
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
METHACRIFOS
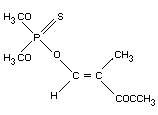 Explanation
Methacrifos was evaluated at the 1980 Joint Meeting (FAO/WHO
1981) 1, and a temporary ADI of 0.0003 mg/kg body weight was
allocated on the basis of studies conducted by Industrial Bio-Test
Laboratories (IBT). The IBT data were validated and the validation was
accepted by the 1980 JMPR. Further work was required, which included a
teratogenicity study in rats and rabbits, and an appropriate
reproduction study. Additional data have been submitted and are
reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Rabbit
Groups of 20 mated female rabbits (Chinchilla) were
intubated with technical methacrifos as a suspension in sodium
carboxymethylcellulose at 0, 1, 3 or 10 mg/kg bw/day from day 6
through day 18 inclusive of pregnancy (day of mating = day 0 of
pregnancy). The does were sacrificed on day 28 of pregnancy and
foetuses were removed by caesarean section for examination for
external, skeletal and visceral abnormalities. One doe (pregnant) at
10 mg/kg bw died on day 13 of pregnancy. Pregnancy rate was low in all
treated groups (50-68.5%) as compared to concurrent controls (85%).
(The pregnancy rate encountered in the breed of rabbits used was given
in the report to be between 30-87%.) Maternal body weight and food
1 See Annex 2 for WHO and FAO documentation.
consumption were not significantly different between control and
treated groups. There were no treatment-related effects with respect
to mean number of corpora lutea, mean number of implantations, live
litter size, early and late resorptions, sex ratio and foetal weight.
Irregular ossification of the 6th sternebrae occurred in 1/77 foetuses
at 1 mg/kg bw/day and 1/91 foetuses at 10 mg/kg bw/day, but in none
of the foetuses at 3 mg/kg bw/day or of the control group. This
particular anomaly appeared unlikely to be related to the compound. No
visceral or gross abnormalities were noted in any of the treated
groups (Fritz et al 1980).
Special Studies on Mutagenicity
In a test for non-disjunction on Saccharomyces cerevisiae D61,
methacrifos, at concentrations ranging from 2 to 2 000 µg/ml, did not
induce an increase in the incidence of monosomic colonies, as compared
to the controls, in the 16-hour and the 72-hour tests (Arni and Müller
1980a).
The mutagenic potential of methacriphos was evaluated in the
multi-purpose MP-1 strain of S. cerevisiae. Concentrations of
methacriphos at 125 to 4 000 µg/ml did not cause any significant
increase in the number of recombinants or convertants. However,
concentrations at 500 µg/ml and above produced a slight increase in
the number of forward mutants (cycloheximide resistant cells) (Arni
and Müller 1980b).
Methacrifos was tested for its mutagenic activity in the D7
strain of S. cerevisiae in the presence or absence of metabolic
activation preparation (S-9 fraction of liver from rats induced with
Aroclor 1254). The concentrations used, 640-10 000 µg/ml, all led to a
decrease in the colony count in the presence or absence of the S-9
fraction. An increase in the number of recombinants, convertants and
mutants was noted at 640 µg/ml and above without the metabolic
activation preparation. With the inclusion of microsomal activation in
the test system, only an increase in the number of convertants was
apparent. In a repeat experiment using concentrations of 384 to
6 000 µg/ml (growth inhibitory effect noted at 960 µg/ml and above), a
mutagenic response (as suggested by an observed increase in the number
of recombinants and convertants) was induced in the absence but not in
the presence of the S-9 fraction (Arni and Müller 1982).
In two separate DNA damage and repair tests, cultures of freshly
isolated hepatocytes and of an established human fibroblast line were
exposed for 5 hours to methacrifos at concentrations ranging from 0.32
to 40 nl/ml and 0.4 to 50 nl/ml, respectively, in the presence of
3H-thymidine. Results indicated that methacrifos, at the
concentrations used, did not induce unscheduled DNA synthesis in
either of the exposed cultures (Puri and Müller 1982a, b).
Groups generally of 6 mice (16-hour fasted) were treated orally
with methacrifos 3 times at hourly intervals, at 0, 7, 14 or
28 mg/kg bw (mice serving as hosts to strain TA 1537 were given 2.5, 5
or 10 mg/kg bw) and then injected via the lateral vein of the tail
immediately after the last dose with the indicator organisms
( Salmonella typhimurium TA 98, TA 100, TA 1535, TA 1537 or TA 1538)
in a host-mediated assay. One hour later, the bacteria were recovered
from the liver of the mice. A slight increase in the number of black
mutant colonies per plate was observed at 28 mg/kg bw with strain TA
100 in the first experiment, but was not confirmed in two replicate
experiments. There was no significant increase in mutation frequency
of the other indicator strains (Arni and Müller 1981).
Groups of male and female Chinese hamsters were treated orally
with methacrifos at 0, 13.5, 27 or 54 mg/kg bw/day for two consecutive
days in a nucleus anomaly test. The animals were sacrificed 24 hours
after the second dose and bone marrow smears were evaluated for
nucleus anomalies in interphase cells. There was no significant
difference between control and treated groups in the incidence of bone
marrow cells with anomalies of nuclei (Hool et al 1981).
In an in vivo sister chromatid exchange test, groups of Chinese
hamsters (each subcutaneously implanted with a single 45 mg tablet of
5-bromodeoxy-uridine 2 hours earlier) were given single oral doses of
methacrifos at 0, 27, 54 or 108 mg/kg bw and sacrificed 24 hours later
for the preparation of bone marrow cell slides (each animal was given
intraperitoneally 10 mg/kg body weight of colcemide 2 hours prior to
sacrifice). There was no significant difference between control and
treated groups in the incidence of sister chromatid exchanges (Hool
and Müller 1981).
Short-Term Studies
Dog
Two female pedigreed beagles were given methacrifos in gelatin
capsules 7 days/ week for 4 consecutive weeks at 3 mg/kg bw/day
(week 1), 6 mg/kg bw/day (week 2) and 12 mg/kg bw/day (weeks 3 and 4).
