
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
2-PHENYLPHENOL AND ITS SODIUM SALT
First draft prepared by
Jens-Jorgen Larsen
Institute of Food Safety and Toxicology
Ministry of Food, Agriculture and Fisheries, Soborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multugeneration reproductive toxicity
Developmental toxicity
Special studies: Mechanisms of carcinogenicity in rat
urinary bladder
Observations in humans
Comments
Toxicological evaluation
References
Explanation
2-Phenylphenol and its sodium salt were evaluated by the 1969,
1983, 1985, 1989, and 1990 Joint Meetings (Annex 1, references 12,
40, 44, 56, and 59). A temporary ADI of 0-0.02 mg/kg bw was
allocated in 1983, which was extended in 1985 and 1989. An ADI of
0-0.02 mg/kg bw was established in 1990. Since that Meeting, studies
have become available on biochemical aspects, biotransformation,
effects on enzymes and other biochemical parameters, acute toxicity,
short-term toxicity, long-term toxicity, genotoxicity, reproductive
toxicity, dermal and ocular irritation and dermal sensitization, and
on the mechanism of the carcinogenic effect in the rat urinary
bladder. The compound was reviewed by the present Meeting within the
periodic review programme of the Codex Committee on Pesticide
Residues.
The toxicological data on the sodium salt of 2-phenylphenol were
not used to establish the ADI, since the salt rapidly dissociates to
2-phenylphenol. These data were, however, considered of value for the
review and are therefore included.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Groups of four male Fischer 344 rats were given single oral doses
of [14C]2-phenylphenol (purity, 99.8%; specific activity, 19
mCi/mmol) or [14C]sodium 2-phenylphenol (purity, 98.7%) by gavage at
a dose of 500 mg/kg bw and were immediately placed in glass metabolism
cages. About 90-95% of the administered radiolabel on both compounds
was recovered in urine and 5-6% in faeces, mainly during the first 24
h. The rates of urinary excretion were virtually identical in the two
groups. In a second experiment, animals were fed diets containing
13 000 ppm of 2-phenylphenol or 20 000 ppm of the sodium salt
(equimolar amounts) for 2 weeks before administration of single oral
doses of the labelled compounds. The animals still eliminated most of
the radiolabel (88-94%) in urine and small amounts in faeces (3-5%).
Preconditioning did not greatly affect the disposition of radiolabel,
although the sodium salt appeared to have been eliminated somewhat
more rapidly than 2-phenylphenol (Reitz et al., 1983).
Groups of four male Fischer 344 rats were given single oral doses
of [14C]2-phenylphenol (purity not given; specific activity, 1.6
mCi/mmol) at 160 mg/kg bw or [14C]sodium 2-phenylphenol at 250 mg/kg
bw (equimolar levels; purity not given; specific activity, 1.6
mCi/mmol). The animals were fasted overnight before and for 6 h after
dosing. Urine and faeces were collected daily for 7 days. The
excretion patterns in the two groups did not differ significantly, and
82-98% of the dose was recovered in urine and only 2-5% in faeces
within 24 h of dosing. Two male rats received bile duct cannulae, and
bile was collected for 3 days after a single oral dose of 250 mg/kg bw
of radiolabelled sodium salt. Excretion of radiolabel in the bile
began within the first hour of dosing, reached a peak within 3-6 h,
and was almost complete by 8 h' about one-fourth of the dose was
recovered in bile over the 3-day collection period. The authors
interpreted these results as indicating rapid absorption from the
intestine and enterohepatic circulation of 2-phenylphenol metabolites.
The pattern of distribution in organs and tissues, examined on days 1,
3, and 7 after administration of the sodium salt and on days 1 and 7
after administration of 2-phenylphenol, showed little difference.
Little radiolabel was retained in organs and tissues. including the
urinary bladder (Yamaha et al., 1983; Sato et al., 1988).
In a comparative study, [12C/13C/14C]2-phenylphenol
(purity, 99.5%; specific activity, 48 mCi/mmol) was given to 10 male
B6C3F1 mice as a single oral dose of 15 or 800 mg/kg bw, to 10 male
and 10 female Fischer 344 rats as a single oral dose of 28 or 27 mg/kg
bw, and to six male volunteers as a dermal dose of approximately 6
µg/kg bw on the forearm for 8 h. The compound was well absorbed in the
mice, 84% and 98% of the two doses being recovered in urine collected
over 48 h. Extensive absorption and rapid elimination were also seen
in the rats, 89 and 86% of the dose being found in the urine of males
and females, respectively, within 24 h. 2-Phenylphenol was also
rapidly eliminated by the volunteers, 99% of the absorbed dose being
collected in urine within the first 48 h of exposure (Bartels et al.,
1998).
The skin of the forearm of six male volunteers aged 19-27 and
weighing 58-98 kg was exposed to 100 µl of a 0.4% isopropanol solution
of [13C/14C]2-phenylphenol providing a dose of approximately 6
µg/kg bw and 42 µCi for 8 h. Samples of blood, urine, and faeces were
collected at various times for five days, and blood samples were also
taken during exposure. High concentrations of radiolabel in blood were
observed within the first 2 h of the start of exposure in all
subjects, indicating rapid absorption. The rate diminished fairly
rapidly at the end of the exposure period, and little or no radiolabel
was present in venous blood samples collected 2 days after termination
of exposure. About 43% of the applied dose of 2-phenylphenol was
absorbed, about 58% of which was recovered in swabs, skin rinse,
gauze, and the protective enclosure. The majority (99%) of the
absorbed compound was excreted in urine, and faeces represented a
minor route of elimination (1% within 5 days). A mean of 0.04% of the
administered radiolabel was found in the tape strips covering the
application site, indicating no accumulation in the superficial layers
of the skin (Selim, 1996).
The plasma concentrations peaked within 4 h of dosing and then
declined rapidly, virtually all of the absorbed dose being excreted in
urine within 24 h. A one-compartment model was used to describe the
pharmacokinetics of absorption and clearance of [14C]2phenylphenol
in these volunteers. Approximately 43% of the applied dose was
absorbed through the skin, with an average absorption half-time of
10 h. Once absorbed, its renal clearance was rapid, with an average
elimination half-time of 0.8 h. Overall, the pharmacokinetics of
[14C]2-phenylphenol was similar in the individual volunteers, and
the model parameters were in excellent agreement with the experimental
data. The rapid excretion in urine indicates that 2-phenylphenol is
unlikely to accumulate in humans exposed repeatedly (Timchalk, 1996).
(b) Biotransformation
Groups of 10 male B6C3F1 mice were given a single oral dose of
[14C]2-phenylphenol (specific activity, 48 mCi/mmol) at 25 or 1000
mg/kg bw or five daily doses of unlabelled compound (purity, 99.5%) at
1000 mg/kg bw per day followed by a single oral dose of labelled
compound at 1000 mg/kg bw and were killed 48 h after dosing. For
comparison, groups of two male and two female Fischer 344 rats were
given a single oral dose of labelled compound at 25 or 125 mg/kg bw
and were killed 24 h after dosing. The excretion of
[14C]2-phenylphenol in mice was rapid and was complete by 12-24 h
after dosing, with 74-98% of the recovered radiolabel in urine and
6-13% in faeces; < 1% was recovered in the tissues and carcass. Eight
radiolabelled metabolites were detected in the urine of both mice and
rats, with no major differences in distribution by species, by sex in
the rats, or single or repeated dosing in mice. A small amount (0.4%)
of free 2-phenylphenol was detected only in urine of female rats given
the single dose of 125 mg/kg bw. Four major urinary metabolites were
identified: phenylhydroquinone glucuronide, phenylhydroquinone
sulfate, 2-phenylphenol sulfate, and 2-phenylphenol glucuronide,
accounting for about 98% of the recovered dose in mice and 102% in
rats. An additional metabolite which accounted for about 2.7% of the
recovered dose in rat urine was tentatively identified as the sulfate
conjugate of 2,4'-dihydroxybiphenyl. No qualitative difference in
metabolites was observed in male mice, but a dose-dependent,
quantitative difference was noted in the extent of sulfation and
glucuronidation of 2-phenylphenol. After a single dose of 25 mg/kg bw
to mice, the sulfate was the major urinary metabolite, accounting for
56% of the recovered radiolabel, while the glucuronide accounted for
29%. After single or repeated doses of 1000 mgkg bw, the glucuronide
was the major metabolite, accounting for 48-60% of the urinary
radiolabel, while the sulfate accounted for 20-27%. In rats given a
single oral dose of 25 mg/kg bw, 2-phenylphenol sulfate was the major
metabolite, accounting for 91% of the recovered radiolabel, while the
glucuronide accounted for only 7%. Formation of phenylhydroquinone
glucuronide and sulfate represented minor metabolic pathways,
accounting for 11-23% and 2-7% of the radiolabel in mice and rats,
respectively. The extent of conjugation was not dose-dependent in mice
given a single oral dose of 25 or 1000 mg/kg bw of 2-phenylphenol. The
authors concluded that 2phenylphenol is completely metabolized in mice
and rapidly eliminated in the urine, predominantly as the sulfate and
glucuronide conjugates. The extent of metabolism was qualitatively
comparable in mice and rats, although quantitative differences were
seen in the extent of conjugation (McNett et al., 1997).
In the comparative study of Bartels et al. (1998) described
above, sulfation of 2-phenylphenol was found to be the major metabolic
pathway at low doses in all three species, accounting for 57% of the
urinary radiolabel in male mice given 15 mg/kg bw, 82% in male rats
given 28 mg/kg bw, and 69% in the male volunteers given 0.006 mg/kg
bw. The glucuronide was also found, representing 29, 7, and 4% of the
total urinary metabolites at these low doses in the three species,
respectively. Conjugates of phenylhydroquinone accounted for 12, 5,
and 15% of the dose in mice, rats, and humans, respectively. Little or
no free 2-phenylphenol was found in any species, and no free
phenylhydroquinone or phenylbenzoquinone was found in any species,
with a limit of detection of 0.1-0.6%. A novel metabolite, the sulfate
conjugate of 2,4'-dihydroxybiphenyl, was identified in rats and
humans, comprising 3 and 13% of the low doses, respectively.
Dose-dependent shifts in the conjugation of parent 2-phenylphenol were
seen in mice, indicating saturation of the sulfation pathway after the
high dose of 800 mg/kg bw. Dose-dependent increases in the total
amount of phenylhydroquinone were also observed in the mice.
The major metabolites identified in the urine of five male and
five female Fischer 344 rats fed 20 000 ppm of sodium 2-phenylphenol
(purity not given) in the diet for 136 days were glucuronide
conjugates of 2-phenylphenol and 2,5-dihydroxybiphenyl. Trace amounts
of phenyl-1,4-benzoquinone were also tentatively identified.
Unconjugated phenolic metabolites accounted for only 1% of the
phenolic metabolites excreted; no other metabolites were found. By 24
h after feeding, 55% of the dose had been recovered in males and 40%
in females. A sex difference was found in the proportion of urinary
metabolites, male rats excreting 1.8 times as much conjugated
2-phenylphenol and more than 7 times as much conjugated
2,5-dihydroxybiphenyl as female rats in 24-h urine samples. No
explanation was given for the inability to find the sulfate ester of
2-phenylphenol in urine in this study. As only 40-55% of the
administered dose was recovered, it may have been present but not
identified (Nakao et al., 1983).
Single oral doses of 5, 50, or 500 mg/kg bw of
[14C]2-phenylphenol (purity, 99.8%; specific activity, 19 mCi/mmol
per L) or [14C]sodium 2-phenylphenol (purity, 98.7%; specific
activity, 19 mCi/mmol/L) were administered to groups of four male
rats, and the urinary metabolites were identified and quantitified. At
the two lower doses, the major metabolites of both compounds were the
glucuronide and sulfate ester conjugates of 2-phenylphenol, and
unconjugated 2-phenylphenol and 2,5-dihydroxybiphenyl accounted for
< 2% of the total radiolabel recovered in urine at a limit of
detection of 1-2%. Nearly identical high-performance liquid
chromatograms were obtained for the two compounds. At 500 mg/kg bw, a
further metabolite of both compounds was identified, which accounted
for 20-30% of the urinary radiolabel and appeared to be a conjugated
dihydroxybiphenyl molecule, most likely with glucuronide and/or
sulfate groups. The authors hypothesized that this metabolite is
formed only at high doses as a result of saturation of normal
glucuronide and sulfate ester conjugation pathways. Incubation of
[14C]2-phenylphenol with purified microsomes in vitro in the
absence of conjugating substrates yielded large amounts of a material
which co-chromatographed with 2,5-dihydroxybiphenyl. The semiquinone
and quinone were not identified in these studies, but their formation
was proposed on the basis of the results of similar studies on benzene
(Reitz et al., 1983).
In a study of toxicity in male Fischer 344 rats given diets
containing 0, 800, 4000, 8000, or 12 500 ppm of 2-phenylphenol
(purity, 99.5%) for 13 weeks, the DNA of the urothelium was isolated
at the end of the study and examined for covalent adducts of
2-phenylphenol by the 32P-postlabelling assay. The concentrations of
2-phenylphenol metabolites were also measured in overnight urine
samples collected from the animals at the end of the study. The
glucuronide and sulfate conjugates of 2-phenylphenol and the
hydroxylated metabolite, 2,5-phenylhydroquinone, were found to be the
major metabolites. The major conjugation in all samples was with
sulfate. The formation of this metabolite appeared to be saturated at
8000 ppm, while the concentrations of the remaining three conjugated
metabolites increased in a dose-dependent fashion up to the high dose.
Traces of free 2-phenylphenol and phenylhydroquinone were observed at
all doses, free phenylhydroquinone comprising 0.6-1.5% of the total
metabolites measured. The concentrations of creatinine were comparable
in all groups (Bartels & McNett, 1996).
Mature cats and dogs were given [14C]sodium 2-phenylphenol
(purity and specific activity not given) at single oral doses < 3
g/kg bw. The amount of radiolabel in plasma was higher in dogs than in
cats, and the dogs metabolized and excreted three times more
radiolabel in urine than cats. The urinary metabolites were unchanged
2-phenylphenol, glucuronide and sulfate conjugates, and phenol derived
from cleavage of the phenylphenol bond and ring hydroxylation. The
phenol metabolites were derived from both 2-phenylphenol ring moieties
(Oehme & Smith, 1972).
Urine samples were collected weekly after single oral doses every
second day of [14C]2-phenylphenol (purity, 95%) to three male and
three female mature beagle dogs (0.3 mg/kg bw per day), three male and
three female immature beagle dogs (2.0 mg/kg bw per day), three male
and three female mature domestic cats (1.2 mg/kg bw per day), and
three male and three female immature domestic cats (2.0 mg/kg bw per
day) for 8 weeks. The main urinary excretion product was unchanged
2-phenylphenol, representing 70-90% of the radiolabel in dogs and
95-98% in cats. Dogs excreted significantly more glucuronide- and
sulfate ester-conjugated 2-phenylphenol than cats, and immature dogs
excreted four times as much glucuronide conjugate as mature dogs. The
age differences did not affect the rate of excretion of the sulfate
ester conjugate in either species (Savides & Oehme, 1980).
In the study of Selim (1996) in volunteers treated dermally,
described above, 99% of the absorbed dose of 2-phenylphenol was
eliminated in urine, primarily as polar conjugates or hydroxylated
metabolites. The major urinary metabolite was the sulfate conjugate,
which accounted for 68% of the absorbed dose; conjugation with
glucuronic acid accounted for only 3%. Hydroxylation of the phenol or
phenyl ring, followed by conjugation, was also significant,
phenylhydroquinone glucuronide representing 14% of the absorbed dose
and 2,4œ-dihydroxybiphenyl sulfate, 12%. Traces of unmetabolized
parent compound (0.5% of absorbed dose) were found only in samples
taken shortly after administration. No free phenylhydroquinone or
phenylhydroquinone-sulfate was found in urine (Bartels et al., 1997;
Timchalk et al., 1998).
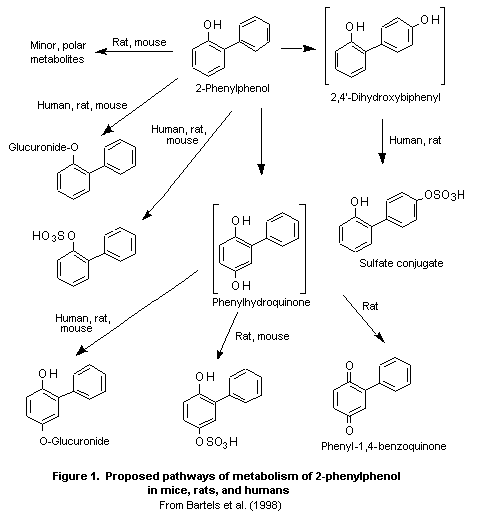
The proposed metabolic pathways of 2-phenylphenol in rodents and
humans are shown in Figure 1.
(c) Effects on enzymes and other biochemical parameters
2-Phenylphenol
2-Phenylphenol was converted to 2,5-dihydroxy biphenyl
(phenylhydroquinone) by microsomal cytochrome P450 enzymes. Depending
on the cofactor used, the microsomal enzymes catalysed either
oxidation and/or reduction of the metabolite. Phenylhydroquinone was
oxidized to phenyl-1,4-benzoquinone by cumene
hydroperoxide-supported enzymes, and this compound was reduced to
phenylhydroquinone by cytochrome P450 reductase. This study provides
direct evidence of cytochrome P450-catalysed redox cycling of
 2phenylphenol, which may play a role in the induction of bladder
cancer by this substance (Roy, 1990).
Activation of the 2-phenylphenol metabolite phenylhydroquinone by
prostaglandin (H) synthase in the presence of arachidonic acid and
hydrogen peroxide was studied to test the hypothesis that
prostaglandin synthase in rat urinary bladder transitional epithelium
and kidney medullar papilla is responsible for activation of the
metabolite to reactive intermediates in the bladder and kidney.
Phenylhydroquinone was found to be metabolized by the peroxidase
activity of prostaglandin synthase and by other peroxidases such as
horseradish peroxidase and myeloperoxidase, suggesting that the
peroxidative metabolism of phenylhydroquinone could play a role in
urinary bladder and kidney carcinogenesis in rats (Kolachana et al.,
1991).
In a study of the effect of pH on nonenzymatic oxidation of
phenylhydroquinone, the effects of phenylbenzoquinone and oxygen
concentration on autoxidation of phenylhydroquinone, and the
nonenzymatic conversion of phenylbenzoquinone to phenylhydroquinone, a
curvilinear relationship was found between the rate of oxidation of
phenylhydroquinone and pH over the range 6.3-7.6. Phenylbenzoquinone
was formed during autoxidation of phenylhydroquinone, with a yield of
0.92 ± 0.02. The results indicate that the production of reactive
metabolites from phenylhydroquinone involves both a pH-independent
(i.e. oxygen-dependent) and a pH-dependent pathway and that the
presence of phenylbenzoquinone enhances the rate of pH-dependent
phenylhydroquinone autoxidation. The authors suggested that ionization
of phenylhydroquinone semiquinone is a key step in production of
reactive species in the pH-dependent pathway. They found a good
correlation between the proposed reaction pathway and the induction by
2-phenylphenol of bladder lesions in rats. Thus, pH-dependent
autoxidation of free phenylhydroquinone in urine may be responsible
for the tumorigenic effects of 2-phenylphenol and sodium
2-phenylphenol in the rat bladder (Kwok & Eastmond, 1997).
Groups of eight female B6C3F1 mice were given 0, 1, 10, or 200
mg/kg bw per day of 2-phenylphenol (purity, > 98%) by gavage on 5
days per week for 2 weeks. As a positive control, mice were given 45
mg/kg bw of cyclophosphamide intraperitoneally for 4 days. The weights
of the body, liver, spleen, kidney, and thymus were recorded, and
samples were prepared for histopathological examination. Haematology
and clinical chemistry were conducted, and bone-marrow cellularity and
colony formation, lymphoproliferative responses, delayed
hypersensitivity responses, immunoglobulin, antibodies, response to
Listeria monocytogenes challenge, and tumour susceptibility were
studied. None of the treated animals died or showed signs of toxicity.
Histopathological examination revealed no significant lesion in any
tissues. The weight of the thymus and the relative weight of the
spleen were slightly increased at 200 mg/kg bw per day. The slight
haematological alterations seen did not show a dose-response
relationship and were within the normal range of biological variation.
A slight increase in serum cholesterol concentration and a
corresponding decrease in triglyceride concentration were seen in mice
at 200 mg/kg bw per day. The activity of alanine aminotransferase and
total protein in serum were not affected, although the
albumin:globulin ratio was slightly decreased at the high dose.
Bone-marrow cellularity, lymphoproliferative responses, immune
function, and host susceptibility were not altered. In contrast,
treatment with cyclophosphamide resulted in marked alterations. The
authors concluded that 2-phenylphenol, even at relatively high doses,
did not alter immune function or host susceptibility (Luster et al.,
1981).
Sodium 2-phenylphenol
Binding of sodium 2-phenylphenol metabolites to macromolecules
in vitro was studied by incubating [14C]sodium 2-phenylphenol
(specific activity, 19 mCi/mmol) with purified liver microsomes from
male rats in the presence of a NADPH regenerating system and bovine
serum albumin, which served as a 'protein acceptor'. Macromolecular
binding of radiolabel to protein, which was dependent on the presence
of both active microsomes and NADP, was observed. In order to study
the binding of metabolites of 2-phenylphenol and its sodium salt to
macromolecules in the liver, kidney, and urinary bladder in vivo,
groups of four male rats were given single oral doses of
14C-labelled compounds at doses of 50, 100, 200, or 500 mg/kg bw,
and tissues were excised 16-18 h later for measurement of
macromolecular binding, which was determined as nanomoles of bound
material per milligram of protein. The extent of binding was not
linearly related to the administered dose. Disproportionate increases
were seen in each tissue at doses of sodium 2-phenylphenol > 200
mg/kg bw and in liver and bladder at doses of 2-phenylphenol at
200-500 mg/kg bw (Reitz et al., 1984).
Sodium 2-phenylphenol (purity not given) was administered in the
diet at a concentration of 20 000 ppm to 4-week-old male and female
Fischer 344 rats for 136 days. Urine was collected periodically. At
the end of treatment, the rats were killed, blood samples were
collected, and livers and kidneys were removed. The amounts of cyclic
nucleotides (cAMP and c-GMP) were determined in urine, plasma, liver,
and kidneys, and adenylate cyclase activity was measured in liver and
kidneys. In male rats, the c-AMP levels in urine and plasma were
decreased whereas the c-GMP levels were increased. In females, c-AMP
levels were decreased only during the first 3 days of feeding, and the
levels of c-AMP and cGMP in liver and kidneys were unchanged. The
decreased urinary c-AMP in male rats was probably the result of
decreased adenylate cyclase activity in liver and kidneys. A similar
change in adenylate cyclase activity was observed in liver but not in
kidneys of female rats treated with sodium 2-phenylphenol. The
sex-related alterations in cyclic nucleotide levels were postulated to
be involved in the sex-dependent induction of urinary bladder tumours
by sodium 2-phenylphenol (Nakagawa et al., 1984).
In male and female Fischer 344 Du Crj rats given 20 000 ppm of
sodium 2-phenylphenol in the diet for 20 weeks, urinary gamma-glutamyl
transpeptidase activity decreased immediately after the start of
treatment and remained low throughout the study. The activities of
this enzyme and of alkaline phosphatase in kidney homogenate were
found to have decreased to about 80% of the control values at 20
weeks, but the activity of glucose-6phosphate dehydrogenase was
significantly increased; that of Na/K-ATPase was unchanged. In liver
homogenate, however, gamma-glutamyl transpeptidase activity was
increased by about eight times and that of glucose-6-phosphate
dehydrogenase was significantly increased, but the activities of
alkaline phosphatase and Na/K-ATPase were not significantly different
from the control values. The glutathione concentration in the livers
of treated rats was significantly reduced (Nagai & Nakao, 1984).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of 2-phenylphenol
and its sodium salt are summarized in Table 1. The clinical signs of
toxicity were generally nonspecific.
Table 1. Acute toxicity of 2-phenylphenol and its sodium salt
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/L)
2-Phenylphenol
Mouse M Oral 1200 Taniguchi et al. (1981)
F 1100
Mouse M Oral 3500 Tayama et al. (1983, 1984)
F 3200
Rat M Oral 2600 Tayama et al. (1980)
F 2900
Rat M Oral 2800 Gilbert & Crissman (1994)
F 2800
Rat M&F Inhalation
(4 h) > 36 Landry et al. (1992)
Rabbit M&F Dermal > 5000 Carreon & New (1981)
Sodium 2-phenylphenol
Mouse M Oral 900 Ogata et al. (1979)
F 800
Rat M Oral 1700 Taniguchi et al. (1981)
F 1600
Rat M Oral 1100 Tayama et al. (1979)
F 1100
Rat M Oral 850 Gilbert & Stebbins (1994)
F 590
(b) Short-term studies of toxicity
2-Phenylphenol
Rats
2-Phenylphenol (purity, 99.8%) was administered to 30 male
Fischer 344 rats (Charles River) in the diet at a concentration of
20 000 ppm, equal to 1000 mg/kg bw per day, for up to 90 days. Interim
sacrifices were performed at 3, 7, 30, and 65 days. Only seven rats at
each dose were permitted to live to 90 days, at which time they were
killed. Food consumption and body weight were markedly reduced within
the first week and remained low throughout the study. The renal
lesions observed in these rats included focal cortical cysts,
significantly decreased urine specific gravity (at 65 and 90 days),
small amounts of blood in the urine, focal tubular collapse and
atrophy in the cortex, and cystic degeneration (at 65 and 90 days). No
treatment-related urinary bladder lesions were observed. A NOAEL could
not be identified since the body weight was reduced at the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1300, 3100, 6300, 13 000, or 25 000 ppm of
2-phenylphenol (purity not given), equal to 0, 180, 390, 760, 1700,
and 2800 mg/kg bw per day for males and 0, 200, 410, 800, 1700, and
3000 mg/kg bw per day for females, for 12 weeks. Body weight and
body-weight gain were severely depressed in males and females at
25 000 ppm and to a lesser extent (14%) in males fed 13 000 ppm. No
significant treatment-related effects were seen in analyses of urine
performed at weeks 9 and 13. Haematological and blood chemical values
were generally normal, other than a slight decrease in haemoglobin
concentration in male and female rats at the highest dose. The
absolute and relative weight of many organs in male rats at this dose
were significantly decreased. The NOAEL was 6300 ppm, equal to 760
mg/kg bw per day, on the basis of reduced body weight and body-weight
gain at 13 000 ppm (Iguchi et al., 1984).
Groups of five male and five female Fischer 344 rats received
dermal applications of 0, 100, 500, or 1000 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) once daily on 5 days per week for a
total of 15 applications over 21 days. The amount applied per animal
was adjusted weekly on the basis of the body weight of individual
animals and was applied to a 5 cm × 5 cm area of clipped skin on the
back, covered with a nonabsorbent cotton patch held in place by an
elastic wrap secured with adhesive tape. The wraps and patches were
removed not less than 6 h after treatment, and the treated area was
wiped with a wettened gauze pad to remove residual test material.
Control animals were handled in the same way. All animals were
acclimated to the wraps for 2 days before the beginning of treatment.
They were observed at least daily and were given a complete clinical
examination weekly. The skin at the site of treatment was examined
after removal of the wrap on the last day of dosing, each week, and on
the day before necropsy. Body weights were measured weekly and feed
consumption and feed efficiency were calculated weekly. Urine was
analysed on day 19. The rats were fasted overnight before necropsy,
when haematological and serum clinical chemical parameters were
evaluated, all animals were examined for gross pathological changes,
selected organs were weighed, and tissues were preserved. Selected
tissues and all gross lesions from animals in the control and
high-dose groups were examined histologically.
No deaths occurred at any dose. Treatment-related effects
indicative of dermal irritation were observed at the site of
application in animals of each sex at 500 and 1000 mg/kg bw per day.
Female rats appeared to be slightly more sensitive than males, but the
severity of lesions increased with duration of exposure and dose in
both sexes. The irritating effects ranged from scaling to fissures.
Body weights and feed consumption were not affected, and no
significant treatment-related effects were found in the
haematological, clinical chemical, and urinary parameters evaluated.
No treatment-associated alterations were found in the liver or kidneys
(Zempel & Szabo, 1993).
Guinea-pigs
2-Phenylphenol was evaluated in 10 male Hartley albino
guinea-pigs for dermal sensitization potential by a modified Buehler
method. The animals received three dermal applications of 0.4 g of
2-phenylphenol (purity, 99.9%) during a 3-week induction period and
were challenged with 0.4 g of the compound 2 weeks after the last
induction. The condition of the test sites was assessed approximately
24 and 48 h after the challenge. No erythema or oedema was seen in any
of the animals. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Berdasco, 1991).
Ten male Hartley albino guinea-pigs were clipped free of hair on
the day before dosing and received three dermal applications of 0.4 g
of 2-phenylphenol (purity, 99.9%) moistened with 0.20 ml of distilled
water during a 3-week induction period. Two weeks after the last
induction application, they were given a challenge application of
0.4 ml of a 7.5% suspension of the compound in water on another site
for 6 h. Five animals received no induction but a 0.4-ml aliquot of a
7.5% suspension of 2-phenylphenol in water. The condition of the test
sites was assessed approximately 24 and 48 h after challenge. No
erythema was seen, and none of the uninduced animals showed
irritation. The animals were in good health and gained weight during
the study. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Gilbert, 1994b).
Rabbits
The ability of 2-phenylphenol (purity, 99.9%) to cause primary
dermal irritation was studied in three male and three female New
Zealand white rabbits that received applications of aliquots of 0.5 ml
of the substance moistened with 0.3 ml of distilled water for 4 h on
intact skin on a clipped, 10 cm × 10 cm area of the back. The
application sites were graded for erythema and oedema within 30 min
and 24, 48, and 72 h of removal of the patch and on days 7, 8, 9,
10,11, 14, and 15. The animals were weighed on the day of treatment
and at the end of the study. Very slight erythema was observed at the
application site in one of the six rabbits within 30 min of removal of
the test material and in two rabbits 24 h later. Severe to slight
eschar formation was observed in four rabbits within 30 min of
treatment which persisted throughout the remainder of the study. Four
animals had burns at the site of application within 30 min, which
resolved as scabs and then scars by the end of the study. Four rabbits
had very slight-to-severe oedema 30 min and 24 h after removal of the
test material, and slight-to-severe oedema was observed on three
animals 48 and 72 h after removal. Body weight was not affected
(Gilbert, 1994a).
Instillation of 0.1 g of 2-phenylphenol (purity not given) into
the right eye of six New Zealand white rabbits resulted in moderate
corneal injury, iritis, and moderate-to-severe conjunctival redness
and chemosis in all animals 24, 48, and 72 h and 7 days after dosing
(Norris, 1971).
Dogs
In studies to assess the palatability and toxicity of
2-phenylphenol (purity, 99.8%) in beagle dogs, males and females were
treated with several regimens. For palatability, one female was given
feed containing 2-phenylphenol to give a dose of 300 mg/kg bw per day
for 5 days. In a study to assess toxicity, groups of two males and
three females were given 300-1000 mg/kg bw per day as a solution in
peanut oil by gastric intubation for up to 9 days or 400-700 mg/kg bw
per day by capsule for 1-2 days. In a 4-week study, groups of two
males and two females were given 0, 100, 200, or 300 mg/kg bw per day
as a solution in peanut oil by gastric intubation on 5 days per week.
In a 1-year study, groups of four males and four females were given
2-phenylphenol as a solution in peanut oil by gastric intubation at
doses of 0, 30, 100, or 300 mg/kg bw per day for 5 days per week for
1 year. The animals were examined daily for clinical signs, and body
weight, feed consumption, haematological, urinary, and clinical
chemical parameters, organ weights, ophthalmologic endpoints, and
gross and histopathology were determined.
Administration of 300 mg/kg bw per day in feed or by intubation
for 5 days resulted in decreased body weights and feed consumption
that correlated with the unpalatability of 2-phenylphenol. Repeated
emesis was seen at doses > 400 mg/kg bw per day administered in
gelatin capsules or in peanut oil by intubation and at doses > 200
mg/kg bw per day by intubation throughout the 4-week study.
Dose-related emesis was also seen in animals given 2-phenylphenol for
1 year. In general, more frequent emesis and ejection of greater
volumes of gastric content was seen in dogs given 300 mg/kg bw per day
than in those given lower doses. While emesis effectively limited the
dose that could be retained, the degree of emesis did not appear to
compromise the health of the animals over 1 year. The reaction was
categorized as a local, transitory response of the mucosal lining of
the upper alimentary tract rather than a reaction of the central
nervous system. No adverse effects were seen on body weight, feed
consumption, haematological, urinary, clinical chemical, or
ophthalmological parameters, organ weights, or gross or histological
appearance of a range of tissues from all dogs. The only deaths were
of two males at the high dose in the 1-year study which died
subsequent to inadvertent deposition of the test solution into the
lungs after approximately 4.5 months of dosing. The NOAEL was 300
mg/kg bw per day (Cosse et al., 1990).
Sodium 2-phenylphenol
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing 0, 2500, 5000, 10 000, 20 000, or 40 000 ppm of sodium
2-phenylphenol as the tetrahydrate (purity not given), equivalent to
0, 270, 550, 1100, 2200, and 4400 mg/kg bw per day, for 13 weeks.
Body-weight gain was significantly depressed in males fed 10 000 or
20 000 ppm and in animals of each sex fed 40 000 ppm. Urinary analysis
showed increased pH and decreased specific gravity at the highest
dose. The relative weights of the livers of animals given 10 000,
20 000, or 40 000 ppm were significantly greater than those of
controls, but no treatment-related histopathological findings were
made. Light and scanning electron microscope examination of the
bladder epithelium at 4, 8, and 13 weeks in three males and three
females in the control group and at 20 000 ppm showed no abnormaility
in the appearance of the bladder epithelium of treated mice at any
time. The NOAEL was 5000 ppm, equivalent to 550 mg/kg bw per day, on
the basis of reduced body-weight gain and increased relative liver
weight at 10 000 ppm (Shibata et al., 1985).
Rats
Sodium 2-phenylphenol (purity, 98.7%) was administered in the
diet to 30 male Fischer 344 rats at a concentration of 20 000 ppm for
up to 90 days. Interim sacrifices were performed at 3, 7, 14, 30, and
65 days. Only seven rats per group were permitted to live to 90 days,
at which time they were killed. The lesions seen in the urinary
bladder epithelium were increased mitosis beginning at 3 days and
thickening (i.e. simple hyperplasia) beginning at 14 days. No tumours
were observed in the bladder. A NOAEL could not be identified since
lesions were observed in the bladder at 20 000 ppm, the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol (purity, > 95%), equal to 0, 85, 180, 350, 710,
1400, and 2500 for males and 0, 87, 180, 350, 690, 1300, and 2400
mg/kg bw per day for females, for 13 weeks. The rats were observed
daily for changes in general condition and were weighed weekly; the
amounts of feed and water consumed were measured on 3 days every other
week. No deaths occurred during the study. A 15-17% decrease in
body-weight gain was seen in animals at doses > 5000 ppm. Urinary
bladder tumours occurred in male rats at frequencies of 1/10 at 10 000
ppm, 9/10 (five transitional-cell carcinomas) at 20 000 ppm, and 1/10
at 40 000 ppm. Six rats at 40 000 ppm had pyelonephritis. In female
rats, the frequencies of tumours were 0/10 at 20 000 ppm and 2/10
(papillomas only) at 40 000 ppm. No bladder calculi were observed in
this experiment. The NOAEL was 2500 ppm, equal to 180 mg/kg bw per
day, on the basis of reduced body-weight gain at 5000 ppm (Iguchi et
al., 1979; Hiraga & Fujii, 1981).
Guinea-pigs
Sodium 2-phenylphenol (purity, 99.1%) was applied to the clipped
skin of 10 male Hartley albino guinea-pigs as a 0.4-ml aliquot of a
0.5% suspension in distilled water at 3-week intervals for induction.
Two weeks after the last dose, a challenge of 0.4 ml of a 0.1%
solution of the compound in distilled water was applied to the other
side of the animals. No erythema occurred at the test site. The
animals gained weight throughout the study. The author concluded that
sodium 2-phenylphenol did not cause delayed contact hypersensitivity
(Gilbert, 1994c).
Rabbits
Sodium 2-phenylphenol diluted 1:200 with distilled water was
instilled into the eyes of six rabbits (strain not given). Temporary,
mild conjunctival reactions were observed in three animals. The
authors concluded that the compound slightly irritated the eye (Davies
& Liggett, 1973).
(c) Long-term studies of toxicity and carcinogenicity
2-Phenylphenol
Mice
Groups of 50 male and 50 female B6C3F1 mice were fed diets
(concentrations not given) supplying 2-phenylphenol (purity, 99.9%) at
doses of 0, 250, 500, or 1000 mg/kg bw per day for 2 years. A
satellite group of 10 male and 10 female mice at each dose was
maintained on the diets for 12 months and then necropsied. All mice
were observed at least once daily for overt signs of toxicity, and a
thorough clinical examination was performed at least once a week
throughout the study. Body weights and feed consumption were recorded
weekly for the first 13 weeks and monthly thereafter. The diets were
prepared weekly or every other week, with adjustments of the
2-phenylphenol concentration according to group mean body weights and
feed consumption to maintain the desired doses for each group.
Clinical signs and mortality rates were unaffected by treatment,
but decreased body weights (by 6-20%) and weight gain (by 10-38%) were
seen in all treated groups except for males fed 250 mg/kg bw.
Haematological, clinical chemical, and urinary parameters in mice
necropsied at 12 and 24 months showed no consistent, toxicologically
significant alterations indicative of target organ toxicity. Changes
in the weights of the adrenal glands, brain, heart, kidneys, liver,
testis, and spleen which were found to be statistically significant
were confounded by the marked decrease in body weight. Nevertheless,
the consistent increases in the absolute and/or relative weights of
the liver at all doses suggests a treatment-related effect. Gross
observations at necropsy in males at the high dose at 12 months and in
males at the intermediate and high doses at 24 months showed a slight
increase in the number of mice with liver masses or nodules.
Microscopic examination of the livers of mice at 12 and 24 months
revealed treatment-related effects at all doses. The cytoplasm of
hepatocytes stained homogeneously, indicating liver enzyme induction,
but there was no evidence of degeneration or necrosis. The microscopic
changes were dose-related and resembled those associated with
adaptation to metabolic demands. An increased incidence of
eosinophilic hepatocellular foci was also observed in males at 100 and
500 mg/kg bw per day.
Males fed 1000 mg/kg bw per day and necropsied at 12 months had a
slightly increased incidence of hepatocellular adenoma. At 24 months,
a statistically significant increase in the number of males with
hepatocellular adenoma was seen at 500 ( n = 40) and 1000 ( n = 41)
mg/kg bw per day, the incidence in controls being 27/50. Low
incidences of a variant form of hepatocellular carcinoma
(hepatoblastoma) were observed in all treated groups of males (2/50 at
250 mg/kg bw per day, 6/50 at 500 mg/kg bw per day, and 3/50 at 1000
mg/kg bw per day versus 0/50 in controls), but the incidence of
hepatocellular carcinoma was not significantly increased at any dose.
The combined incidence of hepatoblastoma and hepatocellular carcinoma
was also not significantly increased in the male mice. The primary
non-tumourous microscopic changes in the livers of male mice, which
appeared to have been adaptive, ultimately resulted in the promotion
of hepatocellular adenomas. The incidences of tumours in other tissues
were not statistically significantly increased. The livers of female
mice showed similar microscopic adaptive changes, but none of them had
hepatoblastoma, and no statistically significant increase in the
incidence of tumours in any tissues was found. Decreased incidences of
microscopic lesions when compared with controls were found in the
adrenals, kidneys, lungs, oral tissues, pancreas, peripheral nerve,
spleen, and testis of males and in the kidneys, lungs, and nasal
tissues of females. These findings were considered to reflect normal
variation and the decreased body weights of the mice and not a primary
response to 2-phenylphenol. A NOAEL for toxicity could not be
identified. The NOAEL for carcinogenicity was 250 mg/kg bw per day on
the basis of an increased incidence of hepatocellular adenomas at 500
mg/kg bw per day (Quast & McGuirk, 1995).
Rats
2-Phenylphenol (purity, 98%) was administered in the diet at
concentrations of 0, 6300, 13 000, or 25 000 ppm, equal to 0, 320,
650, and 1300 mg/kg bw per day to groups of 20-24 male Fischer 344
rats (Charles River) for 91 weeks. The percentage survival was 96, 90,
71, and 65% in the four groups, respectively. In the rats that died
during the study, the incidences of urinary bladder tumours were 0/1
in controls, 0/2 at 6300 ppm, 7/7 at 13 000 ppm, and 0/8 at 25 000
ppm. Bladder lesions were found in 10%, 96%, and 48% of rats at the
three doses, respectively. The incidences of urinary bladder
papillomas and transitional-cell carcinomas were 23/24 at 13 000 ppm
and 4/23 at 25 000 ppm. A NOAEL could not be identified since bladder
lesions were seen at all doses tested (Hiraga, 1983a; Hiraga & Fujii,
1984).
Groups of 70-75 male and 70-75 female Fischer 344 rats were fed
diets containing 2-phenylphenol (purity, 99.5%) at concentrations of
0, 800, 4000, or 8000/10 000 ppm, equal to 0, 39, 200, and 400 mg/kg
bw per day for males and 0, 49, 240, and 650 mg/kg bw per day for
females, for 1 year before interim sacrifice of satellite groups of 20
rats per dose and for 2 years for the remaining 50 rats of each sex.
The animals were observed daily and were examined weekly for clinical
signs of toxicity. Each animal was weighed once a week and also
immediately before necropsy to allow calculation of organ:body weight
ratios. Food consumption was measured weekly. Blood and overnight
urine samples were collected at 3, 6, 12, 18, and 24 months from the
first 20 surviving rats of each sex in the group scheduled for
sacrifice at 2 years. Any dead or moribund animals were prepared for
necropsy, and all surviving animals were killed at the end of the test
periods.
A 5% decrease in body-weight gain was seen in animals at 4000
ppm, and a decrease of 11% was seen in males at 8000 ppm and in
females at 10 000 ppm. Food consumption was unaffected in all groups.
Minor clinical and gross observations included an increased incidence
of abnormally coloured urine, urine stains, and red stains in male
rats given 8000 ppm 2-phenylphenol and an increased incidence of urine
and brown stains in female rats given 4000 or 10 000 ppm. There were
no treatment-related changes in ophthalmological, haematological,
clinical chemical, or urinary parameters, except for an increased
incidence of blood in the urine of males at 8000 ppm. The mortality
rate was slightly increased among males at 8000 ppm. Gross
pathological examination showed increased incidences of urinary
bladder masses in males fed 4000 ppm for 2 years or 8000 ppm for 1 or
2 years and increased incidence of pitted zones and abnormal texture
of the kidney in females fed 10 000 ppm for 2 years. Histopathological
examination showed hyperplasia and transitional-cell carcinoma in the
urinary bladders of males fed 4000 or 8000 ppm for 1 or 2 years, the
increase being statistically significant at 8000 ppm and of borderline
significance at 4000 ppm. The NOAEL for toxicity was 800 ppm, equal to
39 mg/kg bw per day, on the basis of reduced body-weight gain and
hyperplasia in the urinary bladder at all doses. The NOAEL for
carcinogenicity was 800 ppm, equal to 39 mg/kg bw per day (Wahle &
Christenson, 1996).
Sodium 2-phenylphenol
Mice
Sodium 2-phenylphenol (purity, 97%) was administered in the diet
to groups of 50 male and 50 female B6C3F1 mice (Charles River) at
concentrations of 0, 5000, 10 000, or 20 000 ppm, equal to 0, 590,
1400, and 3000 mg/kg bw per day for males and 0, 780, 1500, and 3100
mg/kg bw per day for females, for 96 weeks. The mice were then given
control diet for an additional 8 weeks. The survival rate of males at
the high dose was slightly decreased. Decreased body weight was
observed in males and females at 20 000 ppm and in females at 5000 and
10 000 ppm. Alkaline phosphatase activity was increased in females at
5000, 10 000, and 20 000 ppm. No urinary bladder stones, tumours, or
extensive renal damage were observed in any of the mice. The NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, the
highest dose tested (Ito, 1983a; Hagiwara et al., 1984).
Rats
Groups of 20-21 male and 20-21 female Fischer 344 rats were fed
diets containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol as the tetrahydrate (purity, > 95%), equivalent
to 0, 70, 140, 270, 550, 1100, or 2200 mg/kg bw per day, for 91 weeks.
The rats were observed daily for changes in general condition. The
survival rates were 90, 90, 95, 90, 90, 57, and 71% for the seven
groups, respectively. Increased incidences of urinary bladder
papil-lomas and transitional-cell carcinomas were seen, with 1/21 at
5000 ppm, 7/21 at 10 000 ppm, 20/21 at 20 000 ppm, and 17/20 at 40 000
ppm. Transitional-cell carcinomas of the kidney were also observed at
doses > 5000 ppm. The NOAEL was 2500 ppm, equivalent to 270 mg/kg
bw per day, on the basis of the increased incidence of urinary bladder
tumours (Hiraga & Fujii, 1981).
Groups of 50 male and 50 female Fischer 344/DuCrj rats were fed
diets containing 0, 7000, or 20 000 ppm (males) or 0, 5000, or 10 000
(females) of sodium 2-phenylphenol (purity, 95.5%) for 104 weeks
followed by control diet for 2 weeks. In a second study, groups of 25
male and 25 female rats were fed diets containing the compound at 0,
2500, 7000, or 20 000 ppm, equal to 0, 95, 270, and 770 mg/kg bw per
day, for males, and 0, 2500, 5000, or 10 000 ppm, equal to 0, 110,
220, and 470 mg/kg bw per day, for females, for 104 weeks followed by
control diet for life.
The survival rate at week 104 was 20% in males at 20 000 ppm in
the first study and 24% in the second study, while those in the other
groups were > 50%. Urinary bladder tumours were observed in the first
study in 2/50 males at 7000 ppm, 47/50 males at 20 000 ppm, 1/50
females at 5000 ppm, and 4/50 females at 10 000 ppm. In the second
study, the bladder tumour incidence was 3/25 in males at 7000 ppm,
23/25 in males at 20 000 ppm, and 2/25 in females at 10 000 ppm. In
the first study, transitional-cell carcinomas were found in 2/2 males
at 7000 ppm, 46/47 males at 20 000 ppm, and 1/4 females at 10 000 ppm.
In the second study, carcinomas were found in 1/3 males at 7000 ppm,
21/23 males at 20 000 ppm, and 1/2 females at 10 000 ppm. The
incidence of bladder tumours was thus dose-dependent. The NOAEL was
2500 ppm, equal to 95 mg/kg bw per day, on the basis of urinary
bladder tumours at all doses (Hiraga, 1983b; Fujii & Hiraga, 1985).
A working group convened by the International Agency for Research
on Cancer (IARC) classified sodium 2-phenylphenol as possibly
carcinogenic to humans and 2-phenylphenol as not classifiable as to
its carcinogenicity to humans (IARC, 1987, 1999).
(d) Genotoxicity
The results of tests for the genotoxicity of 2-phenylphenol,
sodium 2-phenylphenol, and the metabolites phenylhydroquinone and
phenylbenzoquinone are summarized in Table 2.
Covalent binding to urinary bladder DNA was determined in vivo
in pooled samples from eight male rats dosed with 500 mg/kg bw of
[14C]2-phenylphenol (purity, 99.8%) or [14C]sodium 2-phenylphenol
(purity, 98.7%). No radiolabel was detected in DNA from bladders
excised 16 h after dosing with either compound. The detection limit
was less than one alkylation per 106 nucleotides. Identical results
were obtained in a second experiment (Reitz et al., 1983).
The reactions of 2-phenylphenol and its metabolites
phenylhydroquinone and phenylbenzo-quinone with DNA were investigated
by a sequencing technique and by ultraviolet-visible and electron spin
resonance spectroscopy. In the presence of Cu(II), phenylhydroquinone
caused extensive DNA damage. Catalase, methionine, and methional
inhibited the DNA damage completely, whereas mannitol, sodium formate,
ethanol, tert-butyl alcohol, and superoxide dismutase did not.
Phenylhydroquinone plus Cu(II) frequently induced a piperidine-labile
site at thymine and guanine residues. Addition of Fe(III), Mn(II),
Co(II), Ni(II), Zn(II), Cd(II), or Pb(II) to phenylhydroquinone did
not induce DNA damage. This metabolite also induced DNA damage in the
presence of Cu(II) when peroxide was added, and Cu(II) accelerated the
autoxidation of phenylhydroquinone to quinone. Electron spin resonance
spectroscopy revealed that the semiquinone radical is an intermediate
in the autoxidation. Catalase did not inhibit the acceleration by
Cu(II). Superoxide dismutase promoted both the autoxidation of
phenylhydroquinone and the initial rate of semiquinone radical
production. Electron spin resonance trapping showed that addition of
Fe(III) produced hydroxyl radicals during the autoxidation of
phenylhydroquinone, whereas addition of Cu(II) did so sparingly. The
results suggest that DNA damage induced by phenylhydroquinone plus
Cu(II) is due to active species other than hydroxyl free radicals
(Inoue et al., 1990).
Table 2. Results of studies of the genotoxicity of 2-phenylphenol, sodium 2-phenylphenol, and the metabolites phenylhydroquinone
and phenylbenzoquinone
End-point Test object Concentration Purity Result Reference
(%)
2-Phenylphenol
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative - S9 Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive + S9 Ushiyama et al. (1992)
Negative - S9
Gene mutation B. subtilis H17, M45 NR NR Negative Shirasu et al. (1978)
Gene mutation S. typhimurium 10 -1000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA92, TA1535, Negative - S9
TA100, TA1537,
TA94, TA98
Gene mutation E. coli WP2 hcr NR NR Negative + S9 Shirasu et al. (1978)
Negative - S9
Gene mutation S. typhimurium 3-200 µg/plate > 99 Negative + S9 National Toxicology
TA100, TA1535, Weakly positive + S9 Program (1986)
TA1537, TA98
Gene mutation Mouse lymphoma 0.3-60 µg/ml > 99 Positive + S9 National Toxicology
(L5178Y) cells, Positive - S9 Program (1986)
Tk locus
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation Human Rsa cells, 1-30 µg/ml NR Positive Suzuki et al. (1985)
repair deficient,
HPRT locus
Chromosomal CHO-K1 cells 50-175 µg/ml > 99 Positive - S9 Tayama-Nawai et al.
aberration (1984)
Chromosomal CHO fibroblasts 12-125 µg/ml NR Weakly positive + S9 Ishidate et al. (1983)
aberration Weakly positive - S9
Chromosomal CHO-K1 cells 60-90 µg/ml > 99 Negative + S9 National Toxicology
aberration Negative - S9 Program (1986)
Chromosomal CHO-K1 cells 25-175 µg/ml > 99 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Chromosomal CHO-K1 cells 100-200 µg/ml > 99 Positive + S9 Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Host-mediated S. typhimurium G46 200 or 600 mg/kg NR Negative Shirasu et al. (1978)
gene mutation in male JCL-ICR mice bw orally for 5 days
In vivo
Sex-linked D. melanogaster 250 ppm in feed > 99 Negative National Toxicology
recessive lethal for 3 days or Program (1986)
mutation injection of 500 ppm
DNA binding Male rat urinary 500 mg/kg bw > 99 Negative Reitz et al. (1983)
bladder orally
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
DNA Rat urinary bladder 60-940 mg/kg > 99 Positive at Christenson et al.
32P-postlabelling bw per day 570 and 940 (1996a)
mg/kg bw per day
Chromosomal Male rat bone 800 mg/kg bw NR Negative Shirasu et al. (1978)
aberration marrow over 5 days or
single doses
< 4000 mg/kg
bw orally
Dominant lethal Male mice 100 or 500 mg/kg > 99 Negative Kaneda et al. (1978)
mutation bw per day for 5 days
Dominant lethal Male mice 100 or 500 mg/kg NR Negative Shirasu et al. (1978)
mutation bw per day for 5
days
Sodium 2-phenylphenol
In vitro
Gene mutation S. typhimurium 50-5000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA100, TA98 Negative - S9
Gene mutation S. typhimurium 0.025-250 µg/plate 99 Negative + S9 Reitz et al. (1983)
TA100, TA98, Negative - S9
TA1535, TA1537,
TA1538
Uncheduled DNA Male rat primary 10-7-10-4 mol/L 99 Negative Reitz et al. (1983)
synthesis hepatocytes
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
DNA Male rat urinary 2% in diet for > 99 Positive Ushiyama et al. (1992)
32P-postlabelling bladder 13 weeks
DNA Mouse skin 10 or 20 mg/animal 97 Positive Pathak & Roy (1993)
32P-postlabelling topically
Phenylhydroquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Positive Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Chromosomal CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Chromosomal CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Sister chromatid CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
exchange Positive - S9
Sister chromatid CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
exchange inhibited by (1991)
cysteine or
glutathione
Micronucleus V79 CH lung 6-125 µmol/L NR Positive + S9 Lambert & Eastmond
formation fibroblast cells Negative - S9 (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA Mouse skin 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
Phenylbenzoquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative Nagai et al. (1990)
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation S. typhimurium 0.05-1000 NR PBQ negative/ Ishidate et al. (1983)
TA100, TA2637, µg/plate PBQ negative
TA98
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Micronucleus V79 CH lung 6-125 µmol/L NR Negative Lambert & Eastmond
formation fibroblast cells (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA adduct formation in HL-60 cells treated with the
2-phenylphenol metabolites 2-phenylhydroquinone and
2-phenylbenzoquinone was studied by 32P-postlabelling. Treatment
with 25-500 µmol/L of 2-phenylhydroquinone for 8 h produced one
principal and three minor adducts, with a relative distribution of 80,
10, 6, and 4%. The relative adduct frequencies were 0.26-2.3
adducts/107 nucleotides. Treatment with 25-250 µmol/L of
2-phenylbenzoquinone for 2 h resulted in a similar level of DNA
modification and adduct distribution. Reaction of purified calf thymus
DNA with 2-phenylbenzo-quinone produced one DNA adduct, which did not
correspond to the major adduct produced in HL-60 cells. These results
show that both metabolites can form DNA adducts. Peroxidase activation
of 2-phenylphenol may therefore play a role in its carcinogenic effect
(Horvath et al., 1992).
In a similar study of covalent binding to DNA,
32P-postlabelling analysis of the products of reaction of DNA with
phenylbenzoquinone revealed four major and several minor adducts.
Chemical reaction with deoxyguanosine 3'-phosphate also resulted in
four major adducts, and their chromatographic mobility was identical
to that of major adducts of phenylbenzoquinone-DNA, which were shown
to be stable. More total covalent binding was found in deoxyguanosine
3'-phosphate than in DNA. Reaction of DNA with 2-phenylphenol or
phenylhydroquinone in the presence of microsomes and NADPH or cumene
hydroperoxide also resulted in four major adducts, and their formation
was drastically decreased by known inhibitors of cytochrome P450. The
chromatographic mobility of these adducts matched that of the adducts
observed in deoxyguanosine 3'-phosphate and DNA reacted with
phenylbenzoquinone. Thus, both 2-phenyl-phenol and phenylhydroquinone
can bind covalently to DNA in the presence of a microsomal cytochrome
P450 activation system, and phenylbenzoquinone is one of the
DNA-binding metabolites of 2-phenylphenol (Pathak & Roy, 1992).
Covalent modification of skin DNA by sodium 2-phenylphenol
in vivo was studied by the 32P-postlabelling method to elucidate
the biochemical mechanism of promotion of chemically induced skin
carcinogenesis by this compound. Topical application of sodium
2-phenylphenol or phenylhydroquinone to the skin of CD-1 mice produced
four distinct major and several minor adducts in skin DNA. Total
covalent binding in skin DNA was 0.31 fmol/µg DNA after treatment with
10 mg of sodium 2-phenylphenol and 0.62 fmol/µg DNA with 20 mg. The
adducts were not observed in skin DNA of untreated animals.
Pretreatment of the mice with a-naphthylisothiocyanate, an inhibitor
of cytochrome P450, or indomethacin, an inhibitor of prostaglandin
synthase, resulted in lower numbers of DNA adducts. Incubation of DNA
with 2-phenylphenol or phenylhydroquinone in vitro in the presence
of cytochrome P450 or prostaglandin synthase activation systems
resulted in four major adducts. The pattern of chromatographic
mobility observed in vitro in the presence of these enzymatic
systems appeared to be similar to that of adducts in vivo. The
chemical reaction of DNA or deoxyguanosine monophosphate with
phenylbenzoquinone also resulted in four major and several minor
adducts. The four major adducts were identical in chromatographic
mobility to the four major adducts produced in vivo and in vitro.
The results show that 2-phenylphenol and phenylhydroquinone can bind
covalently to DNA and that one of the DNA-binding metabolites of
2-phenylphenol may be phenylbenzoquinone (Pathak & Roy, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
2-Phenylphenol
Rats
Groups of 35 albino Sprague-Dawley rats, nine weeks old at the
start of the study, were fed diets containing technical-grade
2-phenylphenol (purity, 99.4-99.5%) at concentrations of
200-10 000 ppm, equal to 0, 36, 120, and 460 mg/kg bw per day, for two
generations. The dose was adjusted during the premating period
according to changes in body weight, and adjustments were made during
lactation to avoid overdosing the pups, although this was later
considered unnecessary. Two F1 females at the high dose and 12 F2a
pups were removed from the study for examination of the heritability
of hypotrichosis. Parents (F0) for the F1 generation were assigned
randomly to dose groups. The genealogy of the F1b pups was checked
to prevent the mating of littermates. For production of the F2
generation, 132 male and 134 female F1b pups were selected
randomly,one or more pups of each sex per litter being used as parents
(F1), divided into 35 pairs of rats per dose, except for the
controls which consisted of 27 males and 29 females. Estrus cycles
were studied by vaginal smears. The animals were examined daily, and
routine observations of birth statistics and pup weights were made;
the litters were culled to eight pups when necessary. Standard
observations and extensive histological examination of the urinary
tract were carried out at sacrifice.
Treatment did not affect clinical signs, body-weight gain during
gestation or lactation, or any of the reproductive variables examined.
Histological examination of the adults and pups revealed no
significant changes in the reproductive tracts. F0 and F1 adults
at 460 mg/kg bw per day showed a treatment-related decrease in body
weight which was not clearly related to a decrease in food
consumption, and F1b, F2a, and F2b pups showed a statistically
significant decrease in body weight on days 14 and/or 21 of lactation,
an effect not seen during the first week of lactation, indicating that
development had not been disturbed. The relative weight of the kidneys
was increased in a dose-dependent manner, in the absence of changes in
other organ weights, in males of the F0 and F1 generations. Male
rats at 120 and 460 mg/kg bw per day had an increased incidence of
calculi in the urinary tract. Transitional-cell hyperplasia was found
in the urinary bladder, defined in the report as 'an area (focal or
diffuse) of at least three to four cells thick (of cuboidal cells) in
an inflated bladder', whereas normal bladders had a cell thickness of
one or two flattened cells. Quantification by simple morphometry
indicated a compound-related effect in the bladder in F0 males and
females at 120 and 460 mg/kg bw per day and in F1 males at 457 mg/kg
bw per day. Neoplasms of the urinary tract were found in four rats:
one bladder and one ureteric tumour in rats at 125 mg/kg bw per day
and two bladder tumours in rats at 457 mg/kg bw per day. The NOAEL for
reproductive toxicity was 460 mg/kg bw per day and that for
carcinogenicity was 36 mg/kg bw per day (Eigenberg, 1990).
In a similar study, groups of 30 CD Sprague-Dawley rats were fed
diets containing technical-grade 2-phenylphenol (purity, 99.5-100%) at
concentrations of 200-10 000 ppm, equal to 0, 17, 92, and 460 mg/kg bw
per day, for two generations. The dose was adjusted during the
premating period according to changes in body weight. The F0 and
F1 adults received the compound in the diet throughout the study,
beginning at 7 weeks of age for the F0 adults and at weaning for the
F1 adults. The animals received treated feed for 10 weeks before
breeding, beginning approximately 2 weeks after weaning of the last
F1b litter for F1 parents. F0 adults were mated to produce the
F1a and F1b litters, and F1 adults (consisting of randomly
selected F1b pups) were mated to produce the F2a and F2b
litters.Adult animals were evaluated during the study for effects of
2-phenylphenol on body weight, food consumption, clinical signs,
estrous cycling, mating, fertility, length of gestation, and litter
size. The offspring were evaluated for effects on sex ratio,
viability, body-weight gain, and clinical signs. Gross necropsy was
performed on all adults and pups, and the reproductive organs,
pituitary, kidneys with ureter attached, urinary bladder, and gross
lesions of all F0 and F1 adults were evaluated histologically.
At 460 mg/kg bw per day, urine staining was observed in F0
males and females and F1 males, urinary bladder calculi were found
at necropsy in F1 adult males, and one F0 male died from renal
failure. At this dose, there was an increase in food consumption by
females during lactation, decreased pup weight, and a decrease in the
terminal body weight of F0 and F1 adult males and females.
Histopathological examination of the kidneys revealed debris in the
renal pelvis, chronic active inflammation, and increased severity of
background lesions in F0 and F1 males. Further, transitional-cell
hyperplasia (simple, nodular, or papilary) of the bladder, calculi,
chronic inflammation of the bladder, and dilatation and hyperplasia of
the ureter were seen in F0 and F1 males. Two F1 males at this
dose had malignant lymphomas in several tissues. One F1 female at 92
mg/kg bw per day had a nephroblastoma, and one F0 male at the high
dose and one control F1 female had a pituitary adenoma. All of these
lesions were considered to be incidental to treatment.
There were no treatment-related effects on adult reproductive
parameters and no effect on litter size, sex ratio, the number of
stillborn pups, pup viability, or clinical signs, no gross lesions in
the pups, and no treatment-related effects on the organ weights of
adults. The mean live birth indexes (with standard error) were 98
(0.91) in the controls, 99 (0.77) at 17 mg/kg bw per day, 98 (1.3) at
92 mg/kg bw per day, and 98 (0.85) at 460 mg/kg bw per day in the
F1a generation; 98 (0.85) in the controls, 98 (1.5) at 17 mg/kg bw
per day, 99 (0.71) at 92 mg/kg bw per day, and 99 (0.63) at 460 mg/kg
bw per day in the F1b generation; 99 (0.84) in the controls, 98
(1.0) at 17 mg/kg bw per day, 97 (1.4) at 92 mg/kg bw per day, and 100
(0.38) at 460 mg/kg bw per day in the F2a generation; and 97 (1.2)
in the controls, 99 (0.78) at 17 mg/kg bw per day, 96 (1.9) at 92
mg/kg bw per day, and 99 (0.46) at 460 mg/kg bw per day in the F2b
generation. The differences between the groups were not statistically
significant. The NOAEL for reproductive toxicity was 460 mg/kg bw per
day, the highest dose tested. The NOAEL for systemic and developmental
toxicity was 92 mg/kg bw per day, on the basis of decreased body
weight and morphological lesions in the kidneys, urinary bladder, and
ureter and a decrease in pup body weight (Eigenberg, 1995).
(ii) Developmental toxicity
2-Phenylphenol and sodium 2-phenylphenol
Mice
Groups of 20-21 pregnant JCL-ICR mice were given 0, 1500, 1700,
or 2100 mg/kg bw per day of 2-phenylphenol (purity not given) or 100,
200, or 400 mg/kg bw per day of sodium 2-phenylphenol (purity not
given) by gavage on days 7-15 of gestation. The animals were weighed
daily, and any change in their general condition was noted. On day 18
of gestation, the dams were killed and their uteri opened, and the
numbers of implantation scars, fetuses that died in early and late
stages, and live fetuses were counted. The live fetuses were weighed
and sexed and observed for external abnormalities. The number of
corpora lutea in each ovary was counted, the major organs were
weighed, and examinations were made to determine whether any
macroscopic abnormalities were present.
With 2-phenylphenol, body-weight gain was reduced at 1700 and
2100 mg/kg bw per day. Four dams at 1500 mg/kg bw per day, seven at
1700 mg/kg bw per day, and 16 at 2100 mg/kg bw per day died. Pregnancy
was confirmed in 5/5 surviving dams at 2100 mg/kg bw per day, 14/14 at
1700 mg/kg bw per day, 14/17 at 1500 mg/kg bw per day, and 20/21
controls, in which implantation was confirmed at the time of sacrifice
and laparotomy on day 18 of gestation. The only significant changes
found at autopsy of these mice were reduced heart weights at 1700 and
2100 mg/kg bw per day and significantly increased liver weights at
1500 and 1700 mg/kg bw per day, a tendency that was also seen at 2100
mg/kg bw per day. Live fetuses were found in all pregnant dams. The
body weights of male and female fetuses at all three doses of
2-phenylphenol were significantly reduced, and the decrease was
dose-related in males. No unique external or internal deformities or
abnormalities were found in the fetuses, and the skeletal
abnormalities found were compatible with delayed development. No NOAEL
could be identified for maternal or fetotoxicity. The NOAEL for
developmental toxicity was 2100 mg/kg bw per day, the highest dose
tested.
With sodium 2-phenylphenol, body-weight gain was statistically
significantly reduced in a dose-dependent manner in dams at all doses.
Four animals at 200 mg/kg bw per day and 16 at 400 mg/kg bw per day
died. No abnormalities were seen at autopsy of dams on day 18 of
gestation. Significantly reduced liver, heart, and spleen weights were
recorded in animals at 400 mg/kg bw per day, while the weight of the
lungs was increased in dams at 200 mg/kg bw per day. Dams at 200 mg/kg
bw per day had a low average number of implantations and a low average
number of live fetuses. No NOAEL could be identified for maternal
toxicity. The NOAEL was 100 mg/kg bw per day for fetotoxicity and 400
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Ogata et al., 1978).
2-Phenylphenol
Rats
Groups of 18-20 pregnant Wistar rats were given 0, 150, 300, or
600 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by gavage on
days 6-15 of gestation. An additional group of 11 rats was given 1200
mg/kg bw per day, but this dose proved to be lethal. No untoward signs
of toxicity were observed in the controls or at 150 mg/kg bw per day.
At doses > 300 mg/kg bw per day, dose-related ataxia and decreased
mean body-weight gains were observed. All surviving rats were killed
on day 20 of gestation, and the uterine contents were examined.
Fetuses were grossly examined; the skeletons were examined with
Alizarin red S and the viscera by a modified Wilson method. The mean
numbers of implantation sites, live fetuses, resorptions, and fetal
weights in animals at 150 and 300 mg/kg bw per day were comparable to
those of controls, but at 600 mg/kg bw per day the number of fetal
resorptions was increased and fetal weight was decreased. Although a
few fetal anomalies were observed in all groups, they did not appear
to be related to treatment. The NOAEL was 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 600
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Kaneda et al., 1978).
In a similar study, groups of 25-35 pregnant rats were given 0,
100, 300, or 700 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by
gavage on days 6-15 of gestation. They were killed on day 21, and the
fetuses were removed surgically. All fetuses were weighed, sexed, and
examined externally and skeletally, and the soft tissues of
approximately one-third of the fetuses were examined. One rat at the
high dose died as a result of a dosing accident. Pregnant rats given
700 mg/kg bw per day gained significantly less body weight during the
first 4 days of treatment (days 6-9 of gestation) than did controls,
and their food consumption was significantly decreased on days 9-11 of
gestation. At necropsy, the weights of the liver (but not the
liver:body weight ratios) were significantly decreased. There was no
effect on the number of implantation sites per dam, mean litter size,
incidences of resorptions, or fetal body weight or crown-rump length.
