
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
METHACRIFOS
IDENTITY
Chemical name
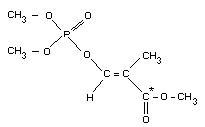
O-2-methoxycarbonylprop-1-enyl O,O-dimethyl phosphorothioate
(IUPAC)
Synonyms CGA-20168 (trans), C-23763 (trans), Damfin(R)
OMS-2005
Chemical structure
S CH3
CH3O " '
\ " '
\" '
P - O - CH = C - COOCH3
/
CH3O /
Molecular formula: C7H13O5PS
Molecular weight: 240.21
Description: colourless liquid
Specific gravity: 1.225 g/cm3 at 20°C
Boiling point: 90°C at 0.01 mm Hg
Volatility: saturated vapour conc. at 20°C: 16 mg/m3
Solubility: 400 ppm in water at 20°C; soluble in methanol,
methylene chloride, benzene, hexane and other
organic solvents
Stability: half-lives at 20°C in water were:
9.5 days at pH 9
29 days at pH 7
44 days at pH 5
66 days at pH 1
at ± 200°C decomposition of both isomers
Purity: >93%
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY INTAKE
BIOLOGICAL ASPECTS
Absorption, distribution, excretion and biotransformation
The fate of methacrifos was followed in male and female RAI-SPF
rats by using methacrifos 14C-labelled in the carboxy group:
 Following a single oral dose of about 5 mg/kg 14C-methacrifos, the
excretion in urine, faeces and expired air was in males 30.2, 10.2
and 54.7% of the administered dose, respectively, and in females
43.8, 8.7, 52.7%, respectively. Most of the radioactive label was
excreted in the first 24 hours. Total recovery was 97.4 and 106.6%
for males and females, respectively. Excretion half life time was
8 hours.
The animals were killed five days after dosing, the tissues
investigated showed the following residues:
Tissue residue
(in mg/kg methacrifos eq.)
males females
(n=4) (n=4)
Liver 0.306 0.287
fat 0.110 0.040
kidneys 0.087 0.089
muscles 0.035 0.020
blood 0.041 0.040
brain 0.027 0.023
spleen 0.052 0.048
testes 0.033 -
ovary - 0.043
(Ifflaender and Müke, 1975)
In a similar experiment, in which about 25 mg/kg 14C-methacrifos
was orally administered, the mean excretion pattern for males and
females was 41.5, 10.5 and 38.0% of the given dose in urine, faeces
and expired air respectively. The main urinary metabolite,
representing 12% of the dose, was isolated and identified as
N-acetyl-S-(2-methoxycarbonylprop-1-enyl) cysteine. It was
demonstrated that this cysteine conjugate when injected i.v. to
rats is excreted via the kidney mainly unchanged.
Another urinary metabolite, representing 4% of the dose was
probably the monodemethylated phosphoric acid derivative of
methacrifos. Both metabolites were also found in the faeces.
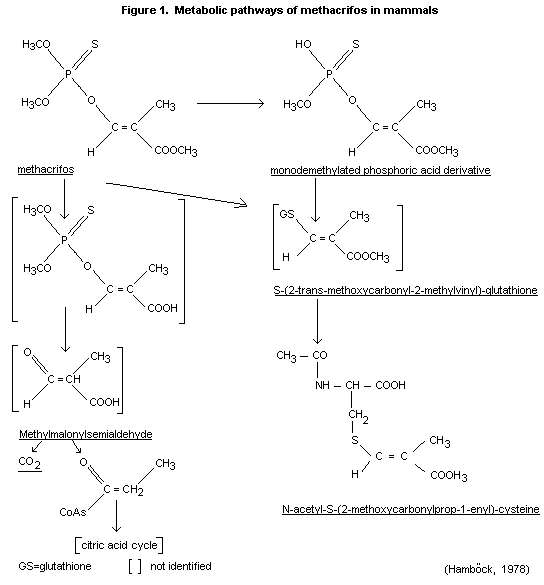
Most, if not all, of the radioactivity expired was 14CO2. The
following metabolic pathways of methacrifos in the rat are
proposed: see Figure 1.
Following a single oral dose of about 5 mg/kg 14C-methacrifos, the
excretion in urine, faeces and expired air was in males 30.2, 10.2
and 54.7% of the administered dose, respectively, and in females
43.8, 8.7, 52.7%, respectively. Most of the radioactive label was
excreted in the first 24 hours. Total recovery was 97.4 and 106.6%
for males and females, respectively. Excretion half life time was
8 hours.
The animals were killed five days after dosing, the tissues
investigated showed the following residues:
Tissue residue
(in mg/kg methacrifos eq.)
males females
(n=4) (n=4)
Liver 0.306 0.287
fat 0.110 0.040
kidneys 0.087 0.089
muscles 0.035 0.020
blood 0.041 0.040
brain 0.027 0.023
spleen 0.052 0.048
testes 0.033 -
ovary - 0.043
(Ifflaender and Müke, 1975)
In a similar experiment, in which about 25 mg/kg 14C-methacrifos
was orally administered, the mean excretion pattern for males and
females was 41.5, 10.5 and 38.0% of the given dose in urine, faeces
and expired air respectively. The main urinary metabolite,
representing 12% of the dose, was isolated and identified as
N-acetyl-S-(2-methoxycarbonylprop-1-enyl) cysteine. It was
demonstrated that this cysteine conjugate when injected i.v. to
rats is excreted via the kidney mainly unchanged.
Another urinary metabolite, representing 4% of the dose was
probably the monodemethylated phosphoric acid derivative of
methacrifos. Both metabolites were also found in the faeces.
Most, if not all, of the radioactivity expired was 14CO2. The
following metabolic pathways of methacrifos in the rat are
proposed: see Figure 1.
 TOXICOLOGICAL STUDIES
Special studies on teratogenicity and reproduction
Rat
After successfully being mated with male Sprague-Dawley albino rats
four groups of 2-month old female rats (25 animals/group) of the
same strain were orally incubated with methacrifos at doses of 0,
5, 25 or 50 mg/kg bw. In a preliminary study 6 out of 10 dams
died, when the compound was administered at the dose of 150 mg/kg
bw.
Treatment started at day 6 of pregnancy and was continued through
day 15. During the experiment a reduction in body weight gain was
found in the dams of the 50 mg group, starting half-way through the
dosing period. This reduction was accompanied by a temporary
decrease in food consumption at the 11th and 16th day of dosing
(food consumption was measured at day 6, 11, 16 and 21 of dosing).
Further a dose-related increase in the percentages of embryonal
deaths (early resorptions) was found, which already started at the
5 mg/kg level. No structural abnormalities related to the
methacrifos treatment were observed in the pups. The number of
foetuses with still incomplete ossified sternebrae was slightly
higher in the 50 and 25 mg/kg dose groups. (35.8% in control
versus 35.0, 43.0 and 52.8% for the 5, 25 and 50 mg/kg groups,
respectively). In the treated groups no foetal deaths or dead
foetuses were found (Fritz et al, 1978).
Groups of rats (8 males and 16 females/group) were fed methacrifos
in the diet at dosage levels of 0, 1, 10 or 100 mg/kg (mean
analytical concentrations of <0.1, 0.79, 7.4 and 73 mg/kg), and
subjected to a standard 3-generation, 2-litter per generation
study.
Gross and histopathologic examinations were conducted on the
parenteral animals of the F0, F1, F2 and F3. Gross
pathological evaluations were made on at least 10 male and 10
female weanlings of the 0 and 100 mg/kg group. No
treatment-related changes were noted in the tissues and organs
examined. Throughout the study a dose-related increase in
mortality of the male parent animals was observed in all treated
groups, whereas the mortality was also higher in the females of the
treated groups. The cause of mortalities was ascribed to acute
bronchopneumonia superimposed on lesions of chronic murine
pneumonia. There was no clear effect on the body weight gain or
organ weights of the treated groups.
The total number of pups delivered was dose-related decreased in
almost all litters, where the mean number was not clearly affected.
In addition somewhat reduced survival percentages after 21 days
were noted in comparison to the controls in the 10 and 100 mg/kg
F1a and F1b, 1 mg/kg F3b and 100 mg/kg F3a and F3b litters
(Charles et al, 1980a).
Special studies on mutagenicity
Bacteria
Histidine-autotrophic mutants of Salmonella typhimurium were
treated with methacrifos in concentrations of 0.25, 0.75, 2.25,
6.75 or 20.25 mg/ml with or without microsomal activation, in order
to study the possible induction of point mutations by methacrifos.
The test results were negative (Arni and Müller, 1979).
Mice
Twenty male albino mice were administered methacrifos orally in
single doses of O, 7, and 21 mg/kg and then mated to two untreated
female albino mice over a period of six weeks. At the end of each
week the females were replaced by new ones. The effects on mating
ratio and the number of implantations and embryonic deaths as well
as the effects on the loss of pre-implantation zygotes and the rate
of deaths of post-implantation stages of embryonic development were
studied. No evidence of a dominant lethal effect was observed
(Hool and Müller, 1980).
Special studies on carcinogenicity
Groups of 5-7 week old Swiss, white mice (60/sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (purity 94.6%) for
21-22 months (sacrifice of male and females at 50% mortality).
Body weight, food consumption and mortality were not clearly
affected. Haematological investigations carried out at the end of
the study revealed increases in total leucocyte count for males of
the 1 and 10 mg/kg groups. Urinalysis, performed also on 10
animals/sex/group, were normal at sacrifice but blood chemistry
revealed a significant increase in total protein in females and in
cholesterol in males at 100 mg/kg. In females an obvious
dose-related, but not significant, increase was found in absolute
and relative weight of gonads at 100 and 10 mg/kg. At 1 mg/kg a
tendency to an increase was found. The other organ weights were
within normal limits.
At sacrifice the activity of cholinesterase in plasma, appeared
inhibited at 100 and 10 mg/kg in both males and females; in
erythrocytes it was inhibited in females at 100 mg/kg. Brain
cholinesterase was not dose-related affected.
At histopathology no effects on tissues were apparent.
Carcinogenicity has not been found in this experiment (Charles et
al, 1980b).
Special studies on inhalation
Groups of 7/8 week-old rats (9/sex/group) were exposed to 0, 90,
191 or 467 mg methacrifos/m3 air, 15 times over a period of 21
days.
Thereafter 4 animals/sex of the control and highest dose group were
kept for a recovery period of 21 days. The compound was injected
into an air stream discharged into the exposure chamber through a
spray nozzle under a pressure of 2 atmospheres at a rate of 10
1/min.; the exposure concentrations were determined. The animals
were kept separately in PVC tubes, which were positioned radially
around the exposure chamber. The animals at the highest dose group
showed a significant decrease in food consumption and body weight
gain, but returned to normal during the recovery period. In the
191 mg/m3 group the body weight was also decreased at the end of
the study. Haematology and blood biochemistry revealed a
significant increase in SGPT in the 467 mg/m3 group. No clear
effects on the weight of the organs were observed. Blood urea
concentration was increased in all doses tested, which disappeared
after the recovery period. The cholinesterase activity of plasmal
erythrocytes and brain was significantly depressed in all treated
groups. After the recovery period, brain cholinesterase activity
was still decreased.
One male and one female rat died during the treatment: congestion
often with haemorrhages were observed in various tissues. All
other gross or microscopic findings were not related to the
treatment (Ullmann et al, 1977).
Special studies on dermal toxicity
Groups of KA 46, Himalayan strain rabbits (3/sex/group: 1.3-2 kg)
were dermally exposed to 0, 10, 50 or 250 mg/kg methacrifos, once
a day, 5 times a week for a period of 3 weeks. Thereafter 1
animal/sex/group was kept for a recovery period of 3 weeks. The
compound was diluted in PEG 400 and saline and applied to the
shaven skin of the back, with occlusive dressings. All treated
groups showed reversible erythema at the application site. The
animals of the 50 and 250 mg/kg groups showed clinical symptoms
(tremors, ataxy, especially during the first week). At 250 mg, 5
animals died within 9 days of the study. In the other groups no
effects on food consumption, body weight gain, haematological and
clinical chemistry were observed.
All the treated groups showed after 3 weeks a dose-related
inhibition of the cholinesterase activity of plasma and
erythrocytes of 30-80%, and 10-75% respectively. Brain
cholinesterase values revealed an inhibition of approximately
10-30, 55 and 85% of the control values in the treated groups. At
the end of the 3-week recovery period the blood and brain
cholinesterase activities of the 10 and 50 mg/kg groups had almost
returned to normal values.
Due to the small number of animals the effects on the organ weights
are difficult to evaluate.
Histopathological examinations revealed not pathological changes
which could be attributed to the treatment (Sachsse et al, 1978).
Special studies on skin sensitisation
Groups of guinea pigs of the Pirbright White strain (10/sex/group)
were intradermally injected saline, methacrifos and
dinitrochlorobenzene (DNCB) (positive control) (0.1 ml of the test
substance, 0.1%) every other day for 3 treatments.
During the second and third week of induction 6 sensitizing doses
of 0.1 ml, with Freund's adjuvants, were injected intracutaneously.
Two weeks after the last exposure, the animals were challenged and
examined for sensitisation. Ten days thereafter a second challenge
was given. There were indications that methacrifos induced a
sensitisation reaction, but it was less severe than DNCB (Ullmann
and Sachsse, 1975).
Special studies on neurotoxicity
In hens of the White Leghorn strain, over 12 months of age, which
were orally dosed by gavage with 0, 25, 50, 101 or 202 mg/kg
methacrifos (10, 15, 20 and 30 animals/group resp.) on day 0 and
21, no symptoms of delayed neurotoxicity were described in the
intermediate observation period of 21 days nor in the 42-day
observation period thereafter. At all doses the treatment resulted
within 1 hour to 4 days in ataxy, curved or ventral position,
sedation and salivation. Histopathological lesions in the spinal
cord and the sciatic nerve were not observed.
In the positive control animals, which had been treated with TOCP,
delayed neurotoxicity was found, obviously with slight to severe
lesions in the spinal cord and the peripheral nerves (Sachsse et
al, 1979).
Special studies on potentiation
In a study with RAI f-SPF rats (5 animals/sex/group) in which acute
oral LD50 values were determined no potentiation was found with the
insecticides dichlorvos, phosphamidon, diazinon and CGA 15324;
however a potentiation did occur with the insecticides methidathion
and malathion.
LD50 in mg/kg
techn. compound techn. compound techn. compound + CGA 201681
theoretically experimentally
phosphamidon 12 347 400
dichlorvos 54 395 611
CGA 15324 426 581 568
diazinon 534 635 520
methidathion 43 389.5 201
malthion 1397 1066.5 454
1 acute oral LD50 of CGA 20168: 745 mg/kg (Sachsse and Bathe,
1978)
TABLE 1. Acute toxicity of methacrifos
Mammals
route of LD50 in mg/kg reference
species sex administration or mg/m3
Chicken oral 1011 Sachsse et al, 1979
Rat M + F oral 6782 Bathe, 1974a
Mouse M + F oral 662 Bathe, 1974b
Mouse M + F oral 582 Bathe and Sachsse, 1978
Dog M + F oral 5731 Bathe and Sachsse, 1975
Rat M + F dermal >31002 Bathe, 1974c
Rabbit M + F dermal 27321 Ullmann and Sachsse, 1977
Rat M + F inhalation >2500 Ullmann and Sachsse, 1976
1 observation period of 14 days
2 observation period 7 days
Fish
exp. temp. 96 hour LC50
Species in C mg/kg Reference
Rainbow trout 14 0.4
Crucian carp 14 30
Catfish 21 6 Sachsse and Ullmann, 1974a
Bluegill 14 2
Guppy 21 3
Short-term studies
Quail
Groups of adult Japanese quails (3 males and 7 females/group) were
given, 0, 1000, 6000, or 10,000 mg/kg methacrifos in the feed for a
5-day period. They were held for observation during a further 3 days.
The mortality observed during the entire 8-day period was used to
calculate the LC50 which appeared to be approximately 10,000 mg/kg.
Observation of symptoms, egg production, food consumption and body
weight revealed sedation and ruffled feathers, partly to complete
refusal of food and a decrease in body weight over the whole dosing
period and a stop in egg production (Sachsse and Ullmann, 1974b).
Chicken
Groups of one-day old Hubbard chickens (32 sex/group) were dietary fed
0, 10, 100, 250, 500 or 1000 mg/kg methacrifos for 63 days.
Mortality was found to be 1.6, 0, 1.6, 3.1, 37.6 and 98.2% in the 0,
10, 100, 250, 500 and 1000 mg/kg groups, respectively. Most animals
died in the first period (0-14 days). The dying animals of the 500
and 1000 mg/kg groups showed signs of ataxia, lack of appetite,
somnolence and ruffled plumage. Body weight and food consumption were
significantly decreased at 250 and 500 mg/kg during the whole
experiment, at 100 mg/kg temporarily up to at least 28 days. No
effects on haematology and histopathology were found.
Final cholinesterase activity measurement in the brain on 8 animals/
sex/group revealed a dose-related inhibition at 100, 250 and 500 mg/kg
(Strittmatter and Gfeller, 1975).
Rat
Groups of Sprague-Dawley derived rats (150-170 g; 5 or 10, sex/group)
were given 0, 10, 100 or 1000 mg/kg methacrifos in the feed for 30
days, followed by a recovery period of 28 days for the control and
1000 mg/kg groups but nearly complete recovery was found in food
consumption at about 25 days; loss of hair in males was also noted.
In the males of the 1000 mg/kg group abnormalities in blood
composition were observed: a significant decrease in Hb, PCV, number
of erythrocytes and lymphocytes and prothrombin time and a slight
increase in neutrophils. At 100 mg/kg in the males the number of
lymphocytes and neutrophils appeared affected, also.
A decrease in cholinesterase activity in plasma and erythrocytes was
found at the end of the dosing period in both males and females of the
100 and 1000 mg/kg groups; plasma cholinesterase activity was also
decreased in males of the 10 mg/kg groups. At the end of the
experiment partial recovery was observed. Cholinesterase activity in
plasma and erythrocytes in the 1000 mg/kg group was still about 20%
decreased in both males and females. Brain cholinesterase has not
been determined. Further effects on clinical-chemical parameters and
urinalysis were not found. In the male animals of the 1000 mg/kg
groups the weight of the kidneys was decreased in comparison to the
control value (Drake, 1975).
Dog
Groups of pure bred beagle dogs (6.5-11 kg; 2 or 4 sex/group) were
dietary fed 0, 10 or 100 mg/kg for 28 days. Another group of dogs
received 1000 mg/kg for 11 days but treatment had to be stopped
because of a deterioration in the bodily condition of the dogs. After
7 days the animals then received 500 mg/kg for 4 weeks. Thereafter 4
animals were sacrificed and 4 were allowed a 4-week recovery period.
Due to dose changes evaluation of the effects of 1000 or 500 mg/kg
methacrifos is difficult. However body weight and food intake
drastically decreased and two animals showed higher SGPT values.
There was a marked depression of plasma and red cell cholinesterase
values, with a partial recovery. One animal was sacrificed in poor
bodily condition. Macroscopically a whitening of the splenic capsule
and a small amount of free blood beneath the membranes of the spinal
cord was observed.
In the other treated groups food consumption, body weights and organ
weights were measured and haematology, blood biochemistry, urinalysis,
ophthalmoscopy and histopathology performed but no toxic effects were
found except for a reduced water intake at 100 mg/kg and 10 mg/kg in
the males and a final inhibition of cholinesterase activity both in
the erythrocytes and plasma at 10 and 100 mg/kg in males and females.
Brain cholinesterase activity was not affected (Chesterman et al,
1975).
Groups of adult pure bred beagle dogs (8.5-15 kg; 8 sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (94.6%) for 26 weeks.
Thereafter 2 animals/sex/group were kept for a recovery period of 4
weeks.
No effects were found on food consumption and body weight gain but the
animals in the dose groups vomited more often and showed more
pronounced diarrhoea, when compared with the controls. Haematology
and urinalysis after 4, 13 and 26 weeks, were normal but blood
biochemistry showed a decrease in blood glucose at 26 weeks in all
dose groups, which fully recovered in the 1 mg/kg groups in the 4-week
recovery period but which only partially recovered in the 100 and 10
mg/kg groups in this period.
The main effects were found on cholinesterase activity. Plasma
cholinesterase was dose-related inhibited in the 100 and 10 mg/kg
groups at 4, 13 and 26 weeks and in the 1 mg/kg groups after 4 and 13
weeks only, with about 30%. Complete recovery was found after 4
weeks.
Erythrocyte cholinesterase was also dose-related inhibited in the 100
and 10 mg/kg groups at 4, 13 and 26 weeks but recovery was incomplete
after 4 weeks, inhibition was still present in males and females at
100 mg/kg and in females at 10 mg/kg. Brain cholinesterase was not
affected. Gross and microscopic examinations of organs and tissues
showed no effects attributed to methacrifos (Bathe et al,1977).
Pig
Groups of large white pigs (about 44 kg; 2/sex/group) were dietary fed
0, 10, 100 or 1000 mg/kg for about 4 weeks. No effects were found on
food consumption, body weights, blood chemistry, and haematology,
except for an influence on cholinesterase activity. At day 15 and 29
an inhibition of cholinesterase activity in plasma and erythrocytes
was found at 1000 mg/kg, at day 29 also in brain at 1000 mg/kg and in
erythrocytes also at 100 mg/kg. No histopathological changes were
found (Gfeller, 1974).
Long-term studies
Rat
Groups of about 40-day old CR strain rats (65/sex/group) were dietary
fed 0, 1, 10 or 100 mg/kg methacrifos for 2 years. Haematology,
coagulation, clinical chemistry and urinalysis studies were carried
out in 10 rats/sex from the control and 100 mg/kg group.
No clear effects were found on mortality, food consumption, body and
organ weights, haematology, clinical chemistry and urinalysis.
Cholinesterase activity in plasma and erythrocytes, which was measured
at 3, 6, 12, 18 and 24 months, was influenced. Cholinesterase
activity in plasma was inhibited in females of the 100 mg/kg group at
3, 6, 12 and 24 months, in males of the same group at 3, 6 and 12
months and in females of the 10 mg/kg group at 3 and 24 months.
Cholinesterase activity in erythrocytes was inhibited in females of
the 100 mg/kg groups at 6 months and in males of the same group at 12
months. Finally, cholinesterase activity in brain, which was measured
at 24 months, was not affected. The results of the microscopic
examination indicated that methacrifos did not produce
histopathological changes. A no-effect level in this study is 1 mg/kg
in the diet (Basler et al, 1980).
RESIDUES IN FOOD
USE PATTERN
Methacrifos is an organophosphorus insecticide and acaricide, with a
rapid and long-lasting action on a number of major pests of stored
products. It is mainly used as a grain protectant but also against
insect pests in other stored products. It is effective against
insects showing resistance to commonly used stored product
insecticides, including other organophosphorus compounds.
The main fields of application are cereal grains (maize, rice,
sorghum), cocoa, coffee, peanuts, pulses and tobacco.
The material shows a wide spectrum of activity notably against
Sitophilus spp., Rhizopertha dominica, Tribolium spp., Oryzaephilus
surinamensis, Lasioderma serricorna, Dermestes frisehii,
Anugasta Kuhniella, Plodio interpunetella, Acarus siro,
Glycophagus domesticus (Wyniger et al, 1977).
The major formulations are emulsifiable concentrate, solution in
organic solvents and dusts.
The dosage rate under usual conditions of storage and infestation is
10 mg ai/kg, which gives protection to stored products for several
months. At lower storage temperatures, or if infestation is moderate,
satisfactory protection can be obtained with 5 mg/kg. In some
situations (e.g. in Australia against a resistant strain of
Rhizopertha dominica) a rate of 20 mg ai/kg may be needed to
achieve satisfactory control.
Methacrifos is also used for space treatment in empty silos at 50-75
mg ai/m3, for surface treatment of stored products and as a wall
treatment in storage premises.
Methacrifos application does not affect germination, baking quality,
taste or odour.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
various countries, including Australia, France, Spain, Switzerland,
Mali and South Africa, on a variety of cereal grains, dry pulses,
tropical seeds and products of animal origin. The data were obtained
from both large and small-scale trials performed under practical
conditions.
Wheat, barley, oats
ln laboratory experiments, simulating grain storage, in Australia, it
was shown that the residue of methacrifos decreased more rapidly with
increased temperature and moisture content (Table 2); increased rate
and/or repeated application did not influence the dissipation rate
(Table 3); some evidence was obtained that enzymic activity in the
grain was at least partially responsible for the more rapid initial
breakdown (Table 3). The rate of residue decrease after treatment of
wheat, barley and oats is similar. On all variety loss during storage
at 36°C is more rapid than at 31°C as shown in Table 3. (McDougall,
1974; Moore, 1973; Moore et al, 1974).
Several small-scale trials in which wheat, barley or oats were stored
in mini-silos (5´ ton of each grain) or in drums (120 l) showed
similar results. As in the laboratory experiment, the application
rate of methacrifos does not affect the rate of dissipation in any of
the grains investigated (McDougall, 1975; McDougall, 1976) (Table 4
and 5). From these trials half-life periods could be calculated using
the formula t´ = (t2 - t1) log 2 / [2 - (logC2/C1) × 100], where
C1, C2 are concentrations of the insecticide at times t1 and t2
respectively. Only data for 84 days storage were used since the
temperature dropped after that period.
TABLE 2. Laboratory trials on the dissipation of methacrifos in stored grains
Storage Conditions Application Residues of methacrifos, in mg/kg, after storage
rate
Commodity Moisture % Temp. °C mg/kg 0/1 7 14 28 42 56 70 84
Wheat 9.3 28 24 17.3 16.1 14.7 12.3 12.1
10.3 " " 14.8 14.0 12.0 8.8 7.6
11.3 " " 13.4 12.4 9.5 6.7 4.9
12.3 " " 13.4 10.7 7.7 5.0 3.0
9.3 35 24 14.5 13.8 12.5 8.5 6.3
10.3 " " 13.8 11.5 8.1 4.8 3.2
11.3 " " 13.3 10.2 6.1 - -
12.3 " " 12.9 8.2 4.4 - -
9.6 42 24 13.1 8.5 4.8 - -
10.4 " " 12.6 6.7 3.1 - -
12.3 " " 4.9 2.0 - - -
12.4 " " 11.1 4.1 1.2 - -
Wheat 11.9 31 20 16.4 12.8 9.6 7.6 5.4 4.1
11.8 36 " 16.2 11.4 8.9 4.5 3.2 -
Barley 11.7 31 " 16.9 14.2 12.0 8.0 5.8 3.4
11.8 36 " 18.9 11.6 8.7 4.4 2.5 -
Oats 11.7 31 " 19.2 13.2 11.6 6.9 5.1 -
11.9 36 " 18.8 11.6 8.4 3.7 2.5 -
TABLE 3. Laboratory trials on the relative disspation of methacrifos in wheat
Storage Condition Application Residues of methacrifos as %
rate of initial residue (=100)
commodity moisture % temp. °C mg/kg 0 2 14 28 42 Reference
Wheat 11.2 35 24 100 82 48 30 22 Moore & McDougall, 1974
11.2 " 50 100 73 45 28 21
11.1 " 1501 100 74 47 34 25
1O.91 "1 24 100 98 60 36 22
11.2 " 24 100 93 44 29 21
12.5 " 4 + 15 100 79 49 33 -
1 applied in "dead" grain
TABLE 4. Mini-silo storage trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2/3 6-8 13-15 28 56 84 105 126 147 168 Reference
Wheat 10.2 22-26.5 10 4.7 5.8 5.0 5.0 4.2 4.1 - 3.0 - 2.9 McDougall, 1975
Barley 12.8 " " 9.4 8.6 8.6 8.0 5.7 3.9 2.9 - 2.0 - 1.8
Maize 12.9 " " 7.2 7.0 7.0 6.8 7.0 3.9 4.0 - 2.4 - 2.9
Sunflower 7.2 " " 7.7 7.5 7.2 6.7 7.4 5.0 4.9 - 3.6 - 2.7
TABLE 5. Drum trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos in mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2 5 7 12 28 56 84 126 175 210 Reference
Wheat 8.3-10.2 32 5 5.3 - - 5.5 5.4 4.0 3.1 2.5 1.6 1.2 1.1 McDougall, 1976
10 8.8 - - 9.9 8.2 7.1 5.3 4.3 3.1 2.4 1.8
Oats 7.4-8.0 10 10 4.1 - 4.4 - 3.6 2.8 2.4 1.7 1.5 - 0.4
(in sealed
bags) 7-8-8.4 32 10 4.1 - 4.8 - 4.4 3.4 2.4 1.7 1.3 - 0.4
Maize 8.6-10.3 32 5 2.6 2.9 - - 2.4 2.0 1.8 1.4 1.0 0.6 0.5
10 4.2 6.0 - - 6.0 4.5 4.0 2.5 1.9 1.5 1.3
Peanuts 4.4-7.9 32 10 7.3 - - 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
20 16.0 - - 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
Soybeans 5.5-7.4 32 5 2.3 2.6 - - 2.5 2.1 1.6 1.4 1.1 0.9 0.9
10 7.1 7.6 - - 7.1 6.3 5.7 4.8 3.8 3.6 3.2
TABLE 7. Half-life of methacrifos on various grains stored in mini
silos at 26°C
Commodity Storage conditions Half-life (days)
moisture temp.°C
Wheat 10.2 26 105
Barley 12.9 26 44
Maize 12.8 26 100
Sunflower seed 7.2 26 90
From the trial in which the grains were stored in drums at 32°C the
following half-life periods were calculated; for the moisture content
(see table 5).
Commodity Half-life(days)
Wheat 70
Oats 60
Maize 85
Peanuts 55
Soybeans 100
Large-scale trials
Wheat and barley
Large-scale trials in silos were carried out under a range of storage
conditions in various countries e.g. Australia, Argentina, Morocco,
Spain, Switzerland and France. As in the small-scale trials it was
found that the application rate did not affect the rate of dissipation
of the residues in any of the grain investigated (McDougall 1976)
(table 8).
The experiments under practical conditions confirmed that the
dissipation of methacrifos is mainly dependant on time and
temperature. The residue decrease under storage conditions prevailing
normally in Europe (temperature 15-25°C) is much slower than in
regions where storage temperatures are generally higher e.g. Australia
(Formica, 1975).
In the trials carried out in Australia in which the grain was aerated
during the storage period, there were indications of a decreased rate
of dissipation, compared with silos in which the grain was stored
under the same conditions of temperature and moisture at the start of
the experiment, but not aerated. Aeration was started 24 hours after
treatment and functioned only during the cooler hours of the day for a
total of 84 hours a week for the first 4 weeks and about 30 hours
weekly thereafter (Table 9) (Moor and McDougall 1975). In other
experiments aeration had little or no effect on residue dissipation
(Formica, 1978g).
TABLE 8. Dissipation of methacrifos in small grain
Large-scale trials in silo. (Moore and McDougall, 1974a, 1974b and 1975).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0,15 1 6 11/12 16 21 22/23 26 34 41
Wheat Australia 100 11.3 ± 30 20 0.6 21.8 11.8 7.5 5.6 4.1 4.2
(Queensl.) 10.3 20 1.5 14.2 9.6 5.3 3.9 2.9 2.6
1974 10.8 20 6.0 15.5 8.6 5.2 3.0 1.8 1.6
Australia 800 11.5 26-28 20 0.6 9.3 6.4 4.8 4.0 4.1 3.7
(N.S.W.) 11.3 20 1.5 8.6 6.3 4.9 3.8 3.0 2.5
1974 10.9 20 6.0 10.7 8.1 5.7 4.9 4.6 3.3
(aerated) Australia 550 12.4 (note 1) 15 0.1 9.7 5.0 3.6 2.3 1.4 1.6
(Queensl.) 11.4 15 2 9.0 5.6 4.2 2.6 2.1 2.6
11.0 15 4 9.6 5.7 4.8 3.6 2.8 2.2
11.0 15 6 11.9 6.0 5.3 3.3
not 10.9 (note 2) 15 0.1 11.4 6.0 3.7 2.3 1.6 1.5
aerated 10.2 2 9.8 6.1 3.6 1.6 0.75 0.8
10.4 4 10.0 3.8 1.7 1.6 0.6 0.6
9.9 6 10.4 4.8 2.6 1.8
1 Mean temperature in silo first 18 weeks 25-31°C; from 23-41 week 14-23°C.
2 Mean temperature in silo first 18 weeks 27.5-34°C; from 23-41 week 17-20°C.
TABLE 9. Dissipation of methacrifos in small grains
Large scale trials in silos. (Formica, 1978a, 1978b).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0 45 70 87 171/ 322/ 430 463 504 560
180 339
Barley Switzerland 51 2-25 22.3 2 13.8 7.6 2.6 3.8 2.5 1.5 0.86
(3.0- (7.8- (2.9- (2.9-
1.75) 1.6) 1.7) 0.3)
Barley Spain 100 11.6 13-26 10 2 4.1 3.7 3.2 1.3 2.8 1.7
Wheat Spain 100 11.0 13-26 10 2 9.4 5.6 3.0 2.8 2.1 1.0
The effect of aeration was also evaluated in a residue trial carried
out under Swiss conditions. A bin of barley treated with methacrifos
at 10 mg/kg was aerated twice during the storage period (after one and
three weeks) and another bin in the same warehouse was not aerated.
The residue on the aerated barley was 12.4 mg/kg at the beginning of
the experiment, 6.6 mg/kg after one week; 4.6 mg/kg after 32 weeks and
2.0 mg/kg after 74 weeks. The residues in the non-aerated bin were
11.3, 8.6, 4.2 and 2.5 mg/kg at the same sampling times, showing that
aeration had little or no effect on the residue dissipation. The
residue in oats stored at the same farm (without aeration ) were 10.2,
9.0, 6.0 and 3.7 mg/kg at these sampling times. Comparable results
were obtained in a similar trial on wheat in Switzerland in which one
bin was aerated after 54, 103 and 142 days post-treatment, by
circulating the wheat through the conveyer system and back to the
silo, and another bin not aerated. The initial residues were 6.0 and
6.1. Residues after the three aerations were 4.2, 3.7 and 3.4 mg/kg
on the aerated wheat and 4.8, 4.2 and 3.9 mg/kg on the nonaerated.
