
2-PHENYLPHENOL AND ITS SODIUM SALT
EXPLANATION
The toxicology of 2-phenylphenol (OPP) and its sodium salt (SOPP)
was previously reviewed by the Joint Meetings in 1969 and 1983 (Annex
1, FAO/WHO, 1970a, 1984). A toxicological monograph was prepared by
the Joint Meeting in 1969 (Annex 1, FAO/WHO, 1970b). Just prior to the
1983 Meeting, new information consisting of biochemical, teratogenic,
carcinogenic and mutagenic data became available and was evaluated at
the meeting. A monograph addendum reviewing the recently-submitted
data was not completed, however, at that time.
In 1983, the JMPR agreed that OPP and SOPP should be considered
as equivalent for the purposes of toxicological evaluation and should
be dealt with together since the use of SOPP results in OPP residues
in agricultural commodities. Furthermore, SOPP was established as a
bladder carcinogen in the rat. Although fewer data on OPP were
available, a similar pattern of neoplastic response in rats was noted.
The existing ADI for OPP and SOPP was converted to a temporary ADI and
the safety factor was simultaneously increased from 100 to 5000 to
reflect the concerns of the Meeting. In addition, further information
to permit adequate evaluation in the future was required to be
submitted to the Joint Meeting by 1985. The additional required
toxicological information included:
1. Information on the progress of a multigeneration reproduction
study.
2. Information on the progress of a carcinogenicity/chronic toxicity
study in a rat strain known to be sensitive to induction of
bladder carcinomas (a strain other than F344).
3. Metabolic, pharmacokinetic and other related studies, as
appropriate, in the strains of animals tested in long-term
studies for carcinogenicity.
4. Additional metabolic and pharmacokinetic studies in other species
and strains of animals.
This monograph addendum reviews the data previously submitted in
1983 together with additional information submitted in 1985.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Radioactivity in urine and faeces was monitored for up to 96
hours following single oral doses of 14C-2-phenylphenol or 14C-sodium
2-phenylphenate to groups of 4 male rats at 500 mg/kg b.w.
Approximately 90-95% of the administered radioactivity was recovered
in urine and 5-6% in faeces for both compounds. Most of the
radioactivity was recovered within 24 hours; therefore, both compounds
were nearly completely and rapidly absorbed, metabolized and excreted.
In preliminary experiments, no radioactivity was found in expired
air. Urinary and faecal excretory patterns in groups of four male rats
preconditioned by the feeding of 1.3% 2-phenylphenol or 2.0% sodium
2-phenylphenate in the diet for 2 weeks were essentially unchanged when
14C-2-phenylphenol or 14C-sodium 2-phenylphenate was administered as
before (Reitz et al., 1983).
Biliary excretion of radioactivity, demonstrated in male rats
following a single oral dose of 14C-sodium 2-phenylphenate, suggested
an enterohepatic circulation of metabolites. Lack of retention of
significant amounts of radioactivity in numerous organs and tissues
(including urinary bladder) following single oral doses of
14C-2-phenylphenol or 14C-sodium 2-phenylphenate was demonstrated by
liquid scintillation counting of solubilized organs and tissues and by
whole-body autoradiography (Yamaha et al., 1983).
Biotransformation
Major metabolites identified in the urine of five male and five
female rats fed 2.0% sodium 2-phenylphenate in the diet for 136
days were glucuronide conjugates of 2-phenylphenol and
2,5-dihydroxybiphenyl. Trace amounts of 2,5-diquinonebiphenyl were
also tentatively identified. Unconjugated phenolic metabolites
accounted for only 1% of the phenolic metabolites excreted. No other
metabolites could be found. Recovery during the 24 hours after feeding
was 55% of the dose in males and 40% in females. A sex difference in
the proportions of urinary metabolites was demonstrated. Male rats
excreted 1.8 times as much conjugated 2-phenylphenol and more than 7
times as much conjugated 2,5-dihydroxybiphenyl as did female rats in
24-hour urine samples. There is no explanation as to why the sulfate
ester of 2-phenylphenol was not identified in the urine in this study.
The recovery of only 40-55% of the administered dose in 24-hour
samples suggests that it may have been present but not identified
(Nakao et al., 1983).
Single oral doses of 5, 50 or 500 mg/kg b.w. of 14C-2-
phenylphenol or 14C-sodium 2-phenylphenate were administered to
groups of 4 male rats and urinary metabolites were identified and
quantitated. At 5 and 50 mg/kg b.w., the major metabolites for both
compounds were glucuronide and sulfate ester conjugates of
2-phenylphenol. Unconjugated 2-phenylphenol and 2,5-dihydroxy-biphenyl
accounted for less than 2% of the total radioactivity recovered in the
urine. Nearly identical HPLC chromatograms were obtained for both
compounds. At 500 mg/kg b.w. for both compounds, in addition to
metabolites identified at the lower doses, a new metabolite was
identified. This metabolite was not present at the lower dosage levels
at a detection limit of 1-2% of the total radioactivity in the urine.
The new metabolite, which accounted for 20-30% of the urinary
radioactivity at 500 mg/kg b.w., appeared to be a conjugated
dihydroxybiphenyl molecule, most likely with glucuronide and/or
sulfate groups. The formation of this metabolite appeared to be
markedly dose-dependant.
The authors hypothesized that the new metabolite may be formed
only at high-dosage levels as a result of saturation of normal
glucuronide and sulfate ester conjugation pathways. In an in vitro
experiment, incubation of 14C-2-phenylphenol with purified
microsomes, in the absence of conjugating substrates, yielded
large amounts of a material which co-chromatographed with
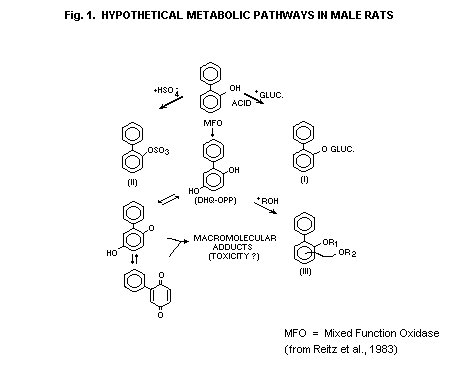
2,5-dihydroxybiphenyl. A hypothetical scheme for the metabolism of
these compounds in male rats, based on identification of metabolic
products and dose-response studies, is presented in Fig. 1. The
semiquinone and quinone were not identified in these studies, but
speculation on their formation was based on similar studies on benzene
(Reitz et al., 1983).
Mature dogs and cats were given single oral doses of sodium
14C-2-phenylphenate (14C-OPP) at dosage levels up to 3 g/kg b.w.
Resulting plasma radioactivity levels were higher in dogs than in
cats. Dogs also metabolized and excreted 3 times more radioactivity in
the urine than cats. Urinary metabolites were unchanged OPP,
glucuronide conjugates, sulfate conjugates and phenol (derived from
cleavage of the phenylphenol bond followed by ring hydroxylation). The
phenol metabolites resulted from both OPP ring moieties (Oehme and
Smith, 1972).
Fig. 1. HYPOTHETICAL METABOLIC PATHWAYS IN MALE RATS
 Weekly urine samples were collected from three mature and three
immature male and female dogs and cats given single oral doses of
3.7 mg of 14C-2-phenylphenol every other day for 25 doses, regardless
of body weight. Average single doses for mature and immature dogs were
0.27 and 2.03 mg/kg b.w., respectively, and for mature and immature
cats, 1.16 and 2.04 mg/kg b.w., respectively. The dominant urinary
excretion product in both species was unchanged 2-phenylphenol, which
accounted for 68-90% of the urinary radioactivity in dogs and 95-98%
in cats. Dogs excreted significantly more glucuronide- and sulfate
ester-conjugated 2-phenylphenol as did cats. Immature dogs excreted
four times as much glucuronide conjugate than did mature dogs. Age
differences for both species did not affect the rate of excretion of
the sulfate ester conjugate (Savides and Oehme, 1980).
Effects on enzymes and other biochemical parameters
In vivo covalent binding to urinary bladder DNA was determined
in pooled samples from 8 male rats dosed with 500 mg/kg b.w. of
14C-2-phenylphenol or 14C-sodium 2-phenylphenate. No increased
radioactivity was observed in DNA isolated and purified from bladders
excised at 16 hours after dosing for either compound. The detection
limit was < 1 alkylation/106 nucleotides. Identical results were
obtained in a repeat experiment (Reitz et al., 1983).
In vitro binding of sodium 2-phenylphenate metabolites to
macromolecules was studied by incubating 14C-sodium 2-phenylphenate
with purified male rat liver microsomes in the presence of a NADPH
regenerating system and bovine serum albumin which served as a
"protein acceptor". Macromolecular binding of radioactivity to
protein, dependent on the presence of both active microsomes and NADP,
was observed. In vivo binding of 2-phenylphenol and sodium
2-phenylphenate metabolites to macromolecules in the liver, kidney and
urinary bladder of male rats was also studied. Groups of 4 animals
were given single oral doses of the 14C-labeled compounds at dosage
levels of 50, 100, 200 or 500 mg/kg b.w. Tissues were excised 16-18
hours later for measurement of macromolecular binding, which was
determined as nanomoles of bound material/mg protein. Levels of
binding were not linearly related to the administered dose.
Disproportionate increases observed in each tissue at dosage levels of
> 200 mg/kg b.w. of sodium 2-phenylphenate and in liver and bladder
at levels of 200-500 mg/kg b.w. of 2-phenylphenol indicated
substantially-increased binding of metabolites to macromolecules at
these dosage levels (Reitz et al., 1984).
SOPP was administered in the diet to 4-week-old male and female
F344 rats at dosage levels of 0 (control) and 2% for 136 days. Urine
was periodically collected. At 136 days, the rats were sacrificed,
blood samples were collected and livers and kidneys were removed.
Cyclic nucleotide levels (c-AMP and c-GMP) were determined in urine,
plasma, liver and kidneys. Adenylate cyclase activity was also assayed
in liver and kidney. In SOPP-treated male rats, c-AMP levels in the
urine and plasma decreased, whereas c-GMP levels increased. In SOPP-
treated female rats, similar changes were not observed except for
decreased c-AMP during the first 3 days of feeding. Levels of c-AMP
and c-GMP in liver and kidney were unchanged. The decrease in urinary
c-AMP in male rats was likely the result of decreased adenylate
cyclase activities in liver and kidney. A similar change in adenylate
cyclase activity was also observed in liver but not in kidneys of
female rats treated with SOPP. The sex-related alterations in cyclic
nucleotide levels were postulated to be related to the sex-related
induction of urinary bladder tumors by dietary SOPP (Nakagawa,
et al., 1984).
Toxicological studies
Special studies on teratogenicity
OPP of 99.7% purity was administered daily by gavage to groups of
18-20 pregnant Wistar rats on days 6-15 of gestation. Dosage levels
were 0, 150, 300 or 600 mg/kg b.w. An additional group of 11 rats was
also dosed at 1200 mg/kg b.w., but this dosage level proved to be
lethal. No untoward signs of toxicity were observed in the control or
150 mg/kg b.w. rats. At 300 mg/kg b.w. and higher, dose-related ataxia
and decreased mean body-weight gains were observed. All surviving rats
were sacrificed on day 20 of gestation and uterine contents were
examined. Foetuses were grossly examined. Skeletal examinations
utilizing the Alizarin red S technique and visceral examinations using
a modified method of Wilson were performed. Mean numbers of
implantation sites, live foetuses, resorptions and foetal weights were
comparable to control numbers for the 150 and 300 mg/kg b.w. groups.
At 600 mg/kg b.w., foetal resorptions were increased and foetal
weights were decreased. Although small numbers of foetal anomalies
were observed in all groups, no relation to the test material was
detected. OPP was negative for teratogenic effects in this study
(Kaneda et al., 1978).
OPP was administered by gavage to groups of 25-27 pregnant rats
on days 6-15 of gestation at daily dosage levels of 100, 300 or
700 mg/kg b.w. A group of 35 pregnant rats served as vehicle controls.
Dams were sacrificed on day 21 and foetuses were removed by Cesarean
section. All foetuses were weighed, sexed and subjected to external
and skeletal examination. The soft tissues of approximately one-third
of the foetuses were examined.
One high-dosage level rat died as a result of a dosing accident.
Pregnant rats given 700 mg/kg b.w. gained significantly less body
weight during the first 4 days of treatment (days 6-9 of gestation)
than did control rats. Food consumption was also significantly
decreased from days 9-11 of gestation at 700 mg/kg b.w. In addition,
liver weights (but not liver/body-weight ratios) were found to be
significantly decreased at necropsy. There was no effect of
2-phenylphenol on the number of implantation sites per dam, mean
litter size, incidences of resorptions, foetal body weights or crown-
rump measurements.
A major malformation (hypoplastic tail and missing sacral and
caudal vertebrae) was observed in a single foetus from the 300 mg/kg
b.w. group. There were no other major malformations observed in any
foetus. An increase in delayed ossification of sternebrae and
unossified sternebrae were observed in the 700 mg/kg b.w. group.
Foramina (small holes) in the skull and bony islands in the skull were
also slightly increased in this group. With the exception of the
single major malformation, all of these effects were considered to be
minor skeletal variants. No adverse effects on embryonal or foetal
development were observed that were considered to be due to
2-phenylphenol. The authors concluded that OPP was not embryotoxic or
teratogenic in rats at dosage levels up to and including 700 mg/kg
b.w. (John et al., 1981).
Special studies on carcinogenicity
Mouse
OPP
A mouse skin-painting study was conducted to determine whether
OPP might be a complete skin carcinogen or possibly a promoter in a
two-stage initiation/promotion process. OPP was dermally applied to
the interscapular area of the backs of 50 male and 50 female Swiss
CD-1 mice at a dosage level of 55.5 mg (in 0.1 ml acetone), 3 times
per week, for 2 years (OPP group). A second group of 50 male and 50
female mice was treated identically except that their backs were
pretreated one time with a dermal dose of 0.05 mg (in 0.1 ml acetone)
of 7,12-dimethylbenz(a)anthracene, or DMBA, a known initiator of skin
carcinogenicity (DMBA/OPP group). Additional groups of 50 male and 50
female mice served as control groups and included an acetone vehicle
control group, treated only with acetone (acetone group); an initiator
control group, treated once with DMBA and thereafter only with acetone
(DMBA/acetone group); and a positive control group, treated once with
DMBA and thereafter with 12-o-tetradecanoylphorbol-13-acetate, or TPA,
a known promoter of skin carcinogenicity (DMBA/TPA group). TPA was
dermally applied at a dosage level of 0.005 mg (in 0.1 ml acetone), 3
times per week, for 2 years.
Mean body weights and survival of the mice treated with OPP or
with DMBA/OPP were generally similar to those of the respective
negative control groups. Survival was substantially decreased in the
DMBA/TPA group. In the DMBA/TPA group, the incidence of neoplastic
skin lesions at the site of application (squamous cell papillomas/
carcinomas, keratocanthomas, basal cell tumours/carcinomas) was
clearly incrased (52/100) over that in the DMBA/acetone control group
(15/100). Time-to-tumour was also substantially decreased in the
DMBA/TPA group. Similar neoplastic skin lesions were also observed in
the DMBA/OPP group (17/100), but at an incidence equivalent to that in
the DMBA/acetone control group (15/100). No neoplastic skin lesions
were observed in the OPP group. The author concluded that there is no
evidence of carcinogenicity in male or female Swiss CD-1 mice
administered OPP alone or as a promoter following initiation with DMBA
(Luster, 1985).
SOPP
SOPP was administered in the diet to groups (50 male and 50
female) of B6C3F1 mice (Charles River, Japan) for 96 weeks at dosage
levels of 0, 0.5, 1.0 or 2.0%. At the end of the 96-week period of
administration, the mice were given the control diet for an additional
8 weeks. Survival rate was slightly decreased only in the high-dose
group male mice.
Decreased body weights were observed in males and females at 2.0%
and in females at 1.0% and at 0.5%. Increased alkaline phosphatase
activity was observed in females at 2.0%, 1.0% and 0.5%. There were no
urinary bladder stones observed in any of the mice. Tumours of the
urinary bladder did not occur in any mice treated with SOPP. Extensive
renal damage due to SOPP was not observed. The authors concluded that
SOPP did not induce an increased incidence of neoplasms (of any kind)
considered to be related to treatment in male or female mice at dosage
levels up to 2.0% when administered in the diet for 96 weeks (Hagiwara
et al., 1984; Ito, 1983a).
Rat
OPP
OPP was administered in the diet to a group of 14 male F344 rats
(Charles-River, Japan) for 32 weeks at a dosage level of 2.0%. No
hyperplasias, papillomas or cancers were observed in any of the
treated rats. Pretreatment of additional rats with 0.05% N-butyl-N-
(4-hydroxybutyl) nitrosamine (BBN) did not significantly increase the
incidence of urinary bladder lesions over that of rats treated with
BBN alone (Fukushima et al., 1983).
OPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. No papillomas or
carcinomas of the urinary bladder were observed, although one
papillary/nodular (PN) hyperplasia was observed (with no stones). Six
rats having small stones were observed to have simple hyperplasia of
the urinary bladder epithelium. Pre-treatment of additional rats with
BBN did not significantly increase the incidence of urinary bladder
lesions over that of rats treated with BBN alone.
In another experiment, OPP was administered in the diet to male F344
rats for up to 12 weeks at dosage levels up to 2.0%. No urinary stones
or tumours were observed in these animals (Ito, 1983b).
OPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 30 and 65 days. Only 7 rats
per group were permitted to live to 90 days, at which time they were
sacrificed.
Kidney pathology was observed in these rats as follows: focal
cortical cysts, significantly decreased urine specific gravity (at 65
and 90 days), small amounts of blood in the urine, focal tubular
collapse and atrophy in the cortex, and cystic degeneration (at 65 and
90 days). No treatment-related urinary bladder lesions were observed
(Reitz et al., 1983).
OPP was administered in the diet to male F344 rats (Charles-
River, Japan) for 91 weeks at dosage levels of 0 (control), 0.625%,
1.25% or 2.5% (2.5% OPP is equivalent to about 4.0% SOPP on a molar
basis). For survival data and non-neoplastic lesions of the urethelial
system, see Table 1.
