
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
ISOFENPHOS
IDENTITY
Common name: isofenphos
IUPAC chemical name: O-ethyl- 0-2-isopropoxy-carbonylphenyl
isopropylphosphoramidothioate
Synonyms: C.A. name: 1-methylethyl 2-[ethoxy [1-methylethyl)-
amino] phosphinothionyl] oxy] benzoates BAY
SRA 12869, OFTANOLR, AMAZER
Structural formula:
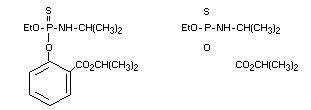 Other information on identity and properties
Empirical formula: C15H24NO4PS
Molecular weight: 345.4
Appearance: colourless oil (pure active ingredient)
yellow-brown liquid (technical active
ingredient)
Boiling point: not distillable (pure active ingredient)
at and above 200°C decomposition (techn.
a.i.)
Vapour pressure: 4 × 10-6mbar at 20°C (pure active
ingredient)
Specific gravity: 1.134 at 20°C
4°
Solubility: water 0.002 (pure active ingredient)
(g/100 g solvent
at 20°C) cyclohecanone > 60
isopropyl alcohol > 60
methylene chloride > 60
ligroin (80 -110°C) > 60
toluene > 60
Minimum degree of
purity: 88%
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, elimination and biotransformation
Rat
After a single oral dose of 15 mg/kg bw of ring-uL-(14C)-
isofenphos, male and female rats excreted 88% of the administered
radioactivity in the urine and 4% in the faeces within 72h of dosing.
No 14CO2 was detectable in the expired air. The rate of elimination
during the first 24 h appeared to be slower in females than in males,
but the total percentage of administered radioactive dose recovered in
the excreta of both sexes in the 72h post-treatment period was
comparable. The major urinary metabolite identified was isopropyl
salicylate (in conjugated and non-conjugated forms), which accounts
for 52% of the 14C-dose given to the animals. Other metabolites found
in the urine included conjugated and unconjugated O-hydroxyhippuric
acid, salicylic acid and deaminated isofenphos oxygen analogue, which
together amounts to approximately another 10% of the administered
radioactivity. There was no significant sex difference in metabolites,
either qualitatively or quantitatively (Shaw et al 1977).
Female rats were intubated with ring uL(14C)-isofenphos at
15 mg/kg bw/day for 6 consecutive days. Radioactive residues in the
tissues analysed (liver, fat, kidney and muscle) remained fairly
constant during the dosing period and declined sharply upon cessation
of treatment. At the end of an 8-day withdrawal period, the kidney was
the only tissue still containing small amounts of 14C-residues. In
the rats sacrificed 1 1/2 h after the sixth dose, unchanged
isofenphos, isofenphos oxygen analogue, isopropyl salicylate and
O-hydroxyhippuric acid were detected in all four tissues examined. The
major identified component in liver, kidney and fat was unchanged
isofenphos and in muscle was isopropyl salicylate. Deaminated
isofenphos oxygen analogue was also found in liver, kidney and muscle
(Strankowski et al 1977a).
Pig
A male pig was fed ring-uL-(14C)-isofenphos-treated feed pellets
at a rate of 2 mg/kg bw/day for 5 consecutive days. Consumption of the
treated feed was monitored to ensure complete intake of the daily
dose. Within 24h of the first dose, 81% of the administered 14C was
eliminated in the urine and 14% in the faeces. Of the total
radioactivity administered during the 5-day dosing period, 56% was
recovered in the urine and 21% in the faeces. At sacrifice 3h after
treatment on day 5 of the study, the kidney had the highest
14C-residue level and the brain had the lowest, as compared to 5
other tissues (liver, heart, bacon, ham and fat) examined.
A total of 76% 14C-residue in a composite urine sample was
identified. The urinary metabolites included conjugated and
unconjugated isopropyl salicylate (55%), deaminated isofenphos oxygen
analogue glucuronide (10%), salicylic acid (5%), O-hydroxyhippuric
acid (4%) and cyclic isofenphos (2%). Unchanged isofenphos, found in
all 4 tissues (liver, kidney, muscle, fat) analysed was the primary
component identified in muscle and fat. Isopropyl salicylate
(conjugated and unconjugated) was the major metabolite in liver and
kidney. Other tissue metabolites detected included deaminated
isofenphos oxygen analogue O-hydroxyhippuric acid, isofenphos oxygen
analogue and des N-isopropyl isofenphos (Strankowski et al 1977b).
Cow
In a lactating dairy cow treated acutely, via bolus, with ring-uL
(14C)-isofenphos at 0.2 mg/kg bw, 14C concentration in the blood
plasma peaked 2h after the dose and then dropped sharply to one fourth
of the maximum level within 24h of treatment. Approximately 90% of the
administered radioactive dose was eliminated in the urine and 4% in
the faeces within 48 h of dosing. Less than 1% of the 14C dose was
recovered in the milk. The major metabolite in both urine and milk was
O-hydroxyhippuric acid. Other metabolites identified in urine and milk
were salicylic acid, isopropyl salicylate and deaminated isofenphos
oxygen analogue. In urine and the milk, about 78% and 66% respectively
of the radioactive residues were identified (Strankowski and Murphy
1977a).
A lactating cow receiving, via bolus, 0.2 mg/kg bw/day of ring-
uL(14C)-isofenphos for 5 days had excreted 63% of the total
administered radioactivity in the urine at sacrifice 2 h after the
fifth dose. No 14C residues were indicated in the report to
"accumulate" in the milk. Of the 11 tissues analysed for radioactive
tissues at sacrifice, liver and kidney were the only tissues found to
have significant radioactivity. The major metabolite in liver and
kidney was salicylic acid, both conjugated and unconjugated. Other
metabolites identified in both tissues were isofenphos oxygen analogue
and conjugated and non-conjugated forms of O-hydroxyhippuric acid and
isopropyl salicylate (Strankowski and Murphy 1977b).
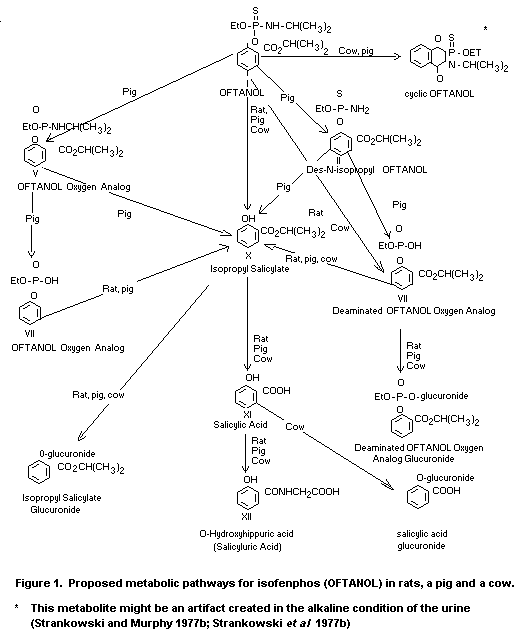
Figure 1 presents the proposed metabolic pathways for isofenphos
in mammals (rat, pig and cow) (Shaw et al 1977; Strankowski et al
1977b; Strankowski and Murphy 1977a).
Hen
In hens orally treated with ring-uL(14C)-isofenphos at 4 mg/kg
bw/day for 3 consecutive days, 41% of the total administered dose was
stated to be eliminated in the excreta "within 51 h" presumably of the
last dose. The major components found in the excreta were isofenphos
and isopropyl salicylate. The total organphosphate residue in tissues
and eggs accounted for less than 3% of the total dose administered. In
the tissues and eggs, isofenphos was also the major compound
identified, with isopropyl salicylate, isofenphos oxygen analogue and
des N-isopropyl isofenphos being the minor metabolites (Kurtz and Shaw
II 1977).
Fish
Channel catfish were exposed continuously to ring-uL (14C)-
isofenphos in water at a concentration of about 10 ppb for 28 days.
During the exposure period, the 14C residues accumulated to a peak of
approximately 75 times the level of the water in 7 days and slowly
declined thereafter. About 88% of the total 14C-residues in the whole
fish could be extracted by acetonitrile, and the non-edible portion of
the fish contained 76% of the extractable residues. One hundred
percent of these 14C-residues were identified as unchanged
isofenphos. Upon treatment withdrawal, approximately 63% of the
accumulated 14C-residues were excreted within 5 h and 87% within one
day. Ten days after discontinuation of exposure only 4% of the
accumulated radioactivity still remained in the fish (Nelson and Roney
1977).
Effects on enzymes and other biochemical parameters
The 50% depression of cholinesterase activity in serum,
erythrocyte and brain of male rats by isofenphos ("technically pure
grade") was determined in vitro. The I50 values were: serum:
8.2 × 10-4 mole, erythrocyte: 3.1 × 10-4 mole, and brain:
2.87 × 10-4 mole (Solmecke and Kimmerle 1972b).
Groups of 5 male rats were given acute oral doses of isofenphos
at levels ranging from 0.5 to 35 mg/kg bw. Activity of plasma and
erythrocyte cholinesterase in the males was depressed (20 to 81%), in
a dose-dependent pattern, above 0.5 mg/kg bw at both 2 and 24 h post-
treatment, with maximum inhibition being noted at 2 h. Plasma
cholinesterase was no longer affected at any dosage level 3 days after
dosing, while erythrocyte cholinesterase was still inhibited by 26% at
35 mg/kg bw 7 days after treatment. In female rats, a single oral dose
of isofenphos at 2.5 mg/kg bw but not at 0.5 mg/kg bw caused a
Other information on identity and properties
Empirical formula: C15H24NO4PS
Molecular weight: 345.4
Appearance: colourless oil (pure active ingredient)
yellow-brown liquid (technical active
ingredient)
Boiling point: not distillable (pure active ingredient)
at and above 200°C decomposition (techn.
a.i.)
Vapour pressure: 4 × 10-6mbar at 20°C (pure active
ingredient)
Specific gravity: 1.134 at 20°C
4°
Solubility: water 0.002 (pure active ingredient)
(g/100 g solvent
at 20°C) cyclohecanone > 60
isopropyl alcohol > 60
methylene chloride > 60
ligroin (80 -110°C) > 60
toluene > 60
Minimum degree of
purity: 88%
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, elimination and biotransformation
Rat
After a single oral dose of 15 mg/kg bw of ring-uL-(14C)-
isofenphos, male and female rats excreted 88% of the administered
radioactivity in the urine and 4% in the faeces within 72h of dosing.
No 14CO2 was detectable in the expired air. The rate of elimination
during the first 24 h appeared to be slower in females than in males,
but the total percentage of administered radioactive dose recovered in
the excreta of both sexes in the 72h post-treatment period was
comparable. The major urinary metabolite identified was isopropyl
salicylate (in conjugated and non-conjugated forms), which accounts
for 52% of the 14C-dose given to the animals. Other metabolites found
in the urine included conjugated and unconjugated O-hydroxyhippuric
acid, salicylic acid and deaminated isofenphos oxygen analogue, which
together amounts to approximately another 10% of the administered
radioactivity. There was no significant sex difference in metabolites,
either qualitatively or quantitatively (Shaw et al 1977).
Female rats were intubated with ring uL(14C)-isofenphos at
15 mg/kg bw/day for 6 consecutive days. Radioactive residues in the
tissues analysed (liver, fat, kidney and muscle) remained fairly
constant during the dosing period and declined sharply upon cessation
of treatment. At the end of an 8-day withdrawal period, the kidney was
the only tissue still containing small amounts of 14C-residues. In
the rats sacrificed 1 1/2 h after the sixth dose, unchanged
isofenphos, isofenphos oxygen analogue, isopropyl salicylate and
O-hydroxyhippuric acid were detected in all four tissues examined. The
major identified component in liver, kidney and fat was unchanged
isofenphos and in muscle was isopropyl salicylate. Deaminated
isofenphos oxygen analogue was also found in liver, kidney and muscle
(Strankowski et al 1977a).
Pig
A male pig was fed ring-uL-(14C)-isofenphos-treated feed pellets
at a rate of 2 mg/kg bw/day for 5 consecutive days. Consumption of the
treated feed was monitored to ensure complete intake of the daily
dose. Within 24h of the first dose, 81% of the administered 14C was
eliminated in the urine and 14% in the faeces. Of the total
radioactivity administered during the 5-day dosing period, 56% was
recovered in the urine and 21% in the faeces. At sacrifice 3h after
treatment on day 5 of the study, the kidney had the highest
14C-residue level and the brain had the lowest, as compared to 5
other tissues (liver, heart, bacon, ham and fat) examined.
A total of 76% 14C-residue in a composite urine sample was
identified. The urinary metabolites included conjugated and
unconjugated isopropyl salicylate (55%), deaminated isofenphos oxygen
analogue glucuronide (10%), salicylic acid (5%), O-hydroxyhippuric
acid (4%) and cyclic isofenphos (2%). Unchanged isofenphos, found in
all 4 tissues (liver, kidney, muscle, fat) analysed was the primary
component identified in muscle and fat. Isopropyl salicylate
(conjugated and unconjugated) was the major metabolite in liver and
kidney. Other tissue metabolites detected included deaminated
isofenphos oxygen analogue O-hydroxyhippuric acid, isofenphos oxygen
analogue and des N-isopropyl isofenphos (Strankowski et al 1977b).
Cow
In a lactating dairy cow treated acutely, via bolus, with ring-uL
(14C)-isofenphos at 0.2 mg/kg bw, 14C concentration in the blood
plasma peaked 2h after the dose and then dropped sharply to one fourth
of the maximum level within 24h of treatment. Approximately 90% of the
administered radioactive dose was eliminated in the urine and 4% in
the faeces within 48 h of dosing. Less than 1% of the 14C dose was
recovered in the milk. The major metabolite in both urine and milk was
O-hydroxyhippuric acid. Other metabolites identified in urine and milk
were salicylic acid, isopropyl salicylate and deaminated isofenphos
oxygen analogue. In urine and the milk, about 78% and 66% respectively
of the radioactive residues were identified (Strankowski and Murphy
1977a).
A lactating cow receiving, via bolus, 0.2 mg/kg bw/day of ring-
uL(14C)-isofenphos for 5 days had excreted 63% of the total
administered radioactivity in the urine at sacrifice 2 h after the
fifth dose. No 14C residues were indicated in the report to
"accumulate" in the milk. Of the 11 tissues analysed for radioactive
tissues at sacrifice, liver and kidney were the only tissues found to
have significant radioactivity. The major metabolite in liver and
kidney was salicylic acid, both conjugated and unconjugated. Other
metabolites identified in both tissues were isofenphos oxygen analogue
and conjugated and non-conjugated forms of O-hydroxyhippuric acid and
isopropyl salicylate (Strankowski and Murphy 1977b).
Figure 1 presents the proposed metabolic pathways for isofenphos
in mammals (rat, pig and cow) (Shaw et al 1977; Strankowski et al
1977b; Strankowski and Murphy 1977a).
Hen
In hens orally treated with ring-uL(14C)-isofenphos at 4 mg/kg
bw/day for 3 consecutive days, 41% of the total administered dose was
stated to be eliminated in the excreta "within 51 h" presumably of the
last dose. The major components found in the excreta were isofenphos
and isopropyl salicylate. The total organphosphate residue in tissues
and eggs accounted for less than 3% of the total dose administered. In
the tissues and eggs, isofenphos was also the major compound
identified, with isopropyl salicylate, isofenphos oxygen analogue and
des N-isopropyl isofenphos being the minor metabolites (Kurtz and Shaw
II 1977).
Fish
Channel catfish were exposed continuously to ring-uL (14C)-
isofenphos in water at a concentration of about 10 ppb for 28 days.
During the exposure period, the 14C residues accumulated to a peak of
approximately 75 times the level of the water in 7 days and slowly
declined thereafter. About 88% of the total 14C-residues in the whole
fish could be extracted by acetonitrile, and the non-edible portion of
the fish contained 76% of the extractable residues. One hundred
percent of these 14C-residues were identified as unchanged
isofenphos. Upon treatment withdrawal, approximately 63% of the
accumulated 14C-residues were excreted within 5 h and 87% within one
day. Ten days after discontinuation of exposure only 4% of the
accumulated radioactivity still remained in the fish (Nelson and Roney
1977).
Effects on enzymes and other biochemical parameters
The 50% depression of cholinesterase activity in serum,
erythrocyte and brain of male rats by isofenphos ("technically pure
grade") was determined in vitro. The I50 values were: serum:
8.2 × 10-4 mole, erythrocyte: 3.1 × 10-4 mole, and brain:
2.87 × 10-4 mole (Solmecke and Kimmerle 1972b).
Groups of 5 male rats were given acute oral doses of isofenphos
at levels ranging from 0.5 to 35 mg/kg bw. Activity of plasma and
erythrocyte cholinesterase in the males was depressed (20 to 81%), in
a dose-dependent pattern, above 0.5 mg/kg bw at both 2 and 24 h post-
treatment, with maximum inhibition being noted at 2 h. Plasma
cholinesterase was no longer affected at any dosage level 3 days after
dosing, while erythrocyte cholinesterase was still inhibited by 26% at
35 mg/kg bw 7 days after treatment. In female rats, a single oral dose
of isofenphos at 2.5 mg/kg bw but not at 0.5 mg/kg bw caused a
 reduction in erythrocyte cholinesterase level by 20% at 2 h post
dosing but not thereafter. Plasma cholinesterase was not affected.
Male rats sacrificed 24h after acute oral doses of isofenphos above
1 mg/kg bw displayed a dose-related decrease (23-74%) in activity of
brain cholinesterase (Solmecke and Kimmerle 1972b).
Groups of 5 male and 5 female rats (Wistar II strain of
unspecified age) were intubated acutely with the oxygen analogue of
isofenphos at 0.1, 0.25, 0.5, 1.0, 2.5 or 5 mg/kg bw (males) or at
0.25, 0.5 or 1 mg/kg bw (females). Cholinesterase in plasma and
erythrocytes was assayed at 2, 5, 24, 48h and 7 days (in males only)
post treatment. No control groups were included in the experiment and
the pre-treatment cholinesterase activities of individual groups were
used as the basis of comparison. In males, there was a depression
(>20%), generally dose-dependent, of plasma cholinesterase at
0.5 mg/kg bw and above and of erythrocyte cholinesterase at 2.5 mg/kg
bw and above after 2, 5 and 24 h. (Males at 1 mg/kg bw showed a
decrease (29%) of erythrocyte cholinesterase after a single interval
of 5 h.) Complete or almost complete recovery to pre-treatment values
was seen after 48 h for plasma cholinesterase and after 7 days for
erythrocyte cholinesterase. In females, both plasma and erythrocyte
cholinesterase was inhibited (20 to 43%) at 0.25 mg/kg bw and above
during the first 24 h. Activities of cholinesterase in plasma and
erythrocyte were close to the pre-treatment values 48 h after dosing.
The inhibitory effect of the oxygen analogue on plasma and erythrocyte
cholinesterase appeared to peak about 2 or 5 h after treatment in both
sexes (Thyssen 1974a).
Groups of 5 male and 5 female rats were orally treated with
oxygen analogue of isofenphos and then sacrificed 24 h later for
determination of brain cholinesterase activity. It was stated (no data
were provided) that brain cholinesterase activity in males was
moderately depressed at both 10 and 20 mg/kg bw but was unaffected at
5 mg/kg bw. In the case of females, brain cholinesterase activity was
stated to be within physiological ranges at dosages ranging from 1 to
5 mg/kg bw (Thyssen 1974a).
TOXICOLOGICAL STUDIES
Acute toxicity
In experiments with mice, rats, hamsters and rabbits exposed in
inhalation chambers to isofenphos (dissolved in a mixture of alcohol
and polyethylene glycol 400) as a spray at 30-minute intervals during
a 4-h period, rats and hamsters were found to be more sensitive than
mice and rabbits as shown in Table 1 (Solmecke and Kimmerle 1972c).
TABLE 1. Acute toxicity of isofenphos in animals
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Mouse M Oral PG 127 Solmecke and Kimmerle 1972c
F 91.3
M Inhalation Ethanol & > 0.272 mg/l Solmecke and Kimmerle 1972c
(4-hour PG (1:1)
exposure)
Rat M Oral PG 38.7-45 ) Solmecke and Kimmerle 1972c
F 28 - 32 ) Lamb et al 1977
M Oral Water &
Cremophor EL 33 - 48.2 Flucke 1980a;Flucke 1981
M Oral " 19.9 Flucke 1980b
M I.P. PG 35.8 Solmecke and Kimmerle 1972c
F 29.5
M Dermal None >1000 µl/kg bw) Solmecke and Kimmerle 1972c
(4-hour
exposure) None
M Dermal(24-h) None 705 µl/kg bw Kimmerle 1972a
Dermal None 188 µl/kg bw Solmecke and Kimmerle 1972a
(7-day
exposure) (undiluted)
M&F Inhalation Ethanol & >1300 mg/l air Kimmerle 1976
(1-hour PG (1:1)
exposure)
M Inhalation Ethanol & 0.21 mg/l air Solmecke and Kimmerle
F (4-hour PG (1.1) 0.144 mg/l air 1972c
exposure)
TABLE 1. (con't)
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Hamster M Inhalation Ethanol & 0.23 mg/l air Solmecke and Kimmerle
(4-hour PG (1:1) 1972c
exposure)
Rabbit F Oral PG approx. 150 Solmecke and Kimmerle 1972c
M Dermal Ethanol & 162 Nelson and Burke 1977a
F (24-hour PG (1:1) 315
exposure)
Dog F Oral PG > 25 Solmecke and Kimmerle 1972c
Bobwhite quail M&F Oral PG 8.7 Lamb and Burke 1979
Mallard duck M Oral PG 36.0 Nelson and Burke 1977b
F 32.0
Quail M Oral Water & 10 Thyssen 1978
Cremophor EL 10
Starling Oral Corn oil 972 Ross et al 1976
Hen F Oral Water & 16 Thyssen 1978
Cremophor EL
PG - polyethylene glycol 400.
Signs of oral poisoning in mammals
Cholinergic symptoms (e.g. muscle twitching, breathing disorders)
induced by toxic acute oral doses of isofenphos were typical of those
of cholinesterase inhibitors and were similar in mice, rats and
rabbits. Toxic signs usually developed within 2 h of dosing in mice
and rats and 4 h in rabbits and lasted for 2 to 10 days in survivors.
Mortalities from lethal doses usually occurred 1 to 4 days after
treatment. Cholinergic signs were not observed in dogs treated at
25 mg/kg bw, the top dosage level tested (Solmecke and Kimmerle
1972c).
Short-term studies
Rat
Groups of 15 male and 15 female rats (SPF Wistar II strain of
unspecified age) were orally treated by gavage with isofenphos ("pure
technical grade") in polyethylene glycol 400 at 0, 0.1, 0.25, 1.0 or
2.5 mg/kg bw/day for 30 days. Mortality, behaviour, growth, organ
weights and liver and kidney function were unaffected. Dose-related
inhibition (>20%) of cholinesterase in plasma and erythrocytes was
apparent at and above 1 mg/kg bw in females at 9, 16 and 30 days of
the study. In males, depression (dose related, >20%) of erythrocyte
cholinesterase was noted at both 1 and 2.5 mg/kg bw at 16 and 30 days
and of plasma cholinesterase at and above 1 mg/kg bw at 16 days and at
2.5 mg/kg bw also at 9 and 30 days (Solmecke and Kimmerle 1972a).
Groups of 10 male and 10 female rats (SPF Wistar W74 strain, 8
weeks old) were fed isofenphos (91.9% pure) in the diet at the dosage
levels of 0, 0.3, 1.0 or 3 ppm for 4 weeks. There was no mortality.
Behaviour, body weight and food consumption were not significantly
different between control and treated animals. Determinations of blood
cholinesterase activity after 3, 7, 10, 14, 17, 21, 24 and 28 days of
dietary feeding indicated dose-related inhibition (>20%) of plasma
cholinesterase in females at 1 ppm after 7, 17 and 28 days and at
3 ppm at all intervals. Only a slight depression (13-16%) of plasma
cholinesterase was noted in males even at 3 ppm. Activity of
erythrocyte cholinesterase determined over the course of the study and
of brain cholinesterase measured terminally was not affected in all
treated groups (both sexes). The no-effect level on plasma
cholinesterase was 0.3 ppm, which was equivalent to 0.024 mg/kg bw,
according to body weight and food consumption data (Löser 1978).
Groups of 15 male and 15 female rats (SPF Wistar strain, 28 to 32 days
old) were fed isofenphos (86.6% pure) in their diet at levels of 0.5,
1, 5, 25 or 125 ppm for 3 months. The control group consisted of 30
males and 30 females. One male at 125 ppm died after 9 days, due to
pneumonia. Toxic signs, such as salivation and muscle twitching, were
observed in animals receiving 125 ppm during the first 2 weeks of
feeding. Body weight was adversely affected in the highest dosage
group (both sexes). Food consumption was comparable to controls.
Haematological and biochemical studies and urinalysis conducted after
one and three months of the study indicated no significant dose-
related findings other than an increase in SGOT in males at 125 ppm at
the 1-month interval. Measurements of cholinesterase activity after 1,
4, 8 and 13 weeks of feeding revealed depression (dose-related >20%)
of the enzyme in both plasma and erythrocytes in females at 5 ppm and
above and in males at 25 ppm and above at all intervals. (Males at
5 ppm showed a 29% decrease of plasma cholinesterase level after one
week of feeding, but not thereafter.) The brain cholinesterase level
determined terminally was reduced at 25 ppm and above in both sexes.
At sacrifice upon termination of the study, an increase in absolute
weight and organ/body weight ratio of thymus was seen in females at
125 ppm. There were also variations in absolute weights of several
organs in males of the top dosage group. No compound-induced
histopathological alterations were observed in a variety of tissues
evaluated, including the thymus (Löser 1973; Urwin and Newman 1973).
Dog
Groups of dogs (4 males and 4 females per group, 5 months old)
were fed isofenphos (of unspecified purity) in the diet daily at 0,
0.3, 1.0, 10 or 30 ppm for 3 months. There were no treatment-related
effects on mortality, clinical symptoms, reflexes, body weight, food
consumption, ophthalmoscopic, haematological, clinical chemistry and
urinalysis parameters. Cholinesterase in plasma and erythrocytes was
depressed (by >20%), in a dose-dependent manner, at and above
10 ppm in both sexes after 3, 6 and 13 weeks of the study. (Plasma
cholinesterase in these dosage groups was inhibited also after 1 week
of the study.) At the conclusion of the study, absolute weight and
organ/body weight ratio of the liver was elevated at 10 ppm and
above in males (non-dose related) and females (dose related).
Histopathological examination of about 30 tissues, including the
liver, from each dog revealed no abnormalities or variations
associated with inclusion of isofenphos in the diet. The no-effect
level for the study was 1.0 ppm (Murmann 1973; Thomson and Newman
1973).
Groups of dogs (4 males and 4 females per group) were fed a daily
diet containing the following levels of isofenphos (89.3% pure) in a
2-year study: Control: 0 ppm; Group 1: males - 3 ppm from week 1
to week 83 and 2 ppm from week 84 to week 104, and females - 3 ppm
from week 1 to week 104; Group 2: 15 ppm throughout the 104 weeks,
and Group 3: 75 ppm from week 1 to week 53, 150 ppm from week 54 to
week 99 and 300 ppm from week 100 to week 104. No compound-related
findings were noted with respect to general condition (reflexes, body
temperature and pulse rate), water intake, ophthalmoscopic and
urinalysis parameters. Assay of cholinesterase 12 times over the
course of the study indicated dose-dependent inhibition (>20%) of
plasma cholinesterase in Groups 2 and 3 (both sexes) and of
erythrocyte cholinesterase in Group 3 at all intervals. Depression
(approx. or equal to 20%) of plasma cholinesterase was also found in
Group 1 males at weeks 7, 39, 66 and 79 but was not evident at weeks
92 and 103 after the dietary level was reduced from 3 ppm to 2 ppm at
week 84. Animals of Group 3 also showed depression (>60%) of brain
cholinesterase activity measured terminally.
Adverse effects on parameters other than cholinesterase activity
in plasma and erythrocytes were observed only in Group 3. In this
group, one male died at week 104 and another male was sacrificed
terminally in moribund condition. Toxic symptoms, such as salivation,
vomiting, diarrhoea, weakness in the hind extremities, unsteady gait,
etc., began to appear after 78 weeks and increased in severity with
time. The symptoms were more marked in males than in females.
Depression of body weight gain (both sexes) and of food consumption
(females) was obvious after 100 weeks. An increase in the SAP level at
several intervals during the study and a reduction of the terminal
serum A/G ratio were observed in males and females. The one male that
died at 104 weeks showed significant changes in several haematological
parameters. At terminal sacrifice, elevated organ/body weight ratios
of lung, liver, kidney, prostate, brain and pituitary and depressed
organ/body weight ratios of adrenal and testis were detected in the
males but, except in the testis, microscopic abnormalities of the
tissues in question were not noted. Histopathologically, hypoplasia of
the testis and erosion of esophageal mucosa, partially accompanied by
cellular reaction of propria mucosa, were observed in several males of
Group 3 but none in the other groups, including the control. Two
animals (Group 3) also exhibited foci of softening ("degeneration
processes") in the brain stem with a degree of severity greater than
the one case seen in the control group. The no-effect level in the
study was 2 ppm (Hoffman and Kaliner 1977).
Hen and quail
In adult hens and female quail intubated with isofenphos as an
emulsion in water and polyethylene glycol 400 daily for 5 consecutive
days, and then observed for 14 additional days, the 5 day LD50 was
found to be 6 mg/kg bw/day and 5 mg/kg bw/day respectively. Major
symptoms observed were inactivity in both species, ruffled feathers
and breathing disorders in the quail (Thyssen 1978).
Bobwhite quail and mallard duck
The dietary LC50 in bobwhite quail and mallard ducks fed
isofenphos in their diet for 5 days, followed by untreated diet for
3 days, was determined to be 145 ppm (Nelson and Burke 1977) and
>1000 ppm respectively (Nelson and Burke 1977c; Lamb and Burke 1977).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females per
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to isofenphos (as an emulsion in water and
Cremophor EL) at 0, 1 or 5 mg/kg bw/day 7 h per day, 5 times per week
for a total of 15 applications in a 21-day period. The treated sites,
not covered with bandages, were washed with soap and water after each
daily exposure period. One male at 5 mg/kg bw died of a cause
unrelated to treatment after 10 applications. There were no toxic
signs or adverse effects on body weight or on terminal haematological
and clinical chemistry parameters. Local irritation on treated sites,
generally transitory in nature and characterized by erythema on intact
skin and erythema, oedema and eschar formation on abraded skin, were
seen in some animals at 5 mg/kg bw. Changes in weights of several
organs were seen at 5 mg/kg bw without any accompanying
histopathological changes. Plasma and erythrocyte cholinesterase
activities measured after the 10th and 15th exposures and brain
cholinesterase level determined terminally in both sexes were
significantly depressed at 5 mg/kg bw (Thyssen and Kaliner 1977).
Rat - inhalation
In male and female rats exposed to isofenphos as an aerosol (of
unspecified particle sizes) 4h per day for 5 days, the LC50 was
>0.055 mg/l in males and 0.029-0.055 mg/l in females ((Solmecke and
Kimmerle 1972c).
Groups of 10 male and 10 female rats were exposed to an aerosol
of isofenphos at 0, 0.72 or 2.93 mg/m3 air 6h per day for 5
consecutive days per week over a 3-week period. (No information was
given on the particle size of the aerosol.) The treated animals were
not different from the controls as judged by general condition, body
weight gain, terminal haematological values, kidney and liver
function, gross pathological changes and organ weights. Cholinesterase
in plasma and erythrocytes was inhibited (>20%) at 2.93 mg/m3 air at
each weekly determination (Solmecke and Kimmerle 1972a).
Long-term studies
Mouse
Groups of 40 male and 40 female mice (6 to 7 weeks old, SPF NMRI
strain) were fed diets containing technical isofenphos (89.3% pure) at
0, 1, 10 or 100 ppm for 108 weeks. Additionally, each group included 3
sub-groups of 10 males and 10 females. One sub-group, fed the treated
diet for 108 weeks, was used for haematological tests and urinalysis
every 3 months over the course of the study. Another sub-group
maintained on dietary feedings for 9 months, was subjected to liver
function tests (plasma alkaline phosphatase and GPT) every 3 months.
The third sub-group was given isofenphos in the diet for 24 weeks and
activity of cholinesterase in plasma and erythrocyte was measured
after 2, 4, 8, 12 and 24 weeks and in brain after 24 weeks. All
animals that died during the study or were sacrificed terminally were
subjected to gross and histopathological examination. Mortality, which
was high in all groups, including the control, especially in the
females, was not attributed to treatment. At the end of the study,
only 42 to 57% of the males and 12 to 20% of the females per group
were still alive. Nevertheless, 53 to 73% of females per group
survived at least 80 weeks. There were no significant differences
between control and treated groups in clinical symptoms, body weight
and food consumption. Data on differential white blood cell counts
showed an increase at 100 ppm of eosinophil (males) and segmented cell
counts (females) after 21 months and of segmented cell counts in males
after 24 months. Liver function tests and urinalysis parameters were
normal. Dose-related inhibition (72 to 94%) of plasma cholinesterase
occurred in both sexes at and above 10 ppm at all intervals.
Erythrocyte cholinesterase was unaffected in the treated groups, with
depression consistently below 20%. Terminal brain cholinesterase
activity was reduced at 100 ppm by 46% in males and 31% in females. At
terminal sacrifice, absolute weight of lung in males was significantly
elevated at 100 ppm but no concomitant histological lesion of the
particular tissue was evident. An apparent dose-related increase, as
compared to concurrent controls, in frequency of mild gastritis (in
glandular mucosa of the stomach) was observed in males of all treated
groups which was, however, not statistically significant, especially
in the case of the 1 ppm and 10 ppm groups. No histopathological
changes related to treatment was observed in a variety of other
tissues evaluated.
Based on the tumour data, isofenphos was not carcinogenic in the
mouse, as indicated by the absence of a dose-related increase in
incidence of a) any particular type of tumours, b) animals with
tumours, c) animals with multiple tumours, and d) animals with
malignant tumours. The time of appearance of tumours was comparable
between control and treated groups.
The no-effect level for the study was 1 ppm (Brune et al.
1978).
Rat
Groups of 50 male and 50 female rats (SPF Wistar strain, 28 to 32
days old) were fed diets containing isofenphos (of unspecified purity)
at 1, 10 or 100 ppm for 2 years. The control group consisted of 100
males and 100 females. Mortality was unaffected by treatment, and
between 69 and 90% of animals per group were still alive at the end of
the study. At 100 ppm, cholinergic signs were seen during the first
week and depression of growth in both sexes and of food consumption in
males was apparent throughout the feeding period. Haematological and
biochemical studies and urinalysis conducted at five intervals over
the course of the study failed to reveal any consistent effect related
to presence of isofenphos in the diet. Assay of cholinesterase after
1, 2, 4, 13, 26, 52, 78 and 105 weeks of the study indicated dose-
related inhibition (>20%) of both plasm and erythrocyte
cholinesterase at 10 ppm and above in both sexes at all intervals and
of plasma cholinesterase in males at 1 ppm after 4 and 78 weeks. The
extent of depression of cholinesterase in plasma and erythrocytes
remained fairly constant over the course of the study. At the
conclusion of the experiment, brain cholinesterase activity was
reduced at 100 ppm in both sexes. Variations in absolute weights of
several organs were seen at 100 ppm but histological changes of the
particular organs were absent. Except for an apparent increase in
incidence of ulceration of the forestomach in females at 100 ppm,
as compared to the concurrent controls, no treatment-related
alterations were evident in a set of about 30 tissues examined
histopathologically. Analysis of tumour data revealed no indications
suggestive of carcinogenic activity of isofenphos under the conditions
of the experiment.
The study demonstrated 1 ppm as a marginal no-effect level with
respect to plasma cholinesterase. On other parameters monitored,
10 ppm was without any significant adverse effect (Bomhard and Löser
1977).
Special studies on reproduction
Groups of 20 male and 20 female weanling rats (SPF CFY strain)
were fed dietary levels of technical isofenphos (of unspecified
purity) at 0, 1, 10 or 100 ppm for 60 days prior to mating to initiate
a reproduction study originally designed to cover 3 generations with
one litter per generation. Three litters (F1a, F2a and F3a) were
obtained as expected for control, 1 ppm and 10 ppm groups. In the case
of the highest dosage group, owing to poor survival of pups, there
were insufficient F1a weanlings to form an adequate second parental
generation (F1). F0 animals at 100 ppm, as well as those of other
groups including the control, were therefore re-mated. This second
mating again failed to provide sufficient F1b offspring at 100 ppm as
potential parents. In an attempt to determine the causes of the
initial adverse effects at 100 ppm, F0 animals of control and top
dosage groups were allowed two additional matings, with the fourth
mating using treated males and untreated females and vice-versa. In
the third mating trial, the number of litters (F1c) at 100 ppm
obtained was too small to permit any meaningful interpretation of
results. Based on results of the fourth mating, fertility appeared to
be adversely affected, primarily in the females. This summary will
refer only to data on control, 1 ppm and 10 ppm groups.
In parental generations, there were no mortality and clinical
symptoms attributable to treatment. Body weight gain during pre-
mating, gestation and lactation periods, mating performance, duration
of gestation and terminal gross pathological findings were not
significantly different from controls. Pregnancy rate was 90%, 70% and
60% respectively in control, 1 ppm and 10 ppm groups in the F0
generation at the second mating suggesting a slight effect at 10 ppm
and a marginal effect at 1 ppm. (The pregnancy rate at 100 ppm was 40%
for the second mating trial.) Assay of plasma and erythrocyte
cholinesterase in F0 animals after 37 weeks of dietary feeding
revealed inhibition (>20%) at 10 ppm of erythrocyte cholinesterase in
both sexes and of plasma cholinesterase in females. Over the three
progeny generations (F1a, F2a and F3a litters), litter size, pup
mortality and mean pup weight from birth through lactation to weaning
were comparable to controls at both 1 and 10 ppm. In F1b litters, pup
weight at 10 ppm seemed to be slightly decreased at days 12 and 21.
Pups observed grossly at weaning displayed no apparent compound-
induced abnormalities. The available data tend to indicate 1 ppm as a
marginal no-effect level with respect to reproductive capability
(Palmer et at 1977).
Special studies on teratogenicity
Rat
Groups of 20 to 21 pregnant rats (Long Evans FB30 strain) were
intubated with isofenphos (86.6% pure) in polyethylene glycol 400 at
doses of 0, 0.3, 1 or 3 mg/kg bw/day from gestation day 6 to day 15.
The dams were sacrificed on gestation day 20 and foetuses were removed
by caesarean section for external, visceral and skeletal examination.
No treatment-related mortality or abnormal behavioural reactions
were noted, and maternal body weight was normal during the dosing
period. An increase in the average number of resorbed foetuses per
litter occurred at 3 mg/kg bw, but a dose-related elevation in the
percentage of litters with rasorptions was not apparent at this dosage
level. There were no significant differences between control and
treated groups with respect to fertility rate, mean number of
implantation sites, mean litter size, foetal weight, placental weight
and incidence of stunted foetuses. The frequency of foetuses showing
slight bone variations and/or malformation was very low and not
significantly different from that in the control group (Machemer
1972).
Groups of 21 to 23 pregnant rats (Long Evans FB30 strain) were
exposed dermally to isofenphos (dissolved in polyethylene glycol 400)
at 0, 0.3, 1.0, 3 or 10 mg/kg bw/day for 5 h per day from gestation
day 6 to day 15. The treated (clipped) sites were washed with soap
solution after each daily exposure period. Animals' necks were
immobilized. On day 20 of pregnancy, the dams were sacrificed and
foetuses were removed by caesarean section. A decrease in maternal
body weight gain during and after treatment period was observed at
10 mg/kg bw. Dams at this level also showed an increased incidence of
ruffled fur. There was a slight increase in the mean number of
resorptions per pregnant dam at 10 mg/kg bw, but the percentage of
pregnant dams with resorbed foetus(es) was not significantly elevated.
No treatment-related effects were observed on other tested parameters,
viz. fertility rate, mean number of implantations, mean litter size,
foetal weight, placental weight, frequency of stunted foetuses and
incidence of skeletal variations. A number of foetal malformations
were seen in all groups, including the control, but they were
generally non-dose-related. The only abnormalities observed at
10 mg/kg bw, but not in the lower dosage groups or controls, were
multiple defects manifested as hypoplasia of telencephalon and
unspecified eye malformation in 3 foetuses from a single litter. These
particular findings were not believed to be compound induced. Under
conditions of the experiment, isofenphos was not teratogenic, although
a dosage level of 10 mg/kg bw was maternally toxic (Schlüter 1981).
Groups of 22 to 25 pregnant rats (Long Evans FB30 strain) were
exposed in an inhalation chamber to an aerosol of isofenphos
(formulated with a 1:1 mixture of polyethylene glycol 400 and ethanol)
at actual chamber concentrations of 0, 0.25, 0.75 or 3.1 mg/m3 air,
for 6 h per day from day 6 through day 15 of gestation. More than 97%
of the particles counted were of sizes within respirable range in the
rat. On day 20 of gestation, the dams were sacrificed and the foetuses
were removed for gross, skeletal and visceral examination. No maternal
toxicity was observed. Pregnancy rate, mean number of implantations,
mean number of resorptions, mean litter size, foetal weight and
incidence of stunted foetuses were not adversely affected. An increase
in placental weight was noted at 3.1 mg/m3 air, which was of doubtful
biological significance and unlikely to be treatment-related in view
of the absence of any embryotoxic activity. There was no dose-related
effect on the incidence of skeletal and visceral malformations. Based
on the data, isofenphos was not teratogenic under the conditions of
the experiment (Schlüter and Thyssen 1981).
Rabbit
Groups of pregnant (Himalayan) rabbits (11 to 13 rabbits per
group) were orally treated with isofenphos at 0, 1, 2 or 5 mg/kg
bw/day from gestation day 6 through day 18. The females were
sacrificed on gestation day 29 and uterine contents were examined.
Foetuses were subjected to evaluation for external, skeletal and
internal malformations.
Compound-related mortalities and toxic symptoms (diarrhoea) were
seen in does at 5 mg/kg bw. A marked decrease (non-dose-related) in
maternal body weight gain during the treatment period occurred in all
treated groups. One of the 13 does aborted at 2 mg/kg bw. There were
no significant differences between control and treated groups in
pregnancy rate, litter size, mean number of implantations, mean number
of resorptions, sex ratio, foetal weight, placental weight and
incidence of stunted foetuses. "Slight bone variations" were not
indicated to occur in any foetuses from control or treated groups. The
only malformation observed in the study was indicated to be
arthrogryphosis of both front extremities in a foetus at the dose
level of 5 mg/kg bw (Machemer 1975).
Special studies on mutagenicity
In two similar but separate studies, isofenphos was evaluated for
its mutagenic activity using two microbial assay systems (rec-assay
and reversion test). Indicator micro-organisms used in the former were
Bacillus subtilis strains N1G17, N1G45, H 17 and M 45. For the
latter, Salmonella typhimurium strains TA 1535, TA 1537, TA 1538, TA
98 and TA 100 and Escherichia coli strain WP2 hcr were employed.
The reversion test was conducted in the presence or absence of a
mammalian metabolic activation system (S-9 homogenates of liver from
rats or mice induced by phenobarbital or Aroclor 1254) while the
rec-assay was carried out without the activation preparation. Under
the conditions of the experiments, isofenphos was demonstrated not to
be mutagenic at concentrations up to 0.02 ml/disc of undilated
isofenphos and 5000 µg/plate (Shirasu et al 1980) or 300 µg/disc
and 1000 µg/plate (Inukai and Iyatomi 1977), respectively, in the
rec-assay and the reversion test. Mutagenic responses were obtained
with several positive control compounds, such as mitomycin C,
9-amino-acridine HCl, furylfuramide, acetylaminofluorene and
beta-propiolactone (Inukai and Iyatomi 1977; Shirasu et al 1980).
Another Ames test conducted with S. typhimurium strains
TA 100, TA 1537, and TA 98, with or without the S-9 mix and using
concentrations as high as 3150 µg/plate, also confirmed the non-
mutagenicity of isofenphos (Oesch 1977).
In a dominant lethal assay, groups of 20 male NMRI mice (10 weeks
old) were given by gavage a single dose of 0 or 15 mg/kg bw of
isofenphos. Each male was then mated with three untreated female mice,
which were replaced weekly for 8 consecutive weeks. The females were
sacrificed around the 14th day of gestation or 14 days after
separation from the males and the uteri were removed for examination.
The treated males exhibited no signs of toxicity. There were no
significant differences between the control and the treated groups
with respect to fertility rate, living implants and dead implants.
Pre-implantation loss (no. of corpora lutea and no. of implantations)
was significantly increased at 15 mg/kg bw during the first week after
dosing, but not thereafter. It was indicated in the report that the
values for pre-implantation loss observed in the treated group were
"completely within the norm of the strain" (Machemer 1973).
Special studies on carcinogenicity
See under "Long-term studies".
Special studies on neurotoxicity
Hen
Groups generally comprising 10 adult hens (15 to 18 months old)
were given acute oral or intraperitoneal doses of technical
isofenphos. The oral and intraperitoneal LD50 was found to be
21 mg/kg bw and 11.4 mg/kg bw respectively. No delayed neurotoxic
signs were observed in any of the treated animals during a 6-week
post-exposure observation period. Pre-treatment of hens with atropine
(50 mg/kg bw) intraperitoneally elevated oral LD50 of isofenphos to
74 mg/kg bw. Five hens surviving an oral dose of 20 mg/kg bw of
isofenphos and 6 atropinized hens that were still alive after
receiving 74 mg/kg bw of the insecticide were sacrificed 21 days after
dosing for histopathological examination of the brain, spinal cord and
peripheral nerve. These hens showed no compound-related morphological
change or variation from normal in any of the nervous tissues
examined. Hens treated with an oral dose of TOCP at 350 mg/kg bw
displayed typical neurotoxic signs and showed degenerative nerve
fibres in the spinal cord and the sciatic nerve (Kimmerle 1972; Cherry
et al 1972).
Special studies on antidotes
In 2 separate experiments, atropine sulphate (50 mg/kg bw), 2-PAM
(50 mg/kg bw) or Toxogonin (20 mg/kg bw) was given singly or in
combination (atropine plus PAM or Toxogonin) i.p. to male rats: a) 45
to 120 minutes after single oral toxic doses of isofenphos (and before
onset of severe toxic symptoms), and b) 5 minutes after acute oral
toxic doses of the oxygen analogue of isofenphos and before onset of
toxic symptoms. Results indicated that, under the conditions of the
experiments, none of the three compounds tested was effective as an
antidote for isofenphos or its oxygen analogue (Solmecke and Kimmerle
1972b, Thyssen 1974a).
Simultaneous administration of atropine sulphate at 100 mg/kg bw
and obidoxime at 20 mg/kg bw to male rats i.p. after onset of toxic
symptoms (at 25 minutes) resulting from acute oral toxic doses of
isofenphos elevated LD50 of the insecticide from 43.1 mg/kg bw to
84.2 mg/kg bw. No data were provided on the effect of the antidotes on
the toxic signs (Kimmerle and Gröning 1975).
Special studies on potentiation
When the acute oral LD50 values in male rats, determined
experimentally for equitoxic mixtures of isofenphos and malathion or
of isofenphos and EPN, were compared with the expected LD50 values
for the mixtures, the acute toxicity of isofenphos was found to be
potentiated by malathion. An additive effect resulted when isofenphos
was combined with EPN (Solmecke and Kimmerle 1972b).
In further studies with male rats, it was demonstrated with
isofenphos did not potentiate the acute oral toxicity of phenamiphos
or phoxim (both organophorous insecticides) (Thyssen 1976; Thyssen
1977).
Special studies on the acute toxicity of the oxygen analogue
Rat
The LD50 of the oxygen analogue of isofenphos was determined in
6 female and 6 male rats and is reported in Table 2. Thyssen and
Kimmerle 1974).
TABLE 2. Acute toxicity of the oxygen analogue of isofenphos in rats
Sex Route Vehicle LD50
(mg/kg bw)
M Oral Water & Cremophor EL 30.8
F 16.1
M i.p. Water & Cremophor EL 15.9
F 7.8
M Dermal None 117.6 µl/kg bw
F (24 h) (undiluted) 25 µl/kg bw
M Dermal Water & Cremophor EL 97.9
F (24 h) 29.5
M Inhalation Ethanol & polyethylene >353 mg/m3 air
(1 h) glycol 400 (1:1)
F approx. 353 mg/m3 air
M Inhalation Ethanol & polyethylene >195 mg/m3 air
(4 h) glycol 400 (1:1)
F 63.8 - 131 mg/m3 air
Signs of oral poisoning
Acutely toxic doses of the oxygen analogue of isofenphos to
rats produced cholinergic symptoms typical of those seen with
cholinesterase inhibitors. These toxic signs occurred within 1 h of
dosing and persisted for a maximum of 2 days in survivors. Deaths from
lethal doses were observed from about 30 minutes to 3 days after
treatment (Thyssen and Kimmerle 1974).
The acute LD50 of the oxygen analogue of isofenphos in hens was
>10 <25 mg/kg bw by the oral route and about 10 mg/kg bw i.p. No
neurotoxic signs were observed in any of the treated hens over a
28-day post exposure observation period (Thyssen 1974c).
Special studies on the subacute oral toxicity of the oxygen analogue
and of a mixture of isofenphos and its metabolites
Groups of 15 male and 15 female rats (Wistar II strain) were
treated daily by gavage with the oxygen analogue of isofenphos (HOL
0654) (as an emulsion in water and Cremophor EL) at 0, 0.2, 0.6 or
1.8 mg/kg bw for 30 consecutive days. Activity of cholinesterase in
plasma and erythrocytes was determined five times over the course of
the study and brain cholinesterase was assayed at terminal sacrifice.
Physical appearance, behaviour and body weight were comparable to
controls. Dose-dependent inhibition (>20%) of plasma cholinesterase
was observed in males and females at 1.8 mg/kg bw throughout the
feeding period, at 0.6 mg/kg bw in males after 3 weeks only and
in females at 3, 10, 17 and 23 days of the study. The level of
erythrocyte cholinesterase was reduced by >20% in both sexes at
1.8 mg/kg bw. In general, the degree of depression of both plasma and
erythrocyte cholinesterase was greater in the females than in the
males. Terminal brain cholinesterase was unaffected in both sexes.
There were no significant gross pathological alterations in treated
animals. The no-effect level on plasma cholinesterase was 0.2 mg/kg bw
(Thyssen 1974b).
Cow
Groups of 3 dairy cows were fed alfalfa pellets treated with a
mixture of isofenphos, isofenphos oxygen analogue and des-N-isopropyl
isofenphos oxygen analogue (1:8:1) at 2, 6 or 20 ppm for 28 days and
then sacrificed for analyses of tissue residues. One cow was used as a
control. Whole blood cholinesterase was assayed at weekly intervals.
Other than the observation of a more sedated or docile appearance in
cows at 6 ppm and above, as compared to the control animal or animals
at 2 ppm, there were no adverse effects noted with respect to body
weight, feed consumption and milk production. A reduction (>20%) of
whole blood cholinesterase activity occurred at 20 ppm at days 14, 21
and 28 of the study and at 6 ppm at days 21 and 28. The inhibition of
whole blood cholinesterase was dose-related and increased with time
over the course of the study (Strankowski et al 1977c).
Hen
Groups of 4 hens were fed diets containing a mixture of
isofenphos, isofenphos oxygen analogue and des N-isopropyl isofenphos
oxygen analogue (1:8:1) at the levels of 0, 5, 15, 50 or 150 ppm for
28 days and then sacrificed for analyses of tissue residues. Body
weight and food consumption at both 50 and 150 ppm and egg production
at 150 ppm were adversely affected. Dose-related depression (22 to
98%) of plasma cholinesterase determined at terminal sacrifice was
observed in all treated groups (Strankowski 1977).
OBSERVATIONS IN HUMANS
In 3 male workers (wearing standard protective clothing) involved
for 5 to 11 working days (generally 7.5 to 8 h per day and not more
than 3 consecutive days at one time) in the application of a 7.5%
granulated formulation of isofenphos through a standard applicator
fixed to carrot seed drilling equipment, the whole blood
cholinesterase activity was not significantly depressed, as compared
to 2 male and 1 female control subjects. There was no mention in the
report with respect to adverse effects or symptoms of exposure. No
other clinical measurements were made (Bagnall 1976).
RESIDUES IN FOOD
USE PATTERN
Isofenphos is an insecticide possessing good activity against
soil insects, such as Diabrotica spp., wireworms, chafer grubs,
vegetable flies and flea beetles. It has proved to be highly effective
against leaf-eating pests, such as pear sucker and Colorado potato
beetle, and also against rice stem borers.
The compound acts as a contact and stomach poison. It has a root-
systemic action, i.e. the active ingredient is absorbed by the roots
of the plants and translocated to a limited degree within the plant.
The active ingredient is notable for its long residual activity
and its good dispersibility in soil. In numerous trials, isofenphos
has displayed generally good crop plant tolerance, whether used as a
seed treatment or applied as granular and emulsifiable concentrate
formulations. Isofenphos is formulated as an emulsifiable concentrate,
granular formulation and seed dressing powder.
It is mainly applied in row at or immediately after seeding. Two
applications are registered only for corn in the United States. The
crops, pests and recommended or registered dose rates are listed in
Table 3.
TABLE 3. Recommended application rates for isofenphos
Crop Pests Formulation Dose (a.i.)
Onion, carrot, brassica
leafy vegetables vegetable flies GR 1.5-2.5 kg/ha in row
Onion onion fly GR 5 kg/ha broadcast or spray
and overall
EC 0.076 g/m in row
Brassica leafy vegetables cabbage root fly GR 0.05 % a.i. (at 100 cm3/plant
and as soil drench after
EC transplanting)
0.0375-0.075 % a.i. at a rate
of 0.0375-0.15g/m row
Potato wireworm GR 5 kg/ha broadcast overall
before planting
Maize Diabrotica sp. GR 0.11 g/m in row at seeding +
and lay by (50 cm row spacing)
EC
Oilseeds, rape, flea beetle Seed 12-16 g/kg seed
swedes, marrowstem dressing seed treatment
kale, turnips powder
RESIDUES RESULTING FROM SUPERVISED TRIALS
Experiments were run on various plants at different locations in
Canada, Europe and USA. Isofenphos was applied at about the
recommended, and in certain cases somewhat higher, dosage rates.
The residues looked for included isofenphos, isofenphos oxygen
analogue (IOA) in European experiments and des-N-isopropyl isofenphos
(DNI) and des N-isopropyl isofenphos oxygen analogue (DNIOA) were
additionally included in the analyses carried out in USA. The residues
measured in brassicas, oilseed, rape, root and tuber vegetables were
low, generally at or about the limit of determination for the
recommended pre-harvest interval, as well as those found in kernel of
corn at the milk and dry stages. In green forage, however, residues
were as much as 2 mg/kg. Onions and potatoes also contained detectable
residues. The major part of the residue consisted of isofenphos and
IOA in all crops.
Brassica leafy vegetables
Brussels sprouts were treated by applying a soil drench at a rate
of 0.05 g a.i./plant shortly or 1 day after planting. No residue was
measurable 99-119 days after treatment (Bayer 1978). isofenphos was
applied on cabbages, savoy and white, and cauliflower either in row or
with overall treatment at dose rates of 0.075 g a.i./m in a 10 cm band
or 1.5 to 3 kg/a.i./ha, respectively. The experiments were carried out
in Federal Republic of Germany (FRG) and UK and results are summarized
in Table 4 (Bayer 1973-74, 1977).
Celery
Oftanol 200 EC was worked in the soil at rate of 4 kg a.i./ha one
day before planting. Samples were taken 84 and 90 days after treatment
and analysed for isofenphos and IOA. The residues were below the limit
of determination (0.01 mg/kg) (Netherlands 1981).
Maize
Supervised trials were carried out in various states of the USA
in 1979 and 1980.
AMAZE 15 G or 20 G were used for the treatment of the plots in a
15 cm band at planting plus either a double side dress at the 4-leaf
growth stage or band treatment at the base of the plant at growth
stage of 45 cm. A dosage rate of 0.19 g a.i./m was applied in all
treatments (equal to 2.5 kg a.i./ha). Samples were taken from green
forage, kernel, cob and husk in the milk stage and from the dried
parts of plant. Tests were made for residues of isofenphos and its
three metabolites. The limit of determination was 0.01 mg/kg for all
compounds individually.
TABLE 4. Residues of isofenphos in brassica leafy vegetables
Application Residues 1 (mg/kg) at intervals (days) after application
Crop Country Year No. Rate
(kg a.i./ha) Formulation 0/42 49 55-56 60 64-70 80-87
Cabbage FRG 1974 1 3 kg/ha 5 G 0.01 0.01 <0.01
FRG 1974 1 " 5 G 0.08 0.02 0.01
savoy FRG 1974 1 " 5 G <0.01 <0.01 <0.01
FRG 1977 1 )0.075 g/m 500 EC 0.02 0.01 0.01 <0.01
FRG 1977 1 ) in row 500 EC 0.12 0.03 0.02 <0.01
FRG 1977 1 ) 500 EC 0.09 0.03 0.02 0.01
Cabbage UK 1974 1 1.5 kg/ha 5 G 0.007
FRG 1973 1 2.5 kg/ha 5 G 0.1 0.01
white FRG 1973 1 2.5 kg/ha 5 G 0.06 0.03
FRG 1973 1 2.5 kg/ha 5 G 0.08 0.02
Cauliflower FRG 1977 1 0.075 g/m 500 EC 0.11 0.04 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.01 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.02 <0.01 <0.01 <0.01
in row
FRG 1973 1 2-2.5 kg/ha 5 G <0.012 <0.011 <0.013
1 Sum of isofenphos and its oxygen analogue;
2 Results were obtained by analysing 3 samples taken from different plots;
3 Results of analyses of 6 samples.
Isofenphos, IOA and DNIOA were detected in forage. The results
are summarized in Table 5. Levels of DNIOA ranged from 0.01 to
0.08 mg/kg, with an average of 0.04 mg/kg. No measurable residues were
found in kernels either in tile milk or dry stage. Residues in the
cob were at or below the limit of determination (total residue
< 0.02 mg/kg). The husk contained residues of less than 0.2 mg/kg
at all times and stages (Mobay 1979-80).
