
MANEB
First draft prepared by
A. Kocialski
Office of Pesticide Programs,
US Environmental Protection Agency, Washington, DC, USA
EXPLANATION
Maneb was evaluated by the Joint Meetings in 1963, 1965, 1967,
1970, 1974, 1977 and 1980 (Annex I, references 2, 4, 8, 14, 22, 28,
34). An ADI of 0-0.05 mg/kg bw was established at the 1980 Meeting
for maneb, or the sum of any combination of maneb, mancozeb and
zineb, of which not more than 0.002 mg/kg bw may be present as
ethylenethiourea (ETU).
This monograph summarizes new or not previously reviewed data
on maneb, as well as relevant data from previous monographs and
monograph addenda on this substance.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biological data
Biochemical aspects
Absorption, distribution and excretion
Rats
Sprague-Dawley rats (CD-Crl:CD[SD]BR) were divided into three
experimental groups of 5 animals/sex/dose and treated as follows:
Group B received a single oral dose by gavage of the radiolabelled
test compound (purity not stated) at 25 mg/kg bw, Group C received
oral gavage doses of unlabelled test compound (80% wettable powder
formulation) daily for 14 days at 25 mg/kg bw followed by a single
oral gavage dose of radiolabelled test compound at 25 mg/kg bw on
day 15, and Group D received a single oral gavage dose of
radiolabelled compound at 2235 mg/kg bw. Animals were then placed
in individual metabolism cages and observed daily. Expired carbon
dioxide, urine and faecal samples were collected at 6, 12, 24, 48,
72, 96 and 120 hours from all groups and from the high-dose group
only at 144 and 168 hours. Blood samples were collected at
termination and the following tissues excised for determination of
14C residues, bone (femur), brain, fat, gonads, heart, kidney,
thyroid, liver, lung, blood, muscle (thigh), spleen and residual
carcass.
There were no treatment-related effects in animals receiving 25
mg/kg bw. Hypoactivity, excessive salivation, and soft faeces were
observed in animals given the high dose. One male died and one male
was sacrificed in a moribund condition at the high dose. There were
no differences between sexes within groups with regard to excretion
patterns, with the maximum difference between sexes within each
group for urine and faeces generally not greater than 3%. 14C
urine and faeces values for sexes combined at 120 hours were 51% and
29% and 60% and 28% for groups B and C, respectively. Greater than
90% of the absorbed 14C was eliminated in urine by 24 hours in
males and females. Mean urine and faeces values for sexes combined
at the high dose (Group D) at 7 days were 40% and 38%, respectively.
Less than 1% of the dose was eliminated as carbon dioxide in all
dose groups. The 14C concentration in rat blood at termination was
less than 0.02% of the administered dose. The average 14C
concentration (as percent of dose per gram of tissue) was greatest
for the thyroid followed by the kidney and liver in Groups B and C.
The percent of 14C present in urine as ETU at 12 hours after
oral administration was 21.3%-25.7% in males and 27.7%-30.4% in
females, on a mole/mole basis, for all groups. The percent of 14C
present in faeces as ETU at 24 hours was 3.7% in males and females
of Group B, 12% and 6% in males and females, respectively in Group
C, and 50% in males and 42% in females in the high-dose group. The
percent of 14C in urine present as maneb (and/or other carbon
disulfide generators) was 0.08%-0.14% in males and 0.12%-0.34% in
females, for all groups (Puhl, 1985).
Groups of male Charles River rats (32 animals/group) were
treated with a single dermal application of 14C maneb technical
formulation (98% radio-purity, 91% chemical purity) at doses of
0.08, 0.71 or 8.9 mg/rat. A fourth group was given 0.08 mg/rat of
14C maneb in deionized water. Doses were applied to 10 square
centimetres of the shaved anterior dorsal area of each animal. Four
animals from each group were exposed to 14C maneb for either 0.5,
1.0, 2.0, 4.0, 10 or 24 hours and sacrificed at the end of the
exposure period. Two additional groups were exposed at each dose
level and then sacrificed at 48 and 72 hours after the initial
exposure. Animals treated with 0.08, 0.71 or 8.9 mg/rat and exposed
from 0.5 to 10.0 hours showed skin absorbtion values ranging from
0.1% to 0.5%. 14C in blood and urine was less than the limit of
quantitation (LOQ). The amount of 14C bound to skin ranged from
1.56 to 7.76%. These same groups of animals exposed for 24 hours
and sacrificed at 24 hours showed skin absorbtion values ranging
from about or below the LOQ to 0.25%. The amount of 14C found in
blood was at or below 0.01%. The amount detected in urine was less
than 0.26%. 14C bound to skin ranged from a low of 2.56% to 6.99%.
Group four animals exposed to 0.08 mg/rat for 10 hours and
sacrificed at 10 hours showed skin absorbtion values ranging from
0.19% to 3.18%. Blood values ranged from 0.01% to 0.06%, and urine
values ranged from 0.28% to 1.56%. The percent bound to skin was
between 22.79 to 34.53%. Group four animals exposed to 0.08 mg/rat
and sacrificed at 24 hours had the following values - 6.5% absorbed
through skin, 0.01% found in blood, 3.05% detected in urine and
26.8% bound to skin (Craine, 1991).
Biotransformation
The biotransformation pathway for maneb in rats is shown in
Figure 1.
Toxicological studies
Acute toxicity studies
Summaries of acute toxicity data for maneb technical and maneb
75% dust are given in Tables 1 and 2. WHO has classified maneb as
unlikely to present acute hazard in normal use (WHO, 1992).
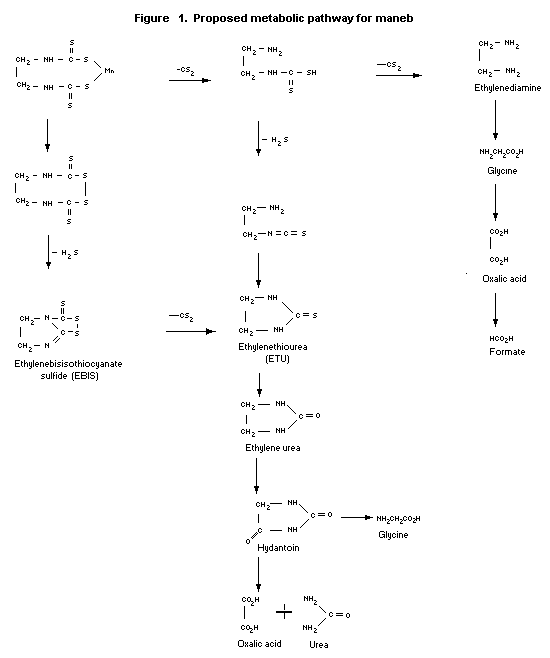 Table 1. Acute toxicity of maneb technical
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat1 Crl:CD BR M/F oral > 5000 Naas, 1989a
Rat2 Crl:CD BR M/F inhal. 7.38 Terrill, 1990
Rabbit3 NZW M/F dermal > 2000 Naas, 1989b
1 Deionized water as vehicle; 3/5 males died, 0/5 females died.
2 Dose only 4 hour exposure, respirable dust of 3.8 microns. Analytical LC50 of 5.34 mg/l
for males and greater than 7.38 mg/l for females. 14 day observation period.
Respiratory distress and decreased body-weight were the major clinical signs.
3 Deionized water as vehicle.
Table 2. Acute toxicity of maneb DF1
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat2 Sprague-Dawley M/F oral > 5000 Glaza, 1988a
CD(R)
Rat3 Sprague-Dawley M/F inhal. 4 h > 2.0 Hoffmann, 1991
CD(R) exposure
Rabbit4 NZW M/F dermal > 2000 Glaza, 1988b
1 Maneb DF (75% Dust)
2 Uniform suspension in deionized water
3 Mean exposure concentration 2.0 mg/l with a nominal concentration of 53 mg/l.
Mass Median Aerodynamic Diameter was 3.3 microns with an average
Geometric Standard Deviation of 2.4, and 8.8% of particles were
less than one (1) micron in size. There were no deaths during the whole body exposure.
4 0.9% saline vehicle
Short-term toxicity studies
Rats
Sprague-Dawley (Crl:CD BR) rats (32/sex/dose; control 16/sex)
received a nose-only exposure to maneb technical (86.8% purity
adjusted to 100%) for 6 hours/day, five days a week for 4 weeks at a
target level of 100 mg/m3 (particle size ranged from 3.45-3.83
microns). All animals survived to scheduled sacrifice and compound-
related signs were unremarkable. T4 and TSH values in males and
TSH values in females were decreased during week one only. However,
results for treated males were generally less than control values
for the remainder of the study and were not considered biologically
meaningful. Results for females were generally comparable to
controls. Gross necropsy was not remarkable between treated and
controls. Mean absolute lung weights for both sexes were comparable
to controls but relative weights increased. Mean terminal body-
weights were decreased for both sexes and paralleled the increased
relative lung to body-weight ratio. Residue levels of maneb,
manganese and ETU generally remained fairly constant during the
exposure period and was less than the limit of detection after the
recovery period. During weeks 1-4, residue levels in lungs for
males ranged between 3.9-7.9 ppm (7.9-6.8 ppm for weeks 2 and 4) for
maneb; 4.8-6.0 ppm for manganese and 0.12-0.19 ppm for ETU; for
females 2.1-4.2 ppm for maneb, 1.8-2.8 for manganese and < 0.1-0.12
ppm for ETU. Residue levels in the urine of females was generally
less than the limit of detection for maneb and manganese while
values for ETU generally ranged between 1.3 and 2.15 ppm during the
exposure period and less than the limit of detection during the
recovery phase. Manganese was not detected in males and maneb and
ETU were not detected during recovery. Maneb ranged from 1.3-6.6 ppm
during exposure and ETU from 5-29 ppm (5.0-5.1 for weeks 2 and 4)
(Terrill, 1991).
Charles River Sprague-Dawley albino rats (28/sex/dose) received
nose-only target inhalation exposures of 0, 10, 30 or 100 mg/m3 (0,
10, 32, or 98 mg/m3 gravimetric concentration with an equivalent
mean aerodynamic diameter of 3.5 [+/-2.0] microns) of technical
maneb (purity not stated) for 6 hours a day, 5 days a week for 13
consecutive weeks. Measured concentrations of ETU for the low,
middle, and high-dose groups were 0.12, 0.71 and 2.5 mg/m3.
Fourteen animals per sex per dose were sacrificed after 13 weeks of
exposure and the remaining animals allowed to recover for 13 weeks
and then sacrificed.
There were no exposure-related effects on appearance or
behaviour and no compound-related deaths. Body weight of females
exposed to the inter-mediate and high dose were depressed during the
exposure period, whereas males had decreased body weights only at
100 mg/m3 for weeks 1-3. There were no exposure-related effects
observed in ophthalmologic, haematologic (10 parameters) or serum
biochemical measurements (21 parameters including T3 and T4) after
the exposure period. Absolute organ weights were comparable to
control group. There was no evidence of any dose-related
macroscopic or microscopic changes in animals sacrificed at 13
weeks. ETU was detected in the urine of all exposed rats, in the
thyroids of all high-dose animals, in the liver of two females of
the intermediate-dose group and the plasma of one intermediate dose
female. ETU concentration in the urine was dose-related in both
sexes but was much greater in females. ETU mean residues in the
thyroid for males were however much greater in males (23.6 µg/ml)
than females (8.4 µg/ml). Maneb residues were also found in urine
of both sexes.
At the end of the 13-week recovery period no differences were
observed among the treated groups that could be attributed to test
material exposure when compared to controls. ETU and maneb residues
were not detected in any tissue or body fluids measured after a 13-
week recovery period. The NOAEL was 10 mg/m3 based on decreased
body weight at 30 mg/m3 (Ulrich, 1986, 1987).
Sprague-Dawley derived Crl:CDBR rats (15 animals/sex/dose)
received dietary concentrations of 0, 80, 400 or 1300 ppm of maneb
technical (78% purity). Ten animals/sex/dose were sacrificed at 14
weeks and 5 animals/sex/dose were placed on a 4-week recovery period
and sacrificed at 17 weeks. There were no overt signs of toxicity
or dose-related effects on mortality, ophthalmology or haematology.
Body-weight gains for males and females at 1300 ppm were
significantly decreased at 13 weeks. At week 17, body weights of
high-dose males were comparable to the control group, while female
body weight was 10% lower than controls. Food consumption was
comparable to controls.
A dose-related decrease in T4 levels and a concomitant
increase in TSH levels were seen in dosed females at 14 weeks. The
decrease in T4 levels was significant at the high dose. In males
T4 values were slightly decreased and TSH values slightly increased
at 1300 ppm. T4 values for males and females were similar to or
higher than concurrent controls at the end of the recovery period,
while TSH values were similar to or lower than controls. T3 values
were increased, but not statistically significant in high-dose males
and females at 14 weeks and in females at the end of the recovery
period. T3 values for males at the high dose were comparable to
controls at the end of the recovery period. There were no changes
of toxicological significance in other clinical chemistry
parameters. A dose-related trend (dose-related increase) was
evident for thyroid/parathyroid weights of dosed males and females.
Absolute weights were significantly increased in males at 400 and
1300 ppm, and relative weights were significantly increased in high-
dose males. Thyroid/parathyroid weights of dosed and control
animals were similar following a 4-week recovery period. There were
no compound-related changes in macroscopic pathology in dosed
animals at 14 or 18 weeks. Follicular cell hyperplasia of the
thyroid was observed in 1/10 mid-dosed males, 10/10 high-dosed males
and 2/10 high-dosed females compared with none in the controls.
Increased colloid of the thyroid was also exhibited in 4/10 high-
dose males. Thyroid changes regressed following the recovery phase.
However one high-dose male manifested a follicular cell adenoma.
Granular renal pigment was observed in 2/10 low-dose males and all
animals in the mid and high-dose groups. However, the incidence of
chronic progressive nephropathy was similar in dosed and control
males and females. At the end of the recovery period a reduction in
the incidence and amount of renal pigment was reported in 3/5 mid-
dose and 3/5 high-dose males and 4/5 mid-dose and 4/5 high-dose
females. The NOAEL was 80 ppm, equal to 5.0 mg/kg bw/day, based on
an increase in absolute thyroid weight and thyroid follicular cell
hyperplasia at 400 ppm (Trutter, 1988).
Rabbits
New Zeeland white rabbits (five animals/sex/dose) were
administered 0 (sham treated), 100, 300 or 1000 mg/kg bw/day of 86%
maneb technical (adjusted to 100%) and applied to the shaved (10%)
dorsal intact skin area. Applications were made for 6 hours a day,
5 days a week for 3 weeks. Clinical findings, body weight, food
consumption and organ weights were comparable to controls. Values
for haematology and serum chemistry were comparable between treated
and control groups. Gross signs of skin irritation were observed in
2/10, 8/10 and 10/10 animals at the low, mid and high-dose.
Erythema was not observed at 100 mg/kg bw/day and edema was not
observed at 100 or 300 mg/kg bw/day (Draize method). Erythema when
present was generally graded slight as was edema. Epidermal scaling
was observed in 2, 8, and 10 animals at the low-, mid- and high-
doses. Histopathology showed epidermal irritation at the site of
application in all dose groups, which was characterized by minimal
to slight acanthosis and hyperkeratosis. Follicular cell
hypertrophy of the thyroid was observed in 7/10 animals at 1000
mg/kg/day. Increased colloid material was observed in 2 and 4
females at 300 and 1000 mg/kg/day, respectively (Trutter, 1988).
Dogs
Beagle dogs (2 animals/sex/dose) received dietary
concentrations of technical maneb (91.58% purity) of 0, 100, 400 or
1600 ppm for 13 weeks. At the high dose, diarrhoea was observed in
1 male and 1 female with attendant decreased activity in the female.
Body weight was decreased 20% and food consumption 30% in high-dose
females. Body weight and food consumption were comparable between
treated and control groups for males. Slight to marked anaemia was
observed in 1 male and 1 female at 6 and 13 weeks with enhanced
erythropoietic activity in all dogs at the high dose. T3 and T4
values were decreased in both sexes at 1600 ppm at six weeks but not
13 weeks. Total lipid, cholesterol, triglyceride and phospholipid
concentrations were slightly increased in males at 6 and 13 weeks.
Fluctuations for these parameters were also observed in one high-
dose female along with slight to moderate increases in glutamate
dehydrogenase and GGT activity and slight decreases in plasma
calcium, creatinine and total protein concentrations at 6 weeks.
Post-mortem examination revealed increased thyroid weight in 1 male
and 1 female at 1600 ppm. Increased liver to body-weight ratio was
also observed in the second female. One male and 1 female of the
high-dose group also manifested enlargement or thickening of the
thyroid gland upon macroscopic examination. Microscopic examination
revealed moderate to severe thyroid follicular cell hyperplasia in
high-dose males. One female at 400 ppm manifested slight thyroid
follicular cell hyperplasia; both females in the 1600 ppm group
showed thyroid follicular cell hyperplasia ranging from slight to
minimal. A minimal degree of thyroid follicular cell hypertrophy
was recorded for 1 male at 400 ppm and 1 female at 100 ppm. A
slight focus of hypertrophic cells in the adenohypophysis of the
high-dose male, which had a severe thyroid follicular cell
hyperplasia was also observed. The NOAEL was 100 ppm, equal to 3.7
mg/kg bw/day, based on thyroid follicular cell hyperplasia at 400
ppm (Allen et al., 1989).
Beagle dogs (5/sex/dose) were given dietary concentrations of
0, 50, 200, 1000 or 2200 ppm of maneb (89.2% purity) for 52 weeks.
No deaths occurred during the study. Diarrhoea was observed in dogs
at 1000 ppm (1/5 females) and 2200 ppm (3/5 males and 1/5 females).
Body weight for males was generally comparable between treated and
control groups. High-dose females displayed lower body weights
compared to controls. Body-weight gain was decreased in both males
and females at 2200 ppm. Group mean food intake at 2200 ppm was
decreased in males and females.
Ophthalmological examination revealed no adverse effects at any
dose level. Mean erythrocyte count, haemoglobin concentration, and
haematocrit at 200, 1000 and 2200 ppm were significantly decreased
at 13 weeks only in females. Increased mean cell volumes were
observed at 2200 ppm in males and 1000 ppm in females. Decreased
mean cell haemoglobin concentration was reported in both sexes at
2200 ppm along with reticulocytosis and polychromasia. Platelet
counts were increased in both sexes at 2200 ppm and in males at 1000
ppm. T4 values were decreased at 2200 ppm in males and females.
T3 was markedly depressed at 2200 ppm for both sexes. Increased
group mean values for cholesterol, total lipids, and phospholipids
were dose-related and statistically significant but only at 2200 ppm
for some of the parameters of either sex. Bilirubin values were
increased in males and females at 2200 ppm as was ALP. Urinalysis
findings were comparable between treated and controls. Neurological
examinations of postural reactions, spinal reflexes and of cranial
nerves revealed no treatment-related effects. The absolute and
relative weight of the thyroids was increased in both sexes at 2200
ppm as was the adrenal weight. Thyroid enlargement and/or
thickening was observed in all dogs at 2200 ppm and 7/10 dogs at
1000 ppm. Thyroid follicular hyperplasia was reported for all dogs
at 1000 and 2200 ppm. Thyroid histopathology was unremarkable at
lower dose levels. There were no other findings suggestive of any
treatment-related effect. The NOAEL was 200 ppm, equal to 6.4 mg/kg
bw/day for males and 7.2 mg/kg bw/day for females, based on thyroid
enlargement and thickening and thyroid follicular cell hyperplasia
at 1000 ppm (Corney et al., 1992).
Monkeys
Rhesus monkeys, (4/sex/group) received 0, 100, 300 or 3000 ppm
of maneb technical (90% purity) in the diet for 6 months. At 6, 13
and 26 weeks, all animals were administered 10 µCi of 131I (on an
empty stomach) and determinations were made for 131I absorption into
the thyroid at 2 and 48 hours post-dosing in conjunction with
determinations for serum T4, T3, protein-bound 131I in serum and
loss of 131I from the thyroid (i.e. half-life in days).
Behaviour and external appearance of treated and control
animals were comparable, and all females demonstrated normal
menstrual discharges. Mean body-weight gains in animals (sexes
combined) given 3000 ppm maneb were lower than those of controls and
the low- and mid-dose groups. Food consumption was unaffected.
Haematology and clinical biochemistry revealed no meaningful
differences between controls and treated groups. However, studies
conducted with 131I revealed significantly decreased 131I absorption
by the thyroid in animals (sexes combined) receiving 3000 ppm at 26
weeks. 131I uptake was decreased 40% in males and 35% in females.
Protein-bound 131I was also decreased in animals (sexes combined) at
3000 ppm with the decrease largely attributable to males. Results
of urinalysis and electrocardiograms were comparable between control
and treated groups. Gross necropsy was unremarkable between groups.
Absolute organ weights for thyroids were increased in monkeys (sexes
combined) at 300 and 3000 ppm. However, statistical significance was
reached only at the high dose and largely attributable to males.
Males also showed a greater increase at 300 ppm than did females.
Enlarged thyroid with large follicles, flat epithelium, and colloid
described as somewhat darker than usual was reported for 7 animals
at 3000 ppm. Moderate proliferation of the epithelium was also
present in two high-dose animals. Only 1 animal at the high-dose
had no thyroid-associated pathology. The NOAEL was 100 ppm, equal
to 7.3 mg/kg bw/day, based on an increase in thyroid weight at 300
ppm (Leuschner et al., 1977).
Long-term toxicity/carcinogenicity studies
Mice
Crl:CD-1 (ICR) BR mice (75/sex/group) received dietary
concentrations of 0, 60, 240 or 2400 ppm maneb technical (89.5%
purity) for 79 weeks. An interim sacrifice and necropsy was
performed on 20 animals/sex/dose group at 52 weeks. The mortality
rate, clinical signs, and palpable masses were comparable between
treated and control groups for both sexes. Female body weight was
decreased at 240 ppm and decreased for males and females at 2400
ppm. Erythrocytes, haemoglobin, and haematocrit were decreased at
2400 ppm in both sexes at interim and terminal sacrifice. T4
levels were decreased at 2400 ppm in both sexes. T4 levels were
also significantly decreased in females at 60 ppm and 240 ppm at
terminal sacrifice. A dose-related trend was evident across all
three doses. At terminal necropsy hepatic masses were evident and
occurred at a greater frequency in high-dose males and females than
controls. Organ-weight changes at 2400 ppm were biologically and
statistically significant only for thyroid and only at terminal
sacrifice. However, histopathology of the thyroid was unremarkable.
A significant increase was observed in high-dose males of
hepatocellular adenomas at terminal necropsy. The number of females
with hepatocellular adenomas at terminal necropsy were comparable to
controls. However, the overall incidence of hepatocellular adenomas
was significantly increased for both sexes at 2400 ppm. The NOAEL
was 60 ppm, equal to 11 mg/kg bw/day, based on decreased body weight
and decreased T4 levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes (Tompkins, 1992).
Rats
Sprague-Dawley (SIV 50) rats (90/sex/group) were fed dietary
concentrations of maneb (90% purity) of 0, 30, 100, 300 or 1000 ppm
for 31 months. Interim sacrifices were conducted at 3, 6, and 12
months on 5 animals/sex/dose and all survivors terminated at 31
months. Beginning at 26 weeks all animals were palpated once a
week. Haematology and clinical biochemistry parameters were
evaluated at pre-test and 3, 6, 12, 18 and 24 months in 10
animals/sex/dose. At 3, 6, 12 and 24 months, 10 animals/sex/dose
were gavaged with 131I (10 µCi) and determinations made for the
biological half-life of 131I in thyroid over a 10-day period, 131I
serum activity, protein bound 131I in serum, serum T4, and the
binding index of T3. Tumour and mortality rates were compared
statistically by means of analysis of variance according to the
method of Peto.
Behaviour, appearance, food consumption and mortality were
comparable between treated and control groups. Body weights at 31
months were within the normal range. However, body weights were
decreased 25% in males and 40% in females at 12 and 24 months. There
were no significant differences between groups or any apparent dose-
related trends in any of the haematology or clinical biochemistry
parameters measured. 131I retention time in the thyroid was
significantly increased only at the high dose tested for males at 6
and 12 months and females at 6 months. Values for 1000 ppm animals
were slightly increased but not statistically significant. Mean
serum T4 was not significantly different between groups for any
time period. However, T4 values at 1000 ppm at 6 and 12 months
were decreased 10% in males and 20% in females. Urinalysis
comparisons between groups were unremarkable. Thyroid weights were
increased at 1000 ppm in males and in females when compared to
controls at 31 months.
All other absolute and relative organ weights appeared
comparable to control values. Macroscopic and microscopic
examination of animals sacrificed during the interim periods
(5/sex/dose) revealed no compound-related changes. Gross and
histopathology of 75 animals (inter-current deaths and terminal
sacrifice) per sex in the control group and high-dose group did not
reveal compound-related non-neoplastic or neoplastic changes.
Additional histological examination of liver, kidney and urinary
bladder in both sexes at all lower doses also revealed no compound-
related non-neoplastic or neoplastic changes. The NOAEL was 300 ppm,
equal to 20 mg/kg bw/day, based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm (Leuschner et al., 1979, 1986a,b; Leuschner 1991).
