
BITERTANOL JMPR 1998
First draft prepared by
R. Solecki
Pesticide and Biocide Division, Federal Insitute
for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Effects on the central nervous system
Toxicity in combinations
Effect on the liver
Studies on metabolites
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-
3,3-dimethylbutan-2-one (plants, soil)
Bitertanol benzoic acid (soil)
1,2,4-triazole (photodegradation, soil)
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Bitertanol was previously evaluated toxicologically by the Joint
Meeting in 1983, 1987, and 1988 (Annex 1, references 40, 50, and 53).
The 1983 JMPR allocated a temporary ADI of 0-0.005 mg/kg bw and
requested studies on metabolism in order to clarify the metabolic
pathway of bitertanol, a study of toxicity in dogs treated orally for
a minimum of one year, and a long-term study of toxicity and
carcinogenicity in rats at appropriate doses. Relevant data were
submitted for evaluation by the 1987 JMPR, when an ADI of 0-0.003
mg/kg bw was established on the basis of a NOAEL of 10 ppm in a
one-year study in dogs. In 1988, the Meeting concluded that, upon
further consideration of the data from two-year and one-year studies
of toxicity in dogs, the NOAEL was 25 ppm (equal to 1 mg/kg bw per
day). Therefore, an ADI of 0-0.01 mg/kg bw was allocated using a
safety factor of 100. The compound was reviewed at the present Meeting
within the CCPR periodic review programme. This monograph summarizes
new data on bitertanol and data that were not previously reviewed and
includes relevant data from the previous monographs (Annex 1,
references 41 and 52).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Groups of five male and five female Wistar rats received
[14C-phenyl]-bitertanol as a solution in ethylene glycol as a single
oral dose of 100 or 1000 mg/kg bw or a single intravenous dose of
100 mg/kg bw as well as 14 daily oral doses of unlabelled chemical at
100 mg/kg bw, followed by a single oral dose of 14C-bitertanol at
100 mg/kg bw. Urine and faeces were collected 6 and 24 h after dosing
and then at subsequent 24-h intervals until sacrifice seven days
later. Serial blood samples were collected during this period.
Total absorption of radiolabel was found to depend on the dose
over the range studied. Faecal excretion of radiolabel after
intravenous administration showed that biliary excretion was
predominant. Urinary excretion represented 4-11% of the administered
dose, while no radiolabel was detected in expired air. The total
recovery of radiolabel was > 92%. Pharmaco-kinetic analysis indicated
that absorption of bitertanol after oral administration follows a
first-order pattern for single and repeated doses of 100 mg/kg bw but
not at the higher dose (1000 mg/kg), suggesting saturation of
absorption, distribution, or elimination. The highest concentrations
detected in tissues were in the liver (17.9 ppm, females) and kidney
(5.9 ppm, males) of animals at the high dose. Seven days after dosing,
0.2-0.4% of the administered radiolabel remained in the body (Puhl &
Hurley, 1983).
The biokinetic behaviour of bitertanol in rats was investigated
in studies requested by the Japanese registration authority, which
were conducted in compliance with standards of good laboratory
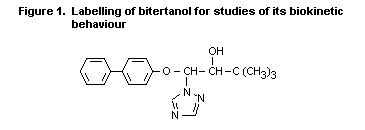
practice. The compound was uniformly labelled with 14C in the phenyl
moiety, as shown in Figure 1 and was administered orally to male and
female rats at a dose of 10 mg/kg bw. Radiolabel was determined over
time in the excreta and plasma, and the body and individual organs and
tissues were assayed for total radiolabel at sacrifice. Male rats were
also given the same dose intraduodenally after bile fistulation, and
total radiolabel was assayed in excreta, including the bile, and in
the body at sacrifice. Total radiolabel was also determined over time
in various organs of male rats after oral administration of a single
dose of 10 mg/kg bw.
 About 84% of the dose was absorbed after oral administration.
Absorption commenced immediately, and the plasma concentration
increased from 25 to 75% of the peak value within 1-2 h. The
radiolabel was eliminated rapidly and almost completely from the body
within the 72-h test period. More than 90% of the recovered radiolabel
was excreted with the faeces, and about 7% with the urine. On average,
the residual radiolabel in the body (excluding the gastrointestinal
tract) after 72 h represented 0.5% of the administered dose. The
results obtained after bile fistulation indicate that the largest
fraction of faecally eliminated radioactivity was first absorbed and
then eliminated into the gut lumen with the bile (about 77%). Most of
the radiolabel eliminated in the bile underwent enterohepatic
circulation. The radiolabel was rapidly distributed from the
intravascular space to the peripheral tissues. The maximum
concentration in plasma was reached after 3-8 h. The terminal phase of
elimination of radiolabel from the plasma was described by linear
regression analysis, which yielded a half-life of about 26 h. The
distribution of total radiolabel in individual organs of male rats at
various times after administration of a single oral dose of 10 mg/kg
was generally similar to that observed 72 h after administration. The
highest concentrations were found in the liver and kidney (Klein,
1988a,b, 1989).
After a 50% formulation of 14C-labelled bitertanol was applied
to the skin of adult male and female albino rabbits, the mean level of
dermal penetration was around 10%. There were no marked sex-specific
differences. Since this experiment was designed to simulate
'worst-case' conditions, lower absorption rates might expected in
practice (Hixson, 1984).
(b) Biotransformation
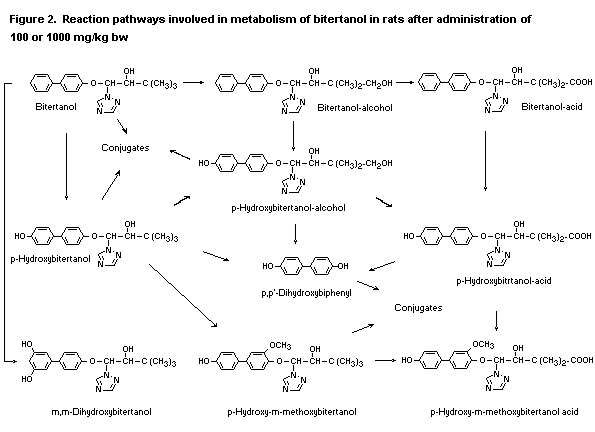
In the first study described above, bitertanol was extensively
metabolized, and the metabolite profile was similar in the groups
receiving 100 or 1000 mg/kg bw. The relative amounts of metabolites
were also similar, except that the animals receiving the highest oral
dose eliminated much more unchanged parent compound than the others.
Fourteen metabolites (plus bitertanol), representing 38-76% of the
recovered radiolabel, were identified or characterized. Hydroxylation
of the para position of the biphenyl and methyl groups of the
tert-butyl moieties gave rise to phenolic and diol metabolites.
Although one diastereomer of the latter was detected, it underwent
oxidation to the corresponding butanoic acid and subsequent ring
hydroxylation. The parahydroxylated diol was also detected, as was
4,4-dihydroxy-biophenyl, and other hydroxylated metabolites were also
tentatively identified. The metabolic reactions thus included ring
monohydroxylation, ring dihydroxylation, aryl O-methylation,
aliphatic hydroxylation, aliphatic oxidation to carboxylic acids, and
ether cleavage. In addition to the metabolites shown in Figure 2,
glucuronide and sulfate conjugates of some metabolites, including
para-hydroxybitertanol, were also detected in faeces and urine (Puhl
& Hurley, 1983).
In the second set of studies described above, the structures and
amounts of metabolites were determined in faeces, urine, and bile and
in the liver, kidneys, and perirenal fat at various times after
treatment. The metabolism of bitertanol began immediately after
absorption from the gastrointestinal tract lumen. The parent compound
was not detected in urine or bile, and the only metabolite identified
in the bile was para-hydroxybitertanol. Excretion of the unchanged
parent compound in the urine was unlikely because of its lipophilic
character. Metabolic degradation of bitertanol in the organs was
rapid: within 8 h, the concentration in the liver fell from around 15%
to about 2% of the total radiolabel in the organs. The main metabolite
in the liver was also para-hydroxybitertanol; smaller amounts of
para-hydroxy-bitertanol acid, para-hydroxybitertanol alcohol, and
bitertanol acid were also identified. The distribution of metabolites
in the kidneys was similar: total organ radiolabel fell from around
14% to about 2.5% within 8 h; the main metabolite was again
para-hydroxybitertanol, which represented 30-50% of the organ
radiolabel. Furthermore, the amounts of metabolites in the liver and
kidneys were similar. The total amount of radiolabel in fat samples
was too low to permit reliable quantification or identification of
possible metabolites, due mainly to the small amount of fat available
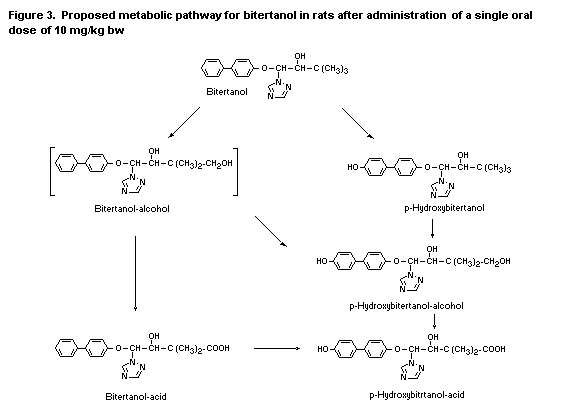
in the young animals. A proposed metabolic pathway for bitertanol in
rats is given in Figure 3. The parent compound found in the faeces of
orally treated rats was probably due to the unabsorbed fraction of
administered radiolabel, representing about 15% of the original dose.
The main metabolite in the liver and kidneys,
para-hydroxy-bitertanol, was also identified in the faeces. The
amounts of the other biotransformation products in the organs were
probably too low for detection in the excreta (Klein, 1988b, 1989).
2. Toxicological studies
(a) Acute toxicity
The methods used in the studies summarized below complied to a
certain extent with OECD guidelines. At the time that most of the
studies were performed, compliance with good laboratory practice (GLP)
was not compulsory. The results of studies on the acute toxicity of
About 84% of the dose was absorbed after oral administration.
Absorption commenced immediately, and the plasma concentration
increased from 25 to 75% of the peak value within 1-2 h. The
radiolabel was eliminated rapidly and almost completely from the body
within the 72-h test period. More than 90% of the recovered radiolabel
was excreted with the faeces, and about 7% with the urine. On average,
the residual radiolabel in the body (excluding the gastrointestinal
tract) after 72 h represented 0.5% of the administered dose. The
results obtained after bile fistulation indicate that the largest
fraction of faecally eliminated radioactivity was first absorbed and
then eliminated into the gut lumen with the bile (about 77%). Most of
the radiolabel eliminated in the bile underwent enterohepatic
circulation. The radiolabel was rapidly distributed from the
intravascular space to the peripheral tissues. The maximum
concentration in plasma was reached after 3-8 h. The terminal phase of
elimination of radiolabel from the plasma was described by linear
regression analysis, which yielded a half-life of about 26 h. The
distribution of total radiolabel in individual organs of male rats at
various times after administration of a single oral dose of 10 mg/kg
was generally similar to that observed 72 h after administration. The
highest concentrations were found in the liver and kidney (Klein,
1988a,b, 1989).
After a 50% formulation of 14C-labelled bitertanol was applied
to the skin of adult male and female albino rabbits, the mean level of
dermal penetration was around 10%. There were no marked sex-specific
differences. Since this experiment was designed to simulate
'worst-case' conditions, lower absorption rates might expected in
practice (Hixson, 1984).
(b) Biotransformation
In the first study described above, bitertanol was extensively
metabolized, and the metabolite profile was similar in the groups
receiving 100 or 1000 mg/kg bw. The relative amounts of metabolites
were also similar, except that the animals receiving the highest oral
dose eliminated much more unchanged parent compound than the others.
Fourteen metabolites (plus bitertanol), representing 38-76% of the
recovered radiolabel, were identified or characterized. Hydroxylation
of the para position of the biphenyl and methyl groups of the
tert-butyl moieties gave rise to phenolic and diol metabolites.
Although one diastereomer of the latter was detected, it underwent
oxidation to the corresponding butanoic acid and subsequent ring
hydroxylation. The parahydroxylated diol was also detected, as was
4,4-dihydroxy-biophenyl, and other hydroxylated metabolites were also
tentatively identified. The metabolic reactions thus included ring
monohydroxylation, ring dihydroxylation, aryl O-methylation,
aliphatic hydroxylation, aliphatic oxidation to carboxylic acids, and
ether cleavage. In addition to the metabolites shown in Figure 2,
glucuronide and sulfate conjugates of some metabolites, including
para-hydroxybitertanol, were also detected in faeces and urine (Puhl
& Hurley, 1983).
In the second set of studies described above, the structures and
amounts of metabolites were determined in faeces, urine, and bile and
in the liver, kidneys, and perirenal fat at various times after
treatment. The metabolism of bitertanol began immediately after
absorption from the gastrointestinal tract lumen. The parent compound
was not detected in urine or bile, and the only metabolite identified
in the bile was para-hydroxybitertanol. Excretion of the unchanged
parent compound in the urine was unlikely because of its lipophilic
character. Metabolic degradation of bitertanol in the organs was
rapid: within 8 h, the concentration in the liver fell from around 15%
to about 2% of the total radiolabel in the organs. The main metabolite
in the liver was also para-hydroxybitertanol; smaller amounts of
para-hydroxy-bitertanol acid, para-hydroxybitertanol alcohol, and
bitertanol acid were also identified. The distribution of metabolites
in the kidneys was similar: total organ radiolabel fell from around
14% to about 2.5% within 8 h; the main metabolite was again
para-hydroxybitertanol, which represented 30-50% of the organ
radiolabel. Furthermore, the amounts of metabolites in the liver and
kidneys were similar. The total amount of radiolabel in fat samples
was too low to permit reliable quantification or identification of
possible metabolites, due mainly to the small amount of fat available
in the young animals. A proposed metabolic pathway for bitertanol in
rats is given in Figure 3. The parent compound found in the faeces of
orally treated rats was probably due to the unabsorbed fraction of
administered radiolabel, representing about 15% of the original dose.
The main metabolite in the liver and kidneys,
para-hydroxy-bitertanol, was also identified in the faeces. The
amounts of the other biotransformation products in the organs were
probably too low for detection in the excreta (Klein, 1988b, 1989).
2. Toxicological studies
(a) Acute toxicity
The methods used in the studies summarized below complied to a
certain extent with OECD guidelines. At the time that most of the
studies were performed, compliance with good laboratory practice (GLP)
was not compulsory. The results of studies on the acute toxicity of
 bitertanol are summarized in Table 1. Bitertanol had very low acute
toxicity in rats, mice, and dogs treated orally. No significant sex
difference was observed. The LD50 values are 4000-5000 mg/kg bw or
higher. The toxicological properties of the A and B isomers are
similar. The symptoms included nonspecific signs, such as deteriorated
general condition, isolation from the group, and piloerection;
however, central nervous system effects were also seen, including
sedation, spasms, and respiratory disturbances. Lobulation of the
liver and irritation of the glandular stomach mucosa were observed at
necropsy of orally treated rats. Further signs in dogs were vomiting
and diarrhoea. Sheep were more susceptible, with an oral LD50 of
about 1000 mg/kg bw. Bitertanol was moderately toxic to rats and mice
after intraperitoneal injection.
Dermal exposure to a dose of 5000 mg/kg bw was tolerated by rats
with no observed signs. It was not toxic to rats after inhalation in
an aerosol, even at the highest test concentration. An initial test
showed no primary irritation of the intact or abraded skin of rabbits
inspected 24 and 72 h after the beginning of exposure (Thyssen &
Kimmerle, 1977a). In a study in rabbits, the active ingredient was
found to be slightly irritating to intact and abraded skin (Iyatomy,
1981).
A five-minute treatment of the eye of rabbits did not cause
primary irritation of the mucous membranes. Slight to moderate
reddening of the conjunctivae, which persisted for about 48 h, was
observed after a 24-h exposure (Thyssen & Kimmerle, 1977a). A reaction
that was reversible within four days was seen in rabbit conjunctivae;
the cornea and iris were unaffected (Iyatomy, 1981).
After an initial administration of 0.05 mg bitertanol per animal
to female Pirbright guinea-pigs, nine repeated intracutaneous
injections of emulsified bitertanol (0.1 mg) over three consecutive
weeks had no sensitizing effect. Intracutaneous injection of an
additional 0.05 mg after a further two weeks also failed to produce
signs of dermal sensitization (Thyssen, 1977).
Magnusson and Kligman tests in 20 male and 20 female Pirbright
guinea-pigs with Freund's adjuvant provided no evidence of sensitizing
effects of bitertanol at a concentration of 1% for intradermal
induction, 25% for topical induction, and 25% for challenge (Flucke,
1981).
(b) Short-term studies of toxicity
Rats
Groups of 20 male and 20 female Wistar rats were given bitertanol
(purity, 96.5%) by gavage at doses of 0, 30, 100, or 300 mg/kg bw per
day for 28 days. Half of the animals were then sacrificed, and the
other half were observed for a further 28 days. The method used in
Table 1. Acute toxicity of bitertanol
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat NR M Oral > 5000 99.1 A Mihail (1978a)
Rat NR M Oral > 5000 97.1 B Mihail (1978b)
Rat Wistar M/F Oral (fasted) > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M Oral > 5000 97.3 Flucke (1978)
Rat NR M Oral 4000 95.0 Flucke (1979)
Rat NR M Oral 3700 95.0 Iyatomy (1980)
F Oral 3900
Rat Wistar F Oral (fasted) > 5000 95.1 Flucke (1980)
Rat NR M Oral > 5000 96.7 Heimann (1981)
M Oral (fasted) > 5000
Rat NR M Oral (fasted) 4800 99.1 Mihail (1982a)
Rat NR M Oral (fasted) 4800 97.1 Mihail (1982b)
Rat NR M Oral > 5000 NR Heimann (1983a)
Rat NR M Oral (fasted) > 5000 97.6 Heimann (1984a)
Mouse NMRI M Oral (fasted) 4500 96.5 Thyssen & Kimmerle (1977a)
F Oral (fasted) 4200
Mouse NR M Oral 3500 95.0 Iyatomy (1980)
F Oral 3200
Dog Beagle M/F Oral (fasted) > 5000 95.0 Hoffmann (1981a)
Sheep Blackface M/F Oral ~ 1000 95.0 Hoffmann (1981b)
Rat Wistar M/F Dermal > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M/F Dermal > 5000a 95.0 Iyatomy (1980)
Mouse NR M/F Dermal > 5000 95.0 Iyatomy (1980)
Rabbit New Zealand M/F Dermal > 2000 94.9 Hixson (1979)
Rat Wistar M/F Inhalation 1 h > 720 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 4 h > 550 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 5 x 4 h > 380 96.5 Thyssen & Kimmerle (1977a)
Ratb Sprague-Dawley M/F Inhalation 4 h > 1200 95.7 Shiotsuka (1987)
Table 1. (continued)
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat Wistar M Intraperitoneal 1200 96.5 Thyssen & Kimmerle (1977a)
F Intraperitoneal 720
Rat NR M Intraperitoneal 700 95.0 Iyatomy (1980)
F Intraperitoneal 560
Mouse NR M Intraperitoneal 570 95.0 Iyatomy (1980)
F Intraperitoneal 610
Rat NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
Mouse NMRI M/F Subcutaneous > 5000 96.5 Thyssen & Kimmerle (1977a)
Mouse NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
NR, not reported; M, male; F, female; A, diastereomer A; B, diastereomer B
a Slight local irritation present
b The study was conducted in compliance with good laboratory practive
this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
Doses of 100 mg/kg per day and higher had a dose-related adverse
effect on body-weight development. At 300 mg/kg per day, behavioural
disturbances (isolation from the other animals, dirty coat) and hair
loss were observed in the female rats; however, all females had normal
behaviour near the end of the treatment period. Animals at this dose
had moderate leukocytosis, and females had reduced haemoglobin and
thrombocyte counts. Increased alkaline phosphatase activity was
observed only in the females. The relative liver weights of animals of
each sex at 100 mg/kg bw per day and above were increased. At
300 mg/kg bw per day, the absolute liver weights were elevated and the
absolute weights of the heart, kidneys, adrenals, and ovaries
depressed in female rats at the termination of treatment. The relative
weights of the thyroid and testes in males and of the spleen in
females were also elevated, whereas the relative weights of the heart,
adrenals, and ovaries were depressed in females. Hyperkeratoses and
parakeratoses, distended epithelial cells, slight inward growth of the
papillary body, and cellular infiltration in the epithelial and
subepithelial layers were observed histopathologically in the
forestomachs of four female animals at 300 mg/kg bw per day that were
sacrificed at the end of treatment. The appearance of the digestive
tract was unexceptional at the end of the recovery period., and all of
the changes in the rats at the high and intermediate doses had
likewise reverted. The NOAEL was 30 mg/kg bw per day (Thyssen &
Kaliner, 1977).
Groups of 20 male and 20 female Sprague-Dawley rats were given
bitertanol (purity, 95%) at doses of 0, 100, 400, or 1600 ppm, equal
to 7, 28, and 110 mg/kg bw per day in males and 7.4, 30, and 110 mg/kg
bw per day in females, for 28 days. The test procedures complied to a
certain extent with OECD guideline 407; at the time the study was
performed, compliance with GLP was not compulsory. Half of the animals
were sacrificed and examined after four weeks, whereas the other half
were observed without treatment for a further four weeks.
The dose of 400 ppm had an adverse effect on body-weight
development and food intake, and an adverse effect on the red blood
cell population was seen from decreases in the haematocrit and
haemoglobin readings. The relative liver weight was slightly elevated
in animals of each sex. Those at 1600 ppm had ataxia, reduced food
intake and body weight, and depressed red blood cell parameters
(haemoglobin, haematocrit, erythrocyte count), with a simultaneous
increase in the reticulocyte count. A slight increase in the serum
cholesterol concentration and a significant increase in the relative
liver weight were found in animals of each sex. Histopathological
examination revealed weak irritation of the gastric mucosa and
slightly altered ovaries, adrenals, and pituitaries. All of the
observed effects were reversible within the four-week recovery period.
The NOAEL was 100 ppm, equal to 7 mg/kg bw per day (Hatanaka et al.,
1981).
Bitertanol (purity, 90.2%) was administered to groups of 20 male
and 20 female Wistar rats at concentrations of 0, 150, 600, or 2400
ppm, equal to 12, 48 and 300 mg/kg bw per day in males and 13, 58, and
310 mg/kg bw per day in females, for three months. This study was
conducted before enactment of prevailing regulatory guidelines;
however, the procedures complied to certain extent with OECD guideline
408.
Although appearance and behaviour were unaffected at doses up to
600 ppm, 2400 ppm caused decreased motility and reduced food intakes.
Dose-related growth retardation was observed in females at 600 and
2400 ppm and in male animals at all concentrations; however, the 150
ppm dose decreased body weights only temporarily. The dose of 2400 ppm
had adverse effects on various haematological parameters, including
reduced erythrocyte and leukocyte counts, decreased haemoglobin
content and haematocrit reading, increased reticulocyte counts, a
relative increase in the polymorphonuclear leukocyte count, and a
relative decrease in the lymphocyte count. The changes in clinical
chemical parameters of the blood also found at this concentration were
elevated alkaline phosphatase, aspartate aminotransferase, and
glutamate dehydrogenase activities in the female animals. The blood
protein level was slightly depressed and the cholesterol level
slightly elevated. The finding of increased liver weight in females at
2400 ppm also indicated an effect on the liver. The NOAEL was 150 ppm,
equal to 12 mg/kg bw per day (Bomhard & Löser, 1978).
In a supplementary study, bitertanol (purity, 90.8%) was
administered to groups of 15 male and 15 female Wistar rats at
concentrations of 0, 30, 100, or 300 ppm, equal to 2.5, 8.1, and 25
mg/kg bw per day in males and 3.3, 10, and 32 mg/kg bw per day in
females, for three months. The method used in this study complied to a
certain extent with OECD guideline 408; at the time the study was
performed, compliance with GLP was not compulsory.
The appearance, behaviour, and mortality rate were unaffected at
all concentrations. At 300 ppm, the rats gained less weight than the
controls. Haematological, clinical chemical, gross and
histopathological examination, and urinary analyses provided no
evidence of adverse effects. The NOAEL was 100 ppm, equal to 8 mg/kg
bw per day (Krötlinger et al., 1978).
In a further study, bitertanol (purity, 95%) was administered to
groups of 15 male and 15 female Sprague-Dawley rats at concentrations
of 0, 40, 200, or 1000 ppm, equal to 3.1, 16, and 82 mg/kg bw per day
in males and 3.6, 19, and 88 mg/kg bw per day in females, for three
months. Five males and five females were subjected to urinary,
haematological, and clinical chemical determinations. The method used
in this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
The dose of 200 ppm retarded weight gain in male and female
animals and slightly increased the activities of alkaline phosphatase
and aspartate aminotransferase in the serum of females. The dose of
1000 ppm reduced body-weight gain and decreased food and water intakes
in animals of each sex. The erythrocyte count was also depressed, and
the reticulocyte count elevated in male and female animals. In
addition, the leukocyte and thrombocyte counts were elevated, and the
haemoglobin content and haematocrit reading were decreased in females.
Elevated lactate dehydrogenase, aspartate aminotransferase, and
alkaline phosphatase activities and a slightly elevated cholesterol
concentration were found in the serum of females. The absolute weights
of the liver and spleen were increased in females and the relative
weights in males and females. The following lesions were found
histopathologically in animals of each sex: increased swelling and
fatty degeneration of hepatocytes, hyperkeratoses of the oesophageal
and gastric mucosal epithelium and/or erosions of the glandular
stomach, swelling, and fatty degeneration of adrenal cortical cells.
Isolated bile-duct proliferation was also seen in females. The NOAEL
was 40 ppm, equal to 3 mg/kg bw per day (Yonemura et al., 1981).
Groups of 10 male and 10 female Wistar rats were exposed to
bitertanol at mean analytically determined concentrations of 18, 63,
or 200 mg/m3 air for 6 h per day, five days per week for three weeks.
Only the head and nose of the animals were exposed, under dynamic
conditions, and the inhaled bitertanol was dissolved in a 1:1 blend of
ethanol:polyethylene glycol 400.The method used in this study complied
to a certain extent with OECD guideline 412; at the time the study was
performed, compliance with GLP was not compulsory.
The concentrations of 18 and 63 mg/m3 air were tolerated by male
and female rats with no observed effect. Exposure to 200 mg/m3 air
caused deterioration of the general condition of female rats, and the
growth of male rats in this group was significantly decreased.
Although the relative weights of the lung, liver, kidney, and adrenal
in males and females at the high concentration were sometimes higher
than control values, no evidence of damage due to exposure to
bitertanol was seen histopathologically. The NOAEL was 63 mg/m3 air
(Mihail & Kimmerle, 1977).
Dogs
Groups of four male and four female beagles received bitertanol
(purity, 90.2%) at a dose of 0, 1, 5, or 25 mg/kg bw per day in
gelatin capsules. The method used in this study complied to a certain
extent with OECD guideline 409; at the time the study was performed,
compliance with GLP was not compulsory.
Doses of 5 mg/kg bw per day and above led to signs of
dose-related adverse effects on the skin and mucous membranes. The
effects on the skin were manifested by increased reddening, with local
inflammation, desquamation, and hair loss; and those on the mucous
membranes by increased reddening and slight inflammatory phenomena in
regions of the oral cavity (gingiva) and eyes (conjunctiva). They were
evident histologically as slight broadening of the epithelial layer of
the skin and slightly enhanced cornification in some cases. Evidence
for superficial involvement of the cornea (keratitis), apparently
resulting from conjunctival irritation, was observed in a few animals
at 25 mg/kg bw per day. The behaviour of the controls and treated
animals was similar; in contrast, the food intake and body-weight
development of animals at the high dose showed an adverse effect. The
results of laboratory tests indicated treatment-related interference
with liver function (elevated alkaline phosphatase and alanine
aminotransferase activities) in dogs at 5 and 25 mg/kg bw per day.
Elevated N-demethylase activity and an increase in the cytochrome
P450 concentration in liver homogenates were found in the high-dose
group at study termination. The weights of the thymus in females at
25 mg/kg per day and of the prostate (relative at 5 mg/kg per day and
both relative and absolute at 25 mg/kg per day) were statistically
significantly lower than those of the controls. The changes in
prostate weights correlated with dose-related histopathological
findings of retarded development. The NOAEL was 1 mg/kg bw per day
(Hoffmann & Schilde, 1979).
In view of the dermal phenomena observed in the previous study,
tests were performed to determine whether they represented a
sensitization effect. Beagle dogs were first treated orally 10-42
times with 35 or 70 mg/kg bw bitertanol, which led to marked
irritation and dermal lesions, particularly in the area of the head.
After a treatment-free interval of four to six weeks, when the lesions
had healed, the animals were treated with a dose of 1.75 mg/kg bw,
previously found to induce no reaction, for 14 days. Hair loss and a
slightly increased incidence of reddened gingiva were determined in
isolated animals only at termination of treatment. The study gave no
evidence for sensitization, and the delay in development of the effect
specifically argues against such an effect (Hoffmann, 1977).
No evidence for sensitization was found in a study in which
beagles were exposed to a concentration of 28.8 mg/m3 air for 4 h per
day, five days per week for three weeks, left untreated for 10 days,
and subsequently re-exposed to a concentration of 47.1 mg/m3 air for
one week. All dogs tolerated the exposures with no signs, and no
evidence was found for either an irritating effect on visible mucous
membranes or for a Type I (immediate anaphylactic type) allergy
(Thyssen & Kimmerle, 1977b).
Cats
Groups of three male and three female cats weighing 2-3 kg
received whole-body exposure to aerosols of bitertanol (active
ingredient, 95.8%) in a 3-m3 inhalation chamber for 6 h per day for
four weeks (20 x). The mean analytically determined bitertanol
concentration was 27 mg/m3 air. Analysis of the generated aerosols
showed that more than 90% of the aerosol particles had a mass-related
and more than 99% a particle-related aerodynamic diameter smaller than
5 µm. The exposed animals were compared with negative controls (air)
and positive controls (dichlozoline, 28 mg/m3 air). The animals were
observed over a recovery period of 12 weeks after termination of the
exposure. Their eyes were examined with an ophthalmoscope before
exposure and each week throughout the four-week exposure and the
recovery period and for gross and histopathological alterations at the
end of the study. The method used complied with OECD guideline 412; at
the time the study was performed, compliance with GLP was not
compulsory.
The exposed cats tolerated the treatment with no signs of
toxicity, damage to the eyes, or cataractogenesis. Under comparable
experimental conditions, the animals exposed to dichlozoline showed
signs of lenticular opacity (cataracts), starting from the end of the
second week of exposure. Lenticular opacity that had not reverted up
to the end of the recovery period was observed in all animals of this
group at the end of the second week of the recovery period. Cats
exposed to bitertanol had no cataracts (Pauluhn et al., 1983).
Rabbits
Groups of six male and six female New Zealand rabbits received
applications of bitertanol (purity, 95.8%) in an aqueous suspension
(0.5 ml) at concentrations of 0, 50, or 250 mg/kg bw on the intact or
abraded dorsal and lateral skin for 6 h per day, five times per week
for three weeks. The method used in this study complied to a certain
extent with OECD guideline 411; at the time the study was performed,
compliance with GLP was not compulsory.
Bitertanol had no apparent effect on the appearance, behaviour,
body weight, or survival of the rabbits. Transient erythema developed
on the exposed areas, initially on abraded skin but later on intact
skin. There was no effect on skin-fold thickness or on haematological,
clinical chemical, or urinary parameters. At necropsy, there were no
gross pathological findings. Histopathological examination showed
slight epidermal thickening only in exposed areas of all treated
animals. Preparations of liver showed no evidence of induction of
microsomal N- or O-demethylation or cytochrome P450 content. The
dermal application had no effect on any of the parameters
investigated. The NOAEL was 250 mg/kg bw (Heimann & Vogel, 1984).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Bitertanol (purity, 94-95%) was administered to groups of 50 male
and 50 female CF1/W74 mice at doses of 0, 20, 100, or 500 ppm, equal
to 2, 25, and 130 mg/kg bw per day in males and 7, 37, and 180 mg/kg
bw per day in females, for two years. The study was conducted before
enactment of prevailing regulatory guidelines; however, the test
procedures complied to a certain extent with OECD guideline 453 but
with no attempt to monitor ocular changes.
Behaviour, feed consumption, mortality, and haematological
parameters were not affected by treatment. In animals at 500 ppm, body
weights were reduced and serum alkaline phosphatase activity was
significantly elevated; females at this dose had an increased
incidence of enlarged and greenish-coloured livers at necropsy. The
liver weights were also increased, particularly in the female mice,
and the livers had increased numbers of eosinophilic foci. No evidence
was found for a carcinogenic effect at any concentration, which
extended into the toxic range. The NOAEL was 100 ppm, equal to 25
mg/kg bw per day (Bomhard & Löser, 1981a).
Rats
Bitertanol (purity, 94% in weeks 1-18; 95% from week 19) was
administered to groups of 50 male and 50 female SPF Wistar rats at
doses of 0, 20, 100, or 500 ppm, equal to 1, 4.9, and 26 mg/kg bw per
day in males and 1.3, 6.6, and 34 mg/kg bw per day in females, for two
years. The study was conducted before the enactment of prevailing
regulatory guidelines; however, the test procedures complied to a
certain extent with OECD guideline 453.
