
VINCLOZOLIN
EXPLANATION
Vinclozolin is a fungicide that is registered for uses on fruits,
vegetables, and ornamental plants, and is formulated as a solo product
and as a mixture with other fungicides. It is commonly sold under the
trade name Ronilan(R). This chemical has not been previously
evaluated by the WHO Expert Group.
IDENTITY AND PROPERTIES
CHEMICAL NAME 3-(3, 5-dichlorophenyl)-5-ethenyl-5-methyl-2,4-
oxazolidinedione
CASE NUMBER: 50471-44-8
SYNONYMS Ronilan(R); BAS 352 F; Reg. No. 83 258
EMPIRICAL FORMULA C12H9Cl2O3
STRUCTURAL FORMULA
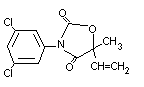 MOLECULAR WEIGHT 286.1
PHYSICAL STATE Crystalline solid
COLOUR AND ODOUR White, odorless
MELTING POINT 108°C
VAPOUR PRESSURE < 0.1 × 10-6mbar at 20°C
SOLUBILITY
Solvent g compound/100 g solvent at 20°C
Water < 0.1
ethyl ether 6.3
ethyl alcohol 1.4
chloroform 31.9
lutrol 2.0 (approx.)
acetone 43.5
ethyl acetate 25.3
cyclohexane 0.9
benzene 14.6
olive oil 1.5 (approx.)
OCTANOL/WATER PARTITION COEFFICIENT
1000 (approx.)
SPECIFIC GRAVITY approx. 1.513 kg/l at 20°C
BULK DENSITY 640 g/l loose (WHO method)
900 g/l compact (WHO method)
COMMERCIAL FORMULATIONS AVAILABLE
Too numerous to list; most are available under
the trade name Ronilan(R), and are formulated
as wettable powders, suspension conentrates, or
dust and smoke formulations.
STABILITY Stable at temperatures up to 50°C; stable in
water and dilute acids; half-life in 0.1 N
NaOH = 3.8 hours; stable to light.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Data were not submitted to adequately characterize the absorption
and excretion of vinclozolin.
A group of 5 male rats (strain unknown) was administered 5 doses
by gavage of 40 mg/kg b.w. 14C-vinclozolin (universal label on the
phenyl ring, s.a. = 8.07 µCi/mg). Excreta were collected daily and
frozen. Four hours after the last treatment, the rats were sacrificed
and tissues were collected and frozen. Urine was measured directly for
radioactivity, whereas samples of faeces, blood and tissues were
combusted and levels of 14CO2 were determined.
Data were expressed as the concentration of vinclozolin in
excreta, blood and tissues. The highest average concentration of
chemical was found in faeces (1300 µg/g), followed by urine
(450 µg/g), kidney (76.4 µg/g), liver (75.2 µg/g), fat (56.8 µg/g),
muscle (30.8 µg/g), and blood (13.3 µg/g). Levels of radioactivity in
urine and faeces appeared to reach a plateau by the second day of
treatment (Otto et al., 1977).
In a second study, 14C-vinclozolin was administered orally for
7 days at a dose of 40 mg/kg b.w./day. Six days after the last dose,
47% and 54% (mean values) of the total administered dose had been
eliminated in urine and feaces, respectively, for a total of 101%. No
radioactivity was detectable in carcasses at this time, nor was any
radioactivity detected in expired air.
Cannulation of bile ducts after a single oral dose revealed that
65% of administered radioactivity was excreted into the bile, whereas
only 19% and 15% were eliminated in the urine and faeces,
respectively.
Peak plasma levels were detected after about 1 hour; the plasma
half-life was 20 hours. As dosing continued, baseline plasma levels
tended to increase. After 7 doses, the highest levels of tissue
radioactivity were detected in the liver, kidneys, gastrointestinal
tract, fat, adrenals, and ovaries. By 96 hours after the last dose,
levels of radioactivity in tissues were not different from those of
plasma. These findings were confirmed by whole-body autoradiography
(Chasseaud et al., 1976).
Biotransformation
Samples of urine and faeces were analyzed to determine the
identities of metabolites. Vinclozolin was metabolized extensively, as
apparently no parent compound was detected in the urine, and about
8 - 40% of the parent compound was detected in the faeces.
N-(3,5-Dichlorophenyl)-2-methyl-2,3,4-trihydroxybutanoic acid amide
(metabolite F) was the major excreted metabolite, which accounted for
42% of the urinary radioactivity and 60 - 90% of the faecal
radioactivity. Metabolite F was excreted in urine as either a
glucuronide or sulfate conjugate, and was excreted in free form in the
faeces. This metabolite was also the major species found in the blood,
kidneys, and liver. Other metabolites, resulting from further
degradation of metabolite F, were formed in insignificant amounts
(Otto et al., 1977).
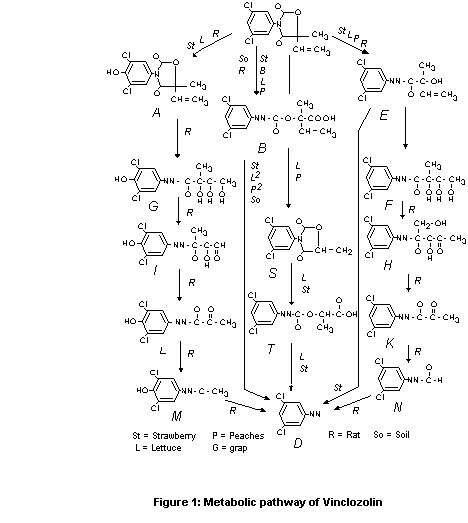
A proposed metabolic pathway is described in Figure 1.
Toxicological studies
Special study on carcinogenicity
Mice
Groups of NMRI mice (50/sex/dose) were fed diets containing
0, 162, 486, 1460, or 4370 ppm vinclozolin (purity unspecified) for
110 weeks. Control or test diet and water were offered ad libitum.
The dietary concentrations of vinclozolin were equal to 27, 82, 275,
or 818 mg/kg b.w./day in males and 31, 92, 287, or 912 mg/kg b.w./day
in females. The results of analyses of the test material or of the
test diets were not presented. Animals were examined twice daily for
abnormalities of behaviour or appearance, and were palpated weekly
beginning at week 26 for evidence of tumours. Eyes, ears, and
dentition were examined at necropsy. Mice were weighed weekly. The
schedule and manner of measurement of food consumption were not
specified.
Blood was sampled at 0, 6, 13, 26, 52, and 78 weeks, from 10
mice/sex/dose, for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted.
Urine was collected on a similar schedule for standard urinalysis
determinations.
MOLECULAR WEIGHT 286.1
PHYSICAL STATE Crystalline solid
COLOUR AND ODOUR White, odorless
MELTING POINT 108°C
VAPOUR PRESSURE < 0.1 × 10-6mbar at 20°C
SOLUBILITY
Solvent g compound/100 g solvent at 20°C
Water < 0.1
ethyl ether 6.3
ethyl alcohol 1.4
chloroform 31.9
lutrol 2.0 (approx.)
acetone 43.5
ethyl acetate 25.3
cyclohexane 0.9
benzene 14.6
olive oil 1.5 (approx.)
OCTANOL/WATER PARTITION COEFFICIENT
1000 (approx.)
SPECIFIC GRAVITY approx. 1.513 kg/l at 20°C
BULK DENSITY 640 g/l loose (WHO method)
900 g/l compact (WHO method)
COMMERCIAL FORMULATIONS AVAILABLE
Too numerous to list; most are available under
the trade name Ronilan(R), and are formulated
as wettable powders, suspension conentrates, or
dust and smoke formulations.
STABILITY Stable at temperatures up to 50°C; stable in
water and dilute acids; half-life in 0.1 N
NaOH = 3.8 hours; stable to light.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Data were not submitted to adequately characterize the absorption
and excretion of vinclozolin.
A group of 5 male rats (strain unknown) was administered 5 doses
by gavage of 40 mg/kg b.w. 14C-vinclozolin (universal label on the
phenyl ring, s.a. = 8.07 µCi/mg). Excreta were collected daily and
frozen. Four hours after the last treatment, the rats were sacrificed
and tissues were collected and frozen. Urine was measured directly for
radioactivity, whereas samples of faeces, blood and tissues were
combusted and levels of 14CO2 were determined.
Data were expressed as the concentration of vinclozolin in
excreta, blood and tissues. The highest average concentration of
chemical was found in faeces (1300 µg/g), followed by urine
(450 µg/g), kidney (76.4 µg/g), liver (75.2 µg/g), fat (56.8 µg/g),
muscle (30.8 µg/g), and blood (13.3 µg/g). Levels of radioactivity in
urine and faeces appeared to reach a plateau by the second day of
treatment (Otto et al., 1977).
In a second study, 14C-vinclozolin was administered orally for
7 days at a dose of 40 mg/kg b.w./day. Six days after the last dose,
47% and 54% (mean values) of the total administered dose had been
eliminated in urine and feaces, respectively, for a total of 101%. No
radioactivity was detectable in carcasses at this time, nor was any
radioactivity detected in expired air.
Cannulation of bile ducts after a single oral dose revealed that
65% of administered radioactivity was excreted into the bile, whereas
only 19% and 15% were eliminated in the urine and faeces,
respectively.
Peak plasma levels were detected after about 1 hour; the plasma
half-life was 20 hours. As dosing continued, baseline plasma levels
tended to increase. After 7 doses, the highest levels of tissue
radioactivity were detected in the liver, kidneys, gastrointestinal
tract, fat, adrenals, and ovaries. By 96 hours after the last dose,
levels of radioactivity in tissues were not different from those of
plasma. These findings were confirmed by whole-body autoradiography
(Chasseaud et al., 1976).
Biotransformation
Samples of urine and faeces were analyzed to determine the
identities of metabolites. Vinclozolin was metabolized extensively, as
apparently no parent compound was detected in the urine, and about
8 - 40% of the parent compound was detected in the faeces.
N-(3,5-Dichlorophenyl)-2-methyl-2,3,4-trihydroxybutanoic acid amide
(metabolite F) was the major excreted metabolite, which accounted for
42% of the urinary radioactivity and 60 - 90% of the faecal
radioactivity. Metabolite F was excreted in urine as either a
glucuronide or sulfate conjugate, and was excreted in free form in the
faeces. This metabolite was also the major species found in the blood,
kidneys, and liver. Other metabolites, resulting from further
degradation of metabolite F, were formed in insignificant amounts
(Otto et al., 1977).
A proposed metabolic pathway is described in Figure 1.
Toxicological studies
Special study on carcinogenicity
Mice
Groups of NMRI mice (50/sex/dose) were fed diets containing
0, 162, 486, 1460, or 4370 ppm vinclozolin (purity unspecified) for
110 weeks. Control or test diet and water were offered ad libitum.
The dietary concentrations of vinclozolin were equal to 27, 82, 275,
or 818 mg/kg b.w./day in males and 31, 92, 287, or 912 mg/kg b.w./day
in females. The results of analyses of the test material or of the
test diets were not presented. Animals were examined twice daily for
abnormalities of behaviour or appearance, and were palpated weekly
beginning at week 26 for evidence of tumours. Eyes, ears, and
dentition were examined at necropsy. Mice were weighed weekly. The
schedule and manner of measurement of food consumption were not
specified.
Blood was sampled at 0, 6, 13, 26, 52, and 78 weeks, from 10
mice/sex/dose, for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted.
Urine was collected on a similar schedule for standard urinalysis
determinations.
 Mice that died on test, moribund animals, and all mice surviving
to 112 weeks were subjected to complete mecropsies. Mice were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs were apparent. Survival
was somewhat lower in high-dose males than in controls (76% mortality
vs. 48% in the control group); however, mortality in all female groups
was about 70%, with no effects of treatment. Mean body weights were
reduced in males in the 2 highest dose groups; these decreases emerged
in the second year of treatment and persisted until termination. Food
intake did not appear to be affected by treatment. No effects of
treatment oil haematology, clinical chemistry, or urinalysis
parameters were noted.
At necropsy, significant increases of 150 - 250% in the absolute
and relative weights of the liver were noted in females from the 1460
and 4370 ppm groups. An increase in liver weight of about 40% and an
increase in testes weight of about 50% were noted in high-dose males.
Absolute weights of other organs were altered in males; however, these
changes probably were related to decreases in body weight.
After microscopic examination, no toxicologically significant
differences in the incidences of neoplastic or non-neoplastic lesions
were noted. Vinclozolin was therefore negative for oncogenic potential
in mice (Leuschner et al., 1977a).
Special studies on mutagenicity
Vinclozolin was negative in a number of acceptable mutagenicity
studies, which included assessments of gene mutations (bacteria and
mammalian cells), direct DNA damage (bacteria and mammalian cells),
and clastogenic effects (in vivo mammalian cells).
Summary results are presnted in Table 1.
Special study on reproduction
Rats
Groups of male and female Sprague-Dawley rats (20/sex/dose) were
fed diets containing 0, 162, 486, or 1485 ppm vinclozolin (purity
unspecified) over 3 generations. Control or test diet and water were
offered ad libitum. The dietary concentrations of vinclozolin were
equal to 12, 37, or 113 mg/kg b.w./day in males and 17, 51, or
152 mg/kg b.w./day in females. The results of analyses of the test
material or of the test diets were not presented.
Table 1: Results of mutagenicity studies on vinclozolin
Test system Test object Concentration of Purity Results Reference
vinclozolin
Ames test1 S. typhimurium 0, 100, 500 98.1% Negative2 Gelbke & Engelhardt, 1983
TA98, TA100, TA1535, 2500, 5000,
10,000, TA1537, 7500, and
and TA1538. µg/plate
CHO/HGPRT gene Chinese hamster 0,0.32,1.0, > 99.5% Negative3 Gelbke & Jäckh, 1985
mutation ovary cells, 3.2, or 10.0
assay1 CHO-K1 mg/ml
Unscheduled Primary hepatocytes 0, 5, 10, > 99.5% Negative4 Cifone & Myhr, 1984
DNA from male Fischer 25, 50, 100,
synthesis 344 rats 250, 500, &
1000 µg/ml
Mouse lymphoma L5178Y mouse Nonactivated: Technical Negative3 Witterland & Hoorn, 1984
forward lymphoma 0 - 1000 µg/ml
mutation cells (TK+/-) (8 dose levels)
assay1 Activated:
0 - 600 µg/ml
(13 dose levels)
DNA repair B. subtilis 0, 1, 10, Technical Negative5 Hoorn, 1983
assay1 H17 and M45 100, 500,
(rec +/-) 1000, 2500,
5000 & 10,000
µg/plate
Table 1: (cont'd).
Test system Test object Concentration of Purity Results Reference
vinclozolin
In vivo sister Chinese hamster 0, 3830, & 98.1% Negative6 Gelbke, et al., 1982
chromatid bone marrow 5620 mg/kg
exchange assay
Mouse dominant NMRI mice 0 & 2000 Technical Negative7 Hofmann & Peh, 1975a
lethal assay mg/kg
1 With and without activation.
2 The positive controls gave the expected positive results.
3 The positive controls, EMS and MCA, gave the expected positive results.
4 The positive control, 0.05 µg/ml 2-AAF, gave the expected positive result.
5 The positive controls, MMS and sterigmatocystin, gave the expected positive results.
6 The positive control, cyclophosphamide, gave the expected positive response.
7 This study is not acceptable. A positive control was not included; therefore, the sensitivity of the test system was not
established.
Two litters were obtained from each generation. Rats from the
parental generation (F0) were bred after 8 and 16 weeks of treatment
(1:1 male to female) to form the F1a and F1b litters, respectively.
Twenty rats/sex/dose were selected from the F1b litters to become
parents of the F2a and F2b litters. This process was repeated
through the F3 generation. Twenty rats of the F3b generation were
maintained on test diets for 9 weeks, at which time they were
sacrificed and histopathological evaluations were conducted on 10
rats/sex/dose.
Animals were examined daily for abnormalities of behaviour or
appearance, and food consumption was measured daily. Rats were weighed
weekly. Fertility, litter size, pup growth, and pup survival for each
litter were determined. At weaning, selected pups were evaluated for
consciousness, emotional behaviour, activity and reactivity,
coordination, and reflexes. Parental rats of each generation were
sacrificed after completion of the second litter and subjected to
gross necropsies. Pups not selected for breeding of the next
generation were sacrificed at weaning with the exception of 10 F3b
pups/sex/dose, which were maintained for 9 weeks on test diets and
then subjected to complete histopathological evaluation.
No effects of treatment on clinical signs, food consumption, or
body weight of parental rats were noted. Similarly, no alterations in
fertility, gestation length, litter size, sex ratio of pups, fetal
birth weight, weight gain of pups, or survival of pups during
lactation were noted in any of the litter intervals. No treatment-
related malformations or developmental defects were noted. No
treatment-related pathological findings were noted in either parental
rats or pups at necropsy. The NOEL for reproductive effects was
determined to be greater than the highest dose tested (Leuschner
et al., 1977b).
