
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
VAMIDOTHION
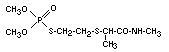 Explanation
Vamidothion was evaluated for an ADI by the 1973 Joint FAO/WHO
Meeting (FAO/WHO 1974).1 The data were not adequate for the
estimation of an acceptable daily intake for man.
Since the previous evaluation was done, a metabolic study and two
long-term studies have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Data on the metabolic fate of vamidothion have been reviewed by
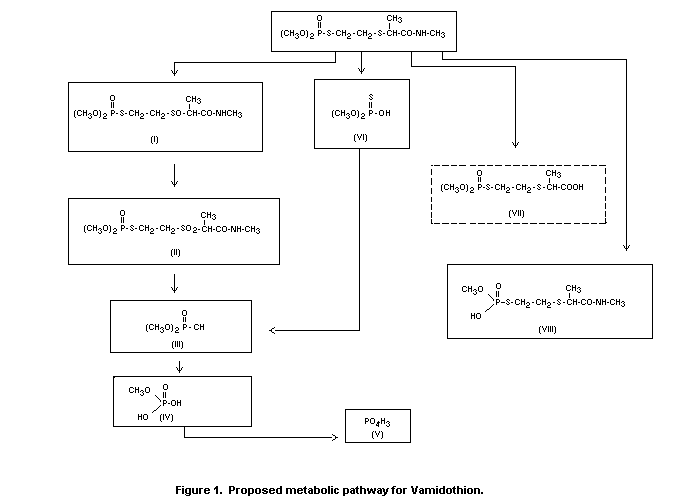
Metivier (1974). The proposed metabolic pathway in plants is shown in
Figure 1. Vamidothion sulphoxide (I) is the main metabolite, whereas
vamidothion sulphone is formed in very small amounts. Desmethyl
vamidothion (VIII) has been observed in plants but not in animals. The
acid (VII) is a hypothetical metabolite observed neither in plants nor
in animals. Acidic fragments (III, IV, V and VI) are formed in both
animals and plants.
Vamidothion sulphoxide appears to be the main metabolite of
toxicological importance.
1 See Annex 2 for WHO and FAO documentation.
Explanation
Vamidothion was evaluated for an ADI by the 1973 Joint FAO/WHO
Meeting (FAO/WHO 1974).1 The data were not adequate for the
estimation of an acceptable daily intake for man.
Since the previous evaluation was done, a metabolic study and two
long-term studies have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Data on the metabolic fate of vamidothion have been reviewed by
Metivier (1974). The proposed metabolic pathway in plants is shown in
Figure 1. Vamidothion sulphoxide (I) is the main metabolite, whereas
vamidothion sulphone is formed in very small amounts. Desmethyl
vamidothion (VIII) has been observed in plants but not in animals. The
acid (VII) is a hypothetical metabolite observed neither in plants nor
in animals. Acidic fragments (III, IV, V and VI) are formed in both
animals and plants.
Vamidothion sulphoxide appears to be the main metabolite of
toxicological importance.
1 See Annex 2 for WHO and FAO documentation.
 TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
(See under Long-Term Studies)
Acute Toxicity
A summary of results of acute oral toxicity studies on
Vamidothion and its metabolites is shown in Table 1 (Metivier 1974).
Table 1. The Acute Toxicity of Vamidothion and Its Metabolites
LD50
Compounds (mg/kg) Species Sex Route
Vamidothion 35 mouse oral
Vamidothion 105 rat M oral
Vamidothion 85 guinea pig oral
Vamidothion sulphoxide 80 mouse oral
Vamidothion sulphoxide 160 rat M oral
Vamidothion sulphoxide 205 guinea pig oral
Vamidothion sulphone 75 mouse M + F oral
Vamidothion sulphone 140 rat F oral
Vamidothion desmethyl > 10 000 mouse oral
Ac.(VI)NH+4 salt ca.5 600 mouse
oral
AC.(III)K+ salt > 3 000 rat
oral
Acid (IV >2 000 rat oral
disodium salt
Acid (V) ca.5 300 mouse oral
monoCa++ salt
Long-Term Studies
Mouse
Groups of mice (60 male and 60 female ICR mice/group) were
administered vamidothion (purity not stated) in the diet for 24 months
at dosage levels of 0, 0.1, 1, 10 or 100 ppm. Observations were made
with respect to abnormal symptoms and mortality. At and after 54 weeks
(12 months), special attention was paid to the occurrence of tumours.
