
VINCLOZOLIN
First draft prepared by
M. Watson
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries
and Food,
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily retake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Hormonal effects
Receptor binding
Nephrotoxicity
Haemoglobin adduct formation
Review of ophthalmoscopic findings
Observations in humans
Comments
Toxicological
References
Explanation
Vinclozolin was previously evaluated by the Joint Meeting in 1986
and 1988 (Annex I, references 47 and 53). In 1986, although the data
were considered to be incomplete, sufficient information was provided
to estimate a temporary ADI. It was noted that a plant metabolite
(Metabolite T) had been identified that was not found in rats. It was
concluded that vinclozolin had a low order of acute toxicity, that
studies of carcinogenicity demonstrated no potential for oncogenicity,
and that it had no specific mutagenic, teratogenic, or developmental
effects. A temporary ADI of 0-0.04 mg/kg bw was allocated on the basis
of an NOAEL of 7 mg/kg bw per day for histological changes in spleen,
prostate, and bone marrow in a six-month study in dogs and a 200-fold
safety factor. In 1988, the Meeting evaluated limited data on the
acute toxicity and mutagenicity of metabolite T and noted that the
chemical was a transient residue in only two commodities. An ADI of
0-0.07 mg/kg bw was allocated using the same NOAEL as that used in
1986 and a safety factor of 100.
The compound was reviewed at the present Meeting within the CCPR
periodic review programme. This monograph summarizes new data and that
not previously reviewed and relevant data from previous monographs on
this pesticide.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Five male rats (strain unspecified) were given five daily doses
of 40 mg/kg bw [U-14C-phenyl]-vinclozolin by gavage. Excreta were
collected daily and frozen, and 4 h after the last treatment the rats
were sacrificed and tissues were collected and frozen. Urine was
measured directly for radiolabel, whereas samples of faeces, blood,
and tissues were combusted and levels of 14C-carbon dioxide were
determined. Data were expressed as the concentration of vinclozolin in
excreta, blood, and tissues. The highest average concentration was
found in faeces, followed by urine, kidney, liver, fat, muscle, and
blood. The levels in urine and faeces appeared to reach a plateau by
the second day of treatment (Otto et al., 1977)
[U-14C-phenyl]-Vinclozolin was administered orally for seven
days at a dose of 40 mg/kg bw per day. Six days after the last dose, a
mean of 47% of the total administered dose had been eliminated in
urine and 54% in faeces. No radiolabel was detected in carcasses at
this time, and none was detected in expired air. Cannulation of bile
ducts after a single oral dose resulted in excretion of 65% of the
administered radiolabel in the bile and only 19% in urine and 15% in
faeces. Peak plasma levels were detected after about 1 h; the plasma
half-life was 20 h. As treatment continued, the baseline plasma levels
tended to increase. After seven doses, the highest levels of
radiolabel were detected in the liver, kidneys, gastrointestinal
tract, fat, adrenals, and ovaries. By 196 h after the last dose, the
levels in tissues were no different from those in plasma. These
findings were confirmed by whole-body autoradiography (Chasseaud
et al., 1976)
The biokinetics of [U-14C-phenyl]-vinclozolin was studied in
male and female Wistar rats. For studies of excretion balance and
plasma kinetics, five animals of each sex per test group were used;
for studies of extended tissue distribution and accumulation, biliary
excretion, and plasma kinetics after dietary administration of
14C-vinclozolin, three animals per group were used. In a pilot
study, no detectable radiolabel was excreted in expired air. There
were no apparent sex differences in the routes of excretion of
radiolabel. For five days after a single oral dose at a nominal level
of 10 mg/kg bw (with or without a 14-day pretreatment with
non-radiolabelled vinclozolin), the mean urinary excretion of
radiolabel was 52-55% of the dose and the mean faecal excretion was
34-46%; 0.7-1.4% was retained. For five days after a single oral dose
of 14C-vinclozolin at a nominal level of 100 mg/kg bw, males
excreted mean levels of 48% of the dose in urine and 49% in faeces and
females excreted 54% in urine and 40% in faeces; males retained 0.6%
of the dose and females 1.1%. For five days after a single intravenous
dose of 14C-vinclozolin at a nominal level of 1 mg/kg bw, the
overall mean levels (for animals of each sex) excreted were 72% in
urine and 23% in faeces. In rats with cannulated bile ducts, males
excreted a mean of 73% of the radiolabel in bile and females 64% up to
48 h after a single oral dose of 10 mg/kg bw 14C-vinclozolin. After
a single oral dose of 100 mg/kg bw, means of 62% in males and 39% in
females were excreted in bile. These results indicate that pronounced
enterohepatic recirculation of radiolabel occurs in intact rats. After
single oral doses of 14C-vinclozolin at nominal levels of 10, 100,
or 200 mg/kg bw, the time taken to reach peak plasma concentrations of
radiolabel (Cmax) tended to increase with increasing dose. Once peak
levels had been reached, the concentrations declined in an apparently
biphasic manner, with overall mean half-lives of 23 h for male rats
and 36 h for females. The Cmax values and the areas under the plasma
radiolabel concentration-time curves (AUC) were apparently linearly
related to dose at the higher levels, whereas they were propor-
tionately higher at the lowest dose, probably due to a greater extent
of absorption. During ingestion of diet containing 14C-vinclozolin
at 5000 ppm over 24 h (equivalent to about 45 mg/kg bw), the plasma
radiolabel concentrations increased over the initial 12 h, in
accordance with a constant (zero-order) absorption model. After the
animals were withdrawn from treated diet, the concentrations declined,
with a mean half-life of about 40 h. The systemic availability of
radiolabel appeared to be equivalent after administration of
14C-vinclozolin by gavage or in the diet. After oral doses of
14C-vinclozolin, radiolabel was widely distributed in tissues. In
general, the tissue concentrations were higher in female rats than in
males. After a single dose of 10 mg/kg bw, peak tissue concentrations
occurred at 2 h in males and 6 h in females, the highest levels being
found in liver, kidneys, fat, adrenals, and Harderian gland. The
concentrations in all tissues declined in a generally linear manner
with time. Five days after a single dose of 100 mg/kg bw of
14C-vinclozolin, the tissue concentrations of radiolabel were
highest in liver, kidneys, and female fat. With oral administration of
14C-vinclozolin once daily for seven days at 10 mg/kg bw, the
highest concentrations occurred mainly 6 h after the final dose, and
the highest mean concentrations were again present in liver, kidneys,
fat, adrenals, and Harderian gland. The concentrations in all tissues
declined in a linear fashion (Hawkins et al., 1990a).
In order to determine the tissue distribution in female Wistar
rats of 14C-vinclozolin administered orally once daily for seven
days at a dose of 100 mg/kg bw, duplicate animals were studied by
whole-body autoradiography 2, 6, 24, 69, and 168 h after the final
administration. Radiolabel was absorbed from the gastrointestinal
tract and widely distributed. After 2 h, high tissue concentrations
were found in the organs involved in excretion and metabolism
(gastrointestinal tract, bladder, liver, and kidneys) and in fat,
adrenals, and glands in the region of the eye (intra- and exorbidal
and especially the Harderian gland). After 168 h, radiolabel was
detected at low levels only in the nasal mucosa, liver, kidneys, and
intestinal contents. In comparison with the results of whole-body
autoradiography after the single administration in the previous study,
the distribution of radiolabel was comparable, with the possible
exception that more radioactivity was detected in the vicinity of the
eye (Hawkins et al., 1991a).
The absorption, distribution, and excretion of radiolabel were
examined in groups of 24 male Wistar rats after dermal administration
of single doses of 0.002, 0.02, 0.2, or 2 mg/cm2 of [U-14C-
phenyl]-vinclozolin, equivalent to 0.13, 1.3, 13, and 130 mg/kg bw.
The doses were applied for 10 h under a semi-occlusive dressing, and
animals were sacrificed 0.5, 1, 2, 4, 10, and 72 h after the start of
treatment. Absorption decreased as a percentage of increasing dose but
increased with longer duration of exposure. Absorbed radiolabel was
excreted in the urine and faeces, with means of 17, 12, 2, and 0.4% in
urine and 8, 5, 1, and 0.2% in faeces over 72 h at the four doses,
respectively. At sacrifice or 10 h after the start of treatment, the
treated skin was washed with water. Unabsorbed radioactivity amounted
to 53-99% of the dose. Treated skin of animals sacrificed up to 10 h
contained 4-23% of the dose and that of animals sacrificed at 72 h
contained 0.6-4%. At all doses, the liver contained the highest levels
of radiolabel, followed by kidneys, adrenals, plasma, brain, blood,
and testes (Hawkins et al., 1991b).
The percutaneous absorption through rat and human epidermis of
[U-14C-phenyl]-vinclozolin was assessed in vitro in flow-through
diffusion cells. The test substance was applied over 24 h at 2 or
200 µg/cm2. Cumulative absorption after 8 h was 1.2% through human
skin and 20% through rat skin after the high dose and 16% through
human skin and 69% through rat skin after the low dose (Cameron &
Jack, 1991).
(b) Biotransformation
Samples of urine and faeces were analysed to determine the
identities of metabolites. Vinclozolin was metabolized extensively, as
no parent compound was detected in the urine and 8-40% was detected in
the faeces. N-(3,5-Dichlorophenyl)-2-methyl-2,3,4-trihydroxy-
butanoic acid amide was the major metabolite, accounting for 42% of
the urinary radiolabel and 60-90% of that in faeces. It was excreted
as either a glucuronide or sulfate conjugate in urine and in free form
in the faeces. This metabolite was also the major species found in
blood, kidneys, and liver. Other metabolites resulting from further
degradation of this metabolite were formed in insignificant amounts
(Otto et al., 1977).
The metabolism of [U-14C-phenyl]-vinclozolin was investigated
after oral administration of single doses of 10 or 100 mg/kg bw to
intact and bile duct-cannulated animals, administration of 10 mg/kg bw
for seven days, or a 14-day pretreatment followed by a single dose of
10 mg/kg bw. Further groups were given an intravenous injection of
10 mg/kg bw or a single dose of 200 mg/kg bw and a 5000-ppm dietary
concentration for 6 h. Vinclozolin was extensively metabolized after
administration by either route. At least 15 phase-I and phase-II
metabolites of vinclozolin were excreted in urine, and some were also
excreted in bile and faeces. Nine of the more important urinary
metabolites were characterized by mass spectrometry, enzyme
deconjugation, and comparison with reference standards. These
metabolites, together with unchanged vinclozolin, accounted for about
80% of the urinary and faecal radiolabel and for more than 60% of
single oral doses of 10 or 100 mg/kg bw of 14C-vinclozolin. The
structures of these metabolites show that several competing pathways
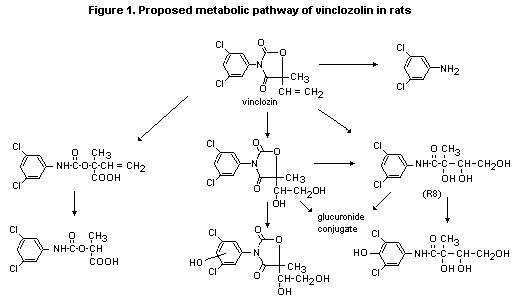
of biotransformation of vinclozolin exist in the rat (Figure 1). The
phase-I pathways include: (1) hydrolytic opening of the oxazolidine
ring system, involving cleavage of the 2-, 3-, or 3,4-nitrogen-carbon
bonds, the former being followed by decarboxylation; (2) addition of
two hydroxyl groups to the vinyl group, presumably via an epoxide
intermediate; and (3) aromatic hydroxylation. The polyhydroxy
compounds resulting from pathways (1) and (2) were extensively
conjugated with glucuronic acid, while phenolic metabolites appeared
to be conjugated with sulfate. The major phase-I metabolite of
vinclozolin in the rat (R8) was the product of cleavage of the
2,3-bond in the oxazolidine ring and dihydroxylation of the vinyl
group. This metabolite was excreted mainly in urine as the glucuronide
conjugate, although the free aglycone was also detected. Owing to
deconjugation by intestinal microflora, the free aglycone was a major
component in faeces (Hawkins et al., 1990b).
A proposed metabolic pathway for vinclozolin is shown in Figure
1.
(c) Effects on enzymes and other biochemical parameters
The acute pharmacological effects of vinclozolin were
investigated in a series of studies in vitro and in vivo designed
to assess effects on the central nervous system, respiratory and
circulatory systems, autonomic nervous system, skeletal muscle
innervation, and blood. Vinclozolin increased the mean sleeping time
induced by hexobarbitone in mice and delayed pentetrazole- and
strychnine-induced convulsions in mice. It had no effect on body
temperature in rats or rabbits, on heart rate, respiratory rate, or
blood pressure in rabbits, or on electrically stimulated muscle
response in rats. Intestinal motility (charcoal propulsion in mice)
was also not affected. Blood coagulation parameters were not affected
in rats, but moderate haemolysis of an erythrocyte suspension was seen
(Block et al., 1987).
 After oral administration of 2000 or 5000 mg/kg bw vinclozolin to
male Sprague-Dawley rats, the effects on the cortical electroence-
phalogram were examined. At both doses, sleeping stages were prolonged
and the number and duration of rapid eye movement phases were slightly
reduced; however, these alterations were not pronounced and suggest
only a moderate sedative effect (Kretzschmar et al., 1987).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of vinclozolin are
summarized in Table 1. The clinical signs of toxicity after treatment
with vinclozolin were generally nonspecific, and no consistent,
treatment-related effects were seen at autopsy.
The LD50 of metabolite T,3,5-dichlorophenylcarbamoyl-2-
propionic acid, was reported to be 2740 mg/kg bw in rats and >
2000 mg/kg bw in mice. Clinical signs of reaction to treatment
included dyspnoea, staggering, piloerection, and poor general state
(Kirsch, 1986a,b).
Table 1. Acute toxicity of vinclozolin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse Oral > 15 000 92.8 Shirasu et al. (1978a)
Mouse Intraperitoneal 1570-1640 92.8 Shirasu et al. (1978a)
Mouse Subcutaneous > 15 000 92.8 Shirasu et al. (1978a)
Rat Oral > 15 000 92.8 Shirasu et al. (1978b)
Rat Intraperitoneal 4220-8300 92.8 Shirasu et al. (1978a)
Rat Dermal > 5000 92.8 Shirasu et al. (1978b)
Rat Inhalation > 29.1 NR Leuschner (1979)
Guinea-pig Oral 8000 90-97 Hofmann (1973a)
Guinea-pig Intraperitoneal 3000 90-97 Hofmann (1973b)
Rabbit Oral > 5620 98.1 Gelbke & Kirsch (1981)
Dog Oral > 10 000 97 Gelbke & Kirsch (1979)
NR, not reported
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing vinclozolin at doses of 0, 100, 1000, 2500, or 5000 ppm for
three months. Food consumption and body weight were determined weekly;
clinical signs were checked daily, with a weekly comprehensive
clinical examination. At the end of the study, clinicochemical and
haematological examinations were performed, and all animals were
assessed grossly and histopathologically. Reduced body-weight gain was
seen in males at the high dose at the end of the study. Clinico-
chemical parameters were affected at doses > 1000 ppm and
haematological parameters only at the highest dose. Decreased
triglyceride and cholesterol levels were seen in animals at 1000 ppm,
and decreased glucose levels in males and albumin levels in females at
the three higher doses. Increased alkaline phosphatase activity was
seen in males and increased globulins in males and females at 2500 and
5000 ppm; alanine aminotransferase activity was increased only in
males at 5000 ppm. Increases were seen in the mean corpuscular
haemoglobin value in females at the two highest doses, the mean
corpuscular volume in animals of each sex, haemoglobin in males, and
reticulocyte counts in females at the highest dose. Absolute and
relative liver weights were found to be increased at the three higher
doses. Centrilobular hypertrophy of hepatocytes was seen in males at
2500 and 5000 ppm; testicular weights were increased at these doses,
and multifocal hyperplasia in Leydig cells was noted at 1000 ppm or
more. Absolute and relative adrenal gland weights were increased in
males at 2500 and 5000 ppm, and lipogenic pigment and lipid vacuoles
in the adrenals were noted in animals of each sex at these doses. In
females at 1000 ppm, increased lipogenic pigment was seen in the
adrenals. Hyperplasia in stromal cells of the ovaries was observed at
the highest dose only. No treatment-related adverse effects were seen
at 100 ppm. The NOAEL was 100 ppm, equivalent to about 20 mg/kg bw per
day (Schilling et al., 1990a).
The B6C3F1 mouse strain has a relatively high frequency of
spontaneous liver lesions, including tumours. In the three-month study
with this strain, clear increases in liver weight and hepatocellular
hypertrophy were observed, indicating that the liver is one of the
target organs of vinclozolin. In order to assess more accurately its
potential proliferative effect on mouse liver, the toxicity of
vinclozolin was investigated in C57B1 mice, which have a low
background incidence of spontaneous liver neoplasia. Groups of 10 male
and 10 female mice were fed diets containing vinclozolin at doses of
0, 100, 1000, or 5000 ppm. Food consumption and body weight were
determined weekly, and clinical signs were checked daily, with a
weekly comprehensive clinical examination. At the end of the study,
clinicochemical and haematological examinations were performed. All
animals were assessed macroscopically and histologically. A reduction
in body-weight gain was seen in males at the high dose. Decreased
triglyceride and cholesterol values were seen in animals at 1000 and
5000 ppm, and glucose was decreased only in males at the high dose.
Increased alanine aminotransferase activity was seen in animals of
each sex, and total protein and alkaline phosphatase were increased in
males at the high dose. Haemoglobin, mean cell volume, and mean
corpuscular haemoglobin were increased in animals of each sex at
5000 ppm and in females at 1000 ppm; haematocrit and leukocyte and
lymphocyte counts were increased in animals of each sex and the
erythrocyte count in males at the highest dose. Increased liver
weights were seen at 1000 and 5000 ppm, and centrilobular hypertrophy
of hepatocytes was seen at 5000 ppm. Increased adrenal weights were
seen males at this dose; an increase in lipogenic pigment was noted
histologically in animals of each sex at 1000 ppm and 5000 ppm and
lipid vacuoles were seen at 5000 ppm. Hyperplasia and hypertrophy of
the stromal cells of the ovaries were seen at the middle and high
doses, and focal hyperplasia of the Leydig cells of the testis was
observed at the high dose. There were no compound-related findings at
100 ppm. The NOAEL was 100 ppm, equivalent to 25 mg/kg bw per day
(Schilling et al., 1990b).
Rats
Groups of 16 male and 16 female Sprague-Dawley rats were fed
diets containing 0,100, 300, 1000, or 2000 ppm technical-grade
vinclozolin for three months, and six rats from each group were
further maintained on control diets for six weeks after the end of the
study. Rats were examined daily for mortality, abnormal appearance or
behaviour, and food consumption. Body weights were determined weekly,
when rats were palpated and the eyes examined. Blood and urine were
sampled before treatment, after six and 12 weeks of treatment, at
termination, and at the end of the observation period. At termination,
rats were sacrificed, dissected, and examined for gross pathological
changes. The absolute and relative weights of major organs were
determined, and a complete set of tissues from each animal was saved
for future histopathological examination. The results of microscopic
examinations of tissues were not reported, except for the eyes, which
were examined in serial sections. A single death occurred, in a female
at the high dose on day 42; all other rats survived to scheduled
termination. No abnormalities of appearance or behaviour were noted,
and the eyes were normal at all examinations. The body weights of
treated rats were comparable to those of controls throughout the
study. Occasional statistically significant increases in food
consumption were seen in treated females, but that of males was not
affected. Haematological changes consistent with decreased erythrocyte
mass-decreased erythrocyte count, haematocrit, and haemoglobin and
increased mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentration-were seen at the six-week sampling time in
males and females fed doses > 300 ppm; however, these changes were
seen at termination only in females at 1000 and 2000 ppm. Clinical
chemical parameters were not altered in a toxicologically significant
manner. At necropsy, no effects of treatment on the gross appearance
of tissues were apparent. Statistically significant, dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were seen in males and females at 1000 and 2000 ppm; at
2000 ppm, increased relative weights of kidneys were seen in males and
females and increased relative spleen weights in females. These
effects were apparently reversible, as they were not observed in
treated rats that received control diets for an additional six weeks.
Microscopic examination of the eyes revealed no treatment-related
abnormalities (Hofmann, 1974).
Groups of rats received vinclozolin at dietary levels of 0, 900,
1800, 3000, or 15 000 ppm for four weeks. There were no clinical signs
of toxicity, and food intake and body-weight gain were reduced only in
animals at the high dose. Erythrocyte parameters were reduced in all
treated female rats, and urinalysis revealed reduced osmolality at the
highest dose. Liver and adrenal weights were increased in all treated
rats, and necropsy revealed a grey-white discolouration of the
adrenals. The ascorbic acid content of the adrenals was increased in
all treated animals, and the glycogen content of the liver was reduced
in all treated females and in males at 15 000 ppm. As progressive,
dose-related transformation of the adrenal cortex was seen in all
treated rats, there was no NOAEL (Hoffman & Munk, 1975a).
Groups of 50 male and 50 female Sprague-Dawley rats received
vinclozolin in the diet at levels of 0, 150, or 450 ppm for three
months. Clinical behaviour, food and drinking-water consumption, and
body-weight gain were not affected, and clinicochemical examinations
and urinalyses revealed no substance-induced changes. Histopatho-
logical examination of the liver, adrenals, and pituitary at the end
of treatment gave no reliable indication of substance-induced changes.
The NOAEL was > 450 ppm, equal to 44 mg/kg bw per day for males and
40 mg/kg bw per day for females (Leuschner et al., 1975).
Groups of 10 male and 10 female Wistar rats received vinclozolin
in the diet at levels of 0, 300, 1000, or 3000 ppm for three months.
There were no clinical signs of reaction to treatment, and no rats
died before the scheduled sacrifice. Body-weight gain and food intake
were unaffected by treatment, but water intake was increased in rats
at 3000 ppm. Ophthalmoscopic examination revealed two cataracts, one
unilateral and one bilateral, in rats receiving 3000 ppm, and other
lenticular changes were seen in all groups; it was concluded that the
ocular lesions were spontaneous and unrelated to treatment. Evidence
of anaemia and decreased serum alkaline phosphatase activity were seen
in animals at the high dose, with decreases in leukocyte counts at
1000 and 3000 ppm. Examination post mortem revealed white, enlarged
adrenals in all treated rats. The weights of the liver, adrenal, and
testis were increased at 1000 and 3000 ppm, and histopathological
examination revealed hypertrophy of the adrenal cortex, cystoid
degeneration in the pituitary, Leydig-cell hyperplasia, cloudy
swelling of hepatocytes, single live cell necrosis, and vacuolization
of luteal cells in the ovaries at these doses. Acinar vacuolization in
the pancreas was seen in all treated rats. Vinclozolin was thus toxic
at all doses; the lowest dietary level of 300 ppm is equal to 22 mg/kg
bw per day (Mellert et al., 1993a).
In order to define a clear no-effect level, groups of 10 male and
10 female Wistar rats received vinclozolin in the diet at levels of 0
or 50 ppm for three months, and investigations similar to those in the
previous study were performed. In particular, laboratory investi-
gations and ophthalmoscopic examinations were carried out, and all
animals underwent detailed pathological examination. No treatment-
related changes were seen. The NOAEL was 50 ppm, equal to 4 mg/kg bw
per day (Mellert et al., 1993b).
Rabbits
Groups of six New Zealand white rabbits received dermal
applications of vinclozolin for 8 h per day for three weeks at doses
of 0, 111, 333, or 1000 mg/kg bw. There were no deaths and no clinical
signs of reaction to treatment; gross pathology and histopathology
revealed no evidence of dermal irritation (Leuschner et al., 1977a).
Dogs
Groups of four beagle dogs of each sex were fed diets containing
vinclozolin at doses of 0, 100, 300, 1000, or 2000 ppm for three
months. There were no signs of toxicity and no compound-induced
deaths. Repeated ophthalmological examinations revealed no effects.
The body-weight gain of treated animals was not affected, and
urinalysis showed no treatment-related findings. Females at 2000 ppm
had a reduced haemoglobin content after four and eight weeks and a
decreased erythrocyte count throughout the study. An increased
platelet count was seen in female animals at 1000 and 2000 ppm.
Howell-Jolly bodies were found in a differential blood count in males
and females at these doses, suggesting a compensatory reaction
elicited by an anaemic process. The relative liver weights and the
relative and absolute adrenal weights were increased in females at the
highest dose. Histopathological assessment revealed compound-induced
cholestasis of the liver. In a further evaluation, there was a slight
to moderate, dose-related increase in the haemosiderin content of the
liver, particularly in females, at 300, 1000, and 2000 ppm. Females
had a higher pigment content than males, and the spleens of females
also had an increased haemosiderin content. It was assumed that the
haemosiderosis in the liver was caused by increased haemolysis, in
contrast to the original interpretation that the pigment deposits in
the liver were due to cholestasis. The NOAEL was 100 ppm, on the basis
of the increased haemosiderin content of the liver (Hoffman & Munk,
1975b).
Pairs of one male and one female beagle dog were given technical-
grade vinclozolin at doses of 0, 1000, or 2000 ppm for three months.
There were no compound-induced deaths or other signs of toxicity
during treatment. The animals underwent ophthalmological examinations
twice a week and were additionally examined by an eye specialist
towards the end of the treatment period. There was no indication of
cataract formation. The NOAEL was thus > 2000 ppm (Kirsch et al.,
1974).
Groups of six beagle dogs of each sex received diets containing
technical-grade vinclozolin at doses of 0, 100, 300, 600, or 2000 ppm
for six months. Clinicochemical, haematological, and urinalyses were
carried out at regular intervals; ophthalmoscopy was performed before
the beginning of the study, after about three months, and towards the
end of treatment. At the end of the study, all animals were examined
grossly and histopathologically. No deaths occurred, food consumption
and body-weight gain were unchanged in comparison with the control
group, and there were no clinical signs of toxicity. Males at the
highest dose had pronounced, and females slight, haemolytic anaemia.
Increased incidences of Howell-Jolly bodies and reticulocytes in males
suggested a compensatory bone-marrow reaction to the compound-induced
loss of blood cells. Slight increases in bilirubin level in males at
600 and 2000 ppm, in the haemoglobin concentration of individual
erythrocytes, and in lactic dehydrogenase activity in males at
2000 ppm were further consequences of increased decomposition of
erythrocytes. The considerably increased platelet counts in males at
2000 ppm and the slight increase in females at 600 and 2000 ppm are
probably due to the anaemic process, since hyper-regenerative anaemia
is frequently accompanied by thrombocytosis. The absolute and relative
adrenal weights showed a dose-related increase in groups treated with
2000, 600, or 300 ppm, and histopathological examination showed
vacuolization of the zona fasciculata and severe lipid incorporation
and birefringence of the adrenal cortex (in females) at the highest
dose. Increased absolute and relative spleen weights were observed at
2000 ppm; at 600 ppm, only the absolute spleen weight was increased in
females. Histopathological examination revealed dilated splenic
sinuses and hyperaemia in females at both doses. Reduced relative
weights of the kidney (at 2000 ppm) and pituitary (at 600 and
2000 ppm), haemosiderin deposits in the liver (at 600 and 2000 ppm),
and severe prostatic atrophy (at 2000 ppm) were also found.
Ophthalmological examinations revealed no compound-related findings.
The NOAEL was 100 ppm, equivalent to 4.0 mg/kg bw per day (Kirsch
et al., 1982).
Groups of six beagle dogs of each sex were fed diets containing
0, 35, 75, 150, or 1500 ppm vinclozolin for 12 months. There were no
deaths and no clinical signs of toxicity. Food consumption and
body-weight gains were not significantly affected by treatment.
Haematological, clinical chemical, and urinalyses were conducted
before treatment and in weeks 13, 26, and 52. At the highest dose,
reticulocyte counts were increased in animals of each sex, platelet
counts were increased in males, the mean cell volume was increased in
females, and total bilirubin concentrations were increased in animals
of each sex. Adrenal weights were increased at 1500 ppm and slightly
increased at 150 ppm in animals of each sex; the adrenals were
enlarged in all males and in five females at 1500 ppm, and the mean
width of the adrenal cortex was increased in animals of each sex at
1500 ppm and, to a lesser degree, in females at lower doses. No
dose-response relationship was identified. Progressive transformation
and increased lipid content were seen in the adrenals of all males and
five females at 1500 ppm. Testicular weights were increased in a
dose-related fashion; at 1500 ppm, the weights were markedly
increased, and at 150 ppm the relative weights were significantly
increased. At 1500 ppm, diffuse hyperplasia of the Leydig cells was
observed in five males. The prostates of one male at 150 ppm and two
at 1500 ppm appeared smaller macroscopically. Histopathological
examination revealed that the prostates of two males at 150 ppm were
slightly or moderately atrophied and those of five males at 1500 ppm
were slightly (one) or severely (four) atrophied; similar but minimal
effects were seen in one male at 0, one at 35, and one at 75 ppm.
Liver weights were increased in animals at doses up to 150 ppm and
were markedly increased in males at 1500 ppm. Increased haemosiderin
deposition was observed in the livers of males at 1500 ppm and of
females at 150 and 1500 ppm. Spleen and thyroid weights were increased
in males at 1500 ppm, and spleen weights were also slightly increased
in females at 1500 ppm. The NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological effects at 150 and 1.500 ppm
(Hellwig et al., 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were fed diets
containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 112 weeks.
The results of analyses of the diets were not reported. There were no
clinical signs of reaction to treatment and no effect on food intake.
The weight gain of males at 1460 or 4370 ppm was reduced, and the
survival of males at the highest dose was adversely affected; no such
changes were seen in females. The weights of the liver and testis were
increased at the highest dose, and liver weight was increased in
females at 1460 ppm. Histopathological examination revealed no
treatment-related changes, and no tumours were seen (Leuschner
et al., 1977b).
Groups of 100 male and 100 female control C57Bl/6/JICO mice and
60 male and 60 female treated animals received vinclozolin in the diet
at levels of 0, 15, 150, 3000, or 8000 ppm for 18 months. Ten animals
of each sex were taken from each group for interim sacrifice after 12
months of treatment. There were no clinical signs. The mortality rates
of males and females at the highest dose were greater than those of
controls, and weight gain and food intake were reduced in animals at
3000 and 8000 ppm. Examination of blood smears revealed an increased
polymorphonuclear neutrophil count and decreased lymphocyte count in
animals of each sex at 8000 ppm. Pathological examinations revealed
similar findings at the interim and terminal kills. Increased liver
and adrenal weights were seen in animals at 3000 and 8000 ppm, with
smaller epididymides, seminal vesicles, and prostate. Focal necrosis,
bile-duct proliferation, and pigment deposition were seen in the
livers of animals at 3000 or 8000 ppm. At 8000 ppm, these changes were
accompanied by diffuse hepatocyte hypertrophy, decreased lipid
storage, and increased focal fatty infiltration in the liver, hepatic
single-cell necrosis, an increased prevalence of biliary cysts in the
livers of males, focal cellular alterations and focal hyperplasia of
the livers of males and females, hepatocellular carcinomas in three
males and 22 females, and hepatocellular adenomas in three females. In
males at 3000 and 8000 ppm, diffuse Leydig-cell hyperplasia of the
testis was seen, with atrophy of the seminal vesicles and coagulation
glands. Females at these doses had atrophic uteri, accompanied in
animals at the highest dose by diffuse stromal hyperplasia, an
increased incidence of pigmented interstitial cells in the ovaries,
and loss of ovarian follicles. The adrenal cortexes of animals at 3000
or 8000 ppm had increased lipogenic pigment in the cortico-medullary
region and lipidosis. In addition, in animals at 8000 ppm, foam cells,
eosinophilic crystals, and pneumonitis were seen in the lungs of males
and erosions or ulcers in the glandular stomachs of males and females.
Vinclozolin thus caused hepatocellular carcinomas at a dose of
8000 ppm, equal to 1300 mg/kg bw per day, a dose associated with clear
toxicity; there were no treatment-related tumours at 3000 ppm, equal
to 495 mg/kg bw per day, although evidence of toxicity was seen. The
NOAEL was 150 ppm, equal to 24 mg/kg bw per day (Mellert et al.,
1994a).
Rats
Groups of 50 male and 50 female Sprague-Dawley rats were fed
diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 130
weeks; the study was terminated when survival in the control group
reached 70%. The results of analyses of the diets were not reported.
There were no clinical signs of reaction to treatment. Dose-related
reductions in food intake and weight gain were seen in animals at 1460
and 4370 ppm, but their survival was better than that of controls.
Clinical chemical and histological investigations showed no reaction
to treatment. Interpretation of the data on organ weights was hampered
by disparities in body weight between groups. No tumours were observed
(Leuschner et al., 1977c).
Groups of 20 male and 20 female Wistar rats received vinclozolin
in the diet at levels of 0, 150, 500, 1500, or 4500 ppm for 24 months.
The only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in all treated males and
cataracts in all treated rats. Although the mortality rate of females
at the high dose was higher than that in concurrent controls, the
increase was marginal and may have been unrelated to treatment. Weight
gain and food intake were adversely affected in animals at 1500 and
4500 ppm. Water intake was increased in males at 4500 ppm and
decreased in females at this dose and in males and females at
1500 ppm. Ophthalmoscopic examination revealed a treatment-related
incidence of cataracts. Bilateral cataracts were present in all
animals at 1500 and 4500 ppm that survived to termination, and
cataracts were also seen at 500 ppm. The ophthalmoscopic changes at
150 ppm were confined to lenticular degeneration and calcification in
a few animals. Animals at 1500 and 4500 ppm showed evidence of
anaemia, decreased serum alanine aminotransferase and alkaline
phosphatase activities, and increased creatinine, total protein,
cholesterol, and gamma-glutamyl transferase activity. Increased liver
and adrenal weights were seen in animals at 4500 ppm and increased
testicular weights in all treated males. Leydig-cell tumours were seen
in almost all animals treated with 500, 1500, or 4500 ppm and were
increased in incidence in rats at 150 ppm in comparison with controls.
Focal hyperplasia and cystic ducts were seen in the rete testis of
rats at 4500 ppm, and two males at this dose but no controls had rete
testicular adenomas. Cystic ducts in the rete testis were also seen in
animals at 1500 ppm. Atrophy of the seminal vesicles, coagulating
gland, and epididymides were seen in almost all animals at 1500 and
4500 ppm, similar, less marked effects being seen in animals at 150
and 500 ppm. Dose-related reduced secretion and increased fibrosis in
the prostate were also seen in all treated males. Benign stromal
tumours of the sex cord in the ovaries were seen in 10 rats treated
with 4500 ppm, four at 1500 ppm, and two at 500 ppm; none were seen in
animals at 150 ppm or in controls. Five adenomas and one metastatic
carcinoma of the adrenal cortex were seen in females at 4500 ppm and
one adenocarcinoma of the adrenal cortex in a female at 1500 ppm; no
adrenal tumours were seen at 150 or 500 ppm or in controls. A
dose-related incidence of lipidosis in the adrenal cortex was seen in
all treated animals. Hepatocellular carcinomas occurred in nine male
rats at 4500 ppm, with none in controls. Although hepatic tumours were
found only in animals at the high dose, hepatic necrosis, hypertrophy,
and eosinophilic foci were seen in a dose-related fashion at doses
down to 150 ppm; the only changes in animals at the low dose were
eosinophilic foci in one female. Vacuolation of the pancreatic
exocrine cells was seen in all treated groups. Treatment-related
changes were also seen in the pituitary in animals at 1500 and
4500 ppm, consisting of a reduction in focal hyperplasia and an
increase in diffuse hyperplasia in males and a reduction in the
incidence of pituitary adenomas in females. Vinclozolin was toxic at
all doses tested. The lowest dietary level of 150 ppm was equal to
8 mg/kg bw per day (Mellert et al., 1994b).
In order to define a clear no-effect level, groups of 20 male and
20 female Wistar rats received vinclozolin in the diet at levels of 0,
25, or 50 ppm for 24 months, with investigations similar to those
performed in the previous study. In particular, laboratory
investigations and ophthalmoscopic examinations were carried out every
three months, and all animals underwent detailed pathological
examination. No treatment-related changes were seen. The NOAEL was
50 ppm, equal to 2.8 mg/kg bw per day (Mellert et al., 1993b).
Groups of 50 male and 50 female Wistar rats received vinclozolin
in the diet at levels of 0, 50, 500, or 3000 ppm for 24 months. The
only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in males at 500 and 3000 ppm
and cataracts in all treated rats. Mortality was not affected. Weight
gain and food intake were decreased in animals at 3000 ppm. Clinical
examination showed an increased incidence of cataracts in animals at
500 and 3000 ppm, but ophthalmoscopy of control animals and those at
the low dose revealed an increased incidence of lenticular
degeneration and one case of lenticular calcification in animals at 50
ppm. Animals at 3000 ppm had increased liver, adrenal, and testicular
weights, and histopathological examination revealed Leydig-cell
tumours in almost all males at 500 or 3000 ppm. Focal hyperplasia and
cystic ducts in the rete testis were seen in males at 3000 ppm, and an
adenoma in the rete testis was seen in one treated and no control
males. Atrophy of the seminal vesicles and coagulating gland was seen
in almost all males at 3000 ppm and in some at 500 ppm. Dose-related
reduced secretion and increased fibrosis in the prostate were seen in
all treated groups. Benign stromal tumours of the sex cord in the
ovaries were seen in 29 rats at 3000 ppm and four controls. Two
animals at the high dose had malignant thecomas of the ovary, with
none in controls. Lipidosis of ovarian interstitial cells and an
increased incidence of abnormal ovarian follicles were seen in all
treated groups. Seven animals at the high dose had adenocarcinomas of
the uterus (none in controls), and three of these had metastases.
Adenomas of the adrenal gland were seen in 21 females at 3000 ppm, and
one had a metastatic carcinoma of the adrenal cortex; there was no
increase in the incidence of adrenal tumours in animals at 50 or
500 ppm. A dose-related incidence of lipidosis and focal hyperplasia
in the adrenal cortex was seen in all treated groups, and hepatic
hypertrophy and eosinophilic foci were seen in a dose-related fashion
at doses down to 50 ppm. Vacuolation of the pancreatic exocrine cells
was seen in rats at 500 or 3000 ppm. At 3000 ppm, males had an
increased incidence of focal hyperplasia in the pituitary, and females
had a reduced incidence of pituitary adenomas. Thus, increased
incidences of tumours were seen in animals of each sex at 3000 ppm and
in males at 500 ppm. The NOAEL was 50 ppm, equal to 2.7 mg/kg bw per
day, for tumour formation and < 50 ppm for overall toxicity
(Mellert et al., 1994d).
