
TECNAZENE
First draft prepared by
J.-J. Larsen,
Institute of Toxicology, National Food Agency,
Ministry of Health, Soborg, Denmark
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Tecnazene was evaluated by the Joint Meeting in 1974, 1978,
1981 and 1983 (Annex I, references 22, 30, 36 and 40). A temporary
ADI (0-0.01 mg/kg bw) was allocated in 1978, which was confirmed as
a full ADI in 1983. Since review of the toxicology of tecnazene in
1983, several new studies have become available. This monograph
summarizes the data received since the previous evaluation and
contains relevant summaries from the previous monograph and
monograph addenda on tecnazene (Annex I, references 23, 31 and 37).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Two rats weighing about 200 g each and two guinea-pigs weighing
about 500 g each were treated orally by stomach tube with
suspensions of tecnazene in water, at 750 µmol/kg bw for rats and
450 µmol/kg bw for guinea-pigs. About 20% was recovered in the
faeces of both species over 72 h, indicating a high degree of
absorption (Bray et al., 1959).
In a preliminary experiment in rats, each of three males and
three females was given a single oral dose of 1 mg/kg bw
14C-tecnazene. Excretion of radioactivity in urine, faeces and
expired air was measured for up to 48 h, and the distribution of
radioactivity in tissues was determined by whole-body
autoradiography in one male and one female killed at 24 and 48 h
after dosing. In addition, each of five male and five female rats
was given a single oral dose of 1 mg/kg bw 14C-tecnazene. Urinary
and faecal excretion of radioactivity was monitored for seven days,
at which time residual radioactivity was measured in blood, selected
tissues and carcasses. The male rats excreted 42% of the
administered radiolabel in the urine and 47% in the faeces during
the 0-48-h period after dosing, whereas females excreted 80% in
urine and 12% in faeces during the same period. The remaining
portion of the dose was excreted more slowly; radiolabel was still
detected in urine and faeces seven days after treatment. Negligible
levels were detected in expired carbon dioxide. Whole-body
autoradiography showed the highest concentrations of radiolabel 24 h
after treatment in the intestinal contents of male and female rats.
The highest tissue concentrations were found in kidney, liver and
nasal passages; radiolabel was also present in blood, lungs and
skin. The distribution of radioactive residues in tissues 48 h after
treatment was similar to that at 24 h, although the amounts were
much lower in all tissues except the nasal passages. There was no
significant difference in tissue distribution between male and
female rats. Seven days after treatment, the concentrations of
radiolabel in the tissues were low but were generally slightly
higher in males (0.13%) than in females (0.05%). In males, the
highest concentrations were found in abdominal fat, kidney, lung,
blood and heart. In female rats, the highest concentrations were
found in abdominal fat, blood and ovaries. The amounts of radiolabel
in the residual carcasses did not exceed 0.5% of the dose in rats of
either sex (Bratt, 1991).
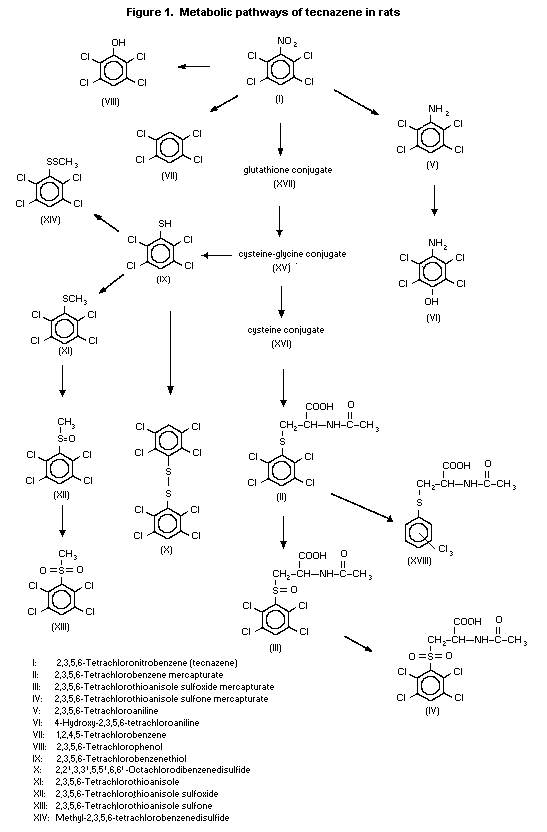
(b) Biotransformation
The distribution of 14C-tecnazene was studied in male and
female rats (strain and number not specified) after oral
administration of 1 mg/kg bw. Pooled urine and faeces were taken
over 0-48 h and analysed chromatographically. The pattern of
metabolites in urine appeared to be the same in males and females,
but the quantities differed. The major component in urine was a
tetrachlorophenyl-mercapturate conjugate; tecnazene,
tetrachloroaniline and tetrachlorothioanisole were identified in
faeces (Bentley & Powles, 1992).
Alpk:APfSD rats of each sex received 14C-tecnazene as one
oral dose of 1 mg/kg bw or four daily doses of 135 mg/kg bw, and
their urine and faeces were collected. Metabolites were also
determined in bile from one male and one female rat given one oral
dose of 135 mg/kg bw. Females given 1 mg/kg bw excreted 75% of the
radiolabel in urine and 25% in faeces, while males excreted 50% in
urine and 50% in faeces. After a single dose of 135 mg/kg bw, males
excreted 80% of the radiolabel in bile, 5% in urine and 5% in
faeces, and females excreted 35% in bile, 35% in urine and 15% in
faeces. Tecnazene was extensively metabolized: 16 metabolites were
identified in urine and bile, and the chemical structures of an
additional two metabolites were partially elucidated (Figure 1). A
total of 42 metabolites were separated as radiolabelled peaks by
high-performance liquid chromatography, although many of these
represented less than 0.1% of the administered dose. The principal
route of metabolism was via the tetrachlorobenzene-glutathione
conjugate pathway. There was evidence of enterohepatic circulation,
leading to the excretion of tetrachlorobenzene mercapturate and
corresponding S-oxidation products in the urine. Tecnazene was also
metabolized by minor pathways, including ß-lyase-mediated metabolism
and formation of tetrachlorobenzenethiol, probably from the cysteine
conjugate, which resulted in a number of minor sulfur-containing
metabolites. Tecnazene also underwent loss of the nitro moiety,
resulting in tetrachlorobenzene and replacement of the nitro moiety
with a hydroxyl group, to give tetrachlorophenol. The nitro moiety
of tecnazene was also reduced, resulting in the formation of
tetrachloroaniline which is hydroxylated to form
4-hydroxytetrachloroaniline (Lappin & Pritchard, 1992).
Tecnazene was administered to groups of one to six female
rabbits, weighing 2-3 kg, at single doses of 0.01, 0.1, 0.5, 1.5 or
3 g by stomach tube as suspensions in water. Most of the material
(60-70%) was recovered in the faeces within three days; the
remainder (35-38%) was excreted in the urine, primarily as
conjugated products. The average percentages of the dose excreted in
urine were 12% as 2,3,5,6-tetrachloroaniline, 12% as glucuronide, 1%
as ethereal sulfate and 11% as mercapturic acid. Some
4-amino-2,3,5,6-tetrachlorophenol was excreted unconjugated (Bray
et al., 1953).
 Other polychlorinated nitrobenzenes, such as
2,3,4,6-tetrachloronitrobenzene and pentachloro-nitrobenzene, also
form mercapturic acids in vivo. Pentachlorophenyl mercapturic acid
was the major metabolite of pentachloronitrobenzene in the urine of
rats (Renner, 1980) and rabbits (Betts et al., 1955).
2. Toxicological studies
Aromatic nitro compounds are of considerable importance not
only as pesticides, e.g. tecnazene and quintozene
(pentachloronitrobenzene), but also as solvents and synthetic
intermediates for many technical products. Nitrobenzene is toxic and
is readily absorbed through the skin. In cases of acute poisoning,
haematological changes occur, such as decreased haemoglobin level
and methaemoglobinaemia; and symptoms of intoxication, such as
vomiting, colic, headaches, breathlessness and cyanosis, are seen.
Additional effects on the central nervous system result in anxiety
and convulsions. A variety of nitrophenols affect the release of
acetylcholine at nerve endings in the small intestine and certain
muscles. Individual nitro aromatic compounds can cause specific
symptoms of poisoning in addition to the general ones described
above. Chronic poisoning with nitroaromatic compounds results in
cirrhosis or acute atrophy of the liver. Allergic reactions are
caused by 1-chloro-2,4-dinitrobenzene, probably by an
antigen-antibody reaction which results after the dinitrophenyl
group is bound covalently to natural proteins. In early studies,
pentachloronitrobenzene caused liver abnormalities, such as
single-cell necrosis, fatty metamorphosis of hepatocytes and
enlarged centrilobular hepatocytes.
(a) Acute toxicity
Two early studies in which rats were given an aqueous
suspension of tecnazene orally or intraperitoneally indicated little
acute toxicity, the LD50 values being 7500 and 3500 mg/kg bw,
respectively (Annex I, references 23 and 31). The LD50 values for
tecnazene (purity, 96%) administered orally to rats in corn oil were
2000 mg/kg bw in males and 1300 mg/kg bw in females (Barber, 1985).
(b) Short-term toxicity
Mice
Groups of 12 mice were fed tecnazene in the diet at levels of
0, 1344 or 13 440 ppm, equivalent to 0, 202 or 2016 mg/kg bw per
day, for 31 days, after which they were killed. Body weights were
measured at the start and end of the study, and food consumption was
determined daily. Mice receiving the high dose did not gain body
weight during the 31-day period. The NOAEL was 1344 ppm, equivalent
to 202 mg/kg bw per day (Buttle & Dyer, 1950).
A group of 24 mice were fed tecnazene in the diet at a dose of
10 000 mg/kg bw per day. When deaths occurred within three to four
days, the study was discontinued. Animals were found to have fatty
degeneration of the liver and fatty changes in the spleen and kidney
(Buttle & Dyer, 1950).
Rats
Groups of five rats were fed tecnazene in the diet at doses of
0, 800, 4000 or 20 000 ppm, equivalent to 0, 40, 200 or 1000 mg/kg
bw per day, for 10 weeks. Deaths occurred at the highest dose, and
growth was reduced at 4000 ppm. The NOAEL was 800 ppm, equivalent to
40 mg/kg bw per day (Buttle & Dyer, 1950).
Groups of 12 male and 12 female randomly selected Alpk:APfSD
rats weighing 120-124 g (males) and 111-116 g (females) were fed
diets containing tecnazene (purity, 98.7%) at concentrations of 0,
50, 500 or 5000 ppm, equal to 0, 4.5, 45 or 500 mg/kg bw per day in
males and 0, 4.9, 49 or 500 mg/kg bw per day in females, for 90
days. All animals were observed daily for mortality and signs of
toxicity, and body weight and food consumption were recorded weekly.
Animals in the control and high-dose groups underwent
ophthalmoscopic examinations during the week before sacrifice. After
sacrifice, haematology, clinical chemistry, gross pathology and
histopathology were undertaken on all rats. Administration of 5000
ppm tecnazene was associated with reduced body-weight gain
throughout the study and with an associated reduction in food
consumption and efficiency of food use during week 1. The marked
effect on body weight was reflected in reduced plasma triglyceride
levels, plasma alanine transaminase and creatine kinase activities
and erythrocyte parameters and changes in the levels of total plasma
protein and urea. The liver was identified as a target organ:
Increased liver weight and hepatic aminopyrine-N-demethylase
activity were seen at 500 and 5000 ppm, with histological evidence
of hepatic hypertrophy at 5000 ppm, indicating an adaptive change.
Increased plasma alkaline phosphatase activity and cholesterol
levels were noted at 5000 ppm, and to a lesser extent for males at
500 ppm. In the absence of adverse histopathological findings,
however, these changes were considered not to be of toxicological
significance. The kidney was also identified as a target organ.
Increased kidney weight was seen in males and females given 5000
ppm, which, in the absence of histopathological change, reflects a
possible adaptive response. Mild renal functional changes, indicated
by signs of urinary incontinence (wetness or staining around the
urogenital area), decreased specific gravity and cloudy urine, were
noted at 5000 ppm. Urinary incontinence was also observed at 500 ppm
in females. On the basis of general toxicity, including the adaptive
effects in the liver and kidneys, the NOAEL was 500 ppm, equal to 45
mg/kg bw per day (Horner, 1992).
Dogs
Groups of four male and four female beagle dogs were given
tecnazene (purity, 98.7%) at 0, 2, 15 or 200 mg/kg bw per day for 90
days. Blood samples were taken before treatment and at weeks 4, 8
and 13 and tested for changes in haematological parameters and
clinical chemistry. Food consumption and clinical observations were
recorded daily, body weight was recorded weekly, and ophthalmoscopic
examinations were done before treatment and before sacrifice. All
dogs were examined by gross pathology and histopathology. Body
weight was reduced in animals of each sex at 200 mg/kg bw per day,
but food consumption was unaffected by treatment. The liver was
identified as the target organ, since evidence for an adaptive
response was seen in all treated groups in the form of a
dose-related increase in liver aminopyrine- N-demethylase activity.