Table 1. Survival and non-neoplastic lesions of the urothelial system in male rats
administered 2-phenylphenol in the diet
Dose Number Kidney Urinary
(% in of animals Survival (inflammatory bladder
diet) treated (at 91 weeks) lesions) (hyperplasia)
0 24 23/24 (96%) 0/24 (0%)
0.625 20 18/20 (90%) 0/20 (0%)*
1.25 24 17/24 (71%) 3/24 (13%) 0/24 (0%)
2.5 23 15/23 (65%) 23/23 (100%) 7/23 (30%)
* May be 2/20 (10%)(discrepancy in report).
As shown in Table 2, OPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in the experiment at dosage levels greater than 1.25% (Hiraga & Fujii,
1984; Hiraga, 1983a).
SOPP
SOPP was administered in the diet to groups of 9-10 male and 9-10
female F344 rats (Charles-River, Japan) for 13 weeks at dosage levels
up to 4.0% There were no mortalities during the study. Urinary bladder
neoplasms were observed in male and female rats as described below in
Table 3. No bladder calculi were observed in this experiment.
Table 2. Neoplastic lesions of the urothelial system in rats
administered 2-phenylphenol.
Urinary bladder
Dose Papillomas(1) Transitional Total
(% in diet) cell carcinomas (2) neoplasms
0 0/24 0/24 0/24 (0%)
0.625 0/20 0/20 0/20 (0%)
1.25 3/24(3) 20/24(5) 23/24* (96%)
2.5 2/23(4) 2/23(6) 4/23 (17%)(7)
*p < 0.001
(1) first papilloma at 65 weeks (1.25% group)
(2) first carcinoma at 82 weeks (1.25% group)
(3) 1/3 rats had calculi
(4) 2/2 rats had calculi
(5) 16/20 rats had calculi
(6) 1/2 rats had calculi
(7) An additional 5 rats in the 2.5% group had calculi but no
neoplasms and 6 had calculi and only hyperplasia.
In a second experiment, SOPP was administered in the diet to
groups of 20-21 male F344 rats for 91 weeks at dosage levels up to
4.0% (Table 4).
The authors concluded that SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional-cell carcinomas)
in male F344 rats at dosage levels of 1.0% and above when administered
in the diet for 13 weeks or for 91 weeks. In the 91-day study,
transitional cell carcinomas of the kidney were also observed at
dosage levels of > 0.5%. SOPP also induced an increased incidence
of urinary-bladder tumours (papillomas only) in female F344 rats at a
dosage level of 4.0% when administered in the diet for 13 weeks
(Hiraga & Fujii, 1981).
Table 3. Urinary bladder neoplasms in rats administered sodium
2-phenylphenate for 13 weeks
Urinary bladder
Dose Papillomas Transitional cell Total
(% in diet) carcinomas neoplasms
Males
0 0/10 0/10 0/10 (0%)
0.125 0/10 0/10 0/10 (0%)
0.25 0/10 0/10 0/10 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 1/10 0/10 1/10 (10%)
2.0 4/10 5/10 9/10 (90%)
4.0(1) 0/10 1/10 1/10 (10%)
Females
0 0/10 0/10 0/10 (0%)
0.125 0/9 0/9 0/9 (0%)
0.25 0/9 0/9 0/9 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 0/10 0/10 0/10 (0%)
2.0 0/10 0/10 0/10 (0%)
4.0(2) 2/10 0/10 2/10 (20%)
(1) 6 rats in this group had moderate pyelonephritis.
(2) 1 rat in this group had slight pyelonephritis.
Table 4. Neoplastic lesions of the urothelial system in rats administered sodium
2-phenylphenate for 91 weeks
Transitional cell carcinomas
Dose Number of Survival
(% in animals treated (at Urinary Kidney(2) Total(3)
diet) (all males) 91 weeks) bladder(1)
0 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.125 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.25 20 19/20 (95)% 0/20 0/20 0/20 (0%)
0.5 21 18/21 (86%) 0/21 1/21 1/21 (5%)
1.0 21 18/21 (86%) 6/21 1/21 7/21 (33%)
2.0(4) 21 12/21 (57%) 19/21(6) 1/21 20/21 (95%)
4.0(5) 20 14/20 (70%) 8/20 13/20 17/20 (85%)
(1) bladder calculi were observed in 8 of the rats with bladder tumours.
(2) first kidney tumour at 79 weeks.
(3) total number of animals with neoplasms in the urothelial system.
(4) 4 rats in this group had pyelonephritis.
(5) 20 rats in this group had pyelonephritis.
(6) one neoplasm was a carcinosarcoma.
SOPP was administered in the diet to male and female F344 rats
(Charles-River, Japan) for 104 weeks at dosage levels up to 2.0%. The
rats were then given control diet for an additional 2 weeks at which
time they were sacrificed and examined (referred to as the "106-week
study"). For survival data see Table 5, for non-neoplastic lesions
Table 6, and for neoplastic lesions Table 7.
Table 5. Survival rates of rats administered sodium
2-phenylphenate (106-week study)
Dose Number of animals treated Survival
(% in diet) Males Females (at 104 weeks)
0 50 50 >50%
0.5 - 50 >50%
0.7 50 - >50%
1.0 - 50 >50%
2.0 50 - 10/50 (20%)(1)
(1) urinary bladder tumours were largely responsible for the
decreased survival in this group.
Table 6. Non-neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Dose Kidney(1) Urinary bladder
(% in diet) (inflammatory lesions) (hyperplasia)
Males
0 0/50 (0%) 0/50 (0%)
0.7 1/50 (2%) 0/50 (0%)
2.0 5/50 (10%)* 1/50 (2%)
Females
0 0/50 (0%) 0/50 (0%)
0.5 3/50 (6%) 1/50 (2%)
1.0 20/50 (40%)** 4/50 (8%)
p < 0.05
** p < 0.001
(1) kidney lesions other than age-related lesions
(i.e. chronic nephropathy).
Table 7. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Urinary bladder
Dose Papillomas Transitional Total
(% in diet) cell carcinomas neoplasms
Males
0 0/50 0/50 0/50 (0%)
0.7(1) 0/50 2/50 2/50 (4%)
2.0(2) 1/50 46/50 47/50 (94%)*
Females
0 0/50 0/50 0/50 (0%)
0.5 1/50 0/50 1/50 (2%)
1.0 3/50 1/50 4/50 (8%)
* p < 0.001, first tumour (transitional cell carcinoma)
at 40 weeks.
(1) 2/50 (4%) rats had calculi.
(2) 3 kidney tumours were also observed in this group
(1 papilloma and 2 transitional cell carcinomas);
27/50 (54%) rats had calculi.
In this 106-week study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male rats at dosage levels higher than 0.7%. All rats with calculi
had urinary bladder tumours. There is a possibility that the presence
of calculi may exacerbate tumour formation in the urinary bladder.
It also appears that the incidence of urinary bladder tumours may
be inversely related to the incidence of nephritic lesions in male
rats given 2% or 4% SOPP (See the studies described above by Hiraga
and Fujii (1981) for similar results in a 91-week study with SOPP and
by Hiraga and Fujii (1984) for similar results in a 91-week study with
OPP). It is possible that severe lesions in the kidney may somehow
inhibit the formation of bladder tumours in male rats (Hiraga, 1983b).
In a second experiment, SOPP was administered in the diet to male
and female F344 rats for 104 weeks at dosage levels up to 2.0% The
rats were then given control diet until they either died or became
moribund, at which time they were sacrificed (referred to as the
"lifetime study"). For survival data see Table 8.
Table 8. Suvival rates of rats administered sodium
2-phenylphenate (lifetime study)
Dose Number of treated animals Survival
(% in diet) males females (at 104 weeks)
0 25 25 > 50%
0.25 25 25 > 50%
0.5 - 25 > 50%
0.7 25 - > 50%
1.0 - 25 > 50%
2.0 25 - 6/25(25%)(1)
(1) urinary bladder tumours were likely responsible for
the decreased survival in this group (at 104 weeks).
Hyperplasia of the urinary bladder was not observed in any of the
rats in this experiment. Urinary bladder neoplasms were observed in
male and female rats shown in Table 9.
Table 9. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (lifetime study)
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms
Males
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.7 2/25 1/25 3/25 (12%)
2.0(1) 2/25 21/25 23/25 (92%)*
Females
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.5 0/25 0/25 0/25 (0%)
1.0(2) 1/25 1/25 2/25 (8%)
* p < 0.001; first tumour (transitional cell carcinoma)
at 54 weeks.
(1) 8/25 (32%) rats had calculi.
(2) 1/25 (4%) rats had calculi.
In this lifetime study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male F344 rats at dosage levels of > 0.7% and in female F344 rats
at dosage levels of > 0.5% when administered in the diet for 104
weeks. Urinary bladder neoplasms were not observed in either sex at a
dosage level of 0.25% (Table 9). All rats with calculi had urinary
bladder tumours (Hiraga, 1983b).
SOPP was administered in the diet to a group of 29 male F344 rats
(Charles-River, Japan) and to another group of 15 male rats for 32
weeks at a dosage level of 2.0%. Urinary bladder lesions reported were
as in Table 10.
Table 10. Urinary bladder lesions in rats administered sodium 2-phenylphenate
at 2.0% in the diet for 32 weeks
Urinary bladder
Effective Simple PN Total
no. of rats hyperplasia hyperplasia papillomas cancer neoplasms
29 29/29 25/29 5/29 0/29 5/29 (17%)
15 13/15 14/15 2/15 1/15 3/15 (20%)
Pretreatment of additional rats with 0.01% BBN significantly
increased the incidence of simple hyperplasia and papillary/nodular
(PN) hyperplasia, but not of papillomas or cancers over that of rats
treated with BBN alone. Pretreatment of additional rats with 0.05% BBN
significantly increased the incidence of PN hyperplasia, papillomas
and cancers over that of rats treated with BBN alone (Fukushima
et al., 1983).
SOPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. One rat had
small stone formations in the urinary bladder. SOPP induced simple
hyperplasia, PN hyperplasia (19/28, 68%), papillomas (5/28, 18%) and
cancers (6/28, 21%) of the urinary bladder. Pretreatment of additional
rats with BBN increased the incidence of PN hyperplasia (p < 0.05),
papillomas (non-significant), and cancers (non-significant) over that
of rats treated with BBN alone.
In another experiment, SOPP was administered in the diet to male
F344 rats for up to 104 weeks at dosage levels of 0, 0.25%, 0.5%,
1.0%, or 2.0%. Animals from each group were periodically sacrificed
and examined at 4, 8, 12, 24, 36 and 104 weeks. No stone formation was
observed in the urinary bladder of any rats treated with SOPP. In the
2.0% group, simple hyperplasia was observed at 4 weeks and beyond and
PN hyperplasia at 36 weeks and beyond (in all rats at 104 weeks).
Papillomas (2/5, 40%) and cancers (2/5, 40%) of the urinary bladder
were observed in rats administered 2.0% SOPP at 104 weeks. In the 1.0%
group, only simple hyperplasia was observed (> 36 weeks) (Ito,
1983b).
phi-Phenylphenol, o-phenylphenol (OPP), o-phenylphenol sodium
salt tetrahydrate (SOPP), and some biphenyl derivatives were examined
for their bladder carcinogenicity to rats by a short-term assay for
agglutinability of bladder epithelial cells with concanavalin A.
Increased agglutinability was observed after 1-week treatment with
2.0% and 1.0% OPP or 2.0% and 1.0% SOPP, suggesting the bladder
carcinogenicity of these compounds. Such an increase in
agglutinability was not observed in rats fed diets containing
phi-phenylphenol or biphenyl derivatives at 2.0%. An in vivo
carcinogenesis experiment was perfomed with SOPP. As shown in Table
11, bladder carcinomas developed in 14 out of 36 male Fisher rats
(Charles-River, Japan) fed a diet containing 2% SOPP for 50 weeks
(Honma et al., 1983).
Table 11. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms(1)
0 0/11 0/11 0/11 (0%)
2.0(2) 19/36** 14/36* 33/36 (92%)**
* p < 0.05
** p < 0.01
(1) No calculi were observed in any of the treated or control rats.
(2) 31/36 of these rats had PN hykperplasia in the urinary bladder.
In addition, 3/36 and 9/36 of these rats had papillomas and
PN hyperplasia, respectively, in the renal pelvis.
SOPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 14, 30 and 65 days. Only 7
rats per group were permitted to live to 90 days, at which time they
were sacrificed.
Urinary bladder pathology was observed in these rats as follows:
increased mitosis, beginning at 3 days, in the epithelium; thickening
(i.e. simple hyperplasia), beginning at 14 days, in the epithelium. No
tumours of any kind were observed in the urinary bladder (Reitz
et al., 1983).
Special studies on mutagenicity
OPP
Ames tests utilizing S. typhimurium strains TA92, TA1535,
TA100, TA1537, TA94 and TA98, without and with metabolic activation
derived from livers of male Fischer rats, were uniformly negative.
Chromosomal aberration tests using Chinese hamster fibroblasts in
culture gave ambiguous results without and with metabolic activation
(Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA1537, TA98 and
TA100 were negative without and with metabolic activation derived from
male rats. Strain TA1535, however, gave a weakly-positive response
without activation, but a negative response with activation. A mouse
lymphoma assay (L5178Y/TK+/-) was weakly positive without and with
metabolic activation. Chromosomal aberration assays using Chinese
hamster ovary cells, without and with activation, were negative. A
sister chromatid-exchange assay using Chinese hamster ovary cells was
weakly positive without activation, but negative with activation. A
sex-linked recessive-lethal assay in Drosophila melanogaster was
negative (Luster, 1985).
In a dominant-lethal study, OPP of 99.7% purity was administered
by gavage to 10-week-old male C3H mice each day for 5 days at dosage
levels of 0 (control), 100, or 500 mg/kg/day. Fifteen mice were
treated at each dosage level. An additional positive control group of
15 male mice was given a single intraperitoneal dose of 300 mg/kg of
ethylmethanesulfonate (EMS). Immediately following dosing, each male
mouse was mated with 2 untreated virgin female mice for 7 days. At the
end of 7 days, the female mice were replaced by another 2 female mice.
The replacement procedure was repeated for a total of 6 weeks. All
female mice were sacrificed on days 12-13 of gestation and scored for
numbers of corpora lutea, implants, living embryos, and early or late
embryonic deaths. Frequencies of induced dominant-lethal mutations
were calculated. Scores for control and treatment groups were compared
for each 7-day period. For the positive control group, the frequency
of early embryonic deaths was increased in weeks 1, 2, 3, and 6. No
untoward differences from control values were detected for mice
treated with OPP. Dominant-lethal mutations were not induced in this
study by treatment with OPP (Kaneda et al., 1978).
SOPP
Ames tests using S. typhimurium strains TA98 and TA100 were
negative without and with metabolic activation derived from livers of
male Fischer rats. Chromosomal aberration tests using Chinese hamster
fibroblasts in culture gave ambiguous results without and with
metabolic activation (Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA98, TA100,
TA1535, TA1537, and TA1538 were negative without and with metabolic
activation. An unscheduled DNA synthesis assay in primary rat
hepatocytes in culture was negative (Reitz et al., 1983).
Possible metabolites of OPP and SOPP
Two possible metabolites of OPP and SOPP, 2,5-dihydroxybiphenyl
and 2-phenyl-l,4-benzoquinone, were tested in Ames S. typhimurium
strains TA98, TA100 and TA1537. Both compounds gave negative results
without and with metabolic activation derived from livers of male
Fischer rats. Chromosomal aberration tests on both compounds using
Chinese hamster fibroblasts in culture gave negative results without
and with metabolic activation (Ishidate et al., 1983).
COMMENTS
In response to the requirement for further information, a
protocol for a proposed 2-generation reproduction study in rats using
OPP as the test material has been received (Rao, 1985). No information
on the progress of a new carcinogenicity/chronic toxicity study in a
strain of rats other than F344 has been received. Some new metabolic
and pharmacokinetic data have been received, but they are very limited
with respect to elucidating possible biochemical mechanisms of action
of carcinogenicity.
An IARC monograph on OPP and SOPP was published in 1983 (IARC,
1983). This document summarizes chemical and physical data,
production, use and occurence of these chemicals. It also evaluates
biological data, relevant to the oncogenic risk to humans, that was
available prior to 1983.
Recent carcinogenicity studies on sodium 2-phenylphenate (SOPP)
have reported statistically-significant increased incidences of
carcinomas in the urinary bladder, and some in the renal pelvis, of
male F344 rats following administration of 1% and 2% SOPP in the diet.
In female F344 rats, an increased, but lower, incidence of urinary
bladder neoplasms was also reported at a dietary dosage level of 1%.
Similar tumours observed at lower dietary dosage levels (0.5% and
0.7%) in male and female rats, although not statistically significant,
were considered to be biologically relevant owing to the rarity of
these tumours in this rat strain and their absence in control animals.
There does not appear to be a cause-effect relationship between stones
and neoplasms in the urinary bladders of rats. Therefore, SOPP has
been determined to be a carcinogen to the urothelium of F344 rats.
Although fewer carcinogenicity studies on 2-phenylphenol (OPP)
are available than on SOPP, some recent evidence suggest that OPP may
also be a carcinogen on the urinary bladder of male F344 rats at
dietary dosage levels of 1.25% and 2.5%. Another earlier study did
not, however, demonstrate a similar response in male or female Wistar
rats. OPP has not been adequately tested to permit a definitive
conclusion regarding its carcinogenicity.
Carcinogenicity studies in mice with both OPP and SOPP were
negative for tumourigenic effects. The dosage level in the OPP study
may have been too low to permit expression of tumours.
Mutagenicity assays with OPP and SOPP in bacteria have been
overwhelmingly negative, although weakly positive results have been
occasionally noted in isolated instances. Results of studies in
cultured mammalian cells have been mixed. Both negative and weakly
positive responses in several assays have been reported. Results from
in vivo studies have been regularly negative.
A series of in vitro and in vivo biochemical studies suggest
the possibility that an altered metabolic pathway for OPP/SOPP,
occurring at high dosage levels only, may be related to (and
presumably responsible for) the occurrence of urothelial tumours at
similar dosage levels in long-term studies. Although this speculation
appears plausible at this time, considerably more direct experimental
support will be required before it can be accepted as fact.