(d) Reproductive toxicity
In a three-generation study, groups of 20 Sprague-Dawley rats of
each sex were fed diets containing vinclozolin at 0, 162, 486, or
1458 ppm. Two litters were produced per generation, and the second
litters were used as parental animals (F0, F1, and F2). The
period of treatment before mating was about eight weeks for F0
males, 16 weeks for F0 females, 15 weeks for F1 and F2 males,
and 24 weeks for F1 and F2 females (including lactation). No
treatment-related deaths or clinical signs of toxicity were seen in
pups or parents, and there were no treatment-related effects on food
consumption, body weights, litter size, malformations, birth weight of
pups, sex ratio, or behaviour. In parents, there were no treatment-
related effects on fertility, pregnancy rate, duration of pregnancy,
lactation, or viability. Auditory acuity and ophthalmic parameters
were not affected by treatment. There were no treatment-related
effects on organ weights or gross or microscopic appearance. The
no-effect level was thus > 1458 ppm, equivalent to about 73 mg/kg bw
per day. The results are not in accordance with more recent work, but
this study did not include investigations of anogenital distance in
males, and diets were not analysed for vinclozolin (Leuschner, 1977).
Groups of 24 Wistar rats of each sex were fed diets containing 0,
50, 300, 1000, or 3000 ppm vinclozolin. After a pre-mating period of
10 weeks, these F0 animals were mated twice to produce F1a and
F1b pups. F1a animals were used as F1 parents to produce F2a
and F2b animals. Some rats were raised to adulthood in order to
observe the development of their sexual organs, resulting in an FX
group of F1b rats, an FY group of F2a rats, and an FZ group of
F2b rats. The results of the study are summarized in Table 2. All
F1 males and females at 300 ppm were fertile either at the F2b
mating or at further matings for those animals that did not prove
their fertility in at least one of the scheduled matings for the
F2a/F2b litters. The authors considered that 'clear adverse
effects on fertility were noted only at 1000 and 3000 ppm, whereas 300
and 50 ppm were without any adverse effects on male and female
fertility in both parental generations.' It must be noted, however,
that rats are particularly fertile, and the effects observed,
particularly at 300 ppm, may indicate sub-fertility. The Meeting
concluded that the dietary level of 50 ppm, equivalent to 4.5 mg/kg bw
per day, is a marginal-effect level, on the basis of a possible
treatment-related reduction in the fertility of F1 males at 300 ppm,
signs of delayed development at 300 ppm (reduction in numbers of pups
with pinna unfolding and eye opening at the expected time), and
reduced epididymal weight in F2 animals at 50 ppm (Hellwig et al.,
1990a).
Groups of 25 male and 25 female Wistar rats were fed diets
containing vinclozolin at doses of 0, 20, or 40 ppm. After a 70-day
premating period, these F0 parents were mated to produce F1a and
F1b animals, and F1a rats were mated to produce F2a and F2b
animals. Randomly selected F1b, F2a, and F2b pups were
additionally raised to adulthood, resulting in FX, FY, and FZ groups,
respectively. No effect on clinical signs, weight, food intake, or
reproduction was observed, and gross pathological examination and
organ weight analysis also revealed no reaction to treatment. In
particular, there was no treatment-related effect on epididymal weight
in FX, FY, or FZ animals. The NOAEL was thus 40 ppm, equal to
approximately 4 mg/kg bw per day (Hellwig et al., 1994).
Vinclozolin was administered to groups of pregnant rats by gavage
at doses of 0, 100, or 200 mg/kg bw per day from day 14 of gestation
to postnatal day 3. Male pups in both groups of offspring displayed
feminine characteristics and their reproductive capacity was adversely
affected. About 25% of the treated males died during the study as a
result of bladder stones, hydroureter, or hydronephrosis. Malformations
noted at necropsy, when the males were approximately one year of age,
included the presence of a vaginal pouch, suprainguinal ectopic scrotum
or testes, cleft phallus with hypospadias, and small to absent
accessory sex glands. The authors proposed that vinclozolin is an
androgen receptor antagonist (Gray et al., 1994a).
In a study reported only in brief summary form, pregnant rats
were treated with 0, 3.12, 6.25, 12.5, 25, 50, or 100 mg/kg bw per day
vinclozolin from day 14 of gestation to postnatal day 3. Fertility was
adversely affected at 50 and 100 mg/kg bw per day, and subtle changes
in anogenital distance were observed at all doses (Gray et al.,
1994b).
(e) Developmental toxicity
Mice
Female NMRI albino mice were fed diets containing vinclozolin on
days 1-19 of gestation in two tests. In the first test, 24 mice
received 0 and 28 received 60 000 ppm vinclozolin; in the second,
groups of 30 mice received 0, 600, or 6000 ppm. All animals were
killed on day 19 of gestation. Females at 6000 ppm did not gain weight
during gestation and had decreased food consumption during the first
six days of treatment. Females at 60 000 ppm lost weight and had
decreased food consumption. One female at 6000 ppm died on day 11, and
all females treated at 60 000 ppm died within the first nine days.
Clinical signs of toxicity were observed only in mice at 60 000 ppm
and included ruffled coats and, before death, apathy and signs of
pronounced diuresis. Gross pathology revealed emaciation, atrophy of
musculature, and considerable loss of perirenal fatty tissue in
Table 2. Results of a multigeneration study in Wistar rats fed diets
containing vinclozolin
Dietary level Generation Finding
(ppm)
General toxicity
50 --
300 F0 increased relative liver weight,
marginal signs of anaemia (females),
lenticular degeneration
F1 As F0, plus increased adrenal
weight, Leydig-cell hyperplasia
F2 As F1
1000 F0 Reduced food intake and weight gain,
anaemia (females), increased liver,
adrenal, and testicular weights,
lenticular degeneration, hepatic
single-cell necrosis, Leydig-cell
hyperplasia
F1 As F0, plus vacuolation of pituitary
cells, lipidosis, and hypertrophy of
adrenal cells
3000 F0 Reduced food intake and weight gain,
lenticular degeneration, anaemia
(females), increased liver, adrenal
and testicular weights, hepatic
single-cell necrosis, lipidosis and
hyperplasia of adrenal cells,
vacuolation of pituitary cells,
Leydig-cell hyperplasia
F1 As F0, pills benign Leydig-cell
tumours in some animals
Effects on reproductive performance
50 --
300 F0 --
F1 Possible reduction in fertility in
males (all males eventually proved
fertile)
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
1000 F0 --
F1 Infertility of all males due to
feminization of outer genital organs
3000 F0 Increased total litter loss,
decreased number of delivered pups,
infertility of males
F1 Infertility, of all males
(feminization), infertility of six
females
Signs of developmental toxicity
50 F1 --
F2 Reduced epididymal weight (no
morphological changes)
300 F1 Slight functional reduction of
prostate and coagulating gland,
reduced epididymal
weight
F2 As F1, plus slight interstitial-cell
hyperplasia in testes and ovaries,
some pups with reduced morphological
development (delayed eye opening and
pinna unfolding)
1000 F1 Decreased pup survival, feminization
of males, reduced body-weight gain,
slightly delayed pup development,
reduced size and function of
secondary male genital organs,
atrophy of seminiferous tubules,
interstitial-cell hyperplasia in
testes and ovaries
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
3000 F1 Increased number of stillborn pups,
decreased pup survival, feminization
of males, reduced body-weight gain,
retarded morphological development,
atrophy of primary and secondary
male genital organs,
interstitial-cell hyperplasia in
testes and ovaries
From Hellwig et al. (1990a)
animals that died. No implantation sites were detected in any female
at 6000 or 60 000 ppm, and hence no fetuses were observed at these
doses. Fetuses of dams at 600 ppm had no treatment-related adverse
effects. The NOAEL for maternal toxicity and fetotoxicity was 600 ppm,
equivalent to 90 mg/kg bw per day (Hofman & Peh, 1975a).
Rats
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 15, 50, or 150 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
effects, and no adverse findings were noted at gross necropsy. There
were no treatment-related effects on pre- or post-implantation losses,
resorptions, numbers of live fetuses, or fetal or placental weights.
Anogenital distances were decreased in male fetuses at 150 and, to a
lesser extent, 50 mg/kg bw per day. This effect was considered to
indicate the beginning of feminization of male fetuses, perhaps due to
a hormonal (anti-androgenic) action on sexual differentiation. There
were no other treatment-related effects on male or female fetuses. As
no maternal toxicity, embryotoxicity, or fetotoxicity was observed,
the NOAEL was > 150 mg/kg bw per day. The NOAEL for teratogenicity
was 15 mg/kg bw per day, on the basis of reductions in anogenital
distance at 50 and 150 mg/kg bw per day (Hellwig et al., 1989a).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 50, 100, or 200 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, or
placental or fetal weights. The only possibly treatment-related
morphological effect observed in the fetuses was a significantly
increased incidence of symmetrically dumb-bell-shaped thoracic
vertebral bodies at 200 mg/kg bw per day, which is indicative of
retarded development. A slight, not statistically significant increase
was observed at 100 mg/kg bw per day. Similar effects were observed in
a follow-up study. The NOAEL for maternal toxicity was > 200 mg/kg bw
per day, and that for fetotoxicity was 100 mg/kg bw per day, on the
basis of a possible treatment-related increase in symmetrically
dumb-bell-shaped thoracic vertebral bodies at 200 mg/kg bw per day.
There was no NOAEL for teratogenicity, as the anogenital distance in
fetuses was not measured (Hellwig et al., 1989b).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 200, or 400 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at gross
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, fetal
or placental weights, or sex ratio. The anogenital distances of male
fetuses were decreased in a dose-related fashion at both 200 and
400 mg/kg bw per day, indicating the beginning of feminization. A
treatment-related increase in dilated renal pelvis and hydroureter was
also observed in fetuses of animals of each sex, which were
statistically significant in fetuses at 400 mg/kg bw per day. These
findings are considered to indicate a transient developmental delay.
There was also a significant increase in the number of fetuses with
symmetrical dumb-bell-shaped thoracic vertebral bodies at 200 and
400 mg/kg bw per day, indicative of retarded development. At 400 mg/kg
bw per day, the numbers of fetuses with accessory 14th ribs were also
significantly increased. The NOAEL for maternal toxicity was >
400 mg/kg bw per day, and that for fetotoxicity was < 200 mg/kg bw
per day, on the basis of the increased incidences of symmetrical
dumb-bell-shaped thoracic vertebral bodies at 200 and 400 mg/kg bw per
day in animals of each sex. The NOAEL for teratogenicity was <
200 mg/kg bw per day, on the basis of reductions in anogenital
distance in male fetuses at 200 and 400 mg/kg bw per day (Hellwig
et al., 1989c).
Groups of 10 female Wistar rats were treated orally with
vinclozolin at doses of 0, 600, or 1000 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths and no
abortions. Clinical signs of toxicity included unsteady gait in one
female at 600 mg/kg bw per day and in seven females at 1000 mg/kg bw
per day, and piloerection in two females at 1000 mg/kg bw per day.
Food consumption was reduced in animals at 1000 mg/kg bw per day on
days 6-13 of gestation, and the water consumption of animals at 600
and 1000 mg/kg bw per day was increased. The body-weight gain of
females at 1000 mg/kg bw per day was impaired on days 8-10.
Haematological investigations performed at day 20 revealed no
treatment-related findings. Liver and adrenal weights were increased
in a dose-related fashion. There were no treatment-related effects on
pre- or post-implantation losses, resorptions, numbers of live
fetuses, or placental weights. Fetal weights were decreased at
1000 mg/kg bw per day. When the sex ratio was determined by measuring
the anogenital distance, 68% of fetuses at 600 and 99% at 1000 mg/kg
bw per day were deemed to be female; however, internal examination
showed that 39% at 600 and 52% at 1000 mg/kg bw per day were female.
The anogenital distances were decreased in treated male fetuses. At
1000 mg/kg bw per day, the incidences of symmetrical dumb-bell-shaped
thoracic vertebral bodies were increased. The NOAEL for maternal
toxicity was < 600 mg/kg bw per day, on the basis of increased liver
and adrenal weights and findings at necropsy at 600 and 1000 mg/kg bw
per day. The NOAEL for fetotoxicity was < 600 mg/kg bw per day, on
the basis of increased incidences of hydroureter at 600 and 1000 mg/kg
bw per day. The NOAEL for teratogenicity was < 600 mg/kg bw per day,
on the basis of reductions in anogenital distance in male fetuses at
600 and 1000 mg/kg bw per day (Hellwig et al., 1989d).
In a range finding study, groups of 10 female Wistar rats
received dermal applications of vinclozolin at doses of 0, 300, 900,
or 2500 mg/kg bw per day for 6 h/day on days 6-19 of gestation. The
substance was applied onto the dorsal area of the trunk under an
occlusive dressing, and the animals were killed on day 20. Adrenal and
liver weights were increased, but not in a dose-related fashion, in
all treated animals. The anogenital distances were reduced in male
fetuses but the effect was not dose-related. The incidences of dilated
renal pelvis in fetuses were increased in a dose-related fashion at
all doses, with statistical significance at 900 and 2500 mg/kg bw per
day. The absence of a dose-response relationship for some of the
effects observed in this study is probably attributable to limited
dermal absorption of the rather pasty suspension of vinclozolin at
2500 mg/kg bw per day.
In the main study, groups of female Wistar rats received dermal
applications of 0, 60, 180, or 360 mg/kg bw per day for 6 h/day on
days 6-19 of gestation. The substance was applied on the dorsal area
of the trunk under an occlusive dressing, and the animals were killed
on day 20. There were no deaths, no abortions, no signs of toxicity,
no treatment-related effects on body weight or food consumption, and
no treatment-related findings in haematological investigations
performed on day 20. Absolute liver weights were increased at 180 and
360 mg/kg bw per day; absolute adrenal weights were significantly
increased at these doses but not dose-dependently. There were no
treatment-related macroscopic pathological findings or effects on
implantation losses, resorptions, numbers of fetuses, sex ratio, or
weights of fetuses or placentae. The anogenital distances of males at
180 and 360 mg/kg bw per day were decreased, but there were no other
treatment-related malformations, variations, or retardations in
treated fetuses. The NOAEL for maternal toxicity was 60 mg/kg bw per
day, on the basis of increased absolute adrenal and liver weights at
180 and 360 mg/kg bw per day. The NOAEL for fetotoxicity was >
360 mg/kg bw per day, and that for teratogenicity was 60 mg/kg bw per
day, on the basis of reductions in anogenital distances in male
fetuses at 180 and 360 mg/kg bw per day (Hellwig et al., 1990b).
Rabbits
Groups of 15 New Zealand white female rabbits were treated orally
with vinclozolin at doses of 0, 20, 80, or 300 mg/kg bw per day on
days 6-18 of gestation and were killed on day 29. There were no signs
of toxicity and no deaths that were obviously attributable to
treatment. Body-weight gain was not affected, and there were no
treatment-related effects on numbers of live young, sex ratio,
embryonic deaths, pre-implantation losses, or litter or fetal weights.
The frequency of post-implantation losses was slightly increased but
was within historical control values. There were no treatment-related
major malformations or visceral anomalies. There was a slight,
non-dose-dependent increase in the incidence of minor skeletal
anomalies, which was within historical control values. The NOAEL for
maternal toxicity and fetotoxicity was > 300 mg/kg bw per day (Cozens
et al., 1981; Cozens & Palmer, 1987).
Groups of 15 female Himalayan rabbits were treated orally with
vinclozolin at 0, 50, 200, or 800 mg/kg bw per day on days 7-28 of
gestation and were killed on day 29. One female at 200 mg/kg bw per
day aborted on day 26 and was killed; at 800 mg/kg bw per day, one
female died, one aborted and died, and 11 aborted and were killed. The
abortions occurred between days 21 and 27. Clinical signs of toxicity
included reddish-brown discolouration of the urine in 13 females and
apathy, hunched posture, conjunctivitis, and urine-smeared fur in one
or two animals at 800 mg/kg bw per day. The female at 200 mg/kg bw per
day which aborted had vaginal haemorrhage and discoloured urine. A
second female at this dose had vaginal haemorrhage on day 24. The food
consumption of animals at 800 mg/kg bw per day was significantly
reduced during the treatment period, and that of animals at 200 mg/kg
bw per day was reduced mainly on days 7-19. Animals at 50 and
200 mg/kg bw per day had no treatment-related increases in pre- or
post-implantation losses. The only dam at 800 mg/kg bw per day with
viable fetuses had three post-implantation losses, as early
resorptions. There were no treatment-related resorptions in animals at
50 or 200 mg/kg bw per day. The sex ratios, numbers of live fetuses,
and placental and fetal weights were not affected by treatment at 50
or 200 mg/kg bw per day. There were no treatment-related external
malformations or visceral abnormalities. At 800 mg/kg bw per day, one
of four viable fetuses had malformations of the sternebrae, but the
relevance of this finding is questionable owing to the small number of
fetuses. One of 94 fetuses at 50 mg/kg bw per day also had
malformations of the sternebrae, but the incidence was within the
historical control range of 0-1% (mean, 0.2%). No treatment-related
changes in the male fetal genital organs were observed. The NOAEL for
maternal toxicity was 50 mg/kg bw per day, on the basis of reduced
food consumption, abortion, and clinical signs of toxicity at
200 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity was
200 mg/kg bw per day, on the basis of three resorptions as a
consequence of maternal toxicity in the female alive at termination in
the high-dose group. There was no evidence of teratogenicity at 50 or
200 mg/kg bw per day (Hellwig et al., 1990c).
Groups of 20 female Himalayan rabbits were treated orally with
vinclozolin at 0 or 400 mg/kg bw per day on days 7-8 of gestation and
were killed on day 29. One female at 400 mg/kg bw per day aborted and
died on day 19, and nine aborted and were killed between days 20 and
27. Clinical signs of toxicity included blood in the bedding of two
treated females, one of which aborted. Eight had red-brown urine. The
food consumption of treated animals was reduced on days 7-26, and
body-weight gain was impaired on days 7-25. Pre- and post-implantation
losses were increased, and the number of early resorptions was thus
also increased. The numbers of live fetuses per litter were slightly
decreased, and the ratio of males:females was 1:1.8 at 400 mg/kg bw
per day in comparison with 1:1.16 in the controls; however, no
treatment-related changes were seen in male fetal genital organs.
Fetal weights were increased in the treated animals, probably as a
consequence of the slightly decreased litter sizes. There were no
external or soft-tissue malformations, but the frequency of separated
origins of the carotids was 36% in the treated group in comparison
with 10% in concurrent controls and a mean of 19% in historical
controls (range, 10-31%). There were no skeletal malformations and no
treatment-related skeletal variations or retardations; in particular,
no malformed sternebrae were observed, indicating that the effect
observed in the previous study at 50 mg/kg bw per day was not
treatment-related. The NOAEL for maternal, embryo-, and fetotoxicity
was < 400 mg/kg bw per day. There was no clear evidence of
teratogenicity, but the increased incidence of separated origins of
the carotids indicates potential teratogenicity at this severely
maternally toxic dose (Hellwig et al., 1990d).
(f) Genotoxicity
The results of standard regulatory assays for genotoxicity are
summarized in Table 3.
A medium-term bioassay based on preneoplastic glutathione
S-transferase placental form (GST-P)-positive foci in rat liver was
performed with vinclozolin. Rats were injected with N-nitrosodi-
ethylamine and two weeks later were fed a diet containing 2000 ppm
vinclozolin for six weeks, with partial hepatectomy at week 3; they
were then killed. In one group that received only vinclozolin,
negative results were obtained, but administration after initiation
with N-nitrosodiethylamine gave positive results. The authors
reported that the test is highly predictive for genotoxic hepato-
carcinogens but less predictive for carcinogens that have target
organs other than the liver. They suggested that since the assay is
based on the two-stage hypothesis of carcinogenesis, chemicals for
which the results are positive are tumour promoters in rat liver (Ito
& Hasegawa, 1992; Ito et al., 1993, 1994).
Vinclozolin was cytotoxic in BALB/c3T3 cells in the absence but
not in the presence of an exogenous metabolic system. It induced
transformation in this cell line in both the presence and the absence
of metabolic activation (Perocco et al., 1993).
As shown in Table 3, negative results were obtained in a range of
assays in vivo and in vitro. Although positive results were
obtained in the medium-term bioassay and in the test for cell
transformation, these studies are not well validated.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The intact and abraded dorsal skin of four male and two female
white Vienna rabbits was treated with a 50% aqueous suspension of
vinclozolin under an occlusive covering for 24 h. The intact skin of
five of the six animals had well-defined erythema, and slight oedema
was seen in one animal. All of the reactions subsided completely
within 72 h. The erythema that formed on the abraded skin of all
animals after 24 h was more severe than that on the intact skin; it
had completely subsided in one-half the animals by 72 h but remained
unchanged in the other half. One animal in this group had slight
oedema after 24 h but no longer at 72 h. Vinclozolin cannot be
classified as a skin irritant, however, a slight, transient irritation
potential was evident (Hildebrand, 1977a).
Table 3. Results of tests for the genotoxicity of vinclozolin
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S typhimurium TA98, < 1000 µg/plate 92.8 Negativea Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA98, TA100, < 3000 µg/plate 92.8 Negativea Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP hrc
Reverse mutation S. typhimurium TA98, TA100 < 10 000 µg/plate 98.1 Negativea Gelbke & Engelhardt
TA1535, TA1537, TA1538 (1983)
Gene mutation hprt L5178Y cells < 1000 µg/ml NR Negativea Witterland & Hoorn
(1984)
Gene mutation hprt Chinese hamster ovary cells < 10 mg/ml > 99.5 Negativea Gelbke & Jackh (1975)
Chromosomal aberration Chinese hamster ovary cells < 500 µg/ml NR Negativea Murli (1989)
DNA repair B. subtilis M45 and H17 rec < 2000 µg/plate 92.8 Negative Shirasu et al. (1977)
DNA repair B. subtilis M45 and H17 rec < 10 000 µg/plate NR Negativea Hoorn (1983)
Unscheduled DNA Rat hepatocytes < 1000 µg/ml > 99.5 Negative Cifone & Myhr (1984)
synthesis
In vivo
Host-mediated mutation S. typhimurium G46 his- 2 × 200 and 1000 92.8 Negative Shirasu et al. (1977)
mg/kg bw
Dominant lethal mutation NMRI male mice 5 × 2000 mg/kg bw NR Negative Hofmann & Peh
(1975b)
Sister chromatid Male and female Chinese 3830 and 5620 98.1 Negative Gelbke & Engelhardt
exchange hamsters mg/kg bw (1981)
NR, not reported
a With and without metabolic activation
Eye irritation was investigated in three male and three female
New Zealand white rabbits. After 24 h, the only finding was slight
redness of the conjunctivae, which was not completely reversible
within 72 h Vinclozolin may be regarded as not irritating to the eyes
(Hildebrand, 1977b).
Technical-grade vinclozolin was tested in groups of 12 male
Pirbright white guinea-pigs and five controls in the Magnusson-Kligman
maximization test. The treated animals had questionable dermal changes
after the first challenge and distinct changes after the second,
suggesting sensitization (Gelbke, 1979).
No dermal sensitization was observed in an open epicutaneous test
in guinea-pigs with BAS 352 04 F formulation (Ronilan), containing 50%
vinclozolin. A 60% preparation in water caused slight erythema and
oedema at the beginning of the induction period; a 20% preparation
elicited sporadic skin irritation. Since no differences were found
between the control and test groups, these concentrations are assumed
not to be sensitizing. Neither skin irritation nor sensitization was
observed with 2 or 6% preparations. These concentrations can be
assumed not to have sensitizing potential that would be of importance
under field conditions (Grundler & Gelbke, 1980).
(ii) Hormonal effects
Groups of 20 male and 20 female Wistar rats were fed diets
containing 4500 ppm vinclozolin for six months; the control group
consisted of 10 males and 10 females. After sacrifice, blood was
collected and the levels of the following hormones were determined:
adrenocorticotrophic hormone (ACTH), corticosterone, dehydroepian-
dosterone (DHEA), testosterone, estradiol, hydroxy-progesterone, and
luteinizing hormone (LH). Males showed more effects than females: in
males, the level of LH was increased by about 10-fold and those of
testosterone and DHEA doubled; in females, the level of LH was
increased by 2.5-fold, but there was no change in the concentrations
of sex steroids. That of ACTH increased in animals of each sex, but
the results were variable and the relationship of this change to
treatment is uncertain (Knuppen, 1989).
Groups of 20 male and 20 female Wistar rats were treated with 0
or 4500 ppm vinclozolin for three months, and groups of 10 of each sex
were then allowed to recover for three months to study the
reversibility of any changes. The hormones that were measured were LH,
follicle-stimulating hormone, testosterone, estradiol, DHEA, ACTH,
aldosterone, and corticosterone. The results basically confirmed those
of the previous study. The levels of LH, follicle-stimulating hormone,
testosterone, and DHEA were increased in males, LH most markedly,
while only that of LH was increased in females. The concentration of
ACTH was increased in both males and females, and those of aldosterone
and corticosterone were marginally increased in males but decreased in
females. All of these changes, except perhaps that of ACTH in females,
was reversible within two months (Mellert et al., 1992).
(iii) Receptor binding
In a study of binding to the androgen receptor, vinclozolin was
incubated in vitro with MCF-7 cells derived from a human mammary
carcinoma which contains a large amount of androgen receptor. A clear
affinity with cytosolic and nuclear androgen receptors was seen
(Knuppen, 1990). In order to confirm this result and to investigate
whether the binding is due to vinclozolin or its main rat metabolite
(Reg. No. 119 208), their effects were compared with those of the
anti-androgenic drug Flutamide and the synthetic androgen Mibolerone.
Vinclozolin bound to the androgen receptor with an affinity of
approximately 50% of that of Flutamide, and the binding affinity of
the metabolite was virtually zero (Knuppen & Schutze, 1991). In a
comparison of the effects of vinclozolin on receptors in vitro and
in vivo, not reported in detail, vinclozolin was shown to have a
binding affinity similar to that of Flutamide for androgen receptors
in the LNCAP cell line, which is derived from human prostate, a
typical target tissue for vinclozolin-mediated anti-androgenic
effects. Vinclozolin was also capable of binding to androgen receptors
in castrated Wistar rats (Knuppen & Schutze, 1992).
The ability of vinclozolin to inhibit 5-alpha-reductase and to
compete with androgen for binding at the androgen receptor was
investigated. Neither vinclozolin nor its degradation products
2-{[(3,5-dichlorophenyl)carbamoyl]oxy}-2-methyl-3-butenoic acid and
3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide inhibited 5-alpha-
reductase. Although vinclozolin competed only weakly with androgen for
binding at the androgen receptor, the two metabolites were effective
antagonists. The authors reported that the concentrations of the first
metabolite in the serum of pregnant rats after treatment with
100 mg/kg bw per day vinclozolin could be sufficient to meet or exceed
the Ki for androgen receptor inhibition in vitro (Kelce et al.,
1994).
(iv) Nephrotoxicity
The acute nephrotoxic potential of vinclozolin was compared with
that of two other N-(3,5-dichlorophenyl) carboximide fungicides:
N-(3,5-dichlorophenyl) succimide and iprodione. Groups of four male
Fischer 344 rats received a single intraperitoneal injection of 0.4 or
1.0 mmol/kg bw or vehicle, and renal function was monitored at 24 and
48 h. N-(3,5-Dichlorophenyl) succimide induced renal effects
characterized by marked diuresis, increased proteinuria, elevated
blood urea nitrogen, increased kidney weights, and proximal tubule
necrosis. Iprodione and vinclozolin caused only minor or no
alterations in renal function (Rankin et al., 1989).
(v) Haemoglobin adduct formation
Female Wistar rats were treated orally with various pesticides at
doses of up to 1 mmol/kg bw. Blood was taken 24 h after treatment,
haemoglobin was isolated and hydrolysed with 1N sodium hydroxide, and
aromatic amines extracted and quantified. No adducts with 3,5-di-
chloroaniline from vinclozolin were found (Sabbioni & Neumann, 1990).
(vi) Review of ophthalmoscopic findings
In a review of the cataracts and other lenticular changes seen at
ophthalmoscopy in short- and long-term studies with vinclozolin in
rats, an analysis was carried out to determine whether these changes
were due to acceleration of normal, age-related changes, since
lenticular changes are seen commonly in old rats and cataracts also
occur spontaneously in untreated rats. The clear NOAELs are shown in
Table 4. Vinclozolin induced cataracts and other lenticular changes
only in rats, and there were no treatment-related lenticular changes
in B6C3F1 or C57B1 mice or in dogs. In the studies of absorption and
distribution, a high concentration of radiolabel was found in the
Harderian gland, which is situated close to the eye and secretes a
fluid onto its surface. The presence of radiolabel in this secretion
was confirmed by examination of enlargements of whole-body
autoradiographs (Schilling, 1993).
3. Observations in humans
A cross-sectional study was performed of 67 men who had handled
vinclozolin for 1-13 years during its synthesis and formulation and 52
unexposed controls. The men were monitored by determining urinary
metabolites containing a 3,5-dichloraniline moiety and observation for
reversible changes in the levels of hormones of the adrenocortico-
tropic and gonadotropic feedback systems, signs of liver injury,
haemolytic anaemia, cataract formation, and hormonally induced
hyperplasia and tumours at high doses. The clinical investigation
consisted of a medical and occupational history questionnaire,
physical examination, laboratory determinations, including
measurements of testosterone, LH, and follicle-stimulating hormone,
ultrasonography of the liver and prostate, a detailed examination, and
routine spirometry. The mean 3,5-dichloraniline concentration in
exposed workers was 235-422 µg/g creatinine, depending on the work
area, in comparison with a mean of 7 µg/g creatinine in controls. On
the basis of a series of assumptions, including 40% excretion through
the kidneys, 1.5-litre urine output per day, and 70-kg body weight,
the authors estimated that the occupational exposure of two-thirds of
the employees to vinclozolin exceeded 25 µg/kg bw per day. The
physical examinations and laboratory tests provided no evidence of
vinclozolin-induced hormonal responses, liver injury, prostatic
changes, cataract formation, or haemolytic anaemia. The authors
concluded that vinclozolin induced no health effects and, in
particular, no anti-androgenic effects (Zober et al., 1995).
Table 4. NOAELs for ophthalmoscopic effects in short- and long-term
studies of the toxicity of vinclozolin in rats
Effect NOAEL (ppm)
Short-term study Long-term study
Cataracts 1000 150
Striations 300 150
Bosselated lens structure 1000 150
Bulbiform thickening 3000 150
Opacities 3000 150
Comments
Vinclozolin is well absorbed after oral administration to rats
and extensively metabolized. The majority of the administered
radiolabel was found in the bile, and no unchanged vinclozolin was
excreted in the urine. After single oral doses of radiolabelled
vinclozolin, excretion was rapid; after multiple doses there was no
significant accumulation. Vinclozolin is only moderately absorbed via
the dermal route in rats: over 72 h, about 17% of a dose of 0.13 mg/kg
bw was excreted in the urine.
Vinclozolin has low acute toxicity, with an oral LD50 in rats
of > 15 000 mg/kg bw. The clinical signs of toxicity after acute
dosing with vinclozolin were generally non-specific and there were no
consistent treatment-related findings at necropsy. Vinclozolin is not
irritating to rabbit skin or eyes, but induced skin sensitization in a
maximization study in guinea-pigs. WHO has classified vinclozolin as
unlikely to present an acute hazard in normal use.
Studies of repeated administration were carried out in mice,
rats, rabbits and dogs, in which vinclozolin and/or its metabolites
caused toxic effects indicative of anti-androgenic activity. In two
three-month feeding studies in different strains of mice at dietary
levels of 100-5000 ppm, the NOAEL was equivalent to 20 mg/kg bw per
day, on the basis of signs of hepatotoxicity, signs consistent with
anti-androgenicity, and changes in the adrenal glands. In two recent
three-month feeding studies in rats (at levels of 0, 300, 1000, and
3000 ppm and 0 and 50 ppm, respectively) vinclozolin caused changes
qualitatively similar to those seen in mice; however, effects on the
adrenal glands (including lipidosis) were seen at 300 ppm, and the
NOAEL was confirmed in the second study as 50 ppm, equal to 4 mg/kg bw
per day. In a 12-month feeding study in dogs at dietary levels of 0,
35, 75 150, or 1500 ppm, the NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological changes in the liver, spleen,
prostate, testis, and adrenals. The results of studies incorporating
withdrawal periods indicate that the anti-androgenic effects of
vinclozolin are reversible on cessation of treatment.
In a recent study of carcinogenicity in C57Bl/6 mice at dietary
levels of 0, 15, 150, 3000, or 8000 ppm, hepatocellular carcinomas
were seen at 8000 ppm. There was evidence of toxicity at 3000 ppm,
including hepatotoxicity, Leydig-cell hyperplasia, atrophy of
accessory sex glands, atrophic uteri, and lipidosis in the
cortico-medullary region of the adrenals. The NOAEL was 150 ppm, equal
to 24 mg/kg bw per day. In an earlier study in NMRI mice at levels of
0, 160, 490, 1460, or 4370 ppm, survival was adversely affected at the
highest dose, and the NOAEL was 490 ppm on the basis of increased
liver weight, without histological change. In rats, the long-term
toxicity and carcinogenicity of vinclozolin has recently been
investigated in three studies with dietary levels of 25-4500 ppm.
Cataracts and other lenticular changes were seen in rats treated with
50 ppm or more. (Mice and dogs were closely examined for ocular
changes, but vinclozolin did not affect the eyes in these species.) An
increased incidence of Leydig-cell tumours was seen in rats treated
with 150 ppm and more, together with atrophy of accessory sex glands.
Benign sex cord stromal tumours in the ovaries were seen in rats
treated at 500 ppm and above, and uterine adenocarcinomas were
detected at 3000 ppm (the highest dose tested in the carcinogenicity
study). Adrenal tumours were seen at 1500 ppm and above.
Hepatocellular carcinomas were seen in males treated with 4500 ppm,
and signs of hepatotoxicity were seen in rats treated with 150 ppm or
more. The NOAEL was 25 ppm, equal to 1.4 mg/kg bw per day.
In multi-generation studies, vinclozolin led to infertility of
males, owing to feminization of the outer genital organs, at dietary
levels of 1000 ppm or more. At 300 ppm, although all males were
eventually proved fertile, the observed effects may have indicated
sub-fertility. At 50 ppm, the only adverse effect was a reduction in
epididymal weight (with no associated morphological changes) in F2
offspring. The NOAEL was 40 ppm, equivalent to approximately 4 mg/kg
bw per day. Recent investigations of developmental toxicity have been
conducted in rats and rabbits. In rats, the most sensitive indicator
of teratogenicity was a reduction in the anogenital distance; in a
series of studies, the NOAEL for a change in anogenital distance was
15 mg/kg bw per day. The NOAEL for fetotoxicity was about 100 mg/kg bw
per day, on the basis of signs of developmental delay, while the NOAEL
for maternal toxicity was about 400 mg/kg bw per day, on the basis of
clinical signs of toxicity.
Three studies of developmental toxicity have been conducted in
rabbits. In the first, there were no signs of maternal toxicity,
fetotoxicity, or teratogenicity at doses up to and including 300 mg/kg
bw per day. In the second study, with doses up to and including
800 mg/kg bw per day, toxicity led to extensive mortality at the
highest dose, precluding any reliable assessment at this dose. The
NOAEL for maternal toxicity was 50 mg/kg bw per day, and that for
fetotoxicity was 200 mg/kg bw per day; there was no evidence of
teratogenicity at this dose (the highest dose available for
assessment). The third study involved only one dose, 400 mg/kg bw per
day. The number of female offspring exceeded the number of males, but
there was no treatment-related change in the appearance of the male
fetal genital organs. An increase in the incidence of separated
origins of the carotid arteries indicated potential teratogenicity at
this maternally toxic dose.
Vinclozolin has been tested for genotoxicity in a range of tests
in vivo and in vitro. The Meeting concluded that vinclozolin is
not genotoxic. It noted that positive results were obtained in a study
of cell transformation, but the process giving rise to this effect is
unknown. One study suggests that vinclozolin may be a promoter in rat
liver in vivo, which may indicate the mechanism by which liver
tumours were induced in rats at a high dose.
Studies have been conducted that confirm the anti-androgenic
properties of vinclozolin, which are likely to be associated with
binding to the androgen receptor. This proposed mechanism of action
could account for the results seen in studies of the reproductive
toxicity and long-term toxicity of vinclozolin.
In an epidemiological study of manufacturing plant personnel, it
was concluded that there was no evidence that vinclozolin had induced
health effects in employees with possible long-term exposure.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1.4 mg/kg bw per day in the two-year study of carcinogenicity
in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 20 mg/kg bw per day (three-month study of
toxicity)
490 ppm, equivalent to 63 mg/kg bw per day (112-week study
of toxicity and carcinogenicity in NMRI mice)
150 ppm equal to 24 mg/kg bw per day (18-month study of
toxicity and carcinogenicity in C57Bl/6 mice)
Rat: 50 ppm, equal to 4 mg/kg bw per day (three-month study of
toxicity)
25 ppm, equal to 1.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
40 ppm, equivalent to 4 mg/kg bw per day (study of
reproductive toxicity)
15 mg/kg bw per day (study of developmental toxicity)
100 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
400 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 50 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
200 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 75 ppm, equal to 2.4 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to vinclozolin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 15 000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test
Inhalation, toxicity, rat LC50 > 29 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat NOAEL = 15 mg/kg bw per day; teratogenicity
Long-term (> one year) Dietary, two years, toxicity and NOAEL = 1.4 mg/kg bw per day; signs of
carcinogenicity, rat antiandrogenicity
Dietary, one year, toxicity, dog NOAEL = 2.4 mg/kg bw per day; signs of
antiandrogenicity
References
Block, I. et al. (1987) Study of effect on vital functions of
animals -- general pharmacology -- of vinclozolin. Unpublished
report from Research & Consulting Co. AG, Itingen, Switzerland.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cameron, B.D. & Jack, L. (1991) In vitro percutaneous absorption of
14C-Reg. No. 83 258. A comparison using rat and human
epidermis. Unpublished report from Inveresk Research
International, Tranent, Scotland. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B. &
Franklin, E.R. (1976) The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral administration to
rats. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cifone, M.A. & Myhr, B.C. (1984) Evaluation of vinclozolin (831233) in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Kensington, Maryland,
USA. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cozens, D.D. & Palmer, A.K. (1987) Statement on maternal toxicity;
effect of vinclozolin on pregnancy of the New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R. & Offer, J.M.
(1981) Effect of vinclozolin on pregnancy of New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P (1979) Study of sensitization effect on guinea pigs
maximization test. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1981) Cytogenetic investigation in
Chinese hamsters after a single oral administration of Reg.
No. 83 258. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of
vinclozolin (Reg. No. 83 258) in the Ames test. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Gelbke, H.P & Jackh, R. (1975) Report on a point mutation test carried
out on CHO cells (HGPRT locus) with the test substance
vinclozolin (substance No. 841382). Unpublished report from BASF
AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1979) Report on the study of the acute oral
toxicity of vinclozolin (Reg. No. 83 258) in beagle dogs.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1981) Acute oral toxicity of vinclozolin in
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gray, L.E., Jr, Ostby, J.S. & Kelce, W.R. (1994a) Developmental
effects of an environmental antiandrogen: The fungicide
vinclozolin alters sex differentiation in the male rat. Toxicol.
Appl. Pharmacol., 129, 46-52.
Gray, L.E, Jr, Ostby, J.S., Monosson, E. & Kelce, W.R. (1994b)
Alterations of sex differentiation in male rats following
perinatal exposure to low doses of the antiandrogenic pesticide
vinclozolin. Biol. Reprod., 50 (Suppl. 1), 101.
