seen. No specific pathological lesions were induced. The
lowest dose, 4 mg/kg bw, affected temperature and pulse rate only
slightly . The profile of toxic reactions was consistent with
depression of hypothalamic function. All of the effects were
reversible.
Table 1. Acute toxicity of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
Mouse Oral > 1600 Patton & Sutton (1971)
Rat Oral 600 Patton & Sutton (1971);
Shaw (1973a)
Rat Dermal >1600 Patton & Sutton (1971)
Rat Intraperitoneal 800 Shaw (1971, 1973a)
Rat Inhalation (6 h) 65 Berczy et al. (1972)
Guinea-pig Oral 400-800 Patton & Sutton (1971)
Rabbit Oral > 100 Patton & Sutton (1971)
Rabbit Dermal > 200 Sutton & Williams (1972)
Dog Oral 100 Patton & Sutton (1971)
Baboon Oral 100-250 Patton (1973)
The dermal irritation potential of amitraz (purity, 99%) was
studied in six New Zealand white rabbits weighing 1.9-2.5 kg.
Approximately 24 h after hair had been removed from the dorso-lumbar
region, 0.5 g of technical-grade amitraz was applied under a 2.5-cm
gauze pad moistened with 0.5 ml distilled water, and each treatment
site was occluded with an elastic adhesive dressing for 4 h. At the
end of the exposure period, the dressing was removed and the treatment
site was washed with water. The treated skin was examined on days 1,
2, 3, and 4 after treatment. There was no response to treatment
(Liggett & Smith, 1987a).
The ocular irritation potential of amitraz (purity unspecified)
was studied in six New Zealand white rabbits weighing 2.1-2.8 kg. The
eyes of each animal were examined before instillation of 45 mg (0.1 ml
crystalline powder) of technical-grade amitraz (purity, 98.4%) into
one eye. Both eyes were examined 1 h before and 1, 2, 3, 4, and 7 days
after instillation. Only minimal or slight conjunctival irritation was
observed (Liggett & Smith, 1987b).
Groups of 12 guinea-pigs weighing 300-350 g were treated daily
with 0.1 ml of acetone or 10% amitraz (purity unspecified) or with 1%
dinitrochlorobenzene on the outer surface of each ear for three days.
One week after the start of treatment, the backs and flanks were
clipped and treated with challenge doses of 0.2 ml acetone, amitraz
(10%), or dinitrochlorobenzene (0.25%). The degree of erythema was
assessed after 24 h. Sensitizing activity was observed after treatment
with the positive control but not with amitraz (Sutton, 1971).
The dermal sensitizing effect of amitraz was studied in the
Buehler test. During induction, five male and five female guinea-pigs
received 500 mg technical-grade amitraz (purity, 99%) on the anterior
right flank for 6 h under a dry compress on days 1, 8, and 15. A dry
compress alone was applied on the anterior left flank. After a
two-week rest period, each animal received a challenge of 500 mg
technical-grade amitraz to the right flank and a dry compress alone to
the left flank. The cutaneous reactions were evaluated 24 and 48 h
after challenge. The animals were then sacrificed, and cutaneous
samples were taken from the challenge site in all animals. No clinical
signs or deaths related to treatment were observed, and no cutaneous
reactions were found 24 and 48 h after the challenge with amitraz
(Clouzeau, 1992).
Delayed contact hypersensivity in response to amitraz (purity,
98.4%) was tested in the maximization test described by Magnusson and
Kligman. After random allocation of 20 female guinea-pigs to a control
group and 20 to a test group, the animals were induced by intradermal
injection of 5% w/w amitraz in Alembicol D (a coconut oil) into a
4 × 6-cm clipped area of the dorsal skin on the scapular region. One
week after the injections, the same area was clipped again and given a
topical application of 15 or 30% w/w amitraz in Alembicol D on a
2 × 4-cm patch saturated with the test solution, which was placed on
the skin, covered with impermeable plastic adhesive tape, secured by
an elastic adhesive bandage, and left in place for 48 h. The test and
control animals were challenged topically as described above, two
weeks after the induction, with 15 or 30% w/w technical-grade amitraz
in Alembicol D. The challenge sites were evaluated 24, 48, and 72 h
after removal of the patches, and an arbitrary scale of 0-4 was used
to score the reactions. The dermal reactions observed in the test
animals were more marked and persistent than those seen in the
controls (Kynoch & Parcell, 1988).
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female B6C3F1 mice were fed diets
containing 0, 100, 200, 400, 600, or 800 ppm amitraz (purity, 98.3%),
equal to 0, 13, 26, 53, 96, and 110 mg/kg bw per day for males and 0,
17, 35, 68, 110, and 150 mg/kg bw per day for females, for 13 weeks.
Clinical signs, body weight, and food consumption were recorded
throughout the study. All mice were killed at the end of treatment and
examined grossly but not histopathologically. The only overt sign of
reaction to treatment was an increase in aggressive behaviour, as
evidenced by fighting and resultant cutaneous lesions, among males
treated at doses > 400 ppm. The overall body-weight gain was
statistically significant lower in the males (40%) treated at > 400
ppm and in females (34%) at > 200 ppm. Food consumption did not
differ statistically significantly between the control and treated
groups. The NOAEL was 100 ppm, equal to 17 mg/kg bw per day, on the
basis of the overall reduced body-weight gain of females (Colley et
al., 1981).
Rats
Groups of 21 male and 21 female Ash-Wistar rats were given
amitraz (purity unspecified) suspended in 0.4% Cellosize solution by
oral intubation at a dose of 0, 3, or 12 mg/kg bw per day for 90 days.
They were then killed either immediately or after a three-week
recovery. Treatment of rats with 50 mg/kg bw per day was discontinued
after seven days because of depressed growth and behavioural
disturbances, and treatment with 200 mg/kg bw per day was discontinued
after seven days because of irritability and debilitation. Body
weights were recorded three times per week. Haematological
examinations were made on some control rats and on some rats receiving
12 mg/kg bw per day at 4, 8, and 12 weeks. At autopsy, blood was
withdrawn from the heart to estimate plasma alkaline phosphatase
activity, bilirubin, aspartate and alanine aminotransferase activity,
and sodium and potassium concentrations. Organs were weighed and
prepared for microscopic examination. Significant reductions were seen
in the overall body-weight gain (8%) and absolute (8%) and relative
weights (6%) of the liver of male rats receiving 12 mg/kg bw per day.
The NOAEL was 3 mg/kg bw per day on the basis of these last effects
(Sutton & Williams, 1971).
Groups of 12 rats (strain not given) were exposed daily for 6 h
to dusts of amitraz (purity unspecified) at a concentration of 0,
0.01, 0.1, or 1 mg/L air, on 14 days over three weeks. During exposure
to 0.1 mg/L, signs of mild dyspnoea, slight eye irritation, and
hyposensitivity to noise were recorded. After termination of exposure,
the rats were hypersensitive to touch and became aggressive. Exposure
to 1 mg/L elicited signs similar to but more severe than those seen in
the previous group. In addition, ataxia, increased nasal secretion,
polyuria, body tremors, and coma were observed in the exposed rats.
Consumption of food and water was reduced, and there was body-weight
loss. Reductions in packed cell volume, haemoglobin, red cells, and
plasma protein concentrations in blood may have been related to
treatment. The body tremors, aggressive behaviour, and coma indicate
that the central nervous system was affected (Berczy et al., 1973).
Rabbits
Groups of four to eight male and female New Zealand white rabbits
weighing 2.5-4.5 kg (the health status of which must be regarded as
suspect due to general infection) were treated on the intact skin of
the back (without occlusion) with amitraz (purity unspecified) in
acetone at a dose of 0, 50, or 200 mg/kg bw per day for a total of 15
doses over 21 days. Body weight, food consumption, heart rate, and
rectal temperature were determined. Haematological and blood
biochemical parameters and organ weights were measured, and
histopathological examinations were carried out. A transient sedative
effect and local skin reactions were seen in animals of each sex at 50
and 200 mg/kg bw per day. Body weights and food consumption were
adversely affected, and some deaths occurred that were possibly
related to treatment. Variable tubular degeneration was seen in the
testes, which were underweight. At 50 mg/kg bw per day, body weights
and food consumption were less adversely affected than at the high
dose, and the death of only one male was considered related to
treatment (Sutton, 1973a).
Dogs
Groups of two male and two female beagles received amitraz
(purity unspecified) in capsules at a dose of 0, 0.25, 1, or 4 mg/kg
bw per day for 90 days. The animals were examined clinically before
dosing began and during weeks 2, 4, 8, and 13 of dosing. Food
consumption was recorded daily and body weights weekly.
Haematological, blood biochemical, and urinary parameters were
measured before and at intervals during treatment. During week 13 of
dosing, control animals and those at 4 mg/kg bw per day were placed in
metabolism cages, and their water intake and urine excretion over 24 h
were recorded. The faeces were tested daily for occult blood during
the first week of dosing. At the end of treatment, the animals were
sacrificed, and several organs were weighed and prepared for
histological examination.
On the three first days of dosing, all four animals at 4 mg/kg bw
per day showed central nervous system depression, vomiting, ataxia,
and reduced rectal temperature and pulse rate, which recurred
consistently within 6 h. Hyperglycaemia and occasional glycosuria were
also induced. The livers were slightly enlarged and showed low-grade
microscopic changes. The dose of 1 mg/kg bw per day had similar but
less severe effects. At 0.25 mg/kg bw per day, only minor changes were
seen, although central nervous system depression was seen in one dog
on one occasion 3 h after dosing during week 8 of treatment. The NOAEL
was 0.25 mg/kg bw per day on the basis of central nervous system
depression and reduced rectal temperature and pulse rate (Patton &
Williams, 1971).
Groups of four male and four female beagles were given 0, 0.1,
0.25, or 1 mg/kg bw per day amitraz (purity unspecified) in gelatine
capsules for two years. They were examined clinically before and
immediately after dosing on the first two days and again during weeks
4, 13, 26, 39, 52, 78, and 102. Heart rate and rectal temperature were
monitored serially for up to 24 h after dosing on day l and during
weeks 39, 52, 79, and 103. Signs of toxicity and faecal appearance
were recorded daily and body weights weekly. Haematological, blood
biochemical, and urinary parameters were measured before dosing and
during weeks 4, 13, 26, 52, 78, and 102. In addition, serial blood
samples were obtained during weeks 40 and 53 for estimating blood
sugar and during weeks 91 to 96 for estimating methaemoglobin. The
animals were sacrificed and examined macroscopically on the day after
the final dose, and their organs were weighed and prepared for
histopathological examination.
All eight animals given 1 mg/kg bw per day showed signs of slight
central nervous system depression 3 h after dosing on days 1 and 2,
but all appeared normal by the following morning. On both days, one
dog had a slightly subnormal temperature (38.3°C) at 3 h, which had
returned to normal (38.7°C) by 24 h. Subsequently, all of the animals
in this group appeared clinically normal, apart from one bitch that
was slightly hypothermic 3 h after dosing during weeks 52 and 79. The
NOAEL was 0.25 mg/kg bw per day on the basis of central nervous system
depression (Morgan et al., 1973).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CFLP mice were fed diets
containing 0, 25, 100, or 400 ppm amitraz (purity unspecified),
equivalent to 3, 8, 15, and 60 mg/kg bw per day (wrongly given as 25,
100, and 400 mg/kg bw per day in reference 34, Annex 1), for 80 weeks.
Animals at 100 or 400 ppm gained less weight than the controls over
the first 40 weeks, whereas those at 25 ppm gained more weight than
the controls from week 10 onwards and showed a 20% greater body-weight
gain at the end of the experiment. Calculated over 80 weeks, food
consumption was increased for male mice at 100 and 400 ppm and female
mice at 400 ppm, and decreased for males at 25 and 100 ppm. The
survival rate was similar in all groups. An increased incidence of
lymphoreticular tumours was observed in female mice at 400 ppm (49%
compared with 23% in controls). A slight, not statistically
significant increase in the incidence of all types of liver-cell
tumour was found in animals of each sex fed 400 ppm. No other
pathological finding related to treatment was reported. The NOAEL for
carcinogenicity was 100 ppm, equivalent to 15 mg/kg bw per day, on the
basis of an increased incidence of lymphoreticular tumours in females
at 400 ppm (Burnett et al, 1976; Kakuk, 1979).
Groups of 75 male and 75 female B6C3F1 hybrid mice, 33 days old,
were given diets containing 25, 100, or 400 ppm amitraz (purity,
97.9%), equal to 2.3, 9.6, and 45 mg/kg bw per day for males and 2.6,
11, and 50 mg/kg bw per day for females, for 104 weeks. The untreated
controls consisted of 100 male and 100 female mice. Clinical signs,
food consumption, and body weight were recorded throughout the study.
Survivors were killed at the end of the dosing period and all animals
were subjected to a complete gross autopsy. All tissues were collected
from all animals and preserved for future examination.
Histopathological examination was performed on all tissues from the
high-dose group and controls, and on the liver, pancreas, spleen,
lung, stomach, pituitary, thyroid, adrenals, ovaries, uterus, sternum,
and all abnormal tissues found grossly from animals at the low and
intermediate doses.
Males at 400 ppm were hyperactive and behaved aggressively. The
incidence of cutaneous ulceration and inflammation of the perigenital
and perianal areas was greater in mice at 100 and 400 ppm than in
those at 25 ppm or in controls, and the incidence of urogenital
swelling was greater in all treated mice than in controls. The
incidences of gross adverse effects in treated females were comparable
to the control incidences. There was clearly increased mortality of
males at 400 ppm (20/75) and of males and females combined (37/150).
Mean body-weight gain was reduced in mice at 400 ppm (by 30-50%)
throughout the study, with a significant decrease in females at 100
ppm over the last 74 weeks of the study. Food consumption was
marginally reduced initially in animals at 100 and 400 ppm but was
comparable to that of controls from week 25 to termination. Increased
liver and lymph node involvement was seen in males and females at 400
ppm, and the incidence of preputial gland enlargement in males at 400
ppm was greater than that in controls (20/75 vs 12/100). The
prominence of the limiting ridge of the stomach was greater than in
controls for females at all doses and for males at 100 and 400 ppm.
Decreased production of myeloid elements accompanied by an increase in
erythroid elements resulted in a significant decrease in the
myeloid:erythroid ratio for males at 400 ppm and females at 100 or
400 ppm. The incidences of spleen haematopoiesis in males and of
stomach focal hyperkeratosis in males and females at all three doses
were greater than that in controls. There was apparent liver
involvement in females, with an increased incidence of hepatocellular
carcinoma at 400 ppm (15/75 vs 2/100 hepatocarcinoma; 16/75 vs 4/100
hepatocellular adenoma). This was accompanied by a dose-related
increase in the incidence of hyperplastic nodules and telangiectatic
and basophilic foci of the liver in animals at 100 and 400 ppm. Males
at 400 ppm also had an apparent increase in the incidence of
hyperplastic nodules and telangiectatic and basophilic foci of the
liver. The NOAEL for carcinogenicity was 100 ppm, equal to 11 mg/kg bw
per day, on the basis of hepatocellular carcinoma in females at
400 ppm. This dose was considered to be greater than the conventional
maximum tolerated dose. The NOAEL for long-term toxicity was 25 ppm,
equal to 2.3 mg/kg bw per day, on the basis of general toxicity
(Colley et al., 1983).
Rats
Groups of 40 male and 40 female Ash-Wistar rats received amitraz
(purity, 97.8%) at a dietary concentration of 0, 15, 50, or 200 ppm
(equal to 0, 0.77, 2.5, or 10 mg/kg bw per day in males and 0, 0.97,
3.1, or 13 mg/kg bw per day in females) for two years. Body weights
were measured twice per week during the period of maximal growth and
later weekly. Food consumption was determined weekly during this
growth period and later monthly. Haemato-logical, biochemical, and
urinary analysis were performed during and at the end of the study,
and organs were weighed and examined grossly and histologically at the
end of study.
Rats at 200 ppm were occasionally nervous, excitable, and
aggressive, and their food consumption was temporarily reduced. The
overall body-weight gain of males was significantly reduced (10%).
Rats at 50 and 15 ppm showed no adverse reactions to treatment. The
incidence, type, and time to appearance of tumours were not
significantly different in treated and control groups. The NOAEL for
toxicity was 50 ppm, equal to 2.5 mg/kg bw per day, on the basis of
effects on the central nervous system and reduced overall body-weight
gain in males (Sutton & Offer, 1973).
(d) Genotoxicity
The results of tests for the genotoxicity of amitraz are
summarized in Table 2. The results of the tests in vitro and
in vivo were negative.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a three-generation study of reproductive toxicity, groups of
10 male and 20 female newly weaned Boots-Wistar rats were fed amitraz
(purity, 99.8%) at a dietary concentration of 0, 15, 50, or 200 ppm,
equal to 0, 1.3, 4.4, and 16 mg/kg bw per day in males and 0, 1.5,
5.1, and 20 mg/kg bw per day in females. After the F1 generation had
been weaned, 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. Amitraz at 200 ppm decreased the
growth, food consumption, fertility, and viability of offspring of the
F0 generation, and this dose was eliminated when the F1 generation
had been weaned, because of the very low survival. No effect was found
on the number of litters or mean litter size at 50 ppm; however, a
decreased number of young alive at 21 days was observed in all
generations. No further effects due to treatment were found in this or
the other group. The NOAEL was 50 ppm, equal to 4.4 mg/kg bw per day,
for maternal toxicity and 15 ppm, equal to 1.3 mg/kg bw per day, for
developmental toxicity (Sutton, 1973b).