The only major malformation -- hypoplastic tail and missing sacral and
caudal vertebrae -- was observed in a single fetus at 300 mg/kg bw per
day. An increase in the incidence of delayed ossification of
sternebrae and unossified sternebrae was observed at 700 mg/kg bw per
day. The incidences of foramina and bony islands in the skull were
also slightly increased in this group. No adverse effects on embryonic
or fetal development were observed that were considered to be due to
2-phenylphenol. The NOAEL was 300 mg/kg bw per day for maternal
toxicity and 700 mg/kg bw per day, the highest dose tested, for
fetotoxicity and developmental toxicity (John et al., 1981).
Rabbits
In a range-finding study, groups of two non-pregnant New Zealand
white rabbits were given doses of 0, 100, 500, or 1000 mg/kg bw per
day of 2-phenylphenol (purity, 99.8%) in corn oil for 13 consecutive
days and were submitted to gross necropsy after the last day. The
animals were examined for clinical signs, body weights, body-weight
gain, kidney and liver weights, and gross appearance. The rabbits at
1000 mg/kg bw per day appeared to have stopped eating and had lost 24%
of their body weight by day 7. One rabbit at this dose died on day 8,
and the second was killed in moribund condition on day 10 with
nonspecific lesions or lesions secondary to anorexia. Rabbits given
500 mg/kg per day showed a slight decrease in body-weight gain. All
the other rabbits survived to the end of the study with no other
treatment-related effects. The dose of 100 mg/kg bw per day was
tolerated over the course of treatment.
In the second study, groups of seven artificially inseminated
females were given 0, 250, 500, or 750 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) in corn oil by gavage on days 7-19 of
gestation. They were observed for clinical signs, body weight, and
body-weight gain. On day 20 of gestation, all surviving animals were
killed and examined for gross pathological alterations and changes in
liver and kidney weights. The uteri and ovaries were examined for
implantations, resorptions, and corpora lutea, and the liver, kidneys,
and stomach were examined histologically. Dose-related signs of
maternal toxicity were seen at all doses. One rabbit at 250 mg/kg bw
per day, two at 500 mg/kg bw per day, and six at 750 mg/kg bw per day
died. The dose-related effects observed included increased incidences
of haemorrhage, gaseous distension, and erosions of the stomach,
decreased or soft ingesta in the gastrointestinal tract, decreased
body weight and body-weight gain, increased absolute and relative mean
weights of the kidney, and increased incidence and/or severity of
renal tubular degeneration and inflammation. Treatment-related effects
were observed on reproductive, embryonal, or fetal parameters at 750
mg/kg bw per day.
In the third study, groups of 16-24 artificially inseminated
adult female New Zealand white rabbits were given 0, 25, 100, or 250
mg/kg bw per day of 2-phenylphenol (purity, 99.8%) in corn oil by
gavage on days 7-19 of gestation. They were observed for clinical
signs, body weight, and body-weight gain. On day 28 of gestation, all
surviving rabbits were killed and necropsied, when the weights of the
liver, kidney, and gravid uterus and the numbers of corpora lutea,
implantations, resorptions, and live and dead fetuses were recorded.
All fetuses were removed from the uterus, weighed, sexed, and examined
for external, visceral, and skeletal alterations. The kidneys of all
animals were examined histologically. Administration at 250 mg/kg bw
per day resulted in maternal toxicity evidenced by treatment-related
mortality (13%), gross pathological alterations (ulceration and
haemorrhage of the gastric mucosa, haemolysed blood in the intestinal
tract, and decreased ingesta), and histopathological alterations
(renal tubular degeneration and inflammation). No significant maternal
effects were observed at 25 or 100 mg/kg bw per day, and no adverse
embryonal or fetal effects were observed at any dose. The overall
NOAELs were 100 mg/kg bw per day for maternal toxicity, 500 mg/kg per
day for fetotoxicity, and 750 mg/kg per day, the highest dose tested,
for developmental toxicity (Zablotny et al., 1991).
(f) Special studies: Mechanisms of carcinogenicity in rat urinary
bladder
In a study to determine whether 2-phenylphenol is a complete skin
carcinogen or a promoter in a two-stage initiation and promotion
process, the compound was applied to the interscapular area of the
backs of 50 male and 50 female Swiss CD-1 mice at a dose of 55.5 mg in
0.1 ml acetone, three times per week for 2 years. A second group of 50
male and 50 female mice was treated identically except that their
backs were pretreated once with 0.05 mg in 0.1 ml acetone of
7,12-dimethylbenz[ a]anthracene (DMBA), a known initiator of skin
cancer. Additional groups of 50 male and 50 female mice served as
acetone vehicle controls, controls treated once with DMBA and
thereafter only with acetone, and a positive control group treated
once with DMBA and thereafter with 12- O-tetradecanylphorbol
13-acetate (TPA), a known promoter of skin cancer, at a dose of 0.005
mg in 0.1 ml acetone, three times per week for 2 years.
The mean body weights and survival of the mice treated with
2-phenylphenol or with DMBA plus 2-phenylphenol were generally similar
to those of the respective negative control groups, but the survival
of the group given DMBA plus TPA was substantially decreased. In this
group, the incidences of squamous-cell papillomas and carcinomas,
keratocanthomas, and basal-cell carcinomas at the site of application
were clearly increased (52/100) over that in the group given DMBA plus
acetone(15/100). The time to tumour was also substantially decreased
in the group given DMBA plus TPA. Similar neoplastic skin lesions were
observed with DMBA plus acetone (17/100), but at an incidence
equivalent to that in the control group (15/100). No neoplastic skin
lesions were observed in the group given 2-phenylphenol. The author
concluded that 2-phenylphenol is not carcinogenic alone or as a
promoter (Luster, 1986).
The promoting effect of 2-phenylphenol (purity, 98%) and sodium
2-phenylphenol (purity, 97%) in the urinary bladder was studied in
male Fischer 344 rat initiated with
N-nitrosobutyl- N-(4-hydroxybutyl)amine (NBHBA). Groups of 30 rats
were given drinking-water containing 0.01% NBHBA for 4 weeks and then
diets containing 20 000 ppm of sodium 2-phenylphenol (equivalent to
1000 mg/kg bw per day) for 32 weeks, NBHBA for 4 weeks followed by
untreated feed for 32 weeks, or drinking-water without NBHBA for 4
weeks followed by diet containing 20 000 ppm of sodium 2-phenylphenol
for 32 weeks. In another experiment, groups of 30 male rats were given
0.05% NBHBA in drinking-water for 4 weeks followed by diets containing
20 000 ppm of sodium 2-phenylphenol or 20 000 ppm of 2-phenylphenol
for 32 weeks, no NBHBA for 4 weeks, and then 20 000 ppm of sodium
2-phenylphenol (15 rats) or 20 000 ppm of 2-phenylphenol (15 rats) in
the diet for 32 weeks. In a third experiment, groups of 15 rats were
given diets containing 0, 20 000 ppm of sodium 2-phenylphenol, or
20 000 ppm of 2-phenylphenol. Urine samples were obtained from these
rats by forced urination on days 27, 29, and 32.
Administration of 20 000 ppm sodium 2-phenylphenol in the diet
significantly increased the incidence and number of preneoplastic
lesions (papillary or nodular hyperplasia) per 10 cm of basement
membrane of the urinary bladder in male rats pretreated with 100 ppm
NBHBA, and the incidence and number of papillomas and carcinomas of
the urinary bladder in the group pretreated with 500 ppm NBHBA.
Moreover, treatment with sodium 2-phenylphenol alone, without
initiation, induced papillary or nodular hyperplasia, papillomaa, and
carcinoma. In contrast, administration of 2-phenylphenol in the diet
after initiation only slightly increased the incidence of urinary
bladder lesions over that with NBHBA alone, and its effect was not
statistically significant. No tumours of the urinary bladder were
induced by 2-phenylphenol alone. The authors concluded that sodium
2-phenylphenol, and not 2-phenylphenol, has tumour promoting activity
and might be a complete carcinogen in rat urinary bladder. Since the
sodium salt increased the pH of the urine, the authors speculated that
an active metabolite reaches the urinary bladder at a higher
concentration than with 2-phenylphenol. They suggested that sodium
2-phenylphenol is a carcinogens that acts by a non-genotoxic mechanism
(Fukushima et al., 1983).
In a similar study, 2-phenylphenol or sodium 2-phenylphenol
(purity of neither given) was administered in the diet at a
concentration of 20 000 ppm to 28 male Fischer 344 rats for 64 weeks.
One rat receiving sodium 2-phenylphenol had small stones in the
urinary bladder, and this compound, but not 2-phenylphenol, induced
papillary or nodular hyperplasia (19/28), papillomas (5/28), and
carcinomas (6/28) of the urinary bladder. Pretreatment of additional
rats with NBHBA increased the incidence of papillary or nodular
hyperplasia ( p < 0.05), papillomas (not significant), and
carcinomas (not significant) over that in rats treated with NBHBA
alone. In another experiment, 2-phenylphenol or sodium 2-phenylphenol
was administered in the diet at concentrations of 2500, 5000, 10 000,
or 20 000 ppm to groups of five to nine male Fischer 344 rats for up
to 104 weeks. Animals from each group were killed and examined at 4,
8, 12, 24, 36, and 104 weeks. No stone formation was observed in the
urinary bladders of rats treated with sodium 2-phenylphenol. At 20 000
ppm, simple hyperplasia of the urinary bladder was observed from 4
weeks in 5/5 animals, papillary or nodular hyperplasia from 36 weeks
in 5/5 animals, and papillomas in 2/5 and carcinomas in 2/5 at 104
weeks. At 10 000 ppm of sodium 2-phenylphenol, only simple hyperplasia
was observed from 36 weeks. 2-Phenylphenol alone did not cause bladder
tumours and did not enhance the bladder lesions induced by NBHBA (Ito,
1983b).
In a short-term assay for bladder carcinogenicity in rats,
increased agglutinability of bladder epithelial cells with
concanavalin A was observed after a 1-week treatment with 10 000 or
20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol (purity of
neither given), suggesting that these compounds cause bladder cancer.
No such increase was observed in rats fed diets containing
rho-phenylphenol or biphenyl derivatives at 20 000 ppm. In male Fisher
rats fed diets containing 20 000 ppm of sodium 2-phenylphenol for 50
weeks, bladder papillomas developed in 19 of 36 rats and bladder
carcinomas in 14 of 36 rats (Honma et al., 1983).
Groups of 20 male Fischer 344 rats were fed diets containing
20 000 ppm of 2-phenylphenol, 20 000 ppm of sodium 2-phenylphenol
(purity of neither given), or 5000 ppm of biphenyl for up to 24 weeks.
Changes in the amounts of DNA synthesis and in the morphology of the
renal papilla and renal pelvis were recorded under light and scanning
electron microscopes. Increased DNA synthesis in both renal papilla
and pelvis and distinct morphological alterations in the cell surface
were seen with 2-phenylphenol and its sodium salt by 4 weeks.
Sequential light microscopy revealed renal papillary necrosis in
animals fed 2-phenylphenol from week 4, followed by regenerative
hyperplasia at weeks 16 (1/5) and 24 (3/5), but no changes in the
renal pelvis. Feeding of sodium 2-phenylphenol caused similar changes
in the renal papillae and also hyperplasia in the renal pelvis (2/5).
No proliferative response of the kidney was apparent in rats fed
biphenyl. The authors concluded that the proliferative responses
caused by sodium 2-phenylphenol in the renal pelvic epithelium were
similar to those induced by this compound in the urinary bladder
(Shibata et al., 1989a).
The interactive effects of ascorbic acid, saccharin, and hippuric
acid on the carcinogenicity of 2-phenylphenol and sodium
2-phenylphenol were studied in groups of 20 male Fischer 344 rats. The
animals were given 2-phenylphenol (purity, 99.5%) or sodium
2-phenylphenol in the diet at a concentration of 20 000 ppm
(equivalent to 1000 mg/kg bw per day) for 24 weeks with or without
ascorbic acid, sodium ascorbate, acid saccharin, sodium saccharin,
hippuric acid, or sodium hippurate at 50 000 ppm. The urinary sodium
concentration was increased in all animals receiving sodium salts
and/or sodium 2-phenylphenol. The pH of the urine was increased in
those given sodium 2-phenylphenol, sodium ascorbate, or sodium
saccharin, and the osmolality was decreased in those given sodium
2-phenylphenol, sodium ascorbate, or sodium hippurate. 2-Phenylphenol
decreased the osmolality but did not affect the pH or sodium
concentration of urine. Histopathologically, the bladders of rats
given sodium 2-phenylphenol showed epithelial thickening (epithelial
thickness, four to eight cells) at 8, 16, and 24 weeks and papillary
and nodular changes at 16 and 24 weeks. Treatment with the other
sodium salts provoked 'slight to moderate' hyperplasia at 8 and 16
weeks but no papillary or nodular changes; the changes had regressed
by 24 weeks. The combination of raised urinary pH and sodium promoted
the effects of sodium 2-phenylphenol, while sodium hippurate raised
urinary sodium but not pH and had no effect (Fukushima et al., 1989).
In an essentially similar study, groups of 31 male Fischer 344
rats received NaHCO3 to raise the urinary pH or NH4Cl to lower it.
2-Phenylphenol was given at a dietary concentration of 12 500 ppm
(equivalent to 625 mg/kg bw per day) and sodium 2-phenylphenol at 20
000 ppm (equivalent to 1000 mg/kg bw per day or 625 mg/kg bw per day
of 2-phenylphenol). Hyperplasia of the bladder epithelium was seen in
animals given 2-phenylphenol, 2-phenylphenol plus NaHCO3, or sodium
2phenylphenol. Administration of the sodium salt with NH4Cl had no
significant effect. The incidence of tumours was significantly
increased with 2-phenylphenol (12/31), sodium 2-phenylphenol (22/31),
and 2-phenylphenol plus NaHCO3 (20/31), but only three tumours were
seen in 31 rats given sodium 2-phenylphenol plus NH4Cl. Thus, the
carcinogenic effects of 2-phenylphenol were promoted in alkaline
urine, and those of sodium 2-phenylphenol were inhibited in acid urine
(Fujii et al., 1987).
Changes in urinary parameters, particularly electrolyte levels
and pH, DNA synthesis, and the morphology of the bladder epithelium
were investigated in Fischer 344 rats fed diets containing various
sodium, potassium, magnesium, and calcium carbonate salts at a
concentration of 30 000 ppm, with or without L-ascorbic acid at 50 000
ppm, for 4 or 8 weeks. The effects of treatment with NH4Cl at 10 000
ppm (to acidify urine) and of combined treatment with sodium ascorbate
at 50 000 ppm and NH4Cl were also investigated. Urinary pH was
significantly raised in groups given NaHCO3, K2CO3, ascorbic
acid plus NaHCO3, ascorbic acid plus K2CO3, or sodium ascorbate,
whereas treatment with ascorbic acid or NH4Cl alone caused a
significant decrease in urinary pH. Increases in urinary electrolyte
or ascorbic acid contents were associated with the corresponding
dosing regimen. DNA synthesis in the bladder epithelium was increased
in groups given NaHCO3, K2CO3, ascorbic acid plus NaHCO3,
ascorbic acid plus K2CO3, or sodium ascorbate. Furthermore, all
treatments that increased DNA synthesis also induced some
morphological alterations in the bladder epithelium. Administration of
ascorbic acid in conjunction with NaHCO3 or K2CO3 induced more
changes than those with either salt alone. In contrast, the degree of
response of the bladder epithelium of rats given sodium ascorbate was
reduced by simultaneous administration of NH4Cl. These results
suggest that the degree of DNA synthesis and/or morphological
alteration in rat bladder epithelium after treatment with various
bases depends on changes in the urinary concentrations of Na+ or
K+ and/or pH and the presence of ascorbic acid in the urine (Shibata
et al., 1989b).
The role of urinary pH and Na+ concentration on the
carcinogenic effect of 2-phenylphenol and sodium 2-phenylphenol on rat
urinary bladder was studied in two experiments. In the first, groups
of 36 male Fischer 344 rats were fed diets containing 20 000 ppm of
sodium 2-phenylphenol (equivalent to 1000 mg/kg bw per day), 12 500
ppm of 2-phenylphenol (equivalent to 625 mg/kg bw per day), 6400 ppm
of NaHCO3, 12 500 ppm of 2-phenylphenol plus 6400 ppm of NaHCO3,
12 500 ppm of 2-phenylphenol plus 3200 ppm of NaHCO3, or 12 500 ppm
of 2-phenylphenol plus 1600 ppm of NaHCO3 for 104 weeks. Body
weights were measured weekly up to week 14 and monthly thereafter.
Food consumption was measured on 2 consecutive days per week on a
per-cage basis. Urine samples were obtained from four to six rats in
each group by forced urination, and the urinary pH was determined 10
times during the 2-year experiment. For measurement of urinary
electrolytes, three or four rats in each group were housed
individually in metal metabolic cages without food or water for 4 h in
the morning during weeks 58, 80, and 96. All surviving animals were
killed at the end of the experiment and were examined carefully for
gross abnormalities at autopsy. The liver, kidney, and tissues with
macroscopic lesions were removed and fixed for histological
examination. Autopsies were also performed on all animals that died or
became moribund and were killed during the experiment.
Body-weight gain was reduced throughout the study in all treated
groups, but the reduction was less and started later in animals given
6400 ppm of NaHCO3 alone. The absolute and relative weights of the
bladder were significantly higher in treated groups than in controls,
especially in rats given 2-phenylphenol plus 6400 ppm of NaHCO3. The
relative weights of the kidneys and liver in all treated groups were
significantly higher than in controls. At week 104, 58% of rats given
sodium 2-phenylphenol and 68-84% of those in other groups were still
alive compared with 73% of controls. Macroscopically, more tumours
were found in bladders of rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm of NaHCO3 than in rats fed
2-phenylphenol plus 3200 ppm or 1600 ppm NaHCO3, and no tumours were
found in rats fed 2-phenylphenol alone or in controls. No stone
formation related to tumours was seen in any group. The bladder
lesions were classified as simple hyperplasia, papillary or nodular
hyperplasia, papilloma, and carcinoma. The incidences of bladder
carcinomas were significantly higher than controls in rats fed sodium
2-phenylphenol or 2-phenylphenol plus 6400 ppm NaHCO3.
In the other experiment, groups of five rats were given diets
supplemented with test chemicals as in the first experiment for only 8
weeks before being killed. Urinary electrolytes and pH were determined
at weeks 2, 4, 6, and 8, and osmolality was measured at weeks 4 and 8.
When all animals were killed at week 8, no stone formation was
observed macroscopically in any groups. Various changes in the luminal
surface of the bladder, particularly in rats fed sodium 2-phenylphenol
or 2-phenylphenol plus 6400 ppm of NaHCO3, were revealed by scanning
electron microscopy. The authors concluded that sodium 2-phenylphenol
is carcinogenic to the male rat bladder at 20 000 ppm in the diet.
2-Phenylphenol was not carcinogenic, although it induced a low
incidence of papillary or nodular hyperplasia (Fukushima et al.,
1989).
Species differences in the induction of urinary bladder lesions
by sodium 2-phenylphenol were studied in groups of 30 male Fischer 344
rats, B6C3F1 mice, Syrian golden hamsters, and Hartley guinea-pigs
fed diets containing 20 000 ppm of sodium 2-phenylphenol (purity not
given). Body weight and food consumption were determined periodically.
Groups of five animals from each group were killed at weeks 4, 8, 12,
24, 36, and 48, and five control animals were killed at weeks 12 and
48. Urine was collected in metabolism cages for 4 h from all animals
at weeks 12 and 48 for measurement of urine volume, pH, osmolality,
and microscopic appearance. Although food consumption did not differ
between the treated and untreated groups, retardation of growth was
associated with administration of sodium 2-phenylphenol in the diet,
especially during the first 8 weeks of the test. Although the absolute
weights of the liver were similar in both treated and untreated
groups, the relative weights were slightly increased in all species.
Morphological changes in the urinary bladder were remarkable only in
rats, which showed simple hyperplasia at week 4, increasing in
incidence and density to week 48. Lesions classified as papillary
nodular hyperplasia were observed in rats from week 36 of treatment,
but no papillomas were found. Scanning electron microscopy revealed
pleomorphic microvilli only in rats, which increased in grade with
time. In the other species, no changes indicative of proliferation
were observed, except for a slight effect in mice at weeks 24 and 48.
The pH of the urine was slightly increased in rats, while the
background pH in the other species was usually high, except in mice at
48 weeks. Osmolality was not affected by administration of sodium
2-phenylphenol, but crystal formation was seen in rats and
guinea-pigs, which increased slightly in rats with time. The authors
concluded that sodium 2-phenylphenol is likely to be a urinary bladder
carcinogen in rats but not in mice, guinea-pigs, or hamsters (Hasegawa
et al., 1990a).
Sex differences in the carcinogenic effect in rat urinary bladder
associated with administration of 2-phenylphenol (purity, > 99%) and
sodium 2-phenylphenol (purity, > 99%) were investigated in groups of
five or six male and five or six female Fischer 344 rats fed diets
containing 12 500 ppm of 2-phenylphenol (equivalent to 625 mg/kg bw
per day), 20 000 ppm of sodium 2-phenylphenol, equivalent to 1000
mg/kg bw per day), 30 000 ppm of NaHCO3, 10 000 ppm of NH4Cl,
2-phenylphenol plus NaHCO3, or sodium 2-phenylphenol plus NH4Cl
for 8 weeks. Body weights and food and water consumption were
determined weekly. Fresh urine specimens were obtained from all rats
by forced urination at week 8 and examined for pH and osmolality.
During the last week of the study, the rats were transferred to metal
metabolism cages without food or water, and urine samples were
collected from 07:00-13:00 h over 3 consecutive days to obtain enough
pooled urine for analysis and determination of metabolites, although
only pooled samples from the groups treated with 2-phenylphenol or its
sodium salt were examined.
No animals died before the end of the experiment, and food intake
was not significantly different between groups. The body weights at
week 8 were significantly lower in all treated groups of male rats and
in female rats given 2-phenylphenol or sodium 2-phenylphenol. The
urinary pH values for all groups were significantly different from the
7.0 found in untreated males and the 6.8 found in untreated females,
except in the groups given sodium 2-phenylphenol alone in which the pH
values were comparable to those of controls. The pH values were
highest in the groups given NaHCO3 alone, followed by the groups
given 2-phenylphenol plus NaHCO3, while the pH was lower than in
controls for groups given 2-phenylphenol alone, sodium 2-phenylphenol
plus NH4Cl, or NH4Cl alone. The Na+ concentrations were higher
in males than in females in all groups except controls and those given
NH4Cl. In the groups given sodium 2-phenylphenol alone, the Na+
concentration was slightly increased over control values in males but
not in females. The depressive effect of NH4Cl on Na+
concentration was also less pronounced in females. Only unconjugated
urinary metabolites were identified. No urothelial hyperplastic
changes were observed with 2-phenylphenol alone in either sex, while
an equimolar dose of sodium 2-phenyl-phenol induced mild papillary and
nodular hyperplasia or simple hyperplasia in male rats only. The
possible mechanisms underlying the differences in response between the
sexes might include excretion of other types of conjugated forms and
the formation of microcrystals such as the silicate crystals found in
the urine of rats fed sodium saccharin (Hasegawa et al., 1991).
The physiological effects of 2-phenylphenol (purity, 99.5%) on
urothelial cells and potential formation of DNA adducts were studied
in male Fischer 344 rats. In an initial experiment, rats were fed
dietary concentrations of 0, 1000, 4000, or 12 500 ppm for 13 weeks.
There was no evidence of urinary calculi, microcrystalluria, or
calcium phosphate-containing precipitate, but urothelial cytotoxicity
and hyperplasia were seen at the highest dose. In a second experiment,
rats were fed dietary concentrations of 0, 800, 4000, 8000, or 12 500
ppm for 13 weeks. The urinary pH was > 7 in all groups. The urinary
volume was increased at the highest dose, with consequent decreases in
osmolality and the concentrations of creatinine and other solutes. The
urinary excretion of total 2-phenylphenol metabolites was increased.
Most of the metabolites were conjugates of 2-phenylphenol and of
phenylhydroquinone, and free 2-phenylphenol and metabolites accounted
for < 2% at each dose. Urothelial toxicity and hyperplasia occurred
only at 8000 and 12 500 ppm. No 2-phenylphenol-DNA adducts were
detected in the urothelium at any dose. The small percentage of
unconjugated metabolites and the absence of DNA adducts suggest that
2-phenylphenol acts as a bladder carcinogen in male rats by inducing
cytotoxicity and hyperplasia without direct binding of the compound or
its metabolites to DNA (Smith et al., 1998).
The carcinogenic effect of sodium 2-phenylphenol and its
metabolites on female rat urinary bladder after intravesicular
instillation was studied in groups of nine 6-week-old Fischer 344 rats
that received 0.2 ml of a saline solution of 0.1% sodium
2-phenylphenol (purity, > 99%), phenylbenzoquinone (purity, > 99%),
or phenylhydroquinone (purity, > 99%) through a catheter into the
urethra once, twice, or four times. The pH values of the solutions
were 11 for sodium 2-phenylphenol, 6.5 for phenylbenzoquinone, and 6.4
for phenylhydroquinone. Saline or a solution of NaOH (pH 11) were
given to controls. The animals were maintained under light ether
anaesthesia during instillation and for a further 10 min thereafter to
prevent spontaneous urination. Two or three animals from each group
were killed under ether anaesthesia at 24 h and 4 and 7 days after the
last instillation. The histopathological findings in rats killed 24 h
after a single injection of saline, phenylbenzoquinone, or
phenylhydroquinone included swelling and vacuolation of urothelial
cells. The bladder epithelium of rats treated with alkaline solutions
of sodium 2-phenylphenol or NaOH showed minimal hyperplasia associated
with mild oedema and inflammatory-cell infiltration of the epithelial
and submucosal tissues. Moderate epithelial hyperplasia was seen in
rats killed 7 days after treatment with phenylbenzoquinone. In rats
given two or four instillations of this metabolite, the grading of the
hyperplastic changes was clearly dependent on the number of
instillations and the time between the last treatment and death. The
epithelial hyperplasia was marked and was classified as papillary
and/or nodular in rats treated with four instillations of
phenylbenzoquinone and killed 4 days after the last instillation. No
carcinomas were induced.
In another experiment, groups of 20 female rats were treated in
the same way but twice a week for 5 weeks. From week 6, some rats were
fed the basal diet supplemented with 5% sodium saccharin for 31 weeks
as a promotion treatment, while the other rats were maintained on
basal diet during this period. A separate group was given 500 ppm of
NBHBA for 4 weeks, and then 50 000 ppm of sodium saccharin as a
positive control. Body weights and food consumption were determined
periodically. The histopathological findings in the positive control
group included papillomas in two rats, papillary and/or nodular
hyperplasia in nine rats, and simple hyperplasia in 11 rats. In
contrast, no hyperplastic changes were seen in rats treated first with
sodium 2-phenylphenol or its metabolites followed by promotion with
sodium saccharin, except in nine rats given phenylbenzoquinone, which
had papillary and/or nodular hyperplasia and/or simple hyperplasia.
Formation of lymph follicles in the submucosa of the urinary bladder
was seen in particular with phenylbenzoquinone and in the positive
control group. The authors concluded that phenylbenzoquinone plays an
essential role in the urinary bladder carcinogenesis induced by sodium
2-phenylphenol (Hasegawa et al., 1990b).
A series of studies was carried out on the carcinogenicity of
2-phenylphenol and sodium 2-phenyl-phenol (purity of neither given) in
rat urinary bladder. In the first study, groups of 10 male Fischer 344
rats were fed diets containing sodium 2-phenylphenol at concentrations
of 0, 2500, 5000, 10 000, or 20 000 ppm for 36 weeks. The rats were
observed daily and were weighed periodically. Body-weight gain was
suppressed at 20 000 ppm. No calculi or mucosal tumours were found
grossly, but histological analysis revealed a statistically
significant increase in the frequency of bladder lesions in rats at
20 000 ppm, in which simple hyperplasia was seen in 10/10 and
papillary or nodular hyperplasia in 4/10 animals. Simple hyperplasia
was seen in 1/10 rats at 10 000 ppm.
In the second study, groups of five male rats were fed diets
containing 20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol for 4
weeks. The animals were weighed at the end of treatment, at which time
the mean body weights of both treated groups were reduced, to 86% of
the control value with 2-phenylphenol and to 96% with sodium
2-phenylphenol. One hour before sacrifice, each rat was given an
intraperitoneal injection of 100 mg/kg bw of 5-bromo-2'-deoxyuridine
(BrdU), and the urinary bladders were stained immunohistochemically
with anti-BrdU antibodies to investigate the capacity of the
epithelial cells for proliferation. The number of cells that had taken
up BrdU per 1000 urinary bladder epithelial cells was determined by
light microscopy and expressed as per cent labelled cells. Rats fed
sodium 2-phenylphenol showed increased urinary pH and extensive
BrdU-labelling in urinary bladder epithelial cells, indicating
increased DNA synthesis. Rats fed 2-phenylphenol also showed a
tendency to increased BrdU-labelling, suggested that it also can
cause, albeit weak, urinary bladder epithelium proliferation.
In the third study, groups of five male Fischer 344 rats were
given diets containing 6400 ppm of NaHCO3; 13 000 ppm of
2-phenylphenol; 13 000 ppm of 2-phenylphenol plus NaHCO3 at 1600,
3200, or 6400 ppm; or 20 000 ppm of sodium 2-phenylphenol for 8 weeks.
The urinary pH at week 8 was significantly increased in animals fed
NaHCO3 alone, 2-phenylphenol plus 3200 or 6400 ppm NaHCO3, or
sodium 2-phenylphenol. The urinary concentrations of Na+ were
significantly increased at 2, 4, 6, and 8 weeks in rats fed sodium
2-phenylphenol and at 8 weeks in rats fed NaHCO3 alone or
2-phenylphenol plus 3200 or 6400 ppm NaHCO3. At 8 weeks, a
significant increase or a tendency to an increase in urine volume and
significantly lower osmotic pressure were seen in pooled urine samples
from all treated groups when compared with controls. When the bladders
were examined by scanning electron microscopy, the surface of the
epithelium appeared normal in the controls and in animals given only
NaHCO3, and was made up of polygonal cells of uniform dimensions
with reticular peaked microridges at the surface. In treated animals,
the cells in the outermost layer of the bladder epithelium assumed a
cobblestone configuration in pavement form; at high magnification,
pleomorphic microvilli, short uniform microvilli, and ropy or leafy
microridges were seen at the surface of these cells. These alterations
were observed mainly in rats given sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. The extent of the changes and
the frequency of their appearance was correlated with the
concentration of NaHCO3 given with 2-phenylphenol.
In the fourth study, groups of 30-31 male Fischer 344 rats were
given 20 000 ppm of sodium 2-phenylphenol or 13 000 ppm of
2-phenylphenol with or without NaHCO3 at 1600, 3200, or 6400 ppm for
104 weeks. The animals were observed daily for deaths, and body weight
and feed consumption were measured at regular intervals. Pooled 4-h
urine samples were collected from three or four rats at each dose at
weeks 58, 80, and 96 for measurement of electrolytes. The urinary pH
was significantly increased in rats fed sodium 2-phenylphenol or
2-phenylphenol alone or in combination with 3200 or 6400 ppm NaHCO3
or 6400 ppm NaHCO3 alone. The Na+ concentrations were
significantly increased in rats fed sodium 2-phenylphenol,
2-phenylphenol plus 6400 ppm NaHCO3, or 6400 ppm NaHCO3 alone. No
difference was seen in rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. Urinary bladder tumours were
found in all groups except those given 2-phenylphenol alone, and the
frequencies were highest with sodium 2-phenylphenol and with
2-phenylphenol plus 6400 ppm NaHCO3. The presence of calculi could
not be confirmed. Histological examination of the urinary bladder
epithelium revealed simple hyperplasia, papillary or nodular
hyperplasia, papillomas, and carcinomas. Carcinomas occurred in rats
fed sodium 2-phenylphenol (41%), 2-phenylphenol plus 6400 ppm NaHCO3
(31%), and 6400 ppm NaHCO3 alone. The results confirm that
administration of 20 000 ppm of sodium 2-phenylphenol is carcinogenic
in male rats, while an equimolar concentration of 2-phenylphenol
causes only a low frequency of papillary or nodular hyperplasia and no
papillomas or cancers. Administration of NaHCO3 in conjunction with
2-phenylphenol caused carcinomas, correlated to the NaHCO3
concentration, which also increased urinary pH and Na+ concentration
(Inoue, 1993).
The induction of DNA damage in the urinary bladder epithelium of
male and female Fischer 344 rats by 2-phenylphenol and its metabolites
was studied by the alkaline elution assay after an intravesicalar
injection. Phenylbenzoquinone at 0.05-0.1% had weak DNA-damaging
activity in animals of each sex, whereas 2-phenylphenol and
phenylhydroquinone had no effect at the same dose. Histopathological
examination revealed diffuse, moderate, simple hyperplasia 5 days
after injection of 0.1% phenylbenzoquinone in male rats. The lesions
were associated with submucosal infiltration, small round cells, and
slight oedema. The only change in the bladders of rats injected with
0.1% phenylhydroquinone was slight swelling and/or vacuolization of
the epithelial cells, and the bladders of rats injected with 0.1%
2-phenylphenol were normal (Morimoto et al., 1989).
Groups of 5-10 Fischer 344 rats received diets containing sodium
2-phenylphenol at concentrations of 0, 2500, 5000, 10 000, or 20 000
ppm (equivalent to 0, 250, 500, 1000, and 2000 mg/kg bw per day) for
up to 5 months to investigate the correlation between urinary
phenylbenzoquinone and DNA damage in the bladder epithelium. Slight
but dose-dependent DNA damage was observed in the epithelium of male
rats fed 10 000 or 20 000 ppm for 3-5 months. A plot of the
dose-response relationship for DNA damage at 3 months showed a
threshold at 5000 ppm of sodium 2-phenylphenol. The amounts of
unconjugated 2-phenylphenol, phenylhydroquinone, and
phenylbenzoquinone in 24-h urine samples collected from males and
females after 5 months correlated well with the dietary concentrations
of sodium 2-phenylphenol. The total amounts of free metabolites in the
urine of males given 5000 ppm were similar to those in the urine of
females given 20 000 ppm. Free metabolites represented 0.3% of the
total average intake of male rats fed 5000 ppm, 0.8% of the intake of
10 000 ppm, and 1% of the intake of 20 000 ppm of sodium
2-phenylphenol. The average concentrations of free phenylhydroquinone
in the urine of males given 20 000 ppm of sodium 2-phenylphenol were
significantly higher than those in males fed 5000 ppm or in females
fed 20 000 ppm. The concentrations of phenylbenzoquinone were much
lower than those of phenylhydroquinone. Only 10% of phenylbenzoquinone
was recovered from spiked urine, indicating that this metabolite may
react with urinary nucleophilic groups. The authors concluded that
phenylbenzoquinone is the reactive species in the initiation of
bladder tumours induced by 2-phenylphenol and sodium 2-phenylphenol
(Morimoto et al., 1989).
The interaction of 2-phenylphenol and its metabolites with pUC18
DNA from Escherichia coli plasmids was studied in vitro. The major
metabolite formed from 2-phenylphenol by mixed-function oxidases was
phenylhydroquinone. This finding corroborates earlier reports that
phenylhydroquinone in the form of a glucuronide conjugate is the main
product in the bladders of rats fed 2-phenylphenol. When pUC18 DNA was
incubated with phenylhydroquinone, DNA strand scission was observed,
whereas barely detectable DNA cleavage was seen with 2-phenylphenol
and phenylbenzoquinone. DNA cleavage by phenylhydroquinone was
inhibited by superoxide dismutase, catalase, and several oxygen
radical scavengers, indicating that the oxygen radicals generated in
the process of oxidation of phenylhydroquinone in aqueous solution are
responsible for the DNA cleavage. The attack seemed to occur at
guanine residues in general and was not restricted to guanines with
specific residues, indicating no hot spots (Nagai et al., 1990).
The generation of 8-hydroxydeoxyguanosine in calf thymus DNA
treated with 2-phenylphenol, phenylhydroquinone, or
phenylbenzoquinone, was studied in vitro. The content of
8-hydroxydeoxy-guanosine residues was increased in DNA treated with
phenylhydroquinone in a concentration-dependent manner, but
phenylbenzoquinone had little effect, and 2-phenylphenol had no
effect. The formation of 8-hydroxydeoxyguanosine by phenylhydroquinone
was reduced by oxygen radical scavengers and accelerated by the
addition of CuCl or CuCl2. Hydroxyl radicals generated during
oxidation of phenylhydroquinone thus contribute to the formation of
8-hydroxydeoxyguanosine in DNA, and copper ions facilitate the
oxidative DNA damage. Copper ions greatly accelerated
phenylhydroquinone-induced DNA cleavage in vitro, although they had
no effect on cleavage without phenylhydroquinone. In contrast, DNA
cleavage occurred with the addition of FeCl2 in the absence and
presence of phenylhydroquinone. The formation of
8-hydroxydeoxyguanosine in bladder DNA is likely to be one of a series
of events in the carcinogenesis induced by 2-phenylphenol (Nagai et
al., 1995).
The effect of the selective gamma-glutamylcysteine synthetase
inhibitor, buthionine sulfoximine, on the hepatotoxic and nephrotoxic
potential of 2-phenylphenol and its metabolites was studied in groups
of four male Fischer 344/DuCrj rats. The animals were given an
intraperitoneal injection of 0 or 900 mg/kg bw of buthionine
sulfoximine and 1 h later received 2-phenylphenol, phenylhydroquinone,
or phenylbenzoquinone at single oral doses of 0, 700, or 1400 mg/kg
bw. The rats were killed 6 and 24 h later, and serum was collected for
measurement of alanine and aspartate aminotransferase activities and
urea nitrogen. The liver and kidneys were removed and weighed, and
hepatic and renal glutathione were assayed.
2-Phenylphenol caused acute hepatocellular damage, as shown by
necrotic centrilobular hepatocytes accompanied by increased serum
aminotransferase activity. Pretreatment with buthionine sulfoximine
potentiated the hepatic and renal toxicity of 2-phenylphenol,
indicating that the liver and kidneys are its target organs of at high
doses. 2-Phenylphenol depleted hepatic and renal glutathione by 6 h
after administration, and this effect was enhanced by pretreatment
with buthionine sulfoximine. Recovery of glutathione concentrations in
both organs was slower in rats given 1400 mg/kg bw of 2-phenylphenol
than in those given 700 mg/kg bw, suggesting that the hepatic and
renal damage caused by this compound is associated with prolonged
depletion of glutathione and that it acts indirectly on the liver.
Within 24 h, 75% of the rats treated with phenylbenzoquinone at 1400
mg/kg bw had died. Administration of phenylbenzoquinone at 700 m/kg bw
or phenylhydroquinone at 1400 mg/kg bw significantly increased
aminotransferase activities. The activity of alanine aminotransferase
in both groups was about twice that of rats given 1400 mg/kg bw
2-phenylphenol. A slight decrease in liver weight, nuclear pyknosis,
eosinophilic degeneration of periportal hepatocytes, increased
relative kidney weight, slight renal papillary necrosis, dilatation of
renal tubules, and increased serum urea nitrogen concentration were
observed at 700 mg/kg bw of phenylbenzoquinone. The relative weight of
the kidneys was increased at 1400 mg/kg bw of phenylhydroquinone.
These results indicate that phenylbenzoquinone is more toxic to liver
and kidney than phenylhydroquinone (Nakagawa & Tayama, 1988).
In a study of the conjugation of 2-phenylphenol with glutathione
in rat liver in vitro and in vivo, radiolabel derived from
[14C]2-phenylphenol bound irreversibly to hepatic microsomal
macromolecules in an NADPH-generating system, and the binding was
inhibited by cysteine and glutathione. When [14C]2-phenylphenol and
glutathione were incubated in a microsomal NADPH-generating system,
the radiolabelled material derived from the aqueous phase of the
incubation mixture was similar to a synthetic, water-soluble
phenylhydroquinone-glutathione conjugate produced by a nonenzymic
reaction between phenylbenzoquinone and glutathione.
Phenylhydroquinone-glutathione was excreted as a minor conjugate in
the bile after oral administration of 2-phenylphenol to rats at a dose
of 1000 mg/kg bw. The cumulative biliary excretion of the conjugate
over 6 h represented about 4% of the dose. The results show that a
reactive intermediate of 2-phenylphenol can form adducts with
glutathione to produce water-soluble conjugates. The reactive
intermediate is probably phenylbenzoquinone derived from
phenylhydroquinone. Since glutathione protects against cellular
injury, the acute hepatic damage caused by high doses of
2-phenylphenol is probably associated with the formation of an active
intermediate (phenylbenzoquinone) which depletes cellular glutathione
(Nakagawa & Tayama, 1989).
The relationship between the metabolism and cytotoxicity of
2-phenylphenol was studied in isolated rat hepatocytes. Addition of
high concentrations of 2-phenylphenol to the cells caused
dose-dependent toxicity, with death at the highest dose of 1.0 mmol/L.
Pretreatment of the hepatocytes with a non-toxic dose of 5 µmol/L of
SKF-525A enhanced the cytotoxicity of 2-phenylphenol at 0.5-1.0 mmol/L
and inhibited its metabolism. At lower concentrations (0.5 or 0.75
mmol/L), 2-phenylphenol was converted sequentially to
phenylhydroquinone and then to its glutathione conjugate. The
concentrations of both metabolites and especially of the conjugate,
were very low in hepatocytes exposed to 2-phenylphenol at 1.0 mmol/L
alone or with SKF-525A. The cytotoxicity induced by 2-phenylphenol at
0.5 mmol/L was enhanced by the addition of 1.25 mmol/L of
diethylmaleate, which continuously depletes cellular glutathione. In
contrast, the cytotoxicity induced by phenylhydroquinone at 0.5 mmol/L
was significantly inhibited by addition to the hepatocytes of 5 mmol/L
of dithiothreitol, cysteine, N-acetyl-L-cysteine, or ascorbic acid.
Loss of glutathione, protein thiols, and ATP was also prevented. These
results indicate that the acute cytotoxicity of 2-phenylphenol at 1.0
mmol/L is a direct action and that prolonged depletion of cellular
glutathione enhances the cytotoxicity of low concentrations of
2-phenylphenol metabolites. The cytotoxicity of phenylhydroquinone is
prevented significantly by addition of cysteine, glutathione, or
ascorbic acid (Nakagawa et al., 1992).
Groups of 22 male CDF (Fischer 344)/BR rats were given diets
containing 2phenylphenol (purity, 99.5%) to provide concentrations of
0, 800, 4000, 8000, or 12 500 ppm, equal to 0, 56, 280, 560, and 920
mg/kg bw per day, for 13 weeks. During weeks 12-13 and 13-14 of the
study, urine was collected for determination of metabolites and
urinary characteristics, respectively. In addition, urinary bladders
were collected from 12 animals per group during week 14 for analysisof
the urothelium by 32P-postlabelling, while histopathological
evaluation of 10 animals group included determination of a labelling
index and light and scanning electron microscopy. The body-weight gain
was reduced by about 10% at 8000 and 12 500 ppm, but food intake was
unaffected at all doses tested. Histological examination showed simple
hyperplasia of the urothelium at concentrations of 8000 and 12 500 ppm
with significant changes in the bladder. The glucuronide and sulfate
conjugates of 2-phenylphenol and the hydroxylated metabolite
phenylhydroquinone were the major urinary metabolites, although the
major conjugate at all doses was the sulfate. Minute levels of free
2phenylphenol and phenylhydroquinone were found at all doses, free
phenylhydroquinone comprising 0.6-1.5% of the total metabolites
measured. An increase in the labelling index of the bladder epithelium
was observed at 8000 and 12 500 ppm. 32P-Postlabelled urothelial DNA
showed no evidence of formation of 2-phenylphenol-DNA adducts.
The authors concluded that a hyperplastic response of the urinary
bladder epithelium occurs after exposure to 2-phenylphenol at 8000 or
12 500 ppm, which are unequivocal carcinogenic doses for the bladder
of male rats, which is due to mild cytotoxicity with consequent
regenerative hyperplasia. The increased mitotic activity (labelling
index), the presence of very small amounts of free phenylhydroquinone
in the urine, and the absence of DNA adducts in the bladder epithelium
further suggest that the bladder carcinogenesis in male rats exposed
to 2-phenylphenol is probably mediated by an indirect, dose-dependent
cytotoxic effect on the bladder epithelium leading to regenerative
hyperplasia and subsequent tumorigenesis of epigenetic origin, rather
than to direct metabolic activation of 2-phenylphenol to reactive
metabolites capable of forming 2-phenylphenol-DNA adducts (Christenson
et al., 1996a).
Groups of 20-30 male CDF (Fischer344)/BR rats were given diets
containing 2-phenylphenol (purity, 99.9%) at concentrations of 0,
1000, 4000, or 12 500 ppm, equal to 0, 54, 220, and 680 mg/kg bw per
day, for 13 weeks. Animals from the control and high-dose groups were
allowed to recover for 4 weeks. Urine was collected for chemical and
and electron microscopic evaluation at various times, and urinary
bladders were collected from animals in the recovery groups during
weeks 4, 13, and 17 for histological evaluations which included
determination of a labelling indexes and light and electron
microscopy.
Body-weight gain was reduced only in rats at 12 500 ppm, and food
intake was unaffected at all doses. Weekly clinical examinations
showed an increased incidence of urine staining at 4000 and 12 500
ppm. No unusual precipitate or crystal was found in the urinary
sediment of treated animals. Urothelial hyperplasia was observed only
after 13 weeks at 12 500 ppm, and the effect was reversed by 4 weeks
on control diet. After 4 and 13 weeks of exposure to 2-phenylphenol,
necrotic foci were observed in the bladders of rats at 12 500 ppm, and
at 13 weeks the bladders also showed evidence of regenerative
hyperplasia. Increased labelling indexes were observed in the bladders
of animals at the high dose at 4 and 13 weeks, but the index had
returned to control values after 4 weeks' recovery, confirming the
reversibility of the proliferative changes in the urothelium. The
results of this study suggest that 2-phenylphenol acts by a mechanism
involving a cytotoxic action on the urothelium leading to the
formation of regenerative, reversible hyperplasia. The origin of the
cytotoxicity remains unclear, however, as no evidence was found of
either abnormal crystalluria or a calcium phosphate-containing
amorphous precipitate (Christenson et al. 1996b).
The relative importance of bladder distension, urinary pH, and
Na+ concentration in the induction of cell proliferation in the
bladder epithelium of rats fed various sodium salts was investigated.
In male rats fed a diet containing 5% NaHCO3, the bladder epithelium
showed an increased number of replicating cells, distension, increased
urinary pH, and a high urinary Na+ concentration. Cell proliferation
also occurred when the bladders were subjected to distension in
vivo by mechanical (female) or physiological (male) means.
Inclusion of CaCO3 in the diet increased the urinary pH without
altering other factors and did not induce cell proliferation, but
proliferation was increased when CaCO3 was combined with the
mechanical or physiological treatment. Thus, high urinary pH was of
secondary importance to bladder distension as a causative factor but
acted to enhance cell proliferation when distension occurred. Similar
findings were obtained with regard to the Na+ concentration. The
authors concluded that bladder distension is a prerequisite for
proliferation of epithelial cells in the bladders of rats fed diets
containing high concentrations of sodium salts and that changes in
urinary pH and Na+ concentration also determine the degree of
proliferation (Shioya et al., 1994).
3. Observations in humans
In one of the earliest studies on the toxicity of 2-phenylphenol,
skin irritation and sensitization due to exposure to this compound and
its sodium salt were evaluated in 100 male and 100 female, unselected
persons. A patch impregnated with the test material was placed in
direct contact with the skin of the back of each person, covered with
an impervious film, and taped securely in place. The first patch was
kept in constant contact with the skin for 5 days, at which time the
patch was removed and the reaction noted. A second patch was applied
in the same way 3 weeks after removal of the first patch and was kept
in direct contact with the skin for 48 h. Each subject was examined
immediately and again 3 and 8 days after removal of the second patch.
2-Phenylphenol as a 5.0% solution in sesame oil did not cause primary
irritation or sensitization, but sodium 2-phenylphenol was
significantly irritating when applied as a 5% or a 1% aqueous
solution. A 0.5% solution caused very slight, simple irritation,
whereas a 0.1% solution produced no irritation and no sensitization
(Hodge et al., 1952).
Comments
After oral administration to mice and rats, 2-phenylphenol and
its sodium salt are rapidly and extensively absorbed (95%) and
distributed. Excretion is also rapid in these species, being almost
complete within 48 h, and occurs mainly in urine (about 90%) and in
faeces (about 5%). Little radiolabel (< 1%) is retained in organs and
tissues, including the urinary bladder. After dermal application of
2-phenylphenol to humans, about 43% of the applied dose was absorbed
through the skin and about 58% was recovered in skin rinse and the
protective enclosure. Most of the absorbed radiolabel was recovered in
urine (99%, and only 1% was recovered in faeces. The absorption
half-time was 10 h, and the elimination half-time was 0.8 h. The rapid
excretion of the radiolabel into urine indicates that 2-phenylphenol
is unlikely to accumulate in humans exposed repeatedly. The metabolic
profiles of both compounds were similar in mice, rats, and humans at
the various doses tested. The main metabolic pathways are conjugation
of 2-phenylphenol or hydroxylation at the 5 position of the phenol
ring, followed by conjugation with glucuronide or sulfate. The parent
compound was detected in only very small amounts (0.4%) in urine. The
metabolic profile in plants raised no toxicological concern, since
about 90% of the residue found in oranges and pears is 2-phenylphenol
or its conjugates.
2-Phenylphenol and its sodium salt have low acute toxicity in
mice and rats treated orally, the LD50 values ranging from 600 to
3500 mg/kg bw. Neither 2-phenylphenol nor its sodium salt has been
classified by WHO for acute toxicity.
2-Phenylphenol and its sodium salt caused severe dermal
irritation in rabbits, and the sodium salt caused severe dermal
irritation in humans. 2-Phenylphenol irritated the eye of rabbits,
whereas the sodium salt caused only moderate ocular irritation.
Neither substance caused delayed contact hypersensitivity in
guinea-pigs or humans.
In medium- and long-term tests for toxicity, the urinary bladder
was regarded as the main toxicological target organ of both
2-phenylphenol and its sodium salt in male and female rats. At doses
of 200 mg/kg bw per day and above, hyperplasia, papillomas, and
transitional-cell carcinomas were seen with both compounds in male
rats. Increased mitosis was observed in the bladder epithelium three
days after the start of dosing, and thickening, i.e. simple
hyperplasia, was seen at 14 days. In female rats, hyperplasia and
papillomas were observed, but to a far lower degree than in males. In
male and female mice, the liver was the primary target organ.
Increased relative liver weights and an increased incidence of
hepatocellular adenomas were seen with 2-phenylphenol at doses of 500
mg/kg bw per day and above. Reduced body-weight gain was a common
finding in mice and rats. In 90-day studies, the NOAELs for
2-phenylphenol were 6300 ppm, equal to 760 mg/kg bw per day, in rats
and 300 mg/kg bw per day (the highest dose tested for up to 1 year) in
dogs. The NOAEL for the sodium salt was 5000 ppm, equivalent to 550
mg/kg bw per day, in mice and 2500 ppm, equal to 180 mg/kg bw per day,
in rats. In a 1-year study of toxicity, the NOAEL for 2-phenylphenol
was 800 ppm, equal to 39 mg/kg bw per day, in rats. In 2-year studies
of carcinogenicity, the NOAEL for 2-phenylphenol was 250 mg/kg bw per
day in mice and 800 ppm, equal to 39 mg/kg bw per day, in rats. In
2-year carcinogenicity studies with the sodium salt, the NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, in
mice and 2500 ppm, equivalent to 95 mg/kg bw per day, in rats. The
Meeting concluded that both 2-phenylphenol and its sodium salt are
carcinogenic in male rats and that 2-phenylphenol is carcinogenic in
male mice.
2-Phenylphenol has been more extensively tested for genotoxic
activity than its sodium salt. Within that limitation, the results for
the two compounds were similar. Data regarding covalent binding to DNA
in the urinary bladder of rats dosed with either compound were
conflicting. 2-Phenylphenol induced chromosomal aberrations in
cultured mammalian cells, but negative results were obtained in
vivo. The Meeting concluded that there are unresolved questions
about the genotoxic potential of 2-phenylphenol.
Several studies have been conducted to elucidate the mechanism of
the carcinogenic action of 2-phenylphenol and its sodium salt on the
male rat urinary bladder, since neither compound has a carcinogenic
effect on the urinary bladder of female rats or in mice, guinea-pigs,
or hamsters of either sex. No clear mechanisms have been found,
although raising the urinary pH or sodium concentration has a
promoting effect. There was some evidence from studies with the sodium
salt that initial irritation followed by hyperplasia might be involved
in the bladder carcinogenicity in male rats. In addition,
32P-postlabelling showed binding of 2-phenylphenol and its sodium
salt to DNA in the male rat urinary bladder in some but not in other
studies. The genotoxicity of the metabolites phenylhydroquinone and
dihydroxybiphenyl appears to be similar to that of the parent
molecules.
The Meeting concluded that the urinary bladder tumours observed
in male rats and the liver tumours observed in male mice exposed to
2-phenylphenol are threshold phenomena that are species- and
sex-specific, and that 2-phenylphenol is therefore unlikely to
represent a carcinogenic risk to humans. In coming to this conclusion,
the Meeting was aware that a working group convened by IARC had
classified 2-phenylphenol, sodium salt, in Group 2B (possibly
carcinogenic to humans) and 2-phenylphenol in Group 3 (not
classifiable as to its carcinogenicity to humans). The Meeting noted,
however, that the IARC classification is based on hazard
identification, not on risk assessment, and is furthermore limited to
published literature, with the exclusion of unpublished studies on
toxicity and carcinogenicity.
In two two-generation studies of reproductive toxicity in rats,
2-phenylphenol had no reproductive toxicity, even at 460 mg/kg bw per
day, the highest dose tested. The overall NOAEL for carcinogenicity
was 92 mg/kg bw per day, since urinary bladder tumours were found in
male rats at doses of 120 mg/bw per day and above.
In a study of developmental toxicity in mice with 2-phenylphenol
and its sodium salt, the NOAELs for 2-phenylphenol were below 1500
mg/kg bw per day (lowest dose tested) for maternal toxicity and
fetotoxicity and 2100 mg/kg bw per day (highest dose tested) for
teratogenicity. The NOAELs for the sodium salt were below 100 mg/kg bw
per day (lowest dose tested) for maternal toxicity, 100 mg/kg bw per
day for fetotoxicity, and 400 mg/kg bw per day (highest dose tested)
for teratogenicity. In two studies of developmental toxicity in rats,
the overall NOAELs for 2-phenylphenol were 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 700
mg/kg bw per day (highest dose tested) for teratogenicity. In two
studies of developmental toxicity in rabbits, the overall NOAELs for
2-phenylphenol were 100 mg/kg bw per day for maternal toxicity, 500
mg/kg bw per day for fetotoxicity, and 750 mg/kg bw per day (highest
dose tested) for teratogenicity.
The Meeting established an ADI of 0-0.4 mg/kg bw for
2-phenylphenol, on the basis of the NOAEL of 39 mg/kg per day in the
2-year study of toxicity (based on decreased body-weight gain and
hyperplasia of the urinary bladder) and carcinogenicity of the urinary
bladder in male rats and a safety factor of 100.
The Meeting determined that it was unnecessary to establish an
acute reference dose because of the low acute toxicity of
2-phenylphenol.
Toxicological Evaluation
Levels of 2-phenylphenol that cause no toxic effect
Mouse: < 250 mg/kg bw per day for carcinogenicity (lowest dose
tested; 2-year study of toxicity and carcinogenicity)
< 1500 mg/kg bw per day (lowest dose tested; study of
developmental toxicity; maternal toxicity)
2100 mg/kg bw per day (highest dose tested; study of
developmental toxicity; not teratogenic)
Rat: 800 ppm, equal to 39 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
460 mg/kg bw per day (two-generation study of reproductive
toxicity; no reproductive toxicity; highest dose tested)
92 mg/kg bw per day (two-generation study of reproductive
toxicity; carcinogenicity)
150 mg/kg bw per day (study of developmental toxicity;
maternal toxicity)
300 mg/kg bw per day (study of developmental toxicity;
developmental toxicity)
700 mg/kg bw per day (study of developmental toxicity;
teratogenicity)
Rabbit: 100 mg/kg bw per day (two studies of developmental toxicity;
maternal toxicity)
500 mg/kg bw per day (two studies of developmental toxicity;
fetotoxicity)
750 mg/kg bw per day (two studies of developmental toxicity;
teratogenicity)
Dog: 750 mg/bw per day (highest dose tested; 1-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.4 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Mechanistic studies on urinary bladder tumours in male rats
2. Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to
2-phenylphenol (unless otherwise specified)
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Rapid (24 h) and complete (95-100%), mice and rats
Dermal absorption Rapid and well absorbed (43%), humans
Distribution Small concentrations (< 1%) in tissues, mice and rats
Potential for accumulation No accumulation, mice, rats, and humans
Rate and extent of excretion Rapid and complete (95-100%), mice, rats, and humans
Metabolism in animals Glucuronide and sulfate of 2-phenylphenol and phenylhydroquinone,
mice and rats
Toxicologically significant compounds 2-Phenylphenol
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 5000 mg/kg bw
Rabbit, LC50, inhalation (4 h) > 36 mg/m3 air (aerosol)
Dermal irritation 2-Phenylphenol and its sodium salt: severe dermal irritation,
rabbits
Ocular irritation 2-Phenylphenol: occular irritation, rabbits
Sodium salt: slight ocular irritation, rabbits
Dermal sensitization 2-Phenylphenol and its sodium salt: no dermal sensitization,
guinea-pigs and humans
Short-term toxicity
Target/critical effect Body-weight decrease, mice and rats, and urinary bladder tumours,
male rats
Lowest relevant oral NOAEL 300 mg/kg bw per day, dogs
Lowest relevant dermal NOAEL No NOAEL, 1000 mg/kg bw per day, highest dose tested, rats
Lowest relevant inhalation NOAEL Not investigated
Genotoxicity Unresolved questions
Long-term toxicity and carcinogenicity
Target/critical effect Urinary bladder, male rats
Liver, male and female mice
Lowest relevant NOAEL 39 mg/kg bw per day, male rats
Carcinogenicity Urinary bladder tumours, male rats
Liver tumours, male and female mice
Reproductive toxicity
Reproductive target/critical effect No reproductive toxicity, rats
Lowest relevant reproductive NOAEL 460 mg/kg bw per day, highest dose tested, rats
Developmental target/critical effect Developmental toxicity at maternally toxic doses, mice
Lowest relevant developmental NOAEL 300 mg/kg bw per day, rabbits
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rats.
No evidence of neurotoxicity or neuropathology in medium- and
long-term studies, mice, rats, dogs, or in developmental toxicity
studies, mice, rats, and rabbits
Other toxicological studies
Medical data Dermal irritation with the sodium salt, not with 2-phenylphenol
Summary Value Study Safety factor
ADI 0-0.4 mg/kg bw Long-term study of toxicity 100
and carcinogenicity, rat
Acute reference dose Unnecessary
References
Bartels, M.J. & McNett, D.A. (1996) Quantitation of orthophenylphenol
metabolites in rat urine samples from a 32P-postlabeling study.
Unpublished report No. HET K-001024-062 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Bartels, M.J., Brzak, K.A., McNett, D.A. & Shabrang, S.N. (1997)
Ortho-phenylphenol (OPP): Limited metabolism study in human.
Unpublished report No. HET K-001024-059 from Dow Chemical
Company, Midland, Michigan, USA. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Bartels, M.J., McNett, D.A., Timchalk, C., Mendrala, A.L.,
Christenson, W.R., Sangha, G.K., Brzak, K.A. & Shabrang, S.N.
(1998) Comparative metabolism of orthophenylphenol in mouse,
rat and man. Xenobiotica, 28, 579-594.
Berdasco, N.M. (1991) Orthophenylphenol: Dermal sensitization
potential in the Hartley albino guinea pig. Unpublished report
No. K-001024-048 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Carreon, R.E. & New M.A. (1981) Dowicide*1: Acute percutaneous
absorption potential. Unpublished report No. HET K-1024-(37) from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S., Bartels, M.J. & Cohen, S.M. (1996a)
Technical grade orthophenylphenol: A 32P-postlabeling study
to examine the potential for the formation of DNA adducts in the
urinary bladder of the male rat. Unpublished report No. 94-972-AV
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S. & Cohen, S.M. (1996b) Technical grade
ortho-phenylphenol: A special subchronic dietary study to
examine the mechanism of urinary bladder carcinogenesis in the
male rat. Unpublished report No. 92-972-MS from Bayer
Corporation, Agriculture Division, Toxicology, Stilwell, Kansas,
and University of Nebraska Medical Center, Department of
Pathology & Micropathology, Omaha, Nebraska, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Cosse, P.F., Stebbins, K.E., Stott, W.T., Johnson, K.A. & Atkin, L.
(1990) Orthophenylphenol: Palatability/probe, four-week and
one-year oral toxicity studies in beagle dogs. Unpublished report
No. K-001024-039 from Dow Chemical Co., Midland, Michigan , USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Davies, R.E. & Liggett, M.P. (1973) Irritant effects of agricultural
disinfectant C2 on rabbit eye mucosa. Unpublished report No.
2215/169D/73 from Huntingdon Research Centre, England. Submitted
to WHO by Leng Associates, Midland, Michigan, USA,
Eigenberg, D.A. (1990) Two-generation dietary reproduction study in
rats using orthophenylphenol. Unpublished revised report No
85-671-02 from Mobay Corporation Health, Environment, Safety &
Plant Management, Corporate Toxicology Department, South Metcalf,
Stilwell, Kansas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Eigenberg, D.A. (1995) A two-generation dietary reproduction study in
Sprague Dawley rats using technical grade ortho-phenylphenol.
Unpublished report No. 93-672-VX from Bayer Corporation
Agriculture Division, Toxicology, South Metcalf, Stilwell,
Kansas, USA. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Fujii, T. & Hiraga, K. (1985) Carcinogenicity testing of sodium
orthophenylphenate in F344 rats. J. Saitana Med. School, 12,
277-287.
Fujii, T., Nakamura, K. & Hiraga, K. (1987) Effects of pH on the
carcinogenicity of ophenylphenol and sodium o-phenylphenate
in the rat urinary bladder. Food Chem. Toxicol., 25, 359-362.
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. (1983)
Promoting effect of sodium o-phenylphenate and o-phenylphenol
on two-stage urinary bladder carcinogenesis in rats. Jpn. J.
Cancer Res. (Gann), 74, 625-632.
Fukushima, S., Inoue, T., Uwagawa, S., Shibata, M. & Ito N. (1989)
Co-carcinogenic effects of NaHCO3 on o-phenylphenol-induced
rat bladder carcinogenesis. Carcinogenesis, 10, 1635-1640.
Gilbert, K.S. (1994a) Dowicide(R) 1 antimicrobial: Primary dermal
irritation study in New Zealand white rabbits. Unpublished report
No. K-001024-057B from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert, K.S. (1994b) Dowicide(R) 1 antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001024-57E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. (1994c) Dowicide(R) A antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001025-014E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. & Crissman, J.W. (1994) Dowicide(R) 1 antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
no. K-001024-057A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert K.S. & Stebbins, K. (1994) Dowicide(R) A antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
No. K-001025-014A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Hagiwara, A., Shibata, M., Hirose, M., Fukushima, S. & Ito, N. (1984)
Long-term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Food Chem. Toxicol., 22,
809-814.
Hasegawa, R., Takahashi, S., Asamoto, M., Shirai, T. & Fukushima, S.
(1990a) Species differences in sodium o-phenylphenate induction
of urinary bladder lesions. Cancer Lett., 50, 87-91.
Hasegawa, R., Furukawa, F., Toyoda K., Sato, H., Takahashi, M. &
Hayashi, Y. (1990b) Urothelial damage and tumor initiation by
urinary metabolites of sodium o-phenylphenate in the urinary
bladder of female rats. Jpn. J. Cancer Res., 81, 483-488.
Hasegawa, R., Fukuoka, M., Takahashi, T., Yamamoto, A., Yamaguchi, S.,
Shibata, M.A., Tanaka, A. & Fukushima, S. (1991) Sex differences
in o-phenylphenol and sodium ophenylphenate rat urinary
bladder carcinogenesis: Urinary metabolites and electrolytes
under conditions of aciduria and alkalinuria. Jpn. J. Cancer
Res., 82, 657-664.
Hiraga, K. (1983a) Short report of a 91-week feeding study of
orthophenylphenol (OPP) in male rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. (1983b) Carcinogenicity testing of sodium
orthophenylphenate in F-344/DuCrj rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. & Fujii, T. (1981) Induction of tumours of the urinary
system in F344 rats by dietary administration of sodium
o-phenylphenate. Food Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. (1984) Induction of tumours of the urinary
bladder in F344 rats by dietary administration of o-phenylphenol.
Food Cosmet. Toxicol., 22, 865-870.
Hodge, H.C., Maynard, E.A., Blanchet, H.J., Jr, Spencer, H.C. & Rowe,
V.K. (1952) Toxicological studies of orthophenylphenol (Dowicide
1). J. Pharmacol. Exp. Ther., 104, 202-210.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T. & Sugimura, T. (1983)
Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
Horvath, E., Levay, G. Pongracz, K. & Bodell, W.J. (1992) Peroxidative
activation of ophenylhydroquinone leads to the formation of DNA
adducts in HL-60 cells. Carcinogenesis, 13, 1937-1939.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to
42, Lyon, International Agency for Research on Cancer, pp.
37-41.
IARC (1999) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 73, Miscellaneous
Pesticides, Lyon, International Agency for Research on Cancer,
pp. 451-480.
Iguchi, S., Takahashi, H., Fujii, T., Fukumori, N., Mikuriya, H.,
Tada, Y., Yuzawa, K. & Hiraga, K. (1984) Subchronic toxicity of
o-phenylphenol (OPP) by food administration to rats. Ann.
Rep. Tokyo Metr. Res. Lab. Public Health, 35, 407-415.
Iguchi, S., Tayama, K. & Hiraga, K. (1979) Subacute toxicity of sodium
o-phenylphenate by food administration to rats. Ann. Rep.
Tokyo Metropolitan Res. Lab. Public Health, 30, 67-79.
Inoue, T. (1993) Pathological studies on rat urinary bladder
carcinogenicity of ophenylphenol and its sodium salt. J.
Nagoya City Univ. Medical Assoc. (Nagoya Shiritsu Daigaku
Igakkai Zasshi), 44, 463-477.
Inoue, S., Yamamoto, K. & Kawanishi, S. (1990) DNA damage induced by
metabolites of ophenylphenol in the presence of copper (II)
ion. Chem. Res. Toxicol., 3, 144-149.
Ishidate, M., Jr, Yoshikawa, K. & Sofuni, T. (1983) Mutagenicity tests
on OPP, OPPsodium salt, and their possible metabolites.
Unpublished report from Division of Mutagenesis, Biological
Safety Research Center, National Institute of Hygienic Sciences,
Tokyo, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983a) Long-term toxicity and carcinogenicity study of sodium
o-phenyl-phenate in B6C3F1 mice. Unpublished report from First
Department of Pathology, Nagoya City University Medical School,
Nagoya, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983b) Pathological analysis of the carcinogenicity of sodium
o-phenyl-phenate and o-phenylphenol. Unpublished report from
First Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz B.A. (1981)
Teratological evaluation of orthophenylphenol in rats. Fundam.
Appl. Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. (1978)
Teratogenicity and dominantlethal studies with o-phenylphenol.
J. Pestic. Sci., 3, 365-370.
Kolachana, P., Subrahmanyam, V.V., Eastmond, D.A. & Smith, M.T. (1991)
Metabolism of phenyhydroquinone by prostaglandin (H) synthase:
Possible implications in ophenylphenol carcinogenesis.
Carcinogenesis, 12, 145-149.