Again the rotation and resulting aeration of the wheat had little, if
any, influence on the dissipation rate (Formica, 1978d, 1978e). The
residue dissipation at different levels in a silo was studied in
Switzerland under typical European continental storage conditions with
temperatures of about 5°C in winter and 22°C in summer. Methacrifos
was mixed with the grain in a closed conveyer system, at a rate of 11
mg/kg; there was no ventilation during the storage period. The
initial concentration was 50 - 70% of the nominal value calculated
from the application rate and the volume treated. The residue at 2 m
depth increased during the following 5 weeks to almost the nominal
value and slowly decreased thereafter. It is assumed that the active
ingredient was not homogeneously dispersed immediately after
application but became more uniformly distributed through migration.
From a comparison of the residues in the samples taken from the
surface and at 2 m depth, it seems evident that the dissipation was
somewhat slower in the interior than on the surface of the stored
grain (Formica, 1976f). (Table 10).
In a study of the influence of the formulation on dissipation an
aqueous formulation showed more rapid dissipation than a formulation
with kerosene. After treatment with a nominal 10 mg/kg the residues
of the aqueous formulation were 0.2/0.16 and 0.15/0.25 mg/kg after 2
and 15 weeks respectively, whereas the residues of the kerosene
formulation were 9.1/9.3 and 4.0/3.5 mg/kg.
TABLE 10. Dissipation of methacrifos in wheat (Switzerland)
Days after
treatment 1 7 21 35 77 162 252 324 470 526 653
samples from
Surface 5.3 3.3 3.2 8.6/6.1 5.9/5.9 4.0/4.0 3.5 2.4 1.3 0.7 -
2 metres 4.6/3.9 5.2 6.0 10.1/7.4 6.7/5.6 6.2/6.5 5.2 4.0 2.6 2.8 1.4/2.25
Maize
Several small-scale trials were carried out with maize kernels and/or
cobs stored under various conditions of temperature and moisture
content. Although the distribution of the active ingredient was
rather inhomogeneous, it was evident, as with the other grains, that
the dissipation is dependant on time and temperature.
Under tropical conditions (temperature 35°C and higher) the residue
decreased much more rapidly than at moderate temperatures (Giannone
and Formica, 1979b).
The dissipation of methacrifos in maize was studied in a large scale
trial in Switzerland; 80 tons of maize kernels (moisture content
14.5%) were treated at 10 mg/kg on a conveyer system during the
filling of concrete silo cells, which were closed without ventilation.
Results are shown in Table 11.
TABLE 11. Dissipation of methacrifos in maize
Temp. in silos °C1 19 9 14 27
Days after treatment 6 76 300 376
Residue, mg/kg 7.8 3.62 3.9/3.4 0.4/0.7
(5.7-9.6) 3.1-4.2
1 Temperature at a depth of 2 metres.
2 Mean value of 4 samples; two samples analysed 5 months later than
the other two samples; samples stored at -20°C.
Rice
Small-scale trials on the dissipation of methacrifos under various
storage conditions were carried out in Australia. Brown and polished
rice were treated at the rate of 10 mg/kg and stored in bags, at
temperatures of 13-26°C. The moisture content of the brown rice was
approximately 14.2% and of the polished rice 14.4%. The initial
residue of 9.1 mg/kg in brown rice decreased to 5.0 mg/kg after 12
weeks and to 3.9 mg/kg after 24 weeks. In the polished rice the
corresponding residue levels were 7.9, 6.3 and 4.4 mg/kg respectively.
In other trials paddy rice, brown rice and polished rice were stored
in cardboard drums, and paddy rice also in mini-silos of about six
tons capacity. The decrease of the residue in paddy rice stored at
about 32°C was faster than in brown or polished rice. Under the
conditions occurring in the trials the dissipation rate of methacrifos
was independent of the application rate (Hart and Moore 1975;
McDougall 1976b) (Table 12).
TABLE 12. Dissipation of methacrifos in rice (McDougall, 1976b; Hart and Moore, 1976
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
mini-silo moisture temp. °C mg/kg
or cardboard %
Type of rice drums 0 12 23 54/51 124/121 166 205
paddy rice silo 13.7 17-26 10 8.2 5.8 4.7 3.7 2.6
paddy rice drums 8.8-12.8 32 10 10.8 6.2 4.4 2.2 1.2
drums 8.8-12.8 32 5 4.5 2.6 1.9 0.9 0.5
brown rice drums 8.8-12.8 32 10 9.1 7.0 5.5 3.8 2.7
drums 8.8-12.8 32 5 3.7 3.2 2.4 1.2 1.0
polished rice drums 8.8-12.8 32 10 13.0 9.6 7.2 4.6 2.7
drums 8.8-12.8 32 5 4.7 3.9 3.2 1.6 1.0
TABLE 13. Dissipation of methacrifos in sorghum
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
silo or moisture temp. °C mg/kg
Country bags % 0 15 21 45 56 90 147
Australia silo 13 27 15 13.6 9.3 7.7 6.3
13 27 15 9.1 4.1 7.1 4.3
13 27 7.5 8.0 4.7 4.9 2.7
Mali bags 25-30 10(dust) 3.6 3.0 1.9
25-30 10(solution) 6.1 4.2 2.1
TABLE 14. Dissipation of methacrifos in peanuts, stored in drums (McDougall, 1976a)
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
moisture % temp. °C mg/kg
Country % 7 12 28 56 84 126 175 210
Peanut
whole 7.8-4.4 32 20 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
kernel 0.6 1.5 1.7 0.3 0.3 0.4 1.0 0.4
shell 50.8 49.5 30.6 30.5 29.2 10.3 8.8 7.7
Peanut
whole 7.8-4.4 32 10 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
kernel 0.6 0.3 <0.3 0.3 <0.3 <0.3 <0.3 <0.3
shell 41.5 21.5 23.7 12.9 7.3 7.3 4.1 5.7
Sorghum
Data are available from large-scale trials in Australia where sorghum
treated at 7.5 and 15 mg/kg was stored in silo bins and from Mali
where sorghum treated according to the so-called "sandwich method"
with a dust or a spray was stored in bags. The rate of dissipation
was similar to that in the other grains (Hart and Moore 1976, Giannone
and Formica, 1980) (Table 13).
Pulses (dried legume vegetables)
In a small-scale trial in Switzerland, a mixture of beans and peas
(Vicia faba, Phaseolus nanus and Pisum sativum) was
treated with a dust (10 or 20 mg/kg ai/kg) or a liquid formulation (10
mg ai/kg) and stored in drums at 25 ± 1°C; moisture content before
treatment was 9.8%. Three hours after the application the residues in
the lots treated with the dusts at 20 and 10 mg ai/kg and the solution
at 10 mg ai/kg were 5.5, 3.9 and 2.7 mg/kg respectively. After 27
days the residue levels in these lots were 6.5, 2.6 and 2.6 mg/kg and
after 142 days 3.5, 1.7 and 1.7 mg/kg respectively.
In a small-scale trial in Nigeria, cowpeas were treated with 10 mg/kg
methacrifos and stored in open jute bags under tropical conditions.
No residues (<0.12 mg/kg) were found 65 and 92 days after treatment.
Peanuts
A mini-silo (drum) trial was carried out in Australia. Methacrifos
was applied at 10 and 20 mg/kg to whole peanuts, which were stored in
120 l cardboard drums at 32°C. On all sampling dates kernels and the
shells were analysed separately. The results show that nearly all the
residue is contained in the shell (McDougall, 1976).
In Mali, peanut kernels were treated with 10 mg/kg methacrifos and
stored in closed plastic bags under tropical conditions (25-30°C).
Residues after 15, 60 and 90 days were 7.6, 5.6, 5.2 mg/kg
respectively (McDougall 1976).
Cocoa-beans
Cocoa-beans stored in closed jute bags in a warehouse were treated
with methacrifos at 0.4 g ai/m2 on the outside of the bags. Samples
were taken at four sampling dates from the outside layer of cocoa-
beans, which had been exposed to the treatment, and from the interior
of the bags. The residues in samples from near the outside of the
bags were higher than in the internal samples; 34 days after treatment
the residues in the outer samples were 1.0 and 1.2 mg/kg and in the
inner samples <0.025. After 98 days the levels were 0.83 and 0.32
mg/kg respectively.
The methacrifos residues were found mainly in the peel. 34 days after
treatment the residues in the whole beans were 1.0 and 1.2, in the
peel 5.6 and in the kernel 0.5 mg/kg (Formica, 1978c).
Coffee beans
Raw coffee beans imported from Nigeria were treated in Switzerland
with methacrifos in 10 and 20 mg/kg. The treated beans were stored in
closed drums at 20° ± 2°C. After 4 months storage the residues from
the 20 mg/kg treatment were 2 mg/kg but 0.5 mg/kg in the lot treated
with 10 mg/kg (Renfer, 1976).
FATE OF RESIDUES
In animals
General
As grain treated with methacrifos and grain products derived from
treated grain may be used as animal feed, it was necessary to study
the fate of methacrifos in livestock animals and to demonstrate that
no significant residue would remain in livestock and poultry, milk or
eggs. Special trials therefore, were carried out to study possible
carry-over from residues in feed to tissues or products of animal
origin.
Chickens
In a preliminary experiment, four young male broilers were given a
single oral dose of 20 mg/kg methacrifos by capsule. The birds were
slaughtered 6, 12, 24 and 48 hours after dosing. Samples of fat,
muscle, liver, kidney, heart, gizzard and skin were analysed. The
residues in all muscle and heart samples were 0.01 mg/kg or less.
Residues in the other samples decreased very rapidly (Moore and
McDougall, 1974c) (Table 15).
TABLE 15. Residues of methacrifos in chicken tissues after single
oral treatment with 20 mg/kg
Tissue Residues in mg/kg
time (hours post-treatment)
6 12 24 48
Fat 0.22 0.06 <0.01 <0.01
Liver 0.02 0.01 0.01 <0.01
kidney 0.08 <0.03 <0.03 <0.03
Gizzard 0.42 <0.01 <0.01 <0.01
Skin 0.06 0.02 <0.01 <0.01
Muscle <0.01 <0.01 <0.01 <0.01
Heart 0.01 <0.01 <0.01 <O.01
In a further experiment 5 groups of 77 one-day old chicks each were
given feed fortified with methacrifos at levels of 0, 10, 100, 250 or
500 mg/kg for 63 days. At the end of the feeding period 20 chickens
from each group (10 males and 10 females) were slaughtered and the
combined muscles, skin, kidney and liver used for analysis. In all
tissues examined residues were less than 0.01 mg/kg (Formica, 1974).
Eggs
Three groups of 15 laying hens were fed a diet containing 0, 10 or 20
mg/kg methacrifos. Egg samples were collected one day before
treatment, on the 16th day of treatment and 0, 2, 3, 7 days after the
end of the treatment. In none of the samples were residues found
(<0.01 mg/kg) (Schnabel and Formica, 1979).
Milk
Two lactating cows were given 30 mg methacrifos by capsule daily for
10 days. This dose corresponds to about 3 kg of cereals treated at a
rate of 10 mg/kg daily in the total ration. Pooled morning and
evening milk samples were taken before, during the feeding period and
three days after its completion. No residues (<0.001 mg/kg) could be
detected in the milk of the treated cows before, during or after the
10 day period (Formica, 1977).
In plants
Stored grain
The degradation of carbonyl 14C methacrifos (specific radioactivity
31.1 µCi/mg) in grain treated with 10 mg/kg was studied under
laboratory conditions for one year; 84-94% of the applied
radioactivity was recovered at all sampling times, indicating very
limited volatility of methacrifos or its degradation products. After
one year about 25% of the then recovered radioactivity was present as
parent compound. The fact that little volatilisation of the
radioactivity occurred during the storage indicates that methacrifos
and its degradation products were strongly absorbed to the wheat
grain. Less than 15% of the recovered radioactivity could be washed
off the surface of the grain with methylene chloride. The bulk of the
radioactivity could be extracted only after grinding of grain. The
non-extractable portion of radioactivity was low at the early sampling
dates but rose to 9-14% after 140 and 385 days. The low but
continuous evolution of 14C during the experiment may demonstrate
that the insecticide is eventually metabolised by the wheat grain.
The organosoluble radioactivity on or in the grains consisted mainly
of the parent compound. The oxon was not detected. Mono- and
di-demethylated methacrifos were detected by ethylation followed by
GLC analysis. Their identity was confirmed by methylation with
deuterated diazomethane (CD2N2) followed by GCMS.
The results give strong evidence for the degradation pathways shown in
figure 2 (Blattmann 1978).
Figure 2. Degradation of methacrifos in grain
TOXICOLOGICAL STUDIES
Special studies on teratogenicity and reproduction
Rat
After successfully being mated with male Sprague-Dawley albino rats
four groups of 2-month old female rats (25 animals/group) of the
same strain were orally incubated with methacrifos at doses of 0,
5, 25 or 50 mg/kg bw. In a preliminary study 6 out of 10 dams
died, when the compound was administered at the dose of 150 mg/kg
bw.
Treatment started at day 6 of pregnancy and was continued through
day 15. During the experiment a reduction in body weight gain was
found in the dams of the 50 mg group, starting half-way through the
dosing period. This reduction was accompanied by a temporary
decrease in food consumption at the 11th and 16th day of dosing
(food consumption was measured at day 6, 11, 16 and 21 of dosing).
Further a dose-related increase in the percentages of embryonal
deaths (early resorptions) was found, which already started at the
5 mg/kg level. No structural abnormalities related to the
methacrifos treatment were observed in the pups. The number of
foetuses with still incomplete ossified sternebrae was slightly
higher in the 50 and 25 mg/kg dose groups. (35.8% in control
versus 35.0, 43.0 and 52.8% for the 5, 25 and 50 mg/kg groups,
respectively). In the treated groups no foetal deaths or dead
foetuses were found (Fritz et al, 1978).
Groups of rats (8 males and 16 females/group) were fed methacrifos
in the diet at dosage levels of 0, 1, 10 or 100 mg/kg (mean
analytical concentrations of <0.1, 0.79, 7.4 and 73 mg/kg), and
subjected to a standard 3-generation, 2-litter per generation
study.
Gross and histopathologic examinations were conducted on the
parenteral animals of the F0, F1, F2 and F3. Gross
pathological evaluations were made on at least 10 male and 10
female weanlings of the 0 and 100 mg/kg group. No
treatment-related changes were noted in the tissues and organs
examined. Throughout the study a dose-related increase in
mortality of the male parent animals was observed in all treated
groups, whereas the mortality was also higher in the females of the
treated groups. The cause of mortalities was ascribed to acute
bronchopneumonia superimposed on lesions of chronic murine
pneumonia. There was no clear effect on the body weight gain or
organ weights of the treated groups.
The total number of pups delivered was dose-related decreased in
almost all litters, where the mean number was not clearly affected.
In addition somewhat reduced survival percentages after 21 days
were noted in comparison to the controls in the 10 and 100 mg/kg
F1a and F1b, 1 mg/kg F3b and 100 mg/kg F3a and F3b litters
(Charles et al, 1980a).
Special studies on mutagenicity
Bacteria
Histidine-autotrophic mutants of Salmonella typhimurium were
treated with methacrifos in concentrations of 0.25, 0.75, 2.25,
6.75 or 20.25 mg/ml with or without microsomal activation, in order
to study the possible induction of point mutations by methacrifos.
The test results were negative (Arni and Müller, 1979).
Mice
Twenty male albino mice were administered methacrifos orally in
single doses of O, 7, and 21 mg/kg and then mated to two untreated
female albino mice over a period of six weeks. At the end of each
week the females were replaced by new ones. The effects on mating
ratio and the number of implantations and embryonic deaths as well
as the effects on the loss of pre-implantation zygotes and the rate
of deaths of post-implantation stages of embryonic development were
studied. No evidence of a dominant lethal effect was observed
(Hool and Müller, 1980).
Special studies on carcinogenicity
Groups of 5-7 week old Swiss, white mice (60/sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (purity 94.6%) for
21-22 months (sacrifice of male and females at 50% mortality).
Body weight, food consumption and mortality were not clearly
affected. Haematological investigations carried out at the end of
the study revealed increases in total leucocyte count for males of
the 1 and 10 mg/kg groups. Urinalysis, performed also on 10
animals/sex/group, were normal at sacrifice but blood chemistry
revealed a significant increase in total protein in females and in
cholesterol in males at 100 mg/kg. In females an obvious
dose-related, but not significant, increase was found in absolute
and relative weight of gonads at 100 and 10 mg/kg. At 1 mg/kg a
tendency to an increase was found. The other organ weights were
within normal limits.
At sacrifice the activity of cholinesterase in plasma, appeared
inhibited at 100 and 10 mg/kg in both males and females; in
erythrocytes it was inhibited in females at 100 mg/kg. Brain
cholinesterase was not dose-related affected.
At histopathology no effects on tissues were apparent.
Carcinogenicity has not been found in this experiment (Charles et
al, 1980b).
Special studies on inhalation
Groups of 7/8 week-old rats (9/sex/group) were exposed to 0, 90,
191 or 467 mg methacrifos/m3 air, 15 times over a period of 21
days.
Thereafter 4 animals/sex of the control and highest dose group were
kept for a recovery period of 21 days. The compound was injected
into an air stream discharged into the exposure chamber through a
spray nozzle under a pressure of 2 atmospheres at a rate of 10
1/min.; the exposure concentrations were determined. The animals
were kept separately in PVC tubes, which were positioned radially
around the exposure chamber. The animals at the highest dose group
showed a significant decrease in food consumption and body weight
gain, but returned to normal during the recovery period. In the
191 mg/m3 group the body weight was also decreased at the end of
the study. Haematology and blood biochemistry revealed a
significant increase in SGPT in the 467 mg/m3 group. No clear
effects on the weight of the organs were observed. Blood urea
concentration was increased in all doses tested, which disappeared
after the recovery period. The cholinesterase activity of plasmal
erythrocytes and brain was significantly depressed in all treated
groups. After the recovery period, brain cholinesterase activity
was still decreased.
One male and one female rat died during the treatment: congestion
often with haemorrhages were observed in various tissues. All
other gross or microscopic findings were not related to the
treatment (Ullmann et al, 1977).
Special studies on dermal toxicity
Groups of KA 46, Himalayan strain rabbits (3/sex/group: 1.3-2 kg)
were dermally exposed to 0, 10, 50 or 250 mg/kg methacrifos, once
a day, 5 times a week for a period of 3 weeks. Thereafter 1
animal/sex/group was kept for a recovery period of 3 weeks. The
compound was diluted in PEG 400 and saline and applied to the
shaven skin of the back, with occlusive dressings. All treated
groups showed reversible erythema at the application site. The
animals of the 50 and 250 mg/kg groups showed clinical symptoms
(tremors, ataxy, especially during the first week). At 250 mg, 5
animals died within 9 days of the study. In the other groups no
effects on food consumption, body weight gain, haematological and
clinical chemistry were observed.
All the treated groups showed after 3 weeks a dose-related
inhibition of the cholinesterase activity of plasma and
erythrocytes of 30-80%, and 10-75% respectively. Brain
cholinesterase values revealed an inhibition of approximately
10-30, 55 and 85% of the control values in the treated groups. At
the end of the 3-week recovery period the blood and brain
cholinesterase activities of the 10 and 50 mg/kg groups had almost
returned to normal values.
Due to the small number of animals the effects on the organ weights
are difficult to evaluate.
Histopathological examinations revealed not pathological changes
which could be attributed to the treatment (Sachsse et al, 1978).
Special studies on skin sensitisation
Groups of guinea pigs of the Pirbright White strain (10/sex/group)
were intradermally injected saline, methacrifos and
dinitrochlorobenzene (DNCB) (positive control) (0.1 ml of the test
substance, 0.1%) every other day for 3 treatments.
During the second and third week of induction 6 sensitizing doses
of 0.1 ml, with Freund's adjuvants, were injected intracutaneously.
Two weeks after the last exposure, the animals were challenged and
examined for sensitisation. Ten days thereafter a second challenge
was given. There were indications that methacrifos induced a
sensitisation reaction, but it was less severe than DNCB (Ullmann
and Sachsse, 1975).
Special studies on neurotoxicity
In hens of the White Leghorn strain, over 12 months of age, which
were orally dosed by gavage with 0, 25, 50, 101 or 202 mg/kg
methacrifos (10, 15, 20 and 30 animals/group resp.) on day 0 and
21, no symptoms of delayed neurotoxicity were described in the
intermediate observation period of 21 days nor in the 42-day
observation period thereafter. At all doses the treatment resulted
within 1 hour to 4 days in ataxy, curved or ventral position,
sedation and salivation. Histopathological lesions in the spinal
cord and the sciatic nerve were not observed.
In the positive control animals, which had been treated with TOCP,
delayed neurotoxicity was found, obviously with slight to severe
lesions in the spinal cord and the peripheral nerves (Sachsse et
al, 1979).
Special studies on potentiation
In a study with RAI f-SPF rats (5 animals/sex/group) in which acute
oral LD50 values were determined no potentiation was found with the
insecticides dichlorvos, phosphamidon, diazinon and CGA 15324;
however a potentiation did occur with the insecticides methidathion
and malathion.
LD50 in mg/kg
techn. compound techn. compound techn. compound + CGA 201681
theoretically experimentally
phosphamidon 12 347 400
dichlorvos 54 395 611
CGA 15324 426 581 568
diazinon 534 635 520
methidathion 43 389.5 201
malthion 1397 1066.5 454
1 acute oral LD50 of CGA 20168: 745 mg/kg (Sachsse and Bathe,
1978)
TABLE 1. Acute toxicity of methacrifos
Mammals
route of LD50 in mg/kg reference
species sex administration or mg/m3
Chicken oral 1011 Sachsse et al, 1979
Rat M + F oral 6782 Bathe, 1974a
Mouse M + F oral 662 Bathe, 1974b
Mouse M + F oral 582 Bathe and Sachsse, 1978
Dog M + F oral 5731 Bathe and Sachsse, 1975
Rat M + F dermal >31002 Bathe, 1974c
Rabbit M + F dermal 27321 Ullmann and Sachsse, 1977
Rat M + F inhalation >2500 Ullmann and Sachsse, 1976
1 observation period of 14 days
2 observation period 7 days
Fish
exp. temp. 96 hour LC50
Species in C mg/kg Reference
Rainbow trout 14 0.4
Crucian carp 14 30
Catfish 21 6 Sachsse and Ullmann, 1974a
Bluegill 14 2
Guppy 21 3
Short-term studies
Quail
Groups of adult Japanese quails (3 males and 7 females/group) were
given, 0, 1000, 6000, or 10,000 mg/kg methacrifos in the feed for a
5-day period. They were held for observation during a further 3 days.
The mortality observed during the entire 8-day period was used to
calculate the LC50 which appeared to be approximately 10,000 mg/kg.
Observation of symptoms, egg production, food consumption and body
weight revealed sedation and ruffled feathers, partly to complete
refusal of food and a decrease in body weight over the whole dosing
period and a stop in egg production (Sachsse and Ullmann, 1974b).
Chicken
Groups of one-day old Hubbard chickens (32 sex/group) were dietary fed
0, 10, 100, 250, 500 or 1000 mg/kg methacrifos for 63 days.
Mortality was found to be 1.6, 0, 1.6, 3.1, 37.6 and 98.2% in the 0,
10, 100, 250, 500 and 1000 mg/kg groups, respectively. Most animals
died in the first period (0-14 days). The dying animals of the 500
and 1000 mg/kg groups showed signs of ataxia, lack of appetite,
somnolence and ruffled plumage. Body weight and food consumption were
significantly decreased at 250 and 500 mg/kg during the whole
experiment, at 100 mg/kg temporarily up to at least 28 days. No
effects on haematology and histopathology were found.
Final cholinesterase activity measurement in the brain on 8 animals/
sex/group revealed a dose-related inhibition at 100, 250 and 500 mg/kg
(Strittmatter and Gfeller, 1975).
Rat
Groups of Sprague-Dawley derived rats (150-170 g; 5 or 10, sex/group)
were given 0, 10, 100 or 1000 mg/kg methacrifos in the feed for 30
days, followed by a recovery period of 28 days for the control and
1000 mg/kg groups but nearly complete recovery was found in food
consumption at about 25 days; loss of hair in males was also noted.
In the males of the 1000 mg/kg group abnormalities in blood
composition were observed: a significant decrease in Hb, PCV, number
of erythrocytes and lymphocytes and prothrombin time and a slight
increase in neutrophils. At 100 mg/kg in the males the number of
lymphocytes and neutrophils appeared affected, also.
A decrease in cholinesterase activity in plasma and erythrocytes was
found at the end of the dosing period in both males and females of the
100 and 1000 mg/kg groups; plasma cholinesterase activity was also
decreased in males of the 10 mg/kg groups. At the end of the
experiment partial recovery was observed. Cholinesterase activity in
plasma and erythrocytes in the 1000 mg/kg group was still about 20%
decreased in both males and females. Brain cholinesterase has not
been determined. Further effects on clinical-chemical parameters and
urinalysis were not found. In the male animals of the 1000 mg/kg
groups the weight of the kidneys was decreased in comparison to the
control value (Drake, 1975).
Dog
Groups of pure bred beagle dogs (6.5-11 kg; 2 or 4 sex/group) were
dietary fed 0, 10 or 100 mg/kg for 28 days. Another group of dogs
received 1000 mg/kg for 11 days but treatment had to be stopped
because of a deterioration in the bodily condition of the dogs. After
7 days the animals then received 500 mg/kg for 4 weeks. Thereafter 4
animals were sacrificed and 4 were allowed a 4-week recovery period.
Due to dose changes evaluation of the effects of 1000 or 500 mg/kg
methacrifos is difficult. However body weight and food intake
drastically decreased and two animals showed higher SGPT values.
There was a marked depression of plasma and red cell cholinesterase
values, with a partial recovery. One animal was sacrificed in poor
bodily condition. Macroscopically a whitening of the splenic capsule
and a small amount of free blood beneath the membranes of the spinal
cord was observed.
In the other treated groups food consumption, body weights and organ
weights were measured and haematology, blood biochemistry, urinalysis,
ophthalmoscopy and histopathology performed but no toxic effects were
found except for a reduced water intake at 100 mg/kg and 10 mg/kg in
the males and a final inhibition of cholinesterase activity both in
the erythrocytes and plasma at 10 and 100 mg/kg in males and females.
Brain cholinesterase activity was not affected (Chesterman et al,
1975).
Groups of adult pure bred beagle dogs (8.5-15 kg; 8 sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (94.6%) for 26 weeks.
Thereafter 2 animals/sex/group were kept for a recovery period of 4
weeks.
No effects were found on food consumption and body weight gain but the
animals in the dose groups vomited more often and showed more
pronounced diarrhoea, when compared with the controls. Haematology
and urinalysis after 4, 13 and 26 weeks, were normal but blood
biochemistry showed a decrease in blood glucose at 26 weeks in all
dose groups, which fully recovered in the 1 mg/kg groups in the 4-week
recovery period but which only partially recovered in the 100 and 10
mg/kg groups in this period.
The main effects were found on cholinesterase activity. Plasma
cholinesterase was dose-related inhibited in the 100 and 10 mg/kg
groups at 4, 13 and 26 weeks and in the 1 mg/kg groups after 4 and 13
weeks only, with about 30%. Complete recovery was found after 4
weeks.
Erythrocyte cholinesterase was also dose-related inhibited in the 100
and 10 mg/kg groups at 4, 13 and 26 weeks but recovery was incomplete
after 4 weeks, inhibition was still present in males and females at
100 mg/kg and in females at 10 mg/kg. Brain cholinesterase was not
affected. Gross and microscopic examinations of organs and tissues
showed no effects attributed to methacrifos (Bathe et al,1977).
Pig
Groups of large white pigs (about 44 kg; 2/sex/group) were dietary fed
0, 10, 100 or 1000 mg/kg for about 4 weeks. No effects were found on
food consumption, body weights, blood chemistry, and haematology,
except for an influence on cholinesterase activity. At day 15 and 29
an inhibition of cholinesterase activity in plasma and erythrocytes
was found at 1000 mg/kg, at day 29 also in brain at 1000 mg/kg and in
erythrocytes also at 100 mg/kg. No histopathological changes were
found (Gfeller, 1974).
Long-term studies
Rat
Groups of about 40-day old CR strain rats (65/sex/group) were dietary
fed 0, 1, 10 or 100 mg/kg methacrifos for 2 years. Haematology,
coagulation, clinical chemistry and urinalysis studies were carried
out in 10 rats/sex from the control and 100 mg/kg group.
No clear effects were found on mortality, food consumption, body and
organ weights, haematology, clinical chemistry and urinalysis.
Cholinesterase activity in plasma and erythrocytes, which was measured
at 3, 6, 12, 18 and 24 months, was influenced. Cholinesterase
activity in plasma was inhibited in females of the 100 mg/kg group at
3, 6, 12 and 24 months, in males of the same group at 3, 6 and 12
months and in females of the 10 mg/kg group at 3 and 24 months.
Cholinesterase activity in erythrocytes was inhibited in females of
the 100 mg/kg groups at 6 months and in males of the same group at 12
months. Finally, cholinesterase activity in brain, which was measured
at 24 months, was not affected. The results of the microscopic
examination indicated that methacrifos did not produce
histopathological changes. A no-effect level in this study is 1 mg/kg
in the diet (Basler et al, 1980).
RESIDUES IN FOOD
USE PATTERN
Methacrifos is an organophosphorus insecticide and acaricide, with a
rapid and long-lasting action on a number of major pests of stored
products. It is mainly used as a grain protectant but also against
insect pests in other stored products. It is effective against
insects showing resistance to commonly used stored product
insecticides, including other organophosphorus compounds.
The main fields of application are cereal grains (maize, rice,
sorghum), cocoa, coffee, peanuts, pulses and tobacco.
The material shows a wide spectrum of activity notably against
Sitophilus spp., Rhizopertha dominica, Tribolium spp., Oryzaephilus
surinamensis, Lasioderma serricorna, Dermestes frisehii,
Anugasta Kuhniella, Plodio interpunetella, Acarus siro,
Glycophagus domesticus (Wyniger et al, 1977).
The major formulations are emulsifiable concentrate, solution in
organic solvents and dusts.
The dosage rate under usual conditions of storage and infestation is
10 mg ai/kg, which gives protection to stored products for several
months. At lower storage temperatures, or if infestation is moderate,
satisfactory protection can be obtained with 5 mg/kg. In some
situations (e.g. in Australia against a resistant strain of
Rhizopertha dominica) a rate of 20 mg ai/kg may be needed to
achieve satisfactory control.
Methacrifos is also used for space treatment in empty silos at 50-75
mg ai/m3, for surface treatment of stored products and as a wall
treatment in storage premises.
Methacrifos application does not affect germination, baking quality,
taste or odour.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
various countries, including Australia, France, Spain, Switzerland,
Mali and South Africa, on a variety of cereal grains, dry pulses,
tropical seeds and products of animal origin. The data were obtained
from both large and small-scale trials performed under practical
conditions.
Wheat, barley, oats
ln laboratory experiments, simulating grain storage, in Australia, it
was shown that the residue of methacrifos decreased more rapidly with
increased temperature and moisture content (Table 2); increased rate
and/or repeated application did not influence the dissipation rate
(Table 3); some evidence was obtained that enzymic activity in the
grain was at least partially responsible for the more rapid initial
breakdown (Table 3). The rate of residue decrease after treatment of
wheat, barley and oats is similar. On all variety loss during storage
at 36°C is more rapid than at 31°C as shown in Table 3. (McDougall,
1974; Moore, 1973; Moore et al, 1974).
Several small-scale trials in which wheat, barley or oats were stored
in mini-silos (5´ ton of each grain) or in drums (120 l) showed
similar results. As in the laboratory experiment, the application
rate of methacrifos does not affect the rate of dissipation in any of
the grains investigated (McDougall, 1975; McDougall, 1976) (Table 4
and 5). From these trials half-life periods could be calculated using
the formula t´ = (t2 - t1) log 2 / [2 - (logC2/C1) × 100], where
C1, C2 are concentrations of the insecticide at times t1 and t2
respectively. Only data for 84 days storage were used since the
temperature dropped after that period.