Reproduction studies
Rats
Male and female rats (Crl:CD(SD)BR VAF/Plus) were randomly
assigned by weight into 4 groups of 28 (F0) and 24 (F1) males and
females per group and received 0, 75, 300 or 1200 ppm of maneb (80-
90% purity) in the diet. Animals were bred for 1 litter in each of
2 consecutive filial generations. There were no treatment-related
deaths or clinical signs at the low-or mid-dose. One high-dose
female in the F0 and F1 generation died. Both females had
locomotor difficulties and appeared thin. The second generation
female also suffered from hind limb paralysis. Signs were
considered treatment-related. Overall food consumption was decreased
for the F0 and F1 males and females. There was an increase in the
food conversion ratio for females of both generations during the
early part of mating. There was also a dose-related increase in
water consumption at the mid and high-dose for males and females of
the F0 and F1 generation during the final two weeks prior to
mating. No change in food or water consumption was observed at the
low dose. F1 females had a 20% decrease in food consumption at
1200 ppm and a 10% decrease at 300 but values were not statistically
significant. Body-weight gains were significantly lower for males
and females of the F0/F1 generations at 1200 ppm and for females
of the F0 generation at 300 ppm. Body weight for dams of the F0
and F1 generations during the periods of gestation and lactation
was decreased only at 1200 ppm of the F1 generation. Body-weight
gain, however, during the lactation period for the F0 and F1 dams
was substantially increased at 1200 ppm.
The mating performance, pregnancy rate and gestational period
for treated groups was comparable to the control group. Adult gross
pathology revealed a slight but apparently dose-related increase in
the number of enlarged cervical lymph nodes for males of the mid-
and high-doses of the F0 and F1 generation. The increase was not
reported as statistically significant. Slight but apparently dose-
related increases were also reported for fluid distention of the
uterus in both generations of females, but the values were not
statistically significant. Minimal diffuse follicular epithelial
hypertrophy/hyperplasia and centrilobular hepatocyte enlargement
were observed in males at 1200 ppm in both generations. Thyroid
follicular cell adenomas were also observed in F1 males at 1200
ppm. High-dose females of both generations also manifested minimal
diffuse follicular cell hyperplasia/hypertrophy. At 300 ppm 2/23
males of the F1 generation manifested thyroid follicular cell
hyperplasia. There were no apparent adverse effects of treatment on
implantation rates, litter size or pup mortality. A significant
decrease in mean pup and litter weights was observed only at 1200
ppm of the F1 generation and F2 generation. The startle response
(reflex) was slightly delayed at the mid and high dose of both
generations. Macroscopic examination of selected tissues showed no
compound-related effects. Liver weights were increased (organ
weight/body-weight ratio) in both sexes of the F1 generation at 75,
300 and 1200 ppm, as well as for females of the F0 generation at
300 and 1200 pm. Kidney weight was also increased in the F0
generation males (1200 ppm) and females (300 and 1200 ppm). The
NOAEL was 75 ppm, equal to 5.6 mg/kg bw/day for males and 6.2 mg/kg
bw/day for females, based on increased organ to body-weight ratios
for liver and kidney, and thyroid follicular cell hyperplasia at 300
ppm (Ryle et al., 1991).
Special studies on embryotoxicity/teratogenicity
Rats
Sexually mature female Sprague-Dawley Crl:CD BR rats were
inseminated by sexually mature males and randomly divided into 4
groups of 25 dams each. Pregnant dams were gavaged with 0, 20, 100
or 500 mg/kg bw/day of maneb technical (90.4% purity) on days 6-15
of gestation. No dams died on study. Neurobehavioural clinical
signs (impaired mobility, hind limb paralysis, excessive
mastication) were observed on day 11 of gestation and persisted
throughout the study in high-dose treated females. Soft stools and
decreased defecation was also reported. Two high-dose females also
exhibited pallor and decreased body temperature. At 100 mg/kg
bw/day soft stool was the only compound-related sign observed.
Clinical signs for low-dose treated animals were comparable to
control animals. At 100 and 500 mg/kg bw/day, maternal body weights
and body-weight gain were significantly reduced. Low-dose values
were comparable to controls. Terminal body weights were decreased
at 100 and 500 mg/kg bw/day. However, net body weight (i.e.
terminal weight minus gravid uterine weight) was decreased only at
the high dose, as was gravid uterine weight. Food consumption was
comparable between control group and low-dose females. Food
consumption was decreased at higher doses. Caesarean section
observations indicated no fetal deaths but, dose-related resorptions
at the mid and high-dose levels which paralleled a dose-related and
statistically significant increase in early resorptions. Post-
implantation losses were also increased at the mid and high dose
with concurrent decreases in the number of viable fetuses at the
same dose levels. Low-dose observations were comparable to
controls. External fetal observations were comparable to control
group for all dose levels as were visceral malformations and
variations. Skeletal malformations were comparable between all
experimental groups when compared on a litter or fetal basis.
Numerous skeletal variations were observed only in the high-dose
group and were generally indicative of developmental delay as
evidenced by reduced ossification of various skeletal structures.
These findings at the high dose also exceeded maximum values of the
historical control data. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 20 mg/kg bw/day. Maternal toxicity was seen
at 100 mg/kg bw/day as decreased body weight and decreased food
consumption. Embryo/fetotoxicity was observed as increased (early)
resorptions, increased post-implantation losses and a decrease in
the number of viable fetuses at 100 mg/kg bw/day. No teratogenicity
was observed (Nemec, 1992).
Female Sprague-Dawley rats (23-25 dams/group) were gavaged with
20, 100, or 500 mg/kg bw/day of technical maneb (99.99% purity with
less than 0.01% ETU) in 0.5% CMC on days 6-15 of gestation. Two
additional groups serving as controls received either CMC (24 dams)
or were left untreated (24 dams).
There were no compound-related deaths and no clinical signs
below 500 mg/kg bw/day. High-dose dams manifested unsteady gait,
dragging of the rear limbs, diminished sensitivity to pain in the
affected limbs and paresis of the rear limbs. Body weights and
body-weight gain were decreased at the high dose. Gross pathology
was unremarkable in all treated dams. There were no statistically
significant differences between treated and control groups for
implantations, conception rate, corpora lutea, and the number of
live fetuses per litter. At 500 mg/kg bw/day, however, the number
of litters with dead fetuses was increased. Fetal observations at
the time of caesarean section revealed no remarkable findings at
dose levels of 20 and 100 mg/kg bw/day. However, animals receiving
500 mg/kg bw/day showed numerous changes including short tail,
meningocele, accessory toe, shortened toes, macroglossia,
syndactyly, kyphosis, oligodactyly and shortened splayed toes. The
mean fetal body weight per litter was also decreased as was mean
fetal body length per litter at 500 mg/kg bw/day. The number of
anomalous litters was significantly increased at the high dose
(25/25, 100%) for all malformations combined. Of the 304 live
fetuses examined at the high dose, 246 (80.9%) had anomalies. A
statistically significant increase in the number of fetal variants
was also observed at 500 mg/kg bw/day for all variations and
retardations combined. Maternal toxicity was seen at 500 mg/kg
bw/day as clinical signs and decreased body weight and body-weight
gain. Embryo/fetotoxicity and teratogenicity were seen at 500 mg/kg
bw/day based on decreased fetal body weight and body length, and an
increase in the number of anomalous litters and fetuses for all
malformations combined and for all variations and retardations
combined (Kapp et al., 1991).
Rabbits
Himalayan Chbb:HM rabbits were randomly assigned into four
groups (15 does/group). The study was divided into 3 phases. Five
does per dose group were artificially inseminated at each time
period. Gavage doses of 5, 20 or 80 mg/kg bw/day of maneb technical
(90.6% purity with 2% ETU contamination) were administered in CMC on
days 6 through 18 inclusive. The control group received CMC only.
At the high dose, 1 animal died and 3 were sacrificed - 1 moribund,
1 after aborting on day 21, and 1 after premature delivery on day
28. At the mid-dose, 1 animal was sacrificed in a moribund state
and 1 control animal delivered prematurely.
There were no compound-related signs of toxicity observed
during the study. Body weight and body-weight gain were decreased
at the high dose. Food consumption was decreased on days 7-9 and
10-11. Gross pathology revealed no compound-related abnormalities
in dams at terminal sacrifice. Pre-implantation loss and pregnancy
rates were comparable between control and test groups as were the
number of corpora lutea or implantations among control and test
groups. Post-implantation loss was non-significantly increased at
the high dose; however, a significant decrease in the number of
viable fetuses and an increase in the number of resorptions were
observed. Fetal body weights, crown-rump length and sex ratio were
comparable between treated and control groups. The placental weight
of female fetuses only was significantly greater at the high dose
compared to controls. Uterine weight was significantly reduced in
does administered 80 mg/kg bw/day. There were no fetal external
abnormalities observed. There were no compound-related visceral or
skeletal abnormalities in fetuses. NOAELs could not be determined
due to study deficiencies (Merkle, 1983).
Special study on hydrolytic stability of maneb in DMSO
14C-maneb (1.7 mg) was dissolved in 1.7 ml of DMSO and 30
minutes later added (dosing) to teflon-lined vials containing
sterilized buffer solutions (pH 5, 7 and 9) and stirred under
aseptic conditions. The final maneb concentration was approximately
10 ppm and the DMSO concentration was 1% (v/v). Hydrolysis was
performed in the dark at 25 °C. Aliquots were analyzed on day 0
(i.e. immediately after dosing), and on days 1, 2, 4, 7, 14 and 30.
Parent chemical, maneb, was not detected at any sampling time. A
mixture of several identified and unidentified compounds was
reported at all pH levels on day 0 including, but not limited to,
ETU, EBIS and Jaffe's base. ETU was generally the highest on day 0
and increased in concentration with time while the remaining
compounds decreased with time. The proportionality of the compounds
varied with changing pH (Rudel, 1990).
Special studies on genotoxicity
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The results are summarized in Table
3. A number of available studies were not considered, either
because DMSO was used as a solvent in which maneb is very unstable
or because of important omissions from the reports. The Meeting
concluded that maneb is not genotoxic.
Special studies on sensitization and irritation
Guinea-pigs
Hartley albino guinea-pigs (6/sex) were dosed topically with
technical maneb (purity not stated) for 6 hours a day, three times a
week for three weeks (modified Buehler method). Animals were
challenged two weeks after the last induction and re-challenged one
week later. Three males and three females received
dinitrochlorobenzene (DNCB; positive control group) and were treated
in a similar manner as the test group but were not re-challenged.
Reactions were scored at 24 and 48 hours after a challenge. DNCB
was determined to be an extreme sensitizing agent and maneb a
moderate sensitizing agent under the test conditions (Naas, 1989e).
Table 3. Results of genotoxicity assays on maneb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium TA1535, 8.3-675 µg/plate; Not specified Negative Arni, 1981
reversion assay TA1537, TA98, TA100 in acetone
S. typhimurium 3-100 µg/plate; 88.1% a.i. Negative Thomas, 1985
TA1535, TA1537, in deionized water
TA1538, TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.1-30 µg/ml; 88.1% a.i. Negative Thomas, 1986b,
mutation assay (CHO)/hprt in deionized water 1988
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations
In vivo aberrations Chick embryos 0.5-27 g/l in 80% a.i. Negative Arias, 1988
aqueous solution
Complete and partial D. melanogaster 10 000 ppm saturated Not specified Negative Woodruff et al.,
chromosome loss mus-302 mutant aqueous solution with 1983
ethanol and sucrose
Bone marrow Albino mouse 100 mg/kg Not specified Negative Kondratenko &
cytogenetics Kurinnyi, 1972
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
Bone marrow Male and female 46.4 mg/kg/day for 2 days; Not specified Negative Zeller &
cytogenetics Chinese hamsters in carboxy-methylcellulose (increased Engelhardt, 1980
(cont'd) gaps)
Male Fischer 344 rat 4.9 g/kg (acute); 88.1% a.i. Negative Ivett & Lebowitz,
1.64 g/kg/day for 5 days; 1985
in carboxy-methylcellulose
Dominant lethal assay Male NMRI mice 10-100 mg/kg/day for Not specified Negative Leuschner, 1978
5 days; in saline
Micronucleus assay Male or female ICR mouse 1 g/kg with 100 mg/kg Not specified Negative Seiler, 1977
with sodium nitrite NaNO2; in gum arabic soln.
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro unscheduled Primary rat hepatocytes 0.5-100 µg/ml; 88.1% a.i. Negative Loveday, 1986,
DNA synthesis (UDS) from male Fischer 344 rat in culture medium 1988
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro SCE assays Chinese hamster ovary 0.5-30 µg/ml ± rat 88.1% a.i. Weak Thomas, 1986a
(CHO) cells or mouse S9; positive
in deionized water with
activation
In vivo SCE assays Chick embryos 0.5-27 g/l in aqueous 80% a.i. Positive Arias, 1988
solution
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 0.05-0.2 µg/ml; 88.1% a.i. Negative Tu et al., 1985
in water?;
no activation used
C3H/10T 1/2 cells with 0.2 µg/ml as 88.1% a.i. Negative Tu et al., 1986
"promotion" "promoter"; in water?
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not
normally use such supplementation; solvent is provided if specified in the report
Maneb DF (75% dust) was administered to male albino guinea-pigs
of the Dunkin-Hartley strain following a modified method of Buehler.
No delayed hypersensitivity reaction was observed. Animals
receiving DNCB as positive control showed positive sensitization
reactions (Glaza, 1988c).
Rabbits
Single, 0.5 gram doses of maneb technical (purity not stated)
were applied to the clipped, intact skin of 6 New Zeeland white
rabbits under a semi-occlusive dressing for a 4-hour exposure
period. Application sites were evaluated in accordance with the
method of Draize at approximately 30-60 minutes and 24, 48 and 72
hours after patch removal and daily thereafter for 14 days.
Technical maneb induced a very slight to slight erythema and edema.
Other dermal findings were not remarkable. Technical maneb was
concluded to be slightly irritating (Draize score 1.5) (Naas,
1989c).
Single, 100 mg doses of maneb technical were instilled into the
lower conjunctival sac of the right eyes of 6 New Zeeland white
rabbits. The eyelids were held closed for approximately one second
and released. The left eye served as the contralateral control.
Eyes were left unwashed and examined at 1, 24, 48 and 72 hours and
at 4 and 7 days. Sodium fluorescein was used in evaluating corneal
damage at 72 hours and 7 days. No corneal involvement was observed.
Four of six rabbits cleared of all ocular reactions by day 7. Minor
conjunctival reactions were present in two rabbits on day 7. Maneb
technical was considered to be a moderate irritant based on
persistent conjunctival irritation in two rabbits for 7 days and the
mean response of 8-19 out of a maximum of 20 for conjunctival
irritation over the initial 72-hour period (Naas, 1989d).
Maneb DF (75% dust) produced corneal and iridial irritation and
slight to severe conjunctival irritation in the unwashed eyes of New
Zeeland white rabbits. Iridial irritation and slight to moderate
conjunctival irritation were observed in the washed eyes (60 second
flush with water at 30 seconds post-compound administration) of
companion New Zeeland white rabbits. Ocular irritation cleared by
72 hours in washed eyes and in less than 7 days in unwashed eyes
(Glaza, 1988d).
Maneb DF (75% dust) applied to the intact skin of New Zeeland
white rabbits for 4 hours and scored according to the method of
Draize at 4, 24, 48, 72 and 96 hours post-administration produced a
slight irritation (Draize score < 1.0 for all readings) (Glaza,
1988e).
COMMENTS
Male and female rats given 25 mg/kg bw/day of 14C-labelled
maneb orally showed no differences between sexes with regard to
excretion patterns. Greater than 90% of the absorbed 14C was
eliminated in urine by 24 hours. Less than 1% was eliminated as
carbon dioxide. Average 14C concentration as percent of dose per
gram of tissue was greatest for the thyroid, followed by kidney and
liver. The percent of 14C present in urine as ETU at 12 hours was
21-30% on a mole/mole basis and less than 0.4% as maneb.
The acute oral, dermal and inhalation toxicity of maneb
technical and maneb 75% dust is low. WHO has classified maneb as
unlikely to present acute hazard in normal use.
Rats were fed dietary concentrations of 0, 80, 400 or 1300 ppm
maneb technical for 13 weeks. The NOAEL was 80 ppm (equal to 5.0
mg/kg bw/day) based on an increase in absolute thyroid weight and
thyroid follicular cell hyperplasia at 400 ppm.
In dogs fed dietary concentrations of maneb technical at 0,
100, 400 or 1600 ppm for 13 weeks, the NOAEL was 100 ppm (equal to
3.7 mg/kg bw/day) based on thyroid follicular cell hyperplasia at
400 ppm.
In a 52-week study in dogs, maneb was administered at dietary
concentrations of 0, 50, 200, 1000 or 2200 ppm. The NOAEL was 200
ppm (equal to 6.4 mg/kg bw/day) based on thyroid enlargement and
thickening and thyroid follicular cell hyperplasia at 1000 ppm.
The overall NOAEL in dogs, based on the evaluation of all of
the data on this species, was 6.4 mg/kg bw/day.
In a six-month study in monkeys, maneb was administered at
dietary concentrations of 0, 100, 300 or 3000 ppm. The NOAEL was 100
ppm (equal to 7.3 mg/kg bw/day) based on an increase in thyroid
weight at 300 ppm.
In a 79-week carcinogenicity study in mice at dietary
concentrations of 0, 60, 240 or 2400 ppm, the NOAEL was 60 ppm,
equal to 11 mg/kg bw/day, based on decreased body weight and
decreased thyroxine levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes.
In a 31-month toxicity/carcinogenicity study in rats at dietary
concentrations of 0, 30, 100, 300 or 1000 ppm, the NOAEL was 300 ppm
(equal to 20 mg/kg bw/day) based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm. There was no evidence of carcinogenicity.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 75, 300 or 1200 ppm, the NOAEL was 75 ppm
(equal to 5.6 mg/kg bw/day) based on increased organ to body-weight
ratios for liver and kidney, and thyroid follicular cell hyperplasia
at 300 ppm.
An oral teratogenicity study in rats was conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day. The NOAEL was 20 mg/kg
bw/day for maternal toxicity and embryo/fetotoxicity. Maternal
toxicity was seen at 100 mg/kg bw/day as decreased body weight and
decreased food consumption. Embryo/fetotoxicity was observed as
increased (early) resorptions, increased post-implantation losses
and a decrease in viable fetuses at 100 mg/kg bw/day. No
teratogenicity was observed.
In a second oral teratogenicity study in rats conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day the NOAEL for maternal
toxicity and embryo/fetotoxic and teratogenic effects was 100 mg/kg
bw/day. Maternal toxicity was seen at the highest dose as decreased
body weight and clinical signs. Embryo/fetotoxicity and
teratogenicity were seen at the highest dose as decreased fetal body
weight and body length, and an increase in the number of anomalous
litters and fetuses for all malformations combined and for all
variations and retardations combined.
An oral teratogenicity study in rabbits was conducted at dose
levels of 0, 5, 20 or 80 mg/kg bw/day. Due to study deficiencies a
NOAEL could not be determined.
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The Meeting concluded that maneb is
not genotoxic. A number of available studies were not considered,
either because DMSO was used as a solvent in which maneb is very
unstable or because of important omissions from the reports.
The data on maneb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 5.0 mg/kg bw/day for thyroid effects in rats,
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for maneb, alone or in combination with
mancozeb, metiram and/or zineb, because of the similarity of the
chemical structures of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differential using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 60 ppm in the diet, equal to 11 mg/kg bw/day
(79-week study)
Rat: 80 ppm in the diet, equal to 5.0 mg/kg bw/day
(13-week study)
300 ppm in the diet, equal to 20 mg/kg bw/day
(31-month study)
75 ppm in the diet, equal to 5.6 mg/kg bw/day
(reproduction study)
Dog: 200 ppm in the diet, equal to 6.4 mg/kg bw/day
(52-week study)
Monkey: 100 ppm in the diet, equal to 7.3 mg/kg bw/day
(6-month study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, metiram, and zineb).
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Allen, T.R., Frei, Th., Biedermann, K., Luetkemeier, H., Terrier,
Ch., Vogel, O., Wilson, J. (1989). 13-week oral toxicity (feeding)
study with maneb technical in the dog. Unpublished report No. 206605
from Research and Consulting Company, Ltd., Itingen, Switzerland.
Submitted to WHO by Elf Atochem.
Arias, E. (1988). Sister chromatid exchanges and chromosomal
aberrations in chick embryos after treatment with the fungicide
maneb. Mutation Res. 206: 271-273.
Arni, P. (1981). Salmonella/mammalian-microsome mutagenicity test
with CGA 31 364 = maneb. Unpublished experiment No. 810466 from
Ciba-Geigy Limited, Basle, Switzerland.
Corney, S.J., Allen, T.R., Janiak, T., Frei, Th., Leutkemeier, H.,
Biedermann, K., Vogel., O. & Springall, C. (1992). A 52-week oral
toxicity study (feeding) with maneb technical in the dog.
Unpublished report No. 206616 from Research and Consulting Co.,
Ltd., Itingen, Switzerland. Submitted to WHO by Elf Atochem.
Craine, E.M. (1991). A dermal radiotracer absorbtion study in rats
with 14C maneb. Unpublished report No. WIL-134010 (reissued final
report March 13, 1991) from WIL Research Laboratories, Inc.,
Ashland, Ohio, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988a). Acute oral toxicity study of Maneb DF in rats.
Unpublished report No. HLA 80104922 from Hazleton Labs America Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988b). Acute dermal toxicity study of Maneb DF in
rabbits. Unpublished report No. HLA 80104923 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988c). Dermal sensitization study of Maneb DF in guinea-
pigs. Unpublished report No. HLA 80104926 from Hazleton Labs America
Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988d). Primary eye irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104924 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988e). Primary dermal irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104925 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Hoffman, G. (1991). An acute inhalation toxicity study of Maneb DF
in the rat. Unpublished report No. 90-8287 from Bio/dynamics, Inc.,
East Millstone, New Jersey, USA. Submitted to WHO by Elf Atochem.
Ivett, J. & Lebowitz, H. (1985). Clastogenic evaluation of maneb
technical lot MT 01 (88.1% ai.) in the rat bone marrow cytogenic
assay. Unpublished final report No. LBI 22202 from Litton Bionetics
Inc., Kensington, Maryland, USA. Submitted to WHO by Elf Atochem.
Kapp, R.W., Schellhaas, L.J., Piccirillo, V.J. (1991). Prenatal
toxicity study of maneb in rats. Unpublished report Nos. BASF
88/0522; 88/0523; 88/0524 from BASF Gewerbehygiene und Toxikologie,
Ludwigshaven, Germany. Submitted to WHO by Elf Atochem.
Kondratenko, T. & Kurinnyi, A. (1972). Effects of some fungicides
(dithiocarbamic acid derivatives) on chromosomes of bone marrow
cells in mice. Tsitol. Genet. 6: 225-228.
Leuschner, F. (1991). Chronic oral toxicity of manganese-ethylene-
1,2-bis-dithiocarbamate, 90% - called for short "maneb" - in Sprague-
Dawley (SIV) - rats (with special attention to carcinogenic
properties). Unpublished registration document No. (BASF) 86/0430
Amendment No. 3 dated April 19, 1991 from Laboratory of Pharmacology
and Toxicology, Hamburg, West Germany. Submitted to WHO by Elf
Atochem.
Leuschner, F. (1978). Dominant lethal test on male mice with
manganese ethylene-1,2-bis-dithiocarbamate. Unpublished report No.
BASF 78/298 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Dontenwill, W. & Hubschner, F. (1986a). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" -in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 1 dated October 2, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A. & Hubschner, F. (1986b). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" - in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 2 dated October 20, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Klie, R., Dontenwill, W. & Rogulja, P.
(1979). Chronic oral toxicity of manganese-ethylene-1,2-bis-
dithiocarbamate, 90% - called for short "maneb" - in Sprague-Dawley
(SIV) - rats (with special attention to carcinogenic properties).
Unpublished registration document No. (BASF) 86/0430 from Laboratory
of Pharmacology and Toxicology, Hamburg, West Germany. Submitted to
WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Schneider, C., Schwerdtfeger, W. &
Dontenwill, W. (1977). Oral toxicity of maneb in the Rhesus monkey:
six months dietary dosing. Unpublished report No. WF 1172 from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Loveday, K. (1986). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Unpublished
report No. 850047-20 from American Biogenics Corporation, Woburn,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Loveday, K. (1988). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Supplementary
report PW-118 to Unpublished report No. 850047-20 from American
Biogenics Corporation, Woburn, Massachusetts, USA. Submitted to WHO
by Elf Atochem.
Merkle, J. (1983). Study to determine the prenatal toxicity of
manganous ethylenebis (dithiocarbamate) in rabbits. Unpublished
study RZ-No. 83/094 from BASF AG, Ludwigshafen Rhein, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Naas, D.J. (1989a). Acute oral toxicity (LD50) study in albino rats
with maneb technical. Unpublished report No. WIL-134003 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J., (1989b). Acute dermal toxicity (LD50) study in albino
rabbits with maneb technical. Unpublished report No. WIL-134004 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989c). Primary dermal irritation study in albino
rabbits with maneb technical. Unpublished report No. WIL-134005 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989d). Primary eye irritation study in albino rabbits
with maneb technical. Unpublished report No. WIL-134006 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J. (1989e). Skin sensitization study in albino guinea-pigs
with maneb technical. Unpublished report No. WIL-134007 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Nemec, M., (1992). A developmental toxicity study of maneb technical
in rats. Unpublished report No. WIL-134011 from WIL Research
Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by Elf
Atochem.