Kwok, E.S.C. & Eastmond, D.A. (1997) Effects of pH on nonenzymatic
oxidation of phenylhydroquinone: Potential role in urinary
bladder carcinogenesis induced by ophenylphenol in Fischer 344
rats. Chem. Res. Toxicol., 10, 742-749.
Lambert, A.C. & Eastmond, D.A. (1994) Genotoxic effects of the
o-phenylphenol metabolites phenylhydroquinone and
phenylbenzoquinone in V79 cells. Mutat. Res., 322, 243-256.
Landry, T.D., Stebbins, K.E. & Battjes, J.E. (1992)
Ortho-phenylphenol: Acute aerosol inhalation toxicity study in
Fischer 344 rats. Unpublished report No. K-001024-049 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Luster, M. (1986) NTP Technical Report on the Toxicology and
Carcinogenesis Studies of ortho-Phenylphenol (CAS No. 90-43-7)
Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No.
57-97-6) in Swiss CD-1 Mice (Dermal Studies) (NIH publication
No. 86-2557, NTP TR 301), US Department of Health and Human
Services, National Toxicology Program, Research Triangle Park,
North Carolina, USA.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A. & Wilson, R.E. (1981) The effects of
orthophenylphenol, tris(2,3dichloropropyl)phosphate and
cyclophosphamide on the immune system and host susceptibility of
mice following subchronic exposure. Toxicol. Appl. Pharmacol.,
58, 252-261.
McNett, D.A., Timchalk, C. & Mendrala, A.L. (1997) Ortho-phenylphenol
(OPP): Metabolism of 14C-labelled OPP in B6C3F1 mice and
Fischer 344 rats. Unpublished report No. HET K-001024-060 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Morimoto, K., Sato, M., Fukuoka, M., Hasegawa, R., Takahashi, T.,
Tsuchiya, T., Tanaka, A., Takahashi, A. & Hayashi, Y. (1989)
Correlation between the DNA damage in urinary bladder epithelium
and the urinary 2-phenyl-1,4-benzoquinone levels from F344 rats
fed sodium o-phenylphenate in the diet. Carcinogenesis, 10,
1823-1827.
Nagai, F. & Nakao, T. (1984) Changes in enzyme activities in the urine
and tissues of rats fed sodium o-phenylphenate. Food Chem.
Toxicol., 22, 361-364.
Nagai, F., Ushiyama, K., Satho, K. & Kano, I. (1990) DNA cleavage by
phenylhydroquinone: The major metabolite of a fungicide
o-phenylphenhol. Chem.-Biol. Interactions, 76, 163-179.
Nagai, F., Ushiyama, K., Satoh, K., Kasai, H. & Kano, I. (1995)
Formation of 8hydroxyguanosine in calf thymus DNA treated
in vitro with phenylhydroquinone, the major metabolite of
o-phenylphenol. Carcinogenesis, 16, 837-840.
Nakagawa, Y. & Tayama, K. (1988) Effect of buthionine sulfoximine on
orthophenylphenolinduced hepato- and nephrotoxic potential in
male rats. Arch. Toxicol., 62, 452-457.
Nakagawa, Y. & Tayama, S. (1989) Formation of ortho-phenylphenol
glutathione conjugates in the rat liver. Xenobiotica, 19,
499-507.
Nakagawa, A., Nakao, T. & Nakao, M. (1984) Altered levels of cyclic
nucleotides in F344 rats fed sodium o-phenylphenate. Food
Chem. Toxicol., 22, 217-221.
Nakagawa Y., Tayama, S., Moore, G.A. & Moldéus, P. (1992) Relationship
between metabolism and cytotoxicity of ortho-phenylphenol in
isolated rat hepatocytes. Biochem. Pharmacol., 43, 1431-1437.
Nakao, T., Kabashima, U.J., Nagai, F., Nakagawa, A., Ohno, T.,
Ichikawa, H., Kabayashi, H. & Hiraga, K. (1983) The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Food Chem. Toxicol., 21, 325-329.
National Toxicology Program (1986) Toxicology and Carcinogenesis
Studies of orthoPhenylphenol (CAS No. 90-43-7) Alone and with
7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1
Mice (Dermal Studies) (NTP Technical Report No. 301, NIH
publication No. 86-2557), US Department of Health and Human
Services, Research Triangle Park, North Carolina, USA.
Norris, J..M. (1971) Eye irritation test conducted on Dowicide l
preservative. Unpublished report from Chemical Biology Research,
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Oehme, F.W. & Smith, T.H.F. (1972) The metabolism and urinary
excretion of ophenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1978) Teratological tests
of ortho-phenylphenol and its sodium salt in mice. Ann. Rep.
Tokyo Metr. Res. Lab. Public Health, 29, 89-96.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1979) Acute oral toxicity
of sodiumphenylphenate (OPP-Na) in mice. Ann. Rep. Tokyo Metr.
Res. Lab. ublic Health, 30, 54.
Pathak, D.N. & Roy, D. (1992) Examination of microsomal cytochrome
P450-catalyzed in vitro activation of o-phenylphenol to DNA
binding metabolite(s) by 32P-postlabeling technique.
Carcinogenesis, 13, 1593-1597.
Pathak, D.N. & Roy, D. (1993) In vivo genotoxicity of sodium
ortho-phenylphenol: phenylbenzoquinone is one of the
DNA-binding metabolite(s) of sodium orthophenylphenol.
Mutat. Res., 286, 309-319.
Quast, J.F. & McGuirk, R.J. (1995) ortho-Phenylphenol: Two-year
dietary chronic toxicity/oncogenicity study in B6C3F1 mice.
Unpublished report No. K-001024-047 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1983) Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem.-Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1984) Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP) on
the urinary tract of male F344 rats. Toxicol. Appl.
Pharmacol., 73, 345-349.
Roy, D. (1990) Cytochrome P-450 catalyzed redox cycling of
orthopenylphenol. Biochem. Int., 22, 849-857.
Sato, M., Tanaka, A., Tsuchiya, T., Yamaha, T., Nakaura, S. & Tanaka,
S. (1988) Excretion, distribution and metabolic fate of sodium
o-phenylphenate and o- phenylphenol in the rat. J. Food
Hyg. Soc. Jpn, 29, 7-12.
Savides, M.C. & Oehme, F.W. (1980) Urinary metabolism of orally
administered orthophenyl phenol in dogs and cats.
Toxicology, 17, 355-363.
Selim, S.A. (1996) A single dose open label study to investigate the
absorption and excretion of 14C/13C-labeled orthophenylphenol
formulation after dermal application to healthy volunteers.
Unpublished report No. P0995002 from Pharma Bio-Research Clinics
BV, Assen, Netherlands. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Shibata, M.-A., Hagiwara, A., Tamano, S., Fukushima, S. & Ito, N.
(1985) Subchronic toxicity study of sodium o-phenylphenate in
mice. Toxicol. Lett., 25, 239-246.
Shibata, M.-A., Tanaka, H., Yamada, M., Tamano, S. & Fukushima, S.
(1989a) Proliferative response of renal pelvic epithelium in male
rats to oral administration of orthophenylphenol, sodium
ortho-phenylphenate and diphenyl. Cancer Lett., 48, 19-28.
Shibata, M.-A., Tamano, S., Kurata, Y., Hagiwara, A. & Fukushima, S.
(1989b) Participation of urinary Na+, K+, pH, and L-ascorbic
acid in the proliferative response of the bladder epithelium
after the oral administration of various salts and/or ascorbic
acid to rats. Food Chem. Toxicol., 27, 403-413.
Shioya, S., Nagami-Oguihara, R., Oguihara, S., Kimura, T., Imaida, K.
& Fukushima, S. (1994) Roles of bladder distension, urinary pH
and urinary sodium ion concentration in cell proliferation of
urinary bladder epithelium in rats ingesting sodium salts.
Food Chem. Toxicol., 32, 165-171.
Shirasu, Y., Moriya, M., Kato, K., Tezuka, H., Henmi, R., Shingu, A.,
Kaneda, M. & Teramoto, S. (1978) Mutagenicity testing on
o-phenylphenol. Mutat. Res. 54, 227 (abstract).
Smith, R.A., Christenson, W.R., Bartels, M.J., Arnold, L.L., St John,
M.K., Cano, M., Garland, E.M., Lake, S.G., Wahle, B.S., McNett,
D.A. & Cohen, S.M. (1998) Urinary physiologic and chemical
metabolic effects on the urothelial cytotoxicity and potential
DNA adducts of o-phenylphenol in male rats. Toxicol. Appl.
Pharmacol., 150, 402-413.
Suzuki, H., Suzuki, N., Sasaki, M. & Hiraga, K. (1985)
Orthophenylphenol mutagenicity in a human cell strain. Mutat.
Res., 156, 123-127.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981a)
Toxicological study of o-phenylphenol (OPP) in mice. J. Nara
Med. Assoc., 32, 425.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981b)
Toxicological study of o-phenylphenylphenate (OPP-Na) in rats II.
Acute oral toxicity in Fischer 344 DUCRJ rats. J. Nara Med.
Assoc., 32, 709.
Tayama, S. & Nakagawa, Y. (1991) Sulfhydryl compounds inhibit the
cyto- and genotoxicity of o-phenylphenol metabolites in CHO-K1
cells. Mutat. Res., 259, 1-12.
Tayama, K., Iguchi, S. & Hiraga, K. (1979) Acute oral toxicity of
sodium o-phenylphenol (OPP-Na) in rats. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 30, 57.
Tayama, K., Iguchi, S. & Hiraga, K. (1980) Acute oral toxicity of
o-phenylphenol in rats. Ann. Rep. Tokyo Metr. Res. Lab.
Public Health, 31, 1.
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1983) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 34, 325
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1984) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Toxicology, 101,
187 (abstract).
Tayama, S., Kamiya, N. & Nakagawa, Y. (1989) Genotoxic effects of
o-phenylphenol metabolites in CHO-K1 cells. Mutat. Res., 223,
23-33.
Tayama-Nawai, S., Yoshida, S., Nakao, T. & Hiraga, K. (1984) Induction
of chromosome aberrations and sister-chromatid exchanges in
CHO-K1 cells by o-phenylphenol. Mutat. Res., 141, 95-99.
Timchalk, C. (1996) 14C-Orthophenylphenol: Pharmacokinetics
following dermal application in male human volunteers.
Unpublished report No. HET-K-001024-064 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Timchalk, C., Selim, S., Sangha, G. & Bartels, M.J. (1998) The
pharmacokinetics and metabolism of 14C/13C-labeled
ortho-phenylphenol formulation following dermal application to
human volunteers. Hum. Exp. Toxicol., 17, 411-417.
Ushiyama, K., Nagai, F., Nakagawa, A. & Kano, I. (1992) DNA adduct
formation by ophenylphenol metabolite in vivo and in
vitro. Carcinogenesis, 13, 1469-1473.
Wahle, B.S. & Christenson, W.R. (1996) Technical grade
ortho-phenylphenol: A combined chronic toxicity/oncogenicity
testing study in the rat. Unpublished report No. 92-272-SC from
Bayer Corp., Agriculture Division, Toxicology, Stilwell, Kansas,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Yamaha, T., Tanaka, A., Sato, M. & Tsuchiya, T. (1983) Excretion,
distribution and metabolic fate of sodium o-phenylphenol in the
rat. Unpublished report from Division of Medical Chemistry,
National Institute of Hygienic Sciences, Tokyo, Japan. Submitted
to WHO by Leng Associates, Midland, Michigan, USA.
Zablotny, C.L., Breslin, W.J. & Kociba, R.J. (1991) Ortho-phenylphenol
(OPP): Gavage teratology study in New Zealand white rabbits.
Unpublished report No. K-001024-045 from Toxicology Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Zempel, J.A. & Szabo, J.R. (1993) Ortho-phenylphenol: 21-day repeated
dermal dose study of systemic toxicity in Fischer 344 rats.
Unpublished report No. K-001024-056 from Health & Environmental
Sciences-Texas, Lake Jackson Research Center, Dow Chemical Co.,
Freeport, Texas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
2phenylphenol, which may play a role in the induction of bladder
cancer by this substance (Roy, 1990).
Activation of the 2-phenylphenol metabolite phenylhydroquinone by
prostaglandin (H) synthase in the presence of arachidonic acid and
hydrogen peroxide was studied to test the hypothesis that
prostaglandin synthase in rat urinary bladder transitional epithelium
and kidney medullar papilla is responsible for activation of the
metabolite to reactive intermediates in the bladder and kidney.
Phenylhydroquinone was found to be metabolized by the peroxidase
activity of prostaglandin synthase and by other peroxidases such as
horseradish peroxidase and myeloperoxidase, suggesting that the
peroxidative metabolism of phenylhydroquinone could play a role in
urinary bladder and kidney carcinogenesis in rats (Kolachana et al.,
1991).
In a study of the effect of pH on nonenzymatic oxidation of
phenylhydroquinone, the effects of phenylbenzoquinone and oxygen
concentration on autoxidation of phenylhydroquinone, and the
nonenzymatic conversion of phenylbenzoquinone to phenylhydroquinone, a
curvilinear relationship was found between the rate of oxidation of
phenylhydroquinone and pH over the range 6.3-7.6. Phenylbenzoquinone
was formed during autoxidation of phenylhydroquinone, with a yield of
0.92 ± 0.02. The results indicate that the production of reactive
metabolites from phenylhydroquinone involves both a pH-independent
(i.e. oxygen-dependent) and a pH-dependent pathway and that the
presence of phenylbenzoquinone enhances the rate of pH-dependent
phenylhydroquinone autoxidation. The authors suggested that ionization
of phenylhydroquinone semiquinone is a key step in production of
reactive species in the pH-dependent pathway. They found a good
correlation between the proposed reaction pathway and the induction by
2-phenylphenol of bladder lesions in rats. Thus, pH-dependent
autoxidation of free phenylhydroquinone in urine may be responsible
for the tumorigenic effects of 2-phenylphenol and sodium
2-phenylphenol in the rat bladder (Kwok & Eastmond, 1997).
Groups of eight female B6C3F1 mice were given 0, 1, 10, or 200
mg/kg bw per day of 2-phenylphenol (purity, > 98%) by gavage on 5
days per week for 2 weeks. As a positive control, mice were given 45
mg/kg bw of cyclophosphamide intraperitoneally for 4 days. The weights
of the body, liver, spleen, kidney, and thymus were recorded, and
samples were prepared for histopathological examination. Haematology
and clinical chemistry were conducted, and bone-marrow cellularity and
colony formation, lymphoproliferative responses, delayed
hypersensitivity responses, immunoglobulin, antibodies, response to
Listeria monocytogenes challenge, and tumour susceptibility were
studied. None of the treated animals died or showed signs of toxicity.
Histopathological examination revealed no significant lesion in any
tissues. The weight of the thymus and the relative weight of the
spleen were slightly increased at 200 mg/kg bw per day. The slight
haematological alterations seen did not show a dose-response
relationship and were within the normal range of biological variation.
A slight increase in serum cholesterol concentration and a
corresponding decrease in triglyceride concentration were seen in mice
at 200 mg/kg bw per day. The activity of alanine aminotransferase and
total protein in serum were not affected, although the
albumin:globulin ratio was slightly decreased at the high dose.
Bone-marrow cellularity, lymphoproliferative responses, immune
function, and host susceptibility were not altered. In contrast,
treatment with cyclophosphamide resulted in marked alterations. The
authors concluded that 2-phenylphenol, even at relatively high doses,
did not alter immune function or host susceptibility (Luster et al.,
1981).
Sodium 2-phenylphenol
Binding of sodium 2-phenylphenol metabolites to macromolecules
in vitro was studied by incubating [14C]sodium 2-phenylphenol
(specific activity, 19 mCi/mmol) with purified liver microsomes from
male rats in the presence of a NADPH regenerating system and bovine
serum albumin, which served as a 'protein acceptor'. Macromolecular
binding of radiolabel to protein, which was dependent on the presence
of both active microsomes and NADP, was observed. In order to study
the binding of metabolites of 2-phenylphenol and its sodium salt to
macromolecules in the liver, kidney, and urinary bladder in vivo,
groups of four male rats were given single oral doses of
14C-labelled compounds at doses of 50, 100, 200, or 500 mg/kg bw,
and tissues were excised 16-18 h later for measurement of
macromolecular binding, which was determined as nanomoles of bound
material per milligram of protein. The extent of binding was not
linearly related to the administered dose. Disproportionate increases
were seen in each tissue at doses of sodium 2-phenylphenol > 200
mg/kg bw and in liver and bladder at doses of 2-phenylphenol at
200-500 mg/kg bw (Reitz et al., 1984).
Sodium 2-phenylphenol (purity not given) was administered in the
diet at a concentration of 20 000 ppm to 4-week-old male and female
Fischer 344 rats for 136 days. Urine was collected periodically. At
the end of treatment, the rats were killed, blood samples were
collected, and livers and kidneys were removed. The amounts of cyclic
nucleotides (cAMP and c-GMP) were determined in urine, plasma, liver,
and kidneys, and adenylate cyclase activity was measured in liver and
kidneys. In male rats, the c-AMP levels in urine and plasma were
decreased whereas the c-GMP levels were increased. In females, c-AMP
levels were decreased only during the first 3 days of feeding, and the
levels of c-AMP and cGMP in liver and kidneys were unchanged. The
decreased urinary c-AMP in male rats was probably the result of
decreased adenylate cyclase activity in liver and kidneys. A similar
change in adenylate cyclase activity was observed in liver but not in
kidneys of female rats treated with sodium 2-phenylphenol. The
sex-related alterations in cyclic nucleotide levels were postulated to
be involved in the sex-dependent induction of urinary bladder tumours
by sodium 2-phenylphenol (Nakagawa et al., 1984).
In male and female Fischer 344 Du Crj rats given 20 000 ppm of
sodium 2-phenylphenol in the diet for 20 weeks, urinary gamma-glutamyl
transpeptidase activity decreased immediately after the start of
treatment and remained low throughout the study. The activities of
this enzyme and of alkaline phosphatase in kidney homogenate were
found to have decreased to about 80% of the control values at 20
weeks, but the activity of glucose-6phosphate dehydrogenase was
significantly increased; that of Na/K-ATPase was unchanged. In liver
homogenate, however, gamma-glutamyl transpeptidase activity was
increased by about eight times and that of glucose-6-phosphate
dehydrogenase was significantly increased, but the activities of
alkaline phosphatase and Na/K-ATPase were not significantly different
from the control values. The glutathione concentration in the livers
of treated rats was significantly reduced (Nagai & Nakao, 1984).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of 2-phenylphenol
and its sodium salt are summarized in Table 1. The clinical signs of
toxicity were generally nonspecific.
Table 1. Acute toxicity of 2-phenylphenol and its sodium salt
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/L)
2-Phenylphenol
Mouse M Oral 1200 Taniguchi et al. (1981)
F 1100
Mouse M Oral 3500 Tayama et al. (1983, 1984)
F 3200
Rat M Oral 2600 Tayama et al. (1980)
F 2900
Rat M Oral 2800 Gilbert & Crissman (1994)
F 2800
Rat M&F Inhalation
(4 h) > 36 Landry et al. (1992)
Rabbit M&F Dermal > 5000 Carreon & New (1981)
Sodium 2-phenylphenol
Mouse M Oral 900 Ogata et al. (1979)
F 800
Rat M Oral 1700 Taniguchi et al. (1981)
F 1600
Rat M Oral 1100 Tayama et al. (1979)
F 1100
Rat M Oral 850 Gilbert & Stebbins (1994)
F 590
(b) Short-term studies of toxicity
2-Phenylphenol
Rats
2-Phenylphenol (purity, 99.8%) was administered to 30 male
Fischer 344 rats (Charles River) in the diet at a concentration of
20 000 ppm, equal to 1000 mg/kg bw per day, for up to 90 days. Interim
sacrifices were performed at 3, 7, 30, and 65 days. Only seven rats at
each dose were permitted to live to 90 days, at which time they were
killed. Food consumption and body weight were markedly reduced within
the first week and remained low throughout the study. The renal
lesions observed in these rats included focal cortical cysts,
significantly decreased urine specific gravity (at 65 and 90 days),
small amounts of blood in the urine, focal tubular collapse and
atrophy in the cortex, and cystic degeneration (at 65 and 90 days). No
treatment-related urinary bladder lesions were observed. A NOAEL could
not be identified since the body weight was reduced at the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1300, 3100, 6300, 13 000, or 25 000 ppm of
2-phenylphenol (purity not given), equal to 0, 180, 390, 760, 1700,
and 2800 mg/kg bw per day for males and 0, 200, 410, 800, 1700, and
3000 mg/kg bw per day for females, for 12 weeks. Body weight and
body-weight gain were severely depressed in males and females at
25 000 ppm and to a lesser extent (14%) in males fed 13 000 ppm. No
significant treatment-related effects were seen in analyses of urine
performed at weeks 9 and 13. Haematological and blood chemical values
were generally normal, other than a slight decrease in haemoglobin
concentration in male and female rats at the highest dose. The
absolute and relative weight of many organs in male rats at this dose
were significantly decreased. The NOAEL was 6300 ppm, equal to 760
mg/kg bw per day, on the basis of reduced body weight and body-weight
gain at 13 000 ppm (Iguchi et al., 1984).
Groups of five male and five female Fischer 344 rats received
dermal applications of 0, 100, 500, or 1000 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) once daily on 5 days per week for a
total of 15 applications over 21 days. The amount applied per animal
was adjusted weekly on the basis of the body weight of individual
animals and was applied to a 5 cm × 5 cm area of clipped skin on the
back, covered with a nonabsorbent cotton patch held in place by an
elastic wrap secured with adhesive tape. The wraps and patches were
removed not less than 6 h after treatment, and the treated area was
wiped with a wettened gauze pad to remove residual test material.
Control animals were handled in the same way. All animals were
acclimated to the wraps for 2 days before the beginning of treatment.
They were observed at least daily and were given a complete clinical
examination weekly. The skin at the site of treatment was examined
after removal of the wrap on the last day of dosing, each week, and on
the day before necropsy. Body weights were measured weekly and feed
consumption and feed efficiency were calculated weekly. Urine was
analysed on day 19. The rats were fasted overnight before necropsy,
when haematological and serum clinical chemical parameters were
evaluated, all animals were examined for gross pathological changes,
selected organs were weighed, and tissues were preserved. Selected
tissues and all gross lesions from animals in the control and
high-dose groups were examined histologically.
No deaths occurred at any dose. Treatment-related effects
indicative of dermal irritation were observed at the site of
application in animals of each sex at 500 and 1000 mg/kg bw per day.
Female rats appeared to be slightly more sensitive than males, but the
severity of lesions increased with duration of exposure and dose in
both sexes. The irritating effects ranged from scaling to fissures.
Body weights and feed consumption were not affected, and no
significant treatment-related effects were found in the
haematological, clinical chemical, and urinary parameters evaluated.
No treatment-associated alterations were found in the liver or kidneys
(Zempel & Szabo, 1993).
Guinea-pigs
2-Phenylphenol was evaluated in 10 male Hartley albino
guinea-pigs for dermal sensitization potential by a modified Buehler
method. The animals received three dermal applications of 0.4 g of
2-phenylphenol (purity, 99.9%) during a 3-week induction period and
were challenged with 0.4 g of the compound 2 weeks after the last
induction. The condition of the test sites was assessed approximately
24 and 48 h after the challenge. No erythema or oedema was seen in any
of the animals. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Berdasco, 1991).
Ten male Hartley albino guinea-pigs were clipped free of hair on
the day before dosing and received three dermal applications of 0.4 g
of 2-phenylphenol (purity, 99.9%) moistened with 0.20 ml of distilled
water during a 3-week induction period. Two weeks after the last
induction application, they were given a challenge application of
0.4 ml of a 7.5% suspension of the compound in water on another site
for 6 h. Five animals received no induction but a 0.4-ml aliquot of a
7.5% suspension of 2-phenylphenol in water. The condition of the test
sites was assessed approximately 24 and 48 h after challenge. No
erythema was seen, and none of the uninduced animals showed
irritation. The animals were in good health and gained weight during
the study. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Gilbert, 1994b).
Rabbits
The ability of 2-phenylphenol (purity, 99.9%) to cause primary
dermal irritation was studied in three male and three female New
Zealand white rabbits that received applications of aliquots of 0.5 ml
of the substance moistened with 0.3 ml of distilled water for 4 h on
intact skin on a clipped, 10 cm × 10 cm area of the back. The
application sites were graded for erythema and oedema within 30 min
and 24, 48, and 72 h of removal of the patch and on days 7, 8, 9,
10,11, 14, and 15. The animals were weighed on the day of treatment
and at the end of the study. Very slight erythema was observed at the
application site in one of the six rabbits within 30 min of removal of
the test material and in two rabbits 24 h later. Severe to slight
eschar formation was observed in four rabbits within 30 min of
treatment which persisted throughout the remainder of the study. Four
animals had burns at the site of application within 30 min, which
resolved as scabs and then scars by the end of the study. Four rabbits
had very slight-to-severe oedema 30 min and 24 h after removal of the
test material, and slight-to-severe oedema was observed on three
animals 48 and 72 h after removal. Body weight was not affected
(Gilbert, 1994a).
Instillation of 0.1 g of 2-phenylphenol (purity not given) into
the right eye of six New Zealand white rabbits resulted in moderate
corneal injury, iritis, and moderate-to-severe conjunctival redness
and chemosis in all animals 24, 48, and 72 h and 7 days after dosing
(Norris, 1971).
Dogs
In studies to assess the palatability and toxicity of
2-phenylphenol (purity, 99.8%) in beagle dogs, males and females were
treated with several regimens. For palatability, one female was given
feed containing 2-phenylphenol to give a dose of 300 mg/kg bw per day
for 5 days. In a study to assess toxicity, groups of two males and
three females were given 300-1000 mg/kg bw per day as a solution in
peanut oil by gastric intubation for up to 9 days or 400-700 mg/kg bw
per day by capsule for 1-2 days. In a 4-week study, groups of two
males and two females were given 0, 100, 200, or 300 mg/kg bw per day
as a solution in peanut oil by gastric intubation on 5 days per week.
In a 1-year study, groups of four males and four females were given
2-phenylphenol as a solution in peanut oil by gastric intubation at
doses of 0, 30, 100, or 300 mg/kg bw per day for 5 days per week for
1 year. The animals were examined daily for clinical signs, and body
weight, feed consumption, haematological, urinary, and clinical
chemical parameters, organ weights, ophthalmologic endpoints, and
gross and histopathology were determined.
Administration of 300 mg/kg bw per day in feed or by intubation
for 5 days resulted in decreased body weights and feed consumption
that correlated with the unpalatability of 2-phenylphenol. Repeated
emesis was seen at doses > 400 mg/kg bw per day administered in
gelatin capsules or in peanut oil by intubation and at doses > 200
mg/kg bw per day by intubation throughout the 4-week study.
Dose-related emesis was also seen in animals given 2-phenylphenol for
1 year. In general, more frequent emesis and ejection of greater
volumes of gastric content was seen in dogs given 300 mg/kg bw per day
than in those given lower doses. While emesis effectively limited the
dose that could be retained, the degree of emesis did not appear to
compromise the health of the animals over 1 year. The reaction was
categorized as a local, transitory response of the mucosal lining of
the upper alimentary tract rather than a reaction of the central
nervous system. No adverse effects were seen on body weight, feed
consumption, haematological, urinary, clinical chemical, or
ophthalmological parameters, organ weights, or gross or histological
appearance of a range of tissues from all dogs. The only deaths were
of two males at the high dose in the 1-year study which died
subsequent to inadvertent deposition of the test solution into the
lungs after approximately 4.5 months of dosing. The NOAEL was 300
mg/kg bw per day (Cosse et al., 1990).
Sodium 2-phenylphenol
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing 0, 2500, 5000, 10 000, 20 000, or 40 000 ppm of sodium
2-phenylphenol as the tetrahydrate (purity not given), equivalent to
0, 270, 550, 1100, 2200, and 4400 mg/kg bw per day, for 13 weeks.
Body-weight gain was significantly depressed in males fed 10 000 or
20 000 ppm and in animals of each sex fed 40 000 ppm. Urinary analysis
showed increased pH and decreased specific gravity at the highest
dose. The relative weights of the livers of animals given 10 000,
20 000, or 40 000 ppm were significantly greater than those of
controls, but no treatment-related histopathological findings were
made. Light and scanning electron microscope examination of the
bladder epithelium at 4, 8, and 13 weeks in three males and three
females in the control group and at 20 000 ppm showed no abnormaility
in the appearance of the bladder epithelium of treated mice at any
time. The NOAEL was 5000 ppm, equivalent to 550 mg/kg bw per day, on
the basis of reduced body-weight gain and increased relative liver
weight at 10 000 ppm (Shibata et al., 1985).
Rats
Sodium 2-phenylphenol (purity, 98.7%) was administered in the
diet to 30 male Fischer 344 rats at a concentration of 20 000 ppm for
up to 90 days. Interim sacrifices were performed at 3, 7, 14, 30, and
65 days. Only seven rats per group were permitted to live to 90 days,
at which time they were killed. The lesions seen in the urinary
bladder epithelium were increased mitosis beginning at 3 days and
thickening (i.e. simple hyperplasia) beginning at 14 days. No tumours
were observed in the bladder. A NOAEL could not be identified since
lesions were observed in the bladder at 20 000 ppm, the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol (purity, > 95%), equal to 0, 85, 180, 350, 710,
1400, and 2500 for males and 0, 87, 180, 350, 690, 1300, and 2400
mg/kg bw per day for females, for 13 weeks. The rats were observed
daily for changes in general condition and were weighed weekly; the
amounts of feed and water consumed were measured on 3 days every other
week. No deaths occurred during the study. A 15-17% decrease in
body-weight gain was seen in animals at doses > 5000 ppm. Urinary
bladder tumours occurred in male rats at frequencies of 1/10 at 10 000
ppm, 9/10 (five transitional-cell carcinomas) at 20 000 ppm, and 1/10
at 40 000 ppm. Six rats at 40 000 ppm had pyelonephritis. In female
rats, the frequencies of tumours were 0/10 at 20 000 ppm and 2/10
(papillomas only) at 40 000 ppm. No bladder calculi were observed in
this experiment. The NOAEL was 2500 ppm, equal to 180 mg/kg bw per
day, on the basis of reduced body-weight gain at 5000 ppm (Iguchi et
al., 1979; Hiraga & Fujii, 1981).
Guinea-pigs
Sodium 2-phenylphenol (purity, 99.1%) was applied to the clipped
skin of 10 male Hartley albino guinea-pigs as a 0.4-ml aliquot of a
0.5% suspension in distilled water at 3-week intervals for induction.
Two weeks after the last dose, a challenge of 0.4 ml of a 0.1%
solution of the compound in distilled water was applied to the other
side of the animals. No erythema occurred at the test site. The
animals gained weight throughout the study. The author concluded that
sodium 2-phenylphenol did not cause delayed contact hypersensitivity
(Gilbert, 1994c).
Rabbits
Sodium 2-phenylphenol diluted 1:200 with distilled water was
instilled into the eyes of six rabbits (strain not given). Temporary,
mild conjunctival reactions were observed in three animals. The
authors concluded that the compound slightly irritated the eye (Davies
& Liggett, 1973).
(c) Long-term studies of toxicity and carcinogenicity
2-Phenylphenol
Mice
Groups of 50 male and 50 female B6C3F1 mice were fed diets
(concentrations not given) supplying 2-phenylphenol (purity, 99.9%) at
doses of 0, 250, 500, or 1000 mg/kg bw per day for 2 years. A
satellite group of 10 male and 10 female mice at each dose was
maintained on the diets for 12 months and then necropsied. All mice
were observed at least once daily for overt signs of toxicity, and a
thorough clinical examination was performed at least once a week
throughout the study. Body weights and feed consumption were recorded
weekly for the first 13 weeks and monthly thereafter. The diets were
prepared weekly or every other week, with adjustments of the
2-phenylphenol concentration according to group mean body weights and
feed consumption to maintain the desired doses for each group.
Clinical signs and mortality rates were unaffected by treatment,
but decreased body weights (by 6-20%) and weight gain (by 10-38%) were
seen in all treated groups except for males fed 250 mg/kg bw.
Haematological, clinical chemical, and urinary parameters in mice
necropsied at 12 and 24 months showed no consistent, toxicologically
significant alterations indicative of target organ toxicity. Changes
in the weights of the adrenal glands, brain, heart, kidneys, liver,
testis, and spleen which were found to be statistically significant
were confounded by the marked decrease in body weight. Nevertheless,
the consistent increases in the absolute and/or relative weights of
the liver at all doses suggests a treatment-related effect. Gross
observations at necropsy in males at the high dose at 12 months and in
males at the intermediate and high doses at 24 months showed a slight
increase in the number of mice with liver masses or nodules.
Microscopic examination of the livers of mice at 12 and 24 months
revealed treatment-related effects at all doses. The cytoplasm of
hepatocytes stained homogeneously, indicating liver enzyme induction,
but there was no evidence of degeneration or necrosis. The microscopic
changes were dose-related and resembled those associated with
adaptation to metabolic demands. An increased incidence of
eosinophilic hepatocellular foci was also observed in males at 100 and
500 mg/kg bw per day.
Males fed 1000 mg/kg bw per day and necropsied at 12 months had a
slightly increased incidence of hepatocellular adenoma. At 24 months,
a statistically significant increase in the number of males with
hepatocellular adenoma was seen at 500 ( n = 40) and 1000 ( n = 41)
mg/kg bw per day, the incidence in controls being 27/50. Low
incidences of a variant form of hepatocellular carcinoma
(hepatoblastoma) were observed in all treated groups of males (2/50 at
250 mg/kg bw per day, 6/50 at 500 mg/kg bw per day, and 3/50 at 1000
mg/kg bw per day versus 0/50 in controls), but the incidence of
hepatocellular carcinoma was not significantly increased at any dose.
The combined incidence of hepatoblastoma and hepatocellular carcinoma
was also not significantly increased in the male mice. The primary
non-tumourous microscopic changes in the livers of male mice, which
appeared to have been adaptive, ultimately resulted in the promotion
of hepatocellular adenomas. The incidences of tumours in other tissues
were not statistically significantly increased. The livers of female
mice showed similar microscopic adaptive changes, but none of them had
hepatoblastoma, and no statistically significant increase in the
incidence of tumours in any tissues was found. Decreased incidences of
microscopic lesions when compared with controls were found in the
adrenals, kidneys, lungs, oral tissues, pancreas, peripheral nerve,
spleen, and testis of males and in the kidneys, lungs, and nasal
tissues of females. These findings were considered to reflect normal
variation and the decreased body weights of the mice and not a primary
response to 2-phenylphenol. A NOAEL for toxicity could not be
identified. The NOAEL for carcinogenicity was 250 mg/kg bw per day on
the basis of an increased incidence of hepatocellular adenomas at 500
mg/kg bw per day (Quast & McGuirk, 1995).
Rats
2-Phenylphenol (purity, 98%) was administered in the diet at
concentrations of 0, 6300, 13 000, or 25 000 ppm, equal to 0, 320,
650, and 1300 mg/kg bw per day to groups of 20-24 male Fischer 344
rats (Charles River) for 91 weeks. The percentage survival was 96, 90,
71, and 65% in the four groups, respectively. In the rats that died
during the study, the incidences of urinary bladder tumours were 0/1
in controls, 0/2 at 6300 ppm, 7/7 at 13 000 ppm, and 0/8 at 25 000
ppm. Bladder lesions were found in 10%, 96%, and 48% of rats at the
three doses, respectively. The incidences of urinary bladder
papillomas and transitional-cell carcinomas were 23/24 at 13 000 ppm
and 4/23 at 25 000 ppm. A NOAEL could not be identified since bladder
lesions were seen at all doses tested (Hiraga, 1983a; Hiraga & Fujii,
1984).
Groups of 70-75 male and 70-75 female Fischer 344 rats were fed
diets containing 2-phenylphenol (purity, 99.5%) at concentrations of
0, 800, 4000, or 8000/10 000 ppm, equal to 0, 39, 200, and 400 mg/kg
bw per day for males and 0, 49, 240, and 650 mg/kg bw per day for
females, for 1 year before interim sacrifice of satellite groups of 20
rats per dose and for 2 years for the remaining 50 rats of each sex.
The animals were observed daily and were examined weekly for clinical
signs of toxicity. Each animal was weighed once a week and also
immediately before necropsy to allow calculation of organ:body weight
ratios. Food consumption was measured weekly. Blood and overnight
urine samples were collected at 3, 6, 12, 18, and 24 months from the
first 20 surviving rats of each sex in the group scheduled for
sacrifice at 2 years. Any dead or moribund animals were prepared for
necropsy, and all surviving animals were killed at the end of the test
periods.
A 5% decrease in body-weight gain was seen in animals at 4000
ppm, and a decrease of 11% was seen in males at 8000 ppm and in
females at 10 000 ppm. Food consumption was unaffected in all groups.
Minor clinical and gross observations included an increased incidence
of abnormally coloured urine, urine stains, and red stains in male
rats given 8000 ppm 2-phenylphenol and an increased incidence of urine
and brown stains in female rats given 4000 or 10 000 ppm. There were
no treatment-related changes in ophthalmological, haematological,
clinical chemical, or urinary parameters, except for an increased
incidence of blood in the urine of males at 8000 ppm. The mortality
rate was slightly increased among males at 8000 ppm. Gross
pathological examination showed increased incidences of urinary
bladder masses in males fed 4000 ppm for 2 years or 8000 ppm for 1 or
2 years and increased incidence of pitted zones and abnormal texture
of the kidney in females fed 10 000 ppm for 2 years. Histopathological
examination showed hyperplasia and transitional-cell carcinoma in the
urinary bladders of males fed 4000 or 8000 ppm for 1 or 2 years, the
increase being statistically significant at 8000 ppm and of borderline
significance at 4000 ppm. The NOAEL for toxicity was 800 ppm, equal to
39 mg/kg bw per day, on the basis of reduced body-weight gain and
hyperplasia in the urinary bladder at all doses. The NOAEL for
carcinogenicity was 800 ppm, equal to 39 mg/kg bw per day (Wahle &
Christenson, 1996).
Sodium 2-phenylphenol
Mice
Sodium 2-phenylphenol (purity, 97%) was administered in the diet
to groups of 50 male and 50 female B6C3F1 mice (Charles River) at
concentrations of 0, 5000, 10 000, or 20 000 ppm, equal to 0, 590,
1400, and 3000 mg/kg bw per day for males and 0, 780, 1500, and 3100
mg/kg bw per day for females, for 96 weeks. The mice were then given
control diet for an additional 8 weeks. The survival rate of males at
the high dose was slightly decreased. Decreased body weight was
observed in males and females at 20 000 ppm and in females at 5000 and
10 000 ppm. Alkaline phosphatase activity was increased in females at
5000, 10 000, and 20 000 ppm. No urinary bladder stones, tumours, or
extensive renal damage were observed in any of the mice. The NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, the
highest dose tested (Ito, 1983a; Hagiwara et al., 1984).
Rats
Groups of 20-21 male and 20-21 female Fischer 344 rats were fed
diets containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol as the tetrahydrate (purity, > 95%), equivalent
to 0, 70, 140, 270, 550, 1100, or 2200 mg/kg bw per day, for 91 weeks.
The rats were observed daily for changes in general condition. The
survival rates were 90, 90, 95, 90, 90, 57, and 71% for the seven
groups, respectively. Increased incidences of urinary bladder
papil-lomas and transitional-cell carcinomas were seen, with 1/21 at
5000 ppm, 7/21 at 10 000 ppm, 20/21 at 20 000 ppm, and 17/20 at 40 000
ppm. Transitional-cell carcinomas of the kidney were also observed at
doses > 5000 ppm. The NOAEL was 2500 ppm, equivalent to 270 mg/kg
bw per day, on the basis of the increased incidence of urinary bladder
tumours (Hiraga & Fujii, 1981).
Groups of 50 male and 50 female Fischer 344/DuCrj rats were fed
diets containing 0, 7000, or 20 000 ppm (males) or 0, 5000, or 10 000
(females) of sodium 2-phenylphenol (purity, 95.5%) for 104 weeks
followed by control diet for 2 weeks. In a second study, groups of 25
male and 25 female rats were fed diets containing the compound at 0,
2500, 7000, or 20 000 ppm, equal to 0, 95, 270, and 770 mg/kg bw per
day, for males, and 0, 2500, 5000, or 10 000 ppm, equal to 0, 110,
220, and 470 mg/kg bw per day, for females, for 104 weeks followed by
control diet for life.
The survival rate at week 104 was 20% in males at 20 000 ppm in
the first study and 24% in the second study, while those in the other
groups were > 50%. Urinary bladder tumours were observed in the first
study in 2/50 males at 7000 ppm, 47/50 males at 20 000 ppm, 1/50
females at 5000 ppm, and 4/50 females at 10 000 ppm. In the second
study, the bladder tumour incidence was 3/25 in males at 7000 ppm,
23/25 in males at 20 000 ppm, and 2/25 in females at 10 000 ppm. In
the first study, transitional-cell carcinomas were found in 2/2 males
at 7000 ppm, 46/47 males at 20 000 ppm, and 1/4 females at 10 000 ppm.
In the second study, carcinomas were found in 1/3 males at 7000 ppm,
21/23 males at 20 000 ppm, and 1/2 females at 10 000 ppm. The
incidence of bladder tumours was thus dose-dependent. The NOAEL was
2500 ppm, equal to 95 mg/kg bw per day, on the basis of urinary
bladder tumours at all doses (Hiraga, 1983b; Fujii & Hiraga, 1985).
A working group convened by the International Agency for Research
on Cancer (IARC) classified sodium 2-phenylphenol as possibly
carcinogenic to humans and 2-phenylphenol as not classifiable as to
its carcinogenicity to humans (IARC, 1987, 1999).
(d) Genotoxicity
The results of tests for the genotoxicity of 2-phenylphenol,
sodium 2-phenylphenol, and the metabolites phenylhydroquinone and
phenylbenzoquinone are summarized in Table 2.
Covalent binding to urinary bladder DNA was determined in vivo
in pooled samples from eight male rats dosed with 500 mg/kg bw of
[14C]2-phenylphenol (purity, 99.8%) or [14C]sodium 2-phenylphenol
(purity, 98.7%). No radiolabel was detected in DNA from bladders
excised 16 h after dosing with either compound. The detection limit
was less than one alkylation per 106 nucleotides. Identical results
were obtained in a second experiment (Reitz et al., 1983).
The reactions of 2-phenylphenol and its metabolites
phenylhydroquinone and phenylbenzo-quinone with DNA were investigated
by a sequencing technique and by ultraviolet-visible and electron spin
resonance spectroscopy. In the presence of Cu(II), phenylhydroquinone
caused extensive DNA damage. Catalase, methionine, and methional
inhibited the DNA damage completely, whereas mannitol, sodium formate,
ethanol, tert-butyl alcohol, and superoxide dismutase did not.
Phenylhydroquinone plus Cu(II) frequently induced a piperidine-labile
site at thymine and guanine residues. Addition of Fe(III), Mn(II),
Co(II), Ni(II), Zn(II), Cd(II), or Pb(II) to phenylhydroquinone did
not induce DNA damage. This metabolite also induced DNA damage in the
presence of Cu(II) when peroxide was added, and Cu(II) accelerated the
autoxidation of phenylhydroquinone to quinone. Electron spin resonance
spectroscopy revealed that the semiquinone radical is an intermediate
in the autoxidation. Catalase did not inhibit the acceleration by
Cu(II). Superoxide dismutase promoted both the autoxidation of
phenylhydroquinone and the initial rate of semiquinone radical
production. Electron spin resonance trapping showed that addition of
Fe(III) produced hydroxyl radicals during the autoxidation of
phenylhydroquinone, whereas addition of Cu(II) did so sparingly. The
results suggest that DNA damage induced by phenylhydroquinone plus
Cu(II) is due to active species other than hydroxyl free radicals
(Inoue et al., 1990).
Table 2. Results of studies of the genotoxicity of 2-phenylphenol, sodium 2-phenylphenol, and the metabolites phenylhydroquinone
and phenylbenzoquinone
End-point Test object Concentration Purity Result Reference
(%)
2-Phenylphenol
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative - S9 Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive + S9 Ushiyama et al. (1992)
Negative - S9
Gene mutation B. subtilis H17, M45 NR NR Negative Shirasu et al. (1978)
Gene mutation S. typhimurium 10 -1000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA92, TA1535, Negative - S9
TA100, TA1537,
TA94, TA98
Gene mutation E. coli WP2 hcr NR NR Negative + S9 Shirasu et al. (1978)
Negative - S9
Gene mutation S. typhimurium 3-200 µg/plate > 99 Negative + S9 National Toxicology
TA100, TA1535, Weakly positive + S9 Program (1986)
TA1537, TA98
Gene mutation Mouse lymphoma 0.3-60 µg/ml > 99 Positive + S9 National Toxicology
(L5178Y) cells, Positive - S9 Program (1986)
Tk locus
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation Human Rsa cells, 1-30 µg/ml NR Positive Suzuki et al. (1985)
repair deficient,
HPRT locus
Chromosomal CHO-K1 cells 50-175 µg/ml > 99 Positive - S9 Tayama-Nawai et al.
aberration (1984)
Chromosomal CHO fibroblasts 12-125 µg/ml NR Weakly positive + S9 Ishidate et al. (1983)
aberration Weakly positive - S9
Chromosomal CHO-K1 cells 60-90 µg/ml > 99 Negative + S9 National Toxicology
aberration Negative - S9 Program (1986)
Chromosomal CHO-K1 cells 25-175 µg/ml > 99 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Chromosomal CHO-K1 cells 100-200 µg/ml > 99 Positive + S9 Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Host-mediated S. typhimurium G46 200 or 600 mg/kg NR Negative Shirasu et al. (1978)
gene mutation in male JCL-ICR mice bw orally for 5 days
In vivo
Sex-linked D. melanogaster 250 ppm in feed > 99 Negative National Toxicology
recessive lethal for 3 days or Program (1986)
mutation injection of 500 ppm
DNA binding Male rat urinary 500 mg/kg bw > 99 Negative Reitz et al. (1983)
bladder orally
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
DNA Rat urinary bladder 60-940 mg/kg > 99 Positive at Christenson et al.
32P-postlabelling bw per day 570 and 940 (1996a)
mg/kg bw per day
Chromosomal Male rat bone 800 mg/kg bw NR Negative Shirasu et al. (1978)
aberration marrow over 5 days or
single doses
< 4000 mg/kg
bw orally
Dominant lethal Male mice 100 or 500 mg/kg > 99 Negative Kaneda et al. (1978)
mutation bw per day for 5 days
Dominant lethal Male mice 100 or 500 mg/kg NR Negative Shirasu et al. (1978)
mutation bw per day for 5
days
Sodium 2-phenylphenol
In vitro
Gene mutation S. typhimurium 50-5000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA100, TA98 Negative - S9
Gene mutation S. typhimurium 0.025-250 µg/plate 99 Negative + S9 Reitz et al. (1983)
TA100, TA98, Negative - S9
TA1535, TA1537,
TA1538
Uncheduled DNA Male rat primary 10-7-10-4 mol/L 99 Negative Reitz et al. (1983)
synthesis hepatocytes
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
DNA Male rat urinary 2% in diet for > 99 Positive Ushiyama et al. (1992)
32P-postlabelling bladder 13 weeks
DNA Mouse skin 10 or 20 mg/animal 97 Positive Pathak & Roy (1993)
32P-postlabelling topically
Phenylhydroquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Positive Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Chromosomal CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Chromosomal CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Sister chromatid CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
exchange Positive - S9
Sister chromatid CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
exchange inhibited by (1991)
cysteine or
glutathione
Micronucleus V79 CH lung 6-125 µmol/L NR Positive + S9 Lambert & Eastmond
formation fibroblast cells Negative - S9 (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA Mouse skin 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
Phenylbenzoquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative Nagai et al. (1990)
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation S. typhimurium 0.05-1000 NR PBQ negative/ Ishidate et al. (1983)
TA100, TA2637, µg/plate PBQ negative
TA98
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Micronucleus V79 CH lung 6-125 µmol/L NR Negative Lambert & Eastmond
formation fibroblast cells (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA adduct formation in HL-60 cells treated with the
2-phenylphenol metabolites 2-phenylhydroquinone and
2-phenylbenzoquinone was studied by 32P-postlabelling. Treatment
with 25-500 µmol/L of 2-phenylhydroquinone for 8 h produced one
principal and three minor adducts, with a relative distribution of 80,
10, 6, and 4%. The relative adduct frequencies were 0.26-2.3
adducts/107 nucleotides. Treatment with 25-250 µmol/L of
2-phenylbenzoquinone for 2 h resulted in a similar level of DNA
modification and adduct distribution. Reaction of purified calf thymus
DNA with 2-phenylbenzo-quinone produced one DNA adduct, which did not
correspond to the major adduct produced in HL-60 cells. These results
show that both metabolites can form DNA adducts. Peroxidase activation
of 2-phenylphenol may therefore play a role in its carcinogenic effect
(Horvath et al., 1992).
In a similar study of covalent binding to DNA,
32P-postlabelling analysis of the products of reaction of DNA with
phenylbenzoquinone revealed four major and several minor adducts.
Chemical reaction with deoxyguanosine 3'-phosphate also resulted in
four major adducts, and their chromatographic mobility was identical
to that of major adducts of phenylbenzoquinone-DNA, which were shown
to be stable. More total covalent binding was found in deoxyguanosine
3'-phosphate than in DNA. Reaction of DNA with 2-phenylphenol or
phenylhydroquinone in the presence of microsomes and NADPH or cumene
hydroperoxide also resulted in four major adducts, and their formation
was drastically decreased by known inhibitors of cytochrome P450. The
chromatographic mobility of these adducts matched that of the adducts
observed in deoxyguanosine 3'-phosphate and DNA reacted with
phenylbenzoquinone. Thus, both 2-phenyl-phenol and phenylhydroquinone
can bind covalently to DNA in the presence of a microsomal cytochrome
P450 activation system, and phenylbenzoquinone is one of the
DNA-binding metabolites of 2-phenylphenol (Pathak & Roy, 1992).
Covalent modification of skin DNA by sodium 2-phenylphenol
in vivo was studied by the 32P-postlabelling method to elucidate
the biochemical mechanism of promotion of chemically induced skin
carcinogenesis by this compound. Topical application of sodium
2-phenylphenol or phenylhydroquinone to the skin of CD-1 mice produced
four distinct major and several minor adducts in skin DNA. Total
covalent binding in skin DNA was 0.31 fmol/µg DNA after treatment with
10 mg of sodium 2-phenylphenol and 0.62 fmol/µg DNA with 20 mg. The
adducts were not observed in skin DNA of untreated animals.
Pretreatment of the mice with a-naphthylisothiocyanate, an inhibitor
of cytochrome P450, or indomethacin, an inhibitor of prostaglandin
synthase, resulted in lower numbers of DNA adducts. Incubation of DNA
with 2-phenylphenol or phenylhydroquinone in vitro in the presence
of cytochrome P450 or prostaglandin synthase activation systems
resulted in four major adducts. The pattern of chromatographic
mobility observed in vitro in the presence of these enzymatic
systems appeared to be similar to that of adducts in vivo. The
chemical reaction of DNA or deoxyguanosine monophosphate with
phenylbenzoquinone also resulted in four major and several minor
adducts. The four major adducts were identical in chromatographic
mobility to the four major adducts produced in vivo and in vitro.
The results show that 2-phenylphenol and phenylhydroquinone can bind
covalently to DNA and that one of the DNA-binding metabolites of
2-phenylphenol may be phenylbenzoquinone (Pathak & Roy, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
2-Phenylphenol
Rats
Groups of 35 albino Sprague-Dawley rats, nine weeks old at the
start of the study, were fed diets containing technical-grade
2-phenylphenol (purity, 99.4-99.5%) at concentrations of
200-10 000 ppm, equal to 0, 36, 120, and 460 mg/kg bw per day, for two
generations. The dose was adjusted during the premating period
according to changes in body weight, and adjustments were made during
lactation to avoid overdosing the pups, although this was later
considered unnecessary. Two F1 females at the high dose and 12 F2a
pups were removed from the study for examination of the heritability
of hypotrichosis. Parents (F0) for the F1 generation were assigned
randomly to dose groups. The genealogy of the F1b pups was checked
to prevent the mating of littermates. For production of the F2
generation, 132 male and 134 female F1b pups were selected
randomly,one or more pups of each sex per litter being used as parents
(F1), divided into 35 pairs of rats per dose, except for the
controls which consisted of 27 males and 29 females. Estrus cycles
were studied by vaginal smears. The animals were examined daily, and
routine observations of birth statistics and pup weights were made;
the litters were culled to eight pups when necessary. Standard
observations and extensive histological examination of the urinary
tract were carried out at sacrifice.
Treatment did not affect clinical signs, body-weight gain during
gestation or lactation, or any of the reproductive variables examined.
Histological examination of the adults and pups revealed no
significant changes in the reproductive tracts. F0 and F1 adults
at 460 mg/kg bw per day showed a treatment-related decrease in body
weight which was not clearly related to a decrease in food
consumption, and F1b, F2a, and F2b pups showed a statistically
significant decrease in body weight on days 14 and/or 21 of lactation,
an effect not seen during the first week of lactation, indicating that
development had not been disturbed. The relative weight of the kidneys
was increased in a dose-dependent manner, in the absence of changes in
other organ weights, in males of the F0 and F1 generations. Male
rats at 120 and 460 mg/kg bw per day had an increased incidence of
calculi in the urinary tract. Transitional-cell hyperplasia was found
in the urinary bladder, defined in the report as 'an area (focal or
diffuse) of at least three to four cells thick (of cuboidal cells) in
an inflated bladder', whereas normal bladders had a cell thickness of
one or two flattened cells. Quantification by simple morphometry
indicated a compound-related effect in the bladder in F0 males and
females at 120 and 460 mg/kg bw per day and in F1 males at 457 mg/kg
bw per day. Neoplasms of the urinary tract were found in four rats:
one bladder and one ureteric tumour in rats at 125 mg/kg bw per day
and two bladder tumours in rats at 457 mg/kg bw per day. The NOAEL for
reproductive toxicity was 460 mg/kg bw per day and that for
carcinogenicity was 36 mg/kg bw per day (Eigenberg, 1990).
In a similar study, groups of 30 CD Sprague-Dawley rats were fed
diets containing technical-grade 2-phenylphenol (purity, 99.5-100%) at
concentrations of 200-10 000 ppm, equal to 0, 17, 92, and 460 mg/kg bw
per day, for two generations. The dose was adjusted during the
premating period according to changes in body weight. The F0 and
F1 adults received the compound in the diet throughout the study,
beginning at 7 weeks of age for the F0 adults and at weaning for the
F1 adults. The animals received treated feed for 10 weeks before
breeding, beginning approximately 2 weeks after weaning of the last
F1b litter for F1 parents. F0 adults were mated to produce the
F1a and F1b litters, and F1 adults (consisting of randomly
selected F1b pups) were mated to produce the F2a and F2b
litters.Adult animals were evaluated during the study for effects of
2-phenylphenol on body weight, food consumption, clinical signs,
estrous cycling, mating, fertility, length of gestation, and litter
size. The offspring were evaluated for effects on sex ratio,
viability, body-weight gain, and clinical signs. Gross necropsy was
performed on all adults and pups, and the reproductive organs,
pituitary, kidneys with ureter attached, urinary bladder, and gross
lesions of all F0 and F1 adults were evaluated histologically.
At 460 mg/kg bw per day, urine staining was observed in F0
males and females and F1 males, urinary bladder calculi were found
at necropsy in F1 adult males, and one F0 male died from renal
failure. At this dose, there was an increase in food consumption by
females during lactation, decreased pup weight, and a decrease in the
terminal body weight of F0 and F1 adult males and females.
Histopathological examination of the kidneys revealed debris in the
renal pelvis, chronic active inflammation, and increased severity of
background lesions in F0 and F1 males. Further, transitional-cell
hyperplasia (simple, nodular, or papilary) of the bladder, calculi,
chronic inflammation of the bladder, and dilatation and hyperplasia of
the ureter were seen in F0 and F1 males. Two F1 males at this
dose had malignant lymphomas in several tissues. One F1 female at 92
mg/kg bw per day had a nephroblastoma, and one F0 male at the high
dose and one control F1 female had a pituitary adenoma. All of these
lesions were considered to be incidental to treatment.
There were no treatment-related effects on adult reproductive
parameters and no effect on litter size, sex ratio, the number of
stillborn pups, pup viability, or clinical signs, no gross lesions in
the pups, and no treatment-related effects on the organ weights of
adults. The mean live birth indexes (with standard error) were 98
(0.91) in the controls, 99 (0.77) at 17 mg/kg bw per day, 98 (1.3) at
92 mg/kg bw per day, and 98 (0.85) at 460 mg/kg bw per day in the
F1a generation; 98 (0.85) in the controls, 98 (1.5) at 17 mg/kg bw
per day, 99 (0.71) at 92 mg/kg bw per day, and 99 (0.63) at 460 mg/kg
bw per day in the F1b generation; 99 (0.84) in the controls, 98
(1.0) at 17 mg/kg bw per day, 97 (1.4) at 92 mg/kg bw per day, and 100
(0.38) at 460 mg/kg bw per day in the F2a generation; and 97 (1.2)
in the controls, 99 (0.78) at 17 mg/kg bw per day, 96 (1.9) at 92
mg/kg bw per day, and 99 (0.46) at 460 mg/kg bw per day in the F2b
generation. The differences between the groups were not statistically
significant. The NOAEL for reproductive toxicity was 460 mg/kg bw per
day, the highest dose tested. The NOAEL for systemic and developmental
toxicity was 92 mg/kg bw per day, on the basis of decreased body
weight and morphological lesions in the kidneys, urinary bladder, and
ureter and a decrease in pup body weight (Eigenberg, 1995).
(ii) Developmental toxicity
2-Phenylphenol and sodium 2-phenylphenol
Mice
Groups of 20-21 pregnant JCL-ICR mice were given 0, 1500, 1700,
or 2100 mg/kg bw per day of 2-phenylphenol (purity not given) or 100,
200, or 400 mg/kg bw per day of sodium 2-phenylphenol (purity not
given) by gavage on days 7-15 of gestation. The animals were weighed
daily, and any change in their general condition was noted. On day 18
of gestation, the dams were killed and their uteri opened, and the
numbers of implantation scars, fetuses that died in early and late
stages, and live fetuses were counted. The live fetuses were weighed
and sexed and observed for external abnormalities. The number of
corpora lutea in each ovary was counted, the major organs were
weighed, and examinations were made to determine whether any
macroscopic abnormalities were present.
With 2-phenylphenol, body-weight gain was reduced at 1700 and
2100 mg/kg bw per day. Four dams at 1500 mg/kg bw per day, seven at
1700 mg/kg bw per day, and 16 at 2100 mg/kg bw per day died. Pregnancy
was confirmed in 5/5 surviving dams at 2100 mg/kg bw per day, 14/14 at
1700 mg/kg bw per day, 14/17 at 1500 mg/kg bw per day, and 20/21
controls, in which implantation was confirmed at the time of sacrifice
and laparotomy on day 18 of gestation. The only significant changes
found at autopsy of these mice were reduced heart weights at 1700 and
2100 mg/kg bw per day and significantly increased liver weights at
1500 and 1700 mg/kg bw per day, a tendency that was also seen at 2100
mg/kg bw per day. Live fetuses were found in all pregnant dams. The
body weights of male and female fetuses at all three doses of
2-phenylphenol were significantly reduced, and the decrease was
dose-related in males. No unique external or internal deformities or
abnormalities were found in the fetuses, and the skeletal
abnormalities found were compatible with delayed development. No NOAEL
could be identified for maternal or fetotoxicity. The NOAEL for
developmental toxicity was 2100 mg/kg bw per day, the highest dose
tested.
With sodium 2-phenylphenol, body-weight gain was statistically
significantly reduced in a dose-dependent manner in dams at all doses.
Four animals at 200 mg/kg bw per day and 16 at 400 mg/kg bw per day
died. No abnormalities were seen at autopsy of dams on day 18 of
gestation. Significantly reduced liver, heart, and spleen weights were
recorded in animals at 400 mg/kg bw per day, while the weight of the
lungs was increased in dams at 200 mg/kg bw per day. Dams at 200 mg/kg
bw per day had a low average number of implantations and a low average
number of live fetuses. No NOAEL could be identified for maternal
toxicity. The NOAEL was 100 mg/kg bw per day for fetotoxicity and 400
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Ogata et al., 1978).
2-Phenylphenol
Rats
Groups of 18-20 pregnant Wistar rats were given 0, 150, 300, or
600 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by gavage on
days 6-15 of gestation. An additional group of 11 rats was given 1200
mg/kg bw per day, but this dose proved to be lethal. No untoward signs
of toxicity were observed in the controls or at 150 mg/kg bw per day.
At doses > 300 mg/kg bw per day, dose-related ataxia and decreased
mean body-weight gains were observed. All surviving rats were killed
on day 20 of gestation, and the uterine contents were examined.
Fetuses were grossly examined; the skeletons were examined with
Alizarin red S and the viscera by a modified Wilson method. The mean
numbers of implantation sites, live fetuses, resorptions, and fetal
weights in animals at 150 and 300 mg/kg bw per day were comparable to
those of controls, but at 600 mg/kg bw per day the number of fetal
resorptions was increased and fetal weight was decreased. Although a
few fetal anomalies were observed in all groups, they did not appear
to be related to treatment. The NOAEL was 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 600
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Kaneda et al., 1978).
In a similar study, groups of 25-35 pregnant rats were given 0,
100, 300, or 700 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by
gavage on days 6-15 of gestation. They were killed on day 21, and the
fetuses were removed surgically. All fetuses were weighed, sexed, and
examined externally and skeletally, and the soft tissues of
approximately one-third of the fetuses were examined. One rat at the
high dose died as a result of a dosing accident. Pregnant rats given
700 mg/kg bw per day gained significantly less body weight during the
first 4 days of treatment (days 6-9 of gestation) than did controls,
and their food consumption was significantly decreased on days 9-11 of
gestation. At necropsy, the weights of the liver (but not the
liver:body weight ratios) were significantly decreased. There was no
effect on the number of implantation sites per dam, mean litter size,
incidences of resorptions, or fetal body weight or crown-rump length.
The only major malformation -- hypoplastic tail and missing sacral and
caudal vertebrae -- was observed in a single fetus at 300 mg/kg bw per
day. An increase in the incidence of delayed ossification of
sternebrae and unossified sternebrae was observed at 700 mg/kg bw per
day. The incidences of foramina and bony islands in the skull were
also slightly increased in this group. No adverse effects on embryonic
or fetal development were observed that were considered to be due to
2-phenylphenol. The NOAEL was 300 mg/kg bw per day for maternal
toxicity and 700 mg/kg bw per day, the highest dose tested, for
fetotoxicity and developmental toxicity (John et al., 1981).
Rabbits
In a range-finding study, groups of two non-pregnant New Zealand
white rabbits were given doses of 0, 100, 500, or 1000 mg/kg bw per
day of 2-phenylphenol (purity, 99.8%) in corn oil for 13 consecutive
days and were submitted to gross necropsy after the last day. The
animals were examined for clinical signs, body weights, body-weight
gain, kidney and liver weights, and gross appearance. The rabbits at
1000 mg/kg bw per day appeared to have stopped eating and had lost 24%
of their body weight by day 7. One rabbit at this dose died on day 8,
and the second was killed in moribund condition on day 10 with
nonspecific lesions or lesions secondary to anorexia. Rabbits given
500 mg/kg per day showed a slight decrease in body-weight gain. All
the other rabbits survived to the end of the study with no other
treatment-related effects. The dose of 100 mg/kg bw per day was
tolerated over the course of treatment.
In the second study, groups of seven artificially inseminated
females were given 0, 250, 500, or 750 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) in corn oil by gavage on days 7-19 of
gestation. They were observed for clinical signs, body weight, and
body-weight gain. On day 20 of gestation, all surviving animals were
killed and examined for gross pathological alterations and changes in
liver and kidney weights. The uteri and ovaries were examined for
implantations, resorptions, and corpora lutea, and the liver, kidneys,
and stomach were examined histologically. Dose-related signs of
maternal toxicity were seen at all doses. One rabbit at 250 mg/kg bw
per day, two at 500 mg/kg bw per day, and six at 750 mg/kg bw per day
died. The dose-related effects observed included increased incidences
of haemorrhage, gaseous distension, and erosions of the stomach,
decreased or soft ingesta in the gastrointestinal tract, decreased
body weight and body-weight gain, increased absolute and relative mean
weights of the kidney, and increased incidence and/or severity of
renal tubular degeneration and inflammation. Treatment-related effects
were observed on reproductive, embryonal, or fetal parameters at 750
mg/kg bw per day.
In the third study, groups of 16-24 artificially inseminated
adult female New Zealand white rabbits were given 0, 25, 100, or 250
mg/kg bw per day of 2-phenylphenol (purity, 99.8%) in corn oil by
gavage on days 7-19 of gestation. They were observed for clinical
signs, body weight, and body-weight gain. On day 28 of gestation, all
surviving rabbits were killed and necropsied, when the weights of the
liver, kidney, and gravid uterus and the numbers of corpora lutea,
implantations, resorptions, and live and dead fetuses were recorded.
All fetuses were removed from the uterus, weighed, sexed, and examined
for external, visceral, and skeletal alterations. The kidneys of all
animals were examined histologically. Administration at 250 mg/kg bw
per day resulted in maternal toxicity evidenced by treatment-related
mortality (13%), gross pathological alterations (ulceration and
haemorrhage of the gastric mucosa, haemolysed blood in the intestinal
tract, and decreased ingesta), and histopathological alterations
(renal tubular degeneration and inflammation). No significant maternal
effects were observed at 25 or 100 mg/kg bw per day, and no adverse
embryonal or fetal effects were observed at any dose. The overall
NOAELs were 100 mg/kg bw per day for maternal toxicity, 500 mg/kg per
day for fetotoxicity, and 750 mg/kg per day, the highest dose tested,
for developmental toxicity (Zablotny et al., 1991).
(f) Special studies: Mechanisms of carcinogenicity in rat urinary
bladder
In a study to determine whether 2-phenylphenol is a complete skin
carcinogen or a promoter in a two-stage initiation and promotion
process, the compound was applied to the interscapular area of the
backs of 50 male and 50 female Swiss CD-1 mice at a dose of 55.5 mg in
0.1 ml acetone, three times per week for 2 years. A second group of 50
male and 50 female mice was treated identically except that their
backs were pretreated once with 0.05 mg in 0.1 ml acetone of
7,12-dimethylbenz[ a]anthracene (DMBA), a known initiator of skin
cancer. Additional groups of 50 male and 50 female mice served as
acetone vehicle controls, controls treated once with DMBA and
thereafter only with acetone, and a positive control group treated
once with DMBA and thereafter with 12- O-tetradecanylphorbol
13-acetate (TPA), a known promoter of skin cancer, at a dose of 0.005
mg in 0.1 ml acetone, three times per week for 2 years.
The mean body weights and survival of the mice treated with
2-phenylphenol or with DMBA plus 2-phenylphenol were generally similar
to those of the respective negative control groups, but the survival
of the group given DMBA plus TPA was substantially decreased. In this
group, the incidences of squamous-cell papillomas and carcinomas,
keratocanthomas, and basal-cell carcinomas at the site of application
were clearly increased (52/100) over that in the group given DMBA plus
acetone(15/100). The time to tumour was also substantially decreased
in the group given DMBA plus TPA. Similar neoplastic skin lesions were
observed with DMBA plus acetone (17/100), but at an incidence
equivalent to that in the control group (15/100). No neoplastic skin
lesions were observed in the group given 2-phenylphenol. The author
concluded that 2-phenylphenol is not carcinogenic alone or as a
promoter (Luster, 1986).
