
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 54
First draft prepared by R.G. Liteplo and M.E. Meek, Health Canada, Ottawa, Canada; and
M. Lewis, Environment Canada, Ottawa, Canada
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Ethylene oxide.
(Concise international chemical assessment document ; 54)
1.Ethylene oxide - toxicity 2.Risk assessment 3.Environmental exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153054 5 (LC/NLM Classification: QD 305.E7)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: ). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on ethylene oxide was prepared jointly by the Environmental Health Directorate of Health Canada and the Commercial Chemicals Evaluation Branch of Environment Canada, based on documentation prepared as part of the Priority Substances Program under the Canadian Environmental Protection Act (CEPA). The objective of assessments on priority substances under CEPA is to assess potential effects of indirect exposure in the general environment on human health as well as environmental effects. Data identified as of the end of May 1998 (environmental effects) and August 1999 (human health effects) were considered in this review.2 Information on the nature of the peer review and availability of the source document (Environment Canada & Health Canada, 2001) is presented in Appendix 2. Other reviews that were also consulted include ATSDR (1990), BUA (1995), IARC (1976, 1994), US EPA (1985), and an earlier EHC monograph on this chemical (IPCS, 1985). Information on the peer review of this CICAD is presented in Appendix 3. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Monks Wood, United Kingdom, on 16–19 September 2002. Participants at the Final Review Board meeting are presented in Appendix 4. The International Chemical Safety Card for ethylene oxide (ICSC 0155), produced by the International Programme on Chemical Safety (IPCS, 1999), has also been reproduced in this document.
Ethylene oxide (CAS No.
Production of ethylene oxide in the source country for this CICAD (i.e., Canada) in 1996 was 625 kilotonnes, 95% of which was used in the manufacture of ethylene glycol. An estimated 4% was used in the manufacture of surfactants. Ethylene oxide is also used as a sterilant for health care materials and other heat-sensitive products.
Most ethylene oxide is released to the atmosphere. Releases of ethylene oxide from natural sources, such as waterlogged soil, are expected to be negligible. In the source country (Canada), anthropogenic sources, not including sterilization, released an estimated 23.0 tonnes, all to the atmosphere, in 1996. An estimated additional 3.0 tonnes/year were lost to the atmosphere in 1996 from the servicing of medical facilities using ethylene oxide in sterilization processes and commercial sterilization operations.
Release of ethylene oxide to the atmosphere is unlikely to result in transfer to other environmental compartments in significant quantities. Atmospheric half-lives based on reaction with photogenerated hydroxyl radicals range from 38 to 382 days. In the event of release or spill to water, ethylene oxide is expected to be susceptible to evaporation, hydrolysis, and aerobic and, to a lesser extent, anaerobic biodegradation. In water, the volatilization half-life is about 1 h, the hydrolysis half-life about 12–14 days, the aerobic biodegradation half-life 20 days to 6 months, and the anaerobic biodegradation half-life 4 months to 2 years. In soil, ethylene oxide is expected to volatilize rapidly. Hydrolysis half-lives for soil and groundwater are estimated to be between 10.5 and 11.9 days. Ethylene oxide is not expected to bioaccumulate on the basis of its very low log octanol/water partition coefficient (Kow).
Ethylene oxide is rapidly taken up via the lungs, distributed, and metabolized to ethylene glycol and to glutathione conjugates. Ethylene oxide can be absorbed through the skin from the gas phase or from aqueous solutions and is uniformly distributed throughout the body. Ethylene oxide is an alkylating agent and forms protein and DNA adducts. Haemoglobin adducts have been used for biomonitoring.
The acute inhalation toxicity of ethylene oxide in rodents and dogs is low, with 4-h LC50s generally being greater than 1500 mg/m3. Available data on the non-neoplastic effects of repeated exposure to ethylene oxide in studies are limited, with past focus being primarily on the carcinogenicity of the compound. Reported effects in studies in animals were restricted primarily to those on the haematological and nervous systems.
Based on studies primarily in occupationally exposed populations, ethylene oxide is an ocular, respiratory, and dermal irritant and a sensitizing agent. Neurological effects (primarily sensorimotor polyneuropathy) have been observed in workers exposed to relatively high concentrations and in animals exposed to levels greater than those at which increases in tumours have been reported.
The route of likely greatest exposure and focus of the human health assessment is inhalation from air. Based on studies in animals, cancer is considered the critical end-point for effects of ethylene oxide on human health for long-term exposure of the general population. In inhalation studies, ethylene oxide has induced a wide range of tumours (e.g., leukaemia, lymphoma, brain, lung), with a strong likelihood that the mode of action involves direct interaction with genetic material, for which there is consistent and convincing evidence. While there is some evidence of an association between exposure to ethylene oxide and the development of haematological cancers in epidemiological studies of occupationally exposed populations, limitations of the data preclude definitive conclusions.
Ethylene oxide induces gene mutations at all phylogenetic levels tested in vitro and in vivo. It also induces germ cell mutations and clastogenic effects in experimental animals. There is consistent evidence that ethylene oxide has induced clastogenic changes in exposed workers.
In experimental animals, ethylene oxide is fetotoxic in the presence and absence of maternal toxicity at concentrations higher than those associated with cancer and other non-cancer (i.e., neurological) effects; it is teratogenic only at very high concentrations (above about 1600 mg/m3). Evidence from epidemiological studies of reproductive effects (primarily spontaneous abortions) of ethylene oxide in humans is limited. In experimental animals, among non-neoplastic effects, reproductive effects occur at lowest concentration (>90 mg/m3). These include reductions in litter size, increased post-implantation losses, alterations in sperm morphology, and changes in sperm count and motility.
Cancer is considered the critical end-point for quantification of exposure–response for risk characterization for ethylene oxide. The lowest concentration causing a 5% increase in tumour incidence above background (TC05) from a study in rats or mice that had optimal characterization of exposure–response was 2.2 mg/m3 (unit risk = 0.05/2.2 mg/m3 = 0.023 per mg/m3) for the development of mononuclear leukaemias in female F344 rats exposed via inhalation to ethylene oxide; the lower 95% confidence limit was 1.5 mg/m3. Primarily as a basis for comparison with the tumorigenic potencies for cancer, the concentration associated with a 5% increase in the incidence of germ cell mutations (BMC05) is also presented (46 mg/m3), although it is based on dominant visible mutations only and does not take into account other genetic end-points in live offspring. Similarly, tolerable concentrations based on observed neurological or reproductive effects would be in the range of tens of micrograms per cubic metre.
On this basis, predicted cancer risks in the vicinity of industrial point sources, based on limited modelling and monitoring data, are greater than 10–5.
Since ethylene oxide is expected to be present primarily in air, the potential for adverse effects is greatest for terrestrial organisms, for which available data are limited. The most significant end-point with the greatest potential to result in population-level effects in wildlife was the induction of adverse reproductive effects. Comparison of the worst-case average concentration in air with the estimated no-effects value indicates that it is unlikely that terrestrial organisms are exposed to harmful levels of ethylene oxide in air.
Ethylene oxide (CAS registry number
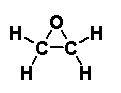
Fig. 1: Chemical structure of ethylene oxide
The molecular formula for ethylene oxide is H2COCH2, and its relative molecular mass is 44.05. At room temperature (25 °C) and normal atmospheric pressure, ethylene oxide is a colourless, highly reactive, and flammable gas with a characteristic ethereal odour. It has a high vapour pressure (~146 kPa) and high water solubility (completely miscible). It is reactive in both the liquid and vapour phases (IPCS, 1985). Table 1 summarizes the physical and chemical properties of ethylene oxide. Additional properties are presented in ICSC 0155, which is reproduced in this document.
Table 1: Physical and chemical properties of ethylene oxide.
|
Property |
Parameter |
Reference |
|
Boiling point (°C) |
10.7 |
WHO (1985) |
|
Vapour pressure (kPa) |
66 (0°C) |
Verschueren (1983) |
|
Henry’s law constant (Pa·m3/mol) |
14 |
BUA (1995) |
|
Sorption partition coefficient (log Koc) |
1.204 |
KemI (1995) |
|
Octanol/water partition coefficient (log Kow) |
–0.22 |
WHO (1985) |
|
Solubility in water (g/litre) |
infinitely soluble |
WHO (1985) |
The conversion factors3 for ethylene oxide in air (at 20 °C and 101.3 kPa) are as follows:
1 ppm = 1.83 mg/m3
1 mg/m3 = 0.55 ppm
The most common method for the identification of ethylene oxide in various media is gas chromatography (GC). GC with an electron capture detector (ECD) is often used to measure concentrations in workplace air. The sample is first adsorbed on hydrobromic acid-coated charcoal, desorbed with dimethylformamide, and then derivatized to 2-bromoethylheptafluorobutyrate for analysis. This method (NIOSH Method 1614) has an estimated detection limit of 1 µg ethylene oxide per sample (Eller, 1987a). The US Occupational Health and Safety Administration delineates a method with slight modification to sample collection: adsorption onto charcoal, desorption with a benzene:carbon disulfide solution, and conversion to 2-bromoethanol prior to analysis (Tucker & Arnold, 1984). NIOSH Method 3702 describes analysis with a portable gas chromatograph and a photoionization detector for ethylene oxide in workplace air. The sample is drawn directly into a syringe or collected as a bag sample and injected directly into the gas chromatograph. This method has an estimated detection limit of 2.5 pg/ml injection (Eller, 1987b). Passive samplers are also available for ethylene oxide sample collection.
Haemoglobin adducts of ethylene oxide (hydroxyethyl valine and hydroxyethyl histidine) have been determined by radioimmunological techniques, a modified Edman degradation procedure with GC/mass spectrometry (MS), GC with selective ion MS, and GC/ECD (IARC, 1994).
Ethylene oxide has been determined in emissions from production plants and commercial sterilizers by GC/flame ionization detection. GC and headspace GC have also been used to analyse ethylene oxide residues in sterilants, drugs, plastics, ethoxylated surfactants and demulsifiers, packaging materials, and processed food products (IARC, 1994).
Data on production, sources, and emissions primarily from the source country of the national assessment on which the CICAD is based (i.e., Canada) are presented here as an example. Sources and patterns of emissions in other countries are expected to be similar, although quantitative values may vary.
Ethylene oxide is produced from a few natural sources. In certain plants, ethylene (a natural plant growth regulator) is degraded to ethylene oxide (Abeles & Dunn, 1985). It is also a product of ethylene catabolism in certain microorganisms (De Bont & Albers, 1976). Ethylene oxide can be generated from waterlogged soil (Smith & Jackson, 1974; Jackson et al., 1978), manure, and sewage sludge (Wong et al., 1983). Quantitative estimates of production from these natural sources are not available, but emissions are expected to be negligible.
Canadian production of ethylene oxide was estimated at 625 kilotonnes in 1996, and the forecasted total estimated for 1999 was 682 kilotonnes (CIS, 1997).
Virtually all of the ethylene oxide produced is used as an intermediate in the production of various chemicals (ATSDR, 1990). In 1993, 89% of the total Canadian production of ethylene oxide was used in the production of ethylene glycol (SRI, 1993); in 1996, 95% was used for this purpose (CIS, 1997). An estimated 4% (26 kilotonnes) is used in the manufacture of surfactants (CIS, 1997). Ethylene oxide, alone or in combination with other gases, such as carbon dioxide and nitrogen, is used to sterilize instruments from the health care, publication, and wood products sectors. Ethylene oxide is also used in other industries where heat-sensitive goods are sterilized (How-Grant, 1991; BUA, 1995) and in the manufacture of choline chloride, glycol ethers, and polyglycols (CIS, 1997). Other minor uses worldwide include its application in the manufacture of rocket propellant and petroleum demulsifiers (Lewis, 1993).
Ethylene oxide is used for the control of insects in stored products and for the control of bacteria in spices and natural seasonings (J. Ballantine, personal communication, 1997). Ethylene oxide is also present as a formulant or component of a formulant in pest control products at concentrations up to 0.4%. The formulants include fungicides, insecticides, herbicides, and an adjuvant (J. Ballantine, personal communication, 1997).
Non-point sources of release of ethylene oxide include its formation from fossil fuel combustion (US EPA, 1984) and presence in tobacco smoke (Howard, 1989). Neither source is expected to be significant (US EPA, 1984). Ethylene oxide is used as a component in the production of polyoxyethylene surfactants (Gaskin & Holloway, 1992). Ethylene oxide in this form is bound within the surfactant molecule, and any release is expected to be minimal. Similarly, ethylene oxide may be present in nonylphenol ethoxylate formulations at concentrations below 10 mg/litre (Talmage, 1994), and ethylene oxide may remain as a contaminant at 10 mg/kg in liquid detergents. A variety of other products, including paints and coatings, were reported to contain ethylene oxide at levels ranging from trace to <0.5%.
Ethylene oxide is used for the control of insect (i.e., fumigation) and microbial (i.e., sterilization) infestations (Agriculture and Agri-Food Canada, 1996; Health Canada, 1999a; S. Conviser, personal communication, 1999). Following fumigation, concentrations of ethylene oxide generally fall to negligible levels within a few hours (IARC, 1976).
Gaseous and liquid forms of ethylene oxide can be released during production and use, as well as during the manufacture of ethylene glycol, ethoxylates, ethers, and ethanolamines (Howard, 1989). Ethylene oxide releases to the Canadian environment totalled 23.0 tonnes in 1996, with the reporting sectors being plastics and synthetics (0.24 tonnes), inorganic chemicals (6.1 tonnes), industrial organic chemicals (8.7 tonnes), and soap and cleaning compounds (8.0 tonnes) (NPRI, 1996). By 1997, emissions were reduced by 82% from the 1993 levels (ARET, 1999). An estimated additional 3.0 tonnes were lost to the atmosphere in 1996 from the servicing of medical facilities using ethylene oxide in sterilization processes and commercial sterilization operations.
Although sterilization is not a major use of ethylene oxide in terms of volumes consumed, it may be a very significant source of release to the environment (IPCS, 1985). Based on a survey of hospitals using ethylene oxide as a sterilizing agent, conducted in April 1994, the amount of ethylene oxide used as a sterilant was estimated to be 40 tonnes/year. Because many facilities now have improved control measures in place (Havlicek et al., 1992; Canadian Hospital Association & Environment Canada, 1994) and because of the use of alternative equipment that does not involve the use of ethylene oxide (S. Smyth-Plewes, personal communication, 1998), the current volumes of ethylene oxide used and released may be significantly less than the 1994 estimates.
In an examination of the primary US sources of ethylene oxide releases, sterilization/fumigation sites, production/captive use, medical facilities, and ethoxylation accounted for 57%, 31%, 8%, and 4% of total emissions, respectively (Markwordt, 1985). In an early US study, it was estimated that <0.1% of ethylene oxide produced is used as a sterilizing agent or fumigant, yet this accounts for the majority of ethylene oxide released into the atmosphere (Markwordt, 1985). Similarly, Berkopec & Vidic (1996) reported that in Slovenia, emissions to the atmosphere during sterilization were higher than emissions from other processes, such as synthesis of glycols and other derivatives in the chemical industry, although the sterilization process accounts for only 2% of total ethylene oxide use. In Belgium, an estimated 0.07% of the total consumption of ethylene oxide was used in sterilization operations in health care and medical products industries (Wolfs et al., 1983).
In facilities that have recirculating-water vacuum pumps, there is practically no loss of ethylene oxide through the water drain (Meiners & Nicholson, 1988; US EPA, 1992, 1994). In those facilities that use once-through water-sealed vacuum pumps, some ethylene oxide dissolved in the water will be directed to a floor drain and will likely volatilize to the atmosphere at an outdoor ground-level drain near the facility or wastewater treatment facility (US EPA, 1992; WCB, 1994).
Based on empirical data on fate, release of ethylene oxide to the atmosphere is unlikely to result in transfer to other environmental compartments in significant quantities. Reaction half-lives in the atmosphere may be significantly long (between 38 and 382 days). Although water solubility suggests that washout from the atmosphere by precipitation could be important, volatilization from the water is too rapid to suggest this to be a significant fate process. On the basis of a low log Kow (–0.30), the potential for bioaccumulation of ethylene oxide is considered to be very low. As a result of its high water solubility and vapour pressure, ethylene oxide is not expected to bioaccumulate or accumulate in sediment or soil.
The atmospheric half-lives for ethylene oxide following vapour-phase reactions with photochemically produced hydroxyl radicals, assuming an atmospheric concentration of 1 × 106 radicals/cm3, were estimated to be 120 days (Atkinson, 1986), 99 days (Lorenz & Zellner, 1984), 151 days (C. Zetzsch, personal communication, 1985, cited in Atkinson, 1986), and between 38 and 382 days (Howard et al., 1991).
The theoretical atmospheric lifetimes (approximately 1.43 × t˝) for ethylene oxide were estimated at ~200 days (Bunce, 1996) and 330 days (Winer et al., 1987) and were calculated based on the reaction with hydroxyl radicals at a concentration of 8.0 × 105 and 1.0 × 106 radicals/cm3, respectively. Such lifetimes are expected to be long enough to allow a very small percentage of the amount emitted to reach the stratosphere (Bunce, 1996).
Ethylene oxide has a very high water solubility (completely miscible), which would suggest that some washout via precipitation can be expected; however, its high vapour pressure (~146 kPa) and rapid volatilization rate may limit the effectiveness of this process. An examination of the effect of atmospheric precipitation was conducted in a laboratory setting (Winer et al., 1987), resulting in evidence that washout has little impact on reducing atmospheric concentrations.
Ethylene oxide is expected to undergo numerous fate processes in water, including evaporation, hydrolysis, and aerobic and anaerobic degradation. The reported experimental aquatic half-life for evaporation of ethylene oxide in water is 1 h with no wind and 0.8 h with a 5 m/s wind (Conway et al., 1983). Ethylene oxide degrades in water by hydrolysis and other nucleophilic reactions (US EPA, 1985). Ethylene oxide is hydrolysed in fresh water to ethylene glycol and in salt water to ethylene glycol and ethylene chlorohydrin. The half-life was estimated experimentally to be 12–14 days for hydrolysis at pH 5–7 in fresh water and 9–11 days for hydrolysis in salt water (Conway et al., 1983). The aqueous aerobic biodegradation half-life of ethylene oxide was approximately 20 days from a lightly seeded biological oxygen demand (BOD) test, and the rate in a biological waste treatment system is expected to be much faster (Conway et al., 1983). Based on the BOD test results of Bridié et al. (1979a) and Conway et al. (1983), Howard et al. (1991) estimated the unacclimated aqueous biodegradation half-life to be from 1 to 6 months. The aqueous anaerobic half-life, based on the estimated aerobic biodegradation half-life, is 4–24 months (Howard et al., 1991). The 5-day BOD was 3% of the theoretical oxygen demand of 1.82 g/g (Bridié et al., 1979a).
Ethylene oxide is miscible in water and poorly adsorbed to soil; however, owing to its high vapour pressure (146 kPa), a spill of ethylene oxide to soil will result in most volatilizing to the atmosphere, with only a small fraction infiltrating the soil. Evaporation will continue within the soil, but at a reduced rate (Environment Canada, 1985). Dilution with water will reduce the velocity at which the ethylene oxide moves downward and at the same time diminish the vapour pressure and reduce the rate of evaporation. Upon reaching the groundwater table, ethylene oxide will move in the direction of groundwater flow. The half-lives for hydrolysis in groundwater and soil are estimated to be between 10.5 and 11.9 days, based on measured rate constants at pH 5, 7, and 9 (Mabey & Mill, 1978; Howard et al., 1991). In general, volatilization is the primary removal mechanism, but ethylene oxide is expected to hydrolyse and be biodegraded relatively rapidly in most soils.
Information on the environmental fate of ethylene oxide in sediment has not been identified. Because of its physical and chemical properties, ethylene oxide is not expected to be sorbed by sediment or soil.
Reported levels of ethylene oxide in environmental biota have not been identified. On the basis of a low log Kow (–0.30), the potential for bioaccumulation of ethylene oxide is expected to be very low (Verschueren, 1983; Howard, 1989).
Fugacity modelling was conducted to characterize key reaction, intercompartment, and advection (movement out of a system) pathways for ethylene oxide and its overall distribution in the environment in the source country (Canada). A steady-state, non-equilibrium model (Level III fugacity model) was run using the methods developed by Mackay (1991) and Mackay & Paterson (1991). All physical and chemical property input values were selected from a compilation of literature values based on criteria for integrity (for details, see DMER & AEL, 1996).
Based on the ChemCAN Level III fugacity model, which depicts a mixedwood plain region of a densely populated area of southern Ontario, it is estimated that ethylene oxide will have an overall persistence of 3 days in that region from a reaction persistence estimated at 70 days. Owing to its short overall persistence, higher ethylene oxide concentrations will likely be centralized in areas close to discharges. Based on the 1993 release volume of 53 200 kg to the atmosphere in southern Ontario (NPRI, 1993), the average steady-state levels in the southern Ontario region are estimated to be 1.02 ng/m3 in air (344 kg), 0.067 ng/litre in water (99.0 kg), 6.03 × 10–5 ng/g in soil (0.858 kg), and 3.27 × 10–5 ng/g in sediment (0.034 kg). Bioaccumulation is not expected (DMER & AEL, 1996).
The concentrations of ethylene oxide predicted above are based on the assumption that air entering southern Ontario from neighbouring regions contains no ethylene oxide. Estimates of concentrations of ethylene oxide in air in the 48 contiguous states of the USA, derived from atmospheric dispersion modelling and US emission inventories, are available (Woodruff et al., 1998). Mean concentrations predicted for 1990 in Michigan and New York, which border southern Ontario, were 4.9 ng/m3 and 5.9 ng/m3, respectively. When the average of these concentrations was assumed for the concentration of ethylene oxide in air advected into southern Ontario, the concentrations predicted by the ChemCAN model increased approximately 6-fold, to 6.2 ng/m3 in air, 0.4 ng/litre in water, 3.7 × 10–4 ng/g in soil, and 2.0 × 10–4 ng/g in sediment. Concentrations predicted in the ChemCAN auxiliary compartments of terrestrial animals and terrestrial plants were 4.3 × 10–5 ng/g and 1.4 × 10–3 ng/g, respectively, when this additional advective input was included in the fugacity modelling (Health Canada, 1999a).
Data on concentrations in the environment from the source country of the national assessment on which the CICAD is based (i.e., Canada) are presented here as a basis for the sample risk characterization. Patterns of exposure in other countries are expected to be similar, although quantitative values may vary.
Data on concentrations of ethylene oxide in emissions or ambient air are very limited.
Ethylene oxide was detected at concentrations of 3.7, 3.9, and 4.9 µg/m3 in 3 of 50 24-h samples of air collected outside of randomly selected residences during a multimedia exposure study conducted in Canada (Health Canada, 1999a). The censored mean value was 0.34 µg/m3 when a concentration equivalent to one-half the limit of detection (i.e., ˝ × 0.19 µg/m3 = 0.095 µg/m3) was assumed for the 47 samples in which ethylene oxide was not detected. Ethylene oxide was detected at 3 (or 33%) of 9 locations in Alberta, but at none of the 35 locations in Ontario or the 6 locations in Nova Scotia during this study (Health Canada, 1999a).
Based on data on 1993 air quality modelling predictions from a Canadian production facility, (Environment Canada, 1997), it was estimated that 1-h average ground-level concentrations of ethylene oxide would exceed 12 µg/m3 for a total of 17 h a year in the immediate vicinity of the plant. Predicted maximum 1-h-average ground-level concentrations ranged from 3.7 to 20.1 µg/m3 at distances of 5 km and 2.7 km from the plant, respectively. No measurements were available to validate these predictions.
Estimated maximum average daily concentrations of ethylene oxide in the vicinity of Canadian hospitals were 0.26, 0.83, 1.3, and 2.12 µg/m3 between 100 and 70 m from the emission source and from stack heights of 30 m, 18 m, 15 m, and 12 m, respectively (Environment Canada, 1999). Concentrations closer to or farther from the source are predicted to be less. The estimate was based on the US EPA "SCREEN3" Gaussian plume model, which incorporates source-related and meteorological factors to estimate pollutant concentrations from continuous sources. The model assumes that the pollutant does not undergo any chemical reactions and that no other removal processes, such as wet or dry deposition, act on the plume during its transport from the source (for input parameters, see US EPA, 1995).
In an assessment of emissions and concentrations of ethylene oxide throughout California, USA, mean 24-h ambient air concentrations sampled in Los Angeles ranged from 0.038 to 955.7 µg/m3 (n = 128) (Havlicek et al., 1992). The authors reported that heavy usage of ethylene oxide within the Los Angeles basin coupled with restricted airflow out of the basin likely led to the large range of concentrations. There was a high degree of local variability that would be consistent with the release of ethylene oxide during a sterilization cycle. Concentrations in air sampled in northern California ranged from 0.032 to 0.40 µg/m3 (n = 36). At remote coastal locations in California, concentrations ranged from 0.029 to 0.36 µg/m3 (n = 22). The authors cautioned that it was not possible to draw definitive conclusions regarding the spatial and temporal distribution of ethylene oxide based on the samples collected. Levels were highly variable, especially in urban areas, with 100-fold shifts in airborne concentrations over a few minutes.
Peak short-term and long-term ambient concentrations of ethylene oxide as a result of emissions from four sterilization facilities in Duval County, Florida, USA, were estimated based on the US EPA SCREEN and Industrial Source Complex Short-Term dispersion models (Tutt & Tilley, 1993). These included a commercial spice fumigation facility (with estimated annual emissions of 1959.5 kg ethylene oxide) and three hospitals of decreasing emission profiles (i.e., from 210.9 to 2.1 kg/year). The predicted maximum average annual concentrations from the two highest emitters were 11 µg/m3 for a sterilization facility in Florida and 2 µg/m3 in the vicinity of a hospital in Florida, both at a distance of 32 m from their respective point sources.
Ethylene oxide was detected at a concentration of 4 µg/m3 in only 1 of 50 24-h samples of air collected inside randomly selected residences during a multimedia exposure study conducted in Canada (Health Canada, 1999a). The censored mean value was 0.17 µg/m3 when a concentration equivalent to one-half the limit of detection (i.e., ˝ × 0.19 µg/m3 = 0.095 µg/m3) was assumed for the 49 samples in which ethylene oxide was not detected. Ethylene oxide was detected at concentrations of 5 µg/m3 in 3 of 24 personal air samples collected from an occupant of each of the 50 residences (Conor Pacific Environmental, 1998).
Data on concentrations of ethylene oxide in drinking-water, surface water, groundwater, sediment, soil, or biota were not identified.
Ethylene oxide was detected in 96 (or 47%) of 204 samples of food products taken from retail stores in Denmark in 1985 (Jensen, 1988). The reported concentrations reflect the total amount of ethylene chlorohydrin and ethylene oxide present at the time of analysis. These concentrations ranged from <0.05 to 1800 µg/g in the individual samples, without correction for recoveries. Ethylene oxide was detected frequently among 24 samples of spices (Jensen, 1988), at a mean concentration of 84 µg/g and a maximum concentration of 580 µg/g.
Ethylene oxide was detected, but not quantified, in 1 of 2372 samples of eggs and in 1 of 3262 samples of fish collected in the USA in 1975 (Duggan et al., 1983).
Ethylene oxide may be present in tobacco as a result of its use as a fumigant and sterilizing agent (ATSDR, 1990). It has been detected in smoke from fumigated and unfumigated tobacco at levels of 0.3 µg/ml and 0.02 µg/ml, respectively (Binder, 1974).
Ethylene oxide may also be present as a contaminant of skin care products. Current commercial preparations of polyglycol ethers may contain residues of ethylene oxide monomer up to approximately 1 µg/g, according to a European study (Filser et al., 1994). Kreuzer (1992) reported concentrations of ethylene oxide monomer in skin care products ranging from 1.9 to 34 nmol/cm3 (0.08–1.5 mg/litre) and a range of maximum skin penetration of ethylene oxide of 1.0–14% in various product formulations.
Ethylene oxide is the most common agent currently used for sterilizing disposable dialysers, blood tubing, and heat-sensitive medical items (Henne et al., 1984; Babich, 1985). Ethylene oxide may be absorbed by medical equipment during sterilization and may remain there as the unchanged compound or as one of its reaction products (IPCS, 1985). Concentrations of residual ethylene oxide in medical devices immediately following their sterilization have ranged up to 1 or 2% (Gillespie et al., 1979; Gilding et al., 1980). These concentrations generally declined rapidly after a few days’ aeration, although levels exceeding 180 mg/m3 were sometimes measured following aeration.
The focus of this section and the basis for risk characterization is exposure in air, for which there are at least some data as a basis to estimate exposure. This is justified on the basis that most ethylene oxide is released to air and is unlikely to be transferred to other media. Moreover, ethylene oxide is not expected to accumulate in sediment or soil or to bioaccumulate, as a result of its high water solubility and vapour pressure.
