
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 55
ETHYLENE OXIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1985
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154195 4
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1985
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENE OXIDE
1. SUMMARY
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Chemical and physical properties of ethylene oxide
2.3. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT
AND DISTRIBUTION
3.1. Production, uses, disposal of wastes
3.1.1. Production levels and processes
3.1.2. Uses
3.1.3. Disposal of wastes
3.2. Transport and fate in the environment
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Occurrence in the environment
4.2. General population exposure
4.2.1. Exposure via food and tobacco
4.2.2. Exposure via medical equipment
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Absorption
5.2. Distribution
5.3. Metabolic transformation and excretion
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7. EFFECTS ON ANIMALS
7.1. Acute exposures
7.1.1. Oral, intravenous, and inhalation studies
7.1.2. Acute effects on eyes and skin
7.2. Short-term studies
7.2.1. Inhalation exposure
7.2.2. Oral exposure
7.3. Long-term inhalation studies
7.4. Carcinogenicity
7.4.1. Inhalation exposure
7.4.2. Oral exposure
7.4.3. Subcutaneous exposure
7.4.4. Dermal exposure
7.5. Mutagenicity and related end-points
7.6. Effects on reproduction
7.7. Teratogenicity
8. EFFECTS ON MAN
8.1. Exposure of the skin and eyes
8.2. Sensitization
8.3. Accidental inhalation exposure
8.4. Other accidental exposures
8.5. Occupational inhalation exposure
8.6. Mortality studies
8.7. Mutagenicity and related end-points
8.8. Effects on reproduction
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
10. RECOMMENDATIONS FOR FURTHER RESEARCH
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON ETHYLENE OXIDE
Members
Dr R. Bruce, Environmental and Criteria Assessment Office, US
Environmental Protection Agency, Research Triangle Park,
North Carolina, USA (Rapporteur)
Mr T.P. Bwititi, Hazardous Substances and Articles Department,
Ministry of Health, Harare, Zimbabwe
Dr B. Gilbert, CODETEC, University City, Campinas, Brazil
Prof P. Grasso, Robens Institute, University of Surrey,
Guildford, Surrey, United Kingdom
Prof M. Ikeda, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan (Chairman)
Dr T. Lewis, US National Institute for Occupational Safety and
Health, Cincinnati, Ohio, USA
Dr B. Malek, Prague Hygiene Station, Department of Industrial
Hygiene, Prague, Czechoslovakia
Prof N.C. Nayak, Department of Pathology, All-India Institute
of Medical Sciences, New Delhi, India
Prof M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, Alexandria, Egypt (Vice-
Chairman)
Dr G.J. Van Esch, Bilthoven, The Netherlands
Members of Other Organizations
Dr A. Berlin, Health and Safety Directorate, Commission of the
European Communities, Luxembourg
Dr R. Steger, International Commission on Occupational Health,
Geneva, Switzerland
Mme M.Th. Van der Venne, Health and Safety Directorate,
Commission of the European Communities, Luxembourg
Observers
Dr E. Longstaff (European Chemical Industry Ecology and
Toxicology Centre), ICI Central Toxicology Laboratory,
Genetic Toxicology Section, Macclesfield, United Kingdom
Dr M. Martens, Institute of Hygiene and Epidemiology, Division
of Toxicology, Brussels, Belgium
Observers (contd.)
Dr W. Moens, Institute of Hygiene and Epidemiology, Division
of Toxicology, Brussels, Belgium
Dr M. Wooder (European Chemical Industry Ecology and
Toxicology Centre), Shell International Petroleum Company,
Health, Safety and Environment Division, London, United
Kingdom
Secretariat
Prof F. Valic, Andrija Stampar School of Public Health,
University of Zagreb, Zagreb, Yugoslavia (Secretary)a
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, The Netherlands (Temporary
Adviser)
Mr J. Wilbourn, International Agency for Research on Cancer,
Lyons, France
-------------------------------------------------------------------
a IPCS Consultant.
PREFACE
Although only key references essential for the evaluation of
the risks for human health and the environment are cited, this
document is based on a comprehensive search of the available,
original scientific literature, while valuable information has also
been obtained from various reviews.
A detailed data profile on ethylene oxide can be obtained from
the International Register of Potentially Toxic Chemicals
(UNEP/IRPTC, Palais des Nations, CH-1211 Geneva 10, Switzerland,
telephone number 988400 - 985850).
The document focuses on describing and evaluating the risks of
ethylene oxide for human health and the environment.
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors, which may have occurred, to
the Manager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENE OXIDE
The WHO Task Group for the Environmental Health Criteria for
Ethylene Oxide met at the Institute of Hygiene and Epidemiology, in
Brussels, Belgium, on 21 - 26 October 1985. Dr G. Thiers, who
opened the meeting, welcomed the participants on behalf of the host
government, and Dr F. Valic welcomed them on behalf of the heads of
the three IPCS co-sponsoring organizations (ILO/WHO/UNEP). The
Group reviewed and revised the second draft criteria document and
made an evaluation of the health risks of exposure to ethylene
oxide.
The efforts of DR T. VERMEIRE, of the NATIONAL INSTITUTE OF
PUBLIC HEALTH AND ENVIRONMENTAL HYGIENE, Bilthoven, the
Netherlands, who was responsible for the preparation of the draft,
and of all who helped in the preparation and the finalization of
the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY
Ethylene oxide is a colourless, highly reactive, and flammable
gas at room temperature and ambient pressure. The current world
production is greater than 5.5 million tonnes. Its major use is as
an intermediate in the production of various chemicals. Since
ethylene oxide is a reactive epoxide and potent biocide, a small
quantity (less than 1%) is used for the fumigation and
sterilization of foodstuffs and medical equipment. Because of its
high odour threshold (900 - 1260 mg/m3), sensory recognition does
not offer adequate warning of a health hazard.
Detection limits of 0.024 mg/m3, 2 mg/litre, and 0.15 mg/kg
have been reported for gas chromatographic determinations in air,
water, and food, respectively. A total loss to the atmosphere of
1 - 2% of production occurs during its manufacture and use. Its
removal from the atmosphere and neutral water is slow, but it is
more rapid under acidic or basic catalysis. Aerobic biodegradation
is slow.
Human exposure mainly occurs through inhalation in
sterilization facilities and in production plants. In
sterilization facilities, 8-h time-weighted average levels have
usually been below 36 mg/m3, with short-term exposures of about
100 mg/m3, and peak levels of up to 1800 mg/m3. In production
plants, the time-weighted average has usually been below 4 mg/m3.
Ambient levels at a distance from point sources of emission have
been estimated to be below the limit of detection.
Exposure to residues of ethylene oxide or its reaction
products, halohydrins and ethylene glycol, also occurs from
fumigated foods, pharmaceutical products, and sterilized medical
equipment. 2-Chloroethanol levels as high as several g/kg have
been measured in food and levels of several hundred mg/kg in
medical equipment.
Ethylene oxide is not expected to bioaccumulate in the
environment. Fish are the most susceptible aquatic organisms. An
LC50 of 90 mg/litre was observed for goldfish exposed for 24 h.
2-Chloroethanol, a degradation product in saline water, is equally
toxic but, 1,2-ethanediol, a major degradation product, is much
less toxic.
When inhaled, ethylene oxide is readily absorbed, distributed
throughout the body, and rapidly metabolized. Accordingly, most
organs receive equivalent doses of the chemical and its
metabolites. The degree of alkylation of proteins and DNA varies
slightly between the different organs and blood. In man and
rodents, the half-life of the compound in tissues has been
estimated to be 9 - 10 min. Two metabolic pathways have been
identified including hydrolysis to 1,2-ethanediol and conjugation
with glutathione. Excretion is primarily via the urine.
Ethylene oxide is moderately toxic for mammals (the LD50 for
the rat is 280 - 365 mg/kg body weight; the 4-h LC50 is 2630
mg/m3). Both experimental animal and human data show that aqueous
solutions of ethylene oxide are irritating for the skin and eyes;
the irritant effects of ethylene oxide vapour or residues in
medical equipment on the eyes and the respiratory tract have also
been observed. These effects are often delayed. Severe skin
irritation is characterized by the formation of vesicles. A
concentration of 10 mg/litre produced mild irritation of the human
skin; a concentration of 500 g/litre was most injurious to the
human skin. Allergic contact dermatitis has been reported;
systemic immunologically mediated allergy is considered rare.
Respiratory tract irritation increases with inhaled vapour
concentration and may result in severe life-threatening pulmonary
disease. Repeated exposure (2 - 8 weeks) to ethylene oxide vapour
at or above 900 mg/m3 produced sensory and motor neurological
impairment and may result in a peripheral neuropathy. In animals,
the latter was often accompanied by muscular atrophy. Lesions in
the medulla oblongata of monkeys, following 2 years of intermittent
exposure (7 h/day, 5 days/week) to 90 and 180 mg/m3 indicated
neuropathy in the brain, which may be related to the neuropathies
observed in man and other animal species. Cardiovascular collapse
and renal failure have been attributed to residues of ethylene
oxide in medical equipment.
Ethylene oxide alkylates DNA and is mutagenic for plants,
microorganisms, insects, and mammals. Cytogenetic studies on man
have shown dose-related increased frequencies of both sister
chromatid exchanges (SCEs) and chromosomal aberrations; in one
study, SCEs developed following daily exposure for less than 5 min
per day.
The evidence that ethylene oxide is a reproductive toxin is
less conclusive. Where fetal developmental effects have occurred,
the doses of ethylene oxide approached or equalled those producing
maternal toxicity. To date, impaired male reproductive function in
animals has been demonstrated only at concentrations of 90 mg/m3 or
more in long-term intermittent exposures or at higher air
concentrations for brief exposures. In pregnant women, the results
of one study suggest that occupational exposure estimated to be an
8-h time-weighted average of 0.18 - 0.90 mg/m3, with peak
concentrations up to 450 mg/m3, was associated with spontaneous
abortions. However, limited exposure data prevents the
establishment of a relationship between abortion rates and exposure
levels.
Ethylene oxide is carcinogenic for animals when administered by
the intragastric, subcutaneous injection, and inhalation routes of
exposure. In man, 2 studies have shown an association between
ethylene oxide exposure and an excess risk of cancer, but both
studies have limitations. Airborne concentrations of ethylene
oxide in the 2 studies were reported to be time-weighted averages
of 36 ± 18 mg/m3 and 10 - 50 mg/m3, with occasional brief exposures
in excess of the odour threshold (900 - 1260 mg/m3).
Taking into account available data concerning the alkylating
nature of ethylene oxide, the demonstration of DNA adducts, and the
overwhelmingly positive in vivo responses in mutagenic and
clastogenic assays, the reproducible positive carcinogenic findings
in animals, and the epidemiological findings suggesting an increase
in the incidence of human cancer, ethylene oxide should be
considered as a probable human carcinogen, and its levels in the
environment should be kept as low as feasible.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
Structural formula: O
/ \
/ \
/ \
H--C-------C--H
| |
| |
H H
Molecular formula: C2H4O
Abbreviation: EO, ETO
Common synonyms: dihydrooxirene; dimethylene oxide;
1,2-epoxyethane; ethene oxide;
oxane; alpha, beta-oxidoethane;
oxirane (CAS and IUPAC name)
Common trade names: Anprolene; Melgas; Merpal; Sterigas
P (pure products); Carboxide;
Cartox; Etox; Oxyfume 20; Oxyfume
30; Sterigas 90/10; Steroxide 20;
T-gas (formulations with carbon
dioxide); Oxyfume 12; Sterigas
12/88; Steroxide 12/88 (formulations
with fluorocarbons); Etoxiat
CAS registry number: 75-21-8
RTECS registry number: KX 2450000
2.2. Chemical and Physical Properties of Ethylene Oxide
Ethylene oxide is a gas at room temperature and normal
atmospheric pressure. It condenses to a liquid at 10 °C. The
vapour is highly flammable and subject to explosive decomposition.
The liquid is stable to common detonating agents, but may
polymerize violently after initiation by acids, bases, or heat.
Polymerization is catalysed by metal chlorides and oxides.
Ethylene oxide is very reactive in both the liquid and vapour
phase. Ring opening readily occurs with release of energy,
particularly in reactions with nucleophiles such as water,
alcohols, halides, amines, and sulfhydryl compounds.
Some physical and chemical data on ethylene oxide are given in
Table 1.
Table 1. Some physical and chemical data on ethylene oxide
-------------------------------------------------------------------
Physical state gas
Colour colourless
Odour ethereal
Odour threshold 470 mg/m3 for perception and
900 - 1260 mg/m3 for recognitiona
Relative molecular mass 44.05
Melting point -111 °C
Boiling point 10.4 °C
Water solubility infinitely soluble
log n-Octanol-water partition -0.30
coefficient
Density 0.87 g/ml, 20 °C
Relative vapour density 1.5
Vapour pressure 146 kPa (1095 mm Hg), 20 °C
Flash point < -18 °C (open-cup)
Flammable limits 3 - 100% by volume
-------------------------------------------------------------------
a From: Jacobson et al. (1956) and Hellman & Small (1974).
Conversion factor
ethylene oxide 1 ppm = 1.80 mg/m3 air at 25 °C
and 101.3 kPa (760 mm Hg)
2.3. Analytical Methods
Methods for the sampling and determination of ethylene oxide in
air, water, food, plastic materials, blood, and urine are
summarized in Table 2. Some methods are also suitable for
measuring important reaction products such as 2-chloroethanol
(ethylene chlorohydrin) and 1,2-ethanediol (monoethylene glycol).