Grundler, P. & Gelbke, H.P. (1980) Report on the sensitizing effect of
BAS 352 04 F - Ronilan (WNT No. 791661) in the guinea pig -- open
epicutaneous test (OET). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990a) The biokinetics of 14C-vinclozolin in
the rat. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990b) The biotransformation of 14C-vin-
clozolin in the rat. Unpublished report from Huntingdon Research
Centre, Huntingdon, Cambs, United Kingdom. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991a) The determination by whole body
autoradiography of the tissue distribution of radioactivity in
female rats after oral administration of 14C-vinclozolin.
Unpublished report from Huntingdon Research Centre, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991b) The dermal absorption of
14C-vinclozolin in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1987) Report on the study of the toxicity of
Reg. No. 83 258 (vinclozolin) in beagle dogs after 12-month
administration via the diet. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989a) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- first study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989b) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- second study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF: AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- third study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989d) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- test study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1990a) Report: Reproduction study with Reg.
No. 83 258 in rats. Continuous administration over 2 generations.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990b) Report: Study of the prenatal toxicity of
Reg. No. 83 258 in rats after dermal application. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rabbits after oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990d) Report on the supplementary steady of the
prenatal toxicity of Reg. No. 83 258 in rabbits after oral
administration (gavage). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1994) Report: Second reproduction study with
Reg. No. 83 258 in rats. Continuous administration over 2
generations. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977a) Primary skin irritation of Reg. No. 83 258
(vinclozolin) on the intact and scarified dorsal skin of white
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977b) Primary irritation of Reg. No. 83 258
(vinclozolin) to the eye of white rabbits. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. (1973a) Report on acute oral toxicity trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1973b) Report on the acute intraperitoneal trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1974) Report on the testing of 3-(3,5-dichiorophenyl)-
5-methyl-5-vinyl-l,3-oxazolidine-2,4-dione in a 3-month feeding
experiment on rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975a) Report on the supplementary
toxicological study of 3-(3,5-dichiorophenyl)-5-methyl-
5-vinyl-1,3-oxazolidine-2,4-dione in a 4-week feeding study in
the rat. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975b) Report on the toxicological testing
of 3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-
2,4-dione in a three-month feeding trial on the dog. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975a) Study on the prenatal toxicity of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on mice. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975b) Study of the mutagenic effect of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on the male mouse following repeated oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hoorn, A.J.W. (1983) Mutagenicity of vinclozolin, compound No. 831233,
in the rec assay with Bacillus subtilis. Unpublished report
from Litton Bionetics, Veenendaal, Netherlands. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Ito, N. & Hasegawa, R. (1992) Liver medium term bioassay in rats for
screening of carcinogenesis and modifying factors in
hepatocarcinogenesis. Food Chem. Toxicol., 30, 979-992.
Ito, N., Hoshiya, T., Hasegawa, R., Hakoi, K., Cui, L., Ogiso, T. &
Cabral, R. (1993) Enhancement by non-mutagenic pesticides of
GST-P positive hepatic foci development initiated with
diethylnitrosamine in the rat. Cancer Lett., 72, 59-64.
Ito, N., Hasegawa, R., Imaida, K., Takahashi, S. & Shirai, T. (1994)
Medium term rat liver bioassay for rapid detection of carcinogens
and modifiers of hepatocarcinogenesis. Drug Metab. Rev.,
26, 431-442.
Kelce, W.R., Monosson, E., Gamcsik, M.P., Laws, S.C. & Gray, L.E., Jr
(1994) Environmental hormone disruptors: Evidence that
vinclozolin developmental toxicity is mediated by antiandrogenic
metabolites. Toxicol. Appl. Pharmacol., 126, 276-285.
Kirsch, P. (1986a) Report of the study of the acute oral toxicity on
the rat based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. (1986b) Report of the study of the acute oral toxicity on
the mouse based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. et al. (1974) Report on the study of 3-(3,5-dichloro-
phenyl)-5-methyl-5-vinyl-1,3-oxazoildine-2,4-dione for cataract
formation in a 3-month feeding study in dogs. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Kirsch, P., Deckhardt, M. & Hellwig, J. (1982) Report on the study of
the toxicity of Reg. No. 83 258 (vinclozolin) in beagle dogs
after 6-month administration in the diet. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. (1989) Examination of the hormone status. Unpublished
report from University of Lübeck, Lübeck, Germany. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Knuppen, R. (1990) Final report: Study of the binding of
3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-2,4-dione to the androgen
receptor in MCF-7 cells. Unpublished report from University of
Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1991) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) -- Reg. No. 119 208
(metabolite BF 352-22) to the androgen and glucocortiroid
receptors in the cytosol from MC-F-7 cells and from the prostate
and liver tissues of the rat. Unpublished report from University
of Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1992) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) to the androgen receptor
in the cytosol from a cell line expressing the androgen receptor
and from the prostate tissue of the rat. Unpublished report from
University of Lübeck, Lübeck, Germany. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Kretzschmar, R. et al. (1987) Study on the EEG effects in the
conscious rat of vinclozolin. Unpublished report from Knoll AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Leuschner, F. (1977) Chronic oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in a
reproduction study covering three generations of Sprague-Dawley
rats. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikoiogie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. (1979) Acute inhalation toxicity study on the
preparation vinclozolin. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1975) Oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in
Sprague-Dawley rats. Unpublished report from Laboratorium für
Pharmakoiogie und Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977a) 3-weeks-toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in NZW
rabbits by local application. Unpublished report from
Laboratorium fur Pharmakologie und Toxikoiogie (LPT), Hamburg,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977b) Oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in NMRI
mice. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikologie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. et al. (1977c) Chronic oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in the
Sprague-Dawley rat. Unpublished report from Laboratorium fur
Pharmakoiogie and Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1992) Report: Study on the influence of Reg.
No. 83 258 (vinclozolin) on the hormone status of Wistar rats.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1993a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in Wistar rats. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Mellert, W. et al. (1993b) Report: Supplementary study on the oral
toxicity of Reg. No. 83 258 (vinclozolin) in Wistar rats.
Administration in the diet over 3 months. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994a) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in C57BL mice. Administration in the
diet for 18 months Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994b) Report: Study of the chronic toxicity of
Reg. No. 83 258 (Vinclozolin) in rats. Administration via the
diet over 24 months. Unpublished report from BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994c) Report: Chronic toxicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994d) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Murli, H. (1989) Mutagenicity test with Reg. No. 83 258, Batch No. 183
(= ZST No. 881375) in an in vitro cytogenetics assay measuring
chromosome aberration frequency in Chinese hamsters ovary cells
(CHO cells). Unpublished report from Hazleton, Kensington,
Maryland, USA. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Oesch, F. (1977) Ames test for vinclozolin. Unpublished report from
University of Mainz, Mainz, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Otto, S., Bentel, P., Elzner, J. & Ohnsorg, U. (1977) Metabolism of
14C-vinclozolin in rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Perocco, P., Collaci, A. & Grilli, S. (1993) In vitro cytotoxic and
cell transforming activities exerted by the pesticides cyanazine,
dithianon, diflubenzuron, procymidone and vinclozolin on BALB/c
3T3 cells. Environ. Mol. Mutag., 21, 81-86.
Rankin, G.O., Teets, V.J., Nicoll, D.W. & Brown, P.I. (1989)
Comparative acute renal effects of three N(3,5-dichlorophenyl)
carboximide fungicides: N-(3,5-dichlorophenyl) succimide,
vinclozolin and iprodione. Toxicology, 56, 263-272.
Sabbioni G. & Neumann H.-G. (1990) Biomonitoring of arylamines:
Haemoglobin adducts of urea and carbamate pesticides.
Carcinogenesis, 11, 111-116.
Schilling, K. (1993) Evaluation of ophthalmology findings recognised
within various rat feeding studies with Reg. No. 83 258
(vinclozolin). Unpublished report from K. Schilling, Consultant,
Kirchheimbolanden, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in B6C3F1 mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990b) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in C57BL mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Shirasu, Y. et al. (1977) Mutagenicity testing on BAS 352 04 F in
microbial systems. Unpublished report from Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Shirasu, Y., Takahashi, K. & Saito, T. (1978a) Report of acute
toxicity tests with BAS 352 F in mice. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany
Shirasu, Y., Takahashi, K. & Saito, T. (1978b) Report of acute
toxicity tests with BAS 352 F in rats. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Witterland, W.F. & Hoorn, A.J.W. (1984) Mutagenicity evaluation of
vinclozolin (831233) in the mouse lymphoma forward mutation
assay. Unpublished report from Litton Bionetics, Veenendal,
Netherlands. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Zober, A. et al. (1995) Morbidity study of personnel with potential
exposure to vinclozolin. Occup. Environ. Med., 52, 233-241.
After oral administration of 2000 or 5000 mg/kg bw vinclozolin to
male Sprague-Dawley rats, the effects on the cortical electroence-
phalogram were examined. At both doses, sleeping stages were prolonged
and the number and duration of rapid eye movement phases were slightly
reduced; however, these alterations were not pronounced and suggest
only a moderate sedative effect (Kretzschmar et al., 1987).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of vinclozolin are
summarized in Table 1. The clinical signs of toxicity after treatment
with vinclozolin were generally nonspecific, and no consistent,
treatment-related effects were seen at autopsy.
The LD50 of metabolite T,3,5-dichlorophenylcarbamoyl-2-
propionic acid, was reported to be 2740 mg/kg bw in rats and >
2000 mg/kg bw in mice. Clinical signs of reaction to treatment
included dyspnoea, staggering, piloerection, and poor general state
(Kirsch, 1986a,b).
Table 1. Acute toxicity of vinclozolin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse Oral > 15 000 92.8 Shirasu et al. (1978a)
Mouse Intraperitoneal 1570-1640 92.8 Shirasu et al. (1978a)
Mouse Subcutaneous > 15 000 92.8 Shirasu et al. (1978a)
Rat Oral > 15 000 92.8 Shirasu et al. (1978b)
Rat Intraperitoneal 4220-8300 92.8 Shirasu et al. (1978a)
Rat Dermal > 5000 92.8 Shirasu et al. (1978b)
Rat Inhalation > 29.1 NR Leuschner (1979)
Guinea-pig Oral 8000 90-97 Hofmann (1973a)
Guinea-pig Intraperitoneal 3000 90-97 Hofmann (1973b)
Rabbit Oral > 5620 98.1 Gelbke & Kirsch (1981)
Dog Oral > 10 000 97 Gelbke & Kirsch (1979)
NR, not reported
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing vinclozolin at doses of 0, 100, 1000, 2500, or 5000 ppm for
three months. Food consumption and body weight were determined weekly;
clinical signs were checked daily, with a weekly comprehensive
clinical examination. At the end of the study, clinicochemical and
haematological examinations were performed, and all animals were
assessed grossly and histopathologically. Reduced body-weight gain was
seen in males at the high dose at the end of the study. Clinico-
chemical parameters were affected at doses > 1000 ppm and
haematological parameters only at the highest dose. Decreased
triglyceride and cholesterol levels were seen in animals at 1000 ppm,
and decreased glucose levels in males and albumin levels in females at
the three higher doses. Increased alkaline phosphatase activity was
seen in males and increased globulins in males and females at 2500 and
5000 ppm; alanine aminotransferase activity was increased only in
males at 5000 ppm. Increases were seen in the mean corpuscular
haemoglobin value in females at the two highest doses, the mean
corpuscular volume in animals of each sex, haemoglobin in males, and
reticulocyte counts in females at the highest dose. Absolute and
relative liver weights were found to be increased at the three higher
doses. Centrilobular hypertrophy of hepatocytes was seen in males at
2500 and 5000 ppm; testicular weights were increased at these doses,
and multifocal hyperplasia in Leydig cells was noted at 1000 ppm or
more. Absolute and relative adrenal gland weights were increased in
males at 2500 and 5000 ppm, and lipogenic pigment and lipid vacuoles
in the adrenals were noted in animals of each sex at these doses. In
females at 1000 ppm, increased lipogenic pigment was seen in the
adrenals. Hyperplasia in stromal cells of the ovaries was observed at
the highest dose only. No treatment-related adverse effects were seen
at 100 ppm. The NOAEL was 100 ppm, equivalent to about 20 mg/kg bw per
day (Schilling et al., 1990a).
The B6C3F1 mouse strain has a relatively high frequency of
spontaneous liver lesions, including tumours. In the three-month study
with this strain, clear increases in liver weight and hepatocellular
hypertrophy were observed, indicating that the liver is one of the
target organs of vinclozolin. In order to assess more accurately its
potential proliferative effect on mouse liver, the toxicity of
vinclozolin was investigated in C57B1 mice, which have a low
background incidence of spontaneous liver neoplasia. Groups of 10 male
and 10 female mice were fed diets containing vinclozolin at doses of
0, 100, 1000, or 5000 ppm. Food consumption and body weight were
determined weekly, and clinical signs were checked daily, with a
weekly comprehensive clinical examination. At the end of the study,
clinicochemical and haematological examinations were performed. All
animals were assessed macroscopically and histologically. A reduction
in body-weight gain was seen in males at the high dose. Decreased
triglyceride and cholesterol values were seen in animals at 1000 and
5000 ppm, and glucose was decreased only in males at the high dose.
Increased alanine aminotransferase activity was seen in animals of
each sex, and total protein and alkaline phosphatase were increased in
males at the high dose. Haemoglobin, mean cell volume, and mean
corpuscular haemoglobin were increased in animals of each sex at
5000 ppm and in females at 1000 ppm; haematocrit and leukocyte and
lymphocyte counts were increased in animals of each sex and the
erythrocyte count in males at the highest dose. Increased liver
weights were seen at 1000 and 5000 ppm, and centrilobular hypertrophy
of hepatocytes was seen at 5000 ppm. Increased adrenal weights were
seen males at this dose; an increase in lipogenic pigment was noted
histologically in animals of each sex at 1000 ppm and 5000 ppm and
lipid vacuoles were seen at 5000 ppm. Hyperplasia and hypertrophy of
the stromal cells of the ovaries were seen at the middle and high
doses, and focal hyperplasia of the Leydig cells of the testis was
observed at the high dose. There were no compound-related findings at
100 ppm. The NOAEL was 100 ppm, equivalent to 25 mg/kg bw per day
(Schilling et al., 1990b).
Rats
Groups of 16 male and 16 female Sprague-Dawley rats were fed
diets containing 0,100, 300, 1000, or 2000 ppm technical-grade
vinclozolin for three months, and six rats from each group were
further maintained on control diets for six weeks after the end of the
study. Rats were examined daily for mortality, abnormal appearance or
behaviour, and food consumption. Body weights were determined weekly,
when rats were palpated and the eyes examined. Blood and urine were
sampled before treatment, after six and 12 weeks of treatment, at
termination, and at the end of the observation period. At termination,
rats were sacrificed, dissected, and examined for gross pathological
changes. The absolute and relative weights of major organs were
determined, and a complete set of tissues from each animal was saved
for future histopathological examination. The results of microscopic
examinations of tissues were not reported, except for the eyes, which
were examined in serial sections. A single death occurred, in a female
at the high dose on day 42; all other rats survived to scheduled
termination. No abnormalities of appearance or behaviour were noted,
and the eyes were normal at all examinations. The body weights of
treated rats were comparable to those of controls throughout the
study. Occasional statistically significant increases in food
consumption were seen in treated females, but that of males was not
affected. Haematological changes consistent with decreased erythrocyte
mass-decreased erythrocyte count, haematocrit, and haemoglobin and
increased mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentration-were seen at the six-week sampling time in
males and females fed doses > 300 ppm; however, these changes were
seen at termination only in females at 1000 and 2000 ppm. Clinical
chemical parameters were not altered in a toxicologically significant
manner. At necropsy, no effects of treatment on the gross appearance
of tissues were apparent. Statistically significant, dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were seen in males and females at 1000 and 2000 ppm; at
2000 ppm, increased relative weights of kidneys were seen in males and
females and increased relative spleen weights in females. These
effects were apparently reversible, as they were not observed in
treated rats that received control diets for an additional six weeks.
Microscopic examination of the eyes revealed no treatment-related
abnormalities (Hofmann, 1974).
Groups of rats received vinclozolin at dietary levels of 0, 900,
1800, 3000, or 15 000 ppm for four weeks. There were no clinical signs
of toxicity, and food intake and body-weight gain were reduced only in
animals at the high dose. Erythrocyte parameters were reduced in all
treated female rats, and urinalysis revealed reduced osmolality at the
highest dose. Liver and adrenal weights were increased in all treated
rats, and necropsy revealed a grey-white discolouration of the
adrenals. The ascorbic acid content of the adrenals was increased in
all treated animals, and the glycogen content of the liver was reduced
in all treated females and in males at 15 000 ppm. As progressive,
dose-related transformation of the adrenal cortex was seen in all
treated rats, there was no NOAEL (Hoffman & Munk, 1975a).
Groups of 50 male and 50 female Sprague-Dawley rats received
vinclozolin in the diet at levels of 0, 150, or 450 ppm for three
months. Clinical behaviour, food and drinking-water consumption, and
body-weight gain were not affected, and clinicochemical examinations
and urinalyses revealed no substance-induced changes. Histopatho-
logical examination of the liver, adrenals, and pituitary at the end
of treatment gave no reliable indication of substance-induced changes.
The NOAEL was > 450 ppm, equal to 44 mg/kg bw per day for males and
40 mg/kg bw per day for females (Leuschner et al., 1975).
Groups of 10 male and 10 female Wistar rats received vinclozolin
in the diet at levels of 0, 300, 1000, or 3000 ppm for three months.
There were no clinical signs of reaction to treatment, and no rats
died before the scheduled sacrifice. Body-weight gain and food intake
were unaffected by treatment, but water intake was increased in rats
at 3000 ppm. Ophthalmoscopic examination revealed two cataracts, one
unilateral and one bilateral, in rats receiving 3000 ppm, and other
lenticular changes were seen in all groups; it was concluded that the
ocular lesions were spontaneous and unrelated to treatment. Evidence
of anaemia and decreased serum alkaline phosphatase activity were seen
in animals at the high dose, with decreases in leukocyte counts at
1000 and 3000 ppm. Examination post mortem revealed white, enlarged
adrenals in all treated rats. The weights of the liver, adrenal, and
testis were increased at 1000 and 3000 ppm, and histopathological
examination revealed hypertrophy of the adrenal cortex, cystoid
degeneration in the pituitary, Leydig-cell hyperplasia, cloudy
swelling of hepatocytes, single live cell necrosis, and vacuolization
of luteal cells in the ovaries at these doses. Acinar vacuolization in
the pancreas was seen in all treated rats. Vinclozolin was thus toxic
at all doses; the lowest dietary level of 300 ppm is equal to 22 mg/kg
bw per day (Mellert et al., 1993a).
In order to define a clear no-effect level, groups of 10 male and
10 female Wistar rats received vinclozolin in the diet at levels of 0
or 50 ppm for three months, and investigations similar to those in the
previous study were performed. In particular, laboratory investi-
gations and ophthalmoscopic examinations were carried out, and all
animals underwent detailed pathological examination. No treatment-
related changes were seen. The NOAEL was 50 ppm, equal to 4 mg/kg bw
per day (Mellert et al., 1993b).
Rabbits
Groups of six New Zealand white rabbits received dermal
applications of vinclozolin for 8 h per day for three weeks at doses
of 0, 111, 333, or 1000 mg/kg bw. There were no deaths and no clinical
signs of reaction to treatment; gross pathology and histopathology
revealed no evidence of dermal irritation (Leuschner et al., 1977a).
Dogs
Groups of four beagle dogs of each sex were fed diets containing
vinclozolin at doses of 0, 100, 300, 1000, or 2000 ppm for three
months. There were no signs of toxicity and no compound-induced
deaths. Repeated ophthalmological examinations revealed no effects.
The body-weight gain of treated animals was not affected, and
urinalysis showed no treatment-related findings. Females at 2000 ppm
had a reduced haemoglobin content after four and eight weeks and a
decreased erythrocyte count throughout the study. An increased
platelet count was seen in female animals at 1000 and 2000 ppm.
Howell-Jolly bodies were found in a differential blood count in males
and females at these doses, suggesting a compensatory reaction
elicited by an anaemic process. The relative liver weights and the
relative and absolute adrenal weights were increased in females at the
highest dose. Histopathological assessment revealed compound-induced
cholestasis of the liver. In a further evaluation, there was a slight
to moderate, dose-related increase in the haemosiderin content of the
liver, particularly in females, at 300, 1000, and 2000 ppm. Females
had a higher pigment content than males, and the spleens of females
also had an increased haemosiderin content. It was assumed that the
haemosiderosis in the liver was caused by increased haemolysis, in
contrast to the original interpretation that the pigment deposits in
the liver were due to cholestasis. The NOAEL was 100 ppm, on the basis
of the increased haemosiderin content of the liver (Hoffman & Munk,
1975b).
Pairs of one male and one female beagle dog were given technical-
grade vinclozolin at doses of 0, 1000, or 2000 ppm for three months.
There were no compound-induced deaths or other signs of toxicity
during treatment. The animals underwent ophthalmological examinations
twice a week and were additionally examined by an eye specialist
towards the end of the treatment period. There was no indication of
cataract formation. The NOAEL was thus > 2000 ppm (Kirsch et al.,
1974).
Groups of six beagle dogs of each sex received diets containing
technical-grade vinclozolin at doses of 0, 100, 300, 600, or 2000 ppm
for six months. Clinicochemical, haematological, and urinalyses were
carried out at regular intervals; ophthalmoscopy was performed before
the beginning of the study, after about three months, and towards the
end of treatment. At the end of the study, all animals were examined
grossly and histopathologically. No deaths occurred, food consumption
and body-weight gain were unchanged in comparison with the control
group, and there were no clinical signs of toxicity. Males at the
highest dose had pronounced, and females slight, haemolytic anaemia.
Increased incidences of Howell-Jolly bodies and reticulocytes in males
suggested a compensatory bone-marrow reaction to the compound-induced
loss of blood cells. Slight increases in bilirubin level in males at
600 and 2000 ppm, in the haemoglobin concentration of individual
erythrocytes, and in lactic dehydrogenase activity in males at
2000 ppm were further consequences of increased decomposition of
erythrocytes. The considerably increased platelet counts in males at
2000 ppm and the slight increase in females at 600 and 2000 ppm are
probably due to the anaemic process, since hyper-regenerative anaemia
is frequently accompanied by thrombocytosis. The absolute and relative
adrenal weights showed a dose-related increase in groups treated with
2000, 600, or 300 ppm, and histopathological examination showed
vacuolization of the zona fasciculata and severe lipid incorporation
and birefringence of the adrenal cortex (in females) at the highest
dose. Increased absolute and relative spleen weights were observed at
2000 ppm; at 600 ppm, only the absolute spleen weight was increased in
females. Histopathological examination revealed dilated splenic
sinuses and hyperaemia in females at both doses. Reduced relative
weights of the kidney (at 2000 ppm) and pituitary (at 600 and
2000 ppm), haemosiderin deposits in the liver (at 600 and 2000 ppm),
and severe prostatic atrophy (at 2000 ppm) were also found.
Ophthalmological examinations revealed no compound-related findings.
The NOAEL was 100 ppm, equivalent to 4.0 mg/kg bw per day (Kirsch
et al., 1982).
Groups of six beagle dogs of each sex were fed diets containing
0, 35, 75, 150, or 1500 ppm vinclozolin for 12 months. There were no
deaths and no clinical signs of toxicity. Food consumption and
body-weight gains were not significantly affected by treatment.
Haematological, clinical chemical, and urinalyses were conducted
before treatment and in weeks 13, 26, and 52. At the highest dose,
reticulocyte counts were increased in animals of each sex, platelet
counts were increased in males, the mean cell volume was increased in
females, and total bilirubin concentrations were increased in animals
of each sex. Adrenal weights were increased at 1500 ppm and slightly
increased at 150 ppm in animals of each sex; the adrenals were
enlarged in all males and in five females at 1500 ppm, and the mean
width of the adrenal cortex was increased in animals of each sex at
1500 ppm and, to a lesser degree, in females at lower doses. No
dose-response relationship was identified. Progressive transformation
and increased lipid content were seen in the adrenals of all males and
five females at 1500 ppm. Testicular weights were increased in a
dose-related fashion; at 1500 ppm, the weights were markedly
increased, and at 150 ppm the relative weights were significantly
increased. At 1500 ppm, diffuse hyperplasia of the Leydig cells was
observed in five males. The prostates of one male at 150 ppm and two
at 1500 ppm appeared smaller macroscopically. Histopathological
examination revealed that the prostates of two males at 150 ppm were
slightly or moderately atrophied and those of five males at 1500 ppm
were slightly (one) or severely (four) atrophied; similar but minimal
effects were seen in one male at 0, one at 35, and one at 75 ppm.
Liver weights were increased in animals at doses up to 150 ppm and
were markedly increased in males at 1500 ppm. Increased haemosiderin
deposition was observed in the livers of males at 1500 ppm and of
females at 150 and 1500 ppm. Spleen and thyroid weights were increased
in males at 1500 ppm, and spleen weights were also slightly increased
in females at 1500 ppm. The NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological effects at 150 and 1.500 ppm
(Hellwig et al., 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were fed diets
containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 112 weeks.
The results of analyses of the diets were not reported. There were no
clinical signs of reaction to treatment and no effect on food intake.
The weight gain of males at 1460 or 4370 ppm was reduced, and the
survival of males at the highest dose was adversely affected; no such
changes were seen in females. The weights of the liver and testis were
increased at the highest dose, and liver weight was increased in
females at 1460 ppm. Histopathological examination revealed no
treatment-related changes, and no tumours were seen (Leuschner
et al., 1977b).
Groups of 100 male and 100 female control C57Bl/6/JICO mice and
60 male and 60 female treated animals received vinclozolin in the diet
at levels of 0, 15, 150, 3000, or 8000 ppm for 18 months. Ten animals
of each sex were taken from each group for interim sacrifice after 12
months of treatment. There were no clinical signs. The mortality rates
of males and females at the highest dose were greater than those of
controls, and weight gain and food intake were reduced in animals at
3000 and 8000 ppm. Examination of blood smears revealed an increased
polymorphonuclear neutrophil count and decreased lymphocyte count in
animals of each sex at 8000 ppm. Pathological examinations revealed
similar findings at the interim and terminal kills. Increased liver
and adrenal weights were seen in animals at 3000 and 8000 ppm, with
smaller epididymides, seminal vesicles, and prostate. Focal necrosis,
bile-duct proliferation, and pigment deposition were seen in the
livers of animals at 3000 or 8000 ppm. At 8000 ppm, these changes were
accompanied by diffuse hepatocyte hypertrophy, decreased lipid
storage, and increased focal fatty infiltration in the liver, hepatic
single-cell necrosis, an increased prevalence of biliary cysts in the
livers of males, focal cellular alterations and focal hyperplasia of
the livers of males and females, hepatocellular carcinomas in three
males and 22 females, and hepatocellular adenomas in three females. In
males at 3000 and 8000 ppm, diffuse Leydig-cell hyperplasia of the
testis was seen, with atrophy of the seminal vesicles and coagulation
glands. Females at these doses had atrophic uteri, accompanied in
animals at the highest dose by diffuse stromal hyperplasia, an
increased incidence of pigmented interstitial cells in the ovaries,
and loss of ovarian follicles. The adrenal cortexes of animals at 3000
or 8000 ppm had increased lipogenic pigment in the cortico-medullary
region and lipidosis. In addition, in animals at 8000 ppm, foam cells,
eosinophilic crystals, and pneumonitis were seen in the lungs of males
and erosions or ulcers in the glandular stomachs of males and females.
Vinclozolin thus caused hepatocellular carcinomas at a dose of
8000 ppm, equal to 1300 mg/kg bw per day, a dose associated with clear
toxicity; there were no treatment-related tumours at 3000 ppm, equal
to 495 mg/kg bw per day, although evidence of toxicity was seen. The
NOAEL was 150 ppm, equal to 24 mg/kg bw per day (Mellert et al.,
1994a).
Rats
Groups of 50 male and 50 female Sprague-Dawley rats were fed
diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 130
weeks; the study was terminated when survival in the control group
reached 70%. The results of analyses of the diets were not reported.
There were no clinical signs of reaction to treatment. Dose-related
reductions in food intake and weight gain were seen in animals at 1460
and 4370 ppm, but their survival was better than that of controls.
Clinical chemical and histological investigations showed no reaction
to treatment. Interpretation of the data on organ weights was hampered
by disparities in body weight between groups. No tumours were observed
(Leuschner et al., 1977c).
Groups of 20 male and 20 female Wistar rats received vinclozolin
in the diet at levels of 0, 150, 500, 1500, or 4500 ppm for 24 months.
The only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in all treated males and
cataracts in all treated rats. Although the mortality rate of females
at the high dose was higher than that in concurrent controls, the
increase was marginal and may have been unrelated to treatment. Weight
gain and food intake were adversely affected in animals at 1500 and
4500 ppm. Water intake was increased in males at 4500 ppm and
decreased in females at this dose and in males and females at
1500 ppm. Ophthalmoscopic examination revealed a treatment-related
incidence of cataracts. Bilateral cataracts were present in all
animals at 1500 and 4500 ppm that survived to termination, and
cataracts were also seen at 500 ppm. The ophthalmoscopic changes at
150 ppm were confined to lenticular degeneration and calcification in
a few animals. Animals at 1500 and 4500 ppm showed evidence of
anaemia, decreased serum alanine aminotransferase and alkaline
phosphatase activities, and increased creatinine, total protein,
cholesterol, and gamma-glutamyl transferase activity. Increased liver
and adrenal weights were seen in animals at 4500 ppm and increased
testicular weights in all treated males. Leydig-cell tumours were seen
in almost all animals treated with 500, 1500, or 4500 ppm and were
increased in incidence in rats at 150 ppm in comparison with controls.
Focal hyperplasia and cystic ducts were seen in the rete testis of
rats at 4500 ppm, and two males at this dose but no controls had rete
testicular adenomas. Cystic ducts in the rete testis were also seen in
animals at 1500 ppm. Atrophy of the seminal vesicles, coagulating
gland, and epididymides were seen in almost all animals at 1500 and
4500 ppm, similar, less marked effects being seen in animals at 150
and 500 ppm. Dose-related reduced secretion and increased fibrosis in
the prostate were also seen in all treated males. Benign stromal
tumours of the sex cord in the ovaries were seen in 10 rats treated
with 4500 ppm, four at 1500 ppm, and two at 500 ppm; none were seen in
animals at 150 ppm or in controls. Five adenomas and one metastatic
carcinoma of the adrenal cortex were seen in females at 4500 ppm and
one adenocarcinoma of the adrenal cortex in a female at 1500 ppm; no
adrenal tumours were seen at 150 or 500 ppm or in controls. A
dose-related incidence of lipidosis in the adrenal cortex was seen in
all treated animals. Hepatocellular carcinomas occurred in nine male
rats at 4500 ppm, with none in controls. Although hepatic tumours were
found only in animals at the high dose, hepatic necrosis, hypertrophy,
and eosinophilic foci were seen in a dose-related fashion at doses
down to 150 ppm; the only changes in animals at the low dose were
eosinophilic foci in one female. Vacuolation of the pancreatic
exocrine cells was seen in all treated groups. Treatment-related
changes were also seen in the pituitary in animals at 1500 and
4500 ppm, consisting of a reduction in focal hyperplasia and an
increase in diffuse hyperplasia in males and a reduction in the
incidence of pituitary adenomas in females. Vinclozolin was toxic at
all doses tested. The lowest dietary level of 150 ppm was equal to
8 mg/kg bw per day (Mellert et al., 1994b).
In order to define a clear no-effect level, groups of 20 male and
20 female Wistar rats received vinclozolin in the diet at levels of 0,
25, or 50 ppm for 24 months, with investigations similar to those
performed in the previous study. In particular, laboratory
investigations and ophthalmoscopic examinations were carried out every
three months, and all animals underwent detailed pathological
examination. No treatment-related changes were seen. The NOAEL was
50 ppm, equal to 2.8 mg/kg bw per day (Mellert et al., 1993b).
Groups of 50 male and 50 female Wistar rats received vinclozolin
in the diet at levels of 0, 50, 500, or 3000 ppm for 24 months. The
only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in males at 500 and 3000 ppm
and cataracts in all treated rats. Mortality was not affected. Weight
gain and food intake were decreased in animals at 3000 ppm. Clinical
examination showed an increased incidence of cataracts in animals at
500 and 3000 ppm, but ophthalmoscopy of control animals and those at
the low dose revealed an increased incidence of lenticular
degeneration and one case of lenticular calcification in animals at 50
ppm. Animals at 3000 ppm had increased liver, adrenal, and testicular
weights, and histopathological examination revealed Leydig-cell
tumours in almost all males at 500 or 3000 ppm. Focal hyperplasia and
cystic ducts in the rete testis were seen in males at 3000 ppm, and an
adenoma in the rete testis was seen in one treated and no control
males. Atrophy of the seminal vesicles and coagulating gland was seen
in almost all males at 3000 ppm and in some at 500 ppm. Dose-related
reduced secretion and increased fibrosis in the prostate were seen in
all treated groups. Benign stromal tumours of the sex cord in the
ovaries were seen in 29 rats at 3000 ppm and four controls. Two
animals at the high dose had malignant thecomas of the ovary, with
none in controls. Lipidosis of ovarian interstitial cells and an
increased incidence of abnormal ovarian follicles were seen in all
treated groups. Seven animals at the high dose had adenocarcinomas of
the uterus (none in controls), and three of these had metastases.
Adenomas of the adrenal gland were seen in 21 females at 3000 ppm, and
one had a metastatic carcinoma of the adrenal cortex; there was no
increase in the incidence of adrenal tumours in animals at 50 or
500 ppm. A dose-related incidence of lipidosis and focal hyperplasia
in the adrenal cortex was seen in all treated groups, and hepatic
hypertrophy and eosinophilic foci were seen in a dose-related fashion
at doses down to 50 ppm. Vacuolation of the pancreatic exocrine cells
was seen in rats at 500 or 3000 ppm. At 3000 ppm, males had an
increased incidence of focal hyperplasia in the pituitary, and females
had a reduced incidence of pituitary adenomas. Thus, increased
incidences of tumours were seen in animals of each sex at 3000 ppm and
in males at 500 ppm. The NOAEL was 50 ppm, equal to 2.7 mg/kg bw per
day, for tumour formation and < 50 ppm for overall toxicity
(Mellert et al., 1994d).
(d) Reproductive toxicity
In a three-generation study, groups of 20 Sprague-Dawley rats of
each sex were fed diets containing vinclozolin at 0, 162, 486, or
1458 ppm. Two litters were produced per generation, and the second
litters were used as parental animals (F0, F1, and F2). The
period of treatment before mating was about eight weeks for F0
males, 16 weeks for F0 females, 15 weeks for F1 and F2 males,
and 24 weeks for F1 and F2 females (including lactation). No
treatment-related deaths or clinical signs of toxicity were seen in
pups or parents, and there were no treatment-related effects on food
consumption, body weights, litter size, malformations, birth weight of
pups, sex ratio, or behaviour. In parents, there were no treatment-
related effects on fertility, pregnancy rate, duration of pregnancy,
lactation, or viability. Auditory acuity and ophthalmic parameters
were not affected by treatment. There were no treatment-related
effects on organ weights or gross or microscopic appearance. The
no-effect level was thus > 1458 ppm, equivalent to about 73 mg/kg bw
per day. The results are not in accordance with more recent work, but
this study did not include investigations of anogenital distance in
males, and diets were not analysed for vinclozolin (Leuschner, 1977).
Groups of 24 Wistar rats of each sex were fed diets containing 0,
50, 300, 1000, or 3000 ppm vinclozolin. After a pre-mating period of
10 weeks, these F0 animals were mated twice to produce F1a and
F1b pups. F1a animals were used as F1 parents to produce F2a
and F2b animals. Some rats were raised to adulthood in order to
observe the development of their sexual organs, resulting in an FX
group of F1b rats, an FY group of F2a rats, and an FZ group of
F2b rats. The results of the study are summarized in Table 2. All
F1 males and females at 300 ppm were fertile either at the F2b
mating or at further matings for those animals that did not prove
their fertility in at least one of the scheduled matings for the
F2a/F2b litters. The authors considered that 'clear adverse
effects on fertility were noted only at 1000 and 3000 ppm, whereas 300
and 50 ppm were without any adverse effects on male and female
fertility in both parental generations.' It must be noted, however,
that rats are particularly fertile, and the effects observed,
particularly at 300 ppm, may indicate sub-fertility. The Meeting
concluded that the dietary level of 50 ppm, equivalent to 4.5 mg/kg bw
per day, is a marginal-effect level, on the basis of a possible
treatment-related reduction in the fertility of F1 males at 300 ppm,
signs of delayed development at 300 ppm (reduction in numbers of pups
with pinna unfolding and eye opening at the expected time), and
reduced epididymal weight in F2 animals at 50 ppm (Hellwig et al.,
1990a).
Groups of 25 male and 25 female Wistar rats were fed diets
containing vinclozolin at doses of 0, 20, or 40 ppm. After a 70-day
premating period, these F0 parents were mated to produce F1a and
F1b animals, and F1a rats were mated to produce F2a and F2b
animals. Randomly selected F1b, F2a, and F2b pups were
additionally raised to adulthood, resulting in FX, FY, and FZ groups,
respectively. No effect on clinical signs, weight, food intake, or
reproduction was observed, and gross pathological examination and
organ weight analysis also revealed no reaction to treatment. In
particular, there was no treatment-related effect on epididymal weight
in FX, FY, or FZ animals. The NOAEL was thus 40 ppm, equal to
approximately 4 mg/kg bw per day (Hellwig et al., 1994).
Vinclozolin was administered to groups of pregnant rats by gavage
at doses of 0, 100, or 200 mg/kg bw per day from day 14 of gestation
to postnatal day 3. Male pups in both groups of offspring displayed
feminine characteristics and their reproductive capacity was adversely
affected. About 25% of the treated males died during the study as a
result of bladder stones, hydroureter, or hydronephrosis. Malformations
noted at necropsy, when the males were approximately one year of age,
included the presence of a vaginal pouch, suprainguinal ectopic scrotum
or testes, cleft phallus with hypospadias, and small to absent
accessory sex glands. The authors proposed that vinclozolin is an
androgen receptor antagonist (Gray et al., 1994a).
In a study reported only in brief summary form, pregnant rats
were treated with 0, 3.12, 6.25, 12.5, 25, 50, or 100 mg/kg bw per day
vinclozolin from day 14 of gestation to postnatal day 3. Fertility was
adversely affected at 50 and 100 mg/kg bw per day, and subtle changes
in anogenital distance were observed at all doses (Gray et al.,
1994b).
(e) Developmental toxicity
Mice
Female NMRI albino mice were fed diets containing vinclozolin on
days 1-19 of gestation in two tests. In the first test, 24 mice
received 0 and 28 received 60 000 ppm vinclozolin; in the second,
groups of 30 mice received 0, 600, or 6000 ppm. All animals were
killed on day 19 of gestation. Females at 6000 ppm did not gain weight
during gestation and had decreased food consumption during the first
six days of treatment. Females at 60 000 ppm lost weight and had
decreased food consumption. One female at 6000 ppm died on day 11, and
all females treated at 60 000 ppm died within the first nine days.