Special studies on teratology
Mice
Groups of female NMRI [SPF] mice (24-30/dose) with vaginal plugs
were fed diets containing technical vinclozolin (purity unspecified)
from days 0 - 18 of presumed gestation. Two studies were conducted, 1
with test diets containing 0 and 60,000 ppm vinclozolin, and a second
study with test diets containing 0, 600, and 6000 ppm vinclozolin.
Identical protocols were followed in both studies. Mice were examined
daily for clinical signs of toxicity, and food consumption and body
weights were determined periodically. On day 18 of gestation, the mice
were sacrificed and the number of implantation and resorption sites
and the number of live and dead fetuses were determined. Body weights,
lengths, and the sex of live fetuses were determined, and placentas
were weighed. Fetuses were examined for external abnormalities, and
one-third of the fetuses were examined for visceral abnormalities, and
the remaining two-thirds were examined for skeletal defects.
The appearance and behaviour of mice from the low-dose (600 ppm)
and mid-dose (6000 ppm) groups were not affected by treatment. In
contrast, all mice from the high-dose (60,000 ppm) group died during
the treatment interval. Since these mice also refused food and lost
considerable body weight, it was not clear whether death was the
result of toxicity or of starvation due to food avoidance. Mice fed
600 ppm vinclozolin had a mean body-weight gain during gestation that
was similar to that of mice in the control groups, whereas animals fed
6000 ppm ate about 25% less food than control or low-dose mice, and
gained significantly less body weight. Mice offered the 60,000 ppm
diets refused food, and lost body weight until death.
At necropsy, it was found that none of the mice from the 6000
or 60,000 ppm dose groups had any implantations. The number of
implantations and resorptions, fetal weights and lengths, and
placental weights were similar in control and low-dose mice. Visceral
and skeletal examinations of control and low-dose fetuses revealed
no treatment-related abnormalities. The NOEL for maternal and
developmental toxicity in mice in this study therefore was 600 ppm
(equal to 110 mg/kg b.w./day) (Hofmann & Peh, 1975b).
Rabbits
Groups of female New Zealand white rabbits (15/dose) were
administered doses of 0, 20, 80, or 300 mg/kg b.w./day of technical
vinclozolin (98.1% purity) by gavage from days 6 - 18 of presumed
gestation. Doses were based on a preliminary range-finding assay which
demonstrated that a dose of 900 mg/kg b.w./day was not well tolerated,
while a dose of 300 mg/kg b.w./day produced moderate signs of
toxicity. Rabbits were observed daily for clinical signs of toxicity,
and were weighed periodically during gestation. On day 29 of
gestation, the rabbits were sacrificed by cervical dislocation, and
uteri and the ovaries were examined to determine the number of corpora
lutea and resorptions, live and dead fetuses, gravid uterine weights,
and fetal body weights and lengths. Live fetuses were examined for
external abnormalities, then sacrificed by injection of pheno-
barbitone. Fetuses were weighed, then dissected and examined for
visceral abnormalities. Fetuses were then processed and stained for
skeletal examinations.
No signs of toxicity were observed in does during gestation (the
findings observed in the range-finding study were not reproduced), and
the 4 recorded deaths were considered unrelated to treatment. No
effects of treatment on maternal body weights were apparent. At
necropsy, no effects of treatment on gravid uterine weight or on
litter size or frequency were noted. An approximately 3-fold increase
in the number of resorptions was noted in the high-dose group
(compared to the control group); however, this increase was not
statistically significant. Mean fetal weight was decreased in the
high-dose group by about 6%; this change was not statistically
significant. No effects of treatment on the incidences of visceral or
skeletal abnormalities were apparent. The authors concluded that the
highest dose tested of 300 mg/kg b.w./day had no statistically-
significant effects on litter parameters, nor on maternal health
(Cozens et al., 1981).
Acute toxicity
Vinclozolin possesses a low order of acute toxicity. The results
of acute toxicity studies are summarized in Table 1.
Table 2. Acute toxicity of vinclozolin1
LD50 LD50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M > 15,000 - Shirasu et al.,
F > 15,000 1978a
s.c. M > 15,000 - Shirasu et al.,
F > 15,000 1978a
i.p. M 1570 - Shirasu et al.,
F 1640 1978a
Rats oral M > 15,000 - Shirasu et al.,
F > 15,000 1978b
i.p. M 8300 - Shirasu et al.,
F 4220 1978a
dermal M > 5000 - Shirasu et al.,
F > 5000 1978b
inhalation M - > 29.1 Leuschner, 1979
(4 hr. exp.) F > 29.1
Guinea-pig oral M/F 8000 - Hofmann, 1973
1 Technical vinclozolin was tested in these studies.
Technical vinclozolin was shown to cause mild reversible
irritation to the conjunctivae of the eye (Hildebrand, 1977a) and skin
(Hildebrand, 1977b).
Short-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (16
rats/sex/dose) were fed diets containing 0, 100, 300, 1000, or
2000 ppm technical vinclozolin for 3 months. Six rats from each group
were further maintained on control diets for a post-observation period
of 6 weeks at the end of the study. Rats were examined daily for
mortality, abnormalities of appearance or behaviour, and food
consumption. Body weights were determined weekly. Rats were palpated
at weekly weighings, and the eyes were examined. Blood was sampled for
haematology and clinical chemistry examinations prior to initiation,
after 6 and 12 weeks of treatment, at termination, and at the end of
the post-observation period. Urine was collected on a similar
schedule. At termination, rats were sacrificed by CO2 asphyxiation
and decapitated. Animals were dissected and examined for gross
pathological changes. Absolute and relative weights of major organs
were determined, and a complete set of tissues from each animal was
saved for future histopathological examinations. The results of
microscopic examinations of tissues were not reported in this study,
with the exception of eyes, which were examined in serial sections.
A single death was noted on day 42 in the female high-dose
treatment group; all other rats survived to scheduled termination. No
disturbances of appearance or behaviour were noted, and eyes appeared
normal at all examinations. Body weights of rats in all treatment
groups were comparable to those of controls throughout the study
period. Food consumption was not affected in males, although
occasional statistically-significant increases were noted in the
female treatment groups. Changes in haematology consistent with
decreased red cell mass (decreased numbers of erythrocytes, heama-
tocrits, and haemoglobin, with increased MCH and MCHC) were noted
at the 6-week sampling time in males and females fed diets of 300 ppm
and higher; however, these changes were apparent at termination only
in the female 1000 and 2000 ppm groups. Clinical chemistry parameters
were not altered in a toxicologically-significant manner.
At necropsy, no effects of treatment on the gross appearance of
tissues were apparent. Statistically-significant dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were noted in males and females from the 1000 and 2000 ppm
groups. Increased relative weights of kidneys were noted in 2000 ppm
males and females, and increased relative spleen weight was noted in
2000 ppm females. These organ-weight effects were apparently
reversible, as they were not observed in treated rats that were
continued on control diets for an additional 6 weeks. Microscopic
examinations of eyes did not reveal any treatment-related
abnormalities. The results of microscopic examinations of the
remaining tissues were not included in the study report (Hofmann,
1974).
Another 3-month feeding study in rats has been performed, but it
was not reviewed, because the study report was incomplete. In the
study narrative the authors concluded that 300 ppm (equal to
21.3 mg/kg b.w./day in males and 24.1 mg/kg b.w./day in females) was
the NOEL based on decreased body-weight gain and altered haematology
and clinical chemistry parameters at 1500 ppm and 7500 ppm, and on
altered organ weights and histopathological findings in the liver,
kidneys, ovaries, and pancreas at 7500 ppm (Takehara et al., 1978).
Rabbits
Groups of New Zealand white rabbits (6/sex/dose, 3 of each group
with intact skin, 3 with abraded skin) were treated by dermal
application for 21 consecutive days (8 hours/day) with doses of 111,
333, or 1000 mg/kg b.w. vinclozolin. All animals survived the
treatment period without effects on appearance, behaviour, body
weight, food or water intake, haematology, clinical chemistry, or
necropsy parameters. The highest dose was considered to be the NOEL in
this study (Leuschner et al., 1977c).
Dogs
Groups of purebred beagle dogs (6/sex/dose) were fed diets
containing 0, 100, 300, 600, or 2000 ppm technical vinclozolin
(98.1% purity) for 6 months. Dogs were offered a total of 700 grams of
food for 3 hours/day, and consumption was measured daily. Daily
dosages were equal to 0, 7.0, 20, 41, or 135 mg/kg b.w./day in males
and 0, 7.4, 21, 41, or 141 mg/kg b.w./day in females. Dogs were
examined daily for clinical signs of toxicity, and body weights were
determined weekly. Eyes were examined at 3-month intervals for changes
in refracting media or the fundus. Blood was sampled at approximately
monthly intervals for measurement of haematology and clinical
chemistry parameters. Urine was collected approximately every 2 months
for urinalysis determinations.
At termination, dogs were anaesthetized and sacrificed by
exsanguination. Standard necropsy techniques were followed. The
following tissues were examined (those indicated by an asterisk * were
also weighed) for gross and microscopic changes: abnormalities,
adrenals*, aorta, bone (sternum & marrow), brain*, colon, duodenum,
esophagus, eye (with optic nerve), gall, bladder, heart*, ileum,
jejunum, kidneys*, liver*, lungs (with mainstem bronchi), lymph nodes
(axillary & mesenteric), mammary glands, ovaries*, pancreas, parotid,
pituitary*, prostate, sciatic nerve, skeletal muscle, skin, spleen*,
spinal cord (thoracic, lumbar), stomach, testes* (with epididymides),
thymus, thyroid* (with parathyroid), trachea, urinary bladder, and
uterus (corpus, cervix, and cornu).
No effects of treatment on clinical signs, survival, food
consumption or body-weight gain were apparent. Ophthalmoscopic
examinations did not reveal any treatment-related abnormalities.
Evidence of haemolytic anaemia was noted in high-dose males and
females in the form of increased MCHC, increased numbers of
reticulocytes and red cells containing Howell-Jolly bodies, and
increased serum bilirubin, lactate dehydrogenase, and urea levels.
However, decreases in erythrocyte counts or haematocrits were not
observed, apparently due to compensatory phenomena. Increased numbers
of platelets noted in these dogs also suggested a compensatory
response to anaemia. These effects were more profound in males than in
females. Other clinical chemistry parameters were not affected.
Statistically-significant decreases in SGPT, which were observed in
females, are of questionable toxicological significance. Urinalysis
parameters were not affected by treatment.
At necropsy, statistically-significant dose-related increases in
absolute and relative weights of adrenals were noted in males and
females fed 300 ppm or more vinclozolin. Other changes in organ
weights included decreases in absolute weights of kidneys in males at
300 ppm and above, decreases in absolute brain weights, and increases
in relative spleen weights in high-dose males. Other changes noted in
females included decreased relative pituitary weights at 300 ppm and
higher.
Potential treatment-related gross findings were restricted to
reduced size of the prostate in high-dose males. Upon microscopic
examination, the following potential treatment-related findings were
observed in males (numbers and incidences in control, 100, 300, 600,
and 2000 ppm groups, respectively, of 6 dogs examined): increased
erythropoiesis of bone marrow (0, 0, 0, 1, 4); sinusoidal dilatation/
accumulation of erythrocytes in the spleen (1, 2, 2, 2, 4);
atrophy/stromal proliferation of the prostate (0, 0, 2, 4, 6); and
vacuolization of the zona fasciculata of the adrenals (0, 0, 1, 1, 4).
No gross findings were evident in females. Incidences of
microscopic lesions (as described above) were: increased erythro-
poiesis of bone marrow (1, 0, 2, 2, 5), sideropexia of the bone
marrow (1, 0, 4, 5, 6); sinusoidal dilatation/accumulation of
erythrocytes in the spleen (0, 1, 3, 4, 5); and vacuolization of zona
fasciculata of the adrenals (0, 0, 0, 3, 6).
The authors concluded that the no adverse-effect level in this
study was between 300 and 600 ppm vinclozolin in the diet (Kirsch
et al., 1982).
Long-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (50/sex/dose)
were fed diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin
(purity unspecified) for 130 weeks. Control or test diet and water
were offered ad libitum. The dietary concentrations were equal to
9.4, 27, 83, or 257 mg/kg b.w./day in males and 9.1, 28, 84, or
278 mg/kg b.w./day in females. The results of analyses of the test
material or of test diets were not presented. Animals were examined
twice/day for abnormalities of behaviour or appearance, and were
palpated weekly beginning at week 26 for evidence of rumours. Eyes,
ears, and dentition were examined "regularly". Rats were weighed
weekly. The schedule and manner of measurement of food consumption
were not specified.
Blood was sampled at 0, 4, 8, 13, 26, 52, and 104 weeks from 10
rats/sex/dose for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted,
and in addition, at week 104, glycogen in heart, liver, and skeletal
muscle, total lipids in liver, and ascorbic acid in the adrenals were
measured. Urine was collected on a similar schedule for standard
urinalysis determinations.
Rats that died on test, moribund animals, and all rats surviving
to 130 weeks were subjected to complete necropsies. Rats were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, kidneys, liver, lungs, lymph node,
ovaries, pancreas, parotid, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs or mortality were
apparent. Eye, hearing, and dentition parameters were within normal
limits. The study was terminated at week 130 when combined male and
female mortality in the control group reached 70%. Survival in test
groups was inversely related to dose, as the lowest mortality was
noted in the high-dose group. Body weights were decreased by about 10%
in males and females fed the 1460 ppm diet, and by about 40 and 25%,
respectively, in males and females fed the 4370 ppm diet. These
changes emerged within the first month of treatment and persisted
until study termination. Food consumption was decreased in a similar
manner in the 2 highest-dose groups.
No effects of treatment on haematology or clinical chemistry
parameters were apparent. A decrease of about 20% total bilirubin in
high-dose females is of doubtful toxicological significance.
Occasional increases in the urinary excretion of 17 ketosteroids and
of ascorbic acid were noted in high-dose females in the first year of
treatment; however, these changes were not evident in the final year
of treatment.
At necropsy, organ weights were altered in a manner related to
the body-weight changes. Absolute weights of several organs were
decreased in males and females from the 1460 ppm and 4370 ppm groups,
whereas relative organ weights tended to increase in these groups.
Other necropsy parameters were not affected by treatment. The
incidences of macroscopic and microscopic findings were randomly
distributed among all test groups, and did not appear to be related to
treatment with the test material. The total number of rumours, as well
as the distribution of specific rumour types, was random throughout
the test groups, and not affected by treatment. Therefore, vinclozolin
was determined not to be oncogenic in the rat. The NOEL for chronic
toxicity was 486 ppm, equal to 27 and 28 mg/kg b.w./day in males and
females, respectively, based on decreases in food consumption and
body-weight gain (Leuschner et al., 1977d).
Observations in humans
No information available.
COMMENTS
Data were not submitted to completely characterize the rates of
absorption or elimination of vinclozolin. In studies that were
submitted, all of the administered dose was eliminated, and no
potential for bioaccumulation was apparent. The plasma half-life was
about 20 hours.
Vinclozolin is extensively metabolized, and its major metabolite
(metabolite F) is excreted as a glucuronide or sulfate conjugate.
Specific interaction with enzymes is not known.
A plant metabolite has been identified that is not formed in rats
(metabolite T). Additional testing is necessary to evaluate the
toxicity of this compound.
Vinclozolin has a low order of acute toxicity.
Special studies on carcinogenicity were negative for oncogenic
potential in rats and mice. Similarly, this chemical has no known
specific potential for mutagenicity, teratogenicity, or reproductive
toxicity.
A sub-chronic feeding study in rats suggested possible anaemia
after treatment with diets containing 1000 or 2000 ppm vinclozolin.
However, this finding was not reproduced in a 2-year feeding study in
rats. Chronic effects noted in the long-term rat study were restricted
to decreases in food consumption and body-weight gain of rats fed
1460 ppm and higher (equal to 83 mg/kg b.w./day), with a NOEL for this
effect of 486 ppm (equal to 27 and 28 mg/kg b.w./day in males and
females, respectively).
A feeding study in dogs demonstrated evidence of haemolytic
anaemia in animals fed 2000 ppm vinclozolin for 6 months (equal to 135
and 141 mg/kg b.w./day in males and females, respectively). Other
relevant findings included significant increases in relative and
absolute adrenal weights in males and females at doses of 300 ppm and
higher (equal to 20 and 21 mg/kg b.w./day in males and females,
respectively). A clear dose-related trend for vacuolization of the
zona fasciculata of the adrenals was noted in males and females at
doses of 600 and 2000 ppm. Thus, the changes in adrenal weights
observed at the lower doses can be related to pathology at higher
doses. Other dose-related histopathological findings noted in the
300 ppm groups included accumulation of erythrocytes in the spleen,
atrophy of the prostate, and sideropexia of the bone marrow
(accumulation of haemosiderin within reticuloendothelial elements of
the bone marrow). On the basis of these findings, the NOEL in this
study was 100 ppm, equal to 7.0 mg/kg b.w./day in males and 7.4 mg/kg
b.w./day in females.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECTS
Averages for males and females:
Mice: 486 ppm, equal to 87 /kg b.w./day.
