
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
ETHOPROPHOS
IDENTITY
Chemical Name
O-ethyl S,S-dipropyl phosphorodithioate (IUPAC and CAS)
Synonyms: ethoprop (common name adopted by the American National
Standards Institute); Mocap (R); Prophos (R); VC 9-104
Structural Formula
O S - CH2 - CH2 - CH3
" /
CH3 - CH2 - O - P
\
S - CH2 - CH2 - CH3
Other information on identity and properties
Molecular weight 242.3
Description clear pale yellow liquid
Density d20 1.094
4
Boiling point 86 - 91°C/0.2 mm Hg
Vapour pressure 46.5 mPa at 26°C
Solubility 750 mg/l water; 50% wt/wt in acetone,
1,2-dichloroethane, diethyl ether, ethanol,
acetate, petroleum spirit and xylene.
Partition coefficient 140
octanol/water
Hydrolysis Rapidly hydrolysed at 25°C and pH 9
Thermal stability Stable 8 hours at 150°C, 12 weeks; at 50°C
Chemical stability In acid media: stable up to 100°C. In alkalin
media: moderate hydrolysis at 25°C, rapid at
100°C.
Purity of the technical > 94%*
material *n-propyl isomer 93.7%
isopropyl isomer 0.3%/
satisfactory information on the other
impurities was provided by the manufacturer.
Formulations Granular 5, 10 and 20%
EC about 60, 200, 500 and 700 g/l
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Ethoprophos is a non-systemic, non-fumigant nematicide, which is
also effective against several soil-borne insects. It is applied to
several annual and perennial crops, in general at sowing or planting.
Owing to its contact and non-fumigant action it has to be worked into
the soil immediately after application and to be brought to the soil
level where the pest population in the following crop will mainly
occur, i.e. in the root zone.
The dosage range is 3 - 10 kg a.i./ha, depending on soil type,
crop, pest organism and soil management (see Table 1).
Because of the absence of systemic action the residues in the
aerial parts of crops at harvest are, with very few exceptions very
low, of the order of 0.02 mg/kg or less.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data were supplied from a large number of trials in which
soil applications were carried out to control pests in several main
crops planted or sown immediately after the application. Data were
from several countries in Europe, America and some other regions. The
data are summarized in Table 2.
General comment on data
The residues in crops whose edible parts grow in the top soil
layer are in general low except in the case of carrots. However,
interferences are found and levels may exceed 0.092 mg/kg, depending
on how thoroughly adhering soil particles are removed by washing or
otherwise removing soil particles from the samples.
Fruit Crops
Grapes. Grapes grown in soils treated with ethoprophos dosages up
to 30 kg a.i./ha did not show measurable residues (<0.02 mg/kg) even
when relatively short pre-harvest intervals for this crop (80 days)
were observed.
In normal growing practice the period between application and harvest
will be longer than 80 days.
Bananas, treated at planting or both at planting and during
development with dosages of about 10 kg a.i./ha or 2 × 7 g a.i./plant
stool, did not show residues in fruits harvested 8-170 days after the
last application.
Table 1. Recommended uses and dosage rates
Application at sowing or planting, if not otherwise stated.
Type and group Crop Dosage rate,
kg a.i./ha
Fruit:
Citrus fruits citrus sp. 10
Berries and other small fruits Grapes 6-10
Strawberries 5-10
Tropical fruit,inedible peel Bananas 10 (2x6 g per stool)
Pineapple 10-15
Vegetables:
Bulb vegetables Onions 8-10
Brassica vegetables Cabbage 4-10
Fruiting vegetables,cucurbits Cucumbers 3-10
Gherkins 6-10
Melons(cantaloupes) 8-10
Fruiting vegetables, other
than cucurbits Eggplant 8-10
Peppers 6
Tomatoes 8-10
Leafy vegetables Lettuce 4
Legume vegetables Common bean 1 8-10
Lima bean 8-10
Soybean 6-10
Garden pea, field
pea 6-10
Root vegetables Carrots 4
Potatoes 4-10
Radish 4-6
Sweet potato 6-10
Sugar beet 8
Turnip 6-10
Stem and stalk vegetables Sugarcane 4-10
Cereal grains Maize 6-10
Other cereal grains 3 2
Oil seeds Peanuts 6-10
Miscellaneous crops Tobacco 8
1 Synonyms: French bean, string bean, snap bean.
2 Dosage against wireworms.
Table 2. Ethopropos residues resulting from supervised trials
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Fruits
Grapes France 1970 15 10 G 1 80 <0.01 CNRA
30 10 G 1 80 <0.01
Strawberries Netherlands 1979 10 10 G 2(8) 310-327 <0.01 Duphar
10 10 G 1(4) 362 0.01 (<0.01-0.02)
1981 10 10 G 2(6) 82-159 <0.01 Duphar
(glass-house) 1980 20 10 G 1(3) 198 0.1 (0.07-0.13) Duphar
Bananas Costa 1969 5-13 g/pl 10 G 3 8-77 <0.02 Mobil
Rica 1x
1969 3-6.5 g/pl 10 G 4 10-111 <0.02 Mobil
1-2x
1969 3-6.5 g/pl 10 G 6 8-170 <0.02 Mobil
1-2x
1969 3-7 g/pl 10 G 2 60-120 <0.02 Mobil
2x
Ivory 1968/69 7.6-10 g/pl 10 G 5 119 <0.02 Mobil
Coast 2x
Pineapple Hawaii 1966/68 1 10 G 2 565-619 <0.02
fruit of 2 10 G 3 532-619 <0.02
plant crop 4 10 G 3 532-619 <0.02
8 10 G 1 565 <0.02
fruit of 1 10 G 1 930 <0.02
ratoon crop 2 10 G 1 930 <0.02
4 10 G 1 930 <0.02
8 10 G 1 930 <0.02
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
The tops and leaves of the plant crop and ratoon crop at all dosages after the above PHIs showed residues of less than 0.02 mg/kg
(at our about limit of determination)
Fruit tops Ivory 1968 4.5 10 G 5 360 <0.02 Mobil
Tops Coast 4.5 10 G 5 360 <0.02 Mobil
Vegetables
Bulb vegetables France 1977 6 10 G 2 <0.005 INRA
Onions 10 10 G 1 <0.005 INRA
USA 1976 3.4 10 G 1(4) 108 0.0009 Hunt,
6.7 10 G 1(4) 108 0.035 1981
13.4 10 G 1(4) 108 0.068
USA 1976 3 10 G 1(4) 42 0.12±0.03 (0.07-0.19) Argauer,
6 10 G 1(4) 42 0.52±0.13 (0.32-0.89) 1978
12 10 G 1(4) 42 1.34±0.13 (1.09-1.64)
Brassicas USA 1976 3.4 10 G 1(4) 56 <0.01 Argauer,
Cabbage 6.7 10 G 1(4) 56 <0.01 1978
13.4 10 G 1(4) 56 <0.01
Fruiting,
vegetables,
cucurbits
Cucumber Netherlands 1978 7.5 10 G 2(8) 31-37 <0.01 Duphar
(glass-house) 10 10 G 2(8) 31-37 <0.01
15 10 G 2(8) 31-37 <0.01
1978 7.5 10 G 3(12) 47 <0.01
10 10 G 3(12) 47 <0.01
15 10 G 3(12) 47 <0.01
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
France 1982 10 G 0 <0.05 Rhône-Poulenc
7 <0.05
22-28 <0.05
(outdoor) USA 1976 3.4 10 G 1(4) 58-62 <0.05 Hunt,
6.7 10 G 1(4) 58-62 <0.05 1982
13.4 10 G 1(4) 58-62 <0.05
Gherkin Netherlands 1978 15 10 G 3(12) 42-45 <0.01 Duphar
Melon France 1977 10 10 G 2 <0.005 INRA
Melon USA 1976 3.4 10 G 1 (4) 98 <0.01 Argauer,
(cantaloupes) 6.7 10 G 1(4) 98 <0.01 1978
13.4 10 G 1(4) 98 <0.01
Fruiting
vegetables, non
cucurbits
Eggplant USA 1976 3.4 10 G 1(4) 77 0.027±0.004 (0014-0.034) Argauer,
6.7 10 G 1(4) 77 0.044±0.007 (0.036-0.05) 1978
13.4 10 G 1(4) 77 0.086±0.011 (0.061-0.116)
Pepper USA 1977 2.2 10 G 1 <0.01
4.5 10 G 1 <0.01
6.7 10 G 1(2) <0.01
13.4 10 G 1 <0.01
20.1 10 G 1 <0.01
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Tomato France 1970 15 10 G 1(2) 127 <0.001 INRA
1977 10 10 G 1(2) <0.005 INRA
(glass-house) Netherlands 1978 15 10 G 1(3) 48 <0.01) Duphar
1979 501 20 G 3(2-4) 113-129 <0.01 Duphar
(outdoor) USA 1976 3.4 10 G 1(4) 80-86 <0.005 Hunt,
6.7 10 G 1(4) 80-86 <0.005 1981
13.4 10 G 1(4) 80-86 <0.005
USA 1976 3.4 10 G 1(4) 91 <0.01 Argauer,
6.7 10 G 1(4) 91 <0.01 1978
13.4 10 G 1(4) 91 <0.01
Leafy
vegetables
Lettuce USA 1976 3.4 10 G 1(4) 87 <0.005 Hunt,
6.7 10 G 1(4) <0.005 1981
13.4 10 G 1(4) <0.005
USA 1976 3.4 10 G 1(3) 56 0.02 (<0.01-0.05) Argauer,
1978
6.7 10 G 1(4) 0.04 (0.02-0.08)
13.4 10 G 1(3) 0.1 (0.03-0.23)
roots of 13.4 10 G 1(3) 56 max. 0.14
lettuce
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Lettuce Netherlands 1979 30 10 G 1(3) 310 <0.01 Duphar
(rotational 1979/80 30 10 G 1 327 <0.01
crop after
Gherkins)
Legume
vegetables
Common bean USA 1977 3.4 10 G 1(4) 0.02 Narain,
(string b.) 6.7 10 G 0.03 1980
13.4 10 G 0.07
USA 1978 3.4 10 G 1(4) 0.01
6.7 10 G 0.09
13.4 10 G 0.11
(snap b.) USA 1976 3.4 10 G 1(4) 66-77 <0.005 Hunt,
6.7 66-77 <0.005-0.008 1981
13.4 66-77 0.005-0.018
(French b.) France 1977 6 10 G 2 <0.005 INRA
Lima bean USA 1977 3.4 10 G 1(4) 0.02 Narain,
6.7 10 G 1(4) 0.13 1980
13.4 10 G 1(4) 0.02
USA 1978 3.4 10 G 1(4) 3.34 Narain,
6.7 10 G 1(4) 4.16 1980
13.4 10 G 1(4) 3.38
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Soybean USA 1966 2.2 10 G 3(4) 158-165 <0.02 Mobil
4.5 10 G
6.7 10 G
1966 2 10 G 2(1+2) 171-172 <0.02 Mobil
2.6
3.3
1968 1.7 10 G 1(2) 186 <0.02 Mobil
1979 6 EC 60/l 1(2) 81 <0.02 Mobil
1979 3 EC 60g/l 1(2) 175 <0.02 Mobil
6 EC 60 g/l
Soybean hay 1979 3 EC 60g/l 1(2) 175 <0.02 Mobil
6 EC 60g/l
Pea USA 1976 3.4 10 G 1(4) 56 <0.01 Argauer,
6.7 10 G 1(4) 1978
13.4 10 G 1(4)
