
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
BITERTANOL
Explanation
Date for the estimation of an acceptable daily intake for
bitertanol were evaluated by the 1983 Joint Meeting which estimated a
temporary acceptable daily intake. This evaluation considers residues
in food and gives details of the identity of the compound.
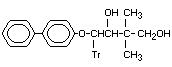
Chemical name: all-rac-1-(biphenyl-4-yloxy)-3,3-
dimethyl-1-(1H-1,2,4-triazol-
1-yl)butan-2-ol
Registry number [55179-31-2]
Synonyms: Baycor, Sibutol
Structural formula: KWG 0599
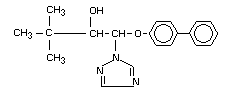 Empirical formula: C20H23N3O2
Molecular weight: 337.4
RESIDUES IN FOOD
USE PATTERN
Bitertanol is a broad-spectrum fungicide said to be effective for
the control of scab and Monilinia fruit diseases, banana and peanut
leaf spot diseases, and rusts and mildews on a variety of crops. It is
available as a wettable powder, emulsifiable concentrate and
suspension concentrate, all applied by spraying. It is also used as a
seed treatment for cereals. Only the wettable powder is recommended
for pome fruit owing to possible foliage or fruit injury by the EC
formulation.
Bitertanol is said to be registered and marketed in over 20
countries in Europe, Africa and South America and registration is
being sought in additional countries. Recommendations by geographical
areas are listed in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue field trials have been conducted on a variety of crops,
primarily fruits and vegetables. Data provided to the JMPR are
summarized in Table 2.
Pome Fruit
Residues trials in apples have been conducted in four countries,
although according to Table 1 bitertanol is currently approved for use
in only one of the four. The trial in this country reflects European
uses. Where reported, controls were <0.01 mg/kg. With maximum
residues of 1.0 mg/kg at the recommended 14-day interval, these data
and the supporting data from other countries indicate that a 2 mg/kg
limit would be required with a 14-day pre-harvest interval. Additional
data are desirable from countries with approved uses.
Cucumber
Trials on cucumbers (under glass) were available from one country
with WP application at approximately the approved rate and EC at twice
the approved rate. Maximum residues were 0.41 mg/kg at the normal
harvest interval. Residues from the two EC applications were similar
to those from six with the WP at similar intervals. These limited data
indicate a possible need for a 0.5 mg/kg limit at a 3-day pre-harvest
interval, but are not sufficient to support a limit.
Peanuts
In trials in one country reflecting approved application rates
(but excessive numbers of applications) no residues were detected
(<0.1 mg/kg) 9-14 days after the last application. Analyses were
apparently on shelled nuts. Data are not sufficient to support a
limit.
Beans
Data were available from trials in three countries, but approved
use information was available from only one of these. From the country
with approved uses residues in dry bean pods at 16 days (28-day safety
interval required) were 0.1 - 0.18 mg/kg at the normal application
rate. This compares with 0.1 mg/kg in untreated controls. Similar
application rates in South America resulted in <0.05 mg/kg residues
(3 samples) at 26-43 days post-treatment, but it is not clear whether
the beans were shelled or in pod. Data are insufficient to support a
limit.
Table 1. Use Pattern - spray treatment
Region and Number of Safety interval,
Commodity g a.i./ha treatments days
Europe
Pome fruit 1, 2 187 - 375 6 - 12 14 - 42
Stone fruit 375 - 562 2 - 3 *
Snake gourd )
(Cucumber ) under
Peppers ) glass 9003 2 - 6 3
Melons )
South Africa
Peanuts 195 - 240 2 - 4 as dry feed 42
Stone fruit 140 - 240 2 - 3 28
Beans 126 - 250** 2 (as dry feed 42)
Asia
Bananas 125 - 150 26
South America
Bananas 125 18 - 26
* Not later than at the end of flowering stage; following this application no residues are
expected.
** aerial application
1 Registration (pre-harvest WP on apples) at 0.05 - 1.0% and a 28-day PHI is under way in
Finland.
2 Netherlands - 25 WP at 50-80 g a.i./100 1 and 300EC at 22.5 g/l. Both 14-day pre-harvest
interval.
3 300 EC in The Netherlands.
Seed treatment - Federal Republic of Germany
Wheat 75 g a.i./100 kg seed
Rye 56 g a.i./100 kg seed
Oat 56 g a.i./100 kg seed
Table 2. Bitertanol residues in crops resulting from supervised trials
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Pome fruit Bayer
Apples 187 - 375 g/ha 10-12 FRG 14 0.13 - 1.8 0.65 (6) 1 10303/79
(25% WP) (1979-1982) 21 0.12 - 1.0 0.43 (6) 10305/79
10313/80
10325/80
10304-5/82
Mobay
1-4 oz/100 gal. 6-14 USA 0 0.53 - 2.54 1.62 (5) 64382+83
(50% WP) (1979) 14 0.24 - 1.5 0.9 (5)
21 0.18 - 1.2 0.8 (4)
28 0.27 - 0.91 0.6 (4)
1-2 oz/100 gal. 5-8 Canada 66-67 0.06 - 0.10 0.07 (4) Mobay
(50% WP) (1983) 74-92 < 0.01 - 0.04 0.02 (12) 68282-86
0.8 g/tree 4 Finland Siltanen
(1979) 32 0.2 (1) and Makinen
(1980= 49 0.1 1980
5 (1981) 67 0.04 Siltanen
6 35 0.1 and Makinen
8 24 0.4 1981
Cucumbers 875 - 1200 g/ha 2(EC) NL 0- 1 0.4 - 0.7 0.54 (4) Bayer
(Snake gourds, (200 g/1 EC or 6(WP) (1979-1980) 3- 4 0.25 - 0.41 0.34 (3) 10326/79
under glass) 50% WP) 7-10 0.06 - 0.25 0.15 (5) 10314-15/80
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Peanuts 250 - 300 g/ha 4-5 S.Africa 9-14 < 0.1 < 0.1 (3) Bayer
(Shelled?) (300 g/L EC or (1979-1983) 10301/77
25% WP) 10313/78
10328/80
Beans 225 - 250 g/ha 4 Costa 26 0.05 0.05 (1) Bayer
(mature, dry) (300 EC) Rica 37 0.05 0.05 (1) 10332-33/80
Columbia 43 0.05 0.05 (1) 10342/81
(1981)
Beans 125 g a.i./ha S. Africa S. Africa
(dry) (300 EC) (not avail.) submission
whole plt. 1 1 23 : 30
4 2.5 : 3.4
8 3.7 : 3.8
16 0.36 : 0.3
250 g a.i./ha 1 43 : 39.4
(300 EC; 4 6 : 6.8
double rate) 8 4.5 : 5.0
16 0.3 : 0.32
pods 125 g a.i. /ha 1 11 sic? 2.7 : 2.4
(300 EC) 4 0.13: 9.19
8 0.15: 0.13
16 0.18: 0.10
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
250 g a.i./ha 11? 3.2 : 2.9
(300 EC; 4 0.16: 0.1
double rate) 8 0.4 : 0.4
16 0.23: 0.21
A B
Stone fruit A
Nectarines 100- 100 g/ha 4 S.Africa 0 0.53 - 1.5 SABS
(300 EC) (1982) 3 0.47 - 0.68 (1)* 311/88419
data: 10 0.21 - 0.54 (1) W 212
A = 0.125% a.i. 14 0.18 - 0.51
B = 0.25% a.i. 24 0.18 - 0.37 (1)
Peaches 100 - 200 g/ha 3 S. Africa 0 0.6 - 1.8 (1)* SABS
(300 EC) (1982-1983) 5 0.4 - 1.2 (1)* 311/88421/
data: 11 0.3 - - (1)* W214
A = 0.125% a.i. 19 0.2 - 0.5 (1)*
B = 0.25% a.i. 26 < 0.1 - - (1)*
ripe 375 g/ha 3 France 0 1.7
(25& WP) (1979) 7 1.2
14 1.3
21 0.91
300 & 400 g/ha 3 Italy 0 0.8 - 1.2(1)* (3.5cm fruit) Bayer
(300 EC) (1981) 7 0.3 - 0.4(1)* 10346-47/81
14 0.2 - 0.2(1)*
20 0.11- 0.13(1)* (5-7cmfruit)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Plums 250 - 370 g/ha 1 S. Africa 0 0.7 - 0.8(1)*
(300 EC) (1982) 3 0.8 - 0.8(1)*
data: 9 0.6 - 0.5(1)*
A = 0.025% a.i. 16 0.5 - 0.6(1)*
B = 0.0375% a.i.
Bananas 120 - 300 g/ha 6 - 26 Costa Rica Bayer
unbagged (EC 200-300; Columbia 2 0.015 0.015(1) 10309-11/78
pulp WP 25%)
Taiwan 4 ND ND (1) 10320-21/80
Kamerun 8 0.014-0.019 0.017(2) 10330-31/80
(1978-1980) 23 < 0.1 < 0.1 (1) 10350-80
40 ND ND (1) 10344-45/81
78 ND ND (1) 10366/81
peel 2 0.14 0.14(1)
4 ND ND (1)
8 0.12 - 0.15 0.14(2)
23 0.025 0.025(1)
40 ND ND (1)
78 ND ND (1)
total (calculated) 2 0.06 0.06(1)
4 ND ND (1)
8 0.047-0.055 0.051 (2)
23 < 0.01 < 0.01 (1)
40 ND ND (1)
78 ND ND (1)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged 0 ND ND (2)
pulp 2 ND ND (1)
6 ND ND (1)
8 < 0.01 < 0.01 (2)
11 ND ND (2)
12 ND ND (1)
peel 0 ND ND (2)
2 0.019 0.019 (1)
6 0.1 0.1 (1)
11 ND ND (2)
12 ND ND (1)
Total (calculated) 0 ND
2 < 0.01
6 < 0.01
8 < 0.01-0.015
11 ND
12 ND
Bananas, green 125 - 300 g/ha 9 - 12 Honduras 0/3 Mobay
unbagged, (300 EC) or Costa 68896-900
unwashed 25 or 50% WP) Rica - 80469-473
pulp (1980-1982 0.03-0.13 0.07(6)
peel 0.10-0.76 0.39(6)
total 0.06-0.33 0.19(6)
unbagged, washed pulp 0.03-0.17 0.09(5)
peel 0.09-0.73 0.37(5)
total 0.05-0.36 0.19(5)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, unwashed
pulp < 0.01-0.01 < 0.01(4)
peel < 0.01-0.05 0.03(4)
total < 0.01-0.03 0.02(4)
bagged, washed
pulp < 0.01-0.02 0.01(5)
peel 0.03-0.15 0.06(5)
total 0.02-0.06 0.03(5)
Bananas, ripe
unbagged, unwashed
pulp < 0.01-0.08 0.05(3)
peel 0.03-0.32 0.19(3)
total 0.01-0.17 0.10(3)
Bananas, ripe
unbagged, unwashed pulp 0.02-0.14 0.09(3)
peel 0.05-0.51 0.34(3)
total 0.03-0.28 0.18(3)
Bananas, ripe
bagged-unwashed
pulp < 0.01-0.01 < 0.01(3)
peel < 0.01-0.12 0.05(3)
total < 0.01-0.05 0.02(3)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, washed
pulp < 0.01-0.02 0.02(3)
peel 0.03-0.08 0.05(3)
total 0.01-0.04 0.03(3)
Oats, Green forage 56 g/100 kg seed 1 FRG 94/97 < 0.05 < 0.05(2) Bayer
Grain (375 FS) (1983) 140-141 < 0.05 < 0.05(2) 10212+13/83
Straw 140/I41 < 0.05 < 0.05(2)
Rye, Green Forage 56 ml/100 kg seed 1 FRG 220/224 < 0.05 < 0.05(2) 10210+11/83
Grain (375 FS) (1983) 291/295 < 0.05 < 0.05(2)
Straw 291/295 < 0.05 < 0.05(2)
Wheat, Green Forage 75 ml (g)/100 kg seed 1 FRG 56-77 < 0.1 < 0.1 (7) 10341+43/80
Ears (1980-1982) 73-90 < 0.05-0.1 < 0.05-0.1(5) 10370+71/80
Grain q (375 FS) 130-154 < 0.05-0.1 < 0.05-0.1(5)
Straw 87-154 < 0.05-0.1 < 0.05-0.1(7)
Oats, Green Forage 200g product/100 1 FRG 63-86 < 0.1 < 0.1 (1) 10366+67/82
Ears kg seed (0.132 (1982) 75-86 < 0.1 < 0.1 (2)
Grain q kg a.i./ha) 131-139 < 0.05 < 0.05(2)
Straw (37.5% DS) 131-139 < 0.05 < 0.05(2)
Rye Green Forage 0.08-0.11 kg 1 FRG 219-254 < 0.1 < 0.1 (7) 10363-65/82
Ears a.i./ha(150 g (1981-1982) 228-254 < 0.1 < 0.1 (4) 10372-82
Grain production/100 kg - 291-308 < 0.05 < 0.05(4)
Straw seed) (37.5 or 291-308 < 0.05 < 0.05 (4)
3.75 DS)
1 Number in parenthesis = number of samples
* One sample per dose level (mean of duplicate analyses)
Stone Fruit
Data were available from three countries, all of which have
approved uses, or are in close geographical proximity to countries
with them. While European trials on peaches reflect recommended
European dosage rates, applications were apparently made after the end
of flowering, and therefore do not appear to reflect the recommended
time of application (Table 1) for Europe. South African trials also
reflect recommended application rates in that country, but the pre-
harvest interval was not available to the meeting. The only available
PHI for stone fruit was 21 days for cherries in one European country
(see "National maximum residue limits". A 1 mg/kg limit for stone
fruit can be supported at a 21-day pre-harvest interval. Additional
residue data and good agricultural practice information for stone
fruit are desirable, especially for small fruit such as cherries.
Residues on peaches were significantly more persistent from WP
applications than from EC formulations.
Bananas
Supervised trials data were available for three Asian and three
South American countries and generally reflected the application rates
and number of applications (WP or EC) recommended in those
geographical areas. Since no national pre-harvest intervals were
provided, it must be assumed that application on the day of harvest,
as included in the trials, reflects practice. Data were available for
both green and ripe, bagged and unbagged, and washed and unwashed
bananas and on pulp and peel. In general, average residues were
approximately 5-10 times higher in peel than in pulp, whether ripe or
green, bagged or unbagged, washed or unwashed. As might be expected,
residues were higher on unbagged fruit. Washing (in the field) does
not appear to affect average residues significantly.
Maximum residues on bagged bananas (whole, green, washed) were
0.06 mg/kg. Maximum residues on whole ripe, unbagged, washed bananas
were 0.28 mg/kg as compared to 0.36 mg/kg for green, unbagged, washed
bananas.
Untreated controls (peel, pulp or whole) were all <0.01 mg/kg. A 0.5
mg/kg limit was estimated.
Cereal crops
No residues of bitertanol were detected in the green forage,
ears, grain or straw of oats, rye or wheat when the seeds were treated
pre-plant with recommended rates. The limit of determination ranged
from 0.05 - 0.1 mg/kg among the 15 studies. The available data would
support a limit of 0.1 mg/kg (at the limit of determination) limit for
seed treatments only. Additional data from other countries with
approved uses for cereal grains are desirable.
FATE OF RESIDUES
General
The fate of bitertanol has been studied in animals, plants, soil
water and light. A proposed metabolic pathway is given in Figure 1.
The structures and chemical names are presented in Table 3 which also
indicates where the compounds have been identified. Although the
metabolic picture is somewhat limited in scope, the data in general
demonstrate that metabolism in animals is much more extensive than
degradation by other routes. Bitertanol is the predominant residue in
plants. Bitertanol and p-hydroxybitertanol are the major residues in
animals.
In plants
Uptake and translocation. Golden Delicious variety apples were
individually syringe-treated to run-off with a 50% wettable powder
formulation at 0.15 g a.i./l of 14C-bitertanol labelled
uniformly (UL) in the biphenyl ring. Sampling was immediately after
drying, at 3 and 7-days and thereafter at 7-day intervals through 49
days (Phul and Hurley, 1979c). Analysis was by TLC using
radiochromatogram scanning and autoradiography techniques. The peel
contained 95% of the total recovered radioactivity even after 49 days,
the rest being in the pulp, showing that little migration had
occurred. Surface residues decreased from 92% at day 0 to 43% at 49
days while the radioactivity in the peel organic extract increased
from 7 to 43.5% of the total. Peel solids increased to 12% in the same
interval.
The two diasterioisomers of bitertanol were the major residues,
in roughly equal proportions throughout the 49-day study. The level of
the two combined in peel and pulp ranged from 98.4% of the total
radioactivity at day 0 to 83% at 49 days, with lower surface residues
and greater peel uptake by the end of that time. The two had
penetrated the pulp by day 7 from which time residues were relatively
constant at about 3% over the remaining test period. Minor metabolites
were bitertanol ketone and 4-hydroxy-biphenyl, together never
exceeding 3% of the total radioactivity. All of these metabolites were
in the peel. The half-life of bitertanol in this experiment was
estimated at approximately 150 days.
The metabolism of bitertanol by peanut plants has also been
studied (Phul and Hurley, 1981). In preliminary studies, the authors
obtained evidence that foliar applications result in poor leaf
absorption (96% unchanged bitertanol on the leaf surface after 14
days), with no observable volatility losses. Only very little
translocation (acro- and basi-petal) was observed, as well as little
translocation to opposite leaves Bitertanol - 14C uniformly labelled
in the biphenyl ring was sprayed as a 50% WP formulation to both young
(1 mo.) and old (2 mo.) potted peanut plants at an approximate
equivalent field rate of 0.5 kg a.i./ha. Young plants were sampled at
Empirical formula: C20H23N3O2
Molecular weight: 337.4
RESIDUES IN FOOD
USE PATTERN
Bitertanol is a broad-spectrum fungicide said to be effective for
the control of scab and Monilinia fruit diseases, banana and peanut
leaf spot diseases, and rusts and mildews on a variety of crops. It is
available as a wettable powder, emulsifiable concentrate and
suspension concentrate, all applied by spraying. It is also used as a
seed treatment for cereals. Only the wettable powder is recommended
for pome fruit owing to possible foliage or fruit injury by the EC
formulation.
Bitertanol is said to be registered and marketed in over 20
countries in Europe, Africa and South America and registration is
being sought in additional countries. Recommendations by geographical
areas are listed in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue field trials have been conducted on a variety of crops,
primarily fruits and vegetables. Data provided to the JMPR are
summarized in Table 2.
Pome Fruit
Residues trials in apples have been conducted in four countries,
although according to Table 1 bitertanol is currently approved for use
in only one of the four. The trial in this country reflects European
uses. Where reported, controls were <0.01 mg/kg. With maximum
residues of 1.0 mg/kg at the recommended 14-day interval, these data
and the supporting data from other countries indicate that a 2 mg/kg
limit would be required with a 14-day pre-harvest interval. Additional
data are desirable from countries with approved uses.
Cucumber
Trials on cucumbers (under glass) were available from one country
with WP application at approximately the approved rate and EC at twice
the approved rate. Maximum residues were 0.41 mg/kg at the normal
harvest interval. Residues from the two EC applications were similar
to those from six with the WP at similar intervals. These limited data
indicate a possible need for a 0.5 mg/kg limit at a 3-day pre-harvest
interval, but are not sufficient to support a limit.
Peanuts
In trials in one country reflecting approved application rates
(but excessive numbers of applications) no residues were detected
(<0.1 mg/kg) 9-14 days after the last application. Analyses were
apparently on shelled nuts. Data are not sufficient to support a
limit.
Beans
Data were available from trials in three countries, but approved
use information was available from only one of these. From the country
with approved uses residues in dry bean pods at 16 days (28-day safety
interval required) were 0.1 - 0.18 mg/kg at the normal application
rate. This compares with 0.1 mg/kg in untreated controls. Similar
application rates in South America resulted in <0.05 mg/kg residues
(3 samples) at 26-43 days post-treatment, but it is not clear whether
the beans were shelled or in pod. Data are insufficient to support a
limit.
Table 1. Use Pattern - spray treatment
Region and Number of Safety interval,
Commodity g a.i./ha treatments days
Europe
Pome fruit 1, 2 187 - 375 6 - 12 14 - 42
Stone fruit 375 - 562 2 - 3 *
Snake gourd )
(Cucumber ) under
Peppers ) glass 9003 2 - 6 3
Melons )
South Africa
Peanuts 195 - 240 2 - 4 as dry feed 42
Stone fruit 140 - 240 2 - 3 28
Beans 126 - 250** 2 (as dry feed 42)
Asia
Bananas 125 - 150 26
South America
Bananas 125 18 - 26
* Not later than at the end of flowering stage; following this application no residues are
expected.
** aerial application
1 Registration (pre-harvest WP on apples) at 0.05 - 1.0% and a 28-day PHI is under way in
Finland.
2 Netherlands - 25 WP at 50-80 g a.i./100 1 and 300EC at 22.5 g/l. Both 14-day pre-harvest
interval.
3 300 EC in The Netherlands.
Seed treatment - Federal Republic of Germany
Wheat 75 g a.i./100 kg seed
Rye 56 g a.i./100 kg seed
Oat 56 g a.i./100 kg seed
Table 2. Bitertanol residues in crops resulting from supervised trials
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Pome fruit Bayer
Apples 187 - 375 g/ha 10-12 FRG 14 0.13 - 1.8 0.65 (6) 1 10303/79
(25% WP) (1979-1982) 21 0.12 - 1.0 0.43 (6) 10305/79
10313/80
10325/80
10304-5/82
Mobay
1-4 oz/100 gal. 6-14 USA 0 0.53 - 2.54 1.62 (5) 64382+83
(50% WP) (1979) 14 0.24 - 1.5 0.9 (5)
21 0.18 - 1.2 0.8 (4)
28 0.27 - 0.91 0.6 (4)
1-2 oz/100 gal. 5-8 Canada 66-67 0.06 - 0.10 0.07 (4) Mobay
(50% WP) (1983) 74-92 < 0.01 - 0.04 0.02 (12) 68282-86
0.8 g/tree 4 Finland Siltanen
(1979) 32 0.2 (1) and Makinen
(1980= 49 0.1 1980
5 (1981) 67 0.04 Siltanen
6 35 0.1 and Makinen
8 24 0.4 1981
Cucumbers 875 - 1200 g/ha 2(EC) NL 0- 1 0.4 - 0.7 0.54 (4) Bayer
(Snake gourds, (200 g/1 EC or 6(WP) (1979-1980) 3- 4 0.25 - 0.41 0.34 (3) 10326/79
under glass) 50% WP) 7-10 0.06 - 0.25 0.15 (5) 10314-15/80
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Peanuts 250 - 300 g/ha 4-5 S.Africa 9-14 < 0.1 < 0.1 (3) Bayer
(Shelled?) (300 g/L EC or (1979-1983) 10301/77
25% WP) 10313/78
10328/80
Beans 225 - 250 g/ha 4 Costa 26 0.05 0.05 (1) Bayer
(mature, dry) (300 EC) Rica 37 0.05 0.05 (1) 10332-33/80
Columbia 43 0.05 0.05 (1) 10342/81
(1981)
Beans 125 g a.i./ha S. Africa S. Africa
(dry) (300 EC) (not avail.) submission
whole plt. 1 1 23 : 30
4 2.5 : 3.4
8 3.7 : 3.8
16 0.36 : 0.3
250 g a.i./ha 1 43 : 39.4
(300 EC; 4 6 : 6.8
double rate) 8 4.5 : 5.0
16 0.3 : 0.32
pods 125 g a.i. /ha 1 11 sic? 2.7 : 2.4
(300 EC) 4 0.13: 9.19
8 0.15: 0.13
16 0.18: 0.10
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
250 g a.i./ha 11? 3.2 : 2.9
(300 EC; 4 0.16: 0.1
double rate) 8 0.4 : 0.4
16 0.23: 0.21
A B
Stone fruit A
Nectarines 100- 100 g/ha 4 S.Africa 0 0.53 - 1.5 SABS
(300 EC) (1982) 3 0.47 - 0.68 (1)* 311/88419
data: 10 0.21 - 0.54 (1) W 212
A = 0.125% a.i. 14 0.18 - 0.51
B = 0.25% a.i. 24 0.18 - 0.37 (1)
Peaches 100 - 200 g/ha 3 S. Africa 0 0.6 - 1.8 (1)* SABS
(300 EC) (1982-1983) 5 0.4 - 1.2 (1)* 311/88421/
data: 11 0.3 - - (1)* W214
A = 0.125% a.i. 19 0.2 - 0.5 (1)*
B = 0.25% a.i. 26 < 0.1 - - (1)*
ripe 375 g/ha 3 France 0 1.7
(25& WP) (1979) 7 1.2
14 1.3
21 0.91
300 & 400 g/ha 3 Italy 0 0.8 - 1.2(1)* (3.5cm fruit) Bayer
(300 EC) (1981) 7 0.3 - 0.4(1)* 10346-47/81
14 0.2 - 0.2(1)*
20 0.11- 0.13(1)* (5-7cmfruit)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Plums 250 - 370 g/ha 1 S. Africa 0 0.7 - 0.8(1)*
(300 EC) (1982) 3 0.8 - 0.8(1)*
data: 9 0.6 - 0.5(1)*
A = 0.025% a.i. 16 0.5 - 0.6(1)*
B = 0.0375% a.i.
Bananas 120 - 300 g/ha 6 - 26 Costa Rica Bayer
unbagged (EC 200-300; Columbia 2 0.015 0.015(1) 10309-11/78
pulp WP 25%)
Taiwan 4 ND ND (1) 10320-21/80
Kamerun 8 0.014-0.019 0.017(2) 10330-31/80
(1978-1980) 23 < 0.1 < 0.1 (1) 10350-80
40 ND ND (1) 10344-45/81
78 ND ND (1) 10366/81
peel 2 0.14 0.14(1)
4 ND ND (1)
8 0.12 - 0.15 0.14(2)
23 0.025 0.025(1)
40 ND ND (1)
78 ND ND (1)
total (calculated) 2 0.06 0.06(1)
4 ND ND (1)
8 0.047-0.055 0.051 (2)
23 < 0.01 < 0.01 (1)
40 ND ND (1)
78 ND ND (1)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged 0 ND ND (2)
pulp 2 ND ND (1)
6 ND ND (1)
8 < 0.01 < 0.01 (2)
11 ND ND (2)
12 ND ND (1)
peel 0 ND ND (2)
2 0.019 0.019 (1)
6 0.1 0.1 (1)
11 ND ND (2)
12 ND ND (1)
Total (calculated) 0 ND
2 < 0.01
6 < 0.01
8 < 0.01-0.015
11 ND
12 ND
Bananas, green 125 - 300 g/ha 9 - 12 Honduras 0/3 Mobay
unbagged, (300 EC) or Costa 68896-900
unwashed 25 or 50% WP) Rica - 80469-473
pulp (1980-1982 0.03-0.13 0.07(6)
peel 0.10-0.76 0.39(6)
total 0.06-0.33 0.19(6)
unbagged, washed pulp 0.03-0.17 0.09(5)
peel 0.09-0.73 0.37(5)
total 0.05-0.36 0.19(5)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, unwashed
pulp < 0.01-0.01 < 0.01(4)
peel < 0.01-0.05 0.03(4)
total < 0.01-0.03 0.02(4)
bagged, washed
pulp < 0.01-0.02 0.01(5)
peel 0.03-0.15 0.06(5)
total 0.02-0.06 0.03(5)
Bananas, ripe
unbagged, unwashed
pulp < 0.01-0.08 0.05(3)
peel 0.03-0.32 0.19(3)
total 0.01-0.17 0.10(3)
Bananas, ripe
unbagged, unwashed pulp 0.02-0.14 0.09(3)
peel 0.05-0.51 0.34(3)
total 0.03-0.28 0.18(3)
Bananas, ripe
bagged-unwashed
pulp < 0.01-0.01 < 0.01(3)
peel < 0.01-0.12 0.05(3)
total < 0.01-0.05 0.02(3)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, washed
pulp < 0.01-0.02 0.02(3)
peel 0.03-0.08 0.05(3)
total 0.01-0.04 0.03(3)
Oats, Green forage 56 g/100 kg seed 1 FRG 94/97 < 0.05 < 0.05(2) Bayer
Grain (375 FS) (1983) 140-141 < 0.05 < 0.05(2) 10212+13/83
Straw 140/I41 < 0.05 < 0.05(2)
Rye, Green Forage 56 ml/100 kg seed 1 FRG 220/224 < 0.05 < 0.05(2) 10210+11/83
Grain (375 FS) (1983) 291/295 < 0.05 < 0.05(2)
Straw 291/295 < 0.05 < 0.05(2)
Wheat, Green Forage 75 ml (g)/100 kg seed 1 FRG 56-77 < 0.1 < 0.1 (7) 10341+43/80
Ears (1980-1982) 73-90 < 0.05-0.1 < 0.05-0.1(5) 10370+71/80
Grain q (375 FS) 130-154 < 0.05-0.1 < 0.05-0.1(5)
Straw 87-154 < 0.05-0.1 < 0.05-0.1(7)
Oats, Green Forage 200g product/100 1 FRG 63-86 < 0.1 < 0.1 (1) 10366+67/82
Ears kg seed (0.132 (1982) 75-86 < 0.1 < 0.1 (2)
Grain q kg a.i./ha) 131-139 < 0.05 < 0.05(2)
Straw (37.5% DS) 131-139 < 0.05 < 0.05(2)
Rye Green Forage 0.08-0.11 kg 1 FRG 219-254 < 0.1 < 0.1 (7) 10363-65/82
Ears a.i./ha(150 g (1981-1982) 228-254 < 0.1 < 0.1 (4) 10372-82
Grain production/100 kg - 291-308 < 0.05 < 0.05(4)
Straw seed) (37.5 or 291-308 < 0.05 < 0.05 (4)
3.75 DS)
1 Number in parenthesis = number of samples
* One sample per dose level (mean of duplicate analyses)
Stone Fruit
Data were available from three countries, all of which have
approved uses, or are in close geographical proximity to countries
with them. While European trials on peaches reflect recommended
European dosage rates, applications were apparently made after the end
of flowering, and therefore do not appear to reflect the recommended
time of application (Table 1) for Europe. South African trials also
reflect recommended application rates in that country, but the pre-
harvest interval was not available to the meeting. The only available
PHI for stone fruit was 21 days for cherries in one European country
(see "National maximum residue limits". A 1 mg/kg limit for stone
fruit can be supported at a 21-day pre-harvest interval. Additional
residue data and good agricultural practice information for stone
fruit are desirable, especially for small fruit such as cherries.
