
DIMETHIPIN
EXPLANATION
Dimethipin is a harvest-aid dessicant or moisture-reduction
chemical used on oilseeds, potatoes, and tomatoes. It was evaluated
for the first time by the present Meeting.
IDENTITY
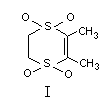
CHEMICAL NAME: 2,3-Dihydro-5,6-dimethyl-l,4-dithiin
1,1,4,4-tetraoxde
SYNONYMS: Harvade(R), UB1-N252
STRUCTURAL FORMULA:
 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 210
STATE: A white crystalline solid with mild odour
MELTING RANGE: 162-167°C
SOLUBILITY (gram solute/100 grams solvent at 25°C):
Distilled water 0.3; dimethyl formamide 32.4;
benzene 1.96; xylene 0.57; methanol 0.05;
chloroform 7.92; ethylene dichloride 7.59
STABILITY: Stable for at least 2 years at +30°C and -30°C
OCTANOL/WATER PARTITION COEFFICIENT:
1.0 at 1 X concentration
2.0 at 5 X concentration
DENSITY: 0.47 g/ml at 20°C
Specifications of technical material
Technical dimethipin has a purity of about 98%. Depending on the
starting material used in the manufacturing process, as many as 4
impurities greater than 0.1% could be present. The impurities include:
(i) 1,4-dithiin, 2,3-dihydro-5,6-dimethyl-l,1,4-trioxide;
(ii) 1,4-dithiin, 5-ethyl-2,3-dihydro-l,1,4,4-tetraoxide;
(iii) 1,4-dithiane, 2-methyl-3-methylene-l,1,4,4-tetraoxide; and
(iv) 1,3-dithiolane, 2-ethyl-2-methyl-l,1,3,3-tetraoxide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, elimination, and biotransformation
Rat
In rats (3 males and 2 females) given a single oral dose of
approximately 3.8 mg/kg b.w. of 14C-dimethipin (labelled in the
2,3-position of the dithiin ring; 96% radiochemically pure) in
distilled water, an average of about 89% of the administered
radioactivity was excreted in 48 hours via the urine and faeces. (One
female rat, identically treated, eliminated only about 42% of the
given dose via these routes over the same period. Reason for the low
14C recovery was said to be unclear, as tissue and blood levels in
this animal were similar to those in the other treated animals.) In
general, faecal elimination slightly exceeded urinary excretion. Less
than 0.1% of the administered 14C was detected in the expired air. At
sacrifice 96 hours after treatment, mean total-residue levels in the
tissues analyzed (excluding the blood) amounted to about 1% of the
given dose. Residue concentrations were highest in lung, heart, liver,
and kidney, and lowest in the gastrointestinal tract, brain, muscle,
and fat. Blood-residue levels ranged from 2 to 7.7% of the
administered 14C dose. No significant sex difference was apparent in
rate, route of elimination, or tissue-residue levels of the compound
(Caplan & Merricks, 1978).
Analysis of pooled urine and faceal samples from the
above-treated rats (excluding the female rat with unusually-low
excretary rate) indicated the presence of about 5% of the 14C
recovered in the urine or in the faeces (corresponding, respectively,
to 2% and 1% of the administered dose) as the unchanged parent
compound. The other readioactive components in the urine and the
faeces were highly polar (Smilo et al., 1978).
Goat
Two goats, 1 of them with a cannulated bile duct, were fed
14C-dimethipin (98% radiochemically pure) at 500 ppm in the diet for
3 days. Samples of urine, liver, bile, and kidney were collected for
characterization and identification of metabolites. (No details of
experimental conditions were provided. Data were presented apparently
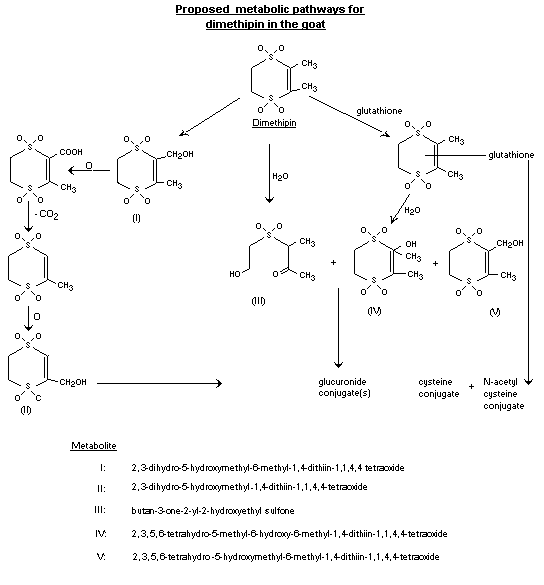
on only one of the treated animals.) Figure 1 presents the proposed
metabolic pathways for dimethipin in the goat.
Invariably, in urine, bile, liver and kidney, metabolites III and
IV were identified together with the unchanged parent compound. The
latter accounted for 2-8% of the 14C recovered in each case. Some
alcohol metabolites of dimethipin, viz. metabolites I, II, and V,
were identified only in the urine and bile. Most of the radioactive
components in urine, bile, liver, and kidney were of a highly polar
nature, and were present as conjugates such as glucuronide, cysteine,
and acetylcysteine conjugates (McManus, 1984).
Proposed metabolic pathways for dimethipin in the goat
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 210
STATE: A white crystalline solid with mild odour
MELTING RANGE: 162-167°C
SOLUBILITY (gram solute/100 grams solvent at 25°C):
Distilled water 0.3; dimethyl formamide 32.4;
benzene 1.96; xylene 0.57; methanol 0.05;
chloroform 7.92; ethylene dichloride 7.59
STABILITY: Stable for at least 2 years at +30°C and -30°C
OCTANOL/WATER PARTITION COEFFICIENT:
1.0 at 1 X concentration
2.0 at 5 X concentration
DENSITY: 0.47 g/ml at 20°C
Specifications of technical material
Technical dimethipin has a purity of about 98%. Depending on the
starting material used in the manufacturing process, as many as 4
impurities greater than 0.1% could be present. The impurities include:
(i) 1,4-dithiin, 2,3-dihydro-5,6-dimethyl-l,1,4-trioxide;
(ii) 1,4-dithiin, 5-ethyl-2,3-dihydro-l,1,4,4-tetraoxide;
(iii) 1,4-dithiane, 2-methyl-3-methylene-l,1,4,4-tetraoxide; and
(iv) 1,3-dithiolane, 2-ethyl-2-methyl-l,1,3,3-tetraoxide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, elimination, and biotransformation
Rat
In rats (3 males and 2 females) given a single oral dose of
approximately 3.8 mg/kg b.w. of 14C-dimethipin (labelled in the
2,3-position of the dithiin ring; 96% radiochemically pure) in
distilled water, an average of about 89% of the administered
radioactivity was excreted in 48 hours via the urine and faeces. (One
female rat, identically treated, eliminated only about 42% of the
given dose via these routes over the same period. Reason for the low
14C recovery was said to be unclear, as tissue and blood levels in
this animal were similar to those in the other treated animals.) In
general, faecal elimination slightly exceeded urinary excretion. Less
than 0.1% of the administered 14C was detected in the expired air. At
sacrifice 96 hours after treatment, mean total-residue levels in the
tissues analyzed (excluding the blood) amounted to about 1% of the
given dose. Residue concentrations were highest in lung, heart, liver,
and kidney, and lowest in the gastrointestinal tract, brain, muscle,
and fat. Blood-residue levels ranged from 2 to 7.7% of the
administered 14C dose. No significant sex difference was apparent in
rate, route of elimination, or tissue-residue levels of the compound
(Caplan & Merricks, 1978).
Analysis of pooled urine and faceal samples from the
above-treated rats (excluding the female rat with unusually-low
excretary rate) indicated the presence of about 5% of the 14C
recovered in the urine or in the faeces (corresponding, respectively,
to 2% and 1% of the administered dose) as the unchanged parent
compound. The other readioactive components in the urine and the
faeces were highly polar (Smilo et al., 1978).
Goat
Two goats, 1 of them with a cannulated bile duct, were fed
14C-dimethipin (98% radiochemically pure) at 500 ppm in the diet for
3 days. Samples of urine, liver, bile, and kidney were collected for
characterization and identification of metabolites. (No details of
experimental conditions were provided. Data were presented apparently
on only one of the treated animals.) Figure 1 presents the proposed
metabolic pathways for dimethipin in the goat.
Invariably, in urine, bile, liver and kidney, metabolites III and
IV were identified together with the unchanged parent compound. The
latter accounted for 2-8% of the 14C recovered in each case. Some
alcohol metabolites of dimethipin, viz. metabolites I, II, and V,
were identified only in the urine and bile. Most of the radioactive
components in urine, bile, liver, and kidney were of a highly polar
nature, and were present as conjugates such as glucuronide, cysteine,
and acetylcysteine conjugates (McManus, 1984).
Proposed metabolic pathways for dimethipin in the goat
 TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical dimethipin (99.7% pure)
at 0, 50, 200, or 800 ppm for 105 days prior to mating (1 male and 2
females; "sibling and half-sibling mating avoided") to initiate a
2-generation (2 litters/generation) reproduction study (day 0 of
gestation = day when positive vaginal smear or copulatory plug
detected). Weanlings of the second litter (F1b) were selected to
become parents of the next generation and mated after being placed on
the test diets for 125 days. In each generation, the second mating
trial took place at least 14 days after weaning at 21 days of age of
the first litters, i.e. F1a and F2a.
In the parental generations, mortality (the incidence of which
was not dose-related) occured only in the females. No compound-related
behavioural abnormalities were apparent. F0 and F1b female adults at
800 ppm weighed less than the concurrent controls during the premating
period, throughout gestation, and practically throughout the lactation
periods. Food consumption was depressed at 800 ppm in the pre-mating
period in F0 females from weeks 6-10, in F1b females from weeks
6-17, and in F1b males at weeks 1, 4, and 9. Fertility in males ("a
demonstrated ability to impregnate at least 1 female"), mating index
(% females mated), gestation index (% mated females with viable
litters), the number of days required by females to mate, and the
duration of gestation period in treated groups were all comparable to
control values. With respect to the progeny generations, the mean
number of pups per litter born alive, survival of pups to days 4, 7,
14, and 21, sex ratio, and behaviour of pups were not adversely
affected. Pup weight was reduced at 800 ppm in F1a and F1b litters on
days 7, 4, and 21. Pup weights on day 21 were decreased in F1a
litters at 200 ppm and in F2a litters at 800 ppm. Gross external
examination of all pups, including those found dead, revealed only one
abnormal pup in the study, which was a stillbirth in a F1a litter at
200 ppm.
Findings from gross pathological examination of all parental
animals sacrificed after weaning (F0) or 30 days after weaning (F1b)
of the second litters (i.e. after 32 weeks and 39 weeks of dietary
feeding, respectively) and weanlings from each progeny generation were
not significantly different between control and treated groups.
Organ-weight determinations on all F1b adults and on 5 male and 5
female weanlings per group of F1b and F2b litters showed increased
organ/body-weight ratios of the liver at both 200 and 800 ppm, and of
the kidney and brain at 800 ppm in F1b adult females. The
organ/body-weight ratio of the liver was, however, depressed at 50 ppm
in F1b adult females. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults plus 5
male and 5 female weanlings per group from F1b and F2b litters, and
of gross lesions and gonads from F0 adults, indicated no significant
changes attributable to treatment.
The study showed 200 ppm as a no-effect level for reproduction
(The finding of a decrease in pup weight on day 21 at 200 ppm in F1a
litters was unlikely to be compound-induced, as it occurred only in a
single generation and was not recurrent in nature) (Kehoe & MacKenzie,
1982).
Special studies on teratogenicity
Rat
Groups of sexually-mature mated female rats (BLU:(SD)BR) were
intubated with technical dimethipin (97.5% purity) as a suspension in
corn oil at 0, 80, 400, or 800 mg/kg b.w./day from day 6 through day
15 of gestation (Day 0 = day vaginal plug observed). An additional
group of mated female rats, treated with 250 mg/kg b.w./day of
acetylsalicylic acid (aspirin), was used as the positive control. Due
to "excessive deaths" of dams at both 400 and 800 mg/kg b.w./day
within 8 days of initiation of treatment, these dosage groups were
terminated and were not investigated further. Two new dosage groups,
30 and 160 mg/kg b.w./day, were then added to the test 2 weeks after
the study began, with the original dosage group of 80 mg/kg b.w./day
being maintained as the intermediate level (no concurrent control
groups were included for the 2 new dosage groups). The dams were
sacrificed on day 20 of gestation and the foetuses were removed by
Caesarean section for gross external, visceral, and skeletal
examination.
At dosage levels up to 160 mg/kg b.w./day, no compound-related
mortality or clinical signs were observed. Growth rate of dams during
gestation was comparable in all groups. The total number of dams per
group that were pregnant and alive on day 20 ranged from 20 to 22. The
mean number of implantation sites or live foetuses, foetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, without a concomitant rise in the incidence of
pregnant dams with resorption(s), was noted at 160 mg/kg b.w. There
were no significant differences between control and treated groups in
skeletal or visceral malformations of foetuses. The positive control
group exhibited a number of foetal abnormalities such as
encephalomenigocele and gastroschisis.
The study demonstrated that the compound was non-teratogenic,
non-fetotoxic, and non-toxic to the dams at levels as high as
160 mg/kg b.w. (Knickerboker et al., 1977).
Rabbit
Groups of 16 sexually-mature female Dutch Belted rabbits,
artificially inseminated, were intubated with technical dimethipin
(98.3% purity) as a suspension in 0.5% carboxymethyl cellulose at 0,
7.5, 20, or 40 mg/kg b.w./day (at a constant volume of 1 ml/kg b.w.)
on days 6-27 of gestation (day 0 of gestation = the day of
insemination). The does were sacrificed on day 28 of pregnancy, and
the uterine contents were examined. Foetuses (including those aborted
or dead) were subjected to examination for gross, skeletal, and
visceral abnormalities.
No deaths were noted. A slight increase, as compared to
concurrent controls, "in the number of females with a reduction in the
amount of faeces observed beneath the cage" was reportedly seen at
both 20 and 40 mg/kg b.w. at various intervals during gestation. Food
consumption data were not available. Does at 40 mg/kg b.w. showed an
actual weight loss between days 6 and 12, and maternal weight gain was
depressed in a dose-response pattern in all treated groups between
days 6 and 28. Fertility rate ranged from approximately 88 to 94% in
control and treated groups. One doe each at 0, 20, and 40 mg/kg b.w.
"aborted" on day 28. Seven non-viable foetuses were found in the one
doe each at 0 and 20 mg/kg b.w. that "aborted". The doe at 40 mg/kg
b.w. which "aborted" had 3 late resorptions.
At terminal sacrifice, gross pathological findings in treated
does were comparable to those in the contols. No significant
differences between controls and treated groups were found in the mean
number of corpora lutea, implantations, early or late resorptions,
viable or non-viable foetuses, or foetal weight. A non-dose-related
increase in post-implantation loss due mainly to a non-dose-dependent
increase in early resorptions was noted in all treated groups. The
values of this particular parameter in the treated groups, however,
were within the range of historical control values submitted. The sex
ratio (M/F) of foetuses was elevated at 40 mg/kg b.w., with the mean
number of female foetuses being reduced. An increase in incidence of
foetuses (as well as litters containing foetuses) with 27 presacral
vertebrae and with scoliosis (with or without associated rib
anomalies) was observed at 40 mg/kg b.w., as compared to concurrent
control or historical control incidences presented in the report from
a total of 951 foetuses from 149 litters (from an unspecified number
of studies with Dutch Belted rabbits over an unspecified period of
time). There was no apparent dose- or compound-related increase in
frequency of foetal soft-tissue abnormalities.
Although the possibility exists that the increase in incidence of
the particular skeletal anomalies seen at 40 mg/kg b.w. might be
related to maternal toxicity, prudence dictates that 20 mg/kg b.w. be
considered as a clear-cut teratogenic no-effect level (McMeekin
et al., 1981).
Special studies on mutagenicity
Dimethipin was without mutagenic activity in a number of assays
with micro-organisms and mammalian cells, except with the mouse
lymphoma forward-mutation assay in the presence of metabolic
activation (Table 1).
Special studies on eye and skin irritation
In an eye irritation study with New Zealand White rabbits,
dimethipin (presumably technical grade of unspecified purity) was
found to be extremely irritating to the eye (Baker et al., 1976).
Dimethipin (presumably technical grade of unspecified purity) was
shown to be slightly irritating to the skin of New Zealand White
rabbits in a primary skin-irritation test (Baker et al., 1976).
Special study on skin sensitization
Results of a dermal-sensitization study in male guinea-pigs
(Hartley strain) indicated technical dimethipin (purity not given) to
be a weak skin sensitizer (Madison, 1983).
Table 1. Results of mutagenicity assays on dimethipin*
Test Organisms/ Dosage levels Results References
tissues tested
In vitro
Reverse S. typhimurium 1-1000 Negative Jagannath &
mutation strains TA1538, µg/plate (1) Brusick,
TA1537, TA1535, 1978, 1981
TA98, & TAlO0
S. cerevisiae 1-1000 Negative Jagannath &
strain D4 µg/plate (1) Brusick, 1978
Mitotic non- S. cerevisiae 1-2000 Negative Bootman & Lodge,
dysjunction, strain D6 µg/plate (1) 1982
recombination,
& mutation
Mitotic gene S. cerevisiae 125-2000 Negative Forster et al.,
conversion strain D4 µg/ml (1) 1984a
Chromosome Chinese hamster 5-50 Negative Sorg et al., 1983
aberration ovary cells µg/ml (1)
Sister chromatid Chinese hamster 1.56-25 Negative Galloway & Brusick,
exchange ovary cells µg/ml(3) (1) 1981
3.1-200
µg/ml(4)
Mouse lymphoma L5178Y TK+/- 1.56-75 (2) Myhr & Brusick,
forward mouse lymphoma µg/ml(3) 1981
mutation cells 12.5-200
µg/ml(4)
Table 1. (Con't)
Test Organisms/ Dosage levels Results References
tissues tested
In vivo
Micronucleus Mouse 2 successive Negative Forster et al.,
daily oral (5) 1984b
doses at
22,73.3, or
220 mg/
kg b.w./day
(males) or
at 30, 100, or
300 mg/ kg b.w./
day (females)
* Technical dimethipin (> 98% purity) was used in all the above studies.
(1) With or without metabolic activation.
(2) Negative without metabolic activation but positive with metabolic activation at 75 µg/ml and above.
(3) Non-activated.
(4) Activated.
(5) 1/5 Males and 5/5 females at the top dosage level died after the first dose.
Acute toxicity
Table 2. Acute toxicity of dimethipin*
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 440 Shapiro, 1977a
F 600
Rat M&F oral 1180 Varner &
Matthews, 1977
M i.p. 235 Shapiro, 1977b
F 236
M&F inhalation LC50 Babish, 1977
(1h exposure) > 20 mg/l
Rabbit M&F dermal > 12,000 Reagen & Becci,
(24h exposure) 1982
* Technical dimethipin of > 97.5% purity was used in the
oral studies.
In both mice and rats, death occurred 24 to 48 hours after
treatment. The principal toxic effects in rats were a decrease in body
tone and slowed respiration. Data were not provided on mice with
respect to toxic signs (Shapiro, 1977a; Varner & Matthews, 1977).
Dog
Groups of 24-hour fasted crossbred dogs were given single oral
doses of technical dimethipin (98.3% purity) in gelatin capsules at 0
(4 males), 464 (4 females), 600 (3 males and 1 female), 1000 (2 males
and 2 females), or 1560 mg/kg b.w. (1 male and 3 females). All 4
animals at 1560 mg/kg b.w., 1 male and 2 females at 1000 mg/kg b.w.,
and 1 male and 1 female at 600 mg/kg b.w. died 8-28 hours post-dosing.
Emesis, observed in all animals of all treated groups, usually within
1 hour of dosing, was recurrent in many of the affected animals,
generally during the next 2 to 4 hours. Other frequently-noted toxic
signs included general "depression" and inappetance, salivation,
tremor, and loose blood-stained stools. Survivors recovered from the
toxic signs 2 to 10 days after treatment. The major finding at
necropsy was marked irritation to the stomach and the intestine at and
above 600 mg/kg b.w. The author calculated the oral LD50 of the
compound in dogs to be 690 mg/kg b.w. (Strong, 1981).