The animals were then maintained for a recovery period of 3 weeks
(cholinesterase and carboxy esterase data were, however, available for
a total of 8 weeks). The control group comprised 2 female dogs. There
was no mortality. Diarrhoea, observed in both control and treated
groups, was more pronounced and more frequent in the latter. Vomiting
was also noted in both treated dogs, but the time of occurrence was
not given. Body weight and food consumption were adversely affected
from week 2 onward, with recovery being evident for food consumption
but not for body weight 3 weeks after treatment withdrawal.
Erythrocyte cholinesterase was inhibited by 63-75% and plasma
cholinesterase by 21-57% throughout the treatment period, with the
magnitude of depression increasing with time. At the end of the
recovery period, plasma cholinesterase almost completely recovered,
while erythrocyte cholinesterase remained depressed by nearly 50%.
Plasma carboxylesterase activity was reduced (>20%) in one treated
dog through the 4-week dosing period and in another only at week 4.
Complete recovery of the enzyme was apparent 2 to 3 weeks after
treatment withdrawal. Brain cholinesterase assayed 4 weeks
after termination of the treatment period was not depressed.
Neurophysiological examination (including determination of maximum
nerve conduction velocity in sensory and motor pathways), observation
of behaviour and neurological examination conducted at the end of
dosing period all failed to reveal any significant difference between
treated and control dogs. Histopathological examination of the teased
peripheral nerve fibres (tibial nerve and plantar nerve) from animals
sacrificed at the end of the 4-week recovery period showed no abnormal
morphological changes (Gfeller et al 1982).
Observations in Humans
Two healthy male volunteers were given technical methacrifos
(96.2%) orally at approximately 0.073 mg/kg bw/day for 7 consecutive
days followed by a dose of 0.145 mg/kg bw on the 8th day. There were
no toxic signs. Haematological, biochemical and urinalysis values
determined before and after the treatment period were not
significantly different. The activity of cholinesterase in plasma and
erythrocyte was not inhibited at any time during the treatment or the
1-week recovery period (Strittmatter and Gfeller 1979).
A male volunteer was given daily 5 mg or approximately
0.067 mg/kg bw technical methacrifos (95% pure, "absorbed" in 150 mg
of lactose) in gelatin capsule, 7 days/week for 4 weeks. No adverse
reaction was reported. The activities of plasma and erythrocyte
cholinesterase measured on day 19 of treatment and on day 12 post-
treatment were not significantly different from those before treatment
(Loosli et al 1982).
COMMENTS
The available teratology study in (Chinchilla) rabbits failed to
indicate any teratogenic activity of methacrifos under the conditions
of the experiment. However, sensitivity of this particular strain of
rabbits to known teratogens was not established. Mutagenicity studies
including unscheduled DNA synthesis in rat hepatocytes and human
fibroblasts, a host-mediated assay, a nucleus anomaly test and an
in vivo sister chromatid exchange test were all negative.
Nevertheless, there were some suggestions of positive mutagenic
response in 2 of the 3 strains of Saccharomyces cerevisiae employed
in the in vitro tests. Observations in humans involving a very
limited number (a total of 3) of volunteers seemed to indicate
subacute (7-28 days) oral treatment with methacrifos in the vicinity
of 0.07 mg/kg bw/day resulted in no adverse effects or any significant
inhibition of plasma or erythrocyte cholinesterase.
In the absence of an appropriate reproduction study and a
teratology study in rats, the Meeting could only agree to extend the
temporary ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 mg/kg in the diet, equivalent to 0.05 mg/kg bw
Dog : 1 mg/kg in the diet, equivalent to 0.025 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Teratogenicity study in rats.
2. An appropriate reproduction study
Desirable
1. Information on sensitivity of chinchilla rabbits to known
teratogens.
2. Further observations in humans.
REFERENCES
Arni, P. and Müller, D. Test for non-disjunction on Saccharomyces
1980a cerevisiae D61 with CGA 20168. (Test for mutagenic
properties in yeast cells). Report from Ciba-Geigy Limited,
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
1980b Mutagenicity test on Saccharomyces cerevisiae MP-1
in vitro with CGA 20168. (Test for mutagenic properties in
yeast cells). Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
1981 Intrasanguine host-mediated assay with Saccharomyces
cerevisiae with CGA 20168, (Test for the demonstration of
point mutations in bacteria in vivo.) Report from Ciba-Geigy
Limited submitted to the World Health Organization by Ciba-
Geigy. (Unpublished)
1982 D7/mammalian-microsome mutagenicity test in vitro with CGA
20168. (Test for mutagenic properties in yeast cells).
Report from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Fritz, H., Giese, K. and Hess, R. Report on CGA 20168 tech. Teratology
1980 study (Seg. II) in rabbits. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Gfeller, W., Krinke, G. and Schaeppi, U. Final report CGA 20168.
1982 Neurotoxicity study in dogs. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Hool, G. and Müller, D. Sister chromatid exchange study CGA 20168.