Residues on peaches were significantly more persistent from WP
applications than from EC formulations.
Bananas
Supervised trials data were available for three Asian and three
South American countries and generally reflected the application rates
and number of applications (WP or EC) recommended in those
geographical areas. Since no national pre-harvest intervals were
provided, it must be assumed that application on the day of harvest,
as included in the trials, reflects practice. Data were available for
both green and ripe, bagged and unbagged, and washed and unwashed
bananas and on pulp and peel. In general, average residues were
approximately 5-10 times higher in peel than in pulp, whether ripe or
green, bagged or unbagged, washed or unwashed. As might be expected,
residues were higher on unbagged fruit. Washing (in the field) does
not appear to affect average residues significantly.
Maximum residues on bagged bananas (whole, green, washed) were
0.06 mg/kg. Maximum residues on whole ripe, unbagged, washed bananas
were 0.28 mg/kg as compared to 0.36 mg/kg for green, unbagged, washed
bananas.
Untreated controls (peel, pulp or whole) were all <0.01 mg/kg. A 0.5
mg/kg limit was estimated.
Cereal crops
No residues of bitertanol were detected in the green forage,
ears, grain or straw of oats, rye or wheat when the seeds were treated
pre-plant with recommended rates. The limit of determination ranged
from 0.05 - 0.1 mg/kg among the 15 studies. The available data would
support a limit of 0.1 mg/kg (at the limit of determination) limit for
seed treatments only. Additional data from other countries with
approved uses for cereal grains are desirable.
FATE OF RESIDUES
General
The fate of bitertanol has been studied in animals, plants, soil
water and light. A proposed metabolic pathway is given in Figure 1.
The structures and chemical names are presented in Table 3 which also
indicates where the compounds have been identified. Although the
metabolic picture is somewhat limited in scope, the data in general
demonstrate that metabolism in animals is much more extensive than
degradation by other routes. Bitertanol is the predominant residue in
plants. Bitertanol and p-hydroxybitertanol are the major residues in
animals.
In plants
Uptake and translocation. Golden Delicious variety apples were
individually syringe-treated to run-off with a 50% wettable powder
formulation at 0.15 g a.i./l of 14C-bitertanol labelled
uniformly (UL) in the biphenyl ring. Sampling was immediately after
drying, at 3 and 7-days and thereafter at 7-day intervals through 49
days (Phul and Hurley, 1979c). Analysis was by TLC using
radiochromatogram scanning and autoradiography techniques. The peel
contained 95% of the total recovered radioactivity even after 49 days,
the rest being in the pulp, showing that little migration had
occurred. Surface residues decreased from 92% at day 0 to 43% at 49
days while the radioactivity in the peel organic extract increased
from 7 to 43.5% of the total. Peel solids increased to 12% in the same
interval.
The two diasterioisomers of bitertanol were the major residues,
in roughly equal proportions throughout the 49-day study. The level of
the two combined in peel and pulp ranged from 98.4% of the total
radioactivity at day 0 to 83% at 49 days, with lower surface residues
and greater peel uptake by the end of that time. The two had
penetrated the pulp by day 7 from which time residues were relatively
constant at about 3% over the remaining test period. Minor metabolites
were bitertanol ketone and 4-hydroxy-biphenyl, together never
exceeding 3% of the total radioactivity. All of these metabolites were
in the peel. The half-life of bitertanol in this experiment was
estimated at approximately 150 days.
The metabolism of bitertanol by peanut plants has also been
studied (Phul and Hurley, 1981). In preliminary studies, the authors
obtained evidence that foliar applications result in poor leaf
absorption (96% unchanged bitertanol on the leaf surface after 14
days), with no observable volatility losses. Only very little
translocation (acro- and basi-petal) was observed, as well as little
translocation to opposite leaves Bitertanol - 14C uniformly labelled
in the biphenyl ring was sprayed as a 50% WP formulation to both young
(1 mo.) and old (2 mo.) potted peanut plants at an approximate
equivalent field rate of 0.5 kg a.i./ha. Young plants were sampled at
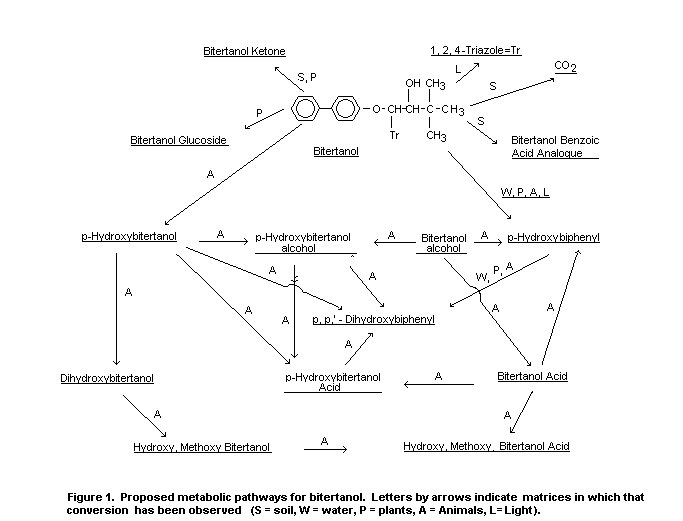 Table 3. Bitertanol, its degradation products and their occurrence a
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
Table 3. Bitertanol, its degradation products and their occurrence a
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
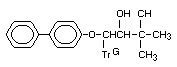 P-(1,1'-Biphenyl)-4-yloxy-o-(1,1- Bitertanol X X X X X
dimethytethyl)-1H-1.2.4-triazole-1-
ethanol. Bitertanol
P-(1,1'-Biphenyl)-4-yloxy-o-(1,1- Bitertanol X X X X X
dimethytethyl)-1H-1.2.4-triazole-1-
ethanol. Bitertanol
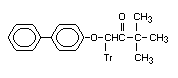 1-[1,1-Biphenyl]-4-yloxy),3,3- Bitertanol ketone X X
dimethyl-1-(1H-1,2,4-triazol-1-yl)- BUE 1662
2-butanone
1-(o)-3,3-dimethyl-1-(tr)butan-2-one
1-[1,1-Biphenyl]-4-yloxy),3,3- Bitertanol ketone X X
dimethyl-1-(1H-1,2,4-triazol-1-yl)- BUE 1662
2-butanone
1-(o)-3,3-dimethyl-1-(tr)butan-2-one
 4-(1,1'-Biphenyl)-4-yloxy)-2,2- Bitertanol alcohol
dimethyl-4-(1H-1,2,4-triazol-1-yl) KWG 1714 X
Note 1. 1,3-Butanodiol-1,3-butanediol
4-(o)-2,2.dimethyl-4-tr)butane-1,3-
diol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-(1,1'-Biphenyl)-4-yloxy)-2,2- Bitertanol alcohol
dimethyl-4-(1H-1,2,4-triazol-1-yl) KWG 1714 X
Note 1. 1,3-Butanodiol-1,3-butanediol
4-(o)-2,2.dimethyl-4-tr)butane-1,3-
diol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
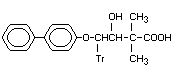 -([1,1'-Biphenyl]-4-yloxy)-B-hydroxy- Bitertanol acid X
dimethyl-1H-1,2,4-triazol-1-butanoic
acid4-(Biphenyl-4-yloxy)-3-hydroxy-
2,2-dimethyl-4,4-(1H-1,2,4.triazol.1.
1-yl)butyric acid
-([1,1'-Biphenyl]-4-yloxy)-B-hydroxy- Bitertanol acid X
dimethyl-1H-1,2,4-triazol-1-butanoic
acid4-(Biphenyl-4-yloxy)-3-hydroxy-
2,2-dimethyl-4,4-(1H-1,2,4.triazol.1.
1-yl)butyric acid
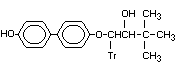 -(1,1-Dimethylethyl)- -(4'-hydroxy p-hydroxy-bitertanol X
-(1,1'-biphenyl]-4-yloxy)-1H-1,2,4-
triazole-1-ethanol
1-(4'-hydroxy o)-3,3-dimethyl-1-(tr)
butan-2-ol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-(1,1-Dimethylethyl)- -(4'-hydroxy p-hydroxy-bitertanol X
-(1,1'-biphenyl]-4-yloxy)-1H-1,2,4-
triazole-1-ethanol
1-(4'-hydroxy o)-3,3-dimethyl-1-(tr)
butan-2-ol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
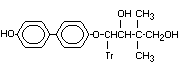 4-(4'Hydroxy-[1-1'-biphenyl]-4-yloxy) X
-2,2-dimethyl-4-((1H)-1,2,4-triazol-1-
yl)-1,3-butanediol
4-(4'-hydroxyo)-2,2-dimethyl-4-
(tr)butane.1,3.diol
4-(4'Hydroxy-[1-1'-biphenyl]-4-yloxy) X
-2,2-dimethyl-4-((1H)-1,2,4-triazol-1-
yl)-1,3-butanediol
4-(4'-hydroxyo)-2,2-dimethyl-4-
(tr)butane.1,3.diol
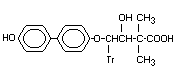 -Hydroxy- -(4'-hydroxy-[1,1'- p-Hydroxy-bitertanol
biphenyl]-4-yloxy- -dimethyl-1H.1,2 alcohol X
4-triazole-1-butanoic acid
3-hydroxy(4'-hydroxyo)-2,2-
dimethyl-4-(tr)butyric acid
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-Hydroxy- -(4'-hydroxy-[1,1'- p-Hydroxy-bitertanol
biphenyl]-4-yloxy- -dimethyl-1H.1,2 alcohol X
4-triazole-1-butanoic acid
3-hydroxy(4'-hydroxyo)-2,2-
dimethyl-4-(tr)butyric acid
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
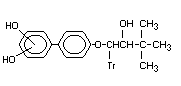 - (1,1-dimethylethyl)-p-(x'-di- Dihydroxy-bitertanol X
hydroxy-[1,1'-biphenyl]-4-yloxy)-1H-1,
2,4-triazol-ethanol
1-(dihyvroxyo)-3,3-dimethyl-1-(tr)
butan-2-olc(distal biphenyl ring
substituted)
- (1,1-dimethylethyl)-p-(x'-di- Dihydroxy-bitertanol X
hydroxy-[1,1'-biphenyl]-4-yloxy)-1H-1,
2,4-triazol-ethanol
1-(dihyvroxyo)-3,3-dimethyl-1-(tr)
butan-2-olc(distal biphenyl ring
substituted)
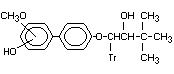 -(1,1-Dimethylethyl)-B-(x-hydroxy- Hydroxy, methoxyd
x'-methoxy-[1,1'-biphenyl]-4-yloxy)- bitertanol X
1H-1,2,4-triazole-1-ethanol
1-(hydroxymethoxyo)-3,3.dimethyl-1-
(tr)butan-2-old (distal biphenyl ring
substituted)
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-(1,1-Dimethylethyl)-B-(x-hydroxy- Hydroxy, methoxyd
x'-methoxy-[1,1'-biphenyl]-4-yloxy)- bitertanol X
1H-1,2,4-triazole-1-ethanol
1-(hydroxymethoxyo)-3,3.dimethyl-1-
(tr)butan-2-old (distal biphenyl ring
substituted)
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
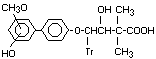 - Hydroxy- -(x'hydroxy-x'-methoxy- Hydroxy,methoxyd
[1,1'-biphenyl]-4-yloxy)-d,d-dimethyl bitertanol acid. X
1H-1,2,4-triazole-1-butanoic acid
3-hydroxy-4-(hydroxymethoxyo)-2-2-
dimethyl-4,4-(tr)butyric acidd
(distal biphenyl ring substituted)
- Hydroxy- -(x'hydroxy-x'-methoxy- Hydroxy,methoxyd
[1,1'-biphenyl]-4-yloxy)-d,d-dimethyl bitertanol acid. X
1H-1,2,4-triazole-1-butanoic acid
3-hydroxy-4-(hydroxymethoxyo)-2-2-
dimethyl-4,4-(tr)butyric acidd
(distal biphenyl ring substituted)
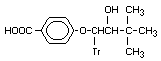 4-([2-Hydroxy-3,3-dimethyl-1-[1H- Bitertanol benzoic X
1,2,4-triazol-l-yl]butoxy)=benzoic acid analogue
acid BUE 2684
Note: Butoxybenzoic is one word
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-([2-Hydroxy-3,3-dimethyl-1-[1H- Bitertanol benzoic X
1,2,4-triazol-l-yl]butoxy)=benzoic acid analogue
acid BUE 2684
Note: Butoxybenzoic is one word
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
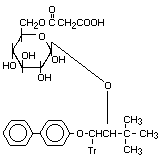 Bitertanol glucoside X
Bitertanol glucoside X
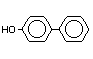 4-Hydroxybiphenyl p-Hydroxybiphenyl X X X X
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-Hydroxybiphenyl p-Hydroxybiphenyl X X X X
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
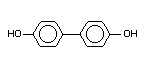 4,4'-Dihydroxybiphenyl p,p,'-dihydroxy-
biphenyl X X X X
4,4'-Dihydroxybiphenyl p,p,'-dihydroxy-
biphenyl X X X X
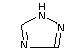 1,2,4-triazol X
CO2
Carbon dioxide X
Table 3. (continued)
a An X indicates that the compound has been found to occur as a degradation product of bitertanol in the substrate
concerned (or on irradiation). A blank indicates it has not been found there, but does not exclude its presence in
amounts too small to detect.
b tr = 1-H-1,2,4-Triazol-l-yl
c The position of the hydroxy groups is uncertain
d The positions of the hydroxy and methoxy groups are uncertain.
12 and 28 days after treatment and the older plants and nuts after 2.5
months. In young plants, 61-72% of the total recovered radioactivity
was surface residue, the remainder being mostly (27-36%) extractable.
In the older plants the distribution was 46% surface and 50%
extractable. In both cases most of the extractables were
organo-soluble. The composition of the residues is shown in Table 4.
As in the case of apples most of the residue, both surface and
extractable, was bitertanol I and II and a metabolite (R-1)
tentatively identified as a bitertanol I glucoside. Low levels of 4-
hydroxybiphenyl (0.6% of the total shoot residues) were also detected.
The authors conclude that the lower I:II isomer ratios in the surface
residues in the older plants compared to the 1.33 ratio of the
starting material indicates faster absorption of the I isomer and that
the 1.10 ratio of I:II in the combined surface and absorbed residues
gives evidence that isomer I is metabolized faster than II. Faster
isomer I absorption is supported further by the 1.64 ratio of
(bitertanol I + metabolite R-1):bitertanol II in the absorbed residues
in the old plants and experimental evidence suggesting increased
levels of the tentatively identified glucoside of isomer I (R-1) as
opposed to isomer II. There is no convincing evidence as to whether
isomeric conversion also occurs to account for the lower bitertanol
I:II ratio in the total residue. The half-life in peanuts has been
estimated at 141 days.
In a similar and related experiment in the same set of studies
with 8.9 mg a.i./plant (approximately twice the rate per plant in the
above), residues in nut meats were 0.06 mg/kg bitertanol equivalent
(0.008% of the total plant residue) 2.5 months after treatment. A
similar level was found in the shells. It cannot be concluded from
this study whether nut residues resulted from the foliar application
or from soil uptake.
Seven days after growing peanuts in a 1 mg/l
14C-biphenyl-labelled bitertanol nutrient solution followed by 7 days
in an unfortified nutrient solution 94.3% of administered
radioactivity was recovered with 28.8% in the roots, 14.9% in the
shoots, 40% in the fortified nutrient solution and 10.6% in the
non-fortified solution. Radioactivity was concentrated in the veins,
but distributed throughout the plant. No translocation to new growth
occurred after removal of the fortified solution. Radioactivity in the
non-fortified solution suggests desorption from within or from the
surface.
Of the absorbed radioactivity, 32.1% was in the shoots and 67.9%
in the roots with the distribution in the organo-soluble fraction
shown in Table 5.
Table 4. Composition of bitertanol residues in peanut shoots following spray application of
14C-biphenyl-laballed bitertanol
YOUNG PLANTS (1 month) OLD PLANTS (2 months)
12 day2 Ratio3 28 day2 Ratio3 2.5 mos.2 Ratio3
Surface Residues
bitertanol II 31.3 1.25 26.7 1.24 20.7 0.98
bitertanol I 39 33 20.5
metabolite R-11 -
Absorbed (organosoluble)
bitertanol II 11.2 1.64
bitertanol I 11.9 1.27 13.4 1.3 15
metabolite R-1 2.4 13.1 18.9
4.3 5.8
1 Tentatively identified as the 6-0-malonyl-B-D-glucoside of bitertanol I.
2 Interval after treatment.
3 Ratio of bitertanol I to bitertanol II in surface residues; ratio of
(bitertanol I + metabolite R-1) to bitertanol II in absorbed residues.
Table 5. Distribution of organo-soluble radioactivity absorbed by peanut plants.
Compound Shoots Roots Total
% of Ratio* % of Ratio* % of Ratio*
absorbed absorbed absorbed
14C 14C 14C
bitertanol II 10.7 20.4 31.1 1.32
bitertanol I 8.6 1.17 16.1 1.39 24.7
metalite R-1 3.9 12.3 16.2
23.2 48.8 72.0
Ratio of (bitertanol I + metabolite R-i) to bitertanol II.
Unextracted residues were 18.3% of the absorbed activity.
Assuming R-1 is a glucoside of bitertanol I as postulated the ratio of
(I + R-1) to II (1.32) n the total absorbed residue is essentially
identical to that in the starting material. This suggests equal root
absorption rates of bitertanol I and II. The finding of higher levels
of both R-1 and bitertanol I in the roots than in the shoots suggests
faster translocation of bitertanol II through-out the plant and/or
more rapid metabolism of bitertanol I. There was no evidence of
volatility from leaf surfaces.
Of the 18.3% unextracted but absorbed residue, 5 and
13.3%, respectively, was in shoots and roots. The metabolite
4-hydroxybiphenyl was detected in both (64% of the root-concentrated
residue).
Other peanut studies (Scheinpflug and Van den Boom, 1981) also
show bitertanol to be slowly absorbed by peanut leaves from surface
application with little translocation from petiole applications.
In animals
The fate of residues in rats and other non-food animals was
evaluated by the 1983 meeting. This evaluation considers the fate in
cows and chickens.
Cow. A single dose of phenyl-UL-14C bitertanol was fed to a
341 kg Holstein dairy cow at 0.2 mg/kg body weight by gelatin capsule,
and excreta and milk were analyzed periodically for six days. The I:II
isomer ratio was 53:47. Food consumption was not recorded (Obrist et
al. 1981). Eighty per cent of the dose was eliminated within 48
hours: urine 8.3%, faeces 70.4% and milk 0.2%, with milk residues
becoming steady after 32 hours. Ninety two per cent was eliminated by
6 days. Residues in the blood peaked at
0.13 mg/l after 12 hours.
Six days after the single dose the same cow was dosed daily at
the same rate for an additional 5 days. The 14C residues (as
bitertanol equivalent) in tissues and milk, 2.5 hours after the last
dose were: liver 0.82 mg/kg; kidney 0.11 mg/kg; muscle
0.01 mg/kg; fat 0.03 mg/kg; milk 0.008 mg/l.
The 0.008 mg/l in milk from the multiple dosing is comparable to
0.009 mg/l found from the single dosing. None of the residues were
identified. Summary data were provided on the identity and
distribution of residues in the tissues, milk and urine of cows fed
UL-14C-phenyl-labelled bitertanol at 0.2 mg/kg, but the study itself
was not provided (Obrist, et al, 1983).
Chickens. Laying hens (average weight 1170g) were administered
single oral doses of 14C-phenyl-UL bitertanol (I:II isomer ratio
53:47) by gelatin capsule at 2.5 mg/kg body weight for excretion
studies (5 trials) and blood level determinations (10 birds). Three
birds were dosed daily for 5 days at a rate of 8 mg/kg body weight for
tissue analyses. Food consumption data were not provided (Obrist et
al, 1982).
From a single dose, 92% was eliminated in the excreta within 24
hours with <0.1% in eggs (0.2% by 96 hours). Bitertanol equivalents
after 4 days were 0.1 and 0.02 mg/kg in liver and kidney respectively
(both 2X standard deviation) and <0.006 mg/kg in other tissues. In
eggs, residues as bitertanol equivalent from a single dose ranged from
<0.002 mg/kg (limit of determination) after 24 hours to 0.061 mg/kg
(2X standard deviation) after
96 hours. Dose-dependent residues from the multiple doses ranged from
<0.002 to 0.38 mg/kg for the same time interval.
Tissue and blood plasma residues, as bitertanol equivalent,
45 minutes after the last of the 5 daily doses varied significantly
(by factors of 5-20) among the 3 birds. They are shown in Table 6.
The composition of the chicken tissue residues is given in Table
7, where it can be seen that bitertanol and p-hydroxy-bitertanol were
by far the main residues, with the latter predominating in liver,
kidney and eggs, whereas bitertanol was predominant in the other
tissues. Conjugation was also greater in liver, kidney and eggs.
Combined unidentified and unextracted residues accounted for up to 19%
of the chicken liver residue and less in other tissues.
Residues in excreta (single dose) were 69.5%
p-hydroxy-bitertanol, 6.7% bitertanol, 13.7% unidentified and <3%
each of other identified compounds. The same residues were identified
in excreta as in tissues with the exception of dihydroxy-bitertanol,
found only in the liver. Bitertanol acid and
p-hydroxy-methoxy-bitertanol acid found in rat faeces (Phul and
Hurley, 1983) were not identified in chicken excreta or tissues.
Summary data provided to the meeting (from Obrist et al 1983)
suggest that residues in cow tissues and milk are similar
qualitatively, and in some respects quantitatively, to those in
chicken tissues except that the dihydroxy-bitertanol found in chicken
livers was reportedly not identified in dairy cow tissues or excreta.
Cow urine reportedly contained bitertanol acid and
p-hydroxy-bitertanol acid, also identified in rat urine (Phul et
al 1979b). The cow study itself was not provided. Further evaluation
must await availability of the study.
Table 6. Tissue and plasma residues in chickens dosed with 14C
bitertanol
14C residue
Tissue Bitertanol equivalent,mg/kg % organo-soluble
Mean Max
liver 6.2 12.6 86
kidney 4.3 10.3 91
breast muscle 0.35 0.91 95.2
leg muscle 0.48 1.1
fat 1.4 3.01 93.2
gizzard 0.72 1.7 90.8
heart 1.3 3.2 92.9
skin 0.63 1.4 94.7
blood plasma 1.8 4.5 --
Table 7. Composition of residues in chicken tissues following administration of multiple oral doses of
bitertanol-phenyl-UL 14C at 8.0 mg/kg (from Obrist and Phul, 1982)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
Bitertanol 34.7 27.2 67.8 62.4 66.0 78.2 74.9 36.6
p-Hydroxy-bitertanol
Free 14.7 27.7 23.2 14.8 17.1 12.1 13.2 6.4
Conjugateda 25.4 24.8 1.5 10.4 4.4 - 4.2 37.9
Hydroxy, Methoxy-bitertanol
Free 0.2 1.0 - - - - - 0.3
Conjugateda 1.5 1.2 - - - - - 0.7
Bitertanol alcohol
Free 1.2 1.8 - - - - - 0.5
Conjugated 0.8 1.1 - - - - - -
Dihydroxy-bitertanolb 0.9 - - - - - - -
p-Hydroxy-bitertanol alcohol
Free 0.4 0.9 - - - - - 0.3
Conjugateda 0.2 - - - - - - -
p-Hydroxy bipbenyl 0.2 0.2 - - - - - 13
Table 7. (continued)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
p,p'-Dihydroxybiphenyl 0.7 0.2 - - - - - -
Unidentified residue 11.9 12.0 4.9 5.9 5.4 2.9 2.4 9.3
Unextracted 7.2 1.9 2.6 6.5 7.1 6.8 5.3 6.7
TOTALS 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
The accumulation, persistence and composition of residues of
14C-bitertanol in bluegill fish maintained in aquarium water
fortified at 10 and 10 mg/l for 30 days has been investigated (Lamb,
1979). Residues on a whole fish basis reached a plateau in both cases
by three days at 2.1 and 16.2 mg/kg at the 10 and 100 mg/l
concentrations respectively, with maximum concentration factors of 203
and 174 respectively. Residues were quantitatively extracted with
acetonitrile with 57-66% of the radioactivity in non-edible parts
(head, viscera, scales) and 34-43% in edible parts. Residues were
characterized by TLC as bitertanol II 40%; bitertanol I 34%;
metabolite R-2 (unidentified) 7%; remaining at origin 13%; other
unidentified 6%.
Approximately 96% of the residues were excreted within 3 days
when the fish were transferred to untreated water.
In soil
Adsorption/desorption. In soil adsorption/desorption studies
bitertanol was shown to be strongly adsorbed to three soils in the
decreasing order: sand, loam and silty clay with adsorption
coefficients (K values) of 39.9, 35.8 and 19.6 (Phul and Hurley,
1979a). The data conformed to the Freundlich equation and in each case
>84% was adsorbed under experimental conditions. Only 4-10% of the
adsorbed compound was desorbed from sand.
Leaching. In TLC leaching experiments six soil types were examined
by comparing Rf values (Obrist and Thornton, 1979). The Rf values
ranged from 0.04 to 0.22, which places bitertanol in the two lowest
mobility ranges of the five classes which have been proposed (Helling
and Turner, 1968; Helling et al 1971). This is consistent with the
high adsorption determined in adsorption experiments. No significant
difference was observed between the two bitertanol isomers.
In aged soils (moist sandy loam) 44% of the initial radioactivity
was lost during room temperature incubation for 30 days, probably by
volatilization (Obrist, 1979). Less than 2% of the aged applied
bitertanol was found in the leachate from 30 cm soil columns, leached
at an equivalent of 13 mm/day for 45 days. Except for 55% further
losses during leaching, most of the remaining residue was in the upper
1.3 cm of the column.
Similar results were found in 19 studies with either Dutch polder
soil or a standard loamy sand containing aged bitertanol residues
leached at an equivalent of 200 mm for 48 hours or up to 500 mm in
polder soil after 72 additional hours (Brennecke, 1983). Only 0.8% of
the aged soil residue was found in the leachate of polder soil after
48 hours, and 3% after 120 hours. In experiments with 11 standard
soils, the leachates contained <6.6% of the original radioactivity in
the aged soil in all but one sample, where 48.9% was in the leachate
and was identified as the bitertanol soil metabolite
4-[2-hydroxy-3,3-dimethyl-(1H-1,2,4-triazol-1-yl)butoxybenzoic acid
(BUE 2684). A repeat of this experiment resulted in only 2.1% or less
of aged residue in the leachate, although less leachate (approximately
400 ml) was collected in the repeat, compared with approximately 650
ml in the original experiment. No unchanged bitertanol was found in
any leachate. Most leachate residues were BUE 2684 and traces of its
methyl ester.