Rats: 486 ppm, equal to 27 /kg b.w./day.
Dogs: 100 ppm, equal to 7.2 /kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DALLY INTAKE FOR MAN
0 - 0.04 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE, TO
BE SUBMITTED TO WHO BY 1987:
Additional studies to evaluate the toxicity of the plant
metabolite, metabolite T.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND:
1. Data to demonstrate the rate of absorption and elimination
after a single oral dose of vinclozolin.
2. Data on the potential effects to humans of exposure to
vinclozolin.
REFERENCES
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B., &
1976 Franklin, E.R. The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral adminstration to
rats. Not submitted for review.
Cifone, M.A. & Myhr, B.C. Report on the evaluation of Vinclozolin in
1984 the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 814/072 from Litton Bionetics, Inc.,
Kensington, MD, USA. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R., & Offer, J.M.
1981 Effect of vinclozolin on pregnancy of the the New Zealand
white rabbit. Unpublished report No. BSF/3810/381/81357 from
Huntingdon Research Centre, Huntingdon, England. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P., Engelhardt, G., & Ruff, M. Cytogenetic investigations
1982 in Chinese hamsters after a single oral administration of
Reg. No. 83 258 - sister chromatid exchange. Unpublished
report No. 82/085, from BASF Gewerbehygiene und Toxikologie,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P. & Engelhardt, G. Report on the study of Vinclozolin in
1983 the Ames test. Unpublished report No. 85/228, from BASF
Gewerbehygiene und Toxikologie, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Gelbke, H.-P. & Jäckh, R. Report on a point mutation test carried out
1985 on CHO cells (HGPRT locus) with the test substance
vinclozolin. Unpublished report No. 85/352 from BASF Dept.
Toxicology, Crop Protection Division. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) to
1977a the eye of white rabbits. Unpublished report No. 77/020,
from BASF Gewerbehygiene und Toxikologie, Ludwigshafen/
Rhein, FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) on
1977b the intact and scarified dorsal skin of white rabbits.
Unpublished report No. 77/022, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. Report on the acute oral toxicity trial of 3-(3,5-di-
1973 chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report No. XXII/337 from
Agricultural Research Station, BASF, Limburgerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. Report on the testing of 3-(3,5-dichlorophenyl)-
1974 5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione in a three month
feeding experiment on rats. Unpublished report No. 74/001
from Medizinisch- Biologische Forschungslaboratorium, BASF.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the mutagenic effect of 3-(3,5-di-
1975a chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in mice. Unpublished report No. 75/003 from Medizinisch-
Biologische Forschungslaboratorium, BASF. Submitted to WHO
by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the prenatal toxicity of
1975b 3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-
dione in mice. Unpublished report No. 75/004 from
Medizinisch-Biologische Forschungslaboratorium, BASF.
Ssubmitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hoorn, A.J.W. Report on the mutagenicity evaluation of Vinclozolin in
1983 the rec assay with Bacillus subtilis. Unpublished report
No. 83/236 from Litton Bionetics, 3905 Pe Veenendaal,
Netherlands. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Kirsch, Deckhardt, Mirea, Hellwig, Hildebrand, & Ruff. Report on the
1982 study of the toxicity of Reg. No. 83 258 (vinclozolin) in
beagle dogs after 6-month administration in the diet.
Unpublished report No. 82/177, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., Dontenwill, W., & Rogulja,
1977a P.V. Oral toxicity of an oxazolidine derivative, batch
813258 - called for short "OXA" - in NMRI mice (with special
attention to carcinogenic properties). Unpublished report
No. 77/017 from Laboratorium fur Pharmakologie und
Toxikologie, Hamburg, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Hubscher, F., Neumann, W., Neumann, B., Rogulja, P.V. &
1977b Dontenwill, W. Chronic oral toxicity of an oxazolidine
derivative, batch 813258 - called for short "OXA" - in a
reproductive study covering three generations in
Sprague-Dawley rats. Unpublished report No. 77/019 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg,
FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., & Dontenwill, W.
1977c 3-Weeks toxicity of an oxazolidine derivative, batch 813258
- called for short "OXA" - in NZW rabbits by local
application. Unpublished report No. 77/015 from Laboratorium
fur Pharmakologie und Toxikologie, Hamburg, FRG. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., & Dontenwill, W. Chronic
1977d oral toxicity of an oxazolidine derivative, batch 813258 -
called for short "OXA" - in the Sprague-Dawley rat.
Unpublished report No. 77/016 from Laboratorium fur
Pharmakologie und Toxikologie, hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F. Report on the acute toxicity of the preparation Reg.
1979 No. 813258 when administered to rats by inhalation.
Unpublished report No. 79/029 from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Otto, S., Beutel, P., Elzner, J., & Ohnsorge, U. Metabolism of
1977 14C-vinclozolin in rats. Unpublished report No. 1474 from
Agricultural Research Station, BASF, Limbergerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978a with BAS 352 F in mice. Unpublished report No. 78/031 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978b with BAS 352 F in rats. Unpublished report No. 78/030 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Takehara, K., Kudoh, S., Maruyama, Y., Hayashi, K., Itabashi, M., &
1978 Tajima, M. Three-month oral toxicity study of BAS-352-F in
rats. Unpublished report No. 78/029 from Nippon Institute
for Biological Science, Tokyo, Japan. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Witterland, W.F. & Hoorn, A.J.W. Report on the mutagenicity evaluation
1984 of vinclozolin in the mouse lymphoma forward mutation assay.
Unpublished report No. 84/267 from Litton Bionetics, 3905 Pe
Veenendaal, Netherlands. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Mice that died on test, moribund animals, and all mice surviving
to 112 weeks were subjected to complete mecropsies. Mice were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs were apparent. Survival
was somewhat lower in high-dose males than in controls (76% mortality
vs. 48% in the control group); however, mortality in all female groups
was about 70%, with no effects of treatment. Mean body weights were
reduced in males in the 2 highest dose groups; these decreases emerged
in the second year of treatment and persisted until termination. Food
intake did not appear to be affected by treatment. No effects of
treatment oil haematology, clinical chemistry, or urinalysis
parameters were noted.
At necropsy, significant increases of 150 - 250% in the absolute
and relative weights of the liver were noted in females from the 1460
and 4370 ppm groups. An increase in liver weight of about 40% and an
increase in testes weight of about 50% were noted in high-dose males.
Absolute weights of other organs were altered in males; however, these
changes probably were related to decreases in body weight.
After microscopic examination, no toxicologically significant
differences in the incidences of neoplastic or non-neoplastic lesions
were noted. Vinclozolin was therefore negative for oncogenic potential
in mice (Leuschner et al., 1977a).
Special studies on mutagenicity
Vinclozolin was negative in a number of acceptable mutagenicity
studies, which included assessments of gene mutations (bacteria and
mammalian cells), direct DNA damage (bacteria and mammalian cells),
and clastogenic effects (in vivo mammalian cells).
Summary results are presnted in Table 1.
Special study on reproduction
Rats
Groups of male and female Sprague-Dawley rats (20/sex/dose) were
fed diets containing 0, 162, 486, or 1485 ppm vinclozolin (purity
unspecified) over 3 generations. Control or test diet and water were
offered ad libitum. The dietary concentrations of vinclozolin were
equal to 12, 37, or 113 mg/kg b.w./day in males and 17, 51, or
152 mg/kg b.w./day in females. The results of analyses of the test
material or of the test diets were not presented.
Table 1: Results of mutagenicity studies on vinclozolin
Test system Test object Concentration of Purity Results Reference
vinclozolin
Ames test1 S. typhimurium 0, 100, 500 98.1% Negative2 Gelbke & Engelhardt, 1983
TA98, TA100, TA1535, 2500, 5000,
10,000, TA1537, 7500, and
and TA1538. µg/plate
CHO/HGPRT gene Chinese hamster 0,0.32,1.0, > 99.5% Negative3 Gelbke & Jäckh, 1985
mutation ovary cells, 3.2, or 10.0
assay1 CHO-K1 mg/ml
Unscheduled Primary hepatocytes 0, 5, 10, > 99.5% Negative4 Cifone & Myhr, 1984
DNA from male Fischer 25, 50, 100,
synthesis 344 rats 250, 500, &
1000 µg/ml
Mouse lymphoma L5178Y mouse Nonactivated: Technical Negative3 Witterland & Hoorn, 1984
forward lymphoma 0 - 1000 µg/ml
mutation cells (TK+/-) (8 dose levels)
assay1 Activated:
0 - 600 µg/ml
(13 dose levels)
DNA repair B. subtilis 0, 1, 10, Technical Negative5 Hoorn, 1983
assay1 H17 and M45 100, 500,
(rec +/-) 1000, 2500,
5000 & 10,000
µg/plate
Table 1: (cont'd).
Test system Test object Concentration of Purity Results Reference
vinclozolin
In vivo sister Chinese hamster 0, 3830, & 98.1% Negative6 Gelbke, et al., 1982
chromatid bone marrow 5620 mg/kg
exchange assay
Mouse dominant NMRI mice 0 & 2000 Technical Negative7 Hofmann & Peh, 1975a
lethal assay mg/kg
1 With and without activation.
2 The positive controls gave the expected positive results.
3 The positive controls, EMS and MCA, gave the expected positive results.
4 The positive control, 0.05 µg/ml 2-AAF, gave the expected positive result.
5 The positive controls, MMS and sterigmatocystin, gave the expected positive results.
6 The positive control, cyclophosphamide, gave the expected positive response.
7 This study is not acceptable. A positive control was not included; therefore, the sensitivity of the test system was not
established.
Two litters were obtained from each generation. Rats from the
parental generation (F0) were bred after 8 and 16 weeks of treatment
(1:1 male to female) to form the F1a and F1b litters, respectively.
Twenty rats/sex/dose were selected from the F1b litters to become
parents of the F2a and F2b litters. This process was repeated
through the F3 generation. Twenty rats of the F3b generation were
maintained on test diets for 9 weeks, at which time they were
sacrificed and histopathological evaluations were conducted on 10
rats/sex/dose.
Animals were examined daily for abnormalities of behaviour or
appearance, and food consumption was measured daily. Rats were weighed
weekly. Fertility, litter size, pup growth, and pup survival for each
litter were determined. At weaning, selected pups were evaluated for
consciousness, emotional behaviour, activity and reactivity,
coordination, and reflexes. Parental rats of each generation were
sacrificed after completion of the second litter and subjected to
gross necropsies. Pups not selected for breeding of the next
generation were sacrificed at weaning with the exception of 10 F3b
pups/sex/dose, which were maintained for 9 weeks on test diets and
then subjected to complete histopathological evaluation.
No effects of treatment on clinical signs, food consumption, or
body weight of parental rats were noted. Similarly, no alterations in
fertility, gestation length, litter size, sex ratio of pups, fetal
birth weight, weight gain of pups, or survival of pups during
lactation were noted in any of the litter intervals. No treatment-
related malformations or developmental defects were noted. No
treatment-related pathological findings were noted in either parental
rats or pups at necropsy. The NOEL for reproductive effects was
determined to be greater than the highest dose tested (Leuschner
et al., 1977b).
Special studies on teratology
Mice
Groups of female NMRI [SPF] mice (24-30/dose) with vaginal plugs
were fed diets containing technical vinclozolin (purity unspecified)
from days 0 - 18 of presumed gestation. Two studies were conducted, 1
with test diets containing 0 and 60,000 ppm vinclozolin, and a second
study with test diets containing 0, 600, and 6000 ppm vinclozolin.
Identical protocols were followed in both studies. Mice were examined
daily for clinical signs of toxicity, and food consumption and body
weights were determined periodically. On day 18 of gestation, the mice
were sacrificed and the number of implantation and resorption sites
and the number of live and dead fetuses were determined. Body weights,
lengths, and the sex of live fetuses were determined, and placentas
were weighed. Fetuses were examined for external abnormalities, and
one-third of the fetuses were examined for visceral abnormalities, and
the remaining two-thirds were examined for skeletal defects.
The appearance and behaviour of mice from the low-dose (600 ppm)
and mid-dose (6000 ppm) groups were not affected by treatment. In
contrast, all mice from the high-dose (60,000 ppm) group died during
the treatment interval. Since these mice also refused food and lost
considerable body weight, it was not clear whether death was the
result of toxicity or of starvation due to food avoidance. Mice fed
600 ppm vinclozolin had a mean body-weight gain during gestation that
was similar to that of mice in the control groups, whereas animals fed
6000 ppm ate about 25% less food than control or low-dose mice, and
gained significantly less body weight. Mice offered the 60,000 ppm
diets refused food, and lost body weight until death.
At necropsy, it was found that none of the mice from the 6000
or 60,000 ppm dose groups had any implantations. The number of
implantations and resorptions, fetal weights and lengths, and
placental weights were similar in control and low-dose mice. Visceral
and skeletal examinations of control and low-dose fetuses revealed
no treatment-related abnormalities. The NOEL for maternal and
developmental toxicity in mice in this study therefore was 600 ppm
(equal to 110 mg/kg b.w./day) (Hofmann & Peh, 1975b).
Rabbits
Groups of female New Zealand white rabbits (15/dose) were
administered doses of 0, 20, 80, or 300 mg/kg b.w./day of technical
vinclozolin (98.1% purity) by gavage from days 6 - 18 of presumed
gestation. Doses were based on a preliminary range-finding assay which
demonstrated that a dose of 900 mg/kg b.w./day was not well tolerated,
while a dose of 300 mg/kg b.w./day produced moderate signs of
toxicity. Rabbits were observed daily for clinical signs of toxicity,
and were weighed periodically during gestation. On day 29 of
gestation, the rabbits were sacrificed by cervical dislocation, and
uteri and the ovaries were examined to determine the number of corpora
lutea and resorptions, live and dead fetuses, gravid uterine weights,
and fetal body weights and lengths. Live fetuses were examined for
external abnormalities, then sacrificed by injection of pheno-
barbitone. Fetuses were weighed, then dissected and examined for
visceral abnormalities. Fetuses were then processed and stained for
skeletal examinations.
No signs of toxicity were observed in does during gestation (the
findings observed in the range-finding study were not reproduced), and
the 4 recorded deaths were considered unrelated to treatment. No
effects of treatment on maternal body weights were apparent. At
necropsy, no effects of treatment on gravid uterine weight or on
litter size or frequency were noted. An approximately 3-fold increase
in the number of resorptions was noted in the high-dose group
(compared to the control group); however, this increase was not
statistically significant. Mean fetal weight was decreased in the
high-dose group by about 6%; this change was not statistically
significant. No effects of treatment on the incidences of visceral or
skeletal abnormalities were apparent. The authors concluded that the
highest dose tested of 300 mg/kg b.w./day had no statistically-
significant effects on litter parameters, nor on maternal health
(Cozens et al., 1981).
Acute toxicity
Vinclozolin possesses a low order of acute toxicity. The results
of acute toxicity studies are summarized in Table 1.
Table 2. Acute toxicity of vinclozolin1
LD50 LD50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M > 15,000 - Shirasu et al.,
F > 15,000 1978a
s.c. M > 15,000 - Shirasu et al.,
F > 15,000 1978a
i.p. M 1570 - Shirasu et al.,
F 1640 1978a
Rats oral M > 15,000 - Shirasu et al.,
F > 15,000 1978b
i.p. M 8300 - Shirasu et al.,
F 4220 1978a
dermal M > 5000 - Shirasu et al.,
F > 5000 1978b
inhalation M - > 29.1 Leuschner, 1979
(4 hr. exp.) F > 29.1
Guinea-pig oral M/F 8000 - Hofmann, 1973
1 Technical vinclozolin was tested in these studies.
Technical vinclozolin was shown to cause mild reversible
irritation to the conjunctivae of the eye (Hildebrand, 1977a) and skin
(Hildebrand, 1977b).
Short-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (16
rats/sex/dose) were fed diets containing 0, 100, 300, 1000, or
2000 ppm technical vinclozolin for 3 months. Six rats from each group
were further maintained on control diets for a post-observation period
of 6 weeks at the end of the study. Rats were examined daily for
mortality, abnormalities of appearance or behaviour, and food
consumption. Body weights were determined weekly. Rats were palpated
at weekly weighings, and the eyes were examined. Blood was sampled for
haematology and clinical chemistry examinations prior to initiation,
after 6 and 12 weeks of treatment, at termination, and at the end of
the post-observation period. Urine was collected on a similar
schedule. At termination, rats were sacrificed by CO2 asphyxiation
and decapitated. Animals were dissected and examined for gross
pathological changes. Absolute and relative weights of major organs
were determined, and a complete set of tissues from each animal was
saved for future histopathological examinations. The results of
microscopic examinations of tissues were not reported in this study,
with the exception of eyes, which were examined in serial sections.