The 500 ppm concentration led to retarded growth in animals of
each sex. Ocular changes were not recorded. Haematological, blood
chemical, and urinary parameters were not significantly affected, and
the mortality rate was not adversely influenced. No treatment-related
effects were seen on organ weights or the gross or microscopic
appearance of the tissues. In particular, the type, location,
incidence, and time to occurrence of the tumours observed in the
treated groups were comparable to the control findings and to the
spontaneous findings for this strain of rat. The NOAEL was 100 ppm,
equal to 4.9 mg/kg bw per day (Bomhard & Löser, 1981b).
Dogs
Groups of four male and four female beagle dogs received
bitertanol (purity, 95-97.3%) in the diet at concentrations of 0, 10,
40, or 160 ppm, equal to 0.3, 1.2, and 4.9 mg/kg bw per day, for two
years. The method used in this study complied to a certain extent with
OECD guideline 452; at the time the study was performed, compliance
with GLP was not compulsory.
Although the control dogs gained more weight than did treated
dogs, there was no association with treatment. A good nutritional
status was maintained by dogs in all groups, and there was no
significant difference between groups in food or water consumption. No
abnormalities were seen in selected reflexes, body temperature, or
pulse rate; however, three of eight dogs at the high dose developed
bilateral cataracts. Urinary and haematological examinations conducted
at approximately three-month intervals revealed no abnormalities,
although serum alanine aminotransferase and alkaline phosphatase
activities were increased at 40 and 160 ppm. At necropsy, the mean
liver weight of dogs at the high dose was markedly increased.
Histological examination showed mild to moderate vacuolation of the
adrenal zona reticularis epithelia at doses at and above 40 ppm. The
NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day (Hoffmann & Gröning,
1983).
Groups of six male and six female beagles were fed diets
containing 0, 3, or 25 ppm bitertanol (purity, 96.3-96.7%) for 12
months, equal to 0.1, 1, and 7.6 mg/kg bw per day. A further group was
maintained on a diet containing 200 ppm bitertanol for 20 months to
permit continued ophthalmoscopic examination. The method used in this
study complied to a certain extent with OECD guideline 452; at the
time the study was performed, compliance with GLP was not compulsory.
Treatment had no apparent effect on behaviour, appearance, or
nutritional status, and food consumption and body-weight gain were
also unaffected. Pulse rates, body temperature, and selected reflexes
were unchanged. Ophthalmoscopy, conducted on animals at 0, 3, or 25
ppm at three-month intervals, showed no changes; however, one dog at
the high dose developed severe bilateral lenticular cataracts, which
were observable from week 58. Four other animals had slight lenticular
opacification by week 85. Dogs in this group also had signs of
conjunctivitis, with nasociliary discharge and incrustation.
Intermittent increases in serum alanine aminotransferase, glutamate
dehydrogenase, and alkaline phosphatase activities were also seen in
the dogs at the high dose. Other clinical chemical, haematological,
and urinary parameters were unaffected by treatment. At necropsy,
there were no gross abnormalities. The weights of the adrenals of the
dogs at the high dose were apparently greater than those of controls,
and lipoid vacuolation was observed in the zona reticularis epithelia.
The NOAEL was 25 ppm, equal to 1 mg/kg bw per day (Hoffmann & Vogel,
1983).
(d) Genotoxicity
No genotoxic or mutagenic potential of bitertanol was found in
lower organisms or in mammalian cells or systems in vivo or
in vitro. The results of assays for the genotoxicity of bitertanol
are summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
The effect of bitertanol (purity, 95.0%) on fertility, lactation
performance, and pup development was examined in a three-generation
study in Long-Evans FB 30 rats with two litters per generation. The
test substance was administered throughout the study period at
concentrations of 0, 20, 100, or 500 ppm, equivalent to 1, 5, and 25
mg/kg bw per day. Each of the mating groups consisted of 10 male and
20 female rats. The animals were five to six weeks old at the
beginning of the study and were treated for 70 days before the first
mating. The F3b generation pups and their parents (F2b generation)
were sacrificed and examined histopathologically after a four-week
lactation period. The method used in this study complied to a certain
Table 2. Results of assays for the genotoxicity of bitertanol
Test system Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, TA100, 4-2500 µg/plate 93.7 Negative Herbold (1979)
TA1535, TA1537
Reverse mutationa E. coli WP2 hcr- 1-5000 µg/plate 95.0 Negative Shirasu et al. (1981)
S. typhimurium TA98, TA100,
TA1535, TA1537, TA1538
Forward mutationa Mouse lymphoma L5178Y tk +/- 1-25 µg/mlb 95.0 Negative Bootman & Rees
1-20 mg/mlc (1983)
DNA repaird B. subtilis H17 rec+, M45 rec- 20-5000 µg/disc 95.0 Negative Shirasu et al. (1981)
DNA repaira,d E. coli W3110 pol A+; P3470 pol A- 0.1-33.3 mg/plate 95.0 Negative Riach (1981)
Aneuploidy Sordaria brevicollis 0.1-5.0 mg/L 95.0 Negative Bond & McGregor (1981)
Cytogenetic Chinese hamster lung cells 3.3 × 10-6-3.3 × 10-4 97.1 Negative Sasaki (1987)
alterationsa,d mol/Lb
1.0 × 10-5-1.0 ×.10-3
mol/Lc
In vivo
Micronucleus NMRI mice 2 × 1000 mg/kg bw 93.7 Negative Herbold (1978a)
formation 2 × 2000 mg/kg bw
Dominant lethal Male NMRI mice 1000 mg/kg bw 93.7 Negative Herbold (1978b)
mutation
a With and without exogenous metabolic activation
b Without exogenous metabolic activation
c With exogenous metabolic activation
d Conducted in compliance with good laboratory practice and to a certain extent with OECD guidelines
extent with OECD guideline 416; at the time the study was performed,
compliance with GLP was not compulsory.
No adverse effects were seen on appearance, behaviour, or
mortality in any group or generation. Bitertanol at a concentration of
20 ppm in the diet did not affect reproductive performance.
Administration of 100 or 500 ppm led to reductions in pup survival
rates during the lactation period in several matings and to pups with
lower birth weights. At 100 ppm, retarded pup growth was also seen in
the F2a and F2b generations. At 500 ppm, general retardation of
growth was seen. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Löser & Eiben, 1981).
(ii) Developmental toxicity
Rats
Groups of 20-23 Long-Evans rats were given bitertanol (purity,
96.5%) orally at doses of 0, 10, 30, or 100 mg/kg bw per day on days
6-15 post coitum. The method used in this study complied to a
certain extent with OECD guideline 414; at the time the study was
performed, compliance with GLP was not compulsory. The body-weight
gain of dams at 30 or 100 mg/kg bw per day was significantly reduced
during treatment and throughout the gestation period for those at 100
mg/kg bw per day. Resorption rates, fetal deaths, placental weights,
and sex ratio were unaffected by treatment; however, fetal weights
were significantly reduced at 100 mg/kg bw per day and significant
fetal stunting occurred at doses of 30 mg/kg bw per day and higher. At
100 mg/kg bw per day, skeletal ossification was retarded, and a
significantly increased incidence of malformations was observed, which
included cleft palate, hydrocephalus, malformed tails, dysplasia, and
synostosis of the ribs. A simple case of hydrocephalus occurred at 30
mg/kg bw per day. The NOAEL was 10 mg/kg bw per day (Machemer, 1977).
Groups of 22 or 23 Sprague-Dawley rats were given bitertanol
(purity, 96%) orally at doses of 0, 10, 25, or 65 mg/kg bw per day on
days 6-15 post coitum. The study was conducted in compliance with
GLP standards and with OECD guideline 414. Reduced weight gain was
seen in dams at daily doses of 25 mg/kg bw and higher, which persisted
throughout the gestation period in those at 65 mg/kg bw per day. The
number of corpora lutea, implantation rates, resorption rates, live
fetuses, sex ratio, and fetal and placental weights were unaffected by
treatment. An increased incidence of a fourteenth (lumbar) rib
relative to controls was observed at daily doses of 25 mg/kg bw (32%
incidence) and 65 mg/kg bw (70% incidence). No teratogenic effects
were observed. The NOAEL was 10 mg/kg bw (Nagumo et al., 1987).
Pregnant Wistar rats were given single oral doses of 100, 500, or
1000 mg/kg bw bitertanol on day 9, 10, 11, or 13 of gestation. The
doses were calculated to represent 1/5, 1/10, and 1/50 of the reported
LD50 value of 5000 mg/kg bw. The method used complied to a limited
extent to OECD guideline 414. The largest deviation was the short
application period; no information was given on compliance with GLP.
The study was available only in abstract form and is therefore of only
limited value for risk evaluation. Bitertanol induced congenital
anomalies when given on day 9, 10, or 11 at 500 or 1000 mg/kg bw. The
malformations consisted of microcaudia and acaudia and, in rare cases,
exophthalmus, hypognathia, and cleft palte. The NOAEL was 100 mg/kg bw
(Vergieva, 1990).
In two studies, groups of 25 Long-Evans rats were exposed to
bitertanol (purity, 93.7%) by inhalation for 4 h per day on days 6-15
post coitum at a mean analytical concentration of 0, 2.9, 6.4, or 22
mg/m3 air in one study and 0, 27, 60, or 120 mg/m3 air in the other.
The method used complied to a certain extent with OECD guideline 414;
at the time the study was performed, compliance with GLP was not
compulsory. No adverse effects were observed on dams exposed at any
concentration, but the average fetal weights were reduced
significantly by exposure to 120 mg/m3. Fetuses with below-average
weights were found increasingly at aerosol concentra-tions greater
than 22 mg/m3 air, without a clear dose-response relationship.
Implantation rates, resorption rates, placental weights, and sex ratio
were unaffected by treatment. No embryolethal or teratogenic effects
were observed. The NOAEL was 22 mg/m3 air (Machemer & Thyssen, 1979).
Rabbits
Twelve Himalayan rabbits, were given bitertanol (purity, 96.7%)
at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method used complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. The dose of 100 mg/kg bw led to reduced weight
gain and isolated clinical signs, such as reduced food intake,
diarrhoea, and blood in the urine; one dam died on day 29
post coitum. This dose also resulted in increased embryonal and
fetal mortality and reduced fetal and placental weights, and three
individual malformations occurred, which deviated from those seen in
controls in their nature (epignathus, pulmonary hypoplasia, and
aplasia) but not in their number. The NOAEL was 30 mg/kg bw per day
(Roetz, 1982).
Groups of 15 Himalayan rabbits were given bitertanol (purity,
93.9%) at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. Treatment had no effect on the behaviour,
appearance, or body-weight gain of the dams. At 100 mg/kg bw per day,
a significantly higher resorption rate and reduced numbers and weights
of fetuses were seen, and the number of malformed fetuses was
increased, with rare types of malformation such as cleft palate and
pigeon chest. The NOAEL was 30 mg/kg bw per day (Schlüter, 1983).
Groups of 16 fertilized chinchilla rabbits were given bitertanol
(purity, 96.9%) at a daily oral dose of 0, 10, 50, or 250 mg/kg bw on
days 6-18 of gestation. Because of the very high rate of post-
implantation loss at 250 mg/kg bw per day, doses of 0 and 150 mg/kg bw
per day were used in a supplementary study. The study was conducted in
compliance with OECD guideline 414 and with GLP standards. A
dose-related reduction in food consumption was seen in dams at > 50
mg/kg bw per day. The dose of 250 mg/kg bw per day significantly
reduced body weights from day 7 to the end of the experiment and
significantly reduced corrected body-weight gain. One dam at 150 mg/kg
bw per day and two at 250 mg/kg bw per day died during the study.
Isolated hair loss and enlarged and heavier livers at necropsy were
observed in dams at the high dose. A dose-related increase in the rate
of post-implantation losses was observed at > 150 mg/kg bw per day.
Two dams at 150 mg/kg bw per day and 13 at 250 mg/kg bw per day
completely resorbed their embryos, and the fetuses at these doses
showed dose-related reductions in body weight and a dose-related
increased in the incidence of incompletely or unossified phalangeal
nuclei and calcanei. The NOAEL was 50 mg/kg bw per day (Becker et al.,
1987).
(f) Special studies
As the studies in this section were published in the open
literature, the level of detail needed for full evaluation was not
available.
(i) Effects on the central nervous system
The effect of bitertanol on the central nervous system was
examined in pharmacological screening tests in mice and rats, which
included tests for potentiation of anaesthesia in mice (hexobarbital
sleep period), stimulation of spontaneous motility in mice, an Irwin
behaviour test in mice, the novel box response in rats, the open field
test in mice, and a test for reserpine ptosis in mice. In these
investigations, bitertanol was administered as a single oral dose of
0.075, 0.6, or 4.8 mg/kg bw. The results showed a slight stimulating
effect of bitertanol on the central nervous system in mice, but no
specific pharmacological effects, such as potentiation of amphetamine
effects or antagonism to reserpine ptosis, were determined (Polacek,
1983).
In a pharmacological test programme, groups of 10 male mice were
given a single oral dose of 0.02, 0.2, 2, 20, or 200 mg/kg bw
bitertanol. The highest dose significantly increased spontaneous motor
activity, with marked effects when treatment was given during the dark
period. In mice treated with 20 mg/kg bw per day, motor activity
tended to increase but not significantly, and lower doses had no
effect. No further effects on behaviour were observed at any dose
(Kaneto, 1986a). In a further test, male mice received bitertanol at 1
or 100 ppm for one week or one month. No increase in spontaneous motor
activity and no potentiating effect in amphetamine-pretreated animals
were seen after repeated dosing (Kaneto, 1986b).
In a study designed to determine whether bitertanol has similar
behavioural effects on the fixed-interval response rate and motor
activity of rats, doses of 10-300 mg/kg bw were given
intraperitoneally to rats maintained under a multiple fixed-interval
1-min schedule of reinforcement. Intermediate doses increased the
response rates and disrupted response patterning in both
fixed-interval components. The same doses of bitertanol did not
increase motor activity (Allen & MacPhail, 1983).
In rats placed in an actographic device designed for continuous
measurement of the locomotor component of spontaneous motor activity,
bitertanol increased motor activity at 200 mg/kg bw after
administration either orally or intraperitoneally. The activity peaks
at the low dose of 100 mg/kg bw coincided with normal night and
morning activity maxima (Frantik et al., 1996).
One the basis of previous results showing that acute exposure to
the triazole fungicide triadimefon affects central nervous system
catecholamines and induces a transient syndrome in rats that consists
of hyperactivity and stereotyped behaviour, a study was designed to
determine whether this type of toxicity is characteristic of other
triazoles. Dose-effect functions were determined for 14 triazoles or
structurally related pesticides, including bitertanol, in adult male
Long-Evans rats. All of the chemicals were administered orally in corn
oil. Hyperactivity was measured for 2 h in figure-eight mazes. Only
triadimefon and triadimenol induced hyperactivity, suggesting a very
rigid structure-activity relationship for the hyper-activity syndrome.
The absence of an effect of bitertanol may be due to steric hindrance
of benzene-ring substitution for the halogen on the benzene-ring
structure of triadimefon and/or triadimenol. Alternatively, bitertanol
may lack halogen substituents on the benzene rings and thus be less
polarized. The lack of activity of bitertanol is probably not due to
differences in absorption kinetics (Crofton, 1996).
(ii) Toxicity in combinations
Acute tests were performed to determine whether bitertanol has
superadditive (potentiating) effects when administered in combination
with triadimenol, captan, fuberidazole, or dodine. At the time the
studies were performed, OECD methods were not available and compliance
with GLP was not compulsory. The study involved administration of
single equitoxic oral (with triadimenol, captan, or fuberidazole) or
intraperitoneal (with dodine) doses of the active ingredients to male
rats. A factor greater than 1 between observed and expected LD50
values indicates a superadditive effect. Bitertanol plus triadimenol,
bitertanol plus fuberidazole, and bitertanol plus dodine had no
superadditive effects but only additive toxic effects (Mihail, 1982b;
Flucke, 1980; Heimann, 1984b). In contrast, the active ingredient
combination of bitertanol plus captan had slightly superadditive
action, with a potentiation factor of 1.9 (Mihail, 1982a).
(iii) Effect on the liver
Groups of 10 male and 10 female Wistar rats received bitertanol
(purity, 95.8%) suspended in distilled water with Cremorph EL by
gavage at doses of 0, 30, 100, or 300 mg/kg bw per day for 14 days. On
sacrifice, blood was collected for detailed haematological and
clinical chemical testing, and liver samples were taken for detailed
histopathological and enzyme studies. During treatment, 1/10 female
rats at the intermediate dose and 9/10 at the high dose showed hair
loss, and some lost weight, while females at the low dose had reduced
body-weight gain in comparison with control animals. Female rats also
had a dose-related tendency to mild thrombocytosis, which was
significant at the intermediate and high doses. Serum gamma-glutamyl
transpeptidase activity and bilirubin concentration were slightly
increased in females at the high dose. There were no gross
pathological findings at necropsy. The liver weights tended to be
increased at the intermediate and high doses, especially in females.
Slight-to-moderate bile-duct proliferation with peribiliary
infiltration of monocytes or polynucleocytes was seen histologically
in animals at the intermediate and high doses. These changes were
sometimes accompanied by parenchymal Councilman bodies or, more
occasionally, mitoses. The hepatocytes of animals at the high dose
were occasionally swollen, with finely granular cytoplasm. Little
fatty infiltration was seen. The results of studies in vitro were
consistent with induction of hepatic microsomal enzymes, as the
cytochrome P450 content increased in a dose-related manner, especially
in males. Aminopyrene N-demethylase activity was increased in males
and females at the high dose and in males at the intermediate dose,
while O-demethylase activity was increased in males at the
intermediate and high doses and in females at the high dose. The
hepatic triglyceride content was not affected by treatment. Bitertanol
thus caused mild hepatotoxicity, with modest induction of hepatic
microsomal activity in rats at 100 and 300 mg/kg bw per day. The NOAEL
was 30 mg/kg bw per day (Mihail & Luckhaus, 1985).
3. Studies on metabolites
(a) 1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one
(plants, soil)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one, a
keto analogue of bitertanol, has very little acute toxicity in rats
when given orally or dermally. It induced signs of effects on the
central nervous system, with initial sedation and respiratory
disturbances and later stimulation. The highest technically
administrable oral dose, 1750 mg/kg bw, caused no deaths. A dermal
dose of 5000 mg/kg bw and the highest dose administered by inhalation
(dynamic dust nebulization) were tolerated with no observed signs. The
LC50 value in male and female rats was > 500 mg/m3 air after a 1-h
exposure or a 4-h exposure, respectively. In rabbits, the compound was
slightly irritating to the skin only after contact for 24 h and was
mildly irritating to the mucous membranes of the eyes (Thyssen &
Kimmerle, 1978).
(b) Bitertanol benzoic acid (soil)
Bitertanol benzoic acid had little acute toxicity when given
orally. The highest administered dose, 5000 mg/kg bw, was tolerated by
fasted male rats with no observed signs or deaths (Heimann, 1983b).
The compound was not mutagenic to Salmonella typhimurium in the
presence of an exogenous metabolic activation system (Herbold, 1983).
(c) 1,2,4-Triazole (photodegradation, soil)
1,2,4-Triazole has moderate or low acute toxicity when given
orally (LD50 value, 1600 mg/kg bw in males and females) or dermally
(LD50 values, 4200 mg/kg bw in males and 3100 mg/kg bw in females).
At high oral and dermal doses, the compound had effects on the central
nervous system. In tests of inhalation of air enriched with vapours of
the test substance, male rats and mice tolerated exposure for 4 and
6 h, respectively, with no observed signs. No dermal irritation was
observed on rabbits exposed for 24 h or on the skin of five male
volunteers exposed for 8 h. 1,2,4-Triazole was strongly irritating to
the mucous membranes of the rabbit eye (Thyssen & Kimmerle, 1976).
Groups of 15 male and 15 female rats were given 1,2,4-triazole
(purity, 99.6%) at a dose of 0, 100, 500, or 2500 ppm for three
months. The study was conducted before enactment of prevailing
regulatory guidelines, but the test procedures complied to a certain
extent with OECD guideline 408. Treatment at 2500 ppm resulted in a
temporary decrease in food intake, a reduction in body weight, and
transient, slight palmospasms in isolated animals. Significant
reductions in the haemoglobin, haematocrit, mean corpuscular volume,
and mean corpuscular haemoglobin values in male rats at 2500 ppm
indicated an effect on the blood. In this group, slight to moderate
fat accumulation was found in the cells of the liver parenchyma in
three of 15 males, which was attributed to the treatment. The NOAEL
was 500 ppm, equal to 38 mg/kg bw per day (Bomhard et al., 1979).
1,2,4-Triazole at concentrations of 10-5000 µg/plate did not
induce point mutations in Salmonella typhimurium TA1535, TA1537,
TA98, or TA100, with or without metabolic activation (Poth, 1989).
In two studies, groups of 25 fertilized Wistar rats were given
daily oral doses of 1,2,4-triazole (purity, 95.3% and 94.0%,
respectively) on days 6-15 of gestation at doses of 0, 10, 30, or 100
mg/kg bw in one study and 0, 100, or 200 mg/kg bw in the other. The
studies were conducted in compliance with GLP standards and OECD
guideline 414. Maternal toxicity was indicated by decreased weight
gain of dams at 100 and 200 ppm relative to those in the control
group. Reduced fetal weights, retarded osteogenesis, and increased
numbers of runts were found at > 100 mg/kg bw per day. An increased
resorption rate was found in dams at 200 mg/kg per day, but the rate
of fetuses with retarded ossification was not increased. The types and
incidences of the malformations observed in this group (including
cleft palate and malformed extremities) indicate that 1,2,4-triazole
has teratogenic potential. The NOAEL was 30 mg/kg bw per day (Renhof,
1988a,b).
4. Observations in humans
No health impairment was observed in male or female employees
engaged in formulating bitertanol and using the customary safety
precautions, who had regular medical examinations (Miksche, 1981).
Slight, transient prurient reddening of the forearms, which
regressed spontaneously after a few days, developed in rare, isolated
cases after direct dermal contact during packaging of a powder of the
pure active ingredient. Unequivocal differentiation between an
allergic dermal reaction and mechanical-toxic skin irritation was not
possible. There was no tendency to relapse (Faul, 1986).
Comments
After oral administration bitertanol is rapidly and extensively
absorbed (about 84%) and distributed. Excretion is also rapid (rats,
almost complete within 72 h) and occurs mainly in the faeces (about
90%) by biliary excretion, owing to the lipophilic nature of the
parent substance. The liver and kidneys are the main sites of tissue
accumulation in both male and female rats. Although some statistically
significant sex-related differences were seen, they were of minor
physiological importance. The substance has a relatively low rate of
dermal penetration. The metabolic profile was similar at the various
doses tested. The main metabolic pathways are hydroxylation of the
phenyl ring in the para position and oxidation of the tert-butyl
moiety, leading to bitertanol alcohol and the corresponding carboxylic
acid; metabolites derived from the two pathways combined were also
observed. The metabolites occur in both free and conjugated forms. The
parent compound was not detected in urine or bile. There was no
toxicological concern with regard to the metabolic profile in plants.
Bitertanol had very low acute toxicity in rats, mice, and dogs
when given orally and after dermal application or by inhalation. It
was of moderate to low toxicity in rats and mice after intraperitoneal
injection. Females appeared to be slightly more sensitive than males,
but only in some studies. This was perhaps due to slightly different
absorption characteristics in animals of the two sexes, as seen after
oral administration. In view of the toxic signs (including respiratory
disturbances, sedation, spasms, and tremor) and the findings of the
pharmacological screening tests, it may be inferred that bitertanol
has central nervous system activity; however, no specific
pharmacological effects, such as potentiation of amphetamine action,
antagonism of reserpine ptosis, or an effect on hexobarbital
anaesthesia, were observed.
Bitertanol induces no, or only very slight, dermal irritation. It
induces slight to moderate reactions of the ocular mucosa but has no
effect on the cornea or iris. No evidence for a sensitizing effect was
observed in any study
Bitertanol has been classified by WHO as unlikely to present an
acute hazard in normal use (WHO, 1996).
In medium- and long-term tests for toxicity, the liver is
regarded as the main toxicological target organ in dogs and rats at
doses of 1.2 and 28 mg/kg bw per day and above, respectively. Liver
weight was found to be the most sensitive indicator. Corresponding
patterns of disruption of liver function were observed in rats and
dogs. The activities of the transaminases, alkaline phosphatase, and
glutamate dehydrogenase in the serum were increased. In addition, a
rise in cholesterol level was observed in several studies in rats at
doses of 61 mg/kg bw per day and above. The ability of bitertanol to
induce mixed-function oxidases was verified in both species. It is
therefore likely that the effect on weight is essentially due to
hypertrophy of the endoplasmic reticulum in hepatocytes at doses of
100 mg/kg bw per day and above. Morphological changes in the liver
were seen only at relatively high doses and consisted of hepatocytic
swelling, bile-duct proliferation, perilobular fatty degeneration,
eosinophilic foci, and fibrous structures. The induced effects
corresponded to toxic liver damage with bile-duct involvement.
Evidence for haemotoxicity was found at doses of 28 mg/kg bw per
day and above in rats; this may be classified as an effect on the
peripheral red blood cell population. The decreases in erythrocyte
count, haemoglobin concentration, and packed cell volume and the
compensatory rise in the reticulocyte count argue for this
interpretation. No evidence was found for damage to the haematogenic
organs.
Slight increases in the leukocyte count were also seen in a few
studies in rats at doses of 61 mg/kg bw per day and above. The
increased leukocyte counts are probably attributable to inflammatory
processes, since the increase occurred in studies and at doses at
which inflammatory processes were also observed. Hyperkeratosis of the
oesophageal epithelium and glandular stomach and/or erosions of the
glandular stomach as well as parakeratosis of the stomach wall were
observed in rats. The histopathological picture included distended
epithelial cells, slight inward growth of the papillary body, and
cellular infiltration of the epithelial and subepithelial layers in
the affected animals. These changes are probably attributable to
irritation of the mucous membranes by the active ingredient.
Effects on the skin were observed in dogs, sheep and rats. The
effects in dogs at doses of 5 mg/kg bw per day and above consisted of
reddening, with localized inflammation, desquamation, and hair loss,
and increased reddening and slight inflammatory phenomena in the
mucous membranes of the oral cavity and eyes. Histological examination
showed broadening of the epithelial layer, enhanced cornification, and
minor erosions. The dermal effects were apparently accompanied by
pruritus. The keratitis observed in dogs was considered to be
secondary to conjunctivitis. Hair loss was also observed in rats and
sheep, which occurred after administration of bitertanol by capsule or
gavage at doses of 300 mg/kg per day and above; this was considered to
be a systemic effect.
Pathological changes were seen in the adrenals of dogs and rats
at 1.2 and 81 mg/kg bw per day and above, respectively, consisting of
swelling and fatty degeneration of the adrenal cortical cells,
particularly in the zona reticularis and zona fasciculata. These
alterations were considered to be due to inhibition of sterol
biosynthesis by triazole derivatives. It is highly probable that this
effect, which also represents the biological, antimycotic action of
the substance, leads to an effect on corticoid metabolism with
corresponding morphological effects in the cells of the adrenal
cortex.
In feeding studies in dogs, lenticular opacity seen at doses of
4.9 mg/kg bw per day and above were considered to be related to
treatment. No lenticular alterations attributable to administration of
bitertanol were seen in any other study or species. The exact
mechanism of the cataractogenesis resulting from long-term
administration of triazole fungicides is presently unknown. The ocular
lens undertakes its own de-novo synthesis of cholesterol, which is
isolated from lipoproteins circulating in the blood; other substances
that inhibit cholesterol synthesis can induce cataracts.
No evidence for any carcinogenic potential of bitertanol was
found in long-term studies of toxicity and carcinogenicity in rats and
mice treated in the diet. The highest doses tested were 130 mg/kg bw
per day in mice and 26 mg/kg bw per day in rats.
In a series of studies, bitertanol had no genotoxic potential in
lower organisms or in mammalian cells or systems in vitro or
in vivo.
In a three-generation study of reproductive toxicity in rats,
adverse effects on the pups (reductions in survival rates during the
four-week lactation period, reduced weight at birth, and retarded
growth) were observed at parentally toxic doses of 100 or 500 ppm,
equivalent to 5 or 25 mg/kg bw per day. The NOAEL was 1 mg/kg bw per
day.
In studies of developmental toxicity, treatment with bitertanol
led to several embryotoxic and teratogenic effects, depending on the
animal species, route of administration, and dose. In rats, an oral
dose of 10 mg/kg bw per day was tolerated with no observed effect.
Doses of 25 mg/kg bw per day and above led to retardations and
variations (e.g. increased incidence of the 14th rib). Malformations
were observed at an oral dose of 100 mg/kg bw per day, which was
clearly maternally toxic. Exposure of pregnant rats to concentrations
of 27 mg/m3 air and above by inhalation resulted in retardation
effects; no malformations were observed. In rabbits, doses of 50 mg/kg
bw per day and above were maternally toxic; fetotoxic effects were
seen at doses of 100 mg/kg bw per day and above. Teratogenic effects
were observed in Himalayan rabbits at 100 mg/kg bw per day, while in
the second strain tested (Chinchilla), even a dose of 250 mg/kg bw per
day did not lead to malformations.
The ADI established at the 1988 Meeting of 0-0.01 mg/kg bw, based
on a combined NOAEL of 1 mg/kg bw per day from the two-year and the
one-year study in dogs, was maintained. The ADI is supported by the
NOAEL of 20 ppm (equivalent to 1 mg/kg bw per day) in a
three-generation study in rats.
An acute RfD was not allocated because bitertanol has been
classified by WHO as unlikely to present an acute hazard in normal use
and it has not shown any specific adverse effects (teratogenicity,
neurotoxicity) after single doses 100 times the lowest relevant NOAEL
in long- and short-term studies that were used to establish the ADI.
Therefore, the Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 25 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 100 ppm, equal to 4.9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
20 ppm, equivalent to 1 mg/kg bw per day (reproductive
and parental toxicity in a three-generation study)
10 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 1 mg/kg bw per day (overall NOAEL in one-year and
two-year studies of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Commenced immediately, about 84% absorbed
Dermal absorption About 10%
Distribution Highest concentrations in liver and kidneys
Potential for accumulation None
Rate and extent of excretion About 90% excreted with bile, 10% with urine
Metabolism in animals No parent compound in bile or faeces; extensively
metabolized to 14 metabolites (ring
monohydroxylation, ring dihydroxylation, aryl
O-methylation, aliphatic hydroxylation, aliphatic
oxidation to carboxylic acids, and ether cleavage)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral > 5000 mg/kg bw
Rat: LD50,, dermal > 5000 mg/kg bw
Rat: LC50, inhalation > 550 mg/m3 (4 h)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not a sensitizer (Magnussen & Kligman test)
Short-term toxicity
Target/critical effect Liver, red blood cells, adrenals, digestive tract
Lowest relevant oral NOAEL Dog: 90 days: 1 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 3 weeks; 250 mg/kg per day
Lowest relevant inhalation NOAEL Rat: 3 weeks, 63 mg/m3
Genotoxicity No genotoxic or mutagenic potential
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL Dog: 1 year and 2 years: 1 mg/kg bw per day
Carcinogenicity No evidence of carcinogenic potential
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced litter size, pup
growth rate, and pup survival) at parentally toxic
doses
Lowest relevant reproductive NOAEL Rat: 1 mg/kg bw per day
Developmental target/critical effect Fetotoxic and teratogenic effects at maternally
toxic doses
Lowest relevant developmental NOAEL Rat: 10 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No relevant effects
Other toxicological studies Induction of hepatic microsomal activity
Medical data No health impairments observed in employees
subjected to regular medical examinations
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 1 and 2 years in dogs 100
Acute reference dose None allocated
(unnecessary)
References
Allen, A.R. & MacPhail, R.C. (1993) Bitertanol, a triazole fungicide,
increases operant responding but not motor activity. Neurotoxicol.
Teratol., 15, 237-242.
Becker, H., Mueller, E., Vogel, W. & Terrier, C. (1987) Embryotoxicity
(including teratogenicity) study with KWG 0599 in the rabbit.
Unpublished report No. R4081 from Research & Consulting Company AG,
Itingen, Switzerland. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Löser, E. (1978) KWG 0599: Subchronic toxicological
study on rats (feeding experiment over three months). Unpublished
report No. 7322 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Löser, E. (1981a) KWG 0599 (Bitertanol, the active
ingredient of Baycor): Chronic toxicity study on mice (2-year feeding
experiment). Unpublished report No. 10103 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Bomhard, E. & Löser, E. (1981b) KWG 0599: Chronic toxicity study on
rats (2-year feeding experiment). Unpublished report No. 10104 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole: Subchronic
toxicological study with rats (feeding study over three months).
Unpublished report No. 8667 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bond, J. & McGregor, D. (1981) Testing for aneuploid induction of HE
1004 in Sordaria brevicollis. Unpublished report No. R2063 from
Inveresk Research International, Musselburgh, Scotland. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bootman, J. & Rees, R. (1983) KWG 0599: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report No. R2406 from Life Science Research. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Crofton, K.M. (1996) A structure-activity relationship for the
neurotoxicity of triazole fungicides. Toxicol. Lett., 84, 155-159.
Faul, J. (1986) Baycor active ingredient and formulations. Unpublished
report from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50) -- rat p.o.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1979) KWG 0599--Determination of acute toxicity (LD50):
rat p.o./mixed batch (from 5 batches). Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) KWG 0599 and W VII/117: Study for acute combination
toxicity. Unpublished report No. 9456 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1981) KWG 0599: Evaluation for sensitization in guinea
pigs (maximization test after Magnusson and Kligman). Unpublished
report No. 9934 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Frantik, E., Hornychova, M., Dvorak, J. & Mraz, J. (1996) Spontaneous
motor activity in rats enhanced by 1,2,4-triazole derivatives:
Triadimefon, triadimenol and biteranol. Homeostasis, 37, 3.