The promoting effect of 2-phenylphenol (purity, 98%) and sodium
2-phenylphenol (purity, 97%) in the urinary bladder was studied in
male Fischer 344 rat initiated with
N-nitrosobutyl- N-(4-hydroxybutyl)amine (NBHBA). Groups of 30 rats
were given drinking-water containing 0.01% NBHBA for 4 weeks and then
diets containing 20 000 ppm of sodium 2-phenylphenol (equivalent to
1000 mg/kg bw per day) for 32 weeks, NBHBA for 4 weeks followed by
untreated feed for 32 weeks, or drinking-water without NBHBA for 4
weeks followed by diet containing 20 000 ppm of sodium 2-phenylphenol
for 32 weeks. In another experiment, groups of 30 male rats were given
0.05% NBHBA in drinking-water for 4 weeks followed by diets containing
20 000 ppm of sodium 2-phenylphenol or 20 000 ppm of 2-phenylphenol
for 32 weeks, no NBHBA for 4 weeks, and then 20 000 ppm of sodium
2-phenylphenol (15 rats) or 20 000 ppm of 2-phenylphenol (15 rats) in
the diet for 32 weeks. In a third experiment, groups of 15 rats were
given diets containing 0, 20 000 ppm of sodium 2-phenylphenol, or
20 000 ppm of 2-phenylphenol. Urine samples were obtained from these
rats by forced urination on days 27, 29, and 32.
Administration of 20 000 ppm sodium 2-phenylphenol in the diet
significantly increased the incidence and number of preneoplastic
lesions (papillary or nodular hyperplasia) per 10 cm of basement
membrane of the urinary bladder in male rats pretreated with 100 ppm
NBHBA, and the incidence and number of papillomas and carcinomas of
the urinary bladder in the group pretreated with 500 ppm NBHBA.
Moreover, treatment with sodium 2-phenylphenol alone, without
initiation, induced papillary or nodular hyperplasia, papillomaa, and
carcinoma. In contrast, administration of 2-phenylphenol in the diet
after initiation only slightly increased the incidence of urinary
bladder lesions over that with NBHBA alone, and its effect was not
statistically significant. No tumours of the urinary bladder were
induced by 2-phenylphenol alone. The authors concluded that sodium
2-phenylphenol, and not 2-phenylphenol, has tumour promoting activity
and might be a complete carcinogen in rat urinary bladder. Since the
sodium salt increased the pH of the urine, the authors speculated that
an active metabolite reaches the urinary bladder at a higher
concentration than with 2-phenylphenol. They suggested that sodium
2-phenylphenol is a carcinogens that acts by a non-genotoxic mechanism
(Fukushima et al., 1983).
In a similar study, 2-phenylphenol or sodium 2-phenylphenol
(purity of neither given) was administered in the diet at a
concentration of 20 000 ppm to 28 male Fischer 344 rats for 64 weeks.
One rat receiving sodium 2-phenylphenol had small stones in the
urinary bladder, and this compound, but not 2-phenylphenol, induced
papillary or nodular hyperplasia (19/28), papillomas (5/28), and
carcinomas (6/28) of the urinary bladder. Pretreatment of additional
rats with NBHBA increased the incidence of papillary or nodular
hyperplasia ( p < 0.05), papillomas (not significant), and
carcinomas (not significant) over that in rats treated with NBHBA
alone. In another experiment, 2-phenylphenol or sodium 2-phenylphenol
was administered in the diet at concentrations of 2500, 5000, 10 000,
or 20 000 ppm to groups of five to nine male Fischer 344 rats for up
to 104 weeks. Animals from each group were killed and examined at 4,
8, 12, 24, 36, and 104 weeks. No stone formation was observed in the
urinary bladders of rats treated with sodium 2-phenylphenol. At 20 000
ppm, simple hyperplasia of the urinary bladder was observed from 4
weeks in 5/5 animals, papillary or nodular hyperplasia from 36 weeks
in 5/5 animals, and papillomas in 2/5 and carcinomas in 2/5 at 104
weeks. At 10 000 ppm of sodium 2-phenylphenol, only simple hyperplasia
was observed from 36 weeks. 2-Phenylphenol alone did not cause bladder
tumours and did not enhance the bladder lesions induced by NBHBA (Ito,
1983b).
In a short-term assay for bladder carcinogenicity in rats,
increased agglutinability of bladder epithelial cells with
concanavalin A was observed after a 1-week treatment with 10 000 or
20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol (purity of
neither given), suggesting that these compounds cause bladder cancer.
No such increase was observed in rats fed diets containing
rho-phenylphenol or biphenyl derivatives at 20 000 ppm. In male Fisher
rats fed diets containing 20 000 ppm of sodium 2-phenylphenol for 50
weeks, bladder papillomas developed in 19 of 36 rats and bladder
carcinomas in 14 of 36 rats (Honma et al., 1983).
Groups of 20 male Fischer 344 rats were fed diets containing
20 000 ppm of 2-phenylphenol, 20 000 ppm of sodium 2-phenylphenol
(purity of neither given), or 5000 ppm of biphenyl for up to 24 weeks.
Changes in the amounts of DNA synthesis and in the morphology of the
renal papilla and renal pelvis were recorded under light and scanning
electron microscopes. Increased DNA synthesis in both renal papilla
and pelvis and distinct morphological alterations in the cell surface
were seen with 2-phenylphenol and its sodium salt by 4 weeks.
Sequential light microscopy revealed renal papillary necrosis in
animals fed 2-phenylphenol from week 4, followed by regenerative
hyperplasia at weeks 16 (1/5) and 24 (3/5), but no changes in the
renal pelvis. Feeding of sodium 2-phenylphenol caused similar changes
in the renal papillae and also hyperplasia in the renal pelvis (2/5).
No proliferative response of the kidney was apparent in rats fed
biphenyl. The authors concluded that the proliferative responses
caused by sodium 2-phenylphenol in the renal pelvic epithelium were
similar to those induced by this compound in the urinary bladder
(Shibata et al., 1989a).
The interactive effects of ascorbic acid, saccharin, and hippuric
acid on the carcinogenicity of 2-phenylphenol and sodium
2-phenylphenol were studied in groups of 20 male Fischer 344 rats. The
animals were given 2-phenylphenol (purity, 99.5%) or sodium
2-phenylphenol in the diet at a concentration of 20 000 ppm
(equivalent to 1000 mg/kg bw per day) for 24 weeks with or without
ascorbic acid, sodium ascorbate, acid saccharin, sodium saccharin,
hippuric acid, or sodium hippurate at 50 000 ppm. The urinary sodium
concentration was increased in all animals receiving sodium salts
and/or sodium 2-phenylphenol. The pH of the urine was increased in
those given sodium 2-phenylphenol, sodium ascorbate, or sodium
saccharin, and the osmolality was decreased in those given sodium
2-phenylphenol, sodium ascorbate, or sodium hippurate. 2-Phenylphenol
decreased the osmolality but did not affect the pH or sodium
concentration of urine. Histopathologically, the bladders of rats
given sodium 2-phenylphenol showed epithelial thickening (epithelial
thickness, four to eight cells) at 8, 16, and 24 weeks and papillary
and nodular changes at 16 and 24 weeks. Treatment with the other
sodium salts provoked 'slight to moderate' hyperplasia at 8 and 16
weeks but no papillary or nodular changes; the changes had regressed
by 24 weeks. The combination of raised urinary pH and sodium promoted
the effects of sodium 2-phenylphenol, while sodium hippurate raised
urinary sodium but not pH and had no effect (Fukushima et al., 1989).
In an essentially similar study, groups of 31 male Fischer 344
rats received NaHCO3 to raise the urinary pH or NH4Cl to lower it.
2-Phenylphenol was given at a dietary concentration of 12 500 ppm
(equivalent to 625 mg/kg bw per day) and sodium 2-phenylphenol at 20
000 ppm (equivalent to 1000 mg/kg bw per day or 625 mg/kg bw per day
of 2-phenylphenol). Hyperplasia of the bladder epithelium was seen in
animals given 2-phenylphenol, 2-phenylphenol plus NaHCO3, or sodium
2phenylphenol. Administration of the sodium salt with NH4Cl had no
significant effect. The incidence of tumours was significantly
increased with 2-phenylphenol (12/31), sodium 2-phenylphenol (22/31),
and 2-phenylphenol plus NaHCO3 (20/31), but only three tumours were
seen in 31 rats given sodium 2-phenylphenol plus NH4Cl. Thus, the
carcinogenic effects of 2-phenylphenol were promoted in alkaline
urine, and those of sodium 2-phenylphenol were inhibited in acid urine
(Fujii et al., 1987).
Changes in urinary parameters, particularly electrolyte levels
and pH, DNA synthesis, and the morphology of the bladder epithelium
were investigated in Fischer 344 rats fed diets containing various
sodium, potassium, magnesium, and calcium carbonate salts at a
concentration of 30 000 ppm, with or without L-ascorbic acid at 50 000
ppm, for 4 or 8 weeks. The effects of treatment with NH4Cl at 10 000
ppm (to acidify urine) and of combined treatment with sodium ascorbate
at 50 000 ppm and NH4Cl were also investigated. Urinary pH was
significantly raised in groups given NaHCO3, K2CO3, ascorbic
acid plus NaHCO3, ascorbic acid plus K2CO3, or sodium ascorbate,
whereas treatment with ascorbic acid or NH4Cl alone caused a
significant decrease in urinary pH. Increases in urinary electrolyte
or ascorbic acid contents were associated with the corresponding
dosing regimen. DNA synthesis in the bladder epithelium was increased
in groups given NaHCO3, K2CO3, ascorbic acid plus NaHCO3,
ascorbic acid plus K2CO3, or sodium ascorbate. Furthermore, all
treatments that increased DNA synthesis also induced some
morphological alterations in the bladder epithelium. Administration of
ascorbic acid in conjunction with NaHCO3 or K2CO3 induced more
changes than those with either salt alone. In contrast, the degree of
response of the bladder epithelium of rats given sodium ascorbate was
reduced by simultaneous administration of NH4Cl. These results
suggest that the degree of DNA synthesis and/or morphological
alteration in rat bladder epithelium after treatment with various
bases depends on changes in the urinary concentrations of Na+ or
K+ and/or pH and the presence of ascorbic acid in the urine (Shibata
et al., 1989b).
The role of urinary pH and Na+ concentration on the
carcinogenic effect of 2-phenylphenol and sodium 2-phenylphenol on rat
urinary bladder was studied in two experiments. In the first, groups
of 36 male Fischer 344 rats were fed diets containing 20 000 ppm of
sodium 2-phenylphenol (equivalent to 1000 mg/kg bw per day), 12 500
ppm of 2-phenylphenol (equivalent to 625 mg/kg bw per day), 6400 ppm
of NaHCO3, 12 500 ppm of 2-phenylphenol plus 6400 ppm of NaHCO3,
12 500 ppm of 2-phenylphenol plus 3200 ppm of NaHCO3, or 12 500 ppm
of 2-phenylphenol plus 1600 ppm of NaHCO3 for 104 weeks. Body
weights were measured weekly up to week 14 and monthly thereafter.
Food consumption was measured on 2 consecutive days per week on a
per-cage basis. Urine samples were obtained from four to six rats in
each group by forced urination, and the urinary pH was determined 10
times during the 2-year experiment. For measurement of urinary
electrolytes, three or four rats in each group were housed
individually in metal metabolic cages without food or water for 4 h in
the morning during weeks 58, 80, and 96. All surviving animals were
killed at the end of the experiment and were examined carefully for
gross abnormalities at autopsy. The liver, kidney, and tissues with
macroscopic lesions were removed and fixed for histological
examination. Autopsies were also performed on all animals that died or
became moribund and were killed during the experiment.
Body-weight gain was reduced throughout the study in all treated
groups, but the reduction was less and started later in animals given
6400 ppm of NaHCO3 alone. The absolute and relative weights of the
bladder were significantly higher in treated groups than in controls,
especially in rats given 2-phenylphenol plus 6400 ppm of NaHCO3. The
relative weights of the kidneys and liver in all treated groups were
significantly higher than in controls. At week 104, 58% of rats given
sodium 2-phenylphenol and 68-84% of those in other groups were still
alive compared with 73% of controls. Macroscopically, more tumours
were found in bladders of rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm of NaHCO3 than in rats fed
2-phenylphenol plus 3200 ppm or 1600 ppm NaHCO3, and no tumours were
found in rats fed 2-phenylphenol alone or in controls. No stone
formation related to tumours was seen in any group. The bladder
lesions were classified as simple hyperplasia, papillary or nodular
hyperplasia, papilloma, and carcinoma. The incidences of bladder
carcinomas were significantly higher than controls in rats fed sodium
2-phenylphenol or 2-phenylphenol plus 6400 ppm NaHCO3.
In the other experiment, groups of five rats were given diets
supplemented with test chemicals as in the first experiment for only 8
weeks before being killed. Urinary electrolytes and pH were determined
at weeks 2, 4, 6, and 8, and osmolality was measured at weeks 4 and 8.
When all animals were killed at week 8, no stone formation was
observed macroscopically in any groups. Various changes in the luminal
surface of the bladder, particularly in rats fed sodium 2-phenylphenol
or 2-phenylphenol plus 6400 ppm of NaHCO3, were revealed by scanning
electron microscopy. The authors concluded that sodium 2-phenylphenol
is carcinogenic to the male rat bladder at 20 000 ppm in the diet.
2-Phenylphenol was not carcinogenic, although it induced a low
incidence of papillary or nodular hyperplasia (Fukushima et al.,
1989).
Species differences in the induction of urinary bladder lesions
by sodium 2-phenylphenol were studied in groups of 30 male Fischer 344
rats, B6C3F1 mice, Syrian golden hamsters, and Hartley guinea-pigs
fed diets containing 20 000 ppm of sodium 2-phenylphenol (purity not
given). Body weight and food consumption were determined periodically.
Groups of five animals from each group were killed at weeks 4, 8, 12,
24, 36, and 48, and five control animals were killed at weeks 12 and
48. Urine was collected in metabolism cages for 4 h from all animals
at weeks 12 and 48 for measurement of urine volume, pH, osmolality,
and microscopic appearance. Although food consumption did not differ
between the treated and untreated groups, retardation of growth was
associated with administration of sodium 2-phenylphenol in the diet,
especially during the first 8 weeks of the test. Although the absolute
weights of the liver were similar in both treated and untreated
groups, the relative weights were slightly increased in all species.
Morphological changes in the urinary bladder were remarkable only in
rats, which showed simple hyperplasia at week 4, increasing in
incidence and density to week 48. Lesions classified as papillary
nodular hyperplasia were observed in rats from week 36 of treatment,
but no papillomas were found. Scanning electron microscopy revealed
pleomorphic microvilli only in rats, which increased in grade with
time. In the other species, no changes indicative of proliferation
were observed, except for a slight effect in mice at weeks 24 and 48.
The pH of the urine was slightly increased in rats, while the
background pH in the other species was usually high, except in mice at
48 weeks. Osmolality was not affected by administration of sodium
2-phenylphenol, but crystal formation was seen in rats and
guinea-pigs, which increased slightly in rats with time. The authors
concluded that sodium 2-phenylphenol is likely to be a urinary bladder
carcinogen in rats but not in mice, guinea-pigs, or hamsters (Hasegawa
et al., 1990a).
Sex differences in the carcinogenic effect in rat urinary bladder
associated with administration of 2-phenylphenol (purity, > 99%) and
sodium 2-phenylphenol (purity, > 99%) were investigated in groups of
five or six male and five or six female Fischer 344 rats fed diets
containing 12 500 ppm of 2-phenylphenol (equivalent to 625 mg/kg bw
per day), 20 000 ppm of sodium 2-phenylphenol, equivalent to 1000
mg/kg bw per day), 30 000 ppm of NaHCO3, 10 000 ppm of NH4Cl,
2-phenylphenol plus NaHCO3, or sodium 2-phenylphenol plus NH4Cl
for 8 weeks. Body weights and food and water consumption were
determined weekly. Fresh urine specimens were obtained from all rats
by forced urination at week 8 and examined for pH and osmolality.
During the last week of the study, the rats were transferred to metal
metabolism cages without food or water, and urine samples were
collected from 07:00-13:00 h over 3 consecutive days to obtain enough
pooled urine for analysis and determination of metabolites, although
only pooled samples from the groups treated with 2-phenylphenol or its
sodium salt were examined.
No animals died before the end of the experiment, and food intake
was not significantly different between groups. The body weights at
week 8 were significantly lower in all treated groups of male rats and
in female rats given 2-phenylphenol or sodium 2-phenylphenol. The
urinary pH values for all groups were significantly different from the
7.0 found in untreated males and the 6.8 found in untreated females,
except in the groups given sodium 2-phenylphenol alone in which the pH
values were comparable to those of controls. The pH values were
highest in the groups given NaHCO3 alone, followed by the groups
given 2-phenylphenol plus NaHCO3, while the pH was lower than in
controls for groups given 2-phenylphenol alone, sodium 2-phenylphenol
plus NH4Cl, or NH4Cl alone. The Na+ concentrations were higher
in males than in females in all groups except controls and those given
NH4Cl. In the groups given sodium 2-phenylphenol alone, the Na+
concentration was slightly increased over control values in males but
not in females. The depressive effect of NH4Cl on Na+
concentration was also less pronounced in females. Only unconjugated
urinary metabolites were identified. No urothelial hyperplastic
changes were observed with 2-phenylphenol alone in either sex, while
an equimolar dose of sodium 2-phenyl-phenol induced mild papillary and
nodular hyperplasia or simple hyperplasia in male rats only. The
possible mechanisms underlying the differences in response between the
sexes might include excretion of other types of conjugated forms and
the formation of microcrystals such as the silicate crystals found in
the urine of rats fed sodium saccharin (Hasegawa et al., 1991).
The physiological effects of 2-phenylphenol (purity, 99.5%) on
urothelial cells and potential formation of DNA adducts were studied
in male Fischer 344 rats. In an initial experiment, rats were fed
dietary concentrations of 0, 1000, 4000, or 12 500 ppm for 13 weeks.
There was no evidence of urinary calculi, microcrystalluria, or
calcium phosphate-containing precipitate, but urothelial cytotoxicity
and hyperplasia were seen at the highest dose. In a second experiment,
rats were fed dietary concentrations of 0, 800, 4000, 8000, or 12 500
ppm for 13 weeks. The urinary pH was > 7 in all groups. The urinary
volume was increased at the highest dose, with consequent decreases in
osmolality and the concentrations of creatinine and other solutes. The
urinary excretion of total 2-phenylphenol metabolites was increased.
Most of the metabolites were conjugates of 2-phenylphenol and of
phenylhydroquinone, and free 2-phenylphenol and metabolites accounted
for < 2% at each dose. Urothelial toxicity and hyperplasia occurred
only at 8000 and 12 500 ppm. No 2-phenylphenol-DNA adducts were
detected in the urothelium at any dose. The small percentage of
unconjugated metabolites and the absence of DNA adducts suggest that
2-phenylphenol acts as a bladder carcinogen in male rats by inducing
cytotoxicity and hyperplasia without direct binding of the compound or
its metabolites to DNA (Smith et al., 1998).
The carcinogenic effect of sodium 2-phenylphenol and its
metabolites on female rat urinary bladder after intravesicular
instillation was studied in groups of nine 6-week-old Fischer 344 rats
that received 0.2 ml of a saline solution of 0.1% sodium
2-phenylphenol (purity, > 99%), phenylbenzoquinone (purity, > 99%),
or phenylhydroquinone (purity, > 99%) through a catheter into the
urethra once, twice, or four times. The pH values of the solutions
were 11 for sodium 2-phenylphenol, 6.5 for phenylbenzoquinone, and 6.4
for phenylhydroquinone. Saline or a solution of NaOH (pH 11) were
given to controls. The animals were maintained under light ether
anaesthesia during instillation and for a further 10 min thereafter to
prevent spontaneous urination. Two or three animals from each group
were killed under ether anaesthesia at 24 h and 4 and 7 days after the
last instillation. The histopathological findings in rats killed 24 h
after a single injection of saline, phenylbenzoquinone, or
phenylhydroquinone included swelling and vacuolation of urothelial
cells. The bladder epithelium of rats treated with alkaline solutions
of sodium 2-phenylphenol or NaOH showed minimal hyperplasia associated
with mild oedema and inflammatory-cell infiltration of the epithelial
and submucosal tissues. Moderate epithelial hyperplasia was seen in
rats killed 7 days after treatment with phenylbenzoquinone. In rats
given two or four instillations of this metabolite, the grading of the
hyperplastic changes was clearly dependent on the number of
instillations and the time between the last treatment and death. The
epithelial hyperplasia was marked and was classified as papillary
and/or nodular in rats treated with four instillations of
phenylbenzoquinone and killed 4 days after the last instillation. No
carcinomas were induced.
In another experiment, groups of 20 female rats were treated in
the same way but twice a week for 5 weeks. From week 6, some rats were
fed the basal diet supplemented with 5% sodium saccharin for 31 weeks
as a promotion treatment, while the other rats were maintained on
basal diet during this period. A separate group was given 500 ppm of
NBHBA for 4 weeks, and then 50 000 ppm of sodium saccharin as a
positive control. Body weights and food consumption were determined
periodically. The histopathological findings in the positive control
group included papillomas in two rats, papillary and/or nodular
hyperplasia in nine rats, and simple hyperplasia in 11 rats. In
contrast, no hyperplastic changes were seen in rats treated first with
sodium 2-phenylphenol or its metabolites followed by promotion with
sodium saccharin, except in nine rats given phenylbenzoquinone, which
had papillary and/or nodular hyperplasia and/or simple hyperplasia.
Formation of lymph follicles in the submucosa of the urinary bladder
was seen in particular with phenylbenzoquinone and in the positive
control group. The authors concluded that phenylbenzoquinone plays an
essential role in the urinary bladder carcinogenesis induced by sodium
2-phenylphenol (Hasegawa et al., 1990b).
A series of studies was carried out on the carcinogenicity of
2-phenylphenol and sodium 2-phenyl-phenol (purity of neither given) in
rat urinary bladder. In the first study, groups of 10 male Fischer 344
rats were fed diets containing sodium 2-phenylphenol at concentrations
of 0, 2500, 5000, 10 000, or 20 000 ppm for 36 weeks. The rats were
observed daily and were weighed periodically. Body-weight gain was
suppressed at 20 000 ppm. No calculi or mucosal tumours were found
grossly, but histological analysis revealed a statistically
significant increase in the frequency of bladder lesions in rats at
20 000 ppm, in which simple hyperplasia was seen in 10/10 and
papillary or nodular hyperplasia in 4/10 animals. Simple hyperplasia
was seen in 1/10 rats at 10 000 ppm.
In the second study, groups of five male rats were fed diets
containing 20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol for 4
weeks. The animals were weighed at the end of treatment, at which time
the mean body weights of both treated groups were reduced, to 86% of
the control value with 2-phenylphenol and to 96% with sodium
2-phenylphenol. One hour before sacrifice, each rat was given an
intraperitoneal injection of 100 mg/kg bw of 5-bromo-2'-deoxyuridine
(BrdU), and the urinary bladders were stained immunohistochemically
with anti-BrdU antibodies to investigate the capacity of the
epithelial cells for proliferation. The number of cells that had taken
up BrdU per 1000 urinary bladder epithelial cells was determined by
light microscopy and expressed as per cent labelled cells. Rats fed
sodium 2-phenylphenol showed increased urinary pH and extensive
BrdU-labelling in urinary bladder epithelial cells, indicating
increased DNA synthesis. Rats fed 2-phenylphenol also showed a
tendency to increased BrdU-labelling, suggested that it also can
cause, albeit weak, urinary bladder epithelium proliferation.
In the third study, groups of five male Fischer 344 rats were
given diets containing 6400 ppm of NaHCO3; 13 000 ppm of
2-phenylphenol; 13 000 ppm of 2-phenylphenol plus NaHCO3 at 1600,
3200, or 6400 ppm; or 20 000 ppm of sodium 2-phenylphenol for 8 weeks.
The urinary pH at week 8 was significantly increased in animals fed
NaHCO3 alone, 2-phenylphenol plus 3200 or 6400 ppm NaHCO3, or
sodium 2-phenylphenol. The urinary concentrations of Na+ were
significantly increased at 2, 4, 6, and 8 weeks in rats fed sodium
2-phenylphenol and at 8 weeks in rats fed NaHCO3 alone or
2-phenylphenol plus 3200 or 6400 ppm NaHCO3. At 8 weeks, a
significant increase or a tendency to an increase in urine volume and
significantly lower osmotic pressure were seen in pooled urine samples
from all treated groups when compared with controls. When the bladders
were examined by scanning electron microscopy, the surface of the
epithelium appeared normal in the controls and in animals given only
NaHCO3, and was made up of polygonal cells of uniform dimensions
with reticular peaked microridges at the surface. In treated animals,
the cells in the outermost layer of the bladder epithelium assumed a
cobblestone configuration in pavement form; at high magnification,
pleomorphic microvilli, short uniform microvilli, and ropy or leafy
microridges were seen at the surface of these cells. These alterations
were observed mainly in rats given sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. The extent of the changes and
the frequency of their appearance was correlated with the
concentration of NaHCO3 given with 2-phenylphenol.
In the fourth study, groups of 30-31 male Fischer 344 rats were
given 20 000 ppm of sodium 2-phenylphenol or 13 000 ppm of
2-phenylphenol with or without NaHCO3 at 1600, 3200, or 6400 ppm for
104 weeks. The animals were observed daily for deaths, and body weight
and feed consumption were measured at regular intervals. Pooled 4-h
urine samples were collected from three or four rats at each dose at
weeks 58, 80, and 96 for measurement of electrolytes. The urinary pH
was significantly increased in rats fed sodium 2-phenylphenol or
2-phenylphenol alone or in combination with 3200 or 6400 ppm NaHCO3
or 6400 ppm NaHCO3 alone. The Na+ concentrations were
significantly increased in rats fed sodium 2-phenylphenol,
2-phenylphenol plus 6400 ppm NaHCO3, or 6400 ppm NaHCO3 alone. No
difference was seen in rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. Urinary bladder tumours were
found in all groups except those given 2-phenylphenol alone, and the
frequencies were highest with sodium 2-phenylphenol and with
2-phenylphenol plus 6400 ppm NaHCO3. The presence of calculi could
not be confirmed. Histological examination of the urinary bladder
epithelium revealed simple hyperplasia, papillary or nodular
hyperplasia, papillomas, and carcinomas. Carcinomas occurred in rats
fed sodium 2-phenylphenol (41%), 2-phenylphenol plus 6400 ppm NaHCO3
(31%), and 6400 ppm NaHCO3 alone. The results confirm that
administration of 20 000 ppm of sodium 2-phenylphenol is carcinogenic
in male rats, while an equimolar concentration of 2-phenylphenol
causes only a low frequency of papillary or nodular hyperplasia and no
papillomas or cancers. Administration of NaHCO3 in conjunction with
2-phenylphenol caused carcinomas, correlated to the NaHCO3
concentration, which also increased urinary pH and Na+ concentration
(Inoue, 1993).
The induction of DNA damage in the urinary bladder epithelium of
male and female Fischer 344 rats by 2-phenylphenol and its metabolites
was studied by the alkaline elution assay after an intravesicalar
injection. Phenylbenzoquinone at 0.05-0.1% had weak DNA-damaging
activity in animals of each sex, whereas 2-phenylphenol and
phenylhydroquinone had no effect at the same dose. Histopathological
examination revealed diffuse, moderate, simple hyperplasia 5 days
after injection of 0.1% phenylbenzoquinone in male rats. The lesions
were associated with submucosal infiltration, small round cells, and
slight oedema. The only change in the bladders of rats injected with
0.1% phenylhydroquinone was slight swelling and/or vacuolization of
the epithelial cells, and the bladders of rats injected with 0.1%
2-phenylphenol were normal (Morimoto et al., 1989).
Groups of 5-10 Fischer 344 rats received diets containing sodium
2-phenylphenol at concentrations of 0, 2500, 5000, 10 000, or 20 000
ppm (equivalent to 0, 250, 500, 1000, and 2000 mg/kg bw per day) for
up to 5 months to investigate the correlation between urinary
phenylbenzoquinone and DNA damage in the bladder epithelium. Slight
but dose-dependent DNA damage was observed in the epithelium of male
rats fed 10 000 or 20 000 ppm for 3-5 months. A plot of the
dose-response relationship for DNA damage at 3 months showed a
threshold at 5000 ppm of sodium 2-phenylphenol. The amounts of
unconjugated 2-phenylphenol, phenylhydroquinone, and
phenylbenzoquinone in 24-h urine samples collected from males and
females after 5 months correlated well with the dietary concentrations
of sodium 2-phenylphenol. The total amounts of free metabolites in the
urine of males given 5000 ppm were similar to those in the urine of
females given 20 000 ppm. Free metabolites represented 0.3% of the
total average intake of male rats fed 5000 ppm, 0.8% of the intake of
10 000 ppm, and 1% of the intake of 20 000 ppm of sodium
2-phenylphenol. The average concentrations of free phenylhydroquinone
in the urine of males given 20 000 ppm of sodium 2-phenylphenol were
significantly higher than those in males fed 5000 ppm or in females
fed 20 000 ppm. The concentrations of phenylbenzoquinone were much
lower than those of phenylhydroquinone. Only 10% of phenylbenzoquinone
was recovered from spiked urine, indicating that this metabolite may
react with urinary nucleophilic groups. The authors concluded that
phenylbenzoquinone is the reactive species in the initiation of
bladder tumours induced by 2-phenylphenol and sodium 2-phenylphenol
(Morimoto et al., 1989).
The interaction of 2-phenylphenol and its metabolites with pUC18
DNA from Escherichia coli plasmids was studied in vitro. The major
metabolite formed from 2-phenylphenol by mixed-function oxidases was
phenylhydroquinone. This finding corroborates earlier reports that
phenylhydroquinone in the form of a glucuronide conjugate is the main
product in the bladders of rats fed 2-phenylphenol. When pUC18 DNA was
incubated with phenylhydroquinone, DNA strand scission was observed,
whereas barely detectable DNA cleavage was seen with 2-phenylphenol
and phenylbenzoquinone. DNA cleavage by phenylhydroquinone was
inhibited by superoxide dismutase, catalase, and several oxygen
radical scavengers, indicating that the oxygen radicals generated in
the process of oxidation of phenylhydroquinone in aqueous solution are
responsible for the DNA cleavage. The attack seemed to occur at
guanine residues in general and was not restricted to guanines with
specific residues, indicating no hot spots (Nagai et al., 1990).
The generation of 8-hydroxydeoxyguanosine in calf thymus DNA
treated with 2-phenylphenol, phenylhydroquinone, or
phenylbenzoquinone, was studied in vitro. The content of
8-hydroxydeoxy-guanosine residues was increased in DNA treated with
phenylhydroquinone in a concentration-dependent manner, but
phenylbenzoquinone had little effect, and 2-phenylphenol had no
effect. The formation of 8-hydroxydeoxyguanosine by phenylhydroquinone
was reduced by oxygen radical scavengers and accelerated by the
addition of CuCl or CuCl2. Hydroxyl radicals generated during
oxidation of phenylhydroquinone thus contribute to the formation of
8-hydroxydeoxyguanosine in DNA, and copper ions facilitate the
oxidative DNA damage. Copper ions greatly accelerated
phenylhydroquinone-induced DNA cleavage in vitro, although they had
no effect on cleavage without phenylhydroquinone. In contrast, DNA
cleavage occurred with the addition of FeCl2 in the absence and
presence of phenylhydroquinone. The formation of
8-hydroxydeoxyguanosine in bladder DNA is likely to be one of a series
of events in the carcinogenesis induced by 2-phenylphenol (Nagai et
al., 1995).
The effect of the selective gamma-glutamylcysteine synthetase
inhibitor, buthionine sulfoximine, on the hepatotoxic and nephrotoxic
potential of 2-phenylphenol and its metabolites was studied in groups
of four male Fischer 344/DuCrj rats. The animals were given an
intraperitoneal injection of 0 or 900 mg/kg bw of buthionine
sulfoximine and 1 h later received 2-phenylphenol, phenylhydroquinone,
or phenylbenzoquinone at single oral doses of 0, 700, or 1400 mg/kg
bw. The rats were killed 6 and 24 h later, and serum was collected for
measurement of alanine and aspartate aminotransferase activities and
urea nitrogen. The liver and kidneys were removed and weighed, and
hepatic and renal glutathione were assayed.
2-Phenylphenol caused acute hepatocellular damage, as shown by
necrotic centrilobular hepatocytes accompanied by increased serum
aminotransferase activity. Pretreatment with buthionine sulfoximine
potentiated the hepatic and renal toxicity of 2-phenylphenol,
indicating that the liver and kidneys are its target organs of at high
doses. 2-Phenylphenol depleted hepatic and renal glutathione by 6 h
after administration, and this effect was enhanced by pretreatment
with buthionine sulfoximine. Recovery of glutathione concentrations in
both organs was slower in rats given 1400 mg/kg bw of 2-phenylphenol
than in those given 700 mg/kg bw, suggesting that the hepatic and
renal damage caused by this compound is associated with prolonged
depletion of glutathione and that it acts indirectly on the liver.
Within 24 h, 75% of the rats treated with phenylbenzoquinone at 1400
mg/kg bw had died. Administration of phenylbenzoquinone at 700 m/kg bw
or phenylhydroquinone at 1400 mg/kg bw significantly increased
aminotransferase activities. The activity of alanine aminotransferase
in both groups was about twice that of rats given 1400 mg/kg bw
2-phenylphenol. A slight decrease in liver weight, nuclear pyknosis,
eosinophilic degeneration of periportal hepatocytes, increased
relative kidney weight, slight renal papillary necrosis, dilatation of
renal tubules, and increased serum urea nitrogen concentration were
observed at 700 mg/kg bw of phenylbenzoquinone. The relative weight of
the kidneys was increased at 1400 mg/kg bw of phenylhydroquinone.
These results indicate that phenylbenzoquinone is more toxic to liver
and kidney than phenylhydroquinone (Nakagawa & Tayama, 1988).
In a study of the conjugation of 2-phenylphenol with glutathione
in rat liver in vitro and in vivo, radiolabel derived from
[14C]2-phenylphenol bound irreversibly to hepatic microsomal
macromolecules in an NADPH-generating system, and the binding was
inhibited by cysteine and glutathione. When [14C]2-phenylphenol and
glutathione were incubated in a microsomal NADPH-generating system,
the radiolabelled material derived from the aqueous phase of the
incubation mixture was similar to a synthetic, water-soluble
phenylhydroquinone-glutathione conjugate produced by a nonenzymic
reaction between phenylbenzoquinone and glutathione.
Phenylhydroquinone-glutathione was excreted as a minor conjugate in
the bile after oral administration of 2-phenylphenol to rats at a dose
of 1000 mg/kg bw. The cumulative biliary excretion of the conjugate
over 6 h represented about 4% of the dose. The results show that a
reactive intermediate of 2-phenylphenol can form adducts with
glutathione to produce water-soluble conjugates. The reactive
intermediate is probably phenylbenzoquinone derived from
phenylhydroquinone. Since glutathione protects against cellular
injury, the acute hepatic damage caused by high doses of
2-phenylphenol is probably associated with the formation of an active
intermediate (phenylbenzoquinone) which depletes cellular glutathione
(Nakagawa & Tayama, 1989).
The relationship between the metabolism and cytotoxicity of
2-phenylphenol was studied in isolated rat hepatocytes. Addition of
high concentrations of 2-phenylphenol to the cells caused
dose-dependent toxicity, with death at the highest dose of 1.0 mmol/L.
Pretreatment of the hepatocytes with a non-toxic dose of 5 µmol/L of
SKF-525A enhanced the cytotoxicity of 2-phenylphenol at 0.5-1.0 mmol/L
and inhibited its metabolism. At lower concentrations (0.5 or 0.75
mmol/L), 2-phenylphenol was converted sequentially to
phenylhydroquinone and then to its glutathione conjugate. The
concentrations of both metabolites and especially of the conjugate,
were very low in hepatocytes exposed to 2-phenylphenol at 1.0 mmol/L
alone or with SKF-525A. The cytotoxicity induced by 2-phenylphenol at
0.5 mmol/L was enhanced by the addition of 1.25 mmol/L of
diethylmaleate, which continuously depletes cellular glutathione. In
contrast, the cytotoxicity induced by phenylhydroquinone at 0.5 mmol/L
was significantly inhibited by addition to the hepatocytes of 5 mmol/L
of dithiothreitol, cysteine, N-acetyl-L-cysteine, or ascorbic acid.
Loss of glutathione, protein thiols, and ATP was also prevented. These
results indicate that the acute cytotoxicity of 2-phenylphenol at 1.0
mmol/L is a direct action and that prolonged depletion of cellular
glutathione enhances the cytotoxicity of low concentrations of
2-phenylphenol metabolites. The cytotoxicity of phenylhydroquinone is
prevented significantly by addition of cysteine, glutathione, or
ascorbic acid (Nakagawa et al., 1992).
Groups of 22 male CDF (Fischer 344)/BR rats were given diets
containing 2phenylphenol (purity, 99.5%) to provide concentrations of
0, 800, 4000, 8000, or 12 500 ppm, equal to 0, 56, 280, 560, and 920
mg/kg bw per day, for 13 weeks. During weeks 12-13 and 13-14 of the
study, urine was collected for determination of metabolites and
urinary characteristics, respectively. In addition, urinary bladders
were collected from 12 animals per group during week 14 for analysisof
the urothelium by 32P-postlabelling, while histopathological
evaluation of 10 animals group included determination of a labelling
index and light and scanning electron microscopy. The body-weight gain
was reduced by about 10% at 8000 and 12 500 ppm, but food intake was
unaffected at all doses tested. Histological examination showed simple
hyperplasia of the urothelium at concentrations of 8000 and 12 500 ppm
with significant changes in the bladder. The glucuronide and sulfate
conjugates of 2-phenylphenol and the hydroxylated metabolite
phenylhydroquinone were the major urinary metabolites, although the
major conjugate at all doses was the sulfate. Minute levels of free
2phenylphenol and phenylhydroquinone were found at all doses, free
phenylhydroquinone comprising 0.6-1.5% of the total metabolites
measured. An increase in the labelling index of the bladder epithelium
was observed at 8000 and 12 500 ppm. 32P-Postlabelled urothelial DNA
showed no evidence of formation of 2-phenylphenol-DNA adducts.
The authors concluded that a hyperplastic response of the urinary
bladder epithelium occurs after exposure to 2-phenylphenol at 8000 or
12 500 ppm, which are unequivocal carcinogenic doses for the bladder
of male rats, which is due to mild cytotoxicity with consequent
regenerative hyperplasia. The increased mitotic activity (labelling
index), the presence of very small amounts of free phenylhydroquinone
in the urine, and the absence of DNA adducts in the bladder epithelium
further suggest that the bladder carcinogenesis in male rats exposed
to 2-phenylphenol is probably mediated by an indirect, dose-dependent
cytotoxic effect on the bladder epithelium leading to regenerative
hyperplasia and subsequent tumorigenesis of epigenetic origin, rather
than to direct metabolic activation of 2-phenylphenol to reactive
metabolites capable of forming 2-phenylphenol-DNA adducts (Christenson
et al., 1996a).
Groups of 20-30 male CDF (Fischer344)/BR rats were given diets
containing 2-phenylphenol (purity, 99.9%) at concentrations of 0,
1000, 4000, or 12 500 ppm, equal to 0, 54, 220, and 680 mg/kg bw per
day, for 13 weeks. Animals from the control and high-dose groups were
allowed to recover for 4 weeks. Urine was collected for chemical and
and electron microscopic evaluation at various times, and urinary
bladders were collected from animals in the recovery groups during
weeks 4, 13, and 17 for histological evaluations which included
determination of a labelling indexes and light and electron
microscopy.
Body-weight gain was reduced only in rats at 12 500 ppm, and food
intake was unaffected at all doses. Weekly clinical examinations
showed an increased incidence of urine staining at 4000 and 12 500
ppm. No unusual precipitate or crystal was found in the urinary
sediment of treated animals. Urothelial hyperplasia was observed only
after 13 weeks at 12 500 ppm, and the effect was reversed by 4 weeks
on control diet. After 4 and 13 weeks of exposure to 2-phenylphenol,
necrotic foci were observed in the bladders of rats at 12 500 ppm, and
at 13 weeks the bladders also showed evidence of regenerative
hyperplasia. Increased labelling indexes were observed in the bladders
of animals at the high dose at 4 and 13 weeks, but the index had
returned to control values after 4 weeks' recovery, confirming the
reversibility of the proliferative changes in the urothelium. The
results of this study suggest that 2-phenylphenol acts by a mechanism
involving a cytotoxic action on the urothelium leading to the
formation of regenerative, reversible hyperplasia. The origin of the
cytotoxicity remains unclear, however, as no evidence was found of
either abnormal crystalluria or a calcium phosphate-containing
amorphous precipitate (Christenson et al. 1996b).
The relative importance of bladder distension, urinary pH, and
Na+ concentration in the induction of cell proliferation in the
bladder epithelium of rats fed various sodium salts was investigated.
In male rats fed a diet containing 5% NaHCO3, the bladder epithelium
showed an increased number of replicating cells, distension, increased
urinary pH, and a high urinary Na+ concentration. Cell proliferation
also occurred when the bladders were subjected to distension in
vivo by mechanical (female) or physiological (male) means.
Inclusion of CaCO3 in the diet increased the urinary pH without
altering other factors and did not induce cell proliferation, but
proliferation was increased when CaCO3 was combined with the
mechanical or physiological treatment. Thus, high urinary pH was of
secondary importance to bladder distension as a causative factor but
acted to enhance cell proliferation when distension occurred. Similar
findings were obtained with regard to the Na+ concentration. The
authors concluded that bladder distension is a prerequisite for
proliferation of epithelial cells in the bladders of rats fed diets
containing high concentrations of sodium salts and that changes in
urinary pH and Na+ concentration also determine the degree of
proliferation (Shioya et al., 1994).
3. Observations in humans
In one of the earliest studies on the toxicity of 2-phenylphenol,
skin irritation and sensitization due to exposure to this compound and
its sodium salt were evaluated in 100 male and 100 female, unselected
persons. A patch impregnated with the test material was placed in
direct contact with the skin of the back of each person, covered with
an impervious film, and taped securely in place. The first patch was
kept in constant contact with the skin for 5 days, at which time the
patch was removed and the reaction noted. A second patch was applied
in the same way 3 weeks after removal of the first patch and was kept
in direct contact with the skin for 48 h. Each subject was examined
immediately and again 3 and 8 days after removal of the second patch.
2-Phenylphenol as a 5.0% solution in sesame oil did not cause primary
irritation or sensitization, but sodium 2-phenylphenol was
significantly irritating when applied as a 5% or a 1% aqueous
solution. A 0.5% solution caused very slight, simple irritation,
whereas a 0.1% solution produced no irritation and no sensitization
(Hodge et al., 1952).
Comments
After oral administration to mice and rats, 2-phenylphenol and
its sodium salt are rapidly and extensively absorbed (95%) and
distributed. Excretion is also rapid in these species, being almost
complete within 48 h, and occurs mainly in urine (about 90%) and in
faeces (about 5%). Little radiolabel (< 1%) is retained in organs and
tissues, including the urinary bladder. After dermal application of
2-phenylphenol to humans, about 43% of the applied dose was absorbed
through the skin and about 58% was recovered in skin rinse and the
protective enclosure. Most of the absorbed radiolabel was recovered in
urine (99%, and only 1% was recovered in faeces. The absorption
half-time was 10 h, and the elimination half-time was 0.8 h. The rapid
excretion of the radiolabel into urine indicates that 2-phenylphenol
is unlikely to accumulate in humans exposed repeatedly. The metabolic
profiles of both compounds were similar in mice, rats, and humans at
the various doses tested. The main metabolic pathways are conjugation
of 2-phenylphenol or hydroxylation at the 5 position of the phenol
ring, followed by conjugation with glucuronide or sulfate. The parent
compound was detected in only very small amounts (0.4%) in urine. The
metabolic profile in plants raised no toxicological concern, since
about 90% of the residue found in oranges and pears is 2-phenylphenol
or its conjugates.
2-Phenylphenol and its sodium salt have low acute toxicity in
mice and rats treated orally, the LD50 values ranging from 600 to
3500 mg/kg bw. Neither 2-phenylphenol nor its sodium salt has been
classified by WHO for acute toxicity.
2-Phenylphenol and its sodium salt caused severe dermal
irritation in rabbits, and the sodium salt caused severe dermal
irritation in humans. 2-Phenylphenol irritated the eye of rabbits,
whereas the sodium salt caused only moderate ocular irritation.
Neither substance caused delayed contact hypersensitivity in
guinea-pigs or humans.
In medium- and long-term tests for toxicity, the urinary bladder
was regarded as the main toxicological target organ of both
2-phenylphenol and its sodium salt in male and female rats. At doses
of 200 mg/kg bw per day and above, hyperplasia, papillomas, and
transitional-cell carcinomas were seen with both compounds in male
rats. Increased mitosis was observed in the bladder epithelium three
days after the start of dosing, and thickening, i.e. simple
hyperplasia, was seen at 14 days. In female rats, hyperplasia and
papillomas were observed, but to a far lower degree than in males. In
male and female mice, the liver was the primary target organ.
Increased relative liver weights and an increased incidence of
hepatocellular adenomas were seen with 2-phenylphenol at doses of 500
mg/kg bw per day and above. Reduced body-weight gain was a common
finding in mice and rats. In 90-day studies, the NOAELs for
2-phenylphenol were 6300 ppm, equal to 760 mg/kg bw per day, in rats
and 300 mg/kg bw per day (the highest dose tested for up to 1 year) in
dogs. The NOAEL for the sodium salt was 5000 ppm, equivalent to 550
mg/kg bw per day, in mice and 2500 ppm, equal to 180 mg/kg bw per day,
in rats. In a 1-year study of toxicity, the NOAEL for 2-phenylphenol
was 800 ppm, equal to 39 mg/kg bw per day, in rats. In 2-year studies
of carcinogenicity, the NOAEL for 2-phenylphenol was 250 mg/kg bw per
day in mice and 800 ppm, equal to 39 mg/kg bw per day, in rats. In
2-year carcinogenicity studies with the sodium salt, the NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, in
mice and 2500 ppm, equivalent to 95 mg/kg bw per day, in rats. The
Meeting concluded that both 2-phenylphenol and its sodium salt are
carcinogenic in male rats and that 2-phenylphenol is carcinogenic in
male mice.
2-Phenylphenol has been more extensively tested for genotoxic
activity than its sodium salt. Within that limitation, the results for
the two compounds were similar. Data regarding covalent binding to DNA
in the urinary bladder of rats dosed with either compound were
conflicting. 2-Phenylphenol induced chromosomal aberrations in
cultured mammalian cells, but negative results were obtained in
vivo. The Meeting concluded that there are unresolved questions
about the genotoxic potential of 2-phenylphenol.
Several studies have been conducted to elucidate the mechanism of
the carcinogenic action of 2-phenylphenol and its sodium salt on the
male rat urinary bladder, since neither compound has a carcinogenic
effect on the urinary bladder of female rats or in mice, guinea-pigs,
or hamsters of either sex. No clear mechanisms have been found,
although raising the urinary pH or sodium concentration has a
promoting effect. There was some evidence from studies with the sodium
salt that initial irritation followed by hyperplasia might be involved
in the bladder carcinogenicity in male rats. In addition,
32P-postlabelling showed binding of 2-phenylphenol and its sodium
salt to DNA in the male rat urinary bladder in some but not in other
studies. The genotoxicity of the metabolites phenylhydroquinone and
dihydroxybiphenyl appears to be similar to that of the parent
molecules.
The Meeting concluded that the urinary bladder tumours observed
in male rats and the liver tumours observed in male mice exposed to
2-phenylphenol are threshold phenomena that are species- and
sex-specific, and that 2-phenylphenol is therefore unlikely to
represent a carcinogenic risk to humans. In coming to this conclusion,
the Meeting was aware that a working group convened by IARC had
classified 2-phenylphenol, sodium salt, in Group 2B (possibly
carcinogenic to humans) and 2-phenylphenol in Group 3 (not
classifiable as to its carcinogenicity to humans). The Meeting noted,
however, that the IARC classification is based on hazard
identification, not on risk assessment, and is furthermore limited to
published literature, with the exclusion of unpublished studies on
toxicity and carcinogenicity.
In two two-generation studies of reproductive toxicity in rats,
2-phenylphenol had no reproductive toxicity, even at 460 mg/kg bw per
day, the highest dose tested. The overall NOAEL for carcinogenicity
was 92 mg/kg bw per day, since urinary bladder tumours were found in
male rats at doses of 120 mg/bw per day and above.
In a study of developmental toxicity in mice with 2-phenylphenol
and its sodium salt, the NOAELs for 2-phenylphenol were below 1500
mg/kg bw per day (lowest dose tested) for maternal toxicity and
fetotoxicity and 2100 mg/kg bw per day (highest dose tested) for
teratogenicity. The NOAELs for the sodium salt were below 100 mg/kg bw
per day (lowest dose tested) for maternal toxicity, 100 mg/kg bw per
day for fetotoxicity, and 400 mg/kg bw per day (highest dose tested)
for teratogenicity. In two studies of developmental toxicity in rats,
the overall NOAELs for 2-phenylphenol were 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 700
mg/kg bw per day (highest dose tested) for teratogenicity. In two
studies of developmental toxicity in rabbits, the overall NOAELs for
2-phenylphenol were 100 mg/kg bw per day for maternal toxicity, 500
mg/kg bw per day for fetotoxicity, and 750 mg/kg bw per day (highest
dose tested) for teratogenicity.
The Meeting established an ADI of 0-0.4 mg/kg bw for
2-phenylphenol, on the basis of the NOAEL of 39 mg/kg per day in the
2-year study of toxicity (based on decreased body-weight gain and
hyperplasia of the urinary bladder) and carcinogenicity of the urinary
bladder in male rats and a safety factor of 100.
The Meeting determined that it was unnecessary to establish an
acute reference dose because of the low acute toxicity of
2-phenylphenol.
Toxicological Evaluation
Levels of 2-phenylphenol that cause no toxic effect
Mouse: < 250 mg/kg bw per day for carcinogenicity (lowest dose
tested; 2-year study of toxicity and carcinogenicity)
< 1500 mg/kg bw per day (lowest dose tested; study of
developmental toxicity; maternal toxicity)
2100 mg/kg bw per day (highest dose tested; study of
developmental toxicity; not teratogenic)
Rat: 800 ppm, equal to 39 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
460 mg/kg bw per day (two-generation study of reproductive
toxicity; no reproductive toxicity; highest dose tested)
92 mg/kg bw per day (two-generation study of reproductive
toxicity; carcinogenicity)
150 mg/kg bw per day (study of developmental toxicity;
maternal toxicity)
300 mg/kg bw per day (study of developmental toxicity;
developmental toxicity)
700 mg/kg bw per day (study of developmental toxicity;
teratogenicity)
Rabbit: 100 mg/kg bw per day (two studies of developmental toxicity;
maternal toxicity)
500 mg/kg bw per day (two studies of developmental toxicity;
fetotoxicity)
750 mg/kg bw per day (two studies of developmental toxicity;
teratogenicity)
Dog: 750 mg/bw per day (highest dose tested; 1-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.4 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Mechanistic studies on urinary bladder tumours in male rats
2. Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to
2-phenylphenol (unless otherwise specified)
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Rapid (24 h) and complete (95-100%), mice and rats
Dermal absorption Rapid and well absorbed (43%), humans
Distribution Small concentrations (< 1%) in tissues, mice and rats
Potential for accumulation No accumulation, mice, rats, and humans
Rate and extent of excretion Rapid and complete (95-100%), mice, rats, and humans
Metabolism in animals Glucuronide and sulfate of 2-phenylphenol and phenylhydroquinone,
mice and rats
Toxicologically significant compounds 2-Phenylphenol
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 5000 mg/kg bw
Rabbit, LC50, inhalation (4 h) > 36 mg/m3 air (aerosol)
Dermal irritation 2-Phenylphenol and its sodium salt: severe dermal irritation,
rabbits
Ocular irritation 2-Phenylphenol: occular irritation, rabbits
Sodium salt: slight ocular irritation, rabbits
Dermal sensitization 2-Phenylphenol and its sodium salt: no dermal sensitization,
guinea-pigs and humans
Short-term toxicity
Target/critical effect Body-weight decrease, mice and rats, and urinary bladder tumours,
male rats
Lowest relevant oral NOAEL 300 mg/kg bw per day, dogs
Lowest relevant dermal NOAEL No NOAEL, 1000 mg/kg bw per day, highest dose tested, rats
Lowest relevant inhalation NOAEL Not investigated
Genotoxicity Unresolved questions
Long-term toxicity and carcinogenicity
Target/critical effect Urinary bladder, male rats
Liver, male and female mice
Lowest relevant NOAEL 39 mg/kg bw per day, male rats
Carcinogenicity Urinary bladder tumours, male rats
Liver tumours, male and female mice
Reproductive toxicity
Reproductive target/critical effect No reproductive toxicity, rats
Lowest relevant reproductive NOAEL 460 mg/kg bw per day, highest dose tested, rats
Developmental target/critical effect Developmental toxicity at maternally toxic doses, mice
Lowest relevant developmental NOAEL 300 mg/kg bw per day, rabbits
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rats.
No evidence of neurotoxicity or neuropathology in medium- and
long-term studies, mice, rats, dogs, or in developmental toxicity
studies, mice, rats, and rabbits
Other toxicological studies
Medical data Dermal irritation with the sodium salt, not with 2-phenylphenol
Summary Value Study Safety factor
ADI 0-0.4 mg/kg bw Long-term study of toxicity 100
and carcinogenicity, rat
Acute reference dose Unnecessary
References
Bartels, M.J. & McNett, D.A. (1996) Quantitation of orthophenylphenol
metabolites in rat urine samples from a 32P-postlabeling study.
Unpublished report No. HET K-001024-062 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Bartels, M.J., Brzak, K.A., McNett, D.A. & Shabrang, S.N. (1997)
Ortho-phenylphenol (OPP): Limited metabolism study in human.
Unpublished report No. HET K-001024-059 from Dow Chemical
Company, Midland, Michigan, USA. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Bartels, M.J., McNett, D.A., Timchalk, C., Mendrala, A.L.,
Christenson, W.R., Sangha, G.K., Brzak, K.A. & Shabrang, S.N.
(1998) Comparative metabolism of orthophenylphenol in mouse,
rat and man. Xenobiotica, 28, 579-594.
Berdasco, N.M. (1991) Orthophenylphenol: Dermal sensitization
potential in the Hartley albino guinea pig. Unpublished report
No. K-001024-048 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Carreon, R.E. & New M.A. (1981) Dowicide*1: Acute percutaneous
absorption potential. Unpublished report No. HET K-1024-(37) from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S., Bartels, M.J. & Cohen, S.M. (1996a)
Technical grade orthophenylphenol: A 32P-postlabeling study
to examine the potential for the formation of DNA adducts in the
urinary bladder of the male rat. Unpublished report No. 94-972-AV
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S. & Cohen, S.M. (1996b) Technical grade
ortho-phenylphenol: A special subchronic dietary study to
examine the mechanism of urinary bladder carcinogenesis in the
male rat. Unpublished report No. 92-972-MS from Bayer
Corporation, Agriculture Division, Toxicology, Stilwell, Kansas,
and University of Nebraska Medical Center, Department of
Pathology & Micropathology, Omaha, Nebraska, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Cosse, P.F., Stebbins, K.E., Stott, W.T., Johnson, K.A. & Atkin, L.
(1990) Orthophenylphenol: Palatability/probe, four-week and
one-year oral toxicity studies in beagle dogs. Unpublished report
No. K-001024-039 from Dow Chemical Co., Midland, Michigan , USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Davies, R.E. & Liggett, M.P. (1973) Irritant effects of agricultural
disinfectant C2 on rabbit eye mucosa. Unpublished report No.
2215/169D/73 from Huntingdon Research Centre, England. Submitted
to WHO by Leng Associates, Midland, Michigan, USA,
Eigenberg, D.A. (1990) Two-generation dietary reproduction study in
rats using orthophenylphenol. Unpublished revised report No
85-671-02 from Mobay Corporation Health, Environment, Safety &
Plant Management, Corporate Toxicology Department, South Metcalf,
Stilwell, Kansas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Eigenberg, D.A. (1995) A two-generation dietary reproduction study in
Sprague Dawley rats using technical grade ortho-phenylphenol.
Unpublished report No. 93-672-VX from Bayer Corporation
Agriculture Division, Toxicology, South Metcalf, Stilwell,
Kansas, USA. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Fujii, T. & Hiraga, K. (1985) Carcinogenicity testing of sodium
orthophenylphenate in F344 rats. J. Saitana Med. School, 12,
277-287.
Fujii, T., Nakamura, K. & Hiraga, K. (1987) Effects of pH on the
carcinogenicity of ophenylphenol and sodium o-phenylphenate
in the rat urinary bladder. Food Chem. Toxicol., 25, 359-362.
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. (1983)
Promoting effect of sodium o-phenylphenate and o-phenylphenol
on two-stage urinary bladder carcinogenesis in rats. Jpn. J.
Cancer Res. (Gann), 74, 625-632.
Fukushima, S., Inoue, T., Uwagawa, S., Shibata, M. & Ito N. (1989)
Co-carcinogenic effects of NaHCO3 on o-phenylphenol-induced
rat bladder carcinogenesis. Carcinogenesis, 10, 1635-1640.
Gilbert, K.S. (1994a) Dowicide(R) 1 antimicrobial: Primary dermal
irritation study in New Zealand white rabbits. Unpublished report
No. K-001024-057B from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert, K.S. (1994b) Dowicide(R) 1 antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001024-57E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. (1994c) Dowicide(R) A antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001025-014E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. & Crissman, J.W. (1994) Dowicide(R) 1 antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
no. K-001024-057A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert K.S. & Stebbins, K. (1994) Dowicide(R) A antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
No. K-001025-014A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Hagiwara, A., Shibata, M., Hirose, M., Fukushima, S. & Ito, N. (1984)
Long-term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Food Chem. Toxicol., 22,
809-814.
Hasegawa, R., Takahashi, S., Asamoto, M., Shirai, T. & Fukushima, S.
(1990a) Species differences in sodium o-phenylphenate induction
of urinary bladder lesions. Cancer Lett., 50, 87-91.
Hasegawa, R., Furukawa, F., Toyoda K., Sato, H., Takahashi, M. &
Hayashi, Y. (1990b) Urothelial damage and tumor initiation by
urinary metabolites of sodium o-phenylphenate in the urinary
bladder of female rats. Jpn. J. Cancer Res., 81, 483-488.
Hasegawa, R., Fukuoka, M., Takahashi, T., Yamamoto, A., Yamaguchi, S.,
Shibata, M.A., Tanaka, A. & Fukushima, S. (1991) Sex differences
in o-phenylphenol and sodium ophenylphenate rat urinary
bladder carcinogenesis: Urinary metabolites and electrolytes
under conditions of aciduria and alkalinuria. Jpn. J. Cancer
Res., 82, 657-664.
Hiraga, K. (1983a) Short report of a 91-week feeding study of
orthophenylphenol (OPP) in male rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. (1983b) Carcinogenicity testing of sodium
orthophenylphenate in F-344/DuCrj rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. & Fujii, T. (1981) Induction of tumours of the urinary
system in F344 rats by dietary administration of sodium
o-phenylphenate. Food Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. (1984) Induction of tumours of the urinary
bladder in F344 rats by dietary administration of o-phenylphenol.
Food Cosmet. Toxicol., 22, 865-870.
Hodge, H.C., Maynard, E.A., Blanchet, H.J., Jr, Spencer, H.C. & Rowe,
V.K. (1952) Toxicological studies of orthophenylphenol (Dowicide
1). J. Pharmacol. Exp. Ther., 104, 202-210.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T. & Sugimura, T. (1983)
Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
Horvath, E., Levay, G. Pongracz, K. & Bodell, W.J. (1992) Peroxidative
activation of ophenylhydroquinone leads to the formation of DNA
adducts in HL-60 cells. Carcinogenesis, 13, 1937-1939.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to
42, Lyon, International Agency for Research on Cancer, pp.
37-41.
IARC (1999) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 73, Miscellaneous
Pesticides, Lyon, International Agency for Research on Cancer,
pp. 451-480.
Iguchi, S., Takahashi, H., Fujii, T., Fukumori, N., Mikuriya, H.,
Tada, Y., Yuzawa, K. & Hiraga, K. (1984) Subchronic toxicity of
o-phenylphenol (OPP) by food administration to rats. Ann.
Rep. Tokyo Metr. Res. Lab. Public Health, 35, 407-415.
Iguchi, S., Tayama, K. & Hiraga, K. (1979) Subacute toxicity of sodium
o-phenylphenate by food administration to rats. Ann. Rep.
Tokyo Metropolitan Res. Lab. Public Health, 30, 67-79.
Inoue, T. (1993) Pathological studies on rat urinary bladder
carcinogenicity of ophenylphenol and its sodium salt. J.
Nagoya City Univ. Medical Assoc. (Nagoya Shiritsu Daigaku
Igakkai Zasshi), 44, 463-477.
Inoue, S., Yamamoto, K. & Kawanishi, S. (1990) DNA damage induced by
metabolites of ophenylphenol in the presence of copper (II)
ion. Chem. Res. Toxicol., 3, 144-149.
Ishidate, M., Jr, Yoshikawa, K. & Sofuni, T. (1983) Mutagenicity tests
on OPP, OPPsodium salt, and their possible metabolites.
Unpublished report from Division of Mutagenesis, Biological
Safety Research Center, National Institute of Hygienic Sciences,
Tokyo, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983a) Long-term toxicity and carcinogenicity study of sodium
o-phenyl-phenate in B6C3F1 mice. Unpublished report from First
Department of Pathology, Nagoya City University Medical School,
Nagoya, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983b) Pathological analysis of the carcinogenicity of sodium
o-phenyl-phenate and o-phenylphenol. Unpublished report from
First Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz B.A. (1981)
Teratological evaluation of orthophenylphenol in rats. Fundam.
Appl. Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. (1978)
Teratogenicity and dominantlethal studies with o-phenylphenol.
J. Pestic. Sci., 3, 365-370.
Kolachana, P., Subrahmanyam, V.V., Eastmond, D.A. & Smith, M.T. (1991)
Metabolism of phenyhydroquinone by prostaglandin (H) synthase:
Possible implications in ophenylphenol carcinogenesis.
Carcinogenesis, 12, 145-149.
Kwok, E.S.C. & Eastmond, D.A. (1997) Effects of pH on nonenzymatic
oxidation of phenylhydroquinone: Potential role in urinary
bladder carcinogenesis induced by ophenylphenol in Fischer 344
rats. Chem. Res. Toxicol., 10, 742-749.
Lambert, A.C. & Eastmond, D.A. (1994) Genotoxic effects of the
o-phenylphenol metabolites phenylhydroquinone and
phenylbenzoquinone in V79 cells. Mutat. Res., 322, 243-256.
Landry, T.D., Stebbins, K.E. & Battjes, J.E. (1992)
Ortho-phenylphenol: Acute aerosol inhalation toxicity study in
Fischer 344 rats. Unpublished report No. K-001024-049 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Luster, M. (1986) NTP Technical Report on the Toxicology and
Carcinogenesis Studies of ortho-Phenylphenol (CAS No. 90-43-7)
Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No.
57-97-6) in Swiss CD-1 Mice (Dermal Studies) (NIH publication
No. 86-2557, NTP TR 301), US Department of Health and Human
Services, National Toxicology Program, Research Triangle Park,
North Carolina, USA.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A. & Wilson, R.E. (1981) The effects of
orthophenylphenol, tris(2,3dichloropropyl)phosphate and
cyclophosphamide on the immune system and host susceptibility of
mice following subchronic exposure. Toxicol. Appl. Pharmacol.,
58, 252-261.
McNett, D.A., Timchalk, C. & Mendrala, A.L. (1997) Ortho-phenylphenol
(OPP): Metabolism of 14C-labelled OPP in B6C3F1 mice and
Fischer 344 rats. Unpublished report No. HET K-001024-060 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Morimoto, K., Sato, M., Fukuoka, M., Hasegawa, R., Takahashi, T.,
Tsuchiya, T., Tanaka, A., Takahashi, A. & Hayashi, Y. (1989)
Correlation between the DNA damage in urinary bladder epithelium
and the urinary 2-phenyl-1,4-benzoquinone levels from F344 rats
fed sodium o-phenylphenate in the diet. Carcinogenesis, 10,
1823-1827.
Nagai, F. & Nakao, T. (1984) Changes in enzyme activities in the urine
and tissues of rats fed sodium o-phenylphenate. Food Chem.
Toxicol., 22, 361-364.
Nagai, F., Ushiyama, K., Satho, K. & Kano, I. (1990) DNA cleavage by
phenylhydroquinone: The major metabolite of a fungicide
o-phenylphenhol. Chem.-Biol. Interactions, 76, 163-179.
Nagai, F., Ushiyama, K., Satoh, K., Kasai, H. & Kano, I. (1995)
Formation of 8hydroxyguanosine in calf thymus DNA treated
in vitro with phenylhydroquinone, the major metabolite of
o-phenylphenol. Carcinogenesis, 16, 837-840.
Nakagawa, Y. & Tayama, K. (1988) Effect of buthionine sulfoximine on
orthophenylphenolinduced hepato- and nephrotoxic potential in
male rats. Arch. Toxicol., 62, 452-457.
Nakagawa, Y. & Tayama, S. (1989) Formation of ortho-phenylphenol
glutathione conjugates in the rat liver. Xenobiotica, 19,
499-507.
Nakagawa, A., Nakao, T. & Nakao, M. (1984) Altered levels of cyclic
nucleotides in F344 rats fed sodium o-phenylphenate. Food
Chem. Toxicol., 22, 217-221.
Nakagawa Y., Tayama, S., Moore, G.A. & Moldéus, P. (1992) Relationship
between metabolism and cytotoxicity of ortho-phenylphenol in
isolated rat hepatocytes. Biochem. Pharmacol., 43, 1431-1437.
Nakao, T., Kabashima, U.J., Nagai, F., Nakagawa, A., Ohno, T.,
Ichikawa, H., Kabayashi, H. & Hiraga, K. (1983) The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Food Chem. Toxicol., 21, 325-329.
National Toxicology Program (1986) Toxicology and Carcinogenesis
Studies of orthoPhenylphenol (CAS No. 90-43-7) Alone and with
7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1
Mice (Dermal Studies) (NTP Technical Report No. 301, NIH
publication No. 86-2557), US Department of Health and Human
Services, Research Triangle Park, North Carolina, USA.
Norris, J..M. (1971) Eye irritation test conducted on Dowicide l
preservative. Unpublished report from Chemical Biology Research,
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Oehme, F.W. & Smith, T.H.F. (1972) The metabolism and urinary
excretion of ophenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1978) Teratological tests
of ortho-phenylphenol and its sodium salt in mice. Ann. Rep.
Tokyo Metr. Res. Lab. Public Health, 29, 89-96.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1979) Acute oral toxicity
of sodiumphenylphenate (OPP-Na) in mice. Ann. Rep. Tokyo Metr.
Res. Lab. ublic Health, 30, 54.
Pathak, D.N. & Roy, D. (1992) Examination of microsomal cytochrome
P450-catalyzed in vitro activation of o-phenylphenol to DNA
binding metabolite(s) by 32P-postlabeling technique.
Carcinogenesis, 13, 1593-1597.
Pathak, D.N. & Roy, D. (1993) In vivo genotoxicity of sodium
ortho-phenylphenol: phenylbenzoquinone is one of the
DNA-binding metabolite(s) of sodium orthophenylphenol.
Mutat. Res., 286, 309-319.
Quast, J.F. & McGuirk, R.J. (1995) ortho-Phenylphenol: Two-year
dietary chronic toxicity/oncogenicity study in B6C3F1 mice.
Unpublished report No. K-001024-047 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1983) Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem.-Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1984) Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP) on
the urinary tract of male F344 rats. Toxicol. Appl.
Pharmacol., 73, 345-349.
Roy, D. (1990) Cytochrome P-450 catalyzed redox cycling of
orthopenylphenol. Biochem. Int., 22, 849-857.