The animals dying during the experiment were subjected to autopsy for
investigation on the cause of death. The animals were weighed once a
week between weeks 1 and 55, and every 2 weeks thereafter. Feed and
water consumption were determined once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the average daily
feed consumption. Haematologic, clinical chemistry and urinalysis
examinations were carried out on 6 animals of each sex in each
group in weeks 26 and 53, and on 10 animals of each sex in each
group in week 110. Major organs were weighed and anatomical and
histopathological examinations were performed on major tissues and
organs. Those mice surviving after 110 weeks were sacrificed in week
112 for pathological examinations.
There was no remarkable difference in mortality between the
control and treated groups. Growth, food and water consumption and
feeding efficiency were similar in the control and any treated group,
as were urinalysis parameters observed over the course of the study.
No significant differences between control and treated groups
were seen in respect to haematological examinations except for i) a
decrease in the leucocyte count in males and females of the 100 ppm
group in week 23; ii) a decrease in the leucocyte count in males of
the 100 ppm group and in females of the 10 ppm and 100 ppm groups in
week 53. However, haematological examinations in week 100 did not
reveal any significant changes in male and female of the treated
groups as compared with those in the control.
Clinical chemistry values, with the exception of cholinesterase
activity, were normal, Serum and erythrocyte cholinesterase were
depressed at dietary levels of 10 ppm, whereas brain cholinesterase
was depressed only at 100 ppm levels in weeks 26 and 53, but not in
week 100. The no-effect level with respect to serum and erythrocyte
cholinesterase was 1 ppm.
Although slight changes in weights of some organs were recorded
in weeks 26 and 53, they were neither consistent between males and
females nor dose related. Thus, it seems unlikely that they were
related to the toxic effect of administered vamidothion. However,
measurements of organ weights in week 110 revealed no remarkable
changes in either absolute organ weights or their ratios to body
weight.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion.
Data on the examination of animals for tumours did not suggest
carcinogenic potential (Toyoshima et al 1975).
Rat
Groups of rats (48 male and 48 female Wistar-strain rats/group)
were administered vamidothion (purity not stated) in the diet for 24
months at dosage levels of 0, 0.1, 1, 10 and 100 ppm. Observations
were made with respect to abnormal symptoms and mortality. In and
after week 54, special attention was paid to the occurrence of
tumours. The animals dying during the experiment were subjected to
anatomical and histopathological investigations to determine the cause
of death. Body weight of both males and females was measured once a
week until week 53 and every 2 weeks thereafter. Feed and water
consumption was measured once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the daily average
feed consumption. Haematological, clinical chemistry and urinalysis
examinations were carried out in weeks 26 and 53 (6 animals/sex/group)
and in week 110 (10 animals/sex/group). Major organs were weighed and
anatomical and histopathological examinations on major tissues and
organs were carried out.
Those rats surviving after 110 weeks were sacrificed in week 112
for anatomopathological examinations.
No significant differences between control and test groups were
observed with respect to toxicological symptoms, mortality, feed
consumption, water consumption, feeding efficiency, body weight gain
and haematological and urinalysis parameters. The only difference in
clinical chemistry values between control and treated groups were
i) lower GOT activity in females of the 100 ppm group in week 26;
ii) lower cholesterol level and lower total protein in males of the
10 ppm group in week 53; iii) depression of serum and erythrocyte
cholinesterase activities, starting with 10 ppm group; iv) depression
of brain cholinesterase activity only in males of the 10 ppm and
100 ppm in week 53. The no-effect level for serum and erythrocyte
cholinesterase was 1 ppm.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion. Data on the examination of animals
for tumours did not suggest a carcinogenic potential (Toyoshima
et al 1975).