(ii) Developmental toxicity
In a study of developmental toxicity, groups of 11-13 female
Boots-Wistar rats received amitraz (purity, 99.8%) at a dose of 0, 1,
3, or 12 mg/kg bw per day on days 8-20 of gestation. The rats were
killed on day 21, and the uterine contents were examined. There were
no deaths or clinical signs of toxicity. Food consumption, body-weight
gain, average litter size, fetal viability, and the implantation index
were not affected. At 12 mg/kg bw, fetal weight was lower than in the
controls, and the calcification of the sternebrae was less advanced.
The NOAEL for maternal toxicity was 12 mg/kg bw per day, and that for
developmental toxicity was 3 mg/kg bw per day, on the basis of reduced
fetal weight and reduced calcification of the sternebrae (Sutton,
1973c).
Table 2. Results of tests for the genotoxicity of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium 31-500 µg/plate 99.9 Negative Everest &
TA1535, TA1537, Wilcox (1976)
TA1558
Reverse mutation S. typhimurium 0, 33, 100, 333, 1000, 98.4 Negative McGregor &
TA98, TA100, TA1535, 3300, 10 000 µg/plate Printice (1983)
TA1537, TA1538
Chromosomal Human lymphocytes 0, 5, 10, 20 µg/ml -S9; 99.5 Negative Brooker et al.
aberration 0, 3, 5, 30 µg/ml +S9 (1988)
Cell mutation Mouse L5178Y tk+/- 0.06-33 µg/ml +S9; 98.4 Negative McGregor &
cells 0.06-20 µg/ml -S9 Riach (1983a)
Unscheduled Human embryonic 20, 60, 100, 140, 180, 100 Negative McGregor &
DNA synthesis fibroblasts 220, 260, 300 µg/ml ±S9 Riach (1983b)
DNA damage Chinese hamster 0.01-0.3 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Reverse mutation S. typhimurium 0, 100, 200, 400 mg/kg NR Negative Everest (1976);
(host-mediated, G46, TA1532 bw, single dose Wilcox (1976)
mouse) TA1964
Dominant lethal Female CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977a)
for 5 days
Table 2. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
Dominant lethal Male CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977b)
for 5 days
NR, not reported; S9, 9000 × g microsomal fraction from rodent liver
In another study of developmental toxicity, groups of 24 mated
Sprague-Dawley rats weighing 215-280 g were given 0, 7.5, 15, or 30
mg/kg bw per day amitraz (purity, 99.7%) by gavage on days 6-15 of
gestation. Immediately after mating, the females were assigned to
treatment groups by a randomization process based on stratified body
weight. Each female was then individually identified by ear notching.
All females were examined once or twice daily for clinical signs of
ill health, toxicity, or behavioural changes, and body weights and
food intake were recorded on days 0, 6, 10, 15, and 20 of gestation.
On day 20 of gestation, the females were killed and their uterine
contents examined.
One control female was found dead on day 10 of gestation; there
were no other deaths. The principal clinical sign was fur staining,
which was slightly more frequent at 30 mg/kg bw per day. At this dose,
a slight loss of body weight up to day 10 of gestation was followed by
reduced body-weight gain at termination. The body-weight gain of rats
at 15 mg/kg bw per day was slightly reduced (10%), but that of animals
at 7.5 mg/kg bw per day was not adversely affected by treatment. Food
intake of rats at 30 mg/kg bw per day, and to a lesser extent those at
15 mg/kg bw per day, was initially lower than in the control group;
there was no effect on food intake at 7.5 mg/kg bw per day. The
pregnancy rate was high in all groups, and no adverse findings were
made at necropsy. The treatment did not adversely affect the number of
implantations, the incidence of post-implantation loss, or the number
or sex ratio of fetuses. Animals at 30 mg/kg bw per day had a
statistically significantly increased incidence of dilated ureters and
increased bilateral renal pelvic cavitation. Although the incidence of
the latter lesion at 15 mg/kg bw per day was statistically
significantly higher than that of the concurrent controls
(p < 0.05), the group percentage increase (5.7%) was comparable to
the upper range of the relevant historical control values (5.4%). The
NOAEL was 7.5 mg/kg bw per day for both maternal toxicity, on the
basis of reduced body-weight gain, and for developmental toxicity, on
the basis of increased incidences of dilated ureters and renal pelvic
cavitation (McIntyre, 1987a).
Rabbits
In a study of developmental toxicity, groups of 8-10 New Zealand
rabbits were treated with amitraz (purity unspecified) at a dose of 0,
1, 5, or 25 mg/kg bw per day on days 6-18 of pregnancy and were killed
on day 30. At 25 mg/kg bw per day, the number of litters and mean
litter size were decreased and abortions were observed. No increase in
the incidence of congenital abnormalities was found. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, the highest dose tested,
and the NOAEL for developmental toxicity was 5 mg/kg bw per day on the
basis of a reduced number of litters and litter size (Sutton, 1973d).
In another study of developmental toxicity, groups of 16 mated
female New Zealand white rabbits weighing 3-4 kg were given amitraz
(purity, 99.7%) by gavage at a dose of 0, 3, 6, or 12 mg/kg bw per day
on days 7-19 of gestation. All females were examined once or twice
daily for clinical signs of ill health, toxicity, or behavioural
changes, and body weight and food intake were recorded on days 0, 7,
13, 19, 24, and 28 of gestation. On day 28 of gestation, the surviving
females were killed and their uterine contents examined.
One female at 12 mg/kg bw per day died, and three females at this
dose were killed because of poor clinical condition or abortion. Two
of the deaths were considered to be directly related to treatment. Two
females at 6 mg/kg bw per day died; at 3 mg/kg bw per day, one female
was killed and a further two died. Two control animals also died.
Langour, polypnoea, and squinting were observed in all treated groups,
and the incidence, severity, and duration of these signs appeared to
be dose-related. At 12 mg/kg bw per day, body-weight loss followed by
a reduction in body-weight gain were observed during dosing, but the
weight gain was similar to that of controls on cessation of dosing.
Treatment at 3 or 6 mg/kg bw per day had no effect on body weight.
Food intake was reduced during dosing and up to day 24 at 12 mg/kg bw
per day, but no adverse effect was seen at 6 or 3 mg/kg bw per day. No
adverse effects were seen at necropsy. Three of the surviving females
at 12 mg/kg bw per day lost their litters on day 28 of gestation.
Litter paraments were not affected at 6 or 3 mg/kg bw per day, as
assessed by the numbers of corpora lutea, implantation sites, and
viable fetuses. The mean litter and fetal weights and sex ratio of
fetuses were not adversely affected by treatment. No major fetal
defects were recorded that were considered to be related to treatment,
and there were no treatment-related effects on the incidence of minor
or variant anomalies. There was no NOAEL for maternal toxicity because
of deaths of animals at all doses and in the control group. The NOAEL
for developmental toxicity was 6 mg/kg bw per day, on the basis of
litter loss (McIntyre, 1987b).
(f) Special studies
(i) Effects on the thymus and hormone concentrations
Diets containing 400 ppm amitraz (purity, 97.1%), equal to 110
mg/kg bw per day for males and 150 mg/kg bw per day for females, were
given to 24 male CFLP mice for up to 18 weeks and to 52 female CFLP
mice for up to 33 weeks. A group of 36 males and 64 females given
plain diet served as controls. Body weight and food consumption were
measured regularly throughout the study, and the animals were examined
for overt signs of toxicity at least once a week. Vaginal smears were
monitored every morning during weeks 6-9, 15-18, 23-26, and 30-33.
Twelve male and 12 female controls were killed during the first week,
and 12 males and 12 females from both treated and control groups were
killed after 9 and 18 weeks. The remaining animals were killed after
33 weeks. The ß-estradiol concentration of the blood of female mice
was estimated at weeks 9 and 18, and the thymuses from all animals
were weighed at autopsy. Detailed histological examinations were
conducted on a selected range of tissues.
Body-weight gain was reduced in amitraz-treated mice, to a
slightly greater degre in the males, and food consumption was markedly
increased, particularly in the females. Examination of vaginal smears
indicated prolongation of estrus in treated mice, but there was no
measurable effect on the circulating concentrations of ß-estradiol.
The thymus weights and histological appearance were not affected by
treatment. Histopathological examination revealed two lymphoreticular
tumours in the treated females and two in controls. In comparison with
the controls, females given amitraz for 33 weeks had a higher
incidence of foci of inflammatory cells in the liver (Brown et al.
1978).
(ii) Effects on the estrus cycle and hormone concentrations
Mice
The effects of amitraz on the estrus cycle and hormone
concentrations were evaluated in groups of 70 female B6C3F1 mice fed
diets containing amitraz (purity, 98-100%) at 0, 25, 100, or 400 ppm,
equivalent to 0, 3.8, 15, and 60 mg/kg bw per day, for up to 28 weeks.
Permanently stained and mounted vaginal smears were prepared from each
animal daily for 30 days after 13 weeks of treatment, and the stage of
the oestrus cycle on each day was determined by microscopic
examination of the cell population on each slide. Blood samples were
taken at necropsy after overnight starvation and assayed for
dehydroepiandrosterone sulfate, estradiol, progesterone, testosterone,
lutropin, follitropin, prolactin, thyroxine, triiodothyronine, and
thyroid hormone uptake, as indicators of hormonal status and routine
clinical chemical parameters. The fresh liver weight was recorded at
necropsy.
Pro-estrus was prolonged, and a trend towards reduced duration of
diestrus was evident in animals at 400 ppm. The blood concentrations
of progesterone were lower and the concentrations of
dehydroepiandrosterone sulfate were higher in animals at 400 ppm and
to a lesser degree in those at 100 ppm when compared with controls.
The relative liver weights were increased at 400 (by 4%) and 100 ppm
(by 5%). There were no treatment-related effects at 25 ppm. The NOAEL
was 25 ppm, equivalent to 3.8 mg/kg bw per day, on the basis of lower
blood concentrations of progesterone, higher concentrations of
dehydroepiandrosterone sulfate, and increased relative liver weight
(Hounsell & Rush, 1984).
Rats
Groups of 20 female 22-week-old Boots-Wistar rats were fed diets
containing 0 or 200 ppm (equivalent to 0 and 12 mg/kg bw per day)
amitraz (purity unspecified) for 18 weeks. Vaginal smears were taken
routinely over 32 days. After fixation in methanol, the smears were
stained with 1% aqueous methylene blue and examined for keratinized
cells under 60 × magnification. Estrus was characterized by the
presence of keratinized cells only, and the cycle length was taken as
the interval between the first day of estrus in successive cycles.
The average cycle length of the controls was 4.3 days; two of the
controls had prolonged periods in estrus (four and six days), and a
third had a period of prolonged diestrus with a cycle lasting 18 days.
In the remaining animals, the shortest cycles were two days and the
longest six days. The low incidence of prolonged estrus among these
animals confirms that the technique of smearing did not affect vaginal
cytology. In the treated rats, the average cycle length was 6.1 days,
and the range was 2-16 days. Six rats had one or more periods of
prolonged diestrus; four of these had been acyclic in a preliminary
test. Estrus lasted for three to eight consecutive days in seven rats,
two of which had shown a similar tendency in a preliminary test. The
second test showed that the cycle length was significantly altered by
treatment, either estrus or diestrus being prolonged. Treated rats
thus had longer oestrus cycles than controls, resulting from prolonged
periods of estrus or diestrus (Merryman & Sutton, 1972).
(iii) Mechanism of action
The effects of amitraz and its major metabolite,
N-methyl- N'-(2,4-xylyl)formamidine, given orally or intravenously
on the cardiovascular system, pupil diameter, and the respiratory
system were studied in conscious and anaesthetized rats, cats, and
dogs. Both compounds caused a fall in blood pressure, sometimes
preceded by hypertension and bradycardia. The threshold for the effect
in conscious rats dosed orally with amitraz was 1 mg/kg bw. Amitraz
also caused mydriasis, sedation, and a reduced respiratory rate. The
bradycardia, sedation, and mydriatic effect was antagonized by the
alpha2-blocking agent yohimbine but not by the alpha1-blocking agent
prazosin, indicating that the effects were caused by stimulation of
presynaptic alpha2-adrenoceptors. The lethal effect of amitraz in mice
and dogs has also been shown to be inhibited by yohimbine (Parkinson &
Sim, 1970; Cullen & Reynoldson, 1983; Hsu & Kakuk, 1984; Moser &
MacPhail, 1985; Hsu et al., 1986; Hovda & McManus, 1993).
Amitraz and N-methyl- N'-(2,4-xylyl)formamidine were tested
for their ability to potentiate the pressor responses to tyramine in a
pithed rat preparation, since a structurally related compound,
chlordimeform, inhibited monoamine oxidase in vitro in rat liver
homogenates and centrally in vivo through an effect on brain
serotonin concentrations. These effects were obvious only with nearly
toxic oral doses of 80 mg/kg bw amitraz and 40 mg/kg bw
N-methyl- N'-(2,4-xylyl)formamidine (Parkinson, 1974).
Amitraz and chlordimeform were tested for their ability to
inhibit prostaglandin synthesis after intraperitoneal administration
in rats. Both compounds had antipyretic and antiinflam-matory effects
at doses of 5-80 mg/kg bw. They reduced yeast-induced fever, with
potencies intermediate between those of indomethacin and aspirin, and
antagonized carrageenan-induced swelling of the hind paw. They also
inhibited the synthesis of prostaglandin E2 from arachidonic acid by
bovine seminal vesicle microsomes. The potency of amitraz in this
assay was the same as that of aspirin (Yim et al., 1978).
(g) Studies on metabolites
(i) Acute toxicity
The acute toxicity of metabolites of amitraz (purity unspecified)
has been investigated in several species (Table 3).
Table 3. Acute toxicity of metabolites of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
N-Methyl-N'-(2,4-xylyl)formamidine
Mouse Oral 150 Sutton (1970a)
Rat Oral 200 Sutton (1970b)
Dog Oral >20 Morgan (1973);
Morgan & Williams (1974)
4-Amino-meta-toluic acid
Mouse Oral >1600 Shaw & Williams (1973a)
Rat Oral >1600 Shaw & Williams (1973b)
Form-2',4'-xylidide
Rat Oral 1600 Shaw (1973b)
(ii) Short-term toxicity
Rats
Groups of 10 male and 10 female newly-weaned Boots-Wistar rats
were dosed by gastric intubation with
N-methyl- N'-(2,4-xylyl)formamidine (purity unspecified) for 90
days at 0, 0.25, 1, 3, or 12 mg/kg bw per day. Body weights were
recorded three times per week, and the rats were observed each day for
signs of toxicity. At 3 mg/kg bw per day, an initial reduction in
body-weight gain was seen in males. At 12 mg/kg bw per day, the rats
became nervous and difficult to handle, and two deaths occurred. The
growth rate was reduced in animals of each sex but to a greater extent
in the males. At the end of experiment, haemoglobin and haematocrit
values were decreased in males, and the number of erythrocytes was
decreased in females. Slight biochemical changes were seen. At
autopsy, the weights of the adrenals, ovaries, uterus, and liver in
females and spleen and testes in males were increased, but the only
histopathological changes were a slight increase in lymphoid
infiltration, some sinusoidal leukocytosis, and loss of glycogen in
the livers of most males and slight cellular accumulations in the
hearts of some females. The NOAEL was 1 mg/kg bw per day on the basis
of the reduced body-weight gain and increased organ weights (Shaw &
Williams, 1975).
Groups of five male and five female Wistar rats were dosed by
gastric intubation with 4-amino- meta-toluic acid (purity
unspecified) at a dose of 0, 40, 100, or 250 mg/kg bw per day for 21
days. At 250 mg/kg bw per day, slight decreases in weight gain and in
blood urea nitrogen concentration were observed in males and an
increased relative weight of the spleen in females. No gross
pathological changes due to treatment were found. The NOAEL was
250 mg/kg bw per day, the highest dose tested (Shaw, 1975).
Dogs
Groups of four male and four female beagles were given gelatin
capsules containing N-methyl- N'-(2,4-xylyl)formamidine (purity
unspecified) as free base diluted in lactose to 1% at a dose of 0,
0.1, 0.25, or 1 mg/kg bw per day for 90 days. Clinical signs were
recorded daily, food consumption twice daily, and body weight once a
week. Ophthalmoscopic examination and recordings of temperature and
heart rate were carried out before dosing and after 6 and 12 weeks.
Haematological, clinical chemical, and urinary analyses were perfomed,
and gross and histopathological examinations were carried out.
At 0.25 and 1 mg/kg bw per day, abnormal quietness and drowsiness
and significantly lower body temperature (by up to 16.2°C) were
observed 0.5-4 h after dosing. At 1 mg/kg bw per day, the heart rate
was significantly reduced (by up to 40 beats/min) 1-2 h after dosing,
and the liver weight and urine volume were increased. A slight
reduction in thymus weight was observed at 0.25 and 1 mg/kg bw per
day. No histopathological anomalies were found. The NOAEL was 0.1
mg/kg bw per day on the basis of central nervous system depression and
lowered body temperature (Chesterman et al., 1973).
Groups of four male and four female beagles were given gelatin
capsules containing 4-amino- meta-toluic acid (purity unspecified) at
a dose of 0, 16, 40, or 100 mg/kg bw per day for 90 days. Slightly
increased urinary concentrations of total reducing substances other
than glucose were found at the highest dose. No dose-related effects
were observed on behaviour, electrocardiogram, heart rate, rectal
temperature, body weight, food consumption, haematological or blood
chemical parameters, organ weights, or histopathological appearance
(Morgan et al., 1974).