Clinical signs of toxicity were observed only in mice at 60 000 ppm
and included ruffled coats and, before death, apathy and signs of
pronounced diuresis. Gross pathology revealed emaciation, atrophy of
musculature, and considerable loss of perirenal fatty tissue in
Table 2. Results of a multigeneration study in Wistar rats fed diets
containing vinclozolin
Dietary level Generation Finding
(ppm)
General toxicity
50 --
300 F0 increased relative liver weight,
marginal signs of anaemia (females),
lenticular degeneration
F1 As F0, plus increased adrenal
weight, Leydig-cell hyperplasia
F2 As F1
1000 F0 Reduced food intake and weight gain,
anaemia (females), increased liver,
adrenal, and testicular weights,
lenticular degeneration, hepatic
single-cell necrosis, Leydig-cell
hyperplasia
F1 As F0, plus vacuolation of pituitary
cells, lipidosis, and hypertrophy of
adrenal cells
3000 F0 Reduced food intake and weight gain,
lenticular degeneration, anaemia
(females), increased liver, adrenal
and testicular weights, hepatic
single-cell necrosis, lipidosis and
hyperplasia of adrenal cells,
vacuolation of pituitary cells,
Leydig-cell hyperplasia
F1 As F0, pills benign Leydig-cell
tumours in some animals
Effects on reproductive performance
50 --
300 F0 --
F1 Possible reduction in fertility in
males (all males eventually proved
fertile)
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
1000 F0 --
F1 Infertility of all males due to
feminization of outer genital organs
3000 F0 Increased total litter loss,
decreased number of delivered pups,
infertility of males
F1 Infertility, of all males
(feminization), infertility of six
females
Signs of developmental toxicity
50 F1 --
F2 Reduced epididymal weight (no
morphological changes)
300 F1 Slight functional reduction of
prostate and coagulating gland,
reduced epididymal
weight
F2 As F1, plus slight interstitial-cell
hyperplasia in testes and ovaries,
some pups with reduced morphological
development (delayed eye opening and
pinna unfolding)
1000 F1 Decreased pup survival, feminization
of males, reduced body-weight gain,
slightly delayed pup development,
reduced size and function of
secondary male genital organs,
atrophy of seminiferous tubules,
interstitial-cell hyperplasia in
testes and ovaries
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
3000 F1 Increased number of stillborn pups,
decreased pup survival, feminization
of males, reduced body-weight gain,
retarded morphological development,
atrophy of primary and secondary
male genital organs,
interstitial-cell hyperplasia in
testes and ovaries
From Hellwig et al. (1990a)
animals that died. No implantation sites were detected in any female
at 6000 or 60 000 ppm, and hence no fetuses were observed at these
doses. Fetuses of dams at 600 ppm had no treatment-related adverse
effects. The NOAEL for maternal toxicity and fetotoxicity was 600 ppm,
equivalent to 90 mg/kg bw per day (Hofman & Peh, 1975a).
Rats
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 15, 50, or 150 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
effects, and no adverse findings were noted at gross necropsy. There
were no treatment-related effects on pre- or post-implantation losses,
resorptions, numbers of live fetuses, or fetal or placental weights.
Anogenital distances were decreased in male fetuses at 150 and, to a
lesser extent, 50 mg/kg bw per day. This effect was considered to
indicate the beginning of feminization of male fetuses, perhaps due to
a hormonal (anti-androgenic) action on sexual differentiation. There
were no other treatment-related effects on male or female fetuses. As
no maternal toxicity, embryotoxicity, or fetotoxicity was observed,
the NOAEL was > 150 mg/kg bw per day. The NOAEL for teratogenicity
was 15 mg/kg bw per day, on the basis of reductions in anogenital
distance at 50 and 150 mg/kg bw per day (Hellwig et al., 1989a).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 50, 100, or 200 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, or
placental or fetal weights. The only possibly treatment-related
morphological effect observed in the fetuses was a significantly
increased incidence of symmetrically dumb-bell-shaped thoracic
vertebral bodies at 200 mg/kg bw per day, which is indicative of
retarded development. A slight, not statistically significant increase
was observed at 100 mg/kg bw per day. Similar effects were observed in
a follow-up study. The NOAEL for maternal toxicity was > 200 mg/kg bw
per day, and that for fetotoxicity was 100 mg/kg bw per day, on the
basis of a possible treatment-related increase in symmetrically
dumb-bell-shaped thoracic vertebral bodies at 200 mg/kg bw per day.
There was no NOAEL for teratogenicity, as the anogenital distance in
fetuses was not measured (Hellwig et al., 1989b).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 200, or 400 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at gross
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, fetal
or placental weights, or sex ratio. The anogenital distances of male
fetuses were decreased in a dose-related fashion at both 200 and
400 mg/kg bw per day, indicating the beginning of feminization. A
treatment-related increase in dilated renal pelvis and hydroureter was
also observed in fetuses of animals of each sex, which were
statistically significant in fetuses at 400 mg/kg bw per day. These
findings are considered to indicate a transient developmental delay.
There was also a significant increase in the number of fetuses with
symmetrical dumb-bell-shaped thoracic vertebral bodies at 200 and
400 mg/kg bw per day, indicative of retarded development. At 400 mg/kg
bw per day, the numbers of fetuses with accessory 14th ribs were also
significantly increased. The NOAEL for maternal toxicity was >
400 mg/kg bw per day, and that for fetotoxicity was < 200 mg/kg bw
per day, on the basis of the increased incidences of symmetrical
dumb-bell-shaped thoracic vertebral bodies at 200 and 400 mg/kg bw per
day in animals of each sex. The NOAEL for teratogenicity was <
200 mg/kg bw per day, on the basis of reductions in anogenital
distance in male fetuses at 200 and 400 mg/kg bw per day (Hellwig
et al., 1989c).
Groups of 10 female Wistar rats were treated orally with
vinclozolin at doses of 0, 600, or 1000 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths and no
abortions. Clinical signs of toxicity included unsteady gait in one
female at 600 mg/kg bw per day and in seven females at 1000 mg/kg bw
per day, and piloerection in two females at 1000 mg/kg bw per day.
Food consumption was reduced in animals at 1000 mg/kg bw per day on
days 6-13 of gestation, and the water consumption of animals at 600
and 1000 mg/kg bw per day was increased. The body-weight gain of
females at 1000 mg/kg bw per day was impaired on days 8-10.
Haematological investigations performed at day 20 revealed no
treatment-related findings. Liver and adrenal weights were increased
in a dose-related fashion. There were no treatment-related effects on
pre- or post-implantation losses, resorptions, numbers of live
fetuses, or placental weights. Fetal weights were decreased at
1000 mg/kg bw per day. When the sex ratio was determined by measuring
the anogenital distance, 68% of fetuses at 600 and 99% at 1000 mg/kg
bw per day were deemed to be female; however, internal examination
showed that 39% at 600 and 52% at 1000 mg/kg bw per day were female.
The anogenital distances were decreased in treated male fetuses. At
1000 mg/kg bw per day, the incidences of symmetrical dumb-bell-shaped
thoracic vertebral bodies were increased. The NOAEL for maternal
toxicity was < 600 mg/kg bw per day, on the basis of increased liver
and adrenal weights and findings at necropsy at 600 and 1000 mg/kg bw
per day. The NOAEL for fetotoxicity was < 600 mg/kg bw per day, on
the basis of increased incidences of hydroureter at 600 and 1000 mg/kg
bw per day. The NOAEL for teratogenicity was < 600 mg/kg bw per day,
on the basis of reductions in anogenital distance in male fetuses at
600 and 1000 mg/kg bw per day (Hellwig et al., 1989d).
In a range finding study, groups of 10 female Wistar rats
received dermal applications of vinclozolin at doses of 0, 300, 900,
or 2500 mg/kg bw per day for 6 h/day on days 6-19 of gestation. The
substance was applied onto the dorsal area of the trunk under an
occlusive dressing, and the animals were killed on day 20. Adrenal and
liver weights were increased, but not in a dose-related fashion, in
all treated animals. The anogenital distances were reduced in male
fetuses but the effect was not dose-related. The incidences of dilated
renal pelvis in fetuses were increased in a dose-related fashion at
all doses, with statistical significance at 900 and 2500 mg/kg bw per
day. The absence of a dose-response relationship for some of the
effects observed in this study is probably attributable to limited
dermal absorption of the rather pasty suspension of vinclozolin at
2500 mg/kg bw per day.
In the main study, groups of female Wistar rats received dermal
applications of 0, 60, 180, or 360 mg/kg bw per day for 6 h/day on
days 6-19 of gestation. The substance was applied on the dorsal area
of the trunk under an occlusive dressing, and the animals were killed
on day 20. There were no deaths, no abortions, no signs of toxicity,
no treatment-related effects on body weight or food consumption, and
no treatment-related findings in haematological investigations
performed on day 20. Absolute liver weights were increased at 180 and
360 mg/kg bw per day; absolute adrenal weights were significantly
increased at these doses but not dose-dependently. There were no
treatment-related macroscopic pathological findings or effects on
implantation losses, resorptions, numbers of fetuses, sex ratio, or
weights of fetuses or placentae. The anogenital distances of males at
180 and 360 mg/kg bw per day were decreased, but there were no other
treatment-related malformations, variations, or retardations in
treated fetuses. The NOAEL for maternal toxicity was 60 mg/kg bw per
day, on the basis of increased absolute adrenal and liver weights at
180 and 360 mg/kg bw per day. The NOAEL for fetotoxicity was >
360 mg/kg bw per day, and that for teratogenicity was 60 mg/kg bw per
day, on the basis of reductions in anogenital distances in male
fetuses at 180 and 360 mg/kg bw per day (Hellwig et al., 1990b).
Rabbits
Groups of 15 New Zealand white female rabbits were treated orally
with vinclozolin at doses of 0, 20, 80, or 300 mg/kg bw per day on
days 6-18 of gestation and were killed on day 29. There were no signs
of toxicity and no deaths that were obviously attributable to
treatment. Body-weight gain was not affected, and there were no
treatment-related effects on numbers of live young, sex ratio,
embryonic deaths, pre-implantation losses, or litter or fetal weights.
The frequency of post-implantation losses was slightly increased but
was within historical control values. There were no treatment-related
major malformations or visceral anomalies. There was a slight,
non-dose-dependent increase in the incidence of minor skeletal
anomalies, which was within historical control values. The NOAEL for
maternal toxicity and fetotoxicity was > 300 mg/kg bw per day (Cozens
et al., 1981; Cozens & Palmer, 1987).
Groups of 15 female Himalayan rabbits were treated orally with
vinclozolin at 0, 50, 200, or 800 mg/kg bw per day on days 7-28 of
gestation and were killed on day 29. One female at 200 mg/kg bw per
day aborted on day 26 and was killed; at 800 mg/kg bw per day, one
female died, one aborted and died, and 11 aborted and were killed. The
abortions occurred between days 21 and 27. Clinical signs of toxicity
included reddish-brown discolouration of the urine in 13 females and
apathy, hunched posture, conjunctivitis, and urine-smeared fur in one
or two animals at 800 mg/kg bw per day. The female at 200 mg/kg bw per
day which aborted had vaginal haemorrhage and discoloured urine. A
second female at this dose had vaginal haemorrhage on day 24. The food
consumption of animals at 800 mg/kg bw per day was significantly
reduced during the treatment period, and that of animals at 200 mg/kg
bw per day was reduced mainly on days 7-19. Animals at 50 and
200 mg/kg bw per day had no treatment-related increases in pre- or
post-implantation losses. The only dam at 800 mg/kg bw per day with
viable fetuses had three post-implantation losses, as early
resorptions. There were no treatment-related resorptions in animals at
50 or 200 mg/kg bw per day. The sex ratios, numbers of live fetuses,
and placental and fetal weights were not affected by treatment at 50
or 200 mg/kg bw per day. There were no treatment-related external
malformations or visceral abnormalities. At 800 mg/kg bw per day, one
of four viable fetuses had malformations of the sternebrae, but the
relevance of this finding is questionable owing to the small number of
fetuses. One of 94 fetuses at 50 mg/kg bw per day also had
malformations of the sternebrae, but the incidence was within the
historical control range of 0-1% (mean, 0.2%). No treatment-related
changes in the male fetal genital organs were observed. The NOAEL for
maternal toxicity was 50 mg/kg bw per day, on the basis of reduced
food consumption, abortion, and clinical signs of toxicity at
200 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity was
200 mg/kg bw per day, on the basis of three resorptions as a
consequence of maternal toxicity in the female alive at termination in
the high-dose group. There was no evidence of teratogenicity at 50 or
200 mg/kg bw per day (Hellwig et al., 1990c).
Groups of 20 female Himalayan rabbits were treated orally with
vinclozolin at 0 or 400 mg/kg bw per day on days 7-8 of gestation and
were killed on day 29. One female at 400 mg/kg bw per day aborted and
died on day 19, and nine aborted and were killed between days 20 and
27. Clinical signs of toxicity included blood in the bedding of two
treated females, one of which aborted. Eight had red-brown urine. The
food consumption of treated animals was reduced on days 7-26, and
body-weight gain was impaired on days 7-25. Pre- and post-implantation
losses were increased, and the number of early resorptions was thus
also increased. The numbers of live fetuses per litter were slightly
decreased, and the ratio of males:females was 1:1.8 at 400 mg/kg bw
per day in comparison with 1:1.16 in the controls; however, no
treatment-related changes were seen in male fetal genital organs.
Fetal weights were increased in the treated animals, probably as a
consequence of the slightly decreased litter sizes. There were no
external or soft-tissue malformations, but the frequency of separated
origins of the carotids was 36% in the treated group in comparison
with 10% in concurrent controls and a mean of 19% in historical
controls (range, 10-31%). There were no skeletal malformations and no
treatment-related skeletal variations or retardations; in particular,
no malformed sternebrae were observed, indicating that the effect
observed in the previous study at 50 mg/kg bw per day was not
treatment-related. The NOAEL for maternal, embryo-, and fetotoxicity
was < 400 mg/kg bw per day. There was no clear evidence of
teratogenicity, but the increased incidence of separated origins of
the carotids indicates potential teratogenicity at this severely
maternally toxic dose (Hellwig et al., 1990d).
(f) Genotoxicity
The results of standard regulatory assays for genotoxicity are
summarized in Table 3.
A medium-term bioassay based on preneoplastic glutathione
S-transferase placental form (GST-P)-positive foci in rat liver was
performed with vinclozolin. Rats were injected with N-nitrosodi-
ethylamine and two weeks later were fed a diet containing 2000 ppm
vinclozolin for six weeks, with partial hepatectomy at week 3; they
were then killed. In one group that received only vinclozolin,
negative results were obtained, but administration after initiation
with N-nitrosodiethylamine gave positive results. The authors
reported that the test is highly predictive for genotoxic hepato-
carcinogens but less predictive for carcinogens that have target
organs other than the liver. They suggested that since the assay is
based on the two-stage hypothesis of carcinogenesis, chemicals for
which the results are positive are tumour promoters in rat liver (Ito
& Hasegawa, 1992; Ito et al., 1993, 1994).
Vinclozolin was cytotoxic in BALB/c3T3 cells in the absence but
not in the presence of an exogenous metabolic system. It induced
transformation in this cell line in both the presence and the absence
of metabolic activation (Perocco et al., 1993).
As shown in Table 3, negative results were obtained in a range of
assays in vivo and in vitro. Although positive results were
obtained in the medium-term bioassay and in the test for cell
transformation, these studies are not well validated.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The intact and abraded dorsal skin of four male and two female
white Vienna rabbits was treated with a 50% aqueous suspension of
vinclozolin under an occlusive covering for 24 h. The intact skin of
five of the six animals had well-defined erythema, and slight oedema
was seen in one animal. All of the reactions subsided completely
within 72 h. The erythema that formed on the abraded skin of all
animals after 24 h was more severe than that on the intact skin; it
had completely subsided in one-half the animals by 72 h but remained
unchanged in the other half. One animal in this group had slight
oedema after 24 h but no longer at 72 h. Vinclozolin cannot be
classified as a skin irritant, however, a slight, transient irritation
potential was evident (Hildebrand, 1977a).
Table 3. Results of tests for the genotoxicity of vinclozolin
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S typhimurium TA98, < 1000 µg/plate 92.8 Negativea Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA98, TA100, < 3000 µg/plate 92.8 Negativea Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP hrc
Reverse mutation S. typhimurium TA98, TA100 < 10 000 µg/plate 98.1 Negativea Gelbke & Engelhardt
TA1535, TA1537, TA1538 (1983)
Gene mutation hprt L5178Y cells < 1000 µg/ml NR Negativea Witterland & Hoorn
(1984)
Gene mutation hprt Chinese hamster ovary cells < 10 mg/ml > 99.5 Negativea Gelbke & Jackh (1975)
Chromosomal aberration Chinese hamster ovary cells < 500 µg/ml NR Negativea Murli (1989)
DNA repair B. subtilis M45 and H17 rec < 2000 µg/plate 92.8 Negative Shirasu et al. (1977)
DNA repair B. subtilis M45 and H17 rec < 10 000 µg/plate NR Negativea Hoorn (1983)
Unscheduled DNA Rat hepatocytes < 1000 µg/ml > 99.5 Negative Cifone & Myhr (1984)
synthesis
In vivo
Host-mediated mutation S. typhimurium G46 his- 2 × 200 and 1000 92.8 Negative Shirasu et al. (1977)
mg/kg bw
Dominant lethal mutation NMRI male mice 5 × 2000 mg/kg bw NR Negative Hofmann & Peh
(1975b)
Sister chromatid Male and female Chinese 3830 and 5620 98.1 Negative Gelbke & Engelhardt
exchange hamsters mg/kg bw (1981)
NR, not reported
a With and without metabolic activation
Eye irritation was investigated in three male and three female
New Zealand white rabbits. After 24 h, the only finding was slight
redness of the conjunctivae, which was not completely reversible
within 72 h Vinclozolin may be regarded as not irritating to the eyes
(Hildebrand, 1977b).
Technical-grade vinclozolin was tested in groups of 12 male
Pirbright white guinea-pigs and five controls in the Magnusson-Kligman
maximization test. The treated animals had questionable dermal changes
after the first challenge and distinct changes after the second,
suggesting sensitization (Gelbke, 1979).
No dermal sensitization was observed in an open epicutaneous test
in guinea-pigs with BAS 352 04 F formulation (Ronilan), containing 50%
vinclozolin. A 60% preparation in water caused slight erythema and
oedema at the beginning of the induction period; a 20% preparation
elicited sporadic skin irritation. Since no differences were found
between the control and test groups, these concentrations are assumed
not to be sensitizing. Neither skin irritation nor sensitization was
observed with 2 or 6% preparations. These concentrations can be
assumed not to have sensitizing potential that would be of importance
under field conditions (Grundler & Gelbke, 1980).
(ii) Hormonal effects
Groups of 20 male and 20 female Wistar rats were fed diets
containing 4500 ppm vinclozolin for six months; the control group
consisted of 10 males and 10 females. After sacrifice, blood was
collected and the levels of the following hormones were determined:
adrenocorticotrophic hormone (ACTH), corticosterone, dehydroepian-
dosterone (DHEA), testosterone, estradiol, hydroxy-progesterone, and
luteinizing hormone (LH). Males showed more effects than females: in
males, the level of LH was increased by about 10-fold and those of
testosterone and DHEA doubled; in females, the level of LH was
increased by 2.5-fold, but there was no change in the concentrations
of sex steroids. That of ACTH increased in animals of each sex, but
the results were variable and the relationship of this change to
treatment is uncertain (Knuppen, 1989).
Groups of 20 male and 20 female Wistar rats were treated with 0
or 4500 ppm vinclozolin for three months, and groups of 10 of each sex
were then allowed to recover for three months to study the
reversibility of any changes. The hormones that were measured were LH,
follicle-stimulating hormone, testosterone, estradiol, DHEA, ACTH,
aldosterone, and corticosterone. The results basically confirmed those
of the previous study. The levels of LH, follicle-stimulating hormone,
testosterone, and DHEA were increased in males, LH most markedly,
while only that of LH was increased in females. The concentration of
ACTH was increased in both males and females, and those of aldosterone
and corticosterone were marginally increased in males but decreased in
females. All of these changes, except perhaps that of ACTH in females,
was reversible within two months (Mellert et al., 1992).
(iii) Receptor binding
In a study of binding to the androgen receptor, vinclozolin was
incubated in vitro with MCF-7 cells derived from a human mammary
carcinoma which contains a large amount of androgen receptor. A clear
affinity with cytosolic and nuclear androgen receptors was seen
(Knuppen, 1990). In order to confirm this result and to investigate
whether the binding is due to vinclozolin or its main rat metabolite
(Reg. No. 119 208), their effects were compared with those of the
anti-androgenic drug Flutamide and the synthetic androgen Mibolerone.
Vinclozolin bound to the androgen receptor with an affinity of
approximately 50% of that of Flutamide, and the binding affinity of
the metabolite was virtually zero (Knuppen & Schutze, 1991). In a
comparison of the effects of vinclozolin on receptors in vitro and
in vivo, not reported in detail, vinclozolin was shown to have a
binding affinity similar to that of Flutamide for androgen receptors
in the LNCAP cell line, which is derived from human prostate, a
typical target tissue for vinclozolin-mediated anti-androgenic
effects. Vinclozolin was also capable of binding to androgen receptors
in castrated Wistar rats (Knuppen & Schutze, 1992).
The ability of vinclozolin to inhibit 5-alpha-reductase and to
compete with androgen for binding at the androgen receptor was
investigated. Neither vinclozolin nor its degradation products
2-{[(3,5-dichlorophenyl)carbamoyl]oxy}-2-methyl-3-butenoic acid and
3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide inhibited 5-alpha-
reductase. Although vinclozolin competed only weakly with androgen for
binding at the androgen receptor, the two metabolites were effective
antagonists. The authors reported that the concentrations of the first
metabolite in the serum of pregnant rats after treatment with
100 mg/kg bw per day vinclozolin could be sufficient to meet or exceed
the Ki for androgen receptor inhibition in vitro (Kelce et al.,
1994).
(iv) Nephrotoxicity
The acute nephrotoxic potential of vinclozolin was compared with
that of two other N-(3,5-dichlorophenyl) carboximide fungicides:
N-(3,5-dichlorophenyl) succimide and iprodione. Groups of four male
Fischer 344 rats received a single intraperitoneal injection of 0.4 or
1.0 mmol/kg bw or vehicle, and renal function was monitored at 24 and
48 h. N-(3,5-Dichlorophenyl) succimide induced renal effects
characterized by marked diuresis, increased proteinuria, elevated
blood urea nitrogen, increased kidney weights, and proximal tubule
necrosis. Iprodione and vinclozolin caused only minor or no
alterations in renal function (Rankin et al., 1989).
(v) Haemoglobin adduct formation
Female Wistar rats were treated orally with various pesticides at
doses of up to 1 mmol/kg bw. Blood was taken 24 h after treatment,
haemoglobin was isolated and hydrolysed with 1N sodium hydroxide, and
aromatic amines extracted and quantified. No adducts with 3,5-di-
chloroaniline from vinclozolin were found (Sabbioni & Neumann, 1990).
(vi) Review of ophthalmoscopic findings
In a review of the cataracts and other lenticular changes seen at
ophthalmoscopy in short- and long-term studies with vinclozolin in
rats, an analysis was carried out to determine whether these changes
were due to acceleration of normal, age-related changes, since
lenticular changes are seen commonly in old rats and cataracts also
occur spontaneously in untreated rats. The clear NOAELs are shown in
Table 4. Vinclozolin induced cataracts and other lenticular changes
only in rats, and there were no treatment-related lenticular changes
in B6C3F1 or C57B1 mice or in dogs. In the studies of absorption and
distribution, a high concentration of radiolabel was found in the
Harderian gland, which is situated close to the eye and secretes a
fluid onto its surface. The presence of radiolabel in this secretion
was confirmed by examination of enlargements of whole-body
autoradiographs (Schilling, 1993).
3. Observations in humans
A cross-sectional study was performed of 67 men who had handled
vinclozolin for 1-13 years during its synthesis and formulation and 52
unexposed controls. The men were monitored by determining urinary
metabolites containing a 3,5-dichloraniline moiety and observation for
reversible changes in the levels of hormones of the adrenocortico-
tropic and gonadotropic feedback systems, signs of liver injury,
haemolytic anaemia, cataract formation, and hormonally induced
hyperplasia and tumours at high doses. The clinical investigation
consisted of a medical and occupational history questionnaire,
physical examination, laboratory determinations, including
measurements of testosterone, LH, and follicle-stimulating hormone,
ultrasonography of the liver and prostate, a detailed examination, and
routine spirometry. The mean 3,5-dichloraniline concentration in
exposed workers was 235-422 µg/g creatinine, depending on the work
area, in comparison with a mean of 7 µg/g creatinine in controls. On
the basis of a series of assumptions, including 40% excretion through
the kidneys, 1.5-litre urine output per day, and 70-kg body weight,
the authors estimated that the occupational exposure of two-thirds of
the employees to vinclozolin exceeded 25 µg/kg bw per day. The
physical examinations and laboratory tests provided no evidence of
vinclozolin-induced hormonal responses, liver injury, prostatic
changes, cataract formation, or haemolytic anaemia. The authors
concluded that vinclozolin induced no health effects and, in
particular, no anti-androgenic effects (Zober et al., 1995).
Table 4. NOAELs for ophthalmoscopic effects in short- and long-term
studies of the toxicity of vinclozolin in rats
Effect NOAEL (ppm)
Short-term study Long-term study
Cataracts 1000 150
Striations 300 150
Bosselated lens structure 1000 150
Bulbiform thickening 3000 150
Opacities 3000 150
Comments
Vinclozolin is well absorbed after oral administration to rats
and extensively metabolized. The majority of the administered
radiolabel was found in the bile, and no unchanged vinclozolin was
excreted in the urine. After single oral doses of radiolabelled
vinclozolin, excretion was rapid; after multiple doses there was no
significant accumulation. Vinclozolin is only moderately absorbed via
the dermal route in rats: over 72 h, about 17% of a dose of 0.13 mg/kg
bw was excreted in the urine.
Vinclozolin has low acute toxicity, with an oral LD50 in rats
of > 15 000 mg/kg bw. The clinical signs of toxicity after acute
dosing with vinclozolin were generally non-specific and there were no
consistent treatment-related findings at necropsy. Vinclozolin is not
irritating to rabbit skin or eyes, but induced skin sensitization in a
maximization study in guinea-pigs. WHO has classified vinclozolin as
unlikely to present an acute hazard in normal use.
Studies of repeated administration were carried out in mice,
rats, rabbits and dogs, in which vinclozolin and/or its metabolites
caused toxic effects indicative of anti-androgenic activity. In two
three-month feeding studies in different strains of mice at dietary
levels of 100-5000 ppm, the NOAEL was equivalent to 20 mg/kg bw per
day, on the basis of signs of hepatotoxicity, signs consistent with
anti-androgenicity, and changes in the adrenal glands. In two recent
three-month feeding studies in rats (at levels of 0, 300, 1000, and
3000 ppm and 0 and 50 ppm, respectively) vinclozolin caused changes
qualitatively similar to those seen in mice; however, effects on the
adrenal glands (including lipidosis) were seen at 300 ppm, and the
NOAEL was confirmed in the second study as 50 ppm, equal to 4 mg/kg bw
per day. In a 12-month feeding study in dogs at dietary levels of 0,
35, 75 150, or 1500 ppm, the NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological changes in the liver, spleen,
prostate, testis, and adrenals. The results of studies incorporating
withdrawal periods indicate that the anti-androgenic effects of
vinclozolin are reversible on cessation of treatment.
In a recent study of carcinogenicity in C57Bl/6 mice at dietary
levels of 0, 15, 150, 3000, or 8000 ppm, hepatocellular carcinomas
were seen at 8000 ppm. There was evidence of toxicity at 3000 ppm,
including hepatotoxicity, Leydig-cell hyperplasia, atrophy of
accessory sex glands, atrophic uteri, and lipidosis in the
cortico-medullary region of the adrenals. The NOAEL was 150 ppm, equal
to 24 mg/kg bw per day. In an earlier study in NMRI mice at levels of
0, 160, 490, 1460, or 4370 ppm, survival was adversely affected at the
highest dose, and the NOAEL was 490 ppm on the basis of increased
liver weight, without histological change. In rats, the long-term
toxicity and carcinogenicity of vinclozolin has recently been
investigated in three studies with dietary levels of 25-4500 ppm.
Cataracts and other lenticular changes were seen in rats treated with
50 ppm or more. (Mice and dogs were closely examined for ocular
changes, but vinclozolin did not affect the eyes in these species.) An
increased incidence of Leydig-cell tumours was seen in rats treated
with 150 ppm and more, together with atrophy of accessory sex glands.
Benign sex cord stromal tumours in the ovaries were seen in rats
treated at 500 ppm and above, and uterine adenocarcinomas were
detected at 3000 ppm (the highest dose tested in the carcinogenicity
study). Adrenal tumours were seen at 1500 ppm and above.
Hepatocellular carcinomas were seen in males treated with 4500 ppm,
and signs of hepatotoxicity were seen in rats treated with 150 ppm or
more. The NOAEL was 25 ppm, equal to 1.4 mg/kg bw per day.
In multi-generation studies, vinclozolin led to infertility of
males, owing to feminization of the outer genital organs, at dietary
levels of 1000 ppm or more. At 300 ppm, although all males were
eventually proved fertile, the observed effects may have indicated
sub-fertility. At 50 ppm, the only adverse effect was a reduction in
epididymal weight (with no associated morphological changes) in F2
offspring. The NOAEL was 40 ppm, equivalent to approximately 4 mg/kg
bw per day. Recent investigations of developmental toxicity have been
conducted in rats and rabbits. In rats, the most sensitive indicator
of teratogenicity was a reduction in the anogenital distance; in a
series of studies, the NOAEL for a change in anogenital distance was
15 mg/kg bw per day. The NOAEL for fetotoxicity was about 100 mg/kg bw
per day, on the basis of signs of developmental delay, while the NOAEL
for maternal toxicity was about 400 mg/kg bw per day, on the basis of
clinical signs of toxicity.
Three studies of developmental toxicity have been conducted in
rabbits. In the first, there were no signs of maternal toxicity,
fetotoxicity, or teratogenicity at doses up to and including 300 mg/kg
bw per day. In the second study, with doses up to and including
800 mg/kg bw per day, toxicity led to extensive mortality at the
highest dose, precluding any reliable assessment at this dose. The
NOAEL for maternal toxicity was 50 mg/kg bw per day, and that for
fetotoxicity was 200 mg/kg bw per day; there was no evidence of
teratogenicity at this dose (the highest dose available for
assessment). The third study involved only one dose, 400 mg/kg bw per
day. The number of female offspring exceeded the number of males, but
there was no treatment-related change in the appearance of the male
fetal genital organs. An increase in the incidence of separated
origins of the carotid arteries indicated potential teratogenicity at
this maternally toxic dose.
Vinclozolin has been tested for genotoxicity in a range of tests
in vivo and in vitro. The Meeting concluded that vinclozolin is
not genotoxic. It noted that positive results were obtained in a study
of cell transformation, but the process giving rise to this effect is
unknown. One study suggests that vinclozolin may be a promoter in rat
liver in vivo, which may indicate the mechanism by which liver
tumours were induced in rats at a high dose.
Studies have been conducted that confirm the anti-androgenic
properties of vinclozolin, which are likely to be associated with
binding to the androgen receptor. This proposed mechanism of action
could account for the results seen in studies of the reproductive
toxicity and long-term toxicity of vinclozolin.
In an epidemiological study of manufacturing plant personnel, it
was concluded that there was no evidence that vinclozolin had induced
health effects in employees with possible long-term exposure.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1.4 mg/kg bw per day in the two-year study of carcinogenicity
in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 20 mg/kg bw per day (three-month study of
toxicity)
490 ppm, equivalent to 63 mg/kg bw per day (112-week study
of toxicity and carcinogenicity in NMRI mice)
150 ppm equal to 24 mg/kg bw per day (18-month study of
toxicity and carcinogenicity in C57Bl/6 mice)
Rat: 50 ppm, equal to 4 mg/kg bw per day (three-month study of
toxicity)
25 ppm, equal to 1.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
40 ppm, equivalent to 4 mg/kg bw per day (study of
reproductive toxicity)
15 mg/kg bw per day (study of developmental toxicity)
100 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
400 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 50 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
200 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 75 ppm, equal to 2.4 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to vinclozolin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 15 000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test
Inhalation, toxicity, rat LC50 > 29 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat NOAEL = 15 mg/kg bw per day; teratogenicity
Long-term (> one year) Dietary, two years, toxicity and NOAEL = 1.4 mg/kg bw per day; signs of
carcinogenicity, rat antiandrogenicity
Dietary, one year, toxicity, dog NOAEL = 2.4 mg/kg bw per day; signs of
antiandrogenicity
References
Block, I. et al. (1987) Study of effect on vital functions of
animals -- general pharmacology -- of vinclozolin. Unpublished
report from Research & Consulting Co. AG, Itingen, Switzerland.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cameron, B.D. & Jack, L. (1991) In vitro percutaneous absorption of
14C-Reg. No. 83 258. A comparison using rat and human
epidermis. Unpublished report from Inveresk Research
International, Tranent, Scotland. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B. &
Franklin, E.R. (1976) The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral administration to
rats. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cifone, M.A. & Myhr, B.C. (1984) Evaluation of vinclozolin (831233) in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Kensington, Maryland,
USA. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cozens, D.D. & Palmer, A.K. (1987) Statement on maternal toxicity;
effect of vinclozolin on pregnancy of the New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R. & Offer, J.M.
(1981) Effect of vinclozolin on pregnancy of New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P (1979) Study of sensitization effect on guinea pigs
maximization test. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1981) Cytogenetic investigation in
Chinese hamsters after a single oral administration of Reg.
No. 83 258. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of
vinclozolin (Reg. No. 83 258) in the Ames test. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Gelbke, H.P & Jackh, R. (1975) Report on a point mutation test carried
out on CHO cells (HGPRT locus) with the test substance
vinclozolin (substance No. 841382). Unpublished report from BASF
AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1979) Report on the study of the acute oral
toxicity of vinclozolin (Reg. No. 83 258) in beagle dogs.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1981) Acute oral toxicity of vinclozolin in
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gray, L.E., Jr, Ostby, J.S. & Kelce, W.R. (1994a) Developmental
effects of an environmental antiandrogen: The fungicide
vinclozolin alters sex differentiation in the male rat. Toxicol.
Appl. Pharmacol., 129, 46-52.
Gray, L.E, Jr, Ostby, J.S., Monosson, E. & Kelce, W.R. (1994b)
Alterations of sex differentiation in male rats following
perinatal exposure to low doses of the antiandrogenic pesticide
vinclozolin. Biol. Reprod., 50 (Suppl. 1), 101.
Grundler, P. & Gelbke, H.P. (1980) Report on the sensitizing effect of
BAS 352 04 F - Ronilan (WNT No. 791661) in the guinea pig -- open
epicutaneous test (OET). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990a) The biokinetics of 14C-vinclozolin in
the rat. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990b) The biotransformation of 14C-vin-
clozolin in the rat. Unpublished report from Huntingdon Research
Centre, Huntingdon, Cambs, United Kingdom. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991a) The determination by whole body
autoradiography of the tissue distribution of radioactivity in
female rats after oral administration of 14C-vinclozolin.
Unpublished report from Huntingdon Research Centre, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991b) The dermal absorption of
14C-vinclozolin in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1987) Report on the study of the toxicity of
Reg. No. 83 258 (vinclozolin) in beagle dogs after 12-month
administration via the diet. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989a) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- first study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989b) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- second study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF: AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- third study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989d) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- test study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1990a) Report: Reproduction study with Reg.
No. 83 258 in rats. Continuous administration over 2 generations.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990b) Report: Study of the prenatal toxicity of
Reg. No. 83 258 in rats after dermal application. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rabbits after oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990d) Report on the supplementary steady of the
prenatal toxicity of Reg. No. 83 258 in rabbits after oral
administration (gavage). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1994) Report: Second reproduction study with
Reg. No. 83 258 in rats. Continuous administration over 2
generations. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977a) Primary skin irritation of Reg. No. 83 258
(vinclozolin) on the intact and scarified dorsal skin of white
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977b) Primary irritation of Reg. No. 83 258
(vinclozolin) to the eye of white rabbits. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. (1973a) Report on acute oral toxicity trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1973b) Report on the acute intraperitoneal trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1974) Report on the testing of 3-(3,5-dichiorophenyl)-
5-methyl-5-vinyl-l,3-oxazolidine-2,4-dione in a 3-month feeding
experiment on rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975a) Report on the supplementary
toxicological study of 3-(3,5-dichiorophenyl)-5-methyl-
5-vinyl-1,3-oxazolidine-2,4-dione in a 4-week feeding study in
the rat. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975b) Report on the toxicological testing
of 3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-
2,4-dione in a three-month feeding trial on the dog. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975a) Study on the prenatal toxicity of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on mice. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975b) Study of the mutagenic effect of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on the male mouse following repeated oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hoorn, A.J.W. (1983) Mutagenicity of vinclozolin, compound No. 831233,
in the rec assay with Bacillus subtilis. Unpublished report
from Litton Bionetics, Veenendaal, Netherlands. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Ito, N. & Hasegawa, R. (1992) Liver medium term bioassay in rats for
screening of carcinogenesis and modifying factors in
hepatocarcinogenesis. Food Chem. Toxicol., 30, 979-992.
Ito, N., Hoshiya, T., Hasegawa, R., Hakoi, K., Cui, L., Ogiso, T. &
Cabral, R. (1993) Enhancement by non-mutagenic pesticides of
GST-P positive hepatic foci development initiated with
diethylnitrosamine in the rat. Cancer Lett., 72, 59-64.
Ito, N., Hasegawa, R., Imaida, K., Takahashi, S. & Shirai, T. (1994)
Medium term rat liver bioassay for rapid detection of carcinogens
and modifiers of hepatocarcinogenesis. Drug Metab. Rev.,
26, 431-442.
Kelce, W.R., Monosson, E., Gamcsik, M.P., Laws, S.C. & Gray, L.E., Jr
(1994) Environmental hormone disruptors: Evidence that
vinclozolin developmental toxicity is mediated by antiandrogenic
metabolites. Toxicol. Appl. Pharmacol., 126, 276-285.
