
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
CARBON TETRACHLORIDE
Compound
Carbon tetrachloride
Chemical name
Carbon tetrachloride
Synonym
Tetrachloromethane
Empirical formula
CCl4
Structural formula
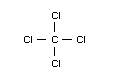 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 76.8°C
Odour: sweetish, chloroform-like
Lowest concentration in air which is detectable by odour: 70 ppm
Flash point: non-flammable
Solubility:
Water: insoluble
Organic solvents: miscible with most organic solvents
Specific gravity (liquid): 1.60
Specific gravity (gas): 5.32
Uses
Carbon tetrachloride is used extensively as a fumigant, either
alone or mixed with other more toxic fumigants including ethylene
dichloride, ethylene dibromide, acrylonitrile, carbon disulfide and
methyl bromide. Carbon tetrachloride is not highly toxic to insects
but since it penetrates to great depths is used for the treatment of
grain in deep silo storages where the disadvantage of its relatively
low toxicity can be overcome by a long exposure period, e.g., of seven
or even 14 days. It is applied as evenly as possible over the grain
surface by hand or preferably by a mechanically driven pump. A typical
rate of application is one gallon per 12 tons of grain.
Residues
Carbon tetrachloride is taken up physically and without chemical
action. The amount taken up by unground whole wheat can be accounted
for by assuming it forms an ideal solution in the wheat oils. Ground
wheat absorbs additional amounts of carbon tetrachloride evidently not
held in solution (Pepper et al., 1947).
Wheat treated on a laboratory scale with a high concentration of
carbon tetrachloride (approximately 300 mg per litre at 20°C for 24
hours) contained 49 ppm four days after treatment and 10 ppm after 55
days. Flour produced from wheat containing 10 ppm of carbon
tetrachloride had 5.7 ppm. No carbon tetrachloride was found in bread
baked from this flour (sensitivity of method 0.5 ppm) (Deshusses and
Desbaumes, 1950).
In an extensive study of the persistence of fumigant residues in
wheat and its milled products (Conroy et al., 1957), it was shown that
grain fumigated in the laboratory with a commonly used application
rate of carbon tetrachloride:carbon disulfide mixture (80:20) had the
following residues of carbon tetrachloride: original wheat 115 ppm;
after milling, flour 21 ppm, shorts 39 ppm, bran 88 ppm. Maximum
levels of carbon tetrachloride in 30 bushel-batches of wheat after
treatment with normal and triple USDA dosage levels of carbon
tetrachloride: carbon disulfide mixture (80:20) were 15 and 25 ppm
respectively. After cleaning and tempering, the residues of the wheat
fumigated at the triple dosage level fell to 9 ppm and after milling
the bran contained 12 ppm. Flour milled from the wheat treated with a
triple dosage contained 1.5 ppm of carbon tetrachloride. During
shipment, residues of carbon tetrachloride in flour decreased from 1.5
ppm to 0.7 ppm.
Maximum residue of carbon tetrachloride in 33 samples of
commercially treated wheat one to five months after fumigation was 50
ppm. The maximum residues in corn, rough rice, oats and grain sorghum
were all lower than for wheat.
Wheat fumigated with carbon tetrachloride: ethylene dichloride
mixture (25:75) showed an average residue of 200 ppm of carbon
tetrachloride 24 hours after fumigation. The first turning reduced the
level to 145 ppm (at 65°F) and the residue, after two additional
turnings was 100 ppm. Two more turnings resulted in only about 10%
further reduction. The highest persistent residues of carbon
tetrachloride in commercial usage would be about 130 ppm.
Carbon tetrachloride added to baking flour (Munsey et al., 1957)
at approximately 10 times the maximum levels found in the flour after
normal fumigation did not persist in the bread. (Sensitivity of
method, 1 ppm.)
Effect of fumigant on treated crop
Carbon tetrachloride does not appear to combine chemically with
the constituents of food crops. The level of residues in the wheat
germ which has a high fat content was found to be less than 50% of
that in the bran (Conroy et al., 1957).
BIOLOGICAL DATA
Biochemical aspects
Carbon tetrachloride causes widespread liver damage. When fed
orally to dogs most of it is excreted unchanged by the lungs (Robbins,
1929). Monkeys inhaling 14CCl4 absorbed about 30% of the amount
inhaled and excreted at least 51% of the dose unchanged in the expired
air. Some was metabolized since 14CO2 was detected in the expired
air and radioactive carbon was present in the urea and carbonate
fractions of the urine. Most of the radioactivity in the urine was
present as unidentified metabolites. Body fat contained the highest
concentration of radioactive material (McCollister et al., 1951).
After subcutaneous administration to rats only traces were
excreted in urine and faeoes, over 90% being excreted through the
lungs. Carbon tetrachloride was detected in the expired air 49 hours
after a dose of 43 mg/kg. Red blood cells retained about 2.5 times
more carbon tetrachloride than did plasma (Soucek, 1961).
After administration to rabbits, 51% was eliminated by
respiration and 49% in urine and faeces. Highest concentrations were
found in nerve, bone marrow and the suprarenal gland (Fabre et al.,
1961).
Toxicological studies
1. The fumigant
Because of the high toxicity of carbon tetrachloride vapour,
stringent precautions must be taken to protect those handling it.