The low leaching potential of bitertanol under experimental
conditions was supported by 30 additional reports provided to the
meeting.
Soil persistence. The soil persistence of bitertanol applied to 7
soil types as the 25 WP formulation at 11 kg a.i./ha to field plots in
9 studies in scattered locations in North America was reported in 9
Mobay reports (68296-68304). Half-lives ranged from 10 to 39 days with
an average of 25 days, except in one study with Oregon loam in which
the half-life was 131 days. The exceptionally long half-life did not
appear to be accounted for by temperature, rainfall, pH or
geographical area when compared to other studies.
Under laboratory aerobic conditions the half-life on silt loam
ranged from 14 to 20 days (Phul and Hurley, 1979b). Degradation was
slower under anaerobic conditions and there was no significant
degradation in sterilized soil after 31 days, showing the importance
of soil micro-organisms in bitertanol degradation. Both
diasterioisomers of bitertanol were degraded at approximately the same
rates.
Under laboratory conditions bitertanol decomposed faster in
alluvial loam soil (12 day half-life) than volcanic ash loam soil (30
days half-life), while under field conditions decomposition was faster
with volcanic ash/sandy loam than with alluvial loam soil (Takase and
Yoshimoto, 1980). In other laboratory tests bitertanol had a
half-life of 26 days in type 1 soil and 72-days in type 2 (Mobay
Report No. 10.317/18), while in field tests half-lives ranged from 19
to 27 days (Mobay Reports NO. 10.324/79, 10.325/79, 10 311/80 and
10.31280).
Rotational Crops. The uptake of 14C-biphenyl ring-labelled WP
bitertanol by rotational crops planted 31, 118 and 364 days after the
last of eight applications to outdoor tub-planted peanuts at an
equivalent of 0.56 kg a.i./ha resulted in 14C residues in leafy
vegetables at harvest ranging from 0.1 mg/kg bitertanol equivalent in
the 118-day planting to 0.02 mg/kg in the 364-day planting (Phul et
al. 1981). In sugar beets residues were 0.38 and 0.01 mg/kg for the
same plantings, and in wheat heads 0.23 and 0.01 mg/kg for 31-day and
364-day plantings. Residues in wheat straw were 1.4 mg/kg from the
31-day planting. The organo-soluble portion of the residue was
<0.04 mg/kg bitertanol equivalent in all at-harvest samples except
for 0.51 mg/kg in wheat straw. The benzoic acid derivative of
bitertanol, BUE 2684 was identified in the 31-day wheat samples.
Soil transformation. The fate of bitertanol has been investigated
in silt loam soil (pH 7.4) under aerobic and anaerobic conditions and
in sterile soil (Phul and Hurley, 1979b). Soil was fortified at 1 and
10 mg/kg for aerobic conditions and 1 mg/kg for anaerobic and sterile
conditions. One mg/kg approximates the maximum residues expected from
recommended field rates of 0.56 kg a.i./ha applied to peanuts. In the
1 mg/kg experiment, bitertanol (I + II) and total organo-solubles
decreased from 98.6 and 99.8% of the total 14C on the first day to 6
and 8.4% after 121 days, while 14CO2 and unextracted residues
increased from 0.8 and 4.6% on day 3 to 45.8 and 45% of the total
radioactivity, respectively, after 121 days. A similar trend was
observed with the 10 mg/kg fortification over the 29-day period
examined. The two diasterioisomers of the bitertanol benzoic acid
metabolite were observed at the 10 mg/kg fortification level (8.6% of
the total radioactivity) but not at the 1 mg/kg level.
Degradation was somewhat slower under anaerobic conditions than
aerobic, but the distribution (bitertanol, total organic, 14CO2 and
unextracted) was not markedly different at comparable intervals.
Bitertanol still accounted for 95.4% of the total 14C in sterile
soil after 31 days compared to 11% in non-sterile soil, indicating the
importance of soil microbes in the metabolism of bitertanol; this was
further demonstrated in another study (Phul et al 1979a). The
identity of the microbes present in the soil of the latter study was
investigated by Smedly and Hepler (1979).
In other studies (Brennecke, 1982) 14C-biphenyl-labelled
bitertanol was shown to be mineralized primarily into 14CO2, up to
68% in "standard soil 2.1" and 49% in Netherlands polder soil. The
bitertanol benzoic acid metabolite BUE 2684 (<19.1%) and an unknown
(<1%) were also found in one of 5 experiments in standard soil.
Bitertanol ketone (BUE 1662) was identified by TLC at <1.7% of the
total radioactivity in polder soil.
In water
The stability of bitertanol in sterile aqueous solutions has been
studied (Nichols and Thornton, 1979). No apparent degradation was
observed after thirty days in sterile aqueous solutions, buffers at pH
4, 7 and 9 and maintained at 25 and 40°C, which contained 0.25 and 2.5
mg/l of bitertanol. After 30 days, 94-109% of the bitertanol was
accounted for at the higher concentrations and 85-98% at the lower
level. The 85% recovery was from 0.25 mg/l at pH 9 and 40°C. The
slightly lower recovery at the lower fortification level was
attributed to adsorption to the vials. This is consistent with the
adsorption observed at low concentration in other studies (Phul and
Hurley, 1979). After using a correction factor to account for vial
glass adsorption, the recovery was 100% under all conditions of this
study.
Additional information on the hydrolytic stability of bitertanol
was provided (Stegh, 1980) but not in the working language of the
meeting.
Photolysis
The photolysis of bitertanol has been investigated on soil and
silica surfaces and in water. In the soil surface experiment,
bitertanol-treated silt loam of 1 mm thickness irradiated for 35 days
did not show substantially different extractability (91.5%) from dark
controls (96.6%), (Sietsema, 1982). Volatile radioactivity accounted
for <0.1% of the original amount. The extracted residue was shown to
be bitertanol by mass spectrometry.
A study on the photolysis of bitertanol on silica gel places
(Stegh and Wilmes, 1982) and two reports on the photolysis in water
and on silica gel (Wilmes, 1980; Stegh, 1980) were provided, but not
in the working language of the meeting. Another report (Wilmes, 1981)
discussed the hydrolytic behaviour and stability of bitertanol in
solution. No data were provided, but reference was made to the two
studies above which included data. Another bitertanol photolysis study
(Sietsema, 1983) was brought to the attention of the meeting, but was
not included in the data provided.
METHODS OF RESIDUE ANALYSIS
An analytical method developed for the analysis of triadimefon in
apples (Thornton, 1977) has been used for bitertanol in apples. The
sample is ground with dry ice, blended with acetone and filtered. The
filter cake is blended with dichloromethane and filtered, and the
combined filtrates are partitioned with water, taken to dryness and
cleaned up on a Florisil column with 60:40 hexane:ethyl acetate as
eluant. The eluate is concentrated and bitertanol determined by GLC
using an alkali flame detector.
The procedure was validated for bitertanol in apples at
0.05-0.1 mg/kg with recoveries of 86-108% and a blank of
<0.01 mg/kg (Morris, 1979a) Although detection was easily attainable
at 0.05 mg/kg and probably 0.01 mg/kg, chromatograms showed
interferences which would make quantitation below 0.05 mg/kg
questionable.
Interference studies have demonstrated that the basic procedure
above permits analysis of bitertanol at levels equivalent to 0.1 mg/kg
in samples in the presence of tolerance levels of 75 pesticides
registered in the U.S.A. for apples or peanuts (1979), five compounds
registered for bananas (1980) and three compounds registered for
cherries, plums, peaches and pears (1981) (Obrist and Nichols,1979).
Several compounds interfered, but could be eliminated by a 1-hour
reflux with 1 N NaOH and zinc or by partitioning with 0.1 N HC1.
However, analytical recoveries from a crop matrix with these steps
incorporated were not provided.
Another analytical procedure (also suitable for other crop
protectants) has been tested for bitertanol on apples, bananas, beans,
currants, cherries, damsons, wheat (green, grain and straw) and soil
(Specht and Tillkes, 1980). Many of the analyses in the field trials
were conducted with this basic procedure. Samples are extracted with
2:1 acetone: water, partitioned with dichloromethane, cleaned up by
gel permeation chromatography and analyzed by GLC utilizing a flame
alkali detector. Where necessary (e.g. for straw) an additional silica
gel column clean-up is used. Analytical recoveries were 74-112%,
although fortification levels were described only as "0.02-0.1 ppm
(lower limit of determination), and 1.0-3.0 mg/kg". For the compounds
tested, the authors concluded that the lower limit of determination
was 0.02 to 0.05 mg/kg for plants with >70% water content and 0.1
mg/kg for cereals and soil. The information was not sufficient for the
meeting to draw a conclusion on the limit of determination.
A method developed for triadimefon in soil and water (Thornton
and Lloyd, 1977) is also suitable for bitertanol in soil and water
with minor modifications in the GLC column stationary phase (Morris,
1979b). Water is simply extracted with chloroform, the extract is
concentrated (a Florisil column clean-up is optional), and analyzed by
GLC using a flame-alkali detector. Soil is extracted by refluxing with
methanol/water, partitioned with chloroform, washed with water and
cleaned up on a Florisil column. Recoveries from water fortified at
0.005 and 0.01 mg/l were 94% and 78% respectively with a blank of
<0.001 mg/l. Recoveries from six soils fortified at 0.05-0.5 mg/kg
ranged from 76 to 121% (most were >90%) with blanks <0.01 mg/kg
(Morris, 1979b). Chromatograms were consistent with the data.
In a similar procedure for the analysis of bitertanol and
numerous other fungicides in water (Brennecke and Vogeler, 1980),
extraction is with dichloromethane and clean-up by gel permeation
chromatography. The two recoveries for bitertanol were 108% and 104%
for 0.005 and 0.5 mg/l fortifications respectively. The lower limit of
determination was said to be 0.005 mg/l, although the meeting could
not confirm this with the available data.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs were reported to the meeting.
Safety
interval MRL
Country Crop (days) mg/kg
Australia Apples 14 1.0 1)
Peanuts 14 0.2 1) 2)
Belgium Apples 42 0.25
Pears 42 0.25
France Apples 15 1.0
Pears 15 1.0
Germany, Federal Apples 14
Republic Cherries 21
Great Britain Apples 21
Pears 21
Greece Apples 21
Beans 4 crops 14
Pears 5 PHIs 7
Stone fruit 14
10
Israel Apples 14
Italy Pears 14
Beets 30 1.0
Fruit 1.0
Pome fruit 21
Stone fruit 21
Vegetables 14 0.5
Morocco Beans 15
Netherlands Apples 14
Blackberries 0.05 2)
Cucumbers 3
Fruiting Vegetables 1.0
Melons 3
Pears 14
Peppers 3
Other commodities 03)(0.05)
New Zealand Pome fruit 14
Strawberries 21
Safety
interval MRL
Country Crop (days) mg/kg
South Africa Apricots 56 0.5
Beans 28 0.1
Beans (fodder) 42
Peaches 56 0.5
Peanuts 0.05
Peanuts (fodder) 42
Plums 56 0.5
Spain Pome fruit 15 1)
Stone fruit 15 1)
Switzerland Pome fruit 42 0.6
Venezuela Apples 15 1.0
Broad beans 28 1.0
Peanuts 0.2 (1)
Pears 15 1.0
Stone fruit 42 0.6
Yugoslavia General 28
1) = Preliminary
2) = Level at or about the limit of determination.
3) = No residues should be present. Limit of determination 0.05 mg/kg.
APPRAISAL
Bitertanol is a relatively new broad-spectrum fungicide
introduced and being introduced in a number of countries. Its
toxicology was evaluated by the 1983 JMPR which estimated a temporary
ADI. The present meeting evaluated residues in food.
To date only limited residue trials data have been provided and
these have been evaluated. Data were insufficient to estimate residue
levels in cucumbers and beans, but temporary limits were estimated for
several commodities.
The fate of bitertanol has been investigated in plants (apples
and peanuts), animals, soil, light and water.
In the plant studies bitertanol penetration from surface
applications was minimal and there was little volatility. Residues are
taken up by roots, although translocation into the plant is relatively
low. There are some measurable but not major differences in
absorption, translocation and plant metabolism rates of the
two diasterioisomeric forms of bitertanol. Bitertanol is by far
the main plant residue, with low levels of its ketone and
4-hydroxybiphenyl. In apples, most of the residue in the peel.
The fate of residues in non-food animals was evaluated by the
1983 JMPR. The present meeting evaluated metabolism studies on
poultry, cows and fish. Bitertanol is metabolized substantially more
in animals than in plants. The products are rapidly excreted but low
residues can occur in milk, eggs and tissues. These are predominantly
in the liver and kidney, as demonstrated with exaggerated feeding
levels. Bitertanol and p-hydroxy-bitertanol are by far the predominant
residues in chicken tissues and eggs and are reported to be the major
residues in cow tissues and milk, although other metabolites occur at
significant levels in the latter. Although cow metabolism studies were
available to the meeting the only one detailing the distribution of
identified residues was received too late for evaluation, until it has
been reviewed at a future meeting, no final conclusion can be drawn on
the adequacy of information on the fate of residues in animals. The
available data indicate that metabolism appears to be similar among
rats, poultry and cows.
Conventional feeding studies on poultry and ruminants, an
analytical method for the analysis of bitertanol in animal products,
and information on the fate of residues in apples during processing
were all received too late for evaluation.
Extensive information was available to the meeting on the fate of
bitertanol residues in soil, in which it is of low mobility, and some
data were available on water stability and photolysis. The water and
photolysis data available in the working language of the meeting
indicate a high degree of stability in each case. Other studies
provided, but not in the working language of the meeting, were not
evaluated.
Several analytical methods utilizing solvent extraction, GPC or
Florisil clean-up followed by gas chromatography are available for
determining residues in crops, soil and water. Further confirmation of
the limit of determination is desirable.
No information was provided on the fate of residues in storage,
in processing, in commerce or at consumption.
The meeting examined residue data from supervised trials
reflecting good agricultural practice on a number of crops and was
able to estimate the maximum residue levels which were likely to occur
when bitertanol was used in practice and when the reported intervals
between the last application and harvest were observed. These levels
refer only to the parent compound.
RECOMMENDATIONS
COMMODITY MRL (mg/kg)1 Pre-harvest interval on
which recommendations
are based
Apples 2 14
Stone fruit 1 21
Bananas 0.5 0
Oats:
grain 0.1* )
forage 0.1* )
straw 0.1* )
Rye:
grain 0.1* )
forage 0.1* )
straw 0.1* ) from seed treatment
Wheat:
grain 0.1* )
forage 0.1* )
straw 0.1* )
* At or about the limit of determination.
1 Temporary irrespective of the status of the ADI.
FURTHER WORK OR INFORMATION
1. Except for bananas for which substantial data reflecting approved
uses were available, additional information on good agricultural
practice and residue data reflecting approved uses for all
commodities for which temporary limits have been estimated.
Residue data should be from countries from which details of
approved uses have been or will be provided, or those in close
proximity.
2. Additional plant metabolism studies.
3. Additional residue data reflecting good agricultural practice for
additional plant products likely to be used as animal feeds.
Desirable
1. Analytical recoveries through the Thornton analytical method
utilizing the NaOH and HC1 steps designed to remove
interferences.
2. Confirmation of limits of determination for the Specht method.
3. Information on the stability of residues in crops during cold
storage.
4. Additional national maximum residue limits and safety intervals.
5. Photolysis/hydrolysis studies in English (Stegh and Wilmes, 1982;
Wilmes, 1980; Stegh 1980 and Sietsema, 1983).
REFERENCES
Mobay Reports are unpublished reports prepared by the Research and
Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, USA.
Nitokuno Reports are unpublished reports prepared by Nihon Tokushu
Noyaku Seizo KK., No. 8, 2-chome, Nihonbashi, Chuo-ku, Tokyo, Japan.
Bayer Reports:
Leaching in Soil
10.314 - 10.316/78 10.342 - 10.334/82
10.318 - 10.320/78 10.348 - 10.350/82a
10.334 - 10.336/80 10.374 - 10.376/82
10.334A - 10.346A/8010.379 - 10.381/82b
10.309 - 10.311/81 10.388 - 10.390/82b
Soil Persistence
10.317/78 10.324/7910.3257910.311/8010.312/80
68 296 68 29868 30068 30268 304
68 297 68 29968 30168 303
Brennecke, R. Leaching characteristics of Aged [biphenyl-UL-14C]
1983 bitertanol Soil Residues. Unpublished Bayer Report RA
981/209B, October 1983.
Brennecke, R. and Vogeler, K. Method for the Gas Chromatographic
1982 Determination of Residues of Various Fungicides in Water.
Bayer AG, Pflanzenschutz-Anwendungstechnik, unpublished
Report RA-80, January, 1982.
Helling, C.S. and Turner, B.C. Pesticide Mobility: Determination by
1968 Soil Thin-Layer Chromatography, Science 162, 562 (1968).
Helling, C.S., Kearney, P.C. and Alexander, M. Behaviour of Pesticides
1971 in soils, Advan. Agron. 23; 147, 1971.
Lamb, D.W., BAYCOR-14C Accumulation and Persistence of Residues in
1979 Bluegill. Mobay Report No. 68 343 December 13, 1979.
Mobay, Recovery Reports, Apples 68 249, Soil and Water 69 250.
Morris, R.A. Recovery of Baycor from Apples. Unpublished Mobay Report
1979a 68 249, November 19, 1979.
Morris, R.A. Recovery of Baycor from Soil and Water. Unpublished Mobay
1979b Report 68 250, 1979.
Nichols, S.S. and Thornton, J.S. The Behaviour of BAYCOR in Sterile
1979 Aqueous Solutions. Mobay Report No. 68 273. October 22,
1979.
Christ, J.J. Leaching Characteristics of Aged BAYCOR Soil Residues
1979a Mobay Report No. 68 271. September 25, 1979.
Obrist, J.J. and Nichols, S.S. An Interference Study for the BAYCOR
1979 Residue Method for Apples and Peanuts. Mobay Report No. 68
311. November 27, 1979.
Obrist, J.J., Phul, R.J. and Thornton, J.S. Excretion and Tissue
1981 Levels of 14C Following Oral Administration of BAYCOR-
Phenyl-UL-14C to a Dairy Cow. Mobay Report NO. 69 400.
March 24, 1981.
Obrist, J.J., Phul, R.J. and Thornton, J.S. The Metabolism and
1982 Excretion of BAYCOR-Phenyl-UL-14C by Chickens. Mobay Report
No 82614. October 8, 1982.
Obrist, J.J., Phul R.J. and Thornton, J.S. The Metabolism and
1983 Excretion of BAYCOR-Phenyl-UL-14C Following Oral
Administration to a Dairy Cow. Mobay Report, Manuscript in
Preparation. 1983.
Obrist, J.J. and Thornton, J.S. Soil Thin-Layer Mobility of BAYCOR,
1979 BAYTAN, DYRENE, and PEROPAL. Mobay Report No. 68 272.
October 22, 1979.
Phul, R.J. and Hurley, J.B. Soil Adsorption and Desorption of BAYCOR.
1979a Mobay Report No. 68 003. July 18, 1979.
Phul, R.J. and Hurley, J.B. The Metabolism and Degradation of BAYCOR-
1979b Biphenyl-UL-14C on Soil. Mobay Report NO. 67 998. August 6,
1979.
Phul, R.J. and Hurley, J.B. The Metabolism of BAYCOR-Biphenyl-UL-14C
1979c in Apple Fruit. Mobay Report No. 68 305. November 5, 1979,
Revised February 3, 1981A.
Phul, R.J. and Hurley, J.B. Metabolism of BAYCOR-UL-14C in Peanut
1981 Plants. Mobay Report No. 69 310. October 23, 1979, Revised
June 18, 1981b.
Phul, R.J. and Hurley, J.B. The Absorption, Excretion and Metabolism
1983 of BAYCOR-Phenyl-UL-14C by Rats. Mobay Report, Manuscript
in Preparation, 1983.
Phul, R.J., Hurley, J.B. and Close, C.L. Total Radioactive Residues in
1981 Rotational Crops Following a Target Drop of Peanuts Treated
with BAYCOR-14C. Mobay Report No.80 059. September 11,
1981.
Phul, R.J., Hurley, J.B. and Thornton, J.S. The effect of Soil
1979a Microorganisms on the Degradation of BAYCOR. Mobay Report
No. 69 012. July 2, 1979.
Phul, R.J., Obrist, J.J. and Pither, K.M. The excretion of
1979b BAYCOR-Phenyl-UL-14C Following Administration of a Single
Oral Dose to Rats. Mobay Report NO. 68 307. November 19,
1979.
Scheinpflug, H. and van den Boom, T. BAYCOR, A New Fungicide for
1981 Tropical and Subtropical Crops. PflanzenschutzNachrichten
Bayer 34, 8-28.1981.
Sietsema, W.K. Photodecomposition of BAYCOR on a Soil Surface. Mobay
1982 Report No. 82 572. November 16, 1982.
Sietsema, W.K. Photodegradation of BAYCOR in Water. Mobay Report (in
1983 Preparation). 1983.
Smedly, L.A. and Hepler, D.I. Identification of Microorganisms in
1979 Samples of Kansas Loam Soil. Mobay Report No. 67 814. May 1,
1979.
Specht, W. and Tillkes, M. Determination of Agrochemicals After
1980 Clean-up using Gel Permeation Chromatography and Mini-Silica
Gel Column Chromatography. Pflanzenschutz-Nachrichten Bayer
33, 61 - 85. 1980.
Stegh, R. Versuche zum Abbau von KWG 0599 in wässerigen Salzlosungen.
1980 Bayer Experimental Notice. January 24, 1980.
Stegh, R. and Wilmes, R. Light Induced Degradation of Agrochemical
1982 Parent Compounds on Silica Gel as a Model for Degradation on
Soil. Bayer Ag. Pflanzenschutz-Anwendungstechnik unpublished
report PF1666. February 10, 1982.
Takase, I. and Yoshimoto, Y. Residues of KWG 0599 in Upland Soils
1980 Under Laboratory and Field Conditions. Nitokuno Report No.
1134. December 5, 1980.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and its
1977a Metabolite in Apples. Mobay Report No. 54 166. December 30,
1977.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and KWG 0599
1977 in Soil and Water. Mobay Report NO. 51 231. January 31,
1977.
Wilmes, R. Preliminary Studies on Stability to Light. Bayer Pharma
1980 Unpublished Report, 1980.
Wilmes, R. Abiotic Degradation of Bitertanol. Bayer summary Report.
1981 March 31, 1981.
1,2,4-triazol X
CO2
Carbon dioxide X
Table 3. (continued)
a An X indicates that the compound has been found to occur as a degradation product of bitertanol in the substrate
concerned (or on irradiation). A blank indicates it has not been found there, but does not exclude its presence in
amounts too small to detect.
b tr = 1-H-1,2,4-Triazol-l-yl
c The position of the hydroxy groups is uncertain
d The positions of the hydroxy and methoxy groups are uncertain.
12 and 28 days after treatment and the older plants and nuts after 2.5
months. In young plants, 61-72% of the total recovered radioactivity
was surface residue, the remainder being mostly (27-36%) extractable.
In the older plants the distribution was 46% surface and 50%
extractable. In both cases most of the extractables were
organo-soluble. The composition of the residues is shown in Table 4.
As in the case of apples most of the residue, both surface and
extractable, was bitertanol I and II and a metabolite (R-1)
tentatively identified as a bitertanol I glucoside. Low levels of 4-
hydroxybiphenyl (0.6% of the total shoot residues) were also detected.
The authors conclude that the lower I:II isomer ratios in the surface
residues in the older plants compared to the 1.33 ratio of the
starting material indicates faster absorption of the I isomer and that
the 1.10 ratio of I:II in the combined surface and absorbed residues
gives evidence that isomer I is metabolized faster than II. Faster
isomer I absorption is supported further by the 1.64 ratio of
(bitertanol I + metabolite R-1):bitertanol II in the absorbed residues
in the old plants and experimental evidence suggesting increased
levels of the tentatively identified glucoside of isomer I (R-1) as
opposed to isomer II. There is no convincing evidence as to whether
isomeric conversion also occurs to account for the lower bitertanol
I:II ratio in the total residue. The half-life in peanuts has been
estimated at 141 days.
In a similar and related experiment in the same set of studies
with 8.9 mg a.i./plant (approximately twice the rate per plant in the
above), residues in nut meats were 0.06 mg/kg bitertanol equivalent
(0.008% of the total plant residue) 2.5 months after treatment. A
similar level was found in the shells. It cannot be concluded from
this study whether nut residues resulted from the foliar application
or from soil uptake.
Seven days after growing peanuts in a 1 mg/l
14C-biphenyl-labelled bitertanol nutrient solution followed by 7 days
in an unfortified nutrient solution 94.3% of administered
radioactivity was recovered with 28.8% in the roots, 14.9% in the
shoots, 40% in the fortified nutrient solution and 10.6% in the
non-fortified solution. Radioactivity was concentrated in the veins,
but distributed throughout the plant. No translocation to new growth
occurred after removal of the fortified solution. Radioactivity in the
non-fortified solution suggests desorption from within or from the
surface.
Of the absorbed radioactivity, 32.1% was in the shoots and 67.9%
in the roots with the distribution in the organo-soluble fraction
shown in Table 5.
Table 4. Composition of bitertanol residues in peanut shoots following spray application of
14C-biphenyl-laballed bitertanol
YOUNG PLANTS (1 month) OLD PLANTS (2 months)
12 day2 Ratio3 28 day2 Ratio3 2.5 mos.2 Ratio3
Surface Residues
bitertanol II 31.3 1.25 26.7 1.24 20.7 0.98
bitertanol I 39 33 20.5
metabolite R-11 -
Absorbed (organosoluble)
bitertanol II 11.2 1.64
bitertanol I 11.9 1.27 13.4 1.3 15
metabolite R-1 2.4 13.1 18.9
4.3 5.8
1 Tentatively identified as the 6-0-malonyl-B-D-glucoside of bitertanol I.
2 Interval after treatment.
3 Ratio of bitertanol I to bitertanol II in surface residues; ratio of
(bitertanol I + metabolite R-1) to bitertanol II in absorbed residues.
Table 5. Distribution of organo-soluble radioactivity absorbed by peanut plants.
Compound Shoots Roots Total
% of Ratio* % of Ratio* % of Ratio*
absorbed absorbed absorbed
14C 14C 14C
bitertanol II 10.7 20.4 31.1 1.32
bitertanol I 8.6 1.17 16.1 1.39 24.7
metalite R-1 3.9 12.3 16.2
23.2 48.8 72.0
Ratio of (bitertanol I + metabolite R-i) to bitertanol II.
Unextracted residues were 18.3% of the absorbed activity.
Assuming R-1 is a glucoside of bitertanol I as postulated the ratio of
(I + R-1) to II (1.32) n the total absorbed residue is essentially
identical to that in the starting material. This suggests equal root
absorption rates of bitertanol I and II. The finding of higher levels
of both R-1 and bitertanol I in the roots than in the shoots suggests
faster translocation of bitertanol II through-out the plant and/or
more rapid metabolism of bitertanol I. There was no evidence of
volatility from leaf surfaces.
Of the 18.3% unextracted but absorbed residue, 5 and
13.3%, respectively, was in shoots and roots. The metabolite
4-hydroxybiphenyl was detected in both (64% of the root-concentrated
residue).
Other peanut studies (Scheinpflug and Van den Boom, 1981) also
show bitertanol to be slowly absorbed by peanut leaves from surface
application with little translocation from petiole applications.
In animals
The fate of residues in rats and other non-food animals was
evaluated by the 1983 meeting. This evaluation considers the fate in
cows and chickens.
Cow. A single dose of phenyl-UL-14C bitertanol was fed to a
341 kg Holstein dairy cow at 0.2 mg/kg body weight by gelatin capsule,
and excreta and milk were analyzed periodically for six days. The I:II
isomer ratio was 53:47. Food consumption was not recorded (Obrist et
al. 1981). Eighty per cent of the dose was eliminated within 48
hours: urine 8.3%, faeces 70.4% and milk 0.2%, with milk residues
becoming steady after 32 hours. Ninety two per cent was eliminated by
6 days. Residues in the blood peaked at
0.13 mg/l after 12 hours.
Six days after the single dose the same cow was dosed daily at
the same rate for an additional 5 days. The 14C residues (as
bitertanol equivalent) in tissues and milk, 2.5 hours after the last
dose were: liver 0.82 mg/kg; kidney 0.11 mg/kg; muscle
0.01 mg/kg; fat 0.03 mg/kg; milk 0.008 mg/l.
The 0.008 mg/l in milk from the multiple dosing is comparable to
0.009 mg/l found from the single dosing. None of the residues were
identified. Summary data were provided on the identity and
distribution of residues in the tissues, milk and urine of cows fed
UL-14C-phenyl-labelled bitertanol at 0.2 mg/kg, but the study itself
was not provided (Obrist, et al, 1983).
