
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
CARBOPHENOTHION
Explanation
Carbophenothion was evaluated at the 1972 (FAO/WHO, 1973), 1976
(FAO/WHO,1977), 1977 (FAO/WHO, 1978) and 1979 (FAO/WHO, 1980) Joint
Meetings. In 1979 a full-ADI of 0.0005 mg/kg b.w. was allocated.
Studies on the identity and the relative toxicity of metabolites were
considered desirable.
No additional studies concerning the relative toxicity of these
metabolites have been received. However, new studies have become
available on acute delayed neurotoxicity in adult hens, subacute oral
toxicity in beagle dogs and metabolism of carbophenothion and are
included in this monograph addendum.
The 1977 Joint Meeting listed as desirable additional elucidation of
the nature of terminal residues on crops under field conditions,
particularly as to the possible presence of photolysis products which
had been reported in laboratory experiments. In response to this
request for information a greenhouse study on the metabolism of
carbophenothion on orange trees has been submitted and is evaluated in
this addendum. Additional information on national use patterns and
tolerances were also provided.
DATA CONSIDERED FOR DERIVATION OF ACCEPTABLE DAILY
INTAKE
BIOCHEMICAL ASPECTS
Excretion via faeces, urine and expired air was studied in rats after
oral administration of carbophenothion [14C]-phenyl (3 mg/kg bw),
carbophenothion [14C]-thiomethyl (3 mg/kg bw), carbophenothion
sulphoxide [14C]-phenyl (3 mg/kg bw), and [14C]-4-chlorothiophenol
(8.3 mg/kg bw).
The radiocarbon recovered after 66 - 144 hours as % of the
administered dose in the carbophenothion [14C]-phenyl,
carbophenothion sulphoxide [14C]-phenyl and on the [14C]-4-
chlorothiophenol-treated animals were much the same: in faeces
(17-24%), urine (71-80%), and expired air (0%). The major route of
excretion of [14C] in rats receiving carbophenothion [14C]-thiomethyl
group was via expired air as CO2: 59%, faeces: 4%, and urine 31%.
The metabolic products following administration of carbophenothion or
carbophenothion sulphoxide were quantitatively and qualitatively very
similar. Therefore it was concluded that there is an equilibrium
between carbophenothion and the sulphoxide in vivo. However, no
sulphoxidation products were detected (Menn et al, 1975).
TOXICOLOGICAL STUDIES
Special study on neurotoxicity
Groups of 10-14 adult hens were administered orally corn oil (2 ml/kg
bw), carbophenothion 92.4% (330 mg/kg bw) or TOCP (500 mg/kg bw). The
treatment was repeated for surviving hens after 21 days. The animals
were observed for 42 days. The toxic signs of the carbophenothion
treated hens were most severe up to 7 days after either administration
and they included signs of cholinergic influence, e.g. ataxia,
salivation and diarrhoea, loss of body weight and hampered egg
production. The severity of these signs diminished with time.
Minimal leg weakness was noted only one occasion for one hen given c
carbophenothion. Histopathological changes in nerve tissue (brain
stem, cervical, thoracic and lumbal spinal cord distal and proximal
sciatic nerve) of hens given carbophenothion were similar to those in
the group given corn oil. However, one of 14 chickens receiving
carbophenothion had nerve fibre degeneration, but no specific lesions
in spinal cord and medulla. The TOCP treated birds showed progressive
loss of body weight and paralysis. Axonal degeneration was observed
by light microscopy in nerve tissue, including sciatic nerves
(bilateral), of this group (Miller and Sprague, 1978).
Short-term study
Four male and 4 female beagle dogs per dose group were orally
administered carbophenothion (purity 92.8%) at dose levels of 0, 0.02,
0.2 or 2 mg/kg bw per day for 22-25 days (Bier, 1979). The parameters
evaluated were mortality, general appearance and behaviour, body
weight, food consumption, growth, gross pathology and plasma,
erythrocyte and brain cholinesterase activity (white and grey matter).
The animals in the intermediate and high dose group showed
dose-dependent emesis and diarrhoea.
Plasma butyrylthiocholinesterase activity was depressed throughout the
study in both males and females; from day 1 at the 0.2 mg/kg bw per
day level, with a plateau of about 60%, and at the 2 mg/kg bw per day
level a plateau of about 85% decrease was reached on day 3. The
acetylcholinesterase activity of erythrocytes was extremely variable.
In the high dose groups however the depression was consistent
throughout the study. The acetylcholinesterase activity of the grey
matter of the brain measured at necropsy was decreased at the 0.2 and
the 2 mg/kg bw group in both males and females, whereas the activity
was unaffected in white matter.
RESIDUES IN FOOD
USE PATTERN
Additional information was provided on carbophenothion use patterns in
the Netherlands:
Extent of Use
Pest and Economic Application
Crop Controlled Importance rate Formulation Treatment
Grapes1 Red spider small 0.6 g/m3 aerosol, 14.8% Apply only
mite scale and 25.5% before
blossom
and after
Peaches1 harvest
Plums
1 Glasshouse
FATE OF RESIDUES
Plants
Available data on the plant metabolism of carbophenothion were
reviewed by the Joint Meeting in 1972, 1976 (FAO/WHO, 1973, 1977).
