
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
DIMETHIPIN
First draft prepared by
W. Dykstra
Environmental Protection Agency, Washington DC, United States
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Comments
Toxicological evaluation
References
Explanation
Dimethipin was first evaluated by the 1985 Joint Meeting (Annex
1, reference 44), when a temporary ADI of 0-0.003 mg/kg bw was
established on the basis of a NOAEL of 100 ppm, equivalent to 2.5
mg/kg bw per day, in a 90-day study in dogs treated in the diet.
Dimethipin was evaluated again by the 1988 Joint Meeting (Annex 1,
reference 53), which reviewed additional data and established an ADI
of 0-0.02 mg/kg bw on the basis of a NOAEL of 2 mg/kg bw per day for
increases in the absolute and relative (to body weight) weights of the
liver in female rats fed dimethipin in the diet in a 2-year study.
Further data have been provided. The compound was re-evaluated within
the periodic review programme of the Codex Committee on Pesticide
Residues.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
Three male and two female CD rats received a single oral dose of
approximately 3.8 mg/kg bw of [2,3-14C-dithiin ring]dimethipin
(radiochemical purity, 96%) in distilled water. About 89% of the
administered radiolabel was excreted in urine and faeces within 48 h,
although one of the females eliminated only about 42% of the dose by
these routes over the same period. The reason for the low recovery of
label in this animal was unclear, as the tissue and blood
concentrations were similar to those in the other treated animals. In
general, faecal elimination slightly exceeded urinary excretion. Less
than 0.1% of the administered label was detected in expired air. At
sacrifice 96 h after treatment, the mean concentration of total
residue in the tissues analysed (excluding blood) amounted to about 1%
of the administered dose. The concentrations of residue were highest
in the lung, heart, liver, and kidney and lowest in the
gastrointestinal tract, brain, muscle, and fat. The concentration in
blood represented 2-7.7% of the administered dose. No significant sex
difference was apparent in the rate, route of elimination, or
concentrations of residue in tissues (Caplan & Merricks, 1978; JMPR,
1985).
Groups of four to five male and female CD Sprague-Dawley rats
received a single oral dose of 1.2 mg/kg bw of labelled dimethipin; a
single intravenous dose of 2 mg/kg bw (targeted at 1.2 mg/kg bw) of
labelled dimethipin; a single oral dose of 50 mg/kg bw of labelled
dimethipin; or 1000 ppm of unlabelled dimethipin in the diet
continuously for 14 days followed immediately on day 15 by a single
oral dose of 50 mg/kg bw radiolabelled dimethipin. Urine, faeces,
blood, and tissue samples were analysed at numerous intervals after
treatment. Significant absorption and rapid excretion were found
(Billings, 1987; JMPR, 1988).
(b) Biotransformation
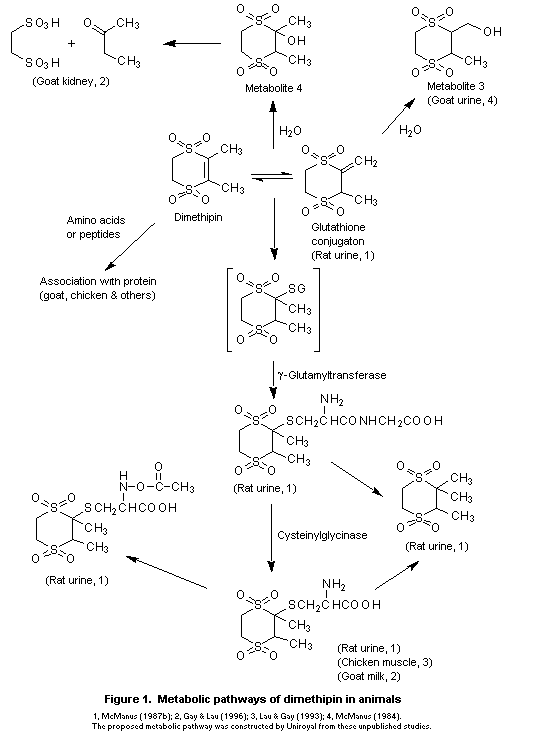
The metabolic pathways of dimethipin in animals are shown in
Figure 1.
Rats
In the study of Caplan & Merricks (1978) described above,
analysis of pooled urinary and faecal samples from the treated rats,
except for the female rat with an unusually low excretory rate, showed
that about 5% of the label was in the urine or faeces as the unchanged
parent compound. The other radiolabelled components were highly polar
(Smilo, et al., 1978; JMPR 1985).
In the study of Billings et al. (1987) described above, urine
samples collected from rats treated in the diet were analysed by
high-performance liquid chromatography (HPLC). During the first 24-h
collection period, the amounts of the reduced product,
N-acetylcysteine, and polar fractions increased while those of
cysteinylglycine conjugates decreased. There were no sex differences.
These results supported the proposed metabolic pathway involving
glutathione conjugation. The glutathione concentrations in liver and
blood from three male and three female rats treated in the diet were
no different from control values (McManus, 1987a; JMPR, 1988).
 Three young male Charles River CD rats weighing approximately
225 g each received a single oral dose of 800 mg/kg bw of
14C-dimethipin. Urine and faeces were collected separately 24, 48,
and 72 h after dosing. The samples collected at 24 h were used for
identification of metabolites. Only trace amounts of dimethipin were
detected in the urine by HPLC, but seven metabolites were detected.
The three identified polar products, which accounted for 77% of the
urinary radioactivity, were a cysteinylglycine conjugate (54%), an
N-acetylcysteine conjugate (12%), and a reduced metabolite,
2,3-dimethyl-5,6-dihydro-1,4-dithiane-1,1,4,4-tetraoxide (11%). The
proposed metabolic pathway involves conjugation with glutathione
followed by degradation to cysteinylglycine and cysteine conjugates
and formation of mercapturic acid conjugates (McManus, 1987b; JMPR
1988).
Goats
A goat with a cannulated bile duct received 14C-dimethipin
(radiochemical purity, 98%) at a dietary concentration of 500 ppm for
three days. Samples of urine, liver, bile, and kidney were collected
for characterization and identification of metabolites. No details of
the experimental conditions were provided, and although two animals
appear to have been treated, data were presented on only one. Only 18%
of the labelled residues in liver and 15% of those in kidney were
identified: (butan-3-one-2-yl)-2-hydroxyethyl sulfone (due to ring
cleavage) and 2,3,5,6,-tetrahydro-5-hydroxy-5,6-dimethyl-1,4-dithiine
1,1,4,4,-tetraoxide being the predominant residues. Metabolites 3 and
4 were invariably identified together with unchanged parent compound
in urine, bile, liver, and kidney. Metabolite 4 accounted for 2-8% of
the radiolabel recovered in each case. Some alcohol metabolites of
dimethipin were identified only in urine and bile. Most of the
radiolabelled components in urine, bile, liver, and kidney were highly
polar and were present as glucuronide, cysteine, and acetylcysteine
conjugates. Studies in vitro confirmed that these are the likely
major metabolic steps. It was not reported whether total radioactivity
was measured or whether residues were identified in muscle, fat, or
milk (McManus, 1984; FAO/WHO, 1985).
Goats received a normal ration supplemented with 14C-dimethipin
at 1 or 10 mg/kg of feed until a plateau was reached in the milk after
17 days. After 18 days of dosing, about 97% of the total administered
radiolabel was excreted in the urine and faeces, but none were
detected in muscle and the concentrations were low in milk, liver, and
kidney. The concentrations added to the feed were higher than the
so-called 'worst case' situation in which every component of the diet
contains residues at the tolerable maximum residue level (TMRL)
proposed by the JMPR in 1985. Thus, if animals are exposed to a normal
feeding regime, the concentrations in meat, milk, and edible offal
will not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP standards and with a
signed and dated quality assurance statement, two lactating goats
received 14C-dimethipin (radiochemical purity, > 98%) at a
concentration of 0.15 or 50 mg/kg bw per day for 5 days before being
killed about 22 h after the last dose. The doses were equivalent to
dietary concentrations of 3.1 ppm (10 times actual exposure) and
1000 ppm (3200 times actual exposure), respectively. Urine and faeces
were collected once daily, and milk was collected twice daily.
Overall, 97% of the total radiolabel administered was recovered from
the animal given the low dose and 96% from that given the high dose.
More than 95% of the total administered dose was identified in the
excreta, and 0.1-0.2% was eliminated in the milk. The average daily
concentrations in milk were 0.005 ppm at the low dose and 1 ppm at the
high dose. Fat, muscle, and whole blood contained the lowest
concentrations of radiolabel at both the low dose (0.001 ppm in fat,
0.002 ppm in muscle, and 0.006 ppm in whole blood and the high dose
(0.32 ppm in fat, 0.64 ppm in muscle, and 2.7 ppm in whole blood),
whereas the liver and kidney contained the highest concentrations,
with 0.27 and 0.14 ppm at the low dose and 79 and 28 ppm at the high
dose, respectively. The sensitivity of the method was < 0.0004 ppm
for the low dose and < 0.011 ppm for the high dose (Byrd, 1992).
Enzymatic digestion followed by acid hydrolysis of liver tissue
from this study suggested that the radiolabelled residues of
dimethipin were covalently bound to tissue proteins. After acid
hydrolysis, acetyldithiane was identified as the single radiolabelled
peak in liver. Kidney samples from the animals at the low dose
contained ethane disulfonic acid as the only metabolite, whereas renal
tissue from the animal at the high dose also contained ethane
disulfonic acid; acid hydrolysis of the bound material from this
kidney showed that acetyl dithiane was also present. Milk from both
animals contained dimethipin cysteine conjugate as the major
metabolite. Acid hydrolysis of muscle from the goat at the high dose,
which contained 0.64 ppm of radiolabelled material, yielded reduced
dimethipin as the major metabolite. The results of this study suggests
that dimethipin is metabolized primarily via Michael addition to
glutathione or its derivatives or to protein (Gay & Lau, 1996).
Chickens
Laying hens were fed a normal diet fortified with
14C-dimethipin at concentrations of 1, 6, or 30 mg/kg for 30 days,
when half of the birds were slaughtered; the remainder were killed
after a withdrawal period of 11 days. By day 30, more than 95% of the
total administered radiolabel had been excreted in the faeces. No
residues were detectable in muscle or fat at the end of dosing with
1 mg/kg or in fat after administration of 6 mg/kg. The concentrations
found in tissues corresponded roughly to the doses. The concentrations
found in the tissues of birds after 11 days of withdrawal were
considerably lower, and none of the tissues except blood from hens at
1 mg/kg contained measurable residues. The birds fed 6 or 30 mg/kg
14C-dimethipin had measurable residues in all tissues except fat. In
eggs, the residual radiocarbon increased slowly over 10 days and
remained fairly constant for the remainder of the dosing period.
Except in birds given 30 mg/kg, the concentrations in eggs decreased
to below the limit of determination during the 11 days of withdrawal.
Extrapolation from these data indicates that residues in birds fed a
hypothetical 'worst case' diet in which all or nearly all of the
components contain residues at the level of the MRL proposed by the
1985 JMPR would not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP, with a signed and
dated quality assurance statement, [14C-6]dimethipin (radiochemical
purity, > 95%) was administered orally to white Leghorn laying hens
daily for five consecutive days at a dose of 15.8 mg/kg bw per day
(five hens) or 152 mg/kg bw per day (two hens), equivalent to
concentrations of 203 ppm (7000 times that caclulated to result from
use of dimethipin at the permitted level on the cereals in the diet)
and 2770 ppm (92 000 times the calculated concentration),
respectively. Five untreated birds served as controls. Excreta and
eggs were collected once daily during treatment. More than 90% of the
total administered radiolabel was found in the excreta. The hens were
killed within 24 h of the last dose, and tissues were collected,
examined, and weighed. Edible tissues and eggs contained 5.5% and 5.1%
of the total radiolabel at the low and high doses, respectively. The
liver and kidney had the highest concentrations, with 9.7 and 65 ppm
in liver and 4.5 and 39 ppm in kidney at the low and high doses,
respectively. Breast and thigh muscle each contained 10 ppm at the
high dose and 0.63 and 0.72 ppm at the low dose, respectively. Egg
yolk and egg white contained 6.9 and 6.6 ppm, respectively, at the
high dose and 1.1 and 0.68 ppm at the low dose. Fat had the lowest
concentrations, with 0.20 ppm at the low dose and 2.4 ppm at the high
dose. Multiple metabolites were identified in eggs and tissues, all
formed as conjugates of glutathione followed by degradation, resulting
in the following compounds: glutathionyl-dimethipin,
gamma-glutamyl-cysteinyl-dimethipin, cysteinyl-dimethipin,
mercaptodimethipin, thioacetyl-dimethipin, thiomethyl-dimethipin, and
methyl sulfoxide-dimethipin. The major metabolite was
gamma-glutamylcysteinyl-dimethipinin most tissues and
cysteinyl-dimethipin in liver (Lau & Gay, 1993).
While the metabolism of dimethipin in plants was found to be
negligible, because it is applied at or close to the harvesting stage
when the biochemical activities of the plant are at a minimum, its
metabolism in animals is quite extensive. As a result, the main
residues in crops are the parent product, while in animals dimethipin
undergoes glutathione conjugation with subsequent degradation. In a
second pathway, dimethipin undergoes hydration followed by ring
cleavage.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of dimethipin after administration by the
oral, dermal, and inhalation routes and its ocular and dermal
irritation and dermal sensitizing capacity are summarized in Table 1.
Table 1. Acute toxicity of dimethipin
Species Purity Sex Route LD50 or LC50 Reference
(%) (mg/kg bw or mg/L)
Mouse > 97.5 M Oral 440 Shapiro (1977a)
F 600
Rat > 97.5 M&F Oral 1 200 Varner & Matthews
(1977)
Rat 98 M Oral 460 Blaszcak (1992a)
F 550
Rat NR M Intraperitoneal 240 Shapiro (1977b)
F 240
Rat NR M&F Inhalation (1 h) > 20 Babish (1977)
Rat 98.9 M Inhalation (4 h) 1.5 Hoffman (1992)
F 0.88
Rabbit NR M&F Dermal (24 h) > 12 000 Reagen & Becci (1982)
Rabbit 98 M&F Dermal (24 h) > 5 000 Blaszcak (1992b)
NR, not reported
In rats (strain unspecified), the LD50 for dimethipin (purity,
> 97.5%) administered orally was 1200 mg/kg bw for animals of each
sex (Varner & Matthews, 1977; JMPR, 1985). In another study, the
LD50 for dimethipin (purity, 98.0%) was 550 mg/kg bw in male and 460
mg/kg bw in female Sprague Dawley CD rats (Blaszcak, 1992a). Surviving
animals lost weight during week 1, but gained weight during the
remainder of the observation period. The signs of toxicity included
nasal discharge, hypoactivity, and rales. At necropsy, discoloured
lungs, red distended stomachs, and red fluid in the intestines were
noted.
The LC50 in Sprague-Dawley CD rats exposed by inhalation by
nose only for 4 h was 1.5 mg/L for males and 0.88 mg/L for females
(Hoffman, 1992). The clinical signs of toxicity included respiratory
distress. Body weights were substantially decreased (41% in males and
18% in females) during the first week, but weight was gained after
that time. At necropsy, discoloured lungs were observed.
The LD50 in rabbits was > 5000 mg/kg bw, as no deaths were
seen (Blaszcak, 1992b). It severely irritated the eyes (Griffiths &
Koschier, 1980), but the material tested was a recrystallized form of
dimethipin, which may not be representative of the technical-grade
material. Technical-grade dimethipin was not irritating to rabbit skin
(Blaszcak, 1992c). It was a weak dermal sensitizer in Hartley
guinea-pigs (Madison, 1983).
WHO (1999) has classified dimethipin as 'slightly hazardous'.
(b) Short-term studies of toxicity
Rats
Groups of six male and six female Charles River CD Sprague-Dawley
rats, eight weeks of age, received technical-grade dimethipin (purity,
98%) moistened with distilled water dermally for 6 h/day at doses of
0, 10, 100, or 1000 mg/kg bw per day for 21 days. The study was
conducted under GLP requirements, and a signed and dated quality
assurance statement was available. There were no treatment-related
alterations in mortality rate, body weight, food consumption, clinical
signs, clinical pathology, or gross and histopathological appearance.
Traces of hyperkeratosis were present in the skin of treated males at
all doses and in females at the intermediate and high doses. The
weights of the liver relative to body weights were statistically
significantly increased in males (20%) and females (15%) at 1000 mg/kg
bw per day, but the absolute liver weights of these animals were
statistically nonsignificantly increased, by 18% in males and 12% in
females. In the absence of clinical pathological or histopathological
effects, the NOAEL for systemic toxicity was 1000 mg/kg bw per day,
the highest dose tested (Goldenthal, 1991).
Groups of 15 male and 15 female Charles River rats, 28 days old,
were fed diets containing technical-grade dimethipin (purity,
> 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for 95 days.
There was no treatment-related change in mortality rate or abnormal
behaviour. Food consumption was slightly depressed in females at the
highest dose throughout the study, but body weights were unaffected.
Haematology, blood chemistry, and urinalysis performed in 10 males and
10 females from the control and high-dose groups after 45 and 85 days
of treatment indicated no significant compound-related effects. At
termination, the ratios of organ:body weight of liver and kidney were
increased in females at the highest dose. No significant differences
in gross pathological changes were observed between control and
treated groups. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females from the
control and high-dose groups showed no lesions attributable to
treatment. The NOAEL was 300 ppm, equivalent to 15 mg/kg bw per day
(Marias et al., 1976; JMPR, 1985).
Groups of 10 male and 10 female Charles River CD Sprague-Dawley
rats aged four week were fed diets containing technical-grade
dimethipin (purity, 98.5%) at concentrations of 0, 40, 1750, or 3500
ppm (equal to 2.5, 110, and 220 mg/kg bw per day in males and 3.1,
130, and 260 mg/kg bw per day in females) for 13 weeks. The study was
conducted under GLP requirements, and a signed quality assurance
statement was available. The animals were observed for deaths and
clinical signs of toxicity twice daily, and body weight and food
consumption were measured weekly. Ophthalmoscopic examinations were
made before treatment and at the end of the study. Clinical data were
collected on all surviving animals at 13 weeks. The weights of the
brain, kidneys, liver, and testis were recorded for all animals, and
all tissues were examined microscopically.
One male at the low dose was killed in extremis, but this
animal showed no significant gross or microscopic changes, and the
cause of death was not identified. No clinical signs of toxicity or
ophthalmoscopic findings were reported. Males at the high dose had
significantly decreased food consumption (11%), body weight (6%), and
body-weight gain (8%) in comparison with controls; and females at the
intermediate and high doses had significantly decreased food
consumption (12% at the intermediate and 16% at the high dose), body
weight (10% and 11%), and body-weight gain (20% and 28%). Males at the
high dose had slightly decreased erythrocyte volume fractions (8%) and
haemoglobin values (7%). Significantly increased cholesterol
concentrations were found in females at the intermediate (37%) and
high doses (41%), and those at the high dose had increased serum
activity of aspartate aminotransferase (30%). There were no gross
findings at necropsy that were considered to be related to treatment.
The weight of the liver relative to body weight was significantly
increased in males at the high dose (18%) and was correlated with
microscopic hepatocellular hypertrophy in 5 of 10 animals. Females at
the intermediate and high doses showed increased relative weights of
the brain, kidney, and liver, in the absence of microscopic changes,
and these changes were considered to be related to the decreased body
weights of those animals. The NOAEL was 40 ppm, equal to 2.5 mg/kg bw
per day (Goldenthal, 1993).
Dogs
Groups of four male and four female pure-bred beagles, about six
months old, were given diets containing technical-grade dimethipin
(purity, > 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for
90 days. No deaths occurred, and no treatment-related effects were
seen on behaviour, body weight, or food consumption or in blood
chemistry or haematology conducted after 42 and 85 days of treatment.
Urinary analysis at the same two intervals indicated an increase (not
dose-related) in the incidence of moderate-to-large amounts of
'crystals' in the urinary sediments of all treated females after 85
days. No other urinary parameters were affected. At terminal
sacrifice, the organ weights and gross appearance were not affected by
treatment. Microscopic examination of a large number of tissues,
including the testis, from each animal revealed oesophageal lesions
characterized by focal mucosal vesicles containing a few acute
inflammatory cells in one of eight animals at 300 ppm and three of
eight animals at 1000 ppm, but in none of the concurrent controls or
those at the lowest dose. The NOAEL was 100 ppm, equivalent to 2.5
mg/kg bw per day (Burtner et al., 1976).
Groups of six male and six female pure-bred beagles, about 7.5
months old and caged individually, were given diets containing
technical-grade dimethipin (purity, 99.7%) at concentrations of 0,
300, 1000, or 3000 ppm for one year. One male and three females at the
highest dose died or were sacrificed in extremis between weeks 13
and 52. 'Thinness', a major clinical sign, was seen frequently in most
animals at 3000 ppm and infrequently in one animal at 1000 ppm. Other
signs, including dehydration and paleness of the gums, were also noted
infrequently at 3000 ppm. Weight loss or growth depression and
decreased food consumption were seen in animals of each sex at 3000
ppm throughout most of the study. Bitches at 300 or 1000 ppm showed a
marginal (10%) but not consistently dose-dependent reduction in growth
between weeks 12 and 48. Animals of the highest dose had abnormalities
in the T-wave on electrocardiograms at the end of the study, and
ophthalmoscopic examination showed increased incidences of
conjunctival discharge, inflammation and corneal irregularities and
roughening at week 27 but not at week 52. 'Severe to slight thinness
and irregular or erratic heartbeat' were seen almost exclusively in
animals at 3000 ppm throughout the study.
Monthly haematological and blood chemical determinations revealed
deviations from control values in many parameters mainly in animals of
each sex at 3000 ppm, including decreased erythrocyte volume fraction,
increased platelet count, and depressed values of total protein,
albumin, globulin, calcium, blood urea nitrogen, and creatinine at
most sampling intervals. Animals at 1000 ppm had decreased values of
blood urea nitrogen (both sexes) and creatinine (dogs) at many
sampling intervals. When compared with concurrent controls, treated
dogs showed a slight but consistent, generally dose-related decrease
in erythrocyte counts and haemoglobin levels. These findings were
considered unlikely to be related to treatment, because the values
were within the normal ranges for control beagles in the published
literature (Bushby, 1970) and those recorded for control beagle dogs
maintained in the testing laboratory. Urinalysis, including microscopy
of urinary sediments, conducted twice monthly showed no significant
changes related to treatment.
At termination, the gross pathological appearance in the treated
groups was not significantly different from that in the controls. The
ratio of organ:body weight for the kidneys was increased at both 1000
and 3000 ppm (both sexes), for liver in dogs at 3000 ppm and in
bitches at > 1000 ppm, for brain at 3000 ppm (both sexes), and for
testis at 3000 ppm. Histopathological evaluation of a large number of
tissues from each animal showed testicular degeneration in 0/6, 2/6,
1/6, and 3/6 dogs at 0, 300, 1000, and 3000 ppm, respectively. Severe
and diffuse testicular degeneration was seen in one affected dog at
300 ppm and one at 3000 ppm, while generally mild focal degeneration
of the testis was seen in the other affected animals. Although the
incidence and severity of testicular degeneration did not show a
dose-response relationship, the complete absence of this lesion in
concurrent controls and in seven similar 1-year studies conducted in
the same laboratory and comprising over 20 control dogs (McGee, 1983)
indicated that the possibility that the testicular lesion is related
to treatment cannot be ruled out. Nevertheless, the testicular lesions
were considered not to be a direct effect of dimethipin on the testes,
but rather the result of the prolonged poor nutritional status of the
dogs or an incidental finding, as they were similar to incidental
findings in other studies in dogs in the same laboratory.
Additionally, no testicular lesions were seen in dogs in a 90-day
study. Other microscopic findings likely to be attributable to
treatment included hypocellularity of the bone marrow, lesions in the
gastrointestinal tract (gastritis, oedema, and ulceration) and heart
(haemorrhage), and thymic atrophy at the high dose, and an increased
incidence of nephritis, centrilobular degeneration in the liver,
lymphadenitis, and splenic hyperplasia at both 1000 and 3000 ppm. The
NOAEL was 300 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
the increased relative liver weight, increased alanine
aminotransferase and alkaline phosphatase activities, and
hepatocellular degeneration in bitches at 1000 ppm (Benson, 1981;
JMPR, 1985, 1999).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CD-1 mice, about 50 days old,
housed five per sex per cage, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at 0, 80, 400, or 2000 ppm
for 78 weeks. The animals fed dimethipin were kept for about 35 weeks
in the same room as animals receiving a highly photodegradable
compound identified only by a code name. It was stated, but
unsubstantiated by data, that dietary analysis showed that dimethipin
was stable in the diet for 7 days and that the mixture of dimethipin
and basal diet was satisfactorily homogeneous. All animals killed
in extremis or that died during the study and those killed at the
end were examined grossly, and a wide range of tissues, including the
brain, were examined microscopically.
The mortality rate was not affected by treatment: 58-78% of males
and 76-86% of females in all groups were still alive at the conclusion
of the study. A slight (< 10%), non-dose-related decrease in
body-weight gain was seen in males at doses > 400 ppm during the
first 13 weeks. Food consumption was not affected in a consistent
dose-related pattern. There were no significant differences between
control and treated groups in the incidence of clinical signs or
palpable nodules or tissue masses. Haematological examination of five
males and five females per group at three intervals during the study
revealed a significant increase in erythrocyte volume fraction in
males at 2000 ppm at week 13 and in erythrocyte volume fraction,
haemoglobin, and erythrocyte values at week 78. At termination, the
erythrocyte volume fraction was elevated in females at both 400 and
2000 ppm. All treated females showed a statistically significant,
albeit not strictly dose-dependent, increase in erythrocyte counts at
week 78. Blood chemical and urinary parameters were not evaluated. No
significant gross pathological alterations or changes in organ weights
were seen. No detailed histopathological data with morphological
descriptions of lesions in individual animals were available, but
tabulated 'individual histopathology findings' indicated that no
compound-induced non-neoplastic changes were found.
The only notable neoplastic finding was an increased incidence of
pulmonary (alveolar and bronchiolar) tumours in males at 2000 ppm. The
incidence of lung adenocarcinomas, but not of adenomas alone, was
significantly increased in males at 2000 ppm when compared with
concurrent or historical controls from five studies ( p < 0.05,
Fisher exact test), but not when compared with the maximum incidence
of lung adenocarcinomas in historical controls. The time to appearance
of adenocarcinomas and the multiplicity of tumours were not modified
by treatment. Additionally, the pulmonary tumours were not associated
with an increase in hyperplastic pulmonary changes. The combined
incidence of lung adenocarcinoma and adenoma was significantly
increased in comparison with the incidence in historical controls from
five studies but not when compared with the incidence in concurrent
controls or the maximum incidence in historical controls. There was no
dose-related increase in the incidence of benign or malignant lung
tumours in females. The incidence, location, and type of tumours other
than lung tumours were comparable to those in controls. About 30% of
the male and 40% of the female concurrent controls were found to have
tumours. Lymphoma (in males), lung tumours (in both sexes), and
hepatocellular carcinoma (in males) were the most frequently observed
spontaneous tumours. The fact that the animals were about 50 days old
at initiation of the study may have compromised the sensitivity of the
test. The NOAEL was 80 ppm, equal to 12 mg/kg bw per day, on the basis
of criteria other than tumours. The data on lung tumours are unclear
(Serota et al., 1981a; JMPR, 1985). As lung adenomas and
adenocarcinomas occur commonly in this strain of mice, this finding
was considered to be of no toxicological relevance (JMPR, 1988).
Rats
Groups of 50 male and 50 female Sprague-Dawley CD rats, about 40
days old and caged individually, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at concentrations of 0,
40, 200, or 1000 ppm for 104 weeks. The control group was used for
both this study and another study on a chemical identified only by a
code, and it was not reported whether treated animals in the two
studies were kept in the same room. All animals that were killed in
moribund condition or died during the study and all survivors killed
at the end of the study during weeks 105 and 106 were necropsied, and
a variety of tissues including the brain and any 'unusual' lesions
were examined histopathologically. Sections of the spinal cord and
'head' of 10 male and 10 female survivors per group were also
evaluated microscopically. Five males and five females per group were
studied for haematological and blood chemical parameters at five
intervals during the study and similar numbers at the same intervals
for urinary indices.
The survival of animals at the highest dose appeared to be better
than that in other groups. By 104 weeks, the survival rates were 44%
for males at 200 ppm and 50-72% for males and females in all groups,
including controls. No clinical signs related to treatment were seen,
and there were no dose- or compound-related effects on food
consumption or on the incidence of palpable nodules, tissue masses, or
wart-like lesions. A slight but consistent depression of growth
(< 5% in males and < 10% in females) was seen at 1000 ppm
between weeks 43 and 95 in males and between weeks 51 and 87 in
females. Females in all treated groups had increased total protein at
week 13 and decreased platelet counts at week 104, the only time at
which this parameter was measured. Other deviations from control
values were observed in certain haematological and blood chemical
parameters, but essentially only in animals at the highest dose. No
significant differences were seen between control and treated groups
in urinary parameters. The gross pathological changes seen in treated
animals were not significantly different from those in controls. A
non-dose-related increase in the ratio of liver:body weight was seen
in males in all treated groups, and a dose-related increase was seen
in females at 200 and 1000 ppm.The absolute weight of the adrenals and
that relative to body weight were decreased in females in all treated
groups. Histopathological examination showed focally dilated bile
ducts containing basophilic homogeneous material in one male and one
female control, two males and three females at 40 ppm, five males and
nine females at 200 ppm, and 33 males and 18 females at 1000 ppm. This
finding was presumed to be related to treatment. The microscopic
changes in other tissues, including the adrenal gland, were similar to
those in controls. The finding that 9-27% of the males in the control
and treated groups showed lactation and/or galactocoele may have been
associated with the increased incidence of mammary fibroadenoma in
males at the highest dose. The only other noteworthy neoplastic
findings were increased incidences of astrocytoma in males and
hepatocellular carcinoma in females at 200 and 1000 ppm. The
incidences of these tumours were not, however, significantly different
from those in concurrent controls (Fisher exact test). The incidence
of hepatocellular carcinoma was not significantly different from that
in historical controls, but that of astrocytoma in males was
significantly increased at both 200 and 1000 ppm ( p < 0.05) when
compared with the incidence in historical controls from seven studies.