At 200 mg/kg bw per day, there was a more marked response, including
increased alkaline phosphatase activity, increased liver weight (>
60%) and histopathological changes typical of increased
proliferation of the smooth endoplasmic reticulum. Altered liver
function at this dose was indicated by changes in most clinical
chemical parameters and small changes in some haematological
parameters, including increased clotting times, platelet counts and
mean cell volumes. Minimal alterations in liver weight (19%) and
clinical chemical parameters were also seen in females receiving 15
mg/kg bw per day. A slight increase in liver weight in males at 15
mg/kg bw per day was due mainly to the response in one dog. Changes
in organ weights of animals at 200 mg/kg bw per day were not
associated with histopathological abnormalities induced by
tecnazene, although there was evidence of delayed sexual maturation
in males that was consistent with the reduced growth of these
animals. On the basis of body-weight reduction and the adaptive
effect on the liver, the NOAEL in this study was 15 mg/kg bw per day
(Hodge, 1992).
Two male and two female beagle dogs were given capsules
containing tecnazene at concentrations of 0, 3.8, 15, 60 or 240
mg/kg bw per day, six days per week for two years. All of the dogs
receiving the highest dose died during the first year of the study,
and microscopic changes were noted in the liver, kidney and bone
marrow. At 60 mg/kg bw per day, growth was normal, but serum
alkaline phosphatase activity was increased. No effects were seen on
haematological, urological or electrocardiographic parameters. The
NOAEL was 15 mg/kg bw per day for animals of each sex (summarized in
Annex I, reference 23).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 65 male and 65 female CD-1 mice were fed 0, 750 or
1500 ppm tecnazene (purity, > 99.0%) in the diet for 80 weeks,
equal to 0, 78 or 155 mg/kg bw per day in males and 0, 86 or 174
mg/kg bw per day in females. The mice were observed daily for
clinical signs. Those that died during the first 13 weeks of the
study were submitted to a macroscopic necropsy and replaced by
reserve animals on the same treatment. Body weights were recorded
weekly during the first 12 weeks and every two weeks thereafter.
Food consumption was assessed weekly, and water intake was assessed
daily by visual inspection. At the end of study, all mice were
examined by gross pathology and histopathology. A total of 237 mice
(131 males and 106 females) died or were killed in extremis, but
the group distribution, time and cause of death were not related to
treatment. Body weights and food and water intakes of treated mice
were similar to those of the untreated controls throughout the
study. The pattern of non-neoplastic pathological effects was
unchanged by treatment, and treatment had no effect on tumour type
or incidence. The NOAEL was 1500 ppm, equal to 155 mg/kg bw per day
(Ben-Dyke et al., 1978a).
Rats
Groups of 65 male and 65 female CD rats were fed tecnazene
(purity, > 99.0%) in the diet at 0, 750 or 1500 ppm for 104 weeks,
equal to 0, 27 or 56 mg/kg bw per day in males and 0, 32 or 63 mg/kg
bw per day in females. Animals were observed daily for clinical
signs. Those that died during the first 13 weeks of the study were
submitted to macroscopic necropsy and replaced by reserve animals on
the same treatment. Body weights were recorded weekly during the
first 12 weeks and every two weeks thereafter. Food consumption was
assessed weekly, and water intake was assessed daily by visual
inspection. At the end of study, all mice were examined by gross
pathology and histopathology. The few signs seen during treatment
were considered not to be of biological significance. Mortality
rates, body weights, food intakes and efficiency of food use were
not affected by treatment. The range of macroscopic changes seen at
necropsy were those commonly seen in CD rats. There were some
differences between groups in the incidences of pituitary adenomas
and mammary gland tumours, but these were considered not to be
related to treatment. The incidences of other tumour types were
similar in treated and control groups. The NOAEL was 1500 ppm, equal
to 56 mg/kg bw per day (Ben-Dyke et al., 1978b).
(d) Reproductive toxicity
Rats
Groups of 30 male and 30 female Alpk:APfSD (Wistar-derived)
rats, four to five weeks old, were fed diets containing tecnazene
(purity, 98.7%) at 0, 300, 1000 or 5000 ppm, equal to 32, 106 or 571
mg/kg bw per day in males and 34, 113 or 576 mg/kg bw per day in
females. After 12 weeks, the animals were mated and allowed to rear
the ensuing F1a litters to weaning. During the pre-weaning phase,
it became evident that the F1a pups could not tolerate the
5000-ppm dose, and they and their dams were given control diet from
days 17-20 for the remainder of the lactation period. The F1a
offspring were considered to be unsuitable to form the next parental
generation, so an F1b litter was produced after the parental dose
had been reduced to 3000 ppm. The F1b offspring and dams at the
highest dose were also given control diet during the pre-weaning
phase because of poor weight gain of the pups (from days 21-25, and
earlier for a few litters born later); however, the F1 parents
were selected from among the F1b offspring. The highest dietary
level was further reduced to 2000 ppm, and these animals were
allowed to produce the F2a litter after a 12-week pre-mating
period. Diets containing 0, 300, 1000 or 2000 ppm tecnazene, equal
to 31, 103 or 220 mg/kg bw per day in males and 34, 111 or 235 mg/kg
bw per day in females, were then fed continuously throughout the
remainder of the study.
Dietary administration of 5000 ppm tecnazene was associated
with reduced body-weight gain and food consumption during the
pre-mating period, but 300 or 1000 ppm had no adverse effects. A
dose-related incidence of signs of urinary incontinence (wetness or
staining around the urogenital area) was seen in treated animals,
but the significance of this finding was uncertain. A clear,
dose-related effect on the offspring was associated with
administration of 3000 or 5000 ppm tecnazene. At the highest dietary
level, pup survival, clinical condition and growth were adversely
affected; only pup growth was affected by 3000 ppm. Doses of 2000
ppm or less had no adverse effect on pup growth or survival.
Treatment induced a dose-related increase in kidney weight in males
of both generations, but no corresponding histological change was
observed. Increased liver weight was seen in both parents and
offspring at all dietary levels of tecnazene; histological changes
indicative of an adaptive response were observed in adults receiving
1000 ppm or more but not in those receiving 300 ppm or in any of the
offspring. The increased organ weights were considered to reflect an
adaptive response and, in the absence of histological change, were
considered to be of no toxicological significance. Tecnazene did not
affect reproductive performance in either generation. The NOAEL for
maternal toxicity was 1000 ppm, equal to 103 mg/kg bw per day. The
NOAEL for filial toxicity and reproductive toxicity was 2000 ppm,
equal to 220 mg/kg bw per day (Moxon, 1992).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant Alpk:APfSD (Wistar-derived) rats, 12
weeks of age, were treated by gavage with tecnazene (purity, 98.7%)
at 0, 15, 50 or 150 mg/kg bw per day on days 7-16 of gestation. The
females were observed daily for clinical signs of toxicity and were
killed on day 22 of gestation. They were examined by gross
pathology, and their uteri were examined for live fetuses and
intra-uterine deaths. The fetuses were weighed, examined for
external visceral abnormalities, sexed, eviscerated and stained for
skeletal examination, and the number of implantations was
determined. Administration of 150 mg/kg bw per day resulted in a
statistically significant reduction in body-weight gain of dams,
particularly on days 7-10; no maternal toxicity was apparent at
lower doses. Overall, there was an increased number of fetuses with
minor skeletal defects (unossified centra of the third and fourth
cervical vertebrae) and an increased incidence of some skeletal
variants (partially ossified transverse processes of the seventh
cervical vertebrae and of the fourth lumbar vertebrae) at 150 mg/kg
bw per day. There was no evidence of embryo- or fetotoxicity or
teratogenicity at any dose. The NOAEL for maternal toxicity and for
embryo- and fetotoxicity was 50 mg/kg bw per day, on the basis of
effects on body weight and minor skeletal defects, respectively
(Whiles, 1991).
Rabbits
Tecnazene (purity, 98.7%) was administered in corn oil by
gavage to groups of 20 pregnant New Zealand white rabbits at 0, 15,
45 or 135 mg/kg bw per day on days 7-19 of gestation. The animals
were killed on day 30 of gestation and their uteri examined for live
fetuses and intra-uterine deaths. The fetuses were weighed, examined
for external and visceral abnormalities, sexed, eviscerated and
processed for skeletal examination. Two animals given 135 mg/kg bw
per day were killed when moribund on days 20 and 27 of gestation;
the death that occurred on day 20 was considered to be related to
treatment, as it was associated with adverse clinical signs, poor
food consumption and body-weight loss. There was no evidence of
overt toxicity in the remaining animals in this group or in animals
given 15 or 45 mg/kg bw per day, and there was no treatment-related
effect on the litters. A total of 27 major defects were seen in 18
fetuses from 14 litters. Of these defects, 13 occurred in controls,
and treatment did not appear to have affected the overall incidence
of major defects. Four minor skeletal defects or variants were noted
at 45 or 135 mg/kg bw per day, which were considered to represent a
minimal effect of tecnazene on fetal development. These defects
included slightly increased manus scores at 45 and 135 mg/kg bw per
day, marginally increased incidences of 27 pre-sacral vertebrae and
extra thirteenth ribs and a slightly increased incidence of
misshapen hyoid at 135 mg/kg bw per day. The NOAEL for maternal
toxicity was 45 mg/kg bw per day on the basis of adverse clinical
signs and body-weight loss, and the NOAEL for embryo- and
fetotoxicity was 15 mg/kg bw per day, on the basis of minor skeletal
defects (Hopkins, 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tecnazene are
summarized in Table 1.
Table 1. Results of tests for the genotoxicity of tecnazene
End-point Test system Concentration Purity Results Reference
of tecnazene (%)
In vitro
Reverse S. typhimurium TA98, 0.32-1000 µg/plate NR Negativea Cattanach & Riach, 1989
mutation 100, 1535, 1538
Forward Mouse lymphoma 1-50 µg/ml 97.1 Negativeb Adams et al., 1989
mutation cells (L5178Y) Positivec
at tk locus
Chromosomal Human 20 µg/ml 97.1 Negativea Brooker et al., 1989
aberration lymphocytes 80 µg/ml Negativec
80 µg/ml Positiveb
160 µg/ml Positivea
In vivo
Micronucleus C57Bl/6 1160-1860 mg/kg bw 97.1 Negative Randall & Howard, 1989
formation JfCD 1/Alpk
mice
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
3. Observations in humans
No information was available.
Comments
After oral administration of radiolabelled tecnazene to rats,
about 90% of the dose was recovered within 48 h. Excretion was
divided almost equally between urine and faeces in males but
occurred predominantly (80%) via the urine in females. The remainder
of the dose was excreted more slowly, and radioactivity was still
detectable in urine and faeces seven days after dosing. Negligible
levels of radioactivity were detected in expired carbon dioxide.
Whole-body autoradiography showed that 24 h after dosing the highest
concentrations of radioactivity were located in the intestinal
contents of animals of each sex; the highest tissue concentrations
were found in the kidney, liver and the nasal passages.
Tecnazene is extensively metabolized in rats. A total of 42
metabolites have been separated from urine and bile, of which 16,
including the parent compound, have been identified. The principal
route of metabolism is via the tetrachlorobenzene glutathione
conjugate pathway. Metabolites present in urine and faeces include
the tetrachloro-phenyl-mercapturate conjugate, tetrachloroaniline
and tetrachlorothioanisole. The pattern of metabolites in urine and
faeces is the same in male and female rats, but the quantities
differ.
In female rabbits, about 70% of an oral dose of tecnazene was
recovered in the faeces within three days. The remainder was
excreted in urine, primarily as glucuronide, sulfate and mercapturic
acid conjugates of 2,3,5,6-tetrachloroaniline. Some unconjugated
4-amino-2,3,5,6-tetrachlorophenol was also excreted.
Tecnazene has low oral toxicity in rats. WHO (1992) has
classified tecnazene as unlikely to present an acute hazard in
normal use.
In a 90-day study in rats fed dietary concentrations of 0, 50,
500 or 5000 ppm, the NOAEL was 500 ppm, equal to 45 mg/kg bw per
day, on the basis of effects on body-weight gain and on the liver
and kidneys.
In a 90-day study in dogs given 0, 2, 15 or 200 mg/kg bw per
day orally, the NOAEL was 15 mg/kg bw per day on the basis of
effects on body and liver weights.
In a two-year study in which dogs were given tecnazene at 0,
3.8, 15, 60 or 240 mg/kg bw per day orally, the NOAEL was 15 mg/kg
bw per day, on the basis of elevation of serum alkaline phosphatase
activity. Owing to the small number of animals and lack of access to
the original report, data from this study could not be considered in
establishing an ADI.
In an 80-week study of carcinogenicity in mice fed dietary
concentrations of 0, 750 or 1500 ppm, the NOAEL was 1500 ppm, equal
to 155 mg/kg bw per day. There was no evidence of carcinogenicity
and no effect on body weight, mortality or clinical signs.
In a 104-week study of carcinogenicity in rats fed 0, 750 or
1500 ppm, the NOAEL was also 1500 ppm, equal to 56 mg/kg bw per day.
There was no evidence of carcinogenicity. As no clinical chemical or
haematological parameters were evaluated in this study, the Meeting
considered it inadequate for an evaluation of long-term toxicity.
In a two-generation study of reproductive toxicity in rats fed
dietary concentrations of 0, 300, 1000 or 5000/2000 ppm, the NOAEL
for parental toxicity was 1000 ppm, equal to 106 mg/kg bw per day.
The NOAEL for filial toxicity was 2000 ppm, equal to 220 mg/kg bw
per day. There were no adverse effects on reproduction.
In a study of teratogenicity in rats administered 0, 15, 50 or
150 mg/kg bw per day by gavage, the NOAEL was 50 mg/kg bw per day
for both maternal toxicity and embryo- and fetotoxicity on the basis
of reduced body-weight gain and minor skeletal defects,
respectively. No teratogenic effects were observed.
In a study of teratogenicity in rabbits administered 0, 15, 45
or 135 mg/kg bw per day by gavage, the NOAEL was 45 mg/kg bw per day
for maternal toxicity on the basis of body-weight loss and reduced
food consumption. The NOAEL was 15 mg/kg bw per day for embryo- and
fetotoxicity on the basis of minor skeletal defects. No teratogenic
effects were observed.