1977 3.4 10 G 1(4) - 0.01 Narain,
6.7 10 G 1(4) 0.01 1980
13.4 10 G 1(4) 0.02
1978 3.4 10 G 1(4) - 1.65 Narain,
6.7 10 G 1(4) 2.12 1980
13.4 10 G 1(4) 1.06
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Root
vegetables
Carrot France 1976 4 10 G 1 0.105 INRA
6 10 G 1 0.255
USA 1976 3.4 10 G 1(4) 84 0.14±0.035 (0.08-0.23 Argauer,
6.7 10 G 1(4) 84 0.34±0.14 (0.15-0.74) 1978
13.4 10 G 1(4) 84 0.81±0.186(0.28-1.14)
1977 3.4 10 G 1(4) 0.06 Narain,
6.7 10 G 1(4) 0.78 1980
13.4 10 G 1(4) 0.52
Garden beet USA 1976 3.4 10 G 1(4) 84 <0.01 Argauer,
6.7 10 G 1(4) 84 <0.01 1978
13.4 10 G 1(4) 84 <0.01
Potato France 1975 6 (broodcast) 10 G 147 <0.01 INRA
2 (plant-hole) 10 G 147 <0.01
France 1982 1 10 G 2 118-147 <0.02 Rhône-
3 10 G 2 118-147 <0.02 Poulenc
6 10 G 2 118-147 <0.02
1982 0.4 (plant-hole) 10 G 2 157-170 <0.05 Rhône-
Poulenc
Fed. Rep. 1979 10 20 G 1(2-4) 58 0.1 Schering
Germany 87 0.07
134 0.02
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
1979 10 20 G 1(2-4) 98 0.04 Schering
118 0.06
139 0.02
1979 10 20 G 1(2) 82 <0.01 Schering
118 <0.01
1979 10 20 G 1(2) 61 0.06 Schering
75 0.03
Netherlands 1975 10 10 G 4(12) 132-153 0.01 CIVO
1980 10 20 G 1(4) 170 <0.003 (<0.002-0.006) KvW A'dam
1980 10 20 G 1(4) 160 <0.006 (<0.002-0.012)
1980 10 20 G 1(4) 148 <0.004 (<0.002-0.008)
Jersey 1980 11 10 G 2(6) 64-65 0.021 (0.015-0.031) Murphy
peel U.K. 1980 10 10 G 4(12) 128-129 0.018 (0.08-0.031) Murphy
flesh 0.0013 (<0. 001-0.005)
Potato U.K. 1971 11 10 G 1(2) 131 0.021 (0.02-0.022) Fisons,
11 10 G 2(3) 92 0.022 (0.018-0.027) Chester-
11 10 G 2(3) 120 0.025 (0.020-0.029) ford Park
11 10 G 2(3) 157 0.004 (0.004-0.005)
11 10 G 2 80 0.032 (0.023-0.042)
11 10 G 2 113 0.014 (0.007-0.022)
11 10 G 2 136 0.005 (0.004-0.006)
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
USA 1969/ 2.2 broodcast 10 G 1 161 <0.02 Mobil
1971 4.5 " 10 G 2 164 <0.02
1973 6.6 " 10 G 1(5) 120-164 <0.02
9 " 10 G en 1(8) 108-133 <0.02
EC 60 g/l
3.4 row tr 10 G 2(6) <0.02
4.5 10 G 1(3) <0.02
3.4 row post 10 G 1(4) 86 <0.02
4.4l emergence 10 G 1(3) 94 <0.02
Sweet USA 1966/ 2.2 10 G 1(4) 124 <0.02 Mobil
potato 1968 3.4 10 G 2(5) 124-156 <0.02
4.5 10 G 2(5) 124-156 <0.02
6.7 10 G 2(10) 127-153 <0.02
1968 2.8 10 G 1 150 <0.02
1967 4.5 10 G 1(2) 116 <0.02
9 10 G 1(2) 116 <0.02
13.4 10 G 1 116 <0.02
Radish USA 1976 3.4 10 G 1(4) 59 0.038 Hunt,
66 0.018 1981
6.7 10 G 1(4) 59 0.079
66 0.031
13.4 10 G 1(4) 59 0.345
66 0.163
3.4 10 G 1(4) 42 0.12±0.023(0.008-0.18) Argauer,
6.7 10 G 1(4) 42 0.33±0.039(0.26-0.44) 1978
13.4 10 G 1(4) 42 0.66±0.023(0.60-0.71)
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
USA 1977 3.4 10 G 1(4) 0.01 Narain,
6.7 10 G 1(4) 0.07 1980
13.4 10 G 1(4) 0.03
1978 3.4 10 G 1(4) 0.09
6.7 10 G 1(4) 0.14
13.4 10 G 1(4) 0.09
1977 6 10 G 2(2) <0.005 INRA
8 10 G 2(2) <0.005
Turnip USA 1976 3.4 10 G 1(4) 87 0.017 Hunt,
roots 3.4 10 G 97 0.010 1981
6.7 10 G 1(4) 87 0.019
6.7 10 G 97 0.012
13.4 10 G 1(4) 87 0.035
13.4 10 G 97 0.013
leaves 1976 3.4 10 G 1 (4) 87 0.018 Hunt,
3.4 10 G 97 <0.005 1981
6.7 10 G 87 0.041
6.7 10 G 97 <0.005
6.7 10 G 87 0.281
6.7 10 G 97 0.036
roots USA 1977 3.4 10 G 1(4) 0.01 Narain
6.7 10 G 0.01 1980
13.4 10 G 0.02
1978 3.4 10 G 1(4) 0.15
6.7 10 G 0.05
13.4 10 G 0.06
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
tops USA 1977 3.4 10 G 1(4) 0.01 Narain,
6.7 10 G 1(4) 0.02 1980
13.4 10 G 1(4) 0.13
1978 3.4 10 G 1(4) 0.05
6.7 10 G 1(4) 0.05
13.4 10 G 1(4) 0.05
Stems and
stalk
vegetables
Sugarcane USA 1966/67 4.2 10 G 1 320 <0.02 Mobil
stalks 8.4 10 G 1 320 <0.02
16.8 10 G 1 320 <0.02
stalks USA 1967/68 4.5 10 G 1(2) 315 <0.02
leaves 4.5 10 G 1(2) 315 <0.02
stalks USA 1968/69 4.5 10 G 1(4) 366 <0.02
leaves USA 1968 5.6 19 G 1(6) 246 <0.92
stalks 5.6 10 G 1(6) 246 <0.02
leaves USA 1966/67 6.7 10 G 1(4) 360 <0.02
stalks USA 1967/68 11.2 10 G 1(3) 350 <0.02
leaves 11.2 10 G 1(3) 350 <0.02
Cereal
grain
Maize France 1982 3 ( EC 480 1 (4) 195 <0.05 (<0.05-<0.05) Rhône-
4 (pre-emergence g/l 1 (4) 195 <0.05 (<0.05-<0.05) Poulenc
6 (spraying 1 (4) 195 <0.05 (<0.05-<0.05)
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
ears USA 1966 1.1 10 G 2(7) 160-165 <0.025 Mobil
2.2 1(6) 165 <0.025
ears + husk 1.1 10 G 1(8) 155 <0.025
2.2 1(8) 155 <0.025
Silage 1.1 10 G 3(15) 80-114 <0.025
2.2 2(14) 108-114 <0.025
Oilseeds
Peanut USA 1968 2.2 10 G 2(5) 118-154 <0.02 Mobil
3.4 10 G 4(13) 138-166 <0.02
4.5 10 G 3(12) 138-166 <0.02
5.6 10 G 3(12) 138-166 <0.02
1966 4.5 EC 1(3) 154 <0.02
8.8 EC 1(2) 154 <0.02
13.4 EC 1(2) 154 <0.02
1973 2.2 10 G 1 143 <0.02
3.4 10 G 1 164 <0.02
6.7 10 G 1 164 <0.02
8.8 10 G 1 143 <0.02
Peanut hay 1973 3.4 10 G 1 164 <0.02
4.5 10 G 1 143 <0.02
6.7 10 G 2(2) 143-164 <0.02
8.8 10 G 1 143 <0.02
Table 2. (continued)
Application Number Residues, mg/kg
of (ethoprophos only)
rate, trials P H I average and
Crop Country Year kg a.i./ha formulation (replicates) (days) (range) Ref.
Tobacco France 1982 5 10 G 1(2-3) 110 0.07 (0.05-0.08) Rhône-
(green) 10 10 G 1(2-3) 110 0.16 (0.14-0.19) Poulenc
20 10 G 1(2-3) 110 0.38 (0.17-0.53)
1982 5 (pre- EC 480 g/l 1(2-3) 110 0.17 (0.16-0.20) Rhône
10 (emergence EC 480 g/l 1(2-3) 110 0.27 (0.26-0.29) Poulenc
20 (spraying EC 480 g/l 1(2-3) 110 1.01 (0.68-1.48)
(green) USA 1966 4.5 10 G 1(2) <0.02 Mobil
5.6 10 G 1(2) <0.02
8.4 10 G 5(14) <0.02
9.0 10 G 2(14) <0.02
(cured) USA 1965 5.7 10 G 5(7) 2 0.08(<0.02-0.15) Mobil
1970 6.7 EC 2(6) 2 0.02(<0.02-0.025)
1967 11 EC 1 82 <0.02 Mobil
102 0.036
116 0.024
22 EC 1 82 0.1
102 <0.02
116 <0.02
1) excessive dosage
2) composite leaf samples during growing season
Pineapple. Soil treatments shortly before or at planting did not
result in residues in fruit of the planted crop (pre-harvest interval
565-619 days) or of the ratoon crops (<0.02 mg/kg). In the latter
crops the period between application and harvest was more than 900
days. Residues in the tops and leaves of both the planted and the
ratoon crops did not exceed 0.02 mg/kg (at or about the limit of
determination).
Vegetables
Onions. In some of the supervised trials with normal application
rates (6-10 kg a.i./ha) residues above 0.02 mg/kg were found (for
example Hunt et al 1981, Argauer and Feldmesser, 1978). Owing to the
parchment structure and the development of successive coat layers soil
particles are easily entrapped. It has to be recognised that complete
removal of these soil particles is difficult.
Beans, peas. Residues are in general below 0.02 mg/kg on crops
grown in soil treated at normal dosages shortly before or at planting.
Residues exceeded 0.02 mg/kg only in very few cases (Narain, 1980) but
these results can be explained as being due to soil particles with
attached ethoprophos residues, since adhering soil particles were not
removed.
Fruiting vegetables
Cucumbers, gherkins, melons (cantaloupes), tomatoes and cabbage.
Residues at harvest on crops grown in ethoprophos-treated soil at
normal dosages (6-10 kg a.i./ha) did not exceed 0.01 mg/kg. One series
of supervised trials on eggplant showed residues above 0.02 mg/kg
(Argauer and Feldmesser, 1978). There was no apparent explanation for
these results: the samples were washed before analysis to remove
adhering soil.
Lettuce. In most supervised trials, residues at harvest did not
exceed 0.02 mg/kg in lettuce grown on soil treated with dosages up to
13 kg a.i./ha. Some results (Argauer and Feldmesser, 1978) showed
higher residues. These may have been due to soil particles containing
ethoprophos.
Root vegetables
Potatoes. Extensive data were available from supervised trials on
this crop from several countries in Europe and America. With very few
exceptions residues at harvest were of the order of 0.02 mg/kg or
less. Depending on the extent of washing or otherwise removing
adhering soil particles from the samples some soil contamination was
found, giving "residue" levels slightly above 0.02 mg/kg. In general,
however, residues in samples which were thoroughly washed or brushed
to remove adhering soil particles did not exceed 0.02 mg/kg.
Carrots. Residues at harvest were distinctly higher than in other
crops in which the edible part grows in the top soil layer. Carrots
grown in soil following soil application at normal dosages of 6-10 kg
a.i./ha shortly before or at sowing showed residues at harvest of the
order of 0.5-1 mg/kg. In view of these higher residues, the
manufacturer does not recommend the use of ethoprophos for carrots.
Radishes. Residues in radishes at harvest were higher than
0.02 mg/kg in several supervised trials. Entrapment of soil particles
may have been partly responsible.