A single death was noted on day 42 in the female high-dose
treatment group; all other rats survived to scheduled termination. No
disturbances of appearance or behaviour were noted, and eyes appeared
normal at all examinations. Body weights of rats in all treatment
groups were comparable to those of controls throughout the study
period. Food consumption was not affected in males, although
occasional statistically-significant increases were noted in the
female treatment groups. Changes in haematology consistent with
decreased red cell mass (decreased numbers of erythrocytes, heama-
tocrits, and haemoglobin, with increased MCH and MCHC) were noted
at the 6-week sampling time in males and females fed diets of 300 ppm
and higher; however, these changes were apparent at termination only
in the female 1000 and 2000 ppm groups. Clinical chemistry parameters
were not altered in a toxicologically-significant manner.
At necropsy, no effects of treatment on the gross appearance of
tissues were apparent. Statistically-significant dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were noted in males and females from the 1000 and 2000 ppm
groups. Increased relative weights of kidneys were noted in 2000 ppm
males and females, and increased relative spleen weight was noted in
2000 ppm females. These organ-weight effects were apparently
reversible, as they were not observed in treated rats that were
continued on control diets for an additional 6 weeks. Microscopic
examinations of eyes did not reveal any treatment-related
abnormalities. The results of microscopic examinations of the
remaining tissues were not included in the study report (Hofmann,
1974).
Another 3-month feeding study in rats has been performed, but it
was not reviewed, because the study report was incomplete. In the
study narrative the authors concluded that 300 ppm (equal to
21.3 mg/kg b.w./day in males and 24.1 mg/kg b.w./day in females) was
the NOEL based on decreased body-weight gain and altered haematology
and clinical chemistry parameters at 1500 ppm and 7500 ppm, and on
altered organ weights and histopathological findings in the liver,
kidneys, ovaries, and pancreas at 7500 ppm (Takehara et al., 1978).
Rabbits
Groups of New Zealand white rabbits (6/sex/dose, 3 of each group
with intact skin, 3 with abraded skin) were treated by dermal
application for 21 consecutive days (8 hours/day) with doses of 111,
333, or 1000 mg/kg b.w. vinclozolin. All animals survived the
treatment period without effects on appearance, behaviour, body
weight, food or water intake, haematology, clinical chemistry, or
necropsy parameters. The highest dose was considered to be the NOEL in
this study (Leuschner et al., 1977c).
Dogs
Groups of purebred beagle dogs (6/sex/dose) were fed diets
containing 0, 100, 300, 600, or 2000 ppm technical vinclozolin
(98.1% purity) for 6 months. Dogs were offered a total of 700 grams of
food for 3 hours/day, and consumption was measured daily. Daily
dosages were equal to 0, 7.0, 20, 41, or 135 mg/kg b.w./day in males
and 0, 7.4, 21, 41, or 141 mg/kg b.w./day in females. Dogs were
examined daily for clinical signs of toxicity, and body weights were
determined weekly. Eyes were examined at 3-month intervals for changes
in refracting media or the fundus. Blood was sampled at approximately
monthly intervals for measurement of haematology and clinical
chemistry parameters. Urine was collected approximately every 2 months
for urinalysis determinations.
At termination, dogs were anaesthetized and sacrificed by
exsanguination. Standard necropsy techniques were followed. The
following tissues were examined (those indicated by an asterisk * were
also weighed) for gross and microscopic changes: abnormalities,
adrenals*, aorta, bone (sternum & marrow), brain*, colon, duodenum,
esophagus, eye (with optic nerve), gall, bladder, heart*, ileum,
jejunum, kidneys*, liver*, lungs (with mainstem bronchi), lymph nodes
(axillary & mesenteric), mammary glands, ovaries*, pancreas, parotid,
pituitary*, prostate, sciatic nerve, skeletal muscle, skin, spleen*,
spinal cord (thoracic, lumbar), stomach, testes* (with epididymides),
thymus, thyroid* (with parathyroid), trachea, urinary bladder, and
uterus (corpus, cervix, and cornu).
No effects of treatment on clinical signs, survival, food
consumption or body-weight gain were apparent. Ophthalmoscopic
examinations did not reveal any treatment-related abnormalities.
Evidence of haemolytic anaemia was noted in high-dose males and
females in the form of increased MCHC, increased numbers of
reticulocytes and red cells containing Howell-Jolly bodies, and
increased serum bilirubin, lactate dehydrogenase, and urea levels.
However, decreases in erythrocyte counts or haematocrits were not
observed, apparently due to compensatory phenomena. Increased numbers
of platelets noted in these dogs also suggested a compensatory
response to anaemia. These effects were more profound in males than in
females. Other clinical chemistry parameters were not affected.
Statistically-significant decreases in SGPT, which were observed in
females, are of questionable toxicological significance. Urinalysis
parameters were not affected by treatment.
At necropsy, statistically-significant dose-related increases in
absolute and relative weights of adrenals were noted in males and
females fed 300 ppm or more vinclozolin. Other changes in organ
weights included decreases in absolute weights of kidneys in males at
300 ppm and above, decreases in absolute brain weights, and increases
in relative spleen weights in high-dose males. Other changes noted in
females included decreased relative pituitary weights at 300 ppm and
higher.
Potential treatment-related gross findings were restricted to
reduced size of the prostate in high-dose males. Upon microscopic
examination, the following potential treatment-related findings were
observed in males (numbers and incidences in control, 100, 300, 600,
and 2000 ppm groups, respectively, of 6 dogs examined): increased
erythropoiesis of bone marrow (0, 0, 0, 1, 4); sinusoidal dilatation/
accumulation of erythrocytes in the spleen (1, 2, 2, 2, 4);
atrophy/stromal proliferation of the prostate (0, 0, 2, 4, 6); and
vacuolization of the zona fasciculata of the adrenals (0, 0, 1, 1, 4).
No gross findings were evident in females. Incidences of
microscopic lesions (as described above) were: increased erythro-
poiesis of bone marrow (1, 0, 2, 2, 5), sideropexia of the bone
marrow (1, 0, 4, 5, 6); sinusoidal dilatation/accumulation of
erythrocytes in the spleen (0, 1, 3, 4, 5); and vacuolization of zona
fasciculata of the adrenals (0, 0, 0, 3, 6).
The authors concluded that the no adverse-effect level in this
study was between 300 and 600 ppm vinclozolin in the diet (Kirsch
et al., 1982).
Long-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (50/sex/dose)
were fed diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin
(purity unspecified) for 130 weeks. Control or test diet and water
were offered ad libitum. The dietary concentrations were equal to
9.4, 27, 83, or 257 mg/kg b.w./day in males and 9.1, 28, 84, or
278 mg/kg b.w./day in females. The results of analyses of the test
material or of test diets were not presented. Animals were examined
twice/day for abnormalities of behaviour or appearance, and were
palpated weekly beginning at week 26 for evidence of rumours. Eyes,
ears, and dentition were examined "regularly". Rats were weighed
weekly. The schedule and manner of measurement of food consumption
were not specified.
Blood was sampled at 0, 4, 8, 13, 26, 52, and 104 weeks from 10
rats/sex/dose for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted,
and in addition, at week 104, glycogen in heart, liver, and skeletal
muscle, total lipids in liver, and ascorbic acid in the adrenals were
measured. Urine was collected on a similar schedule for standard
urinalysis determinations.
Rats that died on test, moribund animals, and all rats surviving
to 130 weeks were subjected to complete necropsies. Rats were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, kidneys, liver, lungs, lymph node,
ovaries, pancreas, parotid, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs or mortality were
apparent. Eye, hearing, and dentition parameters were within normal
limits. The study was terminated at week 130 when combined male and
female mortality in the control group reached 70%. Survival in test
groups was inversely related to dose, as the lowest mortality was
noted in the high-dose group. Body weights were decreased by about 10%
in males and females fed the 1460 ppm diet, and by about 40 and 25%,
respectively, in males and females fed the 4370 ppm diet. These
changes emerged within the first month of treatment and persisted
until study termination. Food consumption was decreased in a similar
manner in the 2 highest-dose groups.
No effects of treatment on haematology or clinical chemistry
parameters were apparent. A decrease of about 20% total bilirubin in
high-dose females is of doubtful toxicological significance.
Occasional increases in the urinary excretion of 17 ketosteroids and
of ascorbic acid were noted in high-dose females in the first year of
treatment; however, these changes were not evident in the final year
of treatment.
At necropsy, organ weights were altered in a manner related to
the body-weight changes. Absolute weights of several organs were
decreased in males and females from the 1460 ppm and 4370 ppm groups,
whereas relative organ weights tended to increase in these groups.
Other necropsy parameters were not affected by treatment. The
incidences of macroscopic and microscopic findings were randomly
distributed among all test groups, and did not appear to be related to
treatment with the test material. The total number of rumours, as well
as the distribution of specific rumour types, was random throughout
the test groups, and not affected by treatment. Therefore, vinclozolin
was determined not to be oncogenic in the rat. The NOEL for chronic
toxicity was 486 ppm, equal to 27 and 28 mg/kg b.w./day in males and
females, respectively, based on decreases in food consumption and
body-weight gain (Leuschner et al., 1977d).
Observations in humans
No information available.
COMMENTS
Data were not submitted to completely characterize the rates of
absorption or elimination of vinclozolin. In studies that were
submitted, all of the administered dose was eliminated, and no
potential for bioaccumulation was apparent. The plasma half-life was
about 20 hours.
Vinclozolin is extensively metabolized, and its major metabolite
(metabolite F) is excreted as a glucuronide or sulfate conjugate.
Specific interaction with enzymes is not known.
A plant metabolite has been identified that is not formed in rats
(metabolite T). Additional testing is necessary to evaluate the
toxicity of this compound.
Vinclozolin has a low order of acute toxicity.
Special studies on carcinogenicity were negative for oncogenic
potential in rats and mice. Similarly, this chemical has no known
specific potential for mutagenicity, teratogenicity, or reproductive
toxicity.
A sub-chronic feeding study in rats suggested possible anaemia
after treatment with diets containing 1000 or 2000 ppm vinclozolin.
However, this finding was not reproduced in a 2-year feeding study in
rats. Chronic effects noted in the long-term rat study were restricted
to decreases in food consumption and body-weight gain of rats fed
1460 ppm and higher (equal to 83 mg/kg b.w./day), with a NOEL for this
effect of 486 ppm (equal to 27 and 28 mg/kg b.w./day in males and
females, respectively).
A feeding study in dogs demonstrated evidence of haemolytic
anaemia in animals fed 2000 ppm vinclozolin for 6 months (equal to 135
and 141 mg/kg b.w./day in males and females, respectively). Other
relevant findings included significant increases in relative and
absolute adrenal weights in males and females at doses of 300 ppm and
higher (equal to 20 and 21 mg/kg b.w./day in males and females,
respectively). A clear dose-related trend for vacuolization of the
zona fasciculata of the adrenals was noted in males and females at
doses of 600 and 2000 ppm. Thus, the changes in adrenal weights
observed at the lower doses can be related to pathology at higher
doses. Other dose-related histopathological findings noted in the
300 ppm groups included accumulation of erythrocytes in the spleen,
atrophy of the prostate, and sideropexia of the bone marrow
(accumulation of haemosiderin within reticuloendothelial elements of
the bone marrow). On the basis of these findings, the NOEL in this
study was 100 ppm, equal to 7.0 mg/kg b.w./day in males and 7.4 mg/kg
b.w./day in females.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECTS
Averages for males and females:
Mice: 486 ppm, equal to 87 /kg b.w./day.
Rats: 486 ppm, equal to 27 /kg b.w./day.
Dogs: 100 ppm, equal to 7.2 /kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DALLY INTAKE FOR MAN
0 - 0.04 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE, TO
BE SUBMITTED TO WHO BY 1987:
Additional studies to evaluate the toxicity of the plant
metabolite, metabolite T.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND:
1. Data to demonstrate the rate of absorption and elimination
after a single oral dose of vinclozolin.
2. Data on the potential effects to humans of exposure to
vinclozolin.
REFERENCES
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B., &
1976 Franklin, E.R. The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral adminstration to
rats. Not submitted for review.
Cifone, M.A. & Myhr, B.C. Report on the evaluation of Vinclozolin in
1984 the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 814/072 from Litton Bionetics, Inc.,
Kensington, MD, USA. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R., & Offer, J.M.
1981 Effect of vinclozolin on pregnancy of the the New Zealand
white rabbit. Unpublished report No. BSF/3810/381/81357 from
Huntingdon Research Centre, Huntingdon, England. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P., Engelhardt, G., & Ruff, M. Cytogenetic investigations
1982 in Chinese hamsters after a single oral administration of
Reg. No. 83 258 - sister chromatid exchange. Unpublished
report No. 82/085, from BASF Gewerbehygiene und Toxikologie,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P. & Engelhardt, G. Report on the study of Vinclozolin in
1983 the Ames test. Unpublished report No. 85/228, from BASF
Gewerbehygiene und Toxikologie, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Gelbke, H.-P. & Jäckh, R. Report on a point mutation test carried out
1985 on CHO cells (HGPRT locus) with the test substance
vinclozolin. Unpublished report No. 85/352 from BASF Dept.
Toxicology, Crop Protection Division. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) to
1977a the eye of white rabbits. Unpublished report No. 77/020,
from BASF Gewerbehygiene und Toxikologie, Ludwigshafen/
Rhein, FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) on
1977b the intact and scarified dorsal skin of white rabbits.
Unpublished report No. 77/022, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. Report on the acute oral toxicity trial of 3-(3,5-di-
1973 chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report No. XXII/337 from
Agricultural Research Station, BASF, Limburgerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. Report on the testing of 3-(3,5-dichlorophenyl)-
1974 5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione in a three month
feeding experiment on rats. Unpublished report No. 74/001
from Medizinisch- Biologische Forschungslaboratorium, BASF.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the mutagenic effect of 3-(3,5-di-
1975a chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in mice. Unpublished report No. 75/003 from Medizinisch-
Biologische Forschungslaboratorium, BASF. Submitted to WHO
by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the prenatal toxicity of
1975b 3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-
dione in mice. Unpublished report No. 75/004 from
Medizinisch-Biologische Forschungslaboratorium, BASF.
Ssubmitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hoorn, A.J.W. Report on the mutagenicity evaluation of Vinclozolin in
1983 the rec assay with Bacillus subtilis. Unpublished report
No. 83/236 from Litton Bionetics, 3905 Pe Veenendaal,
Netherlands. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Kirsch, Deckhardt, Mirea, Hellwig, Hildebrand, & Ruff. Report on the
1982 study of the toxicity of Reg. No. 83 258 (vinclozolin) in
beagle dogs after 6-month administration in the diet.
Unpublished report No. 82/177, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., Dontenwill, W., & Rogulja,
1977a P.V. Oral toxicity of an oxazolidine derivative, batch
813258 - called for short "OXA" - in NMRI mice (with special
attention to carcinogenic properties). Unpublished report
No. 77/017 from Laboratorium fur Pharmakologie und
Toxikologie, Hamburg, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Hubscher, F., Neumann, W., Neumann, B., Rogulja, P.V. &
1977b Dontenwill, W. Chronic oral toxicity of an oxazolidine
derivative, batch 813258 - called for short "OXA" - in a
reproductive study covering three generations in
Sprague-Dawley rats. Unpublished report No. 77/019 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg,
FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., & Dontenwill, W.
1977c 3-Weeks toxicity of an oxazolidine derivative, batch 813258
- called for short "OXA" - in NZW rabbits by local
application. Unpublished report No. 77/015 from Laboratorium
fur Pharmakologie und Toxikologie, Hamburg, FRG. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., & Dontenwill, W. Chronic
1977d oral toxicity of an oxazolidine derivative, batch 813258 -
called for short "OXA" - in the Sprague-Dawley rat.
Unpublished report No. 77/016 from Laboratorium fur
Pharmakologie und Toxikologie, hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F. Report on the acute toxicity of the preparation Reg.
1979 No. 813258 when administered to rats by inhalation.
Unpublished report No. 79/029 from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Otto, S., Beutel, P., Elzner, J., & Ohnsorge, U. Metabolism of
1977 14C-vinclozolin in rats. Unpublished report No. 1474 from
Agricultural Research Station, BASF, Limbergerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978a with BAS 352 F in mice. Unpublished report No. 78/031 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978b with BAS 352 F in rats. Unpublished report No. 78/030 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Takehara, K., Kudoh, S., Maruyama, Y., Hayashi, K., Itabashi, M., &
1978 Tajima, M. Three-month oral toxicity study of BAS-352-F in
rats. Unpublished report No. 78/029 from Nippon Institute
for Biological Science, Tokyo, Japan. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Witterland, W.F. & Hoorn, A.J.W. Report on the mutagenicity evaluation
1984 of vinclozolin in the mouse lymphoma forward mutation assay.