Chickens. Laying hens (average weight 1170g) were administered
single oral doses of 14C-phenyl-UL bitertanol (I:II isomer ratio
53:47) by gelatin capsule at 2.5 mg/kg body weight for excretion
studies (5 trials) and blood level determinations (10 birds). Three
birds were dosed daily for 5 days at a rate of 8 mg/kg body weight for
tissue analyses. Food consumption data were not provided (Obrist et
al, 1982).
From a single dose, 92% was eliminated in the excreta within 24
hours with <0.1% in eggs (0.2% by 96 hours). Bitertanol equivalents
after 4 days were 0.1 and 0.02 mg/kg in liver and kidney respectively
(both 2X standard deviation) and <0.006 mg/kg in other tissues. In
eggs, residues as bitertanol equivalent from a single dose ranged from
<0.002 mg/kg (limit of determination) after 24 hours to 0.061 mg/kg
(2X standard deviation) after
96 hours. Dose-dependent residues from the multiple doses ranged from
<0.002 to 0.38 mg/kg for the same time interval.
Tissue and blood plasma residues, as bitertanol equivalent,
45 minutes after the last of the 5 daily doses varied significantly
(by factors of 5-20) among the 3 birds. They are shown in Table 6.
The composition of the chicken tissue residues is given in Table
7, where it can be seen that bitertanol and p-hydroxy-bitertanol were
by far the main residues, with the latter predominating in liver,
kidney and eggs, whereas bitertanol was predominant in the other
tissues. Conjugation was also greater in liver, kidney and eggs.
Combined unidentified and unextracted residues accounted for up to 19%
of the chicken liver residue and less in other tissues.
Residues in excreta (single dose) were 69.5%
p-hydroxy-bitertanol, 6.7% bitertanol, 13.7% unidentified and <3%
each of other identified compounds. The same residues were identified
in excreta as in tissues with the exception of dihydroxy-bitertanol,
found only in the liver. Bitertanol acid and
p-hydroxy-methoxy-bitertanol acid found in rat faeces (Phul and
Hurley, 1983) were not identified in chicken excreta or tissues.
Summary data provided to the meeting (from Obrist et al 1983)
suggest that residues in cow tissues and milk are similar
qualitatively, and in some respects quantitatively, to those in
chicken tissues except that the dihydroxy-bitertanol found in chicken
livers was reportedly not identified in dairy cow tissues or excreta.
Cow urine reportedly contained bitertanol acid and
p-hydroxy-bitertanol acid, also identified in rat urine (Phul et
al 1979b). The cow study itself was not provided. Further evaluation
must await availability of the study.
Table 6. Tissue and plasma residues in chickens dosed with 14C
bitertanol
14C residue
Tissue Bitertanol equivalent,mg/kg % organo-soluble
Mean Max
liver 6.2 12.6 86
kidney 4.3 10.3 91
breast muscle 0.35 0.91 95.2
leg muscle 0.48 1.1
fat 1.4 3.01 93.2
gizzard 0.72 1.7 90.8
heart 1.3 3.2 92.9
skin 0.63 1.4 94.7
blood plasma 1.8 4.5 --
Table 7. Composition of residues in chicken tissues following administration of multiple oral doses of
bitertanol-phenyl-UL 14C at 8.0 mg/kg (from Obrist and Phul, 1982)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
Bitertanol 34.7 27.2 67.8 62.4 66.0 78.2 74.9 36.6
p-Hydroxy-bitertanol
Free 14.7 27.7 23.2 14.8 17.1 12.1 13.2 6.4
Conjugateda 25.4 24.8 1.5 10.4 4.4 - 4.2 37.9
Hydroxy, Methoxy-bitertanol
Free 0.2 1.0 - - - - - 0.3
Conjugateda 1.5 1.2 - - - - - 0.7
Bitertanol alcohol
Free 1.2 1.8 - - - - - 0.5
Conjugated 0.8 1.1 - - - - - -
Dihydroxy-bitertanolb 0.9 - - - - - - -
p-Hydroxy-bitertanol alcohol
Free 0.4 0.9 - - - - - 0.3
Conjugateda 0.2 - - - - - - -
p-Hydroxy bipbenyl 0.2 0.2 - - - - - 13
Table 7. (continued)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
p,p'-Dihydroxybiphenyl 0.7 0.2 - - - - - -
Unidentified residue 11.9 12.0 4.9 5.9 5.4 2.9 2.4 9.3
Unextracted 7.2 1.9 2.6 6.5 7.1 6.8 5.3 6.7
TOTALS 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
The accumulation, persistence and composition of residues of
14C-bitertanol in bluegill fish maintained in aquarium water
fortified at 10 and 10 mg/l for 30 days has been investigated (Lamb,
1979). Residues on a whole fish basis reached a plateau in both cases
by three days at 2.1 and 16.2 mg/kg at the 10 and 100 mg/l
concentrations respectively, with maximum concentration factors of 203
and 174 respectively. Residues were quantitatively extracted with
acetonitrile with 57-66% of the radioactivity in non-edible parts
(head, viscera, scales) and 34-43% in edible parts. Residues were
characterized by TLC as bitertanol II 40%; bitertanol I 34%;
metabolite R-2 (unidentified) 7%; remaining at origin 13%; other
unidentified 6%.
Approximately 96% of the residues were excreted within 3 days
when the fish were transferred to untreated water.
In soil
Adsorption/desorption. In soil adsorption/desorption studies
bitertanol was shown to be strongly adsorbed to three soils in the
decreasing order: sand, loam and silty clay with adsorption
coefficients (K values) of 39.9, 35.8 and 19.6 (Phul and Hurley,
1979a). The data conformed to the Freundlich equation and in each case
>84% was adsorbed under experimental conditions. Only 4-10% of the
adsorbed compound was desorbed from sand.
Leaching. In TLC leaching experiments six soil types were examined
by comparing Rf values (Obrist and Thornton, 1979). The Rf values
ranged from 0.04 to 0.22, which places bitertanol in the two lowest
mobility ranges of the five classes which have been proposed (Helling
and Turner, 1968; Helling et al 1971). This is consistent with the
high adsorption determined in adsorption experiments. No significant
difference was observed between the two bitertanol isomers.
In aged soils (moist sandy loam) 44% of the initial radioactivity
was lost during room temperature incubation for 30 days, probably by
volatilization (Obrist, 1979). Less than 2% of the aged applied
bitertanol was found in the leachate from 30 cm soil columns, leached
at an equivalent of 13 mm/day for 45 days. Except for 55% further
losses during leaching, most of the remaining residue was in the upper
1.3 cm of the column.
Similar results were found in 19 studies with either Dutch polder
soil or a standard loamy sand containing aged bitertanol residues
leached at an equivalent of 200 mm for 48 hours or up to 500 mm in
polder soil after 72 additional hours (Brennecke, 1983). Only 0.8% of
the aged soil residue was found in the leachate of polder soil after
48 hours, and 3% after 120 hours. In experiments with 11 standard
soils, the leachates contained <6.6% of the original radioactivity in
the aged soil in all but one sample, where 48.9% was in the leachate
and was identified as the bitertanol soil metabolite
4-[2-hydroxy-3,3-dimethyl-(1H-1,2,4-triazol-1-yl)butoxybenzoic acid
(BUE 2684). A repeat of this experiment resulted in only 2.1% or less
of aged residue in the leachate, although less leachate (approximately
400 ml) was collected in the repeat, compared with approximately 650
ml in the original experiment. No unchanged bitertanol was found in
any leachate. Most leachate residues were BUE 2684 and traces of its
methyl ester.
The low leaching potential of bitertanol under experimental
conditions was supported by 30 additional reports provided to the
meeting.
Soil persistence. The soil persistence of bitertanol applied to 7
soil types as the 25 WP formulation at 11 kg a.i./ha to field plots in
9 studies in scattered locations in North America was reported in 9
Mobay reports (68296-68304). Half-lives ranged from 10 to 39 days with
an average of 25 days, except in one study with Oregon loam in which
the half-life was 131 days. The exceptionally long half-life did not
appear to be accounted for by temperature, rainfall, pH or
geographical area when compared to other studies.
Under laboratory aerobic conditions the half-life on silt loam
ranged from 14 to 20 days (Phul and Hurley, 1979b). Degradation was
slower under anaerobic conditions and there was no significant
degradation in sterilized soil after 31 days, showing the importance
of soil micro-organisms in bitertanol degradation. Both
diasterioisomers of bitertanol were degraded at approximately the same
rates.
Under laboratory conditions bitertanol decomposed faster in
alluvial loam soil (12 day half-life) than volcanic ash loam soil (30
days half-life), while under field conditions decomposition was faster
with volcanic ash/sandy loam than with alluvial loam soil (Takase and
Yoshimoto, 1980). In other laboratory tests bitertanol had a
half-life of 26 days in type 1 soil and 72-days in type 2 (Mobay
Report No. 10.317/18), while in field tests half-lives ranged from 19
to 27 days (Mobay Reports NO. 10.324/79, 10.325/79, 10 311/80 and
10.31280).
Rotational Crops. The uptake of 14C-biphenyl ring-labelled WP
bitertanol by rotational crops planted 31, 118 and 364 days after the
last of eight applications to outdoor tub-planted peanuts at an
equivalent of 0.56 kg a.i./ha resulted in 14C residues in leafy
vegetables at harvest ranging from 0.1 mg/kg bitertanol equivalent in
the 118-day planting to 0.02 mg/kg in the 364-day planting (Phul et
al. 1981). In sugar beets residues were 0.38 and 0.01 mg/kg for the
same plantings, and in wheat heads 0.23 and 0.01 mg/kg for 31-day and
364-day plantings. Residues in wheat straw were 1.4 mg/kg from the
31-day planting. The organo-soluble portion of the residue was
<0.04 mg/kg bitertanol equivalent in all at-harvest samples except
for 0.51 mg/kg in wheat straw. The benzoic acid derivative of
bitertanol, BUE 2684 was identified in the 31-day wheat samples.
Soil transformation. The fate of bitertanol has been investigated
in silt loam soil (pH 7.4) under aerobic and anaerobic conditions and
in sterile soil (Phul and Hurley, 1979b). Soil was fortified at 1 and
10 mg/kg for aerobic conditions and 1 mg/kg for anaerobic and sterile
conditions. One mg/kg approximates the maximum residues expected from
recommended field rates of 0.56 kg a.i./ha applied to peanuts. In the
1 mg/kg experiment, bitertanol (I + II) and total organo-solubles
decreased from 98.6 and 99.8% of the total 14C on the first day to 6
and 8.4% after 121 days, while 14CO2 and unextracted residues
increased from 0.8 and 4.6% on day 3 to 45.8 and 45% of the total
radioactivity, respectively, after 121 days. A similar trend was
observed with the 10 mg/kg fortification over the 29-day period
examined. The two diasterioisomers of the bitertanol benzoic acid
metabolite were observed at the 10 mg/kg fortification level (8.6% of
the total radioactivity) but not at the 1 mg/kg level.
Degradation was somewhat slower under anaerobic conditions than
aerobic, but the distribution (bitertanol, total organic, 14CO2 and
unextracted) was not markedly different at comparable intervals.
Bitertanol still accounted for 95.4% of the total 14C in sterile
soil after 31 days compared to 11% in non-sterile soil, indicating the
importance of soil microbes in the metabolism of bitertanol; this was
further demonstrated in another study (Phul et al 1979a). The
identity of the microbes present in the soil of the latter study was
investigated by Smedly and Hepler (1979).
In other studies (Brennecke, 1982) 14C-biphenyl-labelled
bitertanol was shown to be mineralized primarily into 14CO2, up to
68% in "standard soil 2.1" and 49% in Netherlands polder soil. The
bitertanol benzoic acid metabolite BUE 2684 (<19.1%) and an unknown
(<1%) were also found in one of 5 experiments in standard soil.
Bitertanol ketone (BUE 1662) was identified by TLC at <1.7% of the
total radioactivity in polder soil.
In water
The stability of bitertanol in sterile aqueous solutions has been
studied (Nichols and Thornton, 1979). No apparent degradation was
observed after thirty days in sterile aqueous solutions, buffers at pH
4, 7 and 9 and maintained at 25 and 40°C, which contained 0.25 and 2.5
mg/l of bitertanol. After 30 days, 94-109% of the bitertanol was
accounted for at the higher concentrations and 85-98% at the lower
level. The 85% recovery was from 0.25 mg/l at pH 9 and 40°C. The
slightly lower recovery at the lower fortification level was
attributed to adsorption to the vials. This is consistent with the
adsorption observed at low concentration in other studies (Phul and
Hurley, 1979). After using a correction factor to account for vial
glass adsorption, the recovery was 100% under all conditions of this
study.
Additional information on the hydrolytic stability of bitertanol
was provided (Stegh, 1980) but not in the working language of the
meeting.
Photolysis
The photolysis of bitertanol has been investigated on soil and
silica surfaces and in water. In the soil surface experiment,
bitertanol-treated silt loam of 1 mm thickness irradiated for 35 days
did not show substantially different extractability (91.5%) from dark
controls (96.6%), (Sietsema, 1982). Volatile radioactivity accounted
for <0.1% of the original amount. The extracted residue was shown to
be bitertanol by mass spectrometry.
A study on the photolysis of bitertanol on silica gel places
(Stegh and Wilmes, 1982) and two reports on the photolysis in water
and on silica gel (Wilmes, 1980; Stegh, 1980) were provided, but not
in the working language of the meeting. Another report (Wilmes, 1981)
discussed the hydrolytic behaviour and stability of bitertanol in
solution. No data were provided, but reference was made to the two
studies above which included data. Another bitertanol photolysis study
(Sietsema, 1983) was brought to the attention of the meeting, but was
not included in the data provided.
METHODS OF RESIDUE ANALYSIS
An analytical method developed for the analysis of triadimefon in
apples (Thornton, 1977) has been used for bitertanol in apples. The
sample is ground with dry ice, blended with acetone and filtered. The
filter cake is blended with dichloromethane and filtered, and the
combined filtrates are partitioned with water, taken to dryness and
cleaned up on a Florisil column with 60:40 hexane:ethyl acetate as
eluant. The eluate is concentrated and bitertanol determined by GLC
using an alkali flame detector.
The procedure was validated for bitertanol in apples at
0.05-0.1 mg/kg with recoveries of 86-108% and a blank of
<0.01 mg/kg (Morris, 1979a) Although detection was easily attainable
at 0.05 mg/kg and probably 0.01 mg/kg, chromatograms showed
interferences which would make quantitation below 0.05 mg/kg
questionable.
Interference studies have demonstrated that the basic procedure
above permits analysis of bitertanol at levels equivalent to 0.1 mg/kg
in samples in the presence of tolerance levels of 75 pesticides
registered in the U.S.A. for apples or peanuts (1979), five compounds
registered for bananas (1980) and three compounds registered for
cherries, plums, peaches and pears (1981) (Obrist and Nichols,1979).
Several compounds interfered, but could be eliminated by a 1-hour
reflux with 1 N NaOH and zinc or by partitioning with 0.1 N HC1.
However, analytical recoveries from a crop matrix with these steps
incorporated were not provided.
Another analytical procedure (also suitable for other crop
protectants) has been tested for bitertanol on apples, bananas, beans,
currants, cherries, damsons, wheat (green, grain and straw) and soil
(Specht and Tillkes, 1980). Many of the analyses in the field trials
were conducted with this basic procedure. Samples are extracted with
2:1 acetone: water, partitioned with dichloromethane, cleaned up by
gel permeation chromatography and analyzed by GLC utilizing a flame
alkali detector. Where necessary (e.g. for straw) an additional silica
gel column clean-up is used. Analytical recoveries were 74-112%,
although fortification levels were described only as "0.02-0.1 ppm
(lower limit of determination), and 1.0-3.0 mg/kg". For the compounds
tested, the authors concluded that the lower limit of determination
was 0.02 to 0.05 mg/kg for plants with >70% water content and 0.1
mg/kg for cereals and soil. The information was not sufficient for the
meeting to draw a conclusion on the limit of determination.
A method developed for triadimefon in soil and water (Thornton
and Lloyd, 1977) is also suitable for bitertanol in soil and water
with minor modifications in the GLC column stationary phase (Morris,
1979b). Water is simply extracted with chloroform, the extract is
concentrated (a Florisil column clean-up is optional), and analyzed by
GLC using a flame-alkali detector. Soil is extracted by refluxing with
methanol/water, partitioned with chloroform, washed with water and
cleaned up on a Florisil column. Recoveries from water fortified at
0.005 and 0.01 mg/l were 94% and 78% respectively with a blank of
<0.001 mg/l. Recoveries from six soils fortified at 0.05-0.5 mg/kg
ranged from 76 to 121% (most were >90%) with blanks <0.01 mg/kg
(Morris, 1979b). Chromatograms were consistent with the data.
In a similar procedure for the analysis of bitertanol and
numerous other fungicides in water (Brennecke and Vogeler, 1980),
extraction is with dichloromethane and clean-up by gel permeation
chromatography. The two recoveries for bitertanol were 108% and 104%
for 0.005 and 0.5 mg/l fortifications respectively. The lower limit of
determination was said to be 0.005 mg/l, although the meeting could
not confirm this with the available data.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs were reported to the meeting.
Safety
interval MRL
Country Crop (days) mg/kg
Australia Apples 14 1.0 1)
Peanuts 14 0.2 1) 2)
Belgium Apples 42 0.25
Pears 42 0.25
France Apples 15 1.0
Pears 15 1.0
Germany, Federal Apples 14
Republic Cherries 21
Great Britain Apples 21
Pears 21
Greece Apples 21
Beans 4 crops 14
Pears 5 PHIs 7
Stone fruit 14
10
Israel Apples 14
Italy Pears 14
Beets 30 1.0
Fruit 1.0
Pome fruit 21
Stone fruit 21
Vegetables 14 0.5
Morocco Beans 15
Netherlands Apples 14
Blackberries 0.05 2)
Cucumbers 3
Fruiting Vegetables 1.0
Melons 3
Pears 14
Peppers 3
Other commodities 03)(0.05)
New Zealand Pome fruit 14
Strawberries 21
Safety
interval MRL
Country Crop (days) mg/kg
South Africa Apricots 56 0.5
Beans 28 0.1
Beans (fodder) 42
Peaches 56 0.5
Peanuts 0.05
Peanuts (fodder) 42
Plums 56 0.5
Spain Pome fruit 15 1)
Stone fruit 15 1)
Switzerland Pome fruit 42 0.6
Venezuela Apples 15 1.0
Broad beans 28 1.0
Peanuts 0.2 (1)
Pears 15 1.0
Stone fruit 42 0.6
Yugoslavia General 28
1) = Preliminary
2) = Level at or about the limit of determination.
3) = No residues should be present. Limit of determination 0.05 mg/kg.
APPRAISAL
Bitertanol is a relatively new broad-spectrum fungicide
introduced and being introduced in a number of countries. Its
toxicology was evaluated by the 1983 JMPR which estimated a temporary
ADI. The present meeting evaluated residues in food.
To date only limited residue trials data have been provided and
these have been evaluated. Data were insufficient to estimate residue
levels in cucumbers and beans, but temporary limits were estimated for
several commodities.
The fate of bitertanol has been investigated in plants (apples
and peanuts), animals, soil, light and water.
In the plant studies bitertanol penetration from surface
applications was minimal and there was little volatility. Residues are
taken up by roots, although translocation into the plant is relatively
low. There are some measurable but not major differences in
absorption, translocation and plant metabolism rates of the
two diasterioisomeric forms of bitertanol. Bitertanol is by far
the main plant residue, with low levels of its ketone and
4-hydroxybiphenyl. In apples, most of the residue in the peel.
The fate of residues in non-food animals was evaluated by the
1983 JMPR. The present meeting evaluated metabolism studies on
poultry, cows and fish. Bitertanol is metabolized substantially more
in animals than in plants. The products are rapidly excreted but low
residues can occur in milk, eggs and tissues. These are predominantly
in the liver and kidney, as demonstrated with exaggerated feeding
levels. Bitertanol and p-hydroxy-bitertanol are by far the predominant
residues in chicken tissues and eggs and are reported to be the major
residues in cow tissues and milk, although other metabolites occur at
significant levels in the latter. Although cow metabolism studies were
available to the meeting the only one detailing the distribution of
identified residues was received too late for evaluation, until it has
been reviewed at a future meeting, no final conclusion can be drawn on
the adequacy of information on the fate of residues in animals. The
available data indicate that metabolism appears to be similar among
rats, poultry and cows.
Conventional feeding studies on poultry and ruminants, an
analytical method for the analysis of bitertanol in animal products,
and information on the fate of residues in apples during processing
were all received too late for evaluation.
Extensive information was available to the meeting on the fate of
bitertanol residues in soil, in which it is of low mobility, and some
data were available on water stability and photolysis. The water and
photolysis data available in the working language of the meeting
indicate a high degree of stability in each case. Other studies
provided, but not in the working language of the meeting, were not
evaluated.
Several analytical methods utilizing solvent extraction, GPC or
Florisil clean-up followed by gas chromatography are available for
determining residues in crops, soil and water. Further confirmation of
the limit of determination is desirable.
No information was provided on the fate of residues in storage,
in processing, in commerce or at consumption.
The meeting examined residue data from supervised trials
reflecting good agricultural practice on a number of crops and was
able to estimate the maximum residue levels which were likely to occur
when bitertanol was used in practice and when the reported intervals
between the last application and harvest were observed. These levels
refer only to the parent compound.
RECOMMENDATIONS
COMMODITY MRL (mg/kg)1 Pre-harvest interval on
which recommendations
are based
Apples 2 14
Stone fruit 1 21
Bananas 0.5 0
Oats:
grain 0.1* )
forage 0.1* )
straw 0.1* )
Rye:
grain 0.1* )
forage 0.1* )
straw 0.1* ) from seed treatment
Wheat:
grain 0.1* )
forage 0.1* )
straw 0.1* )
* At or about the limit of determination.
1 Temporary irrespective of the status of the ADI.
FURTHER WORK OR INFORMATION
1. Except for bananas for which substantial data reflecting approved
uses were available, additional information on good agricultural
practice and residue data reflecting approved uses for all
commodities for which temporary limits have been estimated.
Residue data should be from countries from which details of
approved uses have been or will be provided, or those in close
proximity.
2. Additional plant metabolism studies.
3. Additional residue data reflecting good agricultural practice for
additional plant products likely to be used as animal feeds.
Desirable
1. Analytical recoveries through the Thornton analytical method
utilizing the NaOH and HC1 steps designed to remove
interferences.
2. Confirmation of limits of determination for the Specht method.
3. Information on the stability of residues in crops during cold
storage.
4. Additional national maximum residue limits and safety intervals.
5. Photolysis/hydrolysis studies in English (Stegh and Wilmes, 1982;
Wilmes, 1980; Stegh 1980 and Sietsema, 1983).
REFERENCES
Mobay Reports are unpublished reports prepared by the Research and
Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, USA.
Nitokuno Reports are unpublished reports prepared by Nihon Tokushu
Noyaku Seizo KK., No. 8, 2-chome, Nihonbashi, Chuo-ku, Tokyo, Japan.
Bayer Reports:
Leaching in Soil
10.314 - 10.316/78 10.342 - 10.334/82
10.318 - 10.320/78 10.348 - 10.350/82a
10.334 - 10.336/80 10.374 - 10.376/82
10.334A - 10.346A/8010.379 - 10.381/82b
10.309 - 10.311/81 10.388 - 10.390/82b
Soil Persistence
10.317/78 10.324/7910.3257910.311/8010.312/80
68 296 68 29868 30068 30268 304
68 297 68 29968 30168 303
Brennecke, R. Leaching characteristics of Aged [biphenyl-UL-14C]
1983 bitertanol Soil Residues. Unpublished Bayer Report RA
981/209B, October 1983.
Brennecke, R. and Vogeler, K. Method for the Gas Chromatographic
1982 Determination of Residues of Various Fungicides in Water.
Bayer AG, Pflanzenschutz-Anwendungstechnik, unpublished
Report RA-80, January, 1982.
Helling, C.S. and Turner, B.C. Pesticide Mobility: Determination by
1968 Soil Thin-Layer Chromatography, Science 162, 562 (1968).
Helling, C.S., Kearney, P.C. and Alexander, M. Behaviour of Pesticides
1971 in soils, Advan. Agron. 23; 147, 1971.
Lamb, D.W., BAYCOR-14C Accumulation and Persistence of Residues in
1979 Bluegill. Mobay Report No. 68 343 December 13, 1979.
Mobay, Recovery Reports, Apples 68 249, Soil and Water 69 250.
Morris, R.A. Recovery of Baycor from Apples. Unpublished Mobay Report
1979a 68 249, November 19, 1979.
Morris, R.A. Recovery of Baycor from Soil and Water. Unpublished Mobay
1979b Report 68 250, 1979.
Nichols, S.S. and Thornton, J.S. The Behaviour of BAYCOR in Sterile
1979 Aqueous Solutions. Mobay Report No. 68 273. October 22,
1979.
Christ, J.J. Leaching Characteristics of Aged BAYCOR Soil Residues
1979a Mobay Report No. 68 271. September 25, 1979.
Obrist, J.J. and Nichols, S.S. An Interference Study for the BAYCOR
1979 Residue Method for Apples and Peanuts. Mobay Report No. 68
311. November 27, 1979.
Obrist, J.J., Phul, R.J. and Thornton, J.S. Excretion and Tissue
1981 Levels of 14C Following Oral Administration of BAYCOR-
Phenyl-UL-14C to a Dairy Cow. Mobay Report NO. 69 400.
March 24, 1981.
Obrist, J.J., Phul, R.J. and Thornton, J.S. The Metabolism and
1982 Excretion of BAYCOR-Phenyl-UL-14C by Chickens. Mobay Report
No 82614. October 8, 1982.
Obrist, J.J., Phul R.J. and Thornton, J.S. The Metabolism and
1983 Excretion of BAYCOR-Phenyl-UL-14C Following Oral
Administration to a Dairy Cow. Mobay Report, Manuscript in
Preparation. 1983.
Obrist, J.J. and Thornton, J.S. Soil Thin-Layer Mobility of BAYCOR,
1979 BAYTAN, DYRENE, and PEROPAL. Mobay Report No. 68 272.
October 22, 1979.
Phul, R.J. and Hurley, J.B. Soil Adsorption and Desorption of BAYCOR.
1979a Mobay Report No. 68 003. July 18, 1979.
Phul, R.J. and Hurley, J.B. The Metabolism and Degradation of BAYCOR-
1979b Biphenyl-UL-14C on Soil. Mobay Report NO. 67 998. August 6,
1979.
Phul, R.J. and Hurley, J.B. The Metabolism of BAYCOR-Biphenyl-UL-14C
1979c in Apple Fruit. Mobay Report No. 68 305. November 5, 1979,
Revised February 3, 1981A.
Phul, R.J. and Hurley, J.B. Metabolism of BAYCOR-UL-14C in Peanut
1981 Plants. Mobay Report No. 69 310. October 23, 1979, Revised
June 18, 1981b.
Phul, R.J. and Hurley, J.B. The Absorption, Excretion and Metabolism
1983 of BAYCOR-Phenyl-UL-14C by Rats. Mobay Report, Manuscript
in Preparation, 1983.
Phul, R.J., Hurley, J.B. and Close, C.L. Total Radioactive Residues in
1981 Rotational Crops Following a Target Drop of Peanuts Treated
with BAYCOR-14C. Mobay Report No.80 059. September 11,
1981.
Phul, R.J., Hurley, J.B. and Thornton, J.S. The effect of Soil
1979a Microorganisms on the Degradation of BAYCOR. Mobay Report
No. 69 012. July 2, 1979.
Phul, R.J., Obrist, J.J. and Pither, K.M. The excretion of
1979b BAYCOR-Phenyl-UL-14C Following Administration of a Single
Oral Dose to Rats. Mobay Report NO. 68 307. November 19,
1979.
Scheinpflug, H. and van den Boom, T. BAYCOR, A New Fungicide for
1981 Tropical and Subtropical Crops. PflanzenschutzNachrichten
Bayer 34, 8-28.1981.
Sietsema, W.K. Photodecomposition of BAYCOR on a Soil Surface. Mobay
1982 Report No. 82 572. November 16, 1982.
Sietsema, W.K. Photodegradation of BAYCOR in Water. Mobay Report (in
1983 Preparation). 1983.
Smedly, L.A. and Hepler, D.I. Identification of Microorganisms in
1979 Samples of Kansas Loam Soil. Mobay Report No. 67 814. May 1,
1979.
Specht, W. and Tillkes, M. Determination of Agrochemicals After
1980 Clean-up using Gel Permeation Chromatography and Mini-Silica
Gel Column Chromatography. Pflanzenschutz-Nachrichten Bayer
33, 61 - 85. 1980.
Stegh, R. Versuche zum Abbau von KWG 0599 in wässerigen Salzlosungen.