Comparison with the maximum incidence in historical controls showed no
significant difference, even at 1000 ppm. An additional glioma was
reportedly found in the control group by a consultant to the company
who evaluated three additional brain sections from each control and
treated male. No preneoplastic lesions (gliosis) were seen in the
original or additional brain slides (Squire, 1984). The latency to
appearance of astrocytoma was not reduced by treatment. The
minimum-effect level on parameters other than tumours was 40 ppm, the
lowest dose tested, equivalent to 2.0 mg/kg bw per day (Serota et al.,
1981b; JMPR, 1985).
Further data on the changes in organ weights, provided
subsequently by the testing laboratory, gave a NOAEL of 200 ppm for
the decrease in relative and absolute weights of the adrenal glands in
female rats, and a NOAEL of 200 ppm for the increase in absolute and
relative liver weights in male rats. The NOAEL for the increase in
absolute and relative liver weights in females was 40 ppm (JMPR, 1998)
Groups of 60 young Sprague-Dawley rats (Crl:CD BR (VAF/Plus)) of
each sex were fed diets containing technical-grade dimethipin (purity,
98.5%) at concentrations of 0, 40, 1750, or 3500 ppm for males (equal
to 0, 1.8, 78, or 160 mg/kg bw per day) and 0, 40, 875, or 1750 ppm
for females (equal to 0, 2.2, 50, or 100 mg/kg bw per day) for 104
weeks. The study was conducted under GLP requirements, and a signed
quality assurance statement was available. Ten animals of each sex per
dose were killed after 12 months for interim evaluations of clinical
signs, deaths, body weight, food consumption, ophthalmological,
haematological, clinical chemical, and urinary parameters, organ
weights, and gross and histopathological appearance. The survival of
females at the high dose was decreased but not statistically
significantly. Of the 50 rats per sex allocated to the main study,
only 20, 24, 19, and 21 of the males and 20, 18, 18, and 13 of the
females in the control, low-, intermediate-, and high-dose groups,
respectively, survived to week 104. There were no treatment-related
clinical signs of toxicity, ophthalmic or haematological effects, or
changes in urinary parameters in any treated group in comparison with
controls. The mean body weight of males at the high dose was decreased
by 13-20% during the study; females at the intermediate dose weighed
< 16% less than controls and those at the high dose weighed
< 19% less. The mean body-weight gain of males at the high dose was
consistently decreased by 24%, and those of females at the
intermediate and high doses by 13 and 29% in comparison with controls.
No accompanying decrease in food consumption was seen.
Significantly increased serum aspartate and alanine
aminotransferase activities were observed in females at the
intermediate and high doses at 12, 18, and 24 months and in males at
the high dose at 18 months. Animals of each sex at the intermediate
and high doses also had elevated cholesterol levels at all sampling
intervals, and males at these doses had increased serum concentrations
of urea nitrogen and creatinine. The findings indicate renal and
hepatic toxicity. Macroscopic alterations were seen in the liver and
kidneys of animals that died during the study or were killed at the
end. Males at the high dose and females at the intermediate and high
doses had an increased incidence of liver cysts, and males at the
highest dose had an increased incidence of tan discolouration of the
liver and small testes. Males at the two higher doses had a slight
increase in the incidence of enlarged kidneys. Tan discolouration and
tan foci in the liver and a granular surface on the kidneys were seen
in females at the highest dose. Significant increases in organ weights
( p < 0.05) in males at the highest dose that were considered to be
related to treatment were in the absolute (115%) and relative (166%)
weights of the liver and the absolute (132%) and relative (152%)
weights of the kidney.
At interim sacrifice at 12 months, the incidence and/or grade of
bile-duct hyperplasia was increased in treated males (4/12, 5/13, and
5/12 at the low, intermediate, and high doses, respectively) when
compared with controls (2/13). In females, the incidence and/or grade
was increased at both 875 ppm (70%) and 1750 ppm (43%) when compared
with controls (8%). Epithelial hyperplasia of the duodenum was seen in
11/12 males and 10/14 females at the high dose and in none of the
controls by 12 months. In animals killed at the end of the study, the
incidence and/or grade of biliary cysts in the liver was increased in
females at the intermediate (12%) and high doses (37%) and in males at
the high dose (10%) in comparison with controls (2% in females and 0%
in males). Bile-duct hyperplasia in the liver was increased in
incidence and/or severity in males at the intermediate (25/47) and
high doses (29/48) in comparison with controls (21/47), and in females
at the intermediate (68%) and high doses (61%) when compared with
controls (33%). Dose-related increases in the severity of the lesion
were seen in animals of each sex. Male rats fed the high dose also
showed a significantly increased incidence (33%) of eosinophilic foci
in comparison with controls (13%).
The incidence and severity of chronic progressive nephropathy was
increased in 47/47 males and 37/50 females at the intermediate dose
and 46/48 males and 38/46 females at the high dose in comparison with
controls (33/47 in males and 18/48 in females). The incidence and
grade of epithelial hyperplasia of the duodenum was increased in males
at the intermediate (38%) and high doses (46%) in comparison with
controls (2%), and in females at the intermediate (14%) and high doses
(33%) in comparison with controls (0%). Signs of gastrointestinal
tract toxicity were seen in animals of each sex at the high dose;
males at this dose had an increased incidence and severity of
epithelial hyperplasia of the nonglandular stomach (15%, 2% in
controls), and females showed demineralization of the glandular
stomach (7/46, 0/48 in controls). Males at the high dose had an
increased incidence (21/47) and grade of seminiferous tubular
degeneration of the testis (eight had grades of moderate and six
severe; 12/47 in controls of which 0 were moderate and four severe)
and hypospermia of the epididymis (13/47, 5/47 in controls).The
incidence of seminiferous tubular degeneration was 13/27 (three
moderate and two severe) in males at the low dose and 16/34 (four
moderate and three severe) at the intermediate dose. Although the
testes of all rats at the low and intermediate doses were not
examined, a treatment-related increase in the incidence of testicular
lesions at the intermediate dose could be discerned. The occurrence of
testicular degeneration at the intermediate and high doses was
considered to be related to treatment, as was the increased incidence
of epididymal hypospermia, which was probably a result of the
seminiferous tubular degeneration. Females at the high dose showed an
increased incidence and severity of vascular mineralization of the
heart (5/46, 0/46 in controls) and aortic artery (6/45, 0/48 in
controls). The other histological lesions reported were considered not
to be related to treatment.
The doses tested were adequate to assess the tumorigenic
potential of dimethipin. The only statistically significant increase
in neoplastic lesions was in the incidence of benign
phaeochromocytomas (17%, 4% in concurrent controls) in the adrenal
medulla of male rats at the high dose. There was no accompanying
increase in hyperplasia in this tissue and no increase in the
incidence of malignant phaeochromocytomas, and the combined incidence
of benign and malignant neoplasms was not increased significantly.
Furthermore, the incidence of benign phaeochromocytomas in historical
controls ranged from about 0 to 18%, and the combined incidence of
malignant and benign tumours ranged from about 0 to 20% in similar
studies conducted in the same laboratory during the 5 years preceding
termination of this study. The increased incidence of benign
phaeochromocytomas was therefore considered not to be related to
treatment. Slight but biologically insignificant increases or
decreases in the incidences of other tumours were seen in treated
groups in comparison with controls. The tumour incidences in this
study are presented in Table 2. During the first 12 months of the
study, additional fibroadenomas and adenocarcinomas were found in
females in both control and treated groups and an additional
fibroadenoma was found in a male at the intermediate dose. The other
tumour types listed in Table 2 were not found during the first 12
months. The increased incidences of astrocytomas in males at 200 and
1000 ppm, hepatocellular carcinomas in females at 200 and 1000 ppm,
and mammary fibroadenomas in males at 1000 ppm in the study of Serota
et al. (1981b) were not found in this study, even though the doses and
the number of rats per sex at each dose exceeded those in the earlier
study. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day
(Goldenthal, 1996).
(d) Genotoxicity
Dimethipin had no mutagenic activity in a number of assays in
microorganisms, mammalian cells, and rodents in vitro and in
vivo. The only exception was the induction of forward mutation in
mouse lymphoma cells in the presence of metabolic activation
(Table 3).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical-grade dimethipin
(purity, 99.7%) at 0, 50, 200, or 800 ppm for 105 days before mating
(one male:two females; sibling and half-sibling mating avoided). he
day on which a positive vaginal smear or copulatory plug was detected
was considered to be day 0 of gestation. Weanlings of the second
litter (F1b) were selected to become parents of the next generation
and mated after receiving the test diets for 125 days. In each
generation, the second mating was allowed at least 14 days after the
first litter (F1a and F2a) had been weaned at 21 days of age.
Table 2. Tumour incidencea in rats dying between weeks 52 and 104 in a 2-year study of dimethipin
Tumour site Males (dose, ppm) Females (dose, ppm)
0 40 1750 3500 0 40 875 1750
Mammary gland
Adenoma 1/7 0/3 1/2 2/7 4/48 3/41 7/46 2/46
Fibroadenoma 28/48 23/41 27/46 16/46
Adenocarcinoma 9/48 6/41 10/46 4/46
Brain
Astrocytoma 1/44 0/24 0/28 1/48 0/48 0/31 0/33 0/46
Granular-cell tumour 1/44 0/24 0/28 1/48
Liver
Adenoma 1/47 0/48 3/47 2/48 1/48 0/49 1/50 3/46
Carcinoma 1/47 1/48 2/47 1/48 0/48 0/49 0/50 0/46
Interstitial-cell tumour of the testis
Benign 2/47 1/27 2/34 5/47
Malignant 0/47 0/27 1/34 0/47
Combined 2/47 1/27 3/34 5/47
Phaeochromocytoma of the adrenal medulla
Benign 2/47 4/28 0/28 8/48* 1/48 1/32 0/35 0/46
Malignant 2/47 1/28 1/28 0/48 0/48 0/32 0/35 0/46
Combined 4/47 5/28 1/28 8/48
a Number of rats with tumours/Number of rats examined microscopically
* Significantly different from incidemce in concurrent controls at p < 0.05
Table 3. Results of assays for the genotoxicity of dimethipin (purity, > 98%)
End-point Test system Dose Result Reference
In vitro
Reverse mutation S. typhimurium TA1538, 1-1000 mg/plate Negativea Jagannath & Brusick
TA1537, TA1535, TA98, (1978, 1981)
Mitotic non-disjunction, S. cerevisiae D4 1-1000 mg/plate Negativea Jagannath & Brusick
recombination, and (1978)
mutation S. cerevisiae D6 1-2000 mg/plate Negativea Bootman & Lodge
(1982)
Mitotic gene conversion S. cerevisiae D4 125-2000 mg/ml Negativea Forster et al. (1984a)
Chromosomal aberration Chinese hamster ovary cells 5-50 mg/ml Negativea Sorg et al. (1983)
Sister chromatid exchange Chinese hamster ovary cells 1.56-24 mg/mlb Negativea Galloway & Brusick
3.1-200 mg/mlc (1981)
Forward mutation L5178Y Tk+/- mouse 1.56-75 mg/mlb ? Myhr & Brusick
lymphoma cells 125-200 mg/mlc (1981)
In vivo
In vivo/in vitro unscheduled Wistar rat 100, 300, or 1000 Negative McManus (1987c)
DNA synthesis mg/kg bw
Micronucleus formation Swiss CD-1 mouse 220 mg/kg bw Negative McManus (1986)
Micronucleus formation Mouse Two successive Negatived Forster et al. (1984b)
daily oral doses of
22, 73.3, or 220
mg/kg bw per day
(males) or at 30,
100, or 300 mg/kg
bw per day (females)
In the parental generations, deaths (the incidence of which was
not dose-related) occurred only among females. No compound-related
behavioural abnormalities were seen. F0 and F1b adult females at
800 ppm weighed less than the concurrent controls before mating,
throughout gestation, and throughout most of the the lactation
periods. Food consumption was depressed in animals at 800 ppm, in F0
females before mating in weeks 6-10, in F1b females in weeks 6-17,
and in F1b males in weeks, 1, 4, and 9. Fertility in males, as
determined by a demonstrated ability to impregnate at least one
female, mating index (% females mated), gestation index (% mated
females with viable litters), the number of days required by females
to mate, and the duration of gestation in treated groups were all
comparable to the control values. In the progeny, the mean number of
pups per litter born alive, survival of pups to days 4, 7, 14, and 21,
the sex ratio, and the behaviour of pups were not adversely affected.
The weights of pups in the F1a and F1b litters at 800 ppm were
reduced on days 7, 4, and 21, and the weights of those in the F1a
litters at 200 ppm and in the F2a litters at 800 ppm were decreased
on day 21. Gross external examination of all pups, including those
found dead, revealed only one abnormal pup, which was a stillborn in
an F1a litter at 200 ppm.
Gross pathological examination of all parental animals of the
second litters killed after weaning (F0) or 30 days after weaning
(F1b) (i.e. after 32 weeks and 39 weeks of dietary feeding,
respectively) and weanlings in each generation revealed no significant
difference between control and treated groups. Determinations of the
weights of organs from all F1b adults and five male and five female
weanlings from the F1b and the F2b litters showed increased
organ:body-weight ratios for the liver at 200 and 800 ppm and for the
kidney and brain at 800 ppm in adult F1b females; however, the
organ:body-weight ratio of the liver in F1b adult females was
depressed at 50 ppm. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults and
five male and five female weanlings from the F1b and the F2b
litters and of gross lesions and gonads from F0 adults indicated no
significant changes attributable to treatment.
The NOAEL was 200 ppm, equivalent to 10 mg/kg bw per day, as the
finding of a decrease in pup weight on day 21 in F1a litters at 200
ppm was unlikely to be treatment-related, as it occurred in only a
single generation and was not recurrent (Kehoe & Mackenzie, 1982;
JMPR, 1985).
(ii) Developmental toxicity
Rats
Groups of sexually mature mated female rats (BLU:(SD)BR) received
technical-grade dimethipin (purity, 97.5%) by intubation as a
suspension in corn oil at a dose of 0, 80, 400, or 800 mg/kg bw per
day on days 6-15 of gestation, the day on which a vaginal plug was
observed being considered day 0. An additional group of mated female
rats treated with 250 mg/kg bw per day of acetylsalicylic acid was
used as the positive control. The groups at 400 and 800 mg/kg bw per
day were terminated within 8 days of initiation of treatment owing to
'excessive deaths' and were not investigated further. Two new groups,
at 30 and 160 mg/kg bw per day, were added 2 weeks after the study
began, but no concurrent control groups were included for the two new
doses. The dams were killed on day 20 of gestation and their fetuses
were removed surgically for gross external, visceral, and skeletal
examination.
No compound-related deaths or clinical signs were observed at
doses up to 160 mg/kg bw per day, and the growth rates of dams during
gestation were comparable in all groups. The number of dams in each
group that became pregnant and were alive on day 20 was 20-22. The
mean number of implantation sites or live fetuses, fetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, with no concomitant increase in the incidence of
pregnant dams with resorptions, was seen at 160 mg/kg bw per day. The
incidence of skeletal or visceral malformations of fetuses did not
differ significantly between control and treated groups. The positive
control group had a number of fetal abnormalities, including
encephalomenigocoele and gastroschisis. The NOAEL for both maternal
and developmental toxicity was 160 mg/kg bw per day (Knickerboker et
al., 1977; JMPR, 1985).
Rabbits
Groups of 16 sexually mature female Dutch belted rabbits were
artificially inseminated and were intubated with technical-grade
dimethipin (purity, 98.3%) as a suspension in 0.5% carboxymethyl
cellulose at 0, 7.5, 20, or 40 mg/kg bw per day at a constant volume
of 1 ml/kg bw, on days 6-27 of gestation, day 0 of gestation being
considered the day of insemination. They were killed on day 28 of
gestation, and the uterine contents were examined. All fetuses,
including those that were aborted or dead, were examined grossly and
for skeletal and visceral abnormalities.
No deaths occurred. A slight increase in the number of females at
20 and 40 mg/kg bw per day that had a reduced amount of faeces beneath
the cage was seen at various intervals during gestation as compared
with concurrent controls. No data on food consumption were available.
Does at 40 mg/kg bw per day showed weight loss between days 6 and 12,
and maternal weight gain was depressed in a dose-response pattern in
all treated groups between days 6 and 28. The fertility rate was
88-94% in control and treated groups. One doe each at 0, 20, and 40
mg/kg bw per day aborted on day 28; seven non-viable fetuses were
found in does at 0 and 20 mg/kg bw per day; and three late resorptions
occurred in the doe at 40 mg/kg bw per day. At terminal sacrifice, the
gross pathological findings in treated does were comparable to those
in the controls. No significant differences were found between
controls and treated groups in the mean numbers of corpora lutea,
implantations, early or late resorptions, or viable or non-viable
fetuses, or in fetal weight.
A non-dose-related increase in postimplantation loss, due mainly
to an increased number of early resorptions, was seen in all treated
groups, although the values for this parameter were within the range
of historical controls. The sex ratio of fetuses at 40 mg/kg bw per
day was increased, the mean number of females being reduced. Increases
in the incidence of fetuses and of litters containing fetuses with 27
presacral vertebrae and with scoliosis (with or without associated rib
anomalies) were observed at 40 mg/kg bw per day, when compared with
concurrent or historical control incidences, in a total of 951 fetuses
in 149 litters from an unspecified number of studies with Dutch belted
rabbits over an unspecified period. There was no apparent dose- or
compound-related increase in the frequency of fetal soft-tissue
abnormalities. The NOAEL for maternal and developmental toxicity was
20 mg/kg bw per day (McMeekin et al., 1981; JMPR, 1985).
Comments
After oral administration to rats, goats and hens,
14C-dimethipin was extensively absorbed (69% within 24 h) and
rapidly excreted (89% within 48 h), mainly in the urine. Unchanged
dimethipin represented only a small fraction of the residue in
animals. In one metabolic pathway, dimethipin undergoes glutathione
conjugation and subsequent degradation to several metabolites,
including its mercapturic acid. In another pathway, dimethipin is
hydrated and then undergoes ring cleavage. Dimethipin also binds
covalently to amino acids, peptides and proteins, although the extent
to which this binding is catalysed by enzymes is unknown.
Dimethipin (purity, 98.5%) was moderately toxic to rats given
single oral doses, with LD50 values of 460 mg/kg bw in males and 550
mg/kg bw in females, or after exposure by inhalation, with LC50
values of 1.5 mg/L in males and 0.88 mg/L in females. It showed little
toxicity in rabbits exposed dermally, with an LD50 value greater
than 5000 mg/kg bw. A recrystallized form of dimethipin was severely
irritating to the eye in rabbits. Technical-grade dimethipin was not
irritating to rabbit skin but weakly sensitized the skin of
guinea-pigs.
WHO (1999) has classified dimethipin as 'slightly hazardous'.
In 90-day and long-term tests for toxicity in rats, the liver was
the main target at doses of 10 mg/kg bw per day and above. The
clinical findings consisted of increased absolute and relative weights
of the liver and increased serum cholesterol concentration and
transaminase activity. At doses greater than 85 mg/kg bw per day,
hepatocellular hypertrophy was seen in 90-day studies, whereas in
long-term studies the hepatocellular effects included focal dilatation
of bile ducts, biliary cysts and bile-duct hyperplasia.
The testis was identified as another target organ. In a one-year
study in dogs given dimethipin, testicular changes were seen at all
doses, the lowest dose being 300 ppm (equivalent to 7.5 mg/kg bw per
day), but these were considered not to be related to treatment but to
be a result of poor nutritional status or incidental findings, as they
were similar to testicular lesions seen in other studies in dogs in
the same laboratory. Additionally, no testicular lesions were seen in
dogs in a 90-day study. In contrast, Sprague-Dawley rats fed diets
containing technical-grade dimethipin for two years showed increased
incidences and severity of seminiferous tubular degeneration at the
two highest doses, 1750 and 3500 ppm (equal to 78 and 160 mg/kg bw per
day), associated at the high dose with hypospermia in the
epididymides. The NOAEL for testicular degeneration was 40 ppm
(2 mg/kg bw per day).
In a 90-day study in dogs given dimethipin in the diet, the
lowest dose of 100 ppm (equivalent to 2.5 mg/kg bw per day) was the
NOAEL, on the basis of oesophageal lesions at the LOAEL of 300 ppm
(equivalent to 7.5 mg/kg bw per day).
In the 1-year study in dogs described above, the effects seen at
1000 and 3000 ppm (equal to 25 and 75 mg/kg bw per day) included
'thinness' and increased relative kidney weights in animals of each
sex. At this dose, males had decreased blood urea nitrogen and
creatinine concentrations and females had increased relative liver
weights. One male and three females at the highest dose died, and
animals of each sex had decreased body weights and food consumption,
hypocellularity of the bone marrow, gastritis, oedema, ulceration of
the gastrointestinal tract, thymic atrophy, nephritis, centrilobular
degeneration of the liver, splenic hyperplasia and lymphadenitis. The
NOAEL was 300 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of
increased relative liver weights, increased alanine aminotransferase
and alkaline phosphatase activities, and hepatocellular degeneration
in females at 1000 ppm (equivalent to 25 mg/kg bw per day).
In a 78-week study of carcinogenicity in mice, a statistically
significant increase in the incidence of alveloar and bronchiolar
carcinomas was seen in males at the highest dose (2000 ppm, equal to
300 mg/kg bw per day). The combined incidence of lung adenocarcinoma
and adenoma was significantly greater than the mean for controls in
five previous studies but not when compared with that for concurrent
controls or with the mean maximum incidence in controls in previous
studies. As lung adenomas and adenocarcinomas occur commonly in this
strain of mice, this finding was not considered to be of toxicological
relevance. The NOAEL for systemic toxicity was 80 ppm (equal to 12
mg/kg bw per day) on the basis of increased erythrocyte volume
fraction at the LOAEL of 400 ppm (equal to 60 mg/kg bw per day).
In two 2-year studies in rats, the NOAEL for systemic toxicity
was 40 ppm (equal to 2 mg/kg bw per day) on the basis of decreased
body weights, increased absolute and relative weights of the liver, an
increased incidence of biliary hyperplasia, and testicular
degeneration at higher doses. No increase in tumour incidence was
observed in rats at any dose. The Meeting concluded that dimethipin is
not carcinogenic in mice or rats and is unlikely to pose a
carcinogenic risk to humans.
Dimethipin has been tested in an adequate range of tests for
genotoxicity in vitro and in vivo. Negative results were obtained
in most assays. It induced a weak mutagenic response in one test for
forward mutation in mouse lymphoma cells in the presence of metabolic
activation. The Meeting concluded that dimethipin is unlikely to be
genotoxic.
In a two-generation study of reproductive toxicity in rats, the
highest dose of 800 ppm (equivalent to 40 mg/kg bw per day) caused
decreased body weights and food consumption in parental animals of
each sex and decreased body weights in pups on days 7, 14, and 21 of
lactation. The NOAEL for both systemic toxicity in the parental
generation and developmental toxicity in the pups was 200 ppm
(equivalent to 10 mg/kg bw per day).
In a study of developmental toxicity in rats, excess mortality
occurred at doses of 400 and 800 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 160 mg/kg bw per day. In
rabbits, the NOAEL for both maternal and developmental toxicity was 20
mg/kg bw per day. Does at the maternal LOAEL of 40 mg/kg bw per day
showed body-weight loss on days 6-12 of gestation and decreased weight
gain on days 6-28 of gestation. The LOAEL for developmental toxicity
was 40 mg/kg bw per day on the basis of an increased incidence of
fetuses with skeletal malformations (scoliosis).
The present Meeting confirmed the ADI of 0-0.02 mg/kg bw
established by the 1988 Joint Meeting on the basis of the NOAEL of 2
mg/kg bw per day in the 2-year study in rats conducted in 1981 and a
safety factor of 100. This ADI is supported by the NOAEL of 40 ppm,
equivalent to 2 mg/kg bw per day, in the 2-year study in rats
conducted in 1996. The ADI provides a 1000-fold margin of safety with
respect to the NOAEL of 20 mg/kg bw per day for developmental toxicity
in rabbits, which showed skeletal malformations at the LOAEL of 40
mg/kg bw per day.
An acute reference dose of 0.02 mg/kg bw was established on the
basis of the NOAEL of 20 mg/kg bw per day for skeletal malformations
in the study of developmental toxicity in rabbits and a safety factor
of 1000. This high safety factor was used because of the nature of the
effect.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 80 ppm, equivalent to 12 mg/kg bw per day (toxicity in
a 78-week study of toxicity and carcinogenicity)
Rat: 40 ppm, equivalent to 2 mg/kg bw per day (toxicity in
two 2-year studies of toxicity and carcinogenicity)
160 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
10 mg/kg bw per day (parental and reproductive toxicity
in a two-generation study of reproductive toxicity)
Rabbit: 20 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 100 ppm, equivalent to 2.5 mg/kg bw per day (toxicity
in a 90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to dimethipin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 69% within 24 h, rats
Dermal absorption Low dermal penetration, rabbits
Distribution Widely distributed, rats
Potential for accumulation No evidence of accumulation
Rate and extent of excretion 89% within 48 h mainly via urine
Metabolism in animals Parent < 5%; metabolites consist of glutathione conjugates and degradates, rats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity Oral toxicity is moderate, but only slightly toxic by dermal and inhalation
routes of exposure
Rat, LD50, oral 440 mg/kg bw (males) and 600 mg/kg bw (females)
Rabbit, LD50, dermal > 5000 mg/kg bw
Rat, LC50, inhalation 0.88 mg/L, 4 h (female) and 1.5 mg/L, 4 h (males)
Rabbit, dermal irritation Not irritating
Rabbit, ocular irritation Severely irritating
Guinea-pig, dermal sensitization Weakly sensitizing
Short-term toxicity
Target/critical effect Liver: hepatotoxicity, hepatic hypertrophy, rats
Lowest relevant oral NOAEL 2 mg/kg bw per day, rats
Lowest relevant dermal NOAEL 1000 mg/kg bw per day (highest dose tested), rats
Lowest relevant inhalation NOAEL Not determined
Long-term toxicity and carcinogenicity
Target/critical effect Rat liver: increased weight, liver enzymes, bile-duct hyperplasia; rat
testis: degeneration
Lowest relevant NOAEL 2 mg/kg bw per day in two 2-year studies, rat
Carcinogenicity Not carcinogenic in mice or rats
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect None. Decreased pup body weight on days 7, 14, and 21 of lactation at
maternally toxic doses, rats
Lowest relevant reproductive NOAEL 10 mg/kg bw per day
Developmental target/critical effect Increased incidence of skeletal malformations, rabbit
Lowest relevant developmental NOAEL Rabbit, 20 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Medical data None
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Two 2-year studies in rats 100
Acute reference dose 0.02 mg/kg bw Skeletal malformations in rabbits 1000
References
Babish, J.G. (1977) Harvade(R) (N252) Tech. C-8-1162-00. Acute
inhalation study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Benson, B.W. (1981) 1-Year dietary toxicity study in dogs with N252.
Unpublished report from International Research and Development
Corp., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA
Billings, T.J. (1987) A (C14)-radiolabeled pharmacokinetics and
metabolism study in the rat using Harvade. Southwest Bio-Labs,
Inc. (Project No. 8656r). Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L (1992a) Acute oral toxicity study in rats with Harvade
technical (Project No. 6169-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992b) Acute dermal toxicity study in rabbits with
Harvade technical (Project No. 6170-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992c) Primary dermal irritation study in rabbits with
Harvade technical (Project No. 6171-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Bootman, J. & Lodge, D.C. (1982) ARS7728 (technical grade
Harvade(R)): Assessment of its ability to induce genetic damage
in Saccharomyces cerevisiae. Unpublished report from Life
Science Research, United Kingdom. Submitted to WHO by Uniroyal
Inc., USA.
Byrd, J.W. (1992), Nature of the residue of radiolabeled Harvade in
lactating goat; Part I: Dosing, specimen collection, and
quantitation of Harvade residues in lactating goats (Project No.
9202), unpublished study. Submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 61108063) from Industrial
Bio-Test Laboratories, Inc., USA (validated by the Canadian
Health Protection Branch). Submitted to WHO by Uniroyal Inc.,
USA.
Bushby, S.R.M. (1970) Hematological studies during toxicity tests. In:
Paget, G.E., ed., Methods in Toxicology, Oxford, Blackwell
Scientific Publications.
Caplan, J. & Merricks, D.L. (1978) 14C-Harvade(R) radiocarbon
study in rats. Unpublished report from Biospherics, Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Forster, R., Edwards, N.& Nunziata, A. (1984a) Mitotic gene conversion
in Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from Life
Science Research, Roma Toxicology Centre, Italy. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Forster, R., Mosesso, P. & Nunziata, A. (1984b) Micronucleus test.
Test substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Galloway, S.M. & Brusick, D. J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 90% D-11401 in the sister chromatid exchange
assay with Chinese hamster ovary (CHO) cells. Final report.
Unpublished report from Litton Bionetics, Inc., USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gay, M.H. & Lau, R.C.M. (1996) Nature of the residue of dimethipin in
lactating goat (Project No. 9202), unpublished study. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1991) 21-Day dermal toxicity study in rats with
Harvade technical (Report No. 399-116), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal Inc., USA.
Goldenthal, E.I. (1993) 13 Week dietary toxicity study in rats with
Harvade technical (Project No. 399-133), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal, Inc. USA.
Goldenthal, E.I. (1996) Two year dietary chronic toxicity and
oncogenicity study in rats (with) Harvade technical (dimethipin)
(Project No. 399-134), unpublished study from MPI Research.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Griffiths, J.T. & Koschier, F.J. (1980) Rabbit eye irritation study
with Harvade technical (recrystallized) (Project No. 6413a),
unpublished report from Food and Drug Research Laboratories, Inc.
Submitted to WHO by Uniroyal Inc., USA
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
Harvade technical in the rat (Project No. 91-8376), unpublished
study from Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Jagannath, D.R. & Brusick, D.J. (1978) Mutagenicity evaluation of
N-252, technical lot D10406 BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Jagannath, D.R. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 98% D-11401 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kehoe, D.F. & Mackenzie, K.M. (1982) Final report. Two-generation rat
reproduction study with N252. Unpublished report from Hazleton
Raltech, Inc., USA. Submitted to WHO by Uniroyal Inc., USA.