Short-term studies
Rat
Male and female Charles-River rats (15 per sex per group, 28 days
old) were fed dietary levels of technical dimethipin (> 99.2% purity)
at 0, 100, 300, or 1000 ppm for 95 days. There was no treatment-
related mortality or abnormal behaviour. Food consumption was slightly
depressed in females at 1000 ppm throughout the study. Body weight was
unaffected. Haematology, blood chemistry, and urinalysis, determined
in 10 males and 10 females per control and top-dosage group after 45
and 85 days of the study, indicated no significant compound-related
effects. At termination, organ/body-weight ratios of liver and kidney
were elevated in females at 1000 ppm. Significant differences were not
observed between control and treated groups in gross pathological
changes. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females per control
and top-dosage group failed to show any lesions attributable to
inclusion of the compound in the diet. The no-effect level appeared to
be 300 ppm (Marias et al., 1976).
Dog
Groups of 4 male and 4 female purebred Beagle dogs (about 6
months old) were given technical dimethipin (> 99.2% purity) in their
diet at 0, 100, 300, or 1000 ppm for 90 days. No mortality occurred.
Compound-related effects were not apparent on behaviour, body weight,
or food consumption during the study or on blood chemistry or
haematology conducted after 42 and 85 days of dietary feeding.
Urinalysis determined at the same 2 intervals indicated an increase
(not dose-related) in incidence of females of all treated groups with
moderate to large amounts of "crystals" in their urinary sediments
after 85 days. Other urinalysis parameters were not affected (that the
particular urinary finding in females of all treated groups was not
likely to be compound-related was supported by the absence of effect
on any of the urinalysis parameters monitored in the 1-year feeding
study in dogs, summarized below). At terminal sacrifice, organ weights
and gross pathology were not altered by treatment. Microscopic
examination of a large number of tissues, including the testis, from
each animal in the study revealed esophagus lesions characterized by
"focal mucosal vesicles containing a few acute inflammatory cells" in
1/8 animals at 300 ppm and 3/8 animals at 1000 ppm, but in none of the
concurrent control or of the lowest-dosage (100 ppm) groups. Based on
the data, 100 ppm could be considered as the no-effect level (Burtner
et al., 1976).
Male and female purebred Beagle dogs (6 males and 6 females per
group; about 7.5 months old), individually caged, were given technical
dimethipin (99.7% purity) in their diet at 0, 300, 1000, or 3000 ppm
for one year. One male and three females at 3000 ppm died or were
sacrificed in extremis between weeks 13 and 52. "Thinness", a major
clinical sign, was seen frequently in most animals at 3000 ppm and
infrequently in one animal at 1000 ppm. Other signs such as
dehydration and paleness of the gums were also noted infrequently at
3000 ppm. Actual weight loss or growth depression and a decrease in
food consumption were evident in both sexes at 3000 ppm throughout, or
practically throughout, the study. Females at both 300 and 1000 ppm
exhibited a marginal, but not consistently dose-dependent, growth
reduction (= 10%) between weeks 12 and 48. Animals of the top-dosage
group exhibited abnormalities in the T-wave in the electrocardiograms
taken at termination and, upon ophthalmoscopic examination, increased
incidence of conjunctival discharge and inflammation as well as
corneal irregularities and roughening at week 27, but not at week 52.
Findings at physical examination described as "severe to slight
thinness and irregular or erratic heart beat" were noted almost
exclusively in animals at 3000 ppm throughout the study.
Monthly determinations of haematology and blood chemistry
revealed deviations from control values, mainly at 3000 ppm (both
sexes), in many parameters including decreased haematocrits, increased
platelet counts, and depressed values of total protein, albumin,
globulin, calcium, BUN, and creatinine at most sampling intervals.
Animals at 1000 ppm displayed decreased values of BUN (both sexes) and
creatinine (males) at many of the sampling intervals. As compared to
concurrent controls, males of all treated groups seemingly showed a
slight but consistent and generally dose-related decrease in
erythrocyte counts and haemoglobin levels. However, these findings
were unlikely to be treatment-associated, because values of these 2
haematological parameters for males even at 3000 ppm were within the
normal ranges given both for control Beagles in the published
literature (Bushby, 1970) and those reportedly recorded for control
Beagle dogs maintained in the testing laboratory. Urinalysis,
including microscopy of urinary sediments, conducted bi-monthly gave
no significant findings related to treatment.
At termination, gross pathological findings in the treated groups
were not significantly different from those in the controls. There was
an increase in organ/body-weight ratio of kidneys at both 1000 and
3000 ppm (both sexes), of liver in males at 3000 ppm and in females at
1000 ppm and above, of brain at 3000 ppm (both sexes), and of testes
at 3000 ppm. Histopathological evaluation of an extensive number of
tissues from each animal showed the occurrence of testicular
degeneration in 0/6, 2/6, 1/6, and 3/6 males at 0, 300, 1000, and
3000 ppm respectively. Severe and diffuse testicular degeneration was
seen in one affected male each at 300 ppm and 3000 ppm, while
generally mild and focal degeneration of the testes was present in the
other affected males. Admittedly, the incidence and severity of
testicular degeneration did not follow a dose-response relationship.
However, on account of the complete absence of the lesion from the
concurrent controls, and from a total of 7 similar 1-year studies
conducted at the testing laboratory comprising over 20 control male
dogs (McGee, 1983), the possibility of the testicular lesion being
related to the compound could not be completely ruled out. Other
microscopic findings likely to be attributable to treatment included
the presence in the top-dosage group of hypocellularity of the bone
marrow, lesions in the gastrointestinal tract (gastritis, edema,
ulceration) and heart (haemorrhage), and thymus atrophy, as well as an
increased incidence of lesions in the kidney (nephritis), liver
(centrilobular degeneration), lymph node (lymphadenitis), and spleen
(hyperplasia) at both 1000 and 3000 ppm.
The study did not permit the setting of a no-effect level, mainly
because of uncertainty concerning the occurrence of testicular
degeneration, which occurred at all dose levels (Benson, 1981).
Long-term studies
Mouse
Male and female CD-1 mice (50 animals per sex per group, about 50
days old), housed 5 per sex per cage, were fed dietary levels of
technical dimethipin (97-98% purity) at 0, 80, 400, or 2000 ppm for 78
weeks to evaluate the chronic toxicity and carcinogenic potential of
the compound. (Animals fed dimethipin were kept in the same room for
about 35 weeks as animals in a companion study receiving a highly
photodegradable compound identified only with a code name. It was
stated, but unsubstantiated with data, that, based on results of diet
analyses, dimethipin was stable in the diet for 7 days and that the
mixture of dimethipin and basal diet was satisfactorily homogeneous.)
All animals in the study, viz., those sacrificed in extremis or
that died during the study, and those sacrificed terminally, were
subjected to gross pathological examination and to microscopic
evaluation of a wide range of tissues, including the brain.
Mortality was not influenced by treatment. Between 58 and 78% of
the males and 76 and 86% of the females of the control and treated
groups were still alive at the conclusion of the study. A slight
(< 10%) and non-dose-related decrease in body-weight gain was noted
in males at 400 ppm and above during the first 13 weeks. Food
consumption was not affected in any consistent dose-related pattern.
There were no significant differences between control and treated
groups in clinical signs and incidence of palpable nodules or tissue
masses. Haematology determined on 5 males and 5 females per group at 3
intervals of the study revealed a significant increase in males at
2000 ppm of haematocrit levels at week 13 and of haemotocrit,
haemoglobin, and erythrocyte values at week 78. Terminal haematocrit
levels were elevated in females at both 400 and 2000 ppm. Females of
all treated groups showed a statistically-significant increase, albeit
not strictly dose-dependent, of erythrocyte counts at week 78. Blood-
chemistry and urinalysis parameters were not studied. Gross
pathological alterations and weights of selected organs were not
significantly different between control and treated groups.
Based on tabulated data on "individual histopathology findings"
(no detailed histopathological data with morphological descriptions of
lesions on individual animals were available), compound-induced non-
neoplastic changes were not evident. The only notable neoplastic
finding appeared to be an elevated incidence of pulmonary
(aveolar/bronchiolar) tumours in males at 2000 ppm, as shown in
Table 3.
Table 3 Number of males with lung tumours/number of males with the lung
examined microscopicallya
Pulmonary tumour Control 80 ppm 400 ppm 2000 ppm Historical controlb
(alveolar/bronchiolar) Total Range
adenocarcinomac 1/49 3/49 3/50 7/50 6/168 0/13(0)-
(2) (6.1) (6) (14)d (3.6) 2/24(8.3)
adenoma 5/49 3/49 3/50 5/50 15/168 1/24(4.2)-
(10.2)e (6.1) (6) (10)f (8.9) 2/13(15.4)
adenocarcinoma 6/49 6/49 6/50 12/50 21/168 7/61(11.5)or
or adenoma (12.2) (12.2) (12) (24) (12.5) 2/13(15.4)
a Figure in parenthesis indicates incidence in %.
b Data presented in the submitted report on a total of 168 CD-1 control male
mice from five 78-week studies over an unspecified timeframe.
c Multiple adenocarcinomas noted in 1 control and 2 animals at 2000 ppm.
d 1/7, ...
e 1/5, or ...
f 2/5 of the lung-tumour bearers died or were sacrificed at an unspecified time
during the study. All other bearers of pulmonary tumours were terminal
survivors.
When compared to concurrent-control or historical-control
incidences from a total of 5 studies, the incidence of lung
adenocarcinoma, but not of adenoma alone, was significantly increased
in males at 2000 ppm (P < 0.05, Fisher exact probability). However,
comparison with the maximum historical-control incidence with respect
to lung adenocarcinoma at 2000 ppm was not significant (P > 0.05).
Time-to-tumour (adenocarcinoma) or the multiplicity of tumours was not
modified by treatment. The combined incidence of lung adenocarcinoma
and adenoma was significantly elevated when compared to the
historical-control incidence from a total of 5 studies, but not when
compared with concurrent-control or maximum historical-control
incidences. There was no dose-related increase in the incidence of
benign and/or malignant lung tumours in the females. Other than lung
tumours, the incidence, location, and type of tumours in treated
groups were comparable to controls. About 30% of the male and 40% of
the female concurrent controls were found to have tumours. Lymphoma
(in males), lung tumours (in both sexes), and hepatocellular carcinoma
(in males) were the most frequently-observed spontaneous tumours. It
should be noted that the animals were about 50 days old at the
initiation of the study. This could compromise the sensitivity of the
test as a carcinogenicity study.
The study demonstrated that 80 ppm, equal to 12.3 mg/kg b.w./day,
is a virtual no-effect level, based on the monitored criteria other
than tumours. The lung-tumour data are unclear (Serota et al.,
1981a).
Rat
Groups of 50 male and 50 female rats (Sprague-Dawley CD strain,
about 40 days old), individually caged, were fed technical dimethipin
(97-98% purity) in their diet at 0, 40, 200, or 1000 ppm for 104 weeks
to assess the chronic toxicity and carcinogenic potential of the
compound. (The control group served as common controls for this study
and for a companion study on a chemical identified only by a code
name. Whether treated animals from the two studies were kept in the
same room was not specified.) All animals sacrified in moribund
condition or dying during the study, and all survivors sacrificed
terminally (during weeks 105 and 106), were subjected to necropsy and
histopathological examination of a variety of tissues, including the
brain plus any "unusual" lesions. Sections of spinal cord and "head"
from 10 male and 10 female terminal survivors per group were also
evaluated microscopically.
Survival, not adversely affected by treatment, appeared to be
better in the top-dosage group than in the concurrent control or in
lower-dosage groups. At the end of 104 weeks, with the exception of
males at 200 ppm having a survival rate on only 44%, between 50 and
72% of the males and females of all groups, including the control,
were still alive. Clincial signs related to treatment were not
evident. There were no dose- or compound-related effects on food
consumption or incidences of palpable nodules, tissue masses, or wart-
like lesions. A slight but consistent growth depression (< 5% in
males and < 10% in females) was seen at 1000 ppm between weeks 43
and 95 in males and between weeks 51 through 87 in females.
Haematology and blood chemistry conducted on 5 males and 5 females per
group at 5 intervals over the course of the study indicated that
females of all treated groups displayed an increase of total-protein
values at week 13 only, and a decrease of platelet counts at week 104,
the only sampling interval for this particular parameter. Other
deviations from controls were also observed in certain haematological
and blood-chemistry values, but these were essentially confined to the
top-dosage group. Significant differences were not apparent between
control and treated groups in urinalysis parameters monitored on 5
males and 5 females per group at the same intervals as the blood
studies. Gross pathological changes in treated animals were not
significantly different from those in the controls. Organ-weight
determinations showed an increase (non-dose-related in males and dose-
related in females) of the organ/body-weight ratio of the liver in
males of all treated groups and in females at both 200 and 1000 ppm.
In addition, absolute weight and organ/body-weight ratios of the
adrenal gland were decreased in females of all treated groups.
Histopathologically, "focally-dilated bile ducts containing basophilic
homogeneous material" occurred in 1 male and 1 female in the control
group, 2 males and 3 females at 40 ppm, 5 males and 9 females at 200
ppm, and 33 males and 18 females at 1000 ppm. This finding was likely
to be related to inclusion of the compound in the diet. Microscopic
changes in other tissues, including the adrenal gland, were comparable
in treated animals to those in the controls. An interesting but non-
dose- or compound-related microscopic finding was that 9-27% of the
males in the control and treated groups showed lactation and/or
galactocoele. Whether this might be connected with the observation of
an increased incidence of mammary fibroadenoma in males of the top-
dosage group, as pointed out later, was not certain. An evaluation of
the tumour data revealed that the only noteworthy findings were those
tabulated in Table 4.
The data in Table 4 seem to indicate increased incidences of
astrocytoma in males and hepatocellular carcinoma in females at both
200 and 1000 ppm and of mammary fibroadenoma in males at 1000 ppm. The
incidence of none of these tumours was, however, significantly
different from the concurrent controls (P > 0.05, Fisher exact
probability). This result was confirmed for hepatocellular carcinoma
when compared with historical control incidences. However, with
respect to astrocytoma in males, the incidence was significantly
increased at both 200 and 1000 ppm (P< 0.05) when compared with
historical-control incidence from a total of 7 studies. Comparison
with maximum historical-control incidence was nevertheless not
significant (P > 0.05), even at 1000 ppm. It might be noted that one
additional glioma was reportedly found (by a consultant to the
company) in the control group upon microscopic evaluation of 3
additional brain sections from each animal of the male control and
treated groups. In addition, no pre-neoplastic lesions (gliosis) were
seen in the original or additional brain slides (Squire, 1984). The
latency period of astrocytoma was apparently not reduced by treatment.
Overall, no significant differences between control and treated groups
were noted in the incidence of animals with tumours (benign and/or
malignant), benign tumours, malignant tumours, or multiple primary
tumours. Over 80% of the males and 90% of the females of the
concurrent control group were tumour bearers, with pituitary adenoma
and adrenal tumours in both sexes and mammary tumours in females being
the most prevalent tumours.
The study indicated that 40 ppm, the lowest tested-level, was a
minimum-effect level on parameters other than tumours (Serota
et al., 1981b).
EVALUATION
COMMENTS
In rats, the compound is readily absorbed, metabolized, and
eliminated via the urine and faeces. It is degraded primarily to polar
metabolites, with less than 5% of a single oral dose being recovered
as unchanged dimethipin in the excreta. In goats, dimethipin is
extensively degraded, also primarily to polar metabolites.
Biotransformation of the compound includes hydrolysis, oxidation,
decarboxylation, a ring opening, and conjugation.
Dimethipin has an oral LC50 value of about 500 mg/kg b.w. in
mice and 1200 mg/kg b.w. in rats.
A 2-generation (2 litters/generation) reproduction study in rats,
as well as teratology studies in rats and rabbits, were negative.
Practically all of the mutagenic studies available were negative. The
only positive mutagenic response was seen with the mouse lymphoma
forward mutation assay and only in the presence of metabolic
activation.
A 1-year feeding study in dogs failed to show a no-effect level,
mainly because of the uncertainty concerning the presence of
testicular degeneration even at 300 ppm, the lowest tested level.
However, taking into consideration the lack of a dose-response
relationship regarding incidence and severity of the testicular
lesion, the absence of effect of the compound on the testes of rats in
No. of animals with the tumour/No. of animals with the tissue examined
microscopically
Location *Historical Control
& type of
tumour Control 40 ppm 200 ppm 1000 ppm Total Range
Male Female Male Female Male Female Male Female Male Female Male Female
Brain
Astrocytoma 1a/-50(2) 1/49(2) 0/49(0) 2/50(4) 3b/-50(6) 0/50(0) 5c/50(10) 0/50(0) 1/279 0/266 0/43-1/21 0/21-0/37
** 2/50(4) (0.4) (0) (0)-(4.8) (0)
Mammary
gland ***
Fibroadenoma 0/43(0) N.P. 0/31(0) N.P. 0/33(0) N.P. 3/45(6.7) N.P. N.A. N.A. N.A. N.A.
Liver
Hepatocellular
cardnoma 2/50(4) 1/50(2) 1/50(2) 1/50(2) 5/50(10) 4/50(8) 2/50(4) 4/50(8) 13/305 8/285 0/35-3/34 0/32-2/31
(4.3) (2.8) (0)-(8.8) (0)-(6.5)
Neoplastic
nodule **** 2/50 6/50 2/50 5/50 5/50 5/50 2/50 5/50 N.A. N.A. N.A. N.A.
Figure in parenthesis indicates incidence in %
a astrocytoma present in one animal found dead at 20 weeks. This incidence (1/50 or 2%) was used as a basis of comparison in statistical
analyses.
b all 3 brain tumours found in terminal survivors.
c the time of detection of the brain tumour ranging irom 71 to 101 weeks with the mean being 83.4 weeks.
N.P. data not presented due to the complete absence of a dose-response relationship re incidence
N.A. not available
(Con't)
* Data included in the submitted report from a total of seven 104-week studies In Sprague-Dawley rats over an unspecified time-frame.
** The histopathological examination of 3 additional brain section from each male of control and all treated groups reportedly yielded
one additional glioma which was found in one control terminal survivor. It was also indicated that no preneoplastic lesions (gliosis)
were found in the original and the additional brain slides (Squire, 1984)
*** The historical control incidence of mammary fibroadenoma in an unspecified number of male Sprague-Dawley rats maintained in the
testing laboratory was stated to range from 0 to 3% (Serota 1985).
**** Not considered as tumour per se but included for information only.
the 2-generation reproduction study, and the similarly-negative effect
on the testes of mice and rats in the long-term studies, plus the
virtual absence of other toxic effects at the lowest-tested level, it
was concluded that a level somewhat below 300 ppm could reasonably be
taken as a no-effect level for this species. In this connection, the
Meeting agreed that 100 ppm, the no-effect level established in an
available 90-day dog-feeding study, be accepted as a 1-year no-effect
level for this species.
In the long-term mouse and rat studies, there was seemingly an
increase in the incidence of lung adenocarcinoma in male mice of the
top-dosage group (2000 ppm) and of astrocytoma in male rats of both
the intermediate- and top-dosage groups (i.e. 200 ppm and 1000 ppm).
Although the evidence for a causal relationship between these
particular tumour findings and treatment did not appear to be
convincing, such a possibility could not be excluded at this time.
Before a more definitive opinion on the tumourigenic/carcinogenic
potential of the compound can be expressed, additional information, as
indicated under "Further work or information required", is needed on
those mouse and rat studies from which data on historical control
incidences of certain tumours (specifically lung tumours in mice and
astrocytoma and liver tumours in rats) were obtained.
In view of the concerns with respect to increased incidence of
lung adenocarcinoma in male mice and of astrocytoma in male rats of
the long-term studies, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 80 ppm in the diet, equal to 12.3 mg/kg b.w.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.003 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
1. Information on those mouse and rat studies from which data on
historical control incidences of certain tumours, viz. lung
tumours in CD-1 mice and astrocytoma and liver tumours in
Sprague-Dawley rats, were obtained and presented in the submitted
reports. (Information for each of the studies should include date
of each study, age of animals at initiation, mortality rate of
animals, and experimental conditions such as diet, number of
animals per cage, etc.)
2. Further pharmacokinetic and metabolic studies in rats and/or a
non-rodent mammalian species using appropriate multiple-dosage
levels.
3. Acute oral toxicity studies on major plant metabolites which are
not found in animals if these are liable to occur at substantial
residues.
DESIRED
1. Results of diet analysis for the 18-month mouse and 2-year rat
feeding studies.
2. Studies on any potential oestrogenic or other hormonal effect of
dimethipin, including the determination of the affinity of the
compound for steroid receptors.
3. Further studies in dogs to assess effects, if any, of dimethipin
on the testis.
4. Observations in man.
REFERENCES
Babish, J.G. Harvade(R) (N252) Tech. C-8-1162-00. Acute inhalation
(1977) study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc., submitted to WHO by Uniroyal
Inc., USA.
Baker, R.C., Mastri, C.W., Kinoshita, F.K., & Keplinger, M.L. Acute
(1976) irritation tests with a sample coded N252-C10406, Lot No.