Sato, M., Tanaka, A., Tsuchiya, T., Yamaha, T., Nakaura, S. & Tanaka,
S. (1988) Excretion, distribution and metabolic fate of sodium
o-phenylphenate and o- phenylphenol in the rat. J. Food
Hyg. Soc. Jpn, 29, 7-12.
Savides, M.C. & Oehme, F.W. (1980) Urinary metabolism of orally
administered orthophenyl phenol in dogs and cats.
Toxicology, 17, 355-363.
Selim, S.A. (1996) A single dose open label study to investigate the
absorption and excretion of 14C/13C-labeled orthophenylphenol
formulation after dermal application to healthy volunteers.
Unpublished report No. P0995002 from Pharma Bio-Research Clinics
BV, Assen, Netherlands. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Shibata, M.-A., Hagiwara, A., Tamano, S., Fukushima, S. & Ito, N.
(1985) Subchronic toxicity study of sodium o-phenylphenate in
mice. Toxicol. Lett., 25, 239-246.
Shibata, M.-A., Tanaka, H., Yamada, M., Tamano, S. & Fukushima, S.
(1989a) Proliferative response of renal pelvic epithelium in male
rats to oral administration of orthophenylphenol, sodium
ortho-phenylphenate and diphenyl. Cancer Lett., 48, 19-28.
Shibata, M.-A., Tamano, S., Kurata, Y., Hagiwara, A. & Fukushima, S.
(1989b) Participation of urinary Na+, K+, pH, and L-ascorbic
acid in the proliferative response of the bladder epithelium
after the oral administration of various salts and/or ascorbic
acid to rats. Food Chem. Toxicol., 27, 403-413.
Shioya, S., Nagami-Oguihara, R., Oguihara, S., Kimura, T., Imaida, K.
& Fukushima, S. (1994) Roles of bladder distension, urinary pH
and urinary sodium ion concentration in cell proliferation of
urinary bladder epithelium in rats ingesting sodium salts.
Food Chem. Toxicol., 32, 165-171.
Shirasu, Y., Moriya, M., Kato, K., Tezuka, H., Henmi, R., Shingu, A.,
Kaneda, M. & Teramoto, S. (1978) Mutagenicity testing on
o-phenylphenol. Mutat. Res. 54, 227 (abstract).
Smith, R.A., Christenson, W.R., Bartels, M.J., Arnold, L.L., St John,
M.K., Cano, M., Garland, E.M., Lake, S.G., Wahle, B.S., McNett,
D.A. & Cohen, S.M. (1998) Urinary physiologic and chemical
metabolic effects on the urothelial cytotoxicity and potential
DNA adducts of o-phenylphenol in male rats. Toxicol. Appl.
Pharmacol., 150, 402-413.
Suzuki, H., Suzuki, N., Sasaki, M. & Hiraga, K. (1985)
Orthophenylphenol mutagenicity in a human cell strain. Mutat.
Res., 156, 123-127.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981a)
Toxicological study of o-phenylphenol (OPP) in mice. J. Nara
Med. Assoc., 32, 425.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981b)
Toxicological study of o-phenylphenylphenate (OPP-Na) in rats II.
Acute oral toxicity in Fischer 344 DUCRJ rats. J. Nara Med.
Assoc., 32, 709.
Tayama, S. & Nakagawa, Y. (1991) Sulfhydryl compounds inhibit the
cyto- and genotoxicity of o-phenylphenol metabolites in CHO-K1
cells. Mutat. Res., 259, 1-12.
Tayama, K., Iguchi, S. & Hiraga, K. (1979) Acute oral toxicity of
sodium o-phenylphenol (OPP-Na) in rats. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 30, 57.
Tayama, K., Iguchi, S. & Hiraga, K. (1980) Acute oral toxicity of
o-phenylphenol in rats. Ann. Rep. Tokyo Metr. Res. Lab.
Public Health, 31, 1.
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1983) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 34, 325
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1984) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Toxicology, 101,
187 (abstract).
Tayama, S., Kamiya, N. & Nakagawa, Y. (1989) Genotoxic effects of
o-phenylphenol metabolites in CHO-K1 cells. Mutat. Res., 223,
23-33.
Tayama-Nawai, S., Yoshida, S., Nakao, T. & Hiraga, K. (1984) Induction
of chromosome aberrations and sister-chromatid exchanges in
CHO-K1 cells by o-phenylphenol. Mutat. Res., 141, 95-99.
Timchalk, C. (1996) 14C-Orthophenylphenol: Pharmacokinetics
following dermal application in male human volunteers.
Unpublished report No. HET-K-001024-064 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Timchalk, C., Selim, S., Sangha, G. & Bartels, M.J. (1998) The
pharmacokinetics and metabolism of 14C/13C-labeled
ortho-phenylphenol formulation following dermal application to
human volunteers. Hum. Exp. Toxicol., 17, 411-417.
Ushiyama, K., Nagai, F., Nakagawa, A. & Kano, I. (1992) DNA adduct
formation by ophenylphenol metabolite in vivo and in
vitro. Carcinogenesis, 13, 1469-1473.
Wahle, B.S. & Christenson, W.R. (1996) Technical grade
ortho-phenylphenol: A combined chronic toxicity/oncogenicity
testing study in the rat. Unpublished report No. 92-272-SC from
Bayer Corp., Agriculture Division, Toxicology, Stilwell, Kansas,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Yamaha, T., Tanaka, A., Sato, M. & Tsuchiya, T. (1983) Excretion,
distribution and metabolic fate of sodium o-phenylphenol in the
rat. Unpublished report from Division of Medical Chemistry,
National Institute of Hygienic Sciences, Tokyo, Japan. Submitted
to WHO by Leng Associates, Midland, Michigan, USA.
Zablotny, C.L., Breslin, W.J. & Kociba, R.J. (1991) Ortho-phenylphenol
(OPP): Gavage teratology study in New Zealand white rabbits.
Unpublished report No. K-001024-045 from Toxicology Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Zempel, J.A. & Szabo, J.R. (1993) Ortho-phenylphenol: 21-day repeated
dermal dose study of systemic toxicity in Fischer 344 rats.
Unpublished report No. K-001024-056 from Health & Environmental
Sciences-Texas, Lake Jackson Research Center, Dow Chemical Co.,
Freeport, Texas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
 2phenylphenol, which may play a role in the induction of bladder
cancer by this substance (Roy, 1990).
Activation of the 2-phenylphenol metabolite phenylhydroquinone by
prostaglandin (H) synthase in the presence of arachidonic acid and
hydrogen peroxide was studied to test the hypothesis that
prostaglandin synthase in rat urinary bladder transitional epithelium
and kidney medullar papilla is responsible for activation of the
metabolite to reactive intermediates in the bladder and kidney.
Phenylhydroquinone was found to be metabolized by the peroxidase
activity of prostaglandin synthase and by other peroxidases such as
horseradish peroxidase and myeloperoxidase, suggesting that the
peroxidative metabolism of phenylhydroquinone could play a role in
urinary bladder and kidney carcinogenesis in rats (Kolachana et al.,
1991).
In a study of the effect of pH on nonenzymatic oxidation of
phenylhydroquinone, the effects of phenylbenzoquinone and oxygen
concentration on autoxidation of phenylhydroquinone, and the
nonenzymatic conversion of phenylbenzoquinone to phenylhydroquinone, a
curvilinear relationship was found between the rate of oxidation of
phenylhydroquinone and pH over the range 6.3-7.6. Phenylbenzoquinone
was formed during autoxidation of phenylhydroquinone, with a yield of
0.92 ± 0.02. The results indicate that the production of reactive
metabolites from phenylhydroquinone involves both a pH-independent
(i.e. oxygen-dependent) and a pH-dependent pathway and that the
presence of phenylbenzoquinone enhances the rate of pH-dependent
phenylhydroquinone autoxidation. The authors suggested that ionization
of phenylhydroquinone semiquinone is a key step in production of
reactive species in the pH-dependent pathway. They found a good
correlation between the proposed reaction pathway and the induction by
2-phenylphenol of bladder lesions in rats. Thus, pH-dependent
autoxidation of free phenylhydroquinone in urine may be responsible
for the tumorigenic effects of 2-phenylphenol and sodium
2-phenylphenol in the rat bladder (Kwok & Eastmond, 1997).
Groups of eight female B6C3F1 mice were given 0, 1, 10, or 200
mg/kg bw per day of 2-phenylphenol (purity, > 98%) by gavage on 5
days per week for 2 weeks. As a positive control, mice were given 45
mg/kg bw of cyclophosphamide intraperitoneally for 4 days. The weights
of the body, liver, spleen, kidney, and thymus were recorded, and
samples were prepared for histopathological examination. Haematology
and clinical chemistry were conducted, and bone-marrow cellularity and
colony formation, lymphoproliferative responses, delayed
hypersensitivity responses, immunoglobulin, antibodies, response to
Listeria monocytogenes challenge, and tumour susceptibility were
studied. None of the treated animals died or showed signs of toxicity.
Histopathological examination revealed no significant lesion in any
tissues. The weight of the thymus and the relative weight of the
spleen were slightly increased at 200 mg/kg bw per day. The slight
haematological alterations seen did not show a dose-response
relationship and were within the normal range of biological variation.
A slight increase in serum cholesterol concentration and a
corresponding decrease in triglyceride concentration were seen in mice
at 200 mg/kg bw per day. The activity of alanine aminotransferase and
total protein in serum were not affected, although the
albumin:globulin ratio was slightly decreased at the high dose.
Bone-marrow cellularity, lymphoproliferative responses, immune
function, and host susceptibility were not altered. In contrast,
treatment with cyclophosphamide resulted in marked alterations. The
authors concluded that 2-phenylphenol, even at relatively high doses,
did not alter immune function or host susceptibility (Luster et al.,
1981).
Sodium 2-phenylphenol
Binding of sodium 2-phenylphenol metabolites to macromolecules
in vitro was studied by incubating [14C]sodium 2-phenylphenol
(specific activity, 19 mCi/mmol) with purified liver microsomes from
male rats in the presence of a NADPH regenerating system and bovine
serum albumin, which served as a 'protein acceptor'. Macromolecular
binding of radiolabel to protein, which was dependent on the presence
of both active microsomes and NADP, was observed. In order to study
the binding of metabolites of 2-phenylphenol and its sodium salt to
macromolecules in the liver, kidney, and urinary bladder in vivo,
groups of four male rats were given single oral doses of
14C-labelled compounds at doses of 50, 100, 200, or 500 mg/kg bw,
and tissues were excised 16-18 h later for measurement of
macromolecular binding, which was determined as nanomoles of bound
material per milligram of protein. The extent of binding was not
linearly related to the administered dose. Disproportionate increases
were seen in each tissue at doses of sodium 2-phenylphenol > 200
mg/kg bw and in liver and bladder at doses of 2-phenylphenol at
200-500 mg/kg bw (Reitz et al., 1984).
Sodium 2-phenylphenol (purity not given) was administered in the
diet at a concentration of 20 000 ppm to 4-week-old male and female
Fischer 344 rats for 136 days. Urine was collected periodically. At
the end of treatment, the rats were killed, blood samples were
collected, and livers and kidneys were removed. The amounts of cyclic
nucleotides (cAMP and c-GMP) were determined in urine, plasma, liver,
and kidneys, and adenylate cyclase activity was measured in liver and
kidneys. In male rats, the c-AMP levels in urine and plasma were
decreased whereas the c-GMP levels were increased. In females, c-AMP
levels were decreased only during the first 3 days of feeding, and the
levels of c-AMP and cGMP in liver and kidneys were unchanged. The
decreased urinary c-AMP in male rats was probably the result of
decreased adenylate cyclase activity in liver and kidneys. A similar
change in adenylate cyclase activity was observed in liver but not in
kidneys of female rats treated with sodium 2-phenylphenol. The
sex-related alterations in cyclic nucleotide levels were postulated to
be involved in the sex-dependent induction of urinary bladder tumours
by sodium 2-phenylphenol (Nakagawa et al., 1984).
In male and female Fischer 344 Du Crj rats given 20 000 ppm of
sodium 2-phenylphenol in the diet for 20 weeks, urinary gamma-glutamyl
transpeptidase activity decreased immediately after the start of
treatment and remained low throughout the study. The activities of
this enzyme and of alkaline phosphatase in kidney homogenate were
found to have decreased to about 80% of the control values at 20
weeks, but the activity of glucose-6phosphate dehydrogenase was
significantly increased; that of Na/K-ATPase was unchanged. In liver
homogenate, however, gamma-glutamyl transpeptidase activity was
increased by about eight times and that of glucose-6-phosphate
dehydrogenase was significantly increased, but the activities of
alkaline phosphatase and Na/K-ATPase were not significantly different
from the control values. The glutathione concentration in the livers
of treated rats was significantly reduced (Nagai & Nakao, 1984).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of 2-phenylphenol
and its sodium salt are summarized in Table 1. The clinical signs of
toxicity were generally nonspecific.
Table 1. Acute toxicity of 2-phenylphenol and its sodium salt
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/L)
2-Phenylphenol
Mouse M Oral 1200 Taniguchi et al. (1981)
F 1100
Mouse M Oral 3500 Tayama et al. (1983, 1984)
F 3200
Rat M Oral 2600 Tayama et al. (1980)
F 2900
Rat M Oral 2800 Gilbert & Crissman (1994)
F 2800
Rat M&F Inhalation
(4 h) > 36 Landry et al. (1992)
Rabbit M&F Dermal > 5000 Carreon & New (1981)
Sodium 2-phenylphenol
Mouse M Oral 900 Ogata et al. (1979)
F 800
Rat M Oral 1700 Taniguchi et al. (1981)
F 1600
Rat M Oral 1100 Tayama et al. (1979)
F 1100
Rat M Oral 850 Gilbert & Stebbins (1994)
F 590
(b) Short-term studies of toxicity
2-Phenylphenol
Rats
2-Phenylphenol (purity, 99.8%) was administered to 30 male
Fischer 344 rats (Charles River) in the diet at a concentration of
20 000 ppm, equal to 1000 mg/kg bw per day, for up to 90 days. Interim
sacrifices were performed at 3, 7, 30, and 65 days. Only seven rats at
each dose were permitted to live to 90 days, at which time they were
killed. Food consumption and body weight were markedly reduced within
the first week and remained low throughout the study. The renal
lesions observed in these rats included focal cortical cysts,
significantly decreased urine specific gravity (at 65 and 90 days),
small amounts of blood in the urine, focal tubular collapse and
atrophy in the cortex, and cystic degeneration (at 65 and 90 days). No
treatment-related urinary bladder lesions were observed. A NOAEL could
not be identified since the body weight was reduced at the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1300, 3100, 6300, 13 000, or 25 000 ppm of
2-phenylphenol (purity not given), equal to 0, 180, 390, 760, 1700,
and 2800 mg/kg bw per day for males and 0, 200, 410, 800, 1700, and
3000 mg/kg bw per day for females, for 12 weeks. Body weight and
body-weight gain were severely depressed in males and females at
25 000 ppm and to a lesser extent (14%) in males fed 13 000 ppm. No
significant treatment-related effects were seen in analyses of urine
performed at weeks 9 and 13. Haematological and blood chemical values
were generally normal, other than a slight decrease in haemoglobin
concentration in male and female rats at the highest dose. The
absolute and relative weight of many organs in male rats at this dose
were significantly decreased. The NOAEL was 6300 ppm, equal to 760
mg/kg bw per day, on the basis of reduced body weight and body-weight
gain at 13 000 ppm (Iguchi et al., 1984).
Groups of five male and five female Fischer 344 rats received
dermal applications of 0, 100, 500, or 1000 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) once daily on 5 days per week for a
total of 15 applications over 21 days. The amount applied per animal
was adjusted weekly on the basis of the body weight of individual
animals and was applied to a 5 cm × 5 cm area of clipped skin on the
back, covered with a nonabsorbent cotton patch held in place by an
elastic wrap secured with adhesive tape. The wraps and patches were
removed not less than 6 h after treatment, and the treated area was
wiped with a wettened gauze pad to remove residual test material.
Control animals were handled in the same way. All animals were
acclimated to the wraps for 2 days before the beginning of treatment.
They were observed at least daily and were given a complete clinical
examination weekly. The skin at the site of treatment was examined
after removal of the wrap on the last day of dosing, each week, and on
the day before necropsy. Body weights were measured weekly and feed
consumption and feed efficiency were calculated weekly. Urine was
analysed on day 19. The rats were fasted overnight before necropsy,
when haematological and serum clinical chemical parameters were
evaluated, all animals were examined for gross pathological changes,
selected organs were weighed, and tissues were preserved. Selected
tissues and all gross lesions from animals in the control and
high-dose groups were examined histologically.
No deaths occurred at any dose. Treatment-related effects
indicative of dermal irritation were observed at the site of
application in animals of each sex at 500 and 1000 mg/kg bw per day.
Female rats appeared to be slightly more sensitive than males, but the
severity of lesions increased with duration of exposure and dose in
both sexes. The irritating effects ranged from scaling to fissures.
Body weights and feed consumption were not affected, and no
significant treatment-related effects were found in the
haematological, clinical chemical, and urinary parameters evaluated.
No treatment-associated alterations were found in the liver or kidneys
(Zempel & Szabo, 1993).
Guinea-pigs
2-Phenylphenol was evaluated in 10 male Hartley albino
guinea-pigs for dermal sensitization potential by a modified Buehler
method. The animals received three dermal applications of 0.4 g of
2-phenylphenol (purity, 99.9%) during a 3-week induction period and
were challenged with 0.4 g of the compound 2 weeks after the last
induction. The condition of the test sites was assessed approximately
24 and 48 h after the challenge. No erythema or oedema was seen in any
of the animals. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Berdasco, 1991).
Ten male Hartley albino guinea-pigs were clipped free of hair on
the day before dosing and received three dermal applications of 0.4 g
of 2-phenylphenol (purity, 99.9%) moistened with 0.20 ml of distilled
water during a 3-week induction period. Two weeks after the last
induction application, they were given a challenge application of
0.4 ml of a 7.5% suspension of the compound in water on another site
for 6 h. Five animals received no induction but a 0.4-ml aliquot of a
7.5% suspension of 2-phenylphenol in water. The condition of the test
sites was assessed approximately 24 and 48 h after challenge. No
erythema was seen, and none of the uninduced animals showed
irritation. The animals were in good health and gained weight during
the study. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Gilbert, 1994b).
Rabbits
The ability of 2-phenylphenol (purity, 99.9%) to cause primary
dermal irritation was studied in three male and three female New
Zealand white rabbits that received applications of aliquots of 0.5 ml
of the substance moistened with 0.3 ml of distilled water for 4 h on
intact skin on a clipped, 10 cm × 10 cm area of the back. The
application sites were graded for erythema and oedema within 30 min
and 24, 48, and 72 h of removal of the patch and on days 7, 8, 9,
10,11, 14, and 15. The animals were weighed on the day of treatment
and at the end of the study. Very slight erythema was observed at the
application site in one of the six rabbits within 30 min of removal of
the test material and in two rabbits 24 h later. Severe to slight
eschar formation was observed in four rabbits within 30 min of
treatment which persisted throughout the remainder of the study. Four
animals had burns at the site of application within 30 min, which
resolved as scabs and then scars by the end of the study. Four rabbits
had very slight-to-severe oedema 30 min and 24 h after removal of the
test material, and slight-to-severe oedema was observed on three
animals 48 and 72 h after removal. Body weight was not affected
(Gilbert, 1994a).
Instillation of 0.1 g of 2-phenylphenol (purity not given) into
the right eye of six New Zealand white rabbits resulted in moderate
corneal injury, iritis, and moderate-to-severe conjunctival redness
and chemosis in all animals 24, 48, and 72 h and 7 days after dosing
(Norris, 1971).
Dogs
In studies to assess the palatability and toxicity of
2-phenylphenol (purity, 99.8%) in beagle dogs, males and females were
treated with several regimens. For palatability, one female was given
feed containing 2-phenylphenol to give a dose of 300 mg/kg bw per day
for 5 days. In a study to assess toxicity, groups of two males and
three females were given 300-1000 mg/kg bw per day as a solution in
peanut oil by gastric intubation for up to 9 days or 400-700 mg/kg bw
per day by capsule for 1-2 days. In a 4-week study, groups of two
males and two females were given 0, 100, 200, or 300 mg/kg bw per day
as a solution in peanut oil by gastric intubation on 5 days per week.
In a 1-year study, groups of four males and four females were given
2-phenylphenol as a solution in peanut oil by gastric intubation at
doses of 0, 30, 100, or 300 mg/kg bw per day for 5 days per week for
1 year. The animals were examined daily for clinical signs, and body
weight, feed consumption, haematological, urinary, and clinical
chemical parameters, organ weights, ophthalmologic endpoints, and
gross and histopathology were determined.
Administration of 300 mg/kg bw per day in feed or by intubation
for 5 days resulted in decreased body weights and feed consumption
that correlated with the unpalatability of 2-phenylphenol. Repeated
emesis was seen at doses > 400 mg/kg bw per day administered in
gelatin capsules or in peanut oil by intubation and at doses > 200
mg/kg bw per day by intubation throughout the 4-week study.
Dose-related emesis was also seen in animals given 2-phenylphenol for
1 year. In general, more frequent emesis and ejection of greater
volumes of gastric content was seen in dogs given 300 mg/kg bw per day
than in those given lower doses. While emesis effectively limited the
dose that could be retained, the degree of emesis did not appear to
compromise the health of the animals over 1 year. The reaction was
categorized as a local, transitory response of the mucosal lining of
the upper alimentary tract rather than a reaction of the central
nervous system. No adverse effects were seen on body weight, feed
consumption, haematological, urinary, clinical chemical, or
ophthalmological parameters, organ weights, or gross or histological
appearance of a range of tissues from all dogs. The only deaths were
of two males at the high dose in the 1-year study which died
subsequent to inadvertent deposition of the test solution into the
lungs after approximately 4.5 months of dosing. The NOAEL was 300
mg/kg bw per day (Cosse et al., 1990).
Sodium 2-phenylphenol
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing 0, 2500, 5000, 10 000, 20 000, or 40 000 ppm of sodium
2-phenylphenol as the tetrahydrate (purity not given), equivalent to
0, 270, 550, 1100, 2200, and 4400 mg/kg bw per day, for 13 weeks.
Body-weight gain was significantly depressed in males fed 10 000 or
20 000 ppm and in animals of each sex fed 40 000 ppm. Urinary analysis
showed increased pH and decreased specific gravity at the highest
dose. The relative weights of the livers of animals given 10 000,
20 000, or 40 000 ppm were significantly greater than those of
controls, but no treatment-related histopathological findings were
made. Light and scanning electron microscope examination of the
bladder epithelium at 4, 8, and 13 weeks in three males and three
females in the control group and at 20 000 ppm showed no abnormaility
in the appearance of the bladder epithelium of treated mice at any
time. The NOAEL was 5000 ppm, equivalent to 550 mg/kg bw per day, on
the basis of reduced body-weight gain and increased relative liver
weight at 10 000 ppm (Shibata et al., 1985).
Rats
Sodium 2-phenylphenol (purity, 98.7%) was administered in the
diet to 30 male Fischer 344 rats at a concentration of 20 000 ppm for
up to 90 days. Interim sacrifices were performed at 3, 7, 14, 30, and
65 days. Only seven rats per group were permitted to live to 90 days,
at which time they were killed. The lesions seen in the urinary
bladder epithelium were increased mitosis beginning at 3 days and
thickening (i.e. simple hyperplasia) beginning at 14 days. No tumours
were observed in the bladder. A NOAEL could not be identified since
lesions were observed in the bladder at 20 000 ppm, the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol (purity, > 95%), equal to 0, 85, 180, 350, 710,
1400, and 2500 for males and 0, 87, 180, 350, 690, 1300, and 2400
mg/kg bw per day for females, for 13 weeks. The rats were observed
daily for changes in general condition and were weighed weekly; the
amounts of feed and water consumed were measured on 3 days every other
week. No deaths occurred during the study. A 15-17% decrease in
body-weight gain was seen in animals at doses > 5000 ppm. Urinary
bladder tumours occurred in male rats at frequencies of 1/10 at 10 000
ppm, 9/10 (five transitional-cell carcinomas) at 20 000 ppm, and 1/10
at 40 000 ppm. Six rats at 40 000 ppm had pyelonephritis. In female
rats, the frequencies of tumours were 0/10 at 20 000 ppm and 2/10
(papillomas only) at 40 000 ppm. No bladder calculi were observed in
this experiment. The NOAEL was 2500 ppm, equal to 180 mg/kg bw per
day, on the basis of reduced body-weight gain at 5000 ppm (Iguchi et
al., 1979; Hiraga & Fujii, 1981).
Guinea-pigs
Sodium 2-phenylphenol (purity, 99.1%) was applied to the clipped
skin of 10 male Hartley albino guinea-pigs as a 0.4-ml aliquot of a
0.5% suspension in distilled water at 3-week intervals for induction.
Two weeks after the last dose, a challenge of 0.4 ml of a 0.1%
solution of the compound in distilled water was applied to the other
side of the animals. No erythema occurred at the test site. The
animals gained weight throughout the study. The author concluded that
sodium 2-phenylphenol did not cause delayed contact hypersensitivity
(Gilbert, 1994c).
Rabbits
Sodium 2-phenylphenol diluted 1:200 with distilled water was
instilled into the eyes of six rabbits (strain not given). Temporary,
mild conjunctival reactions were observed in three animals. The
authors concluded that the compound slightly irritated the eye (Davies
& Liggett, 1973).
(c) Long-term studies of toxicity and carcinogenicity
2-Phenylphenol
Mice
Groups of 50 male and 50 female B6C3F1 mice were fed diets
(concentrations not given) supplying 2-phenylphenol (purity, 99.9%) at
doses of 0, 250, 500, or 1000 mg/kg bw per day for 2 years. A
satellite group of 10 male and 10 female mice at each dose was
maintained on the diets for 12 months and then necropsied. All mice
were observed at least once daily for overt signs of toxicity, and a
thorough clinical examination was performed at least once a week
throughout the study. Body weights and feed consumption were recorded
weekly for the first 13 weeks and monthly thereafter. The diets were
prepared weekly or every other week, with adjustments of the
2-phenylphenol concentration according to group mean body weights and
feed consumption to maintain the desired doses for each group.
Clinical signs and mortality rates were unaffected by treatment,
but decreased body weights (by 6-20%) and weight gain (by 10-38%) were
seen in all treated groups except for males fed 250 mg/kg bw.
Haematological, clinical chemical, and urinary parameters in mice
necropsied at 12 and 24 months showed no consistent, toxicologically
significant alterations indicative of target organ toxicity. Changes
in the weights of the adrenal glands, brain, heart, kidneys, liver,
testis, and spleen which were found to be statistically significant
were confounded by the marked decrease in body weight. Nevertheless,
the consistent increases in the absolute and/or relative weights of
the liver at all doses suggests a treatment-related effect. Gross
observations at necropsy in males at the high dose at 12 months and in
males at the intermediate and high doses at 24 months showed a slight
increase in the number of mice with liver masses or nodules.
Microscopic examination of the livers of mice at 12 and 24 months
revealed treatment-related effects at all doses. The cytoplasm of
hepatocytes stained homogeneously, indicating liver enzyme induction,
but there was no evidence of degeneration or necrosis. The microscopic
changes were dose-related and resembled those associated with
adaptation to metabolic demands. An increased incidence of
eosinophilic hepatocellular foci was also observed in males at 100 and
500 mg/kg bw per day.
Males fed 1000 mg/kg bw per day and necropsied at 12 months had a
slightly increased incidence of hepatocellular adenoma. At 24 months,
a statistically significant increase in the number of males with
hepatocellular adenoma was seen at 500 ( n = 40) and 1000 ( n = 41)
mg/kg bw per day, the incidence in controls being 27/50. Low
incidences of a variant form of hepatocellular carcinoma
(hepatoblastoma) were observed in all treated groups of males (2/50 at
250 mg/kg bw per day, 6/50 at 500 mg/kg bw per day, and 3/50 at 1000
mg/kg bw per day versus 0/50 in controls), but the incidence of
hepatocellular carcinoma was not significantly increased at any dose.
The combined incidence of hepatoblastoma and hepatocellular carcinoma
was also not significantly increased in the male mice. The primary
non-tumourous microscopic changes in the livers of male mice, which
appeared to have been adaptive, ultimately resulted in the promotion
of hepatocellular adenomas. The incidences of tumours in other tissues
were not statistically significantly increased. The livers of female
mice showed similar microscopic adaptive changes, but none of them had
hepatoblastoma, and no statistically significant increase in the
incidence of tumours in any tissues was found. Decreased incidences of
microscopic lesions when compared with controls were found in the
adrenals, kidneys, lungs, oral tissues, pancreas, peripheral nerve,
spleen, and testis of males and in the kidneys, lungs, and nasal
tissues of females. These findings were considered to reflect normal
variation and the decreased body weights of the mice and not a primary
response to 2-phenylphenol. A NOAEL for toxicity could not be
identified. The NOAEL for carcinogenicity was 250 mg/kg bw per day on
the basis of an increased incidence of hepatocellular adenomas at 500
mg/kg bw per day (Quast & McGuirk, 1995).
Rats
2-Phenylphenol (purity, 98%) was administered in the diet at
concentrations of 0, 6300, 13 000, or 25 000 ppm, equal to 0, 320,
650, and 1300 mg/kg bw per day to groups of 20-24 male Fischer 344
rats (Charles River) for 91 weeks. The percentage survival was 96, 90,
71, and 65% in the four groups, respectively. In the rats that died
during the study, the incidences of urinary bladder tumours were 0/1
in controls, 0/2 at 6300 ppm, 7/7 at 13 000 ppm, and 0/8 at 25 000
ppm. Bladder lesions were found in 10%, 96%, and 48% of rats at the
three doses, respectively. The incidences of urinary bladder
papillomas and transitional-cell carcinomas were 23/24 at 13 000 ppm
and 4/23 at 25 000 ppm. A NOAEL could not be identified since bladder
lesions were seen at all doses tested (Hiraga, 1983a; Hiraga & Fujii,
1984).
Groups of 70-75 male and 70-75 female Fischer 344 rats were fed
diets containing 2-phenylphenol (purity, 99.5%) at concentrations of
0, 800, 4000, or 8000/10 000 ppm, equal to 0, 39, 200, and 400 mg/kg
bw per day for males and 0, 49, 240, and 650 mg/kg bw per day for
females, for 1 year before interim sacrifice of satellite groups of 20
rats per dose and for 2 years for the remaining 50 rats of each sex.
The animals were observed daily and were examined weekly for clinical
signs of toxicity. Each animal was weighed once a week and also
immediately before necropsy to allow calculation of organ:body weight
ratios. Food consumption was measured weekly. Blood and overnight
urine samples were collected at 3, 6, 12, 18, and 24 months from the
first 20 surviving rats of each sex in the group scheduled for
sacrifice at 2 years. Any dead or moribund animals were prepared for
necropsy, and all surviving animals were killed at the end of the test
periods.
A 5% decrease in body-weight gain was seen in animals at 4000
ppm, and a decrease of 11% was seen in males at 8000 ppm and in
females at 10 000 ppm. Food consumption was unaffected in all groups.
Minor clinical and gross observations included an increased incidence
of abnormally coloured urine, urine stains, and red stains in male
rats given 8000 ppm 2-phenylphenol and an increased incidence of urine
and brown stains in female rats given 4000 or 10 000 ppm. There were
no treatment-related changes in ophthalmological, haematological,
clinical chemical, or urinary parameters, except for an increased
incidence of blood in the urine of males at 8000 ppm. The mortality
rate was slightly increased among males at 8000 ppm. Gross
pathological examination showed increased incidences of urinary
bladder masses in males fed 4000 ppm for 2 years or 8000 ppm for 1 or
2 years and increased incidence of pitted zones and abnormal texture
of the kidney in females fed 10 000 ppm for 2 years. Histopathological
examination showed hyperplasia and transitional-cell carcinoma in the
urinary bladders of males fed 4000 or 8000 ppm for 1 or 2 years, the
increase being statistically significant at 8000 ppm and of borderline
significance at 4000 ppm. The NOAEL for toxicity was 800 ppm, equal to
39 mg/kg bw per day, on the basis of reduced body-weight gain and
hyperplasia in the urinary bladder at all doses. The NOAEL for
carcinogenicity was 800 ppm, equal to 39 mg/kg bw per day (Wahle &
Christenson, 1996).
Sodium 2-phenylphenol
Mice
Sodium 2-phenylphenol (purity, 97%) was administered in the diet
to groups of 50 male and 50 female B6C3F1 mice (Charles River) at
concentrations of 0, 5000, 10 000, or 20 000 ppm, equal to 0, 590,
1400, and 3000 mg/kg bw per day for males and 0, 780, 1500, and 3100
mg/kg bw per day for females, for 96 weeks. The mice were then given
control diet for an additional 8 weeks. The survival rate of males at
the high dose was slightly decreased. Decreased body weight was
observed in males and females at 20 000 ppm and in females at 5000 and
10 000 ppm. Alkaline phosphatase activity was increased in females at
5000, 10 000, and 20 000 ppm. No urinary bladder stones, tumours, or
extensive renal damage were observed in any of the mice. The NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, the
highest dose tested (Ito, 1983a; Hagiwara et al., 1984).
Rats
Groups of 20-21 male and 20-21 female Fischer 344 rats were fed
diets containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol as the tetrahydrate (purity, > 95%), equivalent
to 0, 70, 140, 270, 550, 1100, or 2200 mg/kg bw per day, for 91 weeks.
The rats were observed daily for changes in general condition. The
survival rates were 90, 90, 95, 90, 90, 57, and 71% for the seven
groups, respectively. Increased incidences of urinary bladder
papil-lomas and transitional-cell carcinomas were seen, with 1/21 at
5000 ppm, 7/21 at 10 000 ppm, 20/21 at 20 000 ppm, and 17/20 at 40 000
ppm. Transitional-cell carcinomas of the kidney were also observed at
doses > 5000 ppm. The NOAEL was 2500 ppm, equivalent to 270 mg/kg
bw per day, on the basis of the increased incidence of urinary bladder
tumours (Hiraga & Fujii, 1981).
Groups of 50 male and 50 female Fischer 344/DuCrj rats were fed
diets containing 0, 7000, or 20 000 ppm (males) or 0, 5000, or 10 000
(females) of sodium 2-phenylphenol (purity, 95.5%) for 104 weeks
followed by control diet for 2 weeks. In a second study, groups of 25
male and 25 female rats were fed diets containing the compound at 0,
2500, 7000, or 20 000 ppm, equal to 0, 95, 270, and 770 mg/kg bw per
day, for males, and 0, 2500, 5000, or 10 000 ppm, equal to 0, 110,
220, and 470 mg/kg bw per day, for females, for 104 weeks followed by
control diet for life.
The survival rate at week 104 was 20% in males at 20 000 ppm in
the first study and 24% in the second study, while those in the other
groups were > 50%. Urinary bladder tumours were observed in the first
study in 2/50 males at 7000 ppm, 47/50 males at 20 000 ppm, 1/50
females at 5000 ppm, and 4/50 females at 10 000 ppm. In the second
study, the bladder tumour incidence was 3/25 in males at 7000 ppm,
23/25 in males at 20 000 ppm, and 2/25 in females at 10 000 ppm. In
the first study, transitional-cell carcinomas were found in 2/2 males
at 7000 ppm, 46/47 males at 20 000 ppm, and 1/4 females at 10 000 ppm.
In the second study, carcinomas were found in 1/3 males at 7000 ppm,
21/23 males at 20 000 ppm, and 1/2 females at 10 000 ppm. The
incidence of bladder tumours was thus dose-dependent. The NOAEL was
2500 ppm, equal to 95 mg/kg bw per day, on the basis of urinary
bladder tumours at all doses (Hiraga, 1983b; Fujii & Hiraga, 1985).
A working group convened by the International Agency for Research
on Cancer (IARC) classified sodium 2-phenylphenol as possibly
carcinogenic to humans and 2-phenylphenol as not classifiable as to
its carcinogenicity to humans (IARC, 1987, 1999).
(d) Genotoxicity
The results of tests for the genotoxicity of 2-phenylphenol,
sodium 2-phenylphenol, and the metabolites phenylhydroquinone and
phenylbenzoquinone are summarized in Table 2.
Covalent binding to urinary bladder DNA was determined in vivo
in pooled samples from eight male rats dosed with 500 mg/kg bw of
[14C]2-phenylphenol (purity, 99.8%) or [14C]sodium 2-phenylphenol
(purity, 98.7%). No radiolabel was detected in DNA from bladders
excised 16 h after dosing with either compound. The detection limit
was less than one alkylation per 106 nucleotides. Identical results
were obtained in a second experiment (Reitz et al., 1983).
The reactions of 2-phenylphenol and its metabolites
phenylhydroquinone and phenylbenzo-quinone with DNA were investigated
by a sequencing technique and by ultraviolet-visible and electron spin
resonance spectroscopy. In the presence of Cu(II), phenylhydroquinone
caused extensive DNA damage. Catalase, methionine, and methional
inhibited the DNA damage completely, whereas mannitol, sodium formate,
ethanol, tert-butyl alcohol, and superoxide dismutase did not.
Phenylhydroquinone plus Cu(II) frequently induced a piperidine-labile
site at thymine and guanine residues. Addition of Fe(III), Mn(II),
Co(II), Ni(II), Zn(II), Cd(II), or Pb(II) to phenylhydroquinone did
not induce DNA damage. This metabolite also induced DNA damage in the
presence of Cu(II) when peroxide was added, and Cu(II) accelerated the
autoxidation of phenylhydroquinone to quinone. Electron spin resonance
spectroscopy revealed that the semiquinone radical is an intermediate
in the autoxidation. Catalase did not inhibit the acceleration by
Cu(II). Superoxide dismutase promoted both the autoxidation of
phenylhydroquinone and the initial rate of semiquinone radical
production. Electron spin resonance trapping showed that addition of
Fe(III) produced hydroxyl radicals during the autoxidation of
phenylhydroquinone, whereas addition of Cu(II) did so sparingly. The
results suggest that DNA damage induced by phenylhydroquinone plus
Cu(II) is due to active species other than hydroxyl free radicals
(Inoue et al., 1990).
Table 2. Results of studies of the genotoxicity of 2-phenylphenol, sodium 2-phenylphenol, and the metabolites phenylhydroquinone
and phenylbenzoquinone
End-point Test object Concentration Purity Result Reference
(%)
2-Phenylphenol
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative - S9 Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive + S9 Ushiyama et al. (1992)
Negative - S9
Gene mutation B. subtilis H17, M45 NR NR Negative Shirasu et al. (1978)
Gene mutation S. typhimurium 10 -1000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA92, TA1535, Negative - S9
TA100, TA1537,
TA94, TA98
Gene mutation E. coli WP2 hcr NR NR Negative + S9 Shirasu et al. (1978)
Negative - S9
Gene mutation S. typhimurium 3-200 µg/plate > 99 Negative + S9 National Toxicology
TA100, TA1535, Weakly positive + S9 Program (1986)
TA1537, TA98
Gene mutation Mouse lymphoma 0.3-60 µg/ml > 99 Positive + S9 National Toxicology
(L5178Y) cells, Positive - S9 Program (1986)
Tk locus
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation Human Rsa cells, 1-30 µg/ml NR Positive Suzuki et al. (1985)
repair deficient,
HPRT locus
Chromosomal CHO-K1 cells 50-175 µg/ml > 99 Positive - S9 Tayama-Nawai et al.
aberration (1984)
Chromosomal CHO fibroblasts 12-125 µg/ml NR Weakly positive + S9 Ishidate et al. (1983)
aberration Weakly positive - S9
Chromosomal CHO-K1 cells 60-90 µg/ml > 99 Negative + S9 National Toxicology
aberration Negative - S9 Program (1986)
Chromosomal CHO-K1 cells 25-175 µg/ml > 99 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Chromosomal CHO-K1 cells 100-200 µg/ml > 99 Positive + S9 Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Host-mediated S. typhimurium G46 200 or 600 mg/kg NR Negative Shirasu et al. (1978)
gene mutation in male JCL-ICR mice bw orally for 5 days
In vivo
Sex-linked D. melanogaster 250 ppm in feed > 99 Negative National Toxicology
recessive lethal for 3 days or Program (1986)
mutation injection of 500 ppm
DNA binding Male rat urinary 500 mg/kg bw > 99 Negative Reitz et al. (1983)
bladder orally
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
DNA Rat urinary bladder 60-940 mg/kg > 99 Positive at Christenson et al.
32P-postlabelling bw per day 570 and 940 (1996a)
mg/kg bw per day
Chromosomal Male rat bone 800 mg/kg bw NR Negative Shirasu et al. (1978)
aberration marrow over 5 days or
single doses
< 4000 mg/kg
bw orally
Dominant lethal Male mice 100 or 500 mg/kg > 99 Negative Kaneda et al. (1978)
mutation bw per day for 5 days
Dominant lethal Male mice 100 or 500 mg/kg NR Negative Shirasu et al. (1978)
mutation bw per day for 5
days
Sodium 2-phenylphenol
In vitro
Gene mutation S. typhimurium 50-5000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA100, TA98 Negative - S9
Gene mutation S. typhimurium 0.025-250 µg/plate 99 Negative + S9 Reitz et al. (1983)
TA100, TA98, Negative - S9
TA1535, TA1537,
TA1538
Uncheduled DNA Male rat primary 10-7-10-4 mol/L 99 Negative Reitz et al. (1983)
synthesis hepatocytes
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
DNA Male rat urinary 2% in diet for > 99 Positive Ushiyama et al. (1992)
32P-postlabelling bladder 13 weeks
DNA Mouse skin 10 or 20 mg/animal 97 Positive Pathak & Roy (1993)
32P-postlabelling topically
Phenylhydroquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Positive Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Chromosomal CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Chromosomal CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Sister chromatid CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
exchange Positive - S9
Sister chromatid CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
exchange inhibited by (1991)
cysteine or
glutathione
Micronucleus V79 CH lung 6-125 µmol/L NR Positive + S9 Lambert & Eastmond
formation fibroblast cells Negative - S9 (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA Mouse skin 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
Phenylbenzoquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative Nagai et al. (1990)
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation S. typhimurium 0.05-1000 NR PBQ negative/ Ishidate et al. (1983)
TA100, TA2637, µg/plate PBQ negative
TA98
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Micronucleus V79 CH lung 6-125 µmol/L NR Negative Lambert & Eastmond
formation fibroblast cells (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA adduct formation in HL-60 cells treated with the
2-phenylphenol metabolites 2-phenylhydroquinone and
2-phenylbenzoquinone was studied by 32P-postlabelling. Treatment
with 25-500 µmol/L of 2-phenylhydroquinone for 8 h produced one
principal and three minor adducts, with a relative distribution of 80,
10, 6, and 4%. The relative adduct frequencies were 0.26-2.3
adducts/107 nucleotides. Treatment with 25-250 µmol/L of
2-phenylbenzoquinone for 2 h resulted in a similar level of DNA
modification and adduct distribution. Reaction of purified calf thymus
DNA with 2-phenylbenzo-quinone produced one DNA adduct, which did not
correspond to the major adduct produced in HL-60 cells. These results
show that both metabolites can form DNA adducts. Peroxidase activation
of 2-phenylphenol may therefore play a role in its carcinogenic effect
(Horvath et al., 1992).
In a similar study of covalent binding to DNA,
32P-postlabelling analysis of the products of reaction of DNA with
phenylbenzoquinone revealed four major and several minor adducts.
Chemical reaction with deoxyguanosine 3'-phosphate also resulted in
four major adducts, and their chromatographic mobility was identical
to that of major adducts of phenylbenzoquinone-DNA, which were shown
to be stable. More total covalent binding was found in deoxyguanosine
3'-phosphate than in DNA. Reaction of DNA with 2-phenylphenol or
phenylhydroquinone in the presence of microsomes and NADPH or cumene
hydroperoxide also resulted in four major adducts, and their formation
was drastically decreased by known inhibitors of cytochrome P450. The
chromatographic mobility of these adducts matched that of the adducts
observed in deoxyguanosine 3'-phosphate and DNA reacted with
phenylbenzoquinone. Thus, both 2-phenyl-phenol and phenylhydroquinone
can bind covalently to DNA in the presence of a microsomal cytochrome
P450 activation system, and phenylbenzoquinone is one of the
DNA-binding metabolites of 2-phenylphenol (Pathak & Roy, 1992).
Covalent modification of skin DNA by sodium 2-phenylphenol
in vivo was studied by the 32P-postlabelling method to elucidate
the biochemical mechanism of promotion of chemically induced skin
carcinogenesis by this compound. Topical application of sodium
2-phenylphenol or phenylhydroquinone to the skin of CD-1 mice produced
four distinct major and several minor adducts in skin DNA. Total
covalent binding in skin DNA was 0.31 fmol/µg DNA after treatment with
10 mg of sodium 2-phenylphenol and 0.62 fmol/µg DNA with 20 mg. The
adducts were not observed in skin DNA of untreated animals.
Pretreatment of the mice with a-naphthylisothiocyanate, an inhibitor
of cytochrome P450, or indomethacin, an inhibitor of prostaglandin
synthase, resulted in lower numbers of DNA adducts. Incubation of DNA
with 2-phenylphenol or phenylhydroquinone in vitro in the presence
of cytochrome P450 or prostaglandin synthase activation systems
resulted in four major adducts. The pattern of chromatographic
mobility observed in vitro in the presence of these enzymatic
systems appeared to be similar to that of adducts in vivo. The
chemical reaction of DNA or deoxyguanosine monophosphate with
phenylbenzoquinone also resulted in four major and several minor
adducts. The four major adducts were identical in chromatographic
mobility to the four major adducts produced in vivo and in vitro.
The results show that 2-phenylphenol and phenylhydroquinone can bind
covalently to DNA and that one of the DNA-binding metabolites of
2-phenylphenol may be phenylbenzoquinone (Pathak & Roy, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
2-Phenylphenol
Rats
Groups of 35 albino Sprague-Dawley rats, nine weeks old at the
start of the study, were fed diets containing technical-grade
2-phenylphenol (purity, 99.4-99.5%) at concentrations of
200-10 000 ppm, equal to 0, 36, 120, and 460 mg/kg bw per day, for two
generations. The dose was adjusted during the premating period
according to changes in body weight, and adjustments were made during
lactation to avoid overdosing the pups, although this was later
considered unnecessary. Two F1 females at the high dose and 12 F2a
pups were removed from the study for examination of the heritability
of hypotrichosis. Parents (F0) for the F1 generation were assigned
randomly to dose groups. The genealogy of the F1b pups was checked
to prevent the mating of littermates. For production of the F2
generation, 132 male and 134 female F1b pups were selected
randomly,one or more pups of each sex per litter being used as parents
(F1), divided into 35 pairs of rats per dose, except for the
controls which consisted of 27 males and 29 females. Estrus cycles
were studied by vaginal smears. The animals were examined daily, and
routine observations of birth statistics and pup weights were made;
the litters were culled to eight pups when necessary. Standard
observations and extensive histological examination of the urinary
tract were carried out at sacrifice.
Treatment did not affect clinical signs, body-weight gain during
gestation or lactation, or any of the reproductive variables examined.
Histological examination of the adults and pups revealed no
significant changes in the reproductive tracts. F0 and F1 adults
at 460 mg/kg bw per day showed a treatment-related decrease in body
weight which was not clearly related to a decrease in food
consumption, and F1b, F2a, and F2b pups showed a statistically
significant decrease in body weight on days 14 and/or 21 of lactation,
an effect not seen during the first week of lactation, indicating that
development had not been disturbed. The relative weight of the kidneys
was increased in a dose-dependent manner, in the absence of changes in
other organ weights, in males of the F0 and F1 generations. Male
rats at 120 and 460 mg/kg bw per day had an increased incidence of
calculi in the urinary tract. Transitional-cell hyperplasia was found
in the urinary bladder, defined in the report as 'an area (focal or
diffuse) of at least three to four cells thick (of cuboidal cells) in
an inflated bladder', whereas normal bladders had a cell thickness of
one or two flattened cells. Quantification by simple morphometry
indicated a compound-related effect in the bladder in F0 males and
females at 120 and 460 mg/kg bw per day and in F1 males at 457 mg/kg
bw per day. Neoplasms of the urinary tract were found in four rats:
one bladder and one ureteric tumour in rats at 125 mg/kg bw per day
and two bladder tumours in rats at 457 mg/kg bw per day. The NOAEL for
reproductive toxicity was 460 mg/kg bw per day and that for
carcinogenicity was 36 mg/kg bw per day (Eigenberg, 1990).
In a similar study, groups of 30 CD Sprague-Dawley rats were fed
diets containing technical-grade 2-phenylphenol (purity, 99.5-100%) at
concentrations of 200-10 000 ppm, equal to 0, 17, 92, and 460 mg/kg bw
per day, for two generations. The dose was adjusted during the
premating period according to changes in body weight. The F0 and
F1 adults received the compound in the diet throughout the study,
beginning at 7 weeks of age for the F0 adults and at weaning for the
F1 adults. The animals received treated feed for 10 weeks before
breeding, beginning approximately 2 weeks after weaning of the last
F1b litter for F1 parents. F0 adults were mated to produce the
F1a and F1b litters, and F1 adults (consisting of randomly
selected F1b pups) were mated to produce the F2a and F2b
litters.Adult animals were evaluated during the study for effects of
2-phenylphenol on body weight, food consumption, clinical signs,
estrous cycling, mating, fertility, length of gestation, and litter
size. The offspring were evaluated for effects on sex ratio,
viability, body-weight gain, and clinical signs. Gross necropsy was
performed on all adults and pups, and the reproductive organs,
pituitary, kidneys with ureter attached, urinary bladder, and gross
lesions of all F0 and F1 adults were evaluated histologically.
At 460 mg/kg bw per day, urine staining was observed in F0
males and females and F1 males, urinary bladder calculi were found
at necropsy in F1 adult males, and one F0 male died from renal
failure. At this dose, there was an increase in food consumption by
females during lactation, decreased pup weight, and a decrease in the
terminal body weight of F0 and F1 adult males and females.
Histopathological examination of the kidneys revealed debris in the
renal pelvis, chronic active inflammation, and increased severity of
background lesions in F0 and F1 males. Further, transitional-cell
hyperplasia (simple, nodular, or papilary) of the bladder, calculi,
chronic inflammation of the bladder, and dilatation and hyperplasia of
the ureter were seen in F0 and F1 males. Two F1 males at this
dose had malignant lymphomas in several tissues. One F1 female at 92
mg/kg bw per day had a nephroblastoma, and one F0 male at the high
dose and one control F1 female had a pituitary adenoma. All of these
lesions were considered to be incidental to treatment.
There were no treatment-related effects on adult reproductive
parameters and no effect on litter size, sex ratio, the number of
stillborn pups, pup viability, or clinical signs, no gross lesions in
the pups, and no treatment-related effects on the organ weights of
adults. The mean live birth indexes (with standard error) were 98
(0.91) in the controls, 99 (0.77) at 17 mg/kg bw per day, 98 (1.3) at
92 mg/kg bw per day, and 98 (0.85) at 460 mg/kg bw per day in the
F1a generation; 98 (0.85) in the controls, 98 (1.5) at 17 mg/kg bw
per day, 99 (0.71) at 92 mg/kg bw per day, and 99 (0.63) at 460 mg/kg
bw per day in the F1b generation; 99 (0.84) in the controls, 98
(1.0) at 17 mg/kg bw per day, 97 (1.4) at 92 mg/kg bw per day, and 100
(0.38) at 460 mg/kg bw per day in the F2a generation; and 97 (1.2)
in the controls, 99 (0.78) at 17 mg/kg bw per day, 96 (1.9) at 92
mg/kg bw per day, and 99 (0.46) at 460 mg/kg bw per day in the F2b
generation. The differences between the groups were not statistically
significant. The NOAEL for reproductive toxicity was 460 mg/kg bw per
day, the highest dose tested. The NOAEL for systemic and developmental
toxicity was 92 mg/kg bw per day, on the basis of decreased body
weight and morphological lesions in the kidneys, urinary bladder, and
ureter and a decrease in pup body weight (Eigenberg, 1995).
(ii) Developmental toxicity
2-Phenylphenol and sodium 2-phenylphenol
Mice
Groups of 20-21 pregnant JCL-ICR mice were given 0, 1500, 1700,
or 2100 mg/kg bw per day of 2-phenylphenol (purity not given) or 100,
200, or 400 mg/kg bw per day of sodium 2-phenylphenol (purity not
given) by gavage on days 7-15 of gestation. The animals were weighed
daily, and any change in their general condition was noted. On day 18
of gestation, the dams were killed and their uteri opened, and the
numbers of implantation scars, fetuses that died in early and late
stages, and live fetuses were counted. The live fetuses were weighed
and sexed and observed for external abnormalities. The number of
corpora lutea in each ovary was counted, the major organs were
weighed, and examinations were made to determine whether any
macroscopic abnormalities were present.
With 2-phenylphenol, body-weight gain was reduced at 1700 and
2100 mg/kg bw per day. Four dams at 1500 mg/kg bw per day, seven at
1700 mg/kg bw per day, and 16 at 2100 mg/kg bw per day died. Pregnancy
was confirmed in 5/5 surviving dams at 2100 mg/kg bw per day, 14/14 at
1700 mg/kg bw per day, 14/17 at 1500 mg/kg bw per day, and 20/21
controls, in which implantation was confirmed at the time of sacrifice
and laparotomy on day 18 of gestation. The only significant changes
found at autopsy of these mice were reduced heart weights at 1700 and
2100 mg/kg bw per day and significantly increased liver weights at
1500 and 1700 mg/kg bw per day, a tendency that was also seen at 2100
mg/kg bw per day. Live fetuses were found in all pregnant dams. The
body weights of male and female fetuses at all three doses of
2-phenylphenol were significantly reduced, and the decrease was
dose-related in males. No unique external or internal deformities or
abnormalities were found in the fetuses, and the skeletal
abnormalities found were compatible with delayed development. No NOAEL
could be identified for maternal or fetotoxicity. The NOAEL for
developmental toxicity was 2100 mg/kg bw per day, the highest dose
tested.
With sodium 2-phenylphenol, body-weight gain was statistically
significantly reduced in a dose-dependent manner in dams at all doses.
Four animals at 200 mg/kg bw per day and 16 at 400 mg/kg bw per day
died. No abnormalities were seen at autopsy of dams on day 18 of
gestation. Significantly reduced liver, heart, and spleen weights were
recorded in animals at 400 mg/kg bw per day, while the weight of the
lungs was increased in dams at 200 mg/kg bw per day. Dams at 200 mg/kg
bw per day had a low average number of implantations and a low average
number of live fetuses. No NOAEL could be identified for maternal
toxicity. The NOAEL was 100 mg/kg bw per day for fetotoxicity and 400
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Ogata et al., 1978).
2-Phenylphenol
Rats
Groups of 18-20 pregnant Wistar rats were given 0, 150, 300, or
600 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by gavage on
days 6-15 of gestation. An additional group of 11 rats was given 1200
mg/kg bw per day, but this dose proved to be lethal. No untoward signs
of toxicity were observed in the controls or at 150 mg/kg bw per day.
At doses > 300 mg/kg bw per day, dose-related ataxia and decreased
mean body-weight gains were observed. All surviving rats were killed
on day 20 of gestation, and the uterine contents were examined.
Fetuses were grossly examined; the skeletons were examined with
Alizarin red S and the viscera by a modified Wilson method. The mean
numbers of implantation sites, live fetuses, resorptions, and fetal
weights in animals at 150 and 300 mg/kg bw per day were comparable to
those of controls, but at 600 mg/kg bw per day the number of fetal
resorptions was increased and fetal weight was decreased. Although a
few fetal anomalies were observed in all groups, they did not appear
to be related to treatment. The NOAEL was 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 600
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Kaneda et al., 1978).
In a similar study, groups of 25-35 pregnant rats were given 0,
100, 300, or 700 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by
gavage on days 6-15 of gestation. They were killed on day 21, and the
fetuses were removed surgically. All fetuses were weighed, sexed, and
examined externally and skeletally, and the soft tissues of
approximately one-third of the fetuses were examined. One rat at the
high dose died as a result of a dosing accident. Pregnant rats given
700 mg/kg bw per day gained significantly less body weight during the
first 4 days of treatment (days 6-9 of gestation) than did controls,
and their food consumption was significantly decreased on days 9-11 of
gestation. At necropsy, the weights of the liver (but not the
liver:body weight ratios) were significantly decreased. There was no
effect on the number of implantation sites per dam, mean litter size,
incidences of resorptions, or fetal body weight or crown-rump length.
The only major malformation -- hypoplastic tail and missing sacral and
caudal vertebrae -- was observed in a single fetus at 300 mg/kg bw per
day. An increase in the incidence of delayed ossification of
sternebrae and unossified sternebrae was observed at 700 mg/kg bw per
day. The incidences of foramina and bony islands in the skull were
also slightly increased in this group. No adverse effects on embryonic
or fetal development were observed that were considered to be due to
2-phenylphenol. The NOAEL was 300 mg/kg bw per day for maternal
toxicity and 700 mg/kg bw per day, the highest dose tested, for
fetotoxicity and developmental toxicity (John et al., 1981).
Rabbits
In a range-finding study, groups of two non-pregnant New Zealand
white rabbits were given doses of 0, 100, 500, or 1000 mg/kg bw per
day of 2-phenylphenol (purity, 99.8%) in corn oil for 13 consecutive
days and were submitted to gross necropsy after the last day. The
animals were examined for clinical signs, body weights, body-weight
gain, kidney and liver weights, and gross appearance. The rabbits at
1000 mg/kg bw per day appeared to have stopped eating and had lost 24%
of their body weight by day 7. One rabbit at this dose died on day 8,
and the second was killed in moribund condition on day 10 with
nonspecific lesions or lesions secondary to anorexia. Rabbits given
500 mg/kg per day showed a slight decrease in body-weight gain. All
the other rabbits survived to the end of the study with no other
treatment-related effects. The dose of 100 mg/kg bw per day was
tolerated over the course of treatment.
In the second study, groups of seven artificially inseminated
females were given 0, 250, 500, or 750 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) in corn oil by gavage on days 7-19 of
gestation. They were observed for clinical signs, body weight, and
body-weight gain. On day 20 of gestation, all surviving animals were
killed and examined for gross pathological alterations and changes in
liver and kidney weights. The uteri and ovaries were examined for
implantations, resorptions, and corpora lutea, and the liver, kidneys,
and stomach were examined histologically. Dose-related signs of
maternal toxicity were seen at all doses. One rabbit at 250 mg/kg bw
per day, two at 500 mg/kg bw per day, and six at 750 mg/kg bw per day
died. The dose-related effects observed included increased incidences
of haemorrhage, gaseous distension, and erosions of the stomach,
decreased or soft ingesta in the gastrointestinal tract, decreased
body weight and body-weight gain, increased absolute and relative mean
weights of the kidney, and increased incidence and/or severity of
renal tubular degeneration and inflammation. Treatment-related effects
were observed on reproductive, embryonal, or fetal parameters at 750
mg/kg bw per day.
In the third study, groups of 16-24 artificially inseminated
adult female New Zealand white rabbits were given 0, 25, 100, or 250
mg/kg bw per day of 2-phenylphenol (purity, 99.8%) in corn oil by
gavage on days 7-19 of gestation. They were observed for clinical
signs, body weight, and body-weight gain. On day 28 of gestation, all
surviving rabbits were killed and necropsied, when the weights of the
liver, kidney, and gravid uterus and the numbers of corpora lutea,
implantations, resorptions, and live and dead fetuses were recorded.
All fetuses were removed from the uterus, weighed, sexed, and examined
for external, visceral, and skeletal alterations. The kidneys of all
animals were examined histologically. Administration at 250 mg/kg bw
per day resulted in maternal toxicity evidenced by treatment-related
mortality (13%), gross pathological alterations (ulceration and
haemorrhage of the gastric mucosa, haemolysed blood in the intestinal
tract, and decreased ingesta), and histopathological alterations
(renal tubular degeneration and inflammation). No significant maternal
effects were observed at 25 or 100 mg/kg bw per day, and no adverse
embryonal or fetal effects were observed at any dose. The overall
NOAELs were 100 mg/kg bw per day for maternal toxicity, 500 mg/kg per
day for fetotoxicity, and 750 mg/kg per day, the highest dose tested,
for developmental toxicity (Zablotny et al., 1991).
(f) Special studies: Mechanisms of carcinogenicity in rat urinary
bladder
In a study to determine whether 2-phenylphenol is a complete skin
carcinogen or a promoter in a two-stage initiation and promotion
process, the compound was applied to the interscapular area of the
backs of 50 male and 50 female Swiss CD-1 mice at a dose of 55.5 mg in
0.1 ml acetone, three times per week for 2 years. A second group of 50
male and 50 female mice was treated identically except that their
backs were pretreated once with 0.05 mg in 0.1 ml acetone of
7,12-dimethylbenz[ a]anthracene (DMBA), a known initiator of skin
cancer. Additional groups of 50 male and 50 female mice served as
acetone vehicle controls, controls treated once with DMBA and
thereafter only with acetone, and a positive control group treated
once with DMBA and thereafter with 12- O-tetradecanylphorbol
13-acetate (TPA), a known promoter of skin cancer, at a dose of 0.005
mg in 0.1 ml acetone, three times per week for 2 years.
The mean body weights and survival of the mice treated with
2-phenylphenol or with DMBA plus 2-phenylphenol were generally similar
to those of the respective negative control groups, but the survival
of the group given DMBA plus TPA was substantially decreased. In this
group, the incidences of squamous-cell papillomas and carcinomas,
keratocanthomas, and basal-cell carcinomas at the site of application
were clearly increased (52/100) over that in the group given DMBA plus
acetone(15/100). The time to tumour was also substantially decreased
in the group given DMBA plus TPA. Similar neoplastic skin lesions were
observed with DMBA plus acetone (17/100), but at an incidence
equivalent to that in the control group (15/100). No neoplastic skin
lesions were observed in the group given 2-phenylphenol. The author
concluded that 2-phenylphenol is not carcinogenic alone or as a
promoter (Luster, 1986).
The promoting effect of 2-phenylphenol (purity, 98%) and sodium
2-phenylphenol (purity, 97%) in the urinary bladder was studied in
male Fischer 344 rat initiated with
N-nitrosobutyl- N-(4-hydroxybutyl)amine (NBHBA). Groups of 30 rats
were given drinking-water containing 0.01% NBHBA for 4 weeks and then
diets containing 20 000 ppm of sodium 2-phenylphenol (equivalent to
1000 mg/kg bw per day) for 32 weeks, NBHBA for 4 weeks followed by
untreated feed for 32 weeks, or drinking-water without NBHBA for 4
weeks followed by diet containing 20 000 ppm of sodium 2-phenylphenol
for 32 weeks. In another experiment, groups of 30 male rats were given
0.05% NBHBA in drinking-water for 4 weeks followed by diets containing
20 000 ppm of sodium 2-phenylphenol or 20 000 ppm of 2-phenylphenol
for 32 weeks, no NBHBA for 4 weeks, and then 20 000 ppm of sodium
2-phenylphenol (15 rats) or 20 000 ppm of 2-phenylphenol (15 rats) in
the diet for 32 weeks. In a third experiment, groups of 15 rats were
given diets containing 0, 20 000 ppm of sodium 2-phenylphenol, or
20 000 ppm of 2-phenylphenol. Urine samples were obtained from these
rats by forced urination on days 27, 29, and 32.
Administration of 20 000 ppm sodium 2-phenylphenol in the diet
significantly increased the incidence and number of preneoplastic
lesions (papillary or nodular hyperplasia) per 10 cm of basement
membrane of the urinary bladder in male rats pretreated with 100 ppm
NBHBA, and the incidence and number of papillomas and carcinomas of
the urinary bladder in the group pretreated with 500 ppm NBHBA.
Moreover, treatment with sodium 2-phenylphenol alone, without
initiation, induced papillary or nodular hyperplasia, papillomaa, and
carcinoma. In contrast, administration of 2-phenylphenol in the diet
after initiation only slightly increased the incidence of urinary
bladder lesions over that with NBHBA alone, and its effect was not
statistically significant. No tumours of the urinary bladder were
induced by 2-phenylphenol alone. The authors concluded that sodium
2-phenylphenol, and not 2-phenylphenol, has tumour promoting activity
and might be a complete carcinogen in rat urinary bladder. Since the
sodium salt increased the pH of the urine, the authors speculated that
an active metabolite reaches the urinary bladder at a higher
concentration than with 2-phenylphenol. They suggested that sodium
2-phenylphenol is a carcinogens that acts by a non-genotoxic mechanism
(Fukushima et al., 1983).
In a similar study, 2-phenylphenol or sodium 2-phenylphenol
(purity of neither given) was administered in the diet at a
concentration of 20 000 ppm to 28 male Fischer 344 rats for 64 weeks.
One rat receiving sodium 2-phenylphenol had small stones in the
urinary bladder, and this compound, but not 2-phenylphenol, induced
papillary or nodular hyperplasia (19/28), papillomas (5/28), and
carcinomas (6/28) of the urinary bladder. Pretreatment of additional
rats with NBHBA increased the incidence of papillary or nodular
hyperplasia ( p < 0.05), papillomas (not significant), and
carcinomas (not significant) over that in rats treated with NBHBA
alone. In another experiment, 2-phenylphenol or sodium 2-phenylphenol
was administered in the diet at concentrations of 2500, 5000, 10 000,
or 20 000 ppm to groups of five to nine male Fischer 344 rats for up
to 104 weeks. Animals from each group were killed and examined at 4,
8, 12, 24, 36, and 104 weeks. No stone formation was observed in the
urinary bladders of rats treated with sodium 2-phenylphenol. At 20 000
ppm, simple hyperplasia of the urinary bladder was observed from 4
weeks in 5/5 animals, papillary or nodular hyperplasia from 36 weeks
in 5/5 animals, and papillomas in 2/5 and carcinomas in 2/5 at 104
weeks. At 10 000 ppm of sodium 2-phenylphenol, only simple hyperplasia
was observed from 36 weeks. 2-Phenylphenol alone did not cause bladder
tumours and did not enhance the bladder lesions induced by NBHBA (Ito,
1983b).
In a short-term assay for bladder carcinogenicity in rats,
increased agglutinability of bladder epithelial cells with
concanavalin A was observed after a 1-week treatment with 10 000 or
20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol (purity of
neither given), suggesting that these compounds cause bladder cancer.
No such increase was observed in rats fed diets containing
rho-phenylphenol or biphenyl derivatives at 20 000 ppm. In male Fisher
rats fed diets containing 20 000 ppm of sodium 2-phenylphenol for 50
weeks, bladder papillomas developed in 19 of 36 rats and bladder
carcinomas in 14 of 36 rats (Honma et al., 1983).
Groups of 20 male Fischer 344 rats were fed diets containing
20 000 ppm of 2-phenylphenol, 20 000 ppm of sodium 2-phenylphenol
(purity of neither given), or 5000 ppm of biphenyl for up to 24 weeks.
Changes in the amounts of DNA synthesis and in the morphology of the
renal papilla and renal pelvis were recorded under light and scanning
electron microscopes. Increased DNA synthesis in both renal papilla
and pelvis and distinct morphological alterations in the cell surface
were seen with 2-phenylphenol and its sodium salt by 4 weeks.
Sequential light microscopy revealed renal papillary necrosis in
animals fed 2-phenylphenol from week 4, followed by regenerative
hyperplasia at weeks 16 (1/5) and 24 (3/5), but no changes in the
renal pelvis. Feeding of sodium 2-phenylphenol caused similar changes
in the renal papillae and also hyperplasia in the renal pelvis (2/5).
No proliferative response of the kidney was apparent in rats fed
biphenyl. The authors concluded that the proliferative responses
caused by sodium 2-phenylphenol in the renal pelvic epithelium were
similar to those induced by this compound in the urinary bladder
(Shibata et al., 1989a).