Sweet corn
Experimental plots were treated in a 15 cm band at planting and
with either a 15 cm band at the base of the plant or a basal spray at
lay by 4 to 8 weeks later. OFTANOL 6 EC and 15 G formulations were
used in the trials (Mobay 1978a).
The active ingredient was applied at a dose rate of 0.19 g/m,
equal to 3.72 kg a.i/ha. The residues (parent compound and
3 metabolites) were analysed separately in 10 milk stage kernel
samples. The oxygen analogue was detected twice at levels of
0.02 mg/kg and 0.05 mg/kg; the other residues were below the limit of
determination (0.01 mg/kg for all compounds). No residues were
detected in dry kernels (3 samples).
The green and dry forages were also analysed, and levels of
isofenphos and IOA are listed in Table 5. The cob in the milk stage
did not contain detectable residues, with the exception of 0.02 mg/kg
of oxygen analogue. The oxygen analogue was also the only detectable
residue in husk at the milk stage, with a maximum level of 0.16 mg/kg.
The dry husk contained both IOA and DNIOA. Maximum levels were
0.27 mg/kg and 0.02 mg/kg respectively.
Onion
At various locations in the USA in 1978, 17 experimental plots
were treated in furrow at planting with either 15 G, 10 G, 5 G or 4 EC
formulations at a dose rate of 0.1 g isofenphos/m. The dose rate
expressed in kg/na varied depending on the row spacing, which was
between 30 to 100 cm (Mobay 1978b). Both mature bulbs and immature
bulbs were sampled and analysed for isofenphos, IOA, DNI and DNIOA.
The residues were generally low in the bulb.
DNI and DNIOA were only detected in one sample at levels of
0.01 mg/kg and 0.08 mg/kg respectively. Supervised trials were also
carried out in Federal Republic of Germany, France and in The
Netherlands. Five kg a.i./ha was applied from both 5 G and 500 EC
formulations one day before sowing or at the 4-leaf stage. Samples
were taken 92 to 158 days after application and the levels of
isofenphos and IOA were measured (Bayer 1972-73, 1975, 1978). The
number of samples taken from experimental plots both in USA and in
Europe are listed in Table 6.
TABLE 5. Residues of isofenphos in forage of maize and sweet corn
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Maize USA 0.03 0.02 0.02
1980 2 2.5 15 G
Indiana 0.141 0.071 0.111
" 1980 2 2.5 15 G 0.05 0.04 0.04
0.141 0.191 0.111
" 1980 2 2.5 20 G 0.03 0.07 <0.01
0.111 0.231 <0.011
Nebraska 1979 2 2.5 20 G <0.01 <0.01 <0.01 <0.01 <0.01
<0.011 <0.011 <0.011 <0.011 <0.011
" 1979 2 2.5 15 G <0.01 0.02 <0.01 <0.01 <0.01
<0.011 0.01 <0.01 <0.01 <0.01
Sweet corn USA 1978 2 3.72 6 EC 0.22
Missouri 0.741
Oregon 1978 2 3.72 6 EC <0.01 <0.01 <0.01 <0.01 <0.01
<0.031 0.031 0.041 0.031 <0.011
Nebraska 78 2 3.72 6 EC 7.86 0.01 <0.01 0.04
9.261 0.251 0.061 0.051
Texas 1978 2 3.72 6 EC 0.2 0.05 0.08 0.04
2.091 1.611 1.991 0.071
Florida 1978 2 3.72 6 EC 0.03 0.02 0.04 <0.01
0.751 1.371 0.671 0.071
Missouri 78 2 3.72 15 G 0.94 0.07 0.1
0.741 0.231 0.191
TABLE 5. (con't)
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Oregon 1978 2 3.72 15 G 0.02 0.02 0.02 <0.01 <0.01
0.081 0.081 0.061 0.06 0.011
Texas 1978 2 3.72 15 G 0.01 0.03 0.05
0.721 1.041 0.151
Florida 1978 2 3.72 15 G <0.01
0.041
Missouri 78 2 3.72 15 G 0.39 0.03 <0.01
0.871 0.14 0.11
1 Residues of oxygen analogue.
TABLE 6. Supervised trials measuring isofenphos and its oxygen
analogue in onions
Compound Residue (mg/kg) No. of samples
Isofenphos < 0.01 12
< 0.05 0
< 0.1 1
< 0.2 1
< 0.6 3
IOA < 0.01 4
< 0.05 8
< 0.1 2
< 0.2 2
< 0.6 1
Isofenphos + < 0.01 2
IOA 1 < 0.05 4
< 0.1 3
1 Result of experiments in Europe.
The sum of the residue detected was always below 1 mg/kg in
mature bulb samples. Immature bulbs were analysed in two occasions and
the residues found were: isofenphos-0.32 and 0.95 mg/kg; isofenphos
oxygen analogue - 0.18 and 0.26 mg/kg.
Rapeseed
Seeds of spring and autumn varieties of rape were seed dressed
with BAY 6643 B at a rate of 15 g isofenphos/kg seed in 1973-74. The
crops were sampled at various locations in the FRG. Residues were
below the limit of determination (0.01 mg/kg) in all of the 13 trials
(Bayer 1974a).
Supervised trials with three varieties were carried out at three
locations in Canada in 1989 (Mobay 1980a). AMAZE 40% was used for seed
dressing prior to seeding at a rate of 25 g a.i./kg seed. The seeds
were treated either with formulation or the powder was applied to seed
after coating with oil. The harvested seed was analysed for
isofenphos, IOA, DNI and DNIOA 94-113 days after treatment. Residues
of active ingredient and its metabolites were below the limit of
determination (0.01-0.02 mg/kg).
Root and tuber vegetables
Celeriac
Supervised trials, in which 4 kg a.i./ha of Oftanol 200 EC was
applied before planting, were carried out on celeriac fields at two
locations of The Netherlands in 1978. The mature crops were samples
158 days after application. Residues of isofenphos were not detected
(Netherlands 1981).
Potato
In an experiment in FRG, a 5 G formulation of isofenphos was
worked into the soil at a rate of 5 kg a.i./ha before planting. At 110
days after application, a residue of 0.11 mg/kg was detected in the
tubers that had been washed before analysis (Bayer 1973).
Potato fields were treated with isofenphos at three different
locations in Spain in 1980. The broadcast applications were made at a
rate of 2.5 kg a.i./ha at the time of planting. Samples were taken
111, 112 and 159 days later at the time of harvest and their residue
contents were found to be 0.45, 0.04 and 0.05 mg/kg respectively
(Bayer 1980).
Swedes and turnips
Oftanol T was used for seed dressing at a rate of 16 to
20 g a.i./kg seed. Experimental plots from 8 locations in FRG were
sampled 109 to 179 days after sowing in case of swedes, while three
plots of turnips were sampled 89 to 103 days after sowing. Leaves and
roots were analysed separately. No residue was detected (<0.01 mg/kg)
(Bayer 1974-76).
Availability of soil residues to rotational crops
Wheat, green beans and sugarbeets were planted as rotational
crops 3.5 months after a sandy soil had been treated with ring-µL
(14C)-isofenphos at a field application rate of 5.6 kg a.i./ha (Kurtz
1977). Corn had been used as the original crop in this isofenphos-
treated soil. When the rotational crops were planted, the soil
contained approximately 4 mg/kg isofenphos. The rotational crops were
sampled at 2, 8 and 32 weeks after planting. At the time of harvest,
8 weeks for green beans and 32 weeks for sugarbeet and wheat, the
residue level was measured in different parts of the crops (Table 7).
No des-N-isopropyl isofenphos was detected in any of the crops.
TABLE 7. Residues of isofenphos detected in rotational
crops grown in soil treated with isofenphos 1
Crop Sample Residue (mg/kg)
I IOA DNIOA
Green bean Forage < 0.01 0.05 < 0.01
Sugarbeets Roots 0.04 0.02 < 0.01
Tops < 0.01 0.02 0.06
Wheat Heads < 0.01 0.24 0.03
Straw 0.14 0.79 0.2
Forage 0.05 1.08 0.23
1 Application rate - 5.6 kg a.i./ha.
In the investigation of field rotational crops, wheat, oats or
sorghum, spinach turnips and soybean were planted in plots treated the
previous year with isofenphos (Mobay 1980b). The plots had been used
previously for performance tests and hence were cropped using normal
agricultural practices. Rotational crop planting dates ranged from 214
to 390 days (7 to 13 months) post-treatment. Treatment was with either
AMAZE 20 G in an 18cm band over the row at 1.2 kg a.i./ha to 1.5 kg
a.i./ha broadcast equivalent-with presswheel incorporation or with
AMAZE 6 E or 20 G applied in furrow at 1.1 to 2 kg a.i./ha broadcast
equivalent. Prior to planting the rotational crops, the plots were
rototilled or disced. The crops were then planted across and
perpendicular to the direction of the originally treated rows. To
insure homogeneity of sampling, the entire lengths of the crop rows
were harvested. Each of the harvested crops were analysed for residues
of isofenphos, IOA, DNI and DNIOA. The results of the study are given
in Tables 8 and 9.
TABLE 8. Residues of isofenphos in grain (wheat, oats or sorghum) rotational crops
Treatment Plant Back Residue (mg/kg 1
Crop Location Rate Interval
(USA) (kg a.i./ha) (days) Forage Grain Straw
Winter
Wheat Kansas 1.5 77 <0.01 <0.01 <0.01
Sorghum Texas 1.2 214 <0.012 <0.01 0.02
<0.033
Spring
wheat Kansas 1.5 295 0.064 NA5 NA
0.026
Spring
Wheat Nebraska 1.5 350 <0.014 <0.01 <0.01
<0.016
Oats Illinois 1.2 362 <0.014 <0.01 <0.01
<0.016
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos equivalents;
2 Immature crop, 42 days post-planting;
3 Immature crop, 57 days post-planting;
4 Immature crop, 45 days post-planting;
5 NA - no mature crop available for analyses;
6 Immature crop, 60 days post-planting.
TABLE 9. Residues of isofenphos in rotational crops
Treatment Plant back Residue
Crop Location rate Interval (mg/kg) 1
(kg a.i./ha) (days)
Spinach Arizona 2 279 <0.01
Spinach Arizona 2 279 <0.01
Turnip tops Kansas 1.5 316 <0.01
Spinach Nebraska 1.5 363 <0.01
Turnip tops Nebraska 1.5 363 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Kansas 1.5 316 <0.01
Turnip root Nebraska 1.5 363 <0.01
Soybean Kansas 1.5 295 0.03
Soybean Nebraska 1.5 363 <0.01
Soybean Illinois 1.14 390 <0.01
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos
equivalents.
Residues in all rotational crops studied consisted mainly of
isofenphos and IOA and were <0.05 mg/kg; most were <0.01 mg/kg. In
the four mature grain crops, the grain had no detectable residue and
the straw showed a detectable residue of 0.02 mg/kg in only one
sample. In the five vegetable crops, only one had a detectable residue
(0.01 mg/kg) was seed in only one of the bean samples and in two of
the dry vine samples. Based on these results, little or no isofenphos
residue would be expected in grain, vegetable, root or oil seed crops
grown in fields treated 9 or more months earlier with isofenphos at
rates up to 2 kg a.i./ha.
FATE OF RESIDUES
General comments
The degradation and metabolism of isofenphos was studied in
animals, plants, soils, water and during frozen storage. The
structural formula, chemical name, identifying symbol and occurrence
of its degradation products are shown in Figure 2. Metabolic pathways
for isofenphos in animals (A) plants (P) and Soil (S) are shown in
Figure 3.
In plants
The metabolism of isofenphos was studied in maize grown in ring -
µL (14C)-isofenphos treated soil both outdoors and in a greenhouse
(Stanley et al 1977).
For the outdoor experiment the labelled isofenphos was prepared
as a 6 EC formulation and incorporated at the rate of 5.6 kg a.i./ha
(ca 4 mg/kg soil) into soil contained in a tub. Sweet corn was planted
in the soil. Plant samples above the soil line were harvested at 28,
56 and 94 days after planting. The 94-day crop was divided into
kernels, cobs, husks and stalks in the laboratory.
In the greenhouse experiment the labelled isofenphos was
incorporated into soil at a rate of 4 mg/kg. The soil was placed in
pots and field corn was planted in it. The crop was harvested at
maturity, 141 days after treatment, and the plants were divided as in
the other experiment. The root samples were washed with water to
remove soil particles.
The compounds identified in the corn stalks were qualitatively
the same throughout the study, although their relative quantities
changed as the plant matured.
reduction in erythrocyte cholinesterase level by 20% at 2 h post
dosing but not thereafter. Plasma cholinesterase was not affected.
Male rats sacrificed 24h after acute oral doses of isofenphos above
1 mg/kg bw displayed a dose-related decrease (23-74%) in activity of
brain cholinesterase (Solmecke and Kimmerle 1972b).
Groups of 5 male and 5 female rats (Wistar II strain of
unspecified age) were intubated acutely with the oxygen analogue of
isofenphos at 0.1, 0.25, 0.5, 1.0, 2.5 or 5 mg/kg bw (males) or at
0.25, 0.5 or 1 mg/kg bw (females). Cholinesterase in plasma and
erythrocytes was assayed at 2, 5, 24, 48h and 7 days (in males only)
post treatment. No control groups were included in the experiment and
the pre-treatment cholinesterase activities of individual groups were
used as the basis of comparison. In males, there was a depression
(>20%), generally dose-dependent, of plasma cholinesterase at
0.5 mg/kg bw and above and of erythrocyte cholinesterase at 2.5 mg/kg
bw and above after 2, 5 and 24 h. (Males at 1 mg/kg bw showed a
decrease (29%) of erythrocyte cholinesterase after a single interval
of 5 h.) Complete or almost complete recovery to pre-treatment values
was seen after 48 h for plasma cholinesterase and after 7 days for
erythrocyte cholinesterase. In females, both plasma and erythrocyte
cholinesterase was inhibited (20 to 43%) at 0.25 mg/kg bw and above
during the first 24 h. Activities of cholinesterase in plasma and
erythrocyte were close to the pre-treatment values 48 h after dosing.
The inhibitory effect of the oxygen analogue on plasma and erythrocyte
cholinesterase appeared to peak about 2 or 5 h after treatment in both
sexes (Thyssen 1974a).
Groups of 5 male and 5 female rats were orally treated with
oxygen analogue of isofenphos and then sacrificed 24 h later for
determination of brain cholinesterase activity. It was stated (no data
were provided) that brain cholinesterase activity in males was
moderately depressed at both 10 and 20 mg/kg bw but was unaffected at
5 mg/kg bw. In the case of females, brain cholinesterase activity was
stated to be within physiological ranges at dosages ranging from 1 to
5 mg/kg bw (Thyssen 1974a).
TOXICOLOGICAL STUDIES
Acute toxicity
In experiments with mice, rats, hamsters and rabbits exposed in
inhalation chambers to isofenphos (dissolved in a mixture of alcohol
and polyethylene glycol 400) as a spray at 30-minute intervals during
a 4-h period, rats and hamsters were found to be more sensitive than
mice and rabbits as shown in Table 1 (Solmecke and Kimmerle 1972c).
TABLE 1. Acute toxicity of isofenphos in animals
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Mouse M Oral PG 127 Solmecke and Kimmerle 1972c
F 91.3
M Inhalation Ethanol & > 0.272 mg/l Solmecke and Kimmerle 1972c
(4-hour PG (1:1)
exposure)
Rat M Oral PG 38.7-45 ) Solmecke and Kimmerle 1972c
F 28 - 32 ) Lamb et al 1977
M Oral Water &
Cremophor EL 33 - 48.2 Flucke 1980a;Flucke 1981
M Oral " 19.9 Flucke 1980b
M I.P. PG 35.8 Solmecke and Kimmerle 1972c
F 29.5
M Dermal None >1000 µl/kg bw) Solmecke and Kimmerle 1972c
(4-hour
exposure) None
M Dermal(24-h) None 705 µl/kg bw Kimmerle 1972a
Dermal None 188 µl/kg bw Solmecke and Kimmerle 1972a
(7-day
exposure) (undiluted)
M&F Inhalation Ethanol & >1300 mg/l air Kimmerle 1976
(1-hour PG (1:1)
exposure)
M Inhalation Ethanol & 0.21 mg/l air Solmecke and Kimmerle
F (4-hour PG (1.1) 0.144 mg/l air 1972c
exposure)
TABLE 1. (con't)
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Hamster M Inhalation Ethanol & 0.23 mg/l air Solmecke and Kimmerle
(4-hour PG (1:1) 1972c
exposure)
Rabbit F Oral PG approx. 150 Solmecke and Kimmerle 1972c
M Dermal Ethanol & 162 Nelson and Burke 1977a
F (24-hour PG (1:1) 315
exposure)
Dog F Oral PG > 25 Solmecke and Kimmerle 1972c
Bobwhite quail M&F Oral PG 8.7 Lamb and Burke 1979
Mallard duck M Oral PG 36.0 Nelson and Burke 1977b
F 32.0
Quail M Oral Water & 10 Thyssen 1978
Cremophor EL 10
Starling Oral Corn oil 972 Ross et al 1976
Hen F Oral Water & 16 Thyssen 1978
Cremophor EL
PG - polyethylene glycol 400.
Signs of oral poisoning in mammals
Cholinergic symptoms (e.g. muscle twitching, breathing disorders)
induced by toxic acute oral doses of isofenphos were typical of those
of cholinesterase inhibitors and were similar in mice, rats and
rabbits. Toxic signs usually developed within 2 h of dosing in mice
and rats and 4 h in rabbits and lasted for 2 to 10 days in survivors.
Mortalities from lethal doses usually occurred 1 to 4 days after
treatment. Cholinergic signs were not observed in dogs treated at
25 mg/kg bw, the top dosage level tested (Solmecke and Kimmerle
1972c).
Short-term studies
Rat
Groups of 15 male and 15 female rats (SPF Wistar II strain of
unspecified age) were orally treated by gavage with isofenphos ("pure
technical grade") in polyethylene glycol 400 at 0, 0.1, 0.25, 1.0 or
2.5 mg/kg bw/day for 30 days. Mortality, behaviour, growth, organ
weights and liver and kidney function were unaffected. Dose-related
inhibition (>20%) of cholinesterase in plasma and erythrocytes was
apparent at and above 1 mg/kg bw in females at 9, 16 and 30 days of
the study. In males, depression (dose related, >20%) of erythrocyte
cholinesterase was noted at both 1 and 2.5 mg/kg bw at 16 and 30 days
and of plasma cholinesterase at and above 1 mg/kg bw at 16 days and at
2.5 mg/kg bw also at 9 and 30 days (Solmecke and Kimmerle 1972a).
Groups of 10 male and 10 female rats (SPF Wistar W74 strain, 8
weeks old) were fed isofenphos (91.9% pure) in the diet at the dosage
levels of 0, 0.3, 1.0 or 3 ppm for 4 weeks. There was no mortality.
Behaviour, body weight and food consumption were not significantly
different between control and treated animals. Determinations of blood
cholinesterase activity after 3, 7, 10, 14, 17, 21, 24 and 28 days of
dietary feeding indicated dose-related inhibition (>20%) of plasma
cholinesterase in females at 1 ppm after 7, 17 and 28 days and at
3 ppm at all intervals. Only a slight depression (13-16%) of plasma
cholinesterase was noted in males even at 3 ppm. Activity of
erythrocyte cholinesterase determined over the course of the study and
of brain cholinesterase measured terminally was not affected in all
treated groups (both sexes). The no-effect level on plasma
cholinesterase was 0.3 ppm, which was equivalent to 0.024 mg/kg bw,
according to body weight and food consumption data (Löser 1978).
Groups of 15 male and 15 female rats (SPF Wistar strain, 28 to 32 days
old) were fed isofenphos (86.6% pure) in their diet at levels of 0.5,
1, 5, 25 or 125 ppm for 3 months. The control group consisted of 30
males and 30 females. One male at 125 ppm died after 9 days, due to
pneumonia. Toxic signs, such as salivation and muscle twitching, were
observed in animals receiving 125 ppm during the first 2 weeks of
feeding. Body weight was adversely affected in the highest dosage
group (both sexes). Food consumption was comparable to controls.
Haematological and biochemical studies and urinalysis conducted after
one and three months of the study indicated no significant dose-
related findings other than an increase in SGOT in males at 125 ppm at
the 1-month interval. Measurements of cholinesterase activity after 1,
4, 8 and 13 weeks of feeding revealed depression (dose-related >20%)
of the enzyme in both plasma and erythrocytes in females at 5 ppm and
above and in males at 25 ppm and above at all intervals. (Males at
5 ppm showed a 29% decrease of plasma cholinesterase level after one
week of feeding, but not thereafter.) The brain cholinesterase level
determined terminally was reduced at 25 ppm and above in both sexes.
At sacrifice upon termination of the study, an increase in absolute
weight and organ/body weight ratio of thymus was seen in females at
125 ppm. There were also variations in absolute weights of several
organs in males of the top dosage group. No compound-induced
histopathological alterations were observed in a variety of tissues
evaluated, including the thymus (Löser 1973; Urwin and Newman 1973).
Dog
Groups of dogs (4 males and 4 females per group, 5 months old)
were fed isofenphos (of unspecified purity) in the diet daily at 0,
0.3, 1.0, 10 or 30 ppm for 3 months. There were no treatment-related
effects on mortality, clinical symptoms, reflexes, body weight, food
consumption, ophthalmoscopic, haematological, clinical chemistry and
urinalysis parameters. Cholinesterase in plasma and erythrocytes was
depressed (by >20%), in a dose-dependent manner, at and above
10 ppm in both sexes after 3, 6 and 13 weeks of the study. (Plasma
cholinesterase in these dosage groups was inhibited also after 1 week
of the study.) At the conclusion of the study, absolute weight and
organ/body weight ratio of the liver was elevated at 10 ppm and
above in males (non-dose related) and females (dose related).
Histopathological examination of about 30 tissues, including the
liver, from each dog revealed no abnormalities or variations
associated with inclusion of isofenphos in the diet. The no-effect
level for the study was 1.0 ppm (Murmann 1973; Thomson and Newman
1973).
Groups of dogs (4 males and 4 females per group) were fed a daily
diet containing the following levels of isofenphos (89.3% pure) in a
2-year study: Control: 0 ppm; Group 1: males - 3 ppm from week 1
to week 83 and 2 ppm from week 84 to week 104, and females - 3 ppm
from week 1 to week 104; Group 2: 15 ppm throughout the 104 weeks,
and Group 3: 75 ppm from week 1 to week 53, 150 ppm from week 54 to
week 99 and 300 ppm from week 100 to week 104. No compound-related
findings were noted with respect to general condition (reflexes, body
temperature and pulse rate), water intake, ophthalmoscopic and
urinalysis parameters. Assay of cholinesterase 12 times over the
course of the study indicated dose-dependent inhibition (>20%) of
plasma cholinesterase in Groups 2 and 3 (both sexes) and of
erythrocyte cholinesterase in Group 3 at all intervals. Depression
(approx. or equal to 20%) of plasma cholinesterase was also found in
Group 1 males at weeks 7, 39, 66 and 79 but was not evident at weeks
92 and 103 after the dietary level was reduced from 3 ppm to 2 ppm at
week 84. Animals of Group 3 also showed depression (>60%) of brain
cholinesterase activity measured terminally.
Adverse effects on parameters other than cholinesterase activity
in plasma and erythrocytes were observed only in Group 3. In this
group, one male died at week 104 and another male was sacrificed
terminally in moribund condition. Toxic symptoms, such as salivation,
vomiting, diarrhoea, weakness in the hind extremities, unsteady gait,
etc., began to appear after 78 weeks and increased in severity with
time. The symptoms were more marked in males than in females.
Depression of body weight gain (both sexes) and of food consumption
(females) was obvious after 100 weeks. An increase in the SAP level at
several intervals during the study and a reduction of the terminal
serum A/G ratio were observed in males and females. The one male that
died at 104 weeks showed significant changes in several haematological
parameters. At terminal sacrifice, elevated organ/body weight ratios
of lung, liver, kidney, prostate, brain and pituitary and depressed
organ/body weight ratios of adrenal and testis were detected in the
males but, except in the testis, microscopic abnormalities of the
tissues in question were not noted. Histopathologically, hypoplasia of
the testis and erosion of esophageal mucosa, partially accompanied by
cellular reaction of propria mucosa, were observed in several males of
Group 3 but none in the other groups, including the control. Two
animals (Group 3) also exhibited foci of softening ("degeneration
processes") in the brain stem with a degree of severity greater than
the one case seen in the control group. The no-effect level in the
study was 2 ppm (Hoffman and Kaliner 1977).
Hen and quail
In adult hens and female quail intubated with isofenphos as an
emulsion in water and polyethylene glycol 400 daily for 5 consecutive
days, and then observed for 14 additional days, the 5 day LD50 was
found to be 6 mg/kg bw/day and 5 mg/kg bw/day respectively. Major
symptoms observed were inactivity in both species, ruffled feathers
and breathing disorders in the quail (Thyssen 1978).
Bobwhite quail and mallard duck
The dietary LC50 in bobwhite quail and mallard ducks fed
isofenphos in their diet for 5 days, followed by untreated diet for
3 days, was determined to be 145 ppm (Nelson and Burke 1977) and
>1000 ppm respectively (Nelson and Burke 1977c; Lamb and Burke 1977).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females per
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to isofenphos (as an emulsion in water and
Cremophor EL) at 0, 1 or 5 mg/kg bw/day 7 h per day, 5 times per week
for a total of 15 applications in a 21-day period. The treated sites,
not covered with bandages, were washed with soap and water after each
daily exposure period. One male at 5 mg/kg bw died of a cause
unrelated to treatment after 10 applications. There were no toxic
signs or adverse effects on body weight or on terminal haematological
and clinical chemistry parameters. Local irritation on treated sites,
generally transitory in nature and characterized by erythema on intact
skin and erythema, oedema and eschar formation on abraded skin, were
seen in some animals at 5 mg/kg bw. Changes in weights of several
organs were seen at 5 mg/kg bw without any accompanying
histopathological changes. Plasma and erythrocyte cholinesterase
activities measured after the 10th and 15th exposures and brain
cholinesterase level determined terminally in both sexes were
significantly depressed at 5 mg/kg bw (Thyssen and Kaliner 1977).
Rat - inhalation
In male and female rats exposed to isofenphos as an aerosol (of
unspecified particle sizes) 4h per day for 5 days, the LC50 was
>0.055 mg/l in males and 0.029-0.055 mg/l in females ((Solmecke and
Kimmerle 1972c).
Groups of 10 male and 10 female rats were exposed to an aerosol
of isofenphos at 0, 0.72 or 2.93 mg/m3 air 6h per day for 5
consecutive days per week over a 3-week period. (No information was
given on the particle size of the aerosol.) The treated animals were
not different from the controls as judged by general condition, body
weight gain, terminal haematological values, kidney and liver
function, gross pathological changes and organ weights. Cholinesterase
in plasma and erythrocytes was inhibited (>20%) at 2.93 mg/m3 air at
each weekly determination (Solmecke and Kimmerle 1972a).
Long-term studies
Mouse
Groups of 40 male and 40 female mice (6 to 7 weeks old, SPF NMRI
strain) were fed diets containing technical isofenphos (89.3% pure) at
0, 1, 10 or 100 ppm for 108 weeks. Additionally, each group included 3
sub-groups of 10 males and 10 females. One sub-group, fed the treated
diet for 108 weeks, was used for haematological tests and urinalysis
every 3 months over the course of the study. Another sub-group
maintained on dietary feedings for 9 months, was subjected to liver
function tests (plasma alkaline phosphatase and GPT) every 3 months.
The third sub-group was given isofenphos in the diet for 24 weeks and
activity of cholinesterase in plasma and erythrocyte was measured
after 2, 4, 8, 12 and 24 weeks and in brain after 24 weeks. All
animals that died during the study or were sacrificed terminally were
subjected to gross and histopathological examination. Mortality, which
was high in all groups, including the control, especially in the
females, was not attributed to treatment. At the end of the study,
only 42 to 57% of the males and 12 to 20% of the females per group
were still alive. Nevertheless, 53 to 73% of females per group
survived at least 80 weeks. There were no significant differences
between control and treated groups in clinical symptoms, body weight
and food consumption. Data on differential white blood cell counts
showed an increase at 100 ppm of eosinophil (males) and segmented cell
counts (females) after 21 months and of segmented cell counts in males
after 24 months. Liver function tests and urinalysis parameters were
normal. Dose-related inhibition (72 to 94%) of plasma cholinesterase
occurred in both sexes at and above 10 ppm at all intervals.
Erythrocyte cholinesterase was unaffected in the treated groups, with
depression consistently below 20%. Terminal brain cholinesterase
activity was reduced at 100 ppm by 46% in males and 31% in females. At
terminal sacrifice, absolute weight of lung in males was significantly
elevated at 100 ppm but no concomitant histological lesion of the
particular tissue was evident. An apparent dose-related increase, as
compared to concurrent controls, in frequency of mild gastritis (in
glandular mucosa of the stomach) was observed in males of all treated
groups which was, however, not statistically significant, especially
in the case of the 1 ppm and 10 ppm groups. No histopathological
changes related to treatment was observed in a variety of other
tissues evaluated.
Based on the tumour data, isofenphos was not carcinogenic in the
mouse, as indicated by the absence of a dose-related increase in
incidence of a) any particular type of tumours, b) animals with
tumours, c) animals with multiple tumours, and d) animals with
malignant tumours. The time of appearance of tumours was comparable
between control and treated groups.
The no-effect level for the study was 1 ppm (Brune et al.
1978).
Rat
Groups of 50 male and 50 female rats (SPF Wistar strain, 28 to 32
days old) were fed diets containing isofenphos (of unspecified purity)
at 1, 10 or 100 ppm for 2 years. The control group consisted of 100
males and 100 females. Mortality was unaffected by treatment, and
between 69 and 90% of animals per group were still alive at the end of
the study. At 100 ppm, cholinergic signs were seen during the first
week and depression of growth in both sexes and of food consumption in
males was apparent throughout the feeding period. Haematological and
biochemical studies and urinalysis conducted at five intervals over
the course of the study failed to reveal any consistent effect related
to presence of isofenphos in the diet. Assay of cholinesterase after
1, 2, 4, 13, 26, 52, 78 and 105 weeks of the study indicated dose-
related inhibition (>20%) of both plasm and erythrocyte
cholinesterase at 10 ppm and above in both sexes at all intervals and
of plasma cholinesterase in males at 1 ppm after 4 and 78 weeks. The
extent of depression of cholinesterase in plasma and erythrocytes
remained fairly constant over the course of the study. At the
conclusion of the experiment, brain cholinesterase activity was
reduced at 100 ppm in both sexes. Variations in absolute weights of
several organs were seen at 100 ppm but histological changes of the
particular organs were absent. Except for an apparent increase in
incidence of ulceration of the forestomach in females at 100 ppm,
as compared to the concurrent controls, no treatment-related
alterations were evident in a set of about 30 tissues examined
histopathologically. Analysis of tumour data revealed no indications
suggestive of carcinogenic activity of isofenphos under the conditions
of the experiment.
The study demonstrated 1 ppm as a marginal no-effect level with
respect to plasma cholinesterase. On other parameters monitored,
10 ppm was without any significant adverse effect (Bomhard and Löser
1977).
Special studies on reproduction
Groups of 20 male and 20 female weanling rats (SPF CFY strain)
were fed dietary levels of technical isofenphos (of unspecified
purity) at 0, 1, 10 or 100 ppm for 60 days prior to mating to initiate
a reproduction study originally designed to cover 3 generations with
one litter per generation. Three litters (F1a, F2a and F3a) were
obtained as expected for control, 1 ppm and 10 ppm groups. In the case
of the highest dosage group, owing to poor survival of pups, there
were insufficient F1a weanlings to form an adequate second parental
generation (F1). F0 animals at 100 ppm, as well as those of other
groups including the control, were therefore re-mated. This second
mating again failed to provide sufficient F1b offspring at 100 ppm as
potential parents. In an attempt to determine the causes of the
initial adverse effects at 100 ppm, F0 animals of control and top
dosage groups were allowed two additional matings, with the fourth
mating using treated males and untreated females and vice-versa. In
the third mating trial, the number of litters (F1c) at 100 ppm
obtained was too small to permit any meaningful interpretation of
results. Based on results of the fourth mating, fertility appeared to
be adversely affected, primarily in the females. This summary will
refer only to data on control, 1 ppm and 10 ppm groups.
In parental generations, there were no mortality and clinical
symptoms attributable to treatment. Body weight gain during pre-
mating, gestation and lactation periods, mating performance, duration
of gestation and terminal gross pathological findings were not
significantly different from controls. Pregnancy rate was 90%, 70% and
60% respectively in control, 1 ppm and 10 ppm groups in the F0
generation at the second mating suggesting a slight effect at 10 ppm
and a marginal effect at 1 ppm. (The pregnancy rate at 100 ppm was 40%
for the second mating trial.) Assay of plasma and erythrocyte
cholinesterase in F0 animals after 37 weeks of dietary feeding
revealed inhibition (>20%) at 10 ppm of erythrocyte cholinesterase in
both sexes and of plasma cholinesterase in females. Over the three
progeny generations (F1a, F2a and F3a litters), litter size, pup
mortality and mean pup weight from birth through lactation to weaning
were comparable to controls at both 1 and 10 ppm. In F1b litters, pup
weight at 10 ppm seemed to be slightly decreased at days 12 and 21.
Pups observed grossly at weaning displayed no apparent compound-
induced abnormalities. The available data tend to indicate 1 ppm as a
marginal no-effect level with respect to reproductive capability
(Palmer et at 1977).
Special studies on teratogenicity
Rat
Groups of 20 to 21 pregnant rats (Long Evans FB30 strain) were
intubated with isofenphos (86.6% pure) in polyethylene glycol 400 at
doses of 0, 0.3, 1 or 3 mg/kg bw/day from gestation day 6 to day 15.
The dams were sacrificed on gestation day 20 and foetuses were removed
by caesarean section for external, visceral and skeletal examination.
No treatment-related mortality or abnormal behavioural reactions
were noted, and maternal body weight was normal during the dosing
period. An increase in the average number of resorbed foetuses per
litter occurred at 3 mg/kg bw, but a dose-related elevation in the
percentage of litters with rasorptions was not apparent at this dosage
level. There were no significant differences between control and
treated groups with respect to fertility rate, mean number of
implantation sites, mean litter size, foetal weight, placental weight
and incidence of stunted foetuses. The frequency of foetuses showing
slight bone variations and/or malformation was very low and not
significantly different from that in the control group (Machemer
1972).
Groups of 21 to 23 pregnant rats (Long Evans FB30 strain) were
exposed dermally to isofenphos (dissolved in polyethylene glycol 400)
at 0, 0.3, 1.0, 3 or 10 mg/kg bw/day for 5 h per day from gestation
day 6 to day 15. The treated (clipped) sites were washed with soap
solution after each daily exposure period. Animals' necks were
immobilized. On day 20 of pregnancy, the dams were sacrificed and
foetuses were removed by caesarean section. A decrease in maternal
body weight gain during and after treatment period was observed at
10 mg/kg bw. Dams at this level also showed an increased incidence of
ruffled fur. There was a slight increase in the mean number of
resorptions per pregnant dam at 10 mg/kg bw, but the percentage of
pregnant dams with resorbed foetus(es) was not significantly elevated.
No treatment-related effects were observed on other tested parameters,
viz. fertility rate, mean number of implantations, mean litter size,
foetal weight, placental weight, frequency of stunted foetuses and
incidence of skeletal variations. A number of foetal malformations
were seen in all groups, including the control, but they were
generally non-dose-related. The only abnormalities observed at
10 mg/kg bw, but not in the lower dosage groups or controls, were
multiple defects manifested as hypoplasia of telencephalon and
unspecified eye malformation in 3 foetuses from a single litter. These
particular findings were not believed to be compound induced. Under
conditions of the experiment, isofenphos was not teratogenic, although
a dosage level of 10 mg/kg bw was maternally toxic (Schlüter 1981).
Groups of 22 to 25 pregnant rats (Long Evans FB30 strain) were
exposed in an inhalation chamber to an aerosol of isofenphos
(formulated with a 1:1 mixture of polyethylene glycol 400 and ethanol)
at actual chamber concentrations of 0, 0.25, 0.75 or 3.1 mg/m3 air,
for 6 h per day from day 6 through day 15 of gestation. More than 97%
of the particles counted were of sizes within respirable range in the
rat. On day 20 of gestation, the dams were sacrificed and the foetuses
were removed for gross, skeletal and visceral examination. No maternal
toxicity was observed. Pregnancy rate, mean number of implantations,
mean number of resorptions, mean litter size, foetal weight and
incidence of stunted foetuses were not adversely affected. An increase
in placental weight was noted at 3.1 mg/m3 air, which was of doubtful
biological significance and unlikely to be treatment-related in view
of the absence of any embryotoxic activity. There was no dose-related
effect on the incidence of skeletal and visceral malformations. Based
on the data, isofenphos was not teratogenic under the conditions of
the experiment (Schlüter and Thyssen 1981).
Rabbit
Groups of pregnant (Himalayan) rabbits (11 to 13 rabbits per
group) were orally treated with isofenphos at 0, 1, 2 or 5 mg/kg
bw/day from gestation day 6 through day 18. The females were
sacrificed on gestation day 29 and uterine contents were examined.
Foetuses were subjected to evaluation for external, skeletal and
internal malformations.
Compound-related mortalities and toxic symptoms (diarrhoea) were
seen in does at 5 mg/kg bw. A marked decrease (non-dose-related) in
maternal body weight gain during the treatment period occurred in all
treated groups. One of the 13 does aborted at 2 mg/kg bw. There were
no significant differences between control and treated groups in
pregnancy rate, litter size, mean number of implantations, mean number
of resorptions, sex ratio, foetal weight, placental weight and
incidence of stunted foetuses. "Slight bone variations" were not
indicated to occur in any foetuses from control or treated groups. The
only malformation observed in the study was indicated to be
arthrogryphosis of both front extremities in a foetus at the dose
level of 5 mg/kg bw (Machemer 1975).
Special studies on mutagenicity
In two similar but separate studies, isofenphos was evaluated for
its mutagenic activity using two microbial assay systems (rec-assay
and reversion test). Indicator micro-organisms used in the former were
Bacillus subtilis strains N1G17, N1G45, H 17 and M 45. For the
latter, Salmonella typhimurium strains TA 1535, TA 1537, TA 1538, TA
98 and TA 100 and Escherichia coli strain WP2 hcr were employed.
The reversion test was conducted in the presence or absence of a
mammalian metabolic activation system (S-9 homogenates of liver from
rats or mice induced by phenobarbital or Aroclor 1254) while the
rec-assay was carried out without the activation preparation. Under
the conditions of the experiments, isofenphos was demonstrated not to
be mutagenic at concentrations up to 0.02 ml/disc of undilated
isofenphos and 5000 µg/plate (Shirasu et al 1980) or 300 µg/disc
and 1000 µg/plate (Inukai and Iyatomi 1977), respectively, in the
rec-assay and the reversion test. Mutagenic responses were obtained
with several positive control compounds, such as mitomycin C,
9-amino-acridine HCl, furylfuramide, acetylaminofluorene and
beta-propiolactone (Inukai and Iyatomi 1977; Shirasu et al 1980).
Another Ames test conducted with S. typhimurium strains
TA 100, TA 1537, and TA 98, with or without the S-9 mix and using
concentrations as high as 3150 µg/plate, also confirmed the non-
mutagenicity of isofenphos (Oesch 1977).
In a dominant lethal assay, groups of 20 male NMRI mice (10 weeks
old) were given by gavage a single dose of 0 or 15 mg/kg bw of
isofenphos. Each male was then mated with three untreated female mice,
which were replaced weekly for 8 consecutive weeks. The females were
sacrificed around the 14th day of gestation or 14 days after
separation from the males and the uteri were removed for examination.
The treated males exhibited no signs of toxicity. There were no
significant differences between the control and the treated groups
with respect to fertility rate, living implants and dead implants.
Pre-implantation loss (no. of corpora lutea and no. of implantations)
was significantly increased at 15 mg/kg bw during the first week after
dosing, but not thereafter. It was indicated in the report that the
values for pre-implantation loss observed in the treated group were
"completely within the norm of the strain" (Machemer 1973).
Special studies on carcinogenicity
See under "Long-term studies".
Special studies on neurotoxicity
Hen
Groups generally comprising 10 adult hens (15 to 18 months old)
were given acute oral or intraperitoneal doses of technical
isofenphos. The oral and intraperitoneal LD50 was found to be
21 mg/kg bw and 11.4 mg/kg bw respectively. No delayed neurotoxic
signs were observed in any of the treated animals during a 6-week
post-exposure observation period. Pre-treatment of hens with atropine
(50 mg/kg bw) intraperitoneally elevated oral LD50 of isofenphos to
74 mg/kg bw. Five hens surviving an oral dose of 20 mg/kg bw of
isofenphos and 6 atropinized hens that were still alive after
receiving 74 mg/kg bw of the insecticide were sacrificed 21 days after
dosing for histopathological examination of the brain, spinal cord and
peripheral nerve. These hens showed no compound-related morphological
change or variation from normal in any of the nervous tissues
examined. Hens treated with an oral dose of TOCP at 350 mg/kg bw
displayed typical neurotoxic signs and showed degenerative nerve
fibres in the spinal cord and the sciatic nerve (Kimmerle 1972; Cherry
et al 1972).
Special studies on antidotes
In 2 separate experiments, atropine sulphate (50 mg/kg bw), 2-PAM
(50 mg/kg bw) or Toxogonin (20 mg/kg bw) was given singly or in
combination (atropine plus PAM or Toxogonin) i.p. to male rats: a) 45
to 120 minutes after single oral toxic doses of isofenphos (and before
onset of severe toxic symptoms), and b) 5 minutes after acute oral
toxic doses of the oxygen analogue of isofenphos and before onset of
toxic symptoms. Results indicated that, under the conditions of the
experiments, none of the three compounds tested was effective as an
antidote for isofenphos or its oxygen analogue (Solmecke and Kimmerle
1972b, Thyssen 1974a).
Simultaneous administration of atropine sulphate at 100 mg/kg bw
and obidoxime at 20 mg/kg bw to male rats i.p. after onset of toxic
symptoms (at 25 minutes) resulting from acute oral toxic doses of
isofenphos elevated LD50 of the insecticide from 43.1 mg/kg bw to
84.2 mg/kg bw. No data were provided on the effect of the antidotes on
the toxic signs (Kimmerle and Gröning 1975).
Special studies on potentiation
When the acute oral LD50 values in male rats, determined
experimentally for equitoxic mixtures of isofenphos and malathion or
of isofenphos and EPN, were compared with the expected LD50 values
for the mixtures, the acute toxicity of isofenphos was found to be
potentiated by malathion. An additive effect resulted when isofenphos
was combined with EPN (Solmecke and Kimmerle 1972b).
In further studies with male rats, it was demonstrated with
isofenphos did not potentiate the acute oral toxicity of phenamiphos
or phoxim (both organophorous insecticides) (Thyssen 1976; Thyssen
1977).
Special studies on the acute toxicity of the oxygen analogue
Rat
The LD50 of the oxygen analogue of isofenphos was determined in
6 female and 6 male rats and is reported in Table 2. Thyssen and
Kimmerle 1974).
TABLE 2. Acute toxicity of the oxygen analogue of isofenphos in rats
Sex Route Vehicle LD50
(mg/kg bw)
M Oral Water & Cremophor EL 30.8
F 16.1
M i.p. Water & Cremophor EL 15.9
F 7.8
M Dermal None 117.6 µl/kg bw
F (24 h) (undiluted) 25 µl/kg bw
M Dermal Water & Cremophor EL 97.9
F (24 h) 29.5
M Inhalation Ethanol & polyethylene >353 mg/m3 air
(1 h) glycol 400 (1:1)
F approx. 353 mg/m3 air
M Inhalation Ethanol & polyethylene >195 mg/m3 air
(4 h) glycol 400 (1:1)
F 63.8 - 131 mg/m3 air
Signs of oral poisoning
Acutely toxic doses of the oxygen analogue of isofenphos to
rats produced cholinergic symptoms typical of those seen with
cholinesterase inhibitors. These toxic signs occurred within 1 h of
dosing and persisted for a maximum of 2 days in survivors. Deaths from
lethal doses were observed from about 30 minutes to 3 days after
treatment (Thyssen and Kimmerle 1974).
The acute LD50 of the oxygen analogue of isofenphos in hens was
>10 <25 mg/kg bw by the oral route and about 10 mg/kg bw i.p. No
neurotoxic signs were observed in any of the treated hens over a
28-day post exposure observation period (Thyssen 1974c).
Special studies on the subacute oral toxicity of the oxygen analogue
and of a mixture of isofenphos and its metabolites
Groups of 15 male and 15 female rats (Wistar II strain) were
treated daily by gavage with the oxygen analogue of isofenphos (HOL
0654) (as an emulsion in water and Cremophor EL) at 0, 0.2, 0.6 or
1.8 mg/kg bw for 30 consecutive days. Activity of cholinesterase in
plasma and erythrocytes was determined five times over the course of
the study and brain cholinesterase was assayed at terminal sacrifice.
Physical appearance, behaviour and body weight were comparable to
controls. Dose-dependent inhibition (>20%) of plasma cholinesterase
was observed in males and females at 1.8 mg/kg bw throughout the
feeding period, at 0.6 mg/kg bw in males after 3 weeks only and
in females at 3, 10, 17 and 23 days of the study. The level of
erythrocyte cholinesterase was reduced by >20% in both sexes at
1.8 mg/kg bw. In general, the degree of depression of both plasma and
erythrocyte cholinesterase was greater in the females than in the
males. Terminal brain cholinesterase was unaffected in both sexes.
There were no significant gross pathological alterations in treated
animals. The no-effect level on plasma cholinesterase was 0.2 mg/kg bw
(Thyssen 1974b).
Cow
Groups of 3 dairy cows were fed alfalfa pellets treated with a
mixture of isofenphos, isofenphos oxygen analogue and des-N-isopropyl
isofenphos oxygen analogue (1:8:1) at 2, 6 or 20 ppm for 28 days and
then sacrificed for analyses of tissue residues. One cow was used as a
control. Whole blood cholinesterase was assayed at weekly intervals.
Other than the observation of a more sedated or docile appearance in
cows at 6 ppm and above, as compared to the control animal or animals
at 2 ppm, there were no adverse effects noted with respect to body
weight, feed consumption and milk production. A reduction (>20%) of
whole blood cholinesterase activity occurred at 20 ppm at days 14, 21
and 28 of the study and at 6 ppm at days 21 and 28. The inhibition of
whole blood cholinesterase was dose-related and increased with time
over the course of the study (Strankowski et al 1977c).
Hen
Groups of 4 hens were fed diets containing a mixture of
isofenphos, isofenphos oxygen analogue and des N-isopropyl isofenphos
oxygen analogue (1:8:1) at the levels of 0, 5, 15, 50 or 150 ppm for
28 days and then sacrificed for analyses of tissue residues. Body
weight and food consumption at both 50 and 150 ppm and egg production
at 150 ppm were adversely affected. Dose-related depression (22 to
98%) of plasma cholinesterase determined at terminal sacrifice was
observed in all treated groups (Strankowski 1977).
OBSERVATIONS IN HUMANS
In 3 male workers (wearing standard protective clothing) involved
for 5 to 11 working days (generally 7.5 to 8 h per day and not more
than 3 consecutive days at one time) in the application of a 7.5%
granulated formulation of isofenphos through a standard applicator
fixed to carrot seed drilling equipment, the whole blood
cholinesterase activity was not significantly depressed, as compared
to 2 male and 1 female control subjects. There was no mention in the
report with respect to adverse effects or symptoms of exposure. No
other clinical measurements were made (Bagnall 1976).
RESIDUES IN FOOD
USE PATTERN
Isofenphos is an insecticide possessing good activity against
soil insects, such as Diabrotica spp., wireworms, chafer grubs,
vegetable flies and flea beetles. It has proved to be highly effective
against leaf-eating pests, such as pear sucker and Colorado potato
beetle, and also against rice stem borers.
The compound acts as a contact and stomach poison. It has a root-
systemic action, i.e. the active ingredient is absorbed by the roots
of the plants and translocated to a limited degree within the plant.
The active ingredient is notable for its long residual activity
and its good dispersibility in soil. In numerous trials, isofenphos
has displayed generally good crop plant tolerance, whether used as a
seed treatment or applied as granular and emulsifiable concentrate
formulations. Isofenphos is formulated as an emulsifiable concentrate,
granular formulation and seed dressing powder.
It is mainly applied in row at or immediately after seeding. Two
applications are registered only for corn in the United States. The
crops, pests and recommended or registered dose rates are listed in
Table 3.
TABLE 3. Recommended application rates for isofenphos
Crop Pests Formulation Dose (a.i.)
Onion, carrot, brassica
leafy vegetables vegetable flies GR 1.5-2.5 kg/ha in row
Onion onion fly GR 5 kg/ha broadcast or spray
and overall
EC 0.076 g/m in row
Brassica leafy vegetables cabbage root fly GR 0.05 % a.i. (at 100 cm3/plant
and as soil drench after
EC transplanting)
0.0375-0.075 % a.i. at a rate
of 0.0375-0.15g/m row
Potato wireworm GR 5 kg/ha broadcast overall
before planting
Maize Diabrotica sp. GR 0.11 g/m in row at seeding +
and lay by (50 cm row spacing)
EC
Oilseeds, rape, flea beetle Seed 12-16 g/kg seed
swedes, marrowstem dressing seed treatment
kale, turnips powder
RESIDUES RESULTING FROM SUPERVISED TRIALS
Experiments were run on various plants at different locations in
Canada, Europe and USA. Isofenphos was applied at about the
recommended, and in certain cases somewhat higher, dosage rates.
The residues looked for included isofenphos, isofenphos oxygen
analogue (IOA) in European experiments and des-N-isopropyl isofenphos
(DNI) and des N-isopropyl isofenphos oxygen analogue (DNIOA) were
additionally included in the analyses carried out in USA. The residues
measured in brassicas, oilseed, rape, root and tuber vegetables were
low, generally at or about the limit of determination for the
recommended pre-harvest interval, as well as those found in kernel of
corn at the milk and dry stages. In green forage, however, residues
were as much as 2 mg/kg. Onions and potatoes also contained detectable
residues. The major part of the residue consisted of isofenphos and
IOA in all crops.
Brassica leafy vegetables
Brussels sprouts were treated by applying a soil drench at a rate
of 0.05 g a.i./plant shortly or 1 day after planting. No residue was
measurable 99-119 days after treatment (Bayer 1978). isofenphos was
applied on cabbages, savoy and white, and cauliflower either in row or
with overall treatment at dose rates of 0.075 g a.i./m in a 10 cm band
or 1.5 to 3 kg/a.i./ha, respectively. The experiments were carried out
in Federal Republic of Germany (FRG) and UK and results are summarized
in Table 4 (Bayer 1973-74, 1977).
Celery
Oftanol 200 EC was worked in the soil at rate of 4 kg a.i./ha one
day before planting. Samples were taken 84 and 90 days after treatment
and analysed for isofenphos and IOA. The residues were below the limit
of determination (0.01 mg/kg) (Netherlands 1981).
Maize
Supervised trials were carried out in various states of the USA
in 1979 and 1980.
AMAZE 15 G or 20 G were used for the treatment of the plots in a
15 cm band at planting plus either a double side dress at the 4-leaf
growth stage or band treatment at the base of the plant at growth
stage of 45 cm. A dosage rate of 0.19 g a.i./m was applied in all
treatments (equal to 2.5 kg a.i./ha). Samples were taken from green
forage, kernel, cob and husk in the milk stage and from the dried
parts of plant. Tests were made for residues of isofenphos and its
three metabolites. The limit of determination was 0.01 mg/kg for all
compounds individually.
TABLE 4. Residues of isofenphos in brassica leafy vegetables
Application Residues 1 (mg/kg) at intervals (days) after application
Crop Country Year No. Rate
(kg a.i./ha) Formulation 0/42 49 55-56 60 64-70 80-87
Cabbage FRG 1974 1 3 kg/ha 5 G 0.01 0.01 <0.01
FRG 1974 1 " 5 G 0.08 0.02 0.01
savoy FRG 1974 1 " 5 G <0.01 <0.01 <0.01
FRG 1977 1 )0.075 g/m 500 EC 0.02 0.01 0.01 <0.01
FRG 1977 1 ) in row 500 EC 0.12 0.03 0.02 <0.01
FRG 1977 1 ) 500 EC 0.09 0.03 0.02 0.01
Cabbage UK 1974 1 1.5 kg/ha 5 G 0.007
FRG 1973 1 2.5 kg/ha 5 G 0.1 0.01
white FRG 1973 1 2.5 kg/ha 5 G 0.06 0.03
FRG 1973 1 2.5 kg/ha 5 G 0.08 0.02
Cauliflower FRG 1977 1 0.075 g/m 500 EC 0.11 0.04 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.01 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.02 <0.01 <0.01 <0.01
in row
FRG 1973 1 2-2.5 kg/ha 5 G <0.012 <0.011 <0.013
1 Sum of isofenphos and its oxygen analogue;
2 Results were obtained by analysing 3 samples taken from different plots;
3 Results of analyses of 6 samples.
Isofenphos, IOA and DNIOA were detected in forage. The results
are summarized in Table 5. Levels of DNIOA ranged from 0.01 to
0.08 mg/kg, with an average of 0.04 mg/kg. No measurable residues were
found in kernels either in tile milk or dry stage. Residues in the
cob were at or below the limit of determination (total residue
< 0.02 mg/kg). The husk contained residues of less than 0.2 mg/kg
at all times and stages (Mobay 1979-80).
Sweet corn
Experimental plots were treated in a 15 cm band at planting and
with either a 15 cm band at the base of the plant or a basal spray at
lay by 4 to 8 weeks later. OFTANOL 6 EC and 15 G formulations were
used in the trials (Mobay 1978a).
The active ingredient was applied at a dose rate of 0.19 g/m,
equal to 3.72 kg a.i/ha. The residues (parent compound and
3 metabolites) were analysed separately in 10 milk stage kernel
samples. The oxygen analogue was detected twice at levels of
0.02 mg/kg and 0.05 mg/kg; the other residues were below the limit of
determination (0.01 mg/kg for all compounds). No residues were
detected in dry kernels (3 samples).
The green and dry forages were also analysed, and levels of
isofenphos and IOA are listed in Table 5. The cob in the milk stage
did not contain detectable residues, with the exception of 0.02 mg/kg
of oxygen analogue. The oxygen analogue was also the only detectable
residue in husk at the milk stage, with a maximum level of 0.16 mg/kg.
The dry husk contained both IOA and DNIOA. Maximum levels were
0.27 mg/kg and 0.02 mg/kg respectively.
Onion
At various locations in the USA in 1978, 17 experimental plots
were treated in furrow at planting with either 15 G, 10 G, 5 G or 4 EC
formulations at a dose rate of 0.1 g isofenphos/m. The dose rate
expressed in kg/na varied depending on the row spacing, which was
between 30 to 100 cm (Mobay 1978b). Both mature bulbs and immature
bulbs were sampled and analysed for isofenphos, IOA, DNI and DNIOA.
The residues were generally low in the bulb.
DNI and DNIOA were only detected in one sample at levels of
0.01 mg/kg and 0.08 mg/kg respectively. Supervised trials were also
carried out in Federal Republic of Germany, France and in The
Netherlands. Five kg a.i./ha was applied from both 5 G and 500 EC
formulations one day before sowing or at the 4-leaf stage. Samples
were taken 92 to 158 days after application and the levels of
isofenphos and IOA were measured (Bayer 1972-73, 1975, 1978). The
number of samples taken from experimental plots both in USA and in
Europe are listed in Table 6.
TABLE 5. Residues of isofenphos in forage of maize and sweet corn
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Maize USA 0.03 0.02 0.02
1980 2 2.5 15 G
Indiana 0.141 0.071 0.111
" 1980 2 2.5 15 G 0.05 0.04 0.04
0.141 0.191 0.111
" 1980 2 2.5 20 G 0.03 0.07 <0.01
0.111 0.231 <0.011
Nebraska 1979 2 2.5 20 G <0.01 <0.01 <0.01 <0.01 <0.01
<0.011 <0.011 <0.011 <0.011 <0.011
" 1979 2 2.5 15 G <0.01 0.02 <0.01 <0.01 <0.01
<0.011 0.01 <0.01 <0.01 <0.01
Sweet corn USA 1978 2 3.72 6 EC 0.22
Missouri 0.741
Oregon 1978 2 3.72 6 EC <0.01 <0.01 <0.01 <0.01 <0.01
<0.031 0.031 0.041 0.031 <0.011
Nebraska 78 2 3.72 6 EC 7.86 0.01 <0.01 0.04
9.261 0.251 0.061 0.051
Texas 1978 2 3.72 6 EC 0.2 0.05 0.08 0.04
2.091 1.611 1.991 0.071
Florida 1978 2 3.72 6 EC 0.03 0.02 0.04 <0.01
0.751 1.371 0.671 0.071
Missouri 78 2 3.72 15 G 0.94 0.07 0.1
0.741 0.231 0.191
TABLE 5. (con't)
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Oregon 1978 2 3.72 15 G 0.02 0.02 0.02 <0.01 <0.01
0.081 0.081 0.061 0.06 0.011
Texas 1978 2 3.72 15 G 0.01 0.03 0.05
0.721 1.041 0.151
Florida 1978 2 3.72 15 G <0.01
0.041
Missouri 78 2 3.72 15 G 0.39 0.03 <0.01
0.871 0.14 0.11
1 Residues of oxygen analogue.
TABLE 6. Supervised trials measuring isofenphos and its oxygen
analogue in onions
Compound Residue (mg/kg) No. of samples
Isofenphos < 0.01 12
< 0.05 0
< 0.1 1
< 0.2 1
< 0.6 3
IOA < 0.01 4
< 0.05 8
< 0.1 2
< 0.2 2
< 0.6 1
Isofenphos + < 0.01 2
IOA 1 < 0.05 4
< 0.1 3
1 Result of experiments in Europe.
The sum of the residue detected was always below 1 mg/kg in
mature bulb samples. Immature bulbs were analysed in two occasions and
the residues found were: isofenphos-0.32 and 0.95 mg/kg; isofenphos
oxygen analogue - 0.18 and 0.26 mg/kg.