The principal route of metabolism was judged to be thioether oxidation
of carbophenothion to form the sulphoxide and sulphone, followed by
phosphorothionate oxidation to form the oxygen analogue sulphone. Each
of these products as well as the oxygen analogue of carbophenothion
and the oxygen analogue sulphoxide were identified in plants.
Laboratory photolysis experiments have also shown that ethion and four
"other related organophosphorus products" can be formed although there
was no evidence to indicate whether those would occur under field
conditions.
In the study evaluated in this Monograph Addendum (McBain et al,
1977) water-emulsified [phenyl-14C] carbophenothion was brush-applied
to the surfaces of selected fruits and foliage of a greenhouse-grown
dwarf variety orange tree at a field concentration equivalent of 2.4 g
active ingredient (labelled + unlabelled) per liter of spray solution.
0.5 ml aliquots ( ca 0.94 mg after adjusting for losses) were
applied to each fruit or foliage sample. Samples were taken at 0, 3,
5, 10, 26 and 42 days following treatment.
Residues were separated, characterised and quantified using sequential
solvent extractions, column chromatography, two-dimensional TLC, LSC
and incubation with enzymes and acid. Residues were categorised as
external (surface) or internal. Internal residues were further
characterized as methanol-soluble (water-soluble + methylene
chloride-soluble) and bound.
The distribution of 14C residues in internal and external fractions
as a percentage of those applied are summarised in Table 1. At
harvest, 42 days after application, the surface radioactivity had
fallen to 11.7% and 5.3% of that applied to the fruit and leaves
respectively. These surface residues were 32% and 23% of the total
residues at harvest. The total internal radioactivity at harvest was
17.4% of that applied to the leaves (77% of the whole leaf harvest
residue). For fruit at 26 days, 28% of the applied radioactivity was
within the fruit (59% of the total whole fruit radioactivity) and at
42 days 25% of the applied radioactivity remained within the fruit
(68% of the total whole fruit radioactivity).
14C recovered from fruit and foliage decreased from 98.4 and 102% at
0 day to 36.2 and 22.6% respectively at 42 days. Bound radioactivity
was none to negligible at 0 day and 3.4 and 4.5% of applied (9.3 and
19.8% of terminal) at 42 days for fruit and foliage respectively.
There was little translocation of 14C from the surface of fruit to
pulp and juice (<1% of applied). It was shown that only a little
14C translocation occurs from treated foliage to adjacent untreated
foliage and fruit but it was not possible to determine how much of the
applied 14C could have translocated to unanalysed fruit and foliage.
The disappearance of radioactivity is attributed primarily to
volatilisation, which is consistent with an experiment showing 50%
dissipation of 14C within 80 h when [14C]-carbophenothion was coated
on to glass slides as a thin layer. As would be expected, however,
this was a faster dissipation rate than was observed on the fruit and
foliage.
TABLE 1. Distribution of 14C Recovered Following Topical Application of [14C]
Carbophenothion to Orange Fruit and Foliage
Distribution of 14C From Plant Harvest1
External2 Internal
Methanol-Soluble
Samples and methylene
time of harvest water- chloride- total bound Total
(days) soluble soluble recovered
Treated foliage
0 102.3 0.0 0.0 0.0 0.01 102.3
3 73.1 6.8 1.3 8.1 0.4 81.6
5 37.2 7.2 1.5 8.7 0.7 46.6
10 39.0 9.0 1.7 10.7 0.4 50.1
26 19.0 8.1 4.8 12.7 3.9 35.8
42 5.3 5.0 7.9 12.9 4.5 22.6
Treated fruit
0 98.4 0.0 0.0 0.0 0.0 98.4
3 70.7 3.0 2.1 5.1 0.9 76.7
5 74.7 7.1 2.2 9.3 0.6 84.6
10 64.0 8.7 3.3 12.0 1.3 77.3
26 19.7 20.3 5.2 25.5 2.7 47.9
42 11.7 12.6 8.5 21.1 3.4 36.2
Pulp and juice
0 0.00 0.00
3 0.09 0.01 0.11
5 0.13 0.02 0.12
10 0.09 0.03 0.13
26 0.22 0.04 0.24
42 0.49 0.11 0.61
1 Expressed as % of applied
2 External (surface) residues from acetone rinses.
Individual components identified in peel and foliage external residues
and in peel and foliage internal organosoluble residues were
carbophenothion, its oxygen analogue and their respective sulphoxides
and sulphones as shown in tables 2 and 3 (McBain et al, 1977).
Unidentified polar and non-polar materials were also detected.
Carbophenothion is quantitatively the most important individual
component identified except in the 42-day external foliage fraction
where the carbophenothion sulphoxide and sulphone predominate.
However, in this same fraction unknown polar compounds exceed any
individually identified residue.
Unidentified surface residues have been reported previously (FAO/WHO,
1973).
