
PROPINEB
First draft prepared by
M. Watson
Pesticide Safety Directorate, Ministry of Agriculture, Fisheries and
Food
Harpenden, Hertfordshire, United Kingdom
EXPLANATION
Propineb was first evaluated by the Joint Meeting in 1977, when
a temporary ADI of 0-0.005 mg/kg bw was established (Annex I,
reference 28). The temporary ADI was extended in 1980 and 1983
(Annex I, references 34 and 40). At the 1985 Joint Meeting (Annex
I, reference 44), the temporary ADI was not extended in view of the
carcinogenic response in the liver of mice to propylene thiourea
(PTU) and the lack of NOAELs for thyroid effects of propineb and
PTU. This monograph summarizes new or not previously reviewed data
on propineb, as well as relevant data from previous monographs and
monograph addenda on this pesticide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
In male Sprague-Dawley rats, dosed orally with 5 or 50 mg 14C-
propineb/kg bw, 60-70% of the radioactivity was absorbed in 48
hours. About 50% of the activity was excreted via urine and 40% via
faeces within this period. Exhalation during the first 24 hours
after administration amounted to 7% and excretion in the bile to 3%
of the administered dose (Ecker, 1975; Weber, 1980).
Peak levels in blood and most organs were reached 3-5 hours
after administration, whereas in the thyroid the maximum
concentration was attained after 24 hours. Within 4 days the total
elimination exceeded 99%. The half-life of elimination from the
tissues (except the thyroid) was first rapid, than slower, varying
between 5 and 20 days. Four days post-dosing (50 mg/kg bw), the
concentration of radioactive material in the thyroid was about 100
times and in the kidney and pituitary 3-4 times higher than the
average concentration for all other organs except the
gastrointestinal tract. After 16 days the relative concentration
factor for the thyroid was about 30 (Patschke & Wegner, 1975).
Biotransformation
The main metabolites found in the urine of dosed rats were
propylene diamine (accounting for 12-15% of the renally excreted
activity), PTU and propylene urea (PU) (accounting together for 40-
45%). A minor metabolite was 4-methylimidazoline (less than 5%). No
compounds could be considered as potential intermediates for
complete degradation to CO2 (Ecker, 1975).
The metabolic pathway of propineb in rats is illustrated in
Figure 1.
Toxicological studies
Acute toxicity studies
Technical grade propineb of 84-87% purity was tested in the
acute toxicity studies summarised in Table 1. WHO has classified
propineb as unlikely to present acute hazard in normal use (WHO,
1992).
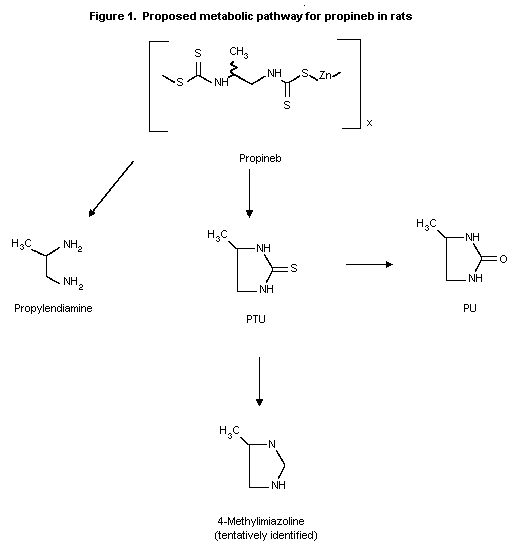 Table 1. Acute toxicity of propineb
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/m3)
Mouse M/F oral > 5000 Thyssen & Kimmerle, 1978
Mouse M/F s.c. 1500-2000 Thyssen & Kimmerle, 1978
Mouse M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F oral > 5000 Thyssen & Kimmerle, 1978
Rat F oral 5900 Flucke & Kimmerle, 1977
Rat M/F dermal > 5000 Thyssen & Kimmerle, 1978
Rat M/F i.p. 102-141 Thyssen & Kimmerle, 1978
Rat M/F inhalation > 522 (1 x 1 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 693 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 193 (5 x 4 h) Thyssen & Kimmerle, 1978
Hamster M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Cat M oral > 500 Thyssen & Kimmerle, 1978
Sheep M/F oral 2500 Hoffmann, 1983
Short-term toxicity studies
Rats
Groups of 50 male and 50 female Wistar rats received diets
containing propineb (technical material, 93.5% purity) at
concentrations of 0, 100 or 500 ppm. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. Half of the rats from each group were
sacrificed after 6 months, the remainder were sacrificed after a
subsequent withdrawal period of 8 weeks. Treatment with 100 ppm did
not cause thyroid enlargement in either sex, whereas treatment with
500 ppm caused marked thyroid enlargement in both males and females.
During the 8-week withdrawal period the enlargement receded in male
rats, but relative thyroid weights were still higher than control
values in females, indicating a tendency to reversibility (Loeser,
1968).
In a 3-month feeding study in Wistar rats, groups of 15 males
and 15 females received dietary levels of 0, 5, 10, 25, 50 or 100
ppm propineb (87% purity). The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. There was no treatment-related effect on
behaviour, survival, food intake or weight gain. Haematological and
urinalysis investigations revealed no indication of any reaction to
treatment. Clinical chemistry investigations revealed increased
sorbitol dehydrogenase activity in serum in male rats treated with
50 or 100 ppm and increased serum lactate dehydrogenase activity in
males and females treated with 100 ppm. Gross pathology and organ
weight analysis revealed no indication of any reaction to treatment.
No histopathological examination of tissues was performed and it was
thus considered inappropriate to derive an NOAEL from the results of
this study (Loeser, 1969).
Groups of 10 male and 10 female Wistar TNO/W74 rats were
exposed to propineb aerosols at analytically-determined
concentrations of 0, 5, 8, 29, or 44 mg/m3, five times per week for
6 hours, for 3 weeks. Rats exposed to 44 mg/m3 exhibited severely
disturbed behaviour including paralysis of the extremities. All the
female rats and 1 male rat at this exposure level died by the 13th
exposure. Animals that died had sharp losses in body weight and
were cachectic. Gross necropsies revealed small spleens and livers,
enlarged adrenals, and inflamed lungs. Histopathology revealed
acute vasculature congestion in the lungs, liver, kidneys, and
bronchial lymph nodes, indicating that the cause of death was
cardiovascular failure. One male rat at 29 mg/m3 also exhibited
hind limb paralysis but survived to the end of the study. The
remaining test animals did not differ from control animals with
respect to appearance, behaviour or body-weight gains.
Haematological examinations, clinical chemistry, and urinalyses
(performed just prior to termination), and results of gross
necropsies, organ weight determinations, and histopathological
examinations revealed no indication of any reaction to treatment in
animals surviving to termination (Thyssen & Mohr, 1979).
In a study to compare the effects of propineb with various
other thyro-suppressive agents, groups of 50 male and 50 female
Wistar rats were treated orally with 50 mg/kg bw/day of the
following compounds: pure propineb, two technical samples of
propineb, PTU, ETU, zineb or methyl thiouracil as a positive
control. An untreated control was also included and 10 male and 10
female rats were killed after 7, 14 or 21 days treatment and after
14 or 28 days recovery. Investigations of thyroid activity was
limited to recording of thyroid weight, no biochemical analyses or
histopathology were performed. PTU, ETU and methyl thiouracil caused
significant increases in absolute and relative thyroid weight in
rats of both sexes, while the three propineb samples caused an
increase in the relative weight in females only. The thyroid
enlargement induced by propineb was reversible during the withdrawal
period, the effect of the other agents was partially reversible.
Propineb had only a moderate effect compared to methyl thiouracil,
whereas PTU had an equivalent effect to that of methyl thiouracil
and was somewhat stronger than ETU (Kimmerle, 1972).
Propineb of unknown purity was administered in the feed for 62
days to 5 groups of 80 male Wistar TNO/W74 rats at dosage levels of
0, 2, 10, 50, or 250 ppm. The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. Ten rats per group were killed for interim
sacrifice at 7 days or at 21 days. An additional 10 rats per group
were also sacrificed at 7, 21, or 62 days for "thyroid function
tests", which was reported separately (see Weber & Dressler, 1980).
The remaining animals were sacrificed at 62 days. No mortalities
occurred during the study and daily examinations indicated no
effects that could be attributed to the test material. Mean body
weights of animals in the 250 ppm group were slightly but
consistently lower than those of the control group throughout the
entire study, but food consumption was unaffected. Total thyroxin
was decreased in the 50 and 250 ppm groups at 21 and 62 days.
Thyroid weights were decreased in the 250 ppm group at 7 days, but
later increased in the 50 and 250 ppm groups at 62 days.
Histopathology did not reveal any treatment-related changes in the
thyroid. Organ weight analysis and histopathological examinations
of liver and adrenals revealed no effects that could be attributed
to the test material. The NOAEL in this study was 10 ppm (equal to
0.74 mg/kg bw/day), based on changes in thyroxin concentration and
increased thyroid weight at 50 and 250 ppm (Kroetlinger et al.,
1980).
Ten male rats per group from the above-described study were
used for "thyroid function tests" at 7, 21, or 62 days. In
addition, iodine accumulation in the thyroid was assayed 24 hours
after oral intubation of 0.2 µCi of 131I-NaI tracer by measuring
radioactivity in excised and weighed thyroid tissue. Concentrations
of T3 and T4 in the serum were also determined by use of a
commercially-available 125I radioimmunoassay test system. Mean
thyroid weights were increased above control values in the 50 ppm
group at 62 days, and in the 250 ppm group at 21 days and at 62
days. A decrease in mean thyroid weights was observed in the 2 ppm
group at 7 days. Mean iodine accumulation in the thyroid, expressed
as the amount of radioactive iodine per mg of tissue, was decreased
below control values in the 50 ppm group at 62 days and in the 250
ppm group at 21 and 62 days. Mean T3 concentrations were increased
above control values in the 2 ppm group and in the 10 ppm group at 7
days. A decrease in mean T3 was observed in the 250 ppm group at
62 days. Mean T4 concentrations were decreased below control
values in the 250 ppm group at 7, 21 and 62 days. The study results
indicate that propineb affects thyroid function at all dosage levels
tested. The effect appears to be dose-related. At 50 ppm and 250
ppm, iodine accumulation in the thyroid was reduced and at 250 ppm,
T3 and T4 serum levels were also reduced. A compensatory reaction
occurred, however, at these dosage levels, as evidenced by increased
thyroid weights and a return toward control values for several of
the other parameters. At 2 and 10 ppm, an effect on thyroid
function was indicated by the increased levels at 7 days of T3, the
more biologically-active form of the thyroid hormone. However, this
is considered to be a normal physiological response, without lasting
adverse effect. The NOAEL in this study was thus 10 ppm, equal to
0.74 mg/kg bw/day (Weber & Dressler, 1980).
The effect of propineb on thyroid function was investigated in
a 63-day feeding study in Wistar rats. Groups of 36 males received
dietary concentrations of 0, 0.2, 0.6, 2 or 10 ppm propineb.
Technical material of 83.3% purity was used, but dietary levels
refer to pure active ingredient. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
checked during the study and found to be adequate. On days 7, 21
and 63 thyroid function was investigated by determination of thyroid
weight, measurement of 131I incorporation into the thyroid and by
radio immunoassay determination of T3, T4 and TSH in the serum.
Food and water intake was not affected by treatment, but body-weight
gain was slightly depressed at 2 and 10 ppm in comparison with
controls. Thyroid weights were unaffected by treatment at any of
the examinations. Although minor inter-group differences in results
of other investigations attained a level of statistical significance
when compared with controls, there were no consistent, dose-related
effects. Histopathological examination of thyroids was conducted
later and this revealed a minimal to slight hypertrophy in the
follicular epithelium of 2 of 10 rats treated with 10 ppm for 63
days. No similar changes were seen at the same dose level after
shorter treatment periods or at lower doses at any of the treatment
periods. The NOAEL in this study was 10 ppm since the minor
histological changes seen in thyroid at 10 ppm were not considered
to be a permanent adverse effect in rats. The apparent changes in
body-weight gain were disregarded, since this effect was not
supported by results of any other feeding study with propineb in
rats, and there were large inter-group differences in initial body
weight in this study, which hampered interpretation of the weight
gain data (Weber et al., 1991).
Rabbits
Technical grade propineb of 87.3% purity was dermally applied
to the shaven backs of groups of 6/sex New Zeeland white rabbits
(one half of which had intact and one half abraded skin test sites),
5 times per week for 7 hours for 3 weeks at dosage levels of 0, 50,
or 250 mg/kg bw/day. Three male rabbits died during the study of
severe pneumonitis. Daily observations, including skin reactions,
revealed no effects attributable to the test material. No
differences in body weights between control and test animals were
observed. All clinical laboratory tests, gross necropsies and
histopathological examinations revealed no indication of any
reaction to treatment with propineb. With the exception of slightly
increased liver weights in female rabbits treated with 250 mg/kg
bw/day, organ weights in control and test animals were similar
(Mihail & Kaliner, 1979).
Dogs
In a 4-month feeding study, groups of beagle dogs (2/sex)
received dietary concentrations of 0, 100, 400 or 1600 ppm propineb
(86% purity). The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The results of this study revealed no indication of any
reaction to treatment in terms of survival, behaviour, food intake,
weight gain, clinical laboratory investigations, gross pathology and
organ weight analysis. No histopathological examination of tissues
was carried out and it is thus considered inappropriate to derive an
NOAEL from the results of this study (Loeser, 1967).
In a 2-year feeding study, groups of 4 male and 4 female beagle
dogs received dietary concentrations of 0, 100, 300, 1000 or 3000
ppm propineb (purity approximately 86%). The summarized results of
this study revealed no indication of any reaction to treatment in
terms of survival, behaviour, food intake, weight gain, clinical
laboratory investigations, gross pathology, organ weight analysis or
histopathology. The NOAEL for this study may therefore be defined
as 3000 ppm (equivalent to 75 mg/kg bw/day), the highest dose level
tested. A complete detailed report of this study was not available
for evaluation (Loser, 1973a).
Long-term toxicity/carcinogenicity studies
Mice
Propineb of 82.9% purity was administered in the feed to groups
of NMRI mice (50/sex) for 104 weeks at dietary levels of 0, 50, 200,
or 800 ppm. The homogeneity, stability and accuracy of admixture of
the test material into the test diet was not checked during the
study. No substantial differences in mortality were observed
between male and female control and test groups, although the best
survival of any group was seen in the high-dose males. Clinical
observations did not indicate any differences between test and
control mice and food consumption and body-weight gain was similar
in male and female control and test groups. Haematological,
clinical chemistry, and urinalysis examinations did not reveal any
effects attributable to the test material. Gross necropsies were
reported to be negative for lesions attributable to the test
material. Organ weight analysis did not reveal any differences that
could be related to the test material. Non-neoplastic lesions were
not reported in the histopathological evaluation. Percentages of
female mice with tumours of any kind were 65, 66, 73, and 88% for
the control, low-, mid-, and high-dose groups, respectively. A
similar increase was not observed in treated male mice. Frequently-
observed neoplasms were those commonly seen in NMRI mice and
consisted of pulmonary adenomas and malignant lymphomas in male and
female mice and benign granulosa cell tumours in the ovaries of
female mice. Thyroid or adrenal tumours were not increased in
treated male or female mice. An increased incidence of
hepatocellular adenomas was observed in the high-dose male group.
Percentages were 6, 7, 0, and 20 for the control, low, mid-, and
high-dose male groups, respectively. Hepatocellular carcinomas in
male mice ranged from 0 to 4%, but their incidence was not dose-
related. Historical control data presented by the testing
laboratory for hepatocellular adenomas in NMRI mice ranged from 4 to
12%. The increase in hepatocellular adenomas in the high-dose male
group may possibly be related to treatment with the test material,
but may have been affected by the higher survival rate in this group
compared to the controls. Hepatocellular tumours were not increased
in treated female mice. The NOAEL in this study was 200 ppm (equal
to 26 mg/kg bw/day), based on the equivocal alteration in hepatic
tumour incidence at 800 ppm (Brune et al., 1980).