COMMENTS
Vamidothion was considered by the 1973 JMPR. An ADI was not
determined. A metabolic study and two long-term studies (mouse, rat)
were examined by the 1982 JMPR. The metabolic sequence is similar to
that observed in other organophosphorus compounds and includes both
oxidative and hydrolytic degradation.
Two long-term studies failed to reveal adverse effects other than
moderate plasma and erythrocyte (but not brain) cholinesterase
inhibition, and demonstrated no oncogenicity,
However, the Meeting agreed that only a temporary ADI could be
allocated because of the absence or inadequacy of the multigeneration
reproduction study, a teratogenicity study, a delayed neurotoxicity
study and a non-rodent study.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.137 mg/kg bw.
Rat : 1 ppm in the diet, equivalent to 0.054 mg/kg bw.
Dog : 5 ppm in the diet, equivalent to 0.125 mg/kg bw.
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. Teratogenicity studies.
2. Multigeneration reproduction studies.
3. Delayed neurotoxicity studies.
4. A non-rodent (dog) study of adequate duration.
Desirable
1. Mutagenicity tests.
2. Observations in humans.
REFERENCES
Metivier, J. Vamidothion. Etude du métabolisme. (S.U.C.R:P-D,S.Ph.No.
1974 17-929). Report from Laboratoires Recherches de la Société
des Usines Chimiques Rhône-Poulenc submitted to the World
Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
Toyoshima, S., Sato, H., Sato, R., Sato, S., Kashima, M. and Motoyama,
1975a M. 24-month chronic oral toxicity study with vamidothion in
mice. Report from Keio University Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
1975b 24-month chronic oral toxicity study with vamidothion in
rats. Report from Keio University, Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
(See under Long-Term Studies)
Acute Toxicity
A summary of results of acute oral toxicity studies on
Vamidothion and its metabolites is shown in Table 1 (Metivier 1974).
Table 1. The Acute Toxicity of Vamidothion and Its Metabolites
LD50
Compounds (mg/kg) Species Sex Route
Vamidothion 35 mouse oral
Vamidothion 105 rat M oral
Vamidothion 85 guinea pig oral
Vamidothion sulphoxide 80 mouse oral
Vamidothion sulphoxide 160 rat M oral
Vamidothion sulphoxide 205 guinea pig oral
Vamidothion sulphone 75 mouse M + F oral
Vamidothion sulphone 140 rat F oral
Vamidothion desmethyl > 10 000 mouse oral
Ac.(VI)NH+4 salt ca.5 600 mouse
oral
AC.(III)K+ salt > 3 000 rat
oral
Acid (IV >2 000 rat oral
disodium salt
Acid (V) ca.5 300 mouse oral
monoCa++ salt
Long-Term Studies
Mouse
Groups of mice (60 male and 60 female ICR mice/group) were
administered vamidothion (purity not stated) in the diet for 24 months
at dosage levels of 0, 0.1, 1, 10 or 100 ppm. Observations were made
with respect to abnormal symptoms and mortality. At and after 54 weeks
(12 months), special attention was paid to the occurrence of tumours.
The animals dying during the experiment were subjected to autopsy for
investigation on the cause of death. The animals were weighed once a
week between weeks 1 and 55, and every 2 weeks thereafter. Feed and
water consumption were determined once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the average daily
feed consumption. Haematologic, clinical chemistry and urinalysis
examinations were carried out on 6 animals of each sex in each
group in weeks 26 and 53, and on 10 animals of each sex in each
group in week 110. Major organs were weighed and anatomical and
histopathological examinations were performed on major tissues and
organs. Those mice surviving after 110 weeks were sacrificed in week
112 for pathological examinations.
There was no remarkable difference in mortality between the
control and treated groups. Growth, food and water consumption and
feeding efficiency were similar in the control and any treated group,
as were urinalysis parameters observed over the course of the study.
No significant differences between control and treated groups
were seen in respect to haematological examinations except for i) a
decrease in the leucocyte count in males and females of the 100 ppm
group in week 23; ii) a decrease in the leucocyte count in males of
the 100 ppm group and in females of the 10 ppm and 100 ppm groups in
week 53. However, haematological examinations in week 100 did not
reveal any significant changes in male and female of the treated
groups as compared with those in the control.