The concentration of ethylene oxide predicted for ambient air (i.e., 6.2 × 10–3 µg/m3) by ChemCAN fugacity modelling, assuming that air entering southern Ontario from the USA contains ethylene oxide at a concentration of 5.4 × 10–3 µg/m3, was considered the basis for the minimum estimate of exposure via inhalation. Censored mean concentrations of ethylene oxide in outdoor and indoor air (i.e., 0.34 µg/m3 and 0.17 µg/m3, respectively), derived from the multimedia exposure study, were considered to represent the maximum concentrations to which the general population is exposed daily outdoors and indoors, respectively. Upper-bounding estimates of exposure via inhalation for the general population in Canada were based upon the maximum concentrations of ethylene oxide in outdoor and indoor air (i.e., 4.9 µg/m3 and 4.0 µg/m3, respectively) reported from the multimedia exposure study (Conor Pacific Environmental, 1998). Mean concentrations in ambient air sampled in Los Angeles, California, ranged from 0.038 to 955.7 µg/m3 (Havlicek et al., 1992).
Exposure to ethylene oxide in ambient air may be substantially higher for populations residing in the vicinity of point sources. An ethylene oxide concentration of 2 µg/m3 was predicted for outdoor air in close proximity to hospitals in Canada (Environment Canada, 1999) and Florida (Tutt & Tilley, 1993). A concentration of 11 µg ethylene oxide/m3 was predicted for outdoor air in close proximity to a sterilization facility in Florida (Tutt & Tilley, 1993). A maximum 1-h concentration of 20.1 µg ethylene oxide/m3 was predicted for outdoor air near a production facility for ethylene glycol in Alberta (Environment Canada, 1997).
Limitations of the data preclude development of meaningful probabilistic estimates of exposure of the general population to ethylene oxide in air.
Workers may be exposed to ethylene oxide during its production or use in the manufacture of other chemicals. Because ethylene oxide is highly explosive and reactive, the processing equipment generally consists of tightly closed and highly automated systems, which limit occupational exposure. Exposures occur primarily during the loading or unloading of transport tanks, product sampling procedures, and equipment maintenance and repair (CHIP, 1982). The Toxic Chemical Release Inventory listed 197 industrial facilities that produced, processed, or otherwise used ethylene oxide in 1988 (US EPA, 1990).
Industrial workers may also be exposed to ethylene oxide during sterilization of a variety of products, such as medical equipment and products (surgical products, single-use medical devices, etc.), disposable health care products, pharmaceutical and veterinary products, spices, and animal feed. Although much smaller amounts of ethylene oxide are used in sterilizing medical instruments and supplies in hospitals and for the fumigation of spices, it is during these uses that the highest occupational exposure levels have been measured (IARC, 1994). There was a wide range in reported concentrations (from 0 to about 1500 mg/m3), depending on operation, conditions, and duration of sampling for workers in US hospitals where ethylene oxide is used as a gaseous sterilant for heat-sensitive medical items, surgical instruments, and other objects and fluids coming in contact with biological tissues. Based on a limited field survey of hospitals, it was reported that concentrations of ethylene oxide near malfunctioning or improperly designed equipment may reach transitory levels of hundreds or even a few thousand milligrams per cubic metre, but time-weighted average (TWA) ambient and breathing zone concentrations were generally below about 90 mg/m3 (CHIP, 1982).
Information on the kinetics and metabolism of ethylene oxide has been derived primarily from studies conducted with laboratory animals exposed via inhalation, although some limited data from humans have been identified.
Ethylene oxide is very soluble in blood, and pulmonary uptake is expected to be rapid, dependent only upon the alveolar ventilation rate and concentration in the inspired air (IPCS, 1985). There is a lack of quantitative data on the absorption of ethylene oxide in various species; however, studies have revealed that ethylene oxide is absorbed rapidly through the respiratory tract in rats (Filser & Bolt, 1984; Koga et al., 1987; Tardif et al., 1987), mice (Ehrenberg et al., 1974; Tardif et al., 1987), and rabbits (Tardif et al., 1987). Ehrenberg et al. (1974) estimated that close to 100% of the inhaled dose of ethylene oxide was absorbed by mice exposed for 1–2 h to average concentrations between 2 and 55 mg/m3.
Quantitative information on the absorption of ethylene oxide in laboratory animals following ingestion or dermal exposure was not identified.
Ethylene oxide and its metabolites are rapidly distributed throughout the body. In the study performed by Ehrenberg et al. (1974) with mice exposed to [14C]ethylene oxide at concentrations of 2–55 mg/m3 for between 1 and 2 h, the greatest amount of radioactivity was detected in the liver, kidneys, and lungs, with smaller amounts in the spleen, testes, and brain. In rats exposed to [14C]ethylene oxide vapour at concentrations of 18.3, 183, or 1830 mg/m3 for 6 h (estimated mean absorbed doses of 2.7, 20.2, and 106.8 mg/kg body weight, respectively), the greatest amounts of radioactivity were found in the urinary bladder, liver, packed blood cells, and adrenal glands; the lowest levels were in fat (Tyler & McKelvey, 1982).
Ehrenberg et al. (1974) reported that in mice, about 78% of an inhaled dose was eliminated in the urine within 48 h, with the majority excreted within the first 24 h. Tyler & McKelvey (1982) observed that at all exposure levels tested, the primary route of [14C]ethylene oxide elimination in rats following inhalation exposure was in the urine (mean value of 59% recovered radioactivity), with lesser amounts expired as carbon dioxide (12%) and ethylene oxide (1%) or eliminated in the faeces (4.5%). After a single dose of 2 mg [14C]ethylene oxide/kg body weight (in propanediol) was administered intraperitoneally to rats, 43% of the radioactivity was excreted in the urine within 50 h, most (approximately 40%) appearing within the first 18 h following injection (Jones & Wells, 1981); 9% was identified as S-(2-hydroxyethyl)cysteine, and 33% was identified as N-acetyl-S-(2-hydroxyethyl)cysteine (both products of glutathione conjugation). In addition, 1.5% was exhaled via the lungs as carbon dioxide, and 1% was exhaled as unmetabolized ethylene oxide.
Brown et al. (1996) examined the distribution and elimination of ethylene oxide in rats and mice following inhalation exposure. Loss of ethylene oxide from the blood (and other tissues) was approximately 3- to 4-fold faster in mice than in rats. Following exposure, similar levels of ethylene oxide were measured in the brain, blood, and muscle within each species. However, concentrations in the testes of rats were 20% of those in other tissues; in mice, levels in the testes were 50% of those measured in other tissues.
In animals and humans, there are two routes of ethylene oxide catabolism, both of which are considered to be detoxification pathways. The first involves hydrolysis to ethylene glycol, with subsequent conversion to oxalic acid, formic acid, and carbon dioxide. The second involves conjugation with glutathione, with subsequent metabolic steps yielding S-(2-hydroxyethyl)cysteine [S-(2-carboxymethyl)cysteine] and N-acetylated derivatives (i.e., N-acetyl-S-(2-hydroxyethyl)cysteine [and N-acetyl-S-(2-carboxymethyl)cysteine]) (Wolfs et al., 1983; IPCS, 1985; ATSDR, 1990; Popp et al., 1994). Based upon available data, the route involving conjugation with glutathione appears to predominate in rats and mice; in larger species (rabbits, dogs), the conversion of ethylene oxide is primarily via hydrolysis through ethylene glycol (Jones & Wells, 1981; Martis et al., 1982; Gérin & Tardif, 1986; Tardif et al., 1987; Brown et al., 1996). Ethylene oxide may also be formed from the metabolism of ethylene (IARC, 1994).
A physiologically based pharmacokinetic (PBPK) model for the dosimetry of inhaled ethylene oxide was first developed for rats and included binding of ethylene oxide to haemoglobin and DNA in addition to tissue distribution, metabolic pathways (i.e., hydrolysis by epoxide hydrolase and conjugation by glutathione-S-transferase), and depletion of hepatic and extra-hepatic glutathione (Krishnan et al., 1992). The model was then refined and extended to mice and humans (Fennell & Brown, 2001). Simulations indicate that in mice, rats, and humans, about 80%, 60%, and 20%, respectively, would be metabolized via glutathione conjugation (Fennell & Brown, 2001).
This is consistent with observed levels of theta-class glutathione S-transferase (GSTT1) enzyme activity in the order mice > rats > humans.4 In rats and mice, GSTT1 activity was highest in the liver, followed by the kidney and testes. Rat brain and rat and mouse lung contained small amounts of activity compared with other tissues (enzyme activity in mouse brain was not examined).5 Ethylene oxide is a substrate for the human GSTT1 enzyme (Hallier et al., 1993; Pemble et al., 1994; Hayes & Pulford, 1995).
Ethylene oxide is an electrophilic agent that alkylates nucleophilic groups in biological macromolecules, including DNA and proteins. In haemoglobin, for example, adducts can be formed at cysteine residues, N-terminal valine, as well as N’- and N’-histidine (Segerbäck, 1990). Since ethylene oxide is formed during the metabolism of ethylene, a natural body constituent, endogenous as well as exogenous sources of ethylene and ethylene oxide contribute to background alkylation of proteins such as haemoglobin and albumin, as well as DNA (Bolt, 1996). N-(2-Hydroxyethyl)valine (HEVal) and hydroxyethylhistidine (HEHis) adducts have been frequently monitored in tissues of workers exposed to ethylene oxide in occupational settings (see IARC, 1994). Background levels of HEVal in non-smokers ranged from 9 to 188 pmol/g globin (Törnqvist et al., 1986, 1989; Bailey et al., 1988; Hagmar et al., 1991; Sarto et al., 1991; Tates et al., 1991, 1992; van Sittert et al., 1993; van Sittert & van Vliet, 1994; Farmer et al., 1996; Granath et al., 1996). Ethylene oxide binding to DNA results primarily in the formation of 7-(2-hydroxyethyl)guanine (7-HEGua) (Föst et al., 1989; Li et al., 1992); other adducts have also been identified at much lower levels. In DNA extracted from the lymphocytes of unexposed individuals, mean background levels of 7-HEGua ranged from 2 to 8.5 pmol/mg DNA (Föst et al., 1989; Bolt et al., 1997). Although these levels were similar to those measured in rodents not exposed to ethylene oxide (Föst et al., 1989; Walker et al., 1992), Wu et al. (1999a), using a more sensitive technique, reported that human tissue contains 10- to 15-fold higher levels of endogenous 7-HEGua than rodent tissue.
Studies of smokers exposed to ethylene oxide in cigarette smoke (Fennell et al., 2000) and occupationally exposed workers (Yong et al., 2001) have revealed higher levels of haemoglobin HEVal adducts among individuals with a GSTT1 "null genotype" (i.e., homozygous deletion of GSTT1 gene) than among those with a GSTT1 "positive genotype" (i.e., having at least one copy of the GSTT1 gene). In mice, half-lives for the removal of 7-HEGua in DNA from a variety of tissues (brain, lung, spleen, liver, and testes) were 1.5- to 3.9-fold lower than in rats (Walker et al., 1992). In both rats and mice, substantive depletion of glutathione pools has been observed following single exposure to high levels (i.e., >550 mg/m3) of ethylene oxide (McKelvey & Zemaitis, 1986; Brown et al., 1998), although it should be noted that increases in tumour incidence have been observed at lower concentrations. Reports on two PBPK models for ethylene oxide in rodents and humans have recently appeared (Csanády et al., 2000; Fennell & Brown, 2001). The models for rats, mice, and humans are qualitatively similar in their elements and provide for interspecies comparisons of internal ethylene oxide dose. The models are consistent with the conclusion that ethylene oxide is acting as a direct-acting alkylating agent in humans and rodents. Quantitative differences in response in biomarkers of exposure and effect are accounted for by differences in basic physiology between rodents and humans, rather than by factors suggesting a different mode of action.
Ethylene oxide is of low acute toxicity following inhalation, with 4-h LC50s of about 2700, 1500, and 1800 mg/m3 for rats, mice, and dogs, respectively (Jacobson et al., 1956). LD50s for ethylene oxide administered orally (in water) were 330 mg/kg body weight for male rats, 280 and 365 mg/kg body weight for female and male mice, respectively, and 270 mg/kg body weight for guinea-pigs (both sexes) (Smyth et al., 1941; Woodard & Woodard, 1971).
The lungs (oedema, congestion, haemorrhage) and nervous system (convulsions, prostration) are the principal organs affected following inhalation exposure to acutely toxic levels of ethylene oxide.
Available data on the repeated-dose toxicity of ethylene oxide are limited, being restricted primarily to inhalation studies in which animals were exposed to single concentrations.
Mortality was increased following the inhalation exposure of rats, mice, guinea-pigs, rabbits, and monkeys to concentrations of ethylene oxide ranging from about 730 to 1500 mg/m3 for 10 days to 8 weeks (Hollingsworth et al., 1956; Jacobson et al., 1956; Snellings, 1982; NTP, 1987). In rats exposed to ethylene oxide at concentrations between 180 and 915 mg/m3 for several weeks, there were haematological effects, changes in clinical chemistry, as well as histopathological alterations in various tissues (Jacobson et al., 1956; Snellings, 1982; Mori et al., 1990). Effects in mice exposed to ethylene oxide at 810 mg/m3 for 3 weeks included reduced body weight gain, poor coordination of the hindquarters, irregular breathing, convulsions, and red urine. In both rats and mice, body weight gain was reduced following repeated exposure to levels of ethylene oxide as low as 90 mg/m3 for approximately 7 weeks (Snellings, 1982).
Reductions in haemoglobin concentration, haematocrit, and red blood cell counts, accompanied by increases in reticulocytes, were observed in rats exposed to 915 mg ethylene oxide/m3 for 13 weeks (Fujishiro et al., 1990; Mori et al., 1990). This exposure regimen also produced declines in the activities of glutathione reductase and creatine kinase in blood and various tissues (Katoh et al., 1988, 1989; Matsuoka et al., 1990; Mori et al., 1990; Fujishiro et al., 1991), as well as increased hepatic lipid peroxidation (Katoh et al., 1988, 1989). Other effects observed in rats following medium-term exposure to ethylene oxide at concentrations ranging from 370 to 915 mg/m3 included those on the nervous system (Hollingsworth et al., 1956; Ohnishi et al., 1985, 1986; Matsuoka et al., 1990; Mori et al., 1990), disturbances in hepatic porphyrin-haem metabolism (Fujishiro et al., 1990), and histopathological changes in the testes, kidneys, and lungs (Hollingsworth et al., 1956). Effects in mice exposed to ethylene oxide are similar to those in rats (Snellings et al., 1984a; Popp et al., 1986); renal tubular degeneration was observed at concentrations as low as 183 mg/m3 (NTP, 1987). Exposure to concentrations as low as 86 mg/m3 reduced locomotor activity (Snellings et al., 1984a).
No differences in haematological parameters (red or white blood cell counts, haematocrit, haemoglobin, or white cell differential) were observed in rabbits exposed to 458 mg ethylene oxide/m3 for 12 weeks, compared with unexposed controls (Yager & Benz, 1982).
In the only short- or medium-term study identified on the oral toxicity of ethylene oxide, there was a loss of body weight, gastric irritation, and slight liver damage following exposure of rats to 100 mg ethylene oxide/kg body weight, 5 times/week, for a total of 15 doses in 21 days (Hollingsworth et al., 1956).
Non-neoplastic effects associated with long-term exposure to ethylene oxide have not been investigated extensively, most studies having focused on the carcinogenicity of this substance. In several investigations conducted with rats exposed to ethylene oxide for 2 years, significant reductions in body weight gain at concentrations as low as 60.4 mg/m3 and decreased survival time at exposures of >92 mg/m3 have been observed (Lynch et al., 1984a,b; Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986). Additional non-neoplastic effects observed at exposures of >92 mg ethylene oxide/m3 include increased levels of aspartate aminotransferase in serum, reduced absolute kidney and adrenal weights, an increased incidence of inflammatory lesions in the lungs, nasal cavity, trachea, and internal ear, proliferative and degenerative lesions in the adrenal cortex, increased splenic extramedullary haematopoiesis, as well as an increased incidence of multifocal mineralization of the posterior layers of the choroid/sclera portion of the eye (Lynch et al., 1984a,b). Skeletal muscular atrophy (in the absence of sciatic nerve neuropathy) was noted following exposure to 183 mg ethylene oxide/m3 (Lynch et al., 1984a,b).
No exposure-related effects upon survival, body weight gain, clinical signs, or other non-neoplastic end-points (examined in a wide range of tissues) were observed in B6C3F1 mice exposed to 92 or 183 mg ethylene oxide/m3 for 2 years (NTP, 1987).
Monkeys exposed for 2 years to >92 mg ethylene oxide/m3 developed axonal dystrophy in the nucleus gracilis of the medulla oblongata of the brain, along with demyelination in the distal portion of the fasciculus gracilis (Sprinz et al., 1982; Lynch et al., 1984b). Decreased nerve conduction velocity was observed in only 2 of 12 monkeys exposed to 183 mg/m3. Weight gain was significantly reduced following exposure to 183 mg ethylene oxide/m3 (Lynch et al., 1984a,b; Setzer et al., 1996). Lynch et al. (1992) indicated subsequently that in animals exposed to 0, 92, or 183 mg ethylene oxide/m3, the incidence of lens opacities was 0/12, 2/11, and 3/11, respectively, when assessed during the last month of exposure, or 2/4, 2/3, and 4/4, respectively, when assessed 10 years after the cessation of exposure.
Substance-related increases in a variety of tumour types have been observed in rodents exposed to ethylene oxide. Descriptions of study protocols and results (including incidence of tumours) are presented in Table 2 (rats) and Table 3 (mice). In two studies, inhalation exposure increased the incidence of mononuclear cell leukaemia6 and gliomas of the brain in F344 rats of both sexes and of peritoneal mesotheliomas in male rats. In mice, increased incidences of alveolar/bronchiolar adenomas or carcinomas and Harderian gland papillary cystadenomas were observed in both sexes, while the incidences of malignant lymphomas, uterine and mammary gland adenocarcinomas, and mammary gland adenocarcinomas or adenosquamous carcinomas (combined) were increased in females. An increase in squamous cell carcinomas of the forestomach was observed in female rats following the administration (by gavage) of ethylene oxide; the subcutaneous injection of ethylene oxide to female mice induced local fibrosarcomas.
Table 2: Incidence of tumours in Fischer 344 rats exposed to ethylene oxide by inhalation.
|
Sex, n, exposure pattern |
Exposure (ppm)a |
Incidence of tumoursb |
> Reference; comments |
||||
|
Mononuclear cell leukaemia |
Peritoneal mesothelioma |
Brain tumoursc |
|||||
|
Male, 80/group, 7 h/day, 5 days/week, 104 weeks |
0 |
24/77 |
3/78 |
0/76 |
Lynch et al., 1984a,b; trend significant for mesothelioma |
||
|
50 |
38/79* |
9/79 |
2/77 |
||||
|
100 |
30/76 |
21/79** |
5/79* |
||||
|
Male and female, 120/group, 6 h/day, 5 days/week, 104 weeks |
M |
F |
M |
M |
F |
Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986; trend significant for leukaemias in males and females and for mesotheliomas in males |
|
|
0 |
13/97 |
11/116 |
2/97 |
1/181 |
1/188 |
||
|
10 |
9/51 |
11/54 |
2/51 |
1/92 |
1/94 |
||
|
33 |
12/39 |
14/48 |
4/39 |
5/85* |
3/92 |
||
|
100 |
9/30 |
15/26*** |
4/30 |
7/87** |
4/80 |
||
|
a |
1 ppm = 1.83 mg/m3. |
|
b |
*, P < 0.05; **, P = 0.01; ***, P < 0.001. |
|
c |
Brain tumours defined as "mixed cell gliomas" in the Lynch et al. (1984a,b) studies, as gliomas, malignant reticuloses, and granular cell tumours in the Snellings and Garman studies (Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986). |
Table 3: Incidence of tumours in B6C3F1 mice exposed to ethylene oxide.a
|
Sex, n, exposure pattern |
Exposure (ppm)b |
Incidence of tumoursc |
||||||
|
Alveolar/bronchial carcinoma |
Papillary cystadenoma of Harderian gland |
Malignant lymphoma in haematopoietic system |
Uterine adenocarcinoma |
Mammary adeno- and adenosquamous carcinoma |
||||
|
Males and females, 60/group, |
M |
F |
M |
F |
F |
F |
F |
|
|
0 |
6/50 |
0/49 |
1/43 |
1/46 |
9/49 |
0/49 |
1/49 |
|
|
50 |
10/50 |
1/48 |
9/44* |
6/46 |
6/48 |
1/47 |
8/48 |
|
|
100 |
16/50* |
7/49* |
8/42* |
8/47* |
22/49** |
5/49 |
6/49 |
|
|
a |
From NTP (1987). |
|
b |
1 ppm = 1.83 mg/m3. |
|
c |
* P < 0.05; ** P < 0.01. |
In male Fischer 344 rats exposed to 0, 92, or 183 mg ethylene oxide/m3, the incidence of mononuclear cell leukaemia was increased, notably in the low exposure group (Lynch et al., 1984a,b). The incidence of peritoneal mesotheliomas and mixed cell gliomas in brain tissue was increased in an exposure-related fashion.
Results were similar when groups (n = 120 per sex) of male and female Fischer 344 rats were exposed to 0, 18.3, 60.4, or 183 mg ethylene oxide/m3 (Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986). Trend analysis of the incidence of mononuclear cell leukaemia revealed a significant association for both sexes, although the increase was clearly concentration related only in females and was significantly different from the control group in females at the highest concentration only (Snellings et al., 1984b). Among males, trend analysis of the incidence of peritoneal mesotheliomas indicated a relationship between exposure to ethylene oxide and tumour induction after adjustment for mortality (Snellings et al., 1984b). Concentration-related increases in primary brain tumours (gliomas, malignant reticuloses, and granular cell tumours) were observed in both sexes (Garman et al., 1985; Garman & Snellings, 1986). The incidence of subcutaneous fibroma (15/58) was significantly increased in male rats in the highest exposure group (i.e., 183 mg/m3) (Snellings et al., 1984b). The increase in the incidence of mononuclear leukaemia, mesothelioma, and brain tumours in these animals occurred during the later stages of this study (i.e., after about 20–24 months of exposure to ethylene oxide) (Snellings et al., 1984b; Golberg, 1986).
In male and female B6C3F1 mice exposed to 0, 92, or 183 mg ethylene oxide/m3, there was a significant concentration-related increase in the incidence of alveolar/bronchiolar carcinomas and papillary cystadenomas in the Harderian gland (NTP, 1987). In females, there were concentration-related increases in the incidence of malignant lymphomas of the haematopoietic system and uterine adenocarcinoma; the incidence of mammary adenocarcinoma and adenosquamous carcinoma was elevated in both treated groups (NTP, 1987).
In a strain A mouse short-term test for carcinogenicity, a concentration-related increase in the incidence of pulmonary adenomas was observed following exposure (6 h/day, 5 days/week) to 128 and 366 mg ethylene oxide/m3 for 6 months (Adkins et al., 1986).
In a carcinogenicity study involving oral exposure, the intragastric administration of 7.5 or 30 mg ethylene oxide/kg body weight to female Sprague-Dawley rats twice weekly for 150 weeks produced a dose-related increase in the incidence of forestomach tumours (mainly squamous cell carcinomas) (Dunkelberg, 1982).
In female NMRI mice, the subcutaneous injection of ethylene oxide for 95 weeks (mean total doses up to 64.4 mg/mouse) resulted in a significant dose-dependent increase in the number of tumours (i.e., sarcomas) at the site of injection (Dunkelberg, 1981). No skin tumours were observed in female ICR/Ha Swiss mice following the dermal application of approximately 100 mg ethylene oxide (10% in acetone) 3 times weekly for life (Van Duuren et al., 1965).
The genotoxicity of ethylene oxide has been reviewed extensively (IARC, 1994). Owing to the consistency of the results, only a brief summary of studies conducted with in vitro systems or with laboratory animals is provided here. Ethylene oxide is a potent alkylating agent that has been genotoxic in virtually all studies in which it was examined (reviewed in IARC, 1994). In in vitro testing, it induced DNA damage and gene mutations in bacteria, yeast, and fungi and gene conversion in yeast. In mammalian cells, observed effects include gene mutations, micronucleus formation, chromosomal aberrations, cell transformation, unscheduled DNA synthesis, sister chromatid exchange, and DNA strand breaks. Notably, Hallier et al. (1993) observed that the frequency of sister chromatid exchange in human peripheral blood lymphocytes exposed in vitro to ethylene oxide was higher in cells isolated from individuals expressing low levels of GSTT1 than in cells from subjects expressing higher levels of this enzyme.
The results of in vivo studies on the genotoxicity of ethylene oxide have also been consistently positive (see IARC, 1994) following ingestion, inhalation, or injection. In vivo exposure to ethylene oxide induced gene mutation at the hypoxanthine phosphoribosyl transferase (Hprt) locus in mouse and rat splenic T-lymphocytes; sister chromatid exchange was induced in lymphocytes from rabbit, rat, and monkey, in bone marrow cells from mouse and rat, and in rat spleen. Increases in the frequency of gene mutations in the lung (lacI locus) (Sisk et al., 1997) and in T-lymphocytes (Hprt locus) (Walker et al., 1997a) have been observed in transgenic mice exposed to ethylene oxide via inhalation, at concentrations similar to those in carcinogenesis bioassays with this species (NTP, 1987).
In male Big Blue® (lacI transgenic) B6C3F1 mice exposed to 0, 92, 183, or 366 mg ethylene oxide/m3 for 6 h/day, 5 days/week, for 4 weeks, the observed mean (±SE) frequency of mutation at the Hprt locus in splenic T-lymphocytes was 2.2 (±0.03) × 10–6, 3.8 (±0.5) × 10–6 (P = 0.009), 6.8 (±0.9) × 10–6 (P = 0.001), and 14.1 (±1.1) × 10–6 (P < 0.001), respectively (Walker et al., 1997a). The frequency of Hprt mutations in splenic T-lymphocytes was increased (compared with unexposed controls) 5.0- to 5.6-fold in male F344 rats and (non-transgenic) male B6C3F1 mice exposed to 366 mg ethylene oxide/m3 for 6 h/day, 5 days/week, for 4 weeks (Walker et al., 1997b). Similarly, the frequency of lacI mutations in the lungs, bone marrow, and spleen, but not in germ cells, was increased in male Big Blue® (lacI transgenic) B6C3F1 mice exposed to 0 or 366 mg ethylene oxide/m3 (Sisk et al., 1997; Recio et al., 1999).
In vivo exposure to ethylene oxide also induced heritable mutations or effects in germ cells in rodents (IARC, 1994). Ethylene oxide induced dominant lethal effects in mice and rats and heritable translocations in mice. There were dominant visible and electrophoretically detectable mutations in the offspring of male mice exposed (by inhalation) to 366 mg ethylene oxide/m3 for 6 h/day, 5 days/week, for 7 weeks and then mated. This exposure regimen was adopted to ensure that all progeny originated from sperm exposed during the entire spermatogenic process (Lewis et al., 1986). In a study in which male (C3H × 101)F1 mice were exposed by inhalation to 0, 302, 373, 458, or 549 mg ethylene oxide/m3, 6 h/day, 5 days/week, for 6 weeks, then daily for an additional 2.5 weeks, and subsequently mated to T-stock (or [SEC × 101]F1) females, the percent dominant lethals (P < 0.01 at concentrations >373 mg/m3, compared with controls) was 0 (0), 6 (8), 14 (13), 23 (24), and 60 (45), respectively (Generoso et al., 1990). The frequency of translocation carriers (P < 0.01 at all concentrations, compared with controls) among the progeny of these groups of ethylene oxide-exposed male mice mated to T-stock (or [SEC × C57BL]F1) females (data combined) was 1/2068 (0.05%), 32/1143 (2.8%), 52/1021 (5.1%), 88/812 (10.8%), and 109/427 (25.5%), respectively (Generoso et al., 1990).
Degeneration of the seminiferous tubules and germ cells, decreased epididymal weight, decreased sperm count, and an increase in the percentage of abnormal sperm were observed in Wistar rats exposed to >458 mg ethylene oxide/m3 for 13 weeks (Mori et al., 1989, 1991). When abnormal sperm heads were classified into immature and teratic types, the frequency of teratic types was increased at exposures of >92 mg/m3, although it was not concentration dependent (Mori et al., 1991). Decreased relative testicular weight was observed in rats after exposure to 915 mg ethylene oxide/m3 (Mori et al., 1989). In a limited study in rats, slight degeneration of the tubules in the testes was observed after exposure to 370 mg ethylene oxide/m3 for 25–32 weeks (Hollingsworth et al., 1956). Embryotoxic and fetotoxic effects were observed in reproductive studies with rats after exposure of the dams via inhalation to concentrations of ethylene oxide between 183 and 275 mg/m3, prior to mating and throughout gestation. These effects included a decrease in the number of implantation sites per pregnant female, an increase in the incidence of resorptions, a decrease in the median number of pups born on day 0 postpartum per litter, as well as a lower ratio of the number of fetuses born to the number of implantation sites per female (Hackett et al., 1982; Snellings et al., 1982a,b; Hardin et al., 1983). Under these exposure conditions, adverse effects on the dams were not observed (based simply upon clinical appearance and demeanour).