Puhl, J.R. (1985). Metabolism of radiolabeled maneb in rats.
Unpublished report No. 6181-101 from Hazleton Labs., America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Rudel, H. (1990). Hydrolysis of maneb at pH 5, 7, and 9. Unpublished
project No. MRG-O1/7-04 from Fraunhofer-Institut für Umweltchemie
und Okotoxikologie, D-5948 Schmallenberg, Federal Republic of
Germany. Submitted to WHO by Elf Atochem.
Ryle, P.R., Bell, P.F., Parker, C., Farmer, H., Offer, J.M.,
Anderson, A. & Dawe, I.S., (1991). A study of the effect of maneb
(technical) on reproductive function of two generations in the rat.
Unpublished report No. MNB1/9072 from Huntington Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Elf Atochem.
Seiler, J. (1977). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Terrill, J.B. (1990). Acute inhalation toxicity study with maneb in
the rat. Unpublished report No. HLA 2567-100 from Hazleton Labs.,
Rockville, Maryland, USA. Submitted to WHO by Elf Atochem.
Terrill, J.B., (1991). A four-week study in the rat to determine the
lung tissue residue and effect upon the thyroid function with maneb.
Unpublished report No. HLA 2567-101 from Hazleton Labs., Rockville,
Maryland, USA. Submitted to WHO by Elf Atochem.
Thomas, M. (1985). Salmonella/microsome mutagenesis assay on
technical grade maneb. Unpublished final report No. ABC 850047-40
from American Biogenics Corp., Woburn, Massachusetts, USA. Submitted
to WHO by Elf Atochem.
Thomas, M. (1986a). In vitro sister chromatid exchange assay in
cultured Chinese hamster ovary (CHO) cells treated with technical
grade maneb. Unpublished final report No. ABC 850047-30 from
American Biogenics Corp., Woburn, Massachusetts, USA. Submitted to
WHO by Elf Atochem.
Thomas, M. (1986b). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Unpublished final report No. ABC
850047-10 from American Biogenics Corp., Woburn, Massachusetts, USA.
Submitted to WHO by Elf Atochem.
Thomas, M. (1988). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Supplementary report PW-117 to
Unpublished final report No. ABC 850047-10 from American Biogenics
Corp., Woburn, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tompkins, C.E. (1992). An 18-month dietary oncogenicity study in
mice with maneb technical. Unpublished report No. WIL-134008 from
WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to
WHO by Elf Atochem.
Trutter, J.A. (1988a). Subchronic toxicity study with maneb
technical in the rat. Unpublished report No. HLA 153-140 from
Hazleton Laboratories America, Inc., Rockville, Maryland, USA.
Submitted to WHO by Elf Atochem.
Trutter, J.A. (1988b). A 21-day dermal toxicity study in rabbits
with maneb technical. Unpublished report No. HLA 153-139 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA.
Submitted to WHO by Elf Atochem.
Tu, A., Breen, P. & Sivak, A. (1986). Evaluation of maneb in the
C3H-10T ´ cell transformation assay for promotion activity.
Unpublished report No. ADL 88720-44 from Arthur D. Little, Inc.,
Cambridge, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tu, A., Sivak, A., Hatch, K. & Breen, P. (1985). Evaluation of maneb
in the C3H-10T ´ cell transformation assay. Unpublished final report
No. ADL 88720-44 (1-0860) from Arthur D. Little, Inc., Cambridge,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1986). Thirteen-week subchronic inhalation toxicity
study on maneb in rats (final report). Unpublished report No. 550-
001 from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1987). Thirteen-week subchronic inhalation toxicity
study on maneb in rats - addendum to the final report covering the
recovery phase. Unpublished report No. 550-001 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Elf Atochem.
WHO (1992). The WH0 recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/91.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Woodruff, R., Phillips, J. & Irwin, D. (1983). Pesticide induced
complete and partial chromosome loss in screens with repair-
defective females of Drosophila melanogaster. Environ. Mutagen. 5:
835-846.
Zeller, H. & Engelhardt, G. (1980). Cytogenic investigations in
Chinese hamsters after two intraperitoneal administrations of maneb.
Bone marrow chromosome analysis. Unpublished report dated December
19, 1980 from Gewerbehygiene und Toxikologie of BASF, Germany.
Submitted to WHO by Elf Atochem.
Table 1. Acute toxicity of maneb technical
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat1 Crl:CD BR M/F oral > 5000 Naas, 1989a
Rat2 Crl:CD BR M/F inhal. 7.38 Terrill, 1990
Rabbit3 NZW M/F dermal > 2000 Naas, 1989b
1 Deionized water as vehicle; 3/5 males died, 0/5 females died.
2 Dose only 4 hour exposure, respirable dust of 3.8 microns. Analytical LC50 of 5.34 mg/l
for males and greater than 7.38 mg/l for females. 14 day observation period.
Respiratory distress and decreased body-weight were the major clinical signs.
3 Deionized water as vehicle.
Table 2. Acute toxicity of maneb DF1
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat2 Sprague-Dawley M/F oral > 5000 Glaza, 1988a
CD(R)
Rat3 Sprague-Dawley M/F inhal. 4 h > 2.0 Hoffmann, 1991
CD(R) exposure
Rabbit4 NZW M/F dermal > 2000 Glaza, 1988b
1 Maneb DF (75% Dust)
2 Uniform suspension in deionized water
3 Mean exposure concentration 2.0 mg/l with a nominal concentration of 53 mg/l.
Mass Median Aerodynamic Diameter was 3.3 microns with an average
Geometric Standard Deviation of 2.4, and 8.8% of particles were
less than one (1) micron in size. There were no deaths during the whole body exposure.
4 0.9% saline vehicle
Short-term toxicity studies
Rats
Sprague-Dawley (Crl:CD BR) rats (32/sex/dose; control 16/sex)
received a nose-only exposure to maneb technical (86.8% purity
adjusted to 100%) for 6 hours/day, five days a week for 4 weeks at a
target level of 100 mg/m3 (particle size ranged from 3.45-3.83
microns). All animals survived to scheduled sacrifice and compound-
related signs were unremarkable. T4 and TSH values in males and
TSH values in females were decreased during week one only. However,
results for treated males were generally less than control values
for the remainder of the study and were not considered biologically
meaningful. Results for females were generally comparable to
controls. Gross necropsy was not remarkable between treated and
controls. Mean absolute lung weights for both sexes were comparable
to controls but relative weights increased. Mean terminal body-
weights were decreased for both sexes and paralleled the increased
relative lung to body-weight ratio. Residue levels of maneb,
manganese and ETU generally remained fairly constant during the
exposure period and was less than the limit of detection after the
recovery period. During weeks 1-4, residue levels in lungs for
males ranged between 3.9-7.9 ppm (7.9-6.8 ppm for weeks 2 and 4) for
maneb; 4.8-6.0 ppm for manganese and 0.12-0.19 ppm for ETU; for
females 2.1-4.2 ppm for maneb, 1.8-2.8 for manganese and < 0.1-0.12
ppm for ETU. Residue levels in the urine of females was generally
less than the limit of detection for maneb and manganese while
values for ETU generally ranged between 1.3 and 2.15 ppm during the
exposure period and less than the limit of detection during the
recovery phase. Manganese was not detected in males and maneb and
ETU were not detected during recovery. Maneb ranged from 1.3-6.6 ppm
during exposure and ETU from 5-29 ppm (5.0-5.1 for weeks 2 and 4)
(Terrill, 1991).
Charles River Sprague-Dawley albino rats (28/sex/dose) received
nose-only target inhalation exposures of 0, 10, 30 or 100 mg/m3 (0,
10, 32, or 98 mg/m3 gravimetric concentration with an equivalent
mean aerodynamic diameter of 3.5 [+/-2.0] microns) of technical
maneb (purity not stated) for 6 hours a day, 5 days a week for 13
consecutive weeks. Measured concentrations of ETU for the low,
middle, and high-dose groups were 0.12, 0.71 and 2.5 mg/m3.
Fourteen animals per sex per dose were sacrificed after 13 weeks of
exposure and the remaining animals allowed to recover for 13 weeks
and then sacrificed.
There were no exposure-related effects on appearance or
behaviour and no compound-related deaths. Body weight of females
exposed to the inter-mediate and high dose were depressed during the
exposure period, whereas males had decreased body weights only at
100 mg/m3 for weeks 1-3. There were no exposure-related effects
observed in ophthalmologic, haematologic (10 parameters) or serum
biochemical measurements (21 parameters including T3 and T4) after
the exposure period. Absolute organ weights were comparable to
control group. There was no evidence of any dose-related
macroscopic or microscopic changes in animals sacrificed at 13
weeks. ETU was detected in the urine of all exposed rats, in the
thyroids of all high-dose animals, in the liver of two females of
the intermediate-dose group and the plasma of one intermediate dose
female. ETU concentration in the urine was dose-related in both
sexes but was much greater in females. ETU mean residues in the
thyroid for males were however much greater in males (23.6 µg/ml)
than females (8.4 µg/ml). Maneb residues were also found in urine
of both sexes.
At the end of the 13-week recovery period no differences were
observed among the treated groups that could be attributed to test
material exposure when compared to controls. ETU and maneb residues
were not detected in any tissue or body fluids measured after a 13-
week recovery period. The NOAEL was 10 mg/m3 based on decreased
body weight at 30 mg/m3 (Ulrich, 1986, 1987).
Sprague-Dawley derived Crl:CDBR rats (15 animals/sex/dose)
received dietary concentrations of 0, 80, 400 or 1300 ppm of maneb
technical (78% purity). Ten animals/sex/dose were sacrificed at 14
weeks and 5 animals/sex/dose were placed on a 4-week recovery period
and sacrificed at 17 weeks. There were no overt signs of toxicity
or dose-related effects on mortality, ophthalmology or haematology.
Body-weight gains for males and females at 1300 ppm were
significantly decreased at 13 weeks. At week 17, body weights of
high-dose males were comparable to the control group, while female
body weight was 10% lower than controls. Food consumption was
comparable to controls.
A dose-related decrease in T4 levels and a concomitant
increase in TSH levels were seen in dosed females at 14 weeks. The
decrease in T4 levels was significant at the high dose. In males
T4 values were slightly decreased and TSH values slightly increased
at 1300 ppm. T4 values for males and females were similar to or
higher than concurrent controls at the end of the recovery period,
while TSH values were similar to or lower than controls. T3 values
were increased, but not statistically significant in high-dose males
and females at 14 weeks and in females at the end of the recovery
period. T3 values for males at the high dose were comparable to
controls at the end of the recovery period. There were no changes
of toxicological significance in other clinical chemistry
parameters. A dose-related trend (dose-related increase) was
evident for thyroid/parathyroid weights of dosed males and females.
Absolute weights were significantly increased in males at 400 and
1300 ppm, and relative weights were significantly increased in high-
dose males. Thyroid/parathyroid weights of dosed and control
animals were similar following a 4-week recovery period. There were
no compound-related changes in macroscopic pathology in dosed
animals at 14 or 18 weeks. Follicular cell hyperplasia of the
thyroid was observed in 1/10 mid-dosed males, 10/10 high-dosed males
and 2/10 high-dosed females compared with none in the controls.
Increased colloid of the thyroid was also exhibited in 4/10 high-
dose males. Thyroid changes regressed following the recovery phase.
However one high-dose male manifested a follicular cell adenoma.
Granular renal pigment was observed in 2/10 low-dose males and all
animals in the mid and high-dose groups. However, the incidence of
chronic progressive nephropathy was similar in dosed and control
males and females. At the end of the recovery period a reduction in
the incidence and amount of renal pigment was reported in 3/5 mid-
dose and 3/5 high-dose males and 4/5 mid-dose and 4/5 high-dose
females. The NOAEL was 80 ppm, equal to 5.0 mg/kg bw/day, based on
an increase in absolute thyroid weight and thyroid follicular cell
hyperplasia at 400 ppm (Trutter, 1988).
Rabbits
New Zeeland white rabbits (five animals/sex/dose) were
administered 0 (sham treated), 100, 300 or 1000 mg/kg bw/day of 86%
maneb technical (adjusted to 100%) and applied to the shaved (10%)
dorsal intact skin area. Applications were made for 6 hours a day,
5 days a week for 3 weeks. Clinical findings, body weight, food
consumption and organ weights were comparable to controls. Values
for haematology and serum chemistry were comparable between treated
and control groups. Gross signs of skin irritation were observed in
2/10, 8/10 and 10/10 animals at the low, mid and high-dose.
Erythema was not observed at 100 mg/kg bw/day and edema was not
observed at 100 or 300 mg/kg bw/day (Draize method). Erythema when
present was generally graded slight as was edema. Epidermal scaling
was observed in 2, 8, and 10 animals at the low-, mid- and high-
doses. Histopathology showed epidermal irritation at the site of
application in all dose groups, which was characterized by minimal
to slight acanthosis and hyperkeratosis. Follicular cell
hypertrophy of the thyroid was observed in 7/10 animals at 1000
mg/kg/day. Increased colloid material was observed in 2 and 4
females at 300 and 1000 mg/kg/day, respectively (Trutter, 1988).
Dogs
Beagle dogs (2 animals/sex/dose) received dietary
concentrations of technical maneb (91.58% purity) of 0, 100, 400 or
1600 ppm for 13 weeks. At the high dose, diarrhoea was observed in
1 male and 1 female with attendant decreased activity in the female.
Body weight was decreased 20% and food consumption 30% in high-dose
females. Body weight and food consumption were comparable between
treated and control groups for males. Slight to marked anaemia was
observed in 1 male and 1 female at 6 and 13 weeks with enhanced
erythropoietic activity in all dogs at the high dose. T3 and T4
values were decreased in both sexes at 1600 ppm at six weeks but not
13 weeks. Total lipid, cholesterol, triglyceride and phospholipid
concentrations were slightly increased in males at 6 and 13 weeks.
Fluctuations for these parameters were also observed in one high-
dose female along with slight to moderate increases in glutamate
dehydrogenase and GGT activity and slight decreases in plasma
calcium, creatinine and total protein concentrations at 6 weeks.
Post-mortem examination revealed increased thyroid weight in 1 male
and 1 female at 1600 ppm. Increased liver to body-weight ratio was
also observed in the second female. One male and 1 female of the
high-dose group also manifested enlargement or thickening of the
thyroid gland upon macroscopic examination. Microscopic examination
revealed moderate to severe thyroid follicular cell hyperplasia in
high-dose males. One female at 400 ppm manifested slight thyroid
follicular cell hyperplasia; both females in the 1600 ppm group
showed thyroid follicular cell hyperplasia ranging from slight to
minimal. A minimal degree of thyroid follicular cell hypertrophy
was recorded for 1 male at 400 ppm and 1 female at 100 ppm. A
slight focus of hypertrophic cells in the adenohypophysis of the
high-dose male, which had a severe thyroid follicular cell
hyperplasia was also observed. The NOAEL was 100 ppm, equal to 3.7
mg/kg bw/day, based on thyroid follicular cell hyperplasia at 400
ppm (Allen et al., 1989).
Beagle dogs (5/sex/dose) were given dietary concentrations of
0, 50, 200, 1000 or 2200 ppm of maneb (89.2% purity) for 52 weeks.
No deaths occurred during the study. Diarrhoea was observed in dogs
at 1000 ppm (1/5 females) and 2200 ppm (3/5 males and 1/5 females).
Body weight for males was generally comparable between treated and
control groups. High-dose females displayed lower body weights
compared to controls. Body-weight gain was decreased in both males
and females at 2200 ppm. Group mean food intake at 2200 ppm was
decreased in males and females.
Ophthalmological examination revealed no adverse effects at any
dose level. Mean erythrocyte count, haemoglobin concentration, and
haematocrit at 200, 1000 and 2200 ppm were significantly decreased
at 13 weeks only in females. Increased mean cell volumes were
observed at 2200 ppm in males and 1000 ppm in females. Decreased
mean cell haemoglobin concentration was reported in both sexes at
2200 ppm along with reticulocytosis and polychromasia. Platelet
counts were increased in both sexes at 2200 ppm and in males at 1000
ppm. T4 values were decreased at 2200 ppm in males and females.
T3 was markedly depressed at 2200 ppm for both sexes. Increased
group mean values for cholesterol, total lipids, and phospholipids
were dose-related and statistically significant but only at 2200 ppm
for some of the parameters of either sex. Bilirubin values were
increased in males and females at 2200 ppm as was ALP. Urinalysis
findings were comparable between treated and controls. Neurological
examinations of postural reactions, spinal reflexes and of cranial
nerves revealed no treatment-related effects. The absolute and
relative weight of the thyroids was increased in both sexes at 2200
ppm as was the adrenal weight. Thyroid enlargement and/or
thickening was observed in all dogs at 2200 ppm and 7/10 dogs at
1000 ppm. Thyroid follicular hyperplasia was reported for all dogs
at 1000 and 2200 ppm. Thyroid histopathology was unremarkable at
lower dose levels. There were no other findings suggestive of any
treatment-related effect. The NOAEL was 200 ppm, equal to 6.4 mg/kg
bw/day for males and 7.2 mg/kg bw/day for females, based on thyroid
enlargement and thickening and thyroid follicular cell hyperplasia
at 1000 ppm (Corney et al., 1992).
Monkeys
Rhesus monkeys, (4/sex/group) received 0, 100, 300 or 3000 ppm
of maneb technical (90% purity) in the diet for 6 months. At 6, 13
and 26 weeks, all animals were administered 10 µCi of 131I (on an
empty stomach) and determinations were made for 131I absorption into
the thyroid at 2 and 48 hours post-dosing in conjunction with
determinations for serum T4, T3, protein-bound 131I in serum and
loss of 131I from the thyroid (i.e. half-life in days).
Behaviour and external appearance of treated and control
animals were comparable, and all females demonstrated normal
menstrual discharges. Mean body-weight gains in animals (sexes
combined) given 3000 ppm maneb were lower than those of controls and
the low- and mid-dose groups. Food consumption was unaffected.
Haematology and clinical biochemistry revealed no meaningful
differences between controls and treated groups. However, studies
conducted with 131I revealed significantly decreased 131I absorption
by the thyroid in animals (sexes combined) receiving 3000 ppm at 26
weeks. 131I uptake was decreased 40% in males and 35% in females.
Protein-bound 131I was also decreased in animals (sexes combined) at
3000 ppm with the decrease largely attributable to males. Results
of urinalysis and electrocardiograms were comparable between control
and treated groups. Gross necropsy was unremarkable between groups.
Absolute organ weights for thyroids were increased in monkeys (sexes
combined) at 300 and 3000 ppm. However, statistical significance was
reached only at the high dose and largely attributable to males.
Males also showed a greater increase at 300 ppm than did females.
Enlarged thyroid with large follicles, flat epithelium, and colloid
described as somewhat darker than usual was reported for 7 animals
at 3000 ppm. Moderate proliferation of the epithelium was also
present in two high-dose animals. Only 1 animal at the high-dose
had no thyroid-associated pathology. The NOAEL was 100 ppm, equal
to 7.3 mg/kg bw/day, based on an increase in thyroid weight at 300
ppm (Leuschner et al., 1977).
Long-term toxicity/carcinogenicity studies
Mice
Crl:CD-1 (ICR) BR mice (75/sex/group) received dietary
concentrations of 0, 60, 240 or 2400 ppm maneb technical (89.5%
purity) for 79 weeks. An interim sacrifice and necropsy was
performed on 20 animals/sex/dose group at 52 weeks. The mortality
rate, clinical signs, and palpable masses were comparable between
treated and control groups for both sexes. Female body weight was
decreased at 240 ppm and decreased for males and females at 2400
ppm. Erythrocytes, haemoglobin, and haematocrit were decreased at
2400 ppm in both sexes at interim and terminal sacrifice. T4
levels were decreased at 2400 ppm in both sexes. T4 levels were
also significantly decreased in females at 60 ppm and 240 ppm at
terminal sacrifice. A dose-related trend was evident across all
three doses. At terminal necropsy hepatic masses were evident and
occurred at a greater frequency in high-dose males and females than
controls. Organ-weight changes at 2400 ppm were biologically and
statistically significant only for thyroid and only at terminal
sacrifice. However, histopathology of the thyroid was unremarkable.
A significant increase was observed in high-dose males of
hepatocellular adenomas at terminal necropsy. The number of females
with hepatocellular adenomas at terminal necropsy were comparable to
controls. However, the overall incidence of hepatocellular adenomas
was significantly increased for both sexes at 2400 ppm. The NOAEL
was 60 ppm, equal to 11 mg/kg bw/day, based on decreased body weight
and decreased T4 levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes (Tompkins, 1992).
Rats
Sprague-Dawley (SIV 50) rats (90/sex/group) were fed dietary
concentrations of maneb (90% purity) of 0, 30, 100, 300 or 1000 ppm
for 31 months. Interim sacrifices were conducted at 3, 6, and 12
months on 5 animals/sex/dose and all survivors terminated at 31
months. Beginning at 26 weeks all animals were palpated once a
week. Haematology and clinical biochemistry parameters were
evaluated at pre-test and 3, 6, 12, 18 and 24 months in 10
animals/sex/dose. At 3, 6, 12 and 24 months, 10 animals/sex/dose
were gavaged with 131I (10 µCi) and determinations made for the
biological half-life of 131I in thyroid over a 10-day period, 131I
serum activity, protein bound 131I in serum, serum T4, and the
binding index of T3. Tumour and mortality rates were compared
statistically by means of analysis of variance according to the
method of Peto.
Behaviour, appearance, food consumption and mortality were
comparable between treated and control groups. Body weights at 31
months were within the normal range. However, body weights were
decreased 25% in males and 40% in females at 12 and 24 months. There
were no significant differences between groups or any apparent dose-
related trends in any of the haematology or clinical biochemistry
parameters measured. 131I retention time in the thyroid was
significantly increased only at the high dose tested for males at 6
and 12 months and females at 6 months. Values for 1000 ppm animals
were slightly increased but not statistically significant. Mean
serum T4 was not significantly different between groups for any
time period. However, T4 values at 1000 ppm at 6 and 12 months
were decreased 10% in males and 20% in females. Urinalysis
comparisons between groups were unremarkable. Thyroid weights were
increased at 1000 ppm in males and in females when compared to
controls at 31 months.
All other absolute and relative organ weights appeared
comparable to control values. Macroscopic and microscopic
examination of animals sacrificed during the interim periods
(5/sex/dose) revealed no compound-related changes. Gross and
histopathology of 75 animals (inter-current deaths and terminal
sacrifice) per sex in the control group and high-dose group did not
reveal compound-related non-neoplastic or neoplastic changes.
Additional histological examination of liver, kidney and urinary
bladder in both sexes at all lower doses also revealed no compound-
related non-neoplastic or neoplastic changes. The NOAEL was 300 ppm,
equal to 20 mg/kg bw/day, based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm (Leuschner et al., 1979, 1986a,b; Leuschner 1991).
Reproduction studies
Rats
Male and female rats (Crl:CD(SD)BR VAF/Plus) were randomly
assigned by weight into 4 groups of 28 (F0) and 24 (F1) males and
females per group and received 0, 75, 300 or 1200 ppm of maneb (80-
90% purity) in the diet. Animals were bred for 1 litter in each of
2 consecutive filial generations. There were no treatment-related
deaths or clinical signs at the low-or mid-dose. One high-dose
female in the F0 and F1 generation died. Both females had
locomotor difficulties and appeared thin. The second generation
female also suffered from hind limb paralysis. Signs were
considered treatment-related. Overall food consumption was decreased
for the F0 and F1 males and females. There was an increase in the
food conversion ratio for females of both generations during the
early part of mating. There was also a dose-related increase in
water consumption at the mid and high-dose for males and females of
the F0 and F1 generation during the final two weeks prior to
mating. No change in food or water consumption was observed at the
low dose. F1 females had a 20% decrease in food consumption at
1200 ppm and a 10% decrease at 300 but values were not statistically
significant. Body-weight gains were significantly lower for males
and females of the F0/F1 generations at 1200 ppm and for females
of the F0 generation at 300 ppm. Body weight for dams of the F0
and F1 generations during the periods of gestation and lactation
was decreased only at 1200 ppm of the F1 generation. Body-weight
gain, however, during the lactation period for the F0 and F1 dams
was substantially increased at 1200 ppm.
The mating performance, pregnancy rate and gestational period
for treated groups was comparable to the control group. Adult gross
pathology revealed a slight but apparently dose-related increase in
the number of enlarged cervical lymph nodes for males of the mid-
and high-doses of the F0 and F1 generation. The increase was not
reported as statistically significant. Slight but apparently dose-
related increases were also reported for fluid distention of the
uterus in both generations of females, but the values were not
statistically significant. Minimal diffuse follicular epithelial
hypertrophy/hyperplasia and centrilobular hepatocyte enlargement
were observed in males at 1200 ppm in both generations. Thyroid
follicular cell adenomas were also observed in F1 males at 1200
ppm. High-dose females of both generations also manifested minimal
diffuse follicular cell hyperplasia/hypertrophy. At 300 ppm 2/23
males of the F1 generation manifested thyroid follicular cell
hyperplasia. There were no apparent adverse effects of treatment on
implantation rates, litter size or pup mortality. A significant
decrease in mean pup and litter weights was observed only at 1200
ppm of the F1 generation and F2 generation. The startle response
(reflex) was slightly delayed at the mid and high dose of both
generations. Macroscopic examination of selected tissues showed no
compound-related effects. Liver weights were increased (organ
weight/body-weight ratio) in both sexes of the F1 generation at 75,
300 and 1200 ppm, as well as for females of the F0 generation at
300 and 1200 pm. Kidney weight was also increased in the F0
generation males (1200 ppm) and females (300 and 1200 ppm). The
NOAEL was 75 ppm, equal to 5.6 mg/kg bw/day for males and 6.2 mg/kg
bw/day for females, based on increased organ to body-weight ratios
for liver and kidney, and thyroid follicular cell hyperplasia at 300
ppm (Ryle et al., 1991).