Hatanaka, J., Asaoka, S., Ikeda, M., Shimizu, M., Inukai, H.,
Iyatomy, A. & Itoh, H. (1981) KWG 0599: Short-term toxicity tests on
rats (4-week feeding and 4-week recovery tests). Unpublished report
No. 195 from Nihon Tokushu Noyaku Seizo K.K. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1981) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983a) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983b) Determination of acute toxicity (LD50): BUE
2684 (Bitertanol-benzoic acid)--rat p.o. Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50): KWG
0599. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984b) Dodine & KWG 0599 (c.n. Dodine & Bitertanol
(proposed)): Combination toxicity study. Unpublished report No. 12608
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Vogel, O. (1984) KWG 0599 (c.n. Bitertanol): Subacute
study of dermal toxicity to rabbits. Unpublished report No. 12571 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978a) KWG 0599: Micronucleus test on mouse to evaluate
KWG 0599 for potential mutagenic effects. Unpublished report No. 7860
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978b) KWG 0599: Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 7964 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979) KWG 0599: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 8152 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) BUE 2684: Bitertanol-benzoic acid:
Salmonella/microsome test to evaluate for point mutation. Unpublished
report No. 12087 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hixson, E.J. (1979) Baycor technical: Acute dermal toxicity to
rabbits. Unpublished report No. 57 from Mobay Chemical Corporation,
Staneley Research Center. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1984) Dermal absorption of 14C-Baycor applied as 50%
wettable powder. Unpublished report No. 484 from Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1977) KWG 0599: Evaluation for sensitization.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1981a) KWG 0599: Determination of the acute toxicity to
the dog after oral administration. Unpublished report No. T 6001023
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1981b) KWG 0599/012: Acute oral toxicity to sheep.
Unpublished report No. 9768 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1983) KWG 0599 (c.n. Bitertanol): Chronic
oral toxicity study on dogs (2-year feeding experiment). Unpublished
report No. 12307 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hoffmann, K. & Schilde, B. (1979) KWG 0599: Subchronic toxicity study
on dogs (thirteen-week administration in capsules). Unpublished report
No. 8053 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Hoffmann, K. & Vogel, O. (1983) KWG 0599 (Bitertanol): Second chronic
toxicity study with dogs on oral administration (feeding study, 0-3-25
ppm 12 months, 200 ppm 20 months). Unpublished report No. 12328 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Iyatomy, A. (1980) KWG 0599: Report of acute toxicity. Unpublished
report No. Sheet A-31 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iyatomy, A. (1981) Report of acute toxicity: KWG 0599. Unpublished
report No. Sheet 22 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986a) Effect of bitertanol on spontaneous motor activity
in mice; Part 1. The experiment on the acute effect. Unpublished
report No. DT-1 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986b) Effect of bitertanol on spontaneous motor activity
in mice; Part 2. The experiment on the subacute effect. Unpublished
report No. DT-2 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Klein, O. (1988a) [U-phenyl-14C]-Bitertanol: Absorption, excretion,
tissue-distribution, and biokinetic profile of the total radioactivity
in the rat following single oral and intraduodenal dosage. Unpublished
report No. PF3076 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Klein, O. (1988b) [U-phenyl-14C]-Bitertanol: Distribution of the
parent compound in the excreta and in some organs at different times
following single oral administration to rats. Unpublished report No.
PF3096 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1989) [U-phenyl-14C]-Bitertanol: Distribution of the
metabolites in some organs at different times following single oral
administration to rats. Unpublished report No. PF3501 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Bomhard, E., Löser, E. & Schilde, B. (1978 ) B. KWG
0599: Subchronic toxicity study on rats (three-month feeding
experiment). Unpublished report No. 8002 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Löser, E. & Eiben, R. (1981) KWG 0599: Multigeneration reproduction
study on rats. Unpublished report No. 10024 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. & Thyssen, J. (1979) KWG 0599 (Baycor active ingredient):
Evaluation for embryotoxic and teratogenic effects on rats after
dynamic inhalational exposure. Unpublished report No. 8610 from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1977) KWG 0599: Evaluation for embryotoxic and
teratogenic effects on rats after oral administration. Unpublished
report No. 6697 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978a) Determination of acute toxicity (LD50) / KWG 0599
Form A: PFU 190978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1978b) Determination of acute toxicity (LD50) / KWG 0599
Form B: PFU 210978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982a) KWG 0599 and captan: Study for acute combination
toxicity. Unpublished report No. 11205 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982b) KWG 0519 and KWG 0599: Study for acute combination
toxicity. Unpublished report No. 11203 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Kimmerle, G. (1977) KWG 0599: Subacute inhalation
toxicity study on rats. Unpublished report No. 6868 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Luckhaus, G. (1985) KWG 0599 (c.n. Bitertanol): Subacute
oral toxicity study with special attention to effect on the liver.
Unpublished report No. 13230 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Miksche, L. (1981) Data on effects on humans/In-company occupational
medical experience. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Nagumo, K., Teraki, Y., Chiba, T. & Miyasaka, M. (1981, revised 1987)
Teratogenicity test of KWG 0599 in pregnant rats. Unpublished report
from St Marianna University School of Medicine. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Pauluhn, J., Thyssen, J. & Vogel, O. (1983) KWG 0599 (Baycor active
ingredient) (c.n. Bitertanol): Study for cataract-inducing effect on
subacute whole-body exposure with cats (exposure 20 × 6 hours).
Unpublished report No. 12006 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Polacek, I. (1983) Central nervous effects of KWG 0599 (pilot study).
Unpublished report No. R2694 from Toxikologisches Institut Regensburg.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with 1H-
1,2,4-triazole. Unpublished report No. R4859 from Cytotest Cell
Research GmbH & Co. KG. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puhl, R.J. & Hurley, J.B. (1983) The absorption, excretion, and
metabolism of Baycor-phenyl-UL-14C by rats. Unpublished report No.
85832 from Mobay Chemical Corporation, Agricultural Chemicals
Division. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) 1,2,4-triazole: Investigations into embryotoxic
effects on rats after oral administration. Unpublished report No.
17401 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Renhof, M. (1988b) 1,2,4-triazole: Investigation into embryotoxic
effects on rats after oral administration. Supplement to study No.
T5019339. Unpublished report No. 17402 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Riach, C.G. (1981) KWG 0599: Testing the modification of cellular DNA
in Escherichia coli. Unpublished report No. R2013 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Roetz, R. (1982) KWG 0599 (Bitertanol, the active ingredient of
Baycor): Study of embryotoxic (and teratogenic) effects on rabbits
after oral administration. Unpublished report No. 10979 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sasaki, Y.F.X. (1987) Bitertanol: In vitro cytogenetics test.
Unpublished report No. DT-5 from The Institute of Environmental
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1983) KWG 0599: Study to determine embryotoxic and
teratogenic effects on rabbits following oral administration.
Unpublished report No. 11548 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987) Acute inhalation toxicity study with Baycor
technical dust in Sprague-Dawley rats. Unpublished report No. 837 from
Mobay Corporation, Staneley Research Center, Report. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Ohta, T. (1981) Bitertanol: Microbial
mutagenicity study. Unpublished report from Department of Toxicology,
Institute of Environmental Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977) KWG 0599: Intracutaneous allergy test on guinea
pigs. Unpublished report No. 7113 from Bayer AG, Leverkusen. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kaliner, G. (1977) KWG 0599: Subacute oral cumulative
toxicity study on rats (four-week treatment). Unpublished report No.
7153 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole: Occupational
toxicology study. Unpublished report No. 5926 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977a) KWG 0599: Acute toxicity studies.
Unpublished report No. 6546 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977b) KWG 0599: Subacute inhalational
toxicity study on dogs. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1978)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one (third
stage KWG 0599, BUE 1662): Industrial toxicology study. Unpublished
report No. 7242 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vergieva, T. (1990) Triazoles teratogenicity in rats. Teratology,
42, 27A-28A.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Yonemura, H., Shimazaki, M., Yamazaki, K., Takahashi, Y. & Hashizume,
M. (1981) Subacute toxicity tests with Bitertanol (KWG 0599) in rats.
Unpublished report from Research Institute for Animal Science in
Biochemistry and Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
bitertanol are summarized in Table 1. Bitertanol had very low acute
toxicity in rats, mice, and dogs treated orally. No significant sex
difference was observed. The LD50 values are 4000-5000 mg/kg bw or
higher. The toxicological properties of the A and B isomers are
similar. The symptoms included nonspecific signs, such as deteriorated
general condition, isolation from the group, and piloerection;
however, central nervous system effects were also seen, including
sedation, spasms, and respiratory disturbances. Lobulation of the
liver and irritation of the glandular stomach mucosa were observed at
necropsy of orally treated rats. Further signs in dogs were vomiting
and diarrhoea. Sheep were more susceptible, with an oral LD50 of
about 1000 mg/kg bw. Bitertanol was moderately toxic to rats and mice
after intraperitoneal injection.
Dermal exposure to a dose of 5000 mg/kg bw was tolerated by rats
with no observed signs. It was not toxic to rats after inhalation in
an aerosol, even at the highest test concentration. An initial test
showed no primary irritation of the intact or abraded skin of rabbits
inspected 24 and 72 h after the beginning of exposure (Thyssen &
Kimmerle, 1977a). In a study in rabbits, the active ingredient was
found to be slightly irritating to intact and abraded skin (Iyatomy,
1981).
A five-minute treatment of the eye of rabbits did not cause
primary irritation of the mucous membranes. Slight to moderate
reddening of the conjunctivae, which persisted for about 48 h, was
observed after a 24-h exposure (Thyssen & Kimmerle, 1977a). A reaction
that was reversible within four days was seen in rabbit conjunctivae;
the cornea and iris were unaffected (Iyatomy, 1981).
After an initial administration of 0.05 mg bitertanol per animal
to female Pirbright guinea-pigs, nine repeated intracutaneous
injections of emulsified bitertanol (0.1 mg) over three consecutive
weeks had no sensitizing effect. Intracutaneous injection of an
additional 0.05 mg after a further two weeks also failed to produce
signs of dermal sensitization (Thyssen, 1977).
Magnusson and Kligman tests in 20 male and 20 female Pirbright
guinea-pigs with Freund's adjuvant provided no evidence of sensitizing
effects of bitertanol at a concentration of 1% for intradermal
induction, 25% for topical induction, and 25% for challenge (Flucke,
1981).
(b) Short-term studies of toxicity
Rats
Groups of 20 male and 20 female Wistar rats were given bitertanol
(purity, 96.5%) by gavage at doses of 0, 30, 100, or 300 mg/kg bw per
day for 28 days. Half of the animals were then sacrificed, and the
other half were observed for a further 28 days. The method used in
Table 1. Acute toxicity of bitertanol
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat NR M Oral > 5000 99.1 A Mihail (1978a)
Rat NR M Oral > 5000 97.1 B Mihail (1978b)
Rat Wistar M/F Oral (fasted) > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M Oral > 5000 97.3 Flucke (1978)
Rat NR M Oral 4000 95.0 Flucke (1979)
Rat NR M Oral 3700 95.0 Iyatomy (1980)
F Oral 3900
Rat Wistar F Oral (fasted) > 5000 95.1 Flucke (1980)
Rat NR M Oral > 5000 96.7 Heimann (1981)
M Oral (fasted) > 5000
Rat NR M Oral (fasted) 4800 99.1 Mihail (1982a)
Rat NR M Oral (fasted) 4800 97.1 Mihail (1982b)
Rat NR M Oral > 5000 NR Heimann (1983a)
Rat NR M Oral (fasted) > 5000 97.6 Heimann (1984a)
Mouse NMRI M Oral (fasted) 4500 96.5 Thyssen & Kimmerle (1977a)
F Oral (fasted) 4200
Mouse NR M Oral 3500 95.0 Iyatomy (1980)
F Oral 3200
Dog Beagle M/F Oral (fasted) > 5000 95.0 Hoffmann (1981a)
Sheep Blackface M/F Oral ~ 1000 95.0 Hoffmann (1981b)
Rat Wistar M/F Dermal > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M/F Dermal > 5000a 95.0 Iyatomy (1980)
Mouse NR M/F Dermal > 5000 95.0 Iyatomy (1980)
Rabbit New Zealand M/F Dermal > 2000 94.9 Hixson (1979)
Rat Wistar M/F Inhalation 1 h > 720 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 4 h > 550 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 5 x 4 h > 380 96.5 Thyssen & Kimmerle (1977a)
Ratb Sprague-Dawley M/F Inhalation 4 h > 1200 95.7 Shiotsuka (1987)
Table 1. (continued)
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat Wistar M Intraperitoneal 1200 96.5 Thyssen & Kimmerle (1977a)
F Intraperitoneal 720
Rat NR M Intraperitoneal 700 95.0 Iyatomy (1980)
F Intraperitoneal 560
Mouse NR M Intraperitoneal 570 95.0 Iyatomy (1980)
F Intraperitoneal 610
Rat NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
Mouse NMRI M/F Subcutaneous > 5000 96.5 Thyssen & Kimmerle (1977a)
Mouse NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
NR, not reported; M, male; F, female; A, diastereomer A; B, diastereomer B
a Slight local irritation present
b The study was conducted in compliance with good laboratory practive
this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
Doses of 100 mg/kg per day and higher had a dose-related adverse
effect on body-weight development. At 300 mg/kg per day, behavioural
disturbances (isolation from the other animals, dirty coat) and hair
loss were observed in the female rats; however, all females had normal
behaviour near the end of the treatment period. Animals at this dose
had moderate leukocytosis, and females had reduced haemoglobin and
thrombocyte counts. Increased alkaline phosphatase activity was
observed only in the females. The relative liver weights of animals of
each sex at 100 mg/kg bw per day and above were increased. At
300 mg/kg bw per day, the absolute liver weights were elevated and the
absolute weights of the heart, kidneys, adrenals, and ovaries
depressed in female rats at the termination of treatment. The relative
weights of the thyroid and testes in males and of the spleen in
females were also elevated, whereas the relative weights of the heart,
adrenals, and ovaries were depressed in females. Hyperkeratoses and
parakeratoses, distended epithelial cells, slight inward growth of the
papillary body, and cellular infiltration in the epithelial and
subepithelial layers were observed histopathologically in the
forestomachs of four female animals at 300 mg/kg bw per day that were
sacrificed at the end of treatment. The appearance of the digestive
tract was unexceptional at the end of the recovery period., and all of
the changes in the rats at the high and intermediate doses had
likewise reverted. The NOAEL was 30 mg/kg bw per day (Thyssen &
Kaliner, 1977).
Groups of 20 male and 20 female Sprague-Dawley rats were given
bitertanol (purity, 95%) at doses of 0, 100, 400, or 1600 ppm, equal
to 7, 28, and 110 mg/kg bw per day in males and 7.4, 30, and 110 mg/kg
bw per day in females, for 28 days. The test procedures complied to a
certain extent with OECD guideline 407; at the time the study was
performed, compliance with GLP was not compulsory. Half of the animals
were sacrificed and examined after four weeks, whereas the other half
were observed without treatment for a further four weeks.
The dose of 400 ppm had an adverse effect on body-weight
development and food intake, and an adverse effect on the red blood
cell population was seen from decreases in the haematocrit and
haemoglobin readings. The relative liver weight was slightly elevated
in animals of each sex. Those at 1600 ppm had ataxia, reduced food
intake and body weight, and depressed red blood cell parameters
(haemoglobin, haematocrit, erythrocyte count), with a simultaneous
increase in the reticulocyte count. A slight increase in the serum
cholesterol concentration and a significant increase in the relative
liver weight were found in animals of each sex. Histopathological
examination revealed weak irritation of the gastric mucosa and
slightly altered ovaries, adrenals, and pituitaries. All of the
observed effects were reversible within the four-week recovery period.
The NOAEL was 100 ppm, equal to 7 mg/kg bw per day (Hatanaka et al.,
1981).
Bitertanol (purity, 90.2%) was administered to groups of 20 male
and 20 female Wistar rats at concentrations of 0, 150, 600, or 2400
ppm, equal to 12, 48 and 300 mg/kg bw per day in males and 13, 58, and
310 mg/kg bw per day in females, for three months. This study was
conducted before enactment of prevailing regulatory guidelines;
however, the procedures complied to certain extent with OECD guideline
408.
Although appearance and behaviour were unaffected at doses up to
600 ppm, 2400 ppm caused decreased motility and reduced food intakes.
Dose-related growth retardation was observed in females at 600 and
2400 ppm and in male animals at all concentrations; however, the 150
ppm dose decreased body weights only temporarily. The dose of 2400 ppm
had adverse effects on various haematological parameters, including
reduced erythrocyte and leukocyte counts, decreased haemoglobin
content and haematocrit reading, increased reticulocyte counts, a
relative increase in the polymorphonuclear leukocyte count, and a
relative decrease in the lymphocyte count. The changes in clinical
chemical parameters of the blood also found at this concentration were
elevated alkaline phosphatase, aspartate aminotransferase, and
glutamate dehydrogenase activities in the female animals. The blood
protein level was slightly depressed and the cholesterol level
slightly elevated. The finding of increased liver weight in females at
2400 ppm also indicated an effect on the liver. The NOAEL was 150 ppm,
equal to 12 mg/kg bw per day (Bomhard & Löser, 1978).
In a supplementary study, bitertanol (purity, 90.8%) was
administered to groups of 15 male and 15 female Wistar rats at
concentrations of 0, 30, 100, or 300 ppm, equal to 2.5, 8.1, and 25
mg/kg bw per day in males and 3.3, 10, and 32 mg/kg bw per day in
females, for three months. The method used in this study complied to a
certain extent with OECD guideline 408; at the time the study was
performed, compliance with GLP was not compulsory.
The appearance, behaviour, and mortality rate were unaffected at
all concentrations. At 300 ppm, the rats gained less weight than the
controls. Haematological, clinical chemical, gross and
histopathological examination, and urinary analyses provided no
evidence of adverse effects. The NOAEL was 100 ppm, equal to 8 mg/kg
bw per day (Krötlinger et al., 1978).
In a further study, bitertanol (purity, 95%) was administered to
groups of 15 male and 15 female Sprague-Dawley rats at concentrations
of 0, 40, 200, or 1000 ppm, equal to 3.1, 16, and 82 mg/kg bw per day
in males and 3.6, 19, and 88 mg/kg bw per day in females, for three
months. Five males and five females were subjected to urinary,
haematological, and clinical chemical determinations. The method used
in this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
The dose of 200 ppm retarded weight gain in male and female
animals and slightly increased the activities of alkaline phosphatase
and aspartate aminotransferase in the serum of females. The dose of
1000 ppm reduced body-weight gain and decreased food and water intakes
in animals of each sex. The erythrocyte count was also depressed, and
the reticulocyte count elevated in male and female animals. In
addition, the leukocyte and thrombocyte counts were elevated, and the
haemoglobin content and haematocrit reading were decreased in females.
Elevated lactate dehydrogenase, aspartate aminotransferase, and
alkaline phosphatase activities and a slightly elevated cholesterol
concentration were found in the serum of females. The absolute weights
of the liver and spleen were increased in females and the relative
weights in males and females. The following lesions were found
histopathologically in animals of each sex: increased swelling and
fatty degeneration of hepatocytes, hyperkeratoses of the oesophageal
and gastric mucosal epithelium and/or erosions of the glandular
stomach, swelling, and fatty degeneration of adrenal cortical cells.
Isolated bile-duct proliferation was also seen in females. The NOAEL
was 40 ppm, equal to 3 mg/kg bw per day (Yonemura et al., 1981).
Groups of 10 male and 10 female Wistar rats were exposed to
bitertanol at mean analytically determined concentrations of 18, 63,
or 200 mg/m3 air for 6 h per day, five days per week for three weeks.
Only the head and nose of the animals were exposed, under dynamic
conditions, and the inhaled bitertanol was dissolved in a 1:1 blend of
ethanol:polyethylene glycol 400.The method used in this study complied
to a certain extent with OECD guideline 412; at the time the study was
performed, compliance with GLP was not compulsory.
The concentrations of 18 and 63 mg/m3 air were tolerated by male
and female rats with no observed effect. Exposure to 200 mg/m3 air
caused deterioration of the general condition of female rats, and the
growth of male rats in this group was significantly decreased.
Although the relative weights of the lung, liver, kidney, and adrenal
in males and females at the high concentration were sometimes higher
than control values, no evidence of damage due to exposure to
bitertanol was seen histopathologically. The NOAEL was 63 mg/m3 air
(Mihail & Kimmerle, 1977).
Dogs
Groups of four male and four female beagles received bitertanol
(purity, 90.2%) at a dose of 0, 1, 5, or 25 mg/kg bw per day in
gelatin capsules. The method used in this study complied to a certain
extent with OECD guideline 409; at the time the study was performed,
compliance with GLP was not compulsory.
Doses of 5 mg/kg bw per day and above led to signs of
dose-related adverse effects on the skin and mucous membranes. The
effects on the skin were manifested by increased reddening, with local
inflammation, desquamation, and hair loss; and those on the mucous
membranes by increased reddening and slight inflammatory phenomena in
regions of the oral cavity (gingiva) and eyes (conjunctiva). They were
evident histologically as slight broadening of the epithelial layer of
the skin and slightly enhanced cornification in some cases. Evidence
for superficial involvement of the cornea (keratitis), apparently
resulting from conjunctival irritation, was observed in a few animals
at 25 mg/kg bw per day. The behaviour of the controls and treated
animals was similar; in contrast, the food intake and body-weight
development of animals at the high dose showed an adverse effect. The
results of laboratory tests indicated treatment-related interference
with liver function (elevated alkaline phosphatase and alanine
aminotransferase activities) in dogs at 5 and 25 mg/kg bw per day.
Elevated N-demethylase activity and an increase in the cytochrome
P450 concentration in liver homogenates were found in the high-dose
group at study termination. The weights of the thymus in females at
25 mg/kg per day and of the prostate (relative at 5 mg/kg per day and
both relative and absolute at 25 mg/kg per day) were statistically
significantly lower than those of the controls. The changes in
prostate weights correlated with dose-related histopathological
findings of retarded development. The NOAEL was 1 mg/kg bw per day
(Hoffmann & Schilde, 1979).
In view of the dermal phenomena observed in the previous study,
tests were performed to determine whether they represented a
sensitization effect. Beagle dogs were first treated orally 10-42
times with 35 or 70 mg/kg bw bitertanol, which led to marked
irritation and dermal lesions, particularly in the area of the head.
After a treatment-free interval of four to six weeks, when the lesions
had healed, the animals were treated with a dose of 1.75 mg/kg bw,
previously found to induce no reaction, for 14 days. Hair loss and a
slightly increased incidence of reddened gingiva were determined in
isolated animals only at termination of treatment. The study gave no
evidence for sensitization, and the delay in development of the effect
specifically argues against such an effect (Hoffmann, 1977).
No evidence for sensitization was found in a study in which
beagles were exposed to a concentration of 28.8 mg/m3 air for 4 h per
day, five days per week for three weeks, left untreated for 10 days,
and subsequently re-exposed to a concentration of 47.1 mg/m3 air for
one week. All dogs tolerated the exposures with no signs, and no
evidence was found for either an irritating effect on visible mucous
membranes or for a Type I (immediate anaphylactic type) allergy
(Thyssen & Kimmerle, 1977b).
Cats
Groups of three male and three female cats weighing 2-3 kg
received whole-body exposure to aerosols of bitertanol (active
ingredient, 95.8%) in a 3-m3 inhalation chamber for 6 h per day for
four weeks (20 x). The mean analytically determined bitertanol
concentration was 27 mg/m3 air. Analysis of the generated aerosols
showed that more than 90% of the aerosol particles had a mass-related
and more than 99% a particle-related aerodynamic diameter smaller than
5 µm. The exposed animals were compared with negative controls (air)
and positive controls (dichlozoline, 28 mg/m3 air). The animals were
observed over a recovery period of 12 weeks after termination of the
exposure. Their eyes were examined with an ophthalmoscope before
exposure and each week throughout the four-week exposure and the
recovery period and for gross and histopathological alterations at the
end of the study. The method used complied with OECD guideline 412; at
the time the study was performed, compliance with GLP was not
compulsory.
The exposed cats tolerated the treatment with no signs of
toxicity, damage to the eyes, or cataractogenesis. Under comparable
experimental conditions, the animals exposed to dichlozoline showed
signs of lenticular opacity (cataracts), starting from the end of the
second week of exposure. Lenticular opacity that had not reverted up
to the end of the recovery period was observed in all animals of this
group at the end of the second week of the recovery period. Cats
exposed to bitertanol had no cataracts (Pauluhn et al., 1983).
Rabbits
Groups of six male and six female New Zealand rabbits received
applications of bitertanol (purity, 95.8%) in an aqueous suspension
(0.5 ml) at concentrations of 0, 50, or 250 mg/kg bw on the intact or
abraded dorsal and lateral skin for 6 h per day, five times per week
for three weeks. The method used in this study complied to a certain
extent with OECD guideline 411; at the time the study was performed,
compliance with GLP was not compulsory.
Bitertanol had no apparent effect on the appearance, behaviour,
body weight, or survival of the rabbits. Transient erythema developed
on the exposed areas, initially on abraded skin but later on intact
skin. There was no effect on skin-fold thickness or on haematological,
clinical chemical, or urinary parameters. At necropsy, there were no
gross pathological findings. Histopathological examination showed
slight epidermal thickening only in exposed areas of all treated
animals. Preparations of liver showed no evidence of induction of
microsomal N- or O-demethylation or cytochrome P450 content. The
dermal application had no effect on any of the parameters
investigated. The NOAEL was 250 mg/kg bw (Heimann & Vogel, 1984).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Bitertanol (purity, 94-95%) was administered to groups of 50 male
and 50 female CF1/W74 mice at doses of 0, 20, 100, or 500 ppm, equal
to 2, 25, and 130 mg/kg bw per day in males and 7, 37, and 180 mg/kg
bw per day in females, for two years. The study was conducted before
enactment of prevailing regulatory guidelines; however, the test
procedures complied to a certain extent with OECD guideline 453 but
with no attempt to monitor ocular changes.
Behaviour, feed consumption, mortality, and haematological
parameters were not affected by treatment. In animals at 500 ppm, body
weights were reduced and serum alkaline phosphatase activity was
significantly elevated; females at this dose had an increased
incidence of enlarged and greenish-coloured livers at necropsy. The
liver weights were also increased, particularly in the female mice,
and the livers had increased numbers of eosinophilic foci. No evidence
was found for a carcinogenic effect at any concentration, which
extended into the toxic range. The NOAEL was 100 ppm, equal to 25
mg/kg bw per day (Bomhard & Löser, 1981a).
Rats
Bitertanol (purity, 94% in weeks 1-18; 95% from week 19) was
administered to groups of 50 male and 50 female SPF Wistar rats at
doses of 0, 20, 100, or 500 ppm, equal to 1, 4.9, and 26 mg/kg bw per
day in males and 1.3, 6.6, and 34 mg/kg bw per day in females, for two
years. The study was conducted before the enactment of prevailing
regulatory guidelines; however, the test procedures complied to a
certain extent with OECD guideline 453.
The 500 ppm concentration led to retarded growth in animals of
each sex. Ocular changes were not recorded. Haematological, blood
chemical, and urinary parameters were not significantly affected, and
the mortality rate was not adversely influenced. No treatment-related
effects were seen on organ weights or the gross or microscopic
appearance of the tissues. In particular, the type, location,
incidence, and time to occurrence of the tumours observed in the
treated groups were comparable to the control findings and to the
spontaneous findings for this strain of rat. The NOAEL was 100 ppm,
equal to 4.9 mg/kg bw per day (Bomhard & Löser, 1981b).
Dogs
Groups of four male and four female beagle dogs received
bitertanol (purity, 95-97.3%) in the diet at concentrations of 0, 10,
40, or 160 ppm, equal to 0.3, 1.2, and 4.9 mg/kg bw per day, for two
years. The method used in this study complied to a certain extent with
OECD guideline 452; at the time the study was performed, compliance
with GLP was not compulsory.
Although the control dogs gained more weight than did treated
dogs, there was no association with treatment. A good nutritional
status was maintained by dogs in all groups, and there was no
significant difference between groups in food or water consumption. No
abnormalities were seen in selected reflexes, body temperature, or
pulse rate; however, three of eight dogs at the high dose developed
bilateral cataracts. Urinary and haematological examinations conducted
at approximately three-month intervals revealed no abnormalities,
although serum alanine aminotransferase and alkaline phosphatase
activities were increased at 40 and 160 ppm. At necropsy, the mean
liver weight of dogs at the high dose was markedly increased.
Histological examination showed mild to moderate vacuolation of the
adrenal zona reticularis epithelia at doses at and above 40 ppm. The
NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day (Hoffmann & Gröning,
1983).
Groups of six male and six female beagles were fed diets
containing 0, 3, or 25 ppm bitertanol (purity, 96.3-96.7%) for 12
months, equal to 0.1, 1, and 7.6 mg/kg bw per day. A further group was
maintained on a diet containing 200 ppm bitertanol for 20 months to
permit continued ophthalmoscopic examination. The method used in this
study complied to a certain extent with OECD guideline 452; at the
time the study was performed, compliance with GLP was not compulsory.
Treatment had no apparent effect on behaviour, appearance, or
nutritional status, and food consumption and body-weight gain were
also unaffected. Pulse rates, body temperature, and selected reflexes
were unchanged. Ophthalmoscopy, conducted on animals at 0, 3, or 25
ppm at three-month intervals, showed no changes; however, one dog at
the high dose developed severe bilateral lenticular cataracts, which
were observable from week 58. Four other animals had slight lenticular
opacification by week 85. Dogs in this group also had signs of
conjunctivitis, with nasociliary discharge and incrustation.
Intermittent increases in serum alanine aminotransferase, glutamate
dehydrogenase, and alkaline phosphatase activities were also seen in
the dogs at the high dose. Other clinical chemical, haematological,
and urinary parameters were unaffected by treatment. At necropsy,
there were no gross abnormalities. The weights of the adrenals of the
dogs at the high dose were apparently greater than those of controls,
and lipoid vacuolation was observed in the zona reticularis epithelia.
The NOAEL was 25 ppm, equal to 1 mg/kg bw per day (Hoffmann & Vogel,
1983).
(d) Genotoxicity
No genotoxic or mutagenic potential of bitertanol was found in
lower organisms or in mammalian cells or systems in vivo or
in vitro. The results of assays for the genotoxicity of bitertanol
are summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
The effect of bitertanol (purity, 95.0%) on fertility, lactation
performance, and pup development was examined in a three-generation
study in Long-Evans FB 30 rats with two litters per generation. The
test substance was administered throughout the study period at
concentrations of 0, 20, 100, or 500 ppm, equivalent to 1, 5, and 25
mg/kg bw per day. Each of the mating groups consisted of 10 male and
20 female rats. The animals were five to six weeks old at the
beginning of the study and were treated for 70 days before the first
mating. The F3b generation pups and their parents (F2b generation)
were sacrificed and examined histopathologically after a four-week
lactation period. The method used in this study complied to a certain
Table 2. Results of assays for the genotoxicity of bitertanol
Test system Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, TA100, 4-2500 µg/plate 93.7 Negative Herbold (1979)
TA1535, TA1537
Reverse mutationa E. coli WP2 hcr- 1-5000 µg/plate 95.0 Negative Shirasu et al. (1981)
S. typhimurium TA98, TA100,
TA1535, TA1537, TA1538
Forward mutationa Mouse lymphoma L5178Y tk +/- 1-25 µg/mlb 95.0 Negative Bootman & Rees
1-20 mg/mlc (1983)
DNA repaird B. subtilis H17 rec+, M45 rec- 20-5000 µg/disc 95.0 Negative Shirasu et al. (1981)
DNA repaira,d E. coli W3110 pol A+; P3470 pol A- 0.1-33.3 mg/plate 95.0 Negative Riach (1981)
Aneuploidy Sordaria brevicollis 0.1-5.0 mg/L 95.0 Negative Bond & McGregor (1981)
Cytogenetic Chinese hamster lung cells 3.3 × 10-6-3.3 × 10-4 97.1 Negative Sasaki (1987)
alterationsa,d mol/Lb
1.0 × 10-5-1.0 ×.10-3
mol/Lc
In vivo
Micronucleus NMRI mice 2 × 1000 mg/kg bw 93.7 Negative Herbold (1978a)
formation 2 × 2000 mg/kg bw
Dominant lethal Male NMRI mice 1000 mg/kg bw 93.7 Negative Herbold (1978b)
mutation
a With and without exogenous metabolic activation
b Without exogenous metabolic activation
c With exogenous metabolic activation
d Conducted in compliance with good laboratory practice and to a certain extent with OECD guidelines
extent with OECD guideline 416; at the time the study was performed,
compliance with GLP was not compulsory.
No adverse effects were seen on appearance, behaviour, or
mortality in any group or generation. Bitertanol at a concentration of
20 ppm in the diet did not affect reproductive performance.
Administration of 100 or 500 ppm led to reductions in pup survival
rates during the lactation period in several matings and to pups with
lower birth weights. At 100 ppm, retarded pup growth was also seen in
the F2a and F2b generations. At 500 ppm, general retardation of
growth was seen. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Löser & Eiben, 1981).