Knickerbocker, M., Re, T.A. & Babish, J.G. (1977) Teratologic
evaluation of N252 (Harvade (R)) technical in Sprague-Dawley
rats. Unpublished report from Food and Drug Research
Laboratories, Inc., USA, submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA
Lau, R.C.M. & Gay, M.H. (1993) Nature of the residue of 14C-Harvade
in laying hens (Uniroyal Chemical Co. Project No. 91121),
unpublished stud. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Madison, W.A. (1983) Technical grade Harvade(R). Dermal
sensitization in guinea pigs (modified closed patch technique).
Unpublished report from Hazleton Raltech, Inc., USA. Submitted to
WHO by Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT NO. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA (validated by the
Canadian Health Protection Branch). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McGee, D.H. (1983) Unpublished letter from International Research
Development Corp., USA, to Uniroyal Chemical Co. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA
McManus, J.P. (1984) Metabolism of Harvade(R) in goats. Unpublished
report from Uniroyal Chemical Co. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1986) Mouse micronucleus test with dimethipin
technical, Life Sciences Research, Rome (Report 180001-M-06886).
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA
McManus, J.P. (1987a) Analysis of urine samples from dimethipin
(Harvade) rat pharmacokinetic study. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1987b) Metabolism of (C14) Dimethipin (Harvade) in the
Rat - Urinary Metabolite Identification. Uniroyal Project No.
85125. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
McManus, J.P. (1987c) In vivo/in vitro UDS study in rats, Robens
Institute (Report No. 4/86/TX). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McMeekin, S.O.., Schardein, J.L. & Blair, M. (1981) N252 (Harvade(R)
technical). Teratology study in rabbits. Unpublished report from
International Research and Development Corp., Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Myhr, B.C. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) in the mouse lymphoma forward mutation assay.
Revised final report. Unpublished report from Litton Bionetics,
Inc., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Reagan, E.L. & Becci, P.J. (1982) Acute dermal toxicity study (LD50)
in albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories, Inc., USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Serota, D.G., Alsaker, R.D. & Banas, D. (1981a) 18-month toxicity and
oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc., USA.
Ssubmitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K. & Kunalzins, W. (1981b)
104-Week chronic toxicity study in rats. N252 (Harvade(R)
technical) final report. Unpublished report from Hazleton
Laboratories America, Inc., USA, submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977a) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977b) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K. & Cardona, R.A.
(1978) Characterization of excretory metabolites from
14C-Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemica. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Sorg, R.M., Naismith, R.W. & Matthews, R.J. (1983) CHO metaphase
analysis in vitro chromosome aberration analysis in Chinese
hamster ovary cells (CHO). PH320-UN-00183 Harvade(R),
unpublished report from Pharmakon Research International, Inc.,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Squire, R.A. (1984) Unpublished letter to Uniroyal Chemical. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Varner, L.L. & Matthews, R J. (1977) Acute oral LD50 in rats.
Uni-N-252 CND-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Three young male Charles River CD rats weighing approximately
225 g each received a single oral dose of 800 mg/kg bw of
14C-dimethipin. Urine and faeces were collected separately 24, 48,
and 72 h after dosing. The samples collected at 24 h were used for
identification of metabolites. Only trace amounts of dimethipin were
detected in the urine by HPLC, but seven metabolites were detected.
The three identified polar products, which accounted for 77% of the
urinary radioactivity, were a cysteinylglycine conjugate (54%), an
N-acetylcysteine conjugate (12%), and a reduced metabolite,
2,3-dimethyl-5,6-dihydro-1,4-dithiane-1,1,4,4-tetraoxide (11%). The
proposed metabolic pathway involves conjugation with glutathione
followed by degradation to cysteinylglycine and cysteine conjugates
and formation of mercapturic acid conjugates (McManus, 1987b; JMPR
1988).
Goats
A goat with a cannulated bile duct received 14C-dimethipin
(radiochemical purity, 98%) at a dietary concentration of 500 ppm for
three days. Samples of urine, liver, bile, and kidney were collected
for characterization and identification of metabolites. No details of
the experimental conditions were provided, and although two animals
appear to have been treated, data were presented on only one. Only 18%
of the labelled residues in liver and 15% of those in kidney were
identified: (butan-3-one-2-yl)-2-hydroxyethyl sulfone (due to ring
cleavage) and 2,3,5,6,-tetrahydro-5-hydroxy-5,6-dimethyl-1,4-dithiine
1,1,4,4,-tetraoxide being the predominant residues. Metabolites 3 and
4 were invariably identified together with unchanged parent compound
in urine, bile, liver, and kidney. Metabolite 4 accounted for 2-8% of
the radiolabel recovered in each case. Some alcohol metabolites of
dimethipin were identified only in urine and bile. Most of the
radiolabelled components in urine, bile, liver, and kidney were highly
polar and were present as glucuronide, cysteine, and acetylcysteine
conjugates. Studies in vitro confirmed that these are the likely
major metabolic steps. It was not reported whether total radioactivity
was measured or whether residues were identified in muscle, fat, or
milk (McManus, 1984; FAO/WHO, 1985).
Goats received a normal ration supplemented with 14C-dimethipin
at 1 or 10 mg/kg of feed until a plateau was reached in the milk after
17 days. After 18 days of dosing, about 97% of the total administered
radiolabel was excreted in the urine and faeces, but none were
detected in muscle and the concentrations were low in milk, liver, and
kidney. The concentrations added to the feed were higher than the
so-called 'worst case' situation in which every component of the diet
contains residues at the tolerable maximum residue level (TMRL)
proposed by the JMPR in 1985. Thus, if animals are exposed to a normal
feeding regime, the concentrations in meat, milk, and edible offal
will not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP standards and with a
signed and dated quality assurance statement, two lactating goats
received 14C-dimethipin (radiochemical purity, > 98%) at a
concentration of 0.15 or 50 mg/kg bw per day for 5 days before being
killed about 22 h after the last dose. The doses were equivalent to
dietary concentrations of 3.1 ppm (10 times actual exposure) and
1000 ppm (3200 times actual exposure), respectively. Urine and faeces
were collected once daily, and milk was collected twice daily.
Overall, 97% of the total radiolabel administered was recovered from
the animal given the low dose and 96% from that given the high dose.
More than 95% of the total administered dose was identified in the
excreta, and 0.1-0.2% was eliminated in the milk. The average daily
concentrations in milk were 0.005 ppm at the low dose and 1 ppm at the
high dose. Fat, muscle, and whole blood contained the lowest
concentrations of radiolabel at both the low dose (0.001 ppm in fat,
0.002 ppm in muscle, and 0.006 ppm in whole blood and the high dose
(0.32 ppm in fat, 0.64 ppm in muscle, and 2.7 ppm in whole blood),
whereas the liver and kidney contained the highest concentrations,
with 0.27 and 0.14 ppm at the low dose and 79 and 28 ppm at the high
dose, respectively. The sensitivity of the method was < 0.0004 ppm
for the low dose and < 0.011 ppm for the high dose (Byrd, 1992).
Enzymatic digestion followed by acid hydrolysis of liver tissue
from this study suggested that the radiolabelled residues of
dimethipin were covalently bound to tissue proteins. After acid
hydrolysis, acetyldithiane was identified as the single radiolabelled
peak in liver. Kidney samples from the animals at the low dose
contained ethane disulfonic acid as the only metabolite, whereas renal
tissue from the animal at the high dose also contained ethane
disulfonic acid; acid hydrolysis of the bound material from this
kidney showed that acetyl dithiane was also present. Milk from both
animals contained dimethipin cysteine conjugate as the major
metabolite. Acid hydrolysis of muscle from the goat at the high dose,
which contained 0.64 ppm of radiolabelled material, yielded reduced
dimethipin as the major metabolite. The results of this study suggests
that dimethipin is metabolized primarily via Michael addition to
glutathione or its derivatives or to protein (Gay & Lau, 1996).
Chickens
Laying hens were fed a normal diet fortified with
14C-dimethipin at concentrations of 1, 6, or 30 mg/kg for 30 days,
when half of the birds were slaughtered; the remainder were killed
after a withdrawal period of 11 days. By day 30, more than 95% of the
total administered radiolabel had been excreted in the faeces. No
residues were detectable in muscle or fat at the end of dosing with
1 mg/kg or in fat after administration of 6 mg/kg. The concentrations
found in tissues corresponded roughly to the doses. The concentrations
found in the tissues of birds after 11 days of withdrawal were
considerably lower, and none of the tissues except blood from hens at
1 mg/kg contained measurable residues. The birds fed 6 or 30 mg/kg
14C-dimethipin had measurable residues in all tissues except fat. In
eggs, the residual radiocarbon increased slowly over 10 days and
remained fairly constant for the remainder of the dosing period.
Except in birds given 30 mg/kg, the concentrations in eggs decreased
to below the limit of determination during the 11 days of withdrawal.
Extrapolation from these data indicates that residues in birds fed a
hypothetical 'worst case' diet in which all or nearly all of the
components contain residues at the level of the MRL proposed by the
1985 JMPR would not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP, with a signed and
dated quality assurance statement, [14C-6]dimethipin (radiochemical
purity, > 95%) was administered orally to white Leghorn laying hens
daily for five consecutive days at a dose of 15.8 mg/kg bw per day
(five hens) or 152 mg/kg bw per day (two hens), equivalent to
concentrations of 203 ppm (7000 times that caclulated to result from
use of dimethipin at the permitted level on the cereals in the diet)
and 2770 ppm (92 000 times the calculated concentration),
respectively. Five untreated birds served as controls. Excreta and
eggs were collected once daily during treatment. More than 90% of the
total administered radiolabel was found in the excreta. The hens were
killed within 24 h of the last dose, and tissues were collected,
examined, and weighed. Edible tissues and eggs contained 5.5% and 5.1%
of the total radiolabel at the low and high doses, respectively. The
liver and kidney had the highest concentrations, with 9.7 and 65 ppm
in liver and 4.5 and 39 ppm in kidney at the low and high doses,
respectively. Breast and thigh muscle each contained 10 ppm at the
high dose and 0.63 and 0.72 ppm at the low dose, respectively. Egg
yolk and egg white contained 6.9 and 6.6 ppm, respectively, at the
high dose and 1.1 and 0.68 ppm at the low dose. Fat had the lowest
concentrations, with 0.20 ppm at the low dose and 2.4 ppm at the high
dose. Multiple metabolites were identified in eggs and tissues, all
formed as conjugates of glutathione followed by degradation, resulting
in the following compounds: glutathionyl-dimethipin,
gamma-glutamyl-cysteinyl-dimethipin, cysteinyl-dimethipin,
mercaptodimethipin, thioacetyl-dimethipin, thiomethyl-dimethipin, and
methyl sulfoxide-dimethipin. The major metabolite was
gamma-glutamylcysteinyl-dimethipinin most tissues and
cysteinyl-dimethipin in liver (Lau & Gay, 1993).
While the metabolism of dimethipin in plants was found to be
negligible, because it is applied at or close to the harvesting stage
when the biochemical activities of the plant are at a minimum, its
metabolism in animals is quite extensive. As a result, the main
residues in crops are the parent product, while in animals dimethipin
undergoes glutathione conjugation with subsequent degradation. In a
second pathway, dimethipin undergoes hydration followed by ring
cleavage.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of dimethipin after administration by the
oral, dermal, and inhalation routes and its ocular and dermal
irritation and dermal sensitizing capacity are summarized in Table 1.
Table 1. Acute toxicity of dimethipin
Species Purity Sex Route LD50 or LC50 Reference
(%) (mg/kg bw or mg/L)
Mouse > 97.5 M Oral 440 Shapiro (1977a)
F 600
Rat > 97.5 M&F Oral 1 200 Varner & Matthews
(1977)
Rat 98 M Oral 460 Blaszcak (1992a)
F 550
Rat NR M Intraperitoneal 240 Shapiro (1977b)
F 240
Rat NR M&F Inhalation (1 h) > 20 Babish (1977)
Rat 98.9 M Inhalation (4 h) 1.5 Hoffman (1992)
F 0.88
Rabbit NR M&F Dermal (24 h) > 12 000 Reagen & Becci (1982)
Rabbit 98 M&F Dermal (24 h) > 5 000 Blaszcak (1992b)
NR, not reported
In rats (strain unspecified), the LD50 for dimethipin (purity,
> 97.5%) administered orally was 1200 mg/kg bw for animals of each
sex (Varner & Matthews, 1977; JMPR, 1985). In another study, the
LD50 for dimethipin (purity, 98.0%) was 550 mg/kg bw in male and 460
mg/kg bw in female Sprague Dawley CD rats (Blaszcak, 1992a). Surviving
animals lost weight during week 1, but gained weight during the
remainder of the observation period. The signs of toxicity included
nasal discharge, hypoactivity, and rales. At necropsy, discoloured
lungs, red distended stomachs, and red fluid in the intestines were
noted.
The LC50 in Sprague-Dawley CD rats exposed by inhalation by
nose only for 4 h was 1.5 mg/L for males and 0.88 mg/L for females
(Hoffman, 1992). The clinical signs of toxicity included respiratory
distress. Body weights were substantially decreased (41% in males and
18% in females) during the first week, but weight was gained after
that time. At necropsy, discoloured lungs were observed.
The LD50 in rabbits was > 5000 mg/kg bw, as no deaths were
seen (Blaszcak, 1992b). It severely irritated the eyes (Griffiths &
Koschier, 1980), but the material tested was a recrystallized form of
dimethipin, which may not be representative of the technical-grade
material. Technical-grade dimethipin was not irritating to rabbit skin
(Blaszcak, 1992c). It was a weak dermal sensitizer in Hartley
guinea-pigs (Madison, 1983).
WHO (1999) has classified dimethipin as 'slightly hazardous'.
(b) Short-term studies of toxicity
Rats
Groups of six male and six female Charles River CD Sprague-Dawley
rats, eight weeks of age, received technical-grade dimethipin (purity,
98%) moistened with distilled water dermally for 6 h/day at doses of
0, 10, 100, or 1000 mg/kg bw per day for 21 days. The study was
conducted under GLP requirements, and a signed and dated quality
assurance statement was available. There were no treatment-related
alterations in mortality rate, body weight, food consumption, clinical
signs, clinical pathology, or gross and histopathological appearance.
Traces of hyperkeratosis were present in the skin of treated males at
all doses and in females at the intermediate and high doses. The
weights of the liver relative to body weights were statistically
significantly increased in males (20%) and females (15%) at 1000 mg/kg
bw per day, but the absolute liver weights of these animals were
statistically nonsignificantly increased, by 18% in males and 12% in
females. In the absence of clinical pathological or histopathological
effects, the NOAEL for systemic toxicity was 1000 mg/kg bw per day,
the highest dose tested (Goldenthal, 1991).
Groups of 15 male and 15 female Charles River rats, 28 days old,
were fed diets containing technical-grade dimethipin (purity,
> 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for 95 days.
There was no treatment-related change in mortality rate or abnormal
behaviour. Food consumption was slightly depressed in females at the
highest dose throughout the study, but body weights were unaffected.
Haematology, blood chemistry, and urinalysis performed in 10 males and
10 females from the control and high-dose groups after 45 and 85 days
of treatment indicated no significant compound-related effects. At
termination, the ratios of organ:body weight of liver and kidney were
increased in females at the highest dose. No significant differences
in gross pathological changes were observed between control and
treated groups. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females from the
control and high-dose groups showed no lesions attributable to
treatment. The NOAEL was 300 ppm, equivalent to 15 mg/kg bw per day
(Marias et al., 1976; JMPR, 1985).
Groups of 10 male and 10 female Charles River CD Sprague-Dawley
rats aged four week were fed diets containing technical-grade
dimethipin (purity, 98.5%) at concentrations of 0, 40, 1750, or 3500
ppm (equal to 2.5, 110, and 220 mg/kg bw per day in males and 3.1,
130, and 260 mg/kg bw per day in females) for 13 weeks. The study was
conducted under GLP requirements, and a signed quality assurance
statement was available. The animals were observed for deaths and
clinical signs of toxicity twice daily, and body weight and food
consumption were measured weekly. Ophthalmoscopic examinations were
made before treatment and at the end of the study. Clinical data were
collected on all surviving animals at 13 weeks. The weights of the
brain, kidneys, liver, and testis were recorded for all animals, and
all tissues were examined microscopically.
One male at the low dose was killed in extremis, but this
animal showed no significant gross or microscopic changes, and the
cause of death was not identified. No clinical signs of toxicity or
ophthalmoscopic findings were reported. Males at the high dose had
significantly decreased food consumption (11%), body weight (6%), and
body-weight gain (8%) in comparison with controls; and females at the
intermediate and high doses had significantly decreased food
consumption (12% at the intermediate and 16% at the high dose), body
weight (10% and 11%), and body-weight gain (20% and 28%). Males at the
high dose had slightly decreased erythrocyte volume fractions (8%) and
haemoglobin values (7%). Significantly increased cholesterol
concentrations were found in females at the intermediate (37%) and
high doses (41%), and those at the high dose had increased serum
activity of aspartate aminotransferase (30%). There were no gross
findings at necropsy that were considered to be related to treatment.
The weight of the liver relative to body weight was significantly
increased in males at the high dose (18%) and was correlated with
microscopic hepatocellular hypertrophy in 5 of 10 animals. Females at
the intermediate and high doses showed increased relative weights of
the brain, kidney, and liver, in the absence of microscopic changes,
and these changes were considered to be related to the decreased body
weights of those animals. The NOAEL was 40 ppm, equal to 2.5 mg/kg bw
per day (Goldenthal, 1993).
Dogs
Groups of four male and four female pure-bred beagles, about six
months old, were given diets containing technical-grade dimethipin
(purity, > 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for
90 days. No deaths occurred, and no treatment-related effects were
seen on behaviour, body weight, or food consumption or in blood
chemistry or haematology conducted after 42 and 85 days of treatment.
Urinary analysis at the same two intervals indicated an increase (not
dose-related) in the incidence of moderate-to-large amounts of
'crystals' in the urinary sediments of all treated females after 85
days. No other urinary parameters were affected. At terminal
sacrifice, the organ weights and gross appearance were not affected by
treatment. Microscopic examination of a large number of tissues,
including the testis, from each animal revealed oesophageal lesions
characterized by focal mucosal vesicles containing a few acute
inflammatory cells in one of eight animals at 300 ppm and three of
eight animals at 1000 ppm, but in none of the concurrent controls or
those at the lowest dose. The NOAEL was 100 ppm, equivalent to 2.5
mg/kg bw per day (Burtner et al., 1976).
Groups of six male and six female pure-bred beagles, about 7.5
months old and caged individually, were given diets containing
technical-grade dimethipin (purity, 99.7%) at concentrations of 0,
300, 1000, or 3000 ppm for one year. One male and three females at the
highest dose died or were sacrificed in extremis between weeks 13
and 52. 'Thinness', a major clinical sign, was seen frequently in most
animals at 3000 ppm and infrequently in one animal at 1000 ppm. Other
signs, including dehydration and paleness of the gums, were also noted
infrequently at 3000 ppm. Weight loss or growth depression and
decreased food consumption were seen in animals of each sex at 3000
ppm throughout most of the study. Bitches at 300 or 1000 ppm showed a
marginal (10%) but not consistently dose-dependent reduction in growth
between weeks 12 and 48. Animals of the highest dose had abnormalities
in the T-wave on electrocardiograms at the end of the study, and
ophthalmoscopic examination showed increased incidences of
conjunctival discharge, inflammation and corneal irregularities and
roughening at week 27 but not at week 52. 'Severe to slight thinness
and irregular or erratic heartbeat' were seen almost exclusively in
animals at 3000 ppm throughout the study.
Monthly haematological and blood chemical determinations revealed
deviations from control values in many parameters mainly in animals of
each sex at 3000 ppm, including decreased erythrocyte volume fraction,
increased platelet count, and depressed values of total protein,
albumin, globulin, calcium, blood urea nitrogen, and creatinine at
most sampling intervals. Animals at 1000 ppm had decreased values of
blood urea nitrogen (both sexes) and creatinine (dogs) at many
sampling intervals. When compared with concurrent controls, treated
dogs showed a slight but consistent, generally dose-related decrease
in erythrocyte counts and haemoglobin levels. These findings were
considered unlikely to be related to treatment, because the values
were within the normal ranges for control beagles in the published
literature (Bushby, 1970) and those recorded for control beagle dogs
maintained in the testing laboratory. Urinalysis, including microscopy
of urinary sediments, conducted twice monthly showed no significant
changes related to treatment.
At termination, the gross pathological appearance in the treated
groups was not significantly different from that in the controls. The
ratio of organ:body weight for the kidneys was increased at both 1000
and 3000 ppm (both sexes), for liver in dogs at 3000 ppm and in
bitches at > 1000 ppm, for brain at 3000 ppm (both sexes), and for
testis at 3000 ppm. Histopathological evaluation of a large number of
tissues from each animal showed testicular degeneration in 0/6, 2/6,
1/6, and 3/6 dogs at 0, 300, 1000, and 3000 ppm, respectively. Severe
and diffuse testicular degeneration was seen in one affected dog at
300 ppm and one at 3000 ppm, while generally mild focal degeneration
of the testis was seen in the other affected animals. Although the
incidence and severity of testicular degeneration did not show a
dose-response relationship, the complete absence of this lesion in
concurrent controls and in seven similar 1-year studies conducted in
the same laboratory and comprising over 20 control dogs (McGee, 1983)
indicated that the possibility that the testicular lesion is related
to treatment cannot be ruled out. Nevertheless, the testicular lesions
were considered not to be a direct effect of dimethipin on the testes,
but rather the result of the prolonged poor nutritional status of the
dogs or an incidental finding, as they were similar to incidental
findings in other studies in dogs in the same laboratory.
Additionally, no testicular lesions were seen in dogs in a 90-day
study. Other microscopic findings likely to be attributable to
treatment included hypocellularity of the bone marrow, lesions in the
gastrointestinal tract (gastritis, oedema, and ulceration) and heart
(haemorrhage), and thymic atrophy at the high dose, and an increased
incidence of nephritis, centrilobular degeneration in the liver,
lymphadenitis, and splenic hyperplasia at both 1000 and 3000 ppm. The
NOAEL was 300 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
the increased relative liver weight, increased alanine
aminotransferase and alkaline phosphatase activities, and
hepatocellular degeneration in bitches at 1000 ppm (Benson, 1981;
JMPR, 1985, 1999).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CD-1 mice, about 50 days old,
housed five per sex per cage, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at 0, 80, 400, or 2000 ppm
for 78 weeks. The animals fed dimethipin were kept for about 35 weeks
in the same room as animals receiving a highly photodegradable
compound identified only by a code name. It was stated, but
unsubstantiated by data, that dietary analysis showed that dimethipin
was stable in the diet for 7 days and that the mixture of dimethipin
and basal diet was satisfactorily homogeneous. All animals killed
in extremis or that died during the study and those killed at the
end were examined grossly, and a wide range of tissues, including the
brain, were examined microscopically.
The mortality rate was not affected by treatment: 58-78% of males
and 76-86% of females in all groups were still alive at the conclusion
of the study. A slight (< 10%), non-dose-related decrease in
body-weight gain was seen in males at doses > 400 ppm during the
first 13 weeks. Food consumption was not affected in a consistent
dose-related pattern. There were no significant differences between
control and treated groups in the incidence of clinical signs or
palpable nodules or tissue masses. Haematological examination of five
males and five females per group at three intervals during the study
revealed a significant increase in erythrocyte volume fraction in
males at 2000 ppm at week 13 and in erythrocyte volume fraction,
haemoglobin, and erythrocyte values at week 78. At termination, the
erythrocyte volume fraction was elevated in females at both 400 and
2000 ppm. All treated females showed a statistically significant,
albeit not strictly dose-dependent, increase in erythrocyte counts at
week 78. Blood chemical and urinary parameters were not evaluated. No
significant gross pathological alterations or changes in organ weights
were seen. No detailed histopathological data with morphological
descriptions of lesions in individual animals were available, but
tabulated 'individual histopathology findings' indicated that no
compound-induced non-neoplastic changes were found.
The only notable neoplastic finding was an increased incidence of
pulmonary (alveolar and bronchiolar) tumours in males at 2000 ppm. The
incidence of lung adenocarcinomas, but not of adenomas alone, was
significantly increased in males at 2000 ppm when compared with
concurrent or historical controls from five studies ( p < 0.05,
Fisher exact test), but not when compared with the maximum incidence
of lung adenocarcinomas in historical controls. The time to appearance
of adenocarcinomas and the multiplicity of tumours were not modified
by treatment. Additionally, the pulmonary tumours were not associated
with an increase in hyperplastic pulmonary changes. The combined
incidence of lung adenocarcinoma and adenoma was significantly
increased in comparison with the incidence in historical controls from
five studies but not when compared with the incidence in concurrent
controls or the maximum incidence in historical controls. There was no
dose-related increase in the incidence of benign or malignant lung
tumours in females. The incidence, location, and type of tumours other
than lung tumours were comparable to those in controls. About 30% of
the male and 40% of the female concurrent controls were found to have
tumours. Lymphoma (in males), lung tumours (in both sexes), and
hepatocellular carcinoma (in males) were the most frequently observed
spontaneous tumours. The fact that the animals were about 50 days old
at initiation of the study may have compromised the sensitivity of the
test. The NOAEL was 80 ppm, equal to 12 mg/kg bw per day, on the basis
of criteria other than tumours. The data on lung tumours are unclear
(Serota et al., 1981a; JMPR, 1985). As lung adenomas and
adenocarcinomas occur commonly in this strain of mice, this finding
was considered to be of no toxicological relevance (JMPR, 1988).
Rats
Groups of 50 male and 50 female Sprague-Dawley CD rats, about 40
days old and caged individually, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at concentrations of 0,
40, 200, or 1000 ppm for 104 weeks. The control group was used for
both this study and another study on a chemical identified only by a
code, and it was not reported whether treated animals in the two
studies were kept in the same room. All animals that were killed in
moribund condition or died during the study and all survivors killed
at the end of the study during weeks 105 and 106 were necropsied, and
a variety of tissues including the brain and any 'unusual' lesions
were examined histopathologically. Sections of the spinal cord and
'head' of 10 male and 10 female survivors per group were also
evaluated microscopically. Five males and five females per group were
studied for haematological and blood chemical parameters at five
intervals during the study and similar numbers at the same intervals
for urinary indices.
The survival of animals at the highest dose appeared to be better
than that in other groups. By 104 weeks, the survival rates were 44%
for males at 200 ppm and 50-72% for males and females in all groups,
including controls. No clinical signs related to treatment were seen,
and there were no dose- or compound-related effects on food
consumption or on the incidence of palpable nodules, tissue masses, or
wart-like lesions. A slight but consistent depression of growth
(< 5% in males and < 10% in females) was seen at 1000 ppm
between weeks 43 and 95 in males and between weeks 51 and 87 in
females. Females in all treated groups had increased total protein at
week 13 and decreased platelet counts at week 104, the only time at
which this parameter was measured. Other deviations from control
values were observed in certain haematological and blood chemical
parameters, but essentially only in animals at the highest dose. No
significant differences were seen between control and treated groups
in urinary parameters. The gross pathological changes seen in treated
animals were not significantly different from those in controls. A
non-dose-related increase in the ratio of liver:body weight was seen
in males in all treated groups, and a dose-related increase was seen
in females at 200 and 1000 ppm.The absolute weight of the adrenals and
that relative to body weight were decreased in females in all treated
groups. Histopathological examination showed focally dilated bile
ducts containing basophilic homogeneous material in one male and one
female control, two males and three females at 40 ppm, five males and
nine females at 200 ppm, and 33 males and 18 females at 1000 ppm. This
finding was presumed to be related to treatment. The microscopic
changes in other tissues, including the adrenal gland, were similar to
those in controls. The finding that 9-27% of the males in the control
and treated groups showed lactation and/or galactocoele may have been
associated with the increased incidence of mammary fibroadenoma in
males at the highest dose. The only other noteworthy neoplastic
findings were increased incidences of astrocytoma in males and
hepatocellular carcinoma in females at 200 and 1000 ppm. The
incidences of these tumours were not, however, significantly different
from those in concurrent controls (Fisher exact test). The incidence
of hepatocellular carcinoma was not significantly different from that
in historical controls, but that of astrocytoma in males was
significantly increased at both 200 and 1000 ppm ( p < 0.05) when
compared with the incidence in historical controls from seven studies.
Comparison with the maximum incidence in historical controls showed no
significant difference, even at 1000 ppm. An additional glioma was
reportedly found in the control group by a consultant to the company
who evaluated three additional brain sections from each control and
treated male. No preneoplastic lesions (gliosis) were seen in the
original or additional brain slides (Squire, 1984). The latency to
appearance of astrocytoma was not reduced by treatment. The
minimum-effect level on parameters other than tumours was 40 ppm, the
lowest dose tested, equivalent to 2.0 mg/kg bw per day (Serota et al.,
1981b; JMPR, 1985).
Further data on the changes in organ weights, provided
subsequently by the testing laboratory, gave a NOAEL of 200 ppm for
the decrease in relative and absolute weights of the adrenal glands in
female rats, and a NOAEL of 200 ppm for the increase in absolute and
relative liver weights in male rats. The NOAEL for the increase in
absolute and relative liver weights in females was 40 ppm (JMPR, 1998)
Groups of 60 young Sprague-Dawley rats (Crl:CD BR (VAF/Plus)) of
each sex were fed diets containing technical-grade dimethipin (purity,
98.5%) at concentrations of 0, 40, 1750, or 3500 ppm for males (equal
to 0, 1.8, 78, or 160 mg/kg bw per day) and 0, 40, 875, or 1750 ppm
for females (equal to 0, 2.2, 50, or 100 mg/kg bw per day) for 104
weeks. The study was conducted under GLP requirements, and a signed
quality assurance statement was available. Ten animals of each sex per
dose were killed after 12 months for interim evaluations of clinical
signs, deaths, body weight, food consumption, ophthalmological,
haematological, clinical chemical, and urinary parameters, organ
weights, and gross and histopathological appearance. The survival of
females at the high dose was decreased but not statistically
significantly. Of the 50 rats per sex allocated to the main study,
only 20, 24, 19, and 21 of the males and 20, 18, 18, and 13 of the
females in the control, low-, intermediate-, and high-dose groups,
respectively, survived to week 104. There were no treatment-related
clinical signs of toxicity, ophthalmic or haematological effects, or
changes in urinary parameters in any treated group in comparison with
controls. The mean body weight of males at the high dose was decreased
by 13-20% during the study; females at the intermediate dose weighed
< 16% less than controls and those at the high dose weighed
< 19% less. The mean body-weight gain of males at the high dose was
consistently decreased by 24%, and those of females at the
intermediate and high doses by 13 and 29% in comparison with controls.