Clinical chemistry values, with the exception of cholinesterase
activity, were normal, Serum and erythrocyte cholinesterase were
depressed at dietary levels of 10 ppm, whereas brain cholinesterase
was depressed only at 100 ppm levels in weeks 26 and 53, but not in
week 100. The no-effect level with respect to serum and erythrocyte
cholinesterase was 1 ppm.
Although slight changes in weights of some organs were recorded
in weeks 26 and 53, they were neither consistent between males and
females nor dose related. Thus, it seems unlikely that they were
related to the toxic effect of administered vamidothion. However,
measurements of organ weights in week 110 revealed no remarkable
changes in either absolute organ weights or their ratios to body
weight.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion.
Data on the examination of animals for tumours did not suggest
carcinogenic potential (Toyoshima et al 1975).
Rat
Groups of rats (48 male and 48 female Wistar-strain rats/group)
were administered vamidothion (purity not stated) in the diet for 24
months at dosage levels of 0, 0.1, 1, 10 and 100 ppm. Observations
were made with respect to abnormal symptoms and mortality. In and
after week 54, special attention was paid to the occurrence of
tumours. The animals dying during the experiment were subjected to
anatomical and histopathological investigations to determine the cause
of death. Body weight of both males and females was measured once a
week until week 53 and every 2 weeks thereafter. Feed and water
consumption was measured once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the daily average
feed consumption. Haematological, clinical chemistry and urinalysis
examinations were carried out in weeks 26 and 53 (6 animals/sex/group)
and in week 110 (10 animals/sex/group). Major organs were weighed and
anatomical and histopathological examinations on major tissues and
organs were carried out.
Those rats surviving after 110 weeks were sacrificed in week 112
for anatomopathological examinations.
No significant differences between control and test groups were
observed with respect to toxicological symptoms, mortality, feed
consumption, water consumption, feeding efficiency, body weight gain
and haematological and urinalysis parameters. The only difference in
clinical chemistry values between control and treated groups were
i) lower GOT activity in females of the 100 ppm group in week 26;
ii) lower cholesterol level and lower total protein in males of the
10 ppm group in week 53; iii) depression of serum and erythrocyte
cholinesterase activities, starting with 10 ppm group; iv) depression
of brain cholinesterase activity only in males of the 10 ppm and
100 ppm in week 53. The no-effect level for serum and erythrocyte
cholinesterase was 1 ppm.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion. Data on the examination of animals
for tumours did not suggest a carcinogenic potential (Toyoshima
et al 1975).
COMMENTS
Vamidothion was considered by the 1973 JMPR. An ADI was not
determined. A metabolic study and two long-term studies (mouse, rat)
were examined by the 1982 JMPR. The metabolic sequence is similar to
that observed in other organophosphorus compounds and includes both
oxidative and hydrolytic degradation.
Two long-term studies failed to reveal adverse effects other than
moderate plasma and erythrocyte (but not brain) cholinesterase
inhibition, and demonstrated no oncogenicity,
However, the Meeting agreed that only a temporary ADI could be
allocated because of the absence or inadequacy of the multigeneration
reproduction study, a teratogenicity study, a delayed neurotoxicity
study and a non-rodent study.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.137 mg/kg bw.
Rat : 1 ppm in the diet, equivalent to 0.054 mg/kg bw.
Dog : 5 ppm in the diet, equivalent to 0.125 mg/kg bw.
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. Teratogenicity studies.
2. Multigeneration reproduction studies.
3. Delayed neurotoxicity studies.
4. A non-rodent (dog) study of adequate duration.
Desirable
1. Mutagenicity tests.
2. Observations in humans.
REFERENCES
Metivier, J. Vamidothion. Etude du métabolisme. (S.U.C.R:P-D,S.Ph.No.
