
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
ETHOPROPHOS
P.H. van Hoeven-Arentzen and J.G.M. van Engelen
Centre For Substances And Risk Assessment
National Institute of Public Health and the Environment,
Bilthoven, The Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: neurotoxicity
Studies on metabolites
Acute toxicity
Cholinesterase inhibition
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Ethoprophos was evaluated for toxicological effects by the Joint
Meeting in 1983 and 1987 (Annex 1, references 40 and 50). An ADI
of 0-0.0003 mg/kg bw was established in 1987 on the basis of a NOAEL
of 0.025 mg/kg bw per day in a 1-year study in dogs. Since that time,
several studies have been conducted on the metabolism, acute,
short-term and long-term toxicity, reproductive and developmental
toxicity, neurotoxicity and mutagenicity of ethoprophos. The compound
was evaluated at the present meeting within the periodic review
programme of the Codex Committee on Pesticide Residues.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
In a study conducted according to GLP with a statement of QA,
groups of five male and five female Crl:CD(SD)BR rats were given
[ethyl-1-14C]ethoprophos (radiochemical purity, 97%) by gavage in a
0.9% solution of sodium chloride or intravenously in a solution of 35%
ethanol in 0.9% sodium chloride. The animals received a single oral or
intravenous dose of 4 mg/kg bw, an oral dose of 12.5 mg/kg bw, or an
oral (pulse) dose of 4 mg/kg bw after 14 daily doses of 4 mg/kg bw of
unlabelled ethoprophos (purity, 99.4%). Samples of urine and faeces,
cage debris, and cage washings were taken for analysis at 6, 12, 24,
48, 72, 96, 120, 144, and 168 h, at which time the animals were killed
for analyses of radiolabel in various tissues. Expired air was
captured in a trapping system at 6, 12, 18, 24, 36, 48, 60, 72, 96,
120, 144, and 168 h. An additional group of five animals of each sex
received a single oral dose of 4 mg/kg bw, a group of five males
received a single oral dose of 25 mg/kg bw, and a group of five
females received a dose of 12.5 mg/kg bw. (The original dose was 25
mg/kg bw but was lowered to 12.5 mg/kg bw because of severe toxic
effects.) Blood samples were taken from the lateral tail vein of these
additional animals, before treatment, at 0.5, 1, 2, 3, 4, 6, 12, and
24 h, and at subsequent 24-h intervals until 168 h for determination
of radiolabel and pharmacokinetic parameters.
No abnormal clinical findings were noted after intravenous
injection or after single or multiple oral administration of the low
dose. Clinical signs were observed during the first few hours after
oral dosing with the high dose, including teeth grinding, dyspnoea,
poor posture, and hypoactivity in males and teeth grinding, severe
tremors, and excessive salivation in females. One female was found
dead and was replaced.
The patterns of excretion after the various dosing regimens are
shown in Table 1. After the single intravenous injection, 94% of the
administered dose was recovered in males and 90% in females. The
primary route of excretion was the urine, which accounted for about
57% of the administered dose, the the majority being excreted within
12 h. Less was found in faeces (7-9%) and expired air (13-17%),
although these were significant routes of elimination. Within 24 h,
5.6% of the radiolabel was detected in faeces in males and 7.7% in
females. The cage washings contained 11% of the administered dose for
males and 8.6% for females. In animals of each sex, 2.0% of the
administered dose was found in the carcass and 0.5% in liver; the
total recovery in tissues, including carcass, was 2.7% of the
administered dose. Residual radiolabel was found in all tissues, with
the highest concentrations (expressed as equivalents) in liver, lung,
kidney, abdominal fat, testis, and blood (0.3-0.5 µg/g),
concentrations of 0.1-0.2 µg/g in heart, spleen, ovaries, and uterus,
and < 0.1 µg/g in other tissues including bone marrow.
Similar profiles and rates of excretion were seen in animals
treated orally with single or multiple doses of 4 mg/kg bw, although
the amount of radiolabel in faeces was slightly higher (10-16%) than
after intravenous injection (7-9%). As in animals treated
intravenously, most of the radiolabel was recovered within 12 h in the
urine and within 24 h in faeces. Less radiolabel was recovered in the
tissues of animals given multiple doses (0.3% of the administered
dose) than those given the single low dose (2%, with 1-1.5% in carcass
and 0.6% in liver). The amounts of radiolabel were highest in liver,
Table 1. Recovery of radiolabel, expressed as equivalents, after administration of 14C-ethoprophos to groups of five rats
of each sex
Treatment Sex Recovery (% of administered dose)
Urine Faeces Expired air Cage wash Cage Tissues Total
debris (+ carcass)
4 mg/kg bw intravenously M 57 6.6 17 11 ND 2.8 94
F 57 8.7 13 8.6 ND 2.7 90
4 mg/kg bw orally M 52 16 19 3.0 ND 2.2 93
F 50 12 12 13 ND 1.9 91
4 mg/kg bw orally; repeated doses M 54 12 14 10 ND 0.3 91
F 59 10 13 10 ND 0.3 93
12.5 mg/kg bw orally M 58 12 13 4.1 0.2 1.5 88
F 54 9.9 11 7.8 0.8 3.6 88
ND, not detected
kidneys, and lungs, the organs associated with excretion, the
concentrations being 0.2-0.8 µg/g after the single low dose and
0.1-0.2 µg/g after mutliple doses. The concentrations were about 0.2
and 0.1 µg/g in abdominal fat for the respective dose groups and
< 0.1 µg/g in all other tissues including bone marrow.
The pattern of excretion of radiolabel after the oral dose of
12.5 mg/kg bw was similar to that with the intravenous and low single
and multiple oral doses and was similar for males and females. Renal
excretion predominated, and elimination in faeces and expired air
accounted for a smaller but significant proportion of the administered
dose. The highest concentrations of radiolabel, expressed as
equivalents, were again found in liver, kidneys, and lungs and ranged
from 0.4 to 0.9 µg/g. Apart from abdominal fat, which contained about
0.5 µg/g, the concentrations in other tissues were 0.1-0.4 µg/g. One
female had a very high concentration in the uterus (190 µg/g), which
was considered to be due to contamination.
Haematological pharmacokinetics also showed no apparent sex
difference. A mean maximum concentration of about 1.5 µg/g was
achieved between 0.5 and 1 h after the single oral dose of 4 mg/kg bw.
Female rats receiving 12.5 mg/kg bw achieved a mean maximal blood
concentration of 4.6 µg/g 0.5 h after dosing, while male rats
receiving 25 mg/kg bw had a maximal blood concentration of 3.8 µg/g
around 0.6 h after dosing. The terminal elimination half-life of the
radiolabelled material was 110-120 h at 4 mg/kg bw, 92 h at 12.5 mg/kg
bw, and 140 h at 25 mg/kg bw. The systemic concentrations of
radiolabel increased with dose but not in a directly proportional
manner (the integrated area under the curve of
concentration-time0-infinity being 60 µg h/g at 4 mg/kg bw, 110 µg
h/g at 12.5 mg/kg bw, and 150 at 25 mg/kg bw µg h/g (Yenne, 1990).
Male and female rats given a single oral dose of
[14C-ethyl]ethoprophos (1.3 × 106 counts/min) or
[14C-propyl]ethoprophos (3 × 106 counts/min) excreted 55-65% of
the administered radiolabel (actual doses not given) in the urine, and
< 1% in the faeces. Most of the radiolabel in the urine was
eliminated during the first 6 h after treatment and was in the form of
hydrolytic products, including O-ethyl- S-propyl phosphorothioic
acid, which was the major urinary metabolite, accounting for
approximately 40% of the administered dose. Other water-soluble
metabolites identified in the urine included O-ethylphosphoric acid,
S-propyl-phosphorothiolic acid, and S,S-dipropyl-phosphorodithioic
acid. The urine of rats treated with the 14C-propyl-labelled
compound also contained metabolites extractable in organic solvents,
consisting of methyl propyl sulfide, methyl propyl sulfoxide, and
methyl propyl sulfone, which accounted for about 2% of the
administered radiolabel (Iqbal & Menzer, 1972).
(b) Biotransformation
Samples of urine and faeces containing > 50 000 dpm/g from rats
in the study of Yenne (1990) were pooled by sex, dose, and dose
interval and examined for metabolites by both thin-layer and
high-performance liquid chromatography. The biotransformation of
[14C]ethroprophos did not appear to be dependent on sex, dose, or
dose frequency. No parent compound was found in up to five
chromatographically resolved fractions of urine and faeces. Analysis
by gas chromatography and mass spectroscopy and 31P-nuclear magnetic
resonance spectromtery confirmed the presence of ethyl phosphate and
O-ethyl- S-propyl phosphorodithioate in the urine. One fraction was
evaluated as a conjugate of ethylphosphate and two other fractions as
conjugates of O-ethyl- S-propyl phosphorothioate. The conjugates
(not specified) of the two metabolites represent the majority (> 60%)
of the total residue found in urine and faeces. Ethyl phosphate was
the major metabolite in faeces. A proposed metabolic pathway for
ethoprophos is shown in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The permeability of human, mouse, rat, and rabbit skin in
vitro to undiluted or diluted [14C]ethoprophos (radiochemical
purity, 95.5%) was compared over 24 h. An emulsified concentrate of
[14C]ethoprophos was mixed with 10 g of unlabelled ethoprophos
(purity, 70%), or one part of 70% unlabelled concentrate was mixed
with 19 parts of water and [14C]ethoprophos was then added. Skin
samples were obtained from laboratory animals and from the abdomens of
human corpses; those from rabbits and humans were stripped of
subcutaneous fat. The dermal sides of the samples were applied to
penetrating wells containing 10 ml of normal saline buffer, and 2 ml
were withdrawn for scintillation counting and replaced by 2 ml of
normal saline at each interval. A 1.4-cm-wide plastic cylinder was
fixed on the epidermal side of each specimen, and 0.02 ml of the test
solutions was applied. The results are shown in Table 2. Less
penetration was found through human skin than through that of the
other species. The penetration rate was highest for mice and was equal
for rats and rabbits. Undiluted ethoprophos penetrated more slowly
than diluted ethoprophos, but a fairly consistent rate was
established, especially in rabbits, mice, and rats, after 1 h
(Stoughton, 1986). As radiolabel in the skin was not determined, this
study provides only a qualitative indication of dermal penetration.
NADPH-dependent microsomal enzymes from the livers of rats and
rabbits biotransformed [14C-ethyl]ethoprophos or
[14C-propyl]ethoprophos to O-ethyl- S-propylphosphorothioic acid,
the major metabolite, and to O-ethyl-phosphoric acid from
[14C-ethyl]ethoprophos, or to S-propyl phosphorothiolic acid from
[14C-propyl]ethoprophos. Incubation of liver supernatant
preparations from rats and rabbits with ethoprophos in the presence of
reduced glutathione led to the formation of
O-ethyl- S-propyl-phosphorothioic acid as the major metabolite.
S-Ethyl glutathione and S-S-dipropyl-phosphorodithioic acid were
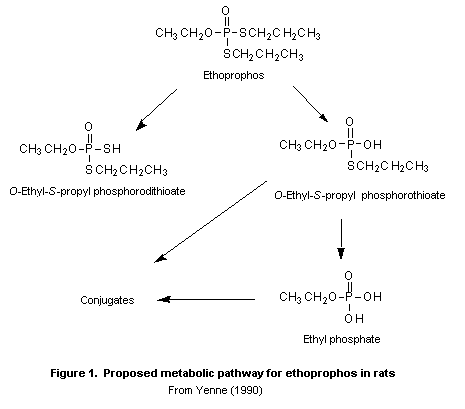 Table 2. Penetration of undiluted or 1:19 diluted 14C-ethoprophos solutions through
the skin of various species (in % of applied dose)
Time Undiluted ethoprophos Diluted ethoprophos
Human Rabbit Mouse Rat Human Rabbit Mouse Rat
15 min 0 0.003 0.003 0.01 0.001 0.004 0.004 0.05
30 min 0.0004 0.008 0.01 0.05 0.01 0.02 0.12 0.15
1 h 0.0008 0.04 0.11 0.15 0.08 0.2 1.9 0.55
2 h 0.002 0.2 0.91 0.4 0.31 1.1 4.2 1.7
4 h 0.004 0.7 2.6 1.0 0.72 3.7 11 4.2
6 h 0.08 1.5 5.0 1.7 1.1 7.7 29 7.8
24 h 1.0 7.8 16 5.4 5.2 27 38 23
also formed when the 14C-ethyl- and the 14C-propyl-labelled
compound, respectively, was the substrate (Iqbal & Menzer, 1972).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ethoprophos in animals is summarized in
Table 3. In 1983, the Meeting reported that "in the studies evaluated
the toxic symptoms in the treated species were characteristic of
anticholinesterase poisoning and usually persisted for up to 48 h in
mice and 72 h in rats surviving treatment. In general, deaths occurred
within 4 h (mice) or 1 h to 3 days (rats) post-dosing. Information on
duration of symptoms and time of death in rabbits was not available."
Ethoprophos is considered to be toxic after single oral and
dermal doses and very toxic after inhalation. The dermal and oral
LD50 values for rabbits and mice are of the same order of magnitude
and indicate efficient absorption of the compound after dermal
exposure in these species, whereas less is absorbed dermally in rats.
A single application of 0.1 ml of undiluted technical-grade
ethoprophos (equivalent to 44 mg/kg bw) into one eye of each of three
New Zealand white rabbits immediately produced moderate erythema and
vascularization of the sclera and nictating membrane. The substance
was severely toxic, since all three animals died within 1 h of
treatment (Weir, 1965).
In a study of primary dermal irritation, all six male New Zealand
white rabbits that received 0.5 ml of undiluted technical-grade
ethoprophos (purity, 93%) at a dose equivalent to 240 mg/kg bw on
clipped, abraded and unabraded skin and kept under an occlusive patch
died within the first 8 h of treatment (Becker & Parke, 1977).
Table 3. Acute toxicity of ethoprophos
Species Strain Sex Purity Vehicle Route LD50/LC50 Reference
(%) (mg/kg bw or
mg/L)
Mousea OF1 (SPF) M & F NR Water Oral 31 Pasquet & Mazuret (1982)
Rat SD M NR Corn oil Oral 62b Powers (1965)
F 33b
Ratc,d Crl:CD (SD) F NR Corn oil Oral 56 Weiler (1998)
BR VAF/ Plus
Rabbite NZW F NR NR Oral 33 Schwartz (1978)
Mouse CD-1 M NR Acetone Dermal (24 h) 18b Auletta & Rinehart (1979)
Rata CD (COBS) M & F NR Water Dermal (24 h) 226 Pasquet & Mazuret
(1982)
Ratd,f SD M NR None Dermal (24 h) 1280 Karcher et al. (1987);
F 424 Blacker (1987)
Rabbitg NZW M & F NR None Dermal (24 h) 8.5 Karcher et al. (1986);
Blacker (1987)
Pigh Yorkshire white M 94% None Dermal 327 Rucci (1979)
Rabbith Albino M & F NR None or 40% Dermal (intact 26b Powers (1965)
solution in and abraded
corn oil skin; 24 h)
Rat Wistar M & F 92% None Inhalation(4 h) 0.250j Kopp et al. (1980)
Table 3 (continued)
NR, not reported
a Ethoprophos lot No. DA 232. Clinical signs: trembling, convulsions, hypomotility, and dyspnoea in both mice and rats; no dermal
irritation in rats. Only 3 male and female mice and 2 male and female rats per dose.
b Doses reported as µl/kg bw. They were converted to mg/kg bw by multiplying them by the specific gravity of ethoprophos at
20°C, 1.094.
c Ethoprophos batch No. 51EAR122. Deaths occurred within 4 days. Clinical signs: thinness, staggered gait, salivation,
hypoactivity, hunched posture, and tremors.
d Statements of compliance with good laboratory practice and quality assurance included.
e Clinical signs: ataxia, diarrhoea, and decreased activity. Only 4 females per group. Information on duration of symptoms and
time of death not available.
f Technical-grade ethoprophos lot No. 302106001. Deaths occurred within 3 days. Clinical signs: urine staining, tremors, excess
salivation, decreased activity, ataxia, soft stools, diarrhoea, laboured breathing, lachrymation, exophthalmus, and prostration,
in general increased with increasing dose. No signs of irritation. Considerable body-weight decreases in animals that died.
g Ethoprophos lot No. 302106001. Deaths occurred within 4 days except for one on day 9. Clinical signs: prostration, laboured
breathing, salivation, loss of locomotor ability, tremor, ataxia, soft stools, diarrhoea, and lachrymation. No signs of
irritation. Considerable body-weight decreases in animals that died. Only statement of quality assurance included.
h Deaths occurred within 4 days. Clinical signs: laboured breathing, salivation, wobbly gait, and decreased activity. Erythema
was reported but no indication of severity was given. Only 2-4 males per group.
i Clinical signs: depression, laboured breathing, unsteadiness, tremors, salivation. Deaths occurred within 24 h. Very slight,
subsiding erythema observed in surviving animals 24-48 h after dosing on abraded (> 10 µl/kg bw) and unabraded skin
(31.6 µg/kg bw). Only 4 animals per group.
j 4-h LC50 expressed as actual chamber concentration and particle size < 10 µm; animals were exposed to the liquid
technical-grade material as an aerosol (nominal concentration, 1500 mg/m3) in a nose-only exposure chamber. Clinical signs:
apathy, dyspnoea, salivation (from JMPR, 1983, and slightly modified by reference to original report).
(b) Short-term studies of toxicity
(i) Oral administration
Rats
Groups of 25 male and 25 female Charles River caesarean-derived
rats were fed diets containing technical-grade ethoprophos (purity not
stated) in acetone at concentrations of 0, 0.3, 1, or 100 ppm
(equivalent to 0, 0.015, 0.05, and 5 mg/kg bw per day) for three
months. The control group was given basal diet containing 'a volume of
acetone equivalent to the volume used for the test diets'. No deaths
or treatment-induced toxic signs were observed. Growth was depressed
by < 10% in males at doses > 1 ppm and in females at 100 ppm
during the last 6 weeks of the study. Food consumption was unaffected.
Haematological, clinical chemical (only blood urea nitrogen, aspartate
aminotransferase, and glucose determined), and urinary examinations in
five males and five females per group after 1 and 3 months indicated
no significant treatment-related findings. Cholinesterase activity in
plasma, erythrocytes, and brain was measured in five rats of each sex
per group at days 4, 8, 16, and 33 and terminally in the remaining
rats. Marked inhibition (25-100%) of the enzyme activity was found at
100 ppm at all sampling intervals, the depression being greatest for
erythrocyte and least for brain acetylcholinesterase activity. Maximum
inhibition of the enzyme occurred on day 8 or 16, in all tissues. Male
rats at 0.3 or 1 ppm showed a 24-28% reduction in erythrocyte and
plasma cholinesterase activity on day 8 and of brain cholinesterase
activity at termination. In females, brain cholinesterase activity was
inhibited by 20-25% at 0.3 and 1 ppm on day 8 and at 1 ppm on day 16,
and plasma and erythrocyte cholinesterase activity was inhibited by
23-26% at 1 ppm on day 16. At the end of the study, the absolute and
relative weights of the adrenals were decreased, but not in relation
to dose, in females in all treated groups. Histopathological
evaluation of about 20 selected tissues, including the adrenals, from
five males and five females in the control and highest-dose groups
showed no significant changes attributable to treatment. No NOAEL
could be identified, as brain acetylcholinesterase activity was
inhibited at all doses (Weir, 1967a). As only five animals of each sex
were examined for haematological, biochemical, and urinary parameters,
instead of the 20 rats of each sex required by the OECD guideline, and
few parameters were tested, the study was considered unreliable and
was not used for further evaluation of the toxicology of ethoprophos.
Dogs
In a study conducted according to GLP and with a statement of QA,
ethoprophos (purity, 95.6%) was administered orally to groups of six
male and six female beagle dogs aged 6-7 months at doses of 0, 0.01,
0.025, or 1 mg/kg bw per day in corn oil in gelatin capsules for 20
weeks.Two dogs of each sex at each dose were then allowed a 4-week
recovery. The animals were observed for deaths and clinical signs.
Body weights and food consumption were recorded, and ophthalmological
examinations were performed before dosing and at sacrifice after the
exposure (week 20) and recovery (week 24) periods. Haematological,
biochemical, and urinary analyses were carried out before treatment
and during weeks 17, 20, and 24. Cholinesterase activity in plasma and
erythrocytes was measured before dosing and about 3 h after dosing
during weeks 2, 4, 8, 12, 20, and 24. Brain cholinesterase activity
was measured at sacrifice; at that time, the weights of about 10
organs were determined and about 40 tissues and all gross lesions from
all animals were examined macroscopically and microscopically.
There were no deaths, and there were no treatment-related
clinical signs or effects on body weight, body-weight gain, food
consumption, ophthalmologic, haematological, biochemical, or urinary
end-points, macroscopic or microscopic findings, or organ weights. No
histopathological changes were found in the heart. The only finding
was a statistically significant increase in platelet count in bitches
at the high dose at weeks 17 and 20. This was considered to be of no
toxicological relevance since the value was increased before
treatment, and an elevated value was observed after recovery.
Significant inhibition of plasma cholinesterase activity was observed
in bitches at the intermediate dose and males and females at the high
dose. In animals at the high dose, erythrocyte acetylcholinesterase
activity was inhibited by more than 20% in males at all times during
exposure and in females only at weeks 8 and 12, when the values
reached statistical significance in males. Brain acetylcholinesterase
activity was not inhibited. After the recovery period, plasma
cholinesterase activity was found to have returned to normal, but the
activity in erythrocytes showed some variation and it could not be
concluded that recovery had occurred. The NOAEL was 1 mg/kg bw per
day, the highest dose tested, in the absence of inhibition of brain
acetylcholinesterase activity (Hamada, 1990).
Groups of three male and three female young adult pure-bred
beagles weighing 6-12 kg were fed diets containing technical-grade
ethoprophos (purity unknown) at concentrations of 0, 1, 3, or 100 ppm
for 13 weeks, equal to 0, 0.034, 0.098, or 3.4 mg/kg bw per day for
males and 0, 0.035, 0.11, or 4.0 mg/kg bw per day for females. There
were no deaths. Emesis occurred once in two animals at 100 ppm. Body
weights, food consumption, haematological, biochemical (glucose, blood
urea nitrogen, and aspartate aminotransferase and alkaline phosphatase
activity), and urinary parameters measured at 1 and 3 months were not
significantly affected by treatment. At termination, however, males
and females at the high dose had slightly decreased erythrocyte counts
and erythrocyte volume fractions. Plasma cholinesterase activity was
measured before dosing and on days 2, 4, 8, 16, 35, 65, and 87 of
administration. The activity was inhibited by 27-88% at doses > 3
ppm in animals of each sex at virtually all of the sampling intervals
and by 20-27% at 1 ppm in males at the two last sampling times. A
> 20% depression of erythrocyte cholinesterase activity was seen in
animals at the highest dose at all but the first two or three sampling
intervals and in females at 3 ppm on one occasion (day 65) only.
Cholinesterase activity in brain was not measured. At termination, no
compound-related effects on organ weights or gross pathological
changes were noted. The only significant finding on histopathological
evaluation of about 20 tissues from each animal in the control and
high-dose groups was foci of perivascular myocardial swelling with
loss of striations in one of three female controls and two of three
males and one of three females at 100 ppm; all of these animals also
showed swelling and vacuolation of Purkinje fibres. These changes were
considered by the pathologist to represent 'an artefact induced in the
processing of the tissues'. The NOAEL was 3 ppm, equal to 0.098 mg/kg
bw per day, on the basis of depression of erythrocyte cholinesterase
activity at 100 ppm (Weir, 1967b).
Groups of four male and four female pure-bred beagle dogs aged
4-6 months were given capsules containing ethoprophos (purity, 96%)
dissolved in peanut oil at doses of 0, 0.025, 1.0, or 10 mg/kg bw per
day orally for 52 weeks. The doses were based on the results of a
4-week range-finding study. The animals were offered 400 g/day of food
and water ad libitum and were observed daily for signs of toxicity;
body weights were recorded weekly and food consumption daily. Blood
and urine were collected before treatment and after 6, 13, 26, and
52 weeks of treatment. The standard haematological, serum chemical,
and urinary examinations were performed, in addition to measurements
of plasma and erythrocyte cholinesterase activity. At the end of the
study, the animals were killed by an overdose of thiopentone sodium
followed by exsanguination. All tissues were examined grossly
in situ, and the major organs were removed and weighed, and brain
cholinesterase activity was measured. The standard set of tissues was
collected for histopathological examination. A statement of QA was
provided.
No effect of treatment on the incidence of clinical signs was
observed. Treatment did not affect the weight gain of treated males,
but a dose-related trend to decreased body weight was seen in treated
bitches throughout the study, and, at termination, bitches at the high
dose weighed about 10% less than controls. Males and females at the
high dose tended to consume about 10% less food than controls.
Erythrocyte count, haemoglobin concentration, and erythrocyte volume
fraction were statistically significantly lower in males at the high
dose than in control males at all intervals. The only change in serum
chemistry was an increase in mean aspartate aminotransferase activity
associated with decreased total cholesterol and serum albumin in males
at the high dose from 6 weeks of treatment throughout the study. This
effect was due largely to an apparent hepatotoxic response in two of
four males at this dose; in one of these dogs, aspartate
aminotransferase activity was increased by 10-fold over that of
controls at study termination, and serum alkaline phosphatase,
gamma-glutamyl transferase, and alanine aminotrans-ferase activities
were also greatly increased. The serum albumin concentration was
decreased in all males at the high dose. Plasma and erythrocyte
cholinesterase activities were depressed in a dose-related manner in
dogs at the intermediate and high doses at all intervals. At the end
of the study, plasma cholinesterase activity was decreased to 33% and
17% of the control value in males and to 58%, 19%, and 17% in females
at the low, intermediate, and high doses, respectively. The
erythrocyte cholinesterase activity in males at the end of the study
was 127%, 88%, and 39% of the control value at the low, intermediate,
and high doses, respectively, whereas the values for females were
103%, 62%, and 38% of control, respectively. Brain cholinesterase
activity was inhibited only in males at the high dose (to 56% of the
control value; significant) and females (72% of control) at the high
dose. Animals at the intermediate dose showed inhibition to 91% and
94% of the control values, respectively, which was considered not to
be toxicologically relevant. Ophthalmoscopic examination conducted
before and at the end of the study revealed no treatment-related
effects. At necropsy, no effect of treatment on absolute or relative
organ weights was seen, nor was there any effect on the incidence of
gross findings. Treatment-related histopathological changes were
restricted to the liver. Selected findings are tabulated in Table 4.
The NOAEL was 0.025 mg/kg bw per day on the basis of findings in the
liver at the low dose (Brown, 1986).
Table 4. Pathological findings in the liver of dogs treated with ethoprophos for 52 weeks
Lesion Dose (mg/kg bw per day)
Males Females
Control 0.02 1 10 Control 0.025 1 10
Vacuolation 0 0 3 4 0 0 3 4
Focal necrosis 0 0 0 2 1 0 0 1
Pigment 0 0 1 4 0 0 0 3
Kupffer-cell pigment 0 0 1 4 0 0 1 4
Fibrosis 0 0 0 4 0 0 0 4
Biliary proliferation 0 0 0 4 0 0 0 4
(ii) Dermal administration
Rats
In a range-finding study for dermal toxicity conducted according
to GLP and with a QA statement, groups of three Crl:CD(R)BR rats of
each sex received dermal applications of ethoprophos (purity, 95.6%)
in mineral oil at doses of 0, 0.3, 1, 10, 30, or 100 mg/kg bw per day
for 6 h per day on 5 days per week for 3 weeks. The animals were
observed for clinical signs and dermal irritation. Body weight and
food consumption were recorded, and cholinesterase activity was
measured in plasma and erythrocytes before dosing, at week 2, and at
the end of study (week 4). At necropsy, brain acetylcholinesterase
activity was measured. Macroscopic examinations were performed and
organ weights recorded.
Deaths occurred in the groups given 30 (2/6 rats on days 7 and 8)
and 100 mg/kg bw per day (6/6 rats, days 2-7). The clinical signs in
these animals were characteristic of cholinesterase poisoning. Small
faeces, hunched posture, and low body temperature were also observed
at 10 mg/kg bw per day, and the latter finding was also observed in
one male and one female at 0.3 mg/kg bw per day and two males and one
female at 1 mg/kg bw per day. Food consumption was lower (reaching
statistical significance in females) at 10 mg/kg bw per day in females
and 30 mg/kg bw per day in males and females during the first week of
exposure. There were no signs of dermal irritation. Statistically
significant inhibition of plasma cholinesterase activity was observed
at weeks 2 and 4 in females at 0.3 and 1 mg/kg bw per day and in
animals of each sex at 10 and 30 mg/kg bw per day. Inhibition of
erythrocyte acetylcholinesterase activity by > 20% was observed in
females at all doses at week 2 and at 10 and 30 mg/kg bw per day at
week 4 and in males at 10 and 30 mg/kg bw per day at both times. Brain
acetylcholinesterase activity was inhibited by > 50% in animals of
each sex at 10 and 30 mg/kg bw per day; the effect was dose-related
and significant at the high dose. No treatment-related changes were
observed in organ weights or in gross appearance (Henwood, 1990a).
In the main study, conducted according to GLP and with a QA
statement, groups of 10 Crl:CD(R)BR rats of each sex aged about 2.5
months received 1.4 ml/kg bw of ethoprophos (purity, 95.6%) dermally
in mineral oil at doses of 0, 0.3, 1, or 10 mg/kg bw per day for 6
h/day on 5 days per week for 3 weeks. The area of exposure on the
dorsal trunk constituted about 10% of the total body surface. The
animals were observed for clinical signs and dermal irritation. Body
weights and food consumption were recorded, and cholinesterase
activity was measured in plasma and erthrocytes before treatment, in
week 2, and at the end of study (week 4). Haematological and clinical
chemical parameters were evaluated on the day of necropsy, when the
brain was collected for analysis of acetylcholinesterase activity.
Macroscopic examinations were performed, and the weights of the brain,
kidneys, and liver were recorded. Kidneys, liver, skin, and tissues
with lesions from all rats in the control and high-dose groups were
examined microscopically.
The deaths of one control female and one at the high dose were
considered to be unrelated to treatment. Clinical signs observed in
some females at the high dose included soft and/or small faeces (three
animals) and hunched posture (one animal). During the first week of
exposure, a slightly lower (not statistically significant) body-weight
gain was observed in animals at the high dose, but food consumption
was not affected. There were no signs of dermal irritation that could
be related to treatment. In all groups, including controls, slight
erythema and desquamation associated with pustules or papules were
observed. Moderate desquamation was observed in one male and one
female at 0.3 mg/kg bw per day and in one male at 1 mg/kg bw per day.
Dose-related, statistically significant inhibition of plasma
cholinesterase activity was observed in weeks 2 and 4 in animals given
1 or 10 mg/kg bw per day. Erythrocyte acetylcholinesterase activity
was inhibited by > 20% in rats at 1 mg/kg bw per day in week 4 and at
10 mg/kg bw per day at both times, with 30% inhibition in week 2 and
37% (males) and 54% (females) in week 4. Brain acetylcholinesterase
activity was statistically significantly inhibited by 50% in males and
70% in females at 10 mg/kg bw per day. At 1 mg/kg bw per day, the
inhibition was 12% in males and 16% in females (not significant). At
0.3 mg/kg bw per day, brain acetylcholinesterase activity was not
inhibited in males and was nonsignificantly inhibited by 11% in
females. In female controls and in those at 0.3 mg/kg bw per day, but
not those at 1 mg/kg bw per day, the standard deviation of the mean
acetylcholinesterase activity was relatively high. As a result, the
inhibition in brain at 0.3 mg/kg bw per day is considered not
toxicologically relevant, whereas that at 1 mg/kg bw per day is
relevant. Other clinical findings in females were a dose-related
decrease in platelet count and a decrease unrelated to dose in the
numbers of neutrophils and lymphocytes in animals at 1 and 10 mg/kg bw
per day. These findings were considered to be due to the excessive
formation of fibrin strands in the blood of these females. The
statistically significant findings of increases in aspartate
aminotransferase activity in males at 1 mg/kg bw per day and slight
decreases in urea nitrogen in males at 0.3 and 10 mg/kg bw per day
were considered not to be related to treatment. Increased plasma
concentrations of sodium and chloride were observed in females at 0.3
mg/kg bw per day and in animals of each sex at 1 and 10 mg/kg bw per
day, but these changes were small and not related to dose. An
increased calcium concentration was seen in males at 1 mg/kg bw per
day and in males and females at 10 mg/kg bw per day. No
compound-related changes were observed in terminal body weights or
organ weights. At macroscopic examination, two of 10 males at the high
dose were found to have crusted areas on untreated skin in the
cervical and/or thoracic region, confirmed microscopically to be
multifocal erosion or ulceration of the epidermis and chronic
inflammation. Three of nine females at the high dose showed dark areas
in the glandular stomach, which was confirmed microscopically in two
rats as oedema or focal erosion or ulceration of the glandular mucosa;
and one of these females and another at the high dose showed
dilatation of the pelvis. The NOAEL was 0.3 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity in females
at 1 mg/kg bw per day. Since no sign of treatment-related dermal
irritation was seen, the NOAEL for this end-point was 10 mg/kg bw per
day, the highest dose tested (Henwood, 1990b).
Rabbits
In a study conducted according to GLP and with a QA statement,
groups of 10 Hra:(NZW)SPF rabbits of each sex, aged about 3 months,
received dermal applications of 1.4 ml/kg bw of ethoprophos (purity,
95.6%) in 4% carboxymethylcellulose at doses of 0, 0.03, 0.1, or 1
mg/kg bw per day for 6 h/day on 5 days per week for 3 weeks. The area
of exposure on the dorsal trunk constituted about 10% of the total
body surface. The animals were observed for clinical signs and dermal
irritation. Body weight and food consumption were recorded and
cholinesterase activity was measured in plasma and erythrocytes before
treatment and at the end of study (week 4). Haematological and
clinical chemical parameters were evaluated on the day of necropsy,
when the brain was collected for measurement of acetylcholinesterase
activity. Macroscopic examinations were performed, and the weights of
the brain, kidneys, and liver were recorded. Kidneys, liver, skin, and
tissues with lesions from all rabbits in the control and high-dose
groups were examined microscopically.
Deaths occurred in one male and three females at 0.03 mg/kg bw
per day and two males at 1 mg/kg bw per day. These animals and a few
others in each group except female controls showed clinical signs that
were associated with an increased incidence of mucoid enteritis
(diarrhoea, mucoid diarrhoea, and reduced food consumption), but this
finding was considered to be unrelated to treatment. Females at the
high dose had significantly lower body weights in weeks 2 and 4. The
groups in which the deaths occurred had lower food consumption, but
this was considered to be of no toxicological importance. An increased
incidence of slight-to-moderate irritation (erythema and sometimes
desquamation) was seen in all treated groups when compared with
controls, and the frequency increased with dose. The only
treatment-related change in haematological or clinical chemical
parameters was statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain at the high dose. The
inhibition in males was 37% in plasma, 42% in erythrocytes, and 49% in
brain, and that in females was 35% in plasma, 42% in erythrocytes, and
49% in brain. No inhibition of brain acetylcholinesterase activity was
found at lower doses; erythrocyte acetylcholinesterase activity was
inhibited by 12% in females at the intermediate dose, but this is
considered to be of no toxicological relevance. The terminal body
weights were significantly lower for females given 0.03 or 1 mg/kg bw
per day, which might be due to their lower food consumption. The
absolute kidney weights were significantly lower in females and
slightly lower in males at the high dose, and the relative kidney
weights were slightly (and not significantly) lower in females at this
dose. There were no treatment-related macroscopic or microscopic
changes. The NOAEL for dermal toxicity was 0.1 mg/kg bw per day on the
basis of inhibition of cholinesterase activity in brain and
erythrocytes and decreased kidney weights at 1 mg/kg bw per day. No
NOAEL could be identified for dermal irritation, as slight irritation
of the skin was observed at all doses (Henwood, 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Randomly assigned groups of 80 male and 80 female B6C3F1 (SPF)
mice were fed diets containing 0, 0.2, 2, or 30 ppm of technical-grade
ethoprophos (purity, 94.6%) for 2 years. The test material and diets
were analysed periodically to ensure that the concentrations were
within acceptable limits of the nominal values. The diets and water
were provided ad libitum. Animals were examined daily for signs of
toxicity, and moribund animals were killed. Body weights were recorded
weekly for the first 26 weeks of treatment and twice weekly
thereafter. Food consumption was determined weekly. Haematological,
clinical chemical, and urinary parameters and cholinesterase activity
were measured after 26, 52, 78, and 104 weeks of treatment. Ten mice
of each sex at each dose were killed after 26, 52, and 78 weeks of
treatment, and all surviving animals were killed after 104 weeks of
treatment. Complete post-mortem examinations were conducted on all
animals killed at scheduled sacrifice and on those that died or were
killed in a moribund condition during the study.
No effect of treatment was seen on survival or the incidence of
clinical signs. Mean body-weight gain was decreased by 5-10% in males
and females at the high dose during the first 80 weeks of treatment,
but by the end of the study there was no decrease in males and only a
slight decrease (6%) in females. Treatment had no effect on food
consumption, the mean intake levels over the course of the study being
0, 0.026, 0.25, and 4.0 mg/kg bw per day for males and 0, 0.032, 0.32,
and 4.9 mg/kg bw per day for females. The only change in
haematological parameters was a decrease in the mean total leukocyte
count in all treated males: at the end of the study, the mean total
counts were: 4.1 ± 1.7, 2.3 ± 0.9, 2.1 ± 1.1, and 1.6 ± 0.5 ×
103/mm3 for controls and males at the low, intermediate, and high
doses, respectively, which were statistically significantly different
from the controls in all groups. The significance of this finding is
unclear as it was not associated with any other toxic effect that
might result from an impaired immune function. Historical control data
would have been useful for evaluatng this finding, but they were not
submitted.
Plasma and erythrocyte cholinesterase activity was inhibited in a
dose-related manner in animals at the intermediate and high doses
during the first 78 weeks of treatment, and in most cases statistical
significance was reached. The cholinesterase activity remaining in
plasma represented 65-90% of control values at the intermediate dose
and 23-34% at the high dose, whereas in erythrocytes it was 83-89% of
control values at the intermediate dose and 19-26% at the high dose.
By the end of the study, the plasma and erythrocyte cholinesterase
activities in the group at the intermediate dose were similar to those
of controls, except that the activity in plasma of females at the
intermediate dose was significantly decreased by about 20%. Brain
cholinesterase activity was inhibited only in males and females at the
high dose, with statistical significance reached in week 26 (64% of
control value for males and 71% for females), week 52 (82% of control
value in males), and week 104 (81% of control value in males and 83%
in females).
Necropsy of animals that died on test or were killed in a
moribund condition did not reveal any treatment-related lesions or an
effect on absolute or relative organ weights. No treatment-related
lesions were found at gross and microscopic examinations at interim
sacrifices. At final sacrifice, an increased incidence of
calcification of the kidney was noted in males at the high dose
(13/42, 2/45 controls) with 'basophilic changes' in the kidneys of
males only (0/45 control, 9/31 at the low dose, 13/38 at the
intermediate dose, and 24/42 at the high dose). The historical control
data that were supplied showed a high spontaneous incidence of these
lesions, with incidences of calcium deposits in males of 2.4-72% and
basophilic changes in males of 0-87%. The study authors reported that
these lesions are of spontaneous origin and do not represent
treatment-related pathological lesions. Treatment had no effect on the
incidences of tumours in specific tissues or on the total tumour
burden.
The NOAEL for toxicity was 2 ppm, equal to 0.25 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinesterase activity at
30 ppm (Yamagata et al., 1984a,b).
Rats
Groups of 10 male and 20 female Fischer 344 rats were fed diets
containing technical-grade ethoprophos (purity, 95.3%) at
concentrations of 0, 60.5, 131, or 262 ppm for 8 weeks before mating
(one male to two females). (The section on methods indicates that the
F0 generation comprised 10 males and 20 females per group, but later
in the report it is stated that approximately 16 males and 32 females
at each dose were mated. The complete absence of data on the
reproduction phase of the study precluded verification of the
information. The report stated that analytical data for dietary
analyses were not available.) Ten days after the detection of a
positive vaginal smear, the females were separated from the males and
were maintained on the test diets until weaning of their pups. This
part of the study constituted the reproductive phase. Weanlings from
the reproductive phase (60 males and females per group) were selected
randomly and fed diets containing ethoprophos at 0, 4.5, 9, or 18 ppm
during weeks 0-12 and at 0, 49, 98, or 196 ppm during weeks 13-109 in
a study of carcinogenicity. The dietary levels during weeks 13-109
were equivalent to doses of 0, 2.5, 4.9, and 9.8 mg/kg bw per day.
(The mean intake over the whole exposure period was not reported.) In
this 109-week study, all animals that died or were killed in a
moribund condition, all survivors at the end of the study, and the 10
males and 10 females per group killed after 52 weeks of treatment were
subjected to gross necropsy and histopathological examination of a
wide range of tissues and all gross lesions.
The mortality rate was increased in males of the highest dose
during the first 7 months, although the rates at the end of the study
(53-63%) were comparable in all groups, including the controls. Other
than emaciation in animals at the high dose, there were no
treatment-related clinical signs. Growth was depressed at the highest
dose throughout the study and at the intermediate dose during most of
the study. Food consumption was reduced in animals at the intermediate
and high doses during the first 52 weeks. Weekly water consumption,
monitored between weeks 102 and 109, was unaffected. Haematological,
blood chemical (glucose, blood urea nitrogen, gamma-glutamyl
transferase, alanine and aspartate aminotransferase and alkaline
phosphatase activities, total protein, and cholesterol concentrations)
and urinary (pH, glucose, ketones, and protein) parameters measured at
the end of weeks 52 and 109 revealed decreased erythrocyte count,
erythrocyte volume fraction, and haemoglobin values in males at the
two higher doses and in females of the highest dose after 52 weeks.
Assay of cholinesterase in plasma, erythrocytes, and brain, conducted
at termination only, indicated a dose-related, statistically
significant 81-93% depression of plasma cholinesterase activity and a
30-68% decrease in brain cholinesterase activity in all treated
groups. Erythrocyte cholinesterase activity was not inhibited. The
weights of the spleen, liver, kidney, and testis deviated from those
of controls at the 52-week and terminal sacrifices, but essentially
only at the highest dose, with no accompanying microscopic lesions.
The gross pathologic changes seen were not significantly different
from those in controls, but histopathological examination of males at
the highest dose showed an increased incidence of 'scleral
mineralization' of the eye. No other microscopic lesions that might be
attributable to treatment were found.
The only notable oncogenic finding was a statistically
significant increase in the incidence of thyroid C-cell adenoma in
males at the highest dose at terminal sacrifice, with incidences of
2/34 in controls, 3/31 at the low dose, 1/29 at the intermediate dose,
and 9/33 at the high dose, expressed as the ratio of the number of
survivors bearing the tumour to the number examined
histopathologically. C-cell adenomas were also observed at the interim
sacrifice in one male in the control group and one at the low and one
at the high dose. The background incidence of the tumour was not
reported. The incidences of animals with benign and/or malignant
tumours, malignant tumours, or multiple primary tumours were not
significantly different in control and treated groups.
Interstitial-cell adenoma of the testis in males and pituitary adenoma
in females were the most frequently observed spontaneous tumours,
occurring in nearly 90% of males and over 50% of females in the
concurrent control group (Barnett et al., 1983). The authors
considered that the increased incidence of C-cell adenomas in males at
the high dose was a direct treatment-related effect as there was no
trend with dose. No NOAEL could be identified since inhibition of
brain acetylcholinesterase activity was seen at all doses. The LOAEL
was 49 ppm, equivalent to 2.5 mg/kg bw per day.
Groups of 70 male and 70 female Fischer 344 rats were assigned
randomly to receive diets containing technical-grade ethoprophos
(purity, 95.9%) at concentrations of 0, 1, 10, or 100 ppm (equivalent
to 0, 0.05, 0.5, or 5 mg/kg bw per day; mean intake over entire
exposure not reported) for 105 consecutive weeks. The test material
and diets were analysed periodically to ensure the stability and
homogeneity of diets. Food and water were provided ad libitum. The
animals were examined daily for mortality and morbidity and underwent
detailed physical examinations every week. The body weights were
recorded weekly for the first 26 weeks and twice weekly thereafter
until the end of the study, as were measurements of food and water
consumption. Ophthalmoscopic examinations were performed on all rats
at the beginning of the study and after 6, 12, 18, and 24 months of
treatment, and routine haematological, serological, and urinary
studies were conducted at the same intervals after the beginning of
treatment. Plasma and erythrocyte cholinesterase activity was measured
at the same intervals as other parameters, and brain
acetylcholinesterase activity was measured at interim sacrifice of 10
rats of each sex per dose after 12 and 18 months and at final
sacrifice at the end of 24 months of treatment. All animals were
killed on schedule, and those that died on test or were killed in a
moribund condition were subjected to a complete post-mortem
examination. A statement of QA was provided.
Treatment had no effect on survival, body-weight gain, or food or
water consumption. The only potential treatment-related finding at
physical examination was an increased incidence of anogenital staining
in females at the high dose during weeks 14-78 of treatment.
Haematological examination revealed decreased erythrocyte counts,
haemoglobin, and erythrocyte volume fraction, with increased mean
corpuscular volume in males and females at the high dose at all
intervals except 18 months. This change was statistically significant
only after 6 and 12 months of treatment, but not at final sacrifice. A
slight (10-20% over controls) increase in blood urea nitrogen was also
seen in these animals, which was statistically significant only at 6
months in females and at 18 months in males. A statistically
significant decrease in serum globulin content was noted after 6 and
12 months of treatment but not at final sacrifice. No toxicologically
significant alterations were found in urinary or ophthalmic
end-points.
Plasma and erythrocyte cholinesterase activity was significantly
inhibited in a dose-related manner at most intervals in males and
females at the intermediate and high doses. Erythrocyte activity was
inhibited by 28-44% in animals at the high dose, by < 20% in males at
the intermediate dose, and by 19-27% in females at 6, 12, and 18
months and 4% at the end of the study. Brain cholinesterase activity
was significantly inhibited in animals at the high dose at all
measured intervals, with inhibition of 27-35% in males and 36-48% in
females.
At the 12-month sacrifice, a statistically significant increase
of about 10% was noted in the relative weight of the spleen of males
and females at the high dose, with decreases of a similar magnitude in
the absolute and relative weights of the kidney in males at the high
dose. No significant treatment-related changes were seen on gross or
microscopic examination. At the 18-month sacrifice, no significant
alterations in organs weights were found, nor were any significant
changes noted on gross or microscopic examination. At final sacrifice,
statistically significant, 16-20% increases in the absolute and
relative weights of the thyroid/parathyroid were seen in males at the
high dose, with non-significant, 14-16% increases in males at the
intermediate dose. Gross examination revealed an increased incidence
of enlarged thyroids in males at these doses: 5/35 and 5/39,
respectively, compared with 1/36 controls. The only potentially
treatment-related lesion found on microscopic examination was an
increased incidence of parafollicular C-cell neoplasms in males at the
high dose (Table 5). The authors concluded that these changes were
statistically significant and the incidence of thyroid neoplasia was
'random and unrelated to the test article'.
Table 5. Incidence of C-cell neoplasms in male rats fed diets containing
ethoprophos
Neoplasm Dose (ppm)
0 1 10 100
C-cell adenoma
Deaths and unscheduled sacrifices 2/13 0/7 0/13 1/9
Final sacrifice 6/36 5/39 5/35 11/39
Total 8/49 5/46 5/48 12/48
C-cell carcinoma
Deaths and unscheduled sacrifices 0/13 0/7 0/13 1/9
Final sacrifice 0/36 0/39 1/35 2/39
Total 0/49 0/46 1/48 3/48
All C-cell neoplasms 8/49 5/46 6/48 15/48
The NOAEL for toxicity was 10 ppm, equivalent to 0.5 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase
activity, effects on erythrocyte parameters, and effects on the
thyroid at 100 ppm (Spicer, 1985).
In a study conducted according to GLP and with a QA statement,
ethoprophos (purity, 95.6%) was mixed into the diet of groups of 70
randomly assigned Crl:CD(R)(SD)BR VAF/Plus(R) rats of each sex at
concentrations of 0, 1, 60, and 600 ppm for 105 weeks. During week 3
of the study, the highest dose was lowered to 400 ppm because of the
occurrence of tremors, ataxia, and death in females. The average doses
throughout treatment were 0, 0.04, 2.7, and 20 mg/kg bw per day for
males and 0, 0.06, 3.4, and 26 mg/kg bw per day for females in the
four groups, respectively. Ten additional animals of each sex per dose
were killed after 54 weeks, while another 10 animals of each sex were
allocated to the control and high-dose groups to study recovery after
dosing for 52 weeks followed by 4 weeks of control diet. The test
material and diets were analysed regularly to ensure that the
concentrations in the diet were within an acceptable range. Food and
water were provided ad libitum, and animals were monitored twice
daily for their clinical condition. Body weights and food consumption
were determined weekly up to week 13 and at least every other week
thereafter. The clinical pathological examinations comprised
haematology, clinical chemistry, including erythrocyte and plasma
cholinesterase activity, and urinalysis at weeks 12, 26, 52, and 78 on
10 animals per group and on all surviving animals at termination of
the study, when brain cholinesterase activity was determined in all
animals. All animals were necropsied, and 41 organs from animals in
the control and high-dose groups and all those that died or were
killed before the end of the study were examined histologically.
Additionally, all macroscopic lesions and lung, liver, and kidneys
from animals at the low and intermediate doses were studied. The
thyroids of all animals were examined, and ophthalmic examinations
were done on all animals at weeks 0, 52. and 104.
Animals at the high dose showed depressed food consumption (up to
week 70 in males and up to week 20 in females) and cumulative
body-weight gain, and in males food efficiency was depressed up to
week 20. The survival rate was essentially the same in all groups up
to week 78, but by week 104 the survival rate of animals at the high
dose was higher than that of other groups. The major causes of death
were renal disease and pituitary tumours. Females at the high dose had
significantly reduced erythrocyte count, haemoglobin, and erythrocyte
volume fraction after 12, 26, 52, and 78 weeks of treatment, although
the decreases were not statistically significant at 53 or 104 weeks.
In males at this dose, these parameters were significantly reduced
only at week 26; at weeks 12, 52, 53, and 78, nonsignificant decreases
were seen, and at week 104 there was no difference from controls.
These effects were reversible in the animals allowed to recover. Males
and females at the high dose had significant reductions in total
plasma protein and globulin at weeks 12 and 26 and males also at week
52 and 53. In females, the decrease was significant only for total
protein at week 52 and was nonsignificant for both total plasma and
globulin at week 53. At week 78, only a significant decrease for total
protein was observed, while in females both parameters showed a
nonsignificant decrease. At week 104, the decreases in total plasma
protein and globulin were no longer significant in animals of either
sex. The effects on total plasma protein and globulin were reversible
after 4 weeks of control diet. Other significant changes in
haematological and blood biochemical parameters were incidental and
considered not to be related to treatment. No changes in
haematological or general blood biochemical parameters were observed
in rats at the other doses. The results of urinary analysis were
summarized in the report and indicated that males at the high dose had
significantly decreased urine volume and urea nitrogen and creatinine
concentrations at the end of the study. These findings were attributed
to a lower incidence of chronic renal disease in these animals.
Plasma cholinesterase activity was significantly inhibited
throughout the experiment, by 48-64% in males at the intermediate
dose, 62-77% in males at the high dose, 61-77% in females at the
intermediate dose, and 75-82% in females at the high dose. Erythrocyte
cholinesterase activity was also significantly inhibited throughout
the study, by 34-44% in males at the intermediate dose, 35-51% in
males at the high dose, 36-48% (36% inhibition not significant) in
females at the intermediate dose, and 41-51% in females at the high
dose. At the low dose, inhibition of plasma and erythrocyte
cholinesterase activity was highly variable but never exceeded 18% in
plasma or 6% in erythrocytes in either males or females and was not
statistically significant. Inhibition of brain cholinesterase activity
was significant at weeks 52 and 104 and amounted to 35-33% of control
values in males at the intermediate dose, 53-64% in males at the high
dose, 28-32% in females at the intermediate dose, and 64-66% in
females at the high dose. At the low dose, inhibition of brain
cholinesterase activity was maximal in females at week 104 (8%), but
the differences (whether increased or decreased) were not
statistically significant in either males or females. Both plasma and
erythrocyte cholinesterase activity recovered to about 80% of the
control activity after 4 weeks of control diet from week 52, and brain
acetylcholinesterase activity was nearly fully restored.
Both males and females at the high dose tended to have lower
terminal body weights, but the differences were not statistically
significant. At week 52, the weight of the left thyroid glands of
females at this dose was reduced both absolutely and relative to the
weight of the brain. Males that were allowed to recover showed reduced
absolute left and right adrenal weights, left thyroid plus parathyroid
weights, and heart weight; the organ:brain weight and the kidney:brain
weight ratios were also reduced. The weight of the left testis
relative to that of the body was increased. At 104 weeks, females at
the high dose had increased brain weights and decreased kidney:brain
weights. The males in this group had reduced absolute and relative (to
body) weights of the right and left kidney, heart, and right adrenal
gland.
Males at the high dose had a decreased incidence and reduced
severity of chronic nephropathy as compared with control animals,
whereas females at this dose had a reduced incidence of mineral
calcareous deposits in the renal pelvis. Males and females at the high
dose showed increased incidences of inflammation of tissues of the
tail (joints and skin) and of alveolar macrophage infiltration.
Probably as a result of this inflammation, higher incidences of
lymphoreticular cell hyperplasia, sinal ectasia, and congestion were
observed in lymph nodes at various sites in the body. The inflammatory
changes were considered to be age-related, and their increased
incidence in animals at the high dose was assumed to reflect the poor
physical condition of these animals. In females at the high dose,
increased incidences of gastric erosion and submucosal oedema were
found at terminal sacrifice. No treatment-related ophthalmic changes
were observed.
Tumours of various cell types occurred in many tissues. The only
changes that showed a clear relationship to treatment were increased
proliferative cell lesions in thyroid C-cells, the adrenal medulla,
and the uterine endometrium (Table 6). A statistical evaluation of the
tumour incidence in all animals was not provided, but according to a
statistical analysis of data on tumours found at final sacrifice,
females at the low and high doses had a significantly reduced
incidence of C-cell hyperplasia at terminal sacrifice, while males at
the low and intermediate doses had significantly increased incidences
of C-cell adenomas. The increase in C-cell carcinomas at termination
Table 6. Occurrence (%) of proliferative lesions in organs of rats given ethoprophos in the diet
Groups of animals and tumour type Dose (ppm)
Males Females
0 1 60 400 0 1 60 400
No. of animals
No. of unscheduled deaths 50 42 42 30 42 47 37 27
No. at terminal sacrifice 20 28 28 41 29 23 33 44
Total 70 70 70 71 71 70 70 71
Thyroid
C-cell hyperplasia 31 29 41 39 61 39 63 46
C-cell adenomas 11 9 13 17 14 11 16 17
C-cell carcinomas 0 0 1 4 1 1 1 3
All tumours 11 9 14 21 15 12 17 20
Adrenal
Benign phaeochromocytomas 20 10 10 7 4 3 1 3
Malignant phaeochromocytomas 0 3 3 7 0 0 0 0
All tumours 20 13 13 14 4 3 1 3
Uterus
Endometrial stromal polyps 1 1 4 8
Data are incidences in percentages of the total number of animals (terminal sacrifice plus intercurrent deaths)
was not significant. Males at the high dose showed a significant
decrease in the incidence of benign phaeochromocytomas but a
significant increase in that of malignant phaeochromocytomas at study
termination. The increased incidence of uterine endometrial stromal
polyps in females at the high dose was also significant. The study
authors indicated that C-cell and uterine proliferative lesions are
more likely to occur in old animals and their increased incidences are
attributable to the greater longevity of the animals at the high dose.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of inhibition of brain acetylcholinesterase activity at the next
highest dose (Williams, 1992).
(d) Genotoxicity
The results of studies on the genotoxicity of ethoprophos are
summarized in Table 7.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity conducted
according to GLP and with a QA statement, groups of 28
Crl:CD(R)(SD)BR rats of each sex, 6 weeks of age, were given diets
containing ethoprophos (purity, 95.3%) in acetone at concentrations of
0, 1, 30, or 300 ppm, equal to 0, 0.04, 1.3, or 23 mg/kg bw per day
for males and 0, 0.09, 2.6, or 27 mg/kg bw per day for females. After
10 weeks of exposure, the F0 parents were mated 1:1 to produce the
F1a generation. Treatment was continued through mating, gestation,
parturition, and lactation. On day 4 after parturition, each litter
was culled to four pups of each sex. After 3 weeks of lactation, the
pups were weaned. Owing to significant mortality among weanlings at
the high dose, pups in the control, low-, and intermediate-dose groups
were not used as parents for the next generation but instead 10 pups
of each sex were selected for necropsy. All weanlings at the high dose
and control F1a weanlings selected as parents were maintained on
their respective diets until day 49 post partum; the weanlings at
the high dose and 10 controls of each sex were then necropsied. From
about 1 week after weaning of the last F1a pups, F0 parents at 300
ppm were fed a diet containing 150 ppm of ethoprophos (equal to 7.1
mg/kg bw per day for males and 14 mg/kg bw per day for females), and
all F0 animals at the other two doses were maintained on their
original diets. After 3 weeks, the F0 parents were mated again to
produce the F1b generation, mating, gestation, parturition,
lactation, and weaning being conducted as described above. One week
after weaning, 28 F1b pups of each sex at each dose were selected as
parents of the F2 generation. The remaining F1b pups and all F2
pups were necropsied. Parental F0 animals aged 35-36 weeks and
parental F1 animals aged 20-21 weeks were killed after weaning of
the F1b and F2 progeny, respectively, and neccropsied grossly.
Blood was taken just before sacrifice, and brain tissue was collected,
for analyses of cholinesterase in plasma, erythrocytes, and brain. The
Table 7. Results of studies of the genotoxicity of ethoprophos
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa,b S. typhimurium 10-1000 µg/plate in NR Negative ± S9 Barfknecht et al. (1985a)
TA98, TA100, TA1535, DMSO
TA1537, TA1538
Gene mutationa Mouse lymphoma 0.24-0.032 µg/ml 95 Negative Thomson et al. (1981)
L5178Y cells, Tk locus (total growth between 18
and 126%, respectively),
- S9
0.024-0.0032 µg/ml
(total growth between 10
and 106%), + S9
Gene mutationc,d Chinese hamster ovary 0-500 µg/ml - S9 NR Negative Stankowski et al. (1985)
cells (CHO-K1-BH4); 0-150 µg/ml + S9
Hprt locus in DMSO
Chromosomal Chinese hamster ovary 0-300 µg/ml - S9 NR Negative SanSebastian et al. (1985)
aberrationsc,e cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivef
Unscheduled DNA Rat hepatocytes 2.5-100 nl/ml in DMSO; NR Negative Myhr & Brusick (1981)
synthesisg,h toxic from 50 nl/ml
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Sister chromatid Chinese hamster ovary 0-350 µg/ml - S9 NR Negative SanSebastian et al. (1986)
exchangec,j cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivek
Table 7. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Chromosomal Rat (SD) bone 0-20 mg/kg bw per day for 5 days 95.7 Negative Skinner et al. (1981)
aberrationl marrow by gavage in Methocel K4M
premium; sacrifice 6 h after dosing
Chromosomal Rat (SD) bone 0-25 mg/kg bw once by gavage; 95.5 Negative Ivett (1989)
aberrationm,n marrow sacrifice 6, 18, and 30 h after
dosing 0-25 mg/kg bw per day for
5 days by gavage; sacrifice 6 h
after dosing
Vehicle, corn oil.