Tecnazene can produce clastogenic effects in vitro but not
in vivo. No mutagenicity was observed in bacteria. The Meeting
concluded that tecnazene is not genotoxic.
An ADI was established on the basis of an NOAEL of 15 mg/kg bw
per day for toxicity in the 90-day study in dogs and for embryo- and
fetotoxicity in the study of teratogenicity in rabbits. Owing to the
lack of adequate data on the long-term toxicity of the compound, the
Meeting applied a 1000-fold safety factor to the NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1500 ppm, equal to 155 mg/kg bw per day (80-week
study of carcinogenicity)
Rat: 500 ppm, equal to 45 mg/kg bw per day (90-day study
of toxicity)
1500 ppm, equal to 56 mg/kg bw per day (104-week
study of carcinogenicity)
Rabbit: 15 mg/kg bw per day (embryo- and fetotoxicity in a
study of teratogenicity)
Dog: 15 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. One-year study of toxicity in dogs
2. Long-term study of toxicity in rats
References
Adams, K., Ransome, S.J., Henly, S.M., Kirkpatrick, D., Skinner, N.
& Bottoms, M.A (1989) An assessement of the mutagenic potential of
tecnazene using the mouse lymphoma TK locus assay. Unpublished
report No. ISN 206/89508 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Barber, J.E. (1985) Tecnazene: acute oral toxicity study.
Unpublished report No. CTL/P/1232 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978a) Tecnazene: oncogenic response in mice to continuous
dietary administration for 80 weeks. Unpublished report No. 78/ILY
19/080 from Life Science Research, Stock, United Kingdom. Submitted
to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United
Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978b) Tecnazene: carcinogenic response in rats to dietary
administration for 104 weeks. Unpublished report No. 78/ILY 20/078
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Bentley, M. & Powles, P. (1992) Tecnazene--preliminary examination
of urinary and faecal metabolites in the rat. Unpublished report No.
7119-72/305 from Hazleton United Kingdom, Harrogate, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and the
formation of mercapturic acids in the rabbit. Biochem. J., 61,
611-617.
Bratt, H. (1991) Tecnazene: Excretion and tissue retention of a
single oral dose (1 mg/kg) in the rat. Unpublished report No.
CTL/P/3202 from ICI Central Toxicology Laboratory. Submitted to WHO
by Zeneca Agrochemicals, Fernshurst, Haslemere, United Kingdom.
Bray, H.G., Hybs, Z., James, S.P. & Thorpe, W.V. (1953) The
metabolism of 2:3:5:6- and 2:3:4:5-tetrachloronitrobenzenes in the
rabbit and the reduction of aromatic nitro compounds in the
intestine. Biochem. J., 53, 266-273.
Bray, H.G., Franklin, T.J. & James, S.P. (1959) The formation of
mercapturic acids. 3. N-Acetylation of S-substituted cysteines in
the rabbit, rat and guinea pig. Biochem. J., 73, 465-473.
Brooker, P.C., Akhurst, L.C. & King, J.D. (1989) Tecnazene:
metaphase chromosome analysis of human lymphocytes cultured in
vitro. Unpublished report No. ISN 207/89289 from Huntingdon
Research Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by
Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Buttle, G.A.H. & Dyer, F.J. (1950) Experiments on the toxicology of
2,3,5,6-tetrachloronitrobenzene. J. Pharm. Pharmacol., 2, 371-375.
Cattanach, P.J. & Riach, C.G. (1989) Tecnazene: testing for
mutagenic activity with Salmonella typhimurium TA 1535, TA 1537, TA
1538, TA 98 and TA 100. Unpublished report No. 4872 from Inveresk
Research International, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Donikian, M., Owen, S.D., Wiland, J. & Drobeck, H.P. (1965) Oral
administration of 2,3,5,6-tetrachloronitrobenzene to beagle dogs for
two years. Unpublished report from Sterling Winthrop Research
Institute (1974 Evaluations of some pesticide residues in food, WHO
1975).
Hodge, M.C.E. (1992) Tecnazene: 90 day oral dosing study in dogs.
Unpublished report No. CTL/P/3614 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Hopkins, M.N. (1991) Tecnazene: Teratogenicity study in the rabbit.
Unpublished report No. CTL/P/3184 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Horner, J.M. (1992) Tecnazene: 90-day feeding study in rats.
Unpublished report No. CTL/P/3648 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Lappin, G.J. & Pritchard, D.J. (1992) Tecnazene: Biotransformation
in the rat. Unpublished report No. CTL/P/3731 from ICI Central
Toxicology Laboratory, Macclesfield, United Kingdom. Submitted to
WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Moxon, M.E. (1992) Tecnazene: multigeneration study in the rat.
Unpublished report No. CTL/P/3596 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Randall, V. & Howard, C.A. (1989) Tecnazene: an evaluation in the
mouse micronucleus test. Unpublished report No. CTL/P/2632 from ICI
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Renner, G. (1980) Metabolic studies on pentachloronitrobenzene
(PCNB) in rats. Xenobiotica, 10, 537-550.
Whiles, A.J. (1991) Tecnazene: Teratogenicity study in the rat.
Unpublished report No. CTL/P/3193 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Other polychlorinated nitrobenzenes, such as
2,3,4,6-tetrachloronitrobenzene and pentachloro-nitrobenzene, also
form mercapturic acids in vivo. Pentachlorophenyl mercapturic acid
was the major metabolite of pentachloronitrobenzene in the urine of
rats (Renner, 1980) and rabbits (Betts et al., 1955).
2. Toxicological studies
Aromatic nitro compounds are of considerable importance not
only as pesticides, e.g. tecnazene and quintozene
(pentachloronitrobenzene), but also as solvents and synthetic
intermediates for many technical products. Nitrobenzene is toxic and
is readily absorbed through the skin. In cases of acute poisoning,
haematological changes occur, such as decreased haemoglobin level
and methaemoglobinaemia; and symptoms of intoxication, such as
vomiting, colic, headaches, breathlessness and cyanosis, are seen.
Additional effects on the central nervous system result in anxiety
and convulsions. A variety of nitrophenols affect the release of
acetylcholine at nerve endings in the small intestine and certain
muscles. Individual nitro aromatic compounds can cause specific
symptoms of poisoning in addition to the general ones described
above. Chronic poisoning with nitroaromatic compounds results in
cirrhosis or acute atrophy of the liver. Allergic reactions are
caused by 1-chloro-2,4-dinitrobenzene, probably by an
antigen-antibody reaction which results after the dinitrophenyl
group is bound covalently to natural proteins. In early studies,
pentachloronitrobenzene caused liver abnormalities, such as
single-cell necrosis, fatty metamorphosis of hepatocytes and
enlarged centrilobular hepatocytes.
(a) Acute toxicity
Two early studies in which rats were given an aqueous
suspension of tecnazene orally or intraperitoneally indicated little
acute toxicity, the LD50 values being 7500 and 3500 mg/kg bw,
respectively (Annex I, references 23 and 31). The LD50 values for
tecnazene (purity, 96%) administered orally to rats in corn oil were
2000 mg/kg bw in males and 1300 mg/kg bw in females (Barber, 1985).
(b) Short-term toxicity
Mice
Groups of 12 mice were fed tecnazene in the diet at levels of
0, 1344 or 13 440 ppm, equivalent to 0, 202 or 2016 mg/kg bw per
day, for 31 days, after which they were killed. Body weights were
measured at the start and end of the study, and food consumption was
determined daily. Mice receiving the high dose did not gain body
weight during the 31-day period. The NOAEL was 1344 ppm, equivalent
to 202 mg/kg bw per day (Buttle & Dyer, 1950).
A group of 24 mice were fed tecnazene in the diet at a dose of
10 000 mg/kg bw per day. When deaths occurred within three to four
days, the study was discontinued. Animals were found to have fatty
degeneration of the liver and fatty changes in the spleen and kidney
(Buttle & Dyer, 1950).
Rats
Groups of five rats were fed tecnazene in the diet at doses of
0, 800, 4000 or 20 000 ppm, equivalent to 0, 40, 200 or 1000 mg/kg
bw per day, for 10 weeks. Deaths occurred at the highest dose, and
growth was reduced at 4000 ppm. The NOAEL was 800 ppm, equivalent to
40 mg/kg bw per day (Buttle & Dyer, 1950).
Groups of 12 male and 12 female randomly selected Alpk:APfSD
rats weighing 120-124 g (males) and 111-116 g (females) were fed
diets containing tecnazene (purity, 98.7%) at concentrations of 0,
50, 500 or 5000 ppm, equal to 0, 4.5, 45 or 500 mg/kg bw per day in
males and 0, 4.9, 49 or 500 mg/kg bw per day in females, for 90
days. All animals were observed daily for mortality and signs of
toxicity, and body weight and food consumption were recorded weekly.
Animals in the control and high-dose groups underwent
ophthalmoscopic examinations during the week before sacrifice. After
sacrifice, haematology, clinical chemistry, gross pathology and
histopathology were undertaken on all rats. Administration of 5000
ppm tecnazene was associated with reduced body-weight gain
throughout the study and with an associated reduction in food
consumption and efficiency of food use during week 1. The marked
effect on body weight was reflected in reduced plasma triglyceride
levels, plasma alanine transaminase and creatine kinase activities
and erythrocyte parameters and changes in the levels of total plasma
protein and urea. The liver was identified as a target organ:
Increased liver weight and hepatic aminopyrine-N-demethylase
activity were seen at 500 and 5000 ppm, with histological evidence
of hepatic hypertrophy at 5000 ppm, indicating an adaptive change.
Increased plasma alkaline phosphatase activity and cholesterol
levels were noted at 5000 ppm, and to a lesser extent for males at
500 ppm. In the absence of adverse histopathological findings,
however, these changes were considered not to be of toxicological
significance. The kidney was also identified as a target organ.
Increased kidney weight was seen in males and females given 5000
ppm, which, in the absence of histopathological change, reflects a
possible adaptive response. Mild renal functional changes, indicated
by signs of urinary incontinence (wetness or staining around the
urogenital area), decreased specific gravity and cloudy urine, were
noted at 5000 ppm. Urinary incontinence was also observed at 500 ppm
in females. On the basis of general toxicity, including the adaptive
effects in the liver and kidneys, the NOAEL was 500 ppm, equal to 45
mg/kg bw per day (Horner, 1992).
Dogs
Groups of four male and four female beagle dogs were given
tecnazene (purity, 98.7%) at 0, 2, 15 or 200 mg/kg bw per day for 90
days. Blood samples were taken before treatment and at weeks 4, 8
and 13 and tested for changes in haematological parameters and
clinical chemistry. Food consumption and clinical observations were
recorded daily, body weight was recorded weekly, and ophthalmoscopic
examinations were done before treatment and before sacrifice. All
dogs were examined by gross pathology and histopathology. Body
weight was reduced in animals of each sex at 200 mg/kg bw per day,
but food consumption was unaffected by treatment. The liver was
identified as the target organ, since evidence for an adaptive
response was seen in all treated groups in the form of a
dose-related increase in liver aminopyrine- N-demethylase activity.
At 200 mg/kg bw per day, there was a more marked response, including
increased alkaline phosphatase activity, increased liver weight (>
60%) and histopathological changes typical of increased
proliferation of the smooth endoplasmic reticulum. Altered liver
function at this dose was indicated by changes in most clinical
chemical parameters and small changes in some haematological
parameters, including increased clotting times, platelet counts and
mean cell volumes. Minimal alterations in liver weight (19%) and
clinical chemical parameters were also seen in females receiving 15
mg/kg bw per day. A slight increase in liver weight in males at 15
mg/kg bw per day was due mainly to the response in one dog. Changes
in organ weights of animals at 200 mg/kg bw per day were not
associated with histopathological abnormalities induced by
tecnazene, although there was evidence of delayed sexual maturation
in males that was consistent with the reduced growth of these
animals. On the basis of body-weight reduction and the adaptive
effect on the liver, the NOAEL in this study was 15 mg/kg bw per day
(Hodge, 1992).
Two male and two female beagle dogs were given capsules
containing tecnazene at concentrations of 0, 3.8, 15, 60 or 240
mg/kg bw per day, six days per week for two years. All of the dogs
receiving the highest dose died during the first year of the study,
and microscopic changes were noted in the liver, kidney and bone
marrow. At 60 mg/kg bw per day, growth was normal, but serum
alkaline phosphatase activity was increased. No effects were seen on
haematological, urological or electrocardiographic parameters. The
NOAEL was 15 mg/kg bw per day for animals of each sex (summarized in
Annex I, reference 23).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 65 male and 65 female CD-1 mice were fed 0, 750 or
1500 ppm tecnazene (purity, > 99.0%) in the diet for 80 weeks,
equal to 0, 78 or 155 mg/kg bw per day in males and 0, 86 or 174
mg/kg bw per day in females. The mice were observed daily for
clinical signs. Those that died during the first 13 weeks of the
study were submitted to a macroscopic necropsy and replaced by
reserve animals on the same treatment. Body weights were recorded
weekly during the first 12 weeks and every two weeks thereafter.
Food consumption was assessed weekly, and water intake was assessed
daily by visual inspection. At the end of study, all mice were
examined by gross pathology and histopathology. A total of 237 mice
(131 males and 106 females) died or were killed in extremis, but
the group distribution, time and cause of death were not related to
treatment. Body weights and food and water intakes of treated mice
were similar to those of the untreated controls throughout the
study. The pattern of non-neoplastic pathological effects was
unchanged by treatment, and treatment had no effect on tumour type
or incidence. The NOAEL was 1500 ppm, equal to 155 mg/kg bw per day
(Ben-Dyke et al., 1978a).