Reproductive effects in mice are similar to those observed in rats. These include increases in the number of resorption bodies and reductions in the number of implants per female and in the number of living embryos per female in female animals exposed to 549 or 2196 mg ethylene oxide/m3 prior to mating (Generoso et al., 1987), concentration-related increases in the percentage of abnormal sperm in animals exposed to 366 mg ethylene oxide/m3 for 5 days (Ribeiro et al., 1987), and a decline in absolute but not relative testicular weight without histological changes in mice exposed to 86 mg ethylene oxide/m3 for 10 weeks (Snellings et al., 1984a).
A decline in sperm count and motility was observed in monkeys exposed to concentrations of ethylene oxide as low as 92 mg/m3 for 24 months (Lynch et al., 1984b,c).
Exposure of Sprague-Dawley rats to a maternally toxic concentration of 275 mg ethylene oxide/m3 either prior to mating and throughout gestation or only during various stages of gestation resulted in reduced fetal body weight and crown-to-rump length, as well as reduced skeletal ossification (Hackett et al., 1982; Hardin et al., 1983). Fetal body weights were reduced in Fischer 344 rats following exposure, only during the period of organogenesis, to 183 mg ethylene oxide/m3, a concentration having no overt toxic effects on the dams (Snellings et al., 1982a). Repeated brief exposures of pregnant Sprague-Dawley rats during gestation to 1464 or 2196 mg ethylene oxide/m3 produced a decline in fetal body weight (at both concentrations) and maternal toxicity (reduced body weight gain) at 2196 mg/m3 (Saillenfait et al., 1996); however, there was no evidence of teratogenicity.
In offspring of female hybrid mice exposed to 2196 mg ethylene oxide/m3 at various intervals shortly after mating, there was a range of congenital malformations, including omphalocoele, hydrops, eye defects, open thorax, cardiac defects, cleft palate, and tail and limb defects (Generoso et al., 1987; Rutledge & Generoso, 1989). There were also increases in the numbers of mid-gestational and late fetal deaths and offspring that did not reach weaning (Generoso et al., 1987; Rutledge & Generoso, 1989; Rutledge et al., 1992). In the offspring of female mice exposed to >1647 mg ethylene oxide/m3 for brief periods shortly after mating, skeletal ossification was reduced and the incidence of axial skeletal anomalies and cleft sternum was increased (Polifka et al., 1991, 1992).
The effect of exposure rate was assessed in pregnant C57BL/6J mice through inhalation exposure to ethylene oxide for 1.5, 3, or 6 h at 3800 or 4900 (mg/m3)-h on gestational day 7 (Weller et al., 1999). Animals with short, high exposures to ethylene oxide had increased adverse effects on fetal death and resorptions, malformations, crown-to-rump length, and fetal weight compared with those exposed to the same total (mg/m3)-h but at longer, lower exposures.
Effects on the nervous system have been observed frequently in laboratory animals exposed to ethylene oxide. The paralysis observed in some animals was reversed upon cessation of exposure (Hollingsworth et al., 1956). Poor coordination of the hindquarters was observed in rats and mice following exposure to 810 mg ethylene oxide/m3 for 7–8 weeks (Snellings, 1982). In subchronic or chronic studies in rats exposed to 458–915 mg ethylene oxide/m3, there was a range of neurological effects, including awkward or ataxic gait, paralysis, and atrophy of the muscles of the hindlimbs, accompanied in some cases by pathological evidence of axonal degeneration of myelinated fibres in nerves of the hind legs (Hollingsworth et al., 1956; Ohnishi et al., 1985, 1986; Matsuoka et al., 1990; Mori et al., 1990). Abnormal posture during gait and reduced locomotor activity were also observed in mice after exposure to ethylene oxide at concentrations ranging from 86 to 425 mg/m3 for 6 h/day, 5 days/week, for 10 or 11 weeks (Snellings et al., 1984a); effects on various reflexes (righting, tail pinch, toe pinch) were also noted at the highest concentration examined (i.e., 425 mg/m3).
Paralysis of the hind limbs and atrophy of the leg muscles have also been reported in rabbits and monkeys following exposure to >370 mg ethylene oxide/m3 (Hollingsworth et al., 1956).
Histological alterations in the axons and demyelination were reported in cynomolgus monkeys exposed to 92 or 183 mg ethylene oxide/m3 for 2 years (Sprinz et al., 1982; Lynch et al., 1984b).
It is likely that the carcinogenicity of ethylene oxide in laboratory animals arises primarily as a result of its direct alkylation of biological macromolecules (i.e., nucleic acids). In vivo exposure to ethylene oxide induced mutations (5- to 5.6-fold) at the Hprt locus in splenic T-lymphocytes in rats and mice (Walker et al., 1997a,b). A statistically significant (i.e., P < 0.05) increase (1.5-fold) in the frequency of lacI mutations was observed in the lungs of transgenic mice exposed to 366 mg ethylene oxide/m3 (Sisk et al., 1997); the frequencies of lacI mutations in the bone marrow and spleen of these animals (1.9- and 1.3-fold, respectively), although increased, were not statistically different from those of the unexposed controls. Currently, there is no clear evidence of a relationship between the mutagenic response observed at these two "indicator" loci and the species- and tissue-specific carcinogenicity of ethylene oxide. Molecular analysis of ethylene oxide-induced mutations at the HPRT locus in human diploid fibroblasts exposed in vitro revealed that a high proportion involved large deletions of this gene (Bastlová et al., 1993).
A potential role of the formation of 7-HEGua in the carcinogenic response has been the focus of many studies, this adduct having been identified in both humans and laboratory animals. In reports by Walker et al. (1992) and Wu et al. (1999b), F344 rats and B6C3F1 mice were exposed (via inhalation for 6 h/day, 5 days/
week, for 4 weeks) to concentrations of ethylene oxide similar to those used in previous carcinogenicity bioassays involving these strains (Lynch et al., 1984a,b; Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986; NTP, 1987). Slightly higher levels of 7-HEGua were measured in tissues (lung, spleen, brain, liver) from rats than from mice; within each species, similar levels of the adduct were measured in the lung, spleen, brain, and liver. Since an increased incidence of brain tumours has been observed in rats but not in mice exposed to ethylene oxide and an increased incidence of lung tumours has been observed in mice but not in rats exposed to this substance, the results provided by Walker et al. (1992) and Wu et al. (1999b) point to no obvious relationship between the overall level of 7-HEGua within various tissues and the observed species-specific carcinogenic response. The potential roles of this and other ethylene oxide-induced DNA adducts, as well as other factors, in mediating the carcinogenicity of ethylene oxide have not been defined.
Exposure to ethylene oxide vapour can cause irritation of the eyes and respiratory tract (Thiess, 1963; ATSDR, 1990). Mild irritation of the skin has been reported after contact with aqueous solutions of ethylene oxide as low as 1% (Sexton & Henson, 1949). Dermal injury is characterized by oedema and erythema, occurring 1–5 h after exposure, followed by the formation of vesicles. Dermal irritation has also been observed after contact with ethylene oxide-sterilized materials and clothing (Royce & Moore, 1955; Marx et al., 1969; Hanifin, 1971; Biro et al., 1974; LaDage, 1979; Bommer & Ritz, 1987; Fisher, 1988; Lerman et al., 1995).
Ethylene oxide is a sensitizing agent. Type I (anaphylaxis) and Type IV (contact dermatitis) hypersensitivity reactions have been observed in individuals exposed to ethylene oxide. Anaphylactic reactions (ranging from mild to severe) have been noted among patients undergoing dialysis involving equipment sterilized by exposure to ethylene oxide (reviewed in Bommer & Ritz, 1987). Asthmatic reactions may occur either alone or in combination with anaphylactic events; case reports of occupational asthma attributed to ethylene oxide exposure have appeared (Dugue et al., 1991; Verraes & Michel, 1995). Reports of contact dermatitis attributed to ethylene oxide are not uncommon; these may be due to allergic reaction or to the irritative effects.
Hemminki et al. (1982) determined the incidence of spontaneous abortion among Finnish hospital staff who had used ethylene oxide, glutaraldehyde, and formaldehyde for instrument sterilization. Sterilizing staff employed in Finnish hospitals in 1980 were included in the analysis, with a total of 1443 pregnancies (545 workers exposed during pregnancy). No measurements of exposure were taken specifically as part of this study (Hemminki et al., 1982). However, independent measurements carried out in 24 Finnish hospitals between 1976 and 1981 revealed 8-h TWA exposures ranging from 0.2 to 0.9 mg ethylene oxide/m3, with peak concentrations up to 458 mg/m3 (Hemminki et al., 1982, 1983), although concentrations of ethylene oxide may have been higher prior to 1976. Nurses from auxiliaries in the same hospitals having no exposure to sterilizing agents, anaesthetic gases, or X-rays served as controls. Information on exposure to sterilizing agents was obtained from the supervising nurses. Information on pregnancy outcome was obtained via a questionnaire and confirmed using a hospital discharge register for all of Finland between 1973 and 1979. The adjusted (for age, parity, decade of reported pregnancy, coffee and alcohol consumption, and smoking) rate of spontaneous abortion for the sterilization staff as a whole (9.7%) was similar to the rate in the control group (10.5%). However, when the pregnancies of the sterilizing staff were analysed according to employment at the time of conception, the rate of spontaneous abortion was significantly (P < 0.001) increased in the exposed (15.1%) versus the unexposed group (4.6%). When the associations between ethylene oxide and the different sterilizing agents were analysed, only exposure to ethylene oxide during early pregnancy was related to an increased frequency of spontaneous abortion (adjusted rate of 16.1% in exposed versus 7.8% in unexposed workers; P < 0.01). Hospital discharge records revealed a similar pattern, with spontaneous abortion rates of 22.6% (significantly higher than controls, P < 0.05), 9.9%, and 9.2% in sterilizing workers exposed to ethylene oxide, unexposed workers, and controls, respectively. In a subsequent analysis, only pregnancies that began during hospital employment were analysed in all groups, with controls chosen from the same hospitals (Hemminki et al., 1983). The rate of spontaneous abortion remained significantly higher (P < 0.05) among the pregnancies associated with exposure to ethylene oxide (20.4%) compared with controls (11.3%).
Rowland et al. (1996) examined the occurrence of spontaneous abortion and pre- and post-term delivery in relation to ethylene oxide exposure among 7000 randomly selected dental assistants (aged 18–39) identified from the 1987 dental assistant registry in California, USA. The most recent pregnancy outcome was chosen for analysis to maximize recall of pregnancy and exposure information, with 1320 women who provided information on age and ethylene oxide exposure contributing to the analysis. A total of 32 women reported ethylene oxide exposure during pregnancy; no quantitative measures or details on timing of exposure during pregnancy were available. The age-adjusted relative risk of spontaneous abortion among ethylene oxide-exposed women was 2.5 (95% confidence interval [CI] = 1.0–6.3); the relative risks of pre-term births (21–37 weeks) and post-term births (>42 weeks) were 2.7 (95% CI = 0.8–8.8) and 2.1 (95% CI = 0.7–5.9), respectively. Using a logistic model, ethylene oxide-exposed women were 2.7 times (95% CI = 1.2–6.1) more likely to have any of the three adverse pregnancy outcomes after adjusting for age. Adjustment for unscavenged nitrous oxide exposure, high amalgam use, and smoking yielded a relative risk of 2.1 (95% CI = 0.7–5.7).
In the only identified study in which the effect of paternal exposure to ethylene oxide on reproductive outcome was assessed, Lindbolm et al. (1991) reported a significantly (P < 0.05) increased risk of spontaneous abortion (odds ratio [OR] = 4.7; 95% CI = 1.2–18.4) among Finnish women whose partners had been exposed to ethylene oxide. In total, 99 186 pregnancies were included in the analysis. Paternal exposure to ethylene oxide was based upon the job and industry in which the men were employed; quantitative data on exposure were not available, and the numbers of spontaneous abortions (n = 3) and pregnancies (n = 10) in the paternal ethylene oxide-exposed group were small. Other potential confounding factors, such as previous abortions and alcohol and tobacco consumption, were not considered in the analysis.
Sensorimotor polyneuropathy was reported for a number of cases following single or long-term exposure to ethylene oxide (exposure concentrations, when reported, ranged from 7.7 to >1281 mg/m3) (Gross et al., 1979; Finelli et al., 1983; Kuzuhara et al., 1983; Zampollo et al., 1984; Schroder et al., 1985; Fukushima et al., 1986; Ristow & Cornelius, 1986; Crystal et al., 1988). Amelioration of the symptoms following cessation of exposure has been commonly observed. In individuals exposed to >1300 mg ethylene oxide/m3, sural nerve biopsies revealed axonal degeneration with mild changes in the myelin sheath; muscle biopsies revealed degeneration atrophy (Kuzuhara et al., 1983). Effects on the central nervous system (e.g., seizures) have been observed following single exposure to 915–1281 mg ethylene oxide/m3 (Gross et al., 1979; Salinas et al., 1981).
Increases in chromosomal aberrations in peripheral blood lymphocytes have been consistently reported in studies of workers exposed to concentrations of ethylene oxide of >9.2 mg/m3 (Table 4). Effects observed at lower concentrations (i.e., <9.2 mg/m3) have been mixed.
Significant increases in the frequency of sister chromatid exchange in peripheral blood cells have also been observed among individuals exposed to elevated levels of ethylene oxide (i.e., usually >9.2 mg/m3). Results of studies of individuals exposed to lower levels (i.e., <0.9 mg/m3) have been mixed. In some studies, increases in the frequency of sister chromatid exchange have been observed to persist after exposure had ceased. Effects have been related to the concentration or duration of exposure to ethylene oxide in a number of studies.
In some studies, the frequency of micronuclei in peripheral blood was increased in workers exposed to relatively high (3.7–60.4 mg/m3) levels of ethylene oxide (Tates et al., 1991; Ribeiro et al., 1994). However, in the majority of the studies involving exposures to lower levels, no effect on the frequency of micronuclei was observed. Apparent inconsistencies in the data could reflect the influence of peak exposures, differences in exposure duration, or smoking status.
Table 4: Cytogenetic effects in humans.a
|
Number exposed |
Exposure time (years) |
Ethylene oxide level in air (ppm)b |
Cytogenetic observationsc |
Reference |
||||
|
Range |
Mean |
Range |
Mean (TWA) |
CA |
SCE |
MN |
||
|
75 (0) |
< 50 |
+ |
+ |
Abrahams (1980) |
||||
|
33 (0) |
1–14 |
<0.05–8 |
<0.01d |
(+) |
Clare et al. (1985) |
|||
|
13 (site I) |
0.5e |
– |
– |
Stolley et al. (1984); Galloway et al. (1986) |
||||
|
12 (12) |
< 36 |
+ |
Garry et al. (1979) |
|||||
|
14 (14) |
<0.07–4.3e |
– |
Hansen et al. (1984) |
|||||
|
18 (factory I) |
0.5–8 |
3.2 |
<1 |
+ |
– |
+f |
Hogstedt et al. (1983) |
|
|
18 (sterilization centres) (10) |
1–8 |
0–2.6 |
+ |
Karelová et al. (1987) |
||||
|
15 (smokers) (7) |
0.5–10 |
5.7 |
20–123 |
+ |
Laurent et al. (1984) |
|||
|
10 (10) |
3 |
60–69e |
+ |
+ |
Lerda and Rizzi (1992) |
|||
|
9 (low dose) (48) |
4 |
2.7–10.9 |
2.7 |
+ |
– |
Major et al. (1996) |
||
|
34 (23) |
8g |
<0.1–2.4e |
<0.3 |
– |
+ |
– |
Mayer et al. (1991) |
|
|
12 |
1–8 |
4 |
0.5–1 |
– |
Pero et al. (1981) |
|||
|
11 (smokers) |
0.5–417h |
– |
Popp et al. (1994) |
|||||
|
75 (22) |
3–14 |
7 |
2–5e |
+ |
+ |
Ribeiro et al. (1994) |
||
|
56 (141) |
1–10 |
1–40e |
+ |
+ |
Richmond et al. (1985) |
|||
|
22 (22) |
0.6–4 |
3 |
0.2–0.5e |
0.35 |
(+) |
+ |
Sarto et al. (1984) |
|
|
10 (10) |
0–9.3e |
1.84 |
+ |
Sarto et al. (1987) |
||||
|
9 (27 controls total) |
0.5–12 |
5 |
0.025–0.38e |
– |
Sarto et al. (1990) |
|||
|
5 (10 controls total) |
0.1–4 |
2 |
|
0.025 |
– |
–k |
Sarto et al. (1991) |
|
|
32 (8 controls total) |
5.1 |
0–0.3e |
0.04 |
+ |
– |
Schulte et al. (1992) |
||
|
9 (hospital workers) (8) |
2–6 |
4 |
20–25 |
+ |
+ |
– |
Tates et al. (1991) |
|
|
7 (7 controls total) |
Accidental |
28–429e |
– |
– |
Tates et al. (1995) |
|||
|
9 (low exposure) |
13l |
– |
Yager et al. (1983) |
|||||
|
19 (35 controls total) |
1–5 |
<0.05–8 |
<0.05 |
– |
van Sittert et al. (1985) |
|||
|
a |
Modified from IARC (1994). |
|
b |
1 ppm = 1.83 mg ethylene oxide/m3. |
|
c |
CA = chromosomal aberrations; SCE = sister chromatid exchange; MN = micronucleus; + = positive; (+) = weakly positive: – = negative. |
|
|
Calculated by linear extrapolation. |
|
e |
TWA (8-h). |
|
f |
Positive for erythroblasts and polychromatic erythrocytes (negative for lymphocytes). |
|
|
Maximum years exposed. |
|
h |
Peak concentrations. |
|
i |
Single exposure to high concentration from sterilizer leakage in addition to long-term exposure. |
|
j |
Nasal mucosa. |
|
k |
Buccal cells. |
|
l |
Average 6-month cumulative exposure (mg). |
In a study involving small numbers (i.e., n = 4–12 per group) of non-smoking males and females exposed to ethylene oxide through the sterilization of medical equipment, Fuchs et al. (1994) reported increases (1.5-, 2.2-, and 1.5-fold; P < 0.05, P < 0.05, P > 0.05, respectively) in single-strand DNA breaks in peripheral mononuclear blood cells obtained from individuals exposed to (4-h TWA) ethylene oxide concentrations of 0.1–0.49 mg/m3, 0.5–2.0 mg/m3, and >2 mg/m3, respectively.
Haematological effects were observed among a group of 59 women exposed to ethylene oxide released from sterilizers while employed at nine hospitals in the USA and one in Mexico (Schulte et al., 1995). Exposure was classified as none, low, or high, based on mean 4-month cumulative exposure categories of 0, >0–60, or >60 (mg/m3)-h, respectively. Monitoring data revealed mean 8-h TWA exposures in the US hospitals of 0.15 mg/m3 (range = 0–0.55 mg/m3) and 0.31 mg/m3 (range = 0.24–0.55 mg/m3) for the low- and high-exposure categories, respectively; the corresponding measurements in the Mexican hospital were 0.04 mg/m3 and 0.99 mg/m3 (range = 0.5–2.5 mg/m3), respectively. Among the US workers, haematocrit and haemoglobin levels were reduced (not statistically significant) in the high-exposure group, compared with the unexposed controls; the levels were significantly lower in the high-exposure group than in the low-exposure group (P = 0.03 and 0.02 for haemoglobin and haematocrit levels, respectively). Compared with unexposed controls, US workers in the high-exposure subgroup exhibited a statistically significant (P = 0.04) increase in the percentage of lymphocytes and a reduction (P = 0.03) in the percentage of neutrophils in the blood. Among the Mexican workers, there were no statistically significant relationships between exposure to ethylene oxide and changes in haematocrit or haemoglobin levels (there was only one worker in the unexposed group), although an exposure-related increase (not statistically significant) in the percentage of neutrophils in the blood was observed. More recently, Shaham et al. (2000) reported that, compared with 88 non-occupationally exposed controls (matched for age, sex, and smoking habits), among 46 Israeli hospital workers exposed (at three locations with a mean period of employment of 6.6 years) to 145- to 210-min TWA concentrations of <0.02–0.1 mg ethylene oxide/m3, there were statistically significant (i.e., P < 0.01) increases in the absolute mean numbers of red blood cells, monocytes, and oesinophils, increases in the percent haematocrit, and reductions in the absolute mean numbers of lymphocytes and platelets. There were no significant differences in the absolute mean numbers of neutrophils and basophils or in haemoglobin levels.
Haematological changes were not observed either in a group of 36 male workers employed at an ethylene oxide manufacturing plant with estimated 8-h TWA exposures below 0.09 mg/m3 (van Sittert et al., 1985) or in a group of 84 male workers involved in the manufacture of ethylene oxide who were exposed to estimated concentrations of <1.83 mg/m3 (Currier et al., 1984).
The prevalence of lens opacities and cataracts was assessed in a group of 55 workers exposed to ethylene oxide (airborne concentrations ranged from 0.11 mg/m3 during a 97-min exposure to 71 mg/m3 during a 2.5-min exposure) at five hospitals in Paris, France (Deschamps et al., 1990). There was no difference between the exposed and control groups in the prevalence, location, importance, or type of lens opacities observed, but cataracts were observed in six exposed individuals, compared with none in the control group.
Associations between occupational exposure to ethylene oxide and various types of cancer have been examined in a number of epidemiological studies. A summary of the risk measures for selected cancers (stomach, pancreas, brain, haematological system) is presented in Table 5.
Table 5: Summary of risk measures for selected cancers (stomach, pancreas, brain, haematopoietic, lymphosarcoma, Hodgkin’s disease) from epidemiological studies.
|
Cancer |
Exposure to ethylene oxide |
Risk measurea |
Reference |
|
Stomach |
male and female ethylene oxide production workers (two plants) and medical equipment sterilizers |
SMR = 546: 10 |
Hogstedt (1988) |
|
Blood and lymphatic |
SMR = 459: 9 |
||
|
Leukaemia |
SMR = 921: 7 |
||
|
Stomach |
workers from older production plant |
SMR = 707: 9 |
|
|
Leukaemia |
workers from older production plant |
SMR = 703: 3 |
|
|
10-year update of male workers producing or using ethylene oxide (excluding chlorohydrin production) studied by Greenberg et al. (1990) |
Teta et al. (1993) |
||
|
Stomach |
entire cohort |
SMR = 160 (95% CI = 69–315): 8 |
|
|
Pancreas |
entire cohort |
SMR = 61 (95% CI = 17–156): 4 |
|
|
Brain and nervous system |
entire cohort |
SMR = 150 (95% CI = 55–327): 6 |
|
|
Leukaemia and aleukaemia |
entire cohort |
SMR = 106 (95% CI = 35–248): 5 |
|
|
Stomach |
intermediate-exposure subgroup |
SMR = 364 (95% CI = 102–957): 4* |
|
|
Stomach |
low-exposure subgroup |
SMR = 222 (95% CI = 61–575): 4 |
|
|
male and female workers in facilities producing sterilized medical equipment and spices |
Steenland et al. (1991) |
||
|
Haematopoietic cancers |
those with >20 years since first exposure |
SMR = 1.76 (95% CI = 0.94–3.01): 34 |
|
|
Haematopoietic cancers |
males only |
SMR = 1.55 (P = 0.05): 27* |
|
|
Lymphosarcoma/reticulosarcoma |
males only |
SMR = 2.6 (P = 0.05): 7* |
|
|
Non-Hodgkin’s lymphoma |
males only |
SMR = 2.16: 7 |
|
|
Haematopoietic cancers |
males with >7 years exposure and >20 years since first exposure |
SMR = 2.63 (95% CI = 1.05–5.42): 7* |
|
|
Haematopoietic cancers |
workers with highest cumulative exposure |
SMR = 124 (95% CI = 66–213): 13 |
Stayner et al. (1993) |
|
Non-Hodgkin’s lymphoma |
workers with highest cumulative exposure |
SMR = 192 (95% CI = 77–395): 7 |
|
|
Leukaemia/aleukaemia |
workers with highest cumulative exposure |
SMR = 75 (95% CI = 15–218): 3 |
|
|
Haematopoietic cancers |
males with highest cumulative exposure |
SMR = 196 (95% CI = 101–343): 12* |
|
|
Haematopoietic cancers |
males with moderate cumulative exposure |
SMR = 143 (95% CI = 62–283): 8 |
|
|
Haematopoietic cancers |
males with lowest cumulative exposure |
SMR = 95 (95% CI = 26–243): 4 |
|
|
Haematopoietic cancers |
workers with >20 years since first exposure |
SMR = 155 (95% CI = 77–277): 11 |
|
|
Lympho-/haematopoietic cancers |
male and female workers sterilizing medical equipment |
SIR = 1.78 (95% CI = 0.65–3.88): 6 |
Hagmar et al. (1995) |
|
Leukaemia |
male and female workers sterilizing medical equipment |
SIR = 2.44 (95% CI = 0.3–8.81): 2 |
|
|
Leukaemia |
workers having a minimum 10 years latency (but excluding those with cumulative exposure <0.13 ppm-years) |
SIR = 7.14 (95% CI = 0.87–25.8): 2 |
|
|
Brain |
SIR = 3.80 (95% CI = 0.78–11.1): |
||
|
Hodgkin’s disease |
male workers at a chemical manufacturing plant |
SIR = 497 (95% CI = 238–915): 10* |
Swaen et al. (1996) |
|
Hodgkin’s disease |
nested case–control analysis of male workers at the chemical manufacturing plant |
OR = 8.5 (95% CI = 1.4–39.9): 3c* |
|
|
Leukaemia |
male workers at chemical plants |
SMR = 0.85 (95% CI = 0.10–3.07): 2 |
Kiesselbach et al. (1990) |
|
Stomach |
male workers at chemical plants |
SMR = 1.38 (95% CI = 0.75–2.31): 14 |
|
|
Leukaemia |
males and females at ethylene oxide production/use facilities |
SMR = 2.25 (95% CI = 0.47–6.59): 3 |
Gardner et al. (1989) |
|
Stomach |
males and females at hospitals |
SMR = 1.19 (95% CI = 0.15–4.32): 2 |
|
|
Pancreas |
male petroleum plant workers |
SMR = 377 (95% CI = 76–1102): 3 |
Morgan et al. (1981) |
|
Brain and central nervous system |
SMR = 285 (95% CI = 32–1030): 2 |
||
|
Hodgkin’s disease |
SMR = 570 (95% CI = 64–2058): 2 |
||
|
Brain and central nervous system |
male workers involved in the production of ethylene chlorohydrin and propylene chlorohydrin |
SMR = 123 (95% CI = 25–358): 3 |
Olsen et al. (1997) |
|
Lympho-/haematopoietic |
SMR = 129 (95% CI = 62–238): 10 |
||
|
Lympho-/haematopoietic |
male workers involved in the production of ethylene chlorohydrin |
SMR = 149 (95% CI = 60–307): 7 |
|
|
Lympho-/haematopoietic |
male workers involved in the production of ethylene chlorohydrin (analysis included a 25-year latency period) |
SMR = 194 (95% CI = 71–423): 6 |
|
|
Haematopoietic cancers |
male workers licensed to handle ethylene oxide and other chemicals |
SMR = 250 (95% CI = 91–545): 6 |
Bisanti et al. (1993) |
|
Lympho/reticulosarcoma |
male workers licensed to handle ethylene oxide and other chemicals |
SMR = 682 (95% CI = 186–1745): 4* |
|
|
Leukaemia/aleukaemia |
male workers licensed to handle ethylene oxide and other chemicals |
SMR = 193 (95% CI = 23–699): 2 |
|
|
Stomach |
male workers licensed to handle ethylene oxide and other chemicals |
SMR = 122 (95% CI = 40–287): 5 |
|
|
Pancreas |
male workers licensed to handle ethylene oxide and other chemicals |
SMR = 254 (95% CI = 52–744): 3 |
|
|
Haematopoietic cancers |
male workers licensed to handle ethylene oxide only |
SMR = 700 (95% CI = 227–1637): 5* |
|
|
Lympho/reticulosarcoma |
male workers licensed to handle ethylene oxide only |
SMR = 1693 (95% CI = 349–4953): 3* |
|
|
Leukaemia/aleukaemia |
male workers licensed to handle ethylene oxide only |
SMR = 650 (95% CI = 79–2349): 2 |
|
|
Leukaemia |
male and female workers using ethylene oxide as a sterilant |
SMR = 1.85 (P = 0.42): 1 |
Norman et al. (1995) |
|
Pancreas |
male and female workers using ethylene oxide as a sterilant |
SMR = 3.92 (P = 0.09): 2 |
|
|
Leukaemia |
meta-analysis of reports published between 1979 and 1993 |
sSMR = 1.06 (95% CI = 0.73–1.48): 31 |
Shore et al. (1993) |
|
Non-Hodgkin’s lymphoma |
sSMR = 1.35 (95% CI = 0.93–1.90): 31 |
||
|
Stomach |
sSMR = 1.28 (95% CI = 0.98–1.65): 57 |
||
|
Pancreas |
sSMR = 0.98 (95% CI = 0.69–1.36): 34 |
||
|
Brain and central nervous system |
sSMR = 0.89 (95% CI = 0.55–1.36): 19 |
||
|
Leukaemia |
update of Shore et al. (1993) analyses, but including two additional studies |
mSMR = 1.08 (95% CI = 0.61–1.93): 35 |
Teta et al. (1999) |
|
Non-Hodgkin’s lymphoma |
mSMR = 1.34 (95% CI = 0.96–1.89): 33 |
||
|
Stomach |
mSMR = 1.23 (95% CI = 0.71–2.13): 59 |
||
|
Pancreas |
mSMR = 0.95 (95% CI = 0.69–1.31): 37 |
||
|
Brain |
mSMR = 0.96 (95% CI = 0.49–1.91): 25 |
|
a |
Unless otherwise noted, value in italics is the number of observed deaths or cases. Asterisk (*) indicates increase reported as statistically significant. SMR = standardized mortality ratio; SIR = standardized incidence ratio; OR = odds ratio; mSMR = meta standardized mortality ratio; sSMR = summary standardized mortality ratio. |
|
b |
1 ppm = 1.83 mg/m3. |
|
c |
Number of cases exposed to ethylene oxide. |
In a cohort study of 709 ethylene oxide production and sterilization workers in Sweden, the incidence of leukaemia (after excluding the three cases in the original cluster; standardized incidence ratio [SIR] 630; 95% CI 200–1500) and stomach cancer (SMR = 546; 10 observed deaths) was increased (Hogstedt, 1988). Excess mortality was greatest in operators and repairmen employed in an old production plant where ethylene oxide had been synthesized by the chlorohydrin method from 1941 to 1947 (mostly in an enclosed building). Levels of ethylene oxide in all of the facilities were estimated to have been relatively high in the early years (average exposure levels were estimated to have been 26 mg/m3 between 1941 and 1947 at the plant producing ethylene oxide by the chlorohydrin method; however, peaks above the odour threshold of 732 mg/m3 had been reported). Workers were also exposed to a variety of other chemicals.