Maximum permissible concentration in the atmosphere recommended for
industrial hygiene is 10 ppm (65 mg/m3) (Anon, 1964). It may be
absorbed through the skin and if ingested can be absorbed from the
alimentary tract.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 12 800 Dybing & Dybing, 1946
Rat oral 7 460 Smyth,
Rat oral 2 920 McCollister et al., 1956
Short- and long-term studies
Carbon tetrachloride has been extensively studied from the point
of view of its biochemical and pathological effects on the liver.
These investigations have been recently reviewed by Rouiller (1964).
Carbon tetrachloride has been shown to produce malignant liver tumours
when given by mouth to mice and hamsters, but this effect is not found
in rats (World Health Organization, 1964).
Groups of three or four male rats were exposed -- single doses of
varying concentrations of carbon tetrachloride vapour for different
lengths of time and an estimate of the single doses having no
observable adverse effects was made (Adams et al., 1952). For 3000
ppm, the maximum time was 0.1 hour; for 800 ppm, 0.5 hour and for 50
ppm, 7.0 hours.
Intermittent exposure of animals to carbon tetrachloride vapour
for seven hours (about 140 exposures in 200 days) showed a no-effect
level (with full macroscopic and microscopic examinations of organs
and tissues) at 5 ppm for rats and guinea-pigs. For monkeys (one
animal only studied) the no-effect level was estimated at 25 ppm and
for rabbits 10 ppm (Adams et al., 1952).
In an earlier study (Smyth et al., 1936) guinea-pigs, rats and
monkeys were exposed to different levels of carbon tetrachloride
vapour for periods up to 10 months. A no-effect level was not
clearly demonstrated in these experiments but an estimate was made
that 100 ppm for eight hours per day indefinitely would be "safe".
Man. Smyth et al. (1936) examined 96 men exposed to average
concentrations of from 5 ppm to 117 ppm of carbon tetrachloride. Of
these, 19 had been exposed for 10 years and 11 of these showed
abnormal clinical findings. Of 88 workers exposed to less than 100
ppm, 43 gave no abnormal results but tests on the remainder gave
evidence of some abnormality.
Thirteen workers were exposed to carbon tetrachloride vapour
averaging 30 ppm (0.19 mg/l) for varying periods. One showed acute
toxic symptoms including subicteric increase of bilirubin which
disappeared after one month. Serum colloidal stability tests were
normal but were found altered one month after the onset, returning to
normal only after five months. Indications of hepatic changes in the
other workers were revealed by santonine and quinine liver function
tests (Sassi and Paruccini, 1954).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 64% (by
weight) of carbon tetrachloride showed no observable effect. The
levels of CCl4 in the grain were not stated and no histopathological
examinations were made (Rowe et al., 1956).
Comments on experimental studies reported
1. Carbon tetrachloride has a relatively high chronic vapour toxicity
and industrial safety levels, calculated from vapour toxicity tests on
several species, have been reduced to 10 ppm in ambient air. Carbon
tetrachloride is actively metabolized when absorbed.
2. Carbon tetrachloride produces tumours in the mouse and hamster, but
not in the rat or other species, and differs from many other
carcinogens in only producing tumours in the organ which is also
damaged by acute exposure, namely the liver. In view of the widespread
industrial experience with this chemical compound, it was considered
that there was no evidence to suggest that man was sensitive to this
action of carbon tetrachloride.
3. When used as a fumigant for grain, most of it is lost during
shipment and storage but residues may persist even in milled products.
No carbon tetrachloride was found in bread baked with flour containing
5.7 ppm carbon tetrachloride.
4. Although it dissolves in the fats present in the grain there is no
evidence of chemical reaction with the food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for carbon tetrachloride. Because
of its toxic effects on the mammalian liver, it should be used as a
fumigant only on condition that no residues (the sensitivity of the
present analytical method being 0.01 ppm) of the unchanged compound
reach the consumer.
Further work required
1. Further investigation of the amount of the residual carbon
tetrachloride remaining in the food after treatment and the effect on
this of processing and cooking.
2. Long-term feeding studies should be carried out on two mammalian
species.
REFERENCES
Adams, E. M., Spencer, H. C., Rowe, V. K., McCollister, D. D. & Irish,
D. D. (1952) Arch. industr. Hyg., 6, 50
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Ass. Off. Agr.
Chemists, 40, 192
Deshusses, J. & Desbaumes, P. (1950) Mitt. Lebensmitt. Hyg., 41,
39
Dybing, F. & Dybing, O. (1946) Acta pharmacol. (Kbh.), 2, 223
Fabre, R., Truhaut, R. & Laham, S. (1961) Proceedings of the
Thirteenth International Congress on Occupational Health, New York,
p. 686
McCollister, D. D., Beamer, W. H., Atchison, G. J. & Spencer, H. C.
(1951) J. Pharmacol. exp. Ther., 102, 112
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Ass. Off. Agr.
Chemists, 40, 201
Pepper, J. H., Hastings, E. & Douglas, T. A. (1947) J. econ. Ent.,
40, 64
Robbins, B. H. (1929) J. Pharmacol. exp. Ther., 37, 203
Rouiller, C. (1964) The liver, Academic Press Inc., New York, vol.