Table 2. Sampling, preparation, analysis
----------------------------------------------------------------------------------------------------------------
Medium Sampling method Analytical method Detection limit Comments Reference
----------------------------------------------------------------------------------------------------------------
Air sampling on gas chromatography 0.024 mg/m3 sample size 1 litre; US Department of
charcoal; with electron capture suitable for personal Labor (1984)
desorption with detection after der- and area monitoring
carbon disulfide ivatization with
hydrogen bromide
Air sampling on gas chromatography 0.27 mg/m3 sample size < 10 Quazi & Ketcham
charcoal; with flame ionization litre; suitable for (1977)
desorption with detection personal and area
carbon disulfide monitoring
Air trapping in gas chromatography 1.8 mg/m3 sample size 12 - 99 Romano & Renner
dilute sulfuric with flame ionization (99-litre litre; suitable for (1979)
acid using detection sample) personal and area
a microimpinger monitoring
Air infrared spectro- 1.8 mg/m3 direct analysis; suitable Korpela et al.
scopy for instantaneous and (1983)
continuous area monitor-
ing; limited specificity
Air colorimetric direct 18 mg/m3 simple, cheap method Mouilleseaux et
reading indicator giving a good correlation al. (1983)
tubes with gas chromatographic
analysis; suitable for
short-term area measure-
ments; limited specificity
Water gas chromatography 2 mg/litre direct analysis; also Hartman & Bowman
with flame ionization suitable for measuring (1977)
detection main reaction products;
drugs and formulations
can be analysed after
extraction with water
----------------------------------------------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------------------------------------------
Medium Sampling method Analytical method Detection limit Comments Reference
----------------------------------------------------------------------------------------------------------------
Food extraction by gas chromatography 0.15 mg/kg sample size 5 - 30 g; Scudamore &
5:1 acetone- with flame ionization wet weight also suitable for meas- Heuser (1971)
water (by vol- detection uring reaction products
ume) for 24 h
Food thermal colorimetry using 0.7 mg/kg simple, cheap method; Rajendran &
desorption in an paper strips with wet weight headspace analysis; Muthu (1981)
airtight bottle sodium sulfite and sample size 75 g; other
at 40 °C for thymol blue-phenol- alkene oxides and alde-
30 min phthalein indicator hydes may interfere
Plastic thermal desorp- gas chromatography 0.1 mg/kg headspace analysis Romano et al.
material tion in an air- with flame ionization (1973)
tight vial at detection
100°C for 15 min
Blood, gas chromatography direct analysis after Martis et al.
Urine with flame ionization centrifugation in air- (1982)
detection tight vials; also suit-
able for measuring
metabolites
----------------------------------------------------------------------------------------------------------------
Instantaneous gas chromatographic measurements of ethylene
oxide in air can be performed after taking grab samples
(Mouilleseaux et al., 1983). The rather complex gas
chromatographic procedure of Scudamore & Heuser (1971) for the
determination of ethylene oxide and reaction products in food could
be replaced by a simpler procedure, using temperature programming
(Pfeilsticker et al., 1975). Titrimetric or colorimetric methods
are available, but these methods are not specific, are often
subject to systematic errors, and are not applicable for continuous
monitoring.
In studies on mice and rats, the degree of alkylation of amino
acids, particulary of histidine, in haemoglobin can be used for
monitoring the tissue doses of ethylene oxide. Assuming even
distribution, tissue dose is defined as the integral of the
calculated concentration of free ethylene oxide in the tissues over
a specified period of time (Ehrenberg et al., 1974; Osterman-Golkar
et al., 1983; Segerbäck, 1983). A sensitive method, based on
derivatization with heptafluorobutyric anhydride followed by gas
chromatography and mass spectrometry was developed to measure the
amount of N3-(2-hydroxyethyl)histidine in haemoglobin (Calleman et
al., 1978). This method has a detection limit of 0.004 µg/g
haemoglobin. It was used in industrial workers by Calleman et al.
(1978) and van Sittert (1985). Human haemoglobin has a life span
of about 4 months and, therefore, may integrate the dose of
ethylene oxide over a long period (Osterman-Golkar et al., 1976).
The resolving power of detection of haemoglobin alkylation due to
exposure to ethylene oxide appears to be limited by the occurrence
of background alkylations. It was estimated that exposures of less
than 9 mg/m3 could be masked by these background alkylations
(Osterman-Golkar, 1983). More validation work in human beings is
still needed.
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND
DISTRIBUTION
3.1. Production, Uses, Disposal of Wastes
3.1.1. Production levels and processes
In 1978, world production of ethylene oxide was estimated to be
4540 kilotonnes (Clayton & Clayton, 1981). The USA production,
which roughly accounted for half of this figure, rose from 1750
kilotonnes in 1970 to 2400 kilotonnes in 1980 (USITC, 1971, 1981).
For 1983, USA production was estimated to be 2540 kilotonnes
(Webber, 1984). In western Europe, 865 kilotonnes were produced in
1972 (Glaser, 1979), while for 1981, production was estimated to be
1370 kilotonnes (IARC, 1985). In Japan, 470 kilotonnes were
produced in 1982 (IARC, 1985). Ethylene oxide is also produced in
Australia, Brazil, Bulgaria, Canada, China, Czechoslovakia, the
German Democratic Republic, India, the Republic of Korea, Mexico,
Poland, Romania, and the USSR (IARC, 1985). From the above data,
it can be derived that the current world production will be far
above 5500 kilotonnes per year.
Ethylene oxide is chiefly produced by the oxidation of ethene
with air or oxygen in the presence of a silver oxide catalyst.
This process has virtually replaced the chlorohydrin process in
which 2-chloroethanol (ethylene chlorohydrin) reacts with potassium
hydroxide or calcium oxide. Common impurities in the oxidation
process are water, acetic acid, acetaldehyde, and organic and
inorganic chlorides (WHO, 1978). Common impurities in the
chlorohydrin process are vinyl chloride, 1,2-dichloroethane,
chloroethane, and ethylene chlorohydrin.
3.1.2. Uses
Virtually all the ethylene oxide produced is used as an
intermediate in the production of various chemicals. In order of
importance in the USA, the principal chemicals are: the antifreeze
1,2-ethanediol; polyethylene terephthalate polyester for fibres,
films, and bottles; non-ionic surface active agents; glycol ethers;
ethanolamines; and choline. A small fraction of the total
consumption (about 1% in the USA in 1976) was used as an
antimicrobial sterilant or as an insecticidal fumigant (WHO, 1978;
Glaser, 1979). Less than 0.02% of this production (500 000 kg) was
used for sterilization in hospitals (Glaser, 1979). In Belgium, an
estimated 0.07% of the total consumption of ethylene oxide (120 000
kg) was used in the health care and medical products industries in
1980 (Wolfs et al., 1983).
3.1.3. Disposal of wastes
Escape through air vents during production and sterilization
appears to be the most important source of release of ethylene
oxide into the environment. The waste gas can be removed from the
air by scrubbing. Emission control of liquid wastes mainly occurs
by incineration in liquid-burning hazardous waste incinerators.
Process waters for the manufacture and use of ethylene oxide are a
minor problem with respect to waste management. Conventional
effluent water treatment including biological treatment is reported
to be sufficient. No specific solid wastes are associated with the
manufacture of ethylene oxide (Bogyo et al., 1980).
3.2. Transport and Fate in the Environment
The main pathway of entry of ethylene oxide into the
environment is through its escape into the atmosphere due to
evaporation and with vented gases during production, handling,
storage, transport, and use. Most of the ethylene oxide applied as
a sterilant or fumigant will enter the atmosphere (Bogyo et al.,
1980). In the USA, production losses were estimated at 13 kg per
tonne of ethylene oxide produced by catalytic oxidation.
Sterilization and fumigation processes were estimated to account
for a loss of 9 kg per tonne of ethylene oxide produced or
approximately 1% of the total consumption (WHO, 1978). In 1980,
this would have meant a combined loss of 53 kilotonnes of ethylene
oxide into the atmosphere in the USA, which is approximately 2% of
the total production in the USA.
At ambient levels, ethylene oxide will be removed from the
atmosphere via oxidation by hydroxyl radicals. On the basis of a
theoretical rate constant for this reaction, the atmospheric
residence time of ethylene oxide was estimated to be 5.8 days
(Cupitt, 1980). However, experimental data have shown the
residence time to be 100 - 215 days, depending on the hydroxyl
radical concentration and the ambient temperature (US EPA, 1985).
Because of its high water solubility, ethylene oxide levels in air
will also be reduced through washout by rain (Conway et al., 1983).
The photochemical reactivity of ethylene oxide, in terms of its
ozone-forming ability, is low (Joshi et al., 1982). Evaporation
from water is a significant removal process. Under specific
conditions, Conway et al. (1983) found a half-life of 1 h for the
evaporation of ethylene oxide from water. In the environment,
chemical degradation in water through ionic reactions appears to be
comparatively slow. In neutral, fresh water at 25 °C, ethylene
oxide is broken down to form 1,2-ethanediol with a half-life of
14 days (Conway et al., 1983). At 0 °C, the half-life is 309 days.
The reaction is acid- and base-catalysed (Virtanen, 1963). In the
presence of halide ions, 2-haloethanol will also be formed. In
neutral water of 3% salinity, at 25 °C, 77% of ethylene oxide was
found to react to form 1,2-ethanediol and 23%, to form 2-
chloroethanol with a half-life of 9 days (Conway et al., 1983).
Ethylene oxide and its possible metabolites can be biodegraded
slowly by aerobic microorganisms. Biological oxygen demands of 3 -
5% and 52% of the theoretical oxygen demand were determined for
ethylene oxide after 5 and 20 days, respectively, using a domestic
sewage seed (Bridié et al., 1979b; Conway et al., 1983).
4.ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Occurrence in the Environment
No data are available concerning levels of ethylene oxide in
air, water, or soil, following emission from production plants, and
there are no data indicating that ethylene oxide occurs as a
natural product. Most of the ethylene oxide used for fumigation or
sterilization finally enters the environment, mainly the air.
Uncontrolled emission of ethylene oxide from a hospital
sterilization chamber led to high levels of the sterilant in the
immediate surroundings. Concentrations of between 7700 and 12 000
mg/m3 were measured, 2 - 3 m from an exhaust pipe on the outside
wall (Dunkelberg & Hartmetz, 1977).
4.2. General Population Exposure
4.2.1. Exposure via food and tobacco
Residue levels, after fumigation or sterilization using
ethylene oxide, depend on a number of factors, such as the
concentration of ethylene oxide, the composition of the gas,
temperature, aeration, and storage conditions after treatment,
the type of commodity and its moisture and lipid content, pH,
permeability, particle size, and packaging. Absorbed ethylene
oxide disappears rapidly. In a variety of commodities,
concentrations between 32 and 7000 mg/kg wet weight, found 1 h
after treatment, dropped to below 1 mg/kg within 14 days of
storage, at ambient conditions. However, sealed storage or storage
at low temperatures will impede desorption considerably (Scudamore
& Heuser, 1971). In spices, ethylene oxide at concentrations of
53 - 116 mg/kg wet weight, measured 2 days after fumigation, fell
to less than 25 mg/kg within another 24 days (Gerhardt & Ladd
Effio, 1983).
Ethylene oxide will react with chloride and bromide ions in
commodities to form 2-chloroethanol and 2-bromoethanol,
respectively. This reaction can continue after treatment. Levels
of 2-chloroethanol of up to several thousands of mg/kg wet weight
have been measured, depending on, among other factors, the chloride
content and pH of the commodity, and the concentration of ethylene
oxide (Wesley et al., 1965; Ragelis et al., 1968; Buquet & Manchon,
1970; Scudamore & Heuser, 1971; Gerhardt & Ladd Effio, 1983).
Under unfavourable (e.g., air-tight) conditions, 2-chloroethanol
can persist much longer than residues of ethylene oxide, even
longer than one year after treatment. 2-Chloroethanol will
disappear rapidly from freely-aerated and finely-divided
commodities. However, 2-bromoethanol decomposes slowly (Scudamore
& Heuser, 1971; Stijve et al., 1976).
The formation of 1,2-ethanediol (monoethylene glycol) and
2,2'oxybisethanol (diethylene glycol) from water and ethylene oxide
are competitive reactions (Gordon & Thornburg, 1959; Buquet &
Manchon, 1970; Scudamore & Heuser, 1971). Levels in food up to
2420 mg/kg wet weight have been reported for 1,2-ethanediol and up
to 65 mg/kg wet weight for 2,2'-oxybisethanol, 6 - 12 months after
sterilization (Scudamore & Heuser, 1971). Food constituents can
also be alkylated. Hydroxyethylated derivatives of amino acids,
vitamins, alkaloids, and sugars have been identified that might
affect the nutritive value of food. A change in organoleptic
properties has been reported for a variety of foodstuffs (Oser &
Hall, 1956; Gordon & Thornburg, 1959; Windmueller et al., 1959;
Kröller, 1966; Pfeilsticker & Siddiqui, 1976).
2-Chloroethanol was detected in cigarette smoke at levels of
350 and 82 mg/m3 trapped smoke, 1 and 49 days, respectively, after
fumigation of the tobacco (Chaigneau & Muraz, 1981).
4.2.2. Exposure via medical equipment
Ethylene oxide may also be absorbed by medical equipment during
sterilization and may remain in the materials for some time, as the
unchanged compound or as its reaction products. Factors affecting
residue levels are similar to those mentioned in section 4.2.1 for
food. Aeration and storage conditions are very important,
particularly with respect to possible worker exposure.
Other conditions being equal, the removal of ethylene oxide
residues by aeration takes longer in plastics such as polyvinyl
chloride, polyether-polyurethane, polyglycolic acid, and glassy
polymers. Desorption periods for ethylene oxide in polyethylene
and rubber materials and textiles are shorter (McGunnigle et al.,
1975; Gillespie et al., 1979; Gilding et al., 1980; Star,
1980b,c,d; Dauvois et al., 1982). Star (1980b) sterilized
polyvinyl chloride and rubber tubes and found that initial residues
of ethylene oxide in polyvinyl chloride tubes of between 3510 and
7300 mg/kg dropped to between 3 and 443 mg/kg after 7 days of
aeration at room temperature. In rubber tubes, ethylene oxide
residues dropped from 291 and 858 mg/kg after sterilization to
levels of between 2 and 24 mg/kg, after 24 h of aeration at room
temperature. Using the same sterilization method, similar levels
in polyvinyl chloride and rubber tubes were reached after 24 and
4 h of aeration, at 62 °C, respectively (Star, 1980c). Ethylene
oxide residues in sterilized cotton wool, adhesive dressings, and
compresses dropped from between 270 and 3600 mg/kg to 2 mg/kg or
less, in 7 - 8 days of storage. In sanitary pads, the latter
residue level was only reached after 14 - 32 days of storage
(Dauvois et al., 1982). In sterilized pharmaceutical products,
ethylene oxide levels ranging from the detection limit up to 16 300
mg/kg wet weight were measured after 2 - 8 h of vacuum treatment;
1,2-ethanediol was also identified (Adler, 1965). Ethylene oxide
levels in water eluates from hollow-fibre dialysers corresponded to
a theoretical residue of 1 mg of ethylene oxide per dialyser (50
mg/kg) after an aeration period of 60 days. During 6 h of elution,
the concentration of ethylene oxide in the hourly water eluates did
not change substantially (Henne et al., 1984).
Maximum residues of 2-chloroethanol in polyvinyl chloride
tubes, ranging between 20 and 40 mg/kg, dropped to below the
detection limit, within 4 days (Star, 1980d; Jordy, 1983). In
rubber, however, the 2-chloroethanol level rose from 300 mg/kg,
directly after sterilization, to a maximum of 800 mg/kg, 3 h later;
desorption was slow (Jordy, 1983). Gamma-irradiaton before
sterilization of polyvinyl chloride by ethylene oxide can increase
the 2-chloroethanol residues considerably (Star, 1980d). For the
toxicology of 2-chloroethanol, see Vettorazzi (1979).