Special studies on embryotoxicity/teratogenicity
Rats
Sexually mature female Sprague-Dawley Crl:CD BR rats were
inseminated by sexually mature males and randomly divided into 4
groups of 25 dams each. Pregnant dams were gavaged with 0, 20, 100
or 500 mg/kg bw/day of maneb technical (90.4% purity) on days 6-15
of gestation. No dams died on study. Neurobehavioural clinical
signs (impaired mobility, hind limb paralysis, excessive
mastication) were observed on day 11 of gestation and persisted
throughout the study in high-dose treated females. Soft stools and
decreased defecation was also reported. Two high-dose females also
exhibited pallor and decreased body temperature. At 100 mg/kg
bw/day soft stool was the only compound-related sign observed.
Clinical signs for low-dose treated animals were comparable to
control animals. At 100 and 500 mg/kg bw/day, maternal body weights
and body-weight gain were significantly reduced. Low-dose values
were comparable to controls. Terminal body weights were decreased
at 100 and 500 mg/kg bw/day. However, net body weight (i.e.
terminal weight minus gravid uterine weight) was decreased only at
the high dose, as was gravid uterine weight. Food consumption was
comparable between control group and low-dose females. Food
consumption was decreased at higher doses. Caesarean section
observations indicated no fetal deaths but, dose-related resorptions
at the mid and high-dose levels which paralleled a dose-related and
statistically significant increase in early resorptions. Post-
implantation losses were also increased at the mid and high dose
with concurrent decreases in the number of viable fetuses at the
same dose levels. Low-dose observations were comparable to
controls. External fetal observations were comparable to control
group for all dose levels as were visceral malformations and
variations. Skeletal malformations were comparable between all
experimental groups when compared on a litter or fetal basis.
Numerous skeletal variations were observed only in the high-dose
group and were generally indicative of developmental delay as
evidenced by reduced ossification of various skeletal structures.
These findings at the high dose also exceeded maximum values of the
historical control data. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 20 mg/kg bw/day. Maternal toxicity was seen
at 100 mg/kg bw/day as decreased body weight and decreased food
consumption. Embryo/fetotoxicity was observed as increased (early)
resorptions, increased post-implantation losses and a decrease in
the number of viable fetuses at 100 mg/kg bw/day. No teratogenicity
was observed (Nemec, 1992).
Female Sprague-Dawley rats (23-25 dams/group) were gavaged with
20, 100, or 500 mg/kg bw/day of technical maneb (99.99% purity with
less than 0.01% ETU) in 0.5% CMC on days 6-15 of gestation. Two
additional groups serving as controls received either CMC (24 dams)
or were left untreated (24 dams).
There were no compound-related deaths and no clinical signs
below 500 mg/kg bw/day. High-dose dams manifested unsteady gait,
dragging of the rear limbs, diminished sensitivity to pain in the
affected limbs and paresis of the rear limbs. Body weights and
body-weight gain were decreased at the high dose. Gross pathology
was unremarkable in all treated dams. There were no statistically
significant differences between treated and control groups for
implantations, conception rate, corpora lutea, and the number of
live fetuses per litter. At 500 mg/kg bw/day, however, the number
of litters with dead fetuses was increased. Fetal observations at
the time of caesarean section revealed no remarkable findings at
dose levels of 20 and 100 mg/kg bw/day. However, animals receiving
500 mg/kg bw/day showed numerous changes including short tail,
meningocele, accessory toe, shortened toes, macroglossia,
syndactyly, kyphosis, oligodactyly and shortened splayed toes. The
mean fetal body weight per litter was also decreased as was mean
fetal body length per litter at 500 mg/kg bw/day. The number of
anomalous litters was significantly increased at the high dose
(25/25, 100%) for all malformations combined. Of the 304 live
fetuses examined at the high dose, 246 (80.9%) had anomalies. A
statistically significant increase in the number of fetal variants
was also observed at 500 mg/kg bw/day for all variations and
retardations combined. Maternal toxicity was seen at 500 mg/kg
bw/day as clinical signs and decreased body weight and body-weight
gain. Embryo/fetotoxicity and teratogenicity were seen at 500 mg/kg
bw/day based on decreased fetal body weight and body length, and an
increase in the number of anomalous litters and fetuses for all
malformations combined and for all variations and retardations
combined (Kapp et al., 1991).
Rabbits
Himalayan Chbb:HM rabbits were randomly assigned into four
groups (15 does/group). The study was divided into 3 phases. Five
does per dose group were artificially inseminated at each time
period. Gavage doses of 5, 20 or 80 mg/kg bw/day of maneb technical
(90.6% purity with 2% ETU contamination) were administered in CMC on
days 6 through 18 inclusive. The control group received CMC only.
At the high dose, 1 animal died and 3 were sacrificed - 1 moribund,
1 after aborting on day 21, and 1 after premature delivery on day
28. At the mid-dose, 1 animal was sacrificed in a moribund state
and 1 control animal delivered prematurely.
There were no compound-related signs of toxicity observed
during the study. Body weight and body-weight gain were decreased
at the high dose. Food consumption was decreased on days 7-9 and
10-11. Gross pathology revealed no compound-related abnormalities
in dams at terminal sacrifice. Pre-implantation loss and pregnancy
rates were comparable between control and test groups as were the
number of corpora lutea or implantations among control and test
groups. Post-implantation loss was non-significantly increased at
the high dose; however, a significant decrease in the number of
viable fetuses and an increase in the number of resorptions were
observed. Fetal body weights, crown-rump length and sex ratio were
comparable between treated and control groups. The placental weight
of female fetuses only was significantly greater at the high dose
compared to controls. Uterine weight was significantly reduced in
does administered 80 mg/kg bw/day. There were no fetal external
abnormalities observed. There were no compound-related visceral or
skeletal abnormalities in fetuses. NOAELs could not be determined
due to study deficiencies (Merkle, 1983).
Special study on hydrolytic stability of maneb in DMSO
14C-maneb (1.7 mg) was dissolved in 1.7 ml of DMSO and 30
minutes later added (dosing) to teflon-lined vials containing
sterilized buffer solutions (pH 5, 7 and 9) and stirred under
aseptic conditions. The final maneb concentration was approximately
10 ppm and the DMSO concentration was 1% (v/v). Hydrolysis was
performed in the dark at 25 °C. Aliquots were analyzed on day 0
(i.e. immediately after dosing), and on days 1, 2, 4, 7, 14 and 30.
Parent chemical, maneb, was not detected at any sampling time. A
mixture of several identified and unidentified compounds was
reported at all pH levels on day 0 including, but not limited to,
ETU, EBIS and Jaffe's base. ETU was generally the highest on day 0
and increased in concentration with time while the remaining
compounds decreased with time. The proportionality of the compounds
varied with changing pH (Rudel, 1990).
Special studies on genotoxicity
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The results are summarized in Table
3. A number of available studies were not considered, either
because DMSO was used as a solvent in which maneb is very unstable
or because of important omissions from the reports. The Meeting
concluded that maneb is not genotoxic.
Special studies on sensitization and irritation
Guinea-pigs
Hartley albino guinea-pigs (6/sex) were dosed topically with
technical maneb (purity not stated) for 6 hours a day, three times a
week for three weeks (modified Buehler method). Animals were
challenged two weeks after the last induction and re-challenged one
week later. Three males and three females received
dinitrochlorobenzene (DNCB; positive control group) and were treated
in a similar manner as the test group but were not re-challenged.
Reactions were scored at 24 and 48 hours after a challenge. DNCB
was determined to be an extreme sensitizing agent and maneb a
moderate sensitizing agent under the test conditions (Naas, 1989e).
Table 3. Results of genotoxicity assays on maneb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium TA1535, 8.3-675 µg/plate; Not specified Negative Arni, 1981
reversion assay TA1537, TA98, TA100 in acetone
S. typhimurium 3-100 µg/plate; 88.1% a.i. Negative Thomas, 1985
TA1535, TA1537, in deionized water
TA1538, TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.1-30 µg/ml; 88.1% a.i. Negative Thomas, 1986b,
mutation assay (CHO)/hprt in deionized water 1988
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations
In vivo aberrations Chick embryos 0.5-27 g/l in 80% a.i. Negative Arias, 1988
aqueous solution
Complete and partial D. melanogaster 10 000 ppm saturated Not specified Negative Woodruff et al.,
chromosome loss mus-302 mutant aqueous solution with 1983
ethanol and sucrose
Bone marrow Albino mouse 100 mg/kg Not specified Negative Kondratenko &
cytogenetics Kurinnyi, 1972
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
Bone marrow Male and female 46.4 mg/kg/day for 2 days; Not specified Negative Zeller &
cytogenetics Chinese hamsters in carboxy-methylcellulose (increased Engelhardt, 1980
(cont'd) gaps)
Male Fischer 344 rat 4.9 g/kg (acute); 88.1% a.i. Negative Ivett & Lebowitz,
1.64 g/kg/day for 5 days; 1985
in carboxy-methylcellulose
Dominant lethal assay Male NMRI mice 10-100 mg/kg/day for Not specified Negative Leuschner, 1978
5 days; in saline
Micronucleus assay Male or female ICR mouse 1 g/kg with 100 mg/kg Not specified Negative Seiler, 1977
with sodium nitrite NaNO2; in gum arabic soln.
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro unscheduled Primary rat hepatocytes 0.5-100 µg/ml; 88.1% a.i. Negative Loveday, 1986,
DNA synthesis (UDS) from male Fischer 344 rat in culture medium 1988
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro SCE assays Chinese hamster ovary 0.5-30 µg/ml ± rat 88.1% a.i. Weak Thomas, 1986a
(CHO) cells or mouse S9; positive
in deionized water with
activation
In vivo SCE assays Chick embryos 0.5-27 g/l in aqueous 80% a.i. Positive Arias, 1988
solution
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 0.05-0.2 µg/ml; 88.1% a.i. Negative Tu et al., 1985
in water?;
no activation used
C3H/10T 1/2 cells with 0.2 µg/ml as 88.1% a.i. Negative Tu et al., 1986
"promotion" "promoter"; in water?
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not
normally use such supplementation; solvent is provided if specified in the report
Maneb DF (75% dust) was administered to male albino guinea-pigs
of the Dunkin-Hartley strain following a modified method of Buehler.
No delayed hypersensitivity reaction was observed. Animals
receiving DNCB as positive control showed positive sensitization
reactions (Glaza, 1988c).
Rabbits
Single, 0.5 gram doses of maneb technical (purity not stated)
were applied to the clipped, intact skin of 6 New Zeeland white
rabbits under a semi-occlusive dressing for a 4-hour exposure
period. Application sites were evaluated in accordance with the
method of Draize at approximately 30-60 minutes and 24, 48 and 72
hours after patch removal and daily thereafter for 14 days.
Technical maneb induced a very slight to slight erythema and edema.
Other dermal findings were not remarkable. Technical maneb was
concluded to be slightly irritating (Draize score 1.5) (Naas,
1989c).
Single, 100 mg doses of maneb technical were instilled into the
lower conjunctival sac of the right eyes of 6 New Zeeland white
rabbits. The eyelids were held closed for approximately one second
and released. The left eye served as the contralateral control.
Eyes were left unwashed and examined at 1, 24, 48 and 72 hours and
at 4 and 7 days. Sodium fluorescein was used in evaluating corneal
damage at 72 hours and 7 days. No corneal involvement was observed.
Four of six rabbits cleared of all ocular reactions by day 7. Minor
conjunctival reactions were present in two rabbits on day 7. Maneb
technical was considered to be a moderate irritant based on
persistent conjunctival irritation in two rabbits for 7 days and the
mean response of 8-19 out of a maximum of 20 for conjunctival
irritation over the initial 72-hour period (Naas, 1989d).
Maneb DF (75% dust) produced corneal and iridial irritation and
slight to severe conjunctival irritation in the unwashed eyes of New
Zeeland white rabbits. Iridial irritation and slight to moderate
conjunctival irritation were observed in the washed eyes (60 second
flush with water at 30 seconds post-compound administration) of
companion New Zeeland white rabbits. Ocular irritation cleared by
72 hours in washed eyes and in less than 7 days in unwashed eyes
(Glaza, 1988d).
Maneb DF (75% dust) applied to the intact skin of New Zeeland
white rabbits for 4 hours and scored according to the method of
Draize at 4, 24, 48, 72 and 96 hours post-administration produced a
slight irritation (Draize score < 1.0 for all readings) (Glaza,
1988e).
COMMENTS
Male and female rats given 25 mg/kg bw/day of 14C-labelled
maneb orally showed no differences between sexes with regard to
excretion patterns. Greater than 90% of the absorbed 14C was
eliminated in urine by 24 hours. Less than 1% was eliminated as
carbon dioxide. Average 14C concentration as percent of dose per
gram of tissue was greatest for the thyroid, followed by kidney and
liver. The percent of 14C present in urine as ETU at 12 hours was
21-30% on a mole/mole basis and less than 0.4% as maneb.
The acute oral, dermal and inhalation toxicity of maneb
technical and maneb 75% dust is low. WHO has classified maneb as
unlikely to present acute hazard in normal use.
Rats were fed dietary concentrations of 0, 80, 400 or 1300 ppm
maneb technical for 13 weeks. The NOAEL was 80 ppm (equal to 5.0
mg/kg bw/day) based on an increase in absolute thyroid weight and
thyroid follicular cell hyperplasia at 400 ppm.
In dogs fed dietary concentrations of maneb technical at 0,
100, 400 or 1600 ppm for 13 weeks, the NOAEL was 100 ppm (equal to
3.7 mg/kg bw/day) based on thyroid follicular cell hyperplasia at
400 ppm.
In a 52-week study in dogs, maneb was administered at dietary
concentrations of 0, 50, 200, 1000 or 2200 ppm. The NOAEL was 200
ppm (equal to 6.4 mg/kg bw/day) based on thyroid enlargement and
thickening and thyroid follicular cell hyperplasia at 1000 ppm.
The overall NOAEL in dogs, based on the evaluation of all of
the data on this species, was 6.4 mg/kg bw/day.
In a six-month study in monkeys, maneb was administered at
dietary concentrations of 0, 100, 300 or 3000 ppm. The NOAEL was 100
ppm (equal to 7.3 mg/kg bw/day) based on an increase in thyroid
weight at 300 ppm.
In a 79-week carcinogenicity study in mice at dietary
concentrations of 0, 60, 240 or 2400 ppm, the NOAEL was 60 ppm,
equal to 11 mg/kg bw/day, based on decreased body weight and
decreased thyroxine levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes.
In a 31-month toxicity/carcinogenicity study in rats at dietary
concentrations of 0, 30, 100, 300 or 1000 ppm, the NOAEL was 300 ppm
(equal to 20 mg/kg bw/day) based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm. There was no evidence of carcinogenicity.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 75, 300 or 1200 ppm, the NOAEL was 75 ppm
(equal to 5.6 mg/kg bw/day) based on increased organ to body-weight
ratios for liver and kidney, and thyroid follicular cell hyperplasia
at 300 ppm.
An oral teratogenicity study in rats was conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day. The NOAEL was 20 mg/kg
bw/day for maternal toxicity and embryo/fetotoxicity. Maternal
toxicity was seen at 100 mg/kg bw/day as decreased body weight and
decreased food consumption. Embryo/fetotoxicity was observed as
increased (early) resorptions, increased post-implantation losses
and a decrease in viable fetuses at 100 mg/kg bw/day. No
teratogenicity was observed.
In a second oral teratogenicity study in rats conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day the NOAEL for maternal
toxicity and embryo/fetotoxic and teratogenic effects was 100 mg/kg
bw/day. Maternal toxicity was seen at the highest dose as decreased
body weight and clinical signs. Embryo/fetotoxicity and
teratogenicity were seen at the highest dose as decreased fetal body
weight and body length, and an increase in the number of anomalous
litters and fetuses for all malformations combined and for all
variations and retardations combined.
An oral teratogenicity study in rabbits was conducted at dose
levels of 0, 5, 20 or 80 mg/kg bw/day. Due to study deficiencies a
NOAEL could not be determined.
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The Meeting concluded that maneb is
not genotoxic. A number of available studies were not considered,
either because DMSO was used as a solvent in which maneb is very
unstable or because of important omissions from the reports.
The data on maneb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 5.0 mg/kg bw/day for thyroid effects in rats,
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for maneb, alone or in combination with
mancozeb, metiram and/or zineb, because of the similarity of the
chemical structures of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differential using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 60 ppm in the diet, equal to 11 mg/kg bw/day
(79-week study)
Rat: 80 ppm in the diet, equal to 5.0 mg/kg bw/day
(13-week study)
300 ppm in the diet, equal to 20 mg/kg bw/day
(31-month study)
75 ppm in the diet, equal to 5.6 mg/kg bw/day
(reproduction study)
Dog: 200 ppm in the diet, equal to 6.4 mg/kg bw/day
(52-week study)
Monkey: 100 ppm in the diet, equal to 7.3 mg/kg bw/day
(6-month study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, metiram, and zineb).
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Allen, T.R., Frei, Th., Biedermann, K., Luetkemeier, H., Terrier,
Ch., Vogel, O., Wilson, J. (1989). 13-week oral toxicity (feeding)
study with maneb technical in the dog. Unpublished report No. 206605
from Research and Consulting Company, Ltd., Itingen, Switzerland.
Submitted to WHO by Elf Atochem.
Arias, E. (1988). Sister chromatid exchanges and chromosomal
aberrations in chick embryos after treatment with the fungicide
maneb. Mutation Res. 206: 271-273.
Arni, P. (1981). Salmonella/mammalian-microsome mutagenicity test
with CGA 31 364 = maneb. Unpublished experiment No. 810466 from
Ciba-Geigy Limited, Basle, Switzerland.
Corney, S.J., Allen, T.R., Janiak, T., Frei, Th., Leutkemeier, H.,
Biedermann, K., Vogel., O. & Springall, C. (1992). A 52-week oral
toxicity study (feeding) with maneb technical in the dog.
Unpublished report No. 206616 from Research and Consulting Co.,
Ltd., Itingen, Switzerland. Submitted to WHO by Elf Atochem.
Craine, E.M. (1991). A dermal radiotracer absorbtion study in rats
with 14C maneb. Unpublished report No. WIL-134010 (reissued final
report March 13, 1991) from WIL Research Laboratories, Inc.,
Ashland, Ohio, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988a). Acute oral toxicity study of Maneb DF in rats.
Unpublished report No. HLA 80104922 from Hazleton Labs America Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988b). Acute dermal toxicity study of Maneb DF in
rabbits. Unpublished report No. HLA 80104923 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988c). Dermal sensitization study of Maneb DF in guinea-
pigs. Unpublished report No. HLA 80104926 from Hazleton Labs America
Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988d). Primary eye irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104924 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988e). Primary dermal irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104925 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Hoffman, G. (1991). An acute inhalation toxicity study of Maneb DF
in the rat. Unpublished report No. 90-8287 from Bio/dynamics, Inc.,
East Millstone, New Jersey, USA. Submitted to WHO by Elf Atochem.
Ivett, J. & Lebowitz, H. (1985). Clastogenic evaluation of maneb
technical lot MT 01 (88.1% ai.) in the rat bone marrow cytogenic
assay. Unpublished final report No. LBI 22202 from Litton Bionetics
Inc., Kensington, Maryland, USA. Submitted to WHO by Elf Atochem.
Kapp, R.W., Schellhaas, L.J., Piccirillo, V.J. (1991). Prenatal
toxicity study of maneb in rats. Unpublished report Nos. BASF
88/0522; 88/0523; 88/0524 from BASF Gewerbehygiene und Toxikologie,
Ludwigshaven, Germany. Submitted to WHO by Elf Atochem.
Kondratenko, T. & Kurinnyi, A. (1972). Effects of some fungicides
(dithiocarbamic acid derivatives) on chromosomes of bone marrow
cells in mice. Tsitol. Genet. 6: 225-228.
Leuschner, F. (1991). Chronic oral toxicity of manganese-ethylene-
1,2-bis-dithiocarbamate, 90% - called for short "maneb" - in Sprague-
Dawley (SIV) - rats (with special attention to carcinogenic
properties). Unpublished registration document No. (BASF) 86/0430
Amendment No. 3 dated April 19, 1991 from Laboratory of Pharmacology
and Toxicology, Hamburg, West Germany. Submitted to WHO by Elf
Atochem.
Leuschner, F. (1978). Dominant lethal test on male mice with
manganese ethylene-1,2-bis-dithiocarbamate. Unpublished report No.
BASF 78/298 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Dontenwill, W. & Hubschner, F. (1986a). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" -in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 1 dated October 2, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A. & Hubschner, F. (1986b). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" - in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 2 dated October 20, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Klie, R., Dontenwill, W. & Rogulja, P.
(1979). Chronic oral toxicity of manganese-ethylene-1,2-bis-
dithiocarbamate, 90% - called for short "maneb" - in Sprague-Dawley
(SIV) - rats (with special attention to carcinogenic properties).
Unpublished registration document No. (BASF) 86/0430 from Laboratory
of Pharmacology and Toxicology, Hamburg, West Germany. Submitted to
WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Schneider, C., Schwerdtfeger, W. &
Dontenwill, W. (1977). Oral toxicity of maneb in the Rhesus monkey:
six months dietary dosing. Unpublished report No. WF 1172 from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Loveday, K. (1986). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Unpublished
report No. 850047-20 from American Biogenics Corporation, Woburn,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Loveday, K. (1988). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Supplementary
report PW-118 to Unpublished report No. 850047-20 from American
Biogenics Corporation, Woburn, Massachusetts, USA. Submitted to WHO
by Elf Atochem.
Merkle, J. (1983). Study to determine the prenatal toxicity of
manganous ethylenebis (dithiocarbamate) in rabbits. Unpublished
study RZ-No. 83/094 from BASF AG, Ludwigshafen Rhein, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Naas, D.J. (1989a). Acute oral toxicity (LD50) study in albino rats
with maneb technical. Unpublished report No. WIL-134003 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J., (1989b). Acute dermal toxicity (LD50) study in albino
rabbits with maneb technical. Unpublished report No. WIL-134004 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989c). Primary dermal irritation study in albino
rabbits with maneb technical. Unpublished report No. WIL-134005 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989d). Primary eye irritation study in albino rabbits
with maneb technical. Unpublished report No. WIL-134006 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J. (1989e). Skin sensitization study in albino guinea-pigs
with maneb technical. Unpublished report No. WIL-134007 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Nemec, M., (1992). A developmental toxicity study of maneb technical
in rats. Unpublished report No. WIL-134011 from WIL Research
Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by Elf
Atochem.
Puhl, J.R. (1985). Metabolism of radiolabeled maneb in rats.
Unpublished report No. 6181-101 from Hazleton Labs., America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Rudel, H. (1990). Hydrolysis of maneb at pH 5, 7, and 9. Unpublished
project No. MRG-O1/7-04 from Fraunhofer-Institut für Umweltchemie
und Okotoxikologie, D-5948 Schmallenberg, Federal Republic of
Germany. Submitted to WHO by Elf Atochem.
Ryle, P.R., Bell, P.F., Parker, C., Farmer, H., Offer, J.M.,
Anderson, A. & Dawe, I.S., (1991). A study of the effect of maneb
(technical) on reproductive function of two generations in the rat.
Unpublished report No. MNB1/9072 from Huntington Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Elf Atochem.
Seiler, J. (1977). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Terrill, J.B. (1990). Acute inhalation toxicity study with maneb in
the rat. Unpublished report No. HLA 2567-100 from Hazleton Labs.,
Rockville, Maryland, USA. Submitted to WHO by Elf Atochem.
Terrill, J.B., (1991). A four-week study in the rat to determine the
lung tissue residue and effect upon the thyroid function with maneb.
Unpublished report No. HLA 2567-101 from Hazleton Labs., Rockville,
Maryland, USA. Submitted to WHO by Elf Atochem.
Thomas, M. (1985). Salmonella/microsome mutagenesis assay on
technical grade maneb. Unpublished final report No. ABC 850047-40
from American Biogenics Corp., Woburn, Massachusetts, USA. Submitted
to WHO by Elf Atochem.
Thomas, M. (1986a). In vitro sister chromatid exchange assay in
cultured Chinese hamster ovary (CHO) cells treated with technical
grade maneb. Unpublished final report No. ABC 850047-30 from
American Biogenics Corp., Woburn, Massachusetts, USA. Submitted to
WHO by Elf Atochem.
Thomas, M. (1986b). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Unpublished final report No. ABC
850047-10 from American Biogenics Corp., Woburn, Massachusetts, USA.
Submitted to WHO by Elf Atochem.
Thomas, M. (1988). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Supplementary report PW-117 to
Unpublished final report No. ABC 850047-10 from American Biogenics
Corp., Woburn, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tompkins, C.E. (1992). An 18-month dietary oncogenicity study in
mice with maneb technical. Unpublished report No. WIL-134008 from
WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to
WHO by Elf Atochem.