1981 Chinese hamster. (Test for mutagenic effects on bone marrow
cells.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
Hool, G., Langauer, M. and Müller, D. Nucleus anomaly test in somatic
1981 interphase nuclei CGA 20168. Chinese hamster. (Test for
mutagenic effects on bone marrow cells.) Report from Ciba-
Geigy Limited submitted to the World Health Organization by
Ciba-Geigy. (Unpublished)
Loosli, R., Hornisch, H.P. and Dubach, P. Final Report CGA 20168
1982 28-day palatability study in a human volunteer. Report from
Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Puri, E. and Müller, D. Autoradiographic DNA repair test on rat
1982a hepatocytes- CGA 20168. (In vitro test for DNA-damaging
properties.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
1982b Autoradiographic DNA repair test on human fibroblasts CGA
20168. (In vitro test for DNA-damaging properties.) Report
from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Strittmatter, J. and Gfeller, W. CGA 20168 technical effect on
1979 cholinesterase activity in man following repeated oral
administration. Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
Explanation
Methacrifos was evaluated at the 1980 Joint Meeting (FAO/WHO
1981) 1, and a temporary ADI of 0.0003 mg/kg body weight was
allocated on the basis of studies conducted by Industrial Bio-Test
Laboratories (IBT). The IBT data were validated and the validation was
accepted by the 1980 JMPR. Further work was required, which included a
teratogenicity study in rats and rabbits, and an appropriate
reproduction study. Additional data have been submitted and are
reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Rabbit
Groups of 20 mated female rabbits (Chinchilla) were
intubated with technical methacrifos as a suspension in sodium
carboxymethylcellulose at 0, 1, 3 or 10 mg/kg bw/day from day 6
through day 18 inclusive of pregnancy (day of mating = day 0 of
pregnancy). The does were sacrificed on day 28 of pregnancy and
foetuses were removed by caesarean section for examination for
external, skeletal and visceral abnormalities. One doe (pregnant) at
10 mg/kg bw died on day 13 of pregnancy. Pregnancy rate was low in all
treated groups (50-68.5%) as compared to concurrent controls (85%).
(The pregnancy rate encountered in the breed of rabbits used was given
in the report to be between 30-87%.) Maternal body weight and food
1 See Annex 2 for WHO and FAO documentation.
consumption were not significantly different between control and
treated groups. There were no treatment-related effects with respect
to mean number of corpora lutea, mean number of implantations, live
litter size, early and late resorptions, sex ratio and foetal weight.
Irregular ossification of the 6th sternebrae occurred in 1/77 foetuses
at 1 mg/kg bw/day and 1/91 foetuses at 10 mg/kg bw/day, but in none
of the foetuses at 3 mg/kg bw/day or of the control group. This
particular anomaly appeared unlikely to be related to the compound. No
visceral or gross abnormalities were noted in any of the treated
groups (Fritz et al 1980).
Special Studies on Mutagenicity
In a test for non-disjunction on Saccharomyces cerevisiae D61,
methacrifos, at concentrations ranging from 2 to 2 000 µg/ml, did not
induce an increase in the incidence of monosomic colonies, as compared
to the controls, in the 16-hour and the 72-hour tests (Arni and Müller
1980a).
The mutagenic potential of methacriphos was evaluated in the
multi-purpose MP-1 strain of S. cerevisiae. Concentrations of
methacriphos at 125 to 4 000 µg/ml did not cause any significant
increase in the number of recombinants or convertants. However,
concentrations at 500 µg/ml and above produced a slight increase in
the number of forward mutants (cycloheximide resistant cells) (Arni
and Müller 1980b).
Methacrifos was tested for its mutagenic activity in the D7
strain of S. cerevisiae in the presence or absence of metabolic
activation preparation (S-9 fraction of liver from rats induced with
Aroclor 1254). The concentrations used, 640-10 000 µg/ml, all led to a
decrease in the colony count in the presence or absence of the S-9
fraction. An increase in the number of recombinants, convertants and
mutants was noted at 640 µg/ml and above without the metabolic
activation preparation. With the inclusion of microsomal activation in
the test system, only an increase in the number of convertants was
apparent. In a repeat experiment using concentrations of 384 to
6 000 µg/ml (growth inhibitory effect noted at 960 µg/ml and above), a
mutagenic response (as suggested by an observed increase in the number
of recombinants and convertants) was induced in the absence but not in
the presence of the S-9 fraction (Arni and Müller 1982).
In two separate DNA damage and repair tests, cultures of freshly
isolated hepatocytes and of an established human fibroblast line were
exposed for 5 hours to methacrifos at concentrations ranging from 0.32
to 40 nl/ml and 0.4 to 50 nl/ml, respectively, in the presence of
3H-thymidine. Results indicated that methacrifos, at the
concentrations used, did not induce unscheduled DNA synthesis in
either of the exposed cultures (Puri and Müller 1982a, b).
Groups generally of 6 mice (16-hour fasted) were treated orally
with methacrifos 3 times at hourly intervals, at 0, 7, 14 or
28 mg/kg bw (mice serving as hosts to strain TA 1537 were given 2.5, 5
or 10 mg/kg bw) and then injected via the lateral vein of the tail
immediately after the last dose with the indicator organisms
( Salmonella typhimurium TA 98, TA 100, TA 1535, TA 1537 or TA 1538)
in a host-mediated assay. One hour later, the bacteria were recovered
from the liver of the mice. A slight increase in the number of black
mutant colonies per plate was observed at 28 mg/kg bw with strain TA
100 in the first experiment, but was not confirmed in two replicate
experiments. There was no significant increase in mutation frequency
of the other indicator strains (Arni and Müller 1981).
Groups of male and female Chinese hamsters were treated orally
with methacrifos at 0, 13.5, 27 or 54 mg/kg bw/day for two consecutive
days in a nucleus anomaly test. The animals were sacrificed 24 hours
after the second dose and bone marrow smears were evaluated for
nucleus anomalies in interphase cells. There was no significant
difference between control and treated groups in the incidence of bone
marrow cells with anomalies of nuclei (Hool et al 1981).
In an in vivo sister chromatid exchange test, groups of Chinese
hamsters (each subcutaneously implanted with a single 45 mg tablet of
5-bromodeoxy-uridine 2 hours earlier) were given single oral doses of
methacrifos at 0, 27, 54 or 108 mg/kg bw and sacrificed 24 hours later
for the preparation of bone marrow cell slides (each animal was given
intraperitoneally 10 mg/kg body weight of colcemide 2 hours prior to
sacrifice). There was no significant difference between control and
treated groups in the incidence of sister chromatid exchanges (Hool
and Müller 1981).
Short-Term Studies
Dog
Two female pedigreed beagles were given methacrifos in gelatin
capsules 7 days/ week for 4 consecutive weeks at 3 mg/kg bw/day
(week 1), 6 mg/kg bw/day (week 2) and 12 mg/kg bw/day (weeks 3 and 4).