Rats
Groups of Wistar rats (25/sex/dose and 50/sex/control group)
were fed diets containing propineb (technical material, 93.5%
purity) at dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000 ppm
for 2 years. The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The mortality rate was increased in comparison with controls
at 1000 ppm and above, such that at 2000 and 8000 ppm in females and
at 8000 ppm in males, no rats survived to termination. Dose-related
reduced weight gain and food intake were also evident at 1000, 2000
and 8000 ppm. Apparent muscular weakness, leading to paralysis
(described as myasthenia) was seen in rats receiving 1000, 2000 or
8000 ppm. The results of limited clinical chemistry investigations
(conducted at 16 and 24 months) did not reveal any indication of
reaction to treatment. At autopsy, increased kidney, liver and
thyroid weights were noted at 100 ppm and above. Histopathology
revealed no changes in kidney and liver, but did show evidence of
degeneration of skeletal muscle at 1000 ppm and above, along with an
increased incidence of TSH-related thyroid tumours in these groups.
The NOAEL in this study was thus 10 ppm (equivalent to 0.5 mg/kg
bw/day) based on the organ weight changes noted at 100 ppm and above
(Loeser, 1974a).
In a second study, carried out in order to more closely define
the NOAEL in long-term administration to rats, groups of 40 male and
40 female Wistar rats (95/sex in the control group) were fed diets
containing propineb (93.5% purity) at dietary levels of 0, 5, 10,
25, 50 or 100 ppm for 2 years. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. There were no clinical signs of
reaction to treatment and no effects on food consumption, growth
rate or mortality at any dose level. Limited clinical chemistry
analyses revealed that serum ALT and AST activities were slightly
higher than control values after 52 weeks in rats receiving 100 ppm,
but no similar effect was seen at termination. Serum PBI levels were
lower than control values, at 52 weeks and prior to termination, in
rats receiving 100 ppm. At autopsy there were no macroscopic
abnormalities attributable to treatment and organ weight analysis
revealed no significant intergroup differences. Histopathology
revealed no changes considered to be related to the administration
of propineb, in particular, the nature, location and frequency of
tumours provided no indication of any carcinogenic effect. The
NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, in this study
(Loeser, 1974b).
Reproduction studies
In a 3-generation study in rats, with two litters per
generation, groups of 10 male and 20 female Long Evans rats received
propineb (93.5% purity) at dietary levels of 0, 20, 60, 200 or 600
ppm. The homogeneity, stability and accuracy of admixture of the
test material into the test diet was not checked during the study.
Treatment with 200 ppm led to slight symptoms of hind limb paralysis
in parental animals, while treatment with 600 ppm led to increased
mortality, decreased weight gain and severe hind limb paralysis.
With regard to the reproductive parameters, treatment with 200 ppm
was associated with slightly lower gestation rates and a lower
number of pups per litter after one mating, while treatment with 600
ppm was associated with substantially lower gestation rates (up to
50%) after nearly all matings and lower birth weight and lower
numbers of pups per litter after some matings. Lactation
performance of dams receiving 600 ppm which did have offspring was
not, however, adversely affected. The NOAEL was 60 ppm (equivalent
to 3 mg/kg bw/day) (Loeser, 1973b).
Special studies on embryo/fetotoxicity
Rats
Groups of 17-23 mated female rats were given propineb by oral
gavage at levels of 0, 3, 10, 30 or 100 mg/kg bw/day on days 6 to 15
of gestation. Treatment with 30 mg/kg bw/day was associated with
slight maternal toxicity (somnolence, ruffled coat, limpness), while
treatment with 100 mg/kg bw/day led to severe maternal toxicity
(paralysis of extremities, ataxia, dyspnoea, tremors), leading to
mortality in some cases. At this high dose, fetal growth was
depressed, and teratogenic effects (dysplasia of extremities) were
also seen. The NOAELs were 10 and 30 mg/kg bw/day for maternal and
embryo/fetotoxicity, respectively (Machemer, 1974a).
These results were supported by studies published in the
literature; in one study, doses greater than 400 mg/kg bw/day caused
abnormalities and maternal toxicity when given to rats on day 11 of
gestation and in another study, using doses of 0, 25, 50 100 or
200 mg/kg bw/day on days 6 to 16 of gestation, abnormalities and
maternal toxicity were observed at 100 and 200 mg/kg bw/day
(Larsson et al., 1976; Vicari et al., 1985).
Rabbits
Groups of 16 mated female rabbits were given propineb (83.9%
purity), by oral gavage, at dose levels of 0, 10, 30 or 100 mg/kg
bw/day from day 6 to day 18 of gestation. Signs of severe maternal
toxicity were seen at 100 mg/kg bw/day, including reduced weight
gain and food intake in comparison with controls, dyspnea and
ventro-lateral recumbency, and signs of abortion in two females.
Body-weight gain and food intake were also reduced at 30
mg/kg bw/day. Evaluation of reproduction parameters revealed a
dose-related increased post-implantation loss at 30 and 100 mg/kg
bw/day. Fetal parameters, including external, skeletal and visceral
examinations, revealed no effects associated with treatment with
propineb. The NOAEL for maternal toxicity was 10 mg/kg bw/day, while
there was no indication of embryo/fetotoxicity or teratogenicity at
100 mg/kg bw/day, the highest dose tested (Becker et al., 1988).
Special studies on genotoxicity
Results of genotoxicity tests on propineb are summarized in
Table 2. The Meeting concluded that propineb was not genotoxic.
Special studies on sensitization
Propineb (83.9% purity) was tested for possible skin
sensitizing potential in guinea-pigs in a Magnusson and Kligman
maximization test, employing a test group of 20 animals and two
Table 2. Results of genotoxicity assays on propineb
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium up to 2.5 mg/ 84.3% Negative Herbold, 1980a
TA98, TA100, TA1535, plate
TA1537
Ames test S. typhimurium up to 0.864 ? Negative Hatano Institute, 1978
TA98, TA100, TA1535, mg/plate
TA1537, TA1538
E. coli WP2
Preferential B. subtilis M45, H17 up to 0.864 ? Negative Hatano Institute, 1978
toxicity mg/plate
HGPRT forward CHO cells 0.11-60 mg/ml ? Negative Lehn, 1988
mutation assay
UDS Rat hepatocytes 5-30 mg/ml ? Negative Cifone, 1987
Micronucleus test Mouse 2 x 1000 or 2 x 85.7% Negative Herbold, 1982
2000 mg/kg bw
Micronucleus test Mouse not known ? Negative Rolandi et al., 1984
Dominant lethal NMRI mice 500 mg/kg bw ? Negative Machemer, 1974b
control groups of 10 animals each. Following intradermal induction
with 0.1% propineb and topical induction with 25% propineb, the
first challenge was carried out using 25% propineb. A positive
response was seen in all 20 test animals, against no positive
reactions in the first control group. A second challenge was
carried out using 2.5% propineb. Positive reactions were seen on
this occasion in 18 test animals against 4 in the control group.
This study therefore detected clear indications of the allergic
potential of propineb in guinea-pigs (Heimann, 1987).
In a further experiment, propineb (premix, 83.3% purity) was
tested for possible skin sensitizing potential in guinea-pigs in a
Buhler patch test employing a test group and two control groups of
12 animals each. Following epicutaneous induction with 50%
propineb, challenge was carried out with 2.5% and 50% propineb. The
test material was tolerated without reaction and this study revealed
no indication of a skin sensitizing potential of propineb in guinea-
pigs (Flucke, 1989).
Irritant and/or allergizing characteristics of propineb in
humans was not confirmed. Between 1964 (when production began) and
1988 no allergies or skin sensitizing reactions associated with
propineb were recorded, "even though skin contact had been frequent
as a result of cleaning work" (Mueller, 1988).
Observations in humans
No information available.
COMMENTS
Orally administered propineb in rats is rapidly absorbed and
excreted largely via urine and faeces. Although there was some
evidence of excretion by exhalation, the available metabolic data
(which detected PTU and propyleneurea as the main urinary
metabolites along with propylenediamine and a small amount of 4-
methylimidazoline) found no metabolites which could be considered as
potential intermediates for degradation to CO2. Study results
indicated that a proportion of the administered dose accumulates
temporarily in the thyroid. Although most elimination occurred
within 4 days of dosing, the half-life of elimination for the
proportion remaining was relatively long. This could be due to
incorporation of portion of the molecule into endogenous substances
following metabolism, which would also account for the radioactivity
eliminated with exhaled air.
Propineb has moderate to low acute toxicity in mice, rats,
hamsters, cats and sheep. WHO has classified propineb as unlikely
to present acute hazard in normal use.
The results of toxicity studies clearly indicate that propineb
has a goitrogenic effect in rats, although no similar finding was
noted in rabbits or dogs. In a 62-day study in male rats using
dietary levels of 0, 2, 10, 50 or 250 ppm, the NOAEL was 10 ppm
(equal to 0.74 mg/kg bw/day), based on changes in thyroxine
concentration and increased thyroid weight at higher doses.
Although in a later 63-day study using dietary levels of 0, 0.2,
0.6, 2 or 10 ppm slight hyperplasia was seen in the thyroid in 2
rats out of 10 treated with 10 ppm, this finding was considered not
to be a permanent adverse effect in rats.
In a comparative study in rats of the effects of propineb, PTU,
ETU, zineb and methyl thiouracil on thyroid weight, propineb had
only a moderate effect compared to methyl thiouracil, whereas PTU
had an equivalent effect to that of methyl thiouracil and was
somewhat stronger than ETU. These results suggest that the effects
of propineb on the thyroid in rats may be caused primarily by the
metabolite, PTU. Dogs tolerated much higher doses of propineb,
dietary administration of 3000 ppm, equivalent to 75 mg/kg bw/day,
causing no adverse effects over 2 years.
In long-term studies, treatment-related alterations in tumour
incidence were seen in rats and mice. In mice treated with 0, 50,
200 or 800 ppm, an increase in hepatocellular adenomas was seen in
males only, at the highest dose tested, but there was no increase in
the incidence of hepatic carcinomas and no similar effect in
females. The NOAEL was 200 ppm, equal to 26 mg/kg bw/day, based on
this change in hepatic tumour incidence. In rats, there were two
studies, using dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000
ppm in the first and 0, 5, 10, 25, 50 or 100 ppm in the second. The
overall NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on
increased kidney and liver weight (without histological correlation)
and increased thyroid weight at 100 ppm and above. An increase in
thyrotropin-related thyroid tumours and skeletal muscle degeneration
was seen in rats at dietary levels of 1000 ppm and greater, but
these doses were accompanied by increased mortality.
In a three-generation reproduction study in rats using dietary
levels of 0, 20, 60, 200 or 600 ppm the NOAEL was 60 ppm, equivalent
to 3 mg/kg bw/day, with adverse effects on maternal health and
impaired reproductive performance seen at higher doses.
Teratogenicity studies indicated that propineb has teratogenic
potential in rats, but no evidence of teratogenicity was seen, even
in the presence of maternal toxicity, in rabbits. In rats, using
dose levels of 0, 3, 10, 30 or 100 mg/kg bw/day, the NOAELs were 10
and 30 mg/kg bw/day for maternal and embryo/fetotoxicity,
respectively, with evidence of teratogenicity at 100 mg/kg bw/day.
In rabbits, using dose levels of 0, 10, 30 or 100 mg/kg bw/day, the
NOAEL for maternal toxicity was 10 mg/kg bw/day, while there was no
evidence of teratogenicity or embryo/fetotoxicity at 100 mg/kg
bw/day, the highest dose tested.
Propineb has been adequately tested in a series of genotoxicity
assays from which the Meeting concluded that it is not genotoxic.
An ADI was allocated to propineb, which was based on the NOAEL
from the short-term thyroid function study in rats (10 ppm, equal to
0.74 mg/kg bw/day) using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 200 ppm in the diet, equal to 26 mg/kg bw/day
(2-year study)
Rat: 10 ppm in the diet, equal to 0.74 mg/kg bw/day
(62-day thyroid function study)
50 ppm, equivalent to 2.5 mg/kg bw/day (2-year study)
Dog: 3000 ppm in the diet, equivalent to 75 mg/kg bw/day
(2-year study).
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Becker, H., Vogel, W. & Terrier, Ch. (1988) Embryotoxicity
(including teratogenicity) study with LH 30/Z in the rabbit. Report
R 4460, Research and Consulting Company AG, Itingen, Switzerland.
Unpublished report submitted to WHO by Bayer AG.
Brune, H., Deutsch-Wenzel, R., & Mohr, U. (1980) Toxicology
examination of propineb in a chronic feeding study on NMRI mice.
Biological laboratory of Dr. Brune (Hamburg), Report No. R 1792.
Unpublished report submitted to WHO by Bayer AG.
Cifone, M.A. (1987) Mutagenicity Test on LH 30/Z in the rat primary
hepatocyte unscheduled DNA synthesis assay. Hazleton Laboratories
America, Inc., Report No. TR 4086. Unpublished report submitted to
WHO by Bayer AG.
Ecker, W. (1975) l4C-Antracol, metabolism studies on orally dosed
rats. Pharma report No. PH 5352. Bayer AG, Isotope Chemistry
Laboratory, Institute for Pharmacokinetics. Unpublished report
submitted to WHO by Bayer AG.
Flucke, W. & Kimmerle, G. (1977) Propineb (LH 30/Z), triadimefon
(MEB 6447). study for acute toxicity after simultaneous
administration of both active ingredients. Bayer AG Institute of
Toxicology, Report No. 7065. Unpublished report submitted to WHO by
Bayer AG.
Flucke, W. (1989) Propineb: Study for skin sensitising effect on
guinea-pigs (Buhler's patch test). Bayer AG, Department of
Toxicology. Report No. 17734. Unpublished report submitted to WHO by
Bayer AG.
Hatano Institute, Food and Drug Safety Centre (1978): Report of the
mutagenicity study of Propineb. Unpublished report submitted to WHO
by Bayer AG.
Heimann, K.-G. (1987) Propineb, Study for skin sensitising effect on
guinea-pigs. Bayer AG, Institute of toxicology. Report No. 15897.
Unpublished report submitted to WHO by Bayer AG.
Herbold, B. (1980a) LH 30/Z (Propineb, Antracol - Active ingredient)
Salmonella/microsome test to evaluate for point mutation. Report
No 9321, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Herbold, B. (1982) LH 30/Z - Propineb - antracol - active ingredient
- micronucleus test in mice. Report No 10974, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Hoffmann, K. (1983) LH 30/Z (Antracol active ingredient), acute
toxicity for sheep after oral administration. Bayer AG Institute of
Toxicology, Report No. 11463. Unpublished report submitted to WHO by
Bayer AG.
Kimmerle, G. (1972) Comparative test of Antracol, Propylene
thiourea, zineb and ethylene thiourea for thyroid action in rats.
Report No 3551, Bayer AG, Institute of Toxicology. Unpublished
report submitted to WHO by Bayer AG.
Kroetlinger, F., Loeser, E., Kalinger, G. (1980) Propineb (antracol
active ingredient) - subchronic toxicology study to establish the
dose-time-effect relationship for the thyroid (feeding study over 9
weeks). Report No 9313, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Larsson, S.K., Arnander, C., Cekanova, E. & Kjellberg, M. (1976)
Studies of teratogenic effects of the Dithiocarbamates Maneb,
Mancozeb, and Propineb. Teratology, 14: 171-183.
Lehn, H. (1988) LH 30/Z - Mutagenicity study for the detection of
induced forward mutations in the CHO/HGPRT assay in vitro. Report
No 16840, Bayer AG, Department of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1967) Bay 46131 - Subchronic feeding study on dogs.
Report No 450, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1968) Bay 46131 - Study of the reversibility of
enlargement of thyroids in rats. Report No 836, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1969) Bay 46131 - Subchronic toxicology study in rats
(investigation over 3 months). Report No 1500, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973a) Bay 46131 - Chronic toxicity study in dogs
(duration 2 years). Report No 4213, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973b) Bay 46131 - Multigeneration study in rats. Report
No 4214, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (l974a) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4927, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (l974b) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4608, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974a) LH 30/Z (antracol active ingredient) -
investigation of embryotoxicity and teratogenicity in rats following
oral administration. Report No 4895, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974b): LH 30/Z (antracol active ingredient) -
dominant lethal test in the male mouse. Report No 4795, Bayer AG,
Institute of Toxicology. Unpublished report submitted to WHO by
Bayer AG.