Unpublished report No. 84/267 from Litton Bionetics, 3905 Pe
Veenendaal, Netherlands. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
 MOLECULAR WEIGHT 286.1
PHYSICAL STATE Crystalline solid
COLOUR AND ODOUR White, odorless
MELTING POINT 108°C
VAPOUR PRESSURE < 0.1 × 10-6mbar at 20°C
SOLUBILITY
Solvent g compound/100 g solvent at 20°C
Water < 0.1
ethyl ether 6.3
ethyl alcohol 1.4
chloroform 31.9
lutrol 2.0 (approx.)
acetone 43.5
ethyl acetate 25.3
cyclohexane 0.9
benzene 14.6
olive oil 1.5 (approx.)
OCTANOL/WATER PARTITION COEFFICIENT
1000 (approx.)
SPECIFIC GRAVITY approx. 1.513 kg/l at 20°C
BULK DENSITY 640 g/l loose (WHO method)
900 g/l compact (WHO method)
COMMERCIAL FORMULATIONS AVAILABLE
Too numerous to list; most are available under
the trade name Ronilan(R), and are formulated
as wettable powders, suspension conentrates, or
dust and smoke formulations.
STABILITY Stable at temperatures up to 50°C; stable in
water and dilute acids; half-life in 0.1 N
NaOH = 3.8 hours; stable to light.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Data were not submitted to adequately characterize the absorption
and excretion of vinclozolin.
A group of 5 male rats (strain unknown) was administered 5 doses
by gavage of 40 mg/kg b.w. 14C-vinclozolin (universal label on the
phenyl ring, s.a. = 8.07 µCi/mg). Excreta were collected daily and
frozen. Four hours after the last treatment, the rats were sacrificed
and tissues were collected and frozen. Urine was measured directly for
radioactivity, whereas samples of faeces, blood and tissues were
combusted and levels of 14CO2 were determined.
Data were expressed as the concentration of vinclozolin in
excreta, blood and tissues. The highest average concentration of
chemical was found in faeces (1300 µg/g), followed by urine
(450 µg/g), kidney (76.4 µg/g), liver (75.2 µg/g), fat (56.8 µg/g),
muscle (30.8 µg/g), and blood (13.3 µg/g). Levels of radioactivity in
urine and faeces appeared to reach a plateau by the second day of
treatment (Otto et al., 1977).
In a second study, 14C-vinclozolin was administered orally for
7 days at a dose of 40 mg/kg b.w./day. Six days after the last dose,
47% and 54% (mean values) of the total administered dose had been
eliminated in urine and feaces, respectively, for a total of 101%. No
radioactivity was detectable in carcasses at this time, nor was any
radioactivity detected in expired air.
Cannulation of bile ducts after a single oral dose revealed that
65% of administered radioactivity was excreted into the bile, whereas
only 19% and 15% were eliminated in the urine and faeces,
respectively.
Peak plasma levels were detected after about 1 hour; the plasma
half-life was 20 hours. As dosing continued, baseline plasma levels
tended to increase. After 7 doses, the highest levels of tissue
radioactivity were detected in the liver, kidneys, gastrointestinal
tract, fat, adrenals, and ovaries. By 96 hours after the last dose,
levels of radioactivity in tissues were not different from those of
plasma. These findings were confirmed by whole-body autoradiography
(Chasseaud et al., 1976).
Biotransformation
Samples of urine and faeces were analyzed to determine the
identities of metabolites. Vinclozolin was metabolized extensively, as
apparently no parent compound was detected in the urine, and about
8 - 40% of the parent compound was detected in the faeces.
N-(3,5-Dichlorophenyl)-2-methyl-2,3,4-trihydroxybutanoic acid amide
(metabolite F) was the major excreted metabolite, which accounted for
42% of the urinary radioactivity and 60 - 90% of the faecal
radioactivity. Metabolite F was excreted in urine as either a
glucuronide or sulfate conjugate, and was excreted in free form in the
faeces. This metabolite was also the major species found in the blood,
kidneys, and liver. Other metabolites, resulting from further
degradation of metabolite F, were formed in insignificant amounts
(Otto et al., 1977).
A proposed metabolic pathway is described in Figure 1.
Toxicological studies
Special study on carcinogenicity
Mice
Groups of NMRI mice (50/sex/dose) were fed diets containing
0, 162, 486, 1460, or 4370 ppm vinclozolin (purity unspecified) for
110 weeks. Control or test diet and water were offered ad libitum.
The dietary concentrations of vinclozolin were equal to 27, 82, 275,
or 818 mg/kg b.w./day in males and 31, 92, 287, or 912 mg/kg b.w./day
in females. The results of analyses of the test material or of the
test diets were not presented. Animals were examined twice daily for
abnormalities of behaviour or appearance, and were palpated weekly
beginning at week 26 for evidence of tumours. Eyes, ears, and
dentition were examined at necropsy. Mice were weighed weekly. The
schedule and manner of measurement of food consumption were not
specified.
Blood was sampled at 0, 6, 13, 26, 52, and 78 weeks, from 10
mice/sex/dose, for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted.
Urine was collected on a similar schedule for standard urinalysis
determinations.
MOLECULAR WEIGHT 286.1
PHYSICAL STATE Crystalline solid
COLOUR AND ODOUR White, odorless
MELTING POINT 108°C
VAPOUR PRESSURE < 0.1 × 10-6mbar at 20°C
SOLUBILITY
Solvent g compound/100 g solvent at 20°C
Water < 0.1
ethyl ether 6.3
ethyl alcohol 1.4
chloroform 31.9
lutrol 2.0 (approx.)
acetone 43.5
ethyl acetate 25.3
cyclohexane 0.9
benzene 14.6
olive oil 1.5 (approx.)
OCTANOL/WATER PARTITION COEFFICIENT
1000 (approx.)
SPECIFIC GRAVITY approx. 1.513 kg/l at 20°C
BULK DENSITY 640 g/l loose (WHO method)
900 g/l compact (WHO method)
COMMERCIAL FORMULATIONS AVAILABLE
Too numerous to list; most are available under
the trade name Ronilan(R), and are formulated
as wettable powders, suspension conentrates, or
dust and smoke formulations.
STABILITY Stable at temperatures up to 50°C; stable in
water and dilute acids; half-life in 0.1 N
NaOH = 3.8 hours; stable to light.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Data were not submitted to adequately characterize the absorption
and excretion of vinclozolin.
A group of 5 male rats (strain unknown) was administered 5 doses
by gavage of 40 mg/kg b.w. 14C-vinclozolin (universal label on the
phenyl ring, s.a. = 8.07 µCi/mg). Excreta were collected daily and
frozen. Four hours after the last treatment, the rats were sacrificed
and tissues were collected and frozen. Urine was measured directly for
radioactivity, whereas samples of faeces, blood and tissues were
combusted and levels of 14CO2 were determined.
Data were expressed as the concentration of vinclozolin in
excreta, blood and tissues. The highest average concentration of
chemical was found in faeces (1300 µg/g), followed by urine
(450 µg/g), kidney (76.4 µg/g), liver (75.2 µg/g), fat (56.8 µg/g),
muscle (30.8 µg/g), and blood (13.3 µg/g). Levels of radioactivity in
urine and faeces appeared to reach a plateau by the second day of
treatment (Otto et al., 1977).
In a second study, 14C-vinclozolin was administered orally for
7 days at a dose of 40 mg/kg b.w./day. Six days after the last dose,
47% and 54% (mean values) of the total administered dose had been
eliminated in urine and feaces, respectively, for a total of 101%. No
radioactivity was detectable in carcasses at this time, nor was any
radioactivity detected in expired air.
Cannulation of bile ducts after a single oral dose revealed that
65% of administered radioactivity was excreted into the bile, whereas
only 19% and 15% were eliminated in the urine and faeces,
respectively.
Peak plasma levels were detected after about 1 hour; the plasma
half-life was 20 hours. As dosing continued, baseline plasma levels
tended to increase. After 7 doses, the highest levels of tissue
radioactivity were detected in the liver, kidneys, gastrointestinal
tract, fat, adrenals, and ovaries. By 96 hours after the last dose,
levels of radioactivity in tissues were not different from those of
plasma. These findings were confirmed by whole-body autoradiography
(Chasseaud et al., 1976).
Biotransformation
Samples of urine and faeces were analyzed to determine the
identities of metabolites. Vinclozolin was metabolized extensively, as
apparently no parent compound was detected in the urine, and about
8 - 40% of the parent compound was detected in the faeces.
N-(3,5-Dichlorophenyl)-2-methyl-2,3,4-trihydroxybutanoic acid amide
(metabolite F) was the major excreted metabolite, which accounted for
42% of the urinary radioactivity and 60 - 90% of the faecal
radioactivity. Metabolite F was excreted in urine as either a
glucuronide or sulfate conjugate, and was excreted in free form in the
faeces. This metabolite was also the major species found in the blood,
kidneys, and liver. Other metabolites, resulting from further
degradation of metabolite F, were formed in insignificant amounts
(Otto et al., 1977).
A proposed metabolic pathway is described in Figure 1.
Toxicological studies
Special study on carcinogenicity
Mice
Groups of NMRI mice (50/sex/dose) were fed diets containing
0, 162, 486, 1460, or 4370 ppm vinclozolin (purity unspecified) for
110 weeks. Control or test diet and water were offered ad libitum.
The dietary concentrations of vinclozolin were equal to 27, 82, 275,
or 818 mg/kg b.w./day in males and 31, 92, 287, or 912 mg/kg b.w./day
in females. The results of analyses of the test material or of the
test diets were not presented. Animals were examined twice daily for
abnormalities of behaviour or appearance, and were palpated weekly
beginning at week 26 for evidence of tumours. Eyes, ears, and
dentition were examined at necropsy. Mice were weighed weekly. The
schedule and manner of measurement of food consumption were not
specified.
Blood was sampled at 0, 6, 13, 26, 52, and 78 weeks, from 10
mice/sex/dose, for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted.
Urine was collected on a similar schedule for standard urinalysis
determinations.
 Mice that died on test, moribund animals, and all mice surviving
to 112 weeks were subjected to complete mecropsies. Mice were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs were apparent. Survival
was somewhat lower in high-dose males than in controls (76% mortality
vs. 48% in the control group); however, mortality in all female groups
was about 70%, with no effects of treatment. Mean body weights were
reduced in males in the 2 highest dose groups; these decreases emerged
in the second year of treatment and persisted until termination. Food
intake did not appear to be affected by treatment. No effects of
treatment oil haematology, clinical chemistry, or urinalysis
parameters were noted.
At necropsy, significant increases of 150 - 250% in the absolute
and relative weights of the liver were noted in females from the 1460
and 4370 ppm groups. An increase in liver weight of about 40% and an
increase in testes weight of about 50% were noted in high-dose males.
Absolute weights of other organs were altered in males; however, these
changes probably were related to decreases in body weight.
After microscopic examination, no toxicologically significant
differences in the incidences of neoplastic or non-neoplastic lesions
were noted. Vinclozolin was therefore negative for oncogenic potential
in mice (Leuschner et al., 1977a).
Special studies on mutagenicity
Vinclozolin was negative in a number of acceptable mutagenicity
studies, which included assessments of gene mutations (bacteria and
mammalian cells), direct DNA damage (bacteria and mammalian cells),
and clastogenic effects (in vivo mammalian cells).
Summary results are presnted in Table 1.
Special study on reproduction
Rats
Groups of male and female Sprague-Dawley rats (20/sex/dose) were
fed diets containing 0, 162, 486, or 1485 ppm vinclozolin (purity
unspecified) over 3 generations. Control or test diet and water were
offered ad libitum. The dietary concentrations of vinclozolin were
equal to 12, 37, or 113 mg/kg b.w./day in males and 17, 51, or
152 mg/kg b.w./day in females. The results of analyses of the test
material or of the test diets were not presented.
Table 1: Results of mutagenicity studies on vinclozolin
Test system Test object Concentration of Purity Results Reference
vinclozolin
Ames test1 S. typhimurium 0, 100, 500 98.1% Negative2 Gelbke & Engelhardt, 1983
TA98, TA100, TA1535, 2500, 5000,
10,000, TA1537, 7500, and
and TA1538. µg/plate
CHO/HGPRT gene Chinese hamster 0,0.32,1.0, > 99.5% Negative3 Gelbke & Jäckh, 1985
mutation ovary cells, 3.2, or 10.0
assay1 CHO-K1 mg/ml
Unscheduled Primary hepatocytes 0, 5, 10, > 99.5% Negative4 Cifone & Myhr, 1984
DNA from male Fischer 25, 50, 100,
synthesis 344 rats 250, 500, &
1000 µg/ml
Mouse lymphoma L5178Y mouse Nonactivated: Technical Negative3 Witterland & Hoorn, 1984
forward lymphoma 0 - 1000 µg/ml
mutation cells (TK+/-) (8 dose levels)
assay1 Activated:
0 - 600 µg/ml
(13 dose levels)
DNA repair B. subtilis 0, 1, 10, Technical Negative5 Hoorn, 1983
assay1 H17 and M45 100, 500,
(rec +/-) 1000, 2500,
5000 & 10,000
µg/plate
Table 1: (cont'd).
Test system Test object Concentration of Purity Results Reference
vinclozolin
In vivo sister Chinese hamster 0, 3830, & 98.1% Negative6 Gelbke, et al., 1982
chromatid bone marrow 5620 mg/kg
exchange assay
Mouse dominant NMRI mice 0 & 2000 Technical Negative7 Hofmann & Peh, 1975a
lethal assay mg/kg
1 With and without activation.
2 The positive controls gave the expected positive results.
3 The positive controls, EMS and MCA, gave the expected positive results.
4 The positive control, 0.05 µg/ml 2-AAF, gave the expected positive result.
5 The positive controls, MMS and sterigmatocystin, gave the expected positive results.
6 The positive control, cyclophosphamide, gave the expected positive response.
7 This study is not acceptable. A positive control was not included; therefore, the sensitivity of the test system was not
established.
Two litters were obtained from each generation. Rats from the
parental generation (F0) were bred after 8 and 16 weeks of treatment
(1:1 male to female) to form the F1a and F1b litters, respectively.
Twenty rats/sex/dose were selected from the F1b litters to become
parents of the F2a and F2b litters. This process was repeated
through the F3 generation. Twenty rats of the F3b generation were
maintained on test diets for 9 weeks, at which time they were
sacrificed and histopathological evaluations were conducted on 10
rats/sex/dose.
Animals were examined daily for abnormalities of behaviour or
appearance, and food consumption was measured daily. Rats were weighed
weekly. Fertility, litter size, pup growth, and pup survival for each
litter were determined. At weaning, selected pups were evaluated for
consciousness, emotional behaviour, activity and reactivity,
coordination, and reflexes. Parental rats of each generation were
sacrificed after completion of the second litter and subjected to
gross necropsies. Pups not selected for breeding of the next
generation were sacrificed at weaning with the exception of 10 F3b
pups/sex/dose, which were maintained for 9 weeks on test diets and
then subjected to complete histopathological evaluation.
No effects of treatment on clinical signs, food consumption, or
body weight of parental rats were noted. Similarly, no alterations in
fertility, gestation length, litter size, sex ratio of pups, fetal
birth weight, weight gain of pups, or survival of pups during
lactation were noted in any of the litter intervals. No treatment-
related malformations or developmental defects were noted. No
treatment-related pathological findings were noted in either parental
rats or pups at necropsy. The NOEL for reproductive effects was
determined to be greater than the highest dose tested (Leuschner
et al., 1977b).
Special studies on teratology
Mice
Groups of female NMRI [SPF] mice (24-30/dose) with vaginal plugs
were fed diets containing technical vinclozolin (purity unspecified)
from days 0 - 18 of presumed gestation. Two studies were conducted, 1
with test diets containing 0 and 60,000 ppm vinclozolin, and a second
study with test diets containing 0, 600, and 6000 ppm vinclozolin.
Identical protocols were followed in both studies. Mice were examined
daily for clinical signs of toxicity, and food consumption and body
weights were determined periodically. On day 18 of gestation, the mice
were sacrificed and the number of implantation and resorption sites
and the number of live and dead fetuses were determined. Body weights,
lengths, and the sex of live fetuses were determined, and placentas
were weighed. Fetuses were examined for external abnormalities, and
one-third of the fetuses were examined for visceral abnormalities, and
the remaining two-thirds were examined for skeletal defects.
The appearance and behaviour of mice from the low-dose (600 ppm)
and mid-dose (6000 ppm) groups were not affected by treatment. In
contrast, all mice from the high-dose (60,000 ppm) group died during
the treatment interval. Since these mice also refused food and lost
considerable body weight, it was not clear whether death was the
result of toxicity or of starvation due to food avoidance. Mice fed
600 ppm vinclozolin had a mean body-weight gain during gestation that
was similar to that of mice in the control groups, whereas animals fed
6000 ppm ate about 25% less food than control or low-dose mice, and
gained significantly less body weight. Mice offered the 60,000 ppm
diets refused food, and lost body weight until death.
At necropsy, it was found that none of the mice from the 6000
or 60,000 ppm dose groups had any implantations. The number of
implantations and resorptions, fetal weights and lengths, and
placental weights were similar in control and low-dose mice. Visceral
and skeletal examinations of control and low-dose fetuses revealed
no treatment-related abnormalities. The NOEL for maternal and
developmental toxicity in mice in this study therefore was 600 ppm
(equal to 110 mg/kg b.w./day) (Hofmann & Peh, 1975b).