TABLE 2. Laboratory trials on the dissipation of methacrifos in stored grains
Storage Conditions Application Residues of methacrifos, in mg/kg, after storage
rate
Commodity Moisture % Temp. °C mg/kg 0/1 7 14 28 42 56 70 84
Wheat 9.3 28 24 17.3 16.1 14.7 12.3 12.1
10.3 " " 14.8 14.0 12.0 8.8 7.6
11.3 " " 13.4 12.4 9.5 6.7 4.9
12.3 " " 13.4 10.7 7.7 5.0 3.0
9.3 35 24 14.5 13.8 12.5 8.5 6.3
10.3 " " 13.8 11.5 8.1 4.8 3.2
11.3 " " 13.3 10.2 6.1 - -
12.3 " " 12.9 8.2 4.4 - -
9.6 42 24 13.1 8.5 4.8 - -
10.4 " " 12.6 6.7 3.1 - -
12.3 " " 4.9 2.0 - - -
12.4 " " 11.1 4.1 1.2 - -
Wheat 11.9 31 20 16.4 12.8 9.6 7.6 5.4 4.1
11.8 36 " 16.2 11.4 8.9 4.5 3.2 -
Barley 11.7 31 " 16.9 14.2 12.0 8.0 5.8 3.4
11.8 36 " 18.9 11.6 8.7 4.4 2.5 -
Oats 11.7 31 " 19.2 13.2 11.6 6.9 5.1 -
11.9 36 " 18.8 11.6 8.4 3.7 2.5 -
TABLE 3. Laboratory trials on the relative disspation of methacrifos in wheat
Storage Condition Application Residues of methacrifos as %
rate of initial residue (=100)
commodity moisture % temp. °C mg/kg 0 2 14 28 42 Reference
Wheat 11.2 35 24 100 82 48 30 22 Moore & McDougall, 1974
11.2 " 50 100 73 45 28 21
11.1 " 1501 100 74 47 34 25
1O.91 "1 24 100 98 60 36 22
11.2 " 24 100 93 44 29 21
12.5 " 4 + 15 100 79 49 33 -
1 applied in "dead" grain
TABLE 4. Mini-silo storage trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2/3 6-8 13-15 28 56 84 105 126 147 168 Reference
Wheat 10.2 22-26.5 10 4.7 5.8 5.0 5.0 4.2 4.1 - 3.0 - 2.9 McDougall, 1975
Barley 12.8 " " 9.4 8.6 8.6 8.0 5.7 3.9 2.9 - 2.0 - 1.8
Maize 12.9 " " 7.2 7.0 7.0 6.8 7.0 3.9 4.0 - 2.4 - 2.9
Sunflower 7.2 " " 7.7 7.5 7.2 6.7 7.4 5.0 4.9 - 3.6 - 2.7
TABLE 5. Drum trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos in mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2 5 7 12 28 56 84 126 175 210 Reference
Wheat 8.3-10.2 32 5 5.3 - - 5.5 5.4 4.0 3.1 2.5 1.6 1.2 1.1 McDougall, 1976
10 8.8 - - 9.9 8.2 7.1 5.3 4.3 3.1 2.4 1.8
Oats 7.4-8.0 10 10 4.1 - 4.4 - 3.6 2.8 2.4 1.7 1.5 - 0.4
(in sealed
bags) 7-8-8.4 32 10 4.1 - 4.8 - 4.4 3.4 2.4 1.7 1.3 - 0.4
Maize 8.6-10.3 32 5 2.6 2.9 - - 2.4 2.0 1.8 1.4 1.0 0.6 0.5
10 4.2 6.0 - - 6.0 4.5 4.0 2.5 1.9 1.5 1.3
Peanuts 4.4-7.9 32 10 7.3 - - 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
20 16.0 - - 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
Soybeans 5.5-7.4 32 5 2.3 2.6 - - 2.5 2.1 1.6 1.4 1.1 0.9 0.9
10 7.1 7.6 - - 7.1 6.3 5.7 4.8 3.8 3.6 3.2
TABLE 7. Half-life of methacrifos on various grains stored in mini
silos at 26°C
Commodity Storage conditions Half-life (days)
moisture temp.°C
Wheat 10.2 26 105
Barley 12.9 26 44
Maize 12.8 26 100
Sunflower seed 7.2 26 90
From the trial in which the grains were stored in drums at 32°C the
following half-life periods were calculated; for the moisture content
(see table 5).
Commodity Half-life(days)
Wheat 70
Oats 60
Maize 85
Peanuts 55
Soybeans 100
Large-scale trials
Wheat and barley
Large-scale trials in silos were carried out under a range of storage
conditions in various countries e.g. Australia, Argentina, Morocco,
Spain, Switzerland and France. As in the small-scale trials it was
found that the application rate did not affect the rate of dissipation
of the residues in any of the grain investigated (McDougall 1976)
(table 8).
The experiments under practical conditions confirmed that the
dissipation of methacrifos is mainly dependant on time and
temperature. The residue decrease under storage conditions prevailing
normally in Europe (temperature 15-25°C) is much slower than in
regions where storage temperatures are generally higher e.g. Australia
(Formica, 1975).
In the trials carried out in Australia in which the grain was aerated
during the storage period, there were indications of a decreased rate
of dissipation, compared with silos in which the grain was stored
under the same conditions of temperature and moisture at the start of
the experiment, but not aerated. Aeration was started 24 hours after
treatment and functioned only during the cooler hours of the day for a
total of 84 hours a week for the first 4 weeks and about 30 hours
weekly thereafter (Table 9) (Moor and McDougall 1975). In other
experiments aeration had little or no effect on residue dissipation
(Formica, 1978g).
TABLE 8. Dissipation of methacrifos in small grain
Large-scale trials in silo. (Moore and McDougall, 1974a, 1974b and 1975).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0,15 1 6 11/12 16 21 22/23 26 34 41
Wheat Australia 100 11.3 ± 30 20 0.6 21.8 11.8 7.5 5.6 4.1 4.2
(Queensl.) 10.3 20 1.5 14.2 9.6 5.3 3.9 2.9 2.6
1974 10.8 20 6.0 15.5 8.6 5.2 3.0 1.8 1.6
Australia 800 11.5 26-28 20 0.6 9.3 6.4 4.8 4.0 4.1 3.7
(N.S.W.) 11.3 20 1.5 8.6 6.3 4.9 3.8 3.0 2.5
1974 10.9 20 6.0 10.7 8.1 5.7 4.9 4.6 3.3
(aerated) Australia 550 12.4 (note 1) 15 0.1 9.7 5.0 3.6 2.3 1.4 1.6
(Queensl.) 11.4 15 2 9.0 5.6 4.2 2.6 2.1 2.6
11.0 15 4 9.6 5.7 4.8 3.6 2.8 2.2
11.0 15 6 11.9 6.0 5.3 3.3
not 10.9 (note 2) 15 0.1 11.4 6.0 3.7 2.3 1.6 1.5
aerated 10.2 2 9.8 6.1 3.6 1.6 0.75 0.8
10.4 4 10.0 3.8 1.7 1.6 0.6 0.6
9.9 6 10.4 4.8 2.6 1.8
1 Mean temperature in silo first 18 weeks 25-31°C; from 23-41 week 14-23°C.
2 Mean temperature in silo first 18 weeks 27.5-34°C; from 23-41 week 17-20°C.
TABLE 9. Dissipation of methacrifos in small grains
Large scale trials in silos. (Formica, 1978a, 1978b).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0 45 70 87 171/ 322/ 430 463 504 560
180 339
Barley Switzerland 51 2-25 22.3 2 13.8 7.6 2.6 3.8 2.5 1.5 0.86
(3.0- (7.8- (2.9- (2.9-
1.75) 1.6) 1.7) 0.3)
Barley Spain 100 11.6 13-26 10 2 4.1 3.7 3.2 1.3 2.8 1.7
Wheat Spain 100 11.0 13-26 10 2 9.4 5.6 3.0 2.8 2.1 1.0
The effect of aeration was also evaluated in a residue trial carried
out under Swiss conditions. A bin of barley treated with methacrifos
at 10 mg/kg was aerated twice during the storage period (after one and
three weeks) and another bin in the same warehouse was not aerated.
The residue on the aerated barley was 12.4 mg/kg at the beginning of
the experiment, 6.6 mg/kg after one week; 4.6 mg/kg after 32 weeks and
2.0 mg/kg after 74 weeks. The residues in the non-aerated bin were
11.3, 8.6, 4.2 and 2.5 mg/kg at the same sampling times, showing that
aeration had little or no effect on the residue dissipation. The
residue in oats stored at the same farm (without aeration ) were 10.2,
9.0, 6.0 and 3.7 mg/kg at these sampling times. Comparable results
were obtained in a similar trial on wheat in Switzerland in which one
bin was aerated after 54, 103 and 142 days post-treatment, by
circulating the wheat through the conveyer system and back to the
silo, and another bin not aerated. The initial residues were 6.0 and
6.1. Residues after the three aerations were 4.2, 3.7 and 3.4 mg/kg
on the aerated wheat and 4.8, 4.2 and 3.9 mg/kg on the nonaerated.
Again the rotation and resulting aeration of the wheat had little, if
any, influence on the dissipation rate (Formica, 1978d, 1978e). The
residue dissipation at different levels in a silo was studied in
Switzerland under typical European continental storage conditions with
temperatures of about 5°C in winter and 22°C in summer. Methacrifos
was mixed with the grain in a closed conveyer system, at a rate of 11
mg/kg; there was no ventilation during the storage period. The
initial concentration was 50 - 70% of the nominal value calculated
from the application rate and the volume treated. The residue at 2 m
depth increased during the following 5 weeks to almost the nominal
value and slowly decreased thereafter. It is assumed that the active
ingredient was not homogeneously dispersed immediately after
application but became more uniformly distributed through migration.
From a comparison of the residues in the samples taken from the
surface and at 2 m depth, it seems evident that the dissipation was
somewhat slower in the interior than on the surface of the stored
grain (Formica, 1976f). (Table 10).
In a study of the influence of the formulation on dissipation an
aqueous formulation showed more rapid dissipation than a formulation
with kerosene. After treatment with a nominal 10 mg/kg the residues
of the aqueous formulation were 0.2/0.16 and 0.15/0.25 mg/kg after 2
and 15 weeks respectively, whereas the residues of the kerosene
formulation were 9.1/9.3 and 4.0/3.5 mg/kg.
TABLE 10. Dissipation of methacrifos in wheat (Switzerland)
Days after
treatment 1 7 21 35 77 162 252 324 470 526 653
samples from
Surface 5.3 3.3 3.2 8.6/6.1 5.9/5.9 4.0/4.0 3.5 2.4 1.3 0.7 -
2 metres 4.6/3.9 5.2 6.0 10.1/7.4 6.7/5.6 6.2/6.5 5.2 4.0 2.6 2.8 1.4/2.25
Maize
Several small-scale trials were carried out with maize kernels and/or
cobs stored under various conditions of temperature and moisture
content. Although the distribution of the active ingredient was
rather inhomogeneous, it was evident, as with the other grains, that
the dissipation is dependant on time and temperature.
Under tropical conditions (temperature 35°C and higher) the residue
decreased much more rapidly than at moderate temperatures (Giannone
and Formica, 1979b).
The dissipation of methacrifos in maize was studied in a large scale
trial in Switzerland; 80 tons of maize kernels (moisture content
14.5%) were treated at 10 mg/kg on a conveyer system during the
filling of concrete silo cells, which were closed without ventilation.
Results are shown in Table 11.
TABLE 11. Dissipation of methacrifos in maize
Temp. in silos °C1 19 9 14 27
Days after treatment 6 76 300 376
Residue, mg/kg 7.8 3.62 3.9/3.4 0.4/0.7
(5.7-9.6) 3.1-4.2
1 Temperature at a depth of 2 metres.
2 Mean value of 4 samples; two samples analysed 5 months later than
the other two samples; samples stored at -20°C.
Rice
Small-scale trials on the dissipation of methacrifos under various
storage conditions were carried out in Australia. Brown and polished
rice were treated at the rate of 10 mg/kg and stored in bags, at
temperatures of 13-26°C. The moisture content of the brown rice was
approximately 14.2% and of the polished rice 14.4%. The initial
residue of 9.1 mg/kg in brown rice decreased to 5.0 mg/kg after 12
weeks and to 3.9 mg/kg after 24 weeks. In the polished rice the
corresponding residue levels were 7.9, 6.3 and 4.4 mg/kg respectively.
In other trials paddy rice, brown rice and polished rice were stored
in cardboard drums, and paddy rice also in mini-silos of about six
tons capacity. The decrease of the residue in paddy rice stored at
about 32°C was faster than in brown or polished rice. Under the
conditions occurring in the trials the dissipation rate of methacrifos
was independent of the application rate (Hart and Moore 1975;
McDougall 1976b) (Table 12).
TABLE 12. Dissipation of methacrifos in rice (McDougall, 1976b; Hart and Moore, 1976
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
mini-silo moisture temp. °C mg/kg
or cardboard %
Type of rice drums 0 12 23 54/51 124/121 166 205
paddy rice silo 13.7 17-26 10 8.2 5.8 4.7 3.7 2.6
paddy rice drums 8.8-12.8 32 10 10.8 6.2 4.4 2.2 1.2
drums 8.8-12.8 32 5 4.5 2.6 1.9 0.9 0.5
brown rice drums 8.8-12.8 32 10 9.1 7.0 5.5 3.8 2.7
drums 8.8-12.8 32 5 3.7 3.2 2.4 1.2 1.0
polished rice drums 8.8-12.8 32 10 13.0 9.6 7.2 4.6 2.7
drums 8.8-12.8 32 5 4.7 3.9 3.2 1.6 1.0
TABLE 13. Dissipation of methacrifos in sorghum
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
silo or moisture temp. °C mg/kg
Country bags % 0 15 21 45 56 90 147
Australia silo 13 27 15 13.6 9.3 7.7 6.3
13 27 15 9.1 4.1 7.1 4.3
13 27 7.5 8.0 4.7 4.9 2.7
Mali bags 25-30 10(dust) 3.6 3.0 1.9
25-30 10(solution) 6.1 4.2 2.1
TABLE 14. Dissipation of methacrifos in peanuts, stored in drums (McDougall, 1976a)
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
moisture % temp. °C mg/kg
Country % 7 12 28 56 84 126 175 210
Peanut
whole 7.8-4.4 32 20 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
kernel 0.6 1.5 1.7 0.3 0.3 0.4 1.0 0.4
shell 50.8 49.5 30.6 30.5 29.2 10.3 8.8 7.7
Peanut
whole 7.8-4.4 32 10 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
kernel 0.6 0.3 <0.3 0.3 <0.3 <0.3 <0.3 <0.3
shell 41.5 21.5 23.7 12.9 7.3 7.3 4.1 5.7
Sorghum
Data are available from large-scale trials in Australia where sorghum
treated at 7.5 and 15 mg/kg was stored in silo bins and from Mali
where sorghum treated according to the so-called "sandwich method"
with a dust or a spray was stored in bags. The rate of dissipation
was similar to that in the other grains (Hart and Moore 1976, Giannone
and Formica, 1980) (Table 13).
Pulses (dried legume vegetables)
In a small-scale trial in Switzerland, a mixture of beans and peas
(Vicia faba, Phaseolus nanus and Pisum sativum) was
treated with a dust (10 or 20 mg/kg ai/kg) or a liquid formulation (10
mg ai/kg) and stored in drums at 25 ± 1°C; moisture content before
treatment was 9.8%. Three hours after the application the residues in
the lots treated with the dusts at 20 and 10 mg ai/kg and the solution
at 10 mg ai/kg were 5.5, 3.9 and 2.7 mg/kg respectively. After 27
days the residue levels in these lots were 6.5, 2.6 and 2.6 mg/kg and
after 142 days 3.5, 1.7 and 1.7 mg/kg respectively.
In a small-scale trial in Nigeria, cowpeas were treated with 10 mg/kg
methacrifos and stored in open jute bags under tropical conditions.
No residues (<0.12 mg/kg) were found 65 and 92 days after treatment.
Peanuts
A mini-silo (drum) trial was carried out in Australia. Methacrifos
was applied at 10 and 20 mg/kg to whole peanuts, which were stored in
120 l cardboard drums at 32°C. On all sampling dates kernels and the
shells were analysed separately. The results show that nearly all the
residue is contained in the shell (McDougall, 1976).
In Mali, peanut kernels were treated with 10 mg/kg methacrifos and
stored in closed plastic bags under tropical conditions (25-30°C).
Residues after 15, 60 and 90 days were 7.6, 5.6, 5.2 mg/kg
respectively (McDougall 1976).
Cocoa-beans
Cocoa-beans stored in closed jute bags in a warehouse were treated
with methacrifos at 0.4 g ai/m2 on the outside of the bags. Samples
were taken at four sampling dates from the outside layer of cocoa-
beans, which had been exposed to the treatment, and from the interior
of the bags. The residues in samples from near the outside of the
bags were higher than in the internal samples; 34 days after treatment
the residues in the outer samples were 1.0 and 1.2 mg/kg and in the
inner samples <0.025. After 98 days the levels were 0.83 and 0.32
mg/kg respectively.
The methacrifos residues were found mainly in the peel. 34 days after
treatment the residues in the whole beans were 1.0 and 1.2, in the
peel 5.6 and in the kernel 0.5 mg/kg (Formica, 1978c).
Coffee beans
Raw coffee beans imported from Nigeria were treated in Switzerland
with methacrifos in 10 and 20 mg/kg. The treated beans were stored in
closed drums at 20° ± 2°C. After 4 months storage the residues from
the 20 mg/kg treatment were 2 mg/kg but 0.5 mg/kg in the lot treated
with 10 mg/kg (Renfer, 1976).
FATE OF RESIDUES
In animals
General
As grain treated with methacrifos and grain products derived from
treated grain may be used as animal feed, it was necessary to study
the fate of methacrifos in livestock animals and to demonstrate that
no significant residue would remain in livestock and poultry, milk or
eggs. Special trials therefore, were carried out to study possible
carry-over from residues in feed to tissues or products of animal
origin.
Chickens
In a preliminary experiment, four young male broilers were given a
single oral dose of 20 mg/kg methacrifos by capsule. The birds were
slaughtered 6, 12, 24 and 48 hours after dosing. Samples of fat,
muscle, liver, kidney, heart, gizzard and skin were analysed. The
residues in all muscle and heart samples were 0.01 mg/kg or less.
Residues in the other samples decreased very rapidly (Moore and
McDougall, 1974c) (Table 15).
TABLE 15. Residues of methacrifos in chicken tissues after single
oral treatment with 20 mg/kg
Tissue Residues in mg/kg
time (hours post-treatment)
6 12 24 48
Fat 0.22 0.06 <0.01 <0.01
Liver 0.02 0.01 0.01 <0.01
kidney 0.08 <0.03 <0.03 <0.03
Gizzard 0.42 <0.01 <0.01 <0.01
Skin 0.06 0.02 <0.01 <0.01
Muscle <0.01 <0.01 <0.01 <0.01
Heart 0.01 <0.01 <0.01 <O.01
In a further experiment 5 groups of 77 one-day old chicks each were
given feed fortified with methacrifos at levels of 0, 10, 100, 250 or
500 mg/kg for 63 days. At the end of the feeding period 20 chickens
from each group (10 males and 10 females) were slaughtered and the
combined muscles, skin, kidney and liver used for analysis. In all
tissues examined residues were less than 0.01 mg/kg (Formica, 1974).
Eggs
Three groups of 15 laying hens were fed a diet containing 0, 10 or 20
mg/kg methacrifos. Egg samples were collected one day before
treatment, on the 16th day of treatment and 0, 2, 3, 7 days after the
end of the treatment. In none of the samples were residues found
(<0.01 mg/kg) (Schnabel and Formica, 1979).
Milk
Two lactating cows were given 30 mg methacrifos by capsule daily for
10 days. This dose corresponds to about 3 kg of cereals treated at a
rate of 10 mg/kg daily in the total ration. Pooled morning and
evening milk samples were taken before, during the feeding period and
three days after its completion. No residues (<0.001 mg/kg) could be
detected in the milk of the treated cows before, during or after the
10 day period (Formica, 1977).
In plants
Stored grain
The degradation of carbonyl 14C methacrifos (specific radioactivity
31.1 µCi/mg) in grain treated with 10 mg/kg was studied under
laboratory conditions for one year; 84-94% of the applied
radioactivity was recovered at all sampling times, indicating very
limited volatility of methacrifos or its degradation products. After
one year about 25% of the then recovered radioactivity was present as
parent compound. The fact that little volatilisation of the
radioactivity occurred during the storage indicates that methacrifos
and its degradation products were strongly absorbed to the wheat
grain. Less than 15% of the recovered radioactivity could be washed
off the surface of the grain with methylene chloride. The bulk of the
radioactivity could be extracted only after grinding of grain. The
non-extractable portion of radioactivity was low at the early sampling
dates but rose to 9-14% after 140 and 385 days. The low but
continuous evolution of 14C during the experiment may demonstrate
that the insecticide is eventually metabolised by the wheat grain.
The organosoluble radioactivity on or in the grains consisted mainly
of the parent compound. The oxon was not detected. Mono- and
di-demethylated methacrifos were detected by ethylation followed by
GLC analysis. Their identity was confirmed by methylation with
deuterated diazomethane (CD2N2) followed by GCMS.
The results give strong evidence for the degradation pathways shown in
figure 2 (Blattmann 1978).
Figure 2. Degradation of methacrifos in grain
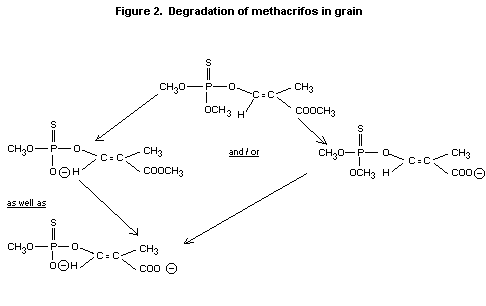 The decrease of the protective effect of methacrifos against insects
is closely related to the decrease of the amount of the unchanged
methacrifos still present. This suggests that the degradation
products are less toxic to insects than the parent compound (Renfer
1977).
TABLE 16. Distribution and characterization of radioactivity in
stored wheat after the application of 10 mg/kg 14C-methacrifos
% of radioactivity recovered after
storage for (days)
Compounds 0 23 140 294 385
Extractable
methacrifos parent 99.7 98.5 51.6 33.3 25.2
demethylated
methacrifos-derivatives - - 34.1 45.4 6O.2
unknowns - - 5.1 6.8 2.9
Non-extractable 0.3 1.5 8.7 13.8 10.9
CO2 - 0.02 0.5 0.7 0.8
The formation of the demethylated degradation products has been
studied in various products stored under practical conditions. The
results are summarized in table 17.
TABLE 17. Formation of the demethylated metabolites during storage (treatment in all
cases 10 mg/kg)
Storage after Residues mg/kg Reference
treatment methacrifos demethylated
days metabolites
Wheat grain 215 2.7/2.9 1.6/1.8 Formica, 1978d
365 1.7/1.8 1.7 Formica, 1978c
430 1.0 2.4 Formica, 1978b
Barley grain 76 4.2 2.0 Giannone and Formica, 1980a
300 3.0 4.2 " " "
370 1.5 3.8 " " "
430 1.3 2.5 Formica, 1978b
Maize grain 76 6.4 1.6 Giannone and Formica, 1980a
300 4.1 2.0 " " "
370 0.7 5.0 " " , 1979
Maize cob1 231 3.5/3.7 11.4/11.6 Giannone and Formica, 1979
Sorghum grain 15 6.1 1.3 Giannone and Formica, 1980b
45 4.2 0.7 " " "
90 2.1 1.4 " " "
Peanut kernels 15 7.6 0.2 Giannone and Formica, 1980c
45 5.6 0.5 " " "
90 5.2 0.6 " " "
Cocoa beans 15 0.44/0.41 O.27/0.29 Giannone and Formica, 1979a
1 Cobs stored in open wired cribs were treated by spraying the surface of cribs with 10 mg
ai/kg related to the total quantity of maize cobs. As the samples were taken from the periphery
of the crib a rate of their treatment higher than 10 mg/kg must be expected.
In processing
Residues of methacrifos have been followed through milling and baking
procedures in several experiments. As the insecticide is primarily
located in the outer layers of the grain, most of the residues are
found in the bran and the low grade flour fractions after milling.
The residue in flour milled 1 month after the treatment of the grain
was about 1/10 of that in whole grain. After prolonged storage the
residues in flour tend to increase, but they do not exceed 1 mg/kg
even after storage for 21 months. In the baking process methacrifos
and its metabolites are degraded to a large extent resulting in low or
undetectable residues (maximum about 0.1 mg/kg or lower). (Table 18).
TABLE 18. Residues of methacrifos in milled wheat fractions and bread
Residues (mg/kg) in
Treatment Storage
mg/kg time whole wheat white flour low grade flour bran white bread Reference
flour
10 1 month 4.0/3.8 0.4/0.4 1.7/1.6 7.6/6.4 <0.03 Formica 1978b
10 7 months 3.1/2.9 0.6/0.6 8.1/8.4 10.0/9.2 0 01/<O.01 " 1978d
10 21 months 1.4/1.6 1.0/0.8 10.3/9.1 7.4/7.5 0.02/0.08 " 1978f
15 1 month 7 8/7.8 0.6/0.6 2.5/2.7 10.4/11.2 <0.03 " 1978b
20 1 month 8.0/7.4 0.9/0.85 3.1/3.2 15.6/18.0 0.03/0.04 " 1978h
10 265 days 0.751 ca. 0.30 2.6 <0.04 Tournayre 1978
5 265 days 0.261 ca. 0.10 0.8 <0.04 " "
10 265 days 2.01 ca. 0.50 6.1 <0.04 " "
1 after 252 days
In experiments with the radio-labelled insecticide it was shown that
the same degradation products are formed during baking as are found in
stored grains (Blattmann 1978).
Further data on the effect of processing rice, oats and barley,
including malted barley are presented in table 19. In these
experiments the initial concentrations are in most cases higher than
recommended. As with wheat, the milling of paddy rice treated with
methacrifos resulted in a considerable loss of insecticide. More than
90% of the initial residue was removed with the hulls (McDougall
1976), resulting in a decrease of the residue in going from paddy to
brown rice by a factor of 5 to 8. Most of the insecticide present in
the brown rice was again removed with the bran fractions.
The amount of methacrifos residue is further decreased on cooking.
Short cooking of about 15 minutes decreased the residue by a factor of
about 2.6 whereas the more usual cooking of 25 minutes resulted in a
reduction by a factor of about 8.
Processing treated barley grain to malt, which was then used to brew
beer, gave very low level in the beer.
The processing of treated cocoa beans to cocoa mass decreased the
initial methacrifos on the beans at least by a factor of 10. Since
further diluting processes take place the overall decrease of
methacrifos from the raw product to the consumed chocolate is even
higher.
METHODS OF RESIDUE ANALYSIS
Residue of the parent compound in:
Cereal grains and grain derivatives. Methanol or acetone extracts can
be used to determine the active ingredient by GLC without cleanup.
Recoveries range from 80-115%; the limit of determination is about
0.05 mg/kg. (Desmarcherlier et al 1977; Moore and McDougall, 1973
and Formica, 1973g).
Chicken tissues. Residues in chicken tissues are determined by GLC
with a phosphorus-specific detector after extraction with acetonitrile
and clean-up by partition (Formica 1974b).
Milk. Milk is coagulated with acetone. After a partition step,
methacrifos is determined by GLC with a phosphorus-specific detector.
'Total residue':
A method has been developed to determine unchanged methacrifos and the
dealkylated metabolites in a two-step procedure in cereal grains, eggs
and cocoa mass. Extraction with aqueous methanol is followed by
partition of the parent compound into hexane. The dealkylated
metabolites are then extracted from the aqueous phase with methyl
acetate and methylated with diasomethane. The residues are determined
by GLC with a phosphorus-specific detector. Limits of determination
are 0.025 mg/kg for the parent compound and 0.04-0.1 mg/kg for the
dealkylated product.
Both the method for residues of the parent compound in cereal grains
and the method for the total residue are suitable or can be adapted
for regulatory purposes.
TABLE 19. Effect of processing on methacrifos residues
Raw product Treatment Storage Residue before Processing or Residue after
level processing processed product processing
mg/kg mg/kg mg/kg
Paddy rice 18 6 months 3.1 brown rice raw 0.65
cooked 15 min. 0.23
white rice raw 0.12
cooked 15 min. <0.02
Paddy rice 10 3 months 3.0 brown rice 0.36
hulls 18.4
white rice <0.2
bran 2.7
Hulled rice 18 3 months 7.1 cooked 5 min. 4.4
cooked 15 min. 2.7
18 6 months 4.8 cooked 25 min. 0.75
Polished rice 18 3 months 7.5 cooked 15 min. 2.6
Oats 18 3 months 4.7 cooked 15 min. 0.9
Barley 18 3 months 4.8 malted barley 0.1
Malt1 10 - 9.8/10.1 brewer's grains 1.7/2.0
wort before boiling 0.27/0.31
yeast <0.1
beer <0.01
Cocoa beans 10 7 days 5.0/5.2 cacao mass 0.46/0.44
surface of
bags with 97 days 0.32 cacao mass 0.02
0.4 g a.i./m2
1 Malt has been directly treated to follow the dissipation during further processing. Under normal
practical conditions only barley grain is to be treated.
EVALUATION
COMMENTS AND APPRAISAL
Methacrifos is an organophosphorous insecticide which is almost
exclusively used as a grain protectant and as a control agent against
insect pests in other stored products. It may be used as an acaricide
in the same stored commodities. It gives good and long-lasting
protection of grain and other stored products against a broad spectrum
of pests including those which show resistance to commonly used stored
products insecticides such as other organophosphorous compounds,
lindane, etc. The major formulations are emulsifiable concentrate,
solutions in othanic solvents and dusts. The normal application rate
is about 10 mg ai/kg grain. Lower rates may be used at lower
temperatures; exceptionally a higher rate up to 20 mg/kg may be
needed.
Methacrifos is rapidly absorbed, metabolised and excreted in mammals.
Two metabolites detected in urine and faeces were identified as
N-acetyl-S(2-methoxycarbonylprop-1-enylcysteine) and P-O-desmethyl
methacrifos. Neither methacrifos nor its metabolites were found to
accumulate in the tissues.
Methacrifos has a moderate acute toxicity following oral
administration to various animal species. Signs of poisoning are
typical of cholinesterase inhibitors. The acute toxicity was found to
be potentiated by several other organophosphorous esters.
Methacrifos did not induce a delayed neurotoxic response in hens. In
short- and long-term studies methacrifos caused signs and symptoms of
cholinesterase inhibition, which were associated with effects on body
weight gain and food consumption. Brain cholinesterase activity was
inhibited at 100 mg/kg. In rats at high dose levels, effects on
several haematological parameters were observed. Cholinesterase
activity in plasma was inhibited at doses above 10 mg/kg and recovery
of cholinesterase activity was relatively slow.
In long and short term studies, cholinesterase depression was observed
at 10 mg/kg and above. A no-effect level of 1 mg/kg was observed.
Because of parental mortality in a 3-generation reproduction study and
foetal death in teratology bioassays, the meeting could not evaluate
fully the potential effects in these areas. Further studies are
required for a full evaluation of this compound.
Mutagenicity studies with bacteria, a mouse carcinogenicity study and
a rat teratology study were all negative.
Acceptable data were available to allow the meeting to establish
no-effect levels in two mammalian species and a temporary ADI was
allocated.
The fate of methacrifos in stored cereals, beans, peanuts and cocoa
has been elucidated in extensive laboratory trials as well an in many
supervised small and large-scale trials under practical conditions in
various countries and under a variety of storage conditions. The
following conclusions can be drawn.
The rate of degradation depends on time and storage conditions. The
residues decrease faster with increasing temperature and moisture
content of the food commodity. Other factors, such as grain variety,
rate of application, application technique and type of formulation,
have little or no influence on the dissipation rate. Aeration of the
stored cereals has only a minor effect on the rate of loss.
The metabolic pathways of methacrifos on grain and other stored
products were elucidated. The compound is demethylated to mono- and
di-desmethylated methacrifos; there is some evidence that these
metabolites are not cholinesterase inhibitors. These are further
metabolised to CO2 at a slow rate.
The residues remain primarily on the outer layers of the grain.
During prolonged storage only limited penetration into the endosperm
takes place. Processing of the raw commodities, such as milling of
cereals, dehusking and polishing rice, removing shells and seed-coats
from peanuts and shells from cocoa, reduce the residue considerably.
During baking and brewing methacrifos and its main metabolites are
further degraded, resulting in residues at or about the limit of
determination in bread and beer. Degradation also occurs during
cooking to an extent that depends upon the time of cooking.
When grains treated at normal rates are fed to livestock animals or
poultry, no residues are found in meat, milk or eggs.
Methods of residue analysis are available for methacrifos residues
(parent compound) in stored products by GLC with a
phosphorous-specific thermionic detector after extraction with
methanol or acetone. The limit of determination is about 0.05 mg/kg.
Methods are also available for methacrifos in meat of poultry, eggs
and milk after extraction with acetonitrile or acetone followed by
various partition steps and GLC with a phosphorous detector; the limit
of determination is 0.01 mg/kg. The methods are suitable or can be
adapted for regulatory purposes.
Residues of the unchanged parent compound and the dealkylated
metabolites can be determined, in a two step procedure, in cereal
grains, eggs and cocoa-mass. The limits of determination are about
0.025 mg/kg for the parent compound and 0.04 - 0.1 for the dealkylated
metabolites. The method can be adapted for regulatory purposes.
When establishing maximum residue limits for methacrifos on grain or
other stored food commodities, the following factors have to be
considered.
1. The residue level depends largely on the application rate, which
has to be chosen according to the nature of the insect or mite
infestation and the desired period of protection. Variation in
residue levels are to be expected among different countries even when
the same dosages are applied and the same storage period is used,
since the rate of dissipation may differ according to the local
climate and storage conditions.
2. The distribution of the insecticides may be heterogeneous,
depending on local application techniques.
3. Stored products may move in international trade at different times
after treatment according to commercial need.
Based on these considerations the maximum residue limits have to be
set at levels that will cover all situations encountered after the
normal applications, even if in some cases the actual residues will be
much lower.
The meeting examined residue data from supervised trials reflecting
established and/or anticipated good storage practice on a number of
stored food or feed commodities. From these data the meeting was able
to estimate the maximum residue levels that were likely to occur when
methacrifos is used in practice.
Level causing no toxicological effect
Rat: 1 mg/kg in the diet equivalent to 0.05 mg/kg bw/day.
Dog: 1 mg/kg in the diet equivalent to 0.025 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.0003 mg/kg bw.
RECOMMENDATION OF RESIDUES LIMITS
The meeting concluded that the maximum residue levels listed below are
suitable for establishing maximum residue limits.
These levels refer to the parent compound only.