4.3. Occupational Exposure
In a total of 8 production plants, the levels of worker
exposure to ethylene oxide, in recent years, were reported
generally to be below 18 mg/m3 (Hogstedt et al., 1979b; Morgan et
al., 1981; Thiess et al., 1981b). In a modern production plant in
western Europe, the geometric mean of 0.5 hour-samples, taken in
1974, was less than 0.02 mg/m3. The geometric mean of 8-h samples
was less than 0.02 mg/m3 in 1978 and 1980, and 0.22 mg/m3 in 1981.
In 89% of the total of 273 samples, the concentration of ethylene
oxide was less than 0.2 mg/m3. In the remaining samples,
concentrations of up to 11.6 mg/m3 were found (van Sittert et al.,
1985). In a plant in the USA, typical average daily exposures were
reported to be 0.3 - 4.0 mg/m3 in 1979 (Flores, 1983).
Occasionally, higher values can occur. Thiess et al. (1981a)
reported a maximum of 3420 mg/m3, during a plant breakdown. Flores
(1983) reported worst-case peak exposures of up to 17 000 mg/m3.
In the past, exposure levels were higher (Joyner, 1964; Hogstedt et
al., 1979b).
Although the volume of ethylene oxide used for sterilization is
relatively small, many workers are involved. In the USA,
approximately 75 000 health-care workers were estimated to be
potentially exposed in 1977 (Glaser, 1979). Peak concentrations of
up to 1800 mg/m3 have been measured and occurred mainly when the
sterilization chambers were opened. Exposure levels very much
depend on the scale and techniques of the process used.
In 4 hospital sterilization units in France, in 1980,
concentrations of between 0.9 and 410 mg/m3 were measured, after
sampling for several minutes. During the loading of the
sterilizers, the average levels per unit ranged from 3 to 45 mg/m3.
During unloading, averages of 8 - 97 mg/m3 were measured. In a
desorption room, 1- or 2-h time-weighted average levels of between
18 and 173 mg/m3 were measured; at other sites, time-weighted
averages were less than 17 mg/m3 (Mouilleseaux et al., 1983).
Exposures, after the opening of sterilizers, ranging from less than
0.2 to 111 mg/m3 were found by personal sampling over several
minutes in 16 hospitals, in Belgium, in 1981 - 83. In one other
hospital, an average of 477 mg/m3 was measured by personal
sampling. In the desorption rooms of a total of 19 hospitals in
Belgium, the time-weighted average concentrations, over 30 min,
ranged from less than 0.02 mg/m3 up to 120.6 mg/m3. In nearby
rooms of several hospitals, average 30-min exposure levels of up to
15 mg/m3 were measured (Lahaye et al., 1984). In 6 hospital
sterilization units in Italy, using pure ethylene oxide, the 8-h
time-weighted average concentrations were 6.7 - 36 mg/m3 with an
average of 19.3 mg/m3. Continuous sampling during the 5-min
interval following the opening of sterilizers revealed time-
weighted average concentrations of 18 - 288 mg/m3 (average 112.5
mg/m3). In 2 other hospitals in Italy, using 11% ethylene oxide in
freon, the 8-h time-weighted average levels were 0.36 - 0.90 mg/m3
with an average of 0.63 mg/m3, and the 5-min exposure levels were
9 - 47 mg/m3 (average 15.5 mg/m3) (Sarto et al., 1984). Time-
weighted average exposures of Swedish personnel involved in
sterilizing medical equipment in 1975 were 14 mg/m3, when the
sterilizer door was open, and 2.3 mg/m3, when the door was closed.
Before working routines were changed, these levels had been 52 and
16 mg/m3, respectively. Instantaneous peak levels had reached
94 mg/m3. In another Swedish factory, in 1978, the time-weighted
average personal exposure, during a total shift, was 4.3 mg/m3
(Högstedt et al., 1983). Pero et al. (1981) reported 1-h time-
weighted average personal exposures of up to 18 mg/m3 for a
sterilization facility in Sweden.
Data from the USA agree with the data from Europe. For workers
in 5 sterilization rooms of a hospital in the USA, 15-min exposures
of up to 86 mg/m3 were found with 8-h time-weighted averages
ranging from less than 0.13 to 7.7 mg/m3 and instantaneous peaks of
up to 1430 mg/m3 (Hansen et al., 1984). At 3 work-sites in the
sterilization facilities of a plant manufacturing health-care
products, 8-h time-weighted averages of 0.9, 9 - 18, and 9 -
36 mg/m3 were measured prior to 1980, but from that year onwards,
the 8-h time-weighted averages were below 1.8 mg/m3 (Stolley et
al., 1984).
5. KINETICS AND METABOLISM
5.1. Absorption
Ethylene oxide is very soluble in blood. Therefore, pulmonary
uptake is expected to be fast and to depend only on the alveolar
ventilation rate and the concentration of ethylene oxide in the
inspired air. Ehrenberg et al. (1974) came to such a conclusion
after inhalation studies on mice. The rate of uptake of ethylene
oxide was 1.1 µg/kg body weight, per min, at an exposure level of
1 mg/m3. This corresponds to nearly 100% absorption of ethylene
oxide from 1.1 litre of air per min and per kg body weight, which
is the reported rate of alveolar ventilation in resting mice
(Altman & Dittmer, 1974). No specific information pertaining to
skin absorption is available, but accidental exposure of the skin
of 3 industrial workers to 1% aqueous solution of ethylene oxide
was reported to have resulted in marked nausea and profuse vomiting
(Sexton & Henson, 1949).
5.2. Distribution
Ethylene oxide is rapidly distributed throughout the body. In
mice, body autoradiography, 2 min after intravenous injection,
showed that concentrations of ethylene oxide in the liver, kidneys,
and pancreas were 3 - 4 times those in the blood. Between 20 min
and 4 h after exposure, radioactivity was distributed throughout
the body (Appelgren et al., 1977). Directly after inhalation by
mice, the highest concentrations of labelled ethylene oxide or its
metabolites were found in the liver, kidney, and lung. The
radioactivity in the liver and kidney dropped exponentially and
approached the levels in the lung, testes, spleen, and brain within
4 h, indicating rapid metabolism and excretion (Ehrenberg et al.,
1974). On the basis of tissue alkylation data (Ehrenberg et al.,
1974) or haemoglobin alkylation data (Osterman-Golkar et al., 1976,
1983), a half-life of approximately 10 min was estimated for the
first-order clearance of ethylene oxide from mouse or rat tissues.
A similar value for man was estimated on the basis of haemoglobin
alkylation data (Calleman et al., 1978). In dogs, intravenously-
administered ethylene oxide cleared from plasma with a mean half-
life of 33 min, which was independent of the dose levels of 25 and
75 mg/kg body weight. Clearance of the confirmed metabolite 1,2-
ethanediol from plasma, following intravenous administration, was
slower with a half-life of between 3 and 4.4 h (Martis et al.,
1982).
When the degree of protein and DNA alkylation was investigated
in mice and rats, only small variations were observed between the
different tissues in the species. Apparently, most organs receive
a more or less equal dose of ethylene oxide after distribution
throughout the body. The extent of protein alkylation was
approximately equal in the lung, liver, kidney, and spleen of mice,
120 min after inhalation of 2 mg ethylene oxide/m3 air, for 75 min,
but in the testes, it was about 50% lower. When the vapour
concentration was increased (up to 59 mg/m3), the degree of protein
alkylation in the liver increased relative to that in the other
tissues. In all the tissues investigated, protein alkylation
increased linearly with the dose up to an exposure level of
59 mg/m3, and was relatively constant for at least 3.5 h following
exposure (Ehrenberg et al., 1974). Haemoglobin alkylation was
previously discussed in section 2.3.
When 0.4 mg ethylene oxide/kg body weight was administered
intraperitoneally to mice, DNA alkylation in the testes and spleen
was, respectively, 50 and 40% of that in the liver, 5 h after
exposure. The approximate half-lives of the alkylation products
were 24 h in the spleen, 10 h in the testes, and 12 h in the liver.
For the spleen, this half-life was found to be shorter in vivo
than in vitro, indicating active removal (Segerbäck, 1983). In a
similar study on rats receiving 0.1 or 0.9 mg ethylene oxide/kg
body weight, DNA alkylation in the testes was about one-third of
that in the liver (Osterman-Golkar et al., 1983). So far, N7-(2-
hydroxyethyl)guanine is the only DNA alkylation product that has
been found in vivo (Ehrenberg et al., 1974; Segerbäck, 1983). This
reaction product has also been identified in vitro (Brookes &
Lawley, 1961). In addition, adenosine also reacted in vitro with
ethylene oxide to form N1-(2-hydroxyethyl)-adenosine (Windmueller
& Kaplan, 1961). When ethylene oxide reacted in vitro with
uridine, N3-(2-hydroxyethyl)uridine was the only product found,
but in the reaction with uridine-5-phosphate, phosphodiester
formation was observed (Ukita et al., 1963).
5.3. Metabolic Transformation and Excretion
Available animal data indicate 2 possible pathways for the
metabolism of ethylene oxide, i.e., hydrolysis and glutathione
conjugation (Fig. 1).
In dogs, peak levels of 13 and 33 mg 1,2-ethanediol/litre
blood-plasma were measured between 1 and 3 h after intravenous
administration of 25 or 75 mg ethylene oxide in water/kg body
weight, respectively. As the half-life for hydrolysis is about
60 h at 40 °C in neutral fresh water (Virtanen, 1963), the
involvement of an epoxide hydrolase (EC 3.3.2.3) has been
suggested, but this has not yet been confirmed. The peak
concentration of 1,2-ethanediol at 25 mg ethylene oxide/kg body
weight represented approximately 25% of the dose of ethylene oxide.
Within 24 h, 7 - 24% of the dose was excreted in the urine as 1,2-
ethanediol. No other compound-related metabolites were identified
(Martis et al., 1982). In the serum of 18 workers occupationally
exposed to ethylene oxide (range 0.54 - 27 mg/m3; average 7.56
mg/m3), for an average of 5.3 years, the blood concentration of
1,2-ethanediol was found to be elevated compared with that in
unexposed controls (Wolfs et al., 1983).
The results of studies on rats, rabbits, and monkeys have shown
that some 1,2-ethanediol is metabolized but that most of it is
excreted unchanged in the urine (Gessner et al., 1961; McChessney
et al., 1971).
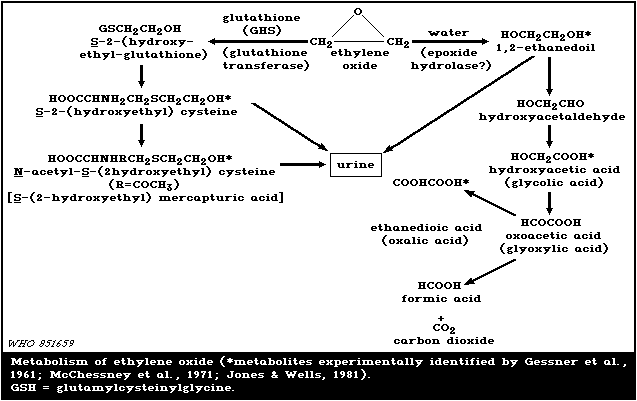 When a single dose of 2 mg labelled ethylene oxide in
propanediol/kg body weight was applied intraperitoneally to rats,
43% of the administered radioactivity was excreted in the urine
within 50 h (41% within 24 h) of exposure, 9% as S-(2-
hydroxyethyl)cysteine and 33% as N-acetyl- S-(2-hydroxyethyl)
cysteine, both products of glutathione conjugation. Via the lungs,
1.5% was excreted as carbon dioxide and 1% as unmetabolized
ethylene oxide (Jones & Wells, 1981). The involvement of
glutathione-epoxide- S-transferase (EC 4.4.1.7) has not been
investigated further. In vitro glutathione conjugation of the
homologue propylene oxide was shown to proceed only in the presence
of an enzyme (Fjellstedt et al., 1973). In rabbits, no effect was
found on liver- and blood-glutathione levels, after 12 weeks of
exposure to concentrations of ethylene oxide at 18, 90, or 450
mg/m3, for 5 days per week, 6 h per day (Yager & Benz, 1982).
As ethylene oxide can react with chloride ions, and this
reaction is acid catalysed, 2-chloroethanol might be expected to be
a metabolite, especially after oral administration. However,
neither 2-chloroethanol, nor its metabolites (Johnson, 1967; Grunow
& Altman, 1982) have been found in the plasma, tissues, or urine of
species exposed to ethylene oxide.
Ehrenberg et al. (1974) found that an average of 74% of
labelled ethylene oxide, inhaled by mice, was excreted in the urine
within 24 h in the form of unidentified metabolites, and only 4%
within the next 24 h. Thus, on the basis of this and previously-
presented excretion data, excretion of metabolites of ethylene
oxide mainly takes place via the urine, within 24 h following
exposure.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
A summary of the acute toxicity of ethylene oxide for aquatic
organisms is presented in Table 3. Data on the most likely
reaction products, 1,2-ethanediol and 2-chloroethanol, are
included.
LC50s of ethylene oxide for aquatic species have been reported
to range from 90 mg/litre (goldfish, 24-h exposure) to 745 mg/litre
(brine shrimp, 48-h exposure). Microorganisms in activated sludge
showed 50% inhibition at concentrations between 10 and 100
mg/litre. Hydrolysis to 1,2-ethanediol results in detoxification.
The toxicity of 2-chloroethanol for aquatic organisms resembles
that of ethylene oxide, though 2-chloroethanol seems to be more
toxic for Daphnia magna. Nevertheless, under environmental
conditions, the conversion of ethylene oxide to 2-chloroethanol or
1,2-ethanediol will be slow.
In a study on the albino guppy, Poecilia reticulata, desorption
of ethylene oxide or its reaction products from non-aerated plastic
materials into water can lead to behavioural disturbances in the
fish almost immediately, and to death within about one h. No such
toxicity was found when the materials were aerated for 24 h
(O'Leary et al., 1969).
Ethylene oxide is very soluble in aqueous media and evaporates
from water to a significant degree. The log n-octanol water-
partition coefficient was reported to be -0.30 (Radding et al.,
1977). Thus, ethylene oxide will not bioaccumulate.