Rapeseed
Seeds of spring and autumn varieties of rape were seed dressed
with BAY 6643 B at a rate of 15 g isofenphos/kg seed in 1973-74. The
crops were sampled at various locations in the FRG. Residues were
below the limit of determination (0.01 mg/kg) in all of the 13 trials
(Bayer 1974a).
Supervised trials with three varieties were carried out at three
locations in Canada in 1989 (Mobay 1980a). AMAZE 40% was used for seed
dressing prior to seeding at a rate of 25 g a.i./kg seed. The seeds
were treated either with formulation or the powder was applied to seed
after coating with oil. The harvested seed was analysed for
isofenphos, IOA, DNI and DNIOA 94-113 days after treatment. Residues
of active ingredient and its metabolites were below the limit of
determination (0.01-0.02 mg/kg).
Root and tuber vegetables
Celeriac
Supervised trials, in which 4 kg a.i./ha of Oftanol 200 EC was
applied before planting, were carried out on celeriac fields at two
locations of The Netherlands in 1978. The mature crops were samples
158 days after application. Residues of isofenphos were not detected
(Netherlands 1981).
Potato
In an experiment in FRG, a 5 G formulation of isofenphos was
worked into the soil at a rate of 5 kg a.i./ha before planting. At 110
days after application, a residue of 0.11 mg/kg was detected in the
tubers that had been washed before analysis (Bayer 1973).
Potato fields were treated with isofenphos at three different
locations in Spain in 1980. The broadcast applications were made at a
rate of 2.5 kg a.i./ha at the time of planting. Samples were taken
111, 112 and 159 days later at the time of harvest and their residue
contents were found to be 0.45, 0.04 and 0.05 mg/kg respectively
(Bayer 1980).
Swedes and turnips
Oftanol T was used for seed dressing at a rate of 16 to
20 g a.i./kg seed. Experimental plots from 8 locations in FRG were
sampled 109 to 179 days after sowing in case of swedes, while three
plots of turnips were sampled 89 to 103 days after sowing. Leaves and
roots were analysed separately. No residue was detected (<0.01 mg/kg)
(Bayer 1974-76).
Availability of soil residues to rotational crops
Wheat, green beans and sugarbeets were planted as rotational
crops 3.5 months after a sandy soil had been treated with ring-µL
(14C)-isofenphos at a field application rate of 5.6 kg a.i./ha (Kurtz
1977). Corn had been used as the original crop in this isofenphos-
treated soil. When the rotational crops were planted, the soil
contained approximately 4 mg/kg isofenphos. The rotational crops were
sampled at 2, 8 and 32 weeks after planting. At the time of harvest,
8 weeks for green beans and 32 weeks for sugarbeet and wheat, the
residue level was measured in different parts of the crops (Table 7).
No des-N-isopropyl isofenphos was detected in any of the crops.
TABLE 7. Residues of isofenphos detected in rotational
crops grown in soil treated with isofenphos 1
Crop Sample Residue (mg/kg)
I IOA DNIOA
Green bean Forage < 0.01 0.05 < 0.01
Sugarbeets Roots 0.04 0.02 < 0.01
Tops < 0.01 0.02 0.06
Wheat Heads < 0.01 0.24 0.03
Straw 0.14 0.79 0.2
Forage 0.05 1.08 0.23
1 Application rate - 5.6 kg a.i./ha.
In the investigation of field rotational crops, wheat, oats or
sorghum, spinach turnips and soybean were planted in plots treated the
previous year with isofenphos (Mobay 1980b). The plots had been used
previously for performance tests and hence were cropped using normal
agricultural practices. Rotational crop planting dates ranged from 214
to 390 days (7 to 13 months) post-treatment. Treatment was with either
AMAZE 20 G in an 18cm band over the row at 1.2 kg a.i./ha to 1.5 kg
a.i./ha broadcast equivalent-with presswheel incorporation or with
AMAZE 6 E or 20 G applied in furrow at 1.1 to 2 kg a.i./ha broadcast
equivalent. Prior to planting the rotational crops, the plots were
rototilled or disced. The crops were then planted across and
perpendicular to the direction of the originally treated rows. To
insure homogeneity of sampling, the entire lengths of the crop rows
were harvested. Each of the harvested crops were analysed for residues
of isofenphos, IOA, DNI and DNIOA. The results of the study are given
in Tables 8 and 9.
TABLE 8. Residues of isofenphos in grain (wheat, oats or sorghum) rotational crops
Treatment Plant Back Residue (mg/kg 1
Crop Location Rate Interval
(USA) (kg a.i./ha) (days) Forage Grain Straw
Winter
Wheat Kansas 1.5 77 <0.01 <0.01 <0.01
Sorghum Texas 1.2 214 <0.012 <0.01 0.02
<0.033
Spring
wheat Kansas 1.5 295 0.064 NA5 NA
0.026
Spring
Wheat Nebraska 1.5 350 <0.014 <0.01 <0.01
<0.016
Oats Illinois 1.2 362 <0.014 <0.01 <0.01
<0.016
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos equivalents;
2 Immature crop, 42 days post-planting;
3 Immature crop, 57 days post-planting;
4 Immature crop, 45 days post-planting;
5 NA - no mature crop available for analyses;
6 Immature crop, 60 days post-planting.
TABLE 9. Residues of isofenphos in rotational crops
Treatment Plant back Residue
Crop Location rate Interval (mg/kg) 1
(kg a.i./ha) (days)
Spinach Arizona 2 279 <0.01
Spinach Arizona 2 279 <0.01
Turnip tops Kansas 1.5 316 <0.01
Spinach Nebraska 1.5 363 <0.01
Turnip tops Nebraska 1.5 363 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Kansas 1.5 316 <0.01
Turnip root Nebraska 1.5 363 <0.01
Soybean Kansas 1.5 295 0.03
Soybean Nebraska 1.5 363 <0.01
Soybean Illinois 1.14 390 <0.01
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos
equivalents.
Residues in all rotational crops studied consisted mainly of
isofenphos and IOA and were <0.05 mg/kg; most were <0.01 mg/kg. In
the four mature grain crops, the grain had no detectable residue and
the straw showed a detectable residue of 0.02 mg/kg in only one
sample. In the five vegetable crops, only one had a detectable residue
(0.01 mg/kg) was seed in only one of the bean samples and in two of
the dry vine samples. Based on these results, little or no isofenphos
residue would be expected in grain, vegetable, root or oil seed crops
grown in fields treated 9 or more months earlier with isofenphos at
rates up to 2 kg a.i./ha.
FATE OF RESIDUES
General comments
The degradation and metabolism of isofenphos was studied in
animals, plants, soils, water and during frozen storage. The
structural formula, chemical name, identifying symbol and occurrence
of its degradation products are shown in Figure 2. Metabolic pathways
for isofenphos in animals (A) plants (P) and Soil (S) are shown in
Figure 3.
In plants
The metabolism of isofenphos was studied in maize grown in ring -
µL (14C)-isofenphos treated soil both outdoors and in a greenhouse
(Stanley et al 1977).
For the outdoor experiment the labelled isofenphos was prepared
as a 6 EC formulation and incorporated at the rate of 5.6 kg a.i./ha
(ca 4 mg/kg soil) into soil contained in a tub. Sweet corn was planted
in the soil. Plant samples above the soil line were harvested at 28,
56 and 94 days after planting. The 94-day crop was divided into
kernels, cobs, husks and stalks in the laboratory.
In the greenhouse experiment the labelled isofenphos was
incorporated into soil at a rate of 4 mg/kg. The soil was placed in
pots and field corn was planted in it. The crop was harvested at
maturity, 141 days after treatment, and the plants were divided as in
the other experiment. The root samples were washed with water to
remove soil particles.
The compounds identified in the corn stalks were qualitatively
the same throughout the study, although their relative quantities
changed as the plant matured.
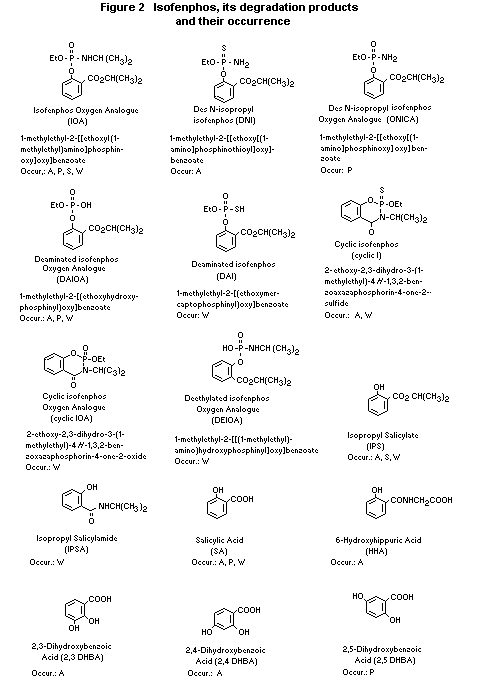
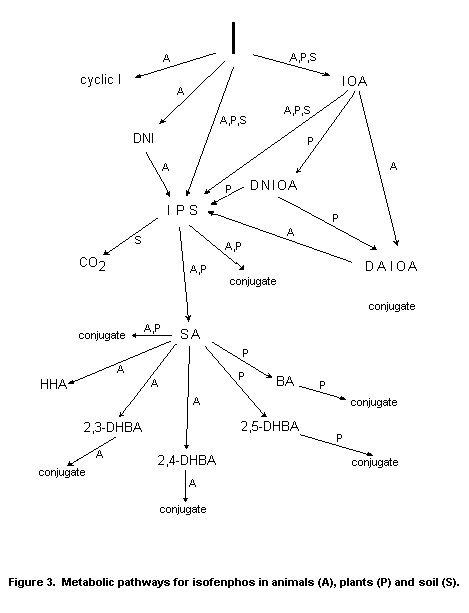 Over 70% of the radioactivity appeared to be non-conjugated and
readily released from the plant matrix of the 28- and 56-day corn
stalk samples by methanol chloroform extraction, while in the 94-day
corn stalks, only 19% of the radioactivity was organosoluble without
hydrolysis. The distribution of radioactivity among isofenphos and its
metabolites in corn stalk grown outdoors is shown in Table 10.
TABLE 10. Distribution of radioactivity among isofenphos and its metabolites inmaize grown
outdoors in ring-µL (14C)-isofenphos-treated soil
Percent Radioactivity Distribution
Organic/Aqueous Water/Solid Fractions
Days after Sample Extract Acid released
Treatment
I IOA DNIOA SA DBHA DAIOA Unk.
28 stalk 19 52 3 nd nd nd nd
56 stalk 8 63 6 <1 12 <1 2
94 stalk 2 15 2 5 35 3 5
cob 0 17 0 nd nd nd nd
The primary organosoluble metabolite was isofenphos oxygen
analogue, but isofenphos and des N-isopropyl isofenphos oxygen
analogue were also detected. Acid hydrolysis of the aqueous and soil
fractions from corn stalk liberated 2,5-dihydroxybenzoic acid (DHBA)
SA and DAIOA.
As the plant matured, DHBA became the predominant metabolite. The
only component identified in the husk and cob samples was IOA.
The small quantity of radioactivity in the organosoluble fraction
of the kernel prevented adequate identifications to be made, but none
of the intact phosphate esters could have been present in an amount
higher than 4% of the kernel's total residue, which accounted for 6.1%
of the activity measured in the whole plant. The concentration of
radioactivity and its distribution in the tissues of mature corn grown
in the greenhouse was similar to that seen in the outdoor study. The
compounds identified were exactly the same.
Bermuda onions were grown in a greenhouse for 60 and 91 days in
soil in which 4 mg/kg ring-µL (14C)-isofenphos had been incorporated.
The harvested plants were divided into tops (above the soil level) and
bulbs (Stanley 1977a). In mature plants, the concentration of residues
increased in the tops and decreased in the bulb, in comparison with
levels measured in 60-day plants, mainly as a result of the changes in
the weight of the plant portions.
Over 84% of the radioactivity in each sample was extracted by
blending and acid hydrolysis. Over 80% of the radioactivity in the
tops and over 50% in the bulbs was organosoluble, indicating the
amount of non-conjugated residues.
The compounds detected in the organosoluble fractions after
blending were isofenphos, IOA and DNIOA. IOA was the major compound in
the tops and the 91-day bulbs, 78-79% and 35% of total activity
respectively, while isofenphos and IOA ratio was 44% and 24% in the
60-day bulbs. DNIOA was present in all samples in small amounts but
was less (<1%) in bulbs and in tops (2-3%) at both intervals. Acid
hydrolysis of the aqueous fraction from the organic extract liberated
salicylic acid (1 to 2%) and an unknown (<1 to 3%). The remaining
radioactivity (6 to 12%) on the TLC plates was at the origin,
indicating very polar compounds, or was present as streaking and not
as discrete spots.
The radioactivity remaining in the solids after organic
extraction constituted about 5 to 8% in tops and 14 to 16% in bulbs of
the total activity of 60 and 91-day samples, respectively. Acid
hydrolysis of the solids released over one half of the radioactivity
remaining in the solids after methanol chloroform extractions. The
radioactivity of solids was not further characterized.
In animals
Pig
The feeding study of a male pig is described previously under
"Biochemical aspects". Four tissues, kidney, liver, muscle and fat
were analysed for isofenphos and its metabolites. The amount of
organosoluble metabolites are given in Table 11.
Enzymatic hydrolysis of neutralized aqueous fractions from kidney
and liver released most of the radioactivity remaining in these
tissues. The major enzyme-released metabolite was IPS. Small amounts
of HHA and DAIOA were also found. Between 30-42% of the kidney and
liver radioactivity extracted after enzyme hydrolysis could not be
identified. Unknown (a) consistuted between 19 and 27% of the total
tissue radioactivity.
TABLE 11. Organosoluble metabolites of isofenphos in pig tissues
Compounds % Radioactivity in tissues on 5th day of feeding
Liver Kidney Muscle Fat
Isofenphos 13 2 52 61
IOA <1 <1 16 10
IPS 1 2 17 15
HHA <1 ND 3 ND
DNI ND ND 5 5
DAIOA 4 1 3 <1
Unknown (a) 9 ND ND ND
Total 30 6 97 92
Cow
Under "Biochemical Aspects", feeding studies are reported in two
lactating dairy cows. In the first animal the only milk sample with
a radioactive residue, >0.01 mg/kg isofenphos equivalents, was
analysed for isofenphos and its metabolites. The 7.5h sample contained
0.014 mg/kg isofenphos equivalents and accounted for 0.07% of the
administered radioactivity. DAIOA and HHA were the main metabolites in
the organosoluble fraction and accounted for 13% and 10% of total
activity in milk, respectively. Small amounts (2 to 4%) of IPS, SA and
several unknowns (12%) were also detected. Enzymatic hydrolysis of the
solvent-extracted, pH 5 milk released an additional 45% of the milk
radioactivity. HHA (27%) was the major constituent in this fraction of
the milk. DAIOA, SA, IPS and several unknowns were also found in small
amounts.
In the second experiment milk was collected prior to each day's
dosing and 8 hours after each dosing. Milk production remained at the
cow's normal levels through the 5 days of the study. The cow was
sacrificed 2 hours after the fifth dose was administered. The
following tissues were collected and analysed, their residue content,
in isofenphos equivalent (mg/kg) were: muscle-loin 0.02, shoulder
0.02, round 0.02; fat-renal 0.07; omental 0.07; subcutaneous 0.06;
kidney 0.53; heart 0.04; liver 0.47; udder 0.05 and brain 0.02.
In kidney and liver tissues, less than 35% of the radioactivity
was organosoluble and consisted of SA 26 to 14%, HHA 3 to 4%, IOA 2 to
1%,, IPS 1% to <1%, respectively. Traces (1%) of unknowns were found
in the liver.
Sulphatase beta-glucuronidase combination was employed in the
enzyme hydrolyses, which released 42 to 45% activity from the kidney
and liver extracts respectively. IPS (7 to 3%), SA (8 to 2%) and HHA
(6 to 8%) were identified in the enzymatic extracts of kidney and
liver.
Several unknowns (20 to 33%) were detected, but a single
compound, unknown (a), accounted for the 90% of activity. The
radioactive residues which were not organosoluble or liberated by
enzyme hydrolysis were not further characterized.
A feeding study was conducted to study the biological effects and
to determine the tissue and milk residues after feeding isofenphos and
its metabolites. The ratio 1:8:1 of isofenphos, IOA and DNIOA in the
mixture was based on plant metabolism studies of isofenphos and should
be representative of field-treated samples. Alfalfa pellets containing
the mixture of compounds at 2, 6 and 20 mg/kg levels were given to
dairy cattle ad libitum for 28 days (Strankowski 1977c). Feed
consumption, body weight and milk production were unaffected by
consumption of the treated feed as compared to the control cow. The
milk of cows fed 20 mg/kg contained detectable residues only in the
range of 0.001 to 0.01 mg/kg of isofenphos equivalents on the 28th day
of feeding. After feeding for 28 days the animals were sacrificed and
the following tissues were taken; liver, kidney, muscle (round, flank
and loin) and fat (omental, renal and subcutaneous). Residues in all
the tissues analysed, except liver and kidney at the 20 mg/kg level,
were below 0.0, mg/kg. The liver and kidney samples contained
residues, in isofenphos equivalents, ranging from 0.01 to 0.02 and
from 0.01 to 0.04 mg/kg, respectively.
Hen
Laying hens were given a single oral dose of ring-µL(14C)-
isofenphos at 2.0 mg/kg (Dupre 1975). Eggs and excreta were collected.
At four sacrifice intervals (6, 24, 48 and 96 h post-treatment) blood,
various organs and tissues were collected from four birds per group.
In eggs, the level of radioactivity reached a maximum level of
0.099 mg/kg with an average of 0.047 mg/kg in isofenphos equivalent at
48 h post-treatment. Approximately 72% and 78% of the radioactivity
was eliminated in the excreta within 24 and 96 h respectively. A
maximum level in the blood plasma of 0.274 mg/kg 14C activity at 6 h
post-treatment fell to 0.009 mg/kg 96 h after treatment; 24 h after
treatment, residue levels in skin, fat, breast, thighs, heart and
gizzard muscle were less than 0.05 mg/kg, while liver and kidney
contained 0.9 mg/kg and 0.68 mg/kg respectively.
In the feeding study in hens described under 'Biochemical
Aspects' residues, expressed as mg/kg isofenphos equivalents, found in
various tissues were: gizzard 17.1, kidney 4.9, liver 3.9, fat 0.83,
skin 0.67, heart 0.42, muscle 0.14, eggs - 51 h 0.25, 48 h 0.06, 24 h
0.032. The distribution of the residues in the organosoluble fractions
of various tissues is given in Table 12.
TABLE 12. Distribution of isofenphos and its metabolites in tissues and eggs
of laying hens
Distribution (%)
Compound Gizzard Kidney Liver Fat Skin Heart Muscle Eggs
Isofenphos 90 14 2 74 53 26 31 37
IDA 0 4 1 4 3 3 4 9
IPS 0 1 1 18 19 10 8 10
DNI 0 0 0 3 3 0 0 0
HHA 0 0 0 0 0 0 5 0
Unknown 0 47 19 0 8 36 28 5
Feed treated with a mixture containing isofenphos, IOA and DNIOA
in the ratio of 1:8:1, at 5, 15, 20 and 150 mg/kg feed was distributed
to laying hens ad libitum for 28 days (Strankowski 1977d). Feed
consumption, body weights and production decreased as the amount of
isofenphos and its metabolites in the feed increased. At sacrifice,
giblets (heart, gizzard, liver), muscle (equal portions of thigh and
breast), fat (equal portions of visceral and subcutaneous) and skin
(without feathers) were collected. No residue was detected in the
muscle at any dose level. Residues in fat, giblets, skin and eggs are
summarized in Table 13.
Fish
Studies on continuous exposure for 28 days of channel catfish to
isofenphos are reported under "Biochemical Aspects".
In soil
Isofenphos degradation under aerobic conditions was investigated
by Minor and Murphy (1977) in sandy loam and silt loam, while the
effects of anaerobic and sterile conditions were studied in sandy
loam. Ring µ-L-(14C) labelled isofenphos was uniformly incorporated
into the soil at a level of 8.7 mg/kg. The extractable residue derived
TABLE 13. Residues in isofenphos equivalents found in tissues and
eggs of laying hens1
Residue (mg/kg)
Dose
Fat Giblet Skin Eggs
50 mg/kg <0.01 (4) <0.01 (2) <0.01 (2) 0.002
0.01 (2) 0.02 0.003
0.08 0.003
0.004
150 mg/kg <0.01 (2) 0.01 <0.01 (2) 0.017
0.01 (2) 0.02 0.03 (2) 0.013
0.04 0.015
0.06 0.003
1 No. of samples in brackets.
from isofenphos decreased with time, while the radioactivity bound to
soil, or lost, increased. Only 10% of the originally applied
radioactivity could be extracted from silt loam at 240 days post-
treatment, whereas 23% of the-radioactivity could still be removed
from the sandy loam one year after traces (<1% of original activity)
of IPS. The concentration of isofenphos decreased continuously with
time, and its half life was found to be 127 days in sandy loam and 59
days in silt loam.
The concentration of isofenphos oxygen analogue reached a maximum
between 60 and 120 days post-treatment. The bound radioactivity
increased to a maximum of approximately 20% and the remainder had
volatilized. The bound radioactivity was distributed among humin,
humic acid and fulvic acid fractions in a fashion similar for both
soils. Even after stringent HCl-NaOH fractionation, approximately 50%
of the bound residues was still unextractable.
The volatile radioactivity from soil was composed of isopropyl
salicylate, 14CO2-isofenphos and cyclic isofenphos. At 134 days
post-treatment, the total volatile radioactivity trapped was
distributed among IPS (67%), 14CO2 (30%), cyclic isofenphos (2%)
and isofenphos (1%) (Minor 1980: The volatile compounds in the
experiments accounted for 16 to 51% of the total activity applied to
soil 134 days before, while the soils contained the remaining part of
activity, indicating the importance of conditions (e.g. temperature
and/or microorganism activity) on degradation rate of isofenphos in
soil. In sandy loam and under anaerobic conditions, isofenphos was not
converted to its oxygen analogue, but some of the radioactivity was
lost. Under sterile conditions in sandy loam, isofenphos was neither
altered nor lost. In isolated soil micro-organisms grown in "shake
culture", isofenphos was not altered. Conversion of isofenphos to its
oxygen analogue appeared to be strictly a chemical reaction occurring
in soil under aerobic conditions. The production of isopropyl
salicylate may also have occurred without microbial activity, but the
isolated 14CO2 was probably the end result of soil micro-organism
cleavage of the isofenphos aromatic ring after the phosphate group had
been removed. Therefore, isofenphos soil degradation appeared to
involve both chemical and microbial alterations, and the chemical
reactions of oxidation and hydrolysis appeared to be prerequisite to
microbial action. Comparison of results from isofenphos treated soil
kept in a greenhouse or maintained outdoors showed that isofenphos
degradation qualitatively is the same under both environments, but the
rate of degradation may vary.
The persistence of isofenphos in soil was investigated in nine
tests set up in various locations in the United States and Canada
(Mobay 1980c). Isofenphos, 6E formulation, was incorporated uniformly
at a rate of 2 mg/kg wet soil (equivalent to 2.24 kg/ha field
application) into the upper 7.6 cm of soils of seven different types.
Samples were taken at 0, 30 to 33, 60 to 61, 89 to 92 and 119 to 124
days post-treatment from 0 to 15 cm and 15 to 30 cm depths. The
residue of isofenphos decreased in all nine studies, and its level in
the upper layer was between 0.11 mg/kg and 0.43 mg/kg 119 to 124 days
after treatment. IOA reached its maximum (0.19 to 0.5 mg/kg) 32 to 123
days post-treatment. Depending on the soil types and possibly on
environmental conditions, the ratio of isofenphos and its oxygen
analogue varied between 0.37 to 14 at the last sampling. Soil samples
from 15 to 30 cm did not contain detectable residues (0.01 mg/kg),
with the exception of rocky sandy silt loam in which 0.02 to 0.15
mg/kg isofenphos or IOA could be detected.
The degradation of isofenphos was studied in the Federal Republic
of Germany (FRG) and in the UK (Bayer 1973-75). The applications were
made with either EC or granular formulation as surface spray or
broadcast at rates of 1, 5 and 7.5 kg a.i./ha. The sampling day varied
from 0 to 534 post-treatment. The results show a pattern similar to
that obtained in the experiments described previously. The results of
laboratory experiments on the persistence of isofenphos carried out in
standard German soil No. 1 and No. 2 (Bayer 1974a) were consistent
with the results of field trials, as were the mobility tests with soil
thin layer chromatography (Thornton 1976) or soil column (Obrist and
Thornton 1977; Bayer 1973-74, 1977b).
The latter experiments indicated that isofenphos and IOA had low
mobility and the majority of the residue remained in the upper 12.5 cm
of the soil, while the leachates contained no intact isofenphos but
did contain a small amount of IOA and traces of IPS (one experiment).
In water
The stability of ring-µL-(14C)-isofenphos in sterile buffer
solutions of various pH's at various temperatures and two
concentrations (1 mg/l and 10 mg/l) was determined (Mc Namara 1977).
Both hydrolysis and volatilization were involved in the dissipation of
isofenphos from the buffered solutions. Isofenphos was not hydrolysed
at temperatures of 20°C and below or at pH's near neutrality, but
volatilization did occur at 20°C and above and increased as the
temperature and isofenphos concentration increased. Hydrolysis of
isofenphos was, however, encountered in both strongly acidic and
alkaline buffers. Hydrolysis products detected in pH3 buffers included
DNI, IOA, DNIOA, IPS, SA. At pH9, the identified hydrolysis products
of isofenphos were IOA, IPS, SA and N-isopropyl salicyclamide. At
37°C, the half life of isofenphos at 1 and 10 mg/kg was 79 and 30 days
respectively, in pH3 buffer, and 88 and 32 days respectively, in pH9
buffer. At 50°C, the half life of isofenphos at 1 and 10 mg/kg was 15
and 9 days respectively, in pH 3 buffers; 34 and 15 days respectively,
in pH 6 buffer; and 6 days for each concentration in pH 9 buffer.
Ring-µL(14C)-isofenphos at an initial concentration of 10 mg/kg
was incubated outdoors in a simulated pond (pH 8) (Mc Namara 1977a).
Within 70 days, 89% of the radioactivity was lost, and the remaining
radioactivity was distributed between water and sediment in an
appropriate 2:1 ratio. The loss of radioactivity was probably due to
volatilization of isofenphos and some of its metabolites. The major
radioactive compounds identified in the pond water were isofenphos,
IOA and cyclic-IOA. Minor components were cyclic isofenphos, IPS and
SA. The amount of radioactivity adsorbed by the pond sediment rose to
20% of the initially applied radioactivity within 7 days, but then
decreased steadily to only 2% at 70 days. The bound radioactivity
never exceeded 2% of the originally applied radioactivity. The major
components in the organosoluble extracts of pond sediment were
isofenphos and IOA. Minor components identified in the sediment were
IPS, SA, cyclic-isofenphos and cyclic-IOA. The overall rate of
isofenphos dissipation was exponential. The half-life of isofenphos
was 13 days. After 70 days, only 2% of the initially applied
isofenphos itself remained in the pond.
In activated sludge
Ring µL-(14C)-isofenphos was studied in a laboratory model of an
activated sewage sludge system (Spare 1979). Activated sludge,
synthetic sewage and (14C)-isofenphos were aerated for 23 h cycle.
During the remaining hour/cycle, the mixture was allowed to settle and
a portion of the supernatant was replaced with fresh synthetic sewage
and an increased amount of (14C)-isofenphos. The concentration of
isofenphos during the first cycle was 0.1 mg/kg and was increased
through 10 cycles to 100 mg/kg.
After the 100 mg/kg cycle concluded, three cycles were carried
out with no addition of test compound. Isofenphos had no detrimental
effect on the bacteria, yeasts or actinmycetes in the system, but at
isofenphos concentrations of 60, 80 and 100 mg/l, protozoa were absent
or few in the treated flasks. The quantity of radioactivity in the
settled solids increased steadily during the course of the study from
10% to 50% of the added radioactivity. (14C)-isofenphos was not
degraded appreciably by the organisms in the activated sludge system.
Greater than 96% of the added (14C)-isofenphos was recovered intact
from the supernatant and solid fractions.
Photodegradation
In aqueous solution
A study of the photodecomposition of ring-uL-(14C)-isofenphos in
aqueous solution was carried out in a photoreactor containing a high
pressure, quartz, mercury-vapour, 200-watt, Hanovia immersion lamp as
the light source (Strankowski 1977e). Decomposition of isofenphos was
slow in the pH 7 solution with only 30% decomposition in 30 days of
continuous exposure to the high intensity light. The isofenphos half-
life was calculated to be 51 days. In an acetone sensitized solution,
the photodecomposition was increased, and the half-life of isofenphos
was reduced to approximately 14 h.
Isofenphos was the only component found in the dark control
solutions from either the unsensitized or sensitized studies. It was
also identified in the photolysis solutions; approximately 75% and 3%
of the total radioactivity remained as isofenphos at the end of the
unsensitized and sensitized studies, respectively.
The major photoproduct, which represented nearly 21% of the total
radioactivity in the sensitized test, was identified by Poje (1979) as
3,3-dimethyl isoindoline-l-one by mass spectroscopy and nuclear
magnetic resonance spectroscopy. This product contains no phosphorous.
The remaining photoproducts, none of which accounted for more than 5%
of the total radioactivity, included IOA, DAI, SA and catechol.
On soil
In a study of the photodegradation of (14C)-isofenphos on soil,
isofenphos had a half-life of 2.6 days when applied to a thin layer of
soil and subjected to light from a 200 watt Hanovia mercury lamp
(Weissenburger and Pollock 1979). Six photoproducts were detected
on the soil. IOA (33% of the applied radioactivity) was the principal
photo-product. Small quantities (approx. 5%) of DNI, DNIOA, des-N-
isopropyl cyclic isofenphos, IPS and phenol were also detected. During
26 days of exposure to the light, 25% of the applied radioactivity was
bound to the soil and 33% was lost through volatility. A trapping
study, carried out for 3 days, showed that the volatile compounds
included isofenphos (91%), DNI (3%), IPS (1%) and phenol (5%). All of
the applied activity was accounted for in the trapping study.
In storage
Stability of isofenphos and its metabolites were studied during
frozen storage (-28°C to -18°C) (Mobay 1980a). Concentration of
isofenphos and IOA did not change significantly in maize kernel,
brassica leafy vegetables, green forage, green beans (pods and vines)
and onions kept deep frozen for 400 to 800 days. However, 24% of
isofenphos decomposed in sugarbeet during 420 days of storage. DNI and
DNIOA residues decreased by 20% add 32% respectively in alfalfa stored
at -23 to -18°C.
Poultry tissues, eggs and giblets, fortified at 1 mg/kg with
isofenphos and its metabolite, were stored frozen for 255, 90 and 60
days respectively without significant changes in concentrations of
residues.
Cattle tissues and milk were fortified at 1.0 mg/kg with
isofenphos and metabolites and placed in frozen storage. Fat and
muscle tissues showed no significant decomposition of the compounds
after 24 to 28 days. Milk showed no significant decomposition of the
compounds after 76 days in frozen storage. In kidney and liver,
isofephos, isofenphos oxygen analogue and des-N-isopropyl isofenphos
were stable for 28 to 35 days. The liver and kidney samples were
extracted within 4 and 8 days, respectively. There was no
decomposition of des-N-isopropyl isofenphos oxygen analogue in kidney
after 10 days of frozen storage; however, it did not disappear
completely in liver after 5 days of frozen storage.
METHODS OF RESIDUE ANALYSIS
A gas chromatographic procedure for the determination of residues
of isofenphos and its oxygen analogue in various crops, soil and water
has been described by Wagner (1976). Plant samples of high water
content and soils are extracted with acetone. The acetone extract is
diluted with water and the residues are partitioned into chloroform or
dichloromethane. Rape, maize and water are extracted with acetonitrile
and chloroform respectively. The concentrated extracts are cleaned on
neutral alumina or on active carbon columns. The residues in the
concentrated extracts are separated on DC-200, QF-1 or OV-17 columns
and detected with a thermionic detector. Recovery data from
experiments run on a large variety of crops by adding known amounts of
isofenphos and its oxygen analogue at the blending step were generally
in the 77% to 105% range. The limit of determination is 0.005 mg/kg
for each compound in plant and soil samples and 0.002 mg/kg in water.
Gas chromatographic methods for the determination of residues of
isofenphos, isofenphos oxygen analogue, des-N-isopropyl isofenphos and
des-N-isopropyl isofenphos oxygen analogue in maize cob, forage, husk
and kernel and in onions are described by Stanley (1977b, 1979a) and
in bovine and poultry tissues, milk and eggs by Shaw II (1977a). For
all four compounds, recoveries were 65 to 70%. The limit of
determination for each compound is 0.01 mg/kg, except for milk and
eggs (0.001 mg/kg). Multiplication factors for converting metabolite
residues to isofenphos equivalents are 1.05 for IOA, 1.139 for DNI and
1.204 for DNIOA.
Interference studies for the determination of residues of
isofenphos and its metabolites as maize and onions were done by
Stanley (1977c, 1979b) and in milk, animal tissues and eggs by Shaw II
(1977b, 1979). All possible interferences by 20 or 27 organophosphate
pesticides registered for use on maize and onions or investigated in
animal products respectively were separated from isofenphos and its
metabolites by GLC on the standard (10% DC-200 + 2% OV-225 on
Chromosorb WHP) or on the confirmatory (5% OV 210 on Supelcoport or 5%
OV 275 on Chromosorb WHP) columns or by clean-up on the silica gel
column used in the residue method. The silica gel column was applied
as follows: 372 g silica gel is deactivated with 28 cm3 water, 10 g
is filled into the column under hexane and settled, and a 1-cm layer
of granular Na2SO4 is placed on top of the column.
Hexane is drained to the top of the sodium sulphate. The
concentrated extract is transferred in the column with 5 to 10 cm3
portions of hexane and finally the column is washed with hexane (total
amount-50 cm3). The portions of hexane is drained through the column
at a rate of 5 to 10 cm3 until 50 cm3 is collected. Eluents (freshly
prepared) and fractions are: (1) 185 cm3 hexane-dichloromethane (1:1)
(isofenphos); (2) 150 cm3 dichloromethane (DNI); (3) 65 cm3
dichloromethane-methanol (99:1) (discarded); (4) 135 cm3
dichloromethane-methanol (99:1) (IOA).
Shaw II (1977b, 1979) applied different conditions: 12 g silica
gel and 6 g Na2SO4; column is washed with 40 cm3 hexane, 100 cm3
hexane-benzene (8:2) 75 cm3 hexane-benzene (6:4); elution: (1)
fraction (isofenphos) 25 cm3 hexane-benzene (6:4) + 100 cm3 hexane-
benzene (4:6) + 100 cm3 hexane-benzene (2:8) + 50 cm benzene; (2)
fraction (IOA + DNI) 50 cm3 benzene + 300 cm3 benzene-acetone (8:2).
A gas chromatographic method for the determination of isofenphos
and isofenphos oxygen analogue in soil and water, using an alkaline
thermionic emission detector, is described by Shaw II (1974).
Recoveries of isofenphos and its oxygen analogue were 93 to 101%; the
sensitivity of the method is approximately 0.005 mg/kg for soil and
0.002 mg/kg for water samples.
The determination of isofenphos and its oxygen analogue residues
in aged soil by alkaline thermionic emission gas chromatography was
investigated by Shaw II (1977c). The soil was extracted in a Soxhlet
apparatus for 16 to 24h with chloroform/methanol (7:3). The extract
was concentrated, partitioned with hexane/acetonitrile (1:1) and
partially purified with three hexane extractions of the residue in a
methanol/water solution. Isofenphos recoveries were >79% and
isofenphos oxygen analogue recoveries were >95% from soils fortified
at 0.05 mg/kg. The sensitivity of this method is 0.01 mg/kg.
Isofenphos and IOA were determined by Brown and Williams (1976)
in cabbage, potato, rapeseed and soil. The rapeseed was extracted with
acetonitrile and cabbage, potato and soil with ethylacetate. The
concentrated extracts were cleaned on a column consisting of the
mixtures of Florisil, silica gel, alumina and Nuchar C. The cleaned-up
extracts were separated on DEGS or OV275 liquid phases and detected
with FPD. The recoveries at 0.01 to 1 mg/kg levels were in the range
of 84 to 98%. The limits of determination were 0.003 mg/kg and 0.005
mg/kg for isofenphos and IOA respectively in potato, rapeseed and
soil, while they were five times higher in cabbage.
The methods described by Wagner (1976) or by Brown and Williams
(1976), in combination with the confirmatory columns and GLC
separation (Stanley 1977b, 1979a), are recommended for regulatory
purposes.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Isofenphos is registered in the following countries: Austria,
Bulgaria, Chile, Denmark, Federal Republic of Germany, German
Democratic Republic, Indonesia, Israel, Italy, Morocco, Mexico,
Norway, The Netherlands, South Africa, Spain, Sweden and the USA.
Maximum residue limits and preharvest intervals were reported
from some of the countries and are summarized in Table 14.
EVALUATIONS
COMMENTS AND APPRAISAL
In the mammalian species tested, the oral LD50 ranged from about
20 mg/kg bw in rats to approximately 150 mg/kg bw in rabbits. In the
rat, the oxygen analogue of isofenphos was shown to be slightly, but
not substantially, more toxic orally than isofenphos, both acutely and
subacutely.
TABLE 14. National MRLs reported to the Meeting
Preharvest
Country Crop/Commodity intervals MRL
(days) (mg/kg)
Fed. Rep. of Leafy and stem
Germany vegetables 0.1
Rapeseed 0.05
Italy Pear 42 0.1
Fruit 0.1
Vegetables 0.1
Sugarbeet 0.1
Netherlands Cauliflower 56 0.1
Cabbages (including
Brussels sprouts) 56 0.1
Onions 0.1
Celery 0.05
Celeriac 0.05
Norway Root, tuber and
bulb vegetables 90
Brassica leafy vegetables Apply not later
than transplanting
South Africa Citrus fruit 180 0.2
Spain Cabbage Apply before sowing
Onions 21
Garlic 21
All crops (except
root vegetables) Apply before sowing
and potato)
USA Maize 75
Corn, forage 1.0
Corn, fodder 1.0
Corn, fresh (including
sweet) 0.1
Corn, grain 0.1
Meat, fat, meat by-
products of cattle,
sheep and poultry
Milk 0.02
Eggs 0.02
Metabolism studies in rats, pigs and cows, treated orally with
ring-µL(14C)-isofenphos indicated that the compound is rapidly
eliminated in both faeces and urine, with the latter being the
predominant route of excretion. It is metabolized mainly by oxidative
desulphuration, dearylation, hydrolysis, deamination and conjugation.
There is no indication that isofenphos and/or its metabolites
accumulate in mammalian tissue. The excretion pattern and proposed
metabolic pathways for isofenphos appear to be similar in the three
mammalian species studied.
A 3-generation reproduction study in rats (with 2 litters in the
first generation (F0) but only one litter each in the second (F1)
and third (F2) generations showed a marginal effect in the F0
generation at the second mating on the pregnancy rate, even at 1 ppm,
the lowest tested level. No indication of teratogenic effect was noted
in a rabbit teratology study at dosages as high as 5 mg/kg bw.
Teratology studies in rats exposed orally, dermally or via inhalation
were all negative.
In vitro microbial assays, including reversion-test and rec-assay
and a dominant lethal study in mice, failed to give any evidency of
mutagenicity. Long-term studies in mice and rats revealed no
carcinogenic activity. An acute delayed neurotoxicity study in hens,
which could be considered as a screen, was negative. The acute oral
toxicity of isofenphos was found to be potentiated by malathion but
not by a number of other organophosphorus insecticides tested.
Plasma cholinesterase inhibition was observed as the most
sensitive indicator of toxicity in the short-term rat studies and the
108-week mouse and 2-year rat feeding studies. A dietary level of
1 ppm was a marginal no-effect level in the rat and a no-effect level
in the mouse with respect to plasma cholinesterase. In the dog, a
90-day study suggested that dietary levels of 10 ppm and above caused
significant plasma cholinesterase depression and an increase in liver
weight, although the latter was not accompanied by histopathological
alteration of the tissue. A 2-year feeding study in the same species
demonstrated 2 ppm to be the no-effect level, based on plasma
cholinesterase. The information provided on observations in humans was
very scanty.
Acceptable data was available to permit the establishment of no-
effect levels in three mammalian species. Owing to the unavailability
of an appropriate neurotoxicity study in hens, only a temporary ADI,
was allocated.
Isofenphos, O-ethyl O-2-isopropoxycarbonylphenyl
isopropylphosphoramidothioate, is an organophosphorus insecticide
applied mainly in soil at a rate of 1.5 to 2.5 kg a.i./ha or 1 to 5 kg
a.i./ha for row or overall surface treatment, respectively.
Supervised trials were carried out on various crops at different
locations in the United States, Canada and Europe. Isofenphos and its
main metabolites, isofenphos oxygen analogue (IOA), des-N-isopropyl
isofenphos (DNI) and des-N-isopropyl isofenphos oxygen analogue
(DNIOA), were analysed. It was found that the residue consisted mainly
of isofenphos and IOA. The residues measured in brassica leafy
vegetables, rapeseed and root and tuber vegetables were below
0.1 mg/kg and usually at or about the limit of determination after the
recommended pre-harvest intervals. No residues were detected in maize
kernels, either in the milk or dry stages, while residues up to
2 mg/kg were found in green forage 3 to 4 weeks after application. The
total residue detected in mature onion bulbs was always below 1 mg/kg.
Rotational crops can also take up residues from previous soil
treatments. The residues, consisting mainly of isofenphos and IOA,
were at or about the limit of determination in cereal grains, leafy
and root vegetables and edible oil crops grown in soil treated with
isofenphos 9 or more months earlier, The residue level might exceed
1 mg/kg in green wheat forage but it is under 0.1 mg/kg in the straw
at the time of harvest.
Isofenphos is degraded in soil, plants and animals, but its route
of degradation in soil and plants is somewhat different from that in
animals. Oxidation seems to be the first reaction in soil and plants.
In animals, hydrolysis and oxidation both appear to be of major
importance. The degradation of isofenphos was not rapid in any of the
systems studied. Soil micro-organisms seem capable of splitting the
aromatic ring to produce CO2 but only after the phosphoramidothioate
rouping has been removed from the molecule. In plants, oxidation is
followed by depropylation, hydrolysis and conjugation. IOA and
isofenphos are the primary residues but DNIOA also appears in smaller
amounts. The approximate ratio of isofenphos, IOA and DNIOA in mature
maize stalks was 1:8:1. 2,5-Dihydroxybenzoic acid (DHBA), salicylic
acid (SA) and deaminated isofenphos oxygen analogue (DAIOA) appear in
traces.
In animals, isofenphos undergoes hydrolysis, oxidation,
N-depropylation, deamination and conjugation. Excretion is rapid
in orally dosed animals. Pigs, cows and hens excreted 81%, 79% and
72% of the applied dose in the urine or excreta within the first
24h after treatment. Urine contained isopropyl salicylate (IPA),
O-hydroxyhippuric acid (HHA) SA and DAIOA in non-conjugated and/or
conjugated forms; 4 to 5% of the administered dose is eliminated in
the faeces over a 72 to 80h period.
During a period of multiple dosing for 6 consecutive days at a
rate of 15 mg/kg/day, rats were able to eliminate residues of
isofenphos fast enough to maintain a relatively constant level of
radioactivity in the tissues. One day after cessation of the
isofenphos treatment a 10-fold decrease in radioactive residue
concentration was detected in the muscle, fat and liver. Dairy cows
and laying hens were kept on feed containing residues of isofenphos,
IOA and DNIOA in the ratio of 1:8:1 at various levels between 2 and
20 mg/kg feed and 5 and 150 mg/kg respectively for 28 days. Residues
in all the bovine tissues, except liver and kidney at the 20 mg/kg
level, were <0.01 mg/kg. The liver and kidney samples collected from
the 20 mg/kg group contained residues of isofenphos ranging from
<0.01 to 0.02 mg/kg and 0.01 to 0.04 mg/kg respectively. Residues of
isofenphos equivalents in milk from all samples, except the 20 mg/kg
group, were below 0.001 mg/kg. The milk samples collected on the 28th
day from the high level group contained residues ranging from 0.001 to
0.01 mg/kg.
Isofenphos residues in poultry tissues and eggs from the control
and 5 mg/kg groups were below the limits of determination of the
analytical method (0.01 mg/kg for tissues, 0.001 mg/kg for eggs). The
hens fed at the 15 mg/kg level had no detectable residues in the
tissues and had residues ranging from <0.001 to 0.002 mg/kg in the
eggs. In the 50 mg/kg group, muscle and fat samples had no measurable
residues, while giblets and skin samples had residues ranging from
0.002 to 0.004 mg/kg. Residues were detected in all tissues, except
muscle, and ranged from <0.01 to 0.05 mg/kg and in eggs from 0.003 to
0.017 mg/kg at the exaggerated 150 mg/kg dietary level. Control values
were <0.01 mg/kg in tissues and <0.001 mg/kg in eggs. No detectable
residues can be expected in products derived from animals fed with
forage that has been treated with isofenphos at recommended
application rates.
Catfish exposed continuously to isofenphos for 28 days in water
containing 10 mg/l isofenphos accumulated residues of 0.75 mg/kg,
consisting entirely of intact isofenphos, within the first 4 days of
exposure. However 87% of this was eliminated within the first day
after transfer of the catfish to uncontaminated water.
Isofenphos is readily absorbed by soil. It is only slowly leached
in all the soils tested, but its degradation products move somewhat
more rapidly. In soil persistence studies with isofenphos incorporated
into the soil at the single-application field-use rate, the residue of
isofenphos itself declined steadily in all soils tested, and the total
organophosphorus residue decreased to half the initial concentration
within 45 to 200 days.
Hydrolysis of isofenphos occurs at acidic and alkaline pH, but it
is stable near neutral pH.
In an activated sludge system, isofenphos has no detrimental
effect on the sludge organisms, and the parent compound was not
degraded.
Photodegradation of isofenphos in aqueous solution is slow and
yields principally a re-arrangement product, 3,3-dimethylisoindoline-
l-one. On soil, photodegradation is rapid and the main product is IOA.
Residue analytical methods for the determination of isofenphos
and IOA, the major residue component, and for DNI and DNIOA are
available. Recoveries are in the range of 77 to 105%.
The limits of determination for isofenphos and IOA in plant
samples, animal tissues, and milk and eggs are 0.005 - 0.01, 0.01 and
0.001 mg/kg respectively, in water 0.002 and in soil 0.01 mg/kg.
Level causing no toxicological effect
Mouse : 1 ppm in the diet equivalent to 0.15 mg/kg bw/day
Rat : 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog : 2 ppm in the diet equivalent to 0.05 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The Meeting estimated the maximum residue levels likely to occur
in various commodities and concluded that they were suitable for
establishing temporary MRLs.
The limits refer to the sum of isofenphos and its oxygen
analogue.
Preharvest intervals (days)
Commodity Limit (mg/kg) on which recommendations
are based
Celeriac 0.02 * 150
Swedes 0.02 * 90
Turnips 0.02 * 90
Brassica leafy vegetables 0.1 60
Celery 0.02 * 90
Maize 0.02 * 90
Maize, fodder 0.5 90
(dry)
Sweet corn 0.02 * 90
Sweet corn fodder (dry) 0.5
Rapeseed 0.02 * 90
Carcass meat 0.02 *
Animal fats 0.02 *
Meat by-products 0.02 *
Cattle milk 0.01 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
An appropriate neurotoxicity study in hens.
Desirable
1. 2-generation (2 litters/generation) reproduction study.
2. In vitro biochemical studies on purified isofenphos with respect
to anticholesterase activity.
3. Further observations in humans.
4. Additional data from supervised trials on potatoes and onions,
including information on soil residues and soil moisture content.
REFERENCES
Bagnall, B.H. Tests to monitor the blood cholinesterase levels of
1976 workers using oftanol granules on carrots. Report from Bayer
UK Limited, Agrochem. Division, submitted by Bayer Ag.
(Unpublished)
Bayer Residue Reports Nos. 4003-4005/74, 4000-4002/77 (Cabbage,
1973-74 savoy), 73-75/73, 4064-/74 (Cabbage, white), 45-47173,
1977a 4000-4002/74, 4003-4005/77 (Cauliflower). (Unpublished).
1974-76 Residue reports nos. 4057/74, 4014-4015/75, 4006-4008/76
(Sweden), 4016/75, 4023-4024/76, (Turnips). (Unpublished)
1972-73 Residue Reports nos. 132/72, 99-101/73, 4003-4004/75,
1975, 4024/78, 4039-4040/78 (Onions). (Unpublished)
1978
1973-75 Residue Reports nos. 214-216/73, 336/73, 4035-4037/74 4031
a-c/75 (Soil, field). (Unpublished)
1973-74 Residue reports nos. 267-268/73, 4038-4040/74, 4043/74,
1977b 4046-4048/74, 4017/74 (Leaching exp.). (Unpublished)
1973 Residue Report no. 105/73 (Potatoes). (Unpublished)
1974 Residue Reports nos. 4032-4034/74, 403211-403411/74,
4049-4051/74, 4049/1-4051/1/74 (Rapes). (Unpublished)
1974 Residue Reports nos. 4044/II.- 4045/74, 4061/74 (Soil,
laboratory). (Unpublished)
1978 Residue Reports nos. 4000-4001/78 (Brussels sprouts).
(Unpublished)
1980 Residue Reports nos. 5001/80, 5002/80, 5202/80 (Potatoes).
(Unpublished)
Bomhard, E. and Löser, E. SRA 12869 chronic toxicity study on rats
1977 (two-year feeding experiment) Report from Bayer AG Institut
Für Toxikologie, submitted by Bayer AG. (Unpublished)
Brown, M.J. and Williams, J.H, Determination of residues of isofenphos
1976 and its phosphoramidate analogue. Pesticide Science,
7: 545-548.
Brune, H., Deutsch-Wenzel, R., Schüman, K. SRA 12869 chronic toxicity
1978 study on NMRI mice (108-week feeding experiment). Report
from Biologisches Laboratorium, Hamburg, submitted by Bayer
AG. (Unpublished)
Cherry, C.P., Newman, A.J. and Urwin, C. Pathology report of SRA 12869
1972 - Acute neurotoxicity experiment in hens. Report from
Huntingdon Research Centre, UK, submitted by Bayer AG.
(Unpublished)
Dupre, G.D. Metabolism and excretion of 14C-labelled SPA 12869 in
1975 poultry. Mobay Report No. 44931. (Unpublished)
Flucke, W. Oftanol bestimmung der akuten toxizität (LD50). Reports
1980a dated 25 July, 29 August, 15 October and 8 December, 1980
from Bayer AG submitted by Bayer AG. (Unpublished)
1980b SRA 12869 Bestimmung der akuten toxizität (LD50). Ratte
p.o. Report dated 7 Nov. 1980 from Bayer AG, submitted by
Bayer AG. (Unpublished)
1981 Oftanol bestimmung der akuten toxizität (LD50). Report
dated 16 February 1981 from Bayer AG, submitted by Bayer AG.
(Unpublished)
Hoffman, K. and Kaliner, G. SPA 12869 chronic toxicity study on dogs
1977 (two-year feeding experiment). Report from Bayer AG Institut
Fur Toxicologie submitted by Bayer AG. (Unpublished)
Inukai, H. and Iyatomi. SRA 12869 mutagenicity test on bacterial
1977 systems. Report from Nitokuno Agricultural Chemicals
Institute, Laboratory of Toxicology, Japan, submitted by
Bayer AG. (Unpublished)
Kimmerle, G. SRA 12869 acute neurotoxicity studies on hens. Report
1972a from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b Kutane toxizitat von Parathion an ratten vesgleich
verschiedener methoden. Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
1976 SPA 12869 acute inhalation toxicity study on rats. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Kimmerle G. and Gröning. SRA 12869 study to evaluate atropine and
1975 oxines for antidotal effect on rats. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Kurtz, S.K. The assimilation and metabolism of 14C Oftanol by
1977 rotational crops. Mobay Report No. 53826. (Unpublished)
Kurtz, S.K. and Shaw II, H.R. Poultry metabolism of (14C) Oftanol.
1977 Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Lamb, D.W., Matzkanin, C.S. and Burke, M.A. Acute oral toxicity of
1977 Oftanol technical. Report from Mobay N. 54000, Ref. 76-282,
submitted by Bayer AG. (Unpublished)
Lamb, D.W. and Burke, M.A. Dietary toxicity of Oftanol technical to
1977 mallard ducks. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 Acute oral toxicity of AmazeTM technical to bobwhite
quail. Report from Mobay Chemical Corp., submitted by Bayer
AG. (Unpublished)
Löser, E. SRA 12869 subchronic toxicity study on rats (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Löser, E. SRA 12869 subacute toxicity study on rats (four-week feeding
1978 experiment). Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Machemer, L. SRA 12869 studies for embryotoxic and teratogenic effects
1972 on rats following oral administration. Report submitted by
Bayer AG. (Unpublished)
1973 SRA 12869 dominant lethal test on the male mouse to test for
mutagenic effect. Report from Bayer AG, submitted by Bayer
AG. (Unpublished)
1975 SRA 12869 studies of embryotoxic and teratogenic effects on
rabbits following oral administration. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
McNamara, F.T. Dissipation of 14C-Oftanol in buffered aqueous
1977a solutions. Mobay Report No. 53617. (Unpublished)
1977b Dissipation of 14C-Oftanol in pond water containing soil.
Mobay Report No. 53618. (Unpublished)
Minor R.G. Identification of volatile isofenphos soil metabolites.
1980 Mobay Report No. 68658. (Unpublished)
Minor R.G. and Murphy, J.J. Metabolism of 14C-Oftanol in soil. Mobay
1977 Report No. 53643. (Unpublished)
Mobay Residue Reports nos. 53819, 53888, 65932, 67505, 67508,
1979-80 68461-68463 (Corn). (Unpublished)
1978a Residue Reports nos. 53820, 53857-53859, 65931, 65933,
65935-65937, 53817, 5382353825, 53853, 65637, 65923, 65924,
65926, 65927 (Sweet corn). (Unpublished)
1978b Residue Reports nos. 65479-65481, 65491-65493, 65657, 65468,
65477, 65484-65487, 65636, 65656, 67394-67395 (Onions).
(Unpublished)
1980a Residue Reports nos. 69025-69027 (Rapes). (Unpublished)
1980b Residue Reports nos. 53620, 53649, 53695, 53698, 53895,
67378 (Stability studies). (Unpublished)
1980c Residue Reports nos. 69101, 69106, 69111, 69128, 69129,
69130, 69133, 69147, 69148 (Degradation in soil).
(Unpublished)
1980d Residue Reports nos. 69101, 60103-69106, 69107, 69-108,
69110, 69111, 69127 69134-69136, 69144, 69145, 69204-69206,
69128-69130, 69133, 69147, 69148 (Residues in rotational
crops). (Unpublished)
Mürmann, P. SRA 12869 subchronic toxicity study on dogs (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Nelson, D.L. and Burke, M.A. Acute dermal toxicity of Oftanol
1977a technical. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG. (Unpublished)
1977b Acute oral toxicity of Oftanol technical to mallard ducks.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
1977c Dietary toxicity of Oftanol technical to bobwhite quail.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Roney, D.J. Accumulation and persistence of residues
1977 in channel catfish exposed to Oftanol-14C. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Anderson, R.H. The skin-sensitizing properties of
1977 Oftanol technical to guinea pigs. Report from
Pharmakologisches Institut der Universität Mainz, submitted
by Bayer AG. (Unpublished)
Netherlands. Information from the Netherlands on pesticides included
1981 in the JMPR priority list.
Obrist, J.J. and Thornton, J.S. Leaching characteristics of aged
1977 Oftanol soil residues. Mobay Report No. 53944. (Unpublished)
Oesch, F. Ames Test for Oftanol (isofenphos). Report from Chemagro
1977 Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Palmer, A.K., Killick, M.E. and Allen, T.R. Effect of SPA 12869 on
1977 reproductive function of multiple generations in the rat.
Report from Huntingdon Research Centre, UK, submitted by
Bayer AG. (Unpublished)
Poje, A.J. AMAZE photoproduct identification. Mobay Report No. 68146.
1979 (Unpublished)
Ross, D.B., Cameron, D.M. and Roberts, N.L. The acute oral toxicity
1976 (LD50) of isofenphos to the starling. Report from
Huntingdon Research Centre, England, submitted by Bayer AG.