Commodity Estimated maximum
residue levels
mg/kg
cereal grains 10
wheat flour (whole meal) 10
wheat flour (white) 2
wheat bran (unprocessed) 10
beans and peas (dry) 5
cocoa beans 10
peanuts (including shell) 10
peanuts 1
poultry meat 0.011
eggs 0.011
milk 0.011
1 The levels refer to the maximum residue levels which might occur
when the animals concerned are fed with commodities treated during
storage with recommended amounts of methacrifos.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Teratogenicity studies in rat and rabbits.
2. An appropriate reproduction study.
REFERENCES
Arni, P. and Müller, B. Samonella/mammalian-microsome mutagenicity
test with CGA 20168. (1979) Unpublished report, May 23, submitted by
Ciba-Geigy to WHO.
Basler, W. et al. Two-year chronic oral toxicity study with
CGA-20168 technical in albino rats. (1980) Unpublished report prepared
by Ciba-Geigy from the original Industrial Bio-test Laboratories
records, May 15, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the rat. (1974a)
Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the mouse.
(1974b) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute dermal LD50 of technical CGA 20168 in the rat.
(1974c) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe R. and Sachsse, K. Acute oral LD50 in the dog of technical CGA
20168. (1975) Unpublished report, November 12, submitted by Ciba-Geigy
to WHO.
Bathe, R. and Sachsse, K. Acute oral LD50 in the mouse of technical
CGA 20168. (1978) Unpublished report, August 15, submitted by
Ciba-Geigy to WHO.
Bathe R. et al. 26 weeks feeding study in dogs with technical CGA
20168. (1977) Unpublished report, March 4, Submitted by Ciba-Geigy to
WHO.
Charles, J.M. et al. Three-generation reproduction study with CGA
20168 technical in albino rats. (1980a) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-Test Laboratories Records,
April 10, submitted by Ciba-Geigy to WHO.
Charles, J.M. et al. A chronic oral carcinogenic toxicity study with
CGA 20168 in Swiss white mice. (1980b) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-test Laboratories records,
May 15, submitted by Ciba-Geigy to WHO.
Chesterman, H. et al. CGA 20168, 28-day dietary feeding study in
beagle dogs. (1975) Unpublished report from Huntingdon Research
Centre. November 28. Submitted by Ciba-Geigy to WHO.
Drake J.C. 1 Month dietary toxicity study in rats with compound CGA
20168. (1975) Unpublished report from Geigy Pharmaceuticals, February
17, Submitted by Ciba-Geigy to WHO.
Fritz H. et al. Reproduction study on CGA 20168 Technical. Rat.
(1978) Unpublished report, June 14, submitted by Ciba-Geigy to WHO.
Gfeller, W. CGA 20168 Oral toxicity in pigs. Daily administration for
28 days. (1974) Unpublished report from Ciba-Geigy Agricultural
Research Centre, January 28, submitted by Ciba-Geigy to WHO.
Hamböck, H. Metabolism of CGA 20168 in the rat. (1978) Unpublished
project report 27/78 from the Agrochemicals Division of Ciba-Geigy,
May 17, submitted by Ciba-Geigy to WHO.
Hool, G. and Müller, D. Dominant lethal study on CGA 20168. (1980)
Unpublished report, February 6, submitted by Ciba-Geigy to WHO.
Ifflaender, V. and Mücke, W. Distribution, degradation and excretion
of CGA 20168 in the rat. (1975) Unpublished report from the
Agrochemicals Division of Ciba-Geigy, April 9, submitted by Ciba-Geigy
to WHO.
Sachsse, K. and Bathe, R. Potentiation study. CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion. (1978)
Unpublished report, June 23, submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Acute oral toxicity and neurotoxicity study of
technical CGA 20168 in the domestic fowl (Gallus domesticus). (1979)
Unpublished report, November 6, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. Acute toxicity to rainbow trout, crucian
carp, catfish, bluegill and guppy of technical CGA 20168. (1974a)
Unpublished report, August 7, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. "8-Day-feeding toxicity" of technical CGA
20168 in the Japanese quail. (1974b) Unpublished report, July 13,
submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Technical CGA 20168. 21-Day percutaneous toxicity
study in rabbits. (1978) Unpublished report, April 24, submitted by
Ciba-Geigy to WHO.
Strittmatter, J. and Gfeller, W. 63 Days dietary toxicity study in
chicks with compound CGA 20168. (1975) Unpublished report from
Ciba-Geigy Agricultural Research Centre, March, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Skin sensitizing (contact allergenic)
effect in guinea pigs of CGA 20168. (1975) Unpublished report, July 1,
submitted by Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute inhalation toxicity in the rat of
technical CGA 20168. (1976) Unpublished report, July 7, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute dermal LD50 in the rabbit of
technical CGA 20168. (1977) Unpublished report, February 2, submitted
by Ciba-Geigy to WHO.
Ullmann, L. et al. 21-Day inhalation study on the rat with technical
CGA 20168. (1977) Unpublished report, December 16, submitted by
Ciba-Geigy to WHO.
REFERENCES ON RESIDUES IN FOOD AND THEIR EVALUATION
Blattmann, P. Degradation of CGA 20168 in stored wheat grains,
including their processing. (1978) Unpublished report PR 42/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Desmarchelier, J.M., Bengston, M., Connel, M., Minett, W., Moore, B.,
Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A collaborative
study of residues on wheat of chlorpyrifos-methyl, fenitrothion,
malathion, methacrifos and pirimiphos-methyl. 1. Method development.
Pestic. Sci. 8: 473-483.
Formica, G. CGA 20168. Gas chromatographic residue determination in
wheat grain, wheat meal, bran, groats and bread. (1973) Unpublished
report HEM 20/73 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. CGA 20168. Gas chromatographic residue determination in
chicken tissues. (1974) Unpublished report REM 9/74 from Ciba-Geigy
Limited, Basle, Switzerland.
Formica, G. CGA 20168. Residues in chicken tissues. (1974a)
Unpublished report RVA 205/74 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Degradation of CGA 20168 in wheat and maize. (1975)
Unpublished report RVA 314/75 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. CGA 20168. Residues in cow's milk after oral medication.
(1977) Unpublished report RVA 612/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Methacrifos (CGA 20168). Determination of CGA 20168 in
feedgrain treated with different formulations and stored in drums.
(1977a) Unpublished report RVA 615/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos in French barley after direct
spray treatment with the formulation EC 950. (1978a) Unpublished
report RVA 620/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat and barley after
direct spray treatment with the formulation EC 950. Carinena, Spain.
(1978b) Unpublished report RVA 626/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Residues of methacrifos in untreated cacao beans stored in
a warehouse and possibly contaminated through cacao beans treated with
DAMFIN(R) 950 EC, stored in the same warehouse. (1978c) Unpublished
report RVA 608/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of residues of methacrifos in stored grain
and its products seven months after direct spray treatment with the
formulation SO 050. (1978d) Unpublished report RVA 610/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat after direct spray
treatment with the formulation SO 050. (1978e) Unpublished report RVA
622/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain, and their products twenty-one months after direct spray
treatment with the formulation EC 950. (1978f) Unpublished report RVA
611/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in barley and oats after
direct spray treatment with the formulation EC 950. (1978g)
Unpublished report RVA 623/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain and their products after direct spray treatment with the
formulation EC 1000. (1978h) Unpublished report RVA 618/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Residue of methacrifos in maize cobs
after surface treatment with DAMFIN(R) 950 EC. (1979) Unpublished
report RVA 4010/79 from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic determination of
total residues in cacao liquor after different processings of cacao
beans treated with DAMFIN(R) SO 050. (1979a) Unpublished report RVA
4009/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic-determination of
methacrifos and its dealkylated metabolites in sorghum after treatment
with DAMFIN(R) SO 050 or DAMFIN(R) P 2. (1980) Unpublished report RVA
4045/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Hart, R.J. and Moore, B. The use of CGA 20168 for the protection of
rice stored in bags. (1975) Unpublished technical report No. 75/10/533
from Ciba-Geigy Australia Limited.
Hart, R.J. and Moors, B. The protection of stored grain sorghum from
insect attack with CGA 20168. (1976) Unpublished technical report No.
76/6/564 from Ciba-Geigy Australia Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168. (1974) Unpublished technical report No. 74/9/476 from
Ciba-Geigy Australia Limited.
McDougall, K.W. Mini silo trials with CGA 20168 - Series 2. (1975)
Unpublished technical report No. 75/8/519 from Ciba-Geigy Australia
Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168 - Part 2. Drum trials. (1976a) Unpublished technical report
No. 76/2/554 from Ciba-Geigy Australia Limited.
McDougall, K.W. The distribution and dissipation of CGA 20168 in
white, brown and paddy rice. (1976b) Unpublished technical report No.
76/2/553 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Determination of CGA 20168 residues in
wheat - Comparison of five analytical procedures. Appendix I: R+D
Department Method No. 129. Appendix II: C.S.I.R.O. Method A. Appendix
III: C.S.I.R.O. Method C. (1973) Unpublished technical report No.
73/11/425 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Further studies on factors affecting the
dissipation of CGA-20168 in wheat. (1974) Unpublished technical report
No. 74/3/433 from Ciba-Geigy Australia Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Insecticide Studies - Determination of residue dissipation. (1974a)
Unpublished technical report No. 74/7/461 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. The Australian Wheat Board Collaborative
Insecticide Studies Determination of residue dissipation. Part II.
(1974b) Unpublished technical report No. 74/9/477 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. CGA 20168 Residues in chickens. (1974c)
Unpublished technical report No. 74/7/462 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Studies 1975: The effect of aeration on the dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. Australia Wheat Board Collaborative
Studies 1975: The effect of aeration on the Dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Renfer, A. Biological evaluation of methacrifos as a grain protectant
under field conditions in Switzerland - I. Basle. (1977) Unpublished
report 9.25 VS 135 from Ciba-Geigy, Basle, Switzerland.
Schnabel, D. and Formica, G. Gas chromatographic determination of CGA
20168 in eggs following oral application via feed. (1979) Unpublished
report RVA 4011/79 from Ciba-Geigy Limited, Basle, Switzerland.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
residues. Proceedings of the First International Working Conference on
Stored-Product Entomology, Savannah, Georgia, USA. (1975).
Tournayre, J.C. Dissipation curve of CGA 20168 in wheat grain and
residues in tissues. (1978) Unpublished reports No. 13/78 A, B, C from
Ciba-Geigy Agrochemicals Division France.
Wyniger. R., Renfer, A., Knuesel, F. and Gfeller, W. Methacrifos - A
new insecticide for the control of insect pests in stored products.
Proc. Brit. Insect. Fungic. Conf. 9, Vol. 3: 1033 -1040.
The decrease of the protective effect of methacrifos against insects
is closely related to the decrease of the amount of the unchanged
methacrifos still present. This suggests that the degradation
products are less toxic to insects than the parent compound (Renfer
1977).
TABLE 16. Distribution and characterization of radioactivity in
stored wheat after the application of 10 mg/kg 14C-methacrifos
% of radioactivity recovered after
storage for (days)
Compounds 0 23 140 294 385
Extractable
methacrifos parent 99.7 98.5 51.6 33.3 25.2
demethylated
methacrifos-derivatives - - 34.1 45.4 6O.2
unknowns - - 5.1 6.8 2.9
Non-extractable 0.3 1.5 8.7 13.8 10.9
CO2 - 0.02 0.5 0.7 0.8
The formation of the demethylated degradation products has been
studied in various products stored under practical conditions. The
results are summarized in table 17.
TABLE 17. Formation of the demethylated metabolites during storage (treatment in all
cases 10 mg/kg)
Storage after Residues mg/kg Reference
treatment methacrifos demethylated
days metabolites
Wheat grain 215 2.7/2.9 1.6/1.8 Formica, 1978d
365 1.7/1.8 1.7 Formica, 1978c
430 1.0 2.4 Formica, 1978b
Barley grain 76 4.2 2.0 Giannone and Formica, 1980a
300 3.0 4.2 " " "
370 1.5 3.8 " " "
430 1.3 2.5 Formica, 1978b
Maize grain 76 6.4 1.6 Giannone and Formica, 1980a
300 4.1 2.0 " " "
370 0.7 5.0 " " , 1979
Maize cob1 231 3.5/3.7 11.4/11.6 Giannone and Formica, 1979
Sorghum grain 15 6.1 1.3 Giannone and Formica, 1980b
45 4.2 0.7 " " "
90 2.1 1.4 " " "
Peanut kernels 15 7.6 0.2 Giannone and Formica, 1980c
45 5.6 0.5 " " "
90 5.2 0.6 " " "
Cocoa beans 15 0.44/0.41 O.27/0.29 Giannone and Formica, 1979a
1 Cobs stored in open wired cribs were treated by spraying the surface of cribs with 10 mg
ai/kg related to the total quantity of maize cobs. As the samples were taken from the periphery
of the crib a rate of their treatment higher than 10 mg/kg must be expected.
In processing
Residues of methacrifos have been followed through milling and baking
procedures in several experiments. As the insecticide is primarily
located in the outer layers of the grain, most of the residues are
found in the bran and the low grade flour fractions after milling.
The residue in flour milled 1 month after the treatment of the grain
was about 1/10 of that in whole grain. After prolonged storage the
residues in flour tend to increase, but they do not exceed 1 mg/kg
even after storage for 21 months. In the baking process methacrifos
and its metabolites are degraded to a large extent resulting in low or
undetectable residues (maximum about 0.1 mg/kg or lower). (Table 18).
TABLE 18. Residues of methacrifos in milled wheat fractions and bread
Residues (mg/kg) in
Treatment Storage
mg/kg time whole wheat white flour low grade flour bran white bread Reference
flour
10 1 month 4.0/3.8 0.4/0.4 1.7/1.6 7.6/6.4 <0.03 Formica 1978b
10 7 months 3.1/2.9 0.6/0.6 8.1/8.4 10.0/9.2 0 01/<O.01 " 1978d
10 21 months 1.4/1.6 1.0/0.8 10.3/9.1 7.4/7.5 0.02/0.08 " 1978f
15 1 month 7 8/7.8 0.6/0.6 2.5/2.7 10.4/11.2 <0.03 " 1978b
20 1 month 8.0/7.4 0.9/0.85 3.1/3.2 15.6/18.0 0.03/0.04 " 1978h
10 265 days 0.751 ca. 0.30 2.6 <0.04 Tournayre 1978
5 265 days 0.261 ca. 0.10 0.8 <0.04 " "
10 265 days 2.01 ca. 0.50 6.1 <0.04 " "
1 after 252 days
In experiments with the radio-labelled insecticide it was shown that
the same degradation products are formed during baking as are found in
stored grains (Blattmann 1978).
Further data on the effect of processing rice, oats and barley,
including malted barley are presented in table 19. In these
experiments the initial concentrations are in most cases higher than
recommended. As with wheat, the milling of paddy rice treated with
methacrifos resulted in a considerable loss of insecticide. More than
90% of the initial residue was removed with the hulls (McDougall
1976), resulting in a decrease of the residue in going from paddy to
brown rice by a factor of 5 to 8. Most of the insecticide present in
the brown rice was again removed with the bran fractions.
The amount of methacrifos residue is further decreased on cooking.
Short cooking of about 15 minutes decreased the residue by a factor of
about 2.6 whereas the more usual cooking of 25 minutes resulted in a
reduction by a factor of about 8.
Processing treated barley grain to malt, which was then used to brew
beer, gave very low level in the beer.
The processing of treated cocoa beans to cocoa mass decreased the
initial methacrifos on the beans at least by a factor of 10. Since
further diluting processes take place the overall decrease of
methacrifos from the raw product to the consumed chocolate is even
higher.
METHODS OF RESIDUE ANALYSIS
Residue of the parent compound in:
Cereal grains and grain derivatives. Methanol or acetone extracts can
be used to determine the active ingredient by GLC without cleanup.
Recoveries range from 80-115%; the limit of determination is about
0.05 mg/kg. (Desmarcherlier et al 1977; Moore and McDougall, 1973
and Formica, 1973g).
Chicken tissues. Residues in chicken tissues are determined by GLC
with a phosphorus-specific detector after extraction with acetonitrile
and clean-up by partition (Formica 1974b).
Milk. Milk is coagulated with acetone. After a partition step,
methacrifos is determined by GLC with a phosphorus-specific detector.
'Total residue':
A method has been developed to determine unchanged methacrifos and the
dealkylated metabolites in a two-step procedure in cereal grains, eggs
and cocoa mass. Extraction with aqueous methanol is followed by
partition of the parent compound into hexane. The dealkylated
metabolites are then extracted from the aqueous phase with methyl
acetate and methylated with diasomethane. The residues are determined
by GLC with a phosphorus-specific detector. Limits of determination
are 0.025 mg/kg for the parent compound and 0.04-0.1 mg/kg for the
dealkylated product.
Both the method for residues of the parent compound in cereal grains
and the method for the total residue are suitable or can be adapted
for regulatory purposes.
TABLE 19. Effect of processing on methacrifos residues
Raw product Treatment Storage Residue before Processing or Residue after
level processing processed product processing
mg/kg mg/kg mg/kg
Paddy rice 18 6 months 3.1 brown rice raw 0.65
cooked 15 min. 0.23
white rice raw 0.12
cooked 15 min. <0.02
Paddy rice 10 3 months 3.0 brown rice 0.36
hulls 18.4
white rice <0.2
bran 2.7
Hulled rice 18 3 months 7.1 cooked 5 min. 4.4
cooked 15 min. 2.7
18 6 months 4.8 cooked 25 min. 0.75
Polished rice 18 3 months 7.5 cooked 15 min. 2.6
Oats 18 3 months 4.7 cooked 15 min. 0.9
Barley 18 3 months 4.8 malted barley 0.1
Malt1 10 - 9.8/10.1 brewer's grains 1.7/2.0
wort before boiling 0.27/0.31
yeast <0.1
beer <0.01
Cocoa beans 10 7 days 5.0/5.2 cacao mass 0.46/0.44
surface of
bags with 97 days 0.32 cacao mass 0.02
0.4 g a.i./m2
1 Malt has been directly treated to follow the dissipation during further processing. Under normal
practical conditions only barley grain is to be treated.
EVALUATION
COMMENTS AND APPRAISAL
Methacrifos is an organophosphorous insecticide which is almost
exclusively used as a grain protectant and as a control agent against
insect pests in other stored products. It may be used as an acaricide
in the same stored commodities. It gives good and long-lasting
protection of grain and other stored products against a broad spectrum
of pests including those which show resistance to commonly used stored
products insecticides such as other organophosphorous compounds,
lindane, etc. The major formulations are emulsifiable concentrate,
solutions in othanic solvents and dusts. The normal application rate
is about 10 mg ai/kg grain. Lower rates may be used at lower
temperatures; exceptionally a higher rate up to 20 mg/kg may be
needed.
Methacrifos is rapidly absorbed, metabolised and excreted in mammals.
Two metabolites detected in urine and faeces were identified as
N-acetyl-S(2-methoxycarbonylprop-1-enylcysteine) and P-O-desmethyl
methacrifos. Neither methacrifos nor its metabolites were found to
accumulate in the tissues.
Methacrifos has a moderate acute toxicity following oral
administration to various animal species. Signs of poisoning are
typical of cholinesterase inhibitors. The acute toxicity was found to
be potentiated by several other organophosphorous esters.
Methacrifos did not induce a delayed neurotoxic response in hens. In
short- and long-term studies methacrifos caused signs and symptoms of
cholinesterase inhibition, which were associated with effects on body
weight gain and food consumption. Brain cholinesterase activity was
inhibited at 100 mg/kg. In rats at high dose levels, effects on
several haematological parameters were observed. Cholinesterase
activity in plasma was inhibited at doses above 10 mg/kg and recovery
of cholinesterase activity was relatively slow.
In long and short term studies, cholinesterase depression was observed
at 10 mg/kg and above. A no-effect level of 1 mg/kg was observed.
Because of parental mortality in a 3-generation reproduction study and
foetal death in teratology bioassays, the meeting could not evaluate
fully the potential effects in these areas. Further studies are
required for a full evaluation of this compound.
Mutagenicity studies with bacteria, a mouse carcinogenicity study and
a rat teratology study were all negative.
Acceptable data were available to allow the meeting to establish
no-effect levels in two mammalian species and a temporary ADI was
allocated.
The fate of methacrifos in stored cereals, beans, peanuts and cocoa
has been elucidated in extensive laboratory trials as well an in many
supervised small and large-scale trials under practical conditions in
various countries and under a variety of storage conditions. The
following conclusions can be drawn.
The rate of degradation depends on time and storage conditions. The
residues decrease faster with increasing temperature and moisture
content of the food commodity. Other factors, such as grain variety,
rate of application, application technique and type of formulation,
have little or no influence on the dissipation rate. Aeration of the
stored cereals has only a minor effect on the rate of loss.
The metabolic pathways of methacrifos on grain and other stored
products were elucidated. The compound is demethylated to mono- and
di-desmethylated methacrifos; there is some evidence that these
metabolites are not cholinesterase inhibitors. These are further
metabolised to CO2 at a slow rate.
The residues remain primarily on the outer layers of the grain.
During prolonged storage only limited penetration into the endosperm
takes place. Processing of the raw commodities, such as milling of
cereals, dehusking and polishing rice, removing shells and seed-coats
from peanuts and shells from cocoa, reduce the residue considerably.
During baking and brewing methacrifos and its main metabolites are
further degraded, resulting in residues at or about the limit of
determination in bread and beer. Degradation also occurs during
cooking to an extent that depends upon the time of cooking.
When grains treated at normal rates are fed to livestock animals or
poultry, no residues are found in meat, milk or eggs.
Methods of residue analysis are available for methacrifos residues
(parent compound) in stored products by GLC with a
phosphorous-specific thermionic detector after extraction with
methanol or acetone. The limit of determination is about 0.05 mg/kg.
Methods are also available for methacrifos in meat of poultry, eggs
and milk after extraction with acetonitrile or acetone followed by
various partition steps and GLC with a phosphorous detector; the limit
of determination is 0.01 mg/kg. The methods are suitable or can be
adapted for regulatory purposes.
Residues of the unchanged parent compound and the dealkylated
metabolites can be determined, in a two step procedure, in cereal
grains, eggs and cocoa-mass. The limits of determination are about
0.025 mg/kg for the parent compound and 0.04 - 0.1 for the dealkylated
metabolites. The method can be adapted for regulatory purposes.
When establishing maximum residue limits for methacrifos on grain or
other stored food commodities, the following factors have to be
considered.
1. The residue level depends largely on the application rate, which
has to be chosen according to the nature of the insect or mite
infestation and the desired period of protection. Variation in
residue levels are to be expected among different countries even when
the same dosages are applied and the same storage period is used,
since the rate of dissipation may differ according to the local
climate and storage conditions.
2. The distribution of the insecticides may be heterogeneous,
depending on local application techniques.
3. Stored products may move in international trade at different times
after treatment according to commercial need.
Based on these considerations the maximum residue limits have to be
set at levels that will cover all situations encountered after the
normal applications, even if in some cases the actual residues will be
much lower.
The meeting examined residue data from supervised trials reflecting
established and/or anticipated good storage practice on a number of
stored food or feed commodities. From these data the meeting was able
to estimate the maximum residue levels that were likely to occur when
methacrifos is used in practice.
Level causing no toxicological effect
Rat: 1 mg/kg in the diet equivalent to 0.05 mg/kg bw/day.
Dog: 1 mg/kg in the diet equivalent to 0.025 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.0003 mg/kg bw.
RECOMMENDATION OF RESIDUES LIMITS
The meeting concluded that the maximum residue levels listed below are
suitable for establishing maximum residue limits.
These levels refer to the parent compound only.
Commodity Estimated maximum
residue levels
mg/kg
cereal grains 10
wheat flour (whole meal) 10
wheat flour (white) 2
wheat bran (unprocessed) 10
beans and peas (dry) 5
cocoa beans 10
peanuts (including shell) 10
peanuts 1
poultry meat 0.011
eggs 0.011
milk 0.011
1 The levels refer to the maximum residue levels which might occur
when the animals concerned are fed with commodities treated during
storage with recommended amounts of methacrifos.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Teratogenicity studies in rat and rabbits.
2. An appropriate reproduction study.
REFERENCES
Arni, P. and Müller, B. Samonella/mammalian-microsome mutagenicity
test with CGA 20168. (1979) Unpublished report, May 23, submitted by
Ciba-Geigy to WHO.
Basler, W. et al. Two-year chronic oral toxicity study with
CGA-20168 technical in albino rats. (1980) Unpublished report prepared
by Ciba-Geigy from the original Industrial Bio-test Laboratories
records, May 15, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the rat. (1974a)
Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the mouse.
(1974b) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute dermal LD50 of technical CGA 20168 in the rat.
(1974c) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe R. and Sachsse, K. Acute oral LD50 in the dog of technical CGA
20168. (1975) Unpublished report, November 12, submitted by Ciba-Geigy
to WHO.
Bathe, R. and Sachsse, K. Acute oral LD50 in the mouse of technical
CGA 20168. (1978) Unpublished report, August 15, submitted by
Ciba-Geigy to WHO.
Bathe R. et al. 26 weeks feeding study in dogs with technical CGA
20168. (1977) Unpublished report, March 4, Submitted by Ciba-Geigy to
WHO.
Charles, J.M. et al. Three-generation reproduction study with CGA
20168 technical in albino rats. (1980a) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-Test Laboratories Records,
April 10, submitted by Ciba-Geigy to WHO.
Charles, J.M. et al. A chronic oral carcinogenic toxicity study with
CGA 20168 in Swiss white mice. (1980b) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-test Laboratories records,
May 15, submitted by Ciba-Geigy to WHO.
Chesterman, H. et al. CGA 20168, 28-day dietary feeding study in
beagle dogs. (1975) Unpublished report from Huntingdon Research
Centre. November 28. Submitted by Ciba-Geigy to WHO.
Drake J.C. 1 Month dietary toxicity study in rats with compound CGA
20168. (1975) Unpublished report from Geigy Pharmaceuticals, February
17, Submitted by Ciba-Geigy to WHO.
Fritz H. et al. Reproduction study on CGA 20168 Technical. Rat.
(1978) Unpublished report, June 14, submitted by Ciba-Geigy to WHO.
Gfeller, W. CGA 20168 Oral toxicity in pigs. Daily administration for
28 days. (1974) Unpublished report from Ciba-Geigy Agricultural
Research Centre, January 28, submitted by Ciba-Geigy to WHO.
Hamböck, H. Metabolism of CGA 20168 in the rat. (1978) Unpublished
project report 27/78 from the Agrochemicals Division of Ciba-Geigy,
May 17, submitted by Ciba-Geigy to WHO.
Hool, G. and Müller, D. Dominant lethal study on CGA 20168. (1980)
Unpublished report, February 6, submitted by Ciba-Geigy to WHO.
Ifflaender, V. and Mücke, W. Distribution, degradation and excretion
of CGA 20168 in the rat. (1975) Unpublished report from the
Agrochemicals Division of Ciba-Geigy, April 9, submitted by Ciba-Geigy
to WHO.
Sachsse, K. and Bathe, R. Potentiation study. CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion. (1978)
Unpublished report, June 23, submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Acute oral toxicity and neurotoxicity study of
technical CGA 20168 in the domestic fowl (Gallus domesticus). (1979)
Unpublished report, November 6, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. Acute toxicity to rainbow trout, crucian
carp, catfish, bluegill and guppy of technical CGA 20168. (1974a)
Unpublished report, August 7, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. "8-Day-feeding toxicity" of technical CGA
20168 in the Japanese quail. (1974b) Unpublished report, July 13,
submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Technical CGA 20168. 21-Day percutaneous toxicity
study in rabbits. (1978) Unpublished report, April 24, submitted by
Ciba-Geigy to WHO.
Strittmatter, J. and Gfeller, W. 63 Days dietary toxicity study in
chicks with compound CGA 20168. (1975) Unpublished report from
Ciba-Geigy Agricultural Research Centre, March, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Skin sensitizing (contact allergenic)
effect in guinea pigs of CGA 20168. (1975) Unpublished report, July 1,
submitted by Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute inhalation toxicity in the rat of
technical CGA 20168. (1976) Unpublished report, July 7, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute dermal LD50 in the rabbit of
technical CGA 20168. (1977) Unpublished report, February 2, submitted
by Ciba-Geigy to WHO.
Ullmann, L. et al. 21-Day inhalation study on the rat with technical
CGA 20168. (1977) Unpublished report, December 16, submitted by
Ciba-Geigy to WHO.
REFERENCES ON RESIDUES IN FOOD AND THEIR EVALUATION
Blattmann, P. Degradation of CGA 20168 in stored wheat grains,
including their processing. (1978) Unpublished report PR 42/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Desmarchelier, J.M., Bengston, M., Connel, M., Minett, W., Moore, B.,
Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A collaborative
study of residues on wheat of chlorpyrifos-methyl, fenitrothion,
malathion, methacrifos and pirimiphos-methyl. 1. Method development.
Pestic. Sci. 8: 473-483.
Formica, G. CGA 20168. Gas chromatographic residue determination in
wheat grain, wheat meal, bran, groats and bread. (1973) Unpublished
report HEM 20/73 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. CGA 20168. Gas chromatographic residue determination in
chicken tissues. (1974) Unpublished report REM 9/74 from Ciba-Geigy
Limited, Basle, Switzerland.
Formica, G. CGA 20168. Residues in chicken tissues. (1974a)
Unpublished report RVA 205/74 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Degradation of CGA 20168 in wheat and maize. (1975)
Unpublished report RVA 314/75 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. CGA 20168. Residues in cow's milk after oral medication.
(1977) Unpublished report RVA 612/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Methacrifos (CGA 20168). Determination of CGA 20168 in
feedgrain treated with different formulations and stored in drums.
(1977a) Unpublished report RVA 615/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos in French barley after direct
spray treatment with the formulation EC 950. (1978a) Unpublished
report RVA 620/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat and barley after
direct spray treatment with the formulation EC 950. Carinena, Spain.
(1978b) Unpublished report RVA 626/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Residues of methacrifos in untreated cacao beans stored in
a warehouse and possibly contaminated through cacao beans treated with
DAMFIN(R) 950 EC, stored in the same warehouse. (1978c) Unpublished
report RVA 608/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of residues of methacrifos in stored grain
and its products seven months after direct spray treatment with the
formulation SO 050. (1978d) Unpublished report RVA 610/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat after direct spray
treatment with the formulation SO 050. (1978e) Unpublished report RVA
622/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain, and their products twenty-one months after direct spray
treatment with the formulation EC 950. (1978f) Unpublished report RVA
611/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in barley and oats after
direct spray treatment with the formulation EC 950. (1978g)
Unpublished report RVA 623/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain and their products after direct spray treatment with the
formulation EC 1000. (1978h) Unpublished report RVA 618/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Residue of methacrifos in maize cobs
after surface treatment with DAMFIN(R) 950 EC. (1979) Unpublished
report RVA 4010/79 from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic determination of
total residues in cacao liquor after different processings of cacao
beans treated with DAMFIN(R) SO 050. (1979a) Unpublished report RVA
4009/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic-determination of
methacrifos and its dealkylated metabolites in sorghum after treatment
with DAMFIN(R) SO 050 or DAMFIN(R) P 2. (1980) Unpublished report RVA
4045/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Hart, R.J. and Moore, B. The use of CGA 20168 for the protection of
rice stored in bags. (1975) Unpublished technical report No. 75/10/533
from Ciba-Geigy Australia Limited.
Hart, R.J. and Moors, B. The protection of stored grain sorghum from
insect attack with CGA 20168. (1976) Unpublished technical report No.
76/6/564 from Ciba-Geigy Australia Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168. (1974) Unpublished technical report No. 74/9/476 from
Ciba-Geigy Australia Limited.
McDougall, K.W. Mini silo trials with CGA 20168 - Series 2. (1975)
Unpublished technical report No. 75/8/519 from Ciba-Geigy Australia
Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168 - Part 2. Drum trials. (1976a) Unpublished technical report
No. 76/2/554 from Ciba-Geigy Australia Limited.
McDougall, K.W. The distribution and dissipation of CGA 20168 in
white, brown and paddy rice. (1976b) Unpublished technical report No.
76/2/553 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Determination of CGA 20168 residues in
wheat - Comparison of five analytical procedures. Appendix I: R+D
Department Method No. 129. Appendix II: C.S.I.R.O. Method A. Appendix
III: C.S.I.R.O. Method C. (1973) Unpublished technical report No.
73/11/425 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Further studies on factors affecting the
dissipation of CGA-20168 in wheat. (1974) Unpublished technical report
No. 74/3/433 from Ciba-Geigy Australia Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Insecticide Studies - Determination of residue dissipation. (1974a)
Unpublished technical report No. 74/7/461 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. The Australian Wheat Board Collaborative
Insecticide Studies Determination of residue dissipation. Part II.
(1974b) Unpublished technical report No. 74/9/477 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. CGA 20168 Residues in chickens. (1974c)
Unpublished technical report No. 74/7/462 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Studies 1975: The effect of aeration on the dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. Australia Wheat Board Collaborative
Studies 1975: The effect of aeration on the Dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Renfer, A. Biological evaluation of methacrifos as a grain protectant
under field conditions in Switzerland - I. Basle. (1977) Unpublished
report 9.25 VS 135 from Ciba-Geigy, Basle, Switzerland.
Schnabel, D. and Formica, G. Gas chromatographic determination of CGA
20168 in eggs following oral application via feed. (1979) Unpublished
report RVA 4011/79 from Ciba-Geigy Limited, Basle, Switzerland.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
residues. Proceedings of the First International Working Conference on
Stored-Product Entomology, Savannah, Georgia, USA. (1975).