Dominant lethal Rat (SD) 0-20 mg/kg bw per day for 5 days NR Equivocal Putman & Schechtman
mutationo by gavage in corn oil (1981)
Dominant lethal Rat (Crl:COBS 0-20 mg/kg bw per day for 5 days 95 Negative Dearlove (1987)
mutationm,p CD(SD)BR) by gavage in carboxymethyl
cellulose
NR, not reported; S9, 9000 × g supernatant of rat liver; DMSO, dimethylsulfoxide; SD, Sprague-Dawley
a Test in triplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
b Cytotoxicity (small colonies) seen in preliminary test at 1666 µg/plate and no growth at 5000 µg/plate in TA1538 and TA100
c Test in duplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
d Cytotoxicity observed at doses > 167 µg/ml; relative initial survival, 18% at 350 µg/ml without S9 and 10% at 150 µg/ml with S9
e Only 100 cells/dose scored instead of 200/dose required by OECD; cytotoxicity at doses > 500 µg/ml without S9 and at 80 µg/ml
with S9, and average proliferation time increased at 400 and 60 µg/ml, respectively
f Statistically significant increase in aberrations seen with metabolic activation at 60 µg/ml. In a second test at concentrations
of 50-70 µg/ml, statistically significant but not dose-related increase at all concentrations (14-22 breaks compared with 2 breaks
in control). The authors considered this a weakly positive result. Although the results of the second test were not dose-related,
they confirm the positive result of the first test.
Table 7 (continued)
g Test in triplicate; positive controls included; GLP and QA statements included
h Purity, 95% (personal communication from Dr Rao, Rhone Poulenc). Toxicity observed as 73% survival at 50 nl/ml and 0% at 100 nl/ml.
i Cytotoxicity (abnormally low nuclear and cytoplasmic grains) at doses > 333 µg/well
j Cytotoxicity at doses > 500 µg/ml without S9 and > 80 µg/ml with S9, and average proliferation time was increased at 400 and
60 µg/ml, respectively.
k Statistically significant, dose-dependent increase with metabolic activation at 50-60 µg/ml. In a second test at concentrations of
50-75 µg/ml, statistically significant, dose-related increase at all concentrations (22-35 aberrations/cell compared with 16 per
cell in controls). Since there was no twofold increase at any dose, the authors considered the compound a weak inducer.
l Aberrations analysed in 5 males/group; positive control included; absorption of compound tested in 1 animal/group and confirmed
by a minimum of a 10-fold depression in cholinesterase activity in plasma at 20 mg/kg bw per day for 5 consecutive days. Clinical
signs: decreased motor activity at high dose (day 2); mitotic index not measured. Not clear that the bone-marrow cells were
exposed to the test substance. Only 50 cells scored instead of 100 cells/animal required by OECD.
m GLP and QA statements included
n Each group of dose and harvest time consisted of 5 rats of each sex. Positive control group included in single-dose study only.
Deaths observed only in females at high dose. Clinical signs at high single dose: dyspnoea, languid appearance, and diarrhoea.
Additional signs at high multiple doses: tremor, rough coat, hunched posture, and dark crusts around eyes. No decrease in mitotic
index after single dose, but slightly decreased (54-75%) in males in short-term study. It is not clear whether the bone-marrow
cells were exposed to the test substance. Aberrations were studied in only 50 cells/animal instead of 100 cells and mitotic
index studied in only 500 cells instead of 1000 as required by OECD.
o 10 male rats/group. Three days after the last dose, males were mated with 2 untreated virgin females. Mating process repeated
another 6 times once a week with new virgin females. Females were killed about 2 weeks after mating; scoring for number of corpora
lutea number of dead and live implants per pregnant female. Absence of body-weight gain in males at high dose. No effect on
fertility index, number of corpora lutea, or number of implantations. Pre-implantation loss statistically significantly increased
in week 3 at high and low doses. Number of dead implants increased in week 1 at low dose, weeks 2-3 at intermediate dose, and weeks
1-6 at high dose, with a positive dose-response relationship. The proportion of females with one or more dead implants was
significantly increased at the high and intermediate doses (week 2 and weeks 2-3, respectively) and at the high dose also of females
with two or more dead implants. The number of dead implants per total number of implants was significantly increased at the high
dose (weeks 2-5) and intermediate dose (week 5) (0.08-0.12 in test groups compared with 0.02-0.05 in control group). The number of
live implants per pregnant female, however, was not affected (change in live implant ratio is the criterion of dominant lethality).
In the positive control group, more marked increases in dead implants per total implants observed in weeks 1-4 (0.6-1.0), and the
number of live implants per pregnant female was significantly decreased (0.0-8.3 compared with 9.9-11.9 in controls). The authors of
the report concluded that the compound was mutagenic; however, the shortcomings of the test and the fact that the number of live
implants was not affected indicate that the result is equivocal. The shortcomings of the study were that only 12-18 pregnant females
were included in each treated group and 15-17 in the control group, whereas according to OECD 478 30-50 pregnant females per mating
interval are required; mating was not confirmed. Only QA statement included.
Table 7 (continued)
p 24 males/group. Doses based on a range-finding study. Two days after the last dose, males were mated with 2 untreated virgin
females. Mating process was repeated another 7 times once a week with new virgin females. Females were killed 14 days after
presumed gestation; scoring for number of corpora lutea and for number of dead and live implants per pregnant female. Other
observations: viability, clinical signs, body weight, food consumption, gross necropsy of males and females, especially reproductive
organs (including histopathological examination). Three males at high dose died showing clear signs of intoxication. One male was
replaced. Clinical signs at high dose were characteristic of cholinesterase poisoning. Body-weight gain was inhibited (dose-related)
during treatment at 5 and 20 mg/kg bw per day, and food consumption was lower at the high dose. No compound-related findings at
gross necropsy except a single finding of an epididymal mass in one male at 5 mg/kg bw per day; no effect on testis weight, mating
index, fertility index, or pregnancy rate. Average numbers of corpora lutea, implantations, litter sizes, resorptions, percentage
of dead conceptuses, and number of females with resorptions similar in treated and control groups, except a single finding of dams
with increased resorptions in week 4 of mating at the intermediate dose.
thyroid glands were weighed, and selected tissues (especially from the
reproductive organs) from control and high-dose animals were examined
histopathologically.
The dietary concentrations of 1, 30, and 300/150 ppm during the
first premating and mating periods were equal to 0.07, 2.2, and 23/12
mg/kg bw per day for F0 and F1 males and 0.09, 2.7, and 27/14
mg/kg bw per day for F0 and F1 females. During the second mating
period, the doses of 1, 30, and 150 ppm given to F0 males were equal
to 0.04, 1.3,and 7.1 mg/kg bw per day; the intake of females,
calculated only for weeks 20-24, was 0.07, 2.0, and 11 mg/kg bw per
day.
The clinical signs in F0 parents at the high dose were soft
faeces during the first 2 weeks of exposure and tremors in females
during gestation and lactation. No clinical signs were observed in
F1 parents. The body weights of F0 males at 300 ppm and F1 males
at 150 ppm were significantly decreased throughout the exposure
period. F0 males at 300 ppm had significantly lower body-weight
gains during several weeks, whereas an increase in body-weight gain
was observed occasionally when the dose was changed to 150 ppm. The
weight gain of F1 males at the high dose was reduced during the
premating period and occasionally thereafter. Significantly decreased
body-weight gains were observed occasionally in males at 30 ppm but
these did not result in significantly lower body weights. F0 females
at the high dose had significantly reduced body-weight gains in weeks
1 and 9, but these did not result in lower body weights during the
premating period for the F1a litter. The total body-weight gain
during the 0-20 days of gestation with the F1a litter was
significantly reduced, and the body weights were lower during
lactation. These lower (but not statistically significant) body
weights of F0 females at the high dose persisted during the
premating and mating periods for F1b progeny and reached statistical
significance on days 4-21 of lactation, although there was no effect
on body-weight gain. The body weights of F1 females at the high dose
were significantly reduced throughout premating, gestation, and
lactation, but with no effect on weight gain. Food intake was affected
only at the high dose, with lower consumption observed for F0 males
in weeks 1, 2, and 20 and for F1 males in weeks 9, 11, and 16-20.
The food consumption of females of the F0 generation at the high
dose was lower during the first week of exposure, but food consumption
was affected only on days 4-14 of gestation of the F1a generation.
Length of gestation and indices of mating, fertility, and gestation
were not affected in either generation. The final body weights of F0
and F1 males at the high dose were significantly reduced, and only
in F1 males at this dose the absolute, but not the relative, weight
of the thyroid was significantly lower. No treatment-related lesions
were observed on gross or histopathological examination.
Cholinesterase activity was significantly inhibited in plasma and
brain of F0 and F1 animals of each sex at the high dose, in F1
animals at 30 ppm, and in F0 females at 30 ppm; plasma
cholinesterase activity was also inhibited in F0 males at 30 ppm.
There was no effect on erythrocyte acetylcholinesterase activity.
Analyses of data for the litters revealed lower body-weight gains
in F1a pups at 300 ppm throughout lactation, resulting in lower body
weights from day 7 onwards in males and from day 4 in female pups. The
survival of F1a pups was not affected up to weaning, but the
survival index was only 51% 1 week after weaning on day 28. Necropsy
of dead F1a pups showed blood in the stomach and/or intestines. The
body weights of surviving F1a pups at the high dose remained reduced
up to sacrifice on day 49, and their body-weight gain was reduced up
to day 35. F1b pups at the high dose of 150 ppm showed lower
body-weight gain, from the second week post partum onwards for
females and from the third week for males, resulting in lower body
weights from day 14 and day 21, respectively. Survival was not
affected. Significant reductions in average litter size at 1 ppm,
beginning on day 4, were considered spurious owing to the lack of a
dose-response relationship. The litter sizes and sex ratios of F2
litters were unaffected by treatment. The decreased litter sizes, and
as a consequence higher pup body weights, at 1 and 30 ppm and the
reduced sex ratio at 30 ppm were considered not to be related to
treatment in the absence of a dose-response relationship. The
body-weight gain of pups at the high dose was reduced from day 4
onwards, resulting in lower weights from lactation day 7. Reduced
weight gain was found on days 4-7 of lactation in the group receiving
30 ppm, with a (probably) compensatory increase on days 14-21, but
this was considered irrelevant toxicologically. Deaths among F2 pups
at the high dose on days 7-14 resulted in a significantly reduced
14-day survival index and lactation index. No treatment-related
lesions were observed in dead F2 pups or in F1a, F1b, and F2
pups subjected to scheduled necropsy 1 week after weaning.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of lower body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in parents of each sex in each
generation. No effects were observed on reproductive parameters. The
NOAEL for toxicity to pups was 30 ppm, equal to 1.3 mg/kg bw per day,
on the basis of effects on body weight and body-weight gain and deaths
in F2 litters at 150 ppm (Neeper-Bradley, 1991).
Groups of 10 male and 20 female Fischer 344 rats, 44 days old,
were fed diets containing technical-grade ethoprophos (purity, 95.3%)
at concentrations of 0, 60.5, 131, or 262 ppm (equivalent to 0, 3.0,
6.6, and 13 mg/kg bw per day) for 56 days before mating. Weanlings
from the second litters were selected to be the parents of the next
generation. All parental animals and weanlings of each generation were
subjected to gross pathological examination. Additionally, all F1b
and F2b parents and five male and five female weanlings from each of
the F1a, F2a, F3a, and F3b litters were evaluated
histopathologically. A statement of QA was provided.
The mortality rate was unaffected but growth was slightly
depressed at the two higher doses in all parental generations. Data on
food consumption were not available. A dose-related increase in the
number of dams that cannibalized their young was noted in all treated
groups of the F0 generation, mainly with regard to the second
litters. The percentage of animals becoming pregnant was reduced in
all treated F1a litters (75, 60, 55, and 55%, respectively, at 0,
60.5, 131, and 262 ppm) and for F3a and F3b litters at 262 ppm.
The lactation index (pup survival on days 4-21) was decreased at both
131 and 262 ppm in the F1b (56% and 40%, respectively, compared with
89% in controls) and the F2a generation (86% and 67%, respectively,
compared with 100% in controls) and at the high dose in the F2b
generation (67% compared with 88% in controls). Weanling weight (not
determined for F1a and F1b litters) was depressed in the F2a and
F2b litters at 131 and 262 ppm and in the F3a litters at 262 ppm.
Other effects that were observed only at the highest dose included a
tendency for decreased litter size at birth in almost all generations
of progeny, prolongation of the mean gestation period of F1b litters
(this parameter was not evaluated in all generations), the occurrence
of bilateral lenticular opacity (cataracts; in 29%) and anophthalmia
(3%) in F2a weanlings (information on the litter distribution of
these abnormalities was not available), and an increased incidence of
multifocal granuloma of the mesenteric lymph nodes in F2b adults.
The increased incidence of cannibalization among F0 dams and the
slight decrease in pregnancy rate in F1a litters at 60.5 ppm did not
recur in all generations and were probably related to maternal
toxicity. This concentration was considered to be the minimal
no-effect level for reproductive toxicity. As enzootic pneumonia was
observed in all parental animals and in some pups, the results of this
test are unreliable (Fletcher et al., 1980).
(ii) Developmental toxicity
Rats
A preliminary dose-range finding study was conducted according to
GLP and with a statement of QA, in which five female Sprague-Dawley
COB SC rats were given ethoprophos (purity, 95.6%) in corn oil at
doses of 0, 0.2, 1, 4, 8, or 16 mg/kg bw per day by gavage on days
7-15 of gestation. Fetuses were removed surgically on day 20 and
examined for external anomalies. The only finding was body-weight loss
in dams at the highest dose during days 6-9 of gestation and reduced
body-weight gains (24%) throughout treatment. There were no signs of
developmental toxicity (Rodwell, 1989a).
In the main study, conducted according to GLP and with a
statement of QA, groups of 25 pregnant female
Crl:COBS(R)CD(R)BR(R)VAF rats aged 3 months were given
ethoprophos (purity, 95.6%) by gavage in corn oil at doses of 0, 2, 9,
or 18 mg/kg bw per day on days 6-15 of gestation. The dams were
observed for clinical signs, and body weight and food consumption were
measured. Fetuses were removed surgically on day 20, weighed, and
examined for external, visceral, and skeletal anomalies.
Dose-dependent maternal toxicity was observed at 9 and 18 mg/kg
bw per day, with treatment-related findings including soft stools at
both doses and faecal staining at the high dose. Significantly lower
body-weight gain was observed at 9 mg/kg bw per day on days 6-9 of
gestation, and during this period females at the high dose showed
body-weight loss which resulted in lower body weights by the end of
the study. The food consumption of animals at the high dose was
reduced on days 6-16 of gestation. No effect was found on survival, no
morphological changes were observed at necropsy, and no changes in
gravid uterine weight or fetal parameters (viability, resorptions,
body weight, and sex ratio) were found. The incidences of external,
visceral, and skeletal variations and of irreversible structural
changes were unchanged. The NOAEL for maternal toxicity was 2 mg/kg bw
per day, and that for developmental toxicity was 18 mg/kg bw per day
(Rodwell, 1989b).
Groups of 25-35 mated female TAC:N(SD)FBR rats were intubated
with technical-grade ethoprophos (purity, 94%) in corn oil at doses of
0, 0.16, 1.6, or 16 mg/kg bw per day on days 6-15 of gestation, the
day a vaginal sperm plug was observed being considered day 0. The dams
were killed on day 20 and the fetuses removed for gross, skeletal, and
visceral examination. A positive control group was included.
The pregnancy rate was comparable in all groups. During
gestation, 1/24 pregnant dams at 1.6 mg/kg bw and 18/30 at 16 mg/kg bw
died or aborted; in addition, three non-pregnant dams at the high dose
were found dead. Growth was depressed on days 15 and 20 of gestation
in dams at 16 mg/kg bw. No significant difference was found between
control and treated groups in the mean numbers of corpora lutea,
implantation sites, live fetuses, dead fetuses or resorptions, mean
fetal weight, or sex ratio. According to a summary of the skeletal
findings and soft-tissue abnormalities in fetuses, the only notable
observation was a significant increase in the incidence of litters
containing fetuses with extra lumbar ribs in animals at 1.6 mg/kg bw.
Since no dose-response relationship was seen, the authors could not
attribute this finding to exposure to ethoprophos. Incomplete
ossification of vertebrae was observed in all groups, including the
controls, with incidences of 18, 10, 7, and 17% in control, low-,
intermediate-, and high-dose groups, respectively). The NOAEL for
maternal toxicity was 1.6 mg/kg bw per day on the basis of deaths and
depressed body-weight gain, and the NOAEL for developmental toxicity
was 16 mg/kg bw per day (Knickerbocker & Re, 1979, and amendment in
1985).
Rabbits
A preliminary dose-range-finding study was conducted according to
GLP and with a statement of QA in which groups of eight female New
Zealand white rabbits received ethoprophos (purity, 95.6%) in corn oil
at doses of 0, 0.1, 0.5, 2, 5, or 10 mg/kg bw per day by gavage on
days 7-18 of gestation. The fetuses were removed surgically on day 29
and observed for external anomalies. Dose-dependent maternal toxicity
was observed at 5 and 10 mg/kg bw per day, the clinical signs
including emaciation, an increased incidence of soft stools, and
faecal and urine staining. Consistent body-weight loss and a
dose-dependent mortality rate were also seen in these two groups.
There were no signs of developmental toxicity (Rodwell, 1989c).
In the main study, conducted according to GLP and with a
statement of QA, groups of 20 artificially inseminated New Zealand
white rabbits aged 6 months received ethoprophos (purity, 95.6%) by
gavage in corn oil at doses of 0, 0.625, 1.25, or 2.5 mg/kg bw per day
on days 6-18 of gestation. The does were observed for clinical signs,
and body weight and food consumption were measured. The fetuses were
removed surgically on day 29, weighed, and examined for external,
visceral, and skeletal anomalies.
There were no clinical signs of maternal toxicity, no adverse
effects on survival, body weight, or food consumption, and no
morphological changes. Gravid uterine weight and fetal parameters
(viability, resorptions, body weight, and sex ratio) were also
unchanged. The finding of a significantly reduced mean number of
viable fetuses per doe at 1.25 mg/kg bw per day was due to the absence
of this effect in the group at the high dose and considered not to be
related to treatment. The incidences of external, visceral, and
skeletal variations were not altered, as the finding of a decreased
incidence of 27 presacral vertebrae in fetuses at the intermediate and
high doses was considered not to be toxicologically relevant.
Increased incidences of cleft palate, flexed paw, gastroschisis, and
cephalocoele seen on external examination and of atelectasis and
hydrocephaly seen on visceral examination of the group at the
intermediate dose when compared with controls and with historical
control values were observed in fetuses of only a single doe and were
considered not to be related to treatment. The incidences of fetuses
with malformations were 2.4, 4.1, 4.0, and 6.6% and those of litters
with malformed fetuses were 12, 11, 11, and 35% in the control, low-,
intermediate-, and high dose groups, respectively. The malformations
seen at the high dose were dissimilar and occurred once or at very low
frequency; furthermore, they were types that occur spontaneously in
this strain of rabbits and the incidences were within the historical
control values. In the absence of effects on fetal weight, the
increased incidences of irreversible structural changes at the high
dose were considered to be unrelated to treatment. The NOAEL for both
maternal and developmental toxicity was 2.5 mg/kg bw per day, the
highest dose tested (Rodwell, 1989d).
Groups of 17 artificially inseminated female New Zealand white
rabbits were intubated with technical-grade ethoprophos (purity,
95.7%) in corn oil at doses of 0, 0.125, 0.5, or 2 mg/kg bw per day on
days 6-18 of gestation, the day of insemination being considered day
0. The surviving does were killed on day 29 of gestation, and their
fetuses were removed for external, visceral, and skeletal
examinations. A statement of QA was provided.
No treatment-related deaths occurred, but does at all doses
showed an increased (but not dose-related) incidence of anorexia
during and after treatment. A dose-related but not statistically
significant decrease in mean body-weight gain was observed between
days 6 and 18 in the does at the two higher doses. Control and treated
groups did not differ significantly with respect to pregnancy rate,
mean numbers of corpora lutea, implantations, resorptions, dead
fetuses, or live fetuses, mean fetal weight, mean fetal crown-rump
length, sex ratio, uterine weight with or without fetuses, or the
frequency of gross and visceral abnormalities. An increased total
incidence of skeletal variants was observed in the litters of all
treated does, with 14% at the low dose, 13% at the intermediate dose,
and 17% at the high dose, and 6% in controls. The increase was not
dose-related, and the frequency of any particular type of skeletal
variant, when considered alone, did not show a dose-response
relationship. The NOAEL for maternal toxicity was 0.12 mg/kg bw per
day on the basis of reduced body-weight gain. The NOAEL for
developmental toxicity was 2 mg/kg bw per day (Wolfe et al., 1981).
(f) Special studies: Neurotoxicity
Rats
In a study conducted according to GLP and with a statement of QA,
groups of 24 Crl:CD(SD)BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single dose of ethoprophos (purity, 95.7%) by gavage
in corn oil. Male rats received doss of 0, 30, or 60 mg/kg bw and
females received 0, 20, or 40 mg/kg bw. The doses were based on a
range-finding study. The animals were observed daily for deaths and
clinical signs, and body weights were recorded weekly. Blood samples
were taken from six animals of each sex per group 2 h and 3, 8, and
15 days after dosing to determine cholinesterase inhibition in plasma
and erythrocytes. Brain acetylcholinesterase activity was determined
in the frontal cortex, caudate/putamen, hippocampus, and cerebellum of
the same animals at the same times.
One male rat given 60 mg/kg bw was found moribund. The clinical
signs observed in females at 40 mg/kg bw and males at 60 mg/kg bw were
tremors and excessive salivation. Males also showed hunched posture,
laboured breathing, rough and stained coats, pale bodies, ocular
discharge, incoordination, hypoactivity, and were cold to touch. Among
animals at the lower dose, the only clinical sign was cold to touch in
one female at 20 mg/kg bw. There were no effects on body weight.
Cholinesterase activity was inhibited in all tissues examined by 2 h
after treatment, and the effects were dose-, time-, and
tissue-dependent (Table 8). Recovery of cholinesterase activity was
apparent by day 3 or 8 after dosing and was complete by day 15 for
plasma and cerebellar cholinesterase activity. Statistically
significant inhibition of acetylcholinesterase activity persisted in
the hippocampus through day 15 at the high dose and in erythrocytes
and the frontal cortex of males at both low and high doses. Persistent
inhibition was observed in the caudate/putamen at both doses, but the
inhibition was not statistically significant (Weiler, 1994a).
In a study conducted according to GLP and with a statement of QA,
groups of 17 Crl:CD(R) BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single oral dose of ethoprophos (purity, 96.2%) by
gavage in corn oil. The doses were 0, 5, 50, or 75 mg/kg bw for males
and 0, 5, 25, or 50 mg/kg bw for females, which were based on
range-finding studies. The animals were observed daily for deaths and
Table 8. Cholinesterase activity in various tissues of rats after a single oral dose of ethoprophos
Tissue Dose Inhibition from control mean (%)a
(mg/kg bw)
Males Females
Day 1b Day 3 Day 8 Day 15 Day 1b Day 3 Day 8 Day 15
Plasma cholinesterase 30/20 83* 43* 6 12 90* 31* 27 6
60/40 93* 68* 19* 14 94* 52* 23 0
Erythrocyte cholinesterase 30/20 43* 46* 31* 23* 44* 48* 29* 17
60/40 53* 46* 33* 22* 49* 50* 35* 17
Brain cholinesterase in 30/20 45* 38 30 9 72* 25 54 25
caudate/putamen 60/40 93* 75* 51 32 92* 63* 39 40
Brain cholinesterase in 30/20 45* 30* 19* 0 50* 14 8 4
hippocampus 60/40 72* 47* 27* 18* 75* 29* 21* 17*
Brain cholinesterase in 30/20 48* 38* 34 19* 55* 10 0 32
frontal cortex 60/40 76* 50* 49* 27* 77* 40* 24* 29
Brain cholinesterase in 30/20 46* 11 0 0 60* 11 0 3
cerebellum 60/40 81* 35* 7 6 80* 29* 9 3a
a 100% - mean treated/mean control × 100%
b 2 h after dosing
* Significantly different from control at p < 0.05
clinical signs, and body weights were recorded before treatment and on
days 1, 8, and 15. Twelve animals of each sex per group were subjected
to a functional observational battery of tests and tests for motor
activity before treatment and at 2 h and 8 and 15 days after dosing.
Cholinesterase activity was determined in plasma and erythrocytes from
the remaining five animals of each sex per group before treatment and
on days 2, 8, and 15 after dosing. After blood collection on day 15,
the animals were killed and their brains collected for determination
of acetylcholinesterase activity. Neuropathological examinations were
conducted at the end of the study on all appropriate tissues from six
rats of each sex per group that had not been used to determine
cholinesterase activity. All animals were examined macroscopically and
only the control and high-dose groups microscopically.
Treatment-related deaths were seen in two males given 75 mg/kg bw
and six females given 50 mg/kg bw; four of the females were replaced.
The deaths of one male at 50 mg/kg bw and one female at 5 mg/kg bw
were considered to be unrelated to treatment. Clinical signs in
animals at the high doses included thin or hunched appearance,
tremors, laboured respiration, protruding eyes, incoordination,
hypoactivity, excessive salivation, and cold to touch. A few males at
50 mg/kg bw also showed some of these signs. The results of the
functional battery showed mild to severe functional or behavioural
changes only on the day of dosing, and the changes were characteristic
of cholinesterase poisoning. No effects were seen at 5 mg/kg bw dose.
Mild effects were seen in two females at 25 mg/kg bw and in several
males at 50 mg/kg bw, which included salivation, lip smacking, ataxia,
negative pupillary response, and tremors. Moderate and severe effects
were observed in females at 50 mg/kg bw and males at 75 mg/kg bw,
which included, in addition to the effects described above, low
carriage and prostrate position, lethargy, changes in the ease of
handling, lachrymation, laboured or gasping respiration, increased
latency until first step, paralytic gait, negative corneal response,
and negative air drop and startle reflexes. The analgesic reflex to a
mild heat stimulus was delayed significantly on day 1 in males and
females at 50 mg/kg bw and in males at 75 mg/kg bw. Body temperature
was statistically significant decreased by 3-4°C in females at 50
mg/kg bw and in males at 75 mg/kg bw; a slight decrease in body
temperature was seen in males at 50 mg/kg bw. Mean forelimb grip
strength was decreased only on day 1 in males at 75 mg/kg bw and
females at 50 mg/kg bw and was statistically significant only in
males. The NOAEL for behavioural and functional effects was 5 mg/kg
bw. Motor activity was markedly and statistically significantly
decreased in males at 50 and 75 mg/kg bw (dose-related) and in females
at 50 mg/kg bw on day 1 (Table 9). Differences were no longer seen on
day 8 or 15. The NOAEL for motor activity was 5 mg/kg bw.
The effects on cholinesterase activity are summarized in Table
10. On day 2, plasma cholinesterase activity was dose-dependently
inhibited for males (45-94%) and females (49-94%) at all doses but had
recovered within 8 days of dosing. Erythrocyte acetylcholinesterase
activity was inhibited in females at all doses (not dose-related) and
in males at 50 and 75 mg/kg bw. Generally nonsignificant inhibition of
Table 9. Motor activity (mean total counts) measured shortly after dosing (day 1) and during 10-min
intervals for 60 min in rats given single doses of ethoprophos
Dose Motor activity (mean (standard deviation))
(mg/kg bw)
Males Females
0-10 min 10-20 min 2-30 min Total 0-10 min 10-20 min 20-30 min Total
(0-60 min) (0-60 min)
0 720 (130) 280 (140) 100 (120) 1300 (510) 580 (86) 280 (140) 98 (100) 1200 (330)
5 630 (110) 300 (160) 51 (64) 1000 (270) 650 (160) 200 (180) 69 (110) 1200 (460)
25 480 (270) 170 (210) 78 (140) 1100 (960)
50 290* (240) 150* (110) 20 (25) 670* (430) 38* (34) 28* (24) 44 (80) 240* (190)
75 33* (28) 25* (22) 17 (19) 180* (100)
* Significantly different at p < 0.05
Table 10. Cholinesterase activity in plasma, erythrocytes, and brain of rats given a single
dose of ethoprophos
Dose Day of Inhibition of cholinesterase from mean control valuea
(mg/kg bw) sampling
Males Females
Plasma Erythrocyte Brain Plasma Erythrocyte Brain
5 2 45* 8 49* 33*
8 -4 27 11 20
15 -8 -7 -3 -3 3 7
25 2 73* 49*
8 -12 21
15 -14 33* 11
50 2 87* 45* 94* 35*
8 -2 26 2 30
15 0 18 17 -3 20 10
75 2 94* 55*
8 6 25
15 -4 31* 18
a 100% - mean treated/mean control × 100%
* Significantly different at p < 0.05
> 20% was still present on days 8 and 15 in females at 25 and 50
mg/kg bw and in males at 50 and 75 mg/kg bw. In these groups, brain
acetylcholinesterase activity was inhibited by about 10% in females
and 18% in males at day 15, but this effect was not statistically
significant. Macro- and microscopic neuropathological examinations
showed no treatment-related changes. As brain acetylcholinesterase
activity was not measured directly after exposure, the NOAEL could not
be based on this end-point. The inhibition in females was not
dose-related, and there was a large standard deviation at 5 mg/kg bw.
The NOAEL for neurotoxicity was 5 mg/kg bw on the basis of behavioural
changes and inhibition of erythrocyte acetylcholinesterase activity
(Weiler, 1994b).
Groups of 27 Crl:CD BR VAF/Plus rats of each sex aged 6 weeks
were fed diets containing ethoprophos (purity, 95.7%) at
concentrations of 0, 4, 40, or 400 ppm, equal to 0, 0.26, 2.6, and 27
mg/kg bw per day for males and 0, 0.31, 3.0, and 31 mg/kg bw per day
for females, for 13 weeks. Twelve rats of each sex at each dose were
selected for functional battery and motor activity testing before
dosing and in weeks 4, 8, and 13. Of these, six of each sex per dose
were selected for neuropathological investigations, and the remaining
rats were observed only macroscopically. Cholinesterase activity was
measured in the plasma and erythrocytes of 10 rats of each sex per
group and acetylcholinesterase activity in four regions of the brain
(frontal cortex, caudate/putamen, hippocampus, and cerebellum) at
sacrifice in five rats of each sex per dose in weeks 4, 8 and 14. All
animals were observed for deaths and clinical signs, and body weight
and food consumption were recorded.
The only clinical sign considered to be related to treatment was
brown staining of the anal region in males at 400 ppm. Significantly
lower body-weight gain was observed in males at 400 ppm throughout
treatment and in females up to week 10, which resulted in
significantly lower body weights in animals at this dose. The mean
food consumption of animals of each sex at the high dose was
significantly lower during the first week and remained low in males
throughout treatment. In males at 40 ppm, the food consumption was
higher than that of controls. Changes in the results of the functional
battery tests considered to be related to treatment were seen only at
the high dose and included mild tremor (one female), vocalization
during handling (two females), slight salivation (one male), unkempt
fur (two males, one female), latency to first step (one male, one
female), and jumping to touch (one male) in week 4; in week 8, the
observations included intermittent vocalization in cage (three
females), slight lachrymation (one female, and one female at 40 ppm),
slight salivation (one male, two females, and one female at 40 ppm),
latency to first step (two females), aggressive behaviour (one
female), jump to touch response (one female), negative air drop reflex
(one male), and negative pupillary response (one female); in week 13,
the observations included aggressive behaviour (one female), latency
to first step (one female, and two males at 40 ppm), and no movement
in open arena (one male). Because the clinical signs observed at 40
ppm also occurred occasionally as single findings in controls, they
were considered to be unrelated to treatment. Other clearly
treatment-related observations in the functional battery test at the
high dose were decreased analgesic reflex in males in weeks 4, 8, and
13, and lower grip strength in fore- and hindlimbs of males and in
forelimbs of females. The motor activity of males at 400 ppm was
generally significantly lower than that of controls at all times;
females showed a slight decrease in activity only in week 4.
Cholinesterase activity in plasma, erythrocytes, and brain is
shown in Table 11. A > 20% inhibition of erythrocyte
acetylcholinesterase activity was found in males and females at doses
> 40 ppm. Inhibition of acetylcholinesterase activity in one or two
regions of the brain by > 20% was observed in males at 40 ppm at
weeks 4 and 8 and in all regions at 400 ppm. In females, inhibition of
acetylcholinesterase activity by > 20% was seen in three brain
regions at weeks 8 and 14 at 4 ppm, which was significant in the
frontal cortex in week 14, and in all regions at all times at higher
doses. No macroscopic or microscopic changes were observed.
An NOAEL could not be identified because brain
acetylcholinesterase was inhibited at all doses. The NOAEL for
neurotoxic effects was 40 ppm, equal to 2.6 mg/kg bw per day, on the
basis of the results of the functional observational battery and motor
activity tests (Weiler, 1994c).
Chickens
Two groups of 10 deep-litter hens weighing 1.4-2.1 kg (age not
specified) were intubated with technical-grade ethoprophos (purity
unknown) at a single dose of 5.6 µl/kg bw, equivalent to 6.2 mg/kg bw
and stated to represent the oral LD50 value in a previous study
performed at the testing laboratory, or tri- ortho-cresyl phosphate
(positive control) at 500 mg/kg bw. Four untreated hens were retained
as untreated controls. The hens given ethoprophos showed no gross
signs of ataxia or paralysis, although symptoms of transient
inactivity and 'depression' were noted between 1 and 48 h. Four hens
died within 48 h of dosing. The positive controls showed clinical
signs characteristic of delayed neurotoxicity. Histopathological
examination of sections of the spinal cord and sciatic nerve, stained
by the Weil-Weigert method (not referenced), from birds in all groups
at the end of a 21-day observation period revealed no evidence of
demyelination (Weir & Murphy, 1967).
In an initial study conducted to determine the LD50 of
ethoprophos in hens, six groups of 10 domestic hens ('a hybrid brown
laying strain') were given ethoprophos (purity, 94.5%) at doses of
0-16 mg/kg bw in a volume of 2 ml/kg bw by gavage. An LD50 of 6.4
mg/kg bw was calculated, with a 95% confidence interval of 4.8-8.8
mg/kg bw. On this basis, a dose of 6.5 mg/kg bw was selected for the
main study. The hens were divided randomly into six groups of 10. The
untreated controls received only corn oil, whereas the positive
controls received 500 mg/kg bw of tri- ortho-cresyl phosphate by
gavage. The treated groups first received 10 mg/kg bw of atropine
Table 11. Cholinesterase activity in plasma, erythrocytes, and brain regions at various
times during 13-week dietary exposure of rats to ethoprophos
Week Tissue Inhibition of cholinesterase activity from mean in controlsa
Males Females
4 ppm 40 ppm 400 ppm 4 ppm 40 ppm 400 ppm
4 Plasma 3 64* 90* 20 80* 97*
Erythrocyte 7 30* 26* -14 19 14
Brain
Caudate/putamen 17 23* 82* 8 32 83*
Hippocampus -7 4 48* 9 18 50*
Frontal cortex 19 -2 71* -28 30 74*
Cerebellum -7 0 52* -3 13 53*
8 Plasma -5 58* 85* 34* 87* 98*
Erythrocyte 10 23 35 19 32 40
Brain
Caudate/putamen 3 11 82* -11 -2 53*
Hippocampus 11 26* 59* 23 33* 67*
Frontal cortex 10 23 61* 33 49 80*
Cerebellum 0 0 46* 21 38* 62*
14 Plasma -2 54* 87* 18 86* 97*
Erythrocyte 19 33* 40* -6 22 11
Brain
Caudate/putamen -3 0 73* 34 49* 88*
Hippocampus 0 -9 54* 23 36* 66*
Frontal cortex 10 7 53* 52* 56* 80*
Cerebellum 9 -11 49* 6 27* 59*
a 100% - mean treated/mean control × 100%
* Significantly different from controls at p < 0.05
sulfate by intramuscular injection and then 6.5 mg/kg bw of
ethoprophos by gavage in corn oil at a constant volume of 2.5 ml/kg
bw. The atropine was found to have had only a minimal protective
effect, as 31/40 of the treated birds died within 4 days, most within
the first 24 h. Because of excessive mortality, two additional groups
were added, consisting of 12 and 11 birds, respectively, but it was
not specified whether they were treated concurrently with the
untreated controls. These birds were given pyridine 2-aldoxime
methiodide at 50 mg/kg bw by intramuscular injection in addition to
atropine before treatment with 6.5 mg/kg bw of ethoprophos. The
surviving birds were given further injections of pyridine 2-aldoxime
methiodide and atropine 24 and 48 h after treatment, and two birds in
the last group were injected 72 h after treatment. This procedure was
only marginally successful, as 14/23 birds died within 48 h of
treatment. On the basis of the deaths after the first dose of
ethoprophos, the LD50 was recalculated as 5.2 mg/kg bw, and this
dose was administered on day 22 to all 18 surviving birds, which were
treated with pyridine 2-aldoxime methiodide and atropine immediately
before administration of ethoprophos and again 5 h after the second
(day 22) treatment with ethoprophos; birds in the initial groups were
given additional injections 24 h after the second treatment. Two of
the 18 treated birds died within 72 h of the second treatment.
No clinical signs of neurotoxicity were noted in untreated
control or ethoprophostreated birds, whereas 9/10 birds treated with
tri- ortho-cresyl phosphate showed slight to marked signs of
neurotoxicity, usually by 11 days after treatment. These birds were
killed on day 21 and examined for histopathological changes. All other
surviving birds were killed on day 43. No remarkable macroscopic
changes were observed at necropsy. Portions of the forebrain, mid- and
hind-brain, cervical, thoracic, and lumbar spine, proximal and distal
sciatic nerve, and tibial nerve were evaluated for microscopic
evidence of neurological change. Lesions were noted in the spinal cord
and peripheral nerves of all positive control birds that reflected
'significant axonal degeneration'. Minimal lesions (grade 2 out of a
maximum of 5) were also found in the spinal cord of 9/10 control
birds, and one control had a minimal lesion in the proximal sciatic
nerve; no lesions were found in the brain or distal sciatic or tibial
nerves. A similar distribution of lesions of the spinal cord was seen
in treated birds, except that minimal lesions of the mid- or
hind-brain and lesions of the proximal sciatic nerve were each found
in 2/16 birds. One of the latter lesions was graded as moderate (grade
3) and occurred in a bird that also had a lesion of the mid- and
hind-brain. Three of 16 birds, including this one, also had minimal
lesions of the distal sciatic nerve. The authors concluded that
treatment with ethoprophos produced no clinical signs of neurotoxicity
and that the result was confirmed by the histological examination,
which showed no treatment-related changes in the nerve tissue. The
changes noted in the proximal sciatic nerve of the bird with multiple
lesions were considered to be unrelated to treatment and to represent
the extreme upper limit of background change (Roberts et al., 1986).
The Committee concluded that the study does not provide clear evidence
of neurotoxicity, but because the high rate of mortality reduced the
sensitivity of the study, the equivocal findings in some birds cannot
be dismissed. Historical control data from the testing facility
suggest that the lesions in proximal sciatic nerve are not
spontaneous.
(g) Studies on metabolites
O-Ethyl- S-propylphosphorothioate, a metabolite in rats, and
O-ethyl- O-methyl- S-propylphosphoro-thioate and
O-ethyl- S-methyl- S-propylphosphorodithioate, two metabolites
identified only in corn and potatoes (Rao, 1999), were tested for
toxicity and for their ability to inhibit cholinesterase activity in
female rats given single oral doses.
(i) Acute toxicity
The LD50 values of the three metabolites of ethoprophos in
female Crl:CD (SD)BR VAF/ Plus rats treated orally with the substances
in corn oil were 50 mg/kg bw for
O-ethyl- S-methyl- S-propylphosphorodithioate, 22 mg/kg bw for
O-ethyl- O-methyl- S-propylphosphorothioate, and 1600 mg/kg bw for
O-ethyl- S-propylphosphorothioate. The clinical signs were similar
for each of the metabolites and differed primarily in time of onset
and duration; the signs were also similar to those caused by the
parent compound. GLP and QA statements were included (Welier, 1998).
(ii) Cholinesterase inhibition
Three groups of 10 female Crl:CD(R)(SD)BR VAF/Plus rats aged
about 6 weeks were given a single dose of ethoprophos at 19 mg/kg bw,
O-ethyl- S-methyl- Spropylphosphorodithioate at 17 mg/kg bw, or
O-ethyl- O-methyl- S-propylphosphorothioate at 8 mg/kg bw by
gavage; a control group received the vehicle, corn oil. The animals
were observed for clinical signs before dosing, 0.5 and 2 h after
dosing, and the next day before sacrifice. Cholinesterase activity was
evaluated in plasma, erythrocytes, and brain 24 h after dosing.
Body-weight gain over the 2-day period was significantly lower in
all treated groups than in controls but resulted in a statistically
significant lower terminal body weight only with
O-ethyl- S-methyl- S-propylphosphorodithioate. In the other two
groups, the only clinical finding was a slightly increased incidence
of soft stools, whereas the animals given
O-ethyl- S-methyl- S-propylphosphorodithioate showed signs of
cholinesterase inhibition consisting of staggered gait and tremors.
All three compounds reduced cholinesterase activity in all three
tissues, the strongest inhibition being due to
O-ethyl- S-methyl- S-propylphosphorodithioate (Table 12; Weiler,
1998).
Table 12. Cholinesterase activity in plasma, erythrocytes, and brain of female rats 24 h
after a single oral dose of ethoprophos and two metabolites
Compound Dose Inhibition from control mean (%)a
(mg/kg bw)
Plasma Erythrocytes Brain
Ethoprophos 19 73* 37* 32*
O-Ethyl-S-methyl-S-propylphosphorodithioate 17 78* 30 71*
O-Ethyl-O-methyl-S-propylphosphorothioate 8 40* 47* 48*
a 100% - mean treated/mean control × 100%
* Significantly different from control at p < 0.05
4. Observations in humans
In a study of occupational exposure to ethoprophos in Salinas
Valley, Monterey County, California, USA, four male volunteers were
recruited among regular employees of an experienced pesticide
application firm who were thoroughly familiar with both the practices
and equipment involved in pesticide application and had not worked
with cholinesterase inhibitors for at least 90 days. The occupation
under consideration was that of mixer-loader-applicator, who applied
an emulsifiable concentrate containing 70% ethoprophos in xylene for 3
days (two men), 4 days (one man), or 5 days (one man) to field plots
of 2-3 ha; the only operator working on day 5 treated a field plot of
about 8.6 ha. During the mixing and loading phase, the concentrate,
supplied in 5-gallon (~ 20-L) containers, was diluted to 5% in two
160-gallon (~ 40-L) tanks. Transfer of the pesticide into the tank,
rinsing the container, and filling the spray tanks were done in a
closed system to minimize exposure. The operators wore long-sleeved,
twill work shirts, denim jeans, and rubber boots. They used rubber
gloves and disposable respirators which were changed each day. Face
shields and hard hats were available, especially for the mixing and
loading operations. The application rate of active ingredient was
about 12 kg/ha (range, 11-15 kg/ha). Blood was collected for
determination of cholinesterase activity in plasma and erythrocytes
three times before exposure and as soon as possible after each man's
work session. During application of a commercial preparation
containing 69.6% ethorprop (as an emulsifiable concentrate in xylene),
dermal exposure was monitored on the hands (three hand washings with
2-propanol in water) and face (passive facial patch collector
consisting of 12-layer surgical gauze), corresponding to a surface of
24 cm2. Inhalation was not monitored. Exposure to ethoprophos was
monitored by a standardized gas chromatographic method.
No clinical signs or adverse effects were noted during exposure,
and the changes in plasma and erythrocyte cholinesterase activity were
not statistically significantly different from the mean baseline
values. The maximal variation around the mean was - 8.9% to 7.9%,
which is considered to be normal day-to-day variation. Exposure on the
head and neck was 3.1-78 µg/h, with an average of 34 µg/h
(calculations based on a default head area of 910 cm2). The total
exposure of the head to ethoprophos was 7.6-153 µg, with means of 96,
53, 67, and 36 µg for the four men. The hands were exposed to 0.2-18
µg/h, with an average value of 6.3 µg/h and a value of 0.52-42 µg for
both hands; the mean values were 29, 2.0, 12, and 6.3 µg for the four
men. The overall exposure during the entire period of observation of
the man who worked for 5 days was 620 µg, and the quantity of active
ingredient handled per day was 31-92.4 kg. The overall exposure of the
other operators was 220 µg for the man who worked for 4 days and
handled 26-44 kg daily; 240 µg for the man who worked for 3 days and
handled 19-27 kg daily; and 130 µg for the man who worked for 3 days
and handled 17-44 kg daily (Leffingwell, 1986).
Comments
After oral administration to rats, 14C-ethoprophos was rapidly
and virtually completely absorbed, metabolized and excreted. The main
route of excretion was the urine (51-56%), but significant proportions
were excreted in expired air (about 15%) and faeces (10-14%). Little
radiolabel was found in tissues at 168 h, representing less than 2.5%
of the dose, and the highest concentrations were found in excretory
organs (liver, kidneys, and lungs). There was no evidence that
bioaccumulation would occur after repeated doses. The kinetics of the
radiolabel and the biotransformation appeared to be independent of the
route of administration (oral or intravenous), magnitude and frequency
of dose and sex. Ethoprophos was metabolized by dealkylation of one or
both S-propyl groups, followed by conjugation. The dermal absorption
of ethoprophos has not been studied in vivo, but radiolabelled
ethoprophos penetrated the skin in vitro, the greatest penetration
being seen through mouse skin, lesser penetration through rat and
rabbit skin (at equal rates), and the least penetration through human
skin. In all of the species tested, the rate of penetration was
greater with ethoprophos (emulsified concentrate) diluted 1:19 in
water than with undiluted material.
Single oral doses of ethoprophos were toxic to rats, mice and
rabbits (LD50 values, 31-62 mg/kg bw), and it was highly toxic to
rats exposed once by inhalation (LC50, 0.25 mg/L). Mice and rabbits
were more sensitive to dermal exposure than rats, and female rats were
more sensitive than males, with LD50 values of 8.5 mg/kg bw for
rabbits, 18 mg/kg bw for mice, 420 mg/kg bw for female rats, and 1300
mg/kg bw for male rats. The toxic signs observed after exposure by any
route were characteristic of cholinesterase inhibition. WHO (1999) has
classified ethoprophos as 'extremely hazardous'.
Ethoprophos not only irritates the eyes of rabbits but is very
toxic after administration into the eye, causing the death of tested
animals within 1 h. In a test for dermal irritation, all treated
animals died within 8 h of exposure to the undiluted compound. In a
three-week study in rabbits, the compound caused slight dermal
irritation at all doses tested (0.03, 0.1, and 1 mg/kg bw per day),
whereas no dermal irritation was observed in a 3-week study in rats
given doses of up to 10 mg/kg bw per day.
In studies in dogs (13, 20 and 52 weeks) and mice and rats (2
years) given ethoprophos orally, inhibition of cholinesterase was the
most sensitive parameter. In dogs, the NOAEL for inhibition of brain
acetylcholinesterase activity was 1 mg/kg bw per day, the highest dose
tested. In mice, brain acetylcholinesterase activity was inhibited at
30 ppm, resulting in a NOAEL of 2 ppm, equal to 0.25 mg/kg bw per day;
in rats, the overall LOAEL was 30 ppm (equal to 1.3 mg/kg bw per day)
and no effects were seen at 10 or 1 ppm (equal to 0.5 and 0.04 mg/kg
bw per day, respectively). In a two-generation study of reproductive
toxicity in rats, inhibition of brain acetylcholinesterase activity
was seen in animals of each sex of both parent generations at 30 ppm,
resulting in a NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day. In a
13-week study of neurotoxicity in rats, the activity of brain
acetylcholinesterase was inhibited in females at all doses tested, the
lowest dose being 4 ppm, equal to 0.26 mg/kg bw per day. Clinical
signs characteristic of cholinesterase inhibition were observed only
in the 2-year study in rats, in which the highest dose was reduced
from 600 to 400 ppm because of clinical signs.
Inhibition of brain acetylcholinesterase activity was also
demonstrated after dermal exposure of rabbits and rats to ethoprophos
for three weeks, with NOAELs of 0.1 mg/kg bw per day (LOAEL, 1 mg/kg
bw per day) in rabbits and 0.3 mg/kg bw per day (LOAEL, 1 mg/kg bw per
day) in rats.
Ethoprophos was not carcinogenic in a long-term study in mice
treated in the diet. The highest dose tested was 30 ppm, equal to 4.9
mg/kg bw per day. In three long-term studies of toxicity and
carcinogenicity in rats, some evidence was obtained that ethoprophos
may stimulate progression of C-cell tumours in the thyroid and adrenal
neoplasms (phaeochromocytomas), but this effect was considered to have
little relevance for human risk. The highest dose tested was 400 ppm,
equal to 26 mg/kg bw per day. The Meeting concluded that ethoprophos
is unlikely to pose a carcinogenic risk to humans.
Ethoprophos was tested in an adequate set of assays for
genotoxicity in vitro and in vivo. Positive results in studies for
chromosomal aberrations and sister chromatid exchanges in vitro
after metabolic activation indicate that the compound has intrinsic
genotoxic activity. Chromosomal aberrations were not induced in rats
in two assays and dominant lethal mutations were not induced in rats
in another study, although a slightly positive or equivocal result was
obtained in a further study for dominant lethality. The Meeting
concluded that ethoprophos is unlikely to be genotoxic in vivo.
In a two-generation study of reproductive toxicity in rats, no
effect was observed on reproductive parameters. Clinical signs were
observed only in the F0 parents at the highest dose (300 ppm). The
NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis of
reduced body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in animals of each sex in both parental
generations. The NOAEL for developmental toxicity was 30 ppm, equal to
1.3 mg/kg bw per day, on the basis of effects on body weight and
body-weight gain in pups of both generations and deaths of pups in the
F2 litters at 150 ppm.
In studies of developmental toxicity, maternal toxicity was
characterized by growth depression in rats in one study (NOAEL,
2 mg/kg bw per day) and in rabbits in one study (NOAEL, 0.12 mg/kg bw
per day), whereas no maternal toxicity was observed in rabbits in
another study (NOAEL, 2.5 mg/kg bw per day, highest dose tested).
Since in the first study in rabbits the effect was only marginal (not
statistically significant), the overall NOAEL for maternal effects in
rabbits was 2.5 mg/kg bw per day. No effects on the fetuses of either
species were observed. Cholinesterase activity was not measured in
these studies.
The relationship between neurotoxicity and cholinesterase
inhibition was examined in rats in two studies. In rats exposed by
gavage to single doses of 0, 20, or 40 mg/kg bw (females) or 0, 30, or
60 mg/kg bw (males), cholinesterase activity in plasma, erythrocytes,
and brain was inhibited, with a maximum effect 2 h after dosing. While
cholinesterase inhibition was found in tissues at the lowest doses
tested, clinical signs characteristic of cholinesterase inhibition
were seen only at the highest doses (60 mg/kg bw for males and
40 mg/kg bw for females). In the second study, in which male rats were
exposed to a single dose of ethoprophos at 5, 50, or 75 mg/kg bw and
females at 5, 25, or 50 mg/kg bw, effects on motor activity were
observed in animals of each sex at doses of 50 mg/kg bw and higher,
and functional and behavioural effects were observed at 25 mg/kg bw
and higher. These neurotoxic signs were seen only on the day of
dosing. Significant inhibition of acetylcholinesterase activity in
erythrocytes, measured 1 day after exposure, was observed in females
at doses of 5 mg/kg bw and higher and in males at 50 mg/kg bw and
higher. The inhibition in females was not dose-related, and there was
a large standard deviation at 5 mg/kg bw . The Meeting concluded that
the NOAEL in this study was 5 mg/kg bw.
In a 13-week study in rats, neurotoxicity was observed in tests
for motor activity and for functional and behavioural abnormalities
only at the highest dose (400 ppm). The NOAEL for neurotoxic effects
was 40 ppm, equal to 2.6 mg/kg bw per day. The activity of brain
acetylcholinesterase was inhibited in females at all doses tested; the
lowest dose was 4 ppm, equal to 0.26 mg/kg bw per day.
A study of delayed neurotoxicity in hens was performed with doses
of ethoprophos that caused a high mortality rate despite antidotal
treatment. Equivocal findings were reported in some of the treated
birds; data on the effect of this compound on neuropathy target
esterase activity in hens would be useful in order to allow full
assessment of the neuropathological potential of ethoprophos.
A study of occupational exposure to ethoprophos did not reveal
significant effects on human plasma or erythrocyte cholinesterase
activity. The calculated rates of exposure of head-and-neck areas were
3.1-78 µg/h (average, 34 µg/h), and the rate of exposure of the hands
was calculated to be 0.2-18 µg/h (average, 6.3 µg/h).
O-Ethyl S-propyl phosphorothioate, a metabolite in rats, and
O-ethyl O-methyl S-propyl phosphorothioate and O-ethyl
S-methyl S-propyl phosphorodithioate, two metabolites that have
been identified only in corn and potatoes, were tested for toxicity
and for their ability to inhibit cholinesterase activity in female
rats given single oral doses. The two plant metabolites were of
approximately the same toxicity as the parent compound, while the
animal metabolite was less toxic (LD50, 1600 mg/kg bw) than the
parent.
The 1987 Meeting derived the ADI on the basis of a slight effect
on the liver in a 52-week study in dogs at a dose of 1 mg/kg bw per
day. The NOAEL for this effect was 0.025 mg/kg bw per day. Since this
marginal effect was not observed in other studies in dogs or in other
species and there is a 40-fold difference between the LOAEL and the
NOAEL, this NOAEL was not used as the overall NOAEL to derive the ADI
in the present evaluation.
The present Meeting established an ADI of 0-0.0004 mg/kg bw on
the basis of the NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day, for
inhibition of brain acetylcholinesterase activity in the 2-year study
of toxicity and carcinogenicity in rats and in the study of
reproductive toxicity in rats, and a 100-fold safety factor.
An acute reference dose of 0.05 mg/kg bw was established on the
basis of the NOAEL of 5 mg/kg bw in the study of acute neurotoxicity
in rats, in which functional and/or behavioural effects and inhibition
of erythrocyte acetylcholinesterase were observed at the next highest
dose, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse 2 ppm, equal to 0.25 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
Rat 1 ppm, equal to 0.04 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
1 ppm, equal to 0.04 mg/kg bw per day (parental toxicity in
a study of reproductive toxicity)
5 mg/kg bw (single dose, study of neurotoxicity by gavage)
2 mg/kg bw (maternal toxicity, study of developmental
toxicity; acetylcholinesterase activity not determined);
18 mg/kg bw (fetotoxicity, highest dose tested, study of
developmental toxicity; acetylcholinesterase activity not
determined)
Rabbit 2.5 mg/kg bw (maternal toxicity and fetotoxicity, study of
developmental toxicity; acetylcholinesterase activity not
determined);
Dog 0.025 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0004 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Information on effects on neuropathy target esterase activity in
hens
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to ethoprophos
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption > 70%, mainly within 12 h (rats)
Dermal absorption Tested in vitro, no quantitative data
Penetration rate Mouse skin > rat, rabbit skin > human skin
Distribution Widely distributed, highest levels in organs of elimination (rats)
Potential for accumulation No
Rate and extent of excretion Excreted in urine (55%), expired air (13-17%), and faeces (7-9%) (rats)
Metabolism in animals In rats, metabolized by dealkylation of one or both S-propyl groups,
followed by conjugation
Toxicologically significant compounds Parent compound and plant metabolites ( O-ethyl O-methyl S-propyl
phosphorothioate and O-ethyl S-methyl S-propyl phosphorodithioate)
Acute toxicity
Rat, LD50, oral 33-62 mg/kg bw (vehicle, corn oil)
Rat, LD50, dermal 420 mg/kg bw, females
Rat, LD50, inhalation 0.25 mg/L
Dermal irritation Not reported, but death occurred within 8 h of dermal exposure
Ocular irritation Yes, but also death within 1 h of ocular exposure
Sensitization Not tested
Short-term toxicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant oral NOAEL < 0.26 mg/kg bw per day (LOAEL, 13-week study of neurotoxicity
in rats)
Lowest relevant dermal NOAEL 0.1 mg/kg bw per day (3-week study of dermal toxicity in rabbits)
Lowest relevant inhalation NOAEL Not tested
Long-term toxicity and carcinogenicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant NOAEL 0.04 mg/kg bw per day (105-week study in rats treated in the diet)
Carcinogenicity Not carcinogenic (mice, rats)
Genotoxicity Not genotoxic in vivo
Reproductive toxicity
Reproductive target/critical effect Reduced body weight and survival at maternally toxic doses
Lowest relevant reproductive NOAEL Maternal toxicity: 1 ppm, equal to 0.04 mg/kg bw per day;
fetotoxicity: 30 ppm, equal to 1.3 mg/kg bw per day
Developmental target/critical effect Only maternal toxicity (highest dose tested), no fetotoxicity
Lowest relevant developmental NOAEL Rabbit: maternal and fetotoxicity: 2.5 mg/kg bw per day (highest
dose tested)
Rat: maternal toxicity: 2 mg/kg bw per day; fetotoxicity:
18 mg/kg bw per day
Neurotoxicity/ Delayed neurotoxicity 5 mg/kg bw per day (acute toxicity in rats)
< 4 ppm, equal to 0.26 mg/kg bw per day (13-week study in rats)
No evidence for delayed neurotoxicity in hens, but some equivocal findings
Other toxicological studies No significant effects on erythrocte acetylcholinesterase activity in
exposed workers
Medical data None
Summary Value Study Safety factor
ADI 0-0.0004 mg/kg bw 2-year study, rat; two-generation 100
study of reproductive toxicity, rats
Acute reference dose 0.05 mg/kg bw Acute neurotoxicity, rats 100
References
Auletta, C.S. & Rinehart, W.E. (1979) A comparative dermal toxicity
evaluation in mice (Ethoprop and nine chemical analogs).
Unpublished report Project No. 5789-79 from Bio/dynamics Inc.,
Princeton, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985a) Ames
Salmonella/microsome plate test (EPA/OECD). Unpublished report
No. PH 301-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by RhônePoulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985b). Rat
hepatocyte primary culture/DNA repair system. Unpublished report
No. PH 311-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Barnett, J.W., Jenkins, L.J. & Parent, R.A. (1983) Evaluation of the
chronic toxicity and oncogenic potential of ethoprop in Fischer
344 rats. Unpublished report from Gulf South Research Institute,
New Iberia, Louisiana, USA. Submitted to WHO by Rhône-Poulenc.
Becker, J. & Parke, G.S.E. (1977) A primary dermal irritation study of
ethoprop 93% technical grade on abraded and non-abraded skin of
New Zealand albino rabbits. Unpublished report Lab. No. 7E-6084
from Cannon Laboratories, Inc., Reading, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
Blacker, A.M. (1987) Executive summary -- Acute dermal LD50 of
ethoprop (technical) in rabbits and rats. Unpublished report No.
87/AMB/035 from Rhone Poulenc. Submitted to WHO by Rhône-Poulenc.
Brown, D. (1986) Ethoprophos: 52 week oral (capsule administration)
toxicity study in the beagle. Unpublished report No. 4923-198/16
from Hazleton Laboratories Europe, Ltd, Harrogate, North
Yorkshire, United Kingdom. Submitted to WHO by Rhône-Poulenc.
Dearlove, G.E. (1987) Dominant lethal study of ethoprop technical
administered orally via gavage to Crl:COBS(R)CD(R)(SD)BR male
rats. Confidential report No. 218-004 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Fletcher, M.J., Springer, S.T., D'Addamio, G.H., Conzelmann, P.C. &
Pullin, T.G. (1980) Evaluation of effects of ethoprop on
reproductive performance by a three-generation reproduction study
in Fischer 344 rats. Unpublished report No. 413-858-41 from Gulf
South Research Institute. Submitted to WHO by Rhône-Poulenc.
Hamada, N.N. (1990) A five-month oral toxicity study with one-month
recovery in beagle dogs with ethoprop technical. Unpublished
report No. HLA 656-143 from Hazleton Laboratories America, Inc.
Vienna, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1989) 3-week dermal toxicity study with ethoprop
technical in rabbits. Unpublished report No. HLA 6224-152 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990a) 3-week dermal toxicity range finding study with
ethoprop technical in rats. Unpublished report No. HLA 6224-160
from Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990b) 3-week dermal toxicity study with ethoprop
technical in rats. Unpublished report No. HLA 6224-162 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Iqbal, Z.M. & Menzer, R.E. (1972) Metabolism of O-ethyl-S,S-dipropyl
phosphorodithioate in rats and liver microsomal systems.
Biochem. Pharmacol., 21, 1569-1584.
Ivett, J.L. (1989) Mutagenicity test on ethoprop technical in the rat
bone marrow cytogenetic assay. Unpublished report No. 10750-0-452
from Hazleton Laboratories America Inc., Kensington, Maryland,
USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1986) Acute dermal LD50
study with ethoprop in New Zealand white rabbits. Unpublished
report No. 3147.2 from Springborn Institute for Bioresearch,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1987) Acute dermal LD50
study with ethoprop (technical) in Sprague Dawley rats.