2, pp. 335-476
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Sassi, C. & Paruccini, C. (1954) Med. d. Lavoro, 45, 93
Smyth, H. F., jr, Chemical Hygiene Fellowship, Mellon Institute,
Pittsburgh (Unpublished data)
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Soucek, B. (1961) Pracov. Lék., 13, 287
World Health Organization (1964) Wld Hlth Org. techn. Rep. Ser.,
276, 44
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 76.8°C
Odour: sweetish, chloroform-like
Lowest concentration in air which is detectable by odour: 70 ppm
Flash point: non-flammable
Solubility:
Water: insoluble
Organic solvents: miscible with most organic solvents
Specific gravity (liquid): 1.60
Specific gravity (gas): 5.32
Uses
Carbon tetrachloride is used extensively as a fumigant, either
alone or mixed with other more toxic fumigants including ethylene
dichloride, ethylene dibromide, acrylonitrile, carbon disulfide and
methyl bromide. Carbon tetrachloride is not highly toxic to insects
but since it penetrates to great depths is used for the treatment of
grain in deep silo storages where the disadvantage of its relatively
low toxicity can be overcome by a long exposure period, e.g., of seven
or even 14 days. It is applied as evenly as possible over the grain
surface by hand or preferably by a mechanically driven pump. A typical
rate of application is one gallon per 12 tons of grain.
Residues
Carbon tetrachloride is taken up physically and without chemical
action. The amount taken up by unground whole wheat can be accounted
for by assuming it forms an ideal solution in the wheat oils. Ground
wheat absorbs additional amounts of carbon tetrachloride evidently not
held in solution (Pepper et al., 1947).
Wheat treated on a laboratory scale with a high concentration of
carbon tetrachloride (approximately 300 mg per litre at 20°C for 24
hours) contained 49 ppm four days after treatment and 10 ppm after 55
days. Flour produced from wheat containing 10 ppm of carbon
tetrachloride had 5.7 ppm. No carbon tetrachloride was found in bread
baked from this flour (sensitivity of method 0.5 ppm) (Deshusses and
Desbaumes, 1950).
In an extensive study of the persistence of fumigant residues in
wheat and its milled products (Conroy et al., 1957), it was shown that
grain fumigated in the laboratory with a commonly used application
rate of carbon tetrachloride:carbon disulfide mixture (80:20) had the
following residues of carbon tetrachloride: original wheat 115 ppm;
after milling, flour 21 ppm, shorts 39 ppm, bran 88 ppm. Maximum
levels of carbon tetrachloride in 30 bushel-batches of wheat after
treatment with normal and triple USDA dosage levels of carbon
tetrachloride: carbon disulfide mixture (80:20) were 15 and 25 ppm
respectively. After cleaning and tempering, the residues of the wheat
fumigated at the triple dosage level fell to 9 ppm and after milling
the bran contained 12 ppm. Flour milled from the wheat treated with a
triple dosage contained 1.5 ppm of carbon tetrachloride. During
shipment, residues of carbon tetrachloride in flour decreased from 1.5
ppm to 0.7 ppm.
Maximum residue of carbon tetrachloride in 33 samples of
commercially treated wheat one to five months after fumigation was 50
ppm. The maximum residues in corn, rough rice, oats and grain sorghum
were all lower than for wheat.
Wheat fumigated with carbon tetrachloride: ethylene dichloride
mixture (25:75) showed an average residue of 200 ppm of carbon
tetrachloride 24 hours after fumigation. The first turning reduced the
level to 145 ppm (at 65°F) and the residue, after two additional
turnings was 100 ppm. Two more turnings resulted in only about 10%
further reduction. The highest persistent residues of carbon
tetrachloride in commercial usage would be about 130 ppm.
Carbon tetrachloride added to baking flour (Munsey et al., 1957)
at approximately 10 times the maximum levels found in the flour after
normal fumigation did not persist in the bread. (Sensitivity of
method, 1 ppm.)
Effect of fumigant on treated crop
Carbon tetrachloride does not appear to combine chemically with
the constituents of food crops. The level of residues in the wheat
germ which has a high fat content was found to be less than 50% of
that in the bran (Conroy et al., 1957).
BIOLOGICAL DATA
Biochemical aspects
Carbon tetrachloride causes widespread liver damage. When fed
orally to dogs most of it is excreted unchanged by the lungs (Robbins,
1929). Monkeys inhaling 14CCl4 absorbed about 30% of the amount
inhaled and excreted at least 51% of the dose unchanged in the expired
air. Some was metabolized since 14CO2 was detected in the expired
air and radioactive carbon was present in the urea and carbonate
fractions of the urine. Most of the radioactivity in the urine was
present as unidentified metabolites. Body fat contained the highest
concentration of radioactive material (McCollister et al., 1951).
After subcutaneous administration to rats only traces were
excreted in urine and faeoes, over 90% being excreted through the
lungs. Carbon tetrachloride was detected in the expired air 49 hours
after a dose of 43 mg/kg. Red blood cells retained about 2.5 times
more carbon tetrachloride than did plasma (Soucek, 1961).
After administration to rabbits, 51% was eliminated by
respiration and 49% in urine and faeces. Highest concentrations were
found in nerve, bone marrow and the suprarenal gland (Fabre et al.,
1961).
Toxicological studies
1. The fumigant
Because of the high toxicity of carbon tetrachloride vapour,
stringent precautions must be taken to protect those handling it.
Maximum permissible concentration in the atmosphere recommended for
industrial hygiene is 10 ppm (65 mg/m3) (Anon, 1964). It may be
absorbed through the skin and if ingested can be absorbed from the
alimentary tract.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 12 800 Dybing & Dybing, 1946
Rat oral 7 460 Smyth,
Rat oral 2 920 McCollister et al., 1956
Short- and long-term studies
Carbon tetrachloride has been extensively studied from the point
of view of its biochemical and pathological effects on the liver.
These investigations have been recently reviewed by Rouiller (1964).