(ii) Developmental toxicity
Rats
Groups of 20-23 Long-Evans rats were given bitertanol (purity,
96.5%) orally at doses of 0, 10, 30, or 100 mg/kg bw per day on days
6-15 post coitum. The method used in this study complied to a
certain extent with OECD guideline 414; at the time the study was
performed, compliance with GLP was not compulsory. The body-weight
gain of dams at 30 or 100 mg/kg bw per day was significantly reduced
during treatment and throughout the gestation period for those at 100
mg/kg bw per day. Resorption rates, fetal deaths, placental weights,
and sex ratio were unaffected by treatment; however, fetal weights
were significantly reduced at 100 mg/kg bw per day and significant
fetal stunting occurred at doses of 30 mg/kg bw per day and higher. At
100 mg/kg bw per day, skeletal ossification was retarded, and a
significantly increased incidence of malformations was observed, which
included cleft palate, hydrocephalus, malformed tails, dysplasia, and
synostosis of the ribs. A simple case of hydrocephalus occurred at 30
mg/kg bw per day. The NOAEL was 10 mg/kg bw per day (Machemer, 1977).
Groups of 22 or 23 Sprague-Dawley rats were given bitertanol
(purity, 96%) orally at doses of 0, 10, 25, or 65 mg/kg bw per day on
days 6-15 post coitum. The study was conducted in compliance with
GLP standards and with OECD guideline 414. Reduced weight gain was
seen in dams at daily doses of 25 mg/kg bw and higher, which persisted
throughout the gestation period in those at 65 mg/kg bw per day. The
number of corpora lutea, implantation rates, resorption rates, live
fetuses, sex ratio, and fetal and placental weights were unaffected by
treatment. An increased incidence of a fourteenth (lumbar) rib
relative to controls was observed at daily doses of 25 mg/kg bw (32%
incidence) and 65 mg/kg bw (70% incidence). No teratogenic effects
were observed. The NOAEL was 10 mg/kg bw (Nagumo et al., 1987).
Pregnant Wistar rats were given single oral doses of 100, 500, or
1000 mg/kg bw bitertanol on day 9, 10, 11, or 13 of gestation. The
doses were calculated to represent 1/5, 1/10, and 1/50 of the reported
LD50 value of 5000 mg/kg bw. The method used complied to a limited
extent to OECD guideline 414. The largest deviation was the short
application period; no information was given on compliance with GLP.
The study was available only in abstract form and is therefore of only
limited value for risk evaluation. Bitertanol induced congenital
anomalies when given on day 9, 10, or 11 at 500 or 1000 mg/kg bw. The
malformations consisted of microcaudia and acaudia and, in rare cases,
exophthalmus, hypognathia, and cleft palte. The NOAEL was 100 mg/kg bw
(Vergieva, 1990).
In two studies, groups of 25 Long-Evans rats were exposed to
bitertanol (purity, 93.7%) by inhalation for 4 h per day on days 6-15
post coitum at a mean analytical concentration of 0, 2.9, 6.4, or 22
mg/m3 air in one study and 0, 27, 60, or 120 mg/m3 air in the other.
The method used complied to a certain extent with OECD guideline 414;
at the time the study was performed, compliance with GLP was not
compulsory. No adverse effects were observed on dams exposed at any
concentration, but the average fetal weights were reduced
significantly by exposure to 120 mg/m3. Fetuses with below-average
weights were found increasingly at aerosol concentra-tions greater
than 22 mg/m3 air, without a clear dose-response relationship.
Implantation rates, resorption rates, placental weights, and sex ratio
were unaffected by treatment. No embryolethal or teratogenic effects
were observed. The NOAEL was 22 mg/m3 air (Machemer & Thyssen, 1979).
Rabbits
Twelve Himalayan rabbits, were given bitertanol (purity, 96.7%)
at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method used complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. The dose of 100 mg/kg bw led to reduced weight
gain and isolated clinical signs, such as reduced food intake,
diarrhoea, and blood in the urine; one dam died on day 29
post coitum. This dose also resulted in increased embryonal and
fetal mortality and reduced fetal and placental weights, and three
individual malformations occurred, which deviated from those seen in
controls in their nature (epignathus, pulmonary hypoplasia, and
aplasia) but not in their number. The NOAEL was 30 mg/kg bw per day
(Roetz, 1982).
Groups of 15 Himalayan rabbits were given bitertanol (purity,
93.9%) at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. Treatment had no effect on the behaviour,
appearance, or body-weight gain of the dams. At 100 mg/kg bw per day,
a significantly higher resorption rate and reduced numbers and weights
of fetuses were seen, and the number of malformed fetuses was
increased, with rare types of malformation such as cleft palate and
pigeon chest. The NOAEL was 30 mg/kg bw per day (Schlüter, 1983).
Groups of 16 fertilized chinchilla rabbits were given bitertanol
(purity, 96.9%) at a daily oral dose of 0, 10, 50, or 250 mg/kg bw on
days 6-18 of gestation. Because of the very high rate of post-
implantation loss at 250 mg/kg bw per day, doses of 0 and 150 mg/kg bw
per day were used in a supplementary study. The study was conducted in
compliance with OECD guideline 414 and with GLP standards. A
dose-related reduction in food consumption was seen in dams at > 50
mg/kg bw per day. The dose of 250 mg/kg bw per day significantly
reduced body weights from day 7 to the end of the experiment and
significantly reduced corrected body-weight gain. One dam at 150 mg/kg
bw per day and two at 250 mg/kg bw per day died during the study.
Isolated hair loss and enlarged and heavier livers at necropsy were
observed in dams at the high dose. A dose-related increase in the rate
of post-implantation losses was observed at > 150 mg/kg bw per day.
Two dams at 150 mg/kg bw per day and 13 at 250 mg/kg bw per day
completely resorbed their embryos, and the fetuses at these doses
showed dose-related reductions in body weight and a dose-related
increased in the incidence of incompletely or unossified phalangeal
nuclei and calcanei. The NOAEL was 50 mg/kg bw per day (Becker et al.,
1987).
(f) Special studies
As the studies in this section were published in the open
literature, the level of detail needed for full evaluation was not
available.
(i) Effects on the central nervous system
The effect of bitertanol on the central nervous system was
examined in pharmacological screening tests in mice and rats, which
included tests for potentiation of anaesthesia in mice (hexobarbital
sleep period), stimulation of spontaneous motility in mice, an Irwin
behaviour test in mice, the novel box response in rats, the open field
test in mice, and a test for reserpine ptosis in mice. In these
investigations, bitertanol was administered as a single oral dose of
0.075, 0.6, or 4.8 mg/kg bw. The results showed a slight stimulating
effect of bitertanol on the central nervous system in mice, but no
specific pharmacological effects, such as potentiation of amphetamine
effects or antagonism to reserpine ptosis, were determined (Polacek,
1983).
In a pharmacological test programme, groups of 10 male mice were
given a single oral dose of 0.02, 0.2, 2, 20, or 200 mg/kg bw
bitertanol. The highest dose significantly increased spontaneous motor
activity, with marked effects when treatment was given during the dark
period. In mice treated with 20 mg/kg bw per day, motor activity
tended to increase but not significantly, and lower doses had no
effect. No further effects on behaviour were observed at any dose
(Kaneto, 1986a). In a further test, male mice received bitertanol at 1
or 100 ppm for one week or one month. No increase in spontaneous motor
activity and no potentiating effect in amphetamine-pretreated animals
were seen after repeated dosing (Kaneto, 1986b).
In a study designed to determine whether bitertanol has similar
behavioural effects on the fixed-interval response rate and motor
activity of rats, doses of 10-300 mg/kg bw were given
intraperitoneally to rats maintained under a multiple fixed-interval
1-min schedule of reinforcement. Intermediate doses increased the
response rates and disrupted response patterning in both
fixed-interval components. The same doses of bitertanol did not
increase motor activity (Allen & MacPhail, 1983).
In rats placed in an actographic device designed for continuous
measurement of the locomotor component of spontaneous motor activity,
bitertanol increased motor activity at 200 mg/kg bw after
administration either orally or intraperitoneally. The activity peaks
at the low dose of 100 mg/kg bw coincided with normal night and
morning activity maxima (Frantik et al., 1996).
One the basis of previous results showing that acute exposure to
the triazole fungicide triadimefon affects central nervous system
catecholamines and induces a transient syndrome in rats that consists
of hyperactivity and stereotyped behaviour, a study was designed to
determine whether this type of toxicity is characteristic of other
triazoles. Dose-effect functions were determined for 14 triazoles or
structurally related pesticides, including bitertanol, in adult male
Long-Evans rats. All of the chemicals were administered orally in corn
oil. Hyperactivity was measured for 2 h in figure-eight mazes. Only
triadimefon and triadimenol induced hyperactivity, suggesting a very
rigid structure-activity relationship for the hyper-activity syndrome.
The absence of an effect of bitertanol may be due to steric hindrance
of benzene-ring substitution for the halogen on the benzene-ring
structure of triadimefon and/or triadimenol. Alternatively, bitertanol
may lack halogen substituents on the benzene rings and thus be less
polarized. The lack of activity of bitertanol is probably not due to
differences in absorption kinetics (Crofton, 1996).
(ii) Toxicity in combinations
Acute tests were performed to determine whether bitertanol has
superadditive (potentiating) effects when administered in combination
with triadimenol, captan, fuberidazole, or dodine. At the time the
studies were performed, OECD methods were not available and compliance
with GLP was not compulsory. The study involved administration of
single equitoxic oral (with triadimenol, captan, or fuberidazole) or
intraperitoneal (with dodine) doses of the active ingredients to male
rats. A factor greater than 1 between observed and expected LD50
values indicates a superadditive effect. Bitertanol plus triadimenol,
bitertanol plus fuberidazole, and bitertanol plus dodine had no
superadditive effects but only additive toxic effects (Mihail, 1982b;
Flucke, 1980; Heimann, 1984b). In contrast, the active ingredient
combination of bitertanol plus captan had slightly superadditive
action, with a potentiation factor of 1.9 (Mihail, 1982a).
(iii) Effect on the liver
Groups of 10 male and 10 female Wistar rats received bitertanol
(purity, 95.8%) suspended in distilled water with Cremorph EL by
gavage at doses of 0, 30, 100, or 300 mg/kg bw per day for 14 days. On
sacrifice, blood was collected for detailed haematological and
clinical chemical testing, and liver samples were taken for detailed
histopathological and enzyme studies. During treatment, 1/10 female
rats at the intermediate dose and 9/10 at the high dose showed hair
loss, and some lost weight, while females at the low dose had reduced
body-weight gain in comparison with control animals. Female rats also
had a dose-related tendency to mild thrombocytosis, which was
significant at the intermediate and high doses. Serum gamma-glutamyl
transpeptidase activity and bilirubin concentration were slightly
increased in females at the high dose. There were no gross
pathological findings at necropsy. The liver weights tended to be
increased at the intermediate and high doses, especially in females.
Slight-to-moderate bile-duct proliferation with peribiliary
infiltration of monocytes or polynucleocytes was seen histologically
in animals at the intermediate and high doses. These changes were
sometimes accompanied by parenchymal Councilman bodies or, more
occasionally, mitoses. The hepatocytes of animals at the high dose
were occasionally swollen, with finely granular cytoplasm. Little
fatty infiltration was seen. The results of studies in vitro were
consistent with induction of hepatic microsomal enzymes, as the
cytochrome P450 content increased in a dose-related manner, especially
in males. Aminopyrene N-demethylase activity was increased in males
and females at the high dose and in males at the intermediate dose,
while O-demethylase activity was increased in males at the
intermediate and high doses and in females at the high dose. The
hepatic triglyceride content was not affected by treatment. Bitertanol
thus caused mild hepatotoxicity, with modest induction of hepatic
microsomal activity in rats at 100 and 300 mg/kg bw per day. The NOAEL
was 30 mg/kg bw per day (Mihail & Luckhaus, 1985).
3. Studies on metabolites
(a) 1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one
(plants, soil)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one, a
keto analogue of bitertanol, has very little acute toxicity in rats
when given orally or dermally. It induced signs of effects on the
central nervous system, with initial sedation and respiratory
disturbances and later stimulation. The highest technically
administrable oral dose, 1750 mg/kg bw, caused no deaths. A dermal
dose of 5000 mg/kg bw and the highest dose administered by inhalation
(dynamic dust nebulization) were tolerated with no observed signs. The
LC50 value in male and female rats was > 500 mg/m3 air after a 1-h
exposure or a 4-h exposure, respectively. In rabbits, the compound was
slightly irritating to the skin only after contact for 24 h and was
mildly irritating to the mucous membranes of the eyes (Thyssen &
Kimmerle, 1978).
(b) Bitertanol benzoic acid (soil)
Bitertanol benzoic acid had little acute toxicity when given
orally. The highest administered dose, 5000 mg/kg bw, was tolerated by
fasted male rats with no observed signs or deaths (Heimann, 1983b).
The compound was not mutagenic to Salmonella typhimurium in the
presence of an exogenous metabolic activation system (Herbold, 1983).
(c) 1,2,4-Triazole (photodegradation, soil)
1,2,4-Triazole has moderate or low acute toxicity when given
orally (LD50 value, 1600 mg/kg bw in males and females) or dermally
(LD50 values, 4200 mg/kg bw in males and 3100 mg/kg bw in females).
At high oral and dermal doses, the compound had effects on the central
nervous system. In tests of inhalation of air enriched with vapours of
the test substance, male rats and mice tolerated exposure for 4 and
6 h, respectively, with no observed signs. No dermal irritation was
observed on rabbits exposed for 24 h or on the skin of five male
volunteers exposed for 8 h. 1,2,4-Triazole was strongly irritating to
the mucous membranes of the rabbit eye (Thyssen & Kimmerle, 1976).
Groups of 15 male and 15 female rats were given 1,2,4-triazole
(purity, 99.6%) at a dose of 0, 100, 500, or 2500 ppm for three
months. The study was conducted before enactment of prevailing
regulatory guidelines, but the test procedures complied to a certain
extent with OECD guideline 408. Treatment at 2500 ppm resulted in a
temporary decrease in food intake, a reduction in body weight, and
transient, slight palmospasms in isolated animals. Significant
reductions in the haemoglobin, haematocrit, mean corpuscular volume,
and mean corpuscular haemoglobin values in male rats at 2500 ppm
indicated an effect on the blood. In this group, slight to moderate
fat accumulation was found in the cells of the liver parenchyma in
three of 15 males, which was attributed to the treatment. The NOAEL
was 500 ppm, equal to 38 mg/kg bw per day (Bomhard et al., 1979).
1,2,4-Triazole at concentrations of 10-5000 µg/plate did not
induce point mutations in Salmonella typhimurium TA1535, TA1537,
TA98, or TA100, with or without metabolic activation (Poth, 1989).
In two studies, groups of 25 fertilized Wistar rats were given
daily oral doses of 1,2,4-triazole (purity, 95.3% and 94.0%,
respectively) on days 6-15 of gestation at doses of 0, 10, 30, or 100
mg/kg bw in one study and 0, 100, or 200 mg/kg bw in the other. The
studies were conducted in compliance with GLP standards and OECD
guideline 414. Maternal toxicity was indicated by decreased weight
gain of dams at 100 and 200 ppm relative to those in the control
group. Reduced fetal weights, retarded osteogenesis, and increased
numbers of runts were found at > 100 mg/kg bw per day. An increased
resorption rate was found in dams at 200 mg/kg per day, but the rate
of fetuses with retarded ossification was not increased. The types and
incidences of the malformations observed in this group (including
cleft palate and malformed extremities) indicate that 1,2,4-triazole
has teratogenic potential. The NOAEL was 30 mg/kg bw per day (Renhof,
1988a,b).
4. Observations in humans
No health impairment was observed in male or female employees
engaged in formulating bitertanol and using the customary safety
precautions, who had regular medical examinations (Miksche, 1981).
Slight, transient prurient reddening of the forearms, which
regressed spontaneously after a few days, developed in rare, isolated
cases after direct dermal contact during packaging of a powder of the
pure active ingredient. Unequivocal differentiation between an
allergic dermal reaction and mechanical-toxic skin irritation was not
possible. There was no tendency to relapse (Faul, 1986).
Comments
After oral administration bitertanol is rapidly and extensively
absorbed (about 84%) and distributed. Excretion is also rapid (rats,
almost complete within 72 h) and occurs mainly in the faeces (about
90%) by biliary excretion, owing to the lipophilic nature of the
parent substance. The liver and kidneys are the main sites of tissue
accumulation in both male and female rats. Although some statistically
significant sex-related differences were seen, they were of minor
physiological importance. The substance has a relatively low rate of
dermal penetration. The metabolic profile was similar at the various
doses tested. The main metabolic pathways are hydroxylation of the
phenyl ring in the para position and oxidation of the tert-butyl
moiety, leading to bitertanol alcohol and the corresponding carboxylic
acid; metabolites derived from the two pathways combined were also
observed. The metabolites occur in both free and conjugated forms. The
parent compound was not detected in urine or bile. There was no
toxicological concern with regard to the metabolic profile in plants.
Bitertanol had very low acute toxicity in rats, mice, and dogs
when given orally and after dermal application or by inhalation. It
was of moderate to low toxicity in rats and mice after intraperitoneal
injection. Females appeared to be slightly more sensitive than males,
but only in some studies. This was perhaps due to slightly different
absorption characteristics in animals of the two sexes, as seen after
oral administration. In view of the toxic signs (including respiratory
disturbances, sedation, spasms, and tremor) and the findings of the
pharmacological screening tests, it may be inferred that bitertanol
has central nervous system activity; however, no specific
pharmacological effects, such as potentiation of amphetamine action,
antagonism of reserpine ptosis, or an effect on hexobarbital
anaesthesia, were observed.
Bitertanol induces no, or only very slight, dermal irritation. It
induces slight to moderate reactions of the ocular mucosa but has no
effect on the cornea or iris. No evidence for a sensitizing effect was
observed in any study
Bitertanol has been classified by WHO as unlikely to present an
acute hazard in normal use (WHO, 1996).
In medium- and long-term tests for toxicity, the liver is
regarded as the main toxicological target organ in dogs and rats at
doses of 1.2 and 28 mg/kg bw per day and above, respectively. Liver
weight was found to be the most sensitive indicator. Corresponding
patterns of disruption of liver function were observed in rats and
dogs. The activities of the transaminases, alkaline phosphatase, and
glutamate dehydrogenase in the serum were increased. In addition, a
rise in cholesterol level was observed in several studies in rats at
doses of 61 mg/kg bw per day and above. The ability of bitertanol to
induce mixed-function oxidases was verified in both species. It is
therefore likely that the effect on weight is essentially due to
hypertrophy of the endoplasmic reticulum in hepatocytes at doses of
100 mg/kg bw per day and above. Morphological changes in the liver
were seen only at relatively high doses and consisted of hepatocytic
swelling, bile-duct proliferation, perilobular fatty degeneration,
eosinophilic foci, and fibrous structures. The induced effects
corresponded to toxic liver damage with bile-duct involvement.
Evidence for haemotoxicity was found at doses of 28 mg/kg bw per
day and above in rats; this may be classified as an effect on the
peripheral red blood cell population. The decreases in erythrocyte
count, haemoglobin concentration, and packed cell volume and the
compensatory rise in the reticulocyte count argue for this
interpretation. No evidence was found for damage to the haematogenic
organs.
Slight increases in the leukocyte count were also seen in a few
studies in rats at doses of 61 mg/kg bw per day and above. The
increased leukocyte counts are probably attributable to inflammatory
processes, since the increase occurred in studies and at doses at
which inflammatory processes were also observed. Hyperkeratosis of the
oesophageal epithelium and glandular stomach and/or erosions of the
glandular stomach as well as parakeratosis of the stomach wall were
observed in rats. The histopathological picture included distended
epithelial cells, slight inward growth of the papillary body, and
cellular infiltration of the epithelial and subepithelial layers in
the affected animals. These changes are probably attributable to
irritation of the mucous membranes by the active ingredient.
Effects on the skin were observed in dogs, sheep and rats. The
effects in dogs at doses of 5 mg/kg bw per day and above consisted of
reddening, with localized inflammation, desquamation, and hair loss,
and increased reddening and slight inflammatory phenomena in the
mucous membranes of the oral cavity and eyes. Histological examination
showed broadening of the epithelial layer, enhanced cornification, and
minor erosions. The dermal effects were apparently accompanied by
pruritus. The keratitis observed in dogs was considered to be
secondary to conjunctivitis. Hair loss was also observed in rats and
sheep, which occurred after administration of bitertanol by capsule or
gavage at doses of 300 mg/kg per day and above; this was considered to
be a systemic effect.
Pathological changes were seen in the adrenals of dogs and rats
at 1.2 and 81 mg/kg bw per day and above, respectively, consisting of
swelling and fatty degeneration of the adrenal cortical cells,
particularly in the zona reticularis and zona fasciculata. These
alterations were considered to be due to inhibition of sterol
biosynthesis by triazole derivatives. It is highly probable that this
effect, which also represents the biological, antimycotic action of
the substance, leads to an effect on corticoid metabolism with
corresponding morphological effects in the cells of the adrenal
cortex.
In feeding studies in dogs, lenticular opacity seen at doses of
4.9 mg/kg bw per day and above were considered to be related to
treatment. No lenticular alterations attributable to administration of
bitertanol were seen in any other study or species. The exact
mechanism of the cataractogenesis resulting from long-term
administration of triazole fungicides is presently unknown. The ocular
lens undertakes its own de-novo synthesis of cholesterol, which is
isolated from lipoproteins circulating in the blood; other substances
that inhibit cholesterol synthesis can induce cataracts.
No evidence for any carcinogenic potential of bitertanol was
found in long-term studies of toxicity and carcinogenicity in rats and
mice treated in the diet. The highest doses tested were 130 mg/kg bw
per day in mice and 26 mg/kg bw per day in rats.
In a series of studies, bitertanol had no genotoxic potential in
lower organisms or in mammalian cells or systems in vitro or
in vivo.
In a three-generation study of reproductive toxicity in rats,
adverse effects on the pups (reductions in survival rates during the
four-week lactation period, reduced weight at birth, and retarded
growth) were observed at parentally toxic doses of 100 or 500 ppm,
equivalent to 5 or 25 mg/kg bw per day. The NOAEL was 1 mg/kg bw per
day.
In studies of developmental toxicity, treatment with bitertanol
led to several embryotoxic and teratogenic effects, depending on the
animal species, route of administration, and dose. In rats, an oral
dose of 10 mg/kg bw per day was tolerated with no observed effect.
Doses of 25 mg/kg bw per day and above led to retardations and
variations (e.g. increased incidence of the 14th rib). Malformations
were observed at an oral dose of 100 mg/kg bw per day, which was
clearly maternally toxic. Exposure of pregnant rats to concentrations
of 27 mg/m3 air and above by inhalation resulted in retardation
effects; no malformations were observed. In rabbits, doses of 50 mg/kg
bw per day and above were maternally toxic; fetotoxic effects were
seen at doses of 100 mg/kg bw per day and above. Teratogenic effects
were observed in Himalayan rabbits at 100 mg/kg bw per day, while in
the second strain tested (Chinchilla), even a dose of 250 mg/kg bw per
day did not lead to malformations.
The ADI established at the 1988 Meeting of 0-0.01 mg/kg bw, based
on a combined NOAEL of 1 mg/kg bw per day from the two-year and the
one-year study in dogs, was maintained. The ADI is supported by the
NOAEL of 20 ppm (equivalent to 1 mg/kg bw per day) in a
three-generation study in rats.
An acute RfD was not allocated because bitertanol has been
classified by WHO as unlikely to present an acute hazard in normal use
and it has not shown any specific adverse effects (teratogenicity,
neurotoxicity) after single doses 100 times the lowest relevant NOAEL
in long- and short-term studies that were used to establish the ADI.
Therefore, the Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 25 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 100 ppm, equal to 4.9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
20 ppm, equivalent to 1 mg/kg bw per day (reproductive
and parental toxicity in a three-generation study)
10 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 1 mg/kg bw per day (overall NOAEL in one-year and
two-year studies of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Commenced immediately, about 84% absorbed
Dermal absorption About 10%
Distribution Highest concentrations in liver and kidneys
Potential for accumulation None
Rate and extent of excretion About 90% excreted with bile, 10% with urine
Metabolism in animals No parent compound in bile or faeces; extensively
metabolized to 14 metabolites (ring
monohydroxylation, ring dihydroxylation, aryl
O-methylation, aliphatic hydroxylation, aliphatic
oxidation to carboxylic acids, and ether cleavage)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral > 5000 mg/kg bw
Rat: LD50,, dermal > 5000 mg/kg bw
Rat: LC50, inhalation > 550 mg/m3 (4 h)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not a sensitizer (Magnussen & Kligman test)
Short-term toxicity
Target/critical effect Liver, red blood cells, adrenals, digestive tract
Lowest relevant oral NOAEL Dog: 90 days: 1 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 3 weeks; 250 mg/kg per day
Lowest relevant inhalation NOAEL Rat: 3 weeks, 63 mg/m3
Genotoxicity No genotoxic or mutagenic potential
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL Dog: 1 year and 2 years: 1 mg/kg bw per day
Carcinogenicity No evidence of carcinogenic potential
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced litter size, pup
growth rate, and pup survival) at parentally toxic
doses
Lowest relevant reproductive NOAEL Rat: 1 mg/kg bw per day
Developmental target/critical effect Fetotoxic and teratogenic effects at maternally
toxic doses
Lowest relevant developmental NOAEL Rat: 10 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No relevant effects
Other toxicological studies Induction of hepatic microsomal activity
Medical data No health impairments observed in employees
subjected to regular medical examinations
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 1 and 2 years in dogs 100
Acute reference dose None allocated
(unnecessary)
References
Allen, A.R. & MacPhail, R.C. (1993) Bitertanol, a triazole fungicide,
increases operant responding but not motor activity. Neurotoxicol.
Teratol., 15, 237-242.
Becker, H., Mueller, E., Vogel, W. & Terrier, C. (1987) Embryotoxicity
(including teratogenicity) study with KWG 0599 in the rabbit.
Unpublished report No. R4081 from Research & Consulting Company AG,
Itingen, Switzerland. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Löser, E. (1978) KWG 0599: Subchronic toxicological
study on rats (feeding experiment over three months). Unpublished
report No. 7322 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Löser, E. (1981a) KWG 0599 (Bitertanol, the active
ingredient of Baycor): Chronic toxicity study on mice (2-year feeding
experiment). Unpublished report No. 10103 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Bomhard, E. & Löser, E. (1981b) KWG 0599: Chronic toxicity study on
rats (2-year feeding experiment). Unpublished report No. 10104 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole: Subchronic
toxicological study with rats (feeding study over three months).
Unpublished report No. 8667 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bond, J. & McGregor, D. (1981) Testing for aneuploid induction of HE
1004 in Sordaria brevicollis. Unpublished report No. R2063 from
Inveresk Research International, Musselburgh, Scotland. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bootman, J. & Rees, R. (1983) KWG 0599: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report No. R2406 from Life Science Research. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Crofton, K.M. (1996) A structure-activity relationship for the
neurotoxicity of triazole fungicides. Toxicol. Lett., 84, 155-159.
Faul, J. (1986) Baycor active ingredient and formulations. Unpublished
report from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50) -- rat p.o.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1979) KWG 0599--Determination of acute toxicity (LD50):
rat p.o./mixed batch (from 5 batches). Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) KWG 0599 and W VII/117: Study for acute combination
toxicity. Unpublished report No. 9456 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1981) KWG 0599: Evaluation for sensitization in guinea
pigs (maximization test after Magnusson and Kligman). Unpublished
report No. 9934 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Frantik, E., Hornychova, M., Dvorak, J. & Mraz, J. (1996) Spontaneous
motor activity in rats enhanced by 1,2,4-triazole derivatives:
Triadimefon, triadimenol and biteranol. Homeostasis, 37, 3.
Hatanaka, J., Asaoka, S., Ikeda, M., Shimizu, M., Inukai, H.,
Iyatomy, A. & Itoh, H. (1981) KWG 0599: Short-term toxicity tests on
rats (4-week feeding and 4-week recovery tests). Unpublished report
No. 195 from Nihon Tokushu Noyaku Seizo K.K. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1981) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983a) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983b) Determination of acute toxicity (LD50): BUE
2684 (Bitertanol-benzoic acid)--rat p.o. Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50): KWG
0599. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984b) Dodine & KWG 0599 (c.n. Dodine & Bitertanol
(proposed)): Combination toxicity study. Unpublished report No. 12608
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Vogel, O. (1984) KWG 0599 (c.n. Bitertanol): Subacute
study of dermal toxicity to rabbits. Unpublished report No. 12571 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978a) KWG 0599: Micronucleus test on mouse to evaluate
KWG 0599 for potential mutagenic effects. Unpublished report No. 7860
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978b) KWG 0599: Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 7964 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979) KWG 0599: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 8152 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) BUE 2684: Bitertanol-benzoic acid:
Salmonella/microsome test to evaluate for point mutation. Unpublished
report No. 12087 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hixson, E.J. (1979) Baycor technical: Acute dermal toxicity to
rabbits. Unpublished report No. 57 from Mobay Chemical Corporation,
Staneley Research Center. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1984) Dermal absorption of 14C-Baycor applied as 50%
wettable powder. Unpublished report No. 484 from Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1977) KWG 0599: Evaluation for sensitization.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1981a) KWG 0599: Determination of the acute toxicity to
the dog after oral administration. Unpublished report No. T 6001023
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1981b) KWG 0599/012: Acute oral toxicity to sheep.
Unpublished report No. 9768 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1983) KWG 0599 (c.n. Bitertanol): Chronic
oral toxicity study on dogs (2-year feeding experiment). Unpublished
report No. 12307 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hoffmann, K. & Schilde, B. (1979) KWG 0599: Subchronic toxicity study
on dogs (thirteen-week administration in capsules). Unpublished report
No. 8053 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Hoffmann, K. & Vogel, O. (1983) KWG 0599 (Bitertanol): Second chronic
toxicity study with dogs on oral administration (feeding study, 0-3-25
ppm 12 months, 200 ppm 20 months). Unpublished report No. 12328 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Iyatomy, A. (1980) KWG 0599: Report of acute toxicity. Unpublished
report No. Sheet A-31 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iyatomy, A. (1981) Report of acute toxicity: KWG 0599. Unpublished
report No. Sheet 22 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986a) Effect of bitertanol on spontaneous motor activity
in mice; Part 1. The experiment on the acute effect. Unpublished
report No. DT-1 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986b) Effect of bitertanol on spontaneous motor activity
in mice; Part 2. The experiment on the subacute effect. Unpublished
report No. DT-2 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Klein, O. (1988a) [U-phenyl-14C]-Bitertanol: Absorption, excretion,
tissue-distribution, and biokinetic profile of the total radioactivity
in the rat following single oral and intraduodenal dosage. Unpublished
report No. PF3076 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Klein, O. (1988b) [U-phenyl-14C]-Bitertanol: Distribution of the
parent compound in the excreta and in some organs at different times
following single oral administration to rats. Unpublished report No.
PF3096 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1989) [U-phenyl-14C]-Bitertanol: Distribution of the
metabolites in some organs at different times following single oral
administration to rats. Unpublished report No. PF3501 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Bomhard, E., Löser, E. & Schilde, B. (1978 ) B. KWG
0599: Subchronic toxicity study on rats (three-month feeding
experiment). Unpublished report No. 8002 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Löser, E. & Eiben, R. (1981) KWG 0599: Multigeneration reproduction
study on rats. Unpublished report No. 10024 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. & Thyssen, J. (1979) KWG 0599 (Baycor active ingredient):
Evaluation for embryotoxic and teratogenic effects on rats after
dynamic inhalational exposure. Unpublished report No. 8610 from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1977) KWG 0599: Evaluation for embryotoxic and
teratogenic effects on rats after oral administration. Unpublished
report No. 6697 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978a) Determination of acute toxicity (LD50) / KWG 0599
Form A: PFU 190978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1978b) Determination of acute toxicity (LD50) / KWG 0599
Form B: PFU 210978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982a) KWG 0599 and captan: Study for acute combination
toxicity. Unpublished report No. 11205 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982b) KWG 0519 and KWG 0599: Study for acute combination
toxicity. Unpublished report No. 11203 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Kimmerle, G. (1977) KWG 0599: Subacute inhalation
toxicity study on rats. Unpublished report No. 6868 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Luckhaus, G. (1985) KWG 0599 (c.n. Bitertanol): Subacute
oral toxicity study with special attention to effect on the liver.
Unpublished report No. 13230 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Miksche, L. (1981) Data on effects on humans/In-company occupational
medical experience. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Nagumo, K., Teraki, Y., Chiba, T. & Miyasaka, M. (1981, revised 1987)
Teratogenicity test of KWG 0599 in pregnant rats. Unpublished report
from St Marianna University School of Medicine. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Pauluhn, J., Thyssen, J. & Vogel, O. (1983) KWG 0599 (Baycor active
ingredient) (c.n. Bitertanol): Study for cataract-inducing effect on
subacute whole-body exposure with cats (exposure 20 × 6 hours).
Unpublished report No. 12006 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Polacek, I. (1983) Central nervous effects of KWG 0599 (pilot study).
Unpublished report No. R2694 from Toxikologisches Institut Regensburg.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with 1H-
1,2,4-triazole. Unpublished report No. R4859 from Cytotest Cell
Research GmbH & Co. KG. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puhl, R.J. & Hurley, J.B. (1983) The absorption, excretion, and
metabolism of Baycor-phenyl-UL-14C by rats. Unpublished report No.
85832 from Mobay Chemical Corporation, Agricultural Chemicals
Division. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) 1,2,4-triazole: Investigations into embryotoxic
effects on rats after oral administration. Unpublished report No.
17401 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Renhof, M. (1988b) 1,2,4-triazole: Investigation into embryotoxic
effects on rats after oral administration. Supplement to study No.
T5019339. Unpublished report No. 17402 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Riach, C.G. (1981) KWG 0599: Testing the modification of cellular DNA
in Escherichia coli. Unpublished report No. R2013 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Roetz, R. (1982) KWG 0599 (Bitertanol, the active ingredient of
Baycor): Study of embryotoxic (and teratogenic) effects on rabbits
after oral administration. Unpublished report No. 10979 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sasaki, Y.F.X. (1987) Bitertanol: In vitro cytogenetics test.