(iii) Genotoxicity
The results of tests for the genotoxicity of potential
metabolites of amitraz are summarized in Table 4. Except for a single
positive response to 2.4-dimethylaniline in the assay for forward
mutation in mouse lymphoma cells, in the presence of metabolic
activation, the results of the tests in vitro and in vivo were
negative.
3. Observations in humans
In a double-blind, randomized cross-over study, six healthy male
volunteers aged 18-45 years and weighing 60-70 kg received sequential
single oral doses of 0, 0.063, and 0.13 mg/kg bw amitraz, two to three
weeks apart. Each dose was given with 150 ml water, 30 min after
breakfast. Pulse rate, respiration rate, blood pressure, and
temperature were measured at - 1, - 0.5, 1, 3, 6, 12, 24, and 36 h,
and electrocardiograms were performed at -1, 1, 3, 6, 12, 24, and 36 h
with respect to dosing. Pupil responsiveness and psychomotor
performance were evaluated before treatment and at 2.5 and 8 h. Urine
was collected at 0-36 h and 36-60 h. There were no clinically
significant changes in vital signs or electrocardiographic parameters.
Moreover, haematological, blood chemical, and urinary parameters,
pupil responsiveness, and psychomotor performance were unaffected by
treatment. The NOAEL was 0.13 mg/kg bw, the highest dose tested (Cass,
1992).
Two human volunteers who received a single oral dose of 0.25
mg/kg bw 14C-amitraz showed drowsiness, disorientation, slurred
speech, decreased pulse rate and blood pressure, and other effects
(Campbell & Needham, 1984c).
In a double-blind cross-over study, four male and two female
volunteers, aged 21-42 years and in normal health, received two doses
of 2 mg (about 0.03 mg/kg bw) of the amitraz metabolite,
N-(2,4-dimethylphenyl)- N'-methylformamidine, one week apart in a
capsule with 100 ml of water, or placebo. Blood pressure, pulse rate,
and temperature were measured at half-hourly intervals over 7 h, and
mental alertness was assessed at 0, 3, and 7 h. An electrocardiograph
was performed, and urine was collected before dosing and at 0-7 h and
7-24 h. The urine samples were analysed to estimate the amount of the
metabolite, 3-methyl-4-aminobenzoic acid, that had been excreted.
Mental alertness was estimated at 0, 3, and 7 h. No difference was
seen from those receiving the placebo. The NOAEL was 0.03 mg/kg bw,
the only dose tested (Hall et al., 1975).
Symptoms of central nervous system depression ranging from
sedation to coma lasting more than 24 h, depressed respiration,
hypotension, and bradycardia have been described in 11 reports after
accidental or intentional ingestion of uncertain amounts of amitraz.
In nine cases, recovery was complete after symptomatic and supportive
treatment (Groppi, 1977; Ros & Aken, 1994; Pronczuk et al., 1995).
Table 4. Results of tests for the genotoxicity of metabolites of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
N-Methyl-N'-(2,4-xylyl)formamidine
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983a)
TA1337, TA1538
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Form-2',4'-xylidide
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983b)
TA1337, TA1538
DNA damage Chinese hamster 0.01-1.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Forward mutation Mouse L5178Y tk+/- 1, 3.3, 10, 33, 100, 200, NR Positive + S9 McGregor &
lymphoma cells 300, 333, 400, 500, Negative -S9 Riach (1984)
600 µg/ml
Cell transformation C3H/10 T1/2 clone 8 5, 10, 20 µg/ml +S9 NR Negative McGregor et al.
mouse embryo fibroblasts 100, 200, 400 µg/ml -S9 (1984)
DNA damage Chinese hamster 0.03-2.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
4-Amino-meta-toluic acid
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Micronucleus Mouse bone 56, 113, 225 mg/kg bw NR Negative Hounsell &
formation marrow twice, 24 h apart Walker (1983)
Comments
Amitraz was well absorbed, extensively metabolized, and rapidly
excreted, mainly in the urine, after oral administration to mice,
rats, dogs, pigs, hens, cows, baboons, and humans. After oral
treatment of mice with 14C-amitraz, 86% of the radiolabelled dose was
excreted, 62% in the urine, within the first 24 h. All of it had been
excreted by 96 h, with 73% in the urine of animals of each sex. The
concentrations of residues were highest in liver, adrenal glands, and
eyes. After oral administration of 14C-amitraz to rats, 94% of the
dose was recovered within three days, with 82% in urine and 12% in
faeces. After oral administration of 14C-amitraz to two humans,
77-87% was recovered within three days. Amitraz is hydrolysed to two
components, N-methyl- N-(2,4-xylyl)formamidine and
form-2',4'-xylidide. The former is the pharmacologically active
compound and accounted for 5-30% of the total urinary excretion in
mice and rats; it was further metabolized to 4-amino- meta-toluic
acid and the acetyl and formyl conjugates, 4-acetamido- and 4-
formamido- meta-toluic acids. These five metabolites were also found
in plants.
Amitraz has low acute oral toxicity in rats but is more toxic in
dogs. The LD50 values ranged from 100 mg/kg bw in dogs to > 1600
mg/kg bw in mice, indicating that dogs are more sensitive. The toxic
signs after oral administration to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Amitraz had no
sensitizing potential in guinea-pigs, and no local irritation was
found in rabbits after a single application to skin or eyes. There was
evidence of delayed contact hypersensitivity after application of
amitraz either topically or intradermally.
WHO (1996) has classified amitraz as slightly hazardous.
In a 13-week study in which mice were fed diets providing 0, 100,
200, 400, 600, or 800 ppm, the NOAEL was 100 ppm, equal to 17 mg/kg bw
per day, on the basis of reduced overall body-weight gain (by 34%).
In a 90-day study, rats were given amitraz at doses of 0, 3, or
12 mg/kg bw per day by gavage. The NOAEL was 3 mg/kg bw per day, on
the basis of reduced terminal body-weight gain, absolute liver weight,
and relative liver weight.
In a 90-day study in dogs, amitraz was administered at doses of
0, 0.25, 1, or 4 mg/kg per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression
and reductions in rectal temperature and pulse rate.
In a two-year study, dogs were given amitraz at doses of 0, 0.1,
0.25, or 1 mg/kg bw per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression.
In two 90-day studies, the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine was administered to rats at
doses of 0, 0.25, 1, 3, or 12 mg/kg bw per day by gastric intubation
or to dogs at 0, 0.1, 0.25, or 1 mg/kg bw per day by gelatine
capsules. The NOAEL was 1 mg/kg bw per day in rats, on the basis of
reduced body-weight gain and increased organ weights, and 0.1 mg/kg bw
per day in dogs, on the basis of central nervous system depression and
lowered body temperature.
In a 21-day study in rats and a 90-day study in dogs, the amitraz
metabolite 4-amino- meta-toluic acid was given at doses of 0, 40,
100, or 250 mg/kg bw per day by gastric intubation to rats or 0, 16,
40, or 100 mg/kg bw per day by gelatin capsules to dogs. The NOAEL was
250 mg/kg bw per day in rats and 100 mg/kg bw per day in dogs, (the
highest doses tested).
In an 80-week study, mice were fed diets containing 0, 25, 100,
or 400 ppm, equivalent to 0, 3.8, 15, and 60 mg/kg bw per day (wrongly
given as 25, 100, or 400 mg/kg bw per day in the 1980 JMPR report).
The NOAEL for carcinogenicity was 100 ppm, equivalent to
15 mg/kg bw per day, on the basis of an increased incidence of
lymphoreticular tumours in females at 400 ppm.
In a two-year study in which mice were fed diets providing 0, 25,
100, or 400 ppm, the NOAEL for carcinogenicity was 100 ppm, equal to
11 mg/kg bw per day, on the basis of the occurrence of hepatocellular
carcinoma in females at 400 ppm. This dose was considered to be
greater than the conventional maximum tolerated dose. The NOAEL for
toxicity was 25 ppm, equal to 2.3 mg/kg bw per day, on the basis of
generalized toxic effects.
In a two-year study, rats were fed diets containing 0, 15, 50, or
200 ppm. The NOAEL for toxicity was 50 ppm, equal to 2.5 mg/kg bw per
day, on the basis of effects on the central nervous system and reduced
overall body-weight gain in males. There was no evidence of
carcinogenicity.
The genotoxic potential of amitraz has been adequately evaluated
in a range of assays in vitro and in vivo. The Meeting concluded
that amitraz is not genotoxic.
In view of the lack of genotoxicity and the finding of tumours
only in mice and only at concentrations at which severe toxicity was
observed, the Meeting concluded that amitraz is not likely to pose a
carcinogenic risk to humans.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 15, 50, or 200 ppm, the NOAEL was 50 ppm,
equal to 4.4 mg/kg bw per day, for maternal toxicity and 15 ppm, equal
to 1.3 mg/kg bw per day for developmental toxicity. No teratogenic
effect was observed.
In two studies of developmental toxicity, pregnant rats were
given amitraz at 0, 1, 3, or 12 mg/kg bw per day by gavage on days
8-20 of gestation or 0, 7.5, 15 or 30 mg/kg bw per day by gavage on
days 6-15. The NOAEL for maternal toxicity was 7.5-12 mg/kg bw per
day, and that for developmental toxicity was 3-7.5 mg/kg bw per day.
In two studies of developmental toxicity in rabbits, amitraz was
given at doses of 0, 1, 5, or 25 mg/kg bw per day by gavage on days
6-18 of gestation or 0, 3, 6, or 12 mg/kg bw per day by gavage on days
7-19. The NOAEL for maternal toxicity was 25 mg/kg bw per day in one
study, but a NOAEL was not identified in the other because all of the
treated animals died. The NOAEL for developmental toxicity was 3-6
mg/kg bw per day.
The effect of amitraz on the estrus cycle and hormone levels was
evaluated in mice fed diets containing 0, 25, 100, or 400 ppm for 28
weeks and in rats fed diets containing 0 or 200 ppm for 18 weeks. In
mice, pro-estrus was prolonged at 400 ppm; blood levels of
progesterone were reduced and those of dehydroepiandrosterone were
increased at 100 and 400 ppm. The NOAEL was 25 ppm, equivalent to
3.8 mg/kg bw per day, on the basis of the changed hormone levels. The
estrus cycles were longer in treated than in control rats, with no
NOAEL.
In a double-blind, randomized, cross-over study of tolerance, six
healthy adult male volunteers received sequential single oral doses of
0, 0.063, and 0.13 mg/kg bw amitraz, two to three weeks apart. The
NOAEL was 0.13 mg/kg bw, the highest dose tested.
Two human volunteers who received single oral doses of 0.25 mg/kg
bw 14C-amitraz showed effects including drowsiness, disorientation,
slurred speech, and decreased pulse rate and blood pressure.
In a double-blind cross-over study of tolerance, six adult
volunteers received two single doses of placebo or 2 mg (about 0.03
mg/kg bw) of the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine one week apart. The NOAEL was
0.03 mg/kg bw, the only dose tested.
The Meeting established an ADI of 0-0.01 mg/kg bw on the basis of
the NOAEL of 1.3 mg/kg bw per day in the study of reproductive
toxicity in rats and a safety factor of 100. The pharmacological
effects on the central nervous system seen in dogs, with a NOAEL of
0.25 mg/kg bw per day, were considered not to be relevant for setting
the ADI because they were reversible and the dogs became tolerant.
Moreover, a NOAEL of 0.13 mg/kg bw per day was seen for such effects
in humans.
The Meeting established an acute RfD of 0.01 mg/kg bw, on the
basis of the NOAEL of 0.13 mg/kg bw per day in the study in humans and
a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 2.3 mg/kg bw per day (toxicity in a
two-year study of carcinogenicity)
Rat: 3 mg/kg bw per day (toxicity in a 90-day study of
toxicity)
50 ppm, equal to 2.5 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
50 ppm, equal to 4.4 mg/kg bw per day (maternal
toxicity in a three-generation study of reproductive
toxicity)
15 ppm, equal to 1.3 mg/kg bw per day (developmental
toxicity in a three-generation study of reproductive
toxicity)
12 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
5 mg/kg bw per day (developmental toxicity)
Dog: 0.25 mg/kg bw per day (toxicity in a two-year study of
toxicity)
Human: 0.13 mg/kg bw (toxicity after single oral doses)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies to further characterize the effects on the
reproductive system of female rodents
2. Further observations in humans
List of end-points relevant for setting guidance values for dietary
and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete
Distribution Liver, adrenals, eyes
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 80-100% in 96 h
Metabolism in animals Metabolites same in rodents, dogs,
humans
Toxicologically significant
compounds (animals, plants, N-Methyl-N'-(2,4-xylyl)formamidine
and environment)
Acute toxicity
Rat: LD50 oral 600 mg/kg bw
Rabbit: LD50 dermal > 200 mg/kg bw
Rat: LC50 inhalation 65 mg/L
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing (Buehler test)
Short-term toxicity
Target/critical effect Central nervous system depression, dog
Lowest relevant oral NOAEL 0.25 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 50 mg/kg bw per day
Lowest relevant inhalation Rat: 0.01 mg/L air
NOAEL
Genotoxicity Unlikely to be genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Lymphoreticular tumours, hepatocellular
carcinomas
Lowest relevant NOAEL Mouse: 11 mg/kg bw per day (80-week and
2-year studies)
Carcinogenicity Unlikely to be carcinogenic
Reproductive toxicity
Reproduction target/critical Decrease in number of young alive
effect at 21 days
Lowest relevant reproductive Rat: 1.3 mg/kg bw per day
NOAEL (developmental toxicity)
Developmental target/critical Reduced fetal weight
effect
Lowest relevant developmental Rat: 3 mg/kg bw per day
NOAEL (developmental toxicity)
Neurotoxicity/Delayed Acute central nervous system
neurotoxicity depression
Other toxicological studies Prolongation of estrus cycles and
reduction of blood concentrationof
progesterone (mouse, rat)
Medical data Central nervous system depression in
two volunteers after a single oral dose
of 0.25 mg/kg bw
Summary Value Study Safety
factor
ADI 0-0.01 mg/kg bw Reproductive toxicity, 100
rat
Acute reference 0.01 mg/kg bw Single oral dose in six
dose volunteers 10
References
Berczy, Z.S., Binns, R. & Newman, A.J. (1972) Acute inhalation
toxicity to the rat of BTS 27419. Unpublished report No. 4971/72/406
from Huntingdon Research Centre Ltd, Huntingdon, Cambridge-shire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. & Whitfield,
R.A.S. (1973) Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report No. 5454/72/850 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brown, D.J., Burnett, R., Crowley, J., Cryer, P.C., Lancaster, M.C. &
Turnbull, G.J. (1978) Amitraz: Investigation of effects on the thymus
gland and oestrous cycle in mice. Unpublished report No. TX 78037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brooker, P.C., Akhurst, L.C. & Gray, V.M. (1988) Technical amitraz,
metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. SMS 127/881149 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Burnett, R., Crowley, J., Lessel, B., Patton, D.S.G., Sutton, M.M. &
Turnbull, G.J. (1976) BTS 27419: 80-week carcinogenicity study in
mice--final report. Unpublished report No. TX 76039 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1981) Excretion and residues of
14C-amitraz in male and female rats given a single oral dose of 10
mg/kg. Unpublished report No. METAB/81/20 from FBC Ltd, Chesterford
Park Research Station, Saffron Walden, Essex, United Kingdom.
Submitted to WHO by Schering Agro-chemicals Ltd.
Campbell, J.K. & Needham, D. (1983) Excretion and tissue residues of
14C amitraz in male and female mice given a single oral dose of 10 mg
14C-amitraz/kg bodyweight. Unpublished report No. METAB/83/34 from
FBC Ltd, Chesterford Park Research Station, Saffron Walden, Essex,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984a) Dermal absorption of 14C-amitraz
in EC formulation by male and female pigs given a single topical
application of 18 mg A.I. Unpublished report No. METAB/84/9 from FBC
Ltd, Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. Needham, D. (1984b) Excretion and tissue residues of
14C-amitraz in a male and a female baboon given a single oral dose of
10 mg 14C-amitraz per kg bodyweight. Unpublished report No.
METAB/83/44 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984c) Urinary excretion of 14C-amitraz
by two male humans following a single oral dose of 0.25 mg/kg
bodyweight. Unpublished report No. METAB/84/10 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984d) The metabolism of 14C-amitraz by
male and female rats. Unpublished report No. METAB/84/2 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984e) A comparison of the metabolism of
14C-amitraz in rat, mouse, baboon and human. Unpublished report No.
METAB/84/1 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Cass, L.M.R. (1992) Report of a double blind tolerance study of
amitraz in six adult healthy volunteers. Unpublished report No.
TOX/92/179-209 from Simbec Research Ltd, Merthyr Tydfil,
Mid-Glamorgan, Wales. Submitted to WHO by Schering Agrochemicals Ltd.
Challis I.R. (1990) Dermal absorption of amitraz in the rat.
Unpublished report No. TOX/90/179-178 from Schering Agrochemicals Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Chesterman, H., Skerrett, K., Street, A.E. & Cherry, C.P. (1973) Boots
27 271, oral toxicity study in beagle dogs (repeated dosage for 13
weeks). Unpublished report No. BTS 38/73511 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO
by Schering Agrochemicals Ltd.
Clouzeau, J. (1992) Technical amitraz: Guinea pig sensitization study
(Buehler test). Unpublished report No. TOX 91/179-203 from Centre
International de Toxicologie, Miserey, Evreux, France. Submitted to
WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Gibson, W.A. & Prentice, D.E.