Kirsch, P. (1986a) Report of the study of the acute oral toxicity on
the rat based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. (1986b) Report of the study of the acute oral toxicity on
the mouse based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. et al. (1974) Report on the study of 3-(3,5-dichloro-
phenyl)-5-methyl-5-vinyl-1,3-oxazoildine-2,4-dione for cataract
formation in a 3-month feeding study in dogs. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Kirsch, P., Deckhardt, M. & Hellwig, J. (1982) Report on the study of
the toxicity of Reg. No. 83 258 (vinclozolin) in beagle dogs
after 6-month administration in the diet. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. (1989) Examination of the hormone status. Unpublished
report from University of Lübeck, Lübeck, Germany. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Knuppen, R. (1990) Final report: Study of the binding of
3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-2,4-dione to the androgen
receptor in MCF-7 cells. Unpublished report from University of
Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1991) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) -- Reg. No. 119 208
(metabolite BF 352-22) to the androgen and glucocortiroid
receptors in the cytosol from MC-F-7 cells and from the prostate
and liver tissues of the rat. Unpublished report from University
of Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1992) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) to the androgen receptor
in the cytosol from a cell line expressing the androgen receptor
and from the prostate tissue of the rat. Unpublished report from
University of Lübeck, Lübeck, Germany. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Kretzschmar, R. et al. (1987) Study on the EEG effects in the
conscious rat of vinclozolin. Unpublished report from Knoll AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Leuschner, F. (1977) Chronic oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in a
reproduction study covering three generations of Sprague-Dawley
rats. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikoiogie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. (1979) Acute inhalation toxicity study on the
preparation vinclozolin. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1975) Oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in
Sprague-Dawley rats. Unpublished report from Laboratorium für
Pharmakoiogie und Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977a) 3-weeks-toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in NZW
rabbits by local application. Unpublished report from
Laboratorium fur Pharmakologie und Toxikoiogie (LPT), Hamburg,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977b) Oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in NMRI
mice. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikologie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. et al. (1977c) Chronic oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in the
Sprague-Dawley rat. Unpublished report from Laboratorium fur
Pharmakoiogie and Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1992) Report: Study on the influence of Reg.
No. 83 258 (vinclozolin) on the hormone status of Wistar rats.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1993a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in Wistar rats. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Mellert, W. et al. (1993b) Report: Supplementary study on the oral
toxicity of Reg. No. 83 258 (vinclozolin) in Wistar rats.
Administration in the diet over 3 months. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994a) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in C57BL mice. Administration in the
diet for 18 months Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994b) Report: Study of the chronic toxicity of
Reg. No. 83 258 (Vinclozolin) in rats. Administration via the
diet over 24 months. Unpublished report from BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994c) Report: Chronic toxicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994d) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Murli, H. (1989) Mutagenicity test with Reg. No. 83 258, Batch No. 183
(= ZST No. 881375) in an in vitro cytogenetics assay measuring
chromosome aberration frequency in Chinese hamsters ovary cells
(CHO cells). Unpublished report from Hazleton, Kensington,
Maryland, USA. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Oesch, F. (1977) Ames test for vinclozolin. Unpublished report from
University of Mainz, Mainz, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Otto, S., Bentel, P., Elzner, J. & Ohnsorg, U. (1977) Metabolism of
14C-vinclozolin in rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Perocco, P., Collaci, A. & Grilli, S. (1993) In vitro cytotoxic and
cell transforming activities exerted by the pesticides cyanazine,
dithianon, diflubenzuron, procymidone and vinclozolin on BALB/c
3T3 cells. Environ. Mol. Mutag., 21, 81-86.
Rankin, G.O., Teets, V.J., Nicoll, D.W. & Brown, P.I. (1989)
Comparative acute renal effects of three N(3,5-dichlorophenyl)
carboximide fungicides: N-(3,5-dichlorophenyl) succimide,
vinclozolin and iprodione. Toxicology, 56, 263-272.
Sabbioni G. & Neumann H.-G. (1990) Biomonitoring of arylamines:
Haemoglobin adducts of urea and carbamate pesticides.
Carcinogenesis, 11, 111-116.
Schilling, K. (1993) Evaluation of ophthalmology findings recognised
within various rat feeding studies with Reg. No. 83 258
(vinclozolin). Unpublished report from K. Schilling, Consultant,
Kirchheimbolanden, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in B6C3F1 mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990b) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in C57BL mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Shirasu, Y. et al. (1977) Mutagenicity testing on BAS 352 04 F in
microbial systems. Unpublished report from Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Shirasu, Y., Takahashi, K. & Saito, T. (1978a) Report of acute
toxicity tests with BAS 352 F in mice. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany
Shirasu, Y., Takahashi, K. & Saito, T. (1978b) Report of acute
toxicity tests with BAS 352 F in rats. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Witterland, W.F. & Hoorn, A.J.W. (1984) Mutagenicity evaluation of
vinclozolin (831233) in the mouse lymphoma forward mutation
assay. Unpublished report from Litton Bionetics, Veenendal,
Netherlands. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Zober, A. et al. (1995) Morbidity study of personnel with potential
exposure to vinclozolin. Occup. Environ. Med., 52, 233-241.
 After oral administration of 2000 or 5000 mg/kg bw vinclozolin to
male Sprague-Dawley rats, the effects on the cortical electroence-
phalogram were examined. At both doses, sleeping stages were prolonged
and the number and duration of rapid eye movement phases were slightly
reduced; however, these alterations were not pronounced and suggest
only a moderate sedative effect (Kretzschmar et al., 1987).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of vinclozolin are
summarized in Table 1. The clinical signs of toxicity after treatment
with vinclozolin were generally nonspecific, and no consistent,
treatment-related effects were seen at autopsy.
The LD50 of metabolite T,3,5-dichlorophenylcarbamoyl-2-
propionic acid, was reported to be 2740 mg/kg bw in rats and >
2000 mg/kg bw in mice. Clinical signs of reaction to treatment
included dyspnoea, staggering, piloerection, and poor general state
(Kirsch, 1986a,b).
Table 1. Acute toxicity of vinclozolin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse Oral > 15 000 92.8 Shirasu et al. (1978a)
Mouse Intraperitoneal 1570-1640 92.8 Shirasu et al. (1978a)
Mouse Subcutaneous > 15 000 92.8 Shirasu et al. (1978a)
Rat Oral > 15 000 92.8 Shirasu et al. (1978b)
Rat Intraperitoneal 4220-8300 92.8 Shirasu et al. (1978a)
Rat Dermal > 5000 92.8 Shirasu et al. (1978b)
Rat Inhalation > 29.1 NR Leuschner (1979)
Guinea-pig Oral 8000 90-97 Hofmann (1973a)
Guinea-pig Intraperitoneal 3000 90-97 Hofmann (1973b)
Rabbit Oral > 5620 98.1 Gelbke & Kirsch (1981)
Dog Oral > 10 000 97 Gelbke & Kirsch (1979)
NR, not reported
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing vinclozolin at doses of 0, 100, 1000, 2500, or 5000 ppm for
three months. Food consumption and body weight were determined weekly;
clinical signs were checked daily, with a weekly comprehensive
clinical examination. At the end of the study, clinicochemical and
haematological examinations were performed, and all animals were
assessed grossly and histopathologically. Reduced body-weight gain was
seen in males at the high dose at the end of the study. Clinico-
chemical parameters were affected at doses > 1000 ppm and
haematological parameters only at the highest dose. Decreased
triglyceride and cholesterol levels were seen in animals at 1000 ppm,
and decreased glucose levels in males and albumin levels in females at
the three higher doses. Increased alkaline phosphatase activity was
seen in males and increased globulins in males and females at 2500 and
5000 ppm; alanine aminotransferase activity was increased only in
males at 5000 ppm. Increases were seen in the mean corpuscular
haemoglobin value in females at the two highest doses, the mean
corpuscular volume in animals of each sex, haemoglobin in males, and
reticulocyte counts in females at the highest dose. Absolute and
relative liver weights were found to be increased at the three higher
doses. Centrilobular hypertrophy of hepatocytes was seen in males at
2500 and 5000 ppm; testicular weights were increased at these doses,
and multifocal hyperplasia in Leydig cells was noted at 1000 ppm or
more. Absolute and relative adrenal gland weights were increased in
males at 2500 and 5000 ppm, and lipogenic pigment and lipid vacuoles
in the adrenals were noted in animals of each sex at these doses. In
females at 1000 ppm, increased lipogenic pigment was seen in the
adrenals. Hyperplasia in stromal cells of the ovaries was observed at
the highest dose only. No treatment-related adverse effects were seen
at 100 ppm. The NOAEL was 100 ppm, equivalent to about 20 mg/kg bw per
day (Schilling et al., 1990a).
The B6C3F1 mouse strain has a relatively high frequency of
spontaneous liver lesions, including tumours. In the three-month study
with this strain, clear increases in liver weight and hepatocellular
hypertrophy were observed, indicating that the liver is one of the
target organs of vinclozolin. In order to assess more accurately its
potential proliferative effect on mouse liver, the toxicity of
vinclozolin was investigated in C57B1 mice, which have a low
background incidence of spontaneous liver neoplasia. Groups of 10 male
and 10 female mice were fed diets containing vinclozolin at doses of
0, 100, 1000, or 5000 ppm. Food consumption and body weight were
determined weekly, and clinical signs were checked daily, with a
weekly comprehensive clinical examination. At the end of the study,
clinicochemical and haematological examinations were performed. All
animals were assessed macroscopically and histologically. A reduction
in body-weight gain was seen in males at the high dose. Decreased
triglyceride and cholesterol values were seen in animals at 1000 and
5000 ppm, and glucose was decreased only in males at the high dose.
Increased alanine aminotransferase activity was seen in animals of
each sex, and total protein and alkaline phosphatase were increased in
males at the high dose. Haemoglobin, mean cell volume, and mean
corpuscular haemoglobin were increased in animals of each sex at
5000 ppm and in females at 1000 ppm; haematocrit and leukocyte and
lymphocyte counts were increased in animals of each sex and the
erythrocyte count in males at the highest dose. Increased liver
weights were seen at 1000 and 5000 ppm, and centrilobular hypertrophy
of hepatocytes was seen at 5000 ppm. Increased adrenal weights were
seen males at this dose; an increase in lipogenic pigment was noted
histologically in animals of each sex at 1000 ppm and 5000 ppm and
lipid vacuoles were seen at 5000 ppm. Hyperplasia and hypertrophy of
the stromal cells of the ovaries were seen at the middle and high
doses, and focal hyperplasia of the Leydig cells of the testis was
observed at the high dose. There were no compound-related findings at
100 ppm. The NOAEL was 100 ppm, equivalent to 25 mg/kg bw per day
(Schilling et al., 1990b).
Rats
Groups of 16 male and 16 female Sprague-Dawley rats were fed
diets containing 0,100, 300, 1000, or 2000 ppm technical-grade
vinclozolin for three months, and six rats from each group were
further maintained on control diets for six weeks after the end of the
study. Rats were examined daily for mortality, abnormal appearance or
behaviour, and food consumption. Body weights were determined weekly,
when rats were palpated and the eyes examined. Blood and urine were
sampled before treatment, after six and 12 weeks of treatment, at
termination, and at the end of the observation period. At termination,
rats were sacrificed, dissected, and examined for gross pathological
changes. The absolute and relative weights of major organs were
determined, and a complete set of tissues from each animal was saved
for future histopathological examination. The results of microscopic
examinations of tissues were not reported, except for the eyes, which
were examined in serial sections. A single death occurred, in a female
at the high dose on day 42; all other rats survived to scheduled
termination. No abnormalities of appearance or behaviour were noted,
and the eyes were normal at all examinations. The body weights of
treated rats were comparable to those of controls throughout the
study. Occasional statistically significant increases in food
consumption were seen in treated females, but that of males was not
affected. Haematological changes consistent with decreased erythrocyte
mass-decreased erythrocyte count, haematocrit, and haemoglobin and
increased mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentration-were seen at the six-week sampling time in
males and females fed doses > 300 ppm; however, these changes were
seen at termination only in females at 1000 and 2000 ppm. Clinical
chemical parameters were not altered in a toxicologically significant
manner. At necropsy, no effects of treatment on the gross appearance
of tissues were apparent. Statistically significant, dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were seen in males and females at 1000 and 2000 ppm; at
2000 ppm, increased relative weights of kidneys were seen in males and
females and increased relative spleen weights in females. These
effects were apparently reversible, as they were not observed in
treated rats that received control diets for an additional six weeks.
Microscopic examination of the eyes revealed no treatment-related
abnormalities (Hofmann, 1974).
Groups of rats received vinclozolin at dietary levels of 0, 900,
1800, 3000, or 15 000 ppm for four weeks. There were no clinical signs
of toxicity, and food intake and body-weight gain were reduced only in
animals at the high dose. Erythrocyte parameters were reduced in all
treated female rats, and urinalysis revealed reduced osmolality at the
highest dose. Liver and adrenal weights were increased in all treated
rats, and necropsy revealed a grey-white discolouration of the
adrenals. The ascorbic acid content of the adrenals was increased in
all treated animals, and the glycogen content of the liver was reduced
in all treated females and in males at 15 000 ppm. As progressive,
dose-related transformation of the adrenal cortex was seen in all
treated rats, there was no NOAEL (Hoffman & Munk, 1975a).
Groups of 50 male and 50 female Sprague-Dawley rats received
vinclozolin in the diet at levels of 0, 150, or 450 ppm for three
months. Clinical behaviour, food and drinking-water consumption, and
body-weight gain were not affected, and clinicochemical examinations
and urinalyses revealed no substance-induced changes. Histopatho-
logical examination of the liver, adrenals, and pituitary at the end
of treatment gave no reliable indication of substance-induced changes.
The NOAEL was > 450 ppm, equal to 44 mg/kg bw per day for males and
40 mg/kg bw per day for females (Leuschner et al., 1975).
Groups of 10 male and 10 female Wistar rats received vinclozolin
in the diet at levels of 0, 300, 1000, or 3000 ppm for three months.
There were no clinical signs of reaction to treatment, and no rats
died before the scheduled sacrifice. Body-weight gain and food intake
were unaffected by treatment, but water intake was increased in rats
at 3000 ppm. Ophthalmoscopic examination revealed two cataracts, one
unilateral and one bilateral, in rats receiving 3000 ppm, and other
lenticular changes were seen in all groups; it was concluded that the
ocular lesions were spontaneous and unrelated to treatment. Evidence
of anaemia and decreased serum alkaline phosphatase activity were seen
in animals at the high dose, with decreases in leukocyte counts at
1000 and 3000 ppm. Examination post mortem revealed white, enlarged
adrenals in all treated rats. The weights of the liver, adrenal, and
testis were increased at 1000 and 3000 ppm, and histopathological
examination revealed hypertrophy of the adrenal cortex, cystoid
degeneration in the pituitary, Leydig-cell hyperplasia, cloudy
swelling of hepatocytes, single live cell necrosis, and vacuolization
of luteal cells in the ovaries at these doses. Acinar vacuolization in
the pancreas was seen in all treated rats. Vinclozolin was thus toxic
at all doses; the lowest dietary level of 300 ppm is equal to 22 mg/kg
bw per day (Mellert et al., 1993a).
In order to define a clear no-effect level, groups of 10 male and
10 female Wistar rats received vinclozolin in the diet at levels of 0
or 50 ppm for three months, and investigations similar to those in the
previous study were performed. In particular, laboratory investi-
gations and ophthalmoscopic examinations were carried out, and all
animals underwent detailed pathological examination. No treatment-
related changes were seen. The NOAEL was 50 ppm, equal to 4 mg/kg bw
per day (Mellert et al., 1993b).
Rabbits
Groups of six New Zealand white rabbits received dermal
applications of vinclozolin for 8 h per day for three weeks at doses
of 0, 111, 333, or 1000 mg/kg bw. There were no deaths and no clinical
signs of reaction to treatment; gross pathology and histopathology
revealed no evidence of dermal irritation (Leuschner et al., 1977a).
Dogs
Groups of four beagle dogs of each sex were fed diets containing
vinclozolin at doses of 0, 100, 300, 1000, or 2000 ppm for three
months. There were no signs of toxicity and no compound-induced
deaths. Repeated ophthalmological examinations revealed no effects.
The body-weight gain of treated animals was not affected, and
urinalysis showed no treatment-related findings. Females at 2000 ppm
had a reduced haemoglobin content after four and eight weeks and a
decreased erythrocyte count throughout the study. An increased
platelet count was seen in female animals at 1000 and 2000 ppm.
Howell-Jolly bodies were found in a differential blood count in males
and females at these doses, suggesting a compensatory reaction
elicited by an anaemic process. The relative liver weights and the
relative and absolute adrenal weights were increased in females at the
highest dose. Histopathological assessment revealed compound-induced
cholestasis of the liver. In a further evaluation, there was a slight
to moderate, dose-related increase in the haemosiderin content of the
liver, particularly in females, at 300, 1000, and 2000 ppm. Females
had a higher pigment content than males, and the spleens of females
also had an increased haemosiderin content. It was assumed that the
haemosiderosis in the liver was caused by increased haemolysis, in
contrast to the original interpretation that the pigment deposits in
the liver were due to cholestasis. The NOAEL was 100 ppm, on the basis
of the increased haemosiderin content of the liver (Hoffman & Munk,
1975b).
Pairs of one male and one female beagle dog were given technical-
grade vinclozolin at doses of 0, 1000, or 2000 ppm for three months.
There were no compound-induced deaths or other signs of toxicity
during treatment. The animals underwent ophthalmological examinations
twice a week and were additionally examined by an eye specialist
towards the end of the treatment period. There was no indication of
cataract formation. The NOAEL was thus > 2000 ppm (Kirsch et al.,
1974).
Groups of six beagle dogs of each sex received diets containing
technical-grade vinclozolin at doses of 0, 100, 300, 600, or 2000 ppm
for six months. Clinicochemical, haematological, and urinalyses were
carried out at regular intervals; ophthalmoscopy was performed before
the beginning of the study, after about three months, and towards the
end of treatment. At the end of the study, all animals were examined
grossly and histopathologically. No deaths occurred, food consumption
and body-weight gain were unchanged in comparison with the control
group, and there were no clinical signs of toxicity. Males at the
highest dose had pronounced, and females slight, haemolytic anaemia.
Increased incidences of Howell-Jolly bodies and reticulocytes in males
suggested a compensatory bone-marrow reaction to the compound-induced
loss of blood cells. Slight increases in bilirubin level in males at
600 and 2000 ppm, in the haemoglobin concentration of individual
erythrocytes, and in lactic dehydrogenase activity in males at
2000 ppm were further consequences of increased decomposition of
erythrocytes. The considerably increased platelet counts in males at
2000 ppm and the slight increase in females at 600 and 2000 ppm are
probably due to the anaemic process, since hyper-regenerative anaemia
is frequently accompanied by thrombocytosis. The absolute and relative
adrenal weights showed a dose-related increase in groups treated with
2000, 600, or 300 ppm, and histopathological examination showed
vacuolization of the zona fasciculata and severe lipid incorporation
and birefringence of the adrenal cortex (in females) at the highest
dose. Increased absolute and relative spleen weights were observed at
2000 ppm; at 600 ppm, only the absolute spleen weight was increased in
females. Histopathological examination revealed dilated splenic
sinuses and hyperaemia in females at both doses. Reduced relative
weights of the kidney (at 2000 ppm) and pituitary (at 600 and
2000 ppm), haemosiderin deposits in the liver (at 600 and 2000 ppm),
and severe prostatic atrophy (at 2000 ppm) were also found.
Ophthalmological examinations revealed no compound-related findings.
The NOAEL was 100 ppm, equivalent to 4.0 mg/kg bw per day (Kirsch
et al., 1982).
Groups of six beagle dogs of each sex were fed diets containing
0, 35, 75, 150, or 1500 ppm vinclozolin for 12 months. There were no
deaths and no clinical signs of toxicity. Food consumption and
body-weight gains were not significantly affected by treatment.
Haematological, clinical chemical, and urinalyses were conducted
before treatment and in weeks 13, 26, and 52. At the highest dose,
reticulocyte counts were increased in animals of each sex, platelet
counts were increased in males, the mean cell volume was increased in
females, and total bilirubin concentrations were increased in animals
of each sex. Adrenal weights were increased at 1500 ppm and slightly
increased at 150 ppm in animals of each sex; the adrenals were
enlarged in all males and in five females at 1500 ppm, and the mean
width of the adrenal cortex was increased in animals of each sex at
1500 ppm and, to a lesser degree, in females at lower doses. No
dose-response relationship was identified. Progressive transformation
and increased lipid content were seen in the adrenals of all males and
five females at 1500 ppm. Testicular weights were increased in a
dose-related fashion; at 1500 ppm, the weights were markedly
increased, and at 150 ppm the relative weights were significantly
increased. At 1500 ppm, diffuse hyperplasia of the Leydig cells was
observed in five males. The prostates of one male at 150 ppm and two
at 1500 ppm appeared smaller macroscopically. Histopathological
examination revealed that the prostates of two males at 150 ppm were
slightly or moderately atrophied and those of five males at 1500 ppm
were slightly (one) or severely (four) atrophied; similar but minimal
effects were seen in one male at 0, one at 35, and one at 75 ppm.
Liver weights were increased in animals at doses up to 150 ppm and
were markedly increased in males at 1500 ppm. Increased haemosiderin
deposition was observed in the livers of males at 1500 ppm and of
females at 150 and 1500 ppm. Spleen and thyroid weights were increased
in males at 1500 ppm, and spleen weights were also slightly increased
in females at 1500 ppm. The NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological effects at 150 and 1.500 ppm
(Hellwig et al., 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were fed diets
containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 112 weeks.
The results of analyses of the diets were not reported. There were no
clinical signs of reaction to treatment and no effect on food intake.
The weight gain of males at 1460 or 4370 ppm was reduced, and the
survival of males at the highest dose was adversely affected; no such
changes were seen in females. The weights of the liver and testis were
increased at the highest dose, and liver weight was increased in
females at 1460 ppm. Histopathological examination revealed no
treatment-related changes, and no tumours were seen (Leuschner
et al., 1977b).
Groups of 100 male and 100 female control C57Bl/6/JICO mice and
60 male and 60 female treated animals received vinclozolin in the diet
at levels of 0, 15, 150, 3000, or 8000 ppm for 18 months. Ten animals
of each sex were taken from each group for interim sacrifice after 12
months of treatment. There were no clinical signs. The mortality rates
of males and females at the highest dose were greater than those of
controls, and weight gain and food intake were reduced in animals at
3000 and 8000 ppm. Examination of blood smears revealed an increased
polymorphonuclear neutrophil count and decreased lymphocyte count in
animals of each sex at 8000 ppm. Pathological examinations revealed
similar findings at the interim and terminal kills. Increased liver
and adrenal weights were seen in animals at 3000 and 8000 ppm, with
smaller epididymides, seminal vesicles, and prostate. Focal necrosis,
bile-duct proliferation, and pigment deposition were seen in the
livers of animals at 3000 or 8000 ppm. At 8000 ppm, these changes were
accompanied by diffuse hepatocyte hypertrophy, decreased lipid
storage, and increased focal fatty infiltration in the liver, hepatic
single-cell necrosis, an increased prevalence of biliary cysts in the
livers of males, focal cellular alterations and focal hyperplasia of
the livers of males and females, hepatocellular carcinomas in three
males and 22 females, and hepatocellular adenomas in three females. In
males at 3000 and 8000 ppm, diffuse Leydig-cell hyperplasia of the
testis was seen, with atrophy of the seminal vesicles and coagulation
glands. Females at these doses had atrophic uteri, accompanied in
animals at the highest dose by diffuse stromal hyperplasia, an
increased incidence of pigmented interstitial cells in the ovaries,
and loss of ovarian follicles. The adrenal cortexes of animals at 3000
or 8000 ppm had increased lipogenic pigment in the cortico-medullary
region and lipidosis. In addition, in animals at 8000 ppm, foam cells,
eosinophilic crystals, and pneumonitis were seen in the lungs of males
and erosions or ulcers in the glandular stomachs of males and females.
Vinclozolin thus caused hepatocellular carcinomas at a dose of
8000 ppm, equal to 1300 mg/kg bw per day, a dose associated with clear
toxicity; there were no treatment-related tumours at 3000 ppm, equal
to 495 mg/kg bw per day, although evidence of toxicity was seen. The
NOAEL was 150 ppm, equal to 24 mg/kg bw per day (Mellert et al.,
1994a).
Rats
Groups of 50 male and 50 female Sprague-Dawley rats were fed
diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 130
weeks; the study was terminated when survival in the control group
reached 70%. The results of analyses of the diets were not reported.
There were no clinical signs of reaction to treatment. Dose-related
reductions in food intake and weight gain were seen in animals at 1460
and 4370 ppm, but their survival was better than that of controls.
Clinical chemical and histological investigations showed no reaction
to treatment. Interpretation of the data on organ weights was hampered
by disparities in body weight between groups. No tumours were observed
(Leuschner et al., 1977c).
Groups of 20 male and 20 female Wistar rats received vinclozolin
in the diet at levels of 0, 150, 500, 1500, or 4500 ppm for 24 months.
The only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in all treated males and
cataracts in all treated rats. Although the mortality rate of females
at the high dose was higher than that in concurrent controls, the
increase was marginal and may have been unrelated to treatment. Weight
gain and food intake were adversely affected in animals at 1500 and
4500 ppm. Water intake was increased in males at 4500 ppm and
decreased in females at this dose and in males and females at
1500 ppm. Ophthalmoscopic examination revealed a treatment-related
incidence of cataracts. Bilateral cataracts were present in all
animals at 1500 and 4500 ppm that survived to termination, and
cataracts were also seen at 500 ppm. The ophthalmoscopic changes at
150 ppm were confined to lenticular degeneration and calcification in
a few animals. Animals at 1500 and 4500 ppm showed evidence of
anaemia, decreased serum alanine aminotransferase and alkaline
phosphatase activities, and increased creatinine, total protein,
cholesterol, and gamma-glutamyl transferase activity. Increased liver
and adrenal weights were seen in animals at 4500 ppm and increased
testicular weights in all treated males. Leydig-cell tumours were seen
in almost all animals treated with 500, 1500, or 4500 ppm and were
increased in incidence in rats at 150 ppm in comparison with controls.
Focal hyperplasia and cystic ducts were seen in the rete testis of
rats at 4500 ppm, and two males at this dose but no controls had rete
testicular adenomas. Cystic ducts in the rete testis were also seen in
animals at 1500 ppm. Atrophy of the seminal vesicles, coagulating
gland, and epididymides were seen in almost all animals at 1500 and
4500 ppm, similar, less marked effects being seen in animals at 150
and 500 ppm. Dose-related reduced secretion and increased fibrosis in
the prostate were also seen in all treated males. Benign stromal
tumours of the sex cord in the ovaries were seen in 10 rats treated
with 4500 ppm, four at 1500 ppm, and two at 500 ppm; none were seen in
animals at 150 ppm or in controls. Five adenomas and one metastatic
carcinoma of the adrenal cortex were seen in females at 4500 ppm and
one adenocarcinoma of the adrenal cortex in a female at 1500 ppm; no
adrenal tumours were seen at 150 or 500 ppm or in controls. A
dose-related incidence of lipidosis in the adrenal cortex was seen in
all treated animals. Hepatocellular carcinomas occurred in nine male
rats at 4500 ppm, with none in controls. Although hepatic tumours were
found only in animals at the high dose, hepatic necrosis, hypertrophy,
and eosinophilic foci were seen in a dose-related fashion at doses
down to 150 ppm; the only changes in animals at the low dose were
eosinophilic foci in one female. Vacuolation of the pancreatic
exocrine cells was seen in all treated groups. Treatment-related
changes were also seen in the pituitary in animals at 1500 and
4500 ppm, consisting of a reduction in focal hyperplasia and an
increase in diffuse hyperplasia in males and a reduction in the
incidence of pituitary adenomas in females. Vinclozolin was toxic at
all doses tested. The lowest dietary level of 150 ppm was equal to
8 mg/kg bw per day (Mellert et al., 1994b).
In order to define a clear no-effect level, groups of 20 male and
20 female Wistar rats received vinclozolin in the diet at levels of 0,
25, or 50 ppm for 24 months, with investigations similar to those
performed in the previous study. In particular, laboratory
investigations and ophthalmoscopic examinations were carried out every
three months, and all animals underwent detailed pathological
examination. No treatment-related changes were seen. The NOAEL was
50 ppm, equal to 2.8 mg/kg bw per day (Mellert et al., 1993b).
Groups of 50 male and 50 female Wistar rats received vinclozolin
in the diet at levels of 0, 50, 500, or 3000 ppm for 24 months. The
only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in males at 500 and 3000 ppm
and cataracts in all treated rats. Mortality was not affected. Weight
gain and food intake were decreased in animals at 3000 ppm. Clinical
examination showed an increased incidence of cataracts in animals at
500 and 3000 ppm, but ophthalmoscopy of control animals and those at
the low dose revealed an increased incidence of lenticular
degeneration and one case of lenticular calcification in animals at 50
ppm. Animals at 3000 ppm had increased liver, adrenal, and testicular
weights, and histopathological examination revealed Leydig-cell
tumours in almost all males at 500 or 3000 ppm. Focal hyperplasia and
cystic ducts in the rete testis were seen in males at 3000 ppm, and an
adenoma in the rete testis was seen in one treated and no control
males. Atrophy of the seminal vesicles and coagulating gland was seen
in almost all males at 3000 ppm and in some at 500 ppm. Dose-related
reduced secretion and increased fibrosis in the prostate were seen in
all treated groups. Benign stromal tumours of the sex cord in the
ovaries were seen in 29 rats at 3000 ppm and four controls. Two
animals at the high dose had malignant thecomas of the ovary, with
none in controls. Lipidosis of ovarian interstitial cells and an
increased incidence of abnormal ovarian follicles were seen in all
treated groups. Seven animals at the high dose had adenocarcinomas of
the uterus (none in controls), and three of these had metastases.
Adenomas of the adrenal gland were seen in 21 females at 3000 ppm, and
one had a metastatic carcinoma of the adrenal cortex; there was no
increase in the incidence of adrenal tumours in animals at 50 or
500 ppm. A dose-related incidence of lipidosis and focal hyperplasia
in the adrenal cortex was seen in all treated groups, and hepatic
hypertrophy and eosinophilic foci were seen in a dose-related fashion
at doses down to 50 ppm. Vacuolation of the pancreatic exocrine cells
was seen in rats at 500 or 3000 ppm. At 3000 ppm, males had an
increased incidence of focal hyperplasia in the pituitary, and females
had a reduced incidence of pituitary adenomas. Thus, increased
incidences of tumours were seen in animals of each sex at 3000 ppm and
in males at 500 ppm. The NOAEL was 50 ppm, equal to 2.7 mg/kg bw per
day, for tumour formation and < 50 ppm for overall toxicity
(Mellert et al., 1994d).
(d) Reproductive toxicity
In a three-generation study, groups of 20 Sprague-Dawley rats of
each sex were fed diets containing vinclozolin at 0, 162, 486, or
1458 ppm. Two litters were produced per generation, and the second
litters were used as parental animals (F0, F1, and F2). The
period of treatment before mating was about eight weeks for F0
males, 16 weeks for F0 females, 15 weeks for F1 and F2 males,
and 24 weeks for F1 and F2 females (including lactation). No
treatment-related deaths or clinical signs of toxicity were seen in
pups or parents, and there were no treatment-related effects on food
consumption, body weights, litter size, malformations, birth weight of
pups, sex ratio, or behaviour. In parents, there were no treatment-
related effects on fertility, pregnancy rate, duration of pregnancy,
lactation, or viability. Auditory acuity and ophthalmic parameters
were not affected by treatment. There were no treatment-related
effects on organ weights or gross or microscopic appearance. The
no-effect level was thus > 1458 ppm, equivalent to about 73 mg/kg bw
per day. The results are not in accordance with more recent work, but
this study did not include investigations of anogenital distance in
males, and diets were not analysed for vinclozolin (Leuschner, 1977).
Groups of 24 Wistar rats of each sex were fed diets containing 0,
50, 300, 1000, or 3000 ppm vinclozolin. After a pre-mating period of
10 weeks, these F0 animals were mated twice to produce F1a and
F1b pups. F1a animals were used as F1 parents to produce F2a
and F2b animals. Some rats were raised to adulthood in order to
observe the development of their sexual organs, resulting in an FX
group of F1b rats, an FY group of F2a rats, and an FZ group of
F2b rats. The results of the study are summarized in Table 2. All
F1 males and females at 300 ppm were fertile either at the F2b
mating or at further matings for those animals that did not prove
their fertility in at least one of the scheduled matings for the
F2a/F2b litters. The authors considered that 'clear adverse
effects on fertility were noted only at 1000 and 3000 ppm, whereas 300
and 50 ppm were without any adverse effects on male and female
fertility in both parental generations.' It must be noted, however,
that rats are particularly fertile, and the effects observed,
particularly at 300 ppm, may indicate sub-fertility. The Meeting
concluded that the dietary level of 50 ppm, equivalent to 4.5 mg/kg bw
per day, is a marginal-effect level, on the basis of a possible
treatment-related reduction in the fertility of F1 males at 300 ppm,
signs of delayed development at 300 ppm (reduction in numbers of pups
with pinna unfolding and eye opening at the expected time), and
reduced epididymal weight in F2 animals at 50 ppm (Hellwig et al.,
1990a).
Groups of 25 male and 25 female Wistar rats were fed diets
containing vinclozolin at doses of 0, 20, or 40 ppm. After a 70-day
premating period, these F0 parents were mated to produce F1a and
F1b animals, and F1a rats were mated to produce F2a and F2b
animals. Randomly selected F1b, F2a, and F2b pups were
additionally raised to adulthood, resulting in FX, FY, and FZ groups,
respectively. No effect on clinical signs, weight, food intake, or
reproduction was observed, and gross pathological examination and
organ weight analysis also revealed no reaction to treatment. In
particular, there was no treatment-related effect on epididymal weight
in FX, FY, or FZ animals. The NOAEL was thus 40 ppm, equal to
approximately 4 mg/kg bw per day (Hellwig et al., 1994).
Vinclozolin was administered to groups of pregnant rats by gavage
at doses of 0, 100, or 200 mg/kg bw per day from day 14 of gestation
to postnatal day 3. Male pups in both groups of offspring displayed
feminine characteristics and their reproductive capacity was adversely
affected. About 25% of the treated males died during the study as a
result of bladder stones, hydroureter, or hydronephrosis. Malformations
noted at necropsy, when the males were approximately one year of age,
included the presence of a vaginal pouch, suprainguinal ectopic scrotum
or testes, cleft phallus with hypospadias, and small to absent
accessory sex glands. The authors proposed that vinclozolin is an
androgen receptor antagonist (Gray et al., 1994a).
In a study reported only in brief summary form, pregnant rats
were treated with 0, 3.12, 6.25, 12.5, 25, 50, or 100 mg/kg bw per day
vinclozolin from day 14 of gestation to postnatal day 3. Fertility was
adversely affected at 50 and 100 mg/kg bw per day, and subtle changes
in anogenital distance were observed at all doses (Gray et al.,
1994b).
(e) Developmental toxicity
Mice
Female NMRI albino mice were fed diets containing vinclozolin on
days 1-19 of gestation in two tests. In the first test, 24 mice
received 0 and 28 received 60 000 ppm vinclozolin; in the second,
groups of 30 mice received 0, 600, or 6000 ppm. All animals were
killed on day 19 of gestation. Females at 6000 ppm did not gain weight
during gestation and had decreased food consumption during the first
six days of treatment. Females at 60 000 ppm lost weight and had
decreased food consumption. One female at 6000 ppm died on day 11, and
all females treated at 60 000 ppm died within the first nine days.
Clinical signs of toxicity were observed only in mice at 60 000 ppm
and included ruffled coats and, before death, apathy and signs of
pronounced diuresis. Gross pathology revealed emaciation, atrophy of
musculature, and considerable loss of perirenal fatty tissue in
Table 2. Results of a multigeneration study in Wistar rats fed diets
containing vinclozolin
Dietary level Generation Finding
(ppm)
General toxicity
50 --
300 F0 increased relative liver weight,
marginal signs of anaemia (females),
lenticular degeneration
F1 As F0, plus increased adrenal
weight, Leydig-cell hyperplasia
F2 As F1
1000 F0 Reduced food intake and weight gain,
anaemia (females), increased liver,
adrenal, and testicular weights,
lenticular degeneration, hepatic
single-cell necrosis, Leydig-cell
hyperplasia
F1 As F0, plus vacuolation of pituitary
cells, lipidosis, and hypertrophy of
adrenal cells
3000 F0 Reduced food intake and weight gain,
lenticular degeneration, anaemia
(females), increased liver, adrenal
and testicular weights, hepatic
single-cell necrosis, lipidosis and
hyperplasia of adrenal cells,
vacuolation of pituitary cells,
Leydig-cell hyperplasia
F1 As F0, pills benign Leydig-cell
tumours in some animals
Effects on reproductive performance
50 --
300 F0 --
F1 Possible reduction in fertility in
males (all males eventually proved
fertile)
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
1000 F0 --
F1 Infertility of all males due to
feminization of outer genital organs
3000 F0 Increased total litter loss,
decreased number of delivered pups,
infertility of males
F1 Infertility, of all males
(feminization), infertility of six
females
Signs of developmental toxicity
50 F1 --
F2 Reduced epididymal weight (no
morphological changes)
300 F1 Slight functional reduction of
prostate and coagulating gland,
reduced epididymal
weight
F2 As F1, plus slight interstitial-cell
hyperplasia in testes and ovaries,
some pups with reduced morphological
development (delayed eye opening and
pinna unfolding)
1000 F1 Decreased pup survival, feminization
of males, reduced body-weight gain,
slightly delayed pup development,
reduced size and function of
secondary male genital organs,
atrophy of seminiferous tubules,
interstitial-cell hyperplasia in
testes and ovaries
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
3000 F1 Increased number of stillborn pups,
decreased pup survival, feminization
of males, reduced body-weight gain,
retarded morphological development,
atrophy of primary and secondary
male genital organs,
interstitial-cell hyperplasia in
testes and ovaries
From Hellwig et al. (1990a)
animals that died. No implantation sites were detected in any female
at 6000 or 60 000 ppm, and hence no fetuses were observed at these
doses. Fetuses of dams at 600 ppm had no treatment-related adverse
effects. The NOAEL for maternal toxicity and fetotoxicity was 600 ppm,
equivalent to 90 mg/kg bw per day (Hofman & Peh, 1975a).
Rats
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 15, 50, or 150 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
effects, and no adverse findings were noted at gross necropsy. There
were no treatment-related effects on pre- or post-implantation losses,
resorptions, numbers of live fetuses, or fetal or placental weights.
Anogenital distances were decreased in male fetuses at 150 and, to a
lesser extent, 50 mg/kg bw per day. This effect was considered to
indicate the beginning of feminization of male fetuses, perhaps due to
a hormonal (anti-androgenic) action on sexual differentiation. There
were no other treatment-related effects on male or female fetuses. As
no maternal toxicity, embryotoxicity, or fetotoxicity was observed,
the NOAEL was > 150 mg/kg bw per day. The NOAEL for teratogenicity
was 15 mg/kg bw per day, on the basis of reductions in anogenital
distance at 50 and 150 mg/kg bw per day (Hellwig et al., 1989a).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 50, 100, or 200 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, or
placental or fetal weights. The only possibly treatment-related
morphological effect observed in the fetuses was a significantly
increased incidence of symmetrically dumb-bell-shaped thoracic
vertebral bodies at 200 mg/kg bw per day, which is indicative of
retarded development. A slight, not statistically significant increase
was observed at 100 mg/kg bw per day. Similar effects were observed in
a follow-up study. The NOAEL for maternal toxicity was > 200 mg/kg bw
per day, and that for fetotoxicity was 100 mg/kg bw per day, on the
basis of a possible treatment-related increase in symmetrically
dumb-bell-shaped thoracic vertebral bodies at 200 mg/kg bw per day.