Trutter, J.A. (1988a). Subchronic toxicity study with maneb
technical in the rat. Unpublished report No. HLA 153-140 from
Hazleton Laboratories America, Inc., Rockville, Maryland, USA.
Submitted to WHO by Elf Atochem.
Trutter, J.A. (1988b). A 21-day dermal toxicity study in rabbits
with maneb technical. Unpublished report No. HLA 153-139 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA.
Submitted to WHO by Elf Atochem.
Tu, A., Breen, P. & Sivak, A. (1986). Evaluation of maneb in the
C3H-10T ´ cell transformation assay for promotion activity.
Unpublished report No. ADL 88720-44 from Arthur D. Little, Inc.,
Cambridge, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tu, A., Sivak, A., Hatch, K. & Breen, P. (1985). Evaluation of maneb
in the C3H-10T ´ cell transformation assay. Unpublished final report
No. ADL 88720-44 (1-0860) from Arthur D. Little, Inc., Cambridge,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1986). Thirteen-week subchronic inhalation toxicity
study on maneb in rats (final report). Unpublished report No. 550-
001 from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1987). Thirteen-week subchronic inhalation toxicity
study on maneb in rats - addendum to the final report covering the
recovery phase. Unpublished report No. 550-001 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Elf Atochem.
WHO (1992). The WH0 recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/91.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Woodruff, R., Phillips, J. & Irwin, D. (1983). Pesticide induced
complete and partial chromosome loss in screens with repair-
defective females of Drosophila melanogaster. Environ. Mutagen. 5:
835-846.
Zeller, H. & Engelhardt, G. (1980). Cytogenic investigations in
Chinese hamsters after two intraperitoneal administrations of maneb.
Bone marrow chromosome analysis. Unpublished report dated December
19, 1980 from Gewerbehygiene und Toxikologie of BASF, Germany.
Submitted to WHO by Elf Atochem.
 Table 1. Acute toxicity of maneb technical
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat1 Crl:CD BR M/F oral > 5000 Naas, 1989a
Rat2 Crl:CD BR M/F inhal. 7.38 Terrill, 1990
Rabbit3 NZW M/F dermal > 2000 Naas, 1989b
1 Deionized water as vehicle; 3/5 males died, 0/5 females died.
2 Dose only 4 hour exposure, respirable dust of 3.8 microns. Analytical LC50 of 5.34 mg/l
for males and greater than 7.38 mg/l for females. 14 day observation period.
Respiratory distress and decreased body-weight were the major clinical signs.
3 Deionized water as vehicle.
Table 2. Acute toxicity of maneb DF1
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat2 Sprague-Dawley M/F oral > 5000 Glaza, 1988a
CD(R)
Rat3 Sprague-Dawley M/F inhal. 4 h > 2.0 Hoffmann, 1991
CD(R) exposure
Rabbit4 NZW M/F dermal > 2000 Glaza, 1988b
1 Maneb DF (75% Dust)
2 Uniform suspension in deionized water
3 Mean exposure concentration 2.0 mg/l with a nominal concentration of 53 mg/l.
Mass Median Aerodynamic Diameter was 3.3 microns with an average
Geometric Standard Deviation of 2.4, and 8.8% of particles were
less than one (1) micron in size. There were no deaths during the whole body exposure.
4 0.9% saline vehicle
Short-term toxicity studies
Rats
Sprague-Dawley (Crl:CD BR) rats (32/sex/dose; control 16/sex)
received a nose-only exposure to maneb technical (86.8% purity
adjusted to 100%) for 6 hours/day, five days a week for 4 weeks at a
target level of 100 mg/m3 (particle size ranged from 3.45-3.83
microns). All animals survived to scheduled sacrifice and compound-
related signs were unremarkable. T4 and TSH values in males and
TSH values in females were decreased during week one only. However,
results for treated males were generally less than control values
for the remainder of the study and were not considered biologically
meaningful. Results for females were generally comparable to
controls. Gross necropsy was not remarkable between treated and
controls. Mean absolute lung weights for both sexes were comparable
to controls but relative weights increased. Mean terminal body-
weights were decreased for both sexes and paralleled the increased
relative lung to body-weight ratio. Residue levels of maneb,
manganese and ETU generally remained fairly constant during the
exposure period and was less than the limit of detection after the
recovery period. During weeks 1-4, residue levels in lungs for
males ranged between 3.9-7.9 ppm (7.9-6.8 ppm for weeks 2 and 4) for
maneb; 4.8-6.0 ppm for manganese and 0.12-0.19 ppm for ETU; for
females 2.1-4.2 ppm for maneb, 1.8-2.8 for manganese and < 0.1-0.12
ppm for ETU. Residue levels in the urine of females was generally
less than the limit of detection for maneb and manganese while
values for ETU generally ranged between 1.3 and 2.15 ppm during the
exposure period and less than the limit of detection during the
recovery phase. Manganese was not detected in males and maneb and
ETU were not detected during recovery. Maneb ranged from 1.3-6.6 ppm
during exposure and ETU from 5-29 ppm (5.0-5.1 for weeks 2 and 4)
(Terrill, 1991).
Charles River Sprague-Dawley albino rats (28/sex/dose) received
nose-only target inhalation exposures of 0, 10, 30 or 100 mg/m3 (0,
10, 32, or 98 mg/m3 gravimetric concentration with an equivalent
mean aerodynamic diameter of 3.5 [+/-2.0] microns) of technical
maneb (purity not stated) for 6 hours a day, 5 days a week for 13
consecutive weeks. Measured concentrations of ETU for the low,
middle, and high-dose groups were 0.12, 0.71 and 2.5 mg/m3.
Fourteen animals per sex per dose were sacrificed after 13 weeks of
exposure and the remaining animals allowed to recover for 13 weeks
and then sacrificed.
There were no exposure-related effects on appearance or
behaviour and no compound-related deaths. Body weight of females
exposed to the inter-mediate and high dose were depressed during the
exposure period, whereas males had decreased body weights only at
100 mg/m3 for weeks 1-3. There were no exposure-related effects
observed in ophthalmologic, haematologic (10 parameters) or serum
biochemical measurements (21 parameters including T3 and T4) after
the exposure period. Absolute organ weights were comparable to
control group. There was no evidence of any dose-related
macroscopic or microscopic changes in animals sacrificed at 13
weeks. ETU was detected in the urine of all exposed rats, in the
thyroids of all high-dose animals, in the liver of two females of
the intermediate-dose group and the plasma of one intermediate dose
female. ETU concentration in the urine was dose-related in both
sexes but was much greater in females. ETU mean residues in the
thyroid for males were however much greater in males (23.6 µg/ml)
than females (8.4 µg/ml). Maneb residues were also found in urine
of both sexes.
At the end of the 13-week recovery period no differences were
observed among the treated groups that could be attributed to test
material exposure when compared to controls. ETU and maneb residues
were not detected in any tissue or body fluids measured after a 13-
week recovery period. The NOAEL was 10 mg/m3 based on decreased
body weight at 30 mg/m3 (Ulrich, 1986, 1987).
Sprague-Dawley derived Crl:CDBR rats (15 animals/sex/dose)
received dietary concentrations of 0, 80, 400 or 1300 ppm of maneb
technical (78% purity). Ten animals/sex/dose were sacrificed at 14
weeks and 5 animals/sex/dose were placed on a 4-week recovery period
and sacrificed at 17 weeks. There were no overt signs of toxicity
or dose-related effects on mortality, ophthalmology or haematology.
Body-weight gains for males and females at 1300 ppm were
significantly decreased at 13 weeks. At week 17, body weights of
high-dose males were comparable to the control group, while female
body weight was 10% lower than controls. Food consumption was
comparable to controls.
A dose-related decrease in T4 levels and a concomitant
increase in TSH levels were seen in dosed females at 14 weeks. The
decrease in T4 levels was significant at the high dose. In males
T4 values were slightly decreased and TSH values slightly increased
at 1300 ppm. T4 values for males and females were similar to or
higher than concurrent controls at the end of the recovery period,
while TSH values were similar to or lower than controls. T3 values
were increased, but not statistically significant in high-dose males
and females at 14 weeks and in females at the end of the recovery
period. T3 values for males at the high dose were comparable to
controls at the end of the recovery period. There were no changes
of toxicological significance in other clinical chemistry
parameters. A dose-related trend (dose-related increase) was
evident for thyroid/parathyroid weights of dosed males and females.
Absolute weights were significantly increased in males at 400 and
1300 ppm, and relative weights were significantly increased in high-
dose males. Thyroid/parathyroid weights of dosed and control
animals were similar following a 4-week recovery period. There were
no compound-related changes in macroscopic pathology in dosed
animals at 14 or 18 weeks. Follicular cell hyperplasia of the
thyroid was observed in 1/10 mid-dosed males, 10/10 high-dosed males
and 2/10 high-dosed females compared with none in the controls.
Increased colloid of the thyroid was also exhibited in 4/10 high-
dose males. Thyroid changes regressed following the recovery phase.
However one high-dose male manifested a follicular cell adenoma.
Granular renal pigment was observed in 2/10 low-dose males and all
animals in the mid and high-dose groups. However, the incidence of
chronic progressive nephropathy was similar in dosed and control
males and females. At the end of the recovery period a reduction in
the incidence and amount of renal pigment was reported in 3/5 mid-
dose and 3/5 high-dose males and 4/5 mid-dose and 4/5 high-dose
females. The NOAEL was 80 ppm, equal to 5.0 mg/kg bw/day, based on
an increase in absolute thyroid weight and thyroid follicular cell
hyperplasia at 400 ppm (Trutter, 1988).
Rabbits
New Zeeland white rabbits (five animals/sex/dose) were
administered 0 (sham treated), 100, 300 or 1000 mg/kg bw/day of 86%
maneb technical (adjusted to 100%) and applied to the shaved (10%)
dorsal intact skin area. Applications were made for 6 hours a day,
5 days a week for 3 weeks. Clinical findings, body weight, food
consumption and organ weights were comparable to controls. Values
for haematology and serum chemistry were comparable between treated
and control groups. Gross signs of skin irritation were observed in
2/10, 8/10 and 10/10 animals at the low, mid and high-dose.
Erythema was not observed at 100 mg/kg bw/day and edema was not
observed at 100 or 300 mg/kg bw/day (Draize method). Erythema when
present was generally graded slight as was edema. Epidermal scaling
was observed in 2, 8, and 10 animals at the low-, mid- and high-
doses. Histopathology showed epidermal irritation at the site of
application in all dose groups, which was characterized by minimal
to slight acanthosis and hyperkeratosis. Follicular cell
hypertrophy of the thyroid was observed in 7/10 animals at 1000
mg/kg/day. Increased colloid material was observed in 2 and 4
females at 300 and 1000 mg/kg/day, respectively (Trutter, 1988).
Dogs
Beagle dogs (2 animals/sex/dose) received dietary
concentrations of technical maneb (91.58% purity) of 0, 100, 400 or
1600 ppm for 13 weeks. At the high dose, diarrhoea was observed in
1 male and 1 female with attendant decreased activity in the female.
Body weight was decreased 20% and food consumption 30% in high-dose
females. Body weight and food consumption were comparable between
treated and control groups for males. Slight to marked anaemia was
observed in 1 male and 1 female at 6 and 13 weeks with enhanced
erythropoietic activity in all dogs at the high dose. T3 and T4
values were decreased in both sexes at 1600 ppm at six weeks but not
13 weeks. Total lipid, cholesterol, triglyceride and phospholipid
concentrations were slightly increased in males at 6 and 13 weeks.
Fluctuations for these parameters were also observed in one high-
dose female along with slight to moderate increases in glutamate
dehydrogenase and GGT activity and slight decreases in plasma
calcium, creatinine and total protein concentrations at 6 weeks.
Post-mortem examination revealed increased thyroid weight in 1 male
and 1 female at 1600 ppm. Increased liver to body-weight ratio was
also observed in the second female. One male and 1 female of the
high-dose group also manifested enlargement or thickening of the
thyroid gland upon macroscopic examination. Microscopic examination
revealed moderate to severe thyroid follicular cell hyperplasia in
high-dose males. One female at 400 ppm manifested slight thyroid
follicular cell hyperplasia; both females in the 1600 ppm group
showed thyroid follicular cell hyperplasia ranging from slight to
minimal. A minimal degree of thyroid follicular cell hypertrophy
was recorded for 1 male at 400 ppm and 1 female at 100 ppm. A
slight focus of hypertrophic cells in the adenohypophysis of the
high-dose male, which had a severe thyroid follicular cell
hyperplasia was also observed. The NOAEL was 100 ppm, equal to 3.7
mg/kg bw/day, based on thyroid follicular cell hyperplasia at 400
ppm (Allen et al., 1989).
Beagle dogs (5/sex/dose) were given dietary concentrations of
0, 50, 200, 1000 or 2200 ppm of maneb (89.2% purity) for 52 weeks.
No deaths occurred during the study. Diarrhoea was observed in dogs
at 1000 ppm (1/5 females) and 2200 ppm (3/5 males and 1/5 females).
Body weight for males was generally comparable between treated and
control groups. High-dose females displayed lower body weights
compared to controls. Body-weight gain was decreased in both males
and females at 2200 ppm. Group mean food intake at 2200 ppm was
decreased in males and females.
Ophthalmological examination revealed no adverse effects at any
dose level. Mean erythrocyte count, haemoglobin concentration, and
haematocrit at 200, 1000 and 2200 ppm were significantly decreased
at 13 weeks only in females. Increased mean cell volumes were
observed at 2200 ppm in males and 1000 ppm in females. Decreased
mean cell haemoglobin concentration was reported in both sexes at
2200 ppm along with reticulocytosis and polychromasia. Platelet
counts were increased in both sexes at 2200 ppm and in males at 1000
ppm. T4 values were decreased at 2200 ppm in males and females.
T3 was markedly depressed at 2200 ppm for both sexes. Increased
group mean values for cholesterol, total lipids, and phospholipids
were dose-related and statistically significant but only at 2200 ppm
for some of the parameters of either sex. Bilirubin values were
increased in males and females at 2200 ppm as was ALP. Urinalysis
findings were comparable between treated and controls. Neurological
examinations of postural reactions, spinal reflexes and of cranial
nerves revealed no treatment-related effects. The absolute and
relative weight of the thyroids was increased in both sexes at 2200
ppm as was the adrenal weight. Thyroid enlargement and/or
thickening was observed in all dogs at 2200 ppm and 7/10 dogs at
1000 ppm. Thyroid follicular hyperplasia was reported for all dogs
at 1000 and 2200 ppm. Thyroid histopathology was unremarkable at
lower dose levels. There were no other findings suggestive of any
treatment-related effect. The NOAEL was 200 ppm, equal to 6.4 mg/kg
bw/day for males and 7.2 mg/kg bw/day for females, based on thyroid
enlargement and thickening and thyroid follicular cell hyperplasia
at 1000 ppm (Corney et al., 1992).
Monkeys
Rhesus monkeys, (4/sex/group) received 0, 100, 300 or 3000 ppm
of maneb technical (90% purity) in the diet for 6 months. At 6, 13
and 26 weeks, all animals were administered 10 µCi of 131I (on an
empty stomach) and determinations were made for 131I absorption into
the thyroid at 2 and 48 hours post-dosing in conjunction with
determinations for serum T4, T3, protein-bound 131I in serum and
loss of 131I from the thyroid (i.e. half-life in days).
Behaviour and external appearance of treated and control
animals were comparable, and all females demonstrated normal
menstrual discharges. Mean body-weight gains in animals (sexes
combined) given 3000 ppm maneb were lower than those of controls and
the low- and mid-dose groups. Food consumption was unaffected.
Haematology and clinical biochemistry revealed no meaningful
differences between controls and treated groups. However, studies
conducted with 131I revealed significantly decreased 131I absorption
by the thyroid in animals (sexes combined) receiving 3000 ppm at 26
weeks. 131I uptake was decreased 40% in males and 35% in females.
Protein-bound 131I was also decreased in animals (sexes combined) at
3000 ppm with the decrease largely attributable to males. Results
of urinalysis and electrocardiograms were comparable between control
and treated groups. Gross necropsy was unremarkable between groups.
Absolute organ weights for thyroids were increased in monkeys (sexes
combined) at 300 and 3000 ppm. However, statistical significance was
reached only at the high dose and largely attributable to males.
Males also showed a greater increase at 300 ppm than did females.
Enlarged thyroid with large follicles, flat epithelium, and colloid
described as somewhat darker than usual was reported for 7 animals
at 3000 ppm. Moderate proliferation of the epithelium was also
present in two high-dose animals. Only 1 animal at the high-dose
had no thyroid-associated pathology. The NOAEL was 100 ppm, equal
to 7.3 mg/kg bw/day, based on an increase in thyroid weight at 300
ppm (Leuschner et al., 1977).
Long-term toxicity/carcinogenicity studies
Mice
Crl:CD-1 (ICR) BR mice (75/sex/group) received dietary
concentrations of 0, 60, 240 or 2400 ppm maneb technical (89.5%
purity) for 79 weeks. An interim sacrifice and necropsy was
performed on 20 animals/sex/dose group at 52 weeks. The mortality
rate, clinical signs, and palpable masses were comparable between
treated and control groups for both sexes. Female body weight was
decreased at 240 ppm and decreased for males and females at 2400
ppm. Erythrocytes, haemoglobin, and haematocrit were decreased at
2400 ppm in both sexes at interim and terminal sacrifice. T4
levels were decreased at 2400 ppm in both sexes. T4 levels were
also significantly decreased in females at 60 ppm and 240 ppm at
terminal sacrifice. A dose-related trend was evident across all
three doses. At terminal necropsy hepatic masses were evident and
occurred at a greater frequency in high-dose males and females than
controls. Organ-weight changes at 2400 ppm were biologically and
statistically significant only for thyroid and only at terminal
sacrifice. However, histopathology of the thyroid was unremarkable.
A significant increase was observed in high-dose males of
hepatocellular adenomas at terminal necropsy. The number of females
with hepatocellular adenomas at terminal necropsy were comparable to
controls. However, the overall incidence of hepatocellular adenomas
was significantly increased for both sexes at 2400 ppm. The NOAEL
was 60 ppm, equal to 11 mg/kg bw/day, based on decreased body weight
and decreased T4 levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes (Tompkins, 1992).
Rats
Sprague-Dawley (SIV 50) rats (90/sex/group) were fed dietary
concentrations of maneb (90% purity) of 0, 30, 100, 300 or 1000 ppm
for 31 months. Interim sacrifices were conducted at 3, 6, and 12
months on 5 animals/sex/dose and all survivors terminated at 31
months. Beginning at 26 weeks all animals were palpated once a
week. Haematology and clinical biochemistry parameters were
evaluated at pre-test and 3, 6, 12, 18 and 24 months in 10
animals/sex/dose. At 3, 6, 12 and 24 months, 10 animals/sex/dose
were gavaged with 131I (10 µCi) and determinations made for the
biological half-life of 131I in thyroid over a 10-day period, 131I
serum activity, protein bound 131I in serum, serum T4, and the
binding index of T3. Tumour and mortality rates were compared
statistically by means of analysis of variance according to the
method of Peto.
Behaviour, appearance, food consumption and mortality were
comparable between treated and control groups. Body weights at 31
months were within the normal range. However, body weights were
decreased 25% in males and 40% in females at 12 and 24 months. There
were no significant differences between groups or any apparent dose-
related trends in any of the haematology or clinical biochemistry
parameters measured. 131I retention time in the thyroid was
significantly increased only at the high dose tested for males at 6
and 12 months and females at 6 months. Values for 1000 ppm animals
were slightly increased but not statistically significant. Mean
serum T4 was not significantly different between groups for any
time period. However, T4 values at 1000 ppm at 6 and 12 months
were decreased 10% in males and 20% in females. Urinalysis
comparisons between groups were unremarkable. Thyroid weights were
increased at 1000 ppm in males and in females when compared to
controls at 31 months.
All other absolute and relative organ weights appeared
comparable to control values. Macroscopic and microscopic
examination of animals sacrificed during the interim periods
(5/sex/dose) revealed no compound-related changes. Gross and
histopathology of 75 animals (inter-current deaths and terminal
sacrifice) per sex in the control group and high-dose group did not
reveal compound-related non-neoplastic or neoplastic changes.
Additional histological examination of liver, kidney and urinary
bladder in both sexes at all lower doses also revealed no compound-
related non-neoplastic or neoplastic changes. The NOAEL was 300 ppm,
equal to 20 mg/kg bw/day, based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm (Leuschner et al., 1979, 1986a,b; Leuschner 1991).
Reproduction studies
Rats
Male and female rats (Crl:CD(SD)BR VAF/Plus) were randomly
assigned by weight into 4 groups of 28 (F0) and 24 (F1) males and
females per group and received 0, 75, 300 or 1200 ppm of maneb (80-
90% purity) in the diet. Animals were bred for 1 litter in each of
2 consecutive filial generations. There were no treatment-related
deaths or clinical signs at the low-or mid-dose. One high-dose
female in the F0 and F1 generation died. Both females had
locomotor difficulties and appeared thin. The second generation
female also suffered from hind limb paralysis. Signs were
considered treatment-related. Overall food consumption was decreased
for the F0 and F1 males and females. There was an increase in the
food conversion ratio for females of both generations during the
early part of mating. There was also a dose-related increase in
water consumption at the mid and high-dose for males and females of
the F0 and F1 generation during the final two weeks prior to
mating. No change in food or water consumption was observed at the
low dose. F1 females had a 20% decrease in food consumption at
1200 ppm and a 10% decrease at 300 but values were not statistically
significant. Body-weight gains were significantly lower for males
and females of the F0/F1 generations at 1200 ppm and for females
of the F0 generation at 300 ppm. Body weight for dams of the F0
and F1 generations during the periods of gestation and lactation
was decreased only at 1200 ppm of the F1 generation. Body-weight
gain, however, during the lactation period for the F0 and F1 dams
was substantially increased at 1200 ppm.
The mating performance, pregnancy rate and gestational period
for treated groups was comparable to the control group. Adult gross
pathology revealed a slight but apparently dose-related increase in
the number of enlarged cervical lymph nodes for males of the mid-
and high-doses of the F0 and F1 generation. The increase was not
reported as statistically significant. Slight but apparently dose-
related increases were also reported for fluid distention of the
uterus in both generations of females, but the values were not
statistically significant. Minimal diffuse follicular epithelial
hypertrophy/hyperplasia and centrilobular hepatocyte enlargement
were observed in males at 1200 ppm in both generations. Thyroid
follicular cell adenomas were also observed in F1 males at 1200
ppm. High-dose females of both generations also manifested minimal
diffuse follicular cell hyperplasia/hypertrophy. At 300 ppm 2/23
males of the F1 generation manifested thyroid follicular cell
hyperplasia. There were no apparent adverse effects of treatment on
implantation rates, litter size or pup mortality. A significant
decrease in mean pup and litter weights was observed only at 1200
ppm of the F1 generation and F2 generation. The startle response
(reflex) was slightly delayed at the mid and high dose of both
generations. Macroscopic examination of selected tissues showed no
compound-related effects. Liver weights were increased (organ
weight/body-weight ratio) in both sexes of the F1 generation at 75,
300 and 1200 ppm, as well as for females of the F0 generation at
300 and 1200 pm. Kidney weight was also increased in the F0
generation males (1200 ppm) and females (300 and 1200 ppm). The
NOAEL was 75 ppm, equal to 5.6 mg/kg bw/day for males and 6.2 mg/kg
bw/day for females, based on increased organ to body-weight ratios
for liver and kidney, and thyroid follicular cell hyperplasia at 300
ppm (Ryle et al., 1991).
Special studies on embryotoxicity/teratogenicity
Rats
Sexually mature female Sprague-Dawley Crl:CD BR rats were
inseminated by sexually mature males and randomly divided into 4
groups of 25 dams each. Pregnant dams were gavaged with 0, 20, 100
or 500 mg/kg bw/day of maneb technical (90.4% purity) on days 6-15
of gestation. No dams died on study. Neurobehavioural clinical
signs (impaired mobility, hind limb paralysis, excessive
mastication) were observed on day 11 of gestation and persisted
throughout the study in high-dose treated females. Soft stools and
decreased defecation was also reported. Two high-dose females also
exhibited pallor and decreased body temperature. At 100 mg/kg
bw/day soft stool was the only compound-related sign observed.
Clinical signs for low-dose treated animals were comparable to
control animals. At 100 and 500 mg/kg bw/day, maternal body weights
and body-weight gain were significantly reduced. Low-dose values
were comparable to controls. Terminal body weights were decreased
at 100 and 500 mg/kg bw/day. However, net body weight (i.e.
terminal weight minus gravid uterine weight) was decreased only at
the high dose, as was gravid uterine weight. Food consumption was
comparable between control group and low-dose females. Food
consumption was decreased at higher doses. Caesarean section
observations indicated no fetal deaths but, dose-related resorptions
at the mid and high-dose levels which paralleled a dose-related and
statistically significant increase in early resorptions. Post-
implantation losses were also increased at the mid and high dose
with concurrent decreases in the number of viable fetuses at the
same dose levels. Low-dose observations were comparable to
controls. External fetal observations were comparable to control
group for all dose levels as were visceral malformations and
variations. Skeletal malformations were comparable between all
experimental groups when compared on a litter or fetal basis.
Numerous skeletal variations were observed only in the high-dose
group and were generally indicative of developmental delay as
evidenced by reduced ossification of various skeletal structures.