Carbon tetrachloride has been shown to produce malignant liver tumours
when given by mouth to mice and hamsters, but this effect is not found
in rats (World Health Organization, 1964).
Groups of three or four male rats were exposed -- single doses of
varying concentrations of carbon tetrachloride vapour for different
lengths of time and an estimate of the single doses having no
observable adverse effects was made (Adams et al., 1952). For 3000
ppm, the maximum time was 0.1 hour; for 800 ppm, 0.5 hour and for 50
ppm, 7.0 hours.
Intermittent exposure of animals to carbon tetrachloride vapour
for seven hours (about 140 exposures in 200 days) showed a no-effect
level (with full macroscopic and microscopic examinations of organs
and tissues) at 5 ppm for rats and guinea-pigs. For monkeys (one
animal only studied) the no-effect level was estimated at 25 ppm and
for rabbits 10 ppm (Adams et al., 1952).
In an earlier study (Smyth et al., 1936) guinea-pigs, rats and
monkeys were exposed to different levels of carbon tetrachloride
vapour for periods up to 10 months. A no-effect level was not
clearly demonstrated in these experiments but an estimate was made
that 100 ppm for eight hours per day indefinitely would be "safe".
Man. Smyth et al. (1936) examined 96 men exposed to average
concentrations of from 5 ppm to 117 ppm of carbon tetrachloride. Of
these, 19 had been exposed for 10 years and 11 of these showed
abnormal clinical findings. Of 88 workers exposed to less than 100
ppm, 43 gave no abnormal results but tests on the remainder gave
evidence of some abnormality.
Thirteen workers were exposed to carbon tetrachloride vapour
averaging 30 ppm (0.19 mg/l) for varying periods. One showed acute
toxic symptoms including subicteric increase of bilirubin which
disappeared after one month. Serum colloidal stability tests were
normal but were found altered one month after the onset, returning to
normal only after five months. Indications of hepatic changes in the
other workers were revealed by santonine and quinine liver function
tests (Sassi and Paruccini, 1954).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 64% (by
weight) of carbon tetrachloride showed no observable effect. The
levels of CCl4 in the grain were not stated and no histopathological
examinations were made (Rowe et al., 1956).
Comments on experimental studies reported
1. Carbon tetrachloride has a relatively high chronic vapour toxicity
and industrial safety levels, calculated from vapour toxicity tests on
several species, have been reduced to 10 ppm in ambient air. Carbon
tetrachloride is actively metabolized when absorbed.
2. Carbon tetrachloride produces tumours in the mouse and hamster, but
not in the rat or other species, and differs from many other
carcinogens in only producing tumours in the organ which is also
damaged by acute exposure, namely the liver. In view of the widespread
industrial experience with this chemical compound, it was considered
that there was no evidence to suggest that man was sensitive to this
action of carbon tetrachloride.
3. When used as a fumigant for grain, most of it is lost during
shipment and storage but residues may persist even in milled products.
No carbon tetrachloride was found in bread baked with flour containing
5.7 ppm carbon tetrachloride.
4. Although it dissolves in the fats present in the grain there is no
evidence of chemical reaction with the food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for carbon tetrachloride. Because
of its toxic effects on the mammalian liver, it should be used as a
fumigant only on condition that no residues (the sensitivity of the
present analytical method being 0.01 ppm) of the unchanged compound
reach the consumer.
Further work required
1. Further investigation of the amount of the residual carbon
tetrachloride remaining in the food after treatment and the effect on
this of processing and cooking.
2. Long-term feeding studies should be carried out on two mammalian
species.
REFERENCES
Adams, E. M., Spencer, H. C., Rowe, V. K., McCollister, D. D. & Irish,
D. D. (1952) Arch. industr. Hyg., 6, 50
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Ass. Off. Agr.
Chemists, 40, 192
Deshusses, J. & Desbaumes, P. (1950) Mitt. Lebensmitt. Hyg., 41,
39
Dybing, F. & Dybing, O. (1946) Acta pharmacol. (Kbh.), 2, 223
Fabre, R., Truhaut, R. & Laham, S. (1961) Proceedings of the
Thirteenth International Congress on Occupational Health, New York,
p. 686
McCollister, D. D., Beamer, W. H., Atchison, G. J. & Spencer, H. C.
(1951) J. Pharmacol. exp. Ther., 102, 112
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Ass. Off. Agr.
Chemists, 40, 201
Pepper, J. H., Hastings, E. & Douglas, T. A. (1947) J. econ. Ent.,
40, 64
Robbins, B. H. (1929) J. Pharmacol. exp. Ther., 37, 203
Rouiller, C. (1964) The liver, Academic Press Inc., New York, vol.
2, pp. 335-476
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Sassi, C. & Paruccini, C. (1954) Med. d. Lavoro, 45, 93
Smyth, H. F., jr, Chemical Hygiene Fellowship, Mellon Institute,
Pittsburgh (Unpublished data)
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Soucek, B. (1961) Pracov. Lék., 13, 287
World Health Organization (1964) Wld Hlth Org. techn. Rep. Ser.,
276, 44
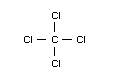 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 76.8°C
Odour: sweetish, chloroform-like
Lowest concentration in air which is detectable by odour: 70 ppm
Flash point: non-flammable
Solubility:
Water: insoluble
Organic solvents: miscible with most organic solvents
Specific gravity (liquid): 1.60
Specific gravity (gas): 5.32
Uses
Carbon tetrachloride is used extensively as a fumigant, either
alone or mixed with other more toxic fumigants including ethylene
dichloride, ethylene dibromide, acrylonitrile, carbon disulfide and
methyl bromide. Carbon tetrachloride is not highly toxic to insects
but since it penetrates to great depths is used for the treatment of
grain in deep silo storages where the disadvantage of its relatively
low toxicity can be overcome by a long exposure period, e.g., of seven
or even 14 days. It is applied as evenly as possible over the grain
surface by hand or preferably by a mechanically driven pump. A typical
rate of application is one gallon per 12 tons of grain.