The animals were then maintained for a recovery period of 3 weeks
(cholinesterase and carboxy esterase data were, however, available for
a total of 8 weeks). The control group comprised 2 female dogs. There
was no mortality. Diarrhoea, observed in both control and treated
groups, was more pronounced and more frequent in the latter. Vomiting
was also noted in both treated dogs, but the time of occurrence was
not given. Body weight and food consumption were adversely affected
from week 2 onward, with recovery being evident for food consumption
but not for body weight 3 weeks after treatment withdrawal.
Erythrocyte cholinesterase was inhibited by 63-75% and plasma
cholinesterase by 21-57% throughout the treatment period, with the
magnitude of depression increasing with time. At the end of the
recovery period, plasma cholinesterase almost completely recovered,
while erythrocyte cholinesterase remained depressed by nearly 50%.
Plasma carboxylesterase activity was reduced (>20%) in one treated
dog through the 4-week dosing period and in another only at week 4.
Complete recovery of the enzyme was apparent 2 to 3 weeks after
treatment withdrawal. Brain cholinesterase assayed 4 weeks
after termination of the treatment period was not depressed.
Neurophysiological examination (including determination of maximum
nerve conduction velocity in sensory and motor pathways), observation
of behaviour and neurological examination conducted at the end of
dosing period all failed to reveal any significant difference between
treated and control dogs. Histopathological examination of the teased
peripheral nerve fibres (tibial nerve and plantar nerve) from animals
sacrificed at the end of the 4-week recovery period showed no abnormal
morphological changes (Gfeller et al 1982).
Observations in Humans
Two healthy male volunteers were given technical methacrifos
(96.2%) orally at approximately 0.073 mg/kg bw/day for 7 consecutive
days followed by a dose of 0.145 mg/kg bw on the 8th day. There were
no toxic signs. Haematological, biochemical and urinalysis values
determined before and after the treatment period were not
significantly different. The activity of cholinesterase in plasma and
erythrocyte was not inhibited at any time during the treatment or the
1-week recovery period (Strittmatter and Gfeller 1979).
A male volunteer was given daily 5 mg or approximately
0.067 mg/kg bw technical methacrifos (95% pure, "absorbed" in 150 mg
of lactose) in gelatin capsule, 7 days/week for 4 weeks. No adverse
reaction was reported. The activities of plasma and erythrocyte
cholinesterase measured on day 19 of treatment and on day 12 post-
treatment were not significantly different from those before treatment
(Loosli et al 1982).
COMMENTS
The available teratology study in (Chinchilla) rabbits failed to
indicate any teratogenic activity of methacrifos under the conditions
of the experiment. However, sensitivity of this particular strain of
rabbits to known teratogens was not established. Mutagenicity studies
including unscheduled DNA synthesis in rat hepatocytes and human
fibroblasts, a host-mediated assay, a nucleus anomaly test and an
in vivo sister chromatid exchange test were all negative.
Nevertheless, there were some suggestions of positive mutagenic
response in 2 of the 3 strains of Saccharomyces cerevisiae employed
in the in vitro tests. Observations in humans involving a very
limited number (a total of 3) of volunteers seemed to indicate
subacute (7-28 days) oral treatment with methacrifos in the vicinity
of 0.07 mg/kg bw/day resulted in no adverse effects or any significant
inhibition of plasma or erythrocyte cholinesterase.
In the absence of an appropriate reproduction study and a
teratology study in rats, the Meeting could only agree to extend the
temporary ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 mg/kg in the diet, equivalent to 0.05 mg/kg bw
Dog : 1 mg/kg in the diet, equivalent to 0.025 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Teratogenicity study in rats.
2. An appropriate reproduction study
Desirable
1. Information on sensitivity of chinchilla rabbits to known
teratogens.
2. Further observations in humans.
REFERENCES
Arni, P. and Müller, D. Test for non-disjunction on Saccharomyces
1980a cerevisiae D61 with CGA 20168. (Test for mutagenic
properties in yeast cells). Report from Ciba-Geigy Limited,
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
1980b Mutagenicity test on Saccharomyces cerevisiae MP-1
in vitro with CGA 20168. (Test for mutagenic properties in
yeast cells). Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
1981 Intrasanguine host-mediated assay with Saccharomyces
cerevisiae with CGA 20168, (Test for the demonstration of
point mutations in bacteria in vivo.) Report from Ciba-Geigy
Limited submitted to the World Health Organization by Ciba-
Geigy. (Unpublished)
1982 D7/mammalian-microsome mutagenicity test in vitro with CGA
20168. (Test for mutagenic properties in yeast cells).
Report from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Fritz, H., Giese, K. and Hess, R. Report on CGA 20168 tech. Teratology
1980 study (Seg. II) in rabbits. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Gfeller, W., Krinke, G. and Schaeppi, U. Final report CGA 20168.
1982 Neurotoxicity study in dogs. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Hool, G. and Müller, D. Sister chromatid exchange study CGA 20168.
1981 Chinese hamster. (Test for mutagenic effects on bone marrow
cells.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
Hool, G., Langauer, M. and Müller, D. Nucleus anomaly test in somatic
1981 interphase nuclei CGA 20168. Chinese hamster. (Test for
mutagenic effects on bone marrow cells.) Report from Ciba-
Geigy Limited submitted to the World Health Organization by
Ciba-Geigy. (Unpublished)
Loosli, R., Hornisch, H.P. and Dubach, P. Final Report CGA 20168
1982 28-day palatability study in a human volunteer. Report from
Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Puri, E. and Müller, D. Autoradiographic DNA repair test on rat
1982a hepatocytes- CGA 20168. (In vitro test for DNA-damaging
properties.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
1982b Autoradiographic DNA repair test on human fibroblasts CGA
20168. (In vitro test for DNA-damaging properties.) Report
from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Strittmatter, J. and Gfeller, W. CGA 20168 technical effect on
1979 cholinesterase activity in man following repeated oral
administration. Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
 Explanation
Methacrifos was evaluated at the 1980 Joint Meeting (FAO/WHO
1981) 1, and a temporary ADI of 0.0003 mg/kg body weight was
allocated on the basis of studies conducted by Industrial Bio-Test
Laboratories (IBT). The IBT data were validated and the validation was
accepted by the 1980 JMPR. Further work was required, which included a
teratogenicity study in rats and rabbits, and an appropriate
reproduction study. Additional data have been submitted and are
reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Rabbit
Groups of 20 mated female rabbits (Chinchilla) were
intubated with technical methacrifos as a suspension in sodium
carboxymethylcellulose at 0, 1, 3 or 10 mg/kg bw/day from day 6
through day 18 inclusive of pregnancy (day of mating = day 0 of
pregnancy). The does were sacrificed on day 28 of pregnancy and
foetuses were removed by caesarean section for examination for
external, skeletal and visceral abnormalities. One doe (pregnant) at
10 mg/kg bw died on day 13 of pregnancy. Pregnancy rate was low in all
treated groups (50-68.5%) as compared to concurrent controls (85%).
