
PHOSMET
First draft prepared by
T.C. Marrs,
Medical Toxicology and Environmental Health,
Department of Health, London, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Delayed neuropathy
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Phosmet was reviewed by the JMPR in 1978 (Annex I, reference
30), when a temporary ADI of 0-0.005 mg/kg bw was allocated. It was
reviewed again in 1979 (Annex I, reference 32), when additional data
on teratogenicity were made available, and an ADI of 0-0.02 mg/kg bw
was established. Further data have become available, and this
monograph summarizes both the new studies and relevant summaries
from the previous monograph and monograph addendum (Annex I,
references 31 and 33). This compound was reviewed at the present
Meeting as a result of the CCPR periodic review programme.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
In a study of the pharmacokinetics and biotransformation of
phosmet in Long-Evans rats, 14C-phosmet was given as a single dose
of 23-35.2 mg/kg bw by gavage to three male and two female rats. The
material was rapidly absorbed, distributed and excreted. Label was
excreted predominantly in the urine: by the time of sacrifice (72 or
120 h after treatment), 79% had been excreted in the urine and 19%
in the faeces, while very little was expired as 14C-carbon
dioxide. Tissue levels of radiolabel were low, especially in fat and
the gonads (Ford et al., 1966).
Groups of five male and five female Sprague-Dawley-derived
Crl:CB(SD)BRVAF/+ rats were given single oral doses of 1 or 25 mg/kg
bw of 14C-phosmet. Further groups were given 14 daily oral doses
of 1 mg/kg bw phosmet followed by a single oral dose of 1 mg/kg bw
labelled compound. The highest blood levels of label were observed
0.5 h after dosing in both groups; thus, the material was readily
absorbed. During the next 8 h, there was a rapid decline in plasma
levels of label, followed by a slower decline. Label was rapidly
excreted in all treated groups (> 70% in 24 h), mainly in the
urine. After 96 h, 88% of the label was recovered in the urine of
the animals given 1 mg/kg bw as a single dose and 81% in the group
given 25 mg/kg bw phosmet. Faecal excretion was minor (6-13% of the
dose). Very little label (1.2-2.1%) was detected in the carcass 96 h
after treatment. The main effect of repeated exposure before
administration of labelled compound was to reduce excretion of the
label, so that about 75% of the label was excreted within 96 h in
the urine. The lowest concentrations of label were found in bone and
fat and the highest in the skin and, to a lesser extent, the
kidneys. The concentrations of label were higher in packed
erythrocytes than in plasma (Fisher, 1989).
Phosmet administered orally to pregnant albino rats (strain
unspecified) in the final stages of pregnancy or injected into the
intra-amniotic sac was absorbed rapidly and crossed the placenta.
The half-life of phosmet in externalized fetuses and newborns was
50-70 min (Ackermann et al., 1976).
(b) Biotransformation
In the study of Ford et al. (1966), described above, < 1% of
the label in the urine was found to be in the form of phosmet or
phosmet oxon. Less than 0.04% of the radiolabel was recovered in
expired air.
Long-Evans rats (sex unspecified) administered
[carbonyl-14C]-phosmet at 27 mg/kg bw excreted 41% of the label in
the urine as phthalamic acid and 21% as phthalic acid; less than
0.04% was present as phosmet or its oxon. Phosmet was readily
converted to phosmet oxon in the rat liver microsome NADPH2 enzyme
system (McBain et al., 1968).
The biotransformation of phosmet in male and female
Sprague-Dawley-derived Crl:CD(SD)BRVAF/+) rats was investigated
using samples from the study of Fisher (1989). Two major urinary
metabolites were observed: N-(methylsulfinylmethyl)phthalamic acid
(52-66%) and N-(methylsulfonylmethyl)phthalamic acid (8-26%);
numerous other metabolites that occurred at low concentrations could
not be identified. The other product of hydrolysis of phosmet would
presumably be O,O-diethylphosphorothioate. Male rats excreted a
greater proportion of the labelled phosmet as N-(methyl
sulfonylmethyl)phthalamic acid (20-26%) than did females (8-13%). A
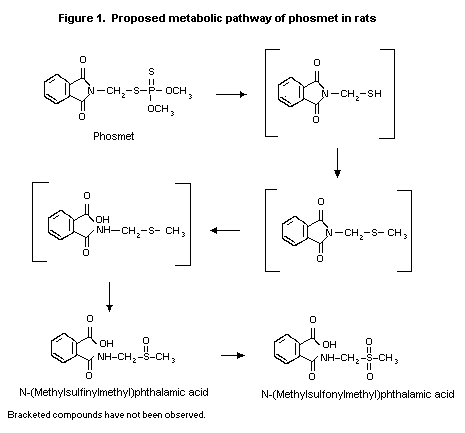
proposed metabolic pathway is given in Figure 1. The differences
between the results of the study conducted in 1968 and that
conducted in 1989-90 may have been due to instability of phthalamic
acid under acidic conditions if, for example, the urine was stored
for a substantial length of time. The metabolic pathway would have
been deamination of phthalamic acid to phthalamic anhydride and
hydrolysis to phthalic acid (Fisher, 1990).
In a study involving two lactating goats (strain unspecified),
[carbonyl-14C]-phosmet was fed at a dietary equivalent of about 8
ppm for four days. About 60% of the dose was excreted in the urine;
the levels of residues in milk were 0.014-0.017 ppm, representing
about 0.2% of the administered dose. Phosmet was not detected in
milk or edible tissues. The metabolites that were detected included
N-(methylthiomethyl)phthalimide and
N-(methylsulfonylmethyl)phthalamic acid. There were considerable
differences in the relative concentrations of metabolites in
different tissues; e.g. there was a high proportion of
N-(methylsulfonylmethyl)phthalamic acid in the milk, kidney and
muscle, whereas the most abundant metabolite in the liver was
N-(methylthiomethyl)phthalimide (Tarr & Hemingway, 1993a).
[carbonyl-14C]-Phosmet was fed in the diet to 15 white
Leghorn laying hens at a concentration of 10.5 ppm for seven days.
Of the cumulative dose, 90% was excreted within 24 h after the end
of feeding. Eggs contained 0.3% of the cumulative dose. Phosmet was
not detected in tissues, but 0.01% was found in egg yolks. The
metabolites detected in edible tissues and egg yolks included
phthalimide and phthalic acid (Tarr & Hemingway, 1993b).
 (c) Effects on enzymes and other biochemical parameters
Erythrocyte and brain cholinesterase are more sensitive to
phosmet in rats than is plasma cholinesterase. Rat aliesterases are
more sensitive to inhibition by phosmet than is
acetylcholinesterase. Other organophosphates (malathion, parathion,
parathion-methyl, schradan, tributyl phosphorotrithioite, diazinon,
EPN, ethion, demeton, mevinphos, carbophenothion, disulfoton
azinphos-methyl) and a carbamate anticholinesterase (carbaryl) did
not potentiate the effect of phosmet in male Sprague-Dawley rats
(Lee & Miaullis, 1969).
Male and female CD rats were treated orally with 0, 10 or 100
mg/kg bw phosmet (purity, 94.7%), and plasma, erythrocyte and brain
cholinesterase activities were measured 4 and 24 h later. The lower
dose had no effect on plasma or erythrocyte cholinesterase
activities at either time or on brain cholinesterase activity at 24
h; however, 4 h after treatment with this dose, brain cholinesterase
activity was inhibited by 14% in males and 21% in females. At 100
mg/kg bw, substantial inhibition was found at both times, except for
plasma enzyme in females at 4 h. At that time, the activity of the
erythrocyte enzyme was the most strongly inhibited (about 85%) and
that of the brain enzyme somewhat less (about 65%); the activity of
plasma cholinesterase was inhibited by only about 35% in males and
not significantly in females. At 24 h, the activity of the brain
enzyme was recovering (34% inhibition in males and 48% in females),
as was that of erythrocyte cholinesterase (40 and 45% inhibition),
while the activity of plasma cholinesterase was further inhibited
(46 and 74% inhibition) (Hendricks & Sprague, 1983).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of phosmet is summarized in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice received
technical-grade phosmet (purity, 95%) in the diet at concentrations
of 0, 5, 15, 50, 150 or 500 ppm, equivalent to 0.75, 2.25, 7.5, 22.5
or 75 mg/kg bw per day, for four weeks. Significant decreases in
food consumption and body-weight gain were seen at 150 and 500 ppm
in males and at 500 ppm in females; females given 150 ppm had only
reduced food intake. Males at the two highest doses had a
significant decrease in absolute liver and kidney weights; relative
liver weights were significantly increased at 150 and 500 ppm in
males and females, while relative kidney weights were significantly
increased only in females at 500 ppm. Erythrocyte cholinesterase
activity was depressed at 50 ppm and above, and brain cholinesterase
activity was statistically significantly depressed by 16% in females
receiving the highest dose. No treatment-related changes were seen
histologically. The NOAEL was 50 ppm, equivalent to 7.5 mg/kg bw per
day, on the basis of reduced food consumption and body-weight gain
and decreased absolute liver and kidney weights at 150 ppm (Jones
et al., 1981).
Rats
Groups of 15 male and 15 female albino rats (strain
unspecified) were given phosmet (purity, 98%) in the diet at 0, 20,
100 or 500 ppm, equivalent to 1, 5 or 25 mg/kg bw per day, for 14
weeks. A second study was started four weeks later, involving four
groups of the same size treated with the same doses. Decreased
weight gain was found in the group receiving the highest dose.
Erythrocyte cholinesterase activity was inhibited by > 20% at the
middle and high doses, while plasma cholinesterase was inhibited by
> 20% at the high dose only; brain cholinesterase activity was
inhibited by > 20% at terminal sacrifice in the high and middle
dose groups. Changes described as 'necrobiotic foci' were observed
in the liver at 500 ppm, which were considered not to be related to
treatment. The NOAEL was 20 ppm, equivalent to 1.0 mg/kg bw per day,
on the basis of inhibition of brain cholinesterase activity at 100
ppm (Johnston, 1962).
Table 1. Acute toxicity of phosmet
Species Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
(95% CI or range)
Mouse Swiss-Webster albino M Oral 50.1 (34.4-73.0) NR Meyding, 1965
Mouse Swiss-Webster albino M Oral 20-43 a Bullock, 1971
Mouse NR M Oral 36.9 (21.7-62.8) 95 Bullock, 1972
Mouse Albino M&F Oral 38 b Johnston, 1966
Mouse Albino M&F Oral 49 c Johnston, 1966
Mouse Albino M&F Oral 43 d Johnston, 1966
Mouse Albino NR Oral 26-60 NR Danilenko, 1969
Mouse Swiss-Webster M Intraperitoneal 40-50 NR Meyding, 1965
Mouse Swiss-Webster M Subcutaneous 300 NR Meyding, 1965
Rat Albino M Oral 310 (267-360) NR Ray, 1964
Rat Sprague-Dawley M Oral 245 (161-367) NR Meyding, 1965
Rat Sprague-Dawley albino M Oral 140 (76-255) 98 Nuclear Science Corp.,
1962
Rat Albino NR Oral 92.5-164 NR Danilenko, 1969
Rat Sprague-Dawley albino M Oral 135-147a a Bullock, 1971
Rat Sprague-Dawley albino M Oral 121.3 (90.6-162.5) 92.5 Castles, 1977
Rat Sprague-Dawley albino F Oral 121.3 (96.7-152.1) 92.5 Castles, 1977
Rat Sprague-Dawley M Intraperitoneal approx. 100 NR Meyding, 1965
Rat Sprague-Dawley M Subcutaneous > 1200 NR Meyding, 1965
Rat Sprague-Dawley albino F Inhalation > 0.15e 92.5 Castles, 1977
Guinea-pig NR NR Oral 200 NR Danilenko, 1969
Rabbit New Zealand white NR Percutaneous > 4600 a Bullock, 1971
Rabbit New Zealand white NR Percutaneous > 5000 92.5 Castles, 1977
Cat NR NR Inhalation 65f NR Cited by Izmerov,
1983
Chicken White Leghorn F Oral 2020 94.7 Sprague, 1982
Table 1 (continued)
NR, not reported
a Precise figure depended on formulation and route of synthesis of active ingredient.
b Technical-grade in corn oil; purity not stated
c Technical-grade in 20% PEG300; purity not stated
d Imidan 50% wettable powder in aqueous suspension; concentration of active ingredient, 50%; results given
for active ingredient
e LC50' 1 h: mg/l
f LC50' time unstated
Three groups of 10 male and 10 female albino rats (strain
unspecified) were treated with phosmet for 16 weeks. Animals in the
'low dose' group initially received 450 ppm, equivalent to 22.5
mg/kg bw per day, which was increased stepwise to 6000 ppm,
equivalent to 300 mg/kg bw per day, by the 12th week. The 'high
dose' group was started on 800 ppm, equivalent to 40 mg/kg bw per
day, which was increased stepwise to 1120 ppm, equivalent to 56
mg/kg bw per day. Clinical signs (nervousness, tremors, diarrhoea)
were observed in all treated animals. Body-weight gain was decreased
in the high dose group in comparison with controls, and marked
hepatic degenerative changes (eosinophilia, vacuolation and
swelling, with little change in nuclear morphology) were also seen
in the high-dose group. Erythrocyte cholinesterase activity was
inhibited by about 100% in both groups, and plasma cholinesterase
activity was inhibited by > 50% in all groups. Brain cholinesterase
was inhibited by 75 and 80% in males at the low and high doses and
by 84 and 82% in females at the low and high doses, respectively.
There was no NOAEL, as brain cholinesterase activity was inhibited
in both treated groups and because of the variable dosing (Johnston,
1963a).
Cats
Veterinary use of phosmet as a dip for treating flea
infestation caused toxic epidermal necrolysis in a female Himalayan
cat. This observation has not been repeated (Frank et al., 1992).
Dogs
Groups of four male and four female beagle dogs were treated
with dietary concentrations of 0, 10, 75 or 563 ppm, equivalent to
0.25, 1.9 or 14 mg/kg bw per day, for 14 weeks. All of the animals
gained weight, except for one given the middle and one given the low
dose. Haematological and clinical chemical parameters were
unaffected by the treatment, except that marked depression of
erythrocyte cholinesterase activity and somewhat less marked
depression of plasma cholinesterase activity were found at the high
dose. Brain cholinesterase activity at terminal sacrifice was also
depressed at the high dose. The levels of plasma, erythrocyte and
brain cholinesterase were normal in the other three groups. At
autopsy, no pathological changes attributable to treatment were
observed. The NOAEL was 75 ppm, equivalent to 1.9 mg/kg bw per day
(Johnston, 1962).
Groups of three male and three female beagle dogs received
phosmet (purity unspecified) in the diet at concentrations of 0, 20,
40 or 400 ppm, equivalent to 0.5, 1 or 10 mg/kg bw per day, for two
years. One male at the highest dose was killed in extremis;
survival was otherwise unaffected. Erythrocyte cholinesterase
activity was depressed at 400 ppm throughout most of the study. At
termination, brain cholinesterase activity was considerably
depressed in the animals at the high dose, to 58% of control
activity in males and 32% in females. Interpretation of the study
was hampered by the small group size. The NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day (Lobdell & Johnston, 1966).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female B6C3F1 mice were fed phosmet
(purity, 94.7%) at dietary concentrations of 0, 5, 25 or 100 ppm,
equal to 0.75, 4 or 15 mg/kg bw per day for two years. Up to 10 mice
of each sex in each group were killed at 12 months, and
cholinesterase activity and haematological parameters were measured
in these animals and in 10 animals of each sex from each group at
terminal sacrifice. Plasma and brain cholinesterase activities were
measured at the time of the interim kill, and plasma, brain and
erythrocyte cholinesterase at termination. Phosmet did not affect
survival. Body weight was slightly but significantly increased for
most of the study in animals receiving the highest dose, and food
consumption was occasionally reduced. In males at the highest dose
there was an increased frequency of convulsions, usually associated
with handling of the animals.
Plasma cholinesterase activity was inhibited by about 50% in
males and females at the highest dose and by about 13% in females at
the middle dose at the time of interim sacrifice. Brain
cholinesterase activity was inhibited by > 20% in all treated
groups at interim sacrifice. Plasma cholinesterase activity was
inhibited at termination in the groups receiving the highest dose,
but the activity in erythrocytes was comparable in all groups. Some
inhibition of brain cholinesterase activity was found in females at
the middle and highest doses at termination, but, while
statistically significant, the depression was < 20% in the middle
dose group and 22% in the highest dose group. Brain cholinesterase
activity was not depressed in males at the terminal kill.
No treatment-related effects were seen on haematological
parameters at interim or final sacrifice, and no treatment-related
changes in organ weights or macroscopic or microscopic appearance
were seen, except in the liver. Relative liver weights were
increased at 12 months in males receiving 100 ppm, and at
termination there was an increase in the prevalence of mild
vacuolation. There was also an increase in the incidence of liver
adenomas (25/50, with 13/49 in controls) in males at the highest
dose. When animals killed at 12 months were included, liver adenomas
were found in 13/60 controls, 10/60 at 5 ppm, 14/60 at 25 ppm and
27/60 at 100 ppm. In an addendum to the report, the prevalence of
liver adenomas in the group given the highest dose was reported to
be comparable to that in historical controls. No increase in the
incidence of liver tumours was seen in females. The NOAEL was 25
ppm, equal to 4 mg/kg bw per day, on the basis of increased
incidences of convulsions, hepatocellular cytoplasmic vacuolation
and liver-cell adenomas in males and decreased brain cholinesterase
activity in females at 100 ppm. The apparent change in brain
cholinesterase activity at the time of the interim kill was
considered not to be a true reaction to treatment with phosmet, in
view of the absence of a similar effect at the time of the terminal
kill, after a longer period of treatment (Katz et al., 1984;
Sprague & Turnier, 1986).
Rats
Groups of 25 male and 25 female albino rats (strain
unspecified) received phosmet (purity unspecified) in the diet at
concentrations of 0, 20, 40 or 400 ppm, equivalent to 1, 2 or 20
mg/kg bw per day, for two years. Body-weight gain and plasma and
erythrocyte cholinesterase activities were depressed at 400 ppm; the
cholinesterase activities were decreased throughout the study, and
brain cholinesterase activity was lowered at termination. Hepatocyte
vacuolation was seen at the highest dose. Pituitary adenomas were
more frequent at 40 ppm (46%) and 400 ppm (56%) than in the controls
(36%) and in those receiving 20 ppm (21%), but there was not a clear
dose-response relationship. The small groups and the small number of
survivors at termination made interpretation of the study somewhat
difficult and precluded a conclusion being drawn about the
carcinogenicity of phosmet. The NOAEL was 40 ppm, equivalent to 2
mg/kg bw per day (Lobdell & Johnston, 1966).
Groups of 60 or 70 Sprague-Dawley Crl:CD SD BR rats of each sex
received phosmet (purity, 94.3%) in the diet at concentrations of 0,
20, 40 or 200 ppm, equal to 1.1, 1.8 or 9.4 mg/kg bw per day for
males and 1.1, 2.1 or 10.9 mg/kg bw per day for females. For interim
evaluation, 20 rats of each sex from the control group and 10 of
each sex from each test group were killed after 12 months, and the
study was continued for a further year. An additional group of 20
rats of each sex received 400 ppm (equal to 23 mg/kg bw per day for
males and 27 mg/kg bw per day for females) for 12 months. Exposure
did not adversely affect survival; indeed, there appeared to be a
dose-related increase in survival. No specific clinical signs were
attributable to treatment. Weight gain was reduced throughout the
study in animals of each sex at 400 ppm and in the early part of the
study at 40 (at 4, 21, 37, 45 and 49 weeks) and 200 ppm (at 1, 2 and
4weeks) in females only. These decreases are unlikely to be
biologically significant. Significant reductions in cholinesterase
activities were observed: plasma cholinesterase activity was reduced
by > 20% at 200 and 400 ppm in males and at 40, 200 and 400 ppm in
females; a reduction of 21% was observed at 18 weeks in females at
20 ppm. Erythrocyte cholinesterase activity was reduced by 15-20% at
20 ppm and by > 20% at higher dietary concentrations in males, and
by 15-20% at 40 ppm and very considerably at higher concentrations
in females. Brain cholinesterase activity was depressed in males at
200 ppm (interim and final kill), although the depression at 24
months was less than 15%. In females, depression of brain
cholinesterase activity by > 20% was observed at 200 ppm at both
interim and final sacrifice. Brain cholinesterase activity was also
depressed in animals of each sex at 400 ppm. An increase in the
incidence and severity of fatty liver was seen at 200 ppm. No
tumours were seen that were attributable to treatment with phosmet.
The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day, on the basis of
an increased incidence of fatty change in the livers of animals of
each sex and depressed brain cholinesterase activity in females at
200 ppm (Chang et al., 1991).
(d) Embryotoxicity and teratogenicity
Rats
In Wistar rats given a single dose of 30 mg/kg bw phosmet
orally once on day 9 of pregnancy or 1.5 mg/kg bw on alternate days
(every day in the summary) throughout pregnancy, post-implantation
mortality of embryos was increased. The dose of 30 mg/kg bw on day 9
or 13 caused developmental abnormalities, such as hypognathia and
hydrocephaly. A dose of 0.06 mg/kg bw on alternate days (every day
in the summary) had no effect. There was no NOAEL (Martson &
Voronina, 1976).