Residues
Carbon tetrachloride is taken up physically and without chemical
action. The amount taken up by unground whole wheat can be accounted
for by assuming it forms an ideal solution in the wheat oils. Ground
wheat absorbs additional amounts of carbon tetrachloride evidently not
held in solution (Pepper et al., 1947).
Wheat treated on a laboratory scale with a high concentration of
carbon tetrachloride (approximately 300 mg per litre at 20°C for 24
hours) contained 49 ppm four days after treatment and 10 ppm after 55
days. Flour produced from wheat containing 10 ppm of carbon
tetrachloride had 5.7 ppm. No carbon tetrachloride was found in bread
baked from this flour (sensitivity of method 0.5 ppm) (Deshusses and
Desbaumes, 1950).
In an extensive study of the persistence of fumigant residues in
wheat and its milled products (Conroy et al., 1957), it was shown that
grain fumigated in the laboratory with a commonly used application
rate of carbon tetrachloride:carbon disulfide mixture (80:20) had the
following residues of carbon tetrachloride: original wheat 115 ppm;
after milling, flour 21 ppm, shorts 39 ppm, bran 88 ppm. Maximum
levels of carbon tetrachloride in 30 bushel-batches of wheat after
treatment with normal and triple USDA dosage levels of carbon
tetrachloride: carbon disulfide mixture (80:20) were 15 and 25 ppm
respectively. After cleaning and tempering, the residues of the wheat
fumigated at the triple dosage level fell to 9 ppm and after milling
the bran contained 12 ppm. Flour milled from the wheat treated with a
triple dosage contained 1.5 ppm of carbon tetrachloride. During
shipment, residues of carbon tetrachloride in flour decreased from 1.5
ppm to 0.7 ppm.
Maximum residue of carbon tetrachloride in 33 samples of
commercially treated wheat one to five months after fumigation was 50
ppm. The maximum residues in corn, rough rice, oats and grain sorghum
were all lower than for wheat.
Wheat fumigated with carbon tetrachloride: ethylene dichloride
mixture (25:75) showed an average residue of 200 ppm of carbon
tetrachloride 24 hours after fumigation. The first turning reduced the
level to 145 ppm (at 65°F) and the residue, after two additional
turnings was 100 ppm. Two more turnings resulted in only about 10%
further reduction. The highest persistent residues of carbon
tetrachloride in commercial usage would be about 130 ppm.
Carbon tetrachloride added to baking flour (Munsey et al., 1957)
at approximately 10 times the maximum levels found in the flour after
normal fumigation did not persist in the bread. (Sensitivity of
method, 1 ppm.)
Effect of fumigant on treated crop
Carbon tetrachloride does not appear to combine chemically with
the constituents of food crops. The level of residues in the wheat
germ which has a high fat content was found to be less than 50% of
that in the bran (Conroy et al., 1957).
BIOLOGICAL DATA
Biochemical aspects
Carbon tetrachloride causes widespread liver damage. When fed
orally to dogs most of it is excreted unchanged by the lungs (Robbins,
1929). Monkeys inhaling 14CCl4 absorbed about 30% of the amount
inhaled and excreted at least 51% of the dose unchanged in the expired
air. Some was metabolized since 14CO2 was detected in the expired
air and radioactive carbon was present in the urea and carbonate
fractions of the urine. Most of the radioactivity in the urine was
present as unidentified metabolites. Body fat contained the highest
concentration of radioactive material (McCollister et al., 1951).
After subcutaneous administration to rats only traces were
excreted in urine and faeoes, over 90% being excreted through the
lungs. Carbon tetrachloride was detected in the expired air 49 hours
after a dose of 43 mg/kg. Red blood cells retained about 2.5 times
more carbon tetrachloride than did plasma (Soucek, 1961).
After administration to rabbits, 51% was eliminated by
respiration and 49% in urine and faeces. Highest concentrations were
found in nerve, bone marrow and the suprarenal gland (Fabre et al.,
1961).
Toxicological studies
1. The fumigant
Because of the high toxicity of carbon tetrachloride vapour,
stringent precautions must be taken to protect those handling it.
Maximum permissible concentration in the atmosphere recommended for
industrial hygiene is 10 ppm (65 mg/m3) (Anon, 1964). It may be
absorbed through the skin and if ingested can be absorbed from the
alimentary tract.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 12 800 Dybing & Dybing, 1946
Rat oral 7 460 Smyth,
Rat oral 2 920 McCollister et al., 1956
Short- and long-term studies
Carbon tetrachloride has been extensively studied from the point
of view of its biochemical and pathological effects on the liver.
These investigations have been recently reviewed by Rouiller (1964).
Carbon tetrachloride has been shown to produce malignant liver tumours
when given by mouth to mice and hamsters, but this effect is not found
in rats (World Health Organization, 1964).