(The pregnancy rate encountered in the breed of rabbits used was given
in the report to be between 30-87%.) Maternal body weight and food
1 See Annex 2 for WHO and FAO documentation.
consumption were not significantly different between control and
treated groups. There were no treatment-related effects with respect
to mean number of corpora lutea, mean number of implantations, live
litter size, early and late resorptions, sex ratio and foetal weight.
Irregular ossification of the 6th sternebrae occurred in 1/77 foetuses
at 1 mg/kg bw/day and 1/91 foetuses at 10 mg/kg bw/day, but in none
of the foetuses at 3 mg/kg bw/day or of the control group. This
particular anomaly appeared unlikely to be related to the compound. No
visceral or gross abnormalities were noted in any of the treated
groups (Fritz et al 1980).
Special Studies on Mutagenicity
In a test for non-disjunction on Saccharomyces cerevisiae D61,
methacrifos, at concentrations ranging from 2 to 2 000 µg/ml, did not
induce an increase in the incidence of monosomic colonies, as compared
to the controls, in the 16-hour and the 72-hour tests (Arni and Müller
1980a).
The mutagenic potential of methacriphos was evaluated in the
multi-purpose MP-1 strain of S. cerevisiae. Concentrations of
methacriphos at 125 to 4 000 µg/ml did not cause any significant
increase in the number of recombinants or convertants. However,
concentrations at 500 µg/ml and above produced a slight increase in
the number of forward mutants (cycloheximide resistant cells) (Arni
and Müller 1980b).
Methacrifos was tested for its mutagenic activity in the D7
strain of S. cerevisiae in the presence or absence of metabolic
activation preparation (S-9 fraction of liver from rats induced with
Aroclor 1254). The concentrations used, 640-10 000 µg/ml, all led to a
decrease in the colony count in the presence or absence of the S-9
fraction. An increase in the number of recombinants, convertants and
mutants was noted at 640 µg/ml and above without the metabolic
activation preparation. With the inclusion of microsomal activation in
the test system, only an increase in the number of convertants was
apparent. In a repeat experiment using concentrations of 384 to
6 000 µg/ml (growth inhibitory effect noted at 960 µg/ml and above), a
mutagenic response (as suggested by an observed increase in the number
of recombinants and convertants) was induced in the absence but not in
the presence of the S-9 fraction (Arni and Müller 1982).
In two separate DNA damage and repair tests, cultures of freshly
isolated hepatocytes and of an established human fibroblast line were
exposed for 5 hours to methacrifos at concentrations ranging from 0.32
to 40 nl/ml and 0.4 to 50 nl/ml, respectively, in the presence of
3H-thymidine. Results indicated that methacrifos, at the
concentrations used, did not induce unscheduled DNA synthesis in
either of the exposed cultures (Puri and Müller 1982a, b).
Groups generally of 6 mice (16-hour fasted) were treated orally
with methacrifos 3 times at hourly intervals, at 0, 7, 14 or
28 mg/kg bw (mice serving as hosts to strain TA 1537 were given 2.5, 5
or 10 mg/kg bw) and then injected via the lateral vein of the tail
immediately after the last dose with the indicator organisms
( Salmonella typhimurium TA 98, TA 100, TA 1535, TA 1537 or TA 1538)
in a host-mediated assay. One hour later, the bacteria were recovered
from the liver of the mice. A slight increase in the number of black
mutant colonies per plate was observed at 28 mg/kg bw with strain TA
100 in the first experiment, but was not confirmed in two replicate
experiments. There was no significant increase in mutation frequency
of the other indicator strains (Arni and Müller 1981).
Groups of male and female Chinese hamsters were treated orally
with methacrifos at 0, 13.5, 27 or 54 mg/kg bw/day for two consecutive
days in a nucleus anomaly test. The animals were sacrificed 24 hours
after the second dose and bone marrow smears were evaluated for
nucleus anomalies in interphase cells. There was no significant
difference between control and treated groups in the incidence of bone
marrow cells with anomalies of nuclei (Hool et al 1981).
In an in vivo sister chromatid exchange test, groups of Chinese
hamsters (each subcutaneously implanted with a single 45 mg tablet of
5-bromodeoxy-uridine 2 hours earlier) were given single oral doses of
methacrifos at 0, 27, 54 or 108 mg/kg bw and sacrificed 24 hours later
for the preparation of bone marrow cell slides (each animal was given
intraperitoneally 10 mg/kg body weight of colcemide 2 hours prior to
sacrifice). There was no significant difference between control and
treated groups in the incidence of sister chromatid exchanges (Hool
and Müller 1981).
Short-Term Studies
Dog
Two female pedigreed beagles were given methacrifos in gelatin
capsules 7 days/ week for 4 consecutive weeks at 3 mg/kg bw/day
(week 1), 6 mg/kg bw/day (week 2) and 12 mg/kg bw/day (weeks 3 and 4).