1974 17-929). Report from Laboratoires Recherches de la Société
des Usines Chimiques Rhône-Poulenc submitted to the World
Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
Toyoshima, S., Sato, H., Sato, R., Sato, S., Kashima, M. and Motoyama,
1975a M. 24-month chronic oral toxicity study with vamidothion in
mice. Report from Keio University Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
1975b 24-month chronic oral toxicity study with vamidothion in
rats. Report from Keio University, Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
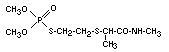 Explanation
Vamidothion was evaluated for an ADI by the 1973 Joint FAO/WHO
Meeting (FAO/WHO 1974).1 The data were not adequate for the
estimation of an acceptable daily intake for man.
Since the previous evaluation was done, a metabolic study and two
long-term studies have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Data on the metabolic fate of vamidothion have been reviewed by
Metivier (1974). The proposed metabolic pathway in plants is shown in
Figure 1. Vamidothion sulphoxide (I) is the main metabolite, whereas
vamidothion sulphone is formed in very small amounts. Desmethyl
vamidothion (VIII) has been observed in plants but not in animals. The
acid (VII) is a hypothetical metabolite observed neither in plants nor
in animals. Acidic fragments (III, IV, V and VI) are formed in both
animals and plants.
Vamidothion sulphoxide appears to be the main metabolite of
toxicological importance.
1 See Annex 2 for WHO and FAO documentation.
Explanation
Vamidothion was evaluated for an ADI by the 1973 Joint FAO/WHO
Meeting (FAO/WHO 1974).1 The data were not adequate for the
estimation of an acceptable daily intake for man.
Since the previous evaluation was done, a metabolic study and two
long-term studies have been provided and are summarized in this
monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
Data on the metabolic fate of vamidothion have been reviewed by
Metivier (1974). The proposed metabolic pathway in plants is shown in
Figure 1. Vamidothion sulphoxide (I) is the main metabolite, whereas
vamidothion sulphone is formed in very small amounts. Desmethyl
vamidothion (VIII) has been observed in plants but not in animals. The
acid (VII) is a hypothetical metabolite observed neither in plants nor
in animals. Acidic fragments (III, IV, V and VI) are formed in both
animals and plants.
Vamidothion sulphoxide appears to be the main metabolite of
toxicological importance.
1 See Annex 2 for WHO and FAO documentation.
 TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
(See under Long-Term Studies)
Acute Toxicity
A summary of results of acute oral toxicity studies on
Vamidothion and its metabolites is shown in Table 1 (Metivier 1974).
Table 1. The Acute Toxicity of Vamidothion and Its Metabolites
LD50
Compounds (mg/kg) Species Sex Route
Vamidothion 35 mouse oral
Vamidothion 105 rat M oral
Vamidothion 85 guinea pig oral
Vamidothion sulphoxide 80 mouse oral
Vamidothion sulphoxide 160 rat M oral
Vamidothion sulphoxide 205 guinea pig oral
Vamidothion sulphone 75 mouse M + F oral
Vamidothion sulphone 140 rat F oral
Vamidothion desmethyl > 10 000 mouse oral
Ac.(VI)NH+4 salt ca.5 600 mouse
oral
AC.(III)K+ salt > 3 000 rat
oral
Acid (IV >2 000 rat oral
disodium salt
Acid (V) ca.5 300 mouse oral
monoCa++ salt
Long-Term Studies
Mouse
Groups of mice (60 male and 60 female ICR mice/group) were
administered vamidothion (purity not stated) in the diet for 24 months
at dosage levels of 0, 0.1, 1, 10 or 100 ppm. Observations were made
with respect to abnormal symptoms and mortality. At and after 54 weeks
(12 months), special attention was paid to the occurrence of tumours.
The animals dying during the experiment were subjected to autopsy for
investigation on the cause of death. The animals were weighed once a
week between weeks 1 and 55, and every 2 weeks thereafter. Feed and
water consumption were determined once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the average daily
feed consumption. Haematologic, clinical chemistry and urinalysis
examinations were carried out on 6 animals of each sex in each
group in weeks 26 and 53, and on 10 animals of each sex in each
group in week 110. Major organs were weighed and anatomical and
histopathological examinations were performed on major tissues and
organs. Those mice surviving after 110 weeks were sacrificed in week
112 for pathological examinations.