Groups of three or four male rats were exposed -- single doses of
varying concentrations of carbon tetrachloride vapour for different
lengths of time and an estimate of the single doses having no
observable adverse effects was made (Adams et al., 1952). For 3000
ppm, the maximum time was 0.1 hour; for 800 ppm, 0.5 hour and for 50
ppm, 7.0 hours.
Intermittent exposure of animals to carbon tetrachloride vapour
for seven hours (about 140 exposures in 200 days) showed a no-effect
level (with full macroscopic and microscopic examinations of organs
and tissues) at 5 ppm for rats and guinea-pigs. For monkeys (one
animal only studied) the no-effect level was estimated at 25 ppm and
for rabbits 10 ppm (Adams et al., 1952).
In an earlier study (Smyth et al., 1936) guinea-pigs, rats and
monkeys were exposed to different levels of carbon tetrachloride
vapour for periods up to 10 months. A no-effect level was not
clearly demonstrated in these experiments but an estimate was made
that 100 ppm for eight hours per day indefinitely would be "safe".
Man. Smyth et al. (1936) examined 96 men exposed to average
concentrations of from 5 ppm to 117 ppm of carbon tetrachloride. Of
these, 19 had been exposed for 10 years and 11 of these showed
abnormal clinical findings. Of 88 workers exposed to less than 100
ppm, 43 gave no abnormal results but tests on the remainder gave
evidence of some abnormality.
Thirteen workers were exposed to carbon tetrachloride vapour
averaging 30 ppm (0.19 mg/l) for varying periods. One showed acute
toxic symptoms including subicteric increase of bilirubin which
disappeared after one month. Serum colloidal stability tests were
normal but were found altered one month after the onset, returning to
normal only after five months. Indications of hepatic changes in the
other workers were revealed by santonine and quinine liver function
tests (Sassi and Paruccini, 1954).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 64% (by
weight) of carbon tetrachloride showed no observable effect. The
levels of CCl4 in the grain were not stated and no histopathological
examinations were made (Rowe et al., 1956).
Comments on experimental studies reported
1. Carbon tetrachloride has a relatively high chronic vapour toxicity
and industrial safety levels, calculated from vapour toxicity tests on
several species, have been reduced to 10 ppm in ambient air. Carbon
tetrachloride is actively metabolized when absorbed.
2. Carbon tetrachloride produces tumours in the mouse and hamster, but
not in the rat or other species, and differs from many other
carcinogens in only producing tumours in the organ which is also
damaged by acute exposure, namely the liver. In view of the widespread
industrial experience with this chemical compound, it was considered
that there was no evidence to suggest that man was sensitive to this
action of carbon tetrachloride.
3. When used as a fumigant for grain, most of it is lost during
shipment and storage but residues may persist even in milled products.
No carbon tetrachloride was found in bread baked with flour containing
5.7 ppm carbon tetrachloride.
4. Although it dissolves in the fats present in the grain there is no
evidence of chemical reaction with the food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for carbon tetrachloride. Because
of its toxic effects on the mammalian liver, it should be used as a
fumigant only on condition that no residues (the sensitivity of the
present analytical method being 0.01 ppm) of the unchanged compound
reach the consumer.
Further work required
1. Further investigation of the amount of the residual carbon
tetrachloride remaining in the food after treatment and the effect on
this of processing and cooking.
2. Long-term feeding studies should be carried out on two mammalian
species.
REFERENCES
Adams, E. M., Spencer, H. C., Rowe, V. K., McCollister, D. D. & Irish,
D. D. (1952) Arch. industr. Hyg., 6, 50
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Ass. Off. Agr.
Chemists, 40, 192
Deshusses, J. & Desbaumes, P. (1950) Mitt. Lebensmitt. Hyg., 41,
39
Dybing, F. & Dybing, O. (1946) Acta pharmacol. (Kbh.), 2, 223
Fabre, R., Truhaut, R. & Laham, S. (1961) Proceedings of the
Thirteenth International Congress on Occupational Health, New York,
p. 686
McCollister, D. D., Beamer, W. H., Atchison, G. J. & Spencer, H. C.
(1951) J. Pharmacol. exp. Ther., 102, 112
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Ass. Off. Agr.
Chemists, 40, 201
Pepper, J. H., Hastings, E. & Douglas, T. A. (1947) J. econ. Ent.,
40, 64
Robbins, B. H. (1929) J. Pharmacol. exp. Ther., 37, 203
Rouiller, C. (1964) The liver, Academic Press Inc., New York, vol.
2, pp. 335-476
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Sassi, C. & Paruccini, C. (1954) Med. d. Lavoro, 45, 93
Smyth, H. F., jr, Chemical Hygiene Fellowship, Mellon Institute,
Pittsburgh (Unpublished data)
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Soucek, B. (1961) Pracov. Lék., 13, 287
World Health Organization (1964) Wld Hlth Org. techn. Rep. Ser.,
276, 44
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): colourless liquid
Boiling-point: 76.8°C
Odour: sweetish, chloroform-like
Lowest concentration in air which is detectable by odour: 70 ppm
Flash point: non-flammable
Solubility:
Water: insoluble
Organic solvents: miscible with most organic solvents
Specific gravity (liquid): 1.60
Specific gravity (gas): 5.32
Uses
Carbon tetrachloride is used extensively as a fumigant, either
alone or mixed with other more toxic fumigants including ethylene
dichloride, ethylene dibromide, acrylonitrile, carbon disulfide and
methyl bromide. Carbon tetrachloride is not highly toxic to insects
but since it penetrates to great depths is used for the treatment of
grain in deep silo storages where the disadvantage of its relatively
low toxicity can be overcome by a long exposure period, e.g., of seven
or even 14 days. It is applied as evenly as possible over the grain
surface by hand or preferably by a mechanically driven pump. A typical
rate of application is one gallon per 12 tons of grain.