Unpublished report No. 3147.5 from Springborn Institute for
Bioresearch, Inc., Spencerville, Ohio, USA. Submitted to WHO by
RhônePoulenc.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of ethoprop
MCTR-603-78 in Sprague-Dawley rats. Unpublished report No. 5850
from Food & Drug Research Laboratories, Inc. Submitted to WHO by
Rhône-Poulenc. Report amended 10 June 1985.
Kopp, R., Gunzel, P., Rosskamp, G., Schmidt, M. & Schuppler, J. (1980)
Acute inhalation toxicity to the rat (M&F) of liquid ethoprophos
following single 4 hours continuous exposure (LC50). Report
(Protocol No. 449/79) from Schering AG. Submitted to WHO by
Rhône-Poulenc (in German).
Leffingwell, J.T. (1986) An exposure study of mixer/loader-applicators
working with MOCAP EC. Unpublished report from Rhône-Poulenc
Inc., New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Myhr, B.C. & Brusick, D.J. (1981) Evaluation of MOBIL No. 01238101 in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 2478-80 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by RhônePoulenc.
Neeper-Bradley, T.L. (1991) Two-generation reproduction study of
ethoprop technical administered in the diet to CD(R) (Sprague
Dawley) rats. Unpublished report No. 53-598 from Bushy Run
Research Center, Export, Pennsylvania, USA. Submitted to WHO
Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1982) Ethoprop (48 095 RP). Acute toxicity
in mice treated orally and rats treated percutaneously.
Unpublished report from Rhône-Poulenc, Vitry sur Seine, France.
Submitted to WHO by Rhône-Poulenc (in French).
Powers, M.B. (1965) V-C 9-104 (technical grade) Acute oral
administration -- Rats. Acute dermal application -- Rabbits.
Unpublished report from Hazleton Laboratories, Inc., Falls
Church, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Putman, D.L. & Schechtman, L.M. (1981) Activity of T1688 in the
dominant lethal assay in rodents. Unpublished report from
Microbial Associates, Bethesda, Maryland, USA. Submitted to WHO
by Rhône-Poulenc.
Rao (1999) Personal communication. Rhône-Poulenc, Research Triangle
Park, North Carolina, USA. Submitted to WHO by Rhône-Poulenc.
Roberts, N.L., Gopinth, C., Anderson, A. & Dawe, I.S. (1986) Acute
delayed neurotoxicity study with ethoprophos in the domestic hen.
Unpublished report No. RNP 244/86189 from Huntingdon Research
Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989a) Range-finding teratology study in rats with
ethoprop. Unpublished report No. 3147.38 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989b) Teratology study in rats with ethoprop.
Unpublished report No. 3147.39 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989c) Range-finding teratology study in rabbits with
ethoprop. Unpublished report No. 3147.40 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989d) Teratology study in rabbits with ethoprop.
Unpublished report No. 3147.41 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rucci, G.. (1979) Ethoprop technical grade; MCTR-60-78. Approximate
acute dermal toxicity (LD50) in young Yorkshire white pigs.
Unpublished report Lab. No. 6074from Food and Drug Research
Laboratories, Inc., Waverly, New York, USA. Submitted to WHO
Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1985) In vitro
chromosome aberration analysis in Chinese hamster ovary (CHO)
cells. Unpublished report No. PH 320-RP-00185 from Pharmakon
Research International Inc., Waverly, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1986) In vitro
sister chromatid exchange in Chinese hamster ovary (CHO) cells.
Unpublished report No. PH 319-RP001-85 from Pharmakon Research
International Inc., Waverly, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Schwartz, C.S. (1978) Acute oral LD50 determination of ethoprop in
female New Zealand white rabbits. Letter report to Mobil Oil
Company on FDRL study No. 5988. Submitted to WHO by
Rhône-Poulenc.
Skinner, M.J., Irwin, S.E., Schreiner, C.A., Mackerer, C.R. & Mehlman,
M.A. (1981) Metaphase analysis of rat bone marrow cells treated
in vivo with ethoprop, technical. Unpublished report No. 2476-80
from Mobil Environmental and Health Science Laboratory,
Plainsboro, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Spicer, E.J.F. (1985) Lifetime dietary toxicity and oncogenicity study
in rats. Unpublished report No. 347-029 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Study reformatted 18 May 1987. Submitted to WHO by Rhône-Poulenc.
Stankowski, L.F., Naismith, R.W. & Matthews, R.J. (1985) CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report
No. PH 314-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Stoughton, R.B. (1986) Penetration of the skin of humans, mice, rats
and rabbits by C14 ethoprop(R) emulsified concentrate and the
emulsified concentrate diluted with distilled water. Unpublished
report from the University of California, San Diego, California,
USA. Submitted to WHO by Rhône-Poulenc.
Thomson, M.A., Blackburn, G.R. & Mackerer, C.R. (1981) A murine
lymphoma mutagenesis assay, heterozygous at the thymidine kinase
locus for the determination of the potential mutagenicity of
ethoprop. Unpublished report No. 2474-80 from Mobil Environmental
& Health Science Laboratory, Plainsboro, New Jersey, USA.
Submitted to WHO by RhônePoulenc.
Weiler, M.S. (1994a) Acute oral gavage study with ethoprop in rats:
Time-related effects of ethoprop on brain, plasma and red blood
cell cholinesterase activities. Unpublished report No. HWI
6224-209 from Hazleton Wisconsin, Inc. Madison, Wisconsin USA.
Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994b) Acute neurotoxicity study with ethoprop in rats.
Unpublished report No. HWI 6224-200 from Hazleton Wisconsin, Inc.
Madison, Wisconsin, USA. Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994c) 13-week dietary neurotoxicity study with ethoprop
in rats. Unpublished report No. HWI 6224-199 from Hazleton
Wisconsin, Inc. Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Weiler, M.S. (1998) Acute oral toxicity study with ethoprop and three
ethoprop metabolites
(O-ethyl-S-methyl-S-propylphosphorodithioate,
O-ethyl-O-methyl-Spropylphosphorothioate, and
O-ethyl-S-propylphosphorothioate) in rats with determination of
effects on cholinesterase activity. Unpublished report No.
6224-246 from Covance Laboratories Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Weir, R.J. (1965) Acute eye application -- Rabbits. V-C 9-104
(technical grade). Unpublished report from Hazleton Laboratories
Inc. Falls Church, Virginia, USA. Submitted to WHO by
Rhône-Poulenc.
Weir, R.J. (1967a) Three-month dietary administration -- Rats.
Technical VC9-104. (Mocap). Unpublished report No. 230-102 from
Hazleton Laboratories Inc, Falls Church, Virginia, USA. Submitted
to WHO by Rhône-Poulenc.
Weir, R.J. (1967b) 13-week dietary administration -- Dogs. Technical
VC9-104. Unpublished report No. 230-110 from Hazleton
Laboratories, Inc, Falls Church, Virginia, USA. Submitted to WHO
by Rhône-Poulenc.
Weir, R.J. & Murphy, J.C. (1967) Neurotoxicity test -- Hens. Technical
VC 9-104. Unpublished report from Hazleton Laboratories Inc.,
Falls Church, Virginia, U.S.A. Submitted to WHO by Rhône-Poulenc.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, K.D. (1992) 104 week combined chronic toxicity and
carcinogenicity study with ethoprop in rats. Unpublished report.
Laboratory project ID: HLA 6224-151 from Hazelton Laboratories
America, Inc., Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Wolfe, G.W., Durloo, R.S. & Phipps, R.B. (1981) Rabbit teratology
study. Ethoprop technical -- 01238101. Unpublished report from
Hazleton Laboratories America, Inc. Vienne, Virginia, USA.
Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Supplementary submission, dated 29 July
1994. Submitted to WHO by Rhône-Poulenc.
Yenne, S.P. (1990) 14C-Ethoprop: Absorption, distribution,
metabolism, and excretion in the rat. Unpublished report HUK
project no. 68/102 from Hazleton UK, North Yorkshire, United
Kingdom. Submitted to WHO by Rhône-Poulenc.
Table 2. Penetration of undiluted or 1:19 diluted 14C-ethoprophos solutions through
the skin of various species (in % of applied dose)
Time Undiluted ethoprophos Diluted ethoprophos
Human Rabbit Mouse Rat Human Rabbit Mouse Rat
15 min 0 0.003 0.003 0.01 0.001 0.004 0.004 0.05
30 min 0.0004 0.008 0.01 0.05 0.01 0.02 0.12 0.15
1 h 0.0008 0.04 0.11 0.15 0.08 0.2 1.9 0.55
2 h 0.002 0.2 0.91 0.4 0.31 1.1 4.2 1.7
4 h 0.004 0.7 2.6 1.0 0.72 3.7 11 4.2
6 h 0.08 1.5 5.0 1.7 1.1 7.7 29 7.8
24 h 1.0 7.8 16 5.4 5.2 27 38 23
also formed when the 14C-ethyl- and the 14C-propyl-labelled
compound, respectively, was the substrate (Iqbal & Menzer, 1972).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ethoprophos in animals is summarized in
Table 3. In 1983, the Meeting reported that "in the studies evaluated
the toxic symptoms in the treated species were characteristic of
anticholinesterase poisoning and usually persisted for up to 48 h in
mice and 72 h in rats surviving treatment. In general, deaths occurred
within 4 h (mice) or 1 h to 3 days (rats) post-dosing. Information on
duration of symptoms and time of death in rabbits was not available."
Ethoprophos is considered to be toxic after single oral and
dermal doses and very toxic after inhalation. The dermal and oral
LD50 values for rabbits and mice are of the same order of magnitude
and indicate efficient absorption of the compound after dermal
exposure in these species, whereas less is absorbed dermally in rats.
A single application of 0.1 ml of undiluted technical-grade
ethoprophos (equivalent to 44 mg/kg bw) into one eye of each of three
New Zealand white rabbits immediately produced moderate erythema and
vascularization of the sclera and nictating membrane. The substance
was severely toxic, since all three animals died within 1 h of
treatment (Weir, 1965).
In a study of primary dermal irritation, all six male New Zealand
white rabbits that received 0.5 ml of undiluted technical-grade
ethoprophos (purity, 93%) at a dose equivalent to 240 mg/kg bw on
clipped, abraded and unabraded skin and kept under an occlusive patch
died within the first 8 h of treatment (Becker & Parke, 1977).
Table 3. Acute toxicity of ethoprophos
Species Strain Sex Purity Vehicle Route LD50/LC50 Reference
(%) (mg/kg bw or
mg/L)
Mousea OF1 (SPF) M & F NR Water Oral 31 Pasquet & Mazuret (1982)
Rat SD M NR Corn oil Oral 62b Powers (1965)
F 33b
Ratc,d Crl:CD (SD) F NR Corn oil Oral 56 Weiler (1998)
BR VAF/ Plus
Rabbite NZW F NR NR Oral 33 Schwartz (1978)
Mouse CD-1 M NR Acetone Dermal (24 h) 18b Auletta & Rinehart (1979)
Rata CD (COBS) M & F NR Water Dermal (24 h) 226 Pasquet & Mazuret
(1982)
Ratd,f SD M NR None Dermal (24 h) 1280 Karcher et al. (1987);
F 424 Blacker (1987)
Rabbitg NZW M & F NR None Dermal (24 h) 8.5 Karcher et al. (1986);
Blacker (1987)
Pigh Yorkshire white M 94% None Dermal 327 Rucci (1979)
Rabbith Albino M & F NR None or 40% Dermal (intact 26b Powers (1965)
solution in and abraded
corn oil skin; 24 h)
Rat Wistar M & F 92% None Inhalation(4 h) 0.250j Kopp et al. (1980)
Table 3 (continued)
NR, not reported
a Ethoprophos lot No. DA 232. Clinical signs: trembling, convulsions, hypomotility, and dyspnoea in both mice and rats; no dermal
irritation in rats. Only 3 male and female mice and 2 male and female rats per dose.
b Doses reported as µl/kg bw. They were converted to mg/kg bw by multiplying them by the specific gravity of ethoprophos at
20°C, 1.094.
c Ethoprophos batch No. 51EAR122. Deaths occurred within 4 days. Clinical signs: thinness, staggered gait, salivation,
hypoactivity, hunched posture, and tremors.
d Statements of compliance with good laboratory practice and quality assurance included.
e Clinical signs: ataxia, diarrhoea, and decreased activity. Only 4 females per group. Information on duration of symptoms and
time of death not available.
f Technical-grade ethoprophos lot No. 302106001. Deaths occurred within 3 days. Clinical signs: urine staining, tremors, excess
salivation, decreased activity, ataxia, soft stools, diarrhoea, laboured breathing, lachrymation, exophthalmus, and prostration,
in general increased with increasing dose. No signs of irritation. Considerable body-weight decreases in animals that died.
g Ethoprophos lot No. 302106001. Deaths occurred within 4 days except for one on day 9. Clinical signs: prostration, laboured
breathing, salivation, loss of locomotor ability, tremor, ataxia, soft stools, diarrhoea, and lachrymation. No signs of
irritation. Considerable body-weight decreases in animals that died. Only statement of quality assurance included.
h Deaths occurred within 4 days. Clinical signs: laboured breathing, salivation, wobbly gait, and decreased activity. Erythema
was reported but no indication of severity was given. Only 2-4 males per group.
i Clinical signs: depression, laboured breathing, unsteadiness, tremors, salivation. Deaths occurred within 24 h. Very slight,
subsiding erythema observed in surviving animals 24-48 h after dosing on abraded (> 10 µl/kg bw) and unabraded skin
(31.6 µg/kg bw). Only 4 animals per group.
j 4-h LC50 expressed as actual chamber concentration and particle size < 10 µm; animals were exposed to the liquid
technical-grade material as an aerosol (nominal concentration, 1500 mg/m3) in a nose-only exposure chamber. Clinical signs:
apathy, dyspnoea, salivation (from JMPR, 1983, and slightly modified by reference to original report).
(b) Short-term studies of toxicity
(i) Oral administration
Rats
Groups of 25 male and 25 female Charles River caesarean-derived
rats were fed diets containing technical-grade ethoprophos (purity not
stated) in acetone at concentrations of 0, 0.3, 1, or 100 ppm
(equivalent to 0, 0.015, 0.05, and 5 mg/kg bw per day) for three
months. The control group was given basal diet containing 'a volume of
acetone equivalent to the volume used for the test diets'. No deaths
or treatment-induced toxic signs were observed. Growth was depressed
by < 10% in males at doses > 1 ppm and in females at 100 ppm
during the last 6 weeks of the study. Food consumption was unaffected.
Haematological, clinical chemical (only blood urea nitrogen, aspartate
aminotransferase, and glucose determined), and urinary examinations in
five males and five females per group after 1 and 3 months indicated
no significant treatment-related findings. Cholinesterase activity in
plasma, erythrocytes, and brain was measured in five rats of each sex
per group at days 4, 8, 16, and 33 and terminally in the remaining
rats. Marked inhibition (25-100%) of the enzyme activity was found at
100 ppm at all sampling intervals, the depression being greatest for
erythrocyte and least for brain acetylcholinesterase activity. Maximum
inhibition of the enzyme occurred on day 8 or 16, in all tissues. Male
rats at 0.3 or 1 ppm showed a 24-28% reduction in erythrocyte and
plasma cholinesterase activity on day 8 and of brain cholinesterase
activity at termination. In females, brain cholinesterase activity was
inhibited by 20-25% at 0.3 and 1 ppm on day 8 and at 1 ppm on day 16,
and plasma and erythrocyte cholinesterase activity was inhibited by
23-26% at 1 ppm on day 16. At the end of the study, the absolute and
relative weights of the adrenals were decreased, but not in relation
to dose, in females in all treated groups. Histopathological
evaluation of about 20 selected tissues, including the adrenals, from
five males and five females in the control and highest-dose groups
showed no significant changes attributable to treatment. No NOAEL
could be identified, as brain acetylcholinesterase activity was
inhibited at all doses (Weir, 1967a). As only five animals of each sex
were examined for haematological, biochemical, and urinary parameters,
instead of the 20 rats of each sex required by the OECD guideline, and
few parameters were tested, the study was considered unreliable and
was not used for further evaluation of the toxicology of ethoprophos.
Dogs
In a study conducted according to GLP and with a statement of QA,
ethoprophos (purity, 95.6%) was administered orally to groups of six
male and six female beagle dogs aged 6-7 months at doses of 0, 0.01,
0.025, or 1 mg/kg bw per day in corn oil in gelatin capsules for 20
weeks.Two dogs of each sex at each dose were then allowed a 4-week
recovery. The animals were observed for deaths and clinical signs.
Body weights and food consumption were recorded, and ophthalmological
examinations were performed before dosing and at sacrifice after the
exposure (week 20) and recovery (week 24) periods. Haematological,
biochemical, and urinary analyses were carried out before treatment
and during weeks 17, 20, and 24. Cholinesterase activity in plasma and
erythrocytes was measured before dosing and about 3 h after dosing
during weeks 2, 4, 8, 12, 20, and 24. Brain cholinesterase activity
was measured at sacrifice; at that time, the weights of about 10
organs were determined and about 40 tissues and all gross lesions from
all animals were examined macroscopically and microscopically.
There were no deaths, and there were no treatment-related
clinical signs or effects on body weight, body-weight gain, food
consumption, ophthalmologic, haematological, biochemical, or urinary
end-points, macroscopic or microscopic findings, or organ weights. No
histopathological changes were found in the heart. The only finding
was a statistically significant increase in platelet count in bitches
at the high dose at weeks 17 and 20. This was considered to be of no
toxicological relevance since the value was increased before
treatment, and an elevated value was observed after recovery.
Significant inhibition of plasma cholinesterase activity was observed
in bitches at the intermediate dose and males and females at the high
dose. In animals at the high dose, erythrocyte acetylcholinesterase
activity was inhibited by more than 20% in males at all times during
exposure and in females only at weeks 8 and 12, when the values
reached statistical significance in males. Brain acetylcholinesterase
activity was not inhibited. After the recovery period, plasma
cholinesterase activity was found to have returned to normal, but the
activity in erythrocytes showed some variation and it could not be
concluded that recovery had occurred. The NOAEL was 1 mg/kg bw per
day, the highest dose tested, in the absence of inhibition of brain
acetylcholinesterase activity (Hamada, 1990).
Groups of three male and three female young adult pure-bred
beagles weighing 6-12 kg were fed diets containing technical-grade
ethoprophos (purity unknown) at concentrations of 0, 1, 3, or 100 ppm
for 13 weeks, equal to 0, 0.034, 0.098, or 3.4 mg/kg bw per day for
males and 0, 0.035, 0.11, or 4.0 mg/kg bw per day for females. There
were no deaths. Emesis occurred once in two animals at 100 ppm. Body
weights, food consumption, haematological, biochemical (glucose, blood
urea nitrogen, and aspartate aminotransferase and alkaline phosphatase
activity), and urinary parameters measured at 1 and 3 months were not
significantly affected by treatment. At termination, however, males
and females at the high dose had slightly decreased erythrocyte counts
and erythrocyte volume fractions. Plasma cholinesterase activity was
measured before dosing and on days 2, 4, 8, 16, 35, 65, and 87 of
administration. The activity was inhibited by 27-88% at doses > 3
ppm in animals of each sex at virtually all of the sampling intervals
and by 20-27% at 1 ppm in males at the two last sampling times. A
> 20% depression of erythrocyte cholinesterase activity was seen in
animals at the highest dose at all but the first two or three sampling
intervals and in females at 3 ppm on one occasion (day 65) only.
Cholinesterase activity in brain was not measured. At termination, no
compound-related effects on organ weights or gross pathological
changes were noted. The only significant finding on histopathological
evaluation of about 20 tissues from each animal in the control and
high-dose groups was foci of perivascular myocardial swelling with
loss of striations in one of three female controls and two of three
males and one of three females at 100 ppm; all of these animals also
showed swelling and vacuolation of Purkinje fibres. These changes were
considered by the pathologist to represent 'an artefact induced in the
processing of the tissues'. The NOAEL was 3 ppm, equal to 0.098 mg/kg
bw per day, on the basis of depression of erythrocyte cholinesterase
activity at 100 ppm (Weir, 1967b).
Groups of four male and four female pure-bred beagle dogs aged
4-6 months were given capsules containing ethoprophos (purity, 96%)
dissolved in peanut oil at doses of 0, 0.025, 1.0, or 10 mg/kg bw per
day orally for 52 weeks. The doses were based on the results of a
4-week range-finding study. The animals were offered 400 g/day of food
and water ad libitum and were observed daily for signs of toxicity;
body weights were recorded weekly and food consumption daily. Blood
and urine were collected before treatment and after 6, 13, 26, and
52 weeks of treatment. The standard haematological, serum chemical,
and urinary examinations were performed, in addition to measurements
of plasma and erythrocyte cholinesterase activity. At the end of the
study, the animals were killed by an overdose of thiopentone sodium
followed by exsanguination. All tissues were examined grossly
in situ, and the major organs were removed and weighed, and brain
cholinesterase activity was measured. The standard set of tissues was
collected for histopathological examination. A statement of QA was
provided.
No effect of treatment on the incidence of clinical signs was
observed. Treatment did not affect the weight gain of treated males,
but a dose-related trend to decreased body weight was seen in treated
bitches throughout the study, and, at termination, bitches at the high
dose weighed about 10% less than controls. Males and females at the
high dose tended to consume about 10% less food than controls.
Erythrocyte count, haemoglobin concentration, and erythrocyte volume
fraction were statistically significantly lower in males at the high
dose than in control males at all intervals. The only change in serum
chemistry was an increase in mean aspartate aminotransferase activity
associated with decreased total cholesterol and serum albumin in males
at the high dose from 6 weeks of treatment throughout the study. This
effect was due largely to an apparent hepatotoxic response in two of
four males at this dose; in one of these dogs, aspartate
aminotransferase activity was increased by 10-fold over that of
controls at study termination, and serum alkaline phosphatase,
gamma-glutamyl transferase, and alanine aminotrans-ferase activities
were also greatly increased. The serum albumin concentration was
decreased in all males at the high dose. Plasma and erythrocyte
cholinesterase activities were depressed in a dose-related manner in
dogs at the intermediate and high doses at all intervals. At the end
of the study, plasma cholinesterase activity was decreased to 33% and
17% of the control value in males and to 58%, 19%, and 17% in females
at the low, intermediate, and high doses, respectively. The
erythrocyte cholinesterase activity in males at the end of the study
was 127%, 88%, and 39% of the control value at the low, intermediate,
and high doses, respectively, whereas the values for females were
103%, 62%, and 38% of control, respectively. Brain cholinesterase
activity was inhibited only in males at the high dose (to 56% of the
control value; significant) and females (72% of control) at the high
dose. Animals at the intermediate dose showed inhibition to 91% and
94% of the control values, respectively, which was considered not to
be toxicologically relevant. Ophthalmoscopic examination conducted
before and at the end of the study revealed no treatment-related
effects. At necropsy, no effect of treatment on absolute or relative
organ weights was seen, nor was there any effect on the incidence of
gross findings. Treatment-related histopathological changes were
restricted to the liver. Selected findings are tabulated in Table 4.
The NOAEL was 0.025 mg/kg bw per day on the basis of findings in the
liver at the low dose (Brown, 1986).
Table 4. Pathological findings in the liver of dogs treated with ethoprophos for 52 weeks
Lesion Dose (mg/kg bw per day)
Males Females
Control 0.02 1 10 Control 0.025 1 10
Vacuolation 0 0 3 4 0 0 3 4
Focal necrosis 0 0 0 2 1 0 0 1
Pigment 0 0 1 4 0 0 0 3
Kupffer-cell pigment 0 0 1 4 0 0 1 4
Fibrosis 0 0 0 4 0 0 0 4
Biliary proliferation 0 0 0 4 0 0 0 4
(ii) Dermal administration
Rats
In a range-finding study for dermal toxicity conducted according
to GLP and with a QA statement, groups of three Crl:CD(R)BR rats of
each sex received dermal applications of ethoprophos (purity, 95.6%)
in mineral oil at doses of 0, 0.3, 1, 10, 30, or 100 mg/kg bw per day
for 6 h per day on 5 days per week for 3 weeks. The animals were
observed for clinical signs and dermal irritation. Body weight and
food consumption were recorded, and cholinesterase activity was
measured in plasma and erythrocytes before dosing, at week 2, and at
the end of study (week 4). At necropsy, brain acetylcholinesterase
activity was measured. Macroscopic examinations were performed and
organ weights recorded.
Deaths occurred in the groups given 30 (2/6 rats on days 7 and 8)
and 100 mg/kg bw per day (6/6 rats, days 2-7). The clinical signs in
these animals were characteristic of cholinesterase poisoning. Small
faeces, hunched posture, and low body temperature were also observed
at 10 mg/kg bw per day, and the latter finding was also observed in
one male and one female at 0.3 mg/kg bw per day and two males and one
female at 1 mg/kg bw per day. Food consumption was lower (reaching
statistical significance in females) at 10 mg/kg bw per day in females
and 30 mg/kg bw per day in males and females during the first week of
exposure. There were no signs of dermal irritation. Statistically
significant inhibition of plasma cholinesterase activity was observed
at weeks 2 and 4 in females at 0.3 and 1 mg/kg bw per day and in
animals of each sex at 10 and 30 mg/kg bw per day. Inhibition of
erythrocyte acetylcholinesterase activity by > 20% was observed in
females at all doses at week 2 and at 10 and 30 mg/kg bw per day at
week 4 and in males at 10 and 30 mg/kg bw per day at both times. Brain
acetylcholinesterase activity was inhibited by > 50% in animals of
each sex at 10 and 30 mg/kg bw per day; the effect was dose-related
and significant at the high dose. No treatment-related changes were
observed in organ weights or in gross appearance (Henwood, 1990a).
In the main study, conducted according to GLP and with a QA
statement, groups of 10 Crl:CD(R)BR rats of each sex aged about 2.5
months received 1.4 ml/kg bw of ethoprophos (purity, 95.6%) dermally
in mineral oil at doses of 0, 0.3, 1, or 10 mg/kg bw per day for 6
h/day on 5 days per week for 3 weeks. The area of exposure on the
dorsal trunk constituted about 10% of the total body surface. The
animals were observed for clinical signs and dermal irritation. Body
weights and food consumption were recorded, and cholinesterase
activity was measured in plasma and erthrocytes before treatment, in
week 2, and at the end of study (week 4). Haematological and clinical
chemical parameters were evaluated on the day of necropsy, when the
brain was collected for analysis of acetylcholinesterase activity.
Macroscopic examinations were performed, and the weights of the brain,
kidneys, and liver were recorded. Kidneys, liver, skin, and tissues
with lesions from all rats in the control and high-dose groups were
examined microscopically.
The deaths of one control female and one at the high dose were
considered to be unrelated to treatment. Clinical signs observed in
some females at the high dose included soft and/or small faeces (three
animals) and hunched posture (one animal). During the first week of
exposure, a slightly lower (not statistically significant) body-weight
gain was observed in animals at the high dose, but food consumption
was not affected. There were no signs of dermal irritation that could
be related to treatment. In all groups, including controls, slight
erythema and desquamation associated with pustules or papules were
observed. Moderate desquamation was observed in one male and one
female at 0.3 mg/kg bw per day and in one male at 1 mg/kg bw per day.
Dose-related, statistically significant inhibition of plasma
cholinesterase activity was observed in weeks 2 and 4 in animals given
1 or 10 mg/kg bw per day. Erythrocyte acetylcholinesterase activity
was inhibited by > 20% in rats at 1 mg/kg bw per day in week 4 and at
10 mg/kg bw per day at both times, with 30% inhibition in week 2 and
37% (males) and 54% (females) in week 4. Brain acetylcholinesterase
activity was statistically significantly inhibited by 50% in males and
70% in females at 10 mg/kg bw per day. At 1 mg/kg bw per day, the
inhibition was 12% in males and 16% in females (not significant). At
0.3 mg/kg bw per day, brain acetylcholinesterase activity was not
inhibited in males and was nonsignificantly inhibited by 11% in
females. In female controls and in those at 0.3 mg/kg bw per day, but
not those at 1 mg/kg bw per day, the standard deviation of the mean
acetylcholinesterase activity was relatively high. As a result, the
inhibition in brain at 0.3 mg/kg bw per day is considered not
toxicologically relevant, whereas that at 1 mg/kg bw per day is
relevant. Other clinical findings in females were a dose-related
decrease in platelet count and a decrease unrelated to dose in the
numbers of neutrophils and lymphocytes in animals at 1 and 10 mg/kg bw
per day. These findings were considered to be due to the excessive
formation of fibrin strands in the blood of these females. The
statistically significant findings of increases in aspartate
aminotransferase activity in males at 1 mg/kg bw per day and slight
decreases in urea nitrogen in males at 0.3 and 10 mg/kg bw per day
were considered not to be related to treatment. Increased plasma
concentrations of sodium and chloride were observed in females at 0.3
mg/kg bw per day and in animals of each sex at 1 and 10 mg/kg bw per
day, but these changes were small and not related to dose. An
increased calcium concentration was seen in males at 1 mg/kg bw per
day and in males and females at 10 mg/kg bw per day. No
compound-related changes were observed in terminal body weights or
organ weights. At macroscopic examination, two of 10 males at the high
dose were found to have crusted areas on untreated skin in the
cervical and/or thoracic region, confirmed microscopically to be
multifocal erosion or ulceration of the epidermis and chronic
inflammation. Three of nine females at the high dose showed dark areas
in the glandular stomach, which was confirmed microscopically in two
rats as oedema or focal erosion or ulceration of the glandular mucosa;
and one of these females and another at the high dose showed
dilatation of the pelvis. The NOAEL was 0.3 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity in females
at 1 mg/kg bw per day. Since no sign of treatment-related dermal
irritation was seen, the NOAEL for this end-point was 10 mg/kg bw per
day, the highest dose tested (Henwood, 1990b).
Rabbits
In a study conducted according to GLP and with a QA statement,
groups of 10 Hra:(NZW)SPF rabbits of each sex, aged about 3 months,
received dermal applications of 1.4 ml/kg bw of ethoprophos (purity,
95.6%) in 4% carboxymethylcellulose at doses of 0, 0.03, 0.1, or 1
mg/kg bw per day for 6 h/day on 5 days per week for 3 weeks. The area
of exposure on the dorsal trunk constituted about 10% of the total
body surface. The animals were observed for clinical signs and dermal
irritation. Body weight and food consumption were recorded and
cholinesterase activity was measured in plasma and erythrocytes before
treatment and at the end of study (week 4). Haematological and
clinical chemical parameters were evaluated on the day of necropsy,
when the brain was collected for measurement of acetylcholinesterase
activity. Macroscopic examinations were performed, and the weights of
the brain, kidneys, and liver were recorded. Kidneys, liver, skin, and
tissues with lesions from all rabbits in the control and high-dose
groups were examined microscopically.
Deaths occurred in one male and three females at 0.03 mg/kg bw
per day and two males at 1 mg/kg bw per day. These animals and a few
others in each group except female controls showed clinical signs that
were associated with an increased incidence of mucoid enteritis
(diarrhoea, mucoid diarrhoea, and reduced food consumption), but this
finding was considered to be unrelated to treatment. Females at the
high dose had significantly lower body weights in weeks 2 and 4. The
groups in which the deaths occurred had lower food consumption, but
this was considered to be of no toxicological importance. An increased
incidence of slight-to-moderate irritation (erythema and sometimes
desquamation) was seen in all treated groups when compared with
controls, and the frequency increased with dose. The only
treatment-related change in haematological or clinical chemical
parameters was statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain at the high dose. The
inhibition in males was 37% in plasma, 42% in erythrocytes, and 49% in
brain, and that in females was 35% in plasma, 42% in erythrocytes, and
49% in brain. No inhibition of brain acetylcholinesterase activity was
found at lower doses; erythrocyte acetylcholinesterase activity was
inhibited by 12% in females at the intermediate dose, but this is
considered to be of no toxicological relevance. The terminal body
weights were significantly lower for females given 0.03 or 1 mg/kg bw
per day, which might be due to their lower food consumption. The
absolute kidney weights were significantly lower in females and
slightly lower in males at the high dose, and the relative kidney
weights were slightly (and not significantly) lower in females at this
dose. There were no treatment-related macroscopic or microscopic
changes. The NOAEL for dermal toxicity was 0.1 mg/kg bw per day on the
basis of inhibition of cholinesterase activity in brain and
erythrocytes and decreased kidney weights at 1 mg/kg bw per day. No
NOAEL could be identified for dermal irritation, as slight irritation
of the skin was observed at all doses (Henwood, 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Randomly assigned groups of 80 male and 80 female B6C3F1 (SPF)
mice were fed diets containing 0, 0.2, 2, or 30 ppm of technical-grade
ethoprophos (purity, 94.6%) for 2 years. The test material and diets
were analysed periodically to ensure that the concentrations were
within acceptable limits of the nominal values. The diets and water
were provided ad libitum. Animals were examined daily for signs of
toxicity, and moribund animals were killed. Body weights were recorded
weekly for the first 26 weeks of treatment and twice weekly
thereafter. Food consumption was determined weekly. Haematological,
clinical chemical, and urinary parameters and cholinesterase activity
were measured after 26, 52, 78, and 104 weeks of treatment. Ten mice
of each sex at each dose were killed after 26, 52, and 78 weeks of
treatment, and all surviving animals were killed after 104 weeks of
treatment. Complete post-mortem examinations were conducted on all
animals killed at scheduled sacrifice and on those that died or were
killed in a moribund condition during the study.
No effect of treatment was seen on survival or the incidence of
clinical signs. Mean body-weight gain was decreased by 5-10% in males
and females at the high dose during the first 80 weeks of treatment,
but by the end of the study there was no decrease in males and only a
slight decrease (6%) in females. Treatment had no effect on food
consumption, the mean intake levels over the course of the study being
0, 0.026, 0.25, and 4.0 mg/kg bw per day for males and 0, 0.032, 0.32,
and 4.9 mg/kg bw per day for females. The only change in
haematological parameters was a decrease in the mean total leukocyte
count in all treated males: at the end of the study, the mean total
counts were: 4.1 ± 1.7, 2.3 ± 0.9, 2.1 ± 1.1, and 1.6 ± 0.5 ×
103/mm3 for controls and males at the low, intermediate, and high
doses, respectively, which were statistically significantly different
from the controls in all groups. The significance of this finding is
unclear as it was not associated with any other toxic effect that
might result from an impaired immune function. Historical control data
would have been useful for evaluatng this finding, but they were not
submitted.
Plasma and erythrocyte cholinesterase activity was inhibited in a
dose-related manner in animals at the intermediate and high doses
during the first 78 weeks of treatment, and in most cases statistical
significance was reached. The cholinesterase activity remaining in
plasma represented 65-90% of control values at the intermediate dose
and 23-34% at the high dose, whereas in erythrocytes it was 83-89% of
control values at the intermediate dose and 19-26% at the high dose.
By the end of the study, the plasma and erythrocyte cholinesterase
activities in the group at the intermediate dose were similar to those
of controls, except that the activity in plasma of females at the
intermediate dose was significantly decreased by about 20%. Brain
cholinesterase activity was inhibited only in males and females at the
high dose, with statistical significance reached in week 26 (64% of
control value for males and 71% for females), week 52 (82% of control
value in males), and week 104 (81% of control value in males and 83%
in females).
Necropsy of animals that died on test or were killed in a
moribund condition did not reveal any treatment-related lesions or an
effect on absolute or relative organ weights. No treatment-related
lesions were found at gross and microscopic examinations at interim
sacrifices. At final sacrifice, an increased incidence of
calcification of the kidney was noted in males at the high dose
(13/42, 2/45 controls) with 'basophilic changes' in the kidneys of
males only (0/45 control, 9/31 at the low dose, 13/38 at the
intermediate dose, and 24/42 at the high dose). The historical control
data that were supplied showed a high spontaneous incidence of these
lesions, with incidences of calcium deposits in males of 2.4-72% and
basophilic changes in males of 0-87%. The study authors reported that
these lesions are of spontaneous origin and do not represent
treatment-related pathological lesions. Treatment had no effect on the
incidences of tumours in specific tissues or on the total tumour
burden.
The NOAEL for toxicity was 2 ppm, equal to 0.25 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinesterase activity at
30 ppm (Yamagata et al., 1984a,b).
Rats
Groups of 10 male and 20 female Fischer 344 rats were fed diets
containing technical-grade ethoprophos (purity, 95.3%) at
concentrations of 0, 60.5, 131, or 262 ppm for 8 weeks before mating
(one male to two females). (The section on methods indicates that the
F0 generation comprised 10 males and 20 females per group, but later
in the report it is stated that approximately 16 males and 32 females
at each dose were mated. The complete absence of data on the
reproduction phase of the study precluded verification of the
information. The report stated that analytical data for dietary
analyses were not available.) Ten days after the detection of a
positive vaginal smear, the females were separated from the males and
were maintained on the test diets until weaning of their pups. This
part of the study constituted the reproductive phase. Weanlings from
the reproductive phase (60 males and females per group) were selected
randomly and fed diets containing ethoprophos at 0, 4.5, 9, or 18 ppm
during weeks 0-12 and at 0, 49, 98, or 196 ppm during weeks 13-109 in
a study of carcinogenicity. The dietary levels during weeks 13-109
were equivalent to doses of 0, 2.5, 4.9, and 9.8 mg/kg bw per day.
(The mean intake over the whole exposure period was not reported.) In
this 109-week study, all animals that died or were killed in a
moribund condition, all survivors at the end of the study, and the 10
males and 10 females per group killed after 52 weeks of treatment were
subjected to gross necropsy and histopathological examination of a
wide range of tissues and all gross lesions.
The mortality rate was increased in males of the highest dose
during the first 7 months, although the rates at the end of the study
(53-63%) were comparable in all groups, including the controls. Other
than emaciation in animals at the high dose, there were no
treatment-related clinical signs. Growth was depressed at the highest
dose throughout the study and at the intermediate dose during most of
the study. Food consumption was reduced in animals at the intermediate
and high doses during the first 52 weeks. Weekly water consumption,
monitored between weeks 102 and 109, was unaffected. Haematological,
blood chemical (glucose, blood urea nitrogen, gamma-glutamyl
transferase, alanine and aspartate aminotransferase and alkaline
phosphatase activities, total protein, and cholesterol concentrations)
and urinary (pH, glucose, ketones, and protein) parameters measured at
the end of weeks 52 and 109 revealed decreased erythrocyte count,
erythrocyte volume fraction, and haemoglobin values in males at the
two higher doses and in females of the highest dose after 52 weeks.
Assay of cholinesterase in plasma, erythrocytes, and brain, conducted
at termination only, indicated a dose-related, statistically
significant 81-93% depression of plasma cholinesterase activity and a
30-68% decrease in brain cholinesterase activity in all treated
groups. Erythrocyte cholinesterase activity was not inhibited. The
weights of the spleen, liver, kidney, and testis deviated from those
of controls at the 52-week and terminal sacrifices, but essentially
only at the highest dose, with no accompanying microscopic lesions.
The gross pathologic changes seen were not significantly different
from those in controls, but histopathological examination of males at
the highest dose showed an increased incidence of 'scleral
mineralization' of the eye. No other microscopic lesions that might be
attributable to treatment were found.
The only notable oncogenic finding was a statistically
significant increase in the incidence of thyroid C-cell adenoma in
males at the highest dose at terminal sacrifice, with incidences of
2/34 in controls, 3/31 at the low dose, 1/29 at the intermediate dose,
and 9/33 at the high dose, expressed as the ratio of the number of
survivors bearing the tumour to the number examined
histopathologically. C-cell adenomas were also observed at the interim
sacrifice in one male in the control group and one at the low and one
at the high dose. The background incidence of the tumour was not
reported. The incidences of animals with benign and/or malignant
tumours, malignant tumours, or multiple primary tumours were not
significantly different in control and treated groups.
Interstitial-cell adenoma of the testis in males and pituitary adenoma
in females were the most frequently observed spontaneous tumours,
occurring in nearly 90% of males and over 50% of females in the
concurrent control group (Barnett et al., 1983). The authors
considered that the increased incidence of C-cell adenomas in males at
the high dose was a direct treatment-related effect as there was no
trend with dose. No NOAEL could be identified since inhibition of
brain acetylcholinesterase activity was seen at all doses. The LOAEL
was 49 ppm, equivalent to 2.5 mg/kg bw per day.
Groups of 70 male and 70 female Fischer 344 rats were assigned
randomly to receive diets containing technical-grade ethoprophos
(purity, 95.9%) at concentrations of 0, 1, 10, or 100 ppm (equivalent
to 0, 0.05, 0.5, or 5 mg/kg bw per day; mean intake over entire
exposure not reported) for 105 consecutive weeks. The test material
and diets were analysed periodically to ensure the stability and
homogeneity of diets. Food and water were provided ad libitum. The
animals were examined daily for mortality and morbidity and underwent
detailed physical examinations every week. The body weights were
recorded weekly for the first 26 weeks and twice weekly thereafter
until the end of the study, as were measurements of food and water
consumption. Ophthalmoscopic examinations were performed on all rats
at the beginning of the study and after 6, 12, 18, and 24 months of
treatment, and routine haematological, serological, and urinary
studies were conducted at the same intervals after the beginning of
treatment. Plasma and erythrocyte cholinesterase activity was measured
at the same intervals as other parameters, and brain
acetylcholinesterase activity was measured at interim sacrifice of 10
rats of each sex per dose after 12 and 18 months and at final
sacrifice at the end of 24 months of treatment. All animals were
killed on schedule, and those that died on test or were killed in a
moribund condition were subjected to a complete post-mortem
examination. A statement of QA was provided.
Treatment had no effect on survival, body-weight gain, or food or
water consumption. The only potential treatment-related finding at
physical examination was an increased incidence of anogenital staining
in females at the high dose during weeks 14-78 of treatment.
Haematological examination revealed decreased erythrocyte counts,
haemoglobin, and erythrocyte volume fraction, with increased mean
corpuscular volume in males and females at the high dose at all
intervals except 18 months. This change was statistically significant
only after 6 and 12 months of treatment, but not at final sacrifice. A
slight (10-20% over controls) increase in blood urea nitrogen was also
seen in these animals, which was statistically significant only at 6
months in females and at 18 months in males. A statistically
significant decrease in serum globulin content was noted after 6 and
12 months of treatment but not at final sacrifice. No toxicologically
significant alterations were found in urinary or ophthalmic
end-points.
Plasma and erythrocyte cholinesterase activity was significantly
inhibited in a dose-related manner at most intervals in males and
females at the intermediate and high doses. Erythrocyte activity was
inhibited by 28-44% in animals at the high dose, by < 20% in males at
the intermediate dose, and by 19-27% in females at 6, 12, and 18
months and 4% at the end of the study. Brain cholinesterase activity
was significantly inhibited in animals at the high dose at all
measured intervals, with inhibition of 27-35% in males and 36-48% in
females.
At the 12-month sacrifice, a statistically significant increase
of about 10% was noted in the relative weight of the spleen of males
and females at the high dose, with decreases of a similar magnitude in
the absolute and relative weights of the kidney in males at the high
dose. No significant treatment-related changes were seen on gross or
microscopic examination. At the 18-month sacrifice, no significant
alterations in organs weights were found, nor were any significant
changes noted on gross or microscopic examination. At final sacrifice,
statistically significant, 16-20% increases in the absolute and
relative weights of the thyroid/parathyroid were seen in males at the
high dose, with non-significant, 14-16% increases in males at the
intermediate dose. Gross examination revealed an increased incidence
of enlarged thyroids in males at these doses: 5/35 and 5/39,
respectively, compared with 1/36 controls. The only potentially
treatment-related lesion found on microscopic examination was an
increased incidence of parafollicular C-cell neoplasms in males at the
high dose (Table 5). The authors concluded that these changes were
statistically significant and the incidence of thyroid neoplasia was
'random and unrelated to the test article'.
Table 5. Incidence of C-cell neoplasms in male rats fed diets containing
ethoprophos
Neoplasm Dose (ppm)
0 1 10 100
C-cell adenoma
Deaths and unscheduled sacrifices 2/13 0/7 0/13 1/9
Final sacrifice 6/36 5/39 5/35 11/39
Total 8/49 5/46 5/48 12/48
C-cell carcinoma
Deaths and unscheduled sacrifices 0/13 0/7 0/13 1/9
Final sacrifice 0/36 0/39 1/35 2/39
Total 0/49 0/46 1/48 3/48
All C-cell neoplasms 8/49 5/46 6/48 15/48
The NOAEL for toxicity was 10 ppm, equivalent to 0.5 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase
activity, effects on erythrocyte parameters, and effects on the
thyroid at 100 ppm (Spicer, 1985).
In a study conducted according to GLP and with a QA statement,
ethoprophos (purity, 95.6%) was mixed into the diet of groups of 70
randomly assigned Crl:CD(R)(SD)BR VAF/Plus(R) rats of each sex at
concentrations of 0, 1, 60, and 600 ppm for 105 weeks. During week 3
of the study, the highest dose was lowered to 400 ppm because of the
occurrence of tremors, ataxia, and death in females. The average doses
throughout treatment were 0, 0.04, 2.7, and 20 mg/kg bw per day for
males and 0, 0.06, 3.4, and 26 mg/kg bw per day for females in the
four groups, respectively. Ten additional animals of each sex per dose
were killed after 54 weeks, while another 10 animals of each sex were
allocated to the control and high-dose groups to study recovery after
dosing for 52 weeks followed by 4 weeks of control diet. The test
material and diets were analysed regularly to ensure that the
concentrations in the diet were within an acceptable range. Food and
water were provided ad libitum, and animals were monitored twice
daily for their clinical condition. Body weights and food consumption
were determined weekly up to week 13 and at least every other week
thereafter. The clinical pathological examinations comprised
haematology, clinical chemistry, including erythrocyte and plasma
cholinesterase activity, and urinalysis at weeks 12, 26, 52, and 78 on
10 animals per group and on all surviving animals at termination of
the study, when brain cholinesterase activity was determined in all
animals. All animals were necropsied, and 41 organs from animals in
the control and high-dose groups and all those that died or were
killed before the end of the study were examined histologically.
Additionally, all macroscopic lesions and lung, liver, and kidneys
from animals at the low and intermediate doses were studied. The
thyroids of all animals were examined, and ophthalmic examinations
were done on all animals at weeks 0, 52. and 104.
Animals at the high dose showed depressed food consumption (up to
week 70 in males and up to week 20 in females) and cumulative
body-weight gain, and in males food efficiency was depressed up to
week 20. The survival rate was essentially the same in all groups up
to week 78, but by week 104 the survival rate of animals at the high
dose was higher than that of other groups. The major causes of death
were renal disease and pituitary tumours. Females at the high dose had
significantly reduced erythrocyte count, haemoglobin, and erythrocyte
volume fraction after 12, 26, 52, and 78 weeks of treatment, although
the decreases were not statistically significant at 53 or 104 weeks.
In males at this dose, these parameters were significantly reduced
only at week 26; at weeks 12, 52, 53, and 78, nonsignificant decreases
were seen, and at week 104 there was no difference from controls.
These effects were reversible in the animals allowed to recover. Males
and females at the high dose had significant reductions in total
plasma protein and globulin at weeks 12 and 26 and males also at week
52 and 53. In females, the decrease was significant only for total
protein at week 52 and was nonsignificant for both total plasma and
globulin at week 53. At week 78, only a significant decrease for total
protein was observed, while in females both parameters showed a
nonsignificant decrease. At week 104, the decreases in total plasma
protein and globulin were no longer significant in animals of either
sex. The effects on total plasma protein and globulin were reversible
after 4 weeks of control diet. Other significant changes in
haematological and blood biochemical parameters were incidental and
considered not to be related to treatment. No changes in
haematological or general blood biochemical parameters were observed
in rats at the other doses. The results of urinary analysis were
summarized in the report and indicated that males at the high dose had
significantly decreased urine volume and urea nitrogen and creatinine
concentrations at the end of the study. These findings were attributed
to a lower incidence of chronic renal disease in these animals.
Plasma cholinesterase activity was significantly inhibited
throughout the experiment, by 48-64% in males at the intermediate
dose, 62-77% in males at the high dose, 61-77% in females at the
intermediate dose, and 75-82% in females at the high dose. Erythrocyte
cholinesterase activity was also significantly inhibited throughout
the study, by 34-44% in males at the intermediate dose, 35-51% in
males at the high dose, 36-48% (36% inhibition not significant) in
females at the intermediate dose, and 41-51% in females at the high
dose. At the low dose, inhibition of plasma and erythrocyte
cholinesterase activity was highly variable but never exceeded 18% in
plasma or 6% in erythrocytes in either males or females and was not
statistically significant. Inhibition of brain cholinesterase activity
was significant at weeks 52 and 104 and amounted to 35-33% of control
values in males at the intermediate dose, 53-64% in males at the high
dose, 28-32% in females at the intermediate dose, and 64-66% in
females at the high dose. At the low dose, inhibition of brain
cholinesterase activity was maximal in females at week 104 (8%), but
the differences (whether increased or decreased) were not
statistically significant in either males or females. Both plasma and
erythrocyte cholinesterase activity recovered to about 80% of the
control activity after 4 weeks of control diet from week 52, and brain
acetylcholinesterase activity was nearly fully restored.
Both males and females at the high dose tended to have lower
terminal body weights, but the differences were not statistically
significant. At week 52, the weight of the left thyroid glands of
females at this dose was reduced both absolutely and relative to the
weight of the brain. Males that were allowed to recover showed reduced
absolute left and right adrenal weights, left thyroid plus parathyroid
weights, and heart weight; the organ:brain weight and the kidney:brain
weight ratios were also reduced. The weight of the left testis
relative to that of the body was increased. At 104 weeks, females at
the high dose had increased brain weights and decreased kidney:brain
weights. The males in this group had reduced absolute and relative (to
body) weights of the right and left kidney, heart, and right adrenal
gland.
Males at the high dose had a decreased incidence and reduced
severity of chronic nephropathy as compared with control animals,
whereas females at this dose had a reduced incidence of mineral
calcareous deposits in the renal pelvis. Males and females at the high
dose showed increased incidences of inflammation of tissues of the
tail (joints and skin) and of alveolar macrophage infiltration.
Probably as a result of this inflammation, higher incidences of
lymphoreticular cell hyperplasia, sinal ectasia, and congestion were
observed in lymph nodes at various sites in the body. The inflammatory
changes were considered to be age-related, and their increased
incidence in animals at the high dose was assumed to reflect the poor
physical condition of these animals. In females at the high dose,
increased incidences of gastric erosion and submucosal oedema were
found at terminal sacrifice. No treatment-related ophthalmic changes
were observed.
Tumours of various cell types occurred in many tissues. The only
changes that showed a clear relationship to treatment were increased
proliferative cell lesions in thyroid C-cells, the adrenal medulla,
and the uterine endometrium (Table 6). A statistical evaluation of the
tumour incidence in all animals was not provided, but according to a
statistical analysis of data on tumours found at final sacrifice,
females at the low and high doses had a significantly reduced
incidence of C-cell hyperplasia at terminal sacrifice, while males at
the low and intermediate doses had significantly increased incidences
of C-cell adenomas. The increase in C-cell carcinomas at termination
Table 6. Occurrence (%) of proliferative lesions in organs of rats given ethoprophos in the diet
Groups of animals and tumour type Dose (ppm)
Males Females
0 1 60 400 0 1 60 400
No. of animals
No. of unscheduled deaths 50 42 42 30 42 47 37 27
No. at terminal sacrifice 20 28 28 41 29 23 33 44
Total 70 70 70 71 71 70 70 71
Thyroid
C-cell hyperplasia 31 29 41 39 61 39 63 46
C-cell adenomas 11 9 13 17 14 11 16 17
C-cell carcinomas 0 0 1 4 1 1 1 3
All tumours 11 9 14 21 15 12 17 20
Adrenal
Benign phaeochromocytomas 20 10 10 7 4 3 1 3
Malignant phaeochromocytomas 0 3 3 7 0 0 0 0
All tumours 20 13 13 14 4 3 1 3
Uterus
Endometrial stromal polyps 1 1 4 8
Data are incidences in percentages of the total number of animals (terminal sacrifice plus intercurrent deaths)
was not significant. Males at the high dose showed a significant
decrease in the incidence of benign phaeochromocytomas but a
significant increase in that of malignant phaeochromocytomas at study
termination. The increased incidence of uterine endometrial stromal
polyps in females at the high dose was also significant. The study
authors indicated that C-cell and uterine proliferative lesions are
more likely to occur in old animals and their increased incidences are
attributable to the greater longevity of the animals at the high dose.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of inhibition of brain acetylcholinesterase activity at the next
highest dose (Williams, 1992).
(d) Genotoxicity
The results of studies on the genotoxicity of ethoprophos are
summarized in Table 7.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity conducted
according to GLP and with a QA statement, groups of 28
Crl:CD(R)(SD)BR rats of each sex, 6 weeks of age, were given diets
containing ethoprophos (purity, 95.3%) in acetone at concentrations of
0, 1, 30, or 300 ppm, equal to 0, 0.04, 1.3, or 23 mg/kg bw per day
for males and 0, 0.09, 2.6, or 27 mg/kg bw per day for females. After
10 weeks of exposure, the F0 parents were mated 1:1 to produce the
F1a generation. Treatment was continued through mating, gestation,
parturition, and lactation. On day 4 after parturition, each litter
was culled to four pups of each sex. After 3 weeks of lactation, the
pups were weaned. Owing to significant mortality among weanlings at
the high dose, pups in the control, low-, and intermediate-dose groups
were not used as parents for the next generation but instead 10 pups
of each sex were selected for necropsy. All weanlings at the high dose
and control F1a weanlings selected as parents were maintained on
their respective diets until day 49 post partum; the weanlings at
the high dose and 10 controls of each sex were then necropsied. From
about 1 week after weaning of the last F1a pups, F0 parents at 300
ppm were fed a diet containing 150 ppm of ethoprophos (equal to 7.1
mg/kg bw per day for males and 14 mg/kg bw per day for females), and
all F0 animals at the other two doses were maintained on their
original diets. After 3 weeks, the F0 parents were mated again to
produce the F1b generation, mating, gestation, parturition,
lactation, and weaning being conducted as described above. One week
after weaning, 28 F1b pups of each sex at each dose were selected as
parents of the F2 generation. The remaining F1b pups and all F2
pups were necropsied. Parental F0 animals aged 35-36 weeks and
parental F1 animals aged 20-21 weeks were killed after weaning of
the F1b and F2 progeny, respectively, and neccropsied grossly.
Blood was taken just before sacrifice, and brain tissue was collected,
for analyses of cholinesterase in plasma, erythrocytes, and brain. The
Table 7. Results of studies of the genotoxicity of ethoprophos
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa,b S. typhimurium 10-1000 µg/plate in NR Negative ± S9 Barfknecht et al. (1985a)
TA98, TA100, TA1535, DMSO
TA1537, TA1538
Gene mutationa Mouse lymphoma 0.24-0.032 µg/ml 95 Negative Thomson et al. (1981)
L5178Y cells, Tk locus (total growth between 18
and 126%, respectively),
- S9
0.024-0.0032 µg/ml
(total growth between 10
and 106%), + S9
Gene mutationc,d Chinese hamster ovary 0-500 µg/ml - S9 NR Negative Stankowski et al. (1985)
cells (CHO-K1-BH4); 0-150 µg/ml + S9
Hprt locus in DMSO
Chromosomal Chinese hamster ovary 0-300 µg/ml - S9 NR Negative SanSebastian et al. (1985)
aberrationsc,e cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivef
Unscheduled DNA Rat hepatocytes 2.5-100 nl/ml in DMSO; NR Negative Myhr & Brusick (1981)
synthesisg,h toxic from 50 nl/ml
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Sister chromatid Chinese hamster ovary 0-350 µg/ml - S9 NR Negative SanSebastian et al. (1986)
exchangec,j cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivek
Table 7. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Chromosomal Rat (SD) bone 0-20 mg/kg bw per day for 5 days 95.7 Negative Skinner et al. (1981)
aberrationl marrow by gavage in Methocel K4M
premium; sacrifice 6 h after dosing
Chromosomal Rat (SD) bone 0-25 mg/kg bw once by gavage; 95.5 Negative Ivett (1989)
aberrationm,n marrow sacrifice 6, 18, and 30 h after
dosing 0-25 mg/kg bw per day for
5 days by gavage; sacrifice 6 h
after dosing
Vehicle, corn oil.
Dominant lethal Rat (SD) 0-20 mg/kg bw per day for 5 days NR Equivocal Putman & Schechtman
mutationo by gavage in corn oil (1981)
Dominant lethal Rat (Crl:COBS 0-20 mg/kg bw per day for 5 days 95 Negative Dearlove (1987)
mutationm,p CD(SD)BR) by gavage in carboxymethyl
cellulose
NR, not reported; S9, 9000 × g supernatant of rat liver; DMSO, dimethylsulfoxide; SD, Sprague-Dawley
a Test in triplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
b Cytotoxicity (small colonies) seen in preliminary test at 1666 µg/plate and no growth at 5000 µg/plate in TA1538 and TA100
c Test in duplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
d Cytotoxicity observed at doses > 167 µg/ml; relative initial survival, 18% at 350 µg/ml without S9 and 10% at 150 µg/ml with S9
e Only 100 cells/dose scored instead of 200/dose required by OECD; cytotoxicity at doses > 500 µg/ml without S9 and at 80 µg/ml
with S9, and average proliferation time increased at 400 and 60 µg/ml, respectively
f Statistically significant increase in aberrations seen with metabolic activation at 60 µg/ml. In a second test at concentrations
of 50-70 µg/ml, statistically significant but not dose-related increase at all concentrations (14-22 breaks compared with 2 breaks
in control). The authors considered this a weakly positive result. Although the results of the second test were not dose-related,
they confirm the positive result of the first test.
Table 7 (continued)
g Test in triplicate; positive controls included; GLP and QA statements included
h Purity, 95% (personal communication from Dr Rao, Rhone Poulenc). Toxicity observed as 73% survival at 50 nl/ml and 0% at 100 nl/ml.
i Cytotoxicity (abnormally low nuclear and cytoplasmic grains) at doses > 333 µg/well
j Cytotoxicity at doses > 500 µg/ml without S9 and > 80 µg/ml with S9, and average proliferation time was increased at 400 and
60 µg/ml, respectively.
k Statistically significant, dose-dependent increase with metabolic activation at 50-60 µg/ml. In a second test at concentrations of
50-75 µg/ml, statistically significant, dose-related increase at all concentrations (22-35 aberrations/cell compared with 16 per
cell in controls). Since there was no twofold increase at any dose, the authors considered the compound a weak inducer.
l Aberrations analysed in 5 males/group; positive control included; absorption of compound tested in 1 animal/group and confirmed
by a minimum of a 10-fold depression in cholinesterase activity in plasma at 20 mg/kg bw per day for 5 consecutive days. Clinical
signs: decreased motor activity at high dose (day 2); mitotic index not measured. Not clear that the bone-marrow cells were
exposed to the test substance. Only 50 cells scored instead of 100 cells/animal required by OECD.
m GLP and QA statements included
n Each group of dose and harvest time consisted of 5 rats of each sex. Positive control group included in single-dose study only.
Deaths observed only in females at high dose. Clinical signs at high single dose: dyspnoea, languid appearance, and diarrhoea.
Additional signs at high multiple doses: tremor, rough coat, hunched posture, and dark crusts around eyes. No decrease in mitotic
index after single dose, but slightly decreased (54-75%) in males in short-term study. It is not clear whether the bone-marrow
cells were exposed to the test substance. Aberrations were studied in only 50 cells/animal instead of 100 cells and mitotic
index studied in only 500 cells instead of 1000 as required by OECD.
o 10 male rats/group. Three days after the last dose, males were mated with 2 untreated virgin females. Mating process repeated
another 6 times once a week with new virgin females. Females were killed about 2 weeks after mating; scoring for number of corpora
lutea number of dead and live implants per pregnant female. Absence of body-weight gain in males at high dose. No effect on
fertility index, number of corpora lutea, or number of implantations. Pre-implantation loss statistically significantly increased
in week 3 at high and low doses. Number of dead implants increased in week 1 at low dose, weeks 2-3 at intermediate dose, and weeks
1-6 at high dose, with a positive dose-response relationship. The proportion of females with one or more dead implants was
significantly increased at the high and intermediate doses (week 2 and weeks 2-3, respectively) and at the high dose also of females
with two or more dead implants. The number of dead implants per total number of implants was significantly increased at the high
dose (weeks 2-5) and intermediate dose (week 5) (0.08-0.12 in test groups compared with 0.02-0.05 in control group). The number of
live implants per pregnant female, however, was not affected (change in live implant ratio is the criterion of dominant lethality).