In general, therefore, carbophenothion and its sulphoxide and sulphone
are the major components of the residues identified with smaller
quantities of the oxon and its sulphoxide and sulphone. This is
consistent with earlier findings.
More specifically, carbophenothion and these 5 oxidative products
account for over 90% of the residual 14C in external and internal
peel extractives at 42 days. They account for only 59 and 87%
respectively of residual 14C in external and internal foliage
extractives at 42 days, reflecting the higher percentage of
unidentified moieties in the foliage surface residues.
Carbophenothion dissipated more rapidly from the surface of foliage
than from peel and the rate of loss from both decreased substantially
with time as more of the remaining material was transported into the
internal tissues. The concentration of oxygen analogues in combined
internal and external extraction plateaued after 10-25 days.
There was no evidence of residues of 4-chlorophenylmethyl sulphoxide
(4-ClPhSOMe), 4-chlorophenylmethyl sulphone (4-ClPhSO2Me), 3-hydroxy,
4-chlorophenylmethylsulphone (3-0H, 4-ClPhSO2Me),
4-chlorophenylsulphenylmethyl methyl sulphone (4-ClPhSMeS02Me), or
4-chlorophenylsulphonylmethyl methyl sulphone (4-ClPhSO2Me), which
are known to occur as animal metabolites (FAO/WHO, 1978).
Water-soluble internal extracts, represented up to 9 and 20% of
applied 14C at 10 and 26 days in foliage and fruit respectively,
followed by a decline. The non-polar fraction contained variable
amounts of carbophenothion sulphoxide and sulphone and unknown
compounds whereas the polar fractions contained unknown compounds and
variable amounts of residues tentatively identified as the
4-chlorobenzene sulphinic and sulphonic acids. Evidence that these
two moieties are present in plants apparently has not previously been
reported to the Joint Meeting although they have been identified in
animals.
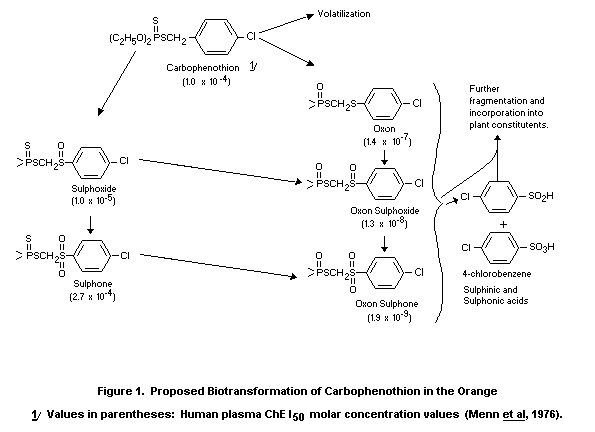
The proposed biotransformation of carbophenothion in the orange is
given in figure 1 (McBain et al, 1977).
TABLE 2. Products Isolated by TLC From the External and Internal Organosoluble
Fraction of [14C] Carbophenothion-treated Orange Fruit Peel
Products1,2 Distribution of 14C as % applied dose, at indicated
days after treatment
0 3 5 10 26 42
Peel, External 14C
Carbophenothion 97.65 68.25 71.67 59.94 16.95 9.04
Carbophenothion-SO 0.17 1.26 1.04 1.91 1.24 1.27
Carbophenothion-SO2 0.10 0.29 0.58 0.67 0.51 0.33
Oxon 0.04 0.23 0.25 0.29 0.11 0.10
Oxon-SO 0.00 0.01 0.02 0.03 0.05 0.09
Oxon-SO2 0.00 0.08 0.04 0.03 0.03 0.04
Non-polar unknowns3 0.41 0.39 0.77 0.72 0.37 0.44
Polar unknowns3 0.03 0.19 0.31 0.43 0.44 0.40
Total 98.40 70.70 74.68 64.02 19.70 11.71
Peel, Internal
Organosoluble
Carbophenothion 0.00 1.24 1.82 2.53 3.97 4.14
Carbophenothion-SO 0.00 0.26 0.11 0.31 0.61 2.21
Carbophenothion-SO2 0.00 0.04 0.03 0.06 0.10 0.24
Oxon 0.00 0.10 0.03 0.07 0.10 0.45
Oxon-SO 0.00 0.08 0.02 0.08 0.15 0.94
Oxon-SO2 0.00 0.00 0.00 0.01 0.01 0.05
Non-polar unknowns3 0.00 0.29 0.14 0.18 0.20 0.38
Polar unknowns3 0.00 0.06 0.03 0.04 0.05 0.10
Total 0.00 2.07 2.18 3.28 5.19 8.51
1 14C-Products were identified by two-dimensional TLC analysis with the
reference standards given in Table 1. The following products did not occur as
metabolites: 4-ClPhSOMe and 4-ClPhSO2Me; 3-OH, 4Cl, PhSO2Me; 4-ClPhSMeSO2Me;
4-ClPhSO2MeSO2Me.