Tournayre, J.C. Dissipation curve of CGA 20168 in wheat grain and
residues in tissues. (1978) Unpublished reports No. 13/78 A, B, C from
Ciba-Geigy Agrochemicals Division France.
Wyniger. R., Renfer, A., Knuesel, F. and Gfeller, W. Methacrifos - A
new insecticide for the control of insect pests in stored products.
Proc. Brit. Insect. Fungic. Conf. 9, Vol. 3: 1033 -1040.
 Following a single oral dose of about 5 mg/kg 14C-methacrifos, the
excretion in urine, faeces and expired air was in males 30.2, 10.2
and 54.7% of the administered dose, respectively, and in females
43.8, 8.7, 52.7%, respectively. Most of the radioactive label was
excreted in the first 24 hours. Total recovery was 97.4 and 106.6%
for males and females, respectively. Excretion half life time was
8 hours.
The animals were killed five days after dosing, the tissues
investigated showed the following residues:
Tissue residue
(in mg/kg methacrifos eq.)
males females
(n=4) (n=4)
Liver 0.306 0.287
fat 0.110 0.040
kidneys 0.087 0.089
muscles 0.035 0.020
blood 0.041 0.040
brain 0.027 0.023
spleen 0.052 0.048
testes 0.033 -
ovary - 0.043
(Ifflaender and Müke, 1975)
In a similar experiment, in which about 25 mg/kg 14C-methacrifos
was orally administered, the mean excretion pattern for males and
females was 41.5, 10.5 and 38.0% of the given dose in urine, faeces
and expired air respectively. The main urinary metabolite,
representing 12% of the dose, was isolated and identified as
N-acetyl-S-(2-methoxycarbonylprop-1-enyl) cysteine. It was
demonstrated that this cysteine conjugate when injected i.v. to
rats is excreted via the kidney mainly unchanged.
Another urinary metabolite, representing 4% of the dose was
probably the monodemethylated phosphoric acid derivative of
methacrifos. Both metabolites were also found in the faeces.
Most, if not all, of the radioactivity expired was 14CO2. The
following metabolic pathways of methacrifos in the rat are
proposed: see Figure 1.
Following a single oral dose of about 5 mg/kg 14C-methacrifos, the
excretion in urine, faeces and expired air was in males 30.2, 10.2
and 54.7% of the administered dose, respectively, and in females
43.8, 8.7, 52.7%, respectively. Most of the radioactive label was
excreted in the first 24 hours. Total recovery was 97.4 and 106.6%
for males and females, respectively. Excretion half life time was
8 hours.
The animals were killed five days after dosing, the tissues
investigated showed the following residues:
Tissue residue
(in mg/kg methacrifos eq.)
males females
(n=4) (n=4)
Liver 0.306 0.287
fat 0.110 0.040
kidneys 0.087 0.089
muscles 0.035 0.020
blood 0.041 0.040
brain 0.027 0.023
spleen 0.052 0.048
testes 0.033 -
ovary - 0.043
(Ifflaender and Müke, 1975)
In a similar experiment, in which about 25 mg/kg 14C-methacrifos
was orally administered, the mean excretion pattern for males and
females was 41.5, 10.5 and 38.0% of the given dose in urine, faeces
and expired air respectively. The main urinary metabolite,
representing 12% of the dose, was isolated and identified as
N-acetyl-S-(2-methoxycarbonylprop-1-enyl) cysteine. It was
demonstrated that this cysteine conjugate when injected i.v. to
rats is excreted via the kidney mainly unchanged.
Another urinary metabolite, representing 4% of the dose was
probably the monodemethylated phosphoric acid derivative of
methacrifos. Both metabolites were also found in the faeces.
Most, if not all, of the radioactivity expired was 14CO2. The
following metabolic pathways of methacrifos in the rat are
proposed: see Figure 1.
 TOXICOLOGICAL STUDIES
Special studies on teratogenicity and reproduction
Rat
After successfully being mated with male Sprague-Dawley albino rats
four groups of 2-month old female rats (25 animals/group) of the
same strain were orally incubated with methacrifos at doses of 0,
5, 25 or 50 mg/kg bw. In a preliminary study 6 out of 10 dams
died, when the compound was administered at the dose of 150 mg/kg
bw.
Treatment started at day 6 of pregnancy and was continued through
day 15. During the experiment a reduction in body weight gain was
found in the dams of the 50 mg group, starting half-way through the
dosing period. This reduction was accompanied by a temporary
decrease in food consumption at the 11th and 16th day of dosing
(food consumption was measured at day 6, 11, 16 and 21 of dosing).
Further a dose-related increase in the percentages of embryonal
deaths (early resorptions) was found, which already started at the
5 mg/kg level. No structural abnormalities related to the
methacrifos treatment were observed in the pups. The number of
foetuses with still incomplete ossified sternebrae was slightly
higher in the 50 and 25 mg/kg dose groups. (35.8% in control
versus 35.0, 43.0 and 52.8% for the 5, 25 and 50 mg/kg groups,
respectively). In the treated groups no foetal deaths or dead
foetuses were found (Fritz et al, 1978).
Groups of rats (8 males and 16 females/group) were fed methacrifos
in the diet at dosage levels of 0, 1, 10 or 100 mg/kg (mean
analytical concentrations of <0.1, 0.79, 7.4 and 73 mg/kg), and
subjected to a standard 3-generation, 2-litter per generation
study.
Gross and histopathologic examinations were conducted on the
parenteral animals of the F0, F1, F2 and F3. Gross
pathological evaluations were made on at least 10 male and 10
female weanlings of the 0 and 100 mg/kg group. No
treatment-related changes were noted in the tissues and organs
examined. Throughout the study a dose-related increase in
mortality of the male parent animals was observed in all treated
groups, whereas the mortality was also higher in the females of the
treated groups. The cause of mortalities was ascribed to acute
bronchopneumonia superimposed on lesions of chronic murine
pneumonia. There was no clear effect on the body weight gain or
organ weights of the treated groups.
The total number of pups delivered was dose-related decreased in
almost all litters, where the mean number was not clearly affected.
In addition somewhat reduced survival percentages after 21 days
were noted in comparison to the controls in the 10 and 100 mg/kg
F1a and F1b, 1 mg/kg F3b and 100 mg/kg F3a and F3b litters
(Charles et al, 1980a).
Special studies on mutagenicity
Bacteria
Histidine-autotrophic mutants of Salmonella typhimurium were
treated with methacrifos in concentrations of 0.25, 0.75, 2.25,
6.75 or 20.25 mg/ml with or without microsomal activation, in order
to study the possible induction of point mutations by methacrifos.
The test results were negative (Arni and Müller, 1979).
Mice
Twenty male albino mice were administered methacrifos orally in
single doses of O, 7, and 21 mg/kg and then mated to two untreated
female albino mice over a period of six weeks. At the end of each
week the females were replaced by new ones. The effects on mating
ratio and the number of implantations and embryonic deaths as well
as the effects on the loss of pre-implantation zygotes and the rate
of deaths of post-implantation stages of embryonic development were
studied. No evidence of a dominant lethal effect was observed
(Hool and Müller, 1980).
Special studies on carcinogenicity
Groups of 5-7 week old Swiss, white mice (60/sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (purity 94.6%) for
21-22 months (sacrifice of male and females at 50% mortality).
Body weight, food consumption and mortality were not clearly
affected. Haematological investigations carried out at the end of
the study revealed increases in total leucocyte count for males of
the 1 and 10 mg/kg groups. Urinalysis, performed also on 10
animals/sex/group, were normal at sacrifice but blood chemistry
revealed a significant increase in total protein in females and in
cholesterol in males at 100 mg/kg. In females an obvious
dose-related, but not significant, increase was found in absolute
and relative weight of gonads at 100 and 10 mg/kg. At 1 mg/kg a
tendency to an increase was found. The other organ weights were
within normal limits.
At sacrifice the activity of cholinesterase in plasma, appeared
inhibited at 100 and 10 mg/kg in both males and females; in
erythrocytes it was inhibited in females at 100 mg/kg. Brain
cholinesterase was not dose-related affected.
At histopathology no effects on tissues were apparent.
Carcinogenicity has not been found in this experiment (Charles et
al, 1980b).
Special studies on inhalation
Groups of 7/8 week-old rats (9/sex/group) were exposed to 0, 90,
191 or 467 mg methacrifos/m3 air, 15 times over a period of 21
days.
Thereafter 4 animals/sex of the control and highest dose group were
kept for a recovery period of 21 days. The compound was injected
into an air stream discharged into the exposure chamber through a
spray nozzle under a pressure of 2 atmospheres at a rate of 10
1/min.; the exposure concentrations were determined. The animals
were kept separately in PVC tubes, which were positioned radially
around the exposure chamber. The animals at the highest dose group
showed a significant decrease in food consumption and body weight
gain, but returned to normal during the recovery period. In the
191 mg/m3 group the body weight was also decreased at the end of
the study. Haematology and blood biochemistry revealed a
significant increase in SGPT in the 467 mg/m3 group. No clear
effects on the weight of the organs were observed. Blood urea
concentration was increased in all doses tested, which disappeared
after the recovery period. The cholinesterase activity of plasmal
erythrocytes and brain was significantly depressed in all treated
groups. After the recovery period, brain cholinesterase activity
was still decreased.
One male and one female rat died during the treatment: congestion
often with haemorrhages were observed in various tissues. All
other gross or microscopic findings were not related to the
treatment (Ullmann et al, 1977).
Special studies on dermal toxicity
Groups of KA 46, Himalayan strain rabbits (3/sex/group: 1.3-2 kg)
were dermally exposed to 0, 10, 50 or 250 mg/kg methacrifos, once
a day, 5 times a week for a period of 3 weeks. Thereafter 1
animal/sex/group was kept for a recovery period of 3 weeks. The
compound was diluted in PEG 400 and saline and applied to the
shaven skin of the back, with occlusive dressings. All treated
groups showed reversible erythema at the application site. The
animals of the 50 and 250 mg/kg groups showed clinical symptoms
(tremors, ataxy, especially during the first week). At 250 mg, 5
animals died within 9 days of the study. In the other groups no
effects on food consumption, body weight gain, haematological and
clinical chemistry were observed.
All the treated groups showed after 3 weeks a dose-related
inhibition of the cholinesterase activity of plasma and
erythrocytes of 30-80%, and 10-75% respectively. Brain
cholinesterase values revealed an inhibition of approximately
10-30, 55 and 85% of the control values in the treated groups. At
the end of the 3-week recovery period the blood and brain
cholinesterase activities of the 10 and 50 mg/kg groups had almost
returned to normal values.
Due to the small number of animals the effects on the organ weights
are difficult to evaluate.
Histopathological examinations revealed not pathological changes
which could be attributed to the treatment (Sachsse et al, 1978).
Special studies on skin sensitisation
Groups of guinea pigs of the Pirbright White strain (10/sex/group)
were intradermally injected saline, methacrifos and
dinitrochlorobenzene (DNCB) (positive control) (0.1 ml of the test
substance, 0.1%) every other day for 3 treatments.
During the second and third week of induction 6 sensitizing doses
of 0.1 ml, with Freund's adjuvants, were injected intracutaneously.
Two weeks after the last exposure, the animals were challenged and
examined for sensitisation. Ten days thereafter a second challenge
was given. There were indications that methacrifos induced a
sensitisation reaction, but it was less severe than DNCB (Ullmann
and Sachsse, 1975).
Special studies on neurotoxicity
In hens of the White Leghorn strain, over 12 months of age, which
were orally dosed by gavage with 0, 25, 50, 101 or 202 mg/kg
methacrifos (10, 15, 20 and 30 animals/group resp.) on day 0 and
21, no symptoms of delayed neurotoxicity were described in the
intermediate observation period of 21 days nor in the 42-day
observation period thereafter. At all doses the treatment resulted
within 1 hour to 4 days in ataxy, curved or ventral position,
sedation and salivation. Histopathological lesions in the spinal
cord and the sciatic nerve were not observed.
In the positive control animals, which had been treated with TOCP,
delayed neurotoxicity was found, obviously with slight to severe
lesions in the spinal cord and the peripheral nerves (Sachsse et
al, 1979).
Special studies on potentiation
In a study with RAI f-SPF rats (5 animals/sex/group) in which acute
oral LD50 values were determined no potentiation was found with the
insecticides dichlorvos, phosphamidon, diazinon and CGA 15324;
however a potentiation did occur with the insecticides methidathion
and malathion.
LD50 in mg/kg
techn. compound techn. compound techn. compound + CGA 201681
theoretically experimentally
phosphamidon 12 347 400
dichlorvos 54 395 611
CGA 15324 426 581 568
diazinon 534 635 520
methidathion 43 389.5 201
malthion 1397 1066.5 454
1 acute oral LD50 of CGA 20168: 745 mg/kg (Sachsse and Bathe,
1978)
TABLE 1. Acute toxicity of methacrifos
Mammals
route of LD50 in mg/kg reference
species sex administration or mg/m3
Chicken oral 1011 Sachsse et al, 1979
Rat M + F oral 6782 Bathe, 1974a
Mouse M + F oral 662 Bathe, 1974b
Mouse M + F oral 582 Bathe and Sachsse, 1978
Dog M + F oral 5731 Bathe and Sachsse, 1975
Rat M + F dermal >31002 Bathe, 1974c
Rabbit M + F dermal 27321 Ullmann and Sachsse, 1977
Rat M + F inhalation >2500 Ullmann and Sachsse, 1976
1 observation period of 14 days
2 observation period 7 days
Fish
exp. temp. 96 hour LC50
Species in C mg/kg Reference
Rainbow trout 14 0.4
Crucian carp 14 30
Catfish 21 6 Sachsse and Ullmann, 1974a
Bluegill 14 2
Guppy 21 3
Short-term studies
Quail
Groups of adult Japanese quails (3 males and 7 females/group) were
given, 0, 1000, 6000, or 10,000 mg/kg methacrifos in the feed for a
5-day period. They were held for observation during a further 3 days.
The mortality observed during the entire 8-day period was used to
calculate the LC50 which appeared to be approximately 10,000 mg/kg.
Observation of symptoms, egg production, food consumption and body
weight revealed sedation and ruffled feathers, partly to complete
refusal of food and a decrease in body weight over the whole dosing
period and a stop in egg production (Sachsse and Ullmann, 1974b).
Chicken
Groups of one-day old Hubbard chickens (32 sex/group) were dietary fed
0, 10, 100, 250, 500 or 1000 mg/kg methacrifos for 63 days.
Mortality was found to be 1.6, 0, 1.6, 3.1, 37.6 and 98.2% in the 0,
10, 100, 250, 500 and 1000 mg/kg groups, respectively. Most animals
died in the first period (0-14 days). The dying animals of the 500
and 1000 mg/kg groups showed signs of ataxia, lack of appetite,
somnolence and ruffled plumage. Body weight and food consumption were
significantly decreased at 250 and 500 mg/kg during the whole
experiment, at 100 mg/kg temporarily up to at least 28 days. No
effects on haematology and histopathology were found.
Final cholinesterase activity measurement in the brain on 8 animals/
sex/group revealed a dose-related inhibition at 100, 250 and 500 mg/kg
(Strittmatter and Gfeller, 1975).
Rat
Groups of Sprague-Dawley derived rats (150-170 g; 5 or 10, sex/group)
were given 0, 10, 100 or 1000 mg/kg methacrifos in the feed for 30
days, followed by a recovery period of 28 days for the control and
1000 mg/kg groups but nearly complete recovery was found in food
consumption at about 25 days; loss of hair in males was also noted.
In the males of the 1000 mg/kg group abnormalities in blood
composition were observed: a significant decrease in Hb, PCV, number
of erythrocytes and lymphocytes and prothrombin time and a slight
increase in neutrophils. At 100 mg/kg in the males the number of
lymphocytes and neutrophils appeared affected, also.
A decrease in cholinesterase activity in plasma and erythrocytes was
found at the end of the dosing period in both males and females of the
100 and 1000 mg/kg groups; plasma cholinesterase activity was also
decreased in males of the 10 mg/kg groups. At the end of the
experiment partial recovery was observed. Cholinesterase activity in
plasma and erythrocytes in the 1000 mg/kg group was still about 20%
decreased in both males and females. Brain cholinesterase has not
been determined. Further effects on clinical-chemical parameters and
urinalysis were not found. In the male animals of the 1000 mg/kg
groups the weight of the kidneys was decreased in comparison to the
control value (Drake, 1975).
Dog
Groups of pure bred beagle dogs (6.5-11 kg; 2 or 4 sex/group) were
dietary fed 0, 10 or 100 mg/kg for 28 days. Another group of dogs
received 1000 mg/kg for 11 days but treatment had to be stopped
because of a deterioration in the bodily condition of the dogs. After
7 days the animals then received 500 mg/kg for 4 weeks. Thereafter 4
animals were sacrificed and 4 were allowed a 4-week recovery period.
Due to dose changes evaluation of the effects of 1000 or 500 mg/kg
methacrifos is difficult. However body weight and food intake
drastically decreased and two animals showed higher SGPT values.
There was a marked depression of plasma and red cell cholinesterase
values, with a partial recovery. One animal was sacrificed in poor
bodily condition. Macroscopically a whitening of the splenic capsule
and a small amount of free blood beneath the membranes of the spinal
cord was observed.
In the other treated groups food consumption, body weights and organ
weights were measured and haematology, blood biochemistry, urinalysis,
ophthalmoscopy and histopathology performed but no toxic effects were
found except for a reduced water intake at 100 mg/kg and 10 mg/kg in
the males and a final inhibition of cholinesterase activity both in
the erythrocytes and plasma at 10 and 100 mg/kg in males and females.
Brain cholinesterase activity was not affected (Chesterman et al,
1975).
Groups of adult pure bred beagle dogs (8.5-15 kg; 8 sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (94.6%) for 26 weeks.
Thereafter 2 animals/sex/group were kept for a recovery period of 4
weeks.
No effects were found on food consumption and body weight gain but the
animals in the dose groups vomited more often and showed more
pronounced diarrhoea, when compared with the controls. Haematology
and urinalysis after 4, 13 and 26 weeks, were normal but blood
biochemistry showed a decrease in blood glucose at 26 weeks in all
dose groups, which fully recovered in the 1 mg/kg groups in the 4-week
recovery period but which only partially recovered in the 100 and 10
mg/kg groups in this period.
The main effects were found on cholinesterase activity. Plasma
cholinesterase was dose-related inhibited in the 100 and 10 mg/kg
groups at 4, 13 and 26 weeks and in the 1 mg/kg groups after 4 and 13
weeks only, with about 30%. Complete recovery was found after 4
weeks.
Erythrocyte cholinesterase was also dose-related inhibited in the 100
and 10 mg/kg groups at 4, 13 and 26 weeks but recovery was incomplete
after 4 weeks, inhibition was still present in males and females at
100 mg/kg and in females at 10 mg/kg. Brain cholinesterase was not
affected. Gross and microscopic examinations of organs and tissues
showed no effects attributed to methacrifos (Bathe et al,1977).
Pig
Groups of large white pigs (about 44 kg; 2/sex/group) were dietary fed
0, 10, 100 or 1000 mg/kg for about 4 weeks. No effects were found on
food consumption, body weights, blood chemistry, and haematology,
except for an influence on cholinesterase activity. At day 15 and 29
an inhibition of cholinesterase activity in plasma and erythrocytes
was found at 1000 mg/kg, at day 29 also in brain at 1000 mg/kg and in
erythrocytes also at 100 mg/kg. No histopathological changes were
found (Gfeller, 1974).
Long-term studies
Rat
Groups of about 40-day old CR strain rats (65/sex/group) were dietary
fed 0, 1, 10 or 100 mg/kg methacrifos for 2 years. Haematology,
coagulation, clinical chemistry and urinalysis studies were carried
out in 10 rats/sex from the control and 100 mg/kg group.
No clear effects were found on mortality, food consumption, body and
organ weights, haematology, clinical chemistry and urinalysis.
Cholinesterase activity in plasma and erythrocytes, which was measured
at 3, 6, 12, 18 and 24 months, was influenced. Cholinesterase
activity in plasma was inhibited in females of the 100 mg/kg group at
3, 6, 12 and 24 months, in males of the same group at 3, 6 and 12
months and in females of the 10 mg/kg group at 3 and 24 months.
Cholinesterase activity in erythrocytes was inhibited in females of
the 100 mg/kg groups at 6 months and in males of the same group at 12
months. Finally, cholinesterase activity in brain, which was measured
at 24 months, was not affected. The results of the microscopic
examination indicated that methacrifos did not produce
histopathological changes. A no-effect level in this study is 1 mg/kg
in the diet (Basler et al, 1980).
RESIDUES IN FOOD
USE PATTERN
Methacrifos is an organophosphorus insecticide and acaricide, with a
rapid and long-lasting action on a number of major pests of stored
products. It is mainly used as a grain protectant but also against
insect pests in other stored products. It is effective against
insects showing resistance to commonly used stored product
insecticides, including other organophosphorus compounds.
The main fields of application are cereal grains (maize, rice,
sorghum), cocoa, coffee, peanuts, pulses and tobacco.
The material shows a wide spectrum of activity notably against
Sitophilus spp., Rhizopertha dominica, Tribolium spp., Oryzaephilus
surinamensis, Lasioderma serricorna, Dermestes frisehii,
Anugasta Kuhniella, Plodio interpunetella, Acarus siro,
Glycophagus domesticus (Wyniger et al, 1977).
The major formulations are emulsifiable concentrate, solution in
organic solvents and dusts.
The dosage rate under usual conditions of storage and infestation is
10 mg ai/kg, which gives protection to stored products for several
months. At lower storage temperatures, or if infestation is moderate,
satisfactory protection can be obtained with 5 mg/kg. In some
situations (e.g. in Australia against a resistant strain of
Rhizopertha dominica) a rate of 20 mg ai/kg may be needed to
achieve satisfactory control.
Methacrifos is also used for space treatment in empty silos at 50-75
mg ai/m3, for surface treatment of stored products and as a wall
treatment in storage premises.
Methacrifos application does not affect germination, baking quality,
taste or odour.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
various countries, including Australia, France, Spain, Switzerland,
Mali and South Africa, on a variety of cereal grains, dry pulses,
tropical seeds and products of animal origin. The data were obtained
from both large and small-scale trials performed under practical
conditions.
Wheat, barley, oats
ln laboratory experiments, simulating grain storage, in Australia, it
was shown that the residue of methacrifos decreased more rapidly with
increased temperature and moisture content (Table 2); increased rate
and/or repeated application did not influence the dissipation rate
(Table 3); some evidence was obtained that enzymic activity in the
grain was at least partially responsible for the more rapid initial
breakdown (Table 3). The rate of residue decrease after treatment of
wheat, barley and oats is similar. On all variety loss during storage
at 36°C is more rapid than at 31°C as shown in Table 3. (McDougall,
1974; Moore, 1973; Moore et al, 1974).
Several small-scale trials in which wheat, barley or oats were stored
in mini-silos (5´ ton of each grain) or in drums (120 l) showed
similar results. As in the laboratory experiment, the application
rate of methacrifos does not affect the rate of dissipation in any of
the grains investigated (McDougall, 1975; McDougall, 1976) (Table 4
and 5). From these trials half-life periods could be calculated using
the formula t´ = (t2 - t1) log 2 / [2 - (logC2/C1) × 100], where
C1, C2 are concentrations of the insecticide at times t1 and t2
respectively. Only data for 84 days storage were used since the
temperature dropped after that period.
TABLE 2. Laboratory trials on the dissipation of methacrifos in stored grains
Storage Conditions Application Residues of methacrifos, in mg/kg, after storage
rate
Commodity Moisture % Temp. °C mg/kg 0/1 7 14 28 42 56 70 84
Wheat 9.3 28 24 17.3 16.1 14.7 12.3 12.1
10.3 " " 14.8 14.0 12.0 8.8 7.6
11.3 " " 13.4 12.4 9.5 6.7 4.9
12.3 " " 13.4 10.7 7.7 5.0 3.0
9.3 35 24 14.5 13.8 12.5 8.5 6.3
10.3 " " 13.8 11.5 8.1 4.8 3.2
11.3 " " 13.3 10.2 6.1 - -
12.3 " " 12.9 8.2 4.4 - -
9.6 42 24 13.1 8.5 4.8 - -
10.4 " " 12.6 6.7 3.1 - -
12.3 " " 4.9 2.0 - - -
12.4 " " 11.1 4.1 1.2 - -
Wheat 11.9 31 20 16.4 12.8 9.6 7.6 5.4 4.1
11.8 36 " 16.2 11.4 8.9 4.5 3.2 -
Barley 11.7 31 " 16.9 14.2 12.0 8.0 5.8 3.4
11.8 36 " 18.9 11.6 8.7 4.4 2.5 -
Oats 11.7 31 " 19.2 13.2 11.6 6.9 5.1 -
11.9 36 " 18.8 11.6 8.4 3.7 2.5 -
TABLE 3. Laboratory trials on the relative disspation of methacrifos in wheat
Storage Condition Application Residues of methacrifos as %
rate of initial residue (=100)
commodity moisture % temp. °C mg/kg 0 2 14 28 42 Reference
Wheat 11.2 35 24 100 82 48 30 22 Moore & McDougall, 1974
11.2 " 50 100 73 45 28 21
11.1 " 1501 100 74 47 34 25
1O.91 "1 24 100 98 60 36 22
11.2 " 24 100 93 44 29 21
12.5 " 4 + 15 100 79 49 33 -
1 applied in "dead" grain
TABLE 4. Mini-silo storage trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2/3 6-8 13-15 28 56 84 105 126 147 168 Reference
Wheat 10.2 22-26.5 10 4.7 5.8 5.0 5.0 4.2 4.1 - 3.0 - 2.9 McDougall, 1975
Barley 12.8 " " 9.4 8.6 8.6 8.0 5.7 3.9 2.9 - 2.0 - 1.8
Maize 12.9 " " 7.2 7.0 7.0 6.8 7.0 3.9 4.0 - 2.4 - 2.9
Sunflower 7.2 " " 7.7 7.5 7.2 6.7 7.4 5.0 4.9 - 3.6 - 2.7
TABLE 5. Drum trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos in mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2 5 7 12 28 56 84 126 175 210 Reference
Wheat 8.3-10.2 32 5 5.3 - - 5.5 5.4 4.0 3.1 2.5 1.6 1.2 1.1 McDougall, 1976
10 8.8 - - 9.9 8.2 7.1 5.3 4.3 3.1 2.4 1.8
Oats 7.4-8.0 10 10 4.1 - 4.4 - 3.6 2.8 2.4 1.7 1.5 - 0.4
(in sealed
bags) 7-8-8.4 32 10 4.1 - 4.8 - 4.4 3.4 2.4 1.7 1.3 - 0.4
Maize 8.6-10.3 32 5 2.6 2.9 - - 2.4 2.0 1.8 1.4 1.0 0.6 0.5
10 4.2 6.0 - - 6.0 4.5 4.0 2.5 1.9 1.5 1.3
Peanuts 4.4-7.9 32 10 7.3 - - 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
20 16.0 - - 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
Soybeans 5.5-7.4 32 5 2.3 2.6 - - 2.5 2.1 1.6 1.4 1.1 0.9 0.9
10 7.1 7.6 - - 7.1 6.3 5.7 4.8 3.8 3.6 3.2
TABLE 7. Half-life of methacrifos on various grains stored in mini
silos at 26°C
Commodity Storage conditions Half-life (days)
moisture temp.°C
Wheat 10.2 26 105
Barley 12.9 26 44
Maize 12.8 26 100
Sunflower seed 7.2 26 90
From the trial in which the grains were stored in drums at 32°C the
following half-life periods were calculated; for the moisture content
(see table 5).
Commodity Half-life(days)
Wheat 70
Oats 60
Maize 85
Peanuts 55
Soybeans 100
Large-scale trials
Wheat and barley
Large-scale trials in silos were carried out under a range of storage
conditions in various countries e.g. Australia, Argentina, Morocco,
Spain, Switzerland and France. As in the small-scale trials it was
found that the application rate did not affect the rate of dissipation
of the residues in any of the grain investigated (McDougall 1976)
(table 8).
The experiments under practical conditions confirmed that the
dissipation of methacrifos is mainly dependant on time and
temperature. The residue decrease under storage conditions prevailing
normally in Europe (temperature 15-25°C) is much slower than in
regions where storage temperatures are generally higher e.g. Australia
(Formica, 1975).
In the trials carried out in Australia in which the grain was aerated
during the storage period, there were indications of a decreased rate
of dissipation, compared with silos in which the grain was stored
under the same conditions of temperature and moisture at the start of
the experiment, but not aerated. Aeration was started 24 hours after
treatment and functioned only during the cooler hours of the day for a
total of 84 hours a week for the first 4 weeks and about 30 hours
weekly thereafter (Table 9) (Moor and McDougall 1975). In other
experiments aeration had little or no effect on residue dissipation
(Formica, 1978g).
TABLE 8. Dissipation of methacrifos in small grain
Large-scale trials in silo. (Moore and McDougall, 1974a, 1974b and 1975).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0,15 1 6 11/12 16 21 22/23 26 34 41
Wheat Australia 100 11.3 ± 30 20 0.6 21.8 11.8 7.5 5.6 4.1 4.2
(Queensl.) 10.3 20 1.5 14.2 9.6 5.3 3.9 2.9 2.6
1974 10.8 20 6.0 15.5 8.6 5.2 3.0 1.8 1.6
Australia 800 11.5 26-28 20 0.6 9.3 6.4 4.8 4.0 4.1 3.7
(N.S.W.) 11.3 20 1.5 8.6 6.3 4.9 3.8 3.0 2.5
1974 10.9 20 6.0 10.7 8.1 5.7 4.9 4.6 3.3
(aerated) Australia 550 12.4 (note 1) 15 0.1 9.7 5.0 3.6 2.3 1.4 1.6
(Queensl.) 11.4 15 2 9.0 5.6 4.2 2.6 2.1 2.6
11.0 15 4 9.6 5.7 4.8 3.6 2.8 2.2
11.0 15 6 11.9 6.0 5.3 3.3
not 10.9 (note 2) 15 0.1 11.4 6.0 3.7 2.3 1.6 1.5
aerated 10.2 2 9.8 6.1 3.6 1.6 0.75 0.8
10.4 4 10.0 3.8 1.7 1.6 0.6 0.6
9.9 6 10.4 4.8 2.6 1.8
1 Mean temperature in silo first 18 weeks 25-31°C; from 23-41 week 14-23°C.
2 Mean temperature in silo first 18 weeks 27.5-34°C; from 23-41 week 17-20°C.
TABLE 9. Dissipation of methacrifos in small grains
Large scale trials in silos. (Formica, 1978a, 1978b).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0 45 70 87 171/ 322/ 430 463 504 560
180 339
Barley Switzerland 51 2-25 22.3 2 13.8 7.6 2.6 3.8 2.5 1.5 0.86
(3.0- (7.8- (2.9- (2.9-
1.75) 1.6) 1.7) 0.3)
Barley Spain 100 11.6 13-26 10 2 4.1 3.7 3.2 1.3 2.8 1.7
Wheat Spain 100 11.0 13-26 10 2 9.4 5.6 3.0 2.8 2.1 1.0
The effect of aeration was also evaluated in a residue trial carried
out under Swiss conditions. A bin of barley treated with methacrifos
at 10 mg/kg was aerated twice during the storage period (after one and
three weeks) and another bin in the same warehouse was not aerated.
The residue on the aerated barley was 12.4 mg/kg at the beginning of
the experiment, 6.6 mg/kg after one week; 4.6 mg/kg after 32 weeks and
2.0 mg/kg after 74 weeks. The residues in the non-aerated bin were
11.3, 8.6, 4.2 and 2.5 mg/kg at the same sampling times, showing that
aeration had little or no effect on the residue dissipation. The
residue in oats stored at the same farm (without aeration ) were 10.2,
9.0, 6.0 and 3.7 mg/kg at these sampling times. Comparable results
were obtained in a similar trial on wheat in Switzerland in which one
bin was aerated after 54, 103 and 142 days post-treatment, by
circulating the wheat through the conveyer system and back to the
silo, and another bin not aerated. The initial residues were 6.0 and
6.1. Residues after the three aerations were 4.2, 3.7 and 3.4 mg/kg
on the aerated wheat and 4.8, 4.2 and 3.9 mg/kg on the nonaerated.
Again the rotation and resulting aeration of the wheat had little, if
any, influence on the dissipation rate (Formica, 1978d, 1978e). The
residue dissipation at different levels in a silo was studied in
Switzerland under typical European continental storage conditions with
temperatures of about 5°C in winter and 22°C in summer. Methacrifos
was mixed with the grain in a closed conveyer system, at a rate of 11
mg/kg; there was no ventilation during the storage period. The
initial concentration was 50 - 70% of the nominal value calculated
from the application rate and the volume treated. The residue at 2 m
depth increased during the following 5 weeks to almost the nominal
value and slowly decreased thereafter. It is assumed that the active
ingredient was not homogeneously dispersed immediately after
application but became more uniformly distributed through migration.
From a comparison of the residues in the samples taken from the
surface and at 2 m depth, it seems evident that the dissipation was
somewhat slower in the interior than on the surface of the stored
grain (Formica, 1976f). (Table 10).
In a study of the influence of the formulation on dissipation an
aqueous formulation showed more rapid dissipation than a formulation
with kerosene. After treatment with a nominal 10 mg/kg the residues
of the aqueous formulation were 0.2/0.16 and 0.15/0.25 mg/kg after 2
and 15 weeks respectively, whereas the residues of the kerosene
formulation were 9.1/9.3 and 4.0/3.5 mg/kg.