Rabbits
Groups of female New Zealand white rabbits (15/dose) were
administered doses of 0, 20, 80, or 300 mg/kg b.w./day of technical
vinclozolin (98.1% purity) by gavage from days 6 - 18 of presumed
gestation. Doses were based on a preliminary range-finding assay which
demonstrated that a dose of 900 mg/kg b.w./day was not well tolerated,
while a dose of 300 mg/kg b.w./day produced moderate signs of
toxicity. Rabbits were observed daily for clinical signs of toxicity,
and were weighed periodically during gestation. On day 29 of
gestation, the rabbits were sacrificed by cervical dislocation, and
uteri and the ovaries were examined to determine the number of corpora
lutea and resorptions, live and dead fetuses, gravid uterine weights,
and fetal body weights and lengths. Live fetuses were examined for
external abnormalities, then sacrificed by injection of pheno-
barbitone. Fetuses were weighed, then dissected and examined for
visceral abnormalities. Fetuses were then processed and stained for
skeletal examinations.
No signs of toxicity were observed in does during gestation (the
findings observed in the range-finding study were not reproduced), and
the 4 recorded deaths were considered unrelated to treatment. No
effects of treatment on maternal body weights were apparent. At
necropsy, no effects of treatment on gravid uterine weight or on
litter size or frequency were noted. An approximately 3-fold increase
in the number of resorptions was noted in the high-dose group
(compared to the control group); however, this increase was not
statistically significant. Mean fetal weight was decreased in the
high-dose group by about 6%; this change was not statistically
significant. No effects of treatment on the incidences of visceral or
skeletal abnormalities were apparent. The authors concluded that the
highest dose tested of 300 mg/kg b.w./day had no statistically-
significant effects on litter parameters, nor on maternal health
(Cozens et al., 1981).
Acute toxicity
Vinclozolin possesses a low order of acute toxicity. The results
of acute toxicity studies are summarized in Table 1.
Table 2. Acute toxicity of vinclozolin1
LD50 LD50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M > 15,000 - Shirasu et al.,
F > 15,000 1978a
s.c. M > 15,000 - Shirasu et al.,
F > 15,000 1978a
i.p. M 1570 - Shirasu et al.,
F 1640 1978a
Rats oral M > 15,000 - Shirasu et al.,
F > 15,000 1978b
i.p. M 8300 - Shirasu et al.,
F 4220 1978a
dermal M > 5000 - Shirasu et al.,
F > 5000 1978b
inhalation M - > 29.1 Leuschner, 1979
(4 hr. exp.) F > 29.1
Guinea-pig oral M/F 8000 - Hofmann, 1973
1 Technical vinclozolin was tested in these studies.
Technical vinclozolin was shown to cause mild reversible
irritation to the conjunctivae of the eye (Hildebrand, 1977a) and skin
(Hildebrand, 1977b).
Short-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (16
rats/sex/dose) were fed diets containing 0, 100, 300, 1000, or
2000 ppm technical vinclozolin for 3 months. Six rats from each group
were further maintained on control diets for a post-observation period
of 6 weeks at the end of the study. Rats were examined daily for
mortality, abnormalities of appearance or behaviour, and food
consumption. Body weights were determined weekly. Rats were palpated
at weekly weighings, and the eyes were examined. Blood was sampled for
haematology and clinical chemistry examinations prior to initiation,
after 6 and 12 weeks of treatment, at termination, and at the end of
the post-observation period. Urine was collected on a similar
schedule. At termination, rats were sacrificed by CO2 asphyxiation
and decapitated. Animals were dissected and examined for gross
pathological changes. Absolute and relative weights of major organs
were determined, and a complete set of tissues from each animal was
saved for future histopathological examinations. The results of
microscopic examinations of tissues were not reported in this study,
with the exception of eyes, which were examined in serial sections.
A single death was noted on day 42 in the female high-dose
treatment group; all other rats survived to scheduled termination. No
disturbances of appearance or behaviour were noted, and eyes appeared
normal at all examinations. Body weights of rats in all treatment
groups were comparable to those of controls throughout the study
period. Food consumption was not affected in males, although
occasional statistically-significant increases were noted in the
female treatment groups. Changes in haematology consistent with
decreased red cell mass (decreased numbers of erythrocytes, heama-
tocrits, and haemoglobin, with increased MCH and MCHC) were noted
at the 6-week sampling time in males and females fed diets of 300 ppm
and higher; however, these changes were apparent at termination only
in the female 1000 and 2000 ppm groups. Clinical chemistry parameters
were not altered in a toxicologically-significant manner.
At necropsy, no effects of treatment on the gross appearance of
tissues were apparent. Statistically-significant dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were noted in males and females from the 1000 and 2000 ppm
groups. Increased relative weights of kidneys were noted in 2000 ppm
males and females, and increased relative spleen weight was noted in
2000 ppm females. These organ-weight effects were apparently
reversible, as they were not observed in treated rats that were
continued on control diets for an additional 6 weeks. Microscopic
examinations of eyes did not reveal any treatment-related
abnormalities. The results of microscopic examinations of the
remaining tissues were not included in the study report (Hofmann,
1974).
Another 3-month feeding study in rats has been performed, but it
was not reviewed, because the study report was incomplete. In the
study narrative the authors concluded that 300 ppm (equal to
21.3 mg/kg b.w./day in males and 24.1 mg/kg b.w./day in females) was
the NOEL based on decreased body-weight gain and altered haematology
and clinical chemistry parameters at 1500 ppm and 7500 ppm, and on
altered organ weights and histopathological findings in the liver,
kidneys, ovaries, and pancreas at 7500 ppm (Takehara et al., 1978).
Rabbits
Groups of New Zealand white rabbits (6/sex/dose, 3 of each group
with intact skin, 3 with abraded skin) were treated by dermal
application for 21 consecutive days (8 hours/day) with doses of 111,
333, or 1000 mg/kg b.w. vinclozolin. All animals survived the
treatment period without effects on appearance, behaviour, body
weight, food or water intake, haematology, clinical chemistry, or
necropsy parameters. The highest dose was considered to be the NOEL in
this study (Leuschner et al., 1977c).
Dogs
Groups of purebred beagle dogs (6/sex/dose) were fed diets
containing 0, 100, 300, 600, or 2000 ppm technical vinclozolin
(98.1% purity) for 6 months. Dogs were offered a total of 700 grams of
food for 3 hours/day, and consumption was measured daily. Daily
dosages were equal to 0, 7.0, 20, 41, or 135 mg/kg b.w./day in males
and 0, 7.4, 21, 41, or 141 mg/kg b.w./day in females. Dogs were
examined daily for clinical signs of toxicity, and body weights were
determined weekly. Eyes were examined at 3-month intervals for changes
in refracting media or the fundus. Blood was sampled at approximately
monthly intervals for measurement of haematology and clinical
chemistry parameters. Urine was collected approximately every 2 months
for urinalysis determinations.
At termination, dogs were anaesthetized and sacrificed by
exsanguination. Standard necropsy techniques were followed. The
following tissues were examined (those indicated by an asterisk * were
also weighed) for gross and microscopic changes: abnormalities,
adrenals*, aorta, bone (sternum & marrow), brain*, colon, duodenum,
esophagus, eye (with optic nerve), gall, bladder, heart*, ileum,
jejunum, kidneys*, liver*, lungs (with mainstem bronchi), lymph nodes
(axillary & mesenteric), mammary glands, ovaries*, pancreas, parotid,
pituitary*, prostate, sciatic nerve, skeletal muscle, skin, spleen*,
spinal cord (thoracic, lumbar), stomach, testes* (with epididymides),
thymus, thyroid* (with parathyroid), trachea, urinary bladder, and
uterus (corpus, cervix, and cornu).
No effects of treatment on clinical signs, survival, food
consumption or body-weight gain were apparent. Ophthalmoscopic
examinations did not reveal any treatment-related abnormalities.
Evidence of haemolytic anaemia was noted in high-dose males and
females in the form of increased MCHC, increased numbers of
reticulocytes and red cells containing Howell-Jolly bodies, and
increased serum bilirubin, lactate dehydrogenase, and urea levels.
However, decreases in erythrocyte counts or haematocrits were not
observed, apparently due to compensatory phenomena. Increased numbers
of platelets noted in these dogs also suggested a compensatory
response to anaemia. These effects were more profound in males than in
females. Other clinical chemistry parameters were not affected.
Statistically-significant decreases in SGPT, which were observed in
females, are of questionable toxicological significance. Urinalysis
parameters were not affected by treatment.
At necropsy, statistically-significant dose-related increases in
absolute and relative weights of adrenals were noted in males and
females fed 300 ppm or more vinclozolin. Other changes in organ
weights included decreases in absolute weights of kidneys in males at
300 ppm and above, decreases in absolute brain weights, and increases
in relative spleen weights in high-dose males. Other changes noted in
females included decreased relative pituitary weights at 300 ppm and
higher.
Potential treatment-related gross findings were restricted to
reduced size of the prostate in high-dose males. Upon microscopic
examination, the following potential treatment-related findings were
observed in males (numbers and incidences in control, 100, 300, 600,
and 2000 ppm groups, respectively, of 6 dogs examined): increased
erythropoiesis of bone marrow (0, 0, 0, 1, 4); sinusoidal dilatation/
accumulation of erythrocytes in the spleen (1, 2, 2, 2, 4);
atrophy/stromal proliferation of the prostate (0, 0, 2, 4, 6); and
vacuolization of the zona fasciculata of the adrenals (0, 0, 1, 1, 4).
No gross findings were evident in females. Incidences of
microscopic lesions (as described above) were: increased erythro-
poiesis of bone marrow (1, 0, 2, 2, 5), sideropexia of the bone
marrow (1, 0, 4, 5, 6); sinusoidal dilatation/accumulation of
erythrocytes in the spleen (0, 1, 3, 4, 5); and vacuolization of zona
fasciculata of the adrenals (0, 0, 0, 3, 6).
The authors concluded that the no adverse-effect level in this
study was between 300 and 600 ppm vinclozolin in the diet (Kirsch
et al., 1982).
Long-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (50/sex/dose)
were fed diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin
(purity unspecified) for 130 weeks. Control or test diet and water
were offered ad libitum. The dietary concentrations were equal to
9.4, 27, 83, or 257 mg/kg b.w./day in males and 9.1, 28, 84, or
278 mg/kg b.w./day in females. The results of analyses of the test
material or of test diets were not presented. Animals were examined
twice/day for abnormalities of behaviour or appearance, and were
palpated weekly beginning at week 26 for evidence of rumours. Eyes,
ears, and dentition were examined "regularly". Rats were weighed
weekly. The schedule and manner of measurement of food consumption
were not specified.
Blood was sampled at 0, 4, 8, 13, 26, 52, and 104 weeks from 10
rats/sex/dose for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted,
and in addition, at week 104, glycogen in heart, liver, and skeletal
muscle, total lipids in liver, and ascorbic acid in the adrenals were
measured. Urine was collected on a similar schedule for standard
urinalysis determinations.
Rats that died on test, moribund animals, and all rats surviving
to 130 weeks were subjected to complete necropsies. Rats were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, kidneys, liver, lungs, lymph node,
ovaries, pancreas, parotid, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs or mortality were
apparent. Eye, hearing, and dentition parameters were within normal
limits. The study was terminated at week 130 when combined male and
female mortality in the control group reached 70%. Survival in test
groups was inversely related to dose, as the lowest mortality was
noted in the high-dose group. Body weights were decreased by about 10%
in males and females fed the 1460 ppm diet, and by about 40 and 25%,
respectively, in males and females fed the 4370 ppm diet. These
changes emerged within the first month of treatment and persisted
until study termination. Food consumption was decreased in a similar
manner in the 2 highest-dose groups.
No effects of treatment on haematology or clinical chemistry
parameters were apparent. A decrease of about 20% total bilirubin in
high-dose females is of doubtful toxicological significance.
Occasional increases in the urinary excretion of 17 ketosteroids and
of ascorbic acid were noted in high-dose females in the first year of
treatment; however, these changes were not evident in the final year
of treatment.
At necropsy, organ weights were altered in a manner related to
the body-weight changes. Absolute weights of several organs were
decreased in males and females from the 1460 ppm and 4370 ppm groups,
whereas relative organ weights tended to increase in these groups.
Other necropsy parameters were not affected by treatment. The
incidences of macroscopic and microscopic findings were randomly
distributed among all test groups, and did not appear to be related to
treatment with the test material. The total number of rumours, as well
as the distribution of specific rumour types, was random throughout
the test groups, and not affected by treatment. Therefore, vinclozolin
was determined not to be oncogenic in the rat. The NOEL for chronic
toxicity was 486 ppm, equal to 27 and 28 mg/kg b.w./day in males and
females, respectively, based on decreases in food consumption and
body-weight gain (Leuschner et al., 1977d).
Observations in humans
No information available.
COMMENTS
Data were not submitted to completely characterize the rates of
absorption or elimination of vinclozolin. In studies that were
submitted, all of the administered dose was eliminated, and no
potential for bioaccumulation was apparent. The plasma half-life was
about 20 hours.
Vinclozolin is extensively metabolized, and its major metabolite
(metabolite F) is excreted as a glucuronide or sulfate conjugate.
Specific interaction with enzymes is not known.
A plant metabolite has been identified that is not formed in rats
(metabolite T). Additional testing is necessary to evaluate the
toxicity of this compound.
Vinclozolin has a low order of acute toxicity.
Special studies on carcinogenicity were negative for oncogenic
potential in rats and mice. Similarly, this chemical has no known
specific potential for mutagenicity, teratogenicity, or reproductive
toxicity.
A sub-chronic feeding study in rats suggested possible anaemia
after treatment with diets containing 1000 or 2000 ppm vinclozolin.
However, this finding was not reproduced in a 2-year feeding study in
rats. Chronic effects noted in the long-term rat study were restricted
to decreases in food consumption and body-weight gain of rats fed
1460 ppm and higher (equal to 83 mg/kg b.w./day), with a NOEL for this
effect of 486 ppm (equal to 27 and 28 mg/kg b.w./day in males and
females, respectively).
A feeding study in dogs demonstrated evidence of haemolytic
anaemia in animals fed 2000 ppm vinclozolin for 6 months (equal to 135
and 141 mg/kg b.w./day in males and females, respectively). Other
relevant findings included significant increases in relative and
absolute adrenal weights in males and females at doses of 300 ppm and
higher (equal to 20 and 21 mg/kg b.w./day in males and females,
respectively). A clear dose-related trend for vacuolization of the
zona fasciculata of the adrenals was noted in males and females at
doses of 600 and 2000 ppm. Thus, the changes in adrenal weights
observed at the lower doses can be related to pathology at higher
doses. Other dose-related histopathological findings noted in the
300 ppm groups included accumulation of erythrocytes in the spleen,
atrophy of the prostate, and sideropexia of the bone marrow
(accumulation of haemosiderin within reticuloendothelial elements of
the bone marrow). On the basis of these findings, the NOEL in this
study was 100 ppm, equal to 7.0 mg/kg b.w./day in males and 7.4 mg/kg
b.w./day in females.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECTS
Averages for males and females:
Mice: 486 ppm, equal to 87 /kg b.w./day.
Rats: 486 ppm, equal to 27 /kg b.w./day.
Dogs: 100 ppm, equal to 7.2 /kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DALLY INTAKE FOR MAN
0 - 0.04 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE, TO
BE SUBMITTED TO WHO BY 1987:
Additional studies to evaluate the toxicity of the plant
metabolite, metabolite T.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND:
1. Data to demonstrate the rate of absorption and elimination
after a single oral dose of vinclozolin.
2. Data on the potential effects to humans of exposure to
vinclozolin.
REFERENCES
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B., &
1976 Franklin, E.R. The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral adminstration to
rats. Not submitted for review.
Cifone, M.A. & Myhr, B.C. Report on the evaluation of Vinclozolin in
1984 the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 814/072 from Litton Bionetics, Inc.,
Kensington, MD, USA. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R., & Offer, J.M.
1981 Effect of vinclozolin on pregnancy of the the New Zealand
white rabbit. Unpublished report No. BSF/3810/381/81357 from
Huntingdon Research Centre, Huntingdon, England. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P., Engelhardt, G., & Ruff, M. Cytogenetic investigations
1982 in Chinese hamsters after a single oral administration of
Reg. No. 83 258 - sister chromatid exchange. Unpublished
report No. 82/085, from BASF Gewerbehygiene und Toxikologie,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P. & Engelhardt, G. Report on the study of Vinclozolin in
1983 the Ames test. Unpublished report No. 85/228, from BASF
Gewerbehygiene und Toxikologie, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Gelbke, H.-P. & Jäckh, R. Report on a point mutation test carried out
1985 on CHO cells (HGPRT locus) with the test substance
vinclozolin. Unpublished report No. 85/352 from BASF Dept.