Phosmet (purity, 95.8%) was administered in the diet of CD rats
at amounts that provided a mean consumption of 0, 10, 22, 27 or 29
mg/kg bw per day on days 6-15 of pregnancy. The size of the groups
varied from 47 controls to 17 and 23 at the two higher doses. The
rats were killed on day 21. At 22 mg/kg bw per day, food intake and
weight gain were reduced. As phosmet was neither teratogenic nor
fetotoxic, the NOAEL for maternal toxicity was 10 mg/kg bw per day
and the NOAEL for fetal toxicity was the highest dose, 29 mg/kg bw
per day. In a similar study, in which phosmet was administered by
gavage at doses of 5, 10, 20, 25 or 30 mg/kg bw per day (with no
separate control group), survival was affected at the two higher
doses. There was a significant reduction in the proportion of rats
at 25 mg/kg bw per day that became pregnant, and there was a
reduction in food intake at > 10 mg/kg bw per day and a reduction
in weight gain at > 20 mg/kg bw per day. In the absence of a
suitable control group, there was no NOAEL in this study (Staples
et al., 1976).
Groups of 24 female alpk:APfSD rats were dosed with phosmet
(purity, 96.4%) in corn oil by gavage at 0, 5, 10 or 15 mg/kg bw per
day on days 7-16 days of gestation. The rats were killed on day 22
of gestation and the uteruses examined for live fetuses and
intrauterine deaths. Maternal toxicity in the form of reduced
body-weight gain, reduced food consumption and clinical signs
(shaking, piloerection) was seen at the highest dose. As there were
smaller but statistically significant effects on body-weight gain at
10 mg/kg bw per day between days 7 and 16, the lowest dose, 5 mg/kg
bw per day, was the NOAEL for maternal toxicity. As no teratogenic
or fetotoxic effects were seen, the NOAEL for developmental toxicity
was 15 mg/kg bw per day (Hodge, 1991).
In a three-generation study in CD rats, the F0 generation
consisted of two groups of 20 males and two groups of 20 females:
One group of each sex received no treatment, while the other
received technical-grade phosmet (purity, 99%) in the feed at 40
ppm, equivalent to 2 mg/kg bw per day, or half this concentration
during the first three weeks. Animals in each group were mated
twice, and the offspring (F1a and F1b) were examined at birth
and at weaning, when the F1a rats were killed. The F0 rats were
killed but not examined by necropsy. The F1b rats were retained to
make up three new groups of 20 rats of each sex, which received
phosmet at dietary concentrations of 0, 40 or 80 ppm from weaning
until sacrifice. Mating of the F1b rats produced F2a and F2b
litters; the F2a rats were sacrificed and the F2b offspring were
used, like the preceding generation, to make three new groups of
F2a and F2b rats. Offspring were sacrificed at weaning. The
treated and control animals were comparable throughout the study. On
histopathological examination of the F3b animals, some mild hepatic
vacuolation and reduced glycogen content were observed more
frequently in treated than in untreated animals; however, the former
finding was not frequent in any dose group, and the latter (in
adults and therefore presumably in fetuses) was strongly dependent
on time since last feed, which may have been different for the
various dose groups. In view of the lack of other findings, the
Meeting considered the NOAEL to be 40 ppm, equivalent to 2 mg/kg bw
per day (Hollingsworth et al., 1965).
In a two-generation study, technical-grade phosmet (purity,
95.2%) was administered to Crl:CD(SD)BRVAF/+tm rats at dietary
concentrations of 0, 20, 80 or 300 ppm, equal to 1.3, 5.0 or 19.4
mg/kg bw per day in F0 males; 1.5, 6.3 or 24.3 mg/kg bw per day in
F1 males; 1.5, 6.0 or 24.4 in F0 females; and 1.5, 6.2 or 26.4
mg/kg bw per day in F1 females. Treatment of the F0 generation
was started at 56 days of age, and mating occurred 56 days later.
The F1a animals were weaned at 21 days and sacrificed. Shortly
afterwards, the F0 rats were again mated to produce the F1b
litters, of which about 25 males and 25 females were used to form
the F1 parents. At about 114 days of age, the F1 parents were
mated to produce the F2 litters. Toxicity was observed in parents
at 80 and 300 ppm in both generations. Reduced body-weight gain and
food consumption were observed in animals of each sex at 300 ppm.
Males had reduced testicular weights in both generations and reduced
spleen weight and hepatocellular vacuolization in the F1
generation. Dehydration was seen in the F0 females at 300 ppm, and
chromorhinorrhoea was seen in F1 females at 300 ppm. At 80 ppm,
there was reduced body-weight gain in F0 male parents and reduced
erythrocyte cholinesterase activity in both generations; in F0
parental females, there was reduced weight gain during lactation and
reduced relative liver and adrenal weights. Reduced relative spleen
and thymus weights were seen in the F1 generation, and reduced
erythrocyte cholinesterase activity was seen in both generations.
Plasma cholinesterase activity was reduced in parental F0 males
only. Erythrocyte cholinesterase activity, although reduced, was >
80% of that of controls at 20 ppm. Mating and fertility were reduced
at 80 and 300 ppm, and there were reduced numbers of pups per
litter, reduced pup weight and reduced survival of pups at 300 ppm.
The NOAEL for toxicity to the parents and for effects on
reproductive performance was 20 ppm (equal to 1.3 mg/kg bw per day),
and the NOAEL for developmental toxicity was 80 ppm (equal to 5.0
mg/kg bw per day) (Meyer & Walberg, 1990).
Rabbits
Phosmet (purity unspecified) was administered by gavage to New
Zealand white rabbits at 35 mg/kg bw per day on days 7-12 of
pregnancy; the animals were killed at day 28. No embryotoxic effects
were detected, although such effects were observed with thalidomide
in the same study. The NOAEL for phosmet was 35 mg/kg bw per day;
however the treatment period was rather short and included only part
of the period of organogenesis (Fabro et al., 1966).
Groups of 20 female New Zealand white rabbits were treated with
phosmet (purity, 96.4%) at doses of 0, 2, 5 or 15 mg/kg bw per day
in corn oil by gavage on days 7-19 of pregnancy. The animals were
killed on day 30 of gestation. The highest dose had a slight effect
on maternal body-weight gain, and clinical signs thought to be
related to treatment (unsteadiness, shaking, salivation and
irregular breathing) were seen in four animals. This dose did not
affect the number or growth of offspring or survival in utero. The
two highest doses increased the number of minor skeletal defects.
The Meeting concluded that the NOAEL for maternal toxicity was 5
mg/kg bw per day and that for fetal toxicity was 2 mg/kg bw per day
(Moxon, 1991).
Monkeys
Phosmet (purity unspecified) was administered at doses of 2, 4
or 8 mg/kg bw per day by gastric intubation to groups of seven
pregnant rhesus macaques (Macaca mulatta) on days 22-32 of
gestation; there were no concurrent controls. Except when resorption
or abortion had occurred, the monkeys were delivered by caesarian
section after 83-87 days of pregnancy. Fetal mortality was observed,
but there was no dose-response relationship and no abnormal fetuses
were observed; abortions or resorptions occurred in 2/7 animals at 2
mg/kg bw per day, 0/7 animals at 4 mg/kg bw per day and 1/7 animals
at 8 mg/kg bw per day. The rate of fetal mortality in untreated
monkeys at the laboratory was stated to be 13.2%. External
examination revealed no abnormal fetuses. Thalidomide, which was
also tested in the study, induced both fetal mortality and abnormal
fetuses, while captan at a dose of 25 mg/kg bw per day induced a
high rate of fetal mortality. The NOAEL for phosmet was thus the
highest dose, 8 mg/kg bw per day (Courtney & Finkelstein, 1968).
(f) Genotoxicity
The results of tests for the genotoxicity of phosmet are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Phosmet is not an irritant in the Draize test. It is a mild eye
irritant (Bullock, 1971).
(ii) Delayed neuropathy in chickens
Three groups of 10 white Leghorn hens were given phosmet at
dietary concentrations of 100, 316 or 1000 ppm, equivalent to 12.5,
39.5 or 125 mg/kg bw per day, for 6-7 weeks; a further group of 10
hens received tri- ortho-cresyl phosphate at 1000 ppm (the oral
LD50 of this compound in hens is about 2 g/kg bw), and a fifth
group of three hens received normal diet. One of the hens receiving
tri- ortho-cresyl phosphate died, and eight of the survivors were
paralysed or severely ataxic by the fourth week. One of the birds
receiving the highest dose of phosmet was considered to be slightly
ataxic, but this was not confirmed by another observer. Spinal
axonal degeneration and myelin degeneration were seen in the birds
treated with tri- ortho-cresyl phosphate but not in those given
phosmet (Johnston, 1963b).
Phosmet (purity, 94.7%) was given to groups of 10 white Leghorn
hens at 0, 0.02, 0.20 or 2.05 g/kg bw (14 birds at the highest dose)
orally in gelatine capsules twice at 21-day intervals, and the birds
were killed 21 days after the second dose. Positive controls
received 0.5 g/kg tri- ortho-cresyl phosphate. Atropine (118 mg/kg
bw) and pralidoxime chloride (55 mg/kg bw) were administered
subcutaneously on days 1 and 22 of the study to all birds treated
with phosmet, and birds that showed severe clinical signs were
further treated with the two antidotes. Transient signs of
cholinergic toxicity were observed at the two higher doses; however,
there were no clinical signs or histopathological findings
suggestive of organophosphate-induced delayed neuropathy, whereas
hens treated with tri- ortho-cresyl phosphate had characteristic
histological changes (Sprague, 1982).
Table 2. Results of tests for the genotoxicity of phosmet
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.156-2.5 mg/plate 95.7 Positivea Majeska & Matheson,
100, 1535, 1537 1986
Reverse mutation S. typhimurium TA1535, Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
1536, 1537, 1538 et al., 1976
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate 95.7 Positivea Moriya et al., 1983
100, 1535, 1537
Reverse mutation B. subtilis H17 rec+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and 45 rec- et al., 1976
Reverse mutation E. coli B/r WP2hcr+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and WP2hcr- et al., 1976
Reverse mutation E. coli WP2 hcr approx. 0.100-5000 mg/plate 95.7 Negative Moriya et al., 1983
Cell BALB/3T3 mouse 0.0005-0.014 mg/ml 95.7 Negative Dickey, 1986
transformation cells
Forward mutation Mouse lymphoma 0.02-0.1 mg/mlb 95.7 Positiveb Hertzel, 1986
at tk locus cells (L1578Y) 0.004--0.04 mg/mlc Negativec
Chromosomal Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Negativea Snyder, 1986a
aberration cells (L1578Y) 0.008-0.04 mg/mlc
Sister chromatid Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Positivea Snyder, 1986a
exchange cells (L1578Y) 0.008-0.040 mg/mlc
DNA damage Human fibroblasts 0.25-1 mg/ml 95.7 Negativea Snyder, 1986b
and repair (foreskin)
Table 2 (contd)
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vivo
Micronucleus Mouse bone marrow 17 mg/kg bw orally 95.5 Negative Gibbs, 1986
formation
NR, not reported
a In the presence and absence of metabolic activation
b In the absence of metabolic activation
c In the presence of metabolic activation
3. Observations in humans
The frequency of chromatid-type aberrations in peripheral blood
lymphocytes taken from workers exposed to phosmet was moderately
increased (Király et al., 1979).
A number of cases of phosmet poisoning have been reported in
the literature. In one, poisoning was associated with decrements in
neuromuscular function and ultrastructural abnormalities in the
motor end-plate. The subject had been exposed during a five-week
spraying operation and had at no time been acutely poisoned. The
changes proved to be reversible (Good et al., 1993).
Comments
Phosmet is rapidly absorbed, distributed and excreted,
predominantly in the urine. Less than 1% of the label in the urine
was in the form of phosmet or phosmet oxon. In rats, there were two
major urinary metabolites, N-(methylsulfinylmethyl)phthalamic acid
and N-(methylsulfonylmethyl)-phthalamic acid.
The LD50 values have been estimated for a variety of species
for most routes. The oral LD50 in mice is 20-50 mg/kg bw and that
in rats is 100-300 mg/kg bw. WHO (1992) has classified phosmet as
moderately hazardous.
In a four-week study of toxicity, mice were fed diets
containing 0, 5, 15, 50, 150 or 500 ppm. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of reduced food
intake, reduced body-weight gain and reduced liver and kidney
weights. In a 14-week study of toxicity, rats were fed diets
containing 0, 20, 100 or 500 ppm. The NOAEL was 20 ppm, equivalent
to 1 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a 14-week study in beagle dogs fed diets
containing 0, 10, 75 or 563 ppm, the NOAEL was 75 ppm, equivalent to
1.9 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a two-year study of toxicity in dogs fed
diets containing 0, 20, 40 or 400 ppm, the NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity. The Meeting concluded, however, that
the last study was inappropriate for estimation of an ADI in view of
the small group size and the large dose interval between the NOAEL
and the effect level.
In a two-year study of carcinogenicity in mice fed levels of 0,
5, 25 or 100 ppm, there was evidence of hepatotoxicity at the high
dose, and a slightly but not statistically significantly increased
incidence of hepatic adenomas in comparison with concurrent
controls. There was, however, no increase in incidence in comparison
with historical controls, and the Meeting concluded that there was
no evidence of carcinogenicity in mice. Although brain
cholinesterase activities were determined in this study, the results
proved difficult to interpret: at the interim kill, brain
cholinesterase activity was apparently reduced in each sex in all
dose groups; at the terminal kill, brain cholinesterase activity was
reduced at the high dietary level in females and not at all in
males. The Meeting concluded that the apparent change seen at the
interim kill did not represent a true reaction to treatment with
phosmet, in view of the absence of a similar effect at the terminal
kill, after a longer treatment period. The NOAEL was 25 ppm (equal
to 4 mg/kg bw per day) on the basis of hepatotoxicity and brain
cholinesterase inhibition at the high dose.
In an early, inadequate, two-year study of toxicity and
carcinogenicity in rats treated via the diet, the NOAEL was 40 ppm,
equivalent to 2 mg/kg bw per day, on the basis of depressed
body-weight gain and brain cholinesterase inhibition. In another
two-year study of toxicity and carcinogenicity, rats were fed diets
containing 0, 20, 40 or 200 ppm phosmet, and a smaller group
received 400 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per
day, on the basis of fatty changes in the liver and reduced brain
cholinesterase activity in females. There was no evidence of
carcinogenicity in rats.
Two multigeneration studies of reproductive toxicity have been
conducted with phosmet in rats. In a three-generation study, animals
were exposed to dietary levels of 0 or 40 ppm phosmet (first
generation) and 0, 40 or 80 ppm (second generation). The NOAEL was
40 ppm, equivalent to 2 mg/kg bw per day. In a two-generation (two
litters per generation) study of reproductive toxicity, rats were
fed dietary concentrations of 0, 20, 80 or 300 ppm phosmet. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of
reduced mating and fertility at higher doses.
In a study of teratogenicity, rats were dosed orally at 0, 5,
10 or 15 mg/kg bw per day. The NOAEL for maternal toxicity was 5
mg/kg bw per day on the basis of reduced body-weight gain; there was
no evidence of fetotoxicity or teratogenicity at the highest dose
tested. In a study of teratogenicity in rabbits dosed at 0, 2, 5 or
15 mg/kg bw per day, the NOAEL for maternal toxicity was 5 mg/kg bw
per day on the basis of reduced body-weight gain, while the NOAEL
for fetotoxicity was 2 mg/kg bw per day on the basis of the presence
of minor skeletal anomalies.
The Meeting concluded that phosmet was not clastogenic but that
its mutagenic potential is unclear. In an attempt to address this
issue, studies of DNA binding in vivo are being requested.
In two studies, phosmet did not cause delayed neuropathy in
chickens; however, the Meeting considered a summary of a study in
which some inhibition of brain neuropathy target esterase was
observed at a dose below the LD50. It therefore concluded that
phosmet may have the potential to cause delayed neuropathy, although
at doses higher than the unprotected LD50. A further study is
being requested to clarify this issue.
An ADI was allocated on the basis of the NOAEL in the
multigeneration study in rats (20 ppm, equal to 1.3 mg/kg bw per
day) and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 4 mg/kg bw per day (two-year study
of carcinogenicity)
Rat: 40 ppm, equal to 1.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
20 ppm, equal to 1.3 mg/kg bw per day (two-generation
study of reproductive toxicity)
5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
15 mg/kg bw per day (study of teratogenicity,
developmental toxicity)
Rabbit: 5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
2 mg/kg bw per day (study of teratogenicity,
fetotoxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Long-term study of toxicity in dogs
2. Study of DNA binding in vivo
3. Study of delayed neurotoxicity in chickens at an appropriately
high dose, with estimation of neuropathy target esterase
4. Further observations in humans
In order to maintain the ADI, these data should be submitted in
1997, in time for review in 1998.
References
Ackermann, H., Seidler, H., Kagan, Y.S. & Voronina, V.M. (1976)
Metabolic and toxic behaviour of phthalimide derivatives. 1. Fate of
Imidan in the fetus. Arch. Toxicol., 36, 127-137.
Bullock, C.H. (1971) Imidan (EDC process). Unpublished report
T-1666, dated 27 May 1971 from Stauffer Chemical Co. Western
Research Center. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Bullock, C.H. (1972) Unpublished report T-4035, dated 18 August 1972
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Castles, T.R. (1977) Unpublished report T-6123, dated 3 August 1977
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Chang, J.C.F., Morrisey, R.L. & Wyand, S. (1991) 2-Year chronic
toxicity/oncogenicity study with R-1504 in rats. Unpublished report
T-13241 dated 29 April 1991 from Ciba-Geigy Corp, Agricultural
Division, Environmental Health Center, Farmington, CT, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Courtney, K.D. & Finkelstein, M. (1968) Teratological investigation
of captan, imidan and thalidomide in Macaca mulatta. Unpublished
report T-2164 to Stauffer Chemical Company, dated 4 April 1968 from
Bionetics Research Laboratories Inc., Falls Church, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Danilenko, L.P. (1969) Maximum permissible concentration of
phthalophos in air of working zone. Hyg. Sanit., 34, 192-197.
Dickey, J.K. (1986) Imidan: morphological transformation of
BALB/3T3. Unpublished report T-12822, dated 8 December 1986 from In
Vitro Toxicology Section, Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Fabro, S., Smith, R.L. & Williams, R.T. (1966) Embryotoxic activity
of some pesticides and drugs related to phthalimide. Food Cosmet.
Toxicol., 3, 587-590.
Fisher, G.D. (1989) R-1504. Metabolism study in rats: recovery of
administered dose. Unpublished report T-13031, dated 16 August 1989
from Ciba-Geigy Corp., Environmental Health Center, Farmington, CT,
USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey,
United Kingdom.
Fisher, G.D. (1990) R-1504. Metabolism study in rats:
biotransformation. Unpublished report T-13031, dated 12 February
1990 from Ciba-Geigy Corp., Environmental Health Center, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ford, I.M., Menn, J.J. & Meyding, G.D. (1966) Metabolism of
N-(mercaptomethyl)phthalimide-carbonyl-C14-(S-(O,O-dimethylphospho
rothioate) (Imidan-C14): balance study in the rat. J. Agric. Food
Chem., 14, 83-86.
Frank, A.A., Ross, J.L. & Sawvell, B.K. (1992) Toxic epidermal
necrolysis associated with flea dips. Vet. Hum. Toxicol., 34,
57-61.
Gibbs, J.A. (1986) Phosmet report of a micronucleus test in the
mouse. Unpublished report No. 2516, dated 18 December 1986 from
Beecham Pharmaceuticals Research Division, Genetic Toxicology Unit,
Stock, United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Good, J.L., Khurana, R.K., Mayer, R.F., Cintra, W.M. & Albuquerque,
E.X. (1993) Pathophysiological studies of neuromuscular function in
subacute organophosphate poisoning induced by phosmet. J. Neurol.
Neurosurg. Psychiatr., 56, 290-294.
Hendricks, M. & Sprague, G.L. (1983) Imidan technical: effect on rat
cholinesterases. Unpublished report No. T-11228, dated 26 April
1983. Richmond Toxicology Laboratory, Stauffer Chemical Co.,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Hertzel, K.M. (1986) Imidan: mutagenicity evaluation in mouse
lymphoma multiple endpoint test forward mutation assay. Unpublished
report No. T-12820, dated 8 May 1986. In Vitro Toxicology Section,
Environmental Health Center, Stauffer Chemical Co., Agrochemical
Division, Farmington, CT, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Hodge, M.C.E. (1991) Phosmet: teratogenicity study in the rat.
Unpublished report No. CTL/P/3360, dated 17 June 1991. ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Hollingsworth, R.L., Johnston, C.D. & Woodard, G. (1965) Imidan
three generation study in rats. Unpublished report, dated 7 May
1965, from Woodard Research Corp., Herndon, VA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Izmerov, N.F. (1983) Scientific Reviews of Soviet Literature on
Toxicity and Hazards of Chemicals, Moscow, Centre of International
Projects. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Johnston, C.D. (1962) Imidan: an evaluation of safety of Imidan in
the rat and the dog. Unpublished report, dated 21 February 1962,
from Woodard Research Corp., Herndon, VA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963a) Further evaluation of the safety of Imidan in
the rat. Unpublished report, dated 27 February 1963, from Woodard
Research Corp., Herndon, VA, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963b) Imidan: demyelination study in the chicken.
Unpublished report, dated 27 February 1963, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1966) Imidan: single dose oral toxicity in mice.
Unpublished report, dated 22 November 1966, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Jones, N.B., Sauerhoff, M.W., Thomassen, R.W. & Zwicker, G.M. (1981)
Four week dietary range-finding study in mice with Imidan technical.