(Unpublished)
Schlüter, G. SRA 12869 Untersuchungen auf embryotoxische und
1981 teratogene wirkungen nach cutaner verabreichung an der
ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Schlüter, D. and Thyssen, J. SRA 12869 untersuchungen auf
1981 embryotoxische und teratogene wirkungen nach inhalation an
der ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Shaw II, H.R. Alkaline emission gas-chromatography method for
1974 determining BAY 92 114 and HOL 0654 in soil and water. Mobay
Report no. 43 072. 1 Oct. (Unpublished)
1977a A gas-chromatographic method for Oftanol, Oftanol oxygen
analogue, des N-isopropyl Oftanol and des-N-isopropyl
Oftanol oxygen analogue in bovine and poultry tissues, milk
and eggs. Mobay Report no. 52 769. (Unpublished)
1977b An interference study for the residue method of Oftanol,
Oftanol oxygen analogue, des-N-isopropyl Oftanol and des
N-isopropyl Oftanol oxygen analogue in bovine and poultry
tissues, milk and eggs. Mobay Report no. 53 512.
(Unpublished)
1977c Gas-chromatographic method for residues of Oftanol and
Oftanol oxygen analogue in soils. Mobay Report no. 53 690.
(Unpublished)
1979 Supplementary methods for eliminating interferences from
Delnav, malathion and Ronnel in the residue methods for
AMAZETM in animal tissues, milk and eggs, Mobay Report
no. 67 652, 23 May 1979. (Unpublished)
Spare, W.C. 14C-AMAZETM activated sludge metabolism. Mobay Report no.
1979 68 201. (Unpublished)
Stanley, C.W., Minor, R.G. and Murphy, I.J. Metabolism of OFTANOLR in
1977 corn. Mobay Report No. 53 110. (Unpublished)
Stanley, C.W. Metabolism of OFTANOLR in onions. Mobay Report no.
1977a 54 043. (Unpublished)
1977b Gas-chromatographic method for residues of OFTANOL and its
metabolites in corn and onions. Mobay Report no. 53 111.
(Unpublished)
1977c An interference study for the residue method for OFTANOL and
its organophosphate metabolites in corn and onions. Mobay
Report no. 53 514, 5 July. (Unpublished)
Stanley, C.W. Gas-chromatographic method for residues of AMAZETM and
1979a its metabolites in corn. Mobay Report no. 67 530.
(Unpublished)
1979b Supplementary methods for eliminating interferences of
diazinon, Di-syston and phorate with AMAZETM residues in
the residues method for crops. Mobay Report no. 67 651.
(Unpublished)
Shaw, H.R. Strankowski, K.J. and Murphy, J.J. Excretion and metabolism
1977 of BAY SRA 12869 by rats. Report from Chemagro Agricultural
Division, Mobay Chemical Corp., submitted by Bayer AG.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, K. Isofenphos mutagenicity test
1980 on bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted by Bayer
AG. (Unpublished)
Solmecke, B. and Kimmerle, G. SRA 12869 subacute toxicity studies.
1972a Report from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b SRA 12869 special toxicological studies. Report from Bayer
AG, submitted by Bayer AG. (Unpublished)
1972c SRA 12869 acute toxicological studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J. Effects of feeding ROftanol/Oftanol/oxygen analogue,
1977 des-N-isopropyl Oftanol oxygen analogue (1:8:1) to chickens
for 28 days. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 AMAZETM octanol/water partition coefficient Mobay Report
no. 68 246. (Unpublished)
1980 AMAZETM water solubility. Mobay Report no. 69 175.
(Unpublished)
Strankowski, K.J. and Murphy, J.J. Metabolism and excretion of (14C)
1977a Oftanol by a lactating dairy cow. Report from Chemical
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
1977b Metabolites of (14C) ROftanol identified in dairy cow
tissues. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG.; (Unpublished)
1977c Photodegradation of 14C-Oftanol in aqueous solution. Mobay
Report no. 53 939 (Unpublished)
Strankowski, K.J., Shaw II, H.R. and Murphy, J.H. Metabolites of (14C)
1977a ROftanol identified in rat tissues. Report from Chemagro
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Strankowski, K.J., Minor, R.G. and Murphy, J.J. Excretion and
1977b metabolism of (14C) ROftanol in a pig. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J., McNamara, F.T. and Minor, R.G. Effects of feeding
1977c ROftanol/Oftanol oxygen analogue,des-N-Isopropyl Oftanol
Oxygen analogue (1:8:1) to dairy cattle for 28 days. Report
from Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Thomson, C. and Newman, A.J. Pathology report of Bayer SRA 12869 sub-
1973 chronic toxicity experiment in dogs (13 weeks administration
in the diet). Report from Hungtindon Research Centre,
England, submitted by Bayer AG. (Unpublished)
Thornton, J.S., Hurley, J.B. and Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticide chemicals. Mobay Report no. 51 016.
(Unpublished)
Thyssen, J. HOL 0654 special toxicological studies. Report from Bayer
1974a AG, submitted by Bayer AG. (Unpublished)
1974b HOL 0654 subacute toxicity studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1974c HOL 0654 akute toxizität bei hühnern. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isofephos or phoxim. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1977 Toxikologische untersuchungen bei gleichzeitiger applikation
von SRA 7502 und SRA 12869. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
1978 SRA 12869 Acute toxicity studies on hens and quail. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Thyssen, J. and Kaliner, G. SRA 12869 subacute dermal cumulative
1977 toxicity study on rabbits. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
Thyssen, J. and Kimmerle, G. HOL 0654 acute toxicity studies. Report
1974 from Bayer, AG, submitted by Bayer AG. (Unpublished)
Urwin, C. and Newman, A.J. Pathology report of SRA 12869 3-month rat
1973 study. Report from Huntingdon Research Centre, UK, submitted
by Bayer AG. (Unpublished)
Wagner, K. Method for the gas-chromatographic determination of
1976 OftanolR residues in plants soil and water. Pflanzenshutz-
Nachrichten Bayer, 29: 67 - 80.
Weissenburger, B. and Pollock, R.J. Photodegradation of 14C AMAZE on a
1979 soil surface Mobay Report no. 68 245. (Unpublished)
Over 70% of the radioactivity appeared to be non-conjugated and
readily released from the plant matrix of the 28- and 56-day corn
stalk samples by methanol chloroform extraction, while in the 94-day
corn stalks, only 19% of the radioactivity was organosoluble without
hydrolysis. The distribution of radioactivity among isofenphos and its
metabolites in corn stalk grown outdoors is shown in Table 10.
TABLE 10. Distribution of radioactivity among isofenphos and its metabolites inmaize grown
outdoors in ring-µL (14C)-isofenphos-treated soil
Percent Radioactivity Distribution
Organic/Aqueous Water/Solid Fractions
Days after Sample Extract Acid released
Treatment
I IOA DNIOA SA DBHA DAIOA Unk.
28 stalk 19 52 3 nd nd nd nd
56 stalk 8 63 6 <1 12 <1 2
94 stalk 2 15 2 5 35 3 5
cob 0 17 0 nd nd nd nd
The primary organosoluble metabolite was isofenphos oxygen
analogue, but isofenphos and des N-isopropyl isofenphos oxygen
analogue were also detected. Acid hydrolysis of the aqueous and soil
fractions from corn stalk liberated 2,5-dihydroxybenzoic acid (DHBA)
SA and DAIOA.
As the plant matured, DHBA became the predominant metabolite. The
only component identified in the husk and cob samples was IOA.
The small quantity of radioactivity in the organosoluble fraction
of the kernel prevented adequate identifications to be made, but none
of the intact phosphate esters could have been present in an amount
higher than 4% of the kernel's total residue, which accounted for 6.1%
of the activity measured in the whole plant. The concentration of
radioactivity and its distribution in the tissues of mature corn grown
in the greenhouse was similar to that seen in the outdoor study. The
compounds identified were exactly the same.
Bermuda onions were grown in a greenhouse for 60 and 91 days in
soil in which 4 mg/kg ring-µL (14C)-isofenphos had been incorporated.
The harvested plants were divided into tops (above the soil level) and
bulbs (Stanley 1977a). In mature plants, the concentration of residues
increased in the tops and decreased in the bulb, in comparison with
levels measured in 60-day plants, mainly as a result of the changes in
the weight of the plant portions.
Over 84% of the radioactivity in each sample was extracted by
blending and acid hydrolysis. Over 80% of the radioactivity in the
tops and over 50% in the bulbs was organosoluble, indicating the
amount of non-conjugated residues.
The compounds detected in the organosoluble fractions after
blending were isofenphos, IOA and DNIOA. IOA was the major compound in
the tops and the 91-day bulbs, 78-79% and 35% of total activity
respectively, while isofenphos and IOA ratio was 44% and 24% in the
60-day bulbs. DNIOA was present in all samples in small amounts but
was less (<1%) in bulbs and in tops (2-3%) at both intervals. Acid
hydrolysis of the aqueous fraction from the organic extract liberated
salicylic acid (1 to 2%) and an unknown (<1 to 3%). The remaining
radioactivity (6 to 12%) on the TLC plates was at the origin,
indicating very polar compounds, or was present as streaking and not
as discrete spots.
The radioactivity remaining in the solids after organic
extraction constituted about 5 to 8% in tops and 14 to 16% in bulbs of
the total activity of 60 and 91-day samples, respectively. Acid
hydrolysis of the solids released over one half of the radioactivity
remaining in the solids after methanol chloroform extractions. The
radioactivity of solids was not further characterized.
In animals
Pig
The feeding study of a male pig is described previously under
"Biochemical aspects". Four tissues, kidney, liver, muscle and fat
were analysed for isofenphos and its metabolites. The amount of
organosoluble metabolites are given in Table 11.
Enzymatic hydrolysis of neutralized aqueous fractions from kidney
and liver released most of the radioactivity remaining in these
tissues. The major enzyme-released metabolite was IPS. Small amounts
of HHA and DAIOA were also found. Between 30-42% of the kidney and
liver radioactivity extracted after enzyme hydrolysis could not be
identified. Unknown (a) consistuted between 19 and 27% of the total
tissue radioactivity.
TABLE 11. Organosoluble metabolites of isofenphos in pig tissues
Compounds % Radioactivity in tissues on 5th day of feeding
Liver Kidney Muscle Fat
Isofenphos 13 2 52 61
IOA <1 <1 16 10
IPS 1 2 17 15
HHA <1 ND 3 ND
DNI ND ND 5 5
DAIOA 4 1 3 <1
Unknown (a) 9 ND ND ND
Total 30 6 97 92
Cow
Under "Biochemical Aspects", feeding studies are reported in two
lactating dairy cows. In the first animal the only milk sample with
a radioactive residue, >0.01 mg/kg isofenphos equivalents, was
analysed for isofenphos and its metabolites. The 7.5h sample contained
0.014 mg/kg isofenphos equivalents and accounted for 0.07% of the
administered radioactivity. DAIOA and HHA were the main metabolites in
the organosoluble fraction and accounted for 13% and 10% of total
activity in milk, respectively. Small amounts (2 to 4%) of IPS, SA and
several unknowns (12%) were also detected. Enzymatic hydrolysis of the
solvent-extracted, pH 5 milk released an additional 45% of the milk
radioactivity. HHA (27%) was the major constituent in this fraction of
the milk. DAIOA, SA, IPS and several unknowns were also found in small
amounts.
In the second experiment milk was collected prior to each day's
dosing and 8 hours after each dosing. Milk production remained at the
cow's normal levels through the 5 days of the study. The cow was
sacrificed 2 hours after the fifth dose was administered. The
following tissues were collected and analysed, their residue content,
in isofenphos equivalent (mg/kg) were: muscle-loin 0.02, shoulder
0.02, round 0.02; fat-renal 0.07; omental 0.07; subcutaneous 0.06;
kidney 0.53; heart 0.04; liver 0.47; udder 0.05 and brain 0.02.
In kidney and liver tissues, less than 35% of the radioactivity
was organosoluble and consisted of SA 26 to 14%, HHA 3 to 4%, IOA 2 to
1%,, IPS 1% to <1%, respectively. Traces (1%) of unknowns were found
in the liver.
Sulphatase beta-glucuronidase combination was employed in the
enzyme hydrolyses, which released 42 to 45% activity from the kidney
and liver extracts respectively. IPS (7 to 3%), SA (8 to 2%) and HHA
(6 to 8%) were identified in the enzymatic extracts of kidney and
liver.
Several unknowns (20 to 33%) were detected, but a single
compound, unknown (a), accounted for the 90% of activity. The
radioactive residues which were not organosoluble or liberated by
enzyme hydrolysis were not further characterized.
A feeding study was conducted to study the biological effects and
to determine the tissue and milk residues after feeding isofenphos and
its metabolites. The ratio 1:8:1 of isofenphos, IOA and DNIOA in the
mixture was based on plant metabolism studies of isofenphos and should
be representative of field-treated samples. Alfalfa pellets containing
the mixture of compounds at 2, 6 and 20 mg/kg levels were given to
dairy cattle ad libitum for 28 days (Strankowski 1977c). Feed
consumption, body weight and milk production were unaffected by
consumption of the treated feed as compared to the control cow. The
milk of cows fed 20 mg/kg contained detectable residues only in the
range of 0.001 to 0.01 mg/kg of isofenphos equivalents on the 28th day
of feeding. After feeding for 28 days the animals were sacrificed and
the following tissues were taken; liver, kidney, muscle (round, flank
and loin) and fat (omental, renal and subcutaneous). Residues in all
the tissues analysed, except liver and kidney at the 20 mg/kg level,
were below 0.0, mg/kg. The liver and kidney samples contained
residues, in isofenphos equivalents, ranging from 0.01 to 0.02 and
from 0.01 to 0.04 mg/kg, respectively.
Hen
Laying hens were given a single oral dose of ring-µL(14C)-
isofenphos at 2.0 mg/kg (Dupre 1975). Eggs and excreta were collected.
At four sacrifice intervals (6, 24, 48 and 96 h post-treatment) blood,
various organs and tissues were collected from four birds per group.
In eggs, the level of radioactivity reached a maximum level of
0.099 mg/kg with an average of 0.047 mg/kg in isofenphos equivalent at
48 h post-treatment. Approximately 72% and 78% of the radioactivity
was eliminated in the excreta within 24 and 96 h respectively. A
maximum level in the blood plasma of 0.274 mg/kg 14C activity at 6 h
post-treatment fell to 0.009 mg/kg 96 h after treatment; 24 h after
treatment, residue levels in skin, fat, breast, thighs, heart and
gizzard muscle were less than 0.05 mg/kg, while liver and kidney
contained 0.9 mg/kg and 0.68 mg/kg respectively.
In the feeding study in hens described under 'Biochemical
Aspects' residues, expressed as mg/kg isofenphos equivalents, found in
various tissues were: gizzard 17.1, kidney 4.9, liver 3.9, fat 0.83,
skin 0.67, heart 0.42, muscle 0.14, eggs - 51 h 0.25, 48 h 0.06, 24 h
0.032. The distribution of the residues in the organosoluble fractions
of various tissues is given in Table 12.
TABLE 12. Distribution of isofenphos and its metabolites in tissues and eggs
of laying hens
Distribution (%)
Compound Gizzard Kidney Liver Fat Skin Heart Muscle Eggs
Isofenphos 90 14 2 74 53 26 31 37
IDA 0 4 1 4 3 3 4 9
IPS 0 1 1 18 19 10 8 10
DNI 0 0 0 3 3 0 0 0
HHA 0 0 0 0 0 0 5 0
Unknown 0 47 19 0 8 36 28 5
Feed treated with a mixture containing isofenphos, IOA and DNIOA
in the ratio of 1:8:1, at 5, 15, 20 and 150 mg/kg feed was distributed
to laying hens ad libitum for 28 days (Strankowski 1977d). Feed
consumption, body weights and production decreased as the amount of
isofenphos and its metabolites in the feed increased. At sacrifice,
giblets (heart, gizzard, liver), muscle (equal portions of thigh and
breast), fat (equal portions of visceral and subcutaneous) and skin
(without feathers) were collected. No residue was detected in the
muscle at any dose level. Residues in fat, giblets, skin and eggs are
summarized in Table 13.
Fish
Studies on continuous exposure for 28 days of channel catfish to
isofenphos are reported under "Biochemical Aspects".
In soil
Isofenphos degradation under aerobic conditions was investigated
by Minor and Murphy (1977) in sandy loam and silt loam, while the
effects of anaerobic and sterile conditions were studied in sandy
loam. Ring µ-L-(14C) labelled isofenphos was uniformly incorporated
into the soil at a level of 8.7 mg/kg. The extractable residue derived
TABLE 13. Residues in isofenphos equivalents found in tissues and
eggs of laying hens1
Residue (mg/kg)
Dose
Fat Giblet Skin Eggs
50 mg/kg <0.01 (4) <0.01 (2) <0.01 (2) 0.002
0.01 (2) 0.02 0.003
0.08 0.003
0.004
150 mg/kg <0.01 (2) 0.01 <0.01 (2) 0.017
0.01 (2) 0.02 0.03 (2) 0.013
0.04 0.015
0.06 0.003
1 No. of samples in brackets.
from isofenphos decreased with time, while the radioactivity bound to
soil, or lost, increased. Only 10% of the originally applied
radioactivity could be extracted from silt loam at 240 days post-
treatment, whereas 23% of the-radioactivity could still be removed
from the sandy loam one year after traces (<1% of original activity)
of IPS. The concentration of isofenphos decreased continuously with
time, and its half life was found to be 127 days in sandy loam and 59
days in silt loam.
The concentration of isofenphos oxygen analogue reached a maximum
between 60 and 120 days post-treatment. The bound radioactivity
increased to a maximum of approximately 20% and the remainder had
volatilized. The bound radioactivity was distributed among humin,
humic acid and fulvic acid fractions in a fashion similar for both
soils. Even after stringent HCl-NaOH fractionation, approximately 50%
of the bound residues was still unextractable.
The volatile radioactivity from soil was composed of isopropyl
salicylate, 14CO2-isofenphos and cyclic isofenphos. At 134 days
post-treatment, the total volatile radioactivity trapped was
distributed among IPS (67%), 14CO2 (30%), cyclic isofenphos (2%)
and isofenphos (1%) (Minor 1980: The volatile compounds in the
experiments accounted for 16 to 51% of the total activity applied to
soil 134 days before, while the soils contained the remaining part of
activity, indicating the importance of conditions (e.g. temperature
and/or microorganism activity) on degradation rate of isofenphos in
soil. In sandy loam and under anaerobic conditions, isofenphos was not
converted to its oxygen analogue, but some of the radioactivity was
lost. Under sterile conditions in sandy loam, isofenphos was neither
altered nor lost. In isolated soil micro-organisms grown in "shake
culture", isofenphos was not altered. Conversion of isofenphos to its
oxygen analogue appeared to be strictly a chemical reaction occurring
in soil under aerobic conditions. The production of isopropyl
salicylate may also have occurred without microbial activity, but the
isolated 14CO2 was probably the end result of soil micro-organism
cleavage of the isofenphos aromatic ring after the phosphate group had
been removed. Therefore, isofenphos soil degradation appeared to
involve both chemical and microbial alterations, and the chemical
reactions of oxidation and hydrolysis appeared to be prerequisite to
microbial action. Comparison of results from isofenphos treated soil
kept in a greenhouse or maintained outdoors showed that isofenphos
degradation qualitatively is the same under both environments, but the
rate of degradation may vary.
The persistence of isofenphos in soil was investigated in nine
tests set up in various locations in the United States and Canada
(Mobay 1980c). Isofenphos, 6E formulation, was incorporated uniformly
at a rate of 2 mg/kg wet soil (equivalent to 2.24 kg/ha field
application) into the upper 7.6 cm of soils of seven different types.
Samples were taken at 0, 30 to 33, 60 to 61, 89 to 92 and 119 to 124
days post-treatment from 0 to 15 cm and 15 to 30 cm depths. The
residue of isofenphos decreased in all nine studies, and its level in
the upper layer was between 0.11 mg/kg and 0.43 mg/kg 119 to 124 days
after treatment. IOA reached its maximum (0.19 to 0.5 mg/kg) 32 to 123
days post-treatment. Depending on the soil types and possibly on
environmental conditions, the ratio of isofenphos and its oxygen
analogue varied between 0.37 to 14 at the last sampling. Soil samples
from 15 to 30 cm did not contain detectable residues (0.01 mg/kg),
with the exception of rocky sandy silt loam in which 0.02 to 0.15
mg/kg isofenphos or IOA could be detected.
The degradation of isofenphos was studied in the Federal Republic
of Germany (FRG) and in the UK (Bayer 1973-75). The applications were
made with either EC or granular formulation as surface spray or
broadcast at rates of 1, 5 and 7.5 kg a.i./ha. The sampling day varied
from 0 to 534 post-treatment. The results show a pattern similar to
that obtained in the experiments described previously. The results of
laboratory experiments on the persistence of isofenphos carried out in
standard German soil No. 1 and No. 2 (Bayer 1974a) were consistent
with the results of field trials, as were the mobility tests with soil
thin layer chromatography (Thornton 1976) or soil column (Obrist and
Thornton 1977; Bayer 1973-74, 1977b).
The latter experiments indicated that isofenphos and IOA had low
mobility and the majority of the residue remained in the upper 12.5 cm
of the soil, while the leachates contained no intact isofenphos but
did contain a small amount of IOA and traces of IPS (one experiment).
In water
The stability of ring-µL-(14C)-isofenphos in sterile buffer
solutions of various pH's at various temperatures and two
concentrations (1 mg/l and 10 mg/l) was determined (Mc Namara 1977).
Both hydrolysis and volatilization were involved in the dissipation of
isofenphos from the buffered solutions. Isofenphos was not hydrolysed
at temperatures of 20°C and below or at pH's near neutrality, but
volatilization did occur at 20°C and above and increased as the
temperature and isofenphos concentration increased. Hydrolysis of
isofenphos was, however, encountered in both strongly acidic and
alkaline buffers. Hydrolysis products detected in pH3 buffers included
DNI, IOA, DNIOA, IPS, SA. At pH9, the identified hydrolysis products
of isofenphos were IOA, IPS, SA and N-isopropyl salicyclamide. At
37°C, the half life of isofenphos at 1 and 10 mg/kg was 79 and 30 days
respectively, in pH3 buffer, and 88 and 32 days respectively, in pH9
buffer. At 50°C, the half life of isofenphos at 1 and 10 mg/kg was 15
and 9 days respectively, in pH 3 buffers; 34 and 15 days respectively,
in pH 6 buffer; and 6 days for each concentration in pH 9 buffer.
Ring-µL(14C)-isofenphos at an initial concentration of 10 mg/kg
was incubated outdoors in a simulated pond (pH 8) (Mc Namara 1977a).
Within 70 days, 89% of the radioactivity was lost, and the remaining
radioactivity was distributed between water and sediment in an
appropriate 2:1 ratio. The loss of radioactivity was probably due to
volatilization of isofenphos and some of its metabolites. The major
radioactive compounds identified in the pond water were isofenphos,
IOA and cyclic-IOA. Minor components were cyclic isofenphos, IPS and
SA. The amount of radioactivity adsorbed by the pond sediment rose to
20% of the initially applied radioactivity within 7 days, but then
decreased steadily to only 2% at 70 days. The bound radioactivity
never exceeded 2% of the originally applied radioactivity. The major
components in the organosoluble extracts of pond sediment were
isofenphos and IOA. Minor components identified in the sediment were
IPS, SA, cyclic-isofenphos and cyclic-IOA. The overall rate of
isofenphos dissipation was exponential. The half-life of isofenphos
was 13 days. After 70 days, only 2% of the initially applied
isofenphos itself remained in the pond.
In activated sludge
Ring µL-(14C)-isofenphos was studied in a laboratory model of an
activated sewage sludge system (Spare 1979). Activated sludge,
synthetic sewage and (14C)-isofenphos were aerated for 23 h cycle.
During the remaining hour/cycle, the mixture was allowed to settle and
a portion of the supernatant was replaced with fresh synthetic sewage
and an increased amount of (14C)-isofenphos. The concentration of
isofenphos during the first cycle was 0.1 mg/kg and was increased
through 10 cycles to 100 mg/kg.
After the 100 mg/kg cycle concluded, three cycles were carried
out with no addition of test compound. Isofenphos had no detrimental
effect on the bacteria, yeasts or actinmycetes in the system, but at
isofenphos concentrations of 60, 80 and 100 mg/l, protozoa were absent
or few in the treated flasks. The quantity of radioactivity in the
settled solids increased steadily during the course of the study from
10% to 50% of the added radioactivity. (14C)-isofenphos was not
degraded appreciably by the organisms in the activated sludge system.
Greater than 96% of the added (14C)-isofenphos was recovered intact
from the supernatant and solid fractions.
Photodegradation
In aqueous solution
A study of the photodecomposition of ring-uL-(14C)-isofenphos in
aqueous solution was carried out in a photoreactor containing a high
pressure, quartz, mercury-vapour, 200-watt, Hanovia immersion lamp as
the light source (Strankowski 1977e). Decomposition of isofenphos was
slow in the pH 7 solution with only 30% decomposition in 30 days of
continuous exposure to the high intensity light. The isofenphos half-
life was calculated to be 51 days. In an acetone sensitized solution,
the photodecomposition was increased, and the half-life of isofenphos
was reduced to approximately 14 h.
Isofenphos was the only component found in the dark control
solutions from either the unsensitized or sensitized studies. It was
also identified in the photolysis solutions; approximately 75% and 3%
of the total radioactivity remained as isofenphos at the end of the
unsensitized and sensitized studies, respectively.
The major photoproduct, which represented nearly 21% of the total
radioactivity in the sensitized test, was identified by Poje (1979) as
3,3-dimethyl isoindoline-l-one by mass spectroscopy and nuclear
magnetic resonance spectroscopy. This product contains no phosphorous.
The remaining photoproducts, none of which accounted for more than 5%
of the total radioactivity, included IOA, DAI, SA and catechol.
On soil
In a study of the photodegradation of (14C)-isofenphos on soil,
isofenphos had a half-life of 2.6 days when applied to a thin layer of
soil and subjected to light from a 200 watt Hanovia mercury lamp
(Weissenburger and Pollock 1979). Six photoproducts were detected
on the soil. IOA (33% of the applied radioactivity) was the principal
photo-product. Small quantities (approx. 5%) of DNI, DNIOA, des-N-
isopropyl cyclic isofenphos, IPS and phenol were also detected. During
26 days of exposure to the light, 25% of the applied radioactivity was
bound to the soil and 33% was lost through volatility. A trapping
study, carried out for 3 days, showed that the volatile compounds
included isofenphos (91%), DNI (3%), IPS (1%) and phenol (5%). All of
the applied activity was accounted for in the trapping study.
In storage
Stability of isofenphos and its metabolites were studied during
frozen storage (-28°C to -18°C) (Mobay 1980a). Concentration of
isofenphos and IOA did not change significantly in maize kernel,
brassica leafy vegetables, green forage, green beans (pods and vines)
and onions kept deep frozen for 400 to 800 days. However, 24% of
isofenphos decomposed in sugarbeet during 420 days of storage. DNI and
DNIOA residues decreased by 20% add 32% respectively in alfalfa stored
at -23 to -18°C.
Poultry tissues, eggs and giblets, fortified at 1 mg/kg with
isofenphos and its metabolite, were stored frozen for 255, 90 and 60
days respectively without significant changes in concentrations of
residues.
Cattle tissues and milk were fortified at 1.0 mg/kg with
isofenphos and metabolites and placed in frozen storage. Fat and
muscle tissues showed no significant decomposition of the compounds
after 24 to 28 days. Milk showed no significant decomposition of the
compounds after 76 days in frozen storage. In kidney and liver,
isofephos, isofenphos oxygen analogue and des-N-isopropyl isofenphos
were stable for 28 to 35 days. The liver and kidney samples were
extracted within 4 and 8 days, respectively. There was no
decomposition of des-N-isopropyl isofenphos oxygen analogue in kidney
after 10 days of frozen storage; however, it did not disappear
completely in liver after 5 days of frozen storage.
METHODS OF RESIDUE ANALYSIS
A gas chromatographic procedure for the determination of residues
of isofenphos and its oxygen analogue in various crops, soil and water
has been described by Wagner (1976). Plant samples of high water
content and soils are extracted with acetone. The acetone extract is
diluted with water and the residues are partitioned into chloroform or
dichloromethane. Rape, maize and water are extracted with acetonitrile
and chloroform respectively. The concentrated extracts are cleaned on
neutral alumina or on active carbon columns. The residues in the
concentrated extracts are separated on DC-200, QF-1 or OV-17 columns
and detected with a thermionic detector. Recovery data from
experiments run on a large variety of crops by adding known amounts of
isofenphos and its oxygen analogue at the blending step were generally
in the 77% to 105% range. The limit of determination is 0.005 mg/kg
for each compound in plant and soil samples and 0.002 mg/kg in water.
Gas chromatographic methods for the determination of residues of
isofenphos, isofenphos oxygen analogue, des-N-isopropyl isofenphos and
des-N-isopropyl isofenphos oxygen analogue in maize cob, forage, husk
and kernel and in onions are described by Stanley (1977b, 1979a) and
in bovine and poultry tissues, milk and eggs by Shaw II (1977a). For
all four compounds, recoveries were 65 to 70%. The limit of
determination for each compound is 0.01 mg/kg, except for milk and
eggs (0.001 mg/kg). Multiplication factors for converting metabolite
residues to isofenphos equivalents are 1.05 for IOA, 1.139 for DNI and
1.204 for DNIOA.
Interference studies for the determination of residues of
isofenphos and its metabolites as maize and onions were done by
Stanley (1977c, 1979b) and in milk, animal tissues and eggs by Shaw II
(1977b, 1979). All possible interferences by 20 or 27 organophosphate
pesticides registered for use on maize and onions or investigated in
animal products respectively were separated from isofenphos and its
metabolites by GLC on the standard (10% DC-200 + 2% OV-225 on
Chromosorb WHP) or on the confirmatory (5% OV 210 on Supelcoport or 5%
OV 275 on Chromosorb WHP) columns or by clean-up on the silica gel
column used in the residue method. The silica gel column was applied
as follows: 372 g silica gel is deactivated with 28 cm3 water, 10 g
is filled into the column under hexane and settled, and a 1-cm layer
of granular Na2SO4 is placed on top of the column.
Hexane is drained to the top of the sodium sulphate. The
concentrated extract is transferred in the column with 5 to 10 cm3
portions of hexane and finally the column is washed with hexane (total
amount-50 cm3). The portions of hexane is drained through the column
at a rate of 5 to 10 cm3 until 50 cm3 is collected. Eluents (freshly
prepared) and fractions are: (1) 185 cm3 hexane-dichloromethane (1:1)
(isofenphos); (2) 150 cm3 dichloromethane (DNI); (3) 65 cm3
dichloromethane-methanol (99:1) (discarded); (4) 135 cm3
dichloromethane-methanol (99:1) (IOA).
Shaw II (1977b, 1979) applied different conditions: 12 g silica
gel and 6 g Na2SO4; column is washed with 40 cm3 hexane, 100 cm3
hexane-benzene (8:2) 75 cm3 hexane-benzene (6:4); elution: (1)
fraction (isofenphos) 25 cm3 hexane-benzene (6:4) + 100 cm3 hexane-
benzene (4:6) + 100 cm3 hexane-benzene (2:8) + 50 cm benzene; (2)
fraction (IOA + DNI) 50 cm3 benzene + 300 cm3 benzene-acetone (8:2).
A gas chromatographic method for the determination of isofenphos
and isofenphos oxygen analogue in soil and water, using an alkaline
thermionic emission detector, is described by Shaw II (1974).
Recoveries of isofenphos and its oxygen analogue were 93 to 101%; the
sensitivity of the method is approximately 0.005 mg/kg for soil and
0.002 mg/kg for water samples.
The determination of isofenphos and its oxygen analogue residues
in aged soil by alkaline thermionic emission gas chromatography was
investigated by Shaw II (1977c). The soil was extracted in a Soxhlet
apparatus for 16 to 24h with chloroform/methanol (7:3). The extract
was concentrated, partitioned with hexane/acetonitrile (1:1) and
partially purified with three hexane extractions of the residue in a
methanol/water solution. Isofenphos recoveries were >79% and
isofenphos oxygen analogue recoveries were >95% from soils fortified
at 0.05 mg/kg. The sensitivity of this method is 0.01 mg/kg.
Isofenphos and IOA were determined by Brown and Williams (1976)
in cabbage, potato, rapeseed and soil. The rapeseed was extracted with
acetonitrile and cabbage, potato and soil with ethylacetate. The
concentrated extracts were cleaned on a column consisting of the
mixtures of Florisil, silica gel, alumina and Nuchar C. The cleaned-up
extracts were separated on DEGS or OV275 liquid phases and detected
with FPD. The recoveries at 0.01 to 1 mg/kg levels were in the range
of 84 to 98%. The limits of determination were 0.003 mg/kg and 0.005
mg/kg for isofenphos and IOA respectively in potato, rapeseed and
soil, while they were five times higher in cabbage.
The methods described by Wagner (1976) or by Brown and Williams
(1976), in combination with the confirmatory columns and GLC
separation (Stanley 1977b, 1979a), are recommended for regulatory
purposes.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Isofenphos is registered in the following countries: Austria,
Bulgaria, Chile, Denmark, Federal Republic of Germany, German
Democratic Republic, Indonesia, Israel, Italy, Morocco, Mexico,
Norway, The Netherlands, South Africa, Spain, Sweden and the USA.
Maximum residue limits and preharvest intervals were reported
from some of the countries and are summarized in Table 14.
EVALUATIONS
COMMENTS AND APPRAISAL
In the mammalian species tested, the oral LD50 ranged from about
20 mg/kg bw in rats to approximately 150 mg/kg bw in rabbits. In the
rat, the oxygen analogue of isofenphos was shown to be slightly, but
not substantially, more toxic orally than isofenphos, both acutely and
subacutely.
TABLE 14. National MRLs reported to the Meeting
Preharvest
Country Crop/Commodity intervals MRL
(days) (mg/kg)
Fed. Rep. of Leafy and stem
Germany vegetables 0.1
Rapeseed 0.05
Italy Pear 42 0.1
Fruit 0.1
Vegetables 0.1
Sugarbeet 0.1
Netherlands Cauliflower 56 0.1
Cabbages (including
Brussels sprouts) 56 0.1
Onions 0.1
Celery 0.05
Celeriac 0.05
Norway Root, tuber and
bulb vegetables 90
Brassica leafy vegetables Apply not later
than transplanting
South Africa Citrus fruit 180 0.2
Spain Cabbage Apply before sowing
Onions 21
Garlic 21
All crops (except
root vegetables) Apply before sowing
and potato)
USA Maize 75
Corn, forage 1.0
Corn, fodder 1.0
Corn, fresh (including
sweet) 0.1
Corn, grain 0.1
Meat, fat, meat by-
products of cattle,
sheep and poultry
Milk 0.02
Eggs 0.02
Metabolism studies in rats, pigs and cows, treated orally with
ring-µL(14C)-isofenphos indicated that the compound is rapidly
eliminated in both faeces and urine, with the latter being the
predominant route of excretion. It is metabolized mainly by oxidative
desulphuration, dearylation, hydrolysis, deamination and conjugation.
There is no indication that isofenphos and/or its metabolites
accumulate in mammalian tissue. The excretion pattern and proposed
metabolic pathways for isofenphos appear to be similar in the three
mammalian species studied.
A 3-generation reproduction study in rats (with 2 litters in the
first generation (F0) but only one litter each in the second (F1)
and third (F2) generations showed a marginal effect in the F0
generation at the second mating on the pregnancy rate, even at 1 ppm,
the lowest tested level. No indication of teratogenic effect was noted
in a rabbit teratology study at dosages as high as 5 mg/kg bw.
Teratology studies in rats exposed orally, dermally or via inhalation
were all negative.
In vitro microbial assays, including reversion-test and rec-assay
and a dominant lethal study in mice, failed to give any evidency of
mutagenicity. Long-term studies in mice and rats revealed no
carcinogenic activity. An acute delayed neurotoxicity study in hens,
which could be considered as a screen, was negative. The acute oral
toxicity of isofenphos was found to be potentiated by malathion but
not by a number of other organophosphorus insecticides tested.
Plasma cholinesterase inhibition was observed as the most
sensitive indicator of toxicity in the short-term rat studies and the
108-week mouse and 2-year rat feeding studies. A dietary level of
1 ppm was a marginal no-effect level in the rat and a no-effect level
in the mouse with respect to plasma cholinesterase. In the dog, a
90-day study suggested that dietary levels of 10 ppm and above caused
significant plasma cholinesterase depression and an increase in liver
weight, although the latter was not accompanied by histopathological
alteration of the tissue. A 2-year feeding study in the same species
demonstrated 2 ppm to be the no-effect level, based on plasma
cholinesterase. The information provided on observations in humans was
very scanty.
Acceptable data was available to permit the establishment of no-
effect levels in three mammalian species. Owing to the unavailability
of an appropriate neurotoxicity study in hens, only a temporary ADI,
was allocated.
Isofenphos, O-ethyl O-2-isopropoxycarbonylphenyl
isopropylphosphoramidothioate, is an organophosphorus insecticide
applied mainly in soil at a rate of 1.5 to 2.5 kg a.i./ha or 1 to 5 kg
a.i./ha for row or overall surface treatment, respectively.
Supervised trials were carried out on various crops at different
locations in the United States, Canada and Europe. Isofenphos and its
main metabolites, isofenphos oxygen analogue (IOA), des-N-isopropyl
isofenphos (DNI) and des-N-isopropyl isofenphos oxygen analogue
(DNIOA), were analysed. It was found that the residue consisted mainly
of isofenphos and IOA. The residues measured in brassica leafy
vegetables, rapeseed and root and tuber vegetables were below
0.1 mg/kg and usually at or about the limit of determination after the
recommended pre-harvest intervals. No residues were detected in maize
kernels, either in the milk or dry stages, while residues up to
2 mg/kg were found in green forage 3 to 4 weeks after application. The
total residue detected in mature onion bulbs was always below 1 mg/kg.
Rotational crops can also take up residues from previous soil
treatments. The residues, consisting mainly of isofenphos and IOA,
were at or about the limit of determination in cereal grains, leafy
and root vegetables and edible oil crops grown in soil treated with
isofenphos 9 or more months earlier, The residue level might exceed
1 mg/kg in green wheat forage but it is under 0.1 mg/kg in the straw
at the time of harvest.
Isofenphos is degraded in soil, plants and animals, but its route
of degradation in soil and plants is somewhat different from that in
animals. Oxidation seems to be the first reaction in soil and plants.
In animals, hydrolysis and oxidation both appear to be of major
importance. The degradation of isofenphos was not rapid in any of the
systems studied. Soil micro-organisms seem capable of splitting the
aromatic ring to produce CO2 but only after the phosphoramidothioate
rouping has been removed from the molecule. In plants, oxidation is
followed by depropylation, hydrolysis and conjugation. IOA and
isofenphos are the primary residues but DNIOA also appears in smaller
amounts. The approximate ratio of isofenphos, IOA and DNIOA in mature
maize stalks was 1:8:1. 2,5-Dihydroxybenzoic acid (DHBA), salicylic
acid (SA) and deaminated isofenphos oxygen analogue (DAIOA) appear in
traces.
In animals, isofenphos undergoes hydrolysis, oxidation,
N-depropylation, deamination and conjugation. Excretion is rapid
in orally dosed animals. Pigs, cows and hens excreted 81%, 79% and
72% of the applied dose in the urine or excreta within the first
24h after treatment. Urine contained isopropyl salicylate (IPA),
O-hydroxyhippuric acid (HHA) SA and DAIOA in non-conjugated and/or
conjugated forms; 4 to 5% of the administered dose is eliminated in
the faeces over a 72 to 80h period.
During a period of multiple dosing for 6 consecutive days at a
rate of 15 mg/kg/day, rats were able to eliminate residues of
isofenphos fast enough to maintain a relatively constant level of
radioactivity in the tissues. One day after cessation of the
isofenphos treatment a 10-fold decrease in radioactive residue
concentration was detected in the muscle, fat and liver. Dairy cows
and laying hens were kept on feed containing residues of isofenphos,
IOA and DNIOA in the ratio of 1:8:1 at various levels between 2 and
20 mg/kg feed and 5 and 150 mg/kg respectively for 28 days. Residues
in all the bovine tissues, except liver and kidney at the 20 mg/kg
level, were <0.01 mg/kg. The liver and kidney samples collected from
the 20 mg/kg group contained residues of isofenphos ranging from
<0.01 to 0.02 mg/kg and 0.01 to 0.04 mg/kg respectively. Residues of
isofenphos equivalents in milk from all samples, except the 20 mg/kg
group, were below 0.001 mg/kg. The milk samples collected on the 28th
day from the high level group contained residues ranging from 0.001 to
0.01 mg/kg.
Isofenphos residues in poultry tissues and eggs from the control
and 5 mg/kg groups were below the limits of determination of the
analytical method (0.01 mg/kg for tissues, 0.001 mg/kg for eggs). The
hens fed at the 15 mg/kg level had no detectable residues in the
tissues and had residues ranging from <0.001 to 0.002 mg/kg in the
eggs. In the 50 mg/kg group, muscle and fat samples had no measurable
residues, while giblets and skin samples had residues ranging from
0.002 to 0.004 mg/kg. Residues were detected in all tissues, except
muscle, and ranged from <0.01 to 0.05 mg/kg and in eggs from 0.003 to
0.017 mg/kg at the exaggerated 150 mg/kg dietary level. Control values
were <0.01 mg/kg in tissues and <0.001 mg/kg in eggs. No detectable
residues can be expected in products derived from animals fed with
forage that has been treated with isofenphos at recommended
application rates.
Catfish exposed continuously to isofenphos for 28 days in water
containing 10 mg/l isofenphos accumulated residues of 0.75 mg/kg,
consisting entirely of intact isofenphos, within the first 4 days of
exposure. However 87% of this was eliminated within the first day
after transfer of the catfish to uncontaminated water.
Isofenphos is readily absorbed by soil. It is only slowly leached
in all the soils tested, but its degradation products move somewhat
more rapidly. In soil persistence studies with isofenphos incorporated
into the soil at the single-application field-use rate, the residue of
isofenphos itself declined steadily in all soils tested, and the total
organophosphorus residue decreased to half the initial concentration
within 45 to 200 days.
Hydrolysis of isofenphos occurs at acidic and alkaline pH, but it
is stable near neutral pH.
In an activated sludge system, isofenphos has no detrimental
effect on the sludge organisms, and the parent compound was not
degraded.
Photodegradation of isofenphos in aqueous solution is slow and
yields principally a re-arrangement product, 3,3-dimethylisoindoline-
l-one. On soil, photodegradation is rapid and the main product is IOA.
Residue analytical methods for the determination of isofenphos
and IOA, the major residue component, and for DNI and DNIOA are
available. Recoveries are in the range of 77 to 105%.
The limits of determination for isofenphos and IOA in plant
samples, animal tissues, and milk and eggs are 0.005 - 0.01, 0.01 and
0.001 mg/kg respectively, in water 0.002 and in soil 0.01 mg/kg.
Level causing no toxicological effect
Mouse : 1 ppm in the diet equivalent to 0.15 mg/kg bw/day
Rat : 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog : 2 ppm in the diet equivalent to 0.05 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The Meeting estimated the maximum residue levels likely to occur
in various commodities and concluded that they were suitable for
establishing temporary MRLs.
The limits refer to the sum of isofenphos and its oxygen
analogue.
Preharvest intervals (days)
Commodity Limit (mg/kg) on which recommendations
are based
Celeriac 0.02 * 150
Swedes 0.02 * 90
Turnips 0.02 * 90
Brassica leafy vegetables 0.1 60
Celery 0.02 * 90
Maize 0.02 * 90
Maize, fodder 0.5 90
(dry)
Sweet corn 0.02 * 90
Sweet corn fodder (dry) 0.5
Rapeseed 0.02 * 90
Carcass meat 0.02 *
Animal fats 0.02 *
Meat by-products 0.02 *
Cattle milk 0.01 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
An appropriate neurotoxicity study in hens.
Desirable
1. 2-generation (2 litters/generation) reproduction study.
2. In vitro biochemical studies on purified isofenphos with respect
to anticholesterase activity.
3. Further observations in humans.
4. Additional data from supervised trials on potatoes and onions,
including information on soil residues and soil moisture content.
REFERENCES
Bagnall, B.H. Tests to monitor the blood cholinesterase levels of
1976 workers using oftanol granules on carrots. Report from Bayer
UK Limited, Agrochem. Division, submitted by Bayer Ag.
(Unpublished)
Bayer Residue Reports Nos. 4003-4005/74, 4000-4002/77 (Cabbage,
1973-74 savoy), 73-75/73, 4064-/74 (Cabbage, white), 45-47173,
1977a 4000-4002/74, 4003-4005/77 (Cauliflower). (Unpublished).
1974-76 Residue reports nos. 4057/74, 4014-4015/75, 4006-4008/76
(Sweden), 4016/75, 4023-4024/76, (Turnips). (Unpublished)
1972-73 Residue Reports nos. 132/72, 99-101/73, 4003-4004/75,
1975, 4024/78, 4039-4040/78 (Onions). (Unpublished)
1978
1973-75 Residue Reports nos. 214-216/73, 336/73, 4035-4037/74 4031
a-c/75 (Soil, field). (Unpublished)
1973-74 Residue reports nos. 267-268/73, 4038-4040/74, 4043/74,
1977b 4046-4048/74, 4017/74 (Leaching exp.). (Unpublished)
1973 Residue Report no. 105/73 (Potatoes). (Unpublished)
1974 Residue Reports nos. 4032-4034/74, 403211-403411/74,
4049-4051/74, 4049/1-4051/1/74 (Rapes). (Unpublished)
1974 Residue Reports nos. 4044/II.- 4045/74, 4061/74 (Soil,
laboratory). (Unpublished)
1978 Residue Reports nos. 4000-4001/78 (Brussels sprouts).
(Unpublished)
1980 Residue Reports nos. 5001/80, 5002/80, 5202/80 (Potatoes).
(Unpublished)
Bomhard, E. and Löser, E. SRA 12869 chronic toxicity study on rats
1977 (two-year feeding experiment) Report from Bayer AG Institut
Für Toxikologie, submitted by Bayer AG. (Unpublished)
Brown, M.J. and Williams, J.H, Determination of residues of isofenphos
1976 and its phosphoramidate analogue. Pesticide Science,
7: 545-548.
Brune, H., Deutsch-Wenzel, R., Schüman, K. SRA 12869 chronic toxicity
1978 study on NMRI mice (108-week feeding experiment). Report
from Biologisches Laboratorium, Hamburg, submitted by Bayer
AG. (Unpublished)
Cherry, C.P., Newman, A.J. and Urwin, C. Pathology report of SRA 12869
1972 - Acute neurotoxicity experiment in hens. Report from
Huntingdon Research Centre, UK, submitted by Bayer AG.
(Unpublished)
Dupre, G.D. Metabolism and excretion of 14C-labelled SPA 12869 in
1975 poultry. Mobay Report No. 44931. (Unpublished)
Flucke, W. Oftanol bestimmung der akuten toxizität (LD50). Reports
1980a dated 25 July, 29 August, 15 October and 8 December, 1980
from Bayer AG submitted by Bayer AG. (Unpublished)
1980b SRA 12869 Bestimmung der akuten toxizität (LD50). Ratte
p.o. Report dated 7 Nov. 1980 from Bayer AG, submitted by
Bayer AG. (Unpublished)
1981 Oftanol bestimmung der akuten toxizität (LD50). Report
dated 16 February 1981 from Bayer AG, submitted by Bayer AG.
(Unpublished)
Hoffman, K. and Kaliner, G. SPA 12869 chronic toxicity study on dogs
1977 (two-year feeding experiment). Report from Bayer AG Institut
Fur Toxicologie submitted by Bayer AG. (Unpublished)
Inukai, H. and Iyatomi. SRA 12869 mutagenicity test on bacterial
1977 systems. Report from Nitokuno Agricultural Chemicals
Institute, Laboratory of Toxicology, Japan, submitted by
Bayer AG. (Unpublished)
Kimmerle, G. SRA 12869 acute neurotoxicity studies on hens. Report
1972a from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b Kutane toxizitat von Parathion an ratten vesgleich
verschiedener methoden. Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
1976 SPA 12869 acute inhalation toxicity study on rats. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Kimmerle G. and Gröning. SRA 12869 study to evaluate atropine and
1975 oxines for antidotal effect on rats. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Kurtz, S.K. The assimilation and metabolism of 14C Oftanol by
1977 rotational crops. Mobay Report No. 53826. (Unpublished)
Kurtz, S.K. and Shaw II, H.R. Poultry metabolism of (14C) Oftanol.
1977 Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Lamb, D.W., Matzkanin, C.S. and Burke, M.A. Acute oral toxicity of
1977 Oftanol technical. Report from Mobay N. 54000, Ref. 76-282,
submitted by Bayer AG. (Unpublished)
Lamb, D.W. and Burke, M.A. Dietary toxicity of Oftanol technical to
1977 mallard ducks. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 Acute oral toxicity of AmazeTM technical to bobwhite
quail. Report from Mobay Chemical Corp., submitted by Bayer
AG. (Unpublished)
Löser, E. SRA 12869 subchronic toxicity study on rats (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Löser, E. SRA 12869 subacute toxicity study on rats (four-week feeding
1978 experiment). Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Machemer, L. SRA 12869 studies for embryotoxic and teratogenic effects
1972 on rats following oral administration. Report submitted by
Bayer AG. (Unpublished)
1973 SRA 12869 dominant lethal test on the male mouse to test for
mutagenic effect. Report from Bayer AG, submitted by Bayer
AG. (Unpublished)
1975 SRA 12869 studies of embryotoxic and teratogenic effects on
rabbits following oral administration. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
McNamara, F.T. Dissipation of 14C-Oftanol in buffered aqueous
1977a solutions. Mobay Report No. 53617. (Unpublished)
1977b Dissipation of 14C-Oftanol in pond water containing soil.
Mobay Report No. 53618. (Unpublished)
Minor R.G. Identification of volatile isofenphos soil metabolites.
1980 Mobay Report No. 68658. (Unpublished)
Minor R.G. and Murphy, J.J. Metabolism of 14C-Oftanol in soil. Mobay
1977 Report No. 53643. (Unpublished)
Mobay Residue Reports nos. 53819, 53888, 65932, 67505, 67508,
1979-80 68461-68463 (Corn). (Unpublished)
1978a Residue Reports nos. 53820, 53857-53859, 65931, 65933,
65935-65937, 53817, 5382353825, 53853, 65637, 65923, 65924,
65926, 65927 (Sweet corn). (Unpublished)
1978b Residue Reports nos. 65479-65481, 65491-65493, 65657, 65468,
65477, 65484-65487, 65636, 65656, 67394-67395 (Onions).
(Unpublished)
1980a Residue Reports nos. 69025-69027 (Rapes). (Unpublished)
1980b Residue Reports nos. 53620, 53649, 53695, 53698, 53895,
67378 (Stability studies). (Unpublished)
1980c Residue Reports nos. 69101, 69106, 69111, 69128, 69129,
69130, 69133, 69147, 69148 (Degradation in soil).
(Unpublished)
1980d Residue Reports nos. 69101, 60103-69106, 69107, 69-108,
69110, 69111, 69127 69134-69136, 69144, 69145, 69204-69206,
69128-69130, 69133, 69147, 69148 (Residues in rotational
crops). (Unpublished)
Mürmann, P. SRA 12869 subchronic toxicity study on dogs (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Nelson, D.L. and Burke, M.A. Acute dermal toxicity of Oftanol
1977a technical. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG. (Unpublished)
1977b Acute oral toxicity of Oftanol technical to mallard ducks.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
1977c Dietary toxicity of Oftanol technical to bobwhite quail.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Roney, D.J. Accumulation and persistence of residues
1977 in channel catfish exposed to Oftanol-14C. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Anderson, R.H. The skin-sensitizing properties of
1977 Oftanol technical to guinea pigs. Report from
Pharmakologisches Institut der Universität Mainz, submitted
by Bayer AG. (Unpublished)
Netherlands. Information from the Netherlands on pesticides included
1981 in the JMPR priority list.
Obrist, J.J. and Thornton, J.S. Leaching characteristics of aged
1977 Oftanol soil residues. Mobay Report No. 53944. (Unpublished)
Oesch, F. Ames Test for Oftanol (isofenphos). Report from Chemagro
1977 Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Palmer, A.K., Killick, M.E. and Allen, T.R. Effect of SPA 12869 on
1977 reproductive function of multiple generations in the rat.
Report from Huntingdon Research Centre, UK, submitted by
Bayer AG. (Unpublished)
Poje, A.J. AMAZE photoproduct identification. Mobay Report No. 68146.
1979 (Unpublished)
Ross, D.B., Cameron, D.M. and Roberts, N.L. The acute oral toxicity
1976 (LD50) of isofenphos to the starling. Report from
Huntingdon Research Centre, England, submitted by Bayer AG.
(Unpublished)
Schlüter, G. SRA 12869 Untersuchungen auf embryotoxische und
1981 teratogene wirkungen nach cutaner verabreichung an der
ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Schlüter, D. and Thyssen, J. SRA 12869 untersuchungen auf
1981 embryotoxische und teratogene wirkungen nach inhalation an
der ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Shaw II, H.R. Alkaline emission gas-chromatography method for
1974 determining BAY 92 114 and HOL 0654 in soil and water. Mobay
Report no. 43 072. 1 Oct. (Unpublished)
1977a A gas-chromatographic method for Oftanol, Oftanol oxygen
analogue, des N-isopropyl Oftanol and des-N-isopropyl
Oftanol oxygen analogue in bovine and poultry tissues, milk
and eggs. Mobay Report no. 52 769. (Unpublished)
1977b An interference study for the residue method of Oftanol,
Oftanol oxygen analogue, des-N-isopropyl Oftanol and des
N-isopropyl Oftanol oxygen analogue in bovine and poultry
tissues, milk and eggs. Mobay Report no. 53 512.
(Unpublished)
1977c Gas-chromatographic method for residues of Oftanol and
Oftanol oxygen analogue in soils. Mobay Report no. 53 690.
(Unpublished)
1979 Supplementary methods for eliminating interferences from
Delnav, malathion and Ronnel in the residue methods for
AMAZETM in animal tissues, milk and eggs, Mobay Report
no. 67 652, 23 May 1979. (Unpublished)
Spare, W.C. 14C-AMAZETM activated sludge metabolism. Mobay Report no.
1979 68 201. (Unpublished)
Stanley, C.W., Minor, R.G. and Murphy, I.J. Metabolism of OFTANOLR in
1977 corn. Mobay Report No. 53 110. (Unpublished)
Stanley, C.W. Metabolism of OFTANOLR in onions. Mobay Report no.
1977a 54 043. (Unpublished)
1977b Gas-chromatographic method for residues of OFTANOL and its
metabolites in corn and onions. Mobay Report no. 53 111.
(Unpublished)
1977c An interference study for the residue method for OFTANOL and
its organophosphate metabolites in corn and onions. Mobay
Report no. 53 514, 5 July. (Unpublished)
Stanley, C.W. Gas-chromatographic method for residues of AMAZETM and
1979a its metabolites in corn. Mobay Report no. 67 530.
(Unpublished)
1979b Supplementary methods for eliminating interferences of
diazinon, Di-syston and phorate with AMAZETM residues in
the residues method for crops. Mobay Report no. 67 651.
(Unpublished)
Shaw, H.R. Strankowski, K.J. and Murphy, J.J. Excretion and metabolism
1977 of BAY SRA 12869 by rats. Report from Chemagro Agricultural
Division, Mobay Chemical Corp., submitted by Bayer AG.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, K. Isofenphos mutagenicity test
1980 on bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted by Bayer
AG. (Unpublished)
Solmecke, B. and Kimmerle, G. SRA 12869 subacute toxicity studies.
1972a Report from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b SRA 12869 special toxicological studies. Report from Bayer
AG, submitted by Bayer AG. (Unpublished)
1972c SRA 12869 acute toxicological studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J. Effects of feeding ROftanol/Oftanol/oxygen analogue,
1977 des-N-isopropyl Oftanol oxygen analogue (1:8:1) to chickens
for 28 days. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 AMAZETM octanol/water partition coefficient Mobay Report
no. 68 246. (Unpublished)
1980 AMAZETM water solubility. Mobay Report no. 69 175.
(Unpublished)
Strankowski, K.J. and Murphy, J.J. Metabolism and excretion of (14C)
1977a Oftanol by a lactating dairy cow. Report from Chemical
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
1977b Metabolites of (14C) ROftanol identified in dairy cow
tissues. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG.; (Unpublished)
1977c Photodegradation of 14C-Oftanol in aqueous solution. Mobay
Report no. 53 939 (Unpublished)
Strankowski, K.J., Shaw II, H.R. and Murphy, J.H. Metabolites of (14C)
1977a ROftanol identified in rat tissues. Report from Chemagro
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Strankowski, K.J., Minor, R.G. and Murphy, J.J. Excretion and
1977b metabolism of (14C) ROftanol in a pig. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J., McNamara, F.T. and Minor, R.G. Effects of feeding
1977c ROftanol/Oftanol oxygen analogue,des-N-Isopropyl Oftanol
Oxygen analogue (1:8:1) to dairy cattle for 28 days. Report
from Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Thomson, C. and Newman, A.J. Pathology report of Bayer SRA 12869 sub-
1973 chronic toxicity experiment in dogs (13 weeks administration
in the diet). Report from Hungtindon Research Centre,
England, submitted by Bayer AG. (Unpublished)
Thornton, J.S., Hurley, J.B. and Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticide chemicals. Mobay Report no. 51 016.
(Unpublished)
Thyssen, J. HOL 0654 special toxicological studies. Report from Bayer
1974a AG, submitted by Bayer AG. (Unpublished)
1974b HOL 0654 subacute toxicity studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1974c HOL 0654 akute toxizität bei hühnern. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isofephos or phoxim. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1977 Toxikologische untersuchungen bei gleichzeitiger applikation
von SRA 7502 und SRA 12869. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
1978 SRA 12869 Acute toxicity studies on hens and quail. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Thyssen, J. and Kaliner, G. SRA 12869 subacute dermal cumulative
1977 toxicity study on rabbits. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
Thyssen, J. and Kimmerle, G. HOL 0654 acute toxicity studies. Report
1974 from Bayer, AG, submitted by Bayer AG. (Unpublished)
Urwin, C. and Newman, A.J. Pathology report of SRA 12869 3-month rat
1973 study. Report from Huntingdon Research Centre, UK, submitted
by Bayer AG. (Unpublished)
Wagner, K. Method for the gas-chromatographic determination of
1976 OftanolR residues in plants soil and water. Pflanzenshutz-
Nachrichten Bayer, 29: 67 - 80.