There was no remarkable difference in mortality between the
control and treated groups. Growth, food and water consumption and
feeding efficiency were similar in the control and any treated group,
as were urinalysis parameters observed over the course of the study.
No significant differences between control and treated groups
were seen in respect to haematological examinations except for i) a
decrease in the leucocyte count in males and females of the 100 ppm
group in week 23; ii) a decrease in the leucocyte count in males of
the 100 ppm group and in females of the 10 ppm and 100 ppm groups in
week 53. However, haematological examinations in week 100 did not
reveal any significant changes in male and female of the treated
groups as compared with those in the control.
Clinical chemistry values, with the exception of cholinesterase
activity, were normal, Serum and erythrocyte cholinesterase were
depressed at dietary levels of 10 ppm, whereas brain cholinesterase
was depressed only at 100 ppm levels in weeks 26 and 53, but not in
week 100. The no-effect level with respect to serum and erythrocyte
cholinesterase was 1 ppm.
Although slight changes in weights of some organs were recorded
in weeks 26 and 53, they were neither consistent between males and
females nor dose related. Thus, it seems unlikely that they were
related to the toxic effect of administered vamidothion. However,
measurements of organ weights in week 110 revealed no remarkable
changes in either absolute organ weights or their ratios to body
weight.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion.
Data on the examination of animals for tumours did not suggest
carcinogenic potential (Toyoshima et al 1975).
Rat
Groups of rats (48 male and 48 female Wistar-strain rats/group)
were administered vamidothion (purity not stated) in the diet for 24
months at dosage levels of 0, 0.1, 1, 10 and 100 ppm. Observations
were made with respect to abnormal symptoms and mortality. In and
after week 54, special attention was paid to the occurrence of
tumours. The animals dying during the experiment were subjected to
anatomical and histopathological investigations to determine the cause
of death. Body weight of both males and females was measured once a
week until week 53 and every 2 weeks thereafter. Feed and water
consumption was measured once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the daily average
feed consumption. Haematological, clinical chemistry and urinalysis
examinations were carried out in weeks 26 and 53 (6 animals/sex/group)
and in week 110 (10 animals/sex/group). Major organs were weighed and
anatomical and histopathological examinations on major tissues and
organs were carried out.
Those rats surviving after 110 weeks were sacrificed in week 112
for anatomopathological examinations.
No significant differences between control and test groups were
observed with respect to toxicological symptoms, mortality, feed
consumption, water consumption, feeding efficiency, body weight gain
and haematological and urinalysis parameters. The only difference in
clinical chemistry values between control and treated groups were
i) lower GOT activity in females of the 100 ppm group in week 26;
ii) lower cholesterol level and lower total protein in males of the
10 ppm group in week 53; iii) depression of serum and erythrocyte
cholinesterase activities, starting with 10 ppm group; iv) depression
of brain cholinesterase activity only in males of the 10 ppm and
100 ppm in week 53. The no-effect level for serum and erythrocyte
cholinesterase was 1 ppm.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion. Data on the examination of animals
for tumours did not suggest a carcinogenic potential (Toyoshima
et al 1975).
COMMENTS
Vamidothion was considered by the 1973 JMPR. An ADI was not
determined. A metabolic study and two long-term studies (mouse, rat)
were examined by the 1982 JMPR. The metabolic sequence is similar to
that observed in other organophosphorus compounds and includes both
oxidative and hydrolytic degradation.
Two long-term studies failed to reveal adverse effects other than
moderate plasma and erythrocyte (but not brain) cholinesterase
inhibition, and demonstrated no oncogenicity,
However, the Meeting agreed that only a temporary ADI could be
allocated because of the absence or inadequacy of the multigeneration
reproduction study, a teratogenicity study, a delayed neurotoxicity
study and a non-rodent study.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.137 mg/kg bw.
Rat : 1 ppm in the diet, equivalent to 0.054 mg/kg bw.
Dog : 5 ppm in the diet, equivalent to 0.125 mg/kg bw.
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. Teratogenicity studies.