BL7668 in albino rabbits. Unpublished report (IBT No. 8530-
08861) from Industrial Bio-Test Laboratories, Inc., USA,
submitted to WHO by Uniroyal Inc., USA (validated by the
Canadian Health Protection Branch).
Benson, B.W. 1-Year dietary toxicity study in dogs with N252.
(1981) Unpublished report from International Research and
Development Corp., USA, submitted to WHO by Uniroyal Inc.,
USA.
Bootman, J. & Lodge, D.C. ARS7728 (technical grade Harvade (R):
(1982) Assessment of its ability to induce genetic damage in
Saccharomyces cerevisiae. Unpublished report from Life
Science Research, England, submitted to WHO by Uniroyal
Inc., USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K, & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 611-08063) from Industrial
Bio-Test Laboratories, Inc., USA, submitted to WHO by
Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
Bushby, S.R.M. "Hematological studies during toxicity tests." In
(1970) Methods in Toxicology, edited by Paget, G.E. Blackwell
Scientific Publications, Oxford and Edinburgh.
Caplan, J. & Merricks, D.L. 14C-Harvade(R) radiocarbon study in rats.
(1978) Unpublished report from Biospherics Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Forster, R., Edwards, N., & Nunziata, A. Mitotic gene conversion in
(1984a) Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from
Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Forster, R., Mosesso, P., & Nunziata, A. Micronucleus test. Test
(1984b) substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Galloway, S.M. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(1981) (N252) 98% D-11401 in the sister chromatid exchange assay
with Chinese hamster ovary (CHO) cells. Final Report.
Unpublished report from Litton Bionetics, Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of N-252,
(1978) technical lot D10406, BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(19981) (N252) 98% D-11401 in the Ames Salmonella/microsome plate
test. Final report. Unpublished report from Litton
Bionetics, Inc., USA, submitted to WHO by Uniroyal Inc.,
USA.
Kehoe, D.F. & MacKenzie, K.M. Final report. Two-generation rat
(1982) reproduction study with N252. Unpublished report from
Hazleton Raltech Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Knickerbocker, M., Re, T.A., & Babish, J.G. Teratologic evaluation of
(1977) N252 (Harvade(R)) technical in Sprague-Dawley rats.
Unpublished report from Food and Drug Research Laboratories,
Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Madison, W.A. Technical grade Harvade(R). Dermal sensitization in
(1983) guinea pigs (modified closed patch technique). Unpublished
report from Hazleton Raltech, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT No. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA, submitted to
WHO by Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
McGee, D.H. Unpublished letter from International Research Development
(1983) Corp., USA, to Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McManus, J.P. Metabolism of Harvade(R) in goats. Unpublished
(1984) report from Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McMeekin, S.O., Schardein, J.L., & Blair, M. N252 (Harvade(R)
(1981) technical). Teratology study in rabbits. Unpublished report
from International Research and Development Corp., USA,
submitted to WHO by Uniroyal Inc., USA.
Myhr, B.C. & Brusick, D.J. Mutagenicity evaluation of Harvade(R) (N252)
(1981) in the mouse lymphoma forward mutation assay. Revised final
report. Unpublished report from Litton Bionetics, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Reagan, E.L. & Becci, P.J. Acute dermal toxicity study (LD50) in
(1982) albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Serota, D.G., Alsaker, R.D., & Banas, D. 18-Month toxicity and
(1981a) oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K., & Kunalzins, W. 104-Week
(1981b) chronic toxicity study in rats. N252 (Harvade(R) technical)
final report. Unpublished report from Hazleton Laboratories
America, Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G. Unpublished letter from Hazleton Laboratories America,
(1985) Inc. to Uniroyal Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977a) USA, submitted to WHO by Uniroyal Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977b) USA, submitted to WHO by Uniroyal Inc., USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K.. & Cardona, R.A.
(1978) Characteriztion of excretory metabolites from a 14C-
Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemical, submitted to WHO by Uniroyal Inc., USA.
Sorg, R.M., Naismith, R.W., & Matthews, R.J. CHO metaphase analysis
(1983) in vitro chromosome aberration analysis in Chinese hamster
ovary cells (CHO). PH320-UN-001-83 Harvade(R). Unpublished
report from Pharmakon Research International Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Squire, R.A. Unpublished letter to Uniroyal Chemical, submitted to WHO
(1984) by Uniroyal Inc., USA.
Strong, M.B. Studies on the acute oral toxicity of N252 (Harvade(R)) in
(1981) crossbred dogs. Unpublished report from Ciba-Geigy Australia
Ltd., submitted to WHO by Uniroyal Inc,, USA,
Varner, L.L. & Matthews, R.J. Acute oral LD50 in rats. Uni-N-252
(1977) C.N.D.-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA, submitted to WHO by
Uniroyal Inc., USA.
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical dimethipin (99.7% pure)
at 0, 50, 200, or 800 ppm for 105 days prior to mating (1 male and 2
females; "sibling and half-sibling mating avoided") to initiate a
2-generation (2 litters/generation) reproduction study (day 0 of
gestation = day when positive vaginal smear or copulatory plug
detected). Weanlings of the second litter (F1b) were selected to
become parents of the next generation and mated after being placed on
the test diets for 125 days. In each generation, the second mating
trial took place at least 14 days after weaning at 21 days of age of
the first litters, i.e. F1a and F2a.
In the parental generations, mortality (the incidence of which
was not dose-related) occured only in the females. No compound-related
behavioural abnormalities were apparent. F0 and F1b female adults at
800 ppm weighed less than the concurrent controls during the premating
period, throughout gestation, and practically throughout the lactation
periods. Food consumption was depressed at 800 ppm in the pre-mating
period in F0 females from weeks 6-10, in F1b females from weeks
6-17, and in F1b males at weeks 1, 4, and 9. Fertility in males ("a
demonstrated ability to impregnate at least 1 female"), mating index
(% females mated), gestation index (% mated females with viable
litters), the number of days required by females to mate, and the
duration of gestation period in treated groups were all comparable to
control values. With respect to the progeny generations, the mean
number of pups per litter born alive, survival of pups to days 4, 7,
14, and 21, sex ratio, and behaviour of pups were not adversely
affected. Pup weight was reduced at 800 ppm in F1a and F1b litters on
days 7, 4, and 21. Pup weights on day 21 were decreased in F1a
litters at 200 ppm and in F2a litters at 800 ppm. Gross external
examination of all pups, including those found dead, revealed only one
abnormal pup in the study, which was a stillbirth in a F1a litter at
200 ppm.
Findings from gross pathological examination of all parental
animals sacrificed after weaning (F0) or 30 days after weaning (F1b)
of the second litters (i.e. after 32 weeks and 39 weeks of dietary
feeding, respectively) and weanlings from each progeny generation were
not significantly different between control and treated groups.
Organ-weight determinations on all F1b adults and on 5 male and 5
female weanlings per group of F1b and F2b litters showed increased
organ/body-weight ratios of the liver at both 200 and 800 ppm, and of
the kidney and brain at 800 ppm in F1b adult females. The
organ/body-weight ratio of the liver was, however, depressed at 50 ppm
in F1b adult females. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults plus 5
male and 5 female weanlings per group from F1b and F2b litters, and
of gross lesions and gonads from F0 adults, indicated no significant
changes attributable to treatment.
The study showed 200 ppm as a no-effect level for reproduction
(The finding of a decrease in pup weight on day 21 at 200 ppm in F1a
litters was unlikely to be compound-induced, as it occurred only in a
single generation and was not recurrent in nature) (Kehoe & MacKenzie,
1982).
Special studies on teratogenicity
Rat
Groups of sexually-mature mated female rats (BLU:(SD)BR) were
intubated with technical dimethipin (97.5% purity) as a suspension in
corn oil at 0, 80, 400, or 800 mg/kg b.w./day from day 6 through day
15 of gestation (Day 0 = day vaginal plug observed). An additional
group of mated female rats, treated with 250 mg/kg b.w./day of
acetylsalicylic acid (aspirin), was used as the positive control. Due
to "excessive deaths" of dams at both 400 and 800 mg/kg b.w./day
within 8 days of initiation of treatment, these dosage groups were
terminated and were not investigated further. Two new dosage groups,
30 and 160 mg/kg b.w./day, were then added to the test 2 weeks after
the study began, with the original dosage group of 80 mg/kg b.w./day
being maintained as the intermediate level (no concurrent control
groups were included for the 2 new dosage groups). The dams were
sacrificed on day 20 of gestation and the foetuses were removed by
Caesarean section for gross external, visceral, and skeletal
examination.
At dosage levels up to 160 mg/kg b.w./day, no compound-related
mortality or clinical signs were observed. Growth rate of dams during
gestation was comparable in all groups. The total number of dams per
group that were pregnant and alive on day 20 ranged from 20 to 22. The
mean number of implantation sites or live foetuses, foetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, without a concomitant rise in the incidence of
pregnant dams with resorption(s), was noted at 160 mg/kg b.w. There
were no significant differences between control and treated groups in
skeletal or visceral malformations of foetuses. The positive control
group exhibited a number of foetal abnormalities such as
encephalomenigocele and gastroschisis.
The study demonstrated that the compound was non-teratogenic,
non-fetotoxic, and non-toxic to the dams at levels as high as
160 mg/kg b.w. (Knickerboker et al., 1977).
Rabbit
Groups of 16 sexually-mature female Dutch Belted rabbits,
artificially inseminated, were intubated with technical dimethipin
(98.3% purity) as a suspension in 0.5% carboxymethyl cellulose at 0,
7.5, 20, or 40 mg/kg b.w./day (at a constant volume of 1 ml/kg b.w.)
on days 6-27 of gestation (day 0 of gestation = the day of
insemination). The does were sacrificed on day 28 of pregnancy, and
the uterine contents were examined. Foetuses (including those aborted
or dead) were subjected to examination for gross, skeletal, and
visceral abnormalities.
No deaths were noted. A slight increase, as compared to
concurrent controls, "in the number of females with a reduction in the
amount of faeces observed beneath the cage" was reportedly seen at
both 20 and 40 mg/kg b.w. at various intervals during gestation. Food
consumption data were not available. Does at 40 mg/kg b.w. showed an
actual weight loss between days 6 and 12, and maternal weight gain was
depressed in a dose-response pattern in all treated groups between
days 6 and 28. Fertility rate ranged from approximately 88 to 94% in
control and treated groups. One doe each at 0, 20, and 40 mg/kg b.w.
"aborted" on day 28. Seven non-viable foetuses were found in the one
doe each at 0 and 20 mg/kg b.w. that "aborted". The doe at 40 mg/kg
b.w. which "aborted" had 3 late resorptions.
At terminal sacrifice, gross pathological findings in treated
does were comparable to those in the contols. No significant
differences between controls and treated groups were found in the mean
number of corpora lutea, implantations, early or late resorptions,
viable or non-viable foetuses, or foetal weight. A non-dose-related
increase in post-implantation loss due mainly to a non-dose-dependent
increase in early resorptions was noted in all treated groups. The
values of this particular parameter in the treated groups, however,
were within the range of historical control values submitted. The sex
ratio (M/F) of foetuses was elevated at 40 mg/kg b.w., with the mean
number of female foetuses being reduced. An increase in incidence of
foetuses (as well as litters containing foetuses) with 27 presacral
vertebrae and with scoliosis (with or without associated rib
anomalies) was observed at 40 mg/kg b.w., as compared to concurrent
control or historical control incidences presented in the report from
a total of 951 foetuses from 149 litters (from an unspecified number
of studies with Dutch Belted rabbits over an unspecified period of
time). There was no apparent dose- or compound-related increase in
frequency of foetal soft-tissue abnormalities.
Although the possibility exists that the increase in incidence of
the particular skeletal anomalies seen at 40 mg/kg b.w. might be
related to maternal toxicity, prudence dictates that 20 mg/kg b.w. be
considered as a clear-cut teratogenic no-effect level (McMeekin
et al., 1981).
Special studies on mutagenicity
Dimethipin was without mutagenic activity in a number of assays
with micro-organisms and mammalian cells, except with the mouse
lymphoma forward-mutation assay in the presence of metabolic
activation (Table 1).
Special studies on eye and skin irritation
In an eye irritation study with New Zealand White rabbits,
dimethipin (presumably technical grade of unspecified purity) was
found to be extremely irritating to the eye (Baker et al., 1976).
Dimethipin (presumably technical grade of unspecified purity) was
shown to be slightly irritating to the skin of New Zealand White
rabbits in a primary skin-irritation test (Baker et al., 1976).
Special study on skin sensitization
Results of a dermal-sensitization study in male guinea-pigs
(Hartley strain) indicated technical dimethipin (purity not given) to
be a weak skin sensitizer (Madison, 1983).
Table 1. Results of mutagenicity assays on dimethipin*
Test Organisms/ Dosage levels Results References
tissues tested
In vitro
Reverse S. typhimurium 1-1000 Negative Jagannath &
mutation strains TA1538, µg/plate (1) Brusick,
TA1537, TA1535, 1978, 1981
TA98, & TAlO0
S. cerevisiae 1-1000 Negative Jagannath &
strain D4 µg/plate (1) Brusick, 1978
Mitotic non- S. cerevisiae 1-2000 Negative Bootman & Lodge,
dysjunction, strain D6 µg/plate (1) 1982
recombination,
& mutation
Mitotic gene S. cerevisiae 125-2000 Negative Forster et al.,
conversion strain D4 µg/ml (1) 1984a
Chromosome Chinese hamster 5-50 Negative Sorg et al., 1983
aberration ovary cells µg/ml (1)
Sister chromatid Chinese hamster 1.56-25 Negative Galloway & Brusick,
exchange ovary cells µg/ml(3) (1) 1981
3.1-200
µg/ml(4)
Mouse lymphoma L5178Y TK+/- 1.56-75 (2) Myhr & Brusick,
forward mouse lymphoma µg/ml(3) 1981
mutation cells 12.5-200
µg/ml(4)
Table 1. (Con't)
Test Organisms/ Dosage levels Results References
tissues tested
In vivo
Micronucleus Mouse 2 successive Negative Forster et al.,
daily oral (5) 1984b
doses at
22,73.3, or
220 mg/
kg b.w./day
(males) or
at 30, 100, or
300 mg/ kg b.w./
day (females)
* Technical dimethipin (> 98% purity) was used in all the above studies.
(1) With or without metabolic activation.
(2) Negative without metabolic activation but positive with metabolic activation at 75 µg/ml and above.
(3) Non-activated.
(4) Activated.
(5) 1/5 Males and 5/5 females at the top dosage level died after the first dose.
Acute toxicity
Table 2. Acute toxicity of dimethipin*
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 440 Shapiro, 1977a
F 600
Rat M&F oral 1180 Varner &
Matthews, 1977
M i.p. 235 Shapiro, 1977b
F 236
M&F inhalation LC50 Babish, 1977
(1h exposure) > 20 mg/l
Rabbit M&F dermal > 12,000 Reagen & Becci,
(24h exposure) 1982
* Technical dimethipin of > 97.5% purity was used in the
oral studies.
In both mice and rats, death occurred 24 to 48 hours after
treatment. The principal toxic effects in rats were a decrease in body
tone and slowed respiration. Data were not provided on mice with
respect to toxic signs (Shapiro, 1977a; Varner & Matthews, 1977).
Dog
Groups of 24-hour fasted crossbred dogs were given single oral
doses of technical dimethipin (98.3% purity) in gelatin capsules at 0
(4 males), 464 (4 females), 600 (3 males and 1 female), 1000 (2 males
and 2 females), or 1560 mg/kg b.w. (1 male and 3 females). All 4
animals at 1560 mg/kg b.w., 1 male and 2 females at 1000 mg/kg b.w.,
and 1 male and 1 female at 600 mg/kg b.w. died 8-28 hours post-dosing.
Emesis, observed in all animals of all treated groups, usually within
1 hour of dosing, was recurrent in many of the affected animals,
generally during the next 2 to 4 hours. Other frequently-noted toxic
signs included general "depression" and inappetance, salivation,
tremor, and loose blood-stained stools. Survivors recovered from the
toxic signs 2 to 10 days after treatment. The major finding at
necropsy was marked irritation to the stomach and the intestine at and
above 600 mg/kg b.w. The author calculated the oral LD50 of the
compound in dogs to be 690 mg/kg b.w. (Strong, 1981).
Short-term studies
Rat
Male and female Charles-River rats (15 per sex per group, 28 days
old) were fed dietary levels of technical dimethipin (> 99.2% purity)
at 0, 100, 300, or 1000 ppm for 95 days. There was no treatment-
related mortality or abnormal behaviour. Food consumption was slightly
depressed in females at 1000 ppm throughout the study. Body weight was
unaffected. Haematology, blood chemistry, and urinalysis, determined
in 10 males and 10 females per control and top-dosage group after 45
and 85 days of the study, indicated no significant compound-related
effects. At termination, organ/body-weight ratios of liver and kidney
were elevated in females at 1000 ppm. Significant differences were not
observed between control and treated groups in gross pathological
changes. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females per control
and top-dosage group failed to show any lesions attributable to
inclusion of the compound in the diet. The no-effect level appeared to
be 300 ppm (Marias et al., 1976).
Dog
Groups of 4 male and 4 female purebred Beagle dogs (about 6
months old) were given technical dimethipin (> 99.2% purity) in their
diet at 0, 100, 300, or 1000 ppm for 90 days. No mortality occurred.
Compound-related effects were not apparent on behaviour, body weight,
or food consumption during the study or on blood chemistry or
haematology conducted after 42 and 85 days of dietary feeding.
Urinalysis determined at the same 2 intervals indicated an increase
(not dose-related) in incidence of females of all treated groups with
moderate to large amounts of "crystals" in their urinary sediments
after 85 days. Other urinalysis parameters were not affected (that the
particular urinary finding in females of all treated groups was not
likely to be compound-related was supported by the absence of effect
on any of the urinalysis parameters monitored in the 1-year feeding
study in dogs, summarized below). At terminal sacrifice, organ weights
and gross pathology were not altered by treatment. Microscopic
examination of a large number of tissues, including the testis, from
each animal in the study revealed esophagus lesions characterized by
"focal mucosal vesicles containing a few acute inflammatory cells" in
1/8 animals at 300 ppm and 3/8 animals at 1000 ppm, but in none of the
concurrent control or of the lowest-dosage (100 ppm) groups. Based on
the data, 100 ppm could be considered as the no-effect level (Burtner
et al., 1976).
Male and female purebred Beagle dogs (6 males and 6 females per
group; about 7.5 months old), individually caged, were given technical
dimethipin (99.7% purity) in their diet at 0, 300, 1000, or 3000 ppm
for one year. One male and three females at 3000 ppm died or were
sacrificed in extremis between weeks 13 and 52. "Thinness", a major
clinical sign, was seen frequently in most animals at 3000 ppm and
infrequently in one animal at 1000 ppm. Other signs such as
dehydration and paleness of the gums were also noted infrequently at
3000 ppm. Actual weight loss or growth depression and a decrease in
food consumption were evident in both sexes at 3000 ppm throughout, or
practically throughout, the study. Females at both 300 and 1000 ppm
exhibited a marginal, but not consistently dose-dependent, growth
reduction (= 10%) between weeks 12 and 48. Animals of the top-dosage
group exhibited abnormalities in the T-wave in the electrocardiograms
taken at termination and, upon ophthalmoscopic examination, increased
incidence of conjunctival discharge and inflammation as well as
corneal irregularities and roughening at week 27, but not at week 52.
Findings at physical examination described as "severe to slight
thinness and irregular or erratic heart beat" were noted almost
exclusively in animals at 3000 ppm throughout the study.
Monthly determinations of haematology and blood chemistry
revealed deviations from control values, mainly at 3000 ppm (both
sexes), in many parameters including decreased haematocrits, increased
platelet counts, and depressed values of total protein, albumin,
globulin, calcium, BUN, and creatinine at most sampling intervals.
Animals at 1000 ppm displayed decreased values of BUN (both sexes) and
creatinine (males) at many of the sampling intervals. As compared to
concurrent controls, males of all treated groups seemingly showed a
slight but consistent and generally dose-related decrease in
erythrocyte counts and haemoglobin levels. However, these findings
were unlikely to be treatment-associated, because values of these 2
haematological parameters for males even at 3000 ppm were within the
normal ranges given both for control Beagles in the published
literature (Bushby, 1970) and those reportedly recorded for control
Beagle dogs maintained in the testing laboratory. Urinalysis,
including microscopy of urinary sediments, conducted bi-monthly gave
no significant findings related to treatment.
At termination, gross pathological findings in the treated groups
were not significantly different from those in the controls. There was
an increase in organ/body-weight ratio of kidneys at both 1000 and
3000 ppm (both sexes), of liver in males at 3000 ppm and in females at
1000 ppm and above, of brain at 3000 ppm (both sexes), and of testes
at 3000 ppm. Histopathological evaluation of an extensive number of
tissues from each animal showed the occurrence of testicular
degeneration in 0/6, 2/6, 1/6, and 3/6 males at 0, 300, 1000, and
3000 ppm respectively. Severe and diffuse testicular degeneration was
seen in one affected male each at 300 ppm and 3000 ppm, while
generally mild and focal degeneration of the testes was present in the
other affected males. Admittedly, the incidence and severity of
testicular degeneration did not follow a dose-response relationship.