In the positive control group, more marked increases in dead implants per total implants observed in weeks 1-4 (0.6-1.0), and the
number of live implants per pregnant female was significantly decreased (0.0-8.3 compared with 9.9-11.9 in controls). The authors of
the report concluded that the compound was mutagenic; however, the shortcomings of the test and the fact that the number of live
implants was not affected indicate that the result is equivocal. The shortcomings of the study were that only 12-18 pregnant females
were included in each treated group and 15-17 in the control group, whereas according to OECD 478 30-50 pregnant females per mating
interval are required; mating was not confirmed. Only QA statement included.
Table 7 (continued)
p 24 males/group. Doses based on a range-finding study. Two days after the last dose, males were mated with 2 untreated virgin
females. Mating process was repeated another 7 times once a week with new virgin females. Females were killed 14 days after
presumed gestation; scoring for number of corpora lutea and for number of dead and live implants per pregnant female. Other
observations: viability, clinical signs, body weight, food consumption, gross necropsy of males and females, especially reproductive
organs (including histopathological examination). Three males at high dose died showing clear signs of intoxication. One male was
replaced. Clinical signs at high dose were characteristic of cholinesterase poisoning. Body-weight gain was inhibited (dose-related)
during treatment at 5 and 20 mg/kg bw per day, and food consumption was lower at the high dose. No compound-related findings at
gross necropsy except a single finding of an epididymal mass in one male at 5 mg/kg bw per day; no effect on testis weight, mating
index, fertility index, or pregnancy rate. Average numbers of corpora lutea, implantations, litter sizes, resorptions, percentage
of dead conceptuses, and number of females with resorptions similar in treated and control groups, except a single finding of dams
with increased resorptions in week 4 of mating at the intermediate dose.
thyroid glands were weighed, and selected tissues (especially from the
reproductive organs) from control and high-dose animals were examined
histopathologically.
The dietary concentrations of 1, 30, and 300/150 ppm during the
first premating and mating periods were equal to 0.07, 2.2, and 23/12
mg/kg bw per day for F0 and F1 males and 0.09, 2.7, and 27/14
mg/kg bw per day for F0 and F1 females. During the second mating
period, the doses of 1, 30, and 150 ppm given to F0 males were equal
to 0.04, 1.3,and 7.1 mg/kg bw per day; the intake of females,
calculated only for weeks 20-24, was 0.07, 2.0, and 11 mg/kg bw per
day.
The clinical signs in F0 parents at the high dose were soft
faeces during the first 2 weeks of exposure and tremors in females
during gestation and lactation. No clinical signs were observed in
F1 parents. The body weights of F0 males at 300 ppm and F1 males
at 150 ppm were significantly decreased throughout the exposure
period. F0 males at 300 ppm had significantly lower body-weight
gains during several weeks, whereas an increase in body-weight gain
was observed occasionally when the dose was changed to 150 ppm. The
weight gain of F1 males at the high dose was reduced during the
premating period and occasionally thereafter. Significantly decreased
body-weight gains were observed occasionally in males at 30 ppm but
these did not result in significantly lower body weights. F0 females
at the high dose had significantly reduced body-weight gains in weeks
1 and 9, but these did not result in lower body weights during the
premating period for the F1a litter. The total body-weight gain
during the 0-20 days of gestation with the F1a litter was
significantly reduced, and the body weights were lower during
lactation. These lower (but not statistically significant) body
weights of F0 females at the high dose persisted during the
premating and mating periods for F1b progeny and reached statistical
significance on days 4-21 of lactation, although there was no effect
on body-weight gain. The body weights of F1 females at the high dose
were significantly reduced throughout premating, gestation, and
lactation, but with no effect on weight gain. Food intake was affected
only at the high dose, with lower consumption observed for F0 males
in weeks 1, 2, and 20 and for F1 males in weeks 9, 11, and 16-20.
The food consumption of females of the F0 generation at the high
dose was lower during the first week of exposure, but food consumption
was affected only on days 4-14 of gestation of the F1a generation.
Length of gestation and indices of mating, fertility, and gestation
were not affected in either generation. The final body weights of F0
and F1 males at the high dose were significantly reduced, and only
in F1 males at this dose the absolute, but not the relative, weight
of the thyroid was significantly lower. No treatment-related lesions
were observed on gross or histopathological examination.
Cholinesterase activity was significantly inhibited in plasma and
brain of F0 and F1 animals of each sex at the high dose, in F1
animals at 30 ppm, and in F0 females at 30 ppm; plasma
cholinesterase activity was also inhibited in F0 males at 30 ppm.
There was no effect on erythrocyte acetylcholinesterase activity.
Analyses of data for the litters revealed lower body-weight gains
in F1a pups at 300 ppm throughout lactation, resulting in lower body
weights from day 7 onwards in males and from day 4 in female pups. The
survival of F1a pups was not affected up to weaning, but the
survival index was only 51% 1 week after weaning on day 28. Necropsy
of dead F1a pups showed blood in the stomach and/or intestines. The
body weights of surviving F1a pups at the high dose remained reduced
up to sacrifice on day 49, and their body-weight gain was reduced up
to day 35. F1b pups at the high dose of 150 ppm showed lower
body-weight gain, from the second week post partum onwards for
females and from the third week for males, resulting in lower body
weights from day 14 and day 21, respectively. Survival was not
affected. Significant reductions in average litter size at 1 ppm,
beginning on day 4, were considered spurious owing to the lack of a
dose-response relationship. The litter sizes and sex ratios of F2
litters were unaffected by treatment. The decreased litter sizes, and
as a consequence higher pup body weights, at 1 and 30 ppm and the
reduced sex ratio at 30 ppm were considered not to be related to
treatment in the absence of a dose-response relationship. The
body-weight gain of pups at the high dose was reduced from day 4
onwards, resulting in lower weights from lactation day 7. Reduced
weight gain was found on days 4-7 of lactation in the group receiving
30 ppm, with a (probably) compensatory increase on days 14-21, but
this was considered irrelevant toxicologically. Deaths among F2 pups
at the high dose on days 7-14 resulted in a significantly reduced
14-day survival index and lactation index. No treatment-related
lesions were observed in dead F2 pups or in F1a, F1b, and F2
pups subjected to scheduled necropsy 1 week after weaning.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of lower body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in parents of each sex in each
generation. No effects were observed on reproductive parameters. The
NOAEL for toxicity to pups was 30 ppm, equal to 1.3 mg/kg bw per day,
on the basis of effects on body weight and body-weight gain and deaths
in F2 litters at 150 ppm (Neeper-Bradley, 1991).
Groups of 10 male and 20 female Fischer 344 rats, 44 days old,
were fed diets containing technical-grade ethoprophos (purity, 95.3%)
at concentrations of 0, 60.5, 131, or 262 ppm (equivalent to 0, 3.0,
6.6, and 13 mg/kg bw per day) for 56 days before mating. Weanlings
from the second litters were selected to be the parents of the next
generation. All parental animals and weanlings of each generation were
subjected to gross pathological examination. Additionally, all F1b
and F2b parents and five male and five female weanlings from each of
the F1a, F2a, F3a, and F3b litters were evaluated
histopathologically. A statement of QA was provided.
The mortality rate was unaffected but growth was slightly
depressed at the two higher doses in all parental generations. Data on
food consumption were not available. A dose-related increase in the
number of dams that cannibalized their young was noted in all treated
groups of the F0 generation, mainly with regard to the second
litters. The percentage of animals becoming pregnant was reduced in
all treated F1a litters (75, 60, 55, and 55%, respectively, at 0,
60.5, 131, and 262 ppm) and for F3a and F3b litters at 262 ppm.
The lactation index (pup survival on days 4-21) was decreased at both
131 and 262 ppm in the F1b (56% and 40%, respectively, compared with
89% in controls) and the F2a generation (86% and 67%, respectively,
compared with 100% in controls) and at the high dose in the F2b
generation (67% compared with 88% in controls). Weanling weight (not
determined for F1a and F1b litters) was depressed in the F2a and
F2b litters at 131 and 262 ppm and in the F3a litters at 262 ppm.
Other effects that were observed only at the highest dose included a
tendency for decreased litter size at birth in almost all generations
of progeny, prolongation of the mean gestation period of F1b litters
(this parameter was not evaluated in all generations), the occurrence
of bilateral lenticular opacity (cataracts; in 29%) and anophthalmia
(3%) in F2a weanlings (information on the litter distribution of
these abnormalities was not available), and an increased incidence of
multifocal granuloma of the mesenteric lymph nodes in F2b adults.
The increased incidence of cannibalization among F0 dams and the
slight decrease in pregnancy rate in F1a litters at 60.5 ppm did not
recur in all generations and were probably related to maternal
toxicity. This concentration was considered to be the minimal
no-effect level for reproductive toxicity. As enzootic pneumonia was
observed in all parental animals and in some pups, the results of this
test are unreliable (Fletcher et al., 1980).
(ii) Developmental toxicity
Rats
A preliminary dose-range finding study was conducted according to
GLP and with a statement of QA, in which five female Sprague-Dawley
COB SC rats were given ethoprophos (purity, 95.6%) in corn oil at
doses of 0, 0.2, 1, 4, 8, or 16 mg/kg bw per day by gavage on days
7-15 of gestation. Fetuses were removed surgically on day 20 and
examined for external anomalies. The only finding was body-weight loss
in dams at the highest dose during days 6-9 of gestation and reduced
body-weight gains (24%) throughout treatment. There were no signs of
developmental toxicity (Rodwell, 1989a).
In the main study, conducted according to GLP and with a
statement of QA, groups of 25 pregnant female
Crl:COBS(R)CD(R)BR(R)VAF rats aged 3 months were given
ethoprophos (purity, 95.6%) by gavage in corn oil at doses of 0, 2, 9,
or 18 mg/kg bw per day on days 6-15 of gestation. The dams were
observed for clinical signs, and body weight and food consumption were
measured. Fetuses were removed surgically on day 20, weighed, and
examined for external, visceral, and skeletal anomalies.
Dose-dependent maternal toxicity was observed at 9 and 18 mg/kg
bw per day, with treatment-related findings including soft stools at
both doses and faecal staining at the high dose. Significantly lower
body-weight gain was observed at 9 mg/kg bw per day on days 6-9 of
gestation, and during this period females at the high dose showed
body-weight loss which resulted in lower body weights by the end of
the study. The food consumption of animals at the high dose was
reduced on days 6-16 of gestation. No effect was found on survival, no
morphological changes were observed at necropsy, and no changes in
gravid uterine weight or fetal parameters (viability, resorptions,
body weight, and sex ratio) were found. The incidences of external,
visceral, and skeletal variations and of irreversible structural
changes were unchanged. The NOAEL for maternal toxicity was 2 mg/kg bw
per day, and that for developmental toxicity was 18 mg/kg bw per day
(Rodwell, 1989b).
Groups of 25-35 mated female TAC:N(SD)FBR rats were intubated
with technical-grade ethoprophos (purity, 94%) in corn oil at doses of
0, 0.16, 1.6, or 16 mg/kg bw per day on days 6-15 of gestation, the
day a vaginal sperm plug was observed being considered day 0. The dams
were killed on day 20 and the fetuses removed for gross, skeletal, and
visceral examination. A positive control group was included.
The pregnancy rate was comparable in all groups. During
gestation, 1/24 pregnant dams at 1.6 mg/kg bw and 18/30 at 16 mg/kg bw
died or aborted; in addition, three non-pregnant dams at the high dose
were found dead. Growth was depressed on days 15 and 20 of gestation
in dams at 16 mg/kg bw. No significant difference was found between
control and treated groups in the mean numbers of corpora lutea,
implantation sites, live fetuses, dead fetuses or resorptions, mean
fetal weight, or sex ratio. According to a summary of the skeletal
findings and soft-tissue abnormalities in fetuses, the only notable
observation was a significant increase in the incidence of litters
containing fetuses with extra lumbar ribs in animals at 1.6 mg/kg bw.
Since no dose-response relationship was seen, the authors could not
attribute this finding to exposure to ethoprophos. Incomplete
ossification of vertebrae was observed in all groups, including the
controls, with incidences of 18, 10, 7, and 17% in control, low-,
intermediate-, and high-dose groups, respectively). The NOAEL for
maternal toxicity was 1.6 mg/kg bw per day on the basis of deaths and
depressed body-weight gain, and the NOAEL for developmental toxicity
was 16 mg/kg bw per day (Knickerbocker & Re, 1979, and amendment in
1985).
Rabbits
A preliminary dose-range-finding study was conducted according to
GLP and with a statement of QA in which groups of eight female New
Zealand white rabbits received ethoprophos (purity, 95.6%) in corn oil
at doses of 0, 0.1, 0.5, 2, 5, or 10 mg/kg bw per day by gavage on
days 7-18 of gestation. The fetuses were removed surgically on day 29
and observed for external anomalies. Dose-dependent maternal toxicity
was observed at 5 and 10 mg/kg bw per day, the clinical signs
including emaciation, an increased incidence of soft stools, and
faecal and urine staining. Consistent body-weight loss and a
dose-dependent mortality rate were also seen in these two groups.
There were no signs of developmental toxicity (Rodwell, 1989c).
In the main study, conducted according to GLP and with a
statement of QA, groups of 20 artificially inseminated New Zealand
white rabbits aged 6 months received ethoprophos (purity, 95.6%) by
gavage in corn oil at doses of 0, 0.625, 1.25, or 2.5 mg/kg bw per day
on days 6-18 of gestation. The does were observed for clinical signs,
and body weight and food consumption were measured. The fetuses were
removed surgically on day 29, weighed, and examined for external,
visceral, and skeletal anomalies.
There were no clinical signs of maternal toxicity, no adverse
effects on survival, body weight, or food consumption, and no
morphological changes. Gravid uterine weight and fetal parameters
(viability, resorptions, body weight, and sex ratio) were also
unchanged. The finding of a significantly reduced mean number of
viable fetuses per doe at 1.25 mg/kg bw per day was due to the absence
of this effect in the group at the high dose and considered not to be
related to treatment. The incidences of external, visceral, and
skeletal variations were not altered, as the finding of a decreased
incidence of 27 presacral vertebrae in fetuses at the intermediate and
high doses was considered not to be toxicologically relevant.
Increased incidences of cleft palate, flexed paw, gastroschisis, and
cephalocoele seen on external examination and of atelectasis and
hydrocephaly seen on visceral examination of the group at the
intermediate dose when compared with controls and with historical
control values were observed in fetuses of only a single doe and were
considered not to be related to treatment. The incidences of fetuses
with malformations were 2.4, 4.1, 4.0, and 6.6% and those of litters
with malformed fetuses were 12, 11, 11, and 35% in the control, low-,
intermediate-, and high dose groups, respectively. The malformations
seen at the high dose were dissimilar and occurred once or at very low
frequency; furthermore, they were types that occur spontaneously in
this strain of rabbits and the incidences were within the historical
control values. In the absence of effects on fetal weight, the
increased incidences of irreversible structural changes at the high
dose were considered to be unrelated to treatment. The NOAEL for both
maternal and developmental toxicity was 2.5 mg/kg bw per day, the
highest dose tested (Rodwell, 1989d).
Groups of 17 artificially inseminated female New Zealand white
rabbits were intubated with technical-grade ethoprophos (purity,
95.7%) in corn oil at doses of 0, 0.125, 0.5, or 2 mg/kg bw per day on
days 6-18 of gestation, the day of insemination being considered day
0. The surviving does were killed on day 29 of gestation, and their
fetuses were removed for external, visceral, and skeletal
examinations. A statement of QA was provided.
No treatment-related deaths occurred, but does at all doses
showed an increased (but not dose-related) incidence of anorexia
during and after treatment. A dose-related but not statistically
significant decrease in mean body-weight gain was observed between
days 6 and 18 in the does at the two higher doses. Control and treated
groups did not differ significantly with respect to pregnancy rate,
mean numbers of corpora lutea, implantations, resorptions, dead
fetuses, or live fetuses, mean fetal weight, mean fetal crown-rump
length, sex ratio, uterine weight with or without fetuses, or the
frequency of gross and visceral abnormalities. An increased total
incidence of skeletal variants was observed in the litters of all
treated does, with 14% at the low dose, 13% at the intermediate dose,
and 17% at the high dose, and 6% in controls. The increase was not
dose-related, and the frequency of any particular type of skeletal
variant, when considered alone, did not show a dose-response
relationship. The NOAEL for maternal toxicity was 0.12 mg/kg bw per
day on the basis of reduced body-weight gain. The NOAEL for
developmental toxicity was 2 mg/kg bw per day (Wolfe et al., 1981).
(f) Special studies: Neurotoxicity
Rats
In a study conducted according to GLP and with a statement of QA,
groups of 24 Crl:CD(SD)BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single dose of ethoprophos (purity, 95.7%) by gavage
in corn oil. Male rats received doss of 0, 30, or 60 mg/kg bw and
females received 0, 20, or 40 mg/kg bw. The doses were based on a
range-finding study. The animals were observed daily for deaths and
clinical signs, and body weights were recorded weekly. Blood samples
were taken from six animals of each sex per group 2 h and 3, 8, and
15 days after dosing to determine cholinesterase inhibition in plasma
and erythrocytes. Brain acetylcholinesterase activity was determined
in the frontal cortex, caudate/putamen, hippocampus, and cerebellum of
the same animals at the same times.
One male rat given 60 mg/kg bw was found moribund. The clinical
signs observed in females at 40 mg/kg bw and males at 60 mg/kg bw were
tremors and excessive salivation. Males also showed hunched posture,
laboured breathing, rough and stained coats, pale bodies, ocular
discharge, incoordination, hypoactivity, and were cold to touch. Among
animals at the lower dose, the only clinical sign was cold to touch in
one female at 20 mg/kg bw. There were no effects on body weight.
Cholinesterase activity was inhibited in all tissues examined by 2 h
after treatment, and the effects were dose-, time-, and
tissue-dependent (Table 8). Recovery of cholinesterase activity was
apparent by day 3 or 8 after dosing and was complete by day 15 for
plasma and cerebellar cholinesterase activity. Statistically
significant inhibition of acetylcholinesterase activity persisted in
the hippocampus through day 15 at the high dose and in erythrocytes
and the frontal cortex of males at both low and high doses. Persistent
inhibition was observed in the caudate/putamen at both doses, but the
inhibition was not statistically significant (Weiler, 1994a).
In a study conducted according to GLP and with a statement of QA,
groups of 17 Crl:CD(R) BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single oral dose of ethoprophos (purity, 96.2%) by
gavage in corn oil. The doses were 0, 5, 50, or 75 mg/kg bw for males
and 0, 5, 25, or 50 mg/kg bw for females, which were based on
range-finding studies. The animals were observed daily for deaths and
Table 8. Cholinesterase activity in various tissues of rats after a single oral dose of ethoprophos
Tissue Dose Inhibition from control mean (%)a
(mg/kg bw)
Males Females
Day 1b Day 3 Day 8 Day 15 Day 1b Day 3 Day 8 Day 15
Plasma cholinesterase 30/20 83* 43* 6 12 90* 31* 27 6
60/40 93* 68* 19* 14 94* 52* 23 0
Erythrocyte cholinesterase 30/20 43* 46* 31* 23* 44* 48* 29* 17
60/40 53* 46* 33* 22* 49* 50* 35* 17
Brain cholinesterase in 30/20 45* 38 30 9 72* 25 54 25
caudate/putamen 60/40 93* 75* 51 32 92* 63* 39 40
Brain cholinesterase in 30/20 45* 30* 19* 0 50* 14 8 4
hippocampus 60/40 72* 47* 27* 18* 75* 29* 21* 17*
Brain cholinesterase in 30/20 48* 38* 34 19* 55* 10 0 32
frontal cortex 60/40 76* 50* 49* 27* 77* 40* 24* 29
Brain cholinesterase in 30/20 46* 11 0 0 60* 11 0 3
cerebellum 60/40 81* 35* 7 6 80* 29* 9 3a
a 100% - mean treated/mean control × 100%
b 2 h after dosing
* Significantly different from control at p < 0.05
clinical signs, and body weights were recorded before treatment and on
days 1, 8, and 15. Twelve animals of each sex per group were subjected
to a functional observational battery of tests and tests for motor
activity before treatment and at 2 h and 8 and 15 days after dosing.
Cholinesterase activity was determined in plasma and erythrocytes from
the remaining five animals of each sex per group before treatment and
on days 2, 8, and 15 after dosing. After blood collection on day 15,
the animals were killed and their brains collected for determination
of acetylcholinesterase activity. Neuropathological examinations were
conducted at the end of the study on all appropriate tissues from six
rats of each sex per group that had not been used to determine
cholinesterase activity. All animals were examined macroscopically and
only the control and high-dose groups microscopically.
Treatment-related deaths were seen in two males given 75 mg/kg bw
and six females given 50 mg/kg bw; four of the females were replaced.
The deaths of one male at 50 mg/kg bw and one female at 5 mg/kg bw
were considered to be unrelated to treatment. Clinical signs in
animals at the high doses included thin or hunched appearance,
tremors, laboured respiration, protruding eyes, incoordination,
hypoactivity, excessive salivation, and cold to touch. A few males at
50 mg/kg bw also showed some of these signs. The results of the
functional battery showed mild to severe functional or behavioural
changes only on the day of dosing, and the changes were characteristic
of cholinesterase poisoning. No effects were seen at 5 mg/kg bw dose.
Mild effects were seen in two females at 25 mg/kg bw and in several
males at 50 mg/kg bw, which included salivation, lip smacking, ataxia,
negative pupillary response, and tremors. Moderate and severe effects
were observed in females at 50 mg/kg bw and males at 75 mg/kg bw,
which included, in addition to the effects described above, low
carriage and prostrate position, lethargy, changes in the ease of
handling, lachrymation, laboured or gasping respiration, increased
latency until first step, paralytic gait, negative corneal response,
and negative air drop and startle reflexes. The analgesic reflex to a
mild heat stimulus was delayed significantly on day 1 in males and
females at 50 mg/kg bw and in males at 75 mg/kg bw. Body temperature
was statistically significant decreased by 3-4°C in females at 50
mg/kg bw and in males at 75 mg/kg bw; a slight decrease in body
temperature was seen in males at 50 mg/kg bw. Mean forelimb grip
strength was decreased only on day 1 in males at 75 mg/kg bw and
females at 50 mg/kg bw and was statistically significant only in
males. The NOAEL for behavioural and functional effects was 5 mg/kg
bw. Motor activity was markedly and statistically significantly
decreased in males at 50 and 75 mg/kg bw (dose-related) and in females
at 50 mg/kg bw on day 1 (Table 9). Differences were no longer seen on
day 8 or 15. The NOAEL for motor activity was 5 mg/kg bw.
The effects on cholinesterase activity are summarized in Table
10. On day 2, plasma cholinesterase activity was dose-dependently
inhibited for males (45-94%) and females (49-94%) at all doses but had
recovered within 8 days of dosing. Erythrocyte acetylcholinesterase
activity was inhibited in females at all doses (not dose-related) and
in males at 50 and 75 mg/kg bw. Generally nonsignificant inhibition of
Table 9. Motor activity (mean total counts) measured shortly after dosing (day 1) and during 10-min
intervals for 60 min in rats given single doses of ethoprophos
Dose Motor activity (mean (standard deviation))
(mg/kg bw)
Males Females
0-10 min 10-20 min 2-30 min Total 0-10 min 10-20 min 20-30 min Total
(0-60 min) (0-60 min)
0 720 (130) 280 (140) 100 (120) 1300 (510) 580 (86) 280 (140) 98 (100) 1200 (330)
5 630 (110) 300 (160) 51 (64) 1000 (270) 650 (160) 200 (180) 69 (110) 1200 (460)
25 480 (270) 170 (210) 78 (140) 1100 (960)
50 290* (240) 150* (110) 20 (25) 670* (430) 38* (34) 28* (24) 44 (80) 240* (190)
75 33* (28) 25* (22) 17 (19) 180* (100)
* Significantly different at p < 0.05
Table 10. Cholinesterase activity in plasma, erythrocytes, and brain of rats given a single
dose of ethoprophos
Dose Day of Inhibition of cholinesterase from mean control valuea
(mg/kg bw) sampling
Males Females
Plasma Erythrocyte Brain Plasma Erythrocyte Brain
5 2 45* 8 49* 33*
8 -4 27 11 20
15 -8 -7 -3 -3 3 7
25 2 73* 49*
8 -12 21
15 -14 33* 11
50 2 87* 45* 94* 35*
8 -2 26 2 30
15 0 18 17 -3 20 10
75 2 94* 55*
8 6 25
15 -4 31* 18
a 100% - mean treated/mean control × 100%
* Significantly different at p < 0.05
> 20% was still present on days 8 and 15 in females at 25 and 50
mg/kg bw and in males at 50 and 75 mg/kg bw. In these groups, brain
acetylcholinesterase activity was inhibited by about 10% in females
and 18% in males at day 15, but this effect was not statistically
significant. Macro- and microscopic neuropathological examinations
showed no treatment-related changes. As brain acetylcholinesterase
activity was not measured directly after exposure, the NOAEL could not
be based on this end-point. The inhibition in females was not
dose-related, and there was a large standard deviation at 5 mg/kg bw.
The NOAEL for neurotoxicity was 5 mg/kg bw on the basis of behavioural
changes and inhibition of erythrocyte acetylcholinesterase activity
(Weiler, 1994b).
Groups of 27 Crl:CD BR VAF/Plus rats of each sex aged 6 weeks
were fed diets containing ethoprophos (purity, 95.7%) at
concentrations of 0, 4, 40, or 400 ppm, equal to 0, 0.26, 2.6, and 27
mg/kg bw per day for males and 0, 0.31, 3.0, and 31 mg/kg bw per day
for females, for 13 weeks. Twelve rats of each sex at each dose were
selected for functional battery and motor activity testing before
dosing and in weeks 4, 8, and 13. Of these, six of each sex per dose
were selected for neuropathological investigations, and the remaining
rats were observed only macroscopically. Cholinesterase activity was
measured in the plasma and erythrocytes of 10 rats of each sex per
group and acetylcholinesterase activity in four regions of the brain
(frontal cortex, caudate/putamen, hippocampus, and cerebellum) at
sacrifice in five rats of each sex per dose in weeks 4, 8 and 14. All
animals were observed for deaths and clinical signs, and body weight
and food consumption were recorded.
The only clinical sign considered to be related to treatment was
brown staining of the anal region in males at 400 ppm. Significantly
lower body-weight gain was observed in males at 400 ppm throughout
treatment and in females up to week 10, which resulted in
significantly lower body weights in animals at this dose. The mean
food consumption of animals of each sex at the high dose was
significantly lower during the first week and remained low in males
throughout treatment. In males at 40 ppm, the food consumption was
higher than that of controls. Changes in the results of the functional
battery tests considered to be related to treatment were seen only at
the high dose and included mild tremor (one female), vocalization
during handling (two females), slight salivation (one male), unkempt
fur (two males, one female), latency to first step (one male, one
female), and jumping to touch (one male) in week 4; in week 8, the
observations included intermittent vocalization in cage (three
females), slight lachrymation (one female, and one female at 40 ppm),
slight salivation (one male, two females, and one female at 40 ppm),
latency to first step (two females), aggressive behaviour (one
female), jump to touch response (one female), negative air drop reflex
(one male), and negative pupillary response (one female); in week 13,
the observations included aggressive behaviour (one female), latency
to first step (one female, and two males at 40 ppm), and no movement
in open arena (one male). Because the clinical signs observed at 40
ppm also occurred occasionally as single findings in controls, they
were considered to be unrelated to treatment. Other clearly
treatment-related observations in the functional battery test at the
high dose were decreased analgesic reflex in males in weeks 4, 8, and
13, and lower grip strength in fore- and hindlimbs of males and in
forelimbs of females. The motor activity of males at 400 ppm was
generally significantly lower than that of controls at all times;
females showed a slight decrease in activity only in week 4.
Cholinesterase activity in plasma, erythrocytes, and brain is
shown in Table 11. A > 20% inhibition of erythrocyte
acetylcholinesterase activity was found in males and females at doses
> 40 ppm. Inhibition of acetylcholinesterase activity in one or two
regions of the brain by > 20% was observed in males at 40 ppm at
weeks 4 and 8 and in all regions at 400 ppm. In females, inhibition of
acetylcholinesterase activity by > 20% was seen in three brain
regions at weeks 8 and 14 at 4 ppm, which was significant in the
frontal cortex in week 14, and in all regions at all times at higher
doses. No macroscopic or microscopic changes were observed.
An NOAEL could not be identified because brain
acetylcholinesterase was inhibited at all doses. The NOAEL for
neurotoxic effects was 40 ppm, equal to 2.6 mg/kg bw per day, on the
basis of the results of the functional observational battery and motor
activity tests (Weiler, 1994c).
Chickens
Two groups of 10 deep-litter hens weighing 1.4-2.1 kg (age not
specified) were intubated with technical-grade ethoprophos (purity
unknown) at a single dose of 5.6 µl/kg bw, equivalent to 6.2 mg/kg bw
and stated to represent the oral LD50 value in a previous study
performed at the testing laboratory, or tri- ortho-cresyl phosphate
(positive control) at 500 mg/kg bw. Four untreated hens were retained
as untreated controls. The hens given ethoprophos showed no gross
signs of ataxia or paralysis, although symptoms of transient
inactivity and 'depression' were noted between 1 and 48 h. Four hens
died within 48 h of dosing. The positive controls showed clinical
signs characteristic of delayed neurotoxicity. Histopathological
examination of sections of the spinal cord and sciatic nerve, stained
by the Weil-Weigert method (not referenced), from birds in all groups
at the end of a 21-day observation period revealed no evidence of
demyelination (Weir & Murphy, 1967).
In an initial study conducted to determine the LD50 of
ethoprophos in hens, six groups of 10 domestic hens ('a hybrid brown
laying strain') were given ethoprophos (purity, 94.5%) at doses of
0-16 mg/kg bw in a volume of 2 ml/kg bw by gavage. An LD50 of 6.4
mg/kg bw was calculated, with a 95% confidence interval of 4.8-8.8
mg/kg bw. On this basis, a dose of 6.5 mg/kg bw was selected for the
main study. The hens were divided randomly into six groups of 10. The
untreated controls received only corn oil, whereas the positive
controls received 500 mg/kg bw of tri- ortho-cresyl phosphate by
gavage. The treated groups first received 10 mg/kg bw of atropine
Table 11. Cholinesterase activity in plasma, erythrocytes, and brain regions at various
times during 13-week dietary exposure of rats to ethoprophos
Week Tissue Inhibition of cholinesterase activity from mean in controlsa
Males Females
4 ppm 40 ppm 400 ppm 4 ppm 40 ppm 400 ppm
4 Plasma 3 64* 90* 20 80* 97*
Erythrocyte 7 30* 26* -14 19 14
Brain
Caudate/putamen 17 23* 82* 8 32 83*
Hippocampus -7 4 48* 9 18 50*
Frontal cortex 19 -2 71* -28 30 74*
Cerebellum -7 0 52* -3 13 53*
8 Plasma -5 58* 85* 34* 87* 98*
Erythrocyte 10 23 35 19 32 40
Brain
Caudate/putamen 3 11 82* -11 -2 53*
Hippocampus 11 26* 59* 23 33* 67*
Frontal cortex 10 23 61* 33 49 80*
Cerebellum 0 0 46* 21 38* 62*
14 Plasma -2 54* 87* 18 86* 97*
Erythrocyte 19 33* 40* -6 22 11
Brain
Caudate/putamen -3 0 73* 34 49* 88*
Hippocampus 0 -9 54* 23 36* 66*
Frontal cortex 10 7 53* 52* 56* 80*
Cerebellum 9 -11 49* 6 27* 59*
a 100% - mean treated/mean control × 100%
* Significantly different from controls at p < 0.05
sulfate by intramuscular injection and then 6.5 mg/kg bw of
ethoprophos by gavage in corn oil at a constant volume of 2.5 ml/kg
bw. The atropine was found to have had only a minimal protective
effect, as 31/40 of the treated birds died within 4 days, most within
the first 24 h. Because of excessive mortality, two additional groups
were added, consisting of 12 and 11 birds, respectively, but it was
not specified whether they were treated concurrently with the
untreated controls. These birds were given pyridine 2-aldoxime
methiodide at 50 mg/kg bw by intramuscular injection in addition to
atropine before treatment with 6.5 mg/kg bw of ethoprophos. The
surviving birds were given further injections of pyridine 2-aldoxime
methiodide and atropine 24 and 48 h after treatment, and two birds in
the last group were injected 72 h after treatment. This procedure was
only marginally successful, as 14/23 birds died within 48 h of
treatment. On the basis of the deaths after the first dose of
ethoprophos, the LD50 was recalculated as 5.2 mg/kg bw, and this
dose was administered on day 22 to all 18 surviving birds, which were
treated with pyridine 2-aldoxime methiodide and atropine immediately
before administration of ethoprophos and again 5 h after the second
(day 22) treatment with ethoprophos; birds in the initial groups were
given additional injections 24 h after the second treatment. Two of
the 18 treated birds died within 72 h of the second treatment.
No clinical signs of neurotoxicity were noted in untreated
control or ethoprophostreated birds, whereas 9/10 birds treated with
tri- ortho-cresyl phosphate showed slight to marked signs of
neurotoxicity, usually by 11 days after treatment. These birds were
killed on day 21 and examined for histopathological changes. All other
surviving birds were killed on day 43. No remarkable macroscopic
changes were observed at necropsy. Portions of the forebrain, mid- and
hind-brain, cervical, thoracic, and lumbar spine, proximal and distal
sciatic nerve, and tibial nerve were evaluated for microscopic
evidence of neurological change. Lesions were noted in the spinal cord
and peripheral nerves of all positive control birds that reflected
'significant axonal degeneration'. Minimal lesions (grade 2 out of a
maximum of 5) were also found in the spinal cord of 9/10 control
birds, and one control had a minimal lesion in the proximal sciatic
nerve; no lesions were found in the brain or distal sciatic or tibial
nerves. A similar distribution of lesions of the spinal cord was seen
in treated birds, except that minimal lesions of the mid- or
hind-brain and lesions of the proximal sciatic nerve were each found
in 2/16 birds. One of the latter lesions was graded as moderate (grade
3) and occurred in a bird that also had a lesion of the mid- and
hind-brain. Three of 16 birds, including this one, also had minimal
lesions of the distal sciatic nerve. The authors concluded that
treatment with ethoprophos produced no clinical signs of neurotoxicity
and that the result was confirmed by the histological examination,
which showed no treatment-related changes in the nerve tissue. The
changes noted in the proximal sciatic nerve of the bird with multiple
lesions were considered to be unrelated to treatment and to represent
the extreme upper limit of background change (Roberts et al., 1986).
The Committee concluded that the study does not provide clear evidence
of neurotoxicity, but because the high rate of mortality reduced the
sensitivity of the study, the equivocal findings in some birds cannot
be dismissed. Historical control data from the testing facility
suggest that the lesions in proximal sciatic nerve are not
spontaneous.
(g) Studies on metabolites
O-Ethyl- S-propylphosphorothioate, a metabolite in rats, and
O-ethyl- O-methyl- S-propylphosphoro-thioate and
O-ethyl- S-methyl- S-propylphosphorodithioate, two metabolites
identified only in corn and potatoes (Rao, 1999), were tested for
toxicity and for their ability to inhibit cholinesterase activity in
female rats given single oral doses.
(i) Acute toxicity
The LD50 values of the three metabolites of ethoprophos in
female Crl:CD (SD)BR VAF/ Plus rats treated orally with the substances
in corn oil were 50 mg/kg bw for
O-ethyl- S-methyl- S-propylphosphorodithioate, 22 mg/kg bw for
O-ethyl- O-methyl- S-propylphosphorothioate, and 1600 mg/kg bw for
O-ethyl- S-propylphosphorothioate. The clinical signs were similar
for each of the metabolites and differed primarily in time of onset
and duration; the signs were also similar to those caused by the
parent compound. GLP and QA statements were included (Welier, 1998).
(ii) Cholinesterase inhibition
Three groups of 10 female Crl:CD(R)(SD)BR VAF/Plus rats aged
about 6 weeks were given a single dose of ethoprophos at 19 mg/kg bw,
O-ethyl- S-methyl- Spropylphosphorodithioate at 17 mg/kg bw, or
O-ethyl- O-methyl- S-propylphosphorothioate at 8 mg/kg bw by
gavage; a control group received the vehicle, corn oil. The animals
were observed for clinical signs before dosing, 0.5 and 2 h after
dosing, and the next day before sacrifice. Cholinesterase activity was
evaluated in plasma, erythrocytes, and brain 24 h after dosing.
Body-weight gain over the 2-day period was significantly lower in
all treated groups than in controls but resulted in a statistically
significant lower terminal body weight only with
O-ethyl- S-methyl- S-propylphosphorodithioate. In the other two
groups, the only clinical finding was a slightly increased incidence
of soft stools, whereas the animals given
O-ethyl- S-methyl- S-propylphosphorodithioate showed signs of
cholinesterase inhibition consisting of staggered gait and tremors.
All three compounds reduced cholinesterase activity in all three
tissues, the strongest inhibition being due to
O-ethyl- S-methyl- S-propylphosphorodithioate (Table 12; Weiler,
1998).
Table 12. Cholinesterase activity in plasma, erythrocytes, and brain of female rats 24 h
after a single oral dose of ethoprophos and two metabolites
Compound Dose Inhibition from control mean (%)a
(mg/kg bw)
Plasma Erythrocytes Brain
Ethoprophos 19 73* 37* 32*
O-Ethyl-S-methyl-S-propylphosphorodithioate 17 78* 30 71*
O-Ethyl-O-methyl-S-propylphosphorothioate 8 40* 47* 48*
a 100% - mean treated/mean control × 100%
* Significantly different from control at p < 0.05
4. Observations in humans
In a study of occupational exposure to ethoprophos in Salinas
Valley, Monterey County, California, USA, four male volunteers were
recruited among regular employees of an experienced pesticide
application firm who were thoroughly familiar with both the practices
and equipment involved in pesticide application and had not worked
with cholinesterase inhibitors for at least 90 days. The occupation
under consideration was that of mixer-loader-applicator, who applied
an emulsifiable concentrate containing 70% ethoprophos in xylene for 3
days (two men), 4 days (one man), or 5 days (one man) to field plots
of 2-3 ha; the only operator working on day 5 treated a field plot of
about 8.6 ha. During the mixing and loading phase, the concentrate,
supplied in 5-gallon (~ 20-L) containers, was diluted to 5% in two
160-gallon (~ 40-L) tanks. Transfer of the pesticide into the tank,
rinsing the container, and filling the spray tanks were done in a
closed system to minimize exposure. The operators wore long-sleeved,
twill work shirts, denim jeans, and rubber boots. They used rubber
gloves and disposable respirators which were changed each day. Face
shields and hard hats were available, especially for the mixing and
loading operations. The application rate of active ingredient was
about 12 kg/ha (range, 11-15 kg/ha). Blood was collected for
determination of cholinesterase activity in plasma and erythrocytes
three times before exposure and as soon as possible after each man's
work session. During application of a commercial preparation
containing 69.6% ethorprop (as an emulsifiable concentrate in xylene),
dermal exposure was monitored on the hands (three hand washings with
2-propanol in water) and face (passive facial patch collector
consisting of 12-layer surgical gauze), corresponding to a surface of
24 cm2. Inhalation was not monitored. Exposure to ethoprophos was
monitored by a standardized gas chromatographic method.
No clinical signs or adverse effects were noted during exposure,
and the changes in plasma and erythrocyte cholinesterase activity were
not statistically significantly different from the mean baseline
values. The maximal variation around the mean was - 8.9% to 7.9%,
which is considered to be normal day-to-day variation. Exposure on the
head and neck was 3.1-78 µg/h, with an average of 34 µg/h
(calculations based on a default head area of 910 cm2). The total
exposure of the head to ethoprophos was 7.6-153 µg, with means of 96,
53, 67, and 36 µg for the four men. The hands were exposed to 0.2-18
µg/h, with an average value of 6.3 µg/h and a value of 0.52-42 µg for
both hands; the mean values were 29, 2.0, 12, and 6.3 µg for the four
men. The overall exposure during the entire period of observation of
the man who worked for 5 days was 620 µg, and the quantity of active
ingredient handled per day was 31-92.4 kg. The overall exposure of the
other operators was 220 µg for the man who worked for 4 days and
handled 26-44 kg daily; 240 µg for the man who worked for 3 days and
handled 19-27 kg daily; and 130 µg for the man who worked for 3 days
and handled 17-44 kg daily (Leffingwell, 1986).
Comments
After oral administration to rats, 14C-ethoprophos was rapidly
and virtually completely absorbed, metabolized and excreted. The main
route of excretion was the urine (51-56%), but significant proportions
were excreted in expired air (about 15%) and faeces (10-14%). Little
radiolabel was found in tissues at 168 h, representing less than 2.5%
of the dose, and the highest concentrations were found in excretory
organs (liver, kidneys, and lungs). There was no evidence that
bioaccumulation would occur after repeated doses. The kinetics of the
radiolabel and the biotransformation appeared to be independent of the
route of administration (oral or intravenous), magnitude and frequency
of dose and sex. Ethoprophos was metabolized by dealkylation of one or
both S-propyl groups, followed by conjugation. The dermal absorption
of ethoprophos has not been studied in vivo, but radiolabelled
ethoprophos penetrated the skin in vitro, the greatest penetration
being seen through mouse skin, lesser penetration through rat and
rabbit skin (at equal rates), and the least penetration through human
skin. In all of the species tested, the rate of penetration was
greater with ethoprophos (emulsified concentrate) diluted 1:19 in
water than with undiluted material.
Single oral doses of ethoprophos were toxic to rats, mice and
rabbits (LD50 values, 31-62 mg/kg bw), and it was highly toxic to
rats exposed once by inhalation (LC50, 0.25 mg/L). Mice and rabbits
were more sensitive to dermal exposure than rats, and female rats were
more sensitive than males, with LD50 values of 8.5 mg/kg bw for
rabbits, 18 mg/kg bw for mice, 420 mg/kg bw for female rats, and 1300
mg/kg bw for male rats. The toxic signs observed after exposure by any
route were characteristic of cholinesterase inhibition. WHO (1999) has
classified ethoprophos as 'extremely hazardous'.
Ethoprophos not only irritates the eyes of rabbits but is very
toxic after administration into the eye, causing the death of tested
animals within 1 h. In a test for dermal irritation, all treated
animals died within 8 h of exposure to the undiluted compound. In a
three-week study in rabbits, the compound caused slight dermal
irritation at all doses tested (0.03, 0.1, and 1 mg/kg bw per day),
whereas no dermal irritation was observed in a 3-week study in rats
given doses of up to 10 mg/kg bw per day.
In studies in dogs (13, 20 and 52 weeks) and mice and rats (2
years) given ethoprophos orally, inhibition of cholinesterase was the
most sensitive parameter. In dogs, the NOAEL for inhibition of brain
acetylcholinesterase activity was 1 mg/kg bw per day, the highest dose
tested. In mice, brain acetylcholinesterase activity was inhibited at
30 ppm, resulting in a NOAEL of 2 ppm, equal to 0.25 mg/kg bw per day;
in rats, the overall LOAEL was 30 ppm (equal to 1.3 mg/kg bw per day)
and no effects were seen at 10 or 1 ppm (equal to 0.5 and 0.04 mg/kg
bw per day, respectively). In a two-generation study of reproductive
toxicity in rats, inhibition of brain acetylcholinesterase activity
was seen in animals of each sex of both parent generations at 30 ppm,
resulting in a NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day. In a
13-week study of neurotoxicity in rats, the activity of brain
acetylcholinesterase was inhibited in females at all doses tested, the
lowest dose being 4 ppm, equal to 0.26 mg/kg bw per day. Clinical
signs characteristic of cholinesterase inhibition were observed only
in the 2-year study in rats, in which the highest dose was reduced
from 600 to 400 ppm because of clinical signs.
Inhibition of brain acetylcholinesterase activity was also
demonstrated after dermal exposure of rabbits and rats to ethoprophos
for three weeks, with NOAELs of 0.1 mg/kg bw per day (LOAEL, 1 mg/kg
bw per day) in rabbits and 0.3 mg/kg bw per day (LOAEL, 1 mg/kg bw per
day) in rats.
Ethoprophos was not carcinogenic in a long-term study in mice
treated in the diet. The highest dose tested was 30 ppm, equal to 4.9
mg/kg bw per day. In three long-term studies of toxicity and
carcinogenicity in rats, some evidence was obtained that ethoprophos
may stimulate progression of C-cell tumours in the thyroid and adrenal
neoplasms (phaeochromocytomas), but this effect was considered to have
little relevance for human risk. The highest dose tested was 400 ppm,
equal to 26 mg/kg bw per day. The Meeting concluded that ethoprophos
is unlikely to pose a carcinogenic risk to humans.
Ethoprophos was tested in an adequate set of assays for
genotoxicity in vitro and in vivo. Positive results in studies for
chromosomal aberrations and sister chromatid exchanges in vitro
after metabolic activation indicate that the compound has intrinsic
genotoxic activity. Chromosomal aberrations were not induced in rats
in two assays and dominant lethal mutations were not induced in rats
in another study, although a slightly positive or equivocal result was
obtained in a further study for dominant lethality. The Meeting
concluded that ethoprophos is unlikely to be genotoxic in vivo.
In a two-generation study of reproductive toxicity in rats, no
effect was observed on reproductive parameters. Clinical signs were
observed only in the F0 parents at the highest dose (300 ppm). The
NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis of
reduced body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in animals of each sex in both parental
generations. The NOAEL for developmental toxicity was 30 ppm, equal to
1.3 mg/kg bw per day, on the basis of effects on body weight and
body-weight gain in pups of both generations and deaths of pups in the
F2 litters at 150 ppm.
In studies of developmental toxicity, maternal toxicity was
characterized by growth depression in rats in one study (NOAEL,
2 mg/kg bw per day) and in rabbits in one study (NOAEL, 0.12 mg/kg bw
per day), whereas no maternal toxicity was observed in rabbits in
another study (NOAEL, 2.5 mg/kg bw per day, highest dose tested).
Since in the first study in rabbits the effect was only marginal (not
statistically significant), the overall NOAEL for maternal effects in
rabbits was 2.5 mg/kg bw per day. No effects on the fetuses of either
species were observed. Cholinesterase activity was not measured in
these studies.
The relationship between neurotoxicity and cholinesterase
inhibition was examined in rats in two studies. In rats exposed by
gavage to single doses of 0, 20, or 40 mg/kg bw (females) or 0, 30, or
60 mg/kg bw (males), cholinesterase activity in plasma, erythrocytes,
and brain was inhibited, with a maximum effect 2 h after dosing. While
cholinesterase inhibition was found in tissues at the lowest doses
tested, clinical signs characteristic of cholinesterase inhibition
were seen only at the highest doses (60 mg/kg bw for males and
40 mg/kg bw for females). In the second study, in which male rats were
exposed to a single dose of ethoprophos at 5, 50, or 75 mg/kg bw and
females at 5, 25, or 50 mg/kg bw, effects on motor activity were
observed in animals of each sex at doses of 50 mg/kg bw and higher,
and functional and behavioural effects were observed at 25 mg/kg bw
and higher. These neurotoxic signs were seen only on the day of
dosing. Significant inhibition of acetylcholinesterase activity in
erythrocytes, measured 1 day after exposure, was observed in females
at doses of 5 mg/kg bw and higher and in males at 50 mg/kg bw and
higher. The inhibition in females was not dose-related, and there was
a large standard deviation at 5 mg/kg bw . The Meeting concluded that
the NOAEL in this study was 5 mg/kg bw.
In a 13-week study in rats, neurotoxicity was observed in tests
for motor activity and for functional and behavioural abnormalities
only at the highest dose (400 ppm). The NOAEL for neurotoxic effects
was 40 ppm, equal to 2.6 mg/kg bw per day. The activity of brain
acetylcholinesterase was inhibited in females at all doses tested; the
lowest dose was 4 ppm, equal to 0.26 mg/kg bw per day.
A study of delayed neurotoxicity in hens was performed with doses
of ethoprophos that caused a high mortality rate despite antidotal
treatment. Equivocal findings were reported in some of the treated
birds; data on the effect of this compound on neuropathy target
esterase activity in hens would be useful in order to allow full
assessment of the neuropathological potential of ethoprophos.
A study of occupational exposure to ethoprophos did not reveal
significant effects on human plasma or erythrocyte cholinesterase
activity. The calculated rates of exposure of head-and-neck areas were
3.1-78 µg/h (average, 34 µg/h), and the rate of exposure of the hands
was calculated to be 0.2-18 µg/h (average, 6.3 µg/h).
O-Ethyl S-propyl phosphorothioate, a metabolite in rats, and
O-ethyl O-methyl S-propyl phosphorothioate and O-ethyl
S-methyl S-propyl phosphorodithioate, two metabolites that have
been identified only in corn and potatoes, were tested for toxicity
and for their ability to inhibit cholinesterase activity in female
rats given single oral doses. The two plant metabolites were of
approximately the same toxicity as the parent compound, while the
animal metabolite was less toxic (LD50, 1600 mg/kg bw) than the
parent.
The 1987 Meeting derived the ADI on the basis of a slight effect
on the liver in a 52-week study in dogs at a dose of 1 mg/kg bw per
day. The NOAEL for this effect was 0.025 mg/kg bw per day. Since this
marginal effect was not observed in other studies in dogs or in other
species and there is a 40-fold difference between the LOAEL and the
NOAEL, this NOAEL was not used as the overall NOAEL to derive the ADI
in the present evaluation.
The present Meeting established an ADI of 0-0.0004 mg/kg bw on
the basis of the NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day, for
inhibition of brain acetylcholinesterase activity in the 2-year study
of toxicity and carcinogenicity in rats and in the study of
reproductive toxicity in rats, and a 100-fold safety factor.
An acute reference dose of 0.05 mg/kg bw was established on the
basis of the NOAEL of 5 mg/kg bw in the study of acute neurotoxicity
in rats, in which functional and/or behavioural effects and inhibition
of erythrocyte acetylcholinesterase were observed at the next highest
dose, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse 2 ppm, equal to 0.25 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
Rat 1 ppm, equal to 0.04 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
1 ppm, equal to 0.04 mg/kg bw per day (parental toxicity in
a study of reproductive toxicity)
5 mg/kg bw (single dose, study of neurotoxicity by gavage)
2 mg/kg bw (maternal toxicity, study of developmental
toxicity; acetylcholinesterase activity not determined);
18 mg/kg bw (fetotoxicity, highest dose tested, study of
developmental toxicity; acetylcholinesterase activity not
determined)
Rabbit 2.5 mg/kg bw (maternal toxicity and fetotoxicity, study of
developmental toxicity; acetylcholinesterase activity not
determined);
Dog 0.025 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0004 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Information on effects on neuropathy target esterase activity in
hens
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to ethoprophos
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption > 70%, mainly within 12 h (rats)
Dermal absorption Tested in vitro, no quantitative data
Penetration rate Mouse skin > rat, rabbit skin > human skin
Distribution Widely distributed, highest levels in organs of elimination (rats)
Potential for accumulation No
Rate and extent of excretion Excreted in urine (55%), expired air (13-17%), and faeces (7-9%) (rats)
Metabolism in animals In rats, metabolized by dealkylation of one or both S-propyl groups,
followed by conjugation
Toxicologically significant compounds Parent compound and plant metabolites ( O-ethyl O-methyl S-propyl
phosphorothioate and O-ethyl S-methyl S-propyl phosphorodithioate)
Acute toxicity
Rat, LD50, oral 33-62 mg/kg bw (vehicle, corn oil)
Rat, LD50, dermal 420 mg/kg bw, females
Rat, LD50, inhalation 0.25 mg/L
Dermal irritation Not reported, but death occurred within 8 h of dermal exposure
Ocular irritation Yes, but also death within 1 h of ocular exposure
Sensitization Not tested
Short-term toxicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant oral NOAEL < 0.26 mg/kg bw per day (LOAEL, 13-week study of neurotoxicity
in rats)
Lowest relevant dermal NOAEL 0.1 mg/kg bw per day (3-week study of dermal toxicity in rabbits)
Lowest relevant inhalation NOAEL Not tested
Long-term toxicity and carcinogenicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant NOAEL 0.04 mg/kg bw per day (105-week study in rats treated in the diet)
Carcinogenicity Not carcinogenic (mice, rats)
Genotoxicity Not genotoxic in vivo
Reproductive toxicity
Reproductive target/critical effect Reduced body weight and survival at maternally toxic doses
Lowest relevant reproductive NOAEL Maternal toxicity: 1 ppm, equal to 0.04 mg/kg bw per day;
fetotoxicity: 30 ppm, equal to 1.3 mg/kg bw per day
Developmental target/critical effect Only maternal toxicity (highest dose tested), no fetotoxicity
Lowest relevant developmental NOAEL Rabbit: maternal and fetotoxicity: 2.5 mg/kg bw per day (highest
dose tested)
Rat: maternal toxicity: 2 mg/kg bw per day; fetotoxicity:
18 mg/kg bw per day
Neurotoxicity/ Delayed neurotoxicity 5 mg/kg bw per day (acute toxicity in rats)
< 4 ppm, equal to 0.26 mg/kg bw per day (13-week study in rats)
No evidence for delayed neurotoxicity in hens, but some equivocal findings
Other toxicological studies No significant effects on erythrocte acetylcholinesterase activity in
exposed workers
Medical data None
Summary Value Study Safety factor
ADI 0-0.0004 mg/kg bw 2-year study, rat; two-generation 100
study of reproductive toxicity, rats
Acute reference dose 0.05 mg/kg bw Acute neurotoxicity, rats 100
References
Auletta, C.S. & Rinehart, W.E. (1979) A comparative dermal toxicity
evaluation in mice (Ethoprop and nine chemical analogs).
Unpublished report Project No. 5789-79 from Bio/dynamics Inc.,
Princeton, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985a) Ames
Salmonella/microsome plate test (EPA/OECD). Unpublished report
No. PH 301-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by RhônePoulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985b). Rat
hepatocyte primary culture/DNA repair system. Unpublished report
No. PH 311-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Barnett, J.W., Jenkins, L.J. & Parent, R.A. (1983) Evaluation of the
chronic toxicity and oncogenic potential of ethoprop in Fischer
344 rats. Unpublished report from Gulf South Research Institute,
New Iberia, Louisiana, USA. Submitted to WHO by Rhône-Poulenc.
Becker, J. & Parke, G.S.E. (1977) A primary dermal irritation study of
ethoprop 93% technical grade on abraded and non-abraded skin of
New Zealand albino rabbits. Unpublished report Lab. No. 7E-6084
from Cannon Laboratories, Inc., Reading, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
Blacker, A.M. (1987) Executive summary -- Acute dermal LD50 of
ethoprop (technical) in rabbits and rats. Unpublished report No.
87/AMB/035 from Rhone Poulenc. Submitted to WHO by Rhône-Poulenc.
Brown, D. (1986) Ethoprophos: 52 week oral (capsule administration)
toxicity study in the beagle. Unpublished report No. 4923-198/16
from Hazleton Laboratories Europe, Ltd, Harrogate, North
Yorkshire, United Kingdom. Submitted to WHO by Rhône-Poulenc.
Dearlove, G.E. (1987) Dominant lethal study of ethoprop technical
administered orally via gavage to Crl:COBS(R)CD(R)(SD)BR male
rats. Confidential report No. 218-004 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Fletcher, M.J., Springer, S.T., D'Addamio, G.H., Conzelmann, P.C. &
Pullin, T.G. (1980) Evaluation of effects of ethoprop on
reproductive performance by a three-generation reproduction study
in Fischer 344 rats. Unpublished report No. 413-858-41 from Gulf
South Research Institute. Submitted to WHO by Rhône-Poulenc.
Hamada, N.N. (1990) A five-month oral toxicity study with one-month
recovery in beagle dogs with ethoprop technical. Unpublished
report No. HLA 656-143 from Hazleton Laboratories America, Inc.
Vienna, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1989) 3-week dermal toxicity study with ethoprop
technical in rabbits. Unpublished report No. HLA 6224-152 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990a) 3-week dermal toxicity range finding study with
ethoprop technical in rats. Unpublished report No. HLA 6224-160
from Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990b) 3-week dermal toxicity study with ethoprop
technical in rats. Unpublished report No. HLA 6224-162 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Iqbal, Z.M. & Menzer, R.E. (1972) Metabolism of O-ethyl-S,S-dipropyl
phosphorodithioate in rats and liver microsomal systems.
Biochem. Pharmacol., 21, 1569-1584.
Ivett, J.L. (1989) Mutagenicity test on ethoprop technical in the rat
bone marrow cytogenetic assay. Unpublished report No. 10750-0-452
from Hazleton Laboratories America Inc., Kensington, Maryland,
USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1986) Acute dermal LD50
study with ethoprop in New Zealand white rabbits. Unpublished
report No. 3147.2 from Springborn Institute for Bioresearch,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1987) Acute dermal LD50
study with ethoprop (technical) in Sprague Dawley rats.
Unpublished report No. 3147.5 from Springborn Institute for
Bioresearch, Inc., Spencerville, Ohio, USA. Submitted to WHO by
RhônePoulenc.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of ethoprop
MCTR-603-78 in Sprague-Dawley rats. Unpublished report No. 5850
from Food & Drug Research Laboratories, Inc. Submitted to WHO by
Rhône-Poulenc. Report amended 10 June 1985.
Kopp, R., Gunzel, P., Rosskamp, G., Schmidt, M. & Schuppler, J. (1980)
Acute inhalation toxicity to the rat (M&F) of liquid ethoprophos
following single 4 hours continuous exposure (LC50). Report
(Protocol No. 449/79) from Schering AG. Submitted to WHO by
Rhône-Poulenc (in German).
Leffingwell, J.T. (1986) An exposure study of mixer/loader-applicators
working with MOCAP EC. Unpublished report from Rhône-Poulenc
Inc., New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Myhr, B.C. & Brusick, D.J. (1981) Evaluation of MOBIL No. 01238101 in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 2478-80 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by RhônePoulenc.
Neeper-Bradley, T.L. (1991) Two-generation reproduction study of
ethoprop technical administered in the diet to CD(R) (Sprague
Dawley) rats. Unpublished report No. 53-598 from Bushy Run
Research Center, Export, Pennsylvania, USA. Submitted to WHO
Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1982) Ethoprop (48 095 RP). Acute toxicity
in mice treated orally and rats treated percutaneously.
Unpublished report from Rhône-Poulenc, Vitry sur Seine, France.
Submitted to WHO by Rhône-Poulenc (in French).
Powers, M.B. (1965) V-C 9-104 (technical grade) Acute oral
administration -- Rats. Acute dermal application -- Rabbits.
Unpublished report from Hazleton Laboratories, Inc., Falls
Church, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Putman, D.L. & Schechtman, L.M. (1981) Activity of T1688 in the
dominant lethal assay in rodents. Unpublished report from
Microbial Associates, Bethesda, Maryland, USA. Submitted to WHO
by Rhône-Poulenc.
Rao (1999) Personal communication. Rhône-Poulenc, Research Triangle
Park, North Carolina, USA. Submitted to WHO by Rhône-Poulenc.
Roberts, N.L., Gopinth, C., Anderson, A. & Dawe, I.S. (1986) Acute
delayed neurotoxicity study with ethoprophos in the domestic hen.
Unpublished report No. RNP 244/86189 from Huntingdon Research
Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989a) Range-finding teratology study in rats with
ethoprop. Unpublished report No. 3147.38 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989b) Teratology study in rats with ethoprop.
Unpublished report No. 3147.39 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989c) Range-finding teratology study in rabbits with
ethoprop. Unpublished report No. 3147.40 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989d) Teratology study in rabbits with ethoprop.
Unpublished report No. 3147.41 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rucci, G.. (1979) Ethoprop technical grade; MCTR-60-78. Approximate
acute dermal toxicity (LD50) in young Yorkshire white pigs.
Unpublished report Lab. No. 6074from Food and Drug Research
Laboratories, Inc., Waverly, New York, USA. Submitted to WHO
Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1985) In vitro
chromosome aberration analysis in Chinese hamster ovary (CHO)
cells. Unpublished report No. PH 320-RP-00185 from Pharmakon
Research International Inc., Waverly, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1986) In vitro
sister chromatid exchange in Chinese hamster ovary (CHO) cells.