TABLE 10. Dissipation of methacrifos in wheat (Switzerland)
Days after
treatment 1 7 21 35 77 162 252 324 470 526 653
samples from
Surface 5.3 3.3 3.2 8.6/6.1 5.9/5.9 4.0/4.0 3.5 2.4 1.3 0.7 -
2 metres 4.6/3.9 5.2 6.0 10.1/7.4 6.7/5.6 6.2/6.5 5.2 4.0 2.6 2.8 1.4/2.25
Maize
Several small-scale trials were carried out with maize kernels and/or
cobs stored under various conditions of temperature and moisture
content. Although the distribution of the active ingredient was
rather inhomogeneous, it was evident, as with the other grains, that
the dissipation is dependant on time and temperature.
Under tropical conditions (temperature 35°C and higher) the residue
decreased much more rapidly than at moderate temperatures (Giannone
and Formica, 1979b).
The dissipation of methacrifos in maize was studied in a large scale
trial in Switzerland; 80 tons of maize kernels (moisture content
14.5%) were treated at 10 mg/kg on a conveyer system during the
filling of concrete silo cells, which were closed without ventilation.
Results are shown in Table 11.
TABLE 11. Dissipation of methacrifos in maize
Temp. in silos °C1 19 9 14 27
Days after treatment 6 76 300 376
Residue, mg/kg 7.8 3.62 3.9/3.4 0.4/0.7
(5.7-9.6) 3.1-4.2
1 Temperature at a depth of 2 metres.
2 Mean value of 4 samples; two samples analysed 5 months later than
the other two samples; samples stored at -20°C.
Rice
Small-scale trials on the dissipation of methacrifos under various
storage conditions were carried out in Australia. Brown and polished
rice were treated at the rate of 10 mg/kg and stored in bags, at
temperatures of 13-26°C. The moisture content of the brown rice was
approximately 14.2% and of the polished rice 14.4%. The initial
residue of 9.1 mg/kg in brown rice decreased to 5.0 mg/kg after 12
weeks and to 3.9 mg/kg after 24 weeks. In the polished rice the
corresponding residue levels were 7.9, 6.3 and 4.4 mg/kg respectively.
In other trials paddy rice, brown rice and polished rice were stored
in cardboard drums, and paddy rice also in mini-silos of about six
tons capacity. The decrease of the residue in paddy rice stored at
about 32°C was faster than in brown or polished rice. Under the
conditions occurring in the trials the dissipation rate of methacrifos
was independent of the application rate (Hart and Moore 1975;
McDougall 1976b) (Table 12).
TABLE 12. Dissipation of methacrifos in rice (McDougall, 1976b; Hart and Moore, 1976
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
mini-silo moisture temp. °C mg/kg
or cardboard %
Type of rice drums 0 12 23 54/51 124/121 166 205
paddy rice silo 13.7 17-26 10 8.2 5.8 4.7 3.7 2.6
paddy rice drums 8.8-12.8 32 10 10.8 6.2 4.4 2.2 1.2
drums 8.8-12.8 32 5 4.5 2.6 1.9 0.9 0.5
brown rice drums 8.8-12.8 32 10 9.1 7.0 5.5 3.8 2.7
drums 8.8-12.8 32 5 3.7 3.2 2.4 1.2 1.0
polished rice drums 8.8-12.8 32 10 13.0 9.6 7.2 4.6 2.7
drums 8.8-12.8 32 5 4.7 3.9 3.2 1.6 1.0
TABLE 13. Dissipation of methacrifos in sorghum
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
silo or moisture temp. °C mg/kg
Country bags % 0 15 21 45 56 90 147
Australia silo 13 27 15 13.6 9.3 7.7 6.3
13 27 15 9.1 4.1 7.1 4.3
13 27 7.5 8.0 4.7 4.9 2.7
Mali bags 25-30 10(dust) 3.6 3.0 1.9
25-30 10(solution) 6.1 4.2 2.1
TABLE 14. Dissipation of methacrifos in peanuts, stored in drums (McDougall, 1976a)
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
moisture % temp. °C mg/kg
Country % 7 12 28 56 84 126 175 210
Peanut
whole 7.8-4.4 32 20 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
kernel 0.6 1.5 1.7 0.3 0.3 0.4 1.0 0.4
shell 50.8 49.5 30.6 30.5 29.2 10.3 8.8 7.7
Peanut
whole 7.8-4.4 32 10 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
kernel 0.6 0.3 <0.3 0.3 <0.3 <0.3 <0.3 <0.3
shell 41.5 21.5 23.7 12.9 7.3 7.3 4.1 5.7
Sorghum
Data are available from large-scale trials in Australia where sorghum
treated at 7.5 and 15 mg/kg was stored in silo bins and from Mali
where sorghum treated according to the so-called "sandwich method"
with a dust or a spray was stored in bags. The rate of dissipation
was similar to that in the other grains (Hart and Moore 1976, Giannone
and Formica, 1980) (Table 13).
Pulses (dried legume vegetables)
In a small-scale trial in Switzerland, a mixture of beans and peas
(Vicia faba, Phaseolus nanus and Pisum sativum) was
treated with a dust (10 or 20 mg/kg ai/kg) or a liquid formulation (10
mg ai/kg) and stored in drums at 25 ± 1°C; moisture content before
treatment was 9.8%. Three hours after the application the residues in
the lots treated with the dusts at 20 and 10 mg ai/kg and the solution
at 10 mg ai/kg were 5.5, 3.9 and 2.7 mg/kg respectively. After 27
days the residue levels in these lots were 6.5, 2.6 and 2.6 mg/kg and
after 142 days 3.5, 1.7 and 1.7 mg/kg respectively.
In a small-scale trial in Nigeria, cowpeas were treated with 10 mg/kg
methacrifos and stored in open jute bags under tropical conditions.
No residues (<0.12 mg/kg) were found 65 and 92 days after treatment.
Peanuts
A mini-silo (drum) trial was carried out in Australia. Methacrifos
was applied at 10 and 20 mg/kg to whole peanuts, which were stored in
120 l cardboard drums at 32°C. On all sampling dates kernels and the
shells were analysed separately. The results show that nearly all the
residue is contained in the shell (McDougall, 1976).
In Mali, peanut kernels were treated with 10 mg/kg methacrifos and
stored in closed plastic bags under tropical conditions (25-30°C).
Residues after 15, 60 and 90 days were 7.6, 5.6, 5.2 mg/kg
respectively (McDougall 1976).
Cocoa-beans
Cocoa-beans stored in closed jute bags in a warehouse were treated
with methacrifos at 0.4 g ai/m2 on the outside of the bags. Samples
were taken at four sampling dates from the outside layer of cocoa-
beans, which had been exposed to the treatment, and from the interior
of the bags. The residues in samples from near the outside of the
bags were higher than in the internal samples; 34 days after treatment
the residues in the outer samples were 1.0 and 1.2 mg/kg and in the
inner samples <0.025. After 98 days the levels were 0.83 and 0.32
mg/kg respectively.
The methacrifos residues were found mainly in the peel. 34 days after
treatment the residues in the whole beans were 1.0 and 1.2, in the
peel 5.6 and in the kernel 0.5 mg/kg (Formica, 1978c).
Coffee beans
Raw coffee beans imported from Nigeria were treated in Switzerland
with methacrifos in 10 and 20 mg/kg. The treated beans were stored in
closed drums at 20° ± 2°C. After 4 months storage the residues from
the 20 mg/kg treatment were 2 mg/kg but 0.5 mg/kg in the lot treated
with 10 mg/kg (Renfer, 1976).
FATE OF RESIDUES
In animals
General
As grain treated with methacrifos and grain products derived from
treated grain may be used as animal feed, it was necessary to study
the fate of methacrifos in livestock animals and to demonstrate that
no significant residue would remain in livestock and poultry, milk or
eggs. Special trials therefore, were carried out to study possible
carry-over from residues in feed to tissues or products of animal
origin.
Chickens
In a preliminary experiment, four young male broilers were given a
single oral dose of 20 mg/kg methacrifos by capsule. The birds were
slaughtered 6, 12, 24 and 48 hours after dosing. Samples of fat,
muscle, liver, kidney, heart, gizzard and skin were analysed. The
residues in all muscle and heart samples were 0.01 mg/kg or less.
Residues in the other samples decreased very rapidly (Moore and
McDougall, 1974c) (Table 15).
TABLE 15. Residues of methacrifos in chicken tissues after single
oral treatment with 20 mg/kg
Tissue Residues in mg/kg
time (hours post-treatment)
6 12 24 48
Fat 0.22 0.06 <0.01 <0.01
Liver 0.02 0.01 0.01 <0.01
kidney 0.08 <0.03 <0.03 <0.03
Gizzard 0.42 <0.01 <0.01 <0.01
Skin 0.06 0.02 <0.01 <0.01
Muscle <0.01 <0.01 <0.01 <0.01
Heart 0.01 <0.01 <0.01 <O.01
In a further experiment 5 groups of 77 one-day old chicks each were
given feed fortified with methacrifos at levels of 0, 10, 100, 250 or
500 mg/kg for 63 days. At the end of the feeding period 20 chickens
from each group (10 males and 10 females) were slaughtered and the
combined muscles, skin, kidney and liver used for analysis. In all
tissues examined residues were less than 0.01 mg/kg (Formica, 1974).
Eggs
Three groups of 15 laying hens were fed a diet containing 0, 10 or 20
mg/kg methacrifos. Egg samples were collected one day before
treatment, on the 16th day of treatment and 0, 2, 3, 7 days after the
end of the treatment. In none of the samples were residues found
(<0.01 mg/kg) (Schnabel and Formica, 1979).
Milk
Two lactating cows were given 30 mg methacrifos by capsule daily for
10 days. This dose corresponds to about 3 kg of cereals treated at a
rate of 10 mg/kg daily in the total ration. Pooled morning and
evening milk samples were taken before, during the feeding period and
three days after its completion. No residues (<0.001 mg/kg) could be
detected in the milk of the treated cows before, during or after the
10 day period (Formica, 1977).
In plants
Stored grain
The degradation of carbonyl 14C methacrifos (specific radioactivity
31.1 µCi/mg) in grain treated with 10 mg/kg was studied under
laboratory conditions for one year; 84-94% of the applied
radioactivity was recovered at all sampling times, indicating very
limited volatility of methacrifos or its degradation products. After
one year about 25% of the then recovered radioactivity was present as
parent compound. The fact that little volatilisation of the
radioactivity occurred during the storage indicates that methacrifos
and its degradation products were strongly absorbed to the wheat
grain. Less than 15% of the recovered radioactivity could be washed
off the surface of the grain with methylene chloride. The bulk of the
radioactivity could be extracted only after grinding of grain. The
non-extractable portion of radioactivity was low at the early sampling
dates but rose to 9-14% after 140 and 385 days. The low but
continuous evolution of 14C during the experiment may demonstrate
that the insecticide is eventually metabolised by the wheat grain.
The organosoluble radioactivity on or in the grains consisted mainly
of the parent compound. The oxon was not detected. Mono- and
di-demethylated methacrifos were detected by ethylation followed by
GLC analysis. Their identity was confirmed by methylation with
deuterated diazomethane (CD2N2) followed by GCMS.
The results give strong evidence for the degradation pathways shown in
figure 2 (Blattmann 1978).
Figure 2. Degradation of methacrifos in grain
TOXICOLOGICAL STUDIES
Special studies on teratogenicity and reproduction
Rat
After successfully being mated with male Sprague-Dawley albino rats
four groups of 2-month old female rats (25 animals/group) of the
same strain were orally incubated with methacrifos at doses of 0,
5, 25 or 50 mg/kg bw. In a preliminary study 6 out of 10 dams
died, when the compound was administered at the dose of 150 mg/kg
bw.
Treatment started at day 6 of pregnancy and was continued through
day 15. During the experiment a reduction in body weight gain was
found in the dams of the 50 mg group, starting half-way through the
dosing period. This reduction was accompanied by a temporary
decrease in food consumption at the 11th and 16th day of dosing
(food consumption was measured at day 6, 11, 16 and 21 of dosing).
Further a dose-related increase in the percentages of embryonal
deaths (early resorptions) was found, which already started at the
5 mg/kg level. No structural abnormalities related to the
methacrifos treatment were observed in the pups. The number of
foetuses with still incomplete ossified sternebrae was slightly
higher in the 50 and 25 mg/kg dose groups. (35.8% in control
versus 35.0, 43.0 and 52.8% for the 5, 25 and 50 mg/kg groups,
respectively). In the treated groups no foetal deaths or dead
foetuses were found (Fritz et al, 1978).
Groups of rats (8 males and 16 females/group) were fed methacrifos
in the diet at dosage levels of 0, 1, 10 or 100 mg/kg (mean
analytical concentrations of <0.1, 0.79, 7.4 and 73 mg/kg), and
subjected to a standard 3-generation, 2-litter per generation
study.
Gross and histopathologic examinations were conducted on the
parenteral animals of the F0, F1, F2 and F3. Gross
pathological evaluations were made on at least 10 male and 10
female weanlings of the 0 and 100 mg/kg group. No
treatment-related changes were noted in the tissues and organs
examined. Throughout the study a dose-related increase in
mortality of the male parent animals was observed in all treated
groups, whereas the mortality was also higher in the females of the
treated groups. The cause of mortalities was ascribed to acute
bronchopneumonia superimposed on lesions of chronic murine
pneumonia. There was no clear effect on the body weight gain or
organ weights of the treated groups.
The total number of pups delivered was dose-related decreased in
almost all litters, where the mean number was not clearly affected.
In addition somewhat reduced survival percentages after 21 days
were noted in comparison to the controls in the 10 and 100 mg/kg
F1a and F1b, 1 mg/kg F3b and 100 mg/kg F3a and F3b litters
(Charles et al, 1980a).
Special studies on mutagenicity
Bacteria
Histidine-autotrophic mutants of Salmonella typhimurium were
treated with methacrifos in concentrations of 0.25, 0.75, 2.25,
6.75 or 20.25 mg/ml with or without microsomal activation, in order
to study the possible induction of point mutations by methacrifos.
The test results were negative (Arni and Müller, 1979).
Mice
Twenty male albino mice were administered methacrifos orally in
single doses of O, 7, and 21 mg/kg and then mated to two untreated
female albino mice over a period of six weeks. At the end of each
week the females were replaced by new ones. The effects on mating
ratio and the number of implantations and embryonic deaths as well
as the effects on the loss of pre-implantation zygotes and the rate
of deaths of post-implantation stages of embryonic development were
studied. No evidence of a dominant lethal effect was observed
(Hool and Müller, 1980).
Special studies on carcinogenicity
Groups of 5-7 week old Swiss, white mice (60/sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (purity 94.6%) for
21-22 months (sacrifice of male and females at 50% mortality).
Body weight, food consumption and mortality were not clearly
affected. Haematological investigations carried out at the end of
the study revealed increases in total leucocyte count for males of
the 1 and 10 mg/kg groups. Urinalysis, performed also on 10
animals/sex/group, were normal at sacrifice but blood chemistry
revealed a significant increase in total protein in females and in
cholesterol in males at 100 mg/kg. In females an obvious
dose-related, but not significant, increase was found in absolute
and relative weight of gonads at 100 and 10 mg/kg. At 1 mg/kg a
tendency to an increase was found. The other organ weights were
within normal limits.
At sacrifice the activity of cholinesterase in plasma, appeared
inhibited at 100 and 10 mg/kg in both males and females; in
erythrocytes it was inhibited in females at 100 mg/kg. Brain
cholinesterase was not dose-related affected.
At histopathology no effects on tissues were apparent.
Carcinogenicity has not been found in this experiment (Charles et
al, 1980b).
Special studies on inhalation
Groups of 7/8 week-old rats (9/sex/group) were exposed to 0, 90,
191 or 467 mg methacrifos/m3 air, 15 times over a period of 21
days.
Thereafter 4 animals/sex of the control and highest dose group were
kept for a recovery period of 21 days. The compound was injected
into an air stream discharged into the exposure chamber through a
spray nozzle under a pressure of 2 atmospheres at a rate of 10
1/min.; the exposure concentrations were determined. The animals
were kept separately in PVC tubes, which were positioned radially
around the exposure chamber. The animals at the highest dose group
showed a significant decrease in food consumption and body weight
gain, but returned to normal during the recovery period. In the
191 mg/m3 group the body weight was also decreased at the end of
the study. Haematology and blood biochemistry revealed a
significant increase in SGPT in the 467 mg/m3 group. No clear
effects on the weight of the organs were observed. Blood urea
concentration was increased in all doses tested, which disappeared
after the recovery period. The cholinesterase activity of plasmal
erythrocytes and brain was significantly depressed in all treated
groups. After the recovery period, brain cholinesterase activity
was still decreased.
One male and one female rat died during the treatment: congestion
often with haemorrhages were observed in various tissues. All
other gross or microscopic findings were not related to the
treatment (Ullmann et al, 1977).
Special studies on dermal toxicity
Groups of KA 46, Himalayan strain rabbits (3/sex/group: 1.3-2 kg)
were dermally exposed to 0, 10, 50 or 250 mg/kg methacrifos, once
a day, 5 times a week for a period of 3 weeks. Thereafter 1
animal/sex/group was kept for a recovery period of 3 weeks. The
compound was diluted in PEG 400 and saline and applied to the
shaven skin of the back, with occlusive dressings. All treated
groups showed reversible erythema at the application site. The
animals of the 50 and 250 mg/kg groups showed clinical symptoms
(tremors, ataxy, especially during the first week). At 250 mg, 5
animals died within 9 days of the study. In the other groups no
effects on food consumption, body weight gain, haematological and
clinical chemistry were observed.
All the treated groups showed after 3 weeks a dose-related
inhibition of the cholinesterase activity of plasma and
erythrocytes of 30-80%, and 10-75% respectively. Brain
cholinesterase values revealed an inhibition of approximately
10-30, 55 and 85% of the control values in the treated groups. At
the end of the 3-week recovery period the blood and brain
cholinesterase activities of the 10 and 50 mg/kg groups had almost
returned to normal values.
Due to the small number of animals the effects on the organ weights
are difficult to evaluate.
Histopathological examinations revealed not pathological changes
which could be attributed to the treatment (Sachsse et al, 1978).
Special studies on skin sensitisation
Groups of guinea pigs of the Pirbright White strain (10/sex/group)
were intradermally injected saline, methacrifos and
dinitrochlorobenzene (DNCB) (positive control) (0.1 ml of the test
substance, 0.1%) every other day for 3 treatments.
During the second and third week of induction 6 sensitizing doses
of 0.1 ml, with Freund's adjuvants, were injected intracutaneously.
Two weeks after the last exposure, the animals were challenged and
examined for sensitisation. Ten days thereafter a second challenge
was given. There were indications that methacrifos induced a
sensitisation reaction, but it was less severe than DNCB (Ullmann
and Sachsse, 1975).
Special studies on neurotoxicity
In hens of the White Leghorn strain, over 12 months of age, which
were orally dosed by gavage with 0, 25, 50, 101 or 202 mg/kg
methacrifos (10, 15, 20 and 30 animals/group resp.) on day 0 and
21, no symptoms of delayed neurotoxicity were described in the
intermediate observation period of 21 days nor in the 42-day
observation period thereafter. At all doses the treatment resulted
within 1 hour to 4 days in ataxy, curved or ventral position,
sedation and salivation. Histopathological lesions in the spinal
cord and the sciatic nerve were not observed.
In the positive control animals, which had been treated with TOCP,
delayed neurotoxicity was found, obviously with slight to severe
lesions in the spinal cord and the peripheral nerves (Sachsse et
al, 1979).
Special studies on potentiation
In a study with RAI f-SPF rats (5 animals/sex/group) in which acute
oral LD50 values were determined no potentiation was found with the
insecticides dichlorvos, phosphamidon, diazinon and CGA 15324;
however a potentiation did occur with the insecticides methidathion
and malathion.
LD50 in mg/kg
techn. compound techn. compound techn. compound + CGA 201681
theoretically experimentally
phosphamidon 12 347 400
dichlorvos 54 395 611
CGA 15324 426 581 568
diazinon 534 635 520
methidathion 43 389.5 201
malthion 1397 1066.5 454
1 acute oral LD50 of CGA 20168: 745 mg/kg (Sachsse and Bathe,
1978)
TABLE 1. Acute toxicity of methacrifos
Mammals
route of LD50 in mg/kg reference
species sex administration or mg/m3
Chicken oral 1011 Sachsse et al, 1979
Rat M + F oral 6782 Bathe, 1974a
Mouse M + F oral 662 Bathe, 1974b
Mouse M + F oral 582 Bathe and Sachsse, 1978
Dog M + F oral 5731 Bathe and Sachsse, 1975
Rat M + F dermal >31002 Bathe, 1974c
Rabbit M + F dermal 27321 Ullmann and Sachsse, 1977
Rat M + F inhalation >2500 Ullmann and Sachsse, 1976
1 observation period of 14 days
2 observation period 7 days
Fish
exp. temp. 96 hour LC50
Species in C mg/kg Reference
Rainbow trout 14 0.4
Crucian carp 14 30
Catfish 21 6 Sachsse and Ullmann, 1974a
Bluegill 14 2
Guppy 21 3
Short-term studies
Quail
Groups of adult Japanese quails (3 males and 7 females/group) were
given, 0, 1000, 6000, or 10,000 mg/kg methacrifos in the feed for a
5-day period. They were held for observation during a further 3 days.
The mortality observed during the entire 8-day period was used to
calculate the LC50 which appeared to be approximately 10,000 mg/kg.
Observation of symptoms, egg production, food consumption and body
weight revealed sedation and ruffled feathers, partly to complete
refusal of food and a decrease in body weight over the whole dosing
period and a stop in egg production (Sachsse and Ullmann, 1974b).
Chicken
Groups of one-day old Hubbard chickens (32 sex/group) were dietary fed
0, 10, 100, 250, 500 or 1000 mg/kg methacrifos for 63 days.
Mortality was found to be 1.6, 0, 1.6, 3.1, 37.6 and 98.2% in the 0,
10, 100, 250, 500 and 1000 mg/kg groups, respectively. Most animals
died in the first period (0-14 days). The dying animals of the 500
and 1000 mg/kg groups showed signs of ataxia, lack of appetite,
somnolence and ruffled plumage. Body weight and food consumption were
significantly decreased at 250 and 500 mg/kg during the whole
experiment, at 100 mg/kg temporarily up to at least 28 days. No
effects on haematology and histopathology were found.
Final cholinesterase activity measurement in the brain on 8 animals/
sex/group revealed a dose-related inhibition at 100, 250 and 500 mg/kg
(Strittmatter and Gfeller, 1975).
Rat
Groups of Sprague-Dawley derived rats (150-170 g; 5 or 10, sex/group)
were given 0, 10, 100 or 1000 mg/kg methacrifos in the feed for 30
days, followed by a recovery period of 28 days for the control and
1000 mg/kg groups but nearly complete recovery was found in food
consumption at about 25 days; loss of hair in males was also noted.
In the males of the 1000 mg/kg group abnormalities in blood
composition were observed: a significant decrease in Hb, PCV, number
of erythrocytes and lymphocytes and prothrombin time and a slight
increase in neutrophils. At 100 mg/kg in the males the number of
lymphocytes and neutrophils appeared affected, also.
A decrease in cholinesterase activity in plasma and erythrocytes was
found at the end of the dosing period in both males and females of the
100 and 1000 mg/kg groups; plasma cholinesterase activity was also
decreased in males of the 10 mg/kg groups. At the end of the
experiment partial recovery was observed. Cholinesterase activity in
plasma and erythrocytes in the 1000 mg/kg group was still about 20%
decreased in both males and females. Brain cholinesterase has not
been determined. Further effects on clinical-chemical parameters and
urinalysis were not found. In the male animals of the 1000 mg/kg
groups the weight of the kidneys was decreased in comparison to the
control value (Drake, 1975).
Dog
Groups of pure bred beagle dogs (6.5-11 kg; 2 or 4 sex/group) were
dietary fed 0, 10 or 100 mg/kg for 28 days. Another group of dogs
received 1000 mg/kg for 11 days but treatment had to be stopped
because of a deterioration in the bodily condition of the dogs. After
7 days the animals then received 500 mg/kg for 4 weeks. Thereafter 4
animals were sacrificed and 4 were allowed a 4-week recovery period.
Due to dose changes evaluation of the effects of 1000 or 500 mg/kg
methacrifos is difficult. However body weight and food intake
drastically decreased and two animals showed higher SGPT values.
There was a marked depression of plasma and red cell cholinesterase
values, with a partial recovery. One animal was sacrificed in poor
bodily condition. Macroscopically a whitening of the splenic capsule
and a small amount of free blood beneath the membranes of the spinal
cord was observed.
In the other treated groups food consumption, body weights and organ
weights were measured and haematology, blood biochemistry, urinalysis,
ophthalmoscopy and histopathology performed but no toxic effects were
found except for a reduced water intake at 100 mg/kg and 10 mg/kg in
the males and a final inhibition of cholinesterase activity both in
the erythrocytes and plasma at 10 and 100 mg/kg in males and females.
Brain cholinesterase activity was not affected (Chesterman et al,
1975).
Groups of adult pure bred beagle dogs (8.5-15 kg; 8 sex/group) were
dietary fed 0, 1, 10 or 100 mg/kg methacrifos (94.6%) for 26 weeks.
Thereafter 2 animals/sex/group were kept for a recovery period of 4
weeks.
No effects were found on food consumption and body weight gain but the
animals in the dose groups vomited more often and showed more
pronounced diarrhoea, when compared with the controls. Haematology
and urinalysis after 4, 13 and 26 weeks, were normal but blood
biochemistry showed a decrease in blood glucose at 26 weeks in all
dose groups, which fully recovered in the 1 mg/kg groups in the 4-week
recovery period but which only partially recovered in the 100 and 10
mg/kg groups in this period.
The main effects were found on cholinesterase activity. Plasma
cholinesterase was dose-related inhibited in the 100 and 10 mg/kg
groups at 4, 13 and 26 weeks and in the 1 mg/kg groups after 4 and 13
weeks only, with about 30%. Complete recovery was found after 4
weeks.
Erythrocyte cholinesterase was also dose-related inhibited in the 100
and 10 mg/kg groups at 4, 13 and 26 weeks but recovery was incomplete
after 4 weeks, inhibition was still present in males and females at
100 mg/kg and in females at 10 mg/kg. Brain cholinesterase was not
affected. Gross and microscopic examinations of organs and tissues
showed no effects attributed to methacrifos (Bathe et al,1977).
Pig
Groups of large white pigs (about 44 kg; 2/sex/group) were dietary fed
0, 10, 100 or 1000 mg/kg for about 4 weeks. No effects were found on
food consumption, body weights, blood chemistry, and haematology,
except for an influence on cholinesterase activity. At day 15 and 29
an inhibition of cholinesterase activity in plasma and erythrocytes
was found at 1000 mg/kg, at day 29 also in brain at 1000 mg/kg and in
erythrocytes also at 100 mg/kg. No histopathological changes were
found (Gfeller, 1974).
Long-term studies
Rat
Groups of about 40-day old CR strain rats (65/sex/group) were dietary
fed 0, 1, 10 or 100 mg/kg methacrifos for 2 years. Haematology,
coagulation, clinical chemistry and urinalysis studies were carried
out in 10 rats/sex from the control and 100 mg/kg group.
No clear effects were found on mortality, food consumption, body and
organ weights, haematology, clinical chemistry and urinalysis.
Cholinesterase activity in plasma and erythrocytes, which was measured
at 3, 6, 12, 18 and 24 months, was influenced. Cholinesterase
activity in plasma was inhibited in females of the 100 mg/kg group at
3, 6, 12 and 24 months, in males of the same group at 3, 6 and 12
months and in females of the 10 mg/kg group at 3 and 24 months.
Cholinesterase activity in erythrocytes was inhibited in females of
the 100 mg/kg groups at 6 months and in males of the same group at 12
months. Finally, cholinesterase activity in brain, which was measured
at 24 months, was not affected. The results of the microscopic
examination indicated that methacrifos did not produce
histopathological changes. A no-effect level in this study is 1 mg/kg
in the diet (Basler et al, 1980).
RESIDUES IN FOOD
USE PATTERN
Methacrifos is an organophosphorus insecticide and acaricide, with a
rapid and long-lasting action on a number of major pests of stored
products. It is mainly used as a grain protectant but also against
insect pests in other stored products. It is effective against
insects showing resistance to commonly used stored product
insecticides, including other organophosphorus compounds.
The main fields of application are cereal grains (maize, rice,
sorghum), cocoa, coffee, peanuts, pulses and tobacco.
The material shows a wide spectrum of activity notably against
Sitophilus spp., Rhizopertha dominica, Tribolium spp., Oryzaephilus
surinamensis, Lasioderma serricorna, Dermestes frisehii,
Anugasta Kuhniella, Plodio interpunetella, Acarus siro,
Glycophagus domesticus (Wyniger et al, 1977).
The major formulations are emulsifiable concentrate, solution in
organic solvents and dusts.
The dosage rate under usual conditions of storage and infestation is
10 mg ai/kg, which gives protection to stored products for several
months. At lower storage temperatures, or if infestation is moderate,
satisfactory protection can be obtained with 5 mg/kg. In some
situations (e.g. in Australia against a resistant strain of
Rhizopertha dominica) a rate of 20 mg ai/kg may be needed to
achieve satisfactory control.
Methacrifos is also used for space treatment in empty silos at 50-75
mg ai/m3, for surface treatment of stored products and as a wall
treatment in storage premises.
Methacrifos application does not affect germination, baking quality,
taste or odour.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
various countries, including Australia, France, Spain, Switzerland,
Mali and South Africa, on a variety of cereal grains, dry pulses,
tropical seeds and products of animal origin. The data were obtained
from both large and small-scale trials performed under practical
conditions.
Wheat, barley, oats
ln laboratory experiments, simulating grain storage, in Australia, it
was shown that the residue of methacrifos decreased more rapidly with
increased temperature and moisture content (Table 2); increased rate
and/or repeated application did not influence the dissipation rate
(Table 3); some evidence was obtained that enzymic activity in the
grain was at least partially responsible for the more rapid initial
breakdown (Table 3). The rate of residue decrease after treatment of
wheat, barley and oats is similar. On all variety loss during storage
at 36°C is more rapid than at 31°C as shown in Table 3. (McDougall,
1974; Moore, 1973; Moore et al, 1974).
Several small-scale trials in which wheat, barley or oats were stored
in mini-silos (5´ ton of each grain) or in drums (120 l) showed
similar results. As in the laboratory experiment, the application
rate of methacrifos does not affect the rate of dissipation in any of
the grains investigated (McDougall, 1975; McDougall, 1976) (Table 4
and 5). From these trials half-life periods could be calculated using
the formula t´ = (t2 - t1) log 2 / [2 - (logC2/C1) × 100], where
C1, C2 are concentrations of the insecticide at times t1 and t2
respectively. Only data for 84 days storage were used since the
temperature dropped after that period.
TABLE 2. Laboratory trials on the dissipation of methacrifos in stored grains
Storage Conditions Application Residues of methacrifos, in mg/kg, after storage
rate
Commodity Moisture % Temp. °C mg/kg 0/1 7 14 28 42 56 70 84
Wheat 9.3 28 24 17.3 16.1 14.7 12.3 12.1
10.3 " " 14.8 14.0 12.0 8.8 7.6
11.3 " " 13.4 12.4 9.5 6.7 4.9
12.3 " " 13.4 10.7 7.7 5.0 3.0
9.3 35 24 14.5 13.8 12.5 8.5 6.3
10.3 " " 13.8 11.5 8.1 4.8 3.2
11.3 " " 13.3 10.2 6.1 - -
12.3 " " 12.9 8.2 4.4 - -
9.6 42 24 13.1 8.5 4.8 - -
10.4 " " 12.6 6.7 3.1 - -
12.3 " " 4.9 2.0 - - -
12.4 " " 11.1 4.1 1.2 - -
Wheat 11.9 31 20 16.4 12.8 9.6 7.6 5.4 4.1
11.8 36 " 16.2 11.4 8.9 4.5 3.2 -
Barley 11.7 31 " 16.9 14.2 12.0 8.0 5.8 3.4
11.8 36 " 18.9 11.6 8.7 4.4 2.5 -
Oats 11.7 31 " 19.2 13.2 11.6 6.9 5.1 -
11.9 36 " 18.8 11.6 8.4 3.7 2.5 -
TABLE 3. Laboratory trials on the relative disspation of methacrifos in wheat
Storage Condition Application Residues of methacrifos as %
rate of initial residue (=100)
commodity moisture % temp. °C mg/kg 0 2 14 28 42 Reference
Wheat 11.2 35 24 100 82 48 30 22 Moore & McDougall, 1974
11.2 " 50 100 73 45 28 21
11.1 " 1501 100 74 47 34 25
1O.91 "1 24 100 98 60 36 22
11.2 " 24 100 93 44 29 21
12.5 " 4 + 15 100 79 49 33 -
1 applied in "dead" grain
TABLE 4. Mini-silo storage trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2/3 6-8 13-15 28 56 84 105 126 147 168 Reference
Wheat 10.2 22-26.5 10 4.7 5.8 5.0 5.0 4.2 4.1 - 3.0 - 2.9 McDougall, 1975
Barley 12.8 " " 9.4 8.6 8.6 8.0 5.7 3.9 2.9 - 2.0 - 1.8
Maize 12.9 " " 7.2 7.0 7.0 6.8 7.0 3.9 4.0 - 2.4 - 2.9
Sunflower 7.2 " " 7.7 7.5 7.2 6.7 7.4 5.0 4.9 - 3.6 - 2.7
TABLE 5. Drum trials (Australia) on the dissipation of methacrifos in stored grains
Storage Condition Application Residues of methacrifos in mg/kg after.....days
Commodity % moisture temp. °C rate mg/kg 0 2 5 7 12 28 56 84 126 175 210 Reference
Wheat 8.3-10.2 32 5 5.3 - - 5.5 5.4 4.0 3.1 2.5 1.6 1.2 1.1 McDougall, 1976
10 8.8 - - 9.9 8.2 7.1 5.3 4.3 3.1 2.4 1.8
Oats 7.4-8.0 10 10 4.1 - 4.4 - 3.6 2.8 2.4 1.7 1.5 - 0.4
(in sealed
bags) 7-8-8.4 32 10 4.1 - 4.8 - 4.4 3.4 2.4 1.7 1.3 - 0.4
Maize 8.6-10.3 32 5 2.6 2.9 - - 2.4 2.0 1.8 1.4 1.0 0.6 0.5
10 4.2 6.0 - - 6.0 4.5 4.0 2.5 1.9 1.5 1.3
Peanuts 4.4-7.9 32 10 7.3 - - 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
20 16.0 - - 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
Soybeans 5.5-7.4 32 5 2.3 2.6 - - 2.5 2.1 1.6 1.4 1.1 0.9 0.9
10 7.1 7.6 - - 7.1 6.3 5.7 4.8 3.8 3.6 3.2
TABLE 7. Half-life of methacrifos on various grains stored in mini
silos at 26°C
Commodity Storage conditions Half-life (days)
moisture temp.°C
Wheat 10.2 26 105
Barley 12.9 26 44
Maize 12.8 26 100
Sunflower seed 7.2 26 90
From the trial in which the grains were stored in drums at 32°C the
following half-life periods were calculated; for the moisture content
(see table 5).