Teratology studies on rats using OPP as the test material were
negative for teratogenic effects.
With respect to the oncogenic potential of OPP and SOPP, the
Meeting compared results from available oncogenic studies to the low
anticipated dietary exposures to OPP and SOPP. On the basis of this
comparison and other information, the Meeting agreed to extend the
temporary ADI for OPP/SOPP to 1989.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg b.w.
Dog: 500 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1989 or earlier)
1. A multigeneration reproduction study.
2. A carcinogenicity/chronic toxicity study in a rat strain known to
be sensitive to induction of bladder carcinomas (a strain other
than F344).
3. Metabolic, pharmacokinetic, and other related studies, as
appropriate, in the strains of animals tested in long-term
studies for carcinogenicity and in other species, including a
consideration of species, sex and dosage-level differences.
4. Qualitative and quantitative monitoring data on the urinary
excretion of OPP and/or its metabolites by industrial workers, or
others, regularly exposed to OPP or SOPP.
5. Mutagenicity studies on urinary metabolites of OPP and/or SOPP.
DESIRED
Additional observations in man.
REFERENCES
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. Promoting
(1983) effect of sodium o-phenylphenate and o-phenylphenol on two-
stage urinary bladder carcinogenesis in rats. Gann 74,
625-632.
Hagiwara, A., Shibata, M., Hirose, M, Fukushima, S. & Ito, N. Long
(1984) term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Fd. Chem. Toxicol. 22,
809-814.
Hiraga, K. Short report of a 91-week feeding study of
(1983a) orthophenylphenon (OPP) in male rats. Unpublished report
from the Department of Toxicology, Tokyo Metropolitan
Research Laboratory of Public Health, Tokyo, Japan.
Submitted to WHO by Dow Chemical Co., Midland, MI, USA.
Hiraga, K. Carcinogenicity testing of sodium orthophenylphenate in
(1983b) F-344/DuCrj rats. Unpublished report from the Department of
Toxicology, Tokyo Metropolitan Research Laboratory of Public
Health, Tokyo, Japan. Submitted to WHO by Dow Chemical Co.,
Midland, MI, USA.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary system in
(1981) F344 rats by dietary administration of sodium
o-phenylphenate.. Fd. Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary bladder in
(1984) F344 rats by dietary administration of o-phenylphenol.
Fd. Cosmet. Toxicol., 22, 865-870.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T., & Sugimura, T.
(1983) Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
IARC. International Agency for Research on Cancer. IARC Monographs on
(1983) the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Miscellaneous Pesticides, 30, 329-344.
Ishidate, M., Jr., Yoshikawa, K., & Sofuni, T. Mutagenicity tests on
(1983) OPP, OPP-sodium salt, and their possible metabolites.
Unpublished report from the Division of Mutagenesis,
Biological Safety Research Center, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Long-term toxicity and carcinogenicity study of sodium
(1983a) o-phenyl-phenate in B6C3F1 mice, Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Pathological analysis carcinogenicity of the sodium
(1983b) o-phenylphenate in B6C3F1 mice. Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz, B.A. Teratological
(1981) evaluation of othophenylphenol in rats. Fund. Appl.
Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. Teratogenicity and
(1978) dominant-lethal studies with o-phenylphenol. J. Pesticide
Sci., 3, 365-370.
Luster, M. NTP technical report on the toxicology and carcinogenesis
(1985) studies of ortho-phenylphenol (CAS No. 90-43-7) alone with
7,12-dimethyl-benz(a)anthracene (CAS No. 57-97-6) in Swiss
CD-1 mice (dermal studies). NIH Publication No. 85-2557, NTP
84-099, U.S. Department of Health and Human Services (Board
Draft, 2/85, National Toxicology Program, NTP TR 301).
Nakagawa, A., Nakao, T., & Nakao, M. Altered levels of cyclic
(1984) nucleotides in F344 rats fed sodium o-phenylphenate.
Fd. Chem. Toxicol., 22, 217-221.
Nakao, T., Ushiyama, K., Kabashima, J., Nagai, F., Nakagawa, A., Ohno,
T., Ichikawa, H., Kobayashi, H., & Hiraga, K. The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Fd. Chem. Toxicol., 21, 325-329.
Oehme, F.W. & Smith, T.H.F. The metabolism and urinary excretion of
(1972) o-phenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Rao, K.S. Proposed reproduction study protocol, orthophenylphenol:
(1985) Two generation dietary reproduction study in rats. Submitted
to WHO by Dow Chemical Co., Midland, MI, U.S.A.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A., & Watanabe, P.G.
Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem. Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP)
on the urinary tract of male F344 rats. Tox. Appl.
Pharmacol. 73,345-349.
Savides, M.C. & Oehme, F.W. Urinary metabolism or orally administered
(1980) o-phenylphenol in dogs and cats. Toxicology, 17,
355-363.
Yamaha, T., Tanaka, A., Sato, M., & Tsuchiya, T. Excretion,
(1983) distribution and metabolic fate of sodium o-phenylphenol and
o-phenylphenol in the rat. Unpublished report from the
Division of Medical Chemistry, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Weekly urine samples were collected from three mature and three
immature male and female dogs and cats given single oral doses of
3.7 mg of 14C-2-phenylphenol every other day for 25 doses, regardless
of body weight. Average single doses for mature and immature dogs were
0.27 and 2.03 mg/kg b.w., respectively, and for mature and immature
cats, 1.16 and 2.04 mg/kg b.w., respectively. The dominant urinary
excretion product in both species was unchanged 2-phenylphenol, which
accounted for 68-90% of the urinary radioactivity in dogs and 95-98%
in cats. Dogs excreted significantly more glucuronide- and sulfate
ester-conjugated 2-phenylphenol as did cats. Immature dogs excreted
four times as much glucuronide conjugate than did mature dogs. Age
differences for both species did not affect the rate of excretion of
the sulfate ester conjugate (Savides and Oehme, 1980).
Effects on enzymes and other biochemical parameters
In vivo covalent binding to urinary bladder DNA was determined
in pooled samples from 8 male rats dosed with 500 mg/kg b.w. of
14C-2-phenylphenol or 14C-sodium 2-phenylphenate. No increased
radioactivity was observed in DNA isolated and purified from bladders
excised at 16 hours after dosing for either compound. The detection
limit was < 1 alkylation/106 nucleotides. Identical results were
obtained in a repeat experiment (Reitz et al., 1983).
In vitro binding of sodium 2-phenylphenate metabolites to
macromolecules was studied by incubating 14C-sodium 2-phenylphenate
with purified male rat liver microsomes in the presence of a NADPH
regenerating system and bovine serum albumin which served as a
"protein acceptor". Macromolecular binding of radioactivity to
protein, dependent on the presence of both active microsomes and NADP,
was observed. In vivo binding of 2-phenylphenol and sodium
2-phenylphenate metabolites to macromolecules in the liver, kidney and
urinary bladder of male rats was also studied. Groups of 4 animals
were given single oral doses of the 14C-labeled compounds at dosage
levels of 50, 100, 200 or 500 mg/kg b.w. Tissues were excised 16-18
hours later for measurement of macromolecular binding, which was
determined as nanomoles of bound material/mg protein. Levels of
binding were not linearly related to the administered dose.
Disproportionate increases observed in each tissue at dosage levels of
> 200 mg/kg b.w. of sodium 2-phenylphenate and in liver and bladder
at levels of 200-500 mg/kg b.w. of 2-phenylphenol indicated
substantially-increased binding of metabolites to macromolecules at
these dosage levels (Reitz et al., 1984).
SOPP was administered in the diet to 4-week-old male and female
F344 rats at dosage levels of 0 (control) and 2% for 136 days. Urine
was periodically collected. At 136 days, the rats were sacrificed,
blood samples were collected and livers and kidneys were removed.
Cyclic nucleotide levels (c-AMP and c-GMP) were determined in urine,
plasma, liver and kidneys. Adenylate cyclase activity was also assayed
in liver and kidney. In SOPP-treated male rats, c-AMP levels in the
urine and plasma decreased, whereas c-GMP levels increased. In SOPP-
treated female rats, similar changes were not observed except for
decreased c-AMP during the first 3 days of feeding. Levels of c-AMP
and c-GMP in liver and kidney were unchanged. The decrease in urinary
c-AMP in male rats was likely the result of decreased adenylate
cyclase activities in liver and kidney. A similar change in adenylate
cyclase activity was also observed in liver but not in kidneys of
female rats treated with SOPP. The sex-related alterations in cyclic
nucleotide levels were postulated to be related to the sex-related
induction of urinary bladder tumors by dietary SOPP (Nakagawa,
et al., 1984).
Toxicological studies
Special studies on teratogenicity
OPP of 99.7% purity was administered daily by gavage to groups of
18-20 pregnant Wistar rats on days 6-15 of gestation. Dosage levels
were 0, 150, 300 or 600 mg/kg b.w. An additional group of 11 rats was
also dosed at 1200 mg/kg b.w., but this dosage level proved to be
lethal. No untoward signs of toxicity were observed in the control or
150 mg/kg b.w. rats. At 300 mg/kg b.w. and higher, dose-related ataxia
and decreased mean body-weight gains were observed. All surviving rats
were sacrificed on day 20 of gestation and uterine contents were
examined. Foetuses were grossly examined. Skeletal examinations
utilizing the Alizarin red S technique and visceral examinations using
a modified method of Wilson were performed. Mean numbers of
implantation sites, live foetuses, resorptions and foetal weights were
comparable to control numbers for the 150 and 300 mg/kg b.w. groups.
At 600 mg/kg b.w., foetal resorptions were increased and foetal
weights were decreased. Although small numbers of foetal anomalies
were observed in all groups, no relation to the test material was
detected. OPP was negative for teratogenic effects in this study
(Kaneda et al., 1978).
OPP was administered by gavage to groups of 25-27 pregnant rats
on days 6-15 of gestation at daily dosage levels of 100, 300 or
700 mg/kg b.w. A group of 35 pregnant rats served as vehicle controls.
Dams were sacrificed on day 21 and foetuses were removed by Cesarean
section. All foetuses were weighed, sexed and subjected to external
and skeletal examination. The soft tissues of approximately one-third
of the foetuses were examined.
One high-dosage level rat died as a result of a dosing accident.
Pregnant rats given 700 mg/kg b.w. gained significantly less body
weight during the first 4 days of treatment (days 6-9 of gestation)
than did control rats. Food consumption was also significantly
decreased from days 9-11 of gestation at 700 mg/kg b.w. In addition,
liver weights (but not liver/body-weight ratios) were found to be
significantly decreased at necropsy. There was no effect of
2-phenylphenol on the number of implantation sites per dam, mean
litter size, incidences of resorptions, foetal body weights or crown-
rump measurements.
A major malformation (hypoplastic tail and missing sacral and
caudal vertebrae) was observed in a single foetus from the 300 mg/kg
b.w. group. There were no other major malformations observed in any
foetus. An increase in delayed ossification of sternebrae and
unossified sternebrae were observed in the 700 mg/kg b.w. group.
Foramina (small holes) in the skull and bony islands in the skull were
also slightly increased in this group. With the exception of the
single major malformation, all of these effects were considered to be
minor skeletal variants. No adverse effects on embryonal or foetal
development were observed that were considered to be due to
2-phenylphenol. The authors concluded that OPP was not embryotoxic or
teratogenic in rats at dosage levels up to and including 700 mg/kg
b.w. (John et al., 1981).
Special studies on carcinogenicity
Mouse
OPP
A mouse skin-painting study was conducted to determine whether
OPP might be a complete skin carcinogen or possibly a promoter in a
two-stage initiation/promotion process. OPP was dermally applied to
the interscapular area of the backs of 50 male and 50 female Swiss
CD-1 mice at a dosage level of 55.5 mg (in 0.1 ml acetone), 3 times
per week, for 2 years (OPP group). A second group of 50 male and 50
female mice was treated identically except that their backs were
pretreated one time with a dermal dose of 0.05 mg (in 0.1 ml acetone)
of 7,12-dimethylbenz(a)anthracene, or DMBA, a known initiator of skin
carcinogenicity (DMBA/OPP group). Additional groups of 50 male and 50
female mice served as control groups and included an acetone vehicle
control group, treated only with acetone (acetone group); an initiator
control group, treated once with DMBA and thereafter only with acetone
(DMBA/acetone group); and a positive control group, treated once with
DMBA and thereafter with 12-o-tetradecanoylphorbol-13-acetate, or TPA,
a known promoter of skin carcinogenicity (DMBA/TPA group). TPA was
dermally applied at a dosage level of 0.005 mg (in 0.1 ml acetone), 3
times per week, for 2 years.
Mean body weights and survival of the mice treated with OPP or
with DMBA/OPP were generally similar to those of the respective
negative control groups. Survival was substantially decreased in the
DMBA/TPA group. In the DMBA/TPA group, the incidence of neoplastic
skin lesions at the site of application (squamous cell papillomas/
carcinomas, keratocanthomas, basal cell tumours/carcinomas) was
clearly incrased (52/100) over that in the DMBA/acetone control group
(15/100). Time-to-tumour was also substantially decreased in the
DMBA/TPA group. Similar neoplastic skin lesions were also observed in
the DMBA/OPP group (17/100), but at an incidence equivalent to that in
the DMBA/acetone control group (15/100). No neoplastic skin lesions
were observed in the OPP group. The author concluded that there is no
evidence of carcinogenicity in male or female Swiss CD-1 mice
administered OPP alone or as a promoter following initiation with DMBA
(Luster, 1985).
SOPP
SOPP was administered in the diet to groups (50 male and 50
female) of B6C3F1 mice (Charles River, Japan) for 96 weeks at dosage
levels of 0, 0.5, 1.0 or 2.0%. At the end of the 96-week period of
administration, the mice were given the control diet for an additional
8 weeks. Survival rate was slightly decreased only in the high-dose
group male mice.
Decreased body weights were observed in males and females at 2.0%
and in females at 1.0% and at 0.5%. Increased alkaline phosphatase
activity was observed in females at 2.0%, 1.0% and 0.5%. There were no
urinary bladder stones observed in any of the mice. Tumours of the
urinary bladder did not occur in any mice treated with SOPP. Extensive
renal damage due to SOPP was not observed. The authors concluded that
SOPP did not induce an increased incidence of neoplasms (of any kind)
considered to be related to treatment in male or female mice at dosage
levels up to 2.0% when administered in the diet for 96 weeks (Hagiwara
et al., 1984; Ito, 1983a).
Rat
OPP
OPP was administered in the diet to a group of 14 male F344 rats
(Charles-River, Japan) for 32 weeks at a dosage level of 2.0%. No
hyperplasias, papillomas or cancers were observed in any of the
treated rats. Pretreatment of additional rats with 0.05% N-butyl-N-
(4-hydroxybutyl) nitrosamine (BBN) did not significantly increase the
incidence of urinary bladder lesions over that of rats treated with
BBN alone (Fukushima et al., 1983).
OPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. No papillomas or
carcinomas of the urinary bladder were observed, although one
papillary/nodular (PN) hyperplasia was observed (with no stones). Six
rats having small stones were observed to have simple hyperplasia of
the urinary bladder epithelium. Pre-treatment of additional rats with
BBN did not significantly increase the incidence of urinary bladder
lesions over that of rats treated with BBN alone.
In another experiment, OPP was administered in the diet to male F344
rats for up to 12 weeks at dosage levels up to 2.0%. No urinary stones
or tumours were observed in these animals (Ito, 1983b).
OPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 30 and 65 days. Only 7 rats
per group were permitted to live to 90 days, at which time they were
sacrificed.
Kidney pathology was observed in these rats as follows: focal
cortical cysts, significantly decreased urine specific gravity (at 65
and 90 days), small amounts of blood in the urine, focal tubular
collapse and atrophy in the cortex, and cystic degeneration (at 65 and
90 days). No treatment-related urinary bladder lesions were observed
(Reitz et al., 1983).
OPP was administered in the diet to male F344 rats (Charles-
River, Japan) for 91 weeks at dosage levels of 0 (control), 0.625%,
1.25% or 2.5% (2.5% OPP is equivalent to about 4.0% SOPP on a molar
basis). For survival data and non-neoplastic lesions of the urethelial
system, see Table 1.
Table 1. Survival and non-neoplastic lesions of the urothelial system in male rats
administered 2-phenylphenol in the diet
Dose Number Kidney Urinary
(% in of animals Survival (inflammatory bladder
diet) treated (at 91 weeks) lesions) (hyperplasia)
0 24 23/24 (96%) 0/24 (0%)
0.625 20 18/20 (90%) 0/20 (0%)*
1.25 24 17/24 (71%) 3/24 (13%) 0/24 (0%)
2.5 23 15/23 (65%) 23/23 (100%) 7/23 (30%)
* May be 2/20 (10%)(discrepancy in report).
As shown in Table 2, OPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in the experiment at dosage levels greater than 1.25% (Hiraga & Fujii,
1984; Hiraga, 1983a).
SOPP
SOPP was administered in the diet to groups of 9-10 male and 9-10
female F344 rats (Charles-River, Japan) for 13 weeks at dosage levels
up to 4.0% There were no mortalities during the study. Urinary bladder
neoplasms were observed in male and female rats as described below in
Table 3. No bladder calculi were observed in this experiment.
Table 2. Neoplastic lesions of the urothelial system in rats
administered 2-phenylphenol.
Urinary bladder
Dose Papillomas(1) Transitional Total
(% in diet) cell carcinomas (2) neoplasms
0 0/24 0/24 0/24 (0%)
0.625 0/20 0/20 0/20 (0%)
1.25 3/24(3) 20/24(5) 23/24* (96%)
2.5 2/23(4) 2/23(6) 4/23 (17%)(7)
*p < 0.001
(1) first papilloma at 65 weeks (1.25% group)
(2) first carcinoma at 82 weeks (1.25% group)
(3) 1/3 rats had calculi
(4) 2/2 rats had calculi
(5) 16/20 rats had calculi
(6) 1/2 rats had calculi
(7) An additional 5 rats in the 2.5% group had calculi but no
neoplasms and 6 had calculi and only hyperplasia.
In a second experiment, SOPP was administered in the diet to
groups of 20-21 male F344 rats for 91 weeks at dosage levels up to
4.0% (Table 4).