No accompanying decrease in food consumption was seen.
Significantly increased serum aspartate and alanine
aminotransferase activities were observed in females at the
intermediate and high doses at 12, 18, and 24 months and in males at
the high dose at 18 months. Animals of each sex at the intermediate
and high doses also had elevated cholesterol levels at all sampling
intervals, and males at these doses had increased serum concentrations
of urea nitrogen and creatinine. The findings indicate renal and
hepatic toxicity. Macroscopic alterations were seen in the liver and
kidneys of animals that died during the study or were killed at the
end. Males at the high dose and females at the intermediate and high
doses had an increased incidence of liver cysts, and males at the
highest dose had an increased incidence of tan discolouration of the
liver and small testes. Males at the two higher doses had a slight
increase in the incidence of enlarged kidneys. Tan discolouration and
tan foci in the liver and a granular surface on the kidneys were seen
in females at the highest dose. Significant increases in organ weights
( p < 0.05) in males at the highest dose that were considered to be
related to treatment were in the absolute (115%) and relative (166%)
weights of the liver and the absolute (132%) and relative (152%)
weights of the kidney.
At interim sacrifice at 12 months, the incidence and/or grade of
bile-duct hyperplasia was increased in treated males (4/12, 5/13, and
5/12 at the low, intermediate, and high doses, respectively) when
compared with controls (2/13). In females, the incidence and/or grade
was increased at both 875 ppm (70%) and 1750 ppm (43%) when compared
with controls (8%). Epithelial hyperplasia of the duodenum was seen in
11/12 males and 10/14 females at the high dose and in none of the
controls by 12 months. In animals killed at the end of the study, the
incidence and/or grade of biliary cysts in the liver was increased in
females at the intermediate (12%) and high doses (37%) and in males at
the high dose (10%) in comparison with controls (2% in females and 0%
in males). Bile-duct hyperplasia in the liver was increased in
incidence and/or severity in males at the intermediate (25/47) and
high doses (29/48) in comparison with controls (21/47), and in females
at the intermediate (68%) and high doses (61%) when compared with
controls (33%). Dose-related increases in the severity of the lesion
were seen in animals of each sex. Male rats fed the high dose also
showed a significantly increased incidence (33%) of eosinophilic foci
in comparison with controls (13%).
The incidence and severity of chronic progressive nephropathy was
increased in 47/47 males and 37/50 females at the intermediate dose
and 46/48 males and 38/46 females at the high dose in comparison with
controls (33/47 in males and 18/48 in females). The incidence and
grade of epithelial hyperplasia of the duodenum was increased in males
at the intermediate (38%) and high doses (46%) in comparison with
controls (2%), and in females at the intermediate (14%) and high doses
(33%) in comparison with controls (0%). Signs of gastrointestinal
tract toxicity were seen in animals of each sex at the high dose;
males at this dose had an increased incidence and severity of
epithelial hyperplasia of the nonglandular stomach (15%, 2% in
controls), and females showed demineralization of the glandular
stomach (7/46, 0/48 in controls). Males at the high dose had an
increased incidence (21/47) and grade of seminiferous tubular
degeneration of the testis (eight had grades of moderate and six
severe; 12/47 in controls of which 0 were moderate and four severe)
and hypospermia of the epididymis (13/47, 5/47 in controls).The
incidence of seminiferous tubular degeneration was 13/27 (three
moderate and two severe) in males at the low dose and 16/34 (four
moderate and three severe) at the intermediate dose. Although the
testes of all rats at the low and intermediate doses were not
examined, a treatment-related increase in the incidence of testicular
lesions at the intermediate dose could be discerned. The occurrence of
testicular degeneration at the intermediate and high doses was
considered to be related to treatment, as was the increased incidence
of epididymal hypospermia, which was probably a result of the
seminiferous tubular degeneration. Females at the high dose showed an
increased incidence and severity of vascular mineralization of the
heart (5/46, 0/46 in controls) and aortic artery (6/45, 0/48 in
controls). The other histological lesions reported were considered not
to be related to treatment.
The doses tested were adequate to assess the tumorigenic
potential of dimethipin. The only statistically significant increase
in neoplastic lesions was in the incidence of benign
phaeochromocytomas (17%, 4% in concurrent controls) in the adrenal
medulla of male rats at the high dose. There was no accompanying
increase in hyperplasia in this tissue and no increase in the
incidence of malignant phaeochromocytomas, and the combined incidence
of benign and malignant neoplasms was not increased significantly.
Furthermore, the incidence of benign phaeochromocytomas in historical
controls ranged from about 0 to 18%, and the combined incidence of
malignant and benign tumours ranged from about 0 to 20% in similar
studies conducted in the same laboratory during the 5 years preceding
termination of this study. The increased incidence of benign
phaeochromocytomas was therefore considered not to be related to
treatment. Slight but biologically insignificant increases or
decreases in the incidences of other tumours were seen in treated
groups in comparison with controls. The tumour incidences in this
study are presented in Table 2. During the first 12 months of the
study, additional fibroadenomas and adenocarcinomas were found in
females in both control and treated groups and an additional
fibroadenoma was found in a male at the intermediate dose. The other
tumour types listed in Table 2 were not found during the first 12
months. The increased incidences of astrocytomas in males at 200 and
1000 ppm, hepatocellular carcinomas in females at 200 and 1000 ppm,
and mammary fibroadenomas in males at 1000 ppm in the study of Serota
et al. (1981b) were not found in this study, even though the doses and
the number of rats per sex at each dose exceeded those in the earlier
study. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day
(Goldenthal, 1996).
(d) Genotoxicity
Dimethipin had no mutagenic activity in a number of assays in
microorganisms, mammalian cells, and rodents in vitro and in
vivo. The only exception was the induction of forward mutation in
mouse lymphoma cells in the presence of metabolic activation
(Table 3).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical-grade dimethipin
(purity, 99.7%) at 0, 50, 200, or 800 ppm for 105 days before mating
(one male:two females; sibling and half-sibling mating avoided). he
day on which a positive vaginal smear or copulatory plug was detected
was considered to be day 0 of gestation. Weanlings of the second
litter (F1b) were selected to become parents of the next generation
and mated after receiving the test diets for 125 days. In each
generation, the second mating was allowed at least 14 days after the
first litter (F1a and F2a) had been weaned at 21 days of age.
Table 2. Tumour incidencea in rats dying between weeks 52 and 104 in a 2-year study of dimethipin
Tumour site Males (dose, ppm) Females (dose, ppm)
0 40 1750 3500 0 40 875 1750
Mammary gland
Adenoma 1/7 0/3 1/2 2/7 4/48 3/41 7/46 2/46
Fibroadenoma 28/48 23/41 27/46 16/46
Adenocarcinoma 9/48 6/41 10/46 4/46
Brain
Astrocytoma 1/44 0/24 0/28 1/48 0/48 0/31 0/33 0/46
Granular-cell tumour 1/44 0/24 0/28 1/48
Liver
Adenoma 1/47 0/48 3/47 2/48 1/48 0/49 1/50 3/46
Carcinoma 1/47 1/48 2/47 1/48 0/48 0/49 0/50 0/46
Interstitial-cell tumour of the testis
Benign 2/47 1/27 2/34 5/47
Malignant 0/47 0/27 1/34 0/47
Combined 2/47 1/27 3/34 5/47
Phaeochromocytoma of the adrenal medulla
Benign 2/47 4/28 0/28 8/48* 1/48 1/32 0/35 0/46
Malignant 2/47 1/28 1/28 0/48 0/48 0/32 0/35 0/46
Combined 4/47 5/28 1/28 8/48
a Number of rats with tumours/Number of rats examined microscopically
* Significantly different from incidemce in concurrent controls at p < 0.05
Table 3. Results of assays for the genotoxicity of dimethipin (purity, > 98%)
End-point Test system Dose Result Reference
In vitro
Reverse mutation S. typhimurium TA1538, 1-1000 mg/plate Negativea Jagannath & Brusick
TA1537, TA1535, TA98, (1978, 1981)
Mitotic non-disjunction, S. cerevisiae D4 1-1000 mg/plate Negativea Jagannath & Brusick
recombination, and (1978)
mutation S. cerevisiae D6 1-2000 mg/plate Negativea Bootman & Lodge
(1982)
Mitotic gene conversion S. cerevisiae D4 125-2000 mg/ml Negativea Forster et al. (1984a)
Chromosomal aberration Chinese hamster ovary cells 5-50 mg/ml Negativea Sorg et al. (1983)
Sister chromatid exchange Chinese hamster ovary cells 1.56-24 mg/mlb Negativea Galloway & Brusick
3.1-200 mg/mlc (1981)
Forward mutation L5178Y Tk+/- mouse 1.56-75 mg/mlb ? Myhr & Brusick
lymphoma cells 125-200 mg/mlc (1981)
In vivo
In vivo/in vitro unscheduled Wistar rat 100, 300, or 1000 Negative McManus (1987c)
DNA synthesis mg/kg bw
Micronucleus formation Swiss CD-1 mouse 220 mg/kg bw Negative McManus (1986)
Micronucleus formation Mouse Two successive Negatived Forster et al. (1984b)
daily oral doses of
22, 73.3, or 220
mg/kg bw per day
(males) or at 30,
100, or 300 mg/kg
bw per day (females)
In the parental generations, deaths (the incidence of which was
not dose-related) occurred only among females. No compound-related
behavioural abnormalities were seen. F0 and F1b adult females at
800 ppm weighed less than the concurrent controls before mating,
throughout gestation, and throughout most of the the lactation
periods. Food consumption was depressed in animals at 800 ppm, in F0
females before mating in weeks 6-10, in F1b females in weeks 6-17,
and in F1b males in weeks, 1, 4, and 9. Fertility in males, as
determined by a demonstrated ability to impregnate at least one
female, mating index (% females mated), gestation index (% mated
females with viable litters), the number of days required by females
to mate, and the duration of gestation in treated groups were all
comparable to the control values. In the progeny, the mean number of
pups per litter born alive, survival of pups to days 4, 7, 14, and 21,
the sex ratio, and the behaviour of pups were not adversely affected.
The weights of pups in the F1a and F1b litters at 800 ppm were
reduced on days 7, 4, and 21, and the weights of those in the F1a
litters at 200 ppm and in the F2a litters at 800 ppm were decreased
on day 21. Gross external examination of all pups, including those
found dead, revealed only one abnormal pup, which was a stillborn in
an F1a litter at 200 ppm.
Gross pathological examination of all parental animals of the
second litters killed after weaning (F0) or 30 days after weaning
(F1b) (i.e. after 32 weeks and 39 weeks of dietary feeding,
respectively) and weanlings in each generation revealed no significant
difference between control and treated groups. Determinations of the
weights of organs from all F1b adults and five male and five female
weanlings from the F1b and the F2b litters showed increased
organ:body-weight ratios for the liver at 200 and 800 ppm and for the
kidney and brain at 800 ppm in adult F1b females; however, the
organ:body-weight ratio of the liver in F1b adult females was
depressed at 50 ppm. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults and
five male and five female weanlings from the F1b and the F2b
litters and of gross lesions and gonads from F0 adults indicated no
significant changes attributable to treatment.
The NOAEL was 200 ppm, equivalent to 10 mg/kg bw per day, as the
finding of a decrease in pup weight on day 21 in F1a litters at 200
ppm was unlikely to be treatment-related, as it occurred in only a
single generation and was not recurrent (Kehoe & Mackenzie, 1982;
JMPR, 1985).
(ii) Developmental toxicity
Rats
Groups of sexually mature mated female rats (BLU:(SD)BR) received
technical-grade dimethipin (purity, 97.5%) by intubation as a
suspension in corn oil at a dose of 0, 80, 400, or 800 mg/kg bw per
day on days 6-15 of gestation, the day on which a vaginal plug was
observed being considered day 0. An additional group of mated female
rats treated with 250 mg/kg bw per day of acetylsalicylic acid was
used as the positive control. The groups at 400 and 800 mg/kg bw per
day were terminated within 8 days of initiation of treatment owing to
'excessive deaths' and were not investigated further. Two new groups,
at 30 and 160 mg/kg bw per day, were added 2 weeks after the study
began, but no concurrent control groups were included for the two new
doses. The dams were killed on day 20 of gestation and their fetuses
were removed surgically for gross external, visceral, and skeletal
examination.
No compound-related deaths or clinical signs were observed at
doses up to 160 mg/kg bw per day, and the growth rates of dams during
gestation were comparable in all groups. The number of dams in each
group that became pregnant and were alive on day 20 was 20-22. The
mean number of implantation sites or live fetuses, fetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, with no concomitant increase in the incidence of
pregnant dams with resorptions, was seen at 160 mg/kg bw per day. The
incidence of skeletal or visceral malformations of fetuses did not
differ significantly between control and treated groups. The positive
control group had a number of fetal abnormalities, including
encephalomenigocoele and gastroschisis. The NOAEL for both maternal
and developmental toxicity was 160 mg/kg bw per day (Knickerboker et
al., 1977; JMPR, 1985).
Rabbits
Groups of 16 sexually mature female Dutch belted rabbits were
artificially inseminated and were intubated with technical-grade
dimethipin (purity, 98.3%) as a suspension in 0.5% carboxymethyl
cellulose at 0, 7.5, 20, or 40 mg/kg bw per day at a constant volume
of 1 ml/kg bw, on days 6-27 of gestation, day 0 of gestation being
considered the day of insemination. They were killed on day 28 of
gestation, and the uterine contents were examined. All fetuses,
including those that were aborted or dead, were examined grossly and
for skeletal and visceral abnormalities.
No deaths occurred. A slight increase in the number of females at
20 and 40 mg/kg bw per day that had a reduced amount of faeces beneath
the cage was seen at various intervals during gestation as compared
with concurrent controls. No data on food consumption were available.
Does at 40 mg/kg bw per day showed weight loss between days 6 and 12,
and maternal weight gain was depressed in a dose-response pattern in
all treated groups between days 6 and 28. The fertility rate was
88-94% in control and treated groups. One doe each at 0, 20, and 40
mg/kg bw per day aborted on day 28; seven non-viable fetuses were
found in does at 0 and 20 mg/kg bw per day; and three late resorptions
occurred in the doe at 40 mg/kg bw per day. At terminal sacrifice, the
gross pathological findings in treated does were comparable to those
in the controls. No significant differences were found between
controls and treated groups in the mean numbers of corpora lutea,
implantations, early or late resorptions, or viable or non-viable
fetuses, or in fetal weight.
A non-dose-related increase in postimplantation loss, due mainly
to an increased number of early resorptions, was seen in all treated
groups, although the values for this parameter were within the range
of historical controls. The sex ratio of fetuses at 40 mg/kg bw per
day was increased, the mean number of females being reduced. Increases
in the incidence of fetuses and of litters containing fetuses with 27
presacral vertebrae and with scoliosis (with or without associated rib
anomalies) were observed at 40 mg/kg bw per day, when compared with
concurrent or historical control incidences, in a total of 951 fetuses
in 149 litters from an unspecified number of studies with Dutch belted
rabbits over an unspecified period. There was no apparent dose- or
compound-related increase in the frequency of fetal soft-tissue
abnormalities. The NOAEL for maternal and developmental toxicity was
20 mg/kg bw per day (McMeekin et al., 1981; JMPR, 1985).
Comments
After oral administration to rats, goats and hens,
14C-dimethipin was extensively absorbed (69% within 24 h) and
rapidly excreted (89% within 48 h), mainly in the urine. Unchanged
dimethipin represented only a small fraction of the residue in
animals. In one metabolic pathway, dimethipin undergoes glutathione
conjugation and subsequent degradation to several metabolites,
including its mercapturic acid. In another pathway, dimethipin is
hydrated and then undergoes ring cleavage. Dimethipin also binds
covalently to amino acids, peptides and proteins, although the extent
to which this binding is catalysed by enzymes is unknown.
Dimethipin (purity, 98.5%) was moderately toxic to rats given
single oral doses, with LD50 values of 460 mg/kg bw in males and 550
mg/kg bw in females, or after exposure by inhalation, with LC50
values of 1.5 mg/L in males and 0.88 mg/L in females. It showed little
toxicity in rabbits exposed dermally, with an LD50 value greater
than 5000 mg/kg bw. A recrystallized form of dimethipin was severely
irritating to the eye in rabbits. Technical-grade dimethipin was not
irritating to rabbit skin but weakly sensitized the skin of
guinea-pigs.
WHO (1999) has classified dimethipin as 'slightly hazardous'.
In 90-day and long-term tests for toxicity in rats, the liver was
the main target at doses of 10 mg/kg bw per day and above. The
clinical findings consisted of increased absolute and relative weights
of the liver and increased serum cholesterol concentration and
transaminase activity. At doses greater than 85 mg/kg bw per day,
hepatocellular hypertrophy was seen in 90-day studies, whereas in
long-term studies the hepatocellular effects included focal dilatation
of bile ducts, biliary cysts and bile-duct hyperplasia.
The testis was identified as another target organ. In a one-year
study in dogs given dimethipin, testicular changes were seen at all
doses, the lowest dose being 300 ppm (equivalent to 7.5 mg/kg bw per
day), but these were considered not to be related to treatment but to
be a result of poor nutritional status or incidental findings, as they
were similar to testicular lesions seen in other studies in dogs in
the same laboratory. Additionally, no testicular lesions were seen in
dogs in a 90-day study. In contrast, Sprague-Dawley rats fed diets
containing technical-grade dimethipin for two years showed increased
incidences and severity of seminiferous tubular degeneration at the
two highest doses, 1750 and 3500 ppm (equal to 78 and 160 mg/kg bw per
day), associated at the high dose with hypospermia in the
epididymides. The NOAEL for testicular degeneration was 40 ppm
(2 mg/kg bw per day).
In a 90-day study in dogs given dimethipin in the diet, the
lowest dose of 100 ppm (equivalent to 2.5 mg/kg bw per day) was the
NOAEL, on the basis of oesophageal lesions at the LOAEL of 300 ppm
(equivalent to 7.5 mg/kg bw per day).
In the 1-year study in dogs described above, the effects seen at
1000 and 3000 ppm (equal to 25 and 75 mg/kg bw per day) included
'thinness' and increased relative kidney weights in animals of each
sex. At this dose, males had decreased blood urea nitrogen and
creatinine concentrations and females had increased relative liver
weights. One male and three females at the highest dose died, and
animals of each sex had decreased body weights and food consumption,
hypocellularity of the bone marrow, gastritis, oedema, ulceration of
the gastrointestinal tract, thymic atrophy, nephritis, centrilobular
degeneration of the liver, splenic hyperplasia and lymphadenitis. The
NOAEL was 300 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of
increased relative liver weights, increased alanine aminotransferase
and alkaline phosphatase activities, and hepatocellular degeneration
in females at 1000 ppm (equivalent to 25 mg/kg bw per day).
In a 78-week study of carcinogenicity in mice, a statistically
significant increase in the incidence of alveloar and bronchiolar
carcinomas was seen in males at the highest dose (2000 ppm, equal to
300 mg/kg bw per day). The combined incidence of lung adenocarcinoma
and adenoma was significantly greater than the mean for controls in
five previous studies but not when compared with that for concurrent
controls or with the mean maximum incidence in controls in previous
studies. As lung adenomas and adenocarcinomas occur commonly in this
strain of mice, this finding was not considered to be of toxicological
relevance. The NOAEL for systemic toxicity was 80 ppm (equal to 12
mg/kg bw per day) on the basis of increased erythrocyte volume
fraction at the LOAEL of 400 ppm (equal to 60 mg/kg bw per day).
In two 2-year studies in rats, the NOAEL for systemic toxicity
was 40 ppm (equal to 2 mg/kg bw per day) on the basis of decreased
body weights, increased absolute and relative weights of the liver, an
increased incidence of biliary hyperplasia, and testicular
degeneration at higher doses. No increase in tumour incidence was
observed in rats at any dose. The Meeting concluded that dimethipin is
not carcinogenic in mice or rats and is unlikely to pose a
carcinogenic risk to humans.
Dimethipin has been tested in an adequate range of tests for
genotoxicity in vitro and in vivo. Negative results were obtained
in most assays. It induced a weak mutagenic response in one test for
forward mutation in mouse lymphoma cells in the presence of metabolic
activation. The Meeting concluded that dimethipin is unlikely to be
genotoxic.
In a two-generation study of reproductive toxicity in rats, the
highest dose of 800 ppm (equivalent to 40 mg/kg bw per day) caused
decreased body weights and food consumption in parental animals of
each sex and decreased body weights in pups on days 7, 14, and 21 of
lactation. The NOAEL for both systemic toxicity in the parental
generation and developmental toxicity in the pups was 200 ppm
(equivalent to 10 mg/kg bw per day).
In a study of developmental toxicity in rats, excess mortality
occurred at doses of 400 and 800 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 160 mg/kg bw per day. In
rabbits, the NOAEL for both maternal and developmental toxicity was 20
mg/kg bw per day. Does at the maternal LOAEL of 40 mg/kg bw per day
showed body-weight loss on days 6-12 of gestation and decreased weight
gain on days 6-28 of gestation. The LOAEL for developmental toxicity
was 40 mg/kg bw per day on the basis of an increased incidence of
fetuses with skeletal malformations (scoliosis).
The present Meeting confirmed the ADI of 0-0.02 mg/kg bw
established by the 1988 Joint Meeting on the basis of the NOAEL of 2
mg/kg bw per day in the 2-year study in rats conducted in 1981 and a
safety factor of 100. This ADI is supported by the NOAEL of 40 ppm,
equivalent to 2 mg/kg bw per day, in the 2-year study in rats
conducted in 1996. The ADI provides a 1000-fold margin of safety with
respect to the NOAEL of 20 mg/kg bw per day for developmental toxicity
in rabbits, which showed skeletal malformations at the LOAEL of 40
mg/kg bw per day.
An acute reference dose of 0.02 mg/kg bw was established on the
basis of the NOAEL of 20 mg/kg bw per day for skeletal malformations
in the study of developmental toxicity in rabbits and a safety factor
of 1000. This high safety factor was used because of the nature of the
effect.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 80 ppm, equivalent to 12 mg/kg bw per day (toxicity in
a 78-week study of toxicity and carcinogenicity)
Rat: 40 ppm, equivalent to 2 mg/kg bw per day (toxicity in
two 2-year studies of toxicity and carcinogenicity)
160 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
10 mg/kg bw per day (parental and reproductive toxicity
in a two-generation study of reproductive toxicity)
Rabbit: 20 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 100 ppm, equivalent to 2.5 mg/kg bw per day (toxicity
in a 90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to dimethipin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 69% within 24 h, rats
Dermal absorption Low dermal penetration, rabbits
Distribution Widely distributed, rats
Potential for accumulation No evidence of accumulation
Rate and extent of excretion 89% within 48 h mainly via urine
Metabolism in animals Parent < 5%; metabolites consist of glutathione conjugates and degradates, rats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity Oral toxicity is moderate, but only slightly toxic by dermal and inhalation
routes of exposure
Rat, LD50, oral 440 mg/kg bw (males) and 600 mg/kg bw (females)
Rabbit, LD50, dermal > 5000 mg/kg bw
Rat, LC50, inhalation 0.88 mg/L, 4 h (female) and 1.5 mg/L, 4 h (males)
Rabbit, dermal irritation Not irritating
Rabbit, ocular irritation Severely irritating
Guinea-pig, dermal sensitization Weakly sensitizing
Short-term toxicity
Target/critical effect Liver: hepatotoxicity, hepatic hypertrophy, rats
Lowest relevant oral NOAEL 2 mg/kg bw per day, rats
Lowest relevant dermal NOAEL 1000 mg/kg bw per day (highest dose tested), rats
Lowest relevant inhalation NOAEL Not determined
Long-term toxicity and carcinogenicity
Target/critical effect Rat liver: increased weight, liver enzymes, bile-duct hyperplasia; rat
testis: degeneration
Lowest relevant NOAEL 2 mg/kg bw per day in two 2-year studies, rat
Carcinogenicity Not carcinogenic in mice or rats
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect None. Decreased pup body weight on days 7, 14, and 21 of lactation at
maternally toxic doses, rats
Lowest relevant reproductive NOAEL 10 mg/kg bw per day
Developmental target/critical effect Increased incidence of skeletal malformations, rabbit
Lowest relevant developmental NOAEL Rabbit, 20 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Medical data None
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Two 2-year studies in rats 100
Acute reference dose 0.02 mg/kg bw Skeletal malformations in rabbits 1000
References
Babish, J.G. (1977) Harvade(R) (N252) Tech. C-8-1162-00. Acute
inhalation study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Benson, B.W. (1981) 1-Year dietary toxicity study in dogs with N252.
Unpublished report from International Research and Development
Corp., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA
Billings, T.J. (1987) A (C14)-radiolabeled pharmacokinetics and
metabolism study in the rat using Harvade. Southwest Bio-Labs,
Inc. (Project No. 8656r). Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L (1992a) Acute oral toxicity study in rats with Harvade
technical (Project No. 6169-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992b) Acute dermal toxicity study in rabbits with
Harvade technical (Project No. 6170-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992c) Primary dermal irritation study in rabbits with
Harvade technical (Project No. 6171-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Bootman, J. & Lodge, D.C. (1982) ARS7728 (technical grade
Harvade(R)): Assessment of its ability to induce genetic damage
in Saccharomyces cerevisiae. Unpublished report from Life
Science Research, United Kingdom. Submitted to WHO by Uniroyal
Inc., USA.
Byrd, J.W. (1992), Nature of the residue of radiolabeled Harvade in
lactating goat; Part I: Dosing, specimen collection, and
quantitation of Harvade residues in lactating goats (Project No.
9202), unpublished study. Submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 61108063) from Industrial
Bio-Test Laboratories, Inc., USA (validated by the Canadian
Health Protection Branch). Submitted to WHO by Uniroyal Inc.,
USA.
Bushby, S.R.M. (1970) Hematological studies during toxicity tests. In:
Paget, G.E., ed., Methods in Toxicology, Oxford, Blackwell
Scientific Publications.
Caplan, J. & Merricks, D.L. (1978) 14C-Harvade(R) radiocarbon
study in rats. Unpublished report from Biospherics, Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Forster, R., Edwards, N.& Nunziata, A. (1984a) Mitotic gene conversion
in Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from Life
Science Research, Roma Toxicology Centre, Italy. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Forster, R., Mosesso, P. & Nunziata, A. (1984b) Micronucleus test.
Test substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Galloway, S.M. & Brusick, D. J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 90% D-11401 in the sister chromatid exchange
assay with Chinese hamster ovary (CHO) cells. Final report.
Unpublished report from Litton Bionetics, Inc., USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gay, M.H. & Lau, R.C.M. (1996) Nature of the residue of dimethipin in
lactating goat (Project No. 9202), unpublished study. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1991) 21-Day dermal toxicity study in rats with
Harvade technical (Report No. 399-116), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal Inc., USA.
Goldenthal, E.I. (1993) 13 Week dietary toxicity study in rats with
Harvade technical (Project No. 399-133), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal, Inc. USA.
Goldenthal, E.I. (1996) Two year dietary chronic toxicity and
oncogenicity study in rats (with) Harvade technical (dimethipin)
(Project No. 399-134), unpublished study from MPI Research.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Griffiths, J.T. & Koschier, F.J. (1980) Rabbit eye irritation study
with Harvade technical (recrystallized) (Project No. 6413a),
unpublished report from Food and Drug Research Laboratories, Inc.
Submitted to WHO by Uniroyal Inc., USA
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
Harvade technical in the rat (Project No. 91-8376), unpublished
study from Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Jagannath, D.R. & Brusick, D.J. (1978) Mutagenicity evaluation of
N-252, technical lot D10406 BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Jagannath, D.R. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 98% D-11401 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kehoe, D.F. & Mackenzie, K.M. (1982) Final report. Two-generation rat
reproduction study with N252. Unpublished report from Hazleton
Raltech, Inc., USA. Submitted to WHO by Uniroyal Inc., USA.
Knickerbocker, M., Re, T.A. & Babish, J.G. (1977) Teratologic
evaluation of N252 (Harvade (R)) technical in Sprague-Dawley
rats. Unpublished report from Food and Drug Research
Laboratories, Inc., USA, submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA
Lau, R.C.M. & Gay, M.H. (1993) Nature of the residue of 14C-Harvade
in laying hens (Uniroyal Chemical Co. Project No. 91121),
unpublished stud. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Madison, W.A. (1983) Technical grade Harvade(R). Dermal
sensitization in guinea pigs (modified closed patch technique).
Unpublished report from Hazleton Raltech, Inc., USA. Submitted to
WHO by Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT NO. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA (validated by the
Canadian Health Protection Branch). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McGee, D.H. (1983) Unpublished letter from International Research
Development Corp., USA, to Uniroyal Chemical Co. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA
McManus, J.P. (1984) Metabolism of Harvade(R) in goats. Unpublished
report from Uniroyal Chemical Co. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1986) Mouse micronucleus test with dimethipin
technical, Life Sciences Research, Rome (Report 180001-M-06886).
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA
McManus, J.P. (1987a) Analysis of urine samples from dimethipin
(Harvade) rat pharmacokinetic study. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1987b) Metabolism of (C14) Dimethipin (Harvade) in the
Rat - Urinary Metabolite Identification. Uniroyal Project No.
85125. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
McManus, J.P. (1987c) In vivo/in vitro UDS study in rats, Robens
Institute (Report No. 4/86/TX). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McMeekin, S.O.., Schardein, J.L. & Blair, M. (1981) N252 (Harvade(R)
technical). Teratology study in rabbits. Unpublished report from
International Research and Development Corp., Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Myhr, B.C. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) in the mouse lymphoma forward mutation assay.
Revised final report. Unpublished report from Litton Bionetics,
Inc., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Reagan, E.L. & Becci, P.J. (1982) Acute dermal toxicity study (LD50)
in albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories, Inc., USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Serota, D.G., Alsaker, R.D. & Banas, D. (1981a) 18-month toxicity and
oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc., USA.
Ssubmitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K. & Kunalzins, W. (1981b)
104-Week chronic toxicity study in rats. N252 (Harvade(R)
technical) final report. Unpublished report from Hazleton
Laboratories America, Inc., USA, submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977a) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977b) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K. & Cardona, R.A.
(1978) Characterization of excretory metabolites from
14C-Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemica. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Sorg, R.M., Naismith, R.W. & Matthews, R.J. (1983) CHO metaphase
analysis in vitro chromosome aberration analysis in Chinese
hamster ovary cells (CHO). PH320-UN-00183 Harvade(R),
unpublished report from Pharmakon Research International, Inc.,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Squire, R.A. (1984) Unpublished letter to Uniroyal Chemical. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Varner, L.L. & Matthews, R J. (1977) Acute oral LD50 in rats.
Uni-N-252 CND-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
 Three young male Charles River CD rats weighing approximately
225 g each received a single oral dose of 800 mg/kg bw of
14C-dimethipin. Urine and faeces were collected separately 24, 48,
and 72 h after dosing. The samples collected at 24 h were used for
identification of metabolites. Only trace amounts of dimethipin were
detected in the urine by HPLC, but seven metabolites were detected.
The three identified polar products, which accounted for 77% of the
urinary radioactivity, were a cysteinylglycine conjugate (54%), an
N-acetylcysteine conjugate (12%), and a reduced metabolite,
2,3-dimethyl-5,6-dihydro-1,4-dithiane-1,1,4,4-tetraoxide (11%). The
proposed metabolic pathway involves conjugation with glutathione
followed by degradation to cysteinylglycine and cysteine conjugates
and formation of mercapturic acid conjugates (McManus, 1987b; JMPR
1988).
Goats
A goat with a cannulated bile duct received 14C-dimethipin
(radiochemical purity, 98%) at a dietary concentration of 500 ppm for
three days. Samples of urine, liver, bile, and kidney were collected
for characterization and identification of metabolites. No details of
the experimental conditions were provided, and although two animals
appear to have been treated, data were presented on only one. Only 18%
of the labelled residues in liver and 15% of those in kidney were
identified: (butan-3-one-2-yl)-2-hydroxyethyl sulfone (due to ring
cleavage) and 2,3,5,6,-tetrahydro-5-hydroxy-5,6-dimethyl-1,4-dithiine
1,1,4,4,-tetraoxide being the predominant residues. Metabolites 3 and
4 were invariably identified together with unchanged parent compound
in urine, bile, liver, and kidney. Metabolite 4 accounted for 2-8% of
the radiolabel recovered in each case. Some alcohol metabolites of
dimethipin were identified only in urine and bile. Most of the
radiolabelled components in urine, bile, liver, and kidney were highly
polar and were present as glucuronide, cysteine, and acetylcysteine
conjugates. Studies in vitro confirmed that these are the likely
major metabolic steps. It was not reported whether total radioactivity
was measured or whether residues were identified in muscle, fat, or
milk (McManus, 1984; FAO/WHO, 1985).
Goats received a normal ration supplemented with 14C-dimethipin
at 1 or 10 mg/kg of feed until a plateau was reached in the milk after
17 days. After 18 days of dosing, about 97% of the total administered
radiolabel was excreted in the urine and faeces, but none were
detected in muscle and the concentrations were low in milk, liver, and
kidney. The concentrations added to the feed were higher than the
so-called 'worst case' situation in which every component of the diet
contains residues at the tolerable maximum residue level (TMRL)
proposed by the JMPR in 1985. Thus, if animals are exposed to a normal
feeding regime, the concentrations in meat, milk, and edible offal
will not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP standards and with a
signed and dated quality assurance statement, two lactating goats
received 14C-dimethipin (radiochemical purity, > 98%) at a
concentration of 0.15 or 50 mg/kg bw per day for 5 days before being
killed about 22 h after the last dose. The doses were equivalent to
dietary concentrations of 3.1 ppm (10 times actual exposure) and
1000 ppm (3200 times actual exposure), respectively. Urine and faeces
were collected once daily, and milk was collected twice daily.
Overall, 97% of the total radiolabel administered was recovered from
the animal given the low dose and 96% from that given the high dose.
More than 95% of the total administered dose was identified in the
excreta, and 0.1-0.2% was eliminated in the milk. The average daily
concentrations in milk were 0.005 ppm at the low dose and 1 ppm at the
high dose. Fat, muscle, and whole blood contained the lowest
concentrations of radiolabel at both the low dose (0.001 ppm in fat,
0.002 ppm in muscle, and 0.006 ppm in whole blood and the high dose
(0.32 ppm in fat, 0.64 ppm in muscle, and 2.7 ppm in whole blood),
whereas the liver and kidney contained the highest concentrations,
with 0.27 and 0.14 ppm at the low dose and 79 and 28 ppm at the high
dose, respectively. The sensitivity of the method was < 0.0004 ppm
for the low dose and < 0.011 ppm for the high dose (Byrd, 1992).
Enzymatic digestion followed by acid hydrolysis of liver tissue
from this study suggested that the radiolabelled residues of
dimethipin were covalently bound to tissue proteins. After acid
hydrolysis, acetyldithiane was identified as the single radiolabelled
peak in liver. Kidney samples from the animals at the low dose
contained ethane disulfonic acid as the only metabolite, whereas renal
tissue from the animal at the high dose also contained ethane
disulfonic acid; acid hydrolysis of the bound material from this
kidney showed that acetyl dithiane was also present. Milk from both
animals contained dimethipin cysteine conjugate as the major
metabolite. Acid hydrolysis of muscle from the goat at the high dose,
which contained 0.64 ppm of radiolabelled material, yielded reduced
dimethipin as the major metabolite. The results of this study suggests
that dimethipin is metabolized primarily via Michael addition to
glutathione or its derivatives or to protein (Gay & Lau, 1996).
Chickens
Laying hens were fed a normal diet fortified with
14C-dimethipin at concentrations of 1, 6, or 30 mg/kg for 30 days,
when half of the birds were slaughtered; the remainder were killed
after a withdrawal period of 11 days. By day 30, more than 95% of the
total administered radiolabel had been excreted in the faeces. No
residues were detectable in muscle or fat at the end of dosing with
1 mg/kg or in fat after administration of 6 mg/kg. The concentrations
found in tissues corresponded roughly to the doses. The concentrations
found in the tissues of birds after 11 days of withdrawal were
considerably lower, and none of the tissues except blood from hens at
1 mg/kg contained measurable residues. The birds fed 6 or 30 mg/kg
14C-dimethipin had measurable residues in all tissues except fat. In
eggs, the residual radiocarbon increased slowly over 10 days and
remained fairly constant for the remainder of the dosing period.
Except in birds given 30 mg/kg, the concentrations in eggs decreased
to below the limit of determination during the 11 days of withdrawal.
Extrapolation from these data indicates that residues in birds fed a
hypothetical 'worst case' diet in which all or nearly all of the
components contain residues at the level of the MRL proposed by the
1985 JMPR would not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP, with a signed and
dated quality assurance statement, [14C-6]dimethipin (radiochemical
purity, > 95%) was administered orally to white Leghorn laying hens
daily for five consecutive days at a dose of 15.8 mg/kg bw per day
(five hens) or 152 mg/kg bw per day (two hens), equivalent to
concentrations of 203 ppm (7000 times that caclulated to result from
use of dimethipin at the permitted level on the cereals in the diet)
and 2770 ppm (92 000 times the calculated concentration),
respectively. Five untreated birds served as controls. Excreta and
eggs were collected once daily during treatment. More than 90% of the
total administered radiolabel was found in the excreta. The hens were
killed within 24 h of the last dose, and tissues were collected,
examined, and weighed. Edible tissues and eggs contained 5.5% and 5.1%
of the total radiolabel at the low and high doses, respectively. The
liver and kidney had the highest concentrations, with 9.7 and 65 ppm
in liver and 4.5 and 39 ppm in kidney at the low and high doses,
respectively. Breast and thigh muscle each contained 10 ppm at the
high dose and 0.63 and 0.72 ppm at the low dose, respectively. Egg
yolk and egg white contained 6.9 and 6.6 ppm, respectively, at the
high dose and 1.1 and 0.68 ppm at the low dose. Fat had the lowest
concentrations, with 0.20 ppm at the low dose and 2.4 ppm at the high
dose. Multiple metabolites were identified in eggs and tissues, all
formed as conjugates of glutathione followed by degradation, resulting
in the following compounds: glutathionyl-dimethipin,
gamma-glutamyl-cysteinyl-dimethipin, cysteinyl-dimethipin,
mercaptodimethipin, thioacetyl-dimethipin, thiomethyl-dimethipin, and
methyl sulfoxide-dimethipin. The major metabolite was
gamma-glutamylcysteinyl-dimethipinin most tissues and
cysteinyl-dimethipin in liver (Lau & Gay, 1993).
While the metabolism of dimethipin in plants was found to be
negligible, because it is applied at or close to the harvesting stage
when the biochemical activities of the plant are at a minimum, its
metabolism in animals is quite extensive. As a result, the main
residues in crops are the parent product, while in animals dimethipin
undergoes glutathione conjugation with subsequent degradation. In a
second pathway, dimethipin undergoes hydration followed by ring
cleavage.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of dimethipin after administration by the
oral, dermal, and inhalation routes and its ocular and dermal
irritation and dermal sensitizing capacity are summarized in Table 1.
Table 1. Acute toxicity of dimethipin
Species Purity Sex Route LD50 or LC50 Reference
(%) (mg/kg bw or mg/L)
Mouse > 97.5 M Oral 440 Shapiro (1977a)
F 600
Rat > 97.5 M&F Oral 1 200 Varner & Matthews
(1977)
Rat 98 M Oral 460 Blaszcak (1992a)
F 550
Rat NR M Intraperitoneal 240 Shapiro (1977b)
F 240
Rat NR M&F Inhalation (1 h) > 20 Babish (1977)
Rat 98.9 M Inhalation (4 h) 1.5 Hoffman (1992)
F 0.88
Rabbit NR M&F Dermal (24 h) > 12 000 Reagen & Becci (1982)
Rabbit 98 M&F Dermal (24 h) > 5 000 Blaszcak (1992b)
NR, not reported
In rats (strain unspecified), the LD50 for dimethipin (purity,
> 97.5%) administered orally was 1200 mg/kg bw for animals of each
sex (Varner & Matthews, 1977; JMPR, 1985). In another study, the
LD50 for dimethipin (purity, 98.0%) was 550 mg/kg bw in male and 460
mg/kg bw in female Sprague Dawley CD rats (Blaszcak, 1992a). Surviving
animals lost weight during week 1, but gained weight during the
remainder of the observation period. The signs of toxicity included
nasal discharge, hypoactivity, and rales. At necropsy, discoloured
lungs, red distended stomachs, and red fluid in the intestines were
noted.
The LC50 in Sprague-Dawley CD rats exposed by inhalation by
nose only for 4 h was 1.5 mg/L for males and 0.88 mg/L for females
(Hoffman, 1992). The clinical signs of toxicity included respiratory
distress. Body weights were substantially decreased (41% in males and
18% in females) during the first week, but weight was gained after
that time. At necropsy, discoloured lungs were observed.
The LD50 in rabbits was > 5000 mg/kg bw, as no deaths were
seen (Blaszcak, 1992b). It severely irritated the eyes (Griffiths &
Koschier, 1980), but the material tested was a recrystallized form of
dimethipin, which may not be representative of the technical-grade
material. Technical-grade dimethipin was not irritating to rabbit skin
(Blaszcak, 1992c). It was a weak dermal sensitizer in Hartley
guinea-pigs (Madison, 1983).
WHO (1999) has classified dimethipin as 'slightly hazardous'.
(b) Short-term studies of toxicity
Rats
Groups of six male and six female Charles River CD Sprague-Dawley
rats, eight weeks of age, received technical-grade dimethipin (purity,
98%) moistened with distilled water dermally for 6 h/day at doses of
0, 10, 100, or 1000 mg/kg bw per day for 21 days. The study was
conducted under GLP requirements, and a signed and dated quality
assurance statement was available. There were no treatment-related
alterations in mortality rate, body weight, food consumption, clinical
signs, clinical pathology, or gross and histopathological appearance.
Traces of hyperkeratosis were present in the skin of treated males at
all doses and in females at the intermediate and high doses. The
weights of the liver relative to body weights were statistically
significantly increased in males (20%) and females (15%) at 1000 mg/kg
bw per day, but the absolute liver weights of these animals were
statistically nonsignificantly increased, by 18% in males and 12% in
females. In the absence of clinical pathological or histopathological
effects, the NOAEL for systemic toxicity was 1000 mg/kg bw per day,
the highest dose tested (Goldenthal, 1991).
Groups of 15 male and 15 female Charles River rats, 28 days old,
were fed diets containing technical-grade dimethipin (purity,
> 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for 95 days.
There was no treatment-related change in mortality rate or abnormal
behaviour. Food consumption was slightly depressed in females at the
highest dose throughout the study, but body weights were unaffected.
Haematology, blood chemistry, and urinalysis performed in 10 males and
10 females from the control and high-dose groups after 45 and 85 days
of treatment indicated no significant compound-related effects. At
termination, the ratios of organ:body weight of liver and kidney were
increased in females at the highest dose. No significant differences
in gross pathological changes were observed between control and
treated groups. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females from the
control and high-dose groups showed no lesions attributable to
treatment. The NOAEL was 300 ppm, equivalent to 15 mg/kg bw per day
(Marias et al., 1976; JMPR, 1985).
Groups of 10 male and 10 female Charles River CD Sprague-Dawley
rats aged four week were fed diets containing technical-grade
dimethipin (purity, 98.5%) at concentrations of 0, 40, 1750, or 3500
ppm (equal to 2.5, 110, and 220 mg/kg bw per day in males and 3.1,
130, and 260 mg/kg bw per day in females) for 13 weeks. The study was
conducted under GLP requirements, and a signed quality assurance
statement was available. The animals were observed for deaths and
clinical signs of toxicity twice daily, and body weight and food
consumption were measured weekly. Ophthalmoscopic examinations were
made before treatment and at the end of the study. Clinical data were
collected on all surviving animals at 13 weeks. The weights of the
brain, kidneys, liver, and testis were recorded for all animals, and
all tissues were examined microscopically.
One male at the low dose was killed in extremis, but this
animal showed no significant gross or microscopic changes, and the
cause of death was not identified. No clinical signs of toxicity or
ophthalmoscopic findings were reported. Males at the high dose had
significantly decreased food consumption (11%), body weight (6%), and
body-weight gain (8%) in comparison with controls; and females at the
intermediate and high doses had significantly decreased food
consumption (12% at the intermediate and 16% at the high dose), body
weight (10% and 11%), and body-weight gain (20% and 28%). Males at the
high dose had slightly decreased erythrocyte volume fractions (8%) and
haemoglobin values (7%). Significantly increased cholesterol
concentrations were found in females at the intermediate (37%) and
high doses (41%), and those at the high dose had increased serum
activity of aspartate aminotransferase (30%). There were no gross
findings at necropsy that were considered to be related to treatment.
The weight of the liver relative to body weight was significantly
increased in males at the high dose (18%) and was correlated with
microscopic hepatocellular hypertrophy in 5 of 10 animals. Females at
the intermediate and high doses showed increased relative weights of
the brain, kidney, and liver, in the absence of microscopic changes,
and these changes were considered to be related to the decreased body
weights of those animals. The NOAEL was 40 ppm, equal to 2.5 mg/kg bw
per day (Goldenthal, 1993).
Dogs
Groups of four male and four female pure-bred beagles, about six
months old, were given diets containing technical-grade dimethipin
(purity, > 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for
90 days. No deaths occurred, and no treatment-related effects were
seen on behaviour, body weight, or food consumption or in blood
chemistry or haematology conducted after 42 and 85 days of treatment.
Urinary analysis at the same two intervals indicated an increase (not
dose-related) in the incidence of moderate-to-large amounts of
'crystals' in the urinary sediments of all treated females after 85
days. No other urinary parameters were affected. At terminal
sacrifice, the organ weights and gross appearance were not affected by
treatment. Microscopic examination of a large number of tissues,
including the testis, from each animal revealed oesophageal lesions
characterized by focal mucosal vesicles containing a few acute
inflammatory cells in one of eight animals at 300 ppm and three of
eight animals at 1000 ppm, but in none of the concurrent controls or
those at the lowest dose. The NOAEL was 100 ppm, equivalent to 2.5
mg/kg bw per day (Burtner et al., 1976).
Groups of six male and six female pure-bred beagles, about 7.5
months old and caged individually, were given diets containing
technical-grade dimethipin (purity, 99.7%) at concentrations of 0,
300, 1000, or 3000 ppm for one year. One male and three females at the
highest dose died or were sacrificed in extremis between weeks 13
and 52. 'Thinness', a major clinical sign, was seen frequently in most
animals at 3000 ppm and infrequently in one animal at 1000 ppm. Other
signs, including dehydration and paleness of the gums, were also noted
infrequently at 3000 ppm. Weight loss or growth depression and
decreased food consumption were seen in animals of each sex at 3000
ppm throughout most of the study. Bitches at 300 or 1000 ppm showed a
marginal (10%) but not consistently dose-dependent reduction in growth
between weeks 12 and 48. Animals of the highest dose had abnormalities
in the T-wave on electrocardiograms at the end of the study, and
ophthalmoscopic examination showed increased incidences of
conjunctival discharge, inflammation and corneal irregularities and
roughening at week 27 but not at week 52. 'Severe to slight thinness
and irregular or erratic heartbeat' were seen almost exclusively in
animals at 3000 ppm throughout the study.
Monthly haematological and blood chemical determinations revealed
deviations from control values in many parameters mainly in animals of
each sex at 3000 ppm, including decreased erythrocyte volume fraction,
increased platelet count, and depressed values of total protein,
albumin, globulin, calcium, blood urea nitrogen, and creatinine at
most sampling intervals. Animals at 1000 ppm had decreased values of
blood urea nitrogen (both sexes) and creatinine (dogs) at many
sampling intervals. When compared with concurrent controls, treated
dogs showed a slight but consistent, generally dose-related decrease
in erythrocyte counts and haemoglobin levels. These findings were
considered unlikely to be related to treatment, because the values
were within the normal ranges for control beagles in the published
literature (Bushby, 1970) and those recorded for control beagle dogs
maintained in the testing laboratory. Urinalysis, including microscopy
of urinary sediments, conducted twice monthly showed no significant
changes related to treatment.
At termination, the gross pathological appearance in the treated
groups was not significantly different from that in the controls. The
ratio of organ:body weight for the kidneys was increased at both 1000
and 3000 ppm (both sexes), for liver in dogs at 3000 ppm and in
bitches at > 1000 ppm, for brain at 3000 ppm (both sexes), and for
testis at 3000 ppm. Histopathological evaluation of a large number of
tissues from each animal showed testicular degeneration in 0/6, 2/6,
1/6, and 3/6 dogs at 0, 300, 1000, and 3000 ppm, respectively. Severe
and diffuse testicular degeneration was seen in one affected dog at
300 ppm and one at 3000 ppm, while generally mild focal degeneration
of the testis was seen in the other affected animals. Although the
incidence and severity of testicular degeneration did not show a
dose-response relationship, the complete absence of this lesion in
concurrent controls and in seven similar 1-year studies conducted in
the same laboratory and comprising over 20 control dogs (McGee, 1983)
indicated that the possibility that the testicular lesion is related
to treatment cannot be ruled out. Nevertheless, the testicular lesions
were considered not to be a direct effect of dimethipin on the testes,
but rather the result of the prolonged poor nutritional status of the
dogs or an incidental finding, as they were similar to incidental
findings in other studies in dogs in the same laboratory.
Additionally, no testicular lesions were seen in dogs in a 90-day
study. Other microscopic findings likely to be attributable to
treatment included hypocellularity of the bone marrow, lesions in the
gastrointestinal tract (gastritis, oedema, and ulceration) and heart
(haemorrhage), and thymic atrophy at the high dose, and an increased
incidence of nephritis, centrilobular degeneration in the liver,
lymphadenitis, and splenic hyperplasia at both 1000 and 3000 ppm. The
NOAEL was 300 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
the increased relative liver weight, increased alanine
aminotransferase and alkaline phosphatase activities, and
hepatocellular degeneration in bitches at 1000 ppm (Benson, 1981;
JMPR, 1985, 1999).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CD-1 mice, about 50 days old,
housed five per sex per cage, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at 0, 80, 400, or 2000 ppm
for 78 weeks. The animals fed dimethipin were kept for about 35 weeks
in the same room as animals receiving a highly photodegradable
compound identified only by a code name. It was stated, but
unsubstantiated by data, that dietary analysis showed that dimethipin
was stable in the diet for 7 days and that the mixture of dimethipin
and basal diet was satisfactorily homogeneous. All animals killed
in extremis or that died during the study and those killed at the
end were examined grossly, and a wide range of tissues, including the
brain, were examined microscopically.
The mortality rate was not affected by treatment: 58-78% of males
and 76-86% of females in all groups were still alive at the conclusion
of the study. A slight (< 10%), non-dose-related decrease in
body-weight gain was seen in males at doses > 400 ppm during the
first 13 weeks. Food consumption was not affected in a consistent
dose-related pattern. There were no significant differences between
control and treated groups in the incidence of clinical signs or
palpable nodules or tissue masses. Haematological examination of five
males and five females per group at three intervals during the study
revealed a significant increase in erythrocyte volume fraction in
males at 2000 ppm at week 13 and in erythrocyte volume fraction,
haemoglobin, and erythrocyte values at week 78. At termination, the
erythrocyte volume fraction was elevated in females at both 400 and
2000 ppm. All treated females showed a statistically significant,
albeit not strictly dose-dependent, increase in erythrocyte counts at
week 78. Blood chemical and urinary parameters were not evaluated. No
significant gross pathological alterations or changes in organ weights
were seen. No detailed histopathological data with morphological
descriptions of lesions in individual animals were available, but
tabulated 'individual histopathology findings' indicated that no
compound-induced non-neoplastic changes were found.
The only notable neoplastic finding was an increased incidence of
pulmonary (alveolar and bronchiolar) tumours in males at 2000 ppm. The
incidence of lung adenocarcinomas, but not of adenomas alone, was
significantly increased in males at 2000 ppm when compared with
concurrent or historical controls from five studies ( p < 0.05,
Fisher exact test), but not when compared with the maximum incidence
of lung adenocarcinomas in historical controls. The time to appearance
of adenocarcinomas and the multiplicity of tumours were not modified
by treatment. Additionally, the pulmonary tumours were not associated
with an increase in hyperplastic pulmonary changes. The combined
incidence of lung adenocarcinoma and adenoma was significantly
increased in comparison with the incidence in historical controls from
five studies but not when compared with the incidence in concurrent
controls or the maximum incidence in historical controls. There was no
dose-related increase in the incidence of benign or malignant lung
tumours in females. The incidence, location, and type of tumours other
than lung tumours were comparable to those in controls. About 30% of
the male and 40% of the female concurrent controls were found to have
tumours. Lymphoma (in males), lung tumours (in both sexes), and
hepatocellular carcinoma (in males) were the most frequently observed
spontaneous tumours. The fact that the animals were about 50 days old
at initiation of the study may have compromised the sensitivity of the
test. The NOAEL was 80 ppm, equal to 12 mg/kg bw per day, on the basis
of criteria other than tumours. The data on lung tumours are unclear
(Serota et al., 1981a; JMPR, 1985). As lung adenomas and
adenocarcinomas occur commonly in this strain of mice, this finding
was considered to be of no toxicological relevance (JMPR, 1988).
Rats
Groups of 50 male and 50 female Sprague-Dawley CD rats, about 40
days old and caged individually, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at concentrations of 0,
40, 200, or 1000 ppm for 104 weeks. The control group was used for
both this study and another study on a chemical identified only by a
code, and it was not reported whether treated animals in the two
studies were kept in the same room. All animals that were killed in
moribund condition or died during the study and all survivors killed
at the end of the study during weeks 105 and 106 were necropsied, and
a variety of tissues including the brain and any 'unusual' lesions
were examined histopathologically. Sections of the spinal cord and
'head' of 10 male and 10 female survivors per group were also
evaluated microscopically. Five males and five females per group were
studied for haematological and blood chemical parameters at five
intervals during the study and similar numbers at the same intervals
for urinary indices.
The survival of animals at the highest dose appeared to be better
than that in other groups. By 104 weeks, the survival rates were 44%
for males at 200 ppm and 50-72% for males and females in all groups,
including controls. No clinical signs related to treatment were seen,
and there were no dose- or compound-related effects on food
consumption or on the incidence of palpable nodules, tissue masses, or
wart-like lesions. A slight but consistent depression of growth
(< 5% in males and < 10% in females) was seen at 1000 ppm
between weeks 43 and 95 in males and between weeks 51 and 87 in
females. Females in all treated groups had increased total protein at
week 13 and decreased platelet counts at week 104, the only time at
which this parameter was measured. Other deviations from control
values were observed in certain haematological and blood chemical
parameters, but essentially only in animals at the highest dose. No
significant differences were seen between control and treated groups
in urinary parameters. The gross pathological changes seen in treated
animals were not significantly different from those in controls. A
non-dose-related increase in the ratio of liver:body weight was seen
in males in all treated groups, and a dose-related increase was seen
in females at 200 and 1000 ppm.The absolute weight of the adrenals and
that relative to body weight were decreased in females in all treated
groups. Histopathological examination showed focally dilated bile
ducts containing basophilic homogeneous material in one male and one
female control, two males and three females at 40 ppm, five males and
nine females at 200 ppm, and 33 males and 18 females at 1000 ppm. This
finding was presumed to be related to treatment. The microscopic
changes in other tissues, including the adrenal gland, were similar to
those in controls. The finding that 9-27% of the males in the control
and treated groups showed lactation and/or galactocoele may have been
associated with the increased incidence of mammary fibroadenoma in
males at the highest dose. The only other noteworthy neoplastic
findings were increased incidences of astrocytoma in males and
hepatocellular carcinoma in females at 200 and 1000 ppm. The
incidences of these tumours were not, however, significantly different
from those in concurrent controls (Fisher exact test). The incidence
of hepatocellular carcinoma was not significantly different from that
in historical controls, but that of astrocytoma in males was
significantly increased at both 200 and 1000 ppm ( p < 0.05) when
compared with the incidence in historical controls from seven studies.
Comparison with the maximum incidence in historical controls showed no
significant difference, even at 1000 ppm. An additional glioma was
reportedly found in the control group by a consultant to the company
who evaluated three additional brain sections from each control and
treated male. No preneoplastic lesions (gliosis) were seen in the
original or additional brain slides (Squire, 1984). The latency to
appearance of astrocytoma was not reduced by treatment. The
minimum-effect level on parameters other than tumours was 40 ppm, the
lowest dose tested, equivalent to 2.0 mg/kg bw per day (Serota et al.,
1981b; JMPR, 1985).
Further data on the changes in organ weights, provided
subsequently by the testing laboratory, gave a NOAEL of 200 ppm for
the decrease in relative and absolute weights of the adrenal glands in
female rats, and a NOAEL of 200 ppm for the increase in absolute and
relative liver weights in male rats. The NOAEL for the increase in
absolute and relative liver weights in females was 40 ppm (JMPR, 1998)
Groups of 60 young Sprague-Dawley rats (Crl:CD BR (VAF/Plus)) of
each sex were fed diets containing technical-grade dimethipin (purity,
98.5%) at concentrations of 0, 40, 1750, or 3500 ppm for males (equal
to 0, 1.8, 78, or 160 mg/kg bw per day) and 0, 40, 875, or 1750 ppm
for females (equal to 0, 2.2, 50, or 100 mg/kg bw per day) for 104
weeks. The study was conducted under GLP requirements, and a signed
quality assurance statement was available. Ten animals of each sex per
dose were killed after 12 months for interim evaluations of clinical
signs, deaths, body weight, food consumption, ophthalmological,
haematological, clinical chemical, and urinary parameters, organ
weights, and gross and histopathological appearance. The survival of
females at the high dose was decreased but not statistically
significantly. Of the 50 rats per sex allocated to the main study,
only 20, 24, 19, and 21 of the males and 20, 18, 18, and 13 of the
females in the control, low-, intermediate-, and high-dose groups,
respectively, survived to week 104. There were no treatment-related
clinical signs of toxicity, ophthalmic or haematological effects, or
changes in urinary parameters in any treated group in comparison with
controls. The mean body weight of males at the high dose was decreased
by 13-20% during the study; females at the intermediate dose weighed
< 16% less than controls and those at the high dose weighed
< 19% less. The mean body-weight gain of males at the high dose was
consistently decreased by 24%, and those of females at the
intermediate and high doses by 13 and 29% in comparison with controls.
No accompanying decrease in food consumption was seen.
Significantly increased serum aspartate and alanine
aminotransferase activities were observed in females at the
intermediate and high doses at 12, 18, and 24 months and in males at
the high dose at 18 months. Animals of each sex at the intermediate
and high doses also had elevated cholesterol levels at all sampling
intervals, and males at these doses had increased serum concentrations
of urea nitrogen and creatinine. The findings indicate renal and
hepatic toxicity. Macroscopic alterations were seen in the liver and
kidneys of animals that died during the study or were killed at the
end. Males at the high dose and females at the intermediate and high
doses had an increased incidence of liver cysts, and males at the
highest dose had an increased incidence of tan discolouration of the
liver and small testes. Males at the two higher doses had a slight
increase in the incidence of enlarged kidneys. Tan discolouration and
tan foci in the liver and a granular surface on the kidneys were seen
in females at the highest dose. Significant increases in organ weights
( p < 0.05) in males at the highest dose that were considered to be
related to treatment were in the absolute (115%) and relative (166%)
weights of the liver and the absolute (132%) and relative (152%)
weights of the kidney.
At interim sacrifice at 12 months, the incidence and/or grade of
bile-duct hyperplasia was increased in treated males (4/12, 5/13, and
5/12 at the low, intermediate, and high doses, respectively) when
compared with controls (2/13). In females, the incidence and/or grade
was increased at both 875 ppm (70%) and 1750 ppm (43%) when compared
with controls (8%). Epithelial hyperplasia of the duodenum was seen in
11/12 males and 10/14 females at the high dose and in none of the
controls by 12 months. In animals killed at the end of the study, the
incidence and/or grade of biliary cysts in the liver was increased in
females at the intermediate (12%) and high doses (37%) and in males at
the high dose (10%) in comparison with controls (2% in females and 0%
in males). Bile-duct hyperplasia in the liver was increased in
incidence and/or severity in males at the intermediate (25/47) and
high doses (29/48) in comparison with controls (21/47), and in females
at the intermediate (68%) and high doses (61%) when compared with
controls (33%). Dose-related increases in the severity of the lesion
were seen in animals of each sex. Male rats fed the high dose also
showed a significantly increased incidence (33%) of eosinophilic foci
in comparison with controls (13%).