These findings at the high dose also exceeded maximum values of the
historical control data. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 20 mg/kg bw/day. Maternal toxicity was seen
at 100 mg/kg bw/day as decreased body weight and decreased food
consumption. Embryo/fetotoxicity was observed as increased (early)
resorptions, increased post-implantation losses and a decrease in
the number of viable fetuses at 100 mg/kg bw/day. No teratogenicity
was observed (Nemec, 1992).
Female Sprague-Dawley rats (23-25 dams/group) were gavaged with
20, 100, or 500 mg/kg bw/day of technical maneb (99.99% purity with
less than 0.01% ETU) in 0.5% CMC on days 6-15 of gestation. Two
additional groups serving as controls received either CMC (24 dams)
or were left untreated (24 dams).
There were no compound-related deaths and no clinical signs
below 500 mg/kg bw/day. High-dose dams manifested unsteady gait,
dragging of the rear limbs, diminished sensitivity to pain in the
affected limbs and paresis of the rear limbs. Body weights and
body-weight gain were decreased at the high dose. Gross pathology
was unremarkable in all treated dams. There were no statistically
significant differences between treated and control groups for
implantations, conception rate, corpora lutea, and the number of
live fetuses per litter. At 500 mg/kg bw/day, however, the number
of litters with dead fetuses was increased. Fetal observations at
the time of caesarean section revealed no remarkable findings at
dose levels of 20 and 100 mg/kg bw/day. However, animals receiving
500 mg/kg bw/day showed numerous changes including short tail,
meningocele, accessory toe, shortened toes, macroglossia,
syndactyly, kyphosis, oligodactyly and shortened splayed toes. The
mean fetal body weight per litter was also decreased as was mean
fetal body length per litter at 500 mg/kg bw/day. The number of
anomalous litters was significantly increased at the high dose
(25/25, 100%) for all malformations combined. Of the 304 live
fetuses examined at the high dose, 246 (80.9%) had anomalies. A
statistically significant increase in the number of fetal variants
was also observed at 500 mg/kg bw/day for all variations and
retardations combined. Maternal toxicity was seen at 500 mg/kg
bw/day as clinical signs and decreased body weight and body-weight
gain. Embryo/fetotoxicity and teratogenicity were seen at 500 mg/kg
bw/day based on decreased fetal body weight and body length, and an
increase in the number of anomalous litters and fetuses for all
malformations combined and for all variations and retardations
combined (Kapp et al., 1991).
Rabbits
Himalayan Chbb:HM rabbits were randomly assigned into four
groups (15 does/group). The study was divided into 3 phases. Five
does per dose group were artificially inseminated at each time
period. Gavage doses of 5, 20 or 80 mg/kg bw/day of maneb technical
(90.6% purity with 2% ETU contamination) were administered in CMC on
days 6 through 18 inclusive. The control group received CMC only.
At the high dose, 1 animal died and 3 were sacrificed - 1 moribund,
1 after aborting on day 21, and 1 after premature delivery on day
28. At the mid-dose, 1 animal was sacrificed in a moribund state
and 1 control animal delivered prematurely.
There were no compound-related signs of toxicity observed
during the study. Body weight and body-weight gain were decreased
at the high dose. Food consumption was decreased on days 7-9 and
10-11. Gross pathology revealed no compound-related abnormalities
in dams at terminal sacrifice. Pre-implantation loss and pregnancy
rates were comparable between control and test groups as were the
number of corpora lutea or implantations among control and test
groups. Post-implantation loss was non-significantly increased at
the high dose; however, a significant decrease in the number of
viable fetuses and an increase in the number of resorptions were
observed. Fetal body weights, crown-rump length and sex ratio were
comparable between treated and control groups. The placental weight
of female fetuses only was significantly greater at the high dose
compared to controls. Uterine weight was significantly reduced in
does administered 80 mg/kg bw/day. There were no fetal external
abnormalities observed. There were no compound-related visceral or
skeletal abnormalities in fetuses. NOAELs could not be determined
due to study deficiencies (Merkle, 1983).
Special study on hydrolytic stability of maneb in DMSO
14C-maneb (1.7 mg) was dissolved in 1.7 ml of DMSO and 30
minutes later added (dosing) to teflon-lined vials containing
sterilized buffer solutions (pH 5, 7 and 9) and stirred under
aseptic conditions. The final maneb concentration was approximately
10 ppm and the DMSO concentration was 1% (v/v). Hydrolysis was
performed in the dark at 25 °C. Aliquots were analyzed on day 0
(i.e. immediately after dosing), and on days 1, 2, 4, 7, 14 and 30.
Parent chemical, maneb, was not detected at any sampling time. A
mixture of several identified and unidentified compounds was
reported at all pH levels on day 0 including, but not limited to,
ETU, EBIS and Jaffe's base. ETU was generally the highest on day 0
and increased in concentration with time while the remaining
compounds decreased with time. The proportionality of the compounds
varied with changing pH (Rudel, 1990).
Special studies on genotoxicity
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The results are summarized in Table
3. A number of available studies were not considered, either
because DMSO was used as a solvent in which maneb is very unstable
or because of important omissions from the reports. The Meeting
concluded that maneb is not genotoxic.
Special studies on sensitization and irritation
Guinea-pigs
Hartley albino guinea-pigs (6/sex) were dosed topically with
technical maneb (purity not stated) for 6 hours a day, three times a
week for three weeks (modified Buehler method). Animals were
challenged two weeks after the last induction and re-challenged one
week later. Three males and three females received
dinitrochlorobenzene (DNCB; positive control group) and were treated
in a similar manner as the test group but were not re-challenged.
Reactions were scored at 24 and 48 hours after a challenge. DNCB
was determined to be an extreme sensitizing agent and maneb a
moderate sensitizing agent under the test conditions (Naas, 1989e).
Table 3. Results of genotoxicity assays on maneb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium TA1535, 8.3-675 µg/plate; Not specified Negative Arni, 1981
reversion assay TA1537, TA98, TA100 in acetone
S. typhimurium 3-100 µg/plate; 88.1% a.i. Negative Thomas, 1985
TA1535, TA1537, in deionized water
TA1538, TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.1-30 µg/ml; 88.1% a.i. Negative Thomas, 1986b,
mutation assay (CHO)/hprt in deionized water 1988
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations
In vivo aberrations Chick embryos 0.5-27 g/l in 80% a.i. Negative Arias, 1988
aqueous solution
Complete and partial D. melanogaster 10 000 ppm saturated Not specified Negative Woodruff et al.,
chromosome loss mus-302 mutant aqueous solution with 1983
ethanol and sucrose
Bone marrow Albino mouse 100 mg/kg Not specified Negative Kondratenko &
cytogenetics Kurinnyi, 1972
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
Bone marrow Male and female 46.4 mg/kg/day for 2 days; Not specified Negative Zeller &
cytogenetics Chinese hamsters in carboxy-methylcellulose (increased Engelhardt, 1980
(cont'd) gaps)
Male Fischer 344 rat 4.9 g/kg (acute); 88.1% a.i. Negative Ivett & Lebowitz,
1.64 g/kg/day for 5 days; 1985
in carboxy-methylcellulose
Dominant lethal assay Male NMRI mice 10-100 mg/kg/day for Not specified Negative Leuschner, 1978
5 days; in saline
Micronucleus assay Male or female ICR mouse 1 g/kg with 100 mg/kg Not specified Negative Seiler, 1977
with sodium nitrite NaNO2; in gum arabic soln.
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro unscheduled Primary rat hepatocytes 0.5-100 µg/ml; 88.1% a.i. Negative Loveday, 1986,
DNA synthesis (UDS) from male Fischer 344 rat in culture medium 1988
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro SCE assays Chinese hamster ovary 0.5-30 µg/ml ± rat 88.1% a.i. Weak Thomas, 1986a
(CHO) cells or mouse S9; positive
in deionized water with
activation
In vivo SCE assays Chick embryos 0.5-27 g/l in aqueous 80% a.i. Positive Arias, 1988
solution
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 0.05-0.2 µg/ml; 88.1% a.i. Negative Tu et al., 1985
in water?;
no activation used
C3H/10T 1/2 cells with 0.2 µg/ml as 88.1% a.i. Negative Tu et al., 1986
"promotion" "promoter"; in water?
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not
normally use such supplementation; solvent is provided if specified in the report
Maneb DF (75% dust) was administered to male albino guinea-pigs
of the Dunkin-Hartley strain following a modified method of Buehler.
No delayed hypersensitivity reaction was observed. Animals
receiving DNCB as positive control showed positive sensitization
reactions (Glaza, 1988c).
Rabbits
Single, 0.5 gram doses of maneb technical (purity not stated)
were applied to the clipped, intact skin of 6 New Zeeland white
rabbits under a semi-occlusive dressing for a 4-hour exposure
period. Application sites were evaluated in accordance with the
method of Draize at approximately 30-60 minutes and 24, 48 and 72
hours after patch removal and daily thereafter for 14 days.
Technical maneb induced a very slight to slight erythema and edema.
Other dermal findings were not remarkable. Technical maneb was
concluded to be slightly irritating (Draize score 1.5) (Naas,
1989c).
Single, 100 mg doses of maneb technical were instilled into the
lower conjunctival sac of the right eyes of 6 New Zeeland white
rabbits. The eyelids were held closed for approximately one second
and released. The left eye served as the contralateral control.
Eyes were left unwashed and examined at 1, 24, 48 and 72 hours and
at 4 and 7 days. Sodium fluorescein was used in evaluating corneal
damage at 72 hours and 7 days. No corneal involvement was observed.
Four of six rabbits cleared of all ocular reactions by day 7. Minor
conjunctival reactions were present in two rabbits on day 7. Maneb
technical was considered to be a moderate irritant based on
persistent conjunctival irritation in two rabbits for 7 days and the
mean response of 8-19 out of a maximum of 20 for conjunctival
irritation over the initial 72-hour period (Naas, 1989d).
Maneb DF (75% dust) produced corneal and iridial irritation and
slight to severe conjunctival irritation in the unwashed eyes of New
Zeeland white rabbits. Iridial irritation and slight to moderate
conjunctival irritation were observed in the washed eyes (60 second
flush with water at 30 seconds post-compound administration) of
companion New Zeeland white rabbits. Ocular irritation cleared by
72 hours in washed eyes and in less than 7 days in unwashed eyes
(Glaza, 1988d).
Maneb DF (75% dust) applied to the intact skin of New Zeeland
white rabbits for 4 hours and scored according to the method of
Draize at 4, 24, 48, 72 and 96 hours post-administration produced a
slight irritation (Draize score < 1.0 for all readings) (Glaza,
1988e).
COMMENTS
Male and female rats given 25 mg/kg bw/day of 14C-labelled
maneb orally showed no differences between sexes with regard to
excretion patterns. Greater than 90% of the absorbed 14C was
eliminated in urine by 24 hours. Less than 1% was eliminated as
carbon dioxide. Average 14C concentration as percent of dose per
gram of tissue was greatest for the thyroid, followed by kidney and
liver. The percent of 14C present in urine as ETU at 12 hours was
21-30% on a mole/mole basis and less than 0.4% as maneb.
The acute oral, dermal and inhalation toxicity of maneb
technical and maneb 75% dust is low. WHO has classified maneb as
unlikely to present acute hazard in normal use.
Rats were fed dietary concentrations of 0, 80, 400 or 1300 ppm
maneb technical for 13 weeks. The NOAEL was 80 ppm (equal to 5.0
mg/kg bw/day) based on an increase in absolute thyroid weight and
thyroid follicular cell hyperplasia at 400 ppm.
In dogs fed dietary concentrations of maneb technical at 0,
100, 400 or 1600 ppm for 13 weeks, the NOAEL was 100 ppm (equal to
3.7 mg/kg bw/day) based on thyroid follicular cell hyperplasia at
400 ppm.
In a 52-week study in dogs, maneb was administered at dietary
concentrations of 0, 50, 200, 1000 or 2200 ppm. The NOAEL was 200
ppm (equal to 6.4 mg/kg bw/day) based on thyroid enlargement and
thickening and thyroid follicular cell hyperplasia at 1000 ppm.
The overall NOAEL in dogs, based on the evaluation of all of
the data on this species, was 6.4 mg/kg bw/day.
In a six-month study in monkeys, maneb was administered at
dietary concentrations of 0, 100, 300 or 3000 ppm. The NOAEL was 100
ppm (equal to 7.3 mg/kg bw/day) based on an increase in thyroid
weight at 300 ppm.
In a 79-week carcinogenicity study in mice at dietary
concentrations of 0, 60, 240 or 2400 ppm, the NOAEL was 60 ppm,
equal to 11 mg/kg bw/day, based on decreased body weight and
decreased thyroxine levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes.
In a 31-month toxicity/carcinogenicity study in rats at dietary
concentrations of 0, 30, 100, 300 or 1000 ppm, the NOAEL was 300 ppm
(equal to 20 mg/kg bw/day) based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm. There was no evidence of carcinogenicity.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 75, 300 or 1200 ppm, the NOAEL was 75 ppm
(equal to 5.6 mg/kg bw/day) based on increased organ to body-weight
ratios for liver and kidney, and thyroid follicular cell hyperplasia
at 300 ppm.
An oral teratogenicity study in rats was conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day. The NOAEL was 20 mg/kg
bw/day for maternal toxicity and embryo/fetotoxicity. Maternal
toxicity was seen at 100 mg/kg bw/day as decreased body weight and
decreased food consumption. Embryo/fetotoxicity was observed as
increased (early) resorptions, increased post-implantation losses
and a decrease in viable fetuses at 100 mg/kg bw/day. No
teratogenicity was observed.
In a second oral teratogenicity study in rats conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day the NOAEL for maternal
toxicity and embryo/fetotoxic and teratogenic effects was 100 mg/kg
bw/day. Maternal toxicity was seen at the highest dose as decreased
body weight and clinical signs. Embryo/fetotoxicity and
teratogenicity were seen at the highest dose as decreased fetal body
weight and body length, and an increase in the number of anomalous
litters and fetuses for all malformations combined and for all
variations and retardations combined.
An oral teratogenicity study in rabbits was conducted at dose
levels of 0, 5, 20 or 80 mg/kg bw/day. Due to study deficiencies a
NOAEL could not be determined.
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The Meeting concluded that maneb is
not genotoxic. A number of available studies were not considered,
either because DMSO was used as a solvent in which maneb is very
unstable or because of important omissions from the reports.
The data on maneb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 5.0 mg/kg bw/day for thyroid effects in rats,
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for maneb, alone or in combination with
mancozeb, metiram and/or zineb, because of the similarity of the
chemical structures of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differential using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 60 ppm in the diet, equal to 11 mg/kg bw/day
(79-week study)
Rat: 80 ppm in the diet, equal to 5.0 mg/kg bw/day
(13-week study)
300 ppm in the diet, equal to 20 mg/kg bw/day
(31-month study)
75 ppm in the diet, equal to 5.6 mg/kg bw/day
(reproduction study)
Dog: 200 ppm in the diet, equal to 6.4 mg/kg bw/day
(52-week study)
Monkey: 100 ppm in the diet, equal to 7.3 mg/kg bw/day
(6-month study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, metiram, and zineb).
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Allen, T.R., Frei, Th., Biedermann, K., Luetkemeier, H., Terrier,
Ch., Vogel, O., Wilson, J. (1989). 13-week oral toxicity (feeding)
study with maneb technical in the dog. Unpublished report No. 206605
from Research and Consulting Company, Ltd., Itingen, Switzerland.
Submitted to WHO by Elf Atochem.
Arias, E. (1988). Sister chromatid exchanges and chromosomal
aberrations in chick embryos after treatment with the fungicide
maneb. Mutation Res. 206: 271-273.
Arni, P. (1981). Salmonella/mammalian-microsome mutagenicity test
with CGA 31 364 = maneb. Unpublished experiment No. 810466 from
Ciba-Geigy Limited, Basle, Switzerland.
Corney, S.J., Allen, T.R., Janiak, T., Frei, Th., Leutkemeier, H.,
Biedermann, K., Vogel., O. & Springall, C. (1992). A 52-week oral
toxicity study (feeding) with maneb technical in the dog.
Unpublished report No. 206616 from Research and Consulting Co.,
Ltd., Itingen, Switzerland. Submitted to WHO by Elf Atochem.
Craine, E.M. (1991). A dermal radiotracer absorbtion study in rats
with 14C maneb. Unpublished report No. WIL-134010 (reissued final
report March 13, 1991) from WIL Research Laboratories, Inc.,
Ashland, Ohio, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988a). Acute oral toxicity study of Maneb DF in rats.
Unpublished report No. HLA 80104922 from Hazleton Labs America Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988b). Acute dermal toxicity study of Maneb DF in
rabbits. Unpublished report No. HLA 80104923 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988c). Dermal sensitization study of Maneb DF in guinea-
pigs. Unpublished report No. HLA 80104926 from Hazleton Labs America
Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988d). Primary eye irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104924 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988e). Primary dermal irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104925 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Hoffman, G. (1991). An acute inhalation toxicity study of Maneb DF
in the rat. Unpublished report No. 90-8287 from Bio/dynamics, Inc.,
East Millstone, New Jersey, USA. Submitted to WHO by Elf Atochem.
Ivett, J. & Lebowitz, H. (1985). Clastogenic evaluation of maneb
technical lot MT 01 (88.1% ai.) in the rat bone marrow cytogenic
assay. Unpublished final report No. LBI 22202 from Litton Bionetics
Inc., Kensington, Maryland, USA. Submitted to WHO by Elf Atochem.
Kapp, R.W., Schellhaas, L.J., Piccirillo, V.J. (1991). Prenatal
toxicity study of maneb in rats. Unpublished report Nos. BASF
88/0522; 88/0523; 88/0524 from BASF Gewerbehygiene und Toxikologie,
Ludwigshaven, Germany. Submitted to WHO by Elf Atochem.
Kondratenko, T. & Kurinnyi, A. (1972). Effects of some fungicides
(dithiocarbamic acid derivatives) on chromosomes of bone marrow
cells in mice. Tsitol. Genet. 6: 225-228.
Leuschner, F. (1991). Chronic oral toxicity of manganese-ethylene-
1,2-bis-dithiocarbamate, 90% - called for short "maneb" - in Sprague-
Dawley (SIV) - rats (with special attention to carcinogenic
properties). Unpublished registration document No. (BASF) 86/0430
Amendment No. 3 dated April 19, 1991 from Laboratory of Pharmacology
and Toxicology, Hamburg, West Germany. Submitted to WHO by Elf
Atochem.
Leuschner, F. (1978). Dominant lethal test on male mice with
manganese ethylene-1,2-bis-dithiocarbamate. Unpublished report No.
BASF 78/298 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Dontenwill, W. & Hubschner, F. (1986a). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" -in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 1 dated October 2, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A. & Hubschner, F. (1986b). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" - in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 2 dated October 20, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Klie, R., Dontenwill, W. & Rogulja, P.
(1979). Chronic oral toxicity of manganese-ethylene-1,2-bis-
dithiocarbamate, 90% - called for short "maneb" - in Sprague-Dawley
(SIV) - rats (with special attention to carcinogenic properties).
Unpublished registration document No. (BASF) 86/0430 from Laboratory
of Pharmacology and Toxicology, Hamburg, West Germany. Submitted to
WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Schneider, C., Schwerdtfeger, W. &
Dontenwill, W. (1977). Oral toxicity of maneb in the Rhesus monkey:
six months dietary dosing. Unpublished report No. WF 1172 from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Loveday, K. (1986). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Unpublished
report No. 850047-20 from American Biogenics Corporation, Woburn,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Loveday, K. (1988). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Supplementary
report PW-118 to Unpublished report No. 850047-20 from American
Biogenics Corporation, Woburn, Massachusetts, USA. Submitted to WHO
by Elf Atochem.
Merkle, J. (1983). Study to determine the prenatal toxicity of
manganous ethylenebis (dithiocarbamate) in rabbits. Unpublished
study RZ-No. 83/094 from BASF AG, Ludwigshafen Rhein, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Naas, D.J. (1989a). Acute oral toxicity (LD50) study in albino rats
with maneb technical. Unpublished report No. WIL-134003 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J., (1989b). Acute dermal toxicity (LD50) study in albino
rabbits with maneb technical. Unpublished report No. WIL-134004 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989c). Primary dermal irritation study in albino
rabbits with maneb technical. Unpublished report No. WIL-134005 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989d). Primary eye irritation study in albino rabbits
with maneb technical. Unpublished report No. WIL-134006 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J. (1989e). Skin sensitization study in albino guinea-pigs
with maneb technical. Unpublished report No. WIL-134007 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Nemec, M., (1992). A developmental toxicity study of maneb technical
in rats. Unpublished report No. WIL-134011 from WIL Research
Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by Elf
Atochem.
Puhl, J.R. (1985). Metabolism of radiolabeled maneb in rats.
Unpublished report No. 6181-101 from Hazleton Labs., America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Rudel, H. (1990). Hydrolysis of maneb at pH 5, 7, and 9. Unpublished
project No. MRG-O1/7-04 from Fraunhofer-Institut für Umweltchemie
und Okotoxikologie, D-5948 Schmallenberg, Federal Republic of
Germany. Submitted to WHO by Elf Atochem.
Ryle, P.R., Bell, P.F., Parker, C., Farmer, H., Offer, J.M.,
Anderson, A. & Dawe, I.S., (1991). A study of the effect of maneb
(technical) on reproductive function of two generations in the rat.
Unpublished report No. MNB1/9072 from Huntington Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Elf Atochem.
Seiler, J. (1977). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Terrill, J.B. (1990). Acute inhalation toxicity study with maneb in
the rat. Unpublished report No. HLA 2567-100 from Hazleton Labs.,
Rockville, Maryland, USA. Submitted to WHO by Elf Atochem.
Terrill, J.B., (1991). A four-week study in the rat to determine the
lung tissue residue and effect upon the thyroid function with maneb.
Unpublished report No. HLA 2567-101 from Hazleton Labs., Rockville,
Maryland, USA. Submitted to WHO by Elf Atochem.
Thomas, M. (1985). Salmonella/microsome mutagenesis assay on
technical grade maneb. Unpublished final report No. ABC 850047-40
from American Biogenics Corp., Woburn, Massachusetts, USA. Submitted
to WHO by Elf Atochem.
Thomas, M. (1986a). In vitro sister chromatid exchange assay in
cultured Chinese hamster ovary (CHO) cells treated with technical
grade maneb. Unpublished final report No. ABC 850047-30 from
American Biogenics Corp., Woburn, Massachusetts, USA. Submitted to
WHO by Elf Atochem.
Thomas, M. (1986b). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Unpublished final report No. ABC
850047-10 from American Biogenics Corp., Woburn, Massachusetts, USA.
Submitted to WHO by Elf Atochem.
Thomas, M. (1988). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Supplementary report PW-117 to
Unpublished final report No. ABC 850047-10 from American Biogenics
Corp., Woburn, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tompkins, C.E. (1992). An 18-month dietary oncogenicity study in
mice with maneb technical. Unpublished report No. WIL-134008 from
WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to
WHO by Elf Atochem.
Trutter, J.A. (1988a). Subchronic toxicity study with maneb
technical in the rat. Unpublished report No. HLA 153-140 from
Hazleton Laboratories America, Inc., Rockville, Maryland, USA.
Submitted to WHO by Elf Atochem.
Trutter, J.A. (1988b). A 21-day dermal toxicity study in rabbits
with maneb technical. Unpublished report No. HLA 153-139 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA.
Submitted to WHO by Elf Atochem.
Tu, A., Breen, P. & Sivak, A. (1986). Evaluation of maneb in the
C3H-10T ´ cell transformation assay for promotion activity.
Unpublished report No. ADL 88720-44 from Arthur D. Little, Inc.,
Cambridge, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tu, A., Sivak, A., Hatch, K. & Breen, P. (1985). Evaluation of maneb
in the C3H-10T ´ cell transformation assay. Unpublished final report
No. ADL 88720-44 (1-0860) from Arthur D. Little, Inc., Cambridge,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1986). Thirteen-week subchronic inhalation toxicity
study on maneb in rats (final report). Unpublished report No. 550-
001 from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1987). Thirteen-week subchronic inhalation toxicity
study on maneb in rats - addendum to the final report covering the
recovery phase. Unpublished report No. 550-001 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Elf Atochem.
WHO (1992). The WH0 recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/91.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Woodruff, R., Phillips, J. & Irwin, D. (1983). Pesticide induced
complete and partial chromosome loss in screens with repair-
defective females of Drosophila melanogaster. Environ. Mutagen. 5:
835-846.
Zeller, H. & Engelhardt, G. (1980). Cytogenic investigations in
Chinese hamsters after two intraperitoneal administrations of maneb.
Bone marrow chromosome analysis. Unpublished report dated December
19, 1980 from Gewerbehygiene und Toxikologie of BASF, Germany.
Submitted to WHO by Elf Atochem.
Table 1. Acute toxicity of maneb technical
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat1 Crl:CD BR M/F oral > 5000 Naas, 1989a
Rat2 Crl:CD BR M/F inhal. 7.38 Terrill, 1990
Rabbit3 NZW M/F dermal > 2000 Naas, 1989b
1 Deionized water as vehicle; 3/5 males died, 0/5 females died.
2 Dose only 4 hour exposure, respirable dust of 3.8 microns. Analytical LC50 of 5.34 mg/l
for males and greater than 7.38 mg/l for females. 14 day observation period.
Respiratory distress and decreased body-weight were the major clinical signs.