Table 3. Acute aquatic toxicitya
-----------------------------------------------------------------------------------------------------------
Organism Description T pH Dissolved Parameter Test Concentration Reference
(°C) oxygen substance (mg/litre)
-----------------------------------------------------------------------------------------------------------
Micro- activated 22 50% growth ethylene oxide 10 - 100 Conway et al.
organisms sludge inhibition 1,2-ethanediol > 10 000 (1983)b
Crustacea water flea 17 7.0 minimal 48-h LC50 ethylene oxide 212 Conway et al.
(Daphnia magna) aeration 1,2-ethanediol > 10 000 (1983)c
2-chloroethanol 100
Fish fathead minnow 22 7.0 no 96-h LC50 ethylene oxide 84 Conway et al.
(Pimephales aeration 1,2-ethanediol > 10 000 (1983)c
promelas) 2-chloroethanol 90
Fish goldfish 20 6-8 no 24-h LC50 ethylene oxide 90 Bridié et al.
(Carassius aeration 1,2-ethanediol > 5000 (1979a)d
auratus) (> 4
mg/litre)
Fish brine shrimp 24 7.0 minimal 48-h LC50 ethylene oxide 745 Conway et al.
(Artemia aeration 1,2-ethanediol > 20 000 (1983)e
salina) 2-chloroethanol 680
-----------------------------------------------------------------------------------------------------------
a All tests were static. Water analysis for the substance under test was reported by all authors.
b Incubation on a shaker for 16 h.
c Medium was fresh water, reconstituted using dechlorinated, carbon-treated tap water; 10 fish per
concentration.
d Medium was local tap water; 6 fish per concentration.
e Medium was sea water, reconstituted using dechlorinated, carbon-treated tap water, 30 - 50 shrimps per
concentration.
7. EFFECTS ON ANIMALS
7.1. Acute Exposures
7.1.1. Oral, intravenous, and inhalation studies
The LD50s for ethylene oxide, administered orally and dissolved
in water, were 330 mg/kg body weight for male rats and 280 and
365 mg/kg body weight for female and male mice, respectively (Smyth
et al., 1941; Woodard & Woodard, 1971). After inhalation, the 4-h
LC50s were 1500 and 1730 mg/m3 for mouse and dog, respectively, and
2630 mg/m3 for rat (Jacobson et al., 1956). 1,2-Ethanediol, a
metabolite, is less toxic: LD50s for rat were above 10 000 mg/kg
body weight, after oral administration, and 5210 mg/kg body weight,
after intravenous administration (Woodard & Woodard, 1971).
The slope of the dose-response curve in relation to the
mortality rate for ethylene oxide was steep. After oral
administration to rats, the difference between 0.1% mortality
(325 mg/kg) and 99.9% mortality (975 mg/kg) was approximately
650 mg/kg body weight (Smyth et al., 1941). After inhalation for
4 h, this difference was approximately 3000 mg/m3, in mice, and
approximately 5000 mg/m3 in rats. No deaths occurred in dogs at
1280 mg/m3 (Jacobson et al., 1956). While no guinea-pigs died
after inhalation of 450 mg ethylene oxide/m3 air for 8 h, the
majority did so at 2400 mg/m3 (Waite et al., 1930). In the above
mortality studies, the lungs and nervous system were the main
targets in rodents and dogs. In dynamic inhalation exposure
studies on guinea-pigs (Waite et al., 1930), rats, mice, and dogs
(Jacobson et al., 1956), nasal irritation was the first clinical
effect, as evidenced by scratching the nose, nasal discharge,
lachrymation, and salivation. Respiratory problems occurred
ranging from gasping to laboured breathing. Dogs exhibited
laboured breathing, vomited, and suffered convulsions. Guinea-
pigs, exposed to a concentration of 13 000 mg ethylene oxide/m3,
for 2.5 h, were found lying on their sides, unable to stand, and
quiet. Gross pathological changes in animals that did not survive
included moderate congestion in the lungs of dogs, minor patchy
oedema in the lungs of rats, and congestion with oedema in the
lungs of guinea-pigs. In rats, moderate congestion with petecchial
haemorrhage of the trachea was also observed. Lobular pneumonia
and hyperaemia of the liver and kidneys were observed in guinea-
pigs. Parenchymatous changes in the kidney of guinea-pigs were
seen at 2300 mg/m3.
Ataxia, prostration, laboured breathing, and occasional tonic
convulsions were effects shown by rats and mice at lethal oral or
intravenous doses of ethylene oxide (Woodard & Woodard, 1971).
Vomiting was the only effect shown by dogs that had received 25 or
75 mg ethylene oxide/kg body weight intravenously (Martis et al.,
1982).
In order to investigate the effects of residues of ethylene
oxide or reaction products in sterilized medical equipment, rabbits
were exposed for 2 h via polyvinyl chloride endotracheal tubes
containing 0, 80, or 600 mg ethylene oxide/kg material. There were
no deaths, but rabbits receiving the tubes with the highest
residues showed increased incidences of hyperaemia, oedema,
leukocyte infiltration, and epithelial erosion of the larynx and
trachea (Star et al., 1980). Two groups of nine dogs each were
exposed through extracorporeal perfusion for 40 min via a polyvinyl
chloride oxygenator containing 12 g ethylene oxide/kg material.
Nine dogs with an interrupted pulmonary circulation died from shock
and pulmonary oedema. Six out of 9 dogs with a preserved pulmonary
circulation died from pulmonary distress. A group of 9 control
dogs was treated in the same manner using a steam-sterilized
oxygenator. Only 1 control dog died (Stanley et al., 1971).
7.1.2. Acute effects on eyes and skin
As noted above, ethylene oxide is an irritating agent for
several different species. A maximum non-damaging concentration of
0.1% ethylene oxide in balanced physiological salt solution
(prepared daily and kept at 0 °C) was established after
instillation of 0.05 ml solution, every 10 min for 6 h, into the
conjunctival sac of rabbits. The concentrations above 1% caused
reversible changes in conjunctiva such as hyperaemia and swelling,
and irreversible opacity, both in the cornea and in the lens.
Possible reaction products, 2-chloroethanol and 1,2-ethanediol,
were less irritating to the eye (McDonald et al., 1973). The
results of in vitro tests with isolated rabbit cornea were in
agreement with the results of these studies. In the in vitro
tests, the endothelia were perfused for 1 - 3 h with a balanced
salt solution containing 250 mg ethylene oxide, 2250 mg
2-chloroethanol, or 5000 mg 1,2-ethanediol/litre. No effects were
observed on corneal thickness and cellular ultrastructure
(Edelhauser et al., 1983).
Skin irritation with hyperaemia, oedema, and scar formation was
observed from 6 min after application of pads of cotton, moistened
with solutions of 100 or 500 g ethylene oxide/litre water, on the
shaved skin of rabbits, under a plastic cover. The intensity of
the response was reported to be roughly proportional to the length
of exposure time (1 - 60 min) and the concentration (Hollingsworth
et al., 1956).
According to Hine & Rowe (1981), liquid ethylene oxide is
apparently without adverse effects on rabbit and human skin, on
single mild exposures, if the material evaporates rapidly. If
large amounts of material are involved, evaporation may cause
sufficient cooling to cause a lesion similar to frost-bite.
7.2. Short-Term Studies
7.2.1. Inhalation exposure
Groups of 10 - 20 Wistar rats per sex, 8 guinea-pigs per sex,
1 - 2 rabbits per sex, and 1 - 2 female rhesus monkeys were each
exposed to concentrations of ethylene oxide at levels of 0, 90,
200, 370, 640, or 1510 mg/m3, for 7 h per day, and 5 days per week.
The female monkeys were not tested at 90 mg/m3, and an additional 3
male monkeys were tested at 640 mg/m3. The test period varied with
the species tested, and the severity of exposure, i.e.,
approximately 26 weeks at 90 mg/m3, 25 - 32 weeks at 200 and
370 mg/m3, 7 - 25 weeks at 640 mg/m3, and 10 days at 1510 mg/m3.
Guinea-pigs, rabbits, and monkeys tolerated 90 and 200 mg/m3, and
rats tolerated exposure to 90 mg/m3 without adverse effects on
general appearance, behaviour, mortality rate, growth, body and
organ weight, gross- and histopathology. Rats showed elevated
mortality rates from 370 mg/m3, rabbits from 640 mg/m3, and all
exposed animals died at 1510 mg/m3. Secondary respiratory
infection caused the deaths of an appreciable number of rats and
mice in these studies.
Surviving rats showed increased relative lung weights after
26 - 27 weeks at 200 and 370 mg/m3. At 370 mg/m3, haemorrhages,
hyperaemia, emphysema, and local alveolar collapse were observed in
these lungs. Lungs of male rabbits also showed hyperaemia and
slight oedema at 370 mg/m3. Even more severe lung injury was seen
in rats at 640 mg/m3 and the higher exposure. Gross respiratory
tract irritation was apparent in all species at 1510 mg/m3.
Delayed reversible effects were observed on the peripheral
nervous system. Monkeys and rabbits exhibited paralysis of the
hind legs at 370 mg/m3 and, together with rats, at 640 mg/m3. This
was accompanied by atrophy of the muscles of the hind legs, except
in rabbits at 370 mg/m3. The effects on the peripheral nervous
system were investigated further in monkeys, and loss of both
sensory and motor function was noted at levels of 370 and
640 mg/m3.
Significant increases in body weight were also observed in
rats, at levels of 200 mg/m3 or more. Rats showed slight but
significant increases in the relative weights of kidney and liver
at 370 mg/m3 (Hollingsworth et al. 1956).
The findings of Jacobson et al. (1956) are in agreement with
these results. Groups, comprising 20 male rats and 30 female mice
each, were exposed to concentrations of ethylene oxide at levels of
0, 180, or 730 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted 26 weeks at 180 mg/m3 and 6 weeks at 730 mg/m3.
Additional groups of 15 rats and mice at the higher, and 60 rats
and mice at the lower, exposure level were used for interim gross
pathology. No clear toxic effects were reported at 180 mg/m3. No
pathological changes were observed except for marked haemosiderosis
in the spleen of a few rats at 730 mg/m3. The highest exposure
(730 mg/m3) resulted in death for both species without clinical
signs in mice. Effects on the respiratory and nervous system were
shown by rats as laboured breathing, reddish nasal discharge,
diarrhoea, tendency towards a side position, and dragging of the
hind-quarters. Rats also lost weight, which was regained by
survivors.
More recently, groups of 30 B6C3F1 mice of each sex were
exposed to concentrations of ethylene oxide (purity 99.9%) at 0,
18, 86, 187, or 425 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted for 10 weeks for males and 11 weeks for females.
No effects were observed in relation to survival, body weight,
clinical signs, white blood cell count, serum clinical chemistry,
urinalysis, and histopathology. At the highest exposure level,
changes at terminal sacrifice included an increased relative liver
weight in female mice, and a decreased testicular weight in males.
A decreased relative spleen weight was observed at 187 and
425 mg/m3 in both sexes. In addition, the red blood cell count,
the packed cell volume, and the haemoglobin concentrations were
decreased at 425 mg/m3. Screening of neuromuscular function in
groups of 5 female mice, in week 6, and 5 mice of both sexes, in
week 10 or 11, revealed altered reflex responses at 425 mg/m3 and a
dose-related trend in alterations of locomotor function from
86 mg/m3 upwards (Snellings et al., 1984a).
Groups of 3 male beagle dogs each were exposed to
concentrations of ethylene oxide (purity 99.7%) of 180 and
530 mg/m3, for 1 - 3 days. No effects were observed on mortality
rate, body weight, electrocardiogram, blood-calcium and -urea,
icteric index, and rectal temperature. Anaemia was noted at both
exposure levels. Effects on the respiratory and nervous systems
were shown at 530 mg/m3, such as hyperaemia and local alveolar
collapse in lungs, vomiting, and occasional slight tremors and
transient weakness in the hind legs. Muscular atrophy was also
observed (Jacobson et al., 1956). No haematological changes were
noted when groups each comprising 3 male New Zealand rabbits were
exposed repeatedly for 12 weeks to 0, 18, 90, or 450 mg/m3 (Yager &
Benz, 1982). The white cell count was depressed in Fischer rats
exposed in groups of 3 or 4, for 3 days, 6 h per day, to 90, 270,
or 810 mg/m3. There was a poor correlation with exposure level
(Kligerman et al., 1983).
The possible neurotoxic effects of ethylene oxide noted in the
above studies were investigated further in groups each comprising
12 male cynomolgus monkeys. These animals were exposed to 0, 90,
or 180 mg ethylene oxide/m3 (purity 99.7%), for 7 h per day, 5 days
per week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related lesions found were in the medulla oblongata of the brain.
Axonal dystrophy was found in the nucleus gracilis, primarily in
the exposed groups. Demyelination of the terminal axons of the
fasciculus gracilis occurred in one monkey at each exposure level,
but not in the controls (Sprinz et al., 1982). Paralysis of the
hind limbs was observed in monkeys repeatedly exposed for up to
32 weeks to 370 mg/m3, for 7 h per day, 5 days per week
(Hollingsworth et al., 1956).
7.2.2. Oral exposure
Groups of 5 Wistar rats each received, by gavage, 22 doses of
3, 10, or 30 mg ethylene oxide/kg body weight in 30 days and 15
doses of 100 mg/kg in 21 days. The vehicle was olive oil. There
were 10 vehicle controls.
No effects on mortality rate, growth, haematology, blood urea-
nitrogen, organ weights, gross- and histopathology were reported at
the 3 lower dose levels. At 100 mg/kg, there was marked loss in
body weight, gastric irritation, and slight (not further specified)
liver damage (Hollingsworth et al., 1956).
7.3. Long-Term Inhalation Studies
In a combined toxicity-carcinogenicity study, groups of 120
male and 120 female Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 18 mg/m3 (10 ppm), 58 mg/m3
(32 ppm), and 173 mg/m3 (96 ppm), for 6 h per day, 5 days per week,
over 25 months. Two control groups each comprising 120 male and
120 female rats were used. There was an exposure-free period of 2
weeks in month 15, because of infection with sialoacryoadenitis
virus. Interim sacrfices occurred at 6, 12, and 18 months.
The mortality rates of male and female rats increased
significantly from the 22nd or 23rd month, at the highest exposure,
with a trend towards an increase at a level of 58 mg/m3. Body
weights in both sexes were depressed at 173 mg/m3, from the end of
the first week onwards, until the end of the study. At 58 mg/m3,
the body weights of female rats were decreased between week 10 and
80. In females, the relative liver weights were increased in the
18th month at 173 mg/m3. This effect on the liver could not be
related to increases in the activities of serum alkaline
phosphatase (EC 3.1.3.1), aspartate aminotransferase (EC 2.6.1.1),
or alanine aminotransferase (EC 2.6.1.2), found mainly at the 2
highest exposures during interim sacrifices. Relative spleen
weights were increased in rats that developed leukaemia (section
7.4.1). Haematological changes were found in rats at all doses,
but mainly at the end of the study in animals exposed to 173 mg/m3;
these included an elevated leukocyte count in both sexes, and a
depressed red blood cell count and haemoglobin value in females.