Toxicology, Crop Protection Division. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) to
1977a the eye of white rabbits. Unpublished report No. 77/020,
from BASF Gewerbehygiene und Toxikologie, Ludwigshafen/
Rhein, FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) on
1977b the intact and scarified dorsal skin of white rabbits.
Unpublished report No. 77/022, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. Report on the acute oral toxicity trial of 3-(3,5-di-
1973 chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report No. XXII/337 from
Agricultural Research Station, BASF, Limburgerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. Report on the testing of 3-(3,5-dichlorophenyl)-
1974 5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione in a three month
feeding experiment on rats. Unpublished report No. 74/001
from Medizinisch- Biologische Forschungslaboratorium, BASF.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the mutagenic effect of 3-(3,5-di-
1975a chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in mice. Unpublished report No. 75/003 from Medizinisch-
Biologische Forschungslaboratorium, BASF. Submitted to WHO
by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the prenatal toxicity of
1975b 3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-
dione in mice. Unpublished report No. 75/004 from
Medizinisch-Biologische Forschungslaboratorium, BASF.
Ssubmitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hoorn, A.J.W. Report on the mutagenicity evaluation of Vinclozolin in
1983 the rec assay with Bacillus subtilis. Unpublished report
No. 83/236 from Litton Bionetics, 3905 Pe Veenendaal,
Netherlands. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Kirsch, Deckhardt, Mirea, Hellwig, Hildebrand, & Ruff. Report on the
1982 study of the toxicity of Reg. No. 83 258 (vinclozolin) in
beagle dogs after 6-month administration in the diet.
Unpublished report No. 82/177, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., Dontenwill, W., & Rogulja,
1977a P.V. Oral toxicity of an oxazolidine derivative, batch
813258 - called for short "OXA" - in NMRI mice (with special
attention to carcinogenic properties). Unpublished report
No. 77/017 from Laboratorium fur Pharmakologie und
Toxikologie, Hamburg, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Hubscher, F., Neumann, W., Neumann, B., Rogulja, P.V. &
1977b Dontenwill, W. Chronic oral toxicity of an oxazolidine
derivative, batch 813258 - called for short "OXA" - in a
reproductive study covering three generations in
Sprague-Dawley rats. Unpublished report No. 77/019 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg,
FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., & Dontenwill, W.
1977c 3-Weeks toxicity of an oxazolidine derivative, batch 813258
- called for short "OXA" - in NZW rabbits by local
application. Unpublished report No. 77/015 from Laboratorium
fur Pharmakologie und Toxikologie, Hamburg, FRG. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., & Dontenwill, W. Chronic
1977d oral toxicity of an oxazolidine derivative, batch 813258 -
called for short "OXA" - in the Sprague-Dawley rat.
Unpublished report No. 77/016 from Laboratorium fur
Pharmakologie und Toxikologie, hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F. Report on the acute toxicity of the preparation Reg.
1979 No. 813258 when administered to rats by inhalation.
Unpublished report No. 79/029 from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Otto, S., Beutel, P., Elzner, J., & Ohnsorge, U. Metabolism of
1977 14C-vinclozolin in rats. Unpublished report No. 1474 from
Agricultural Research Station, BASF, Limbergerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978a with BAS 352 F in mice. Unpublished report No. 78/031 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978b with BAS 352 F in rats. Unpublished report No. 78/030 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Takehara, K., Kudoh, S., Maruyama, Y., Hayashi, K., Itabashi, M., &
1978 Tajima, M. Three-month oral toxicity study of BAS-352-F in
rats. Unpublished report No. 78/029 from Nippon Institute
for Biological Science, Tokyo, Japan. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Witterland, W.F. & Hoorn, A.J.W. Report on the mutagenicity evaluation
1984 of vinclozolin in the mouse lymphoma forward mutation assay.
Unpublished report No. 84/267 from Litton Bionetics, 3905 Pe
Veenendaal, Netherlands. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Mice that died on test, moribund animals, and all mice surviving
to 112 weeks were subjected to complete mecropsies. Mice were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs were apparent. Survival
was somewhat lower in high-dose males than in controls (76% mortality
vs. 48% in the control group); however, mortality in all female groups
was about 70%, with no effects of treatment. Mean body weights were
reduced in males in the 2 highest dose groups; these decreases emerged
in the second year of treatment and persisted until termination. Food
intake did not appear to be affected by treatment. No effects of
treatment oil haematology, clinical chemistry, or urinalysis
parameters were noted.
At necropsy, significant increases of 150 - 250% in the absolute
and relative weights of the liver were noted in females from the 1460
and 4370 ppm groups. An increase in liver weight of about 40% and an
increase in testes weight of about 50% were noted in high-dose males.
Absolute weights of other organs were altered in males; however, these
changes probably were related to decreases in body weight.
After microscopic examination, no toxicologically significant
differences in the incidences of neoplastic or non-neoplastic lesions
were noted. Vinclozolin was therefore negative for oncogenic potential
in mice (Leuschner et al., 1977a).
Special studies on mutagenicity
Vinclozolin was negative in a number of acceptable mutagenicity
studies, which included assessments of gene mutations (bacteria and
mammalian cells), direct DNA damage (bacteria and mammalian cells),
and clastogenic effects (in vivo mammalian cells).
Summary results are presnted in Table 1.
Special study on reproduction
Rats
Groups of male and female Sprague-Dawley rats (20/sex/dose) were
fed diets containing 0, 162, 486, or 1485 ppm vinclozolin (purity
unspecified) over 3 generations. Control or test diet and water were
offered ad libitum. The dietary concentrations of vinclozolin were
equal to 12, 37, or 113 mg/kg b.w./day in males and 17, 51, or
152 mg/kg b.w./day in females. The results of analyses of the test
material or of the test diets were not presented.
Table 1: Results of mutagenicity studies on vinclozolin
Test system Test object Concentration of Purity Results Reference
vinclozolin
Ames test1 S. typhimurium 0, 100, 500 98.1% Negative2 Gelbke & Engelhardt, 1983
TA98, TA100, TA1535, 2500, 5000,
10,000, TA1537, 7500, and
and TA1538. µg/plate
CHO/HGPRT gene Chinese hamster 0,0.32,1.0, > 99.5% Negative3 Gelbke & Jäckh, 1985
mutation ovary cells, 3.2, or 10.0
assay1 CHO-K1 mg/ml
Unscheduled Primary hepatocytes 0, 5, 10, > 99.5% Negative4 Cifone & Myhr, 1984
DNA from male Fischer 25, 50, 100,
synthesis 344 rats 250, 500, &
1000 µg/ml
Mouse lymphoma L5178Y mouse Nonactivated: Technical Negative3 Witterland & Hoorn, 1984
forward lymphoma 0 - 1000 µg/ml
mutation cells (TK+/-) (8 dose levels)
assay1 Activated:
0 - 600 µg/ml
(13 dose levels)
DNA repair B. subtilis 0, 1, 10, Technical Negative5 Hoorn, 1983
assay1 H17 and M45 100, 500,
(rec +/-) 1000, 2500,
5000 & 10,000
µg/plate
Table 1: (cont'd).
Test system Test object Concentration of Purity Results Reference
vinclozolin
In vivo sister Chinese hamster 0, 3830, & 98.1% Negative6 Gelbke, et al., 1982
chromatid bone marrow 5620 mg/kg
exchange assay
Mouse dominant NMRI mice 0 & 2000 Technical Negative7 Hofmann & Peh, 1975a
lethal assay mg/kg
1 With and without activation.
2 The positive controls gave the expected positive results.
3 The positive controls, EMS and MCA, gave the expected positive results.
4 The positive control, 0.05 µg/ml 2-AAF, gave the expected positive result.
5 The positive controls, MMS and sterigmatocystin, gave the expected positive results.
6 The positive control, cyclophosphamide, gave the expected positive response.
7 This study is not acceptable. A positive control was not included; therefore, the sensitivity of the test system was not
established.
Two litters were obtained from each generation. Rats from the
parental generation (F0) were bred after 8 and 16 weeks of treatment
(1:1 male to female) to form the F1a and F1b litters, respectively.
Twenty rats/sex/dose were selected from the F1b litters to become
parents of the F2a and F2b litters. This process was repeated
through the F3 generation. Twenty rats of the F3b generation were
maintained on test diets for 9 weeks, at which time they were
sacrificed and histopathological evaluations were conducted on 10
rats/sex/dose.
Animals were examined daily for abnormalities of behaviour or
appearance, and food consumption was measured daily. Rats were weighed
weekly. Fertility, litter size, pup growth, and pup survival for each
litter were determined. At weaning, selected pups were evaluated for
consciousness, emotional behaviour, activity and reactivity,
coordination, and reflexes. Parental rats of each generation were
sacrificed after completion of the second litter and subjected to
gross necropsies. Pups not selected for breeding of the next
generation were sacrificed at weaning with the exception of 10 F3b
pups/sex/dose, which were maintained for 9 weeks on test diets and
then subjected to complete histopathological evaluation.
No effects of treatment on clinical signs, food consumption, or
body weight of parental rats were noted. Similarly, no alterations in
fertility, gestation length, litter size, sex ratio of pups, fetal
birth weight, weight gain of pups, or survival of pups during
lactation were noted in any of the litter intervals. No treatment-
related malformations or developmental defects were noted. No
treatment-related pathological findings were noted in either parental
rats or pups at necropsy. The NOEL for reproductive effects was
determined to be greater than the highest dose tested (Leuschner
et al., 1977b).
Special studies on teratology
Mice
Groups of female NMRI [SPF] mice (24-30/dose) with vaginal plugs
were fed diets containing technical vinclozolin (purity unspecified)
from days 0 - 18 of presumed gestation. Two studies were conducted, 1
with test diets containing 0 and 60,000 ppm vinclozolin, and a second
study with test diets containing 0, 600, and 6000 ppm vinclozolin.
Identical protocols were followed in both studies. Mice were examined
daily for clinical signs of toxicity, and food consumption and body
weights were determined periodically. On day 18 of gestation, the mice
were sacrificed and the number of implantation and resorption sites
and the number of live and dead fetuses were determined. Body weights,
lengths, and the sex of live fetuses were determined, and placentas
were weighed. Fetuses were examined for external abnormalities, and
one-third of the fetuses were examined for visceral abnormalities, and
the remaining two-thirds were examined for skeletal defects.
The appearance and behaviour of mice from the low-dose (600 ppm)
and mid-dose (6000 ppm) groups were not affected by treatment. In
contrast, all mice from the high-dose (60,000 ppm) group died during
the treatment interval. Since these mice also refused food and lost
considerable body weight, it was not clear whether death was the
result of toxicity or of starvation due to food avoidance. Mice fed
600 ppm vinclozolin had a mean body-weight gain during gestation that
was similar to that of mice in the control groups, whereas animals fed
6000 ppm ate about 25% less food than control or low-dose mice, and
gained significantly less body weight. Mice offered the 60,000 ppm
diets refused food, and lost body weight until death.
At necropsy, it was found that none of the mice from the 6000
or 60,000 ppm dose groups had any implantations. The number of
implantations and resorptions, fetal weights and lengths, and
placental weights were similar in control and low-dose mice. Visceral
and skeletal examinations of control and low-dose fetuses revealed
no treatment-related abnormalities. The NOEL for maternal and
developmental toxicity in mice in this study therefore was 600 ppm
(equal to 110 mg/kg b.w./day) (Hofmann & Peh, 1975b).
Rabbits
Groups of female New Zealand white rabbits (15/dose) were
administered doses of 0, 20, 80, or 300 mg/kg b.w./day of technical
vinclozolin (98.1% purity) by gavage from days 6 - 18 of presumed
gestation. Doses were based on a preliminary range-finding assay which
demonstrated that a dose of 900 mg/kg b.w./day was not well tolerated,
while a dose of 300 mg/kg b.w./day produced moderate signs of
toxicity. Rabbits were observed daily for clinical signs of toxicity,
and were weighed periodically during gestation. On day 29 of
gestation, the rabbits were sacrificed by cervical dislocation, and
uteri and the ovaries were examined to determine the number of corpora
lutea and resorptions, live and dead fetuses, gravid uterine weights,
and fetal body weights and lengths. Live fetuses were examined for
external abnormalities, then sacrificed by injection of pheno-
barbitone. Fetuses were weighed, then dissected and examined for
visceral abnormalities. Fetuses were then processed and stained for
skeletal examinations.
No signs of toxicity were observed in does during gestation (the
findings observed in the range-finding study were not reproduced), and
the 4 recorded deaths were considered unrelated to treatment. No
effects of treatment on maternal body weights were apparent. At
necropsy, no effects of treatment on gravid uterine weight or on
litter size or frequency were noted. An approximately 3-fold increase
in the number of resorptions was noted in the high-dose group
(compared to the control group); however, this increase was not
statistically significant. Mean fetal weight was decreased in the
high-dose group by about 6%; this change was not statistically
significant. No effects of treatment on the incidences of visceral or
skeletal abnormalities were apparent. The authors concluded that the
highest dose tested of 300 mg/kg b.w./day had no statistically-
significant effects on litter parameters, nor on maternal health
(Cozens et al., 1981).
Acute toxicity
Vinclozolin possesses a low order of acute toxicity. The results
of acute toxicity studies are summarized in Table 1.
Table 2. Acute toxicity of vinclozolin1
LD50 LD50
Species Route Sex (mg/kg b.w.) (mg/l) Reference
Mouse oral M > 15,000 - Shirasu et al.,
F > 15,000 1978a
s.c. M > 15,000 - Shirasu et al.,
F > 15,000 1978a
i.p. M 1570 - Shirasu et al.,
F 1640 1978a
Rats oral M > 15,000 - Shirasu et al.,
F > 15,000 1978b
i.p. M 8300 - Shirasu et al.,
F 4220 1978a
dermal M > 5000 - Shirasu et al.,
F > 5000 1978b
inhalation M - > 29.1 Leuschner, 1979
(4 hr. exp.) F > 29.1
Guinea-pig oral M/F 8000 - Hofmann, 1973
1 Technical vinclozolin was tested in these studies.
Technical vinclozolin was shown to cause mild reversible
irritation to the conjunctivae of the eye (Hildebrand, 1977a) and skin
(Hildebrand, 1977b).
Short-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (16
rats/sex/dose) were fed diets containing 0, 100, 300, 1000, or
2000 ppm technical vinclozolin for 3 months. Six rats from each group
were further maintained on control diets for a post-observation period
of 6 weeks at the end of the study. Rats were examined daily for
mortality, abnormalities of appearance or behaviour, and food
consumption. Body weights were determined weekly. Rats were palpated
at weekly weighings, and the eyes were examined. Blood was sampled for
haematology and clinical chemistry examinations prior to initiation,
after 6 and 12 weeks of treatment, at termination, and at the end of
the post-observation period. Urine was collected on a similar
schedule. At termination, rats were sacrificed by CO2 asphyxiation
and decapitated. Animals were dissected and examined for gross
pathological changes. Absolute and relative weights of major organs
were determined, and a complete set of tissues from each animal was
saved for future histopathological examinations. The results of
microscopic examinations of tissues were not reported in this study,
with the exception of eyes, which were examined in serial sections.
A single death was noted on day 42 in the female high-dose
treatment group; all other rats survived to scheduled termination. No
disturbances of appearance or behaviour were noted, and eyes appeared
normal at all examinations. Body weights of rats in all treatment
groups were comparable to those of controls throughout the study
period. Food consumption was not affected in males, although
occasional statistically-significant increases were noted in the
female treatment groups. Changes in haematology consistent with
decreased red cell mass (decreased numbers of erythrocytes, heama-
tocrits, and haemoglobin, with increased MCH and MCHC) were noted
at the 6-week sampling time in males and females fed diets of 300 ppm
and higher; however, these changes were apparent at termination only
in the female 1000 and 2000 ppm groups. Clinical chemistry parameters
were not altered in a toxicologically-significant manner.
At necropsy, no effects of treatment on the gross appearance of
tissues were apparent. Statistically-significant dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were noted in males and females from the 1000 and 2000 ppm
groups. Increased relative weights of kidneys were noted in 2000 ppm
males and females, and increased relative spleen weight was noted in
2000 ppm females. These organ-weight effects were apparently
reversible, as they were not observed in treated rats that were
continued on control diets for an additional 6 weeks. Microscopic
examinations of eyes did not reveal any treatment-related
abnormalities. The results of microscopic examinations of the
remaining tissues were not included in the study report (Hofmann,
1974).
Another 3-month feeding study in rats has been performed, but it
was not reviewed, because the study report was incomplete. In the
study narrative the authors concluded that 300 ppm (equal to
21.3 mg/kg b.w./day in males and 24.1 mg/kg b.w./day in females) was
the NOEL based on decreased body-weight gain and altered haematology
and clinical chemistry parameters at 1500 ppm and 7500 ppm, and on
altered organ weights and histopathological findings in the liver,
kidneys, ovaries, and pancreas at 7500 ppm (Takehara et al., 1978).