The authors concluded that SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional-cell carcinomas)
in male F344 rats at dosage levels of 1.0% and above when administered
in the diet for 13 weeks or for 91 weeks. In the 91-day study,
transitional cell carcinomas of the kidney were also observed at
dosage levels of > 0.5%. SOPP also induced an increased incidence
of urinary-bladder tumours (papillomas only) in female F344 rats at a
dosage level of 4.0% when administered in the diet for 13 weeks
(Hiraga & Fujii, 1981).
Table 3. Urinary bladder neoplasms in rats administered sodium
2-phenylphenate for 13 weeks
Urinary bladder
Dose Papillomas Transitional cell Total
(% in diet) carcinomas neoplasms
Males
0 0/10 0/10 0/10 (0%)
0.125 0/10 0/10 0/10 (0%)
0.25 0/10 0/10 0/10 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 1/10 0/10 1/10 (10%)
2.0 4/10 5/10 9/10 (90%)
4.0(1) 0/10 1/10 1/10 (10%)
Females
0 0/10 0/10 0/10 (0%)
0.125 0/9 0/9 0/9 (0%)
0.25 0/9 0/9 0/9 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 0/10 0/10 0/10 (0%)
2.0 0/10 0/10 0/10 (0%)
4.0(2) 2/10 0/10 2/10 (20%)
(1) 6 rats in this group had moderate pyelonephritis.
(2) 1 rat in this group had slight pyelonephritis.
Table 4. Neoplastic lesions of the urothelial system in rats administered sodium
2-phenylphenate for 91 weeks
Transitional cell carcinomas
Dose Number of Survival
(% in animals treated (at Urinary Kidney(2) Total(3)
diet) (all males) 91 weeks) bladder(1)
0 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.125 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.25 20 19/20 (95)% 0/20 0/20 0/20 (0%)
0.5 21 18/21 (86%) 0/21 1/21 1/21 (5%)
1.0 21 18/21 (86%) 6/21 1/21 7/21 (33%)
2.0(4) 21 12/21 (57%) 19/21(6) 1/21 20/21 (95%)
4.0(5) 20 14/20 (70%) 8/20 13/20 17/20 (85%)
(1) bladder calculi were observed in 8 of the rats with bladder tumours.
(2) first kidney tumour at 79 weeks.
(3) total number of animals with neoplasms in the urothelial system.
(4) 4 rats in this group had pyelonephritis.
(5) 20 rats in this group had pyelonephritis.
(6) one neoplasm was a carcinosarcoma.
SOPP was administered in the diet to male and female F344 rats
(Charles-River, Japan) for 104 weeks at dosage levels up to 2.0%. The
rats were then given control diet for an additional 2 weeks at which
time they were sacrificed and examined (referred to as the "106-week
study"). For survival data see Table 5, for non-neoplastic lesions
Table 6, and for neoplastic lesions Table 7.
Table 5. Survival rates of rats administered sodium
2-phenylphenate (106-week study)
Dose Number of animals treated Survival
(% in diet) Males Females (at 104 weeks)
0 50 50 >50%
0.5 - 50 >50%
0.7 50 - >50%
1.0 - 50 >50%
2.0 50 - 10/50 (20%)(1)
(1) urinary bladder tumours were largely responsible for the
decreased survival in this group.
Table 6. Non-neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Dose Kidney(1) Urinary bladder
(% in diet) (inflammatory lesions) (hyperplasia)
Males
0 0/50 (0%) 0/50 (0%)
0.7 1/50 (2%) 0/50 (0%)
2.0 5/50 (10%)* 1/50 (2%)
Females
0 0/50 (0%) 0/50 (0%)
0.5 3/50 (6%) 1/50 (2%)
1.0 20/50 (40%)** 4/50 (8%)
p < 0.05
** p < 0.001
(1) kidney lesions other than age-related lesions
(i.e. chronic nephropathy).
Table 7. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Urinary bladder
Dose Papillomas Transitional Total
(% in diet) cell carcinomas neoplasms
Males
0 0/50 0/50 0/50 (0%)
0.7(1) 0/50 2/50 2/50 (4%)
2.0(2) 1/50 46/50 47/50 (94%)*
Females
0 0/50 0/50 0/50 (0%)
0.5 1/50 0/50 1/50 (2%)
1.0 3/50 1/50 4/50 (8%)
* p < 0.001, first tumour (transitional cell carcinoma)
at 40 weeks.
(1) 2/50 (4%) rats had calculi.
(2) 3 kidney tumours were also observed in this group
(1 papilloma and 2 transitional cell carcinomas);
27/50 (54%) rats had calculi.
In this 106-week study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male rats at dosage levels higher than 0.7%. All rats with calculi
had urinary bladder tumours. There is a possibility that the presence
of calculi may exacerbate tumour formation in the urinary bladder.
It also appears that the incidence of urinary bladder tumours may
be inversely related to the incidence of nephritic lesions in male
rats given 2% or 4% SOPP (See the studies described above by Hiraga
and Fujii (1981) for similar results in a 91-week study with SOPP and
by Hiraga and Fujii (1984) for similar results in a 91-week study with
OPP). It is possible that severe lesions in the kidney may somehow
inhibit the formation of bladder tumours in male rats (Hiraga, 1983b).
In a second experiment, SOPP was administered in the diet to male
and female F344 rats for 104 weeks at dosage levels up to 2.0% The
rats were then given control diet until they either died or became
moribund, at which time they were sacrificed (referred to as the
"lifetime study"). For survival data see Table 8.
Table 8. Suvival rates of rats administered sodium
2-phenylphenate (lifetime study)
Dose Number of treated animals Survival
(% in diet) males females (at 104 weeks)
0 25 25 > 50%
0.25 25 25 > 50%
0.5 - 25 > 50%
0.7 25 - > 50%
1.0 - 25 > 50%
2.0 25 - 6/25(25%)(1)
(1) urinary bladder tumours were likely responsible for
the decreased survival in this group (at 104 weeks).
Hyperplasia of the urinary bladder was not observed in any of the
rats in this experiment. Urinary bladder neoplasms were observed in
male and female rats shown in Table 9.
Table 9. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (lifetime study)
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms
Males
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.7 2/25 1/25 3/25 (12%)
2.0(1) 2/25 21/25 23/25 (92%)*
Females
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.5 0/25 0/25 0/25 (0%)
1.0(2) 1/25 1/25 2/25 (8%)
* p < 0.001; first tumour (transitional cell carcinoma)
at 54 weeks.
(1) 8/25 (32%) rats had calculi.
(2) 1/25 (4%) rats had calculi.
In this lifetime study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male F344 rats at dosage levels of > 0.7% and in female F344 rats
at dosage levels of > 0.5% when administered in the diet for 104
weeks. Urinary bladder neoplasms were not observed in either sex at a
dosage level of 0.25% (Table 9). All rats with calculi had urinary
bladder tumours (Hiraga, 1983b).
SOPP was administered in the diet to a group of 29 male F344 rats
(Charles-River, Japan) and to another group of 15 male rats for 32
weeks at a dosage level of 2.0%. Urinary bladder lesions reported were
as in Table 10.
Table 10. Urinary bladder lesions in rats administered sodium 2-phenylphenate
at 2.0% in the diet for 32 weeks
Urinary bladder
Effective Simple PN Total
no. of rats hyperplasia hyperplasia papillomas cancer neoplasms
29 29/29 25/29 5/29 0/29 5/29 (17%)
15 13/15 14/15 2/15 1/15 3/15 (20%)
Pretreatment of additional rats with 0.01% BBN significantly
increased the incidence of simple hyperplasia and papillary/nodular
(PN) hyperplasia, but not of papillomas or cancers over that of rats
treated with BBN alone. Pretreatment of additional rats with 0.05% BBN
significantly increased the incidence of PN hyperplasia, papillomas
and cancers over that of rats treated with BBN alone (Fukushima
et al., 1983).
SOPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. One rat had
small stone formations in the urinary bladder. SOPP induced simple
hyperplasia, PN hyperplasia (19/28, 68%), papillomas (5/28, 18%) and
cancers (6/28, 21%) of the urinary bladder. Pretreatment of additional
rats with BBN increased the incidence of PN hyperplasia (p < 0.05),
papillomas (non-significant), and cancers (non-significant) over that
of rats treated with BBN alone.
In another experiment, SOPP was administered in the diet to male
F344 rats for up to 104 weeks at dosage levels of 0, 0.25%, 0.5%,
1.0%, or 2.0%. Animals from each group were periodically sacrificed
and examined at 4, 8, 12, 24, 36 and 104 weeks. No stone formation was
observed in the urinary bladder of any rats treated with SOPP. In the
2.0% group, simple hyperplasia was observed at 4 weeks and beyond and
PN hyperplasia at 36 weeks and beyond (in all rats at 104 weeks).
Papillomas (2/5, 40%) and cancers (2/5, 40%) of the urinary bladder
were observed in rats administered 2.0% SOPP at 104 weeks. In the 1.0%
group, only simple hyperplasia was observed (> 36 weeks) (Ito,
1983b).
phi-Phenylphenol, o-phenylphenol (OPP), o-phenylphenol sodium
salt tetrahydrate (SOPP), and some biphenyl derivatives were examined
for their bladder carcinogenicity to rats by a short-term assay for
agglutinability of bladder epithelial cells with concanavalin A.
Increased agglutinability was observed after 1-week treatment with
2.0% and 1.0% OPP or 2.0% and 1.0% SOPP, suggesting the bladder
carcinogenicity of these compounds. Such an increase in
agglutinability was not observed in rats fed diets containing
phi-phenylphenol or biphenyl derivatives at 2.0%. An in vivo
carcinogenesis experiment was perfomed with SOPP. As shown in Table
11, bladder carcinomas developed in 14 out of 36 male Fisher rats
(Charles-River, Japan) fed a diet containing 2% SOPP for 50 weeks
(Honma et al., 1983).
Table 11. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms(1)
0 0/11 0/11 0/11 (0%)
2.0(2) 19/36** 14/36* 33/36 (92%)**
* p < 0.05
** p < 0.01
(1) No calculi were observed in any of the treated or control rats.
(2) 31/36 of these rats had PN hykperplasia in the urinary bladder.
In addition, 3/36 and 9/36 of these rats had papillomas and
PN hyperplasia, respectively, in the renal pelvis.
SOPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 14, 30 and 65 days. Only 7
rats per group were permitted to live to 90 days, at which time they
were sacrificed.
Urinary bladder pathology was observed in these rats as follows:
increased mitosis, beginning at 3 days, in the epithelium; thickening
(i.e. simple hyperplasia), beginning at 14 days, in the epithelium. No
tumours of any kind were observed in the urinary bladder (Reitz
et al., 1983).
Special studies on mutagenicity
OPP
Ames tests utilizing S. typhimurium strains TA92, TA1535,
TA100, TA1537, TA94 and TA98, without and with metabolic activation
derived from livers of male Fischer rats, were uniformly negative.
Chromosomal aberration tests using Chinese hamster fibroblasts in
culture gave ambiguous results without and with metabolic activation
(Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA1537, TA98 and
TA100 were negative without and with metabolic activation derived from
male rats. Strain TA1535, however, gave a weakly-positive response
without activation, but a negative response with activation. A mouse
lymphoma assay (L5178Y/TK+/-) was weakly positive without and with
metabolic activation. Chromosomal aberration assays using Chinese
hamster ovary cells, without and with activation, were negative. A
sister chromatid-exchange assay using Chinese hamster ovary cells was
weakly positive without activation, but negative with activation. A
sex-linked recessive-lethal assay in Drosophila melanogaster was
negative (Luster, 1985).
In a dominant-lethal study, OPP of 99.7% purity was administered
by gavage to 10-week-old male C3H mice each day for 5 days at dosage
levels of 0 (control), 100, or 500 mg/kg/day. Fifteen mice were
treated at each dosage level. An additional positive control group of
15 male mice was given a single intraperitoneal dose of 300 mg/kg of
ethylmethanesulfonate (EMS). Immediately following dosing, each male
mouse was mated with 2 untreated virgin female mice for 7 days. At the
end of 7 days, the female mice were replaced by another 2 female mice.
The replacement procedure was repeated for a total of 6 weeks. All
female mice were sacrificed on days 12-13 of gestation and scored for
numbers of corpora lutea, implants, living embryos, and early or late
embryonic deaths. Frequencies of induced dominant-lethal mutations
were calculated. Scores for control and treatment groups were compared
for each 7-day period. For the positive control group, the frequency
of early embryonic deaths was increased in weeks 1, 2, 3, and 6. No
untoward differences from control values were detected for mice
treated with OPP. Dominant-lethal mutations were not induced in this
study by treatment with OPP (Kaneda et al., 1978).
SOPP
Ames tests using S. typhimurium strains TA98 and TA100 were
negative without and with metabolic activation derived from livers of
male Fischer rats. Chromosomal aberration tests using Chinese hamster
fibroblasts in culture gave ambiguous results without and with
metabolic activation (Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA98, TA100,
TA1535, TA1537, and TA1538 were negative without and with metabolic
activation. An unscheduled DNA synthesis assay in primary rat
hepatocytes in culture was negative (Reitz et al., 1983).
Possible metabolites of OPP and SOPP
Two possible metabolites of OPP and SOPP, 2,5-dihydroxybiphenyl
and 2-phenyl-l,4-benzoquinone, were tested in Ames S. typhimurium
strains TA98, TA100 and TA1537. Both compounds gave negative results
without and with metabolic activation derived from livers of male
Fischer rats. Chromosomal aberration tests on both compounds using
Chinese hamster fibroblasts in culture gave negative results without
and with metabolic activation (Ishidate et al., 1983).
COMMENTS
In response to the requirement for further information, a
protocol for a proposed 2-generation reproduction study in rats using
OPP as the test material has been received (Rao, 1985). No information
on the progress of a new carcinogenicity/chronic toxicity study in a
strain of rats other than F344 has been received. Some new metabolic
and pharmacokinetic data have been received, but they are very limited
with respect to elucidating possible biochemical mechanisms of action
of carcinogenicity.
An IARC monograph on OPP and SOPP was published in 1983 (IARC,
1983). This document summarizes chemical and physical data,
production, use and occurence of these chemicals. It also evaluates
biological data, relevant to the oncogenic risk to humans, that was
available prior to 1983.
Recent carcinogenicity studies on sodium 2-phenylphenate (SOPP)
have reported statistically-significant increased incidences of
carcinomas in the urinary bladder, and some in the renal pelvis, of
male F344 rats following administration of 1% and 2% SOPP in the diet.
In female F344 rats, an increased, but lower, incidence of urinary
bladder neoplasms was also reported at a dietary dosage level of 1%.
Similar tumours observed at lower dietary dosage levels (0.5% and
0.7%) in male and female rats, although not statistically significant,
were considered to be biologically relevant owing to the rarity of
these tumours in this rat strain and their absence in control animals.
There does not appear to be a cause-effect relationship between stones
and neoplasms in the urinary bladders of rats. Therefore, SOPP has
been determined to be a carcinogen to the urothelium of F344 rats.
Although fewer carcinogenicity studies on 2-phenylphenol (OPP)
are available than on SOPP, some recent evidence suggest that OPP may
also be a carcinogen on the urinary bladder of male F344 rats at
dietary dosage levels of 1.25% and 2.5%. Another earlier study did
not, however, demonstrate a similar response in male or female Wistar
rats. OPP has not been adequately tested to permit a definitive
conclusion regarding its carcinogenicity.
Carcinogenicity studies in mice with both OPP and SOPP were
negative for tumourigenic effects. The dosage level in the OPP study
may have been too low to permit expression of tumours.
Mutagenicity assays with OPP and SOPP in bacteria have been
overwhelmingly negative, although weakly positive results have been
occasionally noted in isolated instances. Results of studies in
cultured mammalian cells have been mixed. Both negative and weakly
positive responses in several assays have been reported. Results from
in vivo studies have been regularly negative.
A series of in vitro and in vivo biochemical studies suggest
the possibility that an altered metabolic pathway for OPP/SOPP,
occurring at high dosage levels only, may be related to (and
presumably responsible for) the occurrence of urothelial tumours at
similar dosage levels in long-term studies. Although this speculation
appears plausible at this time, considerably more direct experimental
support will be required before it can be accepted as fact.
Teratology studies on rats using OPP as the test material were
negative for teratogenic effects.
With respect to the oncogenic potential of OPP and SOPP, the
Meeting compared results from available oncogenic studies to the low
anticipated dietary exposures to OPP and SOPP. On the basis of this
comparison and other information, the Meeting agreed to extend the
temporary ADI for OPP/SOPP to 1989.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg b.w.
Dog: 500 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1989 or earlier)
1. A multigeneration reproduction study.
2. A carcinogenicity/chronic toxicity study in a rat strain known to
be sensitive to induction of bladder carcinomas (a strain other
than F344).
3. Metabolic, pharmacokinetic, and other related studies, as
appropriate, in the strains of animals tested in long-term
studies for carcinogenicity and in other species, including a
consideration of species, sex and dosage-level differences.
4. Qualitative and quantitative monitoring data on the urinary
excretion of OPP and/or its metabolites by industrial workers, or
others, regularly exposed to OPP or SOPP.
5. Mutagenicity studies on urinary metabolites of OPP and/or SOPP.
DESIRED
Additional observations in man.
REFERENCES
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. Promoting
(1983) effect of sodium o-phenylphenate and o-phenylphenol on two-
stage urinary bladder carcinogenesis in rats. Gann 74,
625-632.
Hagiwara, A., Shibata, M., Hirose, M, Fukushima, S. & Ito, N. Long
(1984) term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Fd. Chem. Toxicol. 22,
809-814.
Hiraga, K. Short report of a 91-week feeding study of
(1983a) orthophenylphenon (OPP) in male rats. Unpublished report
from the Department of Toxicology, Tokyo Metropolitan
Research Laboratory of Public Health, Tokyo, Japan.
Submitted to WHO by Dow Chemical Co., Midland, MI, USA.
Hiraga, K. Carcinogenicity testing of sodium orthophenylphenate in
(1983b) F-344/DuCrj rats. Unpublished report from the Department of
Toxicology, Tokyo Metropolitan Research Laboratory of Public
Health, Tokyo, Japan. Submitted to WHO by Dow Chemical Co.,
Midland, MI, USA.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary system in
(1981) F344 rats by dietary administration of sodium
o-phenylphenate.. Fd. Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary bladder in
(1984) F344 rats by dietary administration of o-phenylphenol.