Unpublished report No. PH 319-RP001-85 from Pharmakon Research
International Inc., Waverly, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Schwartz, C.S. (1978) Acute oral LD50 determination of ethoprop in
female New Zealand white rabbits. Letter report to Mobil Oil
Company on FDRL study No. 5988. Submitted to WHO by
Rhône-Poulenc.
Skinner, M.J., Irwin, S.E., Schreiner, C.A., Mackerer, C.R. & Mehlman,
M.A. (1981) Metaphase analysis of rat bone marrow cells treated
in vivo with ethoprop, technical. Unpublished report No. 2476-80
from Mobil Environmental and Health Science Laboratory,
Plainsboro, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Spicer, E.J.F. (1985) Lifetime dietary toxicity and oncogenicity study
in rats. Unpublished report No. 347-029 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Study reformatted 18 May 1987. Submitted to WHO by Rhône-Poulenc.
Stankowski, L.F., Naismith, R.W. & Matthews, R.J. (1985) CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report
No. PH 314-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Stoughton, R.B. (1986) Penetration of the skin of humans, mice, rats
and rabbits by C14 ethoprop(R) emulsified concentrate and the
emulsified concentrate diluted with distilled water. Unpublished
report from the University of California, San Diego, California,
USA. Submitted to WHO by Rhône-Poulenc.
Thomson, M.A., Blackburn, G.R. & Mackerer, C.R. (1981) A murine
lymphoma mutagenesis assay, heterozygous at the thymidine kinase
locus for the determination of the potential mutagenicity of
ethoprop. Unpublished report No. 2474-80 from Mobil Environmental
& Health Science Laboratory, Plainsboro, New Jersey, USA.
Submitted to WHO by RhônePoulenc.
Weiler, M.S. (1994a) Acute oral gavage study with ethoprop in rats:
Time-related effects of ethoprop on brain, plasma and red blood
cell cholinesterase activities. Unpublished report No. HWI
6224-209 from Hazleton Wisconsin, Inc. Madison, Wisconsin USA.
Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994b) Acute neurotoxicity study with ethoprop in rats.
Unpublished report No. HWI 6224-200 from Hazleton Wisconsin, Inc.
Madison, Wisconsin, USA. Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994c) 13-week dietary neurotoxicity study with ethoprop
in rats. Unpublished report No. HWI 6224-199 from Hazleton
Wisconsin, Inc. Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Weiler, M.S. (1998) Acute oral toxicity study with ethoprop and three
ethoprop metabolites
(O-ethyl-S-methyl-S-propylphosphorodithioate,
O-ethyl-O-methyl-Spropylphosphorothioate, and
O-ethyl-S-propylphosphorothioate) in rats with determination of
effects on cholinesterase activity. Unpublished report No.
6224-246 from Covance Laboratories Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Weir, R.J. (1965) Acute eye application -- Rabbits. V-C 9-104
(technical grade). Unpublished report from Hazleton Laboratories
Inc. Falls Church, Virginia, USA. Submitted to WHO by
Rhône-Poulenc.
Weir, R.J. (1967a) Three-month dietary administration -- Rats.
Technical VC9-104. (Mocap). Unpublished report No. 230-102 from
Hazleton Laboratories Inc, Falls Church, Virginia, USA. Submitted
to WHO by Rhône-Poulenc.
Weir, R.J. (1967b) 13-week dietary administration -- Dogs. Technical
VC9-104. Unpublished report No. 230-110 from Hazleton
Laboratories, Inc, Falls Church, Virginia, USA. Submitted to WHO
by Rhône-Poulenc.
Weir, R.J. & Murphy, J.C. (1967) Neurotoxicity test -- Hens. Technical
VC 9-104. Unpublished report from Hazleton Laboratories Inc.,
Falls Church, Virginia, U.S.A. Submitted to WHO by Rhône-Poulenc.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, K.D. (1992) 104 week combined chronic toxicity and
carcinogenicity study with ethoprop in rats. Unpublished report.
Laboratory project ID: HLA 6224-151 from Hazelton Laboratories
America, Inc., Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Wolfe, G.W., Durloo, R.S. & Phipps, R.B. (1981) Rabbit teratology
study. Ethoprop technical -- 01238101. Unpublished report from
Hazleton Laboratories America, Inc. Vienne, Virginia, USA.
Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Supplementary submission, dated 29 July
1994. Submitted to WHO by Rhône-Poulenc.
Yenne, S.P. (1990) 14C-Ethoprop: Absorption, distribution,
metabolism, and excretion in the rat. Unpublished report HUK
project no. 68/102 from Hazleton UK, North Yorkshire, United
Kingdom. Submitted to WHO by Rhône-Poulenc.
 Table 2. Penetration of undiluted or 1:19 diluted 14C-ethoprophos solutions through
the skin of various species (in % of applied dose)
Time Undiluted ethoprophos Diluted ethoprophos
Human Rabbit Mouse Rat Human Rabbit Mouse Rat
15 min 0 0.003 0.003 0.01 0.001 0.004 0.004 0.05
30 min 0.0004 0.008 0.01 0.05 0.01 0.02 0.12 0.15
1 h 0.0008 0.04 0.11 0.15 0.08 0.2 1.9 0.55
2 h 0.002 0.2 0.91 0.4 0.31 1.1 4.2 1.7
4 h 0.004 0.7 2.6 1.0 0.72 3.7 11 4.2
6 h 0.08 1.5 5.0 1.7 1.1 7.7 29 7.8
24 h 1.0 7.8 16 5.4 5.2 27 38 23
also formed when the 14C-ethyl- and the 14C-propyl-labelled
compound, respectively, was the substrate (Iqbal & Menzer, 1972).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ethoprophos in animals is summarized in
Table 3. In 1983, the Meeting reported that "in the studies evaluated
the toxic symptoms in the treated species were characteristic of
anticholinesterase poisoning and usually persisted for up to 48 h in
mice and 72 h in rats surviving treatment. In general, deaths occurred
within 4 h (mice) or 1 h to 3 days (rats) post-dosing. Information on
duration of symptoms and time of death in rabbits was not available."
Ethoprophos is considered to be toxic after single oral and
dermal doses and very toxic after inhalation. The dermal and oral
LD50 values for rabbits and mice are of the same order of magnitude
and indicate efficient absorption of the compound after dermal
exposure in these species, whereas less is absorbed dermally in rats.
A single application of 0.1 ml of undiluted technical-grade
ethoprophos (equivalent to 44 mg/kg bw) into one eye of each of three
New Zealand white rabbits immediately produced moderate erythema and
vascularization of the sclera and nictating membrane. The substance
was severely toxic, since all three animals died within 1 h of
treatment (Weir, 1965).
In a study of primary dermal irritation, all six male New Zealand
white rabbits that received 0.5 ml of undiluted technical-grade
ethoprophos (purity, 93%) at a dose equivalent to 240 mg/kg bw on
clipped, abraded and unabraded skin and kept under an occlusive patch
died within the first 8 h of treatment (Becker & Parke, 1977).
Table 3. Acute toxicity of ethoprophos
Species Strain Sex Purity Vehicle Route LD50/LC50 Reference
(%) (mg/kg bw or
mg/L)
Mousea OF1 (SPF) M & F NR Water Oral 31 Pasquet & Mazuret (1982)
Rat SD M NR Corn oil Oral 62b Powers (1965)
F 33b
Ratc,d Crl:CD (SD) F NR Corn oil Oral 56 Weiler (1998)
BR VAF/ Plus
Rabbite NZW F NR NR Oral 33 Schwartz (1978)
Mouse CD-1 M NR Acetone Dermal (24 h) 18b Auletta & Rinehart (1979)
Rata CD (COBS) M & F NR Water Dermal (24 h) 226 Pasquet & Mazuret
(1982)
Ratd,f SD M NR None Dermal (24 h) 1280 Karcher et al. (1987);
F 424 Blacker (1987)
Rabbitg NZW M & F NR None Dermal (24 h) 8.5 Karcher et al. (1986);
Blacker (1987)
Pigh Yorkshire white M 94% None Dermal 327 Rucci (1979)
Rabbith Albino M & F NR None or 40% Dermal (intact 26b Powers (1965)
solution in and abraded
corn oil skin; 24 h)
Rat Wistar M & F 92% None Inhalation(4 h) 0.250j Kopp et al. (1980)
Table 3 (continued)
NR, not reported
a Ethoprophos lot No. DA 232. Clinical signs: trembling, convulsions, hypomotility, and dyspnoea in both mice and rats; no dermal
irritation in rats. Only 3 male and female mice and 2 male and female rats per dose.
b Doses reported as µl/kg bw. They were converted to mg/kg bw by multiplying them by the specific gravity of ethoprophos at
20°C, 1.094.
c Ethoprophos batch No. 51EAR122. Deaths occurred within 4 days. Clinical signs: thinness, staggered gait, salivation,
hypoactivity, hunched posture, and tremors.
d Statements of compliance with good laboratory practice and quality assurance included.
e Clinical signs: ataxia, diarrhoea, and decreased activity. Only 4 females per group. Information on duration of symptoms and
time of death not available.
f Technical-grade ethoprophos lot No. 302106001. Deaths occurred within 3 days. Clinical signs: urine staining, tremors, excess
salivation, decreased activity, ataxia, soft stools, diarrhoea, laboured breathing, lachrymation, exophthalmus, and prostration,
in general increased with increasing dose. No signs of irritation. Considerable body-weight decreases in animals that died.
g Ethoprophos lot No. 302106001. Deaths occurred within 4 days except for one on day 9. Clinical signs: prostration, laboured
breathing, salivation, loss of locomotor ability, tremor, ataxia, soft stools, diarrhoea, and lachrymation. No signs of
irritation. Considerable body-weight decreases in animals that died. Only statement of quality assurance included.
h Deaths occurred within 4 days. Clinical signs: laboured breathing, salivation, wobbly gait, and decreased activity. Erythema
was reported but no indication of severity was given. Only 2-4 males per group.
i Clinical signs: depression, laboured breathing, unsteadiness, tremors, salivation. Deaths occurred within 24 h. Very slight,
subsiding erythema observed in surviving animals 24-48 h after dosing on abraded (> 10 µl/kg bw) and unabraded skin
(31.6 µg/kg bw). Only 4 animals per group.
j 4-h LC50 expressed as actual chamber concentration and particle size < 10 µm; animals were exposed to the liquid
technical-grade material as an aerosol (nominal concentration, 1500 mg/m3) in a nose-only exposure chamber. Clinical signs:
apathy, dyspnoea, salivation (from JMPR, 1983, and slightly modified by reference to original report).
(b) Short-term studies of toxicity
(i) Oral administration
Rats
Groups of 25 male and 25 female Charles River caesarean-derived
rats were fed diets containing technical-grade ethoprophos (purity not
stated) in acetone at concentrations of 0, 0.3, 1, or 100 ppm
(equivalent to 0, 0.015, 0.05, and 5 mg/kg bw per day) for three
months. The control group was given basal diet containing 'a volume of
acetone equivalent to the volume used for the test diets'. No deaths
or treatment-induced toxic signs were observed. Growth was depressed
by < 10% in males at doses > 1 ppm and in females at 100 ppm
during the last 6 weeks of the study. Food consumption was unaffected.
Haematological, clinical chemical (only blood urea nitrogen, aspartate
aminotransferase, and glucose determined), and urinary examinations in
five males and five females per group after 1 and 3 months indicated
no significant treatment-related findings. Cholinesterase activity in
plasma, erythrocytes, and brain was measured in five rats of each sex
per group at days 4, 8, 16, and 33 and terminally in the remaining
rats. Marked inhibition (25-100%) of the enzyme activity was found at
100 ppm at all sampling intervals, the depression being greatest for
erythrocyte and least for brain acetylcholinesterase activity. Maximum
inhibition of the enzyme occurred on day 8 or 16, in all tissues. Male
rats at 0.3 or 1 ppm showed a 24-28% reduction in erythrocyte and
plasma cholinesterase activity on day 8 and of brain cholinesterase
activity at termination. In females, brain cholinesterase activity was
inhibited by 20-25% at 0.3 and 1 ppm on day 8 and at 1 ppm on day 16,
and plasma and erythrocyte cholinesterase activity was inhibited by
23-26% at 1 ppm on day 16. At the end of the study, the absolute and
relative weights of the adrenals were decreased, but not in relation
to dose, in females in all treated groups. Histopathological
evaluation of about 20 selected tissues, including the adrenals, from
five males and five females in the control and highest-dose groups
showed no significant changes attributable to treatment. No NOAEL
could be identified, as brain acetylcholinesterase activity was
inhibited at all doses (Weir, 1967a). As only five animals of each sex
were examined for haematological, biochemical, and urinary parameters,
instead of the 20 rats of each sex required by the OECD guideline, and
few parameters were tested, the study was considered unreliable and
was not used for further evaluation of the toxicology of ethoprophos.
Dogs
In a study conducted according to GLP and with a statement of QA,
ethoprophos (purity, 95.6%) was administered orally to groups of six
male and six female beagle dogs aged 6-7 months at doses of 0, 0.01,
0.025, or 1 mg/kg bw per day in corn oil in gelatin capsules for 20
weeks.Two dogs of each sex at each dose were then allowed a 4-week
recovery. The animals were observed for deaths and clinical signs.
Body weights and food consumption were recorded, and ophthalmological
examinations were performed before dosing and at sacrifice after the
exposure (week 20) and recovery (week 24) periods. Haematological,
biochemical, and urinary analyses were carried out before treatment
and during weeks 17, 20, and 24. Cholinesterase activity in plasma and
erythrocytes was measured before dosing and about 3 h after dosing
during weeks 2, 4, 8, 12, 20, and 24. Brain cholinesterase activity
was measured at sacrifice; at that time, the weights of about 10
organs were determined and about 40 tissues and all gross lesions from
all animals were examined macroscopically and microscopically.
There were no deaths, and there were no treatment-related
clinical signs or effects on body weight, body-weight gain, food
consumption, ophthalmologic, haematological, biochemical, or urinary
end-points, macroscopic or microscopic findings, or organ weights. No
histopathological changes were found in the heart. The only finding
was a statistically significant increase in platelet count in bitches
at the high dose at weeks 17 and 20. This was considered to be of no
toxicological relevance since the value was increased before
treatment, and an elevated value was observed after recovery.
Significant inhibition of plasma cholinesterase activity was observed
in bitches at the intermediate dose and males and females at the high
dose. In animals at the high dose, erythrocyte acetylcholinesterase
activity was inhibited by more than 20% in males at all times during
exposure and in females only at weeks 8 and 12, when the values
reached statistical significance in males. Brain acetylcholinesterase
activity was not inhibited. After the recovery period, plasma
cholinesterase activity was found to have returned to normal, but the
activity in erythrocytes showed some variation and it could not be
concluded that recovery had occurred. The NOAEL was 1 mg/kg bw per
day, the highest dose tested, in the absence of inhibition of brain
acetylcholinesterase activity (Hamada, 1990).
Groups of three male and three female young adult pure-bred
beagles weighing 6-12 kg were fed diets containing technical-grade
ethoprophos (purity unknown) at concentrations of 0, 1, 3, or 100 ppm
for 13 weeks, equal to 0, 0.034, 0.098, or 3.4 mg/kg bw per day for
males and 0, 0.035, 0.11, or 4.0 mg/kg bw per day for females. There
were no deaths. Emesis occurred once in two animals at 100 ppm. Body
weights, food consumption, haematological, biochemical (glucose, blood
urea nitrogen, and aspartate aminotransferase and alkaline phosphatase
activity), and urinary parameters measured at 1 and 3 months were not
significantly affected by treatment. At termination, however, males
and females at the high dose had slightly decreased erythrocyte counts
and erythrocyte volume fractions. Plasma cholinesterase activity was
measured before dosing and on days 2, 4, 8, 16, 35, 65, and 87 of
administration. The activity was inhibited by 27-88% at doses > 3
ppm in animals of each sex at virtually all of the sampling intervals
and by 20-27% at 1 ppm in males at the two last sampling times. A
> 20% depression of erythrocyte cholinesterase activity was seen in
animals at the highest dose at all but the first two or three sampling
intervals and in females at 3 ppm on one occasion (day 65) only.
Cholinesterase activity in brain was not measured. At termination, no
compound-related effects on organ weights or gross pathological
changes were noted. The only significant finding on histopathological
evaluation of about 20 tissues from each animal in the control and
high-dose groups was foci of perivascular myocardial swelling with
loss of striations in one of three female controls and two of three
males and one of three females at 100 ppm; all of these animals also
showed swelling and vacuolation of Purkinje fibres. These changes were
considered by the pathologist to represent 'an artefact induced in the
processing of the tissues'. The NOAEL was 3 ppm, equal to 0.098 mg/kg
bw per day, on the basis of depression of erythrocyte cholinesterase
activity at 100 ppm (Weir, 1967b).
Groups of four male and four female pure-bred beagle dogs aged
4-6 months were given capsules containing ethoprophos (purity, 96%)
dissolved in peanut oil at doses of 0, 0.025, 1.0, or 10 mg/kg bw per
day orally for 52 weeks. The doses were based on the results of a
4-week range-finding study. The animals were offered 400 g/day of food
and water ad libitum and were observed daily for signs of toxicity;
body weights were recorded weekly and food consumption daily. Blood
and urine were collected before treatment and after 6, 13, 26, and
52 weeks of treatment. The standard haematological, serum chemical,
and urinary examinations were performed, in addition to measurements
of plasma and erythrocyte cholinesterase activity. At the end of the
study, the animals were killed by an overdose of thiopentone sodium
followed by exsanguination. All tissues were examined grossly
in situ, and the major organs were removed and weighed, and brain
cholinesterase activity was measured. The standard set of tissues was
collected for histopathological examination. A statement of QA was
provided.
No effect of treatment on the incidence of clinical signs was
observed. Treatment did not affect the weight gain of treated males,
but a dose-related trend to decreased body weight was seen in treated
bitches throughout the study, and, at termination, bitches at the high
dose weighed about 10% less than controls. Males and females at the
high dose tended to consume about 10% less food than controls.
Erythrocyte count, haemoglobin concentration, and erythrocyte volume
fraction were statistically significantly lower in males at the high
dose than in control males at all intervals. The only change in serum
chemistry was an increase in mean aspartate aminotransferase activity
associated with decreased total cholesterol and serum albumin in males
at the high dose from 6 weeks of treatment throughout the study. This
effect was due largely to an apparent hepatotoxic response in two of
four males at this dose; in one of these dogs, aspartate
aminotransferase activity was increased by 10-fold over that of
controls at study termination, and serum alkaline phosphatase,
gamma-glutamyl transferase, and alanine aminotrans-ferase activities
were also greatly increased. The serum albumin concentration was
decreased in all males at the high dose. Plasma and erythrocyte
cholinesterase activities were depressed in a dose-related manner in
dogs at the intermediate and high doses at all intervals. At the end
of the study, plasma cholinesterase activity was decreased to 33% and
17% of the control value in males and to 58%, 19%, and 17% in females
at the low, intermediate, and high doses, respectively. The
erythrocyte cholinesterase activity in males at the end of the study
was 127%, 88%, and 39% of the control value at the low, intermediate,
and high doses, respectively, whereas the values for females were
103%, 62%, and 38% of control, respectively. Brain cholinesterase
activity was inhibited only in males at the high dose (to 56% of the
control value; significant) and females (72% of control) at the high
dose. Animals at the intermediate dose showed inhibition to 91% and
94% of the control values, respectively, which was considered not to
be toxicologically relevant. Ophthalmoscopic examination conducted
before and at the end of the study revealed no treatment-related
effects. At necropsy, no effect of treatment on absolute or relative
organ weights was seen, nor was there any effect on the incidence of
gross findings. Treatment-related histopathological changes were
restricted to the liver. Selected findings are tabulated in Table 4.
The NOAEL was 0.025 mg/kg bw per day on the basis of findings in the
liver at the low dose (Brown, 1986).
Table 4. Pathological findings in the liver of dogs treated with ethoprophos for 52 weeks
Lesion Dose (mg/kg bw per day)
Males Females
Control 0.02 1 10 Control 0.025 1 10
Vacuolation 0 0 3 4 0 0 3 4
Focal necrosis 0 0 0 2 1 0 0 1
Pigment 0 0 1 4 0 0 0 3
Kupffer-cell pigment 0 0 1 4 0 0 1 4
Fibrosis 0 0 0 4 0 0 0 4
Biliary proliferation 0 0 0 4 0 0 0 4
(ii) Dermal administration
Rats
In a range-finding study for dermal toxicity conducted according
to GLP and with a QA statement, groups of three Crl:CD(R)BR rats of
each sex received dermal applications of ethoprophos (purity, 95.6%)
in mineral oil at doses of 0, 0.3, 1, 10, 30, or 100 mg/kg bw per day
for 6 h per day on 5 days per week for 3 weeks. The animals were
observed for clinical signs and dermal irritation. Body weight and
food consumption were recorded, and cholinesterase activity was
measured in plasma and erythrocytes before dosing, at week 2, and at
the end of study (week 4). At necropsy, brain acetylcholinesterase
activity was measured. Macroscopic examinations were performed and
organ weights recorded.
Deaths occurred in the groups given 30 (2/6 rats on days 7 and 8)
and 100 mg/kg bw per day (6/6 rats, days 2-7). The clinical signs in
these animals were characteristic of cholinesterase poisoning. Small
faeces, hunched posture, and low body temperature were also observed
at 10 mg/kg bw per day, and the latter finding was also observed in
one male and one female at 0.3 mg/kg bw per day and two males and one
female at 1 mg/kg bw per day. Food consumption was lower (reaching
statistical significance in females) at 10 mg/kg bw per day in females
and 30 mg/kg bw per day in males and females during the first week of
exposure. There were no signs of dermal irritation. Statistically
significant inhibition of plasma cholinesterase activity was observed
at weeks 2 and 4 in females at 0.3 and 1 mg/kg bw per day and in
animals of each sex at 10 and 30 mg/kg bw per day. Inhibition of
erythrocyte acetylcholinesterase activity by > 20% was observed in
females at all doses at week 2 and at 10 and 30 mg/kg bw per day at
week 4 and in males at 10 and 30 mg/kg bw per day at both times. Brain
acetylcholinesterase activity was inhibited by > 50% in animals of
each sex at 10 and 30 mg/kg bw per day; the effect was dose-related
and significant at the high dose. No treatment-related changes were
observed in organ weights or in gross appearance (Henwood, 1990a).
In the main study, conducted according to GLP and with a QA
statement, groups of 10 Crl:CD(R)BR rats of each sex aged about 2.5
months received 1.4 ml/kg bw of ethoprophos (purity, 95.6%) dermally
in mineral oil at doses of 0, 0.3, 1, or 10 mg/kg bw per day for 6
h/day on 5 days per week for 3 weeks. The area of exposure on the
dorsal trunk constituted about 10% of the total body surface. The
animals were observed for clinical signs and dermal irritation. Body
weights and food consumption were recorded, and cholinesterase
activity was measured in plasma and erthrocytes before treatment, in
week 2, and at the end of study (week 4). Haematological and clinical
chemical parameters were evaluated on the day of necropsy, when the
brain was collected for analysis of acetylcholinesterase activity.
Macroscopic examinations were performed, and the weights of the brain,
kidneys, and liver were recorded. Kidneys, liver, skin, and tissues
with lesions from all rats in the control and high-dose groups were
examined microscopically.
The deaths of one control female and one at the high dose were
considered to be unrelated to treatment. Clinical signs observed in
some females at the high dose included soft and/or small faeces (three
animals) and hunched posture (one animal). During the first week of
exposure, a slightly lower (not statistically significant) body-weight
gain was observed in animals at the high dose, but food consumption
was not affected. There were no signs of dermal irritation that could
be related to treatment. In all groups, including controls, slight
erythema and desquamation associated with pustules or papules were
observed. Moderate desquamation was observed in one male and one
female at 0.3 mg/kg bw per day and in one male at 1 mg/kg bw per day.
Dose-related, statistically significant inhibition of plasma
cholinesterase activity was observed in weeks 2 and 4 in animals given
1 or 10 mg/kg bw per day. Erythrocyte acetylcholinesterase activity
was inhibited by > 20% in rats at 1 mg/kg bw per day in week 4 and at
10 mg/kg bw per day at both times, with 30% inhibition in week 2 and
37% (males) and 54% (females) in week 4. Brain acetylcholinesterase
activity was statistically significantly inhibited by 50% in males and
70% in females at 10 mg/kg bw per day. At 1 mg/kg bw per day, the
inhibition was 12% in males and 16% in females (not significant). At
0.3 mg/kg bw per day, brain acetylcholinesterase activity was not
inhibited in males and was nonsignificantly inhibited by 11% in
females. In female controls and in those at 0.3 mg/kg bw per day, but
not those at 1 mg/kg bw per day, the standard deviation of the mean
acetylcholinesterase activity was relatively high. As a result, the
inhibition in brain at 0.3 mg/kg bw per day is considered not
toxicologically relevant, whereas that at 1 mg/kg bw per day is
relevant. Other clinical findings in females were a dose-related
decrease in platelet count and a decrease unrelated to dose in the
numbers of neutrophils and lymphocytes in animals at 1 and 10 mg/kg bw
per day. These findings were considered to be due to the excessive
formation of fibrin strands in the blood of these females. The
statistically significant findings of increases in aspartate
aminotransferase activity in males at 1 mg/kg bw per day and slight
decreases in urea nitrogen in males at 0.3 and 10 mg/kg bw per day
were considered not to be related to treatment. Increased plasma
concentrations of sodium and chloride were observed in females at 0.3
mg/kg bw per day and in animals of each sex at 1 and 10 mg/kg bw per
day, but these changes were small and not related to dose. An
increased calcium concentration was seen in males at 1 mg/kg bw per
day and in males and females at 10 mg/kg bw per day. No
compound-related changes were observed in terminal body weights or
organ weights. At macroscopic examination, two of 10 males at the high
dose were found to have crusted areas on untreated skin in the
cervical and/or thoracic region, confirmed microscopically to be
multifocal erosion or ulceration of the epidermis and chronic
inflammation. Three of nine females at the high dose showed dark areas
in the glandular stomach, which was confirmed microscopically in two
rats as oedema or focal erosion or ulceration of the glandular mucosa;
and one of these females and another at the high dose showed
dilatation of the pelvis. The NOAEL was 0.3 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity in females
at 1 mg/kg bw per day. Since no sign of treatment-related dermal
irritation was seen, the NOAEL for this end-point was 10 mg/kg bw per
day, the highest dose tested (Henwood, 1990b).
Rabbits
In a study conducted according to GLP and with a QA statement,
groups of 10 Hra:(NZW)SPF rabbits of each sex, aged about 3 months,
received dermal applications of 1.4 ml/kg bw of ethoprophos (purity,
95.6%) in 4% carboxymethylcellulose at doses of 0, 0.03, 0.1, or 1
mg/kg bw per day for 6 h/day on 5 days per week for 3 weeks. The area
of exposure on the dorsal trunk constituted about 10% of the total
body surface. The animals were observed for clinical signs and dermal
irritation. Body weight and food consumption were recorded and
cholinesterase activity was measured in plasma and erythrocytes before
treatment and at the end of study (week 4). Haematological and
clinical chemical parameters were evaluated on the day of necropsy,
when the brain was collected for measurement of acetylcholinesterase
activity. Macroscopic examinations were performed, and the weights of
the brain, kidneys, and liver were recorded. Kidneys, liver, skin, and
tissues with lesions from all rabbits in the control and high-dose
groups were examined microscopically.
Deaths occurred in one male and three females at 0.03 mg/kg bw
per day and two males at 1 mg/kg bw per day. These animals and a few
others in each group except female controls showed clinical signs that
were associated with an increased incidence of mucoid enteritis
(diarrhoea, mucoid diarrhoea, and reduced food consumption), but this
finding was considered to be unrelated to treatment. Females at the
high dose had significantly lower body weights in weeks 2 and 4. The
groups in which the deaths occurred had lower food consumption, but
this was considered to be of no toxicological importance. An increased
incidence of slight-to-moderate irritation (erythema and sometimes
desquamation) was seen in all treated groups when compared with
controls, and the frequency increased with dose. The only
treatment-related change in haematological or clinical chemical
parameters was statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain at the high dose. The
inhibition in males was 37% in plasma, 42% in erythrocytes, and 49% in
brain, and that in females was 35% in plasma, 42% in erythrocytes, and
49% in brain. No inhibition of brain acetylcholinesterase activity was
found at lower doses; erythrocyte acetylcholinesterase activity was
inhibited by 12% in females at the intermediate dose, but this is
considered to be of no toxicological relevance. The terminal body
weights were significantly lower for females given 0.03 or 1 mg/kg bw
per day, which might be due to their lower food consumption. The
absolute kidney weights were significantly lower in females and
slightly lower in males at the high dose, and the relative kidney
weights were slightly (and not significantly) lower in females at this
dose. There were no treatment-related macroscopic or microscopic
changes. The NOAEL for dermal toxicity was 0.1 mg/kg bw per day on the
basis of inhibition of cholinesterase activity in brain and
erythrocytes and decreased kidney weights at 1 mg/kg bw per day. No
NOAEL could be identified for dermal irritation, as slight irritation
of the skin was observed at all doses (Henwood, 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Randomly assigned groups of 80 male and 80 female B6C3F1 (SPF)
mice were fed diets containing 0, 0.2, 2, or 30 ppm of technical-grade
ethoprophos (purity, 94.6%) for 2 years. The test material and diets
were analysed periodically to ensure that the concentrations were
within acceptable limits of the nominal values. The diets and water
were provided ad libitum. Animals were examined daily for signs of
toxicity, and moribund animals were killed. Body weights were recorded
weekly for the first 26 weeks of treatment and twice weekly
thereafter. Food consumption was determined weekly. Haematological,
clinical chemical, and urinary parameters and cholinesterase activity
were measured after 26, 52, 78, and 104 weeks of treatment. Ten mice
of each sex at each dose were killed after 26, 52, and 78 weeks of
treatment, and all surviving animals were killed after 104 weeks of
treatment. Complete post-mortem examinations were conducted on all
animals killed at scheduled sacrifice and on those that died or were
killed in a moribund condition during the study.
No effect of treatment was seen on survival or the incidence of
clinical signs. Mean body-weight gain was decreased by 5-10% in males
and females at the high dose during the first 80 weeks of treatment,
but by the end of the study there was no decrease in males and only a
slight decrease (6%) in females. Treatment had no effect on food
consumption, the mean intake levels over the course of the study being
0, 0.026, 0.25, and 4.0 mg/kg bw per day for males and 0, 0.032, 0.32,
and 4.9 mg/kg bw per day for females. The only change in
haematological parameters was a decrease in the mean total leukocyte
count in all treated males: at the end of the study, the mean total
counts were: 4.1 ± 1.7, 2.3 ± 0.9, 2.1 ± 1.1, and 1.6 ± 0.5 ×
103/mm3 for controls and males at the low, intermediate, and high
doses, respectively, which were statistically significantly different
from the controls in all groups. The significance of this finding is
unclear as it was not associated with any other toxic effect that
might result from an impaired immune function. Historical control data
would have been useful for evaluatng this finding, but they were not
submitted.
Plasma and erythrocyte cholinesterase activity was inhibited in a
dose-related manner in animals at the intermediate and high doses
during the first 78 weeks of treatment, and in most cases statistical
significance was reached. The cholinesterase activity remaining in
plasma represented 65-90% of control values at the intermediate dose
and 23-34% at the high dose, whereas in erythrocytes it was 83-89% of
control values at the intermediate dose and 19-26% at the high dose.
By the end of the study, the plasma and erythrocyte cholinesterase
activities in the group at the intermediate dose were similar to those
of controls, except that the activity in plasma of females at the
intermediate dose was significantly decreased by about 20%. Brain
cholinesterase activity was inhibited only in males and females at the
high dose, with statistical significance reached in week 26 (64% of
control value for males and 71% for females), week 52 (82% of control
value in males), and week 104 (81% of control value in males and 83%
in females).
Necropsy of animals that died on test or were killed in a
moribund condition did not reveal any treatment-related lesions or an
effect on absolute or relative organ weights. No treatment-related
lesions were found at gross and microscopic examinations at interim
sacrifices. At final sacrifice, an increased incidence of
calcification of the kidney was noted in males at the high dose
(13/42, 2/45 controls) with 'basophilic changes' in the kidneys of
males only (0/45 control, 9/31 at the low dose, 13/38 at the
intermediate dose, and 24/42 at the high dose). The historical control
data that were supplied showed a high spontaneous incidence of these
lesions, with incidences of calcium deposits in males of 2.4-72% and
basophilic changes in males of 0-87%. The study authors reported that
these lesions are of spontaneous origin and do not represent
treatment-related pathological lesions. Treatment had no effect on the
incidences of tumours in specific tissues or on the total tumour
burden.
The NOAEL for toxicity was 2 ppm, equal to 0.25 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinesterase activity at
30 ppm (Yamagata et al., 1984a,b).
Rats
Groups of 10 male and 20 female Fischer 344 rats were fed diets
containing technical-grade ethoprophos (purity, 95.3%) at
concentrations of 0, 60.5, 131, or 262 ppm for 8 weeks before mating
(one male to two females). (The section on methods indicates that the
F0 generation comprised 10 males and 20 females per group, but later
in the report it is stated that approximately 16 males and 32 females
at each dose were mated. The complete absence of data on the
reproduction phase of the study precluded verification of the
information. The report stated that analytical data for dietary
analyses were not available.) Ten days after the detection of a
positive vaginal smear, the females were separated from the males and
were maintained on the test diets until weaning of their pups. This
part of the study constituted the reproductive phase. Weanlings from
the reproductive phase (60 males and females per group) were selected
randomly and fed diets containing ethoprophos at 0, 4.5, 9, or 18 ppm
during weeks 0-12 and at 0, 49, 98, or 196 ppm during weeks 13-109 in
a study of carcinogenicity. The dietary levels during weeks 13-109
were equivalent to doses of 0, 2.5, 4.9, and 9.8 mg/kg bw per day.
(The mean intake over the whole exposure period was not reported.) In
this 109-week study, all animals that died or were killed in a
moribund condition, all survivors at the end of the study, and the 10
males and 10 females per group killed after 52 weeks of treatment were
subjected to gross necropsy and histopathological examination of a
wide range of tissues and all gross lesions.
The mortality rate was increased in males of the highest dose
during the first 7 months, although the rates at the end of the study
(53-63%) were comparable in all groups, including the controls. Other
than emaciation in animals at the high dose, there were no
treatment-related clinical signs. Growth was depressed at the highest
dose throughout the study and at the intermediate dose during most of
the study. Food consumption was reduced in animals at the intermediate
and high doses during the first 52 weeks. Weekly water consumption,
monitored between weeks 102 and 109, was unaffected. Haematological,
blood chemical (glucose, blood urea nitrogen, gamma-glutamyl
transferase, alanine and aspartate aminotransferase and alkaline
phosphatase activities, total protein, and cholesterol concentrations)
and urinary (pH, glucose, ketones, and protein) parameters measured at
the end of weeks 52 and 109 revealed decreased erythrocyte count,
erythrocyte volume fraction, and haemoglobin values in males at the
two higher doses and in females of the highest dose after 52 weeks.
Assay of cholinesterase in plasma, erythrocytes, and brain, conducted
at termination only, indicated a dose-related, statistically
significant 81-93% depression of plasma cholinesterase activity and a
30-68% decrease in brain cholinesterase activity in all treated
groups. Erythrocyte cholinesterase activity was not inhibited. The
weights of the spleen, liver, kidney, and testis deviated from those
of controls at the 52-week and terminal sacrifices, but essentially
only at the highest dose, with no accompanying microscopic lesions.
The gross pathologic changes seen were not significantly different
from those in controls, but histopathological examination of males at
the highest dose showed an increased incidence of 'scleral
mineralization' of the eye. No other microscopic lesions that might be
attributable to treatment were found.
The only notable oncogenic finding was a statistically
significant increase in the incidence of thyroid C-cell adenoma in
males at the highest dose at terminal sacrifice, with incidences of
2/34 in controls, 3/31 at the low dose, 1/29 at the intermediate dose,
and 9/33 at the high dose, expressed as the ratio of the number of
survivors bearing the tumour to the number examined
histopathologically. C-cell adenomas were also observed at the interim
sacrifice in one male in the control group and one at the low and one
at the high dose. The background incidence of the tumour was not
reported. The incidences of animals with benign and/or malignant
tumours, malignant tumours, or multiple primary tumours were not
significantly different in control and treated groups.
Interstitial-cell adenoma of the testis in males and pituitary adenoma
in females were the most frequently observed spontaneous tumours,
occurring in nearly 90% of males and over 50% of females in the
concurrent control group (Barnett et al., 1983). The authors
considered that the increased incidence of C-cell adenomas in males at
the high dose was a direct treatment-related effect as there was no
trend with dose. No NOAEL could be identified since inhibition of
brain acetylcholinesterase activity was seen at all doses. The LOAEL
was 49 ppm, equivalent to 2.5 mg/kg bw per day.
Groups of 70 male and 70 female Fischer 344 rats were assigned
randomly to receive diets containing technical-grade ethoprophos
(purity, 95.9%) at concentrations of 0, 1, 10, or 100 ppm (equivalent
to 0, 0.05, 0.5, or 5 mg/kg bw per day; mean intake over entire
exposure not reported) for 105 consecutive weeks. The test material
and diets were analysed periodically to ensure the stability and
homogeneity of diets. Food and water were provided ad libitum. The
animals were examined daily for mortality and morbidity and underwent
detailed physical examinations every week. The body weights were
recorded weekly for the first 26 weeks and twice weekly thereafter
until the end of the study, as were measurements of food and water
consumption. Ophthalmoscopic examinations were performed on all rats
at the beginning of the study and after 6, 12, 18, and 24 months of
treatment, and routine haematological, serological, and urinary
studies were conducted at the same intervals after the beginning of
treatment. Plasma and erythrocyte cholinesterase activity was measured
at the same intervals as other parameters, and brain
acetylcholinesterase activity was measured at interim sacrifice of 10
rats of each sex per dose after 12 and 18 months and at final
sacrifice at the end of 24 months of treatment. All animals were
killed on schedule, and those that died on test or were killed in a
moribund condition were subjected to a complete post-mortem
examination. A statement of QA was provided.
Treatment had no effect on survival, body-weight gain, or food or
water consumption. The only potential treatment-related finding at
physical examination was an increased incidence of anogenital staining
in females at the high dose during weeks 14-78 of treatment.
Haematological examination revealed decreased erythrocyte counts,
haemoglobin, and erythrocyte volume fraction, with increased mean
corpuscular volume in males and females at the high dose at all
intervals except 18 months. This change was statistically significant
only after 6 and 12 months of treatment, but not at final sacrifice. A
slight (10-20% over controls) increase in blood urea nitrogen was also
seen in these animals, which was statistically significant only at 6
months in females and at 18 months in males. A statistically
significant decrease in serum globulin content was noted after 6 and
12 months of treatment but not at final sacrifice. No toxicologically
significant alterations were found in urinary or ophthalmic
end-points.
Plasma and erythrocyte cholinesterase activity was significantly
inhibited in a dose-related manner at most intervals in males and
females at the intermediate and high doses. Erythrocyte activity was
inhibited by 28-44% in animals at the high dose, by < 20% in males at
the intermediate dose, and by 19-27% in females at 6, 12, and 18
months and 4% at the end of the study. Brain cholinesterase activity
was significantly inhibited in animals at the high dose at all
measured intervals, with inhibition of 27-35% in males and 36-48% in
females.
At the 12-month sacrifice, a statistically significant increase
of about 10% was noted in the relative weight of the spleen of males
and females at the high dose, with decreases of a similar magnitude in
the absolute and relative weights of the kidney in males at the high
dose. No significant treatment-related changes were seen on gross or
microscopic examination. At the 18-month sacrifice, no significant
alterations in organs weights were found, nor were any significant
changes noted on gross or microscopic examination. At final sacrifice,
statistically significant, 16-20% increases in the absolute and
relative weights of the thyroid/parathyroid were seen in males at the
high dose, with non-significant, 14-16% increases in males at the
intermediate dose. Gross examination revealed an increased incidence
of enlarged thyroids in males at these doses: 5/35 and 5/39,
respectively, compared with 1/36 controls. The only potentially
treatment-related lesion found on microscopic examination was an
increased incidence of parafollicular C-cell neoplasms in males at the
high dose (Table 5). The authors concluded that these changes were
statistically significant and the incidence of thyroid neoplasia was
'random and unrelated to the test article'.
Table 5. Incidence of C-cell neoplasms in male rats fed diets containing
ethoprophos
Neoplasm Dose (ppm)
0 1 10 100
C-cell adenoma
Deaths and unscheduled sacrifices 2/13 0/7 0/13 1/9
Final sacrifice 6/36 5/39 5/35 11/39
Total 8/49 5/46 5/48 12/48
C-cell carcinoma
Deaths and unscheduled sacrifices 0/13 0/7 0/13 1/9
Final sacrifice 0/36 0/39 1/35 2/39
Total 0/49 0/46 1/48 3/48
All C-cell neoplasms 8/49 5/46 6/48 15/48
The NOAEL for toxicity was 10 ppm, equivalent to 0.5 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase
activity, effects on erythrocyte parameters, and effects on the
thyroid at 100 ppm (Spicer, 1985).
In a study conducted according to GLP and with a QA statement,
ethoprophos (purity, 95.6%) was mixed into the diet of groups of 70
randomly assigned Crl:CD(R)(SD)BR VAF/Plus(R) rats of each sex at
concentrations of 0, 1, 60, and 600 ppm for 105 weeks. During week 3
of the study, the highest dose was lowered to 400 ppm because of the
occurrence of tremors, ataxia, and death in females. The average doses
throughout treatment were 0, 0.04, 2.7, and 20 mg/kg bw per day for
males and 0, 0.06, 3.4, and 26 mg/kg bw per day for females in the
four groups, respectively. Ten additional animals of each sex per dose
were killed after 54 weeks, while another 10 animals of each sex were
allocated to the control and high-dose groups to study recovery after
dosing for 52 weeks followed by 4 weeks of control diet. The test
material and diets were analysed regularly to ensure that the
concentrations in the diet were within an acceptable range. Food and
water were provided ad libitum, and animals were monitored twice
daily for their clinical condition. Body weights and food consumption
were determined weekly up to week 13 and at least every other week
thereafter. The clinical pathological examinations comprised
haematology, clinical chemistry, including erythrocyte and plasma
cholinesterase activity, and urinalysis at weeks 12, 26, 52, and 78 on
10 animals per group and on all surviving animals at termination of
the study, when brain cholinesterase activity was determined in all
animals. All animals were necropsied, and 41 organs from animals in
the control and high-dose groups and all those that died or were
killed before the end of the study were examined histologically.
Additionally, all macroscopic lesions and lung, liver, and kidneys
from animals at the low and intermediate doses were studied. The
thyroids of all animals were examined, and ophthalmic examinations
were done on all animals at weeks 0, 52. and 104.
Animals at the high dose showed depressed food consumption (up to
week 70 in males and up to week 20 in females) and cumulative
body-weight gain, and in males food efficiency was depressed up to
week 20. The survival rate was essentially the same in all groups up
to week 78, but by week 104 the survival rate of animals at the high
dose was higher than that of other groups. The major causes of death
were renal disease and pituitary tumours. Females at the high dose had
significantly reduced erythrocyte count, haemoglobin, and erythrocyte
volume fraction after 12, 26, 52, and 78 weeks of treatment, although
the decreases were not statistically significant at 53 or 104 weeks.
In males at this dose, these parameters were significantly reduced
only at week 26; at weeks 12, 52, 53, and 78, nonsignificant decreases
were seen, and at week 104 there was no difference from controls.
These effects were reversible in the animals allowed to recover. Males
and females at the high dose had significant reductions in total
plasma protein and globulin at weeks 12 and 26 and males also at week
52 and 53. In females, the decrease was significant only for total
protein at week 52 and was nonsignificant for both total plasma and
globulin at week 53. At week 78, only a significant decrease for total
protein was observed, while in females both parameters showed a
nonsignificant decrease. At week 104, the decreases in total plasma
protein and globulin were no longer significant in animals of either
sex. The effects on total plasma protein and globulin were reversible
after 4 weeks of control diet. Other significant changes in
haematological and blood biochemical parameters were incidental and
considered not to be related to treatment. No changes in
haematological or general blood biochemical parameters were observed
in rats at the other doses. The results of urinary analysis were
summarized in the report and indicated that males at the high dose had
significantly decreased urine volume and urea nitrogen and creatinine
concentrations at the end of the study. These findings were attributed
to a lower incidence of chronic renal disease in these animals.
Plasma cholinesterase activity was significantly inhibited
throughout the experiment, by 48-64% in males at the intermediate
dose, 62-77% in males at the high dose, 61-77% in females at the
intermediate dose, and 75-82% in females at the high dose. Erythrocyte
cholinesterase activity was also significantly inhibited throughout
the study, by 34-44% in males at the intermediate dose, 35-51% in
males at the high dose, 36-48% (36% inhibition not significant) in
females at the intermediate dose, and 41-51% in females at the high
dose. At the low dose, inhibition of plasma and erythrocyte
cholinesterase activity was highly variable but never exceeded 18% in
plasma or 6% in erythrocytes in either males or females and was not
statistically significant. Inhibition of brain cholinesterase activity
was significant at weeks 52 and 104 and amounted to 35-33% of control
values in males at the intermediate dose, 53-64% in males at the high
dose, 28-32% in females at the intermediate dose, and 64-66% in
females at the high dose. At the low dose, inhibition of brain
cholinesterase activity was maximal in females at week 104 (8%), but
the differences (whether increased or decreased) were not
statistically significant in either males or females. Both plasma and
erythrocyte cholinesterase activity recovered to about 80% of the
control activity after 4 weeks of control diet from week 52, and brain
acetylcholinesterase activity was nearly fully restored.
Both males and females at the high dose tended to have lower
terminal body weights, but the differences were not statistically
significant. At week 52, the weight of the left thyroid glands of
females at this dose was reduced both absolutely and relative to the
weight of the brain. Males that were allowed to recover showed reduced
absolute left and right adrenal weights, left thyroid plus parathyroid
weights, and heart weight; the organ:brain weight and the kidney:brain
weight ratios were also reduced. The weight of the left testis
relative to that of the body was increased. At 104 weeks, females at
the high dose had increased brain weights and decreased kidney:brain
weights. The males in this group had reduced absolute and relative (to
body) weights of the right and left kidney, heart, and right adrenal
gland.
Males at the high dose had a decreased incidence and reduced
severity of chronic nephropathy as compared with control animals,
whereas females at this dose had a reduced incidence of mineral
calcareous deposits in the renal pelvis. Males and females at the high
dose showed increased incidences of inflammation of tissues of the
tail (joints and skin) and of alveolar macrophage infiltration.
Probably as a result of this inflammation, higher incidences of
lymphoreticular cell hyperplasia, sinal ectasia, and congestion were
observed in lymph nodes at various sites in the body. The inflammatory
changes were considered to be age-related, and their increased
incidence in animals at the high dose was assumed to reflect the poor
physical condition of these animals. In females at the high dose,
increased incidences of gastric erosion and submucosal oedema were
found at terminal sacrifice. No treatment-related ophthalmic changes
were observed.
Tumours of various cell types occurred in many tissues. The only
changes that showed a clear relationship to treatment were increased
proliferative cell lesions in thyroid C-cells, the adrenal medulla,
and the uterine endometrium (Table 6). A statistical evaluation of the
tumour incidence in all animals was not provided, but according to a
statistical analysis of data on tumours found at final sacrifice,
females at the low and high doses had a significantly reduced
incidence of C-cell hyperplasia at terminal sacrifice, while males at
the low and intermediate doses had significantly increased incidences
of C-cell adenomas. The increase in C-cell carcinomas at termination
Table 6. Occurrence (%) of proliferative lesions in organs of rats given ethoprophos in the diet
Groups of animals and tumour type Dose (ppm)
Males Females
0 1 60 400 0 1 60 400
No. of animals
No. of unscheduled deaths 50 42 42 30 42 47 37 27
No. at terminal sacrifice 20 28 28 41 29 23 33 44
Total 70 70 70 71 71 70 70 71
Thyroid
C-cell hyperplasia 31 29 41 39 61 39 63 46
C-cell adenomas 11 9 13 17 14 11 16 17
C-cell carcinomas 0 0 1 4 1 1 1 3
All tumours 11 9 14 21 15 12 17 20
Adrenal
Benign phaeochromocytomas 20 10 10 7 4 3 1 3
Malignant phaeochromocytomas 0 3 3 7 0 0 0 0
All tumours 20 13 13 14 4 3 1 3
Uterus
Endometrial stromal polyps 1 1 4 8
Data are incidences in percentages of the total number of animals (terminal sacrifice plus intercurrent deaths)
was not significant. Males at the high dose showed a significant
decrease in the incidence of benign phaeochromocytomas but a
significant increase in that of malignant phaeochromocytomas at study
termination. The increased incidence of uterine endometrial stromal
polyps in females at the high dose was also significant. The study
authors indicated that C-cell and uterine proliferative lesions are
more likely to occur in old animals and their increased incidences are
attributable to the greater longevity of the animals at the high dose.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of inhibition of brain acetylcholinesterase activity at the next
highest dose (Williams, 1992).
(d) Genotoxicity
The results of studies on the genotoxicity of ethoprophos are
summarized in Table 7.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity conducted
according to GLP and with a QA statement, groups of 28
Crl:CD(R)(SD)BR rats of each sex, 6 weeks of age, were given diets
containing ethoprophos (purity, 95.3%) in acetone at concentrations of
0, 1, 30, or 300 ppm, equal to 0, 0.04, 1.3, or 23 mg/kg bw per day
for males and 0, 0.09, 2.6, or 27 mg/kg bw per day for females. After
10 weeks of exposure, the F0 parents were mated 1:1 to produce the
F1a generation. Treatment was continued through mating, gestation,
parturition, and lactation. On day 4 after parturition, each litter
was culled to four pups of each sex. After 3 weeks of lactation, the
pups were weaned. Owing to significant mortality among weanlings at
the high dose, pups in the control, low-, and intermediate-dose groups
were not used as parents for the next generation but instead 10 pups
of each sex were selected for necropsy. All weanlings at the high dose
and control F1a weanlings selected as parents were maintained on
their respective diets until day 49 post partum; the weanlings at
the high dose and 10 controls of each sex were then necropsied. From
about 1 week after weaning of the last F1a pups, F0 parents at 300
ppm were fed a diet containing 150 ppm of ethoprophos (equal to 7.1
mg/kg bw per day for males and 14 mg/kg bw per day for females), and
all F0 animals at the other two doses were maintained on their
original diets. After 3 weeks, the F0 parents were mated again to
produce the F1b generation, mating, gestation, parturition,
lactation, and weaning being conducted as described above. One week
after weaning, 28 F1b pups of each sex at each dose were selected as
parents of the F2 generation. The remaining F1b pups and all F2
pups were necropsied. Parental F0 animals aged 35-36 weeks and
parental F1 animals aged 20-21 weeks were killed after weaning of
the F1b and F2 progeny, respectively, and neccropsied grossly.
Blood was taken just before sacrifice, and brain tissue was collected,
for analyses of cholinesterase in plasma, erythrocytes, and brain. The
Table 7. Results of studies of the genotoxicity of ethoprophos
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa,b S. typhimurium 10-1000 µg/plate in NR Negative ± S9 Barfknecht et al. (1985a)
TA98, TA100, TA1535, DMSO
TA1537, TA1538
Gene mutationa Mouse lymphoma 0.24-0.032 µg/ml 95 Negative Thomson et al. (1981)
L5178Y cells, Tk locus (total growth between 18
and 126%, respectively),
- S9
0.024-0.0032 µg/ml
(total growth between 10
and 106%), + S9
Gene mutationc,d Chinese hamster ovary 0-500 µg/ml - S9 NR Negative Stankowski et al. (1985)
cells (CHO-K1-BH4); 0-150 µg/ml + S9
Hprt locus in DMSO
Chromosomal Chinese hamster ovary 0-300 µg/ml - S9 NR Negative SanSebastian et al. (1985)
aberrationsc,e cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivef
Unscheduled DNA Rat hepatocytes 2.5-100 nl/ml in DMSO; NR Negative Myhr & Brusick (1981)
synthesisg,h toxic from 50 nl/ml
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Sister chromatid Chinese hamster ovary 0-350 µg/ml - S9 NR Negative SanSebastian et al. (1986)
exchangec,j cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivek
Table 7. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Chromosomal Rat (SD) bone 0-20 mg/kg bw per day for 5 days 95.7 Negative Skinner et al. (1981)
aberrationl marrow by gavage in Methocel K4M
premium; sacrifice 6 h after dosing
Chromosomal Rat (SD) bone 0-25 mg/kg bw once by gavage; 95.5 Negative Ivett (1989)
aberrationm,n marrow sacrifice 6, 18, and 30 h after
dosing 0-25 mg/kg bw per day for
5 days by gavage; sacrifice 6 h
after dosing
Vehicle, corn oil.
Dominant lethal Rat (SD) 0-20 mg/kg bw per day for 5 days NR Equivocal Putman & Schechtman
mutationo by gavage in corn oil (1981)
Dominant lethal Rat (Crl:COBS 0-20 mg/kg bw per day for 5 days 95 Negative Dearlove (1987)
mutationm,p CD(SD)BR) by gavage in carboxymethyl
cellulose
NR, not reported; S9, 9000 × g supernatant of rat liver; DMSO, dimethylsulfoxide; SD, Sprague-Dawley
a Test in triplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
b Cytotoxicity (small colonies) seen in preliminary test at 1666 µg/plate and no growth at 5000 µg/plate in TA1538 and TA100
c Test in duplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
d Cytotoxicity observed at doses > 167 µg/ml; relative initial survival, 18% at 350 µg/ml without S9 and 10% at 150 µg/ml with S9
e Only 100 cells/dose scored instead of 200/dose required by OECD; cytotoxicity at doses > 500 µg/ml without S9 and at 80 µg/ml
with S9, and average proliferation time increased at 400 and 60 µg/ml, respectively
f Statistically significant increase in aberrations seen with metabolic activation at 60 µg/ml. In a second test at concentrations
of 50-70 µg/ml, statistically significant but not dose-related increase at all concentrations (14-22 breaks compared with 2 breaks
in control). The authors considered this a weakly positive result. Although the results of the second test were not dose-related,
they confirm the positive result of the first test.
Table 7 (continued)
g Test in triplicate; positive controls included; GLP and QA statements included
h Purity, 95% (personal communication from Dr Rao, Rhone Poulenc). Toxicity observed as 73% survival at 50 nl/ml and 0% at 100 nl/ml.
i Cytotoxicity (abnormally low nuclear and cytoplasmic grains) at doses > 333 µg/well
j Cytotoxicity at doses > 500 µg/ml without S9 and > 80 µg/ml with S9, and average proliferation time was increased at 400 and
60 µg/ml, respectively.
k Statistically significant, dose-dependent increase with metabolic activation at 50-60 µg/ml. In a second test at concentrations of
50-75 µg/ml, statistically significant, dose-related increase at all concentrations (22-35 aberrations/cell compared with 16 per
cell in controls). Since there was no twofold increase at any dose, the authors considered the compound a weak inducer.
l Aberrations analysed in 5 males/group; positive control included; absorption of compound tested in 1 animal/group and confirmed
by a minimum of a 10-fold depression in cholinesterase activity in plasma at 20 mg/kg bw per day for 5 consecutive days. Clinical
signs: decreased motor activity at high dose (day 2); mitotic index not measured. Not clear that the bone-marrow cells were
exposed to the test substance. Only 50 cells scored instead of 100 cells/animal required by OECD.
m GLP and QA statements included
n Each group of dose and harvest time consisted of 5 rats of each sex. Positive control group included in single-dose study only.
Deaths observed only in females at high dose. Clinical signs at high single dose: dyspnoea, languid appearance, and diarrhoea.
Additional signs at high multiple doses: tremor, rough coat, hunched posture, and dark crusts around eyes. No decrease in mitotic
index after single dose, but slightly decreased (54-75%) in males in short-term study. It is not clear whether the bone-marrow
cells were exposed to the test substance. Aberrations were studied in only 50 cells/animal instead of 100 cells and mitotic
index studied in only 500 cells instead of 1000 as required by OECD.
o 10 male rats/group. Three days after the last dose, males were mated with 2 untreated virgin females. Mating process repeated
another 6 times once a week with new virgin females. Females were killed about 2 weeks after mating; scoring for number of corpora
lutea number of dead and live implants per pregnant female. Absence of body-weight gain in males at high dose. No effect on
fertility index, number of corpora lutea, or number of implantations. Pre-implantation loss statistically significantly increased
in week 3 at high and low doses. Number of dead implants increased in week 1 at low dose, weeks 2-3 at intermediate dose, and weeks
1-6 at high dose, with a positive dose-response relationship. The proportion of females with one or more dead implants was
significantly increased at the high and intermediate doses (week 2 and weeks 2-3, respectively) and at the high dose also of females
with two or more dead implants. The number of dead implants per total number of implants was significantly increased at the high
dose (weeks 2-5) and intermediate dose (week 5) (0.08-0.12 in test groups compared with 0.02-0.05 in control group). The number of
live implants per pregnant female, however, was not affected (change in live implant ratio is the criterion of dominant lethality).
In the positive control group, more marked increases in dead implants per total implants observed in weeks 1-4 (0.6-1.0), and the
number of live implants per pregnant female was significantly decreased (0.0-8.3 compared with 9.9-11.9 in controls). The authors of
the report concluded that the compound was mutagenic; however, the shortcomings of the test and the fact that the number of live
implants was not affected indicate that the result is equivocal. The shortcomings of the study were that only 12-18 pregnant females
were included in each treated group and 15-17 in the control group, whereas according to OECD 478 30-50 pregnant females per mating
interval are required; mating was not confirmed. Only QA statement included.
Table 7 (continued)
p 24 males/group. Doses based on a range-finding study. Two days after the last dose, males were mated with 2 untreated virgin
females. Mating process was repeated another 7 times once a week with new virgin females. Females were killed 14 days after
presumed gestation; scoring for number of corpora lutea and for number of dead and live implants per pregnant female. Other
observations: viability, clinical signs, body weight, food consumption, gross necropsy of males and females, especially reproductive
organs (including histopathological examination). Three males at high dose died showing clear signs of intoxication. One male was
replaced. Clinical signs at high dose were characteristic of cholinesterase poisoning. Body-weight gain was inhibited (dose-related)
during treatment at 5 and 20 mg/kg bw per day, and food consumption was lower at the high dose. No compound-related findings at
gross necropsy except a single finding of an epididymal mass in one male at 5 mg/kg bw per day; no effect on testis weight, mating
index, fertility index, or pregnancy rate. Average numbers of corpora lutea, implantations, litter sizes, resorptions, percentage
of dead conceptuses, and number of females with resorptions similar in treated and control groups, except a single finding of dams
with increased resorptions in week 4 of mating at the intermediate dose.
thyroid glands were weighed, and selected tissues (especially from the
reproductive organs) from control and high-dose animals were examined
histopathologically.
The dietary concentrations of 1, 30, and 300/150 ppm during the
first premating and mating periods were equal to 0.07, 2.2, and 23/12
mg/kg bw per day for F0 and F1 males and 0.09, 2.7, and 27/14
mg/kg bw per day for F0 and F1 females. During the second mating
period, the doses of 1, 30, and 150 ppm given to F0 males were equal
to 0.04, 1.3,and 7.1 mg/kg bw per day; the intake of females,
calculated only for weeks 20-24, was 0.07, 2.0, and 11 mg/kg bw per
day.
The clinical signs in F0 parents at the high dose were soft
faeces during the first 2 weeks of exposure and tremors in females
during gestation and lactation. No clinical signs were observed in
F1 parents. The body weights of F0 males at 300 ppm and F1 males
at 150 ppm were significantly decreased throughout the exposure
period. F0 males at 300 ppm had significantly lower body-weight
gains during several weeks, whereas an increase in body-weight gain
was observed occasionally when the dose was changed to 150 ppm. The
weight gain of F1 males at the high dose was reduced during the
premating period and occasionally thereafter. Significantly decreased
body-weight gains were observed occasionally in males at 30 ppm but
these did not result in significantly lower body weights. F0 females
at the high dose had significantly reduced body-weight gains in weeks
1 and 9, but these did not result in lower body weights during the
premating period for the F1a litter. The total body-weight gain
during the 0-20 days of gestation with the F1a litter was
significantly reduced, and the body weights were lower during
lactation. These lower (but not statistically significant) body
weights of F0 females at the high dose persisted during the
premating and mating periods for F1b progeny and reached statistical
significance on days 4-21 of lactation, although there was no effect
on body-weight gain. The body weights of F1 females at the high dose
were significantly reduced throughout premating, gestation, and
lactation, but with no effect on weight gain. Food intake was affected
only at the high dose, with lower consumption observed for F0 males
in weeks 1, 2, and 20 and for F1 males in weeks 9, 11, and 16-20.