Unpublished report No. DT-5 from The Institute of Environmental
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1983) KWG 0599: Study to determine embryotoxic and
teratogenic effects on rabbits following oral administration.
Unpublished report No. 11548 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987) Acute inhalation toxicity study with Baycor
technical dust in Sprague-Dawley rats. Unpublished report No. 837 from
Mobay Corporation, Staneley Research Center, Report. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Ohta, T. (1981) Bitertanol: Microbial
mutagenicity study. Unpublished report from Department of Toxicology,
Institute of Environmental Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977) KWG 0599: Intracutaneous allergy test on guinea
pigs. Unpublished report No. 7113 from Bayer AG, Leverkusen. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kaliner, G. (1977) KWG 0599: Subacute oral cumulative
toxicity study on rats (four-week treatment). Unpublished report No.
7153 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole: Occupational
toxicology study. Unpublished report No. 5926 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977a) KWG 0599: Acute toxicity studies.
Unpublished report No. 6546 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977b) KWG 0599: Subacute inhalational
toxicity study on dogs. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1978)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one (third
stage KWG 0599, BUE 1662): Industrial toxicology study. Unpublished
report No. 7242 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vergieva, T. (1990) Triazoles teratogenicity in rats. Teratology,
42, 27A-28A.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Yonemura, H., Shimazaki, M., Yamazaki, K., Takahashi, Y. & Hashizume,
M. (1981) Subacute toxicity tests with Bitertanol (KWG 0599) in rats.
Unpublished report from Research Institute for Animal Science in
Biochemistry and Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
 About 84% of the dose was absorbed after oral administration.
Absorption commenced immediately, and the plasma concentration
increased from 25 to 75% of the peak value within 1-2 h. The
radiolabel was eliminated rapidly and almost completely from the body
within the 72-h test period. More than 90% of the recovered radiolabel
was excreted with the faeces, and about 7% with the urine. On average,
the residual radiolabel in the body (excluding the gastrointestinal
tract) after 72 h represented 0.5% of the administered dose. The
results obtained after bile fistulation indicate that the largest
fraction of faecally eliminated radioactivity was first absorbed and
then eliminated into the gut lumen with the bile (about 77%). Most of
the radiolabel eliminated in the bile underwent enterohepatic
circulation. The radiolabel was rapidly distributed from the
intravascular space to the peripheral tissues. The maximum
concentration in plasma was reached after 3-8 h. The terminal phase of
elimination of radiolabel from the plasma was described by linear
regression analysis, which yielded a half-life of about 26 h. The
distribution of total radiolabel in individual organs of male rats at
various times after administration of a single oral dose of 10 mg/kg
was generally similar to that observed 72 h after administration. The
highest concentrations were found in the liver and kidney (Klein,
1988a,b, 1989).
After a 50% formulation of 14C-labelled bitertanol was applied
to the skin of adult male and female albino rabbits, the mean level of
dermal penetration was around 10%. There were no marked sex-specific
differences. Since this experiment was designed to simulate
'worst-case' conditions, lower absorption rates might expected in
practice (Hixson, 1984).
(b) Biotransformation
In the first study described above, bitertanol was extensively
metabolized, and the metabolite profile was similar in the groups
receiving 100 or 1000 mg/kg bw. The relative amounts of metabolites
were also similar, except that the animals receiving the highest oral
dose eliminated much more unchanged parent compound than the others.
Fourteen metabolites (plus bitertanol), representing 38-76% of the
recovered radiolabel, were identified or characterized. Hydroxylation
of the para position of the biphenyl and methyl groups of the
tert-butyl moieties gave rise to phenolic and diol metabolites.
Although one diastereomer of the latter was detected, it underwent
oxidation to the corresponding butanoic acid and subsequent ring
hydroxylation. The parahydroxylated diol was also detected, as was
4,4-dihydroxy-biophenyl, and other hydroxylated metabolites were also
tentatively identified. The metabolic reactions thus included ring
monohydroxylation, ring dihydroxylation, aryl O-methylation,
aliphatic hydroxylation, aliphatic oxidation to carboxylic acids, and
ether cleavage. In addition to the metabolites shown in Figure 2,
glucuronide and sulfate conjugates of some metabolites, including
para-hydroxybitertanol, were also detected in faeces and urine (Puhl
& Hurley, 1983).
In the second set of studies described above, the structures and
amounts of metabolites were determined in faeces, urine, and bile and
in the liver, kidneys, and perirenal fat at various times after
treatment. The metabolism of bitertanol began immediately after
absorption from the gastrointestinal tract lumen. The parent compound
was not detected in urine or bile, and the only metabolite identified
in the bile was para-hydroxybitertanol. Excretion of the unchanged
parent compound in the urine was unlikely because of its lipophilic
character. Metabolic degradation of bitertanol in the organs was
rapid: within 8 h, the concentration in the liver fell from around 15%
to about 2% of the total radiolabel in the organs. The main metabolite
in the liver was also para-hydroxybitertanol; smaller amounts of
para-hydroxy-bitertanol acid, para-hydroxybitertanol alcohol, and
bitertanol acid were also identified. The distribution of metabolites
in the kidneys was similar: total organ radiolabel fell from around
14% to about 2.5% within 8 h; the main metabolite was again
para-hydroxybitertanol, which represented 30-50% of the organ
radiolabel. Furthermore, the amounts of metabolites in the liver and
kidneys were similar. The total amount of radiolabel in fat samples
was too low to permit reliable quantification or identification of
possible metabolites, due mainly to the small amount of fat available
in the young animals. A proposed metabolic pathway for bitertanol in
rats is given in Figure 3. The parent compound found in the faeces of
orally treated rats was probably due to the unabsorbed fraction of
administered radiolabel, representing about 15% of the original dose.
The main metabolite in the liver and kidneys,
para-hydroxy-bitertanol, was also identified in the faeces. The
amounts of the other biotransformation products in the organs were
probably too low for detection in the excreta (Klein, 1988b, 1989).
2. Toxicological studies
(a) Acute toxicity
The methods used in the studies summarized below complied to a
certain extent with OECD guidelines. At the time that most of the
studies were performed, compliance with good laboratory practice (GLP)
was not compulsory. The results of studies on the acute toxicity of
About 84% of the dose was absorbed after oral administration.
Absorption commenced immediately, and the plasma concentration
increased from 25 to 75% of the peak value within 1-2 h. The
radiolabel was eliminated rapidly and almost completely from the body
within the 72-h test period. More than 90% of the recovered radiolabel
was excreted with the faeces, and about 7% with the urine. On average,
the residual radiolabel in the body (excluding the gastrointestinal
tract) after 72 h represented 0.5% of the administered dose. The
results obtained after bile fistulation indicate that the largest
fraction of faecally eliminated radioactivity was first absorbed and
then eliminated into the gut lumen with the bile (about 77%). Most of
the radiolabel eliminated in the bile underwent enterohepatic
circulation. The radiolabel was rapidly distributed from the
intravascular space to the peripheral tissues. The maximum
concentration in plasma was reached after 3-8 h. The terminal phase of
elimination of radiolabel from the plasma was described by linear
regression analysis, which yielded a half-life of about 26 h. The
distribution of total radiolabel in individual organs of male rats at
various times after administration of a single oral dose of 10 mg/kg
was generally similar to that observed 72 h after administration. The
highest concentrations were found in the liver and kidney (Klein,
1988a,b, 1989).
After a 50% formulation of 14C-labelled bitertanol was applied
to the skin of adult male and female albino rabbits, the mean level of
dermal penetration was around 10%. There were no marked sex-specific
differences. Since this experiment was designed to simulate
'worst-case' conditions, lower absorption rates might expected in
practice (Hixson, 1984).
(b) Biotransformation
In the first study described above, bitertanol was extensively
metabolized, and the metabolite profile was similar in the groups
receiving 100 or 1000 mg/kg bw. The relative amounts of metabolites
were also similar, except that the animals receiving the highest oral
dose eliminated much more unchanged parent compound than the others.
Fourteen metabolites (plus bitertanol), representing 38-76% of the
recovered radiolabel, were identified or characterized. Hydroxylation
of the para position of the biphenyl and methyl groups of the
tert-butyl moieties gave rise to phenolic and diol metabolites.
Although one diastereomer of the latter was detected, it underwent
oxidation to the corresponding butanoic acid and subsequent ring
hydroxylation. The parahydroxylated diol was also detected, as was
4,4-dihydroxy-biophenyl, and other hydroxylated metabolites were also
tentatively identified. The metabolic reactions thus included ring
monohydroxylation, ring dihydroxylation, aryl O-methylation,
aliphatic hydroxylation, aliphatic oxidation to carboxylic acids, and
ether cleavage. In addition to the metabolites shown in Figure 2,
glucuronide and sulfate conjugates of some metabolites, including
para-hydroxybitertanol, were also detected in faeces and urine (Puhl
& Hurley, 1983).
In the second set of studies described above, the structures and
amounts of metabolites were determined in faeces, urine, and bile and
in the liver, kidneys, and perirenal fat at various times after
treatment. The metabolism of bitertanol began immediately after
absorption from the gastrointestinal tract lumen. The parent compound
was not detected in urine or bile, and the only metabolite identified
in the bile was para-hydroxybitertanol. Excretion of the unchanged
parent compound in the urine was unlikely because of its lipophilic
character. Metabolic degradation of bitertanol in the organs was
rapid: within 8 h, the concentration in the liver fell from around 15%
to about 2% of the total radiolabel in the organs. The main metabolite
in the liver was also para-hydroxybitertanol; smaller amounts of
para-hydroxy-bitertanol acid, para-hydroxybitertanol alcohol, and
bitertanol acid were also identified. The distribution of metabolites
in the kidneys was similar: total organ radiolabel fell from around
14% to about 2.5% within 8 h; the main metabolite was again
para-hydroxybitertanol, which represented 30-50% of the organ
radiolabel. Furthermore, the amounts of metabolites in the liver and
kidneys were similar. The total amount of radiolabel in fat samples
was too low to permit reliable quantification or identification of
possible metabolites, due mainly to the small amount of fat available
in the young animals. A proposed metabolic pathway for bitertanol in
rats is given in Figure 3. The parent compound found in the faeces of
orally treated rats was probably due to the unabsorbed fraction of
administered radiolabel, representing about 15% of the original dose.
The main metabolite in the liver and kidneys,
para-hydroxy-bitertanol, was also identified in the faeces. The
amounts of the other biotransformation products in the organs were
probably too low for detection in the excreta (Klein, 1988b, 1989).
2. Toxicological studies
(a) Acute toxicity
The methods used in the studies summarized below complied to a
certain extent with OECD guidelines. At the time that most of the
studies were performed, compliance with good laboratory practice (GLP)
was not compulsory. The results of studies on the acute toxicity of

 bitertanol are summarized in Table 1. Bitertanol had very low acute
toxicity in rats, mice, and dogs treated orally. No significant sex
difference was observed. The LD50 values are 4000-5000 mg/kg bw or
higher. The toxicological properties of the A and B isomers are
similar. The symptoms included nonspecific signs, such as deteriorated
general condition, isolation from the group, and piloerection;
however, central nervous system effects were also seen, including
sedation, spasms, and respiratory disturbances. Lobulation of the
liver and irritation of the glandular stomach mucosa were observed at
necropsy of orally treated rats. Further signs in dogs were vomiting
and diarrhoea. Sheep were more susceptible, with an oral LD50 of
about 1000 mg/kg bw. Bitertanol was moderately toxic to rats and mice
after intraperitoneal injection.
Dermal exposure to a dose of 5000 mg/kg bw was tolerated by rats
with no observed signs. It was not toxic to rats after inhalation in
an aerosol, even at the highest test concentration. An initial test
showed no primary irritation of the intact or abraded skin of rabbits
inspected 24 and 72 h after the beginning of exposure (Thyssen &
Kimmerle, 1977a). In a study in rabbits, the active ingredient was
found to be slightly irritating to intact and abraded skin (Iyatomy,
1981).
A five-minute treatment of the eye of rabbits did not cause
primary irritation of the mucous membranes. Slight to moderate
reddening of the conjunctivae, which persisted for about 48 h, was
observed after a 24-h exposure (Thyssen & Kimmerle, 1977a). A reaction
that was reversible within four days was seen in rabbit conjunctivae;
the cornea and iris were unaffected (Iyatomy, 1981).
After an initial administration of 0.05 mg bitertanol per animal
to female Pirbright guinea-pigs, nine repeated intracutaneous
injections of emulsified bitertanol (0.1 mg) over three consecutive
weeks had no sensitizing effect. Intracutaneous injection of an
additional 0.05 mg after a further two weeks also failed to produce
signs of dermal sensitization (Thyssen, 1977).
Magnusson and Kligman tests in 20 male and 20 female Pirbright
guinea-pigs with Freund's adjuvant provided no evidence of sensitizing
effects of bitertanol at a concentration of 1% for intradermal
induction, 25% for topical induction, and 25% for challenge (Flucke,
1981).
(b) Short-term studies of toxicity
Rats
Groups of 20 male and 20 female Wistar rats were given bitertanol
(purity, 96.5%) by gavage at doses of 0, 30, 100, or 300 mg/kg bw per
day for 28 days. Half of the animals were then sacrificed, and the
other half were observed for a further 28 days. The method used in
Table 1. Acute toxicity of bitertanol
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat NR M Oral > 5000 99.1 A Mihail (1978a)
Rat NR M Oral > 5000 97.1 B Mihail (1978b)
Rat Wistar M/F Oral (fasted) > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M Oral > 5000 97.3 Flucke (1978)
Rat NR M Oral 4000 95.0 Flucke (1979)
Rat NR M Oral 3700 95.0 Iyatomy (1980)
F Oral 3900
Rat Wistar F Oral (fasted) > 5000 95.1 Flucke (1980)
Rat NR M Oral > 5000 96.7 Heimann (1981)
M Oral (fasted) > 5000
Rat NR M Oral (fasted) 4800 99.1 Mihail (1982a)
Rat NR M Oral (fasted) 4800 97.1 Mihail (1982b)
Rat NR M Oral > 5000 NR Heimann (1983a)
Rat NR M Oral (fasted) > 5000 97.6 Heimann (1984a)
Mouse NMRI M Oral (fasted) 4500 96.5 Thyssen & Kimmerle (1977a)
F Oral (fasted) 4200
Mouse NR M Oral 3500 95.0 Iyatomy (1980)
F Oral 3200
Dog Beagle M/F Oral (fasted) > 5000 95.0 Hoffmann (1981a)
Sheep Blackface M/F Oral ~ 1000 95.0 Hoffmann (1981b)
Rat Wistar M/F Dermal > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M/F Dermal > 5000a 95.0 Iyatomy (1980)
Mouse NR M/F Dermal > 5000 95.0 Iyatomy (1980)
Rabbit New Zealand M/F Dermal > 2000 94.9 Hixson (1979)
Rat Wistar M/F Inhalation 1 h > 720 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 4 h > 550 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 5 x 4 h > 380 96.5 Thyssen & Kimmerle (1977a)
Ratb Sprague-Dawley M/F Inhalation 4 h > 1200 95.7 Shiotsuka (1987)
Table 1. (continued)
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat Wistar M Intraperitoneal 1200 96.5 Thyssen & Kimmerle (1977a)
F Intraperitoneal 720
Rat NR M Intraperitoneal 700 95.0 Iyatomy (1980)
F Intraperitoneal 560
Mouse NR M Intraperitoneal 570 95.0 Iyatomy (1980)
F Intraperitoneal 610
Rat NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
Mouse NMRI M/F Subcutaneous > 5000 96.5 Thyssen & Kimmerle (1977a)
Mouse NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
NR, not reported; M, male; F, female; A, diastereomer A; B, diastereomer B
a Slight local irritation present
b The study was conducted in compliance with good laboratory practive
this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
Doses of 100 mg/kg per day and higher had a dose-related adverse
effect on body-weight development. At 300 mg/kg per day, behavioural
disturbances (isolation from the other animals, dirty coat) and hair
loss were observed in the female rats; however, all females had normal
behaviour near the end of the treatment period. Animals at this dose
had moderate leukocytosis, and females had reduced haemoglobin and
thrombocyte counts. Increased alkaline phosphatase activity was
observed only in the females. The relative liver weights of animals of
each sex at 100 mg/kg bw per day and above were increased. At
300 mg/kg bw per day, the absolute liver weights were elevated and the
absolute weights of the heart, kidneys, adrenals, and ovaries
depressed in female rats at the termination of treatment. The relative
weights of the thyroid and testes in males and of the spleen in
females were also elevated, whereas the relative weights of the heart,
adrenals, and ovaries were depressed in females. Hyperkeratoses and
parakeratoses, distended epithelial cells, slight inward growth of the
papillary body, and cellular infiltration in the epithelial and
subepithelial layers were observed histopathologically in the
forestomachs of four female animals at 300 mg/kg bw per day that were
sacrificed at the end of treatment. The appearance of the digestive
tract was unexceptional at the end of the recovery period., and all of
the changes in the rats at the high and intermediate doses had
likewise reverted. The NOAEL was 30 mg/kg bw per day (Thyssen &
Kaliner, 1977).
Groups of 20 male and 20 female Sprague-Dawley rats were given
bitertanol (purity, 95%) at doses of 0, 100, 400, or 1600 ppm, equal
to 7, 28, and 110 mg/kg bw per day in males and 7.4, 30, and 110 mg/kg
bw per day in females, for 28 days. The test procedures complied to a
certain extent with OECD guideline 407; at the time the study was
performed, compliance with GLP was not compulsory. Half of the animals
were sacrificed and examined after four weeks, whereas the other half
were observed without treatment for a further four weeks.
The dose of 400 ppm had an adverse effect on body-weight
development and food intake, and an adverse effect on the red blood
cell population was seen from decreases in the haematocrit and
haemoglobin readings. The relative liver weight was slightly elevated
in animals of each sex. Those at 1600 ppm had ataxia, reduced food
intake and body weight, and depressed red blood cell parameters
(haemoglobin, haematocrit, erythrocyte count), with a simultaneous
increase in the reticulocyte count. A slight increase in the serum
cholesterol concentration and a significant increase in the relative
liver weight were found in animals of each sex. Histopathological
examination revealed weak irritation of the gastric mucosa and
slightly altered ovaries, adrenals, and pituitaries. All of the
observed effects were reversible within the four-week recovery period.
The NOAEL was 100 ppm, equal to 7 mg/kg bw per day (Hatanaka et al.,
1981).
Bitertanol (purity, 90.2%) was administered to groups of 20 male
and 20 female Wistar rats at concentrations of 0, 150, 600, or 2400
ppm, equal to 12, 48 and 300 mg/kg bw per day in males and 13, 58, and
310 mg/kg bw per day in females, for three months. This study was
conducted before enactment of prevailing regulatory guidelines;
however, the procedures complied to certain extent with OECD guideline
408.
Although appearance and behaviour were unaffected at doses up to
600 ppm, 2400 ppm caused decreased motility and reduced food intakes.
Dose-related growth retardation was observed in females at 600 and
2400 ppm and in male animals at all concentrations; however, the 150
ppm dose decreased body weights only temporarily. The dose of 2400 ppm
had adverse effects on various haematological parameters, including
reduced erythrocyte and leukocyte counts, decreased haemoglobin
content and haematocrit reading, increased reticulocyte counts, a
relative increase in the polymorphonuclear leukocyte count, and a
relative decrease in the lymphocyte count. The changes in clinical
chemical parameters of the blood also found at this concentration were
elevated alkaline phosphatase, aspartate aminotransferase, and
glutamate dehydrogenase activities in the female animals. The blood
protein level was slightly depressed and the cholesterol level
slightly elevated. The finding of increased liver weight in females at
2400 ppm also indicated an effect on the liver. The NOAEL was 150 ppm,
equal to 12 mg/kg bw per day (Bomhard & Löser, 1978).
In a supplementary study, bitertanol (purity, 90.8%) was
administered to groups of 15 male and 15 female Wistar rats at
concentrations of 0, 30, 100, or 300 ppm, equal to 2.5, 8.1, and 25
mg/kg bw per day in males and 3.3, 10, and 32 mg/kg bw per day in
females, for three months. The method used in this study complied to a
certain extent with OECD guideline 408; at the time the study was
performed, compliance with GLP was not compulsory.
The appearance, behaviour, and mortality rate were unaffected at
all concentrations. At 300 ppm, the rats gained less weight than the
controls. Haematological, clinical chemical, gross and
histopathological examination, and urinary analyses provided no
evidence of adverse effects. The NOAEL was 100 ppm, equal to 8 mg/kg
bw per day (Krötlinger et al., 1978).
In a further study, bitertanol (purity, 95%) was administered to
groups of 15 male and 15 female Sprague-Dawley rats at concentrations
of 0, 40, 200, or 1000 ppm, equal to 3.1, 16, and 82 mg/kg bw per day
in males and 3.6, 19, and 88 mg/kg bw per day in females, for three
months. Five males and five females were subjected to urinary,
haematological, and clinical chemical determinations. The method used
in this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
The dose of 200 ppm retarded weight gain in male and female
animals and slightly increased the activities of alkaline phosphatase
and aspartate aminotransferase in the serum of females. The dose of
1000 ppm reduced body-weight gain and decreased food and water intakes
in animals of each sex. The erythrocyte count was also depressed, and
the reticulocyte count elevated in male and female animals. In
addition, the leukocyte and thrombocyte counts were elevated, and the
haemoglobin content and haematocrit reading were decreased in females.
Elevated lactate dehydrogenase, aspartate aminotransferase, and
alkaline phosphatase activities and a slightly elevated cholesterol
concentration were found in the serum of females. The absolute weights
of the liver and spleen were increased in females and the relative
weights in males and females. The following lesions were found
histopathologically in animals of each sex: increased swelling and
fatty degeneration of hepatocytes, hyperkeratoses of the oesophageal
and gastric mucosal epithelium and/or erosions of the glandular
stomach, swelling, and fatty degeneration of adrenal cortical cells.
Isolated bile-duct proliferation was also seen in females. The NOAEL
was 40 ppm, equal to 3 mg/kg bw per day (Yonemura et al., 1981).
Groups of 10 male and 10 female Wistar rats were exposed to
bitertanol at mean analytically determined concentrations of 18, 63,
or 200 mg/m3 air for 6 h per day, five days per week for three weeks.
Only the head and nose of the animals were exposed, under dynamic
conditions, and the inhaled bitertanol was dissolved in a 1:1 blend of
ethanol:polyethylene glycol 400.The method used in this study complied
to a certain extent with OECD guideline 412; at the time the study was
performed, compliance with GLP was not compulsory.
The concentrations of 18 and 63 mg/m3 air were tolerated by male
and female rats with no observed effect. Exposure to 200 mg/m3 air
caused deterioration of the general condition of female rats, and the
growth of male rats in this group was significantly decreased.
Although the relative weights of the lung, liver, kidney, and adrenal
in males and females at the high concentration were sometimes higher
than control values, no evidence of damage due to exposure to
bitertanol was seen histopathologically. The NOAEL was 63 mg/m3 air
(Mihail & Kimmerle, 1977).
Dogs
Groups of four male and four female beagles received bitertanol
(purity, 90.2%) at a dose of 0, 1, 5, or 25 mg/kg bw per day in
gelatin capsules. The method used in this study complied to a certain
extent with OECD guideline 409; at the time the study was performed,
compliance with GLP was not compulsory.
Doses of 5 mg/kg bw per day and above led to signs of
dose-related adverse effects on the skin and mucous membranes. The
effects on the skin were manifested by increased reddening, with local
inflammation, desquamation, and hair loss; and those on the mucous
membranes by increased reddening and slight inflammatory phenomena in
regions of the oral cavity (gingiva) and eyes (conjunctiva). They were
evident histologically as slight broadening of the epithelial layer of
the skin and slightly enhanced cornification in some cases. Evidence
for superficial involvement of the cornea (keratitis), apparently
resulting from conjunctival irritation, was observed in a few animals
at 25 mg/kg bw per day. The behaviour of the controls and treated
animals was similar; in contrast, the food intake and body-weight
development of animals at the high dose showed an adverse effect. The
results of laboratory tests indicated treatment-related interference
with liver function (elevated alkaline phosphatase and alanine
aminotransferase activities) in dogs at 5 and 25 mg/kg bw per day.
Elevated N-demethylase activity and an increase in the cytochrome
P450 concentration in liver homogenates were found in the high-dose
group at study termination. The weights of the thymus in females at
25 mg/kg per day and of the prostate (relative at 5 mg/kg per day and
both relative and absolute at 25 mg/kg per day) were statistically
significantly lower than those of the controls. The changes in
prostate weights correlated with dose-related histopathological
findings of retarded development. The NOAEL was 1 mg/kg bw per day
(Hoffmann & Schilde, 1979).
In view of the dermal phenomena observed in the previous study,
tests were performed to determine whether they represented a
sensitization effect. Beagle dogs were first treated orally 10-42
times with 35 or 70 mg/kg bw bitertanol, which led to marked
irritation and dermal lesions, particularly in the area of the head.
After a treatment-free interval of four to six weeks, when the lesions
had healed, the animals were treated with a dose of 1.75 mg/kg bw,
previously found to induce no reaction, for 14 days. Hair loss and a
slightly increased incidence of reddened gingiva were determined in
isolated animals only at termination of treatment. The study gave no
evidence for sensitization, and the delay in development of the effect
specifically argues against such an effect (Hoffmann, 1977).
No evidence for sensitization was found in a study in which
beagles were exposed to a concentration of 28.8 mg/m3 air for 4 h per
day, five days per week for three weeks, left untreated for 10 days,
and subsequently re-exposed to a concentration of 47.1 mg/m3 air for
one week. All dogs tolerated the exposures with no signs, and no
evidence was found for either an irritating effect on visible mucous
membranes or for a Type I (immediate anaphylactic type) allergy
(Thyssen & Kimmerle, 1977b).
Cats
Groups of three male and three female cats weighing 2-3 kg
received whole-body exposure to aerosols of bitertanol (active
ingredient, 95.8%) in a 3-m3 inhalation chamber for 6 h per day for
four weeks (20 x). The mean analytically determined bitertanol
concentration was 27 mg/m3 air. Analysis of the generated aerosols
showed that more than 90% of the aerosol particles had a mass-related
and more than 99% a particle-related aerodynamic diameter smaller than
5 µm. The exposed animals were compared with negative controls (air)
and positive controls (dichlozoline, 28 mg/m3 air). The animals were
observed over a recovery period of 12 weeks after termination of the
exposure. Their eyes were examined with an ophthalmoscope before
exposure and each week throughout the four-week exposure and the
recovery period and for gross and histopathological alterations at the
end of the study. The method used complied with OECD guideline 412; at
the time the study was performed, compliance with GLP was not
compulsory.
The exposed cats tolerated the treatment with no signs of
toxicity, damage to the eyes, or cataractogenesis. Under comparable
experimental conditions, the animals exposed to dichlozoline showed
signs of lenticular opacity (cataracts), starting from the end of the
second week of exposure. Lenticular opacity that had not reverted up
to the end of the recovery period was observed in all animals of this
group at the end of the second week of the recovery period. Cats
exposed to bitertanol had no cataracts (Pauluhn et al., 1983).
Rabbits
Groups of six male and six female New Zealand rabbits received
applications of bitertanol (purity, 95.8%) in an aqueous suspension
(0.5 ml) at concentrations of 0, 50, or 250 mg/kg bw on the intact or
abraded dorsal and lateral skin for 6 h per day, five times per week
for three weeks. The method used in this study complied to a certain
extent with OECD guideline 411; at the time the study was performed,
compliance with GLP was not compulsory.
Bitertanol had no apparent effect on the appearance, behaviour,
body weight, or survival of the rabbits. Transient erythema developed
on the exposed areas, initially on abraded skin but later on intact
skin. There was no effect on skin-fold thickness or on haematological,
clinical chemical, or urinary parameters. At necropsy, there were no
gross pathological findings. Histopathological examination showed
slight epidermal thickening only in exposed areas of all treated
animals. Preparations of liver showed no evidence of induction of
microsomal N- or O-demethylation or cytochrome P450 content. The
dermal application had no effect on any of the parameters
investigated. The NOAEL was 250 mg/kg bw (Heimann & Vogel, 1984).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Bitertanol (purity, 94-95%) was administered to groups of 50 male
and 50 female CF1/W74 mice at doses of 0, 20, 100, or 500 ppm, equal
to 2, 25, and 130 mg/kg bw per day in males and 7, 37, and 180 mg/kg
bw per day in females, for two years. The study was conducted before
enactment of prevailing regulatory guidelines; however, the test
procedures complied to a certain extent with OECD guideline 453 but
with no attempt to monitor ocular changes.
Behaviour, feed consumption, mortality, and haematological
parameters were not affected by treatment. In animals at 500 ppm, body
weights were reduced and serum alkaline phosphatase activity was
significantly elevated; females at this dose had an increased
incidence of enlarged and greenish-coloured livers at necropsy. The
liver weights were also increased, particularly in the female mice,
and the livers had increased numbers of eosinophilic foci. No evidence
was found for a carcinogenic effect at any concentration, which
extended into the toxic range. The NOAEL was 100 ppm, equal to 25
mg/kg bw per day (Bomhard & Löser, 1981a).
Rats
Bitertanol (purity, 94% in weeks 1-18; 95% from week 19) was
administered to groups of 50 male and 50 female SPF Wistar rats at
doses of 0, 20, 100, or 500 ppm, equal to 1, 4.9, and 26 mg/kg bw per
day in males and 1.3, 6.6, and 34 mg/kg bw per day in females, for two
years. The study was conducted before the enactment of prevailing
regulatory guidelines; however, the test procedures complied to a
certain extent with OECD guideline 453.
The 500 ppm concentration led to retarded growth in animals of
each sex. Ocular changes were not recorded. Haematological, blood
chemical, and urinary parameters were not significantly affected, and
the mortality rate was not adversely influenced. No treatment-related
effects were seen on organ weights or the gross or microscopic
appearance of the tissues. In particular, the type, location,
incidence, and time to occurrence of the tumours observed in the
treated groups were comparable to the control findings and to the
spontaneous findings for this strain of rat. The NOAEL was 100 ppm,
equal to 4.9 mg/kg bw per day (Bomhard & Löser, 1981b).
Dogs
Groups of four male and four female beagle dogs received
bitertanol (purity, 95-97.3%) in the diet at concentrations of 0, 10,
40, or 160 ppm, equal to 0.3, 1.2, and 4.9 mg/kg bw per day, for two
years. The method used in this study complied to a certain extent with
OECD guideline 452; at the time the study was performed, compliance
with GLP was not compulsory.
Although the control dogs gained more weight than did treated
dogs, there was no association with treatment. A good nutritional
status was maintained by dogs in all groups, and there was no
significant difference between groups in food or water consumption. No
abnormalities were seen in selected reflexes, body temperature, or
pulse rate; however, three of eight dogs at the high dose developed
bilateral cataracts. Urinary and haematological examinations conducted
at approximately three-month intervals revealed no abnormalities,
although serum alanine aminotransferase and alkaline phosphatase
activities were increased at 40 and 160 ppm. At necropsy, the mean
liver weight of dogs at the high dose was markedly increased.
Histological examination showed mild to moderate vacuolation of the
adrenal zona reticularis epithelia at doses at and above 40 ppm. The
NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day (Hoffmann & Gröning,
1983).
Groups of six male and six female beagles were fed diets
containing 0, 3, or 25 ppm bitertanol (purity, 96.3-96.7%) for 12
months, equal to 0.1, 1, and 7.6 mg/kg bw per day. A further group was
maintained on a diet containing 200 ppm bitertanol for 20 months to
permit continued ophthalmoscopic examination. The method used in this
study complied to a certain extent with OECD guideline 452; at the
time the study was performed, compliance with GLP was not compulsory.
Treatment had no apparent effect on behaviour, appearance, or
nutritional status, and food consumption and body-weight gain were
also unaffected. Pulse rates, body temperature, and selected reflexes
were unchanged. Ophthalmoscopy, conducted on animals at 0, 3, or 25
ppm at three-month intervals, showed no changes; however, one dog at
the high dose developed severe bilateral lenticular cataracts, which
were observable from week 58. Four other animals had slight lenticular
opacification by week 85. Dogs in this group also had signs of
conjunctivitis, with nasociliary discharge and incrustation.
Intermittent increases in serum alanine aminotransferase, glutamate
dehydrogenase, and alkaline phosphatase activities were also seen in
the dogs at the high dose. Other clinical chemical, haematological,
and urinary parameters were unaffected by treatment. At necropsy,
there were no gross abnormalities. The weights of the adrenals of the
dogs at the high dose were apparently greater than those of controls,
and lipoid vacuolation was observed in the zona reticularis epithelia.
The NOAEL was 25 ppm, equal to 1 mg/kg bw per day (Hoffmann & Vogel,
1983).