Residues
Carbon tetrachloride is taken up physically and without chemical
action. The amount taken up by unground whole wheat can be accounted
for by assuming it forms an ideal solution in the wheat oils. Ground
wheat absorbs additional amounts of carbon tetrachloride evidently not
held in solution (Pepper et al., 1947).
Wheat treated on a laboratory scale with a high concentration of
carbon tetrachloride (approximately 300 mg per litre at 20°C for 24
hours) contained 49 ppm four days after treatment and 10 ppm after 55
days. Flour produced from wheat containing 10 ppm of carbon
tetrachloride had 5.7 ppm. No carbon tetrachloride was found in bread
baked from this flour (sensitivity of method 0.5 ppm) (Deshusses and
Desbaumes, 1950).
In an extensive study of the persistence of fumigant residues in
wheat and its milled products (Conroy et al., 1957), it was shown that
grain fumigated in the laboratory with a commonly used application
rate of carbon tetrachloride:carbon disulfide mixture (80:20) had the
following residues of carbon tetrachloride: original wheat 115 ppm;
after milling, flour 21 ppm, shorts 39 ppm, bran 88 ppm. Maximum
levels of carbon tetrachloride in 30 bushel-batches of wheat after
treatment with normal and triple USDA dosage levels of carbon
tetrachloride: carbon disulfide mixture (80:20) were 15 and 25 ppm
respectively. After cleaning and tempering, the residues of the wheat
fumigated at the triple dosage level fell to 9 ppm and after milling
the bran contained 12 ppm. Flour milled from the wheat treated with a
triple dosage contained 1.5 ppm of carbon tetrachloride. During
shipment, residues of carbon tetrachloride in flour decreased from 1.5
ppm to 0.7 ppm.
Maximum residue of carbon tetrachloride in 33 samples of
commercially treated wheat one to five months after fumigation was 50
ppm. The maximum residues in corn, rough rice, oats and grain sorghum
were all lower than for wheat.
Wheat fumigated with carbon tetrachloride: ethylene dichloride
mixture (25:75) showed an average residue of 200 ppm of carbon
tetrachloride 24 hours after fumigation. The first turning reduced the
level to 145 ppm (at 65°F) and the residue, after two additional
turnings was 100 ppm. Two more turnings resulted in only about 10%
further reduction. The highest persistent residues of carbon
tetrachloride in commercial usage would be about 130 ppm.
Carbon tetrachloride added to baking flour (Munsey et al., 1957)
at approximately 10 times the maximum levels found in the flour after
normal fumigation did not persist in the bread. (Sensitivity of
method, 1 ppm.)
Effect of fumigant on treated crop
Carbon tetrachloride does not appear to combine chemically with
the constituents of food crops. The level of residues in the wheat
germ which has a high fat content was found to be less than 50% of
that in the bran (Conroy et al., 1957).
BIOLOGICAL DATA
Biochemical aspects
Carbon tetrachloride causes widespread liver damage. When fed
orally to dogs most of it is excreted unchanged by the lungs (Robbins,
1929). Monkeys inhaling 14CCl4 absorbed about 30% of the amount
inhaled and excreted at least 51% of the dose unchanged in the expired
air. Some was metabolized since 14CO2 was detected in the expired
air and radioactive carbon was present in the urea and carbonate
fractions of the urine. Most of the radioactivity in the urine was
present as unidentified metabolites. Body fat contained the highest
concentration of radioactive material (McCollister et al., 1951).
After subcutaneous administration to rats only traces were
excreted in urine and faeoes, over 90% being excreted through the
lungs. Carbon tetrachloride was detected in the expired air 49 hours
after a dose of 43 mg/kg. Red blood cells retained about 2.5 times
more carbon tetrachloride than did plasma (Soucek, 1961).
After administration to rabbits, 51% was eliminated by
respiration and 49% in urine and faeces. Highest concentrations were
found in nerve, bone marrow and the suprarenal gland (Fabre et al.,
1961).
Toxicological studies
1. The fumigant
Because of the high toxicity of carbon tetrachloride vapour,
stringent precautions must be taken to protect those handling it.
Maximum permissible concentration in the atmosphere recommended for
industrial hygiene is 10 ppm (65 mg/m3) (Anon, 1964). It may be
absorbed through the skin and if ingested can be absorbed from the
alimentary tract.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse oral 12 800 Dybing & Dybing, 1946
Rat oral 7 460 Smyth,
Rat oral 2 920 McCollister et al., 1956
Short- and long-term studies
Carbon tetrachloride has been extensively studied from the point
of view of its biochemical and pathological effects on the liver.
These investigations have been recently reviewed by Rouiller (1964).
Carbon tetrachloride has been shown to produce malignant liver tumours
when given by mouth to mice and hamsters, but this effect is not found
in rats (World Health Organization, 1964).
Groups of three or four male rats were exposed -- single doses of
varying concentrations of carbon tetrachloride vapour for different
lengths of time and an estimate of the single doses having no
observable adverse effects was made (Adams et al., 1952). For 3000
ppm, the maximum time was 0.1 hour; for 800 ppm, 0.5 hour and for 50
ppm, 7.0 hours.