Fd. Cosmet. Toxicol., 22, 865-870.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T., & Sugimura, T.
(1983) Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
IARC. International Agency for Research on Cancer. IARC Monographs on
(1983) the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Miscellaneous Pesticides, 30, 329-344.
Ishidate, M., Jr., Yoshikawa, K., & Sofuni, T. Mutagenicity tests on
(1983) OPP, OPP-sodium salt, and their possible metabolites.
Unpublished report from the Division of Mutagenesis,
Biological Safety Research Center, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Long-term toxicity and carcinogenicity study of sodium
(1983a) o-phenyl-phenate in B6C3F1 mice, Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Pathological analysis carcinogenicity of the sodium
(1983b) o-phenylphenate in B6C3F1 mice. Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz, B.A. Teratological
(1981) evaluation of othophenylphenol in rats. Fund. Appl.
Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. Teratogenicity and
(1978) dominant-lethal studies with o-phenylphenol. J. Pesticide
Sci., 3, 365-370.
Luster, M. NTP technical report on the toxicology and carcinogenesis
(1985) studies of ortho-phenylphenol (CAS No. 90-43-7) alone with
7,12-dimethyl-benz(a)anthracene (CAS No. 57-97-6) in Swiss
CD-1 mice (dermal studies). NIH Publication No. 85-2557, NTP
84-099, U.S. Department of Health and Human Services (Board
Draft, 2/85, National Toxicology Program, NTP TR 301).
Nakagawa, A., Nakao, T., & Nakao, M. Altered levels of cyclic
(1984) nucleotides in F344 rats fed sodium o-phenylphenate.
Fd. Chem. Toxicol., 22, 217-221.
Nakao, T., Ushiyama, K., Kabashima, J., Nagai, F., Nakagawa, A., Ohno,
T., Ichikawa, H., Kobayashi, H., & Hiraga, K. The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Fd. Chem. Toxicol., 21, 325-329.
Oehme, F.W. & Smith, T.H.F. The metabolism and urinary excretion of
(1972) o-phenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Rao, K.S. Proposed reproduction study protocol, orthophenylphenol:
(1985) Two generation dietary reproduction study in rats. Submitted
to WHO by Dow Chemical Co., Midland, MI, U.S.A.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A., & Watanabe, P.G.
Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem. Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP)
on the urinary tract of male F344 rats. Tox. Appl.
Pharmacol. 73,345-349.
Savides, M.C. & Oehme, F.W. Urinary metabolism or orally administered
(1980) o-phenylphenol in dogs and cats. Toxicology, 17,
355-363.
Yamaha, T., Tanaka, A., Sato, M., & Tsuchiya, T. Excretion,
(1983) distribution and metabolic fate of sodium o-phenylphenol and
o-phenylphenol in the rat. Unpublished report from the
Division of Medical Chemistry, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
 Weekly urine samples were collected from three mature and three
immature male and female dogs and cats given single oral doses of
3.7 mg of 14C-2-phenylphenol every other day for 25 doses, regardless
of body weight. Average single doses for mature and immature dogs were
0.27 and 2.03 mg/kg b.w., respectively, and for mature and immature
cats, 1.16 and 2.04 mg/kg b.w., respectively. The dominant urinary
excretion product in both species was unchanged 2-phenylphenol, which
accounted for 68-90% of the urinary radioactivity in dogs and 95-98%
in cats. Dogs excreted significantly more glucuronide- and sulfate
ester-conjugated 2-phenylphenol as did cats. Immature dogs excreted
four times as much glucuronide conjugate than did mature dogs. Age
differences for both species did not affect the rate of excretion of
the sulfate ester conjugate (Savides and Oehme, 1980).
Effects on enzymes and other biochemical parameters
In vivo covalent binding to urinary bladder DNA was determined
in pooled samples from 8 male rats dosed with 500 mg/kg b.w. of
14C-2-phenylphenol or 14C-sodium 2-phenylphenate. No increased
radioactivity was observed in DNA isolated and purified from bladders
excised at 16 hours after dosing for either compound. The detection
limit was < 1 alkylation/106 nucleotides. Identical results were
obtained in a repeat experiment (Reitz et al., 1983).
In vitro binding of sodium 2-phenylphenate metabolites to
macromolecules was studied by incubating 14C-sodium 2-phenylphenate
with purified male rat liver microsomes in the presence of a NADPH
regenerating system and bovine serum albumin which served as a
"protein acceptor". Macromolecular binding of radioactivity to
protein, dependent on the presence of both active microsomes and NADP,
was observed. In vivo binding of 2-phenylphenol and sodium
2-phenylphenate metabolites to macromolecules in the liver, kidney and
urinary bladder of male rats was also studied. Groups of 4 animals
were given single oral doses of the 14C-labeled compounds at dosage
levels of 50, 100, 200 or 500 mg/kg b.w. Tissues were excised 16-18
hours later for measurement of macromolecular binding, which was
determined as nanomoles of bound material/mg protein. Levels of
binding were not linearly related to the administered dose.
Disproportionate increases observed in each tissue at dosage levels of
> 200 mg/kg b.w. of sodium 2-phenylphenate and in liver and bladder
at levels of 200-500 mg/kg b.w. of 2-phenylphenol indicated
substantially-increased binding of metabolites to macromolecules at
these dosage levels (Reitz et al., 1984).
SOPP was administered in the diet to 4-week-old male and female
F344 rats at dosage levels of 0 (control) and 2% for 136 days. Urine
was periodically collected. At 136 days, the rats were sacrificed,
blood samples were collected and livers and kidneys were removed.
Cyclic nucleotide levels (c-AMP and c-GMP) were determined in urine,
plasma, liver and kidneys. Adenylate cyclase activity was also assayed
in liver and kidney. In SOPP-treated male rats, c-AMP levels in the
urine and plasma decreased, whereas c-GMP levels increased. In SOPP-
treated female rats, similar changes were not observed except for
decreased c-AMP during the first 3 days of feeding. Levels of c-AMP
and c-GMP in liver and kidney were unchanged. The decrease in urinary
c-AMP in male rats was likely the result of decreased adenylate
cyclase activities in liver and kidney. A similar change in adenylate
cyclase activity was also observed in liver but not in kidneys of
female rats treated with SOPP. The sex-related alterations in cyclic
nucleotide levels were postulated to be related to the sex-related
induction of urinary bladder tumors by dietary SOPP (Nakagawa,
et al., 1984).
Toxicological studies
Special studies on teratogenicity
OPP of 99.7% purity was administered daily by gavage to groups of
18-20 pregnant Wistar rats on days 6-15 of gestation. Dosage levels
were 0, 150, 300 or 600 mg/kg b.w. An additional group of 11 rats was
also dosed at 1200 mg/kg b.w., but this dosage level proved to be
lethal. No untoward signs of toxicity were observed in the control or
150 mg/kg b.w. rats. At 300 mg/kg b.w. and higher, dose-related ataxia
and decreased mean body-weight gains were observed. All surviving rats
were sacrificed on day 20 of gestation and uterine contents were
examined. Foetuses were grossly examined. Skeletal examinations
utilizing the Alizarin red S technique and visceral examinations using
a modified method of Wilson were performed. Mean numbers of
implantation sites, live foetuses, resorptions and foetal weights were
comparable to control numbers for the 150 and 300 mg/kg b.w. groups.
At 600 mg/kg b.w., foetal resorptions were increased and foetal
weights were decreased. Although small numbers of foetal anomalies
were observed in all groups, no relation to the test material was
detected. OPP was negative for teratogenic effects in this study
(Kaneda et al., 1978).
OPP was administered by gavage to groups of 25-27 pregnant rats
on days 6-15 of gestation at daily dosage levels of 100, 300 or
700 mg/kg b.w. A group of 35 pregnant rats served as vehicle controls.
Dams were sacrificed on day 21 and foetuses were removed by Cesarean
section. All foetuses were weighed, sexed and subjected to external
and skeletal examination. The soft tissues of approximately one-third
of the foetuses were examined.
One high-dosage level rat died as a result of a dosing accident.
Pregnant rats given 700 mg/kg b.w. gained significantly less body
weight during the first 4 days of treatment (days 6-9 of gestation)
than did control rats. Food consumption was also significantly
decreased from days 9-11 of gestation at 700 mg/kg b.w. In addition,
liver weights (but not liver/body-weight ratios) were found to be
significantly decreased at necropsy. There was no effect of
2-phenylphenol on the number of implantation sites per dam, mean
litter size, incidences of resorptions, foetal body weights or crown-
rump measurements.
A major malformation (hypoplastic tail and missing sacral and
caudal vertebrae) was observed in a single foetus from the 300 mg/kg
b.w. group. There were no other major malformations observed in any
foetus. An increase in delayed ossification of sternebrae and
unossified sternebrae were observed in the 700 mg/kg b.w. group.
Foramina (small holes) in the skull and bony islands in the skull were
also slightly increased in this group. With the exception of the
single major malformation, all of these effects were considered to be
minor skeletal variants. No adverse effects on embryonal or foetal
development were observed that were considered to be due to
2-phenylphenol. The authors concluded that OPP was not embryotoxic or
teratogenic in rats at dosage levels up to and including 700 mg/kg
b.w. (John et al., 1981).
Special studies on carcinogenicity
Mouse
OPP
A mouse skin-painting study was conducted to determine whether
OPP might be a complete skin carcinogen or possibly a promoter in a
two-stage initiation/promotion process. OPP was dermally applied to
the interscapular area of the backs of 50 male and 50 female Swiss
CD-1 mice at a dosage level of 55.5 mg (in 0.1 ml acetone), 3 times
per week, for 2 years (OPP group). A second group of 50 male and 50
female mice was treated identically except that their backs were
pretreated one time with a dermal dose of 0.05 mg (in 0.1 ml acetone)
of 7,12-dimethylbenz(a)anthracene, or DMBA, a known initiator of skin
carcinogenicity (DMBA/OPP group). Additional groups of 50 male and 50
female mice served as control groups and included an acetone vehicle
control group, treated only with acetone (acetone group); an initiator
control group, treated once with DMBA and thereafter only with acetone
(DMBA/acetone group); and a positive control group, treated once with
DMBA and thereafter with 12-o-tetradecanoylphorbol-13-acetate, or TPA,
a known promoter of skin carcinogenicity (DMBA/TPA group). TPA was
dermally applied at a dosage level of 0.005 mg (in 0.1 ml acetone), 3
times per week, for 2 years.
Mean body weights and survival of the mice treated with OPP or
with DMBA/OPP were generally similar to those of the respective
negative control groups. Survival was substantially decreased in the
DMBA/TPA group. In the DMBA/TPA group, the incidence of neoplastic
skin lesions at the site of application (squamous cell papillomas/
carcinomas, keratocanthomas, basal cell tumours/carcinomas) was
clearly incrased (52/100) over that in the DMBA/acetone control group
(15/100). Time-to-tumour was also substantially decreased in the
DMBA/TPA group. Similar neoplastic skin lesions were also observed in
the DMBA/OPP group (17/100), but at an incidence equivalent to that in
the DMBA/acetone control group (15/100). No neoplastic skin lesions
were observed in the OPP group. The author concluded that there is no
evidence of carcinogenicity in male or female Swiss CD-1 mice
administered OPP alone or as a promoter following initiation with DMBA
(Luster, 1985).
SOPP
SOPP was administered in the diet to groups (50 male and 50
female) of B6C3F1 mice (Charles River, Japan) for 96 weeks at dosage
levels of 0, 0.5, 1.0 or 2.0%. At the end of the 96-week period of
administration, the mice were given the control diet for an additional
8 weeks. Survival rate was slightly decreased only in the high-dose
group male mice.
Decreased body weights were observed in males and females at 2.0%
and in females at 1.0% and at 0.5%. Increased alkaline phosphatase
activity was observed in females at 2.0%, 1.0% and 0.5%. There were no
urinary bladder stones observed in any of the mice. Tumours of the
urinary bladder did not occur in any mice treated with SOPP. Extensive
renal damage due to SOPP was not observed. The authors concluded that
SOPP did not induce an increased incidence of neoplasms (of any kind)
considered to be related to treatment in male or female mice at dosage
levels up to 2.0% when administered in the diet for 96 weeks (Hagiwara
et al., 1984; Ito, 1983a).
Rat
OPP
OPP was administered in the diet to a group of 14 male F344 rats
(Charles-River, Japan) for 32 weeks at a dosage level of 2.0%. No
hyperplasias, papillomas or cancers were observed in any of the
treated rats. Pretreatment of additional rats with 0.05% N-butyl-N-
(4-hydroxybutyl) nitrosamine (BBN) did not significantly increase the
incidence of urinary bladder lesions over that of rats treated with
BBN alone (Fukushima et al., 1983).
OPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. No papillomas or
carcinomas of the urinary bladder were observed, although one
papillary/nodular (PN) hyperplasia was observed (with no stones). Six
rats having small stones were observed to have simple hyperplasia of
the urinary bladder epithelium. Pre-treatment of additional rats with
BBN did not significantly increase the incidence of urinary bladder
lesions over that of rats treated with BBN alone.
In another experiment, OPP was administered in the diet to male F344
rats for up to 12 weeks at dosage levels up to 2.0%. No urinary stones
or tumours were observed in these animals (Ito, 1983b).
OPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 30 and 65 days. Only 7 rats
per group were permitted to live to 90 days, at which time they were
sacrificed.
Kidney pathology was observed in these rats as follows: focal
cortical cysts, significantly decreased urine specific gravity (at 65
and 90 days), small amounts of blood in the urine, focal tubular
collapse and atrophy in the cortex, and cystic degeneration (at 65 and
90 days). No treatment-related urinary bladder lesions were observed
(Reitz et al., 1983).
OPP was administered in the diet to male F344 rats (Charles-
River, Japan) for 91 weeks at dosage levels of 0 (control), 0.625%,
1.25% or 2.5% (2.5% OPP is equivalent to about 4.0% SOPP on a molar
basis). For survival data and non-neoplastic lesions of the urethelial
system, see Table 1.
Table 1. Survival and non-neoplastic lesions of the urothelial system in male rats
administered 2-phenylphenol in the diet
Dose Number Kidney Urinary
(% in of animals Survival (inflammatory bladder
diet) treated (at 91 weeks) lesions) (hyperplasia)
0 24 23/24 (96%) 0/24 (0%)
0.625 20 18/20 (90%) 0/20 (0%)*
1.25 24 17/24 (71%) 3/24 (13%) 0/24 (0%)
2.5 23 15/23 (65%) 23/23 (100%) 7/23 (30%)
* May be 2/20 (10%)(discrepancy in report).
As shown in Table 2, OPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in the experiment at dosage levels greater than 1.25% (Hiraga & Fujii,
1984; Hiraga, 1983a).
SOPP
SOPP was administered in the diet to groups of 9-10 male and 9-10
female F344 rats (Charles-River, Japan) for 13 weeks at dosage levels
up to 4.0% There were no mortalities during the study. Urinary bladder
neoplasms were observed in male and female rats as described below in
Table 3. No bladder calculi were observed in this experiment.
Table 2. Neoplastic lesions of the urothelial system in rats
administered 2-phenylphenol.
Urinary bladder
Dose Papillomas(1) Transitional Total
(% in diet) cell carcinomas (2) neoplasms
0 0/24 0/24 0/24 (0%)
0.625 0/20 0/20 0/20 (0%)
1.25 3/24(3) 20/24(5) 23/24* (96%)
2.5 2/23(4) 2/23(6) 4/23 (17%)(7)
*p < 0.001
(1) first papilloma at 65 weeks (1.25% group)
(2) first carcinoma at 82 weeks (1.25% group)
(3) 1/3 rats had calculi
(4) 2/2 rats had calculi
(5) 16/20 rats had calculi
(6) 1/2 rats had calculi
(7) An additional 5 rats in the 2.5% group had calculi but no
neoplasms and 6 had calculi and only hyperplasia.
In a second experiment, SOPP was administered in the diet to
groups of 20-21 male F344 rats for 91 weeks at dosage levels up to
4.0% (Table 4).
The authors concluded that SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional-cell carcinomas)
in male F344 rats at dosage levels of 1.0% and above when administered
in the diet for 13 weeks or for 91 weeks. In the 91-day study,
transitional cell carcinomas of the kidney were also observed at
dosage levels of > 0.5%. SOPP also induced an increased incidence
of urinary-bladder tumours (papillomas only) in female F344 rats at a
dosage level of 4.0% when administered in the diet for 13 weeks
(Hiraga & Fujii, 1981).
Table 3. Urinary bladder neoplasms in rats administered sodium
2-phenylphenate for 13 weeks
Urinary bladder
Dose Papillomas Transitional cell Total
(% in diet) carcinomas neoplasms
Males
0 0/10 0/10 0/10 (0%)
0.125 0/10 0/10 0/10 (0%)
0.25 0/10 0/10 0/10 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 1/10 0/10 1/10 (10%)
2.0 4/10 5/10 9/10 (90%)
4.0(1) 0/10 1/10 1/10 (10%)
Females
0 0/10 0/10 0/10 (0%)
0.125 0/9 0/9 0/9 (0%)
0.25 0/9 0/9 0/9 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 0/10 0/10 0/10 (0%)
2.0 0/10 0/10 0/10 (0%)
4.0(2) 2/10 0/10 2/10 (20%)
(1) 6 rats in this group had moderate pyelonephritis.
(2) 1 rat in this group had slight pyelonephritis.
Table 4. Neoplastic lesions of the urothelial system in rats administered sodium
2-phenylphenate for 91 weeks
Transitional cell carcinomas
Dose Number of Survival
(% in animals treated (at Urinary Kidney(2) Total(3)
diet) (all males) 91 weeks) bladder(1)
0 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.125 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.25 20 19/20 (95)% 0/20 0/20 0/20 (0%)
0.5 21 18/21 (86%) 0/21 1/21 1/21 (5%)
1.0 21 18/21 (86%) 6/21 1/21 7/21 (33%)
2.0(4) 21 12/21 (57%) 19/21(6) 1/21 20/21 (95%)
4.0(5) 20 14/20 (70%) 8/20 13/20 17/20 (85%)
(1) bladder calculi were observed in 8 of the rats with bladder tumours.