Turnip roots. Turnips grown in soil treated with normal dosages
(6-10 kg a.i./ha) of ethoprophos showed varying residues. In some
trials residues were 0.02 mg/kg or less, but in several others this
level was exceeded. The highest level found was 0.15 mg/kg, but the
turnip roots in this trial were not brushed or washed to remove
adhering soil particles with ethoprophos residues.
Sugarcane. Sugarcane planted in soil treated at rates up to 15 kg
a.i./ha (the recommended rate is 4-10 kg a.i./ha) did not show
measurable residues at harvest. In an experiment in 1976 all the
intermediate products in the sugar manufacturing process were
analysed. No measurable amounts of ethoprophos (<0.02 mg/kg) were
found in the mixed juice, clarified juice, syrups, raw sugar, molasses
or bagasse. Ethoprophos in the mud could not be determined with the
analytical method used.
Maize
Residues in maize treated immediately before or at sowing did not
exceed 0.02 mg/kg.
Peanuts
Residues in peanuts and peanut hay were in general at or about
the limit of determination of 0.02 mg/kg after soil application before
or at planting.
An extensive series of trials carried out in various regions in
the USA also showed that the level of 0.02 mg/kg was in general not
exceeded if ethoprophos was applied as a spray or drench at or shortly
after pegging.
Tobacco
The residues in tobacco, and in composite samples from leaves
taken at several sampling dates during the growing season were in
general below 0.02 mg/kg.
In an experiment to determine the carry-over in cigarette smoke,
ethoprophos was added to cigarette tobacco. Only about 10% if the
ethoprophos added appeared in the smoke stream. Since a cigarette is
not entirely "consumed" but is in general smoked to a 30 mm butt, only
about 60% of the tobacco is actually burned. The ethoprophos recovered
from the smoke is about 14% of that applied to this amount of tobacco.
The remainder is decomposed and/or volatilized or trapped by the
unburned portion of the tobacco in the butt.
FATE OF RESIDUES
In animals
Dogs were fed for three weeks (3 × 6 days/week) a diet containing
0.025 0.25 and 2.5 ppm ethoprophos. The animals were sacrificed on the
21st day and samples of muscle, kidney, liver and fat immediately
deep-frozen and analysed within a week. No residues (<0.01 mg/kg)
were found in any of the tissues (Hazleton, 1968).
Iqbal et al (1972) studied the metabolism of ethoprophos in
rats and in liver microsomal systems. After oral administration by
gavage of ethyl-14C-and dipropyl-14C-labelled ethoprophos to rats,
chloroform-extractable radioactivity was recovered from urine of rats
treated with the dipropyl-labelled compound. It contained traces of
methyl propyl sulphide, methyl propyl sulphoxide and methyl propyl
sulphone, all products of S-methylation and subsequent oxidation of
the propylthiolate ion released from ethoprophos.
The major water-soluble metabolite isolated from urine, liver
microsomes and the liver supernatant fraction was O-ethyl S-propyl
hydrogen phosphorothioate. The rat urine also contained ethyl
dihydrogen phosphate ethyl dihydrogen phosphate, S-propyl dihydrogen
phosphorothioate and S,S-dipropyl hydrogen phosphorodithioate. (See
Tables 3 and 4).
Rat and rabbit liver supernatant enzymes were able to
O-de-ethylate ethoprophos in the presence of reduced glutathione.
S-ethyl-14C-glutathione was produced when the supernatant preparations
were incubated with ethyl-14C-ethoprophos, indicating that glutathione
acted as an ethyl acceptor.
In view of the metabolites identified in these studies the
pathway of metabolic degradation of ethoprophos shown in Figure 1 can
be proposed.
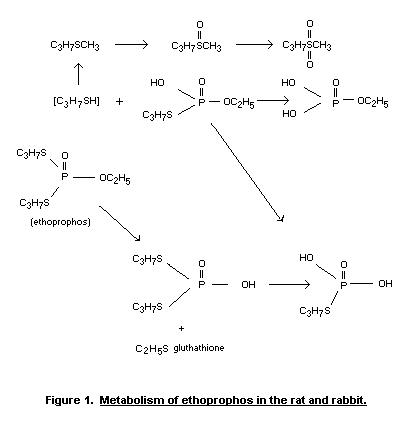 Table 3. Urinary metabolites of S,S-dipropyl-14C-ethoprophos in the rat.
% of administered radoactivity
Male rats Female rats Compounds
0-6 hrs 6-12 hrs 12-24 hrs 0-8 hrs 8-24 hrs
Water fraction
O-ethyl S-propyl
hydrogen phosphorothate 23.06 5.29 5.79 20.79 22.32
S-propyl di-hydrogen
phosphorothioate 7.55 3.15 2.64 3.65 5.88
S,S-dipropyl
hydrogen phosphorodithioate 1.40 1.01 0.97 1.12 0.83
Sum of unknown A,J,K 6.01 2.15 1.00 1.85 0.56
Chloroform extract 0.10 0.10
Methyl propyl sulphide 0.94 0.23 0.18 0.10
Methyl propyl sulphoxide 0.46 0.40 0.73
Methyl propyl sulphone
Total excreted 38.39 12.29 10.77 27.60 30.42
Table 4. Urinary metabolites of O-ethyl-14C-ethoprophos in the rat.
Male rats Female rats
Compounds 0-6 hr 6-12hr 12-24hrs 0-6 hrs 6-12hrs 12-24hrs
O-ethyl S-propyl
hydrogen phosphorothioate 33.71 0.88 0.78 41.54 0.97 0.93
ethyl dihydrogen
phosphate 3.49 0.24 0.12 4.26 0.49 0.21
Sum of unknow A,E,J,K 9.80 0.18 0.07 7.16 0.23 0.05
Chloroform fraction <0.001 <0.001
Total excreted 47.09 1.30 0.96 52.95 1.58 1.19
The abbreviated Tables 3 and 4 indicate the quantitative
distribution of the metabolites found in the urine extracts of rats
(derived from Iqbal and Menzer, 1972).
In plants
Menzer et al (1971) studied the uptake and metabolism of
ethoprophos in bean and maize plants grown on steam-sterilized soil
treated with ethyl-14C-and dipropyl-14C-ethoprophos.
Sterile soil was used to inhibit the biodegradation of the
labelled ethoprophos and to reduce the adsorption capacity of the
soil, so that the maximum amount of ethoprophos would be available for
uptake by the plants. A number of organo-extractable and water-soluble
metabolites formed by dealkylaton of ethoprophos and subsequent
reaction, were detected. Some of these became incorporated into plant
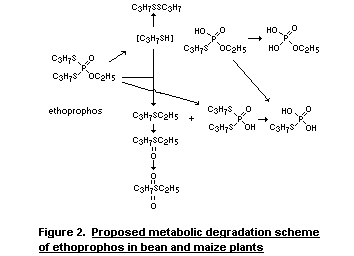
constituents. The proposed degradation pathway of ethoprophos in
plants is shown in Figure 2.
In the proposed metabolic scheme the role of propylthiolate ion
is of critical importance. The S-propyl group has been found to be the
favoured leaving group in a displacement reaction with hydroxide ion.
The propylthiolate ion thus liberated is strongly nucleophilic and can
react with a phosphorothioic ester in an analogous fashion to the
reaction mechanism described by Harvey et al (1963).
[(RO)2P(O)SR'+R'S (RO)(R'S)P(O)O'+ R'SR]
A similar reaction mechanism may be involved in the production of
ethyl propyl sulphide in plants in which it was shown to be an
important metabolite. It was present in significant quantities in
methylene chloride extracts, although it was equivalent to only a very
small percentage of the administered dose.
Kirby and Warren (1967) indicated that the propylthiolate ion
would itself be strongly thiphilic and would be expected to react
further with another thiol thus forming a disulphide. Dipropyl
disulphide was in fact isolated from the methylene chloride extracts
of bean and maize plants grown on dipropyl-14C-ethoprophos-treated
soil.
The fact that O-ethyl S-propyl hydrogen phosphorothioate was the
major component of the methanol-water fraction from maize plants may
also indicate that the propylthiolate ion is formed as an important
metabolic intermediate. In view of its importance and its role in
de-ethylating ethoprophos it is of interest that desethyl-ethoprophos
is not found in plants, although it is present in rat urine (Iqbal and
Menzer, 1972; see Figure 1). Desethyl-ethoprophos was found however to
be rather unstable and it probably loses propyl mercaptan to form
S-propyl dihydrogen phosphorothioate which was found in plants.
Table 3. Urinary metabolites of S,S-dipropyl-14C-ethoprophos in the rat.
% of administered radoactivity
Male rats Female rats Compounds
0-6 hrs 6-12 hrs 12-24 hrs 0-8 hrs 8-24 hrs
Water fraction
O-ethyl S-propyl
hydrogen phosphorothate 23.06 5.29 5.79 20.79 22.32
S-propyl di-hydrogen
phosphorothioate 7.55 3.15 2.64 3.65 5.88
S,S-dipropyl
hydrogen phosphorodithioate 1.40 1.01 0.97 1.12 0.83
Sum of unknown A,J,K 6.01 2.15 1.00 1.85 0.56
Chloroform extract 0.10 0.10
Methyl propyl sulphide 0.94 0.23 0.18 0.10
Methyl propyl sulphoxide 0.46 0.40 0.73
Methyl propyl sulphone
Total excreted 38.39 12.29 10.77 27.60 30.42
Table 4. Urinary metabolites of O-ethyl-14C-ethoprophos in the rat.
Male rats Female rats
Compounds 0-6 hr 6-12hr 12-24hrs 0-6 hrs 6-12hrs 12-24hrs
O-ethyl S-propyl
hydrogen phosphorothioate 33.71 0.88 0.78 41.54 0.97 0.93
ethyl dihydrogen
phosphate 3.49 0.24 0.12 4.26 0.49 0.21
Sum of unknow A,E,J,K 9.80 0.18 0.07 7.16 0.23 0.05
Chloroform fraction <0.001 <0.001
Total excreted 47.09 1.30 0.96 52.95 1.58 1.19
The abbreviated Tables 3 and 4 indicate the quantitative
distribution of the metabolites found in the urine extracts of rats
(derived from Iqbal and Menzer, 1972).
In plants
Menzer et al (1971) studied the uptake and metabolism of
ethoprophos in bean and maize plants grown on steam-sterilized soil
treated with ethyl-14C-and dipropyl-14C-ethoprophos.
Sterile soil was used to inhibit the biodegradation of the
labelled ethoprophos and to reduce the adsorption capacity of the
soil, so that the maximum amount of ethoprophos would be available for
uptake by the plants. A number of organo-extractable and water-soluble
metabolites formed by dealkylaton of ethoprophos and subsequent
reaction, were detected. Some of these became incorporated into plant
constituents. The proposed degradation pathway of ethoprophos in
plants is shown in Figure 2.
In the proposed metabolic scheme the role of propylthiolate ion
is of critical importance. The S-propyl group has been found to be the
favoured leaving group in a displacement reaction with hydroxide ion.
The propylthiolate ion thus liberated is strongly nucleophilic and can
react with a phosphorothioic ester in an analogous fashion to the
reaction mechanism described by Harvey et al (1963).
[(RO)2P(O)SR'+R'S (RO)(R'S)P(O)O'+ R'SR]
A similar reaction mechanism may be involved in the production of
ethyl propyl sulphide in plants in which it was shown to be an
important metabolite. It was present in significant quantities in
methylene chloride extracts, although it was equivalent to only a very
small percentage of the administered dose.
Kirby and Warren (1967) indicated that the propylthiolate ion
would itself be strongly thiphilic and would be expected to react
further with another thiol thus forming a disulphide. Dipropyl
disulphide was in fact isolated from the methylene chloride extracts
of bean and maize plants grown on dipropyl-14C-ethoprophos-treated
soil.