However, on account of the complete absence of the lesion from the
concurrent controls, and from a total of 7 similar 1-year studies
conducted at the testing laboratory comprising over 20 control male
dogs (McGee, 1983), the possibility of the testicular lesion being
related to the compound could not be completely ruled out. Other
microscopic findings likely to be attributable to treatment included
the presence in the top-dosage group of hypocellularity of the bone
marrow, lesions in the gastrointestinal tract (gastritis, edema,
ulceration) and heart (haemorrhage), and thymus atrophy, as well as an
increased incidence of lesions in the kidney (nephritis), liver
(centrilobular degeneration), lymph node (lymphadenitis), and spleen
(hyperplasia) at both 1000 and 3000 ppm.
The study did not permit the setting of a no-effect level, mainly
because of uncertainty concerning the occurrence of testicular
degeneration, which occurred at all dose levels (Benson, 1981).
Long-term studies
Mouse
Male and female CD-1 mice (50 animals per sex per group, about 50
days old), housed 5 per sex per cage, were fed dietary levels of
technical dimethipin (97-98% purity) at 0, 80, 400, or 2000 ppm for 78
weeks to evaluate the chronic toxicity and carcinogenic potential of
the compound. (Animals fed dimethipin were kept in the same room for
about 35 weeks as animals in a companion study receiving a highly
photodegradable compound identified only with a code name. It was
stated, but unsubstantiated with data, that, based on results of diet
analyses, dimethipin was stable in the diet for 7 days and that the
mixture of dimethipin and basal diet was satisfactorily homogeneous.)
All animals in the study, viz., those sacrificed in extremis or
that died during the study, and those sacrificed terminally, were
subjected to gross pathological examination and to microscopic
evaluation of a wide range of tissues, including the brain.
Mortality was not influenced by treatment. Between 58 and 78% of
the males and 76 and 86% of the females of the control and treated
groups were still alive at the conclusion of the study. A slight
(< 10%) and non-dose-related decrease in body-weight gain was noted
in males at 400 ppm and above during the first 13 weeks. Food
consumption was not affected in any consistent dose-related pattern.
There were no significant differences between control and treated
groups in clinical signs and incidence of palpable nodules or tissue
masses. Haematology determined on 5 males and 5 females per group at 3
intervals of the study revealed a significant increase in males at
2000 ppm of haematocrit levels at week 13 and of haemotocrit,
haemoglobin, and erythrocyte values at week 78. Terminal haematocrit
levels were elevated in females at both 400 and 2000 ppm. Females of
all treated groups showed a statistically-significant increase, albeit
not strictly dose-dependent, of erythrocyte counts at week 78. Blood-
chemistry and urinalysis parameters were not studied. Gross
pathological alterations and weights of selected organs were not
significantly different between control and treated groups.
Based on tabulated data on "individual histopathology findings"
(no detailed histopathological data with morphological descriptions of
lesions on individual animals were available), compound-induced non-
neoplastic changes were not evident. The only notable neoplastic
finding appeared to be an elevated incidence of pulmonary
(aveolar/bronchiolar) tumours in males at 2000 ppm, as shown in
Table 3.
Table 3 Number of males with lung tumours/number of males with the lung
examined microscopicallya
Pulmonary tumour Control 80 ppm 400 ppm 2000 ppm Historical controlb
(alveolar/bronchiolar) Total Range
adenocarcinomac 1/49 3/49 3/50 7/50 6/168 0/13(0)-
(2) (6.1) (6) (14)d (3.6) 2/24(8.3)
adenoma 5/49 3/49 3/50 5/50 15/168 1/24(4.2)-
(10.2)e (6.1) (6) (10)f (8.9) 2/13(15.4)
adenocarcinoma 6/49 6/49 6/50 12/50 21/168 7/61(11.5)or
or adenoma (12.2) (12.2) (12) (24) (12.5) 2/13(15.4)
a Figure in parenthesis indicates incidence in %.
b Data presented in the submitted report on a total of 168 CD-1 control male
mice from five 78-week studies over an unspecified timeframe.
c Multiple adenocarcinomas noted in 1 control and 2 animals at 2000 ppm.
d 1/7, ...
e 1/5, or ...
f 2/5 of the lung-tumour bearers died or were sacrificed at an unspecified time
during the study. All other bearers of pulmonary tumours were terminal
survivors.
When compared to concurrent-control or historical-control
incidences from a total of 5 studies, the incidence of lung
adenocarcinoma, but not of adenoma alone, was significantly increased
in males at 2000 ppm (P < 0.05, Fisher exact probability). However,
comparison with the maximum historical-control incidence with respect
to lung adenocarcinoma at 2000 ppm was not significant (P > 0.05).
Time-to-tumour (adenocarcinoma) or the multiplicity of tumours was not
modified by treatment. The combined incidence of lung adenocarcinoma
and adenoma was significantly elevated when compared to the
historical-control incidence from a total of 5 studies, but not when
compared with concurrent-control or maximum historical-control
incidences. There was no dose-related increase in the incidence of
benign and/or malignant lung tumours in the females. Other than lung
tumours, the incidence, location, and type of tumours in treated
groups were comparable to controls. About 30% of the male and 40% of
the female concurrent controls were found to have tumours. Lymphoma
(in males), lung tumours (in both sexes), and hepatocellular carcinoma
(in males) were the most frequently-observed spontaneous tumours. It
should be noted that the animals were about 50 days old at the
initiation of the study. This could compromise the sensitivity of the
test as a carcinogenicity study.
The study demonstrated that 80 ppm, equal to 12.3 mg/kg b.w./day,
is a virtual no-effect level, based on the monitored criteria other
than tumours. The lung-tumour data are unclear (Serota et al.,
1981a).
Rat
Groups of 50 male and 50 female rats (Sprague-Dawley CD strain,
about 40 days old), individually caged, were fed technical dimethipin
(97-98% purity) in their diet at 0, 40, 200, or 1000 ppm for 104 weeks
to assess the chronic toxicity and carcinogenic potential of the
compound. (The control group served as common controls for this study
and for a companion study on a chemical identified only by a code
name. Whether treated animals from the two studies were kept in the
same room was not specified.) All animals sacrified in moribund
condition or dying during the study, and all survivors sacrificed
terminally (during weeks 105 and 106), were subjected to necropsy and
histopathological examination of a variety of tissues, including the
brain plus any "unusual" lesions. Sections of spinal cord and "head"
from 10 male and 10 female terminal survivors per group were also
evaluated microscopically.
Survival, not adversely affected by treatment, appeared to be
better in the top-dosage group than in the concurrent control or in
lower-dosage groups. At the end of 104 weeks, with the exception of
males at 200 ppm having a survival rate on only 44%, between 50 and
72% of the males and females of all groups, including the control,
were still alive. Clincial signs related to treatment were not
evident. There were no dose- or compound-related effects on food
consumption or incidences of palpable nodules, tissue masses, or wart-
like lesions. A slight but consistent growth depression (< 5% in
males and < 10% in females) was seen at 1000 ppm between weeks 43
and 95 in males and between weeks 51 through 87 in females.
Haematology and blood chemistry conducted on 5 males and 5 females per
group at 5 intervals over the course of the study indicated that
females of all treated groups displayed an increase of total-protein
values at week 13 only, and a decrease of platelet counts at week 104,
the only sampling interval for this particular parameter. Other
deviations from controls were also observed in certain haematological
and blood-chemistry values, but these were essentially confined to the
top-dosage group. Significant differences were not apparent between
control and treated groups in urinalysis parameters monitored on 5
males and 5 females per group at the same intervals as the blood
studies. Gross pathological changes in treated animals were not
significantly different from those in the controls. Organ-weight
determinations showed an increase (non-dose-related in males and dose-
related in females) of the organ/body-weight ratio of the liver in
males of all treated groups and in females at both 200 and 1000 ppm.
In addition, absolute weight and organ/body-weight ratios of the
adrenal gland were decreased in females of all treated groups.
Histopathologically, "focally-dilated bile ducts containing basophilic
homogeneous material" occurred in 1 male and 1 female in the control
group, 2 males and 3 females at 40 ppm, 5 males and 9 females at 200
ppm, and 33 males and 18 females at 1000 ppm. This finding was likely
to be related to inclusion of the compound in the diet. Microscopic
changes in other tissues, including the adrenal gland, were comparable
in treated animals to those in the controls. An interesting but non-
dose- or compound-related microscopic finding was that 9-27% of the
males in the control and treated groups showed lactation and/or
galactocoele. Whether this might be connected with the observation of
an increased incidence of mammary fibroadenoma in males of the top-
dosage group, as pointed out later, was not certain. An evaluation of
the tumour data revealed that the only noteworthy findings were those
tabulated in Table 4.
The data in Table 4 seem to indicate increased incidences of
astrocytoma in males and hepatocellular carcinoma in females at both
200 and 1000 ppm and of mammary fibroadenoma in males at 1000 ppm. The
incidence of none of these tumours was, however, significantly
different from the concurrent controls (P > 0.05, Fisher exact
probability). This result was confirmed for hepatocellular carcinoma
when compared with historical control incidences. However, with
respect to astrocytoma in males, the incidence was significantly
increased at both 200 and 1000 ppm (P< 0.05) when compared with
historical-control incidence from a total of 7 studies. Comparison
with maximum historical-control incidence was nevertheless not
significant (P > 0.05), even at 1000 ppm. It might be noted that one
additional glioma was reportedly found (by a consultant to the
company) in the control group upon microscopic evaluation of 3
additional brain sections from each animal of the male control and
treated groups. In addition, no pre-neoplastic lesions (gliosis) were
seen in the original or additional brain slides (Squire, 1984). The
latency period of astrocytoma was apparently not reduced by treatment.
Overall, no significant differences between control and treated groups
were noted in the incidence of animals with tumours (benign and/or
malignant), benign tumours, malignant tumours, or multiple primary
tumours. Over 80% of the males and 90% of the females of the
concurrent control group were tumour bearers, with pituitary adenoma
and adrenal tumours in both sexes and mammary tumours in females being
the most prevalent tumours.
The study indicated that 40 ppm, the lowest tested-level, was a
minimum-effect level on parameters other than tumours (Serota
et al., 1981b).
EVALUATION
COMMENTS
In rats, the compound is readily absorbed, metabolized, and
eliminated via the urine and faeces. It is degraded primarily to polar
metabolites, with less than 5% of a single oral dose being recovered
as unchanged dimethipin in the excreta. In goats, dimethipin is
extensively degraded, also primarily to polar metabolites.
Biotransformation of the compound includes hydrolysis, oxidation,
decarboxylation, a ring opening, and conjugation.
Dimethipin has an oral LC50 value of about 500 mg/kg b.w. in
mice and 1200 mg/kg b.w. in rats.
A 2-generation (2 litters/generation) reproduction study in rats,
as well as teratology studies in rats and rabbits, were negative.
Practically all of the mutagenic studies available were negative. The
only positive mutagenic response was seen with the mouse lymphoma
forward mutation assay and only in the presence of metabolic
activation.
A 1-year feeding study in dogs failed to show a no-effect level,
mainly because of the uncertainty concerning the presence of
testicular degeneration even at 300 ppm, the lowest tested level.
However, taking into consideration the lack of a dose-response
relationship regarding incidence and severity of the testicular
lesion, the absence of effect of the compound on the testes of rats in
No. of animals with the tumour/No. of animals with the tissue examined
microscopically
Location *Historical Control
& type of
tumour Control 40 ppm 200 ppm 1000 ppm Total Range
Male Female Male Female Male Female Male Female Male Female Male Female
Brain
Astrocytoma 1a/-50(2) 1/49(2) 0/49(0) 2/50(4) 3b/-50(6) 0/50(0) 5c/50(10) 0/50(0) 1/279 0/266 0/43-1/21 0/21-0/37
** 2/50(4) (0.4) (0) (0)-(4.8) (0)
Mammary
gland ***
Fibroadenoma 0/43(0) N.P. 0/31(0) N.P. 0/33(0) N.P. 3/45(6.7) N.P. N.A. N.A. N.A. N.A.
Liver
Hepatocellular
cardnoma 2/50(4) 1/50(2) 1/50(2) 1/50(2) 5/50(10) 4/50(8) 2/50(4) 4/50(8) 13/305 8/285 0/35-3/34 0/32-2/31
(4.3) (2.8) (0)-(8.8) (0)-(6.5)
Neoplastic
nodule **** 2/50 6/50 2/50 5/50 5/50 5/50 2/50 5/50 N.A. N.A. N.A. N.A.
Figure in parenthesis indicates incidence in %
a astrocytoma present in one animal found dead at 20 weeks. This incidence (1/50 or 2%) was used as a basis of comparison in statistical
analyses.
b all 3 brain tumours found in terminal survivors.
c the time of detection of the brain tumour ranging irom 71 to 101 weeks with the mean being 83.4 weeks.
N.P. data not presented due to the complete absence of a dose-response relationship re incidence
N.A. not available
(Con't)
* Data included in the submitted report from a total of seven 104-week studies In Sprague-Dawley rats over an unspecified time-frame.
** The histopathological examination of 3 additional brain section from each male of control and all treated groups reportedly yielded
one additional glioma which was found in one control terminal survivor. It was also indicated that no preneoplastic lesions (gliosis)
were found in the original and the additional brain slides (Squire, 1984)
*** The historical control incidence of mammary fibroadenoma in an unspecified number of male Sprague-Dawley rats maintained in the
testing laboratory was stated to range from 0 to 3% (Serota 1985).
**** Not considered as tumour per se but included for information only.
the 2-generation reproduction study, and the similarly-negative effect
on the testes of mice and rats in the long-term studies, plus the
virtual absence of other toxic effects at the lowest-tested level, it
was concluded that a level somewhat below 300 ppm could reasonably be
taken as a no-effect level for this species. In this connection, the
Meeting agreed that 100 ppm, the no-effect level established in an
available 90-day dog-feeding study, be accepted as a 1-year no-effect
level for this species.
In the long-term mouse and rat studies, there was seemingly an
increase in the incidence of lung adenocarcinoma in male mice of the
top-dosage group (2000 ppm) and of astrocytoma in male rats of both
the intermediate- and top-dosage groups (i.e. 200 ppm and 1000 ppm).
Although the evidence for a causal relationship between these
particular tumour findings and treatment did not appear to be
convincing, such a possibility could not be excluded at this time.
Before a more definitive opinion on the tumourigenic/carcinogenic
potential of the compound can be expressed, additional information, as
indicated under "Further work or information required", is needed on
those mouse and rat studies from which data on historical control
incidences of certain tumours (specifically lung tumours in mice and
astrocytoma and liver tumours in rats) were obtained.
In view of the concerns with respect to increased incidence of
lung adenocarcinoma in male mice and of astrocytoma in male rats of
the long-term studies, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 80 ppm in the diet, equal to 12.3 mg/kg b.w.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.003 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
1. Information on those mouse and rat studies from which data on
historical control incidences of certain tumours, viz. lung
tumours in CD-1 mice and astrocytoma and liver tumours in
Sprague-Dawley rats, were obtained and presented in the submitted
reports. (Information for each of the studies should include date
of each study, age of animals at initiation, mortality rate of
animals, and experimental conditions such as diet, number of
animals per cage, etc.)
2. Further pharmacokinetic and metabolic studies in rats and/or a
non-rodent mammalian species using appropriate multiple-dosage
levels.
3. Acute oral toxicity studies on major plant metabolites which are
not found in animals if these are liable to occur at substantial
residues.
DESIRED
1. Results of diet analysis for the 18-month mouse and 2-year rat
feeding studies.
2. Studies on any potential oestrogenic or other hormonal effect of
dimethipin, including the determination of the affinity of the
compound for steroid receptors.
3. Further studies in dogs to assess effects, if any, of dimethipin
on the testis.
4. Observations in man.
REFERENCES
Babish, J.G. Harvade(R) (N252) Tech. C-8-1162-00. Acute inhalation
(1977) study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc., submitted to WHO by Uniroyal
Inc., USA.
Baker, R.C., Mastri, C.W., Kinoshita, F.K., & Keplinger, M.L. Acute
(1976) irritation tests with a sample coded N252-C10406, Lot No.
BL7668 in albino rabbits. Unpublished report (IBT No. 8530-
08861) from Industrial Bio-Test Laboratories, Inc., USA,
submitted to WHO by Uniroyal Inc., USA (validated by the
Canadian Health Protection Branch).
Benson, B.W. 1-Year dietary toxicity study in dogs with N252.
(1981) Unpublished report from International Research and
Development Corp., USA, submitted to WHO by Uniroyal Inc.,
USA.
Bootman, J. & Lodge, D.C. ARS7728 (technical grade Harvade (R):
(1982) Assessment of its ability to induce genetic damage in
Saccharomyces cerevisiae. Unpublished report from Life
Science Research, England, submitted to WHO by Uniroyal
Inc., USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K, & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 611-08063) from Industrial
Bio-Test Laboratories, Inc., USA, submitted to WHO by
Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
Bushby, S.R.M. "Hematological studies during toxicity tests." In
(1970) Methods in Toxicology, edited by Paget, G.E. Blackwell
Scientific Publications, Oxford and Edinburgh.
Caplan, J. & Merricks, D.L. 14C-Harvade(R) radiocarbon study in rats.
(1978) Unpublished report from Biospherics Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Forster, R., Edwards, N., & Nunziata, A. Mitotic gene conversion in
(1984a) Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from
Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Forster, R., Mosesso, P., & Nunziata, A. Micronucleus test. Test
(1984b) substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Galloway, S.M. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(1981) (N252) 98% D-11401 in the sister chromatid exchange assay
with Chinese hamster ovary (CHO) cells. Final Report.
Unpublished report from Litton Bionetics, Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of N-252,
(1978) technical lot D10406, BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(19981) (N252) 98% D-11401 in the Ames Salmonella/microsome plate
test. Final report. Unpublished report from Litton
Bionetics, Inc., USA, submitted to WHO by Uniroyal Inc.,
USA.
Kehoe, D.F. & MacKenzie, K.M. Final report. Two-generation rat
(1982) reproduction study with N252. Unpublished report from
Hazleton Raltech Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Knickerbocker, M., Re, T.A., & Babish, J.G. Teratologic evaluation of
(1977) N252 (Harvade(R)) technical in Sprague-Dawley rats.
Unpublished report from Food and Drug Research Laboratories,
Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Madison, W.A. Technical grade Harvade(R). Dermal sensitization in
(1983) guinea pigs (modified closed patch technique). Unpublished
report from Hazleton Raltech, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT No. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA, submitted to
WHO by Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
McGee, D.H. Unpublished letter from International Research Development
(1983) Corp., USA, to Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McManus, J.P. Metabolism of Harvade(R) in goats. Unpublished
(1984) report from Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McMeekin, S.O., Schardein, J.L., & Blair, M. N252 (Harvade(R)
(1981) technical). Teratology study in rabbits. Unpublished report
from International Research and Development Corp., USA,
submitted to WHO by Uniroyal Inc., USA.
Myhr, B.C. & Brusick, D.J. Mutagenicity evaluation of Harvade(R) (N252)
(1981) in the mouse lymphoma forward mutation assay. Revised final
report. Unpublished report from Litton Bionetics, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Reagan, E.L. & Becci, P.J. Acute dermal toxicity study (LD50) in
(1982) albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Serota, D.G., Alsaker, R.D., & Banas, D. 18-Month toxicity and
(1981a) oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K., & Kunalzins, W. 104-Week
(1981b) chronic toxicity study in rats. N252 (Harvade(R) technical)
final report. Unpublished report from Hazleton Laboratories
America, Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G. Unpublished letter from Hazleton Laboratories America,
(1985) Inc. to Uniroyal Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977a) USA, submitted to WHO by Uniroyal Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977b) USA, submitted to WHO by Uniroyal Inc., USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K.. & Cardona, R.A.
(1978) Characteriztion of excretory metabolites from a 14C-
Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemical, submitted to WHO by Uniroyal Inc., USA.
Sorg, R.M., Naismith, R.W., & Matthews, R.J. CHO metaphase analysis
(1983) in vitro chromosome aberration analysis in Chinese hamster
ovary cells (CHO). PH320-UN-001-83 Harvade(R). Unpublished
report from Pharmakon Research International Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Squire, R.A. Unpublished letter to Uniroyal Chemical, submitted to WHO
(1984) by Uniroyal Inc., USA.