Mihail, F. & Kaliner, G. (1979) LH 30/Z (antracol active
ingredient), subacute dermal cumulative toxicity study on rabbits.
Bayer AG Institute of Toxicology, Report No. 8322. Unpublished
report submitted to WHO by Bayer AG.
Mueller, H. (1988) Internal company memorandum, Bayer AG Medical
Department, to Dr Machemer, Bayer AG, Institute of Toxicology.
Unpublished document submitted to WHO by Bayer AG.
Patschke, K., Wegner, L.A. (1975) Antracol-l4C, biokinetic studies
on rats after oral application. Report No. PH 5268. Bayer AG,
Institute for Pharmacokinetics. Unpublished report submitted to WHO
by Bayer AG.
Rolandi, A., De Marinis, E. & De Caterina, M. (1984) Dithiocarbamate
pesticides, activity of propineb in the micronucleus test in mice.
Mut. Res. 135: 193-197.
Thyssen, J. & Kimmerle, G. (1978) Antracol active ingredient (LH
30/Z), acute toxicological study. Bayer AG Institute of Toxicology,
Report No. 7208. Unpublished report submitted to WHO by Bayer AG.
Thyssen, J. & Mohr (1979) Antracol active ingredient, subacute
inhalation study on rats. Bayer AG Institute of Toxicology, Report
No. 8334. Unpublished report submitted to WHO by Bayer AG.
Vicari, L., De Dominicis, G., Vito, M., Placido, C., de Marinis, E.
(1985) Teratogenic and goitrogenic activity of propineb and
propylene thiourea in the Rat. Boll. Soc. It. Biol. 61: 271-278.
Weber, H. (1980) - Biokinetics of antracol-14C (PH-Report No 5268)
and propylene thiourea (PH-Report No 7397). Bayer AG, Institute. for
Pharmacokinetics. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Dressler, H.F. (1980) Effect on thyroid function of male
rats following subchronic administration of propineb (antracol-
active ingredient). Report No 9268, Bayer AG, Isotope and Biokinetic
Laboratory. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Mager, H., Hartman, E. (1991) Propineb feeding study:
Effect on thyroid function in male Wistar rats at low dose levels up
to 10 ppm for a time interval up to 63 days. Bayer AG Department of
Toxicology. Report No. PF 3475. Unpublished report submitted to WHO
by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Table 1. Acute toxicity of propineb
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/m3)
Mouse M/F oral > 5000 Thyssen & Kimmerle, 1978
Mouse M/F s.c. 1500-2000 Thyssen & Kimmerle, 1978
Mouse M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F oral > 5000 Thyssen & Kimmerle, 1978
Rat F oral 5900 Flucke & Kimmerle, 1977
Rat M/F dermal > 5000 Thyssen & Kimmerle, 1978
Rat M/F i.p. 102-141 Thyssen & Kimmerle, 1978
Rat M/F inhalation > 522 (1 x 1 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 693 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 193 (5 x 4 h) Thyssen & Kimmerle, 1978
Hamster M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Cat M oral > 500 Thyssen & Kimmerle, 1978
Sheep M/F oral 2500 Hoffmann, 1983
Short-term toxicity studies
Rats
Groups of 50 male and 50 female Wistar rats received diets
containing propineb (technical material, 93.5% purity) at
concentrations of 0, 100 or 500 ppm. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. Half of the rats from each group were
sacrificed after 6 months, the remainder were sacrificed after a
subsequent withdrawal period of 8 weeks. Treatment with 100 ppm did
not cause thyroid enlargement in either sex, whereas treatment with
500 ppm caused marked thyroid enlargement in both males and females.
During the 8-week withdrawal period the enlargement receded in male
rats, but relative thyroid weights were still higher than control
values in females, indicating a tendency to reversibility (Loeser,
1968).
In a 3-month feeding study in Wistar rats, groups of 15 males
and 15 females received dietary levels of 0, 5, 10, 25, 50 or 100
ppm propineb (87% purity). The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. There was no treatment-related effect on
behaviour, survival, food intake or weight gain. Haematological and
urinalysis investigations revealed no indication of any reaction to
treatment. Clinical chemistry investigations revealed increased
sorbitol dehydrogenase activity in serum in male rats treated with
50 or 100 ppm and increased serum lactate dehydrogenase activity in
males and females treated with 100 ppm. Gross pathology and organ
weight analysis revealed no indication of any reaction to treatment.
No histopathological examination of tissues was performed and it was
thus considered inappropriate to derive an NOAEL from the results of
this study (Loeser, 1969).
Groups of 10 male and 10 female Wistar TNO/W74 rats were
exposed to propineb aerosols at analytically-determined
concentrations of 0, 5, 8, 29, or 44 mg/m3, five times per week for
6 hours, for 3 weeks. Rats exposed to 44 mg/m3 exhibited severely
disturbed behaviour including paralysis of the extremities. All the
female rats and 1 male rat at this exposure level died by the 13th
exposure. Animals that died had sharp losses in body weight and
were cachectic. Gross necropsies revealed small spleens and livers,
enlarged adrenals, and inflamed lungs. Histopathology revealed
acute vasculature congestion in the lungs, liver, kidneys, and
bronchial lymph nodes, indicating that the cause of death was
cardiovascular failure. One male rat at 29 mg/m3 also exhibited
hind limb paralysis but survived to the end of the study. The
remaining test animals did not differ from control animals with
respect to appearance, behaviour or body-weight gains.
Haematological examinations, clinical chemistry, and urinalyses
(performed just prior to termination), and results of gross
necropsies, organ weight determinations, and histopathological
examinations revealed no indication of any reaction to treatment in
animals surviving to termination (Thyssen & Mohr, 1979).
In a study to compare the effects of propineb with various
other thyro-suppressive agents, groups of 50 male and 50 female
Wistar rats were treated orally with 50 mg/kg bw/day of the
following compounds: pure propineb, two technical samples of
propineb, PTU, ETU, zineb or methyl thiouracil as a positive
control. An untreated control was also included and 10 male and 10
female rats were killed after 7, 14 or 21 days treatment and after
14 or 28 days recovery. Investigations of thyroid activity was
limited to recording of thyroid weight, no biochemical analyses or
histopathology were performed. PTU, ETU and methyl thiouracil caused
significant increases in absolute and relative thyroid weight in
rats of both sexes, while the three propineb samples caused an
increase in the relative weight in females only. The thyroid
enlargement induced by propineb was reversible during the withdrawal
period, the effect of the other agents was partially reversible.
Propineb had only a moderate effect compared to methyl thiouracil,
whereas PTU had an equivalent effect to that of methyl thiouracil
and was somewhat stronger than ETU (Kimmerle, 1972).
Propineb of unknown purity was administered in the feed for 62
days to 5 groups of 80 male Wistar TNO/W74 rats at dosage levels of
0, 2, 10, 50, or 250 ppm. The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. Ten rats per group were killed for interim
sacrifice at 7 days or at 21 days. An additional 10 rats per group
were also sacrificed at 7, 21, or 62 days for "thyroid function
tests", which was reported separately (see Weber & Dressler, 1980).
The remaining animals were sacrificed at 62 days. No mortalities
occurred during the study and daily examinations indicated no
effects that could be attributed to the test material. Mean body
weights of animals in the 250 ppm group were slightly but
consistently lower than those of the control group throughout the
entire study, but food consumption was unaffected. Total thyroxin
was decreased in the 50 and 250 ppm groups at 21 and 62 days.
Thyroid weights were decreased in the 250 ppm group at 7 days, but
later increased in the 50 and 250 ppm groups at 62 days.
Histopathology did not reveal any treatment-related changes in the
thyroid. Organ weight analysis and histopathological examinations
of liver and adrenals revealed no effects that could be attributed
to the test material. The NOAEL in this study was 10 ppm (equal to
0.74 mg/kg bw/day), based on changes in thyroxin concentration and
increased thyroid weight at 50 and 250 ppm (Kroetlinger et al.,
1980).
Ten male rats per group from the above-described study were
used for "thyroid function tests" at 7, 21, or 62 days. In
addition, iodine accumulation in the thyroid was assayed 24 hours
after oral intubation of 0.2 µCi of 131I-NaI tracer by measuring
radioactivity in excised and weighed thyroid tissue. Concentrations
of T3 and T4 in the serum were also determined by use of a
commercially-available 125I radioimmunoassay test system. Mean
thyroid weights were increased above control values in the 50 ppm
group at 62 days, and in the 250 ppm group at 21 days and at 62
days. A decrease in mean thyroid weights was observed in the 2 ppm
group at 7 days. Mean iodine accumulation in the thyroid, expressed
as the amount of radioactive iodine per mg of tissue, was decreased
below control values in the 50 ppm group at 62 days and in the 250
ppm group at 21 and 62 days. Mean T3 concentrations were increased
above control values in the 2 ppm group and in the 10 ppm group at 7
days. A decrease in mean T3 was observed in the 250 ppm group at
62 days. Mean T4 concentrations were decreased below control
values in the 250 ppm group at 7, 21 and 62 days. The study results
indicate that propineb affects thyroid function at all dosage levels
tested. The effect appears to be dose-related. At 50 ppm and 250
ppm, iodine accumulation in the thyroid was reduced and at 250 ppm,
T3 and T4 serum levels were also reduced. A compensatory reaction
occurred, however, at these dosage levels, as evidenced by increased
thyroid weights and a return toward control values for several of
the other parameters. At 2 and 10 ppm, an effect on thyroid
function was indicated by the increased levels at 7 days of T3, the
more biologically-active form of the thyroid hormone. However, this
is considered to be a normal physiological response, without lasting
adverse effect. The NOAEL in this study was thus 10 ppm, equal to
0.74 mg/kg bw/day (Weber & Dressler, 1980).
The effect of propineb on thyroid function was investigated in
a 63-day feeding study in Wistar rats. Groups of 36 males received
dietary concentrations of 0, 0.2, 0.6, 2 or 10 ppm propineb.
Technical material of 83.3% purity was used, but dietary levels
refer to pure active ingredient. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
checked during the study and found to be adequate. On days 7, 21
and 63 thyroid function was investigated by determination of thyroid
weight, measurement of 131I incorporation into the thyroid and by
radio immunoassay determination of T3, T4 and TSH in the serum.
Food and water intake was not affected by treatment, but body-weight
gain was slightly depressed at 2 and 10 ppm in comparison with
controls. Thyroid weights were unaffected by treatment at any of
the examinations. Although minor inter-group differences in results
of other investigations attained a level of statistical significance
when compared with controls, there were no consistent, dose-related
effects. Histopathological examination of thyroids was conducted
later and this revealed a minimal to slight hypertrophy in the
follicular epithelium of 2 of 10 rats treated with 10 ppm for 63
days. No similar changes were seen at the same dose level after
shorter treatment periods or at lower doses at any of the treatment
periods. The NOAEL in this study was 10 ppm since the minor
histological changes seen in thyroid at 10 ppm were not considered
to be a permanent adverse effect in rats. The apparent changes in
body-weight gain were disregarded, since this effect was not
supported by results of any other feeding study with propineb in
rats, and there were large inter-group differences in initial body
weight in this study, which hampered interpretation of the weight
gain data (Weber et al., 1991).
Rabbits
Technical grade propineb of 87.3% purity was dermally applied
to the shaven backs of groups of 6/sex New Zeeland white rabbits
(one half of which had intact and one half abraded skin test sites),
5 times per week for 7 hours for 3 weeks at dosage levels of 0, 50,
or 250 mg/kg bw/day. Three male rabbits died during the study of
severe pneumonitis. Daily observations, including skin reactions,
revealed no effects attributable to the test material. No
differences in body weights between control and test animals were
observed. All clinical laboratory tests, gross necropsies and
histopathological examinations revealed no indication of any
reaction to treatment with propineb. With the exception of slightly
increased liver weights in female rabbits treated with 250 mg/kg
bw/day, organ weights in control and test animals were similar
(Mihail & Kaliner, 1979).
Dogs
In a 4-month feeding study, groups of beagle dogs (2/sex)
received dietary concentrations of 0, 100, 400 or 1600 ppm propineb
(86% purity). The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The results of this study revealed no indication of any
reaction to treatment in terms of survival, behaviour, food intake,
weight gain, clinical laboratory investigations, gross pathology and
organ weight analysis. No histopathological examination of tissues
was carried out and it is thus considered inappropriate to derive an
NOAEL from the results of this study (Loeser, 1967).
In a 2-year feeding study, groups of 4 male and 4 female beagle
dogs received dietary concentrations of 0, 100, 300, 1000 or 3000
ppm propineb (purity approximately 86%). The summarized results of
this study revealed no indication of any reaction to treatment in
terms of survival, behaviour, food intake, weight gain, clinical
laboratory investigations, gross pathology, organ weight analysis or
histopathology. The NOAEL for this study may therefore be defined
as 3000 ppm (equivalent to 75 mg/kg bw/day), the highest dose level
tested. A complete detailed report of this study was not available
for evaluation (Loser, 1973a).
Long-term toxicity/carcinogenicity studies
Mice
Propineb of 82.9% purity was administered in the feed to groups
of NMRI mice (50/sex) for 104 weeks at dietary levels of 0, 50, 200,
or 800 ppm. The homogeneity, stability and accuracy of admixture of
the test material into the test diet was not checked during the
study. No substantial differences in mortality were observed
between male and female control and test groups, although the best
survival of any group was seen in the high-dose males. Clinical
observations did not indicate any differences between test and
control mice and food consumption and body-weight gain was similar
in male and female control and test groups. Haematological,
clinical chemistry, and urinalysis examinations did not reveal any
effects attributable to the test material. Gross necropsies were
reported to be negative for lesions attributable to the test
material. Organ weight analysis did not reveal any differences that
could be related to the test material. Non-neoplastic lesions were
not reported in the histopathological evaluation. Percentages of
female mice with tumours of any kind were 65, 66, 73, and 88% for
the control, low-, mid-, and high-dose groups, respectively. A
similar increase was not observed in treated male mice. Frequently-
observed neoplasms were those commonly seen in NMRI mice and
consisted of pulmonary adenomas and malignant lymphomas in male and
female mice and benign granulosa cell tumours in the ovaries of
female mice. Thyroid or adrenal tumours were not increased in
treated male or female mice. An increased incidence of
hepatocellular adenomas was observed in the high-dose male group.
Percentages were 6, 7, 0, and 20 for the control, low, mid-, and
high-dose male groups, respectively. Hepatocellular carcinomas in
male mice ranged from 0 to 4%, but their incidence was not dose-
related. Historical control data presented by the testing
laboratory for hepatocellular adenomas in NMRI mice ranged from 4 to
12%. The increase in hepatocellular adenomas in the high-dose male
group may possibly be related to treatment with the test material,
but may have been affected by the higher survival rate in this group
compared to the controls. Hepatocellular tumours were not increased
in treated female mice. The NOAEL in this study was 200 ppm (equal
to 26 mg/kg bw/day), based on the equivocal alteration in hepatic
tumour incidence at 800 ppm (Brune et al., 1980).
Rats
Groups of Wistar rats (25/sex/dose and 50/sex/control group)
were fed diets containing propineb (technical material, 93.5%
purity) at dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000 ppm
for 2 years. The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The mortality rate was increased in comparison with controls
at 1000 ppm and above, such that at 2000 and 8000 ppm in females and
at 8000 ppm in males, no rats survived to termination. Dose-related
reduced weight gain and food intake were also evident at 1000, 2000
and 8000 ppm. Apparent muscular weakness, leading to paralysis
(described as myasthenia) was seen in rats receiving 1000, 2000 or
8000 ppm. The results of limited clinical chemistry investigations
(conducted at 16 and 24 months) did not reveal any indication of
reaction to treatment. At autopsy, increased kidney, liver and
thyroid weights were noted at 100 ppm and above. Histopathology
revealed no changes in kidney and liver, but did show evidence of
degeneration of skeletal muscle at 1000 ppm and above, along with an
increased incidence of TSH-related thyroid tumours in these groups.
The NOAEL in this study was thus 10 ppm (equivalent to 0.5 mg/kg
bw/day) based on the organ weight changes noted at 100 ppm and above
(Loeser, 1974a).