Rats
Groups of 65 male and 65 female CD rats were fed tecnazene
(purity, > 99.0%) in the diet at 0, 750 or 1500 ppm for 104 weeks,
equal to 0, 27 or 56 mg/kg bw per day in males and 0, 32 or 63 mg/kg
bw per day in females. Animals were observed daily for clinical
signs. Those that died during the first 13 weeks of the study were
submitted to macroscopic necropsy and replaced by reserve animals on
the same treatment. Body weights were recorded weekly during the
first 12 weeks and every two weeks thereafter. Food consumption was
assessed weekly, and water intake was assessed daily by visual
inspection. At the end of study, all mice were examined by gross
pathology and histopathology. The few signs seen during treatment
were considered not to be of biological significance. Mortality
rates, body weights, food intakes and efficiency of food use were
not affected by treatment. The range of macroscopic changes seen at
necropsy were those commonly seen in CD rats. There were some
differences between groups in the incidences of pituitary adenomas
and mammary gland tumours, but these were considered not to be
related to treatment. The incidences of other tumour types were
similar in treated and control groups. The NOAEL was 1500 ppm, equal
to 56 mg/kg bw per day (Ben-Dyke et al., 1978b).
(d) Reproductive toxicity
Rats
Groups of 30 male and 30 female Alpk:APfSD (Wistar-derived)
rats, four to five weeks old, were fed diets containing tecnazene
(purity, 98.7%) at 0, 300, 1000 or 5000 ppm, equal to 32, 106 or 571
mg/kg bw per day in males and 34, 113 or 576 mg/kg bw per day in
females. After 12 weeks, the animals were mated and allowed to rear
the ensuing F1a litters to weaning. During the pre-weaning phase,
it became evident that the F1a pups could not tolerate the
5000-ppm dose, and they and their dams were given control diet from
days 17-20 for the remainder of the lactation period. The F1a
offspring were considered to be unsuitable to form the next parental
generation, so an F1b litter was produced after the parental dose
had been reduced to 3000 ppm. The F1b offspring and dams at the
highest dose were also given control diet during the pre-weaning
phase because of poor weight gain of the pups (from days 21-25, and
earlier for a few litters born later); however, the F1 parents
were selected from among the F1b offspring. The highest dietary
level was further reduced to 2000 ppm, and these animals were
allowed to produce the F2a litter after a 12-week pre-mating
period. Diets containing 0, 300, 1000 or 2000 ppm tecnazene, equal
to 31, 103 or 220 mg/kg bw per day in males and 34, 111 or 235 mg/kg
bw per day in females, were then fed continuously throughout the
remainder of the study.
Dietary administration of 5000 ppm tecnazene was associated
with reduced body-weight gain and food consumption during the
pre-mating period, but 300 or 1000 ppm had no adverse effects. A
dose-related incidence of signs of urinary incontinence (wetness or
staining around the urogenital area) was seen in treated animals,
but the significance of this finding was uncertain. A clear,
dose-related effect on the offspring was associated with
administration of 3000 or 5000 ppm tecnazene. At the highest dietary
level, pup survival, clinical condition and growth were adversely
affected; only pup growth was affected by 3000 ppm. Doses of 2000
ppm or less had no adverse effect on pup growth or survival.
Treatment induced a dose-related increase in kidney weight in males
of both generations, but no corresponding histological change was
observed. Increased liver weight was seen in both parents and
offspring at all dietary levels of tecnazene; histological changes
indicative of an adaptive response were observed in adults receiving
1000 ppm or more but not in those receiving 300 ppm or in any of the
offspring. The increased organ weights were considered to reflect an
adaptive response and, in the absence of histological change, were
considered to be of no toxicological significance. Tecnazene did not
affect reproductive performance in either generation. The NOAEL for
maternal toxicity was 1000 ppm, equal to 103 mg/kg bw per day. The
NOAEL for filial toxicity and reproductive toxicity was 2000 ppm,
equal to 220 mg/kg bw per day (Moxon, 1992).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant Alpk:APfSD (Wistar-derived) rats, 12
weeks of age, were treated by gavage with tecnazene (purity, 98.7%)
at 0, 15, 50 or 150 mg/kg bw per day on days 7-16 of gestation. The
females were observed daily for clinical signs of toxicity and were
killed on day 22 of gestation. They were examined by gross
pathology, and their uteri were examined for live fetuses and
intra-uterine deaths. The fetuses were weighed, examined for
external visceral abnormalities, sexed, eviscerated and stained for
skeletal examination, and the number of implantations was
determined. Administration of 150 mg/kg bw per day resulted in a
statistically significant reduction in body-weight gain of dams,
particularly on days 7-10; no maternal toxicity was apparent at
lower doses. Overall, there was an increased number of fetuses with
minor skeletal defects (unossified centra of the third and fourth
cervical vertebrae) and an increased incidence of some skeletal
variants (partially ossified transverse processes of the seventh
cervical vertebrae and of the fourth lumbar vertebrae) at 150 mg/kg
bw per day. There was no evidence of embryo- or fetotoxicity or
teratogenicity at any dose. The NOAEL for maternal toxicity and for
embryo- and fetotoxicity was 50 mg/kg bw per day, on the basis of
effects on body weight and minor skeletal defects, respectively
(Whiles, 1991).
Rabbits
Tecnazene (purity, 98.7%) was administered in corn oil by
gavage to groups of 20 pregnant New Zealand white rabbits at 0, 15,
45 or 135 mg/kg bw per day on days 7-19 of gestation. The animals
were killed on day 30 of gestation and their uteri examined for live
fetuses and intra-uterine deaths. The fetuses were weighed, examined
for external and visceral abnormalities, sexed, eviscerated and
processed for skeletal examination. Two animals given 135 mg/kg bw
per day were killed when moribund on days 20 and 27 of gestation;
the death that occurred on day 20 was considered to be related to
treatment, as it was associated with adverse clinical signs, poor
food consumption and body-weight loss. There was no evidence of
overt toxicity in the remaining animals in this group or in animals
given 15 or 45 mg/kg bw per day, and there was no treatment-related
effect on the litters. A total of 27 major defects were seen in 18
fetuses from 14 litters. Of these defects, 13 occurred in controls,
and treatment did not appear to have affected the overall incidence
of major defects. Four minor skeletal defects or variants were noted
at 45 or 135 mg/kg bw per day, which were considered to represent a
minimal effect of tecnazene on fetal development. These defects
included slightly increased manus scores at 45 and 135 mg/kg bw per
day, marginally increased incidences of 27 pre-sacral vertebrae and
extra thirteenth ribs and a slightly increased incidence of
misshapen hyoid at 135 mg/kg bw per day. The NOAEL for maternal
toxicity was 45 mg/kg bw per day on the basis of adverse clinical
signs and body-weight loss, and the NOAEL for embryo- and
fetotoxicity was 15 mg/kg bw per day, on the basis of minor skeletal
defects (Hopkins, 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tecnazene are
summarized in Table 1.
Table 1. Results of tests for the genotoxicity of tecnazene
End-point Test system Concentration Purity Results Reference
of tecnazene (%)
In vitro
Reverse S. typhimurium TA98, 0.32-1000 µg/plate NR Negativea Cattanach & Riach, 1989
mutation 100, 1535, 1538
Forward Mouse lymphoma 1-50 µg/ml 97.1 Negativeb Adams et al., 1989
mutation cells (L5178Y) Positivec
at tk locus
Chromosomal Human 20 µg/ml 97.1 Negativea Brooker et al., 1989
aberration lymphocytes 80 µg/ml Negativec
80 µg/ml Positiveb
160 µg/ml Positivea
In vivo
Micronucleus C57Bl/6 1160-1860 mg/kg bw 97.1 Negative Randall & Howard, 1989
formation JfCD 1/Alpk
mice
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
3. Observations in humans
No information was available.
Comments
After oral administration of radiolabelled tecnazene to rats,
about 90% of the dose was recovered within 48 h. Excretion was
divided almost equally between urine and faeces in males but
occurred predominantly (80%) via the urine in females. The remainder
of the dose was excreted more slowly, and radioactivity was still
detectable in urine and faeces seven days after dosing. Negligible
levels of radioactivity were detected in expired carbon dioxide.
Whole-body autoradiography showed that 24 h after dosing the highest
concentrations of radioactivity were located in the intestinal
contents of animals of each sex; the highest tissue concentrations
were found in the kidney, liver and the nasal passages.
Tecnazene is extensively metabolized in rats. A total of 42
metabolites have been separated from urine and bile, of which 16,
including the parent compound, have been identified. The principal
route of metabolism is via the tetrachlorobenzene glutathione
conjugate pathway. Metabolites present in urine and faeces include
the tetrachloro-phenyl-mercapturate conjugate, tetrachloroaniline
and tetrachlorothioanisole. The pattern of metabolites in urine and
faeces is the same in male and female rats, but the quantities
differ.
In female rabbits, about 70% of an oral dose of tecnazene was
recovered in the faeces within three days. The remainder was
excreted in urine, primarily as glucuronide, sulfate and mercapturic
acid conjugates of 2,3,5,6-tetrachloroaniline. Some unconjugated
4-amino-2,3,5,6-tetrachlorophenol was also excreted.
Tecnazene has low oral toxicity in rats. WHO (1992) has
classified tecnazene as unlikely to present an acute hazard in
normal use.
In a 90-day study in rats fed dietary concentrations of 0, 50,
500 or 5000 ppm, the NOAEL was 500 ppm, equal to 45 mg/kg bw per
day, on the basis of effects on body-weight gain and on the liver
and kidneys.
In a 90-day study in dogs given 0, 2, 15 or 200 mg/kg bw per
day orally, the NOAEL was 15 mg/kg bw per day on the basis of
effects on body and liver weights.
In a two-year study in which dogs were given tecnazene at 0,
3.8, 15, 60 or 240 mg/kg bw per day orally, the NOAEL was 15 mg/kg
bw per day, on the basis of elevation of serum alkaline phosphatase
activity. Owing to the small number of animals and lack of access to
the original report, data from this study could not be considered in
establishing an ADI.
In an 80-week study of carcinogenicity in mice fed dietary
concentrations of 0, 750 or 1500 ppm, the NOAEL was 1500 ppm, equal
to 155 mg/kg bw per day. There was no evidence of carcinogenicity
and no effect on body weight, mortality or clinical signs.
In a 104-week study of carcinogenicity in rats fed 0, 750 or
1500 ppm, the NOAEL was also 1500 ppm, equal to 56 mg/kg bw per day.
There was no evidence of carcinogenicity. As no clinical chemical or
haematological parameters were evaluated in this study, the Meeting
considered it inadequate for an evaluation of long-term toxicity.
In a two-generation study of reproductive toxicity in rats fed
dietary concentrations of 0, 300, 1000 or 5000/2000 ppm, the NOAEL
for parental toxicity was 1000 ppm, equal to 106 mg/kg bw per day.
The NOAEL for filial toxicity was 2000 ppm, equal to 220 mg/kg bw
per day. There were no adverse effects on reproduction.
In a study of teratogenicity in rats administered 0, 15, 50 or
150 mg/kg bw per day by gavage, the NOAEL was 50 mg/kg bw per day
for both maternal toxicity and embryo- and fetotoxicity on the basis
of reduced body-weight gain and minor skeletal defects,
respectively. No teratogenic effects were observed.
In a study of teratogenicity in rabbits administered 0, 15, 45
or 135 mg/kg bw per day by gavage, the NOAEL was 45 mg/kg bw per day
for maternal toxicity on the basis of body-weight loss and reduced
food consumption. The NOAEL was 15 mg/kg bw per day for embryo- and
fetotoxicity on the basis of minor skeletal defects. No teratogenic
effects were observed.
Tecnazene can produce clastogenic effects in vitro but not
in vivo. No mutagenicity was observed in bacteria. The Meeting
concluded that tecnazene is not genotoxic.
An ADI was established on the basis of an NOAEL of 15 mg/kg bw
per day for toxicity in the 90-day study in dogs and for embryo- and
fetotoxicity in the study of teratogenicity in rabbits. Owing to the
lack of adequate data on the long-term toxicity of the compound, the
Meeting applied a 1000-fold safety factor to the NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1500 ppm, equal to 155 mg/kg bw per day (80-week
study of carcinogenicity)
Rat: 500 ppm, equal to 45 mg/kg bw per day (90-day study
of toxicity)
1500 ppm, equal to 56 mg/kg bw per day (104-week
study of carcinogenicity)
Rabbit: 15 mg/kg bw per day (embryo- and fetotoxicity in a
study of teratogenicity)
Dog: 15 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. One-year study of toxicity in dogs
2. Long-term study of toxicity in rats
References
Adams, K., Ransome, S.J., Henly, S.M., Kirkpatrick, D., Skinner, N.
& Bottoms, M.A (1989) An assessement of the mutagenic potential of
tecnazene using the mouse lymphoma TK locus assay. Unpublished
report No. ISN 206/89508 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Barber, J.E. (1985) Tecnazene: acute oral toxicity study.
Unpublished report No. CTL/P/1232 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978a) Tecnazene: oncogenic response in mice to continuous
dietary administration for 80 weeks. Unpublished report No. 78/ILY
19/080 from Life Science Research, Stock, United Kingdom. Submitted
to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United
Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978b) Tecnazene: carcinogenic response in rats to dietary
administration for 104 weeks. Unpublished report No. 78/ILY 20/078
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Bentley, M. & Powles, P. (1992) Tecnazene--preliminary examination
of urinary and faecal metabolites in the rat. Unpublished report No.