The fact that O-ethyl S-propyl hydrogen phosphorothioate was the
major component of the methanol-water fraction from maize plants may
also indicate that the propylthiolate ion is formed as an important
metabolic intermediate. In view of its importance and its role in
de-ethylating ethoprophos it is of interest that desethyl-ethoprophos
is not found in plants, although it is present in rat urine (Iqbal and
Menzer, 1972; see Figure 1). Desethyl-ethoprophos was found however to
be rather unstable and it probably loses propyl mercaptan to form
S-propyl dihydrogen phosphorothioate which was found in plants.
 The study of ethoprophos in plants has shown that the compound is
rapidly converted to metabolites which are likely to be much less
toxic than the parent compound. Its metabolism in plants is similar to
that in animals, with only the slight differences with regard to the
minor metabolite desethyl-ethoprophos mentioned above.
Although ethoprophos is a phosphorodithioate pesticide, it
differs from most of these compounds in not being a phosphorothionate
and hence not needing oxydation to its oxygen analogue to make it an
inhibitor of cholinesterase.
In soil
Soil samples spiked at 2 mg/kg and analysed after five months
storage in a deep-freeze showed only a slight loss of ethoprophos
(immediate recovery 95.2%; recovery after five months 82.3 and 84.5%).
Soil samples were taken at regular intervals from field plots in
the UK treated at normal dosages for potato cyst nematode control,
11 kg/ha, and at the double rate of 22 kg/ha.
One trial showed higher residues than the others: 22.9 mg/kg
after 92 days and 17.5 mg/kg after 157 days. In other three trials,
with treatment at 11 kg/ha residues declined to 4-10 mg/kg after
100-150 days. From two of these trials an initial half-life between 10
and 17 days could be calculated (see Table 5) (Fisons 1972).
In another study on the dissipation of ethoprophos in soil,
Norris and Garlick (1978) reported that the concentration of
ethoprophos parent compound dropped to less than 10% of the initial
concentration in about three to 20 weeks (half-life 5-35 days),
depending on soil type and climatic conditions. Carbon dioxide was the
predominant, if not the only, volatile metabolite or degradation
product found in the headspace over the soils.
Comparison of ethoprophos dissipation in various non-sterile
soils suggests that soil micro-organisms may have a greater effect on
the pesticide than soil characteristics, such as pH, organic matter
content or texture (Norris and Garlick, 1978). Dissipation in soils
with a high organic matter content is however much slower than in
soils low in (low in organic, not low inorganic) organic matter (Smelt
et al 1977, 1981; see below).
Smelt et al (1977) in The Netherlands evaluated the rates of
degradation and downward movement of ethoprophos under spring and
summer field conditions in four soils in aluminium columns 40 cm long.
A 10% granular formulation was incorporated in the top 10 cm at a rate
of 10-10.5 kg a.i./ha. Under outdoor conditions in the periods
mentioned the half-life was about 87 days in a humic sand and a peaty
sand with pH values of 4.5 and 4.6 respectively. In a sandy loam and a
loam soil with pH values of 7.2 and 7.3 respectively, the half-life
ranged between 14 and 20 days. Under experimental conditions with
Table 5. Residues of ethoprophos in soil (air-dried before analysis)
Ethoprophos, mg/kg, in air-dried soil, after interval of (days)
Dosage, kg a.i./ha 0 3 10 17 24 25 32 86 92 113 120 136 157
22 73.0 83.0 42.2 17.9 29.1 18.6 7.6 8.5
71.3 77.1 45.1 19.2 30.5 16.1 8.4 8.6
11 38.4 31 1 11.0 4.4 5.2
40.7 30.7 10.0 4.3 3.4
11 37.7 24.5 22.9 17.5
11 24.9 16.0 16.3 8.1 9.7
Table 6. Calculated half-life of ethoprophos in three soils at 20°C
and 2°C (Smelt et al 1981)
Soil type pH Organic matter, % Half-life (days)
20°C 2°C
Silt loam 7.0-7.2 1.6 12 89
Sandy loam 7.5-7.8 1.8 16 144
Humic loamy sand 5.8-4.7 6.1 73 347 at
6°C 1
1 The half-life of ethoprophos could not be estimated accurately at
2°C in this soil owing to the very low rate of decline.
fallow soils and 35.3 cm rainfall, the downward movement of
substantial amounts of ethoprophos by leaching and diffusion was
restricted to a few cm.
Smelt et al (1981) evaluated similarly the movement and
conversion of ethoprophos in soil in winter in The Netherlands.
Ethoprophos (10% granular) was mixed into the top 9 cm of soil columns
40 cm long and 8.4 cm in diameter. The percolating water was collected
and at regular intervals layers of the soil column were separated and
analysed by GLC with a flame photometric detector in the phosphorus
mode at 526 nm wavelength. In the winter there was a distinct movement
in the two loamy soils, although ethoprophos was retained in the top
30 cm. In a humic loamy sand soil the mobility was comparatively low
and ethoprophos could not be measured in the percolating water. At the
end of April in the year following ethoprophos application to the soil
columns on October 25th, only about 10-20% of the applied dose was
left in the two loamy soils, whereas about 75 percent still remained
in the humic loamy sand soil.
Substantial conversion of ethoprophos was found to occur in
incubation studied in the laboratory in loamy soils (pH 7.5-7.8,
7.0-7.2 and 4.7-5.0) at temperatures down to 2°C. At 10°C the
conversion rate coefficients (kp) in silt loam, sandy loam and humic
loamy sand were 0.023, 0.0187 and 0.0035 respectively, whereas at 2°C
the conversion coefficients were 0.0078, 0.0048 and about 0.0005
respectively. The conversion of ethoprophos in the humic loamy sand
almost stopped at the lowest temperature, but continued at 2°C in the
two other soils. The following half life times could be calculated for
the three soil types.
In processing
Some data were available on the effects of processing, e.g. of
winegrapes into must and wine, and on residues in products such as
sugarcane and intermediate products during the manufacture of sugar
and tobacco. Results are discussed in the section on supervised
residue trials.
No data were available on the effect of the household or
industrial cooking of vegetables or fruit. Such data may not be
important in the view of the low residues at harvest in most fruits
and vegetables (at or below the limit of determination).
METHODS OF RESIDUE ANALYSIS
Several methods have been developed and made available, which
have been modified and improved with time. Most of the samples from
the supervised trials were analysed by GLC, using a flame photometric
detector in the phosphorus mode. Several clean-up procedures have been
proposed, using column chromatography or other physico-chemical
separation techniques. For commodities with a high fat or vegetable
oil content, additional clean-up steps may be needed. The limit of
determination was 0.005-0.02 mg/kg, in some commodities 0.05 mg/kg. In
some experiments, notably with tobacco, analysis by GLC with a
sulphur-mode detector seemed to be more effective. The limit of
determination was about 0.02 mg/kg.
These methods, especially the GLC methods with flame photometric
detection in the phosphorus mode operating at a wavelength of 536 nm,
are suitable or can be adapted for regulatory purposes. The currently
available multi-detection methods for organophosphorus compounds can
also be used for the determination of ethoprophos.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs, established or under consideration,
were reported to the meeting.
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Australia Bananas 0.05
Sugarcane 0.1
Tomatoes 0.01
Austria Potatoes (washed) 0.02
Belgium(Feb. 1982) Potatoes 0.02
Cereal grains 0.02
Brazil Vegetables No residue (at or
about limit of
determination)
France Vegetables, except carrots 0.01
Bananas 0.01
Fed. Rep. of Germany Bananas, Pineapple, ) 0.02
Fruiting vegetables, )
Soybeans, Potatoes. )
Sweet potatoes, Peanuts, )
Maize )
Italy (Feb. 1979) Fruit and vegetables 0.02
Luxembourg (June 1983) Potatoes 0.02
Netherlands Strawberries 0.01*
(March 1984) Fruiting vegetables 0.01*
Potatoes 0.02
Other food commoditie 0 (0.01*)
USA Bananas )
Pineapple fooder and )
forage )
Cabbage )
Cucumbers )
Fresh corn,including )
sweet corn (kernels )
+ cob, husks removed )
Lima bean, Lima bean )
forage )
Snap bean,snap bean )
forage ) 0.02
Soybean,soybean forage ) (negligible
Soybean hay ) residue)
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Potatoes,sweet potatoes )
Maize (corn) grain, )
fodder and forage )
Sugarcane, Sugarcane )
fodder and forage )
Peanut, Peanut hay )
Yugoslavia All food commodities
0.02
* At or about limit of determination.
APPRAISAL
The meeting reviewed substantial information on the residue
levels of ethoprophos from supervised trials on a variety of
commodities in several countries in Europe, America and some other
regions. Ethoprophos is an organophosphorus pesticide which is mainly
used as a soil application at or shortly before planting or sowing
against soil-borne nematodes and some soil-borne insects.
The compound is formulated as 10% and 20% granules and as an EC.
In contract to other organophosphorus or carbamoyl oxime nematicides,
it has only a contact action with no systemic or fumigant effect. It
has therefore to be worked into the top soil layer in which the pest
organism mainly occurs, i.e. the root zone of the crop to be grown on
the treated soil. Owing to the lack of systemic action the residues in
the aerial parts of crops grown on treated soil are in general very
low. Residues in the edible parts of crops which develop in the top
soil layer may show higher and varying residues depending on how
thoroughly soil particles with adhering ethoprophos residues are
removed by washing, brushing or other cleaning procedures. In some
crops soil particles are easily entrapped and complete removal is
almost impossible.
The meeting evaluated qualitative and quantitative aspects of the
fate and metabolism of ethoprophos residues in plants and animals. The
metabolic pathways in plants and animals are in principle similar, the
only difference being a minor metabolite (desethyl-ethoprophos) found
in the urine of rats but not in plants. The expected breakdown product
of desethyl-ethoprophos is, however, found as a constituent of the
plant residue.
Information on analytical methods for ethoprophos and its
metabolites was provided to the meeting. The current method of choice
for residue analysis is GLC with a flame photometric detector operated
in the phosphorus mode at a wavelength of 536 nm. The limit of
determination is 0.005-0.02 mg/kg, depending on crop type, sample size
and interferences not removed in the clean-up procedure. Additional
clean-up steps may be needed for commodities with a high fat or
vegetable oil content. The methods of residue analysis are suitable or
can be adapted for regulatory purposes.
Extensive information was made available on the current
authorization or registration status of ethoprophos in various
countries and the recommended good agricultural practice. Information
was also made available on established national maximum residue
limits.
RECOMMENDATIONS
The meeting examined residue data from supervised trials
reflecting current good agricultural practices on a number of crops,
but considered that the limited data available on egg plant, lima
beans, common beans (i.e. French, snap or string beans) and radishes
were not adequate to estimate maximum residue levels, especially since
these data originated from only one country and the residue levels
differed considerably from those in similar crops, without any
apparent explanation. No maximum residue levels were estimated for
tobacco since this commodity is not a food.
From the extensive data available the meeting was able to
estimate the maximum residue levels listed below. Since no ADI was
allocated, these were recorded as guideline levels. They refer to
ethoprophos only.
Commodity GL (mg/kg)
fruit crops grapes 0.02*
strawberries 0.02*
bananas 0.02*
pineapple 0.02*
vegetables
bulb vegetales onions 0.02*
brassicas cabbage 0.02*
fruiting
vegetables cucumbers 0.02*
gherkins 0.02*
melons 0.02*
Commodity GL (mg/kg)
peppers 0.02*
tomatoes 0œ02*
leafy vegetables lettuce 0.02*
legume vegetables soybeans 0.02*
peas 0.02*
root vegetables beet root 0.02*
potatoes 0.02*
sweet potatoes 0.02*
turnips 0.02*
stem and stalk
vegetables sugarcane 0.02*
cereal grains maize 0.02*
oilseeds peanuts 0.02*
misc.crops pineapple fodder and forage 0.02*
soybean hay 0.02*
sugarcane fodder and forage 0.02*
maize fodder and forage 0.02*
peanut hay 0.02*
* at or about the limit of determination
REFERENCES
Unpublished residue data provided by Industries, Governmental and non-
Governmental Institutes made available by Rhone-Poulenc; reference
codes Duphar, Fisons, Mobil, Rhone-Poulenc, INRA, CIVD, KUW.