There was no NOAEL for teratogenicity, as the anogenital distance in
fetuses was not measured (Hellwig et al., 1989b).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 200, or 400 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at gross
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, fetal
or placental weights, or sex ratio. The anogenital distances of male
fetuses were decreased in a dose-related fashion at both 200 and
400 mg/kg bw per day, indicating the beginning of feminization. A
treatment-related increase in dilated renal pelvis and hydroureter was
also observed in fetuses of animals of each sex, which were
statistically significant in fetuses at 400 mg/kg bw per day. These
findings are considered to indicate a transient developmental delay.
There was also a significant increase in the number of fetuses with
symmetrical dumb-bell-shaped thoracic vertebral bodies at 200 and
400 mg/kg bw per day, indicative of retarded development. At 400 mg/kg
bw per day, the numbers of fetuses with accessory 14th ribs were also
significantly increased. The NOAEL for maternal toxicity was >
400 mg/kg bw per day, and that for fetotoxicity was < 200 mg/kg bw
per day, on the basis of the increased incidences of symmetrical
dumb-bell-shaped thoracic vertebral bodies at 200 and 400 mg/kg bw per
day in animals of each sex. The NOAEL for teratogenicity was <
200 mg/kg bw per day, on the basis of reductions in anogenital
distance in male fetuses at 200 and 400 mg/kg bw per day (Hellwig
et al., 1989c).
Groups of 10 female Wistar rats were treated orally with
vinclozolin at doses of 0, 600, or 1000 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths and no
abortions. Clinical signs of toxicity included unsteady gait in one
female at 600 mg/kg bw per day and in seven females at 1000 mg/kg bw
per day, and piloerection in two females at 1000 mg/kg bw per day.
Food consumption was reduced in animals at 1000 mg/kg bw per day on
days 6-13 of gestation, and the water consumption of animals at 600
and 1000 mg/kg bw per day was increased. The body-weight gain of
females at 1000 mg/kg bw per day was impaired on days 8-10.
Haematological investigations performed at day 20 revealed no
treatment-related findings. Liver and adrenal weights were increased
in a dose-related fashion. There were no treatment-related effects on
pre- or post-implantation losses, resorptions, numbers of live
fetuses, or placental weights. Fetal weights were decreased at
1000 mg/kg bw per day. When the sex ratio was determined by measuring
the anogenital distance, 68% of fetuses at 600 and 99% at 1000 mg/kg
bw per day were deemed to be female; however, internal examination
showed that 39% at 600 and 52% at 1000 mg/kg bw per day were female.
The anogenital distances were decreased in treated male fetuses. At
1000 mg/kg bw per day, the incidences of symmetrical dumb-bell-shaped
thoracic vertebral bodies were increased. The NOAEL for maternal
toxicity was < 600 mg/kg bw per day, on the basis of increased liver
and adrenal weights and findings at necropsy at 600 and 1000 mg/kg bw
per day. The NOAEL for fetotoxicity was < 600 mg/kg bw per day, on
the basis of increased incidences of hydroureter at 600 and 1000 mg/kg
bw per day. The NOAEL for teratogenicity was < 600 mg/kg bw per day,
on the basis of reductions in anogenital distance in male fetuses at
600 and 1000 mg/kg bw per day (Hellwig et al., 1989d).
In a range finding study, groups of 10 female Wistar rats
received dermal applications of vinclozolin at doses of 0, 300, 900,
or 2500 mg/kg bw per day for 6 h/day on days 6-19 of gestation. The
substance was applied onto the dorsal area of the trunk under an
occlusive dressing, and the animals were killed on day 20. Adrenal and
liver weights were increased, but not in a dose-related fashion, in
all treated animals. The anogenital distances were reduced in male
fetuses but the effect was not dose-related. The incidences of dilated
renal pelvis in fetuses were increased in a dose-related fashion at
all doses, with statistical significance at 900 and 2500 mg/kg bw per
day. The absence of a dose-response relationship for some of the
effects observed in this study is probably attributable to limited
dermal absorption of the rather pasty suspension of vinclozolin at
2500 mg/kg bw per day.
In the main study, groups of female Wistar rats received dermal
applications of 0, 60, 180, or 360 mg/kg bw per day for 6 h/day on
days 6-19 of gestation. The substance was applied on the dorsal area
of the trunk under an occlusive dressing, and the animals were killed
on day 20. There were no deaths, no abortions, no signs of toxicity,
no treatment-related effects on body weight or food consumption, and
no treatment-related findings in haematological investigations
performed on day 20. Absolute liver weights were increased at 180 and
360 mg/kg bw per day; absolute adrenal weights were significantly
increased at these doses but not dose-dependently. There were no
treatment-related macroscopic pathological findings or effects on
implantation losses, resorptions, numbers of fetuses, sex ratio, or
weights of fetuses or placentae. The anogenital distances of males at
180 and 360 mg/kg bw per day were decreased, but there were no other
treatment-related malformations, variations, or retardations in
treated fetuses. The NOAEL for maternal toxicity was 60 mg/kg bw per
day, on the basis of increased absolute adrenal and liver weights at
180 and 360 mg/kg bw per day. The NOAEL for fetotoxicity was >
360 mg/kg bw per day, and that for teratogenicity was 60 mg/kg bw per
day, on the basis of reductions in anogenital distances in male
fetuses at 180 and 360 mg/kg bw per day (Hellwig et al., 1990b).
Rabbits
Groups of 15 New Zealand white female rabbits were treated orally
with vinclozolin at doses of 0, 20, 80, or 300 mg/kg bw per day on
days 6-18 of gestation and were killed on day 29. There were no signs
of toxicity and no deaths that were obviously attributable to
treatment. Body-weight gain was not affected, and there were no
treatment-related effects on numbers of live young, sex ratio,
embryonic deaths, pre-implantation losses, or litter or fetal weights.
The frequency of post-implantation losses was slightly increased but
was within historical control values. There were no treatment-related
major malformations or visceral anomalies. There was a slight,
non-dose-dependent increase in the incidence of minor skeletal
anomalies, which was within historical control values. The NOAEL for
maternal toxicity and fetotoxicity was > 300 mg/kg bw per day (Cozens
et al., 1981; Cozens & Palmer, 1987).
Groups of 15 female Himalayan rabbits were treated orally with
vinclozolin at 0, 50, 200, or 800 mg/kg bw per day on days 7-28 of
gestation and were killed on day 29. One female at 200 mg/kg bw per
day aborted on day 26 and was killed; at 800 mg/kg bw per day, one
female died, one aborted and died, and 11 aborted and were killed. The
abortions occurred between days 21 and 27. Clinical signs of toxicity
included reddish-brown discolouration of the urine in 13 females and
apathy, hunched posture, conjunctivitis, and urine-smeared fur in one
or two animals at 800 mg/kg bw per day. The female at 200 mg/kg bw per
day which aborted had vaginal haemorrhage and discoloured urine. A
second female at this dose had vaginal haemorrhage on day 24. The food
consumption of animals at 800 mg/kg bw per day was significantly
reduced during the treatment period, and that of animals at 200 mg/kg
bw per day was reduced mainly on days 7-19. Animals at 50 and
200 mg/kg bw per day had no treatment-related increases in pre- or
post-implantation losses. The only dam at 800 mg/kg bw per day with
viable fetuses had three post-implantation losses, as early
resorptions. There were no treatment-related resorptions in animals at
50 or 200 mg/kg bw per day. The sex ratios, numbers of live fetuses,
and placental and fetal weights were not affected by treatment at 50
or 200 mg/kg bw per day. There were no treatment-related external
malformations or visceral abnormalities. At 800 mg/kg bw per day, one
of four viable fetuses had malformations of the sternebrae, but the
relevance of this finding is questionable owing to the small number of
fetuses. One of 94 fetuses at 50 mg/kg bw per day also had
malformations of the sternebrae, but the incidence was within the
historical control range of 0-1% (mean, 0.2%). No treatment-related
changes in the male fetal genital organs were observed. The NOAEL for
maternal toxicity was 50 mg/kg bw per day, on the basis of reduced
food consumption, abortion, and clinical signs of toxicity at
200 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity was
200 mg/kg bw per day, on the basis of three resorptions as a
consequence of maternal toxicity in the female alive at termination in
the high-dose group. There was no evidence of teratogenicity at 50 or
200 mg/kg bw per day (Hellwig et al., 1990c).
Groups of 20 female Himalayan rabbits were treated orally with
vinclozolin at 0 or 400 mg/kg bw per day on days 7-8 of gestation and
were killed on day 29. One female at 400 mg/kg bw per day aborted and
died on day 19, and nine aborted and were killed between days 20 and
27. Clinical signs of toxicity included blood in the bedding of two
treated females, one of which aborted. Eight had red-brown urine. The
food consumption of treated animals was reduced on days 7-26, and
body-weight gain was impaired on days 7-25. Pre- and post-implantation
losses were increased, and the number of early resorptions was thus
also increased. The numbers of live fetuses per litter were slightly
decreased, and the ratio of males:females was 1:1.8 at 400 mg/kg bw
per day in comparison with 1:1.16 in the controls; however, no
treatment-related changes were seen in male fetal genital organs.
Fetal weights were increased in the treated animals, probably as a
consequence of the slightly decreased litter sizes. There were no
external or soft-tissue malformations, but the frequency of separated
origins of the carotids was 36% in the treated group in comparison
with 10% in concurrent controls and a mean of 19% in historical
controls (range, 10-31%). There were no skeletal malformations and no
treatment-related skeletal variations or retardations; in particular,
no malformed sternebrae were observed, indicating that the effect
observed in the previous study at 50 mg/kg bw per day was not
treatment-related. The NOAEL for maternal, embryo-, and fetotoxicity
was < 400 mg/kg bw per day. There was no clear evidence of
teratogenicity, but the increased incidence of separated origins of
the carotids indicates potential teratogenicity at this severely
maternally toxic dose (Hellwig et al., 1990d).
(f) Genotoxicity
The results of standard regulatory assays for genotoxicity are
summarized in Table 3.
A medium-term bioassay based on preneoplastic glutathione
S-transferase placental form (GST-P)-positive foci in rat liver was
performed with vinclozolin. Rats were injected with N-nitrosodi-
ethylamine and two weeks later were fed a diet containing 2000 ppm
vinclozolin for six weeks, with partial hepatectomy at week 3; they
were then killed. In one group that received only vinclozolin,
negative results were obtained, but administration after initiation
with N-nitrosodiethylamine gave positive results. The authors
reported that the test is highly predictive for genotoxic hepato-
carcinogens but less predictive for carcinogens that have target
organs other than the liver. They suggested that since the assay is
based on the two-stage hypothesis of carcinogenesis, chemicals for
which the results are positive are tumour promoters in rat liver (Ito
& Hasegawa, 1992; Ito et al., 1993, 1994).
Vinclozolin was cytotoxic in BALB/c3T3 cells in the absence but
not in the presence of an exogenous metabolic system. It induced
transformation in this cell line in both the presence and the absence
of metabolic activation (Perocco et al., 1993).
As shown in Table 3, negative results were obtained in a range of
assays in vivo and in vitro. Although positive results were
obtained in the medium-term bioassay and in the test for cell
transformation, these studies are not well validated.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The intact and abraded dorsal skin of four male and two female
white Vienna rabbits was treated with a 50% aqueous suspension of
vinclozolin under an occlusive covering for 24 h. The intact skin of
five of the six animals had well-defined erythema, and slight oedema
was seen in one animal. All of the reactions subsided completely
within 72 h. The erythema that formed on the abraded skin of all
animals after 24 h was more severe than that on the intact skin; it
had completely subsided in one-half the animals by 72 h but remained
unchanged in the other half. One animal in this group had slight
oedema after 24 h but no longer at 72 h. Vinclozolin cannot be
classified as a skin irritant, however, a slight, transient irritation
potential was evident (Hildebrand, 1977a).
Table 3. Results of tests for the genotoxicity of vinclozolin
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S typhimurium TA98, < 1000 µg/plate 92.8 Negativea Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA98, TA100, < 3000 µg/plate 92.8 Negativea Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP hrc
Reverse mutation S. typhimurium TA98, TA100 < 10 000 µg/plate 98.1 Negativea Gelbke & Engelhardt
TA1535, TA1537, TA1538 (1983)
Gene mutation hprt L5178Y cells < 1000 µg/ml NR Negativea Witterland & Hoorn
(1984)
Gene mutation hprt Chinese hamster ovary cells < 10 mg/ml > 99.5 Negativea Gelbke & Jackh (1975)
Chromosomal aberration Chinese hamster ovary cells < 500 µg/ml NR Negativea Murli (1989)
DNA repair B. subtilis M45 and H17 rec < 2000 µg/plate 92.8 Negative Shirasu et al. (1977)
DNA repair B. subtilis M45 and H17 rec < 10 000 µg/plate NR Negativea Hoorn (1983)
Unscheduled DNA Rat hepatocytes < 1000 µg/ml > 99.5 Negative Cifone & Myhr (1984)
synthesis
In vivo
Host-mediated mutation S. typhimurium G46 his- 2 × 200 and 1000 92.8 Negative Shirasu et al. (1977)
mg/kg bw
Dominant lethal mutation NMRI male mice 5 × 2000 mg/kg bw NR Negative Hofmann & Peh
(1975b)
Sister chromatid Male and female Chinese 3830 and 5620 98.1 Negative Gelbke & Engelhardt
exchange hamsters mg/kg bw (1981)
NR, not reported
a With and without metabolic activation
Eye irritation was investigated in three male and three female
New Zealand white rabbits. After 24 h, the only finding was slight
redness of the conjunctivae, which was not completely reversible
within 72 h Vinclozolin may be regarded as not irritating to the eyes
(Hildebrand, 1977b).
Technical-grade vinclozolin was tested in groups of 12 male
Pirbright white guinea-pigs and five controls in the Magnusson-Kligman
maximization test. The treated animals had questionable dermal changes
after the first challenge and distinct changes after the second,
suggesting sensitization (Gelbke, 1979).
No dermal sensitization was observed in an open epicutaneous test
in guinea-pigs with BAS 352 04 F formulation (Ronilan), containing 50%
vinclozolin. A 60% preparation in water caused slight erythema and
oedema at the beginning of the induction period; a 20% preparation
elicited sporadic skin irritation. Since no differences were found
between the control and test groups, these concentrations are assumed
not to be sensitizing. Neither skin irritation nor sensitization was
observed with 2 or 6% preparations. These concentrations can be
assumed not to have sensitizing potential that would be of importance
under field conditions (Grundler & Gelbke, 1980).
(ii) Hormonal effects
Groups of 20 male and 20 female Wistar rats were fed diets
containing 4500 ppm vinclozolin for six months; the control group
consisted of 10 males and 10 females. After sacrifice, blood was
collected and the levels of the following hormones were determined:
adrenocorticotrophic hormone (ACTH), corticosterone, dehydroepian-
dosterone (DHEA), testosterone, estradiol, hydroxy-progesterone, and
luteinizing hormone (LH). Males showed more effects than females: in
males, the level of LH was increased by about 10-fold and those of
testosterone and DHEA doubled; in females, the level of LH was
increased by 2.5-fold, but there was no change in the concentrations
of sex steroids. That of ACTH increased in animals of each sex, but
the results were variable and the relationship of this change to
treatment is uncertain (Knuppen, 1989).
Groups of 20 male and 20 female Wistar rats were treated with 0
or 4500 ppm vinclozolin for three months, and groups of 10 of each sex
were then allowed to recover for three months to study the
reversibility of any changes. The hormones that were measured were LH,
follicle-stimulating hormone, testosterone, estradiol, DHEA, ACTH,
aldosterone, and corticosterone. The results basically confirmed those
of the previous study. The levels of LH, follicle-stimulating hormone,
testosterone, and DHEA were increased in males, LH most markedly,
while only that of LH was increased in females. The concentration of
ACTH was increased in both males and females, and those of aldosterone
and corticosterone were marginally increased in males but decreased in
females. All of these changes, except perhaps that of ACTH in females,
was reversible within two months (Mellert et al., 1992).
(iii) Receptor binding
In a study of binding to the androgen receptor, vinclozolin was
incubated in vitro with MCF-7 cells derived from a human mammary
carcinoma which contains a large amount of androgen receptor. A clear
affinity with cytosolic and nuclear androgen receptors was seen
(Knuppen, 1990). In order to confirm this result and to investigate
whether the binding is due to vinclozolin or its main rat metabolite
(Reg. No. 119 208), their effects were compared with those of the
anti-androgenic drug Flutamide and the synthetic androgen Mibolerone.
Vinclozolin bound to the androgen receptor with an affinity of
approximately 50% of that of Flutamide, and the binding affinity of
the metabolite was virtually zero (Knuppen & Schutze, 1991). In a
comparison of the effects of vinclozolin on receptors in vitro and
in vivo, not reported in detail, vinclozolin was shown to have a
binding affinity similar to that of Flutamide for androgen receptors
in the LNCAP cell line, which is derived from human prostate, a
typical target tissue for vinclozolin-mediated anti-androgenic
effects. Vinclozolin was also capable of binding to androgen receptors
in castrated Wistar rats (Knuppen & Schutze, 1992).
The ability of vinclozolin to inhibit 5-alpha-reductase and to
compete with androgen for binding at the androgen receptor was
investigated. Neither vinclozolin nor its degradation products
2-{[(3,5-dichlorophenyl)carbamoyl]oxy}-2-methyl-3-butenoic acid and
3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide inhibited 5-alpha-
reductase. Although vinclozolin competed only weakly with androgen for
binding at the androgen receptor, the two metabolites were effective
antagonists. The authors reported that the concentrations of the first
metabolite in the serum of pregnant rats after treatment with
100 mg/kg bw per day vinclozolin could be sufficient to meet or exceed
the Ki for androgen receptor inhibition in vitro (Kelce et al.,
1994).
(iv) Nephrotoxicity
The acute nephrotoxic potential of vinclozolin was compared with
that of two other N-(3,5-dichlorophenyl) carboximide fungicides:
N-(3,5-dichlorophenyl) succimide and iprodione. Groups of four male
Fischer 344 rats received a single intraperitoneal injection of 0.4 or
1.0 mmol/kg bw or vehicle, and renal function was monitored at 24 and
48 h. N-(3,5-Dichlorophenyl) succimide induced renal effects
characterized by marked diuresis, increased proteinuria, elevated
blood urea nitrogen, increased kidney weights, and proximal tubule
necrosis. Iprodione and vinclozolin caused only minor or no
alterations in renal function (Rankin et al., 1989).
(v) Haemoglobin adduct formation
Female Wistar rats were treated orally with various pesticides at
doses of up to 1 mmol/kg bw. Blood was taken 24 h after treatment,
haemoglobin was isolated and hydrolysed with 1N sodium hydroxide, and
aromatic amines extracted and quantified. No adducts with 3,5-di-
chloroaniline from vinclozolin were found (Sabbioni & Neumann, 1990).
(vi) Review of ophthalmoscopic findings
In a review of the cataracts and other lenticular changes seen at
ophthalmoscopy in short- and long-term studies with vinclozolin in
rats, an analysis was carried out to determine whether these changes
were due to acceleration of normal, age-related changes, since
lenticular changes are seen commonly in old rats and cataracts also
occur spontaneously in untreated rats. The clear NOAELs are shown in
Table 4. Vinclozolin induced cataracts and other lenticular changes
only in rats, and there were no treatment-related lenticular changes
in B6C3F1 or C57B1 mice or in dogs. In the studies of absorption and
distribution, a high concentration of radiolabel was found in the
Harderian gland, which is situated close to the eye and secretes a
fluid onto its surface. The presence of radiolabel in this secretion
was confirmed by examination of enlargements of whole-body
autoradiographs (Schilling, 1993).
3. Observations in humans
A cross-sectional study was performed of 67 men who had handled
vinclozolin for 1-13 years during its synthesis and formulation and 52
unexposed controls. The men were monitored by determining urinary
metabolites containing a 3,5-dichloraniline moiety and observation for
reversible changes in the levels of hormones of the adrenocortico-
tropic and gonadotropic feedback systems, signs of liver injury,
haemolytic anaemia, cataract formation, and hormonally induced
hyperplasia and tumours at high doses. The clinical investigation
consisted of a medical and occupational history questionnaire,
physical examination, laboratory determinations, including
measurements of testosterone, LH, and follicle-stimulating hormone,
ultrasonography of the liver and prostate, a detailed examination, and
routine spirometry. The mean 3,5-dichloraniline concentration in
exposed workers was 235-422 µg/g creatinine, depending on the work
area, in comparison with a mean of 7 µg/g creatinine in controls. On
the basis of a series of assumptions, including 40% excretion through
the kidneys, 1.5-litre urine output per day, and 70-kg body weight,
the authors estimated that the occupational exposure of two-thirds of
the employees to vinclozolin exceeded 25 µg/kg bw per day. The
physical examinations and laboratory tests provided no evidence of
vinclozolin-induced hormonal responses, liver injury, prostatic
changes, cataract formation, or haemolytic anaemia. The authors
concluded that vinclozolin induced no health effects and, in
particular, no anti-androgenic effects (Zober et al., 1995).
Table 4. NOAELs for ophthalmoscopic effects in short- and long-term
studies of the toxicity of vinclozolin in rats
Effect NOAEL (ppm)
Short-term study Long-term study
Cataracts 1000 150
Striations 300 150
Bosselated lens structure 1000 150
Bulbiform thickening 3000 150
Opacities 3000 150
Comments
Vinclozolin is well absorbed after oral administration to rats
and extensively metabolized. The majority of the administered
radiolabel was found in the bile, and no unchanged vinclozolin was
excreted in the urine. After single oral doses of radiolabelled
vinclozolin, excretion was rapid; after multiple doses there was no
significant accumulation. Vinclozolin is only moderately absorbed via
the dermal route in rats: over 72 h, about 17% of a dose of 0.13 mg/kg
bw was excreted in the urine.
Vinclozolin has low acute toxicity, with an oral LD50 in rats
of > 15 000 mg/kg bw. The clinical signs of toxicity after acute
dosing with vinclozolin were generally non-specific and there were no
consistent treatment-related findings at necropsy. Vinclozolin is not
irritating to rabbit skin or eyes, but induced skin sensitization in a
maximization study in guinea-pigs. WHO has classified vinclozolin as
unlikely to present an acute hazard in normal use.
Studies of repeated administration were carried out in mice,
rats, rabbits and dogs, in which vinclozolin and/or its metabolites
caused toxic effects indicative of anti-androgenic activity. In two
three-month feeding studies in different strains of mice at dietary
levels of 100-5000 ppm, the NOAEL was equivalent to 20 mg/kg bw per
day, on the basis of signs of hepatotoxicity, signs consistent with
anti-androgenicity, and changes in the adrenal glands. In two recent
three-month feeding studies in rats (at levels of 0, 300, 1000, and
3000 ppm and 0 and 50 ppm, respectively) vinclozolin caused changes
qualitatively similar to those seen in mice; however, effects on the
adrenal glands (including lipidosis) were seen at 300 ppm, and the
NOAEL was confirmed in the second study as 50 ppm, equal to 4 mg/kg bw
per day. In a 12-month feeding study in dogs at dietary levels of 0,
35, 75 150, or 1500 ppm, the NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological changes in the liver, spleen,
prostate, testis, and adrenals. The results of studies incorporating
withdrawal periods indicate that the anti-androgenic effects of
vinclozolin are reversible on cessation of treatment.
In a recent study of carcinogenicity in C57Bl/6 mice at dietary
levels of 0, 15, 150, 3000, or 8000 ppm, hepatocellular carcinomas
were seen at 8000 ppm. There was evidence of toxicity at 3000 ppm,
including hepatotoxicity, Leydig-cell hyperplasia, atrophy of
accessory sex glands, atrophic uteri, and lipidosis in the
cortico-medullary region of the adrenals. The NOAEL was 150 ppm, equal
to 24 mg/kg bw per day. In an earlier study in NMRI mice at levels of
0, 160, 490, 1460, or 4370 ppm, survival was adversely affected at the
highest dose, and the NOAEL was 490 ppm on the basis of increased
liver weight, without histological change. In rats, the long-term
toxicity and carcinogenicity of vinclozolin has recently been
investigated in three studies with dietary levels of 25-4500 ppm.
Cataracts and other lenticular changes were seen in rats treated with
50 ppm or more. (Mice and dogs were closely examined for ocular
changes, but vinclozolin did not affect the eyes in these species.) An
increased incidence of Leydig-cell tumours was seen in rats treated
with 150 ppm and more, together with atrophy of accessory sex glands.
Benign sex cord stromal tumours in the ovaries were seen in rats
treated at 500 ppm and above, and uterine adenocarcinomas were
detected at 3000 ppm (the highest dose tested in the carcinogenicity
study). Adrenal tumours were seen at 1500 ppm and above.
Hepatocellular carcinomas were seen in males treated with 4500 ppm,
and signs of hepatotoxicity were seen in rats treated with 150 ppm or
more. The NOAEL was 25 ppm, equal to 1.4 mg/kg bw per day.
In multi-generation studies, vinclozolin led to infertility of
males, owing to feminization of the outer genital organs, at dietary
levels of 1000 ppm or more. At 300 ppm, although all males were
eventually proved fertile, the observed effects may have indicated
sub-fertility. At 50 ppm, the only adverse effect was a reduction in
epididymal weight (with no associated morphological changes) in F2
offspring. The NOAEL was 40 ppm, equivalent to approximately 4 mg/kg
bw per day. Recent investigations of developmental toxicity have been
conducted in rats and rabbits. In rats, the most sensitive indicator
of teratogenicity was a reduction in the anogenital distance; in a
series of studies, the NOAEL for a change in anogenital distance was
15 mg/kg bw per day. The NOAEL for fetotoxicity was about 100 mg/kg bw
per day, on the basis of signs of developmental delay, while the NOAEL
for maternal toxicity was about 400 mg/kg bw per day, on the basis of
clinical signs of toxicity.
Three studies of developmental toxicity have been conducted in
rabbits. In the first, there were no signs of maternal toxicity,
fetotoxicity, or teratogenicity at doses up to and including 300 mg/kg
bw per day. In the second study, with doses up to and including
800 mg/kg bw per day, toxicity led to extensive mortality at the
highest dose, precluding any reliable assessment at this dose. The
NOAEL for maternal toxicity was 50 mg/kg bw per day, and that for
fetotoxicity was 200 mg/kg bw per day; there was no evidence of
teratogenicity at this dose (the highest dose available for
assessment). The third study involved only one dose, 400 mg/kg bw per
day. The number of female offspring exceeded the number of males, but
there was no treatment-related change in the appearance of the male
fetal genital organs. An increase in the incidence of separated
origins of the carotid arteries indicated potential teratogenicity at
this maternally toxic dose.
Vinclozolin has been tested for genotoxicity in a range of tests
in vivo and in vitro. The Meeting concluded that vinclozolin is
not genotoxic. It noted that positive results were obtained in a study
of cell transformation, but the process giving rise to this effect is
unknown. One study suggests that vinclozolin may be a promoter in rat
liver in vivo, which may indicate the mechanism by which liver
tumours were induced in rats at a high dose.
Studies have been conducted that confirm the anti-androgenic
properties of vinclozolin, which are likely to be associated with
binding to the androgen receptor. This proposed mechanism of action
could account for the results seen in studies of the reproductive
toxicity and long-term toxicity of vinclozolin.
In an epidemiological study of manufacturing plant personnel, it
was concluded that there was no evidence that vinclozolin had induced
health effects in employees with possible long-term exposure.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1.4 mg/kg bw per day in the two-year study of carcinogenicity
in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 20 mg/kg bw per day (three-month study of
toxicity)
490 ppm, equivalent to 63 mg/kg bw per day (112-week study
of toxicity and carcinogenicity in NMRI mice)
150 ppm equal to 24 mg/kg bw per day (18-month study of
toxicity and carcinogenicity in C57Bl/6 mice)
Rat: 50 ppm, equal to 4 mg/kg bw per day (three-month study of
toxicity)
25 ppm, equal to 1.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
40 ppm, equivalent to 4 mg/kg bw per day (study of
reproductive toxicity)
15 mg/kg bw per day (study of developmental toxicity)
100 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
400 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 50 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
200 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 75 ppm, equal to 2.4 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to vinclozolin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 15 000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test
Inhalation, toxicity, rat LC50 > 29 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat NOAEL = 15 mg/kg bw per day; teratogenicity
Long-term (> one year) Dietary, two years, toxicity and NOAEL = 1.4 mg/kg bw per day; signs of
carcinogenicity, rat antiandrogenicity
Dietary, one year, toxicity, dog NOAEL = 2.4 mg/kg bw per day; signs of
antiandrogenicity
References
Block, I. et al. (1987) Study of effect on vital functions of
animals -- general pharmacology -- of vinclozolin. Unpublished
report from Research & Consulting Co. AG, Itingen, Switzerland.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cameron, B.D. & Jack, L. (1991) In vitro percutaneous absorption of
14C-Reg. No. 83 258. A comparison using rat and human
epidermis. Unpublished report from Inveresk Research
International, Tranent, Scotland. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B. &
Franklin, E.R. (1976) The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral administration to
rats. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cifone, M.A. & Myhr, B.C. (1984) Evaluation of vinclozolin (831233) in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Kensington, Maryland,
USA. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cozens, D.D. & Palmer, A.K. (1987) Statement on maternal toxicity;
effect of vinclozolin on pregnancy of the New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R. & Offer, J.M.
(1981) Effect of vinclozolin on pregnancy of New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P (1979) Study of sensitization effect on guinea pigs
maximization test. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1981) Cytogenetic investigation in
Chinese hamsters after a single oral administration of Reg.
No. 83 258. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of
vinclozolin (Reg. No. 83 258) in the Ames test. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Gelbke, H.P & Jackh, R. (1975) Report on a point mutation test carried
out on CHO cells (HGPRT locus) with the test substance
vinclozolin (substance No. 841382). Unpublished report from BASF
AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1979) Report on the study of the acute oral
toxicity of vinclozolin (Reg. No. 83 258) in beagle dogs.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1981) Acute oral toxicity of vinclozolin in
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gray, L.E., Jr, Ostby, J.S. & Kelce, W.R. (1994a) Developmental
effects of an environmental antiandrogen: The fungicide
vinclozolin alters sex differentiation in the male rat. Toxicol.
Appl. Pharmacol., 129, 46-52.
Gray, L.E, Jr, Ostby, J.S., Monosson, E. & Kelce, W.R. (1994b)
Alterations of sex differentiation in male rats following
perinatal exposure to low doses of the antiandrogenic pesticide
vinclozolin. Biol. Reprod., 50 (Suppl. 1), 101.
Grundler, P. & Gelbke, H.P. (1980) Report on the sensitizing effect of
BAS 352 04 F - Ronilan (WNT No. 791661) in the guinea pig -- open
epicutaneous test (OET). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990a) The biokinetics of 14C-vinclozolin in
the rat. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990b) The biotransformation of 14C-vin-
clozolin in the rat. Unpublished report from Huntingdon Research
Centre, Huntingdon, Cambs, United Kingdom. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991a) The determination by whole body
autoradiography of the tissue distribution of radioactivity in
female rats after oral administration of 14C-vinclozolin.
Unpublished report from Huntingdon Research Centre, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991b) The dermal absorption of
14C-vinclozolin in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1987) Report on the study of the toxicity of
Reg. No. 83 258 (vinclozolin) in beagle dogs after 12-month
administration via the diet. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989a) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- first study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989b) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- second study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF: AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- third study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989d) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- test study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1990a) Report: Reproduction study with Reg.
No. 83 258 in rats. Continuous administration over 2 generations.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990b) Report: Study of the prenatal toxicity of
Reg. No. 83 258 in rats after dermal application. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rabbits after oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990d) Report on the supplementary steady of the
prenatal toxicity of Reg. No. 83 258 in rabbits after oral
administration (gavage). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1994) Report: Second reproduction study with
Reg. No. 83 258 in rats. Continuous administration over 2
generations. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977a) Primary skin irritation of Reg. No. 83 258
(vinclozolin) on the intact and scarified dorsal skin of white
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977b) Primary irritation of Reg. No. 83 258
(vinclozolin) to the eye of white rabbits. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. (1973a) Report on acute oral toxicity trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1973b) Report on the acute intraperitoneal trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1974) Report on the testing of 3-(3,5-dichiorophenyl)-
5-methyl-5-vinyl-l,3-oxazolidine-2,4-dione in a 3-month feeding
experiment on rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975a) Report on the supplementary
toxicological study of 3-(3,5-dichiorophenyl)-5-methyl-
5-vinyl-1,3-oxazolidine-2,4-dione in a 4-week feeding study in
the rat. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975b) Report on the toxicological testing
of 3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-
2,4-dione in a three-month feeding trial on the dog. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975a) Study on the prenatal toxicity of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on mice. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975b) Study of the mutagenic effect of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on the male mouse following repeated oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hoorn, A.J.W. (1983) Mutagenicity of vinclozolin, compound No. 831233,
in the rec assay with Bacillus subtilis. Unpublished report
from Litton Bionetics, Veenendaal, Netherlands. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Ito, N. & Hasegawa, R. (1992) Liver medium term bioassay in rats for
screening of carcinogenesis and modifying factors in
hepatocarcinogenesis. Food Chem. Toxicol., 30, 979-992.
Ito, N., Hoshiya, T., Hasegawa, R., Hakoi, K., Cui, L., Ogiso, T. &
Cabral, R. (1993) Enhancement by non-mutagenic pesticides of
GST-P positive hepatic foci development initiated with
diethylnitrosamine in the rat. Cancer Lett., 72, 59-64.
Ito, N., Hasegawa, R., Imaida, K., Takahashi, S. & Shirai, T. (1994)
Medium term rat liver bioassay for rapid detection of carcinogens
and modifiers of hepatocarcinogenesis. Drug Metab. Rev.,
26, 431-442.
Kelce, W.R., Monosson, E., Gamcsik, M.P., Laws, S.C. & Gray, L.E., Jr
(1994) Environmental hormone disruptors: Evidence that
vinclozolin developmental toxicity is mediated by antiandrogenic
metabolites. Toxicol. Appl. Pharmacol., 126, 276-285.
Kirsch, P. (1986a) Report of the study of the acute oral toxicity on
the rat based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. (1986b) Report of the study of the acute oral toxicity on
the mouse based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. et al. (1974) Report on the study of 3-(3,5-dichloro-
phenyl)-5-methyl-5-vinyl-1,3-oxazoildine-2,4-dione for cataract
formation in a 3-month feeding study in dogs. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Kirsch, P., Deckhardt, M. & Hellwig, J. (1982) Report on the study of
the toxicity of Reg. No. 83 258 (vinclozolin) in beagle dogs
after 6-month administration in the diet. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. (1989) Examination of the hormone status. Unpublished
report from University of Lübeck, Lübeck, Germany. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Knuppen, R. (1990) Final report: Study of the binding of
3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-2,4-dione to the androgen
receptor in MCF-7 cells. Unpublished report from University of
Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1991) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) -- Reg. No. 119 208
(metabolite BF 352-22) to the androgen and glucocortiroid
receptors in the cytosol from MC-F-7 cells and from the prostate
and liver tissues of the rat. Unpublished report from University
of Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1992) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) to the androgen receptor
in the cytosol from a cell line expressing the androgen receptor
and from the prostate tissue of the rat. Unpublished report from
University of Lübeck, Lübeck, Germany. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Kretzschmar, R. et al. (1987) Study on the EEG effects in the
conscious rat of vinclozolin. Unpublished report from Knoll AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Leuschner, F. (1977) Chronic oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in a
reproduction study covering three generations of Sprague-Dawley
rats. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikoiogie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. (1979) Acute inhalation toxicity study on the
preparation vinclozolin. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1975) Oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in
Sprague-Dawley rats. Unpublished report from Laboratorium für
Pharmakoiogie und Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977a) 3-weeks-toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in NZW
rabbits by local application. Unpublished report from
Laboratorium fur Pharmakologie und Toxikoiogie (LPT), Hamburg,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977b) Oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in NMRI
mice. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikologie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. et al. (1977c) Chronic oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in the
Sprague-Dawley rat. Unpublished report from Laboratorium fur
Pharmakoiogie and Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1992) Report: Study on the influence of Reg.
No. 83 258 (vinclozolin) on the hormone status of Wistar rats.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1993a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in Wistar rats. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Mellert, W. et al. (1993b) Report: Supplementary study on the oral
toxicity of Reg. No. 83 258 (vinclozolin) in Wistar rats.
Administration in the diet over 3 months. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994a) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in C57BL mice. Administration in the
diet for 18 months Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994b) Report: Study of the chronic toxicity of
Reg. No. 83 258 (Vinclozolin) in rats. Administration via the
diet over 24 months. Unpublished report from BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994c) Report: Chronic toxicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994d) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Murli, H. (1989) Mutagenicity test with Reg. No. 83 258, Batch No. 183
(= ZST No. 881375) in an in vitro cytogenetics assay measuring
chromosome aberration frequency in Chinese hamsters ovary cells
(CHO cells). Unpublished report from Hazleton, Kensington,
Maryland, USA. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Oesch, F. (1977) Ames test for vinclozolin. Unpublished report from
University of Mainz, Mainz, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Otto, S., Bentel, P., Elzner, J. & Ohnsorg, U. (1977) Metabolism of
14C-vinclozolin in rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Perocco, P., Collaci, A. & Grilli, S. (1993) In vitro cytotoxic and
cell transforming activities exerted by the pesticides cyanazine,
dithianon, diflubenzuron, procymidone and vinclozolin on BALB/c
3T3 cells. Environ. Mol. Mutag., 21, 81-86.
Rankin, G.O., Teets, V.J., Nicoll, D.W. & Brown, P.I. (1989)
Comparative acute renal effects of three N(3,5-dichlorophenyl)
carboximide fungicides: N-(3,5-dichlorophenyl) succimide,
vinclozolin and iprodione. Toxicology, 56, 263-272.
Sabbioni G. & Neumann H.-G. (1990) Biomonitoring of arylamines:
Haemoglobin adducts of urea and carbamate pesticides.
Carcinogenesis, 11, 111-116.
Schilling, K. (1993) Evaluation of ophthalmology findings recognised
within various rat feeding studies with Reg. No. 83 258
(vinclozolin). Unpublished report from K. Schilling, Consultant,
Kirchheimbolanden, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in B6C3F1 mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990b) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in C57BL mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Shirasu, Y. et al. (1977) Mutagenicity testing on BAS 352 04 F in
microbial systems. Unpublished report from Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Shirasu, Y., Takahashi, K. & Saito, T. (1978a) Report of acute
toxicity tests with BAS 352 F in mice. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany
Shirasu, Y., Takahashi, K. & Saito, T. (1978b) Report of acute
toxicity tests with BAS 352 F in rats. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Witterland, W.F. & Hoorn, A.J.W. (1984) Mutagenicity evaluation of
vinclozolin (831233) in the mouse lymphoma forward mutation
assay. Unpublished report from Litton Bionetics, Veenendal,
Netherlands. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Zober, A. et al. (1995) Morbidity study of personnel with potential
exposure to vinclozolin. Occup. Environ. Med., 52, 233-241.