The animals were then maintained for a recovery period of 3 weeks
(cholinesterase and carboxy esterase data were, however, available for
a total of 8 weeks). The control group comprised 2 female dogs. There
was no mortality. Diarrhoea, observed in both control and treated
groups, was more pronounced and more frequent in the latter. Vomiting
was also noted in both treated dogs, but the time of occurrence was
not given. Body weight and food consumption were adversely affected
from week 2 onward, with recovery being evident for food consumption
but not for body weight 3 weeks after treatment withdrawal.
Erythrocyte cholinesterase was inhibited by 63-75% and plasma
cholinesterase by 21-57% throughout the treatment period, with the
magnitude of depression increasing with time. At the end of the
recovery period, plasma cholinesterase almost completely recovered,
while erythrocyte cholinesterase remained depressed by nearly 50%.
Plasma carboxylesterase activity was reduced (>20%) in one treated
dog through the 4-week dosing period and in another only at week 4.
Complete recovery of the enzyme was apparent 2 to 3 weeks after
treatment withdrawal. Brain cholinesterase assayed 4 weeks
after termination of the treatment period was not depressed.
Neurophysiological examination (including determination of maximum
nerve conduction velocity in sensory and motor pathways), observation
of behaviour and neurological examination conducted at the end of
dosing period all failed to reveal any significant difference between
treated and control dogs. Histopathological examination of the teased
peripheral nerve fibres (tibial nerve and plantar nerve) from animals
sacrificed at the end of the 4-week recovery period showed no abnormal
morphological changes (Gfeller et al 1982).
Observations in Humans
Two healthy male volunteers were given technical methacrifos
(96.2%) orally at approximately 0.073 mg/kg bw/day for 7 consecutive
days followed by a dose of 0.145 mg/kg bw on the 8th day. There were
no toxic signs. Haematological, biochemical and urinalysis values
determined before and after the treatment period were not
significantly different. The activity of cholinesterase in plasma and
erythrocyte was not inhibited at any time during the treatment or the
1-week recovery period (Strittmatter and Gfeller 1979).
A male volunteer was given daily 5 mg or approximately
0.067 mg/kg bw technical methacrifos (95% pure, "absorbed" in 150 mg
of lactose) in gelatin capsule, 7 days/week for 4 weeks. No adverse
reaction was reported. The activities of plasma and erythrocyte
cholinesterase measured on day 19 of treatment and on day 12 post-
treatment were not significantly different from those before treatment
(Loosli et al 1982).
COMMENTS
The available teratology study in (Chinchilla) rabbits failed to
indicate any teratogenic activity of methacrifos under the conditions
of the experiment. However, sensitivity of this particular strain of
rabbits to known teratogens was not established. Mutagenicity studies
including unscheduled DNA synthesis in rat hepatocytes and human
fibroblasts, a host-mediated assay, a nucleus anomaly test and an
in vivo sister chromatid exchange test were all negative.
Nevertheless, there were some suggestions of positive mutagenic
response in 2 of the 3 strains of Saccharomyces cerevisiae employed
in the in vitro tests. Observations in humans involving a very
limited number (a total of 3) of volunteers seemed to indicate
subacute (7-28 days) oral treatment with methacrifos in the vicinity
of 0.07 mg/kg bw/day resulted in no adverse effects or any significant
inhibition of plasma or erythrocyte cholinesterase.
In the absence of an appropriate reproduction study and a
teratology study in rats, the Meeting could only agree to extend the
temporary ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 mg/kg in the diet, equivalent to 0.05 mg/kg bw
Dog : 1 mg/kg in the diet, equivalent to 0.025 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Teratogenicity study in rats.
2. An appropriate reproduction study
Desirable
1. Information on sensitivity of chinchilla rabbits to known
teratogens.
2. Further observations in humans.
REFERENCES
Arni, P. and Müller, D. Test for non-disjunction on Saccharomyces
1980a cerevisiae D61 with CGA 20168. (Test for mutagenic
properties in yeast cells). Report from Ciba-Geigy Limited,
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
1980b Mutagenicity test on Saccharomyces cerevisiae MP-1
in vitro with CGA 20168. (Test for mutagenic properties in
yeast cells). Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
1981 Intrasanguine host-mediated assay with Saccharomyces
cerevisiae with CGA 20168, (Test for the demonstration of
point mutations in bacteria in vivo.) Report from Ciba-Geigy
Limited submitted to the World Health Organization by Ciba-
Geigy. (Unpublished)
1982 D7/mammalian-microsome mutagenicity test in vitro with CGA
20168. (Test for mutagenic properties in yeast cells).
Report from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Fritz, H., Giese, K. and Hess, R. Report on CGA 20168 tech. Teratology
1980 study (Seg. II) in rabbits. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Gfeller, W., Krinke, G. and Schaeppi, U. Final report CGA 20168.
1982 Neurotoxicity study in dogs. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Hool, G. and Müller, D. Sister chromatid exchange study CGA 20168.
1981 Chinese hamster. (Test for mutagenic effects on bone marrow
cells.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
Hool, G., Langauer, M. and Müller, D. Nucleus anomaly test in somatic
1981 interphase nuclei CGA 20168. Chinese hamster. (Test for
mutagenic effects on bone marrow cells.) Report from Ciba-
Geigy Limited submitted to the World Health Organization by
Ciba-Geigy. (Unpublished)
Loosli, R., Hornisch, H.P. and Dubach, P. Final Report CGA 20168
1982 28-day palatability study in a human volunteer. Report from
Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Puri, E. and Müller, D. Autoradiographic DNA repair test on rat
1982a hepatocytes- CGA 20168. (In vitro test for DNA-damaging
properties.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
1982b Autoradiographic DNA repair test on human fibroblasts CGA
20168. (In vitro test for DNA-damaging properties.) Report
from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Strittmatter, J. and Gfeller, W. CGA 20168 technical effect on
1979 cholinesterase activity in man following repeated oral
administration. Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
Explanation
Methacrifos was evaluated at the 1980 Joint Meeting (FAO/WHO
1981) 1, and a temporary ADI of 0.0003 mg/kg body weight was
allocated on the basis of studies conducted by Industrial Bio-Test
Laboratories (IBT). The IBT data were validated and the validation was
accepted by the 1980 JMPR. Further work was required, which included a
teratogenicity study in rats and rabbits, and an appropriate
reproduction study. Additional data have been submitted and are
reviewed in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Teratogenicity
Rabbit
Groups of 20 mated female rabbits (Chinchilla) were
intubated with technical methacrifos as a suspension in sodium
carboxymethylcellulose at 0, 1, 3 or 10 mg/kg bw/day from day 6
through day 18 inclusive of pregnancy (day of mating = day 0 of
pregnancy). The does were sacrificed on day 28 of pregnancy and
foetuses were removed by caesarean section for examination for
external, skeletal and visceral abnormalities. One doe (pregnant) at
10 mg/kg bw died on day 13 of pregnancy. Pregnancy rate was low in all
treated groups (50-68.5%) as compared to concurrent controls (85%).