Greenberg et al. (1990) conducted a study of 2174 workers employed at two ethylene oxide production plants in the USA, for which a 10-year update of this cohort, which excluded 278 chlorohydrin workers, was reported by Teta et al. (1993). Exposure was evaluated using latency and duration of employment in ethylene oxide-related departments, not by the direct analysis of ethylene oxide levels. Comparisons were with both the general population and unexposed workers in the plants In this cohort, there were no statistically significant increases in deaths from any cause for the entire cohort (Teta et al., 1993). Mortality in men from the high-exposure departments was not increased, but an excess of deaths due to stomach cancer was observed in the intermediate-exposure group, and there was an increase (not statistically significant) in the low-exposure group. When risks associated with duration of assignment were examined, there were no significant trends for any cancer, but the numbers of deaths from cancer at any site were small. The relative risk for stomach cancer (2.77; 95% CI = 1.11–6.93; 5 observed deaths) was significantly elevated for those exposed from 2 to 9 years. The SMR for lymphatic/haematopoietic cancers for the 278 ethylene chlorohydrin production workers, considered to have only low, intermittent exposure to ethylene oxide, was 294 (95% CI = 127–580; 8 observed deaths) (Benson & Teta, 1993). A 2- to 3-fold increase in risk for leukaemia was observed among workers with more than 10 years of assignment to ethylene oxide departments.
TWA exposures were estimated over four time periods and three exposure intensity categories (low, medium, high). Average exposures in the most recent time period were based on industrial hygiene monitoring conducted in the plants and were inferred for earlier time periods based on exposure levels in similar manufacturing operations during the period of interest. A separate age-dependent exposure history was developed for each worker, based on the worker’s assignments and estimated exposure levels (i.e., low, medium, high). For exposures between 1974 and 1978, data were collected directly in the area where the subjects worked. For all other time periods (1925–1973), inferences on exposure in the plants in West Virginia were made from data gathered in other facilities in Texas and Sweden. Different ventilation rates in plants located in geographically diverse locations are likely to lead to large variations in exposure.
While there were no quantitative estimates of individual exposure in this investigation (assignments were to low, medium, or high exposure only), the length of follow-up was among the longest in any study (mean duration of follow-up, 27.2 years; mean length of exposure, 5.4 years). Workers were exposed to a variety of other chemicals (approximately 26, including butadiene and benzene) (Shore et al., 1993).
In the largest single cohort studied to date, Steenland et al. (1991) examined mortality in 18 254 male and female workers exposed to ethylene oxide at 14 plants producing sterilized medical supplies and spices in the USA. Comparisons were with the US general population. A more detailed analysis of exposures in this cohort was subsequently conducted by Stayner et al. (1993), being restricted to workers from 13 of the 14 original facilities having adequate information for estimating historical exposures. The database on which these exposure estimates were based was collected in the course of walkthrough surveys of 36 companies in the medical supplies and spice industries and in-depth sampling surveys of 2 of these 36 companies; the database included 2350 individual TWA exposure values acquired from 18 facilities between 1976 and 1985 (Greife et al., 1988). Arithmetic mean exposures were calculated by facility, year, and exposure category. The exposure categories were based on grouping of all sampled jobs into eight categories with similar potential for exposure. Exposure to ethylene oxide was estimated based on an industrial hygiene-based regression model for each exposure category. This model predicted exposures to ethylene oxide within 2.0 mg/m3 of a validation data set (46 measurements not used in the model), with a standard deviation of 6.8 mg/m3 (Hornung et al., 1994). Cumulative exposure for each individual was estimated by integration of estimated ethylene oxide concentration (in parts per million) for each job held multiplied by the duration of time (days) spent on the job.
For the entire cohort, there was no increase in mortality from haematopoietic cancer. There was a slight but significant increase among men, however, but a decrease among women (Steenland et al., 1991). The SMR for deaths due to "all haematopoietic neoplasms" among the group with the highest cumulative exposure was 124 (not statistically significant), and the trend with cumulative exposure was not statistically significant. For the group with the highest cumulative exposure, the SMRs for non-Hodgkin’s lymphoma and leukaemia/aleukaemia were 192 (not statistically significant) and 75, respectively. Increased mortality from kidney cancer (SMR = 322) was observed in the intermediate cumulative exposure group; no trend with exposure was observed (Stayner et al., 1993).
When the results for "all haematopoietic neoplasms" were stratified according to sex, increased mortality was observed among males in the highest exposure category (SMR = 196; 95% CI = 101–343; 12 observed deaths). The authors noted a suggestive positive trend in the SMRs with exposure in males (chi2 = 1.69; P = 0.19; 24 observed deaths) and suggestive negative trend in females (chi2 = 1.01; P = 0.31), the latter based upon a small number of observed deaths (n = 9), particularly in the highest exposure category (n = 1). When results for all workers were stratified according to time since first exposure, the greatest excess in deaths due to "haematopoietic neoplasms" was observed among workers with more than 20 years since their first exposure. Regression analysis revealed a highly statistically significant exposure–response relationship between cumulative exposure to ethylene oxide and mortality from lymphocytic leukaemia and non-Hodgkin’s lymphoma combined. A marginal relationship was also observed between cumulative exposure and mortality from all haematopoietic neoplasms and from non-Hodgkin’s lymphoma. There was a positive, but not statistically significant, exposure–response relationship between cumulative exposure and leukaemia. Inclusion of a 5-year lag period yielded a stronger exposure–response relationship for "lymphoid" neoplasms; the relationship between cumulative exposure and mortality from all haematopoietic neoplasms and from non-Hodgkin’s lymphoma was statistically significant, after inclusion of a 10-year lag period in the analysis. There was a negative exposure–response relationship between cumulative exposure and cancers of the stomach, pancreas, brain, and kidney (Stayner et al., 1993).
While this is one of the few studies for which there are individual estimates of cumulative exposure, the monitoring data on which these estimates are based are limited to those collected after 1978 (however, results for the subgroup of this cohort exposed before 1978 were essentially identical to those for the entire cohort, suggesting that exposures may have been similar). There was no evidence of confounding exposure to other occupational carcinogens. Although this is by far the largest of all of the studies conducted to date, the average follow-up period was short; 28% of workers had attained >20 years since first exposure (average duration of follow-up, 16 years; mean duration of exposure, 4.9 years). In this regard, it should be noted that five of the seven men who died of leukaemia died in the most recent calendar period, generating statistically significant excess mortality for these years (SMR = 345; 95% CI = 111–806), and additional follow-up of this cohort is desirable. The variation in response between men and women in this study cannot be explained on the basis of sex ratio of the study population (i.e., small numbers of women); indeed, a greater proportion of the population was female (55% versus 45%).
In a subsequent analysis of data from the cohort evaluated by Steenland et al. (1991), in which the categorization of non-Hodgkin’s lymphoma included an additional International Classification of Disease category that was omitted in the original Steenland et al. (1991) analysis (i.e., "other neoplasms of lymphoid tissue"), Wong & Trent (1993) reported increased mortality due to non-Hodgkin’s lymphoma in males (SMR = 247; 95% CI = 141–402). No indication of an exposure–response relationship associated with either duration of employment or latency was observed. Similarly, there was no mortality pattern by latency or duration of employment for any cancer site examined. While this study is slightly larger than the corresponding study by Steenland et al. (1991), individual estimates of exposure frequency and intensity were not assigned.
Cancer risks were not significantly increased in a cohort of 2170 male and female Swedish workers exposed to ethylene oxide at two plants producing disposable medical equipment (Hagmar et al., 1995). Non-statistically significant elevations were observed for lymphopoietic/haematopoietic cancers and leukaemia. When the analysis excluded workers with cumulative exposures to ethylene oxide below the median value of 0.24 (mg/m3)-years, but included a minimum 10-year latency period, there was an increased (although not statistically significant) risk of leukaemia. Cases with leukaemia had only a slightly higher cumulative exposure to ethylene oxide than the average cohort member. In this study, the levels of adducts in haemoglobin correlated well with the estimated exposure levels (Hagmar et al., 1995). This is one of the only studies in which there were cumulative estimates of individual exposure; moreover, biological dosimetry of haemoglobin adducts was conducted to corroborate exposure estimates. Exposures were also mainly to ethylene oxide; the only other exposures were to methyl formate or fluorochlorocarbons. However, the median duration of follow-up was relatively short (11.8 years), and exposure levels were relatively low for most workers, since fewer than 200 workers had more than 1.83 (mg/m3)-years of cumulative exposure.
A case–control study in a cluster of 10 cases of Hodgkin’s disease among male employees of a Belgian chemical manufacturing firm revealed a statistically significant increased risk associated with exposure to ethylene oxide (Swaen et al., 1996). The risk remained significantly elevated after restricting the analysis to individuals with durations of exposure of more than 10 years, but estimated exposure was not related to the risk.
No statistically significant increased risks of cancer of the haematopoietic system or other sites were observed in a number of other epidemiological studies in which there were no estimates of individual exposure and small numbers of haematopoietic and other cancers (Morgan et al., 1981; Gardner et al., 1989; Kiesselbach et al., 1990; Olsen et al., 1997). In the study by Kiesselbach et al. (1990), there were no increases in leukaemias or total haematopoietic cancers, nor were there any trends associated with exposure intensity, duration of exposure, or latency in 3658 men from six chemical companies, with median length of exposure of 9.6 years. Gardner et al. (1989) reported a small excess of leukaemia mortality among chemical workers (3 observed/1.33 expected) and a deficit among hospital workers (0 observed/0.76 expected), although neither was statistically significant, in 2876 men and women from four companies that produced or used ethylene oxide and eight hospitals that used ethylene oxide sterilizers (average duration of follow-up not reported). Morgan et al. (1981) observed non-significant excesses of brain cancer, pancreatic cancer, and Hodgkin’s disease, but a deficit of leukaemia, in 767 men at an ethylene oxide production plant. For the entire cohort examined by Olsen et al. (1997), there were non-statistically significant excess deaths from cancer of the large intestine, lung, kidney, lymphopoietic/haematopoietic tissue, and other lymphatic tissues. Among those involved only in the production of ethylene chlorohydrin, the SMR was increased for lymphopoietic/haematopoietic cancer (SMR = 149; 95% CI = 60–307; 7 observed/4.7 expected).
In two additional small studies, significantly increased risks of lymphosarcoma/reticulosarcoma and breast cancer were observed by Bisanti et al. (1993) and Norman et al. (1995), respectively. In the former investigation, the SMR for lymphosarcoma/reticulosarcoma was 682 (4 observed deaths, P < 0.05) among 1971 chemical workers licensed to handle ethylene oxide between 1938 and 1984 in Italy, in comparison with the local population (mean length of follow-up, 9.8 years; no estimates of individual exposure). However, there was no association with duration or latency, although the former could not be accurately determined.
Information on the toxicity of ethylene oxide to natural aquatic and terrestrial organisms is limited. A brief summary of effects is presented below, with an emphasis on the most sensitive end-points in the studies of sufficient quality (e.g., those where concentrations were measured or measures taken to prevent evaporation). In numerous studies, ethylene oxide has induced dose-dependent genetic mutations in various types of biota, including plants, fungi, insects, mammalian cell cultures, and bacteria (IPCS, 1985; US EPA, 1985; Dellarco et al., 1990; IARC, 1994; BUA, 1995; see also section 8.4). The actual population-level impact on wildlife from mutagenic end-points is not clear. In general, however, these effects occur at levels of ethylene oxide exposure similar to or slightly lower than those observed to induce other effects.
The toxicity of ethylene oxide to bacterial cultures has been examined primarily in terms of mutagenicity (Dellarco et al., 1990). In one of the few reported measures of direct toxicity, the IC50 for the effect of ethylene oxide on activated sludge microorganisms in a 16-h bacterial toxicity test at 22 °C was in the range of 10–100 mg/litre (Conway et al., 1983).
In a modified Ames test, direct increases in revertant bacterial mutations in Salmonella typhimurium strains TA1535 and TA100 were observed (Pfeiffer & Dunkelberg, 1980). Similarly, there was a dose–response relationship for mutation induction in Escherichia coli Sd-4 (Hussain, 1984). There was a linear relationship for induction of guanine alkylation at concentrations between 2.6 and ~1000 mg/litre for 1-h exposures (nominal concentrations). Bacterial survival remained essentially constant, at 100%, at all dose levels (Hussain, 1984).
There were dose–response relationships in point mutations in various strains of bacteria following exposure in liquid suspensions up to 4210 mg/litre (Dellarco et al., 1990). From the above studies and others, it is difficult to interpret the effects of ethylene oxide on natural bacterial populations in view of variations in bacterial strains, reproductive potential, natural variability, DNA repair mechanisms, and population resilience.
Ethylene oxide appears to be slightly less toxic to invertebrates than to microorganisms. In a US EPA standard static acute toxicity test on Daphnia magna, 24-h LC50 values based on measured concentrations were 260–300 mg/litre; 48-h LC50 values were 137–300 mg/litre. In acute toxicity tests on brine shrimp (Artemia salinia) under similar conditions, 24-h LC50s were between 350 and 500 mg/litre (n = 3), and 48-h LC50 values were between 490 and 1000 mg/litre (n = 3) (Conway et al., 1983).
Fish are moderately sensitive to ethylene oxide. Bridié et al. (1979b) reported a 24-h LC50 of 90 mg/litre in goldfish (Carassius auratus) at 20 °C based on measured concentrations. In acute static toxicity tests performed according to US EPA standards using fathead minnows (Pimephales promelas) under aerated conditions, under sealed oxygen, or under no aeration, 24-h LC50s were 274, 86, and 90 mg/litre, respectively. With no aeration, the 48- and 96-h LC50s were 89 and 84 mg/litre, respectively (Conway et al., 1983).
Ethylene glycol and ethylene chlorohydrin are the principal breakdown products of ethylene oxide in water. In acute toxicity tests with ethylene glycol, 24-h LC50s were >10 000, >10 000, and >20 000 mg/litre for the fathead minnow, Daphnia magna, and brine shrimp, respectively; for ethylene chlorohydrin, 24-h LC50s were 768, 675, and >1000 mg/litre for the fathead minnow, D. magna, and brine shrimp, respectively (Conway et al., 1983).
Ethylene oxide produces gene mutations in plant cells, including barley, rice, and peas, exposed in vitro (Ehrenberg et al., 1956, 1959; Blixt et al., 1963; Shulovská et al., 1969; Jana & Roy, 1975; Migliore et al., 1982). Chromosome damage and sister chromatid exchange were observed in barley, wheat, spiderwort (Tradescantia paludosa), and pollen (Smith & Lotfy, 1954; Ehrenberg et al., 1956, 1959; Mackey, 1968; Moutschen-Dahmen et al., 1968). Ehrenberg et al. (1956) reported a 5-fold increase in sterility caused by chromosomal aberrations in barley seeds treated at a gaseous concentration of 1.5 × 106 mg/m3 (80%) for 6 days. Second-generation chlorophyll gene mutations increased 33 times over controls under this treatment. Barley seeds soaked for 2 h in solutions of 3084 and 11 894 mg/litre induced second-generation chlorophyll gene mutations 3.7- and 13.8-fold over controls. Jana & Roy (1975) determined that for two genotypes of rice (Oryza sativa), mutagenic efficiency decreased with increasing concentration of ethylene oxide. Concentrations ranged from 888 to 6167 mg/litre, based on nominal concentrations and exposure for 8 h.
In an examination of the control of pathogenic fungi (Fusarium, Alternaria, and Helminthosporium spp.) on sorghum (Sorghum vulgare Pers.), ethylene oxide applied to a filter paper disc at 8 mg/litre was 92.3% effective in controlling fungal growth and 100% effective in inhibiting the viability of the sorghum seed (Raghunathan et al., 1969). Concentrations applied to the filter paper discs are not easily related to air or soil concentrations.
Although data are limited, insects appear to be relatively insensitive to atmospheric exposure to ethylene oxide. In 24-h fumigation experiments, there was an increase in the control-corrected mortality rates from 24.5 to 98.6% in the khapra beetle (Trogoderma granarium) as the nominal concentration of ethylene oxide increased from 1000 to 3000 mg/m3, respectively. Reproduction of the surviving beetles did not differ significantly from that of untreated controls (Rajendran, 1982). Rajendran & Shivaramaiah (1985) studied the effect of 24-h exposure to ethylene oxide at concentrations ranging from 250 to 1500 mg/m3 on the reproductive rate of the lesser grain borer (Rhyzopertha dominica F.). Only concentrations above 500 mg/m3 had any significant effect (P = 0.01). Chromosome damage has been observed in insects exposed to ethylene oxide, including observations of gene mutations in Drosophila melanogaster from a sex-linked recessive lethal test and in vitro evidence of chromosomal breaks and translocations (IPCS, 1985).
Information on the effects of ethylene oxide on birds or wild mammals has not been identified. Laboratory animals are therefore used as surrogates for wildlife. The chronic reproductive effects in rats following inhalation of ethylene oxide at 183 mg/m3, reported by Snellings et al. (1982b), are assumed to represent effects on wild rodent species and are selected as the most environmentally significant measurement end-point for the assessment of the effects of ethylene oxide on the terrestrial environment (see also section 8.5.1).
While increases in mortality due to liver, colon, breast, bladder, oesophageal, stomach, brain, or pancreatic cancer have occasionally been reported in epidemiological studies of workers exposed to ethylene oxide, evidence is not consistent or convincing.
Although generally based on small numbers of observed cases, increased risks of leukaemia, all haematopoietic neoplasms (or non-Hodgkin’s lymphoma in the same cohort), lymphopoietic/haematopoietic cancers, or lymphosarcoma/reticulosarcoma have been reported for production and sterilization workers (Hogstedt, 1988), workers in plants producing sterilized medical supplies and spices (Steenland et al., 1991; Stayner et al., 1993; Wong & Trent, 1993), and those producing disposable medical equipment (Hagmar et al., 1995) (Table 5). These excesses occurred in workers exposed primarily to ethylene oxide in the sterilization of medical supplies and equipment rather than in facilities associated with its production or use, where numerous other substances would have been present.
Risks for lymphopoietic/haematopoietic cancers among the various industrial cohorts have varied, although in general by less than 2-fold (Shore et al., 1993). However, it should be noted that with the single exception of the investigation of workers at ethylene production plants in which there was no increase in haematopoietic cancers reported (Teta et al., 1993), length of follow-up was relatively short in the critical investigations, averaging 11.6 years and 16 years for the more reliable studies in which excesses were observed — namely, Hagmar et al. (1995) and Steenland et al. (1991). In the largest investigation (Steenland et al., 1991; Stayner et al., 1993), only 28% of workers had attained greater than 20 years since first exposure, and five of the seven men who died of leukaemia did so within the most recent calendar period. Therefore, limited strength of the observed associations could be due, at least in part, to the limited period of follow-up.
Risks associated with the frequency or intensity of ethylene oxide exposure could be examined in only three studies, and no trends were observed in either the individual or combined studies. A positive trend by cumulative exposure was, however, noted in the largest investigation (Shore et al., 1993). There were no trends in the individual or combined studies with respect to duration of exposure or latency. However, in the largest cohort examined (n = 18 254), with an extensive characterization of individual exposure and a quantitative estimation of cumulative exposure, regression analysis revealed a significant (P < 0.01) exposure–response relationship between cumulative exposure to ethylene oxide and mortality due to lymphocytic leukaemia and non-Hodgkin’s lymphoma combined (termed "lymphoid" neoplasms) (Steenland et al., 1991; Stayner et al., 1993). An association was also observed between cumulative exposure to ethylene oxide and mortality from all haematopoietic neoplasms and non-Hodgkin’s lymphoma; the exposure–response relationship between cumulative exposure and leukaemia was positive, although not statistically significant. Of interest is the additional observation that none of the other measures of exposure (i.e., duration, average, and maximum) in this analysis were predictors of haematopoietic cancers, consistent with results of other investigations.
Therefore, the available epidemiological studies of the association between haematological cancers and exposure to ethylene oxide in occupationally exposed human populations fulfil, in part only, some of the traditional criteria for causality, including exposure–response and temporal relationship. The risks observed are moderate, but this may be a function of short length of follow-up.
Cytogenetic changes within the peripheral blood cells have been reported in a number of cross-sectional studies, principally of populations exposed occupationally to ethylene oxide (Table 4). Inherent limitations of cross-sectional design make these studies less reliable than cohort or case–control studies as a basis for inference of causality. Nevertheless, observation of cytogenetic effects in some groups of workers exposed to elevated concentrations of ethylene oxide in the most sensitive studies, while not necessarily an indicator of chronic adverse health outcomes, provides some limited additional supporting evidence for the ability of ethylene oxide to interact with the genome in individuals exposed to this substance. An increased occurrence of cytogenetic changes has tended to be observed at exposures to >9.2 mg ethylene oxide/m3, thereby satisfying the criterion of exposure–response. In addition, results were consistently positive in the larger studies of populations exposed to higher concentrations (Stolley et al., 1984; Galloway et al., 1986; high-dose group in Mayer et al., 1991; Ribeiro et al., 1994; Richmond et al., 1985). While there was inadequate control for confounding in several of the early investigations (Garry et al., 1979; Yager et al., 1983), frequencies of clastogenic effects were sufficiently elevated in some cases that they were unlikely to be due to potential confounders (Laurent, 1988).
The carcinogenicity of ethylene oxide in humans is also biologically plausible. The incidence of mononuclear leukaemias in F344 rats and lymphomas in mice (in addition to other types of tumours in both species) is increased following inhalation of ethylene oxide (Lynch et al., 1984a,b; Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986; NTP, 1987). The unequivocal genotoxicity of ethylene oxide almost certainly plays a critical role in tumour induction. Ethylene oxide is a potent alkylating agent that has been genotoxic in almost all available studies in laboratory animals. Gene mutations, DNA damage, and cytogenetic effects have been observed routinely in bacterial, rodent, and human cells exposed in vitro to ethylene oxide and in the somatic cells of laboratory species exposed in vivo to this chemical.
Therefore, there is suggestive but inconclusive evidence for an association between exposure to ethylene oxide and haematological cancers in occupationally exposed populations. There is consistent evidence that ethylene oxide interacts with the genome of cells within the circulatory system in occupationally exposed humans and overwhelming supporting evidence of biological plausibility based on carcinogenicity and genotoxicity in laboratory animals. Based on these considerations and the apparent lack of qualitative differences in metabolism between humans and laboratory animals, ethylene oxide is considered highly likely to be carcinogenic to humans.
Although relevant data in humans are not available, dominant lethal mutations, heritable translocations, chromosomal aberrations, DNA damage, and adduct formation in rodent sperm cells have been observed in a number of studies involving the exposure of rats and mice to ethylene oxide. Based upon the likely role for DNA alkylation in production of the genotoxic effects in germ cells in laboratory animals exposed to ethylene oxide, as well as the lack of qualitative differences in the metabolism of this substance between humans and animals (including DNA adduct formation), ethylene oxide can be considered a likely human germ cell mutagen.
Based on reports in occupationally exposed populations, ethylene oxide is irritating to the eyes, skin, and respiratory tract. It is also a sensitizing agent.
Neurological effects have been clearly documented in workers exposed to relatively high concentrations of ethylene oxide. These include a range of clinical signs related to sensorimotor polyneuropathy, diminished or impaired psychomotor skills, reduced peripheral nerve conduction velocity, and, in individuals exposed to >1281 mg/m3, axonal degeneration with mild changes in the myelin sheath of sural nerves and degeneration atrophy of muscles (Kuzuhara et al., 1983). In monkeys, histological alterations in the axons and demyelination in the central nervous system have been observed after exposure to ethylene oxide (Sprinz et al., 1982; Lynch et al., 1984b). In mice, abnormal gait and reduced locomotor activity have been observed after medium-term exposure to low concentrations (Snellings et al., 1984a).
Evidence from epidemiological studies of reproductive effects (i.e., spontaneous abortions) of ethylene oxide in humans is limited (Hemminki et al., 1982, 1983; Rowland et al., 1996). There is also a single report of increased risk of spontaneous abortion in one investigation of women with partners who had had some potential for exposure to this chemical (Lindbolm et al., 1991). With respect to biological plausibility, the data are supported by studies in animals that indicate that, among non-neoplastic effects, reproductive effects (reductions in litter size, increased post-implantation losses, and alterations in sperm morphology, count, and motility) occur at lowest concentration.
Cancer is considered the critical end-point for quantification of exposure–response for the risk characterization for ethylene oxide.7
Quantification of exposure–response for cancer for ethylene oxide is based on studies in laboratory animals, because of limitations of the existing epidemiological data. Moreover, available data indicate that the metabolism and mode of action of ethylene oxide in humans and laboratory animals do not differ qualitatively.
Data suitable for analysis of exposure–response are available from two carcinogenesis bioassays in F344 rats (Lynch et al., 1984a,b; Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986) and one in B6C3F1 mice (NTP, 1987). In F344 rats, there were dose-related increases in the incidence of mononuclear leukaemias, peritoneal mesotheliomas, and brain tumours; in mice, the incidence of lung carcinomas, malignant lymphomas, uterine adenocarcinomas, mammary adenocarcinomas and adenosquamous carcinomas, and Harderian cystadenomas was increased.
Concentrations of ethylene oxide causing a 5% increase in tumour incidence over background (i.e., tumorigenic concentration05s, or TC05s), calculated as described in Appendix 4, range from 2.2 mg/m3 (unit risk = 0.05/2.2 mg/m3 = 0.023 per mg/m3) (95% lower confidence limit [LCL] = 1.5 mg/m3) for mononuclear leukaemia8 in female F344 rats to 31.0 mg/m3 (95% LCL = 16.1 mg/m3) for brain tumours in female 344 rats. TC05s for comparable tumours in the study in which exposure–response was less well characterized (Lynch et al., 1984a,b)9 were somewhat higher (12.5–31.9 mg/m3, respectively) (Table 6).