The food consumption of females of the F0 generation at the high
dose was lower during the first week of exposure, but food consumption
was affected only on days 4-14 of gestation of the F1a generation.
Length of gestation and indices of mating, fertility, and gestation
were not affected in either generation. The final body weights of F0
and F1 males at the high dose were significantly reduced, and only
in F1 males at this dose the absolute, but not the relative, weight
of the thyroid was significantly lower. No treatment-related lesions
were observed on gross or histopathological examination.
Cholinesterase activity was significantly inhibited in plasma and
brain of F0 and F1 animals of each sex at the high dose, in F1
animals at 30 ppm, and in F0 females at 30 ppm; plasma
cholinesterase activity was also inhibited in F0 males at 30 ppm.
There was no effect on erythrocyte acetylcholinesterase activity.
Analyses of data for the litters revealed lower body-weight gains
in F1a pups at 300 ppm throughout lactation, resulting in lower body
weights from day 7 onwards in males and from day 4 in female pups. The
survival of F1a pups was not affected up to weaning, but the
survival index was only 51% 1 week after weaning on day 28. Necropsy
of dead F1a pups showed blood in the stomach and/or intestines. The
body weights of surviving F1a pups at the high dose remained reduced
up to sacrifice on day 49, and their body-weight gain was reduced up
to day 35. F1b pups at the high dose of 150 ppm showed lower
body-weight gain, from the second week post partum onwards for
females and from the third week for males, resulting in lower body
weights from day 14 and day 21, respectively. Survival was not
affected. Significant reductions in average litter size at 1 ppm,
beginning on day 4, were considered spurious owing to the lack of a
dose-response relationship. The litter sizes and sex ratios of F2
litters were unaffected by treatment. The decreased litter sizes, and
as a consequence higher pup body weights, at 1 and 30 ppm and the
reduced sex ratio at 30 ppm were considered not to be related to
treatment in the absence of a dose-response relationship. The
body-weight gain of pups at the high dose was reduced from day 4
onwards, resulting in lower weights from lactation day 7. Reduced
weight gain was found on days 4-7 of lactation in the group receiving
30 ppm, with a (probably) compensatory increase on days 14-21, but
this was considered irrelevant toxicologically. Deaths among F2 pups
at the high dose on days 7-14 resulted in a significantly reduced
14-day survival index and lactation index. No treatment-related
lesions were observed in dead F2 pups or in F1a, F1b, and F2
pups subjected to scheduled necropsy 1 week after weaning.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of lower body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in parents of each sex in each
generation. No effects were observed on reproductive parameters. The
NOAEL for toxicity to pups was 30 ppm, equal to 1.3 mg/kg bw per day,
on the basis of effects on body weight and body-weight gain and deaths
in F2 litters at 150 ppm (Neeper-Bradley, 1991).
Groups of 10 male and 20 female Fischer 344 rats, 44 days old,
were fed diets containing technical-grade ethoprophos (purity, 95.3%)
at concentrations of 0, 60.5, 131, or 262 ppm (equivalent to 0, 3.0,
6.6, and 13 mg/kg bw per day) for 56 days before mating. Weanlings
from the second litters were selected to be the parents of the next
generation. All parental animals and weanlings of each generation were
subjected to gross pathological examination. Additionally, all F1b
and F2b parents and five male and five female weanlings from each of
the F1a, F2a, F3a, and F3b litters were evaluated
histopathologically. A statement of QA was provided.
The mortality rate was unaffected but growth was slightly
depressed at the two higher doses in all parental generations. Data on
food consumption were not available. A dose-related increase in the
number of dams that cannibalized their young was noted in all treated
groups of the F0 generation, mainly with regard to the second
litters. The percentage of animals becoming pregnant was reduced in
all treated F1a litters (75, 60, 55, and 55%, respectively, at 0,
60.5, 131, and 262 ppm) and for F3a and F3b litters at 262 ppm.
The lactation index (pup survival on days 4-21) was decreased at both
131 and 262 ppm in the F1b (56% and 40%, respectively, compared with
89% in controls) and the F2a generation (86% and 67%, respectively,
compared with 100% in controls) and at the high dose in the F2b
generation (67% compared with 88% in controls). Weanling weight (not
determined for F1a and F1b litters) was depressed in the F2a and
F2b litters at 131 and 262 ppm and in the F3a litters at 262 ppm.
Other effects that were observed only at the highest dose included a
tendency for decreased litter size at birth in almost all generations
of progeny, prolongation of the mean gestation period of F1b litters
(this parameter was not evaluated in all generations), the occurrence
of bilateral lenticular opacity (cataracts; in 29%) and anophthalmia
(3%) in F2a weanlings (information on the litter distribution of
these abnormalities was not available), and an increased incidence of
multifocal granuloma of the mesenteric lymph nodes in F2b adults.
The increased incidence of cannibalization among F0 dams and the
slight decrease in pregnancy rate in F1a litters at 60.5 ppm did not
recur in all generations and were probably related to maternal
toxicity. This concentration was considered to be the minimal
no-effect level for reproductive toxicity. As enzootic pneumonia was
observed in all parental animals and in some pups, the results of this
test are unreliable (Fletcher et al., 1980).
(ii) Developmental toxicity
Rats
A preliminary dose-range finding study was conducted according to
GLP and with a statement of QA, in which five female Sprague-Dawley
COB SC rats were given ethoprophos (purity, 95.6%) in corn oil at
doses of 0, 0.2, 1, 4, 8, or 16 mg/kg bw per day by gavage on days
7-15 of gestation. Fetuses were removed surgically on day 20 and
examined for external anomalies. The only finding was body-weight loss
in dams at the highest dose during days 6-9 of gestation and reduced
body-weight gains (24%) throughout treatment. There were no signs of
developmental toxicity (Rodwell, 1989a).
In the main study, conducted according to GLP and with a
statement of QA, groups of 25 pregnant female
Crl:COBS(R)CD(R)BR(R)VAF rats aged 3 months were given
ethoprophos (purity, 95.6%) by gavage in corn oil at doses of 0, 2, 9,
or 18 mg/kg bw per day on days 6-15 of gestation. The dams were
observed for clinical signs, and body weight and food consumption were
measured. Fetuses were removed surgically on day 20, weighed, and
examined for external, visceral, and skeletal anomalies.
Dose-dependent maternal toxicity was observed at 9 and 18 mg/kg
bw per day, with treatment-related findings including soft stools at
both doses and faecal staining at the high dose. Significantly lower
body-weight gain was observed at 9 mg/kg bw per day on days 6-9 of
gestation, and during this period females at the high dose showed
body-weight loss which resulted in lower body weights by the end of
the study. The food consumption of animals at the high dose was
reduced on days 6-16 of gestation. No effect was found on survival, no
morphological changes were observed at necropsy, and no changes in
gravid uterine weight or fetal parameters (viability, resorptions,
body weight, and sex ratio) were found. The incidences of external,
visceral, and skeletal variations and of irreversible structural
changes were unchanged. The NOAEL for maternal toxicity was 2 mg/kg bw
per day, and that for developmental toxicity was 18 mg/kg bw per day
(Rodwell, 1989b).
Groups of 25-35 mated female TAC:N(SD)FBR rats were intubated
with technical-grade ethoprophos (purity, 94%) in corn oil at doses of
0, 0.16, 1.6, or 16 mg/kg bw per day on days 6-15 of gestation, the
day a vaginal sperm plug was observed being considered day 0. The dams
were killed on day 20 and the fetuses removed for gross, skeletal, and
visceral examination. A positive control group was included.
The pregnancy rate was comparable in all groups. During
gestation, 1/24 pregnant dams at 1.6 mg/kg bw and 18/30 at 16 mg/kg bw
died or aborted; in addition, three non-pregnant dams at the high dose
were found dead. Growth was depressed on days 15 and 20 of gestation
in dams at 16 mg/kg bw. No significant difference was found between
control and treated groups in the mean numbers of corpora lutea,
implantation sites, live fetuses, dead fetuses or resorptions, mean
fetal weight, or sex ratio. According to a summary of the skeletal
findings and soft-tissue abnormalities in fetuses, the only notable
observation was a significant increase in the incidence of litters
containing fetuses with extra lumbar ribs in animals at 1.6 mg/kg bw.
Since no dose-response relationship was seen, the authors could not
attribute this finding to exposure to ethoprophos. Incomplete
ossification of vertebrae was observed in all groups, including the
controls, with incidences of 18, 10, 7, and 17% in control, low-,
intermediate-, and high-dose groups, respectively). The NOAEL for
maternal toxicity was 1.6 mg/kg bw per day on the basis of deaths and
depressed body-weight gain, and the NOAEL for developmental toxicity
was 16 mg/kg bw per day (Knickerbocker & Re, 1979, and amendment in
1985).
Rabbits
A preliminary dose-range-finding study was conducted according to
GLP and with a statement of QA in which groups of eight female New
Zealand white rabbits received ethoprophos (purity, 95.6%) in corn oil
at doses of 0, 0.1, 0.5, 2, 5, or 10 mg/kg bw per day by gavage on
days 7-18 of gestation. The fetuses were removed surgically on day 29
and observed for external anomalies. Dose-dependent maternal toxicity
was observed at 5 and 10 mg/kg bw per day, the clinical signs
including emaciation, an increased incidence of soft stools, and
faecal and urine staining. Consistent body-weight loss and a
dose-dependent mortality rate were also seen in these two groups.
There were no signs of developmental toxicity (Rodwell, 1989c).
In the main study, conducted according to GLP and with a
statement of QA, groups of 20 artificially inseminated New Zealand
white rabbits aged 6 months received ethoprophos (purity, 95.6%) by
gavage in corn oil at doses of 0, 0.625, 1.25, or 2.5 mg/kg bw per day
on days 6-18 of gestation. The does were observed for clinical signs,
and body weight and food consumption were measured. The fetuses were
removed surgically on day 29, weighed, and examined for external,
visceral, and skeletal anomalies.
There were no clinical signs of maternal toxicity, no adverse
effects on survival, body weight, or food consumption, and no
morphological changes. Gravid uterine weight and fetal parameters
(viability, resorptions, body weight, and sex ratio) were also
unchanged. The finding of a significantly reduced mean number of
viable fetuses per doe at 1.25 mg/kg bw per day was due to the absence
of this effect in the group at the high dose and considered not to be
related to treatment. The incidences of external, visceral, and
skeletal variations were not altered, as the finding of a decreased
incidence of 27 presacral vertebrae in fetuses at the intermediate and
high doses was considered not to be toxicologically relevant.
Increased incidences of cleft palate, flexed paw, gastroschisis, and
cephalocoele seen on external examination and of atelectasis and
hydrocephaly seen on visceral examination of the group at the
intermediate dose when compared with controls and with historical
control values were observed in fetuses of only a single doe and were
considered not to be related to treatment. The incidences of fetuses
with malformations were 2.4, 4.1, 4.0, and 6.6% and those of litters
with malformed fetuses were 12, 11, 11, and 35% in the control, low-,
intermediate-, and high dose groups, respectively. The malformations
seen at the high dose were dissimilar and occurred once or at very low
frequency; furthermore, they were types that occur spontaneously in
this strain of rabbits and the incidences were within the historical
control values. In the absence of effects on fetal weight, the
increased incidences of irreversible structural changes at the high
dose were considered to be unrelated to treatment. The NOAEL for both
maternal and developmental toxicity was 2.5 mg/kg bw per day, the
highest dose tested (Rodwell, 1989d).
Groups of 17 artificially inseminated female New Zealand white
rabbits were intubated with technical-grade ethoprophos (purity,
95.7%) in corn oil at doses of 0, 0.125, 0.5, or 2 mg/kg bw per day on
days 6-18 of gestation, the day of insemination being considered day
0. The surviving does were killed on day 29 of gestation, and their
fetuses were removed for external, visceral, and skeletal
examinations. A statement of QA was provided.
No treatment-related deaths occurred, but does at all doses
showed an increased (but not dose-related) incidence of anorexia
during and after treatment. A dose-related but not statistically
significant decrease in mean body-weight gain was observed between
days 6 and 18 in the does at the two higher doses. Control and treated
groups did not differ significantly with respect to pregnancy rate,
mean numbers of corpora lutea, implantations, resorptions, dead
fetuses, or live fetuses, mean fetal weight, mean fetal crown-rump
length, sex ratio, uterine weight with or without fetuses, or the
frequency of gross and visceral abnormalities. An increased total
incidence of skeletal variants was observed in the litters of all
treated does, with 14% at the low dose, 13% at the intermediate dose,
and 17% at the high dose, and 6% in controls. The increase was not
dose-related, and the frequency of any particular type of skeletal
variant, when considered alone, did not show a dose-response
relationship. The NOAEL for maternal toxicity was 0.12 mg/kg bw per
day on the basis of reduced body-weight gain. The NOAEL for
developmental toxicity was 2 mg/kg bw per day (Wolfe et al., 1981).
(f) Special studies: Neurotoxicity
Rats
In a study conducted according to GLP and with a statement of QA,
groups of 24 Crl:CD(SD)BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single dose of ethoprophos (purity, 95.7%) by gavage
in corn oil. Male rats received doss of 0, 30, or 60 mg/kg bw and
females received 0, 20, or 40 mg/kg bw. The doses were based on a
range-finding study. The animals were observed daily for deaths and
clinical signs, and body weights were recorded weekly. Blood samples
were taken from six animals of each sex per group 2 h and 3, 8, and
15 days after dosing to determine cholinesterase inhibition in plasma
and erythrocytes. Brain acetylcholinesterase activity was determined
in the frontal cortex, caudate/putamen, hippocampus, and cerebellum of
the same animals at the same times.
One male rat given 60 mg/kg bw was found moribund. The clinical
signs observed in females at 40 mg/kg bw and males at 60 mg/kg bw were
tremors and excessive salivation. Males also showed hunched posture,
laboured breathing, rough and stained coats, pale bodies, ocular
discharge, incoordination, hypoactivity, and were cold to touch. Among
animals at the lower dose, the only clinical sign was cold to touch in
one female at 20 mg/kg bw. There were no effects on body weight.
Cholinesterase activity was inhibited in all tissues examined by 2 h
after treatment, and the effects were dose-, time-, and
tissue-dependent (Table 8). Recovery of cholinesterase activity was
apparent by day 3 or 8 after dosing and was complete by day 15 for
plasma and cerebellar cholinesterase activity. Statistically
significant inhibition of acetylcholinesterase activity persisted in
the hippocampus through day 15 at the high dose and in erythrocytes
and the frontal cortex of males at both low and high doses. Persistent
inhibition was observed in the caudate/putamen at both doses, but the
inhibition was not statistically significant (Weiler, 1994a).
In a study conducted according to GLP and with a statement of QA,
groups of 17 Crl:CD(R) BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single oral dose of ethoprophos (purity, 96.2%) by
gavage in corn oil. The doses were 0, 5, 50, or 75 mg/kg bw for males
and 0, 5, 25, or 50 mg/kg bw for females, which were based on
range-finding studies. The animals were observed daily for deaths and
Table 8. Cholinesterase activity in various tissues of rats after a single oral dose of ethoprophos
Tissue Dose Inhibition from control mean (%)a
(mg/kg bw)
Males Females
Day 1b Day 3 Day 8 Day 15 Day 1b Day 3 Day 8 Day 15
Plasma cholinesterase 30/20 83* 43* 6 12 90* 31* 27 6
60/40 93* 68* 19* 14 94* 52* 23 0
Erythrocyte cholinesterase 30/20 43* 46* 31* 23* 44* 48* 29* 17
60/40 53* 46* 33* 22* 49* 50* 35* 17
Brain cholinesterase in 30/20 45* 38 30 9 72* 25 54 25
caudate/putamen 60/40 93* 75* 51 32 92* 63* 39 40
Brain cholinesterase in 30/20 45* 30* 19* 0 50* 14 8 4
hippocampus 60/40 72* 47* 27* 18* 75* 29* 21* 17*
Brain cholinesterase in 30/20 48* 38* 34 19* 55* 10 0 32
frontal cortex 60/40 76* 50* 49* 27* 77* 40* 24* 29
Brain cholinesterase in 30/20 46* 11 0 0 60* 11 0 3
cerebellum 60/40 81* 35* 7 6 80* 29* 9 3a
a 100% - mean treated/mean control × 100%
b 2 h after dosing
* Significantly different from control at p < 0.05
clinical signs, and body weights were recorded before treatment and on
days 1, 8, and 15. Twelve animals of each sex per group were subjected
to a functional observational battery of tests and tests for motor
activity before treatment and at 2 h and 8 and 15 days after dosing.
Cholinesterase activity was determined in plasma and erythrocytes from
the remaining five animals of each sex per group before treatment and
on days 2, 8, and 15 after dosing. After blood collection on day 15,
the animals were killed and their brains collected for determination
of acetylcholinesterase activity. Neuropathological examinations were
conducted at the end of the study on all appropriate tissues from six
rats of each sex per group that had not been used to determine
cholinesterase activity. All animals were examined macroscopically and
only the control and high-dose groups microscopically.
Treatment-related deaths were seen in two males given 75 mg/kg bw
and six females given 50 mg/kg bw; four of the females were replaced.
The deaths of one male at 50 mg/kg bw and one female at 5 mg/kg bw
were considered to be unrelated to treatment. Clinical signs in
animals at the high doses included thin or hunched appearance,
tremors, laboured respiration, protruding eyes, incoordination,
hypoactivity, excessive salivation, and cold to touch. A few males at
50 mg/kg bw also showed some of these signs. The results of the
functional battery showed mild to severe functional or behavioural
changes only on the day of dosing, and the changes were characteristic
of cholinesterase poisoning. No effects were seen at 5 mg/kg bw dose.
Mild effects were seen in two females at 25 mg/kg bw and in several
males at 50 mg/kg bw, which included salivation, lip smacking, ataxia,
negative pupillary response, and tremors. Moderate and severe effects
were observed in females at 50 mg/kg bw and males at 75 mg/kg bw,
which included, in addition to the effects described above, low
carriage and prostrate position, lethargy, changes in the ease of
handling, lachrymation, laboured or gasping respiration, increased
latency until first step, paralytic gait, negative corneal response,
and negative air drop and startle reflexes. The analgesic reflex to a
mild heat stimulus was delayed significantly on day 1 in males and
females at 50 mg/kg bw and in males at 75 mg/kg bw. Body temperature
was statistically significant decreased by 3-4°C in females at 50
mg/kg bw and in males at 75 mg/kg bw; a slight decrease in body
temperature was seen in males at 50 mg/kg bw. Mean forelimb grip
strength was decreased only on day 1 in males at 75 mg/kg bw and
females at 50 mg/kg bw and was statistically significant only in
males. The NOAEL for behavioural and functional effects was 5 mg/kg
bw. Motor activity was markedly and statistically significantly
decreased in males at 50 and 75 mg/kg bw (dose-related) and in females
at 50 mg/kg bw on day 1 (Table 9). Differences were no longer seen on
day 8 or 15. The NOAEL for motor activity was 5 mg/kg bw.
The effects on cholinesterase activity are summarized in Table
10. On day 2, plasma cholinesterase activity was dose-dependently
inhibited for males (45-94%) and females (49-94%) at all doses but had
recovered within 8 days of dosing. Erythrocyte acetylcholinesterase
activity was inhibited in females at all doses (not dose-related) and
in males at 50 and 75 mg/kg bw. Generally nonsignificant inhibition of
Table 9. Motor activity (mean total counts) measured shortly after dosing (day 1) and during 10-min
intervals for 60 min in rats given single doses of ethoprophos
Dose Motor activity (mean (standard deviation))
(mg/kg bw)
Males Females
0-10 min 10-20 min 2-30 min Total 0-10 min 10-20 min 20-30 min Total
(0-60 min) (0-60 min)
0 720 (130) 280 (140) 100 (120) 1300 (510) 580 (86) 280 (140) 98 (100) 1200 (330)
5 630 (110) 300 (160) 51 (64) 1000 (270) 650 (160) 200 (180) 69 (110) 1200 (460)
25 480 (270) 170 (210) 78 (140) 1100 (960)
50 290* (240) 150* (110) 20 (25) 670* (430) 38* (34) 28* (24) 44 (80) 240* (190)
75 33* (28) 25* (22) 17 (19) 180* (100)
* Significantly different at p < 0.05
Table 10. Cholinesterase activity in plasma, erythrocytes, and brain of rats given a single
dose of ethoprophos
Dose Day of Inhibition of cholinesterase from mean control valuea
(mg/kg bw) sampling
Males Females
Plasma Erythrocyte Brain Plasma Erythrocyte Brain
5 2 45* 8 49* 33*
8 -4 27 11 20
15 -8 -7 -3 -3 3 7
25 2 73* 49*
8 -12 21
15 -14 33* 11
50 2 87* 45* 94* 35*
8 -2 26 2 30
15 0 18 17 -3 20 10
75 2 94* 55*
8 6 25
15 -4 31* 18
a 100% - mean treated/mean control × 100%
* Significantly different at p < 0.05
> 20% was still present on days 8 and 15 in females at 25 and 50
mg/kg bw and in males at 50 and 75 mg/kg bw. In these groups, brain
acetylcholinesterase activity was inhibited by about 10% in females
and 18% in males at day 15, but this effect was not statistically
significant. Macro- and microscopic neuropathological examinations
showed no treatment-related changes. As brain acetylcholinesterase
activity was not measured directly after exposure, the NOAEL could not
be based on this end-point. The inhibition in females was not
dose-related, and there was a large standard deviation at 5 mg/kg bw.
The NOAEL for neurotoxicity was 5 mg/kg bw on the basis of behavioural
changes and inhibition of erythrocyte acetylcholinesterase activity
(Weiler, 1994b).
Groups of 27 Crl:CD BR VAF/Plus rats of each sex aged 6 weeks
were fed diets containing ethoprophos (purity, 95.7%) at
concentrations of 0, 4, 40, or 400 ppm, equal to 0, 0.26, 2.6, and 27
mg/kg bw per day for males and 0, 0.31, 3.0, and 31 mg/kg bw per day
for females, for 13 weeks. Twelve rats of each sex at each dose were
selected for functional battery and motor activity testing before
dosing and in weeks 4, 8, and 13. Of these, six of each sex per dose
were selected for neuropathological investigations, and the remaining
rats were observed only macroscopically. Cholinesterase activity was
measured in the plasma and erythrocytes of 10 rats of each sex per
group and acetylcholinesterase activity in four regions of the brain
(frontal cortex, caudate/putamen, hippocampus, and cerebellum) at
sacrifice in five rats of each sex per dose in weeks 4, 8 and 14. All
animals were observed for deaths and clinical signs, and body weight
and food consumption were recorded.
The only clinical sign considered to be related to treatment was
brown staining of the anal region in males at 400 ppm. Significantly
lower body-weight gain was observed in males at 400 ppm throughout
treatment and in females up to week 10, which resulted in
significantly lower body weights in animals at this dose. The mean
food consumption of animals of each sex at the high dose was
significantly lower during the first week and remained low in males
throughout treatment. In males at 40 ppm, the food consumption was
higher than that of controls. Changes in the results of the functional
battery tests considered to be related to treatment were seen only at
the high dose and included mild tremor (one female), vocalization
during handling (two females), slight salivation (one male), unkempt
fur (two males, one female), latency to first step (one male, one
female), and jumping to touch (one male) in week 4; in week 8, the
observations included intermittent vocalization in cage (three
females), slight lachrymation (one female, and one female at 40 ppm),
slight salivation (one male, two females, and one female at 40 ppm),
latency to first step (two females), aggressive behaviour (one
female), jump to touch response (one female), negative air drop reflex
(one male), and negative pupillary response (one female); in week 13,
the observations included aggressive behaviour (one female), latency
to first step (one female, and two males at 40 ppm), and no movement
in open arena (one male). Because the clinical signs observed at 40
ppm also occurred occasionally as single findings in controls, they
were considered to be unrelated to treatment. Other clearly
treatment-related observations in the functional battery test at the
high dose were decreased analgesic reflex in males in weeks 4, 8, and
13, and lower grip strength in fore- and hindlimbs of males and in
forelimbs of females. The motor activity of males at 400 ppm was
generally significantly lower than that of controls at all times;
females showed a slight decrease in activity only in week 4.
Cholinesterase activity in plasma, erythrocytes, and brain is
shown in Table 11. A > 20% inhibition of erythrocyte
acetylcholinesterase activity was found in males and females at doses
> 40 ppm. Inhibition of acetylcholinesterase activity in one or two
regions of the brain by > 20% was observed in males at 40 ppm at
weeks 4 and 8 and in all regions at 400 ppm. In females, inhibition of
acetylcholinesterase activity by > 20% was seen in three brain
regions at weeks 8 and 14 at 4 ppm, which was significant in the
frontal cortex in week 14, and in all regions at all times at higher
doses. No macroscopic or microscopic changes were observed.
An NOAEL could not be identified because brain
acetylcholinesterase was inhibited at all doses. The NOAEL for
neurotoxic effects was 40 ppm, equal to 2.6 mg/kg bw per day, on the
basis of the results of the functional observational battery and motor
activity tests (Weiler, 1994c).
Chickens
Two groups of 10 deep-litter hens weighing 1.4-2.1 kg (age not
specified) were intubated with technical-grade ethoprophos (purity
unknown) at a single dose of 5.6 µl/kg bw, equivalent to 6.2 mg/kg bw
and stated to represent the oral LD50 value in a previous study
performed at the testing laboratory, or tri- ortho-cresyl phosphate
(positive control) at 500 mg/kg bw. Four untreated hens were retained
as untreated controls. The hens given ethoprophos showed no gross
signs of ataxia or paralysis, although symptoms of transient
inactivity and 'depression' were noted between 1 and 48 h. Four hens
died within 48 h of dosing. The positive controls showed clinical
signs characteristic of delayed neurotoxicity. Histopathological
examination of sections of the spinal cord and sciatic nerve, stained
by the Weil-Weigert method (not referenced), from birds in all groups
at the end of a 21-day observation period revealed no evidence of
demyelination (Weir & Murphy, 1967).
In an initial study conducted to determine the LD50 of
ethoprophos in hens, six groups of 10 domestic hens ('a hybrid brown
laying strain') were given ethoprophos (purity, 94.5%) at doses of
0-16 mg/kg bw in a volume of 2 ml/kg bw by gavage. An LD50 of 6.4
mg/kg bw was calculated, with a 95% confidence interval of 4.8-8.8
mg/kg bw. On this basis, a dose of 6.5 mg/kg bw was selected for the
main study. The hens were divided randomly into six groups of 10. The
untreated controls received only corn oil, whereas the positive
controls received 500 mg/kg bw of tri- ortho-cresyl phosphate by
gavage. The treated groups first received 10 mg/kg bw of atropine
Table 11. Cholinesterase activity in plasma, erythrocytes, and brain regions at various
times during 13-week dietary exposure of rats to ethoprophos
Week Tissue Inhibition of cholinesterase activity from mean in controlsa
Males Females
4 ppm 40 ppm 400 ppm 4 ppm 40 ppm 400 ppm
4 Plasma 3 64* 90* 20 80* 97*
Erythrocyte 7 30* 26* -14 19 14
Brain
Caudate/putamen 17 23* 82* 8 32 83*
Hippocampus -7 4 48* 9 18 50*
Frontal cortex 19 -2 71* -28 30 74*
Cerebellum -7 0 52* -3 13 53*
8 Plasma -5 58* 85* 34* 87* 98*
Erythrocyte 10 23 35 19 32 40
Brain
Caudate/putamen 3 11 82* -11 -2 53*
Hippocampus 11 26* 59* 23 33* 67*
Frontal cortex 10 23 61* 33 49 80*
Cerebellum 0 0 46* 21 38* 62*
14 Plasma -2 54* 87* 18 86* 97*
Erythrocyte 19 33* 40* -6 22 11
Brain
Caudate/putamen -3 0 73* 34 49* 88*
Hippocampus 0 -9 54* 23 36* 66*
Frontal cortex 10 7 53* 52* 56* 80*
Cerebellum 9 -11 49* 6 27* 59*
a 100% - mean treated/mean control × 100%
* Significantly different from controls at p < 0.05
sulfate by intramuscular injection and then 6.5 mg/kg bw of
ethoprophos by gavage in corn oil at a constant volume of 2.5 ml/kg
bw. The atropine was found to have had only a minimal protective
effect, as 31/40 of the treated birds died within 4 days, most within
the first 24 h. Because of excessive mortality, two additional groups
were added, consisting of 12 and 11 birds, respectively, but it was
not specified whether they were treated concurrently with the
untreated controls. These birds were given pyridine 2-aldoxime
methiodide at 50 mg/kg bw by intramuscular injection in addition to
atropine before treatment with 6.5 mg/kg bw of ethoprophos. The
surviving birds were given further injections of pyridine 2-aldoxime
methiodide and atropine 24 and 48 h after treatment, and two birds in
the last group were injected 72 h after treatment. This procedure was
only marginally successful, as 14/23 birds died within 48 h of
treatment. On the basis of the deaths after the first dose of
ethoprophos, the LD50 was recalculated as 5.2 mg/kg bw, and this
dose was administered on day 22 to all 18 surviving birds, which were
treated with pyridine 2-aldoxime methiodide and atropine immediately
before administration of ethoprophos and again 5 h after the second
(day 22) treatment with ethoprophos; birds in the initial groups were
given additional injections 24 h after the second treatment. Two of
the 18 treated birds died within 72 h of the second treatment.
No clinical signs of neurotoxicity were noted in untreated
control or ethoprophostreated birds, whereas 9/10 birds treated with
tri- ortho-cresyl phosphate showed slight to marked signs of
neurotoxicity, usually by 11 days after treatment. These birds were
killed on day 21 and examined for histopathological changes. All other
surviving birds were killed on day 43. No remarkable macroscopic
changes were observed at necropsy. Portions of the forebrain, mid- and
hind-brain, cervical, thoracic, and lumbar spine, proximal and distal
sciatic nerve, and tibial nerve were evaluated for microscopic
evidence of neurological change. Lesions were noted in the spinal cord
and peripheral nerves of all positive control birds that reflected
'significant axonal degeneration'. Minimal lesions (grade 2 out of a
maximum of 5) were also found in the spinal cord of 9/10 control
birds, and one control had a minimal lesion in the proximal sciatic
nerve; no lesions were found in the brain or distal sciatic or tibial
nerves. A similar distribution of lesions of the spinal cord was seen
in treated birds, except that minimal lesions of the mid- or
hind-brain and lesions of the proximal sciatic nerve were each found
in 2/16 birds. One of the latter lesions was graded as moderate (grade
3) and occurred in a bird that also had a lesion of the mid- and
hind-brain. Three of 16 birds, including this one, also had minimal
lesions of the distal sciatic nerve. The authors concluded that
treatment with ethoprophos produced no clinical signs of neurotoxicity
and that the result was confirmed by the histological examination,
which showed no treatment-related changes in the nerve tissue. The
changes noted in the proximal sciatic nerve of the bird with multiple
lesions were considered to be unrelated to treatment and to represent
the extreme upper limit of background change (Roberts et al., 1986).
The Committee concluded that the study does not provide clear evidence
of neurotoxicity, but because the high rate of mortality reduced the
sensitivity of the study, the equivocal findings in some birds cannot
be dismissed. Historical control data from the testing facility
suggest that the lesions in proximal sciatic nerve are not
spontaneous.
(g) Studies on metabolites
O-Ethyl- S-propylphosphorothioate, a metabolite in rats, and
O-ethyl- O-methyl- S-propylphosphoro-thioate and
O-ethyl- S-methyl- S-propylphosphorodithioate, two metabolites
identified only in corn and potatoes (Rao, 1999), were tested for
toxicity and for their ability to inhibit cholinesterase activity in
female rats given single oral doses.
(i) Acute toxicity
The LD50 values of the three metabolites of ethoprophos in
female Crl:CD (SD)BR VAF/ Plus rats treated orally with the substances
in corn oil were 50 mg/kg bw for
O-ethyl- S-methyl- S-propylphosphorodithioate, 22 mg/kg bw for
O-ethyl- O-methyl- S-propylphosphorothioate, and 1600 mg/kg bw for
O-ethyl- S-propylphosphorothioate. The clinical signs were similar
for each of the metabolites and differed primarily in time of onset
and duration; the signs were also similar to those caused by the
parent compound. GLP and QA statements were included (Welier, 1998).
(ii) Cholinesterase inhibition
Three groups of 10 female Crl:CD(R)(SD)BR VAF/Plus rats aged
about 6 weeks were given a single dose of ethoprophos at 19 mg/kg bw,
O-ethyl- S-methyl- Spropylphosphorodithioate at 17 mg/kg bw, or
O-ethyl- O-methyl- S-propylphosphorothioate at 8 mg/kg bw by
gavage; a control group received the vehicle, corn oil. The animals
were observed for clinical signs before dosing, 0.5 and 2 h after
dosing, and the next day before sacrifice. Cholinesterase activity was
evaluated in plasma, erythrocytes, and brain 24 h after dosing.
Body-weight gain over the 2-day period was significantly lower in
all treated groups than in controls but resulted in a statistically
significant lower terminal body weight only with
O-ethyl- S-methyl- S-propylphosphorodithioate. In the other two
groups, the only clinical finding was a slightly increased incidence
of soft stools, whereas the animals given
O-ethyl- S-methyl- S-propylphosphorodithioate showed signs of
cholinesterase inhibition consisting of staggered gait and tremors.
All three compounds reduced cholinesterase activity in all three
tissues, the strongest inhibition being due to
O-ethyl- S-methyl- S-propylphosphorodithioate (Table 12; Weiler,
1998).
Table 12. Cholinesterase activity in plasma, erythrocytes, and brain of female rats 24 h
after a single oral dose of ethoprophos and two metabolites
Compound Dose Inhibition from control mean (%)a
(mg/kg bw)
Plasma Erythrocytes Brain
Ethoprophos 19 73* 37* 32*
O-Ethyl-S-methyl-S-propylphosphorodithioate 17 78* 30 71*
O-Ethyl-O-methyl-S-propylphosphorothioate 8 40* 47* 48*
a 100% - mean treated/mean control × 100%
* Significantly different from control at p < 0.05
4. Observations in humans
In a study of occupational exposure to ethoprophos in Salinas
Valley, Monterey County, California, USA, four male volunteers were
recruited among regular employees of an experienced pesticide
application firm who were thoroughly familiar with both the practices
and equipment involved in pesticide application and had not worked
with cholinesterase inhibitors for at least 90 days. The occupation
under consideration was that of mixer-loader-applicator, who applied
an emulsifiable concentrate containing 70% ethoprophos in xylene for 3
days (two men), 4 days (one man), or 5 days (one man) to field plots
of 2-3 ha; the only operator working on day 5 treated a field plot of
about 8.6 ha. During the mixing and loading phase, the concentrate,
supplied in 5-gallon (~ 20-L) containers, was diluted to 5% in two
160-gallon (~ 40-L) tanks. Transfer of the pesticide into the tank,
rinsing the container, and filling the spray tanks were done in a
closed system to minimize exposure. The operators wore long-sleeved,
twill work shirts, denim jeans, and rubber boots. They used rubber
gloves and disposable respirators which were changed each day. Face
shields and hard hats were available, especially for the mixing and
loading operations. The application rate of active ingredient was
about 12 kg/ha (range, 11-15 kg/ha). Blood was collected for
determination of cholinesterase activity in plasma and erythrocytes
three times before exposure and as soon as possible after each man's
work session. During application of a commercial preparation
containing 69.6% ethorprop (as an emulsifiable concentrate in xylene),
dermal exposure was monitored on the hands (three hand washings with
2-propanol in water) and face (passive facial patch collector
consisting of 12-layer surgical gauze), corresponding to a surface of
24 cm2. Inhalation was not monitored. Exposure to ethoprophos was
monitored by a standardized gas chromatographic method.
No clinical signs or adverse effects were noted during exposure,
and the changes in plasma and erythrocyte cholinesterase activity were
not statistically significantly different from the mean baseline
values. The maximal variation around the mean was - 8.9% to 7.9%,
which is considered to be normal day-to-day variation. Exposure on the
head and neck was 3.1-78 µg/h, with an average of 34 µg/h
(calculations based on a default head area of 910 cm2). The total
exposure of the head to ethoprophos was 7.6-153 µg, with means of 96,
53, 67, and 36 µg for the four men. The hands were exposed to 0.2-18
µg/h, with an average value of 6.3 µg/h and a value of 0.52-42 µg for
both hands; the mean values were 29, 2.0, 12, and 6.3 µg for the four
men. The overall exposure during the entire period of observation of
the man who worked for 5 days was 620 µg, and the quantity of active
ingredient handled per day was 31-92.4 kg. The overall exposure of the
other operators was 220 µg for the man who worked for 4 days and
handled 26-44 kg daily; 240 µg for the man who worked for 3 days and
handled 19-27 kg daily; and 130 µg for the man who worked for 3 days
and handled 17-44 kg daily (Leffingwell, 1986).
Comments
After oral administration to rats, 14C-ethoprophos was rapidly
and virtually completely absorbed, metabolized and excreted. The main
route of excretion was the urine (51-56%), but significant proportions
were excreted in expired air (about 15%) and faeces (10-14%). Little
radiolabel was found in tissues at 168 h, representing less than 2.5%
of the dose, and the highest concentrations were found in excretory
organs (liver, kidneys, and lungs). There was no evidence that
bioaccumulation would occur after repeated doses. The kinetics of the
radiolabel and the biotransformation appeared to be independent of the
route of administration (oral or intravenous), magnitude and frequency
of dose and sex. Ethoprophos was metabolized by dealkylation of one or
both S-propyl groups, followed by conjugation. The dermal absorption
of ethoprophos has not been studied in vivo, but radiolabelled
ethoprophos penetrated the skin in vitro, the greatest penetration
being seen through mouse skin, lesser penetration through rat and
rabbit skin (at equal rates), and the least penetration through human
skin. In all of the species tested, the rate of penetration was
greater with ethoprophos (emulsified concentrate) diluted 1:19 in
water than with undiluted material.
Single oral doses of ethoprophos were toxic to rats, mice and
rabbits (LD50 values, 31-62 mg/kg bw), and it was highly toxic to
rats exposed once by inhalation (LC50, 0.25 mg/L). Mice and rabbits
were more sensitive to dermal exposure than rats, and female rats were
more sensitive than males, with LD50 values of 8.5 mg/kg bw for
rabbits, 18 mg/kg bw for mice, 420 mg/kg bw for female rats, and 1300
mg/kg bw for male rats. The toxic signs observed after exposure by any
route were characteristic of cholinesterase inhibition. WHO (1999) has
classified ethoprophos as 'extremely hazardous'.
Ethoprophos not only irritates the eyes of rabbits but is very
toxic after administration into the eye, causing the death of tested
animals within 1 h. In a test for dermal irritation, all treated
animals died within 8 h of exposure to the undiluted compound. In a
three-week study in rabbits, the compound caused slight dermal
irritation at all doses tested (0.03, 0.1, and 1 mg/kg bw per day),
whereas no dermal irritation was observed in a 3-week study in rats
given doses of up to 10 mg/kg bw per day.
In studies in dogs (13, 20 and 52 weeks) and mice and rats (2
years) given ethoprophos orally, inhibition of cholinesterase was the
most sensitive parameter. In dogs, the NOAEL for inhibition of brain
acetylcholinesterase activity was 1 mg/kg bw per day, the highest dose
tested. In mice, brain acetylcholinesterase activity was inhibited at
30 ppm, resulting in a NOAEL of 2 ppm, equal to 0.25 mg/kg bw per day;
in rats, the overall LOAEL was 30 ppm (equal to 1.3 mg/kg bw per day)
and no effects were seen at 10 or 1 ppm (equal to 0.5 and 0.04 mg/kg
bw per day, respectively). In a two-generation study of reproductive
toxicity in rats, inhibition of brain acetylcholinesterase activity
was seen in animals of each sex of both parent generations at 30 ppm,
resulting in a NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day. In a
13-week study of neurotoxicity in rats, the activity of brain
acetylcholinesterase was inhibited in females at all doses tested, the
lowest dose being 4 ppm, equal to 0.26 mg/kg bw per day. Clinical
signs characteristic of cholinesterase inhibition were observed only
in the 2-year study in rats, in which the highest dose was reduced
from 600 to 400 ppm because of clinical signs.
Inhibition of brain acetylcholinesterase activity was also
demonstrated after dermal exposure of rabbits and rats to ethoprophos
for three weeks, with NOAELs of 0.1 mg/kg bw per day (LOAEL, 1 mg/kg
bw per day) in rabbits and 0.3 mg/kg bw per day (LOAEL, 1 mg/kg bw per
day) in rats.
Ethoprophos was not carcinogenic in a long-term study in mice
treated in the diet. The highest dose tested was 30 ppm, equal to 4.9
mg/kg bw per day. In three long-term studies of toxicity and
carcinogenicity in rats, some evidence was obtained that ethoprophos
may stimulate progression of C-cell tumours in the thyroid and adrenal
neoplasms (phaeochromocytomas), but this effect was considered to have
little relevance for human risk. The highest dose tested was 400 ppm,
equal to 26 mg/kg bw per day. The Meeting concluded that ethoprophos
is unlikely to pose a carcinogenic risk to humans.
Ethoprophos was tested in an adequate set of assays for
genotoxicity in vitro and in vivo. Positive results in studies for
chromosomal aberrations and sister chromatid exchanges in vitro
after metabolic activation indicate that the compound has intrinsic
genotoxic activity. Chromosomal aberrations were not induced in rats
in two assays and dominant lethal mutations were not induced in rats
in another study, although a slightly positive or equivocal result was
obtained in a further study for dominant lethality. The Meeting
concluded that ethoprophos is unlikely to be genotoxic in vivo.
In a two-generation study of reproductive toxicity in rats, no
effect was observed on reproductive parameters. Clinical signs were
observed only in the F0 parents at the highest dose (300 ppm). The
NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis of
reduced body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in animals of each sex in both parental
generations. The NOAEL for developmental toxicity was 30 ppm, equal to
1.3 mg/kg bw per day, on the basis of effects on body weight and
body-weight gain in pups of both generations and deaths of pups in the
F2 litters at 150 ppm.
In studies of developmental toxicity, maternal toxicity was
characterized by growth depression in rats in one study (NOAEL,
2 mg/kg bw per day) and in rabbits in one study (NOAEL, 0.12 mg/kg bw
per day), whereas no maternal toxicity was observed in rabbits in
another study (NOAEL, 2.5 mg/kg bw per day, highest dose tested).
Since in the first study in rabbits the effect was only marginal (not
statistically significant), the overall NOAEL for maternal effects in
rabbits was 2.5 mg/kg bw per day. No effects on the fetuses of either
species were observed. Cholinesterase activity was not measured in
these studies.
The relationship between neurotoxicity and cholinesterase
inhibition was examined in rats in two studies. In rats exposed by
gavage to single doses of 0, 20, or 40 mg/kg bw (females) or 0, 30, or
60 mg/kg bw (males), cholinesterase activity in plasma, erythrocytes,
and brain was inhibited, with a maximum effect 2 h after dosing. While
cholinesterase inhibition was found in tissues at the lowest doses
tested, clinical signs characteristic of cholinesterase inhibition
were seen only at the highest doses (60 mg/kg bw for males and
40 mg/kg bw for females). In the second study, in which male rats were
exposed to a single dose of ethoprophos at 5, 50, or 75 mg/kg bw and
females at 5, 25, or 50 mg/kg bw, effects on motor activity were
observed in animals of each sex at doses of 50 mg/kg bw and higher,
and functional and behavioural effects were observed at 25 mg/kg bw
and higher. These neurotoxic signs were seen only on the day of
dosing. Significant inhibition of acetylcholinesterase activity in
erythrocytes, measured 1 day after exposure, was observed in females
at doses of 5 mg/kg bw and higher and in males at 50 mg/kg bw and
higher. The inhibition in females was not dose-related, and there was
a large standard deviation at 5 mg/kg bw . The Meeting concluded that
the NOAEL in this study was 5 mg/kg bw.
In a 13-week study in rats, neurotoxicity was observed in tests
for motor activity and for functional and behavioural abnormalities
only at the highest dose (400 ppm). The NOAEL for neurotoxic effects
was 40 ppm, equal to 2.6 mg/kg bw per day. The activity of brain
acetylcholinesterase was inhibited in females at all doses tested; the
lowest dose was 4 ppm, equal to 0.26 mg/kg bw per day.
A study of delayed neurotoxicity in hens was performed with doses
of ethoprophos that caused a high mortality rate despite antidotal
treatment. Equivocal findings were reported in some of the treated
birds; data on the effect of this compound on neuropathy target
esterase activity in hens would be useful in order to allow full
assessment of the neuropathological potential of ethoprophos.
A study of occupational exposure to ethoprophos did not reveal
significant effects on human plasma or erythrocyte cholinesterase
activity. The calculated rates of exposure of head-and-neck areas were
3.1-78 µg/h (average, 34 µg/h), and the rate of exposure of the hands
was calculated to be 0.2-18 µg/h (average, 6.3 µg/h).
O-Ethyl S-propyl phosphorothioate, a metabolite in rats, and
O-ethyl O-methyl S-propyl phosphorothioate and O-ethyl
S-methyl S-propyl phosphorodithioate, two metabolites that have
been identified only in corn and potatoes, were tested for toxicity
and for their ability to inhibit cholinesterase activity in female
rats given single oral doses. The two plant metabolites were of
approximately the same toxicity as the parent compound, while the
animal metabolite was less toxic (LD50, 1600 mg/kg bw) than the
parent.
The 1987 Meeting derived the ADI on the basis of a slight effect
on the liver in a 52-week study in dogs at a dose of 1 mg/kg bw per
day. The NOAEL for this effect was 0.025 mg/kg bw per day. Since this
marginal effect was not observed in other studies in dogs or in other
species and there is a 40-fold difference between the LOAEL and the
NOAEL, this NOAEL was not used as the overall NOAEL to derive the ADI
in the present evaluation.
The present Meeting established an ADI of 0-0.0004 mg/kg bw on
the basis of the NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day, for
inhibition of brain acetylcholinesterase activity in the 2-year study
of toxicity and carcinogenicity in rats and in the study of
reproductive toxicity in rats, and a 100-fold safety factor.
An acute reference dose of 0.05 mg/kg bw was established on the
basis of the NOAEL of 5 mg/kg bw in the study of acute neurotoxicity
in rats, in which functional and/or behavioural effects and inhibition
of erythrocyte acetylcholinesterase were observed at the next highest
dose, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse 2 ppm, equal to 0.25 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
Rat 1 ppm, equal to 0.04 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
1 ppm, equal to 0.04 mg/kg bw per day (parental toxicity in
a study of reproductive toxicity)
5 mg/kg bw (single dose, study of neurotoxicity by gavage)
2 mg/kg bw (maternal toxicity, study of developmental
toxicity; acetylcholinesterase activity not determined);
18 mg/kg bw (fetotoxicity, highest dose tested, study of
developmental toxicity; acetylcholinesterase activity not
determined)
Rabbit 2.5 mg/kg bw (maternal toxicity and fetotoxicity, study of
developmental toxicity; acetylcholinesterase activity not
determined);
Dog 0.025 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0004 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Information on effects on neuropathy target esterase activity in
hens
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to ethoprophos
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption > 70%, mainly within 12 h (rats)
Dermal absorption Tested in vitro, no quantitative data
Penetration rate Mouse skin > rat, rabbit skin > human skin
Distribution Widely distributed, highest levels in organs of elimination (rats)
Potential for accumulation No
Rate and extent of excretion Excreted in urine (55%), expired air (13-17%), and faeces (7-9%) (rats)
Metabolism in animals In rats, metabolized by dealkylation of one or both S-propyl groups,
followed by conjugation
Toxicologically significant compounds Parent compound and plant metabolites ( O-ethyl O-methyl S-propyl
phosphorothioate and O-ethyl S-methyl S-propyl phosphorodithioate)
Acute toxicity
Rat, LD50, oral 33-62 mg/kg bw (vehicle, corn oil)
Rat, LD50, dermal 420 mg/kg bw, females
Rat, LD50, inhalation 0.25 mg/L
Dermal irritation Not reported, but death occurred within 8 h of dermal exposure
Ocular irritation Yes, but also death within 1 h of ocular exposure
Sensitization Not tested
Short-term toxicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant oral NOAEL < 0.26 mg/kg bw per day (LOAEL, 13-week study of neurotoxicity
in rats)
Lowest relevant dermal NOAEL 0.1 mg/kg bw per day (3-week study of dermal toxicity in rabbits)
Lowest relevant inhalation NOAEL Not tested
Long-term toxicity and carcinogenicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant NOAEL 0.04 mg/kg bw per day (105-week study in rats treated in the diet)
Carcinogenicity Not carcinogenic (mice, rats)
Genotoxicity Not genotoxic in vivo
Reproductive toxicity
Reproductive target/critical effect Reduced body weight and survival at maternally toxic doses
Lowest relevant reproductive NOAEL Maternal toxicity: 1 ppm, equal to 0.04 mg/kg bw per day;
fetotoxicity: 30 ppm, equal to 1.3 mg/kg bw per day
Developmental target/critical effect Only maternal toxicity (highest dose tested), no fetotoxicity
Lowest relevant developmental NOAEL Rabbit: maternal and fetotoxicity: 2.5 mg/kg bw per day (highest
dose tested)
Rat: maternal toxicity: 2 mg/kg bw per day; fetotoxicity:
18 mg/kg bw per day
Neurotoxicity/ Delayed neurotoxicity 5 mg/kg bw per day (acute toxicity in rats)
< 4 ppm, equal to 0.26 mg/kg bw per day (13-week study in rats)
No evidence for delayed neurotoxicity in hens, but some equivocal findings
Other toxicological studies No significant effects on erythrocte acetylcholinesterase activity in
exposed workers
Medical data None
Summary Value Study Safety factor
ADI 0-0.0004 mg/kg bw 2-year study, rat; two-generation 100
study of reproductive toxicity, rats
Acute reference dose 0.05 mg/kg bw Acute neurotoxicity, rats 100
References
Auletta, C.S. & Rinehart, W.E. (1979) A comparative dermal toxicity
evaluation in mice (Ethoprop and nine chemical analogs).
Unpublished report Project No. 5789-79 from Bio/dynamics Inc.,
Princeton, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985a) Ames
Salmonella/microsome plate test (EPA/OECD). Unpublished report
No. PH 301-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by RhônePoulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985b). Rat
hepatocyte primary culture/DNA repair system. Unpublished report
No. PH 311-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Barnett, J.W., Jenkins, L.J. & Parent, R.A. (1983) Evaluation of the
chronic toxicity and oncogenic potential of ethoprop in Fischer
344 rats. Unpublished report from Gulf South Research Institute,
New Iberia, Louisiana, USA. Submitted to WHO by Rhône-Poulenc.
Becker, J. & Parke, G.S.E. (1977) A primary dermal irritation study of
ethoprop 93% technical grade on abraded and non-abraded skin of
New Zealand albino rabbits. Unpublished report Lab. No. 7E-6084
from Cannon Laboratories, Inc., Reading, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
Blacker, A.M. (1987) Executive summary -- Acute dermal LD50 of
ethoprop (technical) in rabbits and rats. Unpublished report No.
87/AMB/035 from Rhone Poulenc. Submitted to WHO by Rhône-Poulenc.
Brown, D. (1986) Ethoprophos: 52 week oral (capsule administration)
toxicity study in the beagle. Unpublished report No. 4923-198/16
from Hazleton Laboratories Europe, Ltd, Harrogate, North
Yorkshire, United Kingdom. Submitted to WHO by Rhône-Poulenc.
Dearlove, G.E. (1987) Dominant lethal study of ethoprop technical
administered orally via gavage to Crl:COBS(R)CD(R)(SD)BR male
rats. Confidential report No. 218-004 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Fletcher, M.J., Springer, S.T., D'Addamio, G.H., Conzelmann, P.C. &
Pullin, T.G. (1980) Evaluation of effects of ethoprop on
reproductive performance by a three-generation reproduction study
in Fischer 344 rats. Unpublished report No. 413-858-41 from Gulf
South Research Institute. Submitted to WHO by Rhône-Poulenc.
Hamada, N.N. (1990) A five-month oral toxicity study with one-month
recovery in beagle dogs with ethoprop technical. Unpublished
report No. HLA 656-143 from Hazleton Laboratories America, Inc.
Vienna, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1989) 3-week dermal toxicity study with ethoprop
technical in rabbits. Unpublished report No. HLA 6224-152 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990a) 3-week dermal toxicity range finding study with
ethoprop technical in rats. Unpublished report No. HLA 6224-160
from Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990b) 3-week dermal toxicity study with ethoprop
technical in rats. Unpublished report No. HLA 6224-162 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Iqbal, Z.M. & Menzer, R.E. (1972) Metabolism of O-ethyl-S,S-dipropyl
phosphorodithioate in rats and liver microsomal systems.
Biochem. Pharmacol., 21, 1569-1584.
Ivett, J.L. (1989) Mutagenicity test on ethoprop technical in the rat
bone marrow cytogenetic assay. Unpublished report No. 10750-0-452
from Hazleton Laboratories America Inc., Kensington, Maryland,
USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1986) Acute dermal LD50
study with ethoprop in New Zealand white rabbits. Unpublished
report No. 3147.2 from Springborn Institute for Bioresearch,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1987) Acute dermal LD50
study with ethoprop (technical) in Sprague Dawley rats.
Unpublished report No. 3147.5 from Springborn Institute for
Bioresearch, Inc., Spencerville, Ohio, USA. Submitted to WHO by
RhônePoulenc.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of ethoprop
MCTR-603-78 in Sprague-Dawley rats. Unpublished report No. 5850
from Food & Drug Research Laboratories, Inc. Submitted to WHO by
Rhône-Poulenc. Report amended 10 June 1985.
Kopp, R., Gunzel, P., Rosskamp, G., Schmidt, M. & Schuppler, J. (1980)
Acute inhalation toxicity to the rat (M&F) of liquid ethoprophos
following single 4 hours continuous exposure (LC50). Report
(Protocol No. 449/79) from Schering AG. Submitted to WHO by
Rhône-Poulenc (in German).
Leffingwell, J.T. (1986) An exposure study of mixer/loader-applicators
working with MOCAP EC. Unpublished report from Rhône-Poulenc
Inc., New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Myhr, B.C. & Brusick, D.J. (1981) Evaluation of MOBIL No. 01238101 in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 2478-80 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by RhônePoulenc.
Neeper-Bradley, T.L. (1991) Two-generation reproduction study of
ethoprop technical administered in the diet to CD(R) (Sprague
Dawley) rats. Unpublished report No. 53-598 from Bushy Run
Research Center, Export, Pennsylvania, USA. Submitted to WHO
Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1982) Ethoprop (48 095 RP). Acute toxicity
in mice treated orally and rats treated percutaneously.
Unpublished report from Rhône-Poulenc, Vitry sur Seine, France.
Submitted to WHO by Rhône-Poulenc (in French).
Powers, M.B. (1965) V-C 9-104 (technical grade) Acute oral
administration -- Rats. Acute dermal application -- Rabbits.
Unpublished report from Hazleton Laboratories, Inc., Falls
Church, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Putman, D.L. & Schechtman, L.M. (1981) Activity of T1688 in the
dominant lethal assay in rodents. Unpublished report from
Microbial Associates, Bethesda, Maryland, USA. Submitted to WHO
by Rhône-Poulenc.
Rao (1999) Personal communication. Rhône-Poulenc, Research Triangle
Park, North Carolina, USA. Submitted to WHO by Rhône-Poulenc.
Roberts, N.L., Gopinth, C., Anderson, A. & Dawe, I.S. (1986) Acute
delayed neurotoxicity study with ethoprophos in the domestic hen.
Unpublished report No. RNP 244/86189 from Huntingdon Research
Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989a) Range-finding teratology study in rats with
ethoprop. Unpublished report No. 3147.38 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989b) Teratology study in rats with ethoprop.
Unpublished report No. 3147.39 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989c) Range-finding teratology study in rabbits with
ethoprop. Unpublished report No. 3147.40 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989d) Teratology study in rabbits with ethoprop.
Unpublished report No. 3147.41 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rucci, G.. (1979) Ethoprop technical grade; MCTR-60-78. Approximate
acute dermal toxicity (LD50) in young Yorkshire white pigs.
Unpublished report Lab. No. 6074from Food and Drug Research
Laboratories, Inc., Waverly, New York, USA. Submitted to WHO
Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1985) In vitro
chromosome aberration analysis in Chinese hamster ovary (CHO)
cells. Unpublished report No. PH 320-RP-00185 from Pharmakon
Research International Inc., Waverly, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1986) In vitro
sister chromatid exchange in Chinese hamster ovary (CHO) cells.
Unpublished report No. PH 319-RP001-85 from Pharmakon Research
International Inc., Waverly, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Schwartz, C.S. (1978) Acute oral LD50 determination of ethoprop in
female New Zealand white rabbits. Letter report to Mobil Oil
Company on FDRL study No. 5988. Submitted to WHO by
Rhône-Poulenc.
Skinner, M.J., Irwin, S.E., Schreiner, C.A., Mackerer, C.R. & Mehlman,
M.A. (1981) Metaphase analysis of rat bone marrow cells treated
in vivo with ethoprop, technical. Unpublished report No. 2476-80
from Mobil Environmental and Health Science Laboratory,
Plainsboro, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Spicer, E.J.F. (1985) Lifetime dietary toxicity and oncogenicity study
in rats. Unpublished report No. 347-029 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Study reformatted 18 May 1987. Submitted to WHO by Rhône-Poulenc.
Stankowski, L.F., Naismith, R.W. & Matthews, R.J. (1985) CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report
No. PH 314-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Stoughton, R.B. (1986) Penetration of the skin of humans, mice, rats
and rabbits by C14 ethoprop(R) emulsified concentrate and the
emulsified concentrate diluted with distilled water. Unpublished
report from the University of California, San Diego, California,
USA. Submitted to WHO by Rhône-Poulenc.
Thomson, M.A., Blackburn, G.R. & Mackerer, C.R. (1981) A murine
lymphoma mutagenesis assay, heterozygous at the thymidine kinase
locus for the determination of the potential mutagenicity of
ethoprop. Unpublished report No. 2474-80 from Mobil Environmental
& Health Science Laboratory, Plainsboro, New Jersey, USA.
Submitted to WHO by RhônePoulenc.
Weiler, M.S. (1994a) Acute oral gavage study with ethoprop in rats:
Time-related effects of ethoprop on brain, plasma and red blood
cell cholinesterase activities. Unpublished report No. HWI
6224-209 from Hazleton Wisconsin, Inc. Madison, Wisconsin USA.
Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994b) Acute neurotoxicity study with ethoprop in rats.
Unpublished report No. HWI 6224-200 from Hazleton Wisconsin, Inc.
Madison, Wisconsin, USA. Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994c) 13-week dietary neurotoxicity study with ethoprop
in rats. Unpublished report No. HWI 6224-199 from Hazleton
Wisconsin, Inc. Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Weiler, M.S. (1998) Acute oral toxicity study with ethoprop and three
ethoprop metabolites
(O-ethyl-S-methyl-S-propylphosphorodithioate,
O-ethyl-O-methyl-Spropylphosphorothioate, and
O-ethyl-S-propylphosphorothioate) in rats with determination of
effects on cholinesterase activity. Unpublished report No.
6224-246 from Covance Laboratories Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Weir, R.J. (1965) Acute eye application -- Rabbits. V-C 9-104
(technical grade). Unpublished report from Hazleton Laboratories
Inc. Falls Church, Virginia, USA. Submitted to WHO by
Rhône-Poulenc.
Weir, R.J. (1967a) Three-month dietary administration -- Rats.
Technical VC9-104. (Mocap). Unpublished report No. 230-102 from
Hazleton Laboratories Inc, Falls Church, Virginia, USA. Submitted
to WHO by Rhône-Poulenc.
Weir, R.J. (1967b) 13-week dietary administration -- Dogs. Technical
VC9-104. Unpublished report No. 230-110 from Hazleton
Laboratories, Inc, Falls Church, Virginia, USA. Submitted to WHO
by Rhône-Poulenc.