Some of these rats had leukaemia.
Non-neoplastic histopathological changes observed included an
elevated frequency of focal fatty metamorphosis of the adrenal
cortices in both sexes and bone marrow hyperplasia in females at
173 mg/m3. Although no effect was observed on the hind-quarter
lift reflex, examined monthly, mild skeletal muscular atrophy was
observed, after 2 years of exposure to 173 mg/m3. Observations on
general health and ophthalmology did not reveal anything abnormal.
Neoplastic changes are reported in section 7.4.1 (Snellings et al.,
1981, 1984b).
In another toxicity-carcinogenicity study (Lynch et al.,
1984a), groups of 80 male Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 92 mg/m3 (51 ppm) and 182 mg/m3
(101 ppm), for 7 h per day, 5 days per week, over 2 years. The
control group also comprised 80 rats. There was an exposure-free
period of 2 weeks in month 16 because of a pulmonary infection,
which contributed to the mortality rate.
The mortality rate increased at both exposure levels, the
increase being significant at 182 mg/m3. Only 19% of the rats
survived 2 years of exposure at 182 mg/m3 compared with 49% in the
unexposed group. Body weights were reduced from the 3rd or 4th
month onwards. The relative weights of adrenals and brain were
increased at both exposure levels. The relative weights of lung
and kidney were increased at 92 mg/m3. Serum aspartate
aminotransferase activity was increased in rats exposed to 92 and
182 mg/m3 (section 7.4.1). No other changes were found in
haematology or clinical chemistry.
Non-neoplastic histopathological changes included an elevated
incidence of vacuolization and hyperplasia or hypertrophy in the
adrenals at both exposure levels, and of atrophy and degeneration
of skeletal muscle fibres at 182 mg/m3. There were also increased
incidences of inflammatory lesions of the lungs, nasal cavities,
trachea, and internal ear at both exposure levels. Eye cataracts
developed in 9 out of 78 rats at 182 mg/m3, 3 out of 79 in the
92 mg/m3 group, and 2 out of 77 in the controls.
7.4. Carcinogenicity
7.4.1. Inhalation exposure
In the studies by Snellings et al. (1981, 1984b) (section 7.3)
(Table 4), several neoplasms were induced by ethylene oxide. A
dose-related increased incidence of mononuclear cell leukaemia was
found in both sexes, significant at the 2 highest exposures in
females, from the 18th or 19th month onwards. Trend test revealed
a treatment-related response in both sexes. In males, an increased
incidence of peritoneal mesotheliomas originating from the
testicular mesothelium, occurred at 58 and 173 mg/m3 from the 23rd
month onwards, and an increased incidence of subcutaneous fibroma
was seen in male rats exposed to 173 mg/m3 that had survived for 24
months. Trend analysis showed that there was a treatment-related
increase in peritoneal mesothelioma. There was no increased
incidence of pituitary tumours, but they appeared earlier in the
173 mg/m3 group.
Following the report by Lynch et al. (1984a) of an increased
incidence of brain tumours in Fischer 344 rats exposed to ethylene
oxide (see below), the brain tissue from this study was re-examined
both macro- and microscopically, and a dose-related incidence of
primary brain tumours was observed at 58 and 173 mg/m3 that
appeared to be treatment related in the trend test, but was not
statistically significant. The tumours were mainly diagnosed as
gliomas and malignant reticular tumours. The percentage of rats
with multiple neoplasms was greater than in controls at all
exposure levels in females and at 173 mg/m3 in males. At 58 and
173 mg/m3, the percentage of female rats with at least one
malignancy was increased. The authors considered that a
contribution of the viral outbreak to the toxicity of ethylene
oxide was unlikely (Snellings et al., 1981, 1984b).
Lynch et al. (1984a) (section 7.3, Table 4) also found an
increased incidence of mononuclear cell leukaemia, which was
significant at the lower exposure level. The absence of a dose-
relationship was attributed to the increased mortality rate at 182
mg/m3. Dose-related increased incidences of peritoneal
mesotheliomas, originating from the testicular mesothelium, and of
mixed-cell gliomas in the brain, were found. The increases in both
tumours were significant at 182 mg/m3 (Lynch et al., 1984a).
Table 4. Tumours induced by ethylene oxide in Fischer 344 rats
------------------------------------------------------------------------------
Concen- Leukaemia Meso- Pituitary Subcutaneous Braina
tration (mononuclear) thelioma adenoma fibromaa
(mg/m3)
------------------------------------------------------------------------------
M F M M F M M F
Snellings et al. (1981, 1984b)
173 26(119)b 28(113) 22(119) 27(117) 32(117) 11(30) 3(30) 2(26)
58 25(81) 24(79) 7(91) 16(79) 38(90) 1(39) 1(39) 2(48)
18 21(79) 14(77) 3(89) 27(80) 39(90) 3(51) 0(51) 0
0 20(116) 9(118) 2(114) 28(117) 38(119) 1(48) 1(48) 0
0 18(118) 13(117) 2(116) 22(117) 38(116) 2(49) 0(49) 0
Lynch et al. (1984a)c
182 30(76) NA 21(79) 21(67) NA NR 5(79) NA
92 38(79) NA 9(79) 20(66) NA NR 2(77) NA
0 24(77) NA 3(78) 48(73) NA NR 0(76) NA
------------------------------------------------------------------------------
a Only animals that survived for 24 months were included, because these
tumours appeared after the 18-month interim sacrifice.
b Numbers in brackets refer to the number of rats examined. They include
interim and final kills.
c Only male rats were used.
NA = Not applicable.
NR = Not reported.
7.4.2. Oral exposure
Groups of 50 female Sprague Dawley rats received, in salad oil,
7.5 or 30.0 mg ethylene oxide/kg body weight, by gavage, in the
empty stomach, twice a week, for 110 weeks. There were 50 vehicle
controls, 50 untreated controls, and 50 positive controls. The
rats were observed for their life span. No statistical analysis
was reported. The mean survival period was over 100 weeks for all
groups. The mortality rate increased at 30.0 mg/kg body weight,
from week 100 onward. Elevated incidences of tumours were only
observed in the forestomach, the first tumour appearing in week 79.
The incidences of squamous cell carcinomas were 0/50, 8/50, and
29/50 at 0, 7.5, and 30 mg/kg body weight, respectively. At
30 mg/kg body weight, invasive growth and metastases were observed
in 10 rats. At 30 mg/kg body weight, 2 fibrosarcomas were also
noted. At both doses, the incidences of hyperplasia,
hyperkeratosis, papillomas, and/or carcinomas were increased in the
forestomach (Dunkelberg, 1982).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice were injected once a week with a
tricaprylin solution containing 0.1, 0.3, or 1.0 mg ethylene oxide
per animal, for 106 weeks. There were 200 vehicle controls and 200
untreated controls. From week 35 to week 85, the mortality rate
increased by a maximum of 10% at a dose of 1.0 mg per mouse. The
mean length of survival in this group was 75 weeks. An elevated
incidence of tumours was only observed at the injection site, the
first tumour appearing in week 79. There was a dose-related
increased incidence of sarcomas, mainly fibrosarcomas, which was
significant at 0.3 and 1.0 mg per mouse. The tumour incidence was
11% at the highest dose compared with 2% in vehicle controls
(Dunkelberg, 1981).
7.4.4. Dermal exposure
Each of a group of 30 female Swiss Millerton mice received, for
their lifetime, approximately 100 mg of a 10% solution of ethylene
oxide (purity 99.7%) in acetone, brushed on the clipped dorsal
uncovered skin, 3 times a week. A group of 60 mice did not receive
any treatment, and a group of 60 mice received the vehicle only.
No skin tumours were found, nor was there any sign of skin
irritation. The median length of survival was 493 days for treated
mice and 445 days for controls (Van Duuren et al., 1965). It is
assumed that ethylene oxide, applied in this manner, evaporated
rapidly from the skin.
7.5. Mutagenicity and Related End-Points
Almost all the reports available demonstrate the mutagenic
action of ethylene oxide. A summary of mutagenicity tests with
positive results is presented in Table 5.
Ethylene oxide is an alkylating agent (section 5.2). It has
induced gene mutations in all plants, bacteria, fungi, insects, and
mammalian cells investigated in vitro, with and without metabolic
activation. Chromosome damage and sister chromatid exchanges were
observed in plants, insects, and mammalian somatic cells exposed in
vivo and in vitro. Fomenko & Strekalova (1973) and Strekalova et
al. (1975) reported an increased incidence of chromosomal
aberrations in the bone-marrow cells of rats exposed by inhalation
to concentrations of ethylene oxide vapour at 3.6 and 112 mg/m3.
Unscheduled DNA synthesis, induced by the N-acetoxy-2-acetylamino-
fluorene, was inhibited by ethylene oxide in human lymphocytes in
vitro (Pero et al., 1981). The positive results in the
micronucleus tests are in agreement with those from a distribution
study showing that ethylene oxide, or its metabolites, was retained
in the bone marrow of mice (Appelgren et al., 1977). An increased
incidence of micronuclei was observed after one intraperitoneal
dose of 100 mg/kg body weight in mice (Lyarskii et al., 1983) and
after 4 h of vapour exposure of rats to a concentration of 90 mg/m3
(Embree & Hine, 1975).
Table 5. Mutagenic tests for ethylene oxide with positive resultsa
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Gene mutations
Forward mutations plant barley Ehrenberg et al. (1956,
1959)
Forward mutations barley Shulovsk et al. (1969)
Forward mutations rice Jana & Roy (1975)
Forward mutations pea Blixt et al. (1963)
Reverse mutations bacterium Escherichia coli Hussain & Osterman-Golkar
SD-4 (1984)
Reverse mutations Salmonella typhimurium Rannug et al. (1976);
(base-pair TA1535, TA100 Kauhanen (1978)b;
substitutions) Pfeiffer & Dunkelberg
(1980)
Reverse mutations Bacillus subtilis Tanooka (1979)
(spores) HA101, TKJ5211,
TKJ8201
Reverse mutations fungus Neurospora crassa Kolmark & Westergaard
(macroconidia) (1953); Kolmark & Kilby
(1968)
Forward mutations Aspergillus nidulans Morpurgo (1963)
Forward mutations Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked insect Drosophila melanogaster Bird (1952); Nakao &
recessive lethals Auerbach (1961)
Forward mutations mammal Chinese hamster Tan et al. (1981)b;
on specific locus ( in vitro) ovary cells Zamora et al. (1983)
Chromosome damage
breaks, erosions, plant Tradescantia paludosa Smith & Lofty (1954)
contractions (pollen)
translocations barley Ehrenberg et al. (1956,
1959)
breaks barley Moutschen-Dahmen et al.
(1968)
breaks wheat (hexaploid) MacKey (1968)
translocations insect Drosophila melanogaster Nakao & Auerbach (1961)
small deletions insect Drosophila melanogaster Fahmy & Fahmy (1970)
(min)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Chromosome damage (contd.)
breaks, human anion cells Poirier & Papadopoulo
gaps, exchanges, ( in vitro) (1982)
complexes
sister chromatide human lymphocytes Star (1980a);
exchanges ( in vitro) fibroblasts Garry et al. (1982)
rat lymphocytes Kligerman et al. (1983)
(inhalation)
rabbit lymphocytes Yager & Benz (1982)
(inhalation)
sister chromatide monkey lymphocytes Lynch et al. (1984b)d
exchanges, (inhalation)
breaks, acentric
fragments,
dicentrics,
triradials,
quadriradials,
complex
rearrangements
chromosomal rat bone marrow cells Strekalova et al. (1975)
aberrations (inhalation)
breaks, gaps, rat bone marrow cells Embree & Hine (1975)
rearrangement, (inhalation)
exchanges,
ring formations
micronuclei mouse (ip) polychromatic Conan et al. (1979);
erythrocytes Jenssen & Ramel (1980);
Lyarskii et al. (1983)
micronuclei mouse (iv) polychromatic Appelgren et al. (1978)
erythrocytes
heritable mouse (ip) germ cells Generoso et al. (1980)
translocations
dominant lethals mouse germ cells Cumming & Michaud (1979)
(inhalation)
mouse (ip) Generoso et al. (1980);
Lyarskii et al. (1983);
mouse Generoso et al. (1983)
(inhalation)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
dominant lethals rat Embree et al. (1977)
(contd.) (inhalation)
DNA repair
unscheduled mouse germ cells Cumming & Michaud (1979)
DNA synthesis (inhalation)
unscheduled human lymphocytes Pero et al. (1981)
DNA synthesis ( in vitro)
----------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b A similar effect after metabolic activation by rat liver microsomal fraction.
c A slight reduction in mutagenicity after metabolic activation by mouse liver
microsomal fraction.
d 2-year exposure groups of 12 male monkeys to 0, 90, and 180 mg/m3 for 7 h per day and
5 days per week.
Dose-related damage to germ cells was established in the mid
and late spermatid stages in the dominant lethal assay. One oral
dose of 100 mg/kg body weight in mice generated inconsistent
results (Appelgren et al., 1977), but 150 mg/kg proved positive
(Generoso et al., 1980). After short-term repeated exposures,
dominant lethals were induced in mice at intraperitoneal doses from
40 mg/kg body weight, given over a period of 3 months, 5 times per
week (Lyarskii et al., 1983) and at vapour exposures from
460 mg/m3, for 6 h/day, 5 days per week, over 11 weeks (Generoso et
al., 1983). Heritable translocations were induced in the germ
cells of mice after repeated intraperitoneal exposure, at doses of
30 mg/kg body weight or more, for 5 days/week, over a 5-week period
(Generoso et al., 1980). Recent studies have investigated the
dose-response of inhaled ethylene oxide and have compared effects
of different dose rates (contributions of different concentrations
and durations of exposure, maintaining total exposure (C x t)
constant) on the dominant-lethal response in male mice. In the
dose-reponse study, male mice were exposed by inhalation to
ethylene oxide at concentrations of 540, 720, or 900 mg/m3 (300,
400, or 500 ppm), respectively. Exposures were for 6 h/day, for 4
consecutive days. A dose-related increase in dominant-lethal
mutations was observed; however, the dose-response curve was
nonlinear, i.e., increasing embryonic mortality occurred with
increasing mg/m3 x h. In the dose-rate study, mice had a total
exposure of 3240 mg/m3 (1800 ppm) x h/day for 4 consecutive days,
delivered either at 540 mg/m3 (300 ppm) in 6 h, 1080 mg/m3 in 3 h,
or 2160 mg/m3 (1200 ppm) in 1.5 h. The highest airborne
concentration resulted in the greatest embryonic mortality, 64%,
versus 32 and 11% for the intermediate and lowest airborne
concentrations, respectively (Generoso et al., 1985). According to
an abstract, DNA repair was induced in the germ cells of mice
exposed to 540 mg/m3, for 8 h. The repair seemed inhibited at
higher exposures (Cumming & Michaud, 1979). No details were
available.