(d) Genotoxicity
No genotoxic or mutagenic potential of bitertanol was found in
lower organisms or in mammalian cells or systems in vivo or
in vitro. The results of assays for the genotoxicity of bitertanol
are summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
The effect of bitertanol (purity, 95.0%) on fertility, lactation
performance, and pup development was examined in a three-generation
study in Long-Evans FB 30 rats with two litters per generation. The
test substance was administered throughout the study period at
concentrations of 0, 20, 100, or 500 ppm, equivalent to 1, 5, and 25
mg/kg bw per day. Each of the mating groups consisted of 10 male and
20 female rats. The animals were five to six weeks old at the
beginning of the study and were treated for 70 days before the first
mating. The F3b generation pups and their parents (F2b generation)
were sacrificed and examined histopathologically after a four-week
lactation period. The method used in this study complied to a certain
Table 2. Results of assays for the genotoxicity of bitertanol
Test system Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, TA100, 4-2500 µg/plate 93.7 Negative Herbold (1979)
TA1535, TA1537
Reverse mutationa E. coli WP2 hcr- 1-5000 µg/plate 95.0 Negative Shirasu et al. (1981)
S. typhimurium TA98, TA100,
TA1535, TA1537, TA1538
Forward mutationa Mouse lymphoma L5178Y tk +/- 1-25 µg/mlb 95.0 Negative Bootman & Rees
1-20 mg/mlc (1983)
DNA repaird B. subtilis H17 rec+, M45 rec- 20-5000 µg/disc 95.0 Negative Shirasu et al. (1981)
DNA repaira,d E. coli W3110 pol A+; P3470 pol A- 0.1-33.3 mg/plate 95.0 Negative Riach (1981)
Aneuploidy Sordaria brevicollis 0.1-5.0 mg/L 95.0 Negative Bond & McGregor (1981)
Cytogenetic Chinese hamster lung cells 3.3 × 10-6-3.3 × 10-4 97.1 Negative Sasaki (1987)
alterationsa,d mol/Lb
1.0 × 10-5-1.0 ×.10-3
mol/Lc
In vivo
Micronucleus NMRI mice 2 × 1000 mg/kg bw 93.7 Negative Herbold (1978a)
formation 2 × 2000 mg/kg bw
Dominant lethal Male NMRI mice 1000 mg/kg bw 93.7 Negative Herbold (1978b)
mutation
a With and without exogenous metabolic activation
b Without exogenous metabolic activation
c With exogenous metabolic activation
d Conducted in compliance with good laboratory practice and to a certain extent with OECD guidelines
extent with OECD guideline 416; at the time the study was performed,
compliance with GLP was not compulsory.
No adverse effects were seen on appearance, behaviour, or
mortality in any group or generation. Bitertanol at a concentration of
20 ppm in the diet did not affect reproductive performance.
Administration of 100 or 500 ppm led to reductions in pup survival
rates during the lactation period in several matings and to pups with
lower birth weights. At 100 ppm, retarded pup growth was also seen in
the F2a and F2b generations. At 500 ppm, general retardation of
growth was seen. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Löser & Eiben, 1981).
(ii) Developmental toxicity
Rats
Groups of 20-23 Long-Evans rats were given bitertanol (purity,
96.5%) orally at doses of 0, 10, 30, or 100 mg/kg bw per day on days
6-15 post coitum. The method used in this study complied to a
certain extent with OECD guideline 414; at the time the study was
performed, compliance with GLP was not compulsory. The body-weight
gain of dams at 30 or 100 mg/kg bw per day was significantly reduced
during treatment and throughout the gestation period for those at 100
mg/kg bw per day. Resorption rates, fetal deaths, placental weights,
and sex ratio were unaffected by treatment; however, fetal weights
were significantly reduced at 100 mg/kg bw per day and significant
fetal stunting occurred at doses of 30 mg/kg bw per day and higher. At
100 mg/kg bw per day, skeletal ossification was retarded, and a
significantly increased incidence of malformations was observed, which
included cleft palate, hydrocephalus, malformed tails, dysplasia, and
synostosis of the ribs. A simple case of hydrocephalus occurred at 30
mg/kg bw per day. The NOAEL was 10 mg/kg bw per day (Machemer, 1977).
Groups of 22 or 23 Sprague-Dawley rats were given bitertanol
(purity, 96%) orally at doses of 0, 10, 25, or 65 mg/kg bw per day on
days 6-15 post coitum. The study was conducted in compliance with
GLP standards and with OECD guideline 414. Reduced weight gain was
seen in dams at daily doses of 25 mg/kg bw and higher, which persisted
throughout the gestation period in those at 65 mg/kg bw per day. The
number of corpora lutea, implantation rates, resorption rates, live
fetuses, sex ratio, and fetal and placental weights were unaffected by
treatment. An increased incidence of a fourteenth (lumbar) rib
relative to controls was observed at daily doses of 25 mg/kg bw (32%
incidence) and 65 mg/kg bw (70% incidence). No teratogenic effects
were observed. The NOAEL was 10 mg/kg bw (Nagumo et al., 1987).
Pregnant Wistar rats were given single oral doses of 100, 500, or
1000 mg/kg bw bitertanol on day 9, 10, 11, or 13 of gestation. The
doses were calculated to represent 1/5, 1/10, and 1/50 of the reported
LD50 value of 5000 mg/kg bw. The method used complied to a limited
extent to OECD guideline 414. The largest deviation was the short
application period; no information was given on compliance with GLP.
The study was available only in abstract form and is therefore of only
limited value for risk evaluation. Bitertanol induced congenital
anomalies when given on day 9, 10, or 11 at 500 or 1000 mg/kg bw. The
malformations consisted of microcaudia and acaudia and, in rare cases,
exophthalmus, hypognathia, and cleft palte. The NOAEL was 100 mg/kg bw
(Vergieva, 1990).
In two studies, groups of 25 Long-Evans rats were exposed to
bitertanol (purity, 93.7%) by inhalation for 4 h per day on days 6-15
post coitum at a mean analytical concentration of 0, 2.9, 6.4, or 22
mg/m3 air in one study and 0, 27, 60, or 120 mg/m3 air in the other.
The method used complied to a certain extent with OECD guideline 414;
at the time the study was performed, compliance with GLP was not
compulsory. No adverse effects were observed on dams exposed at any
concentration, but the average fetal weights were reduced
significantly by exposure to 120 mg/m3. Fetuses with below-average
weights were found increasingly at aerosol concentra-tions greater
than 22 mg/m3 air, without a clear dose-response relationship.
Implantation rates, resorption rates, placental weights, and sex ratio
were unaffected by treatment. No embryolethal or teratogenic effects
were observed. The NOAEL was 22 mg/m3 air (Machemer & Thyssen, 1979).
Rabbits
Twelve Himalayan rabbits, were given bitertanol (purity, 96.7%)
at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method used complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. The dose of 100 mg/kg bw led to reduced weight
gain and isolated clinical signs, such as reduced food intake,
diarrhoea, and blood in the urine; one dam died on day 29
post coitum. This dose also resulted in increased embryonal and
fetal mortality and reduced fetal and placental weights, and three
individual malformations occurred, which deviated from those seen in
controls in their nature (epignathus, pulmonary hypoplasia, and
aplasia) but not in their number. The NOAEL was 30 mg/kg bw per day
(Roetz, 1982).
Groups of 15 Himalayan rabbits were given bitertanol (purity,
93.9%) at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. Treatment had no effect on the behaviour,
appearance, or body-weight gain of the dams. At 100 mg/kg bw per day,
a significantly higher resorption rate and reduced numbers and weights
of fetuses were seen, and the number of malformed fetuses was
increased, with rare types of malformation such as cleft palate and
pigeon chest. The NOAEL was 30 mg/kg bw per day (Schlüter, 1983).
Groups of 16 fertilized chinchilla rabbits were given bitertanol
(purity, 96.9%) at a daily oral dose of 0, 10, 50, or 250 mg/kg bw on
days 6-18 of gestation. Because of the very high rate of post-
implantation loss at 250 mg/kg bw per day, doses of 0 and 150 mg/kg bw
per day were used in a supplementary study. The study was conducted in
compliance with OECD guideline 414 and with GLP standards. A
dose-related reduction in food consumption was seen in dams at > 50
mg/kg bw per day. The dose of 250 mg/kg bw per day significantly
reduced body weights from day 7 to the end of the experiment and
significantly reduced corrected body-weight gain. One dam at 150 mg/kg
bw per day and two at 250 mg/kg bw per day died during the study.
Isolated hair loss and enlarged and heavier livers at necropsy were
observed in dams at the high dose. A dose-related increase in the rate
of post-implantation losses was observed at > 150 mg/kg bw per day.
Two dams at 150 mg/kg bw per day and 13 at 250 mg/kg bw per day
completely resorbed their embryos, and the fetuses at these doses
showed dose-related reductions in body weight and a dose-related
increased in the incidence of incompletely or unossified phalangeal
nuclei and calcanei. The NOAEL was 50 mg/kg bw per day (Becker et al.,
1987).
(f) Special studies
As the studies in this section were published in the open
literature, the level of detail needed for full evaluation was not
available.
(i) Effects on the central nervous system
The effect of bitertanol on the central nervous system was
examined in pharmacological screening tests in mice and rats, which
included tests for potentiation of anaesthesia in mice (hexobarbital
sleep period), stimulation of spontaneous motility in mice, an Irwin
behaviour test in mice, the novel box response in rats, the open field
test in mice, and a test for reserpine ptosis in mice. In these
investigations, bitertanol was administered as a single oral dose of
0.075, 0.6, or 4.8 mg/kg bw. The results showed a slight stimulating
effect of bitertanol on the central nervous system in mice, but no
specific pharmacological effects, such as potentiation of amphetamine
effects or antagonism to reserpine ptosis, were determined (Polacek,
1983).
In a pharmacological test programme, groups of 10 male mice were
given a single oral dose of 0.02, 0.2, 2, 20, or 200 mg/kg bw
bitertanol. The highest dose significantly increased spontaneous motor
activity, with marked effects when treatment was given during the dark
period. In mice treated with 20 mg/kg bw per day, motor activity
tended to increase but not significantly, and lower doses had no
effect. No further effects on behaviour were observed at any dose
(Kaneto, 1986a). In a further test, male mice received bitertanol at 1
or 100 ppm for one week or one month. No increase in spontaneous motor
activity and no potentiating effect in amphetamine-pretreated animals
were seen after repeated dosing (Kaneto, 1986b).
In a study designed to determine whether bitertanol has similar
behavioural effects on the fixed-interval response rate and motor
activity of rats, doses of 10-300 mg/kg bw were given
intraperitoneally to rats maintained under a multiple fixed-interval
1-min schedule of reinforcement. Intermediate doses increased the
response rates and disrupted response patterning in both
fixed-interval components. The same doses of bitertanol did not
increase motor activity (Allen & MacPhail, 1983).
In rats placed in an actographic device designed for continuous
measurement of the locomotor component of spontaneous motor activity,
bitertanol increased motor activity at 200 mg/kg bw after
administration either orally or intraperitoneally. The activity peaks
at the low dose of 100 mg/kg bw coincided with normal night and
morning activity maxima (Frantik et al., 1996).
One the basis of previous results showing that acute exposure to
the triazole fungicide triadimefon affects central nervous system
catecholamines and induces a transient syndrome in rats that consists
of hyperactivity and stereotyped behaviour, a study was designed to
determine whether this type of toxicity is characteristic of other
triazoles. Dose-effect functions were determined for 14 triazoles or
structurally related pesticides, including bitertanol, in adult male
Long-Evans rats. All of the chemicals were administered orally in corn
oil. Hyperactivity was measured for 2 h in figure-eight mazes. Only
triadimefon and triadimenol induced hyperactivity, suggesting a very
rigid structure-activity relationship for the hyper-activity syndrome.
The absence of an effect of bitertanol may be due to steric hindrance
of benzene-ring substitution for the halogen on the benzene-ring
structure of triadimefon and/or triadimenol. Alternatively, bitertanol
may lack halogen substituents on the benzene rings and thus be less
polarized. The lack of activity of bitertanol is probably not due to
differences in absorption kinetics (Crofton, 1996).
(ii) Toxicity in combinations
Acute tests were performed to determine whether bitertanol has
superadditive (potentiating) effects when administered in combination
with triadimenol, captan, fuberidazole, or dodine. At the time the
studies were performed, OECD methods were not available and compliance
with GLP was not compulsory. The study involved administration of
single equitoxic oral (with triadimenol, captan, or fuberidazole) or
intraperitoneal (with dodine) doses of the active ingredients to male
rats. A factor greater than 1 between observed and expected LD50
values indicates a superadditive effect. Bitertanol plus triadimenol,
bitertanol plus fuberidazole, and bitertanol plus dodine had no
superadditive effects but only additive toxic effects (Mihail, 1982b;
Flucke, 1980; Heimann, 1984b). In contrast, the active ingredient
combination of bitertanol plus captan had slightly superadditive
action, with a potentiation factor of 1.9 (Mihail, 1982a).
(iii) Effect on the liver
Groups of 10 male and 10 female Wistar rats received bitertanol
(purity, 95.8%) suspended in distilled water with Cremorph EL by
gavage at doses of 0, 30, 100, or 300 mg/kg bw per day for 14 days. On
sacrifice, blood was collected for detailed haematological and
clinical chemical testing, and liver samples were taken for detailed
histopathological and enzyme studies. During treatment, 1/10 female
rats at the intermediate dose and 9/10 at the high dose showed hair
loss, and some lost weight, while females at the low dose had reduced
body-weight gain in comparison with control animals. Female rats also
had a dose-related tendency to mild thrombocytosis, which was
significant at the intermediate and high doses. Serum gamma-glutamyl
transpeptidase activity and bilirubin concentration were slightly
increased in females at the high dose. There were no gross
pathological findings at necropsy. The liver weights tended to be
increased at the intermediate and high doses, especially in females.
Slight-to-moderate bile-duct proliferation with peribiliary
infiltration of monocytes or polynucleocytes was seen histologically
in animals at the intermediate and high doses. These changes were
sometimes accompanied by parenchymal Councilman bodies or, more
occasionally, mitoses. The hepatocytes of animals at the high dose
were occasionally swollen, with finely granular cytoplasm. Little
fatty infiltration was seen. The results of studies in vitro were
consistent with induction of hepatic microsomal enzymes, as the
cytochrome P450 content increased in a dose-related manner, especially
in males. Aminopyrene N-demethylase activity was increased in males
and females at the high dose and in males at the intermediate dose,
while O-demethylase activity was increased in males at the
intermediate and high doses and in females at the high dose. The
hepatic triglyceride content was not affected by treatment. Bitertanol
thus caused mild hepatotoxicity, with modest induction of hepatic
microsomal activity in rats at 100 and 300 mg/kg bw per day. The NOAEL
was 30 mg/kg bw per day (Mihail & Luckhaus, 1985).
3. Studies on metabolites
(a) 1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one
(plants, soil)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one, a
keto analogue of bitertanol, has very little acute toxicity in rats
when given orally or dermally. It induced signs of effects on the
central nervous system, with initial sedation and respiratory
disturbances and later stimulation. The highest technically
administrable oral dose, 1750 mg/kg bw, caused no deaths. A dermal
dose of 5000 mg/kg bw and the highest dose administered by inhalation
(dynamic dust nebulization) were tolerated with no observed signs. The
LC50 value in male and female rats was > 500 mg/m3 air after a 1-h
exposure or a 4-h exposure, respectively. In rabbits, the compound was
slightly irritating to the skin only after contact for 24 h and was
mildly irritating to the mucous membranes of the eyes (Thyssen &
Kimmerle, 1978).
(b) Bitertanol benzoic acid (soil)
Bitertanol benzoic acid had little acute toxicity when given
orally. The highest administered dose, 5000 mg/kg bw, was tolerated by
fasted male rats with no observed signs or deaths (Heimann, 1983b).
The compound was not mutagenic to Salmonella typhimurium in the
presence of an exogenous metabolic activation system (Herbold, 1983).
(c) 1,2,4-Triazole (photodegradation, soil)
1,2,4-Triazole has moderate or low acute toxicity when given
orally (LD50 value, 1600 mg/kg bw in males and females) or dermally
(LD50 values, 4200 mg/kg bw in males and 3100 mg/kg bw in females).
At high oral and dermal doses, the compound had effects on the central
nervous system. In tests of inhalation of air enriched with vapours of
the test substance, male rats and mice tolerated exposure for 4 and
6 h, respectively, with no observed signs. No dermal irritation was
observed on rabbits exposed for 24 h or on the skin of five male
volunteers exposed for 8 h. 1,2,4-Triazole was strongly irritating to
the mucous membranes of the rabbit eye (Thyssen & Kimmerle, 1976).
Groups of 15 male and 15 female rats were given 1,2,4-triazole
(purity, 99.6%) at a dose of 0, 100, 500, or 2500 ppm for three
months. The study was conducted before enactment of prevailing
regulatory guidelines, but the test procedures complied to a certain
extent with OECD guideline 408. Treatment at 2500 ppm resulted in a
temporary decrease in food intake, a reduction in body weight, and
transient, slight palmospasms in isolated animals. Significant
reductions in the haemoglobin, haematocrit, mean corpuscular volume,
and mean corpuscular haemoglobin values in male rats at 2500 ppm
indicated an effect on the blood. In this group, slight to moderate
fat accumulation was found in the cells of the liver parenchyma in
three of 15 males, which was attributed to the treatment. The NOAEL
was 500 ppm, equal to 38 mg/kg bw per day (Bomhard et al., 1979).
1,2,4-Triazole at concentrations of 10-5000 µg/plate did not
induce point mutations in Salmonella typhimurium TA1535, TA1537,
TA98, or TA100, with or without metabolic activation (Poth, 1989).
In two studies, groups of 25 fertilized Wistar rats were given
daily oral doses of 1,2,4-triazole (purity, 95.3% and 94.0%,
respectively) on days 6-15 of gestation at doses of 0, 10, 30, or 100
mg/kg bw in one study and 0, 100, or 200 mg/kg bw in the other. The
studies were conducted in compliance with GLP standards and OECD
guideline 414. Maternal toxicity was indicated by decreased weight
gain of dams at 100 and 200 ppm relative to those in the control
group. Reduced fetal weights, retarded osteogenesis, and increased
numbers of runts were found at > 100 mg/kg bw per day. An increased
resorption rate was found in dams at 200 mg/kg per day, but the rate
of fetuses with retarded ossification was not increased. The types and
incidences of the malformations observed in this group (including
cleft palate and malformed extremities) indicate that 1,2,4-triazole
has teratogenic potential. The NOAEL was 30 mg/kg bw per day (Renhof,
1988a,b).
4. Observations in humans
No health impairment was observed in male or female employees
engaged in formulating bitertanol and using the customary safety
precautions, who had regular medical examinations (Miksche, 1981).
Slight, transient prurient reddening of the forearms, which
regressed spontaneously after a few days, developed in rare, isolated
cases after direct dermal contact during packaging of a powder of the
pure active ingredient. Unequivocal differentiation between an
allergic dermal reaction and mechanical-toxic skin irritation was not
possible. There was no tendency to relapse (Faul, 1986).
Comments
After oral administration bitertanol is rapidly and extensively
absorbed (about 84%) and distributed. Excretion is also rapid (rats,
almost complete within 72 h) and occurs mainly in the faeces (about
90%) by biliary excretion, owing to the lipophilic nature of the
parent substance. The liver and kidneys are the main sites of tissue
accumulation in both male and female rats. Although some statistically
significant sex-related differences were seen, they were of minor
physiological importance. The substance has a relatively low rate of
dermal penetration. The metabolic profile was similar at the various
doses tested. The main metabolic pathways are hydroxylation of the
phenyl ring in the para position and oxidation of the tert-butyl
moiety, leading to bitertanol alcohol and the corresponding carboxylic
acid; metabolites derived from the two pathways combined were also
observed. The metabolites occur in both free and conjugated forms. The
parent compound was not detected in urine or bile. There was no
toxicological concern with regard to the metabolic profile in plants.
Bitertanol had very low acute toxicity in rats, mice, and dogs
when given orally and after dermal application or by inhalation. It
was of moderate to low toxicity in rats and mice after intraperitoneal
injection. Females appeared to be slightly more sensitive than males,
but only in some studies. This was perhaps due to slightly different
absorption characteristics in animals of the two sexes, as seen after
oral administration. In view of the toxic signs (including respiratory
disturbances, sedation, spasms, and tremor) and the findings of the
pharmacological screening tests, it may be inferred that bitertanol
has central nervous system activity; however, no specific
pharmacological effects, such as potentiation of amphetamine action,
antagonism of reserpine ptosis, or an effect on hexobarbital
anaesthesia, were observed.
Bitertanol induces no, or only very slight, dermal irritation. It
induces slight to moderate reactions of the ocular mucosa but has no
effect on the cornea or iris. No evidence for a sensitizing effect was
observed in any study
Bitertanol has been classified by WHO as unlikely to present an
acute hazard in normal use (WHO, 1996).
In medium- and long-term tests for toxicity, the liver is
regarded as the main toxicological target organ in dogs and rats at
doses of 1.2 and 28 mg/kg bw per day and above, respectively. Liver
weight was found to be the most sensitive indicator. Corresponding
patterns of disruption of liver function were observed in rats and
dogs. The activities of the transaminases, alkaline phosphatase, and
glutamate dehydrogenase in the serum were increased. In addition, a
rise in cholesterol level was observed in several studies in rats at
doses of 61 mg/kg bw per day and above. The ability of bitertanol to
induce mixed-function oxidases was verified in both species. It is
therefore likely that the effect on weight is essentially due to
hypertrophy of the endoplasmic reticulum in hepatocytes at doses of
100 mg/kg bw per day and above. Morphological changes in the liver
were seen only at relatively high doses and consisted of hepatocytic
swelling, bile-duct proliferation, perilobular fatty degeneration,
eosinophilic foci, and fibrous structures. The induced effects
corresponded to toxic liver damage with bile-duct involvement.
Evidence for haemotoxicity was found at doses of 28 mg/kg bw per
day and above in rats; this may be classified as an effect on the
peripheral red blood cell population. The decreases in erythrocyte
count, haemoglobin concentration, and packed cell volume and the
compensatory rise in the reticulocyte count argue for this
interpretation. No evidence was found for damage to the haematogenic
organs.
Slight increases in the leukocyte count were also seen in a few
studies in rats at doses of 61 mg/kg bw per day and above. The
increased leukocyte counts are probably attributable to inflammatory
processes, since the increase occurred in studies and at doses at
which inflammatory processes were also observed. Hyperkeratosis of the
oesophageal epithelium and glandular stomach and/or erosions of the
glandular stomach as well as parakeratosis of the stomach wall were
observed in rats. The histopathological picture included distended
epithelial cells, slight inward growth of the papillary body, and
cellular infiltration of the epithelial and subepithelial layers in
the affected animals. These changes are probably attributable to
irritation of the mucous membranes by the active ingredient.
Effects on the skin were observed in dogs, sheep and rats. The
effects in dogs at doses of 5 mg/kg bw per day and above consisted of
reddening, with localized inflammation, desquamation, and hair loss,
and increased reddening and slight inflammatory phenomena in the
mucous membranes of the oral cavity and eyes. Histological examination
showed broadening of the epithelial layer, enhanced cornification, and
minor erosions. The dermal effects were apparently accompanied by
pruritus. The keratitis observed in dogs was considered to be
secondary to conjunctivitis. Hair loss was also observed in rats and
sheep, which occurred after administration of bitertanol by capsule or
gavage at doses of 300 mg/kg per day and above; this was considered to
be a systemic effect.
Pathological changes were seen in the adrenals of dogs and rats
at 1.2 and 81 mg/kg bw per day and above, respectively, consisting of
swelling and fatty degeneration of the adrenal cortical cells,
particularly in the zona reticularis and zona fasciculata. These
alterations were considered to be due to inhibition of sterol
biosynthesis by triazole derivatives. It is highly probable that this
effect, which also represents the biological, antimycotic action of
the substance, leads to an effect on corticoid metabolism with
corresponding morphological effects in the cells of the adrenal
cortex.
In feeding studies in dogs, lenticular opacity seen at doses of
4.9 mg/kg bw per day and above were considered to be related to
treatment. No lenticular alterations attributable to administration of
bitertanol were seen in any other study or species. The exact
mechanism of the cataractogenesis resulting from long-term
administration of triazole fungicides is presently unknown. The ocular
lens undertakes its own de-novo synthesis of cholesterol, which is
isolated from lipoproteins circulating in the blood; other substances
that inhibit cholesterol synthesis can induce cataracts.
No evidence for any carcinogenic potential of bitertanol was
found in long-term studies of toxicity and carcinogenicity in rats and
mice treated in the diet. The highest doses tested were 130 mg/kg bw
per day in mice and 26 mg/kg bw per day in rats.
In a series of studies, bitertanol had no genotoxic potential in
lower organisms or in mammalian cells or systems in vitro or
in vivo.
In a three-generation study of reproductive toxicity in rats,
adverse effects on the pups (reductions in survival rates during the
four-week lactation period, reduced weight at birth, and retarded
growth) were observed at parentally toxic doses of 100 or 500 ppm,
equivalent to 5 or 25 mg/kg bw per day. The NOAEL was 1 mg/kg bw per
day.
In studies of developmental toxicity, treatment with bitertanol
led to several embryotoxic and teratogenic effects, depending on the
animal species, route of administration, and dose. In rats, an oral
dose of 10 mg/kg bw per day was tolerated with no observed effect.
Doses of 25 mg/kg bw per day and above led to retardations and
variations (e.g. increased incidence of the 14th rib). Malformations
were observed at an oral dose of 100 mg/kg bw per day, which was
clearly maternally toxic. Exposure of pregnant rats to concentrations
of 27 mg/m3 air and above by inhalation resulted in retardation
effects; no malformations were observed. In rabbits, doses of 50 mg/kg
bw per day and above were maternally toxic; fetotoxic effects were
seen at doses of 100 mg/kg bw per day and above. Teratogenic effects
were observed in Himalayan rabbits at 100 mg/kg bw per day, while in
the second strain tested (Chinchilla), even a dose of 250 mg/kg bw per
day did not lead to malformations.
The ADI established at the 1988 Meeting of 0-0.01 mg/kg bw, based
on a combined NOAEL of 1 mg/kg bw per day from the two-year and the
one-year study in dogs, was maintained. The ADI is supported by the
NOAEL of 20 ppm (equivalent to 1 mg/kg bw per day) in a
three-generation study in rats.
An acute RfD was not allocated because bitertanol has been
classified by WHO as unlikely to present an acute hazard in normal use
and it has not shown any specific adverse effects (teratogenicity,
neurotoxicity) after single doses 100 times the lowest relevant NOAEL
in long- and short-term studies that were used to establish the ADI.
Therefore, the Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 25 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 100 ppm, equal to 4.9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
20 ppm, equivalent to 1 mg/kg bw per day (reproductive
and parental toxicity in a three-generation study)
10 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 1 mg/kg bw per day (overall NOAEL in one-year and
two-year studies of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Commenced immediately, about 84% absorbed
Dermal absorption About 10%
Distribution Highest concentrations in liver and kidneys
Potential for accumulation None
Rate and extent of excretion About 90% excreted with bile, 10% with urine
Metabolism in animals No parent compound in bile or faeces; extensively
metabolized to 14 metabolites (ring
monohydroxylation, ring dihydroxylation, aryl
O-methylation, aliphatic hydroxylation, aliphatic
oxidation to carboxylic acids, and ether cleavage)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral > 5000 mg/kg bw
Rat: LD50,, dermal > 5000 mg/kg bw
Rat: LC50, inhalation > 550 mg/m3 (4 h)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not a sensitizer (Magnussen & Kligman test)
Short-term toxicity
Target/critical effect Liver, red blood cells, adrenals, digestive tract
Lowest relevant oral NOAEL Dog: 90 days: 1 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 3 weeks; 250 mg/kg per day
Lowest relevant inhalation NOAEL Rat: 3 weeks, 63 mg/m3
Genotoxicity No genotoxic or mutagenic potential
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL Dog: 1 year and 2 years: 1 mg/kg bw per day
Carcinogenicity No evidence of carcinogenic potential
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced litter size, pup
growth rate, and pup survival) at parentally toxic
doses
Lowest relevant reproductive NOAEL Rat: 1 mg/kg bw per day
Developmental target/critical effect Fetotoxic and teratogenic effects at maternally
toxic doses
Lowest relevant developmental NOAEL Rat: 10 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No relevant effects
Other toxicological studies Induction of hepatic microsomal activity
Medical data No health impairments observed in employees
subjected to regular medical examinations
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 1 and 2 years in dogs 100
Acute reference dose None allocated
(unnecessary)
References
Allen, A.R. & MacPhail, R.C. (1993) Bitertanol, a triazole fungicide,
increases operant responding but not motor activity. Neurotoxicol.
Teratol., 15, 237-242.
Becker, H., Mueller, E., Vogel, W. & Terrier, C. (1987) Embryotoxicity
(including teratogenicity) study with KWG 0599 in the rabbit.
Unpublished report No. R4081 from Research & Consulting Company AG,
Itingen, Switzerland. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Löser, E. (1978) KWG 0599: Subchronic toxicological
study on rats (feeding experiment over three months). Unpublished
report No. 7322 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Löser, E. (1981a) KWG 0599 (Bitertanol, the active
ingredient of Baycor): Chronic toxicity study on mice (2-year feeding
experiment). Unpublished report No. 10103 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Bomhard, E. & Löser, E. (1981b) KWG 0599: Chronic toxicity study on
rats (2-year feeding experiment). Unpublished report No. 10104 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole: Subchronic
toxicological study with rats (feeding study over three months).
Unpublished report No. 8667 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bond, J. & McGregor, D. (1981) Testing for aneuploid induction of HE
1004 in Sordaria brevicollis. Unpublished report No. R2063 from
Inveresk Research International, Musselburgh, Scotland. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bootman, J. & Rees, R. (1983) KWG 0599: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report No. R2406 from Life Science Research. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Crofton, K.M. (1996) A structure-activity relationship for the
neurotoxicity of triazole fungicides. Toxicol. Lett., 84, 155-159.
Faul, J. (1986) Baycor active ingredient and formulations. Unpublished
report from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50) -- rat p.o.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1979) KWG 0599--Determination of acute toxicity (LD50):
rat p.o./mixed batch (from 5 batches). Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) KWG 0599 and W VII/117: Study for acute combination
toxicity. Unpublished report No. 9456 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1981) KWG 0599: Evaluation for sensitization in guinea
pigs (maximization test after Magnusson and Kligman). Unpublished
report No. 9934 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Frantik, E., Hornychova, M., Dvorak, J. & Mraz, J. (1996) Spontaneous
motor activity in rats enhanced by 1,2,4-triazole derivatives:
Triadimefon, triadimenol and biteranol. Homeostasis, 37, 3.
Hatanaka, J., Asaoka, S., Ikeda, M., Shimizu, M., Inukai, H.,
Iyatomy, A. & Itoh, H. (1981) KWG 0599: Short-term toxicity tests on
rats (4-week feeding and 4-week recovery tests). Unpublished report
No. 195 from Nihon Tokushu Noyaku Seizo K.K. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1981) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983a) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983b) Determination of acute toxicity (LD50): BUE
2684 (Bitertanol-benzoic acid)--rat p.o. Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50): KWG
0599. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984b) Dodine & KWG 0599 (c.n. Dodine & Bitertanol
(proposed)): Combination toxicity study. Unpublished report No. 12608
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Vogel, O. (1984) KWG 0599 (c.n. Bitertanol): Subacute
study of dermal toxicity to rabbits. Unpublished report No. 12571 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978a) KWG 0599: Micronucleus test on mouse to evaluate
KWG 0599 for potential mutagenic effects. Unpublished report No. 7860
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978b) KWG 0599: Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 7964 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979) KWG 0599: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 8152 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) BUE 2684: Bitertanol-benzoic acid:
Salmonella/microsome test to evaluate for point mutation. Unpublished
report No. 12087 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hixson, E.J. (1979) Baycor technical: Acute dermal toxicity to
rabbits. Unpublished report No. 57 from Mobay Chemical Corporation,
Staneley Research Center. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1984) Dermal absorption of 14C-Baycor applied as 50%
wettable powder. Unpublished report No. 484 from Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1977) KWG 0599: Evaluation for sensitization.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1981a) KWG 0599: Determination of the acute toxicity to
the dog after oral administration. Unpublished report No. T 6001023
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1981b) KWG 0599/012: Acute oral toxicity to sheep.
Unpublished report No. 9768 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1983) KWG 0599 (c.n. Bitertanol): Chronic
oral toxicity study on dogs (2-year feeding experiment). Unpublished
report No. 12307 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hoffmann, K. & Schilde, B. (1979) KWG 0599: Subchronic toxicity study
on dogs (thirteen-week administration in capsules). Unpublished report
No. 8053 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Hoffmann, K. & Vogel, O. (1983) KWG 0599 (Bitertanol): Second chronic
toxicity study with dogs on oral administration (feeding study, 0-3-25
ppm 12 months, 200 ppm 20 months). Unpublished report No. 12328 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Iyatomy, A. (1980) KWG 0599: Report of acute toxicity. Unpublished
report No. Sheet A-31 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iyatomy, A. (1981) Report of acute toxicity: KWG 0599. Unpublished
report No. Sheet 22 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986a) Effect of bitertanol on spontaneous motor activity
in mice; Part 1. The experiment on the acute effect. Unpublished
report No. DT-1 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986b) Effect of bitertanol on spontaneous motor activity
in mice; Part 2. The experiment on the subacute effect. Unpublished
report No. DT-2 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Klein, O. (1988a) [U-phenyl-14C]-Bitertanol: Absorption, excretion,
tissue-distribution, and biokinetic profile of the total radioactivity
in the rat following single oral and intraduodenal dosage. Unpublished
report No. PF3076 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Klein, O. (1988b) [U-phenyl-14C]-Bitertanol: Distribution of the
parent compound in the excreta and in some organs at different times
following single oral administration to rats. Unpublished report No.