(2) first kidney tumour at 79 weeks.
(3) total number of animals with neoplasms in the urothelial system.
(4) 4 rats in this group had pyelonephritis.
(5) 20 rats in this group had pyelonephritis.
(6) one neoplasm was a carcinosarcoma.
SOPP was administered in the diet to male and female F344 rats
(Charles-River, Japan) for 104 weeks at dosage levels up to 2.0%. The
rats were then given control diet for an additional 2 weeks at which
time they were sacrificed and examined (referred to as the "106-week
study"). For survival data see Table 5, for non-neoplastic lesions
Table 6, and for neoplastic lesions Table 7.
Table 5. Survival rates of rats administered sodium
2-phenylphenate (106-week study)
Dose Number of animals treated Survival
(% in diet) Males Females (at 104 weeks)
0 50 50 >50%
0.5 - 50 >50%
0.7 50 - >50%
1.0 - 50 >50%
2.0 50 - 10/50 (20%)(1)
(1) urinary bladder tumours were largely responsible for the
decreased survival in this group.
Table 6. Non-neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Dose Kidney(1) Urinary bladder
(% in diet) (inflammatory lesions) (hyperplasia)
Males
0 0/50 (0%) 0/50 (0%)
0.7 1/50 (2%) 0/50 (0%)
2.0 5/50 (10%)* 1/50 (2%)
Females
0 0/50 (0%) 0/50 (0%)
0.5 3/50 (6%) 1/50 (2%)
1.0 20/50 (40%)** 4/50 (8%)
p < 0.05
** p < 0.001
(1) kidney lesions other than age-related lesions
(i.e. chronic nephropathy).
Table 7. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Urinary bladder
Dose Papillomas Transitional Total
(% in diet) cell carcinomas neoplasms
Males
0 0/50 0/50 0/50 (0%)
0.7(1) 0/50 2/50 2/50 (4%)
2.0(2) 1/50 46/50 47/50 (94%)*
Females
0 0/50 0/50 0/50 (0%)
0.5 1/50 0/50 1/50 (2%)
1.0 3/50 1/50 4/50 (8%)
* p < 0.001, first tumour (transitional cell carcinoma)
at 40 weeks.
(1) 2/50 (4%) rats had calculi.
(2) 3 kidney tumours were also observed in this group
(1 papilloma and 2 transitional cell carcinomas);
27/50 (54%) rats had calculi.
In this 106-week study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male rats at dosage levels higher than 0.7%. All rats with calculi
had urinary bladder tumours. There is a possibility that the presence
of calculi may exacerbate tumour formation in the urinary bladder.
It also appears that the incidence of urinary bladder tumours may
be inversely related to the incidence of nephritic lesions in male
rats given 2% or 4% SOPP (See the studies described above by Hiraga
and Fujii (1981) for similar results in a 91-week study with SOPP and
by Hiraga and Fujii (1984) for similar results in a 91-week study with
OPP). It is possible that severe lesions in the kidney may somehow
inhibit the formation of bladder tumours in male rats (Hiraga, 1983b).
In a second experiment, SOPP was administered in the diet to male
and female F344 rats for 104 weeks at dosage levels up to 2.0% The
rats were then given control diet until they either died or became
moribund, at which time they were sacrificed (referred to as the
"lifetime study"). For survival data see Table 8.
Table 8. Suvival rates of rats administered sodium
2-phenylphenate (lifetime study)
Dose Number of treated animals Survival
(% in diet) males females (at 104 weeks)
0 25 25 > 50%
0.25 25 25 > 50%
0.5 - 25 > 50%
0.7 25 - > 50%
1.0 - 25 > 50%
2.0 25 - 6/25(25%)(1)
(1) urinary bladder tumours were likely responsible for
the decreased survival in this group (at 104 weeks).
Hyperplasia of the urinary bladder was not observed in any of the
rats in this experiment. Urinary bladder neoplasms were observed in
male and female rats shown in Table 9.
Table 9. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (lifetime study)
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms
Males
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.7 2/25 1/25 3/25 (12%)
2.0(1) 2/25 21/25 23/25 (92%)*
Females
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.5 0/25 0/25 0/25 (0%)
1.0(2) 1/25 1/25 2/25 (8%)
* p < 0.001; first tumour (transitional cell carcinoma)
at 54 weeks.
(1) 8/25 (32%) rats had calculi.
(2) 1/25 (4%) rats had calculi.
In this lifetime study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male F344 rats at dosage levels of > 0.7% and in female F344 rats
at dosage levels of > 0.5% when administered in the diet for 104
weeks. Urinary bladder neoplasms were not observed in either sex at a
dosage level of 0.25% (Table 9). All rats with calculi had urinary
bladder tumours (Hiraga, 1983b).
SOPP was administered in the diet to a group of 29 male F344 rats
(Charles-River, Japan) and to another group of 15 male rats for 32
weeks at a dosage level of 2.0%. Urinary bladder lesions reported were
as in Table 10.
Table 10. Urinary bladder lesions in rats administered sodium 2-phenylphenate
at 2.0% in the diet for 32 weeks
Urinary bladder
Effective Simple PN Total
no. of rats hyperplasia hyperplasia papillomas cancer neoplasms
29 29/29 25/29 5/29 0/29 5/29 (17%)
15 13/15 14/15 2/15 1/15 3/15 (20%)
Pretreatment of additional rats with 0.01% BBN significantly
increased the incidence of simple hyperplasia and papillary/nodular
(PN) hyperplasia, but not of papillomas or cancers over that of rats
treated with BBN alone. Pretreatment of additional rats with 0.05% BBN
significantly increased the incidence of PN hyperplasia, papillomas
and cancers over that of rats treated with BBN alone (Fukushima
et al., 1983).
SOPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. One rat had
small stone formations in the urinary bladder. SOPP induced simple
hyperplasia, PN hyperplasia (19/28, 68%), papillomas (5/28, 18%) and
cancers (6/28, 21%) of the urinary bladder. Pretreatment of additional
rats with BBN increased the incidence of PN hyperplasia (p < 0.05),
papillomas (non-significant), and cancers (non-significant) over that
of rats treated with BBN alone.
In another experiment, SOPP was administered in the diet to male
F344 rats for up to 104 weeks at dosage levels of 0, 0.25%, 0.5%,
1.0%, or 2.0%. Animals from each group were periodically sacrificed
and examined at 4, 8, 12, 24, 36 and 104 weeks. No stone formation was
observed in the urinary bladder of any rats treated with SOPP. In the
2.0% group, simple hyperplasia was observed at 4 weeks and beyond and
PN hyperplasia at 36 weeks and beyond (in all rats at 104 weeks).
Papillomas (2/5, 40%) and cancers (2/5, 40%) of the urinary bladder
were observed in rats administered 2.0% SOPP at 104 weeks. In the 1.0%
group, only simple hyperplasia was observed (> 36 weeks) (Ito,
1983b).
phi-Phenylphenol, o-phenylphenol (OPP), o-phenylphenol sodium
salt tetrahydrate (SOPP), and some biphenyl derivatives were examined
for their bladder carcinogenicity to rats by a short-term assay for
agglutinability of bladder epithelial cells with concanavalin A.
Increased agglutinability was observed after 1-week treatment with
2.0% and 1.0% OPP or 2.0% and 1.0% SOPP, suggesting the bladder
carcinogenicity of these compounds. Such an increase in
agglutinability was not observed in rats fed diets containing
phi-phenylphenol or biphenyl derivatives at 2.0%. An in vivo
carcinogenesis experiment was perfomed with SOPP. As shown in Table
11, bladder carcinomas developed in 14 out of 36 male Fisher rats
(Charles-River, Japan) fed a diet containing 2% SOPP for 50 weeks
(Honma et al., 1983).
Table 11. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms(1)
0 0/11 0/11 0/11 (0%)
2.0(2) 19/36** 14/36* 33/36 (92%)**
* p < 0.05
** p < 0.01
(1) No calculi were observed in any of the treated or control rats.
(2) 31/36 of these rats had PN hykperplasia in the urinary bladder.
In addition, 3/36 and 9/36 of these rats had papillomas and
PN hyperplasia, respectively, in the renal pelvis.
SOPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 14, 30 and 65 days. Only 7
rats per group were permitted to live to 90 days, at which time they
were sacrificed.
Urinary bladder pathology was observed in these rats as follows:
increased mitosis, beginning at 3 days, in the epithelium; thickening
(i.e. simple hyperplasia), beginning at 14 days, in the epithelium. No
tumours of any kind were observed in the urinary bladder (Reitz
et al., 1983).
Special studies on mutagenicity
OPP
Ames tests utilizing S. typhimurium strains TA92, TA1535,
TA100, TA1537, TA94 and TA98, without and with metabolic activation
derived from livers of male Fischer rats, were uniformly negative.
Chromosomal aberration tests using Chinese hamster fibroblasts in
culture gave ambiguous results without and with metabolic activation
(Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA1537, TA98 and
TA100 were negative without and with metabolic activation derived from
male rats. Strain TA1535, however, gave a weakly-positive response
without activation, but a negative response with activation. A mouse
lymphoma assay (L5178Y/TK+/-) was weakly positive without and with
metabolic activation. Chromosomal aberration assays using Chinese
hamster ovary cells, without and with activation, were negative. A
sister chromatid-exchange assay using Chinese hamster ovary cells was
weakly positive without activation, but negative with activation. A
sex-linked recessive-lethal assay in Drosophila melanogaster was
negative (Luster, 1985).
In a dominant-lethal study, OPP of 99.7% purity was administered
by gavage to 10-week-old male C3H mice each day for 5 days at dosage
levels of 0 (control), 100, or 500 mg/kg/day. Fifteen mice were
treated at each dosage level. An additional positive control group of
15 male mice was given a single intraperitoneal dose of 300 mg/kg of
ethylmethanesulfonate (EMS). Immediately following dosing, each male
mouse was mated with 2 untreated virgin female mice for 7 days. At the
end of 7 days, the female mice were replaced by another 2 female mice.
The replacement procedure was repeated for a total of 6 weeks. All
female mice were sacrificed on days 12-13 of gestation and scored for
numbers of corpora lutea, implants, living embryos, and early or late
embryonic deaths. Frequencies of induced dominant-lethal mutations
were calculated. Scores for control and treatment groups were compared
for each 7-day period. For the positive control group, the frequency
of early embryonic deaths was increased in weeks 1, 2, 3, and 6. No
untoward differences from control values were detected for mice
treated with OPP. Dominant-lethal mutations were not induced in this
study by treatment with OPP (Kaneda et al., 1978).
SOPP
Ames tests using S. typhimurium strains TA98 and TA100 were
negative without and with metabolic activation derived from livers of
male Fischer rats. Chromosomal aberration tests using Chinese hamster
fibroblasts in culture gave ambiguous results without and with
metabolic activation (Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA98, TA100,
TA1535, TA1537, and TA1538 were negative without and with metabolic
activation. An unscheduled DNA synthesis assay in primary rat
hepatocytes in culture was negative (Reitz et al., 1983).
Possible metabolites of OPP and SOPP
Two possible metabolites of OPP and SOPP, 2,5-dihydroxybiphenyl
and 2-phenyl-l,4-benzoquinone, were tested in Ames S. typhimurium
strains TA98, TA100 and TA1537. Both compounds gave negative results
without and with metabolic activation derived from livers of male
Fischer rats. Chromosomal aberration tests on both compounds using
Chinese hamster fibroblasts in culture gave negative results without
and with metabolic activation (Ishidate et al., 1983).
COMMENTS
In response to the requirement for further information, a
protocol for a proposed 2-generation reproduction study in rats using
OPP as the test material has been received (Rao, 1985). No information
on the progress of a new carcinogenicity/chronic toxicity study in a
strain of rats other than F344 has been received. Some new metabolic
and pharmacokinetic data have been received, but they are very limited
with respect to elucidating possible biochemical mechanisms of action
of carcinogenicity.
An IARC monograph on OPP and SOPP was published in 1983 (IARC,
1983). This document summarizes chemical and physical data,
production, use and occurence of these chemicals. It also evaluates
biological data, relevant to the oncogenic risk to humans, that was
available prior to 1983.
Recent carcinogenicity studies on sodium 2-phenylphenate (SOPP)
have reported statistically-significant increased incidences of
carcinomas in the urinary bladder, and some in the renal pelvis, of
male F344 rats following administration of 1% and 2% SOPP in the diet.
In female F344 rats, an increased, but lower, incidence of urinary
bladder neoplasms was also reported at a dietary dosage level of 1%.
Similar tumours observed at lower dietary dosage levels (0.5% and
0.7%) in male and female rats, although not statistically significant,
were considered to be biologically relevant owing to the rarity of
these tumours in this rat strain and their absence in control animals.
There does not appear to be a cause-effect relationship between stones
and neoplasms in the urinary bladders of rats. Therefore, SOPP has
been determined to be a carcinogen to the urothelium of F344 rats.
Although fewer carcinogenicity studies on 2-phenylphenol (OPP)
are available than on SOPP, some recent evidence suggest that OPP may
also be a carcinogen on the urinary bladder of male F344 rats at
dietary dosage levels of 1.25% and 2.5%. Another earlier study did
not, however, demonstrate a similar response in male or female Wistar
rats. OPP has not been adequately tested to permit a definitive
conclusion regarding its carcinogenicity.
Carcinogenicity studies in mice with both OPP and SOPP were
negative for tumourigenic effects. The dosage level in the OPP study
may have been too low to permit expression of tumours.
Mutagenicity assays with OPP and SOPP in bacteria have been
overwhelmingly negative, although weakly positive results have been
occasionally noted in isolated instances. Results of studies in
cultured mammalian cells have been mixed. Both negative and weakly
positive responses in several assays have been reported. Results from
in vivo studies have been regularly negative.
A series of in vitro and in vivo biochemical studies suggest
the possibility that an altered metabolic pathway for OPP/SOPP,
occurring at high dosage levels only, may be related to (and
presumably responsible for) the occurrence of urothelial tumours at
similar dosage levels in long-term studies. Although this speculation
appears plausible at this time, considerably more direct experimental
support will be required before it can be accepted as fact.
Teratology studies on rats using OPP as the test material were
negative for teratogenic effects.
With respect to the oncogenic potential of OPP and SOPP, the
Meeting compared results from available oncogenic studies to the low
anticipated dietary exposures to OPP and SOPP. On the basis of this
comparison and other information, the Meeting agreed to extend the
temporary ADI for OPP/SOPP to 1989.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg b.w.
Dog: 500 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1989 or earlier)
1. A multigeneration reproduction study.
2. A carcinogenicity/chronic toxicity study in a rat strain known to
be sensitive to induction of bladder carcinomas (a strain other
than F344).
3. Metabolic, pharmacokinetic, and other related studies, as
appropriate, in the strains of animals tested in long-term
studies for carcinogenicity and in other species, including a
consideration of species, sex and dosage-level differences.
4. Qualitative and quantitative monitoring data on the urinary
excretion of OPP and/or its metabolites by industrial workers, or
others, regularly exposed to OPP or SOPP.
5. Mutagenicity studies on urinary metabolites of OPP and/or SOPP.
DESIRED
Additional observations in man.
REFERENCES
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. Promoting
(1983) effect of sodium o-phenylphenate and o-phenylphenol on two-
stage urinary bladder carcinogenesis in rats. Gann 74,
625-632.
Hagiwara, A., Shibata, M., Hirose, M, Fukushima, S. & Ito, N. Long
(1984) term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Fd. Chem. Toxicol. 22,
809-814.
Hiraga, K. Short report of a 91-week feeding study of
(1983a) orthophenylphenon (OPP) in male rats. Unpublished report
from the Department of Toxicology, Tokyo Metropolitan
Research Laboratory of Public Health, Tokyo, Japan.
Submitted to WHO by Dow Chemical Co., Midland, MI, USA.
Hiraga, K. Carcinogenicity testing of sodium orthophenylphenate in
(1983b) F-344/DuCrj rats. Unpublished report from the Department of
Toxicology, Tokyo Metropolitan Research Laboratory of Public
Health, Tokyo, Japan. Submitted to WHO by Dow Chemical Co.,
Midland, MI, USA.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary system in
(1981) F344 rats by dietary administration of sodium
o-phenylphenate.. Fd. Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary bladder in
(1984) F344 rats by dietary administration of o-phenylphenol.
Fd. Cosmet. Toxicol., 22, 865-870.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T., & Sugimura, T.
(1983) Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
IARC. International Agency for Research on Cancer. IARC Monographs on
(1983) the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Miscellaneous Pesticides, 30, 329-344.
Ishidate, M., Jr., Yoshikawa, K., & Sofuni, T. Mutagenicity tests on
(1983) OPP, OPP-sodium salt, and their possible metabolites.
Unpublished report from the Division of Mutagenesis,
Biological Safety Research Center, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Long-term toxicity and carcinogenicity study of sodium
(1983a) o-phenyl-phenate in B6C3F1 mice, Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Pathological analysis carcinogenicity of the sodium
(1983b) o-phenylphenate in B6C3F1 mice. Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz, B.A. Teratological
(1981) evaluation of othophenylphenol in rats. Fund. Appl.
Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. Teratogenicity and
(1978) dominant-lethal studies with o-phenylphenol. J. Pesticide
Sci., 3, 365-370.
Luster, M. NTP technical report on the toxicology and carcinogenesis
(1985) studies of ortho-phenylphenol (CAS No. 90-43-7) alone with
7,12-dimethyl-benz(a)anthracene (CAS No. 57-97-6) in Swiss
CD-1 mice (dermal studies). NIH Publication No. 85-2557, NTP
84-099, U.S. Department of Health and Human Services (Board
Draft, 2/85, National Toxicology Program, NTP TR 301).
Nakagawa, A., Nakao, T., & Nakao, M. Altered levels of cyclic
(1984) nucleotides in F344 rats fed sodium o-phenylphenate.
Fd. Chem. Toxicol., 22, 217-221.
Nakao, T., Ushiyama, K., Kabashima, J., Nagai, F., Nakagawa, A., Ohno,
T., Ichikawa, H., Kobayashi, H., & Hiraga, K. The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Fd. Chem. Toxicol., 21, 325-329.