Strong, M.B. Studies on the acute oral toxicity of N252 (Harvade(R)) in
(1981) crossbred dogs. Unpublished report from Ciba-Geigy Australia
Ltd., submitted to WHO by Uniroyal Inc,, USA,
Varner, L.L. & Matthews, R.J. Acute oral LD50 in rats. Uni-N-252
(1977) C.N.D.-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA, submitted to WHO by
Uniroyal Inc., USA.
 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 210
STATE: A white crystalline solid with mild odour
MELTING RANGE: 162-167°C
SOLUBILITY (gram solute/100 grams solvent at 25°C):
Distilled water 0.3; dimethyl formamide 32.4;
benzene 1.96; xylene 0.57; methanol 0.05;
chloroform 7.92; ethylene dichloride 7.59
STABILITY: Stable for at least 2 years at +30°C and -30°C
OCTANOL/WATER PARTITION COEFFICIENT:
1.0 at 1 X concentration
2.0 at 5 X concentration
DENSITY: 0.47 g/ml at 20°C
Specifications of technical material
Technical dimethipin has a purity of about 98%. Depending on the
starting material used in the manufacturing process, as many as 4
impurities greater than 0.1% could be present. The impurities include:
(i) 1,4-dithiin, 2,3-dihydro-5,6-dimethyl-l,1,4-trioxide;
(ii) 1,4-dithiin, 5-ethyl-2,3-dihydro-l,1,4,4-tetraoxide;
(iii) 1,4-dithiane, 2-methyl-3-methylene-l,1,4,4-tetraoxide; and
(iv) 1,3-dithiolane, 2-ethyl-2-methyl-l,1,3,3-tetraoxide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, elimination, and biotransformation
Rat
In rats (3 males and 2 females) given a single oral dose of
approximately 3.8 mg/kg b.w. of 14C-dimethipin (labelled in the
2,3-position of the dithiin ring; 96% radiochemically pure) in
distilled water, an average of about 89% of the administered
radioactivity was excreted in 48 hours via the urine and faeces. (One
female rat, identically treated, eliminated only about 42% of the
given dose via these routes over the same period. Reason for the low
14C recovery was said to be unclear, as tissue and blood levels in
this animal were similar to those in the other treated animals.) In
general, faecal elimination slightly exceeded urinary excretion. Less
than 0.1% of the administered 14C was detected in the expired air. At
sacrifice 96 hours after treatment, mean total-residue levels in the
tissues analyzed (excluding the blood) amounted to about 1% of the
given dose. Residue concentrations were highest in lung, heart, liver,
and kidney, and lowest in the gastrointestinal tract, brain, muscle,
and fat. Blood-residue levels ranged from 2 to 7.7% of the
administered 14C dose. No significant sex difference was apparent in
rate, route of elimination, or tissue-residue levels of the compound
(Caplan & Merricks, 1978).
Analysis of pooled urine and faceal samples from the
above-treated rats (excluding the female rat with unusually-low
excretary rate) indicated the presence of about 5% of the 14C
recovered in the urine or in the faeces (corresponding, respectively,
to 2% and 1% of the administered dose) as the unchanged parent
compound. The other readioactive components in the urine and the
faeces were highly polar (Smilo et al., 1978).
Goat
Two goats, 1 of them with a cannulated bile duct, were fed
14C-dimethipin (98% radiochemically pure) at 500 ppm in the diet for
3 days. Samples of urine, liver, bile, and kidney were collected for
characterization and identification of metabolites. (No details of
experimental conditions were provided. Data were presented apparently
on only one of the treated animals.) Figure 1 presents the proposed
metabolic pathways for dimethipin in the goat.
Invariably, in urine, bile, liver and kidney, metabolites III and
IV were identified together with the unchanged parent compound. The
latter accounted for 2-8% of the 14C recovered in each case. Some
alcohol metabolites of dimethipin, viz. metabolites I, II, and V,
were identified only in the urine and bile. Most of the radioactive
components in urine, bile, liver, and kidney were of a highly polar
nature, and were present as conjugates such as glucuronide, cysteine,
and acetylcysteine conjugates (McManus, 1984).
Proposed metabolic pathways for dimethipin in the goat
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 210
STATE: A white crystalline solid with mild odour
MELTING RANGE: 162-167°C
SOLUBILITY (gram solute/100 grams solvent at 25°C):
Distilled water 0.3; dimethyl formamide 32.4;
benzene 1.96; xylene 0.57; methanol 0.05;
chloroform 7.92; ethylene dichloride 7.59
STABILITY: Stable for at least 2 years at +30°C and -30°C
OCTANOL/WATER PARTITION COEFFICIENT:
1.0 at 1 X concentration
2.0 at 5 X concentration
DENSITY: 0.47 g/ml at 20°C
Specifications of technical material
Technical dimethipin has a purity of about 98%. Depending on the
starting material used in the manufacturing process, as many as 4
impurities greater than 0.1% could be present. The impurities include:
(i) 1,4-dithiin, 2,3-dihydro-5,6-dimethyl-l,1,4-trioxide;
(ii) 1,4-dithiin, 5-ethyl-2,3-dihydro-l,1,4,4-tetraoxide;
(iii) 1,4-dithiane, 2-methyl-3-methylene-l,1,4,4-tetraoxide; and
(iv) 1,3-dithiolane, 2-ethyl-2-methyl-l,1,3,3-tetraoxide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, elimination, and biotransformation
Rat
In rats (3 males and 2 females) given a single oral dose of
approximately 3.8 mg/kg b.w. of 14C-dimethipin (labelled in the
2,3-position of the dithiin ring; 96% radiochemically pure) in
distilled water, an average of about 89% of the administered
radioactivity was excreted in 48 hours via the urine and faeces. (One
female rat, identically treated, eliminated only about 42% of the
given dose via these routes over the same period. Reason for the low
14C recovery was said to be unclear, as tissue and blood levels in
this animal were similar to those in the other treated animals.) In
general, faecal elimination slightly exceeded urinary excretion. Less
than 0.1% of the administered 14C was detected in the expired air. At
sacrifice 96 hours after treatment, mean total-residue levels in the
tissues analyzed (excluding the blood) amounted to about 1% of the
given dose. Residue concentrations were highest in lung, heart, liver,
and kidney, and lowest in the gastrointestinal tract, brain, muscle,
and fat. Blood-residue levels ranged from 2 to 7.7% of the
administered 14C dose. No significant sex difference was apparent in
rate, route of elimination, or tissue-residue levels of the compound
(Caplan & Merricks, 1978).
Analysis of pooled urine and faceal samples from the
above-treated rats (excluding the female rat with unusually-low
excretary rate) indicated the presence of about 5% of the 14C
recovered in the urine or in the faeces (corresponding, respectively,
to 2% and 1% of the administered dose) as the unchanged parent
compound. The other readioactive components in the urine and the
faeces were highly polar (Smilo et al., 1978).
Goat
Two goats, 1 of them with a cannulated bile duct, were fed
14C-dimethipin (98% radiochemically pure) at 500 ppm in the diet for
3 days. Samples of urine, liver, bile, and kidney were collected for
characterization and identification of metabolites. (No details of
experimental conditions were provided. Data were presented apparently
on only one of the treated animals.) Figure 1 presents the proposed
metabolic pathways for dimethipin in the goat.
Invariably, in urine, bile, liver and kidney, metabolites III and
IV were identified together with the unchanged parent compound. The
latter accounted for 2-8% of the 14C recovered in each case. Some
alcohol metabolites of dimethipin, viz. metabolites I, II, and V,
were identified only in the urine and bile. Most of the radioactive
components in urine, bile, liver, and kidney were of a highly polar
nature, and were present as conjugates such as glucuronide, cysteine,
and acetylcysteine conjugates (McManus, 1984).
Proposed metabolic pathways for dimethipin in the goat
 TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical dimethipin (99.7% pure)
at 0, 50, 200, or 800 ppm for 105 days prior to mating (1 male and 2
females; "sibling and half-sibling mating avoided") to initiate a
2-generation (2 litters/generation) reproduction study (day 0 of
gestation = day when positive vaginal smear or copulatory plug
detected). Weanlings of the second litter (F1b) were selected to
become parents of the next generation and mated after being placed on
the test diets for 125 days. In each generation, the second mating
trial took place at least 14 days after weaning at 21 days of age of
the first litters, i.e. F1a and F2a.
In the parental generations, mortality (the incidence of which
was not dose-related) occured only in the females. No compound-related
behavioural abnormalities were apparent. F0 and F1b female adults at
800 ppm weighed less than the concurrent controls during the premating
period, throughout gestation, and practically throughout the lactation
periods. Food consumption was depressed at 800 ppm in the pre-mating
period in F0 females from weeks 6-10, in F1b females from weeks
6-17, and in F1b males at weeks 1, 4, and 9. Fertility in males ("a
demonstrated ability to impregnate at least 1 female"), mating index
(% females mated), gestation index (% mated females with viable
litters), the number of days required by females to mate, and the
duration of gestation period in treated groups were all comparable to
control values. With respect to the progeny generations, the mean
number of pups per litter born alive, survival of pups to days 4, 7,
14, and 21, sex ratio, and behaviour of pups were not adversely
affected. Pup weight was reduced at 800 ppm in F1a and F1b litters on
days 7, 4, and 21. Pup weights on day 21 were decreased in F1a
litters at 200 ppm and in F2a litters at 800 ppm. Gross external
examination of all pups, including those found dead, revealed only one
abnormal pup in the study, which was a stillbirth in a F1a litter at
200 ppm.
Findings from gross pathological examination of all parental
animals sacrificed after weaning (F0) or 30 days after weaning (F1b)
of the second litters (i.e. after 32 weeks and 39 weeks of dietary
feeding, respectively) and weanlings from each progeny generation were
not significantly different between control and treated groups.
Organ-weight determinations on all F1b adults and on 5 male and 5
female weanlings per group of F1b and F2b litters showed increased
organ/body-weight ratios of the liver at both 200 and 800 ppm, and of
the kidney and brain at 800 ppm in F1b adult females. The
organ/body-weight ratio of the liver was, however, depressed at 50 ppm
in F1b adult females. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults plus 5
male and 5 female weanlings per group from F1b and F2b litters, and
of gross lesions and gonads from F0 adults, indicated no significant
changes attributable to treatment.
The study showed 200 ppm as a no-effect level for reproduction
(The finding of a decrease in pup weight on day 21 at 200 ppm in F1a
litters was unlikely to be compound-induced, as it occurred only in a
single generation and was not recurrent in nature) (Kehoe & MacKenzie,
1982).
Special studies on teratogenicity
Rat
Groups of sexually-mature mated female rats (BLU:(SD)BR) were
intubated with technical dimethipin (97.5% purity) as a suspension in
corn oil at 0, 80, 400, or 800 mg/kg b.w./day from day 6 through day
15 of gestation (Day 0 = day vaginal plug observed). An additional
group of mated female rats, treated with 250 mg/kg b.w./day of
acetylsalicylic acid (aspirin), was used as the positive control. Due
to "excessive deaths" of dams at both 400 and 800 mg/kg b.w./day
within 8 days of initiation of treatment, these dosage groups were
terminated and were not investigated further. Two new dosage groups,
30 and 160 mg/kg b.w./day, were then added to the test 2 weeks after
the study began, with the original dosage group of 80 mg/kg b.w./day
being maintained as the intermediate level (no concurrent control
groups were included for the 2 new dosage groups). The dams were
sacrificed on day 20 of gestation and the foetuses were removed by
Caesarean section for gross external, visceral, and skeletal
examination.
At dosage levels up to 160 mg/kg b.w./day, no compound-related
mortality or clinical signs were observed. Growth rate of dams during
gestation was comparable in all groups. The total number of dams per
group that were pregnant and alive on day 20 ranged from 20 to 22. The
mean number of implantation sites or live foetuses, foetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, without a concomitant rise in the incidence of
pregnant dams with resorption(s), was noted at 160 mg/kg b.w. There
were no significant differences between control and treated groups in
skeletal or visceral malformations of foetuses. The positive control
group exhibited a number of foetal abnormalities such as
encephalomenigocele and gastroschisis.
The study demonstrated that the compound was non-teratogenic,
non-fetotoxic, and non-toxic to the dams at levels as high as
160 mg/kg b.w. (Knickerboker et al., 1977).
Rabbit
Groups of 16 sexually-mature female Dutch Belted rabbits,
artificially inseminated, were intubated with technical dimethipin
(98.3% purity) as a suspension in 0.5% carboxymethyl cellulose at 0,
7.5, 20, or 40 mg/kg b.w./day (at a constant volume of 1 ml/kg b.w.)
on days 6-27 of gestation (day 0 of gestation = the day of
insemination). The does were sacrificed on day 28 of pregnancy, and
the uterine contents were examined. Foetuses (including those aborted
or dead) were subjected to examination for gross, skeletal, and
visceral abnormalities.
No deaths were noted. A slight increase, as compared to
concurrent controls, "in the number of females with a reduction in the
amount of faeces observed beneath the cage" was reportedly seen at
both 20 and 40 mg/kg b.w. at various intervals during gestation. Food
consumption data were not available. Does at 40 mg/kg b.w. showed an
actual weight loss between days 6 and 12, and maternal weight gain was
depressed in a dose-response pattern in all treated groups between
days 6 and 28. Fertility rate ranged from approximately 88 to 94% in
control and treated groups. One doe each at 0, 20, and 40 mg/kg b.w.
"aborted" on day 28. Seven non-viable foetuses were found in the one
doe each at 0 and 20 mg/kg b.w. that "aborted". The doe at 40 mg/kg
b.w. which "aborted" had 3 late resorptions.
At terminal sacrifice, gross pathological findings in treated
does were comparable to those in the contols. No significant
differences between controls and treated groups were found in the mean
number of corpora lutea, implantations, early or late resorptions,
viable or non-viable foetuses, or foetal weight. A non-dose-related
increase in post-implantation loss due mainly to a non-dose-dependent
increase in early resorptions was noted in all treated groups. The
values of this particular parameter in the treated groups, however,
were within the range of historical control values submitted. The sex
ratio (M/F) of foetuses was elevated at 40 mg/kg b.w., with the mean
number of female foetuses being reduced. An increase in incidence of
foetuses (as well as litters containing foetuses) with 27 presacral
vertebrae and with scoliosis (with or without associated rib
anomalies) was observed at 40 mg/kg b.w., as compared to concurrent
control or historical control incidences presented in the report from
a total of 951 foetuses from 149 litters (from an unspecified number
of studies with Dutch Belted rabbits over an unspecified period of
time). There was no apparent dose- or compound-related increase in
frequency of foetal soft-tissue abnormalities.
Although the possibility exists that the increase in incidence of
the particular skeletal anomalies seen at 40 mg/kg b.w. might be
related to maternal toxicity, prudence dictates that 20 mg/kg b.w. be
considered as a clear-cut teratogenic no-effect level (McMeekin
et al., 1981).
Special studies on mutagenicity
Dimethipin was without mutagenic activity in a number of assays
with micro-organisms and mammalian cells, except with the mouse
lymphoma forward-mutation assay in the presence of metabolic
activation (Table 1).
Special studies on eye and skin irritation
In an eye irritation study with New Zealand White rabbits,
dimethipin (presumably technical grade of unspecified purity) was
found to be extremely irritating to the eye (Baker et al., 1976).
Dimethipin (presumably technical grade of unspecified purity) was
shown to be slightly irritating to the skin of New Zealand White
rabbits in a primary skin-irritation test (Baker et al., 1976).
Special study on skin sensitization
Results of a dermal-sensitization study in male guinea-pigs
(Hartley strain) indicated technical dimethipin (purity not given) to
be a weak skin sensitizer (Madison, 1983).
Table 1. Results of mutagenicity assays on dimethipin*
Test Organisms/ Dosage levels Results References
tissues tested
In vitro
Reverse S. typhimurium 1-1000 Negative Jagannath &
mutation strains TA1538, µg/plate (1) Brusick,
TA1537, TA1535, 1978, 1981
TA98, & TAlO0
S. cerevisiae 1-1000 Negative Jagannath &
strain D4 µg/plate (1) Brusick, 1978
Mitotic non- S. cerevisiae 1-2000 Negative Bootman & Lodge,
dysjunction, strain D6 µg/plate (1) 1982
recombination,
& mutation
Mitotic gene S. cerevisiae 125-2000 Negative Forster et al.,
conversion strain D4 µg/ml (1) 1984a
Chromosome Chinese hamster 5-50 Negative Sorg et al., 1983
aberration ovary cells µg/ml (1)
Sister chromatid Chinese hamster 1.56-25 Negative Galloway & Brusick,
exchange ovary cells µg/ml(3) (1) 1981
3.1-200
µg/ml(4)
Mouse lymphoma L5178Y TK+/- 1.56-75 (2) Myhr & Brusick,
forward mouse lymphoma µg/ml(3) 1981
mutation cells 12.5-200
µg/ml(4)
Table 1. (Con't)
Test Organisms/ Dosage levels Results References
tissues tested
In vivo
Micronucleus Mouse 2 successive Negative Forster et al.,
daily oral (5) 1984b
doses at
22,73.3, or
220 mg/
kg b.w./day
(males) or
at 30, 100, or
300 mg/ kg b.w./
day (females)
* Technical dimethipin (> 98% purity) was used in all the above studies.
(1) With or without metabolic activation.
(2) Negative without metabolic activation but positive with metabolic activation at 75 µg/ml and above.
(3) Non-activated.
(4) Activated.
(5) 1/5 Males and 5/5 females at the top dosage level died after the first dose.
Acute toxicity
Table 2. Acute toxicity of dimethipin*
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 440 Shapiro, 1977a
F 600
Rat M&F oral 1180 Varner &
Matthews, 1977
M i.p. 235 Shapiro, 1977b
F 236
M&F inhalation LC50 Babish, 1977
(1h exposure) > 20 mg/l
Rabbit M&F dermal > 12,000 Reagen & Becci,
(24h exposure) 1982
* Technical dimethipin of > 97.5% purity was used in the
oral studies.
In both mice and rats, death occurred 24 to 48 hours after
treatment. The principal toxic effects in rats were a decrease in body
tone and slowed respiration. Data were not provided on mice with
respect to toxic signs (Shapiro, 1977a; Varner & Matthews, 1977).
Dog
Groups of 24-hour fasted crossbred dogs were given single oral
doses of technical dimethipin (98.3% purity) in gelatin capsules at 0
(4 males), 464 (4 females), 600 (3 males and 1 female), 1000 (2 males
and 2 females), or 1560 mg/kg b.w. (1 male and 3 females). All 4
animals at 1560 mg/kg b.w., 1 male and 2 females at 1000 mg/kg b.w.,
and 1 male and 1 female at 600 mg/kg b.w. died 8-28 hours post-dosing.
Emesis, observed in all animals of all treated groups, usually within
1 hour of dosing, was recurrent in many of the affected animals,
generally during the next 2 to 4 hours. Other frequently-noted toxic
signs included general "depression" and inappetance, salivation,
tremor, and loose blood-stained stools. Survivors recovered from the
toxic signs 2 to 10 days after treatment. The major finding at
necropsy was marked irritation to the stomach and the intestine at and
above 600 mg/kg b.w. The author calculated the oral LD50 of the
compound in dogs to be 690 mg/kg b.w. (Strong, 1981).
Short-term studies
Rat
Male and female Charles-River rats (15 per sex per group, 28 days
old) were fed dietary levels of technical dimethipin (> 99.2% purity)
at 0, 100, 300, or 1000 ppm for 95 days. There was no treatment-
related mortality or abnormal behaviour. Food consumption was slightly
depressed in females at 1000 ppm throughout the study. Body weight was
unaffected. Haematology, blood chemistry, and urinalysis, determined
in 10 males and 10 females per control and top-dosage group after 45
and 85 days of the study, indicated no significant compound-related
effects. At termination, organ/body-weight ratios of liver and kidney
were elevated in females at 1000 ppm. Significant differences were not
observed between control and treated groups in gross pathological
changes. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females per control
and top-dosage group failed to show any lesions attributable to
inclusion of the compound in the diet. The no-effect level appeared to
be 300 ppm (Marias et al., 1976).
Dog
Groups of 4 male and 4 female purebred Beagle dogs (about 6
months old) were given technical dimethipin (> 99.2% purity) in their
diet at 0, 100, 300, or 1000 ppm for 90 days. No mortality occurred.
Compound-related effects were not apparent on behaviour, body weight,
or food consumption during the study or on blood chemistry or
haematology conducted after 42 and 85 days of dietary feeding.