Unpublished report No. T-10704, dated July 1981 from Environmental
Health Center, Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Katz, A., Frank, D.W., Zwicker, G.M., Sprague, G.L., Turnier, J.C. &
Freudenthal, R.I. (1984) T(TM)10719. Two-year dietary oncogenicity
study in mice with Imidan technical. Unpublished report, dated May
1984, from Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Király, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. Environ. Contam. Toxicol., 8, 309-319.
Lee, H. & Miaullis, J.B. (1969) The effect of imidan on
cholinesterase and aliesterase activity in rats. Unpublished report
No. 004571 from Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Lobdell, B.J. & Johnston, C.D. (1966) Imidan: safety evaluation by
two-year feeding studies in the rat and the dog. Unpublished report,
dated 21 July 1966, from Woodard Research Corp., Herndon, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
McBain, J.B., Menn, J.J. & Casida, J.E. (1968) Metabolism of
carbonyl-C14-labelled Imidan, N-(mercaptomethyl)phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats and cockroaches. J.
Agric. Food Chem., 16, 813-820.
Majeska, J.B. & Matheson, D.W. (1986) Imidan: mutagenicity
evaluation in Salmonella typhimurium Unpublished report No. T-12819,
dated 4 March 1986 from Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the
effect of a series of phosphoroorganic pesticides (Dipterex and
Imidan) on embryogenesis. Environ. Health Perspectives, 13,
121-125.
Meyer, L.S. & Walberg, J.A. (1990) A two generation reproduction
study in rats with R-1504. Unpublished report No. T-13260, dated 18
May 1990, from Environmental Health Center, Ciba-Geigy Corp.,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Meyding, G.D. (1965) Imidan technical: acute oral subcutaneous and
intraperitoneal toxicity in rats and mice. Unpublished report No.
65-2, dated 26 January 1965 from Richmond Research Center, Animal
Health Toxicology Section, Stauffer Chemical Co., Richmond, CA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Moxon, M.E. (1991) Phosmet: teratogenicity study in the rabbit.
Unpublished report no. CTL/P/3373, dated 20 June 1991, from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, United
Kingdom. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Nuclear Science Corp. (1962) Unpublished report, Project No.
20-0240-32, dated 30 April 1962, from Nuclear Science Corp., Palo
Alto, CA, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ray, D.G. (1964) Imidan RP4-RCT-116: acute oral LD50--rats.
Unpublished report No. 507, dated 18 February 1964, from Biological
Research Center, Toxicology Section, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Shirasu, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4, 226-231.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Microbial screening of pesticides in microbial systems. Mutat.
Res., 40, 19-30.
Snyder, R.D. (1986a) Imidan: Mutagenicity evaluation in mouse
lymphoma multiple endpoint test cytogenetic assay. Unpublished
report No. T-12821, dated 8 May 1986, from In Vitro Toxicology
Section, Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Snyder, R.D. (1986b) Effects of imidan on human fibroblast DNA.
Unpublished report No. T-12823, dated 30 May 1986, from In Vitro
Toxicology Section, Environmental Health Centre, Stauffer Chemical
Co., Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Sprague, G.L. (1982) Acute delayed neurotoxicity study with Imidan
technical in adult hens. Unpublished report No. T-10910, dated 9
August 1982, from Richmond Toxicology Laboratory, Toxicology
Department, Stauffer Chemical Co., de Guigne Technical Center,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Sprague, G.L. & Turnier, J.C. (1986) T-10719. Addendum: Two-year
dietary oncogenicity study in mice with Imidan technical.
Unpublished addendum, dated 22 May 1986, from Environmental Health
Center, Stauffer Chemical Co., Agrochemical Division, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (Dipterex, Imidan) during pregnancy. Environ. Health
Perspectives, 13, 133-140.
Tarr, J.B. & Hemingway, R.J. (1993a) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and milk of
lactating goats. Unpublished report RR 92-103B, study No. PMS-352,
dated 22 January 1983, from Zeneca Agricultural Products, Western
Research Center, ICI Americas Inc., Richmond, CA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Tarr, J.B. & Hemingway, R.J. (1993b) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and eggs of
laying hens. Unpublished report No. 1227-1, RR 93-046B, dated 14
January 1983, from Zeneca Agricultural Products, Western Research
Center, ICI Americas Inc., Richmond, CA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
(c) Effects on enzymes and other biochemical parameters
Erythrocyte and brain cholinesterase are more sensitive to
phosmet in rats than is plasma cholinesterase. Rat aliesterases are
more sensitive to inhibition by phosmet than is
acetylcholinesterase. Other organophosphates (malathion, parathion,
parathion-methyl, schradan, tributyl phosphorotrithioite, diazinon,
EPN, ethion, demeton, mevinphos, carbophenothion, disulfoton
azinphos-methyl) and a carbamate anticholinesterase (carbaryl) did
not potentiate the effect of phosmet in male Sprague-Dawley rats
(Lee & Miaullis, 1969).
Male and female CD rats were treated orally with 0, 10 or 100
mg/kg bw phosmet (purity, 94.7%), and plasma, erythrocyte and brain
cholinesterase activities were measured 4 and 24 h later. The lower
dose had no effect on plasma or erythrocyte cholinesterase
activities at either time or on brain cholinesterase activity at 24
h; however, 4 h after treatment with this dose, brain cholinesterase
activity was inhibited by 14% in males and 21% in females. At 100
mg/kg bw, substantial inhibition was found at both times, except for
plasma enzyme in females at 4 h. At that time, the activity of the
erythrocyte enzyme was the most strongly inhibited (about 85%) and
that of the brain enzyme somewhat less (about 65%); the activity of
plasma cholinesterase was inhibited by only about 35% in males and
not significantly in females. At 24 h, the activity of the brain
enzyme was recovering (34% inhibition in males and 48% in females),
as was that of erythrocyte cholinesterase (40 and 45% inhibition),
while the activity of plasma cholinesterase was further inhibited
(46 and 74% inhibition) (Hendricks & Sprague, 1983).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of phosmet is summarized in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice received
technical-grade phosmet (purity, 95%) in the diet at concentrations
of 0, 5, 15, 50, 150 or 500 ppm, equivalent to 0.75, 2.25, 7.5, 22.5
or 75 mg/kg bw per day, for four weeks. Significant decreases in
food consumption and body-weight gain were seen at 150 and 500 ppm
in males and at 500 ppm in females; females given 150 ppm had only
reduced food intake. Males at the two highest doses had a
significant decrease in absolute liver and kidney weights; relative
liver weights were significantly increased at 150 and 500 ppm in
males and females, while relative kidney weights were significantly
increased only in females at 500 ppm. Erythrocyte cholinesterase
activity was depressed at 50 ppm and above, and brain cholinesterase
activity was statistically significantly depressed by 16% in females
receiving the highest dose. No treatment-related changes were seen
histologically. The NOAEL was 50 ppm, equivalent to 7.5 mg/kg bw per
day, on the basis of reduced food consumption and body-weight gain
and decreased absolute liver and kidney weights at 150 ppm (Jones
et al., 1981).
Rats
Groups of 15 male and 15 female albino rats (strain
unspecified) were given phosmet (purity, 98%) in the diet at 0, 20,
100 or 500 ppm, equivalent to 1, 5 or 25 mg/kg bw per day, for 14
weeks. A second study was started four weeks later, involving four
groups of the same size treated with the same doses. Decreased
weight gain was found in the group receiving the highest dose.
Erythrocyte cholinesterase activity was inhibited by > 20% at the
middle and high doses, while plasma cholinesterase was inhibited by
> 20% at the high dose only; brain cholinesterase activity was
inhibited by > 20% at terminal sacrifice in the high and middle
dose groups. Changes described as 'necrobiotic foci' were observed
in the liver at 500 ppm, which were considered not to be related to
treatment. The NOAEL was 20 ppm, equivalent to 1.0 mg/kg bw per day,
on the basis of inhibition of brain cholinesterase activity at 100
ppm (Johnston, 1962).
Table 1. Acute toxicity of phosmet
Species Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
(95% CI or range)
Mouse Swiss-Webster albino M Oral 50.1 (34.4-73.0) NR Meyding, 1965
Mouse Swiss-Webster albino M Oral 20-43 a Bullock, 1971
Mouse NR M Oral 36.9 (21.7-62.8) 95 Bullock, 1972
Mouse Albino M&F Oral 38 b Johnston, 1966
Mouse Albino M&F Oral 49 c Johnston, 1966
Mouse Albino M&F Oral 43 d Johnston, 1966
Mouse Albino NR Oral 26-60 NR Danilenko, 1969
Mouse Swiss-Webster M Intraperitoneal 40-50 NR Meyding, 1965
Mouse Swiss-Webster M Subcutaneous 300 NR Meyding, 1965
Rat Albino M Oral 310 (267-360) NR Ray, 1964
Rat Sprague-Dawley M Oral 245 (161-367) NR Meyding, 1965
Rat Sprague-Dawley albino M Oral 140 (76-255) 98 Nuclear Science Corp.,
1962
Rat Albino NR Oral 92.5-164 NR Danilenko, 1969
Rat Sprague-Dawley albino M Oral 135-147a a Bullock, 1971
Rat Sprague-Dawley albino M Oral 121.3 (90.6-162.5) 92.5 Castles, 1977
Rat Sprague-Dawley albino F Oral 121.3 (96.7-152.1) 92.5 Castles, 1977
Rat Sprague-Dawley M Intraperitoneal approx. 100 NR Meyding, 1965
Rat Sprague-Dawley M Subcutaneous > 1200 NR Meyding, 1965
Rat Sprague-Dawley albino F Inhalation > 0.15e 92.5 Castles, 1977
Guinea-pig NR NR Oral 200 NR Danilenko, 1969
Rabbit New Zealand white NR Percutaneous > 4600 a Bullock, 1971
Rabbit New Zealand white NR Percutaneous > 5000 92.5 Castles, 1977
Cat NR NR Inhalation 65f NR Cited by Izmerov,
1983
Chicken White Leghorn F Oral 2020 94.7 Sprague, 1982
Table 1 (continued)
NR, not reported
a Precise figure depended on formulation and route of synthesis of active ingredient.
b Technical-grade in corn oil; purity not stated
c Technical-grade in 20% PEG300; purity not stated
d Imidan 50% wettable powder in aqueous suspension; concentration of active ingredient, 50%; results given
for active ingredient
e LC50' 1 h: mg/l
f LC50' time unstated
Three groups of 10 male and 10 female albino rats (strain
unspecified) were treated with phosmet for 16 weeks. Animals in the
'low dose' group initially received 450 ppm, equivalent to 22.5
mg/kg bw per day, which was increased stepwise to 6000 ppm,
equivalent to 300 mg/kg bw per day, by the 12th week. The 'high
dose' group was started on 800 ppm, equivalent to 40 mg/kg bw per
day, which was increased stepwise to 1120 ppm, equivalent to 56
mg/kg bw per day. Clinical signs (nervousness, tremors, diarrhoea)
were observed in all treated animals. Body-weight gain was decreased
in the high dose group in comparison with controls, and marked
hepatic degenerative changes (eosinophilia, vacuolation and
swelling, with little change in nuclear morphology) were also seen
in the high-dose group. Erythrocyte cholinesterase activity was
inhibited by about 100% in both groups, and plasma cholinesterase
activity was inhibited by > 50% in all groups. Brain cholinesterase
was inhibited by 75 and 80% in males at the low and high doses and
by 84 and 82% in females at the low and high doses, respectively.
There was no NOAEL, as brain cholinesterase activity was inhibited
in both treated groups and because of the variable dosing (Johnston,
1963a).
Cats
Veterinary use of phosmet as a dip for treating flea
infestation caused toxic epidermal necrolysis in a female Himalayan
cat. This observation has not been repeated (Frank et al., 1992).
Dogs
Groups of four male and four female beagle dogs were treated
with dietary concentrations of 0, 10, 75 or 563 ppm, equivalent to
0.25, 1.9 or 14 mg/kg bw per day, for 14 weeks. All of the animals
gained weight, except for one given the middle and one given the low
dose. Haematological and clinical chemical parameters were
unaffected by the treatment, except that marked depression of
erythrocyte cholinesterase activity and somewhat less marked
depression of plasma cholinesterase activity were found at the high
dose. Brain cholinesterase activity at terminal sacrifice was also
depressed at the high dose. The levels of plasma, erythrocyte and
brain cholinesterase were normal in the other three groups. At
autopsy, no pathological changes attributable to treatment were
observed. The NOAEL was 75 ppm, equivalent to 1.9 mg/kg bw per day
(Johnston, 1962).
Groups of three male and three female beagle dogs received
phosmet (purity unspecified) in the diet at concentrations of 0, 20,
40 or 400 ppm, equivalent to 0.5, 1 or 10 mg/kg bw per day, for two
years. One male at the highest dose was killed in extremis;
survival was otherwise unaffected. Erythrocyte cholinesterase
activity was depressed at 400 ppm throughout most of the study. At
termination, brain cholinesterase activity was considerably
depressed in the animals at the high dose, to 58% of control
activity in males and 32% in females. Interpretation of the study
was hampered by the small group size. The NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day (Lobdell & Johnston, 1966).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female B6C3F1 mice were fed phosmet
(purity, 94.7%) at dietary concentrations of 0, 5, 25 or 100 ppm,
equal to 0.75, 4 or 15 mg/kg bw per day for two years. Up to 10 mice
of each sex in each group were killed at 12 months, and
cholinesterase activity and haematological parameters were measured
in these animals and in 10 animals of each sex from each group at
terminal sacrifice. Plasma and brain cholinesterase activities were
measured at the time of the interim kill, and plasma, brain and
erythrocyte cholinesterase at termination. Phosmet did not affect
survival. Body weight was slightly but significantly increased for
most of the study in animals receiving the highest dose, and food
consumption was occasionally reduced. In males at the highest dose
there was an increased frequency of convulsions, usually associated
with handling of the animals.
Plasma cholinesterase activity was inhibited by about 50% in
males and females at the highest dose and by about 13% in females at
the middle dose at the time of interim sacrifice. Brain
cholinesterase activity was inhibited by > 20% in all treated
groups at interim sacrifice. Plasma cholinesterase activity was
inhibited at termination in the groups receiving the highest dose,
but the activity in erythrocytes was comparable in all groups. Some
inhibition of brain cholinesterase activity was found in females at
the middle and highest doses at termination, but, while
statistically significant, the depression was < 20% in the middle
dose group and 22% in the highest dose group. Brain cholinesterase
activity was not depressed in males at the terminal kill.
No treatment-related effects were seen on haematological
parameters at interim or final sacrifice, and no treatment-related
changes in organ weights or macroscopic or microscopic appearance
were seen, except in the liver. Relative liver weights were
increased at 12 months in males receiving 100 ppm, and at
termination there was an increase in the prevalence of mild
vacuolation. There was also an increase in the incidence of liver
adenomas (25/50, with 13/49 in controls) in males at the highest
dose. When animals killed at 12 months were included, liver adenomas
were found in 13/60 controls, 10/60 at 5 ppm, 14/60 at 25 ppm and
27/60 at 100 ppm. In an addendum to the report, the prevalence of
liver adenomas in the group given the highest dose was reported to
be comparable to that in historical controls. No increase in the
incidence of liver tumours was seen in females. The NOAEL was 25
ppm, equal to 4 mg/kg bw per day, on the basis of increased
incidences of convulsions, hepatocellular cytoplasmic vacuolation
and liver-cell adenomas in males and decreased brain cholinesterase
activity in females at 100 ppm. The apparent change in brain
cholinesterase activity at the time of the interim kill was
considered not to be a true reaction to treatment with phosmet, in
view of the absence of a similar effect at the time of the terminal
kill, after a longer period of treatment (Katz et al., 1984;
Sprague & Turnier, 1986).
Rats
Groups of 25 male and 25 female albino rats (strain
unspecified) received phosmet (purity unspecified) in the diet at
concentrations of 0, 20, 40 or 400 ppm, equivalent to 1, 2 or 20
mg/kg bw per day, for two years. Body-weight gain and plasma and
erythrocyte cholinesterase activities were depressed at 400 ppm; the
cholinesterase activities were decreased throughout the study, and
brain cholinesterase activity was lowered at termination. Hepatocyte
vacuolation was seen at the highest dose. Pituitary adenomas were
more frequent at 40 ppm (46%) and 400 ppm (56%) than in the controls
(36%) and in those receiving 20 ppm (21%), but there was not a clear
dose-response relationship. The small groups and the small number of
survivors at termination made interpretation of the study somewhat
difficult and precluded a conclusion being drawn about the
carcinogenicity of phosmet. The NOAEL was 40 ppm, equivalent to 2
mg/kg bw per day (Lobdell & Johnston, 1966).
Groups of 60 or 70 Sprague-Dawley Crl:CD SD BR rats of each sex
received phosmet (purity, 94.3%) in the diet at concentrations of 0,
20, 40 or 200 ppm, equal to 1.1, 1.8 or 9.4 mg/kg bw per day for
males and 1.1, 2.1 or 10.9 mg/kg bw per day for females. For interim
evaluation, 20 rats of each sex from the control group and 10 of
each sex from each test group were killed after 12 months, and the
study was continued for a further year. An additional group of 20
rats of each sex received 400 ppm (equal to 23 mg/kg bw per day for
males and 27 mg/kg bw per day for females) for 12 months. Exposure
did not adversely affect survival; indeed, there appeared to be a
dose-related increase in survival. No specific clinical signs were
attributable to treatment. Weight gain was reduced throughout the
study in animals of each sex at 400 ppm and in the early part of the
study at 40 (at 4, 21, 37, 45 and 49 weeks) and 200 ppm (at 1, 2 and
4weeks) in females only. These decreases are unlikely to be
biologically significant. Significant reductions in cholinesterase
activities were observed: plasma cholinesterase activity was reduced
by > 20% at 200 and 400 ppm in males and at 40, 200 and 400 ppm in
females; a reduction of 21% was observed at 18 weeks in females at
20 ppm. Erythrocyte cholinesterase activity was reduced by 15-20% at
20 ppm and by > 20% at higher dietary concentrations in males, and
by 15-20% at 40 ppm and very considerably at higher concentrations
in females. Brain cholinesterase activity was depressed in males at
200 ppm (interim and final kill), although the depression at 24
months was less than 15%. In females, depression of brain
cholinesterase activity by > 20% was observed at 200 ppm at both
interim and final sacrifice. Brain cholinesterase activity was also
depressed in animals of each sex at 400 ppm. An increase in the
incidence and severity of fatty liver was seen at 200 ppm. No
tumours were seen that were attributable to treatment with phosmet.
The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day, on the basis of
an increased incidence of fatty change in the livers of animals of
each sex and depressed brain cholinesterase activity in females at
200 ppm (Chang et al., 1991).
(d) Embryotoxicity and teratogenicity
Rats
In Wistar rats given a single dose of 30 mg/kg bw phosmet
orally once on day 9 of pregnancy or 1.5 mg/kg bw on alternate days
(every day in the summary) throughout pregnancy, post-implantation
mortality of embryos was increased. The dose of 30 mg/kg bw on day 9
or 13 caused developmental abnormalities, such as hypognathia and
hydrocephaly. A dose of 0.06 mg/kg bw on alternate days (every day
in the summary) had no effect. There was no NOAEL (Martson &
Voronina, 1976).
Phosmet (purity, 95.8%) was administered in the diet of CD rats
at amounts that provided a mean consumption of 0, 10, 22, 27 or 29
mg/kg bw per day on days 6-15 of pregnancy. The size of the groups
varied from 47 controls to 17 and 23 at the two higher doses. The
rats were killed on day 21. At 22 mg/kg bw per day, food intake and
weight gain were reduced. As phosmet was neither teratogenic nor
fetotoxic, the NOAEL for maternal toxicity was 10 mg/kg bw per day
and the NOAEL for fetal toxicity was the highest dose, 29 mg/kg bw
per day. In a similar study, in which phosmet was administered by
gavage at doses of 5, 10, 20, 25 or 30 mg/kg bw per day (with no
separate control group), survival was affected at the two higher
doses. There was a significant reduction in the proportion of rats
at 25 mg/kg bw per day that became pregnant, and there was a
reduction in food intake at > 10 mg/kg bw per day and a reduction
in weight gain at > 20 mg/kg bw per day. In the absence of a
suitable control group, there was no NOAEL in this study (Staples
et al., 1976).
Groups of 24 female alpk:APfSD rats were dosed with phosmet
(purity, 96.4%) in corn oil by gavage at 0, 5, 10 or 15 mg/kg bw per
day on days 7-16 days of gestation. The rats were killed on day 22
of gestation and the uteruses examined for live fetuses and
intrauterine deaths. Maternal toxicity in the form of reduced
body-weight gain, reduced food consumption and clinical signs
(shaking, piloerection) was seen at the highest dose. As there were
smaller but statistically significant effects on body-weight gain at
10 mg/kg bw per day between days 7 and 16, the lowest dose, 5 mg/kg
bw per day, was the NOAEL for maternal toxicity. As no teratogenic
or fetotoxic effects were seen, the NOAEL for developmental toxicity
was 15 mg/kg bw per day (Hodge, 1991).