Table 6: TC05s for ethylene oxide.
|
Tumour incidence |
TC05 |
LCL on TC05 |
Chi-square |
df |
P-value |
|
Male rats exposed to 0, 92, or 183 mg ethylene oxide/m3, 7 h/day, 5 days/week |
|||||
|
Incidence of mononuclear cell leukaemia: 24/77, 38/70, 30/76 |
12.5 |
5.1 |
3.5 |
1 |
0.06 |
|
Incidence of peritoneal mesothelioma: 3/78, 9/79, 21/79 |
14.4 |
6.1 |
0 |
0 |
– |
|
Incidence of brain mixed cell glioma: 0/76, 2/77, 5/79 |
31.9 |
18.3 |
0 |
1 |
1.0 |
|
Male and female rats exposed to 0, 18.3, 60.4, or 183 mg ethylene oxide/m3, |
|||||
|
Incidence of mononuclear leukaemia in males: 13/97, 9/51, 12/39, 9/30 |
6.0 |
3.1 |
2.2 |
2 |
0.34 |
|
Incidence of mononuclear leukaemia in females: 11/116, 11/54, 14/48, 15/26 |
2.2 |
1.5 |
0.58 |
2 |
0.75 |
|
Incidence of peritoneal mesothelioma in males: 2/97, 2/51, 4/39, 4/30 |
10.8 |
5.6 |
0.78 |
2 |
0.68 |
|
Incidence of primary brain tumours in males: 1/181, 1/92, 5/85, 7/87 |
17.5 |
10.8 |
1.6 |
2 |
0.50 |
|
Incidence of primary brain tumours in females: 1/188, 1/94, 3/92, 4/80 |
31.0 |
16.1 |
0.45 |
2 |
0.80 |
|
Male and female mice exposed to 0, 92, or 183 mg ethylene oxide/m3, |
|||||
|
Incidence of lung carcinoma in males: 6/50, 10/50, 16/50 |
10.2 |
4.1 |
0 |
0 |
– |
|
Incidence of lung carcinoma in females: 0/49, 1/48, 7/49 |
19.8 |
10.3 |
0.34 |
2 |
0.84 |
|
Incidence of malignant lymphoma in females: 9/49, 6/48, 22/49 |
12.2 |
6.3 |
3.5 |
1 |
0.06 |
|
Incidence of uterine adenocarcinoma: 0/49, 1/47, 5/47 |
22.7 |
11.4 |
0.07 |
2 |
0.97 |
|
Incidence of mammary adenocarcinoma and adenosquamous carcinoma in females: 1/49, 8/48, 6/49 |
10.4 |
6.0 |
3.0 |
1 |
0.08 |
|
Incidence of Harderian cystadenoma in males: 1/43, 9/44, 8/42 |
6.7 |
4.2 |
2.0 |
1 |
0.16 |
|
Incidence of Harderian cystadenoma in females: 1/46, 6/46, 8/47 |
9.1 |
5.5 |
0.30 |
1 |
0.58 |
|
a |
For this study, the resulting TC05s (and LCL on TC05s) were multiplied by (7 h/day / 24 h/day) × (5 days/week / 7 days/week) to adjust for intermittent to continuous exposure. |
|
b |
For this study, the resulting TC05s (and LCL on TC05s) were multiplied by (6 h/day / 24 h/day) × (5 days/week / 7 days/week) to adjust for intermittent to continuous exposure. |
Values of the TC05s in mice ranged from 6.7 mg/m3 (95% LCL = 4.2 mg/m3) for Harderian cystadenomas in males to 22.7 mg/m3 (95% LCL = 11.4 mg/m3) for uterine adenocarcinomas. It should be noted, however, that characterization of exposure–response in the NTP (1987) bioassay on which these values are based was not optimal; there were only two dose groups and controls, with the lowest administered concentration being 92 mg/m3 (Table 6).
Based on modelling (using THC program; Howe, 1995) of the incidence of Hprt mutations in splenic T-lymphocytes of male B6C3F1 mice (Big Blue®, lacI transgenic) exposed to ethylene oxide for 4 weeksfn10 (Walker et al., 1997a), the benchmark concentration05 (BMC05) for somatic cell mutations (i.e., the concentration associated with a 5% increase in the incidence of Hprt mutation) (adjusted for intermittent to continuous exposure) was within the range of the lowest TC05s in rats and mice. It should be noted, however, that characterization of exposure–response in Walker et al. (1997a) was not optimal; although there were three dose groups and controls, the lowest administered concentration was 92 mg/m3.
In the interest of utilizing all available data to inform characterization of exposure–response, the tumorigenic potencies developed based on studies in animals were compared with risks of haematological cancers reported in epidemiological studies in populations occupationally exposed to ethylene oxide. The protocol and results of these analyses are reported elsewhere (Health Canada, 1999b). Results indicated that risks predicted based on the most sensitive outcome in rats (mononuclear cell leukaemia in female F344 rats) were consistent with the confidence intervals of the SMRs observed for both leukaemias overall and all haematopoietic neoplasms in males in the cohort study by Stayner et al. (1993) (i.e., the only epidemiological study in which individual cumulative exposure was characterized). However, the limitations of this comparative exercise preclude its meaningful contribution to quantification of risk. These include uncertainties of the available epidemiological data on ethylene oxide, which prevent adequate consideration of traditional criteria for causality (particularly with respect to periods of follow-up in investigations of greatest sensitivity). Moreover, meaningful direct comparison of potency in laboratory animals with that in humans is precarious at best, in light of the inadequacy of available information on interspecies variations in kinetics and metabolism and mode of action to serve as a basis for characterization of site concordance between animals and humans and the extremely wide range of the confidence limits on the SMRs in the epidemiological studies.
There have been several efforts to quantify the genetic risk to the offspring of humans exposed to ethylene oxide, the most comprehensive of which is that of Natarajan et al. (1995), which documents the outcome of deliberations of an international workshop of experts. This exercise was undertaken to identify data gaps that would permit a more refined estimate of heritable genetic risk from ethylene oxide and to acquire experience with the parallelogram approach to better inform future efforts in this area. The outcome is presented here primarily as a basis for comparison with the tumorigenic potencies for cancer, to ensure that measures developed for this end-point will be protective for other reported effects. However, it meets this objective only in part, since the calculated genetic risk is underestimated; it is based on induced dominant visible mutations only and does not take into consideration recessive mutation, dominant lethal mutations, or heritable translocations. The relevant data for these end-points were judged either not to be sufficiently robust or to result in a very small increment in actual genetic risk to live offspring. An increase in dominant lethal mutations in humans might be manifested in an increase in spontaneous abortions, as reported in some hospital sterilization workers (Hemminki et al., 1982).
The analysis was based on induced dominant visible mutations in mice from a study by Lewis et al. (1986), which was designed to mimic human occupational exposure (i.e., involving exposure for prolonged periods in order to cover all stages of spermatogenesis). Using the parallelogram approach and additional quantitative data on somatic mutations (Hprt in splenocytes) in mice (Walker et al., 1994) and in an occupationally exposed human population (HPRT) (Tates et al., 1991), it was estimated that exposure for one working year (1800 h) to 1.8 mg ethylene oxide/m3 would lead to an incremental risk of 4 × 10–4 above background that a disease with dominant inheritance would be transferred to the offspring. As a basis for comparison with the potency estimates for cancer, the BMC05 for this effect would be 46 mg/m3.11
Identified sources of uncertainty of the estimate were the doubling dose for Hprt mutations in the mouse, the doubling dose for HPRT mutations in humans, the mutation rate in mice, the number of loci involved, the risk from exposure of females, the extrapolation from mutation frequency to dominant disease states, and the possible influence of dose rates (Natarajan et al., 1995). Although there was some attempt by the authors to quantify uncertainty from these sources, such an estimate does not reflect uncertainty associated with reliance on limited (possibly unrepresentative) data, which could be considerably greater.
Non-neoplastic effects of ethylene oxide have been observed only at concentrations greater than those at which increases in tumours have been reported in other studies. In addition, in view of the likely critical role of the genotoxicity of ethylene oxide, for which the weight of evidence is consistent and convincing in the induction of tumours, cancer is clearly the critical end-point for quantification of exposure–response for risk characterization for long-term exposure of the general population, and measures based on this end-point will be protective for other reported effects. For example, a tolerable concentration based upon observed effects on the sperm and brain in monkeys exposed to ethylene oxide over a long period (Sprinz et al., 1982; Lynch et al., 1984b,c; Setzer et al., 1996) or upon reproductive effects observed in rats exposed to ethylene oxide in a subchronic toxicity study (Snellings et al., 1982b) would be in the range of tens of micrograms per cubic metre.
Although limited, available data are consistent with air being the principal medium of exposure of the general population to ethylene oxide; intake from other media is likely to be negligible in comparison. Moreover, with the exception of air in the vicinity of industrial point sources, ethylene oxide has seldom been determined or detected in samples of ambient air, indoor air, or drinking-water.
For compounds such as ethylene oxide, where induction of tumours likely involves direct interaction with genetic material, in view of the consistent and unequivocal evidence of genotoxicity, estimates of exposure are compared with quantitative estimates of cancer potency to characterize risk. The lowest TC05 in the study in rats with optimal characterization of exposure–response was 2.2 mg/m3 for the development of mononuclear leukaemias in female F344 rats exposed via inhalation to ethylene oxide; the 95% LCL was 1.5 mg/m3 (Table 6). The margins between carcinogenic potency and the extremely limited data on measured and predicted concentrations of ethylene oxide in ambient (and indoor) air in Canada (and elsewhere) are presented in Table 7. Based upon margins between censored mean concentrations for monitoring data from a multi-media exposure study conducted in Canada, predicted risks are within the category of equivalent low-dose risk estimatesfn12 of >10–7 to 10–5, although it should be noted that this is based on detection in a very small proportion of samples in the study. The predicted risks in the vicinity of an ethylene oxide production facility or facilities using this substance for sterilization are within the category of equivalent low-dose risk estimates of >10–5. However, it should be noted that this is based on concentrations modelled taking into account information on releases, which have not been validated by monitoring data. Although monitored levels in Los Angeles, California, in 1990 exceeded these values considerably (maximum mean = 956 µg/m3), the 95% confidence interval was wide (0.75–5600 µg/m3), and the sample size (n = 6 samples from single location with highest concentrations) was small (Havlicek et al., 1992).
Table 7: The margins between carcinogenic potency and limited available data on predicted and measured concentrations of ethylene oxide.
|
Concentration of exposure to ethylene oxide |
Margin between exposure and potency estimates: |
Category of equivalent low-dose risk estimatea |
|
|
0.0062 µg/m3; concentration in ambient air in Southern Ontario predicted from ChemCAN fugacity model |
TC05 |
350 000 |
10–7 to 10–5 |
|
0.34 µg/m3; censored mean concentration in ambient air from multi-media survey in Canada (Health Canada, 1999a) |
TC05 |
6 500 |
10–7 to 10–5 |
|
0.17 µg/m3; censored mean concentration in indoor air from multi-media survey in Canada (Health Canada, 1999a) |
TC05 |
13 000 |
10–7 to 10–5 |
|
2.12 µg/m3; predicted maximum average daily concentration in ambient air in the vicinity of Canadian hospitals |
TC05 |
1 040 |
> 10–5 |
|
4.9 µg/m3; maximum concentration in ambient air from multi-media survey in Canada (Health Canada, 1999a) |
TC05 |
450 |
> 10–5 |
|
4.0 µg/m3; maximum concentration in indoor air from multi-media survey in Canada (Health Canada, 1999a) |
TC05 |
550 |
> 10–5 |
|
20.1 µg/m3; predicted maximum 1-h ground-level concentration in ambient air in the vicinity of an ethylene oxide production facility in Canada |
TC05 |
110 |
> 10–5 |
|
2 µg/m3; predicted maximum average annual concentration in ambient air in the vicinity of a sterilization facility in Florida (Tutt & Tilley, 1993) |
TC05 |
1 100 |
> 10–5 |
|
11 µg/m3; predicted maximum average annual concentration in ambient air in the vicinity of a sterilization facility in Florida (Tutt & Tilley, 1993) |
TC05 |
200 |
> 10–5 |
|
956 µg/m3; highest mean 24-h ambient air concentration sampled in Los Angeles (Havlicek et al., 1992) |
TC05 |
2.3 |
> 10–5 |
a Categories of equivalent low-dose risk estimates: <10–7; >10–7 to <10–5; and >10–5 (Health Canada, 1994).
While some limitations of the data on exposure have been indicated above, this text addresses uncertainties in data on effects, since it is this information that is likely most relevant in an international context. Exposure estimates and resulting risk characterizations13 presented in CICADs are examples, only.
The degree of confidence in the database on the toxicity of ethylene oxide is moderate. While the database on non-cancer toxicity in laboratory animals is limited, there is a high degree of confidence that cancer and heritable genotoxicity occur at lowest concentrations, and risk management measures developed on the basis of exposure–response for these effects will be protective for other adverse effects in the general population.
The carcinogenicity of ethylene oxide in humans has been investigated in a number of studies, the largest of which involved a cohort of more than 18 000 individuals. However, limitations of these investigations prevent adequate consideration of traditional criteria for causality (particularly with respect to periods of follow-up in investigations of greatest sensitivity).14 Similarly, epidemiological studies on cytogenetic changes and reproductive effects in human populations are inadequate to allow any inference concerning causality to be drawn.
While there is a high degree of certainty that the genotoxicity of ethylene oxide plays an important role in the carcinogenicity of this substance and that the metabolism and mode of action of ethylene oxide in humans and laboratory animals do not differ qualitatively, the mode of action in inducing cancer or heritable genotoxic effects has not been clearly delineated. Possible quantitative variations between humans and animals have also not been elucidated.
Meaningful direct comparison of carcinogenic potency in laboratory animals with that in humans is precluded due to limitations of the epidemiological database, the inadequacy of available information on interspecies variations in kinetics, metabolism, and mode of action to serve as a basis for characterization of site concordance between animals and humans, and the extremely wide range of the confidence intervals on the SMRs in the epidemiological studies.
There is some uncertainty concerning the relevance to humans of mononuclear cell leukaemias in F344 rats, since this type of tumour is specific to this strain of rat, it arises spontaneously with a significant frequency in older unexposed animals, and its etiology has not been definitively identified. However, TC05s for the tumours with next greatest potency for the studies in rats and mice with optimum characterization of exposure–response would be approximately only 3-fold greater, and the outcome of the sample risk characterization would remain the same. The 95% LCL on the TC05 for mononuclear leukaemia in female rats was 1.5 mg/m3, versus the maximum likelihood estimate of 2.2 mg/m3. Based upon the highest TC05 identified from the study in which exposure–response was best characterized (i.e., 31.0 mg/m3 for primary brain tumours in female F344 rats), the resulting potency would be approximately 14-fold lower than those derived (in section 11.1.3) on the basis of the mononuclear cell leukaemias in female F344 rats.
All reported releases of ethylene oxide are to air, and pathways analysis indicates that, following release to air, ethylene oxide is unlikely to partition to other compartments in significant amounts. Its high water solubility may encourage some washout via precipitation; however, available evidence indicates that this removal mechanism has minimal impact.
Although releases to water and soil are not common, it is understood that some releases to these media may occur in the event of a spill or similar release scenario. Persistence in these media is not expected, as ethylene oxide has a high Henry’s law constant (12.2–19.9 Pa·m3/mol), and the experimental data indicate that volatilization from water will occur rapidly (half-life ~1 h). Although no information was available regarding concentrations of ethylene oxide in wastewater discharged from manufacturing and processing operations, releases from these sources are expected to be minimal, especially when one considers temperatures and retention times in wastewater treatment processes. Based on these considerations, aquatic concentrations are expected to be negligible; therefore, potential for adverse effects on aquatic organisms is also considered negligible.
Given that the primary medium of release for ethylene oxide is the atmosphere and that the chemical’s properties cause it to remain in and react in that compartment, the assessment end-point will be atmospherically exposed organisms. Although evidence is strong concerning ethylene oxide-induced genotoxic and carcinogenic effects (see sections 9.1 and 9.2), the actual population-level impact on wildlife from these end-points is not completely clear when one considers population resilience, dose–response, and induction frequency. Among the observed effects, adverse impacts on reproduction are decidedly the end-point that would have the greatest potential to adversely impact wildlife population levels. Other effects may occur at slightly lower concentrations.
There are only a few ambient measurements of ethylene oxide in Canada. Some limited additional data were identified for the urban area of Los Angeles, California, USA (Havlicek et al., 1992). The maximum mean 24-h ambient air concentration detected in the Los Angeles urban area was 956 µg/m3 (95% CI = 0.75–5600 µg/m3; n = 6 samples from single location with highest concentrations) in May 1990 and will be used as the estimated exposure value (EEV) to represent a worst-case atmospheric concentration.
Data on toxicity are very limited for all of the environmental compartments. The most sensitive terrestrial organisms were laboratory test rodents, which will be considered as surrogates for wild rodent species. The critical toxicity value (CTV) is derived from a reproductive study by Snellings et al. (1982b); the effects reported in this study were determined to represent the most significant ecological end-point in terms of the potential to adversely impact natural wildlife populations. The authors reported that at the highest exposure concentration (183 mg/m3), there was a significant drop in the number of pups born per litter. There were also fewer implantation sites and fewer pups born per implantation site. Thus, the CTV for terrestrial animals is 183 mg/m3. The CTV is based on a relatively small data set and was the maximum concentration tested in the study. In addition, the study was conducted in the laboratory (not the field), and no statistics were applied to determine whether 183 mg/m3 is truly the lowest adverse effect concentration, nor was the study designed to make this determination. For these reasons, and because effects less clearly related to population-level impacts (e.g., decreased weight) were observed at slightly lower levels, a relatively large application factor of 100 is applied to the CTV to derive the estimated no-effects value (ENEV) for terrestrial biota of 1830 µg/m3. The quotient EEV/ENEV thus is 956/1830 = 0.52. Since the quotient is less than 1, the risks posed by long-term exposure of terrestrial biota to ethylene oxide in the environment are expected to be minimal.
Data on toxicity are available for a limited number of species in all environmental compartments. Some level of uncertainty remains for those studies prepared using nominal concentrations as opposed to measured. In addition, although mutagenic effects have been observed in a variety of terrestrial plants and mammals, their population-level impacts are uncertain.
The large variation in atmospheric concentrations in the state of California over a short period of time (see section 6.1.1) suggests that there may be some uncertainty in these ambient values.
IARC (1994) had concluded that there is limited evidence for the carcinogenicity of ethylene oxide in humans, but there is sufficient evidence for the carcino-genicity of ethylene oxide in experimental animals. As ethylene oxide is a powerful mutagen and clastogen at all phylogenetic levels and induces clastogenic changes and DNA and haemoglobin adducts in exposed workers, and as the tumorigenic response in experimental animals is similar to that in humans, IARC (1994) concluded that ethylene oxide is carcinogenic to humans (Group 1).
Abeles FB, Dunn LJ (1985) Ethylene-enhanced ethylene oxidation in Vicia faba. Journal of Plant Growth Regulation, 4:123–128.
Abrahams RH (1980) Recent studies with workers exposed to ethylene oxide. In: Jorkasky JF, ed. The safe use of ethylene oxide. Proceedings of the Educational Seminar, Arlington, VA. Washington, DC, Health Industry Manufacturers Association, pp. 27–38 (HIMA Report No. 80-4).
Adkins B, Van Stee EW, Simmons JE, Eustis SL (1986) Oncogenic response of strain A/J mice to inhaled chemicals. Journal of Toxicology and Environmental Health, 17:311–322.
Agriculture and Agri-Food Canada (1996) Regulatory information on pesticide products. Ethylene oxide fumigant-sterilant gas. Registrant: Proxair Canada Inc.
ARET (1999) Environmental Leaders 3: Voluntary action on toxic substances (A progress report). Environment Canada, Accelerated Reduction/Elimination of Toxics. Ottawa, Ontario, Minister of Public Works and Government Services Canada.
Atkinson R (1986) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chemical Reviews, 86:69–201.
ATSDR (1990) Toxicological profile for ethylene oxide. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (Tp-90-16).
Babich H (1985) Reproductive and carcinogenic health risks to hospital personnel from chemical exposure — A literature review. Journal of Environmental Health, 48:52–56.
Bailey JC, Brooks AGF, Dollery CT, Farmer PB, Passingham BJ, Sleightholm MA, Yates DW (1988) Hydroxyethylvaline adduct formation in haemoglobin as a biological monitor of cigarette smoke intake. Archives of Toxicology, 62:247–253.
Bastlová T, Andersson B, Lambert B, Kolman A (1993) Molecular analysis of ethylene oxide-induced mutations at the HPRT locus in human diploid fibroblasts. Mutation Research, 287:283–292.
Benson LO, Teta MJ (1993) Mortality due to pancreatic and lymphopoietic cancers in chlorohydrin production workers. British Journal of Industrial Medicine, 50:710–716.
Berkopec B, Vidic A (1996) Ecological problems of sterilization with ethylene oxide in Slovenia. Gefahrstoffe-Reinhaltung der Luft, 56:73–76.
Binder H (1974) Ethylene oxide and chlorohydrin in tobacco and its smoke. Fachliche Mitteilungen der Oesterreichischen Tabakregie, 15:294–301 (in German) [cited in Bogyo DA, Lande SS, Meylan WM, Howard PH, Santodonato J (1980) Investigation of selected potential environmental contaminants: Epoxides. Final technical report. Syracuse, NY, Syracuse Research Corporation, Center for Chemical Hazards Assessment (EPA-560/11-80-005)].
Biro L, Fisher AA, Price E (1974) Ethylene oxide burns. Archives of Dermatology, 110:924–925.
Bisanti L, Maggini M, Raschetti R, Spila Alegiani S, Menniti Ippolito F, Caffari B, Segnan N, Ponti A (1993) Cancer mortality in ethylene oxide workers. British Journal of Industrial Medicine, 50:317–324.
Blixt S, Ehrenbergh L, Gelin O (1963) Studies of induced mutations in peas. VII. Mutation spectrum and mutation rate of different mutagenic agents. Agri Hortique Genetica, 21:178–216 [cited in IPCS, 1985].
Bolt HM (1996) Quantification of endogenous carcinogens, the ethylene oxide paradox. Biochemical Pharmacology, 52(1):1–5.
Bolt HM, Leutbecher M, Golka K (1997) A note on the physiological background of the ethylene oxide adduct 7-(2-hydroxyethyl)guanine in DNA from human blood. Archives of Toxicology, 71:719–721.
Bommer J, Ritz E (1987) Ethylene oxide as a major cause of anaphylactoid reactions in dialysis (a review). Artificial Organs, 11:111–117.
Bridié AL, Wolff CJM, Winter M (1979a) BOD and COD of some petrochemicals. Water Research, 13:627–630.
Bridié AL, Wolff CJM, Winter M (1979b) The acute toxicity of some petrochemicals to goldfish. Water Research, 13:623–626.
Brown CD, Wong BA, Fennell TR (1996) In vivo and in vitro kinetics of ethylene oxide metabolism in rats and mice. Toxicology and Applied Pharmacology, 136:8–19.
Brown CD, Asgharian B, Turner MJ, Fennell TR (1998) Ethylene oxide dosimetry in the mouse. Toxicology and Applied Pharmacology, 148:215–221.
BUA (1995) Ethylene oxide. German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (Beratergremium für Umweltrelevante Altstoffe). Stuttgart, Hirzel Verlag (BUA Report 141).
Bunce NJ (1996) Atmospheric properties of substances on the Priority Substances List #2 (PSL2). Report to Environment Canada. Guelph, Ontario, University of Guelph.
Canadian Hospital Association, Environment Canada (1994) The elimination of CFCs in health care facilities. Ottawa, Ontario, CHA Press (ISBN 1-896151-16-7).
CHIP (1982) Chemical hazard information profile (ethylene oxide). Washington, DC, US Environmental Protection Agency, Office of Pesticide Programs and Toxic Substances [cited in NTP, 2000].
CIS (1997) Ethylene oxide (CPI product profiles). Don Mills, Ontario, Camford Information Services Inc.
Clare MG, Dean BJ, de Jong G, van Sittert NJ (1985) Chromosome analysis of lymphocytes from workers at an ethylene oxide plant. Mutation Research, 156:109–116.
Conor Pacific Environmental (1998) A report on multimedia exposures to selected PSL2 substances. Prepared by Conor Pacific Environmental (formerly Bovar Environmental) and Maxxam Analytics Inc. for Health Canada, Ottawa, Ontario (Project No. 741-6705; Contract #DSS File No. 025SS.H4078-6-C574).
Conway RA, Waggy GT, Spiegel MH, Berglund RL (1983) Environmental fate and effects of ethylene oxide. Environmental Science and Technology, 17:107–112.
Crystal HA, Schaumburg HH, Grober E, Fuld PA, Lipton RB (1988) Cognitive impairment and sensory loss associated with chronic low-level ethylene oxide exposure. Neurology, 38:567–569.
Csanády GA, Denk B, Putz C, Kreuzer PE, Kessler W, Baur C, Gargas ML, Filser JG (2000) A physiological toxicokinetic model for exogenous and endogenous ethylene and ethylene oxide in rat, mouse and human: formation of 2-hydroxyethyl adducts with hemoglobin and DNA. Toxicology and Applied Pharmacology, 165:1–26.
Currier MF, Carlo GL, Poston PL, Weledford WE (1984) A cross sectional study of employees with potential occupational exposure to ethylene oxide. British Journal of Industrial Medicine, 41:492–498.
De Bont JAM, Albers RAJM (1976) Microbial metabolism of ethylene. Antonie Van Leeuwenhoek Journal of Microbiology and Serology, 42(1–2):80.
Dellarco VL, Generoso WM, Sega GA, Fowle JR III, Jacobson-Kram D (1990) Review of the mutagenicity of ethylene oxide. Environmental and Molecular Mutagenesis, 16:85–103.
Deschamps D, Leport M, Laurent A-M, Cordier S, Festy B, Conso F (1990) Toxicity of ethylene oxide on the lens and on leukocytes: an epidemiological study in hospital sterilisation installations. British Journal of Industrial Medicine, 47:308–313.
DMER, AEL (1996) Pathways analysis of ethylene oxide for the second Priority Substances List using fugacity modeling. Prepared for Chemicals Evaluation Division, Commercial Chemicals Evaluation Branch, Environment Canada. Peterborough, Ontario, Don Mackay Environmental Research; and Don Mills, Ontario, Angus Environmental Limited.
Duggan RE, Corneliussen PE, Duggan MS, McMahon BM, Martin RJ (1983) Pesticide residue levels in foods in the United States from July 1, 1969 to June 30, 1976. In: Residue monitoring data. Published jointly by the US Food and Drug Administration and the Association of Official Analytical Chemists, pp. 1–34 (TX571.P4 P476).
Dugue P, Faraut C, Figueredo M, Bettendorf A, Salvadori JM (1991) Asthme professionnel à l’oxyde d’éthylène chez une infirmiére. Presse Médicale, 20:1455.
Dunkelberg H (1981) Carcinogenic activity of ethylene oxide and its reaction products 2-chloroethanol, 2-bromoethanol, ethylene glycol and diethylene glycol. I. Carcinogenicity of ethylene oxide in comparison with 1,2-propylene oxide after subcutaneous administration in mice. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene, Abteilung 1, Originale, Reihe B, 174:383–404 (in German) [cited in IARC, 1994].
Dunkelberg H (1982) Carcinogenicity of ethylene oxide and 1,2-propylene oxide upon intragastric administration to rats. British Journal of Cancer, 46:924–933.
Ehrenberg L, Gustafsson A, Lundqvist U (1956) Chemically induced mutation and sterility in barley. Acta Chemica Scandinavica, 10:492–494 [cited in IPCS, 1985; US EPA, 1985].
Ehrenberg L, Gustafsson A, Lundqvist U (1959) The mutagenic effects of ionizing radiations and reactive ethylene derivatives in barley. Hereditas, 45:351–368 [cited in IPCS, 1985].
Ehrenberg L, Hiesche KD, Osterman-Golkar S, Wennberg I (1974) Evaluation of genetic risks of alkylating agents: tissue doses in the mouse from air contaminated with ethylene oxide. Mutation Research, 24:83–103.
Eller PM, ed. (1987a) NIOSH Method 1614. In: NIOSH manual of analytical methods, 3rd ed., Supplement 2. Washington, DC, US Department of Health and Human Services, National Institute for Occupational Safety and Health, pp. 1614-1 – 1614-6 (NIOSH Publication No. 84-100).
Eller PM, ed. (1987b) NIOSH Method 3702. In: NIOSH manual of analytical methods, 3rd ed., Supplement 2. Washington, DC, US Department of Health and Human Services, National Institute for Occupational Safety and Health, pp. 3702-1 – 3702-4 (NIOSH Publication No. 84-100).
Environment Canada (1985) Ethylene oxide: Environmental and technical information for problem spills (TIPS). Ottawa, Ontario, Minister of Supply and Services Canada (ISBN 0-662-14112-1).