7119-72/305 from Hazleton United Kingdom, Harrogate, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and the
formation of mercapturic acids in the rabbit. Biochem. J., 61,
611-617.
Bratt, H. (1991) Tecnazene: Excretion and tissue retention of a
single oral dose (1 mg/kg) in the rat. Unpublished report No.
CTL/P/3202 from ICI Central Toxicology Laboratory. Submitted to WHO
by Zeneca Agrochemicals, Fernshurst, Haslemere, United Kingdom.
Bray, H.G., Hybs, Z., James, S.P. & Thorpe, W.V. (1953) The
metabolism of 2:3:5:6- and 2:3:4:5-tetrachloronitrobenzenes in the
rabbit and the reduction of aromatic nitro compounds in the
intestine. Biochem. J., 53, 266-273.
Bray, H.G., Franklin, T.J. & James, S.P. (1959) The formation of
mercapturic acids. 3. N-Acetylation of S-substituted cysteines in
the rabbit, rat and guinea pig. Biochem. J., 73, 465-473.
Brooker, P.C., Akhurst, L.C. & King, J.D. (1989) Tecnazene:
metaphase chromosome analysis of human lymphocytes cultured in
vitro. Unpublished report No. ISN 207/89289 from Huntingdon
Research Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by
Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Buttle, G.A.H. & Dyer, F.J. (1950) Experiments on the toxicology of
2,3,5,6-tetrachloronitrobenzene. J. Pharm. Pharmacol., 2, 371-375.
Cattanach, P.J. & Riach, C.G. (1989) Tecnazene: testing for
mutagenic activity with Salmonella typhimurium TA 1535, TA 1537, TA
1538, TA 98 and TA 100. Unpublished report No. 4872 from Inveresk
Research International, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Donikian, M., Owen, S.D., Wiland, J. & Drobeck, H.P. (1965) Oral
administration of 2,3,5,6-tetrachloronitrobenzene to beagle dogs for
two years. Unpublished report from Sterling Winthrop Research
Institute (1974 Evaluations of some pesticide residues in food, WHO
1975).
Hodge, M.C.E. (1992) Tecnazene: 90 day oral dosing study in dogs.
Unpublished report No. CTL/P/3614 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Hopkins, M.N. (1991) Tecnazene: Teratogenicity study in the rabbit.
Unpublished report No. CTL/P/3184 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Horner, J.M. (1992) Tecnazene: 90-day feeding study in rats.
Unpublished report No. CTL/P/3648 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Lappin, G.J. & Pritchard, D.J. (1992) Tecnazene: Biotransformation
in the rat. Unpublished report No. CTL/P/3731 from ICI Central
Toxicology Laboratory, Macclesfield, United Kingdom. Submitted to
WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Moxon, M.E. (1992) Tecnazene: multigeneration study in the rat.
Unpublished report No. CTL/P/3596 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Randall, V. & Howard, C.A. (1989) Tecnazene: an evaluation in the
mouse micronucleus test. Unpublished report No. CTL/P/2632 from ICI
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Renner, G. (1980) Metabolic studies on pentachloronitrobenzene
(PCNB) in rats. Xenobiotica, 10, 537-550.
Whiles, A.J. (1991) Tecnazene: Teratogenicity study in the rat.
Unpublished report No. CTL/P/3193 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
 Other polychlorinated nitrobenzenes, such as
2,3,4,6-tetrachloronitrobenzene and pentachloro-nitrobenzene, also
form mercapturic acids in vivo. Pentachlorophenyl mercapturic acid
was the major metabolite of pentachloronitrobenzene in the urine of
rats (Renner, 1980) and rabbits (Betts et al., 1955).
2. Toxicological studies
Aromatic nitro compounds are of considerable importance not
only as pesticides, e.g. tecnazene and quintozene
(pentachloronitrobenzene), but also as solvents and synthetic
intermediates for many technical products. Nitrobenzene is toxic and
is readily absorbed through the skin. In cases of acute poisoning,
haematological changes occur, such as decreased haemoglobin level
and methaemoglobinaemia; and symptoms of intoxication, such as
vomiting, colic, headaches, breathlessness and cyanosis, are seen.
Additional effects on the central nervous system result in anxiety
and convulsions. A variety of nitrophenols affect the release of
acetylcholine at nerve endings in the small intestine and certain
muscles. Individual nitro aromatic compounds can cause specific
symptoms of poisoning in addition to the general ones described
above. Chronic poisoning with nitroaromatic compounds results in
cirrhosis or acute atrophy of the liver. Allergic reactions are
caused by 1-chloro-2,4-dinitrobenzene, probably by an
antigen-antibody reaction which results after the dinitrophenyl
group is bound covalently to natural proteins. In early studies,
pentachloronitrobenzene caused liver abnormalities, such as
single-cell necrosis, fatty metamorphosis of hepatocytes and
enlarged centrilobular hepatocytes.
(a) Acute toxicity
Two early studies in which rats were given an aqueous
suspension of tecnazene orally or intraperitoneally indicated little
acute toxicity, the LD50 values being 7500 and 3500 mg/kg bw,
respectively (Annex I, references 23 and 31). The LD50 values for
tecnazene (purity, 96%) administered orally to rats in corn oil were
2000 mg/kg bw in males and 1300 mg/kg bw in females (Barber, 1985).
(b) Short-term toxicity
Mice
Groups of 12 mice were fed tecnazene in the diet at levels of
0, 1344 or 13 440 ppm, equivalent to 0, 202 or 2016 mg/kg bw per
day, for 31 days, after which they were killed. Body weights were
measured at the start and end of the study, and food consumption was
determined daily. Mice receiving the high dose did not gain body
weight during the 31-day period. The NOAEL was 1344 ppm, equivalent
to 202 mg/kg bw per day (Buttle & Dyer, 1950).
A group of 24 mice were fed tecnazene in the diet at a dose of
10 000 mg/kg bw per day. When deaths occurred within three to four
days, the study was discontinued. Animals were found to have fatty
degeneration of the liver and fatty changes in the spleen and kidney
(Buttle & Dyer, 1950).
Rats
Groups of five rats were fed tecnazene in the diet at doses of
0, 800, 4000 or 20 000 ppm, equivalent to 0, 40, 200 or 1000 mg/kg
bw per day, for 10 weeks. Deaths occurred at the highest dose, and
growth was reduced at 4000 ppm. The NOAEL was 800 ppm, equivalent to
40 mg/kg bw per day (Buttle & Dyer, 1950).
Groups of 12 male and 12 female randomly selected Alpk:APfSD
rats weighing 120-124 g (males) and 111-116 g (females) were fed
diets containing tecnazene (purity, 98.7%) at concentrations of 0,
50, 500 or 5000 ppm, equal to 0, 4.5, 45 or 500 mg/kg bw per day in
males and 0, 4.9, 49 or 500 mg/kg bw per day in females, for 90
days. All animals were observed daily for mortality and signs of
toxicity, and body weight and food consumption were recorded weekly.
Animals in the control and high-dose groups underwent
ophthalmoscopic examinations during the week before sacrifice. After
sacrifice, haematology, clinical chemistry, gross pathology and
histopathology were undertaken on all rats. Administration of 5000
ppm tecnazene was associated with reduced body-weight gain
throughout the study and with an associated reduction in food
consumption and efficiency of food use during week 1. The marked
effect on body weight was reflected in reduced plasma triglyceride
levels, plasma alanine transaminase and creatine kinase activities
and erythrocyte parameters and changes in the levels of total plasma
protein and urea. The liver was identified as a target organ:
Increased liver weight and hepatic aminopyrine-N-demethylase
activity were seen at 500 and 5000 ppm, with histological evidence
of hepatic hypertrophy at 5000 ppm, indicating an adaptive change.
Increased plasma alkaline phosphatase activity and cholesterol
levels were noted at 5000 ppm, and to a lesser extent for males at
500 ppm. In the absence of adverse histopathological findings,
however, these changes were considered not to be of toxicological
significance. The kidney was also identified as a target organ.
Increased kidney weight was seen in males and females given 5000
ppm, which, in the absence of histopathological change, reflects a
possible adaptive response. Mild renal functional changes, indicated
by signs of urinary incontinence (wetness or staining around the
urogenital area), decreased specific gravity and cloudy urine, were
noted at 5000 ppm. Urinary incontinence was also observed at 500 ppm
in females. On the basis of general toxicity, including the adaptive
effects in the liver and kidneys, the NOAEL was 500 ppm, equal to 45
mg/kg bw per day (Horner, 1992).
Dogs
Groups of four male and four female beagle dogs were given
tecnazene (purity, 98.7%) at 0, 2, 15 or 200 mg/kg bw per day for 90
days. Blood samples were taken before treatment and at weeks 4, 8
and 13 and tested for changes in haematological parameters and
clinical chemistry. Food consumption and clinical observations were
recorded daily, body weight was recorded weekly, and ophthalmoscopic
examinations were done before treatment and before sacrifice. All
dogs were examined by gross pathology and histopathology. Body
weight was reduced in animals of each sex at 200 mg/kg bw per day,
but food consumption was unaffected by treatment. The liver was
identified as the target organ, since evidence for an adaptive
response was seen in all treated groups in the form of a
dose-related increase in liver aminopyrine- N-demethylase activity.
At 200 mg/kg bw per day, there was a more marked response, including
increased alkaline phosphatase activity, increased liver weight (>
60%) and histopathological changes typical of increased
proliferation of the smooth endoplasmic reticulum. Altered liver
function at this dose was indicated by changes in most clinical
chemical parameters and small changes in some haematological
parameters, including increased clotting times, platelet counts and
mean cell volumes. Minimal alterations in liver weight (19%) and
clinical chemical parameters were also seen in females receiving 15
mg/kg bw per day. A slight increase in liver weight in males at 15
mg/kg bw per day was due mainly to the response in one dog. Changes
in organ weights of animals at 200 mg/kg bw per day were not
associated with histopathological abnormalities induced by
tecnazene, although there was evidence of delayed sexual maturation
in males that was consistent with the reduced growth of these
animals. On the basis of body-weight reduction and the adaptive
effect on the liver, the NOAEL in this study was 15 mg/kg bw per day
(Hodge, 1992).
Two male and two female beagle dogs were given capsules
containing tecnazene at concentrations of 0, 3.8, 15, 60 or 240
mg/kg bw per day, six days per week for two years. All of the dogs
receiving the highest dose died during the first year of the study,
and microscopic changes were noted in the liver, kidney and bone
marrow. At 60 mg/kg bw per day, growth was normal, but serum
alkaline phosphatase activity was increased. No effects were seen on
haematological, urological or electrocardiographic parameters. The
NOAEL was 15 mg/kg bw per day for animals of each sex (summarized in
Annex I, reference 23).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 65 male and 65 female CD-1 mice were fed 0, 750 or
1500 ppm tecnazene (purity, > 99.0%) in the diet for 80 weeks,
equal to 0, 78 or 155 mg/kg bw per day in males and 0, 86 or 174
mg/kg bw per day in females. The mice were observed daily for
clinical signs. Those that died during the first 13 weeks of the
study were submitted to a macroscopic necropsy and replaced by
reserve animals on the same treatment. Body weights were recorded
weekly during the first 12 weeks and every two weeks thereafter.
Food consumption was assessed weekly, and water intake was assessed
daily by visual inspection. At the end of study, all mice were
examined by gross pathology and histopathology. A total of 237 mice
(131 males and 106 females) died or were killed in extremis, but
the group distribution, time and cause of death were not related to
treatment. Body weights and food and water intakes of treated mice
were similar to those of the untreated controls throughout the
study. The pattern of non-neoplastic pathological effects was
unchanged by treatment, and treatment had no effect on tumour type
or incidence. The NOAEL was 1500 ppm, equal to 155 mg/kg bw per day
(Ben-Dyke et al., 1978a).
Rats
Groups of 65 male and 65 female CD rats were fed tecnazene
(purity, > 99.0%) in the diet at 0, 750 or 1500 ppm for 104 weeks,
equal to 0, 27 or 56 mg/kg bw per day in males and 0, 32 or 63 mg/kg
bw per day in females. Animals were observed daily for clinical
signs. Those that died during the first 13 weeks of the study were
submitted to macroscopic necropsy and replaced by reserve animals on
the same treatment. Body weights were recorded weekly during the
first 12 weeks and every two weeks thereafter. Food consumption was
assessed weekly, and water intake was assessed daily by visual
inspection. At the end of study, all mice were examined by gross
pathology and histopathology. The few signs seen during treatment
were considered not to be of biological significance. Mortality
rates, body weights, food intakes and efficiency of food use were
not affected by treatment. The range of macroscopic changes seen at
necropsy were those commonly seen in CD rats. There were some
differences between groups in the incidences of pituitary adenomas
and mammary gland tumours, but these were considered not to be
related to treatment. The incidences of other tumour types were
similar in treated and control groups. The NOAEL was 1500 ppm, equal
to 56 mg/kg bw per day (Ben-Dyke et al., 1978b).