In a three-generation study in CD rats, the F0 generation
consisted of two groups of 20 males and two groups of 20 females:
One group of each sex received no treatment, while the other
received technical-grade phosmet (purity, 99%) in the feed at 40
ppm, equivalent to 2 mg/kg bw per day, or half this concentration
during the first three weeks. Animals in each group were mated
twice, and the offspring (F1a and F1b) were examined at birth
and at weaning, when the F1a rats were killed. The F0 rats were
killed but not examined by necropsy. The F1b rats were retained to
make up three new groups of 20 rats of each sex, which received
phosmet at dietary concentrations of 0, 40 or 80 ppm from weaning
until sacrifice. Mating of the F1b rats produced F2a and F2b
litters; the F2a rats were sacrificed and the F2b offspring were
used, like the preceding generation, to make three new groups of
F2a and F2b rats. Offspring were sacrificed at weaning. The
treated and control animals were comparable throughout the study. On
histopathological examination of the F3b animals, some mild hepatic
vacuolation and reduced glycogen content were observed more
frequently in treated than in untreated animals; however, the former
finding was not frequent in any dose group, and the latter (in
adults and therefore presumably in fetuses) was strongly dependent
on time since last feed, which may have been different for the
various dose groups. In view of the lack of other findings, the
Meeting considered the NOAEL to be 40 ppm, equivalent to 2 mg/kg bw
per day (Hollingsworth et al., 1965).
In a two-generation study, technical-grade phosmet (purity,
95.2%) was administered to Crl:CD(SD)BRVAF/+tm rats at dietary
concentrations of 0, 20, 80 or 300 ppm, equal to 1.3, 5.0 or 19.4
mg/kg bw per day in F0 males; 1.5, 6.3 or 24.3 mg/kg bw per day in
F1 males; 1.5, 6.0 or 24.4 in F0 females; and 1.5, 6.2 or 26.4
mg/kg bw per day in F1 females. Treatment of the F0 generation
was started at 56 days of age, and mating occurred 56 days later.
The F1a animals were weaned at 21 days and sacrificed. Shortly
afterwards, the F0 rats were again mated to produce the F1b
litters, of which about 25 males and 25 females were used to form
the F1 parents. At about 114 days of age, the F1 parents were
mated to produce the F2 litters. Toxicity was observed in parents
at 80 and 300 ppm in both generations. Reduced body-weight gain and
food consumption were observed in animals of each sex at 300 ppm.
Males had reduced testicular weights in both generations and reduced
spleen weight and hepatocellular vacuolization in the F1
generation. Dehydration was seen in the F0 females at 300 ppm, and
chromorhinorrhoea was seen in F1 females at 300 ppm. At 80 ppm,
there was reduced body-weight gain in F0 male parents and reduced
erythrocyte cholinesterase activity in both generations; in F0
parental females, there was reduced weight gain during lactation and
reduced relative liver and adrenal weights. Reduced relative spleen
and thymus weights were seen in the F1 generation, and reduced
erythrocyte cholinesterase activity was seen in both generations.
Plasma cholinesterase activity was reduced in parental F0 males
only. Erythrocyte cholinesterase activity, although reduced, was >
80% of that of controls at 20 ppm. Mating and fertility were reduced
at 80 and 300 ppm, and there were reduced numbers of pups per
litter, reduced pup weight and reduced survival of pups at 300 ppm.
The NOAEL for toxicity to the parents and for effects on
reproductive performance was 20 ppm (equal to 1.3 mg/kg bw per day),
and the NOAEL for developmental toxicity was 80 ppm (equal to 5.0
mg/kg bw per day) (Meyer & Walberg, 1990).
Rabbits
Phosmet (purity unspecified) was administered by gavage to New
Zealand white rabbits at 35 mg/kg bw per day on days 7-12 of
pregnancy; the animals were killed at day 28. No embryotoxic effects
were detected, although such effects were observed with thalidomide
in the same study. The NOAEL for phosmet was 35 mg/kg bw per day;
however the treatment period was rather short and included only part
of the period of organogenesis (Fabro et al., 1966).
Groups of 20 female New Zealand white rabbits were treated with
phosmet (purity, 96.4%) at doses of 0, 2, 5 or 15 mg/kg bw per day
in corn oil by gavage on days 7-19 of pregnancy. The animals were
killed on day 30 of gestation. The highest dose had a slight effect
on maternal body-weight gain, and clinical signs thought to be
related to treatment (unsteadiness, shaking, salivation and
irregular breathing) were seen in four animals. This dose did not
affect the number or growth of offspring or survival in utero. The
two highest doses increased the number of minor skeletal defects.
The Meeting concluded that the NOAEL for maternal toxicity was 5
mg/kg bw per day and that for fetal toxicity was 2 mg/kg bw per day
(Moxon, 1991).
Monkeys
Phosmet (purity unspecified) was administered at doses of 2, 4
or 8 mg/kg bw per day by gastric intubation to groups of seven
pregnant rhesus macaques (Macaca mulatta) on days 22-32 of
gestation; there were no concurrent controls. Except when resorption
or abortion had occurred, the monkeys were delivered by caesarian
section after 83-87 days of pregnancy. Fetal mortality was observed,
but there was no dose-response relationship and no abnormal fetuses
were observed; abortions or resorptions occurred in 2/7 animals at 2
mg/kg bw per day, 0/7 animals at 4 mg/kg bw per day and 1/7 animals
at 8 mg/kg bw per day. The rate of fetal mortality in untreated
monkeys at the laboratory was stated to be 13.2%. External
examination revealed no abnormal fetuses. Thalidomide, which was
also tested in the study, induced both fetal mortality and abnormal
fetuses, while captan at a dose of 25 mg/kg bw per day induced a
high rate of fetal mortality. The NOAEL for phosmet was thus the
highest dose, 8 mg/kg bw per day (Courtney & Finkelstein, 1968).
(f) Genotoxicity
The results of tests for the genotoxicity of phosmet are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Phosmet is not an irritant in the Draize test. It is a mild eye
irritant (Bullock, 1971).
(ii) Delayed neuropathy in chickens
Three groups of 10 white Leghorn hens were given phosmet at
dietary concentrations of 100, 316 or 1000 ppm, equivalent to 12.5,
39.5 or 125 mg/kg bw per day, for 6-7 weeks; a further group of 10
hens received tri- ortho-cresyl phosphate at 1000 ppm (the oral
LD50 of this compound in hens is about 2 g/kg bw), and a fifth
group of three hens received normal diet. One of the hens receiving
tri- ortho-cresyl phosphate died, and eight of the survivors were
paralysed or severely ataxic by the fourth week. One of the birds
receiving the highest dose of phosmet was considered to be slightly
ataxic, but this was not confirmed by another observer. Spinal
axonal degeneration and myelin degeneration were seen in the birds
treated with tri- ortho-cresyl phosphate but not in those given
phosmet (Johnston, 1963b).
Phosmet (purity, 94.7%) was given to groups of 10 white Leghorn
hens at 0, 0.02, 0.20 or 2.05 g/kg bw (14 birds at the highest dose)
orally in gelatine capsules twice at 21-day intervals, and the birds
were killed 21 days after the second dose. Positive controls
received 0.5 g/kg tri- ortho-cresyl phosphate. Atropine (118 mg/kg
bw) and pralidoxime chloride (55 mg/kg bw) were administered
subcutaneously on days 1 and 22 of the study to all birds treated
with phosmet, and birds that showed severe clinical signs were
further treated with the two antidotes. Transient signs of
cholinergic toxicity were observed at the two higher doses; however,
there were no clinical signs or histopathological findings
suggestive of organophosphate-induced delayed neuropathy, whereas
hens treated with tri- ortho-cresyl phosphate had characteristic
histological changes (Sprague, 1982).
Table 2. Results of tests for the genotoxicity of phosmet
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.156-2.5 mg/plate 95.7 Positivea Majeska & Matheson,
100, 1535, 1537 1986
Reverse mutation S. typhimurium TA1535, Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
1536, 1537, 1538 et al., 1976
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate 95.7 Positivea Moriya et al., 1983
100, 1535, 1537
Reverse mutation B. subtilis H17 rec+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and 45 rec- et al., 1976
Reverse mutation E. coli B/r WP2hcr+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and WP2hcr- et al., 1976
Reverse mutation E. coli WP2 hcr approx. 0.100-5000 mg/plate 95.7 Negative Moriya et al., 1983
Cell BALB/3T3 mouse 0.0005-0.014 mg/ml 95.7 Negative Dickey, 1986
transformation cells
Forward mutation Mouse lymphoma 0.02-0.1 mg/mlb 95.7 Positiveb Hertzel, 1986
at tk locus cells (L1578Y) 0.004--0.04 mg/mlc Negativec
Chromosomal Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Negativea Snyder, 1986a
aberration cells (L1578Y) 0.008-0.04 mg/mlc
Sister chromatid Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Positivea Snyder, 1986a
exchange cells (L1578Y) 0.008-0.040 mg/mlc
DNA damage Human fibroblasts 0.25-1 mg/ml 95.7 Negativea Snyder, 1986b
and repair (foreskin)
Table 2 (contd)
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vivo
Micronucleus Mouse bone marrow 17 mg/kg bw orally 95.5 Negative Gibbs, 1986
formation
NR, not reported
a In the presence and absence of metabolic activation
b In the absence of metabolic activation
c In the presence of metabolic activation
3. Observations in humans
The frequency of chromatid-type aberrations in peripheral blood
lymphocytes taken from workers exposed to phosmet was moderately
increased (Király et al., 1979).
A number of cases of phosmet poisoning have been reported in
the literature. In one, poisoning was associated with decrements in
neuromuscular function and ultrastructural abnormalities in the
motor end-plate. The subject had been exposed during a five-week
spraying operation and had at no time been acutely poisoned. The
changes proved to be reversible (Good et al., 1993).
Comments
Phosmet is rapidly absorbed, distributed and excreted,
predominantly in the urine. Less than 1% of the label in the urine
was in the form of phosmet or phosmet oxon. In rats, there were two
major urinary metabolites, N-(methylsulfinylmethyl)phthalamic acid
and N-(methylsulfonylmethyl)-phthalamic acid.
The LD50 values have been estimated for a variety of species
for most routes. The oral LD50 in mice is 20-50 mg/kg bw and that
in rats is 100-300 mg/kg bw. WHO (1992) has classified phosmet as
moderately hazardous.
In a four-week study of toxicity, mice were fed diets
containing 0, 5, 15, 50, 150 or 500 ppm. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of reduced food
intake, reduced body-weight gain and reduced liver and kidney
weights. In a 14-week study of toxicity, rats were fed diets
containing 0, 20, 100 or 500 ppm. The NOAEL was 20 ppm, equivalent
to 1 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a 14-week study in beagle dogs fed diets
containing 0, 10, 75 or 563 ppm, the NOAEL was 75 ppm, equivalent to
1.9 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a two-year study of toxicity in dogs fed
diets containing 0, 20, 40 or 400 ppm, the NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity. The Meeting concluded, however, that
the last study was inappropriate for estimation of an ADI in view of
the small group size and the large dose interval between the NOAEL
and the effect level.
In a two-year study of carcinogenicity in mice fed levels of 0,
5, 25 or 100 ppm, there was evidence of hepatotoxicity at the high
dose, and a slightly but not statistically significantly increased
incidence of hepatic adenomas in comparison with concurrent
controls. There was, however, no increase in incidence in comparison
with historical controls, and the Meeting concluded that there was
no evidence of carcinogenicity in mice. Although brain
cholinesterase activities were determined in this study, the results
proved difficult to interpret: at the interim kill, brain
cholinesterase activity was apparently reduced in each sex in all
dose groups; at the terminal kill, brain cholinesterase activity was
reduced at the high dietary level in females and not at all in
males. The Meeting concluded that the apparent change seen at the
interim kill did not represent a true reaction to treatment with
phosmet, in view of the absence of a similar effect at the terminal
kill, after a longer treatment period. The NOAEL was 25 ppm (equal
to 4 mg/kg bw per day) on the basis of hepatotoxicity and brain
cholinesterase inhibition at the high dose.
In an early, inadequate, two-year study of toxicity and
carcinogenicity in rats treated via the diet, the NOAEL was 40 ppm,
equivalent to 2 mg/kg bw per day, on the basis of depressed
body-weight gain and brain cholinesterase inhibition. In another
two-year study of toxicity and carcinogenicity, rats were fed diets
containing 0, 20, 40 or 200 ppm phosmet, and a smaller group
received 400 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per
day, on the basis of fatty changes in the liver and reduced brain
cholinesterase activity in females. There was no evidence of
carcinogenicity in rats.
Two multigeneration studies of reproductive toxicity have been
conducted with phosmet in rats. In a three-generation study, animals
were exposed to dietary levels of 0 or 40 ppm phosmet (first
generation) and 0, 40 or 80 ppm (second generation). The NOAEL was
40 ppm, equivalent to 2 mg/kg bw per day. In a two-generation (two
litters per generation) study of reproductive toxicity, rats were
fed dietary concentrations of 0, 20, 80 or 300 ppm phosmet. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of
reduced mating and fertility at higher doses.
In a study of teratogenicity, rats were dosed orally at 0, 5,
10 or 15 mg/kg bw per day. The NOAEL for maternal toxicity was 5
mg/kg bw per day on the basis of reduced body-weight gain; there was
no evidence of fetotoxicity or teratogenicity at the highest dose
tested. In a study of teratogenicity in rabbits dosed at 0, 2, 5 or
15 mg/kg bw per day, the NOAEL for maternal toxicity was 5 mg/kg bw
per day on the basis of reduced body-weight gain, while the NOAEL
for fetotoxicity was 2 mg/kg bw per day on the basis of the presence
of minor skeletal anomalies.
The Meeting concluded that phosmet was not clastogenic but that
its mutagenic potential is unclear. In an attempt to address this
issue, studies of DNA binding in vivo are being requested.
In two studies, phosmet did not cause delayed neuropathy in
chickens; however, the Meeting considered a summary of a study in
which some inhibition of brain neuropathy target esterase was
observed at a dose below the LD50. It therefore concluded that
phosmet may have the potential to cause delayed neuropathy, although
at doses higher than the unprotected LD50. A further study is
being requested to clarify this issue.
An ADI was allocated on the basis of the NOAEL in the
multigeneration study in rats (20 ppm, equal to 1.3 mg/kg bw per
day) and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 4 mg/kg bw per day (two-year study
of carcinogenicity)
Rat: 40 ppm, equal to 1.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
20 ppm, equal to 1.3 mg/kg bw per day (two-generation
study of reproductive toxicity)
5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
15 mg/kg bw per day (study of teratogenicity,
developmental toxicity)
Rabbit: 5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
2 mg/kg bw per day (study of teratogenicity,
fetotoxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Long-term study of toxicity in dogs
2. Study of DNA binding in vivo
3. Study of delayed neurotoxicity in chickens at an appropriately
high dose, with estimation of neuropathy target esterase
4. Further observations in humans
In order to maintain the ADI, these data should be submitted in
1997, in time for review in 1998.
References
Ackermann, H., Seidler, H., Kagan, Y.S. & Voronina, V.M. (1976)
Metabolic and toxic behaviour of phthalimide derivatives. 1. Fate of
Imidan in the fetus. Arch. Toxicol., 36, 127-137.
Bullock, C.H. (1971) Imidan (EDC process). Unpublished report
T-1666, dated 27 May 1971 from Stauffer Chemical Co. Western
Research Center. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Bullock, C.H. (1972) Unpublished report T-4035, dated 18 August 1972
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Castles, T.R. (1977) Unpublished report T-6123, dated 3 August 1977
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Chang, J.C.F., Morrisey, R.L. & Wyand, S. (1991) 2-Year chronic
toxicity/oncogenicity study with R-1504 in rats. Unpublished report
T-13241 dated 29 April 1991 from Ciba-Geigy Corp, Agricultural
Division, Environmental Health Center, Farmington, CT, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Courtney, K.D. & Finkelstein, M. (1968) Teratological investigation
of captan, imidan and thalidomide in Macaca mulatta. Unpublished
report T-2164 to Stauffer Chemical Company, dated 4 April 1968 from
Bionetics Research Laboratories Inc., Falls Church, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Danilenko, L.P. (1969) Maximum permissible concentration of
phthalophos in air of working zone. Hyg. Sanit., 34, 192-197.
Dickey, J.K. (1986) Imidan: morphological transformation of
BALB/3T3. Unpublished report T-12822, dated 8 December 1986 from In
Vitro Toxicology Section, Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Fabro, S., Smith, R.L. & Williams, R.T. (1966) Embryotoxic activity
of some pesticides and drugs related to phthalimide. Food Cosmet.
Toxicol., 3, 587-590.
Fisher, G.D. (1989) R-1504. Metabolism study in rats: recovery of
administered dose. Unpublished report T-13031, dated 16 August 1989
from Ciba-Geigy Corp., Environmental Health Center, Farmington, CT,
USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey,
United Kingdom.
Fisher, G.D. (1990) R-1504. Metabolism study in rats:
biotransformation. Unpublished report T-13031, dated 12 February
1990 from Ciba-Geigy Corp., Environmental Health Center, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ford, I.M., Menn, J.J. & Meyding, G.D. (1966) Metabolism of
N-(mercaptomethyl)phthalimide-carbonyl-C14-(S-(O,O-dimethylphospho
rothioate) (Imidan-C14): balance study in the rat. J. Agric. Food
Chem., 14, 83-86.
Frank, A.A., Ross, J.L. & Sawvell, B.K. (1992) Toxic epidermal
necrolysis associated with flea dips. Vet. Hum. Toxicol., 34,
57-61.
Gibbs, J.A. (1986) Phosmet report of a micronucleus test in the
mouse. Unpublished report No. 2516, dated 18 December 1986 from
Beecham Pharmaceuticals Research Division, Genetic Toxicology Unit,
Stock, United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Good, J.L., Khurana, R.K., Mayer, R.F., Cintra, W.M. & Albuquerque,
E.X. (1993) Pathophysiological studies of neuromuscular function in
subacute organophosphate poisoning induced by phosmet. J. Neurol.
Neurosurg. Psychiatr., 56, 290-294.
Hendricks, M. & Sprague, G.L. (1983) Imidan technical: effect on rat
cholinesterases. Unpublished report No. T-11228, dated 26 April
1983. Richmond Toxicology Laboratory, Stauffer Chemical Co.,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Hertzel, K.M. (1986) Imidan: mutagenicity evaluation in mouse
lymphoma multiple endpoint test forward mutation assay. Unpublished
report No. T-12820, dated 8 May 1986. In Vitro Toxicology Section,
Environmental Health Center, Stauffer Chemical Co., Agrochemical
Division, Farmington, CT, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Hodge, M.C.E. (1991) Phosmet: teratogenicity study in the rat.
Unpublished report No. CTL/P/3360, dated 17 June 1991. ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Hollingsworth, R.L., Johnston, C.D. & Woodard, G. (1965) Imidan
three generation study in rats. Unpublished report, dated 7 May
1965, from Woodard Research Corp., Herndon, VA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Izmerov, N.F. (1983) Scientific Reviews of Soviet Literature on
Toxicity and Hazards of Chemicals, Moscow, Centre of International
Projects. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Johnston, C.D. (1962) Imidan: an evaluation of safety of Imidan in
the rat and the dog. Unpublished report, dated 21 February 1962,
from Woodard Research Corp., Herndon, VA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963a) Further evaluation of the safety of Imidan in
the rat. Unpublished report, dated 27 February 1963, from Woodard
Research Corp., Herndon, VA, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963b) Imidan: demyelination study in the chicken.
Unpublished report, dated 27 February 1963, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1966) Imidan: single dose oral toxicity in mice.
Unpublished report, dated 22 November 1966, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Jones, N.B., Sauerhoff, M.W., Thomassen, R.W. & Zwicker, G.M. (1981)
Four week dietary range-finding study in mice with Imidan technical.
Unpublished report No. T-10704, dated July 1981 from Environmental
Health Center, Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Katz, A., Frank, D.W., Zwicker, G.M., Sprague, G.L., Turnier, J.C. &
Freudenthal, R.I. (1984) T(TM)10719. Two-year dietary oncogenicity
study in mice with Imidan technical. Unpublished report, dated May
1984, from Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Király, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. Environ. Contam. Toxicol., 8, 309-319.
Lee, H. & Miaullis, J.B. (1969) The effect of imidan on
cholinesterase and aliesterase activity in rats. Unpublished report
No. 004571 from Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Lobdell, B.J. & Johnston, C.D. (1966) Imidan: safety evaluation by
two-year feeding studies in the rat and the dog. Unpublished report,
dated 21 July 1966, from Woodard Research Corp., Herndon, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
McBain, J.B., Menn, J.J. & Casida, J.E. (1968) Metabolism of
carbonyl-C14-labelled Imidan, N-(mercaptomethyl)phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats and cockroaches. J.
Agric. Food Chem., 16, 813-820.
Majeska, J.B. & Matheson, D.W. (1986) Imidan: mutagenicity
evaluation in Salmonella typhimurium Unpublished report No. T-12819,
dated 4 March 1986 from Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the
effect of a series of phosphoroorganic pesticides (Dipterex and
Imidan) on embryogenesis. Environ. Health Perspectives, 13,
121-125.
Meyer, L.S. & Walberg, J.A. (1990) A two generation reproduction
study in rats with R-1504. Unpublished report No. T-13260, dated 18
May 1990, from Environmental Health Center, Ciba-Geigy Corp.,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Meyding, G.D. (1965) Imidan technical: acute oral subcutaneous and
intraperitoneal toxicity in rats and mice. Unpublished report No.
65-2, dated 26 January 1965 from Richmond Research Center, Animal
Health Toxicology Section, Stauffer Chemical Co., Richmond, CA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Moxon, M.E. (1991) Phosmet: teratogenicity study in the rabbit.
Unpublished report no. CTL/P/3373, dated 20 June 1991, from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, United
Kingdom. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Nuclear Science Corp. (1962) Unpublished report, Project No.