3 Deionized water as vehicle.
Table 2. Acute toxicity of maneb DF1
Species Strain Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Rat2 Sprague-Dawley M/F oral > 5000 Glaza, 1988a
CD(R)
Rat3 Sprague-Dawley M/F inhal. 4 h > 2.0 Hoffmann, 1991
CD(R) exposure
Rabbit4 NZW M/F dermal > 2000 Glaza, 1988b
1 Maneb DF (75% Dust)
2 Uniform suspension in deionized water
3 Mean exposure concentration 2.0 mg/l with a nominal concentration of 53 mg/l.
Mass Median Aerodynamic Diameter was 3.3 microns with an average
Geometric Standard Deviation of 2.4, and 8.8% of particles were
less than one (1) micron in size. There were no deaths during the whole body exposure.
4 0.9% saline vehicle
Short-term toxicity studies
Rats
Sprague-Dawley (Crl:CD BR) rats (32/sex/dose; control 16/sex)
received a nose-only exposure to maneb technical (86.8% purity
adjusted to 100%) for 6 hours/day, five days a week for 4 weeks at a
target level of 100 mg/m3 (particle size ranged from 3.45-3.83
microns). All animals survived to scheduled sacrifice and compound-
related signs were unremarkable. T4 and TSH values in males and
TSH values in females were decreased during week one only. However,
results for treated males were generally less than control values
for the remainder of the study and were not considered biologically
meaningful. Results for females were generally comparable to
controls. Gross necropsy was not remarkable between treated and
controls. Mean absolute lung weights for both sexes were comparable
to controls but relative weights increased. Mean terminal body-
weights were decreased for both sexes and paralleled the increased
relative lung to body-weight ratio. Residue levels of maneb,
manganese and ETU generally remained fairly constant during the
exposure period and was less than the limit of detection after the
recovery period. During weeks 1-4, residue levels in lungs for
males ranged between 3.9-7.9 ppm (7.9-6.8 ppm for weeks 2 and 4) for
maneb; 4.8-6.0 ppm for manganese and 0.12-0.19 ppm for ETU; for
females 2.1-4.2 ppm for maneb, 1.8-2.8 for manganese and < 0.1-0.12
ppm for ETU. Residue levels in the urine of females was generally
less than the limit of detection for maneb and manganese while
values for ETU generally ranged between 1.3 and 2.15 ppm during the
exposure period and less than the limit of detection during the
recovery phase. Manganese was not detected in males and maneb and
ETU were not detected during recovery. Maneb ranged from 1.3-6.6 ppm
during exposure and ETU from 5-29 ppm (5.0-5.1 for weeks 2 and 4)
(Terrill, 1991).
Charles River Sprague-Dawley albino rats (28/sex/dose) received
nose-only target inhalation exposures of 0, 10, 30 or 100 mg/m3 (0,
10, 32, or 98 mg/m3 gravimetric concentration with an equivalent
mean aerodynamic diameter of 3.5 [+/-2.0] microns) of technical
maneb (purity not stated) for 6 hours a day, 5 days a week for 13
consecutive weeks. Measured concentrations of ETU for the low,
middle, and high-dose groups were 0.12, 0.71 and 2.5 mg/m3.
Fourteen animals per sex per dose were sacrificed after 13 weeks of
exposure and the remaining animals allowed to recover for 13 weeks
and then sacrificed.
There were no exposure-related effects on appearance or
behaviour and no compound-related deaths. Body weight of females
exposed to the inter-mediate and high dose were depressed during the
exposure period, whereas males had decreased body weights only at
100 mg/m3 for weeks 1-3. There were no exposure-related effects
observed in ophthalmologic, haematologic (10 parameters) or serum
biochemical measurements (21 parameters including T3 and T4) after
the exposure period. Absolute organ weights were comparable to
control group. There was no evidence of any dose-related
macroscopic or microscopic changes in animals sacrificed at 13
weeks. ETU was detected in the urine of all exposed rats, in the
thyroids of all high-dose animals, in the liver of two females of
the intermediate-dose group and the plasma of one intermediate dose
female. ETU concentration in the urine was dose-related in both
sexes but was much greater in females. ETU mean residues in the
thyroid for males were however much greater in males (23.6 µg/ml)
than females (8.4 µg/ml). Maneb residues were also found in urine
of both sexes.
At the end of the 13-week recovery period no differences were
observed among the treated groups that could be attributed to test
material exposure when compared to controls. ETU and maneb residues
were not detected in any tissue or body fluids measured after a 13-
week recovery period. The NOAEL was 10 mg/m3 based on decreased
body weight at 30 mg/m3 (Ulrich, 1986, 1987).
Sprague-Dawley derived Crl:CDBR rats (15 animals/sex/dose)
received dietary concentrations of 0, 80, 400 or 1300 ppm of maneb
technical (78% purity). Ten animals/sex/dose were sacrificed at 14
weeks and 5 animals/sex/dose were placed on a 4-week recovery period
and sacrificed at 17 weeks. There were no overt signs of toxicity
or dose-related effects on mortality, ophthalmology or haematology.
Body-weight gains for males and females at 1300 ppm were
significantly decreased at 13 weeks. At week 17, body weights of
high-dose males were comparable to the control group, while female
body weight was 10% lower than controls. Food consumption was
comparable to controls.
A dose-related decrease in T4 levels and a concomitant
increase in TSH levels were seen in dosed females at 14 weeks. The
decrease in T4 levels was significant at the high dose. In males
T4 values were slightly decreased and TSH values slightly increased
at 1300 ppm. T4 values for males and females were similar to or
higher than concurrent controls at the end of the recovery period,
while TSH values were similar to or lower than controls. T3 values
were increased, but not statistically significant in high-dose males
and females at 14 weeks and in females at the end of the recovery
period. T3 values for males at the high dose were comparable to
controls at the end of the recovery period. There were no changes
of toxicological significance in other clinical chemistry
parameters. A dose-related trend (dose-related increase) was
evident for thyroid/parathyroid weights of dosed males and females.
Absolute weights were significantly increased in males at 400 and
1300 ppm, and relative weights were significantly increased in high-
dose males. Thyroid/parathyroid weights of dosed and control
animals were similar following a 4-week recovery period. There were
no compound-related changes in macroscopic pathology in dosed
animals at 14 or 18 weeks. Follicular cell hyperplasia of the
thyroid was observed in 1/10 mid-dosed males, 10/10 high-dosed males
and 2/10 high-dosed females compared with none in the controls.
Increased colloid of the thyroid was also exhibited in 4/10 high-
dose males. Thyroid changes regressed following the recovery phase.
However one high-dose male manifested a follicular cell adenoma.
Granular renal pigment was observed in 2/10 low-dose males and all
animals in the mid and high-dose groups. However, the incidence of
chronic progressive nephropathy was similar in dosed and control
males and females. At the end of the recovery period a reduction in
the incidence and amount of renal pigment was reported in 3/5 mid-
dose and 3/5 high-dose males and 4/5 mid-dose and 4/5 high-dose
females. The NOAEL was 80 ppm, equal to 5.0 mg/kg bw/day, based on
an increase in absolute thyroid weight and thyroid follicular cell
hyperplasia at 400 ppm (Trutter, 1988).
Rabbits
New Zeeland white rabbits (five animals/sex/dose) were
administered 0 (sham treated), 100, 300 or 1000 mg/kg bw/day of 86%
maneb technical (adjusted to 100%) and applied to the shaved (10%)
dorsal intact skin area. Applications were made for 6 hours a day,
5 days a week for 3 weeks. Clinical findings, body weight, food
consumption and organ weights were comparable to controls. Values
for haematology and serum chemistry were comparable between treated
and control groups. Gross signs of skin irritation were observed in
2/10, 8/10 and 10/10 animals at the low, mid and high-dose.
Erythema was not observed at 100 mg/kg bw/day and edema was not
observed at 100 or 300 mg/kg bw/day (Draize method). Erythema when
present was generally graded slight as was edema. Epidermal scaling
was observed in 2, 8, and 10 animals at the low-, mid- and high-
doses. Histopathology showed epidermal irritation at the site of
application in all dose groups, which was characterized by minimal
to slight acanthosis and hyperkeratosis. Follicular cell
hypertrophy of the thyroid was observed in 7/10 animals at 1000
mg/kg/day. Increased colloid material was observed in 2 and 4
females at 300 and 1000 mg/kg/day, respectively (Trutter, 1988).
Dogs
Beagle dogs (2 animals/sex/dose) received dietary
concentrations of technical maneb (91.58% purity) of 0, 100, 400 or
1600 ppm for 13 weeks. At the high dose, diarrhoea was observed in
1 male and 1 female with attendant decreased activity in the female.
Body weight was decreased 20% and food consumption 30% in high-dose
females. Body weight and food consumption were comparable between
treated and control groups for males. Slight to marked anaemia was
observed in 1 male and 1 female at 6 and 13 weeks with enhanced
erythropoietic activity in all dogs at the high dose. T3 and T4
values were decreased in both sexes at 1600 ppm at six weeks but not
13 weeks. Total lipid, cholesterol, triglyceride and phospholipid
concentrations were slightly increased in males at 6 and 13 weeks.
Fluctuations for these parameters were also observed in one high-
dose female along with slight to moderate increases in glutamate
dehydrogenase and GGT activity and slight decreases in plasma
calcium, creatinine and total protein concentrations at 6 weeks.
Post-mortem examination revealed increased thyroid weight in 1 male
and 1 female at 1600 ppm. Increased liver to body-weight ratio was
also observed in the second female. One male and 1 female of the
high-dose group also manifested enlargement or thickening of the
thyroid gland upon macroscopic examination. Microscopic examination
revealed moderate to severe thyroid follicular cell hyperplasia in
high-dose males. One female at 400 ppm manifested slight thyroid
follicular cell hyperplasia; both females in the 1600 ppm group
showed thyroid follicular cell hyperplasia ranging from slight to
minimal. A minimal degree of thyroid follicular cell hypertrophy
was recorded for 1 male at 400 ppm and 1 female at 100 ppm. A
slight focus of hypertrophic cells in the adenohypophysis of the
high-dose male, which had a severe thyroid follicular cell
hyperplasia was also observed. The NOAEL was 100 ppm, equal to 3.7
mg/kg bw/day, based on thyroid follicular cell hyperplasia at 400
ppm (Allen et al., 1989).
Beagle dogs (5/sex/dose) were given dietary concentrations of
0, 50, 200, 1000 or 2200 ppm of maneb (89.2% purity) for 52 weeks.
No deaths occurred during the study. Diarrhoea was observed in dogs
at 1000 ppm (1/5 females) and 2200 ppm (3/5 males and 1/5 females).
Body weight for males was generally comparable between treated and
control groups. High-dose females displayed lower body weights
compared to controls. Body-weight gain was decreased in both males
and females at 2200 ppm. Group mean food intake at 2200 ppm was
decreased in males and females.
Ophthalmological examination revealed no adverse effects at any
dose level. Mean erythrocyte count, haemoglobin concentration, and
haematocrit at 200, 1000 and 2200 ppm were significantly decreased
at 13 weeks only in females. Increased mean cell volumes were
observed at 2200 ppm in males and 1000 ppm in females. Decreased
mean cell haemoglobin concentration was reported in both sexes at
2200 ppm along with reticulocytosis and polychromasia. Platelet
counts were increased in both sexes at 2200 ppm and in males at 1000
ppm. T4 values were decreased at 2200 ppm in males and females.
T3 was markedly depressed at 2200 ppm for both sexes. Increased
group mean values for cholesterol, total lipids, and phospholipids
were dose-related and statistically significant but only at 2200 ppm
for some of the parameters of either sex. Bilirubin values were
increased in males and females at 2200 ppm as was ALP. Urinalysis
findings were comparable between treated and controls. Neurological
examinations of postural reactions, spinal reflexes and of cranial
nerves revealed no treatment-related effects. The absolute and
relative weight of the thyroids was increased in both sexes at 2200
ppm as was the adrenal weight. Thyroid enlargement and/or
thickening was observed in all dogs at 2200 ppm and 7/10 dogs at
1000 ppm. Thyroid follicular hyperplasia was reported for all dogs
at 1000 and 2200 ppm. Thyroid histopathology was unremarkable at
lower dose levels. There were no other findings suggestive of any
treatment-related effect. The NOAEL was 200 ppm, equal to 6.4 mg/kg
bw/day for males and 7.2 mg/kg bw/day for females, based on thyroid
enlargement and thickening and thyroid follicular cell hyperplasia
at 1000 ppm (Corney et al., 1992).
Monkeys
Rhesus monkeys, (4/sex/group) received 0, 100, 300 or 3000 ppm
of maneb technical (90% purity) in the diet for 6 months. At 6, 13
and 26 weeks, all animals were administered 10 µCi of 131I (on an
empty stomach) and determinations were made for 131I absorption into
the thyroid at 2 and 48 hours post-dosing in conjunction with
determinations for serum T4, T3, protein-bound 131I in serum and
loss of 131I from the thyroid (i.e. half-life in days).
Behaviour and external appearance of treated and control
animals were comparable, and all females demonstrated normal
menstrual discharges. Mean body-weight gains in animals (sexes
combined) given 3000 ppm maneb were lower than those of controls and
the low- and mid-dose groups. Food consumption was unaffected.
Haematology and clinical biochemistry revealed no meaningful
differences between controls and treated groups. However, studies
conducted with 131I revealed significantly decreased 131I absorption
by the thyroid in animals (sexes combined) receiving 3000 ppm at 26
weeks. 131I uptake was decreased 40% in males and 35% in females.
Protein-bound 131I was also decreased in animals (sexes combined) at
3000 ppm with the decrease largely attributable to males. Results
of urinalysis and electrocardiograms were comparable between control
and treated groups. Gross necropsy was unremarkable between groups.
Absolute organ weights for thyroids were increased in monkeys (sexes
combined) at 300 and 3000 ppm. However, statistical significance was
reached only at the high dose and largely attributable to males.
Males also showed a greater increase at 300 ppm than did females.
Enlarged thyroid with large follicles, flat epithelium, and colloid
described as somewhat darker than usual was reported for 7 animals
at 3000 ppm. Moderate proliferation of the epithelium was also
present in two high-dose animals. Only 1 animal at the high-dose
had no thyroid-associated pathology. The NOAEL was 100 ppm, equal
to 7.3 mg/kg bw/day, based on an increase in thyroid weight at 300
ppm (Leuschner et al., 1977).
Long-term toxicity/carcinogenicity studies
Mice
Crl:CD-1 (ICR) BR mice (75/sex/group) received dietary
concentrations of 0, 60, 240 or 2400 ppm maneb technical (89.5%
purity) for 79 weeks. An interim sacrifice and necropsy was
performed on 20 animals/sex/dose group at 52 weeks. The mortality
rate, clinical signs, and palpable masses were comparable between
treated and control groups for both sexes. Female body weight was
decreased at 240 ppm and decreased for males and females at 2400
ppm. Erythrocytes, haemoglobin, and haematocrit were decreased at
2400 ppm in both sexes at interim and terminal sacrifice. T4
levels were decreased at 2400 ppm in both sexes. T4 levels were
also significantly decreased in females at 60 ppm and 240 ppm at
terminal sacrifice. A dose-related trend was evident across all
three doses. At terminal necropsy hepatic masses were evident and
occurred at a greater frequency in high-dose males and females than
controls. Organ-weight changes at 2400 ppm were biologically and
statistically significant only for thyroid and only at terminal
sacrifice. However, histopathology of the thyroid was unremarkable.
A significant increase was observed in high-dose males of
hepatocellular adenomas at terminal necropsy. The number of females
with hepatocellular adenomas at terminal necropsy were comparable to
controls. However, the overall incidence of hepatocellular adenomas
was significantly increased for both sexes at 2400 ppm. The NOAEL
was 60 ppm, equal to 11 mg/kg bw/day, based on decreased body weight
and decreased T4 levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes (Tompkins, 1992).
Rats
Sprague-Dawley (SIV 50) rats (90/sex/group) were fed dietary
concentrations of maneb (90% purity) of 0, 30, 100, 300 or 1000 ppm
for 31 months. Interim sacrifices were conducted at 3, 6, and 12
months on 5 animals/sex/dose and all survivors terminated at 31
months. Beginning at 26 weeks all animals were palpated once a
week. Haematology and clinical biochemistry parameters were
evaluated at pre-test and 3, 6, 12, 18 and 24 months in 10
animals/sex/dose. At 3, 6, 12 and 24 months, 10 animals/sex/dose
were gavaged with 131I (10 µCi) and determinations made for the
biological half-life of 131I in thyroid over a 10-day period, 131I
serum activity, protein bound 131I in serum, serum T4, and the
binding index of T3. Tumour and mortality rates were compared
statistically by means of analysis of variance according to the
method of Peto.
Behaviour, appearance, food consumption and mortality were
comparable between treated and control groups. Body weights at 31
months were within the normal range. However, body weights were
decreased 25% in males and 40% in females at 12 and 24 months. There
were no significant differences between groups or any apparent dose-
related trends in any of the haematology or clinical biochemistry
parameters measured. 131I retention time in the thyroid was
significantly increased only at the high dose tested for males at 6
and 12 months and females at 6 months. Values for 1000 ppm animals
were slightly increased but not statistically significant. Mean
serum T4 was not significantly different between groups for any
time period. However, T4 values at 1000 ppm at 6 and 12 months
were decreased 10% in males and 20% in females. Urinalysis
comparisons between groups were unremarkable. Thyroid weights were
increased at 1000 ppm in males and in females when compared to
controls at 31 months.
All other absolute and relative organ weights appeared
comparable to control values. Macroscopic and microscopic
examination of animals sacrificed during the interim periods
(5/sex/dose) revealed no compound-related changes. Gross and
histopathology of 75 animals (inter-current deaths and terminal
sacrifice) per sex in the control group and high-dose group did not
reveal compound-related non-neoplastic or neoplastic changes.
Additional histological examination of liver, kidney and urinary
bladder in both sexes at all lower doses also revealed no compound-
related non-neoplastic or neoplastic changes. The NOAEL was 300 ppm,
equal to 20 mg/kg bw/day, based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm (Leuschner et al., 1979, 1986a,b; Leuschner 1991).
Reproduction studies
Rats
Male and female rats (Crl:CD(SD)BR VAF/Plus) were randomly
assigned by weight into 4 groups of 28 (F0) and 24 (F1) males and
females per group and received 0, 75, 300 or 1200 ppm of maneb (80-
90% purity) in the diet. Animals were bred for 1 litter in each of
2 consecutive filial generations. There were no treatment-related
deaths or clinical signs at the low-or mid-dose. One high-dose
female in the F0 and F1 generation died. Both females had
locomotor difficulties and appeared thin. The second generation
female also suffered from hind limb paralysis. Signs were
considered treatment-related. Overall food consumption was decreased
for the F0 and F1 males and females. There was an increase in the
food conversion ratio for females of both generations during the
early part of mating. There was also a dose-related increase in
water consumption at the mid and high-dose for males and females of
the F0 and F1 generation during the final two weeks prior to
mating. No change in food or water consumption was observed at the
low dose. F1 females had a 20% decrease in food consumption at
1200 ppm and a 10% decrease at 300 but values were not statistically
significant. Body-weight gains were significantly lower for males
and females of the F0/F1 generations at 1200 ppm and for females
of the F0 generation at 300 ppm. Body weight for dams of the F0
and F1 generations during the periods of gestation and lactation
was decreased only at 1200 ppm of the F1 generation. Body-weight
gain, however, during the lactation period for the F0 and F1 dams
was substantially increased at 1200 ppm.
The mating performance, pregnancy rate and gestational period
for treated groups was comparable to the control group. Adult gross
pathology revealed a slight but apparently dose-related increase in
the number of enlarged cervical lymph nodes for males of the mid-
and high-doses of the F0 and F1 generation. The increase was not
reported as statistically significant. Slight but apparently dose-
related increases were also reported for fluid distention of the
uterus in both generations of females, but the values were not
statistically significant. Minimal diffuse follicular epithelial
hypertrophy/hyperplasia and centrilobular hepatocyte enlargement
were observed in males at 1200 ppm in both generations. Thyroid
follicular cell adenomas were also observed in F1 males at 1200
ppm. High-dose females of both generations also manifested minimal
diffuse follicular cell hyperplasia/hypertrophy. At 300 ppm 2/23
males of the F1 generation manifested thyroid follicular cell
hyperplasia. There were no apparent adverse effects of treatment on
implantation rates, litter size or pup mortality. A significant
decrease in mean pup and litter weights was observed only at 1200
ppm of the F1 generation and F2 generation. The startle response
(reflex) was slightly delayed at the mid and high dose of both
generations. Macroscopic examination of selected tissues showed no
compound-related effects. Liver weights were increased (organ
weight/body-weight ratio) in both sexes of the F1 generation at 75,
300 and 1200 ppm, as well as for females of the F0 generation at
300 and 1200 pm. Kidney weight was also increased in the F0
generation males (1200 ppm) and females (300 and 1200 ppm). The
NOAEL was 75 ppm, equal to 5.6 mg/kg bw/day for males and 6.2 mg/kg
bw/day for females, based on increased organ to body-weight ratios
for liver and kidney, and thyroid follicular cell hyperplasia at 300
ppm (Ryle et al., 1991).
Special studies on embryotoxicity/teratogenicity
Rats
Sexually mature female Sprague-Dawley Crl:CD BR rats were
inseminated by sexually mature males and randomly divided into 4
groups of 25 dams each. Pregnant dams were gavaged with 0, 20, 100
or 500 mg/kg bw/day of maneb technical (90.4% purity) on days 6-15
of gestation. No dams died on study. Neurobehavioural clinical
signs (impaired mobility, hind limb paralysis, excessive
mastication) were observed on day 11 of gestation and persisted
throughout the study in high-dose treated females. Soft stools and
decreased defecation was also reported. Two high-dose females also
exhibited pallor and decreased body temperature. At 100 mg/kg
bw/day soft stool was the only compound-related sign observed.
Clinical signs for low-dose treated animals were comparable to
control animals. At 100 and 500 mg/kg bw/day, maternal body weights
and body-weight gain were significantly reduced. Low-dose values
were comparable to controls. Terminal body weights were decreased
at 100 and 500 mg/kg bw/day. However, net body weight (i.e.
terminal weight minus gravid uterine weight) was decreased only at
the high dose, as was gravid uterine weight. Food consumption was
comparable between control group and low-dose females. Food
consumption was decreased at higher doses. Caesarean section
observations indicated no fetal deaths but, dose-related resorptions
at the mid and high-dose levels which paralleled a dose-related and
statistically significant increase in early resorptions. Post-
implantation losses were also increased at the mid and high dose
with concurrent decreases in the number of viable fetuses at the
same dose levels. Low-dose observations were comparable to
controls. External fetal observations were comparable to control
group for all dose levels as were visceral malformations and
variations. Skeletal malformations were comparable between all
experimental groups when compared on a litter or fetal basis.
Numerous skeletal variations were observed only in the high-dose
group and were generally indicative of developmental delay as
evidenced by reduced ossification of various skeletal structures.
These findings at the high dose also exceeded maximum values of the
historical control data. The NOAEL for maternal toxicity and
embryo/fetotoxicity was 20 mg/kg bw/day. Maternal toxicity was seen
at 100 mg/kg bw/day as decreased body weight and decreased food
consumption. Embryo/fetotoxicity was observed as increased (early)
resorptions, increased post-implantation losses and a decrease in
the number of viable fetuses at 100 mg/kg bw/day. No teratogenicity
was observed (Nemec, 1992).
Female Sprague-Dawley rats (23-25 dams/group) were gavaged with
20, 100, or 500 mg/kg bw/day of technical maneb (99.99% purity with
less than 0.01% ETU) in 0.5% CMC on days 6-15 of gestation. Two
additional groups serving as controls received either CMC (24 dams)
or were left untreated (24 dams).
There were no compound-related deaths and no clinical signs
below 500 mg/kg bw/day. High-dose dams manifested unsteady gait,
dragging of the rear limbs, diminished sensitivity to pain in the
affected limbs and paresis of the rear limbs. Body weights and
body-weight gain were decreased at the high dose. Gross pathology
was unremarkable in all treated dams. There were no statistically
significant differences between treated and control groups for
implantations, conception rate, corpora lutea, and the number of
live fetuses per litter. At 500 mg/kg bw/day, however, the number
of litters with dead fetuses was increased. Fetal observations at
the time of caesarean section revealed no remarkable findings at
dose levels of 20 and 100 mg/kg bw/day. However, animals receiving
500 mg/kg bw/day showed numerous changes including short tail,
meningocele, accessory toe, shortened toes, macroglossia,
syndactyly, kyphosis, oligodactyly and shortened splayed toes. The
mean fetal body weight per litter was also decreased as was mean
fetal body length per litter at 500 mg/kg bw/day. The number of
anomalous litters was significantly increased at the high dose
(25/25, 100%) for all malformations combined. Of the 304 live
fetuses examined at the high dose, 246 (80.9%) had anomalies. A
statistically significant increase in the number of fetal variants
was also observed at 500 mg/kg bw/day for all variations and
retardations combined. Maternal toxicity was seen at 500 mg/kg
bw/day as clinical signs and decreased body weight and body-weight
gain. Embryo/fetotoxicity and teratogenicity were seen at 500 mg/kg
bw/day based on decreased fetal body weight and body length, and an
increase in the number of anomalous litters and fetuses for all
malformations combined and for all variations and retardations
combined (Kapp et al., 1991).