2 TLC analyses of the 14C carbophenothion emulsion used to treat the orange tree
revealed the following distribution of 14C-products: Trithion, 98.77%; combined
oxygen analogues 0.14% and two non-polar unknowns positioned near Trithion, 1.10%.
3 14C that failed to migrate significantly from the origin on chromatography was
classified as polar unknowns. The non-polar unknowns represented: (1) minor
impurities present in the 14C carbophenothion treatment medium, (2) several minor
unknown metabolites of carbophenothion and (3) unresolved 14C that smeared from
the identified products along their paths of migration.
TABLE 3. Products Isolated by TLC from the External and Internal Organosoluble
Fractions of 14C Carbophenothion-treated Orange Tree Foliage
Distribution 14C as % applied dose, at indicated
Products1,2 days after treatment
0 3 5 10 26 42
Foliage, External 14C
Carbophenothion 101.13 69.00 32.77 34.48 10.77 0.76
Carbophenothion-SO 0.07 1.05 1.73 1.29 2.68 0.96
Carbophenothion-SO2 0.04 1.04 0.85 1.01 1.61 1.15
Oxon 0.01 0.31 0.25 0.23 0.21 0.04
Oxon-SO 0.00 0.05 0.10 0.08 0.13 0.12
Oxon-SO2 0.00 0.03 0.08 0.05 0.10 0.07
Non-polar unknowns3 0.99 1.02 0.82 0.88 1.05 0.59
Polar unknowns3 0.06 0.64 0.61 1.00 2.44 1.62
Total 102.30 73.14 37.21 39.02 18.99 5.31
Foliage, Internal
Organosoluble 14C
Carbophenothion 0.00 1.11 1.21 1.41 3.27 2.84
Carbophonothion-SO 0.00 0.09 0.15 0.15 0.80 1.82
Carbophenothion-SO2 0.00 0.01 0.02 0.03 0.19 0.49
Oxon 0.00 0.01 0.01 0.02 0.04 0.16
Oxon-SO 0.00 0.02 0.04 0.03 0.14 0.49
Oxon-SO2 0.00 0.00 0.00 0.00 0.01 0.08
Non-Polar unknowns3 0.00 0.02 0.05 0.04 0.24 0.54
Polar unknowns3 0.00 0.01 0.02 0.01 0.11 0.50
Total 0.00 1.27 1.50 1.69 4.80 7.92
1 14C-Products were identified by two-dimensional TLC analysis with the reference
standards given in Table 1. The following products did not occur as metabolites:
4-ClPhSOMe and 4-C1PhSO2Me; 3-OH, 4Cl,PhSO2Me; 4-ClPhSMeSO2Me; 4-ClPhSO2MeSO2Me.
2 TLC analyses of the 14C carbophenothion emulsion used to treat the orange tree
revealed the following distribution of 14C-products: Trithion, 98.77%; combined
oxygen analogues 0.14% and two non-polar unknowns positioned near Trithion, 1.10%.
3 14C that failed to migrate significantly from the origin on chromatography was
classified as polar unknowns. The non-polar unknowns represented: (1) minor
impurities present in the 14C carbophenothion treatment medium, (2) several minor
unknown metabolites of carbophenothion and (3) unresolved 14C that smeared from
the identified products along their paths of migration.
 NATIONAL MAXIMUM RESIDUES LIMITS
The Netherlands reported the following national tolerances to the
Meeting.
Commodity Tolerance (mg/kg)
In force fruits and vegetables 0.01
Under consideration fruit 0.0511/
other commodities
of plant origin 0.05
1/ At or about the limit of determination.
EVALUATION
COMMENTS AND APPRAISAL
Present carbophenothion metabolism studies confirm and extend the
experiments previously reported. Both carbophenothion and its
sulphoxide are metabolised both quantitatively and qualitatively very
similarly. The acute oral toxicity of the majority of the metabolites
of carbophenothion in the rat have been evaluated previously. They
are considerably less toxic than that of the present compound. These
results indicate that carbophenothion is metabolised to less toxic
compounds.
In an acute delayed neurotoxicity study with technical carbophenothion
at an extremely high dose one adult hen out of 14 showed minimal leg
weakness, accompanied by nerve fibre degeneration. However, no
specific histopathological lesions were observed in spinal cord and
medulla. Furthermore, in a previous study with adult hens no
neurotoxic effects had been observed. It seems therefore very
unlikely that carbophenothion induces delayed neurotoxicity.
In a subacute study with dogs a no-effect level, based on
cholinesterase inhibition, of 0.02 mg/kg bw/day was confirmed,
assuring there was no reason to alter the previously established ADI
for man.
In response to requests listed as desirable by the 1977 Joint Meeting,
a carbophenothion metabolism study on orange trees has been submitted
and evaluated. Additional information on national use patterns and
tolerances was also submitted.
In the metabolism study using greenhouse-grown orange trees (14C
phenyl) carbophenothion was applied to selected fruits and foliage,
which were harvested for analysis at 0, 3, 5, 10, 26 and 42 days. It
was found that initial residues were almost entirely surface residues
with a high initial rate of loss, attributed primarily to
volatilisation. After some time, internal residues became the highest
percentage of the total terminal residue in foliage and peels. There
was little translocation of residues from treated foliage to adjacent
untreated foliage and fruit, although there was no basis on which to
determine how much of the total terminal residues would be in the
fruit and foliage not adjacent to that treated. There is minimal
translocation of residues on treated fruit into the pulp and juice.