Urinalysis determined at the same 2 intervals indicated an increase
(not dose-related) in incidence of females of all treated groups with
moderate to large amounts of "crystals" in their urinary sediments
after 85 days. Other urinalysis parameters were not affected (that the
particular urinary finding in females of all treated groups was not
likely to be compound-related was supported by the absence of effect
on any of the urinalysis parameters monitored in the 1-year feeding
study in dogs, summarized below). At terminal sacrifice, organ weights
and gross pathology were not altered by treatment. Microscopic
examination of a large number of tissues, including the testis, from
each animal in the study revealed esophagus lesions characterized by
"focal mucosal vesicles containing a few acute inflammatory cells" in
1/8 animals at 300 ppm and 3/8 animals at 1000 ppm, but in none of the
concurrent control or of the lowest-dosage (100 ppm) groups. Based on
the data, 100 ppm could be considered as the no-effect level (Burtner
et al., 1976).
Male and female purebred Beagle dogs (6 males and 6 females per
group; about 7.5 months old), individually caged, were given technical
dimethipin (99.7% purity) in their diet at 0, 300, 1000, or 3000 ppm
for one year. One male and three females at 3000 ppm died or were
sacrificed in extremis between weeks 13 and 52. "Thinness", a major
clinical sign, was seen frequently in most animals at 3000 ppm and
infrequently in one animal at 1000 ppm. Other signs such as
dehydration and paleness of the gums were also noted infrequently at
3000 ppm. Actual weight loss or growth depression and a decrease in
food consumption were evident in both sexes at 3000 ppm throughout, or
practically throughout, the study. Females at both 300 and 1000 ppm
exhibited a marginal, but not consistently dose-dependent, growth
reduction (= 10%) between weeks 12 and 48. Animals of the top-dosage
group exhibited abnormalities in the T-wave in the electrocardiograms
taken at termination and, upon ophthalmoscopic examination, increased
incidence of conjunctival discharge and inflammation as well as
corneal irregularities and roughening at week 27, but not at week 52.
Findings at physical examination described as "severe to slight
thinness and irregular or erratic heart beat" were noted almost
exclusively in animals at 3000 ppm throughout the study.
Monthly determinations of haematology and blood chemistry
revealed deviations from control values, mainly at 3000 ppm (both
sexes), in many parameters including decreased haematocrits, increased
platelet counts, and depressed values of total protein, albumin,
globulin, calcium, BUN, and creatinine at most sampling intervals.
Animals at 1000 ppm displayed decreased values of BUN (both sexes) and
creatinine (males) at many of the sampling intervals. As compared to
concurrent controls, males of all treated groups seemingly showed a
slight but consistent and generally dose-related decrease in
erythrocyte counts and haemoglobin levels. However, these findings
were unlikely to be treatment-associated, because values of these 2
haematological parameters for males even at 3000 ppm were within the
normal ranges given both for control Beagles in the published
literature (Bushby, 1970) and those reportedly recorded for control
Beagle dogs maintained in the testing laboratory. Urinalysis,
including microscopy of urinary sediments, conducted bi-monthly gave
no significant findings related to treatment.
At termination, gross pathological findings in the treated groups
were not significantly different from those in the controls. There was
an increase in organ/body-weight ratio of kidneys at both 1000 and
3000 ppm (both sexes), of liver in males at 3000 ppm and in females at
1000 ppm and above, of brain at 3000 ppm (both sexes), and of testes
at 3000 ppm. Histopathological evaluation of an extensive number of
tissues from each animal showed the occurrence of testicular
degeneration in 0/6, 2/6, 1/6, and 3/6 males at 0, 300, 1000, and
3000 ppm respectively. Severe and diffuse testicular degeneration was
seen in one affected male each at 300 ppm and 3000 ppm, while
generally mild and focal degeneration of the testes was present in the
other affected males. Admittedly, the incidence and severity of
testicular degeneration did not follow a dose-response relationship.
However, on account of the complete absence of the lesion from the
concurrent controls, and from a total of 7 similar 1-year studies
conducted at the testing laboratory comprising over 20 control male
dogs (McGee, 1983), the possibility of the testicular lesion being
related to the compound could not be completely ruled out. Other
microscopic findings likely to be attributable to treatment included
the presence in the top-dosage group of hypocellularity of the bone
marrow, lesions in the gastrointestinal tract (gastritis, edema,
ulceration) and heart (haemorrhage), and thymus atrophy, as well as an
increased incidence of lesions in the kidney (nephritis), liver
(centrilobular degeneration), lymph node (lymphadenitis), and spleen
(hyperplasia) at both 1000 and 3000 ppm.
The study did not permit the setting of a no-effect level, mainly
because of uncertainty concerning the occurrence of testicular
degeneration, which occurred at all dose levels (Benson, 1981).
Long-term studies
Mouse
Male and female CD-1 mice (50 animals per sex per group, about 50
days old), housed 5 per sex per cage, were fed dietary levels of
technical dimethipin (97-98% purity) at 0, 80, 400, or 2000 ppm for 78
weeks to evaluate the chronic toxicity and carcinogenic potential of
the compound. (Animals fed dimethipin were kept in the same room for
about 35 weeks as animals in a companion study receiving a highly
photodegradable compound identified only with a code name. It was
stated, but unsubstantiated with data, that, based on results of diet
analyses, dimethipin was stable in the diet for 7 days and that the
mixture of dimethipin and basal diet was satisfactorily homogeneous.)
All animals in the study, viz., those sacrificed in extremis or
that died during the study, and those sacrificed terminally, were
subjected to gross pathological examination and to microscopic
evaluation of a wide range of tissues, including the brain.
Mortality was not influenced by treatment. Between 58 and 78% of
the males and 76 and 86% of the females of the control and treated
groups were still alive at the conclusion of the study. A slight
(< 10%) and non-dose-related decrease in body-weight gain was noted
in males at 400 ppm and above during the first 13 weeks. Food
consumption was not affected in any consistent dose-related pattern.
There were no significant differences between control and treated
groups in clinical signs and incidence of palpable nodules or tissue
masses. Haematology determined on 5 males and 5 females per group at 3
intervals of the study revealed a significant increase in males at
2000 ppm of haematocrit levels at week 13 and of haemotocrit,
haemoglobin, and erythrocyte values at week 78. Terminal haematocrit
levels were elevated in females at both 400 and 2000 ppm. Females of
all treated groups showed a statistically-significant increase, albeit
not strictly dose-dependent, of erythrocyte counts at week 78. Blood-
chemistry and urinalysis parameters were not studied. Gross
pathological alterations and weights of selected organs were not
significantly different between control and treated groups.
Based on tabulated data on "individual histopathology findings"
(no detailed histopathological data with morphological descriptions of
lesions on individual animals were available), compound-induced non-
neoplastic changes were not evident. The only notable neoplastic
finding appeared to be an elevated incidence of pulmonary
(aveolar/bronchiolar) tumours in males at 2000 ppm, as shown in
Table 3.
Table 3 Number of males with lung tumours/number of males with the lung
examined microscopicallya
Pulmonary tumour Control 80 ppm 400 ppm 2000 ppm Historical controlb
(alveolar/bronchiolar) Total Range
adenocarcinomac 1/49 3/49 3/50 7/50 6/168 0/13(0)-
(2) (6.1) (6) (14)d (3.6) 2/24(8.3)
adenoma 5/49 3/49 3/50 5/50 15/168 1/24(4.2)-
(10.2)e (6.1) (6) (10)f (8.9) 2/13(15.4)
adenocarcinoma 6/49 6/49 6/50 12/50 21/168 7/61(11.5)or
or adenoma (12.2) (12.2) (12) (24) (12.5) 2/13(15.4)
a Figure in parenthesis indicates incidence in %.
b Data presented in the submitted report on a total of 168 CD-1 control male
mice from five 78-week studies over an unspecified timeframe.
c Multiple adenocarcinomas noted in 1 control and 2 animals at 2000 ppm.
d 1/7, ...
e 1/5, or ...
f 2/5 of the lung-tumour bearers died or were sacrificed at an unspecified time
during the study. All other bearers of pulmonary tumours were terminal
survivors.
When compared to concurrent-control or historical-control
incidences from a total of 5 studies, the incidence of lung
adenocarcinoma, but not of adenoma alone, was significantly increased
in males at 2000 ppm (P < 0.05, Fisher exact probability). However,
comparison with the maximum historical-control incidence with respect
to lung adenocarcinoma at 2000 ppm was not significant (P > 0.05).
Time-to-tumour (adenocarcinoma) or the multiplicity of tumours was not
modified by treatment. The combined incidence of lung adenocarcinoma
and adenoma was significantly elevated when compared to the
historical-control incidence from a total of 5 studies, but not when
compared with concurrent-control or maximum historical-control
incidences. There was no dose-related increase in the incidence of
benign and/or malignant lung tumours in the females. Other than lung
tumours, the incidence, location, and type of tumours in treated
groups were comparable to controls. About 30% of the male and 40% of
the female concurrent controls were found to have tumours. Lymphoma
(in males), lung tumours (in both sexes), and hepatocellular carcinoma
(in males) were the most frequently-observed spontaneous tumours. It
should be noted that the animals were about 50 days old at the
initiation of the study. This could compromise the sensitivity of the
test as a carcinogenicity study.
The study demonstrated that 80 ppm, equal to 12.3 mg/kg b.w./day,
is a virtual no-effect level, based on the monitored criteria other
than tumours. The lung-tumour data are unclear (Serota et al.,
1981a).
Rat
Groups of 50 male and 50 female rats (Sprague-Dawley CD strain,
about 40 days old), individually caged, were fed technical dimethipin
(97-98% purity) in their diet at 0, 40, 200, or 1000 ppm for 104 weeks
to assess the chronic toxicity and carcinogenic potential of the
compound. (The control group served as common controls for this study
and for a companion study on a chemical identified only by a code
name. Whether treated animals from the two studies were kept in the
same room was not specified.) All animals sacrified in moribund
condition or dying during the study, and all survivors sacrificed
terminally (during weeks 105 and 106), were subjected to necropsy and
histopathological examination of a variety of tissues, including the
brain plus any "unusual" lesions. Sections of spinal cord and "head"
from 10 male and 10 female terminal survivors per group were also
evaluated microscopically.
Survival, not adversely affected by treatment, appeared to be
better in the top-dosage group than in the concurrent control or in
lower-dosage groups. At the end of 104 weeks, with the exception of
males at 200 ppm having a survival rate on only 44%, between 50 and
72% of the males and females of all groups, including the control,
were still alive. Clincial signs related to treatment were not
evident. There were no dose- or compound-related effects on food
consumption or incidences of palpable nodules, tissue masses, or wart-
like lesions. A slight but consistent growth depression (< 5% in
males and < 10% in females) was seen at 1000 ppm between weeks 43
and 95 in males and between weeks 51 through 87 in females.
Haematology and blood chemistry conducted on 5 males and 5 females per
group at 5 intervals over the course of the study indicated that
females of all treated groups displayed an increase of total-protein
values at week 13 only, and a decrease of platelet counts at week 104,
the only sampling interval for this particular parameter. Other
deviations from controls were also observed in certain haematological
and blood-chemistry values, but these were essentially confined to the
top-dosage group. Significant differences were not apparent between
control and treated groups in urinalysis parameters monitored on 5
males and 5 females per group at the same intervals as the blood
studies. Gross pathological changes in treated animals were not
significantly different from those in the controls. Organ-weight
determinations showed an increase (non-dose-related in males and dose-
related in females) of the organ/body-weight ratio of the liver in
males of all treated groups and in females at both 200 and 1000 ppm.
In addition, absolute weight and organ/body-weight ratios of the
adrenal gland were decreased in females of all treated groups.
Histopathologically, "focally-dilated bile ducts containing basophilic
homogeneous material" occurred in 1 male and 1 female in the control
group, 2 males and 3 females at 40 ppm, 5 males and 9 females at 200
ppm, and 33 males and 18 females at 1000 ppm. This finding was likely
to be related to inclusion of the compound in the diet. Microscopic
changes in other tissues, including the adrenal gland, were comparable
in treated animals to those in the controls. An interesting but non-
dose- or compound-related microscopic finding was that 9-27% of the
males in the control and treated groups showed lactation and/or
galactocoele. Whether this might be connected with the observation of
an increased incidence of mammary fibroadenoma in males of the top-
dosage group, as pointed out later, was not certain. An evaluation of
the tumour data revealed that the only noteworthy findings were those
tabulated in Table 4.
The data in Table 4 seem to indicate increased incidences of
astrocytoma in males and hepatocellular carcinoma in females at both
200 and 1000 ppm and of mammary fibroadenoma in males at 1000 ppm. The
incidence of none of these tumours was, however, significantly
different from the concurrent controls (P > 0.05, Fisher exact
probability). This result was confirmed for hepatocellular carcinoma
when compared with historical control incidences. However, with
respect to astrocytoma in males, the incidence was significantly
increased at both 200 and 1000 ppm (P< 0.05) when compared with
historical-control incidence from a total of 7 studies. Comparison
with maximum historical-control incidence was nevertheless not
significant (P > 0.05), even at 1000 ppm. It might be noted that one
additional glioma was reportedly found (by a consultant to the
company) in the control group upon microscopic evaluation of 3
additional brain sections from each animal of the male control and
treated groups. In addition, no pre-neoplastic lesions (gliosis) were
seen in the original or additional brain slides (Squire, 1984). The
latency period of astrocytoma was apparently not reduced by treatment.
Overall, no significant differences between control and treated groups
were noted in the incidence of animals with tumours (benign and/or
malignant), benign tumours, malignant tumours, or multiple primary
tumours. Over 80% of the males and 90% of the females of the
concurrent control group were tumour bearers, with pituitary adenoma
and adrenal tumours in both sexes and mammary tumours in females being
the most prevalent tumours.
The study indicated that 40 ppm, the lowest tested-level, was a
minimum-effect level on parameters other than tumours (Serota
et al., 1981b).
EVALUATION
COMMENTS
In rats, the compound is readily absorbed, metabolized, and
eliminated via the urine and faeces. It is degraded primarily to polar
metabolites, with less than 5% of a single oral dose being recovered
as unchanged dimethipin in the excreta. In goats, dimethipin is
extensively degraded, also primarily to polar metabolites.
Biotransformation of the compound includes hydrolysis, oxidation,
decarboxylation, a ring opening, and conjugation.
Dimethipin has an oral LC50 value of about 500 mg/kg b.w. in
mice and 1200 mg/kg b.w. in rats.
A 2-generation (2 litters/generation) reproduction study in rats,
as well as teratology studies in rats and rabbits, were negative.
Practically all of the mutagenic studies available were negative. The
only positive mutagenic response was seen with the mouse lymphoma
forward mutation assay and only in the presence of metabolic
activation.
A 1-year feeding study in dogs failed to show a no-effect level,
mainly because of the uncertainty concerning the presence of
testicular degeneration even at 300 ppm, the lowest tested level.
However, taking into consideration the lack of a dose-response
relationship regarding incidence and severity of the testicular
lesion, the absence of effect of the compound on the testes of rats in
No. of animals with the tumour/No. of animals with the tissue examined
microscopically
Location *Historical Control
& type of
tumour Control 40 ppm 200 ppm 1000 ppm Total Range
Male Female Male Female Male Female Male Female Male Female Male Female
Brain
Astrocytoma 1a/-50(2) 1/49(2) 0/49(0) 2/50(4) 3b/-50(6) 0/50(0) 5c/50(10) 0/50(0) 1/279 0/266 0/43-1/21 0/21-0/37
** 2/50(4) (0.4) (0) (0)-(4.8) (0)
Mammary
gland ***
Fibroadenoma 0/43(0) N.P. 0/31(0) N.P. 0/33(0) N.P. 3/45(6.7) N.P. N.A. N.A. N.A. N.A.
Liver
Hepatocellular
cardnoma 2/50(4) 1/50(2) 1/50(2) 1/50(2) 5/50(10) 4/50(8) 2/50(4) 4/50(8) 13/305 8/285 0/35-3/34 0/32-2/31
(4.3) (2.8) (0)-(8.8) (0)-(6.5)
Neoplastic
nodule **** 2/50 6/50 2/50 5/50 5/50 5/50 2/50 5/50 N.A. N.A. N.A. N.A.
Figure in parenthesis indicates incidence in %
a astrocytoma present in one animal found dead at 20 weeks. This incidence (1/50 or 2%) was used as a basis of comparison in statistical
analyses.
b all 3 brain tumours found in terminal survivors.
c the time of detection of the brain tumour ranging irom 71 to 101 weeks with the mean being 83.4 weeks.
N.P. data not presented due to the complete absence of a dose-response relationship re incidence
N.A. not available
(Con't)
* Data included in the submitted report from a total of seven 104-week studies In Sprague-Dawley rats over an unspecified time-frame.
** The histopathological examination of 3 additional brain section from each male of control and all treated groups reportedly yielded
one additional glioma which was found in one control terminal survivor. It was also indicated that no preneoplastic lesions (gliosis)
were found in the original and the additional brain slides (Squire, 1984)
*** The historical control incidence of mammary fibroadenoma in an unspecified number of male Sprague-Dawley rats maintained in the
testing laboratory was stated to range from 0 to 3% (Serota 1985).
**** Not considered as tumour per se but included for information only.
the 2-generation reproduction study, and the similarly-negative effect
on the testes of mice and rats in the long-term studies, plus the
virtual absence of other toxic effects at the lowest-tested level, it
was concluded that a level somewhat below 300 ppm could reasonably be
taken as a no-effect level for this species. In this connection, the
Meeting agreed that 100 ppm, the no-effect level established in an
available 90-day dog-feeding study, be accepted as a 1-year no-effect
level for this species.
In the long-term mouse and rat studies, there was seemingly an
increase in the incidence of lung adenocarcinoma in male mice of the
top-dosage group (2000 ppm) and of astrocytoma in male rats of both
the intermediate- and top-dosage groups (i.e. 200 ppm and 1000 ppm).
Although the evidence for a causal relationship between these
particular tumour findings and treatment did not appear to be
convincing, such a possibility could not be excluded at this time.
Before a more definitive opinion on the tumourigenic/carcinogenic
potential of the compound can be expressed, additional information, as
indicated under "Further work or information required", is needed on
those mouse and rat studies from which data on historical control
incidences of certain tumours (specifically lung tumours in mice and
astrocytoma and liver tumours in rats) were obtained.
In view of the concerns with respect to increased incidence of
lung adenocarcinoma in male mice and of astrocytoma in male rats of
the long-term studies, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 80 ppm in the diet, equal to 12.3 mg/kg b.w.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.003 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
1. Information on those mouse and rat studies from which data on
historical control incidences of certain tumours, viz. lung
tumours in CD-1 mice and astrocytoma and liver tumours in
Sprague-Dawley rats, were obtained and presented in the submitted
reports. (Information for each of the studies should include date
of each study, age of animals at initiation, mortality rate of
animals, and experimental conditions such as diet, number of
animals per cage, etc.)
2. Further pharmacokinetic and metabolic studies in rats and/or a
non-rodent mammalian species using appropriate multiple-dosage
levels.
3. Acute oral toxicity studies on major plant metabolites which are
not found in animals if these are liable to occur at substantial
residues.
DESIRED
1. Results of diet analysis for the 18-month mouse and 2-year rat
feeding studies.
2. Studies on any potential oestrogenic or other hormonal effect of
dimethipin, including the determination of the affinity of the
compound for steroid receptors.
3. Further studies in dogs to assess effects, if any, of dimethipin
on the testis.
4. Observations in man.
REFERENCES
Babish, J.G. Harvade(R) (N252) Tech. C-8-1162-00. Acute inhalation
(1977) study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc., submitted to WHO by Uniroyal
Inc., USA.
Baker, R.C., Mastri, C.W., Kinoshita, F.K., & Keplinger, M.L. Acute
(1976) irritation tests with a sample coded N252-C10406, Lot No.
BL7668 in albino rabbits. Unpublished report (IBT No. 8530-
08861) from Industrial Bio-Test Laboratories, Inc., USA,
submitted to WHO by Uniroyal Inc., USA (validated by the
Canadian Health Protection Branch).
Benson, B.W. 1-Year dietary toxicity study in dogs with N252.
(1981) Unpublished report from International Research and
Development Corp., USA, submitted to WHO by Uniroyal Inc.,
USA.
Bootman, J. & Lodge, D.C. ARS7728 (technical grade Harvade (R):
(1982) Assessment of its ability to induce genetic damage in
Saccharomyces cerevisiae. Unpublished report from Life
Science Research, England, submitted to WHO by Uniroyal
Inc., USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K, & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 611-08063) from Industrial
Bio-Test Laboratories, Inc., USA, submitted to WHO by
Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
Bushby, S.R.M. "Hematological studies during toxicity tests." In
(1970) Methods in Toxicology, edited by Paget, G.E. Blackwell
Scientific Publications, Oxford and Edinburgh.
Caplan, J. & Merricks, D.L. 14C-Harvade(R) radiocarbon study in rats.
(1978) Unpublished report from Biospherics Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Forster, R., Edwards, N., & Nunziata, A. Mitotic gene conversion in
(1984a) Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from
Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Forster, R., Mosesso, P., & Nunziata, A. Micronucleus test. Test
(1984b) substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Galloway, S.M. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(1981) (N252) 98% D-11401 in the sister chromatid exchange assay
with Chinese hamster ovary (CHO) cells. Final Report.