Oehme, F.W. & Smith, T.H.F. The metabolism and urinary excretion of
(1972) o-phenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Rao, K.S. Proposed reproduction study protocol, orthophenylphenol:
(1985) Two generation dietary reproduction study in rats. Submitted
to WHO by Dow Chemical Co., Midland, MI, U.S.A.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A., & Watanabe, P.G.
Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem. Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP)
on the urinary tract of male F344 rats. Tox. Appl.
Pharmacol. 73,345-349.
Savides, M.C. & Oehme, F.W. Urinary metabolism or orally administered
(1980) o-phenylphenol in dogs and cats. Toxicology, 17,
355-363.
Yamaha, T., Tanaka, A., Sato, M., & Tsuchiya, T. Excretion,
(1983) distribution and metabolic fate of sodium o-phenylphenol and
o-phenylphenol in the rat. Unpublished report from the
Division of Medical Chemistry, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Weekly urine samples were collected from three mature and three
immature male and female dogs and cats given single oral doses of
3.7 mg of 14C-2-phenylphenol every other day for 25 doses, regardless
of body weight. Average single doses for mature and immature dogs were
0.27 and 2.03 mg/kg b.w., respectively, and for mature and immature
cats, 1.16 and 2.04 mg/kg b.w., respectively. The dominant urinary
excretion product in both species was unchanged 2-phenylphenol, which
accounted for 68-90% of the urinary radioactivity in dogs and 95-98%
in cats. Dogs excreted significantly more glucuronide- and sulfate
ester-conjugated 2-phenylphenol as did cats. Immature dogs excreted
four times as much glucuronide conjugate than did mature dogs. Age
differences for both species did not affect the rate of excretion of
the sulfate ester conjugate (Savides and Oehme, 1980).
Effects on enzymes and other biochemical parameters
In vivo covalent binding to urinary bladder DNA was determined
in pooled samples from 8 male rats dosed with 500 mg/kg b.w. of
14C-2-phenylphenol or 14C-sodium 2-phenylphenate. No increased
radioactivity was observed in DNA isolated and purified from bladders
excised at 16 hours after dosing for either compound. The detection
limit was < 1 alkylation/106 nucleotides. Identical results were
obtained in a repeat experiment (Reitz et al., 1983).
In vitro binding of sodium 2-phenylphenate metabolites to
macromolecules was studied by incubating 14C-sodium 2-phenylphenate
with purified male rat liver microsomes in the presence of a NADPH
regenerating system and bovine serum albumin which served as a
"protein acceptor". Macromolecular binding of radioactivity to
protein, dependent on the presence of both active microsomes and NADP,
was observed. In vivo binding of 2-phenylphenol and sodium
2-phenylphenate metabolites to macromolecules in the liver, kidney and
urinary bladder of male rats was also studied. Groups of 4 animals
were given single oral doses of the 14C-labeled compounds at dosage
levels of 50, 100, 200 or 500 mg/kg b.w. Tissues were excised 16-18
hours later for measurement of macromolecular binding, which was
determined as nanomoles of bound material/mg protein. Levels of
binding were not linearly related to the administered dose.
Disproportionate increases observed in each tissue at dosage levels of
> 200 mg/kg b.w. of sodium 2-phenylphenate and in liver and bladder
at levels of 200-500 mg/kg b.w. of 2-phenylphenol indicated
substantially-increased binding of metabolites to macromolecules at
these dosage levels (Reitz et al., 1984).
SOPP was administered in the diet to 4-week-old male and female
F344 rats at dosage levels of 0 (control) and 2% for 136 days. Urine
was periodically collected. At 136 days, the rats were sacrificed,
blood samples were collected and livers and kidneys were removed.
Cyclic nucleotide levels (c-AMP and c-GMP) were determined in urine,
plasma, liver and kidneys. Adenylate cyclase activity was also assayed
in liver and kidney. In SOPP-treated male rats, c-AMP levels in the
urine and plasma decreased, whereas c-GMP levels increased. In SOPP-
treated female rats, similar changes were not observed except for
decreased c-AMP during the first 3 days of feeding. Levels of c-AMP
and c-GMP in liver and kidney were unchanged. The decrease in urinary
c-AMP in male rats was likely the result of decreased adenylate
cyclase activities in liver and kidney. A similar change in adenylate
cyclase activity was also observed in liver but not in kidneys of
female rats treated with SOPP. The sex-related alterations in cyclic
nucleotide levels were postulated to be related to the sex-related
induction of urinary bladder tumors by dietary SOPP (Nakagawa,
et al., 1984).
Toxicological studies
Special studies on teratogenicity
OPP of 99.7% purity was administered daily by gavage to groups of
18-20 pregnant Wistar rats on days 6-15 of gestation. Dosage levels
were 0, 150, 300 or 600 mg/kg b.w. An additional group of 11 rats was
also dosed at 1200 mg/kg b.w., but this dosage level proved to be
lethal. No untoward signs of toxicity were observed in the control or
150 mg/kg b.w. rats. At 300 mg/kg b.w. and higher, dose-related ataxia
and decreased mean body-weight gains were observed. All surviving rats
were sacrificed on day 20 of gestation and uterine contents were
examined. Foetuses were grossly examined. Skeletal examinations
utilizing the Alizarin red S technique and visceral examinations using
a modified method of Wilson were performed. Mean numbers of
implantation sites, live foetuses, resorptions and foetal weights were
comparable to control numbers for the 150 and 300 mg/kg b.w. groups.
At 600 mg/kg b.w., foetal resorptions were increased and foetal
weights were decreased. Although small numbers of foetal anomalies
were observed in all groups, no relation to the test material was
detected. OPP was negative for teratogenic effects in this study
(Kaneda et al., 1978).
OPP was administered by gavage to groups of 25-27 pregnant rats
on days 6-15 of gestation at daily dosage levels of 100, 300 or
700 mg/kg b.w. A group of 35 pregnant rats served as vehicle controls.
Dams were sacrificed on day 21 and foetuses were removed by Cesarean
section. All foetuses were weighed, sexed and subjected to external
and skeletal examination. The soft tissues of approximately one-third
of the foetuses were examined.
One high-dosage level rat died as a result of a dosing accident.
Pregnant rats given 700 mg/kg b.w. gained significantly less body
weight during the first 4 days of treatment (days 6-9 of gestation)
than did control rats. Food consumption was also significantly
decreased from days 9-11 of gestation at 700 mg/kg b.w. In addition,
liver weights (but not liver/body-weight ratios) were found to be
significantly decreased at necropsy. There was no effect of
2-phenylphenol on the number of implantation sites per dam, mean
litter size, incidences of resorptions, foetal body weights or crown-
rump measurements.
A major malformation (hypoplastic tail and missing sacral and
caudal vertebrae) was observed in a single foetus from the 300 mg/kg
b.w. group. There were no other major malformations observed in any
foetus. An increase in delayed ossification of sternebrae and
unossified sternebrae were observed in the 700 mg/kg b.w. group.
Foramina (small holes) in the skull and bony islands in the skull were
also slightly increased in this group. With the exception of the
single major malformation, all of these effects were considered to be
minor skeletal variants. No adverse effects on embryonal or foetal
development were observed that were considered to be due to
2-phenylphenol. The authors concluded that OPP was not embryotoxic or
teratogenic in rats at dosage levels up to and including 700 mg/kg
b.w. (John et al., 1981).
Special studies on carcinogenicity
Mouse
OPP
A mouse skin-painting study was conducted to determine whether
OPP might be a complete skin carcinogen or possibly a promoter in a
two-stage initiation/promotion process. OPP was dermally applied to
the interscapular area of the backs of 50 male and 50 female Swiss
CD-1 mice at a dosage level of 55.5 mg (in 0.1 ml acetone), 3 times
per week, for 2 years (OPP group). A second group of 50 male and 50
female mice was treated identically except that their backs were
pretreated one time with a dermal dose of 0.05 mg (in 0.1 ml acetone)
of 7,12-dimethylbenz(a)anthracene, or DMBA, a known initiator of skin
carcinogenicity (DMBA/OPP group). Additional groups of 50 male and 50
female mice served as control groups and included an acetone vehicle
control group, treated only with acetone (acetone group); an initiator
control group, treated once with DMBA and thereafter only with acetone
(DMBA/acetone group); and a positive control group, treated once with
DMBA and thereafter with 12-o-tetradecanoylphorbol-13-acetate, or TPA,
a known promoter of skin carcinogenicity (DMBA/TPA group). TPA was
dermally applied at a dosage level of 0.005 mg (in 0.1 ml acetone), 3
times per week, for 2 years.
Mean body weights and survival of the mice treated with OPP or
with DMBA/OPP were generally similar to those of the respective
negative control groups. Survival was substantially decreased in the
DMBA/TPA group. In the DMBA/TPA group, the incidence of neoplastic
skin lesions at the site of application (squamous cell papillomas/
carcinomas, keratocanthomas, basal cell tumours/carcinomas) was
clearly incrased (52/100) over that in the DMBA/acetone control group
(15/100). Time-to-tumour was also substantially decreased in the
DMBA/TPA group. Similar neoplastic skin lesions were also observed in
the DMBA/OPP group (17/100), but at an incidence equivalent to that in
the DMBA/acetone control group (15/100). No neoplastic skin lesions
were observed in the OPP group. The author concluded that there is no
evidence of carcinogenicity in male or female Swiss CD-1 mice
administered OPP alone or as a promoter following initiation with DMBA
(Luster, 1985).
SOPP
SOPP was administered in the diet to groups (50 male and 50
female) of B6C3F1 mice (Charles River, Japan) for 96 weeks at dosage
levels of 0, 0.5, 1.0 or 2.0%. At the end of the 96-week period of
administration, the mice were given the control diet for an additional
8 weeks. Survival rate was slightly decreased only in the high-dose
group male mice.
Decreased body weights were observed in males and females at 2.0%
and in females at 1.0% and at 0.5%. Increased alkaline phosphatase
activity was observed in females at 2.0%, 1.0% and 0.5%. There were no
urinary bladder stones observed in any of the mice. Tumours of the
urinary bladder did not occur in any mice treated with SOPP. Extensive
renal damage due to SOPP was not observed. The authors concluded that
SOPP did not induce an increased incidence of neoplasms (of any kind)
considered to be related to treatment in male or female mice at dosage
levels up to 2.0% when administered in the diet for 96 weeks (Hagiwara
et al., 1984; Ito, 1983a).
Rat
OPP
OPP was administered in the diet to a group of 14 male F344 rats
(Charles-River, Japan) for 32 weeks at a dosage level of 2.0%. No
hyperplasias, papillomas or cancers were observed in any of the
treated rats. Pretreatment of additional rats with 0.05% N-butyl-N-
(4-hydroxybutyl) nitrosamine (BBN) did not significantly increase the
incidence of urinary bladder lesions over that of rats treated with
BBN alone (Fukushima et al., 1983).
OPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. No papillomas or
carcinomas of the urinary bladder were observed, although one
papillary/nodular (PN) hyperplasia was observed (with no stones). Six
rats having small stones were observed to have simple hyperplasia of
the urinary bladder epithelium. Pre-treatment of additional rats with
BBN did not significantly increase the incidence of urinary bladder
lesions over that of rats treated with BBN alone.
In another experiment, OPP was administered in the diet to male F344
rats for up to 12 weeks at dosage levels up to 2.0%. No urinary stones
or tumours were observed in these animals (Ito, 1983b).
OPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 30 and 65 days. Only 7 rats
per group were permitted to live to 90 days, at which time they were
sacrificed.
Kidney pathology was observed in these rats as follows: focal
cortical cysts, significantly decreased urine specific gravity (at 65
and 90 days), small amounts of blood in the urine, focal tubular
collapse and atrophy in the cortex, and cystic degeneration (at 65 and
90 days). No treatment-related urinary bladder lesions were observed
(Reitz et al., 1983).
OPP was administered in the diet to male F344 rats (Charles-
River, Japan) for 91 weeks at dosage levels of 0 (control), 0.625%,
1.25% or 2.5% (2.5% OPP is equivalent to about 4.0% SOPP on a molar
basis). For survival data and non-neoplastic lesions of the urethelial
system, see Table 1.
Table 1. Survival and non-neoplastic lesions of the urothelial system in male rats
administered 2-phenylphenol in the diet
Dose Number Kidney Urinary
(% in of animals Survival (inflammatory bladder
diet) treated (at 91 weeks) lesions) (hyperplasia)
0 24 23/24 (96%) 0/24 (0%)
0.625 20 18/20 (90%) 0/20 (0%)*
1.25 24 17/24 (71%) 3/24 (13%) 0/24 (0%)
2.5 23 15/23 (65%) 23/23 (100%) 7/23 (30%)
* May be 2/20 (10%)(discrepancy in report).
As shown in Table 2, OPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in the experiment at dosage levels greater than 1.25% (Hiraga & Fujii,
1984; Hiraga, 1983a).
SOPP
SOPP was administered in the diet to groups of 9-10 male and 9-10
female F344 rats (Charles-River, Japan) for 13 weeks at dosage levels
up to 4.0% There were no mortalities during the study. Urinary bladder
neoplasms were observed in male and female rats as described below in
Table 3. No bladder calculi were observed in this experiment.
Table 2. Neoplastic lesions of the urothelial system in rats
administered 2-phenylphenol.
Urinary bladder
Dose Papillomas(1) Transitional Total
(% in diet) cell carcinomas (2) neoplasms
0 0/24 0/24 0/24 (0%)
0.625 0/20 0/20 0/20 (0%)
1.25 3/24(3) 20/24(5) 23/24* (96%)
2.5 2/23(4) 2/23(6) 4/23 (17%)(7)
*p < 0.001
(1) first papilloma at 65 weeks (1.25% group)
(2) first carcinoma at 82 weeks (1.25% group)
(3) 1/3 rats had calculi
(4) 2/2 rats had calculi
(5) 16/20 rats had calculi
(6) 1/2 rats had calculi
(7) An additional 5 rats in the 2.5% group had calculi but no
neoplasms and 6 had calculi and only hyperplasia.
In a second experiment, SOPP was administered in the diet to
groups of 20-21 male F344 rats for 91 weeks at dosage levels up to
4.0% (Table 4).
The authors concluded that SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional-cell carcinomas)
in male F344 rats at dosage levels of 1.0% and above when administered
in the diet for 13 weeks or for 91 weeks. In the 91-day study,
transitional cell carcinomas of the kidney were also observed at
dosage levels of > 0.5%. SOPP also induced an increased incidence
of urinary-bladder tumours (papillomas only) in female F344 rats at a
dosage level of 4.0% when administered in the diet for 13 weeks
(Hiraga & Fujii, 1981).
Table 3. Urinary bladder neoplasms in rats administered sodium
2-phenylphenate for 13 weeks
Urinary bladder
Dose Papillomas Transitional cell Total
(% in diet) carcinomas neoplasms
Males
0 0/10 0/10 0/10 (0%)
0.125 0/10 0/10 0/10 (0%)
0.25 0/10 0/10 0/10 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 1/10 0/10 1/10 (10%)
2.0 4/10 5/10 9/10 (90%)
4.0(1) 0/10 1/10 1/10 (10%)
Females
0 0/10 0/10 0/10 (0%)
0.125 0/9 0/9 0/9 (0%)
0.25 0/9 0/9 0/9 (0%)
0.5 0/9 0/9 0/9 (0%)
1.0 0/10 0/10 0/10 (0%)
2.0 0/10 0/10 0/10 (0%)
4.0(2) 2/10 0/10 2/10 (20%)
(1) 6 rats in this group had moderate pyelonephritis.
(2) 1 rat in this group had slight pyelonephritis.
Table 4. Neoplastic lesions of the urothelial system in rats administered sodium
2-phenylphenate for 91 weeks
Transitional cell carcinomas
Dose Number of Survival
(% in animals treated (at Urinary Kidney(2) Total(3)
diet) (all males) 91 weeks) bladder(1)
0 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.125 20 18/20 (90%) 0/20 0/20 0/20 (0%)
0.25 20 19/20 (95)% 0/20 0/20 0/20 (0%)
0.5 21 18/21 (86%) 0/21 1/21 1/21 (5%)
1.0 21 18/21 (86%) 6/21 1/21 7/21 (33%)
2.0(4) 21 12/21 (57%) 19/21(6) 1/21 20/21 (95%)
4.0(5) 20 14/20 (70%) 8/20 13/20 17/20 (85%)
(1) bladder calculi were observed in 8 of the rats with bladder tumours.
(2) first kidney tumour at 79 weeks.
(3) total number of animals with neoplasms in the urothelial system.
(4) 4 rats in this group had pyelonephritis.
(5) 20 rats in this group had pyelonephritis.
(6) one neoplasm was a carcinosarcoma.
SOPP was administered in the diet to male and female F344 rats
(Charles-River, Japan) for 104 weeks at dosage levels up to 2.0%. The
rats were then given control diet for an additional 2 weeks at which
time they were sacrificed and examined (referred to as the "106-week
study"). For survival data see Table 5, for non-neoplastic lesions
Table 6, and for neoplastic lesions Table 7.
Table 5. Survival rates of rats administered sodium
2-phenylphenate (106-week study)
Dose Number of animals treated Survival
(% in diet) Males Females (at 104 weeks)
0 50 50 >50%
0.5 - 50 >50%
0.7 50 - >50%
1.0 - 50 >50%
2.0 50 - 10/50 (20%)(1)
(1) urinary bladder tumours were largely responsible for the
decreased survival in this group.
Table 6. Non-neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Dose Kidney(1) Urinary bladder
(% in diet) (inflammatory lesions) (hyperplasia)
Males
0 0/50 (0%) 0/50 (0%)
0.7 1/50 (2%) 0/50 (0%)
2.0 5/50 (10%)* 1/50 (2%)
Females
0 0/50 (0%) 0/50 (0%)
0.5 3/50 (6%) 1/50 (2%)
1.0 20/50 (40%)** 4/50 (8%)
p < 0.05
** p < 0.001
(1) kidney lesions other than age-related lesions
(i.e. chronic nephropathy).
Table 7. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (106-week study).