20-0240-32, dated 30 April 1962, from Nuclear Science Corp., Palo
Alto, CA, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ray, D.G. (1964) Imidan RP4-RCT-116: acute oral LD50--rats.
Unpublished report No. 507, dated 18 February 1964, from Biological
Research Center, Toxicology Section, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Shirasu, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4, 226-231.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Microbial screening of pesticides in microbial systems. Mutat.
Res., 40, 19-30.
Snyder, R.D. (1986a) Imidan: Mutagenicity evaluation in mouse
lymphoma multiple endpoint test cytogenetic assay. Unpublished
report No. T-12821, dated 8 May 1986, from In Vitro Toxicology
Section, Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Snyder, R.D. (1986b) Effects of imidan on human fibroblast DNA.
Unpublished report No. T-12823, dated 30 May 1986, from In Vitro
Toxicology Section, Environmental Health Centre, Stauffer Chemical
Co., Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Sprague, G.L. (1982) Acute delayed neurotoxicity study with Imidan
technical in adult hens. Unpublished report No. T-10910, dated 9
August 1982, from Richmond Toxicology Laboratory, Toxicology
Department, Stauffer Chemical Co., de Guigne Technical Center,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Sprague, G.L. & Turnier, J.C. (1986) T-10719. Addendum: Two-year
dietary oncogenicity study in mice with Imidan technical.
Unpublished addendum, dated 22 May 1986, from Environmental Health
Center, Stauffer Chemical Co., Agrochemical Division, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (Dipterex, Imidan) during pregnancy. Environ. Health
Perspectives, 13, 133-140.
Tarr, J.B. & Hemingway, R.J. (1993a) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and milk of
lactating goats. Unpublished report RR 92-103B, study No. PMS-352,
dated 22 January 1983, from Zeneca Agricultural Products, Western
Research Center, ICI Americas Inc., Richmond, CA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Tarr, J.B. & Hemingway, R.J. (1993b) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and eggs of
laying hens. Unpublished report No. 1227-1, RR 93-046B, dated 14
January 1983, from Zeneca Agricultural Products, Western Research
Center, ICI Americas Inc., Richmond, CA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
 (c) Effects on enzymes and other biochemical parameters
Erythrocyte and brain cholinesterase are more sensitive to
phosmet in rats than is plasma cholinesterase. Rat aliesterases are
more sensitive to inhibition by phosmet than is
acetylcholinesterase. Other organophosphates (malathion, parathion,
parathion-methyl, schradan, tributyl phosphorotrithioite, diazinon,
EPN, ethion, demeton, mevinphos, carbophenothion, disulfoton
azinphos-methyl) and a carbamate anticholinesterase (carbaryl) did
not potentiate the effect of phosmet in male Sprague-Dawley rats
(Lee & Miaullis, 1969).
Male and female CD rats were treated orally with 0, 10 or 100
mg/kg bw phosmet (purity, 94.7%), and plasma, erythrocyte and brain
cholinesterase activities were measured 4 and 24 h later. The lower
dose had no effect on plasma or erythrocyte cholinesterase
activities at either time or on brain cholinesterase activity at 24
h; however, 4 h after treatment with this dose, brain cholinesterase
activity was inhibited by 14% in males and 21% in females. At 100
mg/kg bw, substantial inhibition was found at both times, except for
plasma enzyme in females at 4 h. At that time, the activity of the
erythrocyte enzyme was the most strongly inhibited (about 85%) and
that of the brain enzyme somewhat less (about 65%); the activity of
plasma cholinesterase was inhibited by only about 35% in males and
not significantly in females. At 24 h, the activity of the brain
enzyme was recovering (34% inhibition in males and 48% in females),
as was that of erythrocyte cholinesterase (40 and 45% inhibition),
while the activity of plasma cholinesterase was further inhibited
(46 and 74% inhibition) (Hendricks & Sprague, 1983).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of phosmet is summarized in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice received
technical-grade phosmet (purity, 95%) in the diet at concentrations
of 0, 5, 15, 50, 150 or 500 ppm, equivalent to 0.75, 2.25, 7.5, 22.5
or 75 mg/kg bw per day, for four weeks. Significant decreases in
food consumption and body-weight gain were seen at 150 and 500 ppm
in males and at 500 ppm in females; females given 150 ppm had only
reduced food intake. Males at the two highest doses had a
significant decrease in absolute liver and kidney weights; relative
liver weights were significantly increased at 150 and 500 ppm in
males and females, while relative kidney weights were significantly
increased only in females at 500 ppm. Erythrocyte cholinesterase
activity was depressed at 50 ppm and above, and brain cholinesterase
activity was statistically significantly depressed by 16% in females
receiving the highest dose. No treatment-related changes were seen
histologically. The NOAEL was 50 ppm, equivalent to 7.5 mg/kg bw per
day, on the basis of reduced food consumption and body-weight gain
and decreased absolute liver and kidney weights at 150 ppm (Jones
et al., 1981).
Rats
Groups of 15 male and 15 female albino rats (strain
unspecified) were given phosmet (purity, 98%) in the diet at 0, 20,
100 or 500 ppm, equivalent to 1, 5 or 25 mg/kg bw per day, for 14
weeks. A second study was started four weeks later, involving four
groups of the same size treated with the same doses. Decreased
weight gain was found in the group receiving the highest dose.
Erythrocyte cholinesterase activity was inhibited by > 20% at the
middle and high doses, while plasma cholinesterase was inhibited by
> 20% at the high dose only; brain cholinesterase activity was
inhibited by > 20% at terminal sacrifice in the high and middle
dose groups. Changes described as 'necrobiotic foci' were observed
in the liver at 500 ppm, which were considered not to be related to
treatment. The NOAEL was 20 ppm, equivalent to 1.0 mg/kg bw per day,
on the basis of inhibition of brain cholinesterase activity at 100
ppm (Johnston, 1962).
Table 1. Acute toxicity of phosmet
Species Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
(95% CI or range)
Mouse Swiss-Webster albino M Oral 50.1 (34.4-73.0) NR Meyding, 1965
Mouse Swiss-Webster albino M Oral 20-43 a Bullock, 1971
Mouse NR M Oral 36.9 (21.7-62.8) 95 Bullock, 1972
Mouse Albino M&F Oral 38 b Johnston, 1966
Mouse Albino M&F Oral 49 c Johnston, 1966
Mouse Albino M&F Oral 43 d Johnston, 1966
Mouse Albino NR Oral 26-60 NR Danilenko, 1969
Mouse Swiss-Webster M Intraperitoneal 40-50 NR Meyding, 1965
Mouse Swiss-Webster M Subcutaneous 300 NR Meyding, 1965
Rat Albino M Oral 310 (267-360) NR Ray, 1964
Rat Sprague-Dawley M Oral 245 (161-367) NR Meyding, 1965
Rat Sprague-Dawley albino M Oral 140 (76-255) 98 Nuclear Science Corp.,
1962
Rat Albino NR Oral 92.5-164 NR Danilenko, 1969
Rat Sprague-Dawley albino M Oral 135-147a a Bullock, 1971
Rat Sprague-Dawley albino M Oral 121.3 (90.6-162.5) 92.5 Castles, 1977
Rat Sprague-Dawley albino F Oral 121.3 (96.7-152.1) 92.5 Castles, 1977
Rat Sprague-Dawley M Intraperitoneal approx. 100 NR Meyding, 1965
Rat Sprague-Dawley M Subcutaneous > 1200 NR Meyding, 1965
Rat Sprague-Dawley albino F Inhalation > 0.15e 92.5 Castles, 1977
Guinea-pig NR NR Oral 200 NR Danilenko, 1969
Rabbit New Zealand white NR Percutaneous > 4600 a Bullock, 1971
Rabbit New Zealand white NR Percutaneous > 5000 92.5 Castles, 1977
Cat NR NR Inhalation 65f NR Cited by Izmerov,
1983
Chicken White Leghorn F Oral 2020 94.7 Sprague, 1982
Table 1 (continued)
NR, not reported
a Precise figure depended on formulation and route of synthesis of active ingredient.
b Technical-grade in corn oil; purity not stated
c Technical-grade in 20% PEG300; purity not stated
d Imidan 50% wettable powder in aqueous suspension; concentration of active ingredient, 50%; results given
for active ingredient
e LC50' 1 h: mg/l
f LC50' time unstated
Three groups of 10 male and 10 female albino rats (strain
unspecified) were treated with phosmet for 16 weeks. Animals in the
'low dose' group initially received 450 ppm, equivalent to 22.5
mg/kg bw per day, which was increased stepwise to 6000 ppm,
equivalent to 300 mg/kg bw per day, by the 12th week. The 'high
dose' group was started on 800 ppm, equivalent to 40 mg/kg bw per
day, which was increased stepwise to 1120 ppm, equivalent to 56
mg/kg bw per day. Clinical signs (nervousness, tremors, diarrhoea)
were observed in all treated animals. Body-weight gain was decreased
in the high dose group in comparison with controls, and marked
hepatic degenerative changes (eosinophilia, vacuolation and
swelling, with little change in nuclear morphology) were also seen
in the high-dose group. Erythrocyte cholinesterase activity was
inhibited by about 100% in both groups, and plasma cholinesterase
activity was inhibited by > 50% in all groups. Brain cholinesterase
was inhibited by 75 and 80% in males at the low and high doses and
by 84 and 82% in females at the low and high doses, respectively.
There was no NOAEL, as brain cholinesterase activity was inhibited
in both treated groups and because of the variable dosing (Johnston,
1963a).
Cats
Veterinary use of phosmet as a dip for treating flea
infestation caused toxic epidermal necrolysis in a female Himalayan
cat. This observation has not been repeated (Frank et al., 1992).
Dogs
Groups of four male and four female beagle dogs were treated
with dietary concentrations of 0, 10, 75 or 563 ppm, equivalent to
0.25, 1.9 or 14 mg/kg bw per day, for 14 weeks. All of the animals
gained weight, except for one given the middle and one given the low
dose. Haematological and clinical chemical parameters were
unaffected by the treatment, except that marked depression of
erythrocyte cholinesterase activity and somewhat less marked
depression of plasma cholinesterase activity were found at the high
dose. Brain cholinesterase activity at terminal sacrifice was also
depressed at the high dose. The levels of plasma, erythrocyte and
brain cholinesterase were normal in the other three groups. At
autopsy, no pathological changes attributable to treatment were
observed. The NOAEL was 75 ppm, equivalent to 1.9 mg/kg bw per day
(Johnston, 1962).
Groups of three male and three female beagle dogs received
phosmet (purity unspecified) in the diet at concentrations of 0, 20,
40 or 400 ppm, equivalent to 0.5, 1 or 10 mg/kg bw per day, for two
years. One male at the highest dose was killed in extremis;
survival was otherwise unaffected. Erythrocyte cholinesterase
activity was depressed at 400 ppm throughout most of the study. At
termination, brain cholinesterase activity was considerably
depressed in the animals at the high dose, to 58% of control
activity in males and 32% in females. Interpretation of the study
was hampered by the small group size. The NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day (Lobdell & Johnston, 1966).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female B6C3F1 mice were fed phosmet
(purity, 94.7%) at dietary concentrations of 0, 5, 25 or 100 ppm,
equal to 0.75, 4 or 15 mg/kg bw per day for two years. Up to 10 mice
of each sex in each group were killed at 12 months, and
cholinesterase activity and haematological parameters were measured
in these animals and in 10 animals of each sex from each group at
terminal sacrifice. Plasma and brain cholinesterase activities were
measured at the time of the interim kill, and plasma, brain and
erythrocyte cholinesterase at termination. Phosmet did not affect
survival. Body weight was slightly but significantly increased for
most of the study in animals receiving the highest dose, and food
consumption was occasionally reduced. In males at the highest dose
there was an increased frequency of convulsions, usually associated
with handling of the animals.
Plasma cholinesterase activity was inhibited by about 50% in
males and females at the highest dose and by about 13% in females at
the middle dose at the time of interim sacrifice. Brain
cholinesterase activity was inhibited by > 20% in all treated
groups at interim sacrifice. Plasma cholinesterase activity was
inhibited at termination in the groups receiving the highest dose,
but the activity in erythrocytes was comparable in all groups. Some
inhibition of brain cholinesterase activity was found in females at
the middle and highest doses at termination, but, while
statistically significant, the depression was < 20% in the middle
dose group and 22% in the highest dose group. Brain cholinesterase
activity was not depressed in males at the terminal kill.
No treatment-related effects were seen on haematological
parameters at interim or final sacrifice, and no treatment-related
changes in organ weights or macroscopic or microscopic appearance
were seen, except in the liver. Relative liver weights were
increased at 12 months in males receiving 100 ppm, and at
termination there was an increase in the prevalence of mild
vacuolation. There was also an increase in the incidence of liver
adenomas (25/50, with 13/49 in controls) in males at the highest
dose. When animals killed at 12 months were included, liver adenomas
were found in 13/60 controls, 10/60 at 5 ppm, 14/60 at 25 ppm and
27/60 at 100 ppm. In an addendum to the report, the prevalence of
liver adenomas in the group given the highest dose was reported to
be comparable to that in historical controls. No increase in the
incidence of liver tumours was seen in females. The NOAEL was 25
ppm, equal to 4 mg/kg bw per day, on the basis of increased
incidences of convulsions, hepatocellular cytoplasmic vacuolation
and liver-cell adenomas in males and decreased brain cholinesterase
activity in females at 100 ppm. The apparent change in brain
cholinesterase activity at the time of the interim kill was
considered not to be a true reaction to treatment with phosmet, in
view of the absence of a similar effect at the time of the terminal
kill, after a longer period of treatment (Katz et al., 1984;
Sprague & Turnier, 1986).
Rats
Groups of 25 male and 25 female albino rats (strain
unspecified) received phosmet (purity unspecified) in the diet at
concentrations of 0, 20, 40 or 400 ppm, equivalent to 1, 2 or 20
mg/kg bw per day, for two years. Body-weight gain and plasma and
erythrocyte cholinesterase activities were depressed at 400 ppm; the
cholinesterase activities were decreased throughout the study, and
brain cholinesterase activity was lowered at termination. Hepatocyte
vacuolation was seen at the highest dose. Pituitary adenomas were
more frequent at 40 ppm (46%) and 400 ppm (56%) than in the controls
(36%) and in those receiving 20 ppm (21%), but there was not a clear
dose-response relationship. The small groups and the small number of
survivors at termination made interpretation of the study somewhat
difficult and precluded a conclusion being drawn about the
carcinogenicity of phosmet. The NOAEL was 40 ppm, equivalent to 2
mg/kg bw per day (Lobdell & Johnston, 1966).
Groups of 60 or 70 Sprague-Dawley Crl:CD SD BR rats of each sex
received phosmet (purity, 94.3%) in the diet at concentrations of 0,
20, 40 or 200 ppm, equal to 1.1, 1.8 or 9.4 mg/kg bw per day for
males and 1.1, 2.1 or 10.9 mg/kg bw per day for females. For interim
evaluation, 20 rats of each sex from the control group and 10 of
each sex from each test group were killed after 12 months, and the
study was continued for a further year. An additional group of 20
rats of each sex received 400 ppm (equal to 23 mg/kg bw per day for
males and 27 mg/kg bw per day for females) for 12 months. Exposure
did not adversely affect survival; indeed, there appeared to be a
dose-related increase in survival. No specific clinical signs were
attributable to treatment. Weight gain was reduced throughout the
study in animals of each sex at 400 ppm and in the early part of the
study at 40 (at 4, 21, 37, 45 and 49 weeks) and 200 ppm (at 1, 2 and
4weeks) in females only. These decreases are unlikely to be
biologically significant. Significant reductions in cholinesterase
activities were observed: plasma cholinesterase activity was reduced
by > 20% at 200 and 400 ppm in males and at 40, 200 and 400 ppm in
females; a reduction of 21% was observed at 18 weeks in females at
20 ppm. Erythrocyte cholinesterase activity was reduced by 15-20% at
20 ppm and by > 20% at higher dietary concentrations in males, and
by 15-20% at 40 ppm and very considerably at higher concentrations
in females. Brain cholinesterase activity was depressed in males at
200 ppm (interim and final kill), although the depression at 24
months was less than 15%. In females, depression of brain
cholinesterase activity by > 20% was observed at 200 ppm at both
interim and final sacrifice. Brain cholinesterase activity was also
depressed in animals of each sex at 400 ppm. An increase in the
incidence and severity of fatty liver was seen at 200 ppm. No
tumours were seen that were attributable to treatment with phosmet.
The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day, on the basis of
an increased incidence of fatty change in the livers of animals of
each sex and depressed brain cholinesterase activity in females at
200 ppm (Chang et al., 1991).
(d) Embryotoxicity and teratogenicity
Rats
In Wistar rats given a single dose of 30 mg/kg bw phosmet
orally once on day 9 of pregnancy or 1.5 mg/kg bw on alternate days
(every day in the summary) throughout pregnancy, post-implantation
mortality of embryos was increased. The dose of 30 mg/kg bw on day 9
or 13 caused developmental abnormalities, such as hypognathia and
hydrocephaly. A dose of 0.06 mg/kg bw on alternate days (every day
in the summary) had no effect. There was no NOAEL (Martson &
Voronina, 1976).
Phosmet (purity, 95.8%) was administered in the diet of CD rats
at amounts that provided a mean consumption of 0, 10, 22, 27 or 29
mg/kg bw per day on days 6-15 of pregnancy. The size of the groups
varied from 47 controls to 17 and 23 at the two higher doses. The
rats were killed on day 21. At 22 mg/kg bw per day, food intake and
weight gain were reduced. As phosmet was neither teratogenic nor
fetotoxic, the NOAEL for maternal toxicity was 10 mg/kg bw per day
and the NOAEL for fetal toxicity was the highest dose, 29 mg/kg bw
per day. In a similar study, in which phosmet was administered by
gavage at doses of 5, 10, 20, 25 or 30 mg/kg bw per day (with no
separate control group), survival was affected at the two higher
doses. There was a significant reduction in the proportion of rats
at 25 mg/kg bw per day that became pregnant, and there was a
reduction in food intake at > 10 mg/kg bw per day and a reduction
in weight gain at > 20 mg/kg bw per day. In the absence of a
suitable control group, there was no NOAEL in this study (Staples
et al., 1976).
Groups of 24 female alpk:APfSD rats were dosed with phosmet
(purity, 96.4%) in corn oil by gavage at 0, 5, 10 or 15 mg/kg bw per
day on days 7-16 days of gestation. The rats were killed on day 22
of gestation and the uteruses examined for live fetuses and
intrauterine deaths. Maternal toxicity in the form of reduced
body-weight gain, reduced food consumption and clinical signs
(shaking, piloerection) was seen at the highest dose. As there were
smaller but statistically significant effects on body-weight gain at
10 mg/kg bw per day between days 7 and 16, the lowest dose, 5 mg/kg
bw per day, was the NOAEL for maternal toxicity. As no teratogenic
or fetotoxic effects were seen, the NOAEL for developmental toxicity
was 15 mg/kg bw per day (Hodge, 1991).
In a three-generation study in CD rats, the F0 generation
consisted of two groups of 20 males and two groups of 20 females:
One group of each sex received no treatment, while the other
received technical-grade phosmet (purity, 99%) in the feed at 40
ppm, equivalent to 2 mg/kg bw per day, or half this concentration
during the first three weeks. Animals in each group were mated
twice, and the offspring (F1a and F1b) were examined at birth
and at weaning, when the F1a rats were killed. The F0 rats were
killed but not examined by necropsy. The F1b rats were retained to
make up three new groups of 20 rats of each sex, which received
phosmet at dietary concentrations of 0, 40 or 80 ppm from weaning
until sacrifice. Mating of the F1b rats produced F2a and F2b
litters; the F2a rats were sacrificed and the F2b offspring were
used, like the preceding generation, to make three new groups of
F2a and F2b rats. Offspring were sacrificed at weaning. The
treated and control animals were comparable throughout the study. On
histopathological examination of the F3b animals, some mild hepatic
vacuolation and reduced glycogen content were observed more
frequently in treated than in untreated animals; however, the former
finding was not frequent in any dose group, and the latter (in
adults and therefore presumably in fetuses) was strongly dependent
on time since last feed, which may have been different for the
various dose groups. In view of the lack of other findings, the
Meeting considered the NOAEL to be 40 ppm, equivalent to 2 mg/kg bw
per day (Hollingsworth et al., 1965).
In a two-generation study, technical-grade phosmet (purity,
95.2%) was administered to Crl:CD(SD)BRVAF/+tm rats at dietary
concentrations of 0, 20, 80 or 300 ppm, equal to 1.3, 5.0 or 19.4
mg/kg bw per day in F0 males; 1.5, 6.3 or 24.3 mg/kg bw per day in
F1 males; 1.5, 6.0 or 24.4 in F0 females; and 1.5, 6.2 or 26.4
mg/kg bw per day in F1 females. Treatment of the F0 generation
was started at 56 days of age, and mating occurred 56 days later.
The F1a animals were weaned at 21 days and sacrificed. Shortly
afterwards, the F0 rats were again mated to produce the F1b
litters, of which about 25 males and 25 females were used to form
the F1 parents. At about 114 days of age, the F1 parents were
mated to produce the F2 litters. Toxicity was observed in parents
at 80 and 300 ppm in both generations. Reduced body-weight gain and
food consumption were observed in animals of each sex at 300 ppm.