2. Multigeneration reproduction studies.
3. Delayed neurotoxicity studies.
4. A non-rodent (dog) study of adequate duration.
Desirable
1. Mutagenicity tests.
2. Observations in humans.
REFERENCES
Metivier, J. Vamidothion. Etude du métabolisme. (S.U.C.R:P-D,S.Ph.No.
1974 17-929). Report from Laboratoires Recherches de la Société
des Usines Chimiques Rhône-Poulenc submitted to the World
Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
Toyoshima, S., Sato, H., Sato, R., Sato, S., Kashima, M. and Motoyama,
1975a M. 24-month chronic oral toxicity study with vamidothion in
mice. Report from Keio University Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
1975b 24-month chronic oral toxicity study with vamidothion in
rats. Report from Keio University, Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
TOXICOLOGICAL STUDIES
Special Study for Carcinogenicity
(See under Long-Term Studies)
Acute Toxicity
A summary of results of acute oral toxicity studies on
Vamidothion and its metabolites is shown in Table 1 (Metivier 1974).
Table 1. The Acute Toxicity of Vamidothion and Its Metabolites
LD50
Compounds (mg/kg) Species Sex Route
Vamidothion 35 mouse oral
Vamidothion 105 rat M oral
Vamidothion 85 guinea pig oral
Vamidothion sulphoxide 80 mouse oral
Vamidothion sulphoxide 160 rat M oral
Vamidothion sulphoxide 205 guinea pig oral
Vamidothion sulphone 75 mouse M + F oral
Vamidothion sulphone 140 rat F oral
Vamidothion desmethyl > 10 000 mouse oral
Ac.(VI)NH+4 salt ca.5 600 mouse
oral
AC.(III)K+ salt > 3 000 rat
oral
Acid (IV >2 000 rat oral
disodium salt
Acid (V) ca.5 300 mouse oral
monoCa++ salt
Long-Term Studies
Mouse
Groups of mice (60 male and 60 female ICR mice/group) were
administered vamidothion (purity not stated) in the diet for 24 months
at dosage levels of 0, 0.1, 1, 10 or 100 ppm. Observations were made
with respect to abnormal symptoms and mortality. At and after 54 weeks
(12 months), special attention was paid to the occurrence of tumours.
The animals dying during the experiment were subjected to autopsy for
investigation on the cause of death. The animals were weighed once a
week between weeks 1 and 55, and every 2 weeks thereafter. Feed and
water consumption were determined once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the average daily
feed consumption. Haematologic, clinical chemistry and urinalysis
examinations were carried out on 6 animals of each sex in each
group in weeks 26 and 53, and on 10 animals of each sex in each
group in week 110. Major organs were weighed and anatomical and
histopathological examinations were performed on major tissues and
organs. Those mice surviving after 110 weeks were sacrificed in week
112 for pathological examinations.
There was no remarkable difference in mortality between the
control and treated groups. Growth, food and water consumption and
feeding efficiency were similar in the control and any treated group,
as were urinalysis parameters observed over the course of the study.
No significant differences between control and treated groups
were seen in respect to haematological examinations except for i) a
decrease in the leucocyte count in males and females of the 100 ppm
group in week 23; ii) a decrease in the leucocyte count in males of
the 100 ppm group and in females of the 10 ppm and 100 ppm groups in
week 53. However, haematological examinations in week 100 did not
reveal any significant changes in male and female of the treated
groups as compared with those in the control.
Clinical chemistry values, with the exception of cholinesterase
activity, were normal, Serum and erythrocyte cholinesterase were
depressed at dietary levels of 10 ppm, whereas brain cholinesterase
was depressed only at 100 ppm levels in weeks 26 and 53, but not in
week 100. The no-effect level with respect to serum and erythrocyte
cholinesterase was 1 ppm.
Although slight changes in weights of some organs were recorded
in weeks 26 and 53, they were neither consistent between males and
females nor dose related. Thus, it seems unlikely that they were
related to the toxic effect of administered vamidothion. However,
measurements of organ weights in week 110 revealed no remarkable
changes in either absolute organ weights or their ratios to body
weight.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion.
Data on the examination of animals for tumours did not suggest
carcinogenic potential (Toyoshima et al 1975).