Urinary bladder
Dose Papillomas Transitional Total
(% in diet) cell carcinomas neoplasms
Males
0 0/50 0/50 0/50 (0%)
0.7(1) 0/50 2/50 2/50 (4%)
2.0(2) 1/50 46/50 47/50 (94%)*
Females
0 0/50 0/50 0/50 (0%)
0.5 1/50 0/50 1/50 (2%)
1.0 3/50 1/50 4/50 (8%)
* p < 0.001, first tumour (transitional cell carcinoma)
at 40 weeks.
(1) 2/50 (4%) rats had calculi.
(2) 3 kidney tumours were also observed in this group
(1 papilloma and 2 transitional cell carcinomas);
27/50 (54%) rats had calculi.
In this 106-week study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male rats at dosage levels higher than 0.7%. All rats with calculi
had urinary bladder tumours. There is a possibility that the presence
of calculi may exacerbate tumour formation in the urinary bladder.
It also appears that the incidence of urinary bladder tumours may
be inversely related to the incidence of nephritic lesions in male
rats given 2% or 4% SOPP (See the studies described above by Hiraga
and Fujii (1981) for similar results in a 91-week study with SOPP and
by Hiraga and Fujii (1984) for similar results in a 91-week study with
OPP). It is possible that severe lesions in the kidney may somehow
inhibit the formation of bladder tumours in male rats (Hiraga, 1983b).
In a second experiment, SOPP was administered in the diet to male
and female F344 rats for 104 weeks at dosage levels up to 2.0% The
rats were then given control diet until they either died or became
moribund, at which time they were sacrificed (referred to as the
"lifetime study"). For survival data see Table 8.
Table 8. Suvival rates of rats administered sodium
2-phenylphenate (lifetime study)
Dose Number of treated animals Survival
(% in diet) males females (at 104 weeks)
0 25 25 > 50%
0.25 25 25 > 50%
0.5 - 25 > 50%
0.7 25 - > 50%
1.0 - 25 > 50%
2.0 25 - 6/25(25%)(1)
(1) urinary bladder tumours were likely responsible for
the decreased survival in this group (at 104 weeks).
Hyperplasia of the urinary bladder was not observed in any of the
rats in this experiment. Urinary bladder neoplasms were observed in
male and female rats shown in Table 9.
Table 9. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate (lifetime study)
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms
Males
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.7 2/25 1/25 3/25 (12%)
2.0(1) 2/25 21/25 23/25 (92%)*
Females
0 0/25 0/25 0/25 (0%)
0.25 0/25 0/25 0/25 (0%)
0.5 0/25 0/25 0/25 (0%)
1.0(2) 1/25 1/25 2/25 (8%)
* p < 0.001; first tumour (transitional cell carcinoma)
at 54 weeks.
(1) 8/25 (32%) rats had calculi.
(2) 1/25 (4%) rats had calculi.
In this lifetime study, SOPP induced an increased incidence of
urinary bladder tumours (papillomas and transitional cell carcinomas)
in male F344 rats at dosage levels of > 0.7% and in female F344 rats
at dosage levels of > 0.5% when administered in the diet for 104
weeks. Urinary bladder neoplasms were not observed in either sex at a
dosage level of 0.25% (Table 9). All rats with calculi had urinary
bladder tumours (Hiraga, 1983b).
SOPP was administered in the diet to a group of 29 male F344 rats
(Charles-River, Japan) and to another group of 15 male rats for 32
weeks at a dosage level of 2.0%. Urinary bladder lesions reported were
as in Table 10.
Table 10. Urinary bladder lesions in rats administered sodium 2-phenylphenate
at 2.0% in the diet for 32 weeks
Urinary bladder
Effective Simple PN Total
no. of rats hyperplasia hyperplasia papillomas cancer neoplasms
29 29/29 25/29 5/29 0/29 5/29 (17%)
15 13/15 14/15 2/15 1/15 3/15 (20%)
Pretreatment of additional rats with 0.01% BBN significantly
increased the incidence of simple hyperplasia and papillary/nodular
(PN) hyperplasia, but not of papillomas or cancers over that of rats
treated with BBN alone. Pretreatment of additional rats with 0.05% BBN
significantly increased the incidence of PN hyperplasia, papillomas
and cancers over that of rats treated with BBN alone (Fukushima
et al., 1983).
SOPP was administered in the diet to 28 male F344 rats (Charles-
River, Japan) for 64 weeks at a dosage level of 2.0%. One rat had
small stone formations in the urinary bladder. SOPP induced simple
hyperplasia, PN hyperplasia (19/28, 68%), papillomas (5/28, 18%) and
cancers (6/28, 21%) of the urinary bladder. Pretreatment of additional
rats with BBN increased the incidence of PN hyperplasia (p < 0.05),
papillomas (non-significant), and cancers (non-significant) over that
of rats treated with BBN alone.
In another experiment, SOPP was administered in the diet to male
F344 rats for up to 104 weeks at dosage levels of 0, 0.25%, 0.5%,
1.0%, or 2.0%. Animals from each group were periodically sacrificed
and examined at 4, 8, 12, 24, 36 and 104 weeks. No stone formation was
observed in the urinary bladder of any rats treated with SOPP. In the
2.0% group, simple hyperplasia was observed at 4 weeks and beyond and
PN hyperplasia at 36 weeks and beyond (in all rats at 104 weeks).
Papillomas (2/5, 40%) and cancers (2/5, 40%) of the urinary bladder
were observed in rats administered 2.0% SOPP at 104 weeks. In the 1.0%
group, only simple hyperplasia was observed (> 36 weeks) (Ito,
1983b).
phi-Phenylphenol, o-phenylphenol (OPP), o-phenylphenol sodium
salt tetrahydrate (SOPP), and some biphenyl derivatives were examined
for their bladder carcinogenicity to rats by a short-term assay for
agglutinability of bladder epithelial cells with concanavalin A.
Increased agglutinability was observed after 1-week treatment with
2.0% and 1.0% OPP or 2.0% and 1.0% SOPP, suggesting the bladder
carcinogenicity of these compounds. Such an increase in
agglutinability was not observed in rats fed diets containing
phi-phenylphenol or biphenyl derivatives at 2.0%. An in vivo
carcinogenesis experiment was perfomed with SOPP. As shown in Table
11, bladder carcinomas developed in 14 out of 36 male Fisher rats
(Charles-River, Japan) fed a diet containing 2% SOPP for 50 weeks
(Honma et al., 1983).
Table 11. Neoplastic lesions of the urothelial system in rats
administered sodium 2-phenylphenate
Urinary bladder
Dose Transitional Total
(% in diet) Papillomas cell carcinomas neoplasms(1)
0 0/11 0/11 0/11 (0%)
2.0(2) 19/36** 14/36* 33/36 (92%)**
* p < 0.05
** p < 0.01
(1) No calculi were observed in any of the treated or control rats.
(2) 31/36 of these rats had PN hykperplasia in the urinary bladder.
In addition, 3/36 and 9/36 of these rats had papillomas and
PN hyperplasia, respectively, in the renal pelvis.
SOPP was administered in the diet to 30 male F344 rats (Charles-
River, Wilmington, MA) for up to 90 days at a dosage level of 2.0%.
Interim sacrifices were performed at 3, 7, 14, 30 and 65 days. Only 7
rats per group were permitted to live to 90 days, at which time they
were sacrificed.
Urinary bladder pathology was observed in these rats as follows:
increased mitosis, beginning at 3 days, in the epithelium; thickening
(i.e. simple hyperplasia), beginning at 14 days, in the epithelium. No
tumours of any kind were observed in the urinary bladder (Reitz
et al., 1983).
Special studies on mutagenicity
OPP
Ames tests utilizing S. typhimurium strains TA92, TA1535,
TA100, TA1537, TA94 and TA98, without and with metabolic activation
derived from livers of male Fischer rats, were uniformly negative.
Chromosomal aberration tests using Chinese hamster fibroblasts in
culture gave ambiguous results without and with metabolic activation
(Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA1537, TA98 and
TA100 were negative without and with metabolic activation derived from
male rats. Strain TA1535, however, gave a weakly-positive response
without activation, but a negative response with activation. A mouse
lymphoma assay (L5178Y/TK+/-) was weakly positive without and with
metabolic activation. Chromosomal aberration assays using Chinese
hamster ovary cells, without and with activation, were negative. A
sister chromatid-exchange assay using Chinese hamster ovary cells was
weakly positive without activation, but negative with activation. A
sex-linked recessive-lethal assay in Drosophila melanogaster was
negative (Luster, 1985).
In a dominant-lethal study, OPP of 99.7% purity was administered
by gavage to 10-week-old male C3H mice each day for 5 days at dosage
levels of 0 (control), 100, or 500 mg/kg/day. Fifteen mice were
treated at each dosage level. An additional positive control group of
15 male mice was given a single intraperitoneal dose of 300 mg/kg of
ethylmethanesulfonate (EMS). Immediately following dosing, each male
mouse was mated with 2 untreated virgin female mice for 7 days. At the
end of 7 days, the female mice were replaced by another 2 female mice.
The replacement procedure was repeated for a total of 6 weeks. All
female mice were sacrificed on days 12-13 of gestation and scored for
numbers of corpora lutea, implants, living embryos, and early or late
embryonic deaths. Frequencies of induced dominant-lethal mutations
were calculated. Scores for control and treatment groups were compared
for each 7-day period. For the positive control group, the frequency
of early embryonic deaths was increased in weeks 1, 2, 3, and 6. No
untoward differences from control values were detected for mice
treated with OPP. Dominant-lethal mutations were not induced in this
study by treatment with OPP (Kaneda et al., 1978).
SOPP
Ames tests using S. typhimurium strains TA98 and TA100 were
negative without and with metabolic activation derived from livers of
male Fischer rats. Chromosomal aberration tests using Chinese hamster
fibroblasts in culture gave ambiguous results without and with
metabolic activation (Ishidate et al., 1983).
Ames tests utilizing S. typhimurium strains TA98, TA100,
TA1535, TA1537, and TA1538 were negative without and with metabolic
activation. An unscheduled DNA synthesis assay in primary rat
hepatocytes in culture was negative (Reitz et al., 1983).
Possible metabolites of OPP and SOPP
Two possible metabolites of OPP and SOPP, 2,5-dihydroxybiphenyl
and 2-phenyl-l,4-benzoquinone, were tested in Ames S. typhimurium
strains TA98, TA100 and TA1537. Both compounds gave negative results
without and with metabolic activation derived from livers of male
Fischer rats. Chromosomal aberration tests on both compounds using
Chinese hamster fibroblasts in culture gave negative results without
and with metabolic activation (Ishidate et al., 1983).
COMMENTS
In response to the requirement for further information, a
protocol for a proposed 2-generation reproduction study in rats using
OPP as the test material has been received (Rao, 1985). No information
on the progress of a new carcinogenicity/chronic toxicity study in a
strain of rats other than F344 has been received. Some new metabolic
and pharmacokinetic data have been received, but they are very limited
with respect to elucidating possible biochemical mechanisms of action
of carcinogenicity.
An IARC monograph on OPP and SOPP was published in 1983 (IARC,
1983). This document summarizes chemical and physical data,
production, use and occurence of these chemicals. It also evaluates
biological data, relevant to the oncogenic risk to humans, that was
available prior to 1983.
Recent carcinogenicity studies on sodium 2-phenylphenate (SOPP)
have reported statistically-significant increased incidences of
carcinomas in the urinary bladder, and some in the renal pelvis, of
male F344 rats following administration of 1% and 2% SOPP in the diet.
In female F344 rats, an increased, but lower, incidence of urinary
bladder neoplasms was also reported at a dietary dosage level of 1%.
Similar tumours observed at lower dietary dosage levels (0.5% and
0.7%) in male and female rats, although not statistically significant,
were considered to be biologically relevant owing to the rarity of
these tumours in this rat strain and their absence in control animals.
There does not appear to be a cause-effect relationship between stones
and neoplasms in the urinary bladders of rats. Therefore, SOPP has
been determined to be a carcinogen to the urothelium of F344 rats.
Although fewer carcinogenicity studies on 2-phenylphenol (OPP)
are available than on SOPP, some recent evidence suggest that OPP may
also be a carcinogen on the urinary bladder of male F344 rats at
dietary dosage levels of 1.25% and 2.5%. Another earlier study did
not, however, demonstrate a similar response in male or female Wistar
rats. OPP has not been adequately tested to permit a definitive
conclusion regarding its carcinogenicity.
Carcinogenicity studies in mice with both OPP and SOPP were
negative for tumourigenic effects. The dosage level in the OPP study
may have been too low to permit expression of tumours.
Mutagenicity assays with OPP and SOPP in bacteria have been
overwhelmingly negative, although weakly positive results have been
occasionally noted in isolated instances. Results of studies in
cultured mammalian cells have been mixed. Both negative and weakly
positive responses in several assays have been reported. Results from
in vivo studies have been regularly negative.
A series of in vitro and in vivo biochemical studies suggest
the possibility that an altered metabolic pathway for OPP/SOPP,
occurring at high dosage levels only, may be related to (and
presumably responsible for) the occurrence of urothelial tumours at
similar dosage levels in long-term studies. Although this speculation
appears plausible at this time, considerably more direct experimental
support will be required before it can be accepted as fact.
Teratology studies on rats using OPP as the test material were
negative for teratogenic effects.
With respect to the oncogenic potential of OPP and SOPP, the
Meeting compared results from available oncogenic studies to the low
anticipated dietary exposures to OPP and SOPP. On the basis of this
comparison and other information, the Meeting agreed to extend the
temporary ADI for OPP/SOPP to 1989.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg b.w.
Dog: 500 mg/kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.02 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1989 or earlier)
1. A multigeneration reproduction study.
2. A carcinogenicity/chronic toxicity study in a rat strain known to
be sensitive to induction of bladder carcinomas (a strain other
than F344).
3. Metabolic, pharmacokinetic, and other related studies, as
appropriate, in the strains of animals tested in long-term
studies for carcinogenicity and in other species, including a
consideration of species, sex and dosage-level differences.
4. Qualitative and quantitative monitoring data on the urinary
excretion of OPP and/or its metabolites by industrial workers, or
others, regularly exposed to OPP or SOPP.
5. Mutagenicity studies on urinary metabolites of OPP and/or SOPP.
DESIRED
Additional observations in man.
REFERENCES
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. Promoting
(1983) effect of sodium o-phenylphenate and o-phenylphenol on two-
stage urinary bladder carcinogenesis in rats. Gann 74,
625-632.
Hagiwara, A., Shibata, M., Hirose, M, Fukushima, S. & Ito, N. Long
(1984) term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Fd. Chem. Toxicol. 22,
809-814.
Hiraga, K. Short report of a 91-week feeding study of
(1983a) orthophenylphenon (OPP) in male rats. Unpublished report
from the Department of Toxicology, Tokyo Metropolitan
Research Laboratory of Public Health, Tokyo, Japan.
Submitted to WHO by Dow Chemical Co., Midland, MI, USA.
Hiraga, K. Carcinogenicity testing of sodium orthophenylphenate in
(1983b) F-344/DuCrj rats. Unpublished report from the Department of
Toxicology, Tokyo Metropolitan Research Laboratory of Public
Health, Tokyo, Japan. Submitted to WHO by Dow Chemical Co.,
Midland, MI, USA.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary system in
(1981) F344 rats by dietary administration of sodium
o-phenylphenate.. Fd. Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. Induction of tumours of the urinary bladder in
(1984) F344 rats by dietary administration of o-phenylphenol.
Fd. Cosmet. Toxicol., 22, 865-870.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T., & Sugimura, T.
(1983) Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
IARC. International Agency for Research on Cancer. IARC Monographs on
(1983) the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Miscellaneous Pesticides, 30, 329-344.
Ishidate, M., Jr., Yoshikawa, K., & Sofuni, T. Mutagenicity tests on
(1983) OPP, OPP-sodium salt, and their possible metabolites.
Unpublished report from the Division of Mutagenesis,
Biological Safety Research Center, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Long-term toxicity and carcinogenicity study of sodium
(1983a) o-phenyl-phenate in B6C3F1 mice, Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
Ito, N. Pathological analysis carcinogenicity of the sodium
(1983b) o-phenylphenate in B6C3F1 mice. Unpublished report from the
First Department of Pathology, Nagoya City University
Medical School, Nagoya, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz, B.A. Teratological
(1981) evaluation of othophenylphenol in rats. Fund. Appl.
Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. Teratogenicity and
(1978) dominant-lethal studies with o-phenylphenol. J. Pesticide
Sci., 3, 365-370.
Luster, M. NTP technical report on the toxicology and carcinogenesis
(1985) studies of ortho-phenylphenol (CAS No. 90-43-7) alone with
7,12-dimethyl-benz(a)anthracene (CAS No. 57-97-6) in Swiss
CD-1 mice (dermal studies). NIH Publication No. 85-2557, NTP
84-099, U.S. Department of Health and Human Services (Board
Draft, 2/85, National Toxicology Program, NTP TR 301).
Nakagawa, A., Nakao, T., & Nakao, M. Altered levels of cyclic
(1984) nucleotides in F344 rats fed sodium o-phenylphenate.
Fd. Chem. Toxicol., 22, 217-221.
Nakao, T., Ushiyama, K., Kabashima, J., Nagai, F., Nakagawa, A., Ohno,
T., Ichikawa, H., Kobayashi, H., & Hiraga, K. The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Fd. Chem. Toxicol., 21, 325-329.
Oehme, F.W. & Smith, T.H.F. The metabolism and urinary excretion of
(1972) o-phenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Rao, K.S. Proposed reproduction study protocol, orthophenylphenol:
(1985) Two generation dietary reproduction study in rats. Submitted
to WHO by Dow Chemical Co., Midland, MI, U.S.A.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A., & Watanabe, P.G.
Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem. Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP)
on the urinary tract of male F344 rats. Tox. Appl.
Pharmacol. 73,345-349.
Savides, M.C. & Oehme, F.W. Urinary metabolism or orally administered
(1980) o-phenylphenol in dogs and cats. Toxicology, 17,
355-363.
Yamaha, T., Tanaka, A., Sato, M., & Tsuchiya, T. Excretion,
(1983) distribution and metabolic fate of sodium o-phenylphenol and
o-phenylphenol in the rat. Unpublished report from the
Division of Medical Chemistry, National Institute of
Hygienic Sciences, Tokyo, Japan. Submitted to WHO by Dow
Chemical Co., Midland, MI, USA.