In a second study, carried out in order to more closely define
the NOAEL in long-term administration to rats, groups of 40 male and
40 female Wistar rats (95/sex in the control group) were fed diets
containing propineb (93.5% purity) at dietary levels of 0, 5, 10,
25, 50 or 100 ppm for 2 years. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. There were no clinical signs of
reaction to treatment and no effects on food consumption, growth
rate or mortality at any dose level. Limited clinical chemistry
analyses revealed that serum ALT and AST activities were slightly
higher than control values after 52 weeks in rats receiving 100 ppm,
but no similar effect was seen at termination. Serum PBI levels were
lower than control values, at 52 weeks and prior to termination, in
rats receiving 100 ppm. At autopsy there were no macroscopic
abnormalities attributable to treatment and organ weight analysis
revealed no significant intergroup differences. Histopathology
revealed no changes considered to be related to the administration
of propineb, in particular, the nature, location and frequency of
tumours provided no indication of any carcinogenic effect. The
NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, in this study
(Loeser, 1974b).
Reproduction studies
In a 3-generation study in rats, with two litters per
generation, groups of 10 male and 20 female Long Evans rats received
propineb (93.5% purity) at dietary levels of 0, 20, 60, 200 or 600
ppm. The homogeneity, stability and accuracy of admixture of the
test material into the test diet was not checked during the study.
Treatment with 200 ppm led to slight symptoms of hind limb paralysis
in parental animals, while treatment with 600 ppm led to increased
mortality, decreased weight gain and severe hind limb paralysis.
With regard to the reproductive parameters, treatment with 200 ppm
was associated with slightly lower gestation rates and a lower
number of pups per litter after one mating, while treatment with 600
ppm was associated with substantially lower gestation rates (up to
50%) after nearly all matings and lower birth weight and lower
numbers of pups per litter after some matings. Lactation
performance of dams receiving 600 ppm which did have offspring was
not, however, adversely affected. The NOAEL was 60 ppm (equivalent
to 3 mg/kg bw/day) (Loeser, 1973b).
Special studies on embryo/fetotoxicity
Rats
Groups of 17-23 mated female rats were given propineb by oral
gavage at levels of 0, 3, 10, 30 or 100 mg/kg bw/day on days 6 to 15
of gestation. Treatment with 30 mg/kg bw/day was associated with
slight maternal toxicity (somnolence, ruffled coat, limpness), while
treatment with 100 mg/kg bw/day led to severe maternal toxicity
(paralysis of extremities, ataxia, dyspnoea, tremors), leading to
mortality in some cases. At this high dose, fetal growth was
depressed, and teratogenic effects (dysplasia of extremities) were
also seen. The NOAELs were 10 and 30 mg/kg bw/day for maternal and
embryo/fetotoxicity, respectively (Machemer, 1974a).
These results were supported by studies published in the
literature; in one study, doses greater than 400 mg/kg bw/day caused
abnormalities and maternal toxicity when given to rats on day 11 of
gestation and in another study, using doses of 0, 25, 50 100 or
200 mg/kg bw/day on days 6 to 16 of gestation, abnormalities and
maternal toxicity were observed at 100 and 200 mg/kg bw/day
(Larsson et al., 1976; Vicari et al., 1985).
Rabbits
Groups of 16 mated female rabbits were given propineb (83.9%
purity), by oral gavage, at dose levels of 0, 10, 30 or 100 mg/kg
bw/day from day 6 to day 18 of gestation. Signs of severe maternal
toxicity were seen at 100 mg/kg bw/day, including reduced weight
gain and food intake in comparison with controls, dyspnea and
ventro-lateral recumbency, and signs of abortion in two females.
Body-weight gain and food intake were also reduced at 30
mg/kg bw/day. Evaluation of reproduction parameters revealed a
dose-related increased post-implantation loss at 30 and 100 mg/kg
bw/day. Fetal parameters, including external, skeletal and visceral
examinations, revealed no effects associated with treatment with
propineb. The NOAEL for maternal toxicity was 10 mg/kg bw/day, while
there was no indication of embryo/fetotoxicity or teratogenicity at
100 mg/kg bw/day, the highest dose tested (Becker et al., 1988).
Special studies on genotoxicity
Results of genotoxicity tests on propineb are summarized in
Table 2. The Meeting concluded that propineb was not genotoxic.
Special studies on sensitization
Propineb (83.9% purity) was tested for possible skin
sensitizing potential in guinea-pigs in a Magnusson and Kligman
maximization test, employing a test group of 20 animals and two
Table 2. Results of genotoxicity assays on propineb
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium up to 2.5 mg/ 84.3% Negative Herbold, 1980a
TA98, TA100, TA1535, plate
TA1537
Ames test S. typhimurium up to 0.864 ? Negative Hatano Institute, 1978
TA98, TA100, TA1535, mg/plate
TA1537, TA1538
E. coli WP2
Preferential B. subtilis M45, H17 up to 0.864 ? Negative Hatano Institute, 1978
toxicity mg/plate
HGPRT forward CHO cells 0.11-60 mg/ml ? Negative Lehn, 1988
mutation assay
UDS Rat hepatocytes 5-30 mg/ml ? Negative Cifone, 1987
Micronucleus test Mouse 2 x 1000 or 2 x 85.7% Negative Herbold, 1982
2000 mg/kg bw
Micronucleus test Mouse not known ? Negative Rolandi et al., 1984
Dominant lethal NMRI mice 500 mg/kg bw ? Negative Machemer, 1974b
control groups of 10 animals each. Following intradermal induction
with 0.1% propineb and topical induction with 25% propineb, the
first challenge was carried out using 25% propineb. A positive
response was seen in all 20 test animals, against no positive
reactions in the first control group. A second challenge was
carried out using 2.5% propineb. Positive reactions were seen on
this occasion in 18 test animals against 4 in the control group.
This study therefore detected clear indications of the allergic
potential of propineb in guinea-pigs (Heimann, 1987).
In a further experiment, propineb (premix, 83.3% purity) was
tested for possible skin sensitizing potential in guinea-pigs in a
Buhler patch test employing a test group and two control groups of
12 animals each. Following epicutaneous induction with 50%
propineb, challenge was carried out with 2.5% and 50% propineb. The
test material was tolerated without reaction and this study revealed
no indication of a skin sensitizing potential of propineb in guinea-
pigs (Flucke, 1989).
Irritant and/or allergizing characteristics of propineb in
humans was not confirmed. Between 1964 (when production began) and
1988 no allergies or skin sensitizing reactions associated with
propineb were recorded, "even though skin contact had been frequent
as a result of cleaning work" (Mueller, 1988).
Observations in humans
No information available.
COMMENTS
Orally administered propineb in rats is rapidly absorbed and
excreted largely via urine and faeces. Although there was some
evidence of excretion by exhalation, the available metabolic data
(which detected PTU and propyleneurea as the main urinary
metabolites along with propylenediamine and a small amount of 4-
methylimidazoline) found no metabolites which could be considered as
potential intermediates for degradation to CO2. Study results
indicated that a proportion of the administered dose accumulates
temporarily in the thyroid. Although most elimination occurred
within 4 days of dosing, the half-life of elimination for the
proportion remaining was relatively long. This could be due to
incorporation of portion of the molecule into endogenous substances
following metabolism, which would also account for the radioactivity
eliminated with exhaled air.
Propineb has moderate to low acute toxicity in mice, rats,
hamsters, cats and sheep. WHO has classified propineb as unlikely
to present acute hazard in normal use.
The results of toxicity studies clearly indicate that propineb
has a goitrogenic effect in rats, although no similar finding was
noted in rabbits or dogs. In a 62-day study in male rats using
dietary levels of 0, 2, 10, 50 or 250 ppm, the NOAEL was 10 ppm
(equal to 0.74 mg/kg bw/day), based on changes in thyroxine
concentration and increased thyroid weight at higher doses.
Although in a later 63-day study using dietary levels of 0, 0.2,
0.6, 2 or 10 ppm slight hyperplasia was seen in the thyroid in 2
rats out of 10 treated with 10 ppm, this finding was considered not
to be a permanent adverse effect in rats.
In a comparative study in rats of the effects of propineb, PTU,
ETU, zineb and methyl thiouracil on thyroid weight, propineb had
only a moderate effect compared to methyl thiouracil, whereas PTU
had an equivalent effect to that of methyl thiouracil and was
somewhat stronger than ETU. These results suggest that the effects
of propineb on the thyroid in rats may be caused primarily by the
metabolite, PTU. Dogs tolerated much higher doses of propineb,
dietary administration of 3000 ppm, equivalent to 75 mg/kg bw/day,
causing no adverse effects over 2 years.
In long-term studies, treatment-related alterations in tumour
incidence were seen in rats and mice. In mice treated with 0, 50,
200 or 800 ppm, an increase in hepatocellular adenomas was seen in
males only, at the highest dose tested, but there was no increase in
the incidence of hepatic carcinomas and no similar effect in
females. The NOAEL was 200 ppm, equal to 26 mg/kg bw/day, based on
this change in hepatic tumour incidence. In rats, there were two
studies, using dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000
ppm in the first and 0, 5, 10, 25, 50 or 100 ppm in the second. The
overall NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on
increased kidney and liver weight (without histological correlation)
and increased thyroid weight at 100 ppm and above. An increase in
thyrotropin-related thyroid tumours and skeletal muscle degeneration
was seen in rats at dietary levels of 1000 ppm and greater, but
these doses were accompanied by increased mortality.
In a three-generation reproduction study in rats using dietary
levels of 0, 20, 60, 200 or 600 ppm the NOAEL was 60 ppm, equivalent
to 3 mg/kg bw/day, with adverse effects on maternal health and
impaired reproductive performance seen at higher doses.
Teratogenicity studies indicated that propineb has teratogenic
potential in rats, but no evidence of teratogenicity was seen, even
in the presence of maternal toxicity, in rabbits. In rats, using
dose levels of 0, 3, 10, 30 or 100 mg/kg bw/day, the NOAELs were 10
and 30 mg/kg bw/day for maternal and embryo/fetotoxicity,
respectively, with evidence of teratogenicity at 100 mg/kg bw/day.
In rabbits, using dose levels of 0, 10, 30 or 100 mg/kg bw/day, the
NOAEL for maternal toxicity was 10 mg/kg bw/day, while there was no
evidence of teratogenicity or embryo/fetotoxicity at 100 mg/kg
bw/day, the highest dose tested.
Propineb has been adequately tested in a series of genotoxicity
assays from which the Meeting concluded that it is not genotoxic.
An ADI was allocated to propineb, which was based on the NOAEL
from the short-term thyroid function study in rats (10 ppm, equal to
0.74 mg/kg bw/day) using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 200 ppm in the diet, equal to 26 mg/kg bw/day
(2-year study)
Rat: 10 ppm in the diet, equal to 0.74 mg/kg bw/day
(62-day thyroid function study)
50 ppm, equivalent to 2.5 mg/kg bw/day (2-year study)
Dog: 3000 ppm in the diet, equivalent to 75 mg/kg bw/day
(2-year study).
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Becker, H., Vogel, W. & Terrier, Ch. (1988) Embryotoxicity
(including teratogenicity) study with LH 30/Z in the rabbit. Report
R 4460, Research and Consulting Company AG, Itingen, Switzerland.
Unpublished report submitted to WHO by Bayer AG.
Brune, H., Deutsch-Wenzel, R., & Mohr, U. (1980) Toxicology
examination of propineb in a chronic feeding study on NMRI mice.
Biological laboratory of Dr. Brune (Hamburg), Report No. R 1792.
Unpublished report submitted to WHO by Bayer AG.
Cifone, M.A. (1987) Mutagenicity Test on LH 30/Z in the rat primary
hepatocyte unscheduled DNA synthesis assay. Hazleton Laboratories
America, Inc., Report No. TR 4086. Unpublished report submitted to
WHO by Bayer AG.
Ecker, W. (1975) l4C-Antracol, metabolism studies on orally dosed
rats. Pharma report No. PH 5352. Bayer AG, Isotope Chemistry
Laboratory, Institute for Pharmacokinetics. Unpublished report
submitted to WHO by Bayer AG.
Flucke, W. & Kimmerle, G. (1977) Propineb (LH 30/Z), triadimefon
(MEB 6447). study for acute toxicity after simultaneous
administration of both active ingredients. Bayer AG Institute of
Toxicology, Report No. 7065. Unpublished report submitted to WHO by
Bayer AG.
Flucke, W. (1989) Propineb: Study for skin sensitising effect on
guinea-pigs (Buhler's patch test). Bayer AG, Department of
Toxicology. Report No. 17734. Unpublished report submitted to WHO by
Bayer AG.
Hatano Institute, Food and Drug Safety Centre (1978): Report of the
mutagenicity study of Propineb. Unpublished report submitted to WHO
by Bayer AG.
Heimann, K.-G. (1987) Propineb, Study for skin sensitising effect on
guinea-pigs. Bayer AG, Institute of toxicology. Report No. 15897.
Unpublished report submitted to WHO by Bayer AG.
Herbold, B. (1980a) LH 30/Z (Propineb, Antracol - Active ingredient)
Salmonella/microsome test to evaluate for point mutation. Report
No 9321, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Herbold, B. (1982) LH 30/Z - Propineb - antracol - active ingredient
- micronucleus test in mice. Report No 10974, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Hoffmann, K. (1983) LH 30/Z (Antracol active ingredient), acute
toxicity for sheep after oral administration. Bayer AG Institute of
Toxicology, Report No. 11463. Unpublished report submitted to WHO by
Bayer AG.
Kimmerle, G. (1972) Comparative test of Antracol, Propylene
thiourea, zineb and ethylene thiourea for thyroid action in rats.
Report No 3551, Bayer AG, Institute of Toxicology. Unpublished
report submitted to WHO by Bayer AG.
Kroetlinger, F., Loeser, E., Kalinger, G. (1980) Propineb (antracol
active ingredient) - subchronic toxicology study to establish the
dose-time-effect relationship for the thyroid (feeding study over 9
weeks). Report No 9313, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Larsson, S.K., Arnander, C., Cekanova, E. & Kjellberg, M. (1976)
Studies of teratogenic effects of the Dithiocarbamates Maneb,
Mancozeb, and Propineb. Teratology, 14: 171-183.
Lehn, H. (1988) LH 30/Z - Mutagenicity study for the detection of
induced forward mutations in the CHO/HGPRT assay in vitro. Report
No 16840, Bayer AG, Department of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1967) Bay 46131 - Subchronic feeding study on dogs.
Report No 450, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1968) Bay 46131 - Study of the reversibility of
enlargement of thyroids in rats. Report No 836, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1969) Bay 46131 - Subchronic toxicology study in rats
(investigation over 3 months). Report No 1500, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973a) Bay 46131 - Chronic toxicity study in dogs
(duration 2 years). Report No 4213, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973b) Bay 46131 - Multigeneration study in rats. Report
No 4214, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (l974a) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4927, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (l974b) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4608, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974a) LH 30/Z (antracol active ingredient) -
investigation of embryotoxicity and teratogenicity in rats following
oral administration. Report No 4895, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974b): LH 30/Z (antracol active ingredient) -
dominant lethal test in the male mouse. Report No 4795, Bayer AG,
Institute of Toxicology. Unpublished report submitted to WHO by
Bayer AG.
Mihail, F. & Kaliner, G. (1979) LH 30/Z (antracol active
ingredient), subacute dermal cumulative toxicity study on rabbits.
Bayer AG Institute of Toxicology, Report No. 8322. Unpublished
report submitted to WHO by Bayer AG.
Mueller, H. (1988) Internal company memorandum, Bayer AG Medical
Department, to Dr Machemer, Bayer AG, Institute of Toxicology.
Unpublished document submitted to WHO by Bayer AG.
Patschke, K., Wegner, L.A. (1975) Antracol-l4C, biokinetic studies
on rats after oral application. Report No. PH 5268. Bayer AG,
Institute for Pharmacokinetics. Unpublished report submitted to WHO
by Bayer AG.
Rolandi, A., De Marinis, E. & De Caterina, M. (1984) Dithiocarbamate
pesticides, activity of propineb in the micronucleus test in mice.
Mut. Res. 135: 193-197.
Thyssen, J. & Kimmerle, G. (1978) Antracol active ingredient (LH
30/Z), acute toxicological study. Bayer AG Institute of Toxicology,
Report No. 7208. Unpublished report submitted to WHO by Bayer AG.