(d) Reproductive toxicity
Rats
Groups of 30 male and 30 female Alpk:APfSD (Wistar-derived)
rats, four to five weeks old, were fed diets containing tecnazene
(purity, 98.7%) at 0, 300, 1000 or 5000 ppm, equal to 32, 106 or 571
mg/kg bw per day in males and 34, 113 or 576 mg/kg bw per day in
females. After 12 weeks, the animals were mated and allowed to rear
the ensuing F1a litters to weaning. During the pre-weaning phase,
it became evident that the F1a pups could not tolerate the
5000-ppm dose, and they and their dams were given control diet from
days 17-20 for the remainder of the lactation period. The F1a
offspring were considered to be unsuitable to form the next parental
generation, so an F1b litter was produced after the parental dose
had been reduced to 3000 ppm. The F1b offspring and dams at the
highest dose were also given control diet during the pre-weaning
phase because of poor weight gain of the pups (from days 21-25, and
earlier for a few litters born later); however, the F1 parents
were selected from among the F1b offspring. The highest dietary
level was further reduced to 2000 ppm, and these animals were
allowed to produce the F2a litter after a 12-week pre-mating
period. Diets containing 0, 300, 1000 or 2000 ppm tecnazene, equal
to 31, 103 or 220 mg/kg bw per day in males and 34, 111 or 235 mg/kg
bw per day in females, were then fed continuously throughout the
remainder of the study.
Dietary administration of 5000 ppm tecnazene was associated
with reduced body-weight gain and food consumption during the
pre-mating period, but 300 or 1000 ppm had no adverse effects. A
dose-related incidence of signs of urinary incontinence (wetness or
staining around the urogenital area) was seen in treated animals,
but the significance of this finding was uncertain. A clear,
dose-related effect on the offspring was associated with
administration of 3000 or 5000 ppm tecnazene. At the highest dietary
level, pup survival, clinical condition and growth were adversely
affected; only pup growth was affected by 3000 ppm. Doses of 2000
ppm or less had no adverse effect on pup growth or survival.
Treatment induced a dose-related increase in kidney weight in males
of both generations, but no corresponding histological change was
observed. Increased liver weight was seen in both parents and
offspring at all dietary levels of tecnazene; histological changes
indicative of an adaptive response were observed in adults receiving
1000 ppm or more but not in those receiving 300 ppm or in any of the
offspring. The increased organ weights were considered to reflect an
adaptive response and, in the absence of histological change, were
considered to be of no toxicological significance. Tecnazene did not
affect reproductive performance in either generation. The NOAEL for
maternal toxicity was 1000 ppm, equal to 103 mg/kg bw per day. The
NOAEL for filial toxicity and reproductive toxicity was 2000 ppm,
equal to 220 mg/kg bw per day (Moxon, 1992).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant Alpk:APfSD (Wistar-derived) rats, 12
weeks of age, were treated by gavage with tecnazene (purity, 98.7%)
at 0, 15, 50 or 150 mg/kg bw per day on days 7-16 of gestation. The
females were observed daily for clinical signs of toxicity and were
killed on day 22 of gestation. They were examined by gross
pathology, and their uteri were examined for live fetuses and
intra-uterine deaths. The fetuses were weighed, examined for
external visceral abnormalities, sexed, eviscerated and stained for
skeletal examination, and the number of implantations was
determined. Administration of 150 mg/kg bw per day resulted in a
statistically significant reduction in body-weight gain of dams,
particularly on days 7-10; no maternal toxicity was apparent at
lower doses. Overall, there was an increased number of fetuses with
minor skeletal defects (unossified centra of the third and fourth
cervical vertebrae) and an increased incidence of some skeletal
variants (partially ossified transverse processes of the seventh
cervical vertebrae and of the fourth lumbar vertebrae) at 150 mg/kg
bw per day. There was no evidence of embryo- or fetotoxicity or
teratogenicity at any dose. The NOAEL for maternal toxicity and for
embryo- and fetotoxicity was 50 mg/kg bw per day, on the basis of
effects on body weight and minor skeletal defects, respectively
(Whiles, 1991).
Rabbits
Tecnazene (purity, 98.7%) was administered in corn oil by
gavage to groups of 20 pregnant New Zealand white rabbits at 0, 15,
45 or 135 mg/kg bw per day on days 7-19 of gestation. The animals
were killed on day 30 of gestation and their uteri examined for live
fetuses and intra-uterine deaths. The fetuses were weighed, examined
for external and visceral abnormalities, sexed, eviscerated and
processed for skeletal examination. Two animals given 135 mg/kg bw
per day were killed when moribund on days 20 and 27 of gestation;
the death that occurred on day 20 was considered to be related to
treatment, as it was associated with adverse clinical signs, poor
food consumption and body-weight loss. There was no evidence of
overt toxicity in the remaining animals in this group or in animals
given 15 or 45 mg/kg bw per day, and there was no treatment-related
effect on the litters. A total of 27 major defects were seen in 18
fetuses from 14 litters. Of these defects, 13 occurred in controls,
and treatment did not appear to have affected the overall incidence
of major defects. Four minor skeletal defects or variants were noted
at 45 or 135 mg/kg bw per day, which were considered to represent a
minimal effect of tecnazene on fetal development. These defects
included slightly increased manus scores at 45 and 135 mg/kg bw per
day, marginally increased incidences of 27 pre-sacral vertebrae and
extra thirteenth ribs and a slightly increased incidence of
misshapen hyoid at 135 mg/kg bw per day. The NOAEL for maternal
toxicity was 45 mg/kg bw per day on the basis of adverse clinical
signs and body-weight loss, and the NOAEL for embryo- and
fetotoxicity was 15 mg/kg bw per day, on the basis of minor skeletal
defects (Hopkins, 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tecnazene are
summarized in Table 1.
Table 1. Results of tests for the genotoxicity of tecnazene
End-point Test system Concentration Purity Results Reference
of tecnazene (%)
In vitro
Reverse S. typhimurium TA98, 0.32-1000 µg/plate NR Negativea Cattanach & Riach, 1989
mutation 100, 1535, 1538
Forward Mouse lymphoma 1-50 µg/ml 97.1 Negativeb Adams et al., 1989
mutation cells (L5178Y) Positivec
at tk locus
Chromosomal Human 20 µg/ml 97.1 Negativea Brooker et al., 1989
aberration lymphocytes 80 µg/ml Negativec
80 µg/ml Positiveb
160 µg/ml Positivea
In vivo
Micronucleus C57Bl/6 1160-1860 mg/kg bw 97.1 Negative Randall & Howard, 1989
formation JfCD 1/Alpk
mice
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
3. Observations in humans
No information was available.
Comments
After oral administration of radiolabelled tecnazene to rats,
about 90% of the dose was recovered within 48 h. Excretion was
divided almost equally between urine and faeces in males but
occurred predominantly (80%) via the urine in females. The remainder
of the dose was excreted more slowly, and radioactivity was still
detectable in urine and faeces seven days after dosing. Negligible
levels of radioactivity were detected in expired carbon dioxide.
Whole-body autoradiography showed that 24 h after dosing the highest
concentrations of radioactivity were located in the intestinal
contents of animals of each sex; the highest tissue concentrations
were found in the kidney, liver and the nasal passages.
Tecnazene is extensively metabolized in rats. A total of 42
metabolites have been separated from urine and bile, of which 16,
including the parent compound, have been identified. The principal
route of metabolism is via the tetrachlorobenzene glutathione
conjugate pathway. Metabolites present in urine and faeces include
the tetrachloro-phenyl-mercapturate conjugate, tetrachloroaniline
and tetrachlorothioanisole. The pattern of metabolites in urine and
faeces is the same in male and female rats, but the quantities
differ.
In female rabbits, about 70% of an oral dose of tecnazene was
recovered in the faeces within three days. The remainder was
excreted in urine, primarily as glucuronide, sulfate and mercapturic
acid conjugates of 2,3,5,6-tetrachloroaniline. Some unconjugated
4-amino-2,3,5,6-tetrachlorophenol was also excreted.
Tecnazene has low oral toxicity in rats. WHO (1992) has
classified tecnazene as unlikely to present an acute hazard in
normal use.
In a 90-day study in rats fed dietary concentrations of 0, 50,
500 or 5000 ppm, the NOAEL was 500 ppm, equal to 45 mg/kg bw per
day, on the basis of effects on body-weight gain and on the liver
and kidneys.
In a 90-day study in dogs given 0, 2, 15 or 200 mg/kg bw per
day orally, the NOAEL was 15 mg/kg bw per day on the basis of
effects on body and liver weights.
In a two-year study in which dogs were given tecnazene at 0,
3.8, 15, 60 or 240 mg/kg bw per day orally, the NOAEL was 15 mg/kg
bw per day, on the basis of elevation of serum alkaline phosphatase
activity. Owing to the small number of animals and lack of access to
the original report, data from this study could not be considered in
establishing an ADI.
In an 80-week study of carcinogenicity in mice fed dietary
concentrations of 0, 750 or 1500 ppm, the NOAEL was 1500 ppm, equal
to 155 mg/kg bw per day. There was no evidence of carcinogenicity
and no effect on body weight, mortality or clinical signs.
In a 104-week study of carcinogenicity in rats fed 0, 750 or
1500 ppm, the NOAEL was also 1500 ppm, equal to 56 mg/kg bw per day.
There was no evidence of carcinogenicity. As no clinical chemical or
haematological parameters were evaluated in this study, the Meeting
considered it inadequate for an evaluation of long-term toxicity.
In a two-generation study of reproductive toxicity in rats fed
dietary concentrations of 0, 300, 1000 or 5000/2000 ppm, the NOAEL
for parental toxicity was 1000 ppm, equal to 106 mg/kg bw per day.
The NOAEL for filial toxicity was 2000 ppm, equal to 220 mg/kg bw
per day. There were no adverse effects on reproduction.
In a study of teratogenicity in rats administered 0, 15, 50 or
150 mg/kg bw per day by gavage, the NOAEL was 50 mg/kg bw per day
for both maternal toxicity and embryo- and fetotoxicity on the basis
of reduced body-weight gain and minor skeletal defects,
respectively. No teratogenic effects were observed.
In a study of teratogenicity in rabbits administered 0, 15, 45
or 135 mg/kg bw per day by gavage, the NOAEL was 45 mg/kg bw per day
for maternal toxicity on the basis of body-weight loss and reduced
food consumption. The NOAEL was 15 mg/kg bw per day for embryo- and
fetotoxicity on the basis of minor skeletal defects. No teratogenic
effects were observed.
Tecnazene can produce clastogenic effects in vitro but not
in vivo. No mutagenicity was observed in bacteria. The Meeting
concluded that tecnazene is not genotoxic.
An ADI was established on the basis of an NOAEL of 15 mg/kg bw
per day for toxicity in the 90-day study in dogs and for embryo- and
fetotoxicity in the study of teratogenicity in rabbits. Owing to the
lack of adequate data on the long-term toxicity of the compound, the
Meeting applied a 1000-fold safety factor to the NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1500 ppm, equal to 155 mg/kg bw per day (80-week
study of carcinogenicity)
Rat: 500 ppm, equal to 45 mg/kg bw per day (90-day study
of toxicity)
1500 ppm, equal to 56 mg/kg bw per day (104-week
study of carcinogenicity)
Rabbit: 15 mg/kg bw per day (embryo- and fetotoxicity in a
study of teratogenicity)
Dog: 15 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. One-year study of toxicity in dogs
2. Long-term study of toxicity in rats
References
Adams, K., Ransome, S.J., Henly, S.M., Kirkpatrick, D., Skinner, N.
& Bottoms, M.A (1989) An assessement of the mutagenic potential of
tecnazene using the mouse lymphoma TK locus assay. Unpublished
report No. ISN 206/89508 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Barber, J.E. (1985) Tecnazene: acute oral toxicity study.
Unpublished report No. CTL/P/1232 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978a) Tecnazene: oncogenic response in mice to continuous
dietary administration for 80 weeks. Unpublished report No. 78/ILY
19/080 from Life Science Research, Stock, United Kingdom. Submitted
to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United
Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978b) Tecnazene: carcinogenic response in rats to dietary
administration for 104 weeks. Unpublished report No. 78/ILY 20/078
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Bentley, M. & Powles, P. (1992) Tecnazene--preliminary examination
of urinary and faecal metabolites in the rat. Unpublished report No.
7119-72/305 from Hazleton United Kingdom, Harrogate, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and the
formation of mercapturic acids in the rabbit. Biochem. J., 61,
611-617.
Bratt, H. (1991) Tecnazene: Excretion and tissue retention of a
single oral dose (1 mg/kg) in the rat. Unpublished report No.
CTL/P/3202 from ICI Central Toxicology Laboratory. Submitted to WHO
by Zeneca Agrochemicals, Fernshurst, Haslemere, United Kingdom.
Bray, H.G., Hybs, Z., James, S.P. & Thorpe, W.V. (1953) The
metabolism of 2:3:5:6- and 2:3:4:5-tetrachloronitrobenzenes in the
rabbit and the reduction of aromatic nitro compounds in the
intestine. Biochem. J., 53, 266-273.
Bray, H.G., Franklin, T.J. & James, S.P. (1959) The formation of
mercapturic acids. 3. N-Acetylation of S-substituted cysteines in
the rabbit, rat and guinea pig. Biochem. J., 73, 465-473.
Brooker, P.C., Akhurst, L.C. & King, J.D. (1989) Tecnazene:
metaphase chromosome analysis of human lymphocytes cultured in
vitro. Unpublished report No. ISN 207/89289 from Huntingdon
Research Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by
Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Buttle, G.A.H. & Dyer, F.J. (1950) Experiments on the toxicology of
2,3,5,6-tetrachloronitrobenzene. J. Pharm. Pharmacol., 2, 371-375.
Cattanach, P.J. & Riach, C.G. (1989) Tecnazene: testing for
mutagenic activity with Salmonella typhimurium TA 1535, TA 1537, TA
1538, TA 98 and TA 100. Unpublished report No. 4872 from Inveresk
Research International, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Donikian, M., Owen, S.D., Wiland, J. & Drobeck, H.P. (1965) Oral
administration of 2,3,5,6-tetrachloronitrobenzene to beagle dogs for
two years. Unpublished report from Sterling Winthrop Research
Institute (1974 Evaluations of some pesticide residues in food, WHO
1975).