(The pregnancy rate encountered in the breed of rabbits used was given
in the report to be between 30-87%.) Maternal body weight and food
1 See Annex 2 for WHO and FAO documentation.
consumption were not significantly different between control and
treated groups. There were no treatment-related effects with respect
to mean number of corpora lutea, mean number of implantations, live
litter size, early and late resorptions, sex ratio and foetal weight.
Irregular ossification of the 6th sternebrae occurred in 1/77 foetuses
at 1 mg/kg bw/day and 1/91 foetuses at 10 mg/kg bw/day, but in none
of the foetuses at 3 mg/kg bw/day or of the control group. This
particular anomaly appeared unlikely to be related to the compound. No
visceral or gross abnormalities were noted in any of the treated
groups (Fritz et al 1980).
Special Studies on Mutagenicity
In a test for non-disjunction on Saccharomyces cerevisiae D61,
methacrifos, at concentrations ranging from 2 to 2 000 µg/ml, did not
induce an increase in the incidence of monosomic colonies, as compared
to the controls, in the 16-hour and the 72-hour tests (Arni and Müller
1980a).
The mutagenic potential of methacriphos was evaluated in the
multi-purpose MP-1 strain of S. cerevisiae. Concentrations of
methacriphos at 125 to 4 000 µg/ml did not cause any significant
increase in the number of recombinants or convertants. However,
concentrations at 500 µg/ml and above produced a slight increase in
the number of forward mutants (cycloheximide resistant cells) (Arni
and Müller 1980b).
Methacrifos was tested for its mutagenic activity in the D7
strain of S. cerevisiae in the presence or absence of metabolic
activation preparation (S-9 fraction of liver from rats induced with
Aroclor 1254). The concentrations used, 640-10 000 µg/ml, all led to a
decrease in the colony count in the presence or absence of the S-9
fraction. An increase in the number of recombinants, convertants and
mutants was noted at 640 µg/ml and above without the metabolic
activation preparation. With the inclusion of microsomal activation in
the test system, only an increase in the number of convertants was
apparent. In a repeat experiment using concentrations of 384 to
6 000 µg/ml (growth inhibitory effect noted at 960 µg/ml and above), a
mutagenic response (as suggested by an observed increase in the number
of recombinants and convertants) was induced in the absence but not in
the presence of the S-9 fraction (Arni and Müller 1982).
In two separate DNA damage and repair tests, cultures of freshly
isolated hepatocytes and of an established human fibroblast line were
exposed for 5 hours to methacrifos at concentrations ranging from 0.32
to 40 nl/ml and 0.4 to 50 nl/ml, respectively, in the presence of
3H-thymidine. Results indicated that methacrifos, at the
concentrations used, did not induce unscheduled DNA synthesis in
either of the exposed cultures (Puri and Müller 1982a, b).
Groups generally of 6 mice (16-hour fasted) were treated orally
with methacrifos 3 times at hourly intervals, at 0, 7, 14 or
28 mg/kg bw (mice serving as hosts to strain TA 1537 were given 2.5, 5
or 10 mg/kg bw) and then injected via the lateral vein of the tail
immediately after the last dose with the indicator organisms
( Salmonella typhimurium TA 98, TA 100, TA 1535, TA 1537 or TA 1538)
in a host-mediated assay. One hour later, the bacteria were recovered
from the liver of the mice. A slight increase in the number of black
mutant colonies per plate was observed at 28 mg/kg bw with strain TA
100 in the first experiment, but was not confirmed in two replicate
experiments. There was no significant increase in mutation frequency
of the other indicator strains (Arni and Müller 1981).
Groups of male and female Chinese hamsters were treated orally
with methacrifos at 0, 13.5, 27 or 54 mg/kg bw/day for two consecutive
days in a nucleus anomaly test. The animals were sacrificed 24 hours
after the second dose and bone marrow smears were evaluated for
nucleus anomalies in interphase cells. There was no significant
difference between control and treated groups in the incidence of bone
marrow cells with anomalies of nuclei (Hool et al 1981).
In an in vivo sister chromatid exchange test, groups of Chinese
hamsters (each subcutaneously implanted with a single 45 mg tablet of
5-bromodeoxy-uridine 2 hours earlier) were given single oral doses of
methacrifos at 0, 27, 54 or 108 mg/kg bw and sacrificed 24 hours later
for the preparation of bone marrow cell slides (each animal was given
intraperitoneally 10 mg/kg body weight of colcemide 2 hours prior to
sacrifice). There was no significant difference between control and
treated groups in the incidence of sister chromatid exchanges (Hool
and Müller 1981).
Short-Term Studies
Dog
Two female pedigreed beagles were given methacrifos in gelatin
capsules 7 days/ week for 4 consecutive weeks at 3 mg/kg bw/day
(week 1), 6 mg/kg bw/day (week 2) and 12 mg/kg bw/day (weeks 3 and 4).
The animals were then maintained for a recovery period of 3 weeks
(cholinesterase and carboxy esterase data were, however, available for
a total of 8 weeks). The control group comprised 2 female dogs. There
was no mortality. Diarrhoea, observed in both control and treated
groups, was more pronounced and more frequent in the latter. Vomiting
was also noted in both treated dogs, but the time of occurrence was
not given. Body weight and food consumption were adversely affected
from week 2 onward, with recovery being evident for food consumption
but not for body weight 3 weeks after treatment withdrawal.