Of the individual components identified in the residue,
carbophenothion was at the highest concentration followed by its
sulphoxide and sulphone. The only exception was the external foliage
residue at 42 days from application where the carbophenothion residue
was exceeded by both that of its sulphoxide and sulphone. Residues of
the oxon and its sulphoxide and sulphone occurred in lesser amounts.
In addition to the identified carbophenothion metabolites, relatively
large amounts of unidentified compounds remained, especially in the
external foliage residue where polar unknowns constituted the largest
single part of the terminal surface residue (1.62% of the applied
radioactivity). This is however less than the 3.84% and 1.82%
respectively of carbophenothion and its sulphoxide found in foliage
internal organosolubles. A small portion of the residue was bound
into insoluble components. There was no evidence of the S-methylation
of the 4-chlorothiophenol moiety that has been observed in animals. A
significant proportion of the internal residues was water soluble, and
similarly some of these were identified while others were not. Among
those identified were carbophenothion sulphoxide and sulphone and,
tentatively, the 4-chlorobenzene sulphinic and sulphonic acids. The
latter two have not been previously reported at Joint Meetings as
plant metabolites although they are known to be animal metabolites.
This study is consistent with earlier findings on the metabolism of
carbophenothion in plants and adds significantly to the understanding
of plant metabolism. No additional information was provided to
elucidate the terminal residues or photolysis products of
carbophenothion under field conditions, as previously considered
desirable. However, the metabolism study provided data that, along
with information previously evaluated, enabled the Meeting to conclude
that unidentified terminal residues or photolysis products under field
conditions are unlikely to be more than a very small portion of the
total residues. The meeting confirms that residues currently measured
are sufficient.
Level causing no toxicological effects
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw/day.
Dog: 0.02 mg/kg bw/day.
Human: 0.8 mg/human/day corresponding to 0.01 mg/kg bw/day.
Estimate of acceptable daily intake for man
0 - 0.0005 mg/kg bw/day.
RECOMMENDATION
It is not necessary to revise limits or the expression of limits
previously recommended.
REFERENCES
Bier, C.B. A subacute (21-day) oral toxicity study of Trithion in the
beagle dog. Bio-Research Laboratories TLD, (1979) Unpublished report
submitted to WHO by Stauffer Chemical Company, USA. T-10015.
McBain, J.B., Wren, J.P. and Menn, J.J. Metabolism of 14C phenyl
Carbophenothion by Orange Trees. (1977) Unpublished study provided by
Stauffer Chemical Company, Mountain View Research Center, Mountain
View, Ca. 94042. MRC-B-69, July, 1977.
Menn, J.J., De Baun, J.R., Hoffman, L.J. and Ross, J.H. Metabolism of
thioaryl organo-phosphorus insecticides. Environmental quality and
safety, Supp. Vol III, Pesticides, 382-388, (1975).
Menn, J.J., De Baun, J.R. and McBain, J.B. Recent Advances in the
Metabolism of Organo-phosphorus Insecticides. Fed. Proc. 35, 2598.
Miller, J.L. and Sprague, G.L. Acute delayed neurotoxicity study with
technical TrithionR in adult hens. Report Woodward Research
Corporation, 1978 (unpublished) T-6404.
NATIONAL MAXIMUM RESIDUES LIMITS
The Netherlands reported the following national tolerances to the
Meeting.
Commodity Tolerance (mg/kg)
In force fruits and vegetables 0.01
Under consideration fruit 0.0511/
other commodities
of plant origin 0.05
1/ At or about the limit of determination.
EVALUATION
COMMENTS AND APPRAISAL
Present carbophenothion metabolism studies confirm and extend the
experiments previously reported. Both carbophenothion and its
sulphoxide are metabolised both quantitatively and qualitatively very
similarly. The acute oral toxicity of the majority of the metabolites
of carbophenothion in the rat have been evaluated previously. They
are considerably less toxic than that of the present compound. These
results indicate that carbophenothion is metabolised to less toxic
compounds.
In an acute delayed neurotoxicity study with technical carbophenothion
at an extremely high dose one adult hen out of 14 showed minimal leg
weakness, accompanied by nerve fibre degeneration. However, no
specific histopathological lesions were observed in spinal cord and
medulla. Furthermore, in a previous study with adult hens no
neurotoxic effects had been observed. It seems therefore very
unlikely that carbophenothion induces delayed neurotoxicity.
In a subacute study with dogs a no-effect level, based on
cholinesterase inhibition, of 0.02 mg/kg bw/day was confirmed,
assuring there was no reason to alter the previously established ADI
for man.
In response to requests listed as desirable by the 1977 Joint Meeting,
a carbophenothion metabolism study on orange trees has been submitted
and evaluated. Additional information on national use patterns and
tolerances was also submitted.