Commodity Half-life(days)
Wheat 70
Oats 60
Maize 85
Peanuts 55
Soybeans 100
Large-scale trials
Wheat and barley
Large-scale trials in silos were carried out under a range of storage
conditions in various countries e.g. Australia, Argentina, Morocco,
Spain, Switzerland and France. As in the small-scale trials it was
found that the application rate did not affect the rate of dissipation
of the residues in any of the grain investigated (McDougall 1976)
(table 8).
The experiments under practical conditions confirmed that the
dissipation of methacrifos is mainly dependant on time and
temperature. The residue decrease under storage conditions prevailing
normally in Europe (temperature 15-25°C) is much slower than in
regions where storage temperatures are generally higher e.g. Australia
(Formica, 1975).
In the trials carried out in Australia in which the grain was aerated
during the storage period, there were indications of a decreased rate
of dissipation, compared with silos in which the grain was stored
under the same conditions of temperature and moisture at the start of
the experiment, but not aerated. Aeration was started 24 hours after
treatment and functioned only during the cooler hours of the day for a
total of 84 hours a week for the first 4 weeks and about 30 hours
weekly thereafter (Table 9) (Moor and McDougall 1975). In other
experiments aeration had little or no effect on residue dissipation
(Formica, 1978g).
TABLE 8. Dissipation of methacrifos in small grain
Large-scale trials in silo. (Moore and McDougall, 1974a, 1974b and 1975).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0,15 1 6 11/12 16 21 22/23 26 34 41
Wheat Australia 100 11.3 ± 30 20 0.6 21.8 11.8 7.5 5.6 4.1 4.2
(Queensl.) 10.3 20 1.5 14.2 9.6 5.3 3.9 2.9 2.6
1974 10.8 20 6.0 15.5 8.6 5.2 3.0 1.8 1.6
Australia 800 11.5 26-28 20 0.6 9.3 6.4 4.8 4.0 4.1 3.7
(N.S.W.) 11.3 20 1.5 8.6 6.3 4.9 3.8 3.0 2.5
1974 10.9 20 6.0 10.7 8.1 5.7 4.9 4.6 3.3
(aerated) Australia 550 12.4 (note 1) 15 0.1 9.7 5.0 3.6 2.3 1.4 1.6
(Queensl.) 11.4 15 2 9.0 5.6 4.2 2.6 2.1 2.6
11.0 15 4 9.6 5.7 4.8 3.6 2.8 2.2
11.0 15 6 11.9 6.0 5.3 3.3
not 10.9 (note 2) 15 0.1 11.4 6.0 3.7 2.3 1.6 1.5
aerated 10.2 2 9.8 6.1 3.6 1.6 0.75 0.8
10.4 4 10.0 3.8 1.7 1.6 0.6 0.6
9.9 6 10.4 4.8 2.6 1.8
1 Mean temperature in silo first 18 weeks 25-31°C; from 23-41 week 14-23°C.
2 Mean temperature in silo first 18 weeks 27.5-34°C; from 23-41 week 17-20°C.
TABLE 9. Dissipation of methacrifos in small grains
Large scale trials in silos. (Formica, 1978a, 1978b).
commodity country size storage conditions dosage sampling residues of methacrifos after
of lot % moisture temp. °C rate depth ..... weeks in mg/kg
(tons) mg/kg ..... m 0 45 70 87 171/ 322/ 430 463 504 560
180 339
Barley Switzerland 51 2-25 22.3 2 13.8 7.6 2.6 3.8 2.5 1.5 0.86
(3.0- (7.8- (2.9- (2.9-
1.75) 1.6) 1.7) 0.3)
Barley Spain 100 11.6 13-26 10 2 4.1 3.7 3.2 1.3 2.8 1.7
Wheat Spain 100 11.0 13-26 10 2 9.4 5.6 3.0 2.8 2.1 1.0
The effect of aeration was also evaluated in a residue trial carried
out under Swiss conditions. A bin of barley treated with methacrifos
at 10 mg/kg was aerated twice during the storage period (after one and
three weeks) and another bin in the same warehouse was not aerated.
The residue on the aerated barley was 12.4 mg/kg at the beginning of
the experiment, 6.6 mg/kg after one week; 4.6 mg/kg after 32 weeks and
2.0 mg/kg after 74 weeks. The residues in the non-aerated bin were
11.3, 8.6, 4.2 and 2.5 mg/kg at the same sampling times, showing that
aeration had little or no effect on the residue dissipation. The
residue in oats stored at the same farm (without aeration ) were 10.2,
9.0, 6.0 and 3.7 mg/kg at these sampling times. Comparable results
were obtained in a similar trial on wheat in Switzerland in which one
bin was aerated after 54, 103 and 142 days post-treatment, by
circulating the wheat through the conveyer system and back to the
silo, and another bin not aerated. The initial residues were 6.0 and
6.1. Residues after the three aerations were 4.2, 3.7 and 3.4 mg/kg
on the aerated wheat and 4.8, 4.2 and 3.9 mg/kg on the nonaerated.
Again the rotation and resulting aeration of the wheat had little, if
any, influence on the dissipation rate (Formica, 1978d, 1978e). The
residue dissipation at different levels in a silo was studied in
Switzerland under typical European continental storage conditions with
temperatures of about 5°C in winter and 22°C in summer. Methacrifos
was mixed with the grain in a closed conveyer system, at a rate of 11
mg/kg; there was no ventilation during the storage period. The
initial concentration was 50 - 70% of the nominal value calculated
from the application rate and the volume treated. The residue at 2 m
depth increased during the following 5 weeks to almost the nominal
value and slowly decreased thereafter. It is assumed that the active
ingredient was not homogeneously dispersed immediately after
application but became more uniformly distributed through migration.
From a comparison of the residues in the samples taken from the
surface and at 2 m depth, it seems evident that the dissipation was
somewhat slower in the interior than on the surface of the stored
grain (Formica, 1976f). (Table 10).
In a study of the influence of the formulation on dissipation an
aqueous formulation showed more rapid dissipation than a formulation
with kerosene. After treatment with a nominal 10 mg/kg the residues
of the aqueous formulation were 0.2/0.16 and 0.15/0.25 mg/kg after 2
and 15 weeks respectively, whereas the residues of the kerosene
formulation were 9.1/9.3 and 4.0/3.5 mg/kg.
TABLE 10. Dissipation of methacrifos in wheat (Switzerland)
Days after
treatment 1 7 21 35 77 162 252 324 470 526 653
samples from
Surface 5.3 3.3 3.2 8.6/6.1 5.9/5.9 4.0/4.0 3.5 2.4 1.3 0.7 -
2 metres 4.6/3.9 5.2 6.0 10.1/7.4 6.7/5.6 6.2/6.5 5.2 4.0 2.6 2.8 1.4/2.25
Maize
Several small-scale trials were carried out with maize kernels and/or
cobs stored under various conditions of temperature and moisture
content. Although the distribution of the active ingredient was
rather inhomogeneous, it was evident, as with the other grains, that
the dissipation is dependant on time and temperature.
Under tropical conditions (temperature 35°C and higher) the residue
decreased much more rapidly than at moderate temperatures (Giannone
and Formica, 1979b).
The dissipation of methacrifos in maize was studied in a large scale
trial in Switzerland; 80 tons of maize kernels (moisture content
14.5%) were treated at 10 mg/kg on a conveyer system during the
filling of concrete silo cells, which were closed without ventilation.
Results are shown in Table 11.
TABLE 11. Dissipation of methacrifos in maize
Temp. in silos °C1 19 9 14 27
Days after treatment 6 76 300 376
Residue, mg/kg 7.8 3.62 3.9/3.4 0.4/0.7
(5.7-9.6) 3.1-4.2
1 Temperature at a depth of 2 metres.
2 Mean value of 4 samples; two samples analysed 5 months later than
the other two samples; samples stored at -20°C.
Rice
Small-scale trials on the dissipation of methacrifos under various
storage conditions were carried out in Australia. Brown and polished
rice were treated at the rate of 10 mg/kg and stored in bags, at
temperatures of 13-26°C. The moisture content of the brown rice was
approximately 14.2% and of the polished rice 14.4%. The initial
residue of 9.1 mg/kg in brown rice decreased to 5.0 mg/kg after 12
weeks and to 3.9 mg/kg after 24 weeks. In the polished rice the
corresponding residue levels were 7.9, 6.3 and 4.4 mg/kg respectively.
In other trials paddy rice, brown rice and polished rice were stored
in cardboard drums, and paddy rice also in mini-silos of about six
tons capacity. The decrease of the residue in paddy rice stored at
about 32°C was faster than in brown or polished rice. Under the
conditions occurring in the trials the dissipation rate of methacrifos
was independent of the application rate (Hart and Moore 1975;
McDougall 1976b) (Table 12).
TABLE 12. Dissipation of methacrifos in rice (McDougall, 1976b; Hart and Moore, 1976
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
mini-silo moisture temp. °C mg/kg
or cardboard %
Type of rice drums 0 12 23 54/51 124/121 166 205
paddy rice silo 13.7 17-26 10 8.2 5.8 4.7 3.7 2.6
paddy rice drums 8.8-12.8 32 10 10.8 6.2 4.4 2.2 1.2
drums 8.8-12.8 32 5 4.5 2.6 1.9 0.9 0.5
brown rice drums 8.8-12.8 32 10 9.1 7.0 5.5 3.8 2.7
drums 8.8-12.8 32 5 3.7 3.2 2.4 1.2 1.0
polished rice drums 8.8-12.8 32 10 13.0 9.6 7.2 4.6 2.7
drums 8.8-12.8 32 5 4.7 3.9 3.2 1.6 1.0
TABLE 13. Dissipation of methacrifos in sorghum
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
silo or moisture temp. °C mg/kg
Country bags % 0 15 21 45 56 90 147
Australia silo 13 27 15 13.6 9.3 7.7 6.3
13 27 15 9.1 4.1 7.1 4.3
13 27 7.5 8.0 4.7 4.9 2.7
Mali bags 25-30 10(dust) 3.6 3.0 1.9
25-30 10(solution) 6.1 4.2 2.1
TABLE 14. Dissipation of methacrifos in peanuts, stored in drums (McDougall, 1976a)
Storage conditions dosage Residues of methacrifos in mg/kg
rate after ..... days
moisture % temp. °C mg/kg
Country % 7 12 28 56 84 126 175 210
Peanut
whole 7.8-4.4 32 20 14.2 12.0 9.0 6.6 4.7 3.2 2.2 2.2
kernel 0.6 1.5 1.7 0.3 0.3 0.4 1.0 0.4
shell 50.8 49.5 30.6 30.5 29.2 10.3 8.8 7.7
Peanut
whole 7.8-4.4 32 10 7.1 6.5 4.2 2.4 2.8 1.8 1.2 1.1
kernel 0.6 0.3 <0.3 0.3 <0.3 <0.3 <0.3 <0.3
shell 41.5 21.5 23.7 12.9 7.3 7.3 4.1 5.7
Sorghum
Data are available from large-scale trials in Australia where sorghum
treated at 7.5 and 15 mg/kg was stored in silo bins and from Mali
where sorghum treated according to the so-called "sandwich method"
with a dust or a spray was stored in bags. The rate of dissipation
was similar to that in the other grains (Hart and Moore 1976, Giannone
and Formica, 1980) (Table 13).
Pulses (dried legume vegetables)
In a small-scale trial in Switzerland, a mixture of beans and peas
(Vicia faba, Phaseolus nanus and Pisum sativum) was
treated with a dust (10 or 20 mg/kg ai/kg) or a liquid formulation (10
mg ai/kg) and stored in drums at 25 ± 1°C; moisture content before
treatment was 9.8%. Three hours after the application the residues in
the lots treated with the dusts at 20 and 10 mg ai/kg and the solution
at 10 mg ai/kg were 5.5, 3.9 and 2.7 mg/kg respectively. After 27
days the residue levels in these lots were 6.5, 2.6 and 2.6 mg/kg and
after 142 days 3.5, 1.7 and 1.7 mg/kg respectively.
In a small-scale trial in Nigeria, cowpeas were treated with 10 mg/kg
methacrifos and stored in open jute bags under tropical conditions.
No residues (<0.12 mg/kg) were found 65 and 92 days after treatment.
Peanuts
A mini-silo (drum) trial was carried out in Australia. Methacrifos
was applied at 10 and 20 mg/kg to whole peanuts, which were stored in
120 l cardboard drums at 32°C. On all sampling dates kernels and the
shells were analysed separately. The results show that nearly all the
residue is contained in the shell (McDougall, 1976).
In Mali, peanut kernels were treated with 10 mg/kg methacrifos and
stored in closed plastic bags under tropical conditions (25-30°C).
Residues after 15, 60 and 90 days were 7.6, 5.6, 5.2 mg/kg
respectively (McDougall 1976).
Cocoa-beans
Cocoa-beans stored in closed jute bags in a warehouse were treated
with methacrifos at 0.4 g ai/m2 on the outside of the bags. Samples
were taken at four sampling dates from the outside layer of cocoa-
beans, which had been exposed to the treatment, and from the interior
of the bags. The residues in samples from near the outside of the
bags were higher than in the internal samples; 34 days after treatment
the residues in the outer samples were 1.0 and 1.2 mg/kg and in the
inner samples <0.025. After 98 days the levels were 0.83 and 0.32
mg/kg respectively.
The methacrifos residues were found mainly in the peel. 34 days after
treatment the residues in the whole beans were 1.0 and 1.2, in the
peel 5.6 and in the kernel 0.5 mg/kg (Formica, 1978c).
Coffee beans
Raw coffee beans imported from Nigeria were treated in Switzerland
with methacrifos in 10 and 20 mg/kg. The treated beans were stored in
closed drums at 20° ± 2°C. After 4 months storage the residues from
the 20 mg/kg treatment were 2 mg/kg but 0.5 mg/kg in the lot treated
with 10 mg/kg (Renfer, 1976).
FATE OF RESIDUES
In animals
General
As grain treated with methacrifos and grain products derived from
treated grain may be used as animal feed, it was necessary to study
the fate of methacrifos in livestock animals and to demonstrate that
no significant residue would remain in livestock and poultry, milk or
eggs. Special trials therefore, were carried out to study possible
carry-over from residues in feed to tissues or products of animal
origin.
Chickens
In a preliminary experiment, four young male broilers were given a
single oral dose of 20 mg/kg methacrifos by capsule. The birds were
slaughtered 6, 12, 24 and 48 hours after dosing. Samples of fat,
muscle, liver, kidney, heart, gizzard and skin were analysed. The
residues in all muscle and heart samples were 0.01 mg/kg or less.
Residues in the other samples decreased very rapidly (Moore and
McDougall, 1974c) (Table 15).
TABLE 15. Residues of methacrifos in chicken tissues after single
oral treatment with 20 mg/kg
Tissue Residues in mg/kg
time (hours post-treatment)
6 12 24 48
Fat 0.22 0.06 <0.01 <0.01
Liver 0.02 0.01 0.01 <0.01
kidney 0.08 <0.03 <0.03 <0.03
Gizzard 0.42 <0.01 <0.01 <0.01
Skin 0.06 0.02 <0.01 <0.01
Muscle <0.01 <0.01 <0.01 <0.01
Heart 0.01 <0.01 <0.01 <O.01
In a further experiment 5 groups of 77 one-day old chicks each were
given feed fortified with methacrifos at levels of 0, 10, 100, 250 or
500 mg/kg for 63 days. At the end of the feeding period 20 chickens
from each group (10 males and 10 females) were slaughtered and the
combined muscles, skin, kidney and liver used for analysis. In all
tissues examined residues were less than 0.01 mg/kg (Formica, 1974).
Eggs
Three groups of 15 laying hens were fed a diet containing 0, 10 or 20
mg/kg methacrifos. Egg samples were collected one day before
treatment, on the 16th day of treatment and 0, 2, 3, 7 days after the
end of the treatment. In none of the samples were residues found
(<0.01 mg/kg) (Schnabel and Formica, 1979).
Milk
Two lactating cows were given 30 mg methacrifos by capsule daily for
10 days. This dose corresponds to about 3 kg of cereals treated at a
rate of 10 mg/kg daily in the total ration. Pooled morning and
evening milk samples were taken before, during the feeding period and
three days after its completion. No residues (<0.001 mg/kg) could be
detected in the milk of the treated cows before, during or after the
10 day period (Formica, 1977).
In plants
Stored grain
The degradation of carbonyl 14C methacrifos (specific radioactivity
31.1 µCi/mg) in grain treated with 10 mg/kg was studied under
laboratory conditions for one year; 84-94% of the applied
radioactivity was recovered at all sampling times, indicating very
limited volatility of methacrifos or its degradation products. After
one year about 25% of the then recovered radioactivity was present as
parent compound. The fact that little volatilisation of the
radioactivity occurred during the storage indicates that methacrifos
and its degradation products were strongly absorbed to the wheat
grain. Less than 15% of the recovered radioactivity could be washed
off the surface of the grain with methylene chloride. The bulk of the
radioactivity could be extracted only after grinding of grain. The
non-extractable portion of radioactivity was low at the early sampling
dates but rose to 9-14% after 140 and 385 days. The low but
continuous evolution of 14C during the experiment may demonstrate
that the insecticide is eventually metabolised by the wheat grain.
The organosoluble radioactivity on or in the grains consisted mainly
of the parent compound. The oxon was not detected. Mono- and
di-demethylated methacrifos were detected by ethylation followed by
GLC analysis. Their identity was confirmed by methylation with
deuterated diazomethane (CD2N2) followed by GCMS.
The results give strong evidence for the degradation pathways shown in
figure 2 (Blattmann 1978).
Figure 2. Degradation of methacrifos in grain
 The decrease of the protective effect of methacrifos against insects
is closely related to the decrease of the amount of the unchanged
methacrifos still present. This suggests that the degradation
products are less toxic to insects than the parent compound (Renfer
1977).
TABLE 16. Distribution and characterization of radioactivity in
stored wheat after the application of 10 mg/kg 14C-methacrifos
% of radioactivity recovered after
storage for (days)
Compounds 0 23 140 294 385
Extractable
methacrifos parent 99.7 98.5 51.6 33.3 25.2
demethylated
methacrifos-derivatives - - 34.1 45.4 6O.2
unknowns - - 5.1 6.8 2.9
Non-extractable 0.3 1.5 8.7 13.8 10.9
CO2 - 0.02 0.5 0.7 0.8
The formation of the demethylated degradation products has been
studied in various products stored under practical conditions. The
results are summarized in table 17.
TABLE 17. Formation of the demethylated metabolites during storage (treatment in all
cases 10 mg/kg)
Storage after Residues mg/kg Reference
treatment methacrifos demethylated
days metabolites
Wheat grain 215 2.7/2.9 1.6/1.8 Formica, 1978d
365 1.7/1.8 1.7 Formica, 1978c
430 1.0 2.4 Formica, 1978b
Barley grain 76 4.2 2.0 Giannone and Formica, 1980a
300 3.0 4.2 " " "
370 1.5 3.8 " " "
430 1.3 2.5 Formica, 1978b
Maize grain 76 6.4 1.6 Giannone and Formica, 1980a
300 4.1 2.0 " " "
370 0.7 5.0 " " , 1979
Maize cob1 231 3.5/3.7 11.4/11.6 Giannone and Formica, 1979
Sorghum grain 15 6.1 1.3 Giannone and Formica, 1980b
45 4.2 0.7 " " "
90 2.1 1.4 " " "
Peanut kernels 15 7.6 0.2 Giannone and Formica, 1980c
45 5.6 0.5 " " "
90 5.2 0.6 " " "
Cocoa beans 15 0.44/0.41 O.27/0.29 Giannone and Formica, 1979a
1 Cobs stored in open wired cribs were treated by spraying the surface of cribs with 10 mg
ai/kg related to the total quantity of maize cobs. As the samples were taken from the periphery
of the crib a rate of their treatment higher than 10 mg/kg must be expected.
In processing
Residues of methacrifos have been followed through milling and baking
procedures in several experiments. As the insecticide is primarily
located in the outer layers of the grain, most of the residues are
found in the bran and the low grade flour fractions after milling.
The residue in flour milled 1 month after the treatment of the grain
was about 1/10 of that in whole grain. After prolonged storage the
residues in flour tend to increase, but they do not exceed 1 mg/kg
even after storage for 21 months. In the baking process methacrifos
and its metabolites are degraded to a large extent resulting in low or
undetectable residues (maximum about 0.1 mg/kg or lower). (Table 18).
TABLE 18. Residues of methacrifos in milled wheat fractions and bread
Residues (mg/kg) in
Treatment Storage
mg/kg time whole wheat white flour low grade flour bran white bread Reference
flour
10 1 month 4.0/3.8 0.4/0.4 1.7/1.6 7.6/6.4 <0.03 Formica 1978b
10 7 months 3.1/2.9 0.6/0.6 8.1/8.4 10.0/9.2 0 01/<O.01 " 1978d
10 21 months 1.4/1.6 1.0/0.8 10.3/9.1 7.4/7.5 0.02/0.08 " 1978f
15 1 month 7 8/7.8 0.6/0.6 2.5/2.7 10.4/11.2 <0.03 " 1978b
20 1 month 8.0/7.4 0.9/0.85 3.1/3.2 15.6/18.0 0.03/0.04 " 1978h
10 265 days 0.751 ca. 0.30 2.6 <0.04 Tournayre 1978
5 265 days 0.261 ca. 0.10 0.8 <0.04 " "
10 265 days 2.01 ca. 0.50 6.1 <0.04 " "
1 after 252 days
In experiments with the radio-labelled insecticide it was shown that
the same degradation products are formed during baking as are found in
stored grains (Blattmann 1978).
Further data on the effect of processing rice, oats and barley,
including malted barley are presented in table 19. In these
experiments the initial concentrations are in most cases higher than
recommended. As with wheat, the milling of paddy rice treated with
methacrifos resulted in a considerable loss of insecticide. More than
90% of the initial residue was removed with the hulls (McDougall
1976), resulting in a decrease of the residue in going from paddy to
brown rice by a factor of 5 to 8. Most of the insecticide present in
the brown rice was again removed with the bran fractions.
The amount of methacrifos residue is further decreased on cooking.
Short cooking of about 15 minutes decreased the residue by a factor of
about 2.6 whereas the more usual cooking of 25 minutes resulted in a
reduction by a factor of about 8.
Processing treated barley grain to malt, which was then used to brew
beer, gave very low level in the beer.
The processing of treated cocoa beans to cocoa mass decreased the
initial methacrifos on the beans at least by a factor of 10. Since
further diluting processes take place the overall decrease of
methacrifos from the raw product to the consumed chocolate is even
higher.
METHODS OF RESIDUE ANALYSIS
Residue of the parent compound in:
Cereal grains and grain derivatives. Methanol or acetone extracts can
be used to determine the active ingredient by GLC without cleanup.
Recoveries range from 80-115%; the limit of determination is about
0.05 mg/kg. (Desmarcherlier et al 1977; Moore and McDougall, 1973
and Formica, 1973g).
Chicken tissues. Residues in chicken tissues are determined by GLC
with a phosphorus-specific detector after extraction with acetonitrile
and clean-up by partition (Formica 1974b).
Milk. Milk is coagulated with acetone. After a partition step,
methacrifos is determined by GLC with a phosphorus-specific detector.
'Total residue':
A method has been developed to determine unchanged methacrifos and the
dealkylated metabolites in a two-step procedure in cereal grains, eggs
and cocoa mass. Extraction with aqueous methanol is followed by
partition of the parent compound into hexane. The dealkylated
metabolites are then extracted from the aqueous phase with methyl
acetate and methylated with diasomethane. The residues are determined
by GLC with a phosphorus-specific detector. Limits of determination
are 0.025 mg/kg for the parent compound and 0.04-0.1 mg/kg for the
dealkylated product.
Both the method for residues of the parent compound in cereal grains
and the method for the total residue are suitable or can be adapted
for regulatory purposes.
TABLE 19. Effect of processing on methacrifos residues
Raw product Treatment Storage Residue before Processing or Residue after
level processing processed product processing
mg/kg mg/kg mg/kg
Paddy rice 18 6 months 3.1 brown rice raw 0.65
cooked 15 min. 0.23
white rice raw 0.12
cooked 15 min. <0.02
Paddy rice 10 3 months 3.0 brown rice 0.36
hulls 18.4
white rice <0.2
bran 2.7
Hulled rice 18 3 months 7.1 cooked 5 min. 4.4
cooked 15 min. 2.7
18 6 months 4.8 cooked 25 min. 0.75
Polished rice 18 3 months 7.5 cooked 15 min. 2.6
Oats 18 3 months 4.7 cooked 15 min. 0.9
Barley 18 3 months 4.8 malted barley 0.1
Malt1 10 - 9.8/10.1 brewer's grains 1.7/2.0
wort before boiling 0.27/0.31
yeast <0.1
beer <0.01
Cocoa beans 10 7 days 5.0/5.2 cacao mass 0.46/0.44
surface of
bags with 97 days 0.32 cacao mass 0.02
0.4 g a.i./m2
1 Malt has been directly treated to follow the dissipation during further processing. Under normal
practical conditions only barley grain is to be treated.
EVALUATION
COMMENTS AND APPRAISAL
Methacrifos is an organophosphorous insecticide which is almost
exclusively used as a grain protectant and as a control agent against
insect pests in other stored products. It may be used as an acaricide
in the same stored commodities. It gives good and long-lasting
protection of grain and other stored products against a broad spectrum
of pests including those which show resistance to commonly used stored
products insecticides such as other organophosphorous compounds,
lindane, etc. The major formulations are emulsifiable concentrate,
solutions in othanic solvents and dusts. The normal application rate
is about 10 mg ai/kg grain. Lower rates may be used at lower
temperatures; exceptionally a higher rate up to 20 mg/kg may be
needed.
Methacrifos is rapidly absorbed, metabolised and excreted in mammals.
Two metabolites detected in urine and faeces were identified as
N-acetyl-S(2-methoxycarbonylprop-1-enylcysteine) and P-O-desmethyl
methacrifos. Neither methacrifos nor its metabolites were found to
accumulate in the tissues.
Methacrifos has a moderate acute toxicity following oral
administration to various animal species. Signs of poisoning are
typical of cholinesterase inhibitors. The acute toxicity was found to
be potentiated by several other organophosphorous esters.
Methacrifos did not induce a delayed neurotoxic response in hens. In
short- and long-term studies methacrifos caused signs and symptoms of
cholinesterase inhibition, which were associated with effects on body
weight gain and food consumption. Brain cholinesterase activity was
inhibited at 100 mg/kg. In rats at high dose levels, effects on
several haematological parameters were observed. Cholinesterase
activity in plasma was inhibited at doses above 10 mg/kg and recovery
of cholinesterase activity was relatively slow.
In long and short term studies, cholinesterase depression was observed
at 10 mg/kg and above. A no-effect level of 1 mg/kg was observed.
Because of parental mortality in a 3-generation reproduction study and
foetal death in teratology bioassays, the meeting could not evaluate
fully the potential effects in these areas. Further studies are
required for a full evaluation of this compound.
Mutagenicity studies with bacteria, a mouse carcinogenicity study and
a rat teratology study were all negative.
Acceptable data were available to allow the meeting to establish
no-effect levels in two mammalian species and a temporary ADI was
allocated.
The fate of methacrifos in stored cereals, beans, peanuts and cocoa
has been elucidated in extensive laboratory trials as well an in many
supervised small and large-scale trials under practical conditions in
various countries and under a variety of storage conditions. The
following conclusions can be drawn.
The rate of degradation depends on time and storage conditions. The
residues decrease faster with increasing temperature and moisture
content of the food commodity. Other factors, such as grain variety,
rate of application, application technique and type of formulation,
have little or no influence on the dissipation rate. Aeration of the
stored cereals has only a minor effect on the rate of loss.
The metabolic pathways of methacrifos on grain and other stored
products were elucidated. The compound is demethylated to mono- and
di-desmethylated methacrifos; there is some evidence that these
metabolites are not cholinesterase inhibitors. These are further
metabolised to CO2 at a slow rate.
The residues remain primarily on the outer layers of the grain.
During prolonged storage only limited penetration into the endosperm
takes place. Processing of the raw commodities, such as milling of
cereals, dehusking and polishing rice, removing shells and seed-coats
from peanuts and shells from cocoa, reduce the residue considerably.
During baking and brewing methacrifos and its main metabolites are
further degraded, resulting in residues at or about the limit of
determination in bread and beer. Degradation also occurs during
cooking to an extent that depends upon the time of cooking.
When grains treated at normal rates are fed to livestock animals or
poultry, no residues are found in meat, milk or eggs.
Methods of residue analysis are available for methacrifos residues
(parent compound) in stored products by GLC with a
phosphorous-specific thermionic detector after extraction with
methanol or acetone. The limit of determination is about 0.05 mg/kg.
Methods are also available for methacrifos in meat of poultry, eggs
and milk after extraction with acetonitrile or acetone followed by
various partition steps and GLC with a phosphorous detector; the limit
of determination is 0.01 mg/kg. The methods are suitable or can be
adapted for regulatory purposes.
Residues of the unchanged parent compound and the dealkylated
metabolites can be determined, in a two step procedure, in cereal
grains, eggs and cocoa-mass. The limits of determination are about
0.025 mg/kg for the parent compound and 0.04 - 0.1 for the dealkylated
metabolites. The method can be adapted for regulatory purposes.
When establishing maximum residue limits for methacrifos on grain or
other stored food commodities, the following factors have to be
considered.
1. The residue level depends largely on the application rate, which
has to be chosen according to the nature of the insect or mite
infestation and the desired period of protection. Variation in
residue levels are to be expected among different countries even when
the same dosages are applied and the same storage period is used,
since the rate of dissipation may differ according to the local
climate and storage conditions.
2. The distribution of the insecticides may be heterogeneous,
depending on local application techniques.
3. Stored products may move in international trade at different times
after treatment according to commercial need.
Based on these considerations the maximum residue limits have to be
set at levels that will cover all situations encountered after the
normal applications, even if in some cases the actual residues will be
much lower.
The meeting examined residue data from supervised trials reflecting
established and/or anticipated good storage practice on a number of
stored food or feed commodities. From these data the meeting was able
to estimate the maximum residue levels that were likely to occur when
methacrifos is used in practice.
Level causing no toxicological effect
Rat: 1 mg/kg in the diet equivalent to 0.05 mg/kg bw/day.
Dog: 1 mg/kg in the diet equivalent to 0.025 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.0003 mg/kg bw.
RECOMMENDATION OF RESIDUES LIMITS
The meeting concluded that the maximum residue levels listed below are
suitable for establishing maximum residue limits.
These levels refer to the parent compound only.
Commodity Estimated maximum
residue levels
mg/kg
cereal grains 10
wheat flour (whole meal) 10
wheat flour (white) 2
wheat bran (unprocessed) 10
beans and peas (dry) 5
cocoa beans 10
peanuts (including shell) 10
peanuts 1
poultry meat 0.011
eggs 0.011
milk 0.011
1 The levels refer to the maximum residue levels which might occur
when the animals concerned are fed with commodities treated during
storage with recommended amounts of methacrifos.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Teratogenicity studies in rat and rabbits.
2. An appropriate reproduction study.
REFERENCES
Arni, P. and Müller, B. Samonella/mammalian-microsome mutagenicity
test with CGA 20168. (1979) Unpublished report, May 23, submitted by
Ciba-Geigy to WHO.
Basler, W. et al. Two-year chronic oral toxicity study with
CGA-20168 technical in albino rats. (1980) Unpublished report prepared
by Ciba-Geigy from the original Industrial Bio-test Laboratories
records, May 15, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the rat. (1974a)
Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the mouse.
(1974b) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute dermal LD50 of technical CGA 20168 in the rat.
(1974c) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe R. and Sachsse, K. Acute oral LD50 in the dog of technical CGA
20168. (1975) Unpublished report, November 12, submitted by Ciba-Geigy
to WHO.
Bathe, R. and Sachsse, K. Acute oral LD50 in the mouse of technical
CGA 20168. (1978) Unpublished report, August 15, submitted by
Ciba-Geigy to WHO.
Bathe R. et al. 26 weeks feeding study in dogs with technical CGA
20168. (1977) Unpublished report, March 4, Submitted by Ciba-Geigy to
WHO.
Charles, J.M. et al. Three-generation reproduction study with CGA
20168 technical in albino rats. (1980a) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-Test Laboratories Records,
April 10, submitted by Ciba-Geigy to WHO.