Weissenburger, B. and Pollock, R.J. Photodegradation of 14C AMAZE on a
1979 soil surface Mobay Report no. 68 245. (Unpublished)
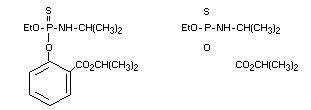 Other information on identity and properties
Empirical formula: C15H24NO4PS
Molecular weight: 345.4
Appearance: colourless oil (pure active ingredient)
yellow-brown liquid (technical active
ingredient)
Boiling point: not distillable (pure active ingredient)
at and above 200°C decomposition (techn.
a.i.)
Vapour pressure: 4 × 10-6mbar at 20°C (pure active
ingredient)
Specific gravity: 1.134 at 20°C
4°
Solubility: water 0.002 (pure active ingredient)
(g/100 g solvent
at 20°C) cyclohecanone > 60
isopropyl alcohol > 60
methylene chloride > 60
ligroin (80 -110°C) > 60
toluene > 60
Minimum degree of
purity: 88%
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, elimination and biotransformation
Rat
After a single oral dose of 15 mg/kg bw of ring-uL-(14C)-
isofenphos, male and female rats excreted 88% of the administered
radioactivity in the urine and 4% in the faeces within 72h of dosing.
No 14CO2 was detectable in the expired air. The rate of elimination
during the first 24 h appeared to be slower in females than in males,
but the total percentage of administered radioactive dose recovered in
the excreta of both sexes in the 72h post-treatment period was
comparable. The major urinary metabolite identified was isopropyl
salicylate (in conjugated and non-conjugated forms), which accounts
for 52% of the 14C-dose given to the animals. Other metabolites found
in the urine included conjugated and unconjugated O-hydroxyhippuric
acid, salicylic acid and deaminated isofenphos oxygen analogue, which
together amounts to approximately another 10% of the administered
radioactivity. There was no significant sex difference in metabolites,
either qualitatively or quantitatively (Shaw et al 1977).
Female rats were intubated with ring uL(14C)-isofenphos at
15 mg/kg bw/day for 6 consecutive days. Radioactive residues in the
tissues analysed (liver, fat, kidney and muscle) remained fairly
constant during the dosing period and declined sharply upon cessation
of treatment. At the end of an 8-day withdrawal period, the kidney was
the only tissue still containing small amounts of 14C-residues. In
the rats sacrificed 1 1/2 h after the sixth dose, unchanged
isofenphos, isofenphos oxygen analogue, isopropyl salicylate and
O-hydroxyhippuric acid were detected in all four tissues examined. The
major identified component in liver, kidney and fat was unchanged
isofenphos and in muscle was isopropyl salicylate. Deaminated
isofenphos oxygen analogue was also found in liver, kidney and muscle
(Strankowski et al 1977a).
Pig
A male pig was fed ring-uL-(14C)-isofenphos-treated feed pellets
at a rate of 2 mg/kg bw/day for 5 consecutive days. Consumption of the
treated feed was monitored to ensure complete intake of the daily
dose. Within 24h of the first dose, 81% of the administered 14C was
eliminated in the urine and 14% in the faeces. Of the total
radioactivity administered during the 5-day dosing period, 56% was
recovered in the urine and 21% in the faeces. At sacrifice 3h after
treatment on day 5 of the study, the kidney had the highest
14C-residue level and the brain had the lowest, as compared to 5
other tissues (liver, heart, bacon, ham and fat) examined.
A total of 76% 14C-residue in a composite urine sample was
identified. The urinary metabolites included conjugated and
unconjugated isopropyl salicylate (55%), deaminated isofenphos oxygen
analogue glucuronide (10%), salicylic acid (5%), O-hydroxyhippuric
acid (4%) and cyclic isofenphos (2%). Unchanged isofenphos, found in
all 4 tissues (liver, kidney, muscle, fat) analysed was the primary
component identified in muscle and fat. Isopropyl salicylate
(conjugated and unconjugated) was the major metabolite in liver and
kidney. Other tissue metabolites detected included deaminated
isofenphos oxygen analogue O-hydroxyhippuric acid, isofenphos oxygen
analogue and des N-isopropyl isofenphos (Strankowski et al 1977b).
Cow
In a lactating dairy cow treated acutely, via bolus, with ring-uL
(14C)-isofenphos at 0.2 mg/kg bw, 14C concentration in the blood
plasma peaked 2h after the dose and then dropped sharply to one fourth
of the maximum level within 24h of treatment. Approximately 90% of the
administered radioactive dose was eliminated in the urine and 4% in
the faeces within 48 h of dosing. Less than 1% of the 14C dose was
recovered in the milk. The major metabolite in both urine and milk was
O-hydroxyhippuric acid. Other metabolites identified in urine and milk
were salicylic acid, isopropyl salicylate and deaminated isofenphos
oxygen analogue. In urine and the milk, about 78% and 66% respectively
of the radioactive residues were identified (Strankowski and Murphy
1977a).
A lactating cow receiving, via bolus, 0.2 mg/kg bw/day of ring-
uL(14C)-isofenphos for 5 days had excreted 63% of the total
administered radioactivity in the urine at sacrifice 2 h after the
fifth dose. No 14C residues were indicated in the report to
"accumulate" in the milk. Of the 11 tissues analysed for radioactive
tissues at sacrifice, liver and kidney were the only tissues found to
have significant radioactivity. The major metabolite in liver and
kidney was salicylic acid, both conjugated and unconjugated. Other
metabolites identified in both tissues were isofenphos oxygen analogue
and conjugated and non-conjugated forms of O-hydroxyhippuric acid and
isopropyl salicylate (Strankowski and Murphy 1977b).
Figure 1 presents the proposed metabolic pathways for isofenphos
in mammals (rat, pig and cow) (Shaw et al 1977; Strankowski et al
1977b; Strankowski and Murphy 1977a).
Hen
In hens orally treated with ring-uL(14C)-isofenphos at 4 mg/kg
bw/day for 3 consecutive days, 41% of the total administered dose was
stated to be eliminated in the excreta "within 51 h" presumably of the
last dose. The major components found in the excreta were isofenphos
and isopropyl salicylate. The total organphosphate residue in tissues
and eggs accounted for less than 3% of the total dose administered. In
the tissues and eggs, isofenphos was also the major compound
identified, with isopropyl salicylate, isofenphos oxygen analogue and
des N-isopropyl isofenphos being the minor metabolites (Kurtz and Shaw
II 1977).
Fish
Channel catfish were exposed continuously to ring-uL (14C)-
isofenphos in water at a concentration of about 10 ppb for 28 days.
During the exposure period, the 14C residues accumulated to a peak of
approximately 75 times the level of the water in 7 days and slowly
declined thereafter. About 88% of the total 14C-residues in the whole
fish could be extracted by acetonitrile, and the non-edible portion of
the fish contained 76% of the extractable residues. One hundred
percent of these 14C-residues were identified as unchanged
isofenphos. Upon treatment withdrawal, approximately 63% of the
accumulated 14C-residues were excreted within 5 h and 87% within one
day. Ten days after discontinuation of exposure only 4% of the
accumulated radioactivity still remained in the fish (Nelson and Roney
1977).
Effects on enzymes and other biochemical parameters
The 50% depression of cholinesterase activity in serum,
erythrocyte and brain of male rats by isofenphos ("technically pure
grade") was determined in vitro. The I50 values were: serum:
8.2 × 10-4 mole, erythrocyte: 3.1 × 10-4 mole, and brain:
2.87 × 10-4 mole (Solmecke and Kimmerle 1972b).
Groups of 5 male rats were given acute oral doses of isofenphos
at levels ranging from 0.5 to 35 mg/kg bw. Activity of plasma and
erythrocyte cholinesterase in the males was depressed (20 to 81%), in
a dose-dependent pattern, above 0.5 mg/kg bw at both 2 and 24 h post-
treatment, with maximum inhibition being noted at 2 h. Plasma
cholinesterase was no longer affected at any dosage level 3 days after
dosing, while erythrocyte cholinesterase was still inhibited by 26% at
35 mg/kg bw 7 days after treatment. In female rats, a single oral dose
of isofenphos at 2.5 mg/kg bw but not at 0.5 mg/kg bw caused a
Other information on identity and properties
Empirical formula: C15H24NO4PS
Molecular weight: 345.4
Appearance: colourless oil (pure active ingredient)
yellow-brown liquid (technical active
ingredient)
Boiling point: not distillable (pure active ingredient)
at and above 200°C decomposition (techn.
a.i.)
Vapour pressure: 4 × 10-6mbar at 20°C (pure active
ingredient)
Specific gravity: 1.134 at 20°C
4°
Solubility: water 0.002 (pure active ingredient)
(g/100 g solvent
at 20°C) cyclohecanone > 60
isopropyl alcohol > 60
methylene chloride > 60
ligroin (80 -110°C) > 60
toluene > 60
Minimum degree of
purity: 88%
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, elimination and biotransformation
Rat
After a single oral dose of 15 mg/kg bw of ring-uL-(14C)-
isofenphos, male and female rats excreted 88% of the administered
radioactivity in the urine and 4% in the faeces within 72h of dosing.
No 14CO2 was detectable in the expired air. The rate of elimination
during the first 24 h appeared to be slower in females than in males,
but the total percentage of administered radioactive dose recovered in
the excreta of both sexes in the 72h post-treatment period was
comparable. The major urinary metabolite identified was isopropyl
salicylate (in conjugated and non-conjugated forms), which accounts
for 52% of the 14C-dose given to the animals. Other metabolites found
in the urine included conjugated and unconjugated O-hydroxyhippuric
acid, salicylic acid and deaminated isofenphos oxygen analogue, which
together amounts to approximately another 10% of the administered
radioactivity. There was no significant sex difference in metabolites,
either qualitatively or quantitatively (Shaw et al 1977).
Female rats were intubated with ring uL(14C)-isofenphos at
15 mg/kg bw/day for 6 consecutive days. Radioactive residues in the
tissues analysed (liver, fat, kidney and muscle) remained fairly
constant during the dosing period and declined sharply upon cessation
of treatment. At the end of an 8-day withdrawal period, the kidney was
the only tissue still containing small amounts of 14C-residues. In
the rats sacrificed 1 1/2 h after the sixth dose, unchanged
isofenphos, isofenphos oxygen analogue, isopropyl salicylate and
O-hydroxyhippuric acid were detected in all four tissues examined. The
major identified component in liver, kidney and fat was unchanged
isofenphos and in muscle was isopropyl salicylate. Deaminated
isofenphos oxygen analogue was also found in liver, kidney and muscle
(Strankowski et al 1977a).
Pig
A male pig was fed ring-uL-(14C)-isofenphos-treated feed pellets
at a rate of 2 mg/kg bw/day for 5 consecutive days. Consumption of the
treated feed was monitored to ensure complete intake of the daily
dose. Within 24h of the first dose, 81% of the administered 14C was
eliminated in the urine and 14% in the faeces. Of the total
radioactivity administered during the 5-day dosing period, 56% was
recovered in the urine and 21% in the faeces. At sacrifice 3h after
treatment on day 5 of the study, the kidney had the highest
14C-residue level and the brain had the lowest, as compared to 5
other tissues (liver, heart, bacon, ham and fat) examined.
A total of 76% 14C-residue in a composite urine sample was
identified. The urinary metabolites included conjugated and
unconjugated isopropyl salicylate (55%), deaminated isofenphos oxygen
analogue glucuronide (10%), salicylic acid (5%), O-hydroxyhippuric
acid (4%) and cyclic isofenphos (2%). Unchanged isofenphos, found in
all 4 tissues (liver, kidney, muscle, fat) analysed was the primary
component identified in muscle and fat. Isopropyl salicylate
(conjugated and unconjugated) was the major metabolite in liver and
kidney. Other tissue metabolites detected included deaminated
isofenphos oxygen analogue O-hydroxyhippuric acid, isofenphos oxygen
analogue and des N-isopropyl isofenphos (Strankowski et al 1977b).
Cow
In a lactating dairy cow treated acutely, via bolus, with ring-uL
(14C)-isofenphos at 0.2 mg/kg bw, 14C concentration in the blood
plasma peaked 2h after the dose and then dropped sharply to one fourth
of the maximum level within 24h of treatment. Approximately 90% of the
administered radioactive dose was eliminated in the urine and 4% in
the faeces within 48 h of dosing. Less than 1% of the 14C dose was
recovered in the milk. The major metabolite in both urine and milk was
O-hydroxyhippuric acid. Other metabolites identified in urine and milk
were salicylic acid, isopropyl salicylate and deaminated isofenphos
oxygen analogue. In urine and the milk, about 78% and 66% respectively
of the radioactive residues were identified (Strankowski and Murphy
1977a).
A lactating cow receiving, via bolus, 0.2 mg/kg bw/day of ring-
uL(14C)-isofenphos for 5 days had excreted 63% of the total
administered radioactivity in the urine at sacrifice 2 h after the
fifth dose. No 14C residues were indicated in the report to
"accumulate" in the milk. Of the 11 tissues analysed for radioactive
tissues at sacrifice, liver and kidney were the only tissues found to
have significant radioactivity. The major metabolite in liver and
kidney was salicylic acid, both conjugated and unconjugated. Other
metabolites identified in both tissues were isofenphos oxygen analogue
and conjugated and non-conjugated forms of O-hydroxyhippuric acid and
isopropyl salicylate (Strankowski and Murphy 1977b).
Figure 1 presents the proposed metabolic pathways for isofenphos
in mammals (rat, pig and cow) (Shaw et al 1977; Strankowski et al
1977b; Strankowski and Murphy 1977a).
Hen
In hens orally treated with ring-uL(14C)-isofenphos at 4 mg/kg
bw/day for 3 consecutive days, 41% of the total administered dose was
stated to be eliminated in the excreta "within 51 h" presumably of the
last dose. The major components found in the excreta were isofenphos
and isopropyl salicylate. The total organphosphate residue in tissues
and eggs accounted for less than 3% of the total dose administered. In
the tissues and eggs, isofenphos was also the major compound
identified, with isopropyl salicylate, isofenphos oxygen analogue and
des N-isopropyl isofenphos being the minor metabolites (Kurtz and Shaw
II 1977).
Fish
Channel catfish were exposed continuously to ring-uL (14C)-
isofenphos in water at a concentration of about 10 ppb for 28 days.
During the exposure period, the 14C residues accumulated to a peak of
approximately 75 times the level of the water in 7 days and slowly
declined thereafter. About 88% of the total 14C-residues in the whole
fish could be extracted by acetonitrile, and the non-edible portion of
the fish contained 76% of the extractable residues. One hundred
percent of these 14C-residues were identified as unchanged
isofenphos. Upon treatment withdrawal, approximately 63% of the
accumulated 14C-residues were excreted within 5 h and 87% within one
day. Ten days after discontinuation of exposure only 4% of the
accumulated radioactivity still remained in the fish (Nelson and Roney
1977).
Effects on enzymes and other biochemical parameters
The 50% depression of cholinesterase activity in serum,
erythrocyte and brain of male rats by isofenphos ("technically pure
grade") was determined in vitro. The I50 values were: serum:
8.2 × 10-4 mole, erythrocyte: 3.1 × 10-4 mole, and brain:
2.87 × 10-4 mole (Solmecke and Kimmerle 1972b).
Groups of 5 male rats were given acute oral doses of isofenphos
at levels ranging from 0.5 to 35 mg/kg bw. Activity of plasma and
erythrocyte cholinesterase in the males was depressed (20 to 81%), in
a dose-dependent pattern, above 0.5 mg/kg bw at both 2 and 24 h post-
treatment, with maximum inhibition being noted at 2 h. Plasma
cholinesterase was no longer affected at any dosage level 3 days after
dosing, while erythrocyte cholinesterase was still inhibited by 26% at
35 mg/kg bw 7 days after treatment. In female rats, a single oral dose
of isofenphos at 2.5 mg/kg bw but not at 0.5 mg/kg bw caused a
 reduction in erythrocyte cholinesterase level by 20% at 2 h post
dosing but not thereafter. Plasma cholinesterase was not affected.
Male rats sacrificed 24h after acute oral doses of isofenphos above
1 mg/kg bw displayed a dose-related decrease (23-74%) in activity of
brain cholinesterase (Solmecke and Kimmerle 1972b).
Groups of 5 male and 5 female rats (Wistar II strain of
unspecified age) were intubated acutely with the oxygen analogue of
isofenphos at 0.1, 0.25, 0.5, 1.0, 2.5 or 5 mg/kg bw (males) or at
0.25, 0.5 or 1 mg/kg bw (females). Cholinesterase in plasma and
erythrocytes was assayed at 2, 5, 24, 48h and 7 days (in males only)
post treatment. No control groups were included in the experiment and
the pre-treatment cholinesterase activities of individual groups were
used as the basis of comparison. In males, there was a depression
(>20%), generally dose-dependent, of plasma cholinesterase at
0.5 mg/kg bw and above and of erythrocyte cholinesterase at 2.5 mg/kg
bw and above after 2, 5 and 24 h. (Males at 1 mg/kg bw showed a
decrease (29%) of erythrocyte cholinesterase after a single interval
of 5 h.) Complete or almost complete recovery to pre-treatment values
was seen after 48 h for plasma cholinesterase and after 7 days for
erythrocyte cholinesterase. In females, both plasma and erythrocyte
cholinesterase was inhibited (20 to 43%) at 0.25 mg/kg bw and above
during the first 24 h. Activities of cholinesterase in plasma and
erythrocyte were close to the pre-treatment values 48 h after dosing.
The inhibitory effect of the oxygen analogue on plasma and erythrocyte
cholinesterase appeared to peak about 2 or 5 h after treatment in both
sexes (Thyssen 1974a).
Groups of 5 male and 5 female rats were orally treated with
oxygen analogue of isofenphos and then sacrificed 24 h later for
determination of brain cholinesterase activity. It was stated (no data
were provided) that brain cholinesterase activity in males was
moderately depressed at both 10 and 20 mg/kg bw but was unaffected at
5 mg/kg bw. In the case of females, brain cholinesterase activity was
stated to be within physiological ranges at dosages ranging from 1 to
5 mg/kg bw (Thyssen 1974a).
TOXICOLOGICAL STUDIES
Acute toxicity
In experiments with mice, rats, hamsters and rabbits exposed in
inhalation chambers to isofenphos (dissolved in a mixture of alcohol
and polyethylene glycol 400) as a spray at 30-minute intervals during
a 4-h period, rats and hamsters were found to be more sensitive than
mice and rabbits as shown in Table 1 (Solmecke and Kimmerle 1972c).
TABLE 1. Acute toxicity of isofenphos in animals
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Mouse M Oral PG 127 Solmecke and Kimmerle 1972c
F 91.3
M Inhalation Ethanol & > 0.272 mg/l Solmecke and Kimmerle 1972c
(4-hour PG (1:1)
exposure)
Rat M Oral PG 38.7-45 ) Solmecke and Kimmerle 1972c
F 28 - 32 ) Lamb et al 1977
M Oral Water &
Cremophor EL 33 - 48.2 Flucke 1980a;Flucke 1981
M Oral " 19.9 Flucke 1980b
M I.P. PG 35.8 Solmecke and Kimmerle 1972c
F 29.5
M Dermal None >1000 µl/kg bw) Solmecke and Kimmerle 1972c
(4-hour
exposure) None
M Dermal(24-h) None 705 µl/kg bw Kimmerle 1972a
Dermal None 188 µl/kg bw Solmecke and Kimmerle 1972a
(7-day
exposure) (undiluted)
M&F Inhalation Ethanol & >1300 mg/l air Kimmerle 1976
(1-hour PG (1:1)
exposure)
M Inhalation Ethanol & 0.21 mg/l air Solmecke and Kimmerle
F (4-hour PG (1.1) 0.144 mg/l air 1972c
exposure)
TABLE 1. (con't)
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Hamster M Inhalation Ethanol & 0.23 mg/l air Solmecke and Kimmerle
(4-hour PG (1:1) 1972c
exposure)
Rabbit F Oral PG approx. 150 Solmecke and Kimmerle 1972c
M Dermal Ethanol & 162 Nelson and Burke 1977a
F (24-hour PG (1:1) 315
exposure)
Dog F Oral PG > 25 Solmecke and Kimmerle 1972c
Bobwhite quail M&F Oral PG 8.7 Lamb and Burke 1979
Mallard duck M Oral PG 36.0 Nelson and Burke 1977b
F 32.0
Quail M Oral Water & 10 Thyssen 1978
Cremophor EL 10
Starling Oral Corn oil 972 Ross et al 1976
Hen F Oral Water & 16 Thyssen 1978
Cremophor EL
PG - polyethylene glycol 400.
Signs of oral poisoning in mammals
Cholinergic symptoms (e.g. muscle twitching, breathing disorders)
induced by toxic acute oral doses of isofenphos were typical of those
of cholinesterase inhibitors and were similar in mice, rats and
rabbits. Toxic signs usually developed within 2 h of dosing in mice
and rats and 4 h in rabbits and lasted for 2 to 10 days in survivors.
Mortalities from lethal doses usually occurred 1 to 4 days after
treatment. Cholinergic signs were not observed in dogs treated at
25 mg/kg bw, the top dosage level tested (Solmecke and Kimmerle
1972c).
Short-term studies
Rat
Groups of 15 male and 15 female rats (SPF Wistar II strain of
unspecified age) were orally treated by gavage with isofenphos ("pure
technical grade") in polyethylene glycol 400 at 0, 0.1, 0.25, 1.0 or
2.5 mg/kg bw/day for 30 days. Mortality, behaviour, growth, organ
weights and liver and kidney function were unaffected. Dose-related
inhibition (>20%) of cholinesterase in plasma and erythrocytes was
apparent at and above 1 mg/kg bw in females at 9, 16 and 30 days of
the study. In males, depression (dose related, >20%) of erythrocyte
cholinesterase was noted at both 1 and 2.5 mg/kg bw at 16 and 30 days
and of plasma cholinesterase at and above 1 mg/kg bw at 16 days and at
2.5 mg/kg bw also at 9 and 30 days (Solmecke and Kimmerle 1972a).
Groups of 10 male and 10 female rats (SPF Wistar W74 strain, 8
weeks old) were fed isofenphos (91.9% pure) in the diet at the dosage
levels of 0, 0.3, 1.0 or 3 ppm for 4 weeks. There was no mortality.
Behaviour, body weight and food consumption were not significantly
different between control and treated animals. Determinations of blood
cholinesterase activity after 3, 7, 10, 14, 17, 21, 24 and 28 days of
dietary feeding indicated dose-related inhibition (>20%) of plasma
cholinesterase in females at 1 ppm after 7, 17 and 28 days and at
3 ppm at all intervals. Only a slight depression (13-16%) of plasma
cholinesterase was noted in males even at 3 ppm. Activity of
erythrocyte cholinesterase determined over the course of the study and
of brain cholinesterase measured terminally was not affected in all
treated groups (both sexes). The no-effect level on plasma
cholinesterase was 0.3 ppm, which was equivalent to 0.024 mg/kg bw,
according to body weight and food consumption data (Löser 1978).
Groups of 15 male and 15 female rats (SPF Wistar strain, 28 to 32 days
old) were fed isofenphos (86.6% pure) in their diet at levels of 0.5,
1, 5, 25 or 125 ppm for 3 months. The control group consisted of 30
males and 30 females. One male at 125 ppm died after 9 days, due to
pneumonia. Toxic signs, such as salivation and muscle twitching, were
observed in animals receiving 125 ppm during the first 2 weeks of
feeding. Body weight was adversely affected in the highest dosage
group (both sexes). Food consumption was comparable to controls.
Haematological and biochemical studies and urinalysis conducted after
one and three months of the study indicated no significant dose-
related findings other than an increase in SGOT in males at 125 ppm at
the 1-month interval. Measurements of cholinesterase activity after 1,
4, 8 and 13 weeks of feeding revealed depression (dose-related >20%)
of the enzyme in both plasma and erythrocytes in females at 5 ppm and
above and in males at 25 ppm and above at all intervals. (Males at
5 ppm showed a 29% decrease of plasma cholinesterase level after one
week of feeding, but not thereafter.) The brain cholinesterase level
determined terminally was reduced at 25 ppm and above in both sexes.
At sacrifice upon termination of the study, an increase in absolute
weight and organ/body weight ratio of thymus was seen in females at
125 ppm. There were also variations in absolute weights of several
organs in males of the top dosage group. No compound-induced
histopathological alterations were observed in a variety of tissues
evaluated, including the thymus (Löser 1973; Urwin and Newman 1973).
Dog
Groups of dogs (4 males and 4 females per group, 5 months old)
were fed isofenphos (of unspecified purity) in the diet daily at 0,
0.3, 1.0, 10 or 30 ppm for 3 months. There were no treatment-related
effects on mortality, clinical symptoms, reflexes, body weight, food
consumption, ophthalmoscopic, haematological, clinical chemistry and
urinalysis parameters. Cholinesterase in plasma and erythrocytes was
depressed (by >20%), in a dose-dependent manner, at and above
10 ppm in both sexes after 3, 6 and 13 weeks of the study. (Plasma
cholinesterase in these dosage groups was inhibited also after 1 week
of the study.) At the conclusion of the study, absolute weight and
organ/body weight ratio of the liver was elevated at 10 ppm and
above in males (non-dose related) and females (dose related).
Histopathological examination of about 30 tissues, including the
liver, from each dog revealed no abnormalities or variations
associated with inclusion of isofenphos in the diet. The no-effect
level for the study was 1.0 ppm (Murmann 1973; Thomson and Newman
1973).
Groups of dogs (4 males and 4 females per group) were fed a daily
diet containing the following levels of isofenphos (89.3% pure) in a
2-year study: Control: 0 ppm; Group 1: males - 3 ppm from week 1
to week 83 and 2 ppm from week 84 to week 104, and females - 3 ppm
from week 1 to week 104; Group 2: 15 ppm throughout the 104 weeks,
and Group 3: 75 ppm from week 1 to week 53, 150 ppm from week 54 to
week 99 and 300 ppm from week 100 to week 104. No compound-related
findings were noted with respect to general condition (reflexes, body
temperature and pulse rate), water intake, ophthalmoscopic and
urinalysis parameters. Assay of cholinesterase 12 times over the
course of the study indicated dose-dependent inhibition (>20%) of
plasma cholinesterase in Groups 2 and 3 (both sexes) and of
erythrocyte cholinesterase in Group 3 at all intervals. Depression
(approx. or equal to 20%) of plasma cholinesterase was also found in
Group 1 males at weeks 7, 39, 66 and 79 but was not evident at weeks
92 and 103 after the dietary level was reduced from 3 ppm to 2 ppm at
week 84. Animals of Group 3 also showed depression (>60%) of brain
cholinesterase activity measured terminally.
Adverse effects on parameters other than cholinesterase activity
in plasma and erythrocytes were observed only in Group 3. In this
group, one male died at week 104 and another male was sacrificed
terminally in moribund condition. Toxic symptoms, such as salivation,
vomiting, diarrhoea, weakness in the hind extremities, unsteady gait,
etc., began to appear after 78 weeks and increased in severity with
time. The symptoms were more marked in males than in females.
Depression of body weight gain (both sexes) and of food consumption
(females) was obvious after 100 weeks. An increase in the SAP level at
several intervals during the study and a reduction of the terminal
serum A/G ratio were observed in males and females. The one male that
died at 104 weeks showed significant changes in several haematological
parameters. At terminal sacrifice, elevated organ/body weight ratios
of lung, liver, kidney, prostate, brain and pituitary and depressed
organ/body weight ratios of adrenal and testis were detected in the
males but, except in the testis, microscopic abnormalities of the
tissues in question were not noted. Histopathologically, hypoplasia of
the testis and erosion of esophageal mucosa, partially accompanied by
cellular reaction of propria mucosa, were observed in several males of
Group 3 but none in the other groups, including the control. Two
animals (Group 3) also exhibited foci of softening ("degeneration
processes") in the brain stem with a degree of severity greater than
the one case seen in the control group. The no-effect level in the
study was 2 ppm (Hoffman and Kaliner 1977).
Hen and quail
In adult hens and female quail intubated with isofenphos as an
emulsion in water and polyethylene glycol 400 daily for 5 consecutive
days, and then observed for 14 additional days, the 5 day LD50 was
found to be 6 mg/kg bw/day and 5 mg/kg bw/day respectively. Major
symptoms observed were inactivity in both species, ruffled feathers
and breathing disorders in the quail (Thyssen 1978).
Bobwhite quail and mallard duck
The dietary LC50 in bobwhite quail and mallard ducks fed
isofenphos in their diet for 5 days, followed by untreated diet for
3 days, was determined to be 145 ppm (Nelson and Burke 1977) and
>1000 ppm respectively (Nelson and Burke 1977c; Lamb and Burke 1977).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females per
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to isofenphos (as an emulsion in water and
Cremophor EL) at 0, 1 or 5 mg/kg bw/day 7 h per day, 5 times per week
for a total of 15 applications in a 21-day period. The treated sites,
not covered with bandages, were washed with soap and water after each
daily exposure period. One male at 5 mg/kg bw died of a cause
unrelated to treatment after 10 applications. There were no toxic
signs or adverse effects on body weight or on terminal haematological
and clinical chemistry parameters. Local irritation on treated sites,
generally transitory in nature and characterized by erythema on intact
skin and erythema, oedema and eschar formation on abraded skin, were
seen in some animals at 5 mg/kg bw. Changes in weights of several
organs were seen at 5 mg/kg bw without any accompanying
histopathological changes. Plasma and erythrocyte cholinesterase
activities measured after the 10th and 15th exposures and brain
cholinesterase level determined terminally in both sexes were
significantly depressed at 5 mg/kg bw (Thyssen and Kaliner 1977).
Rat - inhalation
In male and female rats exposed to isofenphos as an aerosol (of
unspecified particle sizes) 4h per day for 5 days, the LC50 was
>0.055 mg/l in males and 0.029-0.055 mg/l in females ((Solmecke and
Kimmerle 1972c).
Groups of 10 male and 10 female rats were exposed to an aerosol
of isofenphos at 0, 0.72 or 2.93 mg/m3 air 6h per day for 5
consecutive days per week over a 3-week period. (No information was
given on the particle size of the aerosol.) The treated animals were
not different from the controls as judged by general condition, body
weight gain, terminal haematological values, kidney and liver
function, gross pathological changes and organ weights. Cholinesterase
in plasma and erythrocytes was inhibited (>20%) at 2.93 mg/m3 air at
each weekly determination (Solmecke and Kimmerle 1972a).
Long-term studies
Mouse
Groups of 40 male and 40 female mice (6 to 7 weeks old, SPF NMRI
strain) were fed diets containing technical isofenphos (89.3% pure) at
0, 1, 10 or 100 ppm for 108 weeks. Additionally, each group included 3
sub-groups of 10 males and 10 females. One sub-group, fed the treated
diet for 108 weeks, was used for haematological tests and urinalysis
every 3 months over the course of the study. Another sub-group
maintained on dietary feedings for 9 months, was subjected to liver
function tests (plasma alkaline phosphatase and GPT) every 3 months.
The third sub-group was given isofenphos in the diet for 24 weeks and
activity of cholinesterase in plasma and erythrocyte was measured
after 2, 4, 8, 12 and 24 weeks and in brain after 24 weeks. All
animals that died during the study or were sacrificed terminally were
subjected to gross and histopathological examination. Mortality, which
was high in all groups, including the control, especially in the
females, was not attributed to treatment. At the end of the study,
only 42 to 57% of the males and 12 to 20% of the females per group
were still alive. Nevertheless, 53 to 73% of females per group
survived at least 80 weeks. There were no significant differences
between control and treated groups in clinical symptoms, body weight
and food consumption. Data on differential white blood cell counts
showed an increase at 100 ppm of eosinophil (males) and segmented cell
counts (females) after 21 months and of segmented cell counts in males
after 24 months. Liver function tests and urinalysis parameters were
normal. Dose-related inhibition (72 to 94%) of plasma cholinesterase
occurred in both sexes at and above 10 ppm at all intervals.
Erythrocyte cholinesterase was unaffected in the treated groups, with
depression consistently below 20%. Terminal brain cholinesterase
activity was reduced at 100 ppm by 46% in males and 31% in females. At
terminal sacrifice, absolute weight of lung in males was significantly
elevated at 100 ppm but no concomitant histological lesion of the
particular tissue was evident. An apparent dose-related increase, as
compared to concurrent controls, in frequency of mild gastritis (in
glandular mucosa of the stomach) was observed in males of all treated
groups which was, however, not statistically significant, especially
in the case of the 1 ppm and 10 ppm groups. No histopathological
changes related to treatment was observed in a variety of other
tissues evaluated.
Based on the tumour data, isofenphos was not carcinogenic in the
mouse, as indicated by the absence of a dose-related increase in
incidence of a) any particular type of tumours, b) animals with
tumours, c) animals with multiple tumours, and d) animals with
malignant tumours. The time of appearance of tumours was comparable
between control and treated groups.
The no-effect level for the study was 1 ppm (Brune et al.
1978).
Rat
Groups of 50 male and 50 female rats (SPF Wistar strain, 28 to 32
days old) were fed diets containing isofenphos (of unspecified purity)
at 1, 10 or 100 ppm for 2 years. The control group consisted of 100
males and 100 females. Mortality was unaffected by treatment, and
between 69 and 90% of animals per group were still alive at the end of
the study. At 100 ppm, cholinergic signs were seen during the first
week and depression of growth in both sexes and of food consumption in
males was apparent throughout the feeding period. Haematological and
biochemical studies and urinalysis conducted at five intervals over
the course of the study failed to reveal any consistent effect related
to presence of isofenphos in the diet. Assay of cholinesterase after
1, 2, 4, 13, 26, 52, 78 and 105 weeks of the study indicated dose-
related inhibition (>20%) of both plasm and erythrocyte
cholinesterase at 10 ppm and above in both sexes at all intervals and
of plasma cholinesterase in males at 1 ppm after 4 and 78 weeks. The
extent of depression of cholinesterase in plasma and erythrocytes
remained fairly constant over the course of the study. At the
conclusion of the experiment, brain cholinesterase activity was
reduced at 100 ppm in both sexes. Variations in absolute weights of
several organs were seen at 100 ppm but histological changes of the
particular organs were absent. Except for an apparent increase in
incidence of ulceration of the forestomach in females at 100 ppm,
as compared to the concurrent controls, no treatment-related
alterations were evident in a set of about 30 tissues examined
histopathologically. Analysis of tumour data revealed no indications
suggestive of carcinogenic activity of isofenphos under the conditions
of the experiment.
The study demonstrated 1 ppm as a marginal no-effect level with
respect to plasma cholinesterase. On other parameters monitored,
10 ppm was without any significant adverse effect (Bomhard and Löser
1977).
Special studies on reproduction
Groups of 20 male and 20 female weanling rats (SPF CFY strain)
were fed dietary levels of technical isofenphos (of unspecified
purity) at 0, 1, 10 or 100 ppm for 60 days prior to mating to initiate
a reproduction study originally designed to cover 3 generations with
one litter per generation. Three litters (F1a, F2a and F3a) were
obtained as expected for control, 1 ppm and 10 ppm groups. In the case
of the highest dosage group, owing to poor survival of pups, there
were insufficient F1a weanlings to form an adequate second parental
generation (F1). F0 animals at 100 ppm, as well as those of other
groups including the control, were therefore re-mated. This second
mating again failed to provide sufficient F1b offspring at 100 ppm as
potential parents. In an attempt to determine the causes of the
initial adverse effects at 100 ppm, F0 animals of control and top
dosage groups were allowed two additional matings, with the fourth
mating using treated males and untreated females and vice-versa. In
the third mating trial, the number of litters (F1c) at 100 ppm
obtained was too small to permit any meaningful interpretation of
results. Based on results of the fourth mating, fertility appeared to
be adversely affected, primarily in the females. This summary will
refer only to data on control, 1 ppm and 10 ppm groups.
In parental generations, there were no mortality and clinical
symptoms attributable to treatment. Body weight gain during pre-
mating, gestation and lactation periods, mating performance, duration
of gestation and terminal gross pathological findings were not
significantly different from controls. Pregnancy rate was 90%, 70% and
60% respectively in control, 1 ppm and 10 ppm groups in the F0
generation at the second mating suggesting a slight effect at 10 ppm
and a marginal effect at 1 ppm. (The pregnancy rate at 100 ppm was 40%
for the second mating trial.) Assay of plasma and erythrocyte
cholinesterase in F0 animals after 37 weeks of dietary feeding
revealed inhibition (>20%) at 10 ppm of erythrocyte cholinesterase in
both sexes and of plasma cholinesterase in females. Over the three
progeny generations (F1a, F2a and F3a litters), litter size, pup
mortality and mean pup weight from birth through lactation to weaning
were comparable to controls at both 1 and 10 ppm. In F1b litters, pup
weight at 10 ppm seemed to be slightly decreased at days 12 and 21.
Pups observed grossly at weaning displayed no apparent compound-
induced abnormalities. The available data tend to indicate 1 ppm as a
marginal no-effect level with respect to reproductive capability
(Palmer et at 1977).
Special studies on teratogenicity
Rat
Groups of 20 to 21 pregnant rats (Long Evans FB30 strain) were
intubated with isofenphos (86.6% pure) in polyethylene glycol 400 at
doses of 0, 0.3, 1 or 3 mg/kg bw/day from gestation day 6 to day 15.
The dams were sacrificed on gestation day 20 and foetuses were removed
by caesarean section for external, visceral and skeletal examination.
No treatment-related mortality or abnormal behavioural reactions
were noted, and maternal body weight was normal during the dosing
period. An increase in the average number of resorbed foetuses per
litter occurred at 3 mg/kg bw, but a dose-related elevation in the
percentage of litters with rasorptions was not apparent at this dosage
level. There were no significant differences between control and
treated groups with respect to fertility rate, mean number of
implantation sites, mean litter size, foetal weight, placental weight
and incidence of stunted foetuses. The frequency of foetuses showing
slight bone variations and/or malformation was very low and not
significantly different from that in the control group (Machemer
1972).
Groups of 21 to 23 pregnant rats (Long Evans FB30 strain) were
exposed dermally to isofenphos (dissolved in polyethylene glycol 400)
at 0, 0.3, 1.0, 3 or 10 mg/kg bw/day for 5 h per day from gestation
day 6 to day 15. The treated (clipped) sites were washed with soap
solution after each daily exposure period. Animals' necks were
immobilized. On day 20 of pregnancy, the dams were sacrificed and
foetuses were removed by caesarean section. A decrease in maternal
body weight gain during and after treatment period was observed at
10 mg/kg bw. Dams at this level also showed an increased incidence of
ruffled fur. There was a slight increase in the mean number of
resorptions per pregnant dam at 10 mg/kg bw, but the percentage of
pregnant dams with resorbed foetus(es) was not significantly elevated.
No treatment-related effects were observed on other tested parameters,
viz. fertility rate, mean number of implantations, mean litter size,
foetal weight, placental weight, frequency of stunted foetuses and
incidence of skeletal variations. A number of foetal malformations
were seen in all groups, including the control, but they were
generally non-dose-related. The only abnormalities observed at
10 mg/kg bw, but not in the lower dosage groups or controls, were
multiple defects manifested as hypoplasia of telencephalon and
unspecified eye malformation in 3 foetuses from a single litter. These
particular findings were not believed to be compound induced. Under
conditions of the experiment, isofenphos was not teratogenic, although
a dosage level of 10 mg/kg bw was maternally toxic (Schlüter 1981).
Groups of 22 to 25 pregnant rats (Long Evans FB30 strain) were
exposed in an inhalation chamber to an aerosol of isofenphos
(formulated with a 1:1 mixture of polyethylene glycol 400 and ethanol)
at actual chamber concentrations of 0, 0.25, 0.75 or 3.1 mg/m3 air,
for 6 h per day from day 6 through day 15 of gestation. More than 97%
of the particles counted were of sizes within respirable range in the
rat. On day 20 of gestation, the dams were sacrificed and the foetuses
were removed for gross, skeletal and visceral examination. No maternal
toxicity was observed. Pregnancy rate, mean number of implantations,
mean number of resorptions, mean litter size, foetal weight and
incidence of stunted foetuses were not adversely affected. An increase
in placental weight was noted at 3.1 mg/m3 air, which was of doubtful
biological significance and unlikely to be treatment-related in view
of the absence of any embryotoxic activity. There was no dose-related
effect on the incidence of skeletal and visceral malformations. Based
on the data, isofenphos was not teratogenic under the conditions of
the experiment (Schlüter and Thyssen 1981).
Rabbit
Groups of pregnant (Himalayan) rabbits (11 to 13 rabbits per
group) were orally treated with isofenphos at 0, 1, 2 or 5 mg/kg
bw/day from gestation day 6 through day 18. The females were
sacrificed on gestation day 29 and uterine contents were examined.
Foetuses were subjected to evaluation for external, skeletal and
internal malformations.
Compound-related mortalities and toxic symptoms (diarrhoea) were
seen in does at 5 mg/kg bw. A marked decrease (non-dose-related) in
maternal body weight gain during the treatment period occurred in all
treated groups. One of the 13 does aborted at 2 mg/kg bw. There were
no significant differences between control and treated groups in
pregnancy rate, litter size, mean number of implantations, mean number
of resorptions, sex ratio, foetal weight, placental weight and
incidence of stunted foetuses. "Slight bone variations" were not
indicated to occur in any foetuses from control or treated groups. The
only malformation observed in the study was indicated to be
arthrogryphosis of both front extremities in a foetus at the dose
level of 5 mg/kg bw (Machemer 1975).
Special studies on mutagenicity
In two similar but separate studies, isofenphos was evaluated for
its mutagenic activity using two microbial assay systems (rec-assay
and reversion test). Indicator micro-organisms used in the former were
Bacillus subtilis strains N1G17, N1G45, H 17 and M 45. For the
latter, Salmonella typhimurium strains TA 1535, TA 1537, TA 1538, TA
98 and TA 100 and Escherichia coli strain WP2 hcr were employed.
The reversion test was conducted in the presence or absence of a
mammalian metabolic activation system (S-9 homogenates of liver from
rats or mice induced by phenobarbital or Aroclor 1254) while the
rec-assay was carried out without the activation preparation. Under
the conditions of the experiments, isofenphos was demonstrated not to
be mutagenic at concentrations up to 0.02 ml/disc of undilated
isofenphos and 5000 µg/plate (Shirasu et al 1980) or 300 µg/disc
and 1000 µg/plate (Inukai and Iyatomi 1977), respectively, in the
rec-assay and the reversion test. Mutagenic responses were obtained
with several positive control compounds, such as mitomycin C,
9-amino-acridine HCl, furylfuramide, acetylaminofluorene and
beta-propiolactone (Inukai and Iyatomi 1977; Shirasu et al 1980).
Another Ames test conducted with S. typhimurium strains
TA 100, TA 1537, and TA 98, with or without the S-9 mix and using
concentrations as high as 3150 µg/plate, also confirmed the non-
mutagenicity of isofenphos (Oesch 1977).
In a dominant lethal assay, groups of 20 male NMRI mice (10 weeks
old) were given by gavage a single dose of 0 or 15 mg/kg bw of
isofenphos. Each male was then mated with three untreated female mice,
which were replaced weekly for 8 consecutive weeks. The females were
sacrificed around the 14th day of gestation or 14 days after
separation from the males and the uteri were removed for examination.
The treated males exhibited no signs of toxicity. There were no
significant differences between the control and the treated groups
with respect to fertility rate, living implants and dead implants.
Pre-implantation loss (no. of corpora lutea and no. of implantations)
was significantly increased at 15 mg/kg bw during the first week after
dosing, but not thereafter. It was indicated in the report that the
values for pre-implantation loss observed in the treated group were
"completely within the norm of the strain" (Machemer 1973).
Special studies on carcinogenicity
See under "Long-term studies".
Special studies on neurotoxicity
Hen
Groups generally comprising 10 adult hens (15 to 18 months old)
were given acute oral or intraperitoneal doses of technical
isofenphos. The oral and intraperitoneal LD50 was found to be
21 mg/kg bw and 11.4 mg/kg bw respectively. No delayed neurotoxic
signs were observed in any of the treated animals during a 6-week
post-exposure observation period. Pre-treatment of hens with atropine
(50 mg/kg bw) intraperitoneally elevated oral LD50 of isofenphos to
74 mg/kg bw. Five hens surviving an oral dose of 20 mg/kg bw of
isofenphos and 6 atropinized hens that were still alive after
receiving 74 mg/kg bw of the insecticide were sacrificed 21 days after
dosing for histopathological examination of the brain, spinal cord and
peripheral nerve. These hens showed no compound-related morphological
change or variation from normal in any of the nervous tissues
examined. Hens treated with an oral dose of TOCP at 350 mg/kg bw
displayed typical neurotoxic signs and showed degenerative nerve
fibres in the spinal cord and the sciatic nerve (Kimmerle 1972; Cherry
et al 1972).
Special studies on antidotes
In 2 separate experiments, atropine sulphate (50 mg/kg bw), 2-PAM
(50 mg/kg bw) or Toxogonin (20 mg/kg bw) was given singly or in
combination (atropine plus PAM or Toxogonin) i.p. to male rats: a) 45
to 120 minutes after single oral toxic doses of isofenphos (and before
onset of severe toxic symptoms), and b) 5 minutes after acute oral
toxic doses of the oxygen analogue of isofenphos and before onset of
toxic symptoms. Results indicated that, under the conditions of the
experiments, none of the three compounds tested was effective as an
antidote for isofenphos or its oxygen analogue (Solmecke and Kimmerle
1972b, Thyssen 1974a).
Simultaneous administration of atropine sulphate at 100 mg/kg bw
and obidoxime at 20 mg/kg bw to male rats i.p. after onset of toxic
symptoms (at 25 minutes) resulting from acute oral toxic doses of
isofenphos elevated LD50 of the insecticide from 43.1 mg/kg bw to
84.2 mg/kg bw. No data were provided on the effect of the antidotes on
the toxic signs (Kimmerle and Gröning 1975).
Special studies on potentiation
When the acute oral LD50 values in male rats, determined
experimentally for equitoxic mixtures of isofenphos and malathion or
of isofenphos and EPN, were compared with the expected LD50 values
for the mixtures, the acute toxicity of isofenphos was found to be
potentiated by malathion. An additive effect resulted when isofenphos
was combined with EPN (Solmecke and Kimmerle 1972b).
In further studies with male rats, it was demonstrated with
isofenphos did not potentiate the acute oral toxicity of phenamiphos
or phoxim (both organophorous insecticides) (Thyssen 1976; Thyssen
1977).
Special studies on the acute toxicity of the oxygen analogue
Rat
The LD50 of the oxygen analogue of isofenphos was determined in
6 female and 6 male rats and is reported in Table 2. Thyssen and
Kimmerle 1974).
TABLE 2. Acute toxicity of the oxygen analogue of isofenphos in rats
Sex Route Vehicle LD50
(mg/kg bw)
M Oral Water & Cremophor EL 30.8
F 16.1
M i.p. Water & Cremophor EL 15.9
F 7.8
M Dermal None 117.6 µl/kg bw
F (24 h) (undiluted) 25 µl/kg bw
M Dermal Water & Cremophor EL 97.9
F (24 h) 29.5
M Inhalation Ethanol & polyethylene >353 mg/m3 air
(1 h) glycol 400 (1:1)
F approx. 353 mg/m3 air
M Inhalation Ethanol & polyethylene >195 mg/m3 air
(4 h) glycol 400 (1:1)
F 63.8 - 131 mg/m3 air
Signs of oral poisoning
Acutely toxic doses of the oxygen analogue of isofenphos to
rats produced cholinergic symptoms typical of those seen with
cholinesterase inhibitors. These toxic signs occurred within 1 h of
dosing and persisted for a maximum of 2 days in survivors. Deaths from
lethal doses were observed from about 30 minutes to 3 days after
treatment (Thyssen and Kimmerle 1974).
The acute LD50 of the oxygen analogue of isofenphos in hens was
>10 <25 mg/kg bw by the oral route and about 10 mg/kg bw i.p. No
neurotoxic signs were observed in any of the treated hens over a
28-day post exposure observation period (Thyssen 1974c).
Special studies on the subacute oral toxicity of the oxygen analogue
and of a mixture of isofenphos and its metabolites
Groups of 15 male and 15 female rats (Wistar II strain) were
treated daily by gavage with the oxygen analogue of isofenphos (HOL
0654) (as an emulsion in water and Cremophor EL) at 0, 0.2, 0.6 or
1.8 mg/kg bw for 30 consecutive days. Activity of cholinesterase in
plasma and erythrocytes was determined five times over the course of
the study and brain cholinesterase was assayed at terminal sacrifice.
Physical appearance, behaviour and body weight were comparable to
controls. Dose-dependent inhibition (>20%) of plasma cholinesterase
was observed in males and females at 1.8 mg/kg bw throughout the
feeding period, at 0.6 mg/kg bw in males after 3 weeks only and
in females at 3, 10, 17 and 23 days of the study. The level of
erythrocyte cholinesterase was reduced by >20% in both sexes at
1.8 mg/kg bw. In general, the degree of depression of both plasma and
erythrocyte cholinesterase was greater in the females than in the
males. Terminal brain cholinesterase was unaffected in both sexes.
There were no significant gross pathological alterations in treated
animals. The no-effect level on plasma cholinesterase was 0.2 mg/kg bw
(Thyssen 1974b).
Cow
Groups of 3 dairy cows were fed alfalfa pellets treated with a
mixture of isofenphos, isofenphos oxygen analogue and des-N-isopropyl
isofenphos oxygen analogue (1:8:1) at 2, 6 or 20 ppm for 28 days and
then sacrificed for analyses of tissue residues. One cow was used as a
control. Whole blood cholinesterase was assayed at weekly intervals.
Other than the observation of a more sedated or docile appearance in
cows at 6 ppm and above, as compared to the control animal or animals
at 2 ppm, there were no adverse effects noted with respect to body
weight, feed consumption and milk production. A reduction (>20%) of
whole blood cholinesterase activity occurred at 20 ppm at days 14, 21
and 28 of the study and at 6 ppm at days 21 and 28. The inhibition of
whole blood cholinesterase was dose-related and increased with time
over the course of the study (Strankowski et al 1977c).
Hen
Groups of 4 hens were fed diets containing a mixture of
isofenphos, isofenphos oxygen analogue and des N-isopropyl isofenphos
oxygen analogue (1:8:1) at the levels of 0, 5, 15, 50 or 150 ppm for
28 days and then sacrificed for analyses of tissue residues. Body
weight and food consumption at both 50 and 150 ppm and egg production
at 150 ppm were adversely affected. Dose-related depression (22 to
98%) of plasma cholinesterase determined at terminal sacrifice was
observed in all treated groups (Strankowski 1977).
OBSERVATIONS IN HUMANS
In 3 male workers (wearing standard protective clothing) involved
for 5 to 11 working days (generally 7.5 to 8 h per day and not more
than 3 consecutive days at one time) in the application of a 7.5%
granulated formulation of isofenphos through a standard applicator
fixed to carrot seed drilling equipment, the whole blood
cholinesterase activity was not significantly depressed, as compared
to 2 male and 1 female control subjects. There was no mention in the
report with respect to adverse effects or symptoms of exposure. No
other clinical measurements were made (Bagnall 1976).
RESIDUES IN FOOD
USE PATTERN
Isofenphos is an insecticide possessing good activity against
soil insects, such as Diabrotica spp., wireworms, chafer grubs,
vegetable flies and flea beetles. It has proved to be highly effective
against leaf-eating pests, such as pear sucker and Colorado potato
beetle, and also against rice stem borers.
The compound acts as a contact and stomach poison. It has a root-
systemic action, i.e. the active ingredient is absorbed by the roots
of the plants and translocated to a limited degree within the plant.
The active ingredient is notable for its long residual activity
and its good dispersibility in soil. In numerous trials, isofenphos
has displayed generally good crop plant tolerance, whether used as a
seed treatment or applied as granular and emulsifiable concentrate
formulations. Isofenphos is formulated as an emulsifiable concentrate,
granular formulation and seed dressing powder.
It is mainly applied in row at or immediately after seeding. Two
applications are registered only for corn in the United States. The
crops, pests and recommended or registered dose rates are listed in
Table 3.
TABLE 3. Recommended application rates for isofenphos
Crop Pests Formulation Dose (a.i.)
Onion, carrot, brassica
leafy vegetables vegetable flies GR 1.5-2.5 kg/ha in row
Onion onion fly GR 5 kg/ha broadcast or spray
and overall
EC 0.076 g/m in row
Brassica leafy vegetables cabbage root fly GR 0.05 % a.i. (at 100 cm3/plant
and as soil drench after
EC transplanting)
0.0375-0.075 % a.i. at a rate
of 0.0375-0.15g/m row
Potato wireworm GR 5 kg/ha broadcast overall
before planting
Maize Diabrotica sp. GR 0.11 g/m in row at seeding +
and lay by (50 cm row spacing)
EC
Oilseeds, rape, flea beetle Seed 12-16 g/kg seed
swedes, marrowstem dressing seed treatment
kale, turnips powder
RESIDUES RESULTING FROM SUPERVISED TRIALS
Experiments were run on various plants at different locations in
Canada, Europe and USA. Isofenphos was applied at about the
recommended, and in certain cases somewhat higher, dosage rates.
The residues looked for included isofenphos, isofenphos oxygen
analogue (IOA) in European experiments and des-N-isopropyl isofenphos
(DNI) and des N-isopropyl isofenphos oxygen analogue (DNIOA) were
additionally included in the analyses carried out in USA. The residues
measured in brassicas, oilseed, rape, root and tuber vegetables were
low, generally at or about the limit of determination for the
recommended pre-harvest interval, as well as those found in kernel of
corn at the milk and dry stages. In green forage, however, residues
were as much as 2 mg/kg. Onions and potatoes also contained detectable
residues. The major part of the residue consisted of isofenphos and
IOA in all crops.
Brassica leafy vegetables
Brussels sprouts were treated by applying a soil drench at a rate
of 0.05 g a.i./plant shortly or 1 day after planting. No residue was
measurable 99-119 days after treatment (Bayer 1978). isofenphos was
applied on cabbages, savoy and white, and cauliflower either in row or
with overall treatment at dose rates of 0.075 g a.i./m in a 10 cm band
or 1.5 to 3 kg/a.i./ha, respectively. The experiments were carried out
in Federal Republic of Germany (FRG) and UK and results are summarized
in Table 4 (Bayer 1973-74, 1977).
Celery
Oftanol 200 EC was worked in the soil at rate of 4 kg a.i./ha one
day before planting. Samples were taken 84 and 90 days after treatment
and analysed for isofenphos and IOA. The residues were below the limit
of determination (0.01 mg/kg) (Netherlands 1981).
Maize
Supervised trials were carried out in various states of the USA
in 1979 and 1980.
AMAZE 15 G or 20 G were used for the treatment of the plots in a
15 cm band at planting plus either a double side dress at the 4-leaf
growth stage or band treatment at the base of the plant at growth
stage of 45 cm. A dosage rate of 0.19 g a.i./m was applied in all
treatments (equal to 2.5 kg a.i./ha). Samples were taken from green
forage, kernel, cob and husk in the milk stage and from the dried
parts of plant. Tests were made for residues of isofenphos and its
three metabolites. The limit of determination was 0.01 mg/kg for all
compounds individually.
TABLE 4. Residues of isofenphos in brassica leafy vegetables
Application Residues 1 (mg/kg) at intervals (days) after application
Crop Country Year No. Rate
(kg a.i./ha) Formulation 0/42 49 55-56 60 64-70 80-87
Cabbage FRG 1974 1 3 kg/ha 5 G 0.01 0.01 <0.01
FRG 1974 1 " 5 G 0.08 0.02 0.01
savoy FRG 1974 1 " 5 G <0.01 <0.01 <0.01
FRG 1977 1 )0.075 g/m 500 EC 0.02 0.01 0.01 <0.01
FRG 1977 1 ) in row 500 EC 0.12 0.03 0.02 <0.01
FRG 1977 1 ) 500 EC 0.09 0.03 0.02 0.01
Cabbage UK 1974 1 1.5 kg/ha 5 G 0.007
FRG 1973 1 2.5 kg/ha 5 G 0.1 0.01
white FRG 1973 1 2.5 kg/ha 5 G 0.06 0.03
FRG 1973 1 2.5 kg/ha 5 G 0.08 0.02
Cauliflower FRG 1977 1 0.075 g/m 500 EC 0.11 0.04 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.01 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.02 <0.01 <0.01 <0.01
in row
FRG 1973 1 2-2.5 kg/ha 5 G <0.012 <0.011 <0.013
1 Sum of isofenphos and its oxygen analogue;
2 Results were obtained by analysing 3 samples taken from different plots;
3 Results of analyses of 6 samples.
Isofenphos, IOA and DNIOA were detected in forage. The results
are summarized in Table 5. Levels of DNIOA ranged from 0.01 to
0.08 mg/kg, with an average of 0.04 mg/kg. No measurable residues were
found in kernels either in tile milk or dry stage. Residues in the
cob were at or below the limit of determination (total residue
< 0.02 mg/kg). The husk contained residues of less than 0.2 mg/kg
at all times and stages (Mobay 1979-80).
Sweet corn
Experimental plots were treated in a 15 cm band at planting and
with either a 15 cm band at the base of the plant or a basal spray at
lay by 4 to 8 weeks later. OFTANOL 6 EC and 15 G formulations were
used in the trials (Mobay 1978a).