In the metabolism study using greenhouse-grown orange trees (14C
phenyl) carbophenothion was applied to selected fruits and foliage,
which were harvested for analysis at 0, 3, 5, 10, 26 and 42 days. It
was found that initial residues were almost entirely surface residues
with a high initial rate of loss, attributed primarily to
volatilisation. After some time, internal residues became the highest
percentage of the total terminal residue in foliage and peels. There
was little translocation of residues from treated foliage to adjacent
untreated foliage and fruit, although there was no basis on which to
determine how much of the total terminal residues would be in the
fruit and foliage not adjacent to that treated. There is minimal
translocation of residues on treated fruit into the pulp and juice.
Of the individual components identified in the residue,
carbophenothion was at the highest concentration followed by its
sulphoxide and sulphone. The only exception was the external foliage
residue at 42 days from application where the carbophenothion residue
was exceeded by both that of its sulphoxide and sulphone. Residues of
the oxon and its sulphoxide and sulphone occurred in lesser amounts.
In addition to the identified carbophenothion metabolites, relatively
large amounts of unidentified compounds remained, especially in the
external foliage residue where polar unknowns constituted the largest
single part of the terminal surface residue (1.62% of the applied
radioactivity). This is however less than the 3.84% and 1.82%
respectively of carbophenothion and its sulphoxide found in foliage
internal organosolubles. A small portion of the residue was bound
into insoluble components. There was no evidence of the S-methylation
of the 4-chlorothiophenol moiety that has been observed in animals. A
significant proportion of the internal residues was water soluble, and
similarly some of these were identified while others were not. Among
those identified were carbophenothion sulphoxide and sulphone and,
tentatively, the 4-chlorobenzene sulphinic and sulphonic acids. The
latter two have not been previously reported at Joint Meetings as
plant metabolites although they are known to be animal metabolites.
This study is consistent with earlier findings on the metabolism of
carbophenothion in plants and adds significantly to the understanding
of plant metabolism. No additional information was provided to
elucidate the terminal residues or photolysis products of
carbophenothion under field conditions, as previously considered
desirable. However, the metabolism study provided data that, along
with information previously evaluated, enabled the Meeting to conclude
that unidentified terminal residues or photolysis products under field
conditions are unlikely to be more than a very small portion of the
total residues. The meeting confirms that residues currently measured
are sufficient.
Level causing no toxicological effects
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw/day.
Dog: 0.02 mg/kg bw/day.
Human: 0.8 mg/human/day corresponding to 0.01 mg/kg bw/day.
Estimate of acceptable daily intake for man
0 - 0.0005 mg/kg bw/day.
RECOMMENDATION
It is not necessary to revise limits or the expression of limits
previously recommended.
REFERENCES
Bier, C.B. A subacute (21-day) oral toxicity study of Trithion in the
beagle dog. Bio-Research Laboratories TLD, (1979) Unpublished report
submitted to WHO by Stauffer Chemical Company, USA. T-10015.
McBain, J.B., Wren, J.P. and Menn, J.J. Metabolism of 14C phenyl
Carbophenothion by Orange Trees. (1977) Unpublished study provided by
Stauffer Chemical Company, Mountain View Research Center, Mountain
View, Ca. 94042. MRC-B-69, July, 1977.
Menn, J.J., De Baun, J.R., Hoffman, L.J. and Ross, J.H. Metabolism of
thioaryl organo-phosphorus insecticides. Environmental quality and
safety, Supp. Vol III, Pesticides, 382-388, (1975).
Menn, J.J., De Baun, J.R. and McBain, J.B. Recent Advances in the
Metabolism of Organo-phosphorus Insecticides. Fed. Proc. 35, 2598.
Miller, J.L. and Sprague, G.L. Acute delayed neurotoxicity study with
technical TrithionR in adult hens. Report Woodward Research
Corporation, 1978 (unpublished) T-6404.
See Also:
Toxicological Abbreviations
Carbophenothion (ICSC)
Carbophenothion (WHO Pesticide Residues Series 2)
Carbophenothion (Pesticide residues in food: 1976 evaluations)
Carbophenothion (Pesticide residues in food: 1977 evaluations)
Carbophenothion (Pesticide residues in food: 1979 evaluations)
Carbophenothion (Pesticide residues in food: 1983 evaluations)
 NATIONAL MAXIMUM RESIDUES LIMITS
The Netherlands reported the following national tolerances to the
Meeting.
Commodity Tolerance (mg/kg)
In force fruits and vegetables 0.01
Under consideration fruit 0.0511/
other commodities
of plant origin 0.05
1/ At or about the limit of determination.
EVALUATION
COMMENTS AND APPRAISAL
Present carbophenothion metabolism studies confirm and extend the
experiments previously reported. Both carbophenothion and its
sulphoxide are metabolised both quantitatively and qualitatively very
similarly. The acute oral toxicity of the majority of the metabolites
of carbophenothion in the rat have been evaluated previously. They
are considerably less toxic than that of the present compound. These
results indicate that carbophenothion is metabolised to less toxic
compounds.