The interactive effects of ascorbic acid, saccharin, and hippuric
acid on the carcinogenicity of 2-phenylphenol and sodium
2-phenylphenol were studied in groups of 20 male Fischer 344 rats. The
animals were given 2-phenylphenol (purity, 99.5%) or sodium
2-phenylphenol in the diet at a concentration of 20 000 ppm
(equivalent to 1000 mg/kg bw per day) for 24 weeks with or without
ascorbic acid, sodium ascorbate, acid saccharin, sodium saccharin,
hippuric acid, or sodium hippurate at 50 000 ppm. The urinary sodium
concentration was increased in all animals receiving sodium salts
and/or sodium 2-phenylphenol. The pH of the urine was increased in
those given sodium 2-phenylphenol, sodium ascorbate, or sodium
saccharin, and the osmolality was decreased in those given sodium
2-phenylphenol, sodium ascorbate, or sodium hippurate. 2-Phenylphenol
decreased the osmolality but did not affect the pH or sodium
concentration of urine. Histopathologically, the bladders of rats
given sodium 2-phenylphenol showed epithelial thickening (epithelial
thickness, four to eight cells) at 8, 16, and 24 weeks and papillary
and nodular changes at 16 and 24 weeks. Treatment with the other
sodium salts provoked 'slight to moderate' hyperplasia at 8 and 16
weeks but no papillary or nodular changes; the changes had regressed
by 24 weeks. The combination of raised urinary pH and sodium promoted
the effects of sodium 2-phenylphenol, while sodium hippurate raised
urinary sodium but not pH and had no effect (Fukushima et al., 1989).
In an essentially similar study, groups of 31 male Fischer 344
rats received NaHCO3 to raise the urinary pH or NH4Cl to lower it.
2-Phenylphenol was given at a dietary concentration of 12 500 ppm
(equivalent to 625 mg/kg bw per day) and sodium 2-phenylphenol at 20
000 ppm (equivalent to 1000 mg/kg bw per day or 625 mg/kg bw per day
of 2-phenylphenol). Hyperplasia of the bladder epithelium was seen in
animals given 2-phenylphenol, 2-phenylphenol plus NaHCO3, or sodium
2phenylphenol. Administration of the sodium salt with NH4Cl had no
significant effect. The incidence of tumours was significantly
increased with 2-phenylphenol (12/31), sodium 2-phenylphenol (22/31),
and 2-phenylphenol plus NaHCO3 (20/31), but only three tumours were
seen in 31 rats given sodium 2-phenylphenol plus NH4Cl. Thus, the
carcinogenic effects of 2-phenylphenol were promoted in alkaline
urine, and those of sodium 2-phenylphenol were inhibited in acid urine
(Fujii et al., 1987).
Changes in urinary parameters, particularly electrolyte levels
and pH, DNA synthesis, and the morphology of the bladder epithelium
were investigated in Fischer 344 rats fed diets containing various
sodium, potassium, magnesium, and calcium carbonate salts at a
concentration of 30 000 ppm, with or without L-ascorbic acid at 50 000
ppm, for 4 or 8 weeks. The effects of treatment with NH4Cl at 10 000
ppm (to acidify urine) and of combined treatment with sodium ascorbate
at 50 000 ppm and NH4Cl were also investigated. Urinary pH was
significantly raised in groups given NaHCO3, K2CO3, ascorbic
acid plus NaHCO3, ascorbic acid plus K2CO3, or sodium ascorbate,
whereas treatment with ascorbic acid or NH4Cl alone caused a
significant decrease in urinary pH. Increases in urinary electrolyte
or ascorbic acid contents were associated with the corresponding
dosing regimen. DNA synthesis in the bladder epithelium was increased
in groups given NaHCO3, K2CO3, ascorbic acid plus NaHCO3,
ascorbic acid plus K2CO3, or sodium ascorbate. Furthermore, all
treatments that increased DNA synthesis also induced some
morphological alterations in the bladder epithelium. Administration of
ascorbic acid in conjunction with NaHCO3 or K2CO3 induced more
changes than those with either salt alone. In contrast, the degree of
response of the bladder epithelium of rats given sodium ascorbate was
reduced by simultaneous administration of NH4Cl. These results
suggest that the degree of DNA synthesis and/or morphological
alteration in rat bladder epithelium after treatment with various
bases depends on changes in the urinary concentrations of Na+ or
K+ and/or pH and the presence of ascorbic acid in the urine (Shibata
et al., 1989b).
The role of urinary pH and Na+ concentration on the
carcinogenic effect of 2-phenylphenol and sodium 2-phenylphenol on rat
urinary bladder was studied in two experiments. In the first, groups
of 36 male Fischer 344 rats were fed diets containing 20 000 ppm of
sodium 2-phenylphenol (equivalent to 1000 mg/kg bw per day), 12 500
ppm of 2-phenylphenol (equivalent to 625 mg/kg bw per day), 6400 ppm
of NaHCO3, 12 500 ppm of 2-phenylphenol plus 6400 ppm of NaHCO3,
12 500 ppm of 2-phenylphenol plus 3200 ppm of NaHCO3, or 12 500 ppm
of 2-phenylphenol plus 1600 ppm of NaHCO3 for 104 weeks. Body
weights were measured weekly up to week 14 and monthly thereafter.
Food consumption was measured on 2 consecutive days per week on a
per-cage basis. Urine samples were obtained from four to six rats in
each group by forced urination, and the urinary pH was determined 10
times during the 2-year experiment. For measurement of urinary
electrolytes, three or four rats in each group were housed
individually in metal metabolic cages without food or water for 4 h in
the morning during weeks 58, 80, and 96. All surviving animals were
killed at the end of the experiment and were examined carefully for
gross abnormalities at autopsy. The liver, kidney, and tissues with
macroscopic lesions were removed and fixed for histological
examination. Autopsies were also performed on all animals that died or
became moribund and were killed during the experiment.
Body-weight gain was reduced throughout the study in all treated
groups, but the reduction was less and started later in animals given
6400 ppm of NaHCO3 alone. The absolute and relative weights of the
bladder were significantly higher in treated groups than in controls,
especially in rats given 2-phenylphenol plus 6400 ppm of NaHCO3. The
relative weights of the kidneys and liver in all treated groups were
significantly higher than in controls. At week 104, 58% of rats given
sodium 2-phenylphenol and 68-84% of those in other groups were still
alive compared with 73% of controls. Macroscopically, more tumours
were found in bladders of rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm of NaHCO3 than in rats fed
2-phenylphenol plus 3200 ppm or 1600 ppm NaHCO3, and no tumours were
found in rats fed 2-phenylphenol alone or in controls. No stone
formation related to tumours was seen in any group. The bladder
lesions were classified as simple hyperplasia, papillary or nodular
hyperplasia, papilloma, and carcinoma. The incidences of bladder
carcinomas were significantly higher than controls in rats fed sodium
2-phenylphenol or 2-phenylphenol plus 6400 ppm NaHCO3.
In the other experiment, groups of five rats were given diets
supplemented with test chemicals as in the first experiment for only 8
weeks before being killed. Urinary electrolytes and pH were determined
at weeks 2, 4, 6, and 8, and osmolality was measured at weeks 4 and 8.
When all animals were killed at week 8, no stone formation was
observed macroscopically in any groups. Various changes in the luminal
surface of the bladder, particularly in rats fed sodium 2-phenylphenol
or 2-phenylphenol plus 6400 ppm of NaHCO3, were revealed by scanning
electron microscopy. The authors concluded that sodium 2-phenylphenol
is carcinogenic to the male rat bladder at 20 000 ppm in the diet.
2-Phenylphenol was not carcinogenic, although it induced a low
incidence of papillary or nodular hyperplasia (Fukushima et al.,
1989).
Species differences in the induction of urinary bladder lesions
by sodium 2-phenylphenol were studied in groups of 30 male Fischer 344
rats, B6C3F1 mice, Syrian golden hamsters, and Hartley guinea-pigs
fed diets containing 20 000 ppm of sodium 2-phenylphenol (purity not
given). Body weight and food consumption were determined periodically.
Groups of five animals from each group were killed at weeks 4, 8, 12,
24, 36, and 48, and five control animals were killed at weeks 12 and
48. Urine was collected in metabolism cages for 4 h from all animals
at weeks 12 and 48 for measurement of urine volume, pH, osmolality,
and microscopic appearance. Although food consumption did not differ
between the treated and untreated groups, retardation of growth was
associated with administration of sodium 2-phenylphenol in the diet,
especially during the first 8 weeks of the test. Although the absolute
weights of the liver were similar in both treated and untreated
groups, the relative weights were slightly increased in all species.
Morphological changes in the urinary bladder were remarkable only in
rats, which showed simple hyperplasia at week 4, increasing in
incidence and density to week 48. Lesions classified as papillary
nodular hyperplasia were observed in rats from week 36 of treatment,
but no papillomas were found. Scanning electron microscopy revealed
pleomorphic microvilli only in rats, which increased in grade with
time. In the other species, no changes indicative of proliferation
were observed, except for a slight effect in mice at weeks 24 and 48.
The pH of the urine was slightly increased in rats, while the
background pH in the other species was usually high, except in mice at
48 weeks. Osmolality was not affected by administration of sodium
2-phenylphenol, but crystal formation was seen in rats and
guinea-pigs, which increased slightly in rats with time. The authors
concluded that sodium 2-phenylphenol is likely to be a urinary bladder
carcinogen in rats but not in mice, guinea-pigs, or hamsters (Hasegawa
et al., 1990a).
Sex differences in the carcinogenic effect in rat urinary bladder
associated with administration of 2-phenylphenol (purity, > 99%) and
sodium 2-phenylphenol (purity, > 99%) were investigated in groups of
five or six male and five or six female Fischer 344 rats fed diets
containing 12 500 ppm of 2-phenylphenol (equivalent to 625 mg/kg bw
per day), 20 000 ppm of sodium 2-phenylphenol, equivalent to 1000
mg/kg bw per day), 30 000 ppm of NaHCO3, 10 000 ppm of NH4Cl,
2-phenylphenol plus NaHCO3, or sodium 2-phenylphenol plus NH4Cl
for 8 weeks. Body weights and food and water consumption were
determined weekly. Fresh urine specimens were obtained from all rats
by forced urination at week 8 and examined for pH and osmolality.
During the last week of the study, the rats were transferred to metal
metabolism cages without food or water, and urine samples were
collected from 07:00-13:00 h over 3 consecutive days to obtain enough
pooled urine for analysis and determination of metabolites, although
only pooled samples from the groups treated with 2-phenylphenol or its
sodium salt were examined.
No animals died before the end of the experiment, and food intake
was not significantly different between groups. The body weights at
week 8 were significantly lower in all treated groups of male rats and
in female rats given 2-phenylphenol or sodium 2-phenylphenol. The
urinary pH values for all groups were significantly different from the
7.0 found in untreated males and the 6.8 found in untreated females,
except in the groups given sodium 2-phenylphenol alone in which the pH
values were comparable to those of controls. The pH values were
highest in the groups given NaHCO3 alone, followed by the groups
given 2-phenylphenol plus NaHCO3, while the pH was lower than in
controls for groups given 2-phenylphenol alone, sodium 2-phenylphenol
plus NH4Cl, or NH4Cl alone. The Na+ concentrations were higher
in males than in females in all groups except controls and those given
NH4Cl. In the groups given sodium 2-phenylphenol alone, the Na+
concentration was slightly increased over control values in males but
not in females. The depressive effect of NH4Cl on Na+
concentration was also less pronounced in females. Only unconjugated
urinary metabolites were identified. No urothelial hyperplastic
changes were observed with 2-phenylphenol alone in either sex, while
an equimolar dose of sodium 2-phenyl-phenol induced mild papillary and
nodular hyperplasia or simple hyperplasia in male rats only. The
possible mechanisms underlying the differences in response between the
sexes might include excretion of other types of conjugated forms and
the formation of microcrystals such as the silicate crystals found in
the urine of rats fed sodium saccharin (Hasegawa et al., 1991).
The physiological effects of 2-phenylphenol (purity, 99.5%) on
urothelial cells and potential formation of DNA adducts were studied
in male Fischer 344 rats. In an initial experiment, rats were fed
dietary concentrations of 0, 1000, 4000, or 12 500 ppm for 13 weeks.
There was no evidence of urinary calculi, microcrystalluria, or
calcium phosphate-containing precipitate, but urothelial cytotoxicity
and hyperplasia were seen at the highest dose. In a second experiment,
rats were fed dietary concentrations of 0, 800, 4000, 8000, or 12 500
ppm for 13 weeks. The urinary pH was > 7 in all groups. The urinary
volume was increased at the highest dose, with consequent decreases in
osmolality and the concentrations of creatinine and other solutes. The
urinary excretion of total 2-phenylphenol metabolites was increased.
Most of the metabolites were conjugates of 2-phenylphenol and of
phenylhydroquinone, and free 2-phenylphenol and metabolites accounted
for < 2% at each dose. Urothelial toxicity and hyperplasia occurred
only at 8000 and 12 500 ppm. No 2-phenylphenol-DNA adducts were
detected in the urothelium at any dose. The small percentage of
unconjugated metabolites and the absence of DNA adducts suggest that
2-phenylphenol acts as a bladder carcinogen in male rats by inducing
cytotoxicity and hyperplasia without direct binding of the compound or
its metabolites to DNA (Smith et al., 1998).
The carcinogenic effect of sodium 2-phenylphenol and its
metabolites on female rat urinary bladder after intravesicular
instillation was studied in groups of nine 6-week-old Fischer 344 rats
that received 0.2 ml of a saline solution of 0.1% sodium
2-phenylphenol (purity, > 99%), phenylbenzoquinone (purity, > 99%),
or phenylhydroquinone (purity, > 99%) through a catheter into the
urethra once, twice, or four times. The pH values of the solutions
were 11 for sodium 2-phenylphenol, 6.5 for phenylbenzoquinone, and 6.4
for phenylhydroquinone. Saline or a solution of NaOH (pH 11) were
given to controls. The animals were maintained under light ether
anaesthesia during instillation and for a further 10 min thereafter to
prevent spontaneous urination. Two or three animals from each group
were killed under ether anaesthesia at 24 h and 4 and 7 days after the
last instillation. The histopathological findings in rats killed 24 h
after a single injection of saline, phenylbenzoquinone, or
phenylhydroquinone included swelling and vacuolation of urothelial
cells. The bladder epithelium of rats treated with alkaline solutions
of sodium 2-phenylphenol or NaOH showed minimal hyperplasia associated
with mild oedema and inflammatory-cell infiltration of the epithelial
and submucosal tissues. Moderate epithelial hyperplasia was seen in
rats killed 7 days after treatment with phenylbenzoquinone. In rats
given two or four instillations of this metabolite, the grading of the
hyperplastic changes was clearly dependent on the number of
instillations and the time between the last treatment and death. The
epithelial hyperplasia was marked and was classified as papillary
and/or nodular in rats treated with four instillations of
phenylbenzoquinone and killed 4 days after the last instillation. No
carcinomas were induced.
In another experiment, groups of 20 female rats were treated in
the same way but twice a week for 5 weeks. From week 6, some rats were
fed the basal diet supplemented with 5% sodium saccharin for 31 weeks
as a promotion treatment, while the other rats were maintained on
basal diet during this period. A separate group was given 500 ppm of
NBHBA for 4 weeks, and then 50 000 ppm of sodium saccharin as a
positive control. Body weights and food consumption were determined
periodically. The histopathological findings in the positive control
group included papillomas in two rats, papillary and/or nodular
hyperplasia in nine rats, and simple hyperplasia in 11 rats. In
contrast, no hyperplastic changes were seen in rats treated first with
sodium 2-phenylphenol or its metabolites followed by promotion with
sodium saccharin, except in nine rats given phenylbenzoquinone, which
had papillary and/or nodular hyperplasia and/or simple hyperplasia.
Formation of lymph follicles in the submucosa of the urinary bladder
was seen in particular with phenylbenzoquinone and in the positive
control group. The authors concluded that phenylbenzoquinone plays an
essential role in the urinary bladder carcinogenesis induced by sodium
2-phenylphenol (Hasegawa et al., 1990b).
A series of studies was carried out on the carcinogenicity of
2-phenylphenol and sodium 2-phenyl-phenol (purity of neither given) in
rat urinary bladder. In the first study, groups of 10 male Fischer 344
rats were fed diets containing sodium 2-phenylphenol at concentrations
of 0, 2500, 5000, 10 000, or 20 000 ppm for 36 weeks. The rats were
observed daily and were weighed periodically. Body-weight gain was
suppressed at 20 000 ppm. No calculi or mucosal tumours were found
grossly, but histological analysis revealed a statistically
significant increase in the frequency of bladder lesions in rats at
20 000 ppm, in which simple hyperplasia was seen in 10/10 and
papillary or nodular hyperplasia in 4/10 animals. Simple hyperplasia
was seen in 1/10 rats at 10 000 ppm.
In the second study, groups of five male rats were fed diets
containing 20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol for 4
weeks. The animals were weighed at the end of treatment, at which time
the mean body weights of both treated groups were reduced, to 86% of
the control value with 2-phenylphenol and to 96% with sodium
2-phenylphenol. One hour before sacrifice, each rat was given an
intraperitoneal injection of 100 mg/kg bw of 5-bromo-2'-deoxyuridine
(BrdU), and the urinary bladders were stained immunohistochemically
with anti-BrdU antibodies to investigate the capacity of the
epithelial cells for proliferation. The number of cells that had taken
up BrdU per 1000 urinary bladder epithelial cells was determined by
light microscopy and expressed as per cent labelled cells. Rats fed
sodium 2-phenylphenol showed increased urinary pH and extensive
BrdU-labelling in urinary bladder epithelial cells, indicating
increased DNA synthesis. Rats fed 2-phenylphenol also showed a
tendency to increased BrdU-labelling, suggested that it also can
cause, albeit weak, urinary bladder epithelium proliferation.
In the third study, groups of five male Fischer 344 rats were
given diets containing 6400 ppm of NaHCO3; 13 000 ppm of
2-phenylphenol; 13 000 ppm of 2-phenylphenol plus NaHCO3 at 1600,
3200, or 6400 ppm; or 20 000 ppm of sodium 2-phenylphenol for 8 weeks.
The urinary pH at week 8 was significantly increased in animals fed
NaHCO3 alone, 2-phenylphenol plus 3200 or 6400 ppm NaHCO3, or
sodium 2-phenylphenol. The urinary concentrations of Na+ were
significantly increased at 2, 4, 6, and 8 weeks in rats fed sodium
2-phenylphenol and at 8 weeks in rats fed NaHCO3 alone or
2-phenylphenol plus 3200 or 6400 ppm NaHCO3. At 8 weeks, a
significant increase or a tendency to an increase in urine volume and
significantly lower osmotic pressure were seen in pooled urine samples
from all treated groups when compared with controls. When the bladders
were examined by scanning electron microscopy, the surface of the
epithelium appeared normal in the controls and in animals given only
NaHCO3, and was made up of polygonal cells of uniform dimensions
with reticular peaked microridges at the surface. In treated animals,
the cells in the outermost layer of the bladder epithelium assumed a
cobblestone configuration in pavement form; at high magnification,
pleomorphic microvilli, short uniform microvilli, and ropy or leafy
microridges were seen at the surface of these cells. These alterations
were observed mainly in rats given sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. The extent of the changes and
the frequency of their appearance was correlated with the
concentration of NaHCO3 given with 2-phenylphenol.
In the fourth study, groups of 30-31 male Fischer 344 rats were
given 20 000 ppm of sodium 2-phenylphenol or 13 000 ppm of
2-phenylphenol with or without NaHCO3 at 1600, 3200, or 6400 ppm for
104 weeks. The animals were observed daily for deaths, and body weight
and feed consumption were measured at regular intervals. Pooled 4-h
urine samples were collected from three or four rats at each dose at
weeks 58, 80, and 96 for measurement of electrolytes. The urinary pH
was significantly increased in rats fed sodium 2-phenylphenol or
2-phenylphenol alone or in combination with 3200 or 6400 ppm NaHCO3
or 6400 ppm NaHCO3 alone. The Na+ concentrations were
significantly increased in rats fed sodium 2-phenylphenol,
2-phenylphenol plus 6400 ppm NaHCO3, or 6400 ppm NaHCO3 alone. No
difference was seen in rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. Urinary bladder tumours were
found in all groups except those given 2-phenylphenol alone, and the
frequencies were highest with sodium 2-phenylphenol and with
2-phenylphenol plus 6400 ppm NaHCO3. The presence of calculi could
not be confirmed. Histological examination of the urinary bladder
epithelium revealed simple hyperplasia, papillary or nodular
hyperplasia, papillomas, and carcinomas. Carcinomas occurred in rats
fed sodium 2-phenylphenol (41%), 2-phenylphenol plus 6400 ppm NaHCO3
(31%), and 6400 ppm NaHCO3 alone. The results confirm that
administration of 20 000 ppm of sodium 2-phenylphenol is carcinogenic
in male rats, while an equimolar concentration of 2-phenylphenol
causes only a low frequency of papillary or nodular hyperplasia and no
papillomas or cancers. Administration of NaHCO3 in conjunction with
2-phenylphenol caused carcinomas, correlated to the NaHCO3
concentration, which also increased urinary pH and Na+ concentration
(Inoue, 1993).
The induction of DNA damage in the urinary bladder epithelium of
male and female Fischer 344 rats by 2-phenylphenol and its metabolites
was studied by the alkaline elution assay after an intravesicalar
injection. Phenylbenzoquinone at 0.05-0.1% had weak DNA-damaging
activity in animals of each sex, whereas 2-phenylphenol and
phenylhydroquinone had no effect at the same dose. Histopathological
examination revealed diffuse, moderate, simple hyperplasia 5 days
after injection of 0.1% phenylbenzoquinone in male rats. The lesions
were associated with submucosal infiltration, small round cells, and
slight oedema. The only change in the bladders of rats injected with
0.1% phenylhydroquinone was slight swelling and/or vacuolization of
the epithelial cells, and the bladders of rats injected with 0.1%
2-phenylphenol were normal (Morimoto et al., 1989).
Groups of 5-10 Fischer 344 rats received diets containing sodium
2-phenylphenol at concentrations of 0, 2500, 5000, 10 000, or 20 000
ppm (equivalent to 0, 250, 500, 1000, and 2000 mg/kg bw per day) for
up to 5 months to investigate the correlation between urinary
phenylbenzoquinone and DNA damage in the bladder epithelium. Slight
but dose-dependent DNA damage was observed in the epithelium of male
rats fed 10 000 or 20 000 ppm for 3-5 months. A plot of the
dose-response relationship for DNA damage at 3 months showed a
threshold at 5000 ppm of sodium 2-phenylphenol. The amounts of
unconjugated 2-phenylphenol, phenylhydroquinone, and
phenylbenzoquinone in 24-h urine samples collected from males and
females after 5 months correlated well with the dietary concentrations
of sodium 2-phenylphenol. The total amounts of free metabolites in the
urine of males given 5000 ppm were similar to those in the urine of
females given 20 000 ppm. Free metabolites represented 0.3% of the
total average intake of male rats fed 5000 ppm, 0.8% of the intake of
10 000 ppm, and 1% of the intake of 20 000 ppm of sodium
2-phenylphenol. The average concentrations of free phenylhydroquinone
in the urine of males given 20 000 ppm of sodium 2-phenylphenol were
significantly higher than those in males fed 5000 ppm or in females
fed 20 000 ppm. The concentrations of phenylbenzoquinone were much
lower than those of phenylhydroquinone. Only 10% of phenylbenzoquinone
was recovered from spiked urine, indicating that this metabolite may
react with urinary nucleophilic groups. The authors concluded that
phenylbenzoquinone is the reactive species in the initiation of
bladder tumours induced by 2-phenylphenol and sodium 2-phenylphenol
(Morimoto et al., 1989).
The interaction of 2-phenylphenol and its metabolites with pUC18
DNA from Escherichia coli plasmids was studied in vitro. The major
metabolite formed from 2-phenylphenol by mixed-function oxidases was
phenylhydroquinone. This finding corroborates earlier reports that
phenylhydroquinone in the form of a glucuronide conjugate is the main
product in the bladders of rats fed 2-phenylphenol. When pUC18 DNA was
incubated with phenylhydroquinone, DNA strand scission was observed,
whereas barely detectable DNA cleavage was seen with 2-phenylphenol
and phenylbenzoquinone. DNA cleavage by phenylhydroquinone was
inhibited by superoxide dismutase, catalase, and several oxygen
radical scavengers, indicating that the oxygen radicals generated in
the process of oxidation of phenylhydroquinone in aqueous solution are
responsible for the DNA cleavage. The attack seemed to occur at
guanine residues in general and was not restricted to guanines with
specific residues, indicating no hot spots (Nagai et al., 1990).
The generation of 8-hydroxydeoxyguanosine in calf thymus DNA
treated with 2-phenylphenol, phenylhydroquinone, or
phenylbenzoquinone, was studied in vitro. The content of
8-hydroxydeoxy-guanosine residues was increased in DNA treated with
phenylhydroquinone in a concentration-dependent manner, but
phenylbenzoquinone had little effect, and 2-phenylphenol had no
effect. The formation of 8-hydroxydeoxyguanosine by phenylhydroquinone
was reduced by oxygen radical scavengers and accelerated by the
addition of CuCl or CuCl2. Hydroxyl radicals generated during
oxidation of phenylhydroquinone thus contribute to the formation of
8-hydroxydeoxyguanosine in DNA, and copper ions facilitate the
oxidative DNA damage. Copper ions greatly accelerated
phenylhydroquinone-induced DNA cleavage in vitro, although they had
no effect on cleavage without phenylhydroquinone. In contrast, DNA
cleavage occurred with the addition of FeCl2 in the absence and
presence of phenylhydroquinone. The formation of
8-hydroxydeoxyguanosine in bladder DNA is likely to be one of a series
of events in the carcinogenesis induced by 2-phenylphenol (Nagai et
al., 1995).
The effect of the selective gamma-glutamylcysteine synthetase
inhibitor, buthionine sulfoximine, on the hepatotoxic and nephrotoxic
potential of 2-phenylphenol and its metabolites was studied in groups
of four male Fischer 344/DuCrj rats. The animals were given an
intraperitoneal injection of 0 or 900 mg/kg bw of buthionine
sulfoximine and 1 h later received 2-phenylphenol, phenylhydroquinone,
or phenylbenzoquinone at single oral doses of 0, 700, or 1400 mg/kg
bw. The rats were killed 6 and 24 h later, and serum was collected for
measurement of alanine and aspartate aminotransferase activities and
urea nitrogen. The liver and kidneys were removed and weighed, and
hepatic and renal glutathione were assayed.
2-Phenylphenol caused acute hepatocellular damage, as shown by
necrotic centrilobular hepatocytes accompanied by increased serum
aminotransferase activity. Pretreatment with buthionine sulfoximine
potentiated the hepatic and renal toxicity of 2-phenylphenol,
indicating that the liver and kidneys are its target organs of at high
doses. 2-Phenylphenol depleted hepatic and renal glutathione by 6 h
after administration, and this effect was enhanced by pretreatment
with buthionine sulfoximine. Recovery of glutathione concentrations in
both organs was slower in rats given 1400 mg/kg bw of 2-phenylphenol
than in those given 700 mg/kg bw, suggesting that the hepatic and
renal damage caused by this compound is associated with prolonged
depletion of glutathione and that it acts indirectly on the liver.
Within 24 h, 75% of the rats treated with phenylbenzoquinone at 1400
mg/kg bw had died. Administration of phenylbenzoquinone at 700 m/kg bw
or phenylhydroquinone at 1400 mg/kg bw significantly increased
aminotransferase activities. The activity of alanine aminotransferase
in both groups was about twice that of rats given 1400 mg/kg bw
2-phenylphenol. A slight decrease in liver weight, nuclear pyknosis,
eosinophilic degeneration of periportal hepatocytes, increased
relative kidney weight, slight renal papillary necrosis, dilatation of
renal tubules, and increased serum urea nitrogen concentration were
observed at 700 mg/kg bw of phenylbenzoquinone. The relative weight of
the kidneys was increased at 1400 mg/kg bw of phenylhydroquinone.
These results indicate that phenylbenzoquinone is more toxic to liver
and kidney than phenylhydroquinone (Nakagawa & Tayama, 1988).
In a study of the conjugation of 2-phenylphenol with glutathione
in rat liver in vitro and in vivo, radiolabel derived from
[14C]2-phenylphenol bound irreversibly to hepatic microsomal
macromolecules in an NADPH-generating system, and the binding was
inhibited by cysteine and glutathione. When [14C]2-phenylphenol and
glutathione were incubated in a microsomal NADPH-generating system,
the radiolabelled material derived from the aqueous phase of the
incubation mixture was similar to a synthetic, water-soluble
phenylhydroquinone-glutathione conjugate produced by a nonenzymic
reaction between phenylbenzoquinone and glutathione.
Phenylhydroquinone-glutathione was excreted as a minor conjugate in
the bile after oral administration of 2-phenylphenol to rats at a dose
of 1000 mg/kg bw. The cumulative biliary excretion of the conjugate
over 6 h represented about 4% of the dose. The results show that a
reactive intermediate of 2-phenylphenol can form adducts with
glutathione to produce water-soluble conjugates. The reactive
intermediate is probably phenylbenzoquinone derived from
phenylhydroquinone. Since glutathione protects against cellular
injury, the acute hepatic damage caused by high doses of
2-phenylphenol is probably associated with the formation of an active
intermediate (phenylbenzoquinone) which depletes cellular glutathione
(Nakagawa & Tayama, 1989).
The relationship between the metabolism and cytotoxicity of
2-phenylphenol was studied in isolated rat hepatocytes. Addition of
high concentrations of 2-phenylphenol to the cells caused
dose-dependent toxicity, with death at the highest dose of 1.0 mmol/L.
Pretreatment of the hepatocytes with a non-toxic dose of 5 µmol/L of
SKF-525A enhanced the cytotoxicity of 2-phenylphenol at 0.5-1.0 mmol/L
and inhibited its metabolism. At lower concentrations (0.5 or 0.75
mmol/L), 2-phenylphenol was converted sequentially to
phenylhydroquinone and then to its glutathione conjugate. The
concentrations of both metabolites and especially of the conjugate,
were very low in hepatocytes exposed to 2-phenylphenol at 1.0 mmol/L
alone or with SKF-525A. The cytotoxicity induced by 2-phenylphenol at
0.5 mmol/L was enhanced by the addition of 1.25 mmol/L of
diethylmaleate, which continuously depletes cellular glutathione. In
contrast, the cytotoxicity induced by phenylhydroquinone at 0.5 mmol/L
was significantly inhibited by addition to the hepatocytes of 5 mmol/L
of dithiothreitol, cysteine, N-acetyl-L-cysteine, or ascorbic acid.
Loss of glutathione, protein thiols, and ATP was also prevented. These
results indicate that the acute cytotoxicity of 2-phenylphenol at 1.0
mmol/L is a direct action and that prolonged depletion of cellular
glutathione enhances the cytotoxicity of low concentrations of
2-phenylphenol metabolites. The cytotoxicity of phenylhydroquinone is
prevented significantly by addition of cysteine, glutathione, or
ascorbic acid (Nakagawa et al., 1992).
Groups of 22 male CDF (Fischer 344)/BR rats were given diets
containing 2phenylphenol (purity, 99.5%) to provide concentrations of
0, 800, 4000, 8000, or 12 500 ppm, equal to 0, 56, 280, 560, and 920
mg/kg bw per day, for 13 weeks. During weeks 12-13 and 13-14 of the
study, urine was collected for determination of metabolites and
urinary characteristics, respectively. In addition, urinary bladders
were collected from 12 animals per group during week 14 for analysisof
the urothelium by 32P-postlabelling, while histopathological
evaluation of 10 animals group included determination of a labelling
index and light and scanning electron microscopy. The body-weight gain
was reduced by about 10% at 8000 and 12 500 ppm, but food intake was
unaffected at all doses tested. Histological examination showed simple
hyperplasia of the urothelium at concentrations of 8000 and 12 500 ppm
with significant changes in the bladder. The glucuronide and sulfate
conjugates of 2-phenylphenol and the hydroxylated metabolite
phenylhydroquinone were the major urinary metabolites, although the
major conjugate at all doses was the sulfate. Minute levels of free
2phenylphenol and phenylhydroquinone were found at all doses, free
phenylhydroquinone comprising 0.6-1.5% of the total metabolites
measured. An increase in the labelling index of the bladder epithelium
was observed at 8000 and 12 500 ppm. 32P-Postlabelled urothelial DNA
showed no evidence of formation of 2-phenylphenol-DNA adducts.
The authors concluded that a hyperplastic response of the urinary
bladder epithelium occurs after exposure to 2-phenylphenol at 8000 or
12 500 ppm, which are unequivocal carcinogenic doses for the bladder
of male rats, which is due to mild cytotoxicity with consequent
regenerative hyperplasia. The increased mitotic activity (labelling
index), the presence of very small amounts of free phenylhydroquinone
in the urine, and the absence of DNA adducts in the bladder epithelium
further suggest that the bladder carcinogenesis in male rats exposed
to 2-phenylphenol is probably mediated by an indirect, dose-dependent
cytotoxic effect on the bladder epithelium leading to regenerative
hyperplasia and subsequent tumorigenesis of epigenetic origin, rather
than to direct metabolic activation of 2-phenylphenol to reactive
metabolites capable of forming 2-phenylphenol-DNA adducts (Christenson
et al., 1996a).
Groups of 20-30 male CDF (Fischer344)/BR rats were given diets
containing 2-phenylphenol (purity, 99.9%) at concentrations of 0,
1000, 4000, or 12 500 ppm, equal to 0, 54, 220, and 680 mg/kg bw per
day, for 13 weeks. Animals from the control and high-dose groups were
allowed to recover for 4 weeks. Urine was collected for chemical and
and electron microscopic evaluation at various times, and urinary
bladders were collected from animals in the recovery groups during
weeks 4, 13, and 17 for histological evaluations which included
determination of a labelling indexes and light and electron
microscopy.
Body-weight gain was reduced only in rats at 12 500 ppm, and food
intake was unaffected at all doses. Weekly clinical examinations
showed an increased incidence of urine staining at 4000 and 12 500
ppm. No unusual precipitate or crystal was found in the urinary
sediment of treated animals. Urothelial hyperplasia was observed only
after 13 weeks at 12 500 ppm, and the effect was reversed by 4 weeks
on control diet. After 4 and 13 weeks of exposure to 2-phenylphenol,
necrotic foci were observed in the bladders of rats at 12 500 ppm, and
at 13 weeks the bladders also showed evidence of regenerative
hyperplasia. Increased labelling indexes were observed in the bladders
of animals at the high dose at 4 and 13 weeks, but the index had
returned to control values after 4 weeks' recovery, confirming the
reversibility of the proliferative changes in the urothelium. The
results of this study suggest that 2-phenylphenol acts by a mechanism
involving a cytotoxic action on the urothelium leading to the
formation of regenerative, reversible hyperplasia. The origin of the
cytotoxicity remains unclear, however, as no evidence was found of
either abnormal crystalluria or a calcium phosphate-containing
amorphous precipitate (Christenson et al. 1996b).
The relative importance of bladder distension, urinary pH, and
Na+ concentration in the induction of cell proliferation in the
bladder epithelium of rats fed various sodium salts was investigated.
In male rats fed a diet containing 5% NaHCO3, the bladder epithelium
showed an increased number of replicating cells, distension, increased
urinary pH, and a high urinary Na+ concentration. Cell proliferation
also occurred when the bladders were subjected to distension in
vivo by mechanical (female) or physiological (male) means.
Inclusion of CaCO3 in the diet increased the urinary pH without
altering other factors and did not induce cell proliferation, but
proliferation was increased when CaCO3 was combined with the
mechanical or physiological treatment. Thus, high urinary pH was of
secondary importance to bladder distension as a causative factor but
acted to enhance cell proliferation when distension occurred. Similar
findings were obtained with regard to the Na+ concentration. The
authors concluded that bladder distension is a prerequisite for
proliferation of epithelial cells in the bladders of rats fed diets
containing high concentrations of sodium salts and that changes in
urinary pH and Na+ concentration also determine the degree of
proliferation (Shioya et al., 1994).
3. Observations in humans
In one of the earliest studies on the toxicity of 2-phenylphenol,
skin irritation and sensitization due to exposure to this compound and
its sodium salt were evaluated in 100 male and 100 female, unselected
persons. A patch impregnated with the test material was placed in
direct contact with the skin of the back of each person, covered with
an impervious film, and taped securely in place. The first patch was
kept in constant contact with the skin for 5 days, at which time the
patch was removed and the reaction noted. A second patch was applied
in the same way 3 weeks after removal of the first patch and was kept
in direct contact with the skin for 48 h. Each subject was examined
immediately and again 3 and 8 days after removal of the second patch.
2-Phenylphenol as a 5.0% solution in sesame oil did not cause primary
irritation or sensitization, but sodium 2-phenylphenol was
significantly irritating when applied as a 5% or a 1% aqueous
solution. A 0.5% solution caused very slight, simple irritation,
whereas a 0.1% solution produced no irritation and no sensitization
(Hodge et al., 1952).
Comments
After oral administration to mice and rats, 2-phenylphenol and
its sodium salt are rapidly and extensively absorbed (95%) and
distributed. Excretion is also rapid in these species, being almost
complete within 48 h, and occurs mainly in urine (about 90%) and in
faeces (about 5%). Little radiolabel (< 1%) is retained in organs and
tissues, including the urinary bladder. After dermal application of
2-phenylphenol to humans, about 43% of the applied dose was absorbed
through the skin and about 58% was recovered in skin rinse and the
protective enclosure. Most of the absorbed radiolabel was recovered in
urine (99%, and only 1% was recovered in faeces. The absorption
half-time was 10 h, and the elimination half-time was 0.8 h. The rapid
excretion of the radiolabel into urine indicates that 2-phenylphenol
is unlikely to accumulate in humans exposed repeatedly. The metabolic
profiles of both compounds were similar in mice, rats, and humans at
the various doses tested. The main metabolic pathways are conjugation
of 2-phenylphenol or hydroxylation at the 5 position of the phenol
ring, followed by conjugation with glucuronide or sulfate. The parent
compound was detected in only very small amounts (0.4%) in urine. The
metabolic profile in plants raised no toxicological concern, since
about 90% of the residue found in oranges and pears is 2-phenylphenol
or its conjugates.
2-Phenylphenol and its sodium salt have low acute toxicity in
mice and rats treated orally, the LD50 values ranging from 600 to
3500 mg/kg bw. Neither 2-phenylphenol nor its sodium salt has been
classified by WHO for acute toxicity.
2-Phenylphenol and its sodium salt caused severe dermal
irritation in rabbits, and the sodium salt caused severe dermal
irritation in humans. 2-Phenylphenol irritated the eye of rabbits,
whereas the sodium salt caused only moderate ocular irritation.
Neither substance caused delayed contact hypersensitivity in
guinea-pigs or humans.
In medium- and long-term tests for toxicity, the urinary bladder
was regarded as the main toxicological target organ of both
2-phenylphenol and its sodium salt in male and female rats. At doses
of 200 mg/kg bw per day and above, hyperplasia, papillomas, and
transitional-cell carcinomas were seen with both compounds in male
rats. Increased mitosis was observed in the bladder epithelium three
days after the start of dosing, and thickening, i.e. simple
hyperplasia, was seen at 14 days. In female rats, hyperplasia and
papillomas were observed, but to a far lower degree than in males. In
male and female mice, the liver was the primary target organ.
Increased relative liver weights and an increased incidence of
hepatocellular adenomas were seen with 2-phenylphenol at doses of 500
mg/kg bw per day and above. Reduced body-weight gain was a common
finding in mice and rats. In 90-day studies, the NOAELs for
2-phenylphenol were 6300 ppm, equal to 760 mg/kg bw per day, in rats
and 300 mg/kg bw per day (the highest dose tested for up to 1 year) in
dogs. The NOAEL for the sodium salt was 5000 ppm, equivalent to 550
mg/kg bw per day, in mice and 2500 ppm, equal to 180 mg/kg bw per day,
in rats. In a 1-year study of toxicity, the NOAEL for 2-phenylphenol
was 800 ppm, equal to 39 mg/kg bw per day, in rats. In 2-year studies
of carcinogenicity, the NOAEL for 2-phenylphenol was 250 mg/kg bw per
day in mice and 800 ppm, equal to 39 mg/kg bw per day, in rats. In
2-year carcinogenicity studies with the sodium salt, the NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, in
mice and 2500 ppm, equivalent to 95 mg/kg bw per day, in rats. The
Meeting concluded that both 2-phenylphenol and its sodium salt are
carcinogenic in male rats and that 2-phenylphenol is carcinogenic in
male mice.
2-Phenylphenol has been more extensively tested for genotoxic
activity than its sodium salt. Within that limitation, the results for
the two compounds were similar. Data regarding covalent binding to DNA
in the urinary bladder of rats dosed with either compound were
conflicting. 2-Phenylphenol induced chromosomal aberrations in
cultured mammalian cells, but negative results were obtained in
vivo. The Meeting concluded that there are unresolved questions
about the genotoxic potential of 2-phenylphenol.
Several studies have been conducted to elucidate the mechanism of
the carcinogenic action of 2-phenylphenol and its sodium salt on the
male rat urinary bladder, since neither compound has a carcinogenic
effect on the urinary bladder of female rats or in mice, guinea-pigs,
or hamsters of either sex. No clear mechanisms have been found,
although raising the urinary pH or sodium concentration has a
promoting effect. There was some evidence from studies with the sodium
salt that initial irritation followed by hyperplasia might be involved
in the bladder carcinogenicity in male rats. In addition,
32P-postlabelling showed binding of 2-phenylphenol and its sodium
salt to DNA in the male rat urinary bladder in some but not in other
studies. The genotoxicity of the metabolites phenylhydroquinone and
dihydroxybiphenyl appears to be similar to that of the parent
molecules.
The Meeting concluded that the urinary bladder tumours observed
in male rats and the liver tumours observed in male mice exposed to
2-phenylphenol are threshold phenomena that are species- and
sex-specific, and that 2-phenylphenol is therefore unlikely to
represent a carcinogenic risk to humans. In coming to this conclusion,
the Meeting was aware that a working group convened by IARC had
classified 2-phenylphenol, sodium salt, in Group 2B (possibly
carcinogenic to humans) and 2-phenylphenol in Group 3 (not
classifiable as to its carcinogenicity to humans). The Meeting noted,
however, that the IARC classification is based on hazard
identification, not on risk assessment, and is furthermore limited to
published literature, with the exclusion of unpublished studies on
toxicity and carcinogenicity.
In two two-generation studies of reproductive toxicity in rats,
2-phenylphenol had no reproductive toxicity, even at 460 mg/kg bw per
day, the highest dose tested. The overall NOAEL for carcinogenicity
was 92 mg/kg bw per day, since urinary bladder tumours were found in
male rats at doses of 120 mg/bw per day and above.
In a study of developmental toxicity in mice with 2-phenylphenol
and its sodium salt, the NOAELs for 2-phenylphenol were below 1500
mg/kg bw per day (lowest dose tested) for maternal toxicity and
fetotoxicity and 2100 mg/kg bw per day (highest dose tested) for
teratogenicity. The NOAELs for the sodium salt were below 100 mg/kg bw
per day (lowest dose tested) for maternal toxicity, 100 mg/kg bw per
day for fetotoxicity, and 400 mg/kg bw per day (highest dose tested)
for teratogenicity. In two studies of developmental toxicity in rats,
the overall NOAELs for 2-phenylphenol were 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 700
mg/kg bw per day (highest dose tested) for teratogenicity. In two
studies of developmental toxicity in rabbits, the overall NOAELs for
2-phenylphenol were 100 mg/kg bw per day for maternal toxicity, 500
mg/kg bw per day for fetotoxicity, and 750 mg/kg bw per day (highest
dose tested) for teratogenicity.
The Meeting established an ADI of 0-0.4 mg/kg bw for
2-phenylphenol, on the basis of the NOAEL of 39 mg/kg per day in the
2-year study of toxicity (based on decreased body-weight gain and
hyperplasia of the urinary bladder) and carcinogenicity of the urinary
bladder in male rats and a safety factor of 100.
The Meeting determined that it was unnecessary to establish an
acute reference dose because of the low acute toxicity of
2-phenylphenol.
Toxicological Evaluation
Levels of 2-phenylphenol that cause no toxic effect
Mouse: < 250 mg/kg bw per day for carcinogenicity (lowest dose
tested; 2-year study of toxicity and carcinogenicity)
< 1500 mg/kg bw per day (lowest dose tested; study of
developmental toxicity; maternal toxicity)
2100 mg/kg bw per day (highest dose tested; study of
developmental toxicity; not teratogenic)
Rat: 800 ppm, equal to 39 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
460 mg/kg bw per day (two-generation study of reproductive
toxicity; no reproductive toxicity; highest dose tested)
92 mg/kg bw per day (two-generation study of reproductive
toxicity; carcinogenicity)
150 mg/kg bw per day (study of developmental toxicity;
maternal toxicity)
300 mg/kg bw per day (study of developmental toxicity;
developmental toxicity)
700 mg/kg bw per day (study of developmental toxicity;
teratogenicity)
Rabbit: 100 mg/kg bw per day (two studies of developmental toxicity;
maternal toxicity)
500 mg/kg bw per day (two studies of developmental toxicity;
fetotoxicity)
750 mg/kg bw per day (two studies of developmental toxicity;
teratogenicity)
Dog: 750 mg/bw per day (highest dose tested; 1-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.4 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Mechanistic studies on urinary bladder tumours in male rats
2. Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to
2-phenylphenol (unless otherwise specified)
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Rapid (24 h) and complete (95-100%), mice and rats
Dermal absorption Rapid and well absorbed (43%), humans
Distribution Small concentrations (< 1%) in tissues, mice and rats
Potential for accumulation No accumulation, mice, rats, and humans
Rate and extent of excretion Rapid and complete (95-100%), mice, rats, and humans
Metabolism in animals Glucuronide and sulfate of 2-phenylphenol and phenylhydroquinone,
mice and rats
Toxicologically significant compounds 2-Phenylphenol
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 5000 mg/kg bw
Rabbit, LC50, inhalation (4 h) > 36 mg/m3 air (aerosol)
Dermal irritation 2-Phenylphenol and its sodium salt: severe dermal irritation,
rabbits
Ocular irritation 2-Phenylphenol: occular irritation, rabbits
Sodium salt: slight ocular irritation, rabbits
Dermal sensitization 2-Phenylphenol and its sodium salt: no dermal sensitization,
guinea-pigs and humans
Short-term toxicity
Target/critical effect Body-weight decrease, mice and rats, and urinary bladder tumours,
male rats
Lowest relevant oral NOAEL 300 mg/kg bw per day, dogs
Lowest relevant dermal NOAEL No NOAEL, 1000 mg/kg bw per day, highest dose tested, rats
Lowest relevant inhalation NOAEL Not investigated
Genotoxicity Unresolved questions
Long-term toxicity and carcinogenicity
Target/critical effect Urinary bladder, male rats
Liver, male and female mice
Lowest relevant NOAEL 39 mg/kg bw per day, male rats
Carcinogenicity Urinary bladder tumours, male rats
Liver tumours, male and female mice
Reproductive toxicity
Reproductive target/critical effect No reproductive toxicity, rats
Lowest relevant reproductive NOAEL 460 mg/kg bw per day, highest dose tested, rats
Developmental target/critical effect Developmental toxicity at maternally toxic doses, mice
Lowest relevant developmental NOAEL 300 mg/kg bw per day, rabbits
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rats.
No evidence of neurotoxicity or neuropathology in medium- and
long-term studies, mice, rats, dogs, or in developmental toxicity
studies, mice, rats, and rabbits
Other toxicological studies
Medical data Dermal irritation with the sodium salt, not with 2-phenylphenol
Summary Value Study Safety factor
ADI 0-0.4 mg/kg bw Long-term study of toxicity 100
and carcinogenicity, rat
Acute reference dose Unnecessary
References
Bartels, M.J. & McNett, D.A. (1996) Quantitation of orthophenylphenol
metabolites in rat urine samples from a 32P-postlabeling study.
Unpublished report No. HET K-001024-062 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Bartels, M.J., Brzak, K.A., McNett, D.A. & Shabrang, S.N. (1997)
Ortho-phenylphenol (OPP): Limited metabolism study in human.
Unpublished report No. HET K-001024-059 from Dow Chemical
Company, Midland, Michigan, USA. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Bartels, M.J., McNett, D.A., Timchalk, C., Mendrala, A.L.,
Christenson, W.R., Sangha, G.K., Brzak, K.A. & Shabrang, S.N.
(1998) Comparative metabolism of orthophenylphenol in mouse,
rat and man. Xenobiotica, 28, 579-594.
Berdasco, N.M. (1991) Orthophenylphenol: Dermal sensitization
potential in the Hartley albino guinea pig. Unpublished report
No. K-001024-048 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Carreon, R.E. & New M.A. (1981) Dowicide*1: Acute percutaneous
absorption potential. Unpublished report No. HET K-1024-(37) from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S., Bartels, M.J. & Cohen, S.M. (1996a)
Technical grade orthophenylphenol: A 32P-postlabeling study
to examine the potential for the formation of DNA adducts in the
urinary bladder of the male rat. Unpublished report No. 94-972-AV
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S. & Cohen, S.M. (1996b) Technical grade
ortho-phenylphenol: A special subchronic dietary study to
examine the mechanism of urinary bladder carcinogenesis in the
male rat. Unpublished report No. 92-972-MS from Bayer
Corporation, Agriculture Division, Toxicology, Stilwell, Kansas,
and University of Nebraska Medical Center, Department of
Pathology & Micropathology, Omaha, Nebraska, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Cosse, P.F., Stebbins, K.E., Stott, W.T., Johnson, K.A. & Atkin, L.
(1990) Orthophenylphenol: Palatability/probe, four-week and
one-year oral toxicity studies in beagle dogs. Unpublished report
No. K-001024-039 from Dow Chemical Co., Midland, Michigan , USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Davies, R.E. & Liggett, M.P. (1973) Irritant effects of agricultural
disinfectant C2 on rabbit eye mucosa. Unpublished report No.
2215/169D/73 from Huntingdon Research Centre, England. Submitted
to WHO by Leng Associates, Midland, Michigan, USA,
Eigenberg, D.A. (1990) Two-generation dietary reproduction study in
rats using orthophenylphenol. Unpublished revised report No
85-671-02 from Mobay Corporation Health, Environment, Safety &
Plant Management, Corporate Toxicology Department, South Metcalf,
Stilwell, Kansas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Eigenberg, D.A. (1995) A two-generation dietary reproduction study in
Sprague Dawley rats using technical grade ortho-phenylphenol.
Unpublished report No. 93-672-VX from Bayer Corporation
Agriculture Division, Toxicology, South Metcalf, Stilwell,
Kansas, USA. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Fujii, T. & Hiraga, K. (1985) Carcinogenicity testing of sodium
orthophenylphenate in F344 rats. J. Saitana Med. School, 12,
277-287.
Fujii, T., Nakamura, K. & Hiraga, K. (1987) Effects of pH on the
carcinogenicity of ophenylphenol and sodium o-phenylphenate
in the rat urinary bladder. Food Chem. Toxicol., 25, 359-362.
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. (1983)
Promoting effect of sodium o-phenylphenate and o-phenylphenol
on two-stage urinary bladder carcinogenesis in rats. Jpn. J.
Cancer Res. (Gann), 74, 625-632.
Fukushima, S., Inoue, T., Uwagawa, S., Shibata, M. & Ito N. (1989)
Co-carcinogenic effects of NaHCO3 on o-phenylphenol-induced
rat bladder carcinogenesis. Carcinogenesis, 10, 1635-1640.
Gilbert, K.S. (1994a) Dowicide(R) 1 antimicrobial: Primary dermal
irritation study in New Zealand white rabbits. Unpublished report
No. K-001024-057B from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert, K.S. (1994b) Dowicide(R) 1 antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001024-57E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. (1994c) Dowicide(R) A antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001025-014E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. & Crissman, J.W. (1994) Dowicide(R) 1 antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
no. K-001024-057A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert K.S. & Stebbins, K. (1994) Dowicide(R) A antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
No. K-001025-014A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Hagiwara, A., Shibata, M., Hirose, M., Fukushima, S. & Ito, N. (1984)
Long-term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Food Chem. Toxicol., 22,
809-814.
Hasegawa, R., Takahashi, S., Asamoto, M., Shirai, T. & Fukushima, S.
(1990a) Species differences in sodium o-phenylphenate induction
of urinary bladder lesions. Cancer Lett., 50, 87-91.
Hasegawa, R., Furukawa, F., Toyoda K., Sato, H., Takahashi, M. &
Hayashi, Y. (1990b) Urothelial damage and tumor initiation by
urinary metabolites of sodium o-phenylphenate in the urinary
bladder of female rats. Jpn. J. Cancer Res., 81, 483-488.
Hasegawa, R., Fukuoka, M., Takahashi, T., Yamamoto, A., Yamaguchi, S.,
Shibata, M.A., Tanaka, A. & Fukushima, S. (1991) Sex differences
in o-phenylphenol and sodium ophenylphenate rat urinary
bladder carcinogenesis: Urinary metabolites and electrolytes
under conditions of aciduria and alkalinuria. Jpn. J. Cancer
Res., 82, 657-664.
Hiraga, K. (1983a) Short report of a 91-week feeding study of
orthophenylphenol (OPP) in male rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. (1983b) Carcinogenicity testing of sodium
orthophenylphenate in F-344/DuCrj rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. & Fujii, T. (1981) Induction of tumours of the urinary
system in F344 rats by dietary administration of sodium
o-phenylphenate. Food Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. (1984) Induction of tumours of the urinary
bladder in F344 rats by dietary administration of o-phenylphenol.
Food Cosmet. Toxicol., 22, 865-870.
Hodge, H.C., Maynard, E.A., Blanchet, H.J., Jr, Spencer, H.C. & Rowe,
V.K. (1952) Toxicological studies of orthophenylphenol (Dowicide
1). J. Pharmacol. Exp. Ther., 104, 202-210.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T. & Sugimura, T. (1983)
Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
Horvath, E., Levay, G. Pongracz, K. & Bodell, W.J. (1992) Peroxidative
activation of ophenylhydroquinone leads to the formation of DNA
adducts in HL-60 cells. Carcinogenesis, 13, 1937-1939.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to
42, Lyon, International Agency for Research on Cancer, pp.
37-41.
IARC (1999) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 73, Miscellaneous
Pesticides, Lyon, International Agency for Research on Cancer,
pp. 451-480.
Iguchi, S., Takahashi, H., Fujii, T., Fukumori, N., Mikuriya, H.,
Tada, Y., Yuzawa, K. & Hiraga, K. (1984) Subchronic toxicity of
o-phenylphenol (OPP) by food administration to rats. Ann.
Rep. Tokyo Metr. Res. Lab. Public Health, 35, 407-415.
Iguchi, S., Tayama, K. & Hiraga, K. (1979) Subacute toxicity of sodium
o-phenylphenate by food administration to rats. Ann. Rep.
Tokyo Metropolitan Res. Lab. Public Health, 30, 67-79.
Inoue, T. (1993) Pathological studies on rat urinary bladder
carcinogenicity of ophenylphenol and its sodium salt. J.
Nagoya City Univ. Medical Assoc. (Nagoya Shiritsu Daigaku
Igakkai Zasshi), 44, 463-477.
Inoue, S., Yamamoto, K. & Kawanishi, S. (1990) DNA damage induced by
metabolites of ophenylphenol in the presence of copper (II)
ion. Chem. Res. Toxicol., 3, 144-149.
Ishidate, M., Jr, Yoshikawa, K. & Sofuni, T. (1983) Mutagenicity tests
on OPP, OPPsodium salt, and their possible metabolites.
Unpublished report from Division of Mutagenesis, Biological
Safety Research Center, National Institute of Hygienic Sciences,
Tokyo, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983a) Long-term toxicity and carcinogenicity study of sodium
o-phenyl-phenate in B6C3F1 mice. Unpublished report from First
Department of Pathology, Nagoya City University Medical School,
Nagoya, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983b) Pathological analysis of the carcinogenicity of sodium
o-phenyl-phenate and o-phenylphenol. Unpublished report from
First Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz B.A. (1981)
Teratological evaluation of orthophenylphenol in rats. Fundam.
Appl. Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. (1978)
Teratogenicity and dominantlethal studies with o-phenylphenol.
J. Pestic. Sci., 3, 365-370.
Kolachana, P., Subrahmanyam, V.V., Eastmond, D.A. & Smith, M.T. (1991)
Metabolism of phenyhydroquinone by prostaglandin (H) synthase:
Possible implications in ophenylphenol carcinogenesis.
Carcinogenesis, 12, 145-149.
Kwok, E.S.C. & Eastmond, D.A. (1997) Effects of pH on nonenzymatic
oxidation of phenylhydroquinone: Potential role in urinary
bladder carcinogenesis induced by ophenylphenol in Fischer 344
rats. Chem. Res. Toxicol., 10, 742-749.
Lambert, A.C. & Eastmond, D.A. (1994) Genotoxic effects of the
o-phenylphenol metabolites phenylhydroquinone and
phenylbenzoquinone in V79 cells. Mutat. Res., 322, 243-256.
Landry, T.D., Stebbins, K.E. & Battjes, J.E. (1992)
Ortho-phenylphenol: Acute aerosol inhalation toxicity study in
Fischer 344 rats. Unpublished report No. K-001024-049 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Luster, M. (1986) NTP Technical Report on the Toxicology and
Carcinogenesis Studies of ortho-Phenylphenol (CAS No. 90-43-7)
Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No.
57-97-6) in Swiss CD-1 Mice (Dermal Studies) (NIH publication
No. 86-2557, NTP TR 301), US Department of Health and Human
Services, National Toxicology Program, Research Triangle Park,
North Carolina, USA.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A. & Wilson, R.E. (1981) The effects of
orthophenylphenol, tris(2,3dichloropropyl)phosphate and
cyclophosphamide on the immune system and host susceptibility of
mice following subchronic exposure. Toxicol. Appl. Pharmacol.,
58, 252-261.
McNett, D.A., Timchalk, C. & Mendrala, A.L. (1997) Ortho-phenylphenol
(OPP): Metabolism of 14C-labelled OPP in B6C3F1 mice and
Fischer 344 rats. Unpublished report No. HET K-001024-060 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Morimoto, K., Sato, M., Fukuoka, M., Hasegawa, R., Takahashi, T.,
Tsuchiya, T., Tanaka, A., Takahashi, A. & Hayashi, Y. (1989)
Correlation between the DNA damage in urinary bladder epithelium
and the urinary 2-phenyl-1,4-benzoquinone levels from F344 rats
fed sodium o-phenylphenate in the diet. Carcinogenesis, 10,
1823-1827.
Nagai, F. & Nakao, T. (1984) Changes in enzyme activities in the urine
and tissues of rats fed sodium o-phenylphenate. Food Chem.
Toxicol., 22, 361-364.
Nagai, F., Ushiyama, K., Satho, K. & Kano, I. (1990) DNA cleavage by
phenylhydroquinone: The major metabolite of a fungicide
o-phenylphenhol. Chem.-Biol. Interactions, 76, 163-179.
Nagai, F., Ushiyama, K., Satoh, K., Kasai, H. & Kano, I. (1995)
Formation of 8hydroxyguanosine in calf thymus DNA treated
in vitro with phenylhydroquinone, the major metabolite of
o-phenylphenol. Carcinogenesis, 16, 837-840.
Nakagawa, Y. & Tayama, K. (1988) Effect of buthionine sulfoximine on
orthophenylphenolinduced hepato- and nephrotoxic potential in
male rats. Arch. Toxicol., 62, 452-457.
Nakagawa, Y. & Tayama, S. (1989) Formation of ortho-phenylphenol
glutathione conjugates in the rat liver. Xenobiotica, 19,
499-507.
Nakagawa, A., Nakao, T. & Nakao, M. (1984) Altered levels of cyclic
nucleotides in F344 rats fed sodium o-phenylphenate. Food
Chem. Toxicol., 22, 217-221.
Nakagawa Y., Tayama, S., Moore, G.A. & Moldéus, P. (1992) Relationship
between metabolism and cytotoxicity of ortho-phenylphenol in
isolated rat hepatocytes. Biochem. Pharmacol., 43, 1431-1437.
Nakao, T., Kabashima, U.J., Nagai, F., Nakagawa, A., Ohno, T.,
Ichikawa, H., Kabayashi, H. & Hiraga, K. (1983) The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Food Chem. Toxicol., 21, 325-329.
National Toxicology Program (1986) Toxicology and Carcinogenesis
Studies of orthoPhenylphenol (CAS No. 90-43-7) Alone and with
7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1
Mice (Dermal Studies) (NTP Technical Report No. 301, NIH
publication No. 86-2557), US Department of Health and Human
Services, Research Triangle Park, North Carolina, USA.
Norris, J..M. (1971) Eye irritation test conducted on Dowicide l
preservative. Unpublished report from Chemical Biology Research,
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Oehme, F.W. & Smith, T.H.F. (1972) The metabolism and urinary
excretion of ophenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1978) Teratological tests
of ortho-phenylphenol and its sodium salt in mice. Ann. Rep.
Tokyo Metr. Res. Lab. Public Health, 29, 89-96.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1979) Acute oral toxicity
of sodiumphenylphenate (OPP-Na) in mice. Ann. Rep. Tokyo Metr.
Res. Lab. ublic Health, 30, 54.
Pathak, D.N. & Roy, D. (1992) Examination of microsomal cytochrome
P450-catalyzed in vitro activation of o-phenylphenol to DNA
binding metabolite(s) by 32P-postlabeling technique.
Carcinogenesis, 13, 1593-1597.
Pathak, D.N. & Roy, D. (1993) In vivo genotoxicity of sodium
ortho-phenylphenol: phenylbenzoquinone is one of the
DNA-binding metabolite(s) of sodium orthophenylphenol.
Mutat. Res., 286, 309-319.
Quast, J.F. & McGuirk, R.J. (1995) ortho-Phenylphenol: Two-year
dietary chronic toxicity/oncogenicity study in B6C3F1 mice.
Unpublished report No. K-001024-047 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1983) Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem.-Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1984) Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP) on
the urinary tract of male F344 rats. Toxicol. Appl.
Pharmacol., 73, 345-349.
Roy, D. (1990) Cytochrome P-450 catalyzed redox cycling of
orthopenylphenol. Biochem. Int., 22, 849-857.
Sato, M., Tanaka, A., Tsuchiya, T., Yamaha, T., Nakaura, S. & Tanaka,
S. (1988) Excretion, distribution and metabolic fate of sodium
o-phenylphenate and o- phenylphenol in the rat. J. Food
Hyg. Soc. Jpn, 29, 7-12.
Savides, M.C. & Oehme, F.W. (1980) Urinary metabolism of orally
administered orthophenyl phenol in dogs and cats.
Toxicology, 17, 355-363.
Selim, S.A. (1996) A single dose open label study to investigate the
absorption and excretion of 14C/13C-labeled orthophenylphenol
formulation after dermal application to healthy volunteers.
Unpublished report No. P0995002 from Pharma Bio-Research Clinics
BV, Assen, Netherlands. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Shibata, M.-A., Hagiwara, A., Tamano, S., Fukushima, S. & Ito, N.
(1985) Subchronic toxicity study of sodium o-phenylphenate in
mice. Toxicol. Lett., 25, 239-246.
Shibata, M.-A., Tanaka, H., Yamada, M., Tamano, S. & Fukushima, S.
(1989a) Proliferative response of renal pelvic epithelium in male
rats to oral administration of orthophenylphenol, sodium
ortho-phenylphenate and diphenyl. Cancer Lett., 48, 19-28.
Shibata, M.-A., Tamano, S., Kurata, Y., Hagiwara, A. & Fukushima, S.
(1989b) Participation of urinary Na+, K+, pH, and L-ascorbic
acid in the proliferative response of the bladder epithelium
after the oral administration of various salts and/or ascorbic
acid to rats. Food Chem. Toxicol., 27, 403-413.
Shioya, S., Nagami-Oguihara, R., Oguihara, S., Kimura, T., Imaida, K.
& Fukushima, S. (1994) Roles of bladder distension, urinary pH
and urinary sodium ion concentration in cell proliferation of
urinary bladder epithelium in rats ingesting sodium salts.
Food Chem. Toxicol., 32, 165-171.
Shirasu, Y., Moriya, M., Kato, K., Tezuka, H., Henmi, R., Shingu, A.,
Kaneda, M. & Teramoto, S. (1978) Mutagenicity testing on
o-phenylphenol. Mutat. Res. 54, 227 (abstract).
Smith, R.A., Christenson, W.R., Bartels, M.J., Arnold, L.L., St John,
M.K., Cano, M., Garland, E.M., Lake, S.G., Wahle, B.S., McNett,
D.A. & Cohen, S.M. (1998) Urinary physiologic and chemical
metabolic effects on the urothelial cytotoxicity and potential
DNA adducts of o-phenylphenol in male rats. Toxicol. Appl.
Pharmacol., 150, 402-413.
Suzuki, H., Suzuki, N., Sasaki, M. & Hiraga, K. (1985)
Orthophenylphenol mutagenicity in a human cell strain. Mutat.
Res., 156, 123-127.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981a)
Toxicological study of o-phenylphenol (OPP) in mice. J. Nara
Med. Assoc., 32, 425.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981b)
Toxicological study of o-phenylphenylphenate (OPP-Na) in rats II.
Acute oral toxicity in Fischer 344 DUCRJ rats. J. Nara Med.
Assoc., 32, 709.
Tayama, S. & Nakagawa, Y. (1991) Sulfhydryl compounds inhibit the
cyto- and genotoxicity of o-phenylphenol metabolites in CHO-K1
cells. Mutat. Res., 259, 1-12.
Tayama, K., Iguchi, S. & Hiraga, K. (1979) Acute oral toxicity of
sodium o-phenylphenol (OPP-Na) in rats. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 30, 57.
Tayama, K., Iguchi, S. & Hiraga, K. (1980) Acute oral toxicity of
o-phenylphenol in rats. Ann. Rep. Tokyo Metr. Res. Lab.
Public Health, 31, 1.
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1983) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 34, 325
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1984) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Toxicology, 101,
187 (abstract).
Tayama, S., Kamiya, N. & Nakagawa, Y. (1989) Genotoxic effects of
o-phenylphenol metabolites in CHO-K1 cells. Mutat. Res., 223,
23-33.
Tayama-Nawai, S., Yoshida, S., Nakao, T. & Hiraga, K. (1984) Induction
of chromosome aberrations and sister-chromatid exchanges in
CHO-K1 cells by o-phenylphenol. Mutat. Res., 141, 95-99.
Timchalk, C. (1996) 14C-Orthophenylphenol: Pharmacokinetics
following dermal application in male human volunteers.
Unpublished report No. HET-K-001024-064 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Timchalk, C., Selim, S., Sangha, G. & Bartels, M.J. (1998) The
pharmacokinetics and metabolism of 14C/13C-labeled
ortho-phenylphenol formulation following dermal application to
human volunteers. Hum. Exp. Toxicol., 17, 411-417.
Ushiyama, K., Nagai, F., Nakagawa, A. & Kano, I. (1992) DNA adduct
formation by ophenylphenol metabolite in vivo and in
vitro. Carcinogenesis, 13, 1469-1473.
Wahle, B.S. & Christenson, W.R. (1996) Technical grade
ortho-phenylphenol: A combined chronic toxicity/oncogenicity
testing study in the rat. Unpublished report No. 92-272-SC from
Bayer Corp., Agriculture Division, Toxicology, Stilwell, Kansas,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Yamaha, T., Tanaka, A., Sato, M. & Tsuchiya, T. (1983) Excretion,
distribution and metabolic fate of sodium o-phenylphenol in the
rat. Unpublished report from Division of Medical Chemistry,
National Institute of Hygienic Sciences, Tokyo, Japan. Submitted
to WHO by Leng Associates, Midland, Michigan, USA.