Weir, R.J. & Murphy, J.C. (1967) Neurotoxicity test -- Hens. Technical
VC 9-104. Unpublished report from Hazleton Laboratories Inc.,
Falls Church, Virginia, U.S.A. Submitted to WHO by Rhône-Poulenc.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, K.D. (1992) 104 week combined chronic toxicity and
carcinogenicity study with ethoprop in rats. Unpublished report.
Laboratory project ID: HLA 6224-151 from Hazelton Laboratories
America, Inc., Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Wolfe, G.W., Durloo, R.S. & Phipps, R.B. (1981) Rabbit teratology
study. Ethoprop technical -- 01238101. Unpublished report from
Hazleton Laboratories America, Inc. Vienne, Virginia, USA.
Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Supplementary submission, dated 29 July
1994. Submitted to WHO by Rhône-Poulenc.
Yenne, S.P. (1990) 14C-Ethoprop: Absorption, distribution,
metabolism, and excretion in the rat. Unpublished report HUK
project no. 68/102 from Hazleton UK, North Yorkshire, United
Kingdom. Submitted to WHO by Rhône-Poulenc.
Table 2. Penetration of undiluted or 1:19 diluted 14C-ethoprophos solutions through
the skin of various species (in % of applied dose)
Time Undiluted ethoprophos Diluted ethoprophos
Human Rabbit Mouse Rat Human Rabbit Mouse Rat
15 min 0 0.003 0.003 0.01 0.001 0.004 0.004 0.05
30 min 0.0004 0.008 0.01 0.05 0.01 0.02 0.12 0.15
1 h 0.0008 0.04 0.11 0.15 0.08 0.2 1.9 0.55
2 h 0.002 0.2 0.91 0.4 0.31 1.1 4.2 1.7
4 h 0.004 0.7 2.6 1.0 0.72 3.7 11 4.2
6 h 0.08 1.5 5.0 1.7 1.1 7.7 29 7.8
24 h 1.0 7.8 16 5.4 5.2 27 38 23
also formed when the 14C-ethyl- and the 14C-propyl-labelled
compound, respectively, was the substrate (Iqbal & Menzer, 1972).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of ethoprophos in animals is summarized in
Table 3. In 1983, the Meeting reported that "in the studies evaluated
the toxic symptoms in the treated species were characteristic of
anticholinesterase poisoning and usually persisted for up to 48 h in
mice and 72 h in rats surviving treatment. In general, deaths occurred
within 4 h (mice) or 1 h to 3 days (rats) post-dosing. Information on
duration of symptoms and time of death in rabbits was not available."
Ethoprophos is considered to be toxic after single oral and
dermal doses and very toxic after inhalation. The dermal and oral
LD50 values for rabbits and mice are of the same order of magnitude
and indicate efficient absorption of the compound after dermal
exposure in these species, whereas less is absorbed dermally in rats.
A single application of 0.1 ml of undiluted technical-grade
ethoprophos (equivalent to 44 mg/kg bw) into one eye of each of three
New Zealand white rabbits immediately produced moderate erythema and
vascularization of the sclera and nictating membrane. The substance
was severely toxic, since all three animals died within 1 h of
treatment (Weir, 1965).
In a study of primary dermal irritation, all six male New Zealand
white rabbits that received 0.5 ml of undiluted technical-grade
ethoprophos (purity, 93%) at a dose equivalent to 240 mg/kg bw on
clipped, abraded and unabraded skin and kept under an occlusive patch
died within the first 8 h of treatment (Becker & Parke, 1977).
Table 3. Acute toxicity of ethoprophos
Species Strain Sex Purity Vehicle Route LD50/LC50 Reference
(%) (mg/kg bw or
mg/L)
Mousea OF1 (SPF) M & F NR Water Oral 31 Pasquet & Mazuret (1982)
Rat SD M NR Corn oil Oral 62b Powers (1965)
F 33b
Ratc,d Crl:CD (SD) F NR Corn oil Oral 56 Weiler (1998)
BR VAF/ Plus
Rabbite NZW F NR NR Oral 33 Schwartz (1978)
Mouse CD-1 M NR Acetone Dermal (24 h) 18b Auletta & Rinehart (1979)
Rata CD (COBS) M & F NR Water Dermal (24 h) 226 Pasquet & Mazuret
(1982)
Ratd,f SD M NR None Dermal (24 h) 1280 Karcher et al. (1987);
F 424 Blacker (1987)
Rabbitg NZW M & F NR None Dermal (24 h) 8.5 Karcher et al. (1986);
Blacker (1987)
Pigh Yorkshire white M 94% None Dermal 327 Rucci (1979)
Rabbith Albino M & F NR None or 40% Dermal (intact 26b Powers (1965)
solution in and abraded
corn oil skin; 24 h)
Rat Wistar M & F 92% None Inhalation(4 h) 0.250j Kopp et al. (1980)
Table 3 (continued)
NR, not reported
a Ethoprophos lot No. DA 232. Clinical signs: trembling, convulsions, hypomotility, and dyspnoea in both mice and rats; no dermal
irritation in rats. Only 3 male and female mice and 2 male and female rats per dose.
b Doses reported as µl/kg bw. They were converted to mg/kg bw by multiplying them by the specific gravity of ethoprophos at
20°C, 1.094.
c Ethoprophos batch No. 51EAR122. Deaths occurred within 4 days. Clinical signs: thinness, staggered gait, salivation,
hypoactivity, hunched posture, and tremors.
d Statements of compliance with good laboratory practice and quality assurance included.
e Clinical signs: ataxia, diarrhoea, and decreased activity. Only 4 females per group. Information on duration of symptoms and
time of death not available.
f Technical-grade ethoprophos lot No. 302106001. Deaths occurred within 3 days. Clinical signs: urine staining, tremors, excess
salivation, decreased activity, ataxia, soft stools, diarrhoea, laboured breathing, lachrymation, exophthalmus, and prostration,
in general increased with increasing dose. No signs of irritation. Considerable body-weight decreases in animals that died.
g Ethoprophos lot No. 302106001. Deaths occurred within 4 days except for one on day 9. Clinical signs: prostration, laboured
breathing, salivation, loss of locomotor ability, tremor, ataxia, soft stools, diarrhoea, and lachrymation. No signs of
irritation. Considerable body-weight decreases in animals that died. Only statement of quality assurance included.
h Deaths occurred within 4 days. Clinical signs: laboured breathing, salivation, wobbly gait, and decreased activity. Erythema
was reported but no indication of severity was given. Only 2-4 males per group.
i Clinical signs: depression, laboured breathing, unsteadiness, tremors, salivation. Deaths occurred within 24 h. Very slight,
subsiding erythema observed in surviving animals 24-48 h after dosing on abraded (> 10 µl/kg bw) and unabraded skin
(31.6 µg/kg bw). Only 4 animals per group.
j 4-h LC50 expressed as actual chamber concentration and particle size < 10 µm; animals were exposed to the liquid
technical-grade material as an aerosol (nominal concentration, 1500 mg/m3) in a nose-only exposure chamber. Clinical signs:
apathy, dyspnoea, salivation (from JMPR, 1983, and slightly modified by reference to original report).
(b) Short-term studies of toxicity
(i) Oral administration
Rats
Groups of 25 male and 25 female Charles River caesarean-derived
rats were fed diets containing technical-grade ethoprophos (purity not
stated) in acetone at concentrations of 0, 0.3, 1, or 100 ppm
(equivalent to 0, 0.015, 0.05, and 5 mg/kg bw per day) for three
months. The control group was given basal diet containing 'a volume of
acetone equivalent to the volume used for the test diets'. No deaths
or treatment-induced toxic signs were observed. Growth was depressed
by < 10% in males at doses > 1 ppm and in females at 100 ppm
during the last 6 weeks of the study. Food consumption was unaffected.
Haematological, clinical chemical (only blood urea nitrogen, aspartate
aminotransferase, and glucose determined), and urinary examinations in
five males and five females per group after 1 and 3 months indicated
no significant treatment-related findings. Cholinesterase activity in
plasma, erythrocytes, and brain was measured in five rats of each sex
per group at days 4, 8, 16, and 33 and terminally in the remaining
rats. Marked inhibition (25-100%) of the enzyme activity was found at
100 ppm at all sampling intervals, the depression being greatest for
erythrocyte and least for brain acetylcholinesterase activity. Maximum
inhibition of the enzyme occurred on day 8 or 16, in all tissues. Male
rats at 0.3 or 1 ppm showed a 24-28% reduction in erythrocyte and
plasma cholinesterase activity on day 8 and of brain cholinesterase
activity at termination. In females, brain cholinesterase activity was
inhibited by 20-25% at 0.3 and 1 ppm on day 8 and at 1 ppm on day 16,
and plasma and erythrocyte cholinesterase activity was inhibited by
23-26% at 1 ppm on day 16. At the end of the study, the absolute and
relative weights of the adrenals were decreased, but not in relation
to dose, in females in all treated groups. Histopathological
evaluation of about 20 selected tissues, including the adrenals, from
five males and five females in the control and highest-dose groups
showed no significant changes attributable to treatment. No NOAEL
could be identified, as brain acetylcholinesterase activity was
inhibited at all doses (Weir, 1967a). As only five animals of each sex
were examined for haematological, biochemical, and urinary parameters,
instead of the 20 rats of each sex required by the OECD guideline, and
few parameters were tested, the study was considered unreliable and
was not used for further evaluation of the toxicology of ethoprophos.
Dogs
In a study conducted according to GLP and with a statement of QA,
ethoprophos (purity, 95.6%) was administered orally to groups of six
male and six female beagle dogs aged 6-7 months at doses of 0, 0.01,
0.025, or 1 mg/kg bw per day in corn oil in gelatin capsules for 20
weeks.Two dogs of each sex at each dose were then allowed a 4-week
recovery. The animals were observed for deaths and clinical signs.
Body weights and food consumption were recorded, and ophthalmological
examinations were performed before dosing and at sacrifice after the
exposure (week 20) and recovery (week 24) periods. Haematological,
biochemical, and urinary analyses were carried out before treatment
and during weeks 17, 20, and 24. Cholinesterase activity in plasma and
erythrocytes was measured before dosing and about 3 h after dosing
during weeks 2, 4, 8, 12, 20, and 24. Brain cholinesterase activity
was measured at sacrifice; at that time, the weights of about 10
organs were determined and about 40 tissues and all gross lesions from
all animals were examined macroscopically and microscopically.
There were no deaths, and there were no treatment-related
clinical signs or effects on body weight, body-weight gain, food
consumption, ophthalmologic, haematological, biochemical, or urinary
end-points, macroscopic or microscopic findings, or organ weights. No
histopathological changes were found in the heart. The only finding
was a statistically significant increase in platelet count in bitches
at the high dose at weeks 17 and 20. This was considered to be of no
toxicological relevance since the value was increased before
treatment, and an elevated value was observed after recovery.
Significant inhibition of plasma cholinesterase activity was observed
in bitches at the intermediate dose and males and females at the high
dose. In animals at the high dose, erythrocyte acetylcholinesterase
activity was inhibited by more than 20% in males at all times during
exposure and in females only at weeks 8 and 12, when the values
reached statistical significance in males. Brain acetylcholinesterase
activity was not inhibited. After the recovery period, plasma
cholinesterase activity was found to have returned to normal, but the
activity in erythrocytes showed some variation and it could not be
concluded that recovery had occurred. The NOAEL was 1 mg/kg bw per
day, the highest dose tested, in the absence of inhibition of brain
acetylcholinesterase activity (Hamada, 1990).
Groups of three male and three female young adult pure-bred
beagles weighing 6-12 kg were fed diets containing technical-grade
ethoprophos (purity unknown) at concentrations of 0, 1, 3, or 100 ppm
for 13 weeks, equal to 0, 0.034, 0.098, or 3.4 mg/kg bw per day for
males and 0, 0.035, 0.11, or 4.0 mg/kg bw per day for females. There
were no deaths. Emesis occurred once in two animals at 100 ppm. Body
weights, food consumption, haematological, biochemical (glucose, blood
urea nitrogen, and aspartate aminotransferase and alkaline phosphatase
activity), and urinary parameters measured at 1 and 3 months were not
significantly affected by treatment. At termination, however, males
and females at the high dose had slightly decreased erythrocyte counts
and erythrocyte volume fractions. Plasma cholinesterase activity was
measured before dosing and on days 2, 4, 8, 16, 35, 65, and 87 of
administration. The activity was inhibited by 27-88% at doses > 3
ppm in animals of each sex at virtually all of the sampling intervals
and by 20-27% at 1 ppm in males at the two last sampling times. A
> 20% depression of erythrocyte cholinesterase activity was seen in
animals at the highest dose at all but the first two or three sampling
intervals and in females at 3 ppm on one occasion (day 65) only.
Cholinesterase activity in brain was not measured. At termination, no
compound-related effects on organ weights or gross pathological
changes were noted. The only significant finding on histopathological
evaluation of about 20 tissues from each animal in the control and
high-dose groups was foci of perivascular myocardial swelling with
loss of striations in one of three female controls and two of three
males and one of three females at 100 ppm; all of these animals also
showed swelling and vacuolation of Purkinje fibres. These changes were
considered by the pathologist to represent 'an artefact induced in the
processing of the tissues'. The NOAEL was 3 ppm, equal to 0.098 mg/kg
bw per day, on the basis of depression of erythrocyte cholinesterase
activity at 100 ppm (Weir, 1967b).
Groups of four male and four female pure-bred beagle dogs aged
4-6 months were given capsules containing ethoprophos (purity, 96%)
dissolved in peanut oil at doses of 0, 0.025, 1.0, or 10 mg/kg bw per
day orally for 52 weeks. The doses were based on the results of a
4-week range-finding study. The animals were offered 400 g/day of food
and water ad libitum and were observed daily for signs of toxicity;
body weights were recorded weekly and food consumption daily. Blood
and urine were collected before treatment and after 6, 13, 26, and
52 weeks of treatment. The standard haematological, serum chemical,
and urinary examinations were performed, in addition to measurements
of plasma and erythrocyte cholinesterase activity. At the end of the
study, the animals were killed by an overdose of thiopentone sodium
followed by exsanguination. All tissues were examined grossly
in situ, and the major organs were removed and weighed, and brain
cholinesterase activity was measured. The standard set of tissues was
collected for histopathological examination. A statement of QA was
provided.
No effect of treatment on the incidence of clinical signs was
observed. Treatment did not affect the weight gain of treated males,
but a dose-related trend to decreased body weight was seen in treated
bitches throughout the study, and, at termination, bitches at the high
dose weighed about 10% less than controls. Males and females at the
high dose tended to consume about 10% less food than controls.
Erythrocyte count, haemoglobin concentration, and erythrocyte volume
fraction were statistically significantly lower in males at the high
dose than in control males at all intervals. The only change in serum
chemistry was an increase in mean aspartate aminotransferase activity
associated with decreased total cholesterol and serum albumin in males
at the high dose from 6 weeks of treatment throughout the study. This
effect was due largely to an apparent hepatotoxic response in two of
four males at this dose; in one of these dogs, aspartate
aminotransferase activity was increased by 10-fold over that of
controls at study termination, and serum alkaline phosphatase,
gamma-glutamyl transferase, and alanine aminotrans-ferase activities
were also greatly increased. The serum albumin concentration was
decreased in all males at the high dose. Plasma and erythrocyte
cholinesterase activities were depressed in a dose-related manner in
dogs at the intermediate and high doses at all intervals. At the end
of the study, plasma cholinesterase activity was decreased to 33% and
17% of the control value in males and to 58%, 19%, and 17% in females
at the low, intermediate, and high doses, respectively. The
erythrocyte cholinesterase activity in males at the end of the study
was 127%, 88%, and 39% of the control value at the low, intermediate,
and high doses, respectively, whereas the values for females were
103%, 62%, and 38% of control, respectively. Brain cholinesterase
activity was inhibited only in males at the high dose (to 56% of the
control value; significant) and females (72% of control) at the high
dose. Animals at the intermediate dose showed inhibition to 91% and
94% of the control values, respectively, which was considered not to
be toxicologically relevant. Ophthalmoscopic examination conducted
before and at the end of the study revealed no treatment-related
effects. At necropsy, no effect of treatment on absolute or relative
organ weights was seen, nor was there any effect on the incidence of
gross findings. Treatment-related histopathological changes were
restricted to the liver. Selected findings are tabulated in Table 4.
The NOAEL was 0.025 mg/kg bw per day on the basis of findings in the
liver at the low dose (Brown, 1986).
Table 4. Pathological findings in the liver of dogs treated with ethoprophos for 52 weeks
Lesion Dose (mg/kg bw per day)
Males Females
Control 0.02 1 10 Control 0.025 1 10
Vacuolation 0 0 3 4 0 0 3 4
Focal necrosis 0 0 0 2 1 0 0 1
Pigment 0 0 1 4 0 0 0 3
Kupffer-cell pigment 0 0 1 4 0 0 1 4
Fibrosis 0 0 0 4 0 0 0 4
Biliary proliferation 0 0 0 4 0 0 0 4
(ii) Dermal administration
Rats
In a range-finding study for dermal toxicity conducted according
to GLP and with a QA statement, groups of three Crl:CD(R)BR rats of
each sex received dermal applications of ethoprophos (purity, 95.6%)
in mineral oil at doses of 0, 0.3, 1, 10, 30, or 100 mg/kg bw per day
for 6 h per day on 5 days per week for 3 weeks. The animals were
observed for clinical signs and dermal irritation. Body weight and
food consumption were recorded, and cholinesterase activity was
measured in plasma and erythrocytes before dosing, at week 2, and at
the end of study (week 4). At necropsy, brain acetylcholinesterase
activity was measured. Macroscopic examinations were performed and
organ weights recorded.
Deaths occurred in the groups given 30 (2/6 rats on days 7 and 8)
and 100 mg/kg bw per day (6/6 rats, days 2-7). The clinical signs in
these animals were characteristic of cholinesterase poisoning. Small
faeces, hunched posture, and low body temperature were also observed
at 10 mg/kg bw per day, and the latter finding was also observed in
one male and one female at 0.3 mg/kg bw per day and two males and one
female at 1 mg/kg bw per day. Food consumption was lower (reaching
statistical significance in females) at 10 mg/kg bw per day in females
and 30 mg/kg bw per day in males and females during the first week of
exposure. There were no signs of dermal irritation. Statistically
significant inhibition of plasma cholinesterase activity was observed
at weeks 2 and 4 in females at 0.3 and 1 mg/kg bw per day and in
animals of each sex at 10 and 30 mg/kg bw per day. Inhibition of
erythrocyte acetylcholinesterase activity by > 20% was observed in
females at all doses at week 2 and at 10 and 30 mg/kg bw per day at
week 4 and in males at 10 and 30 mg/kg bw per day at both times. Brain
acetylcholinesterase activity was inhibited by > 50% in animals of
each sex at 10 and 30 mg/kg bw per day; the effect was dose-related
and significant at the high dose. No treatment-related changes were
observed in organ weights or in gross appearance (Henwood, 1990a).
In the main study, conducted according to GLP and with a QA
statement, groups of 10 Crl:CD(R)BR rats of each sex aged about 2.5
months received 1.4 ml/kg bw of ethoprophos (purity, 95.6%) dermally
in mineral oil at doses of 0, 0.3, 1, or 10 mg/kg bw per day for 6
h/day on 5 days per week for 3 weeks. The area of exposure on the
dorsal trunk constituted about 10% of the total body surface. The
animals were observed for clinical signs and dermal irritation. Body
weights and food consumption were recorded, and cholinesterase
activity was measured in plasma and erthrocytes before treatment, in
week 2, and at the end of study (week 4). Haematological and clinical
chemical parameters were evaluated on the day of necropsy, when the
brain was collected for analysis of acetylcholinesterase activity.
Macroscopic examinations were performed, and the weights of the brain,
kidneys, and liver were recorded. Kidneys, liver, skin, and tissues
with lesions from all rats in the control and high-dose groups were
examined microscopically.
The deaths of one control female and one at the high dose were
considered to be unrelated to treatment. Clinical signs observed in
some females at the high dose included soft and/or small faeces (three
animals) and hunched posture (one animal). During the first week of
exposure, a slightly lower (not statistically significant) body-weight
gain was observed in animals at the high dose, but food consumption
was not affected. There were no signs of dermal irritation that could
be related to treatment. In all groups, including controls, slight
erythema and desquamation associated with pustules or papules were
observed. Moderate desquamation was observed in one male and one
female at 0.3 mg/kg bw per day and in one male at 1 mg/kg bw per day.
Dose-related, statistically significant inhibition of plasma
cholinesterase activity was observed in weeks 2 and 4 in animals given
1 or 10 mg/kg bw per day. Erythrocyte acetylcholinesterase activity
was inhibited by > 20% in rats at 1 mg/kg bw per day in week 4 and at
10 mg/kg bw per day at both times, with 30% inhibition in week 2 and
37% (males) and 54% (females) in week 4. Brain acetylcholinesterase
activity was statistically significantly inhibited by 50% in males and
70% in females at 10 mg/kg bw per day. At 1 mg/kg bw per day, the
inhibition was 12% in males and 16% in females (not significant). At
0.3 mg/kg bw per day, brain acetylcholinesterase activity was not
inhibited in males and was nonsignificantly inhibited by 11% in
females. In female controls and in those at 0.3 mg/kg bw per day, but
not those at 1 mg/kg bw per day, the standard deviation of the mean
acetylcholinesterase activity was relatively high. As a result, the
inhibition in brain at 0.3 mg/kg bw per day is considered not
toxicologically relevant, whereas that at 1 mg/kg bw per day is
relevant. Other clinical findings in females were a dose-related
decrease in platelet count and a decrease unrelated to dose in the
numbers of neutrophils and lymphocytes in animals at 1 and 10 mg/kg bw
per day. These findings were considered to be due to the excessive
formation of fibrin strands in the blood of these females. The
statistically significant findings of increases in aspartate
aminotransferase activity in males at 1 mg/kg bw per day and slight
decreases in urea nitrogen in males at 0.3 and 10 mg/kg bw per day
were considered not to be related to treatment. Increased plasma
concentrations of sodium and chloride were observed in females at 0.3
mg/kg bw per day and in animals of each sex at 1 and 10 mg/kg bw per
day, but these changes were small and not related to dose. An
increased calcium concentration was seen in males at 1 mg/kg bw per
day and in males and females at 10 mg/kg bw per day. No
compound-related changes were observed in terminal body weights or
organ weights. At macroscopic examination, two of 10 males at the high
dose were found to have crusted areas on untreated skin in the
cervical and/or thoracic region, confirmed microscopically to be
multifocal erosion or ulceration of the epidermis and chronic
inflammation. Three of nine females at the high dose showed dark areas
in the glandular stomach, which was confirmed microscopically in two
rats as oedema or focal erosion or ulceration of the glandular mucosa;
and one of these females and another at the high dose showed
dilatation of the pelvis. The NOAEL was 0.3 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity in females
at 1 mg/kg bw per day. Since no sign of treatment-related dermal
irritation was seen, the NOAEL for this end-point was 10 mg/kg bw per
day, the highest dose tested (Henwood, 1990b).
Rabbits
In a study conducted according to GLP and with a QA statement,
groups of 10 Hra:(NZW)SPF rabbits of each sex, aged about 3 months,
received dermal applications of 1.4 ml/kg bw of ethoprophos (purity,
95.6%) in 4% carboxymethylcellulose at doses of 0, 0.03, 0.1, or 1
mg/kg bw per day for 6 h/day on 5 days per week for 3 weeks. The area
of exposure on the dorsal trunk constituted about 10% of the total
body surface. The animals were observed for clinical signs and dermal
irritation. Body weight and food consumption were recorded and
cholinesterase activity was measured in plasma and erythrocytes before
treatment and at the end of study (week 4). Haematological and
clinical chemical parameters were evaluated on the day of necropsy,
when the brain was collected for measurement of acetylcholinesterase
activity. Macroscopic examinations were performed, and the weights of
the brain, kidneys, and liver were recorded. Kidneys, liver, skin, and
tissues with lesions from all rabbits in the control and high-dose
groups were examined microscopically.
Deaths occurred in one male and three females at 0.03 mg/kg bw
per day and two males at 1 mg/kg bw per day. These animals and a few
others in each group except female controls showed clinical signs that
were associated with an increased incidence of mucoid enteritis
(diarrhoea, mucoid diarrhoea, and reduced food consumption), but this
finding was considered to be unrelated to treatment. Females at the
high dose had significantly lower body weights in weeks 2 and 4. The
groups in which the deaths occurred had lower food consumption, but
this was considered to be of no toxicological importance. An increased
incidence of slight-to-moderate irritation (erythema and sometimes
desquamation) was seen in all treated groups when compared with
controls, and the frequency increased with dose. The only
treatment-related change in haematological or clinical chemical
parameters was statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain at the high dose. The
inhibition in males was 37% in plasma, 42% in erythrocytes, and 49% in
brain, and that in females was 35% in plasma, 42% in erythrocytes, and
49% in brain. No inhibition of brain acetylcholinesterase activity was
found at lower doses; erythrocyte acetylcholinesterase activity was
inhibited by 12% in females at the intermediate dose, but this is
considered to be of no toxicological relevance. The terminal body
weights were significantly lower for females given 0.03 or 1 mg/kg bw
per day, which might be due to their lower food consumption. The
absolute kidney weights were significantly lower in females and
slightly lower in males at the high dose, and the relative kidney
weights were slightly (and not significantly) lower in females at this
dose. There were no treatment-related macroscopic or microscopic
changes. The NOAEL for dermal toxicity was 0.1 mg/kg bw per day on the
basis of inhibition of cholinesterase activity in brain and
erythrocytes and decreased kidney weights at 1 mg/kg bw per day. No
NOAEL could be identified for dermal irritation, as slight irritation
of the skin was observed at all doses (Henwood, 1989).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Randomly assigned groups of 80 male and 80 female B6C3F1 (SPF)
mice were fed diets containing 0, 0.2, 2, or 30 ppm of technical-grade
ethoprophos (purity, 94.6%) for 2 years. The test material and diets
were analysed periodically to ensure that the concentrations were
within acceptable limits of the nominal values. The diets and water
were provided ad libitum. Animals were examined daily for signs of
toxicity, and moribund animals were killed. Body weights were recorded
weekly for the first 26 weeks of treatment and twice weekly
thereafter. Food consumption was determined weekly. Haematological,
clinical chemical, and urinary parameters and cholinesterase activity
were measured after 26, 52, 78, and 104 weeks of treatment. Ten mice
of each sex at each dose were killed after 26, 52, and 78 weeks of
treatment, and all surviving animals were killed after 104 weeks of
treatment. Complete post-mortem examinations were conducted on all
animals killed at scheduled sacrifice and on those that died or were
killed in a moribund condition during the study.
No effect of treatment was seen on survival or the incidence of
clinical signs. Mean body-weight gain was decreased by 5-10% in males
and females at the high dose during the first 80 weeks of treatment,
but by the end of the study there was no decrease in males and only a
slight decrease (6%) in females. Treatment had no effect on food
consumption, the mean intake levels over the course of the study being
0, 0.026, 0.25, and 4.0 mg/kg bw per day for males and 0, 0.032, 0.32,
and 4.9 mg/kg bw per day for females. The only change in
haematological parameters was a decrease in the mean total leukocyte
count in all treated males: at the end of the study, the mean total
counts were: 4.1 ± 1.7, 2.3 ± 0.9, 2.1 ± 1.1, and 1.6 ± 0.5 ×
103/mm3 for controls and males at the low, intermediate, and high
doses, respectively, which were statistically significantly different
from the controls in all groups. The significance of this finding is
unclear as it was not associated with any other toxic effect that
might result from an impaired immune function. Historical control data
would have been useful for evaluatng this finding, but they were not
submitted.
Plasma and erythrocyte cholinesterase activity was inhibited in a
dose-related manner in animals at the intermediate and high doses
during the first 78 weeks of treatment, and in most cases statistical
significance was reached. The cholinesterase activity remaining in
plasma represented 65-90% of control values at the intermediate dose
and 23-34% at the high dose, whereas in erythrocytes it was 83-89% of
control values at the intermediate dose and 19-26% at the high dose.
By the end of the study, the plasma and erythrocyte cholinesterase
activities in the group at the intermediate dose were similar to those
of controls, except that the activity in plasma of females at the
intermediate dose was significantly decreased by about 20%. Brain
cholinesterase activity was inhibited only in males and females at the
high dose, with statistical significance reached in week 26 (64% of
control value for males and 71% for females), week 52 (82% of control
value in males), and week 104 (81% of control value in males and 83%
in females).
Necropsy of animals that died on test or were killed in a
moribund condition did not reveal any treatment-related lesions or an
effect on absolute or relative organ weights. No treatment-related
lesions were found at gross and microscopic examinations at interim
sacrifices. At final sacrifice, an increased incidence of
calcification of the kidney was noted in males at the high dose
(13/42, 2/45 controls) with 'basophilic changes' in the kidneys of
males only (0/45 control, 9/31 at the low dose, 13/38 at the
intermediate dose, and 24/42 at the high dose). The historical control
data that were supplied showed a high spontaneous incidence of these
lesions, with incidences of calcium deposits in males of 2.4-72% and
basophilic changes in males of 0-87%. The study authors reported that
these lesions are of spontaneous origin and do not represent
treatment-related pathological lesions. Treatment had no effect on the
incidences of tumours in specific tissues or on the total tumour
burden.
The NOAEL for toxicity was 2 ppm, equal to 0.25 mg/kg bw per day,
on the basis of inhibition of brain acetylcholinesterase activity at
30 ppm (Yamagata et al., 1984a,b).
Rats
Groups of 10 male and 20 female Fischer 344 rats were fed diets
containing technical-grade ethoprophos (purity, 95.3%) at
concentrations of 0, 60.5, 131, or 262 ppm for 8 weeks before mating
(one male to two females). (The section on methods indicates that the
F0 generation comprised 10 males and 20 females per group, but later
in the report it is stated that approximately 16 males and 32 females
at each dose were mated. The complete absence of data on the
reproduction phase of the study precluded verification of the
information. The report stated that analytical data for dietary
analyses were not available.) Ten days after the detection of a
positive vaginal smear, the females were separated from the males and
were maintained on the test diets until weaning of their pups. This
part of the study constituted the reproductive phase. Weanlings from
the reproductive phase (60 males and females per group) were selected
randomly and fed diets containing ethoprophos at 0, 4.5, 9, or 18 ppm
during weeks 0-12 and at 0, 49, 98, or 196 ppm during weeks 13-109 in
a study of carcinogenicity. The dietary levels during weeks 13-109
were equivalent to doses of 0, 2.5, 4.9, and 9.8 mg/kg bw per day.
(The mean intake over the whole exposure period was not reported.) In
this 109-week study, all animals that died or were killed in a
moribund condition, all survivors at the end of the study, and the 10
males and 10 females per group killed after 52 weeks of treatment were
subjected to gross necropsy and histopathological examination of a
wide range of tissues and all gross lesions.
The mortality rate was increased in males of the highest dose
during the first 7 months, although the rates at the end of the study
(53-63%) were comparable in all groups, including the controls. Other
than emaciation in animals at the high dose, there were no
treatment-related clinical signs. Growth was depressed at the highest
dose throughout the study and at the intermediate dose during most of
the study. Food consumption was reduced in animals at the intermediate
and high doses during the first 52 weeks. Weekly water consumption,
monitored between weeks 102 and 109, was unaffected. Haematological,
blood chemical (glucose, blood urea nitrogen, gamma-glutamyl
transferase, alanine and aspartate aminotransferase and alkaline
phosphatase activities, total protein, and cholesterol concentrations)
and urinary (pH, glucose, ketones, and protein) parameters measured at
the end of weeks 52 and 109 revealed decreased erythrocyte count,
erythrocyte volume fraction, and haemoglobin values in males at the
two higher doses and in females of the highest dose after 52 weeks.
Assay of cholinesterase in plasma, erythrocytes, and brain, conducted
at termination only, indicated a dose-related, statistically
significant 81-93% depression of plasma cholinesterase activity and a
30-68% decrease in brain cholinesterase activity in all treated
groups. Erythrocyte cholinesterase activity was not inhibited. The
weights of the spleen, liver, kidney, and testis deviated from those
of controls at the 52-week and terminal sacrifices, but essentially
only at the highest dose, with no accompanying microscopic lesions.
The gross pathologic changes seen were not significantly different
from those in controls, but histopathological examination of males at
the highest dose showed an increased incidence of 'scleral
mineralization' of the eye. No other microscopic lesions that might be
attributable to treatment were found.
The only notable oncogenic finding was a statistically
significant increase in the incidence of thyroid C-cell adenoma in
males at the highest dose at terminal sacrifice, with incidences of
2/34 in controls, 3/31 at the low dose, 1/29 at the intermediate dose,
and 9/33 at the high dose, expressed as the ratio of the number of
survivors bearing the tumour to the number examined
histopathologically. C-cell adenomas were also observed at the interim
sacrifice in one male in the control group and one at the low and one
at the high dose. The background incidence of the tumour was not
reported. The incidences of animals with benign and/or malignant
tumours, malignant tumours, or multiple primary tumours were not
significantly different in control and treated groups.
Interstitial-cell adenoma of the testis in males and pituitary adenoma
in females were the most frequently observed spontaneous tumours,
occurring in nearly 90% of males and over 50% of females in the
concurrent control group (Barnett et al., 1983). The authors
considered that the increased incidence of C-cell adenomas in males at
the high dose was a direct treatment-related effect as there was no
trend with dose. No NOAEL could be identified since inhibition of
brain acetylcholinesterase activity was seen at all doses. The LOAEL
was 49 ppm, equivalent to 2.5 mg/kg bw per day.
Groups of 70 male and 70 female Fischer 344 rats were assigned
randomly to receive diets containing technical-grade ethoprophos
(purity, 95.9%) at concentrations of 0, 1, 10, or 100 ppm (equivalent
to 0, 0.05, 0.5, or 5 mg/kg bw per day; mean intake over entire
exposure not reported) for 105 consecutive weeks. The test material
and diets were analysed periodically to ensure the stability and
homogeneity of diets. Food and water were provided ad libitum. The
animals were examined daily for mortality and morbidity and underwent
detailed physical examinations every week. The body weights were
recorded weekly for the first 26 weeks and twice weekly thereafter
until the end of the study, as were measurements of food and water
consumption. Ophthalmoscopic examinations were performed on all rats
at the beginning of the study and after 6, 12, 18, and 24 months of
treatment, and routine haematological, serological, and urinary
studies were conducted at the same intervals after the beginning of
treatment. Plasma and erythrocyte cholinesterase activity was measured
at the same intervals as other parameters, and brain
acetylcholinesterase activity was measured at interim sacrifice of 10
rats of each sex per dose after 12 and 18 months and at final
sacrifice at the end of 24 months of treatment. All animals were
killed on schedule, and those that died on test or were killed in a
moribund condition were subjected to a complete post-mortem
examination. A statement of QA was provided.
Treatment had no effect on survival, body-weight gain, or food or
water consumption. The only potential treatment-related finding at
physical examination was an increased incidence of anogenital staining
in females at the high dose during weeks 14-78 of treatment.
Haematological examination revealed decreased erythrocyte counts,
haemoglobin, and erythrocyte volume fraction, with increased mean
corpuscular volume in males and females at the high dose at all
intervals except 18 months. This change was statistically significant
only after 6 and 12 months of treatment, but not at final sacrifice. A
slight (10-20% over controls) increase in blood urea nitrogen was also
seen in these animals, which was statistically significant only at 6
months in females and at 18 months in males. A statistically
significant decrease in serum globulin content was noted after 6 and
12 months of treatment but not at final sacrifice. No toxicologically
significant alterations were found in urinary or ophthalmic
end-points.
Plasma and erythrocyte cholinesterase activity was significantly
inhibited in a dose-related manner at most intervals in males and
females at the intermediate and high doses. Erythrocyte activity was
inhibited by 28-44% in animals at the high dose, by < 20% in males at
the intermediate dose, and by 19-27% in females at 6, 12, and 18
months and 4% at the end of the study. Brain cholinesterase activity
was significantly inhibited in animals at the high dose at all
measured intervals, with inhibition of 27-35% in males and 36-48% in
females.
At the 12-month sacrifice, a statistically significant increase
of about 10% was noted in the relative weight of the spleen of males
and females at the high dose, with decreases of a similar magnitude in
the absolute and relative weights of the kidney in males at the high
dose. No significant treatment-related changes were seen on gross or
microscopic examination. At the 18-month sacrifice, no significant
alterations in organs weights were found, nor were any significant
changes noted on gross or microscopic examination. At final sacrifice,
statistically significant, 16-20% increases in the absolute and
relative weights of the thyroid/parathyroid were seen in males at the
high dose, with non-significant, 14-16% increases in males at the
intermediate dose. Gross examination revealed an increased incidence
of enlarged thyroids in males at these doses: 5/35 and 5/39,
respectively, compared with 1/36 controls. The only potentially
treatment-related lesion found on microscopic examination was an
increased incidence of parafollicular C-cell neoplasms in males at the
high dose (Table 5). The authors concluded that these changes were
statistically significant and the incidence of thyroid neoplasia was
'random and unrelated to the test article'.
Table 5. Incidence of C-cell neoplasms in male rats fed diets containing
ethoprophos
Neoplasm Dose (ppm)
0 1 10 100
C-cell adenoma
Deaths and unscheduled sacrifices 2/13 0/7 0/13 1/9
Final sacrifice 6/36 5/39 5/35 11/39
Total 8/49 5/46 5/48 12/48
C-cell carcinoma
Deaths and unscheduled sacrifices 0/13 0/7 0/13 1/9
Final sacrifice 0/36 0/39 1/35 2/39
Total 0/49 0/46 1/48 3/48
All C-cell neoplasms 8/49 5/46 6/48 15/48
The NOAEL for toxicity was 10 ppm, equivalent to 0.5 mg/kg bw per
day, on the basis of inhibition of brain acetylcholinesterase
activity, effects on erythrocyte parameters, and effects on the
thyroid at 100 ppm (Spicer, 1985).
In a study conducted according to GLP and with a QA statement,
ethoprophos (purity, 95.6%) was mixed into the diet of groups of 70
randomly assigned Crl:CD(R)(SD)BR VAF/Plus(R) rats of each sex at
concentrations of 0, 1, 60, and 600 ppm for 105 weeks. During week 3
of the study, the highest dose was lowered to 400 ppm because of the
occurrence of tremors, ataxia, and death in females. The average doses
throughout treatment were 0, 0.04, 2.7, and 20 mg/kg bw per day for
males and 0, 0.06, 3.4, and 26 mg/kg bw per day for females in the
four groups, respectively. Ten additional animals of each sex per dose
were killed after 54 weeks, while another 10 animals of each sex were
allocated to the control and high-dose groups to study recovery after
dosing for 52 weeks followed by 4 weeks of control diet. The test
material and diets were analysed regularly to ensure that the
concentrations in the diet were within an acceptable range. Food and
water were provided ad libitum, and animals were monitored twice
daily for their clinical condition. Body weights and food consumption
were determined weekly up to week 13 and at least every other week
thereafter. The clinical pathological examinations comprised
haematology, clinical chemistry, including erythrocyte and plasma
cholinesterase activity, and urinalysis at weeks 12, 26, 52, and 78 on
10 animals per group and on all surviving animals at termination of
the study, when brain cholinesterase activity was determined in all
animals. All animals were necropsied, and 41 organs from animals in
the control and high-dose groups and all those that died or were
killed before the end of the study were examined histologically.
Additionally, all macroscopic lesions and lung, liver, and kidneys
from animals at the low and intermediate doses were studied. The
thyroids of all animals were examined, and ophthalmic examinations
were done on all animals at weeks 0, 52. and 104.
Animals at the high dose showed depressed food consumption (up to
week 70 in males and up to week 20 in females) and cumulative
body-weight gain, and in males food efficiency was depressed up to
week 20. The survival rate was essentially the same in all groups up
to week 78, but by week 104 the survival rate of animals at the high
dose was higher than that of other groups. The major causes of death
were renal disease and pituitary tumours. Females at the high dose had
significantly reduced erythrocyte count, haemoglobin, and erythrocyte
volume fraction after 12, 26, 52, and 78 weeks of treatment, although
the decreases were not statistically significant at 53 or 104 weeks.
In males at this dose, these parameters were significantly reduced
only at week 26; at weeks 12, 52, 53, and 78, nonsignificant decreases
were seen, and at week 104 there was no difference from controls.
These effects were reversible in the animals allowed to recover. Males
and females at the high dose had significant reductions in total
plasma protein and globulin at weeks 12 and 26 and males also at week
52 and 53. In females, the decrease was significant only for total
protein at week 52 and was nonsignificant for both total plasma and
globulin at week 53. At week 78, only a significant decrease for total
protein was observed, while in females both parameters showed a
nonsignificant decrease. At week 104, the decreases in total plasma
protein and globulin were no longer significant in animals of either
sex. The effects on total plasma protein and globulin were reversible
after 4 weeks of control diet. Other significant changes in
haematological and blood biochemical parameters were incidental and
considered not to be related to treatment. No changes in
haematological or general blood biochemical parameters were observed
in rats at the other doses. The results of urinary analysis were
summarized in the report and indicated that males at the high dose had
significantly decreased urine volume and urea nitrogen and creatinine
concentrations at the end of the study. These findings were attributed
to a lower incidence of chronic renal disease in these animals.
Plasma cholinesterase activity was significantly inhibited
throughout the experiment, by 48-64% in males at the intermediate
dose, 62-77% in males at the high dose, 61-77% in females at the
intermediate dose, and 75-82% in females at the high dose. Erythrocyte
cholinesterase activity was also significantly inhibited throughout
the study, by 34-44% in males at the intermediate dose, 35-51% in
males at the high dose, 36-48% (36% inhibition not significant) in
females at the intermediate dose, and 41-51% in females at the high
dose. At the low dose, inhibition of plasma and erythrocyte
cholinesterase activity was highly variable but never exceeded 18% in
plasma or 6% in erythrocytes in either males or females and was not
statistically significant. Inhibition of brain cholinesterase activity
was significant at weeks 52 and 104 and amounted to 35-33% of control
values in males at the intermediate dose, 53-64% in males at the high
dose, 28-32% in females at the intermediate dose, and 64-66% in
females at the high dose. At the low dose, inhibition of brain
cholinesterase activity was maximal in females at week 104 (8%), but
the differences (whether increased or decreased) were not
statistically significant in either males or females. Both plasma and
erythrocyte cholinesterase activity recovered to about 80% of the
control activity after 4 weeks of control diet from week 52, and brain
acetylcholinesterase activity was nearly fully restored.
Both males and females at the high dose tended to have lower
terminal body weights, but the differences were not statistically
significant. At week 52, the weight of the left thyroid glands of
females at this dose was reduced both absolutely and relative to the
weight of the brain. Males that were allowed to recover showed reduced
absolute left and right adrenal weights, left thyroid plus parathyroid
weights, and heart weight; the organ:brain weight and the kidney:brain
weight ratios were also reduced. The weight of the left testis
relative to that of the body was increased. At 104 weeks, females at
the high dose had increased brain weights and decreased kidney:brain
weights. The males in this group had reduced absolute and relative (to
body) weights of the right and left kidney, heart, and right adrenal
gland.
Males at the high dose had a decreased incidence and reduced
severity of chronic nephropathy as compared with control animals,
whereas females at this dose had a reduced incidence of mineral
calcareous deposits in the renal pelvis. Males and females at the high
dose showed increased incidences of inflammation of tissues of the
tail (joints and skin) and of alveolar macrophage infiltration.
Probably as a result of this inflammation, higher incidences of
lymphoreticular cell hyperplasia, sinal ectasia, and congestion were
observed in lymph nodes at various sites in the body. The inflammatory
changes were considered to be age-related, and their increased
incidence in animals at the high dose was assumed to reflect the poor
physical condition of these animals. In females at the high dose,
increased incidences of gastric erosion and submucosal oedema were
found at terminal sacrifice. No treatment-related ophthalmic changes
were observed.
Tumours of various cell types occurred in many tissues. The only
changes that showed a clear relationship to treatment were increased
proliferative cell lesions in thyroid C-cells, the adrenal medulla,
and the uterine endometrium (Table 6). A statistical evaluation of the
tumour incidence in all animals was not provided, but according to a
statistical analysis of data on tumours found at final sacrifice,
females at the low and high doses had a significantly reduced
incidence of C-cell hyperplasia at terminal sacrifice, while males at
the low and intermediate doses had significantly increased incidences
of C-cell adenomas. The increase in C-cell carcinomas at termination
Table 6. Occurrence (%) of proliferative lesions in organs of rats given ethoprophos in the diet
Groups of animals and tumour type Dose (ppm)
Males Females
0 1 60 400 0 1 60 400
No. of animals
No. of unscheduled deaths 50 42 42 30 42 47 37 27
No. at terminal sacrifice 20 28 28 41 29 23 33 44
Total 70 70 70 71 71 70 70 71
Thyroid
C-cell hyperplasia 31 29 41 39 61 39 63 46
C-cell adenomas 11 9 13 17 14 11 16 17
C-cell carcinomas 0 0 1 4 1 1 1 3
All tumours 11 9 14 21 15 12 17 20
Adrenal
Benign phaeochromocytomas 20 10 10 7 4 3 1 3
Malignant phaeochromocytomas 0 3 3 7 0 0 0 0
All tumours 20 13 13 14 4 3 1 3
Uterus
Endometrial stromal polyps 1 1 4 8
Data are incidences in percentages of the total number of animals (terminal sacrifice plus intercurrent deaths)
was not significant. Males at the high dose showed a significant
decrease in the incidence of benign phaeochromocytomas but a
significant increase in that of malignant phaeochromocytomas at study
termination. The increased incidence of uterine endometrial stromal
polyps in females at the high dose was also significant. The study
authors indicated that C-cell and uterine proliferative lesions are
more likely to occur in old animals and their increased incidences are
attributable to the greater longevity of the animals at the high dose.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of inhibition of brain acetylcholinesterase activity at the next
highest dose (Williams, 1992).
(d) Genotoxicity
The results of studies on the genotoxicity of ethoprophos are
summarized in Table 7.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a two-generation study of reproductive toxicity conducted
according to GLP and with a QA statement, groups of 28
Crl:CD(R)(SD)BR rats of each sex, 6 weeks of age, were given diets
containing ethoprophos (purity, 95.3%) in acetone at concentrations of
0, 1, 30, or 300 ppm, equal to 0, 0.04, 1.3, or 23 mg/kg bw per day
for males and 0, 0.09, 2.6, or 27 mg/kg bw per day for females. After
10 weeks of exposure, the F0 parents were mated 1:1 to produce the
F1a generation. Treatment was continued through mating, gestation,
parturition, and lactation. On day 4 after parturition, each litter
was culled to four pups of each sex. After 3 weeks of lactation, the
pups were weaned. Owing to significant mortality among weanlings at
the high dose, pups in the control, low-, and intermediate-dose groups
were not used as parents for the next generation but instead 10 pups
of each sex were selected for necropsy. All weanlings at the high dose
and control F1a weanlings selected as parents were maintained on
their respective diets until day 49 post partum; the weanlings at
the high dose and 10 controls of each sex were then necropsied. From
about 1 week after weaning of the last F1a pups, F0 parents at 300
ppm were fed a diet containing 150 ppm of ethoprophos (equal to 7.1
mg/kg bw per day for males and 14 mg/kg bw per day for females), and
all F0 animals at the other two doses were maintained on their
original diets. After 3 weeks, the F0 parents were mated again to
produce the F1b generation, mating, gestation, parturition,
lactation, and weaning being conducted as described above. One week
after weaning, 28 F1b pups of each sex at each dose were selected as
parents of the F2 generation. The remaining F1b pups and all F2
pups were necropsied. Parental F0 animals aged 35-36 weeks and
parental F1 animals aged 20-21 weeks were killed after weaning of
the F1b and F2 progeny, respectively, and neccropsied grossly.
Blood was taken just before sacrifice, and brain tissue was collected,
for analyses of cholinesterase in plasma, erythrocytes, and brain. The
Table 7. Results of studies of the genotoxicity of ethoprophos
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutationa,b S. typhimurium 10-1000 µg/plate in NR Negative ± S9 Barfknecht et al. (1985a)
TA98, TA100, TA1535, DMSO
TA1537, TA1538
Gene mutationa Mouse lymphoma 0.24-0.032 µg/ml 95 Negative Thomson et al. (1981)
L5178Y cells, Tk locus (total growth between 18
and 126%, respectively),
- S9
0.024-0.0032 µg/ml
(total growth between 10
and 106%), + S9
Gene mutationc,d Chinese hamster ovary 0-500 µg/ml - S9 NR Negative Stankowski et al. (1985)
cells (CHO-K1-BH4); 0-150 µg/ml + S9
Hprt locus in DMSO
Chromosomal Chinese hamster ovary 0-300 µg/ml - S9 NR Negative SanSebastian et al. (1985)
aberrationsc,e cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivef
Unscheduled DNA Rat hepatocytes 2.5-100 nl/ml in DMSO; NR Negative Myhr & Brusick (1981)
synthesisg,h toxic from 50 nl/ml
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Unscheduled DNA Rat hepatocytes 0-333 µg/well in DMSO NR Negative Barfknecht et al. (1985b)
synthesisg,i (male Fischer 344)
Sister chromatid Chinese hamster ovary 0-350 µg/ml - S9 NR Negative SanSebastian et al. (1986)
exchangec,j cells (CHO-K1-BH4) 0-60 µg/ml + S9 in DMSO Positivek
Table 7. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Chromosomal Rat (SD) bone 0-20 mg/kg bw per day for 5 days 95.7 Negative Skinner et al. (1981)
aberrationl marrow by gavage in Methocel K4M
premium; sacrifice 6 h after dosing
Chromosomal Rat (SD) bone 0-25 mg/kg bw once by gavage; 95.5 Negative Ivett (1989)
aberrationm,n marrow sacrifice 6, 18, and 30 h after
dosing 0-25 mg/kg bw per day for
5 days by gavage; sacrifice 6 h
after dosing
Vehicle, corn oil.
Dominant lethal Rat (SD) 0-20 mg/kg bw per day for 5 days NR Equivocal Putman & Schechtman
mutationo by gavage in corn oil (1981)
Dominant lethal Rat (Crl:COBS 0-20 mg/kg bw per day for 5 days 95 Negative Dearlove (1987)
mutationm,p CD(SD)BR) by gavage in carboxymethyl
cellulose
NR, not reported; S9, 9000 × g supernatant of rat liver; DMSO, dimethylsulfoxide; SD, Sprague-Dawley
a Test in triplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
b Cytotoxicity (small colonies) seen in preliminary test at 1666 µg/plate and no growth at 5000 µg/plate in TA1538 and TA100
c Test in duplicate; positive controls included; S9 fraction of Aroclor 1254-induced rat liver; GLP and QA statements included
d Cytotoxicity observed at doses > 167 µg/ml; relative initial survival, 18% at 350 µg/ml without S9 and 10% at 150 µg/ml with S9
e Only 100 cells/dose scored instead of 200/dose required by OECD; cytotoxicity at doses > 500 µg/ml without S9 and at 80 µg/ml
with S9, and average proliferation time increased at 400 and 60 µg/ml, respectively
f Statistically significant increase in aberrations seen with metabolic activation at 60 µg/ml. In a second test at concentrations
of 50-70 µg/ml, statistically significant but not dose-related increase at all concentrations (14-22 breaks compared with 2 breaks
in control). The authors considered this a weakly positive result. Although the results of the second test were not dose-related,
they confirm the positive result of the first test.
Table 7 (continued)
g Test in triplicate; positive controls included; GLP and QA statements included
h Purity, 95% (personal communication from Dr Rao, Rhone Poulenc). Toxicity observed as 73% survival at 50 nl/ml and 0% at 100 nl/ml.
i Cytotoxicity (abnormally low nuclear and cytoplasmic grains) at doses > 333 µg/well
j Cytotoxicity at doses > 500 µg/ml without S9 and > 80 µg/ml with S9, and average proliferation time was increased at 400 and
60 µg/ml, respectively.
k Statistically significant, dose-dependent increase with metabolic activation at 50-60 µg/ml. In a second test at concentrations of
50-75 µg/ml, statistically significant, dose-related increase at all concentrations (22-35 aberrations/cell compared with 16 per
cell in controls). Since there was no twofold increase at any dose, the authors considered the compound a weak inducer.
l Aberrations analysed in 5 males/group; positive control included; absorption of compound tested in 1 animal/group and confirmed
by a minimum of a 10-fold depression in cholinesterase activity in plasma at 20 mg/kg bw per day for 5 consecutive days. Clinical
signs: decreased motor activity at high dose (day 2); mitotic index not measured. Not clear that the bone-marrow cells were
exposed to the test substance. Only 50 cells scored instead of 100 cells/animal required by OECD.
m GLP and QA statements included
n Each group of dose and harvest time consisted of 5 rats of each sex. Positive control group included in single-dose study only.
Deaths observed only in females at high dose. Clinical signs at high single dose: dyspnoea, languid appearance, and diarrhoea.
Additional signs at high multiple doses: tremor, rough coat, hunched posture, and dark crusts around eyes. No decrease in mitotic
index after single dose, but slightly decreased (54-75%) in males in short-term study. It is not clear whether the bone-marrow
cells were exposed to the test substance. Aberrations were studied in only 50 cells/animal instead of 100 cells and mitotic
index studied in only 500 cells instead of 1000 as required by OECD.
o 10 male rats/group. Three days after the last dose, males were mated with 2 untreated virgin females. Mating process repeated
another 6 times once a week with new virgin females. Females were killed about 2 weeks after mating; scoring for number of corpora
lutea number of dead and live implants per pregnant female. Absence of body-weight gain in males at high dose. No effect on
fertility index, number of corpora lutea, or number of implantations. Pre-implantation loss statistically significantly increased
in week 3 at high and low doses. Number of dead implants increased in week 1 at low dose, weeks 2-3 at intermediate dose, and weeks
1-6 at high dose, with a positive dose-response relationship. The proportion of females with one or more dead implants was
significantly increased at the high and intermediate doses (week 2 and weeks 2-3, respectively) and at the high dose also of females
with two or more dead implants. The number of dead implants per total number of implants was significantly increased at the high
dose (weeks 2-5) and intermediate dose (week 5) (0.08-0.12 in test groups compared with 0.02-0.05 in control group). The number of
live implants per pregnant female, however, was not affected (change in live implant ratio is the criterion of dominant lethality).
In the positive control group, more marked increases in dead implants per total implants observed in weeks 1-4 (0.6-1.0), and the
number of live implants per pregnant female was significantly decreased (0.0-8.3 compared with 9.9-11.9 in controls). The authors of
the report concluded that the compound was mutagenic; however, the shortcomings of the test and the fact that the number of live
implants was not affected indicate that the result is equivocal. The shortcomings of the study were that only 12-18 pregnant females
were included in each treated group and 15-17 in the control group, whereas according to OECD 478 30-50 pregnant females per mating
interval are required; mating was not confirmed. Only QA statement included.
Table 7 (continued)
p 24 males/group. Doses based on a range-finding study. Two days after the last dose, males were mated with 2 untreated virgin
females. Mating process was repeated another 7 times once a week with new virgin females. Females were killed 14 days after
presumed gestation; scoring for number of corpora lutea and for number of dead and live implants per pregnant female. Other
observations: viability, clinical signs, body weight, food consumption, gross necropsy of males and females, especially reproductive
organs (including histopathological examination). Three males at high dose died showing clear signs of intoxication. One male was
replaced. Clinical signs at high dose were characteristic of cholinesterase poisoning. Body-weight gain was inhibited (dose-related)
during treatment at 5 and 20 mg/kg bw per day, and food consumption was lower at the high dose. No compound-related findings at
gross necropsy except a single finding of an epididymal mass in one male at 5 mg/kg bw per day; no effect on testis weight, mating
index, fertility index, or pregnancy rate. Average numbers of corpora lutea, implantations, litter sizes, resorptions, percentage
of dead conceptuses, and number of females with resorptions similar in treated and control groups, except a single finding of dams
with increased resorptions in week 4 of mating at the intermediate dose.
thyroid glands were weighed, and selected tissues (especially from the
reproductive organs) from control and high-dose animals were examined
histopathologically.
The dietary concentrations of 1, 30, and 300/150 ppm during the
first premating and mating periods were equal to 0.07, 2.2, and 23/12
mg/kg bw per day for F0 and F1 males and 0.09, 2.7, and 27/14
mg/kg bw per day for F0 and F1 females. During the second mating
period, the doses of 1, 30, and 150 ppm given to F0 males were equal
to 0.04, 1.3,and 7.1 mg/kg bw per day; the intake of females,
calculated only for weeks 20-24, was 0.07, 2.0, and 11 mg/kg bw per
day.
The clinical signs in F0 parents at the high dose were soft
faeces during the first 2 weeks of exposure and tremors in females
during gestation and lactation. No clinical signs were observed in
F1 parents. The body weights of F0 males at 300 ppm and F1 males
at 150 ppm were significantly decreased throughout the exposure
period. F0 males at 300 ppm had significantly lower body-weight
gains during several weeks, whereas an increase in body-weight gain
was observed occasionally when the dose was changed to 150 ppm. The
weight gain of F1 males at the high dose was reduced during the
premating period and occasionally thereafter. Significantly decreased
body-weight gains were observed occasionally in males at 30 ppm but
these did not result in significantly lower body weights. F0 females
at the high dose had significantly reduced body-weight gains in weeks
1 and 9, but these did not result in lower body weights during the
premating period for the F1a litter. The total body-weight gain
during the 0-20 days of gestation with the F1a litter was
significantly reduced, and the body weights were lower during
lactation. These lower (but not statistically significant) body
weights of F0 females at the high dose persisted during the
premating and mating periods for F1b progeny and reached statistical
significance on days 4-21 of lactation, although there was no effect
on body-weight gain. The body weights of F1 females at the high dose
were significantly reduced throughout premating, gestation, and
lactation, but with no effect on weight gain. Food intake was affected
only at the high dose, with lower consumption observed for F0 males
in weeks 1, 2, and 20 and for F1 males in weeks 9, 11, and 16-20.
The food consumption of females of the F0 generation at the high
dose was lower during the first week of exposure, but food consumption
was affected only on days 4-14 of gestation of the F1a generation.
Length of gestation and indices of mating, fertility, and gestation
were not affected in either generation. The final body weights of F0
and F1 males at the high dose were significantly reduced, and only
in F1 males at this dose the absolute, but not the relative, weight
of the thyroid was significantly lower. No treatment-related lesions
were observed on gross or histopathological examination.
Cholinesterase activity was significantly inhibited in plasma and
brain of F0 and F1 animals of each sex at the high dose, in F1
animals at 30 ppm, and in F0 females at 30 ppm; plasma
cholinesterase activity was also inhibited in F0 males at 30 ppm.
There was no effect on erythrocyte acetylcholinesterase activity.
Analyses of data for the litters revealed lower body-weight gains
in F1a pups at 300 ppm throughout lactation, resulting in lower body
weights from day 7 onwards in males and from day 4 in female pups. The
survival of F1a pups was not affected up to weaning, but the
survival index was only 51% 1 week after weaning on day 28. Necropsy
of dead F1a pups showed blood in the stomach and/or intestines. The
body weights of surviving F1a pups at the high dose remained reduced
up to sacrifice on day 49, and their body-weight gain was reduced up
to day 35. F1b pups at the high dose of 150 ppm showed lower
body-weight gain, from the second week post partum onwards for
females and from the third week for males, resulting in lower body
weights from day 14 and day 21, respectively. Survival was not
affected. Significant reductions in average litter size at 1 ppm,
beginning on day 4, were considered spurious owing to the lack of a
dose-response relationship. The litter sizes and sex ratios of F2
litters were unaffected by treatment. The decreased litter sizes, and
as a consequence higher pup body weights, at 1 and 30 ppm and the
reduced sex ratio at 30 ppm were considered not to be related to
treatment in the absence of a dose-response relationship. The
body-weight gain of pups at the high dose was reduced from day 4
onwards, resulting in lower weights from lactation day 7. Reduced
weight gain was found on days 4-7 of lactation in the group receiving
30 ppm, with a (probably) compensatory increase on days 14-21, but
this was considered irrelevant toxicologically. Deaths among F2 pups
at the high dose on days 7-14 resulted in a significantly reduced
14-day survival index and lactation index. No treatment-related
lesions were observed in dead F2 pups or in F1a, F1b, and F2
pups subjected to scheduled necropsy 1 week after weaning.
The NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis
of lower body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in parents of each sex in each
generation. No effects were observed on reproductive parameters. The
NOAEL for toxicity to pups was 30 ppm, equal to 1.3 mg/kg bw per day,
on the basis of effects on body weight and body-weight gain and deaths
in F2 litters at 150 ppm (Neeper-Bradley, 1991).