Males had reduced testicular weights in both generations and reduced
spleen weight and hepatocellular vacuolization in the F1
generation. Dehydration was seen in the F0 females at 300 ppm, and
chromorhinorrhoea was seen in F1 females at 300 ppm. At 80 ppm,
there was reduced body-weight gain in F0 male parents and reduced
erythrocyte cholinesterase activity in both generations; in F0
parental females, there was reduced weight gain during lactation and
reduced relative liver and adrenal weights. Reduced relative spleen
and thymus weights were seen in the F1 generation, and reduced
erythrocyte cholinesterase activity was seen in both generations.
Plasma cholinesterase activity was reduced in parental F0 males
only. Erythrocyte cholinesterase activity, although reduced, was >
80% of that of controls at 20 ppm. Mating and fertility were reduced
at 80 and 300 ppm, and there were reduced numbers of pups per
litter, reduced pup weight and reduced survival of pups at 300 ppm.
The NOAEL for toxicity to the parents and for effects on
reproductive performance was 20 ppm (equal to 1.3 mg/kg bw per day),
and the NOAEL for developmental toxicity was 80 ppm (equal to 5.0
mg/kg bw per day) (Meyer & Walberg, 1990).
Rabbits
Phosmet (purity unspecified) was administered by gavage to New
Zealand white rabbits at 35 mg/kg bw per day on days 7-12 of
pregnancy; the animals were killed at day 28. No embryotoxic effects
were detected, although such effects were observed with thalidomide
in the same study. The NOAEL for phosmet was 35 mg/kg bw per day;
however the treatment period was rather short and included only part
of the period of organogenesis (Fabro et al., 1966).
Groups of 20 female New Zealand white rabbits were treated with
phosmet (purity, 96.4%) at doses of 0, 2, 5 or 15 mg/kg bw per day
in corn oil by gavage on days 7-19 of pregnancy. The animals were
killed on day 30 of gestation. The highest dose had a slight effect
on maternal body-weight gain, and clinical signs thought to be
related to treatment (unsteadiness, shaking, salivation and
irregular breathing) were seen in four animals. This dose did not
affect the number or growth of offspring or survival in utero. The
two highest doses increased the number of minor skeletal defects.
The Meeting concluded that the NOAEL for maternal toxicity was 5
mg/kg bw per day and that for fetal toxicity was 2 mg/kg bw per day
(Moxon, 1991).
Monkeys
Phosmet (purity unspecified) was administered at doses of 2, 4
or 8 mg/kg bw per day by gastric intubation to groups of seven
pregnant rhesus macaques (Macaca mulatta) on days 22-32 of
gestation; there were no concurrent controls. Except when resorption
or abortion had occurred, the monkeys were delivered by caesarian
section after 83-87 days of pregnancy. Fetal mortality was observed,
but there was no dose-response relationship and no abnormal fetuses
were observed; abortions or resorptions occurred in 2/7 animals at 2
mg/kg bw per day, 0/7 animals at 4 mg/kg bw per day and 1/7 animals
at 8 mg/kg bw per day. The rate of fetal mortality in untreated
monkeys at the laboratory was stated to be 13.2%. External
examination revealed no abnormal fetuses. Thalidomide, which was
also tested in the study, induced both fetal mortality and abnormal
fetuses, while captan at a dose of 25 mg/kg bw per day induced a
high rate of fetal mortality. The NOAEL for phosmet was thus the
highest dose, 8 mg/kg bw per day (Courtney & Finkelstein, 1968).
(f) Genotoxicity
The results of tests for the genotoxicity of phosmet are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Phosmet is not an irritant in the Draize test. It is a mild eye
irritant (Bullock, 1971).
(ii) Delayed neuropathy in chickens
Three groups of 10 white Leghorn hens were given phosmet at
dietary concentrations of 100, 316 or 1000 ppm, equivalent to 12.5,
39.5 or 125 mg/kg bw per day, for 6-7 weeks; a further group of 10
hens received tri- ortho-cresyl phosphate at 1000 ppm (the oral
LD50 of this compound in hens is about 2 g/kg bw), and a fifth
group of three hens received normal diet. One of the hens receiving
tri- ortho-cresyl phosphate died, and eight of the survivors were
paralysed or severely ataxic by the fourth week. One of the birds
receiving the highest dose of phosmet was considered to be slightly
ataxic, but this was not confirmed by another observer. Spinal
axonal degeneration and myelin degeneration were seen in the birds
treated with tri- ortho-cresyl phosphate but not in those given
phosmet (Johnston, 1963b).
Phosmet (purity, 94.7%) was given to groups of 10 white Leghorn
hens at 0, 0.02, 0.20 or 2.05 g/kg bw (14 birds at the highest dose)
orally in gelatine capsules twice at 21-day intervals, and the birds
were killed 21 days after the second dose. Positive controls
received 0.5 g/kg tri- ortho-cresyl phosphate. Atropine (118 mg/kg
bw) and pralidoxime chloride (55 mg/kg bw) were administered
subcutaneously on days 1 and 22 of the study to all birds treated
with phosmet, and birds that showed severe clinical signs were
further treated with the two antidotes. Transient signs of
cholinergic toxicity were observed at the two higher doses; however,
there were no clinical signs or histopathological findings
suggestive of organophosphate-induced delayed neuropathy, whereas
hens treated with tri- ortho-cresyl phosphate had characteristic
histological changes (Sprague, 1982).
Table 2. Results of tests for the genotoxicity of phosmet
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.156-2.5 mg/plate 95.7 Positivea Majeska & Matheson,
100, 1535, 1537 1986
Reverse mutation S. typhimurium TA1535, Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
1536, 1537, 1538 et al., 1976
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate 95.7 Positivea Moriya et al., 1983
100, 1535, 1537
Reverse mutation B. subtilis H17 rec+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and 45 rec- et al., 1976
Reverse mutation E. coli B/r WP2hcr+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and WP2hcr- et al., 1976
Reverse mutation E. coli WP2 hcr approx. 0.100-5000 mg/plate 95.7 Negative Moriya et al., 1983
Cell BALB/3T3 mouse 0.0005-0.014 mg/ml 95.7 Negative Dickey, 1986
transformation cells
Forward mutation Mouse lymphoma 0.02-0.1 mg/mlb 95.7 Positiveb Hertzel, 1986
at tk locus cells (L1578Y) 0.004--0.04 mg/mlc Negativec
Chromosomal Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Negativea Snyder, 1986a
aberration cells (L1578Y) 0.008-0.04 mg/mlc
Sister chromatid Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Positivea Snyder, 1986a
exchange cells (L1578Y) 0.008-0.040 mg/mlc
DNA damage Human fibroblasts 0.25-1 mg/ml 95.7 Negativea Snyder, 1986b
and repair (foreskin)
Table 2 (contd)
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vivo
Micronucleus Mouse bone marrow 17 mg/kg bw orally 95.5 Negative Gibbs, 1986
formation
NR, not reported
a In the presence and absence of metabolic activation
b In the absence of metabolic activation
c In the presence of metabolic activation
3. Observations in humans
The frequency of chromatid-type aberrations in peripheral blood
lymphocytes taken from workers exposed to phosmet was moderately
increased (Király et al., 1979).
A number of cases of phosmet poisoning have been reported in
the literature. In one, poisoning was associated with decrements in
neuromuscular function and ultrastructural abnormalities in the
motor end-plate. The subject had been exposed during a five-week
spraying operation and had at no time been acutely poisoned. The
changes proved to be reversible (Good et al., 1993).
Comments
Phosmet is rapidly absorbed, distributed and excreted,
predominantly in the urine. Less than 1% of the label in the urine
was in the form of phosmet or phosmet oxon. In rats, there were two
major urinary metabolites, N-(methylsulfinylmethyl)phthalamic acid
and N-(methylsulfonylmethyl)-phthalamic acid.
The LD50 values have been estimated for a variety of species
for most routes. The oral LD50 in mice is 20-50 mg/kg bw and that
in rats is 100-300 mg/kg bw. WHO (1992) has classified phosmet as
moderately hazardous.
In a four-week study of toxicity, mice were fed diets
containing 0, 5, 15, 50, 150 or 500 ppm. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of reduced food
intake, reduced body-weight gain and reduced liver and kidney
weights. In a 14-week study of toxicity, rats were fed diets
containing 0, 20, 100 or 500 ppm. The NOAEL was 20 ppm, equivalent
to 1 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a 14-week study in beagle dogs fed diets
containing 0, 10, 75 or 563 ppm, the NOAEL was 75 ppm, equivalent to
1.9 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a two-year study of toxicity in dogs fed
diets containing 0, 20, 40 or 400 ppm, the NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity. The Meeting concluded, however, that
the last study was inappropriate for estimation of an ADI in view of
the small group size and the large dose interval between the NOAEL
and the effect level.
In a two-year study of carcinogenicity in mice fed levels of 0,
5, 25 or 100 ppm, there was evidence of hepatotoxicity at the high
dose, and a slightly but not statistically significantly increased
incidence of hepatic adenomas in comparison with concurrent
controls. There was, however, no increase in incidence in comparison
with historical controls, and the Meeting concluded that there was
no evidence of carcinogenicity in mice. Although brain
cholinesterase activities were determined in this study, the results
proved difficult to interpret: at the interim kill, brain
cholinesterase activity was apparently reduced in each sex in all
dose groups; at the terminal kill, brain cholinesterase activity was
reduced at the high dietary level in females and not at all in
males. The Meeting concluded that the apparent change seen at the
interim kill did not represent a true reaction to treatment with
phosmet, in view of the absence of a similar effect at the terminal
kill, after a longer treatment period. The NOAEL was 25 ppm (equal
to 4 mg/kg bw per day) on the basis of hepatotoxicity and brain
cholinesterase inhibition at the high dose.
In an early, inadequate, two-year study of toxicity and
carcinogenicity in rats treated via the diet, the NOAEL was 40 ppm,
equivalent to 2 mg/kg bw per day, on the basis of depressed
body-weight gain and brain cholinesterase inhibition. In another
two-year study of toxicity and carcinogenicity, rats were fed diets
containing 0, 20, 40 or 200 ppm phosmet, and a smaller group
received 400 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per
day, on the basis of fatty changes in the liver and reduced brain
cholinesterase activity in females. There was no evidence of
carcinogenicity in rats.
Two multigeneration studies of reproductive toxicity have been
conducted with phosmet in rats. In a three-generation study, animals
were exposed to dietary levels of 0 or 40 ppm phosmet (first
generation) and 0, 40 or 80 ppm (second generation). The NOAEL was
40 ppm, equivalent to 2 mg/kg bw per day. In a two-generation (two
litters per generation) study of reproductive toxicity, rats were
fed dietary concentrations of 0, 20, 80 or 300 ppm phosmet. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of
reduced mating and fertility at higher doses.
In a study of teratogenicity, rats were dosed orally at 0, 5,
10 or 15 mg/kg bw per day. The NOAEL for maternal toxicity was 5
mg/kg bw per day on the basis of reduced body-weight gain; there was
no evidence of fetotoxicity or teratogenicity at the highest dose
tested. In a study of teratogenicity in rabbits dosed at 0, 2, 5 or
15 mg/kg bw per day, the NOAEL for maternal toxicity was 5 mg/kg bw
per day on the basis of reduced body-weight gain, while the NOAEL
for fetotoxicity was 2 mg/kg bw per day on the basis of the presence
of minor skeletal anomalies.
The Meeting concluded that phosmet was not clastogenic but that
its mutagenic potential is unclear. In an attempt to address this
issue, studies of DNA binding in vivo are being requested.
In two studies, phosmet did not cause delayed neuropathy in
chickens; however, the Meeting considered a summary of a study in
which some inhibition of brain neuropathy target esterase was
observed at a dose below the LD50. It therefore concluded that
phosmet may have the potential to cause delayed neuropathy, although
at doses higher than the unprotected LD50. A further study is
being requested to clarify this issue.
An ADI was allocated on the basis of the NOAEL in the
multigeneration study in rats (20 ppm, equal to 1.3 mg/kg bw per
day) and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 4 mg/kg bw per day (two-year study
of carcinogenicity)
Rat: 40 ppm, equal to 1.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
20 ppm, equal to 1.3 mg/kg bw per day (two-generation
study of reproductive toxicity)
5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
15 mg/kg bw per day (study of teratogenicity,
developmental toxicity)
Rabbit: 5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
2 mg/kg bw per day (study of teratogenicity,
fetotoxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Long-term study of toxicity in dogs
2. Study of DNA binding in vivo
3. Study of delayed neurotoxicity in chickens at an appropriately
high dose, with estimation of neuropathy target esterase
4. Further observations in humans
In order to maintain the ADI, these data should be submitted in
1997, in time for review in 1998.
References
Ackermann, H., Seidler, H., Kagan, Y.S. & Voronina, V.M. (1976)
Metabolic and toxic behaviour of phthalimide derivatives. 1. Fate of
Imidan in the fetus. Arch. Toxicol., 36, 127-137.
Bullock, C.H. (1971) Imidan (EDC process). Unpublished report
T-1666, dated 27 May 1971 from Stauffer Chemical Co. Western
Research Center. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Bullock, C.H. (1972) Unpublished report T-4035, dated 18 August 1972
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Castles, T.R. (1977) Unpublished report T-6123, dated 3 August 1977
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Chang, J.C.F., Morrisey, R.L. & Wyand, S. (1991) 2-Year chronic
toxicity/oncogenicity study with R-1504 in rats. Unpublished report
T-13241 dated 29 April 1991 from Ciba-Geigy Corp, Agricultural
Division, Environmental Health Center, Farmington, CT, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Courtney, K.D. & Finkelstein, M. (1968) Teratological investigation
of captan, imidan and thalidomide in Macaca mulatta. Unpublished
report T-2164 to Stauffer Chemical Company, dated 4 April 1968 from
Bionetics Research Laboratories Inc., Falls Church, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Danilenko, L.P. (1969) Maximum permissible concentration of
phthalophos in air of working zone. Hyg. Sanit., 34, 192-197.
Dickey, J.K. (1986) Imidan: morphological transformation of
BALB/3T3. Unpublished report T-12822, dated 8 December 1986 from In
Vitro Toxicology Section, Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Fabro, S., Smith, R.L. & Williams, R.T. (1966) Embryotoxic activity
of some pesticides and drugs related to phthalimide. Food Cosmet.
Toxicol., 3, 587-590.
Fisher, G.D. (1989) R-1504. Metabolism study in rats: recovery of
administered dose. Unpublished report T-13031, dated 16 August 1989
from Ciba-Geigy Corp., Environmental Health Center, Farmington, CT,
USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey,
United Kingdom.
Fisher, G.D. (1990) R-1504. Metabolism study in rats:
biotransformation. Unpublished report T-13031, dated 12 February
1990 from Ciba-Geigy Corp., Environmental Health Center, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ford, I.M., Menn, J.J. & Meyding, G.D. (1966) Metabolism of
N-(mercaptomethyl)phthalimide-carbonyl-C14-(S-(O,O-dimethylphospho
rothioate) (Imidan-C14): balance study in the rat. J. Agric. Food
Chem., 14, 83-86.
Frank, A.A., Ross, J.L. & Sawvell, B.K. (1992) Toxic epidermal
necrolysis associated with flea dips. Vet. Hum. Toxicol., 34,
57-61.
Gibbs, J.A. (1986) Phosmet report of a micronucleus test in the
mouse. Unpublished report No. 2516, dated 18 December 1986 from
Beecham Pharmaceuticals Research Division, Genetic Toxicology Unit,
Stock, United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Good, J.L., Khurana, R.K., Mayer, R.F., Cintra, W.M. & Albuquerque,
E.X. (1993) Pathophysiological studies of neuromuscular function in
subacute organophosphate poisoning induced by phosmet. J. Neurol.
Neurosurg. Psychiatr., 56, 290-294.
Hendricks, M. & Sprague, G.L. (1983) Imidan technical: effect on rat
cholinesterases. Unpublished report No. T-11228, dated 26 April
1983. Richmond Toxicology Laboratory, Stauffer Chemical Co.,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Hertzel, K.M. (1986) Imidan: mutagenicity evaluation in mouse
lymphoma multiple endpoint test forward mutation assay. Unpublished
report No. T-12820, dated 8 May 1986. In Vitro Toxicology Section,
Environmental Health Center, Stauffer Chemical Co., Agrochemical
Division, Farmington, CT, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Hodge, M.C.E. (1991) Phosmet: teratogenicity study in the rat.
Unpublished report No. CTL/P/3360, dated 17 June 1991. ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Hollingsworth, R.L., Johnston, C.D. & Woodard, G. (1965) Imidan
three generation study in rats. Unpublished report, dated 7 May
1965, from Woodard Research Corp., Herndon, VA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Izmerov, N.F. (1983) Scientific Reviews of Soviet Literature on
Toxicity and Hazards of Chemicals, Moscow, Centre of International
Projects. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Johnston, C.D. (1962) Imidan: an evaluation of safety of Imidan in
the rat and the dog. Unpublished report, dated 21 February 1962,
from Woodard Research Corp., Herndon, VA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963a) Further evaluation of the safety of Imidan in
the rat. Unpublished report, dated 27 February 1963, from Woodard
Research Corp., Herndon, VA, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963b) Imidan: demyelination study in the chicken.
Unpublished report, dated 27 February 1963, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1966) Imidan: single dose oral toxicity in mice.
Unpublished report, dated 22 November 1966, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Jones, N.B., Sauerhoff, M.W., Thomassen, R.W. & Zwicker, G.M. (1981)
Four week dietary range-finding study in mice with Imidan technical.
Unpublished report No. T-10704, dated July 1981 from Environmental
Health Center, Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Katz, A., Frank, D.W., Zwicker, G.M., Sprague, G.L., Turnier, J.C. &
Freudenthal, R.I. (1984) T(TM)10719. Two-year dietary oncogenicity
study in mice with Imidan technical. Unpublished report, dated May
1984, from Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Király, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. Environ. Contam. Toxicol., 8, 309-319.
Lee, H. & Miaullis, J.B. (1969) The effect of imidan on
cholinesterase and aliesterase activity in rats. Unpublished report
No. 004571 from Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Lobdell, B.J. & Johnston, C.D. (1966) Imidan: safety evaluation by
two-year feeding studies in the rat and the dog. Unpublished report,
dated 21 July 1966, from Woodard Research Corp., Herndon, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
McBain, J.B., Menn, J.J. & Casida, J.E. (1968) Metabolism of
carbonyl-C14-labelled Imidan, N-(mercaptomethyl)phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats and cockroaches. J.
Agric. Food Chem., 16, 813-820.
Majeska, J.B. & Matheson, D.W. (1986) Imidan: mutagenicity
evaluation in Salmonella typhimurium Unpublished report No. T-12819,
dated 4 March 1986 from Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the
effect of a series of phosphoroorganic pesticides (Dipterex and
Imidan) on embryogenesis. Environ. Health Perspectives, 13,
121-125.
Meyer, L.S. & Walberg, J.A. (1990) A two generation reproduction
study in rats with R-1504. Unpublished report No. T-13260, dated 18
May 1990, from Environmental Health Center, Ciba-Geigy Corp.,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Meyding, G.D. (1965) Imidan technical: acute oral subcutaneous and
intraperitoneal toxicity in rats and mice. Unpublished report No.
65-2, dated 26 January 1965 from Richmond Research Center, Animal
Health Toxicology Section, Stauffer Chemical Co., Richmond, CA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Moxon, M.E. (1991) Phosmet: teratogenicity study in the rabbit.
Unpublished report no. CTL/P/3373, dated 20 June 1991, from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, United
Kingdom. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Nuclear Science Corp. (1962) Unpublished report, Project No.
20-0240-32, dated 30 April 1962, from Nuclear Science Corp., Palo
Alto, CA, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ray, D.G. (1964) Imidan RP4-RCT-116: acute oral LD50--rats.
Unpublished report No. 507, dated 18 February 1964, from Biological
Research Center, Toxicology Section, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Shirasu, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4, 226-231.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Microbial screening of pesticides in microbial systems. Mutat.
Res., 40, 19-30.
Snyder, R.D. (1986a) Imidan: Mutagenicity evaluation in mouse
lymphoma multiple endpoint test cytogenetic assay. Unpublished
report No. T-12821, dated 8 May 1986, from In Vitro Toxicology
Section, Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Snyder, R.D. (1986b) Effects of imidan on human fibroblast DNA.
Unpublished report No. T-12823, dated 30 May 1986, from In Vitro
Toxicology Section, Environmental Health Centre, Stauffer Chemical
Co., Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Sprague, G.L. (1982) Acute delayed neurotoxicity study with Imidan
technical in adult hens. Unpublished report No. T-10910, dated 9
August 1982, from Richmond Toxicology Laboratory, Toxicology
Department, Stauffer Chemical Co., de Guigne Technical Center,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Sprague, G.L. & Turnier, J.C. (1986) T-10719. Addendum: Two-year
dietary oncogenicity study in mice with Imidan technical.
Unpublished addendum, dated 22 May 1986, from Environmental Health
Center, Stauffer Chemical Co., Agrochemical Division, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (Dipterex, Imidan) during pregnancy. Environ. Health
Perspectives, 13, 133-140.