1980 Bayer Experimental Notice. January 24, 1980.
Stegh, R. and Wilmes, R. Light Induced Degradation of Agrochemical
1982 Parent Compounds on Silica Gel as a Model for Degradation on
Soil. Bayer Ag. Pflanzenschutz-Anwendungstechnik unpublished
report PF1666. February 10, 1982.
Takase, I. and Yoshimoto, Y. Residues of KWG 0599 in Upland Soils
1980 Under Laboratory and Field Conditions. Nitokuno Report No.
1134. December 5, 1980.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and its
1977a Metabolite in Apples. Mobay Report No. 54 166. December 30,
1977.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and KWG 0599
1977 in Soil and Water. Mobay Report NO. 51 231. January 31,
1977.
Wilmes, R. Preliminary Studies on Stability to Light. Bayer Pharma
1980 Unpublished Report, 1980.
Wilmes, R. Abiotic Degradation of Bitertanol. Bayer summary Report.
1981 March 31, 1981.
 Empirical formula: C20H23N3O2
Molecular weight: 337.4
RESIDUES IN FOOD
USE PATTERN
Bitertanol is a broad-spectrum fungicide said to be effective for
the control of scab and Monilinia fruit diseases, banana and peanut
leaf spot diseases, and rusts and mildews on a variety of crops. It is
available as a wettable powder, emulsifiable concentrate and
suspension concentrate, all applied by spraying. It is also used as a
seed treatment for cereals. Only the wettable powder is recommended
for pome fruit owing to possible foliage or fruit injury by the EC
formulation.
Bitertanol is said to be registered and marketed in over 20
countries in Europe, Africa and South America and registration is
being sought in additional countries. Recommendations by geographical
areas are listed in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue field trials have been conducted on a variety of crops,
primarily fruits and vegetables. Data provided to the JMPR are
summarized in Table 2.
Pome Fruit
Residues trials in apples have been conducted in four countries,
although according to Table 1 bitertanol is currently approved for use
in only one of the four. The trial in this country reflects European
uses. Where reported, controls were <0.01 mg/kg. With maximum
residues of 1.0 mg/kg at the recommended 14-day interval, these data
and the supporting data from other countries indicate that a 2 mg/kg
limit would be required with a 14-day pre-harvest interval. Additional
data are desirable from countries with approved uses.
Cucumber
Trials on cucumbers (under glass) were available from one country
with WP application at approximately the approved rate and EC at twice
the approved rate. Maximum residues were 0.41 mg/kg at the normal
harvest interval. Residues from the two EC applications were similar
to those from six with the WP at similar intervals. These limited data
indicate a possible need for a 0.5 mg/kg limit at a 3-day pre-harvest
interval, but are not sufficient to support a limit.
Peanuts
In trials in one country reflecting approved application rates
(but excessive numbers of applications) no residues were detected
(<0.1 mg/kg) 9-14 days after the last application. Analyses were
apparently on shelled nuts. Data are not sufficient to support a
limit.
Beans
Data were available from trials in three countries, but approved
use information was available from only one of these. From the country
with approved uses residues in dry bean pods at 16 days (28-day safety
interval required) were 0.1 - 0.18 mg/kg at the normal application
rate. This compares with 0.1 mg/kg in untreated controls. Similar
application rates in South America resulted in <0.05 mg/kg residues
(3 samples) at 26-43 days post-treatment, but it is not clear whether
the beans were shelled or in pod. Data are insufficient to support a
limit.
Table 1. Use Pattern - spray treatment
Region and Number of Safety interval,
Commodity g a.i./ha treatments days
Europe
Pome fruit 1, 2 187 - 375 6 - 12 14 - 42
Stone fruit 375 - 562 2 - 3 *
Snake gourd )
(Cucumber ) under
Peppers ) glass 9003 2 - 6 3
Melons )
South Africa
Peanuts 195 - 240 2 - 4 as dry feed 42
Stone fruit 140 - 240 2 - 3 28
Beans 126 - 250** 2 (as dry feed 42)
Asia
Bananas 125 - 150 26
South America
Bananas 125 18 - 26
* Not later than at the end of flowering stage; following this application no residues are
expected.
** aerial application
1 Registration (pre-harvest WP on apples) at 0.05 - 1.0% and a 28-day PHI is under way in
Finland.
2 Netherlands - 25 WP at 50-80 g a.i./100 1 and 300EC at 22.5 g/l. Both 14-day pre-harvest
interval.
3 300 EC in The Netherlands.
Seed treatment - Federal Republic of Germany
Wheat 75 g a.i./100 kg seed
Rye 56 g a.i./100 kg seed
Oat 56 g a.i./100 kg seed
Table 2. Bitertanol residues in crops resulting from supervised trials
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Pome fruit Bayer
Apples 187 - 375 g/ha 10-12 FRG 14 0.13 - 1.8 0.65 (6) 1 10303/79
(25% WP) (1979-1982) 21 0.12 - 1.0 0.43 (6) 10305/79
10313/80
10325/80
10304-5/82
Mobay
1-4 oz/100 gal. 6-14 USA 0 0.53 - 2.54 1.62 (5) 64382+83
(50% WP) (1979) 14 0.24 - 1.5 0.9 (5)
21 0.18 - 1.2 0.8 (4)
28 0.27 - 0.91 0.6 (4)
1-2 oz/100 gal. 5-8 Canada 66-67 0.06 - 0.10 0.07 (4) Mobay
(50% WP) (1983) 74-92 < 0.01 - 0.04 0.02 (12) 68282-86
0.8 g/tree 4 Finland Siltanen
(1979) 32 0.2 (1) and Makinen
(1980= 49 0.1 1980
5 (1981) 67 0.04 Siltanen
6 35 0.1 and Makinen
8 24 0.4 1981
Cucumbers 875 - 1200 g/ha 2(EC) NL 0- 1 0.4 - 0.7 0.54 (4) Bayer
(Snake gourds, (200 g/1 EC or 6(WP) (1979-1980) 3- 4 0.25 - 0.41 0.34 (3) 10326/79
under glass) 50% WP) 7-10 0.06 - 0.25 0.15 (5) 10314-15/80
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Peanuts 250 - 300 g/ha 4-5 S.Africa 9-14 < 0.1 < 0.1 (3) Bayer
(Shelled?) (300 g/L EC or (1979-1983) 10301/77
25% WP) 10313/78
10328/80
Beans 225 - 250 g/ha 4 Costa 26 0.05 0.05 (1) Bayer
(mature, dry) (300 EC) Rica 37 0.05 0.05 (1) 10332-33/80
Columbia 43 0.05 0.05 (1) 10342/81
(1981)
Beans 125 g a.i./ha S. Africa S. Africa
(dry) (300 EC) (not avail.) submission
whole plt. 1 1 23 : 30
4 2.5 : 3.4
8 3.7 : 3.8
16 0.36 : 0.3
250 g a.i./ha 1 43 : 39.4
(300 EC; 4 6 : 6.8
double rate) 8 4.5 : 5.0
16 0.3 : 0.32
pods 125 g a.i. /ha 1 11 sic? 2.7 : 2.4
(300 EC) 4 0.13: 9.19
8 0.15: 0.13
16 0.18: 0.10
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
250 g a.i./ha 11? 3.2 : 2.9
(300 EC; 4 0.16: 0.1
double rate) 8 0.4 : 0.4
16 0.23: 0.21
A B
Stone fruit A
Nectarines 100- 100 g/ha 4 S.Africa 0 0.53 - 1.5 SABS
(300 EC) (1982) 3 0.47 - 0.68 (1)* 311/88419
data: 10 0.21 - 0.54 (1) W 212
A = 0.125% a.i. 14 0.18 - 0.51
B = 0.25% a.i. 24 0.18 - 0.37 (1)
Peaches 100 - 200 g/ha 3 S. Africa 0 0.6 - 1.8 (1)* SABS
(300 EC) (1982-1983) 5 0.4 - 1.2 (1)* 311/88421/
data: 11 0.3 - - (1)* W214
A = 0.125% a.i. 19 0.2 - 0.5 (1)*
B = 0.25% a.i. 26 < 0.1 - - (1)*
ripe 375 g/ha 3 France 0 1.7
(25& WP) (1979) 7 1.2
14 1.3
21 0.91
300 & 400 g/ha 3 Italy 0 0.8 - 1.2(1)* (3.5cm fruit) Bayer
(300 EC) (1981) 7 0.3 - 0.4(1)* 10346-47/81
14 0.2 - 0.2(1)*
20 0.11- 0.13(1)* (5-7cmfruit)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Plums 250 - 370 g/ha 1 S. Africa 0 0.7 - 0.8(1)*
(300 EC) (1982) 3 0.8 - 0.8(1)*
data: 9 0.6 - 0.5(1)*
A = 0.025% a.i. 16 0.5 - 0.6(1)*
B = 0.0375% a.i.
Bananas 120 - 300 g/ha 6 - 26 Costa Rica Bayer
unbagged (EC 200-300; Columbia 2 0.015 0.015(1) 10309-11/78
pulp WP 25%)
Taiwan 4 ND ND (1) 10320-21/80
Kamerun 8 0.014-0.019 0.017(2) 10330-31/80
(1978-1980) 23 < 0.1 < 0.1 (1) 10350-80
40 ND ND (1) 10344-45/81
78 ND ND (1) 10366/81
peel 2 0.14 0.14(1)
4 ND ND (1)
8 0.12 - 0.15 0.14(2)
23 0.025 0.025(1)
40 ND ND (1)
78 ND ND (1)
total (calculated) 2 0.06 0.06(1)
4 ND ND (1)
8 0.047-0.055 0.051 (2)
23 < 0.01 < 0.01 (1)
40 ND ND (1)
78 ND ND (1)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged 0 ND ND (2)
pulp 2 ND ND (1)
6 ND ND (1)
8 < 0.01 < 0.01 (2)
11 ND ND (2)
12 ND ND (1)
peel 0 ND ND (2)
2 0.019 0.019 (1)
6 0.1 0.1 (1)
11 ND ND (2)
12 ND ND (1)
Total (calculated) 0 ND
2 < 0.01
6 < 0.01
8 < 0.01-0.015
11 ND
12 ND
Bananas, green 125 - 300 g/ha 9 - 12 Honduras 0/3 Mobay
unbagged, (300 EC) or Costa 68896-900
unwashed 25 or 50% WP) Rica - 80469-473
pulp (1980-1982 0.03-0.13 0.07(6)
peel 0.10-0.76 0.39(6)
total 0.06-0.33 0.19(6)
unbagged, washed pulp 0.03-0.17 0.09(5)
peel 0.09-0.73 0.37(5)
total 0.05-0.36 0.19(5)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, unwashed
pulp < 0.01-0.01 < 0.01(4)
peel < 0.01-0.05 0.03(4)
total < 0.01-0.03 0.02(4)
bagged, washed
pulp < 0.01-0.02 0.01(5)
peel 0.03-0.15 0.06(5)
total 0.02-0.06 0.03(5)
Bananas, ripe
unbagged, unwashed
pulp < 0.01-0.08 0.05(3)
peel 0.03-0.32 0.19(3)
total 0.01-0.17 0.10(3)
Bananas, ripe
unbagged, unwashed pulp 0.02-0.14 0.09(3)
peel 0.05-0.51 0.34(3)
total 0.03-0.28 0.18(3)
Bananas, ripe
bagged-unwashed
pulp < 0.01-0.01 < 0.01(3)
peel < 0.01-0.12 0.05(3)
total < 0.01-0.05 0.02(3)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, washed
pulp < 0.01-0.02 0.02(3)
peel 0.03-0.08 0.05(3)
total 0.01-0.04 0.03(3)
Oats, Green forage 56 g/100 kg seed 1 FRG 94/97 < 0.05 < 0.05(2) Bayer
Grain (375 FS) (1983) 140-141 < 0.05 < 0.05(2) 10212+13/83
Straw 140/I41 < 0.05 < 0.05(2)
Rye, Green Forage 56 ml/100 kg seed 1 FRG 220/224 < 0.05 < 0.05(2) 10210+11/83
Grain (375 FS) (1983) 291/295 < 0.05 < 0.05(2)
Straw 291/295 < 0.05 < 0.05(2)
Wheat, Green Forage 75 ml (g)/100 kg seed 1 FRG 56-77 < 0.1 < 0.1 (7) 10341+43/80
Ears (1980-1982) 73-90 < 0.05-0.1 < 0.05-0.1(5) 10370+71/80
Grain q (375 FS) 130-154 < 0.05-0.1 < 0.05-0.1(5)
Straw 87-154 < 0.05-0.1 < 0.05-0.1(7)
Oats, Green Forage 200g product/100 1 FRG 63-86 < 0.1 < 0.1 (1) 10366+67/82
Ears kg seed (0.132 (1982) 75-86 < 0.1 < 0.1 (2)
Grain q kg a.i./ha) 131-139 < 0.05 < 0.05(2)
Straw (37.5% DS) 131-139 < 0.05 < 0.05(2)
Rye Green Forage 0.08-0.11 kg 1 FRG 219-254 < 0.1 < 0.1 (7) 10363-65/82
Ears a.i./ha(150 g (1981-1982) 228-254 < 0.1 < 0.1 (4) 10372-82
Grain production/100 kg - 291-308 < 0.05 < 0.05(4)
Straw seed) (37.5 or 291-308 < 0.05 < 0.05 (4)
3.75 DS)
1 Number in parenthesis = number of samples
* One sample per dose level (mean of duplicate analyses)
Stone Fruit
Data were available from three countries, all of which have
approved uses, or are in close geographical proximity to countries
with them. While European trials on peaches reflect recommended
European dosage rates, applications were apparently made after the end
of flowering, and therefore do not appear to reflect the recommended
time of application (Table 1) for Europe. South African trials also
reflect recommended application rates in that country, but the pre-
harvest interval was not available to the meeting. The only available
PHI for stone fruit was 21 days for cherries in one European country
(see "National maximum residue limits". A 1 mg/kg limit for stone
fruit can be supported at a 21-day pre-harvest interval. Additional
residue data and good agricultural practice information for stone
fruit are desirable, especially for small fruit such as cherries.
Residues on peaches were significantly more persistent from WP
applications than from EC formulations.
Bananas
Supervised trials data were available for three Asian and three
South American countries and generally reflected the application rates
and number of applications (WP or EC) recommended in those
geographical areas. Since no national pre-harvest intervals were
provided, it must be assumed that application on the day of harvest,
as included in the trials, reflects practice. Data were available for
both green and ripe, bagged and unbagged, and washed and unwashed
bananas and on pulp and peel. In general, average residues were
approximately 5-10 times higher in peel than in pulp, whether ripe or
green, bagged or unbagged, washed or unwashed. As might be expected,
residues were higher on unbagged fruit. Washing (in the field) does
not appear to affect average residues significantly.
Maximum residues on bagged bananas (whole, green, washed) were
0.06 mg/kg. Maximum residues on whole ripe, unbagged, washed bananas
were 0.28 mg/kg as compared to 0.36 mg/kg for green, unbagged, washed
bananas.
Untreated controls (peel, pulp or whole) were all <0.01 mg/kg. A 0.5
mg/kg limit was estimated.
Cereal crops
No residues of bitertanol were detected in the green forage,
ears, grain or straw of oats, rye or wheat when the seeds were treated
pre-plant with recommended rates. The limit of determination ranged
from 0.05 - 0.1 mg/kg among the 15 studies. The available data would
support a limit of 0.1 mg/kg (at the limit of determination) limit for
seed treatments only. Additional data from other countries with
approved uses for cereal grains are desirable.
FATE OF RESIDUES
General
The fate of bitertanol has been studied in animals, plants, soil
water and light. A proposed metabolic pathway is given in Figure 1.
The structures and chemical names are presented in Table 3 which also
indicates where the compounds have been identified. Although the
metabolic picture is somewhat limited in scope, the data in general
demonstrate that metabolism in animals is much more extensive than
degradation by other routes. Bitertanol is the predominant residue in
plants. Bitertanol and p-hydroxybitertanol are the major residues in
animals.
In plants
Uptake and translocation. Golden Delicious variety apples were
individually syringe-treated to run-off with a 50% wettable powder
formulation at 0.15 g a.i./l of 14C-bitertanol labelled
uniformly (UL) in the biphenyl ring. Sampling was immediately after
drying, at 3 and 7-days and thereafter at 7-day intervals through 49
days (Phul and Hurley, 1979c). Analysis was by TLC using
radiochromatogram scanning and autoradiography techniques. The peel
contained 95% of the total recovered radioactivity even after 49 days,
the rest being in the pulp, showing that little migration had
occurred. Surface residues decreased from 92% at day 0 to 43% at 49
days while the radioactivity in the peel organic extract increased
from 7 to 43.5% of the total. Peel solids increased to 12% in the same
interval.
The two diasterioisomers of bitertanol were the major residues,
in roughly equal proportions throughout the 49-day study. The level of
the two combined in peel and pulp ranged from 98.4% of the total
radioactivity at day 0 to 83% at 49 days, with lower surface residues
and greater peel uptake by the end of that time. The two had
penetrated the pulp by day 7 from which time residues were relatively
constant at about 3% over the remaining test period. Minor metabolites
were bitertanol ketone and 4-hydroxy-biphenyl, together never
exceeding 3% of the total radioactivity. All of these metabolites were
in the peel. The half-life of bitertanol in this experiment was
estimated at approximately 150 days.
The metabolism of bitertanol by peanut plants has also been
studied (Phul and Hurley, 1981). In preliminary studies, the authors
obtained evidence that foliar applications result in poor leaf
absorption (96% unchanged bitertanol on the leaf surface after 14
days), with no observable volatility losses. Only very little
translocation (acro- and basi-petal) was observed, as well as little
translocation to opposite leaves Bitertanol - 14C uniformly labelled
in the biphenyl ring was sprayed as a 50% WP formulation to both young
(1 mo.) and old (2 mo.) potted peanut plants at an approximate
equivalent field rate of 0.5 kg a.i./ha. Young plants were sampled at
Empirical formula: C20H23N3O2
Molecular weight: 337.4
RESIDUES IN FOOD
USE PATTERN
Bitertanol is a broad-spectrum fungicide said to be effective for
the control of scab and Monilinia fruit diseases, banana and peanut
leaf spot diseases, and rusts and mildews on a variety of crops. It is
available as a wettable powder, emulsifiable concentrate and
suspension concentrate, all applied by spraying. It is also used as a
seed treatment for cereals. Only the wettable powder is recommended
for pome fruit owing to possible foliage or fruit injury by the EC
formulation.
Bitertanol is said to be registered and marketed in over 20
countries in Europe, Africa and South America and registration is
being sought in additional countries. Recommendations by geographical
areas are listed in Table 1.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue field trials have been conducted on a variety of crops,
primarily fruits and vegetables. Data provided to the JMPR are
summarized in Table 2.
Pome Fruit
Residues trials in apples have been conducted in four countries,
although according to Table 1 bitertanol is currently approved for use
in only one of the four. The trial in this country reflects European
uses. Where reported, controls were <0.01 mg/kg. With maximum
residues of 1.0 mg/kg at the recommended 14-day interval, these data
and the supporting data from other countries indicate that a 2 mg/kg
limit would be required with a 14-day pre-harvest interval. Additional
data are desirable from countries with approved uses.
Cucumber
Trials on cucumbers (under glass) were available from one country
with WP application at approximately the approved rate and EC at twice
the approved rate. Maximum residues were 0.41 mg/kg at the normal
harvest interval. Residues from the two EC applications were similar
to those from six with the WP at similar intervals. These limited data
indicate a possible need for a 0.5 mg/kg limit at a 3-day pre-harvest
interval, but are not sufficient to support a limit.
Peanuts
In trials in one country reflecting approved application rates
(but excessive numbers of applications) no residues were detected
(<0.1 mg/kg) 9-14 days after the last application. Analyses were
apparently on shelled nuts. Data are not sufficient to support a
limit.
Beans
Data were available from trials in three countries, but approved
use information was available from only one of these. From the country
with approved uses residues in dry bean pods at 16 days (28-day safety
interval required) were 0.1 - 0.18 mg/kg at the normal application
rate. This compares with 0.1 mg/kg in untreated controls. Similar
application rates in South America resulted in <0.05 mg/kg residues
(3 samples) at 26-43 days post-treatment, but it is not clear whether
the beans were shelled or in pod. Data are insufficient to support a
limit.
Table 1. Use Pattern - spray treatment
Region and Number of Safety interval,
Commodity g a.i./ha treatments days
Europe
Pome fruit 1, 2 187 - 375 6 - 12 14 - 42
Stone fruit 375 - 562 2 - 3 *
Snake gourd )
(Cucumber ) under
Peppers ) glass 9003 2 - 6 3
Melons )
South Africa
Peanuts 195 - 240 2 - 4 as dry feed 42
Stone fruit 140 - 240 2 - 3 28
Beans 126 - 250** 2 (as dry feed 42)
Asia
Bananas 125 - 150 26
South America
Bananas 125 18 - 26
* Not later than at the end of flowering stage; following this application no residues are
expected.
** aerial application
1 Registration (pre-harvest WP on apples) at 0.05 - 1.0% and a 28-day PHI is under way in
Finland.
2 Netherlands - 25 WP at 50-80 g a.i./100 1 and 300EC at 22.5 g/l. Both 14-day pre-harvest
interval.
3 300 EC in The Netherlands.
Seed treatment - Federal Republic of Germany
Wheat 75 g a.i./100 kg seed
Rye 56 g a.i./100 kg seed
Oat 56 g a.i./100 kg seed
Table 2. Bitertanol residues in crops resulting from supervised trials
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Pome fruit Bayer
Apples 187 - 375 g/ha 10-12 FRG 14 0.13 - 1.8 0.65 (6) 1 10303/79
(25% WP) (1979-1982) 21 0.12 - 1.0 0.43 (6) 10305/79
10313/80
10325/80
10304-5/82
Mobay
1-4 oz/100 gal. 6-14 USA 0 0.53 - 2.54 1.62 (5) 64382+83
(50% WP) (1979) 14 0.24 - 1.5 0.9 (5)
21 0.18 - 1.2 0.8 (4)
28 0.27 - 0.91 0.6 (4)
1-2 oz/100 gal. 5-8 Canada 66-67 0.06 - 0.10 0.07 (4) Mobay
(50% WP) (1983) 74-92 < 0.01 - 0.04 0.02 (12) 68282-86
0.8 g/tree 4 Finland Siltanen
(1979) 32 0.2 (1) and Makinen
(1980= 49 0.1 1980
5 (1981) 67 0.04 Siltanen
6 35 0.1 and Makinen
8 24 0.4 1981
Cucumbers 875 - 1200 g/ha 2(EC) NL 0- 1 0.4 - 0.7 0.54 (4) Bayer
(Snake gourds, (200 g/1 EC or 6(WP) (1979-1980) 3- 4 0.25 - 0.41 0.34 (3) 10326/79
under glass) 50% WP) 7-10 0.06 - 0.25 0.15 (5) 10314-15/80
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Peanuts 250 - 300 g/ha 4-5 S.Africa 9-14 < 0.1 < 0.1 (3) Bayer
(Shelled?) (300 g/L EC or (1979-1983) 10301/77
25% WP) 10313/78
10328/80
Beans 225 - 250 g/ha 4 Costa 26 0.05 0.05 (1) Bayer
(mature, dry) (300 EC) Rica 37 0.05 0.05 (1) 10332-33/80
Columbia 43 0.05 0.05 (1) 10342/81
(1981)
Beans 125 g a.i./ha S. Africa S. Africa
(dry) (300 EC) (not avail.) submission
whole plt. 1 1 23 : 30
4 2.5 : 3.4
8 3.7 : 3.8
16 0.36 : 0.3
250 g a.i./ha 1 43 : 39.4
(300 EC; 4 6 : 6.8
double rate) 8 4.5 : 5.0
16 0.3 : 0.32
pods 125 g a.i. /ha 1 11 sic? 2.7 : 2.4
(300 EC) 4 0.13: 9.19
8 0.15: 0.13
16 0.18: 0.10
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
250 g a.i./ha 11? 3.2 : 2.9
(300 EC; 4 0.16: 0.1
double rate) 8 0.4 : 0.4
16 0.23: 0.21
A B
Stone fruit A
Nectarines 100- 100 g/ha 4 S.Africa 0 0.53 - 1.5 SABS
(300 EC) (1982) 3 0.47 - 0.68 (1)* 311/88419
data: 10 0.21 - 0.54 (1) W 212
A = 0.125% a.i. 14 0.18 - 0.51
B = 0.25% a.i. 24 0.18 - 0.37 (1)
Peaches 100 - 200 g/ha 3 S. Africa 0 0.6 - 1.8 (1)* SABS
(300 EC) (1982-1983) 5 0.4 - 1.2 (1)* 311/88421/
data: 11 0.3 - - (1)* W214
A = 0.125% a.i. 19 0.2 - 0.5 (1)*
B = 0.25% a.i. 26 < 0.1 - - (1)*
ripe 375 g/ha 3 France 0 1.7
(25& WP) (1979) 7 1.2
14 1.3
21 0.91
300 & 400 g/ha 3 Italy 0 0.8 - 1.2(1)* (3.5cm fruit) Bayer
(300 EC) (1981) 7 0.3 - 0.4(1)* 10346-47/81
14 0.2 - 0.2(1)*
20 0.11- 0.13(1)* (5-7cmfruit)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
Plums 250 - 370 g/ha 1 S. Africa 0 0.7 - 0.8(1)*
(300 EC) (1982) 3 0.8 - 0.8(1)*
data: 9 0.6 - 0.5(1)*
A = 0.025% a.i. 16 0.5 - 0.6(1)*
B = 0.0375% a.i.
Bananas 120 - 300 g/ha 6 - 26 Costa Rica Bayer
unbagged (EC 200-300; Columbia 2 0.015 0.015(1) 10309-11/78
pulp WP 25%)
Taiwan 4 ND ND (1) 10320-21/80
Kamerun 8 0.014-0.019 0.017(2) 10330-31/80
(1978-1980) 23 < 0.1 < 0.1 (1) 10350-80
40 ND ND (1) 10344-45/81
78 ND ND (1) 10366/81
peel 2 0.14 0.14(1)
4 ND ND (1)
8 0.12 - 0.15 0.14(2)
23 0.025 0.025(1)
40 ND ND (1)
78 ND ND (1)
total (calculated) 2 0.06 0.06(1)
4 ND ND (1)
8 0.047-0.055 0.051 (2)
23 < 0.01 < 0.01 (1)
40 ND ND (1)
78 ND ND (1)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged 0 ND ND (2)
pulp 2 ND ND (1)
6 ND ND (1)
8 < 0.01 < 0.01 (2)
11 ND ND (2)
12 ND ND (1)
peel 0 ND ND (2)
2 0.019 0.019 (1)
6 0.1 0.1 (1)
11 ND ND (2)
12 ND ND (1)
Total (calculated) 0 ND
2 < 0.01
6 < 0.01
8 < 0.01-0.015
11 ND
12 ND
Bananas, green 125 - 300 g/ha 9 - 12 Honduras 0/3 Mobay
unbagged, (300 EC) or Costa 68896-900
unwashed 25 or 50% WP) Rica - 80469-473
pulp (1980-1982 0.03-0.13 0.07(6)
peel 0.10-0.76 0.39(6)
total 0.06-0.33 0.19(6)
unbagged, washed pulp 0.03-0.17 0.09(5)
peel 0.09-0.73 0.37(5)
total 0.05-0.36 0.19(5)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, unwashed
pulp < 0.01-0.01 < 0.01(4)
peel < 0.01-0.05 0.03(4)
total < 0.01-0.03 0.02(4)
bagged, washed
pulp < 0.01-0.02 0.01(5)
peel 0.03-0.15 0.06(5)
total 0.02-0.06 0.03(5)
Bananas, ripe
unbagged, unwashed
pulp < 0.01-0.08 0.05(3)
peel 0.03-0.32 0.19(3)
total 0.01-0.17 0.10(3)
Bananas, ripe
unbagged, unwashed pulp 0.02-0.14 0.09(3)
peel 0.05-0.51 0.34(3)
total 0.03-0.28 0.18(3)
Bananas, ripe
bagged-unwashed
pulp < 0.01-0.01 < 0.01(3)
peel < 0.01-0.12 0.05(3)
total < 0.01-0.05 0.02(3)
Table 2 (continued)
Dose a i. No. of Days after
Crops (Formulation) Applications Country last Residue (mg/kg) Report
(year) Application Range Average No.
bagged, washed
pulp < 0.01-0.02 0.02(3)
peel 0.03-0.08 0.05(3)
total 0.01-0.04 0.03(3)
Oats, Green forage 56 g/100 kg seed 1 FRG 94/97 < 0.05 < 0.05(2) Bayer
Grain (375 FS) (1983) 140-141 < 0.05 < 0.05(2) 10212+13/83
Straw 140/I41 < 0.05 < 0.05(2)
Rye, Green Forage 56 ml/100 kg seed 1 FRG 220/224 < 0.05 < 0.05(2) 10210+11/83
Grain (375 FS) (1983) 291/295 < 0.05 < 0.05(2)
Straw 291/295 < 0.05 < 0.05(2)
Wheat, Green Forage 75 ml (g)/100 kg seed 1 FRG 56-77 < 0.1 < 0.1 (7) 10341+43/80
Ears (1980-1982) 73-90 < 0.05-0.1 < 0.05-0.1(5) 10370+71/80
Grain q (375 FS) 130-154 < 0.05-0.1 < 0.05-0.1(5)
Straw 87-154 < 0.05-0.1 < 0.05-0.1(7)
Oats, Green Forage 200g product/100 1 FRG 63-86 < 0.1 < 0.1 (1) 10366+67/82
Ears kg seed (0.132 (1982) 75-86 < 0.1 < 0.1 (2)
Grain q kg a.i./ha) 131-139 < 0.05 < 0.05(2)
Straw (37.5% DS) 131-139 < 0.05 < 0.05(2)
Rye Green Forage 0.08-0.11 kg 1 FRG 219-254 < 0.1 < 0.1 (7) 10363-65/82
Ears a.i./ha(150 g (1981-1982) 228-254 < 0.1 < 0.1 (4) 10372-82
Grain production/100 kg - 291-308 < 0.05 < 0.05(4)
Straw seed) (37.5 or 291-308 < 0.05 < 0.05 (4)
3.75 DS)
1 Number in parenthesis = number of samples
* One sample per dose level (mean of duplicate analyses)
Stone Fruit
Data were available from three countries, all of which have
approved uses, or are in close geographical proximity to countries
with them. While European trials on peaches reflect recommended
European dosage rates, applications were apparently made after the end
of flowering, and therefore do not appear to reflect the recommended
time of application (Table 1) for Europe. South African trials also
reflect recommended application rates in that country, but the pre-
harvest interval was not available to the meeting. The only available
PHI for stone fruit was 21 days for cherries in one European country
(see "National maximum residue limits". A 1 mg/kg limit for stone
fruit can be supported at a 21-day pre-harvest interval. Additional
residue data and good agricultural practice information for stone
fruit are desirable, especially for small fruit such as cherries.