After oral administration of 2000 or 5000 mg/kg bw vinclozolin to
male Sprague-Dawley rats, the effects on the cortical electroence-
phalogram were examined. At both doses, sleeping stages were prolonged
and the number and duration of rapid eye movement phases were slightly
reduced; however, these alterations were not pronounced and suggest
only a moderate sedative effect (Kretzschmar et al., 1987).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of vinclozolin are
summarized in Table 1. The clinical signs of toxicity after treatment
with vinclozolin were generally nonspecific, and no consistent,
treatment-related effects were seen at autopsy.
The LD50 of metabolite T,3,5-dichlorophenylcarbamoyl-2-
propionic acid, was reported to be 2740 mg/kg bw in rats and >
2000 mg/kg bw in mice. Clinical signs of reaction to treatment
included dyspnoea, staggering, piloerection, and poor general state
(Kirsch, 1986a,b).
Table 1. Acute toxicity of vinclozolin
Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Mouse Oral > 15 000 92.8 Shirasu et al. (1978a)
Mouse Intraperitoneal 1570-1640 92.8 Shirasu et al. (1978a)
Mouse Subcutaneous > 15 000 92.8 Shirasu et al. (1978a)
Rat Oral > 15 000 92.8 Shirasu et al. (1978b)
Rat Intraperitoneal 4220-8300 92.8 Shirasu et al. (1978a)
Rat Dermal > 5000 92.8 Shirasu et al. (1978b)
Rat Inhalation > 29.1 NR Leuschner (1979)
Guinea-pig Oral 8000 90-97 Hofmann (1973a)
Guinea-pig Intraperitoneal 3000 90-97 Hofmann (1973b)
Rabbit Oral > 5620 98.1 Gelbke & Kirsch (1981)
Dog Oral > 10 000 97 Gelbke & Kirsch (1979)
NR, not reported
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice were fed diets
containing vinclozolin at doses of 0, 100, 1000, 2500, or 5000 ppm for
three months. Food consumption and body weight were determined weekly;
clinical signs were checked daily, with a weekly comprehensive
clinical examination. At the end of the study, clinicochemical and
haematological examinations were performed, and all animals were
assessed grossly and histopathologically. Reduced body-weight gain was
seen in males at the high dose at the end of the study. Clinico-
chemical parameters were affected at doses > 1000 ppm and
haematological parameters only at the highest dose. Decreased
triglyceride and cholesterol levels were seen in animals at 1000 ppm,
and decreased glucose levels in males and albumin levels in females at
the three higher doses. Increased alkaline phosphatase activity was
seen in males and increased globulins in males and females at 2500 and
5000 ppm; alanine aminotransferase activity was increased only in
males at 5000 ppm. Increases were seen in the mean corpuscular
haemoglobin value in females at the two highest doses, the mean
corpuscular volume in animals of each sex, haemoglobin in males, and
reticulocyte counts in females at the highest dose. Absolute and
relative liver weights were found to be increased at the three higher
doses. Centrilobular hypertrophy of hepatocytes was seen in males at
2500 and 5000 ppm; testicular weights were increased at these doses,
and multifocal hyperplasia in Leydig cells was noted at 1000 ppm or
more. Absolute and relative adrenal gland weights were increased in
males at 2500 and 5000 ppm, and lipogenic pigment and lipid vacuoles
in the adrenals were noted in animals of each sex at these doses. In
females at 1000 ppm, increased lipogenic pigment was seen in the
adrenals. Hyperplasia in stromal cells of the ovaries was observed at
the highest dose only. No treatment-related adverse effects were seen
at 100 ppm. The NOAEL was 100 ppm, equivalent to about 20 mg/kg bw per
day (Schilling et al., 1990a).
The B6C3F1 mouse strain has a relatively high frequency of
spontaneous liver lesions, including tumours. In the three-month study
with this strain, clear increases in liver weight and hepatocellular
hypertrophy were observed, indicating that the liver is one of the
target organs of vinclozolin. In order to assess more accurately its
potential proliferative effect on mouse liver, the toxicity of
vinclozolin was investigated in C57B1 mice, which have a low
background incidence of spontaneous liver neoplasia. Groups of 10 male
and 10 female mice were fed diets containing vinclozolin at doses of
0, 100, 1000, or 5000 ppm. Food consumption and body weight were
determined weekly, and clinical signs were checked daily, with a
weekly comprehensive clinical examination. At the end of the study,
clinicochemical and haematological examinations were performed. All
animals were assessed macroscopically and histologically. A reduction
in body-weight gain was seen in males at the high dose. Decreased
triglyceride and cholesterol values were seen in animals at 1000 and
5000 ppm, and glucose was decreased only in males at the high dose.
Increased alanine aminotransferase activity was seen in animals of
each sex, and total protein and alkaline phosphatase were increased in
males at the high dose. Haemoglobin, mean cell volume, and mean
corpuscular haemoglobin were increased in animals of each sex at
5000 ppm and in females at 1000 ppm; haematocrit and leukocyte and
lymphocyte counts were increased in animals of each sex and the
erythrocyte count in males at the highest dose. Increased liver
weights were seen at 1000 and 5000 ppm, and centrilobular hypertrophy
of hepatocytes was seen at 5000 ppm. Increased adrenal weights were
seen males at this dose; an increase in lipogenic pigment was noted
histologically in animals of each sex at 1000 ppm and 5000 ppm and
lipid vacuoles were seen at 5000 ppm. Hyperplasia and hypertrophy of
the stromal cells of the ovaries were seen at the middle and high
doses, and focal hyperplasia of the Leydig cells of the testis was
observed at the high dose. There were no compound-related findings at
100 ppm. The NOAEL was 100 ppm, equivalent to 25 mg/kg bw per day
(Schilling et al., 1990b).
Rats
Groups of 16 male and 16 female Sprague-Dawley rats were fed
diets containing 0,100, 300, 1000, or 2000 ppm technical-grade
vinclozolin for three months, and six rats from each group were
further maintained on control diets for six weeks after the end of the
study. Rats were examined daily for mortality, abnormal appearance or
behaviour, and food consumption. Body weights were determined weekly,
when rats were palpated and the eyes examined. Blood and urine were
sampled before treatment, after six and 12 weeks of treatment, at
termination, and at the end of the observation period. At termination,
rats were sacrificed, dissected, and examined for gross pathological
changes. The absolute and relative weights of major organs were
determined, and a complete set of tissues from each animal was saved
for future histopathological examination. The results of microscopic
examinations of tissues were not reported, except for the eyes, which
were examined in serial sections. A single death occurred, in a female
at the high dose on day 42; all other rats survived to scheduled
termination. No abnormalities of appearance or behaviour were noted,
and the eyes were normal at all examinations. The body weights of
treated rats were comparable to those of controls throughout the
study. Occasional statistically significant increases in food
consumption were seen in treated females, but that of males was not
affected. Haematological changes consistent with decreased erythrocyte
mass-decreased erythrocyte count, haematocrit, and haemoglobin and
increased mean corpuscular haemoglobin and mean corpuscular
haemoglobin concentration-were seen at the six-week sampling time in
males and females fed doses > 300 ppm; however, these changes were
seen at termination only in females at 1000 and 2000 ppm. Clinical
chemical parameters were not altered in a toxicologically significant
manner. At necropsy, no effects of treatment on the gross appearance
of tissues were apparent. Statistically significant, dose-related
increases in the mean absolute and relative weights of the liver and
adrenals were seen in males and females at 1000 and 2000 ppm; at
2000 ppm, increased relative weights of kidneys were seen in males and
females and increased relative spleen weights in females. These
effects were apparently reversible, as they were not observed in
treated rats that received control diets for an additional six weeks.
Microscopic examination of the eyes revealed no treatment-related
abnormalities (Hofmann, 1974).
Groups of rats received vinclozolin at dietary levels of 0, 900,
1800, 3000, or 15 000 ppm for four weeks. There were no clinical signs
of toxicity, and food intake and body-weight gain were reduced only in
animals at the high dose. Erythrocyte parameters were reduced in all
treated female rats, and urinalysis revealed reduced osmolality at the
highest dose. Liver and adrenal weights were increased in all treated
rats, and necropsy revealed a grey-white discolouration of the
adrenals. The ascorbic acid content of the adrenals was increased in
all treated animals, and the glycogen content of the liver was reduced
in all treated females and in males at 15 000 ppm. As progressive,
dose-related transformation of the adrenal cortex was seen in all
treated rats, there was no NOAEL (Hoffman & Munk, 1975a).
Groups of 50 male and 50 female Sprague-Dawley rats received
vinclozolin in the diet at levels of 0, 150, or 450 ppm for three
months. Clinical behaviour, food and drinking-water consumption, and
body-weight gain were not affected, and clinicochemical examinations
and urinalyses revealed no substance-induced changes. Histopatho-
logical examination of the liver, adrenals, and pituitary at the end
of treatment gave no reliable indication of substance-induced changes.
The NOAEL was > 450 ppm, equal to 44 mg/kg bw per day for males and
40 mg/kg bw per day for females (Leuschner et al., 1975).
Groups of 10 male and 10 female Wistar rats received vinclozolin
in the diet at levels of 0, 300, 1000, or 3000 ppm for three months.
There were no clinical signs of reaction to treatment, and no rats
died before the scheduled sacrifice. Body-weight gain and food intake
were unaffected by treatment, but water intake was increased in rats
at 3000 ppm. Ophthalmoscopic examination revealed two cataracts, one
unilateral and one bilateral, in rats receiving 3000 ppm, and other
lenticular changes were seen in all groups; it was concluded that the
ocular lesions were spontaneous and unrelated to treatment. Evidence
of anaemia and decreased serum alkaline phosphatase activity were seen
in animals at the high dose, with decreases in leukocyte counts at
1000 and 3000 ppm. Examination post mortem revealed white, enlarged
adrenals in all treated rats. The weights of the liver, adrenal, and
testis were increased at 1000 and 3000 ppm, and histopathological
examination revealed hypertrophy of the adrenal cortex, cystoid
degeneration in the pituitary, Leydig-cell hyperplasia, cloudy
swelling of hepatocytes, single live cell necrosis, and vacuolization
of luteal cells in the ovaries at these doses. Acinar vacuolization in
the pancreas was seen in all treated rats. Vinclozolin was thus toxic
at all doses; the lowest dietary level of 300 ppm is equal to 22 mg/kg
bw per day (Mellert et al., 1993a).
In order to define a clear no-effect level, groups of 10 male and
10 female Wistar rats received vinclozolin in the diet at levels of 0
or 50 ppm for three months, and investigations similar to those in the
previous study were performed. In particular, laboratory investi-
gations and ophthalmoscopic examinations were carried out, and all
animals underwent detailed pathological examination. No treatment-
related changes were seen. The NOAEL was 50 ppm, equal to 4 mg/kg bw
per day (Mellert et al., 1993b).
Rabbits
Groups of six New Zealand white rabbits received dermal
applications of vinclozolin for 8 h per day for three weeks at doses
of 0, 111, 333, or 1000 mg/kg bw. There were no deaths and no clinical
signs of reaction to treatment; gross pathology and histopathology
revealed no evidence of dermal irritation (Leuschner et al., 1977a).
Dogs
Groups of four beagle dogs of each sex were fed diets containing
vinclozolin at doses of 0, 100, 300, 1000, or 2000 ppm for three
months. There were no signs of toxicity and no compound-induced
deaths. Repeated ophthalmological examinations revealed no effects.
The body-weight gain of treated animals was not affected, and
urinalysis showed no treatment-related findings. Females at 2000 ppm
had a reduced haemoglobin content after four and eight weeks and a
decreased erythrocyte count throughout the study. An increased
platelet count was seen in female animals at 1000 and 2000 ppm.
Howell-Jolly bodies were found in a differential blood count in males
and females at these doses, suggesting a compensatory reaction
elicited by an anaemic process. The relative liver weights and the
relative and absolute adrenal weights were increased in females at the
highest dose. Histopathological assessment revealed compound-induced
cholestasis of the liver. In a further evaluation, there was a slight
to moderate, dose-related increase in the haemosiderin content of the
liver, particularly in females, at 300, 1000, and 2000 ppm. Females
had a higher pigment content than males, and the spleens of females
also had an increased haemosiderin content. It was assumed that the
haemosiderosis in the liver was caused by increased haemolysis, in
contrast to the original interpretation that the pigment deposits in
the liver were due to cholestasis. The NOAEL was 100 ppm, on the basis
of the increased haemosiderin content of the liver (Hoffman & Munk,
1975b).
Pairs of one male and one female beagle dog were given technical-
grade vinclozolin at doses of 0, 1000, or 2000 ppm for three months.
There were no compound-induced deaths or other signs of toxicity
during treatment. The animals underwent ophthalmological examinations
twice a week and were additionally examined by an eye specialist
towards the end of the treatment period. There was no indication of
cataract formation. The NOAEL was thus > 2000 ppm (Kirsch et al.,
1974).
Groups of six beagle dogs of each sex received diets containing
technical-grade vinclozolin at doses of 0, 100, 300, 600, or 2000 ppm
for six months. Clinicochemical, haematological, and urinalyses were
carried out at regular intervals; ophthalmoscopy was performed before
the beginning of the study, after about three months, and towards the
end of treatment. At the end of the study, all animals were examined
grossly and histopathologically. No deaths occurred, food consumption
and body-weight gain were unchanged in comparison with the control
group, and there were no clinical signs of toxicity. Males at the
highest dose had pronounced, and females slight, haemolytic anaemia.
Increased incidences of Howell-Jolly bodies and reticulocytes in males
suggested a compensatory bone-marrow reaction to the compound-induced
loss of blood cells. Slight increases in bilirubin level in males at
600 and 2000 ppm, in the haemoglobin concentration of individual
erythrocytes, and in lactic dehydrogenase activity in males at
2000 ppm were further consequences of increased decomposition of
erythrocytes. The considerably increased platelet counts in males at
2000 ppm and the slight increase in females at 600 and 2000 ppm are
probably due to the anaemic process, since hyper-regenerative anaemia
is frequently accompanied by thrombocytosis. The absolute and relative
adrenal weights showed a dose-related increase in groups treated with
2000, 600, or 300 ppm, and histopathological examination showed
vacuolization of the zona fasciculata and severe lipid incorporation
and birefringence of the adrenal cortex (in females) at the highest
dose. Increased absolute and relative spleen weights were observed at
2000 ppm; at 600 ppm, only the absolute spleen weight was increased in
females. Histopathological examination revealed dilated splenic
sinuses and hyperaemia in females at both doses. Reduced relative
weights of the kidney (at 2000 ppm) and pituitary (at 600 and
2000 ppm), haemosiderin deposits in the liver (at 600 and 2000 ppm),
and severe prostatic atrophy (at 2000 ppm) were also found.
Ophthalmological examinations revealed no compound-related findings.
The NOAEL was 100 ppm, equivalent to 4.0 mg/kg bw per day (Kirsch
et al., 1982).
Groups of six beagle dogs of each sex were fed diets containing
0, 35, 75, 150, or 1500 ppm vinclozolin for 12 months. There were no
deaths and no clinical signs of toxicity. Food consumption and
body-weight gains were not significantly affected by treatment.
Haematological, clinical chemical, and urinalyses were conducted
before treatment and in weeks 13, 26, and 52. At the highest dose,
reticulocyte counts were increased in animals of each sex, platelet
counts were increased in males, the mean cell volume was increased in
females, and total bilirubin concentrations were increased in animals
of each sex. Adrenal weights were increased at 1500 ppm and slightly
increased at 150 ppm in animals of each sex; the adrenals were
enlarged in all males and in five females at 1500 ppm, and the mean
width of the adrenal cortex was increased in animals of each sex at
1500 ppm and, to a lesser degree, in females at lower doses. No
dose-response relationship was identified. Progressive transformation
and increased lipid content were seen in the adrenals of all males and
five females at 1500 ppm. Testicular weights were increased in a
dose-related fashion; at 1500 ppm, the weights were markedly
increased, and at 150 ppm the relative weights were significantly
increased. At 1500 ppm, diffuse hyperplasia of the Leydig cells was
observed in five males. The prostates of one male at 150 ppm and two
at 1500 ppm appeared smaller macroscopically. Histopathological
examination revealed that the prostates of two males at 150 ppm were
slightly or moderately atrophied and those of five males at 1500 ppm
were slightly (one) or severely (four) atrophied; similar but minimal
effects were seen in one male at 0, one at 35, and one at 75 ppm.
Liver weights were increased in animals at doses up to 150 ppm and
were markedly increased in males at 1500 ppm. Increased haemosiderin
deposition was observed in the livers of males at 1500 ppm and of
females at 150 and 1500 ppm. Spleen and thyroid weights were increased
in males at 1500 ppm, and spleen weights were also slightly increased
in females at 1500 ppm. The NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological effects at 150 and 1.500 ppm
(Hellwig et al., 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were fed diets
containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 112 weeks.
The results of analyses of the diets were not reported. There were no
clinical signs of reaction to treatment and no effect on food intake.
The weight gain of males at 1460 or 4370 ppm was reduced, and the
survival of males at the highest dose was adversely affected; no such
changes were seen in females. The weights of the liver and testis were
increased at the highest dose, and liver weight was increased in
females at 1460 ppm. Histopathological examination revealed no
treatment-related changes, and no tumours were seen (Leuschner
et al., 1977b).
Groups of 100 male and 100 female control C57Bl/6/JICO mice and
60 male and 60 female treated animals received vinclozolin in the diet
at levels of 0, 15, 150, 3000, or 8000 ppm for 18 months. Ten animals
of each sex were taken from each group for interim sacrifice after 12
months of treatment. There were no clinical signs. The mortality rates
of males and females at the highest dose were greater than those of
controls, and weight gain and food intake were reduced in animals at
3000 and 8000 ppm. Examination of blood smears revealed an increased
polymorphonuclear neutrophil count and decreased lymphocyte count in
animals of each sex at 8000 ppm. Pathological examinations revealed
similar findings at the interim and terminal kills. Increased liver
and adrenal weights were seen in animals at 3000 and 8000 ppm, with
smaller epididymides, seminal vesicles, and prostate. Focal necrosis,
bile-duct proliferation, and pigment deposition were seen in the
livers of animals at 3000 or 8000 ppm. At 8000 ppm, these changes were
accompanied by diffuse hepatocyte hypertrophy, decreased lipid
storage, and increased focal fatty infiltration in the liver, hepatic
single-cell necrosis, an increased prevalence of biliary cysts in the
livers of males, focal cellular alterations and focal hyperplasia of
the livers of males and females, hepatocellular carcinomas in three
males and 22 females, and hepatocellular adenomas in three females. In
males at 3000 and 8000 ppm, diffuse Leydig-cell hyperplasia of the
testis was seen, with atrophy of the seminal vesicles and coagulation
glands. Females at these doses had atrophic uteri, accompanied in
animals at the highest dose by diffuse stromal hyperplasia, an
increased incidence of pigmented interstitial cells in the ovaries,
and loss of ovarian follicles. The adrenal cortexes of animals at 3000
or 8000 ppm had increased lipogenic pigment in the cortico-medullary
region and lipidosis. In addition, in animals at 8000 ppm, foam cells,
eosinophilic crystals, and pneumonitis were seen in the lungs of males
and erosions or ulcers in the glandular stomachs of males and females.
Vinclozolin thus caused hepatocellular carcinomas at a dose of
8000 ppm, equal to 1300 mg/kg bw per day, a dose associated with clear
toxicity; there were no treatment-related tumours at 3000 ppm, equal
to 495 mg/kg bw per day, although evidence of toxicity was seen. The
NOAEL was 150 ppm, equal to 24 mg/kg bw per day (Mellert et al.,
1994a).
Rats
Groups of 50 male and 50 female Sprague-Dawley rats were fed
diets containing 0, 162, 486, 1460, or 4370 ppm vinclozolin for 130
weeks; the study was terminated when survival in the control group
reached 70%. The results of analyses of the diets were not reported.
There were no clinical signs of reaction to treatment. Dose-related
reductions in food intake and weight gain were seen in animals at 1460
and 4370 ppm, but their survival was better than that of controls.
Clinical chemical and histological investigations showed no reaction
to treatment. Interpretation of the data on organ weights was hampered
by disparities in body weight between groups. No tumours were observed
(Leuschner et al., 1977c).
Groups of 20 male and 20 female Wistar rats received vinclozolin
in the diet at levels of 0, 150, 500, 1500, or 4500 ppm for 24 months.
The only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in all treated males and
cataracts in all treated rats. Although the mortality rate of females
at the high dose was higher than that in concurrent controls, the
increase was marginal and may have been unrelated to treatment. Weight
gain and food intake were adversely affected in animals at 1500 and
4500 ppm. Water intake was increased in males at 4500 ppm and
decreased in females at this dose and in males and females at
1500 ppm. Ophthalmoscopic examination revealed a treatment-related
incidence of cataracts. Bilateral cataracts were present in all
animals at 1500 and 4500 ppm that survived to termination, and
cataracts were also seen at 500 ppm. The ophthalmoscopic changes at
150 ppm were confined to lenticular degeneration and calcification in
a few animals. Animals at 1500 and 4500 ppm showed evidence of
anaemia, decreased serum alanine aminotransferase and alkaline
phosphatase activities, and increased creatinine, total protein,
cholesterol, and gamma-glutamyl transferase activity. Increased liver
and adrenal weights were seen in animals at 4500 ppm and increased
testicular weights in all treated males. Leydig-cell tumours were seen
in almost all animals treated with 500, 1500, or 4500 ppm and were
increased in incidence in rats at 150 ppm in comparison with controls.
Focal hyperplasia and cystic ducts were seen in the rete testis of
rats at 4500 ppm, and two males at this dose but no controls had rete
testicular adenomas. Cystic ducts in the rete testis were also seen in
animals at 1500 ppm. Atrophy of the seminal vesicles, coagulating
gland, and epididymides were seen in almost all animals at 1500 and
4500 ppm, similar, less marked effects being seen in animals at 150
and 500 ppm. Dose-related reduced secretion and increased fibrosis in
the prostate were also seen in all treated males. Benign stromal
tumours of the sex cord in the ovaries were seen in 10 rats treated
with 4500 ppm, four at 1500 ppm, and two at 500 ppm; none were seen in
animals at 150 ppm or in controls. Five adenomas and one metastatic
carcinoma of the adrenal cortex were seen in females at 4500 ppm and
one adenocarcinoma of the adrenal cortex in a female at 1500 ppm; no
adrenal tumours were seen at 150 or 500 ppm or in controls. A
dose-related incidence of lipidosis in the adrenal cortex was seen in
all treated animals. Hepatocellular carcinomas occurred in nine male
rats at 4500 ppm, with none in controls. Although hepatic tumours were
found only in animals at the high dose, hepatic necrosis, hypertrophy,
and eosinophilic foci were seen in a dose-related fashion at doses
down to 150 ppm; the only changes in animals at the low dose were
eosinophilic foci in one female. Vacuolation of the pancreatic
exocrine cells was seen in all treated groups. Treatment-related
changes were also seen in the pituitary in animals at 1500 and
4500 ppm, consisting of a reduction in focal hyperplasia and an
increase in diffuse hyperplasia in males and a reduction in the
incidence of pituitary adenomas in females. Vinclozolin was toxic at
all doses tested. The lowest dietary level of 150 ppm was equal to
8 mg/kg bw per day (Mellert et al., 1994b).
In order to define a clear no-effect level, groups of 20 male and
20 female Wistar rats received vinclozolin in the diet at levels of 0,
25, or 50 ppm for 24 months, with investigations similar to those
performed in the previous study. In particular, laboratory
investigations and ophthalmoscopic examinations were carried out every
three months, and all animals underwent detailed pathological
examination. No treatment-related changes were seen. The NOAEL was
50 ppm, equal to 2.8 mg/kg bw per day (Mellert et al., 1993b).
Groups of 50 male and 50 female Wistar rats received vinclozolin
in the diet at levels of 0, 50, 500, or 3000 ppm for 24 months. The
only clinical signs of reaction to treatment were an increased
incidence of palpable, enlarged testes in males at 500 and 3000 ppm
and cataracts in all treated rats. Mortality was not affected. Weight
gain and food intake were decreased in animals at 3000 ppm. Clinical
examination showed an increased incidence of cataracts in animals at
500 and 3000 ppm, but ophthalmoscopy of control animals and those at
the low dose revealed an increased incidence of lenticular
degeneration and one case of lenticular calcification in animals at 50
ppm. Animals at 3000 ppm had increased liver, adrenal, and testicular
weights, and histopathological examination revealed Leydig-cell
tumours in almost all males at 500 or 3000 ppm. Focal hyperplasia and
cystic ducts in the rete testis were seen in males at 3000 ppm, and an
adenoma in the rete testis was seen in one treated and no control
males. Atrophy of the seminal vesicles and coagulating gland was seen
in almost all males at 3000 ppm and in some at 500 ppm. Dose-related
reduced secretion and increased fibrosis in the prostate were seen in
all treated groups. Benign stromal tumours of the sex cord in the
ovaries were seen in 29 rats at 3000 ppm and four controls. Two
animals at the high dose had malignant thecomas of the ovary, with
none in controls. Lipidosis of ovarian interstitial cells and an
increased incidence of abnormal ovarian follicles were seen in all
treated groups. Seven animals at the high dose had adenocarcinomas of
the uterus (none in controls), and three of these had metastases.
Adenomas of the adrenal gland were seen in 21 females at 3000 ppm, and
one had a metastatic carcinoma of the adrenal cortex; there was no
increase in the incidence of adrenal tumours in animals at 50 or
500 ppm. A dose-related incidence of lipidosis and focal hyperplasia
in the adrenal cortex was seen in all treated groups, and hepatic
hypertrophy and eosinophilic foci were seen in a dose-related fashion
at doses down to 50 ppm. Vacuolation of the pancreatic exocrine cells
was seen in rats at 500 or 3000 ppm. At 3000 ppm, males had an
increased incidence of focal hyperplasia in the pituitary, and females
had a reduced incidence of pituitary adenomas. Thus, increased
incidences of tumours were seen in animals of each sex at 3000 ppm and
in males at 500 ppm. The NOAEL was 50 ppm, equal to 2.7 mg/kg bw per
day, for tumour formation and < 50 ppm for overall toxicity
(Mellert et al., 1994d).
(d) Reproductive toxicity
In a three-generation study, groups of 20 Sprague-Dawley rats of
each sex were fed diets containing vinclozolin at 0, 162, 486, or
1458 ppm. Two litters were produced per generation, and the second
litters were used as parental animals (F0, F1, and F2). The
period of treatment before mating was about eight weeks for F0
males, 16 weeks for F0 females, 15 weeks for F1 and F2 males,
and 24 weeks for F1 and F2 females (including lactation). No
treatment-related deaths or clinical signs of toxicity were seen in
pups or parents, and there were no treatment-related effects on food
consumption, body weights, litter size, malformations, birth weight of
pups, sex ratio, or behaviour. In parents, there were no treatment-
related effects on fertility, pregnancy rate, duration of pregnancy,
lactation, or viability. Auditory acuity and ophthalmic parameters
were not affected by treatment. There were no treatment-related
effects on organ weights or gross or microscopic appearance. The
no-effect level was thus > 1458 ppm, equivalent to about 73 mg/kg bw
per day. The results are not in accordance with more recent work, but
this study did not include investigations of anogenital distance in
males, and diets were not analysed for vinclozolin (Leuschner, 1977).
Groups of 24 Wistar rats of each sex were fed diets containing 0,
50, 300, 1000, or 3000 ppm vinclozolin. After a pre-mating period of
10 weeks, these F0 animals were mated twice to produce F1a and
F1b pups. F1a animals were used as F1 parents to produce F2a
and F2b animals. Some rats were raised to adulthood in order to
observe the development of their sexual organs, resulting in an FX
group of F1b rats, an FY group of F2a rats, and an FZ group of
F2b rats. The results of the study are summarized in Table 2. All
F1 males and females at 300 ppm were fertile either at the F2b
mating or at further matings for those animals that did not prove
their fertility in at least one of the scheduled matings for the
F2a/F2b litters. The authors considered that 'clear adverse
effects on fertility were noted only at 1000 and 3000 ppm, whereas 300
and 50 ppm were without any adverse effects on male and female
fertility in both parental generations.' It must be noted, however,
that rats are particularly fertile, and the effects observed,
particularly at 300 ppm, may indicate sub-fertility. The Meeting
concluded that the dietary level of 50 ppm, equivalent to 4.5 mg/kg bw
per day, is a marginal-effect level, on the basis of a possible
treatment-related reduction in the fertility of F1 males at 300 ppm,
signs of delayed development at 300 ppm (reduction in numbers of pups
with pinna unfolding and eye opening at the expected time), and
reduced epididymal weight in F2 animals at 50 ppm (Hellwig et al.,
1990a).
Groups of 25 male and 25 female Wistar rats were fed diets
containing vinclozolin at doses of 0, 20, or 40 ppm. After a 70-day
premating period, these F0 parents were mated to produce F1a and
F1b animals, and F1a rats were mated to produce F2a and F2b
animals. Randomly selected F1b, F2a, and F2b pups were
additionally raised to adulthood, resulting in FX, FY, and FZ groups,
respectively. No effect on clinical signs, weight, food intake, or
reproduction was observed, and gross pathological examination and
organ weight analysis also revealed no reaction to treatment. In
particular, there was no treatment-related effect on epididymal weight
in FX, FY, or FZ animals. The NOAEL was thus 40 ppm, equal to
approximately 4 mg/kg bw per day (Hellwig et al., 1994).
Vinclozolin was administered to groups of pregnant rats by gavage
at doses of 0, 100, or 200 mg/kg bw per day from day 14 of gestation
to postnatal day 3. Male pups in both groups of offspring displayed
feminine characteristics and their reproductive capacity was adversely
affected. About 25% of the treated males died during the study as a
result of bladder stones, hydroureter, or hydronephrosis. Malformations
noted at necropsy, when the males were approximately one year of age,
included the presence of a vaginal pouch, suprainguinal ectopic scrotum
or testes, cleft phallus with hypospadias, and small to absent
accessory sex glands. The authors proposed that vinclozolin is an
androgen receptor antagonist (Gray et al., 1994a).
In a study reported only in brief summary form, pregnant rats
were treated with 0, 3.12, 6.25, 12.5, 25, 50, or 100 mg/kg bw per day
vinclozolin from day 14 of gestation to postnatal day 3. Fertility was
adversely affected at 50 and 100 mg/kg bw per day, and subtle changes
in anogenital distance were observed at all doses (Gray et al.,
1994b).
(e) Developmental toxicity
Mice
Female NMRI albino mice were fed diets containing vinclozolin on
days 1-19 of gestation in two tests. In the first test, 24 mice
received 0 and 28 received 60 000 ppm vinclozolin; in the second,
groups of 30 mice received 0, 600, or 6000 ppm. All animals were
killed on day 19 of gestation. Females at 6000 ppm did not gain weight
during gestation and had decreased food consumption during the first
six days of treatment. Females at 60 000 ppm lost weight and had
decreased food consumption. One female at 6000 ppm died on day 11, and
all females treated at 60 000 ppm died within the first nine days.
Clinical signs of toxicity were observed only in mice at 60 000 ppm
and included ruffled coats and, before death, apathy and signs of
pronounced diuresis. Gross pathology revealed emaciation, atrophy of
musculature, and considerable loss of perirenal fatty tissue in
Table 2. Results of a multigeneration study in Wistar rats fed diets
containing vinclozolin
Dietary level Generation Finding
(ppm)
General toxicity
50 --
300 F0 increased relative liver weight,
marginal signs of anaemia (females),
lenticular degeneration
F1 As F0, plus increased adrenal
weight, Leydig-cell hyperplasia
F2 As F1
1000 F0 Reduced food intake and weight gain,
anaemia (females), increased liver,
adrenal, and testicular weights,
lenticular degeneration, hepatic
single-cell necrosis, Leydig-cell
hyperplasia
F1 As F0, plus vacuolation of pituitary
cells, lipidosis, and hypertrophy of
adrenal cells
3000 F0 Reduced food intake and weight gain,
lenticular degeneration, anaemia
(females), increased liver, adrenal
and testicular weights, hepatic
single-cell necrosis, lipidosis and
hyperplasia of adrenal cells,
vacuolation of pituitary cells,
Leydig-cell hyperplasia
F1 As F0, pills benign Leydig-cell
tumours in some animals
Effects on reproductive performance
50 --
300 F0 --
F1 Possible reduction in fertility in
males (all males eventually proved
fertile)
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
1000 F0 --
F1 Infertility of all males due to
feminization of outer genital organs
3000 F0 Increased total litter loss,
decreased number of delivered pups,
infertility of males
F1 Infertility, of all males
(feminization), infertility of six
females
Signs of developmental toxicity
50 F1 --
F2 Reduced epididymal weight (no
morphological changes)
300 F1 Slight functional reduction of
prostate and coagulating gland,
reduced epididymal
weight
F2 As F1, plus slight interstitial-cell
hyperplasia in testes and ovaries,
some pups with reduced morphological
development (delayed eye opening and
pinna unfolding)
1000 F1 Decreased pup survival, feminization
of males, reduced body-weight gain,
slightly delayed pup development,
reduced size and function of
secondary male genital organs,
atrophy of seminiferous tubules,
interstitial-cell hyperplasia in
testes and ovaries
Table 2. (cont'd).
Dietary level Generation Finding
(ppm)
3000 F1 Increased number of stillborn pups,
decreased pup survival, feminization
of males, reduced body-weight gain,
retarded morphological development,
atrophy of primary and secondary
male genital organs,
interstitial-cell hyperplasia in
testes and ovaries
From Hellwig et al. (1990a)
animals that died. No implantation sites were detected in any female
at 6000 or 60 000 ppm, and hence no fetuses were observed at these
doses. Fetuses of dams at 600 ppm had no treatment-related adverse
effects. The NOAEL for maternal toxicity and fetotoxicity was 600 ppm,
equivalent to 90 mg/kg bw per day (Hofman & Peh, 1975a).
Rats
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 15, 50, or 150 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
effects, and no adverse findings were noted at gross necropsy. There
were no treatment-related effects on pre- or post-implantation losses,
resorptions, numbers of live fetuses, or fetal or placental weights.
Anogenital distances were decreased in male fetuses at 150 and, to a
lesser extent, 50 mg/kg bw per day. This effect was considered to
indicate the beginning of feminization of male fetuses, perhaps due to
a hormonal (anti-androgenic) action on sexual differentiation. There
were no other treatment-related effects on male or female fetuses. As
no maternal toxicity, embryotoxicity, or fetotoxicity was observed,
the NOAEL was > 150 mg/kg bw per day. The NOAEL for teratogenicity
was 15 mg/kg bw per day, on the basis of reductions in anogenital
distance at 50 and 150 mg/kg bw per day (Hellwig et al., 1989a).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 50, 100, or 200 mg/kg bw per day on days
6-19 of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, or
placental or fetal weights. The only possibly treatment-related
morphological effect observed in the fetuses was a significantly
increased incidence of symmetrically dumb-bell-shaped thoracic
vertebral bodies at 200 mg/kg bw per day, which is indicative of
retarded development. A slight, not statistically significant increase
was observed at 100 mg/kg bw per day. Similar effects were observed in
a follow-up study. The NOAEL for maternal toxicity was > 200 mg/kg bw
per day, and that for fetotoxicity was 100 mg/kg bw per day, on the
basis of a possible treatment-related increase in symmetrically
dumb-bell-shaped thoracic vertebral bodies at 200 mg/kg bw per day.
There was no NOAEL for teratogenicity, as the anogenital distance in
fetuses was not measured (Hellwig et al., 1989b).
Groups of 25 female Wistar rats were treated orally with
vinclozolin at doses of 0, 200, or 400 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths, no
clinical signs of toxicity, no abortions, and no treatment-related
effects on food consumption or body weight. Haematological
investigations performed at day 20 revealed no treatment-related
findings, and no treatment-related adverse signs were noted at gross
necropsy. There were no treatment-related effects on pre- or
post-implantation losses, resorptions, numbers of live fetuses, fetal
or placental weights, or sex ratio. The anogenital distances of male
fetuses were decreased in a dose-related fashion at both 200 and
400 mg/kg bw per day, indicating the beginning of feminization. A
treatment-related increase in dilated renal pelvis and hydroureter was
also observed in fetuses of animals of each sex, which were
statistically significant in fetuses at 400 mg/kg bw per day. These
findings are considered to indicate a transient developmental delay.
There was also a significant increase in the number of fetuses with
symmetrical dumb-bell-shaped thoracic vertebral bodies at 200 and
400 mg/kg bw per day, indicative of retarded development. At 400 mg/kg
bw per day, the numbers of fetuses with accessory 14th ribs were also
significantly increased. The NOAEL for maternal toxicity was >
400 mg/kg bw per day, and that for fetotoxicity was < 200 mg/kg bw
per day, on the basis of the increased incidences of symmetrical
dumb-bell-shaped thoracic vertebral bodies at 200 and 400 mg/kg bw per
day in animals of each sex. The NOAEL for teratogenicity was <
200 mg/kg bw per day, on the basis of reductions in anogenital
distance in male fetuses at 200 and 400 mg/kg bw per day (Hellwig
et al., 1989c).
Groups of 10 female Wistar rats were treated orally with
vinclozolin at doses of 0, 600, or 1000 mg/kg bw per day on days 6-19
of gestation and were killed on day 20. There were no deaths and no
abortions. Clinical signs of toxicity included unsteady gait in one
female at 600 mg/kg bw per day and in seven females at 1000 mg/kg bw
per day, and piloerection in two females at 1000 mg/kg bw per day.
Food consumption was reduced in animals at 1000 mg/kg bw per day on
days 6-13 of gestation, and the water consumption of animals at 600
and 1000 mg/kg bw per day was increased. The body-weight gain of
females at 1000 mg/kg bw per day was impaired on days 8-10.
Haematological investigations performed at day 20 revealed no
treatment-related findings. Liver and adrenal weights were increased
in a dose-related fashion. There were no treatment-related effects on
pre- or post-implantation losses, resorptions, numbers of live
fetuses, or placental weights. Fetal weights were decreased at
1000 mg/kg bw per day. When the sex ratio was determined by measuring
the anogenital distance, 68% of fetuses at 600 and 99% at 1000 mg/kg
bw per day were deemed to be female; however, internal examination
showed that 39% at 600 and 52% at 1000 mg/kg bw per day were female.
The anogenital distances were decreased in treated male fetuses. At
1000 mg/kg bw per day, the incidences of symmetrical dumb-bell-shaped
thoracic vertebral bodies were increased. The NOAEL for maternal
toxicity was < 600 mg/kg bw per day, on the basis of increased liver
and adrenal weights and findings at necropsy at 600 and 1000 mg/kg bw
per day. The NOAEL for fetotoxicity was < 600 mg/kg bw per day, on
the basis of increased incidences of hydroureter at 600 and 1000 mg/kg
bw per day. The NOAEL for teratogenicity was < 600 mg/kg bw per day,
on the basis of reductions in anogenital distance in male fetuses at
600 and 1000 mg/kg bw per day (Hellwig et al., 1989d).