Thyssen, J. & Mohr (1979) Antracol active ingredient, subacute
inhalation study on rats. Bayer AG Institute of Toxicology, Report
No. 8334. Unpublished report submitted to WHO by Bayer AG.
Vicari, L., De Dominicis, G., Vito, M., Placido, C., de Marinis, E.
(1985) Teratogenic and goitrogenic activity of propineb and
propylene thiourea in the Rat. Boll. Soc. It. Biol. 61: 271-278.
Weber, H. (1980) - Biokinetics of antracol-14C (PH-Report No 5268)
and propylene thiourea (PH-Report No 7397). Bayer AG, Institute. for
Pharmacokinetics. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Dressler, H.F. (1980) Effect on thyroid function of male
rats following subchronic administration of propineb (antracol-
active ingredient). Report No 9268, Bayer AG, Isotope and Biokinetic
Laboratory. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Mager, H., Hartman, E. (1991) Propineb feeding study:
Effect on thyroid function in male Wistar rats at low dose levels up
to 10 ppm for a time interval up to 63 days. Bayer AG Department of
Toxicology. Report No. PF 3475. Unpublished report submitted to WHO
by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Table 1. Acute toxicity of propineb
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/m3)
Mouse M/F oral > 5000 Thyssen & Kimmerle, 1978
Mouse M/F s.c. 1500-2000 Thyssen & Kimmerle, 1978
Mouse M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F oral > 5000 Thyssen & Kimmerle, 1978
Rat F oral 5900 Flucke & Kimmerle, 1977
Rat M/F dermal > 5000 Thyssen & Kimmerle, 1978
Rat M/F i.p. 102-141 Thyssen & Kimmerle, 1978
Rat M/F inhalation > 522 (1 x 1 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 693 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 193 (5 x 4 h) Thyssen & Kimmerle, 1978
Hamster M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Cat M oral > 500 Thyssen & Kimmerle, 1978
Sheep M/F oral 2500 Hoffmann, 1983
Short-term toxicity studies
Rats
Groups of 50 male and 50 female Wistar rats received diets
containing propineb (technical material, 93.5% purity) at
concentrations of 0, 100 or 500 ppm. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. Half of the rats from each group were
sacrificed after 6 months, the remainder were sacrificed after a
subsequent withdrawal period of 8 weeks. Treatment with 100 ppm did
not cause thyroid enlargement in either sex, whereas treatment with
500 ppm caused marked thyroid enlargement in both males and females.
During the 8-week withdrawal period the enlargement receded in male
rats, but relative thyroid weights were still higher than control
values in females, indicating a tendency to reversibility (Loeser,
1968).
In a 3-month feeding study in Wistar rats, groups of 15 males
and 15 females received dietary levels of 0, 5, 10, 25, 50 or 100
ppm propineb (87% purity). The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. There was no treatment-related effect on
behaviour, survival, food intake or weight gain. Haematological and
urinalysis investigations revealed no indication of any reaction to
treatment. Clinical chemistry investigations revealed increased
sorbitol dehydrogenase activity in serum in male rats treated with
50 or 100 ppm and increased serum lactate dehydrogenase activity in
males and females treated with 100 ppm. Gross pathology and organ
weight analysis revealed no indication of any reaction to treatment.
No histopathological examination of tissues was performed and it was
thus considered inappropriate to derive an NOAEL from the results of
this study (Loeser, 1969).
Groups of 10 male and 10 female Wistar TNO/W74 rats were
exposed to propineb aerosols at analytically-determined
concentrations of 0, 5, 8, 29, or 44 mg/m3, five times per week for
6 hours, for 3 weeks. Rats exposed to 44 mg/m3 exhibited severely
disturbed behaviour including paralysis of the extremities. All the
female rats and 1 male rat at this exposure level died by the 13th
exposure. Animals that died had sharp losses in body weight and
were cachectic. Gross necropsies revealed small spleens and livers,
enlarged adrenals, and inflamed lungs. Histopathology revealed
acute vasculature congestion in the lungs, liver, kidneys, and
bronchial lymph nodes, indicating that the cause of death was
cardiovascular failure. One male rat at 29 mg/m3 also exhibited
hind limb paralysis but survived to the end of the study. The
remaining test animals did not differ from control animals with
respect to appearance, behaviour or body-weight gains.
Haematological examinations, clinical chemistry, and urinalyses
(performed just prior to termination), and results of gross
necropsies, organ weight determinations, and histopathological
examinations revealed no indication of any reaction to treatment in
animals surviving to termination (Thyssen & Mohr, 1979).
In a study to compare the effects of propineb with various
other thyro-suppressive agents, groups of 50 male and 50 female
Wistar rats were treated orally with 50 mg/kg bw/day of the
following compounds: pure propineb, two technical samples of
propineb, PTU, ETU, zineb or methyl thiouracil as a positive
control. An untreated control was also included and 10 male and 10
female rats were killed after 7, 14 or 21 days treatment and after
14 or 28 days recovery. Investigations of thyroid activity was
limited to recording of thyroid weight, no biochemical analyses or
histopathology were performed. PTU, ETU and methyl thiouracil caused
significant increases in absolute and relative thyroid weight in
rats of both sexes, while the three propineb samples caused an
increase in the relative weight in females only. The thyroid
enlargement induced by propineb was reversible during the withdrawal
period, the effect of the other agents was partially reversible.
Propineb had only a moderate effect compared to methyl thiouracil,
whereas PTU had an equivalent effect to that of methyl thiouracil
and was somewhat stronger than ETU (Kimmerle, 1972).
Propineb of unknown purity was administered in the feed for 62
days to 5 groups of 80 male Wistar TNO/W74 rats at dosage levels of
0, 2, 10, 50, or 250 ppm. The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. Ten rats per group were killed for interim
sacrifice at 7 days or at 21 days. An additional 10 rats per group
were also sacrificed at 7, 21, or 62 days for "thyroid function
tests", which was reported separately (see Weber & Dressler, 1980).
The remaining animals were sacrificed at 62 days. No mortalities
occurred during the study and daily examinations indicated no
effects that could be attributed to the test material. Mean body
weights of animals in the 250 ppm group were slightly but
consistently lower than those of the control group throughout the
entire study, but food consumption was unaffected. Total thyroxin
was decreased in the 50 and 250 ppm groups at 21 and 62 days.
Thyroid weights were decreased in the 250 ppm group at 7 days, but
later increased in the 50 and 250 ppm groups at 62 days.
Histopathology did not reveal any treatment-related changes in the
thyroid. Organ weight analysis and histopathological examinations
of liver and adrenals revealed no effects that could be attributed
to the test material. The NOAEL in this study was 10 ppm (equal to
0.74 mg/kg bw/day), based on changes in thyroxin concentration and
increased thyroid weight at 50 and 250 ppm (Kroetlinger et al.,
1980).
Ten male rats per group from the above-described study were
used for "thyroid function tests" at 7, 21, or 62 days. In
addition, iodine accumulation in the thyroid was assayed 24 hours
after oral intubation of 0.2 µCi of 131I-NaI tracer by measuring
radioactivity in excised and weighed thyroid tissue. Concentrations
of T3 and T4 in the serum were also determined by use of a
commercially-available 125I radioimmunoassay test system. Mean
thyroid weights were increased above control values in the 50 ppm
group at 62 days, and in the 250 ppm group at 21 days and at 62
days. A decrease in mean thyroid weights was observed in the 2 ppm
group at 7 days. Mean iodine accumulation in the thyroid, expressed
as the amount of radioactive iodine per mg of tissue, was decreased
below control values in the 50 ppm group at 62 days and in the 250
ppm group at 21 and 62 days. Mean T3 concentrations were increased
above control values in the 2 ppm group and in the 10 ppm group at 7
days. A decrease in mean T3 was observed in the 250 ppm group at
62 days. Mean T4 concentrations were decreased below control
values in the 250 ppm group at 7, 21 and 62 days. The study results
indicate that propineb affects thyroid function at all dosage levels
tested. The effect appears to be dose-related. At 50 ppm and 250
ppm, iodine accumulation in the thyroid was reduced and at 250 ppm,
T3 and T4 serum levels were also reduced. A compensatory reaction
occurred, however, at these dosage levels, as evidenced by increased
thyroid weights and a return toward control values for several of
the other parameters. At 2 and 10 ppm, an effect on thyroid
function was indicated by the increased levels at 7 days of T3, the
more biologically-active form of the thyroid hormone. However, this
is considered to be a normal physiological response, without lasting
adverse effect. The NOAEL in this study was thus 10 ppm, equal to
0.74 mg/kg bw/day (Weber & Dressler, 1980).
The effect of propineb on thyroid function was investigated in
a 63-day feeding study in Wistar rats. Groups of 36 males received
dietary concentrations of 0, 0.2, 0.6, 2 or 10 ppm propineb.
Technical material of 83.3% purity was used, but dietary levels
refer to pure active ingredient. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
checked during the study and found to be adequate. On days 7, 21
and 63 thyroid function was investigated by determination of thyroid
weight, measurement of 131I incorporation into the thyroid and by
radio immunoassay determination of T3, T4 and TSH in the serum.
Food and water intake was not affected by treatment, but body-weight
gain was slightly depressed at 2 and 10 ppm in comparison with
controls. Thyroid weights were unaffected by treatment at any of
the examinations. Although minor inter-group differences in results
of other investigations attained a level of statistical significance
when compared with controls, there were no consistent, dose-related
effects. Histopathological examination of thyroids was conducted
later and this revealed a minimal to slight hypertrophy in the
follicular epithelium of 2 of 10 rats treated with 10 ppm for 63
days. No similar changes were seen at the same dose level after
shorter treatment periods or at lower doses at any of the treatment
periods. The NOAEL in this study was 10 ppm since the minor
histological changes seen in thyroid at 10 ppm were not considered
to be a permanent adverse effect in rats. The apparent changes in
body-weight gain were disregarded, since this effect was not
supported by results of any other feeding study with propineb in
rats, and there were large inter-group differences in initial body
weight in this study, which hampered interpretation of the weight
gain data (Weber et al., 1991).
Rabbits
Technical grade propineb of 87.3% purity was dermally applied
to the shaven backs of groups of 6/sex New Zeeland white rabbits
(one half of which had intact and one half abraded skin test sites),
5 times per week for 7 hours for 3 weeks at dosage levels of 0, 50,
or 250 mg/kg bw/day. Three male rabbits died during the study of
severe pneumonitis. Daily observations, including skin reactions,
revealed no effects attributable to the test material. No
differences in body weights between control and test animals were
observed. All clinical laboratory tests, gross necropsies and
histopathological examinations revealed no indication of any
reaction to treatment with propineb. With the exception of slightly
increased liver weights in female rabbits treated with 250 mg/kg
bw/day, organ weights in control and test animals were similar
(Mihail & Kaliner, 1979).
Dogs
In a 4-month feeding study, groups of beagle dogs (2/sex)
received dietary concentrations of 0, 100, 400 or 1600 ppm propineb
(86% purity). The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The results of this study revealed no indication of any
reaction to treatment in terms of survival, behaviour, food intake,
weight gain, clinical laboratory investigations, gross pathology and
organ weight analysis. No histopathological examination of tissues
was carried out and it is thus considered inappropriate to derive an
NOAEL from the results of this study (Loeser, 1967).
In a 2-year feeding study, groups of 4 male and 4 female beagle
dogs received dietary concentrations of 0, 100, 300, 1000 or 3000
ppm propineb (purity approximately 86%). The summarized results of
this study revealed no indication of any reaction to treatment in
terms of survival, behaviour, food intake, weight gain, clinical
laboratory investigations, gross pathology, organ weight analysis or
histopathology. The NOAEL for this study may therefore be defined
as 3000 ppm (equivalent to 75 mg/kg bw/day), the highest dose level
tested. A complete detailed report of this study was not available
for evaluation (Loser, 1973a).
Long-term toxicity/carcinogenicity studies
Mice
Propineb of 82.9% purity was administered in the feed to groups
of NMRI mice (50/sex) for 104 weeks at dietary levels of 0, 50, 200,
or 800 ppm. The homogeneity, stability and accuracy of admixture of
the test material into the test diet was not checked during the
study. No substantial differences in mortality were observed
between male and female control and test groups, although the best
survival of any group was seen in the high-dose males. Clinical
observations did not indicate any differences between test and
control mice and food consumption and body-weight gain was similar
in male and female control and test groups. Haematological,
clinical chemistry, and urinalysis examinations did not reveal any
effects attributable to the test material. Gross necropsies were
reported to be negative for lesions attributable to the test
material. Organ weight analysis did not reveal any differences that
could be related to the test material. Non-neoplastic lesions were
not reported in the histopathological evaluation. Percentages of
female mice with tumours of any kind were 65, 66, 73, and 88% for
the control, low-, mid-, and high-dose groups, respectively. A
similar increase was not observed in treated male mice. Frequently-
observed neoplasms were those commonly seen in NMRI mice and
consisted of pulmonary adenomas and malignant lymphomas in male and
female mice and benign granulosa cell tumours in the ovaries of
female mice. Thyroid or adrenal tumours were not increased in
treated male or female mice. An increased incidence of
hepatocellular adenomas was observed in the high-dose male group.
Percentages were 6, 7, 0, and 20 for the control, low, mid-, and
high-dose male groups, respectively. Hepatocellular carcinomas in
male mice ranged from 0 to 4%, but their incidence was not dose-
related. Historical control data presented by the testing
laboratory for hepatocellular adenomas in NMRI mice ranged from 4 to
12%. The increase in hepatocellular adenomas in the high-dose male
group may possibly be related to treatment with the test material,
but may have been affected by the higher survival rate in this group
compared to the controls. Hepatocellular tumours were not increased
in treated female mice. The NOAEL in this study was 200 ppm (equal
to 26 mg/kg bw/day), based on the equivocal alteration in hepatic
tumour incidence at 800 ppm (Brune et al., 1980).
Rats
Groups of Wistar rats (25/sex/dose and 50/sex/control group)
were fed diets containing propineb (technical material, 93.5%
purity) at dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000 ppm
for 2 years. The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The mortality rate was increased in comparison with controls
at 1000 ppm and above, such that at 2000 and 8000 ppm in females and
at 8000 ppm in males, no rats survived to termination. Dose-related
reduced weight gain and food intake were also evident at 1000, 2000
and 8000 ppm. Apparent muscular weakness, leading to paralysis
(described as myasthenia) was seen in rats receiving 1000, 2000 or
8000 ppm. The results of limited clinical chemistry investigations
(conducted at 16 and 24 months) did not reveal any indication of
reaction to treatment. At autopsy, increased kidney, liver and
thyroid weights were noted at 100 ppm and above. Histopathology
revealed no changes in kidney and liver, but did show evidence of
degeneration of skeletal muscle at 1000 ppm and above, along with an
increased incidence of TSH-related thyroid tumours in these groups.
The NOAEL in this study was thus 10 ppm (equivalent to 0.5 mg/kg
bw/day) based on the organ weight changes noted at 100 ppm and above
(Loeser, 1974a).
In a second study, carried out in order to more closely define
the NOAEL in long-term administration to rats, groups of 40 male and
40 female Wistar rats (95/sex in the control group) were fed diets
containing propineb (93.5% purity) at dietary levels of 0, 5, 10,
25, 50 or 100 ppm for 2 years. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. There were no clinical signs of
reaction to treatment and no effects on food consumption, growth
rate or mortality at any dose level. Limited clinical chemistry
analyses revealed that serum ALT and AST activities were slightly
higher than control values after 52 weeks in rats receiving 100 ppm,
but no similar effect was seen at termination. Serum PBI levels were
lower than control values, at 52 weeks and prior to termination, in
rats receiving 100 ppm. At autopsy there were no macroscopic
abnormalities attributable to treatment and organ weight analysis
revealed no significant intergroup differences. Histopathology
revealed no changes considered to be related to the administration
of propineb, in particular, the nature, location and frequency of
tumours provided no indication of any carcinogenic effect. The
NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, in this study
(Loeser, 1974b).
Reproduction studies
In a 3-generation study in rats, with two litters per
generation, groups of 10 male and 20 female Long Evans rats received
propineb (93.5% purity) at dietary levels of 0, 20, 60, 200 or 600
ppm. The homogeneity, stability and accuracy of admixture of the
test material into the test diet was not checked during the study.