Tarr, J.B. & Hemingway, R.J. (1993a) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and milk of
lactating goats. Unpublished report RR 92-103B, study No. PMS-352,
dated 22 January 1983, from Zeneca Agricultural Products, Western
Research Center, ICI Americas Inc., Richmond, CA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Tarr, J.B. & Hemingway, R.J. (1993b) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and eggs of
laying hens. Unpublished report No. 1227-1, RR 93-046B, dated 14
January 1983, from Zeneca Agricultural Products, Western Research
Center, ICI Americas Inc., Richmond, CA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
(c) Effects on enzymes and other biochemical parameters
Erythrocyte and brain cholinesterase are more sensitive to
phosmet in rats than is plasma cholinesterase. Rat aliesterases are
more sensitive to inhibition by phosmet than is
acetylcholinesterase. Other organophosphates (malathion, parathion,
parathion-methyl, schradan, tributyl phosphorotrithioite, diazinon,
EPN, ethion, demeton, mevinphos, carbophenothion, disulfoton
azinphos-methyl) and a carbamate anticholinesterase (carbaryl) did
not potentiate the effect of phosmet in male Sprague-Dawley rats
(Lee & Miaullis, 1969).
Male and female CD rats were treated orally with 0, 10 or 100
mg/kg bw phosmet (purity, 94.7%), and plasma, erythrocyte and brain
cholinesterase activities were measured 4 and 24 h later. The lower
dose had no effect on plasma or erythrocyte cholinesterase
activities at either time or on brain cholinesterase activity at 24
h; however, 4 h after treatment with this dose, brain cholinesterase
activity was inhibited by 14% in males and 21% in females. At 100
mg/kg bw, substantial inhibition was found at both times, except for
plasma enzyme in females at 4 h. At that time, the activity of the
erythrocyte enzyme was the most strongly inhibited (about 85%) and
that of the brain enzyme somewhat less (about 65%); the activity of
plasma cholinesterase was inhibited by only about 35% in males and
not significantly in females. At 24 h, the activity of the brain
enzyme was recovering (34% inhibition in males and 48% in females),
as was that of erythrocyte cholinesterase (40 and 45% inhibition),
while the activity of plasma cholinesterase was further inhibited
(46 and 74% inhibition) (Hendricks & Sprague, 1983).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of phosmet is summarized in Table 1.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female B6C3F1 mice received
technical-grade phosmet (purity, 95%) in the diet at concentrations
of 0, 5, 15, 50, 150 or 500 ppm, equivalent to 0.75, 2.25, 7.5, 22.5
or 75 mg/kg bw per day, for four weeks. Significant decreases in
food consumption and body-weight gain were seen at 150 and 500 ppm
in males and at 500 ppm in females; females given 150 ppm had only
reduced food intake. Males at the two highest doses had a
significant decrease in absolute liver and kidney weights; relative
liver weights were significantly increased at 150 and 500 ppm in
males and females, while relative kidney weights were significantly
increased only in females at 500 ppm. Erythrocyte cholinesterase
activity was depressed at 50 ppm and above, and brain cholinesterase
activity was statistically significantly depressed by 16% in females
receiving the highest dose. No treatment-related changes were seen
histologically. The NOAEL was 50 ppm, equivalent to 7.5 mg/kg bw per
day, on the basis of reduced food consumption and body-weight gain
and decreased absolute liver and kidney weights at 150 ppm (Jones
et al., 1981).
Rats
Groups of 15 male and 15 female albino rats (strain
unspecified) were given phosmet (purity, 98%) in the diet at 0, 20,
100 or 500 ppm, equivalent to 1, 5 or 25 mg/kg bw per day, for 14
weeks. A second study was started four weeks later, involving four
groups of the same size treated with the same doses. Decreased
weight gain was found in the group receiving the highest dose.
Erythrocyte cholinesterase activity was inhibited by > 20% at the
middle and high doses, while plasma cholinesterase was inhibited by
> 20% at the high dose only; brain cholinesterase activity was
inhibited by > 20% at terminal sacrifice in the high and middle
dose groups. Changes described as 'necrobiotic foci' were observed
in the liver at 500 ppm, which were considered not to be related to
treatment. The NOAEL was 20 ppm, equivalent to 1.0 mg/kg bw per day,
on the basis of inhibition of brain cholinesterase activity at 100
ppm (Johnston, 1962).
Table 1. Acute toxicity of phosmet
Species Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
(95% CI or range)
Mouse Swiss-Webster albino M Oral 50.1 (34.4-73.0) NR Meyding, 1965
Mouse Swiss-Webster albino M Oral 20-43 a Bullock, 1971
Mouse NR M Oral 36.9 (21.7-62.8) 95 Bullock, 1972
Mouse Albino M&F Oral 38 b Johnston, 1966
Mouse Albino M&F Oral 49 c Johnston, 1966
Mouse Albino M&F Oral 43 d Johnston, 1966
Mouse Albino NR Oral 26-60 NR Danilenko, 1969
Mouse Swiss-Webster M Intraperitoneal 40-50 NR Meyding, 1965
Mouse Swiss-Webster M Subcutaneous 300 NR Meyding, 1965
Rat Albino M Oral 310 (267-360) NR Ray, 1964
Rat Sprague-Dawley M Oral 245 (161-367) NR Meyding, 1965
Rat Sprague-Dawley albino M Oral 140 (76-255) 98 Nuclear Science Corp.,
1962
Rat Albino NR Oral 92.5-164 NR Danilenko, 1969
Rat Sprague-Dawley albino M Oral 135-147a a Bullock, 1971
Rat Sprague-Dawley albino M Oral 121.3 (90.6-162.5) 92.5 Castles, 1977
Rat Sprague-Dawley albino F Oral 121.3 (96.7-152.1) 92.5 Castles, 1977
Rat Sprague-Dawley M Intraperitoneal approx. 100 NR Meyding, 1965
Rat Sprague-Dawley M Subcutaneous > 1200 NR Meyding, 1965
Rat Sprague-Dawley albino F Inhalation > 0.15e 92.5 Castles, 1977
Guinea-pig NR NR Oral 200 NR Danilenko, 1969
Rabbit New Zealand white NR Percutaneous > 4600 a Bullock, 1971
Rabbit New Zealand white NR Percutaneous > 5000 92.5 Castles, 1977
Cat NR NR Inhalation 65f NR Cited by Izmerov,
1983
Chicken White Leghorn F Oral 2020 94.7 Sprague, 1982
Table 1 (continued)
NR, not reported
a Precise figure depended on formulation and route of synthesis of active ingredient.
b Technical-grade in corn oil; purity not stated
c Technical-grade in 20% PEG300; purity not stated
d Imidan 50% wettable powder in aqueous suspension; concentration of active ingredient, 50%; results given
for active ingredient
e LC50' 1 h: mg/l
f LC50' time unstated
Three groups of 10 male and 10 female albino rats (strain
unspecified) were treated with phosmet for 16 weeks. Animals in the
'low dose' group initially received 450 ppm, equivalent to 22.5
mg/kg bw per day, which was increased stepwise to 6000 ppm,
equivalent to 300 mg/kg bw per day, by the 12th week. The 'high
dose' group was started on 800 ppm, equivalent to 40 mg/kg bw per
day, which was increased stepwise to 1120 ppm, equivalent to 56
mg/kg bw per day. Clinical signs (nervousness, tremors, diarrhoea)
were observed in all treated animals. Body-weight gain was decreased
in the high dose group in comparison with controls, and marked
hepatic degenerative changes (eosinophilia, vacuolation and
swelling, with little change in nuclear morphology) were also seen
in the high-dose group. Erythrocyte cholinesterase activity was
inhibited by about 100% in both groups, and plasma cholinesterase
activity was inhibited by > 50% in all groups. Brain cholinesterase
was inhibited by 75 and 80% in males at the low and high doses and
by 84 and 82% in females at the low and high doses, respectively.
There was no NOAEL, as brain cholinesterase activity was inhibited
in both treated groups and because of the variable dosing (Johnston,
1963a).
Cats
Veterinary use of phosmet as a dip for treating flea
infestation caused toxic epidermal necrolysis in a female Himalayan
cat. This observation has not been repeated (Frank et al., 1992).
Dogs
Groups of four male and four female beagle dogs were treated
with dietary concentrations of 0, 10, 75 or 563 ppm, equivalent to
0.25, 1.9 or 14 mg/kg bw per day, for 14 weeks. All of the animals
gained weight, except for one given the middle and one given the low
dose. Haematological and clinical chemical parameters were
unaffected by the treatment, except that marked depression of
erythrocyte cholinesterase activity and somewhat less marked
depression of plasma cholinesterase activity were found at the high
dose. Brain cholinesterase activity at terminal sacrifice was also
depressed at the high dose. The levels of plasma, erythrocyte and
brain cholinesterase were normal in the other three groups. At
autopsy, no pathological changes attributable to treatment were
observed. The NOAEL was 75 ppm, equivalent to 1.9 mg/kg bw per day
(Johnston, 1962).
Groups of three male and three female beagle dogs received
phosmet (purity unspecified) in the diet at concentrations of 0, 20,
40 or 400 ppm, equivalent to 0.5, 1 or 10 mg/kg bw per day, for two
years. One male at the highest dose was killed in extremis;
survival was otherwise unaffected. Erythrocyte cholinesterase
activity was depressed at 400 ppm throughout most of the study. At
termination, brain cholinesterase activity was considerably
depressed in the animals at the high dose, to 58% of control
activity in males and 32% in females. Interpretation of the study
was hampered by the small group size. The NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day (Lobdell & Johnston, 1966).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female B6C3F1 mice were fed phosmet
(purity, 94.7%) at dietary concentrations of 0, 5, 25 or 100 ppm,
equal to 0.75, 4 or 15 mg/kg bw per day for two years. Up to 10 mice
of each sex in each group were killed at 12 months, and
cholinesterase activity and haematological parameters were measured
in these animals and in 10 animals of each sex from each group at
terminal sacrifice. Plasma and brain cholinesterase activities were
measured at the time of the interim kill, and plasma, brain and
erythrocyte cholinesterase at termination. Phosmet did not affect
survival. Body weight was slightly but significantly increased for
most of the study in animals receiving the highest dose, and food
consumption was occasionally reduced. In males at the highest dose
there was an increased frequency of convulsions, usually associated
with handling of the animals.
Plasma cholinesterase activity was inhibited by about 50% in
males and females at the highest dose and by about 13% in females at
the middle dose at the time of interim sacrifice. Brain
cholinesterase activity was inhibited by > 20% in all treated
groups at interim sacrifice. Plasma cholinesterase activity was
inhibited at termination in the groups receiving the highest dose,
but the activity in erythrocytes was comparable in all groups. Some
inhibition of brain cholinesterase activity was found in females at
the middle and highest doses at termination, but, while
statistically significant, the depression was < 20% in the middle
dose group and 22% in the highest dose group. Brain cholinesterase
activity was not depressed in males at the terminal kill.
No treatment-related effects were seen on haematological
parameters at interim or final sacrifice, and no treatment-related
changes in organ weights or macroscopic or microscopic appearance
were seen, except in the liver. Relative liver weights were
increased at 12 months in males receiving 100 ppm, and at
termination there was an increase in the prevalence of mild
vacuolation. There was also an increase in the incidence of liver
adenomas (25/50, with 13/49 in controls) in males at the highest
dose. When animals killed at 12 months were included, liver adenomas
were found in 13/60 controls, 10/60 at 5 ppm, 14/60 at 25 ppm and
27/60 at 100 ppm. In an addendum to the report, the prevalence of
liver adenomas in the group given the highest dose was reported to
be comparable to that in historical controls. No increase in the
incidence of liver tumours was seen in females. The NOAEL was 25
ppm, equal to 4 mg/kg bw per day, on the basis of increased
incidences of convulsions, hepatocellular cytoplasmic vacuolation
and liver-cell adenomas in males and decreased brain cholinesterase
activity in females at 100 ppm. The apparent change in brain
cholinesterase activity at the time of the interim kill was
considered not to be a true reaction to treatment with phosmet, in
view of the absence of a similar effect at the time of the terminal
kill, after a longer period of treatment (Katz et al., 1984;
Sprague & Turnier, 1986).
Rats
Groups of 25 male and 25 female albino rats (strain
unspecified) received phosmet (purity unspecified) in the diet at
concentrations of 0, 20, 40 or 400 ppm, equivalent to 1, 2 or 20
mg/kg bw per day, for two years. Body-weight gain and plasma and
erythrocyte cholinesterase activities were depressed at 400 ppm; the
cholinesterase activities were decreased throughout the study, and
brain cholinesterase activity was lowered at termination. Hepatocyte
vacuolation was seen at the highest dose. Pituitary adenomas were
more frequent at 40 ppm (46%) and 400 ppm (56%) than in the controls
(36%) and in those receiving 20 ppm (21%), but there was not a clear
dose-response relationship. The small groups and the small number of
survivors at termination made interpretation of the study somewhat
difficult and precluded a conclusion being drawn about the
carcinogenicity of phosmet. The NOAEL was 40 ppm, equivalent to 2
mg/kg bw per day (Lobdell & Johnston, 1966).
Groups of 60 or 70 Sprague-Dawley Crl:CD SD BR rats of each sex
received phosmet (purity, 94.3%) in the diet at concentrations of 0,
20, 40 or 200 ppm, equal to 1.1, 1.8 or 9.4 mg/kg bw per day for
males and 1.1, 2.1 or 10.9 mg/kg bw per day for females. For interim
evaluation, 20 rats of each sex from the control group and 10 of
each sex from each test group were killed after 12 months, and the
study was continued for a further year. An additional group of 20
rats of each sex received 400 ppm (equal to 23 mg/kg bw per day for
males and 27 mg/kg bw per day for females) for 12 months. Exposure
did not adversely affect survival; indeed, there appeared to be a
dose-related increase in survival. No specific clinical signs were
attributable to treatment. Weight gain was reduced throughout the
study in animals of each sex at 400 ppm and in the early part of the
study at 40 (at 4, 21, 37, 45 and 49 weeks) and 200 ppm (at 1, 2 and
4weeks) in females only. These decreases are unlikely to be
biologically significant. Significant reductions in cholinesterase
activities were observed: plasma cholinesterase activity was reduced
by > 20% at 200 and 400 ppm in males and at 40, 200 and 400 ppm in
females; a reduction of 21% was observed at 18 weeks in females at
20 ppm. Erythrocyte cholinesterase activity was reduced by 15-20% at
20 ppm and by > 20% at higher dietary concentrations in males, and
by 15-20% at 40 ppm and very considerably at higher concentrations
in females. Brain cholinesterase activity was depressed in males at
200 ppm (interim and final kill), although the depression at 24
months was less than 15%. In females, depression of brain
cholinesterase activity by > 20% was observed at 200 ppm at both
interim and final sacrifice. Brain cholinesterase activity was also
depressed in animals of each sex at 400 ppm. An increase in the
incidence and severity of fatty liver was seen at 200 ppm. No
tumours were seen that were attributable to treatment with phosmet.
The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per day, on the basis of
an increased incidence of fatty change in the livers of animals of
each sex and depressed brain cholinesterase activity in females at
200 ppm (Chang et al., 1991).
(d) Embryotoxicity and teratogenicity
Rats
In Wistar rats given a single dose of 30 mg/kg bw phosmet
orally once on day 9 of pregnancy or 1.5 mg/kg bw on alternate days
(every day in the summary) throughout pregnancy, post-implantation
mortality of embryos was increased. The dose of 30 mg/kg bw on day 9
or 13 caused developmental abnormalities, such as hypognathia and
hydrocephaly. A dose of 0.06 mg/kg bw on alternate days (every day
in the summary) had no effect. There was no NOAEL (Martson &
Voronina, 1976).
Phosmet (purity, 95.8%) was administered in the diet of CD rats
at amounts that provided a mean consumption of 0, 10, 22, 27 or 29
mg/kg bw per day on days 6-15 of pregnancy. The size of the groups
varied from 47 controls to 17 and 23 at the two higher doses. The
rats were killed on day 21. At 22 mg/kg bw per day, food intake and
weight gain were reduced. As phosmet was neither teratogenic nor
fetotoxic, the NOAEL for maternal toxicity was 10 mg/kg bw per day
and the NOAEL for fetal toxicity was the highest dose, 29 mg/kg bw
per day. In a similar study, in which phosmet was administered by
gavage at doses of 5, 10, 20, 25 or 30 mg/kg bw per day (with no
separate control group), survival was affected at the two higher
doses. There was a significant reduction in the proportion of rats
at 25 mg/kg bw per day that became pregnant, and there was a
reduction in food intake at > 10 mg/kg bw per day and a reduction
in weight gain at > 20 mg/kg bw per day. In the absence of a
suitable control group, there was no NOAEL in this study (Staples
et al., 1976).
Groups of 24 female alpk:APfSD rats were dosed with phosmet
(purity, 96.4%) in corn oil by gavage at 0, 5, 10 or 15 mg/kg bw per
day on days 7-16 days of gestation. The rats were killed on day 22
of gestation and the uteruses examined for live fetuses and
intrauterine deaths. Maternal toxicity in the form of reduced
body-weight gain, reduced food consumption and clinical signs
(shaking, piloerection) was seen at the highest dose. As there were
smaller but statistically significant effects on body-weight gain at
10 mg/kg bw per day between days 7 and 16, the lowest dose, 5 mg/kg
bw per day, was the NOAEL for maternal toxicity. As no teratogenic
or fetotoxic effects were seen, the NOAEL for developmental toxicity
was 15 mg/kg bw per day (Hodge, 1991).
In a three-generation study in CD rats, the F0 generation
consisted of two groups of 20 males and two groups of 20 females:
One group of each sex received no treatment, while the other
received technical-grade phosmet (purity, 99%) in the feed at 40
ppm, equivalent to 2 mg/kg bw per day, or half this concentration
during the first three weeks. Animals in each group were mated
twice, and the offspring (F1a and F1b) were examined at birth
and at weaning, when the F1a rats were killed. The F0 rats were
killed but not examined by necropsy. The F1b rats were retained to
make up three new groups of 20 rats of each sex, which received
phosmet at dietary concentrations of 0, 40 or 80 ppm from weaning
until sacrifice. Mating of the F1b rats produced F2a and F2b
litters; the F2a rats were sacrificed and the F2b offspring were
used, like the preceding generation, to make three new groups of
F2a and F2b rats. Offspring were sacrificed at weaning. The
treated and control animals were comparable throughout the study. On
histopathological examination of the F3b animals, some mild hepatic
vacuolation and reduced glycogen content were observed more
frequently in treated than in untreated animals; however, the former
finding was not frequent in any dose group, and the latter (in
adults and therefore presumably in fetuses) was strongly dependent
on time since last feed, which may have been different for the
various dose groups. In view of the lack of other findings, the
Meeting considered the NOAEL to be 40 ppm, equivalent to 2 mg/kg bw
per day (Hollingsworth et al., 1965).
In a two-generation study, technical-grade phosmet (purity,
95.2%) was administered to Crl:CD(SD)BRVAF/+tm rats at dietary
concentrations of 0, 20, 80 or 300 ppm, equal to 1.3, 5.0 or 19.4
mg/kg bw per day in F0 males; 1.5, 6.3 or 24.3 mg/kg bw per day in
F1 males; 1.5, 6.0 or 24.4 in F0 females; and 1.5, 6.2 or 26.4
mg/kg bw per day in F1 females. Treatment of the F0 generation
was started at 56 days of age, and mating occurred 56 days later.
The F1a animals were weaned at 21 days and sacrificed. Shortly
afterwards, the F0 rats were again mated to produce the F1b
litters, of which about 25 males and 25 females were used to form
the F1 parents. At about 114 days of age, the F1 parents were
mated to produce the F2 litters. Toxicity was observed in parents
at 80 and 300 ppm in both generations. Reduced body-weight gain and
food consumption were observed in animals of each sex at 300 ppm.
Males had reduced testicular weights in both generations and reduced
spleen weight and hepatocellular vacuolization in the F1
generation. Dehydration was seen in the F0 females at 300 ppm, and
chromorhinorrhoea was seen in F1 females at 300 ppm. At 80 ppm,
there was reduced body-weight gain in F0 male parents and reduced
erythrocyte cholinesterase activity in both generations; in F0
parental females, there was reduced weight gain during lactation and
reduced relative liver and adrenal weights. Reduced relative spleen
and thymus weights were seen in the F1 generation, and reduced
erythrocyte cholinesterase activity was seen in both generations.
Plasma cholinesterase activity was reduced in parental F0 males
only. Erythrocyte cholinesterase activity, although reduced, was >
80% of that of controls at 20 ppm. Mating and fertility were reduced
at 80 and 300 ppm, and there were reduced numbers of pups per
litter, reduced pup weight and reduced survival of pups at 300 ppm.
The NOAEL for toxicity to the parents and for effects on
reproductive performance was 20 ppm (equal to 1.3 mg/kg bw per day),
and the NOAEL for developmental toxicity was 80 ppm (equal to 5.0
mg/kg bw per day) (Meyer & Walberg, 1990).
Rabbits
Phosmet (purity unspecified) was administered by gavage to New
Zealand white rabbits at 35 mg/kg bw per day on days 7-12 of
pregnancy; the animals were killed at day 28. No embryotoxic effects
were detected, although such effects were observed with thalidomide
in the same study. The NOAEL for phosmet was 35 mg/kg bw per day;
however the treatment period was rather short and included only part
of the period of organogenesis (Fabro et al., 1966).
Groups of 20 female New Zealand white rabbits were treated with
phosmet (purity, 96.4%) at doses of 0, 2, 5 or 15 mg/kg bw per day
in corn oil by gavage on days 7-19 of pregnancy. The animals were
killed on day 30 of gestation. The highest dose had a slight effect
on maternal body-weight gain, and clinical signs thought to be
related to treatment (unsteadiness, shaking, salivation and
irregular breathing) were seen in four animals. This dose did not
affect the number or growth of offspring or survival in utero. The
two highest doses increased the number of minor skeletal defects.
The Meeting concluded that the NOAEL for maternal toxicity was 5
mg/kg bw per day and that for fetal toxicity was 2 mg/kg bw per day
(Moxon, 1991).