(1981) Amitraz toxicity to B6C3F1 mice by dietary administration for
13 weeks. Unpublished report No. BTS 158/80759 from Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Street, A.E., Gibson W.A. &
Gopinath, C. (1983) Amitraz, 104 week tumorigenicity study in mice.
Unpublished report No. BTS 153/8262/A from Huntingdon Research Centre
plc, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agro-chemicals Ltd.
Cullen, L.K. & Reynoldson, J.K. (1983) Effects of amitraz on the
cardiovascular and respiratory systems in the dog. Unpublished report
No. T242 from School of Veterinary Studies, Murdoch University,
Murdoch, Western Australia. Submitted to WHO by Schering Agrochemicals
Ltd.
Everest, R.P. (1976) BTS 27 419: Mutagenicity study in the male mouse
perivisceral cavity host-mediated assay. Unpublished report No. TX
76056 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Everest, R.P. & Wilcox, P. (1976) BTS 27 419: Mutagenicity testing
against Salmonella typhimurium strains TA1535, TA1537 and TA1538 in
the presence and absence of liver microsomes from male and female
mice. Unpublished report No. TX 76057 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Groppi, L. (1977) Medical report on a patient who ingested Mitac
(Edrizar) in Italy in 1977. Report No. T84 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Hall, J.E., Ahluwalia, H. & Hampson, P. (1975) A study of the effects
of oral administration of a metabolite (BTS 27 271) of amitraz to
volunteers. Unpublished report No. MS 75002 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hornish R.E. & Nappier, J.M. (1983) The absorption, metabolism, and
excretion of Mitaban(R) (U-36,059) in the dog from oral and dermal
exposure. Unpublished report No. 527-9760-83-001 from Agricultural
Research and Development Laboratories, The Upjohn Company, Kalamazoo,
Michigan, USA. Submitted to WHO by Schering Agrochemicals Ltd.
Hounsell, I.A.G & Walker, A.K. (1983) A micronucleus study in mice
using BTS 24868 (2,4-dimethylaniline). Unpublished report No.
TOX/84/179-97 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hounsell, I.A. & Rush, K.C. (1984) Technical amitraz: The effect of
dietary administration on the oestrus cycle and hormones in the mouse.
Unpublished report No. TOX/84/179-97 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Hovda, L.R. & McManus, A.C. (1993) Yohimbine for treatment of amitraz
poisoning in dogs. Vet. Hum. Toxicol., 35, 329.
Hsu, W.H. & Kakuk, T.J. (1984) Effect of amitraz on heart rate and
pupil diameter in rats: Mediated by alpha2-adrenoceptors. Toxicol.
Appl. Pharmacol., 73, 411-415.
Hsu, W.H., Lu, Z.-X. & Hembrough, F.B. (1986) Effect of amitraz on
heart rate and aortic blood pressure in conscious dogs: Influence of
atropine, prazosin, tolazoline, and yohimbine. Toxicol. Appl.
Pharmacol., 84, 418-422.
Kakuk, T.J. (1979) Pathological findings on the matched control and
amitraz 400 ppm females from the Boots 80 week mouse study by various
pathologists with commentary. Unpublished report No. 315-78-9610-005
from Agricultural Research and Development Laboratories, The Upjohn
Company, Kalamazoo, Michigan, USA. Submitted to WHO by Schering
Agrochemicals Ltd.
Kynoch, S.R. & Parcell, B.I. (1988) Technical amitraz: Assessment of
delayed contact hypersensitivity in the guinea-pig. Unpublished report
No. TOX 88/179-145 from Huntingdon Research Centre Ltd, Huntingdon,
Cambridgeshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Lancaster, M.C. & Williams, G.A.H. (1978) Amitraz: Multigeneration
feeding test in rats -- Histopathology. Unpublished report No. TX
78064 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Lewis, D.K. (1971) Fate of [14C]-BTS 27419 applied to rats as a
single oral dose. Unpublished report No. C 71011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987a) Technical amitraz: Irritant
effects on rabbit skin. Unpublished report No. TOX/87/179-137 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987b) Technical amitraz: Irritant
effects on the rabbit eye. Unpublished report No. TOX/87/179-142 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Printice, R.D. (1983) Technical amitraz: Ames
bacterial mutagenicity test. Unpublished report No. 2590 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983a) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. 2669 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by Schering
Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983b) Technical amitraz: Unscheduled
DNA synthesis in human embryonic cells. Unpublished report No. 2634
from Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1984) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. TOX/84/179-97 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B., Brown, A.G. & Riach, G.G. (1984) Technical BTS 24868:
Induction of cell transformation in C3H10T1/2 cells. Unpublished
report No. TOX/84/179-97 from FBC Ltd, Chesterford Park Research
Station, Saffron Walden, Essex, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
McIntyre, M. (1987a) Technical amitraz: Teratogenicity study in the
rat. Unpublished report No. 5562-194/11 from Hazleton UK, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
McIntyre, M. (1987b) Technical amitraz: Teratogenicity study in the
rabbit. Unpublished report No. 5536-194/13 from Hazleton UK,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Merryman, D.C. & Sutton, M.M. (1972) Effects on the oestrus cycle of
the rat. Unpublished report No. PM 72003 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. (1973) BTS 27 271: Acute oral toxicity study in dogs.
Unpublished report No. TX 73004 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. & Williams, G.A.H. (1974) BTS 27 271: Acute oral toxicity
study in dogs--Histopathology. Unpublished report No. TX 73 030 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Morgan, H.E., Patton, D.S.G. & Turnbull, G.J. (1973) BTS 27 419:
Two-year oral toxicity study in dogs. Unpublished report No. TX 73035
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Morgan, H.E., Shepherd, G.M. & Turnbull, G.J. (1974) BTS 28 369:
90-day oral toxicity study in dogs. Unpublished report No. TX 74037
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Moser, V.C. & MacPhail, R.C. (1985) Yohimbine attenuates the delayed
lethality induced in mice by amitraz, a formamidine pesticide.
Toxicol. Lett., 28, 99-104.
Needham, D. (1984) The effect of Amitraz on the hepatic mixed-function
oxidase system of male and female mice following oral administration.
Unpublished report No. METAB/84/8 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Needham, D. & Hemmings, P.A. (1988) The metabolism and distribution of
amitraz residues in the laying hen following the daily oral
administration of 24.5 mg 14C-amitraz/per bird. Unpublished report
No. Envir/88/6 from Schering Agrochemicals Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977a) Dominant lethal assay of amitraz in
the female mouse. Unpublished report No. BTS 81/7758 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977b) Dominant lethal assay of amitraz in
the male mouse. Unpublished report No. BTS 80/7792 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Parkinson, R. (1974) The effects of BTS 27 271, BTS 27 419 and BTS 21
103 (chlordimeform) on the pressor response to tyramine in the pithed
rat. Unpublished report No. P74 032 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Parkinson, R. & Sim, M.F. (1970) Some pharmacological effects of the
acaricide RD 27,271 and related compounds. Unpublished report No.
P70508 from The Boots Pure Drug Company, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Patton, D.S.G. (1973) BTS 27419: Acute toxicity in baboons.
Unpublished report No. TX 73002 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Patton, D.S.G & Sutton, M.M. (1971) Acute toxicity studies on BTS 27
419, an acaricide. Unpublished report No. P71544 from The Boots Pure
Drug Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Patton, D.S.G. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in dogs. Unpublished report No. P71547 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Petzold, G.L., Swenberg, J.A. & Bedell, M. (1977) Evaluation of
amitraz (U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915A
and U-54,914) in the DNA damage/alkaline elution assay. Unpublished
report No. 7268/77/7268/001 from The Boots Company Ltd, Nottingham,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Phillips, M.W.A., Swalwell, L.M. & Needham, D. (1987) Identification
of metabolites of amitraz in the milk and meat of a cow dosed for 4
days with amitraz. Unpublished report No. Envir/87/46 from Schering
Agrochemicals Ltd, Chesterford Park Research Station, Saffron Walden,
Essex, United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Pronczuk, J., Heuhs, L., Scaiola, G., Bogdan, M. & Fogel de Korc, E.
(1995) Clinical cholinergic presentation of acute amitraz poisoning.
Report No. T368 from Department of Toxicology, Hospital de Clinicas,
Montevideo, Uruguay. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983a) Technical BTS 27271,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61A/83580
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983b) Technical BTS 27919,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61B/83581
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Ros, J.J.W. & van Aken, J. (1994) A case of poising with amitraz, an
agricultural pesticide. Ned. Tijdschr. Geneeskd., 138, 776-778.
Shaw, J.W. (1971) BTS 27 419 -- Acute intraperitoneal toxicity to
rats. Unpublished report No. PM 71057 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. (1973a) BTS 27 419: Comparison of the acute oral and
intraperitoneal toxicities to rats. Unpublished report No. TXM 73006
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1973b) BTS 27 419: Comparison of the acute oral toxicities
to rats of BTS 27 419 and BTS 27 919. Unpublished report No. TXM 73010
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1975) BTS 27 419 metabolite: 21 Day chronic oral toxicity
in rats of BTS 28 369. Unpublished report No. TX 75058 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973a) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to mice. Unpublished report No. TXM 73 036 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973b) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to rats. Unpublished report No. TXM 73 037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, G.A.H. (1975) BTS 27 419 metabolite: 90 day
chronic oral toxicity in rats of BTS 27 271. Unpublished report No. TX
75059 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Somerville, L. (1973) Fate of 14C-BTS 27419 administered to rats in
repeated oral doses. Unpublished report No. AX 73011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Stewart, F.P. (1993) (14C)-Amitraz: Dermal absorption in the rat.
Unpublished report No. 194/69-1011 from Hazleton Europe, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. (1970a) RD 27,271. Acute oral toxicity to mice.
Unpublished report No. PM 70043 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agro-
chemicals Ltd.
Sutton, M.M. (1970b) RD 27,271 Acute toxicity to rats. Unpublished
report No. PM 70042 from The Boots Pure Drug Company, Nottingham,
United Kingdom. Submitted to WHO by Schering Agro-chemicals Ltd.
Sutton, M.M. (1971) BTS 27 419. Contact sensitisation in the guinea
pig. Unpublished report No. PM 71919 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973a) BTS 27 419: Three week dermal toxicity to
rabbits. Unpublished report No. TX 73 026 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973b) BTS 27 419: Multigeneration feeding test in rats.
Unpublished report No. TX 73 036 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973c) BTS 27 419: Teratogenicity in the rat.
Unpublished report No. TX 73028 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973d) BTS 27 419: Teratogenicity in the rabbit.
Unpublished report No. TX 73029 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. & Offer, J. (1973) BTS 27 419: Carcinogenicity and
long-term toxicity study in rats. Unpublished report No. TX 73043 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Sutton, M.M. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in rats. Unpublished report No. P71548 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. & Williams, P.A. (1972) BTS 27 419: Acute dermal toxicity
to rabbits. Unpublished report No. YM 72 011 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Wilcox, P. (1976) BTS 27 419: Mutagenicity study in the
intraperitoneal host-mediated assay. Unpublished report No. TX 76028
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yim, G.K.W., Holsapple, M.P., Pfister, W.R. & Hollingworth, R.M.
(1978) Prostaglandin synthesis inhibited by formamidine pesticides.
Life Sci., 23, 2509-2516.
In the study of Challis (1990), described above, the metabolic
profile of rats treated dermally was similar to that of rats treated
orally.
In the study of Hornish & Nappier (1983), described above, the
metabolism of amitraz was essentially the same after oral and dermal
administration. 4-Formamido- meta-toluic acid was the predominant
residue in both blood and urine. The parent compound and the first
hydrolysis products, N-methyl- N'-(2,4-xylyl)formamidine and
form-2',4'-xylidide, were not observed at measurable concentrations in
either blood or urine.
One cow received 0.5 mg/kg bw per day 14C-amitraz (specific
activity, 4.2 mCi/g; radiochemical purity, > 98.8%) by capsule twice
daily for four days. All milk was collected, and samples of urine were
taken. The cow was killed 17 h after the final dose. The urinary
metabolites corresponded to 4-acetamido- meta-toluic acid and
4-formamido- meta-toluic acid, which were converted to
4-amino- meta-toluic acid by acid hydrolysis. The total residue in
milk accounted for only 0.2% of the dose. The greatest concentration
of residue (0.07 ppm) was found after the final dose. The residue was
extracted with methanol (recovery, 90%), and the metabolites present
identified by co-chromatography as 4-acetamido- meta-toluic acid,
4-formamido- meta-toluic acid, and 4-amino- meta-toluic acid (23%);
form-2',4'-xylidide (9%); polar material that was converted to
4-amino- meta-toluic acid by acid hydrolysis (34%); and low-polarity
material (24%). The latter broke down readily to form-2',4'-xylidide
under neutral or basic conditions; it was not present in acetonitrile
extracts of whole milk and was considered to be an artefact produced
by the methanol Soxhlet extraction of the milk. 2,4-Dimethylaniline
was not observed. The edible tissue with the highest concentration of
residue was the liver (3.7 ppm).
Methanol Soxhlet extraction released about 60% of the residue,
and the remainder was solubilized after enzymic digestion and acid
hydrolysis. The residues were identified by co-chromatography as
4-acetamido- meta-toluic acid and 4-formamido- meta-toluic acid
(14%), 4-amino- meta-toluic acid (15%), form-2',4'-xylidide (10%),
and polar material (10%) which on acid hydrolysis was converted to
4-amino- meta-toluic acid and additional low-polarity material. Other
polar material (29% of the residue) was not amenable to
chromatography. 2,4-Dimethylaniline was not observed. The low-polarity
material (14%) broke down on neutral or basic thin-layer
chromatography to a range of compounds, suggesting that it was a
product of condensation between 4-amino- meta-toluic acid and
compounds released from acid-labile conjugates. When the
non-methanol-extractable residue was fed to a dog, analysis of urine
gave similar results to those after enzymic digestion (Phillips et
al., 1987).
Six laying hens (Gallus gallus domesticus) were each dosed
orally with 24.5 mg 14C-amitraz (specific activity, 0.14 mCi/g;
radiochemical purity, > 99%) per day for four days, providing a dose
2200 times greater than the expected daily exposure of birds fed
cotton-meal derived from amitraz-treated plants. The hens were killed
4 or 12 h after the final dose, and the concentrations and nature of
radioactive residues in tissues were determined. The radiolabel was
rapidly excreted, with 68% in 0-24-h excreta. The main route of
metabolism was via 4-amino- meta-toluic acid, since 75% of the
metabolites extracted from the excreta could be accounted for as
either this metabolite or its acid-labile conjugates. The highest
concentrations of tissue residues were found in the liver and kidney
(17 and 25 mg/kg respectively) 4 h after the fourth dose, but these
concentrations had fallen to 12 and 10 mg/kg, respectively, by 12 h
after dosing. The major metabolite detected in the liver was
4-amino- meta-toluic acid, present as both free acid and labile
conjugates, which represented 55% of the total residue.
N-Methyl- N'-(2,4-xylyl)formamidine (4%), form-2',4'-xylidide (4%)
and 2,4-dimethylaniline (2%) were also present. A mixture of at least
seven highly polar compounds, believed to be mono- and di-acids
similar to those previously seen in rats, was a further substantial
residue (27%). Unextractable fibre-bound material represented 2% of
the residue. The concentrations in fat, muscle, and skin represented
0.6-2.7 mg/kg 4 and 12 h after the final dose. In fat, the residues
consisted of form-2',4'-xylidide (42%),
N-methyl- N'-(2,4-xylyl)formamidine (24%), unchanged amitraz (21%),
2,4-dimethylaniline (3%), and 4-amino- meta-toluic acid (3%). In
muscle, 81% of the residues were identified as 4-amino- meta-toluic
acid or its acid-labile conjugates; form-2',4'-xylidide was present as
7% of the residue.
The concentrations of amitraz residues in eggs, collected daily,
were 0.28-0.46 mg/kg throughout the study and did not increase over
the four days of dosing. The residue concentrations in the yolks rose
from 0.1 to 1.4 mg/kg during the study;
N-methyl- N'-(2,4-xylyl)formamidine (54%) and 4-amino- meta-toluic
acid (34%) were the major metabolites. 4-Amino- meta-toluic acid
accounted for 91% of the residue in the egg white as both free acid
and labile conjugates, and form-2',4'-xylidide accounted for 4% of the
residue (Needham & Hemmings, 1988).
Separately reported data on the metabolism of amitraz
in various species were compared. After a single oral dose of
14C-amitraz (specific activity, 9 mCi/g; radiochemical
purity, > 95%) to groups of four male and four female mice,
three male and three female rats, one male and one female
baboon, and two men, the administered radiolabel was rapidly excreted.
In all species examined, urine was the major route of excretion,
accounting for 65-84% of the dose (55-76% within the first 24 h).