Treatment with 200 ppm led to slight symptoms of hind limb paralysis
in parental animals, while treatment with 600 ppm led to increased
mortality, decreased weight gain and severe hind limb paralysis.
With regard to the reproductive parameters, treatment with 200 ppm
was associated with slightly lower gestation rates and a lower
number of pups per litter after one mating, while treatment with 600
ppm was associated with substantially lower gestation rates (up to
50%) after nearly all matings and lower birth weight and lower
numbers of pups per litter after some matings. Lactation
performance of dams receiving 600 ppm which did have offspring was
not, however, adversely affected. The NOAEL was 60 ppm (equivalent
to 3 mg/kg bw/day) (Loeser, 1973b).
Special studies on embryo/fetotoxicity
Rats
Groups of 17-23 mated female rats were given propineb by oral
gavage at levels of 0, 3, 10, 30 or 100 mg/kg bw/day on days 6 to 15
of gestation. Treatment with 30 mg/kg bw/day was associated with
slight maternal toxicity (somnolence, ruffled coat, limpness), while
treatment with 100 mg/kg bw/day led to severe maternal toxicity
(paralysis of extremities, ataxia, dyspnoea, tremors), leading to
mortality in some cases. At this high dose, fetal growth was
depressed, and teratogenic effects (dysplasia of extremities) were
also seen. The NOAELs were 10 and 30 mg/kg bw/day for maternal and
embryo/fetotoxicity, respectively (Machemer, 1974a).
These results were supported by studies published in the
literature; in one study, doses greater than 400 mg/kg bw/day caused
abnormalities and maternal toxicity when given to rats on day 11 of
gestation and in another study, using doses of 0, 25, 50 100 or
200 mg/kg bw/day on days 6 to 16 of gestation, abnormalities and
maternal toxicity were observed at 100 and 200 mg/kg bw/day
(Larsson et al., 1976; Vicari et al., 1985).
Rabbits
Groups of 16 mated female rabbits were given propineb (83.9%
purity), by oral gavage, at dose levels of 0, 10, 30 or 100 mg/kg
bw/day from day 6 to day 18 of gestation. Signs of severe maternal
toxicity were seen at 100 mg/kg bw/day, including reduced weight
gain and food intake in comparison with controls, dyspnea and
ventro-lateral recumbency, and signs of abortion in two females.
Body-weight gain and food intake were also reduced at 30
mg/kg bw/day. Evaluation of reproduction parameters revealed a
dose-related increased post-implantation loss at 30 and 100 mg/kg
bw/day. Fetal parameters, including external, skeletal and visceral
examinations, revealed no effects associated with treatment with
propineb. The NOAEL for maternal toxicity was 10 mg/kg bw/day, while
there was no indication of embryo/fetotoxicity or teratogenicity at
100 mg/kg bw/day, the highest dose tested (Becker et al., 1988).
Special studies on genotoxicity
Results of genotoxicity tests on propineb are summarized in
Table 2. The Meeting concluded that propineb was not genotoxic.
Special studies on sensitization
Propineb (83.9% purity) was tested for possible skin
sensitizing potential in guinea-pigs in a Magnusson and Kligman
maximization test, employing a test group of 20 animals and two
Table 2. Results of genotoxicity assays on propineb
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium up to 2.5 mg/ 84.3% Negative Herbold, 1980a
TA98, TA100, TA1535, plate
TA1537
Ames test S. typhimurium up to 0.864 ? Negative Hatano Institute, 1978
TA98, TA100, TA1535, mg/plate
TA1537, TA1538
E. coli WP2
Preferential B. subtilis M45, H17 up to 0.864 ? Negative Hatano Institute, 1978
toxicity mg/plate
HGPRT forward CHO cells 0.11-60 mg/ml ? Negative Lehn, 1988
mutation assay
UDS Rat hepatocytes 5-30 mg/ml ? Negative Cifone, 1987
Micronucleus test Mouse 2 x 1000 or 2 x 85.7% Negative Herbold, 1982
2000 mg/kg bw
Micronucleus test Mouse not known ? Negative Rolandi et al., 1984
Dominant lethal NMRI mice 500 mg/kg bw ? Negative Machemer, 1974b
control groups of 10 animals each. Following intradermal induction
with 0.1% propineb and topical induction with 25% propineb, the
first challenge was carried out using 25% propineb. A positive
response was seen in all 20 test animals, against no positive
reactions in the first control group. A second challenge was
carried out using 2.5% propineb. Positive reactions were seen on
this occasion in 18 test animals against 4 in the control group.
This study therefore detected clear indications of the allergic
potential of propineb in guinea-pigs (Heimann, 1987).
In a further experiment, propineb (premix, 83.3% purity) was
tested for possible skin sensitizing potential in guinea-pigs in a
Buhler patch test employing a test group and two control groups of
12 animals each. Following epicutaneous induction with 50%
propineb, challenge was carried out with 2.5% and 50% propineb. The
test material was tolerated without reaction and this study revealed
no indication of a skin sensitizing potential of propineb in guinea-
pigs (Flucke, 1989).
Irritant and/or allergizing characteristics of propineb in
humans was not confirmed. Between 1964 (when production began) and
1988 no allergies or skin sensitizing reactions associated with
propineb were recorded, "even though skin contact had been frequent
as a result of cleaning work" (Mueller, 1988).
Observations in humans
No information available.
COMMENTS
Orally administered propineb in rats is rapidly absorbed and
excreted largely via urine and faeces. Although there was some
evidence of excretion by exhalation, the available metabolic data
(which detected PTU and propyleneurea as the main urinary
metabolites along with propylenediamine and a small amount of 4-
methylimidazoline) found no metabolites which could be considered as
potential intermediates for degradation to CO2. Study results
indicated that a proportion of the administered dose accumulates
temporarily in the thyroid. Although most elimination occurred
within 4 days of dosing, the half-life of elimination for the
proportion remaining was relatively long. This could be due to
incorporation of portion of the molecule into endogenous substances
following metabolism, which would also account for the radioactivity
eliminated with exhaled air.
Propineb has moderate to low acute toxicity in mice, rats,
hamsters, cats and sheep. WHO has classified propineb as unlikely
to present acute hazard in normal use.
The results of toxicity studies clearly indicate that propineb
has a goitrogenic effect in rats, although no similar finding was
noted in rabbits or dogs. In a 62-day study in male rats using
dietary levels of 0, 2, 10, 50 or 250 ppm, the NOAEL was 10 ppm
(equal to 0.74 mg/kg bw/day), based on changes in thyroxine
concentration and increased thyroid weight at higher doses.
Although in a later 63-day study using dietary levels of 0, 0.2,
0.6, 2 or 10 ppm slight hyperplasia was seen in the thyroid in 2
rats out of 10 treated with 10 ppm, this finding was considered not
to be a permanent adverse effect in rats.
In a comparative study in rats of the effects of propineb, PTU,
ETU, zineb and methyl thiouracil on thyroid weight, propineb had
only a moderate effect compared to methyl thiouracil, whereas PTU
had an equivalent effect to that of methyl thiouracil and was
somewhat stronger than ETU. These results suggest that the effects
of propineb on the thyroid in rats may be caused primarily by the
metabolite, PTU. Dogs tolerated much higher doses of propineb,
dietary administration of 3000 ppm, equivalent to 75 mg/kg bw/day,
causing no adverse effects over 2 years.
In long-term studies, treatment-related alterations in tumour
incidence were seen in rats and mice. In mice treated with 0, 50,
200 or 800 ppm, an increase in hepatocellular adenomas was seen in
males only, at the highest dose tested, but there was no increase in
the incidence of hepatic carcinomas and no similar effect in
females. The NOAEL was 200 ppm, equal to 26 mg/kg bw/day, based on
this change in hepatic tumour incidence. In rats, there were two
studies, using dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000
ppm in the first and 0, 5, 10, 25, 50 or 100 ppm in the second. The
overall NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on
increased kidney and liver weight (without histological correlation)
and increased thyroid weight at 100 ppm and above. An increase in
thyrotropin-related thyroid tumours and skeletal muscle degeneration
was seen in rats at dietary levels of 1000 ppm and greater, but
these doses were accompanied by increased mortality.
In a three-generation reproduction study in rats using dietary
levels of 0, 20, 60, 200 or 600 ppm the NOAEL was 60 ppm, equivalent
to 3 mg/kg bw/day, with adverse effects on maternal health and
impaired reproductive performance seen at higher doses.
Teratogenicity studies indicated that propineb has teratogenic
potential in rats, but no evidence of teratogenicity was seen, even
in the presence of maternal toxicity, in rabbits. In rats, using
dose levels of 0, 3, 10, 30 or 100 mg/kg bw/day, the NOAELs were 10
and 30 mg/kg bw/day for maternal and embryo/fetotoxicity,
respectively, with evidence of teratogenicity at 100 mg/kg bw/day.
In rabbits, using dose levels of 0, 10, 30 or 100 mg/kg bw/day, the
NOAEL for maternal toxicity was 10 mg/kg bw/day, while there was no
evidence of teratogenicity or embryo/fetotoxicity at 100 mg/kg
bw/day, the highest dose tested.
Propineb has been adequately tested in a series of genotoxicity
assays from which the Meeting concluded that it is not genotoxic.
An ADI was allocated to propineb, which was based on the NOAEL
from the short-term thyroid function study in rats (10 ppm, equal to
0.74 mg/kg bw/day) using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 200 ppm in the diet, equal to 26 mg/kg bw/day
(2-year study)
Rat: 10 ppm in the diet, equal to 0.74 mg/kg bw/day
(62-day thyroid function study)
50 ppm, equivalent to 2.5 mg/kg bw/day (2-year study)
Dog: 3000 ppm in the diet, equivalent to 75 mg/kg bw/day
(2-year study).
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Becker, H., Vogel, W. & Terrier, Ch. (1988) Embryotoxicity
(including teratogenicity) study with LH 30/Z in the rabbit. Report
R 4460, Research and Consulting Company AG, Itingen, Switzerland.
Unpublished report submitted to WHO by Bayer AG.
Brune, H., Deutsch-Wenzel, R., & Mohr, U. (1980) Toxicology
examination of propineb in a chronic feeding study on NMRI mice.
Biological laboratory of Dr. Brune (Hamburg), Report No. R 1792.
Unpublished report submitted to WHO by Bayer AG.
Cifone, M.A. (1987) Mutagenicity Test on LH 30/Z in the rat primary
hepatocyte unscheduled DNA synthesis assay. Hazleton Laboratories
America, Inc., Report No. TR 4086. Unpublished report submitted to
WHO by Bayer AG.
Ecker, W. (1975) l4C-Antracol, metabolism studies on orally dosed
rats. Pharma report No. PH 5352. Bayer AG, Isotope Chemistry
Laboratory, Institute for Pharmacokinetics. Unpublished report
submitted to WHO by Bayer AG.
Flucke, W. & Kimmerle, G. (1977) Propineb (LH 30/Z), triadimefon
(MEB 6447). study for acute toxicity after simultaneous
administration of both active ingredients. Bayer AG Institute of
Toxicology, Report No. 7065. Unpublished report submitted to WHO by
Bayer AG.
Flucke, W. (1989) Propineb: Study for skin sensitising effect on
guinea-pigs (Buhler's patch test). Bayer AG, Department of
Toxicology. Report No. 17734. Unpublished report submitted to WHO by
Bayer AG.
Hatano Institute, Food and Drug Safety Centre (1978): Report of the
mutagenicity study of Propineb. Unpublished report submitted to WHO
by Bayer AG.
Heimann, K.-G. (1987) Propineb, Study for skin sensitising effect on
guinea-pigs. Bayer AG, Institute of toxicology. Report No. 15897.
Unpublished report submitted to WHO by Bayer AG.
Herbold, B. (1980a) LH 30/Z (Propineb, Antracol - Active ingredient)
Salmonella/microsome test to evaluate for point mutation. Report
No 9321, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Herbold, B. (1982) LH 30/Z - Propineb - antracol - active ingredient
- micronucleus test in mice. Report No 10974, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Hoffmann, K. (1983) LH 30/Z (Antracol active ingredient), acute
toxicity for sheep after oral administration. Bayer AG Institute of
Toxicology, Report No. 11463. Unpublished report submitted to WHO by
Bayer AG.
Kimmerle, G. (1972) Comparative test of Antracol, Propylene
thiourea, zineb and ethylene thiourea for thyroid action in rats.
Report No 3551, Bayer AG, Institute of Toxicology. Unpublished
report submitted to WHO by Bayer AG.
Kroetlinger, F., Loeser, E., Kalinger, G. (1980) Propineb (antracol
active ingredient) - subchronic toxicology study to establish the
dose-time-effect relationship for the thyroid (feeding study over 9
weeks). Report No 9313, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Larsson, S.K., Arnander, C., Cekanova, E. & Kjellberg, M. (1976)
Studies of teratogenic effects of the Dithiocarbamates Maneb,
Mancozeb, and Propineb. Teratology, 14: 171-183.
Lehn, H. (1988) LH 30/Z - Mutagenicity study for the detection of
induced forward mutations in the CHO/HGPRT assay in vitro. Report
No 16840, Bayer AG, Department of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1967) Bay 46131 - Subchronic feeding study on dogs.
Report No 450, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1968) Bay 46131 - Study of the reversibility of
enlargement of thyroids in rats. Report No 836, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1969) Bay 46131 - Subchronic toxicology study in rats
(investigation over 3 months). Report No 1500, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973a) Bay 46131 - Chronic toxicity study in dogs
(duration 2 years). Report No 4213, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973b) Bay 46131 - Multigeneration study in rats. Report
No 4214, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (l974a) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4927, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (l974b) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4608, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974a) LH 30/Z (antracol active ingredient) -
investigation of embryotoxicity and teratogenicity in rats following
oral administration. Report No 4895, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974b): LH 30/Z (antracol active ingredient) -
dominant lethal test in the male mouse. Report No 4795, Bayer AG,
Institute of Toxicology. Unpublished report submitted to WHO by
Bayer AG.
Mihail, F. & Kaliner, G. (1979) LH 30/Z (antracol active
ingredient), subacute dermal cumulative toxicity study on rabbits.
Bayer AG Institute of Toxicology, Report No. 8322. Unpublished
report submitted to WHO by Bayer AG.
Mueller, H. (1988) Internal company memorandum, Bayer AG Medical
Department, to Dr Machemer, Bayer AG, Institute of Toxicology.
Unpublished document submitted to WHO by Bayer AG.
Patschke, K., Wegner, L.A. (1975) Antracol-l4C, biokinetic studies
on rats after oral application. Report No. PH 5268. Bayer AG,
Institute for Pharmacokinetics. Unpublished report submitted to WHO
by Bayer AG.
Rolandi, A., De Marinis, E. & De Caterina, M. (1984) Dithiocarbamate
pesticides, activity of propineb in the micronucleus test in mice.
Mut. Res. 135: 193-197.
Thyssen, J. & Kimmerle, G. (1978) Antracol active ingredient (LH
30/Z), acute toxicological study. Bayer AG Institute of Toxicology,
Report No. 7208. Unpublished report submitted to WHO by Bayer AG.
Thyssen, J. & Mohr (1979) Antracol active ingredient, subacute
inhalation study on rats. Bayer AG Institute of Toxicology, Report
No. 8334. Unpublished report submitted to WHO by Bayer AG.
Vicari, L., De Dominicis, G., Vito, M., Placido, C., de Marinis, E.
(1985) Teratogenic and goitrogenic activity of propineb and
propylene thiourea in the Rat. Boll. Soc. It. Biol. 61: 271-278.