The incidence and severity of chronic progressive nephropathy was
increased in 47/47 males and 37/50 females at the intermediate dose
and 46/48 males and 38/46 females at the high dose in comparison with
controls (33/47 in males and 18/48 in females). The incidence and
grade of epithelial hyperplasia of the duodenum was increased in males
at the intermediate (38%) and high doses (46%) in comparison with
controls (2%), and in females at the intermediate (14%) and high doses
(33%) in comparison with controls (0%). Signs of gastrointestinal
tract toxicity were seen in animals of each sex at the high dose;
males at this dose had an increased incidence and severity of
epithelial hyperplasia of the nonglandular stomach (15%, 2% in
controls), and females showed demineralization of the glandular
stomach (7/46, 0/48 in controls). Males at the high dose had an
increased incidence (21/47) and grade of seminiferous tubular
degeneration of the testis (eight had grades of moderate and six
severe; 12/47 in controls of which 0 were moderate and four severe)
and hypospermia of the epididymis (13/47, 5/47 in controls).The
incidence of seminiferous tubular degeneration was 13/27 (three
moderate and two severe) in males at the low dose and 16/34 (four
moderate and three severe) at the intermediate dose. Although the
testes of all rats at the low and intermediate doses were not
examined, a treatment-related increase in the incidence of testicular
lesions at the intermediate dose could be discerned. The occurrence of
testicular degeneration at the intermediate and high doses was
considered to be related to treatment, as was the increased incidence
of epididymal hypospermia, which was probably a result of the
seminiferous tubular degeneration. Females at the high dose showed an
increased incidence and severity of vascular mineralization of the
heart (5/46, 0/46 in controls) and aortic artery (6/45, 0/48 in
controls). The other histological lesions reported were considered not
to be related to treatment.
The doses tested were adequate to assess the tumorigenic
potential of dimethipin. The only statistically significant increase
in neoplastic lesions was in the incidence of benign
phaeochromocytomas (17%, 4% in concurrent controls) in the adrenal
medulla of male rats at the high dose. There was no accompanying
increase in hyperplasia in this tissue and no increase in the
incidence of malignant phaeochromocytomas, and the combined incidence
of benign and malignant neoplasms was not increased significantly.
Furthermore, the incidence of benign phaeochromocytomas in historical
controls ranged from about 0 to 18%, and the combined incidence of
malignant and benign tumours ranged from about 0 to 20% in similar
studies conducted in the same laboratory during the 5 years preceding
termination of this study. The increased incidence of benign
phaeochromocytomas was therefore considered not to be related to
treatment. Slight but biologically insignificant increases or
decreases in the incidences of other tumours were seen in treated
groups in comparison with controls. The tumour incidences in this
study are presented in Table 2. During the first 12 months of the
study, additional fibroadenomas and adenocarcinomas were found in
females in both control and treated groups and an additional
fibroadenoma was found in a male at the intermediate dose. The other
tumour types listed in Table 2 were not found during the first 12
months. The increased incidences of astrocytomas in males at 200 and
1000 ppm, hepatocellular carcinomas in females at 200 and 1000 ppm,
and mammary fibroadenomas in males at 1000 ppm in the study of Serota
et al. (1981b) were not found in this study, even though the doses and
the number of rats per sex at each dose exceeded those in the earlier
study. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day
(Goldenthal, 1996).
(d) Genotoxicity
Dimethipin had no mutagenic activity in a number of assays in
microorganisms, mammalian cells, and rodents in vitro and in
vivo. The only exception was the induction of forward mutation in
mouse lymphoma cells in the presence of metabolic activation
(Table 3).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical-grade dimethipin
(purity, 99.7%) at 0, 50, 200, or 800 ppm for 105 days before mating
(one male:two females; sibling and half-sibling mating avoided). he
day on which a positive vaginal smear or copulatory plug was detected
was considered to be day 0 of gestation. Weanlings of the second
litter (F1b) were selected to become parents of the next generation
and mated after receiving the test diets for 125 days. In each
generation, the second mating was allowed at least 14 days after the
first litter (F1a and F2a) had been weaned at 21 days of age.
Table 2. Tumour incidencea in rats dying between weeks 52 and 104 in a 2-year study of dimethipin
Tumour site Males (dose, ppm) Females (dose, ppm)
0 40 1750 3500 0 40 875 1750
Mammary gland
Adenoma 1/7 0/3 1/2 2/7 4/48 3/41 7/46 2/46
Fibroadenoma 28/48 23/41 27/46 16/46
Adenocarcinoma 9/48 6/41 10/46 4/46
Brain
Astrocytoma 1/44 0/24 0/28 1/48 0/48 0/31 0/33 0/46
Granular-cell tumour 1/44 0/24 0/28 1/48
Liver
Adenoma 1/47 0/48 3/47 2/48 1/48 0/49 1/50 3/46
Carcinoma 1/47 1/48 2/47 1/48 0/48 0/49 0/50 0/46
Interstitial-cell tumour of the testis
Benign 2/47 1/27 2/34 5/47
Malignant 0/47 0/27 1/34 0/47
Combined 2/47 1/27 3/34 5/47
Phaeochromocytoma of the adrenal medulla
Benign 2/47 4/28 0/28 8/48* 1/48 1/32 0/35 0/46
Malignant 2/47 1/28 1/28 0/48 0/48 0/32 0/35 0/46
Combined 4/47 5/28 1/28 8/48
a Number of rats with tumours/Number of rats examined microscopically
* Significantly different from incidemce in concurrent controls at p < 0.05
Table 3. Results of assays for the genotoxicity of dimethipin (purity, > 98%)
End-point Test system Dose Result Reference
In vitro
Reverse mutation S. typhimurium TA1538, 1-1000 mg/plate Negativea Jagannath & Brusick
TA1537, TA1535, TA98, (1978, 1981)
Mitotic non-disjunction, S. cerevisiae D4 1-1000 mg/plate Negativea Jagannath & Brusick
recombination, and (1978)
mutation S. cerevisiae D6 1-2000 mg/plate Negativea Bootman & Lodge
(1982)
Mitotic gene conversion S. cerevisiae D4 125-2000 mg/ml Negativea Forster et al. (1984a)
Chromosomal aberration Chinese hamster ovary cells 5-50 mg/ml Negativea Sorg et al. (1983)
Sister chromatid exchange Chinese hamster ovary cells 1.56-24 mg/mlb Negativea Galloway & Brusick
3.1-200 mg/mlc (1981)
Forward mutation L5178Y Tk+/- mouse 1.56-75 mg/mlb ? Myhr & Brusick
lymphoma cells 125-200 mg/mlc (1981)
In vivo
In vivo/in vitro unscheduled Wistar rat 100, 300, or 1000 Negative McManus (1987c)
DNA synthesis mg/kg bw
Micronucleus formation Swiss CD-1 mouse 220 mg/kg bw Negative McManus (1986)
Micronucleus formation Mouse Two successive Negatived Forster et al. (1984b)
daily oral doses of
22, 73.3, or 220
mg/kg bw per day
(males) or at 30,
100, or 300 mg/kg
bw per day (females)
In the parental generations, deaths (the incidence of which was
not dose-related) occurred only among females. No compound-related
behavioural abnormalities were seen. F0 and F1b adult females at
800 ppm weighed less than the concurrent controls before mating,
throughout gestation, and throughout most of the the lactation
periods. Food consumption was depressed in animals at 800 ppm, in F0
females before mating in weeks 6-10, in F1b females in weeks 6-17,
and in F1b males in weeks, 1, 4, and 9. Fertility in males, as
determined by a demonstrated ability to impregnate at least one
female, mating index (% females mated), gestation index (% mated
females with viable litters), the number of days required by females
to mate, and the duration of gestation in treated groups were all
comparable to the control values. In the progeny, the mean number of
pups per litter born alive, survival of pups to days 4, 7, 14, and 21,
the sex ratio, and the behaviour of pups were not adversely affected.
The weights of pups in the F1a and F1b litters at 800 ppm were
reduced on days 7, 4, and 21, and the weights of those in the F1a
litters at 200 ppm and in the F2a litters at 800 ppm were decreased
on day 21. Gross external examination of all pups, including those
found dead, revealed only one abnormal pup, which was a stillborn in
an F1a litter at 200 ppm.
Gross pathological examination of all parental animals of the
second litters killed after weaning (F0) or 30 days after weaning
(F1b) (i.e. after 32 weeks and 39 weeks of dietary feeding,
respectively) and weanlings in each generation revealed no significant
difference between control and treated groups. Determinations of the
weights of organs from all F1b adults and five male and five female
weanlings from the F1b and the F2b litters showed increased
organ:body-weight ratios for the liver at 200 and 800 ppm and for the
kidney and brain at 800 ppm in adult F1b females; however, the
organ:body-weight ratio of the liver in F1b adult females was
depressed at 50 ppm. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults and
five male and five female weanlings from the F1b and the F2b
litters and of gross lesions and gonads from F0 adults indicated no
significant changes attributable to treatment.
The NOAEL was 200 ppm, equivalent to 10 mg/kg bw per day, as the
finding of a decrease in pup weight on day 21 in F1a litters at 200
ppm was unlikely to be treatment-related, as it occurred in only a
single generation and was not recurrent (Kehoe & Mackenzie, 1982;
JMPR, 1985).
(ii) Developmental toxicity
Rats
Groups of sexually mature mated female rats (BLU:(SD)BR) received
technical-grade dimethipin (purity, 97.5%) by intubation as a
suspension in corn oil at a dose of 0, 80, 400, or 800 mg/kg bw per
day on days 6-15 of gestation, the day on which a vaginal plug was
observed being considered day 0. An additional group of mated female
rats treated with 250 mg/kg bw per day of acetylsalicylic acid was
used as the positive control. The groups at 400 and 800 mg/kg bw per
day were terminated within 8 days of initiation of treatment owing to
'excessive deaths' and were not investigated further. Two new groups,
at 30 and 160 mg/kg bw per day, were added 2 weeks after the study
began, but no concurrent control groups were included for the two new
doses. The dams were killed on day 20 of gestation and their fetuses
were removed surgically for gross external, visceral, and skeletal
examination.
No compound-related deaths or clinical signs were observed at
doses up to 160 mg/kg bw per day, and the growth rates of dams during
gestation were comparable in all groups. The number of dams in each
group that became pregnant and were alive on day 20 was 20-22. The
mean number of implantation sites or live fetuses, fetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, with no concomitant increase in the incidence of
pregnant dams with resorptions, was seen at 160 mg/kg bw per day. The
incidence of skeletal or visceral malformations of fetuses did not
differ significantly between control and treated groups. The positive
control group had a number of fetal abnormalities, including
encephalomenigocoele and gastroschisis. The NOAEL for both maternal
and developmental toxicity was 160 mg/kg bw per day (Knickerboker et
al., 1977; JMPR, 1985).
Rabbits
Groups of 16 sexually mature female Dutch belted rabbits were
artificially inseminated and were intubated with technical-grade
dimethipin (purity, 98.3%) as a suspension in 0.5% carboxymethyl
cellulose at 0, 7.5, 20, or 40 mg/kg bw per day at a constant volume
of 1 ml/kg bw, on days 6-27 of gestation, day 0 of gestation being
considered the day of insemination. They were killed on day 28 of
gestation, and the uterine contents were examined. All fetuses,
including those that were aborted or dead, were examined grossly and
for skeletal and visceral abnormalities.
No deaths occurred. A slight increase in the number of females at
20 and 40 mg/kg bw per day that had a reduced amount of faeces beneath
the cage was seen at various intervals during gestation as compared
with concurrent controls. No data on food consumption were available.
Does at 40 mg/kg bw per day showed weight loss between days 6 and 12,
and maternal weight gain was depressed in a dose-response pattern in
all treated groups between days 6 and 28. The fertility rate was
88-94% in control and treated groups. One doe each at 0, 20, and 40
mg/kg bw per day aborted on day 28; seven non-viable fetuses were
found in does at 0 and 20 mg/kg bw per day; and three late resorptions
occurred in the doe at 40 mg/kg bw per day. At terminal sacrifice, the
gross pathological findings in treated does were comparable to those
in the controls. No significant differences were found between
controls and treated groups in the mean numbers of corpora lutea,
implantations, early or late resorptions, or viable or non-viable
fetuses, or in fetal weight.
A non-dose-related increase in postimplantation loss, due mainly
to an increased number of early resorptions, was seen in all treated
groups, although the values for this parameter were within the range
of historical controls. The sex ratio of fetuses at 40 mg/kg bw per
day was increased, the mean number of females being reduced. Increases
in the incidence of fetuses and of litters containing fetuses with 27
presacral vertebrae and with scoliosis (with or without associated rib
anomalies) were observed at 40 mg/kg bw per day, when compared with
concurrent or historical control incidences, in a total of 951 fetuses
in 149 litters from an unspecified number of studies with Dutch belted
rabbits over an unspecified period. There was no apparent dose- or
compound-related increase in the frequency of fetal soft-tissue
abnormalities. The NOAEL for maternal and developmental toxicity was
20 mg/kg bw per day (McMeekin et al., 1981; JMPR, 1985).
Comments
After oral administration to rats, goats and hens,
14C-dimethipin was extensively absorbed (69% within 24 h) and
rapidly excreted (89% within 48 h), mainly in the urine. Unchanged
dimethipin represented only a small fraction of the residue in
animals. In one metabolic pathway, dimethipin undergoes glutathione
conjugation and subsequent degradation to several metabolites,
including its mercapturic acid. In another pathway, dimethipin is
hydrated and then undergoes ring cleavage. Dimethipin also binds
covalently to amino acids, peptides and proteins, although the extent
to which this binding is catalysed by enzymes is unknown.
Dimethipin (purity, 98.5%) was moderately toxic to rats given
single oral doses, with LD50 values of 460 mg/kg bw in males and 550
mg/kg bw in females, or after exposure by inhalation, with LC50
values of 1.5 mg/L in males and 0.88 mg/L in females. It showed little
toxicity in rabbits exposed dermally, with an LD50 value greater
than 5000 mg/kg bw. A recrystallized form of dimethipin was severely
irritating to the eye in rabbits. Technical-grade dimethipin was not
irritating to rabbit skin but weakly sensitized the skin of
guinea-pigs.
WHO (1999) has classified dimethipin as 'slightly hazardous'.
In 90-day and long-term tests for toxicity in rats, the liver was
the main target at doses of 10 mg/kg bw per day and above. The
clinical findings consisted of increased absolute and relative weights
of the liver and increased serum cholesterol concentration and
transaminase activity. At doses greater than 85 mg/kg bw per day,
hepatocellular hypertrophy was seen in 90-day studies, whereas in
long-term studies the hepatocellular effects included focal dilatation
of bile ducts, biliary cysts and bile-duct hyperplasia.
The testis was identified as another target organ. In a one-year
study in dogs given dimethipin, testicular changes were seen at all
doses, the lowest dose being 300 ppm (equivalent to 7.5 mg/kg bw per
day), but these were considered not to be related to treatment but to
be a result of poor nutritional status or incidental findings, as they
were similar to testicular lesions seen in other studies in dogs in
the same laboratory. Additionally, no testicular lesions were seen in
dogs in a 90-day study. In contrast, Sprague-Dawley rats fed diets
containing technical-grade dimethipin for two years showed increased
incidences and severity of seminiferous tubular degeneration at the
two highest doses, 1750 and 3500 ppm (equal to 78 and 160 mg/kg bw per
day), associated at the high dose with hypospermia in the
epididymides. The NOAEL for testicular degeneration was 40 ppm
(2 mg/kg bw per day).
In a 90-day study in dogs given dimethipin in the diet, the
lowest dose of 100 ppm (equivalent to 2.5 mg/kg bw per day) was the
NOAEL, on the basis of oesophageal lesions at the LOAEL of 300 ppm
(equivalent to 7.5 mg/kg bw per day).
In the 1-year study in dogs described above, the effects seen at
1000 and 3000 ppm (equal to 25 and 75 mg/kg bw per day) included
'thinness' and increased relative kidney weights in animals of each
sex. At this dose, males had decreased blood urea nitrogen and
creatinine concentrations and females had increased relative liver
weights. One male and three females at the highest dose died, and
animals of each sex had decreased body weights and food consumption,
hypocellularity of the bone marrow, gastritis, oedema, ulceration of
the gastrointestinal tract, thymic atrophy, nephritis, centrilobular
degeneration of the liver, splenic hyperplasia and lymphadenitis. The
NOAEL was 300 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of
increased relative liver weights, increased alanine aminotransferase
and alkaline phosphatase activities, and hepatocellular degeneration
in females at 1000 ppm (equivalent to 25 mg/kg bw per day).
In a 78-week study of carcinogenicity in mice, a statistically
significant increase in the incidence of alveloar and bronchiolar
carcinomas was seen in males at the highest dose (2000 ppm, equal to
300 mg/kg bw per day). The combined incidence of lung adenocarcinoma
and adenoma was significantly greater than the mean for controls in
five previous studies but not when compared with that for concurrent
controls or with the mean maximum incidence in controls in previous
studies. As lung adenomas and adenocarcinomas occur commonly in this
strain of mice, this finding was not considered to be of toxicological
relevance. The NOAEL for systemic toxicity was 80 ppm (equal to 12
mg/kg bw per day) on the basis of increased erythrocyte volume
fraction at the LOAEL of 400 ppm (equal to 60 mg/kg bw per day).
In two 2-year studies in rats, the NOAEL for systemic toxicity
was 40 ppm (equal to 2 mg/kg bw per day) on the basis of decreased
body weights, increased absolute and relative weights of the liver, an
increased incidence of biliary hyperplasia, and testicular
degeneration at higher doses. No increase in tumour incidence was
observed in rats at any dose. The Meeting concluded that dimethipin is
not carcinogenic in mice or rats and is unlikely to pose a
carcinogenic risk to humans.
Dimethipin has been tested in an adequate range of tests for
genotoxicity in vitro and in vivo. Negative results were obtained
in most assays. It induced a weak mutagenic response in one test for
forward mutation in mouse lymphoma cells in the presence of metabolic
activation. The Meeting concluded that dimethipin is unlikely to be
genotoxic.
In a two-generation study of reproductive toxicity in rats, the
highest dose of 800 ppm (equivalent to 40 mg/kg bw per day) caused
decreased body weights and food consumption in parental animals of
each sex and decreased body weights in pups on days 7, 14, and 21 of
lactation. The NOAEL for both systemic toxicity in the parental
generation and developmental toxicity in the pups was 200 ppm
(equivalent to 10 mg/kg bw per day).
In a study of developmental toxicity in rats, excess mortality
occurred at doses of 400 and 800 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 160 mg/kg bw per day. In
rabbits, the NOAEL for both maternal and developmental toxicity was 20
mg/kg bw per day. Does at the maternal LOAEL of 40 mg/kg bw per day
showed body-weight loss on days 6-12 of gestation and decreased weight
gain on days 6-28 of gestation. The LOAEL for developmental toxicity
was 40 mg/kg bw per day on the basis of an increased incidence of
fetuses with skeletal malformations (scoliosis).
The present Meeting confirmed the ADI of 0-0.02 mg/kg bw
established by the 1988 Joint Meeting on the basis of the NOAEL of 2
mg/kg bw per day in the 2-year study in rats conducted in 1981 and a
safety factor of 100. This ADI is supported by the NOAEL of 40 ppm,
equivalent to 2 mg/kg bw per day, in the 2-year study in rats
conducted in 1996. The ADI provides a 1000-fold margin of safety with
respect to the NOAEL of 20 mg/kg bw per day for developmental toxicity
in rabbits, which showed skeletal malformations at the LOAEL of 40
mg/kg bw per day.
An acute reference dose of 0.02 mg/kg bw was established on the
basis of the NOAEL of 20 mg/kg bw per day for skeletal malformations
in the study of developmental toxicity in rabbits and a safety factor
of 1000. This high safety factor was used because of the nature of the
effect.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 80 ppm, equivalent to 12 mg/kg bw per day (toxicity in
a 78-week study of toxicity and carcinogenicity)
Rat: 40 ppm, equivalent to 2 mg/kg bw per day (toxicity in
two 2-year studies of toxicity and carcinogenicity)
160 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
10 mg/kg bw per day (parental and reproductive toxicity
in a two-generation study of reproductive toxicity)
Rabbit: 20 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 100 ppm, equivalent to 2.5 mg/kg bw per day (toxicity
in a 90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to dimethipin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 69% within 24 h, rats
Dermal absorption Low dermal penetration, rabbits
Distribution Widely distributed, rats
Potential for accumulation No evidence of accumulation
Rate and extent of excretion 89% within 48 h mainly via urine
Metabolism in animals Parent < 5%; metabolites consist of glutathione conjugates and degradates, rats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity Oral toxicity is moderate, but only slightly toxic by dermal and inhalation
routes of exposure
Rat, LD50, oral 440 mg/kg bw (males) and 600 mg/kg bw (females)
Rabbit, LD50, dermal > 5000 mg/kg bw
Rat, LC50, inhalation 0.88 mg/L, 4 h (female) and 1.5 mg/L, 4 h (males)
Rabbit, dermal irritation Not irritating
Rabbit, ocular irritation Severely irritating
Guinea-pig, dermal sensitization Weakly sensitizing
Short-term toxicity
Target/critical effect Liver: hepatotoxicity, hepatic hypertrophy, rats
Lowest relevant oral NOAEL 2 mg/kg bw per day, rats
Lowest relevant dermal NOAEL 1000 mg/kg bw per day (highest dose tested), rats
Lowest relevant inhalation NOAEL Not determined
Long-term toxicity and carcinogenicity
Target/critical effect Rat liver: increased weight, liver enzymes, bile-duct hyperplasia; rat
testis: degeneration
Lowest relevant NOAEL 2 mg/kg bw per day in two 2-year studies, rat
Carcinogenicity Not carcinogenic in mice or rats
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect None. Decreased pup body weight on days 7, 14, and 21 of lactation at
maternally toxic doses, rats
Lowest relevant reproductive NOAEL 10 mg/kg bw per day
Developmental target/critical effect Increased incidence of skeletal malformations, rabbit
Lowest relevant developmental NOAEL Rabbit, 20 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Medical data None
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Two 2-year studies in rats 100
Acute reference dose 0.02 mg/kg bw Skeletal malformations in rabbits 1000
References
Babish, J.G. (1977) Harvade(R) (N252) Tech. C-8-1162-00. Acute
inhalation study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Benson, B.W. (1981) 1-Year dietary toxicity study in dogs with N252.
Unpublished report from International Research and Development
Corp., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA
Billings, T.J. (1987) A (C14)-radiolabeled pharmacokinetics and
metabolism study in the rat using Harvade. Southwest Bio-Labs,
Inc. (Project No. 8656r). Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L (1992a) Acute oral toxicity study in rats with Harvade
technical (Project No. 6169-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992b) Acute dermal toxicity study in rabbits with
Harvade technical (Project No. 6170-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992c) Primary dermal irritation study in rabbits with
Harvade technical (Project No. 6171-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Bootman, J. & Lodge, D.C. (1982) ARS7728 (technical grade
Harvade(R)): Assessment of its ability to induce genetic damage
in Saccharomyces cerevisiae. Unpublished report from Life
Science Research, United Kingdom. Submitted to WHO by Uniroyal
Inc., USA.
Byrd, J.W. (1992), Nature of the residue of radiolabeled Harvade in
lactating goat; Part I: Dosing, specimen collection, and
quantitation of Harvade residues in lactating goats (Project No.
9202), unpublished study. Submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 61108063) from Industrial
Bio-Test Laboratories, Inc., USA (validated by the Canadian
Health Protection Branch). Submitted to WHO by Uniroyal Inc.,
USA.
Bushby, S.R.M. (1970) Hematological studies during toxicity tests. In:
Paget, G.E., ed., Methods in Toxicology, Oxford, Blackwell
Scientific Publications.
Caplan, J. & Merricks, D.L. (1978) 14C-Harvade(R) radiocarbon
study in rats. Unpublished report from Biospherics, Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Forster, R., Edwards, N.& Nunziata, A. (1984a) Mitotic gene conversion
in Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from Life
Science Research, Roma Toxicology Centre, Italy. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Forster, R., Mosesso, P. & Nunziata, A. (1984b) Micronucleus test.
Test substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Galloway, S.M. & Brusick, D. J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 90% D-11401 in the sister chromatid exchange
assay with Chinese hamster ovary (CHO) cells. Final report.
Unpublished report from Litton Bionetics, Inc., USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gay, M.H. & Lau, R.C.M. (1996) Nature of the residue of dimethipin in
lactating goat (Project No. 9202), unpublished study. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1991) 21-Day dermal toxicity study in rats with
Harvade technical (Report No. 399-116), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal Inc., USA.
Goldenthal, E.I. (1993) 13 Week dietary toxicity study in rats with
Harvade technical (Project No. 399-133), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal, Inc. USA.
Goldenthal, E.I. (1996) Two year dietary chronic toxicity and
oncogenicity study in rats (with) Harvade technical (dimethipin)
(Project No. 399-134), unpublished study from MPI Research.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Griffiths, J.T. & Koschier, F.J. (1980) Rabbit eye irritation study
with Harvade technical (recrystallized) (Project No. 6413a),
unpublished report from Food and Drug Research Laboratories, Inc.
Submitted to WHO by Uniroyal Inc., USA
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
Harvade technical in the rat (Project No. 91-8376), unpublished
study from Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Jagannath, D.R. & Brusick, D.J. (1978) Mutagenicity evaluation of
N-252, technical lot D10406 BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Jagannath, D.R. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 98% D-11401 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kehoe, D.F. & Mackenzie, K.M. (1982) Final report. Two-generation rat
reproduction study with N252. Unpublished report from Hazleton
Raltech, Inc., USA. Submitted to WHO by Uniroyal Inc., USA.
Knickerbocker, M., Re, T.A. & Babish, J.G. (1977) Teratologic
evaluation of N252 (Harvade (R)) technical in Sprague-Dawley
rats. Unpublished report from Food and Drug Research
Laboratories, Inc., USA, submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA
Lau, R.C.M. & Gay, M.H. (1993) Nature of the residue of 14C-Harvade
in laying hens (Uniroyal Chemical Co. Project No. 91121),
unpublished stud. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Madison, W.A. (1983) Technical grade Harvade(R). Dermal
sensitization in guinea pigs (modified closed patch technique).
Unpublished report from Hazleton Raltech, Inc., USA. Submitted to
WHO by Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT NO. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA (validated by the
Canadian Health Protection Branch). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McGee, D.H. (1983) Unpublished letter from International Research
Development Corp., USA, to Uniroyal Chemical Co. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA
McManus, J.P. (1984) Metabolism of Harvade(R) in goats. Unpublished
report from Uniroyal Chemical Co. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1986) Mouse micronucleus test with dimethipin
technical, Life Sciences Research, Rome (Report 180001-M-06886).
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA
McManus, J.P. (1987a) Analysis of urine samples from dimethipin
(Harvade) rat pharmacokinetic study. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1987b) Metabolism of (C14) Dimethipin (Harvade) in the
Rat - Urinary Metabolite Identification. Uniroyal Project No.
85125. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
McManus, J.P. (1987c) In vivo/in vitro UDS study in rats, Robens
Institute (Report No. 4/86/TX). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McMeekin, S.O.., Schardein, J.L. & Blair, M. (1981) N252 (Harvade(R)
technical). Teratology study in rabbits. Unpublished report from
International Research and Development Corp., Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Myhr, B.C. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) in the mouse lymphoma forward mutation assay.
Revised final report. Unpublished report from Litton Bionetics,
Inc., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Reagan, E.L. & Becci, P.J. (1982) Acute dermal toxicity study (LD50)
in albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories, Inc., USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Serota, D.G., Alsaker, R.D. & Banas, D. (1981a) 18-month toxicity and
oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc., USA.
Ssubmitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K. & Kunalzins, W. (1981b)
104-Week chronic toxicity study in rats. N252 (Harvade(R)
technical) final report. Unpublished report from Hazleton
Laboratories America, Inc., USA, submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977a) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977b) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K. & Cardona, R.A.
(1978) Characterization of excretory metabolites from
14C-Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemica. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Sorg, R.M., Naismith, R.W. & Matthews, R.J. (1983) CHO metaphase
analysis in vitro chromosome aberration analysis in Chinese
hamster ovary cells (CHO). PH320-UN-00183 Harvade(R),
unpublished report from Pharmakon Research International, Inc.,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Squire, R.A. (1984) Unpublished letter to Uniroyal Chemical. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Varner, L.L. & Matthews, R J. (1977) Acute oral LD50 in rats.
Uni-N-252 CND-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Three young male Charles River CD rats weighing approximately
225 g each received a single oral dose of 800 mg/kg bw of
14C-dimethipin. Urine and faeces were collected separately 24, 48,
and 72 h after dosing. The samples collected at 24 h were used for
identification of metabolites. Only trace amounts of dimethipin were
detected in the urine by HPLC, but seven metabolites were detected.
The three identified polar products, which accounted for 77% of the
urinary radioactivity, were a cysteinylglycine conjugate (54%), an
N-acetylcysteine conjugate (12%), and a reduced metabolite,
2,3-dimethyl-5,6-dihydro-1,4-dithiane-1,1,4,4-tetraoxide (11%). The
proposed metabolic pathway involves conjugation with glutathione
followed by degradation to cysteinylglycine and cysteine conjugates
and formation of mercapturic acid conjugates (McManus, 1987b; JMPR
1988).