Analysis of urine obtained from volunteers dosed with 14C-amitraz
indicated that the metabolism of amitraz was qualitatively similar
to that in the other species. The major metabolites were
4-formamido- meta-toluic acid, 4-acetamido- meta-toluic acid and
N-methyl- N'-(2,4-xylyl)formamidine. In addition, 40-60% of the
metabolites excreted in urine was accounted for by a polar fraction
containing conjugates of 4-formamido- meta-toluic acid and
4-acetamido- meta-toluic acid. The minor metabolites included
4-amino- meta-toluic acid and form-2',4'-xylidide. Excretion of
N-methyl- N'-(2,4-xylyl)formamidine was dose-dependent. The
metabolites identified in the volunteers were
4-formamido- meta-toluic acid plus 4-acetamido- meta-toluic acid
(27% of the total radiolabel in urine),
N-methyl- N'-(2,4-xylyl)formamidine (6%), 4-amino- meta-toluic
acid (4%), form-2',4'-xylidide (4 %), the product of acid hydrolysis
of N-methyl- N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
(1%), and polar material (57%). In both rats and mice, increasing the
dose of amitraz from 1 to 100 mg/kg bw increased the excretion of
N-methyl- N'-(2,4-xylyl)formamidine from approximately 5 to 30% of
the total excretion. Prior dosing of five male and five female mice
with amitraz in the diet at 100 ppm for three weeks, followed by
400 ppm for a further three weeks, had no effect on the metabolism of
a single oral dose of 14C-amitraz (Campbell & Needham, 1984e).
(c) Effects on enzymes and other biochemical parameters
Groups of 18 male and 18 female B6C3F1 mice were dosed orally
with corn oil or amitraz at 100 mg/kg bw per day for two days and,
because of toxic symptoms, at 50 mg/kg bw per day for the following
two days. The animals were then killed, and the livers were assayed
for the activity of microsomal oxidases. A significant increase in
liver weight and in the activity of cytochrome b5 were recorded. The
effect did not appear to be related to increased activity of the
hepatic mixed-function oxidase system, since no significant increase
was seen in the activities of cytochrome P450, aniline hydroxylase, or
para-nitroanisole demethylase or in the concentration of microsomal
protein (Needham, 1984).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of amitraz and its metabolites (purity
unspecified) has been investigated in several species (Table 1). The
toxic signs after oral administration of amitraz to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Rats had intestinal
irritation and bladder distension. The lowest effective doses were 400
mg/kg bw in mice and 200 mg/kg bw in rats. Dermal application of 1600
mg/kg bw to rats had no local or systemic effect. Guinea-pigs were
hyperexcitable after receiving 400 mg/kg bw or more. Rabbits had
central nervous system depression, decreased rectal temperature, pulse
rate, and respiration, nasal discharge, and rales after receiving 100
mg/kg bw, with complete recovery by 48 h. The reactions of dogs to
administration of 100 mg/kg bw and, to a lesser extent, 20 mg/kg bw,
were central nervous system depression, ataxia, muscular weakness,
muscular spasm, uncontrolled vocal spasm and micturition, and
decreased rectal temperature and pulse rate. Haemoconcentration and
increased blood sugar, urea nitrogen, and potassion concentrations
were also seen. No specific pathological lesions were induced. The
lowest dose, 4 mg/kg bw, affected temperature and pulse rate only
slightly . The profile of toxic reactions was consistent with
depression of hypothalamic function. All of the effects were
reversible.
Table 1. Acute toxicity of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
Mouse Oral > 1600 Patton & Sutton (1971)
Rat Oral 600 Patton & Sutton (1971);
Shaw (1973a)
Rat Dermal >1600 Patton & Sutton (1971)
Rat Intraperitoneal 800 Shaw (1971, 1973a)
Rat Inhalation (6 h) 65 Berczy et al. (1972)
Guinea-pig Oral 400-800 Patton & Sutton (1971)
Rabbit Oral > 100 Patton & Sutton (1971)
Rabbit Dermal > 200 Sutton & Williams (1972)
Dog Oral 100 Patton & Sutton (1971)
Baboon Oral 100-250 Patton (1973)
The dermal irritation potential of amitraz (purity, 99%) was
studied in six New Zealand white rabbits weighing 1.9-2.5 kg.
Approximately 24 h after hair had been removed from the dorso-lumbar
region, 0.5 g of technical-grade amitraz was applied under a 2.5-cm
gauze pad moistened with 0.5 ml distilled water, and each treatment
site was occluded with an elastic adhesive dressing for 4 h. At the
end of the exposure period, the dressing was removed and the treatment
site was washed with water. The treated skin was examined on days 1,
2, 3, and 4 after treatment. There was no response to treatment
(Liggett & Smith, 1987a).
The ocular irritation potential of amitraz (purity unspecified)
was studied in six New Zealand white rabbits weighing 2.1-2.8 kg. The
eyes of each animal were examined before instillation of 45 mg (0.1 ml
crystalline powder) of technical-grade amitraz (purity, 98.4%) into
one eye. Both eyes were examined 1 h before and 1, 2, 3, 4, and 7 days
after instillation. Only minimal or slight conjunctival irritation was
observed (Liggett & Smith, 1987b).
Groups of 12 guinea-pigs weighing 300-350 g were treated daily
with 0.1 ml of acetone or 10% amitraz (purity unspecified) or with 1%
dinitrochlorobenzene on the outer surface of each ear for three days.
One week after the start of treatment, the backs and flanks were
clipped and treated with challenge doses of 0.2 ml acetone, amitraz
(10%), or dinitrochlorobenzene (0.25%). The degree of erythema was
assessed after 24 h. Sensitizing activity was observed after treatment
with the positive control but not with amitraz (Sutton, 1971).
The dermal sensitizing effect of amitraz was studied in the
Buehler test. During induction, five male and five female guinea-pigs
received 500 mg technical-grade amitraz (purity, 99%) on the anterior
right flank for 6 h under a dry compress on days 1, 8, and 15. A dry
compress alone was applied on the anterior left flank. After a
two-week rest period, each animal received a challenge of 500 mg
technical-grade amitraz to the right flank and a dry compress alone to
the left flank. The cutaneous reactions were evaluated 24 and 48 h
after challenge. The animals were then sacrificed, and cutaneous
samples were taken from the challenge site in all animals. No clinical
signs or deaths related to treatment were observed, and no cutaneous
reactions were found 24 and 48 h after the challenge with amitraz
(Clouzeau, 1992).
Delayed contact hypersensivity in response to amitraz (purity,
98.4%) was tested in the maximization test described by Magnusson and
Kligman. After random allocation of 20 female guinea-pigs to a control
group and 20 to a test group, the animals were induced by intradermal
injection of 5% w/w amitraz in Alembicol D (a coconut oil) into a
4 × 6-cm clipped area of the dorsal skin on the scapular region. One
week after the injections, the same area was clipped again and given a
topical application of 15 or 30% w/w amitraz in Alembicol D on a
2 × 4-cm patch saturated with the test solution, which was placed on
the skin, covered with impermeable plastic adhesive tape, secured by
an elastic adhesive bandage, and left in place for 48 h. The test and
control animals were challenged topically as described above, two
weeks after the induction, with 15 or 30% w/w technical-grade amitraz
in Alembicol D. The challenge sites were evaluated 24, 48, and 72 h
after removal of the patches, and an arbitrary scale of 0-4 was used
to score the reactions. The dermal reactions observed in the test
animals were more marked and persistent than those seen in the
controls (Kynoch & Parcell, 1988).
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female B6C3F1 mice were fed diets
containing 0, 100, 200, 400, 600, or 800 ppm amitraz (purity, 98.3%),
equal to 0, 13, 26, 53, 96, and 110 mg/kg bw per day for males and 0,
17, 35, 68, 110, and 150 mg/kg bw per day for females, for 13 weeks.
Clinical signs, body weight, and food consumption were recorded
throughout the study. All mice were killed at the end of treatment and
examined grossly but not histopathologically. The only overt sign of
reaction to treatment was an increase in aggressive behaviour, as
evidenced by fighting and resultant cutaneous lesions, among males
treated at doses > 400 ppm. The overall body-weight gain was
statistically significant lower in the males (40%) treated at > 400
ppm and in females (34%) at > 200 ppm. Food consumption did not
differ statistically significantly between the control and treated
groups. The NOAEL was 100 ppm, equal to 17 mg/kg bw per day, on the
basis of the overall reduced body-weight gain of females (Colley et
al., 1981).
Rats
Groups of 21 male and 21 female Ash-Wistar rats were given
amitraz (purity unspecified) suspended in 0.4% Cellosize solution by
oral intubation at a dose of 0, 3, or 12 mg/kg bw per day for 90 days.
They were then killed either immediately or after a three-week
recovery. Treatment of rats with 50 mg/kg bw per day was discontinued
after seven days because of depressed growth and behavioural
disturbances, and treatment with 200 mg/kg bw per day was discontinued
after seven days because of irritability and debilitation. Body
weights were recorded three times per week. Haematological
examinations were made on some control rats and on some rats receiving
12 mg/kg bw per day at 4, 8, and 12 weeks. At autopsy, blood was
withdrawn from the heart to estimate plasma alkaline phosphatase
activity, bilirubin, aspartate and alanine aminotransferase activity,
and sodium and potassium concentrations. Organs were weighed and
prepared for microscopic examination. Significant reductions were seen
in the overall body-weight gain (8%) and absolute (8%) and relative
weights (6%) of the liver of male rats receiving 12 mg/kg bw per day.
The NOAEL was 3 mg/kg bw per day on the basis of these last effects
(Sutton & Williams, 1971).
Groups of 12 rats (strain not given) were exposed daily for 6 h
to dusts of amitraz (purity unspecified) at a concentration of 0,
0.01, 0.1, or 1 mg/L air, on 14 days over three weeks. During exposure
to 0.1 mg/L, signs of mild dyspnoea, slight eye irritation, and
hyposensitivity to noise were recorded. After termination of exposure,
the rats were hypersensitive to touch and became aggressive. Exposure
to 1 mg/L elicited signs similar to but more severe than those seen in
the previous group. In addition, ataxia, increased nasal secretion,
polyuria, body tremors, and coma were observed in the exposed rats.
Consumption of food and water was reduced, and there was body-weight
loss. Reductions in packed cell volume, haemoglobin, red cells, and
plasma protein concentrations in blood may have been related to
treatment. The body tremors, aggressive behaviour, and coma indicate
that the central nervous system was affected (Berczy et al., 1973).
Rabbits
Groups of four to eight male and female New Zealand white rabbits
weighing 2.5-4.5 kg (the health status of which must be regarded as
suspect due to general infection) were treated on the intact skin of
the back (without occlusion) with amitraz (purity unspecified) in
acetone at a dose of 0, 50, or 200 mg/kg bw per day for a total of 15
doses over 21 days. Body weight, food consumption, heart rate, and
rectal temperature were determined. Haematological and blood
biochemical parameters and organ weights were measured, and
histopathological examinations were carried out. A transient sedative
effect and local skin reactions were seen in animals of each sex at 50
and 200 mg/kg bw per day. Body weights and food consumption were
adversely affected, and some deaths occurred that were possibly
related to treatment. Variable tubular degeneration was seen in the
testes, which were underweight. At 50 mg/kg bw per day, body weights
and food consumption were less adversely affected than at the high
dose, and the death of only one male was considered related to
treatment (Sutton, 1973a).
Dogs
Groups of two male and two female beagles received amitraz
(purity unspecified) in capsules at a dose of 0, 0.25, 1, or 4 mg/kg
bw per day for 90 days. The animals were examined clinically before
dosing began and during weeks 2, 4, 8, and 13 of dosing. Food
consumption was recorded daily and body weights weekly.
Haematological, blood biochemical, and urinary parameters were
measured before and at intervals during treatment. During week 13 of
dosing, control animals and those at 4 mg/kg bw per day were placed in
metabolism cages, and their water intake and urine excretion over 24 h
were recorded. The faeces were tested daily for occult blood during
the first week of dosing. At the end of treatment, the animals were
sacrificed, and several organs were weighed and prepared for
histological examination.
On the three first days of dosing, all four animals at 4 mg/kg bw
per day showed central nervous system depression, vomiting, ataxia,
and reduced rectal temperature and pulse rate, which recurred
consistently within 6 h. Hyperglycaemia and occasional glycosuria were
also induced. The livers were slightly enlarged and showed low-grade
microscopic changes. The dose of 1 mg/kg bw per day had similar but
less severe effects. At 0.25 mg/kg bw per day, only minor changes were
seen, although central nervous system depression was seen in one dog
on one occasion 3 h after dosing during week 8 of treatment. The NOAEL
was 0.25 mg/kg bw per day on the basis of central nervous system
depression and reduced rectal temperature and pulse rate (Patton &
Williams, 1971).
Groups of four male and four female beagles were given 0, 0.1,
0.25, or 1 mg/kg bw per day amitraz (purity unspecified) in gelatine
capsules for two years. They were examined clinically before and
immediately after dosing on the first two days and again during weeks
4, 13, 26, 39, 52, 78, and 102. Heart rate and rectal temperature were
monitored serially for up to 24 h after dosing on day l and during
weeks 39, 52, 79, and 103. Signs of toxicity and faecal appearance
were recorded daily and body weights weekly. Haematological, blood
biochemical, and urinary parameters were measured before dosing and
during weeks 4, 13, 26, 52, 78, and 102. In addition, serial blood
samples were obtained during weeks 40 and 53 for estimating blood
sugar and during weeks 91 to 96 for estimating methaemoglobin. The
animals were sacrificed and examined macroscopically on the day after
the final dose, and their organs were weighed and prepared for
histopathological examination.
All eight animals given 1 mg/kg bw per day showed signs of slight
central nervous system depression 3 h after dosing on days 1 and 2,
but all appeared normal by the following morning. On both days, one
dog had a slightly subnormal temperature (38.3°C) at 3 h, which had
returned to normal (38.7°C) by 24 h. Subsequently, all of the animals
in this group appeared clinically normal, apart from one bitch that
was slightly hypothermic 3 h after dosing during weeks 52 and 79. The
NOAEL was 0.25 mg/kg bw per day on the basis of central nervous system
depression (Morgan et al., 1973).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CFLP mice were fed diets
containing 0, 25, 100, or 400 ppm amitraz (purity unspecified),
equivalent to 3, 8, 15, and 60 mg/kg bw per day (wrongly given as 25,
100, and 400 mg/kg bw per day in reference 34, Annex 1), for 80 weeks.
Animals at 100 or 400 ppm gained less weight than the controls over
the first 40 weeks, whereas those at 25 ppm gained more weight than
the controls from week 10 onwards and showed a 20% greater body-weight
gain at the end of the experiment. Calculated over 80 weeks, food
consumption was increased for male mice at 100 and 400 ppm and female
mice at 400 ppm, and decreased for males at 25 and 100 ppm. The
survival rate was similar in all groups. An increased incidence of
lymphoreticular tumours was observed in female mice at 400 ppm (49%
compared with 23% in controls). A slight, not statistically
significant increase in the incidence of all types of liver-cell
tumour was found in animals of each sex fed 400 ppm. No other
pathological finding related to treatment was reported. The NOAEL for
carcinogenicity was 100 ppm, equivalent to 15 mg/kg bw per day, on the
basis of an increased incidence of lymphoreticular tumours in females
at 400 ppm (Burnett et al, 1976; Kakuk, 1979).
Groups of 75 male and 75 female B6C3F1 hybrid mice, 33 days old,
were given diets containing 25, 100, or 400 ppm amitraz (purity,
97.9%), equal to 2.3, 9.6, and 45 mg/kg bw per day for males and 2.6,
11, and 50 mg/kg bw per day for females, for 104 weeks. The untreated
controls consisted of 100 male and 100 female mice. Clinical signs,
food consumption, and body weight were recorded throughout the study.
Survivors were killed at the end of the dosing period and all animals
were subjected to a complete gross autopsy. All tissues were collected
from all animals and preserved for future examination.
Histopathological examination was performed on all tissues from the
high-dose group and controls, and on the liver, pancreas, spleen,
lung, stomach, pituitary, thyroid, adrenals, ovaries, uterus, sternum,
and all abnormal tissues found grossly from animals at the low and
intermediate doses.
Males at 400 ppm were hyperactive and behaved aggressively. The
incidence of cutaneous ulceration and inflammation of the perigenital
and perianal areas was greater in mice at 100 and 400 ppm than in
those at 25 ppm or in controls, and the incidence of urogenital
swelling was greater in all treated mice than in controls. The
incidences of gross adverse effects in treated females were comparable
to the control incidences. There was clearly increased mortality of
males at 400 ppm (20/75) and of males and females combined (37/150).
Mean body-weight gain was reduced in mice at 400 ppm (by 30-50%)
throughout the study, with a significant decrease in females at 100
ppm over the last 74 weeks of the study. Food consumption was
marginally reduced initially in animals at 100 and 400 ppm but was
comparable to that of controls from week 25 to termination. Increased
liver and lymph node involvement was seen in males and females at 400
ppm, and the incidence of preputial gland enlargement in males at 400
ppm was greater than that in controls (20/75 vs 12/100). The
prominence of the limiting ridge of the stomach was greater than in
controls for females at all doses and for males at 100 and 400 ppm.
Decreased production of myeloid elements accompanied by an increase in
erythroid elements resulted in a significant decrease in the
myeloid:erythroid ratio for males at 400 ppm and females at 100 or
400 ppm. The incidences of spleen haematopoiesis in males and of
stomach focal hyperkeratosis in males and females at all three doses
were greater than that in controls. There was apparent liver
involvement in females, with an increased incidence of hepatocellular
carcinoma at 400 ppm (15/75 vs 2/100 hepatocarcinoma; 16/75 vs 4/100
hepatocellular adenoma). This was accompanied by a dose-related
increase in the incidence of hyperplastic nodules and telangiectatic
and basophilic foci of the liver in animals at 100 and 400 ppm. Males
at 400 ppm also had an apparent increase in the incidence of
hyperplastic nodules and telangiectatic and basophilic foci of the
liver. The NOAEL for carcinogenicity was 100 ppm, equal to 11 mg/kg bw
per day, on the basis of hepatocellular carcinoma in females at
400 ppm. This dose was considered to be greater than the conventional
maximum tolerated dose. The NOAEL for long-term toxicity was 25 ppm,
equal to 2.3 mg/kg bw per day, on the basis of general toxicity
(Colley et al., 1983).