Argauer, R.J. and Feldmesser. J. Uptake of ethoprophos (Mocap) by ten
1978 vegetables grown in soil treated for nematodes. J. Agr. Food
Chem. 26 (1) 42-44.
Harvey, G.R., Jacobson, H.I. and Jensen E.V. Phosphoric acid VI. The
1963 reaction of trivalent phosphorus with organic disulphides.
J. Amer. Chem. Soc. 85: 1618-1623.
Hazleton. Mocap 21-day oral administration to dogs. Project No.
1968 230/124. Report of Hazleton Laboratories, Fall Church,
Virginia, to Mobil Chemical Co. (Unpublished).
Smelt, J.H., Leistra, M. and Voerman, S. Movement and rate of
1977 decomposition of ethoprophos in soil columns under field
conditions. Pestic. Sci. 8 147-151.
Smelt, J.H., Leistra, M., Dekker, A. and Schut, C.J. Movement and
1981 conversion of ethoprophos in soil in winter: measured
concentration patterns and conversion rates. Soil Science
131 (4) 242-248.
The study of ethoprophos in plants has shown that the compound is
rapidly converted to metabolites which are likely to be much less
toxic than the parent compound. Its metabolism in plants is similar to
that in animals, with only the slight differences with regard to the
minor metabolite desethyl-ethoprophos mentioned above.
Although ethoprophos is a phosphorodithioate pesticide, it
differs from most of these compounds in not being a phosphorothionate
and hence not needing oxydation to its oxygen analogue to make it an
inhibitor of cholinesterase.
In soil
Soil samples spiked at 2 mg/kg and analysed after five months
storage in a deep-freeze showed only a slight loss of ethoprophos
(immediate recovery 95.2%; recovery after five months 82.3 and 84.5%).
Soil samples were taken at regular intervals from field plots in
the UK treated at normal dosages for potato cyst nematode control,
11 kg/ha, and at the double rate of 22 kg/ha.
One trial showed higher residues than the others: 22.9 mg/kg
after 92 days and 17.5 mg/kg after 157 days. In other three trials,
with treatment at 11 kg/ha residues declined to 4-10 mg/kg after
100-150 days. From two of these trials an initial half-life between 10
and 17 days could be calculated (see Table 5) (Fisons 1972).
In another study on the dissipation of ethoprophos in soil,
Norris and Garlick (1978) reported that the concentration of
ethoprophos parent compound dropped to less than 10% of the initial
concentration in about three to 20 weeks (half-life 5-35 days),
depending on soil type and climatic conditions. Carbon dioxide was the
predominant, if not the only, volatile metabolite or degradation
product found in the headspace over the soils.
Comparison of ethoprophos dissipation in various non-sterile
soils suggests that soil micro-organisms may have a greater effect on
the pesticide than soil characteristics, such as pH, organic matter
content or texture (Norris and Garlick, 1978). Dissipation in soils
with a high organic matter content is however much slower than in
soils low in (low in organic, not low inorganic) organic matter (Smelt
et al 1977, 1981; see below).
Smelt et al (1977) in The Netherlands evaluated the rates of
degradation and downward movement of ethoprophos under spring and
summer field conditions in four soils in aluminium columns 40 cm long.
A 10% granular formulation was incorporated in the top 10 cm at a rate
of 10-10.5 kg a.i./ha. Under outdoor conditions in the periods
mentioned the half-life was about 87 days in a humic sand and a peaty
sand with pH values of 4.5 and 4.6 respectively. In a sandy loam and a
loam soil with pH values of 7.2 and 7.3 respectively, the half-life
ranged between 14 and 20 days. Under experimental conditions with
Table 5. Residues of ethoprophos in soil (air-dried before analysis)
Ethoprophos, mg/kg, in air-dried soil, after interval of (days)
Dosage, kg a.i./ha 0 3 10 17 24 25 32 86 92 113 120 136 157
22 73.0 83.0 42.2 17.9 29.1 18.6 7.6 8.5
71.3 77.1 45.1 19.2 30.5 16.1 8.4 8.6
11 38.4 31 1 11.0 4.4 5.2
40.7 30.7 10.0 4.3 3.4
11 37.7 24.5 22.9 17.5
11 24.9 16.0 16.3 8.1 9.7
Table 6. Calculated half-life of ethoprophos in three soils at 20°C
and 2°C (Smelt et al 1981)
Soil type pH Organic matter, % Half-life (days)
20°C 2°C
Silt loam 7.0-7.2 1.6 12 89
Sandy loam 7.5-7.8 1.8 16 144
Humic loamy sand 5.8-4.7 6.1 73 347 at
6°C 1
1 The half-life of ethoprophos could not be estimated accurately at
2°C in this soil owing to the very low rate of decline.
fallow soils and 35.3 cm rainfall, the downward movement of
substantial amounts of ethoprophos by leaching and diffusion was
restricted to a few cm.
Smelt et al (1981) evaluated similarly the movement and
conversion of ethoprophos in soil in winter in The Netherlands.
Ethoprophos (10% granular) was mixed into the top 9 cm of soil columns
40 cm long and 8.4 cm in diameter. The percolating water was collected
and at regular intervals layers of the soil column were separated and
analysed by GLC with a flame photometric detector in the phosphorus
mode at 526 nm wavelength. In the winter there was a distinct movement
in the two loamy soils, although ethoprophos was retained in the top
30 cm. In a humic loamy sand soil the mobility was comparatively low
and ethoprophos could not be measured in the percolating water. At the
end of April in the year following ethoprophos application to the soil
columns on October 25th, only about 10-20% of the applied dose was
left in the two loamy soils, whereas about 75 percent still remained
in the humic loamy sand soil.
Substantial conversion of ethoprophos was found to occur in
incubation studied in the laboratory in loamy soils (pH 7.5-7.8,
7.0-7.2 and 4.7-5.0) at temperatures down to 2°C. At 10°C the
conversion rate coefficients (kp) in silt loam, sandy loam and humic
loamy sand were 0.023, 0.0187 and 0.0035 respectively, whereas at 2°C
the conversion coefficients were 0.0078, 0.0048 and about 0.0005
respectively. The conversion of ethoprophos in the humic loamy sand
almost stopped at the lowest temperature, but continued at 2°C in the
two other soils. The following half life times could be calculated for
the three soil types.
In processing
Some data were available on the effects of processing, e.g. of
winegrapes into must and wine, and on residues in products such as
sugarcane and intermediate products during the manufacture of sugar
and tobacco. Results are discussed in the section on supervised
residue trials.
No data were available on the effect of the household or
industrial cooking of vegetables or fruit. Such data may not be
important in the view of the low residues at harvest in most fruits
and vegetables (at or below the limit of determination).
METHODS OF RESIDUE ANALYSIS
Several methods have been developed and made available, which
have been modified and improved with time. Most of the samples from
the supervised trials were analysed by GLC, using a flame photometric
detector in the phosphorus mode. Several clean-up procedures have been
proposed, using column chromatography or other physico-chemical
separation techniques. For commodities with a high fat or vegetable
oil content, additional clean-up steps may be needed. The limit of
determination was 0.005-0.02 mg/kg, in some commodities 0.05 mg/kg. In
some experiments, notably with tobacco, analysis by GLC with a
sulphur-mode detector seemed to be more effective. The limit of
determination was about 0.02 mg/kg.
These methods, especially the GLC methods with flame photometric
detection in the phosphorus mode operating at a wavelength of 536 nm,
are suitable or can be adapted for regulatory purposes. The currently
available multi-detection methods for organophosphorus compounds can
also be used for the determination of ethoprophos.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs, established or under consideration,
were reported to the meeting.
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Australia Bananas 0.05
Sugarcane 0.1
Tomatoes 0.01
Austria Potatoes (washed) 0.02
Belgium(Feb. 1982) Potatoes 0.02
Cereal grains 0.02
Brazil Vegetables No residue (at or
about limit of
determination)
France Vegetables, except carrots 0.01
Bananas 0.01
Fed. Rep. of Germany Bananas, Pineapple, ) 0.02
Fruiting vegetables, )
Soybeans, Potatoes. )
Sweet potatoes, Peanuts, )
Maize )
Italy (Feb. 1979) Fruit and vegetables 0.02
Luxembourg (June 1983) Potatoes 0.02
Netherlands Strawberries 0.01*
(March 1984) Fruiting vegetables 0.01*
Potatoes 0.02
Other food commoditie 0 (0.01*)
USA Bananas )
Pineapple fooder and )
forage )
Cabbage )
Cucumbers )
Fresh corn,including )
sweet corn (kernels )
+ cob, husks removed )
Lima bean, Lima bean )
forage )
Snap bean,snap bean )
forage ) 0.02
Soybean,soybean forage ) (negligible
Soybean hay ) residue)
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Potatoes,sweet potatoes )
Maize (corn) grain, )
fodder and forage )
Sugarcane, Sugarcane )
fodder and forage )
Peanut, Peanut hay )
Yugoslavia All food commodities
0.02
* At or about limit of determination.
APPRAISAL
The meeting reviewed substantial information on the residue
levels of ethoprophos from supervised trials on a variety of
commodities in several countries in Europe, America and some other
regions. Ethoprophos is an organophosphorus pesticide which is mainly
used as a soil application at or shortly before planting or sowing
against soil-borne nematodes and some soil-borne insects.
The compound is formulated as 10% and 20% granules and as an EC.
In contract to other organophosphorus or carbamoyl oxime nematicides,
it has only a contact action with no systemic or fumigant effect. It
has therefore to be worked into the top soil layer in which the pest
organism mainly occurs, i.e. the root zone of the crop to be grown on
the treated soil. Owing to the lack of systemic action the residues in
the aerial parts of crops grown on treated soil are in general very
low. Residues in the edible parts of crops which develop in the top
soil layer may show higher and varying residues depending on how
thoroughly soil particles with adhering ethoprophos residues are
removed by washing, brushing or other cleaning procedures. In some
crops soil particles are easily entrapped and complete removal is
almost impossible.
The meeting evaluated qualitative and quantitative aspects of the
fate and metabolism of ethoprophos residues in plants and animals. The
metabolic pathways in plants and animals are in principle similar, the
only difference being a minor metabolite (desethyl-ethoprophos) found
in the urine of rats but not in plants. The expected breakdown product
of desethyl-ethoprophos is, however, found as a constituent of the
plant residue.
Information on analytical methods for ethoprophos and its
metabolites was provided to the meeting. The current method of choice
for residue analysis is GLC with a flame photometric detector operated
in the phosphorus mode at a wavelength of 536 nm. The limit of
determination is 0.005-0.02 mg/kg, depending on crop type, sample size
and interferences not removed in the clean-up procedure. Additional
clean-up steps may be needed for commodities with a high fat or
vegetable oil content. The methods of residue analysis are suitable or
can be adapted for regulatory purposes.
Extensive information was made available on the current
authorization or registration status of ethoprophos in various
countries and the recommended good agricultural practice. Information
was also made available on established national maximum residue
limits.
RECOMMENDATIONS
The meeting examined residue data from supervised trials
reflecting current good agricultural practices on a number of crops,
but considered that the limited data available on egg plant, lima
beans, common beans (i.e. French, snap or string beans) and radishes
were not adequate to estimate maximum residue levels, especially since
these data originated from only one country and the residue levels
differed considerably from those in similar crops, without any
apparent explanation. No maximum residue levels were estimated for
tobacco since this commodity is not a food.
From the extensive data available the meeting was able to
estimate the maximum residue levels listed below. Since no ADI was
allocated, these were recorded as guideline levels. They refer to
ethoprophos only.