Environment Canada (1997) Results of the CEPA Section 16 Notice to Industry respecting the second Priority Substances List and di(2-ethylhexyl) phthalate. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch, Use Patterns Section.
Environment Canada (1999) Canadian Environmental Protection Act — Priority Substances List — Supporting document for the environmental assessment of ethylene oxide. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch.
Environment Canada, Health Canada (2001) Canadian Environmental Protection Act, 1999 — Priority Substances List assessment report — Ethylene oxide. Ottawa, Ontario, Minister of Public Works and Government Services.
Farmer PB, Sepai O, Lawrence R, Autrup H, Nielsen PS, Vestergård AB, Waters R, Leuratti C, Jones NJ, Stone J, Baan RA, van Delft JHM, Steenwinkel MJST, Kyrtopoulos SA, Souliotis VL, Theodorakopoulos N, Bacalis NC, Natarajan AT, Tates AD, Haugen A, Andreassen Å, Øvrebø S, Shuker DEG, Amaning KS, Schouft A, Ellul A, Garner RC, Dingley KH, Abbondandolo A, Merlo F, Cole J, Aldrich K, Beare D, Capulas E, Rowley G, Waugh APW, Povey AC, Haque K, Kirsch-Volders M, Van Hummelen P, Castelain P (1996) Biomonitoring human exposure to environmental carcinogenic chemicals. Mutagenesis, 11(4):363–381.
Fennell TR, Brown CD (2001) A physiologically based pharmacokinetic model for ethylene oxide in mouse, rat and human. Toxicology and Applied Pharmacology, 173:161–175.
Fennell TR, MacNeela JP, Morris RW, Watson M, Thompson CL, Bell DA (2000) Hemoglobin adducts from acrylonitrile and ethylene oxide in cigarette smokers: effects of glutathione S-transferase T1- null and M1-null genotypes. Cancer Epidemiology, Biomarkers & Prevention, 9:705–712.
Filser JG, Bolt HM (1984) Inhalation pharmacokinetics based on gas uptake studies. VI. Comparative evaluation of ethylene oxide and butadiene monoxide as exhaled reactive metabolites of ethylene and 1,3-butadiene in rats. Archives of Toxicology, 55:219–223.
Filser JG, Kreuzer PE, Greim H, Bolt HM (1994) New scientific arguments for regulation of ethylene oxide residues in skin-care products. Archives of Toxicology, 68:401–405.
Finelli PF, Morgan TF, Yaar I, Granger CV (1983) Ethylene-oxide-induced polyneuropathy. Archives of Neurology, 40:419–421.
Fisher A (1988) Burns of the hands due to ethylene oxide used to sterilize gloves. Cutis, 42:267–268.
Föst U, Marczynski B, Kasermann R, Peter H (1989) Determination of 7-(2-hydroxyethyl)guanine with gas chromatography/mass spectrometry as a parameter for genotoxicity of ethylene oxide. Archives of Toxicology, Supplement 13:250–253.
Fuchs J, Wullenweber U, Hengstler JG, Bienfait HG, Hiltl G, Oesch F (1994) Genotoxic risk for humans due to workplace exposure to ethylene oxide: remarkable individual differences in susceptibility. Archives of Toxicology, 68:343–348.
Fujishiro K, Mori K, Inoue N (1990) Chronic inhalation effects of ethylene oxide on porphyrin-heme metabolism. Toxicology, 61:1–11.
Fujishiro K, Mori K, Inoue N (1991) Effects of inhaled ethylene oxide on the lens glutathione redox cycle in rats [short communication]. Archives of Toxicology, 65:606–607.
Fukushima T, Abe K, Nakagawa A, Osaki Y, Yoshida N, Yamane Y (1986) Chronic ethylene oxide poisoning in a factory manufacturing medical appliances. Journal of the Society of Occupational Medicine, 36:118–123.
Galloway SM, Berry PK, Nichols WW, Wolman SR, Soper KA, Stolley PD, Archer P (1986) Chromosome aberrations in individuals occupationally exposed to ethylene oxide, and in a large control population. Mutation Research, 170:55–74.
Gardner MJ, Coggon D, Pannett B, Harris EC (1989) Workers exposed to ethylene oxide: a follow up study. British Journal of Industrial Medicine, 46:860–865.
Garman RH, Snellings WM (1986) Frequency, size and location of brain tumours in F-344 rats chronically exposed to ethylene oxide. Food and Chemical Toxicology, 24(2):145–153.
Garman RH, Snellings WM, Maronpot RR (1985) Brain tumors in F344 rats associated with chronic inhalation exposure to ethylene oxide. Neurotoxicology, 6(1):117–138.
Garry VF, Hozier J, Jacobs D, Wade RL, Gray DG (1979) Ethylene oxide: evidence of human chromosomal effects. Environmental Mutagenesis, 1:375–382.
Gaskin RE, Holloway PJ (1992) Some physicochemical factors influencing foliar uptake enhancement of glyphosatemono(isopropyl ammonium) by polyoxyethylene surfactants. Pesticide Science, 34:195–206.
Generoso WM, Rutledge JC, Cain KT, Hughes LA, Braden PW (1987) Exposure of female mice to ethylene oxide within hours after mating leads to fetal malformation and death. Mutation Research, 176:269–274.
Generoso WM, Cain KT, Cornett CV, Cacheiro NLA, Hughes LA (1990) Concentration–response curves for ethylene-oxide-induced heritable translocations and dominant lethal mutations. Environmental and Molecular Mutagenesis, 16:126–131.
Gérin M, Tardif R (1986) Urinary N-acetyl-S-2-hydroxyethyl-L-cysteine in rats as biological indicator of ethylene oxide exposure. Fundamental and Applied Toxicology, 7:419–423.
Gilding DK, Reed AM, Baskett SA (1980) Ethylene oxide sterilization: effect of polymer structure and sterilization conditions on residue levels. Biomaterials, 1:145–148.
Gillespie EH, Jackson JM, Owen GR (1979) Ethylene oxide sterilization: is it safe? Journal of Clinical Pathology, 32:1184–1187.
Golberg L (1986) Hazard assessment of ethylene oxide. Boca Raton, FL, CRC Press, 196 pp.
Granath F, Rohlén O, Göransson C, Hansson L, Magnusson A-L, Törnqvist M (1996) Relationship between dose in vivo of ethylene oxide and exposure to ethene studied in exposed workers. Human and Experimental Toxicology, 15:826–833.
Greenberg HL, Ott MG, Shore RE (1990) Men assigned to ethylene oxide production or other ethylene oxide related chemical manufacturing: a mortality study. British Journal of Industrial Medicine, 47:221–230.
Greife AL, Hornung RW, Stayner LG, Steenland KN (1988) Development of a model for use in estimating exposure to ethylene oxide in a retrospective cohort mortality study. Scandinavian Journal of Work, Environment and Health, 14(Suppl. 1):29–30.
Gross JA, Hass ML, Swift TR (1979) Ethylene oxide neurotoxicity: report of four cases and review of the literature. Neurology, 29:978–983.
Hackett PL, Brown MG, Buschbom RL, Clark ML, Miller RA, Music RL, Rowe SE, Schirmer RE, Sikov MR (1982) Teratogenic study of ethylene and propylene oxide and n-butyl acetate. Richland, WA, Battelle Pacific Northwest Laboratories (NIOSH Contract No. 210-80-0013).
Hagmar L, Welinder H, Lindén K, Attewell R, Osterman-Golkar S, Törnqvist M (1991) An epidemiological study of cancer risk among workers exposed to ethylene oxide using haemoglobin adducts to validate environmental exposure assessments. International Archives of Occupational and Environmental Health, 63:271–277.
Hagmar L, Mikoczy Z, Welinder H (1995) Cancer incidence in Swedish sterilant workers exposed to ethylene oxide. Occupational and Environmental Medicine, 52:154–156.
Hallier E, Langhof T, Dannappel D, Leutbecher M, Schröder K, Goergens HW, Müller A, Bolt HM (1993) Polymorphism of glutathione conjugation of methyl bromide, ethylene oxide and dichloromethane in human blood: influence on the induction of sister chromatid exchanges (SCE) in lymphocytes. Archives of Toxicology, 67:173–178.
Hanifin JM (1971) Ethylene oxide dermatitis. Journal of the American Medical Association, 217:213.
Hansen JP, Allen J, Brock K, Falconer J, Helms MJ, Shaver GC, Strohm B (1984) Normal sister chromatid exchange levels in hospital sterilization employees exposed to ethylene oxide. Journal of Occupational Medicine, 26(1):29–32.
Hardin BD, Niemeier RW, Sikov MR, Hackett PL (1983) Reproductive-toxicologic assessment of the epoxides ethylene oxide, propylene oxide, butylene oxide, and styrene oxide. Scandinavian Journal of Work, Environment and Health, 9:94–102.
Havlicek SC, Hilpert LR, Dai G, Pierotti D (1992) Assessment of ethylene oxide concentrations and emissions from sterilization and fumigation processes. Final report. Prepared for Research Division, California Air Resources Board, Sacramento, CA (Contract No. A832-125).
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology, 30:445–600.
Health Canada (1994) Canadian Environmental Protection Act. Human health risk assessment for priority substances. Ottawa, Ontario, Health Canada, 36 pp.
Health Canada (1999a) Supporting documentation for ethylene oxide (exposure). Ottawa, Ontario, Health Canada, Health Protection Branch, Priority Substances Section, July draft.
Health Canada (1999b) Supporting documentation for ethylene oxide (health-related effects). Ottawa, Ontario, Health Canada, Health Protection Branch, Priority Substances Section, September.
Hemminki K, Mutanen P, Saloniemi I, Niemi M-L, Vainio H (1982) Spontaneous abortions in hospital staff engaged in sterilising instruments with chemical agents. British Medical Journal, 285:1461–1463.
Hemminki K, Mutanen P, Niemi M-L (1983) Spontaneous abortions in hospital sterilising staff [letter to the editor]. British Medical Journal, 286:1976–1977.
Henne W, Dietrich W, Pelger M, von Sengbusch G (1984) Residual ethylene oxide in hollow-fiber dialyzers. Artificial Organs, 8(3):306–309.
Hogstedt LC (1988) Epidemiological studies on ethylene oxide and cancer: an updating. In: Bartsch H, Hemminki K, O’Neill IK, eds. Methods for detecting DNA damaging agents in humans: Applications in cancer epidemiology and prevention. Lyon, International Agency for Research on Cancer, pp. 265–270 (IARC Scientific Publications No. 89).
Hogstedt B, Gullberg B, Hedner K, Dolnig A, Mitelman F, Skerfving S, Widegren B (1983) Chromosome aberrations and micronuclei in bone marrow cells and peripheral blood lymphocytes in humans exposed to ethylene oxide. Hereditas, 98:105–113.
Hollingsworth RL, Rowe VK, Oyen F, McCollister DD, Spencer HC (1956) Toxicity of ethylene oxide determined on experimental animals. Archives of Industrial Health, 13:217–227.
Hornung RW, Greife AL, Stayner LT, Steenland NK, Herrick RF, Elliott LJ, Ringenburg VL, Morawetz J (1994) Statistical model for prediction of retrospective exposure to ethylene oxide in an occupational mortality study. American Journal of Industrial Medicine, 25:825–836.
Howard PH (1989) Handbook of environmental fate and exposure data for organic chemicals. Vol. I. Chelsea, MI, Lewis Publishers.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, Michalenko EM (1991) Handbook of environmental degradation rates. Chelsea, MI, Lewis Publishers.
Howe RB (1995) THC: A computer program to compute a reference dose from continuous animal toxicity data using the benchmark dose method. Ruston, LA, ICF Kaiser Engineers, Inc.
Howe RB, Crump KS (1982) Global82: A computer program to extrapolate quantal animal toxicity data to low doses. Ruston, LA, Science Research Systems.
How-Grant M, ed. (1991) Kirk-Othmer encyclopedia of chemical technology, 4th ed. Vol. 9. New York, NY, John Wiley & Sons Inc.
Hussain S (1984) Dose–response relationship for mutations induced in E. coli by some model compounds. Hereditas, 101:57–68.
IARC (1976) Cadmium, nickel, some epoxides, miscellaneous industrial chemicals and general considerations of volatile anaesthetics. Lyon, International Agency for Research on Cancer, pp. 157–167 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 11).
IARC (1994) Some industrial chemicals. Ethylene oxide. Lyon, International Agency for Research on Cancer, pp. 73–159 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 60).
IPCS (1985) Ethylene oxide. Geneva, World Health Organization, International Programme on Chemical Safety, 79 pp. (Environmental Health Criteria 55).
IPCS (1999) International Chemical Safety Card — Ethylene oxide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0155).
Jackson MB, Gales K, Campbell DJ (1978) Effects of waterlogged soil conditions on the production of ethylene and on water relationships in tomato plants. Journal of Experimental Botany, 29:183–193.
Jacobson KH, Hackley EB, Feinsilver L (1956) The toxicity of inhaled ethylene oxide and propylene oxide vapors. American Medical Association Archives of Industrial Health, 13:237–244.
Jana MK, Roy K (1975) Effectiveness and efficiency of ethyl methane sulphonate and ethylene oxide for the induction of mutations in rice. Mutation Research, 28:211–215 [cited in IPCS, 1985].
Jensen KG (1988) Determination of ethylene oxide residues in processed food products by gas–liquid chromatography. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 187:535–540.
Jones AR, Wells G (1981) The comparative metabolism of 2-bromoethanol and ethylene oxide in the rat. Xenobiotica, 11(11):763–770.
Karelová J, Jablonická A, Vargová M (1987) Results of cytogenetic testing of workers exposed to ethylene oxide. Journal of Hygiene, Epidemiology, Microbiology and Immunology, 31:119–126.
Katoh T, Higashi K, Inoue N, Tanaka I (1988) Effects of chronic inhalation of ethylene oxide on lipid peroxidation and glutathione redox cycle in rat livers. Research Communications in Chemical Pathology and Pharmacology, 61(2):281–284.
Katoh T, Higashi K, Inoue N, Tanaka I (1989) Lipid peroxidation and the metabolism of glutathione in rat liver and brain following ethylene oxide inhalation. Toxicology, 58:1–9.
KemI (1995) Hazard assessments — Chemical substances selected in the Swedish Sunset Project (Ethylene oxide). Solna, National Chemicals Inspectorate (KemI) (Report No. 12).
Kiesselbach N, Ulm K, Lange H-J, Korallus U (1990) A multicentre mortality study of workers exposed to ethylene oxide. British Journal of Industrial Medicine, 47:182–188.
Koga M, Hori H, Tanaka I (1987) Analysis of urinary metabolites of rats exposed to ethylene oxide. Sangyo Ika Daigaku Zasshi, 9:267–270 (in Japanese).
Kreuzer PE (1992) Kinetik der Permeation von gasförmigem und in verschiedenen Matrizes gelöstem Ethylenoxid durch die Haut von Ratte, Meerschweinchen und Mensch. Neuherberg, GSF-Forschungszentrum für Umwelt und Gesundheit (GSF-Bericht 19/92; ISSN 0721-1694).
Krishnan K, Fennell TR, Gargas ML, Andersen ME (1992) A physiologically based description of ethylene oxide dosimetry in the rat. Toxicology and Industrial Health, 8(3):121–140.
Kuzuhara S, Kanazawa I, Nakanishi T, Egash T (1983) Ethylene oxide polyneuropathy. Neurology, 33:377–380.
LaDage LH (1979) Facial "irritation" from ethylene oxide sterilization of anesthesia mask? [letter to the editor] Plastic and Reconstructive Surgery, 45:179.
Laurent C (1988) SCE increases after an accidental acute inhalation exposure to ethylene oxide and recovery to normal after 2 years. Mutation Research, 204:711–717.
Laurent C, Frederic J, Léonard A (1984) Sister chromatid exchange frequency in workers exposed to high levels of ethylene oxide, in a hospital sterilization service. International Archives of Occupational and Environmental Health, 54:33–43.
Lerda D, Rizzi R (1992) Cytogenetic study of persons occupationally exposed to ethylene oxide. Mutation Research, 281:31–37.
Lerman Y, Ribak J, Skulsky M, Ingber A (1995) An outbreak of irritant contact dermatitis from ethylene oxide among pharmaceutical workers. Contact Dermatitis, 33:280–281.
Lewis RJ (1993) Hawley’s condensed chemical dictionary, 12th ed. New York, NY, Van Nostrand Reinhold Company.
Lewis SE, Barnett LB, Felton C, Johnson FM, Skow LC, Cacheiro N, Shelby MD (1986) Dominant visible and electrophoretically expressed mutations induced in male mice exposed to ethylene oxide by inhalation. Environmental Mutagenesis, 8:867–872.
Li F, Segal A, Solomon JJ (1992) In vitro reaction of ethylene oxide with DNA and characterization of DNA adducts. Chemico-Biological Interactions, 83:35–54.
Lindbolm M-L, Hemminki K, Bonhomme MG, Anttila A, Rantala K, Heikkilä P, Rosenberg MJ (1991) Effects of paternal occupational exposure on spontaneous abortions. American Journal of Public Health, 81:1029–1033.
Lorenz K, Zellner R (1984) Rate constants and vinoxy product yield in the reaction OH + ethylene oxide. Berichte der Bunsen-gesellschaft für Physikalische Chemie, 88:1228–1231 [cited in Atkinson, 1986].
Lynch DW, Lewis TR, Moorman WJ, Burg JR, Groth DH, Khan A, Ackerman J, Cockrell BY (1984a) Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicology and Applied Pharmacology, 76:69–84.
Lynch DW, Lewis TR, Moorman WJ, Burg JR, Lal JB, Setzer JV, Groth DH, Gulati DK, Zavos PM, Sabharwal PS, Ackerman LJ, Cockrell BY, Sprinz H (1984b) Effects on monkeys and rats of long-term inhalation exposure to ethylene oxide: Major findings of the NIOSH study. In: Inhospital ethylene oxide sterilization — Current issues in ethylene oxide toxicity and occupational exposure. Arlington, VA, Association for the Advancement of Medical Instrumentation, pp. 7–10 (AAMI Technology Assessment Report No. 8-84).
Lynch DW, Lewis TR, Moorman WJ, Sabharwal PS, Burg JA (1984c) Toxic and mutagenic effects of ethylene oxide and propylene oxide on spermatogenic functions in cynomolgus monkeys. Toxicologist, 3:60.
Lynch DW, Sharpnack DD, Krieg EF, Ketring K, Lewis TR (1992) Chronic inhalation toxicity of ethylene oxide in monkeys — lens opacities at termination of exposure and 10-year follow-up. Toxicologist, 12:354 (abstract).
Mabey W, Mill T (1978) Critical review of hydrolysis of organic compounds in water under environmental conditions. Journal of Physical and Chemical Reference Data, 7(2):383–411.
Mackay D (1991) Multimedia environmental models: The fugacity approach. Chelsea, MI, Lewis Publishers, 257 pp.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic chemicals: A Level III fugacity model. Environmental Science and Technology, 25:427.
Mackey J (1968) Mutagenesis in vulgare wheat. Hereditas, 59:505–517 [cited in IPCS, 1985].
Major J, Jakab M, Tompa A (1996) Genotoxicological investigation of hospital nurses occupationally exposed to ethylene oxide: I. Chromosome aberrations, sister-chromatid exchanges, cell cycle kinetics, and UV-induced DNA synthesis in peripheral blood lymphocytes. Environmental and Molecular Mutagenesis, 27:84–92.
Markwordt DW (1985) Sources of ethylene oxide emissions. Research Triangle Park, NC, US Environmental Protection Agency (EPA-450/3-85-014; PB85 205516).
Martis L, Kroes R, Darby TD, Woods EF (1982) Disposition kinetics of ethylene oxide, ethylene glycol, and 2-chloroethanol in the dog. Journal of Toxicology and Environmental Health, 10:847–856.
Marx GF, Steen SN, Schapira M, Erlanger HL, Arkins RE, Jadwat CM, Kepes E (1969) Hazards associated with ethylene oxide sterilization. New York State Journal of Medicine, 69:1319–1320.
Matsuoka M, Igisu H, Inoue N, Hori H, Tanaka I (1990) Inhibition of creatine kinase activity by ethylene oxide. British Journal of Industrial Medicine, 47:44–47.
Mayer J, Warburton D, Jeffrey AM, Pero R, Walles S, Andrews L, Toor M, Latriano L, Wazneh L, Tang D, Tsai W-Y, Kuroda M, Perera F (1991) Biologic markers in ethylene oxide-exposed workers and controls. Mutation Research, 248:163–176.
McKelvey JA, Zemaitis MA (1986) The effects of ethylene oxide (EO) exposure on tissue glutathione levels in rats and mice. Drug and Chemical Toxicology, 9(1):51–66.
Meiners AF, Nicholson BI (1988) Ethylene oxide control technology for hospital sterilizers. Paper presented at the 81st Annual Meeting of the Association Dedicated to Air Pollution Control and Hazardous Waste Management, Dallas, TX.
Migliore L, Rossi AM, Loperieno N (1982) Mutagenic action of structurally-related alkene oxides on Schizosaccharomyces pombe: the influence "in vitro" of mouse-liver metabolizing system. Mutation Research, 102:425–437 [cited in IPCS, 1985].
Morgan RW, Claxton KW, Divine BJ, Kaplan SD, Harris VB (1981) Mortality among ethylene oxide workers. Journal of Occupational Medicine, 23(11):767–770.
Mori K, Kaido M, Fujishiro K, Inoue N (1989) Testicular toxicity and alterations of glutathione metabolism resulting from chronic inhalation of ethylene oxide in rats. Toxicology and Applied Pharmacology, 101:299–309.
Mori K, Inoue N, Fujishiro K, Kikuchi M, Chiba S (1990) Biochemical changes in rat erythrocytes caused by ethylene oxide exposure. Fundamental and Applied Toxicology, 15:441–447.
Mori K, Kaido M, Fujishiro K, Inoue N, Koide O, Hori H, Tanaka I (1991) Dose dependent effects of inhaled ethylene oxide on spermatogenesis in rats. British Journal of Industrial Medicine, 48:270–274.
Moutschen-Dahmen J, Moutshen-Dahmen M, Ehrenberg L (1968) Note on the chromosome breaking activity of ethylene oxide and ethyleneimine. Hereditas, 60:267–269 [cited in IPCS, 1985].
Natarajan AT, Preston RJ, Dellarco V, Ehrenberg L, Generoso W, Lewis S, Tates SD (1995) Ethylene oxide: evaluation of genotoxicity data and an exploratory assessment of genetic risk. Mutation Research, 330:55–70.
Norman SA, Berlin JA, Soper KA, Middendorf BF, Stolley PD (1995) Cancer incidence in a group of workers potentially exposed to ethylene oxide. International Journal of Epidemiology, 24(2):276–284.
NPRI (1993) Summary report 1993, National Pollutant Release Inventory. Canadian Environmental Protection Act, Environment Canada. Ottawa, Ontario, Minister of Supply and Services Canada (Catalogue No. EN40-495-1/1-1995E).
NPRI (1996) Summary report 1996, National Pollutant Release Inventory. Canadian Environmental Protection Act, Environment Canada. Ottawa, Ontario, Minister of Supply and Services Canada (Catalogue No. EN40-495/1-1996E).
NTP (1987) Toxicology and carcinogenesis studies of ethylene oxide (CAS No.
NTP (2000) 9th report on carcinogens. Research Triangle Park, NC, US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, at website http://ehis.niehs.nih.gov/roc/toc9.html.
Ohnishi A, Inoue N, Yamamoto T, Murai Y, Hori H, Koga M, Tanaka I, Akiyama T (1985) Ethylene oxide induces central-peripheral distal axonal degeneration of the lumbar primary neurones in rats. British Journal of Industrial Medicine, 42:373–379.
Ohnishi A, Inoue N, Yamamoto T, Murai Y, Hori H, Tanaka I, Koga M, Akiyama T (1986) Ethylene oxide neuropathy in rats. Exposure to 250 ppm. Journal of the Neurological Sciences, 74:215–221.
Olsen GW, Lacy SE, Bodner KM, Chau M, Arceneaux TG, Cartmill JB, Ramlow JM, Boswell JM (1997) Mortality from pancreatic and lymphopoietic cancer among workers in ethylene and propylene chlorohydrin production. Occupational and Environmental Medicine, 54:592–598.
Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochemical Journal, 300:271–276.
Pero RW, Widegren B, Högstedt B, Mitelman F (1981) In vivo and in vitro ethylene oxide exposure of human lymphocytes assessed by chemical stimulation of unscheduled DNA synthesis. Mutation Research, 83:271–289.
Pfeiffer EH, Dunkelberg H (1980) Mutagenicity of ethylene oxide and propylene oxide and of the glycols and halohydrins formed from them during the fumigation of foodstuffs. Food and Cosmetics Toxicology, 18:115–118.
Polifka JE, Rutledge JC, Kimmel GL, Dellarco VV, Generoso WM (1991) Skeletal deviations in mice offspring following zygotic exposure to ethylene oxide. Teratology, 43(5):444.
Polifka JE, Rutledge JC, Kimmel GL, Dellarco VV, Generoso WM (1992) Dose-dependent effects of zygotic exposure to ethylene oxide on mouse skeletal development. Toxicologist, 12(1):100.
Popp DM, Popp RA, Lock S, Mann RC, Hand RE Jr (1986) Use of multiparameter analysis to quantitate hematological damage from exposure to a chemical (ethylene oxide). Journal of Toxicology and Environmental Health, 18:543–565.
Popp W, Vahrenholz C, Przygoda H, Brauksiepe A, Goch S, Müller G, Schell C, Norpoth K (1994) DNA–protein cross-links and sister chromatid exchange frequencies in lymphocytes and hydroxyethyl mercapturic acid in urine of ethylene oxide-exposed hospital workers. International Archives of Occupational and Environmental Health, 66:325–332.
Raghunathan AN, Muthu M, Majumder SK (1969) Control of internal fungi of sorghum by fumigation. Journal of Stored Product Research, 5:389–392.
Rajendran S (1982) Post-fumigation productivity of Trogoderma granarium Everts (Coleoptera; Dermestidae). Bulletin of Entomological Research, 72:247–251 [cited in BUA, 1995].
Rajendran S, Shivaramaiah HN (1985) The differential effects of selected fumigants on the multiplicative potential of Rhyzopertha dominica F. (Coleoptera: Bostrichidae). Entomon, 10:7–12 [cited in BUA, 1995].
Recio L, Abernethy DJ, Donner M, Pluta L, Preston J (1999) Assessment of the in vivo mutagenicity of ethylene oxide in the bone marrow of B6C3F1 lacI transgenic mice following a chronic inhalation exposure. Toxicologist, 48(1-S):368.
Ribeiro LR, Salvadori DMF, Pereira CAB, Beçak W (1987) Activity of ethylene oxide in the mouse sperm morphology test. Archives of Toxicology, 60:331–333.
Ribeiro LR, Salvadori DMF, Rios ACC, Costa SL, Tates AD, Törnqvist M, Natarajan AT (1994) Biological monitoring of workers occupationally exposed to ethylene oxide. Mutation Research, 313:81–87.
Richmond GW, Abrahams RH, Nemenzo JH, Hine CH (1985) An evaluation of the possible effects on health following exposure to ethylene oxide. Archives of Environmental Health, 40:20–25.
Ristow GE, Cornelius D (1986) The neurological manifestations of chronic ethylene oxide exposure. Annals of Neurology, 20(1):136.
Rowland AS, Baird DD, Shore DL, Darden B, Wilcox AJ (1996) Ethylene oxide exposure may increase the risk of spontaneous abortion, preterm birth, and postterm birth. Epidemiology, 7(4):363–368.
Royce A, Moore WKS (1955) Occupational dermatitis caused by ethylene oxide. British Journal of Industrial Medicine, 12:169–171.
Rutledge JC, Generoso WM (1989) Fetal pathology produced by ethylene oxide treatment of the murine zygote. Teratology, 39:563–572.
Rutledge JC, Generoso WM, Shourbaji A, Cain KT, Gans M, Oliva J (1992) Developmental anomalies derived from exposure of zygotes and first-cleavage embryos to mutagens. Mutation Research, 296:167–177.
Saillenfait AM, Gallissot F, Bonnet P, Protois JC (1996) Developmental toxicity of inhaled ethylene oxide in rats following short-duration exposure. Fundamental and Applied Toxicology, 34:223–227.
Salinas E, Sasich L, Hall DH, Kennedy RM, Morriss H (1981) Acute ethylene oxide intoxication. Drug Intelligence and Clinical Pharmacy, 15:384–386.
Sarto F, Cominato I, Pinton AM, Brovedani PG, Faccioli CM, Bianchi V, Levis AG (1984) Cytogenetic damage in workers exposed to ethylene oxide. Mutation Research, 138:185–195.