Goats
A goat with a cannulated bile duct received 14C-dimethipin
(radiochemical purity, 98%) at a dietary concentration of 500 ppm for
three days. Samples of urine, liver, bile, and kidney were collected
for characterization and identification of metabolites. No details of
the experimental conditions were provided, and although two animals
appear to have been treated, data were presented on only one. Only 18%
of the labelled residues in liver and 15% of those in kidney were
identified: (butan-3-one-2-yl)-2-hydroxyethyl sulfone (due to ring
cleavage) and 2,3,5,6,-tetrahydro-5-hydroxy-5,6-dimethyl-1,4-dithiine
1,1,4,4,-tetraoxide being the predominant residues. Metabolites 3 and
4 were invariably identified together with unchanged parent compound
in urine, bile, liver, and kidney. Metabolite 4 accounted for 2-8% of
the radiolabel recovered in each case. Some alcohol metabolites of
dimethipin were identified only in urine and bile. Most of the
radiolabelled components in urine, bile, liver, and kidney were highly
polar and were present as glucuronide, cysteine, and acetylcysteine
conjugates. Studies in vitro confirmed that these are the likely
major metabolic steps. It was not reported whether total radioactivity
was measured or whether residues were identified in muscle, fat, or
milk (McManus, 1984; FAO/WHO, 1985).
Goats received a normal ration supplemented with 14C-dimethipin
at 1 or 10 mg/kg of feed until a plateau was reached in the milk after
17 days. After 18 days of dosing, about 97% of the total administered
radiolabel was excreted in the urine and faeces, but none were
detected in muscle and the concentrations were low in milk, liver, and
kidney. The concentrations added to the feed were higher than the
so-called 'worst case' situation in which every component of the diet
contains residues at the tolerable maximum residue level (TMRL)
proposed by the JMPR in 1985. Thus, if animals are exposed to a normal
feeding regime, the concentrations in meat, milk, and edible offal
will not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP standards and with a
signed and dated quality assurance statement, two lactating goats
received 14C-dimethipin (radiochemical purity, > 98%) at a
concentration of 0.15 or 50 mg/kg bw per day for 5 days before being
killed about 22 h after the last dose. The doses were equivalent to
dietary concentrations of 3.1 ppm (10 times actual exposure) and
1000 ppm (3200 times actual exposure), respectively. Urine and faeces
were collected once daily, and milk was collected twice daily.
Overall, 97% of the total radiolabel administered was recovered from
the animal given the low dose and 96% from that given the high dose.
More than 95% of the total administered dose was identified in the
excreta, and 0.1-0.2% was eliminated in the milk. The average daily
concentrations in milk were 0.005 ppm at the low dose and 1 ppm at the
high dose. Fat, muscle, and whole blood contained the lowest
concentrations of radiolabel at both the low dose (0.001 ppm in fat,
0.002 ppm in muscle, and 0.006 ppm in whole blood and the high dose
(0.32 ppm in fat, 0.64 ppm in muscle, and 2.7 ppm in whole blood),
whereas the liver and kidney contained the highest concentrations,
with 0.27 and 0.14 ppm at the low dose and 79 and 28 ppm at the high
dose, respectively. The sensitivity of the method was < 0.0004 ppm
for the low dose and < 0.011 ppm for the high dose (Byrd, 1992).
Enzymatic digestion followed by acid hydrolysis of liver tissue
from this study suggested that the radiolabelled residues of
dimethipin were covalently bound to tissue proteins. After acid
hydrolysis, acetyldithiane was identified as the single radiolabelled
peak in liver. Kidney samples from the animals at the low dose
contained ethane disulfonic acid as the only metabolite, whereas renal
tissue from the animal at the high dose also contained ethane
disulfonic acid; acid hydrolysis of the bound material from this
kidney showed that acetyl dithiane was also present. Milk from both
animals contained dimethipin cysteine conjugate as the major
metabolite. Acid hydrolysis of muscle from the goat at the high dose,
which contained 0.64 ppm of radiolabelled material, yielded reduced
dimethipin as the major metabolite. The results of this study suggests
that dimethipin is metabolized primarily via Michael addition to
glutathione or its derivatives or to protein (Gay & Lau, 1996).
Chickens
Laying hens were fed a normal diet fortified with
14C-dimethipin at concentrations of 1, 6, or 30 mg/kg for 30 days,
when half of the birds were slaughtered; the remainder were killed
after a withdrawal period of 11 days. By day 30, more than 95% of the
total administered radiolabel had been excreted in the faeces. No
residues were detectable in muscle or fat at the end of dosing with
1 mg/kg or in fat after administration of 6 mg/kg. The concentrations
found in tissues corresponded roughly to the doses. The concentrations
found in the tissues of birds after 11 days of withdrawal were
considerably lower, and none of the tissues except blood from hens at
1 mg/kg contained measurable residues. The birds fed 6 or 30 mg/kg
14C-dimethipin had measurable residues in all tissues except fat. In
eggs, the residual radiocarbon increased slowly over 10 days and
remained fairly constant for the remainder of the dosing period.
Except in birds given 30 mg/kg, the concentrations in eggs decreased
to below the limit of determination during the 11 days of withdrawal.
Extrapolation from these data indicates that residues in birds fed a
hypothetical 'worst case' diet in which all or nearly all of the
components contain residues at the level of the MRL proposed by the
1985 JMPR would not exceed the limit of determination (JMPR, 1987).
In a study conducted in compliance with GLP, with a signed and
dated quality assurance statement, [14C-6]dimethipin (radiochemical
purity, > 95%) was administered orally to white Leghorn laying hens
daily for five consecutive days at a dose of 15.8 mg/kg bw per day
(five hens) or 152 mg/kg bw per day (two hens), equivalent to
concentrations of 203 ppm (7000 times that caclulated to result from
use of dimethipin at the permitted level on the cereals in the diet)
and 2770 ppm (92 000 times the calculated concentration),
respectively. Five untreated birds served as controls. Excreta and
eggs were collected once daily during treatment. More than 90% of the
total administered radiolabel was found in the excreta. The hens were
killed within 24 h of the last dose, and tissues were collected,
examined, and weighed. Edible tissues and eggs contained 5.5% and 5.1%
of the total radiolabel at the low and high doses, respectively. The
liver and kidney had the highest concentrations, with 9.7 and 65 ppm
in liver and 4.5 and 39 ppm in kidney at the low and high doses,
respectively. Breast and thigh muscle each contained 10 ppm at the
high dose and 0.63 and 0.72 ppm at the low dose, respectively. Egg
yolk and egg white contained 6.9 and 6.6 ppm, respectively, at the
high dose and 1.1 and 0.68 ppm at the low dose. Fat had the lowest
concentrations, with 0.20 ppm at the low dose and 2.4 ppm at the high
dose. Multiple metabolites were identified in eggs and tissues, all
formed as conjugates of glutathione followed by degradation, resulting
in the following compounds: glutathionyl-dimethipin,
gamma-glutamyl-cysteinyl-dimethipin, cysteinyl-dimethipin,
mercaptodimethipin, thioacetyl-dimethipin, thiomethyl-dimethipin, and
methyl sulfoxide-dimethipin. The major metabolite was
gamma-glutamylcysteinyl-dimethipinin most tissues and
cysteinyl-dimethipin in liver (Lau & Gay, 1993).
While the metabolism of dimethipin in plants was found to be
negligible, because it is applied at or close to the harvesting stage
when the biochemical activities of the plant are at a minimum, its
metabolism in animals is quite extensive. As a result, the main
residues in crops are the parent product, while in animals dimethipin
undergoes glutathione conjugation with subsequent degradation. In a
second pathway, dimethipin undergoes hydration followed by ring
cleavage.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of dimethipin after administration by the
oral, dermal, and inhalation routes and its ocular and dermal
irritation and dermal sensitizing capacity are summarized in Table 1.
Table 1. Acute toxicity of dimethipin
Species Purity Sex Route LD50 or LC50 Reference
(%) (mg/kg bw or mg/L)
Mouse > 97.5 M Oral 440 Shapiro (1977a)
F 600
Rat > 97.5 M&F Oral 1 200 Varner & Matthews
(1977)
Rat 98 M Oral 460 Blaszcak (1992a)
F 550
Rat NR M Intraperitoneal 240 Shapiro (1977b)
F 240
Rat NR M&F Inhalation (1 h) > 20 Babish (1977)
Rat 98.9 M Inhalation (4 h) 1.5 Hoffman (1992)
F 0.88
Rabbit NR M&F Dermal (24 h) > 12 000 Reagen & Becci (1982)
Rabbit 98 M&F Dermal (24 h) > 5 000 Blaszcak (1992b)
NR, not reported
In rats (strain unspecified), the LD50 for dimethipin (purity,
> 97.5%) administered orally was 1200 mg/kg bw for animals of each
sex (Varner & Matthews, 1977; JMPR, 1985). In another study, the
LD50 for dimethipin (purity, 98.0%) was 550 mg/kg bw in male and 460
mg/kg bw in female Sprague Dawley CD rats (Blaszcak, 1992a). Surviving
animals lost weight during week 1, but gained weight during the
remainder of the observation period. The signs of toxicity included
nasal discharge, hypoactivity, and rales. At necropsy, discoloured
lungs, red distended stomachs, and red fluid in the intestines were
noted.
The LC50 in Sprague-Dawley CD rats exposed by inhalation by
nose only for 4 h was 1.5 mg/L for males and 0.88 mg/L for females
(Hoffman, 1992). The clinical signs of toxicity included respiratory
distress. Body weights were substantially decreased (41% in males and
18% in females) during the first week, but weight was gained after
that time. At necropsy, discoloured lungs were observed.
The LD50 in rabbits was > 5000 mg/kg bw, as no deaths were
seen (Blaszcak, 1992b). It severely irritated the eyes (Griffiths &
Koschier, 1980), but the material tested was a recrystallized form of
dimethipin, which may not be representative of the technical-grade
material. Technical-grade dimethipin was not irritating to rabbit skin
(Blaszcak, 1992c). It was a weak dermal sensitizer in Hartley
guinea-pigs (Madison, 1983).
WHO (1999) has classified dimethipin as 'slightly hazardous'.
(b) Short-term studies of toxicity
Rats
Groups of six male and six female Charles River CD Sprague-Dawley
rats, eight weeks of age, received technical-grade dimethipin (purity,
98%) moistened with distilled water dermally for 6 h/day at doses of
0, 10, 100, or 1000 mg/kg bw per day for 21 days. The study was
conducted under GLP requirements, and a signed and dated quality
assurance statement was available. There were no treatment-related
alterations in mortality rate, body weight, food consumption, clinical
signs, clinical pathology, or gross and histopathological appearance.
Traces of hyperkeratosis were present in the skin of treated males at
all doses and in females at the intermediate and high doses. The
weights of the liver relative to body weights were statistically
significantly increased in males (20%) and females (15%) at 1000 mg/kg
bw per day, but the absolute liver weights of these animals were
statistically nonsignificantly increased, by 18% in males and 12% in
females. In the absence of clinical pathological or histopathological
effects, the NOAEL for systemic toxicity was 1000 mg/kg bw per day,
the highest dose tested (Goldenthal, 1991).
Groups of 15 male and 15 female Charles River rats, 28 days old,
were fed diets containing technical-grade dimethipin (purity,
> 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for 95 days.
There was no treatment-related change in mortality rate or abnormal
behaviour. Food consumption was slightly depressed in females at the
highest dose throughout the study, but body weights were unaffected.
Haematology, blood chemistry, and urinalysis performed in 10 males and
10 females from the control and high-dose groups after 45 and 85 days
of treatment indicated no significant compound-related effects. At
termination, the ratios of organ:body weight of liver and kidney were
increased in females at the highest dose. No significant differences
in gross pathological changes were observed between control and
treated groups. Histopathological evaluation of a variety of tissues,
including liver and kidney, from 10 males and 10 females from the
control and high-dose groups showed no lesions attributable to
treatment. The NOAEL was 300 ppm, equivalent to 15 mg/kg bw per day
(Marias et al., 1976; JMPR, 1985).
Groups of 10 male and 10 female Charles River CD Sprague-Dawley
rats aged four week were fed diets containing technical-grade
dimethipin (purity, 98.5%) at concentrations of 0, 40, 1750, or 3500
ppm (equal to 2.5, 110, and 220 mg/kg bw per day in males and 3.1,
130, and 260 mg/kg bw per day in females) for 13 weeks. The study was
conducted under GLP requirements, and a signed quality assurance
statement was available. The animals were observed for deaths and
clinical signs of toxicity twice daily, and body weight and food
consumption were measured weekly. Ophthalmoscopic examinations were
made before treatment and at the end of the study. Clinical data were
collected on all surviving animals at 13 weeks. The weights of the
brain, kidneys, liver, and testis were recorded for all animals, and
all tissues were examined microscopically.
One male at the low dose was killed in extremis, but this
animal showed no significant gross or microscopic changes, and the
cause of death was not identified. No clinical signs of toxicity or
ophthalmoscopic findings were reported. Males at the high dose had
significantly decreased food consumption (11%), body weight (6%), and
body-weight gain (8%) in comparison with controls; and females at the
intermediate and high doses had significantly decreased food
consumption (12% at the intermediate and 16% at the high dose), body
weight (10% and 11%), and body-weight gain (20% and 28%). Males at the
high dose had slightly decreased erythrocyte volume fractions (8%) and
haemoglobin values (7%). Significantly increased cholesterol
concentrations were found in females at the intermediate (37%) and
high doses (41%), and those at the high dose had increased serum
activity of aspartate aminotransferase (30%). There were no gross
findings at necropsy that were considered to be related to treatment.
The weight of the liver relative to body weight was significantly
increased in males at the high dose (18%) and was correlated with
microscopic hepatocellular hypertrophy in 5 of 10 animals. Females at
the intermediate and high doses showed increased relative weights of
the brain, kidney, and liver, in the absence of microscopic changes,
and these changes were considered to be related to the decreased body
weights of those animals. The NOAEL was 40 ppm, equal to 2.5 mg/kg bw
per day (Goldenthal, 1993).
Dogs
Groups of four male and four female pure-bred beagles, about six
months old, were given diets containing technical-grade dimethipin
(purity, > 99.2%) at concentrations of 0, 100, 300, or 1000 ppm for
90 days. No deaths occurred, and no treatment-related effects were
seen on behaviour, body weight, or food consumption or in blood
chemistry or haematology conducted after 42 and 85 days of treatment.
Urinary analysis at the same two intervals indicated an increase (not
dose-related) in the incidence of moderate-to-large amounts of
'crystals' in the urinary sediments of all treated females after 85
days. No other urinary parameters were affected. At terminal
sacrifice, the organ weights and gross appearance were not affected by
treatment. Microscopic examination of a large number of tissues,
including the testis, from each animal revealed oesophageal lesions
characterized by focal mucosal vesicles containing a few acute
inflammatory cells in one of eight animals at 300 ppm and three of
eight animals at 1000 ppm, but in none of the concurrent controls or
those at the lowest dose. The NOAEL was 100 ppm, equivalent to 2.5
mg/kg bw per day (Burtner et al., 1976).
Groups of six male and six female pure-bred beagles, about 7.5
months old and caged individually, were given diets containing
technical-grade dimethipin (purity, 99.7%) at concentrations of 0,
300, 1000, or 3000 ppm for one year. One male and three females at the
highest dose died or were sacrificed in extremis between weeks 13
and 52. 'Thinness', a major clinical sign, was seen frequently in most
animals at 3000 ppm and infrequently in one animal at 1000 ppm. Other
signs, including dehydration and paleness of the gums, were also noted
infrequently at 3000 ppm. Weight loss or growth depression and
decreased food consumption were seen in animals of each sex at 3000
ppm throughout most of the study. Bitches at 300 or 1000 ppm showed a
marginal (10%) but not consistently dose-dependent reduction in growth
between weeks 12 and 48. Animals of the highest dose had abnormalities
in the T-wave on electrocardiograms at the end of the study, and
ophthalmoscopic examination showed increased incidences of
conjunctival discharge, inflammation and corneal irregularities and
roughening at week 27 but not at week 52. 'Severe to slight thinness
and irregular or erratic heartbeat' were seen almost exclusively in
animals at 3000 ppm throughout the study.
Monthly haematological and blood chemical determinations revealed
deviations from control values in many parameters mainly in animals of
each sex at 3000 ppm, including decreased erythrocyte volume fraction,
increased platelet count, and depressed values of total protein,
albumin, globulin, calcium, blood urea nitrogen, and creatinine at
most sampling intervals. Animals at 1000 ppm had decreased values of
blood urea nitrogen (both sexes) and creatinine (dogs) at many
sampling intervals. When compared with concurrent controls, treated
dogs showed a slight but consistent, generally dose-related decrease
in erythrocyte counts and haemoglobin levels. These findings were
considered unlikely to be related to treatment, because the values
were within the normal ranges for control beagles in the published
literature (Bushby, 1970) and those recorded for control beagle dogs
maintained in the testing laboratory. Urinalysis, including microscopy
of urinary sediments, conducted twice monthly showed no significant
changes related to treatment.
At termination, the gross pathological appearance in the treated
groups was not significantly different from that in the controls. The
ratio of organ:body weight for the kidneys was increased at both 1000
and 3000 ppm (both sexes), for liver in dogs at 3000 ppm and in
bitches at > 1000 ppm, for brain at 3000 ppm (both sexes), and for
testis at 3000 ppm. Histopathological evaluation of a large number of
tissues from each animal showed testicular degeneration in 0/6, 2/6,
1/6, and 3/6 dogs at 0, 300, 1000, and 3000 ppm, respectively. Severe
and diffuse testicular degeneration was seen in one affected dog at
300 ppm and one at 3000 ppm, while generally mild focal degeneration
of the testis was seen in the other affected animals. Although the
incidence and severity of testicular degeneration did not show a
dose-response relationship, the complete absence of this lesion in
concurrent controls and in seven similar 1-year studies conducted in
the same laboratory and comprising over 20 control dogs (McGee, 1983)
indicated that the possibility that the testicular lesion is related
to treatment cannot be ruled out. Nevertheless, the testicular lesions
were considered not to be a direct effect of dimethipin on the testes,
but rather the result of the prolonged poor nutritional status of the
dogs or an incidental finding, as they were similar to incidental
findings in other studies in dogs in the same laboratory.
Additionally, no testicular lesions were seen in dogs in a 90-day
study. Other microscopic findings likely to be attributable to
treatment included hypocellularity of the bone marrow, lesions in the
gastrointestinal tract (gastritis, oedema, and ulceration) and heart
(haemorrhage), and thymic atrophy at the high dose, and an increased
incidence of nephritis, centrilobular degeneration in the liver,
lymphadenitis, and splenic hyperplasia at both 1000 and 3000 ppm. The
NOAEL was 300 ppm, equivalent to 7.5 mg/kg bw per day, on the basis of
the increased relative liver weight, increased alanine
aminotransferase and alkaline phosphatase activities, and
hepatocellular degeneration in bitches at 1000 ppm (Benson, 1981;
JMPR, 1985, 1999).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female CD-1 mice, about 50 days old,
housed five per sex per cage, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at 0, 80, 400, or 2000 ppm
for 78 weeks. The animals fed dimethipin were kept for about 35 weeks
in the same room as animals receiving a highly photodegradable
compound identified only by a code name. It was stated, but
unsubstantiated by data, that dietary analysis showed that dimethipin
was stable in the diet for 7 days and that the mixture of dimethipin
and basal diet was satisfactorily homogeneous. All animals killed
in extremis or that died during the study and those killed at the
end were examined grossly, and a wide range of tissues, including the
brain, were examined microscopically.
The mortality rate was not affected by treatment: 58-78% of males
and 76-86% of females in all groups were still alive at the conclusion
of the study. A slight (< 10%), non-dose-related decrease in
body-weight gain was seen in males at doses > 400 ppm during the
first 13 weeks. Food consumption was not affected in a consistent
dose-related pattern. There were no significant differences between
control and treated groups in the incidence of clinical signs or
palpable nodules or tissue masses. Haematological examination of five
males and five females per group at three intervals during the study
revealed a significant increase in erythrocyte volume fraction in
males at 2000 ppm at week 13 and in erythrocyte volume fraction,
haemoglobin, and erythrocyte values at week 78. At termination, the
erythrocyte volume fraction was elevated in females at both 400 and
2000 ppm. All treated females showed a statistically significant,
albeit not strictly dose-dependent, increase in erythrocyte counts at
week 78. Blood chemical and urinary parameters were not evaluated. No
significant gross pathological alterations or changes in organ weights
were seen. No detailed histopathological data with morphological
descriptions of lesions in individual animals were available, but
tabulated 'individual histopathology findings' indicated that no
compound-induced non-neoplastic changes were found.
The only notable neoplastic finding was an increased incidence of
pulmonary (alveolar and bronchiolar) tumours in males at 2000 ppm. The
incidence of lung adenocarcinomas, but not of adenomas alone, was
significantly increased in males at 2000 ppm when compared with
concurrent or historical controls from five studies ( p < 0.05,
Fisher exact test), but not when compared with the maximum incidence
of lung adenocarcinomas in historical controls. The time to appearance
of adenocarcinomas and the multiplicity of tumours were not modified
by treatment. Additionally, the pulmonary tumours were not associated
with an increase in hyperplastic pulmonary changes. The combined
incidence of lung adenocarcinoma and adenoma was significantly
increased in comparison with the incidence in historical controls from
five studies but not when compared with the incidence in concurrent
controls or the maximum incidence in historical controls. There was no
dose-related increase in the incidence of benign or malignant lung
tumours in females. The incidence, location, and type of tumours other
than lung tumours were comparable to those in controls. About 30% of
the male and 40% of the female concurrent controls were found to have
tumours. Lymphoma (in males), lung tumours (in both sexes), and
hepatocellular carcinoma (in males) were the most frequently observed
spontaneous tumours. The fact that the animals were about 50 days old
at initiation of the study may have compromised the sensitivity of the
test. The NOAEL was 80 ppm, equal to 12 mg/kg bw per day, on the basis
of criteria other than tumours. The data on lung tumours are unclear
(Serota et al., 1981a; JMPR, 1985). As lung adenomas and
adenocarcinomas occur commonly in this strain of mice, this finding
was considered to be of no toxicological relevance (JMPR, 1988).
Rats
Groups of 50 male and 50 female Sprague-Dawley CD rats, about 40
days old and caged individually, were fed diets containing
technical-grade dimethipin (purity, 97-98%) at concentrations of 0,
40, 200, or 1000 ppm for 104 weeks. The control group was used for
both this study and another study on a chemical identified only by a
code, and it was not reported whether treated animals in the two
studies were kept in the same room. All animals that were killed in
moribund condition or died during the study and all survivors killed
at the end of the study during weeks 105 and 106 were necropsied, and
a variety of tissues including the brain and any 'unusual' lesions
were examined histopathologically. Sections of the spinal cord and
'head' of 10 male and 10 female survivors per group were also
evaluated microscopically. Five males and five females per group were
studied for haematological and blood chemical parameters at five
intervals during the study and similar numbers at the same intervals
for urinary indices.
The survival of animals at the highest dose appeared to be better
than that in other groups. By 104 weeks, the survival rates were 44%
for males at 200 ppm and 50-72% for males and females in all groups,
including controls. No clinical signs related to treatment were seen,
and there were no dose- or compound-related effects on food
consumption or on the incidence of palpable nodules, tissue masses, or
wart-like lesions. A slight but consistent depression of growth
(< 5% in males and < 10% in females) was seen at 1000 ppm
between weeks 43 and 95 in males and between weeks 51 and 87 in
females. Females in all treated groups had increased total protein at
week 13 and decreased platelet counts at week 104, the only time at
which this parameter was measured. Other deviations from control
values were observed in certain haematological and blood chemical
parameters, but essentially only in animals at the highest dose. No
significant differences were seen between control and treated groups
in urinary parameters. The gross pathological changes seen in treated
animals were not significantly different from those in controls. A
non-dose-related increase in the ratio of liver:body weight was seen
in males in all treated groups, and a dose-related increase was seen
in females at 200 and 1000 ppm.The absolute weight of the adrenals and
that relative to body weight were decreased in females in all treated
groups. Histopathological examination showed focally dilated bile
ducts containing basophilic homogeneous material in one male and one
female control, two males and three females at 40 ppm, five males and
nine females at 200 ppm, and 33 males and 18 females at 1000 ppm. This
finding was presumed to be related to treatment. The microscopic
changes in other tissues, including the adrenal gland, were similar to
those in controls. The finding that 9-27% of the males in the control
and treated groups showed lactation and/or galactocoele may have been
associated with the increased incidence of mammary fibroadenoma in
males at the highest dose. The only other noteworthy neoplastic
findings were increased incidences of astrocytoma in males and
hepatocellular carcinoma in females at 200 and 1000 ppm. The
incidences of these tumours were not, however, significantly different
from those in concurrent controls (Fisher exact test). The incidence
of hepatocellular carcinoma was not significantly different from that
in historical controls, but that of astrocytoma in males was
significantly increased at both 200 and 1000 ppm ( p < 0.05) when
compared with the incidence in historical controls from seven studies.
Comparison with the maximum incidence in historical controls showed no
significant difference, even at 1000 ppm. An additional glioma was
reportedly found in the control group by a consultant to the company
who evaluated three additional brain sections from each control and
treated male. No preneoplastic lesions (gliosis) were seen in the
original or additional brain slides (Squire, 1984). The latency to
appearance of astrocytoma was not reduced by treatment. The
minimum-effect level on parameters other than tumours was 40 ppm, the
lowest dose tested, equivalent to 2.0 mg/kg bw per day (Serota et al.,
1981b; JMPR, 1985).
Further data on the changes in organ weights, provided
subsequently by the testing laboratory, gave a NOAEL of 200 ppm for
the decrease in relative and absolute weights of the adrenal glands in
female rats, and a NOAEL of 200 ppm for the increase in absolute and
relative liver weights in male rats. The NOAEL for the increase in
absolute and relative liver weights in females was 40 ppm (JMPR, 1998)
Groups of 60 young Sprague-Dawley rats (Crl:CD BR (VAF/Plus)) of
each sex were fed diets containing technical-grade dimethipin (purity,
98.5%) at concentrations of 0, 40, 1750, or 3500 ppm for males (equal
to 0, 1.8, 78, or 160 mg/kg bw per day) and 0, 40, 875, or 1750 ppm
for females (equal to 0, 2.2, 50, or 100 mg/kg bw per day) for 104
weeks. The study was conducted under GLP requirements, and a signed
quality assurance statement was available. Ten animals of each sex per
dose were killed after 12 months for interim evaluations of clinical
signs, deaths, body weight, food consumption, ophthalmological,
haematological, clinical chemical, and urinary parameters, organ
weights, and gross and histopathological appearance. The survival of
females at the high dose was decreased but not statistically
significantly. Of the 50 rats per sex allocated to the main study,
only 20, 24, 19, and 21 of the males and 20, 18, 18, and 13 of the
females in the control, low-, intermediate-, and high-dose groups,
respectively, survived to week 104. There were no treatment-related
clinical signs of toxicity, ophthalmic or haematological effects, or
changes in urinary parameters in any treated group in comparison with
controls. The mean body weight of males at the high dose was decreased
by 13-20% during the study; females at the intermediate dose weighed
< 16% less than controls and those at the high dose weighed
< 19% less. The mean body-weight gain of males at the high dose was
consistently decreased by 24%, and those of females at the
intermediate and high doses by 13 and 29% in comparison with controls.
No accompanying decrease in food consumption was seen.
Significantly increased serum aspartate and alanine
aminotransferase activities were observed in females at the
intermediate and high doses at 12, 18, and 24 months and in males at
the high dose at 18 months. Animals of each sex at the intermediate
and high doses also had elevated cholesterol levels at all sampling
intervals, and males at these doses had increased serum concentrations
of urea nitrogen and creatinine. The findings indicate renal and
hepatic toxicity. Macroscopic alterations were seen in the liver and
kidneys of animals that died during the study or were killed at the
end. Males at the high dose and females at the intermediate and high
doses had an increased incidence of liver cysts, and males at the
highest dose had an increased incidence of tan discolouration of the
liver and small testes. Males at the two higher doses had a slight
increase in the incidence of enlarged kidneys. Tan discolouration and
tan foci in the liver and a granular surface on the kidneys were seen
in females at the highest dose. Significant increases in organ weights
( p < 0.05) in males at the highest dose that were considered to be
related to treatment were in the absolute (115%) and relative (166%)
weights of the liver and the absolute (132%) and relative (152%)
weights of the kidney.
At interim sacrifice at 12 months, the incidence and/or grade of
bile-duct hyperplasia was increased in treated males (4/12, 5/13, and
5/12 at the low, intermediate, and high doses, respectively) when
compared with controls (2/13). In females, the incidence and/or grade
was increased at both 875 ppm (70%) and 1750 ppm (43%) when compared
with controls (8%). Epithelial hyperplasia of the duodenum was seen in
11/12 males and 10/14 females at the high dose and in none of the
controls by 12 months. In animals killed at the end of the study, the
incidence and/or grade of biliary cysts in the liver was increased in
females at the intermediate (12%) and high doses (37%) and in males at
the high dose (10%) in comparison with controls (2% in females and 0%
in males). Bile-duct hyperplasia in the liver was increased in
incidence and/or severity in males at the intermediate (25/47) and
high doses (29/48) in comparison with controls (21/47), and in females
at the intermediate (68%) and high doses (61%) when compared with
controls (33%). Dose-related increases in the severity of the lesion
were seen in animals of each sex. Male rats fed the high dose also
showed a significantly increased incidence (33%) of eosinophilic foci
in comparison with controls (13%).
The incidence and severity of chronic progressive nephropathy was
increased in 47/47 males and 37/50 females at the intermediate dose
and 46/48 males and 38/46 females at the high dose in comparison with
controls (33/47 in males and 18/48 in females). The incidence and
grade of epithelial hyperplasia of the duodenum was increased in males
at the intermediate (38%) and high doses (46%) in comparison with
controls (2%), and in females at the intermediate (14%) and high doses
(33%) in comparison with controls (0%). Signs of gastrointestinal
tract toxicity were seen in animals of each sex at the high dose;
males at this dose had an increased incidence and severity of
epithelial hyperplasia of the nonglandular stomach (15%, 2% in
controls), and females showed demineralization of the glandular
stomach (7/46, 0/48 in controls). Males at the high dose had an
increased incidence (21/47) and grade of seminiferous tubular
degeneration of the testis (eight had grades of moderate and six
severe; 12/47 in controls of which 0 were moderate and four severe)
and hypospermia of the epididymis (13/47, 5/47 in controls).The
incidence of seminiferous tubular degeneration was 13/27 (three
moderate and two severe) in males at the low dose and 16/34 (four
moderate and three severe) at the intermediate dose. Although the
testes of all rats at the low and intermediate doses were not
examined, a treatment-related increase in the incidence of testicular
lesions at the intermediate dose could be discerned. The occurrence of
testicular degeneration at the intermediate and high doses was
considered to be related to treatment, as was the increased incidence
of epididymal hypospermia, which was probably a result of the
seminiferous tubular degeneration. Females at the high dose showed an
increased incidence and severity of vascular mineralization of the
heart (5/46, 0/46 in controls) and aortic artery (6/45, 0/48 in
controls). The other histological lesions reported were considered not
to be related to treatment.