Weber, H. (1980) - Biokinetics of antracol-14C (PH-Report No 5268)
and propylene thiourea (PH-Report No 7397). Bayer AG, Institute. for
Pharmacokinetics. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Dressler, H.F. (1980) Effect on thyroid function of male
rats following subchronic administration of propineb (antracol-
active ingredient). Report No 9268, Bayer AG, Isotope and Biokinetic
Laboratory. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Mager, H., Hartman, E. (1991) Propineb feeding study:
Effect on thyroid function in male Wistar rats at low dose levels up
to 10 ppm for a time interval up to 63 days. Bayer AG Department of
Toxicology. Report No. PF 3475. Unpublished report submitted to WHO
by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Table 1. Acute toxicity of propineb
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/m3)
Mouse M/F oral > 5000 Thyssen & Kimmerle, 1978
Mouse M/F s.c. 1500-2000 Thyssen & Kimmerle, 1978
Mouse M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F oral > 5000 Thyssen & Kimmerle, 1978
Rat F oral 5900 Flucke & Kimmerle, 1977
Rat M/F dermal > 5000 Thyssen & Kimmerle, 1978
Rat M/F i.p. 102-141 Thyssen & Kimmerle, 1978
Rat M/F inhalation > 522 (1 x 1 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 693 (1 x 4 h) Thyssen & Kimmerle, 1978
Rat M/F inhalation > 193 (5 x 4 h) Thyssen & Kimmerle, 1978
Hamster M inhalation > 391 (1 x 4 h) Thyssen & Kimmerle, 1978
Cat M oral > 500 Thyssen & Kimmerle, 1978
Sheep M/F oral 2500 Hoffmann, 1983
Short-term toxicity studies
Rats
Groups of 50 male and 50 female Wistar rats received diets
containing propineb (technical material, 93.5% purity) at
concentrations of 0, 100 or 500 ppm. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. Half of the rats from each group were
sacrificed after 6 months, the remainder were sacrificed after a
subsequent withdrawal period of 8 weeks. Treatment with 100 ppm did
not cause thyroid enlargement in either sex, whereas treatment with
500 ppm caused marked thyroid enlargement in both males and females.
During the 8-week withdrawal period the enlargement receded in male
rats, but relative thyroid weights were still higher than control
values in females, indicating a tendency to reversibility (Loeser,
1968).
In a 3-month feeding study in Wistar rats, groups of 15 males
and 15 females received dietary levels of 0, 5, 10, 25, 50 or 100
ppm propineb (87% purity). The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. There was no treatment-related effect on
behaviour, survival, food intake or weight gain. Haematological and
urinalysis investigations revealed no indication of any reaction to
treatment. Clinical chemistry investigations revealed increased
sorbitol dehydrogenase activity in serum in male rats treated with
50 or 100 ppm and increased serum lactate dehydrogenase activity in
males and females treated with 100 ppm. Gross pathology and organ
weight analysis revealed no indication of any reaction to treatment.
No histopathological examination of tissues was performed and it was
thus considered inappropriate to derive an NOAEL from the results of
this study (Loeser, 1969).
Groups of 10 male and 10 female Wistar TNO/W74 rats were
exposed to propineb aerosols at analytically-determined
concentrations of 0, 5, 8, 29, or 44 mg/m3, five times per week for
6 hours, for 3 weeks. Rats exposed to 44 mg/m3 exhibited severely
disturbed behaviour including paralysis of the extremities. All the
female rats and 1 male rat at this exposure level died by the 13th
exposure. Animals that died had sharp losses in body weight and
were cachectic. Gross necropsies revealed small spleens and livers,
enlarged adrenals, and inflamed lungs. Histopathology revealed
acute vasculature congestion in the lungs, liver, kidneys, and
bronchial lymph nodes, indicating that the cause of death was
cardiovascular failure. One male rat at 29 mg/m3 also exhibited
hind limb paralysis but survived to the end of the study. The
remaining test animals did not differ from control animals with
respect to appearance, behaviour or body-weight gains.
Haematological examinations, clinical chemistry, and urinalyses
(performed just prior to termination), and results of gross
necropsies, organ weight determinations, and histopathological
examinations revealed no indication of any reaction to treatment in
animals surviving to termination (Thyssen & Mohr, 1979).
In a study to compare the effects of propineb with various
other thyro-suppressive agents, groups of 50 male and 50 female
Wistar rats were treated orally with 50 mg/kg bw/day of the
following compounds: pure propineb, two technical samples of
propineb, PTU, ETU, zineb or methyl thiouracil as a positive
control. An untreated control was also included and 10 male and 10
female rats were killed after 7, 14 or 21 days treatment and after
14 or 28 days recovery. Investigations of thyroid activity was
limited to recording of thyroid weight, no biochemical analyses or
histopathology were performed. PTU, ETU and methyl thiouracil caused
significant increases in absolute and relative thyroid weight in
rats of both sexes, while the three propineb samples caused an
increase in the relative weight in females only. The thyroid
enlargement induced by propineb was reversible during the withdrawal
period, the effect of the other agents was partially reversible.
Propineb had only a moderate effect compared to methyl thiouracil,
whereas PTU had an equivalent effect to that of methyl thiouracil
and was somewhat stronger than ETU (Kimmerle, 1972).
Propineb of unknown purity was administered in the feed for 62
days to 5 groups of 80 male Wistar TNO/W74 rats at dosage levels of
0, 2, 10, 50, or 250 ppm. The homogeneity, stability and accuracy
of admixture of the test material into the test diet was not checked
during the study. Ten rats per group were killed for interim
sacrifice at 7 days or at 21 days. An additional 10 rats per group
were also sacrificed at 7, 21, or 62 days for "thyroid function
tests", which was reported separately (see Weber & Dressler, 1980).
The remaining animals were sacrificed at 62 days. No mortalities
occurred during the study and daily examinations indicated no
effects that could be attributed to the test material. Mean body
weights of animals in the 250 ppm group were slightly but
consistently lower than those of the control group throughout the
entire study, but food consumption was unaffected. Total thyroxin
was decreased in the 50 and 250 ppm groups at 21 and 62 days.
Thyroid weights were decreased in the 250 ppm group at 7 days, but
later increased in the 50 and 250 ppm groups at 62 days.
Histopathology did not reveal any treatment-related changes in the
thyroid. Organ weight analysis and histopathological examinations
of liver and adrenals revealed no effects that could be attributed
to the test material. The NOAEL in this study was 10 ppm (equal to
0.74 mg/kg bw/day), based on changes in thyroxin concentration and
increased thyroid weight at 50 and 250 ppm (Kroetlinger et al.,
1980).
Ten male rats per group from the above-described study were
used for "thyroid function tests" at 7, 21, or 62 days. In
addition, iodine accumulation in the thyroid was assayed 24 hours
after oral intubation of 0.2 µCi of 131I-NaI tracer by measuring
radioactivity in excised and weighed thyroid tissue. Concentrations
of T3 and T4 in the serum were also determined by use of a
commercially-available 125I radioimmunoassay test system. Mean
thyroid weights were increased above control values in the 50 ppm
group at 62 days, and in the 250 ppm group at 21 days and at 62
days. A decrease in mean thyroid weights was observed in the 2 ppm
group at 7 days. Mean iodine accumulation in the thyroid, expressed
as the amount of radioactive iodine per mg of tissue, was decreased
below control values in the 50 ppm group at 62 days and in the 250
ppm group at 21 and 62 days. Mean T3 concentrations were increased
above control values in the 2 ppm group and in the 10 ppm group at 7
days. A decrease in mean T3 was observed in the 250 ppm group at
62 days. Mean T4 concentrations were decreased below control
values in the 250 ppm group at 7, 21 and 62 days. The study results
indicate that propineb affects thyroid function at all dosage levels
tested. The effect appears to be dose-related. At 50 ppm and 250
ppm, iodine accumulation in the thyroid was reduced and at 250 ppm,
T3 and T4 serum levels were also reduced. A compensatory reaction
occurred, however, at these dosage levels, as evidenced by increased
thyroid weights and a return toward control values for several of
the other parameters. At 2 and 10 ppm, an effect on thyroid
function was indicated by the increased levels at 7 days of T3, the
more biologically-active form of the thyroid hormone. However, this
is considered to be a normal physiological response, without lasting
adverse effect. The NOAEL in this study was thus 10 ppm, equal to
0.74 mg/kg bw/day (Weber & Dressler, 1980).
The effect of propineb on thyroid function was investigated in
a 63-day feeding study in Wistar rats. Groups of 36 males received
dietary concentrations of 0, 0.2, 0.6, 2 or 10 ppm propineb.
Technical material of 83.3% purity was used, but dietary levels
refer to pure active ingredient. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
checked during the study and found to be adequate. On days 7, 21
and 63 thyroid function was investigated by determination of thyroid
weight, measurement of 131I incorporation into the thyroid and by
radio immunoassay determination of T3, T4 and TSH in the serum.
Food and water intake was not affected by treatment, but body-weight
gain was slightly depressed at 2 and 10 ppm in comparison with
controls. Thyroid weights were unaffected by treatment at any of
the examinations. Although minor inter-group differences in results
of other investigations attained a level of statistical significance
when compared with controls, there were no consistent, dose-related
effects. Histopathological examination of thyroids was conducted
later and this revealed a minimal to slight hypertrophy in the
follicular epithelium of 2 of 10 rats treated with 10 ppm for 63
days. No similar changes were seen at the same dose level after
shorter treatment periods or at lower doses at any of the treatment
periods. The NOAEL in this study was 10 ppm since the minor
histological changes seen in thyroid at 10 ppm were not considered
to be a permanent adverse effect in rats. The apparent changes in
body-weight gain were disregarded, since this effect was not
supported by results of any other feeding study with propineb in
rats, and there were large inter-group differences in initial body
weight in this study, which hampered interpretation of the weight
gain data (Weber et al., 1991).
Rabbits
Technical grade propineb of 87.3% purity was dermally applied
to the shaven backs of groups of 6/sex New Zeeland white rabbits
(one half of which had intact and one half abraded skin test sites),
5 times per week for 7 hours for 3 weeks at dosage levels of 0, 50,
or 250 mg/kg bw/day. Three male rabbits died during the study of
severe pneumonitis. Daily observations, including skin reactions,
revealed no effects attributable to the test material. No
differences in body weights between control and test animals were
observed. All clinical laboratory tests, gross necropsies and
histopathological examinations revealed no indication of any
reaction to treatment with propineb. With the exception of slightly
increased liver weights in female rabbits treated with 250 mg/kg
bw/day, organ weights in control and test animals were similar
(Mihail & Kaliner, 1979).
Dogs
In a 4-month feeding study, groups of beagle dogs (2/sex)
received dietary concentrations of 0, 100, 400 or 1600 ppm propineb
(86% purity). The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The results of this study revealed no indication of any
reaction to treatment in terms of survival, behaviour, food intake,
weight gain, clinical laboratory investigations, gross pathology and
organ weight analysis. No histopathological examination of tissues
was carried out and it is thus considered inappropriate to derive an
NOAEL from the results of this study (Loeser, 1967).
In a 2-year feeding study, groups of 4 male and 4 female beagle
dogs received dietary concentrations of 0, 100, 300, 1000 or 3000
ppm propineb (purity approximately 86%). The summarized results of
this study revealed no indication of any reaction to treatment in
terms of survival, behaviour, food intake, weight gain, clinical
laboratory investigations, gross pathology, organ weight analysis or
histopathology. The NOAEL for this study may therefore be defined
as 3000 ppm (equivalent to 75 mg/kg bw/day), the highest dose level
tested. A complete detailed report of this study was not available
for evaluation (Loser, 1973a).
Long-term toxicity/carcinogenicity studies
Mice
Propineb of 82.9% purity was administered in the feed to groups
of NMRI mice (50/sex) for 104 weeks at dietary levels of 0, 50, 200,
or 800 ppm. The homogeneity, stability and accuracy of admixture of
the test material into the test diet was not checked during the
study. No substantial differences in mortality were observed
between male and female control and test groups, although the best
survival of any group was seen in the high-dose males. Clinical
observations did not indicate any differences between test and
control mice and food consumption and body-weight gain was similar
in male and female control and test groups. Haematological,
clinical chemistry, and urinalysis examinations did not reveal any
effects attributable to the test material. Gross necropsies were
reported to be negative for lesions attributable to the test
material. Organ weight analysis did not reveal any differences that
could be related to the test material. Non-neoplastic lesions were
not reported in the histopathological evaluation. Percentages of
female mice with tumours of any kind were 65, 66, 73, and 88% for
the control, low-, mid-, and high-dose groups, respectively. A
similar increase was not observed in treated male mice. Frequently-
observed neoplasms were those commonly seen in NMRI mice and
consisted of pulmonary adenomas and malignant lymphomas in male and
female mice and benign granulosa cell tumours in the ovaries of
female mice. Thyroid or adrenal tumours were not increased in
treated male or female mice. An increased incidence of
hepatocellular adenomas was observed in the high-dose male group.
Percentages were 6, 7, 0, and 20 for the control, low, mid-, and
high-dose male groups, respectively. Hepatocellular carcinomas in
male mice ranged from 0 to 4%, but their incidence was not dose-
related. Historical control data presented by the testing
laboratory for hepatocellular adenomas in NMRI mice ranged from 4 to
12%. The increase in hepatocellular adenomas in the high-dose male
group may possibly be related to treatment with the test material,
but may have been affected by the higher survival rate in this group
compared to the controls. Hepatocellular tumours were not increased
in treated female mice. The NOAEL in this study was 200 ppm (equal
to 26 mg/kg bw/day), based on the equivocal alteration in hepatic
tumour incidence at 800 ppm (Brune et al., 1980).
Rats
Groups of Wistar rats (25/sex/dose and 50/sex/control group)
were fed diets containing propineb (technical material, 93.5%
purity) at dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000 ppm
for 2 years. The homogeneity, stability and accuracy of admixture
of the test material into the test diet was not checked during the
study. The mortality rate was increased in comparison with controls
at 1000 ppm and above, such that at 2000 and 8000 ppm in females and
at 8000 ppm in males, no rats survived to termination. Dose-related
reduced weight gain and food intake were also evident at 1000, 2000
and 8000 ppm. Apparent muscular weakness, leading to paralysis
(described as myasthenia) was seen in rats receiving 1000, 2000 or
8000 ppm. The results of limited clinical chemistry investigations
(conducted at 16 and 24 months) did not reveal any indication of
reaction to treatment. At autopsy, increased kidney, liver and
thyroid weights were noted at 100 ppm and above. Histopathology
revealed no changes in kidney and liver, but did show evidence of
degeneration of skeletal muscle at 1000 ppm and above, along with an
increased incidence of TSH-related thyroid tumours in these groups.
The NOAEL in this study was thus 10 ppm (equivalent to 0.5 mg/kg
bw/day) based on the organ weight changes noted at 100 ppm and above
(Loeser, 1974a).
In a second study, carried out in order to more closely define
the NOAEL in long-term administration to rats, groups of 40 male and
40 female Wistar rats (95/sex in the control group) were fed diets
containing propineb (93.5% purity) at dietary levels of 0, 5, 10,
25, 50 or 100 ppm for 2 years. The homogeneity, stability and
accuracy of admixture of the test material into the test diet was
not checked during the study. There were no clinical signs of
reaction to treatment and no effects on food consumption, growth
rate or mortality at any dose level. Limited clinical chemistry
analyses revealed that serum ALT and AST activities were slightly
higher than control values after 52 weeks in rats receiving 100 ppm,
but no similar effect was seen at termination. Serum PBI levels were
lower than control values, at 52 weeks and prior to termination, in
rats receiving 100 ppm. At autopsy there were no macroscopic
abnormalities attributable to treatment and organ weight analysis
revealed no significant intergroup differences. Histopathology
revealed no changes considered to be related to the administration
of propineb, in particular, the nature, location and frequency of
tumours provided no indication of any carcinogenic effect. The
NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, in this study
(Loeser, 1974b).
Reproduction studies
In a 3-generation study in rats, with two litters per
generation, groups of 10 male and 20 female Long Evans rats received
propineb (93.5% purity) at dietary levels of 0, 20, 60, 200 or 600
ppm. The homogeneity, stability and accuracy of admixture of the
test material into the test diet was not checked during the study.
Treatment with 200 ppm led to slight symptoms of hind limb paralysis
in parental animals, while treatment with 600 ppm led to increased
mortality, decreased weight gain and severe hind limb paralysis.
With regard to the reproductive parameters, treatment with 200 ppm
was associated with slightly lower gestation rates and a lower
number of pups per litter after one mating, while treatment with 600
ppm was associated with substantially lower gestation rates (up to
50%) after nearly all matings and lower birth weight and lower
numbers of pups per litter after some matings. Lactation
performance of dams receiving 600 ppm which did have offspring was
not, however, adversely affected. The NOAEL was 60 ppm (equivalent
to 3 mg/kg bw/day) (Loeser, 1973b).