Hodge, M.C.E. (1992) Tecnazene: 90 day oral dosing study in dogs.
Unpublished report No. CTL/P/3614 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Hopkins, M.N. (1991) Tecnazene: Teratogenicity study in the rabbit.
Unpublished report No. CTL/P/3184 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Horner, J.M. (1992) Tecnazene: 90-day feeding study in rats.
Unpublished report No. CTL/P/3648 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Lappin, G.J. & Pritchard, D.J. (1992) Tecnazene: Biotransformation
in the rat. Unpublished report No. CTL/P/3731 from ICI Central
Toxicology Laboratory, Macclesfield, United Kingdom. Submitted to
WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Moxon, M.E. (1992) Tecnazene: multigeneration study in the rat.
Unpublished report No. CTL/P/3596 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Randall, V. & Howard, C.A. (1989) Tecnazene: an evaluation in the
mouse micronucleus test. Unpublished report No. CTL/P/2632 from ICI
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Renner, G. (1980) Metabolic studies on pentachloronitrobenzene
(PCNB) in rats. Xenobiotica, 10, 537-550.
Whiles, A.J. (1991) Tecnazene: Teratogenicity study in the rat.
Unpublished report No. CTL/P/3193 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Other polychlorinated nitrobenzenes, such as
2,3,4,6-tetrachloronitrobenzene and pentachloro-nitrobenzene, also
form mercapturic acids in vivo. Pentachlorophenyl mercapturic acid
was the major metabolite of pentachloronitrobenzene in the urine of
rats (Renner, 1980) and rabbits (Betts et al., 1955).
2. Toxicological studies
Aromatic nitro compounds are of considerable importance not
only as pesticides, e.g. tecnazene and quintozene
(pentachloronitrobenzene), but also as solvents and synthetic
intermediates for many technical products. Nitrobenzene is toxic and
is readily absorbed through the skin. In cases of acute poisoning,
haematological changes occur, such as decreased haemoglobin level
and methaemoglobinaemia; and symptoms of intoxication, such as
vomiting, colic, headaches, breathlessness and cyanosis, are seen.
Additional effects on the central nervous system result in anxiety
and convulsions. A variety of nitrophenols affect the release of
acetylcholine at nerve endings in the small intestine and certain
muscles. Individual nitro aromatic compounds can cause specific
symptoms of poisoning in addition to the general ones described
above. Chronic poisoning with nitroaromatic compounds results in
cirrhosis or acute atrophy of the liver. Allergic reactions are
caused by 1-chloro-2,4-dinitrobenzene, probably by an
antigen-antibody reaction which results after the dinitrophenyl
group is bound covalently to natural proteins. In early studies,
pentachloronitrobenzene caused liver abnormalities, such as
single-cell necrosis, fatty metamorphosis of hepatocytes and
enlarged centrilobular hepatocytes.
(a) Acute toxicity
Two early studies in which rats were given an aqueous
suspension of tecnazene orally or intraperitoneally indicated little
acute toxicity, the LD50 values being 7500 and 3500 mg/kg bw,
respectively (Annex I, references 23 and 31). The LD50 values for
tecnazene (purity, 96%) administered orally to rats in corn oil were
2000 mg/kg bw in males and 1300 mg/kg bw in females (Barber, 1985).
(b) Short-term toxicity
Mice
Groups of 12 mice were fed tecnazene in the diet at levels of
0, 1344 or 13 440 ppm, equivalent to 0, 202 or 2016 mg/kg bw per
day, for 31 days, after which they were killed. Body weights were
measured at the start and end of the study, and food consumption was
determined daily. Mice receiving the high dose did not gain body
weight during the 31-day period. The NOAEL was 1344 ppm, equivalent
to 202 mg/kg bw per day (Buttle & Dyer, 1950).
A group of 24 mice were fed tecnazene in the diet at a dose of
10 000 mg/kg bw per day. When deaths occurred within three to four
days, the study was discontinued. Animals were found to have fatty
degeneration of the liver and fatty changes in the spleen and kidney
(Buttle & Dyer, 1950).
Rats
Groups of five rats were fed tecnazene in the diet at doses of
0, 800, 4000 or 20 000 ppm, equivalent to 0, 40, 200 or 1000 mg/kg
bw per day, for 10 weeks. Deaths occurred at the highest dose, and
growth was reduced at 4000 ppm. The NOAEL was 800 ppm, equivalent to
40 mg/kg bw per day (Buttle & Dyer, 1950).
Groups of 12 male and 12 female randomly selected Alpk:APfSD
rats weighing 120-124 g (males) and 111-116 g (females) were fed
diets containing tecnazene (purity, 98.7%) at concentrations of 0,
50, 500 or 5000 ppm, equal to 0, 4.5, 45 or 500 mg/kg bw per day in
males and 0, 4.9, 49 or 500 mg/kg bw per day in females, for 90
days. All animals were observed daily for mortality and signs of
toxicity, and body weight and food consumption were recorded weekly.
Animals in the control and high-dose groups underwent
ophthalmoscopic examinations during the week before sacrifice. After
sacrifice, haematology, clinical chemistry, gross pathology and
histopathology were undertaken on all rats. Administration of 5000
ppm tecnazene was associated with reduced body-weight gain
throughout the study and with an associated reduction in food
consumption and efficiency of food use during week 1. The marked
effect on body weight was reflected in reduced plasma triglyceride
levels, plasma alanine transaminase and creatine kinase activities
and erythrocyte parameters and changes in the levels of total plasma
protein and urea. The liver was identified as a target organ:
Increased liver weight and hepatic aminopyrine-N-demethylase
activity were seen at 500 and 5000 ppm, with histological evidence
of hepatic hypertrophy at 5000 ppm, indicating an adaptive change.
Increased plasma alkaline phosphatase activity and cholesterol
levels were noted at 5000 ppm, and to a lesser extent for males at
500 ppm. In the absence of adverse histopathological findings,
however, these changes were considered not to be of toxicological
significance. The kidney was also identified as a target organ.
Increased kidney weight was seen in males and females given 5000
ppm, which, in the absence of histopathological change, reflects a
possible adaptive response. Mild renal functional changes, indicated
by signs of urinary incontinence (wetness or staining around the
urogenital area), decreased specific gravity and cloudy urine, were
noted at 5000 ppm. Urinary incontinence was also observed at 500 ppm
in females. On the basis of general toxicity, including the adaptive
effects in the liver and kidneys, the NOAEL was 500 ppm, equal to 45
mg/kg bw per day (Horner, 1992).
Dogs
Groups of four male and four female beagle dogs were given
tecnazene (purity, 98.7%) at 0, 2, 15 or 200 mg/kg bw per day for 90
days. Blood samples were taken before treatment and at weeks 4, 8
and 13 and tested for changes in haematological parameters and
clinical chemistry. Food consumption and clinical observations were
recorded daily, body weight was recorded weekly, and ophthalmoscopic
examinations were done before treatment and before sacrifice. All
dogs were examined by gross pathology and histopathology. Body
weight was reduced in animals of each sex at 200 mg/kg bw per day,
but food consumption was unaffected by treatment. The liver was
identified as the target organ, since evidence for an adaptive
response was seen in all treated groups in the form of a
dose-related increase in liver aminopyrine- N-demethylase activity.
At 200 mg/kg bw per day, there was a more marked response, including
increased alkaline phosphatase activity, increased liver weight (>
60%) and histopathological changes typical of increased
proliferation of the smooth endoplasmic reticulum. Altered liver
function at this dose was indicated by changes in most clinical
chemical parameters and small changes in some haematological
parameters, including increased clotting times, platelet counts and
mean cell volumes. Minimal alterations in liver weight (19%) and
clinical chemical parameters were also seen in females receiving 15
mg/kg bw per day. A slight increase in liver weight in males at 15
mg/kg bw per day was due mainly to the response in one dog. Changes
in organ weights of animals at 200 mg/kg bw per day were not
associated with histopathological abnormalities induced by
tecnazene, although there was evidence of delayed sexual maturation
in males that was consistent with the reduced growth of these
animals. On the basis of body-weight reduction and the adaptive
effect on the liver, the NOAEL in this study was 15 mg/kg bw per day
(Hodge, 1992).
Two male and two female beagle dogs were given capsules
containing tecnazene at concentrations of 0, 3.8, 15, 60 or 240
mg/kg bw per day, six days per week for two years. All of the dogs
receiving the highest dose died during the first year of the study,
and microscopic changes were noted in the liver, kidney and bone
marrow. At 60 mg/kg bw per day, growth was normal, but serum
alkaline phosphatase activity was increased. No effects were seen on
haematological, urological or electrocardiographic parameters. The
NOAEL was 15 mg/kg bw per day for animals of each sex (summarized in
Annex I, reference 23).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 65 male and 65 female CD-1 mice were fed 0, 750 or
1500 ppm tecnazene (purity, > 99.0%) in the diet for 80 weeks,
equal to 0, 78 or 155 mg/kg bw per day in males and 0, 86 or 174
mg/kg bw per day in females. The mice were observed daily for
clinical signs. Those that died during the first 13 weeks of the
study were submitted to a macroscopic necropsy and replaced by
reserve animals on the same treatment. Body weights were recorded
weekly during the first 12 weeks and every two weeks thereafter.
Food consumption was assessed weekly, and water intake was assessed
daily by visual inspection. At the end of study, all mice were
examined by gross pathology and histopathology. A total of 237 mice
(131 males and 106 females) died or were killed in extremis, but
the group distribution, time and cause of death were not related to
treatment. Body weights and food and water intakes of treated mice
were similar to those of the untreated controls throughout the
study. The pattern of non-neoplastic pathological effects was
unchanged by treatment, and treatment had no effect on tumour type
or incidence. The NOAEL was 1500 ppm, equal to 155 mg/kg bw per day
(Ben-Dyke et al., 1978a).
Rats
Groups of 65 male and 65 female CD rats were fed tecnazene
(purity, > 99.0%) in the diet at 0, 750 or 1500 ppm for 104 weeks,
equal to 0, 27 or 56 mg/kg bw per day in males and 0, 32 or 63 mg/kg
bw per day in females. Animals were observed daily for clinical
signs. Those that died during the first 13 weeks of the study were
submitted to macroscopic necropsy and replaced by reserve animals on
the same treatment. Body weights were recorded weekly during the
first 12 weeks and every two weeks thereafter. Food consumption was
assessed weekly, and water intake was assessed daily by visual
inspection. At the end of study, all mice were examined by gross
pathology and histopathology. The few signs seen during treatment
were considered not to be of biological significance. Mortality
rates, body weights, food intakes and efficiency of food use were
not affected by treatment. The range of macroscopic changes seen at
necropsy were those commonly seen in CD rats. There were some
differences between groups in the incidences of pituitary adenomas
and mammary gland tumours, but these were considered not to be
related to treatment. The incidences of other tumour types were
similar in treated and control groups. The NOAEL was 1500 ppm, equal
to 56 mg/kg bw per day (Ben-Dyke et al., 1978b).
(d) Reproductive toxicity
Rats
Groups of 30 male and 30 female Alpk:APfSD (Wistar-derived)
rats, four to five weeks old, were fed diets containing tecnazene
(purity, 98.7%) at 0, 300, 1000 or 5000 ppm, equal to 32, 106 or 571
mg/kg bw per day in males and 34, 113 or 576 mg/kg bw per day in
females. After 12 weeks, the animals were mated and allowed to rear
the ensuing F1a litters to weaning. During the pre-weaning phase,
it became evident that the F1a pups could not tolerate the
5000-ppm dose, and they and their dams were given control diet from
days 17-20 for the remainder of the lactation period. The F1a
offspring were considered to be unsuitable to form the next parental
generation, so an F1b litter was produced after the parental dose
had been reduced to 3000 ppm. The F1b offspring and dams at the
highest dose were also given control diet during the pre-weaning
phase because of poor weight gain of the pups (from days 21-25, and
earlier for a few litters born later); however, the F1 parents
were selected from among the F1b offspring. The highest dietary
level was further reduced to 2000 ppm, and these animals were
allowed to produce the F2a litter after a 12-week pre-mating
period. Diets containing 0, 300, 1000 or 2000 ppm tecnazene, equal
to 31, 103 or 220 mg/kg bw per day in males and 34, 111 or 235 mg/kg
bw per day in females, were then fed continuously throughout the
remainder of the study.
Dietary administration of 5000 ppm tecnazene was associated
with reduced body-weight gain and food consumption during the
pre-mating period, but 300 or 1000 ppm had no adverse effects. A
dose-related incidence of signs of urinary incontinence (wetness or
staining around the urogenital area) was seen in treated animals,
but the significance of this finding was uncertain. A clear,
dose-related effect on the offspring was associated with
administration of 3000 or 5000 ppm tecnazene. At the highest dietary
level, pup survival, clinical condition and growth were adversely
affected; only pup growth was affected by 3000 ppm. Doses of 2000
ppm or less had no adverse effect on pup growth or survival.