In an acute delayed neurotoxicity study with technical carbophenothion
at an extremely high dose one adult hen out of 14 showed minimal leg
weakness, accompanied by nerve fibre degeneration. However, no
specific histopathological lesions were observed in spinal cord and
medulla. Furthermore, in a previous study with adult hens no
neurotoxic effects had been observed. It seems therefore very
unlikely that carbophenothion induces delayed neurotoxicity.
In a subacute study with dogs a no-effect level, based on
cholinesterase inhibition, of 0.02 mg/kg bw/day was confirmed,
assuring there was no reason to alter the previously established ADI
for man.
In response to requests listed as desirable by the 1977 Joint Meeting,
a carbophenothion metabolism study on orange trees has been submitted
and evaluated. Additional information on national use patterns and
tolerances was also submitted.
In the metabolism study using greenhouse-grown orange trees (14C
phenyl) carbophenothion was applied to selected fruits and foliage,
which were harvested for analysis at 0, 3, 5, 10, 26 and 42 days. It
was found that initial residues were almost entirely surface residues
with a high initial rate of loss, attributed primarily to
volatilisation. After some time, internal residues became the highest
percentage of the total terminal residue in foliage and peels. There
was little translocation of residues from treated foliage to adjacent
untreated foliage and fruit, although there was no basis on which to
determine how much of the total terminal residues would be in the
fruit and foliage not adjacent to that treated. There is minimal
translocation of residues on treated fruit into the pulp and juice.
Of the individual components identified in the residue,
carbophenothion was at the highest concentration followed by its
sulphoxide and sulphone. The only exception was the external foliage
residue at 42 days from application where the carbophenothion residue
was exceeded by both that of its sulphoxide and sulphone. Residues of
the oxon and its sulphoxide and sulphone occurred in lesser amounts.
In addition to the identified carbophenothion metabolites, relatively
large amounts of unidentified compounds remained, especially in the
external foliage residue where polar unknowns constituted the largest
single part of the terminal surface residue (1.62% of the applied
radioactivity). This is however less than the 3.84% and 1.82%
respectively of carbophenothion and its sulphoxide found in foliage
internal organosolubles. A small portion of the residue was bound
into insoluble components. There was no evidence of the S-methylation
of the 4-chlorothiophenol moiety that has been observed in animals. A
significant proportion of the internal residues was water soluble, and
similarly some of these were identified while others were not. Among
those identified were carbophenothion sulphoxide and sulphone and,
tentatively, the 4-chlorobenzene sulphinic and sulphonic acids. The
latter two have not been previously reported at Joint Meetings as
plant metabolites although they are known to be animal metabolites.
This study is consistent with earlier findings on the metabolism of
carbophenothion in plants and adds significantly to the understanding
of plant metabolism. No additional information was provided to
elucidate the terminal residues or photolysis products of
carbophenothion under field conditions, as previously considered
desirable. However, the metabolism study provided data that, along
with information previously evaluated, enabled the Meeting to conclude
that unidentified terminal residues or photolysis products under field
conditions are unlikely to be more than a very small portion of the
total residues. The meeting confirms that residues currently measured
are sufficient.
Level causing no toxicological effects
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw/day.
Dog: 0.02 mg/kg bw/day.
Human: 0.8 mg/human/day corresponding to 0.01 mg/kg bw/day.
Estimate of acceptable daily intake for man
0 - 0.0005 mg/kg bw/day.
RECOMMENDATION
It is not necessary to revise limits or the expression of limits
previously recommended.
REFERENCES
Bier, C.B. A subacute (21-day) oral toxicity study of Trithion in the
beagle dog. Bio-Research Laboratories TLD, (1979) Unpublished report
submitted to WHO by Stauffer Chemical Company, USA. T-10015.
McBain, J.B., Wren, J.P. and Menn, J.J. Metabolism of 14C phenyl
Carbophenothion by Orange Trees. (1977) Unpublished study provided by
Stauffer Chemical Company, Mountain View Research Center, Mountain
View, Ca. 94042. MRC-B-69, July, 1977.
Menn, J.J., De Baun, J.R., Hoffman, L.J. and Ross, J.H. Metabolism of
thioaryl organo-phosphorus insecticides. Environmental quality and
safety, Supp. Vol III, Pesticides, 382-388, (1975).
Menn, J.J., De Baun, J.R. and McBain, J.B. Recent Advances in the
Metabolism of Organo-phosphorus Insecticides. Fed. Proc. 35, 2598.
Miller, J.L. and Sprague, G.L. Acute delayed neurotoxicity study with
technical TrithionR in adult hens. Report Woodward Research
Corporation, 1978 (unpublished) T-6404.
NATIONAL MAXIMUM RESIDUES LIMITS
The Netherlands reported the following national tolerances to the
Meeting.
Commodity Tolerance (mg/kg)
In force fruits and vegetables 0.01
Under consideration fruit 0.0511/
other commodities
of plant origin 0.05
1/ At or about the limit of determination.