Rats
Groups of 40 male and 40 female Ash-Wistar rats received amitraz
(purity, 97.8%) at a dietary concentration of 0, 15, 50, or 200 ppm
(equal to 0, 0.77, 2.5, or 10 mg/kg bw per day in males and 0, 0.97,
3.1, or 13 mg/kg bw per day in females) for two years. Body weights
were measured twice per week during the period of maximal growth and
later weekly. Food consumption was determined weekly during this
growth period and later monthly. Haemato-logical, biochemical, and
urinary analysis were performed during and at the end of the study,
and organs were weighed and examined grossly and histologically at the
end of study.
Rats at 200 ppm were occasionally nervous, excitable, and
aggressive, and their food consumption was temporarily reduced. The
overall body-weight gain of males was significantly reduced (10%).
Rats at 50 and 15 ppm showed no adverse reactions to treatment. The
incidence, type, and time to appearance of tumours were not
significantly different in treated and control groups. The NOAEL for
toxicity was 50 ppm, equal to 2.5 mg/kg bw per day, on the basis of
effects on the central nervous system and reduced overall body-weight
gain in males (Sutton & Offer, 1973).
(d) Genotoxicity
The results of tests for the genotoxicity of amitraz are
summarized in Table 2. The results of the tests in vitro and
in vivo were negative.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a three-generation study of reproductive toxicity, groups of
10 male and 20 female newly weaned Boots-Wistar rats were fed amitraz
(purity, 99.8%) at a dietary concentration of 0, 15, 50, or 200 ppm,
equal to 0, 1.3, 4.4, and 16 mg/kg bw per day in males and 0, 1.5,
5.1, and 20 mg/kg bw per day in females. After the F1 generation had
been weaned, 12 males and 24 females from each group were retained for
breeding and maintained on the diet. The procedure was repeated until
the F3 generation was weaned. Amitraz at 200 ppm decreased the
growth, food consumption, fertility, and viability of offspring of the
F0 generation, and this dose was eliminated when the F1 generation
had been weaned, because of the very low survival. No effect was found
on the number of litters or mean litter size at 50 ppm; however, a
decreased number of young alive at 21 days was observed in all
generations. No further effects due to treatment were found in this or
the other group. The NOAEL was 50 ppm, equal to 4.4 mg/kg bw per day,
for maternal toxicity and 15 ppm, equal to 1.3 mg/kg bw per day, for
developmental toxicity (Sutton, 1973b).
(ii) Developmental toxicity
In a study of developmental toxicity, groups of 11-13 female
Boots-Wistar rats received amitraz (purity, 99.8%) at a dose of 0, 1,
3, or 12 mg/kg bw per day on days 8-20 of gestation. The rats were
killed on day 21, and the uterine contents were examined. There were
no deaths or clinical signs of toxicity. Food consumption, body-weight
gain, average litter size, fetal viability, and the implantation index
were not affected. At 12 mg/kg bw, fetal weight was lower than in the
controls, and the calcification of the sternebrae was less advanced.
The NOAEL for maternal toxicity was 12 mg/kg bw per day, and that for
developmental toxicity was 3 mg/kg bw per day, on the basis of reduced
fetal weight and reduced calcification of the sternebrae (Sutton,
1973c).
Table 2. Results of tests for the genotoxicity of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium 31-500 µg/plate 99.9 Negative Everest &
TA1535, TA1537, Wilcox (1976)
TA1558
Reverse mutation S. typhimurium 0, 33, 100, 333, 1000, 98.4 Negative McGregor &
TA98, TA100, TA1535, 3300, 10 000 µg/plate Printice (1983)
TA1537, TA1538
Chromosomal Human lymphocytes 0, 5, 10, 20 µg/ml -S9; 99.5 Negative Brooker et al.
aberration 0, 3, 5, 30 µg/ml +S9 (1988)
Cell mutation Mouse L5178Y tk+/- 0.06-33 µg/ml +S9; 98.4 Negative McGregor &
cells 0.06-20 µg/ml -S9 Riach (1983a)
Unscheduled Human embryonic 20, 60, 100, 140, 180, 100 Negative McGregor &
DNA synthesis fibroblasts 220, 260, 300 µg/ml ±S9 Riach (1983b)
DNA damage Chinese hamster 0.01-0.3 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Reverse mutation S. typhimurium 0, 100, 200, 400 mg/kg NR Negative Everest (1976);
(host-mediated, G46, TA1532 bw, single dose Wilcox (1976)
mouse) TA1964
Dominant lethal Female CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977a)
for 5 days
Table 2. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
Dominant lethal Male CFLP mice 0, 12, 50 mg by NR Negative Palmer &
mutation intragastric intubation James (1977b)
for 5 days
NR, not reported; S9, 9000 × g microsomal fraction from rodent liver
In another study of developmental toxicity, groups of 24 mated
Sprague-Dawley rats weighing 215-280 g were given 0, 7.5, 15, or 30
mg/kg bw per day amitraz (purity, 99.7%) by gavage on days 6-15 of
gestation. Immediately after mating, the females were assigned to
treatment groups by a randomization process based on stratified body
weight. Each female was then individually identified by ear notching.
All females were examined once or twice daily for clinical signs of
ill health, toxicity, or behavioural changes, and body weights and
food intake were recorded on days 0, 6, 10, 15, and 20 of gestation.
On day 20 of gestation, the females were killed and their uterine
contents examined.
One control female was found dead on day 10 of gestation; there
were no other deaths. The principal clinical sign was fur staining,
which was slightly more frequent at 30 mg/kg bw per day. At this dose,
a slight loss of body weight up to day 10 of gestation was followed by
reduced body-weight gain at termination. The body-weight gain of rats
at 15 mg/kg bw per day was slightly reduced (10%), but that of animals
at 7.5 mg/kg bw per day was not adversely affected by treatment. Food
intake of rats at 30 mg/kg bw per day, and to a lesser extent those at
15 mg/kg bw per day, was initially lower than in the control group;
there was no effect on food intake at 7.5 mg/kg bw per day. The
pregnancy rate was high in all groups, and no adverse findings were
made at necropsy. The treatment did not adversely affect the number of
implantations, the incidence of post-implantation loss, or the number
or sex ratio of fetuses. Animals at 30 mg/kg bw per day had a
statistically significantly increased incidence of dilated ureters and
increased bilateral renal pelvic cavitation. Although the incidence of
the latter lesion at 15 mg/kg bw per day was statistically
significantly higher than that of the concurrent controls
(p < 0.05), the group percentage increase (5.7%) was comparable to
the upper range of the relevant historical control values (5.4%). The
NOAEL was 7.5 mg/kg bw per day for both maternal toxicity, on the
basis of reduced body-weight gain, and for developmental toxicity, on
the basis of increased incidences of dilated ureters and renal pelvic
cavitation (McIntyre, 1987a).
Rabbits
In a study of developmental toxicity, groups of 8-10 New Zealand
rabbits were treated with amitraz (purity unspecified) at a dose of 0,
1, 5, or 25 mg/kg bw per day on days 6-18 of pregnancy and were killed
on day 30. At 25 mg/kg bw per day, the number of litters and mean
litter size were decreased and abortions were observed. No increase in
the incidence of congenital abnormalities was found. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, the highest dose tested,
and the NOAEL for developmental toxicity was 5 mg/kg bw per day on the
basis of a reduced number of litters and litter size (Sutton, 1973d).
In another study of developmental toxicity, groups of 16 mated
female New Zealand white rabbits weighing 3-4 kg were given amitraz
(purity, 99.7%) by gavage at a dose of 0, 3, 6, or 12 mg/kg bw per day
on days 7-19 of gestation. All females were examined once or twice
daily for clinical signs of ill health, toxicity, or behavioural
changes, and body weight and food intake were recorded on days 0, 7,
13, 19, 24, and 28 of gestation. On day 28 of gestation, the surviving
females were killed and their uterine contents examined.
One female at 12 mg/kg bw per day died, and three females at this
dose were killed because of poor clinical condition or abortion. Two
of the deaths were considered to be directly related to treatment. Two
females at 6 mg/kg bw per day died; at 3 mg/kg bw per day, one female
was killed and a further two died. Two control animals also died.
Langour, polypnoea, and squinting were observed in all treated groups,
and the incidence, severity, and duration of these signs appeared to
be dose-related. At 12 mg/kg bw per day, body-weight loss followed by
a reduction in body-weight gain were observed during dosing, but the
weight gain was similar to that of controls on cessation of dosing.
Treatment at 3 or 6 mg/kg bw per day had no effect on body weight.
Food intake was reduced during dosing and up to day 24 at 12 mg/kg bw
per day, but no adverse effect was seen at 6 or 3 mg/kg bw per day. No
adverse effects were seen at necropsy. Three of the surviving females
at 12 mg/kg bw per day lost their litters on day 28 of gestation.
Litter paraments were not affected at 6 or 3 mg/kg bw per day, as
assessed by the numbers of corpora lutea, implantation sites, and
viable fetuses. The mean litter and fetal weights and sex ratio of
fetuses were not adversely affected by treatment. No major fetal
defects were recorded that were considered to be related to treatment,
and there were no treatment-related effects on the incidence of minor
or variant anomalies. There was no NOAEL for maternal toxicity because
of deaths of animals at all doses and in the control group. The NOAEL
for developmental toxicity was 6 mg/kg bw per day, on the basis of
litter loss (McIntyre, 1987b).
(f) Special studies
(i) Effects on the thymus and hormone concentrations
Diets containing 400 ppm amitraz (purity, 97.1%), equal to 110
mg/kg bw per day for males and 150 mg/kg bw per day for females, were
given to 24 male CFLP mice for up to 18 weeks and to 52 female CFLP
mice for up to 33 weeks. A group of 36 males and 64 females given
plain diet served as controls. Body weight and food consumption were
measured regularly throughout the study, and the animals were examined
for overt signs of toxicity at least once a week. Vaginal smears were
monitored every morning during weeks 6-9, 15-18, 23-26, and 30-33.
Twelve male and 12 female controls were killed during the first week,
and 12 males and 12 females from both treated and control groups were
killed after 9 and 18 weeks. The remaining animals were killed after
33 weeks. The ß-estradiol concentration of the blood of female mice
was estimated at weeks 9 and 18, and the thymuses from all animals
were weighed at autopsy. Detailed histological examinations were
conducted on a selected range of tissues.
Body-weight gain was reduced in amitraz-treated mice, to a
slightly greater degre in the males, and food consumption was markedly
increased, particularly in the females. Examination of vaginal smears
indicated prolongation of estrus in treated mice, but there was no
measurable effect on the circulating concentrations of ß-estradiol.
The thymus weights and histological appearance were not affected by
treatment. Histopathological examination revealed two lymphoreticular
tumours in the treated females and two in controls. In comparison with
the controls, females given amitraz for 33 weeks had a higher
incidence of foci of inflammatory cells in the liver (Brown et al.
1978).
(ii) Effects on the estrus cycle and hormone concentrations
Mice
The effects of amitraz on the estrus cycle and hormone
concentrations were evaluated in groups of 70 female B6C3F1 mice fed
diets containing amitraz (purity, 98-100%) at 0, 25, 100, or 400 ppm,
equivalent to 0, 3.8, 15, and 60 mg/kg bw per day, for up to 28 weeks.
Permanently stained and mounted vaginal smears were prepared from each
animal daily for 30 days after 13 weeks of treatment, and the stage of
the oestrus cycle on each day was determined by microscopic
examination of the cell population on each slide. Blood samples were
taken at necropsy after overnight starvation and assayed for
dehydroepiandrosterone sulfate, estradiol, progesterone, testosterone,
lutropin, follitropin, prolactin, thyroxine, triiodothyronine, and
thyroid hormone uptake, as indicators of hormonal status and routine
clinical chemical parameters. The fresh liver weight was recorded at
necropsy.
Pro-estrus was prolonged, and a trend towards reduced duration of
diestrus was evident in animals at 400 ppm. The blood concentrations
of progesterone were lower and the concentrations of
dehydroepiandrosterone sulfate were higher in animals at 400 ppm and
to a lesser degree in those at 100 ppm when compared with controls.
The relative liver weights were increased at 400 (by 4%) and 100 ppm
(by 5%). There were no treatment-related effects at 25 ppm. The NOAEL
was 25 ppm, equivalent to 3.8 mg/kg bw per day, on the basis of lower
blood concentrations of progesterone, higher concentrations of
dehydroepiandrosterone sulfate, and increased relative liver weight
(Hounsell & Rush, 1984).
Rats
Groups of 20 female 22-week-old Boots-Wistar rats were fed diets
containing 0 or 200 ppm (equivalent to 0 and 12 mg/kg bw per day)
amitraz (purity unspecified) for 18 weeks. Vaginal smears were taken
routinely over 32 days. After fixation in methanol, the smears were
stained with 1% aqueous methylene blue and examined for keratinized
cells under 60 × magnification. Estrus was characterized by the
presence of keratinized cells only, and the cycle length was taken as
the interval between the first day of estrus in successive cycles.
The average cycle length of the controls was 4.3 days; two of the
controls had prolonged periods in estrus (four and six days), and a
third had a period of prolonged diestrus with a cycle lasting 18 days.
In the remaining animals, the shortest cycles were two days and the
longest six days. The low incidence of prolonged estrus among these
animals confirms that the technique of smearing did not affect vaginal
cytology. In the treated rats, the average cycle length was 6.1 days,
and the range was 2-16 days. Six rats had one or more periods of
prolonged diestrus; four of these had been acyclic in a preliminary
test. Estrus lasted for three to eight consecutive days in seven rats,
two of which had shown a similar tendency in a preliminary test. The
second test showed that the cycle length was significantly altered by
treatment, either estrus or diestrus being prolonged. Treated rats
thus had longer oestrus cycles than controls, resulting from prolonged
periods of estrus or diestrus (Merryman & Sutton, 1972).
(iii) Mechanism of action
The effects of amitraz and its major metabolite,
N-methyl- N'-(2,4-xylyl)formamidine, given orally or intravenously
on the cardiovascular system, pupil diameter, and the respiratory
system were studied in conscious and anaesthetized rats, cats, and
dogs. Both compounds caused a fall in blood pressure, sometimes
preceded by hypertension and bradycardia. The threshold for the effect
in conscious rats dosed orally with amitraz was 1 mg/kg bw. Amitraz
also caused mydriasis, sedation, and a reduced respiratory rate. The
bradycardia, sedation, and mydriatic effect was antagonized by the
alpha2-blocking agent yohimbine but not by the alpha1-blocking agent
prazosin, indicating that the effects were caused by stimulation of
presynaptic alpha2-adrenoceptors. The lethal effect of amitraz in mice
and dogs has also been shown to be inhibited by yohimbine (Parkinson &
Sim, 1970; Cullen & Reynoldson, 1983; Hsu & Kakuk, 1984; Moser &
MacPhail, 1985; Hsu et al., 1986; Hovda & McManus, 1993).
Amitraz and N-methyl- N'-(2,4-xylyl)formamidine were tested
for their ability to potentiate the pressor responses to tyramine in a
pithed rat preparation, since a structurally related compound,
chlordimeform, inhibited monoamine oxidase in vitro in rat liver
homogenates and centrally in vivo through an effect on brain
serotonin concentrations. These effects were obvious only with nearly
toxic oral doses of 80 mg/kg bw amitraz and 40 mg/kg bw
N-methyl- N'-(2,4-xylyl)formamidine (Parkinson, 1974).
Amitraz and chlordimeform were tested for their ability to
inhibit prostaglandin synthesis after intraperitoneal administration
in rats. Both compounds had antipyretic and antiinflam-matory effects
at doses of 5-80 mg/kg bw. They reduced yeast-induced fever, with
potencies intermediate between those of indomethacin and aspirin, and
antagonized carrageenan-induced swelling of the hind paw. They also
inhibited the synthesis of prostaglandin E2 from arachidonic acid by
bovine seminal vesicle microsomes. The potency of amitraz in this
assay was the same as that of aspirin (Yim et al., 1978).
(g) Studies on metabolites
(i) Acute toxicity
The acute toxicity of metabolites of amitraz (purity unspecified)
has been investigated in several species (Table 3).
Table 3. Acute toxicity of metabolites of amitraz
Species Route LD50 or LC50 Reference
(mg/kg bw or
mg/L air)
N-Methyl-N'-(2,4-xylyl)formamidine
Mouse Oral 150 Sutton (1970a)
Rat Oral 200 Sutton (1970b)
Dog Oral >20 Morgan (1973);
Morgan & Williams (1974)
4-Amino-meta-toluic acid
Mouse Oral >1600 Shaw & Williams (1973a)
Rat Oral >1600 Shaw & Williams (1973b)
Form-2',4'-xylidide
Rat Oral 1600 Shaw (1973b)
(ii) Short-term toxicity
Rats
Groups of 10 male and 10 female newly-weaned Boots-Wistar rats
were dosed by gastric intubation with
N-methyl- N'-(2,4-xylyl)formamidine (purity unspecified) for 90
days at 0, 0.25, 1, 3, or 12 mg/kg bw per day. Body weights were
recorded three times per week, and the rats were observed each day for
signs of toxicity. At 3 mg/kg bw per day, an initial reduction in
body-weight gain was seen in males. At 12 mg/kg bw per day, the rats
became nervous and difficult to handle, and two deaths occurred. The
growth rate was reduced in animals of each sex but to a greater extent
in the males. At the end of experiment, haemoglobin and haematocrit
values were decreased in males, and the number of erythrocytes was
decreased in females. Slight biochemical changes were seen. At
autopsy, the weights of the adrenals, ovaries, uterus, and liver in
females and spleen and testes in males were increased, but the only
histopathological changes were a slight increase in lymphoid
infiltration, some sinusoidal leukocytosis, and loss of glycogen in
the livers of most males and slight cellular accumulations in the
hearts of some females. The NOAEL was 1 mg/kg bw per day on the basis
of the reduced body-weight gain and increased organ weights (Shaw &
Williams, 1975).