PF3096 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1989) [U-phenyl-14C]-Bitertanol: Distribution of the
metabolites in some organs at different times following single oral
administration to rats. Unpublished report No. PF3501 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Bomhard, E., Löser, E. & Schilde, B. (1978 ) B. KWG
0599: Subchronic toxicity study on rats (three-month feeding
experiment). Unpublished report No. 8002 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Löser, E. & Eiben, R. (1981) KWG 0599: Multigeneration reproduction
study on rats. Unpublished report No. 10024 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. & Thyssen, J. (1979) KWG 0599 (Baycor active ingredient):
Evaluation for embryotoxic and teratogenic effects on rats after
dynamic inhalational exposure. Unpublished report No. 8610 from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1977) KWG 0599: Evaluation for embryotoxic and
teratogenic effects on rats after oral administration. Unpublished
report No. 6697 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978a) Determination of acute toxicity (LD50) / KWG 0599
Form A: PFU 190978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1978b) Determination of acute toxicity (LD50) / KWG 0599
Form B: PFU 210978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982a) KWG 0599 and captan: Study for acute combination
toxicity. Unpublished report No. 11205 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982b) KWG 0519 and KWG 0599: Study for acute combination
toxicity. Unpublished report No. 11203 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Kimmerle, G. (1977) KWG 0599: Subacute inhalation
toxicity study on rats. Unpublished report No. 6868 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Luckhaus, G. (1985) KWG 0599 (c.n. Bitertanol): Subacute
oral toxicity study with special attention to effect on the liver.
Unpublished report No. 13230 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Miksche, L. (1981) Data on effects on humans/In-company occupational
medical experience. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Nagumo, K., Teraki, Y., Chiba, T. & Miyasaka, M. (1981, revised 1987)
Teratogenicity test of KWG 0599 in pregnant rats. Unpublished report
from St Marianna University School of Medicine. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Pauluhn, J., Thyssen, J. & Vogel, O. (1983) KWG 0599 (Baycor active
ingredient) (c.n. Bitertanol): Study for cataract-inducing effect on
subacute whole-body exposure with cats (exposure 20 × 6 hours).
Unpublished report No. 12006 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Polacek, I. (1983) Central nervous effects of KWG 0599 (pilot study).
Unpublished report No. R2694 from Toxikologisches Institut Regensburg.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with 1H-
1,2,4-triazole. Unpublished report No. R4859 from Cytotest Cell
Research GmbH & Co. KG. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puhl, R.J. & Hurley, J.B. (1983) The absorption, excretion, and
metabolism of Baycor-phenyl-UL-14C by rats. Unpublished report No.
85832 from Mobay Chemical Corporation, Agricultural Chemicals
Division. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) 1,2,4-triazole: Investigations into embryotoxic
effects on rats after oral administration. Unpublished report No.
17401 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Renhof, M. (1988b) 1,2,4-triazole: Investigation into embryotoxic
effects on rats after oral administration. Supplement to study No.
T5019339. Unpublished report No. 17402 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Riach, C.G. (1981) KWG 0599: Testing the modification of cellular DNA
in Escherichia coli. Unpublished report No. R2013 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Roetz, R. (1982) KWG 0599 (Bitertanol, the active ingredient of
Baycor): Study of embryotoxic (and teratogenic) effects on rabbits
after oral administration. Unpublished report No. 10979 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sasaki, Y.F.X. (1987) Bitertanol: In vitro cytogenetics test.
Unpublished report No. DT-5 from The Institute of Environmental
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1983) KWG 0599: Study to determine embryotoxic and
teratogenic effects on rabbits following oral administration.
Unpublished report No. 11548 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987) Acute inhalation toxicity study with Baycor
technical dust in Sprague-Dawley rats. Unpublished report No. 837 from
Mobay Corporation, Staneley Research Center, Report. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Ohta, T. (1981) Bitertanol: Microbial
mutagenicity study. Unpublished report from Department of Toxicology,
Institute of Environmental Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977) KWG 0599: Intracutaneous allergy test on guinea
pigs. Unpublished report No. 7113 from Bayer AG, Leverkusen. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kaliner, G. (1977) KWG 0599: Subacute oral cumulative
toxicity study on rats (four-week treatment). Unpublished report No.
7153 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole: Occupational
toxicology study. Unpublished report No. 5926 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977a) KWG 0599: Acute toxicity studies.
Unpublished report No. 6546 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977b) KWG 0599: Subacute inhalational
toxicity study on dogs. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1978)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one (third
stage KWG 0599, BUE 1662): Industrial toxicology study. Unpublished
report No. 7242 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vergieva, T. (1990) Triazoles teratogenicity in rats. Teratology,
42, 27A-28A.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Yonemura, H., Shimazaki, M., Yamazaki, K., Takahashi, Y. & Hashizume,
M. (1981) Subacute toxicity tests with Bitertanol (KWG 0599) in rats.
Unpublished report from Research Institute for Animal Science in
Biochemistry and Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
bitertanol are summarized in Table 1. Bitertanol had very low acute
toxicity in rats, mice, and dogs treated orally. No significant sex
difference was observed. The LD50 values are 4000-5000 mg/kg bw or
higher. The toxicological properties of the A and B isomers are
similar. The symptoms included nonspecific signs, such as deteriorated
general condition, isolation from the group, and piloerection;
however, central nervous system effects were also seen, including
sedation, spasms, and respiratory disturbances. Lobulation of the
liver and irritation of the glandular stomach mucosa were observed at
necropsy of orally treated rats. Further signs in dogs were vomiting
and diarrhoea. Sheep were more susceptible, with an oral LD50 of
about 1000 mg/kg bw. Bitertanol was moderately toxic to rats and mice
after intraperitoneal injection.
Dermal exposure to a dose of 5000 mg/kg bw was tolerated by rats
with no observed signs. It was not toxic to rats after inhalation in
an aerosol, even at the highest test concentration. An initial test
showed no primary irritation of the intact or abraded skin of rabbits
inspected 24 and 72 h after the beginning of exposure (Thyssen &
Kimmerle, 1977a). In a study in rabbits, the active ingredient was
found to be slightly irritating to intact and abraded skin (Iyatomy,
1981).
A five-minute treatment of the eye of rabbits did not cause
primary irritation of the mucous membranes. Slight to moderate
reddening of the conjunctivae, which persisted for about 48 h, was
observed after a 24-h exposure (Thyssen & Kimmerle, 1977a). A reaction
that was reversible within four days was seen in rabbit conjunctivae;
the cornea and iris were unaffected (Iyatomy, 1981).
After an initial administration of 0.05 mg bitertanol per animal
to female Pirbright guinea-pigs, nine repeated intracutaneous
injections of emulsified bitertanol (0.1 mg) over three consecutive
weeks had no sensitizing effect. Intracutaneous injection of an
additional 0.05 mg after a further two weeks also failed to produce
signs of dermal sensitization (Thyssen, 1977).
Magnusson and Kligman tests in 20 male and 20 female Pirbright
guinea-pigs with Freund's adjuvant provided no evidence of sensitizing
effects of bitertanol at a concentration of 1% for intradermal
induction, 25% for topical induction, and 25% for challenge (Flucke,
1981).
(b) Short-term studies of toxicity
Rats
Groups of 20 male and 20 female Wistar rats were given bitertanol
(purity, 96.5%) by gavage at doses of 0, 30, 100, or 300 mg/kg bw per
day for 28 days. Half of the animals were then sacrificed, and the
other half were observed for a further 28 days. The method used in
Table 1. Acute toxicity of bitertanol
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat NR M Oral > 5000 99.1 A Mihail (1978a)
Rat NR M Oral > 5000 97.1 B Mihail (1978b)
Rat Wistar M/F Oral (fasted) > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M Oral > 5000 97.3 Flucke (1978)
Rat NR M Oral 4000 95.0 Flucke (1979)
Rat NR M Oral 3700 95.0 Iyatomy (1980)
F Oral 3900
Rat Wistar F Oral (fasted) > 5000 95.1 Flucke (1980)
Rat NR M Oral > 5000 96.7 Heimann (1981)
M Oral (fasted) > 5000
Rat NR M Oral (fasted) 4800 99.1 Mihail (1982a)
Rat NR M Oral (fasted) 4800 97.1 Mihail (1982b)
Rat NR M Oral > 5000 NR Heimann (1983a)
Rat NR M Oral (fasted) > 5000 97.6 Heimann (1984a)
Mouse NMRI M Oral (fasted) 4500 96.5 Thyssen & Kimmerle (1977a)
F Oral (fasted) 4200
Mouse NR M Oral 3500 95.0 Iyatomy (1980)
F Oral 3200
Dog Beagle M/F Oral (fasted) > 5000 95.0 Hoffmann (1981a)
Sheep Blackface M/F Oral ~ 1000 95.0 Hoffmann (1981b)
Rat Wistar M/F Dermal > 5000 96.5 Thyssen & Kimmerle (1977a)
Rat NR M/F Dermal > 5000a 95.0 Iyatomy (1980)
Mouse NR M/F Dermal > 5000 95.0 Iyatomy (1980)
Rabbit New Zealand M/F Dermal > 2000 94.9 Hixson (1979)
Rat Wistar M/F Inhalation 1 h > 720 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 4 h > 550 96.5 Thyssen & Kimmerle (1977a)
Rat Wistar M/F Inhalation 5 x 4 h > 380 96.5 Thyssen & Kimmerle (1977a)
Ratb Sprague-Dawley M/F Inhalation 4 h > 1200 95.7 Shiotsuka (1987)
Table 1. (continued)
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/m3 air)
Rat Wistar M Intraperitoneal 1200 96.5 Thyssen & Kimmerle (1977a)
F Intraperitoneal 720
Rat NR M Intraperitoneal 700 95.0 Iyatomy (1980)
F Intraperitoneal 560
Mouse NR M Intraperitoneal 570 95.0 Iyatomy (1980)
F Intraperitoneal 610
Rat NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
Mouse NMRI M/F Subcutaneous > 5000 96.5 Thyssen & Kimmerle (1977a)
Mouse NR M/F Subcutaneous > 1000 95.0 Iyatomy (1980)
NR, not reported; M, male; F, female; A, diastereomer A; B, diastereomer B
a Slight local irritation present
b The study was conducted in compliance with good laboratory practive
this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
Doses of 100 mg/kg per day and higher had a dose-related adverse
effect on body-weight development. At 300 mg/kg per day, behavioural
disturbances (isolation from the other animals, dirty coat) and hair
loss were observed in the female rats; however, all females had normal
behaviour near the end of the treatment period. Animals at this dose
had moderate leukocytosis, and females had reduced haemoglobin and
thrombocyte counts. Increased alkaline phosphatase activity was
observed only in the females. The relative liver weights of animals of
each sex at 100 mg/kg bw per day and above were increased. At
300 mg/kg bw per day, the absolute liver weights were elevated and the
absolute weights of the heart, kidneys, adrenals, and ovaries
depressed in female rats at the termination of treatment. The relative
weights of the thyroid and testes in males and of the spleen in
females were also elevated, whereas the relative weights of the heart,
adrenals, and ovaries were depressed in females. Hyperkeratoses and
parakeratoses, distended epithelial cells, slight inward growth of the
papillary body, and cellular infiltration in the epithelial and
subepithelial layers were observed histopathologically in the
forestomachs of four female animals at 300 mg/kg bw per day that were
sacrificed at the end of treatment. The appearance of the digestive
tract was unexceptional at the end of the recovery period., and all of
the changes in the rats at the high and intermediate doses had
likewise reverted. The NOAEL was 30 mg/kg bw per day (Thyssen &
Kaliner, 1977).
Groups of 20 male and 20 female Sprague-Dawley rats were given
bitertanol (purity, 95%) at doses of 0, 100, 400, or 1600 ppm, equal
to 7, 28, and 110 mg/kg bw per day in males and 7.4, 30, and 110 mg/kg
bw per day in females, for 28 days. The test procedures complied to a
certain extent with OECD guideline 407; at the time the study was
performed, compliance with GLP was not compulsory. Half of the animals
were sacrificed and examined after four weeks, whereas the other half
were observed without treatment for a further four weeks.
The dose of 400 ppm had an adverse effect on body-weight
development and food intake, and an adverse effect on the red blood
cell population was seen from decreases in the haematocrit and
haemoglobin readings. The relative liver weight was slightly elevated
in animals of each sex. Those at 1600 ppm had ataxia, reduced food
intake and body weight, and depressed red blood cell parameters
(haemoglobin, haematocrit, erythrocyte count), with a simultaneous
increase in the reticulocyte count. A slight increase in the serum
cholesterol concentration and a significant increase in the relative
liver weight were found in animals of each sex. Histopathological
examination revealed weak irritation of the gastric mucosa and
slightly altered ovaries, adrenals, and pituitaries. All of the
observed effects were reversible within the four-week recovery period.
The NOAEL was 100 ppm, equal to 7 mg/kg bw per day (Hatanaka et al.,
1981).
Bitertanol (purity, 90.2%) was administered to groups of 20 male
and 20 female Wistar rats at concentrations of 0, 150, 600, or 2400
ppm, equal to 12, 48 and 300 mg/kg bw per day in males and 13, 58, and
310 mg/kg bw per day in females, for three months. This study was
conducted before enactment of prevailing regulatory guidelines;
however, the procedures complied to certain extent with OECD guideline
408.
Although appearance and behaviour were unaffected at doses up to
600 ppm, 2400 ppm caused decreased motility and reduced food intakes.
Dose-related growth retardation was observed in females at 600 and
2400 ppm and in male animals at all concentrations; however, the 150
ppm dose decreased body weights only temporarily. The dose of 2400 ppm
had adverse effects on various haematological parameters, including
reduced erythrocyte and leukocyte counts, decreased haemoglobin
content and haematocrit reading, increased reticulocyte counts, a
relative increase in the polymorphonuclear leukocyte count, and a
relative decrease in the lymphocyte count. The changes in clinical
chemical parameters of the blood also found at this concentration were
elevated alkaline phosphatase, aspartate aminotransferase, and
glutamate dehydrogenase activities in the female animals. The blood
protein level was slightly depressed and the cholesterol level
slightly elevated. The finding of increased liver weight in females at
2400 ppm also indicated an effect on the liver. The NOAEL was 150 ppm,
equal to 12 mg/kg bw per day (Bomhard & Löser, 1978).
In a supplementary study, bitertanol (purity, 90.8%) was
administered to groups of 15 male and 15 female Wistar rats at
concentrations of 0, 30, 100, or 300 ppm, equal to 2.5, 8.1, and 25
mg/kg bw per day in males and 3.3, 10, and 32 mg/kg bw per day in
females, for three months. The method used in this study complied to a
certain extent with OECD guideline 408; at the time the study was
performed, compliance with GLP was not compulsory.
The appearance, behaviour, and mortality rate were unaffected at
all concentrations. At 300 ppm, the rats gained less weight than the
controls. Haematological, clinical chemical, gross and
histopathological examination, and urinary analyses provided no
evidence of adverse effects. The NOAEL was 100 ppm, equal to 8 mg/kg
bw per day (Krötlinger et al., 1978).
In a further study, bitertanol (purity, 95%) was administered to
groups of 15 male and 15 female Sprague-Dawley rats at concentrations
of 0, 40, 200, or 1000 ppm, equal to 3.1, 16, and 82 mg/kg bw per day
in males and 3.6, 19, and 88 mg/kg bw per day in females, for three
months. Five males and five females were subjected to urinary,
haematological, and clinical chemical determinations. The method used
in this study complied to a certain extent with OECD guideline 408; at
the time the study was performed, compliance with GLP was not
compulsory.
The dose of 200 ppm retarded weight gain in male and female
animals and slightly increased the activities of alkaline phosphatase
and aspartate aminotransferase in the serum of females. The dose of
1000 ppm reduced body-weight gain and decreased food and water intakes
in animals of each sex. The erythrocyte count was also depressed, and
the reticulocyte count elevated in male and female animals. In
addition, the leukocyte and thrombocyte counts were elevated, and the
haemoglobin content and haematocrit reading were decreased in females.
Elevated lactate dehydrogenase, aspartate aminotransferase, and
alkaline phosphatase activities and a slightly elevated cholesterol
concentration were found in the serum of females. The absolute weights
of the liver and spleen were increased in females and the relative
weights in males and females. The following lesions were found
histopathologically in animals of each sex: increased swelling and
fatty degeneration of hepatocytes, hyperkeratoses of the oesophageal
and gastric mucosal epithelium and/or erosions of the glandular
stomach, swelling, and fatty degeneration of adrenal cortical cells.
Isolated bile-duct proliferation was also seen in females. The NOAEL
was 40 ppm, equal to 3 mg/kg bw per day (Yonemura et al., 1981).
Groups of 10 male and 10 female Wistar rats were exposed to
bitertanol at mean analytically determined concentrations of 18, 63,
or 200 mg/m3 air for 6 h per day, five days per week for three weeks.
Only the head and nose of the animals were exposed, under dynamic
conditions, and the inhaled bitertanol was dissolved in a 1:1 blend of
ethanol:polyethylene glycol 400.The method used in this study complied
to a certain extent with OECD guideline 412; at the time the study was
performed, compliance with GLP was not compulsory.
The concentrations of 18 and 63 mg/m3 air were tolerated by male
and female rats with no observed effect. Exposure to 200 mg/m3 air
caused deterioration of the general condition of female rats, and the
growth of male rats in this group was significantly decreased.
Although the relative weights of the lung, liver, kidney, and adrenal
in males and females at the high concentration were sometimes higher
than control values, no evidence of damage due to exposure to
bitertanol was seen histopathologically. The NOAEL was 63 mg/m3 air
(Mihail & Kimmerle, 1977).
Dogs
Groups of four male and four female beagles received bitertanol
(purity, 90.2%) at a dose of 0, 1, 5, or 25 mg/kg bw per day in
gelatin capsules. The method used in this study complied to a certain
extent with OECD guideline 409; at the time the study was performed,
compliance with GLP was not compulsory.
Doses of 5 mg/kg bw per day and above led to signs of
dose-related adverse effects on the skin and mucous membranes. The
effects on the skin were manifested by increased reddening, with local
inflammation, desquamation, and hair loss; and those on the mucous
membranes by increased reddening and slight inflammatory phenomena in
regions of the oral cavity (gingiva) and eyes (conjunctiva). They were
evident histologically as slight broadening of the epithelial layer of
the skin and slightly enhanced cornification in some cases. Evidence
for superficial involvement of the cornea (keratitis), apparently
resulting from conjunctival irritation, was observed in a few animals
at 25 mg/kg bw per day. The behaviour of the controls and treated
animals was similar; in contrast, the food intake and body-weight
development of animals at the high dose showed an adverse effect. The
results of laboratory tests indicated treatment-related interference
with liver function (elevated alkaline phosphatase and alanine
aminotransferase activities) in dogs at 5 and 25 mg/kg bw per day.
Elevated N-demethylase activity and an increase in the cytochrome
P450 concentration in liver homogenates were found in the high-dose
group at study termination. The weights of the thymus in females at
25 mg/kg per day and of the prostate (relative at 5 mg/kg per day and
both relative and absolute at 25 mg/kg per day) were statistically
significantly lower than those of the controls. The changes in
prostate weights correlated with dose-related histopathological
findings of retarded development. The NOAEL was 1 mg/kg bw per day
(Hoffmann & Schilde, 1979).
In view of the dermal phenomena observed in the previous study,
tests were performed to determine whether they represented a
sensitization effect. Beagle dogs were first treated orally 10-42
times with 35 or 70 mg/kg bw bitertanol, which led to marked
irritation and dermal lesions, particularly in the area of the head.
After a treatment-free interval of four to six weeks, when the lesions
had healed, the animals were treated with a dose of 1.75 mg/kg bw,
previously found to induce no reaction, for 14 days. Hair loss and a
slightly increased incidence of reddened gingiva were determined in
isolated animals only at termination of treatment. The study gave no
evidence for sensitization, and the delay in development of the effect
specifically argues against such an effect (Hoffmann, 1977).
No evidence for sensitization was found in a study in which
beagles were exposed to a concentration of 28.8 mg/m3 air for 4 h per
day, five days per week for three weeks, left untreated for 10 days,
and subsequently re-exposed to a concentration of 47.1 mg/m3 air for
one week. All dogs tolerated the exposures with no signs, and no
evidence was found for either an irritating effect on visible mucous
membranes or for a Type I (immediate anaphylactic type) allergy
(Thyssen & Kimmerle, 1977b).
Cats
Groups of three male and three female cats weighing 2-3 kg
received whole-body exposure to aerosols of bitertanol (active
ingredient, 95.8%) in a 3-m3 inhalation chamber for 6 h per day for
four weeks (20 x). The mean analytically determined bitertanol
concentration was 27 mg/m3 air. Analysis of the generated aerosols
showed that more than 90% of the aerosol particles had a mass-related
and more than 99% a particle-related aerodynamic diameter smaller than
5 µm. The exposed animals were compared with negative controls (air)
and positive controls (dichlozoline, 28 mg/m3 air). The animals were
observed over a recovery period of 12 weeks after termination of the
exposure. Their eyes were examined with an ophthalmoscope before
exposure and each week throughout the four-week exposure and the
recovery period and for gross and histopathological alterations at the
end of the study. The method used complied with OECD guideline 412; at
the time the study was performed, compliance with GLP was not
compulsory.
The exposed cats tolerated the treatment with no signs of
toxicity, damage to the eyes, or cataractogenesis. Under comparable
experimental conditions, the animals exposed to dichlozoline showed
signs of lenticular opacity (cataracts), starting from the end of the
second week of exposure. Lenticular opacity that had not reverted up
to the end of the recovery period was observed in all animals of this
group at the end of the second week of the recovery period. Cats
exposed to bitertanol had no cataracts (Pauluhn et al., 1983).
Rabbits
Groups of six male and six female New Zealand rabbits received
applications of bitertanol (purity, 95.8%) in an aqueous suspension
(0.5 ml) at concentrations of 0, 50, or 250 mg/kg bw on the intact or
abraded dorsal and lateral skin for 6 h per day, five times per week
for three weeks. The method used in this study complied to a certain
extent with OECD guideline 411; at the time the study was performed,
compliance with GLP was not compulsory.
Bitertanol had no apparent effect on the appearance, behaviour,
body weight, or survival of the rabbits. Transient erythema developed
on the exposed areas, initially on abraded skin but later on intact
skin. There was no effect on skin-fold thickness or on haematological,
clinical chemical, or urinary parameters. At necropsy, there were no
gross pathological findings. Histopathological examination showed
slight epidermal thickening only in exposed areas of all treated
animals. Preparations of liver showed no evidence of induction of
microsomal N- or O-demethylation or cytochrome P450 content. The
dermal application had no effect on any of the parameters
investigated. The NOAEL was 250 mg/kg bw (Heimann & Vogel, 1984).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Bitertanol (purity, 94-95%) was administered to groups of 50 male
and 50 female CF1/W74 mice at doses of 0, 20, 100, or 500 ppm, equal
to 2, 25, and 130 mg/kg bw per day in males and 7, 37, and 180 mg/kg
bw per day in females, for two years. The study was conducted before
enactment of prevailing regulatory guidelines; however, the test
procedures complied to a certain extent with OECD guideline 453 but
with no attempt to monitor ocular changes.
Behaviour, feed consumption, mortality, and haematological
parameters were not affected by treatment. In animals at 500 ppm, body
weights were reduced and serum alkaline phosphatase activity was
significantly elevated; females at this dose had an increased
incidence of enlarged and greenish-coloured livers at necropsy. The
liver weights were also increased, particularly in the female mice,
and the livers had increased numbers of eosinophilic foci. No evidence
was found for a carcinogenic effect at any concentration, which
extended into the toxic range. The NOAEL was 100 ppm, equal to 25
mg/kg bw per day (Bomhard & Löser, 1981a).
Rats
Bitertanol (purity, 94% in weeks 1-18; 95% from week 19) was
administered to groups of 50 male and 50 female SPF Wistar rats at
doses of 0, 20, 100, or 500 ppm, equal to 1, 4.9, and 26 mg/kg bw per
day in males and 1.3, 6.6, and 34 mg/kg bw per day in females, for two
years. The study was conducted before the enactment of prevailing
regulatory guidelines; however, the test procedures complied to a
certain extent with OECD guideline 453.
The 500 ppm concentration led to retarded growth in animals of
each sex. Ocular changes were not recorded. Haematological, blood
chemical, and urinary parameters were not significantly affected, and
the mortality rate was not adversely influenced. No treatment-related
effects were seen on organ weights or the gross or microscopic
appearance of the tissues. In particular, the type, location,
incidence, and time to occurrence of the tumours observed in the
treated groups were comparable to the control findings and to the
spontaneous findings for this strain of rat. The NOAEL was 100 ppm,
equal to 4.9 mg/kg bw per day (Bomhard & Löser, 1981b).
Dogs
Groups of four male and four female beagle dogs received
bitertanol (purity, 95-97.3%) in the diet at concentrations of 0, 10,
40, or 160 ppm, equal to 0.3, 1.2, and 4.9 mg/kg bw per day, for two
years. The method used in this study complied to a certain extent with
OECD guideline 452; at the time the study was performed, compliance
with GLP was not compulsory.
Although the control dogs gained more weight than did treated
dogs, there was no association with treatment. A good nutritional
status was maintained by dogs in all groups, and there was no
significant difference between groups in food or water consumption. No
abnormalities were seen in selected reflexes, body temperature, or
pulse rate; however, three of eight dogs at the high dose developed
bilateral cataracts. Urinary and haematological examinations conducted
at approximately three-month intervals revealed no abnormalities,
although serum alanine aminotransferase and alkaline phosphatase
activities were increased at 40 and 160 ppm. At necropsy, the mean
liver weight of dogs at the high dose was markedly increased.
Histological examination showed mild to moderate vacuolation of the
adrenal zona reticularis epithelia at doses at and above 40 ppm. The
NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day (Hoffmann & Gröning,
1983).
Groups of six male and six female beagles were fed diets
containing 0, 3, or 25 ppm bitertanol (purity, 96.3-96.7%) for 12
months, equal to 0.1, 1, and 7.6 mg/kg bw per day. A further group was
maintained on a diet containing 200 ppm bitertanol for 20 months to
permit continued ophthalmoscopic examination. The method used in this
study complied to a certain extent with OECD guideline 452; at the
time the study was performed, compliance with GLP was not compulsory.
Treatment had no apparent effect on behaviour, appearance, or
nutritional status, and food consumption and body-weight gain were
also unaffected. Pulse rates, body temperature, and selected reflexes
were unchanged. Ophthalmoscopy, conducted on animals at 0, 3, or 25
ppm at three-month intervals, showed no changes; however, one dog at
the high dose developed severe bilateral lenticular cataracts, which
were observable from week 58. Four other animals had slight lenticular
opacification by week 85. Dogs in this group also had signs of
conjunctivitis, with nasociliary discharge and incrustation.
Intermittent increases in serum alanine aminotransferase, glutamate
dehydrogenase, and alkaline phosphatase activities were also seen in
the dogs at the high dose. Other clinical chemical, haematological,
and urinary parameters were unaffected by treatment. At necropsy,
there were no gross abnormalities. The weights of the adrenals of the
dogs at the high dose were apparently greater than those of controls,
and lipoid vacuolation was observed in the zona reticularis epithelia.
The NOAEL was 25 ppm, equal to 1 mg/kg bw per day (Hoffmann & Vogel,
1983).
(d) Genotoxicity
No genotoxic or mutagenic potential of bitertanol was found in
lower organisms or in mammalian cells or systems in vivo or
in vitro. The results of assays for the genotoxicity of bitertanol
are summarized in Table 2.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
The effect of bitertanol (purity, 95.0%) on fertility, lactation
performance, and pup development was examined in a three-generation
study in Long-Evans FB 30 rats with two litters per generation. The
test substance was administered throughout the study period at
concentrations of 0, 20, 100, or 500 ppm, equivalent to 1, 5, and 25
mg/kg bw per day. Each of the mating groups consisted of 10 male and
20 female rats. The animals were five to six weeks old at the
beginning of the study and were treated for 70 days before the first
mating. The F3b generation pups and their parents (F2b generation)
were sacrificed and examined histopathologically after a four-week
lactation period. The method used in this study complied to a certain
Table 2. Results of assays for the genotoxicity of bitertanol
Test system Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa S. typhimurium TA98, TA100, 4-2500 µg/plate 93.7 Negative Herbold (1979)
TA1535, TA1537
Reverse mutationa E. coli WP2 hcr- 1-5000 µg/plate 95.0 Negative Shirasu et al. (1981)
S. typhimurium TA98, TA100,
TA1535, TA1537, TA1538
Forward mutationa Mouse lymphoma L5178Y tk +/- 1-25 µg/mlb 95.0 Negative Bootman & Rees
1-20 mg/mlc (1983)
DNA repaird B. subtilis H17 rec+, M45 rec- 20-5000 µg/disc 95.0 Negative Shirasu et al. (1981)
DNA repaira,d E. coli W3110 pol A+; P3470 pol A- 0.1-33.3 mg/plate 95.0 Negative Riach (1981)
Aneuploidy Sordaria brevicollis 0.1-5.0 mg/L 95.0 Negative Bond & McGregor (1981)
Cytogenetic Chinese hamster lung cells 3.3 × 10-6-3.3 × 10-4 97.1 Negative Sasaki (1987)
alterationsa,d mol/Lb
1.0 × 10-5-1.0 ×.10-3
mol/Lc
In vivo
Micronucleus NMRI mice 2 × 1000 mg/kg bw 93.7 Negative Herbold (1978a)
formation 2 × 2000 mg/kg bw
Dominant lethal Male NMRI mice 1000 mg/kg bw 93.7 Negative Herbold (1978b)
mutation
a With and without exogenous metabolic activation
b Without exogenous metabolic activation
c With exogenous metabolic activation
d Conducted in compliance with good laboratory practice and to a certain extent with OECD guidelines
extent with OECD guideline 416; at the time the study was performed,
compliance with GLP was not compulsory.
No adverse effects were seen on appearance, behaviour, or
mortality in any group or generation. Bitertanol at a concentration of
20 ppm in the diet did not affect reproductive performance.
Administration of 100 or 500 ppm led to reductions in pup survival
rates during the lactation period in several matings and to pups with
lower birth weights. At 100 ppm, retarded pup growth was also seen in
the F2a and F2b generations. At 500 ppm, general retardation of
growth was seen. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Löser & Eiben, 1981).
(ii) Developmental toxicity
Rats
Groups of 20-23 Long-Evans rats were given bitertanol (purity,
96.5%) orally at doses of 0, 10, 30, or 100 mg/kg bw per day on days
6-15 post coitum. The method used in this study complied to a
certain extent with OECD guideline 414; at the time the study was
performed, compliance with GLP was not compulsory. The body-weight
gain of dams at 30 or 100 mg/kg bw per day was significantly reduced
during treatment and throughout the gestation period for those at 100
mg/kg bw per day. Resorption rates, fetal deaths, placental weights,
and sex ratio were unaffected by treatment; however, fetal weights
were significantly reduced at 100 mg/kg bw per day and significant
fetal stunting occurred at doses of 30 mg/kg bw per day and higher. At
100 mg/kg bw per day, skeletal ossification was retarded, and a
significantly increased incidence of malformations was observed, which
included cleft palate, hydrocephalus, malformed tails, dysplasia, and
synostosis of the ribs. A simple case of hydrocephalus occurred at 30
mg/kg bw per day. The NOAEL was 10 mg/kg bw per day (Machemer, 1977).
Groups of 22 or 23 Sprague-Dawley rats were given bitertanol
(purity, 96%) orally at doses of 0, 10, 25, or 65 mg/kg bw per day on
days 6-15 post coitum. The study was conducted in compliance with
GLP standards and with OECD guideline 414. Reduced weight gain was
seen in dams at daily doses of 25 mg/kg bw and higher, which persisted
throughout the gestation period in those at 65 mg/kg bw per day. The
number of corpora lutea, implantation rates, resorption rates, live
fetuses, sex ratio, and fetal and placental weights were unaffected by
treatment. An increased incidence of a fourteenth (lumbar) rib
relative to controls was observed at daily doses of 25 mg/kg bw (32%
incidence) and 65 mg/kg bw (70% incidence). No teratogenic effects
were observed. The NOAEL was 10 mg/kg bw (Nagumo et al., 1987).
Pregnant Wistar rats were given single oral doses of 100, 500, or
1000 mg/kg bw bitertanol on day 9, 10, 11, or 13 of gestation. The
doses were calculated to represent 1/5, 1/10, and 1/50 of the reported
LD50 value of 5000 mg/kg bw. The method used complied to a limited
extent to OECD guideline 414. The largest deviation was the short
application period; no information was given on compliance with GLP.
The study was available only in abstract form and is therefore of only
limited value for risk evaluation. Bitertanol induced congenital
anomalies when given on day 9, 10, or 11 at 500 or 1000 mg/kg bw. The
malformations consisted of microcaudia and acaudia and, in rare cases,
exophthalmus, hypognathia, and cleft palte. The NOAEL was 100 mg/kg bw
(Vergieva, 1990).
In two studies, groups of 25 Long-Evans rats were exposed to
bitertanol (purity, 93.7%) by inhalation for 4 h per day on days 6-15
post coitum at a mean analytical concentration of 0, 2.9, 6.4, or 22
mg/m3 air in one study and 0, 27, 60, or 120 mg/m3 air in the other.
The method used complied to a certain extent with OECD guideline 414;
at the time the study was performed, compliance with GLP was not
compulsory. No adverse effects were observed on dams exposed at any
concentration, but the average fetal weights were reduced
significantly by exposure to 120 mg/m3. Fetuses with below-average
weights were found increasingly at aerosol concentra-tions greater
than 22 mg/m3 air, without a clear dose-response relationship.
Implantation rates, resorption rates, placental weights, and sex ratio
were unaffected by treatment. No embryolethal or teratogenic effects
were observed. The NOAEL was 22 mg/m3 air (Machemer & Thyssen, 1979).