Groups of 10 male and 20 female Fischer 344 rats, 44 days old,
were fed diets containing technical-grade ethoprophos (purity, 95.3%)
at concentrations of 0, 60.5, 131, or 262 ppm (equivalent to 0, 3.0,
6.6, and 13 mg/kg bw per day) for 56 days before mating. Weanlings
from the second litters were selected to be the parents of the next
generation. All parental animals and weanlings of each generation were
subjected to gross pathological examination. Additionally, all F1b
and F2b parents and five male and five female weanlings from each of
the F1a, F2a, F3a, and F3b litters were evaluated
histopathologically. A statement of QA was provided.
The mortality rate was unaffected but growth was slightly
depressed at the two higher doses in all parental generations. Data on
food consumption were not available. A dose-related increase in the
number of dams that cannibalized their young was noted in all treated
groups of the F0 generation, mainly with regard to the second
litters. The percentage of animals becoming pregnant was reduced in
all treated F1a litters (75, 60, 55, and 55%, respectively, at 0,
60.5, 131, and 262 ppm) and for F3a and F3b litters at 262 ppm.
The lactation index (pup survival on days 4-21) was decreased at both
131 and 262 ppm in the F1b (56% and 40%, respectively, compared with
89% in controls) and the F2a generation (86% and 67%, respectively,
compared with 100% in controls) and at the high dose in the F2b
generation (67% compared with 88% in controls). Weanling weight (not
determined for F1a and F1b litters) was depressed in the F2a and
F2b litters at 131 and 262 ppm and in the F3a litters at 262 ppm.
Other effects that were observed only at the highest dose included a
tendency for decreased litter size at birth in almost all generations
of progeny, prolongation of the mean gestation period of F1b litters
(this parameter was not evaluated in all generations), the occurrence
of bilateral lenticular opacity (cataracts; in 29%) and anophthalmia
(3%) in F2a weanlings (information on the litter distribution of
these abnormalities was not available), and an increased incidence of
multifocal granuloma of the mesenteric lymph nodes in F2b adults.
The increased incidence of cannibalization among F0 dams and the
slight decrease in pregnancy rate in F1a litters at 60.5 ppm did not
recur in all generations and were probably related to maternal
toxicity. This concentration was considered to be the minimal
no-effect level for reproductive toxicity. As enzootic pneumonia was
observed in all parental animals and in some pups, the results of this
test are unreliable (Fletcher et al., 1980).
(ii) Developmental toxicity
Rats
A preliminary dose-range finding study was conducted according to
GLP and with a statement of QA, in which five female Sprague-Dawley
COB SC rats were given ethoprophos (purity, 95.6%) in corn oil at
doses of 0, 0.2, 1, 4, 8, or 16 mg/kg bw per day by gavage on days
7-15 of gestation. Fetuses were removed surgically on day 20 and
examined for external anomalies. The only finding was body-weight loss
in dams at the highest dose during days 6-9 of gestation and reduced
body-weight gains (24%) throughout treatment. There were no signs of
developmental toxicity (Rodwell, 1989a).
In the main study, conducted according to GLP and with a
statement of QA, groups of 25 pregnant female
Crl:COBS(R)CD(R)BR(R)VAF rats aged 3 months were given
ethoprophos (purity, 95.6%) by gavage in corn oil at doses of 0, 2, 9,
or 18 mg/kg bw per day on days 6-15 of gestation. The dams were
observed for clinical signs, and body weight and food consumption were
measured. Fetuses were removed surgically on day 20, weighed, and
examined for external, visceral, and skeletal anomalies.
Dose-dependent maternal toxicity was observed at 9 and 18 mg/kg
bw per day, with treatment-related findings including soft stools at
both doses and faecal staining at the high dose. Significantly lower
body-weight gain was observed at 9 mg/kg bw per day on days 6-9 of
gestation, and during this period females at the high dose showed
body-weight loss which resulted in lower body weights by the end of
the study. The food consumption of animals at the high dose was
reduced on days 6-16 of gestation. No effect was found on survival, no
morphological changes were observed at necropsy, and no changes in
gravid uterine weight or fetal parameters (viability, resorptions,
body weight, and sex ratio) were found. The incidences of external,
visceral, and skeletal variations and of irreversible structural
changes were unchanged. The NOAEL for maternal toxicity was 2 mg/kg bw
per day, and that for developmental toxicity was 18 mg/kg bw per day
(Rodwell, 1989b).
Groups of 25-35 mated female TAC:N(SD)FBR rats were intubated
with technical-grade ethoprophos (purity, 94%) in corn oil at doses of
0, 0.16, 1.6, or 16 mg/kg bw per day on days 6-15 of gestation, the
day a vaginal sperm plug was observed being considered day 0. The dams
were killed on day 20 and the fetuses removed for gross, skeletal, and
visceral examination. A positive control group was included.
The pregnancy rate was comparable in all groups. During
gestation, 1/24 pregnant dams at 1.6 mg/kg bw and 18/30 at 16 mg/kg bw
died or aborted; in addition, three non-pregnant dams at the high dose
were found dead. Growth was depressed on days 15 and 20 of gestation
in dams at 16 mg/kg bw. No significant difference was found between
control and treated groups in the mean numbers of corpora lutea,
implantation sites, live fetuses, dead fetuses or resorptions, mean
fetal weight, or sex ratio. According to a summary of the skeletal
findings and soft-tissue abnormalities in fetuses, the only notable
observation was a significant increase in the incidence of litters
containing fetuses with extra lumbar ribs in animals at 1.6 mg/kg bw.
Since no dose-response relationship was seen, the authors could not
attribute this finding to exposure to ethoprophos. Incomplete
ossification of vertebrae was observed in all groups, including the
controls, with incidences of 18, 10, 7, and 17% in control, low-,
intermediate-, and high-dose groups, respectively). The NOAEL for
maternal toxicity was 1.6 mg/kg bw per day on the basis of deaths and
depressed body-weight gain, and the NOAEL for developmental toxicity
was 16 mg/kg bw per day (Knickerbocker & Re, 1979, and amendment in
1985).
Rabbits
A preliminary dose-range-finding study was conducted according to
GLP and with a statement of QA in which groups of eight female New
Zealand white rabbits received ethoprophos (purity, 95.6%) in corn oil
at doses of 0, 0.1, 0.5, 2, 5, or 10 mg/kg bw per day by gavage on
days 7-18 of gestation. The fetuses were removed surgically on day 29
and observed for external anomalies. Dose-dependent maternal toxicity
was observed at 5 and 10 mg/kg bw per day, the clinical signs
including emaciation, an increased incidence of soft stools, and
faecal and urine staining. Consistent body-weight loss and a
dose-dependent mortality rate were also seen in these two groups.
There were no signs of developmental toxicity (Rodwell, 1989c).
In the main study, conducted according to GLP and with a
statement of QA, groups of 20 artificially inseminated New Zealand
white rabbits aged 6 months received ethoprophos (purity, 95.6%) by
gavage in corn oil at doses of 0, 0.625, 1.25, or 2.5 mg/kg bw per day
on days 6-18 of gestation. The does were observed for clinical signs,
and body weight and food consumption were measured. The fetuses were
removed surgically on day 29, weighed, and examined for external,
visceral, and skeletal anomalies.
There were no clinical signs of maternal toxicity, no adverse
effects on survival, body weight, or food consumption, and no
morphological changes. Gravid uterine weight and fetal parameters
(viability, resorptions, body weight, and sex ratio) were also
unchanged. The finding of a significantly reduced mean number of
viable fetuses per doe at 1.25 mg/kg bw per day was due to the absence
of this effect in the group at the high dose and considered not to be
related to treatment. The incidences of external, visceral, and
skeletal variations were not altered, as the finding of a decreased
incidence of 27 presacral vertebrae in fetuses at the intermediate and
high doses was considered not to be toxicologically relevant.
Increased incidences of cleft palate, flexed paw, gastroschisis, and
cephalocoele seen on external examination and of atelectasis and
hydrocephaly seen on visceral examination of the group at the
intermediate dose when compared with controls and with historical
control values were observed in fetuses of only a single doe and were
considered not to be related to treatment. The incidences of fetuses
with malformations were 2.4, 4.1, 4.0, and 6.6% and those of litters
with malformed fetuses were 12, 11, 11, and 35% in the control, low-,
intermediate-, and high dose groups, respectively. The malformations
seen at the high dose were dissimilar and occurred once or at very low
frequency; furthermore, they were types that occur spontaneously in
this strain of rabbits and the incidences were within the historical
control values. In the absence of effects on fetal weight, the
increased incidences of irreversible structural changes at the high
dose were considered to be unrelated to treatment. The NOAEL for both
maternal and developmental toxicity was 2.5 mg/kg bw per day, the
highest dose tested (Rodwell, 1989d).
Groups of 17 artificially inseminated female New Zealand white
rabbits were intubated with technical-grade ethoprophos (purity,
95.7%) in corn oil at doses of 0, 0.125, 0.5, or 2 mg/kg bw per day on
days 6-18 of gestation, the day of insemination being considered day
0. The surviving does were killed on day 29 of gestation, and their
fetuses were removed for external, visceral, and skeletal
examinations. A statement of QA was provided.
No treatment-related deaths occurred, but does at all doses
showed an increased (but not dose-related) incidence of anorexia
during and after treatment. A dose-related but not statistically
significant decrease in mean body-weight gain was observed between
days 6 and 18 in the does at the two higher doses. Control and treated
groups did not differ significantly with respect to pregnancy rate,
mean numbers of corpora lutea, implantations, resorptions, dead
fetuses, or live fetuses, mean fetal weight, mean fetal crown-rump
length, sex ratio, uterine weight with or without fetuses, or the
frequency of gross and visceral abnormalities. An increased total
incidence of skeletal variants was observed in the litters of all
treated does, with 14% at the low dose, 13% at the intermediate dose,
and 17% at the high dose, and 6% in controls. The increase was not
dose-related, and the frequency of any particular type of skeletal
variant, when considered alone, did not show a dose-response
relationship. The NOAEL for maternal toxicity was 0.12 mg/kg bw per
day on the basis of reduced body-weight gain. The NOAEL for
developmental toxicity was 2 mg/kg bw per day (Wolfe et al., 1981).
(f) Special studies: Neurotoxicity
Rats
In a study conducted according to GLP and with a statement of QA,
groups of 24 Crl:CD(SD)BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single dose of ethoprophos (purity, 95.7%) by gavage
in corn oil. Male rats received doss of 0, 30, or 60 mg/kg bw and
females received 0, 20, or 40 mg/kg bw. The doses were based on a
range-finding study. The animals were observed daily for deaths and
clinical signs, and body weights were recorded weekly. Blood samples
were taken from six animals of each sex per group 2 h and 3, 8, and
15 days after dosing to determine cholinesterase inhibition in plasma
and erythrocytes. Brain acetylcholinesterase activity was determined
in the frontal cortex, caudate/putamen, hippocampus, and cerebellum of
the same animals at the same times.
One male rat given 60 mg/kg bw was found moribund. The clinical
signs observed in females at 40 mg/kg bw and males at 60 mg/kg bw were
tremors and excessive salivation. Males also showed hunched posture,
laboured breathing, rough and stained coats, pale bodies, ocular
discharge, incoordination, hypoactivity, and were cold to touch. Among
animals at the lower dose, the only clinical sign was cold to touch in
one female at 20 mg/kg bw. There were no effects on body weight.
Cholinesterase activity was inhibited in all tissues examined by 2 h
after treatment, and the effects were dose-, time-, and
tissue-dependent (Table 8). Recovery of cholinesterase activity was
apparent by day 3 or 8 after dosing and was complete by day 15 for
plasma and cerebellar cholinesterase activity. Statistically
significant inhibition of acetylcholinesterase activity persisted in
the hippocampus through day 15 at the high dose and in erythrocytes
and the frontal cortex of males at both low and high doses. Persistent
inhibition was observed in the caudate/putamen at both doses, but the
inhibition was not statistically significant (Weiler, 1994a).
In a study conducted according to GLP and with a statement of QA,
groups of 17 Crl:CD(R) BR VAF/Plus rats of each sex, about 6 weeks
old, were given a single oral dose of ethoprophos (purity, 96.2%) by
gavage in corn oil. The doses were 0, 5, 50, or 75 mg/kg bw for males
and 0, 5, 25, or 50 mg/kg bw for females, which were based on
range-finding studies. The animals were observed daily for deaths and
Table 8. Cholinesterase activity in various tissues of rats after a single oral dose of ethoprophos
Tissue Dose Inhibition from control mean (%)a
(mg/kg bw)
Males Females
Day 1b Day 3 Day 8 Day 15 Day 1b Day 3 Day 8 Day 15
Plasma cholinesterase 30/20 83* 43* 6 12 90* 31* 27 6
60/40 93* 68* 19* 14 94* 52* 23 0
Erythrocyte cholinesterase 30/20 43* 46* 31* 23* 44* 48* 29* 17
60/40 53* 46* 33* 22* 49* 50* 35* 17
Brain cholinesterase in 30/20 45* 38 30 9 72* 25 54 25
caudate/putamen 60/40 93* 75* 51 32 92* 63* 39 40
Brain cholinesterase in 30/20 45* 30* 19* 0 50* 14 8 4
hippocampus 60/40 72* 47* 27* 18* 75* 29* 21* 17*
Brain cholinesterase in 30/20 48* 38* 34 19* 55* 10 0 32
frontal cortex 60/40 76* 50* 49* 27* 77* 40* 24* 29
Brain cholinesterase in 30/20 46* 11 0 0 60* 11 0 3
cerebellum 60/40 81* 35* 7 6 80* 29* 9 3a
a 100% - mean treated/mean control × 100%
b 2 h after dosing
* Significantly different from control at p < 0.05
clinical signs, and body weights were recorded before treatment and on
days 1, 8, and 15. Twelve animals of each sex per group were subjected
to a functional observational battery of tests and tests for motor
activity before treatment and at 2 h and 8 and 15 days after dosing.
Cholinesterase activity was determined in plasma and erythrocytes from
the remaining five animals of each sex per group before treatment and
on days 2, 8, and 15 after dosing. After blood collection on day 15,
the animals were killed and their brains collected for determination
of acetylcholinesterase activity. Neuropathological examinations were
conducted at the end of the study on all appropriate tissues from six
rats of each sex per group that had not been used to determine
cholinesterase activity. All animals were examined macroscopically and
only the control and high-dose groups microscopically.
Treatment-related deaths were seen in two males given 75 mg/kg bw
and six females given 50 mg/kg bw; four of the females were replaced.
The deaths of one male at 50 mg/kg bw and one female at 5 mg/kg bw
were considered to be unrelated to treatment. Clinical signs in
animals at the high doses included thin or hunched appearance,
tremors, laboured respiration, protruding eyes, incoordination,
hypoactivity, excessive salivation, and cold to touch. A few males at
50 mg/kg bw also showed some of these signs. The results of the
functional battery showed mild to severe functional or behavioural
changes only on the day of dosing, and the changes were characteristic
of cholinesterase poisoning. No effects were seen at 5 mg/kg bw dose.
Mild effects were seen in two females at 25 mg/kg bw and in several
males at 50 mg/kg bw, which included salivation, lip smacking, ataxia,
negative pupillary response, and tremors. Moderate and severe effects
were observed in females at 50 mg/kg bw and males at 75 mg/kg bw,
which included, in addition to the effects described above, low
carriage and prostrate position, lethargy, changes in the ease of
handling, lachrymation, laboured or gasping respiration, increased
latency until first step, paralytic gait, negative corneal response,
and negative air drop and startle reflexes. The analgesic reflex to a
mild heat stimulus was delayed significantly on day 1 in males and
females at 50 mg/kg bw and in males at 75 mg/kg bw. Body temperature
was statistically significant decreased by 3-4°C in females at 50
mg/kg bw and in males at 75 mg/kg bw; a slight decrease in body
temperature was seen in males at 50 mg/kg bw. Mean forelimb grip
strength was decreased only on day 1 in males at 75 mg/kg bw and
females at 50 mg/kg bw and was statistically significant only in
males. The NOAEL for behavioural and functional effects was 5 mg/kg
bw. Motor activity was markedly and statistically significantly
decreased in males at 50 and 75 mg/kg bw (dose-related) and in females
at 50 mg/kg bw on day 1 (Table 9). Differences were no longer seen on
day 8 or 15. The NOAEL for motor activity was 5 mg/kg bw.
The effects on cholinesterase activity are summarized in Table
10. On day 2, plasma cholinesterase activity was dose-dependently
inhibited for males (45-94%) and females (49-94%) at all doses but had
recovered within 8 days of dosing. Erythrocyte acetylcholinesterase
activity was inhibited in females at all doses (not dose-related) and
in males at 50 and 75 mg/kg bw. Generally nonsignificant inhibition of
Table 9. Motor activity (mean total counts) measured shortly after dosing (day 1) and during 10-min
intervals for 60 min in rats given single doses of ethoprophos
Dose Motor activity (mean (standard deviation))
(mg/kg bw)
Males Females
0-10 min 10-20 min 2-30 min Total 0-10 min 10-20 min 20-30 min Total
(0-60 min) (0-60 min)
0 720 (130) 280 (140) 100 (120) 1300 (510) 580 (86) 280 (140) 98 (100) 1200 (330)
5 630 (110) 300 (160) 51 (64) 1000 (270) 650 (160) 200 (180) 69 (110) 1200 (460)
25 480 (270) 170 (210) 78 (140) 1100 (960)
50 290* (240) 150* (110) 20 (25) 670* (430) 38* (34) 28* (24) 44 (80) 240* (190)
75 33* (28) 25* (22) 17 (19) 180* (100)
* Significantly different at p < 0.05
Table 10. Cholinesterase activity in plasma, erythrocytes, and brain of rats given a single
dose of ethoprophos
Dose Day of Inhibition of cholinesterase from mean control valuea
(mg/kg bw) sampling
Males Females
Plasma Erythrocyte Brain Plasma Erythrocyte Brain
5 2 45* 8 49* 33*
8 -4 27 11 20
15 -8 -7 -3 -3 3 7
25 2 73* 49*
8 -12 21
15 -14 33* 11
50 2 87* 45* 94* 35*
8 -2 26 2 30
15 0 18 17 -3 20 10
75 2 94* 55*
8 6 25
15 -4 31* 18
a 100% - mean treated/mean control × 100%
* Significantly different at p < 0.05
> 20% was still present on days 8 and 15 in females at 25 and 50
mg/kg bw and in males at 50 and 75 mg/kg bw. In these groups, brain
acetylcholinesterase activity was inhibited by about 10% in females
and 18% in males at day 15, but this effect was not statistically
significant. Macro- and microscopic neuropathological examinations
showed no treatment-related changes. As brain acetylcholinesterase
activity was not measured directly after exposure, the NOAEL could not
be based on this end-point. The inhibition in females was not
dose-related, and there was a large standard deviation at 5 mg/kg bw.
The NOAEL for neurotoxicity was 5 mg/kg bw on the basis of behavioural
changes and inhibition of erythrocyte acetylcholinesterase activity
(Weiler, 1994b).
Groups of 27 Crl:CD BR VAF/Plus rats of each sex aged 6 weeks
were fed diets containing ethoprophos (purity, 95.7%) at
concentrations of 0, 4, 40, or 400 ppm, equal to 0, 0.26, 2.6, and 27
mg/kg bw per day for males and 0, 0.31, 3.0, and 31 mg/kg bw per day
for females, for 13 weeks. Twelve rats of each sex at each dose were
selected for functional battery and motor activity testing before
dosing and in weeks 4, 8, and 13. Of these, six of each sex per dose
were selected for neuropathological investigations, and the remaining
rats were observed only macroscopically. Cholinesterase activity was
measured in the plasma and erythrocytes of 10 rats of each sex per
group and acetylcholinesterase activity in four regions of the brain
(frontal cortex, caudate/putamen, hippocampus, and cerebellum) at
sacrifice in five rats of each sex per dose in weeks 4, 8 and 14. All
animals were observed for deaths and clinical signs, and body weight
and food consumption were recorded.
The only clinical sign considered to be related to treatment was
brown staining of the anal region in males at 400 ppm. Significantly
lower body-weight gain was observed in males at 400 ppm throughout
treatment and in females up to week 10, which resulted in
significantly lower body weights in animals at this dose. The mean
food consumption of animals of each sex at the high dose was
significantly lower during the first week and remained low in males
throughout treatment. In males at 40 ppm, the food consumption was
higher than that of controls. Changes in the results of the functional
battery tests considered to be related to treatment were seen only at
the high dose and included mild tremor (one female), vocalization
during handling (two females), slight salivation (one male), unkempt
fur (two males, one female), latency to first step (one male, one
female), and jumping to touch (one male) in week 4; in week 8, the
observations included intermittent vocalization in cage (three
females), slight lachrymation (one female, and one female at 40 ppm),
slight salivation (one male, two females, and one female at 40 ppm),
latency to first step (two females), aggressive behaviour (one
female), jump to touch response (one female), negative air drop reflex
(one male), and negative pupillary response (one female); in week 13,
the observations included aggressive behaviour (one female), latency
to first step (one female, and two males at 40 ppm), and no movement
in open arena (one male). Because the clinical signs observed at 40
ppm also occurred occasionally as single findings in controls, they
were considered to be unrelated to treatment. Other clearly
treatment-related observations in the functional battery test at the
high dose were decreased analgesic reflex in males in weeks 4, 8, and
13, and lower grip strength in fore- and hindlimbs of males and in
forelimbs of females. The motor activity of males at 400 ppm was
generally significantly lower than that of controls at all times;
females showed a slight decrease in activity only in week 4.
Cholinesterase activity in plasma, erythrocytes, and brain is
shown in Table 11. A > 20% inhibition of erythrocyte
acetylcholinesterase activity was found in males and females at doses
> 40 ppm. Inhibition of acetylcholinesterase activity in one or two
regions of the brain by > 20% was observed in males at 40 ppm at
weeks 4 and 8 and in all regions at 400 ppm. In females, inhibition of
acetylcholinesterase activity by > 20% was seen in three brain
regions at weeks 8 and 14 at 4 ppm, which was significant in the
frontal cortex in week 14, and in all regions at all times at higher
doses. No macroscopic or microscopic changes were observed.
An NOAEL could not be identified because brain
acetylcholinesterase was inhibited at all doses. The NOAEL for
neurotoxic effects was 40 ppm, equal to 2.6 mg/kg bw per day, on the
basis of the results of the functional observational battery and motor
activity tests (Weiler, 1994c).
Chickens
Two groups of 10 deep-litter hens weighing 1.4-2.1 kg (age not
specified) were intubated with technical-grade ethoprophos (purity
unknown) at a single dose of 5.6 µl/kg bw, equivalent to 6.2 mg/kg bw
and stated to represent the oral LD50 value in a previous study
performed at the testing laboratory, or tri- ortho-cresyl phosphate
(positive control) at 500 mg/kg bw. Four untreated hens were retained
as untreated controls. The hens given ethoprophos showed no gross
signs of ataxia or paralysis, although symptoms of transient
inactivity and 'depression' were noted between 1 and 48 h. Four hens
died within 48 h of dosing. The positive controls showed clinical
signs characteristic of delayed neurotoxicity. Histopathological
examination of sections of the spinal cord and sciatic nerve, stained
by the Weil-Weigert method (not referenced), from birds in all groups
at the end of a 21-day observation period revealed no evidence of
demyelination (Weir & Murphy, 1967).
In an initial study conducted to determine the LD50 of
ethoprophos in hens, six groups of 10 domestic hens ('a hybrid brown
laying strain') were given ethoprophos (purity, 94.5%) at doses of
0-16 mg/kg bw in a volume of 2 ml/kg bw by gavage. An LD50 of 6.4
mg/kg bw was calculated, with a 95% confidence interval of 4.8-8.8
mg/kg bw. On this basis, a dose of 6.5 mg/kg bw was selected for the
main study. The hens were divided randomly into six groups of 10. The
untreated controls received only corn oil, whereas the positive
controls received 500 mg/kg bw of tri- ortho-cresyl phosphate by
gavage. The treated groups first received 10 mg/kg bw of atropine
Table 11. Cholinesterase activity in plasma, erythrocytes, and brain regions at various
times during 13-week dietary exposure of rats to ethoprophos
Week Tissue Inhibition of cholinesterase activity from mean in controlsa
Males Females
4 ppm 40 ppm 400 ppm 4 ppm 40 ppm 400 ppm
4 Plasma 3 64* 90* 20 80* 97*
Erythrocyte 7 30* 26* -14 19 14
Brain
Caudate/putamen 17 23* 82* 8 32 83*
Hippocampus -7 4 48* 9 18 50*
Frontal cortex 19 -2 71* -28 30 74*
Cerebellum -7 0 52* -3 13 53*
8 Plasma -5 58* 85* 34* 87* 98*
Erythrocyte 10 23 35 19 32 40
Brain
Caudate/putamen 3 11 82* -11 -2 53*
Hippocampus 11 26* 59* 23 33* 67*
Frontal cortex 10 23 61* 33 49 80*
Cerebellum 0 0 46* 21 38* 62*
14 Plasma -2 54* 87* 18 86* 97*
Erythrocyte 19 33* 40* -6 22 11
Brain
Caudate/putamen -3 0 73* 34 49* 88*
Hippocampus 0 -9 54* 23 36* 66*
Frontal cortex 10 7 53* 52* 56* 80*
Cerebellum 9 -11 49* 6 27* 59*
a 100% - mean treated/mean control × 100%
* Significantly different from controls at p < 0.05
sulfate by intramuscular injection and then 6.5 mg/kg bw of
ethoprophos by gavage in corn oil at a constant volume of 2.5 ml/kg
bw. The atropine was found to have had only a minimal protective
effect, as 31/40 of the treated birds died within 4 days, most within
the first 24 h. Because of excessive mortality, two additional groups
were added, consisting of 12 and 11 birds, respectively, but it was
not specified whether they were treated concurrently with the
untreated controls. These birds were given pyridine 2-aldoxime
methiodide at 50 mg/kg bw by intramuscular injection in addition to
atropine before treatment with 6.5 mg/kg bw of ethoprophos. The
surviving birds were given further injections of pyridine 2-aldoxime
methiodide and atropine 24 and 48 h after treatment, and two birds in
the last group were injected 72 h after treatment. This procedure was
only marginally successful, as 14/23 birds died within 48 h of
treatment. On the basis of the deaths after the first dose of
ethoprophos, the LD50 was recalculated as 5.2 mg/kg bw, and this
dose was administered on day 22 to all 18 surviving birds, which were
treated with pyridine 2-aldoxime methiodide and atropine immediately
before administration of ethoprophos and again 5 h after the second
(day 22) treatment with ethoprophos; birds in the initial groups were
given additional injections 24 h after the second treatment. Two of
the 18 treated birds died within 72 h of the second treatment.
No clinical signs of neurotoxicity were noted in untreated
control or ethoprophostreated birds, whereas 9/10 birds treated with
tri- ortho-cresyl phosphate showed slight to marked signs of
neurotoxicity, usually by 11 days after treatment. These birds were
killed on day 21 and examined for histopathological changes. All other
surviving birds were killed on day 43. No remarkable macroscopic
changes were observed at necropsy. Portions of the forebrain, mid- and
hind-brain, cervical, thoracic, and lumbar spine, proximal and distal
sciatic nerve, and tibial nerve were evaluated for microscopic
evidence of neurological change. Lesions were noted in the spinal cord
and peripheral nerves of all positive control birds that reflected
'significant axonal degeneration'. Minimal lesions (grade 2 out of a
maximum of 5) were also found in the spinal cord of 9/10 control
birds, and one control had a minimal lesion in the proximal sciatic
nerve; no lesions were found in the brain or distal sciatic or tibial
nerves. A similar distribution of lesions of the spinal cord was seen
in treated birds, except that minimal lesions of the mid- or
hind-brain and lesions of the proximal sciatic nerve were each found
in 2/16 birds. One of the latter lesions was graded as moderate (grade
3) and occurred in a bird that also had a lesion of the mid- and
hind-brain. Three of 16 birds, including this one, also had minimal
lesions of the distal sciatic nerve. The authors concluded that
treatment with ethoprophos produced no clinical signs of neurotoxicity
and that the result was confirmed by the histological examination,
which showed no treatment-related changes in the nerve tissue. The
changes noted in the proximal sciatic nerve of the bird with multiple
lesions were considered to be unrelated to treatment and to represent
the extreme upper limit of background change (Roberts et al., 1986).
The Committee concluded that the study does not provide clear evidence
of neurotoxicity, but because the high rate of mortality reduced the
sensitivity of the study, the equivocal findings in some birds cannot
be dismissed. Historical control data from the testing facility
suggest that the lesions in proximal sciatic nerve are not
spontaneous.
(g) Studies on metabolites
O-Ethyl- S-propylphosphorothioate, a metabolite in rats, and
O-ethyl- O-methyl- S-propylphosphoro-thioate and
O-ethyl- S-methyl- S-propylphosphorodithioate, two metabolites
identified only in corn and potatoes (Rao, 1999), were tested for
toxicity and for their ability to inhibit cholinesterase activity in
female rats given single oral doses.
(i) Acute toxicity
The LD50 values of the three metabolites of ethoprophos in
female Crl:CD (SD)BR VAF/ Plus rats treated orally with the substances
in corn oil were 50 mg/kg bw for
O-ethyl- S-methyl- S-propylphosphorodithioate, 22 mg/kg bw for
O-ethyl- O-methyl- S-propylphosphorothioate, and 1600 mg/kg bw for
O-ethyl- S-propylphosphorothioate. The clinical signs were similar
for each of the metabolites and differed primarily in time of onset
and duration; the signs were also similar to those caused by the
parent compound. GLP and QA statements were included (Welier, 1998).
(ii) Cholinesterase inhibition
Three groups of 10 female Crl:CD(R)(SD)BR VAF/Plus rats aged
about 6 weeks were given a single dose of ethoprophos at 19 mg/kg bw,
O-ethyl- S-methyl- Spropylphosphorodithioate at 17 mg/kg bw, or
O-ethyl- O-methyl- S-propylphosphorothioate at 8 mg/kg bw by
gavage; a control group received the vehicle, corn oil. The animals
were observed for clinical signs before dosing, 0.5 and 2 h after
dosing, and the next day before sacrifice. Cholinesterase activity was
evaluated in plasma, erythrocytes, and brain 24 h after dosing.
Body-weight gain over the 2-day period was significantly lower in
all treated groups than in controls but resulted in a statistically
significant lower terminal body weight only with
O-ethyl- S-methyl- S-propylphosphorodithioate. In the other two
groups, the only clinical finding was a slightly increased incidence
of soft stools, whereas the animals given
O-ethyl- S-methyl- S-propylphosphorodithioate showed signs of
cholinesterase inhibition consisting of staggered gait and tremors.
All three compounds reduced cholinesterase activity in all three
tissues, the strongest inhibition being due to
O-ethyl- S-methyl- S-propylphosphorodithioate (Table 12; Weiler,
1998).
Table 12. Cholinesterase activity in plasma, erythrocytes, and brain of female rats 24 h
after a single oral dose of ethoprophos and two metabolites
Compound Dose Inhibition from control mean (%)a
(mg/kg bw)
Plasma Erythrocytes Brain
Ethoprophos 19 73* 37* 32*
O-Ethyl-S-methyl-S-propylphosphorodithioate 17 78* 30 71*
O-Ethyl-O-methyl-S-propylphosphorothioate 8 40* 47* 48*
a 100% - mean treated/mean control × 100%
* Significantly different from control at p < 0.05
4. Observations in humans
In a study of occupational exposure to ethoprophos in Salinas
Valley, Monterey County, California, USA, four male volunteers were
recruited among regular employees of an experienced pesticide
application firm who were thoroughly familiar with both the practices
and equipment involved in pesticide application and had not worked
with cholinesterase inhibitors for at least 90 days. The occupation
under consideration was that of mixer-loader-applicator, who applied
an emulsifiable concentrate containing 70% ethoprophos in xylene for 3
days (two men), 4 days (one man), or 5 days (one man) to field plots
of 2-3 ha; the only operator working on day 5 treated a field plot of
about 8.6 ha. During the mixing and loading phase, the concentrate,
supplied in 5-gallon (~ 20-L) containers, was diluted to 5% in two
160-gallon (~ 40-L) tanks. Transfer of the pesticide into the tank,
rinsing the container, and filling the spray tanks were done in a
closed system to minimize exposure. The operators wore long-sleeved,
twill work shirts, denim jeans, and rubber boots. They used rubber
gloves and disposable respirators which were changed each day. Face
shields and hard hats were available, especially for the mixing and
loading operations. The application rate of active ingredient was
about 12 kg/ha (range, 11-15 kg/ha). Blood was collected for
determination of cholinesterase activity in plasma and erythrocytes
three times before exposure and as soon as possible after each man's
work session. During application of a commercial preparation
containing 69.6% ethorprop (as an emulsifiable concentrate in xylene),
dermal exposure was monitored on the hands (three hand washings with
2-propanol in water) and face (passive facial patch collector
consisting of 12-layer surgical gauze), corresponding to a surface of
24 cm2. Inhalation was not monitored. Exposure to ethoprophos was
monitored by a standardized gas chromatographic method.
No clinical signs or adverse effects were noted during exposure,
and the changes in plasma and erythrocyte cholinesterase activity were
not statistically significantly different from the mean baseline
values. The maximal variation around the mean was - 8.9% to 7.9%,
which is considered to be normal day-to-day variation. Exposure on the
head and neck was 3.1-78 µg/h, with an average of 34 µg/h
(calculations based on a default head area of 910 cm2). The total
exposure of the head to ethoprophos was 7.6-153 µg, with means of 96,
53, 67, and 36 µg for the four men. The hands were exposed to 0.2-18
µg/h, with an average value of 6.3 µg/h and a value of 0.52-42 µg for
both hands; the mean values were 29, 2.0, 12, and 6.3 µg for the four
men. The overall exposure during the entire period of observation of
the man who worked for 5 days was 620 µg, and the quantity of active
ingredient handled per day was 31-92.4 kg. The overall exposure of the
other operators was 220 µg for the man who worked for 4 days and
handled 26-44 kg daily; 240 µg for the man who worked for 3 days and
handled 19-27 kg daily; and 130 µg for the man who worked for 3 days
and handled 17-44 kg daily (Leffingwell, 1986).
Comments
After oral administration to rats, 14C-ethoprophos was rapidly
and virtually completely absorbed, metabolized and excreted. The main
route of excretion was the urine (51-56%), but significant proportions
were excreted in expired air (about 15%) and faeces (10-14%). Little
radiolabel was found in tissues at 168 h, representing less than 2.5%
of the dose, and the highest concentrations were found in excretory
organs (liver, kidneys, and lungs). There was no evidence that
bioaccumulation would occur after repeated doses. The kinetics of the
radiolabel and the biotransformation appeared to be independent of the
route of administration (oral or intravenous), magnitude and frequency
of dose and sex. Ethoprophos was metabolized by dealkylation of one or
both S-propyl groups, followed by conjugation. The dermal absorption
of ethoprophos has not been studied in vivo, but radiolabelled
ethoprophos penetrated the skin in vitro, the greatest penetration
being seen through mouse skin, lesser penetration through rat and
rabbit skin (at equal rates), and the least penetration through human
skin. In all of the species tested, the rate of penetration was
greater with ethoprophos (emulsified concentrate) diluted 1:19 in
water than with undiluted material.
Single oral doses of ethoprophos were toxic to rats, mice and
rabbits (LD50 values, 31-62 mg/kg bw), and it was highly toxic to
rats exposed once by inhalation (LC50, 0.25 mg/L). Mice and rabbits
were more sensitive to dermal exposure than rats, and female rats were
more sensitive than males, with LD50 values of 8.5 mg/kg bw for
rabbits, 18 mg/kg bw for mice, 420 mg/kg bw for female rats, and 1300
mg/kg bw for male rats. The toxic signs observed after exposure by any
route were characteristic of cholinesterase inhibition. WHO (1999) has
classified ethoprophos as 'extremely hazardous'.
Ethoprophos not only irritates the eyes of rabbits but is very
toxic after administration into the eye, causing the death of tested
animals within 1 h. In a test for dermal irritation, all treated
animals died within 8 h of exposure to the undiluted compound. In a
three-week study in rabbits, the compound caused slight dermal
irritation at all doses tested (0.03, 0.1, and 1 mg/kg bw per day),
whereas no dermal irritation was observed in a 3-week study in rats
given doses of up to 10 mg/kg bw per day.
In studies in dogs (13, 20 and 52 weeks) and mice and rats (2
years) given ethoprophos orally, inhibition of cholinesterase was the
most sensitive parameter. In dogs, the NOAEL for inhibition of brain
acetylcholinesterase activity was 1 mg/kg bw per day, the highest dose
tested. In mice, brain acetylcholinesterase activity was inhibited at
30 ppm, resulting in a NOAEL of 2 ppm, equal to 0.25 mg/kg bw per day;
in rats, the overall LOAEL was 30 ppm (equal to 1.3 mg/kg bw per day)
and no effects were seen at 10 or 1 ppm (equal to 0.5 and 0.04 mg/kg
bw per day, respectively). In a two-generation study of reproductive
toxicity in rats, inhibition of brain acetylcholinesterase activity
was seen in animals of each sex of both parent generations at 30 ppm,
resulting in a NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day. In a
13-week study of neurotoxicity in rats, the activity of brain
acetylcholinesterase was inhibited in females at all doses tested, the
lowest dose being 4 ppm, equal to 0.26 mg/kg bw per day. Clinical
signs characteristic of cholinesterase inhibition were observed only
in the 2-year study in rats, in which the highest dose was reduced
from 600 to 400 ppm because of clinical signs.
Inhibition of brain acetylcholinesterase activity was also
demonstrated after dermal exposure of rabbits and rats to ethoprophos
for three weeks, with NOAELs of 0.1 mg/kg bw per day (LOAEL, 1 mg/kg
bw per day) in rabbits and 0.3 mg/kg bw per day (LOAEL, 1 mg/kg bw per
day) in rats.
Ethoprophos was not carcinogenic in a long-term study in mice
treated in the diet. The highest dose tested was 30 ppm, equal to 4.9
mg/kg bw per day. In three long-term studies of toxicity and
carcinogenicity in rats, some evidence was obtained that ethoprophos
may stimulate progression of C-cell tumours in the thyroid and adrenal
neoplasms (phaeochromocytomas), but this effect was considered to have
little relevance for human risk. The highest dose tested was 400 ppm,
equal to 26 mg/kg bw per day. The Meeting concluded that ethoprophos
is unlikely to pose a carcinogenic risk to humans.
Ethoprophos was tested in an adequate set of assays for
genotoxicity in vitro and in vivo. Positive results in studies for
chromosomal aberrations and sister chromatid exchanges in vitro
after metabolic activation indicate that the compound has intrinsic
genotoxic activity. Chromosomal aberrations were not induced in rats
in two assays and dominant lethal mutations were not induced in rats
in another study, although a slightly positive or equivocal result was
obtained in a further study for dominant lethality. The Meeting
concluded that ethoprophos is unlikely to be genotoxic in vivo.
In a two-generation study of reproductive toxicity in rats, no
effect was observed on reproductive parameters. Clinical signs were
observed only in the F0 parents at the highest dose (300 ppm). The
NOAEL was 1 ppm, equal to 0.04 mg/kg bw per day, on the basis of
reduced body-weight gain in F0 males and inhibition of brain
acetylcholinesterase activity in animals of each sex in both parental
generations. The NOAEL for developmental toxicity was 30 ppm, equal to
1.3 mg/kg bw per day, on the basis of effects on body weight and
body-weight gain in pups of both generations and deaths of pups in the
F2 litters at 150 ppm.
In studies of developmental toxicity, maternal toxicity was
characterized by growth depression in rats in one study (NOAEL,
2 mg/kg bw per day) and in rabbits in one study (NOAEL, 0.12 mg/kg bw
per day), whereas no maternal toxicity was observed in rabbits in
another study (NOAEL, 2.5 mg/kg bw per day, highest dose tested).
Since in the first study in rabbits the effect was only marginal (not
statistically significant), the overall NOAEL for maternal effects in
rabbits was 2.5 mg/kg bw per day. No effects on the fetuses of either
species were observed. Cholinesterase activity was not measured in
these studies.
The relationship between neurotoxicity and cholinesterase
inhibition was examined in rats in two studies. In rats exposed by
gavage to single doses of 0, 20, or 40 mg/kg bw (females) or 0, 30, or
60 mg/kg bw (males), cholinesterase activity in plasma, erythrocytes,
and brain was inhibited, with a maximum effect 2 h after dosing. While
cholinesterase inhibition was found in tissues at the lowest doses
tested, clinical signs characteristic of cholinesterase inhibition
were seen only at the highest doses (60 mg/kg bw for males and
40 mg/kg bw for females). In the second study, in which male rats were
exposed to a single dose of ethoprophos at 5, 50, or 75 mg/kg bw and
females at 5, 25, or 50 mg/kg bw, effects on motor activity were
observed in animals of each sex at doses of 50 mg/kg bw and higher,
and functional and behavioural effects were observed at 25 mg/kg bw
and higher. These neurotoxic signs were seen only on the day of
dosing. Significant inhibition of acetylcholinesterase activity in
erythrocytes, measured 1 day after exposure, was observed in females
at doses of 5 mg/kg bw and higher and in males at 50 mg/kg bw and
higher. The inhibition in females was not dose-related, and there was
a large standard deviation at 5 mg/kg bw . The Meeting concluded that
the NOAEL in this study was 5 mg/kg bw.
In a 13-week study in rats, neurotoxicity was observed in tests
for motor activity and for functional and behavioural abnormalities
only at the highest dose (400 ppm). The NOAEL for neurotoxic effects
was 40 ppm, equal to 2.6 mg/kg bw per day. The activity of brain
acetylcholinesterase was inhibited in females at all doses tested; the
lowest dose was 4 ppm, equal to 0.26 mg/kg bw per day.
A study of delayed neurotoxicity in hens was performed with doses
of ethoprophos that caused a high mortality rate despite antidotal
treatment. Equivocal findings were reported in some of the treated
birds; data on the effect of this compound on neuropathy target
esterase activity in hens would be useful in order to allow full
assessment of the neuropathological potential of ethoprophos.
A study of occupational exposure to ethoprophos did not reveal
significant effects on human plasma or erythrocyte cholinesterase
activity. The calculated rates of exposure of head-and-neck areas were
3.1-78 µg/h (average, 34 µg/h), and the rate of exposure of the hands
was calculated to be 0.2-18 µg/h (average, 6.3 µg/h).
O-Ethyl S-propyl phosphorothioate, a metabolite in rats, and
O-ethyl O-methyl S-propyl phosphorothioate and O-ethyl
S-methyl S-propyl phosphorodithioate, two metabolites that have
been identified only in corn and potatoes, were tested for toxicity
and for their ability to inhibit cholinesterase activity in female
rats given single oral doses. The two plant metabolites were of
approximately the same toxicity as the parent compound, while the
animal metabolite was less toxic (LD50, 1600 mg/kg bw) than the
parent.
The 1987 Meeting derived the ADI on the basis of a slight effect
on the liver in a 52-week study in dogs at a dose of 1 mg/kg bw per
day. The NOAEL for this effect was 0.025 mg/kg bw per day. Since this
marginal effect was not observed in other studies in dogs or in other
species and there is a 40-fold difference between the LOAEL and the
NOAEL, this NOAEL was not used as the overall NOAEL to derive the ADI
in the present evaluation.
The present Meeting established an ADI of 0-0.0004 mg/kg bw on
the basis of the NOAEL of 1 ppm, equal to 0.04 mg/kg bw per day, for
inhibition of brain acetylcholinesterase activity in the 2-year study
of toxicity and carcinogenicity in rats and in the study of
reproductive toxicity in rats, and a 100-fold safety factor.
An acute reference dose of 0.05 mg/kg bw was established on the
basis of the NOAEL of 5 mg/kg bw in the study of acute neurotoxicity
in rats, in which functional and/or behavioural effects and inhibition
of erythrocyte acetylcholinesterase were observed at the next highest
dose, and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse 2 ppm, equal to 0.25 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
Rat 1 ppm, equal to 0.04 mg/kg bw per day (2-year study of
carcinogenicity and toxicity)
1 ppm, equal to 0.04 mg/kg bw per day (parental toxicity in
a study of reproductive toxicity)
5 mg/kg bw (single dose, study of neurotoxicity by gavage)
2 mg/kg bw (maternal toxicity, study of developmental
toxicity; acetylcholinesterase activity not determined);
18 mg/kg bw (fetotoxicity, highest dose tested, study of
developmental toxicity; acetylcholinesterase activity not
determined)
Rabbit 2.5 mg/kg bw (maternal toxicity and fetotoxicity, study of
developmental toxicity; acetylcholinesterase activity not
determined);
Dog 0.025 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0004 mg/kg bw
Estimate of acute reference dose
0.05 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Information on effects on neuropathy target esterase activity in
hens
2. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to ethoprophos
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption > 70%, mainly within 12 h (rats)
Dermal absorption Tested in vitro, no quantitative data
Penetration rate Mouse skin > rat, rabbit skin > human skin
Distribution Widely distributed, highest levels in organs of elimination (rats)
Potential for accumulation No
Rate and extent of excretion Excreted in urine (55%), expired air (13-17%), and faeces (7-9%) (rats)
Metabolism in animals In rats, metabolized by dealkylation of one or both S-propyl groups,
followed by conjugation
Toxicologically significant compounds Parent compound and plant metabolites ( O-ethyl O-methyl S-propyl
phosphorothioate and O-ethyl S-methyl S-propyl phosphorodithioate)
Acute toxicity
Rat, LD50, oral 33-62 mg/kg bw (vehicle, corn oil)
Rat, LD50, dermal 420 mg/kg bw, females
Rat, LD50, inhalation 0.25 mg/L
Dermal irritation Not reported, but death occurred within 8 h of dermal exposure
Ocular irritation Yes, but also death within 1 h of ocular exposure
Sensitization Not tested
Short-term toxicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant oral NOAEL < 0.26 mg/kg bw per day (LOAEL, 13-week study of neurotoxicity
in rats)
Lowest relevant dermal NOAEL 0.1 mg/kg bw per day (3-week study of dermal toxicity in rabbits)
Lowest relevant inhalation NOAEL Not tested
Long-term toxicity and carcinogenicity
Target/critical effect Acetylcholinesterase inhibition
Lowest relevant NOAEL 0.04 mg/kg bw per day (105-week study in rats treated in the diet)
Carcinogenicity Not carcinogenic (mice, rats)
Genotoxicity Not genotoxic in vivo
Reproductive toxicity
Reproductive target/critical effect Reduced body weight and survival at maternally toxic doses
Lowest relevant reproductive NOAEL Maternal toxicity: 1 ppm, equal to 0.04 mg/kg bw per day;
fetotoxicity: 30 ppm, equal to 1.3 mg/kg bw per day
Developmental target/critical effect Only maternal toxicity (highest dose tested), no fetotoxicity
Lowest relevant developmental NOAEL Rabbit: maternal and fetotoxicity: 2.5 mg/kg bw per day (highest
dose tested)
Rat: maternal toxicity: 2 mg/kg bw per day; fetotoxicity:
18 mg/kg bw per day
Neurotoxicity/ Delayed neurotoxicity 5 mg/kg bw per day (acute toxicity in rats)
< 4 ppm, equal to 0.26 mg/kg bw per day (13-week study in rats)
No evidence for delayed neurotoxicity in hens, but some equivocal findings
Other toxicological studies No significant effects on erythrocte acetylcholinesterase activity in
exposed workers
Medical data None
Summary Value Study Safety factor
ADI 0-0.0004 mg/kg bw 2-year study, rat; two-generation 100
study of reproductive toxicity, rats
Acute reference dose 0.05 mg/kg bw Acute neurotoxicity, rats 100
References
Auletta, C.S. & Rinehart, W.E. (1979) A comparative dermal toxicity
evaluation in mice (Ethoprop and nine chemical analogs).
Unpublished report Project No. 5789-79 from Bio/dynamics Inc.,
Princeton, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985a) Ames
Salmonella/microsome plate test (EPA/OECD). Unpublished report
No. PH 301-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by RhônePoulenc.
Barfknecht, T.R., Naismith, R.W. & Matthews, R.J. (1985b). Rat
hepatocyte primary culture/DNA repair system. Unpublished report
No. PH 311-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Barnett, J.W., Jenkins, L.J. & Parent, R.A. (1983) Evaluation of the
chronic toxicity and oncogenic potential of ethoprop in Fischer
344 rats. Unpublished report from Gulf South Research Institute,
New Iberia, Louisiana, USA. Submitted to WHO by Rhône-Poulenc.
Becker, J. & Parke, G.S.E. (1977) A primary dermal irritation study of
ethoprop 93% technical grade on abraded and non-abraded skin of
New Zealand albino rabbits. Unpublished report Lab. No. 7E-6084
from Cannon Laboratories, Inc., Reading, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
Blacker, A.M. (1987) Executive summary -- Acute dermal LD50 of
ethoprop (technical) in rabbits and rats. Unpublished report No.
87/AMB/035 from Rhone Poulenc. Submitted to WHO by Rhône-Poulenc.
Brown, D. (1986) Ethoprophos: 52 week oral (capsule administration)
toxicity study in the beagle. Unpublished report No. 4923-198/16
from Hazleton Laboratories Europe, Ltd, Harrogate, North
Yorkshire, United Kingdom. Submitted to WHO by Rhône-Poulenc.
Dearlove, G.E. (1987) Dominant lethal study of ethoprop technical
administered orally via gavage to Crl:COBS(R)CD(R)(SD)BR male
rats. Confidential report No. 218-004 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Fletcher, M.J., Springer, S.T., D'Addamio, G.H., Conzelmann, P.C. &
Pullin, T.G. (1980) Evaluation of effects of ethoprop on
reproductive performance by a three-generation reproduction study
in Fischer 344 rats. Unpublished report No. 413-858-41 from Gulf
South Research Institute. Submitted to WHO by Rhône-Poulenc.
Hamada, N.N. (1990) A five-month oral toxicity study with one-month
recovery in beagle dogs with ethoprop technical. Unpublished
report No. HLA 656-143 from Hazleton Laboratories America, Inc.
Vienna, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1989) 3-week dermal toxicity study with ethoprop
technical in rabbits. Unpublished report No. HLA 6224-152 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990a) 3-week dermal toxicity range finding study with
ethoprop technical in rats. Unpublished report No. HLA 6224-160
from Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1990b) 3-week dermal toxicity study with ethoprop
technical in rats. Unpublished report No. HLA 6224-162 from
Hazleton Laboratories America, Inc. Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Iqbal, Z.M. & Menzer, R.E. (1972) Metabolism of O-ethyl-S,S-dipropyl
phosphorodithioate in rats and liver microsomal systems.
Biochem. Pharmacol., 21, 1569-1584.
Ivett, J.L. (1989) Mutagenicity test on ethoprop technical in the rat
bone marrow cytogenetic assay. Unpublished report No. 10750-0-452
from Hazleton Laboratories America Inc., Kensington, Maryland,
USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1986) Acute dermal LD50
study with ethoprop in New Zealand white rabbits. Unpublished
report No. 3147.2 from Springborn Institute for Bioresearch,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Karcher, R., Siglin, J.C. & Becci, P.J. (1987) Acute dermal LD50
study with ethoprop (technical) in Sprague Dawley rats.
Unpublished report No. 3147.5 from Springborn Institute for
Bioresearch, Inc., Spencerville, Ohio, USA. Submitted to WHO by
RhônePoulenc.
Knickerbocker, M. & Re, T.A. (1979) Teratologic evaluation of ethoprop
MCTR-603-78 in Sprague-Dawley rats. Unpublished report No. 5850
from Food & Drug Research Laboratories, Inc. Submitted to WHO by
Rhône-Poulenc. Report amended 10 June 1985.
Kopp, R., Gunzel, P., Rosskamp, G., Schmidt, M. & Schuppler, J. (1980)
Acute inhalation toxicity to the rat (M&F) of liquid ethoprophos
following single 4 hours continuous exposure (LC50). Report
(Protocol No. 449/79) from Schering AG. Submitted to WHO by
Rhône-Poulenc (in German).
Leffingwell, J.T. (1986) An exposure study of mixer/loader-applicators
working with MOCAP EC. Unpublished report from Rhône-Poulenc
Inc., New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Myhr, B.C. & Brusick, D.J. (1981) Evaluation of MOBIL No. 01238101 in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 2478-80 from Litton Bionetics, Inc.,
Kensington, Maryland, USA. Submitted to WHO by RhônePoulenc.
Neeper-Bradley, T.L. (1991) Two-generation reproduction study of
ethoprop technical administered in the diet to CD(R) (Sprague
Dawley) rats. Unpublished report No. 53-598 from Bushy Run
Research Center, Export, Pennsylvania, USA. Submitted to WHO
Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1982) Ethoprop (48 095 RP). Acute toxicity
in mice treated orally and rats treated percutaneously.
Unpublished report from Rhône-Poulenc, Vitry sur Seine, France.
Submitted to WHO by Rhône-Poulenc (in French).
Powers, M.B. (1965) V-C 9-104 (technical grade) Acute oral
administration -- Rats. Acute dermal application -- Rabbits.
Unpublished report from Hazleton Laboratories, Inc., Falls
Church, Virginia, USA. Submitted to WHO by Rhône-Poulenc.
Putman, D.L. & Schechtman, L.M. (1981) Activity of T1688 in the
dominant lethal assay in rodents. Unpublished report from
Microbial Associates, Bethesda, Maryland, USA. Submitted to WHO
by Rhône-Poulenc.
Rao (1999) Personal communication. Rhône-Poulenc, Research Triangle
Park, North Carolina, USA. Submitted to WHO by Rhône-Poulenc.
Roberts, N.L., Gopinth, C., Anderson, A. & Dawe, I.S. (1986) Acute
delayed neurotoxicity study with ethoprophos in the domestic hen.
Unpublished report No. RNP 244/86189 from Huntingdon Research
Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom. Submitted
to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989a) Range-finding teratology study in rats with
ethoprop. Unpublished report No. 3147.38 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989b) Teratology study in rats with ethoprop.
Unpublished report No. 3147.39 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rodwell, D.E. (1989c) Range-finding teratology study in rabbits with
ethoprop. Unpublished report No. 3147.40 from Springborn Life
Sciences, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc.
Rodwell, D.E. (1989d) Teratology study in rabbits with ethoprop.
Unpublished report No. 3147.41 from Springborn Life Sciences,
Inc., Spencerville, Ohio, USA. Submitted to WHO by Rhône-Poulenc.
Rucci, G.. (1979) Ethoprop technical grade; MCTR-60-78. Approximate
acute dermal toxicity (LD50) in young Yorkshire white pigs.
Unpublished report Lab. No. 6074from Food and Drug Research
Laboratories, Inc., Waverly, New York, USA. Submitted to WHO
Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1985) In vitro
chromosome aberration analysis in Chinese hamster ovary (CHO)
cells. Unpublished report No. PH 320-RP-00185 from Pharmakon
Research International Inc., Waverly, Pennsylvania, USA.
Submitted to WHO by Rhône-Poulenc.
SanSebastian, J.R., Naismith, R.W. & Matthews, R.J. (1986) In vitro
sister chromatid exchange in Chinese hamster ovary (CHO) cells.
Unpublished report No. PH 319-RP001-85 from Pharmakon Research
International Inc., Waverly, Pennsylvania, USA. Submitted to WHO
by Rhône-Poulenc.
Schwartz, C.S. (1978) Acute oral LD50 determination of ethoprop in
female New Zealand white rabbits. Letter report to Mobil Oil
Company on FDRL study No. 5988. Submitted to WHO by
Rhône-Poulenc.
Skinner, M.J., Irwin, S.E., Schreiner, C.A., Mackerer, C.R. & Mehlman,
M.A. (1981) Metaphase analysis of rat bone marrow cells treated
in vivo with ethoprop, technical. Unpublished report No. 2476-80
from Mobil Environmental and Health Science Laboratory,
Plainsboro, New Jersey, USA. Submitted to WHO by Rhône-Poulenc.
Spicer, E.J.F. (1985) Lifetime dietary toxicity and oncogenicity study
in rats. Unpublished report No. 347-029 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Study reformatted 18 May 1987. Submitted to WHO by Rhône-Poulenc.
Stankowski, L.F., Naismith, R.W. & Matthews, R.J. (1985) CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report
No. PH 314-RP-001-85 from Pharmakon Research International Inc.,
Waverly, Pennsylvania, USA. Submitted to WHO by Rhône-Poulenc.
Stoughton, R.B. (1986) Penetration of the skin of humans, mice, rats
and rabbits by C14 ethoprop(R) emulsified concentrate and the
emulsified concentrate diluted with distilled water. Unpublished
report from the University of California, San Diego, California,
USA. Submitted to WHO by Rhône-Poulenc.
Thomson, M.A., Blackburn, G.R. & Mackerer, C.R. (1981) A murine
lymphoma mutagenesis assay, heterozygous at the thymidine kinase
locus for the determination of the potential mutagenicity of
ethoprop. Unpublished report No. 2474-80 from Mobil Environmental
& Health Science Laboratory, Plainsboro, New Jersey, USA.
Submitted to WHO by RhônePoulenc.
Weiler, M.S. (1994a) Acute oral gavage study with ethoprop in rats:
Time-related effects of ethoprop on brain, plasma and red blood
cell cholinesterase activities. Unpublished report No. HWI
6224-209 from Hazleton Wisconsin, Inc. Madison, Wisconsin USA.
Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994b) Acute neurotoxicity study with ethoprop in rats.
Unpublished report No. HWI 6224-200 from Hazleton Wisconsin, Inc.
Madison, Wisconsin, USA. Submitted to WHO by Rhône-Poulenc.
Weiler, M.S. (1994c) 13-week dietary neurotoxicity study with ethoprop
in rats. Unpublished report No. HWI 6224-199 from Hazleton
Wisconsin, Inc. Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Weiler, M.S. (1998) Acute oral toxicity study with ethoprop and three
ethoprop metabolites
(O-ethyl-S-methyl-S-propylphosphorodithioate,
O-ethyl-O-methyl-Spropylphosphorothioate, and
O-ethyl-S-propylphosphorothioate) in rats with determination of
effects on cholinesterase activity. Unpublished report No.
6224-246 from Covance Laboratories Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc.
Weir, R.J. (1965) Acute eye application -- Rabbits. V-C 9-104
(technical grade). Unpublished report from Hazleton Laboratories
Inc. Falls Church, Virginia, USA. Submitted to WHO by
Rhône-Poulenc.
Weir, R.J. (1967a) Three-month dietary administration -- Rats.
Technical VC9-104. (Mocap). Unpublished report No. 230-102 from
Hazleton Laboratories Inc, Falls Church, Virginia, USA. Submitted
to WHO by Rhône-Poulenc.
Weir, R.J. (1967b) 13-week dietary administration -- Dogs. Technical
VC9-104. Unpublished report No. 230-110 from Hazleton
Laboratories, Inc, Falls Church, Virginia, USA. Submitted to WHO
by Rhône-Poulenc.
Weir, R.J. & Murphy, J.C. (1967) Neurotoxicity test -- Hens. Technical
VC 9-104. Unpublished report from Hazleton Laboratories Inc.,
Falls Church, Virginia, U.S.A. Submitted to WHO by Rhône-Poulenc.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, K.D. (1992) 104 week combined chronic toxicity and
carcinogenicity study with ethoprop in rats. Unpublished report.
Laboratory project ID: HLA 6224-151 from Hazelton Laboratories
America, Inc., Madison, Wisconsin, USA. Submitted to WHO by
Rhône-Poulenc.
Wolfe, G.W., Durloo, R.S. & Phipps, R.B. (1981) Rabbit teratology
study. Ethoprop technical -- 01238101. Unpublished report from
Hazleton Laboratories America, Inc. Vienne, Virginia, USA.
Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Submitted to WHO by Rhône-Poulenc.
Yamagata, S., Inoue, H. & Enomoto, M. (1984b) Chronic feeding and
oncogenicity studies in mice with ethoprop. Unpublished report
No. 497 from Biosafety Research Center (ANPYO Center),
Shizuoka-ken, Japan. Supplementary submission, dated 29 July
1994. Submitted to WHO by Rhône-Poulenc.
Yenne, S.P. (1990) 14C-Ethoprop: Absorption, distribution,
metabolism, and excretion in the rat. Unpublished report HUK
project no. 68/102 from Hazleton UK, North Yorkshire, United
Kingdom. Submitted to WHO by Rhône-Poulenc.