Intermittent exposure of animals to carbon tetrachloride vapour
for seven hours (about 140 exposures in 200 days) showed a no-effect
level (with full macroscopic and microscopic examinations of organs
and tissues) at 5 ppm for rats and guinea-pigs. For monkeys (one
animal only studied) the no-effect level was estimated at 25 ppm and
for rabbits 10 ppm (Adams et al., 1952).
In an earlier study (Smyth et al., 1936) guinea-pigs, rats and
monkeys were exposed to different levels of carbon tetrachloride
vapour for periods up to 10 months. A no-effect level was not
clearly demonstrated in these experiments but an estimate was made
that 100 ppm for eight hours per day indefinitely would be "safe".
Man. Smyth et al. (1936) examined 96 men exposed to average
concentrations of from 5 ppm to 117 ppm of carbon tetrachloride. Of
these, 19 had been exposed for 10 years and 11 of these showed
abnormal clinical findings. Of 88 workers exposed to less than 100
ppm, 43 gave no abnormal results but tests on the remainder gave
evidence of some abnormality.
Thirteen workers were exposed to carbon tetrachloride vapour
averaging 30 ppm (0.19 mg/l) for varying periods. One showed acute
toxic symptoms including subicteric increase of bilirubin which
disappeared after one month. Serum colloidal stability tests were
normal but were found altered one month after the onset, returning to
normal only after five months. Indications of hepatic changes in the
other workers were revealed by santonine and quinine liver function
tests (Sassi and Paruccini, 1954).
2. The fumigated foodstuff
Chickens fed for five days, pigs for 12 days and cattle for 7.5
days with grain freshly treated with a fumigant containing 64% (by
weight) of carbon tetrachloride showed no observable effect. The
levels of CCl4 in the grain were not stated and no histopathological
examinations were made (Rowe et al., 1956).
Comments on experimental studies reported
1. Carbon tetrachloride has a relatively high chronic vapour toxicity
and industrial safety levels, calculated from vapour toxicity tests on
several species, have been reduced to 10 ppm in ambient air. Carbon
tetrachloride is actively metabolized when absorbed.
2. Carbon tetrachloride produces tumours in the mouse and hamster, but
not in the rat or other species, and differs from many other
carcinogens in only producing tumours in the organ which is also
damaged by acute exposure, namely the liver. In view of the widespread
industrial experience with this chemical compound, it was considered
that there was no evidence to suggest that man was sensitive to this
action of carbon tetrachloride.
3. When used as a fumigant for grain, most of it is lost during
shipment and storage but residues may persist even in milled products.
No carbon tetrachloride was found in bread baked with flour containing
5.7 ppm carbon tetrachloride.
4. Although it dissolves in the fats present in the grain there is no
evidence of chemical reaction with the food constituents.
Evaluation
On the available toxicological evidence it is impossible to
calculate an acceptable daily intake for carbon tetrachloride. Because
of its toxic effects on the mammalian liver, it should be used as a
fumigant only on condition that no residues (the sensitivity of the
present analytical method being 0.01 ppm) of the unchanged compound
reach the consumer.
Further work required
1. Further investigation of the amount of the residual carbon
tetrachloride remaining in the food after treatment and the effect on
this of processing and cooking.
2. Long-term feeding studies should be carried out on two mammalian
species.
REFERENCES
Adams, E. M., Spencer, H. C., Rowe, V. K., McCollister, D. D. & Irish,
D. D. (1952) Arch. industr. Hyg., 6, 50
Anon. (1964) Threshold limit values for 1964, Arch. environm.
Hlth., 9, 545
Conroy, H. W., Walkden, H. H. & Farrell, E. (1957) J. Ass. Off. Agr.
Chemists, 40, 192
Deshusses, J. & Desbaumes, P. (1950) Mitt. Lebensmitt. Hyg., 41,
39
Dybing, F. & Dybing, O. (1946) Acta pharmacol. (Kbh.), 2, 223
Fabre, R., Truhaut, R. & Laham, S. (1961) Proceedings of the
Thirteenth International Congress on Occupational Health, New York,
p. 686
McCollister, D. D., Beamer, W. H., Atchison, G. J. & Spencer, H. C.
(1951) J. Pharmacol. exp. Ther., 102, 112
McCollister, D. D., Hollingsworth, R. L., Oyen, F. & Rowe, V. K.
(1956) Arch. industr. Hlth, 13, 1
Munsey, V. E., Mills, P. A. & Klein, A. K. (1957) J. Ass. Off. Agr.
Chemists, 40, 201
Pepper, J. H., Hastings, E. & Douglas, T. A. (1947) J. econ. Ent.,
40, 64
Robbins, B. H. (1929) J. Pharmacol. exp. Ther., 37, 203
Rouiller, C. (1964) The liver, Academic Press Inc., New York, vol.
2, pp. 335-476
Rowe, V. K., Hollingsworth, R. L. & McCollister, D. D. (1956) J.
Agric. Food Chem., 2, 1318
Sassi, C. & Paruccini, C. (1954) Med. d. Lavoro, 45, 93
Smyth, H. F., jr, Chemical Hygiene Fellowship, Mellon Institute,
Pittsburgh (Unpublished data)
Smyth, H. F., Smyth, H. F., jr & Carpenter, C. P. (1936) J. industr.
Hyg., 18, 277
Soucek, B. (1961) Pracov. Lék., 13, 287
World Health Organization (1964) Wld Hlth Org. techn. Rep. Ser.,
276, 44