Groups of five male and five female Wistar rats were dosed by
gastric intubation with 4-amino- meta-toluic acid (purity
unspecified) at a dose of 0, 40, 100, or 250 mg/kg bw per day for 21
days. At 250 mg/kg bw per day, slight decreases in weight gain and in
blood urea nitrogen concentration were observed in males and an
increased relative weight of the spleen in females. No gross
pathological changes due to treatment were found. The NOAEL was
250 mg/kg bw per day, the highest dose tested (Shaw, 1975).
Dogs
Groups of four male and four female beagles were given gelatin
capsules containing N-methyl- N'-(2,4-xylyl)formamidine (purity
unspecified) as free base diluted in lactose to 1% at a dose of 0,
0.1, 0.25, or 1 mg/kg bw per day for 90 days. Clinical signs were
recorded daily, food consumption twice daily, and body weight once a
week. Ophthalmoscopic examination and recordings of temperature and
heart rate were carried out before dosing and after 6 and 12 weeks.
Haematological, clinical chemical, and urinary analyses were perfomed,
and gross and histopathological examinations were carried out.
At 0.25 and 1 mg/kg bw per day, abnormal quietness and drowsiness
and significantly lower body temperature (by up to 16.2°C) were
observed 0.5-4 h after dosing. At 1 mg/kg bw per day, the heart rate
was significantly reduced (by up to 40 beats/min) 1-2 h after dosing,
and the liver weight and urine volume were increased. A slight
reduction in thymus weight was observed at 0.25 and 1 mg/kg bw per
day. No histopathological anomalies were found. The NOAEL was 0.1
mg/kg bw per day on the basis of central nervous system depression and
lowered body temperature (Chesterman et al., 1973).
Groups of four male and four female beagles were given gelatin
capsules containing 4-amino- meta-toluic acid (purity unspecified) at
a dose of 0, 16, 40, or 100 mg/kg bw per day for 90 days. Slightly
increased urinary concentrations of total reducing substances other
than glucose were found at the highest dose. No dose-related effects
were observed on behaviour, electrocardiogram, heart rate, rectal
temperature, body weight, food consumption, haematological or blood
chemical parameters, organ weights, or histopathological appearance
(Morgan et al., 1974).
(iii) Genotoxicity
The results of tests for the genotoxicity of potential
metabolites of amitraz are summarized in Table 4. Except for a single
positive response to 2.4-dimethylaniline in the assay for forward
mutation in mouse lymphoma cells, in the presence of metabolic
activation, the results of the tests in vitro and in vivo were
negative.
3. Observations in humans
In a double-blind, randomized cross-over study, six healthy male
volunteers aged 18-45 years and weighing 60-70 kg received sequential
single oral doses of 0, 0.063, and 0.13 mg/kg bw amitraz, two to three
weeks apart. Each dose was given with 150 ml water, 30 min after
breakfast. Pulse rate, respiration rate, blood pressure, and
temperature were measured at - 1, - 0.5, 1, 3, 6, 12, 24, and 36 h,
and electrocardiograms were performed at -1, 1, 3, 6, 12, 24, and 36 h
with respect to dosing. Pupil responsiveness and psychomotor
performance were evaluated before treatment and at 2.5 and 8 h. Urine
was collected at 0-36 h and 36-60 h. There were no clinically
significant changes in vital signs or electrocardiographic parameters.
Moreover, haematological, blood chemical, and urinary parameters,
pupil responsiveness, and psychomotor performance were unaffected by
treatment. The NOAEL was 0.13 mg/kg bw, the highest dose tested (Cass,
1992).
Two human volunteers who received a single oral dose of 0.25
mg/kg bw 14C-amitraz showed drowsiness, disorientation, slurred
speech, decreased pulse rate and blood pressure, and other effects
(Campbell & Needham, 1984c).
In a double-blind cross-over study, four male and two female
volunteers, aged 21-42 years and in normal health, received two doses
of 2 mg (about 0.03 mg/kg bw) of the amitraz metabolite,
N-(2,4-dimethylphenyl)- N'-methylformamidine, one week apart in a
capsule with 100 ml of water, or placebo. Blood pressure, pulse rate,
and temperature were measured at half-hourly intervals over 7 h, and
mental alertness was assessed at 0, 3, and 7 h. An electrocardiograph
was performed, and urine was collected before dosing and at 0-7 h and
7-24 h. The urine samples were analysed to estimate the amount of the
metabolite, 3-methyl-4-aminobenzoic acid, that had been excreted.
Mental alertness was estimated at 0, 3, and 7 h. No difference was
seen from those receiving the placebo. The NOAEL was 0.03 mg/kg bw,
the only dose tested (Hall et al., 1975).
Symptoms of central nervous system depression ranging from
sedation to coma lasting more than 24 h, depressed respiration,
hypotension, and bradycardia have been described in 11 reports after
accidental or intentional ingestion of uncertain amounts of amitraz.
In nine cases, recovery was complete after symptomatic and supportive
treatment (Groppi, 1977; Ros & Aken, 1994; Pronczuk et al., 1995).
Table 4. Results of tests for the genotoxicity of metabolites of amitraz
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
N-Methyl-N'-(2,4-xylyl)formamidine
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983a)
TA1337, TA1538
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Form-2',4'-xylidide
Reverse mutation S. typhimurium < 5000 µg/plate NR Negative Richold et al.
TA98, TA100, TA1535, (1983b)
TA1337, TA1538
DNA damage Chinese hamster 0.01-1.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Forward mutation Mouse L5178Y tk+/- 1, 3.3, 10, 33, 100, 200, NR Positive + S9 McGregor &
lymphoma cells 300, 333, 400, 500, Negative -S9 Riach (1984)
600 µg/ml
Cell transformation C3H/10 T1/2 clone 8 5, 10, 20 µg/ml +S9 NR Negative McGregor et al.
mouse embryo fibroblasts 100, 200, 400 µg/ml -S9 (1984)
DNA damage Chinese hamster 0.03-2.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
Table 4. (continued)
End-point Test system Concentration Purity Results Reference
or dose (%)
4-Amino-meta-toluic acid
DNA damage Chinese hamster 0.03-3.0 mmol/L ±S9 NR Negative Petzold et al.
V79 lung fibroblasts (1977)
In vivo
Product of acid hydrolysis of N-methyl-N'-(2,4-xylyl)formamidine and form-2',4'-xylidide
Micronucleus Mouse bone 56, 113, 225 mg/kg bw NR Negative Hounsell &
formation marrow twice, 24 h apart Walker (1983)
Comments
Amitraz was well absorbed, extensively metabolized, and rapidly
excreted, mainly in the urine, after oral administration to mice,
rats, dogs, pigs, hens, cows, baboons, and humans. After oral
treatment of mice with 14C-amitraz, 86% of the radiolabelled dose was
excreted, 62% in the urine, within the first 24 h. All of it had been
excreted by 96 h, with 73% in the urine of animals of each sex. The
concentrations of residues were highest in liver, adrenal glands, and
eyes. After oral administration of 14C-amitraz to rats, 94% of the
dose was recovered within three days, with 82% in urine and 12% in
faeces. After oral administration of 14C-amitraz to two humans,
77-87% was recovered within three days. Amitraz is hydrolysed to two
components, N-methyl- N-(2,4-xylyl)formamidine and
form-2',4'-xylidide. The former is the pharmacologically active
compound and accounted for 5-30% of the total urinary excretion in
mice and rats; it was further metabolized to 4-amino- meta-toluic
acid and the acetyl and formyl conjugates, 4-acetamido- and 4-
formamido- meta-toluic acids. These five metabolites were also found
in plants.
Amitraz has low acute oral toxicity in rats but is more toxic in
dogs. The LD50 values ranged from 100 mg/kg bw in dogs to > 1600
mg/kg bw in mice, indicating that dogs are more sensitive. The toxic
signs after oral administration to mice and rats were
hyperexcitability, ataxia, tremor, and ptosis. Amitraz had no
sensitizing potential in guinea-pigs, and no local irritation was
found in rabbits after a single application to skin or eyes. There was
evidence of delayed contact hypersensitivity after application of
amitraz either topically or intradermally.
WHO (1996) has classified amitraz as slightly hazardous.
In a 13-week study in which mice were fed diets providing 0, 100,
200, 400, 600, or 800 ppm, the NOAEL was 100 ppm, equal to 17 mg/kg bw
per day, on the basis of reduced overall body-weight gain (by 34%).
In a 90-day study, rats were given amitraz at doses of 0, 3, or
12 mg/kg bw per day by gavage. The NOAEL was 3 mg/kg bw per day, on
the basis of reduced terminal body-weight gain, absolute liver weight,
and relative liver weight.
In a 90-day study in dogs, amitraz was administered at doses of
0, 0.25, 1, or 4 mg/kg per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression
and reductions in rectal temperature and pulse rate.
In a two-year study, dogs were given amitraz at doses of 0, 0.1,
0.25, or 1 mg/kg bw per day in gelatin capsules. The NOAEL was 0.25
mg/kg bw per day, on the basis of central nervous system depression.
In two 90-day studies, the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine was administered to rats at
doses of 0, 0.25, 1, 3, or 12 mg/kg bw per day by gastric intubation
or to dogs at 0, 0.1, 0.25, or 1 mg/kg bw per day by gelatine
capsules. The NOAEL was 1 mg/kg bw per day in rats, on the basis of
reduced body-weight gain and increased organ weights, and 0.1 mg/kg bw
per day in dogs, on the basis of central nervous system depression and
lowered body temperature.
In a 21-day study in rats and a 90-day study in dogs, the amitraz
metabolite 4-amino- meta-toluic acid was given at doses of 0, 40,
100, or 250 mg/kg bw per day by gastric intubation to rats or 0, 16,
40, or 100 mg/kg bw per day by gelatin capsules to dogs. The NOAEL was
250 mg/kg bw per day in rats and 100 mg/kg bw per day in dogs, (the
highest doses tested).
In an 80-week study, mice were fed diets containing 0, 25, 100,
or 400 ppm, equivalent to 0, 3.8, 15, and 60 mg/kg bw per day (wrongly
given as 25, 100, or 400 mg/kg bw per day in the 1980 JMPR report).
The NOAEL for carcinogenicity was 100 ppm, equivalent to
15 mg/kg bw per day, on the basis of an increased incidence of
lymphoreticular tumours in females at 400 ppm.
In a two-year study in which mice were fed diets providing 0, 25,
100, or 400 ppm, the NOAEL for carcinogenicity was 100 ppm, equal to
11 mg/kg bw per day, on the basis of the occurrence of hepatocellular
carcinoma in females at 400 ppm. This dose was considered to be
greater than the conventional maximum tolerated dose. The NOAEL for
toxicity was 25 ppm, equal to 2.3 mg/kg bw per day, on the basis of
generalized toxic effects.
In a two-year study, rats were fed diets containing 0, 15, 50, or
200 ppm. The NOAEL for toxicity was 50 ppm, equal to 2.5 mg/kg bw per
day, on the basis of effects on the central nervous system and reduced
overall body-weight gain in males. There was no evidence of
carcinogenicity.
The genotoxic potential of amitraz has been adequately evaluated
in a range of assays in vitro and in vivo. The Meeting concluded
that amitraz is not genotoxic.
In view of the lack of genotoxicity and the finding of tumours
only in mice and only at concentrations at which severe toxicity was
observed, the Meeting concluded that amitraz is not likely to pose a
carcinogenic risk to humans.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 15, 50, or 200 ppm, the NOAEL was 50 ppm,
equal to 4.4 mg/kg bw per day, for maternal toxicity and 15 ppm, equal
to 1.3 mg/kg bw per day for developmental toxicity. No teratogenic
effect was observed.
In two studies of developmental toxicity, pregnant rats were
given amitraz at 0, 1, 3, or 12 mg/kg bw per day by gavage on days
8-20 of gestation or 0, 7.5, 15 or 30 mg/kg bw per day by gavage on
days 6-15. The NOAEL for maternal toxicity was 7.5-12 mg/kg bw per
day, and that for developmental toxicity was 3-7.5 mg/kg bw per day.
In two studies of developmental toxicity in rabbits, amitraz was
given at doses of 0, 1, 5, or 25 mg/kg bw per day by gavage on days
6-18 of gestation or 0, 3, 6, or 12 mg/kg bw per day by gavage on days
7-19. The NOAEL for maternal toxicity was 25 mg/kg bw per day in one
study, but a NOAEL was not identified in the other because all of the
treated animals died. The NOAEL for developmental toxicity was 3-6
mg/kg bw per day.
The effect of amitraz on the estrus cycle and hormone levels was
evaluated in mice fed diets containing 0, 25, 100, or 400 ppm for 28
weeks and in rats fed diets containing 0 or 200 ppm for 18 weeks. In
mice, pro-estrus was prolonged at 400 ppm; blood levels of
progesterone were reduced and those of dehydroepiandrosterone were
increased at 100 and 400 ppm. The NOAEL was 25 ppm, equivalent to
3.8 mg/kg bw per day, on the basis of the changed hormone levels. The
estrus cycles were longer in treated than in control rats, with no
NOAEL.
In a double-blind, randomized, cross-over study of tolerance, six
healthy adult male volunteers received sequential single oral doses of
0, 0.063, and 0.13 mg/kg bw amitraz, two to three weeks apart. The
NOAEL was 0.13 mg/kg bw, the highest dose tested.
Two human volunteers who received single oral doses of 0.25 mg/kg
bw 14C-amitraz showed effects including drowsiness, disorientation,
slurred speech, and decreased pulse rate and blood pressure.
In a double-blind cross-over study of tolerance, six adult
volunteers received two single doses of placebo or 2 mg (about 0.03
mg/kg bw) of the amitraz metabolite
N-methyl- N-(2,4-xylyl)formamidine one week apart. The NOAEL was
0.03 mg/kg bw, the only dose tested.
The Meeting established an ADI of 0-0.01 mg/kg bw on the basis of
the NOAEL of 1.3 mg/kg bw per day in the study of reproductive
toxicity in rats and a safety factor of 100. The pharmacological
effects on the central nervous system seen in dogs, with a NOAEL of
0.25 mg/kg bw per day, were considered not to be relevant for setting
the ADI because they were reversible and the dogs became tolerant.
Moreover, a NOAEL of 0.13 mg/kg bw per day was seen for such effects
in humans.
The Meeting established an acute RfD of 0.01 mg/kg bw, on the
basis of the NOAEL of 0.13 mg/kg bw per day in the study in humans and
a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 2.3 mg/kg bw per day (toxicity in a
two-year study of carcinogenicity)
Rat: 3 mg/kg bw per day (toxicity in a 90-day study of
toxicity)
50 ppm, equal to 2.5 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
50 ppm, equal to 4.4 mg/kg bw per day (maternal
toxicity in a three-generation study of reproductive
toxicity)
15 ppm, equal to 1.3 mg/kg bw per day (developmental
toxicity in a three-generation study of reproductive
toxicity)
12 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
3 mg/kg bw per day (developmental toxicity)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
5 mg/kg bw per day (developmental toxicity)
Dog: 0.25 mg/kg bw per day (toxicity in a two-year study of
toxicity)
Human: 0.13 mg/kg bw (toxicity after single oral doses)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies to further characterize the effects on the
reproductive system of female rodents
2. Further observations in humans
List of end-points relevant for setting guidance values for dietary
and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid/complete
Distribution Liver, adrenals, eyes
Potential for accumulation Minimal
Rate and extent of excretion Rapid/complete, 80-100% in 96 h
Metabolism in animals Metabolites same in rodents, dogs,
humans
Toxicologically significant
compounds (animals, plants, N-Methyl-N'-(2,4-xylyl)formamidine
and environment)
Acute toxicity
Rat: LD50 oral 600 mg/kg bw
Rabbit: LD50 dermal > 200 mg/kg bw
Rat: LC50 inhalation 65 mg/L
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing (Buehler test)
Short-term toxicity
Target/critical effect Central nervous system depression, dog
Lowest relevant oral NOAEL 0.25 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 50 mg/kg bw per day
Lowest relevant inhalation Rat: 0.01 mg/L air
NOAEL
Genotoxicity Unlikely to be genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Lymphoreticular tumours, hepatocellular
carcinomas
Lowest relevant NOAEL Mouse: 11 mg/kg bw per day (80-week and
2-year studies)
Carcinogenicity Unlikely to be carcinogenic
Reproductive toxicity
Reproduction target/critical Decrease in number of young alive
effect at 21 days
Lowest relevant reproductive Rat: 1.3 mg/kg bw per day
NOAEL (developmental toxicity)
Developmental target/critical Reduced fetal weight
effect
Lowest relevant developmental Rat: 3 mg/kg bw per day
NOAEL (developmental toxicity)
Neurotoxicity/Delayed Acute central nervous system
neurotoxicity depression
Other toxicological studies Prolongation of estrus cycles and
reduction of blood concentrationof
progesterone (mouse, rat)
Medical data Central nervous system depression in
two volunteers after a single oral dose
of 0.25 mg/kg bw
Summary Value Study Safety
factor
ADI 0-0.01 mg/kg bw Reproductive toxicity, 100
rat
Acute reference 0.01 mg/kg bw Single oral dose in six
dose volunteers 10
References
Berczy, Z.S., Binns, R. & Newman, A.J. (1972) Acute inhalation
toxicity to the rat of BTS 27419. Unpublished report No. 4971/72/406
from Huntingdon Research Centre Ltd, Huntingdon, Cambridge-shire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Berczy, Z.S., Binns, R., Street, A.E., Newman, A.J. & Whitfield,
R.A.S. (1973) Subacute inhalation toxicity to the rat of BTS 27419.