Erythrocyte cholinesterase was inhibited by 63-75% and plasma
cholinesterase by 21-57% throughout the treatment period, with the
magnitude of depression increasing with time. At the end of the
recovery period, plasma cholinesterase almost completely recovered,
while erythrocyte cholinesterase remained depressed by nearly 50%.
Plasma carboxylesterase activity was reduced (>20%) in one treated
dog through the 4-week dosing period and in another only at week 4.
Complete recovery of the enzyme was apparent 2 to 3 weeks after
treatment withdrawal. Brain cholinesterase assayed 4 weeks
after termination of the treatment period was not depressed.
Neurophysiological examination (including determination of maximum
nerve conduction velocity in sensory and motor pathways), observation
of behaviour and neurological examination conducted at the end of
dosing period all failed to reveal any significant difference between
treated and control dogs. Histopathological examination of the teased
peripheral nerve fibres (tibial nerve and plantar nerve) from animals
sacrificed at the end of the 4-week recovery period showed no abnormal
morphological changes (Gfeller et al 1982).
Observations in Humans
Two healthy male volunteers were given technical methacrifos
(96.2%) orally at approximately 0.073 mg/kg bw/day for 7 consecutive
days followed by a dose of 0.145 mg/kg bw on the 8th day. There were
no toxic signs. Haematological, biochemical and urinalysis values
determined before and after the treatment period were not
significantly different. The activity of cholinesterase in plasma and
erythrocyte was not inhibited at any time during the treatment or the
1-week recovery period (Strittmatter and Gfeller 1979).
A male volunteer was given daily 5 mg or approximately
0.067 mg/kg bw technical methacrifos (95% pure, "absorbed" in 150 mg
of lactose) in gelatin capsule, 7 days/week for 4 weeks. No adverse
reaction was reported. The activities of plasma and erythrocyte
cholinesterase measured on day 19 of treatment and on day 12 post-
treatment were not significantly different from those before treatment
(Loosli et al 1982).
COMMENTS
The available teratology study in (Chinchilla) rabbits failed to
indicate any teratogenic activity of methacrifos under the conditions
of the experiment. However, sensitivity of this particular strain of
rabbits to known teratogens was not established. Mutagenicity studies
including unscheduled DNA synthesis in rat hepatocytes and human
fibroblasts, a host-mediated assay, a nucleus anomaly test and an
in vivo sister chromatid exchange test were all negative.
Nevertheless, there were some suggestions of positive mutagenic
response in 2 of the 3 strains of Saccharomyces cerevisiae employed
in the in vitro tests. Observations in humans involving a very
limited number (a total of 3) of volunteers seemed to indicate
subacute (7-28 days) oral treatment with methacrifos in the vicinity
of 0.07 mg/kg bw/day resulted in no adverse effects or any significant
inhibition of plasma or erythrocyte cholinesterase.
In the absence of an appropriate reproduction study and a
teratology study in rats, the Meeting could only agree to extend the
temporary ADI.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 1 mg/kg in the diet, equivalent to 0.05 mg/kg bw
Dog : 1 mg/kg in the diet, equivalent to 0.025 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Teratogenicity study in rats.
2. An appropriate reproduction study
Desirable
1. Information on sensitivity of chinchilla rabbits to known
teratogens.
2. Further observations in humans.
REFERENCES
Arni, P. and Müller, D. Test for non-disjunction on Saccharomyces
1980a cerevisiae D61 with CGA 20168. (Test for mutagenic
properties in yeast cells). Report from Ciba-Geigy Limited,
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
1980b Mutagenicity test on Saccharomyces cerevisiae MP-1
in vitro with CGA 20168. (Test for mutagenic properties in
yeast cells). Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
1981 Intrasanguine host-mediated assay with Saccharomyces
cerevisiae with CGA 20168, (Test for the demonstration of
point mutations in bacteria in vivo.) Report from Ciba-Geigy
Limited submitted to the World Health Organization by Ciba-
Geigy. (Unpublished)
1982 D7/mammalian-microsome mutagenicity test in vitro with CGA
20168. (Test for mutagenic properties in yeast cells).
Report from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Fritz, H., Giese, K. and Hess, R. Report on CGA 20168 tech. Teratology
1980 study (Seg. II) in rabbits. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Gfeller, W., Krinke, G. and Schaeppi, U. Final report CGA 20168.
1982 Neurotoxicity study in dogs. Report from Ciba-Geigy Limited
submitted to the World Health Organization by Ciba-Geigy.
(Unpublished)
Hool, G. and Müller, D. Sister chromatid exchange study CGA 20168.
1981 Chinese hamster. (Test for mutagenic effects on bone marrow
cells.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
Hool, G., Langauer, M. and Müller, D. Nucleus anomaly test in somatic
1981 interphase nuclei CGA 20168. Chinese hamster. (Test for
mutagenic effects on bone marrow cells.) Report from Ciba-
Geigy Limited submitted to the World Health Organization by
Ciba-Geigy. (Unpublished)
Loosli, R., Hornisch, H.P. and Dubach, P. Final Report CGA 20168
1982 28-day palatability study in a human volunteer. Report from
Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Puri, E. and Müller, D. Autoradiographic DNA repair test on rat
1982a hepatocytes- CGA 20168. (In vitro test for DNA-damaging
properties.) Report from Ciba-Geigy Limited submitted to the
World Health Organization by Ciba-Geigy. (Unpublished)
1982b Autoradiographic DNA repair test on human fibroblasts CGA
20168. (In vitro test for DNA-damaging properties.) Report
from Ciba-Geigy Limited submitted to the World Health
Organization by Ciba-Geigy. (Unpublished)
Strittmatter, J. and Gfeller, W. CGA 20168 technical effect on
1979 cholinesterase activity in man following repeated oral
administration. Report from Ciba-Geigy Limited submitted to
the World Health Organization by Ciba-Geigy. (Unpublished)