The active ingredient was applied at a dose rate of 0.19 g/m,
equal to 3.72 kg a.i/ha. The residues (parent compound and
3 metabolites) were analysed separately in 10 milk stage kernel
samples. The oxygen analogue was detected twice at levels of
0.02 mg/kg and 0.05 mg/kg; the other residues were below the limit of
determination (0.01 mg/kg for all compounds). No residues were
detected in dry kernels (3 samples).
The green and dry forages were also analysed, and levels of
isofenphos and IOA are listed in Table 5. The cob in the milk stage
did not contain detectable residues, with the exception of 0.02 mg/kg
of oxygen analogue. The oxygen analogue was also the only detectable
residue in husk at the milk stage, with a maximum level of 0.16 mg/kg.
The dry husk contained both IOA and DNIOA. Maximum levels were
0.27 mg/kg and 0.02 mg/kg respectively.
Onion
At various locations in the USA in 1978, 17 experimental plots
were treated in furrow at planting with either 15 G, 10 G, 5 G or 4 EC
formulations at a dose rate of 0.1 g isofenphos/m. The dose rate
expressed in kg/na varied depending on the row spacing, which was
between 30 to 100 cm (Mobay 1978b). Both mature bulbs and immature
bulbs were sampled and analysed for isofenphos, IOA, DNI and DNIOA.
The residues were generally low in the bulb.
DNI and DNIOA were only detected in one sample at levels of
0.01 mg/kg and 0.08 mg/kg respectively. Supervised trials were also
carried out in Federal Republic of Germany, France and in The
Netherlands. Five kg a.i./ha was applied from both 5 G and 500 EC
formulations one day before sowing or at the 4-leaf stage. Samples
were taken 92 to 158 days after application and the levels of
isofenphos and IOA were measured (Bayer 1972-73, 1975, 1978). The
number of samples taken from experimental plots both in USA and in
Europe are listed in Table 6.
TABLE 5. Residues of isofenphos in forage of maize and sweet corn
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Maize USA 0.03 0.02 0.02
1980 2 2.5 15 G
Indiana 0.141 0.071 0.111
" 1980 2 2.5 15 G 0.05 0.04 0.04
0.141 0.191 0.111
" 1980 2 2.5 20 G 0.03 0.07 <0.01
0.111 0.231 <0.011
Nebraska 1979 2 2.5 20 G <0.01 <0.01 <0.01 <0.01 <0.01
<0.011 <0.011 <0.011 <0.011 <0.011
" 1979 2 2.5 15 G <0.01 0.02 <0.01 <0.01 <0.01
<0.011 0.01 <0.01 <0.01 <0.01
Sweet corn USA 1978 2 3.72 6 EC 0.22
Missouri 0.741
Oregon 1978 2 3.72 6 EC <0.01 <0.01 <0.01 <0.01 <0.01
<0.031 0.031 0.041 0.031 <0.011
Nebraska 78 2 3.72 6 EC 7.86 0.01 <0.01 0.04
9.261 0.251 0.061 0.051
Texas 1978 2 3.72 6 EC 0.2 0.05 0.08 0.04
2.091 1.611 1.991 0.071
Florida 1978 2 3.72 6 EC 0.03 0.02 0.04 <0.01
0.751 1.371 0.671 0.071
Missouri 78 2 3.72 15 G 0.94 0.07 0.1
0.741 0.231 0.191
TABLE 5. (con't)
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Oregon 1978 2 3.72 15 G 0.02 0.02 0.02 <0.01 <0.01
0.081 0.081 0.061 0.06 0.011
Texas 1978 2 3.72 15 G 0.01 0.03 0.05
0.721 1.041 0.151
Florida 1978 2 3.72 15 G <0.01
0.041
Missouri 78 2 3.72 15 G 0.39 0.03 <0.01
0.871 0.14 0.11
1 Residues of oxygen analogue.
TABLE 6. Supervised trials measuring isofenphos and its oxygen
analogue in onions
Compound Residue (mg/kg) No. of samples
Isofenphos < 0.01 12
< 0.05 0
< 0.1 1
< 0.2 1
< 0.6 3
IOA < 0.01 4
< 0.05 8
< 0.1 2
< 0.2 2
< 0.6 1
Isofenphos + < 0.01 2
IOA 1 < 0.05 4
< 0.1 3
1 Result of experiments in Europe.
The sum of the residue detected was always below 1 mg/kg in
mature bulb samples. Immature bulbs were analysed in two occasions and
the residues found were: isofenphos-0.32 and 0.95 mg/kg; isofenphos
oxygen analogue - 0.18 and 0.26 mg/kg.
Rapeseed
Seeds of spring and autumn varieties of rape were seed dressed
with BAY 6643 B at a rate of 15 g isofenphos/kg seed in 1973-74. The
crops were sampled at various locations in the FRG. Residues were
below the limit of determination (0.01 mg/kg) in all of the 13 trials
(Bayer 1974a).
Supervised trials with three varieties were carried out at three
locations in Canada in 1989 (Mobay 1980a). AMAZE 40% was used for seed
dressing prior to seeding at a rate of 25 g a.i./kg seed. The seeds
were treated either with formulation or the powder was applied to seed
after coating with oil. The harvested seed was analysed for
isofenphos, IOA, DNI and DNIOA 94-113 days after treatment. Residues
of active ingredient and its metabolites were below the limit of
determination (0.01-0.02 mg/kg).
Root and tuber vegetables
Celeriac
Supervised trials, in which 4 kg a.i./ha of Oftanol 200 EC was
applied before planting, were carried out on celeriac fields at two
locations of The Netherlands in 1978. The mature crops were samples
158 days after application. Residues of isofenphos were not detected
(Netherlands 1981).
Potato
In an experiment in FRG, a 5 G formulation of isofenphos was
worked into the soil at a rate of 5 kg a.i./ha before planting. At 110
days after application, a residue of 0.11 mg/kg was detected in the
tubers that had been washed before analysis (Bayer 1973).
Potato fields were treated with isofenphos at three different
locations in Spain in 1980. The broadcast applications were made at a
rate of 2.5 kg a.i./ha at the time of planting. Samples were taken
111, 112 and 159 days later at the time of harvest and their residue
contents were found to be 0.45, 0.04 and 0.05 mg/kg respectively
(Bayer 1980).
Swedes and turnips
Oftanol T was used for seed dressing at a rate of 16 to
20 g a.i./kg seed. Experimental plots from 8 locations in FRG were
sampled 109 to 179 days after sowing in case of swedes, while three
plots of turnips were sampled 89 to 103 days after sowing. Leaves and
roots were analysed separately. No residue was detected (<0.01 mg/kg)
(Bayer 1974-76).
Availability of soil residues to rotational crops
Wheat, green beans and sugarbeets were planted as rotational
crops 3.5 months after a sandy soil had been treated with ring-µL
(14C)-isofenphos at a field application rate of 5.6 kg a.i./ha (Kurtz
1977). Corn had been used as the original crop in this isofenphos-
treated soil. When the rotational crops were planted, the soil
contained approximately 4 mg/kg isofenphos. The rotational crops were
sampled at 2, 8 and 32 weeks after planting. At the time of harvest,
8 weeks for green beans and 32 weeks for sugarbeet and wheat, the
residue level was measured in different parts of the crops (Table 7).
No des-N-isopropyl isofenphos was detected in any of the crops.
TABLE 7. Residues of isofenphos detected in rotational
crops grown in soil treated with isofenphos 1
Crop Sample Residue (mg/kg)
I IOA DNIOA
Green bean Forage < 0.01 0.05 < 0.01
Sugarbeets Roots 0.04 0.02 < 0.01
Tops < 0.01 0.02 0.06
Wheat Heads < 0.01 0.24 0.03
Straw 0.14 0.79 0.2
Forage 0.05 1.08 0.23
1 Application rate - 5.6 kg a.i./ha.
In the investigation of field rotational crops, wheat, oats or
sorghum, spinach turnips and soybean were planted in plots treated the
previous year with isofenphos (Mobay 1980b). The plots had been used
previously for performance tests and hence were cropped using normal
agricultural practices. Rotational crop planting dates ranged from 214
to 390 days (7 to 13 months) post-treatment. Treatment was with either
AMAZE 20 G in an 18cm band over the row at 1.2 kg a.i./ha to 1.5 kg
a.i./ha broadcast equivalent-with presswheel incorporation or with
AMAZE 6 E or 20 G applied in furrow at 1.1 to 2 kg a.i./ha broadcast
equivalent. Prior to planting the rotational crops, the plots were
rototilled or disced. The crops were then planted across and
perpendicular to the direction of the originally treated rows. To
insure homogeneity of sampling, the entire lengths of the crop rows
were harvested. Each of the harvested crops were analysed for residues
of isofenphos, IOA, DNI and DNIOA. The results of the study are given
in Tables 8 and 9.
TABLE 8. Residues of isofenphos in grain (wheat, oats or sorghum) rotational crops
Treatment Plant Back Residue (mg/kg 1
Crop Location Rate Interval
(USA) (kg a.i./ha) (days) Forage Grain Straw
Winter
Wheat Kansas 1.5 77 <0.01 <0.01 <0.01
Sorghum Texas 1.2 214 <0.012 <0.01 0.02
<0.033
Spring
wheat Kansas 1.5 295 0.064 NA5 NA
0.026
Spring
Wheat Nebraska 1.5 350 <0.014 <0.01 <0.01
<0.016
Oats Illinois 1.2 362 <0.014 <0.01 <0.01
<0.016
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos equivalents;
2 Immature crop, 42 days post-planting;
3 Immature crop, 57 days post-planting;
4 Immature crop, 45 days post-planting;
5 NA - no mature crop available for analyses;
6 Immature crop, 60 days post-planting.
TABLE 9. Residues of isofenphos in rotational crops
Treatment Plant back Residue
Crop Location rate Interval (mg/kg) 1
(kg a.i./ha) (days)
Spinach Arizona 2 279 <0.01
Spinach Arizona 2 279 <0.01
Turnip tops Kansas 1.5 316 <0.01
Spinach Nebraska 1.5 363 <0.01
Turnip tops Nebraska 1.5 363 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Kansas 1.5 316 <0.01
Turnip root Nebraska 1.5 363 <0.01
Soybean Kansas 1.5 295 0.03
Soybean Nebraska 1.5 363 <0.01
Soybean Illinois 1.14 390 <0.01
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos
equivalents.
Residues in all rotational crops studied consisted mainly of
isofenphos and IOA and were <0.05 mg/kg; most were <0.01 mg/kg. In
the four mature grain crops, the grain had no detectable residue and
the straw showed a detectable residue of 0.02 mg/kg in only one
sample. In the five vegetable crops, only one had a detectable residue
(0.01 mg/kg) was seed in only one of the bean samples and in two of
the dry vine samples. Based on these results, little or no isofenphos
residue would be expected in grain, vegetable, root or oil seed crops
grown in fields treated 9 or more months earlier with isofenphos at
rates up to 2 kg a.i./ha.
FATE OF RESIDUES
General comments
The degradation and metabolism of isofenphos was studied in
animals, plants, soils, water and during frozen storage. The
structural formula, chemical name, identifying symbol and occurrence
of its degradation products are shown in Figure 2. Metabolic pathways
for isofenphos in animals (A) plants (P) and Soil (S) are shown in
Figure 3.
In plants
The metabolism of isofenphos was studied in maize grown in ring -
µL (14C)-isofenphos treated soil both outdoors and in a greenhouse
(Stanley et al 1977).
For the outdoor experiment the labelled isofenphos was prepared
as a 6 EC formulation and incorporated at the rate of 5.6 kg a.i./ha
(ca 4 mg/kg soil) into soil contained in a tub. Sweet corn was planted
in the soil. Plant samples above the soil line were harvested at 28,
56 and 94 days after planting. The 94-day crop was divided into
kernels, cobs, husks and stalks in the laboratory.
In the greenhouse experiment the labelled isofenphos was
incorporated into soil at a rate of 4 mg/kg. The soil was placed in
pots and field corn was planted in it. The crop was harvested at
maturity, 141 days after treatment, and the plants were divided as in
the other experiment. The root samples were washed with water to
remove soil particles.
The compounds identified in the corn stalks were qualitatively
the same throughout the study, although their relative quantities
changed as the plant matured.
reduction in erythrocyte cholinesterase level by 20% at 2 h post
dosing but not thereafter. Plasma cholinesterase was not affected.
Male rats sacrificed 24h after acute oral doses of isofenphos above
1 mg/kg bw displayed a dose-related decrease (23-74%) in activity of
brain cholinesterase (Solmecke and Kimmerle 1972b).
Groups of 5 male and 5 female rats (Wistar II strain of
unspecified age) were intubated acutely with the oxygen analogue of
isofenphos at 0.1, 0.25, 0.5, 1.0, 2.5 or 5 mg/kg bw (males) or at
0.25, 0.5 or 1 mg/kg bw (females). Cholinesterase in plasma and
erythrocytes was assayed at 2, 5, 24, 48h and 7 days (in males only)
post treatment. No control groups were included in the experiment and
the pre-treatment cholinesterase activities of individual groups were
used as the basis of comparison. In males, there was a depression
(>20%), generally dose-dependent, of plasma cholinesterase at
0.5 mg/kg bw and above and of erythrocyte cholinesterase at 2.5 mg/kg
bw and above after 2, 5 and 24 h. (Males at 1 mg/kg bw showed a
decrease (29%) of erythrocyte cholinesterase after a single interval
of 5 h.) Complete or almost complete recovery to pre-treatment values
was seen after 48 h for plasma cholinesterase and after 7 days for
erythrocyte cholinesterase. In females, both plasma and erythrocyte
cholinesterase was inhibited (20 to 43%) at 0.25 mg/kg bw and above
during the first 24 h. Activities of cholinesterase in plasma and
erythrocyte were close to the pre-treatment values 48 h after dosing.
The inhibitory effect of the oxygen analogue on plasma and erythrocyte
cholinesterase appeared to peak about 2 or 5 h after treatment in both
sexes (Thyssen 1974a).
Groups of 5 male and 5 female rats were orally treated with
oxygen analogue of isofenphos and then sacrificed 24 h later for
determination of brain cholinesterase activity. It was stated (no data
were provided) that brain cholinesterase activity in males was
moderately depressed at both 10 and 20 mg/kg bw but was unaffected at
5 mg/kg bw. In the case of females, brain cholinesterase activity was
stated to be within physiological ranges at dosages ranging from 1 to
5 mg/kg bw (Thyssen 1974a).
TOXICOLOGICAL STUDIES
Acute toxicity
In experiments with mice, rats, hamsters and rabbits exposed in
inhalation chambers to isofenphos (dissolved in a mixture of alcohol
and polyethylene glycol 400) as a spray at 30-minute intervals during
a 4-h period, rats and hamsters were found to be more sensitive than
mice and rabbits as shown in Table 1 (Solmecke and Kimmerle 1972c).
TABLE 1. Acute toxicity of isofenphos in animals
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Mouse M Oral PG 127 Solmecke and Kimmerle 1972c
F 91.3
M Inhalation Ethanol & > 0.272 mg/l Solmecke and Kimmerle 1972c
(4-hour PG (1:1)
exposure)
Rat M Oral PG 38.7-45 ) Solmecke and Kimmerle 1972c
F 28 - 32 ) Lamb et al 1977
M Oral Water &
Cremophor EL 33 - 48.2 Flucke 1980a;Flucke 1981
M Oral " 19.9 Flucke 1980b
M I.P. PG 35.8 Solmecke and Kimmerle 1972c
F 29.5
M Dermal None >1000 µl/kg bw) Solmecke and Kimmerle 1972c
(4-hour
exposure) None
M Dermal(24-h) None 705 µl/kg bw Kimmerle 1972a
Dermal None 188 µl/kg bw Solmecke and Kimmerle 1972a
(7-day
exposure) (undiluted)
M&F Inhalation Ethanol & >1300 mg/l air Kimmerle 1976
(1-hour PG (1:1)
exposure)
M Inhalation Ethanol & 0.21 mg/l air Solmecke and Kimmerle
F (4-hour PG (1.1) 0.144 mg/l air 1972c
exposure)
TABLE 1. (con't)
Species Sex Route Vehicle LD50
(mg/kg bw) Reference
Hamster M Inhalation Ethanol & 0.23 mg/l air Solmecke and Kimmerle
(4-hour PG (1:1) 1972c
exposure)
Rabbit F Oral PG approx. 150 Solmecke and Kimmerle 1972c
M Dermal Ethanol & 162 Nelson and Burke 1977a
F (24-hour PG (1:1) 315
exposure)
Dog F Oral PG > 25 Solmecke and Kimmerle 1972c
Bobwhite quail M&F Oral PG 8.7 Lamb and Burke 1979
Mallard duck M Oral PG 36.0 Nelson and Burke 1977b
F 32.0
Quail M Oral Water & 10 Thyssen 1978
Cremophor EL 10
Starling Oral Corn oil 972 Ross et al 1976
Hen F Oral Water & 16 Thyssen 1978
Cremophor EL
PG - polyethylene glycol 400.
Signs of oral poisoning in mammals
Cholinergic symptoms (e.g. muscle twitching, breathing disorders)
induced by toxic acute oral doses of isofenphos were typical of those
of cholinesterase inhibitors and were similar in mice, rats and
rabbits. Toxic signs usually developed within 2 h of dosing in mice
and rats and 4 h in rabbits and lasted for 2 to 10 days in survivors.
Mortalities from lethal doses usually occurred 1 to 4 days after
treatment. Cholinergic signs were not observed in dogs treated at
25 mg/kg bw, the top dosage level tested (Solmecke and Kimmerle
1972c).
Short-term studies
Rat
Groups of 15 male and 15 female rats (SPF Wistar II strain of
unspecified age) were orally treated by gavage with isofenphos ("pure
technical grade") in polyethylene glycol 400 at 0, 0.1, 0.25, 1.0 or
2.5 mg/kg bw/day for 30 days. Mortality, behaviour, growth, organ
weights and liver and kidney function were unaffected. Dose-related
inhibition (>20%) of cholinesterase in plasma and erythrocytes was
apparent at and above 1 mg/kg bw in females at 9, 16 and 30 days of
the study. In males, depression (dose related, >20%) of erythrocyte
cholinesterase was noted at both 1 and 2.5 mg/kg bw at 16 and 30 days
and of plasma cholinesterase at and above 1 mg/kg bw at 16 days and at
2.5 mg/kg bw also at 9 and 30 days (Solmecke and Kimmerle 1972a).
Groups of 10 male and 10 female rats (SPF Wistar W74 strain, 8
weeks old) were fed isofenphos (91.9% pure) in the diet at the dosage
levels of 0, 0.3, 1.0 or 3 ppm for 4 weeks. There was no mortality.
Behaviour, body weight and food consumption were not significantly
different between control and treated animals. Determinations of blood
cholinesterase activity after 3, 7, 10, 14, 17, 21, 24 and 28 days of
dietary feeding indicated dose-related inhibition (>20%) of plasma
cholinesterase in females at 1 ppm after 7, 17 and 28 days and at
3 ppm at all intervals. Only a slight depression (13-16%) of plasma
cholinesterase was noted in males even at 3 ppm. Activity of
erythrocyte cholinesterase determined over the course of the study and
of brain cholinesterase measured terminally was not affected in all
treated groups (both sexes). The no-effect level on plasma
cholinesterase was 0.3 ppm, which was equivalent to 0.024 mg/kg bw,
according to body weight and food consumption data (Löser 1978).
Groups of 15 male and 15 female rats (SPF Wistar strain, 28 to 32 days
old) were fed isofenphos (86.6% pure) in their diet at levels of 0.5,
1, 5, 25 or 125 ppm for 3 months. The control group consisted of 30
males and 30 females. One male at 125 ppm died after 9 days, due to
pneumonia. Toxic signs, such as salivation and muscle twitching, were
observed in animals receiving 125 ppm during the first 2 weeks of
feeding. Body weight was adversely affected in the highest dosage
group (both sexes). Food consumption was comparable to controls.
Haematological and biochemical studies and urinalysis conducted after
one and three months of the study indicated no significant dose-
related findings other than an increase in SGOT in males at 125 ppm at
the 1-month interval. Measurements of cholinesterase activity after 1,
4, 8 and 13 weeks of feeding revealed depression (dose-related >20%)
of the enzyme in both plasma and erythrocytes in females at 5 ppm and
above and in males at 25 ppm and above at all intervals. (Males at
5 ppm showed a 29% decrease of plasma cholinesterase level after one
week of feeding, but not thereafter.) The brain cholinesterase level
determined terminally was reduced at 25 ppm and above in both sexes.
At sacrifice upon termination of the study, an increase in absolute
weight and organ/body weight ratio of thymus was seen in females at
125 ppm. There were also variations in absolute weights of several
organs in males of the top dosage group. No compound-induced
histopathological alterations were observed in a variety of tissues
evaluated, including the thymus (Löser 1973; Urwin and Newman 1973).
Dog
Groups of dogs (4 males and 4 females per group, 5 months old)
were fed isofenphos (of unspecified purity) in the diet daily at 0,
0.3, 1.0, 10 or 30 ppm for 3 months. There were no treatment-related
effects on mortality, clinical symptoms, reflexes, body weight, food
consumption, ophthalmoscopic, haematological, clinical chemistry and
urinalysis parameters. Cholinesterase in plasma and erythrocytes was
depressed (by >20%), in a dose-dependent manner, at and above
10 ppm in both sexes after 3, 6 and 13 weeks of the study. (Plasma
cholinesterase in these dosage groups was inhibited also after 1 week
of the study.) At the conclusion of the study, absolute weight and
organ/body weight ratio of the liver was elevated at 10 ppm and
above in males (non-dose related) and females (dose related).
Histopathological examination of about 30 tissues, including the
liver, from each dog revealed no abnormalities or variations
associated with inclusion of isofenphos in the diet. The no-effect
level for the study was 1.0 ppm (Murmann 1973; Thomson and Newman
1973).
Groups of dogs (4 males and 4 females per group) were fed a daily
diet containing the following levels of isofenphos (89.3% pure) in a
2-year study: Control: 0 ppm; Group 1: males - 3 ppm from week 1
to week 83 and 2 ppm from week 84 to week 104, and females - 3 ppm
from week 1 to week 104; Group 2: 15 ppm throughout the 104 weeks,
and Group 3: 75 ppm from week 1 to week 53, 150 ppm from week 54 to
week 99 and 300 ppm from week 100 to week 104. No compound-related
findings were noted with respect to general condition (reflexes, body
temperature and pulse rate), water intake, ophthalmoscopic and
urinalysis parameters. Assay of cholinesterase 12 times over the
course of the study indicated dose-dependent inhibition (>20%) of
plasma cholinesterase in Groups 2 and 3 (both sexes) and of
erythrocyte cholinesterase in Group 3 at all intervals. Depression
(approx. or equal to 20%) of plasma cholinesterase was also found in
Group 1 males at weeks 7, 39, 66 and 79 but was not evident at weeks
92 and 103 after the dietary level was reduced from 3 ppm to 2 ppm at
week 84. Animals of Group 3 also showed depression (>60%) of brain
cholinesterase activity measured terminally.
Adverse effects on parameters other than cholinesterase activity
in plasma and erythrocytes were observed only in Group 3. In this
group, one male died at week 104 and another male was sacrificed
terminally in moribund condition. Toxic symptoms, such as salivation,
vomiting, diarrhoea, weakness in the hind extremities, unsteady gait,
etc., began to appear after 78 weeks and increased in severity with
time. The symptoms were more marked in males than in females.
Depression of body weight gain (both sexes) and of food consumption
(females) was obvious after 100 weeks. An increase in the SAP level at
several intervals during the study and a reduction of the terminal
serum A/G ratio were observed in males and females. The one male that
died at 104 weeks showed significant changes in several haematological
parameters. At terminal sacrifice, elevated organ/body weight ratios
of lung, liver, kidney, prostate, brain and pituitary and depressed
organ/body weight ratios of adrenal and testis were detected in the
males but, except in the testis, microscopic abnormalities of the
tissues in question were not noted. Histopathologically, hypoplasia of
the testis and erosion of esophageal mucosa, partially accompanied by
cellular reaction of propria mucosa, were observed in several males of
Group 3 but none in the other groups, including the control. Two
animals (Group 3) also exhibited foci of softening ("degeneration
processes") in the brain stem with a degree of severity greater than
the one case seen in the control group. The no-effect level in the
study was 2 ppm (Hoffman and Kaliner 1977).
Hen and quail
In adult hens and female quail intubated with isofenphos as an
emulsion in water and polyethylene glycol 400 daily for 5 consecutive
days, and then observed for 14 additional days, the 5 day LD50 was
found to be 6 mg/kg bw/day and 5 mg/kg bw/day respectively. Major
symptoms observed were inactivity in both species, ruffled feathers
and breathing disorders in the quail (Thyssen 1978).
Bobwhite quail and mallard duck
The dietary LC50 in bobwhite quail and mallard ducks fed
isofenphos in their diet for 5 days, followed by untreated diet for
3 days, was determined to be 145 ppm (Nelson and Burke 1977) and
>1000 ppm respectively (Nelson and Burke 1977c; Lamb and Burke 1977).
Rabbit - dermal
Groups of 6 male and 6 female rabbits (3 males and 3 females per
group with intact skin and the remainder per group with abraded skin)
were exposed dermally to isofenphos (as an emulsion in water and
Cremophor EL) at 0, 1 or 5 mg/kg bw/day 7 h per day, 5 times per week
for a total of 15 applications in a 21-day period. The treated sites,
not covered with bandages, were washed with soap and water after each
daily exposure period. One male at 5 mg/kg bw died of a cause
unrelated to treatment after 10 applications. There were no toxic
signs or adverse effects on body weight or on terminal haematological
and clinical chemistry parameters. Local irritation on treated sites,
generally transitory in nature and characterized by erythema on intact
skin and erythema, oedema and eschar formation on abraded skin, were
seen in some animals at 5 mg/kg bw. Changes in weights of several
organs were seen at 5 mg/kg bw without any accompanying
histopathological changes. Plasma and erythrocyte cholinesterase
activities measured after the 10th and 15th exposures and brain
cholinesterase level determined terminally in both sexes were
significantly depressed at 5 mg/kg bw (Thyssen and Kaliner 1977).
Rat - inhalation
In male and female rats exposed to isofenphos as an aerosol (of
unspecified particle sizes) 4h per day for 5 days, the LC50 was
>0.055 mg/l in males and 0.029-0.055 mg/l in females ((Solmecke and
Kimmerle 1972c).
Groups of 10 male and 10 female rats were exposed to an aerosol
of isofenphos at 0, 0.72 or 2.93 mg/m3 air 6h per day for 5
consecutive days per week over a 3-week period. (No information was
given on the particle size of the aerosol.) The treated animals were
not different from the controls as judged by general condition, body
weight gain, terminal haematological values, kidney and liver
function, gross pathological changes and organ weights. Cholinesterase
in plasma and erythrocytes was inhibited (>20%) at 2.93 mg/m3 air at
each weekly determination (Solmecke and Kimmerle 1972a).
Long-term studies
Mouse
Groups of 40 male and 40 female mice (6 to 7 weeks old, SPF NMRI
strain) were fed diets containing technical isofenphos (89.3% pure) at
0, 1, 10 or 100 ppm for 108 weeks. Additionally, each group included 3
sub-groups of 10 males and 10 females. One sub-group, fed the treated
diet for 108 weeks, was used for haematological tests and urinalysis
every 3 months over the course of the study. Another sub-group
maintained on dietary feedings for 9 months, was subjected to liver
function tests (plasma alkaline phosphatase and GPT) every 3 months.
The third sub-group was given isofenphos in the diet for 24 weeks and
activity of cholinesterase in plasma and erythrocyte was measured
after 2, 4, 8, 12 and 24 weeks and in brain after 24 weeks. All
animals that died during the study or were sacrificed terminally were
subjected to gross and histopathological examination. Mortality, which
was high in all groups, including the control, especially in the
females, was not attributed to treatment. At the end of the study,
only 42 to 57% of the males and 12 to 20% of the females per group
were still alive. Nevertheless, 53 to 73% of females per group
survived at least 80 weeks. There were no significant differences
between control and treated groups in clinical symptoms, body weight
and food consumption. Data on differential white blood cell counts
showed an increase at 100 ppm of eosinophil (males) and segmented cell
counts (females) after 21 months and of segmented cell counts in males
after 24 months. Liver function tests and urinalysis parameters were
normal. Dose-related inhibition (72 to 94%) of plasma cholinesterase
occurred in both sexes at and above 10 ppm at all intervals.
Erythrocyte cholinesterase was unaffected in the treated groups, with
depression consistently below 20%. Terminal brain cholinesterase
activity was reduced at 100 ppm by 46% in males and 31% in females. At
terminal sacrifice, absolute weight of lung in males was significantly
elevated at 100 ppm but no concomitant histological lesion of the
particular tissue was evident. An apparent dose-related increase, as
compared to concurrent controls, in frequency of mild gastritis (in
glandular mucosa of the stomach) was observed in males of all treated
groups which was, however, not statistically significant, especially
in the case of the 1 ppm and 10 ppm groups. No histopathological
changes related to treatment was observed in a variety of other
tissues evaluated.
Based on the tumour data, isofenphos was not carcinogenic in the
mouse, as indicated by the absence of a dose-related increase in
incidence of a) any particular type of tumours, b) animals with
tumours, c) animals with multiple tumours, and d) animals with
malignant tumours. The time of appearance of tumours was comparable
between control and treated groups.
The no-effect level for the study was 1 ppm (Brune et al.
1978).
Rat
Groups of 50 male and 50 female rats (SPF Wistar strain, 28 to 32
days old) were fed diets containing isofenphos (of unspecified purity)
at 1, 10 or 100 ppm for 2 years. The control group consisted of 100
males and 100 females. Mortality was unaffected by treatment, and
between 69 and 90% of animals per group were still alive at the end of
the study. At 100 ppm, cholinergic signs were seen during the first
week and depression of growth in both sexes and of food consumption in
males was apparent throughout the feeding period. Haematological and
biochemical studies and urinalysis conducted at five intervals over
the course of the study failed to reveal any consistent effect related
to presence of isofenphos in the diet. Assay of cholinesterase after
1, 2, 4, 13, 26, 52, 78 and 105 weeks of the study indicated dose-
related inhibition (>20%) of both plasm and erythrocyte
cholinesterase at 10 ppm and above in both sexes at all intervals and
of plasma cholinesterase in males at 1 ppm after 4 and 78 weeks. The
extent of depression of cholinesterase in plasma and erythrocytes
remained fairly constant over the course of the study. At the
conclusion of the experiment, brain cholinesterase activity was
reduced at 100 ppm in both sexes. Variations in absolute weights of
several organs were seen at 100 ppm but histological changes of the
particular organs were absent. Except for an apparent increase in
incidence of ulceration of the forestomach in females at 100 ppm,
as compared to the concurrent controls, no treatment-related
alterations were evident in a set of about 30 tissues examined
histopathologically. Analysis of tumour data revealed no indications
suggestive of carcinogenic activity of isofenphos under the conditions
of the experiment.
The study demonstrated 1 ppm as a marginal no-effect level with
respect to plasma cholinesterase. On other parameters monitored,
10 ppm was without any significant adverse effect (Bomhard and Löser
1977).
Special studies on reproduction
Groups of 20 male and 20 female weanling rats (SPF CFY strain)
were fed dietary levels of technical isofenphos (of unspecified
purity) at 0, 1, 10 or 100 ppm for 60 days prior to mating to initiate
a reproduction study originally designed to cover 3 generations with
one litter per generation. Three litters (F1a, F2a and F3a) were
obtained as expected for control, 1 ppm and 10 ppm groups. In the case
of the highest dosage group, owing to poor survival of pups, there
were insufficient F1a weanlings to form an adequate second parental
generation (F1). F0 animals at 100 ppm, as well as those of other
groups including the control, were therefore re-mated. This second
mating again failed to provide sufficient F1b offspring at 100 ppm as
potential parents. In an attempt to determine the causes of the
initial adverse effects at 100 ppm, F0 animals of control and top
dosage groups were allowed two additional matings, with the fourth
mating using treated males and untreated females and vice-versa. In
the third mating trial, the number of litters (F1c) at 100 ppm
obtained was too small to permit any meaningful interpretation of
results. Based on results of the fourth mating, fertility appeared to
be adversely affected, primarily in the females. This summary will
refer only to data on control, 1 ppm and 10 ppm groups.
In parental generations, there were no mortality and clinical
symptoms attributable to treatment. Body weight gain during pre-
mating, gestation and lactation periods, mating performance, duration
of gestation and terminal gross pathological findings were not
significantly different from controls. Pregnancy rate was 90%, 70% and
60% respectively in control, 1 ppm and 10 ppm groups in the F0
generation at the second mating suggesting a slight effect at 10 ppm
and a marginal effect at 1 ppm. (The pregnancy rate at 100 ppm was 40%
for the second mating trial.) Assay of plasma and erythrocyte
cholinesterase in F0 animals after 37 weeks of dietary feeding
revealed inhibition (>20%) at 10 ppm of erythrocyte cholinesterase in
both sexes and of plasma cholinesterase in females. Over the three
progeny generations (F1a, F2a and F3a litters), litter size, pup
mortality and mean pup weight from birth through lactation to weaning
were comparable to controls at both 1 and 10 ppm. In F1b litters, pup
weight at 10 ppm seemed to be slightly decreased at days 12 and 21.
Pups observed grossly at weaning displayed no apparent compound-
induced abnormalities. The available data tend to indicate 1 ppm as a
marginal no-effect level with respect to reproductive capability
(Palmer et at 1977).
Special studies on teratogenicity
Rat
Groups of 20 to 21 pregnant rats (Long Evans FB30 strain) were
intubated with isofenphos (86.6% pure) in polyethylene glycol 400 at
doses of 0, 0.3, 1 or 3 mg/kg bw/day from gestation day 6 to day 15.
The dams were sacrificed on gestation day 20 and foetuses were removed
by caesarean section for external, visceral and skeletal examination.
No treatment-related mortality or abnormal behavioural reactions
were noted, and maternal body weight was normal during the dosing
period. An increase in the average number of resorbed foetuses per
litter occurred at 3 mg/kg bw, but a dose-related elevation in the
percentage of litters with rasorptions was not apparent at this dosage
level. There were no significant differences between control and
treated groups with respect to fertility rate, mean number of
implantation sites, mean litter size, foetal weight, placental weight
and incidence of stunted foetuses. The frequency of foetuses showing
slight bone variations and/or malformation was very low and not
significantly different from that in the control group (Machemer
1972).
Groups of 21 to 23 pregnant rats (Long Evans FB30 strain) were
exposed dermally to isofenphos (dissolved in polyethylene glycol 400)
at 0, 0.3, 1.0, 3 or 10 mg/kg bw/day for 5 h per day from gestation
day 6 to day 15. The treated (clipped) sites were washed with soap
solution after each daily exposure period. Animals' necks were
immobilized. On day 20 of pregnancy, the dams were sacrificed and
foetuses were removed by caesarean section. A decrease in maternal
body weight gain during and after treatment period was observed at
10 mg/kg bw. Dams at this level also showed an increased incidence of
ruffled fur. There was a slight increase in the mean number of
resorptions per pregnant dam at 10 mg/kg bw, but the percentage of
pregnant dams with resorbed foetus(es) was not significantly elevated.
No treatment-related effects were observed on other tested parameters,
viz. fertility rate, mean number of implantations, mean litter size,
foetal weight, placental weight, frequency of stunted foetuses and
incidence of skeletal variations. A number of foetal malformations
were seen in all groups, including the control, but they were
generally non-dose-related. The only abnormalities observed at
10 mg/kg bw, but not in the lower dosage groups or controls, were
multiple defects manifested as hypoplasia of telencephalon and
unspecified eye malformation in 3 foetuses from a single litter. These
particular findings were not believed to be compound induced. Under
conditions of the experiment, isofenphos was not teratogenic, although
a dosage level of 10 mg/kg bw was maternally toxic (Schlüter 1981).
Groups of 22 to 25 pregnant rats (Long Evans FB30 strain) were
exposed in an inhalation chamber to an aerosol of isofenphos
(formulated with a 1:1 mixture of polyethylene glycol 400 and ethanol)
at actual chamber concentrations of 0, 0.25, 0.75 or 3.1 mg/m3 air,
for 6 h per day from day 6 through day 15 of gestation. More than 97%
of the particles counted were of sizes within respirable range in the
rat. On day 20 of gestation, the dams were sacrificed and the foetuses
were removed for gross, skeletal and visceral examination. No maternal
toxicity was observed. Pregnancy rate, mean number of implantations,
mean number of resorptions, mean litter size, foetal weight and
incidence of stunted foetuses were not adversely affected. An increase
in placental weight was noted at 3.1 mg/m3 air, which was of doubtful
biological significance and unlikely to be treatment-related in view
of the absence of any embryotoxic activity. There was no dose-related
effect on the incidence of skeletal and visceral malformations. Based
on the data, isofenphos was not teratogenic under the conditions of
the experiment (Schlüter and Thyssen 1981).
Rabbit
Groups of pregnant (Himalayan) rabbits (11 to 13 rabbits per
group) were orally treated with isofenphos at 0, 1, 2 or 5 mg/kg
bw/day from gestation day 6 through day 18. The females were
sacrificed on gestation day 29 and uterine contents were examined.
Foetuses were subjected to evaluation for external, skeletal and
internal malformations.
Compound-related mortalities and toxic symptoms (diarrhoea) were
seen in does at 5 mg/kg bw. A marked decrease (non-dose-related) in
maternal body weight gain during the treatment period occurred in all
treated groups. One of the 13 does aborted at 2 mg/kg bw. There were
no significant differences between control and treated groups in
pregnancy rate, litter size, mean number of implantations, mean number
of resorptions, sex ratio, foetal weight, placental weight and
incidence of stunted foetuses. "Slight bone variations" were not
indicated to occur in any foetuses from control or treated groups. The
only malformation observed in the study was indicated to be
arthrogryphosis of both front extremities in a foetus at the dose
level of 5 mg/kg bw (Machemer 1975).
Special studies on mutagenicity
In two similar but separate studies, isofenphos was evaluated for
its mutagenic activity using two microbial assay systems (rec-assay
and reversion test). Indicator micro-organisms used in the former were
Bacillus subtilis strains N1G17, N1G45, H 17 and M 45. For the
latter, Salmonella typhimurium strains TA 1535, TA 1537, TA 1538, TA
98 and TA 100 and Escherichia coli strain WP2 hcr were employed.
The reversion test was conducted in the presence or absence of a
mammalian metabolic activation system (S-9 homogenates of liver from
rats or mice induced by phenobarbital or Aroclor 1254) while the
rec-assay was carried out without the activation preparation. Under
the conditions of the experiments, isofenphos was demonstrated not to
be mutagenic at concentrations up to 0.02 ml/disc of undilated
isofenphos and 5000 µg/plate (Shirasu et al 1980) or 300 µg/disc
and 1000 µg/plate (Inukai and Iyatomi 1977), respectively, in the
rec-assay and the reversion test. Mutagenic responses were obtained
with several positive control compounds, such as mitomycin C,
9-amino-acridine HCl, furylfuramide, acetylaminofluorene and
beta-propiolactone (Inukai and Iyatomi 1977; Shirasu et al 1980).
Another Ames test conducted with S. typhimurium strains
TA 100, TA 1537, and TA 98, with or without the S-9 mix and using
concentrations as high as 3150 µg/plate, also confirmed the non-
mutagenicity of isofenphos (Oesch 1977).
In a dominant lethal assay, groups of 20 male NMRI mice (10 weeks
old) were given by gavage a single dose of 0 or 15 mg/kg bw of
isofenphos. Each male was then mated with three untreated female mice,
which were replaced weekly for 8 consecutive weeks. The females were
sacrificed around the 14th day of gestation or 14 days after
separation from the males and the uteri were removed for examination.
The treated males exhibited no signs of toxicity. There were no
significant differences between the control and the treated groups
with respect to fertility rate, living implants and dead implants.
Pre-implantation loss (no. of corpora lutea and no. of implantations)
was significantly increased at 15 mg/kg bw during the first week after
dosing, but not thereafter. It was indicated in the report that the
values for pre-implantation loss observed in the treated group were
"completely within the norm of the strain" (Machemer 1973).
Special studies on carcinogenicity
See under "Long-term studies".
Special studies on neurotoxicity
Hen
Groups generally comprising 10 adult hens (15 to 18 months old)
were given acute oral or intraperitoneal doses of technical
isofenphos. The oral and intraperitoneal LD50 was found to be
21 mg/kg bw and 11.4 mg/kg bw respectively. No delayed neurotoxic
signs were observed in any of the treated animals during a 6-week
post-exposure observation period. Pre-treatment of hens with atropine
(50 mg/kg bw) intraperitoneally elevated oral LD50 of isofenphos to
74 mg/kg bw. Five hens surviving an oral dose of 20 mg/kg bw of
isofenphos and 6 atropinized hens that were still alive after
receiving 74 mg/kg bw of the insecticide were sacrificed 21 days after
dosing for histopathological examination of the brain, spinal cord and
peripheral nerve. These hens showed no compound-related morphological
change or variation from normal in any of the nervous tissues
examined. Hens treated with an oral dose of TOCP at 350 mg/kg bw
displayed typical neurotoxic signs and showed degenerative nerve
fibres in the spinal cord and the sciatic nerve (Kimmerle 1972; Cherry
et al 1972).
Special studies on antidotes
In 2 separate experiments, atropine sulphate (50 mg/kg bw), 2-PAM
(50 mg/kg bw) or Toxogonin (20 mg/kg bw) was given singly or in
combination (atropine plus PAM or Toxogonin) i.p. to male rats: a) 45
to 120 minutes after single oral toxic doses of isofenphos (and before
onset of severe toxic symptoms), and b) 5 minutes after acute oral
toxic doses of the oxygen analogue of isofenphos and before onset of
toxic symptoms. Results indicated that, under the conditions of the
experiments, none of the three compounds tested was effective as an
antidote for isofenphos or its oxygen analogue (Solmecke and Kimmerle
1972b, Thyssen 1974a).
Simultaneous administration of atropine sulphate at 100 mg/kg bw
and obidoxime at 20 mg/kg bw to male rats i.p. after onset of toxic
symptoms (at 25 minutes) resulting from acute oral toxic doses of
isofenphos elevated LD50 of the insecticide from 43.1 mg/kg bw to
84.2 mg/kg bw. No data were provided on the effect of the antidotes on
the toxic signs (Kimmerle and Gröning 1975).
Special studies on potentiation
When the acute oral LD50 values in male rats, determined
experimentally for equitoxic mixtures of isofenphos and malathion or
of isofenphos and EPN, were compared with the expected LD50 values
for the mixtures, the acute toxicity of isofenphos was found to be
potentiated by malathion. An additive effect resulted when isofenphos
was combined with EPN (Solmecke and Kimmerle 1972b).
In further studies with male rats, it was demonstrated with
isofenphos did not potentiate the acute oral toxicity of phenamiphos
or phoxim (both organophorous insecticides) (Thyssen 1976; Thyssen
1977).
Special studies on the acute toxicity of the oxygen analogue
Rat
The LD50 of the oxygen analogue of isofenphos was determined in
6 female and 6 male rats and is reported in Table 2. Thyssen and
Kimmerle 1974).
TABLE 2. Acute toxicity of the oxygen analogue of isofenphos in rats
Sex Route Vehicle LD50
(mg/kg bw)
M Oral Water & Cremophor EL 30.8
F 16.1
M i.p. Water & Cremophor EL 15.9
F 7.8
M Dermal None 117.6 µl/kg bw
F (24 h) (undiluted) 25 µl/kg bw
M Dermal Water & Cremophor EL 97.9
F (24 h) 29.5
M Inhalation Ethanol & polyethylene >353 mg/m3 air
(1 h) glycol 400 (1:1)
F approx. 353 mg/m3 air
M Inhalation Ethanol & polyethylene >195 mg/m3 air
(4 h) glycol 400 (1:1)
F 63.8 - 131 mg/m3 air
Signs of oral poisoning
Acutely toxic doses of the oxygen analogue of isofenphos to
rats produced cholinergic symptoms typical of those seen with
cholinesterase inhibitors. These toxic signs occurred within 1 h of
dosing and persisted for a maximum of 2 days in survivors. Deaths from
lethal doses were observed from about 30 minutes to 3 days after
treatment (Thyssen and Kimmerle 1974).
The acute LD50 of the oxygen analogue of isofenphos in hens was
>10 <25 mg/kg bw by the oral route and about 10 mg/kg bw i.p. No
neurotoxic signs were observed in any of the treated hens over a
28-day post exposure observation period (Thyssen 1974c).
Special studies on the subacute oral toxicity of the oxygen analogue
and of a mixture of isofenphos and its metabolites
Groups of 15 male and 15 female rats (Wistar II strain) were
treated daily by gavage with the oxygen analogue of isofenphos (HOL
0654) (as an emulsion in water and Cremophor EL) at 0, 0.2, 0.6 or
1.8 mg/kg bw for 30 consecutive days. Activity of cholinesterase in
plasma and erythrocytes was determined five times over the course of
the study and brain cholinesterase was assayed at terminal sacrifice.
Physical appearance, behaviour and body weight were comparable to
controls. Dose-dependent inhibition (>20%) of plasma cholinesterase
was observed in males and females at 1.8 mg/kg bw throughout the
feeding period, at 0.6 mg/kg bw in males after 3 weeks only and
in females at 3, 10, 17 and 23 days of the study. The level of
erythrocyte cholinesterase was reduced by >20% in both sexes at
1.8 mg/kg bw. In general, the degree of depression of both plasma and
erythrocyte cholinesterase was greater in the females than in the
males. Terminal brain cholinesterase was unaffected in both sexes.
There were no significant gross pathological alterations in treated
animals. The no-effect level on plasma cholinesterase was 0.2 mg/kg bw
(Thyssen 1974b).
Cow
Groups of 3 dairy cows were fed alfalfa pellets treated with a
mixture of isofenphos, isofenphos oxygen analogue and des-N-isopropyl
isofenphos oxygen analogue (1:8:1) at 2, 6 or 20 ppm for 28 days and
then sacrificed for analyses of tissue residues. One cow was used as a
control. Whole blood cholinesterase was assayed at weekly intervals.
Other than the observation of a more sedated or docile appearance in
cows at 6 ppm and above, as compared to the control animal or animals
at 2 ppm, there were no adverse effects noted with respect to body
weight, feed consumption and milk production. A reduction (>20%) of
whole blood cholinesterase activity occurred at 20 ppm at days 14, 21
and 28 of the study and at 6 ppm at days 21 and 28. The inhibition of
whole blood cholinesterase was dose-related and increased with time
over the course of the study (Strankowski et al 1977c).
Hen
Groups of 4 hens were fed diets containing a mixture of
isofenphos, isofenphos oxygen analogue and des N-isopropyl isofenphos
oxygen analogue (1:8:1) at the levels of 0, 5, 15, 50 or 150 ppm for
28 days and then sacrificed for analyses of tissue residues. Body
weight and food consumption at both 50 and 150 ppm and egg production
at 150 ppm were adversely affected. Dose-related depression (22 to
98%) of plasma cholinesterase determined at terminal sacrifice was
observed in all treated groups (Strankowski 1977).
OBSERVATIONS IN HUMANS
In 3 male workers (wearing standard protective clothing) involved
for 5 to 11 working days (generally 7.5 to 8 h per day and not more
than 3 consecutive days at one time) in the application of a 7.5%
granulated formulation of isofenphos through a standard applicator
fixed to carrot seed drilling equipment, the whole blood
cholinesterase activity was not significantly depressed, as compared
to 2 male and 1 female control subjects. There was no mention in the
report with respect to adverse effects or symptoms of exposure. No
other clinical measurements were made (Bagnall 1976).
RESIDUES IN FOOD
USE PATTERN
Isofenphos is an insecticide possessing good activity against
soil insects, such as Diabrotica spp., wireworms, chafer grubs,
vegetable flies and flea beetles. It has proved to be highly effective
against leaf-eating pests, such as pear sucker and Colorado potato
beetle, and also against rice stem borers.
The compound acts as a contact and stomach poison. It has a root-
systemic action, i.e. the active ingredient is absorbed by the roots
of the plants and translocated to a limited degree within the plant.
The active ingredient is notable for its long residual activity
and its good dispersibility in soil. In numerous trials, isofenphos
has displayed generally good crop plant tolerance, whether used as a
seed treatment or applied as granular and emulsifiable concentrate
formulations. Isofenphos is formulated as an emulsifiable concentrate,
granular formulation and seed dressing powder.
It is mainly applied in row at or immediately after seeding. Two
applications are registered only for corn in the United States. The
crops, pests and recommended or registered dose rates are listed in
Table 3.
TABLE 3. Recommended application rates for isofenphos
Crop Pests Formulation Dose (a.i.)
Onion, carrot, brassica
leafy vegetables vegetable flies GR 1.5-2.5 kg/ha in row
Onion onion fly GR 5 kg/ha broadcast or spray
and overall
EC 0.076 g/m in row
Brassica leafy vegetables cabbage root fly GR 0.05 % a.i. (at 100 cm3/plant
and as soil drench after
EC transplanting)
0.0375-0.075 % a.i. at a rate
of 0.0375-0.15g/m row
Potato wireworm GR 5 kg/ha broadcast overall
before planting
Maize Diabrotica sp. GR 0.11 g/m in row at seeding +
and lay by (50 cm row spacing)
EC
Oilseeds, rape, flea beetle Seed 12-16 g/kg seed
swedes, marrowstem dressing seed treatment
kale, turnips powder
RESIDUES RESULTING FROM SUPERVISED TRIALS
Experiments were run on various plants at different locations in
Canada, Europe and USA. Isofenphos was applied at about the
recommended, and in certain cases somewhat higher, dosage rates.
The residues looked for included isofenphos, isofenphos oxygen
analogue (IOA) in European experiments and des-N-isopropyl isofenphos
(DNI) and des N-isopropyl isofenphos oxygen analogue (DNIOA) were
additionally included in the analyses carried out in USA. The residues
measured in brassicas, oilseed, rape, root and tuber vegetables were
low, generally at or about the limit of determination for the
recommended pre-harvest interval, as well as those found in kernel of
corn at the milk and dry stages. In green forage, however, residues
were as much as 2 mg/kg. Onions and potatoes also contained detectable
residues. The major part of the residue consisted of isofenphos and
IOA in all crops.
Brassica leafy vegetables
Brussels sprouts were treated by applying a soil drench at a rate
of 0.05 g a.i./plant shortly or 1 day after planting. No residue was
measurable 99-119 days after treatment (Bayer 1978). isofenphos was
applied on cabbages, savoy and white, and cauliflower either in row or
with overall treatment at dose rates of 0.075 g a.i./m in a 10 cm band
or 1.5 to 3 kg/a.i./ha, respectively. The experiments were carried out
in Federal Republic of Germany (FRG) and UK and results are summarized
in Table 4 (Bayer 1973-74, 1977).
Celery
Oftanol 200 EC was worked in the soil at rate of 4 kg a.i./ha one
day before planting. Samples were taken 84 and 90 days after treatment
and analysed for isofenphos and IOA. The residues were below the limit
of determination (0.01 mg/kg) (Netherlands 1981).
Maize
Supervised trials were carried out in various states of the USA
in 1979 and 1980.
AMAZE 15 G or 20 G were used for the treatment of the plots in a
15 cm band at planting plus either a double side dress at the 4-leaf
growth stage or band treatment at the base of the plant at growth
stage of 45 cm. A dosage rate of 0.19 g a.i./m was applied in all
treatments (equal to 2.5 kg a.i./ha). Samples were taken from green
forage, kernel, cob and husk in the milk stage and from the dried
parts of plant. Tests were made for residues of isofenphos and its
three metabolites. The limit of determination was 0.01 mg/kg for all
compounds individually.
TABLE 4. Residues of isofenphos in brassica leafy vegetables
Application Residues 1 (mg/kg) at intervals (days) after application
Crop Country Year No. Rate
(kg a.i./ha) Formulation 0/42 49 55-56 60 64-70 80-87
Cabbage FRG 1974 1 3 kg/ha 5 G 0.01 0.01 <0.01
FRG 1974 1 " 5 G 0.08 0.02 0.01
savoy FRG 1974 1 " 5 G <0.01 <0.01 <0.01
FRG 1977 1 )0.075 g/m 500 EC 0.02 0.01 0.01 <0.01
FRG 1977 1 ) in row 500 EC 0.12 0.03 0.02 <0.01
FRG 1977 1 ) 500 EC 0.09 0.03 0.02 0.01
Cabbage UK 1974 1 1.5 kg/ha 5 G 0.007
FRG 1973 1 2.5 kg/ha 5 G 0.1 0.01
white FRG 1973 1 2.5 kg/ha 5 G 0.06 0.03
FRG 1973 1 2.5 kg/ha 5 G 0.08 0.02
Cauliflower FRG 1977 1 0.075 g/m 500 EC 0.11 0.04 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.01 <0.01 <0.01
in row
FRG 1977 1 0.075 g/m 500 EC 0.02 <0.01 <0.01 <0.01
in row
FRG 1973 1 2-2.5 kg/ha 5 G <0.012 <0.011 <0.013
1 Sum of isofenphos and its oxygen analogue;
2 Results were obtained by analysing 3 samples taken from different plots;
3 Results of analyses of 6 samples.
Isofenphos, IOA and DNIOA were detected in forage. The results
are summarized in Table 5. Levels of DNIOA ranged from 0.01 to
0.08 mg/kg, with an average of 0.04 mg/kg. No measurable residues were
found in kernels either in tile milk or dry stage. Residues in the
cob were at or below the limit of determination (total residue
< 0.02 mg/kg). The husk contained residues of less than 0.2 mg/kg
at all times and stages (Mobay 1979-80).
Sweet corn
Experimental plots were treated in a 15 cm band at planting and
with either a 15 cm band at the base of the plant or a basal spray at
lay by 4 to 8 weeks later. OFTANOL 6 EC and 15 G formulations were
used in the trials (Mobay 1978a).
The active ingredient was applied at a dose rate of 0.19 g/m,
equal to 3.72 kg a.i/ha. The residues (parent compound and
3 metabolites) were analysed separately in 10 milk stage kernel
samples. The oxygen analogue was detected twice at levels of
0.02 mg/kg and 0.05 mg/kg; the other residues were below the limit of
determination (0.01 mg/kg for all compounds). No residues were
detected in dry kernels (3 samples).
The green and dry forages were also analysed, and levels of
isofenphos and IOA are listed in Table 5. The cob in the milk stage
did not contain detectable residues, with the exception of 0.02 mg/kg
of oxygen analogue. The oxygen analogue was also the only detectable
residue in husk at the milk stage, with a maximum level of 0.16 mg/kg.
The dry husk contained both IOA and DNIOA. Maximum levels were
0.27 mg/kg and 0.02 mg/kg respectively.
Onion
At various locations in the USA in 1978, 17 experimental plots
were treated in furrow at planting with either 15 G, 10 G, 5 G or 4 EC
formulations at a dose rate of 0.1 g isofenphos/m. The dose rate
expressed in kg/na varied depending on the row spacing, which was
between 30 to 100 cm (Mobay 1978b). Both mature bulbs and immature
bulbs were sampled and analysed for isofenphos, IOA, DNI and DNIOA.
The residues were generally low in the bulb.
DNI and DNIOA were only detected in one sample at levels of
0.01 mg/kg and 0.08 mg/kg respectively. Supervised trials were also
carried out in Federal Republic of Germany, France and in The
Netherlands. Five kg a.i./ha was applied from both 5 G and 500 EC
formulations one day before sowing or at the 4-leaf stage. Samples
were taken 92 to 158 days after application and the levels of
isofenphos and IOA were measured (Bayer 1972-73, 1975, 1978). The
number of samples taken from experimental plots both in USA and in
Europe are listed in Table 6.
TABLE 5. Residues of isofenphos in forage of maize and sweet corn
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Maize USA 0.03 0.02 0.02
1980 2 2.5 15 G
Indiana 0.141 0.071 0.111
" 1980 2 2.5 15 G 0.05 0.04 0.04
0.141 0.191 0.111
" 1980 2 2.5 20 G 0.03 0.07 <0.01
0.111 0.231 <0.011
Nebraska 1979 2 2.5 20 G <0.01 <0.01 <0.01 <0.01 <0.01
<0.011 <0.011 <0.011 <0.011 <0.011
" 1979 2 2.5 15 G <0.01 0.02 <0.01 <0.01 <0.01
<0.011 0.01 <0.01 <0.01 <0.01
Sweet corn USA 1978 2 3.72 6 EC 0.22
Missouri 0.741
Oregon 1978 2 3.72 6 EC <0.01 <0.01 <0.01 <0.01 <0.01
<0.031 0.031 0.041 0.031 <0.011
Nebraska 78 2 3.72 6 EC 7.86 0.01 <0.01 0.04
9.261 0.251 0.061 0.051
Texas 1978 2 3.72 6 EC 0.2 0.05 0.08 0.04
2.091 1.611 1.991 0.071
Florida 1978 2 3.72 6 EC 0.03 0.02 0.04 <0.01
0.751 1.371 0.671 0.071
Missouri 78 2 3.72 15 G 0.94 0.07 0.1
0.741 0.231 0.191
TABLE 5. (con't)
Residues (mg/kg) at intervals (days) after application
Application in green forage
Crop Country Year No. Rate in dry
(kg a.i./ha) Formulation 14-15 20 25-26 29-31 35-39 42-45 55-69 forage
Oregon 1978 2 3.72 15 G 0.02 0.02 0.02 <0.01 <0.01
0.081 0.081 0.061 0.06 0.011
Texas 1978 2 3.72 15 G 0.01 0.03 0.05
0.721 1.041 0.151
Florida 1978 2 3.72 15 G <0.01
0.041
Missouri 78 2 3.72 15 G 0.39 0.03 <0.01
0.871 0.14 0.11
1 Residues of oxygen analogue.
TABLE 6. Supervised trials measuring isofenphos and its oxygen
analogue in onions
Compound Residue (mg/kg) No. of samples
Isofenphos < 0.01 12
< 0.05 0
< 0.1 1
< 0.2 1
< 0.6 3
IOA < 0.01 4
< 0.05 8
< 0.1 2
< 0.2 2
< 0.6 1
Isofenphos + < 0.01 2
IOA 1 < 0.05 4
< 0.1 3
1 Result of experiments in Europe.
The sum of the residue detected was always below 1 mg/kg in
mature bulb samples. Immature bulbs were analysed in two occasions and
the residues found were: isofenphos-0.32 and 0.95 mg/kg; isofenphos
oxygen analogue - 0.18 and 0.26 mg/kg.