Rabbits
Twelve Himalayan rabbits, were given bitertanol (purity, 96.7%)
at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method used complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. The dose of 100 mg/kg bw led to reduced weight
gain and isolated clinical signs, such as reduced food intake,
diarrhoea, and blood in the urine; one dam died on day 29
post coitum. This dose also resulted in increased embryonal and
fetal mortality and reduced fetal and placental weights, and three
individual malformations occurred, which deviated from those seen in
controls in their nature (epignathus, pulmonary hypoplasia, and
aplasia) but not in their number. The NOAEL was 30 mg/kg bw per day
(Roetz, 1982).
Groups of 15 Himalayan rabbits were given bitertanol (purity,
93.9%) at daily oral doses of 0, 10, 30, or 100 mg/kg bw on days 6-18
post coitum. The method complied to a certain extent with OECD
guideline 414; at the time the study was performed, compliance with
GLP was not compulsory. Treatment had no effect on the behaviour,
appearance, or body-weight gain of the dams. At 100 mg/kg bw per day,
a significantly higher resorption rate and reduced numbers and weights
of fetuses were seen, and the number of malformed fetuses was
increased, with rare types of malformation such as cleft palate and
pigeon chest. The NOAEL was 30 mg/kg bw per day (Schlüter, 1983).
Groups of 16 fertilized chinchilla rabbits were given bitertanol
(purity, 96.9%) at a daily oral dose of 0, 10, 50, or 250 mg/kg bw on
days 6-18 of gestation. Because of the very high rate of post-
implantation loss at 250 mg/kg bw per day, doses of 0 and 150 mg/kg bw
per day were used in a supplementary study. The study was conducted in
compliance with OECD guideline 414 and with GLP standards. A
dose-related reduction in food consumption was seen in dams at > 50
mg/kg bw per day. The dose of 250 mg/kg bw per day significantly
reduced body weights from day 7 to the end of the experiment and
significantly reduced corrected body-weight gain. One dam at 150 mg/kg
bw per day and two at 250 mg/kg bw per day died during the study.
Isolated hair loss and enlarged and heavier livers at necropsy were
observed in dams at the high dose. A dose-related increase in the rate
of post-implantation losses was observed at > 150 mg/kg bw per day.
Two dams at 150 mg/kg bw per day and 13 at 250 mg/kg bw per day
completely resorbed their embryos, and the fetuses at these doses
showed dose-related reductions in body weight and a dose-related
increased in the incidence of incompletely or unossified phalangeal
nuclei and calcanei. The NOAEL was 50 mg/kg bw per day (Becker et al.,
1987).
(f) Special studies
As the studies in this section were published in the open
literature, the level of detail needed for full evaluation was not
available.
(i) Effects on the central nervous system
The effect of bitertanol on the central nervous system was
examined in pharmacological screening tests in mice and rats, which
included tests for potentiation of anaesthesia in mice (hexobarbital
sleep period), stimulation of spontaneous motility in mice, an Irwin
behaviour test in mice, the novel box response in rats, the open field
test in mice, and a test for reserpine ptosis in mice. In these
investigations, bitertanol was administered as a single oral dose of
0.075, 0.6, or 4.8 mg/kg bw. The results showed a slight stimulating
effect of bitertanol on the central nervous system in mice, but no
specific pharmacological effects, such as potentiation of amphetamine
effects or antagonism to reserpine ptosis, were determined (Polacek,
1983).
In a pharmacological test programme, groups of 10 male mice were
given a single oral dose of 0.02, 0.2, 2, 20, or 200 mg/kg bw
bitertanol. The highest dose significantly increased spontaneous motor
activity, with marked effects when treatment was given during the dark
period. In mice treated with 20 mg/kg bw per day, motor activity
tended to increase but not significantly, and lower doses had no
effect. No further effects on behaviour were observed at any dose
(Kaneto, 1986a). In a further test, male mice received bitertanol at 1
or 100 ppm for one week or one month. No increase in spontaneous motor
activity and no potentiating effect in amphetamine-pretreated animals
were seen after repeated dosing (Kaneto, 1986b).
In a study designed to determine whether bitertanol has similar
behavioural effects on the fixed-interval response rate and motor
activity of rats, doses of 10-300 mg/kg bw were given
intraperitoneally to rats maintained under a multiple fixed-interval
1-min schedule of reinforcement. Intermediate doses increased the
response rates and disrupted response patterning in both
fixed-interval components. The same doses of bitertanol did not
increase motor activity (Allen & MacPhail, 1983).
In rats placed in an actographic device designed for continuous
measurement of the locomotor component of spontaneous motor activity,
bitertanol increased motor activity at 200 mg/kg bw after
administration either orally or intraperitoneally. The activity peaks
at the low dose of 100 mg/kg bw coincided with normal night and
morning activity maxima (Frantik et al., 1996).
One the basis of previous results showing that acute exposure to
the triazole fungicide triadimefon affects central nervous system
catecholamines and induces a transient syndrome in rats that consists
of hyperactivity and stereotyped behaviour, a study was designed to
determine whether this type of toxicity is characteristic of other
triazoles. Dose-effect functions were determined for 14 triazoles or
structurally related pesticides, including bitertanol, in adult male
Long-Evans rats. All of the chemicals were administered orally in corn
oil. Hyperactivity was measured for 2 h in figure-eight mazes. Only
triadimefon and triadimenol induced hyperactivity, suggesting a very
rigid structure-activity relationship for the hyper-activity syndrome.
The absence of an effect of bitertanol may be due to steric hindrance
of benzene-ring substitution for the halogen on the benzene-ring
structure of triadimefon and/or triadimenol. Alternatively, bitertanol
may lack halogen substituents on the benzene rings and thus be less
polarized. The lack of activity of bitertanol is probably not due to
differences in absorption kinetics (Crofton, 1996).
(ii) Toxicity in combinations
Acute tests were performed to determine whether bitertanol has
superadditive (potentiating) effects when administered in combination
with triadimenol, captan, fuberidazole, or dodine. At the time the
studies were performed, OECD methods were not available and compliance
with GLP was not compulsory. The study involved administration of
single equitoxic oral (with triadimenol, captan, or fuberidazole) or
intraperitoneal (with dodine) doses of the active ingredients to male
rats. A factor greater than 1 between observed and expected LD50
values indicates a superadditive effect. Bitertanol plus triadimenol,
bitertanol plus fuberidazole, and bitertanol plus dodine had no
superadditive effects but only additive toxic effects (Mihail, 1982b;
Flucke, 1980; Heimann, 1984b). In contrast, the active ingredient
combination of bitertanol plus captan had slightly superadditive
action, with a potentiation factor of 1.9 (Mihail, 1982a).
(iii) Effect on the liver
Groups of 10 male and 10 female Wistar rats received bitertanol
(purity, 95.8%) suspended in distilled water with Cremorph EL by
gavage at doses of 0, 30, 100, or 300 mg/kg bw per day for 14 days. On
sacrifice, blood was collected for detailed haematological and
clinical chemical testing, and liver samples were taken for detailed
histopathological and enzyme studies. During treatment, 1/10 female
rats at the intermediate dose and 9/10 at the high dose showed hair
loss, and some lost weight, while females at the low dose had reduced
body-weight gain in comparison with control animals. Female rats also
had a dose-related tendency to mild thrombocytosis, which was
significant at the intermediate and high doses. Serum gamma-glutamyl
transpeptidase activity and bilirubin concentration were slightly
increased in females at the high dose. There were no gross
pathological findings at necropsy. The liver weights tended to be
increased at the intermediate and high doses, especially in females.
Slight-to-moderate bile-duct proliferation with peribiliary
infiltration of monocytes or polynucleocytes was seen histologically
in animals at the intermediate and high doses. These changes were
sometimes accompanied by parenchymal Councilman bodies or, more
occasionally, mitoses. The hepatocytes of animals at the high dose
were occasionally swollen, with finely granular cytoplasm. Little
fatty infiltration was seen. The results of studies in vitro were
consistent with induction of hepatic microsomal enzymes, as the
cytochrome P450 content increased in a dose-related manner, especially
in males. Aminopyrene N-demethylase activity was increased in males
and females at the high dose and in males at the intermediate dose,
while O-demethylase activity was increased in males at the
intermediate and high doses and in females at the high dose. The
hepatic triglyceride content was not affected by treatment. Bitertanol
thus caused mild hepatotoxicity, with modest induction of hepatic
microsomal activity in rats at 100 and 300 mg/kg bw per day. The NOAEL
was 30 mg/kg bw per day (Mihail & Luckhaus, 1985).
3. Studies on metabolites
(a) 1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one
(plants, soil)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one, a
keto analogue of bitertanol, has very little acute toxicity in rats
when given orally or dermally. It induced signs of effects on the
central nervous system, with initial sedation and respiratory
disturbances and later stimulation. The highest technically
administrable oral dose, 1750 mg/kg bw, caused no deaths. A dermal
dose of 5000 mg/kg bw and the highest dose administered by inhalation
(dynamic dust nebulization) were tolerated with no observed signs. The
LC50 value in male and female rats was > 500 mg/m3 air after a 1-h
exposure or a 4-h exposure, respectively. In rabbits, the compound was
slightly irritating to the skin only after contact for 24 h and was
mildly irritating to the mucous membranes of the eyes (Thyssen &
Kimmerle, 1978).
(b) Bitertanol benzoic acid (soil)
Bitertanol benzoic acid had little acute toxicity when given
orally. The highest administered dose, 5000 mg/kg bw, was tolerated by
fasted male rats with no observed signs or deaths (Heimann, 1983b).
The compound was not mutagenic to Salmonella typhimurium in the
presence of an exogenous metabolic activation system (Herbold, 1983).
(c) 1,2,4-Triazole (photodegradation, soil)
1,2,4-Triazole has moderate or low acute toxicity when given
orally (LD50 value, 1600 mg/kg bw in males and females) or dermally
(LD50 values, 4200 mg/kg bw in males and 3100 mg/kg bw in females).
At high oral and dermal doses, the compound had effects on the central
nervous system. In tests of inhalation of air enriched with vapours of
the test substance, male rats and mice tolerated exposure for 4 and
6 h, respectively, with no observed signs. No dermal irritation was
observed on rabbits exposed for 24 h or on the skin of five male
volunteers exposed for 8 h. 1,2,4-Triazole was strongly irritating to
the mucous membranes of the rabbit eye (Thyssen & Kimmerle, 1976).
Groups of 15 male and 15 female rats were given 1,2,4-triazole
(purity, 99.6%) at a dose of 0, 100, 500, or 2500 ppm for three
months. The study was conducted before enactment of prevailing
regulatory guidelines, but the test procedures complied to a certain
extent with OECD guideline 408. Treatment at 2500 ppm resulted in a
temporary decrease in food intake, a reduction in body weight, and
transient, slight palmospasms in isolated animals. Significant
reductions in the haemoglobin, haematocrit, mean corpuscular volume,
and mean corpuscular haemoglobin values in male rats at 2500 ppm
indicated an effect on the blood. In this group, slight to moderate
fat accumulation was found in the cells of the liver parenchyma in
three of 15 males, which was attributed to the treatment. The NOAEL
was 500 ppm, equal to 38 mg/kg bw per day (Bomhard et al., 1979).
1,2,4-Triazole at concentrations of 10-5000 µg/plate did not
induce point mutations in Salmonella typhimurium TA1535, TA1537,
TA98, or TA100, with or without metabolic activation (Poth, 1989).
In two studies, groups of 25 fertilized Wistar rats were given
daily oral doses of 1,2,4-triazole (purity, 95.3% and 94.0%,
respectively) on days 6-15 of gestation at doses of 0, 10, 30, or 100
mg/kg bw in one study and 0, 100, or 200 mg/kg bw in the other. The
studies were conducted in compliance with GLP standards and OECD
guideline 414. Maternal toxicity was indicated by decreased weight
gain of dams at 100 and 200 ppm relative to those in the control
group. Reduced fetal weights, retarded osteogenesis, and increased
numbers of runts were found at > 100 mg/kg bw per day. An increased
resorption rate was found in dams at 200 mg/kg per day, but the rate
of fetuses with retarded ossification was not increased. The types and
incidences of the malformations observed in this group (including
cleft palate and malformed extremities) indicate that 1,2,4-triazole
has teratogenic potential. The NOAEL was 30 mg/kg bw per day (Renhof,
1988a,b).
4. Observations in humans
No health impairment was observed in male or female employees
engaged in formulating bitertanol and using the customary safety
precautions, who had regular medical examinations (Miksche, 1981).
Slight, transient prurient reddening of the forearms, which
regressed spontaneously after a few days, developed in rare, isolated
cases after direct dermal contact during packaging of a powder of the
pure active ingredient. Unequivocal differentiation between an
allergic dermal reaction and mechanical-toxic skin irritation was not
possible. There was no tendency to relapse (Faul, 1986).
Comments
After oral administration bitertanol is rapidly and extensively
absorbed (about 84%) and distributed. Excretion is also rapid (rats,
almost complete within 72 h) and occurs mainly in the faeces (about
90%) by biliary excretion, owing to the lipophilic nature of the
parent substance. The liver and kidneys are the main sites of tissue
accumulation in both male and female rats. Although some statistically
significant sex-related differences were seen, they were of minor
physiological importance. The substance has a relatively low rate of
dermal penetration. The metabolic profile was similar at the various
doses tested. The main metabolic pathways are hydroxylation of the
phenyl ring in the para position and oxidation of the tert-butyl
moiety, leading to bitertanol alcohol and the corresponding carboxylic
acid; metabolites derived from the two pathways combined were also
observed. The metabolites occur in both free and conjugated forms. The
parent compound was not detected in urine or bile. There was no
toxicological concern with regard to the metabolic profile in plants.
Bitertanol had very low acute toxicity in rats, mice, and dogs
when given orally and after dermal application or by inhalation. It
was of moderate to low toxicity in rats and mice after intraperitoneal
injection. Females appeared to be slightly more sensitive than males,
but only in some studies. This was perhaps due to slightly different
absorption characteristics in animals of the two sexes, as seen after
oral administration. In view of the toxic signs (including respiratory
disturbances, sedation, spasms, and tremor) and the findings of the
pharmacological screening tests, it may be inferred that bitertanol
has central nervous system activity; however, no specific
pharmacological effects, such as potentiation of amphetamine action,
antagonism of reserpine ptosis, or an effect on hexobarbital
anaesthesia, were observed.
Bitertanol induces no, or only very slight, dermal irritation. It
induces slight to moderate reactions of the ocular mucosa but has no
effect on the cornea or iris. No evidence for a sensitizing effect was
observed in any study
Bitertanol has been classified by WHO as unlikely to present an
acute hazard in normal use (WHO, 1996).
In medium- and long-term tests for toxicity, the liver is
regarded as the main toxicological target organ in dogs and rats at
doses of 1.2 and 28 mg/kg bw per day and above, respectively. Liver
weight was found to be the most sensitive indicator. Corresponding
patterns of disruption of liver function were observed in rats and
dogs. The activities of the transaminases, alkaline phosphatase, and
glutamate dehydrogenase in the serum were increased. In addition, a
rise in cholesterol level was observed in several studies in rats at
doses of 61 mg/kg bw per day and above. The ability of bitertanol to
induce mixed-function oxidases was verified in both species. It is
therefore likely that the effect on weight is essentially due to
hypertrophy of the endoplasmic reticulum in hepatocytes at doses of
100 mg/kg bw per day and above. Morphological changes in the liver
were seen only at relatively high doses and consisted of hepatocytic
swelling, bile-duct proliferation, perilobular fatty degeneration,
eosinophilic foci, and fibrous structures. The induced effects
corresponded to toxic liver damage with bile-duct involvement.
Evidence for haemotoxicity was found at doses of 28 mg/kg bw per
day and above in rats; this may be classified as an effect on the
peripheral red blood cell population. The decreases in erythrocyte
count, haemoglobin concentration, and packed cell volume and the
compensatory rise in the reticulocyte count argue for this
interpretation. No evidence was found for damage to the haematogenic
organs.
Slight increases in the leukocyte count were also seen in a few
studies in rats at doses of 61 mg/kg bw per day and above. The
increased leukocyte counts are probably attributable to inflammatory
processes, since the increase occurred in studies and at doses at
which inflammatory processes were also observed. Hyperkeratosis of the
oesophageal epithelium and glandular stomach and/or erosions of the
glandular stomach as well as parakeratosis of the stomach wall were
observed in rats. The histopathological picture included distended
epithelial cells, slight inward growth of the papillary body, and
cellular infiltration of the epithelial and subepithelial layers in
the affected animals. These changes are probably attributable to
irritation of the mucous membranes by the active ingredient.
Effects on the skin were observed in dogs, sheep and rats. The
effects in dogs at doses of 5 mg/kg bw per day and above consisted of
reddening, with localized inflammation, desquamation, and hair loss,
and increased reddening and slight inflammatory phenomena in the
mucous membranes of the oral cavity and eyes. Histological examination
showed broadening of the epithelial layer, enhanced cornification, and
minor erosions. The dermal effects were apparently accompanied by
pruritus. The keratitis observed in dogs was considered to be
secondary to conjunctivitis. Hair loss was also observed in rats and
sheep, which occurred after administration of bitertanol by capsule or
gavage at doses of 300 mg/kg per day and above; this was considered to
be a systemic effect.
Pathological changes were seen in the adrenals of dogs and rats
at 1.2 and 81 mg/kg bw per day and above, respectively, consisting of
swelling and fatty degeneration of the adrenal cortical cells,
particularly in the zona reticularis and zona fasciculata. These
alterations were considered to be due to inhibition of sterol
biosynthesis by triazole derivatives. It is highly probable that this
effect, which also represents the biological, antimycotic action of
the substance, leads to an effect on corticoid metabolism with
corresponding morphological effects in the cells of the adrenal
cortex.
In feeding studies in dogs, lenticular opacity seen at doses of
4.9 mg/kg bw per day and above were considered to be related to
treatment. No lenticular alterations attributable to administration of
bitertanol were seen in any other study or species. The exact
mechanism of the cataractogenesis resulting from long-term
administration of triazole fungicides is presently unknown. The ocular
lens undertakes its own de-novo synthesis of cholesterol, which is
isolated from lipoproteins circulating in the blood; other substances
that inhibit cholesterol synthesis can induce cataracts.
No evidence for any carcinogenic potential of bitertanol was
found in long-term studies of toxicity and carcinogenicity in rats and
mice treated in the diet. The highest doses tested were 130 mg/kg bw
per day in mice and 26 mg/kg bw per day in rats.
In a series of studies, bitertanol had no genotoxic potential in
lower organisms or in mammalian cells or systems in vitro or
in vivo.
In a three-generation study of reproductive toxicity in rats,
adverse effects on the pups (reductions in survival rates during the
four-week lactation period, reduced weight at birth, and retarded
growth) were observed at parentally toxic doses of 100 or 500 ppm,
equivalent to 5 or 25 mg/kg bw per day. The NOAEL was 1 mg/kg bw per
day.
In studies of developmental toxicity, treatment with bitertanol
led to several embryotoxic and teratogenic effects, depending on the
animal species, route of administration, and dose. In rats, an oral
dose of 10 mg/kg bw per day was tolerated with no observed effect.
Doses of 25 mg/kg bw per day and above led to retardations and
variations (e.g. increased incidence of the 14th rib). Malformations
were observed at an oral dose of 100 mg/kg bw per day, which was
clearly maternally toxic. Exposure of pregnant rats to concentrations
of 27 mg/m3 air and above by inhalation resulted in retardation
effects; no malformations were observed. In rabbits, doses of 50 mg/kg
bw per day and above were maternally toxic; fetotoxic effects were
seen at doses of 100 mg/kg bw per day and above. Teratogenic effects
were observed in Himalayan rabbits at 100 mg/kg bw per day, while in
the second strain tested (Chinchilla), even a dose of 250 mg/kg bw per
day did not lead to malformations.
The ADI established at the 1988 Meeting of 0-0.01 mg/kg bw, based
on a combined NOAEL of 1 mg/kg bw per day from the two-year and the
one-year study in dogs, was maintained. The ADI is supported by the
NOAEL of 20 ppm (equivalent to 1 mg/kg bw per day) in a
three-generation study in rats.
An acute RfD was not allocated because bitertanol has been
classified by WHO as unlikely to present an acute hazard in normal use
and it has not shown any specific adverse effects (teratogenicity,
neurotoxicity) after single doses 100 times the lowest relevant NOAEL
in long- and short-term studies that were used to establish the ADI.
Therefore, the Meeting concluded that the acute intake of residues is
unlikely to present a risk to consumers.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 25 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
Rat: 100 ppm, equal to 4.9 mg/kg bw per day (toxicity in a
two-year study of toxicity and carcinogenicity)
20 ppm, equivalent to 1 mg/kg bw per day (reproductive
and parental toxicity in a three-generation study)
10 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 1 mg/kg bw per day (overall NOAEL in one-year and
two-year studies of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
Not allocated (unnecessary)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Commenced immediately, about 84% absorbed
Dermal absorption About 10%
Distribution Highest concentrations in liver and kidneys
Potential for accumulation None
Rate and extent of excretion About 90% excreted with bile, 10% with urine
Metabolism in animals No parent compound in bile or faeces; extensively
metabolized to 14 metabolites (ring
monohydroxylation, ring dihydroxylation, aryl
O-methylation, aliphatic hydroxylation, aliphatic
oxidation to carboxylic acids, and ether cleavage)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat: LD50, oral > 5000 mg/kg bw
Rat: LD50,, dermal > 5000 mg/kg bw
Rat: LC50, inhalation > 550 mg/m3 (4 h)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not a sensitizer (Magnussen & Kligman test)
Short-term toxicity
Target/critical effect Liver, red blood cells, adrenals, digestive tract
Lowest relevant oral NOAEL Dog: 90 days: 1 mg/kg bw per day
Lowest relevant dermal NOAEL Rabbit: 3 weeks; 250 mg/kg per day
Lowest relevant inhalation NOAEL Rat: 3 weeks, 63 mg/m3
Genotoxicity No genotoxic or mutagenic potential
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL Dog: 1 year and 2 years: 1 mg/kg bw per day
Carcinogenicity No evidence of carcinogenic potential
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced litter size, pup
growth rate, and pup survival) at parentally toxic
doses
Lowest relevant reproductive NOAEL Rat: 1 mg/kg bw per day
Developmental target/critical effect Fetotoxic and teratogenic effects at maternally
toxic doses
Lowest relevant developmental NOAEL Rat: 10 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No relevant effects
Other toxicological studies Induction of hepatic microsomal activity
Medical data No health impairments observed in employees
subjected to regular medical examinations
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw 1 and 2 years in dogs 100
Acute reference dose None allocated
(unnecessary)
References
Allen, A.R. & MacPhail, R.C. (1993) Bitertanol, a triazole fungicide,
increases operant responding but not motor activity. Neurotoxicol.
Teratol., 15, 237-242.
Becker, H., Mueller, E., Vogel, W. & Terrier, C. (1987) Embryotoxicity
(including teratogenicity) study with KWG 0599 in the rabbit.
Unpublished report No. R4081 from Research & Consulting Company AG,
Itingen, Switzerland. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Löser, E. (1978) KWG 0599: Subchronic toxicological
study on rats (feeding experiment over three months). Unpublished
report No. 7322 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Löser, E. (1981a) KWG 0599 (Bitertanol, the active
ingredient of Baycor): Chronic toxicity study on mice (2-year feeding
experiment). Unpublished report No. 10103 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Bomhard, E. & Löser, E. (1981b) KWG 0599: Chronic toxicity study on
rats (2-year feeding experiment). Unpublished report No. 10104 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole: Subchronic
toxicological study with rats (feeding study over three months).
Unpublished report No. 8667 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bond, J. & McGregor, D. (1981) Testing for aneuploid induction of HE
1004 in Sordaria brevicollis. Unpublished report No. R2063 from
Inveresk Research International, Musselburgh, Scotland. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Bootman, J. & Rees, R. (1983) KWG 0599: Investigation of mutagenic
activity in the TK+/- mouse lymphoma cell mutation system.
Unpublished report No. R2406 from Life Science Research. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Crofton, K.M. (1996) A structure-activity relationship for the
neurotoxicity of triazole fungicides. Toxicol. Lett., 84, 155-159.
Faul, J. (1986) Baycor active ingredient and formulations. Unpublished
report from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of acute toxicity (LD50) -- rat p.o.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Flucke, W. (1979) KWG 0599--Determination of acute toxicity (LD50):
rat p.o./mixed batch (from 5 batches). Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1980) KWG 0599 and W VII/117: Study for acute combination
toxicity. Unpublished report No. 9456 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1981) KWG 0599: Evaluation for sensitization in guinea
pigs (maximization test after Magnusson and Kligman). Unpublished
report No. 9934 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Frantik, E., Hornychova, M., Dvorak, J. & Mraz, J. (1996) Spontaneous
motor activity in rats enhanced by 1,2,4-triazole derivatives:
Triadimefon, triadimenol and biteranol. Homeostasis, 37, 3.
Hatanaka, J., Asaoka, S., Ikeda, M., Shimizu, M., Inukai, H.,
Iyatomy, A. & Itoh, H. (1981) KWG 0599: Short-term toxicity tests on
rats (4-week feeding and 4-week recovery tests). Unpublished report
No. 195 from Nihon Tokushu Noyaku Seizo K.K. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. (1981) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983a) Determination of acute toxicity (LD50) -- rat
p.o. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983b) Determination of acute toxicity (LD50): BUE
2684 (Bitertanol-benzoic acid)--rat p.o. Unpublished report from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50): KWG
0599. Unpublished report from Bayer AG, Leverkusen. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1984b) Dodine & KWG 0599 (c.n. Dodine & Bitertanol
(proposed)): Combination toxicity study. Unpublished report No. 12608
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Vogel, O. (1984) KWG 0599 (c.n. Bitertanol): Subacute
study of dermal toxicity to rabbits. Unpublished report No. 12571 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978a) KWG 0599: Micronucleus test on mouse to evaluate
KWG 0599 for potential mutagenic effects. Unpublished report No. 7860
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B. (1978b) KWG 0599: Dominant lethal study on male mouse to
test for mutagenic effects. Unpublished report No. 7964 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1979) KWG 0599: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished report No. 8152 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1983) BUE 2684: Bitertanol-benzoic acid:
Salmonella/microsome test to evaluate for point mutation. Unpublished
report No. 12087 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hixson, E.J. (1979) Baycor technical: Acute dermal toxicity to
rabbits. Unpublished report No. 57 from Mobay Chemical Corporation,
Staneley Research Center. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hixson, E.J. (1984) Dermal absorption of 14C-Baycor applied as 50%
wettable powder. Unpublished report No. 484 from Mobay Chemical
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1977) KWG 0599: Evaluation for sensitization.
Unpublished report from Bayer AG, Leverkusen. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1981a) KWG 0599: Determination of the acute toxicity to
the dog after oral administration. Unpublished report No. T 6001023
from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1981b) KWG 0599/012: Acute oral toxicity to sheep.
Unpublished report No. 9768 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. & Gröning, P. (1983) KWG 0599 (c.n. Bitertanol): Chronic
oral toxicity study on dogs (2-year feeding experiment). Unpublished
report No. 12307 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Hoffmann, K. & Schilde, B. (1979) KWG 0599: Subchronic toxicity study
on dogs (thirteen-week administration in capsules). Unpublished report
No. 8053 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Hoffmann, K. & Vogel, O. (1983) KWG 0599 (Bitertanol): Second chronic
toxicity study with dogs on oral administration (feeding study, 0-3-25
ppm 12 months, 200 ppm 20 months). Unpublished report No. 12328 from
Bayer AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Iyatomy, A. (1980) KWG 0599: Report of acute toxicity. Unpublished
report No. Sheet A-31 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Iyatomy, A. (1981) Report of acute toxicity: KWG 0599. Unpublished
report No. Sheet 22 from Nitokuno Agricultural Chemicals Institute,
Tokyo. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986a) Effect of bitertanol on spontaneous motor activity
in mice; Part 1. The experiment on the acute effect. Unpublished
report No. DT-1 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kaneto, H. (1986b) Effect of bitertanol on spontaneous motor activity
in mice; Part 2. The experiment on the subacute effect. Unpublished
report No. DT-2 from Department of Pharmacology, Nagasaki University.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Klein, O. (1988a) [U-phenyl-14C]-Bitertanol: Absorption, excretion,
tissue-distribution, and biokinetic profile of the total radioactivity
in the rat following single oral and intraduodenal dosage. Unpublished
report No. PF3076 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Klein, O. (1988b) [U-phenyl-14C]-Bitertanol: Distribution of the
parent compound in the excreta and in some organs at different times
following single oral administration to rats. Unpublished report No.
PF3096 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Klein, O. (1989) [U-phenyl-14C]-Bitertanol: Distribution of the
metabolites in some organs at different times following single oral
administration to rats. Unpublished report No. PF3501 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Krötlinger, F., Bomhard, E., Löser, E. & Schilde, B. (1978 ) B. KWG
0599: Subchronic toxicity study on rats (three-month feeding
experiment). Unpublished report No. 8002 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Löser, E. & Eiben, R. (1981) KWG 0599: Multigeneration reproduction
study on rats. Unpublished report No. 10024 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. & Thyssen, J. (1979) KWG 0599 (Baycor active ingredient):
Evaluation for embryotoxic and teratogenic effects on rats after
dynamic inhalational exposure. Unpublished report No. 8610 from Bayer
AG, Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Machemer, L. (1977) KWG 0599: Evaluation for embryotoxic and
teratogenic effects on rats after oral administration. Unpublished
report No. 6697 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978a) Determination of acute toxicity (LD50) / KWG 0599
Form A: PFU 190978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1978b) Determination of acute toxicity (LD50) / KWG 0599
Form B: PFU 210978. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982a) KWG 0599 and captan: Study for acute combination
toxicity. Unpublished report No. 11205 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. (1982b) KWG 0519 and KWG 0599: Study for acute combination
toxicity. Unpublished report No. 11203 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Kimmerle, G. (1977) KWG 0599: Subacute inhalation
toxicity study on rats. Unpublished report No. 6868 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Mihail, F. & Luckhaus, G. (1985) KWG 0599 (c.n. Bitertanol): Subacute
oral toxicity study with special attention to effect on the liver.
Unpublished report No. 13230 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Miksche, L. (1981) Data on effects on humans/In-company occupational
medical experience. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Nagumo, K., Teraki, Y., Chiba, T. & Miyasaka, M. (1981, revised 1987)
Teratogenicity test of KWG 0599 in pregnant rats. Unpublished report
from St Marianna University School of Medicine. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Pauluhn, J., Thyssen, J. & Vogel, O. (1983) KWG 0599 (Baycor active
ingredient) (c.n. Bitertanol): Study for cataract-inducing effect on
subacute whole-body exposure with cats (exposure 20 × 6 hours).
Unpublished report No. 12006 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Polacek, I. (1983) Central nervous effects of KWG 0599 (pilot study).
Unpublished report No. R2694 from Toxikologisches Institut Regensburg.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with 1H-
1,2,4-triazole. Unpublished report No. R4859 from Cytotest Cell
Research GmbH & Co. KG. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Puhl, R.J. & Hurley, J.B. (1983) The absorption, excretion, and
metabolism of Baycor-phenyl-UL-14C by rats. Unpublished report No.
85832 from Mobay Chemical Corporation, Agricultural Chemicals
Division. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) 1,2,4-triazole: Investigations into embryotoxic
effects on rats after oral administration. Unpublished report No.
17401 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Renhof, M. (1988b) 1,2,4-triazole: Investigation into embryotoxic
effects on rats after oral administration. Supplement to study No.
T5019339. Unpublished report No. 17402 from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany
Riach, C.G. (1981) KWG 0599: Testing the modification of cellular DNA
in Escherichia coli. Unpublished report No. R2013 from Inveresk
Research International, Musselburgh, Scotland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Roetz, R. (1982) KWG 0599 (Bitertanol, the active ingredient of
Baycor): Study of embryotoxic (and teratogenic) effects on rabbits
after oral administration. Unpublished report No. 10979 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sasaki, Y.F.X. (1987) Bitertanol: In vitro cytogenetics test.
Unpublished report No. DT-5 from The Institute of Environmental
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Schlüter, G. (1983) KWG 0599: Study to determine embryotoxic and
teratogenic effects on rabbits following oral administration.
Unpublished report No. 11548 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987) Acute inhalation toxicity study with Baycor
technical dust in Sprague-Dawley rats. Unpublished report No. 837 from
Mobay Corporation, Staneley Research Center, Report. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Shirasu, Y., Moriya, M. & Ohta, T. (1981) Bitertanol: Microbial
mutagenicity study. Unpublished report from Department of Toxicology,
Institute of Environmental Toxicology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977) KWG 0599: Intracutaneous allergy test on guinea
pigs. Unpublished report No. 7113 from Bayer AG, Leverkusen. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kaliner, G. (1977) KWG 0599: Subacute oral cumulative
toxicity study on rats (four-week treatment). Unpublished report No.
7153 from Bayer AG, Leverkusen. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole: Occupational
toxicology study. Unpublished report No. 5926 from Bayer AG,
Leverkusen. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977a) KWG 0599: Acute toxicity studies.
Unpublished report No. 6546 from Bayer AG, Leverkusen. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1977b) KWG 0599: Subacute inhalational
toxicity study on dogs. Unpublished report from Bayer AG, Leverkusen.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1978)
1-(Triazol-1-yl)-1-(4'-phenylphenoxy)-3,3-dimethylbutan-2-one (third
stage KWG 0599, BUE 1662): Industrial toxicology study. Unpublished
report No. 7242 from Bayer AG, Leverkusen. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Vergieva, T. (1990) Triazoles teratogenicity in rats. Teratology,
42, 27A-28A.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Yonemura, H., Shimazaki, M., Yamazaki, K., Takahashi, Y. & Hashizume,
M. (1981) Subacute toxicity tests with Bitertanol (KWG 0599) in rats.
Unpublished report from Research Institute for Animal Science in
Biochemistry and Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