Sarto F, Clonfero E, Bartolucci GB, Franceschi C, Chiricolo M, Levis AG (1987) Sister chromatid exchanges and DNA repair capability in sanitary workers exposed to ethylene oxide: evaluation of the dose–effect relationship. American Journal of Industrial Medicine, 12:625–637.
Sarto F, Tomanin R, Giacomelli L, Iannini G, Cupiraggi AR (1990) The micronucleus assay in human exfoliated cells of the nose and mouth: application to occupational exposures to chromic acid and ethylene oxide. Mutation Research, 244:345–351.
Sarto F, Törnqvist MÅ, Tomanin R, Bartolucci GB, Osterman-Golkar SM, Ehrenberg L (1991) Studies of biological and chemical monitoring of low-level exposure to ethylene oxide. Scandinavian Journal of Work, Environment and Health, 17:60–64.
Schroder JM, Hoheneck M, Weis J, Dies H (1985) Ethylene oxide polyneuropathy: clinical follow-up study with morphometric and electron microscopic findings in a sural nerve biopsy. Journal of Neurology, 232:83–90.
Schulte PA, Boeniger M, Walker JT, Schober SE, Pereira MA, Gulati DK, Wojciechowski JP, Garza A, Froelich R, Strauss G, Halperin WE, Herrick R, Griffith J (1992) Biologic markers in hospital workers exposed to low levels of ethylene oxide. Mutation Research, 278:237–251.
Schulte PA, Walker JT, Boeniger MF, Tsuchiya Y, Halperin WE (1995) Molecular, cytogenetic, and hematologic effects of ethylene oxide on female hospital workers. Journal of Occupational and Environmental Medicine, 37(3):313–320.
Segerbäck D (1990) Reaction products in hemoglobin and DNA after in vitro treatment with ethylene oxide and N-(2-hydroxyethyl)-N-nitrosourea. Carcinogenesis, 11:307–312.
Setzer JV, Brightwell WS, Russo JM, Johnson BL, Lynch DW, Madden G, Burg JR, Sprinz H (1996) Neurophysiological and neuropathological evaluation of primates exposed to ethylene oxide and propylene oxide. Toxicology and Industrial Health, 12(5):667–682.
Sexton RJ, Henson EV (1949) Dermatological injuries by ethylene oxide. Journal of Industrial Hygiene and Toxicology, 31:297–300.
Shaham J, Levi Z, Gurvich R, Shain R, Ribak J (2000) Hematological changes in hospital workers due to chronic exposure to low levels of ethylene oxide. Journal of Occupational and Environmental Medicine, 42:843–850.
Shore RE, Gardner MJ, Pannett B (1993) Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. British Journal of Industrial Medicine, 50:971–997.
Shulovská K, Lindgren D, Eriksson G, Ehrenberg L (1969) The mutagenic effect of low concentrations of ethylene oxide in air. Hereditas, 62:264–266 [cited in IPCS, 1985].
Sisk SC, Pluta LJ, Meyer KG, Wong BC, Recio L (1997) Assessment of the in vivo mutagenicity of ethylene oxide in the tissues of B6C3F1 lacI transgenic mice following inhalation exposure. Mutation Research, 391:153–164.
Smith HH, Lotfy TA (1954) Comparative effects of certain chemicals on Tradescantia chromosomes as observed at pollen tube mitosis. American Journal of Botany, 41:489–593 [cited in IPCS, 1985].
Smith KA, Jackson MB (1974) Ethylene, waterlogging and plant growth. Annual report. Wantage, ARC Letcombe Laboratory, pp. 60–75.
Smyth HF, Seaton J, Fisher L (1941) The single dose toxicity of some glycols and derivatives. Journal of Industrial Hygiene and Toxicology, 23:259–268.
Snellings WM (1982) Ethylene oxide seven- to eight-week inhalation probe study on rats and mice. Final report. Export, PA, Bushy Run Research Center (Project Report 45-139).
Snellings WM, Maronpot RR, Zelenak JP, Laffoon CP (1982a) Teratology study in Fischer 344 rats exposed to ethylene oxide by inhalation. Toxicology and Applied Pharmacology, 64:476–481.
Snellings WM, Zelenak JP, Weil CS (1982b) Effects on reproduction in Fischer 344 rats exposed to ethylene oxide by inhalation for one generation. Toxicology and Applied Pharmacology, 63:382–388.
Snellings WM, Weil CS, Maronpot RR (1984a) A subchronic inhalation study on the toxicologic potential of ethylene oxide in B6C3F1 mice. Toxicology and Applied Pharmacology, 76:510–518.
Snellings WM, Weil CS, Maronpot RR (1984b) A two-year inhalation study of the carcinogenic potential of ethylene oxide in Fischer 344 rats. Toxicology and Applied Pharmacology, 75:105–117.
Sprinz H, Matzke H, Carter J (1982) Neuropathological evaluation of monkeys exposed to ethylene and propylene oxide. Final report. Prepared for National Institute for Occupational Safety and Health, Cincinnati, OH, by Midwest Research Institute, Kansas City, MO, February (NIOSH Contract No. 210-81-6004; MRI Project No. 7222-B).
SRI (1993) Chemical economics handbook: Ethylene oxide. Menlo Park, CA, SRI International.
Stayner L, Steenland K, Greife A, Hornung R, Hayes RB, Morawetz J, Ringenburg V, Elliot L, Halperin W (1993) Exposure–response analysis of cancer mortality in a cohort of workers exposed to ethylene oxide. American Journal of Epidemiology, 138(10):787–798.
Steenland K, Stayner L, Greife A, Halperin W, Hayes R, Hornung R, Nowlin S (1991) Mortality among workers exposed to ethylene oxide. New England Journal of Medicine, 324(20):1402–1407.
Stolley PD, Soper KA, Galloway SM, Nichols WW, Norman SA, Wolman SR (1984) Sister-chromatid exchanges in association with occupational exposure to ethylene oxide. Mutation Research, 129:89–102.
Swaen GMH, Slangen JMM, Ott MG, Kusters E, Van Den Langenbergh G, Arends JW, Zober A (1996) Investigation of a cluster of ten cases of Hodgkin’s disease in an occupational setting. International Archives of Occupational and Environmental Health, 68:224–228.
Talmage SS (1994) Environmental and human safety of major surfactants — Alcohol ethoxylates and alkylphenol ethoxylates. Boca Raton, FL, Lewis Publishers, 374+ pp. (ISBN 1-56670-017-5).
Tardif R, Goyal R, Brodeur J, Gérin M (1987) Species differences in the urinary disposition of some metabolites of ethylene oxide. Fundamental and Applied Toxicology, 9:448–453.
Tates AD, Grunnt T, Törnqvist M, Farmer PB, van Dam FJ, van Mossel H, Schoemaker HM, Osterman-Golkar S, Uebel C, Zwinderman AH, Natarajan AT, Ehrenberg L (1991) Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutation Research, 250:483–497.
Tates AD, Törnqvist M, Grummt T (1992) Corrigendum. Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutation Research, 280:73–74.
Tates AD, Boogaard PJ, Darroudi F, Natarajan AT, Caubo ME, van Sittert NJ (1995) Biological effect monitoring in industrial workers following incidental exposure to high concentrations of ethylene oxide. Mutation Research, 329:63–77.
Teta MJ, Benson LO, Vitale JN (1993) Mortality study of ethylene oxide workers in chemical manufacturing: a 10 year update. British Journal of Industrial Medicine, 50:704–709.
Teta MJ, Sielken RL, Valdez-Flores C (1999) Ethylene oxide risk assessment based on epidemiological data: application of revised regulatory guidelines. Risk Analysis, 19:1135–1155.
Thiess AM (1963) Observations on the adverse health effects from ethylene oxide. Archiv für Toxikologie, 20:127–140 (in German) [cited in Glaser ZR (1979) Ethylene oxide: toxicology review and field study results of hospital use. Journal of Environmental Pathology and Toxicology, 2:173–208].
Törnqvist M, Osterman-Golkar S, Kautiainen A, Jensen S, Farmer PB, Ehrenberg L (1986) Tissue doses of ethylene oxide in cigarette smokers determined from adduct levels in hemoglobin. Carcinogenesis 7:1519–1521.
Törnqvist M, Almberg JG, Bergmark EN, Nilsson S, Osterman-Golkar SM (1989) Ethylene oxide doses in ethene-exposed fruit store workers. Scandinavian Journal of Work, Environment and Health, 15:436–438.
Tucker SP, Arnold JE (1984) Evaluation of OSHA method no 84/02/00. Cincinnati, OH, National Institute for Occupational Safety and Health (NTIS PB84-242049).
Tutt WE, Tilley LS (1993) Assessment of ethylene oxide emissions from hospital sterilizers. In: Proceedings of the 86th Annual Meeting of the Air and Waste Management Association, Denver, CO, June 1993. Vol. 16A. Pittsburgh, PA, Air and Waste Management Association (Paper No. 93TP 66.01).
Tyler TR, McKelvey JA (1982) Dose dependent disposition of 14C labelled ethylene oxide in rats. Export, PA, Bushy Run Research Center (TSCATS/017061; EPA/OTS Document No. 878212056).
US EPA (1984) Health assessment document for ethylene oxide (Review draft). Washington, DC, US Environmental Protection Agency, Office of Health and Environmental Assessment, April (EPA-600/8-84/009A).
US EPA (1985) Health assessment document for ethylene oxide (final report). Washington, DC, US Environmental Protection Agency, Office of Health and Environmental Assessment (EPA-600/8-84/009F).
US EPA (1990) Toxic Chemical Release Inventory 1988. Based upon data collected by the US Environmental Protection Agency; available from the US National Library of Medicine’s TOXNET system [cited in NTP, 2000].
US EPA (1992) Ethylene oxide emissions from commercial sterilization/fumigation operations. Background information for proposed standards. Research Triangle Park, NC, US Environmental Protection Agency, Office of Air Quality Planning and Standards (EPA-450/D-93-016).
US EPA (1994) Ethylene oxide emissions standards for sterilization facilities. Washington, DC, US Environmental Protection Agency, 6 December 1994 (59 FR 62589; 40 CFR 63.360; Subpart O).
US EPA (1995) SCREEN3 model user’s guide. Research Triangle Park, NC, US Environmental Protection Agency, Office of Air Quality Planning and Standards (EPA-454/B-95-004).
Van Duuren BL, Orris L, Nelson N (1965) Carcinogenicity of epoxides, lactones, and peroxy compounds. Part II. Journal of the National Cancer Institute, 35:707–717.
van Sittert NJ, van Vliet EWN (1994) Monitoring occupational exposure to some industrial chemicals by determining hemoglobin adducts. Clinical Chemistry, 40(7):1472–1475.
van Sittert NJ, de Jong G, Clare MG, Davies R, Dean BJ, Wren LJ, Wright AS (1985) Cytogenetic, immunological, and haematological effects in workers in an ethylene oxide manufacturing plant. British Journal of Industrial Medicine, 42:19–26.
van Sittert NJ, Beulink GDJ, van Vliet EWN, van der Waal H (1993) Monitoring occupational exposure to ethylene oxide by the determination of hemoglobin adducts. Environmental Health Perspectives, 99:217–220.
Verraes S, Michel O (1995) Occupational asthma induced by ethylene oxide. Lancet, 346:1434–1435.
Verschueren K (1983) Handbook of environmental data on organic chemicals, 2nd ed. New York, NY, Van Nostrand Reinhold Co., p. 653.
Walker VE, Fennell TR, Upton PB, Skopek TR, Prevost V, Shuker DEG, Swenberg JA (1992) Molecular dosimetry of ethylene oxide: formation and persistence of 7-(2-hydroxyethyl)-guanine in DNA following repeated exposures of rats and mice. Cancer Research, 52:4328–4334.
Walker VE, Recio L, Sisk SC, Skopek TR (1994) Mutagenicity at the HPRT locus of T-cells following inhalation exposures of Big BlueTM mice to ethylene oxide. Toxicologist, 14:318.
Walker VE, Sisk SC, Upton PB, Wong BA, Recio L (1997a) In vivo mutagenicity of ethylene oxide at the hprt locus in T-lymphocytes of B6C3F1 lacI transgenic mice following inhalation exposure. Mutation Research, 392:211–222.
Walker VE, Meng Q, Clement NL (1997b) Spectra of mutations in HPRT exon 3 of T-cells from F344 rats and LAC I transgenic and nontransgenic B6C3F1 mice exposed by inhalation to ethylene oxide. Environmental and Molecular Mutagenesis, 29(S28):54.
WCB (1994) Safe use of ethylene oxide in health care facilities. A manual of standard practice. Richmond, British Columbia, Workers Compensation Board (Bk 50).
Weller E, Long N, Smith A, Williams P, Ravi S, Gill J, Henessey R, Skornik W, Brain J, Kimmel C, Kimmel G, Holmes L, Ryan L (1999) Dose-rate effects of ethylene oxide exposure on developmental toxicity. Toxicological Sciences, 50(2):259–270.
Winer AM, Atkinson R, Arey J, Aschmann SM, Goodman MA (1987) Lifetimes and fates of toxic chemicals in California’s atmosphere. Riverside, CA, University of California, Statewide Air Pollution Research Center (Report No. ARB-R-88/345).
Wolfs P, Dutrieux M, Scailteur V, Haxhe J-J, Zumofen M, Lauwerys R (1983) Surveillance des travailleurs exposés á l’oxyde d’éthylène dans une entreprise de distribution de gaz stérilisants et dans des unités de stérilisation de matériel médical. Archives des Maladies Professionnelles de Médecine du Travail et de Sécurité Sociale, 44:321–328.
Wong MH, Cheung YH, Cheung CL (1983) The effects of ammonia and ethylene oxide in animal manure and sewage sludge on the seed germination and root elongation of Brassica parachinensis. Environmental Pollution (Series A), 30:109–123.
Wong O, Trent LS (1993) An epidemiological study of workers potentially exposed to ethylene oxide. British Journal of Industrial Medicine, 50:308–316.
Woodard G, Woodard M (1971) Toxicity of residuals from ethylene oxide gas sterilization. In: Proceedings of the 1971 HIA Technical Symposium. Washington, DC, Health Industries Association [cited in IARC, 1994].
Woodruff TJ, Axelrad DA, Caldwell J, Morello-Frosch R, Rosenbaum A (1998) Public health implications of 1990 air toxics concentrations across the United States. Environmental Health Perspectives, 106(5):245–251.
Wu K-Y, Scheller N, Ranasinghe A, Yen T-Y, Sangaiah R, Giese R, Swenberg JA (1999a) A gas chromatography/electron capture/negative chemical ionization–high resolution mass spectrometry method for analysis of endogenous and exogenous N7-(2-hydroxyethyl)guanine in rodents and its potential for human biological monitoring. Chemical Research in Toxicology, 12:722–729.
Wu K-Y, Scheller N, Ranasinghe A, Upton PB, Walker VE, Swenberg JA (1999b) Molecular dosimetry of endogenous and ethylene oxide-induced N7-(2-hydroxyethyl)guanine formation in tissues of rodents. Carcinogenesis, 20:1787–1792.
Yager JW, Benz RD (1982) Sister chromatid exchanges induced in rabbit lymphocytes by ethylene oxide after inhalation exposure. Environmental Mutagenesis, 4:121–134.
Yager JW, Hines CJ, Spear RC (1983) Exposure to ethylene oxide at work increases sister chromatid exchanges in human peripheral lymphocytes. Science, 219:1221–1223.
Yong LC, Schulte PA, Wiencke JK, Boeniger MF, Connally LB, Walker JT, Whelan EA, Ward EM (2001) Hemoglobin adducts and sister chromatid exchanges in hospital workers exposed to ethylene oxide: effects of glutathione S-transferase T1 and M1 genotypes. Cancer Epidemiology, Biomarkers & Prevention, 10:539–550.
Zampollo A, Zacchetti O, Pisati G (1984) On ethylene oxide neurotoxicity: report of two cases of peripheral neuropathy. Italian Journal of Neurological Sciences, 5:59–62.
Environment Canada & Health Canada (2001)
Copies of the Canadian Environmental Protection Act Priority Substances List assessment report (Environment Canada & Health Canada, 2001) and unpublished supporting documentation for ethylene oxide (Environment Canada, 1999; Health Canada, 1999a,b) may be obtained from:
Commercial Chemicals Evaluation Branch
Environment Canada
14th floor, Place Vincent Massey
351 St. Joseph Blvd.
Gatineau, Quebec
Canada K1A 0H3
or
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney’s Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the supporting documentation and assessment report for ethylene oxide were prepared by staff of Health Canada and Environment Canada. Sections of the supporting documentation and assessment report on genotoxicity were reviewed by G. Douglas (Environmental and Occupational Toxicology Division, Health Canada). H. Hirtle contributed additional information in the preparation of the draft CICAD.
Environmental sections of the assessment report and supporting documentation (Environment Canada, 1999) were reviewed externally: D. Maletski (BUA, Germany) and D. Markwordt (US Environmental Protection Agency).
In order to address primarily adequacy of coverage, sections of the supporting documentation pertaining to human health were reviewed externally by:
T. Fennell, Chemical Industry Institute of Toxicology
R. Gingell, Shell Chemical Co.
L. Recio, Chemical Industry Institute of Toxicology
W.M. Snellings, Union Carbide
M.J. Teta, Union Carbide
V. Walker, New York State Department of Health
Accuracy of reporting, adequacy of coverage, and defensibility of conclusions with respect to hazard characterization and dose–response analysis were considered in written review by staff of the Information Department of BIBRA International and at a panel meeting of the following members, convened by Toxicology Excellence for Risk Assessment (TERA) on 12 August 1999 in Ottawa, Canada:
M. Bogdanffy, DuPont Haskel Laboratory
J. Christopher, California Environmental Protection Agency
M. Dourson, TERA
S. Felter, Procter & Gamble
J. Mandel, Exponent
R. Rudel, Silent Spring Institute
V. Walker, New York State Department of Health
J. Preston (US Environmental Protection Agency) provided written comments on the draft supporting documentation, hazard characterization, and dose–response analysis.
The draft CICAD on ethylene oxide was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, International Programme on Chemical Safety/Institut de Recherche en Santé et en Sécurité du Travail du Québec, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, USA
H.B.S. Conacher, Bureau of Chemical Safety, Food Directorate, Health Canada, Canada
C. Cowles, Industrial Chemicals Unit, Health and Safety Executive, United Kingdom
S. Dobson, Centre for Ecology and Hydrology, United Kingdom
E. Frantik, National Institute of Public Health, Centre of Industrial Hygiene and Occupational Diseases, Czech Republic
K. Hensle, American Chemistry Council, Ethylene Oxide Industry Council, USA
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Germany
C. Hiremath, Office of Research and Development, US Environmental Protection Agency, USA
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Germany
A. Kligerman, Office of Research and Development, US Environmental Protection Agency, USA
Y.H. Lee, US Food and Drug Administration, USA
R. McGaughy, Office of Research and Development, US Environmental Protection Agency, USA
H. Nagy, National Institute for Occupational Safety and Health, USA
R.J. Preston, Office of Research and Development, US Environmental Protection Agency, USA
R.P. Subramanian, Office of Research and Development, US Environmental Protection Agency, USA
K. Victorin, Institute of Environmental Medicine, Sweden
L. Vodickova, National Institute of Public Health, Centre of Industrial Hygiene and Occupational Diseases, Czech Republic
K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umvelt und Gesundheit, Germany
Monks Wood, United Kingdom
16–19 September 2002
Members
Dr R. Benson, US Environmental Protection Agency, Region VIII, Denver, CO, USA
Mr R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA, USA
Dr S. Czerczak, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health, Jozsef Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr Y. Hayashi, Division of Chem-Bio Informatics, National Institute of Health Sciences, Ministry of Health, Labour and Welfare, Tokyo, Japan
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, Division of Risk Assessment, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Prof. J. Jeyaratnam, Colombo, Sri Lanka
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
Prof. Y.-X. Liang, School of Public Health, Fudan University, Shanghai Medical College, Shanghai, People’s Republic of China
Dr R. Liteplo, Existing Substances Division, Environmental Contaminants Bureau, Health Canada, Ottawa, Ontario, Canada
Ms M.E. Meek, Existing Substances Division, Safe Environments Programme, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr F.P. Simeonova, Sofia, Bulgaria
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, Australia
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, European Commission, DG Employment & Social Affairs, Luxembourg
Resource Persons
Dr C. Cowles, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr C. Elliott-Minty, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr K. Fuller, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Observers
Mr A.G. Berends, Solvay S.A., Brussels, Belgium; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr C. Newsome, Dow Chemical Company Limited, West Drayton, Middlesex, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr M.A. Pemberton, Wilmslow, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Stott, Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr J.M. Waechter, Jr, The Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr H. Malcolm, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Ms C. Vickers, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Concentrations of ethylene oxide causing a 5% increase in tumour incidence over background (i.e., tumorigenic concentration05s, or TC05s) were calculated by first fitting the multistage model to the dose–response data (see Figure A-1). The multistage model is given by:
P(d) = 1 eq0 q1d ... qkdk
where d is dose, k is the number of dose groups in the study minus one, P(d) is the probability of the animal developing a tumour at dose d, and qi > 0, i = 1,..., k are parameters to be estimated.
The models were fit using GLOBAL82 (Howe & Crump, 1982), and the TC05s were calculated as the concentration C that satisfies:
P(C) P(0) = 0.05
1 P(0)
A chi-square lack of fitness test was performed for each of the three model fits. The degrees of freedom for this test are equal to k minus the number of qi’s for which estimates are non-zero. A P-value less than 0.05 indicates a significant lack of fit.
The TC05s and the corresponding 95% lower confidence limit (95% LCL) were adjusted for continuous exposure by multiplying the values by either 7/24 × 5/7 (for the study reported by Lynch et al. [1984a,b], in which animals were exposed for 7 h/day, 5 days/week) or 6/24 × 5/7 (for the studies reported by Snellings et al. [1984b], Garman et al. [1985], Garman & Snellings [1986], and NTP [1987], in which animals were exposed for 6 h/day, 5 days/week). Model parameters, the adjusted TC05s, and corresponding 95% LCLs are presented in Table 7 in section 11.1.3.
For the tumours in rats, characterization of exposure–response was optimal in the study reported by Snellings et al. (1984b), Garman et al. (1985), and Garman & Snellings (1986). The number of dose groups was greatest in this bioassay, and two of the three doses were in a lower concentration range than in the study by Lynch et al. (1984a,b) (0, 18.3, 60.4, or 183 mg/m3 versus 0, 92, or 183 mg/m3). Dose spacing was excellent (approximately 3-fold variation between concentrations), both sexes were exposed, and group sizes were slightly larger (120 per sex per group) than in the bioassay of Lynch et al. (1984a,b) (80 males per group).
For the study in rats in which exposure–response was best characterized (Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986), the TC05s range from 2.2 mg/m3 (95% LCL = 1.5 mg/m3) for mononuclear leukaemia15 in female F344 rats to 31.0 mg/m3 (95% LCL = 16.1 mg/m3) for brain tumours in female F344 rats. TC05s for comparable tumours in the study in which exposure–response was less well characterized (Lynch et al., 1984a,b) were somewhat higher, ranging from 12.5 mg/m3 for mononuclear cell leukaemia to 31.9 mg/m3 for mixed brain cell glioma.
Values of the TC05s in mice ranged from 6.7 mg/m3 (95% LCL = 4.2 mg/m3) for Harderian cystadenomas in males to 22.7 mg/m3 (95% LCL = 11.4 mg/m3) for uterine adenocarcinomas. It should be noted, however, that characterization of exposure–response in the NTP (1987) bioassay on which these values are based was not optimal; there were only two dose groups and controls, with the lowest administered concentration being 92 mg/m3.
For none of the modelled TC05s was there significant lack of fit (P > 0.05, Table 7 in section 11.1.3). For the study in rats in which exposure–response was best characterized (Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986) and that in mice (NTP, 1987), fits for malignant lymphomas and mammary adenocarcinomas and adenosquamous carcinomas (combined) in females in the latter investigation were poorest (P = 0.06 and 0.08, respectively).
Based on modelling (using THC program; Howe, 1995) of the incidence of Hprt mutations in splenic T-lymphocytes of male B6C3F1 mice (Big Blue®, lacI transgenic) exposed to ethylene oxide for 4 weeks16 (Walker et al., 1997a), the benchmark concentration05 (BMC05) for somatic cell mutations (i.e., the concentration associated with a 5% increase in the incidence of Hprt mutation) (adjusted for intermittent to continuous exposure) was within the range of the lowest TC05s in rats and mice. It should be noted, however, that characterization of exposure–response in Walker et al. (1997a) was not optimal; although there were three dose groups and controls, the lowest administered concentration was 92 mg/m3.
In the interest of utilizing all available data to inform characterization of exposure–response, the tumorigenic potencies developed based on studies in animals were compared with risks of haematological cancers reported in epidemiological studies in populations occupationally exposed to ethylene oxide. The SMRs for leukaemia reported by Stayner et al. (1993) (the only epidemiological study in which individual cumulative exposure was characterized) have been compared with the risk for the most severe outcome in rats (mononuclear cell leukaemia in females). There were three exposure groups in the cohort: <1200, 1200–8500, and >8500 ppm-days, with corresponding SMRs (95% CI) of 99 (27–252), 85 (23–219), and 75 (15–218). To compare these results with those from the studies in animals, the human exposures were converted to lifetime concentrations in mg/m3, by first converting the cumulative exposures (ppm-days) to ambient occupational levels by multiplying by:
1 / (4.8 years × 240 days/year)
where 4.8 years was the average duration of exposure in the cohort and 240 days is the number of occupational days worked per year. The resulting ambient occupational exposures were converted to lifetime environmental exposures by multiplying by:
(8 h/24 h) × (240 days/365 days) × (4.8 years/70 years)
where it is additionally assumed that cohort members worked for an average of 8 h/day and that the standard human lifespan is 70 years. The resulting environmental exposures in ppm were multiplied by 1.83, the conversion factor for ethylene oxide, to convert them to the units of mg/m3.
The relative risk that would be predicted by the fitted animal model was then compared with the observed SMRs from the human study. The relative risk at a given experimental dose is calculated by P(d)/P(0), where P(d) is the fitted multistage model. The lifetime exposures above were converted to equivalent exposures in the studies in animals so that P(d) could be applied by multiplying them by (24/6) × (7/5) (6 = h/day of exposure, 5 = days/week of exposure).
The midpoints of the estimates of exposure for the lower two categories and the lower limit for the highest exposure group in the Stayner et al. (1993) cohort were converted to ambient environmental exposures, yielding 0.02, 0.18, and 0.31 mg/m3, respectively. At these exposures, the modelled experimental data from animals predict relative risks of 1.00, 1.04, and 1.06, which lie within the 95% confidence interval of the human SMRs. Note that these exposures are very low compared with the exposures used to fit the animal model. The lowest animal dose group was 3.27 mg/m3 (continuous dosage), with a fitted relative risk of 1.65.
These predicted risks are also consistent with the SMRs for all haematopoietic neoplasms in males from the same cohort. These SMRs were 95 (95% CI 26–243), 143 (62–283), and 196 (101–343) for the same exposure groups as above.