Unpublished report No. 5454/72/850 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brown, D.J., Burnett, R., Crowley, J., Cryer, P.C., Lancaster, M.C. &
Turnbull, G.J. (1978) Amitraz: Investigation of effects on the thymus
gland and oestrous cycle in mice. Unpublished report No. TX 78037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Brooker, P.C., Akhurst, L.C. & Gray, V.M. (1988) Technical amitraz,
metaphase chromosome analysis of human lymphocytes cultured in vitro.
Unpublished report No. SMS 127/881149 from Huntingdon Research Centre
Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Burnett, R., Crowley, J., Lessel, B., Patton, D.S.G., Sutton, M.M. &
Turnbull, G.J. (1976) BTS 27419: 80-week carcinogenicity study in
mice--final report. Unpublished report No. TX 76039 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1981) Excretion and residues of
14C-amitraz in male and female rats given a single oral dose of 10
mg/kg. Unpublished report No. METAB/81/20 from FBC Ltd, Chesterford
Park Research Station, Saffron Walden, Essex, United Kingdom.
Submitted to WHO by Schering Agro-chemicals Ltd.
Campbell, J.K. & Needham, D. (1983) Excretion and tissue residues of
14C amitraz in male and female mice given a single oral dose of 10 mg
14C-amitraz/kg bodyweight. Unpublished report No. METAB/83/34 from
FBC Ltd, Chesterford Park Research Station, Saffron Walden, Essex,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984a) Dermal absorption of 14C-amitraz
in EC formulation by male and female pigs given a single topical
application of 18 mg A.I. Unpublished report No. METAB/84/9 from FBC
Ltd, Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. Needham, D. (1984b) Excretion and tissue residues of
14C-amitraz in a male and a female baboon given a single oral dose of
10 mg 14C-amitraz per kg bodyweight. Unpublished report No.
METAB/83/44 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984c) Urinary excretion of 14C-amitraz
by two male humans following a single oral dose of 0.25 mg/kg
bodyweight. Unpublished report No. METAB/84/10 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984d) The metabolism of 14C-amitraz by
male and female rats. Unpublished report No. METAB/84/2 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Campbell, J.K. & Needham, D. (1984e) A comparison of the metabolism of
14C-amitraz in rat, mouse, baboon and human. Unpublished report No.
METAB/84/1 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Cass, L.M.R. (1992) Report of a double blind tolerance study of
amitraz in six adult healthy volunteers. Unpublished report No.
TOX/92/179-209 from Simbec Research Ltd, Merthyr Tydfil,
Mid-Glamorgan, Wales. Submitted to WHO by Schering Agrochemicals Ltd.
Challis I.R. (1990) Dermal absorption of amitraz in the rat.
Unpublished report No. TOX/90/179-178 from Schering Agrochemicals Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Chesterman, H., Skerrett, K., Street, A.E. & Cherry, C.P. (1973) Boots
27 271, oral toxicity study in beagle dogs (repeated dosage for 13
weeks). Unpublished report No. BTS 38/73511 from Huntingdon Research
Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO
by Schering Agrochemicals Ltd.
Clouzeau, J. (1992) Technical amitraz: Guinea pig sensitization study
(Buehler test). Unpublished report No. TOX 91/179-203 from Centre
International de Toxicologie, Miserey, Evreux, France. Submitted to
WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Gibson, W.A. & Prentice, D.E.
(1981) Amitraz toxicity to B6C3F1 mice by dietary administration for
13 weeks. Unpublished report No. BTS 158/80759 from Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Colley, J., Dawe, S., Heywood, R., Street, A.E., Gibson W.A. &
Gopinath, C. (1983) Amitraz, 104 week tumorigenicity study in mice.
Unpublished report No. BTS 153/8262/A from Huntingdon Research Centre
plc, Huntingdon, Cambridgeshire, United Kingdom. Submitted to WHO by
Schering Agro-chemicals Ltd.
Cullen, L.K. & Reynoldson, J.K. (1983) Effects of amitraz on the
cardiovascular and respiratory systems in the dog. Unpublished report
No. T242 from School of Veterinary Studies, Murdoch University,
Murdoch, Western Australia. Submitted to WHO by Schering Agrochemicals
Ltd.
Everest, R.P. (1976) BTS 27 419: Mutagenicity study in the male mouse
perivisceral cavity host-mediated assay. Unpublished report No. TX
76056 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Everest, R.P. & Wilcox, P. (1976) BTS 27 419: Mutagenicity testing
against Salmonella typhimurium strains TA1535, TA1537 and TA1538 in
the presence and absence of liver microsomes from male and female
mice. Unpublished report No. TX 76057 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Groppi, L. (1977) Medical report on a patient who ingested Mitac
(Edrizar) in Italy in 1977. Report No. T84 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Hall, J.E., Ahluwalia, H. & Hampson, P. (1975) A study of the effects
of oral administration of a metabolite (BTS 27 271) of amitraz to
volunteers. Unpublished report No. MS 75002 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hornish R.E. & Nappier, J.M. (1983) The absorption, metabolism, and
excretion of Mitaban(R) (U-36,059) in the dog from oral and dermal
exposure. Unpublished report No. 527-9760-83-001 from Agricultural
Research and Development Laboratories, The Upjohn Company, Kalamazoo,
Michigan, USA. Submitted to WHO by Schering Agrochemicals Ltd.
Hounsell, I.A.G & Walker, A.K. (1983) A micronucleus study in mice
using BTS 24868 (2,4-dimethylaniline). Unpublished report No.
TOX/84/179-97 from FBC Ltd, Chesterford Park Research Station, Saffron
Walden, Essex, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Hounsell, I.A. & Rush, K.C. (1984) Technical amitraz: The effect of
dietary administration on the oestrus cycle and hormones in the mouse.
Unpublished report No. TOX/84/179-97 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Hovda, L.R. & McManus, A.C. (1993) Yohimbine for treatment of amitraz
poisoning in dogs. Vet. Hum. Toxicol., 35, 329.
Hsu, W.H. & Kakuk, T.J. (1984) Effect of amitraz on heart rate and
pupil diameter in rats: Mediated by alpha2-adrenoceptors. Toxicol.
Appl. Pharmacol., 73, 411-415.
Hsu, W.H., Lu, Z.-X. & Hembrough, F.B. (1986) Effect of amitraz on
heart rate and aortic blood pressure in conscious dogs: Influence of
atropine, prazosin, tolazoline, and yohimbine. Toxicol. Appl.
Pharmacol., 84, 418-422.
Kakuk, T.J. (1979) Pathological findings on the matched control and
amitraz 400 ppm females from the Boots 80 week mouse study by various
pathologists with commentary. Unpublished report No. 315-78-9610-005
from Agricultural Research and Development Laboratories, The Upjohn
Company, Kalamazoo, Michigan, USA. Submitted to WHO by Schering
Agrochemicals Ltd.
Kynoch, S.R. & Parcell, B.I. (1988) Technical amitraz: Assessment of
delayed contact hypersensitivity in the guinea-pig. Unpublished report
No. TOX 88/179-145 from Huntingdon Research Centre Ltd, Huntingdon,
Cambridgeshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Lancaster, M.C. & Williams, G.A.H. (1978) Amitraz: Multigeneration
feeding test in rats -- Histopathology. Unpublished report No. TX
78064 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Lewis, D.K. (1971) Fate of [14C]-BTS 27419 applied to rats as a
single oral dose. Unpublished report No. C 71011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987a) Technical amitraz: Irritant
effects on rabbit skin. Unpublished report No. TOX/87/179-137 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Liggett, M.P. & Smith, P.A. (1987b) Technical amitraz: Irritant
effects on the rabbit eye. Unpublished report No. TOX/87/179-142 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Printice, R.D. (1983) Technical amitraz: Ames
bacterial mutagenicity test. Unpublished report No. 2590 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983a) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. 2669 from Inveresk Research
International, Musselburgh, Scotland. Submitted to WHO by Schering
Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1983b) Technical amitraz: Unscheduled
DNA synthesis in human embryonic cells. Unpublished report No. 2634
from Inveresk Research International, Musselburgh, Scotland. Submitted
to WHO by Schering Agrochemicals Ltd.
McGregor, D.B. & Riach, C.G. (1984) Technical amitraz: Mouse lymphoma
mutation assay. Unpublished report No. TOX/84/179-97 from FBC Ltd,
Chesterford Park Research Station, Saffron Walden, Essex, United
Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
McGregor, D.B., Brown, A.G. & Riach, G.G. (1984) Technical BTS 24868:
Induction of cell transformation in C3H10T1/2 cells. Unpublished
report No. TOX/84/179-97 from FBC Ltd, Chesterford Park Research
Station, Saffron Walden, Essex, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
McIntyre, M. (1987a) Technical amitraz: Teratogenicity study in the
rat. Unpublished report No. 5562-194/11 from Hazleton UK, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
McIntyre, M. (1987b) Technical amitraz: Teratogenicity study in the
rabbit. Unpublished report No. 5536-194/13 from Hazleton UK,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Merryman, D.C. & Sutton, M.M. (1972) Effects on the oestrus cycle of
the rat. Unpublished report No. PM 72003 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. (1973) BTS 27 271: Acute oral toxicity study in dogs.
Unpublished report No. TX 73004 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Morgan, H. & Williams, G.A.H. (1974) BTS 27 271: Acute oral toxicity
study in dogs--Histopathology. Unpublished report No. TX 73 030 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Morgan, H.E., Patton, D.S.G. & Turnbull, G.J. (1973) BTS 27 419:
Two-year oral toxicity study in dogs. Unpublished report No. TX 73035
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Morgan, H.E., Shepherd, G.M. & Turnbull, G.J. (1974) BTS 28 369:
90-day oral toxicity study in dogs. Unpublished report No. TX 74037
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Moser, V.C. & MacPhail, R.C. (1985) Yohimbine attenuates the delayed
lethality induced in mice by amitraz, a formamidine pesticide.
Toxicol. Lett., 28, 99-104.
Needham, D. (1984) The effect of Amitraz on the hepatic mixed-function
oxidase system of male and female mice following oral administration.
Unpublished report No. METAB/84/8 from FBC Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Needham, D. & Hemmings, P.A. (1988) The metabolism and distribution of
amitraz residues in the laying hen following the daily oral
administration of 24.5 mg 14C-amitraz/per bird. Unpublished report
No. Envir/88/6 from Schering Agrochemicals Ltd, Chesterford Park
Research Station, Saffron Walden, Essex, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977a) Dominant lethal assay of amitraz in
the female mouse. Unpublished report No. BTS 81/7758 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Palmer A.K. & James, P.A. (1977b) Dominant lethal assay of amitraz in
the male mouse. Unpublished report No. BTS 80/7792 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Schering Agrochemicals Ltd.
Parkinson, R. (1974) The effects of BTS 27 271, BTS 27 419 and BTS 21
103 (chlordimeform) on the pressor response to tyramine in the pithed
rat. Unpublished report No. P74 032 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Parkinson, R. & Sim, M.F. (1970) Some pharmacological effects of the
acaricide RD 27,271 and related compounds. Unpublished report No.
P70508 from The Boots Pure Drug Company, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Patton, D.S.G. (1973) BTS 27419: Acute toxicity in baboons.
Unpublished report No. TX 73002 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Patton, D.S.G & Sutton, M.M. (1971) Acute toxicity studies on BTS 27
419, an acaricide. Unpublished report No. P71544 from The Boots Pure
Drug Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Patton, D.S.G. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in dogs. Unpublished report No. P71547 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Petzold, G.L., Swenberg, J.A. & Bedell, M. (1977) Evaluation of
amitraz (U-36,059) and its metabolites (U-40,481, U-36,893, U-54,915A
and U-54,914) in the DNA damage/alkaline elution assay. Unpublished
report No. 7268/77/7268/001 from The Boots Company Ltd, Nottingham,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Phillips, M.W.A., Swalwell, L.M. & Needham, D. (1987) Identification
of metabolites of amitraz in the milk and meat of a cow dosed for 4
days with amitraz. Unpublished report No. Envir/87/46 from Schering
Agrochemicals Ltd, Chesterford Park Research Station, Saffron Walden,
Essex, United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Pronczuk, J., Heuhs, L., Scaiola, G., Bogdan, M. & Fogel de Korc, E.
(1995) Clinical cholinergic presentation of acute amitraz poisoning.
Report No. T368 from Department of Toxicology, Hospital de Clinicas,
Montevideo, Uruguay. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983a) Technical BTS 27271,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61A/83580
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Richold, M., Jones, E. & Fenner, L.A. (1983b) Technical BTS 27919,
Ames bacterial mutagenicity test. Unpublished report No. FSB 61B/83581
from Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom. Submitted to WHO by Schering Agrochemicals Ltd.
Ros, J.J.W. & van Aken, J. (1994) A case of poising with amitraz, an
agricultural pesticide. Ned. Tijdschr. Geneeskd., 138, 776-778.
Shaw, J.W. (1971) BTS 27 419 -- Acute intraperitoneal toxicity to
rats. Unpublished report No. PM 71057 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. (1973a) BTS 27 419: Comparison of the acute oral and
intraperitoneal toxicities to rats. Unpublished report No. TXM 73006
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1973b) BTS 27 419: Comparison of the acute oral toxicities
to rats of BTS 27 419 and BTS 27 919. Unpublished report No. TXM 73010
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
Shaw, J.W. (1975) BTS 27 419 metabolite: 21 Day chronic oral toxicity
in rats of BTS 28 369. Unpublished report No. TX 75058 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973a) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to mice. Unpublished report No. TXM 73 036 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, P.A. (1973b) BTS 27 419 metabolite, BTS 28 369
acute oral toxicity to rats. Unpublished report No. TXM 73 037 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Shaw, J.W. & Williams, G.A.H. (1975) BTS 27 419 metabolite: 90 day
chronic oral toxicity in rats of BTS 27 271. Unpublished report No. TX
75059 from The Boots Company Ltd, Nottingham, United Kingdom.
Submitted to WHO by Schering Agrochemicals Ltd.
Somerville, L. (1973) Fate of 14C-BTS 27419 administered to rats in
repeated oral doses. Unpublished report No. AX 73011 from The Boots
Company Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Stewart, F.P. (1993) (14C)-Amitraz: Dermal absorption in the rat.
Unpublished report No. 194/69-1011 from Hazleton Europe, Harrogate,
North Yorkshire, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. (1970a) RD 27,271. Acute oral toxicity to mice.
Unpublished report No. PM 70043 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agro-
chemicals Ltd.
Sutton, M.M. (1970b) RD 27,271 Acute toxicity to rats. Unpublished
report No. PM 70042 from The Boots Pure Drug Company, Nottingham,
United Kingdom. Submitted to WHO by Schering Agro-chemicals Ltd.
Sutton, M.M. (1971) BTS 27 419. Contact sensitisation in the guinea
pig. Unpublished report No. PM 71919 from The Boots Pure Drug Company,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973a) BTS 27 419: Three week dermal toxicity to
rabbits. Unpublished report No. TX 73 026 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973b) BTS 27 419: Multigeneration feeding test in rats.
Unpublished report No. TX 73 036 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973c) BTS 27 419: Teratogenicity in the rat.
Unpublished report No. TX 73028 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. (1973d) BTS 27 419: Teratogenicity in the rabbit.
Unpublished report No. TX 73029 from The Boots Company Ltd,
Nottingham, United Kingdom. Submitted to WHO by Schering Agrochemicals
Ltd.
Sutton, M.M. & Offer, J. (1973) BTS 27 419: Carcinogenicity and
long-term toxicity study in rats. Unpublished report No. TX 73043 from
The Boots Company Ltd, Nottingham, United Kingdom. Submitted to WHO by
Schering Agrochemicals Ltd.
Sutton, M.M. & Williams, G.A.H. (1971) BTS 27 419: 90-day toxicity
study in rats. Unpublished report No. P71548 from The Boots Pure Drug
Company, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Sutton, M.M. & Williams, P.A. (1972) BTS 27 419: Acute dermal toxicity
to rabbits. Unpublished report No. YM 72 011 from The Boots Company
Ltd, Nottingham, United Kingdom. Submitted to WHO by Schering
Agrochemicals Ltd.
Wilcox, P. (1976) BTS 27 419: Mutagenicity study in the
intraperitoneal host-mediated assay. Unpublished report No. TX 76028
from The Boots Company Ltd, Nottingham, United Kingdom. Submitted to
WHO by Schering Agrochemicals Ltd.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
Yim, G.K.W., Holsapple, M.P., Pfister, W.R. & Hollingworth, R.M.
(1978) Prostaglandin synthesis inhibited by formamidine pesticides.
Life Sci., 23, 2509-2516.