Zablotny, C.L., Breslin, W.J. & Kociba, R.J. (1991) Ortho-phenylphenol
(OPP): Gavage teratology study in New Zealand white rabbits.
Unpublished report No. K-001024-045 from Toxicology Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Zempel, J.A. & Szabo, J.R. (1993) Ortho-phenylphenol: 21-day repeated
dermal dose study of systemic toxicity in Fischer 344 rats.
Unpublished report No. K-001024-056 from Health & Environmental
Sciences-Texas, Lake Jackson Research Center, Dow Chemical Co.,
Freeport, Texas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
2phenylphenol, which may play a role in the induction of bladder
cancer by this substance (Roy, 1990).
Activation of the 2-phenylphenol metabolite phenylhydroquinone by
prostaglandin (H) synthase in the presence of arachidonic acid and
hydrogen peroxide was studied to test the hypothesis that
prostaglandin synthase in rat urinary bladder transitional epithelium
and kidney medullar papilla is responsible for activation of the
metabolite to reactive intermediates in the bladder and kidney.
Phenylhydroquinone was found to be metabolized by the peroxidase
activity of prostaglandin synthase and by other peroxidases such as
horseradish peroxidase and myeloperoxidase, suggesting that the
peroxidative metabolism of phenylhydroquinone could play a role in
urinary bladder and kidney carcinogenesis in rats (Kolachana et al.,
1991).
In a study of the effect of pH on nonenzymatic oxidation of
phenylhydroquinone, the effects of phenylbenzoquinone and oxygen
concentration on autoxidation of phenylhydroquinone, and the
nonenzymatic conversion of phenylbenzoquinone to phenylhydroquinone, a
curvilinear relationship was found between the rate of oxidation of
phenylhydroquinone and pH over the range 6.3-7.6. Phenylbenzoquinone
was formed during autoxidation of phenylhydroquinone, with a yield of
0.92 ± 0.02. The results indicate that the production of reactive
metabolites from phenylhydroquinone involves both a pH-independent
(i.e. oxygen-dependent) and a pH-dependent pathway and that the
presence of phenylbenzoquinone enhances the rate of pH-dependent
phenylhydroquinone autoxidation. The authors suggested that ionization
of phenylhydroquinone semiquinone is a key step in production of
reactive species in the pH-dependent pathway. They found a good
correlation between the proposed reaction pathway and the induction by
2-phenylphenol of bladder lesions in rats. Thus, pH-dependent
autoxidation of free phenylhydroquinone in urine may be responsible
for the tumorigenic effects of 2-phenylphenol and sodium
2-phenylphenol in the rat bladder (Kwok & Eastmond, 1997).
Groups of eight female B6C3F1 mice were given 0, 1, 10, or 200
mg/kg bw per day of 2-phenylphenol (purity, > 98%) by gavage on 5
days per week for 2 weeks. As a positive control, mice were given 45
mg/kg bw of cyclophosphamide intraperitoneally for 4 days. The weights
of the body, liver, spleen, kidney, and thymus were recorded, and
samples were prepared for histopathological examination. Haematology
and clinical chemistry were conducted, and bone-marrow cellularity and
colony formation, lymphoproliferative responses, delayed
hypersensitivity responses, immunoglobulin, antibodies, response to
Listeria monocytogenes challenge, and tumour susceptibility were
studied. None of the treated animals died or showed signs of toxicity.
Histopathological examination revealed no significant lesion in any
tissues. The weight of the thymus and the relative weight of the
spleen were slightly increased at 200 mg/kg bw per day. The slight
haematological alterations seen did not show a dose-response
relationship and were within the normal range of biological variation.
A slight increase in serum cholesterol concentration and a
corresponding decrease in triglyceride concentration were seen in mice
at 200 mg/kg bw per day. The activity of alanine aminotransferase and
total protein in serum were not affected, although the
albumin:globulin ratio was slightly decreased at the high dose.
Bone-marrow cellularity, lymphoproliferative responses, immune
function, and host susceptibility were not altered. In contrast,
treatment with cyclophosphamide resulted in marked alterations. The
authors concluded that 2-phenylphenol, even at relatively high doses,
did not alter immune function or host susceptibility (Luster et al.,
1981).
Sodium 2-phenylphenol
Binding of sodium 2-phenylphenol metabolites to macromolecules
in vitro was studied by incubating [14C]sodium 2-phenylphenol
(specific activity, 19 mCi/mmol) with purified liver microsomes from
male rats in the presence of a NADPH regenerating system and bovine
serum albumin, which served as a 'protein acceptor'. Macromolecular
binding of radiolabel to protein, which was dependent on the presence
of both active microsomes and NADP, was observed. In order to study
the binding of metabolites of 2-phenylphenol and its sodium salt to
macromolecules in the liver, kidney, and urinary bladder in vivo,
groups of four male rats were given single oral doses of
14C-labelled compounds at doses of 50, 100, 200, or 500 mg/kg bw,
and tissues were excised 16-18 h later for measurement of
macromolecular binding, which was determined as nanomoles of bound
material per milligram of protein. The extent of binding was not
linearly related to the administered dose. Disproportionate increases
were seen in each tissue at doses of sodium 2-phenylphenol > 200
mg/kg bw and in liver and bladder at doses of 2-phenylphenol at
200-500 mg/kg bw (Reitz et al., 1984).
Sodium 2-phenylphenol (purity not given) was administered in the
diet at a concentration of 20 000 ppm to 4-week-old male and female
Fischer 344 rats for 136 days. Urine was collected periodically. At
the end of treatment, the rats were killed, blood samples were
collected, and livers and kidneys were removed. The amounts of cyclic
nucleotides (cAMP and c-GMP) were determined in urine, plasma, liver,
and kidneys, and adenylate cyclase activity was measured in liver and
kidneys. In male rats, the c-AMP levels in urine and plasma were
decreased whereas the c-GMP levels were increased. In females, c-AMP
levels were decreased only during the first 3 days of feeding, and the
levels of c-AMP and cGMP in liver and kidneys were unchanged. The
decreased urinary c-AMP in male rats was probably the result of
decreased adenylate cyclase activity in liver and kidneys. A similar
change in adenylate cyclase activity was observed in liver but not in
kidneys of female rats treated with sodium 2-phenylphenol. The
sex-related alterations in cyclic nucleotide levels were postulated to
be involved in the sex-dependent induction of urinary bladder tumours
by sodium 2-phenylphenol (Nakagawa et al., 1984).
In male and female Fischer 344 Du Crj rats given 20 000 ppm of
sodium 2-phenylphenol in the diet for 20 weeks, urinary gamma-glutamyl
transpeptidase activity decreased immediately after the start of
treatment and remained low throughout the study. The activities of
this enzyme and of alkaline phosphatase in kidney homogenate were
found to have decreased to about 80% of the control values at 20
weeks, but the activity of glucose-6phosphate dehydrogenase was
significantly increased; that of Na/K-ATPase was unchanged. In liver
homogenate, however, gamma-glutamyl transpeptidase activity was
increased by about eight times and that of glucose-6-phosphate
dehydrogenase was significantly increased, but the activities of
alkaline phosphatase and Na/K-ATPase were not significantly different
from the control values. The glutathione concentration in the livers
of treated rats was significantly reduced (Nagai & Nakao, 1984).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of 2-phenylphenol
and its sodium salt are summarized in Table 1. The clinical signs of
toxicity were generally nonspecific.
Table 1. Acute toxicity of 2-phenylphenol and its sodium salt
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/L)
2-Phenylphenol
Mouse M Oral 1200 Taniguchi et al. (1981)
F 1100
Mouse M Oral 3500 Tayama et al. (1983, 1984)
F 3200
Rat M Oral 2600 Tayama et al. (1980)
F 2900
Rat M Oral 2800 Gilbert & Crissman (1994)
F 2800
Rat M&F Inhalation
(4 h) > 36 Landry et al. (1992)
Rabbit M&F Dermal > 5000 Carreon & New (1981)
Sodium 2-phenylphenol
Mouse M Oral 900 Ogata et al. (1979)
F 800
Rat M Oral 1700 Taniguchi et al. (1981)
F 1600
Rat M Oral 1100 Tayama et al. (1979)
F 1100
Rat M Oral 850 Gilbert & Stebbins (1994)
F 590
(b) Short-term studies of toxicity
2-Phenylphenol
Rats
2-Phenylphenol (purity, 99.8%) was administered to 30 male
Fischer 344 rats (Charles River) in the diet at a concentration of
20 000 ppm, equal to 1000 mg/kg bw per day, for up to 90 days. Interim
sacrifices were performed at 3, 7, 30, and 65 days. Only seven rats at
each dose were permitted to live to 90 days, at which time they were
killed. Food consumption and body weight were markedly reduced within
the first week and remained low throughout the study. The renal
lesions observed in these rats included focal cortical cysts,
significantly decreased urine specific gravity (at 65 and 90 days),
small amounts of blood in the urine, focal tubular collapse and
atrophy in the cortex, and cystic degeneration (at 65 and 90 days). No
treatment-related urinary bladder lesions were observed. A NOAEL could
not be identified since the body weight was reduced at the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1300, 3100, 6300, 13 000, or 25 000 ppm of
2-phenylphenol (purity not given), equal to 0, 180, 390, 760, 1700,
and 2800 mg/kg bw per day for males and 0, 200, 410, 800, 1700, and
3000 mg/kg bw per day for females, for 12 weeks. Body weight and
body-weight gain were severely depressed in males and females at
25 000 ppm and to a lesser extent (14%) in males fed 13 000 ppm. No
significant treatment-related effects were seen in analyses of urine
performed at weeks 9 and 13. Haematological and blood chemical values
were generally normal, other than a slight decrease in haemoglobin
concentration in male and female rats at the highest dose. The
absolute and relative weight of many organs in male rats at this dose
were significantly decreased. The NOAEL was 6300 ppm, equal to 760
mg/kg bw per day, on the basis of reduced body weight and body-weight
gain at 13 000 ppm (Iguchi et al., 1984).
Groups of five male and five female Fischer 344 rats received
dermal applications of 0, 100, 500, or 1000 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) once daily on 5 days per week for a
total of 15 applications over 21 days. The amount applied per animal
was adjusted weekly on the basis of the body weight of individual
animals and was applied to a 5 cm × 5 cm area of clipped skin on the
back, covered with a nonabsorbent cotton patch held in place by an
elastic wrap secured with adhesive tape. The wraps and patches were
removed not less than 6 h after treatment, and the treated area was
wiped with a wettened gauze pad to remove residual test material.
Control animals were handled in the same way. All animals were
acclimated to the wraps for 2 days before the beginning of treatment.
They were observed at least daily and were given a complete clinical
examination weekly. The skin at the site of treatment was examined
after removal of the wrap on the last day of dosing, each week, and on
the day before necropsy. Body weights were measured weekly and feed
consumption and feed efficiency were calculated weekly. Urine was
analysed on day 19. The rats were fasted overnight before necropsy,
when haematological and serum clinical chemical parameters were
evaluated, all animals were examined for gross pathological changes,
selected organs were weighed, and tissues were preserved. Selected
tissues and all gross lesions from animals in the control and
high-dose groups were examined histologically.
No deaths occurred at any dose. Treatment-related effects
indicative of dermal irritation were observed at the site of
application in animals of each sex at 500 and 1000 mg/kg bw per day.
Female rats appeared to be slightly more sensitive than males, but the
severity of lesions increased with duration of exposure and dose in
both sexes. The irritating effects ranged from scaling to fissures.
Body weights and feed consumption were not affected, and no
significant treatment-related effects were found in the
haematological, clinical chemical, and urinary parameters evaluated.
No treatment-associated alterations were found in the liver or kidneys
(Zempel & Szabo, 1993).
Guinea-pigs
2-Phenylphenol was evaluated in 10 male Hartley albino
guinea-pigs for dermal sensitization potential by a modified Buehler
method. The animals received three dermal applications of 0.4 g of
2-phenylphenol (purity, 99.9%) during a 3-week induction period and
were challenged with 0.4 g of the compound 2 weeks after the last
induction. The condition of the test sites was assessed approximately
24 and 48 h after the challenge. No erythema or oedema was seen in any
of the animals. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Berdasco, 1991).
Ten male Hartley albino guinea-pigs were clipped free of hair on
the day before dosing and received three dermal applications of 0.4 g
of 2-phenylphenol (purity, 99.9%) moistened with 0.20 ml of distilled
water during a 3-week induction period. Two weeks after the last
induction application, they were given a challenge application of
0.4 ml of a 7.5% suspension of the compound in water on another site
for 6 h. Five animals received no induction but a 0.4-ml aliquot of a
7.5% suspension of 2-phenylphenol in water. The condition of the test
sites was assessed approximately 24 and 48 h after challenge. No
erythema was seen, and none of the uninduced animals showed
irritation. The animals were in good health and gained weight during
the study. The author concluded that 2-phenylphenol did not cause
delayed contact hypersensitivity (Gilbert, 1994b).
Rabbits
The ability of 2-phenylphenol (purity, 99.9%) to cause primary
dermal irritation was studied in three male and three female New
Zealand white rabbits that received applications of aliquots of 0.5 ml
of the substance moistened with 0.3 ml of distilled water for 4 h on
intact skin on a clipped, 10 cm × 10 cm area of the back. The
application sites were graded for erythema and oedema within 30 min
and 24, 48, and 72 h of removal of the patch and on days 7, 8, 9,
10,11, 14, and 15. The animals were weighed on the day of treatment
and at the end of the study. Very slight erythema was observed at the
application site in one of the six rabbits within 30 min of removal of
the test material and in two rabbits 24 h later. Severe to slight
eschar formation was observed in four rabbits within 30 min of
treatment which persisted throughout the remainder of the study. Four
animals had burns at the site of application within 30 min, which
resolved as scabs and then scars by the end of the study. Four rabbits
had very slight-to-severe oedema 30 min and 24 h after removal of the
test material, and slight-to-severe oedema was observed on three
animals 48 and 72 h after removal. Body weight was not affected
(Gilbert, 1994a).
Instillation of 0.1 g of 2-phenylphenol (purity not given) into
the right eye of six New Zealand white rabbits resulted in moderate
corneal injury, iritis, and moderate-to-severe conjunctival redness
and chemosis in all animals 24, 48, and 72 h and 7 days after dosing
(Norris, 1971).
Dogs
In studies to assess the palatability and toxicity of
2-phenylphenol (purity, 99.8%) in beagle dogs, males and females were
treated with several regimens. For palatability, one female was given
feed containing 2-phenylphenol to give a dose of 300 mg/kg bw per day
for 5 days. In a study to assess toxicity, groups of two males and
three females were given 300-1000 mg/kg bw per day as a solution in
peanut oil by gastric intubation for up to 9 days or 400-700 mg/kg bw
per day by capsule for 1-2 days. In a 4-week study, groups of two
males and two females were given 0, 100, 200, or 300 mg/kg bw per day
as a solution in peanut oil by gastric intubation on 5 days per week.
In a 1-year study, groups of four males and four females were given
2-phenylphenol as a solution in peanut oil by gastric intubation at
doses of 0, 30, 100, or 300 mg/kg bw per day for 5 days per week for
1 year. The animals were examined daily for clinical signs, and body
weight, feed consumption, haematological, urinary, and clinical
chemical parameters, organ weights, ophthalmologic endpoints, and
gross and histopathology were determined.
Administration of 300 mg/kg bw per day in feed or by intubation
for 5 days resulted in decreased body weights and feed consumption
that correlated with the unpalatability of 2-phenylphenol. Repeated
emesis was seen at doses > 400 mg/kg bw per day administered in
gelatin capsules or in peanut oil by intubation and at doses > 200
mg/kg bw per day by intubation throughout the 4-week study.
Dose-related emesis was also seen in animals given 2-phenylphenol for
1 year. In general, more frequent emesis and ejection of greater
volumes of gastric content was seen in dogs given 300 mg/kg bw per day
than in those given lower doses. While emesis effectively limited the
dose that could be retained, the degree of emesis did not appear to
compromise the health of the animals over 1 year. The reaction was
categorized as a local, transitory response of the mucosal lining of
the upper alimentary tract rather than a reaction of the central
nervous system. No adverse effects were seen on body weight, feed
consumption, haematological, urinary, clinical chemical, or
ophthalmological parameters, organ weights, or gross or histological
appearance of a range of tissues from all dogs. The only deaths were
of two males at the high dose in the 1-year study which died
subsequent to inadvertent deposition of the test solution into the
lungs after approximately 4.5 months of dosing. The NOAEL was 300
mg/kg bw per day (Cosse et al., 1990).
Sodium 2-phenylphenol
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing 0, 2500, 5000, 10 000, 20 000, or 40 000 ppm of sodium
2-phenylphenol as the tetrahydrate (purity not given), equivalent to
0, 270, 550, 1100, 2200, and 4400 mg/kg bw per day, for 13 weeks.
Body-weight gain was significantly depressed in males fed 10 000 or
20 000 ppm and in animals of each sex fed 40 000 ppm. Urinary analysis
showed increased pH and decreased specific gravity at the highest
dose. The relative weights of the livers of animals given 10 000,
20 000, or 40 000 ppm were significantly greater than those of
controls, but no treatment-related histopathological findings were
made. Light and scanning electron microscope examination of the
bladder epithelium at 4, 8, and 13 weeks in three males and three
females in the control group and at 20 000 ppm showed no abnormaility
in the appearance of the bladder epithelium of treated mice at any
time. The NOAEL was 5000 ppm, equivalent to 550 mg/kg bw per day, on
the basis of reduced body-weight gain and increased relative liver
weight at 10 000 ppm (Shibata et al., 1985).
Rats
Sodium 2-phenylphenol (purity, 98.7%) was administered in the
diet to 30 male Fischer 344 rats at a concentration of 20 000 ppm for
up to 90 days. Interim sacrifices were performed at 3, 7, 14, 30, and
65 days. Only seven rats per group were permitted to live to 90 days,
at which time they were killed. The lesions seen in the urinary
bladder epithelium were increased mitosis beginning at 3 days and
thickening (i.e. simple hyperplasia) beginning at 14 days. No tumours
were observed in the bladder. A NOAEL could not be identified since
lesions were observed in the bladder at 20 000 ppm, the only dose
tested (Reitz et al., 1983).
Groups of 10 male and 10 female Fischer 344 rats were fed diets
containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol (purity, > 95%), equal to 0, 85, 180, 350, 710,
1400, and 2500 for males and 0, 87, 180, 350, 690, 1300, and 2400
mg/kg bw per day for females, for 13 weeks. The rats were observed
daily for changes in general condition and were weighed weekly; the
amounts of feed and water consumed were measured on 3 days every other
week. No deaths occurred during the study. A 15-17% decrease in
body-weight gain was seen in animals at doses > 5000 ppm. Urinary
bladder tumours occurred in male rats at frequencies of 1/10 at 10 000
ppm, 9/10 (five transitional-cell carcinomas) at 20 000 ppm, and 1/10
at 40 000 ppm. Six rats at 40 000 ppm had pyelonephritis. In female
rats, the frequencies of tumours were 0/10 at 20 000 ppm and 2/10
(papillomas only) at 40 000 ppm. No bladder calculi were observed in
this experiment. The NOAEL was 2500 ppm, equal to 180 mg/kg bw per
day, on the basis of reduced body-weight gain at 5000 ppm (Iguchi et
al., 1979; Hiraga & Fujii, 1981).
Guinea-pigs
Sodium 2-phenylphenol (purity, 99.1%) was applied to the clipped
skin of 10 male Hartley albino guinea-pigs as a 0.4-ml aliquot of a
0.5% suspension in distilled water at 3-week intervals for induction.
Two weeks after the last dose, a challenge of 0.4 ml of a 0.1%
solution of the compound in distilled water was applied to the other
side of the animals. No erythema occurred at the test site. The
animals gained weight throughout the study. The author concluded that
sodium 2-phenylphenol did not cause delayed contact hypersensitivity
(Gilbert, 1994c).
Rabbits
Sodium 2-phenylphenol diluted 1:200 with distilled water was
instilled into the eyes of six rabbits (strain not given). Temporary,
mild conjunctival reactions were observed in three animals. The
authors concluded that the compound slightly irritated the eye (Davies
& Liggett, 1973).
(c) Long-term studies of toxicity and carcinogenicity
2-Phenylphenol
Mice
Groups of 50 male and 50 female B6C3F1 mice were fed diets
(concentrations not given) supplying 2-phenylphenol (purity, 99.9%) at
doses of 0, 250, 500, or 1000 mg/kg bw per day for 2 years. A
satellite group of 10 male and 10 female mice at each dose was
maintained on the diets for 12 months and then necropsied. All mice
were observed at least once daily for overt signs of toxicity, and a
thorough clinical examination was performed at least once a week
throughout the study. Body weights and feed consumption were recorded
weekly for the first 13 weeks and monthly thereafter. The diets were
prepared weekly or every other week, with adjustments of the
2-phenylphenol concentration according to group mean body weights and
feed consumption to maintain the desired doses for each group.
Clinical signs and mortality rates were unaffected by treatment,
but decreased body weights (by 6-20%) and weight gain (by 10-38%) were
seen in all treated groups except for males fed 250 mg/kg bw.
Haematological, clinical chemical, and urinary parameters in mice
necropsied at 12 and 24 months showed no consistent, toxicologically
significant alterations indicative of target organ toxicity. Changes
in the weights of the adrenal glands, brain, heart, kidneys, liver,
testis, and spleen which were found to be statistically significant
were confounded by the marked decrease in body weight. Nevertheless,
the consistent increases in the absolute and/or relative weights of
the liver at all doses suggests a treatment-related effect. Gross
observations at necropsy in males at the high dose at 12 months and in
males at the intermediate and high doses at 24 months showed a slight
increase in the number of mice with liver masses or nodules.
Microscopic examination of the livers of mice at 12 and 24 months
revealed treatment-related effects at all doses. The cytoplasm of
hepatocytes stained homogeneously, indicating liver enzyme induction,
but there was no evidence of degeneration or necrosis. The microscopic
changes were dose-related and resembled those associated with
adaptation to metabolic demands. An increased incidence of
eosinophilic hepatocellular foci was also observed in males at 100 and
500 mg/kg bw per day.
Males fed 1000 mg/kg bw per day and necropsied at 12 months had a
slightly increased incidence of hepatocellular adenoma. At 24 months,
a statistically significant increase in the number of males with
hepatocellular adenoma was seen at 500 ( n = 40) and 1000 ( n = 41)
mg/kg bw per day, the incidence in controls being 27/50. Low
incidences of a variant form of hepatocellular carcinoma
(hepatoblastoma) were observed in all treated groups of males (2/50 at
250 mg/kg bw per day, 6/50 at 500 mg/kg bw per day, and 3/50 at 1000
mg/kg bw per day versus 0/50 in controls), but the incidence of
hepatocellular carcinoma was not significantly increased at any dose.
The combined incidence of hepatoblastoma and hepatocellular carcinoma
was also not significantly increased in the male mice. The primary
non-tumourous microscopic changes in the livers of male mice, which
appeared to have been adaptive, ultimately resulted in the promotion
of hepatocellular adenomas. The incidences of tumours in other tissues
were not statistically significantly increased. The livers of female
mice showed similar microscopic adaptive changes, but none of them had
hepatoblastoma, and no statistically significant increase in the
incidence of tumours in any tissues was found. Decreased incidences of
microscopic lesions when compared with controls were found in the
adrenals, kidneys, lungs, oral tissues, pancreas, peripheral nerve,
spleen, and testis of males and in the kidneys, lungs, and nasal
tissues of females. These findings were considered to reflect normal
variation and the decreased body weights of the mice and not a primary
response to 2-phenylphenol. A NOAEL for toxicity could not be
identified. The NOAEL for carcinogenicity was 250 mg/kg bw per day on
the basis of an increased incidence of hepatocellular adenomas at 500
mg/kg bw per day (Quast & McGuirk, 1995).
Rats
2-Phenylphenol (purity, 98%) was administered in the diet at
concentrations of 0, 6300, 13 000, or 25 000 ppm, equal to 0, 320,
650, and 1300 mg/kg bw per day to groups of 20-24 male Fischer 344
rats (Charles River) for 91 weeks. The percentage survival was 96, 90,
71, and 65% in the four groups, respectively. In the rats that died
during the study, the incidences of urinary bladder tumours were 0/1
in controls, 0/2 at 6300 ppm, 7/7 at 13 000 ppm, and 0/8 at 25 000
ppm. Bladder lesions were found in 10%, 96%, and 48% of rats at the
three doses, respectively. The incidences of urinary bladder
papillomas and transitional-cell carcinomas were 23/24 at 13 000 ppm
and 4/23 at 25 000 ppm. A NOAEL could not be identified since bladder
lesions were seen at all doses tested (Hiraga, 1983a; Hiraga & Fujii,
1984).
Groups of 70-75 male and 70-75 female Fischer 344 rats were fed
diets containing 2-phenylphenol (purity, 99.5%) at concentrations of
0, 800, 4000, or 8000/10 000 ppm, equal to 0, 39, 200, and 400 mg/kg
bw per day for males and 0, 49, 240, and 650 mg/kg bw per day for
females, for 1 year before interim sacrifice of satellite groups of 20
rats per dose and for 2 years for the remaining 50 rats of each sex.
The animals were observed daily and were examined weekly for clinical
signs of toxicity. Each animal was weighed once a week and also
immediately before necropsy to allow calculation of organ:body weight
ratios. Food consumption was measured weekly. Blood and overnight
urine samples were collected at 3, 6, 12, 18, and 24 months from the
first 20 surviving rats of each sex in the group scheduled for
sacrifice at 2 years. Any dead or moribund animals were prepared for
necropsy, and all surviving animals were killed at the end of the test
periods.
A 5% decrease in body-weight gain was seen in animals at 4000
ppm, and a decrease of 11% was seen in males at 8000 ppm and in
females at 10 000 ppm. Food consumption was unaffected in all groups.
Minor clinical and gross observations included an increased incidence
of abnormally coloured urine, urine stains, and red stains in male
rats given 8000 ppm 2-phenylphenol and an increased incidence of urine
and brown stains in female rats given 4000 or 10 000 ppm. There were
no treatment-related changes in ophthalmological, haematological,
clinical chemical, or urinary parameters, except for an increased
incidence of blood in the urine of males at 8000 ppm. The mortality
rate was slightly increased among males at 8000 ppm. Gross
pathological examination showed increased incidences of urinary
bladder masses in males fed 4000 ppm for 2 years or 8000 ppm for 1 or
2 years and increased incidence of pitted zones and abnormal texture
of the kidney in females fed 10 000 ppm for 2 years. Histopathological
examination showed hyperplasia and transitional-cell carcinoma in the
urinary bladders of males fed 4000 or 8000 ppm for 1 or 2 years, the
increase being statistically significant at 8000 ppm and of borderline
significance at 4000 ppm. The NOAEL for toxicity was 800 ppm, equal to
39 mg/kg bw per day, on the basis of reduced body-weight gain and
hyperplasia in the urinary bladder at all doses. The NOAEL for
carcinogenicity was 800 ppm, equal to 39 mg/kg bw per day (Wahle &
Christenson, 1996).
Sodium 2-phenylphenol
Mice
Sodium 2-phenylphenol (purity, 97%) was administered in the diet
to groups of 50 male and 50 female B6C3F1 mice (Charles River) at
concentrations of 0, 5000, 10 000, or 20 000 ppm, equal to 0, 590,
1400, and 3000 mg/kg bw per day for males and 0, 780, 1500, and 3100
mg/kg bw per day for females, for 96 weeks. The mice were then given
control diet for an additional 8 weeks. The survival rate of males at
the high dose was slightly decreased. Decreased body weight was
observed in males and females at 20 000 ppm and in females at 5000 and
10 000 ppm. Alkaline phosphatase activity was increased in females at
5000, 10 000, and 20 000 ppm. No urinary bladder stones, tumours, or
extensive renal damage were observed in any of the mice. The NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, the
highest dose tested (Ito, 1983a; Hagiwara et al., 1984).
Rats
Groups of 20-21 male and 20-21 female Fischer 344 rats were fed
diets containing 0, 1250, 2500, 5000, 10 000, 20 000, or 40 000 ppm of
sodium 2-phenylphenol as the tetrahydrate (purity, > 95%), equivalent
to 0, 70, 140, 270, 550, 1100, or 2200 mg/kg bw per day, for 91 weeks.
The rats were observed daily for changes in general condition. The
survival rates were 90, 90, 95, 90, 90, 57, and 71% for the seven
groups, respectively. Increased incidences of urinary bladder
papil-lomas and transitional-cell carcinomas were seen, with 1/21 at
5000 ppm, 7/21 at 10 000 ppm, 20/21 at 20 000 ppm, and 17/20 at 40 000
ppm. Transitional-cell carcinomas of the kidney were also observed at
doses > 5000 ppm. The NOAEL was 2500 ppm, equivalent to 270 mg/kg
bw per day, on the basis of the increased incidence of urinary bladder
tumours (Hiraga & Fujii, 1981).
Groups of 50 male and 50 female Fischer 344/DuCrj rats were fed
diets containing 0, 7000, or 20 000 ppm (males) or 0, 5000, or 10 000
(females) of sodium 2-phenylphenol (purity, 95.5%) for 104 weeks
followed by control diet for 2 weeks. In a second study, groups of 25
male and 25 female rats were fed diets containing the compound at 0,
2500, 7000, or 20 000 ppm, equal to 0, 95, 270, and 770 mg/kg bw per
day, for males, and 0, 2500, 5000, or 10 000 ppm, equal to 0, 110,
220, and 470 mg/kg bw per day, for females, for 104 weeks followed by
control diet for life.
The survival rate at week 104 was 20% in males at 20 000 ppm in
the first study and 24% in the second study, while those in the other
groups were > 50%. Urinary bladder tumours were observed in the first
study in 2/50 males at 7000 ppm, 47/50 males at 20 000 ppm, 1/50
females at 5000 ppm, and 4/50 females at 10 000 ppm. In the second
study, the bladder tumour incidence was 3/25 in males at 7000 ppm,
23/25 in males at 20 000 ppm, and 2/25 in females at 10 000 ppm. In
the first study, transitional-cell carcinomas were found in 2/2 males
at 7000 ppm, 46/47 males at 20 000 ppm, and 1/4 females at 10 000 ppm.
In the second study, carcinomas were found in 1/3 males at 7000 ppm,
21/23 males at 20 000 ppm, and 1/2 females at 10 000 ppm. The
incidence of bladder tumours was thus dose-dependent. The NOAEL was
2500 ppm, equal to 95 mg/kg bw per day, on the basis of urinary
bladder tumours at all doses (Hiraga, 1983b; Fujii & Hiraga, 1985).
A working group convened by the International Agency for Research
on Cancer (IARC) classified sodium 2-phenylphenol as possibly
carcinogenic to humans and 2-phenylphenol as not classifiable as to
its carcinogenicity to humans (IARC, 1987, 1999).
(d) Genotoxicity
The results of tests for the genotoxicity of 2-phenylphenol,
sodium 2-phenylphenol, and the metabolites phenylhydroquinone and
phenylbenzoquinone are summarized in Table 2.
Covalent binding to urinary bladder DNA was determined in vivo
in pooled samples from eight male rats dosed with 500 mg/kg bw of
[14C]2-phenylphenol (purity, 99.8%) or [14C]sodium 2-phenylphenol
(purity, 98.7%). No radiolabel was detected in DNA from bladders
excised 16 h after dosing with either compound. The detection limit
was less than one alkylation per 106 nucleotides. Identical results
were obtained in a second experiment (Reitz et al., 1983).
The reactions of 2-phenylphenol and its metabolites
phenylhydroquinone and phenylbenzo-quinone with DNA were investigated
by a sequencing technique and by ultraviolet-visible and electron spin
resonance spectroscopy. In the presence of Cu(II), phenylhydroquinone
caused extensive DNA damage. Catalase, methionine, and methional
inhibited the DNA damage completely, whereas mannitol, sodium formate,
ethanol, tert-butyl alcohol, and superoxide dismutase did not.
Phenylhydroquinone plus Cu(II) frequently induced a piperidine-labile
site at thymine and guanine residues. Addition of Fe(III), Mn(II),
Co(II), Ni(II), Zn(II), Cd(II), or Pb(II) to phenylhydroquinone did
not induce DNA damage. This metabolite also induced DNA damage in the
presence of Cu(II) when peroxide was added, and Cu(II) accelerated the
autoxidation of phenylhydroquinone to quinone. Electron spin resonance
spectroscopy revealed that the semiquinone radical is an intermediate
in the autoxidation. Catalase did not inhibit the acceleration by
Cu(II). Superoxide dismutase promoted both the autoxidation of
phenylhydroquinone and the initial rate of semiquinone radical
production. Electron spin resonance trapping showed that addition of
Fe(III) produced hydroxyl radicals during the autoxidation of
phenylhydroquinone, whereas addition of Cu(II) did so sparingly. The
results suggest that DNA damage induced by phenylhydroquinone plus
Cu(II) is due to active species other than hydroxyl free radicals
(Inoue et al., 1990).
Table 2. Results of studies of the genotoxicity of 2-phenylphenol, sodium 2-phenylphenol, and the metabolites phenylhydroquinone
and phenylbenzoquinone
End-point Test object Concentration Purity Result Reference
(%)
2-Phenylphenol
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative - S9 Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive + S9 Ushiyama et al. (1992)
Negative - S9
Gene mutation B. subtilis H17, M45 NR NR Negative Shirasu et al. (1978)
Gene mutation S. typhimurium 10 -1000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA92, TA1535, Negative - S9
TA100, TA1537,
TA94, TA98
Gene mutation E. coli WP2 hcr NR NR Negative + S9 Shirasu et al. (1978)
Negative - S9
Gene mutation S. typhimurium 3-200 µg/plate > 99 Negative + S9 National Toxicology
TA100, TA1535, Weakly positive + S9 Program (1986)
TA1537, TA98
Gene mutation Mouse lymphoma 0.3-60 µg/ml > 99 Positive + S9 National Toxicology
(L5178Y) cells, Positive - S9 Program (1986)
Tk locus
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation Human Rsa cells, 1-30 µg/ml NR Positive Suzuki et al. (1985)
repair deficient,
HPRT locus
Chromosomal CHO-K1 cells 50-175 µg/ml > 99 Positive - S9 Tayama-Nawai et al.
aberration (1984)
Chromosomal CHO fibroblasts 12-125 µg/ml NR Weakly positive + S9 Ishidate et al. (1983)
aberration Weakly positive - S9
Chromosomal CHO-K1 cells 60-90 µg/ml > 99 Negative + S9 National Toxicology
aberration Negative - S9 Program (1986)
Chromosomal CHO-K1 cells 25-175 µg/ml > 99 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Chromosomal CHO-K1 cells 100-200 µg/ml > 99 Positive + S9 Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Host-mediated S. typhimurium G46 200 or 600 mg/kg NR Negative Shirasu et al. (1978)
gene mutation in male JCL-ICR mice bw orally for 5 days
In vivo
Sex-linked D. melanogaster 250 ppm in feed > 99 Negative National Toxicology
recessive lethal for 3 days or Program (1986)
mutation injection of 500 ppm
DNA binding Male rat urinary 500 mg/kg bw > 99 Negative Reitz et al. (1983)
bladder orally
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
DNA Rat urinary bladder 60-940 mg/kg > 99 Positive at Christenson et al.
32P-postlabelling bw per day 570 and 940 (1996a)
mg/kg bw per day
Chromosomal Male rat bone 800 mg/kg bw NR Negative Shirasu et al. (1978)
aberration marrow over 5 days or
single doses
< 4000 mg/kg
bw orally
Dominant lethal Male mice 100 or 500 mg/kg > 99 Negative Kaneda et al. (1978)
mutation bw per day for 5 days
Dominant lethal Male mice 100 or 500 mg/kg NR Negative Shirasu et al. (1978)
mutation bw per day for 5
days
Sodium 2-phenylphenol
In vitro
Gene mutation S. typhimurium 50-5000 µg/plate NR Negative + S9 Ishidate et al. (1983)
TA100, TA98 Negative - S9
Gene mutation S. typhimurium 0.025-250 µg/plate 99 Negative + S9 Reitz et al. (1983)
TA100, TA98, Negative - S9
TA1535, TA1537,
TA1538
Uncheduled DNA Male rat primary 10-7-10-4 mol/L 99 Negative Reitz et al. (1983)
synthesis hepatocytes
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
In vivo
DNA Male rat urinary 2% in diet for > 99 Positive Ushiyama et al. (1992)
32P-postlabelling bladder 13 weeks
DNA Mouse skin 10 or 20 mg/animal 97 Positive Pathak & Roy (1993)
32P-postlabelling topically
Phenylhydroquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Positive Nagai et al. (1990)
DNA Rat liver DNA 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Chromosomal CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
aberration Negative - S9
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Chromosomal CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
aberration inhibited by (1991)
cysteine or
glutathione
Sister chromatid CHO-K1 cells 5-150 µg/ml > 98 Positive + S9 Tayama et al. (1989)
exchange Positive - S9
Sister chromatid CHO-K1 cells 0.3-30 µmol/L > 98 Positive, Tayama & Nakagawa
exchange inhibited by (1991)
cysteine or
glutathione
Micronucleus V79 CH lung 6-125 µmol/L NR Positive + S9 Lambert & Eastmond
formation fibroblast cells Negative - S9 (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA Mouse skin 100 µmol/L NR Positive + S9 Pathak & Roy (1992)
32P-postlabelling Negative - S9
Phenylbenzoquinone
In vitro
DNA strand breaks E. coli plasmid 10-6-10-2 mol/L > 99 Negative Nagai et al. (1990)
DNA binding Calf thymus DNA 40 mmol/L > 99 Positive - S9 Ushiyama et al. (1992)
Table 2. (continued)
End-point Test object Concentration Purity Result Reference
(%)
Gene mutation S. typhimurium 0.05-1000 NR PBQ negative/ Ishidate et al. (1983)
TA100, TA2637, µg/plate PBQ negative
TA98
Gene mutation V79 CH lung 6 -125 µmol/L NR Negative Lambert & Eastmond
fibroblast cells, (1994)
Hprt locus,
± arachidonic acid
Chromosomal CHO fibroblast 1-25 µg/ml NR Negative + S9 Ishidate et al. (1983)
aberration cell line Negative - S9
Micronucleus V79 CH lung 6-125 µmol/L NR Negative Lambert & Eastmond
formation fibroblast cells (1994)
± arachidonic acid
In vivo
DNA damage Male rat urinary 0.0005-0.1% 99% Negative Morimoto et al. (1989)
bladder by injection
DNA adduct formation in HL-60 cells treated with the
2-phenylphenol metabolites 2-phenylhydroquinone and
2-phenylbenzoquinone was studied by 32P-postlabelling. Treatment
with 25-500 µmol/L of 2-phenylhydroquinone for 8 h produced one
principal and three minor adducts, with a relative distribution of 80,
10, 6, and 4%. The relative adduct frequencies were 0.26-2.3
adducts/107 nucleotides. Treatment with 25-250 µmol/L of
2-phenylbenzoquinone for 2 h resulted in a similar level of DNA
modification and adduct distribution. Reaction of purified calf thymus
DNA with 2-phenylbenzo-quinone produced one DNA adduct, which did not
correspond to the major adduct produced in HL-60 cells. These results
show that both metabolites can form DNA adducts. Peroxidase activation
of 2-phenylphenol may therefore play a role in its carcinogenic effect
(Horvath et al., 1992).
In a similar study of covalent binding to DNA,
32P-postlabelling analysis of the products of reaction of DNA with
phenylbenzoquinone revealed four major and several minor adducts.
Chemical reaction with deoxyguanosine 3'-phosphate also resulted in
four major adducts, and their chromatographic mobility was identical
to that of major adducts of phenylbenzoquinone-DNA, which were shown
to be stable. More total covalent binding was found in deoxyguanosine
3'-phosphate than in DNA. Reaction of DNA with 2-phenylphenol or
phenylhydroquinone in the presence of microsomes and NADPH or cumene
hydroperoxide also resulted in four major adducts, and their formation
was drastically decreased by known inhibitors of cytochrome P450. The
chromatographic mobility of these adducts matched that of the adducts
observed in deoxyguanosine 3'-phosphate and DNA reacted with
phenylbenzoquinone. Thus, both 2-phenyl-phenol and phenylhydroquinone
can bind covalently to DNA in the presence of a microsomal cytochrome
P450 activation system, and phenylbenzoquinone is one of the
DNA-binding metabolites of 2-phenylphenol (Pathak & Roy, 1992).
Covalent modification of skin DNA by sodium 2-phenylphenol
in vivo was studied by the 32P-postlabelling method to elucidate
the biochemical mechanism of promotion of chemically induced skin
carcinogenesis by this compound. Topical application of sodium
2-phenylphenol or phenylhydroquinone to the skin of CD-1 mice produced
four distinct major and several minor adducts in skin DNA. Total
covalent binding in skin DNA was 0.31 fmol/µg DNA after treatment with
10 mg of sodium 2-phenylphenol and 0.62 fmol/µg DNA with 20 mg. The
adducts were not observed in skin DNA of untreated animals.
Pretreatment of the mice with a-naphthylisothiocyanate, an inhibitor
of cytochrome P450, or indomethacin, an inhibitor of prostaglandin
synthase, resulted in lower numbers of DNA adducts. Incubation of DNA
with 2-phenylphenol or phenylhydroquinone in vitro in the presence
of cytochrome P450 or prostaglandin synthase activation systems
resulted in four major adducts. The pattern of chromatographic
mobility observed in vitro in the presence of these enzymatic
systems appeared to be similar to that of adducts in vivo. The
chemical reaction of DNA or deoxyguanosine monophosphate with
phenylbenzoquinone also resulted in four major and several minor
adducts. The four major adducts were identical in chromatographic
mobility to the four major adducts produced in vivo and in vitro.
The results show that 2-phenylphenol and phenylhydroquinone can bind
covalently to DNA and that one of the DNA-binding metabolites of
2-phenylphenol may be phenylbenzoquinone (Pathak & Roy, 1993).
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
2-Phenylphenol
Rats
Groups of 35 albino Sprague-Dawley rats, nine weeks old at the
start of the study, were fed diets containing technical-grade
2-phenylphenol (purity, 99.4-99.5%) at concentrations of
200-10 000 ppm, equal to 0, 36, 120, and 460 mg/kg bw per day, for two
generations. The dose was adjusted during the premating period
according to changes in body weight, and adjustments were made during
lactation to avoid overdosing the pups, although this was later
considered unnecessary. Two F1 females at the high dose and 12 F2a
pups were removed from the study for examination of the heritability
of hypotrichosis. Parents (F0) for the F1 generation were assigned
randomly to dose groups. The genealogy of the F1b pups was checked
to prevent the mating of littermates. For production of the F2
generation, 132 male and 134 female F1b pups were selected
randomly,one or more pups of each sex per litter being used as parents
(F1), divided into 35 pairs of rats per dose, except for the
controls which consisted of 27 males and 29 females. Estrus cycles
were studied by vaginal smears. The animals were examined daily, and
routine observations of birth statistics and pup weights were made;
the litters were culled to eight pups when necessary. Standard
observations and extensive histological examination of the urinary
tract were carried out at sacrifice.
Treatment did not affect clinical signs, body-weight gain during
gestation or lactation, or any of the reproductive variables examined.
Histological examination of the adults and pups revealed no
significant changes in the reproductive tracts. F0 and F1 adults
at 460 mg/kg bw per day showed a treatment-related decrease in body
weight which was not clearly related to a decrease in food
consumption, and F1b, F2a, and F2b pups showed a statistically
significant decrease in body weight on days 14 and/or 21 of lactation,
an effect not seen during the first week of lactation, indicating that
development had not been disturbed. The relative weight of the kidneys
was increased in a dose-dependent manner, in the absence of changes in
other organ weights, in males of the F0 and F1 generations. Male
rats at 120 and 460 mg/kg bw per day had an increased incidence of
calculi in the urinary tract. Transitional-cell hyperplasia was found
in the urinary bladder, defined in the report as 'an area (focal or
diffuse) of at least three to four cells thick (of cuboidal cells) in
an inflated bladder', whereas normal bladders had a cell thickness of
one or two flattened cells. Quantification by simple morphometry
indicated a compound-related effect in the bladder in F0 males and
females at 120 and 460 mg/kg bw per day and in F1 males at 457 mg/kg
bw per day. Neoplasms of the urinary tract were found in four rats:
one bladder and one ureteric tumour in rats at 125 mg/kg bw per day
and two bladder tumours in rats at 457 mg/kg bw per day. The NOAEL for
reproductive toxicity was 460 mg/kg bw per day and that for
carcinogenicity was 36 mg/kg bw per day (Eigenberg, 1990).
In a similar study, groups of 30 CD Sprague-Dawley rats were fed
diets containing technical-grade 2-phenylphenol (purity, 99.5-100%) at
concentrations of 200-10 000 ppm, equal to 0, 17, 92, and 460 mg/kg bw
per day, for two generations. The dose was adjusted during the
premating period according to changes in body weight. The F0 and
F1 adults received the compound in the diet throughout the study,
beginning at 7 weeks of age for the F0 adults and at weaning for the
F1 adults. The animals received treated feed for 10 weeks before
breeding, beginning approximately 2 weeks after weaning of the last
F1b litter for F1 parents. F0 adults were mated to produce the
F1a and F1b litters, and F1 adults (consisting of randomly
selected F1b pups) were mated to produce the F2a and F2b
litters.Adult animals were evaluated during the study for effects of
2-phenylphenol on body weight, food consumption, clinical signs,
estrous cycling, mating, fertility, length of gestation, and litter
size. The offspring were evaluated for effects on sex ratio,
viability, body-weight gain, and clinical signs. Gross necropsy was
performed on all adults and pups, and the reproductive organs,
pituitary, kidneys with ureter attached, urinary bladder, and gross
lesions of all F0 and F1 adults were evaluated histologically.
At 460 mg/kg bw per day, urine staining was observed in F0
males and females and F1 males, urinary bladder calculi were found
at necropsy in F1 adult males, and one F0 male died from renal
failure. At this dose, there was an increase in food consumption by
females during lactation, decreased pup weight, and a decrease in the
terminal body weight of F0 and F1 adult males and females.
Histopathological examination of the kidneys revealed debris in the
renal pelvis, chronic active inflammation, and increased severity of
background lesions in F0 and F1 males. Further, transitional-cell
hyperplasia (simple, nodular, or papilary) of the bladder, calculi,
chronic inflammation of the bladder, and dilatation and hyperplasia of
the ureter were seen in F0 and F1 males. Two F1 males at this
dose had malignant lymphomas in several tissues. One F1 female at 92
mg/kg bw per day had a nephroblastoma, and one F0 male at the high
dose and one control F1 female had a pituitary adenoma. All of these
lesions were considered to be incidental to treatment.
There were no treatment-related effects on adult reproductive
parameters and no effect on litter size, sex ratio, the number of
stillborn pups, pup viability, or clinical signs, no gross lesions in
the pups, and no treatment-related effects on the organ weights of
adults. The mean live birth indexes (with standard error) were 98
(0.91) in the controls, 99 (0.77) at 17 mg/kg bw per day, 98 (1.3) at
92 mg/kg bw per day, and 98 (0.85) at 460 mg/kg bw per day in the
F1a generation; 98 (0.85) in the controls, 98 (1.5) at 17 mg/kg bw
per day, 99 (0.71) at 92 mg/kg bw per day, and 99 (0.63) at 460 mg/kg
bw per day in the F1b generation; 99 (0.84) in the controls, 98
(1.0) at 17 mg/kg bw per day, 97 (1.4) at 92 mg/kg bw per day, and 100
(0.38) at 460 mg/kg bw per day in the F2a generation; and 97 (1.2)
in the controls, 99 (0.78) at 17 mg/kg bw per day, 96 (1.9) at 92
mg/kg bw per day, and 99 (0.46) at 460 mg/kg bw per day in the F2b
generation. The differences between the groups were not statistically
significant. The NOAEL for reproductive toxicity was 460 mg/kg bw per
day, the highest dose tested. The NOAEL for systemic and developmental
toxicity was 92 mg/kg bw per day, on the basis of decreased body
weight and morphological lesions in the kidneys, urinary bladder, and
ureter and a decrease in pup body weight (Eigenberg, 1995).
(ii) Developmental toxicity
2-Phenylphenol and sodium 2-phenylphenol
Mice
Groups of 20-21 pregnant JCL-ICR mice were given 0, 1500, 1700,
or 2100 mg/kg bw per day of 2-phenylphenol (purity not given) or 100,
200, or 400 mg/kg bw per day of sodium 2-phenylphenol (purity not
given) by gavage on days 7-15 of gestation. The animals were weighed
daily, and any change in their general condition was noted. On day 18
of gestation, the dams were killed and their uteri opened, and the
numbers of implantation scars, fetuses that died in early and late
stages, and live fetuses were counted. The live fetuses were weighed
and sexed and observed for external abnormalities. The number of
corpora lutea in each ovary was counted, the major organs were
weighed, and examinations were made to determine whether any
macroscopic abnormalities were present.
With 2-phenylphenol, body-weight gain was reduced at 1700 and
2100 mg/kg bw per day. Four dams at 1500 mg/kg bw per day, seven at
1700 mg/kg bw per day, and 16 at 2100 mg/kg bw per day died. Pregnancy
was confirmed in 5/5 surviving dams at 2100 mg/kg bw per day, 14/14 at
1700 mg/kg bw per day, 14/17 at 1500 mg/kg bw per day, and 20/21
controls, in which implantation was confirmed at the time of sacrifice
and laparotomy on day 18 of gestation. The only significant changes
found at autopsy of these mice were reduced heart weights at 1700 and
2100 mg/kg bw per day and significantly increased liver weights at
1500 and 1700 mg/kg bw per day, a tendency that was also seen at 2100
mg/kg bw per day. Live fetuses were found in all pregnant dams. The
body weights of male and female fetuses at all three doses of
2-phenylphenol were significantly reduced, and the decrease was
dose-related in males. No unique external or internal deformities or
abnormalities were found in the fetuses, and the skeletal
abnormalities found were compatible with delayed development. No NOAEL
could be identified for maternal or fetotoxicity. The NOAEL for
developmental toxicity was 2100 mg/kg bw per day, the highest dose
tested.
With sodium 2-phenylphenol, body-weight gain was statistically
significantly reduced in a dose-dependent manner in dams at all doses.
Four animals at 200 mg/kg bw per day and 16 at 400 mg/kg bw per day
died. No abnormalities were seen at autopsy of dams on day 18 of
gestation. Significantly reduced liver, heart, and spleen weights were
recorded in animals at 400 mg/kg bw per day, while the weight of the
lungs was increased in dams at 200 mg/kg bw per day. Dams at 200 mg/kg
bw per day had a low average number of implantations and a low average
number of live fetuses. No NOAEL could be identified for maternal
toxicity. The NOAEL was 100 mg/kg bw per day for fetotoxicity and 400
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Ogata et al., 1978).
2-Phenylphenol
Rats
Groups of 18-20 pregnant Wistar rats were given 0, 150, 300, or
600 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by gavage on
days 6-15 of gestation. An additional group of 11 rats was given 1200
mg/kg bw per day, but this dose proved to be lethal. No untoward signs
of toxicity were observed in the controls or at 150 mg/kg bw per day.
At doses > 300 mg/kg bw per day, dose-related ataxia and decreased
mean body-weight gains were observed. All surviving rats were killed
on day 20 of gestation, and the uterine contents were examined.
Fetuses were grossly examined; the skeletons were examined with
Alizarin red S and the viscera by a modified Wilson method. The mean
numbers of implantation sites, live fetuses, resorptions, and fetal
weights in animals at 150 and 300 mg/kg bw per day were comparable to
those of controls, but at 600 mg/kg bw per day the number of fetal
resorptions was increased and fetal weight was decreased. Although a
few fetal anomalies were observed in all groups, they did not appear
to be related to treatment. The NOAEL was 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 600
mg/kg bw per day, the highest dose tested, for developmental toxicity
(Kaneda et al., 1978).
In a similar study, groups of 25-35 pregnant rats were given 0,
100, 300, or 700 mg/kg bw per day of 2-phenylphenol (purity, 99.7%) by
gavage on days 6-15 of gestation. They were killed on day 21, and the
fetuses were removed surgically. All fetuses were weighed, sexed, and
examined externally and skeletally, and the soft tissues of
approximately one-third of the fetuses were examined. One rat at the
high dose died as a result of a dosing accident. Pregnant rats given
700 mg/kg bw per day gained significantly less body weight during the
first 4 days of treatment (days 6-9 of gestation) than did controls,
and their food consumption was significantly decreased on days 9-11 of
gestation. At necropsy, the weights of the liver (but not the
liver:body weight ratios) were significantly decreased. There was no
effect on the number of implantation sites per dam, mean litter size,
incidences of resorptions, or fetal body weight or crown-rump length.
The only major malformation -- hypoplastic tail and missing sacral and
caudal vertebrae -- was observed in a single fetus at 300 mg/kg bw per
day. An increase in the incidence of delayed ossification of
sternebrae and unossified sternebrae was observed at 700 mg/kg bw per
day. The incidences of foramina and bony islands in the skull were
also slightly increased in this group. No adverse effects on embryonic
or fetal development were observed that were considered to be due to
2-phenylphenol. The NOAEL was 300 mg/kg bw per day for maternal
toxicity and 700 mg/kg bw per day, the highest dose tested, for
fetotoxicity and developmental toxicity (John et al., 1981).
Rabbits
In a range-finding study, groups of two non-pregnant New Zealand
white rabbits were given doses of 0, 100, 500, or 1000 mg/kg bw per
day of 2-phenylphenol (purity, 99.8%) in corn oil for 13 consecutive
days and were submitted to gross necropsy after the last day. The
animals were examined for clinical signs, body weights, body-weight
gain, kidney and liver weights, and gross appearance. The rabbits at
1000 mg/kg bw per day appeared to have stopped eating and had lost 24%
of their body weight by day 7. One rabbit at this dose died on day 8,
and the second was killed in moribund condition on day 10 with
nonspecific lesions or lesions secondary to anorexia. Rabbits given
500 mg/kg per day showed a slight decrease in body-weight gain. All
the other rabbits survived to the end of the study with no other
treatment-related effects. The dose of 100 mg/kg bw per day was
tolerated over the course of treatment.
In the second study, groups of seven artificially inseminated
females were given 0, 250, 500, or 750 mg/kg bw per day of
2-phenylphenol (purity, 99.8%) in corn oil by gavage on days 7-19 of
gestation. They were observed for clinical signs, body weight, and
body-weight gain. On day 20 of gestation, all surviving animals were
killed and examined for gross pathological alterations and changes in
liver and kidney weights. The uteri and ovaries were examined for
implantations, resorptions, and corpora lutea, and the liver, kidneys,
and stomach were examined histologically. Dose-related signs of
maternal toxicity were seen at all doses. One rabbit at 250 mg/kg bw
per day, two at 500 mg/kg bw per day, and six at 750 mg/kg bw per day
died. The dose-related effects observed included increased incidences
of haemorrhage, gaseous distension, and erosions of the stomach,
decreased or soft ingesta in the gastrointestinal tract, decreased
body weight and body-weight gain, increased absolute and relative mean
weights of the kidney, and increased incidence and/or severity of
renal tubular degeneration and inflammation. Treatment-related effects
were observed on reproductive, embryonal, or fetal parameters at 750
mg/kg bw per day.
In the third study, groups of 16-24 artificially inseminated
adult female New Zealand white rabbits were given 0, 25, 100, or 250
mg/kg bw per day of 2-phenylphenol (purity, 99.8%) in corn oil by
gavage on days 7-19 of gestation. They were observed for clinical
signs, body weight, and body-weight gain. On day 28 of gestation, all
surviving rabbits were killed and necropsied, when the weights of the
liver, kidney, and gravid uterus and the numbers of corpora lutea,
implantations, resorptions, and live and dead fetuses were recorded.
All fetuses were removed from the uterus, weighed, sexed, and examined
for external, visceral, and skeletal alterations. The kidneys of all
animals were examined histologically. Administration at 250 mg/kg bw
per day resulted in maternal toxicity evidenced by treatment-related
mortality (13%), gross pathological alterations (ulceration and
haemorrhage of the gastric mucosa, haemolysed blood in the intestinal
tract, and decreased ingesta), and histopathological alterations
(renal tubular degeneration and inflammation). No significant maternal
effects were observed at 25 or 100 mg/kg bw per day, and no adverse
embryonal or fetal effects were observed at any dose. The overall
NOAELs were 100 mg/kg bw per day for maternal toxicity, 500 mg/kg per
day for fetotoxicity, and 750 mg/kg per day, the highest dose tested,
for developmental toxicity (Zablotny et al., 1991).
(f) Special studies: Mechanisms of carcinogenicity in rat urinary
bladder
In a study to determine whether 2-phenylphenol is a complete skin
carcinogen or a promoter in a two-stage initiation and promotion
process, the compound was applied to the interscapular area of the
backs of 50 male and 50 female Swiss CD-1 mice at a dose of 55.5 mg in
0.1 ml acetone, three times per week for 2 years. A second group of 50
male and 50 female mice was treated identically except that their
backs were pretreated once with 0.05 mg in 0.1 ml acetone of
7,12-dimethylbenz[ a]anthracene (DMBA), a known initiator of skin
cancer. Additional groups of 50 male and 50 female mice served as
acetone vehicle controls, controls treated once with DMBA and
thereafter only with acetone, and a positive control group treated
once with DMBA and thereafter with 12- O-tetradecanylphorbol
13-acetate (TPA), a known promoter of skin cancer, at a dose of 0.005
mg in 0.1 ml acetone, three times per week for 2 years.
The mean body weights and survival of the mice treated with
2-phenylphenol or with DMBA plus 2-phenylphenol were generally similar
to those of the respective negative control groups, but the survival
of the group given DMBA plus TPA was substantially decreased. In this
group, the incidences of squamous-cell papillomas and carcinomas,
keratocanthomas, and basal-cell carcinomas at the site of application
were clearly increased (52/100) over that in the group given DMBA plus
acetone(15/100). The time to tumour was also substantially decreased
in the group given DMBA plus TPA. Similar neoplastic skin lesions were
observed with DMBA plus acetone (17/100), but at an incidence
equivalent to that in the control group (15/100). No neoplastic skin
lesions were observed in the group given 2-phenylphenol. The author
concluded that 2-phenylphenol is not carcinogenic alone or as a
promoter (Luster, 1986).
The promoting effect of 2-phenylphenol (purity, 98%) and sodium
2-phenylphenol (purity, 97%) in the urinary bladder was studied in
male Fischer 344 rat initiated with
N-nitrosobutyl- N-(4-hydroxybutyl)amine (NBHBA). Groups of 30 rats
were given drinking-water containing 0.01% NBHBA for 4 weeks and then
diets containing 20 000 ppm of sodium 2-phenylphenol (equivalent to
1000 mg/kg bw per day) for 32 weeks, NBHBA for 4 weeks followed by
untreated feed for 32 weeks, or drinking-water without NBHBA for 4
weeks followed by diet containing 20 000 ppm of sodium 2-phenylphenol
for 32 weeks. In another experiment, groups of 30 male rats were given
0.05% NBHBA in drinking-water for 4 weeks followed by diets containing
20 000 ppm of sodium 2-phenylphenol or 20 000 ppm of 2-phenylphenol
for 32 weeks, no NBHBA for 4 weeks, and then 20 000 ppm of sodium
2-phenylphenol (15 rats) or 20 000 ppm of 2-phenylphenol (15 rats) in
the diet for 32 weeks. In a third experiment, groups of 15 rats were
given diets containing 0, 20 000 ppm of sodium 2-phenylphenol, or
20 000 ppm of 2-phenylphenol. Urine samples were obtained from these
rats by forced urination on days 27, 29, and 32.
Administration of 20 000 ppm sodium 2-phenylphenol in the diet
significantly increased the incidence and number of preneoplastic
lesions (papillary or nodular hyperplasia) per 10 cm of basement
membrane of the urinary bladder in male rats pretreated with 100 ppm
NBHBA, and the incidence and number of papillomas and carcinomas of
the urinary bladder in the group pretreated with 500 ppm NBHBA.
Moreover, treatment with sodium 2-phenylphenol alone, without
initiation, induced papillary or nodular hyperplasia, papillomaa, and
carcinoma. In contrast, administration of 2-phenylphenol in the diet
after initiation only slightly increased the incidence of urinary
bladder lesions over that with NBHBA alone, and its effect was not
statistically significant. No tumours of the urinary bladder were
induced by 2-phenylphenol alone. The authors concluded that sodium
2-phenylphenol, and not 2-phenylphenol, has tumour promoting activity
and might be a complete carcinogen in rat urinary bladder. Since the
sodium salt increased the pH of the urine, the authors speculated that
an active metabolite reaches the urinary bladder at a higher
concentration than with 2-phenylphenol. They suggested that sodium
2-phenylphenol is a carcinogens that acts by a non-genotoxic mechanism
(Fukushima et al., 1983).
In a similar study, 2-phenylphenol or sodium 2-phenylphenol
(purity of neither given) was administered in the diet at a
concentration of 20 000 ppm to 28 male Fischer 344 rats for 64 weeks.
One rat receiving sodium 2-phenylphenol had small stones in the
urinary bladder, and this compound, but not 2-phenylphenol, induced
papillary or nodular hyperplasia (19/28), papillomas (5/28), and
carcinomas (6/28) of the urinary bladder. Pretreatment of additional
rats with NBHBA increased the incidence of papillary or nodular
hyperplasia ( p < 0.05), papillomas (not significant), and
carcinomas (not significant) over that in rats treated with NBHBA
alone. In another experiment, 2-phenylphenol or sodium 2-phenylphenol
was administered in the diet at concentrations of 2500, 5000, 10 000,
or 20 000 ppm to groups of five to nine male Fischer 344 rats for up
to 104 weeks. Animals from each group were killed and examined at 4,
8, 12, 24, 36, and 104 weeks. No stone formation was observed in the
urinary bladders of rats treated with sodium 2-phenylphenol. At 20 000
ppm, simple hyperplasia of the urinary bladder was observed from 4
weeks in 5/5 animals, papillary or nodular hyperplasia from 36 weeks
in 5/5 animals, and papillomas in 2/5 and carcinomas in 2/5 at 104
weeks. At 10 000 ppm of sodium 2-phenylphenol, only simple hyperplasia
was observed from 36 weeks. 2-Phenylphenol alone did not cause bladder
tumours and did not enhance the bladder lesions induced by NBHBA (Ito,
1983b).
In a short-term assay for bladder carcinogenicity in rats,
increased agglutinability of bladder epithelial cells with
concanavalin A was observed after a 1-week treatment with 10 000 or
20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol (purity of
neither given), suggesting that these compounds cause bladder cancer.
No such increase was observed in rats fed diets containing
rho-phenylphenol or biphenyl derivatives at 20 000 ppm. In male Fisher
rats fed diets containing 20 000 ppm of sodium 2-phenylphenol for 50
weeks, bladder papillomas developed in 19 of 36 rats and bladder
carcinomas in 14 of 36 rats (Honma et al., 1983).
Groups of 20 male Fischer 344 rats were fed diets containing
20 000 ppm of 2-phenylphenol, 20 000 ppm of sodium 2-phenylphenol
(purity of neither given), or 5000 ppm of biphenyl for up to 24 weeks.
Changes in the amounts of DNA synthesis and in the morphology of the
renal papilla and renal pelvis were recorded under light and scanning
electron microscopes. Increased DNA synthesis in both renal papilla
and pelvis and distinct morphological alterations in the cell surface
were seen with 2-phenylphenol and its sodium salt by 4 weeks.
Sequential light microscopy revealed renal papillary necrosis in
animals fed 2-phenylphenol from week 4, followed by regenerative
hyperplasia at weeks 16 (1/5) and 24 (3/5), but no changes in the
renal pelvis. Feeding of sodium 2-phenylphenol caused similar changes
in the renal papillae and also hyperplasia in the renal pelvis (2/5).
No proliferative response of the kidney was apparent in rats fed
biphenyl. The authors concluded that the proliferative responses
caused by sodium 2-phenylphenol in the renal pelvic epithelium were
similar to those induced by this compound in the urinary bladder
(Shibata et al., 1989a).
The interactive effects of ascorbic acid, saccharin, and hippuric
acid on the carcinogenicity of 2-phenylphenol and sodium
2-phenylphenol were studied in groups of 20 male Fischer 344 rats. The
animals were given 2-phenylphenol (purity, 99.5%) or sodium
2-phenylphenol in the diet at a concentration of 20 000 ppm
(equivalent to 1000 mg/kg bw per day) for 24 weeks with or without
ascorbic acid, sodium ascorbate, acid saccharin, sodium saccharin,
hippuric acid, or sodium hippurate at 50 000 ppm. The urinary sodium
concentration was increased in all animals receiving sodium salts
and/or sodium 2-phenylphenol. The pH of the urine was increased in
those given sodium 2-phenylphenol, sodium ascorbate, or sodium
saccharin, and the osmolality was decreased in those given sodium
2-phenylphenol, sodium ascorbate, or sodium hippurate. 2-Phenylphenol
decreased the osmolality but did not affect the pH or sodium
concentration of urine. Histopathologically, the bladders of rats
given sodium 2-phenylphenol showed epithelial thickening (epithelial
thickness, four to eight cells) at 8, 16, and 24 weeks and papillary
and nodular changes at 16 and 24 weeks. Treatment with the other
sodium salts provoked 'slight to moderate' hyperplasia at 8 and 16
weeks but no papillary or nodular changes; the changes had regressed
by 24 weeks. The combination of raised urinary pH and sodium promoted
the effects of sodium 2-phenylphenol, while sodium hippurate raised
urinary sodium but not pH and had no effect (Fukushima et al., 1989).
In an essentially similar study, groups of 31 male Fischer 344
rats received NaHCO3 to raise the urinary pH or NH4Cl to lower it.
2-Phenylphenol was given at a dietary concentration of 12 500 ppm
(equivalent to 625 mg/kg bw per day) and sodium 2-phenylphenol at 20
000 ppm (equivalent to 1000 mg/kg bw per day or 625 mg/kg bw per day
of 2-phenylphenol). Hyperplasia of the bladder epithelium was seen in
animals given 2-phenylphenol, 2-phenylphenol plus NaHCO3, or sodium
2phenylphenol. Administration of the sodium salt with NH4Cl had no
significant effect. The incidence of tumours was significantly
increased with 2-phenylphenol (12/31), sodium 2-phenylphenol (22/31),
and 2-phenylphenol plus NaHCO3 (20/31), but only three tumours were
seen in 31 rats given sodium 2-phenylphenol plus NH4Cl. Thus, the
carcinogenic effects of 2-phenylphenol were promoted in alkaline
urine, and those of sodium 2-phenylphenol were inhibited in acid urine
(Fujii et al., 1987).
Changes in urinary parameters, particularly electrolyte levels
and pH, DNA synthesis, and the morphology of the bladder epithelium
were investigated in Fischer 344 rats fed diets containing various
sodium, potassium, magnesium, and calcium carbonate salts at a
concentration of 30 000 ppm, with or without L-ascorbic acid at 50 000
ppm, for 4 or 8 weeks. The effects of treatment with NH4Cl at 10 000
ppm (to acidify urine) and of combined treatment with sodium ascorbate
at 50 000 ppm and NH4Cl were also investigated. Urinary pH was
significantly raised in groups given NaHCO3, K2CO3, ascorbic
acid plus NaHCO3, ascorbic acid plus K2CO3, or sodium ascorbate,
whereas treatment with ascorbic acid or NH4Cl alone caused a
significant decrease in urinary pH. Increases in urinary electrolyte
or ascorbic acid contents were associated with the corresponding
dosing regimen. DNA synthesis in the bladder epithelium was increased
in groups given NaHCO3, K2CO3, ascorbic acid plus NaHCO3,
ascorbic acid plus K2CO3, or sodium ascorbate. Furthermore, all
treatments that increased DNA synthesis also induced some
morphological alterations in the bladder epithelium. Administration of
ascorbic acid in conjunction with NaHCO3 or K2CO3 induced more
changes than those with either salt alone. In contrast, the degree of
response of the bladder epithelium of rats given sodium ascorbate was
reduced by simultaneous administration of NH4Cl. These results
suggest that the degree of DNA synthesis and/or morphological
alteration in rat bladder epithelium after treatment with various
bases depends on changes in the urinary concentrations of Na+ or
K+ and/or pH and the presence of ascorbic acid in the urine (Shibata
et al., 1989b).