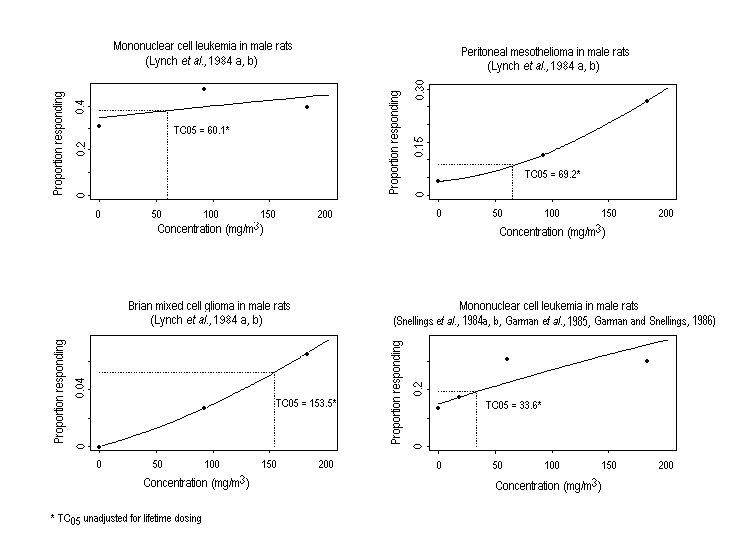
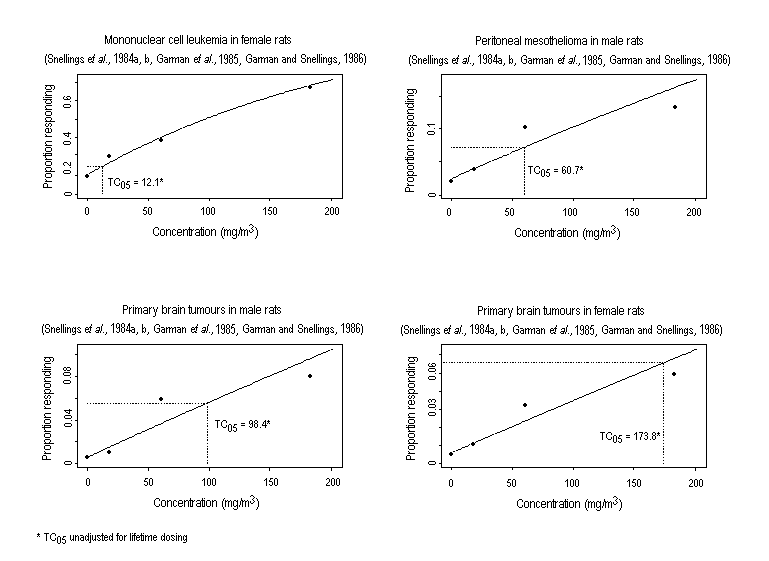
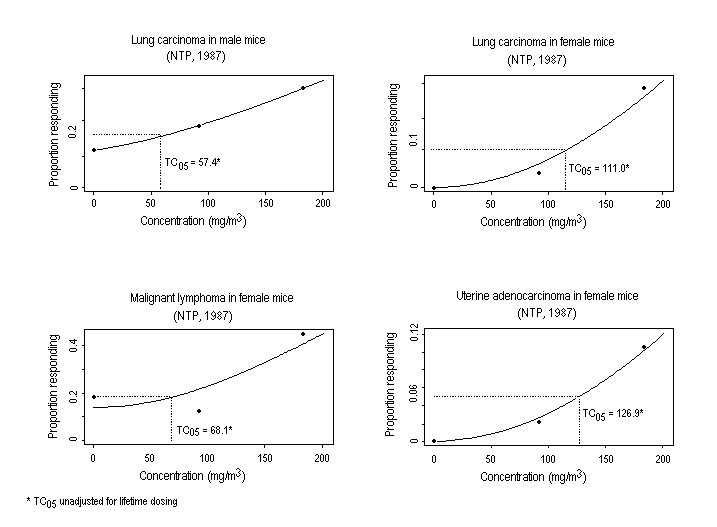
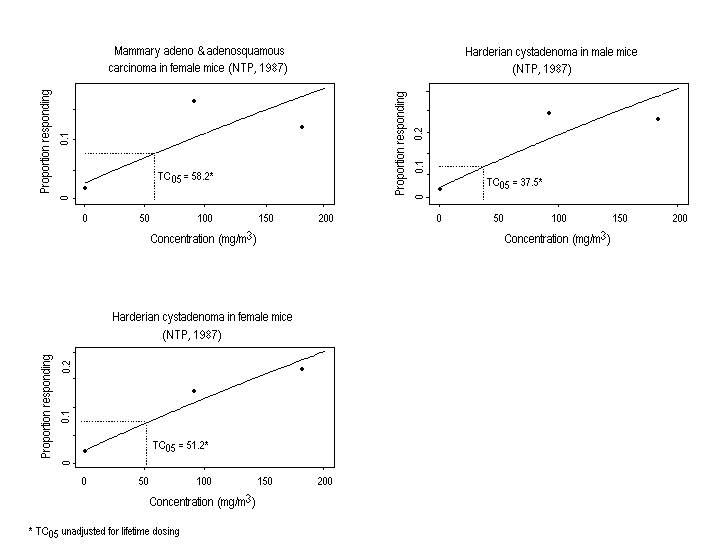
Fig. A-1: TC05s for ethylene oxide
Results indicated that risks predicted based on the most sensitive outcome in rats (mononuclear cell leukaemia in female F344 rats) were consistent with the confidence intervals of the SMRs observed for both leukaemias overall and all haematopoietic neoplasms in males in the cohort study by Stayner et al. (1993) (i.e., the only epidemiological study in which individual cumulative exposure was characterized). However, the limitations of this comparative exercise preclude its meaningful contribution to quantification of risk. These include uncertainties of the available epidemiological data on ethylene oxide, which prevent adequate consideration of traditional criteria for causality (particularly with respect to periods of follow-up in investigations of greatest sensitivity). Moreover, meaningful direct comparison of potency in laboratory animals with that in humans is precarious at best, in light of the inadequacy of available information on interspecies variations in kinetics and metabolism and mode of action to serve as a basis for characterization of site concordance between animals and humans and the extremely wide range of the confidence limits on the SMRs in the epidemiological studies.
|
BMC05 |
benchmark concentration05, concentration associated with a 5% increase in the incidence of an effect above background |
|
BOD |
biological oxygen demand |
|
CAS |
Chemical Abstracts Service |
|
CEPA |
Canadian Environmental Protection Act |
|
CI |
confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
CTV |
critical toxicity value |
|
DNA |
deoxyribonucleic acid |
|
ECD |
electron capture detector |
|
EEV |
estimated exposure value |
|
EHC |
Environmental Health Criteria |
|
ENEV |
estimated no-effects value |
|
GC |
gas chromatography |
|
GSTT1 |
theta-class glutathione S-transferase |
|
7-HEGua |
7-(2-hydroxyethyl)guanine |
|
HEHis |
hydroxyethylhistidine |
|
HEVal |
N-(2-hydroxyethyl)valine |
|
Hprt (HPRT) |
hypoxanthine phosphoribosyl transferase |
|
IC50 |
median inhibitory concentration |
|
ICSC |
International Chemical Safety Card |
|
Koc |
sorption partition coefficient |
|
Kow |
octanol/water partition coefficient |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
MS |
mass spectrometry |
|
mSMR |
meta standardized mortality ratio |
|
NIOSH |
National Institute for Occupational Safety and Health |
|
OR |
odds ratio |
|
PBPK |
physiologically based pharmacokinetic model |
|
SE |
standard error |
|
SI |
International System of Units (Système international d’unités) |
|
SIR |
standardized incidence ratio |
|
SMR |
standardized mortality ratio |
|
sSMR |
summary standardized mortality ratio |
|
t˝ |
half-life |
|
TC05 |
tumorigenic concentration05 (concentration causing a 5% increase in tumour incidence above background) |
|
TWA |
time-weighted average |
|
WHO |
World Health Organization |
Ce CICAD sur l’oxyde d’éthylène a été rédigé conjointement par la Direction de l’hygiène du milieu de Santé Canada et la Division d’évaluation des produits chimiques commerciaux d’Environnement Canada, sur la base d’une documentation préparée dans le cadre du Programme d’évaluation des substances prioritaires, en application de la Loi canadienne sur la protection de l’environnement (LCPE). Les évaluations des substances prioritaires effectuées en application de cette loi portent sur les effets que pourraient avoir ces produits sur la santé humaine en cas d’exposition indirecte dans l’environnement ainsi que sur l’environnement lui-même. La présente mise au point prend en compte les données sur les effets environnementaux jusqu’à mai 1998 et les données sur les effets sanitaires jusqu’à août 1999.17 L’appendice 2 donne des informations sur la nature de l’examen par des pairs et sur la disponibilité des sources documentaires (Environment Canada & Health Canada, 2001). D’autres études ont également été utilisées, à savoir celle de l’ATSDR (1990), de la BUA (1995), du CIRC (1976, 1994), de l’EPA des Etats-Unis (1985), ainsi qu’une précédente monographie sur ce composé parue en 1985 dans la série Critères d’Hygiène de l’Environnement (IPCS, 1985). Des renseignements sur l’examen par des pairs du présent CICAD sont donnés à l’appendice 3. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Monk Woods (Royaume-Uni) du 16 au 19 septembre 2002. La liste des participants à cette réunion figure l’appendice 4. La fiche internationale sur la sécurité chimique de l’oxyde d’éthylène (ICSC 0155), établie par le Programme international sur la sécurité chimique (IPCS, 1999), est également reproduite dans le présent document.
L’oxyde d’éthylène (No CAS
La production d’oxyde d’éthylène dans le pays à l’origine du présent CICAD (à savoir le Canada) a été de 625 kilotonnes en 1996, dont 95 % ont été utilisés pour la production d’éthylène-glycol. On estime que 4 % ont été utilisés pour la fabrication d’agents tensio-actifs. L’oxyde d’éthylène sert également à la stérilisation du matériel médical et autres produits sensibles à la chaleur.
L’oxyde d’éthylène est libéré en majeure partie dans l’atmosphère. Les autres émissions d’oxyde d’éthylène, par exemple à partir de terrains détrempés sont vraisemblablement négligeables. Dans le pays de référence (Canada), on estime que les sources anthropogéniques d’oxyde d’éthylène en ont libéré en 1996 quelque 23,0 tonnes (compte non tenu du tonnage utilisé pour la stérilisation), en totalité dans l’atmosphère. On estime également que 3,0 tonnes supplémentaires se sont dissipées dans l’atmosphère en 1996 lors d’interventions de maintenance dans les établissements de soins utilisant de l’oxyde d’éthylène pour la stérilisation ou encore lors d’opérations de stérilisation à des fins commerciales.
Il est peu probable que l’oxyde d’éthylène libéré dans l’atmosphère passe en quantité importante dans d’autres compartiments de l’environnement. Sa demi-vie atmosphérique établie par référence à sa réaction sur les radicaux hydroxyle engendrés par voie photochimique se situe entre 38 et 382 jours. En cas de décharge ou de déversement dans l’eau, le composé devrait s’évaporer, s’hydrolyser ou encore subir une décomposition aérobie et, dans une moindre mesure, anaérobie. Dans l’eau, sa demi-vie de volatilisation est d’environ 1 h, son temps de demi-hydrolyse d’environ 12 à 14 jours, son temps de demi-dégradation aérobie de 20 jours à 6 mois et son temps de demi-dégradation anaérobie de 4 mois à 2 ans. Dans le sol, l’oxyde d’éthylène devrait se volatiliser rapidement. On estime que son temps de demi-hydrolyse dans le sol et dans les eaux souterraines est compris entre 10,5 et 11,9 jours. Il est peu probable que l’oxyde d’éthylène subisse une bioaccumulation car son coefficient de partage entre l’octanol et l’eau (log Kow) est très faible.
L’oxyde d’éthylène est rapidement résorbé au niveau du poumon, après quoi il se répartit dans l’organisme pour être ensuite métabolisé en éthylène-glycol et en glutathiono-conjugués. Il peut être absorbé par voie transcutanée à partir de la phase gazeuse ou de solutions aqueuses et se répartit ensuite uniformément dans l’organisme. Il se comporte comme un agent alkylant et forme des adduits avec les protéines et l’ADN. On utilise ses adduits avec l’hémoglobine pour la surveillance biologique.
L’oxyde d’éthylène présente une faible toxicité aiguë pour les rongeurs et les chiens, la CL50 à 4 h étant généralement supérieure à 1 500 mg/m3. L’étude des effets non néoplasiques qu’il provoque à l’issue d’expositions répétées n’a fourni que des données limitées, l’accent ayant été mis jusqu’ici sur la cancérogénicité de ce composé. Les effets dont il est fait état chez l’animal sont d’ailleurs principalement de nature hématologique et neurologique.
En s’appuyant sur des études consacrées principalement à des populations exposées de par leur profession, on a constaté que l’oxyde d’éthylène est irritant pour les yeux, les voies respiratoires et la peau et qu’il se comporte en outre comme un agent sensibilisant. Des effets neurologiques (essentiellement une polyneuropathie sensorimotrice) ont été observés chez des travailleurs exposés à des concentrations relativement élevées ainsi que chez des animaux exposés à des concentrations plus fortes que celles qui donnent lieu à une augmentation de l’incidence tumorale.
La voie d’exposition principale chez l’Homme et celle qui est la plus importante sur le plan sanitaire est la voie respiratoire à partir de l’air ambiant. L’expérimentation animale montre que l’on peut considérer le cancer comme le point d’aboutissement essentiel de l’effet toxique d’une exposition prolongée des populations humaines à l’oxyde d’éthylène. Les études comportant une exposition au gaz par inhalation ont permis de mettre en évidence la formation de tumeurs très variées (par exemple, leucémies, lymphomes, tumeurs cérébrales et rénales) et il est très probable, compte tenu des éléments d’appréciation cohérents et convaincants dont on dispose, que le composé agit directement sur le matériel génétique. On est également fondé à penser, dans une certaine mesure, qu’il existe un lien entre l’exposition à l’oxyde d’éthylène et l’apparition de cancers hématologiques chez les personnes exposées de par leur profession, mais les données sont trop limitées pour qu’on puisse en tirer des conclusions définitives.
L’oxyde d’éthylène produit des mutations géniques in vitro et in vivo à tous les niveaux phylogénétiques. Il produit également des mutations dans les cellules germinales et des effets clastogènes chez les animaux de laboratoire. Les données montrent que l’oxyde d’éthylène provoque systématiquement des anomalies clastogènes chez les travailleurs exposés.
Chez les animaux de laboratoire, l’oxyde d’éthylène se révèle foetotoxique à des concentrations supérieures à celles qui provoquent des effets néoplasiques ou non néoplasiques (neurologiques par exemple), que la concentration soit ou non toxique pour la mère; il n’est tératogène qu’à concentration très élevée (supérieure à environ 1 600 mg/m3). Les données fournies par les études épidémiologiques relatives aux effets de l’oxyde d’éthylène sur la reproduction humaine (principalement les avortements spontanés) sont limitées. Chez les animaux de laboratoire, de tous les effets non néoplasiques, ce sont les effets sur la reproduction qui s’observent à la concentration la plus faible (> 90 mg/m3). Il s’agit notamment d’une diminution de la taille des portées, d’une augmentation des pertes foetales post-implantatoires et d’anomalies dans la morphologie, la numération et la mobilité des spermatozoïdes.
On considère que le cancer constitue le point d’aboutissement essentiel pour la quantification de la relation dose-réponse qui sert de base à la caractérisation du risque que représente l’oxyde d’éthylène. La concentration la plus faible déterminant une augmentation de 5 % de l’incidence par rapport à la valeur de référence (CT05) chez des souris ou des rats pour qui la relation dose-réponse avait été caractérisée de manière optimale a été trouvée égale à 2,2 mg/m3 (risque unitaire = 0,05/2,2 mg/m3 = 0,023 par mg/m3) dans le cas de l’apparition de leucémies mononucléaires chez des rattes F344 exposées par voie respiratoire à de l’oxyde d’éthylène; la limite inférieure de l’intervalle de confiance à 95 % était de 1,5 mg/m3. On donne également, principalement dans le but de comparer le pouvoir tumorigène dans le cas du cancer, la concentration (46 mg/m3) qui détermine une augmentation de 5 % de l’incidence des mutations dans les cellules germinales (CMB05), encore que cette valeur n’ait été établie que sur la base de mutations dominantes visibles, sans tenir compte d’autres points d’aboutissement génétiques dans la descendance vivante. De même, les concentrations tolérables basées sur les effets neurologiques ou génésiques seraient de l’ordre de quelques dizaines de microgrammes par mètre cube.
A partir de là, le risque de cancer que l’on peut prévoir au voisinage de sources industrielles ponctuelles en utilisant des données de modélisation et de surveillance limitées, se révèle supérieur à 10–5.
Comme l’oxyde d’éthylène devrait en principe être surtout présent dans l’air, c’est pour les organismes terrestres qu’il est susceptible d’avoir le plus d’effets indésirables, effets au sujet desquels les données disponibles restent d’ailleurs limitées. Le point d’aboutissement le plus significatif de l’action toxique de ce composé, celui qui pourrait avoir le plus de retentissement sur la faune sauvage, concerne essentiellement la reproduction. En comparant la concentration dans l’air ambiant dans le pire des cas à la concentration jugée sans effet, on constate cependant qu’il est peu probable que les organismes terrestres soient exposés à des concentrations atmosphériques dangereuses d’oxyde d’éthylène.
Este CICAD sobre el óxido de etileno, preparado conjuntamente por la Dirección de Higiene del Medio del Ministerio de Sanidad del Canadá y la División de Evaluación de Productos Químicos Comerciales del Ministerio de Medio Ambiente del Canadá, se basó en la documentación preparada al mismo tiempo como parte del Programa de Sustancias Prioritarias en el marco de la Ley Canadiense de Protección del Medio Ambiente (CEPA). Las evaluaciones de sustancias prioritarias previstas en la CEPA tienen por objeto valorar los efectos potenciales para la salud humana de la exposición indirecta en el medio ambiente general, así como los efectos ecológicos. En este examen se analizaron los datos identificados hasta el final de mayo de 1998 (efectos ecológicos) y agosto de 1999 (efectos en la salud humana).18 La información relativa al carácter del examen colegiado y la disponibilidad del documento original (Ministerio de Medio Ambiente del Canadá y Ministerio de Sanidad del Canadá, 2001) figura en el apéndice 2. También se consultaron otros exámenes, entre ellos el del ATSDR (1990), el BUA (1995), el IARC (1976, 1994), la US EPA (1985) y una monografía anterior sobre este producto químico de la serie Criterios de Salud Ambiental (IPCS, 1985). La información sobre el examen colegiado de este CICAD aparece en el apéndice 3. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Monks Wood (Reino Unido) del 16 al 19 de septiembre de 2002. La lista de participantes en esta reunión figura en el apéndice 4. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 0155) para el óxido de etileno, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 1999).
El óxido de etileno (CAS Nş
La producción de óxido de etileno en el país de origen para este CICAD (es decir, el Canadá) en 1996 fue de 625 miles de toneladas, un 95% del cual se utilizó en la fabricación de etilenglicol. Se estima en un 4% el volumen utilizado en la fabricación de sustancias tensioactivas. El óxido de etileno se utiliza asimismo como esterilizante de material sanitario y otros productos sensibles al calor.
La mayor parte del óxido de etileno se libera en la atmósfera. Cabe prever emisiones insignificantes de óxido de etileno procedentes de fuentes naturales, como el suelo encharcado. Las emisiones a la atmósfera de fuentes antropogénicas en el país de origen (el Canadá) en 1996, sin incluir la esterilización, se estiman en 23,0 toneladas. También en 1996 fue a parar a la atmósfera un volumen adicional estimado en tres toneladas/año procedente de la prestación de servicios de las instalaciones médicas que utilizan óxido de etileno en los procesos de esterilización y de las operaciones de esterilización comercial.
Es poco probable que las emisiones de óxido de etileno a la atmósfera den lugar a una transferencia significativa a otros compartimentos del medio ambiente. La semivida atmosférica basada en la reacción con radicales hidroxilo fotogenerados oscila entre 38 y 382 días. En caso de emisión o derrame en agua, se supone que el óxido de etileno sufre un proceso de evaporación, hidrólisis y biodegradación aerobia y en menor medida anaerobia. En el agua, la semivida correspondiente a la volatilización, la hidrólisis, la biodegradación aerobia y la biodegradación anaerobia es, respectivamente, de alrededor de una hora, de unos 12-14 días, de 20 días a seis meses y de cuatro meses a dos años. En el suelo se supone que el óxido de etileno se volatiliza con rapidez. La semivida de la hidrólisis para el suelo y el agua freática se estima que está entre 10,5 y 11,9 días. Habida cuenta de que tiene un log del coeficiente de reparto octanol/agua (Kow) muy bajo, no cabe prever su bioacumulación.
El óxido de etileno se absorbe con rapidez a través de los pulmones, se distribuye y se metaboliza para formar etilenglicol y conjugados del glutatión. El óxido de etileno se puede absorber por la piel a partir de la fase de gas o de soluciones acuosas y se distribuye uniformemente por todo el organismo. Es un agente alquilante y forma aductos de proteínas y ADN. Los aductos de hemoglobina se han utilizado para la biovigilancia.
La toxicidad aguda del dióxido de etileno por inhalación en roedores y perros es baja, con valores de la CL50 a las cuatro horas generalmente superiores a 1500 mg/m3. La información disponible sobre los efectos no neoplásicos de la exposición repetida al óxido de etileno en estudios es limitada, habiéndose concentrado en el pasado sobre todo en la carcinogenicidad del compuesto. Los efectos notificados en estudios con animales se limitaron fundamentalmente a los relativos a los sistemas hematológico y nervioso.
Basándose en estudios realizados principalmente en poblaciones expuestas en el puesto de trabajo, el óxido de etileno tiene actividad irritante ocular, respiratoria y cutánea y es un agente sensibilizador. Se han observado efectos neurológicos (en particular polineuropatía sensitivomotora) en trabajadores expuestos a concentraciones relativamente altas y en animales expuestos a niveles superiores a aquellos en los cuales se han notificado tumores.
La vía de mayor exposición probable y de mayor interés para la evaluación en la salud humana es probablemente la inhalación a partir del aire. Basándose en estudios con animales, el cáncer se considera el efecto final crítico del óxido de etileno en la salud humana en el caso de una exposición prolongada de la población general. En estudios de inhalación, el óxido de etileno ha inducido una gran variedad de tumores (por ejemplo, leucemia y linfoma, de cerebro y de pulmón), con una fuerte probabilidad de que en el mecanismo de acción intervenga una interacción directa con material genético, fenómeno del que existen pruebas sistemáticas y convincentes. Aunque en estudios epidemiológicos de poblaciones profesionalmente expuestas se han obtenido algunas pruebas de una asociación entre la exposición al óxido de etileno y la aparición de cáncer hematológico, lo limitado de los datos impide sacar conclusiones definitivas.
El óxido de etileno induce mutaciones genéticas en todos los niveles filogenéticos sometidos a pruebas in vitro e in vivo. También induce mutaciones en las células germinales y efectos clastogénicos en animales de experimentación. Hay pruebas contundentes de que el óxido de etileno ha inducido cambios clastogénicos en trabajadores expuestos.
En animales de experimentación, el óxido de etileno es fetotóxico en presencia y en ausencia de toxicidad materna en concentraciones superiores a las asociadas con el cáncer y con otros efectos distintos del cáncer (es decir, neurológicos); es teratogénico sólo en concentraciones muy elevadas (por encima de unos 1600 mg/m3). Las pruebas obtenidas de estudios epidemiológicos sobre los efectos reproductivos del óxido de etileno en las personas (en particular abortos espontáneos) son limitadas. Entre los efectos no neoplásicos detectados en animales de experimentación, los reproductivos se producen a la concentración más baja (> 90 mg/m3). Estos consisten en la reducción del tamaño de la camada, el aumento de las pérdidas después de la implantación, la alteración de la morfología de los espermatozoides y cambios en el número y movilidad de los espermatozoides.
El cáncer se considera el efecto final crítico para la cuantificación de la exposición-respuesta en la caracterización del riesgo para el óxido de etileno. La concentración más baja que provoca un aumento de la incidencia de tumores del 5% por encima del nivel habitual (CT05), obtenida de un estudio con ratas o ratones que había optimizado la caracterización de la exposición-respuesta, fue de 2,2 mg/m3 (riesgo unitario = 0,05/2,2 mg/m3 = 0,023 por mg/m3) para la aparición de leucemias mononucleares en ratas hembra F344 expuestas por inhalación al óxido de etileno; el límite de confianza del 95% más bajo fue de 1,5 mg/m3. También se presenta, principalmente como base de la comparación de las potencias tumorígenas para el cáncer, la concentración asociada con un aumento del 5% en la incidencia de mutaciones de las células germinales (BMC05) (46 mg/m3), aunque sólo se basa en mutaciones visibles dominantes y no se tienen en cuenta otros efectos finales genéticos en la progenie viva. Igualmente, las concentraciones tolerables basadas en efectos neurológicos o reproductivos no observados serían del orden de decenas de microgramos por metro cúbico.
Sobre esta base, los riesgos de cáncer previstos en las cercanías de fuentes puntuales industriales, teniendo cuenta los datos limitados obtenidos de modelos y de la vigilancia, son superiores a 10–5.
Puesto que cabe prever la presencia de óxido de etileno fundamentalmente en el aire, los posibles efectos adversos son superiores en los organismos terrestres, para los cuales los datos disponibles son limitados. El efecto final más importante con el mayor potencial para producir efectos en la población silvestre fue la inducción de efectos reproductivos adversos. La comparación de la concentración media en el aire para el caso peor con el valor estimado sin efectos indica que es poco probable que los organismos terrestres se encuentren expuestos a niveles perjudiciales de óxido de etileno en el aire.
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
Critical new information has been scoped to indicate its likely impact on the essential conclusions of this assessment, primarily to establish priority for its consideration in an update. This ensures appropriate consideration in the context of the complete identified database through the several stages of internal and external national review and subsequent international review. More recent information not critical to the hazard characterization or exposure–response analysis, considered by reviewers to add to informational content, has been added. |
|
|
In keeping with WHO policy, which is to provide measurements in SI units, all concentrations of gaseous chemicals in air will be given in SI units in the CICAD series. Where the original study or source document has provided concentrations in SI units, these will be cited here. Where the original study or source document has provided concentrations in volumetric units, conversions will be done using the conversion factors given here, assuming a temperature of 20 °C and a pressure of 101.3 kPa. Conversions are to no more than two significant digits. |
|
|
Although sites at which tumours occur in rats and mice vary, carcinogenic potency is generally greater in rats than in mice. |
|
|
These results are consistent with the observation of tumours in mouse lung and rat brain, if GSTT1 activity is the critical determinant, but not with the lack of observation of tumours in rat lung. |
|
|
Mononuclear cell leukaemias are a common spontaneous tumour in F344 rats. The exact etiology of this tumour type, including cell of origin, has not been definitively identified. |
|
|
However, in situations of short-term or intermittent exposure, other effects could be considered as critical. |
|
|
Mononuclear cell leukaemias are a common spontaneous tumour in F344 rats. The exact etiology of this tumour type, including cell of origin, has not been definitively identified. |
|
|
Notably, the incidence of leukaemia in male rats in the control group in the study by Lynch et al. (1984a,b) was more than twice that in the controls in the study conducted by Snellings and co-workers (Snellings et al., 1984b; Garman et al., 1985; Garman & Snellings, 1986) and was similar to the incidence observed in animals exposed to 183 mg ethylene oxide/m3 in the latter study. |
|
|
The mean frequency (× 10–6) of Hprt mutations was 2.2, 3.8, 6.8, and 14.1 in animals exposed to 0, 92, 183, and 366 mg/m3, respectively. |
|
|
Value has been adjusted for intermittent (occupational) to continuous exposure, but not for reproductive lifetime, due to relatively short period of spermatogenesis. |
|
|
Categories of predicted low-dose risk estimates: <10–7; >10–7 to <10–5; and >10–5 (see Health Canada, 1994). |
|
|
Quantitative estimates of cancer risk based upon the modelling of epidemiological data are lower than those derived on the basis of animal studies (Teta et al., 1999). |
|
|
The Final Review Board became aware that the mortality analysis in this study (Steenland et al., 1991) was recently updated by Steenland and co-workers (unpublished) and that this should be considered in setting priorities for updating. |
|
|
Mononuclear cell leukaemias are a common spontaneous tumour in F344 rats. The exact etiology of this tumour type, including cell of origin, has not been definitively identified. |
|
|
The mean frequency (× 10–6) of Hprt mutations was 2.2, 3.8, 6.8, and 14.1 in animals exposed to 0, 92, 183, and 366 mg/m3, respectively. |
|
|
Les nouvelles données jugées importantes ont été examinées compte tenu de leur influence probable sur les conclusions essentielles de la présente évaluation, le but étant principalement d’établir si leur prise en compte serait prioritaire lors d’une prochaine mise à jour. Elles pourront ainsi être dûment examinées dans le contexte général de la base de données complète lors des différentes phases de l’analyse national interne et externe, puis ultérieurement, lors de l’analyse au niveau international. On a également ajouté des données plus récentes jugées non essentielles pour la caractérisation du risque ou l’établissement d’une relation dose-réponse, mais que les auteurs ont considérées comme informatives. |
|
|
Se ha incluido nueva información crítica para señalar sus probables repercusiones en las conclusiones esenciales de esta evaluación, principalmente con objeto de establecer la prioridad para su examen en una actualización. De esta manera se garantiza un examen adecuado en el marco de la base de datos completa establecida mediante las distintas fases del examen nacional interno y externo y el posterior examen internacional. Se ha añadido información más reciente no decisiva para la caracterización del peligro o el análisis de la exposición-respuesta que los examinadores consideraban que aumentaba el contenido informativo. |
See Also:
Toxicological Abbreviations
Ethylene oxide (EHC 55, 1985)
Ethylene oxide (HSG 16, 1988)
Ethylene oxide (ICSC)
ETHYLENE OXIDE (JECFA Evaluation)
Ethylene oxide (FAO Meeting Report PL/1965/10/2)
Ethylene oxide (FAO/PL:1968/M/9/1)
Ethylene oxide (WHO Pesticide Residues Series 1)
Ethylene Oxide (IARC Summary & Evaluation, Volume 60, 1994)