Residues on peaches were significantly more persistent from WP
applications than from EC formulations.
Bananas
Supervised trials data were available for three Asian and three
South American countries and generally reflected the application rates
and number of applications (WP or EC) recommended in those
geographical areas. Since no national pre-harvest intervals were
provided, it must be assumed that application on the day of harvest,
as included in the trials, reflects practice. Data were available for
both green and ripe, bagged and unbagged, and washed and unwashed
bananas and on pulp and peel. In general, average residues were
approximately 5-10 times higher in peel than in pulp, whether ripe or
green, bagged or unbagged, washed or unwashed. As might be expected,
residues were higher on unbagged fruit. Washing (in the field) does
not appear to affect average residues significantly.
Maximum residues on bagged bananas (whole, green, washed) were
0.06 mg/kg. Maximum residues on whole ripe, unbagged, washed bananas
were 0.28 mg/kg as compared to 0.36 mg/kg for green, unbagged, washed
bananas.
Untreated controls (peel, pulp or whole) were all <0.01 mg/kg. A 0.5
mg/kg limit was estimated.
Cereal crops
No residues of bitertanol were detected in the green forage,
ears, grain or straw of oats, rye or wheat when the seeds were treated
pre-plant with recommended rates. The limit of determination ranged
from 0.05 - 0.1 mg/kg among the 15 studies. The available data would
support a limit of 0.1 mg/kg (at the limit of determination) limit for
seed treatments only. Additional data from other countries with
approved uses for cereal grains are desirable.
FATE OF RESIDUES
General
The fate of bitertanol has been studied in animals, plants, soil
water and light. A proposed metabolic pathway is given in Figure 1.
The structures and chemical names are presented in Table 3 which also
indicates where the compounds have been identified. Although the
metabolic picture is somewhat limited in scope, the data in general
demonstrate that metabolism in animals is much more extensive than
degradation by other routes. Bitertanol is the predominant residue in
plants. Bitertanol and p-hydroxybitertanol are the major residues in
animals.
In plants
Uptake and translocation. Golden Delicious variety apples were
individually syringe-treated to run-off with a 50% wettable powder
formulation at 0.15 g a.i./l of 14C-bitertanol labelled
uniformly (UL) in the biphenyl ring. Sampling was immediately after
drying, at 3 and 7-days and thereafter at 7-day intervals through 49
days (Phul and Hurley, 1979c). Analysis was by TLC using
radiochromatogram scanning and autoradiography techniques. The peel
contained 95% of the total recovered radioactivity even after 49 days,
the rest being in the pulp, showing that little migration had
occurred. Surface residues decreased from 92% at day 0 to 43% at 49
days while the radioactivity in the peel organic extract increased
from 7 to 43.5% of the total. Peel solids increased to 12% in the same
interval.
The two diasterioisomers of bitertanol were the major residues,
in roughly equal proportions throughout the 49-day study. The level of
the two combined in peel and pulp ranged from 98.4% of the total
radioactivity at day 0 to 83% at 49 days, with lower surface residues
and greater peel uptake by the end of that time. The two had
penetrated the pulp by day 7 from which time residues were relatively
constant at about 3% over the remaining test period. Minor metabolites
were bitertanol ketone and 4-hydroxy-biphenyl, together never
exceeding 3% of the total radioactivity. All of these metabolites were
in the peel. The half-life of bitertanol in this experiment was
estimated at approximately 150 days.
The metabolism of bitertanol by peanut plants has also been
studied (Phul and Hurley, 1981). In preliminary studies, the authors
obtained evidence that foliar applications result in poor leaf
absorption (96% unchanged bitertanol on the leaf surface after 14
days), with no observable volatility losses. Only very little
translocation (acro- and basi-petal) was observed, as well as little
translocation to opposite leaves Bitertanol - 14C uniformly labelled
in the biphenyl ring was sprayed as a 50% WP formulation to both young
(1 mo.) and old (2 mo.) potted peanut plants at an approximate
equivalent field rate of 0.5 kg a.i./ha. Young plants were sampled at
 Table 3. Bitertanol, its degradation products and their occurrence a
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
Table 3. Bitertanol, its degradation products and their occurrence a
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
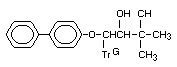 P-(1,1'-Biphenyl)-4-yloxy-o-(1,1- Bitertanol X X X X X
dimethytethyl)-1H-1.2.4-triazole-1-
ethanol. Bitertanol
P-(1,1'-Biphenyl)-4-yloxy-o-(1,1- Bitertanol X X X X X
dimethytethyl)-1H-1.2.4-triazole-1-
ethanol. Bitertanol
 1-[1,1-Biphenyl]-4-yloxy),3,3- Bitertanol ketone X X
dimethyl-1-(1H-1,2,4-triazol-1-yl)- BUE 1662
2-butanone
1-(o)-3,3-dimethyl-1-(tr)butan-2-one
1-[1,1-Biphenyl]-4-yloxy),3,3- Bitertanol ketone X X
dimethyl-1-(1H-1,2,4-triazol-1-yl)- BUE 1662
2-butanone
1-(o)-3,3-dimethyl-1-(tr)butan-2-one
 4-(1,1'-Biphenyl)-4-yloxy)-2,2- Bitertanol alcohol
dimethyl-4-(1H-1,2,4-triazol-1-yl) KWG 1714 X
Note 1. 1,3-Butanodiol-1,3-butanediol
4-(o)-2,2.dimethyl-4-tr)butane-1,3-
diol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-(1,1'-Biphenyl)-4-yloxy)-2,2- Bitertanol alcohol
dimethyl-4-(1H-1,2,4-triazol-1-yl) KWG 1714 X
Note 1. 1,3-Butanodiol-1,3-butanediol
4-(o)-2,2.dimethyl-4-tr)butane-1,3-
diol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 -([1,1'-Biphenyl]-4-yloxy)-B-hydroxy- Bitertanol acid X
dimethyl-1H-1,2,4-triazol-1-butanoic
acid4-(Biphenyl-4-yloxy)-3-hydroxy-
2,2-dimethyl-4,4-(1H-1,2,4.triazol.1.
1-yl)butyric acid
-([1,1'-Biphenyl]-4-yloxy)-B-hydroxy- Bitertanol acid X
dimethyl-1H-1,2,4-triazol-1-butanoic
acid4-(Biphenyl-4-yloxy)-3-hydroxy-
2,2-dimethyl-4,4-(1H-1,2,4.triazol.1.
1-yl)butyric acid
 -(1,1-Dimethylethyl)- -(4'-hydroxy p-hydroxy-bitertanol X
-(1,1'-biphenyl]-4-yloxy)-1H-1,2,4-
triazole-1-ethanol
1-(4'-hydroxy o)-3,3-dimethyl-1-(tr)
butan-2-ol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-(1,1-Dimethylethyl)- -(4'-hydroxy p-hydroxy-bitertanol X
-(1,1'-biphenyl]-4-yloxy)-1H-1,2,4-
triazole-1-ethanol
1-(4'-hydroxy o)-3,3-dimethyl-1-(tr)
butan-2-ol
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 4-(4'Hydroxy-[1-1'-biphenyl]-4-yloxy) X
-2,2-dimethyl-4-((1H)-1,2,4-triazol-1-
yl)-1,3-butanediol
4-(4'-hydroxyo)-2,2-dimethyl-4-
(tr)butane.1,3.diol
4-(4'Hydroxy-[1-1'-biphenyl]-4-yloxy) X
-2,2-dimethyl-4-((1H)-1,2,4-triazol-1-
yl)-1,3-butanediol
4-(4'-hydroxyo)-2,2-dimethyl-4-
(tr)butane.1,3.diol
 -Hydroxy- -(4'-hydroxy-[1,1'- p-Hydroxy-bitertanol
biphenyl]-4-yloxy- -dimethyl-1H.1,2 alcohol X
4-triazole-1-butanoic acid
3-hydroxy(4'-hydroxyo)-2,2-
dimethyl-4-(tr)butyric acid
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-Hydroxy- -(4'-hydroxy-[1,1'- p-Hydroxy-bitertanol
biphenyl]-4-yloxy- -dimethyl-1H.1,2 alcohol X
4-triazole-1-butanoic acid
3-hydroxy(4'-hydroxyo)-2,2-
dimethyl-4-(tr)butyric acid
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 - (1,1-dimethylethyl)-p-(x'-di- Dihydroxy-bitertanol X
hydroxy-[1,1'-biphenyl]-4-yloxy)-1H-1,
2,4-triazol-ethanol
1-(dihyvroxyo)-3,3-dimethyl-1-(tr)
butan-2-olc(distal biphenyl ring
substituted)
- (1,1-dimethylethyl)-p-(x'-di- Dihydroxy-bitertanol X
hydroxy-[1,1'-biphenyl]-4-yloxy)-1H-1,
2,4-triazol-ethanol
1-(dihyvroxyo)-3,3-dimethyl-1-(tr)
butan-2-olc(distal biphenyl ring
substituted)
 -(1,1-Dimethylethyl)-B-(x-hydroxy- Hydroxy, methoxyd
x'-methoxy-[1,1'-biphenyl]-4-yloxy)- bitertanol X
1H-1,2,4-triazole-1-ethanol
1-(hydroxymethoxyo)-3,3.dimethyl-1-
(tr)butan-2-old (distal biphenyl ring
substituted)
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
-(1,1-Dimethylethyl)-B-(x-hydroxy- Hydroxy, methoxyd
x'-methoxy-[1,1'-biphenyl]-4-yloxy)- bitertanol X
1H-1,2,4-triazole-1-ethanol
1-(hydroxymethoxyo)-3,3.dimethyl-1-
(tr)butan-2-old (distal biphenyl ring
substituted)
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 - Hydroxy- -(x'hydroxy-x'-methoxy- Hydroxy,methoxyd
[1,1'-biphenyl]-4-yloxy)-d,d-dimethyl bitertanol acid. X
1H-1,2,4-triazole-1-butanoic acid
3-hydroxy-4-(hydroxymethoxyo)-2-2-
dimethyl-4,4-(tr)butyric acidd
(distal biphenyl ring substituted)
- Hydroxy- -(x'hydroxy-x'-methoxy- Hydroxy,methoxyd
[1,1'-biphenyl]-4-yloxy)-d,d-dimethyl bitertanol acid. X
1H-1,2,4-triazole-1-butanoic acid
3-hydroxy-4-(hydroxymethoxyo)-2-2-
dimethyl-4,4-(tr)butyric acidd
(distal biphenyl ring substituted)
 4-([2-Hydroxy-3,3-dimethyl-1-[1H- Bitertanol benzoic X
1,2,4-triazol-l-yl]butoxy)=benzoic acid analogue
acid BUE 2684
Note: Butoxybenzoic is one word
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-([2-Hydroxy-3,3-dimethyl-1-[1H- Bitertanol benzoic X
1,2,4-triazol-l-yl]butoxy)=benzoic acid analogue
acid BUE 2684
Note: Butoxybenzoic is one word
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 Bitertanol glucoside X
Bitertanol glucoside X
 4-Hydroxybiphenyl p-Hydroxybiphenyl X X X X
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
4-Hydroxybiphenyl p-Hydroxybiphenyl X X X X
Table 3. (continued)
Chemical Name Common, trivial or Soil Water Plant Animal Light
code name
 4,4'-Dihydroxybiphenyl p,p,'-dihydroxy-
biphenyl X X X X
4,4'-Dihydroxybiphenyl p,p,'-dihydroxy-
biphenyl X X X X
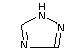 1,2,4-triazol X
CO2
Carbon dioxide X
Table 3. (continued)
a An X indicates that the compound has been found to occur as a degradation product of bitertanol in the substrate
concerned (or on irradiation). A blank indicates it has not been found there, but does not exclude its presence in
amounts too small to detect.
b tr = 1-H-1,2,4-Triazol-l-yl
c The position of the hydroxy groups is uncertain
d The positions of the hydroxy and methoxy groups are uncertain.
12 and 28 days after treatment and the older plants and nuts after 2.5
months. In young plants, 61-72% of the total recovered radioactivity
was surface residue, the remainder being mostly (27-36%) extractable.
In the older plants the distribution was 46% surface and 50%
extractable. In both cases most of the extractables were
organo-soluble. The composition of the residues is shown in Table 4.
As in the case of apples most of the residue, both surface and
extractable, was bitertanol I and II and a metabolite (R-1)
tentatively identified as a bitertanol I glucoside. Low levels of 4-
hydroxybiphenyl (0.6% of the total shoot residues) were also detected.
The authors conclude that the lower I:II isomer ratios in the surface
residues in the older plants compared to the 1.33 ratio of the
starting material indicates faster absorption of the I isomer and that
the 1.10 ratio of I:II in the combined surface and absorbed residues
gives evidence that isomer I is metabolized faster than II. Faster
isomer I absorption is supported further by the 1.64 ratio of
(bitertanol I + metabolite R-1):bitertanol II in the absorbed residues
in the old plants and experimental evidence suggesting increased
levels of the tentatively identified glucoside of isomer I (R-1) as
opposed to isomer II. There is no convincing evidence as to whether
isomeric conversion also occurs to account for the lower bitertanol
I:II ratio in the total residue. The half-life in peanuts has been
estimated at 141 days.
In a similar and related experiment in the same set of studies
with 8.9 mg a.i./plant (approximately twice the rate per plant in the
above), residues in nut meats were 0.06 mg/kg bitertanol equivalent
(0.008% of the total plant residue) 2.5 months after treatment. A
similar level was found in the shells. It cannot be concluded from
this study whether nut residues resulted from the foliar application
or from soil uptake.
Seven days after growing peanuts in a 1 mg/l
14C-biphenyl-labelled bitertanol nutrient solution followed by 7 days
in an unfortified nutrient solution 94.3% of administered
radioactivity was recovered with 28.8% in the roots, 14.9% in the
shoots, 40% in the fortified nutrient solution and 10.6% in the
non-fortified solution. Radioactivity was concentrated in the veins,
but distributed throughout the plant. No translocation to new growth
occurred after removal of the fortified solution. Radioactivity in the
non-fortified solution suggests desorption from within or from the
surface.
Of the absorbed radioactivity, 32.1% was in the shoots and 67.9%
in the roots with the distribution in the organo-soluble fraction
shown in Table 5.
Table 4. Composition of bitertanol residues in peanut shoots following spray application of
14C-biphenyl-laballed bitertanol
YOUNG PLANTS (1 month) OLD PLANTS (2 months)
12 day2 Ratio3 28 day2 Ratio3 2.5 mos.2 Ratio3
Surface Residues
bitertanol II 31.3 1.25 26.7 1.24 20.7 0.98
bitertanol I 39 33 20.5
metabolite R-11 -
Absorbed (organosoluble)
bitertanol II 11.2 1.64
bitertanol I 11.9 1.27 13.4 1.3 15
metabolite R-1 2.4 13.1 18.9
4.3 5.8
1 Tentatively identified as the 6-0-malonyl-B-D-glucoside of bitertanol I.
2 Interval after treatment.
3 Ratio of bitertanol I to bitertanol II in surface residues; ratio of
(bitertanol I + metabolite R-1) to bitertanol II in absorbed residues.
Table 5. Distribution of organo-soluble radioactivity absorbed by peanut plants.
Compound Shoots Roots Total
% of Ratio* % of Ratio* % of Ratio*
absorbed absorbed absorbed
14C 14C 14C
bitertanol II 10.7 20.4 31.1 1.32
bitertanol I 8.6 1.17 16.1 1.39 24.7
metalite R-1 3.9 12.3 16.2
23.2 48.8 72.0
Ratio of (bitertanol I + metabolite R-i) to bitertanol II.
Unextracted residues were 18.3% of the absorbed activity.
Assuming R-1 is a glucoside of bitertanol I as postulated the ratio of
(I + R-1) to II (1.32) n the total absorbed residue is essentially
identical to that in the starting material. This suggests equal root
absorption rates of bitertanol I and II. The finding of higher levels
of both R-1 and bitertanol I in the roots than in the shoots suggests
faster translocation of bitertanol II through-out the plant and/or
more rapid metabolism of bitertanol I. There was no evidence of
volatility from leaf surfaces.
Of the 18.3% unextracted but absorbed residue, 5 and
13.3%, respectively, was in shoots and roots. The metabolite
4-hydroxybiphenyl was detected in both (64% of the root-concentrated
residue).
Other peanut studies (Scheinpflug and Van den Boom, 1981) also
show bitertanol to be slowly absorbed by peanut leaves from surface
application with little translocation from petiole applications.
In animals
The fate of residues in rats and other non-food animals was
evaluated by the 1983 meeting. This evaluation considers the fate in
cows and chickens.
Cow. A single dose of phenyl-UL-14C bitertanol was fed to a
341 kg Holstein dairy cow at 0.2 mg/kg body weight by gelatin capsule,
and excreta and milk were analyzed periodically for six days. The I:II
isomer ratio was 53:47. Food consumption was not recorded (Obrist et
al. 1981). Eighty per cent of the dose was eliminated within 48
hours: urine 8.3%, faeces 70.4% and milk 0.2%, with milk residues
becoming steady after 32 hours. Ninety two per cent was eliminated by
6 days. Residues in the blood peaked at
0.13 mg/l after 12 hours.
Six days after the single dose the same cow was dosed daily at
the same rate for an additional 5 days. The 14C residues (as
bitertanol equivalent) in tissues and milk, 2.5 hours after the last
dose were: liver 0.82 mg/kg; kidney 0.11 mg/kg; muscle
0.01 mg/kg; fat 0.03 mg/kg; milk 0.008 mg/l.
The 0.008 mg/l in milk from the multiple dosing is comparable to
0.009 mg/l found from the single dosing. None of the residues were
identified. Summary data were provided on the identity and
distribution of residues in the tissues, milk and urine of cows fed
UL-14C-phenyl-labelled bitertanol at 0.2 mg/kg, but the study itself
was not provided (Obrist, et al, 1983).
Chickens. Laying hens (average weight 1170g) were administered
single oral doses of 14C-phenyl-UL bitertanol (I:II isomer ratio
53:47) by gelatin capsule at 2.5 mg/kg body weight for excretion
studies (5 trials) and blood level determinations (10 birds). Three
birds were dosed daily for 5 days at a rate of 8 mg/kg body weight for
tissue analyses. Food consumption data were not provided (Obrist et
al, 1982).
From a single dose, 92% was eliminated in the excreta within 24
hours with <0.1% in eggs (0.2% by 96 hours). Bitertanol equivalents
after 4 days were 0.1 and 0.02 mg/kg in liver and kidney respectively
(both 2X standard deviation) and <0.006 mg/kg in other tissues. In
eggs, residues as bitertanol equivalent from a single dose ranged from
<0.002 mg/kg (limit of determination) after 24 hours to 0.061 mg/kg
(2X standard deviation) after
96 hours. Dose-dependent residues from the multiple doses ranged from
<0.002 to 0.38 mg/kg for the same time interval.
Tissue and blood plasma residues, as bitertanol equivalent,
45 minutes after the last of the 5 daily doses varied significantly
(by factors of 5-20) among the 3 birds. They are shown in Table 6.
The composition of the chicken tissue residues is given in Table
7, where it can be seen that bitertanol and p-hydroxy-bitertanol were
by far the main residues, with the latter predominating in liver,
kidney and eggs, whereas bitertanol was predominant in the other
tissues. Conjugation was also greater in liver, kidney and eggs.
Combined unidentified and unextracted residues accounted for up to 19%
of the chicken liver residue and less in other tissues.
Residues in excreta (single dose) were 69.5%
p-hydroxy-bitertanol, 6.7% bitertanol, 13.7% unidentified and <3%
each of other identified compounds. The same residues were identified
in excreta as in tissues with the exception of dihydroxy-bitertanol,
found only in the liver. Bitertanol acid and
p-hydroxy-methoxy-bitertanol acid found in rat faeces (Phul and
Hurley, 1983) were not identified in chicken excreta or tissues.
Summary data provided to the meeting (from Obrist et al 1983)
suggest that residues in cow tissues and milk are similar
qualitatively, and in some respects quantitatively, to those in
chicken tissues except that the dihydroxy-bitertanol found in chicken
livers was reportedly not identified in dairy cow tissues or excreta.
Cow urine reportedly contained bitertanol acid and
p-hydroxy-bitertanol acid, also identified in rat urine (Phul et
al 1979b). The cow study itself was not provided. Further evaluation
must await availability of the study.
Table 6. Tissue and plasma residues in chickens dosed with 14C
bitertanol
14C residue
Tissue Bitertanol equivalent,mg/kg % organo-soluble
Mean Max
liver 6.2 12.6 86
kidney 4.3 10.3 91
breast muscle 0.35 0.91 95.2
leg muscle 0.48 1.1
fat 1.4 3.01 93.2
gizzard 0.72 1.7 90.8
heart 1.3 3.2 92.9
skin 0.63 1.4 94.7
blood plasma 1.8 4.5 --
Table 7. Composition of residues in chicken tissues following administration of multiple oral doses of
bitertanol-phenyl-UL 14C at 8.0 mg/kg (from Obrist and Phul, 1982)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
Bitertanol 34.7 27.2 67.8 62.4 66.0 78.2 74.9 36.6
p-Hydroxy-bitertanol
Free 14.7 27.7 23.2 14.8 17.1 12.1 13.2 6.4
Conjugateda 25.4 24.8 1.5 10.4 4.4 - 4.2 37.9
Hydroxy, Methoxy-bitertanol
Free 0.2 1.0 - - - - - 0.3
Conjugateda 1.5 1.2 - - - - - 0.7
Bitertanol alcohol
Free 1.2 1.8 - - - - - 0.5
Conjugated 0.8 1.1 - - - - - -
Dihydroxy-bitertanolb 0.9 - - - - - - -
p-Hydroxy-bitertanol alcohol
Free 0.4 0.9 - - - - - 0.3
Conjugateda 0.2 - - - - - - -
p-Hydroxy bipbenyl 0.2 0.2 - - - - - 13
Table 7. (continued)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
p,p'-Dihydroxybiphenyl 0.7 0.2 - - - - - -
Unidentified residue 11.9 12.0 4.9 5.9 5.4 2.9 2.4 9.3
Unextracted 7.2 1.9 2.6 6.5 7.1 6.8 5.3 6.7
TOTALS 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
The accumulation, persistence and composition of residues of
14C-bitertanol in bluegill fish maintained in aquarium water
fortified at 10 and 10 mg/l for 30 days has been investigated (Lamb,
1979). Residues on a whole fish basis reached a plateau in both cases
by three days at 2.1 and 16.2 mg/kg at the 10 and 100 mg/l
concentrations respectively, with maximum concentration factors of 203
and 174 respectively. Residues were quantitatively extracted with
acetonitrile with 57-66% of the radioactivity in non-edible parts
(head, viscera, scales) and 34-43% in edible parts. Residues were
characterized by TLC as bitertanol II 40%; bitertanol I 34%;
metabolite R-2 (unidentified) 7%; remaining at origin 13%; other
unidentified 6%.
Approximately 96% of the residues were excreted within 3 days
when the fish were transferred to untreated water.
In soil
Adsorption/desorption. In soil adsorption/desorption studies
bitertanol was shown to be strongly adsorbed to three soils in the
decreasing order: sand, loam and silty clay with adsorption
coefficients (K values) of 39.9, 35.8 and 19.6 (Phul and Hurley,
1979a). The data conformed to the Freundlich equation and in each case
>84% was adsorbed under experimental conditions. Only 4-10% of the
adsorbed compound was desorbed from sand.
Leaching. In TLC leaching experiments six soil types were examined
by comparing Rf values (Obrist and Thornton, 1979). The Rf values
ranged from 0.04 to 0.22, which places bitertanol in the two lowest
mobility ranges of the five classes which have been proposed (Helling
and Turner, 1968; Helling et al 1971). This is consistent with the
high adsorption determined in adsorption experiments. No significant
difference was observed between the two bitertanol isomers.
In aged soils (moist sandy loam) 44% of the initial radioactivity
was lost during room temperature incubation for 30 days, probably by
volatilization (Obrist, 1979). Less than 2% of the aged applied
bitertanol was found in the leachate from 30 cm soil columns, leached
at an equivalent of 13 mm/day for 45 days. Except for 55% further
losses during leaching, most of the remaining residue was in the upper
1.3 cm of the column.
Similar results were found in 19 studies with either Dutch polder
soil or a standard loamy sand containing aged bitertanol residues
leached at an equivalent of 200 mm for 48 hours or up to 500 mm in
polder soil after 72 additional hours (Brennecke, 1983). Only 0.8% of
the aged soil residue was found in the leachate of polder soil after
48 hours, and 3% after 120 hours. In experiments with 11 standard
soils, the leachates contained <6.6% of the original radioactivity in
the aged soil in all but one sample, where 48.9% was in the leachate
and was identified as the bitertanol soil metabolite
4-[2-hydroxy-3,3-dimethyl-(1H-1,2,4-triazol-1-yl)butoxybenzoic acid
(BUE 2684). A repeat of this experiment resulted in only 2.1% or less
of aged residue in the leachate, although less leachate (approximately
400 ml) was collected in the repeat, compared with approximately 650
ml in the original experiment. No unchanged bitertanol was found in
any leachate. Most leachate residues were BUE 2684 and traces of its
methyl ester.
The low leaching potential of bitertanol under experimental
conditions was supported by 30 additional reports provided to the
meeting.
Soil persistence. The soil persistence of bitertanol applied to 7
soil types as the 25 WP formulation at 11 kg a.i./ha to field plots in
9 studies in scattered locations in North America was reported in 9
Mobay reports (68296-68304). Half-lives ranged from 10 to 39 days with
an average of 25 days, except in one study with Oregon loam in which
the half-life was 131 days. The exceptionally long half-life did not
appear to be accounted for by temperature, rainfall, pH or
geographical area when compared to other studies.
Under laboratory aerobic conditions the half-life on silt loam
ranged from 14 to 20 days (Phul and Hurley, 1979b). Degradation was
slower under anaerobic conditions and there was no significant
degradation in sterilized soil after 31 days, showing the importance
of soil micro-organisms in bitertanol degradation. Both
diasterioisomers of bitertanol were degraded at approximately the same
rates.
Under laboratory conditions bitertanol decomposed faster in
alluvial loam soil (12 day half-life) than volcanic ash loam soil (30
days half-life), while under field conditions decomposition was faster
with volcanic ash/sandy loam than with alluvial loam soil (Takase and
Yoshimoto, 1980). In other laboratory tests bitertanol had a
half-life of 26 days in type 1 soil and 72-days in type 2 (Mobay
Report No. 10.317/18), while in field tests half-lives ranged from 19
to 27 days (Mobay Reports NO. 10.324/79, 10.325/79, 10 311/80 and
10.31280).