Commodity GL (mg/kg)
fruit crops grapes 0.02*
strawberries 0.02*
bananas 0.02*
pineapple 0.02*
vegetables
bulb vegetales onions 0.02*
brassicas cabbage 0.02*
fruiting
vegetables cucumbers 0.02*
gherkins 0.02*
melons 0.02*
Commodity GL (mg/kg)
peppers 0.02*
tomatoes 0œ02*
leafy vegetables lettuce 0.02*
legume vegetables soybeans 0.02*
peas 0.02*
root vegetables beet root 0.02*
potatoes 0.02*
sweet potatoes 0.02*
turnips 0.02*
stem and stalk
vegetables sugarcane 0.02*
cereal grains maize 0.02*
oilseeds peanuts 0.02*
misc.crops pineapple fodder and forage 0.02*
soybean hay 0.02*
sugarcane fodder and forage 0.02*
maize fodder and forage 0.02*
peanut hay 0.02*
* at or about the limit of determination
REFERENCES
Unpublished residue data provided by Industries, Governmental and non-
Governmental Institutes made available by Rhone-Poulenc; reference
codes Duphar, Fisons, Mobil, Rhone-Poulenc, INRA, CIVD, KUW.
Argauer, R.J. and Feldmesser. J. Uptake of ethoprophos (Mocap) by ten
1978 vegetables grown in soil treated for nematodes. J. Agr. Food
Chem. 26 (1) 42-44.
Harvey, G.R., Jacobson, H.I. and Jensen E.V. Phosphoric acid VI. The
1963 reaction of trivalent phosphorus with organic disulphides.
J. Amer. Chem. Soc. 85: 1618-1623.
Hazleton. Mocap 21-day oral administration to dogs. Project No.
1968 230/124. Report of Hazleton Laboratories, Fall Church,
Virginia, to Mobil Chemical Co. (Unpublished).
Smelt, J.H., Leistra, M. and Voerman, S. Movement and rate of
1977 decomposition of ethoprophos in soil columns under field
conditions. Pestic. Sci. 8 147-151.
Smelt, J.H., Leistra, M., Dekker, A. and Schut, C.J. Movement and
1981 conversion of ethoprophos in soil in winter: measured
concentration patterns and conversion rates. Soil Science
131 (4) 242-248.
 Table 3. Urinary metabolites of S,S-dipropyl-14C-ethoprophos in the rat.
% of administered radoactivity
Male rats Female rats Compounds
0-6 hrs 6-12 hrs 12-24 hrs 0-8 hrs 8-24 hrs
Water fraction
O-ethyl S-propyl
hydrogen phosphorothate 23.06 5.29 5.79 20.79 22.32
S-propyl di-hydrogen
phosphorothioate 7.55 3.15 2.64 3.65 5.88
S,S-dipropyl
hydrogen phosphorodithioate 1.40 1.01 0.97 1.12 0.83
Sum of unknown A,J,K 6.01 2.15 1.00 1.85 0.56
Chloroform extract 0.10 0.10
Methyl propyl sulphide 0.94 0.23 0.18 0.10
Methyl propyl sulphoxide 0.46 0.40 0.73
Methyl propyl sulphone
Total excreted 38.39 12.29 10.77 27.60 30.42
Table 4. Urinary metabolites of O-ethyl-14C-ethoprophos in the rat.
Male rats Female rats
Compounds 0-6 hr 6-12hr 12-24hrs 0-6 hrs 6-12hrs 12-24hrs
O-ethyl S-propyl
hydrogen phosphorothioate 33.71 0.88 0.78 41.54 0.97 0.93
ethyl dihydrogen
phosphate 3.49 0.24 0.12 4.26 0.49 0.21
Sum of unknow A,E,J,K 9.80 0.18 0.07 7.16 0.23 0.05
Chloroform fraction <0.001 <0.001
Total excreted 47.09 1.30 0.96 52.95 1.58 1.19
The abbreviated Tables 3 and 4 indicate the quantitative
distribution of the metabolites found in the urine extracts of rats
(derived from Iqbal and Menzer, 1972).
In plants
Menzer et al (1971) studied the uptake and metabolism of
ethoprophos in bean and maize plants grown on steam-sterilized soil
treated with ethyl-14C-and dipropyl-14C-ethoprophos.
Sterile soil was used to inhibit the biodegradation of the
labelled ethoprophos and to reduce the adsorption capacity of the
soil, so that the maximum amount of ethoprophos would be available for
uptake by the plants. A number of organo-extractable and water-soluble
metabolites formed by dealkylaton of ethoprophos and subsequent
reaction, were detected. Some of these became incorporated into plant
constituents. The proposed degradation pathway of ethoprophos in
plants is shown in Figure 2.
In the proposed metabolic scheme the role of propylthiolate ion
is of critical importance. The S-propyl group has been found to be the
favoured leaving group in a displacement reaction with hydroxide ion.
The propylthiolate ion thus liberated is strongly nucleophilic and can
react with a phosphorothioic ester in an analogous fashion to the
reaction mechanism described by Harvey et al (1963).
[(RO)2P(O)SR'+R'S (RO)(R'S)P(O)O'+ R'SR]
A similar reaction mechanism may be involved in the production of
ethyl propyl sulphide in plants in which it was shown to be an
important metabolite. It was present in significant quantities in
methylene chloride extracts, although it was equivalent to only a very
small percentage of the administered dose.
Kirby and Warren (1967) indicated that the propylthiolate ion
would itself be strongly thiphilic and would be expected to react
further with another thiol thus forming a disulphide. Dipropyl
disulphide was in fact isolated from the methylene chloride extracts
of bean and maize plants grown on dipropyl-14C-ethoprophos-treated
soil.
The fact that O-ethyl S-propyl hydrogen phosphorothioate was the
major component of the methanol-water fraction from maize plants may
also indicate that the propylthiolate ion is formed as an important
metabolic intermediate. In view of its importance and its role in
de-ethylating ethoprophos it is of interest that desethyl-ethoprophos
is not found in plants, although it is present in rat urine (Iqbal and
Menzer, 1972; see Figure 1). Desethyl-ethoprophos was found however to
be rather unstable and it probably loses propyl mercaptan to form
S-propyl dihydrogen phosphorothioate which was found in plants.
Table 3. Urinary metabolites of S,S-dipropyl-14C-ethoprophos in the rat.
% of administered radoactivity
Male rats Female rats Compounds
0-6 hrs 6-12 hrs 12-24 hrs 0-8 hrs 8-24 hrs
Water fraction
O-ethyl S-propyl
hydrogen phosphorothate 23.06 5.29 5.79 20.79 22.32
S-propyl di-hydrogen
phosphorothioate 7.55 3.15 2.64 3.65 5.88
S,S-dipropyl
hydrogen phosphorodithioate 1.40 1.01 0.97 1.12 0.83
Sum of unknown A,J,K 6.01 2.15 1.00 1.85 0.56
Chloroform extract 0.10 0.10
Methyl propyl sulphide 0.94 0.23 0.18 0.10
Methyl propyl sulphoxide 0.46 0.40 0.73
Methyl propyl sulphone
Total excreted 38.39 12.29 10.77 27.60 30.42
Table 4. Urinary metabolites of O-ethyl-14C-ethoprophos in the rat.
Male rats Female rats
Compounds 0-6 hr 6-12hr 12-24hrs 0-6 hrs 6-12hrs 12-24hrs
O-ethyl S-propyl
hydrogen phosphorothioate 33.71 0.88 0.78 41.54 0.97 0.93
ethyl dihydrogen
phosphate 3.49 0.24 0.12 4.26 0.49 0.21
Sum of unknow A,E,J,K 9.80 0.18 0.07 7.16 0.23 0.05
Chloroform fraction <0.001 <0.001
Total excreted 47.09 1.30 0.96 52.95 1.58 1.19
The abbreviated Tables 3 and 4 indicate the quantitative
distribution of the metabolites found in the urine extracts of rats
(derived from Iqbal and Menzer, 1972).
In plants
Menzer et al (1971) studied the uptake and metabolism of
ethoprophos in bean and maize plants grown on steam-sterilized soil
treated with ethyl-14C-and dipropyl-14C-ethoprophos.
Sterile soil was used to inhibit the biodegradation of the
labelled ethoprophos and to reduce the adsorption capacity of the
soil, so that the maximum amount of ethoprophos would be available for
uptake by the plants. A number of organo-extractable and water-soluble
metabolites formed by dealkylaton of ethoprophos and subsequent
reaction, were detected. Some of these became incorporated into plant
constituents. The proposed degradation pathway of ethoprophos in
plants is shown in Figure 2.
In the proposed metabolic scheme the role of propylthiolate ion
is of critical importance. The S-propyl group has been found to be the
favoured leaving group in a displacement reaction with hydroxide ion.
The propylthiolate ion thus liberated is strongly nucleophilic and can
react with a phosphorothioic ester in an analogous fashion to the
reaction mechanism described by Harvey et al (1963).
[(RO)2P(O)SR'+R'S (RO)(R'S)P(O)O'+ R'SR]
A similar reaction mechanism may be involved in the production of
ethyl propyl sulphide in plants in which it was shown to be an
important metabolite. It was present in significant quantities in
methylene chloride extracts, although it was equivalent to only a very
small percentage of the administered dose.
Kirby and Warren (1967) indicated that the propylthiolate ion
would itself be strongly thiphilic and would be expected to react
further with another thiol thus forming a disulphide. Dipropyl
disulphide was in fact isolated from the methylene chloride extracts
of bean and maize plants grown on dipropyl-14C-ethoprophos-treated
soil.
The fact that O-ethyl S-propyl hydrogen phosphorothioate was the
major component of the methanol-water fraction from maize plants may
also indicate that the propylthiolate ion is formed as an important
metabolic intermediate. In view of its importance and its role in
de-ethylating ethoprophos it is of interest that desethyl-ethoprophos
is not found in plants, although it is present in rat urine (Iqbal and
Menzer, 1972; see Figure 1). Desethyl-ethoprophos was found however to
be rather unstable and it probably loses propyl mercaptan to form
S-propyl dihydrogen phosphorothioate which was found in plants.
 The study of ethoprophos in plants has shown that the compound is
rapidly converted to metabolites which are likely to be much less
toxic than the parent compound. Its metabolism in plants is similar to
that in animals, with only the slight differences with regard to the
minor metabolite desethyl-ethoprophos mentioned above.
Although ethoprophos is a phosphorodithioate pesticide, it
differs from most of these compounds in not being a phosphorothionate
and hence not needing oxydation to its oxygen analogue to make it an
inhibitor of cholinesterase.
In soil
Soil samples spiked at 2 mg/kg and analysed after five months
storage in a deep-freeze showed only a slight loss of ethoprophos
(immediate recovery 95.2%; recovery after five months 82.3 and 84.5%).
Soil samples were taken at regular intervals from field plots in
the UK treated at normal dosages for potato cyst nematode control,
11 kg/ha, and at the double rate of 22 kg/ha.
One trial showed higher residues than the others: 22.9 mg/kg
after 92 days and 17.5 mg/kg after 157 days. In other three trials,
with treatment at 11 kg/ha residues declined to 4-10 mg/kg after
100-150 days. From two of these trials an initial half-life between 10
and 17 days could be calculated (see Table 5) (Fisons 1972).
In another study on the dissipation of ethoprophos in soil,
Norris and Garlick (1978) reported that the concentration of
ethoprophos parent compound dropped to less than 10% of the initial
concentration in about three to 20 weeks (half-life 5-35 days),
depending on soil type and climatic conditions. Carbon dioxide was the
predominant, if not the only, volatile metabolite or degradation
product found in the headspace over the soils.
Comparison of ethoprophos dissipation in various non-sterile
soils suggests that soil micro-organisms may have a greater effect on
the pesticide than soil characteristics, such as pH, organic matter
content or texture (Norris and Garlick, 1978). Dissipation in soils
with a high organic matter content is however much slower than in
soils low in (low in organic, not low inorganic) organic matter (Smelt
et al 1977, 1981; see below).