Special studies on embryo/fetotoxicity
Rats
Groups of 17-23 mated female rats were given propineb by oral
gavage at levels of 0, 3, 10, 30 or 100 mg/kg bw/day on days 6 to 15
of gestation. Treatment with 30 mg/kg bw/day was associated with
slight maternal toxicity (somnolence, ruffled coat, limpness), while
treatment with 100 mg/kg bw/day led to severe maternal toxicity
(paralysis of extremities, ataxia, dyspnoea, tremors), leading to
mortality in some cases. At this high dose, fetal growth was
depressed, and teratogenic effects (dysplasia of extremities) were
also seen. The NOAELs were 10 and 30 mg/kg bw/day for maternal and
embryo/fetotoxicity, respectively (Machemer, 1974a).
These results were supported by studies published in the
literature; in one study, doses greater than 400 mg/kg bw/day caused
abnormalities and maternal toxicity when given to rats on day 11 of
gestation and in another study, using doses of 0, 25, 50 100 or
200 mg/kg bw/day on days 6 to 16 of gestation, abnormalities and
maternal toxicity were observed at 100 and 200 mg/kg bw/day
(Larsson et al., 1976; Vicari et al., 1985).
Rabbits
Groups of 16 mated female rabbits were given propineb (83.9%
purity), by oral gavage, at dose levels of 0, 10, 30 or 100 mg/kg
bw/day from day 6 to day 18 of gestation. Signs of severe maternal
toxicity were seen at 100 mg/kg bw/day, including reduced weight
gain and food intake in comparison with controls, dyspnea and
ventro-lateral recumbency, and signs of abortion in two females.
Body-weight gain and food intake were also reduced at 30
mg/kg bw/day. Evaluation of reproduction parameters revealed a
dose-related increased post-implantation loss at 30 and 100 mg/kg
bw/day. Fetal parameters, including external, skeletal and visceral
examinations, revealed no effects associated with treatment with
propineb. The NOAEL for maternal toxicity was 10 mg/kg bw/day, while
there was no indication of embryo/fetotoxicity or teratogenicity at
100 mg/kg bw/day, the highest dose tested (Becker et al., 1988).
Special studies on genotoxicity
Results of genotoxicity tests on propineb are summarized in
Table 2. The Meeting concluded that propineb was not genotoxic.
Special studies on sensitization
Propineb (83.9% purity) was tested for possible skin
sensitizing potential in guinea-pigs in a Magnusson and Kligman
maximization test, employing a test group of 20 animals and two
Table 2. Results of genotoxicity assays on propineb
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium up to 2.5 mg/ 84.3% Negative Herbold, 1980a
TA98, TA100, TA1535, plate
TA1537
Ames test S. typhimurium up to 0.864 ? Negative Hatano Institute, 1978
TA98, TA100, TA1535, mg/plate
TA1537, TA1538
E. coli WP2
Preferential B. subtilis M45, H17 up to 0.864 ? Negative Hatano Institute, 1978
toxicity mg/plate
HGPRT forward CHO cells 0.11-60 mg/ml ? Negative Lehn, 1988
mutation assay
UDS Rat hepatocytes 5-30 mg/ml ? Negative Cifone, 1987
Micronucleus test Mouse 2 x 1000 or 2 x 85.7% Negative Herbold, 1982
2000 mg/kg bw
Micronucleus test Mouse not known ? Negative Rolandi et al., 1984
Dominant lethal NMRI mice 500 mg/kg bw ? Negative Machemer, 1974b
control groups of 10 animals each. Following intradermal induction
with 0.1% propineb and topical induction with 25% propineb, the
first challenge was carried out using 25% propineb. A positive
response was seen in all 20 test animals, against no positive
reactions in the first control group. A second challenge was
carried out using 2.5% propineb. Positive reactions were seen on
this occasion in 18 test animals against 4 in the control group.
This study therefore detected clear indications of the allergic
potential of propineb in guinea-pigs (Heimann, 1987).
In a further experiment, propineb (premix, 83.3% purity) was
tested for possible skin sensitizing potential in guinea-pigs in a
Buhler patch test employing a test group and two control groups of
12 animals each. Following epicutaneous induction with 50%
propineb, challenge was carried out with 2.5% and 50% propineb. The
test material was tolerated without reaction and this study revealed
no indication of a skin sensitizing potential of propineb in guinea-
pigs (Flucke, 1989).
Irritant and/or allergizing characteristics of propineb in
humans was not confirmed. Between 1964 (when production began) and
1988 no allergies or skin sensitizing reactions associated with
propineb were recorded, "even though skin contact had been frequent
as a result of cleaning work" (Mueller, 1988).
Observations in humans
No information available.
COMMENTS
Orally administered propineb in rats is rapidly absorbed and
excreted largely via urine and faeces. Although there was some
evidence of excretion by exhalation, the available metabolic data
(which detected PTU and propyleneurea as the main urinary
metabolites along with propylenediamine and a small amount of 4-
methylimidazoline) found no metabolites which could be considered as
potential intermediates for degradation to CO2. Study results
indicated that a proportion of the administered dose accumulates
temporarily in the thyroid. Although most elimination occurred
within 4 days of dosing, the half-life of elimination for the
proportion remaining was relatively long. This could be due to
incorporation of portion of the molecule into endogenous substances
following metabolism, which would also account for the radioactivity
eliminated with exhaled air.
Propineb has moderate to low acute toxicity in mice, rats,
hamsters, cats and sheep. WHO has classified propineb as unlikely
to present acute hazard in normal use.
The results of toxicity studies clearly indicate that propineb
has a goitrogenic effect in rats, although no similar finding was
noted in rabbits or dogs. In a 62-day study in male rats using
dietary levels of 0, 2, 10, 50 or 250 ppm, the NOAEL was 10 ppm
(equal to 0.74 mg/kg bw/day), based on changes in thyroxine
concentration and increased thyroid weight at higher doses.
Although in a later 63-day study using dietary levels of 0, 0.2,
0.6, 2 or 10 ppm slight hyperplasia was seen in the thyroid in 2
rats out of 10 treated with 10 ppm, this finding was considered not
to be a permanent adverse effect in rats.
In a comparative study in rats of the effects of propineb, PTU,
ETU, zineb and methyl thiouracil on thyroid weight, propineb had
only a moderate effect compared to methyl thiouracil, whereas PTU
had an equivalent effect to that of methyl thiouracil and was
somewhat stronger than ETU. These results suggest that the effects
of propineb on the thyroid in rats may be caused primarily by the
metabolite, PTU. Dogs tolerated much higher doses of propineb,
dietary administration of 3000 ppm, equivalent to 75 mg/kg bw/day,
causing no adverse effects over 2 years.
In long-term studies, treatment-related alterations in tumour
incidence were seen in rats and mice. In mice treated with 0, 50,
200 or 800 ppm, an increase in hepatocellular adenomas was seen in
males only, at the highest dose tested, but there was no increase in
the incidence of hepatic carcinomas and no similar effect in
females. The NOAEL was 200 ppm, equal to 26 mg/kg bw/day, based on
this change in hepatic tumour incidence. In rats, there were two
studies, using dietary levels of 0, 1, 10, 100, 1000, 2000 or 8000
ppm in the first and 0, 5, 10, 25, 50 or 100 ppm in the second. The
overall NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on
increased kidney and liver weight (without histological correlation)
and increased thyroid weight at 100 ppm and above. An increase in
thyrotropin-related thyroid tumours and skeletal muscle degeneration
was seen in rats at dietary levels of 1000 ppm and greater, but
these doses were accompanied by increased mortality.
In a three-generation reproduction study in rats using dietary
levels of 0, 20, 60, 200 or 600 ppm the NOAEL was 60 ppm, equivalent
to 3 mg/kg bw/day, with adverse effects on maternal health and
impaired reproductive performance seen at higher doses.
Teratogenicity studies indicated that propineb has teratogenic
potential in rats, but no evidence of teratogenicity was seen, even
in the presence of maternal toxicity, in rabbits. In rats, using
dose levels of 0, 3, 10, 30 or 100 mg/kg bw/day, the NOAELs were 10
and 30 mg/kg bw/day for maternal and embryo/fetotoxicity,
respectively, with evidence of teratogenicity at 100 mg/kg bw/day.
In rabbits, using dose levels of 0, 10, 30 or 100 mg/kg bw/day, the
NOAEL for maternal toxicity was 10 mg/kg bw/day, while there was no
evidence of teratogenicity or embryo/fetotoxicity at 100 mg/kg
bw/day, the highest dose tested.
Propineb has been adequately tested in a series of genotoxicity
assays from which the Meeting concluded that it is not genotoxic.
An ADI was allocated to propineb, which was based on the NOAEL
from the short-term thyroid function study in rats (10 ppm, equal to
0.74 mg/kg bw/day) using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 200 ppm in the diet, equal to 26 mg/kg bw/day
(2-year study)
Rat: 10 ppm in the diet, equal to 0.74 mg/kg bw/day
(62-day thyroid function study)
50 ppm, equivalent to 2.5 mg/kg bw/day (2-year study)
Dog: 3000 ppm in the diet, equivalent to 75 mg/kg bw/day
(2-year study).
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Becker, H., Vogel, W. & Terrier, Ch. (1988) Embryotoxicity
(including teratogenicity) study with LH 30/Z in the rabbit. Report
R 4460, Research and Consulting Company AG, Itingen, Switzerland.
Unpublished report submitted to WHO by Bayer AG.
Brune, H., Deutsch-Wenzel, R., & Mohr, U. (1980) Toxicology
examination of propineb in a chronic feeding study on NMRI mice.
Biological laboratory of Dr. Brune (Hamburg), Report No. R 1792.
Unpublished report submitted to WHO by Bayer AG.
Cifone, M.A. (1987) Mutagenicity Test on LH 30/Z in the rat primary
hepatocyte unscheduled DNA synthesis assay. Hazleton Laboratories
America, Inc., Report No. TR 4086. Unpublished report submitted to
WHO by Bayer AG.
Ecker, W. (1975) l4C-Antracol, metabolism studies on orally dosed
rats. Pharma report No. PH 5352. Bayer AG, Isotope Chemistry
Laboratory, Institute for Pharmacokinetics. Unpublished report
submitted to WHO by Bayer AG.
Flucke, W. & Kimmerle, G. (1977) Propineb (LH 30/Z), triadimefon
(MEB 6447). study for acute toxicity after simultaneous
administration of both active ingredients. Bayer AG Institute of
Toxicology, Report No. 7065. Unpublished report submitted to WHO by
Bayer AG.
Flucke, W. (1989) Propineb: Study for skin sensitising effect on
guinea-pigs (Buhler's patch test). Bayer AG, Department of
Toxicology. Report No. 17734. Unpublished report submitted to WHO by
Bayer AG.
Hatano Institute, Food and Drug Safety Centre (1978): Report of the
mutagenicity study of Propineb. Unpublished report submitted to WHO
by Bayer AG.
Heimann, K.-G. (1987) Propineb, Study for skin sensitising effect on
guinea-pigs. Bayer AG, Institute of toxicology. Report No. 15897.
Unpublished report submitted to WHO by Bayer AG.
Herbold, B. (1980a) LH 30/Z (Propineb, Antracol - Active ingredient)
Salmonella/microsome test to evaluate for point mutation. Report
No 9321, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Herbold, B. (1982) LH 30/Z - Propineb - antracol - active ingredient
- micronucleus test in mice. Report No 10974, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Hoffmann, K. (1983) LH 30/Z (Antracol active ingredient), acute
toxicity for sheep after oral administration. Bayer AG Institute of
Toxicology, Report No. 11463. Unpublished report submitted to WHO by
Bayer AG.
Kimmerle, G. (1972) Comparative test of Antracol, Propylene
thiourea, zineb and ethylene thiourea for thyroid action in rats.
Report No 3551, Bayer AG, Institute of Toxicology. Unpublished
report submitted to WHO by Bayer AG.
Kroetlinger, F., Loeser, E., Kalinger, G. (1980) Propineb (antracol
active ingredient) - subchronic toxicology study to establish the
dose-time-effect relationship for the thyroid (feeding study over 9
weeks). Report No 9313, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Larsson, S.K., Arnander, C., Cekanova, E. & Kjellberg, M. (1976)
Studies of teratogenic effects of the Dithiocarbamates Maneb,
Mancozeb, and Propineb. Teratology, 14: 171-183.
Lehn, H. (1988) LH 30/Z - Mutagenicity study for the detection of
induced forward mutations in the CHO/HGPRT assay in vitro. Report
No 16840, Bayer AG, Department of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1967) Bay 46131 - Subchronic feeding study on dogs.
Report No 450, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (1968) Bay 46131 - Study of the reversibility of
enlargement of thyroids in rats. Report No 836, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1969) Bay 46131 - Subchronic toxicology study in rats
(investigation over 3 months). Report No 1500, Bayer AG, Institute
of Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973a) Bay 46131 - Chronic toxicity study in dogs
(duration 2 years). Report No 4213, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (1973b) Bay 46131 - Multigeneration study in rats. Report
No 4214, Bayer AG, Institute of Toxicology. Unpublished report
submitted to WHO by Bayer AG.
Loeser, E. (l974a) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4927, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Loeser, E. (l974b) Chronic toxicity studies on Rats (2-year feeding
experiment). Report No 4608, Bayer AG, Institute of Toxicology.
Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974a) LH 30/Z (antracol active ingredient) -
investigation of embryotoxicity and teratogenicity in rats following
oral administration. Report No 4895, Bayer AG, Institute of
Toxicology. Unpublished report submitted to WHO by Bayer AG.
Machemer, L. (1974b): LH 30/Z (antracol active ingredient) -
dominant lethal test in the male mouse. Report No 4795, Bayer AG,
Institute of Toxicology. Unpublished report submitted to WHO by
Bayer AG.
Mihail, F. & Kaliner, G. (1979) LH 30/Z (antracol active
ingredient), subacute dermal cumulative toxicity study on rabbits.
Bayer AG Institute of Toxicology, Report No. 8322. Unpublished
report submitted to WHO by Bayer AG.
Mueller, H. (1988) Internal company memorandum, Bayer AG Medical
Department, to Dr Machemer, Bayer AG, Institute of Toxicology.
Unpublished document submitted to WHO by Bayer AG.
Patschke, K., Wegner, L.A. (1975) Antracol-l4C, biokinetic studies
on rats after oral application. Report No. PH 5268. Bayer AG,
Institute for Pharmacokinetics. Unpublished report submitted to WHO
by Bayer AG.
Rolandi, A., De Marinis, E. & De Caterina, M. (1984) Dithiocarbamate
pesticides, activity of propineb in the micronucleus test in mice.
Mut. Res. 135: 193-197.
Thyssen, J. & Kimmerle, G. (1978) Antracol active ingredient (LH
30/Z), acute toxicological study. Bayer AG Institute of Toxicology,
Report No. 7208. Unpublished report submitted to WHO by Bayer AG.
Thyssen, J. & Mohr (1979) Antracol active ingredient, subacute
inhalation study on rats. Bayer AG Institute of Toxicology, Report
No. 8334. Unpublished report submitted to WHO by Bayer AG.
Vicari, L., De Dominicis, G., Vito, M., Placido, C., de Marinis, E.
(1985) Teratogenic and goitrogenic activity of propineb and
propylene thiourea in the Rat. Boll. Soc. It. Biol. 61: 271-278.
Weber, H. (1980) - Biokinetics of antracol-14C (PH-Report No 5268)
and propylene thiourea (PH-Report No 7397). Bayer AG, Institute. for
Pharmacokinetics. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Dressler, H.F. (1980) Effect on thyroid function of male
rats following subchronic administration of propineb (antracol-
active ingredient). Report No 9268, Bayer AG, Isotope and Biokinetic
Laboratory. Unpublished report submitted to WHO by Bayer AG.
Weber, H., Mager, H., Hartman, E. (1991) Propineb feeding study:
Effect on thyroid function in male Wistar rats at low dose levels up
to 10 ppm for a time interval up to 63 days. Bayer AG Department of
Toxicology. Report No. PF 3475. Unpublished report submitted to WHO
by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