Rabbits
Himalayan Chbb:HM rabbits were randomly assigned into four
groups (15 does/group). The study was divided into 3 phases. Five
does per dose group were artificially inseminated at each time
period. Gavage doses of 5, 20 or 80 mg/kg bw/day of maneb technical
(90.6% purity with 2% ETU contamination) were administered in CMC on
days 6 through 18 inclusive. The control group received CMC only.
At the high dose, 1 animal died and 3 were sacrificed - 1 moribund,
1 after aborting on day 21, and 1 after premature delivery on day
28. At the mid-dose, 1 animal was sacrificed in a moribund state
and 1 control animal delivered prematurely.
There were no compound-related signs of toxicity observed
during the study. Body weight and body-weight gain were decreased
at the high dose. Food consumption was decreased on days 7-9 and
10-11. Gross pathology revealed no compound-related abnormalities
in dams at terminal sacrifice. Pre-implantation loss and pregnancy
rates were comparable between control and test groups as were the
number of corpora lutea or implantations among control and test
groups. Post-implantation loss was non-significantly increased at
the high dose; however, a significant decrease in the number of
viable fetuses and an increase in the number of resorptions were
observed. Fetal body weights, crown-rump length and sex ratio were
comparable between treated and control groups. The placental weight
of female fetuses only was significantly greater at the high dose
compared to controls. Uterine weight was significantly reduced in
does administered 80 mg/kg bw/day. There were no fetal external
abnormalities observed. There were no compound-related visceral or
skeletal abnormalities in fetuses. NOAELs could not be determined
due to study deficiencies (Merkle, 1983).
Special study on hydrolytic stability of maneb in DMSO
14C-maneb (1.7 mg) was dissolved in 1.7 ml of DMSO and 30
minutes later added (dosing) to teflon-lined vials containing
sterilized buffer solutions (pH 5, 7 and 9) and stirred under
aseptic conditions. The final maneb concentration was approximately
10 ppm and the DMSO concentration was 1% (v/v). Hydrolysis was
performed in the dark at 25 °C. Aliquots were analyzed on day 0
(i.e. immediately after dosing), and on days 1, 2, 4, 7, 14 and 30.
Parent chemical, maneb, was not detected at any sampling time. A
mixture of several identified and unidentified compounds was
reported at all pH levels on day 0 including, but not limited to,
ETU, EBIS and Jaffe's base. ETU was generally the highest on day 0
and increased in concentration with time while the remaining
compounds decreased with time. The proportionality of the compounds
varied with changing pH (Rudel, 1990).
Special studies on genotoxicity
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The results are summarized in Table
3. A number of available studies were not considered, either
because DMSO was used as a solvent in which maneb is very unstable
or because of important omissions from the reports. The Meeting
concluded that maneb is not genotoxic.
Special studies on sensitization and irritation
Guinea-pigs
Hartley albino guinea-pigs (6/sex) were dosed topically with
technical maneb (purity not stated) for 6 hours a day, three times a
week for three weeks (modified Buehler method). Animals were
challenged two weeks after the last induction and re-challenged one
week later. Three males and three females received
dinitrochlorobenzene (DNCB; positive control group) and were treated
in a similar manner as the test group but were not re-challenged.
Reactions were scored at 24 and 48 hours after a challenge. DNCB
was determined to be an extreme sensitizing agent and maneb a
moderate sensitizing agent under the test conditions (Naas, 1989e).
Table 3. Results of genotoxicity assays on maneb
Test system Test object Concentration1 Purity Results Reference
1. GENE MUTATION ASSAYS
1.A. Bacterial Gene Mutation Assays
Salmonella S. typhimurium TA1535, 8.3-675 µg/plate; Not specified Negative Arni, 1981
reversion assay TA1537, TA98, TA100 in acetone
S. typhimurium 3-100 µg/plate; 88.1% a.i. Negative Thomas, 1985
TA1535, TA1537, in deionized water
TA1538, TA98, TA100
1.B. In Vitro Mammalian Gene Mutation Assays
Mammalian gene Chinese hamster ovary 0.1-30 µg/ml; 88.1% a.i. Negative Thomas, 1986b,
mutation assay (CHO)/hprt in deionized water 1988
2. STRUCTURAL CHROMOSOMAL ALTERATIONS
2.A. In Vivo Chromosomal Alterations
In vivo aberrations Chick embryos 0.5-27 g/l in 80% a.i. Negative Arias, 1988
aqueous solution
Complete and partial D. melanogaster 10 000 ppm saturated Not specified Negative Woodruff et al.,
chromosome loss mus-302 mutant aqueous solution with 1983
ethanol and sucrose
Bone marrow Albino mouse 100 mg/kg Not specified Negative Kondratenko &
cytogenetics Kurinnyi, 1972
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
Bone marrow Male and female 46.4 mg/kg/day for 2 days; Not specified Negative Zeller &
cytogenetics Chinese hamsters in carboxy-methylcellulose (increased Engelhardt, 1980
(cont'd) gaps)
Male Fischer 344 rat 4.9 g/kg (acute); 88.1% a.i. Negative Ivett & Lebowitz,
1.64 g/kg/day for 5 days; 1985
in carboxy-methylcellulose
Dominant lethal assay Male NMRI mice 10-100 mg/kg/day for Not specified Negative Leuschner, 1978
5 days; in saline
Micronucleus assay Male or female ICR mouse 1 g/kg with 100 mg/kg Not specified Negative Seiler, 1977
with sodium nitrite NaNO2; in gum arabic soln.
3. OTHER GENOTOXIC EFFECTS
3.A. DNA Damage and/or Repair Assays and Related Tests
In vitro unscheduled Primary rat hepatocytes 0.5-100 µg/ml; 88.1% a.i. Negative Loveday, 1986,
DNA synthesis (UDS) from male Fischer 344 rat in culture medium 1988
3.B. Sister Chromatid Exchange (SCE) Assays
In vitro SCE assays Chinese hamster ovary 0.5-30 µg/ml ± rat 88.1% a.i. Weak Thomas, 1986a
(CHO) cells or mouse S9; positive
in deionized water with
activation
In vivo SCE assays Chick embryos 0.5-27 g/l in aqueous 80% a.i. Positive Arias, 1988
solution
Table 3 (contd)
Test system Test object Concentration1 Purity Results Reference
3.C. Cell Transformation Assays
Cell transformation C3H/10T 1/2 cells 0.05-0.2 µg/ml; 88.1% a.i. Negative Tu et al., 1985
in water?;
no activation used
C3H/10T 1/2 cells with 0.2 µg/ml as 88.1% a.i. Negative Tu et al., 1986
"promotion" "promoter"; in water?
1 In vitro assays performed with and without exogenous activation unless indicated otherwise or the test system does not
normally use such supplementation; solvent is provided if specified in the report
Maneb DF (75% dust) was administered to male albino guinea-pigs
of the Dunkin-Hartley strain following a modified method of Buehler.
No delayed hypersensitivity reaction was observed. Animals
receiving DNCB as positive control showed positive sensitization
reactions (Glaza, 1988c).
Rabbits
Single, 0.5 gram doses of maneb technical (purity not stated)
were applied to the clipped, intact skin of 6 New Zeeland white
rabbits under a semi-occlusive dressing for a 4-hour exposure
period. Application sites were evaluated in accordance with the
method of Draize at approximately 30-60 minutes and 24, 48 and 72
hours after patch removal and daily thereafter for 14 days.
Technical maneb induced a very slight to slight erythema and edema.
Other dermal findings were not remarkable. Technical maneb was
concluded to be slightly irritating (Draize score 1.5) (Naas,
1989c).
Single, 100 mg doses of maneb technical were instilled into the
lower conjunctival sac of the right eyes of 6 New Zeeland white
rabbits. The eyelids were held closed for approximately one second
and released. The left eye served as the contralateral control.
Eyes were left unwashed and examined at 1, 24, 48 and 72 hours and
at 4 and 7 days. Sodium fluorescein was used in evaluating corneal
damage at 72 hours and 7 days. No corneal involvement was observed.
Four of six rabbits cleared of all ocular reactions by day 7. Minor
conjunctival reactions were present in two rabbits on day 7. Maneb
technical was considered to be a moderate irritant based on
persistent conjunctival irritation in two rabbits for 7 days and the
mean response of 8-19 out of a maximum of 20 for conjunctival
irritation over the initial 72-hour period (Naas, 1989d).
Maneb DF (75% dust) produced corneal and iridial irritation and
slight to severe conjunctival irritation in the unwashed eyes of New
Zeeland white rabbits. Iridial irritation and slight to moderate
conjunctival irritation were observed in the washed eyes (60 second
flush with water at 30 seconds post-compound administration) of
companion New Zeeland white rabbits. Ocular irritation cleared by
72 hours in washed eyes and in less than 7 days in unwashed eyes
(Glaza, 1988d).
Maneb DF (75% dust) applied to the intact skin of New Zeeland
white rabbits for 4 hours and scored according to the method of
Draize at 4, 24, 48, 72 and 96 hours post-administration produced a
slight irritation (Draize score < 1.0 for all readings) (Glaza,
1988e).
COMMENTS
Male and female rats given 25 mg/kg bw/day of 14C-labelled
maneb orally showed no differences between sexes with regard to
excretion patterns. Greater than 90% of the absorbed 14C was
eliminated in urine by 24 hours. Less than 1% was eliminated as
carbon dioxide. Average 14C concentration as percent of dose per
gram of tissue was greatest for the thyroid, followed by kidney and
liver. The percent of 14C present in urine as ETU at 12 hours was
21-30% on a mole/mole basis and less than 0.4% as maneb.
The acute oral, dermal and inhalation toxicity of maneb
technical and maneb 75% dust is low. WHO has classified maneb as
unlikely to present acute hazard in normal use.
Rats were fed dietary concentrations of 0, 80, 400 or 1300 ppm
maneb technical for 13 weeks. The NOAEL was 80 ppm (equal to 5.0
mg/kg bw/day) based on an increase in absolute thyroid weight and
thyroid follicular cell hyperplasia at 400 ppm.
In dogs fed dietary concentrations of maneb technical at 0,
100, 400 or 1600 ppm for 13 weeks, the NOAEL was 100 ppm (equal to
3.7 mg/kg bw/day) based on thyroid follicular cell hyperplasia at
400 ppm.
In a 52-week study in dogs, maneb was administered at dietary
concentrations of 0, 50, 200, 1000 or 2200 ppm. The NOAEL was 200
ppm (equal to 6.4 mg/kg bw/day) based on thyroid enlargement and
thickening and thyroid follicular cell hyperplasia at 1000 ppm.
The overall NOAEL in dogs, based on the evaluation of all of
the data on this species, was 6.4 mg/kg bw/day.
In a six-month study in monkeys, maneb was administered at
dietary concentrations of 0, 100, 300 or 3000 ppm. The NOAEL was 100
ppm (equal to 7.3 mg/kg bw/day) based on an increase in thyroid
weight at 300 ppm.
In a 79-week carcinogenicity study in mice at dietary
concentrations of 0, 60, 240 or 2400 ppm, the NOAEL was 60 ppm,
equal to 11 mg/kg bw/day, based on decreased body weight and
decreased thyroxine levels at 240 ppm. Hepatocellular adenomas were
observed at 2400 ppm in both sexes.
In a 31-month toxicity/carcinogenicity study in rats at dietary
concentrations of 0, 30, 100, 300 or 1000 ppm, the NOAEL was 300 ppm
(equal to 20 mg/kg bw/day) based on decreased body weight, an
increase in the half-life retention time of 131I in the thyroid,
decreased T4 values and an increased absolute thyroid weight at
1000 ppm. There was no evidence of carcinogenicity.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 75, 300 or 1200 ppm, the NOAEL was 75 ppm
(equal to 5.6 mg/kg bw/day) based on increased organ to body-weight
ratios for liver and kidney, and thyroid follicular cell hyperplasia
at 300 ppm.
An oral teratogenicity study in rats was conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day. The NOAEL was 20 mg/kg
bw/day for maternal toxicity and embryo/fetotoxicity. Maternal
toxicity was seen at 100 mg/kg bw/day as decreased body weight and
decreased food consumption. Embryo/fetotoxicity was observed as
increased (early) resorptions, increased post-implantation losses
and a decrease in viable fetuses at 100 mg/kg bw/day. No
teratogenicity was observed.
In a second oral teratogenicity study in rats conducted at dose
levels of 0, 20, 100 or 500 mg/kg bw/day the NOAEL for maternal
toxicity and embryo/fetotoxic and teratogenic effects was 100 mg/kg
bw/day. Maternal toxicity was seen at the highest dose as decreased
body weight and clinical signs. Embryo/fetotoxicity and
teratogenicity were seen at the highest dose as decreased fetal body
weight and body length, and an increase in the number of anomalous
litters and fetuses for all malformations combined and for all
variations and retardations combined.
An oral teratogenicity study in rabbits was conducted at dose
levels of 0, 5, 20 or 80 mg/kg bw/day. Due to study deficiencies a
NOAEL could not be determined.
Maneb has been adequately tested in a series of in vitro and
in vivo genotoxicity assays. The Meeting concluded that maneb is
not genotoxic. A number of available studies were not considered,
either because DMSO was used as a solvent in which maneb is very
unstable or because of important omissions from the reports.
The data on maneb would support an ADI of 0-0.05 mg/kg bw,
based on the NOAEL of 5.0 mg/kg bw/day for thyroid effects in rats,
using a 100-fold safety factor. However, the Meeting established a
group ADI of 0-0.03 mg/kg bw for maneb, alone or in combination with
mancozeb, metiram and/or zineb, because of the similarity of the
chemical structures of the EBDCs, the comparable toxicological
profiles of the EBDCs based on the toxic effects of ETU, and the
fact that parent EBDC residues cannot be differential using
presently-available analytical procedures.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 60 ppm in the diet, equal to 11 mg/kg bw/day
(79-week study)
Rat: 80 ppm in the diet, equal to 5.0 mg/kg bw/day
(13-week study)
300 ppm in the diet, equal to 20 mg/kg bw/day
(31-month study)
75 ppm in the diet, equal to 5.6 mg/kg bw/day
(reproduction study)
Dog: 200 ppm in the diet, equal to 6.4 mg/kg bw/day
(52-week study)
Monkey: 100 ppm in the diet, equal to 7.3 mg/kg bw/day
(6-month study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw (group ADI with mancozeb, metiram, and zineb).
Studies which will provide valuable information in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Allen, T.R., Frei, Th., Biedermann, K., Luetkemeier, H., Terrier,
Ch., Vogel, O., Wilson, J. (1989). 13-week oral toxicity (feeding)
study with maneb technical in the dog. Unpublished report No. 206605
from Research and Consulting Company, Ltd., Itingen, Switzerland.
Submitted to WHO by Elf Atochem.
Arias, E. (1988). Sister chromatid exchanges and chromosomal
aberrations in chick embryos after treatment with the fungicide
maneb. Mutation Res. 206: 271-273.
Arni, P. (1981). Salmonella/mammalian-microsome mutagenicity test
with CGA 31 364 = maneb. Unpublished experiment No. 810466 from
Ciba-Geigy Limited, Basle, Switzerland.
Corney, S.J., Allen, T.R., Janiak, T., Frei, Th., Leutkemeier, H.,
Biedermann, K., Vogel., O. & Springall, C. (1992). A 52-week oral
toxicity study (feeding) with maneb technical in the dog.
Unpublished report No. 206616 from Research and Consulting Co.,
Ltd., Itingen, Switzerland. Submitted to WHO by Elf Atochem.
Craine, E.M. (1991). A dermal radiotracer absorbtion study in rats
with 14C maneb. Unpublished report No. WIL-134010 (reissued final
report March 13, 1991) from WIL Research Laboratories, Inc.,
Ashland, Ohio, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988a). Acute oral toxicity study of Maneb DF in rats.
Unpublished report No. HLA 80104922 from Hazleton Labs America Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988b). Acute dermal toxicity study of Maneb DF in
rabbits. Unpublished report No. HLA 80104923 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988c). Dermal sensitization study of Maneb DF in guinea-
pigs. Unpublished report No. HLA 80104926 from Hazleton Labs America
Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Glaza, S. (1988d). Primary eye irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104924 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Glaza, S. (1988e). Primary dermal irritation study of Maneb DF in
rabbits. Unpublished report No. HLA 80104925 from Hazleton Labs
America Inc., Madison, Wisconsin, USA. Submitted to WHO by Elf
Atochem.
Hoffman, G. (1991). An acute inhalation toxicity study of Maneb DF
in the rat. Unpublished report No. 90-8287 from Bio/dynamics, Inc.,
East Millstone, New Jersey, USA. Submitted to WHO by Elf Atochem.
Ivett, J. & Lebowitz, H. (1985). Clastogenic evaluation of maneb
technical lot MT 01 (88.1% ai.) in the rat bone marrow cytogenic
assay. Unpublished final report No. LBI 22202 from Litton Bionetics
Inc., Kensington, Maryland, USA. Submitted to WHO by Elf Atochem.
Kapp, R.W., Schellhaas, L.J., Piccirillo, V.J. (1991). Prenatal
toxicity study of maneb in rats. Unpublished report Nos. BASF
88/0522; 88/0523; 88/0524 from BASF Gewerbehygiene und Toxikologie,
Ludwigshaven, Germany. Submitted to WHO by Elf Atochem.
Kondratenko, T. & Kurinnyi, A. (1972). Effects of some fungicides
(dithiocarbamic acid derivatives) on chromosomes of bone marrow
cells in mice. Tsitol. Genet. 6: 225-228.
Leuschner, F. (1991). Chronic oral toxicity of manganese-ethylene-
1,2-bis-dithiocarbamate, 90% - called for short "maneb" - in Sprague-
Dawley (SIV) - rats (with special attention to carcinogenic
properties). Unpublished registration document No. (BASF) 86/0430
Amendment No. 3 dated April 19, 1991 from Laboratory of Pharmacology
and Toxicology, Hamburg, West Germany. Submitted to WHO by Elf
Atochem.
Leuschner, F. (1978). Dominant lethal test on male mice with
manganese ethylene-1,2-bis-dithiocarbamate. Unpublished report No.
BASF 78/298 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Dontenwill, W. & Hubschner, F. (1986a). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" -in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 1 dated October 2, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A. & Hubschner, F. (1986b). Chronic oral
toxicity of manganese-ethylene-1,2-bis-dithiocarbamate, 90% - called
for short "maneb" - in Sprague-Dawley (SIV) - rats (with special
attention to carcinogenic properties). Unpublished registration
document No. (BASF) 86/0430 Amendment No. 2 dated October 20, 1986
from Laboratory of Pharmacology and Toxicology, Hamburg, West
Germany. Submitted to WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Klie, R., Dontenwill, W. & Rogulja, P.
(1979). Chronic oral toxicity of manganese-ethylene-1,2-bis-
dithiocarbamate, 90% - called for short "maneb" - in Sprague-Dawley
(SIV) - rats (with special attention to carcinogenic properties).
Unpublished registration document No. (BASF) 86/0430 from Laboratory
of Pharmacology and Toxicology, Hamburg, West Germany. Submitted to
WHO by Elf Atochem.
Leuschner, F., Leuschner, A., Schneider, C., Schwerdtfeger, W. &
Dontenwill, W. (1977). Oral toxicity of maneb in the Rhesus monkey:
six months dietary dosing. Unpublished report No. WF 1172 from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Loveday, K. (1986). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Unpublished
report No. 850047-20 from American Biogenics Corporation, Woburn,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Loveday, K. (1988). In vitro unscheduled DNA synthesis assay in
rat hepatocytes: the effect of technical grade maneb. Supplementary
report PW-118 to Unpublished report No. 850047-20 from American
Biogenics Corporation, Woburn, Massachusetts, USA. Submitted to WHO
by Elf Atochem.
Merkle, J. (1983). Study to determine the prenatal toxicity of
manganous ethylenebis (dithiocarbamate) in rabbits. Unpublished
study RZ-No. 83/094 from BASF AG, Ludwigshafen Rhein, Federal
Republic of Germany. Submitted to WHO by Elf Atochem.
Naas, D.J. (1989a). Acute oral toxicity (LD50) study in albino rats
with maneb technical. Unpublished report No. WIL-134003 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J., (1989b). Acute dermal toxicity (LD50) study in albino
rabbits with maneb technical. Unpublished report No. WIL-134004 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989c). Primary dermal irritation study in albino
rabbits with maneb technical. Unpublished report No. WIL-134005 from
WIL Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO
by Elf Atochem.
Naas, D.J., (1989d). Primary eye irritation study in albino rabbits
with maneb technical. Unpublished report No. WIL-134006 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Naas, D.J. (1989e). Skin sensitization study in albino guinea-pigs
with maneb technical. Unpublished report No. WIL-134007 from WIL
Research Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by
Elf Atochem.
Nemec, M., (1992). A developmental toxicity study of maneb technical
in rats. Unpublished report No. WIL-134011 from WIL Research
Laboratories, Inc., Ashland Ohio, USA. Submitted to WHO by Elf
Atochem.
Puhl, J.R. (1985). Metabolism of radiolabeled maneb in rats.
Unpublished report No. 6181-101 from Hazleton Labs., America, Inc.,
Madison, Wisconsin, USA. Submitted to WHO by Elf Atochem.
Rudel, H. (1990). Hydrolysis of maneb at pH 5, 7, and 9. Unpublished
project No. MRG-O1/7-04 from Fraunhofer-Institut für Umweltchemie
und Okotoxikologie, D-5948 Schmallenberg, Federal Republic of
Germany. Submitted to WHO by Elf Atochem.
Ryle, P.R., Bell, P.F., Parker, C., Farmer, H., Offer, J.M.,
Anderson, A. & Dawe, I.S., (1991). A study of the effect of maneb
(technical) on reproductive function of two generations in the rat.
Unpublished report No. MNB1/9072 from Huntington Research Centre
Ltd., Cambridgeshire, England. Submitted to WHO by Elf Atochem.
Seiler, J. (1977). Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Terrill, J.B. (1990). Acute inhalation toxicity study with maneb in
the rat. Unpublished report No. HLA 2567-100 from Hazleton Labs.,
Rockville, Maryland, USA. Submitted to WHO by Elf Atochem.
Terrill, J.B., (1991). A four-week study in the rat to determine the
lung tissue residue and effect upon the thyroid function with maneb.
Unpublished report No. HLA 2567-101 from Hazleton Labs., Rockville,
Maryland, USA. Submitted to WHO by Elf Atochem.
Thomas, M. (1985). Salmonella/microsome mutagenesis assay on
technical grade maneb. Unpublished final report No. ABC 850047-40
from American Biogenics Corp., Woburn, Massachusetts, USA. Submitted
to WHO by Elf Atochem.
Thomas, M. (1986a). In vitro sister chromatid exchange assay in
cultured Chinese hamster ovary (CHO) cells treated with technical
grade maneb. Unpublished final report No. ABC 850047-30 from
American Biogenics Corp., Woburn, Massachusetts, USA. Submitted to
WHO by Elf Atochem.
Thomas, M. (1986b). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Unpublished final report No. ABC
850047-10 from American Biogenics Corp., Woburn, Massachusetts, USA.
Submitted to WHO by Elf Atochem.
Thomas, M. (1988). CHO/HGPRT in vitro mammalian cell mutation
assay on technical grade maneb. Supplementary report PW-117 to
Unpublished final report No. ABC 850047-10 from American Biogenics
Corp., Woburn, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tompkins, C.E. (1992). An 18-month dietary oncogenicity study in
mice with maneb technical. Unpublished report No. WIL-134008 from
WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to
WHO by Elf Atochem.
Trutter, J.A. (1988a). Subchronic toxicity study with maneb
technical in the rat. Unpublished report No. HLA 153-140 from
Hazleton Laboratories America, Inc., Rockville, Maryland, USA.
Submitted to WHO by Elf Atochem.
Trutter, J.A. (1988b). A 21-day dermal toxicity study in rabbits
with maneb technical. Unpublished report No. HLA 153-139 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA.
Submitted to WHO by Elf Atochem.
Tu, A., Breen, P. & Sivak, A. (1986). Evaluation of maneb in the
C3H-10T ´ cell transformation assay for promotion activity.
Unpublished report No. ADL 88720-44 from Arthur D. Little, Inc.,
Cambridge, Massachusetts, USA. Submitted to WHO by Elf Atochem.
Tu, A., Sivak, A., Hatch, K. & Breen, P. (1985). Evaluation of maneb
in the C3H-10T ´ cell transformation assay. Unpublished final report
No. ADL 88720-44 (1-0860) from Arthur D. Little, Inc., Cambridge,
Massachusetts, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1986). Thirteen-week subchronic inhalation toxicity
study on maneb in rats (final report). Unpublished report No. 550-
001 from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Elf Atochem.
Ulrich, C.E. (1987). Thirteen-week subchronic inhalation toxicity
study on maneb in rats - addendum to the final report covering the
recovery phase. Unpublished report No. 550-001 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Elf Atochem.
WHO (1992). The WH0 recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/91.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Woodruff, R., Phillips, J. & Irwin, D. (1983). Pesticide induced
complete and partial chromosome loss in screens with repair-
defective females of Drosophila melanogaster. Environ. Mutagen. 5:
835-846.
Zeller, H. & Engelhardt, G. (1980). Cytogenic investigations in
Chinese hamsters after two intraperitoneal administrations of maneb.
Bone marrow chromosome analysis. Unpublished report dated December
19, 1980 from Gewerbehygiene und Toxikologie of BASF, Germany.
Submitted to WHO by Elf Atochem.