Smelt et al (1977) in The Netherlands evaluated the rates of
degradation and downward movement of ethoprophos under spring and
summer field conditions in four soils in aluminium columns 40 cm long.
A 10% granular formulation was incorporated in the top 10 cm at a rate
of 10-10.5 kg a.i./ha. Under outdoor conditions in the periods
mentioned the half-life was about 87 days in a humic sand and a peaty
sand with pH values of 4.5 and 4.6 respectively. In a sandy loam and a
loam soil with pH values of 7.2 and 7.3 respectively, the half-life
ranged between 14 and 20 days. Under experimental conditions with
Table 5. Residues of ethoprophos in soil (air-dried before analysis)
Ethoprophos, mg/kg, in air-dried soil, after interval of (days)
Dosage, kg a.i./ha 0 3 10 17 24 25 32 86 92 113 120 136 157
22 73.0 83.0 42.2 17.9 29.1 18.6 7.6 8.5
71.3 77.1 45.1 19.2 30.5 16.1 8.4 8.6
11 38.4 31 1 11.0 4.4 5.2
40.7 30.7 10.0 4.3 3.4
11 37.7 24.5 22.9 17.5
11 24.9 16.0 16.3 8.1 9.7
Table 6. Calculated half-life of ethoprophos in three soils at 20°C
and 2°C (Smelt et al 1981)
Soil type pH Organic matter, % Half-life (days)
20°C 2°C
Silt loam 7.0-7.2 1.6 12 89
Sandy loam 7.5-7.8 1.8 16 144
Humic loamy sand 5.8-4.7 6.1 73 347 at
6°C 1
1 The half-life of ethoprophos could not be estimated accurately at
2°C in this soil owing to the very low rate of decline.
fallow soils and 35.3 cm rainfall, the downward movement of
substantial amounts of ethoprophos by leaching and diffusion was
restricted to a few cm.
Smelt et al (1981) evaluated similarly the movement and
conversion of ethoprophos in soil in winter in The Netherlands.
Ethoprophos (10% granular) was mixed into the top 9 cm of soil columns
40 cm long and 8.4 cm in diameter. The percolating water was collected
and at regular intervals layers of the soil column were separated and
analysed by GLC with a flame photometric detector in the phosphorus
mode at 526 nm wavelength. In the winter there was a distinct movement
in the two loamy soils, although ethoprophos was retained in the top
30 cm. In a humic loamy sand soil the mobility was comparatively low
and ethoprophos could not be measured in the percolating water. At the
end of April in the year following ethoprophos application to the soil
columns on October 25th, only about 10-20% of the applied dose was
left in the two loamy soils, whereas about 75 percent still remained
in the humic loamy sand soil.
Substantial conversion of ethoprophos was found to occur in
incubation studied in the laboratory in loamy soils (pH 7.5-7.8,
7.0-7.2 and 4.7-5.0) at temperatures down to 2°C. At 10°C the
conversion rate coefficients (kp) in silt loam, sandy loam and humic
loamy sand were 0.023, 0.0187 and 0.0035 respectively, whereas at 2°C
the conversion coefficients were 0.0078, 0.0048 and about 0.0005
respectively. The conversion of ethoprophos in the humic loamy sand
almost stopped at the lowest temperature, but continued at 2°C in the
two other soils. The following half life times could be calculated for
the three soil types.
In processing
Some data were available on the effects of processing, e.g. of
winegrapes into must and wine, and on residues in products such as
sugarcane and intermediate products during the manufacture of sugar
and tobacco. Results are discussed in the section on supervised
residue trials.
No data were available on the effect of the household or
industrial cooking of vegetables or fruit. Such data may not be
important in the view of the low residues at harvest in most fruits
and vegetables (at or below the limit of determination).
METHODS OF RESIDUE ANALYSIS
Several methods have been developed and made available, which
have been modified and improved with time. Most of the samples from
the supervised trials were analysed by GLC, using a flame photometric
detector in the phosphorus mode. Several clean-up procedures have been
proposed, using column chromatography or other physico-chemical
separation techniques. For commodities with a high fat or vegetable
oil content, additional clean-up steps may be needed. The limit of
determination was 0.005-0.02 mg/kg, in some commodities 0.05 mg/kg. In
some experiments, notably with tobacco, analysis by GLC with a
sulphur-mode detector seemed to be more effective. The limit of
determination was about 0.02 mg/kg.
These methods, especially the GLC methods with flame photometric
detection in the phosphorus mode operating at a wavelength of 536 nm,
are suitable or can be adapted for regulatory purposes. The currently
available multi-detection methods for organophosphorus compounds can
also be used for the determination of ethoprophos.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs, established or under consideration,
were reported to the meeting.
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Australia Bananas 0.05
Sugarcane 0.1
Tomatoes 0.01
Austria Potatoes (washed) 0.02
Belgium(Feb. 1982) Potatoes 0.02
Cereal grains 0.02
Brazil Vegetables No residue (at or
about limit of
determination)
France Vegetables, except carrots 0.01
Bananas 0.01
Fed. Rep. of Germany Bananas, Pineapple, ) 0.02
Fruiting vegetables, )
Soybeans, Potatoes. )
Sweet potatoes, Peanuts, )
Maize )
Italy (Feb. 1979) Fruit and vegetables 0.02
Luxembourg (June 1983) Potatoes 0.02
Netherlands Strawberries 0.01*
(March 1984) Fruiting vegetables 0.01*
Potatoes 0.02
Other food commoditie 0 (0.01*)
USA Bananas )
Pineapple fooder and )
forage )
Cabbage )
Cucumbers )
Fresh corn,including )
sweet corn (kernels )
+ cob, husks removed )
Lima bean, Lima bean )
forage )
Snap bean,snap bean )
forage ) 0.02
Soybean,soybean forage ) (negligible
Soybean hay ) residue)
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Potatoes,sweet potatoes )
Maize (corn) grain, )
fodder and forage )
Sugarcane, Sugarcane )
fodder and forage )
Peanut, Peanut hay )
Yugoslavia All food commodities
0.02
* At or about limit of determination.
APPRAISAL
The meeting reviewed substantial information on the residue
levels of ethoprophos from supervised trials on a variety of
commodities in several countries in Europe, America and some other
regions. Ethoprophos is an organophosphorus pesticide which is mainly
used as a soil application at or shortly before planting or sowing
against soil-borne nematodes and some soil-borne insects.
The compound is formulated as 10% and 20% granules and as an EC.
In contract to other organophosphorus or carbamoyl oxime nematicides,
it has only a contact action with no systemic or fumigant effect. It
has therefore to be worked into the top soil layer in which the pest
organism mainly occurs, i.e. the root zone of the crop to be grown on
the treated soil. Owing to the lack of systemic action the residues in
the aerial parts of crops grown on treated soil are in general very
low. Residues in the edible parts of crops which develop in the top
soil layer may show higher and varying residues depending on how
thoroughly soil particles with adhering ethoprophos residues are
removed by washing, brushing or other cleaning procedures. In some
crops soil particles are easily entrapped and complete removal is
almost impossible.
The meeting evaluated qualitative and quantitative aspects of the
fate and metabolism of ethoprophos residues in plants and animals. The
metabolic pathways in plants and animals are in principle similar, the
only difference being a minor metabolite (desethyl-ethoprophos) found
in the urine of rats but not in plants. The expected breakdown product
of desethyl-ethoprophos is, however, found as a constituent of the
plant residue.
Information on analytical methods for ethoprophos and its
metabolites was provided to the meeting. The current method of choice
for residue analysis is GLC with a flame photometric detector operated
in the phosphorus mode at a wavelength of 536 nm. The limit of
determination is 0.005-0.02 mg/kg, depending on crop type, sample size
and interferences not removed in the clean-up procedure. Additional
clean-up steps may be needed for commodities with a high fat or
vegetable oil content. The methods of residue analysis are suitable or
can be adapted for regulatory purposes.
Extensive information was made available on the current
authorization or registration status of ethoprophos in various
countries and the recommended good agricultural practice. Information
was also made available on established national maximum residue
limits.
RECOMMENDATIONS
The meeting examined residue data from supervised trials
reflecting current good agricultural practices on a number of crops,
but considered that the limited data available on egg plant, lima
beans, common beans (i.e. French, snap or string beans) and radishes
were not adequate to estimate maximum residue levels, especially since
these data originated from only one country and the residue levels
differed considerably from those in similar crops, without any
apparent explanation. No maximum residue levels were estimated for
tobacco since this commodity is not a food.
From the extensive data available the meeting was able to
estimate the maximum residue levels listed below. Since no ADI was
allocated, these were recorded as guideline levels. They refer to
ethoprophos only.
Commodity GL (mg/kg)
fruit crops grapes 0.02*
strawberries 0.02*
bananas 0.02*
pineapple 0.02*
vegetables
bulb vegetales onions 0.02*
brassicas cabbage 0.02*
fruiting
vegetables cucumbers 0.02*
gherkins 0.02*
melons 0.02*
Commodity GL (mg/kg)
peppers 0.02*
tomatoes 0œ02*
leafy vegetables lettuce 0.02*
legume vegetables soybeans 0.02*
peas 0.02*
root vegetables beet root 0.02*
potatoes 0.02*
sweet potatoes 0.02*
turnips 0.02*
stem and stalk
vegetables sugarcane 0.02*
cereal grains maize 0.02*
oilseeds peanuts 0.02*
misc.crops pineapple fodder and forage 0.02*
soybean hay 0.02*
sugarcane fodder and forage 0.02*
maize fodder and forage 0.02*
peanut hay 0.02*
* at or about the limit of determination
REFERENCES
Unpublished residue data provided by Industries, Governmental and non-
Governmental Institutes made available by Rhone-Poulenc; reference
codes Duphar, Fisons, Mobil, Rhone-Poulenc, INRA, CIVD, KUW.
Argauer, R.J. and Feldmesser. J. Uptake of ethoprophos (Mocap) by ten
1978 vegetables grown in soil treated for nematodes. J. Agr. Food
Chem. 26 (1) 42-44.
Harvey, G.R., Jacobson, H.I. and Jensen E.V. Phosphoric acid VI. The
1963 reaction of trivalent phosphorus with organic disulphides.
J. Amer. Chem. Soc. 85: 1618-1623.
Hazleton. Mocap 21-day oral administration to dogs. Project No.
1968 230/124. Report of Hazleton Laboratories, Fall Church,
Virginia, to Mobil Chemical Co. (Unpublished).
Smelt, J.H., Leistra, M. and Voerman, S. Movement and rate of
1977 decomposition of ethoprophos in soil columns under field
conditions. Pestic. Sci. 8 147-151.
Smelt, J.H., Leistra, M., Dekker, A. and Schut, C.J. Movement and
1981 conversion of ethoprophos in soil in winter: measured
concentration patterns and conversion rates. Soil Science
131 (4) 242-248.
The study of ethoprophos in plants has shown that the compound is
rapidly converted to metabolites which are likely to be much less
toxic than the parent compound. Its metabolism in plants is similar to
that in animals, with only the slight differences with regard to the
minor metabolite desethyl-ethoprophos mentioned above.
Although ethoprophos is a phosphorodithioate pesticide, it
differs from most of these compounds in not being a phosphorothionate
and hence not needing oxydation to its oxygen analogue to make it an
inhibitor of cholinesterase.
In soil
Soil samples spiked at 2 mg/kg and analysed after five months
storage in a deep-freeze showed only a slight loss of ethoprophos
(immediate recovery 95.2%; recovery after five months 82.3 and 84.5%).
Soil samples were taken at regular intervals from field plots in
the UK treated at normal dosages for potato cyst nematode control,
11 kg/ha, and at the double rate of 22 kg/ha.