Rapeseed
Seeds of spring and autumn varieties of rape were seed dressed
with BAY 6643 B at a rate of 15 g isofenphos/kg seed in 1973-74. The
crops were sampled at various locations in the FRG. Residues were
below the limit of determination (0.01 mg/kg) in all of the 13 trials
(Bayer 1974a).
Supervised trials with three varieties were carried out at three
locations in Canada in 1989 (Mobay 1980a). AMAZE 40% was used for seed
dressing prior to seeding at a rate of 25 g a.i./kg seed. The seeds
were treated either with formulation or the powder was applied to seed
after coating with oil. The harvested seed was analysed for
isofenphos, IOA, DNI and DNIOA 94-113 days after treatment. Residues
of active ingredient and its metabolites were below the limit of
determination (0.01-0.02 mg/kg).
Root and tuber vegetables
Celeriac
Supervised trials, in which 4 kg a.i./ha of Oftanol 200 EC was
applied before planting, were carried out on celeriac fields at two
locations of The Netherlands in 1978. The mature crops were samples
158 days after application. Residues of isofenphos were not detected
(Netherlands 1981).
Potato
In an experiment in FRG, a 5 G formulation of isofenphos was
worked into the soil at a rate of 5 kg a.i./ha before planting. At 110
days after application, a residue of 0.11 mg/kg was detected in the
tubers that had been washed before analysis (Bayer 1973).
Potato fields were treated with isofenphos at three different
locations in Spain in 1980. The broadcast applications were made at a
rate of 2.5 kg a.i./ha at the time of planting. Samples were taken
111, 112 and 159 days later at the time of harvest and their residue
contents were found to be 0.45, 0.04 and 0.05 mg/kg respectively
(Bayer 1980).
Swedes and turnips
Oftanol T was used for seed dressing at a rate of 16 to
20 g a.i./kg seed. Experimental plots from 8 locations in FRG were
sampled 109 to 179 days after sowing in case of swedes, while three
plots of turnips were sampled 89 to 103 days after sowing. Leaves and
roots were analysed separately. No residue was detected (<0.01 mg/kg)
(Bayer 1974-76).
Availability of soil residues to rotational crops
Wheat, green beans and sugarbeets were planted as rotational
crops 3.5 months after a sandy soil had been treated with ring-µL
(14C)-isofenphos at a field application rate of 5.6 kg a.i./ha (Kurtz
1977). Corn had been used as the original crop in this isofenphos-
treated soil. When the rotational crops were planted, the soil
contained approximately 4 mg/kg isofenphos. The rotational crops were
sampled at 2, 8 and 32 weeks after planting. At the time of harvest,
8 weeks for green beans and 32 weeks for sugarbeet and wheat, the
residue level was measured in different parts of the crops (Table 7).
No des-N-isopropyl isofenphos was detected in any of the crops.
TABLE 7. Residues of isofenphos detected in rotational
crops grown in soil treated with isofenphos 1
Crop Sample Residue (mg/kg)
I IOA DNIOA
Green bean Forage < 0.01 0.05 < 0.01
Sugarbeets Roots 0.04 0.02 < 0.01
Tops < 0.01 0.02 0.06
Wheat Heads < 0.01 0.24 0.03
Straw 0.14 0.79 0.2
Forage 0.05 1.08 0.23
1 Application rate - 5.6 kg a.i./ha.
In the investigation of field rotational crops, wheat, oats or
sorghum, spinach turnips and soybean were planted in plots treated the
previous year with isofenphos (Mobay 1980b). The plots had been used
previously for performance tests and hence were cropped using normal
agricultural practices. Rotational crop planting dates ranged from 214
to 390 days (7 to 13 months) post-treatment. Treatment was with either
AMAZE 20 G in an 18cm band over the row at 1.2 kg a.i./ha to 1.5 kg
a.i./ha broadcast equivalent-with presswheel incorporation or with
AMAZE 6 E or 20 G applied in furrow at 1.1 to 2 kg a.i./ha broadcast
equivalent. Prior to planting the rotational crops, the plots were
rototilled or disced. The crops were then planted across and
perpendicular to the direction of the originally treated rows. To
insure homogeneity of sampling, the entire lengths of the crop rows
were harvested. Each of the harvested crops were analysed for residues
of isofenphos, IOA, DNI and DNIOA. The results of the study are given
in Tables 8 and 9.
TABLE 8. Residues of isofenphos in grain (wheat, oats or sorghum) rotational crops
Treatment Plant Back Residue (mg/kg 1
Crop Location Rate Interval
(USA) (kg a.i./ha) (days) Forage Grain Straw
Winter
Wheat Kansas 1.5 77 <0.01 <0.01 <0.01
Sorghum Texas 1.2 214 <0.012 <0.01 0.02
<0.033
Spring
wheat Kansas 1.5 295 0.064 NA5 NA
0.026
Spring
Wheat Nebraska 1.5 350 <0.014 <0.01 <0.01
<0.016
Oats Illinois 1.2 362 <0.014 <0.01 <0.01
<0.016
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos equivalents;
2 Immature crop, 42 days post-planting;
3 Immature crop, 57 days post-planting;
4 Immature crop, 45 days post-planting;
5 NA - no mature crop available for analyses;
6 Immature crop, 60 days post-planting.
TABLE 9. Residues of isofenphos in rotational crops
Treatment Plant back Residue
Crop Location rate Interval (mg/kg) 1
(kg a.i./ha) (days)
Spinach Arizona 2 279 <0.01
Spinach Arizona 2 279 <0.01
Turnip tops Kansas 1.5 316 <0.01
Spinach Nebraska 1.5 363 <0.01
Turnip tops Nebraska 1.5 363 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Arizona 2 279 <0.01
Turnip root Kansas 1.5 316 <0.01
Turnip root Nebraska 1.5 363 <0.01
Soybean Kansas 1.5 295 0.03
Soybean Nebraska 1.5 363 <0.01
Soybean Illinois 1.14 390 <0.01
1 Sum of isofenphos, IOA, DNI and DNIOA expressed in isofenphos
equivalents.
Residues in all rotational crops studied consisted mainly of
isofenphos and IOA and were <0.05 mg/kg; most were <0.01 mg/kg. In
the four mature grain crops, the grain had no detectable residue and
the straw showed a detectable residue of 0.02 mg/kg in only one
sample. In the five vegetable crops, only one had a detectable residue
(0.01 mg/kg) was seed in only one of the bean samples and in two of
the dry vine samples. Based on these results, little or no isofenphos
residue would be expected in grain, vegetable, root or oil seed crops
grown in fields treated 9 or more months earlier with isofenphos at
rates up to 2 kg a.i./ha.
FATE OF RESIDUES
General comments
The degradation and metabolism of isofenphos was studied in
animals, plants, soils, water and during frozen storage. The
structural formula, chemical name, identifying symbol and occurrence
of its degradation products are shown in Figure 2. Metabolic pathways
for isofenphos in animals (A) plants (P) and Soil (S) are shown in
Figure 3.
In plants
The metabolism of isofenphos was studied in maize grown in ring -
µL (14C)-isofenphos treated soil both outdoors and in a greenhouse
(Stanley et al 1977).
For the outdoor experiment the labelled isofenphos was prepared
as a 6 EC formulation and incorporated at the rate of 5.6 kg a.i./ha
(ca 4 mg/kg soil) into soil contained in a tub. Sweet corn was planted
in the soil. Plant samples above the soil line were harvested at 28,
56 and 94 days after planting. The 94-day crop was divided into
kernels, cobs, husks and stalks in the laboratory.
In the greenhouse experiment the labelled isofenphos was
incorporated into soil at a rate of 4 mg/kg. The soil was placed in
pots and field corn was planted in it. The crop was harvested at
maturity, 141 days after treatment, and the plants were divided as in
the other experiment. The root samples were washed with water to
remove soil particles.
The compounds identified in the corn stalks were qualitatively
the same throughout the study, although their relative quantities
changed as the plant matured.

 Over 70% of the radioactivity appeared to be non-conjugated and
readily released from the plant matrix of the 28- and 56-day corn
stalk samples by methanol chloroform extraction, while in the 94-day
corn stalks, only 19% of the radioactivity was organosoluble without
hydrolysis. The distribution of radioactivity among isofenphos and its
metabolites in corn stalk grown outdoors is shown in Table 10.
TABLE 10. Distribution of radioactivity among isofenphos and its metabolites inmaize grown
outdoors in ring-µL (14C)-isofenphos-treated soil
Percent Radioactivity Distribution
Organic/Aqueous Water/Solid Fractions
Days after Sample Extract Acid released
Treatment
I IOA DNIOA SA DBHA DAIOA Unk.
28 stalk 19 52 3 nd nd nd nd
56 stalk 8 63 6 <1 12 <1 2
94 stalk 2 15 2 5 35 3 5
cob 0 17 0 nd nd nd nd
The primary organosoluble metabolite was isofenphos oxygen
analogue, but isofenphos and des N-isopropyl isofenphos oxygen
analogue were also detected. Acid hydrolysis of the aqueous and soil
fractions from corn stalk liberated 2,5-dihydroxybenzoic acid (DHBA)
SA and DAIOA.
As the plant matured, DHBA became the predominant metabolite. The
only component identified in the husk and cob samples was IOA.
The small quantity of radioactivity in the organosoluble fraction
of the kernel prevented adequate identifications to be made, but none
of the intact phosphate esters could have been present in an amount
higher than 4% of the kernel's total residue, which accounted for 6.1%
of the activity measured in the whole plant. The concentration of
radioactivity and its distribution in the tissues of mature corn grown
in the greenhouse was similar to that seen in the outdoor study. The
compounds identified were exactly the same.
Bermuda onions were grown in a greenhouse for 60 and 91 days in
soil in which 4 mg/kg ring-µL (14C)-isofenphos had been incorporated.
The harvested plants were divided into tops (above the soil level) and
bulbs (Stanley 1977a). In mature plants, the concentration of residues
increased in the tops and decreased in the bulb, in comparison with
levels measured in 60-day plants, mainly as a result of the changes in
the weight of the plant portions.
Over 84% of the radioactivity in each sample was extracted by
blending and acid hydrolysis. Over 80% of the radioactivity in the
tops and over 50% in the bulbs was organosoluble, indicating the
amount of non-conjugated residues.
The compounds detected in the organosoluble fractions after
blending were isofenphos, IOA and DNIOA. IOA was the major compound in
the tops and the 91-day bulbs, 78-79% and 35% of total activity
respectively, while isofenphos and IOA ratio was 44% and 24% in the
60-day bulbs. DNIOA was present in all samples in small amounts but
was less (<1%) in bulbs and in tops (2-3%) at both intervals. Acid
hydrolysis of the aqueous fraction from the organic extract liberated
salicylic acid (1 to 2%) and an unknown (<1 to 3%). The remaining
radioactivity (6 to 12%) on the TLC plates was at the origin,
indicating very polar compounds, or was present as streaking and not
as discrete spots.
The radioactivity remaining in the solids after organic
extraction constituted about 5 to 8% in tops and 14 to 16% in bulbs of
the total activity of 60 and 91-day samples, respectively. Acid
hydrolysis of the solids released over one half of the radioactivity
remaining in the solids after methanol chloroform extractions. The
radioactivity of solids was not further characterized.
In animals
Pig
The feeding study of a male pig is described previously under
"Biochemical aspects". Four tissues, kidney, liver, muscle and fat
were analysed for isofenphos and its metabolites. The amount of
organosoluble metabolites are given in Table 11.
Enzymatic hydrolysis of neutralized aqueous fractions from kidney
and liver released most of the radioactivity remaining in these
tissues. The major enzyme-released metabolite was IPS. Small amounts
of HHA and DAIOA were also found. Between 30-42% of the kidney and
liver radioactivity extracted after enzyme hydrolysis could not be
identified. Unknown (a) consistuted between 19 and 27% of the total
tissue radioactivity.
TABLE 11. Organosoluble metabolites of isofenphos in pig tissues
Compounds % Radioactivity in tissues on 5th day of feeding
Liver Kidney Muscle Fat
Isofenphos 13 2 52 61
IOA <1 <1 16 10
IPS 1 2 17 15
HHA <1 ND 3 ND
DNI ND ND 5 5
DAIOA 4 1 3 <1
Unknown (a) 9 ND ND ND
Total 30 6 97 92
Cow
Under "Biochemical Aspects", feeding studies are reported in two
lactating dairy cows. In the first animal the only milk sample with
a radioactive residue, >0.01 mg/kg isofenphos equivalents, was
analysed for isofenphos and its metabolites. The 7.5h sample contained
0.014 mg/kg isofenphos equivalents and accounted for 0.07% of the
administered radioactivity. DAIOA and HHA were the main metabolites in
the organosoluble fraction and accounted for 13% and 10% of total
activity in milk, respectively. Small amounts (2 to 4%) of IPS, SA and
several unknowns (12%) were also detected. Enzymatic hydrolysis of the
solvent-extracted, pH 5 milk released an additional 45% of the milk
radioactivity. HHA (27%) was the major constituent in this fraction of
the milk. DAIOA, SA, IPS and several unknowns were also found in small
amounts.
In the second experiment milk was collected prior to each day's
dosing and 8 hours after each dosing. Milk production remained at the
cow's normal levels through the 5 days of the study. The cow was
sacrificed 2 hours after the fifth dose was administered. The
following tissues were collected and analysed, their residue content,
in isofenphos equivalent (mg/kg) were: muscle-loin 0.02, shoulder
0.02, round 0.02; fat-renal 0.07; omental 0.07; subcutaneous 0.06;
kidney 0.53; heart 0.04; liver 0.47; udder 0.05 and brain 0.02.
In kidney and liver tissues, less than 35% of the radioactivity
was organosoluble and consisted of SA 26 to 14%, HHA 3 to 4%, IOA 2 to
1%,, IPS 1% to <1%, respectively. Traces (1%) of unknowns were found
in the liver.
Sulphatase beta-glucuronidase combination was employed in the
enzyme hydrolyses, which released 42 to 45% activity from the kidney
and liver extracts respectively. IPS (7 to 3%), SA (8 to 2%) and HHA
(6 to 8%) were identified in the enzymatic extracts of kidney and
liver.
Several unknowns (20 to 33%) were detected, but a single
compound, unknown (a), accounted for the 90% of activity. The
radioactive residues which were not organosoluble or liberated by
enzyme hydrolysis were not further characterized.
A feeding study was conducted to study the biological effects and
to determine the tissue and milk residues after feeding isofenphos and
its metabolites. The ratio 1:8:1 of isofenphos, IOA and DNIOA in the
mixture was based on plant metabolism studies of isofenphos and should
be representative of field-treated samples. Alfalfa pellets containing
the mixture of compounds at 2, 6 and 20 mg/kg levels were given to
dairy cattle ad libitum for 28 days (Strankowski 1977c). Feed
consumption, body weight and milk production were unaffected by
consumption of the treated feed as compared to the control cow. The
milk of cows fed 20 mg/kg contained detectable residues only in the
range of 0.001 to 0.01 mg/kg of isofenphos equivalents on the 28th day
of feeding. After feeding for 28 days the animals were sacrificed and
the following tissues were taken; liver, kidney, muscle (round, flank
and loin) and fat (omental, renal and subcutaneous). Residues in all
the tissues analysed, except liver and kidney at the 20 mg/kg level,
were below 0.0, mg/kg. The liver and kidney samples contained
residues, in isofenphos equivalents, ranging from 0.01 to 0.02 and
from 0.01 to 0.04 mg/kg, respectively.
Hen
Laying hens were given a single oral dose of ring-µL(14C)-
isofenphos at 2.0 mg/kg (Dupre 1975). Eggs and excreta were collected.
At four sacrifice intervals (6, 24, 48 and 96 h post-treatment) blood,
various organs and tissues were collected from four birds per group.
In eggs, the level of radioactivity reached a maximum level of
0.099 mg/kg with an average of 0.047 mg/kg in isofenphos equivalent at
48 h post-treatment. Approximately 72% and 78% of the radioactivity
was eliminated in the excreta within 24 and 96 h respectively. A
maximum level in the blood plasma of 0.274 mg/kg 14C activity at 6 h
post-treatment fell to 0.009 mg/kg 96 h after treatment; 24 h after
treatment, residue levels in skin, fat, breast, thighs, heart and
gizzard muscle were less than 0.05 mg/kg, while liver and kidney
contained 0.9 mg/kg and 0.68 mg/kg respectively.
In the feeding study in hens described under 'Biochemical
Aspects' residues, expressed as mg/kg isofenphos equivalents, found in
various tissues were: gizzard 17.1, kidney 4.9, liver 3.9, fat 0.83,
skin 0.67, heart 0.42, muscle 0.14, eggs - 51 h 0.25, 48 h 0.06, 24 h
0.032. The distribution of the residues in the organosoluble fractions
of various tissues is given in Table 12.
TABLE 12. Distribution of isofenphos and its metabolites in tissues and eggs
of laying hens
Distribution (%)
Compound Gizzard Kidney Liver Fat Skin Heart Muscle Eggs
Isofenphos 90 14 2 74 53 26 31 37
IDA 0 4 1 4 3 3 4 9
IPS 0 1 1 18 19 10 8 10
DNI 0 0 0 3 3 0 0 0
HHA 0 0 0 0 0 0 5 0
Unknown 0 47 19 0 8 36 28 5
Feed treated with a mixture containing isofenphos, IOA and DNIOA
in the ratio of 1:8:1, at 5, 15, 20 and 150 mg/kg feed was distributed
to laying hens ad libitum for 28 days (Strankowski 1977d). Feed
consumption, body weights and production decreased as the amount of
isofenphos and its metabolites in the feed increased. At sacrifice,
giblets (heart, gizzard, liver), muscle (equal portions of thigh and
breast), fat (equal portions of visceral and subcutaneous) and skin
(without feathers) were collected. No residue was detected in the
muscle at any dose level. Residues in fat, giblets, skin and eggs are
summarized in Table 13.
Fish
Studies on continuous exposure for 28 days of channel catfish to
isofenphos are reported under "Biochemical Aspects".
In soil
Isofenphos degradation under aerobic conditions was investigated
by Minor and Murphy (1977) in sandy loam and silt loam, while the
effects of anaerobic and sterile conditions were studied in sandy
loam. Ring µ-L-(14C) labelled isofenphos was uniformly incorporated
into the soil at a level of 8.7 mg/kg. The extractable residue derived
TABLE 13. Residues in isofenphos equivalents found in tissues and
eggs of laying hens1
Residue (mg/kg)
Dose
Fat Giblet Skin Eggs
50 mg/kg <0.01 (4) <0.01 (2) <0.01 (2) 0.002
0.01 (2) 0.02 0.003
0.08 0.003
0.004
150 mg/kg <0.01 (2) 0.01 <0.01 (2) 0.017
0.01 (2) 0.02 0.03 (2) 0.013
0.04 0.015
0.06 0.003
1 No. of samples in brackets.
from isofenphos decreased with time, while the radioactivity bound to
soil, or lost, increased. Only 10% of the originally applied
radioactivity could be extracted from silt loam at 240 days post-
treatment, whereas 23% of the-radioactivity could still be removed
from the sandy loam one year after traces (<1% of original activity)
of IPS. The concentration of isofenphos decreased continuously with
time, and its half life was found to be 127 days in sandy loam and 59
days in silt loam.
The concentration of isofenphos oxygen analogue reached a maximum
between 60 and 120 days post-treatment. The bound radioactivity
increased to a maximum of approximately 20% and the remainder had
volatilized. The bound radioactivity was distributed among humin,
humic acid and fulvic acid fractions in a fashion similar for both
soils. Even after stringent HCl-NaOH fractionation, approximately 50%
of the bound residues was still unextractable.
The volatile radioactivity from soil was composed of isopropyl
salicylate, 14CO2-isofenphos and cyclic isofenphos. At 134 days
post-treatment, the total volatile radioactivity trapped was
distributed among IPS (67%), 14CO2 (30%), cyclic isofenphos (2%)
and isofenphos (1%) (Minor 1980: The volatile compounds in the
experiments accounted for 16 to 51% of the total activity applied to
soil 134 days before, while the soils contained the remaining part of
activity, indicating the importance of conditions (e.g. temperature
and/or microorganism activity) on degradation rate of isofenphos in
soil. In sandy loam and under anaerobic conditions, isofenphos was not
converted to its oxygen analogue, but some of the radioactivity was
lost. Under sterile conditions in sandy loam, isofenphos was neither
altered nor lost. In isolated soil micro-organisms grown in "shake
culture", isofenphos was not altered. Conversion of isofenphos to its
oxygen analogue appeared to be strictly a chemical reaction occurring
in soil under aerobic conditions. The production of isopropyl
salicylate may also have occurred without microbial activity, but the
isolated 14CO2 was probably the end result of soil micro-organism
cleavage of the isofenphos aromatic ring after the phosphate group had
been removed. Therefore, isofenphos soil degradation appeared to
involve both chemical and microbial alterations, and the chemical
reactions of oxidation and hydrolysis appeared to be prerequisite to
microbial action. Comparison of results from isofenphos treated soil
kept in a greenhouse or maintained outdoors showed that isofenphos
degradation qualitatively is the same under both environments, but the
rate of degradation may vary.
The persistence of isofenphos in soil was investigated in nine
tests set up in various locations in the United States and Canada
(Mobay 1980c). Isofenphos, 6E formulation, was incorporated uniformly
at a rate of 2 mg/kg wet soil (equivalent to 2.24 kg/ha field
application) into the upper 7.6 cm of soils of seven different types.
Samples were taken at 0, 30 to 33, 60 to 61, 89 to 92 and 119 to 124
days post-treatment from 0 to 15 cm and 15 to 30 cm depths. The
residue of isofenphos decreased in all nine studies, and its level in
the upper layer was between 0.11 mg/kg and 0.43 mg/kg 119 to 124 days
after treatment. IOA reached its maximum (0.19 to 0.5 mg/kg) 32 to 123
days post-treatment. Depending on the soil types and possibly on
environmental conditions, the ratio of isofenphos and its oxygen
analogue varied between 0.37 to 14 at the last sampling. Soil samples
from 15 to 30 cm did not contain detectable residues (0.01 mg/kg),
with the exception of rocky sandy silt loam in which 0.02 to 0.15
mg/kg isofenphos or IOA could be detected.
The degradation of isofenphos was studied in the Federal Republic
of Germany (FRG) and in the UK (Bayer 1973-75). The applications were
made with either EC or granular formulation as surface spray or
broadcast at rates of 1, 5 and 7.5 kg a.i./ha. The sampling day varied
from 0 to 534 post-treatment. The results show a pattern similar to
that obtained in the experiments described previously. The results of
laboratory experiments on the persistence of isofenphos carried out in
standard German soil No. 1 and No. 2 (Bayer 1974a) were consistent
with the results of field trials, as were the mobility tests with soil
thin layer chromatography (Thornton 1976) or soil column (Obrist and
Thornton 1977; Bayer 1973-74, 1977b).
The latter experiments indicated that isofenphos and IOA had low
mobility and the majority of the residue remained in the upper 12.5 cm
of the soil, while the leachates contained no intact isofenphos but
did contain a small amount of IOA and traces of IPS (one experiment).
In water
The stability of ring-µL-(14C)-isofenphos in sterile buffer
solutions of various pH's at various temperatures and two
concentrations (1 mg/l and 10 mg/l) was determined (Mc Namara 1977).
Both hydrolysis and volatilization were involved in the dissipation of
isofenphos from the buffered solutions. Isofenphos was not hydrolysed
at temperatures of 20°C and below or at pH's near neutrality, but
volatilization did occur at 20°C and above and increased as the
temperature and isofenphos concentration increased. Hydrolysis of
isofenphos was, however, encountered in both strongly acidic and
alkaline buffers. Hydrolysis products detected in pH3 buffers included
DNI, IOA, DNIOA, IPS, SA. At pH9, the identified hydrolysis products
of isofenphos were IOA, IPS, SA and N-isopropyl salicyclamide. At
37°C, the half life of isofenphos at 1 and 10 mg/kg was 79 and 30 days
respectively, in pH3 buffer, and 88 and 32 days respectively, in pH9
buffer. At 50°C, the half life of isofenphos at 1 and 10 mg/kg was 15
and 9 days respectively, in pH 3 buffers; 34 and 15 days respectively,
in pH 6 buffer; and 6 days for each concentration in pH 9 buffer.
Ring-µL(14C)-isofenphos at an initial concentration of 10 mg/kg
was incubated outdoors in a simulated pond (pH 8) (Mc Namara 1977a).
Within 70 days, 89% of the radioactivity was lost, and the remaining
radioactivity was distributed between water and sediment in an
appropriate 2:1 ratio. The loss of radioactivity was probably due to
volatilization of isofenphos and some of its metabolites. The major
radioactive compounds identified in the pond water were isofenphos,
IOA and cyclic-IOA. Minor components were cyclic isofenphos, IPS and
SA. The amount of radioactivity adsorbed by the pond sediment rose to
20% of the initially applied radioactivity within 7 days, but then
decreased steadily to only 2% at 70 days. The bound radioactivity
never exceeded 2% of the originally applied radioactivity. The major
components in the organosoluble extracts of pond sediment were
isofenphos and IOA. Minor components identified in the sediment were
IPS, SA, cyclic-isofenphos and cyclic-IOA. The overall rate of
isofenphos dissipation was exponential. The half-life of isofenphos
was 13 days. After 70 days, only 2% of the initially applied
isofenphos itself remained in the pond.
In activated sludge
Ring µL-(14C)-isofenphos was studied in a laboratory model of an
activated sewage sludge system (Spare 1979). Activated sludge,
synthetic sewage and (14C)-isofenphos were aerated for 23 h cycle.
During the remaining hour/cycle, the mixture was allowed to settle and
a portion of the supernatant was replaced with fresh synthetic sewage
and an increased amount of (14C)-isofenphos. The concentration of
isofenphos during the first cycle was 0.1 mg/kg and was increased
through 10 cycles to 100 mg/kg.
After the 100 mg/kg cycle concluded, three cycles were carried
out with no addition of test compound. Isofenphos had no detrimental
effect on the bacteria, yeasts or actinmycetes in the system, but at
isofenphos concentrations of 60, 80 and 100 mg/l, protozoa were absent
or few in the treated flasks. The quantity of radioactivity in the
settled solids increased steadily during the course of the study from
10% to 50% of the added radioactivity. (14C)-isofenphos was not
degraded appreciably by the organisms in the activated sludge system.
Greater than 96% of the added (14C)-isofenphos was recovered intact
from the supernatant and solid fractions.
Photodegradation
In aqueous solution
A study of the photodecomposition of ring-uL-(14C)-isofenphos in
aqueous solution was carried out in a photoreactor containing a high
pressure, quartz, mercury-vapour, 200-watt, Hanovia immersion lamp as
the light source (Strankowski 1977e). Decomposition of isofenphos was
slow in the pH 7 solution with only 30% decomposition in 30 days of
continuous exposure to the high intensity light. The isofenphos half-
life was calculated to be 51 days. In an acetone sensitized solution,
the photodecomposition was increased, and the half-life of isofenphos
was reduced to approximately 14 h.
Isofenphos was the only component found in the dark control
solutions from either the unsensitized or sensitized studies. It was
also identified in the photolysis solutions; approximately 75% and 3%
of the total radioactivity remained as isofenphos at the end of the
unsensitized and sensitized studies, respectively.
The major photoproduct, which represented nearly 21% of the total
radioactivity in the sensitized test, was identified by Poje (1979) as
3,3-dimethyl isoindoline-l-one by mass spectroscopy and nuclear
magnetic resonance spectroscopy. This product contains no phosphorous.
The remaining photoproducts, none of which accounted for more than 5%
of the total radioactivity, included IOA, DAI, SA and catechol.
On soil
In a study of the photodegradation of (14C)-isofenphos on soil,
isofenphos had a half-life of 2.6 days when applied to a thin layer of
soil and subjected to light from a 200 watt Hanovia mercury lamp
(Weissenburger and Pollock 1979). Six photoproducts were detected
on the soil. IOA (33% of the applied radioactivity) was the principal
photo-product. Small quantities (approx. 5%) of DNI, DNIOA, des-N-
isopropyl cyclic isofenphos, IPS and phenol were also detected. During
26 days of exposure to the light, 25% of the applied radioactivity was
bound to the soil and 33% was lost through volatility. A trapping
study, carried out for 3 days, showed that the volatile compounds
included isofenphos (91%), DNI (3%), IPS (1%) and phenol (5%). All of
the applied activity was accounted for in the trapping study.
In storage
Stability of isofenphos and its metabolites were studied during
frozen storage (-28°C to -18°C) (Mobay 1980a). Concentration of
isofenphos and IOA did not change significantly in maize kernel,
brassica leafy vegetables, green forage, green beans (pods and vines)
and onions kept deep frozen for 400 to 800 days. However, 24% of
isofenphos decomposed in sugarbeet during 420 days of storage. DNI and
DNIOA residues decreased by 20% add 32% respectively in alfalfa stored
at -23 to -18°C.
Poultry tissues, eggs and giblets, fortified at 1 mg/kg with
isofenphos and its metabolite, were stored frozen for 255, 90 and 60
days respectively without significant changes in concentrations of
residues.
Cattle tissues and milk were fortified at 1.0 mg/kg with
isofenphos and metabolites and placed in frozen storage. Fat and
muscle tissues showed no significant decomposition of the compounds
after 24 to 28 days. Milk showed no significant decomposition of the
compounds after 76 days in frozen storage. In kidney and liver,
isofephos, isofenphos oxygen analogue and des-N-isopropyl isofenphos
were stable for 28 to 35 days. The liver and kidney samples were
extracted within 4 and 8 days, respectively. There was no
decomposition of des-N-isopropyl isofenphos oxygen analogue in kidney
after 10 days of frozen storage; however, it did not disappear
completely in liver after 5 days of frozen storage.
METHODS OF RESIDUE ANALYSIS
A gas chromatographic procedure for the determination of residues
of isofenphos and its oxygen analogue in various crops, soil and water
has been described by Wagner (1976). Plant samples of high water
content and soils are extracted with acetone. The acetone extract is
diluted with water and the residues are partitioned into chloroform or
dichloromethane. Rape, maize and water are extracted with acetonitrile
and chloroform respectively. The concentrated extracts are cleaned on
neutral alumina or on active carbon columns. The residues in the
concentrated extracts are separated on DC-200, QF-1 or OV-17 columns
and detected with a thermionic detector. Recovery data from
experiments run on a large variety of crops by adding known amounts of
isofenphos and its oxygen analogue at the blending step were generally
in the 77% to 105% range. The limit of determination is 0.005 mg/kg
for each compound in plant and soil samples and 0.002 mg/kg in water.
Gas chromatographic methods for the determination of residues of
isofenphos, isofenphos oxygen analogue, des-N-isopropyl isofenphos and
des-N-isopropyl isofenphos oxygen analogue in maize cob, forage, husk
and kernel and in onions are described by Stanley (1977b, 1979a) and
in bovine and poultry tissues, milk and eggs by Shaw II (1977a). For
all four compounds, recoveries were 65 to 70%. The limit of
determination for each compound is 0.01 mg/kg, except for milk and
eggs (0.001 mg/kg). Multiplication factors for converting metabolite
residues to isofenphos equivalents are 1.05 for IOA, 1.139 for DNI and
1.204 for DNIOA.
Interference studies for the determination of residues of
isofenphos and its metabolites as maize and onions were done by
Stanley (1977c, 1979b) and in milk, animal tissues and eggs by Shaw II
(1977b, 1979). All possible interferences by 20 or 27 organophosphate
pesticides registered for use on maize and onions or investigated in
animal products respectively were separated from isofenphos and its
metabolites by GLC on the standard (10% DC-200 + 2% OV-225 on
Chromosorb WHP) or on the confirmatory (5% OV 210 on Supelcoport or 5%
OV 275 on Chromosorb WHP) columns or by clean-up on the silica gel
column used in the residue method. The silica gel column was applied
as follows: 372 g silica gel is deactivated with 28 cm3 water, 10 g
is filled into the column under hexane and settled, and a 1-cm layer
of granular Na2SO4 is placed on top of the column.
Hexane is drained to the top of the sodium sulphate. The
concentrated extract is transferred in the column with 5 to 10 cm3
portions of hexane and finally the column is washed with hexane (total
amount-50 cm3). The portions of hexane is drained through the column
at a rate of 5 to 10 cm3 until 50 cm3 is collected. Eluents (freshly
prepared) and fractions are: (1) 185 cm3 hexane-dichloromethane (1:1)
(isofenphos); (2) 150 cm3 dichloromethane (DNI); (3) 65 cm3
dichloromethane-methanol (99:1) (discarded); (4) 135 cm3
dichloromethane-methanol (99:1) (IOA).
Shaw II (1977b, 1979) applied different conditions: 12 g silica
gel and 6 g Na2SO4; column is washed with 40 cm3 hexane, 100 cm3
hexane-benzene (8:2) 75 cm3 hexane-benzene (6:4); elution: (1)
fraction (isofenphos) 25 cm3 hexane-benzene (6:4) + 100 cm3 hexane-
benzene (4:6) + 100 cm3 hexane-benzene (2:8) + 50 cm benzene; (2)
fraction (IOA + DNI) 50 cm3 benzene + 300 cm3 benzene-acetone (8:2).
A gas chromatographic method for the determination of isofenphos
and isofenphos oxygen analogue in soil and water, using an alkaline
thermionic emission detector, is described by Shaw II (1974).
Recoveries of isofenphos and its oxygen analogue were 93 to 101%; the
sensitivity of the method is approximately 0.005 mg/kg for soil and
0.002 mg/kg for water samples.
The determination of isofenphos and its oxygen analogue residues
in aged soil by alkaline thermionic emission gas chromatography was
investigated by Shaw II (1977c). The soil was extracted in a Soxhlet
apparatus for 16 to 24h with chloroform/methanol (7:3). The extract
was concentrated, partitioned with hexane/acetonitrile (1:1) and
partially purified with three hexane extractions of the residue in a
methanol/water solution. Isofenphos recoveries were >79% and
isofenphos oxygen analogue recoveries were >95% from soils fortified
at 0.05 mg/kg. The sensitivity of this method is 0.01 mg/kg.
Isofenphos and IOA were determined by Brown and Williams (1976)
in cabbage, potato, rapeseed and soil. The rapeseed was extracted with
acetonitrile and cabbage, potato and soil with ethylacetate. The
concentrated extracts were cleaned on a column consisting of the
mixtures of Florisil, silica gel, alumina and Nuchar C. The cleaned-up
extracts were separated on DEGS or OV275 liquid phases and detected
with FPD. The recoveries at 0.01 to 1 mg/kg levels were in the range
of 84 to 98%. The limits of determination were 0.003 mg/kg and 0.005
mg/kg for isofenphos and IOA respectively in potato, rapeseed and
soil, while they were five times higher in cabbage.
The methods described by Wagner (1976) or by Brown and Williams
(1976), in combination with the confirmatory columns and GLC
separation (Stanley 1977b, 1979a), are recommended for regulatory
purposes.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Isofenphos is registered in the following countries: Austria,
Bulgaria, Chile, Denmark, Federal Republic of Germany, German
Democratic Republic, Indonesia, Israel, Italy, Morocco, Mexico,
Norway, The Netherlands, South Africa, Spain, Sweden and the USA.
Maximum residue limits and preharvest intervals were reported
from some of the countries and are summarized in Table 14.
EVALUATIONS
COMMENTS AND APPRAISAL
In the mammalian species tested, the oral LD50 ranged from about
20 mg/kg bw in rats to approximately 150 mg/kg bw in rabbits. In the
rat, the oxygen analogue of isofenphos was shown to be slightly, but
not substantially, more toxic orally than isofenphos, both acutely and
subacutely.
TABLE 14. National MRLs reported to the Meeting
Preharvest
Country Crop/Commodity intervals MRL
(days) (mg/kg)
Fed. Rep. of Leafy and stem
Germany vegetables 0.1
Rapeseed 0.05
Italy Pear 42 0.1
Fruit 0.1
Vegetables 0.1
Sugarbeet 0.1
Netherlands Cauliflower 56 0.1
Cabbages (including
Brussels sprouts) 56 0.1
Onions 0.1
Celery 0.05
Celeriac 0.05
Norway Root, tuber and
bulb vegetables 90
Brassica leafy vegetables Apply not later
than transplanting
South Africa Citrus fruit 180 0.2
Spain Cabbage Apply before sowing
Onions 21
Garlic 21
All crops (except
root vegetables) Apply before sowing
and potato)
USA Maize 75
Corn, forage 1.0
Corn, fodder 1.0
Corn, fresh (including
sweet) 0.1
Corn, grain 0.1
Meat, fat, meat by-
products of cattle,
sheep and poultry
Milk 0.02
Eggs 0.02
Metabolism studies in rats, pigs and cows, treated orally with
ring-µL(14C)-isofenphos indicated that the compound is rapidly
eliminated in both faeces and urine, with the latter being the
predominant route of excretion. It is metabolized mainly by oxidative
desulphuration, dearylation, hydrolysis, deamination and conjugation.
There is no indication that isofenphos and/or its metabolites
accumulate in mammalian tissue. The excretion pattern and proposed
metabolic pathways for isofenphos appear to be similar in the three
mammalian species studied.
A 3-generation reproduction study in rats (with 2 litters in the
first generation (F0) but only one litter each in the second (F1)
and third (F2) generations showed a marginal effect in the F0
generation at the second mating on the pregnancy rate, even at 1 ppm,
the lowest tested level. No indication of teratogenic effect was noted
in a rabbit teratology study at dosages as high as 5 mg/kg bw.
Teratology studies in rats exposed orally, dermally or via inhalation
were all negative.
In vitro microbial assays, including reversion-test and rec-assay
and a dominant lethal study in mice, failed to give any evidency of
mutagenicity. Long-term studies in mice and rats revealed no
carcinogenic activity. An acute delayed neurotoxicity study in hens,
which could be considered as a screen, was negative. The acute oral
toxicity of isofenphos was found to be potentiated by malathion but
not by a number of other organophosphorus insecticides tested.
Plasma cholinesterase inhibition was observed as the most
sensitive indicator of toxicity in the short-term rat studies and the
108-week mouse and 2-year rat feeding studies. A dietary level of
1 ppm was a marginal no-effect level in the rat and a no-effect level
in the mouse with respect to plasma cholinesterase. In the dog, a
90-day study suggested that dietary levels of 10 ppm and above caused
significant plasma cholinesterase depression and an increase in liver
weight, although the latter was not accompanied by histopathological
alteration of the tissue. A 2-year feeding study in the same species
demonstrated 2 ppm to be the no-effect level, based on plasma
cholinesterase. The information provided on observations in humans was
very scanty.
Acceptable data was available to permit the establishment of no-
effect levels in three mammalian species. Owing to the unavailability
of an appropriate neurotoxicity study in hens, only a temporary ADI,
was allocated.
Isofenphos, O-ethyl O-2-isopropoxycarbonylphenyl
isopropylphosphoramidothioate, is an organophosphorus insecticide
applied mainly in soil at a rate of 1.5 to 2.5 kg a.i./ha or 1 to 5 kg
a.i./ha for row or overall surface treatment, respectively.
Supervised trials were carried out on various crops at different
locations in the United States, Canada and Europe. Isofenphos and its
main metabolites, isofenphos oxygen analogue (IOA), des-N-isopropyl
isofenphos (DNI) and des-N-isopropyl isofenphos oxygen analogue
(DNIOA), were analysed. It was found that the residue consisted mainly
of isofenphos and IOA. The residues measured in brassica leafy
vegetables, rapeseed and root and tuber vegetables were below
0.1 mg/kg and usually at or about the limit of determination after the
recommended pre-harvest intervals. No residues were detected in maize
kernels, either in the milk or dry stages, while residues up to
2 mg/kg were found in green forage 3 to 4 weeks after application. The
total residue detected in mature onion bulbs was always below 1 mg/kg.
Rotational crops can also take up residues from previous soil
treatments. The residues, consisting mainly of isofenphos and IOA,
were at or about the limit of determination in cereal grains, leafy
and root vegetables and edible oil crops grown in soil treated with
isofenphos 9 or more months earlier, The residue level might exceed
1 mg/kg in green wheat forage but it is under 0.1 mg/kg in the straw
at the time of harvest.
Isofenphos is degraded in soil, plants and animals, but its route
of degradation in soil and plants is somewhat different from that in
animals. Oxidation seems to be the first reaction in soil and plants.
In animals, hydrolysis and oxidation both appear to be of major
importance. The degradation of isofenphos was not rapid in any of the
systems studied. Soil micro-organisms seem capable of splitting the
aromatic ring to produce CO2 but only after the phosphoramidothioate
rouping has been removed from the molecule. In plants, oxidation is
followed by depropylation, hydrolysis and conjugation. IOA and
isofenphos are the primary residues but DNIOA also appears in smaller
amounts. The approximate ratio of isofenphos, IOA and DNIOA in mature
maize stalks was 1:8:1. 2,5-Dihydroxybenzoic acid (DHBA), salicylic
acid (SA) and deaminated isofenphos oxygen analogue (DAIOA) appear in
traces.
In animals, isofenphos undergoes hydrolysis, oxidation,
N-depropylation, deamination and conjugation. Excretion is rapid
in orally dosed animals. Pigs, cows and hens excreted 81%, 79% and
72% of the applied dose in the urine or excreta within the first
24h after treatment. Urine contained isopropyl salicylate (IPA),
O-hydroxyhippuric acid (HHA) SA and DAIOA in non-conjugated and/or
conjugated forms; 4 to 5% of the administered dose is eliminated in
the faeces over a 72 to 80h period.
During a period of multiple dosing for 6 consecutive days at a
rate of 15 mg/kg/day, rats were able to eliminate residues of
isofenphos fast enough to maintain a relatively constant level of
radioactivity in the tissues. One day after cessation of the
isofenphos treatment a 10-fold decrease in radioactive residue
concentration was detected in the muscle, fat and liver. Dairy cows
and laying hens were kept on feed containing residues of isofenphos,
IOA and DNIOA in the ratio of 1:8:1 at various levels between 2 and
20 mg/kg feed and 5 and 150 mg/kg respectively for 28 days. Residues
in all the bovine tissues, except liver and kidney at the 20 mg/kg
level, were <0.01 mg/kg. The liver and kidney samples collected from
the 20 mg/kg group contained residues of isofenphos ranging from
<0.01 to 0.02 mg/kg and 0.01 to 0.04 mg/kg respectively. Residues of
isofenphos equivalents in milk from all samples, except the 20 mg/kg
group, were below 0.001 mg/kg. The milk samples collected on the 28th
day from the high level group contained residues ranging from 0.001 to
0.01 mg/kg.
Isofenphos residues in poultry tissues and eggs from the control
and 5 mg/kg groups were below the limits of determination of the
analytical method (0.01 mg/kg for tissues, 0.001 mg/kg for eggs). The
hens fed at the 15 mg/kg level had no detectable residues in the
tissues and had residues ranging from <0.001 to 0.002 mg/kg in the
eggs. In the 50 mg/kg group, muscle and fat samples had no measurable
residues, while giblets and skin samples had residues ranging from
0.002 to 0.004 mg/kg. Residues were detected in all tissues, except
muscle, and ranged from <0.01 to 0.05 mg/kg and in eggs from 0.003 to
0.017 mg/kg at the exaggerated 150 mg/kg dietary level. Control values
were <0.01 mg/kg in tissues and <0.001 mg/kg in eggs. No detectable
residues can be expected in products derived from animals fed with
forage that has been treated with isofenphos at recommended
application rates.
Catfish exposed continuously to isofenphos for 28 days in water
containing 10 mg/l isofenphos accumulated residues of 0.75 mg/kg,
consisting entirely of intact isofenphos, within the first 4 days of
exposure. However 87% of this was eliminated within the first day
after transfer of the catfish to uncontaminated water.
Isofenphos is readily absorbed by soil. It is only slowly leached
in all the soils tested, but its degradation products move somewhat
more rapidly. In soil persistence studies with isofenphos incorporated
into the soil at the single-application field-use rate, the residue of
isofenphos itself declined steadily in all soils tested, and the total
organophosphorus residue decreased to half the initial concentration
within 45 to 200 days.
Hydrolysis of isofenphos occurs at acidic and alkaline pH, but it
is stable near neutral pH.
In an activated sludge system, isofenphos has no detrimental
effect on the sludge organisms, and the parent compound was not
degraded.
Photodegradation of isofenphos in aqueous solution is slow and
yields principally a re-arrangement product, 3,3-dimethylisoindoline-
l-one. On soil, photodegradation is rapid and the main product is IOA.
Residue analytical methods for the determination of isofenphos
and IOA, the major residue component, and for DNI and DNIOA are
available. Recoveries are in the range of 77 to 105%.
The limits of determination for isofenphos and IOA in plant
samples, animal tissues, and milk and eggs are 0.005 - 0.01, 0.01 and
0.001 mg/kg respectively, in water 0.002 and in soil 0.01 mg/kg.
Level causing no toxicological effect
Mouse : 1 ppm in the diet equivalent to 0.15 mg/kg bw/day
Rat : 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog : 2 ppm in the diet equivalent to 0.05 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The Meeting estimated the maximum residue levels likely to occur
in various commodities and concluded that they were suitable for
establishing temporary MRLs.
The limits refer to the sum of isofenphos and its oxygen
analogue.
Preharvest intervals (days)
Commodity Limit (mg/kg) on which recommendations
are based
Celeriac 0.02 * 150
Swedes 0.02 * 90
Turnips 0.02 * 90
Brassica leafy vegetables 0.1 60
Celery 0.02 * 90
Maize 0.02 * 90
Maize, fodder 0.5 90
(dry)
Sweet corn 0.02 * 90
Sweet corn fodder (dry) 0.5
Rapeseed 0.02 * 90
Carcass meat 0.02 *
Animal fats 0.02 *
Meat by-products 0.02 *
Cattle milk 0.01 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
An appropriate neurotoxicity study in hens.
Desirable
1. 2-generation (2 litters/generation) reproduction study.
2. In vitro biochemical studies on purified isofenphos with respect
to anticholesterase activity.
3. Further observations in humans.
4. Additional data from supervised trials on potatoes and onions,
including information on soil residues and soil moisture content.
REFERENCES
Bagnall, B.H. Tests to monitor the blood cholinesterase levels of
1976 workers using oftanol granules on carrots. Report from Bayer
UK Limited, Agrochem. Division, submitted by Bayer Ag.
(Unpublished)
Bayer Residue Reports Nos. 4003-4005/74, 4000-4002/77 (Cabbage,
1973-74 savoy), 73-75/73, 4064-/74 (Cabbage, white), 45-47173,
1977a 4000-4002/74, 4003-4005/77 (Cauliflower). (Unpublished).
1974-76 Residue reports nos. 4057/74, 4014-4015/75, 4006-4008/76
(Sweden), 4016/75, 4023-4024/76, (Turnips). (Unpublished)
1972-73 Residue Reports nos. 132/72, 99-101/73, 4003-4004/75,
1975, 4024/78, 4039-4040/78 (Onions). (Unpublished)
1978
1973-75 Residue Reports nos. 214-216/73, 336/73, 4035-4037/74 4031
a-c/75 (Soil, field). (Unpublished)
1973-74 Residue reports nos. 267-268/73, 4038-4040/74, 4043/74,
1977b 4046-4048/74, 4017/74 (Leaching exp.). (Unpublished)
1973 Residue Report no. 105/73 (Potatoes). (Unpublished)
1974 Residue Reports nos. 4032-4034/74, 403211-403411/74,
4049-4051/74, 4049/1-4051/1/74 (Rapes). (Unpublished)
1974 Residue Reports nos. 4044/II.- 4045/74, 4061/74 (Soil,
laboratory). (Unpublished)
1978 Residue Reports nos. 4000-4001/78 (Brussels sprouts).
(Unpublished)
1980 Residue Reports nos. 5001/80, 5002/80, 5202/80 (Potatoes).
(Unpublished)
Bomhard, E. and Löser, E. SRA 12869 chronic toxicity study on rats
1977 (two-year feeding experiment) Report from Bayer AG Institut
Für Toxikologie, submitted by Bayer AG. (Unpublished)
Brown, M.J. and Williams, J.H, Determination of residues of isofenphos
1976 and its phosphoramidate analogue. Pesticide Science,
7: 545-548.
Brune, H., Deutsch-Wenzel, R., Schüman, K. SRA 12869 chronic toxicity
1978 study on NMRI mice (108-week feeding experiment). Report
from Biologisches Laboratorium, Hamburg, submitted by Bayer
AG. (Unpublished)
Cherry, C.P., Newman, A.J. and Urwin, C. Pathology report of SRA 12869
1972 - Acute neurotoxicity experiment in hens. Report from
Huntingdon Research Centre, UK, submitted by Bayer AG.
(Unpublished)
Dupre, G.D. Metabolism and excretion of 14C-labelled SPA 12869 in
1975 poultry. Mobay Report No. 44931. (Unpublished)
Flucke, W. Oftanol bestimmung der akuten toxizität (LD50). Reports
1980a dated 25 July, 29 August, 15 October and 8 December, 1980
from Bayer AG submitted by Bayer AG. (Unpublished)
1980b SRA 12869 Bestimmung der akuten toxizität (LD50). Ratte
p.o. Report dated 7 Nov. 1980 from Bayer AG, submitted by
Bayer AG. (Unpublished)
1981 Oftanol bestimmung der akuten toxizität (LD50). Report
dated 16 February 1981 from Bayer AG, submitted by Bayer AG.
(Unpublished)
Hoffman, K. and Kaliner, G. SPA 12869 chronic toxicity study on dogs
1977 (two-year feeding experiment). Report from Bayer AG Institut
Fur Toxicologie submitted by Bayer AG. (Unpublished)
Inukai, H. and Iyatomi. SRA 12869 mutagenicity test on bacterial
1977 systems. Report from Nitokuno Agricultural Chemicals
Institute, Laboratory of Toxicology, Japan, submitted by
Bayer AG. (Unpublished)
Kimmerle, G. SRA 12869 acute neurotoxicity studies on hens. Report
1972a from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b Kutane toxizitat von Parathion an ratten vesgleich
verschiedener methoden. Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
1976 SPA 12869 acute inhalation toxicity study on rats. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Kimmerle G. and Gröning. SRA 12869 study to evaluate atropine and
1975 oxines for antidotal effect on rats. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Kurtz, S.K. The assimilation and metabolism of 14C Oftanol by
1977 rotational crops. Mobay Report No. 53826. (Unpublished)
Kurtz, S.K. and Shaw II, H.R. Poultry metabolism of (14C) Oftanol.
1977 Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Lamb, D.W., Matzkanin, C.S. and Burke, M.A. Acute oral toxicity of
1977 Oftanol technical. Report from Mobay N. 54000, Ref. 76-282,
submitted by Bayer AG. (Unpublished)
Lamb, D.W. and Burke, M.A. Dietary toxicity of Oftanol technical to
1977 mallard ducks. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 Acute oral toxicity of AmazeTM technical to bobwhite
quail. Report from Mobay Chemical Corp., submitted by Bayer
AG. (Unpublished)
Löser, E. SRA 12869 subchronic toxicity study on rats (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Löser, E. SRA 12869 subacute toxicity study on rats (four-week feeding
1978 experiment). Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Machemer, L. SRA 12869 studies for embryotoxic and teratogenic effects
1972 on rats following oral administration. Report submitted by
Bayer AG. (Unpublished)
1973 SRA 12869 dominant lethal test on the male mouse to test for
mutagenic effect. Report from Bayer AG, submitted by Bayer
AG. (Unpublished)
1975 SRA 12869 studies of embryotoxic and teratogenic effects on
rabbits following oral administration. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
McNamara, F.T. Dissipation of 14C-Oftanol in buffered aqueous
1977a solutions. Mobay Report No. 53617. (Unpublished)
1977b Dissipation of 14C-Oftanol in pond water containing soil.
Mobay Report No. 53618. (Unpublished)
Minor R.G. Identification of volatile isofenphos soil metabolites.
1980 Mobay Report No. 68658. (Unpublished)
Minor R.G. and Murphy, J.J. Metabolism of 14C-Oftanol in soil. Mobay
1977 Report No. 53643. (Unpublished)
Mobay Residue Reports nos. 53819, 53888, 65932, 67505, 67508,
1979-80 68461-68463 (Corn). (Unpublished)
1978a Residue Reports nos. 53820, 53857-53859, 65931, 65933,
65935-65937, 53817, 5382353825, 53853, 65637, 65923, 65924,
65926, 65927 (Sweet corn). (Unpublished)
1978b Residue Reports nos. 65479-65481, 65491-65493, 65657, 65468,
65477, 65484-65487, 65636, 65656, 67394-67395 (Onions).
(Unpublished)
1980a Residue Reports nos. 69025-69027 (Rapes). (Unpublished)
1980b Residue Reports nos. 53620, 53649, 53695, 53698, 53895,
67378 (Stability studies). (Unpublished)
1980c Residue Reports nos. 69101, 69106, 69111, 69128, 69129,
69130, 69133, 69147, 69148 (Degradation in soil).
(Unpublished)
1980d Residue Reports nos. 69101, 60103-69106, 69107, 69-108,
69110, 69111, 69127 69134-69136, 69144, 69145, 69204-69206,
69128-69130, 69133, 69147, 69148 (Residues in rotational
crops). (Unpublished)
Mürmann, P. SRA 12869 subchronic toxicity study on dogs (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Nelson, D.L. and Burke, M.A. Acute dermal toxicity of Oftanol
1977a technical. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG. (Unpublished)
1977b Acute oral toxicity of Oftanol technical to mallard ducks.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
1977c Dietary toxicity of Oftanol technical to bobwhite quail.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Roney, D.J. Accumulation and persistence of residues
1977 in channel catfish exposed to Oftanol-14C. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Anderson, R.H. The skin-sensitizing properties of
1977 Oftanol technical to guinea pigs. Report from
Pharmakologisches Institut der Universität Mainz, submitted
by Bayer AG. (Unpublished)
Netherlands. Information from the Netherlands on pesticides included
1981 in the JMPR priority list.
Obrist, J.J. and Thornton, J.S. Leaching characteristics of aged
1977 Oftanol soil residues. Mobay Report No. 53944. (Unpublished)
Oesch, F. Ames Test for Oftanol (isofenphos). Report from Chemagro
1977 Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Palmer, A.K., Killick, M.E. and Allen, T.R. Effect of SPA 12869 on
1977 reproductive function of multiple generations in the rat.
Report from Huntingdon Research Centre, UK, submitted by
Bayer AG. (Unpublished)
Poje, A.J. AMAZE photoproduct identification. Mobay Report No. 68146.
1979 (Unpublished)
Ross, D.B., Cameron, D.M. and Roberts, N.L. The acute oral toxicity
1976 (LD50) of isofenphos to the starling. Report from
Huntingdon Research Centre, England, submitted by Bayer AG.
(Unpublished)
Schlüter, G. SRA 12869 Untersuchungen auf embryotoxische und
1981 teratogene wirkungen nach cutaner verabreichung an der
ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Schlüter, D. and Thyssen, J. SRA 12869 untersuchungen auf
1981 embryotoxische und teratogene wirkungen nach inhalation an
der ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Shaw II, H.R. Alkaline emission gas-chromatography method for
1974 determining BAY 92 114 and HOL 0654 in soil and water. Mobay
Report no. 43 072. 1 Oct. (Unpublished)
1977a A gas-chromatographic method for Oftanol, Oftanol oxygen
analogue, des N-isopropyl Oftanol and des-N-isopropyl
Oftanol oxygen analogue in bovine and poultry tissues, milk
and eggs. Mobay Report no. 52 769. (Unpublished)
1977b An interference study for the residue method of Oftanol,
Oftanol oxygen analogue, des-N-isopropyl Oftanol and des
N-isopropyl Oftanol oxygen analogue in bovine and poultry
tissues, milk and eggs. Mobay Report no. 53 512.
(Unpublished)
1977c Gas-chromatographic method for residues of Oftanol and
Oftanol oxygen analogue in soils. Mobay Report no. 53 690.
(Unpublished)
1979 Supplementary methods for eliminating interferences from
Delnav, malathion and Ronnel in the residue methods for
AMAZETM in animal tissues, milk and eggs, Mobay Report
no. 67 652, 23 May 1979. (Unpublished)
Spare, W.C. 14C-AMAZETM activated sludge metabolism. Mobay Report no.
1979 68 201. (Unpublished)
Stanley, C.W., Minor, R.G. and Murphy, I.J. Metabolism of OFTANOLR in
1977 corn. Mobay Report No. 53 110. (Unpublished)
Stanley, C.W. Metabolism of OFTANOLR in onions. Mobay Report no.
1977a 54 043. (Unpublished)
1977b Gas-chromatographic method for residues of OFTANOL and its
metabolites in corn and onions. Mobay Report no. 53 111.
(Unpublished)
1977c An interference study for the residue method for OFTANOL and
its organophosphate metabolites in corn and onions. Mobay
Report no. 53 514, 5 July. (Unpublished)
Stanley, C.W. Gas-chromatographic method for residues of AMAZETM and
1979a its metabolites in corn. Mobay Report no. 67 530.
(Unpublished)
1979b Supplementary methods for eliminating interferences of
diazinon, Di-syston and phorate with AMAZETM residues in
the residues method for crops. Mobay Report no. 67 651.