Rat
Groups of rats (48 male and 48 female Wistar-strain rats/group)
were administered vamidothion (purity not stated) in the diet for 24
months at dosage levels of 0, 0.1, 1, 10 and 100 ppm. Observations
were made with respect to abnormal symptoms and mortality. In and
after week 54, special attention was paid to the occurrence of
tumours. The animals dying during the experiment were subjected to
anatomical and histopathological investigations to determine the cause
of death. Body weight of both males and females was measured once a
week until week 53 and every 2 weeks thereafter. Feed and water
consumption was measured once a week. Feeding efficiency was
calculated by dividing the weight increase in males and females of
each group in weeks 26, 53 and 105 by their feed consumption at the
respective times. Drug intake was calculated from the daily average
feed consumption. Haematological, clinical chemistry and urinalysis
examinations were carried out in weeks 26 and 53 (6 animals/sex/group)
and in week 110 (10 animals/sex/group). Major organs were weighed and
anatomical and histopathological examinations on major tissues and
organs were carried out.
Those rats surviving after 110 weeks were sacrificed in week 112
for anatomopathological examinations.
No significant differences between control and test groups were
observed with respect to toxicological symptoms, mortality, feed
consumption, water consumption, feeding efficiency, body weight gain
and haematological and urinalysis parameters. The only difference in
clinical chemistry values between control and treated groups were
i) lower GOT activity in females of the 100 ppm group in week 26;
ii) lower cholesterol level and lower total protein in males of the
10 ppm group in week 53; iii) depression of serum and erythrocyte
cholinesterase activities, starting with 10 ppm group; iv) depression
of brain cholinesterase activity only in males of the 10 ppm and
100 ppm in week 53. The no-effect level for serum and erythrocyte
cholinesterase was 1 ppm.
Anatomical and histopathological examinations of tissues and
organs did not reveal any adverse effect attributable to the
administration of vamidothion. Data on the examination of animals
for tumours did not suggest a carcinogenic potential (Toyoshima
et al 1975).
COMMENTS
Vamidothion was considered by the 1973 JMPR. An ADI was not
determined. A metabolic study and two long-term studies (mouse, rat)
were examined by the 1982 JMPR. The metabolic sequence is similar to
that observed in other organophosphorus compounds and includes both
oxidative and hydrolytic degradation.
Two long-term studies failed to reveal adverse effects other than
moderate plasma and erythrocyte (but not brain) cholinesterase
inhibition, and demonstrated no oncogenicity,
However, the Meeting agreed that only a temporary ADI could be
allocated because of the absence or inadequacy of the multigeneration
reproduction study, a teratogenicity study, a delayed neurotoxicity
study and a non-rodent study.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 1 ppm in the diet, equivalent to 0.137 mg/kg bw.
Rat : 1 ppm in the diet, equivalent to 0.054 mg/kg bw.
Dog : 5 ppm in the diet, equivalent to 0.125 mg/kg bw.
Estimate of a Temporary Acceptable Daily Intake for Man
0 - 0.0003 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1985)
1. Teratogenicity studies.
2. Multigeneration reproduction studies.
3. Delayed neurotoxicity studies.
4. A non-rodent (dog) study of adequate duration.
Desirable
1. Mutagenicity tests.
2. Observations in humans.
REFERENCES
Metivier, J. Vamidothion. Etude du métabolisme. (S.U.C.R:P-D,S.Ph.No.
1974 17-929). Report from Laboratoires Recherches de la Société
des Usines Chimiques Rhône-Poulenc submitted to the World
Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
Toyoshima, S., Sato, H., Sato, R., Sato, S., Kashima, M. and Motoyama,
1975a M. 24-month chronic oral toxicity study with vamidothion in
mice. Report from Keio University Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
1975b 24-month chronic oral toxicity study with vamidothion in
rats. Report from Keio University, Tokyo, Japan, Sasaki
Institute, Tokyo, Japan, and Nippon Experimental Medical
Research Institute, Saitama Pref., Japan, submitted to the
World Health Organization by Rhône-Poulenc, Paris, France.
(Unpublished)