Rotational Crops. The uptake of 14C-biphenyl ring-labelled WP
bitertanol by rotational crops planted 31, 118 and 364 days after the
last of eight applications to outdoor tub-planted peanuts at an
equivalent of 0.56 kg a.i./ha resulted in 14C residues in leafy
vegetables at harvest ranging from 0.1 mg/kg bitertanol equivalent in
the 118-day planting to 0.02 mg/kg in the 364-day planting (Phul et
al. 1981). In sugar beets residues were 0.38 and 0.01 mg/kg for the
same plantings, and in wheat heads 0.23 and 0.01 mg/kg for 31-day and
364-day plantings. Residues in wheat straw were 1.4 mg/kg from the
31-day planting. The organo-soluble portion of the residue was
<0.04 mg/kg bitertanol equivalent in all at-harvest samples except
for 0.51 mg/kg in wheat straw. The benzoic acid derivative of
bitertanol, BUE 2684 was identified in the 31-day wheat samples.
Soil transformation. The fate of bitertanol has been investigated
in silt loam soil (pH 7.4) under aerobic and anaerobic conditions and
in sterile soil (Phul and Hurley, 1979b). Soil was fortified at 1 and
10 mg/kg for aerobic conditions and 1 mg/kg for anaerobic and sterile
conditions. One mg/kg approximates the maximum residues expected from
recommended field rates of 0.56 kg a.i./ha applied to peanuts. In the
1 mg/kg experiment, bitertanol (I + II) and total organo-solubles
decreased from 98.6 and 99.8% of the total 14C on the first day to 6
and 8.4% after 121 days, while 14CO2 and unextracted residues
increased from 0.8 and 4.6% on day 3 to 45.8 and 45% of the total
radioactivity, respectively, after 121 days. A similar trend was
observed with the 10 mg/kg fortification over the 29-day period
examined. The two diasterioisomers of the bitertanol benzoic acid
metabolite were observed at the 10 mg/kg fortification level (8.6% of
the total radioactivity) but not at the 1 mg/kg level.
Degradation was somewhat slower under anaerobic conditions than
aerobic, but the distribution (bitertanol, total organic, 14CO2 and
unextracted) was not markedly different at comparable intervals.
Bitertanol still accounted for 95.4% of the total 14C in sterile
soil after 31 days compared to 11% in non-sterile soil, indicating the
importance of soil microbes in the metabolism of bitertanol; this was
further demonstrated in another study (Phul et al 1979a). The
identity of the microbes present in the soil of the latter study was
investigated by Smedly and Hepler (1979).
In other studies (Brennecke, 1982) 14C-biphenyl-labelled
bitertanol was shown to be mineralized primarily into 14CO2, up to
68% in "standard soil 2.1" and 49% in Netherlands polder soil. The
bitertanol benzoic acid metabolite BUE 2684 (<19.1%) and an unknown
(<1%) were also found in one of 5 experiments in standard soil.
Bitertanol ketone (BUE 1662) was identified by TLC at <1.7% of the
total radioactivity in polder soil.
In water
The stability of bitertanol in sterile aqueous solutions has been
studied (Nichols and Thornton, 1979). No apparent degradation was
observed after thirty days in sterile aqueous solutions, buffers at pH
4, 7 and 9 and maintained at 25 and 40°C, which contained 0.25 and 2.5
mg/l of bitertanol. After 30 days, 94-109% of the bitertanol was
accounted for at the higher concentrations and 85-98% at the lower
level. The 85% recovery was from 0.25 mg/l at pH 9 and 40°C. The
slightly lower recovery at the lower fortification level was
attributed to adsorption to the vials. This is consistent with the
adsorption observed at low concentration in other studies (Phul and
Hurley, 1979). After using a correction factor to account for vial
glass adsorption, the recovery was 100% under all conditions of this
study.
Additional information on the hydrolytic stability of bitertanol
was provided (Stegh, 1980) but not in the working language of the
meeting.
Photolysis
The photolysis of bitertanol has been investigated on soil and
silica surfaces and in water. In the soil surface experiment,
bitertanol-treated silt loam of 1 mm thickness irradiated for 35 days
did not show substantially different extractability (91.5%) from dark
controls (96.6%), (Sietsema, 1982). Volatile radioactivity accounted
for <0.1% of the original amount. The extracted residue was shown to
be bitertanol by mass spectrometry.
A study on the photolysis of bitertanol on silica gel places
(Stegh and Wilmes, 1982) and two reports on the photolysis in water
and on silica gel (Wilmes, 1980; Stegh, 1980) were provided, but not
in the working language of the meeting. Another report (Wilmes, 1981)
discussed the hydrolytic behaviour and stability of bitertanol in
solution. No data were provided, but reference was made to the two
studies above which included data. Another bitertanol photolysis study
(Sietsema, 1983) was brought to the attention of the meeting, but was
not included in the data provided.
METHODS OF RESIDUE ANALYSIS
An analytical method developed for the analysis of triadimefon in
apples (Thornton, 1977) has been used for bitertanol in apples. The
sample is ground with dry ice, blended with acetone and filtered. The
filter cake is blended with dichloromethane and filtered, and the
combined filtrates are partitioned with water, taken to dryness and
cleaned up on a Florisil column with 60:40 hexane:ethyl acetate as
eluant. The eluate is concentrated and bitertanol determined by GLC
using an alkali flame detector.
The procedure was validated for bitertanol in apples at
0.05-0.1 mg/kg with recoveries of 86-108% and a blank of
<0.01 mg/kg (Morris, 1979a) Although detection was easily attainable
at 0.05 mg/kg and probably 0.01 mg/kg, chromatograms showed
interferences which would make quantitation below 0.05 mg/kg
questionable.
Interference studies have demonstrated that the basic procedure
above permits analysis of bitertanol at levels equivalent to 0.1 mg/kg
in samples in the presence of tolerance levels of 75 pesticides
registered in the U.S.A. for apples or peanuts (1979), five compounds
registered for bananas (1980) and three compounds registered for
cherries, plums, peaches and pears (1981) (Obrist and Nichols,1979).
Several compounds interfered, but could be eliminated by a 1-hour
reflux with 1 N NaOH and zinc or by partitioning with 0.1 N HC1.
However, analytical recoveries from a crop matrix with these steps
incorporated were not provided.
Another analytical procedure (also suitable for other crop
protectants) has been tested for bitertanol on apples, bananas, beans,
currants, cherries, damsons, wheat (green, grain and straw) and soil
(Specht and Tillkes, 1980). Many of the analyses in the field trials
were conducted with this basic procedure. Samples are extracted with
2:1 acetone: water, partitioned with dichloromethane, cleaned up by
gel permeation chromatography and analyzed by GLC utilizing a flame
alkali detector. Where necessary (e.g. for straw) an additional silica
gel column clean-up is used. Analytical recoveries were 74-112%,
although fortification levels were described only as "0.02-0.1 ppm
(lower limit of determination), and 1.0-3.0 mg/kg". For the compounds
tested, the authors concluded that the lower limit of determination
was 0.02 to 0.05 mg/kg for plants with >70% water content and 0.1
mg/kg for cereals and soil. The information was not sufficient for the
meeting to draw a conclusion on the limit of determination.
A method developed for triadimefon in soil and water (Thornton
and Lloyd, 1977) is also suitable for bitertanol in soil and water
with minor modifications in the GLC column stationary phase (Morris,
1979b). Water is simply extracted with chloroform, the extract is
concentrated (a Florisil column clean-up is optional), and analyzed by
GLC using a flame-alkali detector. Soil is extracted by refluxing with
methanol/water, partitioned with chloroform, washed with water and
cleaned up on a Florisil column. Recoveries from water fortified at
0.005 and 0.01 mg/l were 94% and 78% respectively with a blank of
<0.001 mg/l. Recoveries from six soils fortified at 0.05-0.5 mg/kg
ranged from 76 to 121% (most were >90%) with blanks <0.01 mg/kg
(Morris, 1979b). Chromatograms were consistent with the data.
In a similar procedure for the analysis of bitertanol and
numerous other fungicides in water (Brennecke and Vogeler, 1980),
extraction is with dichloromethane and clean-up by gel permeation
chromatography. The two recoveries for bitertanol were 108% and 104%
for 0.005 and 0.5 mg/l fortifications respectively. The lower limit of
determination was said to be 0.005 mg/l, although the meeting could
not confirm this with the available data.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs were reported to the meeting.
Safety
interval MRL
Country Crop (days) mg/kg
Australia Apples 14 1.0 1)
Peanuts 14 0.2 1) 2)
Belgium Apples 42 0.25
Pears 42 0.25
France Apples 15 1.0
Pears 15 1.0
Germany, Federal Apples 14
Republic Cherries 21
Great Britain Apples 21
Pears 21
Greece Apples 21
Beans 4 crops 14
Pears 5 PHIs 7
Stone fruit 14
10
Israel Apples 14
Italy Pears 14
Beets 30 1.0
Fruit 1.0
Pome fruit 21
Stone fruit 21
Vegetables 14 0.5
Morocco Beans 15
Netherlands Apples 14
Blackberries 0.05 2)
Cucumbers 3
Fruiting Vegetables 1.0
Melons 3
Pears 14
Peppers 3
Other commodities 03)(0.05)
New Zealand Pome fruit 14
Strawberries 21
Safety
interval MRL
Country Crop (days) mg/kg
South Africa Apricots 56 0.5
Beans 28 0.1
Beans (fodder) 42
Peaches 56 0.5
Peanuts 0.05
Peanuts (fodder) 42
Plums 56 0.5
Spain Pome fruit 15 1)
Stone fruit 15 1)
Switzerland Pome fruit 42 0.6
Venezuela Apples 15 1.0
Broad beans 28 1.0
Peanuts 0.2 (1)
Pears 15 1.0
Stone fruit 42 0.6
Yugoslavia General 28
1) = Preliminary
2) = Level at or about the limit of determination.
3) = No residues should be present. Limit of determination 0.05 mg/kg.
APPRAISAL
Bitertanol is a relatively new broad-spectrum fungicide
introduced and being introduced in a number of countries. Its
toxicology was evaluated by the 1983 JMPR which estimated a temporary
ADI. The present meeting evaluated residues in food.
To date only limited residue trials data have been provided and
these have been evaluated. Data were insufficient to estimate residue
levels in cucumbers and beans, but temporary limits were estimated for
several commodities.
The fate of bitertanol has been investigated in plants (apples
and peanuts), animals, soil, light and water.
In the plant studies bitertanol penetration from surface
applications was minimal and there was little volatility. Residues are
taken up by roots, although translocation into the plant is relatively
low. There are some measurable but not major differences in
absorption, translocation and plant metabolism rates of the
two diasterioisomeric forms of bitertanol. Bitertanol is by far
the main plant residue, with low levels of its ketone and
4-hydroxybiphenyl. In apples, most of the residue in the peel.
The fate of residues in non-food animals was evaluated by the
1983 JMPR. The present meeting evaluated metabolism studies on
poultry, cows and fish. Bitertanol is metabolized substantially more
in animals than in plants. The products are rapidly excreted but low
residues can occur in milk, eggs and tissues. These are predominantly
in the liver and kidney, as demonstrated with exaggerated feeding
levels. Bitertanol and p-hydroxy-bitertanol are by far the predominant
residues in chicken tissues and eggs and are reported to be the major
residues in cow tissues and milk, although other metabolites occur at
significant levels in the latter. Although cow metabolism studies were
available to the meeting the only one detailing the distribution of
identified residues was received too late for evaluation, until it has
been reviewed at a future meeting, no final conclusion can be drawn on
the adequacy of information on the fate of residues in animals. The
available data indicate that metabolism appears to be similar among
rats, poultry and cows.
Conventional feeding studies on poultry and ruminants, an
analytical method for the analysis of bitertanol in animal products,
and information on the fate of residues in apples during processing
were all received too late for evaluation.
Extensive information was available to the meeting on the fate of
bitertanol residues in soil, in which it is of low mobility, and some
data were available on water stability and photolysis. The water and
photolysis data available in the working language of the meeting
indicate a high degree of stability in each case. Other studies
provided, but not in the working language of the meeting, were not
evaluated.
Several analytical methods utilizing solvent extraction, GPC or
Florisil clean-up followed by gas chromatography are available for
determining residues in crops, soil and water. Further confirmation of
the limit of determination is desirable.
No information was provided on the fate of residues in storage,
in processing, in commerce or at consumption.
The meeting examined residue data from supervised trials
reflecting good agricultural practice on a number of crops and was
able to estimate the maximum residue levels which were likely to occur
when bitertanol was used in practice and when the reported intervals
between the last application and harvest were observed. These levels
refer only to the parent compound.
RECOMMENDATIONS
COMMODITY MRL (mg/kg)1 Pre-harvest interval on
which recommendations
are based
Apples 2 14
Stone fruit 1 21
Bananas 0.5 0
Oats:
grain 0.1* )
forage 0.1* )
straw 0.1* )
Rye:
grain 0.1* )
forage 0.1* )
straw 0.1* ) from seed treatment
Wheat:
grain 0.1* )
forage 0.1* )
straw 0.1* )
* At or about the limit of determination.
1 Temporary irrespective of the status of the ADI.
FURTHER WORK OR INFORMATION
1. Except for bananas for which substantial data reflecting approved
uses were available, additional information on good agricultural
practice and residue data reflecting approved uses for all
commodities for which temporary limits have been estimated.
Residue data should be from countries from which details of
approved uses have been or will be provided, or those in close
proximity.
2. Additional plant metabolism studies.
3. Additional residue data reflecting good agricultural practice for
additional plant products likely to be used as animal feeds.
Desirable
1. Analytical recoveries through the Thornton analytical method
utilizing the NaOH and HC1 steps designed to remove
interferences.
2. Confirmation of limits of determination for the Specht method.
3. Information on the stability of residues in crops during cold
storage.
4. Additional national maximum residue limits and safety intervals.
5. Photolysis/hydrolysis studies in English (Stegh and Wilmes, 1982;
Wilmes, 1980; Stegh 1980 and Sietsema, 1983).
REFERENCES
Mobay Reports are unpublished reports prepared by the Research and
Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, USA.
Nitokuno Reports are unpublished reports prepared by Nihon Tokushu
Noyaku Seizo KK., No. 8, 2-chome, Nihonbashi, Chuo-ku, Tokyo, Japan.
Bayer Reports:
Leaching in Soil
10.314 - 10.316/78 10.342 - 10.334/82
10.318 - 10.320/78 10.348 - 10.350/82a
10.334 - 10.336/80 10.374 - 10.376/82
10.334A - 10.346A/8010.379 - 10.381/82b
10.309 - 10.311/81 10.388 - 10.390/82b
Soil Persistence
10.317/78 10.324/7910.3257910.311/8010.312/80
68 296 68 29868 30068 30268 304
68 297 68 29968 30168 303
Brennecke, R. Leaching characteristics of Aged [biphenyl-UL-14C]
1983 bitertanol Soil Residues. Unpublished Bayer Report RA
981/209B, October 1983.
Brennecke, R. and Vogeler, K. Method for the Gas Chromatographic
1982 Determination of Residues of Various Fungicides in Water.
Bayer AG, Pflanzenschutz-Anwendungstechnik, unpublished
Report RA-80, January, 1982.
Helling, C.S. and Turner, B.C. Pesticide Mobility: Determination by
1968 Soil Thin-Layer Chromatography, Science 162, 562 (1968).
Helling, C.S., Kearney, P.C. and Alexander, M. Behaviour of Pesticides
1971 in soils, Advan. Agron. 23; 147, 1971.
Lamb, D.W., BAYCOR-14C Accumulation and Persistence of Residues in
1979 Bluegill. Mobay Report No. 68 343 December 13, 1979.
Mobay, Recovery Reports, Apples 68 249, Soil and Water 69 250.
Morris, R.A. Recovery of Baycor from Apples. Unpublished Mobay Report
1979a 68 249, November 19, 1979.
Morris, R.A. Recovery of Baycor from Soil and Water. Unpublished Mobay
1979b Report 68 250, 1979.
Nichols, S.S. and Thornton, J.S. The Behaviour of BAYCOR in Sterile
1979 Aqueous Solutions. Mobay Report No. 68 273. October 22,
1979.
Christ, J.J. Leaching Characteristics of Aged BAYCOR Soil Residues
1979a Mobay Report No. 68 271. September 25, 1979.
Obrist, J.J. and Nichols, S.S. An Interference Study for the BAYCOR
1979 Residue Method for Apples and Peanuts. Mobay Report No. 68
311. November 27, 1979.
Obrist, J.J., Phul, R.J. and Thornton, J.S. Excretion and Tissue
1981 Levels of 14C Following Oral Administration of BAYCOR-
Phenyl-UL-14C to a Dairy Cow. Mobay Report NO. 69 400.
March 24, 1981.
Obrist, J.J., Phul, R.J. and Thornton, J.S. The Metabolism and
1982 Excretion of BAYCOR-Phenyl-UL-14C by Chickens. Mobay Report
No 82614. October 8, 1982.
Obrist, J.J., Phul R.J. and Thornton, J.S. The Metabolism and
1983 Excretion of BAYCOR-Phenyl-UL-14C Following Oral
Administration to a Dairy Cow. Mobay Report, Manuscript in
Preparation. 1983.
Obrist, J.J. and Thornton, J.S. Soil Thin-Layer Mobility of BAYCOR,
1979 BAYTAN, DYRENE, and PEROPAL. Mobay Report No. 68 272.
October 22, 1979.
Phul, R.J. and Hurley, J.B. Soil Adsorption and Desorption of BAYCOR.
1979a Mobay Report No. 68 003. July 18, 1979.
Phul, R.J. and Hurley, J.B. The Metabolism and Degradation of BAYCOR-
1979b Biphenyl-UL-14C on Soil. Mobay Report NO. 67 998. August 6,
1979.
Phul, R.J. and Hurley, J.B. The Metabolism of BAYCOR-Biphenyl-UL-14C
1979c in Apple Fruit. Mobay Report No. 68 305. November 5, 1979,
Revised February 3, 1981A.
Phul, R.J. and Hurley, J.B. Metabolism of BAYCOR-UL-14C in Peanut
1981 Plants. Mobay Report No. 69 310. October 23, 1979, Revised
June 18, 1981b.
Phul, R.J. and Hurley, J.B. The Absorption, Excretion and Metabolism
1983 of BAYCOR-Phenyl-UL-14C by Rats. Mobay Report, Manuscript
in Preparation, 1983.
Phul, R.J., Hurley, J.B. and Close, C.L. Total Radioactive Residues in
1981 Rotational Crops Following a Target Drop of Peanuts Treated
with BAYCOR-14C. Mobay Report No.80 059. September 11,
1981.
Phul, R.J., Hurley, J.B. and Thornton, J.S. The effect of Soil
1979a Microorganisms on the Degradation of BAYCOR. Mobay Report
No. 69 012. July 2, 1979.
Phul, R.J., Obrist, J.J. and Pither, K.M. The excretion of
1979b BAYCOR-Phenyl-UL-14C Following Administration of a Single
Oral Dose to Rats. Mobay Report NO. 68 307. November 19,
1979.
Scheinpflug, H. and van den Boom, T. BAYCOR, A New Fungicide for
1981 Tropical and Subtropical Crops. PflanzenschutzNachrichten
Bayer 34, 8-28.1981.
Sietsema, W.K. Photodecomposition of BAYCOR on a Soil Surface. Mobay
1982 Report No. 82 572. November 16, 1982.
Sietsema, W.K. Photodegradation of BAYCOR in Water. Mobay Report (in
1983 Preparation). 1983.
Smedly, L.A. and Hepler, D.I. Identification of Microorganisms in
1979 Samples of Kansas Loam Soil. Mobay Report No. 67 814. May 1,
1979.
Specht, W. and Tillkes, M. Determination of Agrochemicals After
1980 Clean-up using Gel Permeation Chromatography and Mini-Silica
Gel Column Chromatography. Pflanzenschutz-Nachrichten Bayer
33, 61 - 85. 1980.
Stegh, R. Versuche zum Abbau von KWG 0599 in wässerigen Salzlosungen.
1980 Bayer Experimental Notice. January 24, 1980.
Stegh, R. and Wilmes, R. Light Induced Degradation of Agrochemical
1982 Parent Compounds on Silica Gel as a Model for Degradation on
Soil. Bayer Ag. Pflanzenschutz-Anwendungstechnik unpublished
report PF1666. February 10, 1982.
Takase, I. and Yoshimoto, Y. Residues of KWG 0599 in Upland Soils
1980 Under Laboratory and Field Conditions. Nitokuno Report No.
1134. December 5, 1980.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and its
1977a Metabolite in Apples. Mobay Report No. 54 166. December 30,
1977.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and KWG 0599
1977 in Soil and Water. Mobay Report NO. 51 231. January 31,
1977.
Wilmes, R. Preliminary Studies on Stability to Light. Bayer Pharma
1980 Unpublished Report, 1980.
Wilmes, R. Abiotic Degradation of Bitertanol. Bayer summary Report.
1981 March 31, 1981.
1,2,4-triazol X
CO2
Carbon dioxide X
Table 3. (continued)
a An X indicates that the compound has been found to occur as a degradation product of bitertanol in the substrate
concerned (or on irradiation). A blank indicates it has not been found there, but does not exclude its presence in
amounts too small to detect.
b tr = 1-H-1,2,4-Triazol-l-yl
c The position of the hydroxy groups is uncertain
d The positions of the hydroxy and methoxy groups are uncertain.
12 and 28 days after treatment and the older plants and nuts after 2.5
months. In young plants, 61-72% of the total recovered radioactivity
was surface residue, the remainder being mostly (27-36%) extractable.
In the older plants the distribution was 46% surface and 50%
extractable. In both cases most of the extractables were
organo-soluble. The composition of the residues is shown in Table 4.
As in the case of apples most of the residue, both surface and
extractable, was bitertanol I and II and a metabolite (R-1)
tentatively identified as a bitertanol I glucoside. Low levels of 4-
hydroxybiphenyl (0.6% of the total shoot residues) were also detected.
The authors conclude that the lower I:II isomer ratios in the surface
residues in the older plants compared to the 1.33 ratio of the
starting material indicates faster absorption of the I isomer and that
the 1.10 ratio of I:II in the combined surface and absorbed residues
gives evidence that isomer I is metabolized faster than II. Faster
isomer I absorption is supported further by the 1.64 ratio of
(bitertanol I + metabolite R-1):bitertanol II in the absorbed residues
in the old plants and experimental evidence suggesting increased
levels of the tentatively identified glucoside of isomer I (R-1) as
opposed to isomer II. There is no convincing evidence as to whether
isomeric conversion also occurs to account for the lower bitertanol
I:II ratio in the total residue. The half-life in peanuts has been
estimated at 141 days.
In a similar and related experiment in the same set of studies
with 8.9 mg a.i./plant (approximately twice the rate per plant in the
above), residues in nut meats were 0.06 mg/kg bitertanol equivalent
(0.008% of the total plant residue) 2.5 months after treatment. A
similar level was found in the shells. It cannot be concluded from
this study whether nut residues resulted from the foliar application
or from soil uptake.
Seven days after growing peanuts in a 1 mg/l
14C-biphenyl-labelled bitertanol nutrient solution followed by 7 days
in an unfortified nutrient solution 94.3% of administered
radioactivity was recovered with 28.8% in the roots, 14.9% in the
shoots, 40% in the fortified nutrient solution and 10.6% in the
non-fortified solution. Radioactivity was concentrated in the veins,
but distributed throughout the plant. No translocation to new growth
occurred after removal of the fortified solution. Radioactivity in the
non-fortified solution suggests desorption from within or from the
surface.
Of the absorbed radioactivity, 32.1% was in the shoots and 67.9%
in the roots with the distribution in the organo-soluble fraction
shown in Table 5.
Table 4. Composition of bitertanol residues in peanut shoots following spray application of
14C-biphenyl-laballed bitertanol
YOUNG PLANTS (1 month) OLD PLANTS (2 months)
12 day2 Ratio3 28 day2 Ratio3 2.5 mos.2 Ratio3
Surface Residues
bitertanol II 31.3 1.25 26.7 1.24 20.7 0.98
bitertanol I 39 33 20.5
metabolite R-11 -
Absorbed (organosoluble)
bitertanol II 11.2 1.64
bitertanol I 11.9 1.27 13.4 1.3 15
metabolite R-1 2.4 13.1 18.9
4.3 5.8
1 Tentatively identified as the 6-0-malonyl-B-D-glucoside of bitertanol I.
2 Interval after treatment.
3 Ratio of bitertanol I to bitertanol II in surface residues; ratio of
(bitertanol I + metabolite R-1) to bitertanol II in absorbed residues.
Table 5. Distribution of organo-soluble radioactivity absorbed by peanut plants.
Compound Shoots Roots Total
% of Ratio* % of Ratio* % of Ratio*
absorbed absorbed absorbed
14C 14C 14C
bitertanol II 10.7 20.4 31.1 1.32
bitertanol I 8.6 1.17 16.1 1.39 24.7
metalite R-1 3.9 12.3 16.2
23.2 48.8 72.0
Ratio of (bitertanol I + metabolite R-i) to bitertanol II.
Unextracted residues were 18.3% of the absorbed activity.
Assuming R-1 is a glucoside of bitertanol I as postulated the ratio of
(I + R-1) to II (1.32) n the total absorbed residue is essentially
identical to that in the starting material. This suggests equal root
absorption rates of bitertanol I and II. The finding of higher levels
of both R-1 and bitertanol I in the roots than in the shoots suggests
faster translocation of bitertanol II through-out the plant and/or
more rapid metabolism of bitertanol I. There was no evidence of
volatility from leaf surfaces.
Of the 18.3% unextracted but absorbed residue, 5 and
13.3%, respectively, was in shoots and roots. The metabolite
4-hydroxybiphenyl was detected in both (64% of the root-concentrated
residue).
Other peanut studies (Scheinpflug and Van den Boom, 1981) also
show bitertanol to be slowly absorbed by peanut leaves from surface
application with little translocation from petiole applications.
In animals
The fate of residues in rats and other non-food animals was
evaluated by the 1983 meeting. This evaluation considers the fate in
cows and chickens.
Cow. A single dose of phenyl-UL-14C bitertanol was fed to a
341 kg Holstein dairy cow at 0.2 mg/kg body weight by gelatin capsule,
and excreta and milk were analyzed periodically for six days. The I:II
isomer ratio was 53:47. Food consumption was not recorded (Obrist et
al. 1981). Eighty per cent of the dose was eliminated within 48
hours: urine 8.3%, faeces 70.4% and milk 0.2%, with milk residues
becoming steady after 32 hours. Ninety two per cent was eliminated by
6 days. Residues in the blood peaked at
0.13 mg/l after 12 hours.
Six days after the single dose the same cow was dosed daily at
the same rate for an additional 5 days. The 14C residues (as
bitertanol equivalent) in tissues and milk, 2.5 hours after the last
dose were: liver 0.82 mg/kg; kidney 0.11 mg/kg; muscle
0.01 mg/kg; fat 0.03 mg/kg; milk 0.008 mg/l.
The 0.008 mg/l in milk from the multiple dosing is comparable to
0.009 mg/l found from the single dosing. None of the residues were
identified. Summary data were provided on the identity and
distribution of residues in the tissues, milk and urine of cows fed
UL-14C-phenyl-labelled bitertanol at 0.2 mg/kg, but the study itself
was not provided (Obrist, et al, 1983).
Chickens. Laying hens (average weight 1170g) were administered
single oral doses of 14C-phenyl-UL bitertanol (I:II isomer ratio
53:47) by gelatin capsule at 2.5 mg/kg body weight for excretion
studies (5 trials) and blood level determinations (10 birds). Three
birds were dosed daily for 5 days at a rate of 8 mg/kg body weight for
tissue analyses. Food consumption data were not provided (Obrist et
al, 1982).
From a single dose, 92% was eliminated in the excreta within 24
hours with <0.1% in eggs (0.2% by 96 hours). Bitertanol equivalents
after 4 days were 0.1 and 0.02 mg/kg in liver and kidney respectively
(both 2X standard deviation) and <0.006 mg/kg in other tissues. In
eggs, residues as bitertanol equivalent from a single dose ranged from
<0.002 mg/kg (limit of determination) after 24 hours to 0.061 mg/kg
(2X standard deviation) after
96 hours. Dose-dependent residues from the multiple doses ranged from
<0.002 to 0.38 mg/kg for the same time interval.
Tissue and blood plasma residues, as bitertanol equivalent,
45 minutes after the last of the 5 daily doses varied significantly
(by factors of 5-20) among the 3 birds. They are shown in Table 6.