Negative results were observed on a few occasions only. In one
study, with vapour-exposed rats, chromosome aberrations or slowing
of mitotic activity and cell cycle kinetics were not observed in
lymphocytes at levels at which sister chromatid exchanges occurred
(Kligerman et al., 1983). In a dominant-lethal assay with mice,
relatively high intravenous doses of ethylene oxide (up to
100 mg/kg body weight) did not cause any treatment-related effects
(Appelgren et al., 1977). An intraperitoneal dose of 10 mg/kg body
weight to mice was reported to give a slight increase in the number
of polychromatic erythrocytes with micronuclei, but the statistical
analysis was not adequate (Conan et al., 1979).
7.6. Effects on Reproduction
Rats and guinea-pigs were exposed to vapour concentrations of
370 and 640 mg ethylene oxide/m3, for 7 h per day, 5 days per week,
for up to 32 weeks. Among other effects (section 7.2.1),
degeneration of testes tubules was observed at the higher exposure
level in guinea-pigs, while at 370 mg/m3, there was a decrease in
the relative weights of testes in rats and guinea-pigs, which was
not statistically significant (Hollingsworth et al., 1956).
Significantly-decreased absolute testicular weights were observed
in mice exposed to ethylene oxide at a concentration of 425 mg/m3,
for 6 h/day, 5 days per week, over 10 - 11 weeks (Snellings et al.,
1984a). However, the testicular effects may have been secondary to
toxic effects (e.g., growth inhibition). Male and female Fischer
344 rats exposed repeatedly to concentrations of ethylene oxide of
up to 182 mg/m3, for 6 h/day, 5 days per week, over 25 months, did
not show any histopathological effects on the reproductive tissues
(Snellings et al., 1981).
When groups of 12 male Cynomolgus monkeys were exposed to
concentrations of ethylene oxide at 90 or 180 mg/m3, for 7 h/day,
5 days per week, over 2 years, spermatogenic functions were found
to differ from those of controls. At both exposure levels, sperm
motility and sperm count were decreased and the sperm drive range
was increased, but there was no increase in effect with increase in
dose. The incidence of abnormal sperm heads did not change (Lynch
et al., 1984c).
Groups, each comprising 30 male and 30 female Fischer 344 rats,
were exposed to concentrations of ethylene oxide (purity 99.9%) of
18, 58, or 173 mg/m3, for 6 h/day, 5 days per week, over 12 weeks.
Two control groups of 30 rats per sex each were exposed to air
only. After mating, females were further exposed for 7 days/week,
up to 3 weeks after delivery, with the exception of the first 5
days of lactation. Effects on the reproductive performance were
detected. The number of pups per litter was decreased at 173
mg/m3, as well as the number of implantation sites per female, and
the number of fetuses born per implantation site. The number of
females with a gestation period longer than 22 days was also
increased at this concentration, but no effects were noted on the
average length of the gestation period. Neither parents nor pups
showed signs of toxicity from ethylene oxide. The percentages of
pregnant females and fertile males were not affected (Snellings et
al., 1982a).
7.7. Teratogenicity
Groups of 22 female Fischer 344 rats were exposed to
concentrations of ethylene oxide of 18, 58, or 173 mg/m3, for
6 h/day, on days 6 - 15 of gestation. Two control groups
comprising 22 rats each were exposed to air only. The numbers of
pregnant dams ranged from 17 to 22. Maternal behaviour was normal,
and there were no deaths. The only effect on the fetuses was a 5 -
8% decrease in weight at 180 mg/m3 (Snellings et al., 1982b).
Groups of 32 - 45 female Sprague Dawley rats were exposed to
concentrations of ethylene oxide (purity 99.7%) of 0 or 270 mg/m3,
for 7 h/day, on days 7 - 16 of gestation (Group 1) or on days 1 -
16 of gestation (Group 2) or during 3 weeks before mating (5 days
per week), and on days 1 - 16 of gestation (Group 3). No dams
died, but body weights were decreased in Group 3. In all exposed
groups, the relative and absolute weights of kidney and spleen were
increased. The results of histopathological examination did not
show any abnormalities. There was a significant increase in
resorptions per litter and per implantation site in Group 3, with
no significant effects on the number of implants, live fetuses, and
pregnancies. In all exposed groups, weights and lengths of the
fetuses were decreased. Reduced ossification of sternebrae and
primary skull was observed (Hackett et al., 1982).
New Zealand rabbits were similarly exposed to a concentration
of ethylene oxide of 270 mg/m3 from days 1 - 19 or from days 7 - 19
of gestation. There was no evidence of toxicity in the mothers,
embryos, or fetuses, or of developmental defects (Hackett et al.,
1982).
Groups of 24 - 37 female CD-1 mice each received,
intraveneously, doses of 0, 75, or 150 mg ethylene oxide (purity
not stated)/kg body weight in an aqueous dextrose solution on days
4 - 6, 6 - 8, 8 - 10, or 10 - 12 of pregnancy. Dams exposed on
days 6 - 8 of pregnancy did not show toxic signs. In the other
groups, at the highest dose, toxic signs such as weakness, laboured
respiration, and tremor were observed with a mortality rate of 19 -
48%. In the group without signs of maternal toxicity, fetotoxicity
was observed at 150 mg/kg, as shown by a 20% decrease in mean fetal
weight. Fetal malformations were shown in 19.3% of fetuses in
exposed litters compared with 2% in control groups. These
malformations were mainly fused cervical arches. In addition,
fused thoracic arches, scrambled and fused sternebrae, and fused,
branched, or missing thoracic ribs were observed (Laborde & Kimmel,
1980).
8. EFFECTS ON MAN
8.1. Exposure of the Skin and Eyes
Undiluted ethylene oxide, applied to the skin of volunteers,
evaporated rapidly without leaving any mark or irritation. A
15-min exposure to cotton wool soaked in undiluted ethylene oxide
also did not produce any effects (Greaves Walker & Greeson, 1932).
However, with exposure to larger quantities, there may be
sufficient cooling to produce a lesion similar to frost-bite (Hine
& Rowe, 1981). Skin injury following exposure to aqueous solutions
of ethylene oxide is characterized by the appearance of oedema and
erythema, 1 - 5 h after exposure, followed by the formation of
vesicles. On healing, incrustation, often with itching and
desquamation, is observed. The magnitude of the skin injury seems
to depend on the length of contact and the concentration, a 50%
aqueous solution (500 g/litre) being most hazardous. More
concentrated solutions were less harmful. The lowest concentration
tested, a 1% solution, produced a mild reaction after a 50-min
exposure. Such effects have been observed in a number of accidents
(Sexton & Henson, 1949, 1950; Joyner, 1964; Ippen & Mathies, 1970).
Vapour exposure was found to produce the above dermal effects,
mainly on the humid parts of the skin (Ippen & Mathies, 1970). The
effects were also found, to different extents, after exposure via
ethylene oxide-sterilized materials such as face masks, gloves, and
surgical gowns (Royce & Moor, 1955; Marx et al., 1969; Hanifin,
1971; Biro et al., 1974; Lamy et al., 1974). Patch tests on
volunteers with various sterilized materials containing residues of
ethylene oxide and its reaction products, showed erythema, without
oedema, after 4 - 8 h of contact, from a residue level of 890 mg
ethylene oxide/kg up to 2890 mg/kg of a polyvinyl chloride block.
Most skin types tolerated residues of ethylene oxide of up to
2270 mg/kg polyvinyl chloride film, 2800 mg/kg brown-milled rubber,
and 5100 mg/kg non-woven fabric (Shupack et al., 1981).
Accidental skin exposure to a 1% aqueous solution, from the
waist down, was also reported to result in effects on the nervous
system, such as nausea and repeated vomiting (Sexton & Henson,
1949).
Accidental exposure of the eyes to the vapour of ethylene oxide
can lead to conjunctivitis (Thiess, 1963; Joyner, 1964). Exposure
of 12 men via a leaking sterilizer resulted in neurological
disorders (section 8.3) in 4 of the men, 3 of whom had eye
cataracts; one of the latter also showed an increase in corneal
thickness. Two additional men showed only an increase in corneal
thickness (Gross et al., 1979; Jay et al., 1982). In one case of
accidental exposure of the eyes to pure ethylene oxide, only slight
irritation of the conjunctiva was seen (Thiess, 1963).
The implantation of artificial lenses, sterilized with ethylene
oxide, in 103 eyes was compared with the implantation of lenses,
sterilized with sodium hydroxide (200 control eyes), in a
retrospective study. The follow-up period was 10 months for the
exposed patients. Post-operative inflammatory complications
occurred in 30% of the eyes exposed to residues of ethylene oxide
or its reaction products compared with 9% of the control eyes.
Cystoid macular oedema with reduction in visual acuity developed in
16% of the exposed eyes and in 7% of the control eyes (Stark et
al., 1980).
8.2. Sensitization
No dermal sensitization was observed in a total of 47 workers
frequently exposed to ethylene oxide (Royce & Moor, 1955; Thiess,
1963). In another study using patch tests, one of 12 volunteers
showed a recurrent reaction, 3 weeks after the trial. When
challenged afterwards with a 2 mm-thick patch of polyvinyl chloride
containing 100 mg ethylene oxide/kg, a mild reaction was observed,
which reappeared after 3 weeks (Shupack et al., 1981) (section
8.1). When the skin of 8 workers was exposed repeatedly to aqueous
solutions of ethylene oxide, all sites of previous contact, with
and without a primary reaction, flared up in 3 of them showing
pruritus, erythema, and slight oedema, 5 - 9 days after the last
exposure (Sexton & Henson, 1950). Another case of an apparently
allergic reaction was reported. The patient concerned was exposed
to ethylene oxide via a sterilized face mask (Alomar et al., 1981).
A case of anaphylaxis has been reported in a patient receiving
haemodialysis treatment with equipment that had been sterilized
with ethylene oxide (Poothullil et al., 1975). A cause-effect
relationship with ethylene oxide exposure was demonstrated by
haptan specificity (Dolovich & Bell, 1978).
8.3. Accidental Inhalation Exposure
Respiratory tract irritation was reported as hoarseness
(Thiess, 1963) and cough (Metz, 1939) in 5 cases of acute
accidental exposure to ethylene oxide vapour.
Acute effects on the nervous system in nearly all inhalation
cases were marked by nausea, recurrent vomiting, and headache.
Less frequently reported effects included decreased consciousness
(one case of coma), excitement, sleeplessness, muscular weakness,
diarrhoea, and abdominal discomfort (Blackwood & Erskine, 1938;
Metz, 1939; Thiess, 1963; Capellini & Ghezzi, 1965).
Because of a leaking sterilizer, 4 young men were exposed
intermittently, for 2 - 8 weeks, to ethylene oxide at levels of
approximately 1000 mg/m3. Three of the men developed a reversible
peripheral neuropathy showing abnormal nerve conduction and, in 2
cases, headache, weakness and decreased reflexes in the
extremities, incoordination, and a wide-based gait. The fourth man
developed a reversible acute encephalopathy with headache, nausea,
vomiting, lethargy, recurrent motor seizures, agitation, and a
diffusely slow electroencephalogram (Gross et al., 1979).
Following this, 6 more cases were reported concerning sterilizer
operators, suffering from reversible peripheral neuropathy
following ethylene oxide exposure for 0.5 - 1.5 years. Finelli et
al. (1983) described 3 persons showing subacute polyneuropathy with
bilateral foot-drop, slowing of nerve conduction velocity, and
denervation potential on electromyography as the main findings.
All 3 persons had noticed the smell of ethylene oxide regularly at
work, while 2 persons experienced eye irritation. Polyneuropathy
was also reported in 3 sterilizer operators by Kuzuhara et al.
(1983). Two of these cases were described in detail. Sural nerve
biopsies revealed axonal degeneration with mild changes in the
myeline sheath. Unmyelinated fibres were also involved. Muscle
biopsies showed typical denervation atrophy.
8.4. Other Accidental Exposures
Severe respiratory problems due to inflammatory reactions in
the trachea and larynx were reported in 17 hospital patients who
had received endotracheal intubation. The tubes had been
sterilized with ethylene oxide (Marx et al., 1969; Holley & Gildea,
1971; Lipton et al., 1971; Mantz et al., 1972).
Reversible vocal paralysis was reported to be associated with
ethylene oxide exposure in 5 cases: one woman had been exposed to
vapour (Troisi, 1965), and the other 4 patients were exposed via
sterilized endotracheal tubes (Holley & Gildea, 1971). The vocal
cords showed no or only slight damage. In one of these patients,
who died from a cause unrelated to the intubation, myelin
degeneration of parts of the nervus vagus was noted at autopsy. It
was suggested, therefore, that the paralysis was of neural origin.
Four cases of shock, 1 - 10 h after endovascular examination,
were associated with the catheters used, which contained residues
of ethylene oxide. The presence of bacterial toxins was considered
unlikely. One patient died as a result of renal insufficiency
(Lebrec et al., 1977). Cases of cardiovascular collapse in
children, 3 of which were fatal, were considered by the authors to
be the result of residues of ethylene oxide in a heart-lung machine
on the basis of subsequent studies on dogs (Stanley et al., 1971)
(section 7.1.1). Among others, Hirose et al. (1963) and Clarke et
al. (1966) measured haemolysis due to residues in ethylene oxide-
sterilized plastic tubes in vitro.
8.5. Occupational Inhalation Exposure
The health status of 37 male operators from an ethylene oxide-
producing plant in the USA during the period 1953 - 62 was compared
with that of age-matched operators from other production units.
The average employment period was 11 years for exposed workers and
12 years for controls. The usual average exposure level was
between 9 and 18 mg/m3, with occasional peaks up to 230 mg/m3 for
one particular job (collecting a sample of the product). According
to the medical records, the health of the exposed workers was
somewhat better than that of the controls. A physical examination
and extensive clinical tests did not reveal any exposure-related
effects with the exception of a slightly increased white blood cell
count (Joyner, 1964).