Unpublished report from Litton Bionetics, Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of N-252,
(1978) technical lot D10406, BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(19981) (N252) 98% D-11401 in the Ames Salmonella/microsome plate
test. Final report. Unpublished report from Litton
Bionetics, Inc., USA, submitted to WHO by Uniroyal Inc.,
USA.
Kehoe, D.F. & MacKenzie, K.M. Final report. Two-generation rat
(1982) reproduction study with N252. Unpublished report from
Hazleton Raltech Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Knickerbocker, M., Re, T.A., & Babish, J.G. Teratologic evaluation of
(1977) N252 (Harvade(R)) technical in Sprague-Dawley rats.
Unpublished report from Food and Drug Research Laboratories,
Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Madison, W.A. Technical grade Harvade(R). Dermal sensitization in
(1983) guinea pigs (modified closed patch technique). Unpublished
report from Hazleton Raltech, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT No. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA, submitted to
WHO by Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
McGee, D.H. Unpublished letter from International Research Development
(1983) Corp., USA, to Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McManus, J.P. Metabolism of Harvade(R) in goats. Unpublished
(1984) report from Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McMeekin, S.O., Schardein, J.L., & Blair, M. N252 (Harvade(R)
(1981) technical). Teratology study in rabbits. Unpublished report
from International Research and Development Corp., USA,
submitted to WHO by Uniroyal Inc., USA.
Myhr, B.C. & Brusick, D.J. Mutagenicity evaluation of Harvade(R) (N252)
(1981) in the mouse lymphoma forward mutation assay. Revised final
report. Unpublished report from Litton Bionetics, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Reagan, E.L. & Becci, P.J. Acute dermal toxicity study (LD50) in
(1982) albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Serota, D.G., Alsaker, R.D., & Banas, D. 18-Month toxicity and
(1981a) oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K., & Kunalzins, W. 104-Week
(1981b) chronic toxicity study in rats. N252 (Harvade(R) technical)
final report. Unpublished report from Hazleton Laboratories
America, Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G. Unpublished letter from Hazleton Laboratories America,
(1985) Inc. to Uniroyal Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977a) USA, submitted to WHO by Uniroyal Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977b) USA, submitted to WHO by Uniroyal Inc., USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K.. & Cardona, R.A.
(1978) Characteriztion of excretory metabolites from a 14C-
Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemical, submitted to WHO by Uniroyal Inc., USA.
Sorg, R.M., Naismith, R.W., & Matthews, R.J. CHO metaphase analysis
(1983) in vitro chromosome aberration analysis in Chinese hamster
ovary cells (CHO). PH320-UN-001-83 Harvade(R). Unpublished
report from Pharmakon Research International Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Squire, R.A. Unpublished letter to Uniroyal Chemical, submitted to WHO
(1984) by Uniroyal Inc., USA.
Strong, M.B. Studies on the acute oral toxicity of N252 (Harvade(R)) in
(1981) crossbred dogs. Unpublished report from Ciba-Geigy Australia
Ltd., submitted to WHO by Uniroyal Inc,, USA,
Varner, L.L. & Matthews, R.J. Acute oral LD50 in rats. Uni-N-252
(1977) C.N.D.-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA, submitted to WHO by
Uniroyal Inc., USA.
TOXICOLOGICAL STUDIES
Special study on reproduction
Rat
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical dimethipin (99.7% pure)
at 0, 50, 200, or 800 ppm for 105 days prior to mating (1 male and 2
females; "sibling and half-sibling mating avoided") to initiate a
2-generation (2 litters/generation) reproduction study (day 0 of
gestation = day when positive vaginal smear or copulatory plug
detected). Weanlings of the second litter (F1b) were selected to
become parents of the next generation and mated after being placed on
the test diets for 125 days. In each generation, the second mating
trial took place at least 14 days after weaning at 21 days of age of
the first litters, i.e. F1a and F2a.
In the parental generations, mortality (the incidence of which
was not dose-related) occured only in the females. No compound-related
behavioural abnormalities were apparent. F0 and F1b female adults at
800 ppm weighed less than the concurrent controls during the premating
period, throughout gestation, and practically throughout the lactation
periods. Food consumption was depressed at 800 ppm in the pre-mating
period in F0 females from weeks 6-10, in F1b females from weeks
6-17, and in F1b males at weeks 1, 4, and 9. Fertility in males ("a
demonstrated ability to impregnate at least 1 female"), mating index
(% females mated), gestation index (% mated females with viable
litters), the number of days required by females to mate, and the
duration of gestation period in treated groups were all comparable to
control values. With respect to the progeny generations, the mean
number of pups per litter born alive, survival of pups to days 4, 7,
14, and 21, sex ratio, and behaviour of pups were not adversely
affected. Pup weight was reduced at 800 ppm in F1a and F1b litters on
days 7, 4, and 21. Pup weights on day 21 were decreased in F1a
litters at 200 ppm and in F2a litters at 800 ppm. Gross external
examination of all pups, including those found dead, revealed only one
abnormal pup in the study, which was a stillbirth in a F1a litter at
200 ppm.
Findings from gross pathological examination of all parental
animals sacrificed after weaning (F0) or 30 days after weaning (F1b)
of the second litters (i.e. after 32 weeks and 39 weeks of dietary
feeding, respectively) and weanlings from each progeny generation were
not significantly different between control and treated groups.
Organ-weight determinations on all F1b adults and on 5 male and 5
female weanlings per group of F1b and F2b litters showed increased
organ/body-weight ratios of the liver at both 200 and 800 ppm, and of
the kidney and brain at 800 ppm in F1b adult females. The
organ/body-weight ratio of the liver was, however, depressed at 50 ppm
in F1b adult females. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults plus 5
male and 5 female weanlings per group from F1b and F2b litters, and
of gross lesions and gonads from F0 adults, indicated no significant
changes attributable to treatment.
The study showed 200 ppm as a no-effect level for reproduction
(The finding of a decrease in pup weight on day 21 at 200 ppm in F1a
litters was unlikely to be compound-induced, as it occurred only in a
single generation and was not recurrent in nature) (Kehoe & MacKenzie,
1982).
Special studies on teratogenicity
Rat
Groups of sexually-mature mated female rats (BLU:(SD)BR) were
intubated with technical dimethipin (97.5% purity) as a suspension in
corn oil at 0, 80, 400, or 800 mg/kg b.w./day from day 6 through day
15 of gestation (Day 0 = day vaginal plug observed). An additional
group of mated female rats, treated with 250 mg/kg b.w./day of
acetylsalicylic acid (aspirin), was used as the positive control. Due
to "excessive deaths" of dams at both 400 and 800 mg/kg b.w./day
within 8 days of initiation of treatment, these dosage groups were
terminated and were not investigated further. Two new dosage groups,
30 and 160 mg/kg b.w./day, were then added to the test 2 weeks after
the study began, with the original dosage group of 80 mg/kg b.w./day
being maintained as the intermediate level (no concurrent control
groups were included for the 2 new dosage groups). The dams were
sacrificed on day 20 of gestation and the foetuses were removed by
Caesarean section for gross external, visceral, and skeletal
examination.
At dosage levels up to 160 mg/kg b.w./day, no compound-related
mortality or clinical signs were observed. Growth rate of dams during
gestation was comparable in all groups. The total number of dams per
group that were pregnant and alive on day 20 ranged from 20 to 22. The
mean number of implantation sites or live foetuses, foetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, without a concomitant rise in the incidence of
pregnant dams with resorption(s), was noted at 160 mg/kg b.w. There
were no significant differences between control and treated groups in
skeletal or visceral malformations of foetuses. The positive control
group exhibited a number of foetal abnormalities such as
encephalomenigocele and gastroschisis.
The study demonstrated that the compound was non-teratogenic,
non-fetotoxic, and non-toxic to the dams at levels as high as
160 mg/kg b.w. (Knickerboker et al., 1977).
Rabbit
Groups of 16 sexually-mature female Dutch Belted rabbits,
artificially inseminated, were intubated with technical dimethipin
(98.3% purity) as a suspension in 0.5% carboxymethyl cellulose at 0,
7.5, 20, or 40 mg/kg b.w./day (at a constant volume of 1 ml/kg b.w.)
on days 6-27 of gestation (day 0 of gestation = the day of
insemination). The does were sacrificed on day 28 of pregnancy, and
the uterine contents were examined. Foetuses (including those aborted
or dead) were subjected to examination for gross, skeletal, and
visceral abnormalities.
No deaths were noted. A slight increase, as compared to
concurrent controls, "in the number of females with a reduction in the
amount of faeces observed beneath the cage" was reportedly seen at
both 20 and 40 mg/kg b.w. at various intervals during gestation. Food
consumption data were not available. Does at 40 mg/kg b.w. showed an
actual weight loss between days 6 and 12, and maternal weight gain was
depressed in a dose-response pattern in all treated groups between
days 6 and 28. Fertility rate ranged from approximately 88 to 94% in
control and treated groups. One doe each at 0, 20, and 40 mg/kg b.w.
"aborted" on day 28. Seven non-viable foetuses were found in the one
doe each at 0 and 20 mg/kg b.w. that "aborted". The doe at 40 mg/kg
b.w. which "aborted" had 3 late resorptions.
At terminal sacrifice, gross pathological findings in treated
does were comparable to those in the contols. No significant
differences between controls and treated groups were found in the mean
number of corpora lutea, implantations, early or late resorptions,
viable or non-viable foetuses, or foetal weight. A non-dose-related
increase in post-implantation loss due mainly to a non-dose-dependent
increase in early resorptions was noted in all treated groups. The
values of this particular parameter in the treated groups, however,
were within the range of historical control values submitted. The sex
ratio (M/F) of foetuses was elevated at 40 mg/kg b.w., with the mean
number of female foetuses being reduced. An increase in incidence of
foetuses (as well as litters containing foetuses) with 27 presacral
vertebrae and with scoliosis (with or without associated rib
anomalies) was observed at 40 mg/kg b.w., as compared to concurrent
control or historical control incidences presented in the report from
a total of 951 foetuses from 149 litters (from an unspecified number
of studies with Dutch Belted rabbits over an unspecified period of
time). There was no apparent dose- or compound-related increase in
frequency of foetal soft-tissue abnormalities.
Although the possibility exists that the increase in incidence of
the particular skeletal anomalies seen at 40 mg/kg b.w. might be
related to maternal toxicity, prudence dictates that 20 mg/kg b.w. be
considered as a clear-cut teratogenic no-effect level (McMeekin
et al., 1981).
Special studies on mutagenicity
Dimethipin was without mutagenic activity in a number of assays
with micro-organisms and mammalian cells, except with the mouse
lymphoma forward-mutation assay in the presence of metabolic
activation (Table 1).
Special studies on eye and skin irritation
In an eye irritation study with New Zealand White rabbits,
dimethipin (presumably technical grade of unspecified purity) was
found to be extremely irritating to the eye (Baker et al., 1976).
Dimethipin (presumably technical grade of unspecified purity) was
shown to be slightly irritating to the skin of New Zealand White
rabbits in a primary skin-irritation test (Baker et al., 1976).
Special study on skin sensitization
Results of a dermal-sensitization study in male guinea-pigs
(Hartley strain) indicated technical dimethipin (purity not given) to
be a weak skin sensitizer (Madison, 1983).
Table 1. Results of mutagenicity assays on dimethipin*
Test Organisms/ Dosage levels Results References
tissues tested
In vitro
Reverse S. typhimurium 1-1000 Negative Jagannath &
mutation strains TA1538, µg/plate (1) Brusick,
TA1537, TA1535, 1978, 1981
TA98, & TAlO0
S. cerevisiae 1-1000 Negative Jagannath &
strain D4 µg/plate (1) Brusick, 1978
Mitotic non- S. cerevisiae 1-2000 Negative Bootman & Lodge,
dysjunction, strain D6 µg/plate (1) 1982
recombination,
& mutation
Mitotic gene S. cerevisiae 125-2000 Negative Forster et al.,
conversion strain D4 µg/ml (1) 1984a
Chromosome Chinese hamster 5-50 Negative Sorg et al., 1983
aberration ovary cells µg/ml (1)
Sister chromatid Chinese hamster 1.56-25 Negative Galloway & Brusick,
exchange ovary cells µg/ml(3) (1) 1981
3.1-200
µg/ml(4)
Mouse lymphoma L5178Y TK+/- 1.56-75 (2) Myhr & Brusick,
forward mouse lymphoma µg/ml(3) 1981
mutation cells 12.5-200
µg/ml(4)
Table 1. (Con't)
Test Organisms/ Dosage levels Results References
tissues tested
In vivo
Micronucleus Mouse 2 successive Negative Forster et al.,
daily oral (5) 1984b
doses at
22,73.3, or
220 mg/
kg b.w./day
(males) or
at 30, 100, or
300 mg/ kg b.w./
day (females)
* Technical dimethipin (> 98% purity) was used in all the above studies.
(1) With or without metabolic activation.
(2) Negative without metabolic activation but positive with metabolic activation at 75 µg/ml and above.
(3) Non-activated.
(4) Activated.
(5) 1/5 Males and 5/5 females at the top dosage level died after the first dose.
Acute toxicity
Table 2. Acute toxicity of dimethipin*
LD50
Species Sex Route (mg/kg b.w.) Reference
Mouse M oral 440 Shapiro, 1977a
F 600
Rat M&F oral 1180 Varner &
Matthews, 1977
M i.p. 235 Shapiro, 1977b
F 236
M&F inhalation LC50 Babish, 1977
(1h exposure) > 20 mg/l
Rabbit M&F dermal > 12,000 Reagen & Becci,
(24h exposure) 1982
* Technical dimethipin of > 97.5% purity was used in the
oral studies.
In both mice and rats, death occurred 24 to 48 hours after
treatment. The principal toxic effects in rats were a decrease in body
tone and slowed respiration. Data were not provided on mice with
respect to toxic signs (Shapiro, 1977a; Varner & Matthews, 1977).
Dog
Groups of 24-hour fasted crossbred dogs were given single oral
doses of technical dimethipin (98.3% purity) in gelatin capsules at 0
(4 males), 464 (4 females), 600 (3 males and 1 female), 1000 (2 males
and 2 females), or 1560 mg/kg b.w. (1 male and 3 females). All 4
animals at 1560 mg/kg b.w., 1 male and 2 females at 1000 mg/kg b.w.,
and 1 male and 1 female at 600 mg/kg b.w. died 8-28 hours post-dosing.
Emesis, observed in all animals of all treated groups, usually within
1 hour of dosing, was recurrent in many of the affected animals,
generally during the next 2 to 4 hours. Other frequently-noted toxic
signs included general "depression" and inappetance, salivation,
tremor, and loose blood-stained stools. Survivors recovered from the
toxic signs 2 to 10 days after treatment. The major finding at
necropsy was marked irritation to the stomach and the intestine at and
above 600 mg/kg b.w. The author calculated the oral LD50 of the
compound in dogs to be 690 mg/kg b.w. (Strong, 1981).
Short-term studies
Rat
Male and female Charles-River rats (15 per sex per group, 28 days
old) were fed dietary levels of technical dimethipin (> 99.2% purity)
at 0, 100, 300, or 1000 ppm for 95 days. There was no treatment-
related mortality or abnormal behaviour. Food consumption was slightly
depressed in females at 1000 ppm throughout the study. Body weight was
unaffected. Haematology, blood chemistry, and urinalysis, determined
in 10 males and 10 females per control and top-dosage group after 45
and 85 days of the study, indicated no significant compound-related
effects. At termination, organ/body-weight ratios of liver and kidney
were elevated in females at 1000 ppm. Significant differences were not
observed between control and treated groups in gross pathological
changes. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females per control
and top-dosage group failed to show any lesions attributable to
inclusion of the compound in the diet. The no-effect level appeared to
be 300 ppm (Marias et al., 1976).
Dog
Groups of 4 male and 4 female purebred Beagle dogs (about 6
months old) were given technical dimethipin (> 99.2% purity) in their
diet at 0, 100, 300, or 1000 ppm for 90 days. No mortality occurred.
Compound-related effects were not apparent on behaviour, body weight,
or food consumption during the study or on blood chemistry or
haematology conducted after 42 and 85 days of dietary feeding.
Urinalysis determined at the same 2 intervals indicated an increase
(not dose-related) in incidence of females of all treated groups with
moderate to large amounts of "crystals" in their urinary sediments
after 85 days. Other urinalysis parameters were not affected (that the
particular urinary finding in females of all treated groups was not
likely to be compound-related was supported by the absence of effect
on any of the urinalysis parameters monitored in the 1-year feeding
study in dogs, summarized below). At terminal sacrifice, organ weights
and gross pathology were not altered by treatment. Microscopic
examination of a large number of tissues, including the testis, from
each animal in the study revealed esophagus lesions characterized by
"focal mucosal vesicles containing a few acute inflammatory cells" in
1/8 animals at 300 ppm and 3/8 animals at 1000 ppm, but in none of the
concurrent control or of the lowest-dosage (100 ppm) groups. Based on
the data, 100 ppm could be considered as the no-effect level (Burtner
et al., 1976).
Male and female purebred Beagle dogs (6 males and 6 females per
group; about 7.5 months old), individually caged, were given technical
dimethipin (99.7% purity) in their diet at 0, 300, 1000, or 3000 ppm
for one year. One male and three females at 3000 ppm died or were
sacrificed in extremis between weeks 13 and 52. "Thinness", a major
clinical sign, was seen frequently in most animals at 3000 ppm and
infrequently in one animal at 1000 ppm. Other signs such as
dehydration and paleness of the gums were also noted infrequently at
3000 ppm. Actual weight loss or growth depression and a decrease in
food consumption were evident in both sexes at 3000 ppm throughout, or
practically throughout, the study. Females at both 300 and 1000 ppm
exhibited a marginal, but not consistently dose-dependent, growth
reduction (= 10%) between weeks 12 and 48. Animals of the top-dosage
group exhibited abnormalities in the T-wave in the electrocardiograms
taken at termination and, upon ophthalmoscopic examination, increased
incidence of conjunctival discharge and inflammation as well as
corneal irregularities and roughening at week 27, but not at week 52.
Findings at physical examination described as "severe to slight
thinness and irregular or erratic heart beat" were noted almost
exclusively in animals at 3000 ppm throughout the study.
Monthly determinations of haematology and blood chemistry
revealed deviations from control values, mainly at 3000 ppm (both
sexes), in many parameters including decreased haematocrits, increased
platelet counts, and depressed values of total protein, albumin,
globulin, calcium, BUN, and creatinine at most sampling intervals.
Animals at 1000 ppm displayed decreased values of BUN (both sexes) and
creatinine (males) at many of the sampling intervals. As compared to
concurrent controls, males of all treated groups seemingly showed a
slight but consistent and generally dose-related decrease in
erythrocyte counts and haemoglobin levels. However, these findings
were unlikely to be treatment-associated, because values of these 2
haematological parameters for males even at 3000 ppm were within the
normal ranges given both for control Beagles in the published
literature (Bushby, 1970) and those reportedly recorded for control
Beagle dogs maintained in the testing laboratory. Urinalysis,
including microscopy of urinary sediments, conducted bi-monthly gave
no significant findings related to treatment.
At termination, gross pathological findings in the treated groups
were not significantly different from those in the controls. There was
an increase in organ/body-weight ratio of kidneys at both 1000 and
3000 ppm (both sexes), of liver in males at 3000 ppm and in females at
1000 ppm and above, of brain at 3000 ppm (both sexes), and of testes
at 3000 ppm. Histopathological evaluation of an extensive number of
tissues from each animal showed the occurrence of testicular
degeneration in 0/6, 2/6, 1/6, and 3/6 males at 0, 300, 1000, and
3000 ppm respectively. Severe and diffuse testicular degeneration was
seen in one affected male each at 300 ppm and 3000 ppm, while
generally mild and focal degeneration of the testes was present in the
other affected males. Admittedly, the incidence and severity of
testicular degeneration did not follow a dose-response relationship.
However, on account of the complete absence of the lesion from the
concurrent controls, and from a total of 7 similar 1-year studies
conducted at the testing laboratory comprising over 20 control male
dogs (McGee, 1983), the possibility of the testicular lesion being
related to the compound could not be completely ruled out. Other
microscopic findings likely to be attributable to treatment included
the presence in the top-dosage group of hypocellularity of the bone
marrow, lesions in the gastrointestinal tract (gastritis, edema,
ulceration) and heart (haemorrhage), and thymus atrophy, as well as an
increased incidence of lesions in the kidney (nephritis), liver
(centrilobular degeneration), lymph node (lymphadenitis), and spleen
(hyperplasia) at both 1000 and 3000 ppm.