The role of urinary pH and Na+ concentration on the
carcinogenic effect of 2-phenylphenol and sodium 2-phenylphenol on rat
urinary bladder was studied in two experiments. In the first, groups
of 36 male Fischer 344 rats were fed diets containing 20 000 ppm of
sodium 2-phenylphenol (equivalent to 1000 mg/kg bw per day), 12 500
ppm of 2-phenylphenol (equivalent to 625 mg/kg bw per day), 6400 ppm
of NaHCO3, 12 500 ppm of 2-phenylphenol plus 6400 ppm of NaHCO3,
12 500 ppm of 2-phenylphenol plus 3200 ppm of NaHCO3, or 12 500 ppm
of 2-phenylphenol plus 1600 ppm of NaHCO3 for 104 weeks. Body
weights were measured weekly up to week 14 and monthly thereafter.
Food consumption was measured on 2 consecutive days per week on a
per-cage basis. Urine samples were obtained from four to six rats in
each group by forced urination, and the urinary pH was determined 10
times during the 2-year experiment. For measurement of urinary
electrolytes, three or four rats in each group were housed
individually in metal metabolic cages without food or water for 4 h in
the morning during weeks 58, 80, and 96. All surviving animals were
killed at the end of the experiment and were examined carefully for
gross abnormalities at autopsy. The liver, kidney, and tissues with
macroscopic lesions were removed and fixed for histological
examination. Autopsies were also performed on all animals that died or
became moribund and were killed during the experiment.
Body-weight gain was reduced throughout the study in all treated
groups, but the reduction was less and started later in animals given
6400 ppm of NaHCO3 alone. The absolute and relative weights of the
bladder were significantly higher in treated groups than in controls,
especially in rats given 2-phenylphenol plus 6400 ppm of NaHCO3. The
relative weights of the kidneys and liver in all treated groups were
significantly higher than in controls. At week 104, 58% of rats given
sodium 2-phenylphenol and 68-84% of those in other groups were still
alive compared with 73% of controls. Macroscopically, more tumours
were found in bladders of rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm of NaHCO3 than in rats fed
2-phenylphenol plus 3200 ppm or 1600 ppm NaHCO3, and no tumours were
found in rats fed 2-phenylphenol alone or in controls. No stone
formation related to tumours was seen in any group. The bladder
lesions were classified as simple hyperplasia, papillary or nodular
hyperplasia, papilloma, and carcinoma. The incidences of bladder
carcinomas were significantly higher than controls in rats fed sodium
2-phenylphenol or 2-phenylphenol plus 6400 ppm NaHCO3.
In the other experiment, groups of five rats were given diets
supplemented with test chemicals as in the first experiment for only 8
weeks before being killed. Urinary electrolytes and pH were determined
at weeks 2, 4, 6, and 8, and osmolality was measured at weeks 4 and 8.
When all animals were killed at week 8, no stone formation was
observed macroscopically in any groups. Various changes in the luminal
surface of the bladder, particularly in rats fed sodium 2-phenylphenol
or 2-phenylphenol plus 6400 ppm of NaHCO3, were revealed by scanning
electron microscopy. The authors concluded that sodium 2-phenylphenol
is carcinogenic to the male rat bladder at 20 000 ppm in the diet.
2-Phenylphenol was not carcinogenic, although it induced a low
incidence of papillary or nodular hyperplasia (Fukushima et al.,
1989).
Species differences in the induction of urinary bladder lesions
by sodium 2-phenylphenol were studied in groups of 30 male Fischer 344
rats, B6C3F1 mice, Syrian golden hamsters, and Hartley guinea-pigs
fed diets containing 20 000 ppm of sodium 2-phenylphenol (purity not
given). Body weight and food consumption were determined periodically.
Groups of five animals from each group were killed at weeks 4, 8, 12,
24, 36, and 48, and five control animals were killed at weeks 12 and
48. Urine was collected in metabolism cages for 4 h from all animals
at weeks 12 and 48 for measurement of urine volume, pH, osmolality,
and microscopic appearance. Although food consumption did not differ
between the treated and untreated groups, retardation of growth was
associated with administration of sodium 2-phenylphenol in the diet,
especially during the first 8 weeks of the test. Although the absolute
weights of the liver were similar in both treated and untreated
groups, the relative weights were slightly increased in all species.
Morphological changes in the urinary bladder were remarkable only in
rats, which showed simple hyperplasia at week 4, increasing in
incidence and density to week 48. Lesions classified as papillary
nodular hyperplasia were observed in rats from week 36 of treatment,
but no papillomas were found. Scanning electron microscopy revealed
pleomorphic microvilli only in rats, which increased in grade with
time. In the other species, no changes indicative of proliferation
were observed, except for a slight effect in mice at weeks 24 and 48.
The pH of the urine was slightly increased in rats, while the
background pH in the other species was usually high, except in mice at
48 weeks. Osmolality was not affected by administration of sodium
2-phenylphenol, but crystal formation was seen in rats and
guinea-pigs, which increased slightly in rats with time. The authors
concluded that sodium 2-phenylphenol is likely to be a urinary bladder
carcinogen in rats but not in mice, guinea-pigs, or hamsters (Hasegawa
et al., 1990a).
Sex differences in the carcinogenic effect in rat urinary bladder
associated with administration of 2-phenylphenol (purity, > 99%) and
sodium 2-phenylphenol (purity, > 99%) were investigated in groups of
five or six male and five or six female Fischer 344 rats fed diets
containing 12 500 ppm of 2-phenylphenol (equivalent to 625 mg/kg bw
per day), 20 000 ppm of sodium 2-phenylphenol, equivalent to 1000
mg/kg bw per day), 30 000 ppm of NaHCO3, 10 000 ppm of NH4Cl,
2-phenylphenol plus NaHCO3, or sodium 2-phenylphenol plus NH4Cl
for 8 weeks. Body weights and food and water consumption were
determined weekly. Fresh urine specimens were obtained from all rats
by forced urination at week 8 and examined for pH and osmolality.
During the last week of the study, the rats were transferred to metal
metabolism cages without food or water, and urine samples were
collected from 07:00-13:00 h over 3 consecutive days to obtain enough
pooled urine for analysis and determination of metabolites, although
only pooled samples from the groups treated with 2-phenylphenol or its
sodium salt were examined.
No animals died before the end of the experiment, and food intake
was not significantly different between groups. The body weights at
week 8 were significantly lower in all treated groups of male rats and
in female rats given 2-phenylphenol or sodium 2-phenylphenol. The
urinary pH values for all groups were significantly different from the
7.0 found in untreated males and the 6.8 found in untreated females,
except in the groups given sodium 2-phenylphenol alone in which the pH
values were comparable to those of controls. The pH values were
highest in the groups given NaHCO3 alone, followed by the groups
given 2-phenylphenol plus NaHCO3, while the pH was lower than in
controls for groups given 2-phenylphenol alone, sodium 2-phenylphenol
plus NH4Cl, or NH4Cl alone. The Na+ concentrations were higher
in males than in females in all groups except controls and those given
NH4Cl. In the groups given sodium 2-phenylphenol alone, the Na+
concentration was slightly increased over control values in males but
not in females. The depressive effect of NH4Cl on Na+
concentration was also less pronounced in females. Only unconjugated
urinary metabolites were identified. No urothelial hyperplastic
changes were observed with 2-phenylphenol alone in either sex, while
an equimolar dose of sodium 2-phenyl-phenol induced mild papillary and
nodular hyperplasia or simple hyperplasia in male rats only. The
possible mechanisms underlying the differences in response between the
sexes might include excretion of other types of conjugated forms and
the formation of microcrystals such as the silicate crystals found in
the urine of rats fed sodium saccharin (Hasegawa et al., 1991).
The physiological effects of 2-phenylphenol (purity, 99.5%) on
urothelial cells and potential formation of DNA adducts were studied
in male Fischer 344 rats. In an initial experiment, rats were fed
dietary concentrations of 0, 1000, 4000, or 12 500 ppm for 13 weeks.
There was no evidence of urinary calculi, microcrystalluria, or
calcium phosphate-containing precipitate, but urothelial cytotoxicity
and hyperplasia were seen at the highest dose. In a second experiment,
rats were fed dietary concentrations of 0, 800, 4000, 8000, or 12 500
ppm for 13 weeks. The urinary pH was > 7 in all groups. The urinary
volume was increased at the highest dose, with consequent decreases in
osmolality and the concentrations of creatinine and other solutes. The
urinary excretion of total 2-phenylphenol metabolites was increased.
Most of the metabolites were conjugates of 2-phenylphenol and of
phenylhydroquinone, and free 2-phenylphenol and metabolites accounted
for < 2% at each dose. Urothelial toxicity and hyperplasia occurred
only at 8000 and 12 500 ppm. No 2-phenylphenol-DNA adducts were
detected in the urothelium at any dose. The small percentage of
unconjugated metabolites and the absence of DNA adducts suggest that
2-phenylphenol acts as a bladder carcinogen in male rats by inducing
cytotoxicity and hyperplasia without direct binding of the compound or
its metabolites to DNA (Smith et al., 1998).
The carcinogenic effect of sodium 2-phenylphenol and its
metabolites on female rat urinary bladder after intravesicular
instillation was studied in groups of nine 6-week-old Fischer 344 rats
that received 0.2 ml of a saline solution of 0.1% sodium
2-phenylphenol (purity, > 99%), phenylbenzoquinone (purity, > 99%),
or phenylhydroquinone (purity, > 99%) through a catheter into the
urethra once, twice, or four times. The pH values of the solutions
were 11 for sodium 2-phenylphenol, 6.5 for phenylbenzoquinone, and 6.4
for phenylhydroquinone. Saline or a solution of NaOH (pH 11) were
given to controls. The animals were maintained under light ether
anaesthesia during instillation and for a further 10 min thereafter to
prevent spontaneous urination. Two or three animals from each group
were killed under ether anaesthesia at 24 h and 4 and 7 days after the
last instillation. The histopathological findings in rats killed 24 h
after a single injection of saline, phenylbenzoquinone, or
phenylhydroquinone included swelling and vacuolation of urothelial
cells. The bladder epithelium of rats treated with alkaline solutions
of sodium 2-phenylphenol or NaOH showed minimal hyperplasia associated
with mild oedema and inflammatory-cell infiltration of the epithelial
and submucosal tissues. Moderate epithelial hyperplasia was seen in
rats killed 7 days after treatment with phenylbenzoquinone. In rats
given two or four instillations of this metabolite, the grading of the
hyperplastic changes was clearly dependent on the number of
instillations and the time between the last treatment and death. The
epithelial hyperplasia was marked and was classified as papillary
and/or nodular in rats treated with four instillations of
phenylbenzoquinone and killed 4 days after the last instillation. No
carcinomas were induced.
In another experiment, groups of 20 female rats were treated in
the same way but twice a week for 5 weeks. From week 6, some rats were
fed the basal diet supplemented with 5% sodium saccharin for 31 weeks
as a promotion treatment, while the other rats were maintained on
basal diet during this period. A separate group was given 500 ppm of
NBHBA for 4 weeks, and then 50 000 ppm of sodium saccharin as a
positive control. Body weights and food consumption were determined
periodically. The histopathological findings in the positive control
group included papillomas in two rats, papillary and/or nodular
hyperplasia in nine rats, and simple hyperplasia in 11 rats. In
contrast, no hyperplastic changes were seen in rats treated first with
sodium 2-phenylphenol or its metabolites followed by promotion with
sodium saccharin, except in nine rats given phenylbenzoquinone, which
had papillary and/or nodular hyperplasia and/or simple hyperplasia.
Formation of lymph follicles in the submucosa of the urinary bladder
was seen in particular with phenylbenzoquinone and in the positive
control group. The authors concluded that phenylbenzoquinone plays an
essential role in the urinary bladder carcinogenesis induced by sodium
2-phenylphenol (Hasegawa et al., 1990b).
A series of studies was carried out on the carcinogenicity of
2-phenylphenol and sodium 2-phenyl-phenol (purity of neither given) in
rat urinary bladder. In the first study, groups of 10 male Fischer 344
rats were fed diets containing sodium 2-phenylphenol at concentrations
of 0, 2500, 5000, 10 000, or 20 000 ppm for 36 weeks. The rats were
observed daily and were weighed periodically. Body-weight gain was
suppressed at 20 000 ppm. No calculi or mucosal tumours were found
grossly, but histological analysis revealed a statistically
significant increase in the frequency of bladder lesions in rats at
20 000 ppm, in which simple hyperplasia was seen in 10/10 and
papillary or nodular hyperplasia in 4/10 animals. Simple hyperplasia
was seen in 1/10 rats at 10 000 ppm.
In the second study, groups of five male rats were fed diets
containing 20 000 ppm of 2-phenylphenol or sodium 2-phenylphenol for 4
weeks. The animals were weighed at the end of treatment, at which time
the mean body weights of both treated groups were reduced, to 86% of
the control value with 2-phenylphenol and to 96% with sodium
2-phenylphenol. One hour before sacrifice, each rat was given an
intraperitoneal injection of 100 mg/kg bw of 5-bromo-2'-deoxyuridine
(BrdU), and the urinary bladders were stained immunohistochemically
with anti-BrdU antibodies to investigate the capacity of the
epithelial cells for proliferation. The number of cells that had taken
up BrdU per 1000 urinary bladder epithelial cells was determined by
light microscopy and expressed as per cent labelled cells. Rats fed
sodium 2-phenylphenol showed increased urinary pH and extensive
BrdU-labelling in urinary bladder epithelial cells, indicating
increased DNA synthesis. Rats fed 2-phenylphenol also showed a
tendency to increased BrdU-labelling, suggested that it also can
cause, albeit weak, urinary bladder epithelium proliferation.
In the third study, groups of five male Fischer 344 rats were
given diets containing 6400 ppm of NaHCO3; 13 000 ppm of
2-phenylphenol; 13 000 ppm of 2-phenylphenol plus NaHCO3 at 1600,
3200, or 6400 ppm; or 20 000 ppm of sodium 2-phenylphenol for 8 weeks.
The urinary pH at week 8 was significantly increased in animals fed
NaHCO3 alone, 2-phenylphenol plus 3200 or 6400 ppm NaHCO3, or
sodium 2-phenylphenol. The urinary concentrations of Na+ were
significantly increased at 2, 4, 6, and 8 weeks in rats fed sodium
2-phenylphenol and at 8 weeks in rats fed NaHCO3 alone or
2-phenylphenol plus 3200 or 6400 ppm NaHCO3. At 8 weeks, a
significant increase or a tendency to an increase in urine volume and
significantly lower osmotic pressure were seen in pooled urine samples
from all treated groups when compared with controls. When the bladders
were examined by scanning electron microscopy, the surface of the
epithelium appeared normal in the controls and in animals given only
NaHCO3, and was made up of polygonal cells of uniform dimensions
with reticular peaked microridges at the surface. In treated animals,
the cells in the outermost layer of the bladder epithelium assumed a
cobblestone configuration in pavement form; at high magnification,
pleomorphic microvilli, short uniform microvilli, and ropy or leafy
microridges were seen at the surface of these cells. These alterations
were observed mainly in rats given sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. The extent of the changes and
the frequency of their appearance was correlated with the
concentration of NaHCO3 given with 2-phenylphenol.
In the fourth study, groups of 30-31 male Fischer 344 rats were
given 20 000 ppm of sodium 2-phenylphenol or 13 000 ppm of
2-phenylphenol with or without NaHCO3 at 1600, 3200, or 6400 ppm for
104 weeks. The animals were observed daily for deaths, and body weight
and feed consumption were measured at regular intervals. Pooled 4-h
urine samples were collected from three or four rats at each dose at
weeks 58, 80, and 96 for measurement of electrolytes. The urinary pH
was significantly increased in rats fed sodium 2-phenylphenol or
2-phenylphenol alone or in combination with 3200 or 6400 ppm NaHCO3
or 6400 ppm NaHCO3 alone. The Na+ concentrations were
significantly increased in rats fed sodium 2-phenylphenol,
2-phenylphenol plus 6400 ppm NaHCO3, or 6400 ppm NaHCO3 alone. No
difference was seen in rats fed sodium 2-phenylphenol or
2-phenylphenol plus 6400 ppm NaHCO3. Urinary bladder tumours were
found in all groups except those given 2-phenylphenol alone, and the
frequencies were highest with sodium 2-phenylphenol and with
2-phenylphenol plus 6400 ppm NaHCO3. The presence of calculi could
not be confirmed. Histological examination of the urinary bladder
epithelium revealed simple hyperplasia, papillary or nodular
hyperplasia, papillomas, and carcinomas. Carcinomas occurred in rats
fed sodium 2-phenylphenol (41%), 2-phenylphenol plus 6400 ppm NaHCO3
(31%), and 6400 ppm NaHCO3 alone. The results confirm that
administration of 20 000 ppm of sodium 2-phenylphenol is carcinogenic
in male rats, while an equimolar concentration of 2-phenylphenol
causes only a low frequency of papillary or nodular hyperplasia and no
papillomas or cancers. Administration of NaHCO3 in conjunction with
2-phenylphenol caused carcinomas, correlated to the NaHCO3
concentration, which also increased urinary pH and Na+ concentration
(Inoue, 1993).
The induction of DNA damage in the urinary bladder epithelium of
male and female Fischer 344 rats by 2-phenylphenol and its metabolites
was studied by the alkaline elution assay after an intravesicalar
injection. Phenylbenzoquinone at 0.05-0.1% had weak DNA-damaging
activity in animals of each sex, whereas 2-phenylphenol and
phenylhydroquinone had no effect at the same dose. Histopathological
examination revealed diffuse, moderate, simple hyperplasia 5 days
after injection of 0.1% phenylbenzoquinone in male rats. The lesions
were associated with submucosal infiltration, small round cells, and
slight oedema. The only change in the bladders of rats injected with
0.1% phenylhydroquinone was slight swelling and/or vacuolization of
the epithelial cells, and the bladders of rats injected with 0.1%
2-phenylphenol were normal (Morimoto et al., 1989).
Groups of 5-10 Fischer 344 rats received diets containing sodium
2-phenylphenol at concentrations of 0, 2500, 5000, 10 000, or 20 000
ppm (equivalent to 0, 250, 500, 1000, and 2000 mg/kg bw per day) for
up to 5 months to investigate the correlation between urinary
phenylbenzoquinone and DNA damage in the bladder epithelium. Slight
but dose-dependent DNA damage was observed in the epithelium of male
rats fed 10 000 or 20 000 ppm for 3-5 months. A plot of the
dose-response relationship for DNA damage at 3 months showed a
threshold at 5000 ppm of sodium 2-phenylphenol. The amounts of
unconjugated 2-phenylphenol, phenylhydroquinone, and
phenylbenzoquinone in 24-h urine samples collected from males and
females after 5 months correlated well with the dietary concentrations
of sodium 2-phenylphenol. The total amounts of free metabolites in the
urine of males given 5000 ppm were similar to those in the urine of
females given 20 000 ppm. Free metabolites represented 0.3% of the
total average intake of male rats fed 5000 ppm, 0.8% of the intake of
10 000 ppm, and 1% of the intake of 20 000 ppm of sodium
2-phenylphenol. The average concentrations of free phenylhydroquinone
in the urine of males given 20 000 ppm of sodium 2-phenylphenol were
significantly higher than those in males fed 5000 ppm or in females
fed 20 000 ppm. The concentrations of phenylbenzoquinone were much
lower than those of phenylhydroquinone. Only 10% of phenylbenzoquinone
was recovered from spiked urine, indicating that this metabolite may
react with urinary nucleophilic groups. The authors concluded that
phenylbenzoquinone is the reactive species in the initiation of
bladder tumours induced by 2-phenylphenol and sodium 2-phenylphenol
(Morimoto et al., 1989).
The interaction of 2-phenylphenol and its metabolites with pUC18
DNA from Escherichia coli plasmids was studied in vitro. The major
metabolite formed from 2-phenylphenol by mixed-function oxidases was
phenylhydroquinone. This finding corroborates earlier reports that
phenylhydroquinone in the form of a glucuronide conjugate is the main
product in the bladders of rats fed 2-phenylphenol. When pUC18 DNA was
incubated with phenylhydroquinone, DNA strand scission was observed,
whereas barely detectable DNA cleavage was seen with 2-phenylphenol
and phenylbenzoquinone. DNA cleavage by phenylhydroquinone was
inhibited by superoxide dismutase, catalase, and several oxygen
radical scavengers, indicating that the oxygen radicals generated in
the process of oxidation of phenylhydroquinone in aqueous solution are
responsible for the DNA cleavage. The attack seemed to occur at
guanine residues in general and was not restricted to guanines with
specific residues, indicating no hot spots (Nagai et al., 1990).
The generation of 8-hydroxydeoxyguanosine in calf thymus DNA
treated with 2-phenylphenol, phenylhydroquinone, or
phenylbenzoquinone, was studied in vitro. The content of
8-hydroxydeoxy-guanosine residues was increased in DNA treated with
phenylhydroquinone in a concentration-dependent manner, but
phenylbenzoquinone had little effect, and 2-phenylphenol had no
effect. The formation of 8-hydroxydeoxyguanosine by phenylhydroquinone
was reduced by oxygen radical scavengers and accelerated by the
addition of CuCl or CuCl2. Hydroxyl radicals generated during
oxidation of phenylhydroquinone thus contribute to the formation of
8-hydroxydeoxyguanosine in DNA, and copper ions facilitate the
oxidative DNA damage. Copper ions greatly accelerated
phenylhydroquinone-induced DNA cleavage in vitro, although they had
no effect on cleavage without phenylhydroquinone. In contrast, DNA
cleavage occurred with the addition of FeCl2 in the absence and
presence of phenylhydroquinone. The formation of
8-hydroxydeoxyguanosine in bladder DNA is likely to be one of a series
of events in the carcinogenesis induced by 2-phenylphenol (Nagai et
al., 1995).
The effect of the selective gamma-glutamylcysteine synthetase
inhibitor, buthionine sulfoximine, on the hepatotoxic and nephrotoxic
potential of 2-phenylphenol and its metabolites was studied in groups
of four male Fischer 344/DuCrj rats. The animals were given an
intraperitoneal injection of 0 or 900 mg/kg bw of buthionine
sulfoximine and 1 h later received 2-phenylphenol, phenylhydroquinone,
or phenylbenzoquinone at single oral doses of 0, 700, or 1400 mg/kg
bw. The rats were killed 6 and 24 h later, and serum was collected for
measurement of alanine and aspartate aminotransferase activities and
urea nitrogen. The liver and kidneys were removed and weighed, and
hepatic and renal glutathione were assayed.
2-Phenylphenol caused acute hepatocellular damage, as shown by
necrotic centrilobular hepatocytes accompanied by increased serum
aminotransferase activity. Pretreatment with buthionine sulfoximine
potentiated the hepatic and renal toxicity of 2-phenylphenol,
indicating that the liver and kidneys are its target organs of at high
doses. 2-Phenylphenol depleted hepatic and renal glutathione by 6 h
after administration, and this effect was enhanced by pretreatment
with buthionine sulfoximine. Recovery of glutathione concentrations in
both organs was slower in rats given 1400 mg/kg bw of 2-phenylphenol
than in those given 700 mg/kg bw, suggesting that the hepatic and
renal damage caused by this compound is associated with prolonged
depletion of glutathione and that it acts indirectly on the liver.
Within 24 h, 75% of the rats treated with phenylbenzoquinone at 1400
mg/kg bw had died. Administration of phenylbenzoquinone at 700 m/kg bw
or phenylhydroquinone at 1400 mg/kg bw significantly increased
aminotransferase activities. The activity of alanine aminotransferase
in both groups was about twice that of rats given 1400 mg/kg bw
2-phenylphenol. A slight decrease in liver weight, nuclear pyknosis,
eosinophilic degeneration of periportal hepatocytes, increased
relative kidney weight, slight renal papillary necrosis, dilatation of
renal tubules, and increased serum urea nitrogen concentration were
observed at 700 mg/kg bw of phenylbenzoquinone. The relative weight of
the kidneys was increased at 1400 mg/kg bw of phenylhydroquinone.
These results indicate that phenylbenzoquinone is more toxic to liver
and kidney than phenylhydroquinone (Nakagawa & Tayama, 1988).
In a study of the conjugation of 2-phenylphenol with glutathione
in rat liver in vitro and in vivo, radiolabel derived from
[14C]2-phenylphenol bound irreversibly to hepatic microsomal
macromolecules in an NADPH-generating system, and the binding was
inhibited by cysteine and glutathione. When [14C]2-phenylphenol and
glutathione were incubated in a microsomal NADPH-generating system,
the radiolabelled material derived from the aqueous phase of the
incubation mixture was similar to a synthetic, water-soluble
phenylhydroquinone-glutathione conjugate produced by a nonenzymic
reaction between phenylbenzoquinone and glutathione.
Phenylhydroquinone-glutathione was excreted as a minor conjugate in
the bile after oral administration of 2-phenylphenol to rats at a dose
of 1000 mg/kg bw. The cumulative biliary excretion of the conjugate
over 6 h represented about 4% of the dose. The results show that a
reactive intermediate of 2-phenylphenol can form adducts with
glutathione to produce water-soluble conjugates. The reactive
intermediate is probably phenylbenzoquinone derived from
phenylhydroquinone. Since glutathione protects against cellular
injury, the acute hepatic damage caused by high doses of
2-phenylphenol is probably associated with the formation of an active
intermediate (phenylbenzoquinone) which depletes cellular glutathione
(Nakagawa & Tayama, 1989).
The relationship between the metabolism and cytotoxicity of
2-phenylphenol was studied in isolated rat hepatocytes. Addition of
high concentrations of 2-phenylphenol to the cells caused
dose-dependent toxicity, with death at the highest dose of 1.0 mmol/L.
Pretreatment of the hepatocytes with a non-toxic dose of 5 µmol/L of
SKF-525A enhanced the cytotoxicity of 2-phenylphenol at 0.5-1.0 mmol/L
and inhibited its metabolism. At lower concentrations (0.5 or 0.75
mmol/L), 2-phenylphenol was converted sequentially to
phenylhydroquinone and then to its glutathione conjugate. The
concentrations of both metabolites and especially of the conjugate,
were very low in hepatocytes exposed to 2-phenylphenol at 1.0 mmol/L
alone or with SKF-525A. The cytotoxicity induced by 2-phenylphenol at
0.5 mmol/L was enhanced by the addition of 1.25 mmol/L of
diethylmaleate, which continuously depletes cellular glutathione. In
contrast, the cytotoxicity induced by phenylhydroquinone at 0.5 mmol/L
was significantly inhibited by addition to the hepatocytes of 5 mmol/L
of dithiothreitol, cysteine, N-acetyl-L-cysteine, or ascorbic acid.
Loss of glutathione, protein thiols, and ATP was also prevented. These
results indicate that the acute cytotoxicity of 2-phenylphenol at 1.0
mmol/L is a direct action and that prolonged depletion of cellular
glutathione enhances the cytotoxicity of low concentrations of
2-phenylphenol metabolites. The cytotoxicity of phenylhydroquinone is
prevented significantly by addition of cysteine, glutathione, or
ascorbic acid (Nakagawa et al., 1992).
Groups of 22 male CDF (Fischer 344)/BR rats were given diets
containing 2phenylphenol (purity, 99.5%) to provide concentrations of
0, 800, 4000, 8000, or 12 500 ppm, equal to 0, 56, 280, 560, and 920
mg/kg bw per day, for 13 weeks. During weeks 12-13 and 13-14 of the
study, urine was collected for determination of metabolites and
urinary characteristics, respectively. In addition, urinary bladders
were collected from 12 animals per group during week 14 for analysisof
the urothelium by 32P-postlabelling, while histopathological
evaluation of 10 animals group included determination of a labelling
index and light and scanning electron microscopy. The body-weight gain
was reduced by about 10% at 8000 and 12 500 ppm, but food intake was
unaffected at all doses tested. Histological examination showed simple
hyperplasia of the urothelium at concentrations of 8000 and 12 500 ppm
with significant changes in the bladder. The glucuronide and sulfate
conjugates of 2-phenylphenol and the hydroxylated metabolite
phenylhydroquinone were the major urinary metabolites, although the
major conjugate at all doses was the sulfate. Minute levels of free
2phenylphenol and phenylhydroquinone were found at all doses, free
phenylhydroquinone comprising 0.6-1.5% of the total metabolites
measured. An increase in the labelling index of the bladder epithelium
was observed at 8000 and 12 500 ppm. 32P-Postlabelled urothelial DNA
showed no evidence of formation of 2-phenylphenol-DNA adducts.
The authors concluded that a hyperplastic response of the urinary
bladder epithelium occurs after exposure to 2-phenylphenol at 8000 or
12 500 ppm, which are unequivocal carcinogenic doses for the bladder
of male rats, which is due to mild cytotoxicity with consequent
regenerative hyperplasia. The increased mitotic activity (labelling
index), the presence of very small amounts of free phenylhydroquinone
in the urine, and the absence of DNA adducts in the bladder epithelium
further suggest that the bladder carcinogenesis in male rats exposed
to 2-phenylphenol is probably mediated by an indirect, dose-dependent
cytotoxic effect on the bladder epithelium leading to regenerative
hyperplasia and subsequent tumorigenesis of epigenetic origin, rather
than to direct metabolic activation of 2-phenylphenol to reactive
metabolites capable of forming 2-phenylphenol-DNA adducts (Christenson
et al., 1996a).
Groups of 20-30 male CDF (Fischer344)/BR rats were given diets
containing 2-phenylphenol (purity, 99.9%) at concentrations of 0,
1000, 4000, or 12 500 ppm, equal to 0, 54, 220, and 680 mg/kg bw per
day, for 13 weeks. Animals from the control and high-dose groups were
allowed to recover for 4 weeks. Urine was collected for chemical and
and electron microscopic evaluation at various times, and urinary
bladders were collected from animals in the recovery groups during
weeks 4, 13, and 17 for histological evaluations which included
determination of a labelling indexes and light and electron
microscopy.
Body-weight gain was reduced only in rats at 12 500 ppm, and food
intake was unaffected at all doses. Weekly clinical examinations
showed an increased incidence of urine staining at 4000 and 12 500
ppm. No unusual precipitate or crystal was found in the urinary
sediment of treated animals. Urothelial hyperplasia was observed only
after 13 weeks at 12 500 ppm, and the effect was reversed by 4 weeks
on control diet. After 4 and 13 weeks of exposure to 2-phenylphenol,
necrotic foci were observed in the bladders of rats at 12 500 ppm, and
at 13 weeks the bladders also showed evidence of regenerative
hyperplasia. Increased labelling indexes were observed in the bladders
of animals at the high dose at 4 and 13 weeks, but the index had
returned to control values after 4 weeks' recovery, confirming the
reversibility of the proliferative changes in the urothelium. The
results of this study suggest that 2-phenylphenol acts by a mechanism
involving a cytotoxic action on the urothelium leading to the
formation of regenerative, reversible hyperplasia. The origin of the
cytotoxicity remains unclear, however, as no evidence was found of
either abnormal crystalluria or a calcium phosphate-containing
amorphous precipitate (Christenson et al. 1996b).
The relative importance of bladder distension, urinary pH, and
Na+ concentration in the induction of cell proliferation in the
bladder epithelium of rats fed various sodium salts was investigated.
In male rats fed a diet containing 5% NaHCO3, the bladder epithelium
showed an increased number of replicating cells, distension, increased
urinary pH, and a high urinary Na+ concentration. Cell proliferation
also occurred when the bladders were subjected to distension in
vivo by mechanical (female) or physiological (male) means.
Inclusion of CaCO3 in the diet increased the urinary pH without
altering other factors and did not induce cell proliferation, but
proliferation was increased when CaCO3 was combined with the
mechanical or physiological treatment. Thus, high urinary pH was of
secondary importance to bladder distension as a causative factor but
acted to enhance cell proliferation when distension occurred. Similar
findings were obtained with regard to the Na+ concentration. The
authors concluded that bladder distension is a prerequisite for
proliferation of epithelial cells in the bladders of rats fed diets
containing high concentrations of sodium salts and that changes in
urinary pH and Na+ concentration also determine the degree of
proliferation (Shioya et al., 1994).
3. Observations in humans
In one of the earliest studies on the toxicity of 2-phenylphenol,
skin irritation and sensitization due to exposure to this compound and
its sodium salt were evaluated in 100 male and 100 female, unselected
persons. A patch impregnated with the test material was placed in
direct contact with the skin of the back of each person, covered with
an impervious film, and taped securely in place. The first patch was
kept in constant contact with the skin for 5 days, at which time the
patch was removed and the reaction noted. A second patch was applied
in the same way 3 weeks after removal of the first patch and was kept
in direct contact with the skin for 48 h. Each subject was examined
immediately and again 3 and 8 days after removal of the second patch.
2-Phenylphenol as a 5.0% solution in sesame oil did not cause primary
irritation or sensitization, but sodium 2-phenylphenol was
significantly irritating when applied as a 5% or a 1% aqueous
solution. A 0.5% solution caused very slight, simple irritation,
whereas a 0.1% solution produced no irritation and no sensitization
(Hodge et al., 1952).
Comments
After oral administration to mice and rats, 2-phenylphenol and
its sodium salt are rapidly and extensively absorbed (95%) and
distributed. Excretion is also rapid in these species, being almost
complete within 48 h, and occurs mainly in urine (about 90%) and in
faeces (about 5%). Little radiolabel (< 1%) is retained in organs and
tissues, including the urinary bladder. After dermal application of
2-phenylphenol to humans, about 43% of the applied dose was absorbed
through the skin and about 58% was recovered in skin rinse and the
protective enclosure. Most of the absorbed radiolabel was recovered in
urine (99%, and only 1% was recovered in faeces. The absorption
half-time was 10 h, and the elimination half-time was 0.8 h. The rapid
excretion of the radiolabel into urine indicates that 2-phenylphenol
is unlikely to accumulate in humans exposed repeatedly. The metabolic
profiles of both compounds were similar in mice, rats, and humans at
the various doses tested. The main metabolic pathways are conjugation
of 2-phenylphenol or hydroxylation at the 5 position of the phenol
ring, followed by conjugation with glucuronide or sulfate. The parent
compound was detected in only very small amounts (0.4%) in urine. The
metabolic profile in plants raised no toxicological concern, since
about 90% of the residue found in oranges and pears is 2-phenylphenol
or its conjugates.
2-Phenylphenol and its sodium salt have low acute toxicity in
mice and rats treated orally, the LD50 values ranging from 600 to
3500 mg/kg bw. Neither 2-phenylphenol nor its sodium salt has been
classified by WHO for acute toxicity.
2-Phenylphenol and its sodium salt caused severe dermal
irritation in rabbits, and the sodium salt caused severe dermal
irritation in humans. 2-Phenylphenol irritated the eye of rabbits,
whereas the sodium salt caused only moderate ocular irritation.
Neither substance caused delayed contact hypersensitivity in
guinea-pigs or humans.
In medium- and long-term tests for toxicity, the urinary bladder
was regarded as the main toxicological target organ of both
2-phenylphenol and its sodium salt in male and female rats. At doses
of 200 mg/kg bw per day and above, hyperplasia, papillomas, and
transitional-cell carcinomas were seen with both compounds in male
rats. Increased mitosis was observed in the bladder epithelium three
days after the start of dosing, and thickening, i.e. simple
hyperplasia, was seen at 14 days. In female rats, hyperplasia and
papillomas were observed, but to a far lower degree than in males. In
male and female mice, the liver was the primary target organ.
Increased relative liver weights and an increased incidence of
hepatocellular adenomas were seen with 2-phenylphenol at doses of 500
mg/kg bw per day and above. Reduced body-weight gain was a common
finding in mice and rats. In 90-day studies, the NOAELs for
2-phenylphenol were 6300 ppm, equal to 760 mg/kg bw per day, in rats
and 300 mg/kg bw per day (the highest dose tested for up to 1 year) in
dogs. The NOAEL for the sodium salt was 5000 ppm, equivalent to 550
mg/kg bw per day, in mice and 2500 ppm, equal to 180 mg/kg bw per day,
in rats. In a 1-year study of toxicity, the NOAEL for 2-phenylphenol
was 800 ppm, equal to 39 mg/kg bw per day, in rats. In 2-year studies
of carcinogenicity, the NOAEL for 2-phenylphenol was 250 mg/kg bw per
day in mice and 800 ppm, equal to 39 mg/kg bw per day, in rats. In
2-year carcinogenicity studies with the sodium salt, the NOAEL for
carcinogenicity was 20 000 ppm, equal to 3000 mg/kg bw per day, in
mice and 2500 ppm, equivalent to 95 mg/kg bw per day, in rats. The
Meeting concluded that both 2-phenylphenol and its sodium salt are
carcinogenic in male rats and that 2-phenylphenol is carcinogenic in
male mice.
2-Phenylphenol has been more extensively tested for genotoxic
activity than its sodium salt. Within that limitation, the results for
the two compounds were similar. Data regarding covalent binding to DNA
in the urinary bladder of rats dosed with either compound were
conflicting. 2-Phenylphenol induced chromosomal aberrations in
cultured mammalian cells, but negative results were obtained in
vivo. The Meeting concluded that there are unresolved questions
about the genotoxic potential of 2-phenylphenol.
Several studies have been conducted to elucidate the mechanism of
the carcinogenic action of 2-phenylphenol and its sodium salt on the
male rat urinary bladder, since neither compound has a carcinogenic
effect on the urinary bladder of female rats or in mice, guinea-pigs,
or hamsters of either sex. No clear mechanisms have been found,
although raising the urinary pH or sodium concentration has a
promoting effect. There was some evidence from studies with the sodium
salt that initial irritation followed by hyperplasia might be involved
in the bladder carcinogenicity in male rats. In addition,
32P-postlabelling showed binding of 2-phenylphenol and its sodium
salt to DNA in the male rat urinary bladder in some but not in other
studies. The genotoxicity of the metabolites phenylhydroquinone and
dihydroxybiphenyl appears to be similar to that of the parent
molecules.
The Meeting concluded that the urinary bladder tumours observed
in male rats and the liver tumours observed in male mice exposed to
2-phenylphenol are threshold phenomena that are species- and
sex-specific, and that 2-phenylphenol is therefore unlikely to
represent a carcinogenic risk to humans. In coming to this conclusion,
the Meeting was aware that a working group convened by IARC had
classified 2-phenylphenol, sodium salt, in Group 2B (possibly
carcinogenic to humans) and 2-phenylphenol in Group 3 (not
classifiable as to its carcinogenicity to humans). The Meeting noted,
however, that the IARC classification is based on hazard
identification, not on risk assessment, and is furthermore limited to
published literature, with the exclusion of unpublished studies on
toxicity and carcinogenicity.
In two two-generation studies of reproductive toxicity in rats,
2-phenylphenol had no reproductive toxicity, even at 460 mg/kg bw per
day, the highest dose tested. The overall NOAEL for carcinogenicity
was 92 mg/kg bw per day, since urinary bladder tumours were found in
male rats at doses of 120 mg/bw per day and above.
In a study of developmental toxicity in mice with 2-phenylphenol
and its sodium salt, the NOAELs for 2-phenylphenol were below 1500
mg/kg bw per day (lowest dose tested) for maternal toxicity and
fetotoxicity and 2100 mg/kg bw per day (highest dose tested) for
teratogenicity. The NOAELs for the sodium salt were below 100 mg/kg bw
per day (lowest dose tested) for maternal toxicity, 100 mg/kg bw per
day for fetotoxicity, and 400 mg/kg bw per day (highest dose tested)
for teratogenicity. In two studies of developmental toxicity in rats,
the overall NOAELs for 2-phenylphenol were 150 mg/kg bw per day for
maternal toxicity, 300 mg/kg bw per day for fetotoxicity, and 700
mg/kg bw per day (highest dose tested) for teratogenicity. In two
studies of developmental toxicity in rabbits, the overall NOAELs for
2-phenylphenol were 100 mg/kg bw per day for maternal toxicity, 500
mg/kg bw per day for fetotoxicity, and 750 mg/kg bw per day (highest
dose tested) for teratogenicity.
The Meeting established an ADI of 0-0.4 mg/kg bw for
2-phenylphenol, on the basis of the NOAEL of 39 mg/kg per day in the
2-year study of toxicity (based on decreased body-weight gain and
hyperplasia of the urinary bladder) and carcinogenicity of the urinary
bladder in male rats and a safety factor of 100.
The Meeting determined that it was unnecessary to establish an
acute reference dose because of the low acute toxicity of
2-phenylphenol.
Toxicological Evaluation
Levels of 2-phenylphenol that cause no toxic effect
Mouse: < 250 mg/kg bw per day for carcinogenicity (lowest dose
tested; 2-year study of toxicity and carcinogenicity)
< 1500 mg/kg bw per day (lowest dose tested; study of
developmental toxicity; maternal toxicity)
2100 mg/kg bw per day (highest dose tested; study of
developmental toxicity; not teratogenic)
Rat: 800 ppm, equal to 39 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
460 mg/kg bw per day (two-generation study of reproductive
toxicity; no reproductive toxicity; highest dose tested)
92 mg/kg bw per day (two-generation study of reproductive
toxicity; carcinogenicity)
150 mg/kg bw per day (study of developmental toxicity;
maternal toxicity)
300 mg/kg bw per day (study of developmental toxicity;
developmental toxicity)
700 mg/kg bw per day (study of developmental toxicity;
teratogenicity)
Rabbit: 100 mg/kg bw per day (two studies of developmental toxicity;
maternal toxicity)
500 mg/kg bw per day (two studies of developmental toxicity;
fetotoxicity)
750 mg/kg bw per day (two studies of developmental toxicity;
teratogenicity)
Dog: 750 mg/bw per day (highest dose tested; 1-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.4 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Mechanistic studies on urinary bladder tumours in male rats
2. Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to
2-phenylphenol (unless otherwise specified)
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Rapid (24 h) and complete (95-100%), mice and rats
Dermal absorption Rapid and well absorbed (43%), humans
Distribution Small concentrations (< 1%) in tissues, mice and rats
Potential for accumulation No accumulation, mice, rats, and humans
Rate and extent of excretion Rapid and complete (95-100%), mice, rats, and humans
Metabolism in animals Glucuronide and sulfate of 2-phenylphenol and phenylhydroquinone,
mice and rats
Toxicologically significant compounds 2-Phenylphenol
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 2800 mg/kg bw
Rabbit, LD50, dermal > 5000 mg/kg bw
Rabbit, LC50, inhalation (4 h) > 36 mg/m3 air (aerosol)
Dermal irritation 2-Phenylphenol and its sodium salt: severe dermal irritation,
rabbits
Ocular irritation 2-Phenylphenol: occular irritation, rabbits
Sodium salt: slight ocular irritation, rabbits
Dermal sensitization 2-Phenylphenol and its sodium salt: no dermal sensitization,
guinea-pigs and humans
Short-term toxicity
Target/critical effect Body-weight decrease, mice and rats, and urinary bladder tumours,
male rats
Lowest relevant oral NOAEL 300 mg/kg bw per day, dogs
Lowest relevant dermal NOAEL No NOAEL, 1000 mg/kg bw per day, highest dose tested, rats
Lowest relevant inhalation NOAEL Not investigated
Genotoxicity Unresolved questions
Long-term toxicity and carcinogenicity
Target/critical effect Urinary bladder, male rats
Liver, male and female mice
Lowest relevant NOAEL 39 mg/kg bw per day, male rats
Carcinogenicity Urinary bladder tumours, male rats
Liver tumours, male and female mice
Reproductive toxicity
Reproductive target/critical effect No reproductive toxicity, rats
Lowest relevant reproductive NOAEL 460 mg/kg bw per day, highest dose tested, rats
Developmental target/critical effect Developmental toxicity at maternally toxic doses, mice
Lowest relevant developmental NOAEL 300 mg/kg bw per day, rabbits
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rats.
No evidence of neurotoxicity or neuropathology in medium- and
long-term studies, mice, rats, dogs, or in developmental toxicity
studies, mice, rats, and rabbits
Other toxicological studies
Medical data Dermal irritation with the sodium salt, not with 2-phenylphenol
Summary Value Study Safety factor
ADI 0-0.4 mg/kg bw Long-term study of toxicity 100
and carcinogenicity, rat
Acute reference dose Unnecessary
References
Bartels, M.J. & McNett, D.A. (1996) Quantitation of orthophenylphenol
metabolites in rat urine samples from a 32P-postlabeling study.
Unpublished report No. HET K-001024-062 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Bartels, M.J., Brzak, K.A., McNett, D.A. & Shabrang, S.N. (1997)
Ortho-phenylphenol (OPP): Limited metabolism study in human.
Unpublished report No. HET K-001024-059 from Dow Chemical
Company, Midland, Michigan, USA. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Bartels, M.J., McNett, D.A., Timchalk, C., Mendrala, A.L.,
Christenson, W.R., Sangha, G.K., Brzak, K.A. & Shabrang, S.N.
(1998) Comparative metabolism of orthophenylphenol in mouse,
rat and man. Xenobiotica, 28, 579-594.
Berdasco, N.M. (1991) Orthophenylphenol: Dermal sensitization
potential in the Hartley albino guinea pig. Unpublished report
No. K-001024-048 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Carreon, R.E. & New M.A. (1981) Dowicide*1: Acute percutaneous
absorption potential. Unpublished report No. HET K-1024-(37) from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S., Bartels, M.J. & Cohen, S.M. (1996a)
Technical grade orthophenylphenol: A 32P-postlabeling study
to examine the potential for the formation of DNA adducts in the
urinary bladder of the male rat. Unpublished report No. 94-972-AV
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Leng Associates, Midland, Michigan, USA.
Christenson, W.R., Wahle, B.S. & Cohen, S.M. (1996b) Technical grade
ortho-phenylphenol: A special subchronic dietary study to
examine the mechanism of urinary bladder carcinogenesis in the
male rat. Unpublished report No. 92-972-MS from Bayer
Corporation, Agriculture Division, Toxicology, Stilwell, Kansas,
and University of Nebraska Medical Center, Department of
Pathology & Micropathology, Omaha, Nebraska, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Cosse, P.F., Stebbins, K.E., Stott, W.T., Johnson, K.A. & Atkin, L.
(1990) Orthophenylphenol: Palatability/probe, four-week and
one-year oral toxicity studies in beagle dogs. Unpublished report
No. K-001024-039 from Dow Chemical Co., Midland, Michigan , USA.
Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Davies, R.E. & Liggett, M.P. (1973) Irritant effects of agricultural
disinfectant C2 on rabbit eye mucosa. Unpublished report No.
2215/169D/73 from Huntingdon Research Centre, England. Submitted
to WHO by Leng Associates, Midland, Michigan, USA,
Eigenberg, D.A. (1990) Two-generation dietary reproduction study in
rats using orthophenylphenol. Unpublished revised report No
85-671-02 from Mobay Corporation Health, Environment, Safety &
Plant Management, Corporate Toxicology Department, South Metcalf,
Stilwell, Kansas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Eigenberg, D.A. (1995) A two-generation dietary reproduction study in
Sprague Dawley rats using technical grade ortho-phenylphenol.
Unpublished report No. 93-672-VX from Bayer Corporation
Agriculture Division, Toxicology, South Metcalf, Stilwell,
Kansas, USA. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Fujii, T. & Hiraga, K. (1985) Carcinogenicity testing of sodium
orthophenylphenate in F344 rats. J. Saitana Med. School, 12,
277-287.
Fujii, T., Nakamura, K. & Hiraga, K. (1987) Effects of pH on the
carcinogenicity of ophenylphenol and sodium o-phenylphenate
in the rat urinary bladder. Food Chem. Toxicol., 25, 359-362.
Fukushima, S., Kurata, Y., Shibata, M., Ikawa, E. & Ito, N. (1983)
Promoting effect of sodium o-phenylphenate and o-phenylphenol
on two-stage urinary bladder carcinogenesis in rats. Jpn. J.
Cancer Res. (Gann), 74, 625-632.
Fukushima, S., Inoue, T., Uwagawa, S., Shibata, M. & Ito N. (1989)
Co-carcinogenic effects of NaHCO3 on o-phenylphenol-induced
rat bladder carcinogenesis. Carcinogenesis, 10, 1635-1640.
Gilbert, K.S. (1994a) Dowicide(R) 1 antimicrobial: Primary dermal
irritation study in New Zealand white rabbits. Unpublished report
No. K-001024-057B from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert, K.S. (1994b) Dowicide(R) 1 antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001024-57E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. (1994c) Dowicide(R) A antimicrobial: Dermal
sensitization potential in the Hartley albino guinea pig.
Unpublished report No. K-001025-014E from Toxicological Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Gilbert, K.S. & Crissman, J.W. (1994) Dowicide(R) 1 antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
no. K-001024-057A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Gilbert K.S. & Stebbins, K. (1994) Dowicide(R) A antimicrobial:
Acute oral toxicity study in Fischer 344 rats. Unpublished report
No. K-001025-014A from Toxicological Research Laboratory, Health
& Environmental Sciences, Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Hagiwara, A., Shibata, M., Hirose, M., Fukushima, S. & Ito, N. (1984)
Long-term toxicity and carcinogenicity study of sodium
o-phenylphenate in B6C3F1 mice. Food Chem. Toxicol., 22,
809-814.
Hasegawa, R., Takahashi, S., Asamoto, M., Shirai, T. & Fukushima, S.
(1990a) Species differences in sodium o-phenylphenate induction
of urinary bladder lesions. Cancer Lett., 50, 87-91.
Hasegawa, R., Furukawa, F., Toyoda K., Sato, H., Takahashi, M. &
Hayashi, Y. (1990b) Urothelial damage and tumor initiation by
urinary metabolites of sodium o-phenylphenate in the urinary
bladder of female rats. Jpn. J. Cancer Res., 81, 483-488.
Hasegawa, R., Fukuoka, M., Takahashi, T., Yamamoto, A., Yamaguchi, S.,
Shibata, M.A., Tanaka, A. & Fukushima, S. (1991) Sex differences
in o-phenylphenol and sodium ophenylphenate rat urinary
bladder carcinogenesis: Urinary metabolites and electrolytes
under conditions of aciduria and alkalinuria. Jpn. J. Cancer
Res., 82, 657-664.
Hiraga, K. (1983a) Short report of a 91-week feeding study of
orthophenylphenol (OPP) in male rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. (1983b) Carcinogenicity testing of sodium
orthophenylphenate in F-344/DuCrj rats. Submitted to WHO by Leng
Associates, Midland, Michigan, USA.
Hiraga, K. & Fujii, T. (1981) Induction of tumours of the urinary
system in F344 rats by dietary administration of sodium
o-phenylphenate. Food Cosmet. Toxicol., 19, 303-310.
Hiraga, K. & Fujii, T. (1984) Induction of tumours of the urinary
bladder in F344 rats by dietary administration of o-phenylphenol.
Food Cosmet. Toxicol., 22, 865-870.
Hodge, H.C., Maynard, E.A., Blanchet, H.J., Jr, Spencer, H.C. & Rowe,
V.K. (1952) Toxicological studies of orthophenylphenol (Dowicide
1). J. Pharmacol. Exp. Ther., 104, 202-210.
Honma, Y., Kakizoe, T., Komatsu, H., Niijima, T. & Sugimura, T. (1983)
Increased agglutinability of bladder epithelial cells by
concanavalin A in rats fed several biphenyl derivatives.
J. Cancer Res. Clin. Oncol., 106, 176-178.
Horvath, E., Levay, G. Pongracz, K. & Bodell, W.J. (1992) Peroxidative
activation of ophenylhydroquinone leads to the formation of DNA
adducts in HL-60 cells. Carcinogenesis, 13, 1937-1939.
IARC (1987) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Suppl. 7, Overall Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to
42, Lyon, International Agency for Research on Cancer, pp.
37-41.
IARC (1999) IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 73, Miscellaneous
Pesticides, Lyon, International Agency for Research on Cancer,
pp. 451-480.
Iguchi, S., Takahashi, H., Fujii, T., Fukumori, N., Mikuriya, H.,
Tada, Y., Yuzawa, K. & Hiraga, K. (1984) Subchronic toxicity of
o-phenylphenol (OPP) by food administration to rats. Ann.
Rep. Tokyo Metr. Res. Lab. Public Health, 35, 407-415.
Iguchi, S., Tayama, K. & Hiraga, K. (1979) Subacute toxicity of sodium
o-phenylphenate by food administration to rats. Ann. Rep.
Tokyo Metropolitan Res. Lab. Public Health, 30, 67-79.
Inoue, T. (1993) Pathological studies on rat urinary bladder
carcinogenicity of ophenylphenol and its sodium salt. J.
Nagoya City Univ. Medical Assoc. (Nagoya Shiritsu Daigaku
Igakkai Zasshi), 44, 463-477.
Inoue, S., Yamamoto, K. & Kawanishi, S. (1990) DNA damage induced by
metabolites of ophenylphenol in the presence of copper (II)
ion. Chem. Res. Toxicol., 3, 144-149.
Ishidate, M., Jr, Yoshikawa, K. & Sofuni, T. (1983) Mutagenicity tests
on OPP, OPPsodium salt, and their possible metabolites.
Unpublished report from Division of Mutagenesis, Biological
Safety Research Center, National Institute of Hygienic Sciences,
Tokyo, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983a) Long-term toxicity and carcinogenicity study of sodium
o-phenyl-phenate in B6C3F1 mice. Unpublished report from First
Department of Pathology, Nagoya City University Medical School,
Nagoya, Japan. Submitted to WHO by Leng Associates, Midland,
Michigan, USA.
Ito, N. (1983b) Pathological analysis of the carcinogenicity of sodium
o-phenyl-phenate and o-phenylphenol. Unpublished report from
First Department of Pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
John, J.A., Murray, F.J., Rao, K.S. & Schwetz B.A. (1981)
Teratological evaluation of orthophenylphenol in rats. Fundam.
Appl. Toxicol., 1, 282-285.
Kaneda, M., Teramoto, S., Shingu, A. & Shirasu, Y. (1978)
Teratogenicity and dominantlethal studies with o-phenylphenol.
J. Pestic. Sci., 3, 365-370.
Kolachana, P., Subrahmanyam, V.V., Eastmond, D.A. & Smith, M.T. (1991)
Metabolism of phenyhydroquinone by prostaglandin (H) synthase:
Possible implications in ophenylphenol carcinogenesis.
Carcinogenesis, 12, 145-149.
Kwok, E.S.C. & Eastmond, D.A. (1997) Effects of pH on nonenzymatic
oxidation of phenylhydroquinone: Potential role in urinary
bladder carcinogenesis induced by ophenylphenol in Fischer 344
rats. Chem. Res. Toxicol., 10, 742-749.
Lambert, A.C. & Eastmond, D.A. (1994) Genotoxic effects of the
o-phenylphenol metabolites phenylhydroquinone and
phenylbenzoquinone in V79 cells. Mutat. Res., 322, 243-256.
Landry, T.D., Stebbins, K.E. & Battjes, J.E. (1992)
Ortho-phenylphenol: Acute aerosol inhalation toxicity study in
Fischer 344 rats. Unpublished report No. K-001024-049 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Luster, M. (1986) NTP Technical Report on the Toxicology and
Carcinogenesis Studies of ortho-Phenylphenol (CAS No. 90-43-7)
Alone and with 7,12-Dimethylbenz(a)anthracene (CAS No.
57-97-6) in Swiss CD-1 Mice (Dermal Studies) (NIH publication
No. 86-2557, NTP TR 301), US Department of Health and Human
Services, National Toxicology Program, Research Triangle Park,
North Carolina, USA.
Luster, M.I., Dean, J.H., Boorman, G.A., Archer, D.L., Lauer, L.,
Lawson, L.D., Moore, J.A. & Wilson, R.E. (1981) The effects of
orthophenylphenol, tris(2,3dichloropropyl)phosphate and
cyclophosphamide on the immune system and host susceptibility of
mice following subchronic exposure. Toxicol. Appl. Pharmacol.,
58, 252-261.
McNett, D.A., Timchalk, C. & Mendrala, A.L. (1997) Ortho-phenylphenol
(OPP): Metabolism of 14C-labelled OPP in B6C3F1 mice and
Fischer 344 rats. Unpublished report No. HET K-001024-060 from
Toxicological Research Laboratory, Health & Environmental
Sciences, Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Leng Associates, Midland, Michigan, USA.
Morimoto, K., Sato, M., Fukuoka, M., Hasegawa, R., Takahashi, T.,
Tsuchiya, T., Tanaka, A., Takahashi, A. & Hayashi, Y. (1989)
Correlation between the DNA damage in urinary bladder epithelium
and the urinary 2-phenyl-1,4-benzoquinone levels from F344 rats
fed sodium o-phenylphenate in the diet. Carcinogenesis, 10,
1823-1827.
Nagai, F. & Nakao, T. (1984) Changes in enzyme activities in the urine
and tissues of rats fed sodium o-phenylphenate. Food Chem.
Toxicol., 22, 361-364.
Nagai, F., Ushiyama, K., Satho, K. & Kano, I. (1990) DNA cleavage by
phenylhydroquinone: The major metabolite of a fungicide
o-phenylphenhol. Chem.-Biol. Interactions, 76, 163-179.
Nagai, F., Ushiyama, K., Satoh, K., Kasai, H. & Kano, I. (1995)
Formation of 8hydroxyguanosine in calf thymus DNA treated
in vitro with phenylhydroquinone, the major metabolite of
o-phenylphenol. Carcinogenesis, 16, 837-840.
Nakagawa, Y. & Tayama, K. (1988) Effect of buthionine sulfoximine on
orthophenylphenolinduced hepato- and nephrotoxic potential in
male rats. Arch. Toxicol., 62, 452-457.
Nakagawa, Y. & Tayama, S. (1989) Formation of ortho-phenylphenol
glutathione conjugates in the rat liver. Xenobiotica, 19,
499-507.
Nakagawa, A., Nakao, T. & Nakao, M. (1984) Altered levels of cyclic
nucleotides in F344 rats fed sodium o-phenylphenate. Food
Chem. Toxicol., 22, 217-221.
Nakagawa Y., Tayama, S., Moore, G.A. & Moldéus, P. (1992) Relationship
between metabolism and cytotoxicity of ortho-phenylphenol in
isolated rat hepatocytes. Biochem. Pharmacol., 43, 1431-1437.
Nakao, T., Kabashima, U.J., Nagai, F., Nakagawa, A., Ohno, T.,
Ichikawa, H., Kabayashi, H. & Hiraga, K. (1983) The metabolic
profile of sodium o-phenylphenate after subchronic oral
administration to rats. Food Chem. Toxicol., 21, 325-329.
National Toxicology Program (1986) Toxicology and Carcinogenesis
Studies of orthoPhenylphenol (CAS No. 90-43-7) Alone and with
7,12-Dimethylbenz(a)anthracene (CAS No. 57-97-6) in Swiss CD-1
Mice (Dermal Studies) (NTP Technical Report No. 301, NIH
publication No. 86-2557), US Department of Health and Human
Services, Research Triangle Park, North Carolina, USA.
Norris, J..M. (1971) Eye irritation test conducted on Dowicide l
preservative. Unpublished report from Chemical Biology Research,
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by
Leng Associates, Midland, Michigan, USA.
Oehme, F.W. & Smith, T.H.F. (1972) The metabolism and urinary
excretion of ophenylphenol in dogs and cats. Toxicol. Appl.
Pharmacol., 22, 292.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1978) Teratological tests
of ortho-phenylphenol and its sodium salt in mice. Ann. Rep.
Tokyo Metr. Res. Lab. Public Health, 29, 89-96.
Ogata, A., Ando, H., Kubo, Y. & Hiraga, K. (1979) Acute oral toxicity
of sodiumphenylphenate (OPP-Na) in mice. Ann. Rep. Tokyo Metr.
Res. Lab. ublic Health, 30, 54.
Pathak, D.N. & Roy, D. (1992) Examination of microsomal cytochrome
P450-catalyzed in vitro activation of o-phenylphenol to DNA
binding metabolite(s) by 32P-postlabeling technique.
Carcinogenesis, 13, 1593-1597.
Pathak, D.N. & Roy, D. (1993) In vivo genotoxicity of sodium
ortho-phenylphenol: phenylbenzoquinone is one of the
DNA-binding metabolite(s) of sodium orthophenylphenol.
Mutat. Res., 286, 309-319.
Quast, J.F. & McGuirk, R.J. (1995) ortho-Phenylphenol: Two-year
dietary chronic toxicity/oncogenicity study in B6C3F1 mice.
Unpublished report No. K-001024-047 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1983) Molecular mechanisms involved in the toxicity of
orthophenylphenol and its sodium salt. Chem.-Biol.
Interactions, 43, 99-119.
Reitz, R.H., Fox, T.R., Quast, J.F., Hermann, E.A. & Watanabe, P.G.
(1984) Biochemical factors involved in the effects of
orthophenylphenol (OPP) and sodium orthophenylphenate (SOPP) on
the urinary tract of male F344 rats. Toxicol. Appl.
Pharmacol., 73, 345-349.
Roy, D. (1990) Cytochrome P-450 catalyzed redox cycling of
orthopenylphenol. Biochem. Int., 22, 849-857.
Sato, M., Tanaka, A., Tsuchiya, T., Yamaha, T., Nakaura, S. & Tanaka,
S. (1988) Excretion, distribution and metabolic fate of sodium
o-phenylphenate and o- phenylphenol in the rat. J. Food
Hyg. Soc. Jpn, 29, 7-12.
Savides, M.C. & Oehme, F.W. (1980) Urinary metabolism of orally
administered orthophenyl phenol in dogs and cats.
Toxicology, 17, 355-363.
Selim, S.A. (1996) A single dose open label study to investigate the
absorption and excretion of 14C/13C-labeled orthophenylphenol
formulation after dermal application to healthy volunteers.
Unpublished report No. P0995002 from Pharma Bio-Research Clinics
BV, Assen, Netherlands. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Shibata, M.-A., Hagiwara, A., Tamano, S., Fukushima, S. & Ito, N.
(1985) Subchronic toxicity study of sodium o-phenylphenate in
mice. Toxicol. Lett., 25, 239-246.
Shibata, M.-A., Tanaka, H., Yamada, M., Tamano, S. & Fukushima, S.
(1989a) Proliferative response of renal pelvic epithelium in male
rats to oral administration of orthophenylphenol, sodium
ortho-phenylphenate and diphenyl. Cancer Lett., 48, 19-28.
Shibata, M.-A., Tamano, S., Kurata, Y., Hagiwara, A. & Fukushima, S.
(1989b) Participation of urinary Na+, K+, pH, and L-ascorbic
acid in the proliferative response of the bladder epithelium
after the oral administration of various salts and/or ascorbic
acid to rats. Food Chem. Toxicol., 27, 403-413.
Shioya, S., Nagami-Oguihara, R., Oguihara, S., Kimura, T., Imaida, K.
& Fukushima, S. (1994) Roles of bladder distension, urinary pH
and urinary sodium ion concentration in cell proliferation of
urinary bladder epithelium in rats ingesting sodium salts.
Food Chem. Toxicol., 32, 165-171.
Shirasu, Y., Moriya, M., Kato, K., Tezuka, H., Henmi, R., Shingu, A.,
Kaneda, M. & Teramoto, S. (1978) Mutagenicity testing on
o-phenylphenol. Mutat. Res. 54, 227 (abstract).
Smith, R.A., Christenson, W.R., Bartels, M.J., Arnold, L.L., St John,
M.K., Cano, M., Garland, E.M., Lake, S.G., Wahle, B.S., McNett,
D.A. & Cohen, S.M. (1998) Urinary physiologic and chemical
metabolic effects on the urothelial cytotoxicity and potential
DNA adducts of o-phenylphenol in male rats. Toxicol. Appl.
Pharmacol., 150, 402-413.
Suzuki, H., Suzuki, N., Sasaki, M. & Hiraga, K. (1985)
Orthophenylphenol mutagenicity in a human cell strain. Mutat.
Res., 156, 123-127.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981a)
Toxicological study of o-phenylphenol (OPP) in mice. J. Nara
Med. Assoc., 32, 425.
Taniguchi, Y., Morimoto, J., Okada, K., Imai, S. & Tsubura, Y. (1981b)
Toxicological study of o-phenylphenylphenate (OPP-Na) in rats II.
Acute oral toxicity in Fischer 344 DUCRJ rats. J. Nara Med.
Assoc., 32, 709.
Tayama, S. & Nakagawa, Y. (1991) Sulfhydryl compounds inhibit the
cyto- and genotoxicity of o-phenylphenol metabolites in CHO-K1
cells. Mutat. Res., 259, 1-12.
Tayama, K., Iguchi, S. & Hiraga, K. (1979) Acute oral toxicity of
sodium o-phenylphenol (OPP-Na) in rats. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 30, 57.
Tayama, K., Iguchi, S. & Hiraga, K. (1980) Acute oral toxicity of
o-phenylphenol in rats. Ann. Rep. Tokyo Metr. Res. Lab.
Public Health, 31, 1.
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1983) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Ann. Rep. Tokyo
Metr. Res. Lab. Public Health, 34, 325
Tayama, K., Iguchi, S., Sasaki, M. & Hiraga, K. (1984) Acute oral
toxicity of o-phenylphenol (OPP) in mice. Toxicology, 101,
187 (abstract).
Tayama, S., Kamiya, N. & Nakagawa, Y. (1989) Genotoxic effects of
o-phenylphenol metabolites in CHO-K1 cells. Mutat. Res., 223,
23-33.
Tayama-Nawai, S., Yoshida, S., Nakao, T. & Hiraga, K. (1984) Induction
of chromosome aberrations and sister-chromatid exchanges in
CHO-K1 cells by o-phenylphenol. Mutat. Res., 141, 95-99.
Timchalk, C. (1996) 14C-Orthophenylphenol: Pharmacokinetics
following dermal application in male human volunteers.
Unpublished report No. HET-K-001024-064 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Timchalk, C., Selim, S., Sangha, G. & Bartels, M.J. (1998) The
pharmacokinetics and metabolism of 14C/13C-labeled
ortho-phenylphenol formulation following dermal application to
human volunteers. Hum. Exp. Toxicol., 17, 411-417.
Ushiyama, K., Nagai, F., Nakagawa, A. & Kano, I. (1992) DNA adduct
formation by ophenylphenol metabolite in vivo and in
vitro. Carcinogenesis, 13, 1469-1473.
Wahle, B.S. & Christenson, W.R. (1996) Technical grade
ortho-phenylphenol: A combined chronic toxicity/oncogenicity
testing study in the rat. Unpublished report No. 92-272-SC from
Bayer Corp., Agriculture Division, Toxicology, Stilwell, Kansas,
USA. Submitted to WHO by Leng Associates, Midland, Michigan, USA.
Yamaha, T., Tanaka, A., Sato, M. & Tsuchiya, T. (1983) Excretion,
distribution and metabolic fate of sodium o-phenylphenol in the
rat. Unpublished report from Division of Medical Chemistry,
National Institute of Hygienic Sciences, Tokyo, Japan. Submitted
to WHO by Leng Associates, Midland, Michigan, USA.
Zablotny, C.L., Breslin, W.J. & Kociba, R.J. (1991) Ortho-phenylphenol
(OPP): Gavage teratology study in New Zealand white rabbits.
Unpublished report No. K-001024-045 from Toxicology Research
Laboratory, Health & Environmental Sciences, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
Zempel, J.A. & Szabo, J.R. (1993) Ortho-phenylphenol: 21-day repeated
dermal dose study of systemic toxicity in Fischer 344 rats.
Unpublished report No. K-001024-056 from Health & Environmental
Sciences-Texas, Lake Jackson Research Center, Dow Chemical Co.,
Freeport, Texas, USA. Submitted to WHO by Leng Associates,
Midland, Michigan, USA.