(Unpublished)
Shaw, H.R. Strankowski, K.J. and Murphy, J.J. Excretion and metabolism
1977 of BAY SRA 12869 by rats. Report from Chemagro Agricultural
Division, Mobay Chemical Corp., submitted by Bayer AG.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, K. Isofenphos mutagenicity test
1980 on bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted by Bayer
AG. (Unpublished)
Solmecke, B. and Kimmerle, G. SRA 12869 subacute toxicity studies.
1972a Report from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b SRA 12869 special toxicological studies. Report from Bayer
AG, submitted by Bayer AG. (Unpublished)
1972c SRA 12869 acute toxicological studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J. Effects of feeding ROftanol/Oftanol/oxygen analogue,
1977 des-N-isopropyl Oftanol oxygen analogue (1:8:1) to chickens
for 28 days. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 AMAZETM octanol/water partition coefficient Mobay Report
no. 68 246. (Unpublished)
1980 AMAZETM water solubility. Mobay Report no. 69 175.
(Unpublished)
Strankowski, K.J. and Murphy, J.J. Metabolism and excretion of (14C)
1977a Oftanol by a lactating dairy cow. Report from Chemical
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
1977b Metabolites of (14C) ROftanol identified in dairy cow
tissues. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG.; (Unpublished)
1977c Photodegradation of 14C-Oftanol in aqueous solution. Mobay
Report no. 53 939 (Unpublished)
Strankowski, K.J., Shaw II, H.R. and Murphy, J.H. Metabolites of (14C)
1977a ROftanol identified in rat tissues. Report from Chemagro
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Strankowski, K.J., Minor, R.G. and Murphy, J.J. Excretion and
1977b metabolism of (14C) ROftanol in a pig. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J., McNamara, F.T. and Minor, R.G. Effects of feeding
1977c ROftanol/Oftanol oxygen analogue,des-N-Isopropyl Oftanol
Oxygen analogue (1:8:1) to dairy cattle for 28 days. Report
from Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Thomson, C. and Newman, A.J. Pathology report of Bayer SRA 12869 sub-
1973 chronic toxicity experiment in dogs (13 weeks administration
in the diet). Report from Hungtindon Research Centre,
England, submitted by Bayer AG. (Unpublished)
Thornton, J.S., Hurley, J.B. and Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticide chemicals. Mobay Report no. 51 016.
(Unpublished)
Thyssen, J. HOL 0654 special toxicological studies. Report from Bayer
1974a AG, submitted by Bayer AG. (Unpublished)
1974b HOL 0654 subacute toxicity studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1974c HOL 0654 akute toxizität bei hühnern. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isofephos or phoxim. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1977 Toxikologische untersuchungen bei gleichzeitiger applikation
von SRA 7502 und SRA 12869. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
1978 SRA 12869 Acute toxicity studies on hens and quail. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Thyssen, J. and Kaliner, G. SRA 12869 subacute dermal cumulative
1977 toxicity study on rabbits. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
Thyssen, J. and Kimmerle, G. HOL 0654 acute toxicity studies. Report
1974 from Bayer, AG, submitted by Bayer AG. (Unpublished)
Urwin, C. and Newman, A.J. Pathology report of SRA 12869 3-month rat
1973 study. Report from Huntingdon Research Centre, UK, submitted
by Bayer AG. (Unpublished)
Wagner, K. Method for the gas-chromatographic determination of
1976 OftanolR residues in plants soil and water. Pflanzenshutz-
Nachrichten Bayer, 29: 67 - 80.
Weissenburger, B. and Pollock, R.J. Photodegradation of 14C AMAZE on a
1979 soil surface Mobay Report no. 68 245. (Unpublished)
Over 70% of the radioactivity appeared to be non-conjugated and
readily released from the plant matrix of the 28- and 56-day corn
stalk samples by methanol chloroform extraction, while in the 94-day
corn stalks, only 19% of the radioactivity was organosoluble without
hydrolysis. The distribution of radioactivity among isofenphos and its
metabolites in corn stalk grown outdoors is shown in Table 10.
TABLE 10. Distribution of radioactivity among isofenphos and its metabolites inmaize grown
outdoors in ring-µL (14C)-isofenphos-treated soil
Percent Radioactivity Distribution
Organic/Aqueous Water/Solid Fractions
Days after Sample Extract Acid released
Treatment
I IOA DNIOA SA DBHA DAIOA Unk.
28 stalk 19 52 3 nd nd nd nd
56 stalk 8 63 6 <1 12 <1 2
94 stalk 2 15 2 5 35 3 5
cob 0 17 0 nd nd nd nd
The primary organosoluble metabolite was isofenphos oxygen
analogue, but isofenphos and des N-isopropyl isofenphos oxygen
analogue were also detected. Acid hydrolysis of the aqueous and soil
fractions from corn stalk liberated 2,5-dihydroxybenzoic acid (DHBA)
SA and DAIOA.
As the plant matured, DHBA became the predominant metabolite. The
only component identified in the husk and cob samples was IOA.
The small quantity of radioactivity in the organosoluble fraction
of the kernel prevented adequate identifications to be made, but none
of the intact phosphate esters could have been present in an amount
higher than 4% of the kernel's total residue, which accounted for 6.1%
of the activity measured in the whole plant. The concentration of
radioactivity and its distribution in the tissues of mature corn grown
in the greenhouse was similar to that seen in the outdoor study. The
compounds identified were exactly the same.
Bermuda onions were grown in a greenhouse for 60 and 91 days in
soil in which 4 mg/kg ring-µL (14C)-isofenphos had been incorporated.
The harvested plants were divided into tops (above the soil level) and
bulbs (Stanley 1977a). In mature plants, the concentration of residues
increased in the tops and decreased in the bulb, in comparison with
levels measured in 60-day plants, mainly as a result of the changes in
the weight of the plant portions.
Over 84% of the radioactivity in each sample was extracted by
blending and acid hydrolysis. Over 80% of the radioactivity in the
tops and over 50% in the bulbs was organosoluble, indicating the
amount of non-conjugated residues.
The compounds detected in the organosoluble fractions after
blending were isofenphos, IOA and DNIOA. IOA was the major compound in
the tops and the 91-day bulbs, 78-79% and 35% of total activity
respectively, while isofenphos and IOA ratio was 44% and 24% in the
60-day bulbs. DNIOA was present in all samples in small amounts but
was less (<1%) in bulbs and in tops (2-3%) at both intervals. Acid
hydrolysis of the aqueous fraction from the organic extract liberated
salicylic acid (1 to 2%) and an unknown (<1 to 3%). The remaining
radioactivity (6 to 12%) on the TLC plates was at the origin,
indicating very polar compounds, or was present as streaking and not
as discrete spots.
The radioactivity remaining in the solids after organic
extraction constituted about 5 to 8% in tops and 14 to 16% in bulbs of
the total activity of 60 and 91-day samples, respectively. Acid
hydrolysis of the solids released over one half of the radioactivity
remaining in the solids after methanol chloroform extractions. The
radioactivity of solids was not further characterized.
In animals
Pig
The feeding study of a male pig is described previously under
"Biochemical aspects". Four tissues, kidney, liver, muscle and fat
were analysed for isofenphos and its metabolites. The amount of
organosoluble metabolites are given in Table 11.
Enzymatic hydrolysis of neutralized aqueous fractions from kidney
and liver released most of the radioactivity remaining in these
tissues. The major enzyme-released metabolite was IPS. Small amounts
of HHA and DAIOA were also found. Between 30-42% of the kidney and
liver radioactivity extracted after enzyme hydrolysis could not be
identified. Unknown (a) consistuted between 19 and 27% of the total
tissue radioactivity.
TABLE 11. Organosoluble metabolites of isofenphos in pig tissues
Compounds % Radioactivity in tissues on 5th day of feeding
Liver Kidney Muscle Fat
Isofenphos 13 2 52 61
IOA <1 <1 16 10
IPS 1 2 17 15
HHA <1 ND 3 ND
DNI ND ND 5 5
DAIOA 4 1 3 <1
Unknown (a) 9 ND ND ND
Total 30 6 97 92
Cow
Under "Biochemical Aspects", feeding studies are reported in two
lactating dairy cows. In the first animal the only milk sample with
a radioactive residue, >0.01 mg/kg isofenphos equivalents, was
analysed for isofenphos and its metabolites. The 7.5h sample contained
0.014 mg/kg isofenphos equivalents and accounted for 0.07% of the
administered radioactivity. DAIOA and HHA were the main metabolites in
the organosoluble fraction and accounted for 13% and 10% of total
activity in milk, respectively. Small amounts (2 to 4%) of IPS, SA and
several unknowns (12%) were also detected. Enzymatic hydrolysis of the
solvent-extracted, pH 5 milk released an additional 45% of the milk
radioactivity. HHA (27%) was the major constituent in this fraction of
the milk. DAIOA, SA, IPS and several unknowns were also found in small
amounts.
In the second experiment milk was collected prior to each day's
dosing and 8 hours after each dosing. Milk production remained at the
cow's normal levels through the 5 days of the study. The cow was
sacrificed 2 hours after the fifth dose was administered. The
following tissues were collected and analysed, their residue content,
in isofenphos equivalent (mg/kg) were: muscle-loin 0.02, shoulder
0.02, round 0.02; fat-renal 0.07; omental 0.07; subcutaneous 0.06;
kidney 0.53; heart 0.04; liver 0.47; udder 0.05 and brain 0.02.
In kidney and liver tissues, less than 35% of the radioactivity
was organosoluble and consisted of SA 26 to 14%, HHA 3 to 4%, IOA 2 to
1%,, IPS 1% to <1%, respectively. Traces (1%) of unknowns were found
in the liver.
Sulphatase beta-glucuronidase combination was employed in the
enzyme hydrolyses, which released 42 to 45% activity from the kidney
and liver extracts respectively. IPS (7 to 3%), SA (8 to 2%) and HHA
(6 to 8%) were identified in the enzymatic extracts of kidney and
liver.
Several unknowns (20 to 33%) were detected, but a single
compound, unknown (a), accounted for the 90% of activity. The
radioactive residues which were not organosoluble or liberated by
enzyme hydrolysis were not further characterized.
A feeding study was conducted to study the biological effects and
to determine the tissue and milk residues after feeding isofenphos and
its metabolites. The ratio 1:8:1 of isofenphos, IOA and DNIOA in the
mixture was based on plant metabolism studies of isofenphos and should
be representative of field-treated samples. Alfalfa pellets containing
the mixture of compounds at 2, 6 and 20 mg/kg levels were given to
dairy cattle ad libitum for 28 days (Strankowski 1977c). Feed
consumption, body weight and milk production were unaffected by
consumption of the treated feed as compared to the control cow. The
milk of cows fed 20 mg/kg contained detectable residues only in the
range of 0.001 to 0.01 mg/kg of isofenphos equivalents on the 28th day
of feeding. After feeding for 28 days the animals were sacrificed and
the following tissues were taken; liver, kidney, muscle (round, flank
and loin) and fat (omental, renal and subcutaneous). Residues in all
the tissues analysed, except liver and kidney at the 20 mg/kg level,
were below 0.0, mg/kg. The liver and kidney samples contained
residues, in isofenphos equivalents, ranging from 0.01 to 0.02 and
from 0.01 to 0.04 mg/kg, respectively.
Hen
Laying hens were given a single oral dose of ring-µL(14C)-
isofenphos at 2.0 mg/kg (Dupre 1975). Eggs and excreta were collected.
At four sacrifice intervals (6, 24, 48 and 96 h post-treatment) blood,
various organs and tissues were collected from four birds per group.
In eggs, the level of radioactivity reached a maximum level of
0.099 mg/kg with an average of 0.047 mg/kg in isofenphos equivalent at
48 h post-treatment. Approximately 72% and 78% of the radioactivity
was eliminated in the excreta within 24 and 96 h respectively. A
maximum level in the blood plasma of 0.274 mg/kg 14C activity at 6 h
post-treatment fell to 0.009 mg/kg 96 h after treatment; 24 h after
treatment, residue levels in skin, fat, breast, thighs, heart and
gizzard muscle were less than 0.05 mg/kg, while liver and kidney
contained 0.9 mg/kg and 0.68 mg/kg respectively.
In the feeding study in hens described under 'Biochemical
Aspects' residues, expressed as mg/kg isofenphos equivalents, found in
various tissues were: gizzard 17.1, kidney 4.9, liver 3.9, fat 0.83,
skin 0.67, heart 0.42, muscle 0.14, eggs - 51 h 0.25, 48 h 0.06, 24 h
0.032. The distribution of the residues in the organosoluble fractions
of various tissues is given in Table 12.
TABLE 12. Distribution of isofenphos and its metabolites in tissues and eggs
of laying hens
Distribution (%)
Compound Gizzard Kidney Liver Fat Skin Heart Muscle Eggs
Isofenphos 90 14 2 74 53 26 31 37
IDA 0 4 1 4 3 3 4 9
IPS 0 1 1 18 19 10 8 10
DNI 0 0 0 3 3 0 0 0
HHA 0 0 0 0 0 0 5 0
Unknown 0 47 19 0 8 36 28 5
Feed treated with a mixture containing isofenphos, IOA and DNIOA
in the ratio of 1:8:1, at 5, 15, 20 and 150 mg/kg feed was distributed
to laying hens ad libitum for 28 days (Strankowski 1977d). Feed
consumption, body weights and production decreased as the amount of
isofenphos and its metabolites in the feed increased. At sacrifice,
giblets (heart, gizzard, liver), muscle (equal portions of thigh and
breast), fat (equal portions of visceral and subcutaneous) and skin
(without feathers) were collected. No residue was detected in the
muscle at any dose level. Residues in fat, giblets, skin and eggs are
summarized in Table 13.
Fish
Studies on continuous exposure for 28 days of channel catfish to
isofenphos are reported under "Biochemical Aspects".
In soil
Isofenphos degradation under aerobic conditions was investigated
by Minor and Murphy (1977) in sandy loam and silt loam, while the
effects of anaerobic and sterile conditions were studied in sandy
loam. Ring µ-L-(14C) labelled isofenphos was uniformly incorporated
into the soil at a level of 8.7 mg/kg. The extractable residue derived
TABLE 13. Residues in isofenphos equivalents found in tissues and
eggs of laying hens1
Residue (mg/kg)
Dose
Fat Giblet Skin Eggs
50 mg/kg <0.01 (4) <0.01 (2) <0.01 (2) 0.002
0.01 (2) 0.02 0.003
0.08 0.003
0.004
150 mg/kg <0.01 (2) 0.01 <0.01 (2) 0.017
0.01 (2) 0.02 0.03 (2) 0.013
0.04 0.015
0.06 0.003
1 No. of samples in brackets.
from isofenphos decreased with time, while the radioactivity bound to
soil, or lost, increased. Only 10% of the originally applied
radioactivity could be extracted from silt loam at 240 days post-
treatment, whereas 23% of the-radioactivity could still be removed
from the sandy loam one year after traces (<1% of original activity)
of IPS. The concentration of isofenphos decreased continuously with
time, and its half life was found to be 127 days in sandy loam and 59
days in silt loam.
The concentration of isofenphos oxygen analogue reached a maximum
between 60 and 120 days post-treatment. The bound radioactivity
increased to a maximum of approximately 20% and the remainder had
volatilized. The bound radioactivity was distributed among humin,
humic acid and fulvic acid fractions in a fashion similar for both
soils. Even after stringent HCl-NaOH fractionation, approximately 50%
of the bound residues was still unextractable.
The volatile radioactivity from soil was composed of isopropyl
salicylate, 14CO2-isofenphos and cyclic isofenphos. At 134 days
post-treatment, the total volatile radioactivity trapped was
distributed among IPS (67%), 14CO2 (30%), cyclic isofenphos (2%)
and isofenphos (1%) (Minor 1980: The volatile compounds in the
experiments accounted for 16 to 51% of the total activity applied to
soil 134 days before, while the soils contained the remaining part of
activity, indicating the importance of conditions (e.g. temperature
and/or microorganism activity) on degradation rate of isofenphos in
soil. In sandy loam and under anaerobic conditions, isofenphos was not
converted to its oxygen analogue, but some of the radioactivity was
lost. Under sterile conditions in sandy loam, isofenphos was neither
altered nor lost. In isolated soil micro-organisms grown in "shake
culture", isofenphos was not altered. Conversion of isofenphos to its
oxygen analogue appeared to be strictly a chemical reaction occurring
in soil under aerobic conditions. The production of isopropyl
salicylate may also have occurred without microbial activity, but the
isolated 14CO2 was probably the end result of soil micro-organism
cleavage of the isofenphos aromatic ring after the phosphate group had
been removed. Therefore, isofenphos soil degradation appeared to
involve both chemical and microbial alterations, and the chemical
reactions of oxidation and hydrolysis appeared to be prerequisite to
microbial action. Comparison of results from isofenphos treated soil
kept in a greenhouse or maintained outdoors showed that isofenphos
degradation qualitatively is the same under both environments, but the
rate of degradation may vary.
The persistence of isofenphos in soil was investigated in nine
tests set up in various locations in the United States and Canada
(Mobay 1980c). Isofenphos, 6E formulation, was incorporated uniformly
at a rate of 2 mg/kg wet soil (equivalent to 2.24 kg/ha field
application) into the upper 7.6 cm of soils of seven different types.
Samples were taken at 0, 30 to 33, 60 to 61, 89 to 92 and 119 to 124
days post-treatment from 0 to 15 cm and 15 to 30 cm depths. The
residue of isofenphos decreased in all nine studies, and its level in
the upper layer was between 0.11 mg/kg and 0.43 mg/kg 119 to 124 days
after treatment. IOA reached its maximum (0.19 to 0.5 mg/kg) 32 to 123
days post-treatment. Depending on the soil types and possibly on
environmental conditions, the ratio of isofenphos and its oxygen
analogue varied between 0.37 to 14 at the last sampling. Soil samples
from 15 to 30 cm did not contain detectable residues (0.01 mg/kg),
with the exception of rocky sandy silt loam in which 0.02 to 0.15
mg/kg isofenphos or IOA could be detected.
The degradation of isofenphos was studied in the Federal Republic
of Germany (FRG) and in the UK (Bayer 1973-75). The applications were
made with either EC or granular formulation as surface spray or
broadcast at rates of 1, 5 and 7.5 kg a.i./ha. The sampling day varied
from 0 to 534 post-treatment. The results show a pattern similar to
that obtained in the experiments described previously. The results of
laboratory experiments on the persistence of isofenphos carried out in
standard German soil No. 1 and No. 2 (Bayer 1974a) were consistent
with the results of field trials, as were the mobility tests with soil
thin layer chromatography (Thornton 1976) or soil column (Obrist and
Thornton 1977; Bayer 1973-74, 1977b).
The latter experiments indicated that isofenphos and IOA had low
mobility and the majority of the residue remained in the upper 12.5 cm
of the soil, while the leachates contained no intact isofenphos but
did contain a small amount of IOA and traces of IPS (one experiment).
In water
The stability of ring-µL-(14C)-isofenphos in sterile buffer
solutions of various pH's at various temperatures and two
concentrations (1 mg/l and 10 mg/l) was determined (Mc Namara 1977).
Both hydrolysis and volatilization were involved in the dissipation of
isofenphos from the buffered solutions. Isofenphos was not hydrolysed
at temperatures of 20°C and below or at pH's near neutrality, but
volatilization did occur at 20°C and above and increased as the
temperature and isofenphos concentration increased. Hydrolysis of
isofenphos was, however, encountered in both strongly acidic and
alkaline buffers. Hydrolysis products detected in pH3 buffers included
DNI, IOA, DNIOA, IPS, SA. At pH9, the identified hydrolysis products
of isofenphos were IOA, IPS, SA and N-isopropyl salicyclamide. At
37°C, the half life of isofenphos at 1 and 10 mg/kg was 79 and 30 days
respectively, in pH3 buffer, and 88 and 32 days respectively, in pH9
buffer. At 50°C, the half life of isofenphos at 1 and 10 mg/kg was 15
and 9 days respectively, in pH 3 buffers; 34 and 15 days respectively,
in pH 6 buffer; and 6 days for each concentration in pH 9 buffer.
Ring-µL(14C)-isofenphos at an initial concentration of 10 mg/kg
was incubated outdoors in a simulated pond (pH 8) (Mc Namara 1977a).
Within 70 days, 89% of the radioactivity was lost, and the remaining
radioactivity was distributed between water and sediment in an
appropriate 2:1 ratio. The loss of radioactivity was probably due to
volatilization of isofenphos and some of its metabolites. The major
radioactive compounds identified in the pond water were isofenphos,
IOA and cyclic-IOA. Minor components were cyclic isofenphos, IPS and
SA. The amount of radioactivity adsorbed by the pond sediment rose to
20% of the initially applied radioactivity within 7 days, but then
decreased steadily to only 2% at 70 days. The bound radioactivity
never exceeded 2% of the originally applied radioactivity. The major
components in the organosoluble extracts of pond sediment were
isofenphos and IOA. Minor components identified in the sediment were
IPS, SA, cyclic-isofenphos and cyclic-IOA. The overall rate of
isofenphos dissipation was exponential. The half-life of isofenphos
was 13 days. After 70 days, only 2% of the initially applied
isofenphos itself remained in the pond.
In activated sludge
Ring µL-(14C)-isofenphos was studied in a laboratory model of an
activated sewage sludge system (Spare 1979). Activated sludge,
synthetic sewage and (14C)-isofenphos were aerated for 23 h cycle.
During the remaining hour/cycle, the mixture was allowed to settle and
a portion of the supernatant was replaced with fresh synthetic sewage
and an increased amount of (14C)-isofenphos. The concentration of
isofenphos during the first cycle was 0.1 mg/kg and was increased
through 10 cycles to 100 mg/kg.
After the 100 mg/kg cycle concluded, three cycles were carried
out with no addition of test compound. Isofenphos had no detrimental
effect on the bacteria, yeasts or actinmycetes in the system, but at
isofenphos concentrations of 60, 80 and 100 mg/l, protozoa were absent
or few in the treated flasks. The quantity of radioactivity in the
settled solids increased steadily during the course of the study from
10% to 50% of the added radioactivity. (14C)-isofenphos was not
degraded appreciably by the organisms in the activated sludge system.
Greater than 96% of the added (14C)-isofenphos was recovered intact
from the supernatant and solid fractions.
Photodegradation
In aqueous solution
A study of the photodecomposition of ring-uL-(14C)-isofenphos in
aqueous solution was carried out in a photoreactor containing a high
pressure, quartz, mercury-vapour, 200-watt, Hanovia immersion lamp as
the light source (Strankowski 1977e). Decomposition of isofenphos was
slow in the pH 7 solution with only 30% decomposition in 30 days of
continuous exposure to the high intensity light. The isofenphos half-
life was calculated to be 51 days. In an acetone sensitized solution,
the photodecomposition was increased, and the half-life of isofenphos
was reduced to approximately 14 h.
Isofenphos was the only component found in the dark control
solutions from either the unsensitized or sensitized studies. It was
also identified in the photolysis solutions; approximately 75% and 3%
of the total radioactivity remained as isofenphos at the end of the
unsensitized and sensitized studies, respectively.
The major photoproduct, which represented nearly 21% of the total
radioactivity in the sensitized test, was identified by Poje (1979) as
3,3-dimethyl isoindoline-l-one by mass spectroscopy and nuclear
magnetic resonance spectroscopy. This product contains no phosphorous.
The remaining photoproducts, none of which accounted for more than 5%
of the total radioactivity, included IOA, DAI, SA and catechol.
On soil
In a study of the photodegradation of (14C)-isofenphos on soil,
isofenphos had a half-life of 2.6 days when applied to a thin layer of
soil and subjected to light from a 200 watt Hanovia mercury lamp
(Weissenburger and Pollock 1979). Six photoproducts were detected
on the soil. IOA (33% of the applied radioactivity) was the principal
photo-product. Small quantities (approx. 5%) of DNI, DNIOA, des-N-
isopropyl cyclic isofenphos, IPS and phenol were also detected. During
26 days of exposure to the light, 25% of the applied radioactivity was
bound to the soil and 33% was lost through volatility. A trapping
study, carried out for 3 days, showed that the volatile compounds
included isofenphos (91%), DNI (3%), IPS (1%) and phenol (5%). All of
the applied activity was accounted for in the trapping study.
In storage
Stability of isofenphos and its metabolites were studied during
frozen storage (-28°C to -18°C) (Mobay 1980a). Concentration of
isofenphos and IOA did not change significantly in maize kernel,
brassica leafy vegetables, green forage, green beans (pods and vines)
and onions kept deep frozen for 400 to 800 days. However, 24% of
isofenphos decomposed in sugarbeet during 420 days of storage. DNI and
DNIOA residues decreased by 20% add 32% respectively in alfalfa stored
at -23 to -18°C.
Poultry tissues, eggs and giblets, fortified at 1 mg/kg with
isofenphos and its metabolite, were stored frozen for 255, 90 and 60
days respectively without significant changes in concentrations of
residues.
Cattle tissues and milk were fortified at 1.0 mg/kg with
isofenphos and metabolites and placed in frozen storage. Fat and
muscle tissues showed no significant decomposition of the compounds
after 24 to 28 days. Milk showed no significant decomposition of the
compounds after 76 days in frozen storage. In kidney and liver,
isofephos, isofenphos oxygen analogue and des-N-isopropyl isofenphos
were stable for 28 to 35 days. The liver and kidney samples were
extracted within 4 and 8 days, respectively. There was no
decomposition of des-N-isopropyl isofenphos oxygen analogue in kidney
after 10 days of frozen storage; however, it did not disappear
completely in liver after 5 days of frozen storage.
METHODS OF RESIDUE ANALYSIS
A gas chromatographic procedure for the determination of residues
of isofenphos and its oxygen analogue in various crops, soil and water
has been described by Wagner (1976). Plant samples of high water
content and soils are extracted with acetone. The acetone extract is
diluted with water and the residues are partitioned into chloroform or
dichloromethane. Rape, maize and water are extracted with acetonitrile
and chloroform respectively. The concentrated extracts are cleaned on
neutral alumina or on active carbon columns. The residues in the
concentrated extracts are separated on DC-200, QF-1 or OV-17 columns
and detected with a thermionic detector. Recovery data from
experiments run on a large variety of crops by adding known amounts of
isofenphos and its oxygen analogue at the blending step were generally
in the 77% to 105% range. The limit of determination is 0.005 mg/kg
for each compound in plant and soil samples and 0.002 mg/kg in water.
Gas chromatographic methods for the determination of residues of
isofenphos, isofenphos oxygen analogue, des-N-isopropyl isofenphos and
des-N-isopropyl isofenphos oxygen analogue in maize cob, forage, husk
and kernel and in onions are described by Stanley (1977b, 1979a) and
in bovine and poultry tissues, milk and eggs by Shaw II (1977a). For
all four compounds, recoveries were 65 to 70%. The limit of
determination for each compound is 0.01 mg/kg, except for milk and
eggs (0.001 mg/kg). Multiplication factors for converting metabolite
residues to isofenphos equivalents are 1.05 for IOA, 1.139 for DNI and
1.204 for DNIOA.
Interference studies for the determination of residues of
isofenphos and its metabolites as maize and onions were done by
Stanley (1977c, 1979b) and in milk, animal tissues and eggs by Shaw II
(1977b, 1979). All possible interferences by 20 or 27 organophosphate
pesticides registered for use on maize and onions or investigated in
animal products respectively were separated from isofenphos and its
metabolites by GLC on the standard (10% DC-200 + 2% OV-225 on
Chromosorb WHP) or on the confirmatory (5% OV 210 on Supelcoport or 5%
OV 275 on Chromosorb WHP) columns or by clean-up on the silica gel
column used in the residue method. The silica gel column was applied
as follows: 372 g silica gel is deactivated with 28 cm3 water, 10 g
is filled into the column under hexane and settled, and a 1-cm layer
of granular Na2SO4 is placed on top of the column.
Hexane is drained to the top of the sodium sulphate. The
concentrated extract is transferred in the column with 5 to 10 cm3
portions of hexane and finally the column is washed with hexane (total
amount-50 cm3). The portions of hexane is drained through the column
at a rate of 5 to 10 cm3 until 50 cm3 is collected. Eluents (freshly
prepared) and fractions are: (1) 185 cm3 hexane-dichloromethane (1:1)
(isofenphos); (2) 150 cm3 dichloromethane (DNI); (3) 65 cm3
dichloromethane-methanol (99:1) (discarded); (4) 135 cm3
dichloromethane-methanol (99:1) (IOA).
Shaw II (1977b, 1979) applied different conditions: 12 g silica
gel and 6 g Na2SO4; column is washed with 40 cm3 hexane, 100 cm3
hexane-benzene (8:2) 75 cm3 hexane-benzene (6:4); elution: (1)
fraction (isofenphos) 25 cm3 hexane-benzene (6:4) + 100 cm3 hexane-
benzene (4:6) + 100 cm3 hexane-benzene (2:8) + 50 cm benzene; (2)
fraction (IOA + DNI) 50 cm3 benzene + 300 cm3 benzene-acetone (8:2).
A gas chromatographic method for the determination of isofenphos
and isofenphos oxygen analogue in soil and water, using an alkaline
thermionic emission detector, is described by Shaw II (1974).
Recoveries of isofenphos and its oxygen analogue were 93 to 101%; the
sensitivity of the method is approximately 0.005 mg/kg for soil and
0.002 mg/kg for water samples.
The determination of isofenphos and its oxygen analogue residues
in aged soil by alkaline thermionic emission gas chromatography was
investigated by Shaw II (1977c). The soil was extracted in a Soxhlet
apparatus for 16 to 24h with chloroform/methanol (7:3). The extract
was concentrated, partitioned with hexane/acetonitrile (1:1) and
partially purified with three hexane extractions of the residue in a
methanol/water solution. Isofenphos recoveries were >79% and
isofenphos oxygen analogue recoveries were >95% from soils fortified
at 0.05 mg/kg. The sensitivity of this method is 0.01 mg/kg.
Isofenphos and IOA were determined by Brown and Williams (1976)
in cabbage, potato, rapeseed and soil. The rapeseed was extracted with
acetonitrile and cabbage, potato and soil with ethylacetate. The
concentrated extracts were cleaned on a column consisting of the
mixtures of Florisil, silica gel, alumina and Nuchar C. The cleaned-up
extracts were separated on DEGS or OV275 liquid phases and detected
with FPD. The recoveries at 0.01 to 1 mg/kg levels were in the range
of 84 to 98%. The limits of determination were 0.003 mg/kg and 0.005
mg/kg for isofenphos and IOA respectively in potato, rapeseed and
soil, while they were five times higher in cabbage.
The methods described by Wagner (1976) or by Brown and Williams
(1976), in combination with the confirmatory columns and GLC
separation (Stanley 1977b, 1979a), are recommended for regulatory
purposes.
NATIONAL MAXIMUM RESIDUE LIMITS REPORTED TO THE MEETING
Isofenphos is registered in the following countries: Austria,
Bulgaria, Chile, Denmark, Federal Republic of Germany, German
Democratic Republic, Indonesia, Israel, Italy, Morocco, Mexico,
Norway, The Netherlands, South Africa, Spain, Sweden and the USA.
Maximum residue limits and preharvest intervals were reported
from some of the countries and are summarized in Table 14.
EVALUATIONS
COMMENTS AND APPRAISAL
In the mammalian species tested, the oral LD50 ranged from about
20 mg/kg bw in rats to approximately 150 mg/kg bw in rabbits. In the
rat, the oxygen analogue of isofenphos was shown to be slightly, but
not substantially, more toxic orally than isofenphos, both acutely and
subacutely.
TABLE 14. National MRLs reported to the Meeting
Preharvest
Country Crop/Commodity intervals MRL
(days) (mg/kg)
Fed. Rep. of Leafy and stem
Germany vegetables 0.1
Rapeseed 0.05
Italy Pear 42 0.1
Fruit 0.1
Vegetables 0.1
Sugarbeet 0.1
Netherlands Cauliflower 56 0.1
Cabbages (including
Brussels sprouts) 56 0.1
Onions 0.1
Celery 0.05
Celeriac 0.05
Norway Root, tuber and
bulb vegetables 90
Brassica leafy vegetables Apply not later
than transplanting
South Africa Citrus fruit 180 0.2
Spain Cabbage Apply before sowing
Onions 21
Garlic 21
All crops (except
root vegetables) Apply before sowing
and potato)
USA Maize 75
Corn, forage 1.0
Corn, fodder 1.0
Corn, fresh (including
sweet) 0.1
Corn, grain 0.1
Meat, fat, meat by-
products of cattle,
sheep and poultry
Milk 0.02
Eggs 0.02
Metabolism studies in rats, pigs and cows, treated orally with
ring-µL(14C)-isofenphos indicated that the compound is rapidly
eliminated in both faeces and urine, with the latter being the
predominant route of excretion. It is metabolized mainly by oxidative
desulphuration, dearylation, hydrolysis, deamination and conjugation.
There is no indication that isofenphos and/or its metabolites
accumulate in mammalian tissue. The excretion pattern and proposed
metabolic pathways for isofenphos appear to be similar in the three
mammalian species studied.
A 3-generation reproduction study in rats (with 2 litters in the
first generation (F0) but only one litter each in the second (F1)
and third (F2) generations showed a marginal effect in the F0
generation at the second mating on the pregnancy rate, even at 1 ppm,
the lowest tested level. No indication of teratogenic effect was noted
in a rabbit teratology study at dosages as high as 5 mg/kg bw.
Teratology studies in rats exposed orally, dermally or via inhalation
were all negative.
In vitro microbial assays, including reversion-test and rec-assay
and a dominant lethal study in mice, failed to give any evidency of
mutagenicity. Long-term studies in mice and rats revealed no
carcinogenic activity. An acute delayed neurotoxicity study in hens,
which could be considered as a screen, was negative. The acute oral
toxicity of isofenphos was found to be potentiated by malathion but
not by a number of other organophosphorus insecticides tested.
Plasma cholinesterase inhibition was observed as the most
sensitive indicator of toxicity in the short-term rat studies and the
108-week mouse and 2-year rat feeding studies. A dietary level of
1 ppm was a marginal no-effect level in the rat and a no-effect level
in the mouse with respect to plasma cholinesterase. In the dog, a
90-day study suggested that dietary levels of 10 ppm and above caused
significant plasma cholinesterase depression and an increase in liver
weight, although the latter was not accompanied by histopathological
alteration of the tissue. A 2-year feeding study in the same species
demonstrated 2 ppm to be the no-effect level, based on plasma
cholinesterase. The information provided on observations in humans was
very scanty.
Acceptable data was available to permit the establishment of no-
effect levels in three mammalian species. Owing to the unavailability
of an appropriate neurotoxicity study in hens, only a temporary ADI,
was allocated.
Isofenphos, O-ethyl O-2-isopropoxycarbonylphenyl
isopropylphosphoramidothioate, is an organophosphorus insecticide
applied mainly in soil at a rate of 1.5 to 2.5 kg a.i./ha or 1 to 5 kg
a.i./ha for row or overall surface treatment, respectively.
Supervised trials were carried out on various crops at different
locations in the United States, Canada and Europe. Isofenphos and its
main metabolites, isofenphos oxygen analogue (IOA), des-N-isopropyl
isofenphos (DNI) and des-N-isopropyl isofenphos oxygen analogue
(DNIOA), were analysed. It was found that the residue consisted mainly
of isofenphos and IOA. The residues measured in brassica leafy
vegetables, rapeseed and root and tuber vegetables were below
0.1 mg/kg and usually at or about the limit of determination after the
recommended pre-harvest intervals. No residues were detected in maize
kernels, either in the milk or dry stages, while residues up to
2 mg/kg were found in green forage 3 to 4 weeks after application. The
total residue detected in mature onion bulbs was always below 1 mg/kg.
Rotational crops can also take up residues from previous soil
treatments. The residues, consisting mainly of isofenphos and IOA,
were at or about the limit of determination in cereal grains, leafy
and root vegetables and edible oil crops grown in soil treated with
isofenphos 9 or more months earlier, The residue level might exceed
1 mg/kg in green wheat forage but it is under 0.1 mg/kg in the straw
at the time of harvest.
Isofenphos is degraded in soil, plants and animals, but its route
of degradation in soil and plants is somewhat different from that in
animals. Oxidation seems to be the first reaction in soil and plants.
In animals, hydrolysis and oxidation both appear to be of major
importance. The degradation of isofenphos was not rapid in any of the
systems studied. Soil micro-organisms seem capable of splitting the
aromatic ring to produce CO2 but only after the phosphoramidothioate
rouping has been removed from the molecule. In plants, oxidation is
followed by depropylation, hydrolysis and conjugation. IOA and
isofenphos are the primary residues but DNIOA also appears in smaller
amounts. The approximate ratio of isofenphos, IOA and DNIOA in mature
maize stalks was 1:8:1. 2,5-Dihydroxybenzoic acid (DHBA), salicylic
acid (SA) and deaminated isofenphos oxygen analogue (DAIOA) appear in
traces.
In animals, isofenphos undergoes hydrolysis, oxidation,
N-depropylation, deamination and conjugation. Excretion is rapid
in orally dosed animals. Pigs, cows and hens excreted 81%, 79% and
72% of the applied dose in the urine or excreta within the first
24h after treatment. Urine contained isopropyl salicylate (IPA),
O-hydroxyhippuric acid (HHA) SA and DAIOA in non-conjugated and/or
conjugated forms; 4 to 5% of the administered dose is eliminated in
the faeces over a 72 to 80h period.
During a period of multiple dosing for 6 consecutive days at a
rate of 15 mg/kg/day, rats were able to eliminate residues of
isofenphos fast enough to maintain a relatively constant level of
radioactivity in the tissues. One day after cessation of the
isofenphos treatment a 10-fold decrease in radioactive residue
concentration was detected in the muscle, fat and liver. Dairy cows
and laying hens were kept on feed containing residues of isofenphos,
IOA and DNIOA in the ratio of 1:8:1 at various levels between 2 and
20 mg/kg feed and 5 and 150 mg/kg respectively for 28 days. Residues
in all the bovine tissues, except liver and kidney at the 20 mg/kg
level, were <0.01 mg/kg. The liver and kidney samples collected from
the 20 mg/kg group contained residues of isofenphos ranging from
<0.01 to 0.02 mg/kg and 0.01 to 0.04 mg/kg respectively. Residues of
isofenphos equivalents in milk from all samples, except the 20 mg/kg
group, were below 0.001 mg/kg. The milk samples collected on the 28th
day from the high level group contained residues ranging from 0.001 to
0.01 mg/kg.
Isofenphos residues in poultry tissues and eggs from the control
and 5 mg/kg groups were below the limits of determination of the
analytical method (0.01 mg/kg for tissues, 0.001 mg/kg for eggs). The
hens fed at the 15 mg/kg level had no detectable residues in the
tissues and had residues ranging from <0.001 to 0.002 mg/kg in the
eggs. In the 50 mg/kg group, muscle and fat samples had no measurable
residues, while giblets and skin samples had residues ranging from
0.002 to 0.004 mg/kg. Residues were detected in all tissues, except
muscle, and ranged from <0.01 to 0.05 mg/kg and in eggs from 0.003 to
0.017 mg/kg at the exaggerated 150 mg/kg dietary level. Control values
were <0.01 mg/kg in tissues and <0.001 mg/kg in eggs. No detectable
residues can be expected in products derived from animals fed with
forage that has been treated with isofenphos at recommended
application rates.
Catfish exposed continuously to isofenphos for 28 days in water
containing 10 mg/l isofenphos accumulated residues of 0.75 mg/kg,
consisting entirely of intact isofenphos, within the first 4 days of
exposure. However 87% of this was eliminated within the first day
after transfer of the catfish to uncontaminated water.
Isofenphos is readily absorbed by soil. It is only slowly leached
in all the soils tested, but its degradation products move somewhat
more rapidly. In soil persistence studies with isofenphos incorporated
into the soil at the single-application field-use rate, the residue of
isofenphos itself declined steadily in all soils tested, and the total
organophosphorus residue decreased to half the initial concentration
within 45 to 200 days.
Hydrolysis of isofenphos occurs at acidic and alkaline pH, but it
is stable near neutral pH.
In an activated sludge system, isofenphos has no detrimental
effect on the sludge organisms, and the parent compound was not
degraded.
Photodegradation of isofenphos in aqueous solution is slow and
yields principally a re-arrangement product, 3,3-dimethylisoindoline-
l-one. On soil, photodegradation is rapid and the main product is IOA.
Residue analytical methods for the determination of isofenphos
and IOA, the major residue component, and for DNI and DNIOA are
available. Recoveries are in the range of 77 to 105%.
The limits of determination for isofenphos and IOA in plant
samples, animal tissues, and milk and eggs are 0.005 - 0.01, 0.01 and
0.001 mg/kg respectively, in water 0.002 and in soil 0.01 mg/kg.
Level causing no toxicological effect
Mouse : 1 ppm in the diet equivalent to 0.15 mg/kg bw/day
Rat : 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog : 2 ppm in the diet equivalent to 0.05 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The Meeting estimated the maximum residue levels likely to occur
in various commodities and concluded that they were suitable for
establishing temporary MRLs.
The limits refer to the sum of isofenphos and its oxygen
analogue.
Preharvest intervals (days)
Commodity Limit (mg/kg) on which recommendations
are based
Celeriac 0.02 * 150
Swedes 0.02 * 90
Turnips 0.02 * 90
Brassica leafy vegetables 0.1 60
Celery 0.02 * 90
Maize 0.02 * 90
Maize, fodder 0.5 90
(dry)
Sweet corn 0.02 * 90
Sweet corn fodder (dry) 0.5
Rapeseed 0.02 * 90
Carcass meat 0.02 *
Animal fats 0.02 *
Meat by-products 0.02 *
Cattle milk 0.01 *
Meat of chicken 0.02 *
Chicken by-products 0.02 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
An appropriate neurotoxicity study in hens.
Desirable
1. 2-generation (2 litters/generation) reproduction study.
2. In vitro biochemical studies on purified isofenphos with respect
to anticholesterase activity.
3. Further observations in humans.
4. Additional data from supervised trials on potatoes and onions,
including information on soil residues and soil moisture content.
REFERENCES
Bagnall, B.H. Tests to monitor the blood cholinesterase levels of
1976 workers using oftanol granules on carrots. Report from Bayer
UK Limited, Agrochem. Division, submitted by Bayer Ag.
(Unpublished)
Bayer Residue Reports Nos. 4003-4005/74, 4000-4002/77 (Cabbage,
1973-74 savoy), 73-75/73, 4064-/74 (Cabbage, white), 45-47173,
1977a 4000-4002/74, 4003-4005/77 (Cauliflower). (Unpublished).
1974-76 Residue reports nos. 4057/74, 4014-4015/75, 4006-4008/76
(Sweden), 4016/75, 4023-4024/76, (Turnips). (Unpublished)
1972-73 Residue Reports nos. 132/72, 99-101/73, 4003-4004/75,
1975, 4024/78, 4039-4040/78 (Onions). (Unpublished)
1978
1973-75 Residue Reports nos. 214-216/73, 336/73, 4035-4037/74 4031
a-c/75 (Soil, field). (Unpublished)
1973-74 Residue reports nos. 267-268/73, 4038-4040/74, 4043/74,
1977b 4046-4048/74, 4017/74 (Leaching exp.). (Unpublished)
1973 Residue Report no. 105/73 (Potatoes). (Unpublished)
1974 Residue Reports nos. 4032-4034/74, 403211-403411/74,
4049-4051/74, 4049/1-4051/1/74 (Rapes). (Unpublished)
1974 Residue Reports nos. 4044/II.- 4045/74, 4061/74 (Soil,
laboratory). (Unpublished)
1978 Residue Reports nos. 4000-4001/78 (Brussels sprouts).
(Unpublished)
1980 Residue Reports nos. 5001/80, 5002/80, 5202/80 (Potatoes).
(Unpublished)
Bomhard, E. and Löser, E. SRA 12869 chronic toxicity study on rats
1977 (two-year feeding experiment) Report from Bayer AG Institut
Für Toxikologie, submitted by Bayer AG. (Unpublished)
Brown, M.J. and Williams, J.H, Determination of residues of isofenphos
1976 and its phosphoramidate analogue. Pesticide Science,
7: 545-548.
Brune, H., Deutsch-Wenzel, R., Schüman, K. SRA 12869 chronic toxicity
1978 study on NMRI mice (108-week feeding experiment). Report
from Biologisches Laboratorium, Hamburg, submitted by Bayer
AG. (Unpublished)
Cherry, C.P., Newman, A.J. and Urwin, C. Pathology report of SRA 12869
1972 - Acute neurotoxicity experiment in hens. Report from
Huntingdon Research Centre, UK, submitted by Bayer AG.
(Unpublished)
Dupre, G.D. Metabolism and excretion of 14C-labelled SPA 12869 in
1975 poultry. Mobay Report No. 44931. (Unpublished)
Flucke, W. Oftanol bestimmung der akuten toxizität (LD50). Reports
1980a dated 25 July, 29 August, 15 October and 8 December, 1980
from Bayer AG submitted by Bayer AG. (Unpublished)
1980b SRA 12869 Bestimmung der akuten toxizität (LD50). Ratte
p.o. Report dated 7 Nov. 1980 from Bayer AG, submitted by
Bayer AG. (Unpublished)
1981 Oftanol bestimmung der akuten toxizität (LD50). Report
dated 16 February 1981 from Bayer AG, submitted by Bayer AG.
(Unpublished)
Hoffman, K. and Kaliner, G. SPA 12869 chronic toxicity study on dogs
1977 (two-year feeding experiment). Report from Bayer AG Institut
Fur Toxicologie submitted by Bayer AG. (Unpublished)
Inukai, H. and Iyatomi. SRA 12869 mutagenicity test on bacterial
1977 systems. Report from Nitokuno Agricultural Chemicals
Institute, Laboratory of Toxicology, Japan, submitted by
Bayer AG. (Unpublished)
Kimmerle, G. SRA 12869 acute neurotoxicity studies on hens. Report
1972a from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b Kutane toxizitat von Parathion an ratten vesgleich
verschiedener methoden. Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
1976 SPA 12869 acute inhalation toxicity study on rats. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Kimmerle G. and Gröning. SRA 12869 study to evaluate atropine and
1975 oxines for antidotal effect on rats. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Kurtz, S.K. The assimilation and metabolism of 14C Oftanol by
1977 rotational crops. Mobay Report No. 53826. (Unpublished)
Kurtz, S.K. and Shaw II, H.R. Poultry metabolism of (14C) Oftanol.
1977 Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Lamb, D.W., Matzkanin, C.S. and Burke, M.A. Acute oral toxicity of
1977 Oftanol technical. Report from Mobay N. 54000, Ref. 76-282,
submitted by Bayer AG. (Unpublished)
Lamb, D.W. and Burke, M.A. Dietary toxicity of Oftanol technical to
1977 mallard ducks. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 Acute oral toxicity of AmazeTM technical to bobwhite
quail. Report from Mobay Chemical Corp., submitted by Bayer
AG. (Unpublished)
Löser, E. SRA 12869 subchronic toxicity study on rats (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Löser, E. SRA 12869 subacute toxicity study on rats (four-week feeding
1978 experiment). Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Machemer, L. SRA 12869 studies for embryotoxic and teratogenic effects
1972 on rats following oral administration. Report submitted by
Bayer AG. (Unpublished)
1973 SRA 12869 dominant lethal test on the male mouse to test for
mutagenic effect. Report from Bayer AG, submitted by Bayer
AG. (Unpublished)
1975 SRA 12869 studies of embryotoxic and teratogenic effects on
rabbits following oral administration. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
McNamara, F.T. Dissipation of 14C-Oftanol in buffered aqueous
1977a solutions. Mobay Report No. 53617. (Unpublished)
1977b Dissipation of 14C-Oftanol in pond water containing soil.
Mobay Report No. 53618. (Unpublished)
Minor R.G. Identification of volatile isofenphos soil metabolites.
1980 Mobay Report No. 68658. (Unpublished)
Minor R.G. and Murphy, J.J. Metabolism of 14C-Oftanol in soil. Mobay
1977 Report No. 53643. (Unpublished)
Mobay Residue Reports nos. 53819, 53888, 65932, 67505, 67508,
1979-80 68461-68463 (Corn). (Unpublished)
1978a Residue Reports nos. 53820, 53857-53859, 65931, 65933,
65935-65937, 53817, 5382353825, 53853, 65637, 65923, 65924,
65926, 65927 (Sweet corn). (Unpublished)
1978b Residue Reports nos. 65479-65481, 65491-65493, 65657, 65468,
65477, 65484-65487, 65636, 65656, 67394-67395 (Onions).
(Unpublished)
1980a Residue Reports nos. 69025-69027 (Rapes). (Unpublished)
1980b Residue Reports nos. 53620, 53649, 53695, 53698, 53895,
67378 (Stability studies). (Unpublished)
1980c Residue Reports nos. 69101, 69106, 69111, 69128, 69129,
69130, 69133, 69147, 69148 (Degradation in soil).
(Unpublished)
1980d Residue Reports nos. 69101, 60103-69106, 69107, 69-108,
69110, 69111, 69127 69134-69136, 69144, 69145, 69204-69206,
69128-69130, 69133, 69147, 69148 (Residues in rotational
crops). (Unpublished)
Mürmann, P. SRA 12869 subchronic toxicity study on dogs (three-month
1973 feeding experiment). Report from Bayer AG, submitted by
Bayer AG. (Unpublished)
Nelson, D.L. and Burke, M.A. Acute dermal toxicity of Oftanol
1977a technical. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG. (Unpublished)
1977b Acute oral toxicity of Oftanol technical to mallard ducks.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
1977c Dietary toxicity of Oftanol technical to bobwhite quail.
Report from Chemagro Agricultural Division, Mobay Chemical
Corp., submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Roney, D.J. Accumulation and persistence of residues
1977 in channel catfish exposed to Oftanol-14C. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Nelson, D.L. and Anderson, R.H. The skin-sensitizing properties of
1977 Oftanol technical to guinea pigs. Report from
Pharmakologisches Institut der Universität Mainz, submitted
by Bayer AG. (Unpublished)
Netherlands. Information from the Netherlands on pesticides included
1981 in the JMPR priority list.
Obrist, J.J. and Thornton, J.S. Leaching characteristics of aged
1977 Oftanol soil residues. Mobay Report No. 53944. (Unpublished)
Oesch, F. Ames Test for Oftanol (isofenphos). Report from Chemagro
1977 Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Palmer, A.K., Killick, M.E. and Allen, T.R. Effect of SPA 12869 on
1977 reproductive function of multiple generations in the rat.
Report from Huntingdon Research Centre, UK, submitted by
Bayer AG. (Unpublished)
Poje, A.J. AMAZE photoproduct identification. Mobay Report No. 68146.
1979 (Unpublished)
Ross, D.B., Cameron, D.M. and Roberts, N.L. The acute oral toxicity
1976 (LD50) of isofenphos to the starling. Report from
Huntingdon Research Centre, England, submitted by Bayer AG.
(Unpublished)
Schlüter, G. SRA 12869 Untersuchungen auf embryotoxische und
1981 teratogene wirkungen nach cutaner verabreichung an der
ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Schlüter, D. and Thyssen, J. SRA 12869 untersuchungen auf
1981 embryotoxische und teratogene wirkungen nach inhalation an
der ratte. Report from Bayer AG, submitted by Bayer AG.
(Unpublished)
Shaw II, H.R. Alkaline emission gas-chromatography method for
1974 determining BAY 92 114 and HOL 0654 in soil and water. Mobay
Report no. 43 072. 1 Oct. (Unpublished)
1977a A gas-chromatographic method for Oftanol, Oftanol oxygen
analogue, des N-isopropyl Oftanol and des-N-isopropyl
Oftanol oxygen analogue in bovine and poultry tissues, milk
and eggs. Mobay Report no. 52 769. (Unpublished)
1977b An interference study for the residue method of Oftanol,
Oftanol oxygen analogue, des-N-isopropyl Oftanol and des
N-isopropyl Oftanol oxygen analogue in bovine and poultry
tissues, milk and eggs. Mobay Report no. 53 512.
(Unpublished)
1977c Gas-chromatographic method for residues of Oftanol and
Oftanol oxygen analogue in soils. Mobay Report no. 53 690.
(Unpublished)
1979 Supplementary methods for eliminating interferences from
Delnav, malathion and Ronnel in the residue methods for
AMAZETM in animal tissues, milk and eggs, Mobay Report
no. 67 652, 23 May 1979. (Unpublished)
Spare, W.C. 14C-AMAZETM activated sludge metabolism. Mobay Report no.
1979 68 201. (Unpublished)
Stanley, C.W., Minor, R.G. and Murphy, I.J. Metabolism of OFTANOLR in
1977 corn. Mobay Report No. 53 110. (Unpublished)
Stanley, C.W. Metabolism of OFTANOLR in onions. Mobay Report no.
1977a 54 043. (Unpublished)
1977b Gas-chromatographic method for residues of OFTANOL and its
metabolites in corn and onions. Mobay Report no. 53 111.
(Unpublished)
1977c An interference study for the residue method for OFTANOL and
its organophosphate metabolites in corn and onions. Mobay
Report no. 53 514, 5 July. (Unpublished)
Stanley, C.W. Gas-chromatographic method for residues of AMAZETM and
1979a its metabolites in corn. Mobay Report no. 67 530.
(Unpublished)
1979b Supplementary methods for eliminating interferences of
diazinon, Di-syston and phorate with AMAZETM residues in
the residues method for crops. Mobay Report no. 67 651.
(Unpublished)
Shaw, H.R. Strankowski, K.J. and Murphy, J.J. Excretion and metabolism
1977 of BAY SRA 12869 by rats. Report from Chemagro Agricultural
Division, Mobay Chemical Corp., submitted by Bayer AG.
(Unpublished)
Shirasu, Y., Moriya, M. and Watanabe, K. Isofenphos mutagenicity test
1980 on bacterial systems. Report from Department of Toxicology,
Institute of Environmental Toxicology, submitted by Bayer
AG. (Unpublished)
Solmecke, B. and Kimmerle, G. SRA 12869 subacute toxicity studies.
1972a Report from Bayer AG, submitted by Bayer AG. (Unpublished)
1972b SRA 12869 special toxicological studies. Report from Bayer
AG, submitted by Bayer AG. (Unpublished)
1972c SRA 12869 acute toxicological studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J. Effects of feeding ROftanol/Oftanol/oxygen analogue,
1977 des-N-isopropyl Oftanol oxygen analogue (1:8:1) to chickens
for 28 days. Report from Chemagro Agricultural Division,
Mobay Chemical Corp., submitted by Bayer AG. (Unpublished)
1979 AMAZETM octanol/water partition coefficient Mobay Report
no. 68 246. (Unpublished)
1980 AMAZETM water solubility. Mobay Report no. 69 175.
(Unpublished)
Strankowski, K.J. and Murphy, J.J. Metabolism and excretion of (14C)
1977a Oftanol by a lactating dairy cow. Report from Chemical
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
1977b Metabolites of (14C) ROftanol identified in dairy cow
tissues. Report from Chemagro Agricultural Division, Mobay
Chemical Corp., submitted by Bayer AG.; (Unpublished)
1977c Photodegradation of 14C-Oftanol in aqueous solution. Mobay
Report no. 53 939 (Unpublished)
Strankowski, K.J., Shaw II, H.R. and Murphy, J.H. Metabolites of (14C)
1977a ROftanol identified in rat tissues. Report from Chemagro
Agricultural Division, Mobay Chemical Corp., submitted by
Bayer AG. (Unpublished)
Strankowski, K.J., Minor, R.G. and Murphy, J.J. Excretion and
1977b metabolism of (14C) ROftanol in a pig. Report from
Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Strankowski, K.J., McNamara, F.T. and Minor, R.G. Effects of feeding
1977c ROftanol/Oftanol oxygen analogue,des-N-Isopropyl Oftanol
Oxygen analogue (1:8:1) to dairy cattle for 28 days. Report
from Chemagro Agricultural Division, Mobay Chemical Corp.,
submitted by Bayer AG. (Unpublished)
Thomson, C. and Newman, A.J. Pathology report of Bayer SRA 12869 sub-
1973 chronic toxicity experiment in dogs (13 weeks administration
in the diet). Report from Hungtindon Research Centre,
England, submitted by Bayer AG. (Unpublished)
Thornton, J.S., Hurley, J.B. and Obrist, J.J. Soil thin-layer mobility
1976 of 24 pesticide chemicals. Mobay Report no. 51 016.
(Unpublished)
Thyssen, J. HOL 0654 special toxicological studies. Report from Bayer
1974a AG, submitted by Bayer AG. (Unpublished)
1974b HOL 0654 subacute toxicity studies. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1974c HOL 0654 akute toxizität bei hühnern. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
Thyssen, J. Toxicological studies to evaluate phenamiphos for acute
1976 oral toxicity when administered simultaneously with
fensulfothion, isofephos or phoxim. Report from Bayer AG,
submitted by Bayer AG. (Unpublished)
1977 Toxikologische untersuchungen bei gleichzeitiger applikation
von SRA 7502 und SRA 12869. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
1978 SRA 12869 Acute toxicity studies on hens and quail. Report
from Bayer AG, submitted by Bayer AG. (Unpublished)
Thyssen, J. and Kaliner, G. SRA 12869 subacute dermal cumulative
1977 toxicity study on rabbits. Report from Bayer AG, submitted
by Bayer AG. (Unpublished)
Thyssen, J. and Kimmerle, G. HOL 0654 acute toxicity studies. Report
1974 from Bayer, AG, submitted by Bayer AG. (Unpublished)
Urwin, C. and Newman, A.J. Pathology report of SRA 12869 3-month rat
1973 study. Report from Huntingdon Research Centre, UK, submitted
by Bayer AG. (Unpublished)
Wagner, K. Method for the gas-chromatographic determination of
1976 OftanolR residues in plants soil and water. Pflanzenshutz-
Nachrichten Bayer, 29: 67 - 80.
Weissenburger, B. and Pollock, R.J. Photodegradation of 14C AMAZE on a
1979 soil surface Mobay Report no. 68 245. (Unpublished)