The doses tested were adequate to assess the tumorigenic
potential of dimethipin. The only statistically significant increase
in neoplastic lesions was in the incidence of benign
phaeochromocytomas (17%, 4% in concurrent controls) in the adrenal
medulla of male rats at the high dose. There was no accompanying
increase in hyperplasia in this tissue and no increase in the
incidence of malignant phaeochromocytomas, and the combined incidence
of benign and malignant neoplasms was not increased significantly.
Furthermore, the incidence of benign phaeochromocytomas in historical
controls ranged from about 0 to 18%, and the combined incidence of
malignant and benign tumours ranged from about 0 to 20% in similar
studies conducted in the same laboratory during the 5 years preceding
termination of this study. The increased incidence of benign
phaeochromocytomas was therefore considered not to be related to
treatment. Slight but biologically insignificant increases or
decreases in the incidences of other tumours were seen in treated
groups in comparison with controls. The tumour incidences in this
study are presented in Table 2. During the first 12 months of the
study, additional fibroadenomas and adenocarcinomas were found in
females in both control and treated groups and an additional
fibroadenoma was found in a male at the intermediate dose. The other
tumour types listed in Table 2 were not found during the first 12
months. The increased incidences of astrocytomas in males at 200 and
1000 ppm, hepatocellular carcinomas in females at 200 and 1000 ppm,
and mammary fibroadenomas in males at 1000 ppm in the study of Serota
et al. (1981b) were not found in this study, even though the doses and
the number of rats per sex at each dose exceeded those in the earlier
study. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day
(Goldenthal, 1996).
(d) Genotoxicity
Dimethipin had no mutagenic activity in a number of assays in
microorganisms, mammalian cells, and rodents in vitro and in
vivo. The only exception was the induction of forward mutation in
mouse lymphoma cells in the presence of metabolic activation
(Table 3).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Groups of 15 male and 25 female Charles-River CD(SD)BR rats, 5
weeks old, were fed diets containing technical-grade dimethipin
(purity, 99.7%) at 0, 50, 200, or 800 ppm for 105 days before mating
(one male:two females; sibling and half-sibling mating avoided). he
day on which a positive vaginal smear or copulatory plug was detected
was considered to be day 0 of gestation. Weanlings of the second
litter (F1b) were selected to become parents of the next generation
and mated after receiving the test diets for 125 days. In each
generation, the second mating was allowed at least 14 days after the
first litter (F1a and F2a) had been weaned at 21 days of age.
Table 2. Tumour incidencea in rats dying between weeks 52 and 104 in a 2-year study of dimethipin
Tumour site Males (dose, ppm) Females (dose, ppm)
0 40 1750 3500 0 40 875 1750
Mammary gland
Adenoma 1/7 0/3 1/2 2/7 4/48 3/41 7/46 2/46
Fibroadenoma 28/48 23/41 27/46 16/46
Adenocarcinoma 9/48 6/41 10/46 4/46
Brain
Astrocytoma 1/44 0/24 0/28 1/48 0/48 0/31 0/33 0/46
Granular-cell tumour 1/44 0/24 0/28 1/48
Liver
Adenoma 1/47 0/48 3/47 2/48 1/48 0/49 1/50 3/46
Carcinoma 1/47 1/48 2/47 1/48 0/48 0/49 0/50 0/46
Interstitial-cell tumour of the testis
Benign 2/47 1/27 2/34 5/47
Malignant 0/47 0/27 1/34 0/47
Combined 2/47 1/27 3/34 5/47
Phaeochromocytoma of the adrenal medulla
Benign 2/47 4/28 0/28 8/48* 1/48 1/32 0/35 0/46
Malignant 2/47 1/28 1/28 0/48 0/48 0/32 0/35 0/46
Combined 4/47 5/28 1/28 8/48
a Number of rats with tumours/Number of rats examined microscopically
* Significantly different from incidemce in concurrent controls at p < 0.05
Table 3. Results of assays for the genotoxicity of dimethipin (purity, > 98%)
End-point Test system Dose Result Reference
In vitro
Reverse mutation S. typhimurium TA1538, 1-1000 mg/plate Negativea Jagannath & Brusick
TA1537, TA1535, TA98, (1978, 1981)
Mitotic non-disjunction, S. cerevisiae D4 1-1000 mg/plate Negativea Jagannath & Brusick
recombination, and (1978)
mutation S. cerevisiae D6 1-2000 mg/plate Negativea Bootman & Lodge
(1982)
Mitotic gene conversion S. cerevisiae D4 125-2000 mg/ml Negativea Forster et al. (1984a)
Chromosomal aberration Chinese hamster ovary cells 5-50 mg/ml Negativea Sorg et al. (1983)
Sister chromatid exchange Chinese hamster ovary cells 1.56-24 mg/mlb Negativea Galloway & Brusick
3.1-200 mg/mlc (1981)
Forward mutation L5178Y Tk+/- mouse 1.56-75 mg/mlb ? Myhr & Brusick
lymphoma cells 125-200 mg/mlc (1981)
In vivo
In vivo/in vitro unscheduled Wistar rat 100, 300, or 1000 Negative McManus (1987c)
DNA synthesis mg/kg bw
Micronucleus formation Swiss CD-1 mouse 220 mg/kg bw Negative McManus (1986)
Micronucleus formation Mouse Two successive Negatived Forster et al. (1984b)
daily oral doses of
22, 73.3, or 220
mg/kg bw per day
(males) or at 30,
100, or 300 mg/kg
bw per day (females)
In the parental generations, deaths (the incidence of which was
not dose-related) occurred only among females. No compound-related
behavioural abnormalities were seen. F0 and F1b adult females at
800 ppm weighed less than the concurrent controls before mating,
throughout gestation, and throughout most of the the lactation
periods. Food consumption was depressed in animals at 800 ppm, in F0
females before mating in weeks 6-10, in F1b females in weeks 6-17,
and in F1b males in weeks, 1, 4, and 9. Fertility in males, as
determined by a demonstrated ability to impregnate at least one
female, mating index (% females mated), gestation index (% mated
females with viable litters), the number of days required by females
to mate, and the duration of gestation in treated groups were all
comparable to the control values. In the progeny, the mean number of
pups per litter born alive, survival of pups to days 4, 7, 14, and 21,
the sex ratio, and the behaviour of pups were not adversely affected.
The weights of pups in the F1a and F1b litters at 800 ppm were
reduced on days 7, 4, and 21, and the weights of those in the F1a
litters at 200 ppm and in the F2a litters at 800 ppm were decreased
on day 21. Gross external examination of all pups, including those
found dead, revealed only one abnormal pup, which was a stillborn in
an F1a litter at 200 ppm.
Gross pathological examination of all parental animals of the
second litters killed after weaning (F0) or 30 days after weaning
(F1b) (i.e. after 32 weeks and 39 weeks of dietary feeding,
respectively) and weanlings in each generation revealed no significant
difference between control and treated groups. Determinations of the
weights of organs from all F1b adults and five male and five female
weanlings from the F1b and the F2b litters showed increased
organ:body-weight ratios for the liver at 200 and 800 ppm and for the
kidney and brain at 800 ppm in adult F1b females; however, the
organ:body-weight ratio of the liver in F1b adult females was
depressed at 50 ppm. Microscopic evaluation of a wide range of
tissues, including the liver and kidney, from all F1b adults and
five male and five female weanlings from the F1b and the F2b
litters and of gross lesions and gonads from F0 adults indicated no
significant changes attributable to treatment.
The NOAEL was 200 ppm, equivalent to 10 mg/kg bw per day, as the
finding of a decrease in pup weight on day 21 in F1a litters at 200
ppm was unlikely to be treatment-related, as it occurred in only a
single generation and was not recurrent (Kehoe & Mackenzie, 1982;
JMPR, 1985).
(ii) Developmental toxicity
Rats
Groups of sexually mature mated female rats (BLU:(SD)BR) received
technical-grade dimethipin (purity, 97.5%) by intubation as a
suspension in corn oil at a dose of 0, 80, 400, or 800 mg/kg bw per
day on days 6-15 of gestation, the day on which a vaginal plug was
observed being considered day 0. An additional group of mated female
rats treated with 250 mg/kg bw per day of acetylsalicylic acid was
used as the positive control. The groups at 400 and 800 mg/kg bw per
day were terminated within 8 days of initiation of treatment owing to
'excessive deaths' and were not investigated further. Two new groups,
at 30 and 160 mg/kg bw per day, were added 2 weeks after the study
began, but no concurrent control groups were included for the two new
doses. The dams were killed on day 20 of gestation and their fetuses
were removed surgically for gross external, visceral, and skeletal
examination.
No compound-related deaths or clinical signs were observed at
doses up to 160 mg/kg bw per day, and the growth rates of dams during
gestation were comparable in all groups. The number of dams in each
group that became pregnant and were alive on day 20 was 20-22. The
mean number of implantation sites or live fetuses, fetal weight, and
sex ratio were unaffected. An increase in the mean number of
resorptions per dam, with no concomitant increase in the incidence of
pregnant dams with resorptions, was seen at 160 mg/kg bw per day. The
incidence of skeletal or visceral malformations of fetuses did not
differ significantly between control and treated groups. The positive
control group had a number of fetal abnormalities, including
encephalomenigocoele and gastroschisis. The NOAEL for both maternal
and developmental toxicity was 160 mg/kg bw per day (Knickerboker et
al., 1977; JMPR, 1985).
Rabbits
Groups of 16 sexually mature female Dutch belted rabbits were
artificially inseminated and were intubated with technical-grade
dimethipin (purity, 98.3%) as a suspension in 0.5% carboxymethyl
cellulose at 0, 7.5, 20, or 40 mg/kg bw per day at a constant volume
of 1 ml/kg bw, on days 6-27 of gestation, day 0 of gestation being
considered the day of insemination. They were killed on day 28 of
gestation, and the uterine contents were examined. All fetuses,
including those that were aborted or dead, were examined grossly and
for skeletal and visceral abnormalities.
No deaths occurred. A slight increase in the number of females at
20 and 40 mg/kg bw per day that had a reduced amount of faeces beneath
the cage was seen at various intervals during gestation as compared
with concurrent controls. No data on food consumption were available.
Does at 40 mg/kg bw per day showed weight loss between days 6 and 12,
and maternal weight gain was depressed in a dose-response pattern in
all treated groups between days 6 and 28. The fertility rate was
88-94% in control and treated groups. One doe each at 0, 20, and 40
mg/kg bw per day aborted on day 28; seven non-viable fetuses were
found in does at 0 and 20 mg/kg bw per day; and three late resorptions
occurred in the doe at 40 mg/kg bw per day. At terminal sacrifice, the
gross pathological findings in treated does were comparable to those
in the controls. No significant differences were found between
controls and treated groups in the mean numbers of corpora lutea,
implantations, early or late resorptions, or viable or non-viable
fetuses, or in fetal weight.
A non-dose-related increase in postimplantation loss, due mainly
to an increased number of early resorptions, was seen in all treated
groups, although the values for this parameter were within the range
of historical controls. The sex ratio of fetuses at 40 mg/kg bw per
day was increased, the mean number of females being reduced. Increases
in the incidence of fetuses and of litters containing fetuses with 27
presacral vertebrae and with scoliosis (with or without associated rib
anomalies) were observed at 40 mg/kg bw per day, when compared with
concurrent or historical control incidences, in a total of 951 fetuses
in 149 litters from an unspecified number of studies with Dutch belted
rabbits over an unspecified period. There was no apparent dose- or
compound-related increase in the frequency of fetal soft-tissue
abnormalities. The NOAEL for maternal and developmental toxicity was
20 mg/kg bw per day (McMeekin et al., 1981; JMPR, 1985).
Comments
After oral administration to rats, goats and hens,
14C-dimethipin was extensively absorbed (69% within 24 h) and
rapidly excreted (89% within 48 h), mainly in the urine. Unchanged
dimethipin represented only a small fraction of the residue in
animals. In one metabolic pathway, dimethipin undergoes glutathione
conjugation and subsequent degradation to several metabolites,
including its mercapturic acid. In another pathway, dimethipin is
hydrated and then undergoes ring cleavage. Dimethipin also binds
covalently to amino acids, peptides and proteins, although the extent
to which this binding is catalysed by enzymes is unknown.
Dimethipin (purity, 98.5%) was moderately toxic to rats given
single oral doses, with LD50 values of 460 mg/kg bw in males and 550
mg/kg bw in females, or after exposure by inhalation, with LC50
values of 1.5 mg/L in males and 0.88 mg/L in females. It showed little
toxicity in rabbits exposed dermally, with an LD50 value greater
than 5000 mg/kg bw. A recrystallized form of dimethipin was severely
irritating to the eye in rabbits. Technical-grade dimethipin was not
irritating to rabbit skin but weakly sensitized the skin of
guinea-pigs.
WHO (1999) has classified dimethipin as 'slightly hazardous'.
In 90-day and long-term tests for toxicity in rats, the liver was
the main target at doses of 10 mg/kg bw per day and above. The
clinical findings consisted of increased absolute and relative weights
of the liver and increased serum cholesterol concentration and
transaminase activity. At doses greater than 85 mg/kg bw per day,
hepatocellular hypertrophy was seen in 90-day studies, whereas in
long-term studies the hepatocellular effects included focal dilatation
of bile ducts, biliary cysts and bile-duct hyperplasia.
The testis was identified as another target organ. In a one-year
study in dogs given dimethipin, testicular changes were seen at all
doses, the lowest dose being 300 ppm (equivalent to 7.5 mg/kg bw per
day), but these were considered not to be related to treatment but to
be a result of poor nutritional status or incidental findings, as they
were similar to testicular lesions seen in other studies in dogs in
the same laboratory. Additionally, no testicular lesions were seen in
dogs in a 90-day study. In contrast, Sprague-Dawley rats fed diets
containing technical-grade dimethipin for two years showed increased
incidences and severity of seminiferous tubular degeneration at the
two highest doses, 1750 and 3500 ppm (equal to 78 and 160 mg/kg bw per
day), associated at the high dose with hypospermia in the
epididymides. The NOAEL for testicular degeneration was 40 ppm
(2 mg/kg bw per day).
In a 90-day study in dogs given dimethipin in the diet, the
lowest dose of 100 ppm (equivalent to 2.5 mg/kg bw per day) was the
NOAEL, on the basis of oesophageal lesions at the LOAEL of 300 ppm
(equivalent to 7.5 mg/kg bw per day).
In the 1-year study in dogs described above, the effects seen at
1000 and 3000 ppm (equal to 25 and 75 mg/kg bw per day) included
'thinness' and increased relative kidney weights in animals of each
sex. At this dose, males had decreased blood urea nitrogen and
creatinine concentrations and females had increased relative liver
weights. One male and three females at the highest dose died, and
animals of each sex had decreased body weights and food consumption,
hypocellularity of the bone marrow, gastritis, oedema, ulceration of
the gastrointestinal tract, thymic atrophy, nephritis, centrilobular
degeneration of the liver, splenic hyperplasia and lymphadenitis. The
NOAEL was 300 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of
increased relative liver weights, increased alanine aminotransferase
and alkaline phosphatase activities, and hepatocellular degeneration
in females at 1000 ppm (equivalent to 25 mg/kg bw per day).
In a 78-week study of carcinogenicity in mice, a statistically
significant increase in the incidence of alveloar and bronchiolar
carcinomas was seen in males at the highest dose (2000 ppm, equal to
300 mg/kg bw per day). The combined incidence of lung adenocarcinoma
and adenoma was significantly greater than the mean for controls in
five previous studies but not when compared with that for concurrent
controls or with the mean maximum incidence in controls in previous
studies. As lung adenomas and adenocarcinomas occur commonly in this
strain of mice, this finding was not considered to be of toxicological
relevance. The NOAEL for systemic toxicity was 80 ppm (equal to 12
mg/kg bw per day) on the basis of increased erythrocyte volume
fraction at the LOAEL of 400 ppm (equal to 60 mg/kg bw per day).
In two 2-year studies in rats, the NOAEL for systemic toxicity
was 40 ppm (equal to 2 mg/kg bw per day) on the basis of decreased
body weights, increased absolute and relative weights of the liver, an
increased incidence of biliary hyperplasia, and testicular
degeneration at higher doses. No increase in tumour incidence was
observed in rats at any dose. The Meeting concluded that dimethipin is
not carcinogenic in mice or rats and is unlikely to pose a
carcinogenic risk to humans.
Dimethipin has been tested in an adequate range of tests for
genotoxicity in vitro and in vivo. Negative results were obtained
in most assays. It induced a weak mutagenic response in one test for
forward mutation in mouse lymphoma cells in the presence of metabolic
activation. The Meeting concluded that dimethipin is unlikely to be
genotoxic.
In a two-generation study of reproductive toxicity in rats, the
highest dose of 800 ppm (equivalent to 40 mg/kg bw per day) caused
decreased body weights and food consumption in parental animals of
each sex and decreased body weights in pups on days 7, 14, and 21 of
lactation. The NOAEL for both systemic toxicity in the parental
generation and developmental toxicity in the pups was 200 ppm
(equivalent to 10 mg/kg bw per day).
In a study of developmental toxicity in rats, excess mortality
occurred at doses of 400 and 800 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 160 mg/kg bw per day. In
rabbits, the NOAEL for both maternal and developmental toxicity was 20
mg/kg bw per day. Does at the maternal LOAEL of 40 mg/kg bw per day
showed body-weight loss on days 6-12 of gestation and decreased weight
gain on days 6-28 of gestation. The LOAEL for developmental toxicity
was 40 mg/kg bw per day on the basis of an increased incidence of
fetuses with skeletal malformations (scoliosis).
The present Meeting confirmed the ADI of 0-0.02 mg/kg bw
established by the 1988 Joint Meeting on the basis of the NOAEL of 2
mg/kg bw per day in the 2-year study in rats conducted in 1981 and a
safety factor of 100. This ADI is supported by the NOAEL of 40 ppm,
equivalent to 2 mg/kg bw per day, in the 2-year study in rats
conducted in 1996. The ADI provides a 1000-fold margin of safety with
respect to the NOAEL of 20 mg/kg bw per day for developmental toxicity
in rabbits, which showed skeletal malformations at the LOAEL of 40
mg/kg bw per day.
An acute reference dose of 0.02 mg/kg bw was established on the
basis of the NOAEL of 20 mg/kg bw per day for skeletal malformations
in the study of developmental toxicity in rabbits and a safety factor
of 1000. This high safety factor was used because of the nature of the
effect.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 80 ppm, equivalent to 12 mg/kg bw per day (toxicity in
a 78-week study of toxicity and carcinogenicity)
Rat: 40 ppm, equivalent to 2 mg/kg bw per day (toxicity in
two 2-year studies of toxicity and carcinogenicity)
160 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
10 mg/kg bw per day (parental and reproductive toxicity
in a two-generation study of reproductive toxicity)
Rabbit: 20 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Dog: 100 ppm, equivalent to 2.5 mg/kg bw per day (toxicity
in a 90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Estimate of acute reference dose
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to dimethipin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 69% within 24 h, rats
Dermal absorption Low dermal penetration, rabbits
Distribution Widely distributed, rats
Potential for accumulation No evidence of accumulation
Rate and extent of excretion 89% within 48 h mainly via urine
Metabolism in animals Parent < 5%; metabolites consist of glutathione conjugates and degradates, rats
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity Oral toxicity is moderate, but only slightly toxic by dermal and inhalation
routes of exposure
Rat, LD50, oral 440 mg/kg bw (males) and 600 mg/kg bw (females)
Rabbit, LD50, dermal > 5000 mg/kg bw
Rat, LC50, inhalation 0.88 mg/L, 4 h (female) and 1.5 mg/L, 4 h (males)
Rabbit, dermal irritation Not irritating
Rabbit, ocular irritation Severely irritating
Guinea-pig, dermal sensitization Weakly sensitizing
Short-term toxicity
Target/critical effect Liver: hepatotoxicity, hepatic hypertrophy, rats
Lowest relevant oral NOAEL 2 mg/kg bw per day, rats
Lowest relevant dermal NOAEL 1000 mg/kg bw per day (highest dose tested), rats
Lowest relevant inhalation NOAEL Not determined
Long-term toxicity and carcinogenicity
Target/critical effect Rat liver: increased weight, liver enzymes, bile-duct hyperplasia; rat
testis: degeneration
Lowest relevant NOAEL 2 mg/kg bw per day in two 2-year studies, rat
Carcinogenicity Not carcinogenic in mice or rats
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect None. Decreased pup body weight on days 7, 14, and 21 of lactation at
maternally toxic doses, rats
Lowest relevant reproductive NOAEL 10 mg/kg bw per day
Developmental target/critical effect Increased incidence of skeletal malformations, rabbit
Lowest relevant developmental NOAEL Rabbit, 20 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No evidence of neurotoxicity
Medical data None
Summary Value Study Safety factor
ADI 0-0.02 mg/kg bw Two 2-year studies in rats 100
Acute reference dose 0.02 mg/kg bw Skeletal malformations in rabbits 1000
References
Babish, J.G. (1977) Harvade(R) (N252) Tech. C-8-1162-00. Acute
inhalation study in rats. Unpublished report from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Benson, B.W. (1981) 1-Year dietary toxicity study in dogs with N252.
Unpublished report from International Research and Development
Corp., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA
Billings, T.J. (1987) A (C14)-radiolabeled pharmacokinetics and
metabolism study in the rat using Harvade. Southwest Bio-Labs,
Inc. (Project No. 8656r). Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L (1992a) Acute oral toxicity study in rats with Harvade
technical (Project No. 6169-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992b) Acute dermal toxicity study in rabbits with
Harvade technical (Project No. 6170-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Blaszcak, D.L. (1992c) Primary dermal irritation study in rabbits with
Harvade technical (Project No. 6171-91), unpublished report from
Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc., USA.
Bootman, J. & Lodge, D.C. (1982) ARS7728 (technical grade
Harvade(R)): Assessment of its ability to induce genetic damage
in Saccharomyces cerevisiae. Unpublished report from Life
Science Research, United Kingdom. Submitted to WHO by Uniroyal
Inc., USA.
Byrd, J.W. (1992), Nature of the residue of radiolabeled Harvade in
lactating goat; Part I: Dosing, specimen collection, and
quantitation of Harvade residues in lactating goats (Project No.
9202), unpublished study. Submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA.
Burtner, B.R., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
dogs. Unpublished report (IBT No. 61108063) from Industrial
Bio-Test Laboratories, Inc., USA (validated by the Canadian
Health Protection Branch). Submitted to WHO by Uniroyal Inc.,
USA.
Bushby, S.R.M. (1970) Hematological studies during toxicity tests. In:
Paget, G.E., ed., Methods in Toxicology, Oxford, Blackwell
Scientific Publications.
Caplan, J. & Merricks, D.L. (1978) 14C-Harvade(R) radiocarbon
study in rats. Unpublished report from Biospherics, Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Forster, R., Edwards, N.& Nunziata, A. (1984a) Mitotic gene conversion
in Saccharomyces cerevisiae D4. Test substance: Dimethipin
tech. 131001-M-00284. Final report. Unpublished report from Life
Science Research, Roma Toxicology Centre, Italy. Submitted to WHO
by Uniroyal Chemical Co., Bethany, Connecticut, USA.
Forster, R., Mosesso, P. & Nunziata, A. (1984b) Micronucleus test.
Test substance: Dimithipin tech. Final report. Unpublished report
from Life Science Research, Roma Toxicology Centre, Italy.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Galloway, S.M. & Brusick, D. J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 90% D-11401 in the sister chromatid exchange
assay with Chinese hamster ovary (CHO) cells. Final report.
Unpublished report from Litton Bionetics, Inc., USA. Submitted to
WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Gay, M.H. & Lau, R.C.M. (1996) Nature of the residue of dimethipin in
lactating goat (Project No. 9202), unpublished study. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Goldenthal, E.I. (1991) 21-Day dermal toxicity study in rats with
Harvade technical (Report No. 399-116), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal Inc., USA.
Goldenthal, E.I. (1993) 13 Week dietary toxicity study in rats with
Harvade technical (Project No. 399-133), unpublished study from
International Research and Development Corp. Submitted to WHO by
Uniroyal, Inc. USA.
Goldenthal, E.I. (1996) Two year dietary chronic toxicity and
oncogenicity study in rats (with) Harvade technical (dimethipin)
(Project No. 399-134), unpublished study from MPI Research.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Griffiths, J.T. & Koschier, F.J. (1980) Rabbit eye irritation study
with Harvade technical (recrystallized) (Project No. 6413a),
unpublished report from Food and Drug Research Laboratories, Inc.
Submitted to WHO by Uniroyal Inc., USA
Hoffman, G.M. (1992) An acute nose-only inhalation toxicity study of
Harvade technical in the rat (Project No. 91-8376), unpublished
study from Bio/dynamics, Inc. Submitted to WHO by Uniroyal Inc.,
USA.
Jagannath, D.R. & Brusick, D.J. (1978) Mutagenicity evaluation of
N-252, technical lot D10406 BL8998 CC0005 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Jagannath, D.R. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) 98% D-11401 in the Ames
Salmonella/microsome plate test. Final report. Unpublished
report from Litton Bionetics, Inc., USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Kehoe, D.F. & Mackenzie, K.M. (1982) Final report. Two-generation rat
reproduction study with N252. Unpublished report from Hazleton
Raltech, Inc., USA. Submitted to WHO by Uniroyal Inc., USA.
Knickerbocker, M., Re, T.A. & Babish, J.G. (1977) Teratologic
evaluation of N252 (Harvade (R)) technical in Sprague-Dawley
rats. Unpublished report from Food and Drug Research
Laboratories, Inc., USA, submitted to WHO by Uniroyal Chemical
Co., Inc., Bethany, Connecticut, USA
Lau, R.C.M. & Gay, M.H. (1993) Nature of the residue of 14C-Harvade
in laying hens (Uniroyal Chemical Co. Project No. 91121),
unpublished stud. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Madison, W.A. (1983) Technical grade Harvade(R). Dermal
sensitization in guinea pigs (modified closed patch technique).
Unpublished report from Hazleton Raltech, Inc., USA. Submitted to
WHO by Uniroyal Inc., USA.
Marias, A.J., Kennedy, G.L., Kinoshita, F.K. & Keplinger, M.L. (1976)
90-Day sub-acute oral toxicity study with UBI-N252 technical in
albino rats. Unpublished report (IBT NO. 622-08070) from
Industrial Bio-Test Laboratories, Inc., USA (validated by the
Canadian Health Protection Branch). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McGee, D.H. (1983) Unpublished letter from International Research
Development Corp., USA, to Uniroyal Chemical Co. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA
McManus, J.P. (1984) Metabolism of Harvade(R) in goats. Unpublished
report from Uniroyal Chemical Co. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1986) Mouse micronucleus test with dimethipin
technical, Life Sciences Research, Rome (Report 180001-M-06886).
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA
McManus, J.P. (1987a) Analysis of urine samples from dimethipin
(Harvade) rat pharmacokinetic study. Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McManus, J.P. (1987b) Metabolism of (C14) Dimethipin (Harvade) in the
Rat - Urinary Metabolite Identification. Uniroyal Project No.
85125. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
McManus, J.P. (1987c) In vivo/in vitro UDS study in rats, Robens
Institute (Report No. 4/86/TX). Submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
McMeekin, S.O.., Schardein, J.L. & Blair, M. (1981) N252 (Harvade(R)
technical). Teratology study in rabbits. Unpublished report from
International Research and Development Corp., Inc., USA.
Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Myhr, B.C. & Brusick, D.J. (1981) Mutagenicity evaluation of
Harvade(R) (N252) in the mouse lymphoma forward mutation assay.
Revised final report. Unpublished report from Litton Bionetics,
Inc., USA. Submitted to WHO by Uniroyal Chemical Co., Inc.,
Bethany, Connecticut, USA.
Reagan, E.L. & Becci, P.J. (1982) Acute dermal toxicity study (LD50)
in albino rabbits of Harvade(R) (N252). Unpublished report from
Food and Drug Research Laboratories, Inc., USA. Submitted to WHO
by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Serota, D.G., Alsaker, R.D. & Banas, D. (1981a) 18-month toxicity and
oncogenicity study in mice. N252 technical. Final report.
Unpublished report from Hazleton Laboratories America, Inc., USA.
Ssubmitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Serota, D.G., Alsake, R.D., Dawkins, K.K. & Kunalzins, W. (1981b)
104-Week chronic toxicity study in rats. N252 (Harvade(R)
technical) final report. Unpublished report from Hazleton
Laboratories America, Inc., USA, submitted to WHO by Uniroyal
Chemical Co., Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977a) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Shapiro, R. (1977b) Untitled and unpublished report from Product
Safety Labs, USA. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Smilo, A.R., Fuller, G.B., Tortora, N.J., Curtiss, K. & Cardona, R.A.
(1978) Characterization of excretory metabolites from
14C-Harvade(R) rat balance study. Unpublished report from
Uniroyal Chemica. Submitted to WHO by Uniroyal Chemical Co.,
Inc., Bethany, Connecticut, USA.
Sorg, R.M., Naismith, R.W. & Matthews, R.J. (1983) CHO metaphase
analysis in vitro chromosome aberration analysis in Chinese
hamster ovary cells (CHO). PH320-UN-00183 Harvade(R),
unpublished report from Pharmakon Research International, Inc.,
USA. Submitted to WHO by Uniroyal Chemical Co., Inc., Bethany,
Connecticut, USA.
Squire, R.A. (1984) Unpublished letter to Uniroyal Chemical. Submitted
to WHO by Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
Varner, L.L. & Matthews, R J. (1977) Acute oral LD50 in rats.
Uni-N-252 CND-9801 Lot No. BL-6731. Revised report. Unpublished
report from Pharmakon Laboratories, USA. Submitted to WHO by
Uniroyal Chemical Co., Inc., Bethany, Connecticut, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