The study did not permit the setting of a no-effect level, mainly
because of uncertainty concerning the occurrence of testicular
degeneration, which occurred at all dose levels (Benson, 1981).
Long-term studies
Mouse
Male and female CD-1 mice (50 animals per sex per group, about 50
days old), housed 5 per sex per cage, were fed dietary levels of
technical dimethipin (97-98% purity) at 0, 80, 400, or 2000 ppm for 78
weeks to evaluate the chronic toxicity and carcinogenic potential of
the compound. (Animals fed dimethipin were kept in the same room for
about 35 weeks as animals in a companion study receiving a highly
photodegradable compound identified only with a code name. It was
stated, but unsubstantiated with data, that, based on results of diet
analyses, dimethipin was stable in the diet for 7 days and that the
mixture of dimethipin and basal diet was satisfactorily homogeneous.)
All animals in the study, viz., those sacrificed in extremis or
that died during the study, and those sacrificed terminally, were
subjected to gross pathological examination and to microscopic
evaluation of a wide range of tissues, including the brain.
Mortality was not influenced by treatment. Between 58 and 78% of
the males and 76 and 86% of the females of the control and treated
groups were still alive at the conclusion of the study. A slight
(< 10%) and non-dose-related decrease in body-weight gain was noted
in males at 400 ppm and above during the first 13 weeks. Food
consumption was not affected in any consistent dose-related pattern.
There were no significant differences between control and treated
groups in clinical signs and incidence of palpable nodules or tissue
masses. Haematology determined on 5 males and 5 females per group at 3
intervals of the study revealed a significant increase in males at
2000 ppm of haematocrit levels at week 13 and of haemotocrit,
haemoglobin, and erythrocyte values at week 78. Terminal haematocrit
levels were elevated in females at both 400 and 2000 ppm. Females of
all treated groups showed a statistically-significant increase, albeit
not strictly dose-dependent, of erythrocyte counts at week 78. Blood-
chemistry and urinalysis parameters were not studied. Gross
pathological alterations and weights of selected organs were not
significantly different between control and treated groups.
Based on tabulated data on "individual histopathology findings"
(no detailed histopathological data with morphological descriptions of
lesions on individual animals were available), compound-induced non-
neoplastic changes were not evident. The only notable neoplastic
finding appeared to be an elevated incidence of pulmonary
(aveolar/bronchiolar) tumours in males at 2000 ppm, as shown in
Table 3.
Table 3 Number of males with lung tumours/number of males with the lung
examined microscopicallya
Pulmonary tumour Control 80 ppm 400 ppm 2000 ppm Historical controlb
(alveolar/bronchiolar) Total Range
adenocarcinomac 1/49 3/49 3/50 7/50 6/168 0/13(0)-
(2) (6.1) (6) (14)d (3.6) 2/24(8.3)
adenoma 5/49 3/49 3/50 5/50 15/168 1/24(4.2)-
(10.2)e (6.1) (6) (10)f (8.9) 2/13(15.4)
adenocarcinoma 6/49 6/49 6/50 12/50 21/168 7/61(11.5)or
or adenoma (12.2) (12.2) (12) (24) (12.5) 2/13(15.4)
a Figure in parenthesis indicates incidence in %.
b Data presented in the submitted report on a total of 168 CD-1 control male
mice from five 78-week studies over an unspecified timeframe.
c Multiple adenocarcinomas noted in 1 control and 2 animals at 2000 ppm.
d 1/7, ...
e 1/5, or ...
f 2/5 of the lung-tumour bearers died or were sacrificed at an unspecified time
during the study. All other bearers of pulmonary tumours were terminal
survivors.
When compared to concurrent-control or historical-control
incidences from a total of 5 studies, the incidence of lung
adenocarcinoma, but not of adenoma alone, was significantly increased
in males at 2000 ppm (P < 0.05, Fisher exact probability). However,
comparison with the maximum historical-control incidence with respect
to lung adenocarcinoma at 2000 ppm was not significant (P > 0.05).
Time-to-tumour (adenocarcinoma) or the multiplicity of tumours was not
modified by treatment. The combined incidence of lung adenocarcinoma
and adenoma was significantly elevated when compared to the
historical-control incidence from a total of 5 studies, but not when
compared with concurrent-control or maximum historical-control
incidences. There was no dose-related increase in the incidence of
benign and/or malignant lung tumours in the females. Other than lung
tumours, the incidence, location, and type of tumours in treated
groups were comparable to controls. About 30% of the male and 40% of
the female concurrent controls were found to have tumours. Lymphoma
(in males), lung tumours (in both sexes), and hepatocellular carcinoma
(in males) were the most frequently-observed spontaneous tumours. It
should be noted that the animals were about 50 days old at the
initiation of the study. This could compromise the sensitivity of the
test as a carcinogenicity study.
The study demonstrated that 80 ppm, equal to 12.3 mg/kg b.w./day,
is a virtual no-effect level, based on the monitored criteria other
than tumours. The lung-tumour data are unclear (Serota et al.,
1981a).
Rat
Groups of 50 male and 50 female rats (Sprague-Dawley CD strain,
about 40 days old), individually caged, were fed technical dimethipin
(97-98% purity) in their diet at 0, 40, 200, or 1000 ppm for 104 weeks
to assess the chronic toxicity and carcinogenic potential of the
compound. (The control group served as common controls for this study
and for a companion study on a chemical identified only by a code
name. Whether treated animals from the two studies were kept in the
same room was not specified.) All animals sacrified in moribund
condition or dying during the study, and all survivors sacrificed
terminally (during weeks 105 and 106), were subjected to necropsy and
histopathological examination of a variety of tissues, including the
brain plus any "unusual" lesions. Sections of spinal cord and "head"
from 10 male and 10 female terminal survivors per group were also
evaluated microscopically.
Survival, not adversely affected by treatment, appeared to be
better in the top-dosage group than in the concurrent control or in
lower-dosage groups. At the end of 104 weeks, with the exception of
males at 200 ppm having a survival rate on only 44%, between 50 and
72% of the males and females of all groups, including the control,
were still alive. Clincial signs related to treatment were not
evident. There were no dose- or compound-related effects on food
consumption or incidences of palpable nodules, tissue masses, or wart-
like lesions. A slight but consistent growth depression (< 5% in
males and < 10% in females) was seen at 1000 ppm between weeks 43
and 95 in males and between weeks 51 through 87 in females.
Haematology and blood chemistry conducted on 5 males and 5 females per
group at 5 intervals over the course of the study indicated that
females of all treated groups displayed an increase of total-protein
values at week 13 only, and a decrease of platelet counts at week 104,
the only sampling interval for this particular parameter. Other
deviations from controls were also observed in certain haematological
and blood-chemistry values, but these were essentially confined to the
top-dosage group. Significant differences were not apparent between
control and treated groups in urinalysis parameters monitored on 5
males and 5 females per group at the same intervals as the blood
studies. Gross pathological changes in treated animals were not
significantly different from those in the controls. Organ-weight
determinations showed an increase (non-dose-related in males and dose-
related in females) of the organ/body-weight ratio of the liver in
males of all treated groups and in females at both 200 and 1000 ppm.
In addition, absolute weight and organ/body-weight ratios of the
adrenal gland were decreased in females of all treated groups.
Histopathologically, "focally-dilated bile ducts containing basophilic
homogeneous material" occurred in 1 male and 1 female in the control
group, 2 males and 3 females at 40 ppm, 5 males and 9 females at 200
ppm, and 33 males and 18 females at 1000 ppm. This finding was likely
to be related to inclusion of the compound in the diet. Microscopic
changes in other tissues, including the adrenal gland, were comparable
in treated animals to those in the controls. An interesting but non-
dose- or compound-related microscopic finding was that 9-27% of the
males in the control and treated groups showed lactation and/or
galactocoele. Whether this might be connected with the observation of
an increased incidence of mammary fibroadenoma in males of the top-
dosage group, as pointed out later, was not certain. An evaluation of
the tumour data revealed that the only noteworthy findings were those
tabulated in Table 4.
The data in Table 4 seem to indicate increased incidences of
astrocytoma in males and hepatocellular carcinoma in females at both
200 and 1000 ppm and of mammary fibroadenoma in males at 1000 ppm. The
incidence of none of these tumours was, however, significantly
different from the concurrent controls (P > 0.05, Fisher exact
probability). This result was confirmed for hepatocellular carcinoma
when compared with historical control incidences. However, with
respect to astrocytoma in males, the incidence was significantly
increased at both 200 and 1000 ppm (P< 0.05) when compared with
historical-control incidence from a total of 7 studies. Comparison
with maximum historical-control incidence was nevertheless not
significant (P > 0.05), even at 1000 ppm. It might be noted that one
additional glioma was reportedly found (by a consultant to the
company) in the control group upon microscopic evaluation of 3
additional brain sections from each animal of the male control and
treated groups. In addition, no pre-neoplastic lesions (gliosis) were
seen in the original or additional brain slides (Squire, 1984). The
latency period of astrocytoma was apparently not reduced by treatment.
Overall, no significant differences between control and treated groups
were noted in the incidence of animals with tumours (benign and/or
malignant), benign tumours, malignant tumours, or multiple primary
tumours. Over 80% of the males and 90% of the females of the
concurrent control group were tumour bearers, with pituitary adenoma
and adrenal tumours in both sexes and mammary tumours in females being
the most prevalent tumours.
The study indicated that 40 ppm, the lowest tested-level, was a
minimum-effect level on parameters other than tumours (Serota
et al., 1981b).
EVALUATION
COMMENTS
In rats, the compound is readily absorbed, metabolized, and
eliminated via the urine and faeces. It is degraded primarily to polar
metabolites, with less than 5% of a single oral dose being recovered
as unchanged dimethipin in the excreta. In goats, dimethipin is
extensively degraded, also primarily to polar metabolites.
Biotransformation of the compound includes hydrolysis, oxidation,
decarboxylation, a ring opening, and conjugation.
Dimethipin has an oral LC50 value of about 500 mg/kg b.w. in
mice and 1200 mg/kg b.w. in rats.
A 2-generation (2 litters/generation) reproduction study in rats,
as well as teratology studies in rats and rabbits, were negative.
Practically all of the mutagenic studies available were negative. The
only positive mutagenic response was seen with the mouse lymphoma
forward mutation assay and only in the presence of metabolic
activation.
A 1-year feeding study in dogs failed to show a no-effect level,
mainly because of the uncertainty concerning the presence of
testicular degeneration even at 300 ppm, the lowest tested level.
However, taking into consideration the lack of a dose-response
relationship regarding incidence and severity of the testicular
lesion, the absence of effect of the compound on the testes of rats in
No. of animals with the tumour/No. of animals with the tissue examined
microscopically
Location *Historical Control
& type of
tumour Control 40 ppm 200 ppm 1000 ppm Total Range
Male Female Male Female Male Female Male Female Male Female Male Female
Brain
Astrocytoma 1a/-50(2) 1/49(2) 0/49(0) 2/50(4) 3b/-50(6) 0/50(0) 5c/50(10) 0/50(0) 1/279 0/266 0/43-1/21 0/21-0/37
** 2/50(4) (0.4) (0) (0)-(4.8) (0)
Mammary
gland ***
Fibroadenoma 0/43(0) N.P. 0/31(0) N.P. 0/33(0) N.P. 3/45(6.7) N.P. N.A. N.A. N.A. N.A.
Liver
Hepatocellular
cardnoma 2/50(4) 1/50(2) 1/50(2) 1/50(2) 5/50(10) 4/50(8) 2/50(4) 4/50(8) 13/305 8/285 0/35-3/34 0/32-2/31
(4.3) (2.8) (0)-(8.8) (0)-(6.5)
Neoplastic
nodule **** 2/50 6/50 2/50 5/50 5/50 5/50 2/50 5/50 N.A. N.A. N.A. N.A.
Figure in parenthesis indicates incidence in %
a astrocytoma present in one animal found dead at 20 weeks. This incidence (1/50 or 2%) was used as a basis of comparison in statistical
analyses.
b all 3 brain tumours found in terminal survivors.
c the time of detection of the brain tumour ranging irom 71 to 101 weeks with the mean being 83.4 weeks.
N.P. data not presented due to the complete absence of a dose-response relationship re incidence
N.A. not available
(Con't)
* Data included in the submitted report from a total of seven 104-week studies In Sprague-Dawley rats over an unspecified time-frame.
** The histopathological examination of 3 additional brain section from each male of control and all treated groups reportedly yielded
one additional glioma which was found in one control terminal survivor. It was also indicated that no preneoplastic lesions (gliosis)
were found in the original and the additional brain slides (Squire, 1984)
*** The historical control incidence of mammary fibroadenoma in an unspecified number of male Sprague-Dawley rats maintained in the
testing laboratory was stated to range from 0 to 3% (Serota 1985).
**** Not considered as tumour per se but included for information only.
the 2-generation reproduction study, and the similarly-negative effect
on the testes of mice and rats in the long-term studies, plus the
virtual absence of other toxic effects at the lowest-tested level, it
was concluded that a level somewhat below 300 ppm could reasonably be
taken as a no-effect level for this species. In this connection, the
Meeting agreed that 100 ppm, the no-effect level established in an
available 90-day dog-feeding study, be accepted as a 1-year no-effect
level for this species.
In the long-term mouse and rat studies, there was seemingly an
increase in the incidence of lung adenocarcinoma in male mice of the
top-dosage group (2000 ppm) and of astrocytoma in male rats of both
the intermediate- and top-dosage groups (i.e. 200 ppm and 1000 ppm).
Although the evidence for a causal relationship between these
particular tumour findings and treatment did not appear to be
convincing, such a possibility could not be excluded at this time.
Before a more definitive opinion on the tumourigenic/carcinogenic
potential of the compound can be expressed, additional information, as
indicated under "Further work or information required", is needed on
those mouse and rat studies from which data on historical control
incidences of certain tumours (specifically lung tumours in mice and
astrocytoma and liver tumours in rats) were obtained.
In view of the concerns with respect to increased incidence of
lung adenocarcinoma in male mice and of astrocytoma in male rats of
the long-term studies, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Mouse: 80 ppm in the diet, equal to 12.3 mg/kg b.w.
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.003 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
1. Information on those mouse and rat studies from which data on
historical control incidences of certain tumours, viz. lung
tumours in CD-1 mice and astrocytoma and liver tumours in
Sprague-Dawley rats, were obtained and presented in the submitted
reports. (Information for each of the studies should include date
of each study, age of animals at initiation, mortality rate of
animals, and experimental conditions such as diet, number of
animals per cage, etc.)
2. Further pharmacokinetic and metabolic studies in rats and/or a
non-rodent mammalian species using appropriate multiple-dosage
levels.
3. Acute oral toxicity studies on major plant metabolites which are
not found in animals if these are liable to occur at substantial
residues.
DESIRED
1. Results of diet analysis for the 18-month mouse and 2-year rat
feeding studies.
2. Studies on any potential oestrogenic or other hormonal effect of
dimethipin, including the determination of the affinity of the
compound for steroid receptors.
3. Further studies in dogs to assess effects, if any, of dimethipin
on the testis.
4. Observations in man.
REFERENCES
Babish, J.G. Harvade(R) (N252) Tech. C-8-1162-00. Acute inhalation
(1977) study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc., submitted to WHO by Uniroyal
Inc., USA.
Baker, R.C., Mastri, C.W., Kinoshita, F.K., & Keplinger, M.L. Acute
(1976) irritation tests with a sample coded N252-C10406, Lot No.
BL7668 in albino rabbits. Unpublished report (IBT No. 8530-
08861) from Industrial Bio-Test Laboratories, Inc., USA,
submitted to WHO by Uniroyal Inc., USA (validated by the
Canadian Health Protection Branch).
Benson, B.W. 1-Year dietary toxicity study in dogs with N252.
(1981) Unpublished report from International Research and
Development Corp., USA, submitted to WHO by Uniroyal Inc.,
USA.
Bootman, J. & Lodge, D.C. ARS7728 (technical grade Harvade (R):
(1982) Assessment of its ability to induce genetic damage in
Saccharomyces cerevisiae. Unpublished report from Life
Science Research, England, submitted to WHO by Uniroyal
Inc., USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K, & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 611-08063) from Industrial
Bio-Test Laboratories, Inc., USA, submitted to WHO by
Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
Bushby, S.R.M. "Hematological studies during toxicity tests." In
(1970) Methods in Toxicology, edited by Paget, G.E. Blackwell
Scientific Publications, Oxford and Edinburgh.
Caplan, J. & Merricks, D.L. 14C-Harvade(R) radiocarbon study in rats.
(1978) Unpublished report from Biospherics Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Forster, R., Edwards, N., & Nunziata, A. Mitotic gene conversion in
(1984a) Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from
Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Forster, R., Mosesso, P., & Nunziata, A. Micronucleus test. Test
(1984b) substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy,
submitted to WHO by Uniroyal Inc., USA.
Galloway, S.M. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(1981) (N252) 98% D-11401 in the sister chromatid exchange assay
with Chinese hamster ovary (CHO) cells. Final Report.
Unpublished report from Litton Bionetics, Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of N-252,
(1978) technical lot D10406, BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Jagannath, D.R. & Brusick, D.J. Mutagenicity evaluation of Harvade(R)
(19981) (N252) 98% D-11401 in the Ames Salmonella/microsome plate
test. Final report. Unpublished report from Litton
Bionetics, Inc., USA, submitted to WHO by Uniroyal Inc.,
USA.
Kehoe, D.F. & MacKenzie, K.M. Final report. Two-generation rat
(1982) reproduction study with N252. Unpublished report from
Hazleton Raltech Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Knickerbocker, M., Re, T.A., & Babish, J.G. Teratologic evaluation of
(1977) N252 (Harvade(R)) technical in Sprague-Dawley rats.
Unpublished report from Food and Drug Research Laboratories,
Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Madison, W.A. Technical grade Harvade(R). Dermal sensitization in
(1983) guinea pigs (modified closed patch technique). Unpublished
report from Hazleton Raltech, Inc., USA, submitted to WHO by
Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K., & Keplinger, M.L. 90-Day
(1976) sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT No. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA, submitted to
WHO by Uniroyal Inc., USA (validated by the Canadian Health
Protection Branch).
McGee, D.H. Unpublished letter from International Research Development
(1983) Corp., USA, to Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McManus, J.P. Metabolism of Harvade(R) in goats. Unpublished
(1984) report from Uniroyal Chemical Co., submitted to WHO by
Uniroyal Inc., USA.
McMeekin, S.O., Schardein, J.L., & Blair, M. N252 (Harvade(R)
(1981) technical). Teratology study in rabbits. Unpublished report
from International Research and Development Corp., USA,
submitted to WHO by Uniroyal Inc., USA.
Myhr, B.C. & Brusick, D.J. Mutagenicity evaluation of Harvade(R) (N252)
(1981) in the mouse lymphoma forward mutation assay. Revised final
report. Unpublished report from Litton Bionetics, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Reagan, E.L. & Becci, P.J. Acute dermal toxicity study (LD50) in
(1982) albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories Inc., USA, submitted to
WHO by Uniroyal Inc., USA.
Serota, D.G., Alsaker, R.D., & Banas, D. 18-Month toxicity and
(1981a) oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc.,
USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K., & Kunalzins, W. 104-Week
(1981b) chronic toxicity study in rats. N252 (Harvade(R) technical)
final report. Unpublished report from Hazleton Laboratories
America, Inc., USA, submitted to WHO by Uniroyal Inc., USA.
Serota, D.G. Unpublished letter from Hazleton Laboratories America,
(1985) Inc. to Uniroyal Inc., USA, submitted to WHO by Uniroyal
Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977a) USA, submitted to WHO by Uniroyal Inc., USA.
Shapiro, R. Untitled and unpublished report from Product Safety Labs,
(1977b) USA, submitted to WHO by Uniroyal Inc., USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K.. & Cardona, R.A.
(1978) Characteriztion of excretory metabolites from a 14C-
Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemical, submitted to WHO by Uniroyal Inc., USA.
Sorg, R.M., Naismith, R.W., & Matthews, R.J. CHO metaphase analysis
(1983) in vitro chromosome aberration analysis in Chinese hamster
ovary cells (CHO). PH320-UN-001-83 Harvade(R). Unpublished
report from Pharmakon Research International Inc., USA,
submitted to WHO by Uniroyal Inc., USA.
Squire, R.A. Unpublished letter to Uniroyal Chemical, submitted to WHO
(1984) by Uniroyal Inc., USA.
Strong, M.B. Studies on the acute oral toxicity of N252 (Harvade(R)) in
(1981) crossbred dogs. Unpublished report from Ciba-Geigy Australia
Ltd., submitted to WHO by Uniroyal Inc,, USA,
Varner, L.L. & Matthews, R.J. Acute oral LD50 in rats. Uni-N-252
(1977) C.N.D.-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA, submitted to WHO by
Uniroyal Inc., USA.