In a range finding study, groups of 10 female Wistar rats
received dermal applications of vinclozolin at doses of 0, 300, 900,
or 2500 mg/kg bw per day for 6 h/day on days 6-19 of gestation. The
substance was applied onto the dorsal area of the trunk under an
occlusive dressing, and the animals were killed on day 20. Adrenal and
liver weights were increased, but not in a dose-related fashion, in
all treated animals. The anogenital distances were reduced in male
fetuses but the effect was not dose-related. The incidences of dilated
renal pelvis in fetuses were increased in a dose-related fashion at
all doses, with statistical significance at 900 and 2500 mg/kg bw per
day. The absence of a dose-response relationship for some of the
effects observed in this study is probably attributable to limited
dermal absorption of the rather pasty suspension of vinclozolin at
2500 mg/kg bw per day.
In the main study, groups of female Wistar rats received dermal
applications of 0, 60, 180, or 360 mg/kg bw per day for 6 h/day on
days 6-19 of gestation. The substance was applied on the dorsal area
of the trunk under an occlusive dressing, and the animals were killed
on day 20. There were no deaths, no abortions, no signs of toxicity,
no treatment-related effects on body weight or food consumption, and
no treatment-related findings in haematological investigations
performed on day 20. Absolute liver weights were increased at 180 and
360 mg/kg bw per day; absolute adrenal weights were significantly
increased at these doses but not dose-dependently. There were no
treatment-related macroscopic pathological findings or effects on
implantation losses, resorptions, numbers of fetuses, sex ratio, or
weights of fetuses or placentae. The anogenital distances of males at
180 and 360 mg/kg bw per day were decreased, but there were no other
treatment-related malformations, variations, or retardations in
treated fetuses. The NOAEL for maternal toxicity was 60 mg/kg bw per
day, on the basis of increased absolute adrenal and liver weights at
180 and 360 mg/kg bw per day. The NOAEL for fetotoxicity was >
360 mg/kg bw per day, and that for teratogenicity was 60 mg/kg bw per
day, on the basis of reductions in anogenital distances in male
fetuses at 180 and 360 mg/kg bw per day (Hellwig et al., 1990b).
Rabbits
Groups of 15 New Zealand white female rabbits were treated orally
with vinclozolin at doses of 0, 20, 80, or 300 mg/kg bw per day on
days 6-18 of gestation and were killed on day 29. There were no signs
of toxicity and no deaths that were obviously attributable to
treatment. Body-weight gain was not affected, and there were no
treatment-related effects on numbers of live young, sex ratio,
embryonic deaths, pre-implantation losses, or litter or fetal weights.
The frequency of post-implantation losses was slightly increased but
was within historical control values. There were no treatment-related
major malformations or visceral anomalies. There was a slight,
non-dose-dependent increase in the incidence of minor skeletal
anomalies, which was within historical control values. The NOAEL for
maternal toxicity and fetotoxicity was > 300 mg/kg bw per day (Cozens
et al., 1981; Cozens & Palmer, 1987).
Groups of 15 female Himalayan rabbits were treated orally with
vinclozolin at 0, 50, 200, or 800 mg/kg bw per day on days 7-28 of
gestation and were killed on day 29. One female at 200 mg/kg bw per
day aborted on day 26 and was killed; at 800 mg/kg bw per day, one
female died, one aborted and died, and 11 aborted and were killed. The
abortions occurred between days 21 and 27. Clinical signs of toxicity
included reddish-brown discolouration of the urine in 13 females and
apathy, hunched posture, conjunctivitis, and urine-smeared fur in one
or two animals at 800 mg/kg bw per day. The female at 200 mg/kg bw per
day which aborted had vaginal haemorrhage and discoloured urine. A
second female at this dose had vaginal haemorrhage on day 24. The food
consumption of animals at 800 mg/kg bw per day was significantly
reduced during the treatment period, and that of animals at 200 mg/kg
bw per day was reduced mainly on days 7-19. Animals at 50 and
200 mg/kg bw per day had no treatment-related increases in pre- or
post-implantation losses. The only dam at 800 mg/kg bw per day with
viable fetuses had three post-implantation losses, as early
resorptions. There were no treatment-related resorptions in animals at
50 or 200 mg/kg bw per day. The sex ratios, numbers of live fetuses,
and placental and fetal weights were not affected by treatment at 50
or 200 mg/kg bw per day. There were no treatment-related external
malformations or visceral abnormalities. At 800 mg/kg bw per day, one
of four viable fetuses had malformations of the sternebrae, but the
relevance of this finding is questionable owing to the small number of
fetuses. One of 94 fetuses at 50 mg/kg bw per day also had
malformations of the sternebrae, but the incidence was within the
historical control range of 0-1% (mean, 0.2%). No treatment-related
changes in the male fetal genital organs were observed. The NOAEL for
maternal toxicity was 50 mg/kg bw per day, on the basis of reduced
food consumption, abortion, and clinical signs of toxicity at
200 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity was
200 mg/kg bw per day, on the basis of three resorptions as a
consequence of maternal toxicity in the female alive at termination in
the high-dose group. There was no evidence of teratogenicity at 50 or
200 mg/kg bw per day (Hellwig et al., 1990c).
Groups of 20 female Himalayan rabbits were treated orally with
vinclozolin at 0 or 400 mg/kg bw per day on days 7-8 of gestation and
were killed on day 29. One female at 400 mg/kg bw per day aborted and
died on day 19, and nine aborted and were killed between days 20 and
27. Clinical signs of toxicity included blood in the bedding of two
treated females, one of which aborted. Eight had red-brown urine. The
food consumption of treated animals was reduced on days 7-26, and
body-weight gain was impaired on days 7-25. Pre- and post-implantation
losses were increased, and the number of early resorptions was thus
also increased. The numbers of live fetuses per litter were slightly
decreased, and the ratio of males:females was 1:1.8 at 400 mg/kg bw
per day in comparison with 1:1.16 in the controls; however, no
treatment-related changes were seen in male fetal genital organs.
Fetal weights were increased in the treated animals, probably as a
consequence of the slightly decreased litter sizes. There were no
external or soft-tissue malformations, but the frequency of separated
origins of the carotids was 36% in the treated group in comparison
with 10% in concurrent controls and a mean of 19% in historical
controls (range, 10-31%). There were no skeletal malformations and no
treatment-related skeletal variations or retardations; in particular,
no malformed sternebrae were observed, indicating that the effect
observed in the previous study at 50 mg/kg bw per day was not
treatment-related. The NOAEL for maternal, embryo-, and fetotoxicity
was < 400 mg/kg bw per day. There was no clear evidence of
teratogenicity, but the increased incidence of separated origins of
the carotids indicates potential teratogenicity at this severely
maternally toxic dose (Hellwig et al., 1990d).
(f) Genotoxicity
The results of standard regulatory assays for genotoxicity are
summarized in Table 3.
A medium-term bioassay based on preneoplastic glutathione
S-transferase placental form (GST-P)-positive foci in rat liver was
performed with vinclozolin. Rats were injected with N-nitrosodi-
ethylamine and two weeks later were fed a diet containing 2000 ppm
vinclozolin for six weeks, with partial hepatectomy at week 3; they
were then killed. In one group that received only vinclozolin,
negative results were obtained, but administration after initiation
with N-nitrosodiethylamine gave positive results. The authors
reported that the test is highly predictive for genotoxic hepato-
carcinogens but less predictive for carcinogens that have target
organs other than the liver. They suggested that since the assay is
based on the two-stage hypothesis of carcinogenesis, chemicals for
which the results are positive are tumour promoters in rat liver (Ito
& Hasegawa, 1992; Ito et al., 1993, 1994).
Vinclozolin was cytotoxic in BALB/c3T3 cells in the absence but
not in the presence of an exogenous metabolic system. It induced
transformation in this cell line in both the presence and the absence
of metabolic activation (Perocco et al., 1993).
As shown in Table 3, negative results were obtained in a range of
assays in vivo and in vitro. Although positive results were
obtained in the medium-term bioassay and in the test for cell
transformation, these studies are not well validated.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The intact and abraded dorsal skin of four male and two female
white Vienna rabbits was treated with a 50% aqueous suspension of
vinclozolin under an occlusive covering for 24 h. The intact skin of
five of the six animals had well-defined erythema, and slight oedema
was seen in one animal. All of the reactions subsided completely
within 72 h. The erythema that formed on the abraded skin of all
animals after 24 h was more severe than that on the intact skin; it
had completely subsided in one-half the animals by 72 h but remained
unchanged in the other half. One animal in this group had slight
oedema after 24 h but no longer at 72 h. Vinclozolin cannot be
classified as a skin irritant, however, a slight, transient irritation
potential was evident (Hildebrand, 1977a).
Table 3. Results of tests for the genotoxicity of vinclozolin
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S typhimurium TA98, < 1000 µg/plate 92.8 Negativea Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA98, TA100, < 3000 µg/plate 92.8 Negativea Shirasu et al. (1977)
TA1535, TA1537, TA1538,
E. coli WP hrc
Reverse mutation S. typhimurium TA98, TA100 < 10 000 µg/plate 98.1 Negativea Gelbke & Engelhardt
TA1535, TA1537, TA1538 (1983)
Gene mutation hprt L5178Y cells < 1000 µg/ml NR Negativea Witterland & Hoorn
(1984)
Gene mutation hprt Chinese hamster ovary cells < 10 mg/ml > 99.5 Negativea Gelbke & Jackh (1975)
Chromosomal aberration Chinese hamster ovary cells < 500 µg/ml NR Negativea Murli (1989)
DNA repair B. subtilis M45 and H17 rec < 2000 µg/plate 92.8 Negative Shirasu et al. (1977)
DNA repair B. subtilis M45 and H17 rec < 10 000 µg/plate NR Negativea Hoorn (1983)
Unscheduled DNA Rat hepatocytes < 1000 µg/ml > 99.5 Negative Cifone & Myhr (1984)
synthesis
In vivo
Host-mediated mutation S. typhimurium G46 his- 2 × 200 and 1000 92.8 Negative Shirasu et al. (1977)
mg/kg bw
Dominant lethal mutation NMRI male mice 5 × 2000 mg/kg bw NR Negative Hofmann & Peh
(1975b)
Sister chromatid Male and female Chinese 3830 and 5620 98.1 Negative Gelbke & Engelhardt
exchange hamsters mg/kg bw (1981)
NR, not reported
a With and without metabolic activation
Eye irritation was investigated in three male and three female
New Zealand white rabbits. After 24 h, the only finding was slight
redness of the conjunctivae, which was not completely reversible
within 72 h Vinclozolin may be regarded as not irritating to the eyes
(Hildebrand, 1977b).
Technical-grade vinclozolin was tested in groups of 12 male
Pirbright white guinea-pigs and five controls in the Magnusson-Kligman
maximization test. The treated animals had questionable dermal changes
after the first challenge and distinct changes after the second,
suggesting sensitization (Gelbke, 1979).
No dermal sensitization was observed in an open epicutaneous test
in guinea-pigs with BAS 352 04 F formulation (Ronilan), containing 50%
vinclozolin. A 60% preparation in water caused slight erythema and
oedema at the beginning of the induction period; a 20% preparation
elicited sporadic skin irritation. Since no differences were found
between the control and test groups, these concentrations are assumed
not to be sensitizing. Neither skin irritation nor sensitization was
observed with 2 or 6% preparations. These concentrations can be
assumed not to have sensitizing potential that would be of importance
under field conditions (Grundler & Gelbke, 1980).
(ii) Hormonal effects
Groups of 20 male and 20 female Wistar rats were fed diets
containing 4500 ppm vinclozolin for six months; the control group
consisted of 10 males and 10 females. After sacrifice, blood was
collected and the levels of the following hormones were determined:
adrenocorticotrophic hormone (ACTH), corticosterone, dehydroepian-
dosterone (DHEA), testosterone, estradiol, hydroxy-progesterone, and
luteinizing hormone (LH). Males showed more effects than females: in
males, the level of LH was increased by about 10-fold and those of
testosterone and DHEA doubled; in females, the level of LH was
increased by 2.5-fold, but there was no change in the concentrations
of sex steroids. That of ACTH increased in animals of each sex, but
the results were variable and the relationship of this change to
treatment is uncertain (Knuppen, 1989).
Groups of 20 male and 20 female Wistar rats were treated with 0
or 4500 ppm vinclozolin for three months, and groups of 10 of each sex
were then allowed to recover for three months to study the
reversibility of any changes. The hormones that were measured were LH,
follicle-stimulating hormone, testosterone, estradiol, DHEA, ACTH,
aldosterone, and corticosterone. The results basically confirmed those
of the previous study. The levels of LH, follicle-stimulating hormone,
testosterone, and DHEA were increased in males, LH most markedly,
while only that of LH was increased in females. The concentration of
ACTH was increased in both males and females, and those of aldosterone
and corticosterone were marginally increased in males but decreased in
females. All of these changes, except perhaps that of ACTH in females,
was reversible within two months (Mellert et al., 1992).
(iii) Receptor binding
In a study of binding to the androgen receptor, vinclozolin was
incubated in vitro with MCF-7 cells derived from a human mammary
carcinoma which contains a large amount of androgen receptor. A clear
affinity with cytosolic and nuclear androgen receptors was seen
(Knuppen, 1990). In order to confirm this result and to investigate
whether the binding is due to vinclozolin or its main rat metabolite
(Reg. No. 119 208), their effects were compared with those of the
anti-androgenic drug Flutamide and the synthetic androgen Mibolerone.
Vinclozolin bound to the androgen receptor with an affinity of
approximately 50% of that of Flutamide, and the binding affinity of
the metabolite was virtually zero (Knuppen & Schutze, 1991). In a
comparison of the effects of vinclozolin on receptors in vitro and
in vivo, not reported in detail, vinclozolin was shown to have a
binding affinity similar to that of Flutamide for androgen receptors
in the LNCAP cell line, which is derived from human prostate, a
typical target tissue for vinclozolin-mediated anti-androgenic
effects. Vinclozolin was also capable of binding to androgen receptors
in castrated Wistar rats (Knuppen & Schutze, 1992).
The ability of vinclozolin to inhibit 5-alpha-reductase and to
compete with androgen for binding at the androgen receptor was
investigated. Neither vinclozolin nor its degradation products
2-{[(3,5-dichlorophenyl)carbamoyl]oxy}-2-methyl-3-butenoic acid and
3',5'-dichloro-2-hydroxy-2-methylbut-3-enanilide inhibited 5-alpha-
reductase. Although vinclozolin competed only weakly with androgen for
binding at the androgen receptor, the two metabolites were effective
antagonists. The authors reported that the concentrations of the first
metabolite in the serum of pregnant rats after treatment with
100 mg/kg bw per day vinclozolin could be sufficient to meet or exceed
the Ki for androgen receptor inhibition in vitro (Kelce et al.,
1994).
(iv) Nephrotoxicity
The acute nephrotoxic potential of vinclozolin was compared with
that of two other N-(3,5-dichlorophenyl) carboximide fungicides:
N-(3,5-dichlorophenyl) succimide and iprodione. Groups of four male
Fischer 344 rats received a single intraperitoneal injection of 0.4 or
1.0 mmol/kg bw or vehicle, and renal function was monitored at 24 and
48 h. N-(3,5-Dichlorophenyl) succimide induced renal effects
characterized by marked diuresis, increased proteinuria, elevated
blood urea nitrogen, increased kidney weights, and proximal tubule
necrosis. Iprodione and vinclozolin caused only minor or no
alterations in renal function (Rankin et al., 1989).
(v) Haemoglobin adduct formation
Female Wistar rats were treated orally with various pesticides at
doses of up to 1 mmol/kg bw. Blood was taken 24 h after treatment,
haemoglobin was isolated and hydrolysed with 1N sodium hydroxide, and
aromatic amines extracted and quantified. No adducts with 3,5-di-
chloroaniline from vinclozolin were found (Sabbioni & Neumann, 1990).
(vi) Review of ophthalmoscopic findings
In a review of the cataracts and other lenticular changes seen at
ophthalmoscopy in short- and long-term studies with vinclozolin in
rats, an analysis was carried out to determine whether these changes
were due to acceleration of normal, age-related changes, since
lenticular changes are seen commonly in old rats and cataracts also
occur spontaneously in untreated rats. The clear NOAELs are shown in
Table 4. Vinclozolin induced cataracts and other lenticular changes
only in rats, and there were no treatment-related lenticular changes
in B6C3F1 or C57B1 mice or in dogs. In the studies of absorption and
distribution, a high concentration of radiolabel was found in the
Harderian gland, which is situated close to the eye and secretes a
fluid onto its surface. The presence of radiolabel in this secretion
was confirmed by examination of enlargements of whole-body
autoradiographs (Schilling, 1993).
3. Observations in humans
A cross-sectional study was performed of 67 men who had handled
vinclozolin for 1-13 years during its synthesis and formulation and 52
unexposed controls. The men were monitored by determining urinary
metabolites containing a 3,5-dichloraniline moiety and observation for
reversible changes in the levels of hormones of the adrenocortico-
tropic and gonadotropic feedback systems, signs of liver injury,
haemolytic anaemia, cataract formation, and hormonally induced
hyperplasia and tumours at high doses. The clinical investigation
consisted of a medical and occupational history questionnaire,
physical examination, laboratory determinations, including
measurements of testosterone, LH, and follicle-stimulating hormone,
ultrasonography of the liver and prostate, a detailed examination, and
routine spirometry. The mean 3,5-dichloraniline concentration in
exposed workers was 235-422 µg/g creatinine, depending on the work
area, in comparison with a mean of 7 µg/g creatinine in controls. On
the basis of a series of assumptions, including 40% excretion through
the kidneys, 1.5-litre urine output per day, and 70-kg body weight,
the authors estimated that the occupational exposure of two-thirds of
the employees to vinclozolin exceeded 25 µg/kg bw per day. The
physical examinations and laboratory tests provided no evidence of
vinclozolin-induced hormonal responses, liver injury, prostatic
changes, cataract formation, or haemolytic anaemia. The authors
concluded that vinclozolin induced no health effects and, in
particular, no anti-androgenic effects (Zober et al., 1995).
Table 4. NOAELs for ophthalmoscopic effects in short- and long-term
studies of the toxicity of vinclozolin in rats
Effect NOAEL (ppm)
Short-term study Long-term study
Cataracts 1000 150
Striations 300 150
Bosselated lens structure 1000 150
Bulbiform thickening 3000 150
Opacities 3000 150
Comments
Vinclozolin is well absorbed after oral administration to rats
and extensively metabolized. The majority of the administered
radiolabel was found in the bile, and no unchanged vinclozolin was
excreted in the urine. After single oral doses of radiolabelled
vinclozolin, excretion was rapid; after multiple doses there was no
significant accumulation. Vinclozolin is only moderately absorbed via
the dermal route in rats: over 72 h, about 17% of a dose of 0.13 mg/kg
bw was excreted in the urine.
Vinclozolin has low acute toxicity, with an oral LD50 in rats
of > 15 000 mg/kg bw. The clinical signs of toxicity after acute
dosing with vinclozolin were generally non-specific and there were no
consistent treatment-related findings at necropsy. Vinclozolin is not
irritating to rabbit skin or eyes, but induced skin sensitization in a
maximization study in guinea-pigs. WHO has classified vinclozolin as
unlikely to present an acute hazard in normal use.
Studies of repeated administration were carried out in mice,
rats, rabbits and dogs, in which vinclozolin and/or its metabolites
caused toxic effects indicative of anti-androgenic activity. In two
three-month feeding studies in different strains of mice at dietary
levels of 100-5000 ppm, the NOAEL was equivalent to 20 mg/kg bw per
day, on the basis of signs of hepatotoxicity, signs consistent with
anti-androgenicity, and changes in the adrenal glands. In two recent
three-month feeding studies in rats (at levels of 0, 300, 1000, and
3000 ppm and 0 and 50 ppm, respectively) vinclozolin caused changes
qualitatively similar to those seen in mice; however, effects on the
adrenal glands (including lipidosis) were seen at 300 ppm, and the
NOAEL was confirmed in the second study as 50 ppm, equal to 4 mg/kg bw
per day. In a 12-month feeding study in dogs at dietary levels of 0,
35, 75 150, or 1500 ppm, the NOAEL was 75 ppm, equal to 2.4 mg/kg bw
per day, on the basis of pathological changes in the liver, spleen,
prostate, testis, and adrenals. The results of studies incorporating
withdrawal periods indicate that the anti-androgenic effects of
vinclozolin are reversible on cessation of treatment.
In a recent study of carcinogenicity in C57Bl/6 mice at dietary
levels of 0, 15, 150, 3000, or 8000 ppm, hepatocellular carcinomas
were seen at 8000 ppm. There was evidence of toxicity at 3000 ppm,
including hepatotoxicity, Leydig-cell hyperplasia, atrophy of
accessory sex glands, atrophic uteri, and lipidosis in the
cortico-medullary region of the adrenals. The NOAEL was 150 ppm, equal
to 24 mg/kg bw per day. In an earlier study in NMRI mice at levels of
0, 160, 490, 1460, or 4370 ppm, survival was adversely affected at the
highest dose, and the NOAEL was 490 ppm on the basis of increased
liver weight, without histological change. In rats, the long-term
toxicity and carcinogenicity of vinclozolin has recently been
investigated in three studies with dietary levels of 25-4500 ppm.
Cataracts and other lenticular changes were seen in rats treated with
50 ppm or more. (Mice and dogs were closely examined for ocular
changes, but vinclozolin did not affect the eyes in these species.) An
increased incidence of Leydig-cell tumours was seen in rats treated
with 150 ppm and more, together with atrophy of accessory sex glands.
Benign sex cord stromal tumours in the ovaries were seen in rats
treated at 500 ppm and above, and uterine adenocarcinomas were
detected at 3000 ppm (the highest dose tested in the carcinogenicity
study). Adrenal tumours were seen at 1500 ppm and above.
Hepatocellular carcinomas were seen in males treated with 4500 ppm,
and signs of hepatotoxicity were seen in rats treated with 150 ppm or
more. The NOAEL was 25 ppm, equal to 1.4 mg/kg bw per day.
In multi-generation studies, vinclozolin led to infertility of
males, owing to feminization of the outer genital organs, at dietary
levels of 1000 ppm or more. At 300 ppm, although all males were
eventually proved fertile, the observed effects may have indicated
sub-fertility. At 50 ppm, the only adverse effect was a reduction in
epididymal weight (with no associated morphological changes) in F2
offspring. The NOAEL was 40 ppm, equivalent to approximately 4 mg/kg
bw per day. Recent investigations of developmental toxicity have been
conducted in rats and rabbits. In rats, the most sensitive indicator
of teratogenicity was a reduction in the anogenital distance; in a
series of studies, the NOAEL for a change in anogenital distance was
15 mg/kg bw per day. The NOAEL for fetotoxicity was about 100 mg/kg bw
per day, on the basis of signs of developmental delay, while the NOAEL
for maternal toxicity was about 400 mg/kg bw per day, on the basis of
clinical signs of toxicity.
Three studies of developmental toxicity have been conducted in
rabbits. In the first, there were no signs of maternal toxicity,
fetotoxicity, or teratogenicity at doses up to and including 300 mg/kg
bw per day. In the second study, with doses up to and including
800 mg/kg bw per day, toxicity led to extensive mortality at the
highest dose, precluding any reliable assessment at this dose. The
NOAEL for maternal toxicity was 50 mg/kg bw per day, and that for
fetotoxicity was 200 mg/kg bw per day; there was no evidence of
teratogenicity at this dose (the highest dose available for
assessment). The third study involved only one dose, 400 mg/kg bw per
day. The number of female offspring exceeded the number of males, but
there was no treatment-related change in the appearance of the male
fetal genital organs. An increase in the incidence of separated
origins of the carotid arteries indicated potential teratogenicity at
this maternally toxic dose.
Vinclozolin has been tested for genotoxicity in a range of tests
in vivo and in vitro. The Meeting concluded that vinclozolin is
not genotoxic. It noted that positive results were obtained in a study
of cell transformation, but the process giving rise to this effect is
unknown. One study suggests that vinclozolin may be a promoter in rat
liver in vivo, which may indicate the mechanism by which liver
tumours were induced in rats at a high dose.
Studies have been conducted that confirm the anti-androgenic
properties of vinclozolin, which are likely to be associated with
binding to the androgen receptor. This proposed mechanism of action
could account for the results seen in studies of the reproductive
toxicity and long-term toxicity of vinclozolin.
In an epidemiological study of manufacturing plant personnel, it
was concluded that there was no evidence that vinclozolin had induced
health effects in employees with possible long-term exposure.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1.4 mg/kg bw per day in the two-year study of carcinogenicity
in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 20 mg/kg bw per day (three-month study of
toxicity)
490 ppm, equivalent to 63 mg/kg bw per day (112-week study
of toxicity and carcinogenicity in NMRI mice)
150 ppm equal to 24 mg/kg bw per day (18-month study of
toxicity and carcinogenicity in C57Bl/6 mice)
Rat: 50 ppm, equal to 4 mg/kg bw per day (three-month study of
toxicity)
25 ppm, equal to 1.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
40 ppm, equivalent to 4 mg/kg bw per day (study of
reproductive toxicity)
15 mg/kg bw per day (study of developmental toxicity)
100 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
400 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 50 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
200 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Dog: 75 ppm, equal to 2.4 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to vinclozolin
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 15 000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Not irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test
Inhalation, toxicity, rat LC50 > 29 mg/litre air
Mid-term (1-26 weeks) Oral, developmental toxicity, rat NOAEL = 15 mg/kg bw per day; teratogenicity
Long-term (> one year) Dietary, two years, toxicity and NOAEL = 1.4 mg/kg bw per day; signs of
carcinogenicity, rat antiandrogenicity
Dietary, one year, toxicity, dog NOAEL = 2.4 mg/kg bw per day; signs of
antiandrogenicity
References
Block, I. et al. (1987) Study of effect on vital functions of
animals -- general pharmacology -- of vinclozolin. Unpublished
report from Research & Consulting Co. AG, Itingen, Switzerland.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cameron, B.D. & Jack, L. (1991) In vitro percutaneous absorption of
14C-Reg. No. 83 258. A comparison using rat and human
epidermis. Unpublished report from Inveresk Research
International, Tranent, Scotland. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Chasseaud, L.F., Hawkins, D.R., Kirkpatrick, D., Conway, B. &
Franklin, E.R. (1976) The metabolic fate of the fungicide
Vinclozolin, BAS 352 F, after repeated oral administration to
rats. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cifone, M.A. & Myhr, B.C. (1984) Evaluation of vinclozolin (831233) in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics, Kensington, Maryland,
USA. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Cozens, D.D. & Palmer, A.K. (1987) Statement on maternal toxicity;
effect of vinclozolin on pregnancy of the New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Cozens, D.D., Edwards, J.A., Leeming, N.M., Clark, R. & Offer, J.M.
(1981) Effect of vinclozolin on pregnancy of New Zealand white
rabbit. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P (1979) Study of sensitization effect on guinea pigs
maximization test. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1981) Cytogenetic investigation in
Chinese hamsters after a single oral administration of Reg.
No. 83 258. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Engelhardt, G. (1983) Report on the study of
vinclozolin (Reg. No. 83 258) in the Ames test. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Gelbke, H.P & Jackh, R. (1975) Report on a point mutation test carried
out on CHO cells (HGPRT locus) with the test substance
vinclozolin (substance No. 841382). Unpublished report from BASF
AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1979) Report on the study of the acute oral
toxicity of vinclozolin (Reg. No. 83 258) in beagle dogs.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Gelbke, H.P. & Kirsch, P. (1981) Acute oral toxicity of vinclozolin in
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Gray, L.E., Jr, Ostby, J.S. & Kelce, W.R. (1994a) Developmental
effects of an environmental antiandrogen: The fungicide
vinclozolin alters sex differentiation in the male rat. Toxicol.
Appl. Pharmacol., 129, 46-52.
Gray, L.E, Jr, Ostby, J.S., Monosson, E. & Kelce, W.R. (1994b)
Alterations of sex differentiation in male rats following
perinatal exposure to low doses of the antiandrogenic pesticide
vinclozolin. Biol. Reprod., 50 (Suppl. 1), 101.
Grundler, P. & Gelbke, H.P. (1980) Report on the sensitizing effect of
BAS 352 04 F - Ronilan (WNT No. 791661) in the guinea pig -- open
epicutaneous test (OET). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990a) The biokinetics of 14C-vinclozolin in
the rat. Unpublished report from Huntingdon Research Centre,
Huntingdon, Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1990b) The biotransformation of 14C-vin-
clozolin in the rat. Unpublished report from Huntingdon Research
Centre, Huntingdon, Cambs, United Kingdom. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991a) The determination by whole body
autoradiography of the tissue distribution of radioactivity in
female rats after oral administration of 14C-vinclozolin.
Unpublished report from Huntingdon Research Centre, Huntingdon,
Cambs, United Kingdom. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hawkins, D.R. et al. (1991b) The dermal absorption of
14C-vinclozolin in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1987) Report on the study of the toxicity of
Reg. No. 83 258 (vinclozolin) in beagle dogs after 12-month
administration via the diet. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989a) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- first study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989b) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- second study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF: AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- third study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1989d) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rats after oral administration
(gavage) -- test study. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1990a) Report: Reproduction study with Reg.
No. 83 258 in rats. Continuous administration over 2 generations.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990b) Report: Study of the prenatal toxicity of
Reg. No. 83 258 in rats after dermal application. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990c) Report on the study of the prenatal
toxicity of Reg. No. 83 258 in rabbits after oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany.
Hellwig, J. et al. (1990d) Report on the supplementary steady of the
prenatal toxicity of Reg. No. 83 258 in rabbits after oral
administration (gavage). Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hellwig, J. et al. (1994) Report: Second reproduction study with
Reg. No. 83 258 in rats. Continuous administration over 2
generations. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977a) Primary skin irritation of Reg. No. 83 258
(vinclozolin) on the intact and scarified dorsal skin of white
rabbits. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hildebrand, B. (1977b) Primary irritation of Reg. No. 83 258
(vinclozolin) to the eye of white rabbits. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. (1973a) Report on acute oral toxicity trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1973b) Report on the acute intraperitoneal trial of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
in guinea pigs. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. (1974) Report on the testing of 3-(3,5-dichiorophenyl)-
5-methyl-5-vinyl-l,3-oxazolidine-2,4-dione in a 3-month feeding
experiment on rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975a) Report on the supplementary
toxicological study of 3-(3,5-dichiorophenyl)-5-methyl-
5-vinyl-1,3-oxazolidine-2,4-dione in a 4-week feeding study in
the rat. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Munk, R. (1975b) Report on the toxicological testing
of 3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-
2,4-dione in a three-month feeding trial on the dog. Unpublished
report from BASF AG, Ludwigshafen, Germany. Submitted to WHO by
BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975a) Study on the prenatal toxicity of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on mice. Unpublished report from BASF AG, Ludwigshafen, Germany.
Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Hofmann, H.T. & Peh, J. (1975b) Study of the mutagenic effect of
3-(3,5-dichiorophenyl)-5-methyl-5-vinyl-1,3-oxazolidine-2,4-dione
on the male mouse following repeated oral administration.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Hoorn, A.J.W. (1983) Mutagenicity of vinclozolin, compound No. 831233,
in the rec assay with Bacillus subtilis. Unpublished report
from Litton Bionetics, Veenendaal, Netherlands. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Ito, N. & Hasegawa, R. (1992) Liver medium term bioassay in rats for
screening of carcinogenesis and modifying factors in
hepatocarcinogenesis. Food Chem. Toxicol., 30, 979-992.
Ito, N., Hoshiya, T., Hasegawa, R., Hakoi, K., Cui, L., Ogiso, T. &
Cabral, R. (1993) Enhancement by non-mutagenic pesticides of
GST-P positive hepatic foci development initiated with
diethylnitrosamine in the rat. Cancer Lett., 72, 59-64.
Ito, N., Hasegawa, R., Imaida, K., Takahashi, S. & Shirai, T. (1994)
Medium term rat liver bioassay for rapid detection of carcinogens
and modifiers of hepatocarcinogenesis. Drug Metab. Rev.,
26, 431-442.
Kelce, W.R., Monosson, E., Gamcsik, M.P., Laws, S.C. & Gray, L.E., Jr
(1994) Environmental hormone disruptors: Evidence that
vinclozolin developmental toxicity is mediated by antiandrogenic
metabolites. Toxicol. Appl. Pharmacol., 126, 276-285.
Kirsch, P. (1986a) Report of the study of the acute oral toxicity on
the rat based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. (1986b) Report of the study of the acute oral toxicity on
the mouse based on OECD and EPA (FIFRA) of vinclozolin metabolite
BF 352-42. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Kirsch, P. et al. (1974) Report on the study of 3-(3,5-dichloro-
phenyl)-5-methyl-5-vinyl-1,3-oxazoildine-2,4-dione for cataract
formation in a 3-month feeding study in dogs. Unpublished report
from BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Kirsch, P., Deckhardt, M. & Hellwig, J. (1982) Report on the study of
the toxicity of Reg. No. 83 258 (vinclozolin) in beagle dogs
after 6-month administration in the diet. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. (1989) Examination of the hormone status. Unpublished
report from University of Lübeck, Lübeck, Germany. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Knuppen, R. (1990) Final report: Study of the binding of
3-(3,5-dichlorophenyl)-5-methyl-5-vinyl-2,4-dione to the androgen
receptor in MCF-7 cells. Unpublished report from University of
Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1991) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) -- Reg. No. 119 208
(metabolite BF 352-22) to the androgen and glucocortiroid
receptors in the cytosol from MC-F-7 cells and from the prostate
and liver tissues of the rat. Unpublished report from University
of Lübeck, Lübeck, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Knuppen, R. & Schutze, N. (1992) Final report: Study of a possible
binding of Reg. No. 83 258 (vinclozolin) to the androgen receptor
in the cytosol from a cell line expressing the androgen receptor
and from the prostate tissue of the rat. Unpublished report from
University of Lübeck, Lübeck, Germany. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Kretzschmar, R. et al. (1987) Study on the EEG effects in the
conscious rat of vinclozolin. Unpublished report from Knoll AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Leuschner, F. (1977) Chronic oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in a
reproduction study covering three generations of Sprague-Dawley
rats. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikoiogie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. (1979) Acute inhalation toxicity study on the
preparation vinclozolin. Unpublished report from Laboratorium fur
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO
by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1975) Oral toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in
Sprague-Dawley rats. Unpublished report from Laboratorium für
Pharmakoiogie und Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977a) 3-weeks-toxicity of an oxazolidine
derivative batch No. 83 258 -- called for short 'Oxa' -- in NZW
rabbits by local application. Unpublished report from
Laboratorium fur Pharmakologie und Toxikoiogie (LPT), Hamburg,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Leuschner, F. et al. (1977b) Oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in NMRI
mice. Unpublished report from Laboratorium fur Pharmakoiogie und
Toxikologie (LPT), Hamburg, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Leuschner, F. et al. (1977c) Chronic oral toxicity of an oxazolidine
derivative, batch 83 258 -- called for short 'Oxa' -- in the
Sprague-Dawley rat. Unpublished report from Laboratorium fur
Pharmakoiogie and Toxikoiogie (LPT), Hamburg, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1992) Report: Study on the influence of Reg.
No. 83 258 (vinclozolin) on the hormone status of Wistar rats.
Unpublished report from BASF AG, Ludwigshafen, Germany. Submitted
to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1993a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in Wistar rats. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Mellert, W. et al. (1993b) Report: Supplementary study on the oral
toxicity of Reg. No. 83 258 (vinclozolin) in Wistar rats.
Administration in the diet over 3 months. Unpublished report from
BASF AG, Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994a) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in C57BL mice. Administration in the
diet for 18 months Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994b) Report: Study of the chronic toxicity of
Reg. No. 83 258 (Vinclozolin) in rats. Administration via the
diet over 24 months. Unpublished report from BASF AG,
Ludwigshafen, Germany.
Mellert, W. et al. (1994c) Report: Chronic toxicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Mellert, W. et al. (1994d) Report: Carcinogenicity study with Reg.
No. 83 258 (vinclozolin) in rats. Administration in the diet for
24 months. Unpublished report from BASF AG, Ludwigshafen,
Germany. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Murli, H. (1989) Mutagenicity test with Reg. No. 83 258, Batch No. 183
(= ZST No. 881375) in an in vitro cytogenetics assay measuring
chromosome aberration frequency in Chinese hamsters ovary cells
(CHO cells). Unpublished report from Hazleton, Kensington,
Maryland, USA. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Oesch, F. (1977) Ames test for vinclozolin. Unpublished report from
University of Mainz, Mainz, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Otto, S., Bentel, P., Elzner, J. & Ohnsorg, U. (1977) Metabolism of
14C-vinclozolin in rats. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG, Ludwigshafen,
Germany.
Perocco, P., Collaci, A. & Grilli, S. (1993) In vitro cytotoxic and
cell transforming activities exerted by the pesticides cyanazine,
dithianon, diflubenzuron, procymidone and vinclozolin on BALB/c
3T3 cells. Environ. Mol. Mutag., 21, 81-86.
Rankin, G.O., Teets, V.J., Nicoll, D.W. & Brown, P.I. (1989)
Comparative acute renal effects of three N(3,5-dichlorophenyl)
carboximide fungicides: N-(3,5-dichlorophenyl) succimide,
vinclozolin and iprodione. Toxicology, 56, 263-272.
Sabbioni G. & Neumann H.-G. (1990) Biomonitoring of arylamines:
Haemoglobin adducts of urea and carbamate pesticides.
Carcinogenesis, 11, 111-116.
Schilling, K. (1993) Evaluation of ophthalmology findings recognised
within various rat feeding studies with Reg. No. 83 258
(vinclozolin). Unpublished report from K. Schilling, Consultant,
Kirchheimbolanden, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990a) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in B6C3F1 mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Schilling, K. et al. (1990b) Report: Study on the oral toxicity of
Reg. No. 83 258 (vinclozolin) in C57BL mice. Administration in
the diet over 3 months. Unpublished report from BASF AG,
Ludwigshafen, Germany. Submitted to WHO by BASF AG,
Ludwigshafen, Germany.
Shirasu, Y. et al. (1977) Mutagenicity testing on BAS 352 04 F in
microbial systems. Unpublished report from Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by BASF
AG, Ludwigshafen, Germany.
Shirasu, Y., Takahashi, K. & Saito, T. (1978a) Report of acute
toxicity tests with BAS 352 F in mice. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany
Shirasu, Y., Takahashi, K. & Saito, T. (1978b) Report of acute
toxicity tests with BAS 352 F in rats. Unpublished report from
Institute of Environmental Toxicology, Tokyo, Japan. Submitted to
WHO by BASF AG, Ludwigshafen, Germany.
Witterland, W.F. & Hoorn, A.J.W. (1984) Mutagenicity evaluation of
vinclozolin (831233) in the mouse lymphoma forward mutation
assay. Unpublished report from Litton Bionetics, Veenendal,
Netherlands. Submitted to WHO by BASF AG, Ludwigshafen, Germany.
Zober, A. et al. (1995) Morbidity study of personnel with potential
exposure to vinclozolin. Occup. Environ. Med., 52, 233-241.