Treatment induced a dose-related increase in kidney weight in males
of both generations, but no corresponding histological change was
observed. Increased liver weight was seen in both parents and
offspring at all dietary levels of tecnazene; histological changes
indicative of an adaptive response were observed in adults receiving
1000 ppm or more but not in those receiving 300 ppm or in any of the
offspring. The increased organ weights were considered to reflect an
adaptive response and, in the absence of histological change, were
considered to be of no toxicological significance. Tecnazene did not
affect reproductive performance in either generation. The NOAEL for
maternal toxicity was 1000 ppm, equal to 103 mg/kg bw per day. The
NOAEL for filial toxicity and reproductive toxicity was 2000 ppm,
equal to 220 mg/kg bw per day (Moxon, 1992).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant Alpk:APfSD (Wistar-derived) rats, 12
weeks of age, were treated by gavage with tecnazene (purity, 98.7%)
at 0, 15, 50 or 150 mg/kg bw per day on days 7-16 of gestation. The
females were observed daily for clinical signs of toxicity and were
killed on day 22 of gestation. They were examined by gross
pathology, and their uteri were examined for live fetuses and
intra-uterine deaths. The fetuses were weighed, examined for
external visceral abnormalities, sexed, eviscerated and stained for
skeletal examination, and the number of implantations was
determined. Administration of 150 mg/kg bw per day resulted in a
statistically significant reduction in body-weight gain of dams,
particularly on days 7-10; no maternal toxicity was apparent at
lower doses. Overall, there was an increased number of fetuses with
minor skeletal defects (unossified centra of the third and fourth
cervical vertebrae) and an increased incidence of some skeletal
variants (partially ossified transverse processes of the seventh
cervical vertebrae and of the fourth lumbar vertebrae) at 150 mg/kg
bw per day. There was no evidence of embryo- or fetotoxicity or
teratogenicity at any dose. The NOAEL for maternal toxicity and for
embryo- and fetotoxicity was 50 mg/kg bw per day, on the basis of
effects on body weight and minor skeletal defects, respectively
(Whiles, 1991).
Rabbits
Tecnazene (purity, 98.7%) was administered in corn oil by
gavage to groups of 20 pregnant New Zealand white rabbits at 0, 15,
45 or 135 mg/kg bw per day on days 7-19 of gestation. The animals
were killed on day 30 of gestation and their uteri examined for live
fetuses and intra-uterine deaths. The fetuses were weighed, examined
for external and visceral abnormalities, sexed, eviscerated and
processed for skeletal examination. Two animals given 135 mg/kg bw
per day were killed when moribund on days 20 and 27 of gestation;
the death that occurred on day 20 was considered to be related to
treatment, as it was associated with adverse clinical signs, poor
food consumption and body-weight loss. There was no evidence of
overt toxicity in the remaining animals in this group or in animals
given 15 or 45 mg/kg bw per day, and there was no treatment-related
effect on the litters. A total of 27 major defects were seen in 18
fetuses from 14 litters. Of these defects, 13 occurred in controls,
and treatment did not appear to have affected the overall incidence
of major defects. Four minor skeletal defects or variants were noted
at 45 or 135 mg/kg bw per day, which were considered to represent a
minimal effect of tecnazene on fetal development. These defects
included slightly increased manus scores at 45 and 135 mg/kg bw per
day, marginally increased incidences of 27 pre-sacral vertebrae and
extra thirteenth ribs and a slightly increased incidence of
misshapen hyoid at 135 mg/kg bw per day. The NOAEL for maternal
toxicity was 45 mg/kg bw per day on the basis of adverse clinical
signs and body-weight loss, and the NOAEL for embryo- and
fetotoxicity was 15 mg/kg bw per day, on the basis of minor skeletal
defects (Hopkins, 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tecnazene are
summarized in Table 1.
Table 1. Results of tests for the genotoxicity of tecnazene
End-point Test system Concentration Purity Results Reference
of tecnazene (%)
In vitro
Reverse S. typhimurium TA98, 0.32-1000 µg/plate NR Negativea Cattanach & Riach, 1989
mutation 100, 1535, 1538
Forward Mouse lymphoma 1-50 µg/ml 97.1 Negativeb Adams et al., 1989
mutation cells (L5178Y) Positivec
at tk locus
Chromosomal Human 20 µg/ml 97.1 Negativea Brooker et al., 1989
aberration lymphocytes 80 µg/ml Negativec
80 µg/ml Positiveb
160 µg/ml Positivea
In vivo
Micronucleus C57Bl/6 1160-1860 mg/kg bw 97.1 Negative Randall & Howard, 1989
formation JfCD 1/Alpk
mice
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
3. Observations in humans
No information was available.
Comments
After oral administration of radiolabelled tecnazene to rats,
about 90% of the dose was recovered within 48 h. Excretion was
divided almost equally between urine and faeces in males but
occurred predominantly (80%) via the urine in females. The remainder
of the dose was excreted more slowly, and radioactivity was still
detectable in urine and faeces seven days after dosing. Negligible
levels of radioactivity were detected in expired carbon dioxide.
Whole-body autoradiography showed that 24 h after dosing the highest
concentrations of radioactivity were located in the intestinal
contents of animals of each sex; the highest tissue concentrations
were found in the kidney, liver and the nasal passages.
Tecnazene is extensively metabolized in rats. A total of 42
metabolites have been separated from urine and bile, of which 16,
including the parent compound, have been identified. The principal
route of metabolism is via the tetrachlorobenzene glutathione
conjugate pathway. Metabolites present in urine and faeces include
the tetrachloro-phenyl-mercapturate conjugate, tetrachloroaniline
and tetrachlorothioanisole. The pattern of metabolites in urine and
faeces is the same in male and female rats, but the quantities
differ.
In female rabbits, about 70% of an oral dose of tecnazene was
recovered in the faeces within three days. The remainder was
excreted in urine, primarily as glucuronide, sulfate and mercapturic
acid conjugates of 2,3,5,6-tetrachloroaniline. Some unconjugated
4-amino-2,3,5,6-tetrachlorophenol was also excreted.
Tecnazene has low oral toxicity in rats. WHO (1992) has
classified tecnazene as unlikely to present an acute hazard in
normal use.
In a 90-day study in rats fed dietary concentrations of 0, 50,
500 or 5000 ppm, the NOAEL was 500 ppm, equal to 45 mg/kg bw per
day, on the basis of effects on body-weight gain and on the liver
and kidneys.
In a 90-day study in dogs given 0, 2, 15 or 200 mg/kg bw per
day orally, the NOAEL was 15 mg/kg bw per day on the basis of
effects on body and liver weights.
In a two-year study in which dogs were given tecnazene at 0,
3.8, 15, 60 or 240 mg/kg bw per day orally, the NOAEL was 15 mg/kg
bw per day, on the basis of elevation of serum alkaline phosphatase
activity. Owing to the small number of animals and lack of access to
the original report, data from this study could not be considered in
establishing an ADI.
In an 80-week study of carcinogenicity in mice fed dietary
concentrations of 0, 750 or 1500 ppm, the NOAEL was 1500 ppm, equal
to 155 mg/kg bw per day. There was no evidence of carcinogenicity
and no effect on body weight, mortality or clinical signs.
In a 104-week study of carcinogenicity in rats fed 0, 750 or
1500 ppm, the NOAEL was also 1500 ppm, equal to 56 mg/kg bw per day.
There was no evidence of carcinogenicity. As no clinical chemical or
haematological parameters were evaluated in this study, the Meeting
considered it inadequate for an evaluation of long-term toxicity.
In a two-generation study of reproductive toxicity in rats fed
dietary concentrations of 0, 300, 1000 or 5000/2000 ppm, the NOAEL
for parental toxicity was 1000 ppm, equal to 106 mg/kg bw per day.
The NOAEL for filial toxicity was 2000 ppm, equal to 220 mg/kg bw
per day. There were no adverse effects on reproduction.
In a study of teratogenicity in rats administered 0, 15, 50 or
150 mg/kg bw per day by gavage, the NOAEL was 50 mg/kg bw per day
for both maternal toxicity and embryo- and fetotoxicity on the basis
of reduced body-weight gain and minor skeletal defects,
respectively. No teratogenic effects were observed.
In a study of teratogenicity in rabbits administered 0, 15, 45
or 135 mg/kg bw per day by gavage, the NOAEL was 45 mg/kg bw per day
for maternal toxicity on the basis of body-weight loss and reduced
food consumption. The NOAEL was 15 mg/kg bw per day for embryo- and
fetotoxicity on the basis of minor skeletal defects. No teratogenic
effects were observed.
Tecnazene can produce clastogenic effects in vitro but not
in vivo. No mutagenicity was observed in bacteria. The Meeting
concluded that tecnazene is not genotoxic.
An ADI was established on the basis of an NOAEL of 15 mg/kg bw
per day for toxicity in the 90-day study in dogs and for embryo- and
fetotoxicity in the study of teratogenicity in rabbits. Owing to the
lack of adequate data on the long-term toxicity of the compound, the
Meeting applied a 1000-fold safety factor to the NOAEL.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1500 ppm, equal to 155 mg/kg bw per day (80-week
study of carcinogenicity)
Rat: 500 ppm, equal to 45 mg/kg bw per day (90-day study
of toxicity)
1500 ppm, equal to 56 mg/kg bw per day (104-week
study of carcinogenicity)
Rabbit: 15 mg/kg bw per day (embryo- and fetotoxicity in a
study of teratogenicity)
Dog: 15 mg/kg bw per day (90-day study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. One-year study of toxicity in dogs
2. Long-term study of toxicity in rats
References
Adams, K., Ransome, S.J., Henly, S.M., Kirkpatrick, D., Skinner, N.
& Bottoms, M.A (1989) An assessement of the mutagenic potential of
tecnazene using the mouse lymphoma TK locus assay. Unpublished
report No. ISN 206/89508 from Huntingdon Research Centre Ltd,
Huntingdon, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Barber, J.E. (1985) Tecnazene: acute oral toxicity study.
Unpublished report No. CTL/P/1232 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978a) Tecnazene: oncogenic response in mice to continuous
dietary administration for 80 weeks. Unpublished report No. 78/ILY
19/080 from Life Science Research, Stock, United Kingdom. Submitted
to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United
Kingdom.
Ben-Dyke, R., McSheehy, T.W., Cummins, H.A., Finn, J.P. & Newman,
A.J. (1978b) Tecnazene: carcinogenic response in rats to dietary
administration for 104 weeks. Unpublished report No. 78/ILY 20/078
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Bentley, M. & Powles, P. (1992) Tecnazene--preliminary examination
of urinary and faecal metabolites in the rat. Unpublished report No.
7119-72/305 from Hazleton United Kingdom, Harrogate, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and the
formation of mercapturic acids in the rabbit. Biochem. J., 61,
611-617.
Bratt, H. (1991) Tecnazene: Excretion and tissue retention of a
single oral dose (1 mg/kg) in the rat. Unpublished report No.
CTL/P/3202 from ICI Central Toxicology Laboratory. Submitted to WHO
by Zeneca Agrochemicals, Fernshurst, Haslemere, United Kingdom.
Bray, H.G., Hybs, Z., James, S.P. & Thorpe, W.V. (1953) The
metabolism of 2:3:5:6- and 2:3:4:5-tetrachloronitrobenzenes in the
rabbit and the reduction of aromatic nitro compounds in the
intestine. Biochem. J., 53, 266-273.
Bray, H.G., Franklin, T.J. & James, S.P. (1959) The formation of
mercapturic acids. 3. N-Acetylation of S-substituted cysteines in
the rabbit, rat and guinea pig. Biochem. J., 73, 465-473.
Brooker, P.C., Akhurst, L.C. & King, J.D. (1989) Tecnazene:
metaphase chromosome analysis of human lymphocytes cultured in
vitro. Unpublished report No. ISN 207/89289 from Huntingdon
Research Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by
Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Buttle, G.A.H. & Dyer, F.J. (1950) Experiments on the toxicology of
2,3,5,6-tetrachloronitrobenzene. J. Pharm. Pharmacol., 2, 371-375.
Cattanach, P.J. & Riach, C.G. (1989) Tecnazene: testing for
mutagenic activity with Salmonella typhimurium TA 1535, TA 1537, TA
1538, TA 98 and TA 100. Unpublished report No. 4872 from Inveresk
Research International, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Donikian, M., Owen, S.D., Wiland, J. & Drobeck, H.P. (1965) Oral
administration of 2,3,5,6-tetrachloronitrobenzene to beagle dogs for
two years. Unpublished report from Sterling Winthrop Research
Institute (1974 Evaluations of some pesticide residues in food, WHO
1975).
Hodge, M.C.E. (1992) Tecnazene: 90 day oral dosing study in dogs.
Unpublished report No. CTL/P/3614 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Hopkins, M.N. (1991) Tecnazene: Teratogenicity study in the rabbit.
Unpublished report No. CTL/P/3184 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Horner, J.M. (1992) Tecnazene: 90-day feeding study in rats.
Unpublished report No. CTL/P/3648 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Lappin, G.J. & Pritchard, D.J. (1992) Tecnazene: Biotransformation
in the rat. Unpublished report No. CTL/P/3731 from ICI Central
Toxicology Laboratory, Macclesfield, United Kingdom. Submitted to
WHO by Zeneca Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Moxon, M.E. (1992) Tecnazene: multigeneration study in the rat.
Unpublished report No. CTL/P/3596 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
Randall, V. & Howard, C.A. (1989) Tecnazene: an evaluation in the
mouse micronucleus test. Unpublished report No. CTL/P/2632 from ICI
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Submitted to WHO by Zeneca Agrochemicals, Fernhurst, Haslemere,
United Kingdom.
Renner, G. (1980) Metabolic studies on pentachloronitrobenzene
(PCNB) in rats. Xenobiotica, 10, 537-550.
Whiles, A.J. (1991) Tecnazene: Teratogenicity study in the rat.
Unpublished report No. CTL/P/3193 from ICI Central Toxicology
Laboratory, Macclesfield, United Kingdom. Submitted to WHO by Zeneca
Agrochemicals, Fernhurst, Haslemere, United Kingdom.