EVALUATION
COMMENTS AND APPRAISAL
Present carbophenothion metabolism studies confirm and extend the
experiments previously reported. Both carbophenothion and its
sulphoxide are metabolised both quantitatively and qualitatively very
similarly. The acute oral toxicity of the majority of the metabolites
of carbophenothion in the rat have been evaluated previously. They
are considerably less toxic than that of the present compound. These
results indicate that carbophenothion is metabolised to less toxic
compounds.
In an acute delayed neurotoxicity study with technical carbophenothion
at an extremely high dose one adult hen out of 14 showed minimal leg
weakness, accompanied by nerve fibre degeneration. However, no
specific histopathological lesions were observed in spinal cord and
medulla. Furthermore, in a previous study with adult hens no
neurotoxic effects had been observed. It seems therefore very
unlikely that carbophenothion induces delayed neurotoxicity.
In a subacute study with dogs a no-effect level, based on
cholinesterase inhibition, of 0.02 mg/kg bw/day was confirmed,
assuring there was no reason to alter the previously established ADI
for man.
In response to requests listed as desirable by the 1977 Joint Meeting,
a carbophenothion metabolism study on orange trees has been submitted
and evaluated. Additional information on national use patterns and
tolerances was also submitted.
In the metabolism study using greenhouse-grown orange trees (14C
phenyl) carbophenothion was applied to selected fruits and foliage,
which were harvested for analysis at 0, 3, 5, 10, 26 and 42 days. It
was found that initial residues were almost entirely surface residues
with a high initial rate of loss, attributed primarily to
volatilisation. After some time, internal residues became the highest
percentage of the total terminal residue in foliage and peels. There
was little translocation of residues from treated foliage to adjacent
untreated foliage and fruit, although there was no basis on which to
determine how much of the total terminal residues would be in the
fruit and foliage not adjacent to that treated. There is minimal
translocation of residues on treated fruit into the pulp and juice.
Of the individual components identified in the residue,
carbophenothion was at the highest concentration followed by its
sulphoxide and sulphone. The only exception was the external foliage
residue at 42 days from application where the carbophenothion residue
was exceeded by both that of its sulphoxide and sulphone. Residues of
the oxon and its sulphoxide and sulphone occurred in lesser amounts.
In addition to the identified carbophenothion metabolites, relatively
large amounts of unidentified compounds remained, especially in the
external foliage residue where polar unknowns constituted the largest
single part of the terminal surface residue (1.62% of the applied
radioactivity). This is however less than the 3.84% and 1.82%
respectively of carbophenothion and its sulphoxide found in foliage
internal organosolubles. A small portion of the residue was bound
into insoluble components. There was no evidence of the S-methylation
of the 4-chlorothiophenol moiety that has been observed in animals. A
significant proportion of the internal residues was water soluble, and
similarly some of these were identified while others were not. Among
those identified were carbophenothion sulphoxide and sulphone and,
tentatively, the 4-chlorobenzene sulphinic and sulphonic acids. The
latter two have not been previously reported at Joint Meetings as
plant metabolites although they are known to be animal metabolites.
This study is consistent with earlier findings on the metabolism of
carbophenothion in plants and adds significantly to the understanding
of plant metabolism. No additional information was provided to
elucidate the terminal residues or photolysis products of
carbophenothion under field conditions, as previously considered
desirable. However, the metabolism study provided data that, along
with information previously evaluated, enabled the Meeting to conclude
that unidentified terminal residues or photolysis products under field
conditions are unlikely to be more than a very small portion of the
total residues. The meeting confirms that residues currently measured
are sufficient.
Level causing no toxicological effects
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw/day.
Dog: 0.02 mg/kg bw/day.
Human: 0.8 mg/human/day corresponding to 0.01 mg/kg bw/day.
Estimate of acceptable daily intake for man
0 - 0.0005 mg/kg bw/day.
RECOMMENDATION
It is not necessary to revise limits or the expression of limits
previously recommended.
REFERENCES
Bier, C.B. A subacute (21-day) oral toxicity study of Trithion in the
beagle dog. Bio-Research Laboratories TLD, (1979) Unpublished report
submitted to WHO by Stauffer Chemical Company, USA. T-10015.
McBain, J.B., Wren, J.P. and Menn, J.J. Metabolism of 14C phenyl
Carbophenothion by Orange Trees. (1977) Unpublished study provided by
Stauffer Chemical Company, Mountain View Research Center, Mountain
View, Ca. 94042. MRC-B-69, July, 1977.
Menn, J.J., De Baun, J.R., Hoffman, L.J. and Ross, J.H. Metabolism of
thioaryl organo-phosphorus insecticides. Environmental quality and
safety, Supp. Vol III, Pesticides, 382-388, (1975).
Menn, J.J., De Baun, J.R. and McBain, J.B. Recent Advances in the
Metabolism of Organo-phosphorus Insecticides. Fed. Proc. 35, 2598.
Miller, J.L. and Sprague, G.L. Acute delayed neurotoxicity study with
technical TrithionR in adult hens. Report Woodward Research
Corporation, 1978 (unpublished) T-6404.