One trial showed higher residues than the others: 22.9 mg/kg
after 92 days and 17.5 mg/kg after 157 days. In other three trials,
with treatment at 11 kg/ha residues declined to 4-10 mg/kg after
100-150 days. From two of these trials an initial half-life between 10
and 17 days could be calculated (see Table 5) (Fisons 1972).
In another study on the dissipation of ethoprophos in soil,
Norris and Garlick (1978) reported that the concentration of
ethoprophos parent compound dropped to less than 10% of the initial
concentration in about three to 20 weeks (half-life 5-35 days),
depending on soil type and climatic conditions. Carbon dioxide was the
predominant, if not the only, volatile metabolite or degradation
product found in the headspace over the soils.
Comparison of ethoprophos dissipation in various non-sterile
soils suggests that soil micro-organisms may have a greater effect on
the pesticide than soil characteristics, such as pH, organic matter
content or texture (Norris and Garlick, 1978). Dissipation in soils
with a high organic matter content is however much slower than in
soils low in (low in organic, not low inorganic) organic matter (Smelt
et al 1977, 1981; see below).
Smelt et al (1977) in The Netherlands evaluated the rates of
degradation and downward movement of ethoprophos under spring and
summer field conditions in four soils in aluminium columns 40 cm long.
A 10% granular formulation was incorporated in the top 10 cm at a rate
of 10-10.5 kg a.i./ha. Under outdoor conditions in the periods
mentioned the half-life was about 87 days in a humic sand and a peaty
sand with pH values of 4.5 and 4.6 respectively. In a sandy loam and a
loam soil with pH values of 7.2 and 7.3 respectively, the half-life
ranged between 14 and 20 days. Under experimental conditions with
Table 5. Residues of ethoprophos in soil (air-dried before analysis)
Ethoprophos, mg/kg, in air-dried soil, after interval of (days)
Dosage, kg a.i./ha 0 3 10 17 24 25 32 86 92 113 120 136 157
22 73.0 83.0 42.2 17.9 29.1 18.6 7.6 8.5
71.3 77.1 45.1 19.2 30.5 16.1 8.4 8.6
11 38.4 31 1 11.0 4.4 5.2
40.7 30.7 10.0 4.3 3.4
11 37.7 24.5 22.9 17.5
11 24.9 16.0 16.3 8.1 9.7
Table 6. Calculated half-life of ethoprophos in three soils at 20°C
and 2°C (Smelt et al 1981)
Soil type pH Organic matter, % Half-life (days)
20°C 2°C
Silt loam 7.0-7.2 1.6 12 89
Sandy loam 7.5-7.8 1.8 16 144
Humic loamy sand 5.8-4.7 6.1 73 347 at
6°C 1
1 The half-life of ethoprophos could not be estimated accurately at
2°C in this soil owing to the very low rate of decline.
fallow soils and 35.3 cm rainfall, the downward movement of
substantial amounts of ethoprophos by leaching and diffusion was
restricted to a few cm.
Smelt et al (1981) evaluated similarly the movement and
conversion of ethoprophos in soil in winter in The Netherlands.
Ethoprophos (10% granular) was mixed into the top 9 cm of soil columns
40 cm long and 8.4 cm in diameter. The percolating water was collected
and at regular intervals layers of the soil column were separated and
analysed by GLC with a flame photometric detector in the phosphorus
mode at 526 nm wavelength. In the winter there was a distinct movement
in the two loamy soils, although ethoprophos was retained in the top
30 cm. In a humic loamy sand soil the mobility was comparatively low
and ethoprophos could not be measured in the percolating water. At the
end of April in the year following ethoprophos application to the soil
columns on October 25th, only about 10-20% of the applied dose was
left in the two loamy soils, whereas about 75 percent still remained
in the humic loamy sand soil.
Substantial conversion of ethoprophos was found to occur in
incubation studied in the laboratory in loamy soils (pH 7.5-7.8,
7.0-7.2 and 4.7-5.0) at temperatures down to 2°C. At 10°C the
conversion rate coefficients (kp) in silt loam, sandy loam and humic
loamy sand were 0.023, 0.0187 and 0.0035 respectively, whereas at 2°C
the conversion coefficients were 0.0078, 0.0048 and about 0.0005
respectively. The conversion of ethoprophos in the humic loamy sand
almost stopped at the lowest temperature, but continued at 2°C in the
two other soils. The following half life times could be calculated for
the three soil types.
In processing
Some data were available on the effects of processing, e.g. of
winegrapes into must and wine, and on residues in products such as
sugarcane and intermediate products during the manufacture of sugar
and tobacco. Results are discussed in the section on supervised
residue trials.
No data were available on the effect of the household or
industrial cooking of vegetables or fruit. Such data may not be
important in the view of the low residues at harvest in most fruits
and vegetables (at or below the limit of determination).
METHODS OF RESIDUE ANALYSIS
Several methods have been developed and made available, which
have been modified and improved with time. Most of the samples from
the supervised trials were analysed by GLC, using a flame photometric
detector in the phosphorus mode. Several clean-up procedures have been
proposed, using column chromatography or other physico-chemical
separation techniques. For commodities with a high fat or vegetable
oil content, additional clean-up steps may be needed. The limit of
determination was 0.005-0.02 mg/kg, in some commodities 0.05 mg/kg. In
some experiments, notably with tobacco, analysis by GLC with a
sulphur-mode detector seemed to be more effective. The limit of
determination was about 0.02 mg/kg.
These methods, especially the GLC methods with flame photometric
detection in the phosphorus mode operating at a wavelength of 536 nm,
are suitable or can be adapted for regulatory purposes. The currently
available multi-detection methods for organophosphorus compounds can
also be used for the determination of ethoprophos.
NATIONAL MAXIMUM RESIDUE LIMITS
The following national MRLs, established or under consideration,
were reported to the meeting.
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Australia Bananas 0.05
Sugarcane 0.1
Tomatoes 0.01
Austria Potatoes (washed) 0.02
Belgium(Feb. 1982) Potatoes 0.02
Cereal grains 0.02
Brazil Vegetables No residue (at or
about limit of
determination)
France Vegetables, except carrots 0.01
Bananas 0.01
Fed. Rep. of Germany Bananas, Pineapple, ) 0.02
Fruiting vegetables, )
Soybeans, Potatoes. )
Sweet potatoes, Peanuts, )
Maize )
Italy (Feb. 1979) Fruit and vegetables 0.02
Luxembourg (June 1983) Potatoes 0.02
Netherlands Strawberries 0.01*
(March 1984) Fruiting vegetables 0.01*
Potatoes 0.02
Other food commoditie 0 (0.01*)
USA Bananas )
Pineapple fooder and )
forage )
Cabbage )
Cucumbers )
Fresh corn,including )
sweet corn (kernels )
+ cob, husks removed )
Lima bean, Lima bean )
forage )
Snap bean,snap bean )
forage ) 0.02
Soybean,soybean forage ) (negligible
Soybean hay ) residue)
Country Commodity MRL, mg/kg
(date of establishment
of MRL)
Potatoes,sweet potatoes )
Maize (corn) grain, )
fodder and forage )
Sugarcane, Sugarcane )
fodder and forage )
Peanut, Peanut hay )
Yugoslavia All food commodities
0.02
* At or about limit of determination.
APPRAISAL
The meeting reviewed substantial information on the residue
levels of ethoprophos from supervised trials on a variety of
commodities in several countries in Europe, America and some other
regions. Ethoprophos is an organophosphorus pesticide which is mainly
used as a soil application at or shortly before planting or sowing
against soil-borne nematodes and some soil-borne insects.
The compound is formulated as 10% and 20% granules and as an EC.
In contract to other organophosphorus or carbamoyl oxime nematicides,
it has only a contact action with no systemic or fumigant effect. It
has therefore to be worked into the top soil layer in which the pest
organism mainly occurs, i.e. the root zone of the crop to be grown on
the treated soil. Owing to the lack of systemic action the residues in
the aerial parts of crops grown on treated soil are in general very
low. Residues in the edible parts of crops which develop in the top
soil layer may show higher and varying residues depending on how
thoroughly soil particles with adhering ethoprophos residues are
removed by washing, brushing or other cleaning procedures. In some
crops soil particles are easily entrapped and complete removal is
almost impossible.
The meeting evaluated qualitative and quantitative aspects of the
fate and metabolism of ethoprophos residues in plants and animals. The
metabolic pathways in plants and animals are in principle similar, the
only difference being a minor metabolite (desethyl-ethoprophos) found
in the urine of rats but not in plants. The expected breakdown product
of desethyl-ethoprophos is, however, found as a constituent of the
plant residue.
Information on analytical methods for ethoprophos and its
metabolites was provided to the meeting. The current method of choice
for residue analysis is GLC with a flame photometric detector operated
in the phosphorus mode at a wavelength of 536 nm. The limit of
determination is 0.005-0.02 mg/kg, depending on crop type, sample size
and interferences not removed in the clean-up procedure. Additional
clean-up steps may be needed for commodities with a high fat or
vegetable oil content. The methods of residue analysis are suitable or
can be adapted for regulatory purposes.
Extensive information was made available on the current
authorization or registration status of ethoprophos in various
countries and the recommended good agricultural practice. Information
was also made available on established national maximum residue
limits.
RECOMMENDATIONS
The meeting examined residue data from supervised trials
reflecting current good agricultural practices on a number of crops,
but considered that the limited data available on egg plant, lima
beans, common beans (i.e. French, snap or string beans) and radishes
were not adequate to estimate maximum residue levels, especially since
these data originated from only one country and the residue levels
differed considerably from those in similar crops, without any
apparent explanation. No maximum residue levels were estimated for
tobacco since this commodity is not a food.
From the extensive data available the meeting was able to
estimate the maximum residue levels listed below. Since no ADI was
allocated, these were recorded as guideline levels. They refer to
ethoprophos only.
Commodity GL (mg/kg)
fruit crops grapes 0.02*
strawberries 0.02*
bananas 0.02*
pineapple 0.02*
vegetables
bulb vegetales onions 0.02*
brassicas cabbage 0.02*
fruiting
vegetables cucumbers 0.02*
gherkins 0.02*
melons 0.02*
Commodity GL (mg/kg)
peppers 0.02*
tomatoes 0œ02*
leafy vegetables lettuce 0.02*
legume vegetables soybeans 0.02*
peas 0.02*
root vegetables beet root 0.02*
potatoes 0.02*
sweet potatoes 0.02*
turnips 0.02*
stem and stalk
vegetables sugarcane 0.02*
cereal grains maize 0.02*
oilseeds peanuts 0.02*
misc.crops pineapple fodder and forage 0.02*
soybean hay 0.02*
sugarcane fodder and forage 0.02*
maize fodder and forage 0.02*
peanut hay 0.02*
* at or about the limit of determination
REFERENCES
Unpublished residue data provided by Industries, Governmental and non-
Governmental Institutes made available by Rhone-Poulenc; reference
codes Duphar, Fisons, Mobil, Rhone-Poulenc, INRA, CIVD, KUW.
Argauer, R.J. and Feldmesser. J. Uptake of ethoprophos (Mocap) by ten
1978 vegetables grown in soil treated for nematodes. J. Agr. Food
Chem. 26 (1) 42-44.
Harvey, G.R., Jacobson, H.I. and Jensen E.V. Phosphoric acid VI. The
1963 reaction of trivalent phosphorus with organic disulphides.
J. Amer. Chem. Soc. 85: 1618-1623.
Hazleton. Mocap 21-day oral administration to dogs. Project No.
1968 230/124. Report of Hazleton Laboratories, Fall Church,
Virginia, to Mobil Chemical Co. (Unpublished).
Smelt, J.H., Leistra, M. and Voerman, S. Movement and rate of
1977 decomposition of ethoprophos in soil columns under field
conditions. Pestic. Sci. 8 147-151.
Smelt, J.H., Leistra, M., Dekker, A. and Schut, C.J. Movement and
1981 conversion of ethoprophos in soil in winter: measured
concentration patterns and conversion rates. Soil Science
131 (4) 242-248.