Chromosomal damage was found in a group of 12 workers from a
hospital sterilization facility in the USA (section 8.6). The
maximum exposure concentration measured during sterilization was
65 mg/m3. Another group of 12 persons, who worked in the adjacent
operating room area, volunteered as representatives of an unexposed
or accidentally exposed group. To insure adequate control
throughout the study, unexposed laboratory staff members served as
a third group. Frequently-reported subjective complaints indicated
irritation of the mouth, throat, and eyes, and effects on the
nervous system, such as headache, nausea, speech difficulty, memory
loss, dizziness, and incoordination (Garry et al., 1979).
In Belgium, a group of 18 workers, using or distributing the
sterilant ethylene oxide, was compared with a well-matched control
group by means of a questionnaire, and by analyses for urinary
retinol-binding protein and albumin, beta-microglobulin, and
chromosomal damage in lymphocytes. The overall mean exposure level
was 7.6 mg/m3, and the time-weighted average exposure, over a
working day, ranged between 0.2 and 95 mg/m3. A significant
increase in the incidence of sleeplessness and leg cramps was
recorded, but not irritation or allergy. These studies did not
reveal any abnormalities with the exception of an increase in
sister chromatid exchanges in lymphocytes (Wolfs et al., 1983;
Laurent et al., 1984; section 8.7).
In a plant in Bulgaria, 196 workers engaged in the production
of ethene and ethylene oxide were examined. About 73% of all
concentrations of ethylene oxide measured were 1 mg/m3 or less,
while 27% were between 1 mg/m3 and 3.5 mg/m3. Significant
increases were found in deviations of the autonomous nervous system
and in neurosis-like manifestations, especially in female workers
(Spasovski et al., 1980). Because of a mixed exposure, it is
difficult to evaluate the findings.
Haematological changes were reported in a group of 27 workers
in an ethylene oxide manufacturing and processing plant, in Sweden,
in 1967. The exposure period varied from 2 to 20 years, the
average length being 15 years. Controls worked with ethylene oxide
in other departments, where no leakages were possible. No exposure
data were reported. When 2 cases of anaemia were excluded, there
was still a significantly-decreased haemoglobin value in exposed
workers. There was a 30% increase in the number of lymphocytes,
and one case of chronic lymphatic leukaemia was noted (Ehrenberg &
Hällström, 1967).
In the Federal Republic of Germany, 279 employees from 8 plants
in which alkene oxides were produced or processed, were examined
for morbidity during 1978. They were employed for an average of
10.8 years. Of these workers, 21 had been involved in accidents
with ethylene oxide. Taking into account age and length of
exposure, they were compared with groups of industrial and clerical
workers within the same company. The exposure concentrations were
not reported. The workers were also exposed to many other
chemicals, some of which may be carcinogenic for man.
No abnormalities were found that could be related to exposure
to ethylene oxide or propylene oxide. The investigators related
increases in haemoglobin and mean erythrocyte volume to smoking
habits. Slight lymphocytosis was found to be unrelated to exposure
time, though there was a distinct age influence (Stocker & Thiess,
1979).
8.6. Mortality Studies
Two studies were conducted in Sweden to investigate the
possible neoplastic effects from occupational exposure to ethylene
oxide (Hogstedt et al., 1979a,b, 1984). The first study originally
included 58 male and 172 female workers in a small factory,
sterilizing hospital equipment with a 1:1 mixture of ethylene oxide
and methyl formate, over a period from 1968 to 1977 (Hogstedt et
al., 1979a).
Two cases of leukaemia (one was diagnosed as chronic myeloid
leukaemia and the other as acute myelogenous leukaemia) occurred
among 68 women who were exposed to vapours from sterilized boxes
stored for weekly periods in a factory storage hall where about 30
persons were exposed at any one time. A third case was the local
male manager who developed primary macroglobulinaemia (Morbus
Waldenström; this case was later diagnosed as a non-Hodgkin
lymphoma), 9 years after the installation of the sterilization
equipment; his exposure was estimated to be about 3 h per week in
the storage hall. He is also reported to have had some exposure to
benzene in the past. The concentration of ethylene oxide in the
hall was in the range of 3.6 - 128 mg/m3, and the 8-h time-weighted
average in the breathing zone was calculated to be between 36 ±
18 mg/m3. The other workers had occasional exposure to ethylene
oxide, and 7 operators had relatively high exposure (amount
unspecified) during the sterilization process.
In a follow-up study (Hogstedt et al., 1984), a further case of
leukaemia was found in a woman who had been exposed to ethylene
oxide in the storage hall between 1969 - 72. In this study, the
cohort consisted of 203 workers who had been employed for more than
one year at the plant.
Altogether, 4 deaths from malignancies of the lymphoreticular
system were reported from this factory. The expected number was
0.3.
A second study to investigate the carcinogenic effects of
ethylene oxide was conducted on 241 Swedish male workers in an
ethylene oxide-producing plant (Hogstedt et al., 1979b). These
men were examined medically in 1960. Twenty-three deaths
occurred during the 16-year observation period dating from
1961 - 77 (13.5 expected). The excess mortality was due to cancer
and cardiovascular disease. Three cases of stomach cancer (0.4
expected) and 2 cases of leukaemia (one chronic myeloid and once
acute myeloid leukaemia) (0.14 expected) accounted for the excess
mortality from cancer. No increase in mortality was observed among
86 maintenance workers exposed intermittently to ethylene oxide
among 66 unexposed controls. Average exposure levels during
1941 - 47 were estimated to be below 25 mg/m3 and, during the 1950s
up to 1963, these levels were 10 - 50 mg/m3, but peak exposures
above the odour threshold (about 1000 mg/m3) were known to occur.
The ethylene oxide was manufactured by the chlorohydrin process
so that significant exposure to other chemicals such as 1,2-
dichloroethane, ethylene, ethylene-chlorohydrin, and bis(2-
chloroethyl)ether might have occurred.
This investigation was followed up by a study that extended the
period of observation up to 1982. Seven more deaths had occurred
among the workers exposed to ethylene oxide for the whole of the
working day against 6.6 expected. Three of these were due to
cancer (1.6 expected). Two of these 3 cases were cancer of the
stomach (0.2 expected) and one an oesophageal cancer (0.04
expected). In the period from 1961 - 82, 6 deaths due to stomach
or oesophageal cancer had occurred in workers exposed to ethylene
oxide for the whole of the working day (0.7 expected). Alimentary
tract cancer was observed in 2 maintenance workers (0.8 expected)
and in 1 unexposed worker (0.8 expected). One new case of chronic
myeloid leukaemia was reported during this follow-up period.
During the 20-year period of observation, a total of 17 cases of
cancer were notified to the Cancer Registry against 7.9 expected
(Hogstedt et al., 1984).
The Task Group evaluated these data and concluded that the
evidence was adequate to consider the mixtures of compounds to
which these workers were exposed as carcinogenic for human beings,
but inadequate to label ethylene oxide as a proved human
carcinogen. This conclusion was in agreement with that arrived at
by an International Agency for Research on Cancer Working Group
(IARC, 1985).
A similar study in the USA concerned 767 male workers at an
ethylene oxide producing plant. They were employed for at least
5 years between January 1955 and December 1977 and "potentially
exposed". Concentrations of ethylene oxide were reported to be
below 18 mg/m3, but no further details concerning exposure levels
were reported. Exposure to other chemicals was not reported.
Control data came from national statistics and was adjusted for
sex, age, and calendar time. There were 46 deaths against an
expected 80; there were 11 malignant neoplasms against 15.2
expected. No statistically-significant excess deaths could be
found due to any cause. There were no deaths due to leukaemia, 3
deaths from pancreatic cancer (0.8 expected), 1 death from bladder
cancer (0.3 expected), 2 deaths from brain cancer (0.7 expected),
and 2 deaths from Hodgkin's disease (0.4 expected) (Morgan et al.,
1981).
In the Federal Republic of Germany, 602 workers were
investigated for mortality experience during the period 1928 - 80.
The workers had been employed for at least 6 months in 8 plants
producing or processing ethylene oxide and propylene oxide. A
subcohort of 351 workers was observed for more than 10 years.
Control data came from a styrene plant and from national
statistics. Since 1978, exposure to ethylene oxide had normally
remained below 9 mg/m3. In the past, occasional excursions above
90 mg/m3 (50 ppm) had been reported. On one occasion during plant
breakdown, 3420 mg/m3 (1900 ppm) was measured. No statements were
offered concerning human exposure or the use of personal protective
equipment. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. There were 14 deaths from
cancer compared with 16.6 expected. No statistically-significant
excess deaths could be found due to any cause in the cohort. In
the subcohort of 351 workers, there was a significant increase in
mortality rate due to kidney disease (3 against 0.4 expected).
There was 1 death from gall bladder cancer, 1 death from urinary
bladder cancer, 1 death from brain cancer, and 1 death from myeloid
leukaemia. Two stomach tumours were observed compared with 1.8
expected (Thiess et al., 1981a,b).
8.7. Mutagenicity and Related End-Points
An increase in chromosomal aberrations was found in the
lymphocytes of 3 groups of workers sterilizing medical equipment in
hospitals or factories (Abrahams, 1980; Pero et al., 1981; Högstedt
et al., 1983). A 50% increase in aberration rate was found in 28
workers exposed to 8-h time-weighted average concentrations of
ethylene oxide below 1.8 mg/m3 in the 2.5 years before the study.
Before this period, higher exposures were reported to have
occurred. The workers had been exposed for 0.5 - 8 years. The
mean number of micronuclei in the bone marrow cells of 18 of these
workers was 3 times higher than in the controls (Högstedt et al.,
1983). Pero et al. (1981) found that, while workers exposed to
concentrations of ethylene oxide between 0.9 and 1.8 mg/m3, for
40 h per week, did not show an increased aberration rate; others,
exposed to bursts of ethylene oxide at concentrations between 9 and
18 mg/m3, for 8 h per week, did. The Task Group noted that the
sterilization procedure involved the use of a 50:50 mixture of
ethylene oxide and methyl formate. At the same time, DNA repair,
induced in vitro by the mutagen N-acetoxy-2-acetylamino-fluorene,
was reversibly inhibited in the low exposure group compared with an
unexposed control group, but not affected in the other group. DNA
repair inhibition was positively correlated with duration of
exposure (Pero et al., 1981). In 43 male workers from the cohort
of 602 workers (section 8.5) (Thiess et al., 1981b), an increase in
chromosomal aberration rate was found that was significant for the
workers exposed for more than 20 years, but not for those
accidentally exposed or exposed for average periods of 12 and
17 years (Thiess et al., 1981a).
In another ethylene oxide manufacturing plant, no chromosomal
aberrations were detected in the lymphocytes of 36 male workers.
These men had been exposed for 1 - 14 years to average
concentrations of up to 0.28 mg/m3 (van Sittert et al., 1985).
The sister chromatid exchange rate in lymphocytes was not
increased in groups of 28 and 14 sterilization workers exposed to
8-h time-weighted averages, below 1.8 mg/m3 for 2.5 years before
the study (Högstedt et al., 1983) and below 8 mg/m3 (Hansen et al.,
1984), respectively. In the second study, peaks up to 1430 mg/m3
were also measured. Increases in sister chromatid exchange rate
were found in 4 other studies on sterilization workers (Garry et
al., 1979; Abrahams, 1980; Yager et al., 1983; Laurent et al.,
1984). In one of these studies, in which 75 workers were exposed
to levels generally below an 8-h time-weighted average of 90 mg/m3,
this increase was found in cells together with quadriradial
aberrations (Abrahams, 1980). In the other studies, groups were
small, exposure conditions often unclear, and the number of
metaphases scored, in some cases, limited. Yager et al. (1983)
only found an increased sister chromatid exchange rate at
relatively high calculated integrated doses of more than 100 mg per
person. The length of exposure to ethylene oxide averaged 3.6 min
per day; these tasks were performed between 6 and 120 times during
the 6-month study period. Laurent et al. (1984), studied sister
chromatid exchange rates in 2 groups of ethylene oxide-exposed
workers (estimated ethylene oxide dose in past 2 years, 530 -
715 mg and 1185 - 5800 mg, respectively) as well as unexposed
controls. Although the sister chromatid exchange rates in the
high-exposure group did not differ significantly from the rates in
the low-exposure groups (not adjusted for smoking), the difference
among non-smokers between the exposed (n = 20) and the controls
(n = 15) was significant.
In a study on 41 sterilization workers in 8 hospitals in Italy,
increases in both sister chromatid exchanges and in chromosomal
aberrations were detected in lymphocytes; these effects persisted
for months after exposure was reduced or interrupted. The workers
were exposed to average 8-h time-weighted averages of either
0.63 mg/m3 or 19.3 mg/m3 (section 4.3). A statistically-
significant correlation was found between sister chromatid exchange
frequency and the level of ethylene oxide, as well as a multiple
correlation between sister chromatid exchange frequency and
ethylene oxide exposure, smoking, and age (Sarto et al., 1984).
Similarly, in the USA, the sister chromatid exchange frequencies in
the lymphocytes of 61 sterilization workers involved in sterilizing
health-care products, were monitored over a period of 2 years and
compared with those of 82 unexposed controls. During the study
period, 8-h time-weighted-average exposures were reported to be
less than 1.8 mg/m3. Prior to the start of the study, 8-h time-
weighted averages between 0.9 and 36 mg/m3 were measured. Results
were adjusted for smoking habits, sex, and age. Workers exposed to
low levels of ethylene oxide such as those at a worksite with 8-h
time-weighted-average ethylene oxide levels below 1.8 mg/m3 prior
to and during the study, did not show increased frequencies of
sister chromatid exchange. Workers who had been exposed to levels
of 5 - 36 mg/m3, prior to the study, showed an increased frequency
of sister chromatid exchange that persisted for at least 24 months
after cessation of exposure (Stolley et al., 1984).
8.8. Effects on Reproduction
In a study from the USSR (Yakubova et al., 1976), the course of
pregnancy and birth was followed in 57 operators, 38 laboratory
workers, and 65 adminstrative staff in an ethylene oxide-producing
plant, the majority of the women being between 20 and 29 years of
age. A group of 50 pregnant women working outside the plant served
as additional controls. It was estimated that the operators were
exposed to ethylene oxide concentrations of 0.2 - 0.3 mg/m3 for 80%
of the working time and 1.0 mg/m3 for the remaining 20%, while the
laboratory workers were exposed only to the lower concentrations.
Pregnancy toxaemia in the latter half of pregnancy and other
complications were higher in the operators (14.7%) and laboratory
workers (9.9%) than in the administrative staff (4.6%) and outside
controls (8%). On the other hand, the primiparae among the
operators lost less blood perinatally than those among the other
groups. Spontaneous abortion occurred in 6 out of 57 (10.5%)
operators, 3 out of 38 (7.9%) laboratory workers, and in 5 out of
65 (7.7%) administrative staff. The operators were subjected to
the additional stress of high levels of noise and vibration and
wide variations in atmospheric temperatures.