Charles, J.M. et al. A chronic oral carcinogenic toxicity study with
CGA 20168 in Swiss white mice. (1980b) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-test Laboratories records,
May 15, submitted by Ciba-Geigy to WHO.
Chesterman, H. et al. CGA 20168, 28-day dietary feeding study in
beagle dogs. (1975) Unpublished report from Huntingdon Research
Centre. November 28. Submitted by Ciba-Geigy to WHO.
Drake J.C. 1 Month dietary toxicity study in rats with compound CGA
20168. (1975) Unpublished report from Geigy Pharmaceuticals, February
17, Submitted by Ciba-Geigy to WHO.
Fritz H. et al. Reproduction study on CGA 20168 Technical. Rat.
(1978) Unpublished report, June 14, submitted by Ciba-Geigy to WHO.
Gfeller, W. CGA 20168 Oral toxicity in pigs. Daily administration for
28 days. (1974) Unpublished report from Ciba-Geigy Agricultural
Research Centre, January 28, submitted by Ciba-Geigy to WHO.
Hamböck, H. Metabolism of CGA 20168 in the rat. (1978) Unpublished
project report 27/78 from the Agrochemicals Division of Ciba-Geigy,
May 17, submitted by Ciba-Geigy to WHO.
Hool, G. and Müller, D. Dominant lethal study on CGA 20168. (1980)
Unpublished report, February 6, submitted by Ciba-Geigy to WHO.
Ifflaender, V. and Mücke, W. Distribution, degradation and excretion
of CGA 20168 in the rat. (1975) Unpublished report from the
Agrochemicals Division of Ciba-Geigy, April 9, submitted by Ciba-Geigy
to WHO.
Sachsse, K. and Bathe, R. Potentiation study. CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion. (1978)
Unpublished report, June 23, submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Acute oral toxicity and neurotoxicity study of
technical CGA 20168 in the domestic fowl (Gallus domesticus). (1979)
Unpublished report, November 6, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. Acute toxicity to rainbow trout, crucian
carp, catfish, bluegill and guppy of technical CGA 20168. (1974a)
Unpublished report, August 7, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. "8-Day-feeding toxicity" of technical CGA
20168 in the Japanese quail. (1974b) Unpublished report, July 13,
submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Technical CGA 20168. 21-Day percutaneous toxicity
study in rabbits. (1978) Unpublished report, April 24, submitted by
Ciba-Geigy to WHO.
Strittmatter, J. and Gfeller, W. 63 Days dietary toxicity study in
chicks with compound CGA 20168. (1975) Unpublished report from
Ciba-Geigy Agricultural Research Centre, March, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Skin sensitizing (contact allergenic)
effect in guinea pigs of CGA 20168. (1975) Unpublished report, July 1,
submitted by Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute inhalation toxicity in the rat of
technical CGA 20168. (1976) Unpublished report, July 7, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute dermal LD50 in the rabbit of
technical CGA 20168. (1977) Unpublished report, February 2, submitted
by Ciba-Geigy to WHO.
Ullmann, L. et al. 21-Day inhalation study on the rat with technical
CGA 20168. (1977) Unpublished report, December 16, submitted by
Ciba-Geigy to WHO.
REFERENCES ON RESIDUES IN FOOD AND THEIR EVALUATION
Blattmann, P. Degradation of CGA 20168 in stored wheat grains,
including their processing. (1978) Unpublished report PR 42/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Desmarchelier, J.M., Bengston, M., Connel, M., Minett, W., Moore, B.,
Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A collaborative
study of residues on wheat of chlorpyrifos-methyl, fenitrothion,
malathion, methacrifos and pirimiphos-methyl. 1. Method development.
Pestic. Sci. 8: 473-483.
Formica, G. CGA 20168. Gas chromatographic residue determination in
wheat grain, wheat meal, bran, groats and bread. (1973) Unpublished
report HEM 20/73 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. CGA 20168. Gas chromatographic residue determination in
chicken tissues. (1974) Unpublished report REM 9/74 from Ciba-Geigy
Limited, Basle, Switzerland.
Formica, G. CGA 20168. Residues in chicken tissues. (1974a)
Unpublished report RVA 205/74 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Degradation of CGA 20168 in wheat and maize. (1975)
Unpublished report RVA 314/75 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. CGA 20168. Residues in cow's milk after oral medication.
(1977) Unpublished report RVA 612/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Methacrifos (CGA 20168). Determination of CGA 20168 in
feedgrain treated with different formulations and stored in drums.
(1977a) Unpublished report RVA 615/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos in French barley after direct
spray treatment with the formulation EC 950. (1978a) Unpublished
report RVA 620/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat and barley after
direct spray treatment with the formulation EC 950. Carinena, Spain.
(1978b) Unpublished report RVA 626/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Residues of methacrifos in untreated cacao beans stored in
a warehouse and possibly contaminated through cacao beans treated with
DAMFIN(R) 950 EC, stored in the same warehouse. (1978c) Unpublished
report RVA 608/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of residues of methacrifos in stored grain
and its products seven months after direct spray treatment with the
formulation SO 050. (1978d) Unpublished report RVA 610/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat after direct spray
treatment with the formulation SO 050. (1978e) Unpublished report RVA
622/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain, and their products twenty-one months after direct spray
treatment with the formulation EC 950. (1978f) Unpublished report RVA
611/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in barley and oats after
direct spray treatment with the formulation EC 950. (1978g)
Unpublished report RVA 623/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain and their products after direct spray treatment with the
formulation EC 1000. (1978h) Unpublished report RVA 618/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Residue of methacrifos in maize cobs
after surface treatment with DAMFIN(R) 950 EC. (1979) Unpublished
report RVA 4010/79 from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic determination of
total residues in cacao liquor after different processings of cacao
beans treated with DAMFIN(R) SO 050. (1979a) Unpublished report RVA
4009/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic-determination of
methacrifos and its dealkylated metabolites in sorghum after treatment
with DAMFIN(R) SO 050 or DAMFIN(R) P 2. (1980) Unpublished report RVA
4045/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Hart, R.J. and Moore, B. The use of CGA 20168 for the protection of
rice stored in bags. (1975) Unpublished technical report No. 75/10/533
from Ciba-Geigy Australia Limited.
Hart, R.J. and Moors, B. The protection of stored grain sorghum from
insect attack with CGA 20168. (1976) Unpublished technical report No.
76/6/564 from Ciba-Geigy Australia Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168. (1974) Unpublished technical report No. 74/9/476 from
Ciba-Geigy Australia Limited.
McDougall, K.W. Mini silo trials with CGA 20168 - Series 2. (1975)
Unpublished technical report No. 75/8/519 from Ciba-Geigy Australia
Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168 - Part 2. Drum trials. (1976a) Unpublished technical report
No. 76/2/554 from Ciba-Geigy Australia Limited.
McDougall, K.W. The distribution and dissipation of CGA 20168 in
white, brown and paddy rice. (1976b) Unpublished technical report No.
76/2/553 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Determination of CGA 20168 residues in
wheat - Comparison of five analytical procedures. Appendix I: R+D
Department Method No. 129. Appendix II: C.S.I.R.O. Method A. Appendix
III: C.S.I.R.O. Method C. (1973) Unpublished technical report No.
73/11/425 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Further studies on factors affecting the
dissipation of CGA-20168 in wheat. (1974) Unpublished technical report
No. 74/3/433 from Ciba-Geigy Australia Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Insecticide Studies - Determination of residue dissipation. (1974a)
Unpublished technical report No. 74/7/461 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. The Australian Wheat Board Collaborative
Insecticide Studies Determination of residue dissipation. Part II.
(1974b) Unpublished technical report No. 74/9/477 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. CGA 20168 Residues in chickens. (1974c)
Unpublished technical report No. 74/7/462 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Studies 1975: The effect of aeration on the dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. Australia Wheat Board Collaborative
Studies 1975: The effect of aeration on the Dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Renfer, A. Biological evaluation of methacrifos as a grain protectant
under field conditions in Switzerland - I. Basle. (1977) Unpublished
report 9.25 VS 135 from Ciba-Geigy, Basle, Switzerland.
Schnabel, D. and Formica, G. Gas chromatographic determination of CGA
20168 in eggs following oral application via feed. (1979) Unpublished
report RVA 4011/79 from Ciba-Geigy Limited, Basle, Switzerland.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
residues. Proceedings of the First International Working Conference on
Stored-Product Entomology, Savannah, Georgia, USA. (1975).
Tournayre, J.C. Dissipation curve of CGA 20168 in wheat grain and
residues in tissues. (1978) Unpublished reports No. 13/78 A, B, C from
Ciba-Geigy Agrochemicals Division France.
Wyniger. R., Renfer, A., Knuesel, F. and Gfeller, W. Methacrifos - A
new insecticide for the control of insect pests in stored products.
Proc. Brit. Insect. Fungic. Conf. 9, Vol. 3: 1033 -1040.
The decrease of the protective effect of methacrifos against insects
is closely related to the decrease of the amount of the unchanged
methacrifos still present. This suggests that the degradation
products are less toxic to insects than the parent compound (Renfer
1977).
TABLE 16. Distribution and characterization of radioactivity in
stored wheat after the application of 10 mg/kg 14C-methacrifos
% of radioactivity recovered after
storage for (days)
Compounds 0 23 140 294 385
Extractable
methacrifos parent 99.7 98.5 51.6 33.3 25.2
demethylated
methacrifos-derivatives - - 34.1 45.4 6O.2
unknowns - - 5.1 6.8 2.9
Non-extractable 0.3 1.5 8.7 13.8 10.9
CO2 - 0.02 0.5 0.7 0.8
The formation of the demethylated degradation products has been
studied in various products stored under practical conditions. The
results are summarized in table 17.
TABLE 17. Formation of the demethylated metabolites during storage (treatment in all
cases 10 mg/kg)
Storage after Residues mg/kg Reference
treatment methacrifos demethylated
days metabolites
Wheat grain 215 2.7/2.9 1.6/1.8 Formica, 1978d
365 1.7/1.8 1.7 Formica, 1978c
430 1.0 2.4 Formica, 1978b
Barley grain 76 4.2 2.0 Giannone and Formica, 1980a
300 3.0 4.2 " " "
370 1.5 3.8 " " "
430 1.3 2.5 Formica, 1978b
Maize grain 76 6.4 1.6 Giannone and Formica, 1980a
300 4.1 2.0 " " "
370 0.7 5.0 " " , 1979
Maize cob1 231 3.5/3.7 11.4/11.6 Giannone and Formica, 1979
Sorghum grain 15 6.1 1.3 Giannone and Formica, 1980b
45 4.2 0.7 " " "
90 2.1 1.4 " " "
Peanut kernels 15 7.6 0.2 Giannone and Formica, 1980c
45 5.6 0.5 " " "
90 5.2 0.6 " " "
Cocoa beans 15 0.44/0.41 O.27/0.29 Giannone and Formica, 1979a
1 Cobs stored in open wired cribs were treated by spraying the surface of cribs with 10 mg
ai/kg related to the total quantity of maize cobs. As the samples were taken from the periphery
of the crib a rate of their treatment higher than 10 mg/kg must be expected.
In processing
Residues of methacrifos have been followed through milling and baking
procedures in several experiments. As the insecticide is primarily
located in the outer layers of the grain, most of the residues are
found in the bran and the low grade flour fractions after milling.
The residue in flour milled 1 month after the treatment of the grain
was about 1/10 of that in whole grain. After prolonged storage the
residues in flour tend to increase, but they do not exceed 1 mg/kg
even after storage for 21 months. In the baking process methacrifos
and its metabolites are degraded to a large extent resulting in low or
undetectable residues (maximum about 0.1 mg/kg or lower). (Table 18).
TABLE 18. Residues of methacrifos in milled wheat fractions and bread
Residues (mg/kg) in
Treatment Storage
mg/kg time whole wheat white flour low grade flour bran white bread Reference
flour
10 1 month 4.0/3.8 0.4/0.4 1.7/1.6 7.6/6.4 <0.03 Formica 1978b
10 7 months 3.1/2.9 0.6/0.6 8.1/8.4 10.0/9.2 0 01/<O.01 " 1978d
10 21 months 1.4/1.6 1.0/0.8 10.3/9.1 7.4/7.5 0.02/0.08 " 1978f
15 1 month 7 8/7.8 0.6/0.6 2.5/2.7 10.4/11.2 <0.03 " 1978b
20 1 month 8.0/7.4 0.9/0.85 3.1/3.2 15.6/18.0 0.03/0.04 " 1978h
10 265 days 0.751 ca. 0.30 2.6 <0.04 Tournayre 1978
5 265 days 0.261 ca. 0.10 0.8 <0.04 " "
10 265 days 2.01 ca. 0.50 6.1 <0.04 " "
1 after 252 days
In experiments with the radio-labelled insecticide it was shown that
the same degradation products are formed during baking as are found in
stored grains (Blattmann 1978).
Further data on the effect of processing rice, oats and barley,
including malted barley are presented in table 19. In these
experiments the initial concentrations are in most cases higher than
recommended. As with wheat, the milling of paddy rice treated with
methacrifos resulted in a considerable loss of insecticide. More than
90% of the initial residue was removed with the hulls (McDougall
1976), resulting in a decrease of the residue in going from paddy to
brown rice by a factor of 5 to 8. Most of the insecticide present in
the brown rice was again removed with the bran fractions.
The amount of methacrifos residue is further decreased on cooking.
Short cooking of about 15 minutes decreased the residue by a factor of
about 2.6 whereas the more usual cooking of 25 minutes resulted in a
reduction by a factor of about 8.
Processing treated barley grain to malt, which was then used to brew
beer, gave very low level in the beer.
The processing of treated cocoa beans to cocoa mass decreased the
initial methacrifos on the beans at least by a factor of 10. Since
further diluting processes take place the overall decrease of
methacrifos from the raw product to the consumed chocolate is even
higher.
METHODS OF RESIDUE ANALYSIS
Residue of the parent compound in:
Cereal grains and grain derivatives. Methanol or acetone extracts can
be used to determine the active ingredient by GLC without cleanup.
Recoveries range from 80-115%; the limit of determination is about
0.05 mg/kg. (Desmarcherlier et al 1977; Moore and McDougall, 1973
and Formica, 1973g).
Chicken tissues. Residues in chicken tissues are determined by GLC
with a phosphorus-specific detector after extraction with acetonitrile
and clean-up by partition (Formica 1974b).
Milk. Milk is coagulated with acetone. After a partition step,
methacrifos is determined by GLC with a phosphorus-specific detector.
'Total residue':
A method has been developed to determine unchanged methacrifos and the
dealkylated metabolites in a two-step procedure in cereal grains, eggs
and cocoa mass. Extraction with aqueous methanol is followed by
partition of the parent compound into hexane. The dealkylated
metabolites are then extracted from the aqueous phase with methyl
acetate and methylated with diasomethane. The residues are determined
by GLC with a phosphorus-specific detector. Limits of determination
are 0.025 mg/kg for the parent compound and 0.04-0.1 mg/kg for the
dealkylated product.
Both the method for residues of the parent compound in cereal grains
and the method for the total residue are suitable or can be adapted
for regulatory purposes.
TABLE 19. Effect of processing on methacrifos residues
Raw product Treatment Storage Residue before Processing or Residue after
level processing processed product processing
mg/kg mg/kg mg/kg
Paddy rice 18 6 months 3.1 brown rice raw 0.65
cooked 15 min. 0.23
white rice raw 0.12
cooked 15 min. <0.02
Paddy rice 10 3 months 3.0 brown rice 0.36
hulls 18.4
white rice <0.2
bran 2.7
Hulled rice 18 3 months 7.1 cooked 5 min. 4.4
cooked 15 min. 2.7
18 6 months 4.8 cooked 25 min. 0.75
Polished rice 18 3 months 7.5 cooked 15 min. 2.6
Oats 18 3 months 4.7 cooked 15 min. 0.9
Barley 18 3 months 4.8 malted barley 0.1
Malt1 10 - 9.8/10.1 brewer's grains 1.7/2.0
wort before boiling 0.27/0.31
yeast <0.1
beer <0.01
Cocoa beans 10 7 days 5.0/5.2 cacao mass 0.46/0.44
surface of
bags with 97 days 0.32 cacao mass 0.02
0.4 g a.i./m2
1 Malt has been directly treated to follow the dissipation during further processing. Under normal
practical conditions only barley grain is to be treated.
EVALUATION
COMMENTS AND APPRAISAL
Methacrifos is an organophosphorous insecticide which is almost
exclusively used as a grain protectant and as a control agent against
insect pests in other stored products. It may be used as an acaricide
in the same stored commodities. It gives good and long-lasting
protection of grain and other stored products against a broad spectrum
of pests including those which show resistance to commonly used stored
products insecticides such as other organophosphorous compounds,
lindane, etc. The major formulations are emulsifiable concentrate,
solutions in othanic solvents and dusts. The normal application rate
is about 10 mg ai/kg grain. Lower rates may be used at lower
temperatures; exceptionally a higher rate up to 20 mg/kg may be
needed.
Methacrifos is rapidly absorbed, metabolised and excreted in mammals.
Two metabolites detected in urine and faeces were identified as
N-acetyl-S(2-methoxycarbonylprop-1-enylcysteine) and P-O-desmethyl
methacrifos. Neither methacrifos nor its metabolites were found to
accumulate in the tissues.
Methacrifos has a moderate acute toxicity following oral
administration to various animal species. Signs of poisoning are
typical of cholinesterase inhibitors. The acute toxicity was found to
be potentiated by several other organophosphorous esters.
Methacrifos did not induce a delayed neurotoxic response in hens. In
short- and long-term studies methacrifos caused signs and symptoms of
cholinesterase inhibition, which were associated with effects on body
weight gain and food consumption. Brain cholinesterase activity was
inhibited at 100 mg/kg. In rats at high dose levels, effects on
several haematological parameters were observed. Cholinesterase
activity in plasma was inhibited at doses above 10 mg/kg and recovery
of cholinesterase activity was relatively slow.
In long and short term studies, cholinesterase depression was observed
at 10 mg/kg and above. A no-effect level of 1 mg/kg was observed.
Because of parental mortality in a 3-generation reproduction study and
foetal death in teratology bioassays, the meeting could not evaluate
fully the potential effects in these areas. Further studies are
required for a full evaluation of this compound.
Mutagenicity studies with bacteria, a mouse carcinogenicity study and
a rat teratology study were all negative.
Acceptable data were available to allow the meeting to establish
no-effect levels in two mammalian species and a temporary ADI was
allocated.
The fate of methacrifos in stored cereals, beans, peanuts and cocoa
has been elucidated in extensive laboratory trials as well an in many
supervised small and large-scale trials under practical conditions in
various countries and under a variety of storage conditions. The
following conclusions can be drawn.
The rate of degradation depends on time and storage conditions. The
residues decrease faster with increasing temperature and moisture
content of the food commodity. Other factors, such as grain variety,
rate of application, application technique and type of formulation,
have little or no influence on the dissipation rate. Aeration of the
stored cereals has only a minor effect on the rate of loss.
The metabolic pathways of methacrifos on grain and other stored
products were elucidated. The compound is demethylated to mono- and
di-desmethylated methacrifos; there is some evidence that these
metabolites are not cholinesterase inhibitors. These are further
metabolised to CO2 at a slow rate.
The residues remain primarily on the outer layers of the grain.
During prolonged storage only limited penetration into the endosperm
takes place. Processing of the raw commodities, such as milling of
cereals, dehusking and polishing rice, removing shells and seed-coats
from peanuts and shells from cocoa, reduce the residue considerably.
During baking and brewing methacrifos and its main metabolites are
further degraded, resulting in residues at or about the limit of
determination in bread and beer. Degradation also occurs during
cooking to an extent that depends upon the time of cooking.
When grains treated at normal rates are fed to livestock animals or
poultry, no residues are found in meat, milk or eggs.
Methods of residue analysis are available for methacrifos residues
(parent compound) in stored products by GLC with a
phosphorous-specific thermionic detector after extraction with
methanol or acetone. The limit of determination is about 0.05 mg/kg.
Methods are also available for methacrifos in meat of poultry, eggs
and milk after extraction with acetonitrile or acetone followed by
various partition steps and GLC with a phosphorous detector; the limit
of determination is 0.01 mg/kg. The methods are suitable or can be
adapted for regulatory purposes.
Residues of the unchanged parent compound and the dealkylated
metabolites can be determined, in a two step procedure, in cereal
grains, eggs and cocoa-mass. The limits of determination are about
0.025 mg/kg for the parent compound and 0.04 - 0.1 for the dealkylated
metabolites. The method can be adapted for regulatory purposes.
When establishing maximum residue limits for methacrifos on grain or
other stored food commodities, the following factors have to be
considered.
1. The residue level depends largely on the application rate, which
has to be chosen according to the nature of the insect or mite
infestation and the desired period of protection. Variation in
residue levels are to be expected among different countries even when
the same dosages are applied and the same storage period is used,
since the rate of dissipation may differ according to the local
climate and storage conditions.
2. The distribution of the insecticides may be heterogeneous,
depending on local application techniques.
3. Stored products may move in international trade at different times
after treatment according to commercial need.
Based on these considerations the maximum residue limits have to be
set at levels that will cover all situations encountered after the
normal applications, even if in some cases the actual residues will be
much lower.
The meeting examined residue data from supervised trials reflecting
established and/or anticipated good storage practice on a number of
stored food or feed commodities. From these data the meeting was able
to estimate the maximum residue levels that were likely to occur when
methacrifos is used in practice.
Level causing no toxicological effect
Rat: 1 mg/kg in the diet equivalent to 0.05 mg/kg bw/day.
Dog: 1 mg/kg in the diet equivalent to 0.025 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0-0.0003 mg/kg bw.
RECOMMENDATION OF RESIDUES LIMITS
The meeting concluded that the maximum residue levels listed below are
suitable for establishing maximum residue limits.
These levels refer to the parent compound only.
Commodity Estimated maximum
residue levels
mg/kg
cereal grains 10
wheat flour (whole meal) 10
wheat flour (white) 2
wheat bran (unprocessed) 10
beans and peas (dry) 5
cocoa beans 10
peanuts (including shell) 10
peanuts 1
poultry meat 0.011
eggs 0.011
milk 0.011
1 The levels refer to the maximum residue levels which might occur
when the animals concerned are fed with commodities treated during
storage with recommended amounts of methacrifos.
FURTHER WORK OR INFORMATION
Required (by 1983)
1. Teratogenicity studies in rat and rabbits.
2. An appropriate reproduction study.
REFERENCES
Arni, P. and Müller, B. Samonella/mammalian-microsome mutagenicity
test with CGA 20168. (1979) Unpublished report, May 23, submitted by
Ciba-Geigy to WHO.
Basler, W. et al. Two-year chronic oral toxicity study with
CGA-20168 technical in albino rats. (1980) Unpublished report prepared
by Ciba-Geigy from the original Industrial Bio-test Laboratories
records, May 15, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the rat. (1974a)
Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute oral LD50 of technical CGA 20168 in the mouse.
(1974b) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe, R. Acute dermal LD50 of technical CGA 20168 in the rat.
(1974c) Unpublished report, April 10, submitted by Ciba-Geigy to WHO.
Bathe R. and Sachsse, K. Acute oral LD50 in the dog of technical CGA
20168. (1975) Unpublished report, November 12, submitted by Ciba-Geigy
to WHO.
Bathe, R. and Sachsse, K. Acute oral LD50 in the mouse of technical
CGA 20168. (1978) Unpublished report, August 15, submitted by
Ciba-Geigy to WHO.
Bathe R. et al. 26 weeks feeding study in dogs with technical CGA
20168. (1977) Unpublished report, March 4, Submitted by Ciba-Geigy to
WHO.
Charles, J.M. et al. Three-generation reproduction study with CGA
20168 technical in albino rats. (1980a) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-Test Laboratories Records,
April 10, submitted by Ciba-Geigy to WHO.
Charles, J.M. et al. A chronic oral carcinogenic toxicity study with
CGA 20168 in Swiss white mice. (1980b) Unpublished report prepared by
Ciba-Geigy from the original Industrial Bio-test Laboratories records,
May 15, submitted by Ciba-Geigy to WHO.
Chesterman, H. et al. CGA 20168, 28-day dietary feeding study in
beagle dogs. (1975) Unpublished report from Huntingdon Research
Centre. November 28. Submitted by Ciba-Geigy to WHO.
Drake J.C. 1 Month dietary toxicity study in rats with compound CGA
20168. (1975) Unpublished report from Geigy Pharmaceuticals, February
17, Submitted by Ciba-Geigy to WHO.
Fritz H. et al. Reproduction study on CGA 20168 Technical. Rat.
(1978) Unpublished report, June 14, submitted by Ciba-Geigy to WHO.
Gfeller, W. CGA 20168 Oral toxicity in pigs. Daily administration for
28 days. (1974) Unpublished report from Ciba-Geigy Agricultural
Research Centre, January 28, submitted by Ciba-Geigy to WHO.
Hamböck, H. Metabolism of CGA 20168 in the rat. (1978) Unpublished
project report 27/78 from the Agrochemicals Division of Ciba-Geigy,
May 17, submitted by Ciba-Geigy to WHO.
Hool, G. and Müller, D. Dominant lethal study on CGA 20168. (1980)
Unpublished report, February 6, submitted by Ciba-Geigy to WHO.
Ifflaender, V. and Mücke, W. Distribution, degradation and excretion
of CGA 20168 in the rat. (1975) Unpublished report from the
Agrochemicals Division of Ciba-Geigy, April 9, submitted by Ciba-Geigy
to WHO.
Sachsse, K. and Bathe, R. Potentiation study. CGA 20168 versus 6
insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion. (1978)
Unpublished report, June 23, submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Acute oral toxicity and neurotoxicity study of
technical CGA 20168 in the domestic fowl (Gallus domesticus). (1979)
Unpublished report, November 6, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. Acute toxicity to rainbow trout, crucian
carp, catfish, bluegill and guppy of technical CGA 20168. (1974a)
Unpublished report, August 7, submitted by Ciba-Geigy to WHO.
Sachsse, K. and Ullmann, L. "8-Day-feeding toxicity" of technical CGA
20168 in the Japanese quail. (1974b) Unpublished report, July 13,
submitted by Ciba-Geigy to WHO.
Sachsse, K. et al. Technical CGA 20168. 21-Day percutaneous toxicity
study in rabbits. (1978) Unpublished report, April 24, submitted by
Ciba-Geigy to WHO.
Strittmatter, J. and Gfeller, W. 63 Days dietary toxicity study in
chicks with compound CGA 20168. (1975) Unpublished report from
Ciba-Geigy Agricultural Research Centre, March, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Skin sensitizing (contact allergenic)
effect in guinea pigs of CGA 20168. (1975) Unpublished report, July 1,
submitted by Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute inhalation toxicity in the rat of
technical CGA 20168. (1976) Unpublished report, July 7, submitted by
Ciba-Geigy to WHO.
Ullmann, L. and Sachsse, K. Acute dermal LD50 in the rabbit of
technical CGA 20168. (1977) Unpublished report, February 2, submitted
by Ciba-Geigy to WHO.
Ullmann, L. et al. 21-Day inhalation study on the rat with technical
CGA 20168. (1977) Unpublished report, December 16, submitted by
Ciba-Geigy to WHO.
REFERENCES ON RESIDUES IN FOOD AND THEIR EVALUATION
Blattmann, P. Degradation of CGA 20168 in stored wheat grains,
including their processing. (1978) Unpublished report PR 42/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Desmarchelier, J.M., Bengston, M., Connel, M., Minett, W., Moore, B.,
Phillips, M., Snelson, J., Sticka, R. and Tucker, K. A collaborative
study of residues on wheat of chlorpyrifos-methyl, fenitrothion,
malathion, methacrifos and pirimiphos-methyl. 1. Method development.
Pestic. Sci. 8: 473-483.
Formica, G. CGA 20168. Gas chromatographic residue determination in
wheat grain, wheat meal, bran, groats and bread. (1973) Unpublished
report HEM 20/73 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. CGA 20168. Gas chromatographic residue determination in
chicken tissues. (1974) Unpublished report REM 9/74 from Ciba-Geigy
Limited, Basle, Switzerland.
Formica, G. CGA 20168. Residues in chicken tissues. (1974a)
Unpublished report RVA 205/74 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Degradation of CGA 20168 in wheat and maize. (1975)
Unpublished report RVA 314/75 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. CGA 20168. Residues in cow's milk after oral medication.
(1977) Unpublished report RVA 612/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Methacrifos (CGA 20168). Determination of CGA 20168 in
feedgrain treated with different formulations and stored in drums.
(1977a) Unpublished report RVA 615/77 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos in French barley after direct
spray treatment with the formulation EC 950. (1978a) Unpublished
report RVA 620/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat and barley after
direct spray treatment with the formulation EC 950. Carinena, Spain.
(1978b) Unpublished report RVA 626/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Residues of methacrifos in untreated cacao beans stored in
a warehouse and possibly contaminated through cacao beans treated with
DAMFIN(R) 950 EC, stored in the same warehouse. (1978c) Unpublished
report RVA 608/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of residues of methacrifos in stored grain
and its products seven months after direct spray treatment with the
formulation SO 050. (1978d) Unpublished report RVA 610/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in wheat after direct spray
treatment with the formulation SO 050. (1978e) Unpublished report RVA
622/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain, and their products twenty-one months after direct spray
treatment with the formulation EC 950. (1978f) Unpublished report RVA
611/78 from Ciba-Geigy Limited, Basle, Switzerland.
Formica, G. Determination of methacrifos in barley and oats after
direct spray treatment with the formulation EC 950. (1978g)
Unpublished report RVA 623/78 from Ciba-Geigy Limited, Basle,
Switzerland.
Formica, G. Determination of methacrifos of residues in stored
feedgrain and their products after direct spray treatment with the
formulation EC 1000. (1978h) Unpublished report RVA 618/78 from
Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Residue of methacrifos in maize cobs
after surface treatment with DAMFIN(R) 950 EC. (1979) Unpublished
report RVA 4010/79 from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic determination of
total residues in cacao liquor after different processings of cacao
beans treated with DAMFIN(R) SO 050. (1979a) Unpublished report RVA
4009/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Giannone, C. and Formica, G. Gas chromatographic-determination of
methacrifos and its dealkylated metabolites in sorghum after treatment
with DAMFIN(R) SO 050 or DAMFIN(R) P 2. (1980) Unpublished report RVA
4045/79 A from Ciba-Geigy Limited, Basle, Switzerland.
Hart, R.J. and Moore, B. The use of CGA 20168 for the protection of
rice stored in bags. (1975) Unpublished technical report No. 75/10/533
from Ciba-Geigy Australia Limited.
Hart, R.J. and Moors, B. The protection of stored grain sorghum from
insect attack with CGA 20168. (1976) Unpublished technical report No.
76/6/564 from Ciba-Geigy Australia Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168. (1974) Unpublished technical report No. 74/9/476 from
Ciba-Geigy Australia Limited.
McDougall, K.W. Mini silo trials with CGA 20168 - Series 2. (1975)
Unpublished technical report No. 75/8/519 from Ciba-Geigy Australia
Limited.
McDougall, K.W. The influence of grain variety on the persistence of
CGA 20168 - Part 2. Drum trials. (1976a) Unpublished technical report
No. 76/2/554 from Ciba-Geigy Australia Limited.
McDougall, K.W. The distribution and dissipation of CGA 20168 in
white, brown and paddy rice. (1976b) Unpublished technical report No.
76/2/553 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Determination of CGA 20168 residues in
wheat - Comparison of five analytical procedures. Appendix I: R+D
Department Method No. 129. Appendix II: C.S.I.R.O. Method A. Appendix
III: C.S.I.R.O. Method C. (1973) Unpublished technical report No.
73/11/425 from Ciba-Geigy Australia Limited.
Moore B. and McDougall, K.W. Further studies on factors affecting the
dissipation of CGA-20168 in wheat. (1974) Unpublished technical report
No. 74/3/433 from Ciba-Geigy Australia Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Insecticide Studies - Determination of residue dissipation. (1974a)
Unpublished technical report No. 74/7/461 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. The Australian Wheat Board Collaborative
Insecticide Studies Determination of residue dissipation. Part II.
(1974b) Unpublished technical report No. 74/9/477 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. CGA 20168 Residues in chickens. (1974c)
Unpublished technical report No. 74/7/462 from Ciba-Geigy Australia
Limited.
Moore, B. and McDougall, K.W. Australian Wheat Board Collaborative
Studies 1975: The effect of aeration on the dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Moore, B. and McDougall, K.W. Australia Wheat Board Collaborative
Studies 1975: The effect of aeration on the Dissipation of CGA 20168
in wheat. Unpublished technical report No. 75/12/538 from Ciba-Geigy
Australia Limited.
Renfer, A. Biological evaluation of methacrifos as a grain protectant
under field conditions in Switzerland - I. Basle. (1977) Unpublished
report 9.25 VS 135 from Ciba-Geigy, Basle, Switzerland.
Schnabel, D. and Formica, G. Gas chromatographic determination of CGA
20168 in eggs following oral application via feed. (1979) Unpublished
report RVA 4011/79 from Ciba-Geigy Limited, Basle, Switzerland.
Snelson, J.T. and Desmarchelier, J.M. The significance of pesticide
residues. Proceedings of the First International Working Conference on
Stored-Product Entomology, Savannah, Georgia, USA. (1975).
Tournayre, J.C. Dissipation curve of CGA 20168 in wheat grain and
residues in tissues. (1978) Unpublished reports No. 13/78 A, B, C from
Ciba-Geigy Agrochemicals Division France.
Wyniger. R., Renfer, A., Knuesel, F. and Gfeller, W. Methacrifos - A
new insecticide for the control of insect pests in stored products.
Proc. Brit. Insect. Fungic. Conf. 9, Vol. 3: 1033 -1040.