Rabbits
Groups of New Zealand white rabbits (6/sex/dose, 3 of each group
with intact skin, 3 with abraded skin) were treated by dermal
application for 21 consecutive days (8 hours/day) with doses of 111,
333, or 1000 mg/kg b.w. vinclozolin. All animals survived the
treatment period without effects on appearance, behaviour, body
weight, food or water intake, haematology, clinical chemistry, or
necropsy parameters. The highest dose was considered to be the NOEL in
this study (Leuschner et al., 1977c).
Dogs
Groups of purebred beagle dogs (6/sex/dose) were fed diets
containing 0, 100, 300, 600, or 2000 ppm technical vinclozolin
(98.1% purity) for 6 months. Dogs were offered a total of 700 grams of
food for 3 hours/day, and consumption was measured daily. Daily
dosages were equal to 0, 7.0, 20, 41, or 135 mg/kg b.w./day in males
and 0, 7.4, 21, 41, or 141 mg/kg b.w./day in females. Dogs were
examined daily for clinical signs of toxicity, and body weights were
determined weekly. Eyes were examined at 3-month intervals for changes
in refracting media or the fundus. Blood was sampled at approximately
monthly intervals for measurement of haematology and clinical
chemistry parameters. Urine was collected approximately every 2 months
for urinalysis determinations.
At termination, dogs were anaesthetized and sacrificed by
exsanguination. Standard necropsy techniques were followed. The
following tissues were examined (those indicated by an asterisk * were
also weighed) for gross and microscopic changes: abnormalities,
adrenals*, aorta, bone (sternum & marrow), brain*, colon, duodenum,
esophagus, eye (with optic nerve), gall, bladder, heart*, ileum,
jejunum, kidneys*, liver*, lungs (with mainstem bronchi), lymph nodes
(axillary & mesenteric), mammary glands, ovaries*, pancreas, parotid,
pituitary*, prostate, sciatic nerve, skeletal muscle, skin, spleen*,
spinal cord (thoracic, lumbar), stomach, testes* (with epididymides),
thymus, thyroid* (with parathyroid), trachea, urinary bladder, and
uterus (corpus, cervix, and cornu).
No effects of treatment on clinical signs, survival, food
consumption or body-weight gain were apparent. Ophthalmoscopic
examinations did not reveal any treatment-related abnormalities.
Evidence of haemolytic anaemia was noted in high-dose males and
females in the form of increased MCHC, increased numbers of
reticulocytes and red cells containing Howell-Jolly bodies, and
increased serum bilirubin, lactate dehydrogenase, and urea levels.
However, decreases in erythrocyte counts or haematocrits were not
observed, apparently due to compensatory phenomena. Increased numbers
of platelets noted in these dogs also suggested a compensatory
response to anaemia. These effects were more profound in males than in
females. Other clinical chemistry parameters were not affected.
Statistically-significant decreases in SGPT, which were observed in
females, are of questionable toxicological significance. Urinalysis
parameters were not affected by treatment.
At necropsy, statistically-significant dose-related increases in
absolute and relative weights of adrenals were noted in males and
females fed 300 ppm or more vinclozolin. Other changes in organ
weights included decreases in absolute weights of kidneys in males at
300 ppm and above, decreases in absolute brain weights, and increases
in relative spleen weights in high-dose males. Other changes noted in
females included decreased relative pituitary weights at 300 ppm and
higher.
Potential treatment-related gross findings were restricted to
reduced size of the prostate in high-dose males. Upon microscopic
examination, the following potential treatment-related findings were
observed in males (numbers and incidences in control, 100, 300, 600,
and 2000 ppm groups, respectively, of 6 dogs examined): increased
erythropoiesis of bone marrow (0, 0, 0, 1, 4); sinusoidal dilatation/
accumulation of erythrocytes in the spleen (1, 2, 2, 2, 4);
atrophy/stromal proliferation of the prostate (0, 0, 2, 4, 6); and
vacuolization of the zona fasciculata of the adrenals (0, 0, 1, 1, 4).
No gross findings were evident in females. Incidences of
microscopic lesions (as described above) were: increased erythro-
poiesis of bone marrow (1, 0, 2, 2, 5), sideropexia of the bone
marrow (1, 0, 4, 5, 6); sinusoidal dilatation/accumulation of
erythrocytes in the spleen (0, 1, 3, 4, 5); and vacuolization of zona
fasciculata of the adrenals (0, 0, 0, 3, 6).
The authors concluded that the no adverse-effect level in this
study was between 300 and 600 ppm vinclozolin in the diet (Kirsch
et al., 1982).
Long-term studies
Rats
Groups of male and female Sprague-Dawley [SPF] rats (50/sex/dose)
were fed diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin
(purity unspecified) for 130 weeks. Control or test diet and water
were offered ad libitum. The dietary concentrations were equal to
9.4, 27, 83, or 257 mg/kg b.w./day in males and 9.1, 28, 84, or
278 mg/kg b.w./day in females. The results of analyses of the test
material or of test diets were not presented. Animals were examined
twice/day for abnormalities of behaviour or appearance, and were
palpated weekly beginning at week 26 for evidence of rumours. Eyes,
ears, and dentition were examined "regularly". Rats were weighed
weekly. The schedule and manner of measurement of food consumption
were not specified.
Blood was sampled at 0, 4, 8, 13, 26, 52, and 104 weeks from 10
rats/sex/dose for assessment of haematology and clinical chemistry
parameters. A standard battery of tests/examinations was conducted,
and in addition, at week 104, glycogen in heart, liver, and skeletal
muscle, total lipids in liver, and ascorbic acid in the adrenals were
measured. Urine was collected on a similar schedule for standard
urinalysis determinations.
Rats that died on test, moribund animals, and all rats surviving
to 130 weeks were subjected to complete necropsies. Rats were
sacrificed by decapitation. Animals were dissected, and organs were
weighed. The following tissues were examined for microscopic changes:
adrenals, aorta, bone and marrow, brain, colon, duodenum, esophagus,
eye, heart, ileum, jejunum, kidneys, liver, lungs, lymph node,
ovaries, pancreas, parotid, peripheral nerve, pituitary, prostate,
rectum, skeletal muscle, skin, spleen, spinal cord, stomach, testes,
thymus, thyroid, trachea, urinary bladder, uterus, and any tumours.
No effects of treatment on clinical signs or mortality were
apparent. Eye, hearing, and dentition parameters were within normal
limits. The study was terminated at week 130 when combined male and
female mortality in the control group reached 70%. Survival in test
groups was inversely related to dose, as the lowest mortality was
noted in the high-dose group. Body weights were decreased by about 10%
in males and females fed the 1460 ppm diet, and by about 40 and 25%,
respectively, in males and females fed the 4370 ppm diet. These
changes emerged within the first month of treatment and persisted
until study termination. Food consumption was decreased in a similar
manner in the 2 highest-dose groups.
No effects of treatment on haematology or clinical chemistry
parameters were apparent. A decrease of about 20% total bilirubin in
high-dose females is of doubtful toxicological significance.
Occasional increases in the urinary excretion of 17 ketosteroids and
of ascorbic acid were noted in high-dose females in the first year of
treatment; however, these changes were not evident in the final year
of treatment.
At necropsy, organ weights were altered in a manner related to
the body-weight changes. Absolute weights of several organs were
decreased in males and females from the 1460 ppm and 4370 ppm groups,
whereas relative organ weights tended to increase in these groups.
Other necropsy parameters were not affected by treatment. The
incidences of macroscopic and microscopic findings were randomly
distributed among all test groups, and did not appear to be related to
treatment with the test material. The total number of rumours, as well
as the distribution of specific rumour types, was random throughout
the test groups, and not affected by treatment. Therefore, vinclozolin
was determined not to be oncogenic in the rat. The NOEL for chronic
toxicity was 486 ppm, equal to 27 and 28 mg/kg b.w./day in males and
females, respectively, based on decreases in food consumption and
body-weight gain (Leuschner et al., 1977d).
Observations in humans
No information available.
COMMENTS
Data were not submitted to completely characterize the rates of
absorption or elimination of vinclozolin. In studies that were
submitted, all of the administered dose was eliminated, and no
potential for bioaccumulation was apparent. The plasma half-life was
about 20 hours.
Vinclozolin is extensively metabolized, and its major metabolite
(metabolite F) is excreted as a glucuronide or sulfate conjugate.
Specific interaction with enzymes is not known.
A plant metabolite has been identified that is not formed in rats
(metabolite T). Additional testing is necessary to evaluate the
toxicity of this compound.
Vinclozolin has a low order of acute toxicity.
Special studies on carcinogenicity were negative for oncogenic
potential in rats and mice. Similarly, this chemical has no known
specific potential for mutagenicity, teratogenicity, or reproductive
toxicity.
A sub-chronic feeding study in rats suggested possible anaemia
after treatment with diets containing 1000 or 2000 ppm vinclozolin.
However, this finding was not reproduced in a 2-year feeding study in
rats. Chronic effects noted in the long-term rat study were restricted
to decreases in food consumption and body-weight gain of rats fed
1460 ppm and higher (equal to 83 mg/kg b.w./day), with a NOEL for this
effect of 486 ppm (equal to 27 and 28 mg/kg b.w./day in males and
females, respectively).
A feeding study in dogs demonstrated evidence of haemolytic
anaemia in animals fed 2000 ppm vinclozolin for 6 months (equal to 135
and 141 mg/kg b.w./day in males and females, respectively). Other
relevant findings included significant increases in relative and
absolute adrenal weights in males and females at doses of 300 ppm and
higher (equal to 20 and 21 mg/kg b.w./day in males and females,
respectively). A clear dose-related trend for vacuolization of the
zona fasciculata of the adrenals was noted in males and females at
doses of 600 and 2000 ppm. Thus, the changes in adrenal weights
observed at the lower doses can be related to pathology at higher
doses. Other dose-related histopathological findings noted in the
300 ppm groups included accumulation of erythrocytes in the spleen,
atrophy of the prostate, and sideropexia of the bone marrow
(accumulation of haemosiderin within reticuloendothelial elements of
the bone marrow). On the basis of these findings, the NOEL in this
study was 100 ppm, equal to 7.0 mg/kg b.w./day in males and 7.4 mg/kg
b.w./day in females.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECTS
Averages for males and females:
Mice: 486 ppm, equal to 87 /kg b.w./day.
Rats: 486 ppm, equal to 27 /kg b.w./day.
Dogs: 100 ppm, equal to 7.2 /kg b.w./day.
ESTIMATE OF TEMPORARY ACCEPTABLE DALLY INTAKE FOR MAN
0 - 0.04 mg/kg b.w.
STUDIES WITHOUT WHICH THE DETERMINATION OF AN ADI IS IMPRACTICABLE, TO
BE SUBMITTED TO WHO BY 1987:
Additional studies to evaluate the toxicity of the plant
metabolite, metabolite T.
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND:
1. Data to demonstrate the rate of absorption and elimination
after a single oral dose of vinclozolin.
2. Data on the potential effects to humans of exposure to
vinclozolin.
REFERENCES
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B., &
1976 Franklin, E.R. The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral adminstration to
rats. Not submitted for review.
Cifone, M.A. & Myhr, B.C. Report on the evaluation of Vinclozolin in
1984 the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 814/072 from Litton Bionetics, Inc.,
Kensington, MD, USA. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R., & Offer, J.M.
1981 Effect of vinclozolin on pregnancy of the the New Zealand
white rabbit. Unpublished report No. BSF/3810/381/81357 from
Huntingdon Research Centre, Huntingdon, England. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P., Engelhardt, G., & Ruff, M. Cytogenetic investigations
1982 in Chinese hamsters after a single oral administration of
Reg. No. 83 258 - sister chromatid exchange. Unpublished
report No. 82/085, from BASF Gewerbehygiene und Toxikologie,
Ludwigshafen/Rhein, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Gelbke, H.-P. & Engelhardt, G. Report on the study of Vinclozolin in
1983 the Ames test. Unpublished report No. 85/228, from BASF
Gewerbehygiene und Toxikologie, Ludwigshafen/Rhein, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Gelbke, H.-P. & Jäckh, R. Report on a point mutation test carried out
1985 on CHO cells (HGPRT locus) with the test substance
vinclozolin. Unpublished report No. 85/352 from BASF Dept.
Toxicology, Crop Protection Division. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) to
1977a the eye of white rabbits. Unpublished report No. 77/020,
from BASF Gewerbehygiene und Toxikologie, Ludwigshafen/
Rhein, FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hildebrand. Primary skin irritation of Reg. No. 83258 (vinclozolin) on
1977b the intact and scarified dorsal skin of white rabbits.
Unpublished report No. 77/022, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. Report on the acute oral toxicity trial of 3-(3,5-di-
1973 chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report No. XXII/337 from
Agricultural Research Station, BASF, Limburgerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. Report on the testing of 3-(3,5-dichlorophenyl)-
1974 5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione in a three month
feeding experiment on rats. Unpublished report No. 74/001
from Medizinisch- Biologische Forschungslaboratorium, BASF.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the mutagenic effect of 3-(3,5-di-
1975a chlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in mice. Unpublished report No. 75/003 from Medizinisch-
Biologische Forschungslaboratorium, BASF. Submitted to WHO
by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Hofmann, H.Th. & Peh, J. Study on the prenatal toxicity of
1975b 3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-
dione in mice. Unpublished report No. 75/004 from
Medizinisch-Biologische Forschungslaboratorium, BASF.
Ssubmitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Hoorn, A.J.W. Report on the mutagenicity evaluation of Vinclozolin in
1983 the rec assay with Bacillus subtilis. Unpublished report
No. 83/236 from Litton Bionetics, 3905 Pe Veenendaal,
Netherlands. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Kirsch, Deckhardt, Mirea, Hellwig, Hildebrand, & Ruff. Report on the
1982 study of the toxicity of Reg. No. 83 258 (vinclozolin) in
beagle dogs after 6-month administration in the diet.
Unpublished report No. 82/177, from BASF Gewerbehygiene und
Toxikologie, Ludwigshafen/Rhein, FRG. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., Dontenwill, W., & Rogulja,
1977a P.V. Oral toxicity of an oxazolidine derivative, batch
813258 - called for short "OXA" - in NMRI mice (with special
attention to carcinogenic properties). Unpublished report
No. 77/017 from Laboratorium fur Pharmakologie und
Toxikologie, Hamburg, FRG. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Hubscher, F., Neumann, W., Neumann, B., Rogulja, P.V. &
1977b Dontenwill, W. Chronic oral toxicity of an oxazolidine
derivative, batch 813258 - called for short "OXA" - in a
reproductive study covering three generations in
Sprague-Dawley rats. Unpublished report No. 77/019 from
Laboratorium fur Pharmakologie und Toxikologie, Hamburg,
FRG. Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Schwerdtfeger, W., & Dontenwill, W.
1977c 3-Weeks toxicity of an oxazolidine derivative, batch 813258
- called for short "OXA" - in NZW rabbits by local
application. Unpublished report No. 77/015 from Laboratorium
fur Pharmakologie und Toxikologie, Hamburg, FRG. Submitted
to WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F., Leuschner, A., Hubscher, F., & Dontenwill, W. Chronic
1977d oral toxicity of an oxazolidine derivative, batch 813258 -
called for short "OXA" - in the Sprague-Dawley rat.
Unpublished report No. 77/016 from Laboratorium fur
Pharmakologie und Toxikologie, hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Leuschner, F. Report on the acute toxicity of the preparation Reg.
1979 No. 813258 when administered to rats by inhalation.
Unpublished report No. 79/029 from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, FRG. Submitted to
WHO by BASF Aktiengelsellschaft, Limburgerhof, FRG.
Otto, S., Beutel, P., Elzner, J., & Ohnsorge, U. Metabolism of
1977 14C-vinclozolin in rats. Unpublished report No. 1474 from
Agricultural Research Station, BASF, Limbergerhof, FRG.
Submitted to WHO by BASF Aktiengelsellschaft,
Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978a with BAS 352 F in mice. Unpublished report No. 78/031 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Shirasu, Y., Takahashi, K., & Saito, T. Report of acute toxicity tests
1978b with BAS 352 F in rats. Unpublished report No. 78/030 from
the Toxicology Division, Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
Takehara, K., Kudoh, S., Maruyama, Y., Hayashi, K., Itabashi, M., &
1978 Tajima, M. Three-month oral toxicity study of BAS-352-F in
rats. Unpublished report No. 78/029 from Nippon Institute
for Biological Science, Tokyo, Japan. Submitted to WHO by
BASF Aktiengelsellschaft, Limburgerhof, FRG.
Witterland, W.F. & Hoorn, A.J.W. Report on the mutagenicity evaluation
1984 of vinclozolin in the mouse lymphoma forward mutation assay.
Unpublished report No. 84/267 from Litton Bionetics, 3905 Pe
Veenendaal, Netherlands. Submitted to WHO by BASF
Aktiengelsellschaft, Limburgerhof, FRG.