Monkeys
Phosmet (purity unspecified) was administered at doses of 2, 4
or 8 mg/kg bw per day by gastric intubation to groups of seven
pregnant rhesus macaques (Macaca mulatta) on days 22-32 of
gestation; there were no concurrent controls. Except when resorption
or abortion had occurred, the monkeys were delivered by caesarian
section after 83-87 days of pregnancy. Fetal mortality was observed,
but there was no dose-response relationship and no abnormal fetuses
were observed; abortions or resorptions occurred in 2/7 animals at 2
mg/kg bw per day, 0/7 animals at 4 mg/kg bw per day and 1/7 animals
at 8 mg/kg bw per day. The rate of fetal mortality in untreated
monkeys at the laboratory was stated to be 13.2%. External
examination revealed no abnormal fetuses. Thalidomide, which was
also tested in the study, induced both fetal mortality and abnormal
fetuses, while captan at a dose of 25 mg/kg bw per day induced a
high rate of fetal mortality. The NOAEL for phosmet was thus the
highest dose, 8 mg/kg bw per day (Courtney & Finkelstein, 1968).
(f) Genotoxicity
The results of tests for the genotoxicity of phosmet are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Phosmet is not an irritant in the Draize test. It is a mild eye
irritant (Bullock, 1971).
(ii) Delayed neuropathy in chickens
Three groups of 10 white Leghorn hens were given phosmet at
dietary concentrations of 100, 316 or 1000 ppm, equivalent to 12.5,
39.5 or 125 mg/kg bw per day, for 6-7 weeks; a further group of 10
hens received tri- ortho-cresyl phosphate at 1000 ppm (the oral
LD50 of this compound in hens is about 2 g/kg bw), and a fifth
group of three hens received normal diet. One of the hens receiving
tri- ortho-cresyl phosphate died, and eight of the survivors were
paralysed or severely ataxic by the fourth week. One of the birds
receiving the highest dose of phosmet was considered to be slightly
ataxic, but this was not confirmed by another observer. Spinal
axonal degeneration and myelin degeneration were seen in the birds
treated with tri- ortho-cresyl phosphate but not in those given
phosmet (Johnston, 1963b).
Phosmet (purity, 94.7%) was given to groups of 10 white Leghorn
hens at 0, 0.02, 0.20 or 2.05 g/kg bw (14 birds at the highest dose)
orally in gelatine capsules twice at 21-day intervals, and the birds
were killed 21 days after the second dose. Positive controls
received 0.5 g/kg tri- ortho-cresyl phosphate. Atropine (118 mg/kg
bw) and pralidoxime chloride (55 mg/kg bw) were administered
subcutaneously on days 1 and 22 of the study to all birds treated
with phosmet, and birds that showed severe clinical signs were
further treated with the two antidotes. Transient signs of
cholinergic toxicity were observed at the two higher doses; however,
there were no clinical signs or histopathological findings
suggestive of organophosphate-induced delayed neuropathy, whereas
hens treated with tri- ortho-cresyl phosphate had characteristic
histological changes (Sprague, 1982).
Table 2. Results of tests for the genotoxicity of phosmet
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.156-2.5 mg/plate 95.7 Positivea Majeska & Matheson,
100, 1535, 1537 1986
Reverse mutation S. typhimurium TA1535, Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
1536, 1537, 1538 et al., 1976
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate 95.7 Positivea Moriya et al., 1983
100, 1535, 1537
Reverse mutation B. subtilis H17 rec+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and 45 rec- et al., 1976
Reverse mutation E. coli B/r WP2hcr+ Up to 20 µg/plate NR Negative Shirasu, 1975; Shirasu
and WP2hcr- et al., 1976
Reverse mutation E. coli WP2 hcr approx. 0.100-5000 mg/plate 95.7 Negative Moriya et al., 1983
Cell BALB/3T3 mouse 0.0005-0.014 mg/ml 95.7 Negative Dickey, 1986
transformation cells
Forward mutation Mouse lymphoma 0.02-0.1 mg/mlb 95.7 Positiveb Hertzel, 1986
at tk locus cells (L1578Y) 0.004--0.04 mg/mlc Negativec
Chromosomal Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Negativea Snyder, 1986a
aberration cells (L1578Y) 0.008-0.04 mg/mlc
Sister chromatid Mouse lymphoma 0.04-0.1 mg/mlb 95.7 Positivea Snyder, 1986a
exchange cells (L1578Y) 0.008-0.040 mg/mlc
DNA damage Human fibroblasts 0.25-1 mg/ml 95.7 Negativea Snyder, 1986b
and repair (foreskin)
Table 2 (contd)
End-point Test system Concentration of phosmet Purity (%) Results Reference
In vivo
Micronucleus Mouse bone marrow 17 mg/kg bw orally 95.5 Negative Gibbs, 1986
formation
NR, not reported
a In the presence and absence of metabolic activation
b In the absence of metabolic activation
c In the presence of metabolic activation
3. Observations in humans
The frequency of chromatid-type aberrations in peripheral blood
lymphocytes taken from workers exposed to phosmet was moderately
increased (Király et al., 1979).
A number of cases of phosmet poisoning have been reported in
the literature. In one, poisoning was associated with decrements in
neuromuscular function and ultrastructural abnormalities in the
motor end-plate. The subject had been exposed during a five-week
spraying operation and had at no time been acutely poisoned. The
changes proved to be reversible (Good et al., 1993).
Comments
Phosmet is rapidly absorbed, distributed and excreted,
predominantly in the urine. Less than 1% of the label in the urine
was in the form of phosmet or phosmet oxon. In rats, there were two
major urinary metabolites, N-(methylsulfinylmethyl)phthalamic acid
and N-(methylsulfonylmethyl)-phthalamic acid.
The LD50 values have been estimated for a variety of species
for most routes. The oral LD50 in mice is 20-50 mg/kg bw and that
in rats is 100-300 mg/kg bw. WHO (1992) has classified phosmet as
moderately hazardous.
In a four-week study of toxicity, mice were fed diets
containing 0, 5, 15, 50, 150 or 500 ppm. The NOAEL was 50 ppm,
equivalent to 7.5 mg/kg bw per day, on the basis of reduced food
intake, reduced body-weight gain and reduced liver and kidney
weights. In a 14-week study of toxicity, rats were fed diets
containing 0, 20, 100 or 500 ppm. The NOAEL was 20 ppm, equivalent
to 1 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a 14-week study in beagle dogs fed diets
containing 0, 10, 75 or 563 ppm, the NOAEL was 75 ppm, equivalent to
1.9 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity. In a two-year study of toxicity in dogs fed
diets containing 0, 20, 40 or 400 ppm, the NOAEL was 40 ppm,
equivalent to 1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity. The Meeting concluded, however, that
the last study was inappropriate for estimation of an ADI in view of
the small group size and the large dose interval between the NOAEL
and the effect level.
In a two-year study of carcinogenicity in mice fed levels of 0,
5, 25 or 100 ppm, there was evidence of hepatotoxicity at the high
dose, and a slightly but not statistically significantly increased
incidence of hepatic adenomas in comparison with concurrent
controls. There was, however, no increase in incidence in comparison
with historical controls, and the Meeting concluded that there was
no evidence of carcinogenicity in mice. Although brain
cholinesterase activities were determined in this study, the results
proved difficult to interpret: at the interim kill, brain
cholinesterase activity was apparently reduced in each sex in all
dose groups; at the terminal kill, brain cholinesterase activity was
reduced at the high dietary level in females and not at all in
males. The Meeting concluded that the apparent change seen at the
interim kill did not represent a true reaction to treatment with
phosmet, in view of the absence of a similar effect at the terminal
kill, after a longer treatment period. The NOAEL was 25 ppm (equal
to 4 mg/kg bw per day) on the basis of hepatotoxicity and brain
cholinesterase inhibition at the high dose.
In an early, inadequate, two-year study of toxicity and
carcinogenicity in rats treated via the diet, the NOAEL was 40 ppm,
equivalent to 2 mg/kg bw per day, on the basis of depressed
body-weight gain and brain cholinesterase inhibition. In another
two-year study of toxicity and carcinogenicity, rats were fed diets
containing 0, 20, 40 or 200 ppm phosmet, and a smaller group
received 400 ppm. The NOAEL was 40 ppm, equal to 1.8 mg/kg bw per
day, on the basis of fatty changes in the liver and reduced brain
cholinesterase activity in females. There was no evidence of
carcinogenicity in rats.
Two multigeneration studies of reproductive toxicity have been
conducted with phosmet in rats. In a three-generation study, animals
were exposed to dietary levels of 0 or 40 ppm phosmet (first
generation) and 0, 40 or 80 ppm (second generation). The NOAEL was
40 ppm, equivalent to 2 mg/kg bw per day. In a two-generation (two
litters per generation) study of reproductive toxicity, rats were
fed dietary concentrations of 0, 20, 80 or 300 ppm phosmet. The
NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, on the basis of
reduced mating and fertility at higher doses.
In a study of teratogenicity, rats were dosed orally at 0, 5,
10 or 15 mg/kg bw per day. The NOAEL for maternal toxicity was 5
mg/kg bw per day on the basis of reduced body-weight gain; there was
no evidence of fetotoxicity or teratogenicity at the highest dose
tested. In a study of teratogenicity in rabbits dosed at 0, 2, 5 or
15 mg/kg bw per day, the NOAEL for maternal toxicity was 5 mg/kg bw
per day on the basis of reduced body-weight gain, while the NOAEL
for fetotoxicity was 2 mg/kg bw per day on the basis of the presence
of minor skeletal anomalies.
The Meeting concluded that phosmet was not clastogenic but that
its mutagenic potential is unclear. In an attempt to address this
issue, studies of DNA binding in vivo are being requested.
In two studies, phosmet did not cause delayed neuropathy in
chickens; however, the Meeting considered a summary of a study in
which some inhibition of brain neuropathy target esterase was
observed at a dose below the LD50. It therefore concluded that
phosmet may have the potential to cause delayed neuropathy, although
at doses higher than the unprotected LD50. A further study is
being requested to clarify this issue.
An ADI was allocated on the basis of the NOAEL in the
multigeneration study in rats (20 ppm, equal to 1.3 mg/kg bw per
day) and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 4 mg/kg bw per day (two-year study
of carcinogenicity)
Rat: 40 ppm, equal to 1.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
20 ppm, equal to 1.3 mg/kg bw per day (two-generation
study of reproductive toxicity)
5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
15 mg/kg bw per day (study of teratogenicity,
developmental toxicity)
Rabbit: 5 mg/kg bw per day (study of teratogenicity, maternal
toxicity)
2 mg/kg bw per day (study of teratogenicity,
fetotoxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Long-term study of toxicity in dogs
2. Study of DNA binding in vivo
3. Study of delayed neurotoxicity in chickens at an appropriately
high dose, with estimation of neuropathy target esterase
4. Further observations in humans
In order to maintain the ADI, these data should be submitted in
1997, in time for review in 1998.
References
Ackermann, H., Seidler, H., Kagan, Y.S. & Voronina, V.M. (1976)
Metabolic and toxic behaviour of phthalimide derivatives. 1. Fate of
Imidan in the fetus. Arch. Toxicol., 36, 127-137.
Bullock, C.H. (1971) Imidan (EDC process). Unpublished report
T-1666, dated 27 May 1971 from Stauffer Chemical Co. Western
Research Center. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Bullock, C.H. (1972) Unpublished report T-4035, dated 18 August 1972
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Castles, T.R. (1977) Unpublished report T-6123, dated 3 August 1977
from Stauffer Chemical Co., Western Research Center. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Chang, J.C.F., Morrisey, R.L. & Wyand, S. (1991) 2-Year chronic
toxicity/oncogenicity study with R-1504 in rats. Unpublished report
T-13241 dated 29 April 1991 from Ciba-Geigy Corp, Agricultural
Division, Environmental Health Center, Farmington, CT, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Courtney, K.D. & Finkelstein, M. (1968) Teratological investigation
of captan, imidan and thalidomide in Macaca mulatta. Unpublished
report T-2164 to Stauffer Chemical Company, dated 4 April 1968 from
Bionetics Research Laboratories Inc., Falls Church, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Danilenko, L.P. (1969) Maximum permissible concentration of
phthalophos in air of working zone. Hyg. Sanit., 34, 192-197.
Dickey, J.K. (1986) Imidan: morphological transformation of
BALB/3T3. Unpublished report T-12822, dated 8 December 1986 from In
Vitro Toxicology Section, Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Fabro, S., Smith, R.L. & Williams, R.T. (1966) Embryotoxic activity
of some pesticides and drugs related to phthalimide. Food Cosmet.
Toxicol., 3, 587-590.
Fisher, G.D. (1989) R-1504. Metabolism study in rats: recovery of
administered dose. Unpublished report T-13031, dated 16 August 1989
from Ciba-Geigy Corp., Environmental Health Center, Farmington, CT,
USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey,
United Kingdom.
Fisher, G.D. (1990) R-1504. Metabolism study in rats:
biotransformation. Unpublished report T-13031, dated 12 February
1990 from Ciba-Geigy Corp., Environmental Health Center, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ford, I.M., Menn, J.J. & Meyding, G.D. (1966) Metabolism of
N-(mercaptomethyl)phthalimide-carbonyl-C14-(S-(O,O-dimethylphospho
rothioate) (Imidan-C14): balance study in the rat. J. Agric. Food
Chem., 14, 83-86.
Frank, A.A., Ross, J.L. & Sawvell, B.K. (1992) Toxic epidermal
necrolysis associated with flea dips. Vet. Hum. Toxicol., 34,
57-61.
Gibbs, J.A. (1986) Phosmet report of a micronucleus test in the
mouse. Unpublished report No. 2516, dated 18 December 1986 from
Beecham Pharmaceuticals Research Division, Genetic Toxicology Unit,
Stock, United Kingdom. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Good, J.L., Khurana, R.K., Mayer, R.F., Cintra, W.M. & Albuquerque,
E.X. (1993) Pathophysiological studies of neuromuscular function in
subacute organophosphate poisoning induced by phosmet. J. Neurol.
Neurosurg. Psychiatr., 56, 290-294.
Hendricks, M. & Sprague, G.L. (1983) Imidan technical: effect on rat
cholinesterases. Unpublished report No. T-11228, dated 26 April
1983. Richmond Toxicology Laboratory, Stauffer Chemical Co.,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Hertzel, K.M. (1986) Imidan: mutagenicity evaluation in mouse
lymphoma multiple endpoint test forward mutation assay. Unpublished
report No. T-12820, dated 8 May 1986. In Vitro Toxicology Section,
Environmental Health Center, Stauffer Chemical Co., Agrochemical
Division, Farmington, CT, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Hodge, M.C.E. (1991) Phosmet: teratogenicity study in the rat.
Unpublished report No. CTL/P/3360, dated 17 June 1991. ICI Central
Toxicology Laboratory, Alderley Park, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Hollingsworth, R.L., Johnston, C.D. & Woodard, G. (1965) Imidan
three generation study in rats. Unpublished report, dated 7 May
1965, from Woodard Research Corp., Herndon, VA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Izmerov, N.F. (1983) Scientific Reviews of Soviet Literature on
Toxicity and Hazards of Chemicals, Moscow, Centre of International
Projects. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Johnston, C.D. (1962) Imidan: an evaluation of safety of Imidan in
the rat and the dog. Unpublished report, dated 21 February 1962,
from Woodard Research Corp., Herndon, VA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963a) Further evaluation of the safety of Imidan in
the rat. Unpublished report, dated 27 February 1963, from Woodard
Research Corp., Herndon, VA, USA. Submitted to WHO by Zeneca
Agrochemicals, Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1963b) Imidan: demyelination study in the chicken.
Unpublished report, dated 27 February 1963, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Johnston, C.D. (1966) Imidan: single dose oral toxicity in mice.
Unpublished report, dated 22 November 1966, from Woodard Research
Corp., Herndon, VA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Jones, N.B., Sauerhoff, M.W., Thomassen, R.W. & Zwicker, G.M. (1981)
Four week dietary range-finding study in mice with Imidan technical.
Unpublished report No. T-10704, dated July 1981 from Environmental
Health Center, Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Katz, A., Frank, D.W., Zwicker, G.M., Sprague, G.L., Turnier, J.C. &
Freudenthal, R.I. (1984) T(TM)10719. Two-year dietary oncogenicity
study in mice with Imidan technical. Unpublished report, dated May
1984, from Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Király, J., Szentesi, I., Ruzicska, M. & Czeize, A. (1979)
Chromosome studies in workers producing organophosphate
insecticides. Arch. Environ. Contam. Toxicol., 8, 309-319.
Lee, H. & Miaullis, J.B. (1969) The effect of imidan on
cholinesterase and aliesterase activity in rats. Unpublished report
No. 004571 from Stauffer Chemical Co., Agrochemical Division,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Lobdell, B.J. & Johnston, C.D. (1966) Imidan: safety evaluation by
two-year feeding studies in the rat and the dog. Unpublished report,
dated 21 July 1966, from Woodard Research Corp., Herndon, VA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
McBain, J.B., Menn, J.J. & Casida, J.E. (1968) Metabolism of
carbonyl-C14-labelled Imidan, N-(mercaptomethyl)phthalimide-
S-(O,O-dimethylphosphorodithioate), in rats and cockroaches. J.
Agric. Food Chem., 16, 813-820.
Majeska, J.B. & Matheson, D.W. (1986) Imidan: mutagenicity
evaluation in Salmonella typhimurium Unpublished report No. T-12819,
dated 4 March 1986 from Environmental Health Center, Stauffer
Chemical Co., Agrochemical Division, Farmington, CT, USA. Submitted
to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Martson, L.V. & Voronina, V.M. (1976) Experimental study of the
effect of a series of phosphoroorganic pesticides (Dipterex and
Imidan) on embryogenesis. Environ. Health Perspectives, 13,
121-125.
Meyer, L.S. & Walberg, J.A. (1990) A two generation reproduction
study in rats with R-1504. Unpublished report No. T-13260, dated 18
May 1990, from Environmental Health Center, Ciba-Geigy Corp.,
Farmington, CT, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Meyding, G.D. (1965) Imidan technical: acute oral subcutaneous and
intraperitoneal toxicity in rats and mice. Unpublished report No.
65-2, dated 26 January 1965 from Richmond Research Center, Animal
Health Toxicology Section, Stauffer Chemical Co., Richmond, CA, USA.
Submitted to WHO by Zeneca Agrochemicals, Haslemere, Surrey, United
Kingdom.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Moxon, M.E. (1991) Phosmet: teratogenicity study in the rabbit.
Unpublished report no. CTL/P/3373, dated 20 June 1991, from ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield, United
Kingdom. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Nuclear Science Corp. (1962) Unpublished report, Project No.
20-0240-32, dated 30 April 1962, from Nuclear Science Corp., Palo
Alto, CA, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Ray, D.G. (1964) Imidan RP4-RCT-116: acute oral LD50--rats.
Unpublished report No. 507, dated 18 February 1964, from Biological
Research Center, Toxicology Section, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Shirasu, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4, 226-231.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Microbial screening of pesticides in microbial systems. Mutat.
Res., 40, 19-30.
Snyder, R.D. (1986a) Imidan: Mutagenicity evaluation in mouse
lymphoma multiple endpoint test cytogenetic assay. Unpublished
report No. T-12821, dated 8 May 1986, from In Vitro Toxicology
Section, Environmental Health Center, Stauffer Chemical Co.,
Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Snyder, R.D. (1986b) Effects of imidan on human fibroblast DNA.
Unpublished report No. T-12823, dated 30 May 1986, from In Vitro
Toxicology Section, Environmental Health Centre, Stauffer Chemical
Co., Agrochemical Division, Farmington, CT, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Sprague, G.L. (1982) Acute delayed neurotoxicity study with Imidan
technical in adult hens. Unpublished report No. T-10910, dated 9
August 1982, from Richmond Toxicology Laboratory, Toxicology
Department, Stauffer Chemical Co., de Guigne Technical Center,
Richmond, CA, USA. Submitted to WHO by Zeneca Agrochemicals,
Haslemere, Surrey, United Kingdom.
Sprague, G.L. & Turnier, J.C. (1986) T-10719. Addendum: Two-year
dietary oncogenicity study in mice with Imidan technical.
Unpublished addendum, dated 22 May 1986, from Environmental Health
Center, Stauffer Chemical Co., Agrochemical Division, Farmington,
CT, USA. Submitted to WHO by Zeneca Agrochemicals, Haslemere,
Surrey, United Kingdom.
Staples, R.E., Kellam, R.G. & Haseman, J.K. (1976) Developmental
toxicity in the rat after ingestion or gavage of organophosphate
pesticides (Dipterex, Imidan) during pregnancy. Environ. Health
Perspectives, 13, 133-140.
Tarr, J.B. & Hemingway, R.J. (1993a) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and milk of
lactating goats. Unpublished report RR 92-103B, study No. PMS-352,
dated 22 January 1983, from Zeneca Agricultural Products, Western
Research Center, ICI Americas Inc., Richmond, CA, USA. Submitted to
WHO by Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
Tarr, J.B. & Hemingway, R.J. (1993b) The nature of the residues of
orally administered [carbonyl-14C]-phosmet in tissues and eggs of
laying hens. Unpublished report No. 1227-1, RR 93-046B, dated 14
January 1983, from Zeneca Agricultural Products, Western Research
Center, ICI Americas Inc., Richmond, CA, USA. Submitted to WHO by
Zeneca Agrochemicals, Haslemere, Surrey, United Kingdom.