The composition of the chicken tissue residues is given in Table
7, where it can be seen that bitertanol and p-hydroxy-bitertanol were
by far the main residues, with the latter predominating in liver,
kidney and eggs, whereas bitertanol was predominant in the other
tissues. Conjugation was also greater in liver, kidney and eggs.
Combined unidentified and unextracted residues accounted for up to 19%
of the chicken liver residue and less in other tissues.
Residues in excreta (single dose) were 69.5%
p-hydroxy-bitertanol, 6.7% bitertanol, 13.7% unidentified and <3%
each of other identified compounds. The same residues were identified
in excreta as in tissues with the exception of dihydroxy-bitertanol,
found only in the liver. Bitertanol acid and
p-hydroxy-methoxy-bitertanol acid found in rat faeces (Phul and
Hurley, 1983) were not identified in chicken excreta or tissues.
Summary data provided to the meeting (from Obrist et al 1983)
suggest that residues in cow tissues and milk are similar
qualitatively, and in some respects quantitatively, to those in
chicken tissues except that the dihydroxy-bitertanol found in chicken
livers was reportedly not identified in dairy cow tissues or excreta.
Cow urine reportedly contained bitertanol acid and
p-hydroxy-bitertanol acid, also identified in rat urine (Phul et
al 1979b). The cow study itself was not provided. Further evaluation
must await availability of the study.
Table 6. Tissue and plasma residues in chickens dosed with 14C
bitertanol
14C residue
Tissue Bitertanol equivalent,mg/kg % organo-soluble
Mean Max
liver 6.2 12.6 86
kidney 4.3 10.3 91
breast muscle 0.35 0.91 95.2
leg muscle 0.48 1.1
fat 1.4 3.01 93.2
gizzard 0.72 1.7 90.8
heart 1.3 3.2 92.9
skin 0.63 1.4 94.7
blood plasma 1.8 4.5 --
Table 7. Composition of residues in chicken tissues following administration of multiple oral doses of
bitertanol-phenyl-UL 14C at 8.0 mg/kg (from Obrist and Phul, 1982)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
Bitertanol 34.7 27.2 67.8 62.4 66.0 78.2 74.9 36.6
p-Hydroxy-bitertanol
Free 14.7 27.7 23.2 14.8 17.1 12.1 13.2 6.4
Conjugateda 25.4 24.8 1.5 10.4 4.4 - 4.2 37.9
Hydroxy, Methoxy-bitertanol
Free 0.2 1.0 - - - - - 0.3
Conjugateda 1.5 1.2 - - - - - 0.7
Bitertanol alcohol
Free 1.2 1.8 - - - - - 0.5
Conjugated 0.8 1.1 - - - - - -
Dihydroxy-bitertanolb 0.9 - - - - - - -
p-Hydroxy-bitertanol alcohol
Free 0.4 0.9 - - - - - 0.3
Conjugateda 0.2 - - - - - - -
p-Hydroxy bipbenyl 0.2 0.2 - - - - - 13
Table 7. (continued)
% of 14C in each tissue occurring as named compound
Liver Kidney Muscle Gizzard Heart Fat Skin Eggs
p,p'-Dihydroxybiphenyl 0.7 0.2 - - - - - -
Unidentified residue 11.9 12.0 4.9 5.9 5.4 2.9 2.4 9.3
Unextracted 7.2 1.9 2.6 6.5 7.1 6.8 5.3 6.7
TOTALS 100.0 100.0 100.0 100.0 100.0 100.0 100.0 100.0
The accumulation, persistence and composition of residues of
14C-bitertanol in bluegill fish maintained in aquarium water
fortified at 10 and 10 mg/l for 30 days has been investigated (Lamb,
1979). Residues on a whole fish basis reached a plateau in both cases
by three days at 2.1 and 16.2 mg/kg at the 10 and 100 mg/l
concentrations respectively, with maximum concentration factors of 203
and 174 respectively. Residues were quantitatively extracted with
acetonitrile with 57-66% of the radioactivity in non-edible parts
(head, viscera, scales) and 34-43% in edible parts. Residues were
characterized by TLC as bitertanol II 40%; bitertanol I 34%;
metabolite R-2 (unidentified) 7%; remaining at origin 13%; other
unidentified 6%.
Approximately 96% of the residues were excreted within 3 days
when the fish were transferred to untreated water.
In soil
Adsorption/desorption. In soil adsorption/desorption studies
bitertanol was shown to be strongly adsorbed to three soils in the
decreasing order: sand, loam and silty clay with adsorption
coefficients (K values) of 39.9, 35.8 and 19.6 (Phul and Hurley,
1979a). The data conformed to the Freundlich equation and in each case
>84% was adsorbed under experimental conditions. Only 4-10% of the
adsorbed compound was desorbed from sand.
Leaching. In TLC leaching experiments six soil types were examined
by comparing Rf values (Obrist and Thornton, 1979). The Rf values
ranged from 0.04 to 0.22, which places bitertanol in the two lowest
mobility ranges of the five classes which have been proposed (Helling
and Turner, 1968; Helling et al 1971). This is consistent with the
high adsorption determined in adsorption experiments. No significant
difference was observed between the two bitertanol isomers.
In aged soils (moist sandy loam) 44% of the initial radioactivity
was lost during room temperature incubation for 30 days, probably by
volatilization (Obrist, 1979). Less than 2% of the aged applied
bitertanol was found in the leachate from 30 cm soil columns, leached
at an equivalent of 13 mm/day for 45 days. Except for 55% further
losses during leaching, most of the remaining residue was in the upper
1.3 cm of the column.
Similar results were found in 19 studies with either Dutch polder
soil or a standard loamy sand containing aged bitertanol residues
leached at an equivalent of 200 mm for 48 hours or up to 500 mm in
polder soil after 72 additional hours (Brennecke, 1983). Only 0.8% of
the aged soil residue was found in the leachate of polder soil after
48 hours, and 3% after 120 hours. In experiments with 11 standard
soils, the leachates contained <6.6% of the original radioactivity in
the aged soil in all but one sample, where 48.9% was in the leachate
and was identified as the bitertanol soil metabolite
4-[2-hydroxy-3,3-dimethyl-(1H-1,2,4-triazol-1-yl)butoxybenzoic acid
(BUE 2684). A repeat of this experiment resulted in only 2.1% or less
of aged residue in the leachate, although less leachate (approximately
400 ml) was collected in the repeat, compared with approximately 650
ml in the original experiment. No unchanged bitertanol was found in
any leachate. Most leachate residues were BUE 2684 and traces of its
methyl ester.
The low leaching potential of bitertanol under experimental
conditions was supported by 30 additional reports provided to the
meeting.
Soil persistence. The soil persistence of bitertanol applied to 7
soil types as the 25 WP formulation at 11 kg a.i./ha to field plots in
9 studies in scattered locations in North America was reported in 9
Mobay reports (68296-68304). Half-lives ranged from 10 to 39 days with
an average of 25 days, except in one study with Oregon loam in which
the half-life was 131 days. The exceptionally long half-life did not
appear to be accounted for by temperature, rainfall, pH or
geographical area when compared to other studies.
Under laboratory aerobic conditions the half-life on silt loam
ranged from 14 to 20 days (Phul and Hurley, 1979b). Degradation was
slower under anaerobic conditions and there was no significant
degradation in sterilized soil after 31 days, showing the importance
of soil micro-organisms in bitertanol degradation. Both
diasterioisomers of bitertanol were degraded at approximately the same
rates.
Under laboratory conditions bitertanol decomposed faster in
alluvial loam soil (12 day half-life) than volcanic ash loam soil (30
days half-life), while under field conditions decomposition was faster
with volcanic ash/sandy loam than with alluvial loam soil (Takase and
Yoshimoto, 1980). In other laboratory tests bitertanol had a
half-life of 26 days in type 1 soil and 72-days in type 2 (Mobay
Report No. 10.317/18), while in field tests half-lives ranged from 19
to 27 days (Mobay Reports NO. 10.324/79, 10.325/79, 10 311/80 and
10.31280).
Rotational Crops. The uptake of 14C-biphenyl ring-labelled WP
bitertanol by rotational crops planted 31, 118 and 364 days after the
last of eight applications to outdoor tub-planted peanuts at an
equivalent of 0.56 kg a.i./ha resulted in 14C residues in leafy
vegetables at harvest ranging from 0.1 mg/kg bitertanol equivalent in
the 118-day planting to 0.02 mg/kg in the 364-day planting (Phul et
al. 1981). In sugar beets residues were 0.38 and 0.01 mg/kg for the
same plantings, and in wheat heads 0.23 and 0.01 mg/kg for 31-day and
364-day plantings. Residues in wheat straw were 1.4 mg/kg from the
31-day planting. The organo-soluble portion of the residue was
<0.04 mg/kg bitertanol equivalent in all at-harvest samples except
for 0.51 mg/kg in wheat straw. The benzoic acid derivative of
bitertanol, BUE 2684 was identified in the 31-day wheat samples.
Soil transformation. The fate of bitertanol has been investigated
in silt loam soil (pH 7.4) under aerobic and anaerobic conditions and
in sterile soil (Phul and Hurley, 1979b). Soil was fortified at 1 and
10 mg/kg for aerobic conditions and 1 mg/kg for anaerobic and sterile
conditions. One mg/kg approximates the maximum residues expected from
recommended field rates of 0.56 kg a.i./ha applied to peanuts. In the
1 mg/kg experiment, bitertanol (I + II) and total organo-solubles
decreased from 98.6 and 99.8% of the total 14C on the first day to 6
and 8.4% after 121 days, while 14CO2 and unextracted residues
increased from 0.8 and 4.6% on day 3 to 45.8 and 45% of the total
radioactivity, respectively, after 121 days. A similar trend was
observed with the 10 mg/kg fortification over the 29-day period
examined. The two diasterioisomers of the bitertanol benzoic acid
metabolite were observed at the 10 mg/kg fortification level (8.6% of
the total radioactivity) but not at the 1 mg/kg level.
Degradation was somewhat slower under anaerobic conditions than
aerobic, but the distribution (bitertanol, total organic, 14CO2 and
unextracted) was not markedly different at comparable intervals.
Bitertanol still accounted for 95.4% of the total 14C in sterile
soil after 31 days compared to 11% in non-sterile soil, indicating the
importance of soil microbes in the metabolism of bitertanol; this was
further demonstrated in another study (Phul et al 1979a). The
identity of the microbes present in the soil of the latter study was
investigated by Smedly and Hepler (1979).
In other studies (Brennecke, 1982) 14C-biphenyl-labelled
bitertanol was shown to be mineralized primarily into 14CO2, up to
68% in "standard soil 2.1" and 49% in Netherlands polder soil. The
bitertanol benzoic acid metabolite BUE 2684 (<19.1%) and an unknown
(<1%) were also found in one of 5 experiments in standard soil.
Bitertanol ketone (BUE 1662) was identified by TLC at <1.7% of the
total radioactivity in polder soil.
In water
The stability of bitertanol in sterile aqueous solutions has been
studied (Nichols and Thornton, 1979). No apparent degradation was
observed after thirty days in sterile aqueous solutions, buffers at pH
4, 7 and 9 and maintained at 25 and 40°C, which contained 0.25 and 2.5
mg/l of bitertanol. After 30 days, 94-109% of the bitertanol was
accounted for at the higher concentrations and 85-98% at the lower
level. The 85% recovery was from 0.25 mg/l at pH 9 and 40°C. The
slightly lower recovery at the lower fortification level was
attributed to adsorption to the vials. This is consistent with the
adsorption observed at low concentration in other studies (Phul and
Hurley, 1979). After using a correction factor to account for vial
glass adsorption, the recovery was 100% under all conditions of this
study.
Additional information on the hydrolytic stability of bitertanol
was provided (Stegh, 1980) but not in the working language of the
meeting.
Photolysis
The photolysis of bitertanol has been investigated on soil and
silica surfaces and in water. In the soil surface experiment,
bitertanol-treated silt loam of 1 mm thickness irradiated for 35 days
did not show substantially different extractability (91.5%) from dark
controls (96.6%), (Sietsema, 1982). Volatile radioactivity accounted
for <0.1% of the original amount. The extracted residue was shown to
be bitertanol by mass spectrometry.
A study on the photolysis of bitertanol on silica gel places
(Stegh and Wilmes, 1982) and two reports on the photolysis in water
and on silica gel (Wilmes, 1980; Stegh, 1980) were provided, but not
in the working language of the meeting. Another report (Wilmes, 1981)
discussed the hydrolytic behaviour and stability of bitertanol in
solution. No data were provided, but reference was made to the two
studies above which included data. Another bitertanol photolysis study
(Sietsema, 1983) was brought to the attention of the meeting, but was
not included in the data provided.
METHODS OF RESIDUE ANALYSIS
An analytical method developed for the analysis of triadimefon in
apples (Thornton, 1977) has been used for bitertanol in apples. The
sample is ground with dry ice, blended with acetone and filtered. The
filter cake is blended with dichloromethane and filtered, and the
combined filtrates are partitioned with water, taken to dryness and
cleaned up on a Florisil column with 60:40 hexane:ethyl acetate as
eluant. The eluate is concentrated and bitertanol determined by GLC
using an alkali flame detector.
The procedure was validated for bitertanol in apples at
0.05-0.1 mg/kg with recoveries of 86-108% and a blank of
<0.01 mg/kg (Morris, 1979a) Although detection was easily attainable
at 0.05 mg/kg and probably 0.01 mg/kg, chromatograms showed
interferences which would make quantitation below 0.05 mg/kg
questionable.
Interference studies have demonstrated that the basic procedure
above permits analysis of bitertanol at levels equivalent to 0.1 mg/kg
in samples in the presence of tolerance levels of 75 pesticides
registered in the U.S.A. for apples or peanuts (1979), five compounds
registered for bananas (1980) and three compounds registered for
cherries, plums, peaches and pears (1981) (Obrist and Nichols,1979).
Several compounds interfered, but could be eliminated by a 1-hour
reflux with 1 N NaOH and zinc or by partitioning with 0.1 N HC1.
However, analytical recoveries from a crop matrix with these steps
incorporated were not provided.
Another analytical procedure (also suitable for other crop
protectants) has been tested for bitertanol on apples, bananas, beans,
currants, cherries, damsons, wheat (green, grain and straw) and soil
(Specht and Tillkes, 1980). Many of the analyses in the field trials
were conducted with this basic procedure. Samples are extracted with
2:1 acetone: water, partitioned with dichloromethane, cleaned up by
gel permeation chromatography and analyzed by GLC utilizing a flame
alkali detector. Where necessary (e.g. for straw) an additional silica
gel column clean-up is used. Analytical recoveries were 74-112%,
although fortification levels were described only as "0.02-0.1 ppm
(lower limit of determination), and 1.0-3.0 mg/kg". For the compounds
tested, the authors concluded that the lower limit of determination
was 0.02 to 0.05 mg/kg for plants with >70% water content and 0.1
mg/kg for cereals and soil. The information was not sufficient for the
meeting to draw a conclusion on the limit of determination.
A method developed for triadimefon in soil and water (Thornton
and Lloyd, 1977) is also suitable for bitertanol in soil and water
with minor modifications in the GLC column stationary phase (Morris,
1979b). Water is simply extracted with chloroform, the extract is
concentrated (a Florisil column clean-up is optional), and analyzed by
GLC using a flame-alkali detector. Soil is extracted by refluxing with
methanol/water, partitioned with chloroform, washed with water and
cleaned up on a Florisil column. Recoveries from water fortified at
0.005 and 0.01 mg/l were 94% and 78% respectively with a blank of
<0.001 mg/l. Recoveries from six soils fortified at 0.05-0.5 mg/kg
ranged from 76 to 121% (most were >90%) with blanks <0.01 mg/kg
(Morris, 1979b). Chromatograms were consistent with the data.
In a similar procedure for the analysis of bitertanol and
numerous other fungicides in water (Brennecke and Vogeler, 1980),
extraction is with dichloromethane and clean-up by gel permeation
chromatography. The two recoveries for bitertanol were 108% and 104%
for 0.005 and 0.5 mg/l fortifications respectively. The lower limit of
determination was said to be 0.005 mg/l, although the meeting could
not confirm this with the available data.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs were reported to the meeting.
Safety
interval MRL
Country Crop (days) mg/kg
Australia Apples 14 1.0 1)
Peanuts 14 0.2 1) 2)
Belgium Apples 42 0.25
Pears 42 0.25
France Apples 15 1.0
Pears 15 1.0
Germany, Federal Apples 14
Republic Cherries 21
Great Britain Apples 21
Pears 21
Greece Apples 21
Beans 4 crops 14
Pears 5 PHIs 7
Stone fruit 14
10
Israel Apples 14
Italy Pears 14
Beets 30 1.0
Fruit 1.0
Pome fruit 21
Stone fruit 21
Vegetables 14 0.5
Morocco Beans 15
Netherlands Apples 14
Blackberries 0.05 2)
Cucumbers 3
Fruiting Vegetables 1.0
Melons 3
Pears 14
Peppers 3
Other commodities 03)(0.05)
New Zealand Pome fruit 14
Strawberries 21
Safety
interval MRL
Country Crop (days) mg/kg
South Africa Apricots 56 0.5
Beans 28 0.1
Beans (fodder) 42
Peaches 56 0.5
Peanuts 0.05
Peanuts (fodder) 42
Plums 56 0.5
Spain Pome fruit 15 1)
Stone fruit 15 1)
Switzerland Pome fruit 42 0.6
Venezuela Apples 15 1.0
Broad beans 28 1.0
Peanuts 0.2 (1)
Pears 15 1.0
Stone fruit 42 0.6
Yugoslavia General 28
1) = Preliminary
2) = Level at or about the limit of determination.
3) = No residues should be present. Limit of determination 0.05 mg/kg.
APPRAISAL
Bitertanol is a relatively new broad-spectrum fungicide
introduced and being introduced in a number of countries. Its
toxicology was evaluated by the 1983 JMPR which estimated a temporary
ADI. The present meeting evaluated residues in food.
To date only limited residue trials data have been provided and
these have been evaluated. Data were insufficient to estimate residue
levels in cucumbers and beans, but temporary limits were estimated for
several commodities.
The fate of bitertanol has been investigated in plants (apples
and peanuts), animals, soil, light and water.
In the plant studies bitertanol penetration from surface
applications was minimal and there was little volatility. Residues are
taken up by roots, although translocation into the plant is relatively
low. There are some measurable but not major differences in
absorption, translocation and plant metabolism rates of the
two diasterioisomeric forms of bitertanol. Bitertanol is by far
the main plant residue, with low levels of its ketone and
4-hydroxybiphenyl. In apples, most of the residue in the peel.
The fate of residues in non-food animals was evaluated by the
1983 JMPR. The present meeting evaluated metabolism studies on
poultry, cows and fish. Bitertanol is metabolized substantially more
in animals than in plants. The products are rapidly excreted but low
residues can occur in milk, eggs and tissues. These are predominantly
in the liver and kidney, as demonstrated with exaggerated feeding
levels. Bitertanol and p-hydroxy-bitertanol are by far the predominant
residues in chicken tissues and eggs and are reported to be the major
residues in cow tissues and milk, although other metabolites occur at
significant levels in the latter. Although cow metabolism studies were
available to the meeting the only one detailing the distribution of
identified residues was received too late for evaluation, until it has
been reviewed at a future meeting, no final conclusion can be drawn on
the adequacy of information on the fate of residues in animals. The
available data indicate that metabolism appears to be similar among
rats, poultry and cows.
Conventional feeding studies on poultry and ruminants, an
analytical method for the analysis of bitertanol in animal products,
and information on the fate of residues in apples during processing
were all received too late for evaluation.
Extensive information was available to the meeting on the fate of
bitertanol residues in soil, in which it is of low mobility, and some
data were available on water stability and photolysis. The water and
photolysis data available in the working language of the meeting
indicate a high degree of stability in each case. Other studies
provided, but not in the working language of the meeting, were not
evaluated.
Several analytical methods utilizing solvent extraction, GPC or
Florisil clean-up followed by gas chromatography are available for
determining residues in crops, soil and water. Further confirmation of
the limit of determination is desirable.
No information was provided on the fate of residues in storage,
in processing, in commerce or at consumption.
The meeting examined residue data from supervised trials
reflecting good agricultural practice on a number of crops and was
able to estimate the maximum residue levels which were likely to occur
when bitertanol was used in practice and when the reported intervals
between the last application and harvest were observed. These levels
refer only to the parent compound.
RECOMMENDATIONS
COMMODITY MRL (mg/kg)1 Pre-harvest interval on
which recommendations
are based
Apples 2 14
Stone fruit 1 21
Bananas 0.5 0
Oats:
grain 0.1* )
forage 0.1* )
straw 0.1* )
Rye:
grain 0.1* )
forage 0.1* )
straw 0.1* ) from seed treatment
Wheat:
grain 0.1* )
forage 0.1* )
straw 0.1* )
* At or about the limit of determination.
1 Temporary irrespective of the status of the ADI.
FURTHER WORK OR INFORMATION
1. Except for bananas for which substantial data reflecting approved
uses were available, additional information on good agricultural
practice and residue data reflecting approved uses for all
commodities for which temporary limits have been estimated.
Residue data should be from countries from which details of
approved uses have been or will be provided, or those in close
proximity.
2. Additional plant metabolism studies.
3. Additional residue data reflecting good agricultural practice for
additional plant products likely to be used as animal feeds.
Desirable
1. Analytical recoveries through the Thornton analytical method
utilizing the NaOH and HC1 steps designed to remove
interferences.
2. Confirmation of limits of determination for the Specht method.
3. Information on the stability of residues in crops during cold
storage.
4. Additional national maximum residue limits and safety intervals.
5. Photolysis/hydrolysis studies in English (Stegh and Wilmes, 1982;
Wilmes, 1980; Stegh 1980 and Sietsema, 1983).
REFERENCES
Mobay Reports are unpublished reports prepared by the Research and
Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, USA.
Nitokuno Reports are unpublished reports prepared by Nihon Tokushu
Noyaku Seizo KK., No. 8, 2-chome, Nihonbashi, Chuo-ku, Tokyo, Japan.
Bayer Reports:
Leaching in Soil
10.314 - 10.316/78 10.342 - 10.334/82
10.318 - 10.320/78 10.348 - 10.350/82a
10.334 - 10.336/80 10.374 - 10.376/82
10.334A - 10.346A/8010.379 - 10.381/82b
10.309 - 10.311/81 10.388 - 10.390/82b
Soil Persistence
10.317/78 10.324/7910.3257910.311/8010.312/80
68 296 68 29868 30068 30268 304
68 297 68 29968 30168 303
Brennecke, R. Leaching characteristics of Aged [biphenyl-UL-14C]
1983 bitertanol Soil Residues. Unpublished Bayer Report RA
981/209B, October 1983.
Brennecke, R. and Vogeler, K. Method for the Gas Chromatographic
1982 Determination of Residues of Various Fungicides in Water.
Bayer AG, Pflanzenschutz-Anwendungstechnik, unpublished
Report RA-80, January, 1982.
Helling, C.S. and Turner, B.C. Pesticide Mobility: Determination by
1968 Soil Thin-Layer Chromatography, Science 162, 562 (1968).
Helling, C.S., Kearney, P.C. and Alexander, M. Behaviour of Pesticides
1971 in soils, Advan. Agron. 23; 147, 1971.
Lamb, D.W., BAYCOR-14C Accumulation and Persistence of Residues in
1979 Bluegill. Mobay Report No. 68 343 December 13, 1979.
Mobay, Recovery Reports, Apples 68 249, Soil and Water 69 250.
Morris, R.A. Recovery of Baycor from Apples. Unpublished Mobay Report
1979a 68 249, November 19, 1979.
Morris, R.A. Recovery of Baycor from Soil and Water. Unpublished Mobay
1979b Report 68 250, 1979.
Nichols, S.S. and Thornton, J.S. The Behaviour of BAYCOR in Sterile
1979 Aqueous Solutions. Mobay Report No. 68 273. October 22,
1979.
Christ, J.J. Leaching Characteristics of Aged BAYCOR Soil Residues
1979a Mobay Report No. 68 271. September 25, 1979.
Obrist, J.J. and Nichols, S.S. An Interference Study for the BAYCOR
1979 Residue Method for Apples and Peanuts. Mobay Report No. 68
311. November 27, 1979.
Obrist, J.J., Phul, R.J. and Thornton, J.S. Excretion and Tissue
1981 Levels of 14C Following Oral Administration of BAYCOR-
Phenyl-UL-14C to a Dairy Cow. Mobay Report NO. 69 400.
March 24, 1981.
Obrist, J.J., Phul, R.J. and Thornton, J.S. The Metabolism and
1982 Excretion of BAYCOR-Phenyl-UL-14C by Chickens. Mobay Report
No 82614. October 8, 1982.
Obrist, J.J., Phul R.J. and Thornton, J.S. The Metabolism and
1983 Excretion of BAYCOR-Phenyl-UL-14C Following Oral
Administration to a Dairy Cow. Mobay Report, Manuscript in
Preparation. 1983.
Obrist, J.J. and Thornton, J.S. Soil Thin-Layer Mobility of BAYCOR,
1979 BAYTAN, DYRENE, and PEROPAL. Mobay Report No. 68 272.
October 22, 1979.
Phul, R.J. and Hurley, J.B. Soil Adsorption and Desorption of BAYCOR.
1979a Mobay Report No. 68 003. July 18, 1979.
Phul, R.J. and Hurley, J.B. The Metabolism and Degradation of BAYCOR-
1979b Biphenyl-UL-14C on Soil. Mobay Report NO. 67 998. August 6,
1979.
Phul, R.J. and Hurley, J.B. The Metabolism of BAYCOR-Biphenyl-UL-14C
1979c in Apple Fruit. Mobay Report No. 68 305. November 5, 1979,
Revised February 3, 1981A.
Phul, R.J. and Hurley, J.B. Metabolism of BAYCOR-UL-14C in Peanut
1981 Plants. Mobay Report No. 69 310. October 23, 1979, Revised
June 18, 1981b.
Phul, R.J. and Hurley, J.B. The Absorption, Excretion and Metabolism
1983 of BAYCOR-Phenyl-UL-14C by Rats. Mobay Report, Manuscript
in Preparation, 1983.
Phul, R.J., Hurley, J.B. and Close, C.L. Total Radioactive Residues in
1981 Rotational Crops Following a Target Drop of Peanuts Treated
with BAYCOR-14C. Mobay Report No.80 059. September 11,
1981.
Phul, R.J., Hurley, J.B. and Thornton, J.S. The effect of Soil
1979a Microorganisms on the Degradation of BAYCOR. Mobay Report
No. 69 012. July 2, 1979.
Phul, R.J., Obrist, J.J. and Pither, K.M. The excretion of
1979b BAYCOR-Phenyl-UL-14C Following Administration of a Single
Oral Dose to Rats. Mobay Report NO. 68 307. November 19,
1979.
Scheinpflug, H. and van den Boom, T. BAYCOR, A New Fungicide for
1981 Tropical and Subtropical Crops. PflanzenschutzNachrichten
Bayer 34, 8-28.1981.
Sietsema, W.K. Photodecomposition of BAYCOR on a Soil Surface. Mobay
1982 Report No. 82 572. November 16, 1982.
Sietsema, W.K. Photodegradation of BAYCOR in Water. Mobay Report (in
1983 Preparation). 1983.
Smedly, L.A. and Hepler, D.I. Identification of Microorganisms in
1979 Samples of Kansas Loam Soil. Mobay Report No. 67 814. May 1,
1979.
Specht, W. and Tillkes, M. Determination of Agrochemicals After
1980 Clean-up using Gel Permeation Chromatography and Mini-Silica
Gel Column Chromatography. Pflanzenschutz-Nachrichten Bayer
33, 61 - 85. 1980.
Stegh, R. Versuche zum Abbau von KWG 0599 in wässerigen Salzlosungen.
1980 Bayer Experimental Notice. January 24, 1980.
Stegh, R. and Wilmes, R. Light Induced Degradation of Agrochemical
1982 Parent Compounds on Silica Gel as a Model for Degradation on
Soil. Bayer Ag. Pflanzenschutz-Anwendungstechnik unpublished
report PF1666. February 10, 1982.
Takase, I. and Yoshimoto, Y. Residues of KWG 0599 in Upland Soils
1980 Under Laboratory and Field Conditions. Nitokuno Report No.
1134. December 5, 1980.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and its
1977a Metabolite in Apples. Mobay Report No. 54 166. December 30,
1977.
Thornton, J.S. A Gas Chromatographic Method for BAYLETON and KWG 0599
1977 in Soil and Water. Mobay Report NO. 51 231. January 31,
1977.
Wilmes, R. Preliminary Studies on Stability to Light. Bayer Pharma
1980 Unpublished Report, 1980.
Wilmes, R. Abiotic Degradation of Bitertanol. Bayer summary Report.
1981 March 31, 1981.