Findings in this study do not indicate any unequivocal adverse
effect of ethylene oxide exposure at these concentrations on the
outcome of pregnancy.
An increase in spontaneous abortions was also found in a study
on Finnish hospital sterilizing staff in 1980, using questionnaires
and hospital discharge records. The sterilizing agents were
ethylene oxide, glutaraldehyde, and formaldehyde. Controls were
nursing auxiliaries, from various hospitals, who did not work in
sterilization, anaesthetization, or X-ray recording departments.
Results were adjusted for age, parity, decade of the reported
pregnancy, coffee and alcohol consumption, and smoking habits.
The rate of spontaneous abortions in the sterilization staff
members as a whole (9.7% in 1443 pregnancies) was similar to the
rate in the controls (10.5% in 1179 pregnancies). A significant
increase, however, was observed when the adjusted spontaneous
abortion rate in the sterilization staff who were exposed during
pregnancy (15.1% in 545 pregnancies) was compared with the rate in
the staff members who were not exposed during pregnancy (4.6% in
605 pregnancies). On the basis of a separate study, the time-
weighted average exposure concentration was estimated to be in the
range of 0.18 - 0.90 mg/m3 (0.1 - 0.5 ppm), with peak concentrations
up to 450 mg/m3 (250 ppm). It was considered by the authors that
exposure to ethylene oxide accounted for most of the excess
spontaneous abortions (Hemminki et al., 1982).
In a new analysis of the data, controls were chosen from the
same hospitals, and only pregnancies that started during hospital
employment were analysed in all groups. The spontaneous abortion
rate was still highest for the pregnancies during which exposure to
ethylene oxide took place (20.4%), and the difference compared with
controls (11.3%) was significant. The abortion rate of the group
exposed to glutaraldehyde alone (16.6%) was also significantly
elevated (Hemminki et al., 1983).
Despite any methodological shortcomings of this reproductive
study, such as (a) the statement by Hemminki in 1983 that there was
limited exposure data, which prevented a comparison between
abortion rates and defined exposure levels; and (b) the fact that
the ethylene oxide-exposed and unexposed cohorts were not balanced
with regard to the incidence of prior abortions, there is a
suggestion of an association between ethylene oxide exposure and
adverse pregnancy outcome.
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
Human exposure is mainly through inhalation of the vapour.
Residues in medical equipment that has been sterilized with
ethylene oxide and not sufficiently aerated can migrate into
tissues and blood, producing primarily local effects (section 8.4).
Oral ingestion of ethylene oxide residues in most fumigated or
sterilized foodstuffs is unlikely, as they disappear rapidly
through evaporation or reaction with food constituents. A major
conversion product in foodstuffs is 2-chloroethanol, which is more
persistent than ethylene oxide (section 4.2.1).
Most ethylene oxide is used in the chemical plant in which it
is produced. Because of the explosion hazard, ethylene oxide is
stored and handled in chemical process plants in closed, automated
systems. This equipment is often located outdoors, and, except
during maintenance, workers have a minimal chance of exposure. Air
samples collected in processing areas of chemical production plants
have shown that ethylene oxide vapour concentrations are generally
less than 4 mg/m3 with occasional high peak exposures (section
4.3). Occupational exposure to ethylene oxide tends to be much
higher in health instrument manufacture and in hospitals than in
the chemical processing industries. Ethylene oxide concentrations
near malfunctioning or improperly designed equipment may reach
hundreds of mg/m3 of air for brief periods. However, 8-h time-
weighted average breathing-zone air concentrations in hospitals are
generally less than 36 mg/m3. It should be emphasized that the
exposure of hospital workers to ethylene oxide tends to be of a
short-term and intermittent nature with the likelihood of exposure
to short-term (5 - 120 min) concentrations of about 100 mg/m3 and
to peak concentrations of up to 1800 mg/m3 following the opening of
sterilization chambers (section 4.3).
Ethylene oxide has a high solubility in water but will
evaporate to a great extent. Degradation of ethylene oxide in
neutral water is slow, even in the presence of aerobic
microorganisms. Because of the low log n-octanol water-partition
coefficient, it is unlikely that ethylene oxide and its conversion
products (such as 1,2-ethanediol) will bioaccumulate. The toxicity
of ethylene oxide for aquatic organisms is low (all available LC50s
are above approximately 90 mg/litre) (Table 3). The probable
effects of ethylene oxide on the aquatic environment are,
therefore, considered negligible (sections 3.2, 6). There are no
data concerning the toxicity of ethylene oxide for terrestrial
organisms.
No ambient air monitoring data are available from which the
effects of ethylene oxide on the health of man and the environment
can be assessed. However, the risk for health from exposure to
ethylene oxide in the ambient air, apart from point source
emissions and accidental spillage, is likely to be negligible.
Inhaled ethylene oxide is readily absorbed into the blood,
distributed throughout the body, and rapidly metabolized. The
half-life in the tissues of man and rodents is approximately
10 min; clearance from the blood of dogs occurred with a half-
life of 33 min (section 5.2). Marked nausea and profuse vomiting
following dermal exposure of man to aqueous solutions of ethylene
oxide suggest that absorption can occur through the skin (section
5.1).
Case reports indicate that headache, nausea, vomiting,
dyspnoea, and respiratory tract irritation are common effects of
acute inhalation exposure to ethylene oxide (section 8.3). Case
reports and the results of animal studies indicate that
sensorimotor neuropathies may follow repeated exposure to
concentrations of ethylene oxide recognizable by its odour
(approximately 900 mg/m3 or more) (sections 2.2, 7.2.1, 8.3).
Dermatological effects in man following skin contact with
ethylene oxide include erythema, oedema, and vesiculation, in that
order. The severity of the skin injury is related to concentration
(a 50% (500 g/litre) solution being most hazardous) and duration of
contact. Liquid ethylene oxide, as it vaporizes, can result in a
freeze burn. On repeated exposure, ethylene oxide may cause
delayed allergic contact dermatitis (sections 8.1, 8.2). Ethylene
oxide and its conversion products are irritating to the eyes and
can produce corneal injury. Cataracts have occurred following
repeated exposure to concentrations of the vapour recognizable by
its odour (approximately 900 mg/m3 or more) (Table 1, sections 8.1,
8.3).
Ethylene oxide directly alkylates proteins and DNA and is
mutagenic in microorganisms, plants, insects, mammalian cells in
vitro, and mammals in vivo, including both gene mutations and
chromosomal abnormalities (section 7.5). In man, ethylene oxide
induces chromosomal aberrations and sister chromatid exchanges in
lymphocytes at air concentrations found at the workplace (section
8.7). Tissue distribution studies provide evidence that ethylene
oxide reaches the gonads, supporting the findings of heritable
mutations in insects and rodents (sections 5.2, 7.5). Ethylene
oxide may, therefore, be considered a potential human mutagen for
both somatic and germ cells.
The potential of ethylene oxide to cause teratogenic or adverse
reproductive effects has been examined in 4 animal species (mouse,
rat, rabbit, and monkey) by 2 routes of administration. Results
from these studies showed that ethylene oxide is toxic to
reproductive function in both males (reduced sperm number and sperm
motility, and an increased time to traverse a linear path) and
females (depression of fetal weight gain, fetal death, and fetal
malformation). The levels needed to produce these fetal effects
approach or equal the dose needed to produce maternal toxicity
(section 7.6). The results of animal studies suggest possible
reproductive impairment in human males but are inadequate for
assessing the fetal risk. Data on reproductive effects in human
beings are insufficient; one study, however, suggests an increase
in spontaneous abortion rate in women occupationally exposed to
ethylene oxide (section 8.8). However, the reported time-weighted
average air concentrations may not reflect the exposure levels that
induced the effect.
It has been clearly demonstrated in experimental animal studies
that ethylene oxide is carcinogenic via different routes of
exposure (intragastric, subcutaneous injection, and inhalation).
In 2 inhalation studies, confirmatory data demonstrated dose-
related increases in the incidences of leukaemia, peritoneal
mesothelioma, and cerebral glioma (section 7.4). Although the
evidence for the carcinogenicity of ethylene oxide in man is
inadequate, epidemiological studies indicate that exposure to
ethylene oxide (in mixtures with other chemicals) increases the
risk of malignancies (section 8.6).
Taking into account available data concerning the alkylating
nature of ethylene oxide, the demonstration of DNA adducts, the
overwhelming positive in vivo responses in mutagenic and
clastogenic assays, the reproducible positive carcinogenic findings
in animals, and the epidemiological findings suggesting an increase
in the incidence of human cancer, ethylene oxide should be
considered as a probable human carcinogen, and its levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. The study indicating that exposure to ethylene oxide may be
associated with spontaneous abortion needs to be
corroborated and the implication explored further.
2. The possible effects of ethylene oxide on the reproductive
function of man should be studied.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to ethylene oxide in combination
with other chemicals strongly suggest that additional
epidemiological studies should be carried out on
populations whose exposure has been primarily to ethylene
oxide, including adequate quantification of past exposure.
4. The temporal relationships of air concentrations of
ethylene oxide and duration of exposure must be examined to
determine which of these two factors has the greater impact
on health.
5. Development of methods and research should be conducted to
assess the endogenous occurrence of hydroxyethylation and
the exogenous (environmental) contribution of ethylene
oxide and its precursors to the formation of macromolecular
adducts as markers of internal dose.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of ethylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
ethylene oxide to experimental animals; there is limited
evidence for the carcinogenicity to humans of exposures to
ethylene oxide in combination with other chemicals; there is
inadequate evidence for the carcinogenicity to humans of
exposures to ethylene oxide alone. Taken together, the data
indicate that ethylene oxide is probably carcinogenic to
humans."
REFERENCES
ABRAHAMS, R.H. (1980) Recent studies with workers exposed to
ethylene oxide. In: Jorkasky, J.F., ed. Safe use of ethylene
oxide. Proceedings of the Educational Seminar, Washington DC,
Health Industries Manufacturers Association, pp. 27-38,
211-220 (HIMA Report No. 80-4)
ADLER, N. (1965) Residual ethylene oxide and ethylene glycol
in ethylene oxide sterilized pharmaceuticals. J. Pharm. Sci.,
54: 735-742.
ALOMAR, A., CAMARASA, J.M.G., NOGUERA, J., ASPINOLEA, F.
(1981) Ethylene oxide dermatitis. Contact dermatit., 7:
205-207.
ALTMAN, P.L. & DITTMER, D.S. (1974) Biological data book,
Bethesda, Maryland, Federation of American Societies for
Experimental Biology, Vol. 3.
APPELGREN, L.-E., ENEROTH, G., & GRANT, C. (1977) Studies on
ethylene oxide: whole-body autoradiography and dominant lethal
test in mice. In: Clinical toxicology. Proceedings of the
Meeting held at Edinburgh, June 1976, European Society of
Toxicology, Amsterdam, Excerpta Medica, Vol. 18, pp. 315-317.
APPELGREN, L.-E., ENEROTH, G., GRANT, C., LANDSTROM, L.-E., &
TENGHAGEN, K. (1978) Testing of ethylene oxide for
mutagenicity using the micronucleus test in mice and rats.
Acta pharmacol. toxicol., 43: 69-71.
BINDER, H. & LINDNER, W. (1972) [Determination of ethylene
oxide in the smoke of definitely unfumigated cigarettes.]
Fachliche Mitteilungen der Austria Tabakwerke A.G., 13:
215-220 (in German).
BIRD, M.J. (1952) Chemical production of mutations in
Drosophila: comparison of techniques. J. Genet., 50: 480-485.
BIRO, L., FISHER, A.A., & PRICE, E. (1974) Ethylene oxide
burns. Arch. Dermatol., 110: 924-925.
BLACKWOOD, J.D. & ERSKINE, E.B. (1938) Carboxide poisoning.
US Navy med. Bull., 36: 44-45.
BLIXT, S., EHRENBERGH, L., & GELIN, O. (1963) Studies of
induced mutations in peas. VII. Mutation spectrum and mutation
rate of different mutagenic agents. Agric. Hort. Genet., 21:
178-216.
BOGYO, S., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: epoxides, Syracuse, New York,
Center for Chemical Hazard Assessment, Syracuse Research
Corporation (Report prepared for US EPA) (Report No. EPA
560/11-80-005, PB 80-183197).
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
BROOKES, P. & LAWLEY, P.D. (1961) The alkylation of
guanosine and guanylic acid. J. Chem. Soc., pp. 3923-3928.
BUQUET, A. & MANCHON, P. (1970) Recherche et dosage des
résidus et dérivés dans un pain conservé a l'aide d'oxyde
d'éthylène. Chim. analytique, 52: 978-983.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CAPELLINI, A. & GHEZZI, I. (1965) [Two cases of acute
ethylene oxide poisoning.] Med. Lav., 56: 822-827 (in Italian).
CHAIGNEAU, M. & MURAZ, B. (1981) Action de l'oxyde
d'éthylène sur le tabac ( Nicotiana tabacum L.): absorption et
combinaisons. Ann. Pharm. Fr., 39: 305-311.
CLARKE, C.P., DAVIDSON, W.L., & JOHNSTON, J.B. (1966)
Haemolysis of blood following exposure to an Austrialian
manufactured plastic tubing sterilized by means of ethylene
oxide gas. Aust. NZ J. Surg., 36: 53-55.
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, Vol. 2a: toxicology, 3rd revised ed.,
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
p. 2166.
CONAN, L., FOUCAULT, B., SIOU, G., CHAIGNEAU, M., LE MOAN, G.,
& DOINEL, A. (1979) Contribution à la recherche d'une action
mutagène des résidus d'oxyde d'éthylène, d'éthylène glycol et
de chloro-2-éthanol dans le matériel plastique stérilisé par
l'oxyde d'éthylène. Ann. Fals. exp. Chim., 72: 141-151.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
CUMMING, R.B. & MICHAUD, T.A. (1979) Mutagenic effects of
inhaled ethylene oxide in male mice. Environ. Mutagen., 1:
166-167.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No.
600/3-80-084, PB 80-221948).
DAUVOIS, C., CHAIGNEAU, M., & LE MOAN, G. (1982)
Sterilisation de pansements par l'oxyde d'éthylène. I.
Physisorption. Ann. Pharm. Fr., 40: 125-132.
DOLOVICH, J. & BELL, B. (1978) Allergy to a product(s) of
ethylene oxide gas. Demonstration of IgE and IgG antibodies
and hapten specificity. J. Allergy clin. Immunol., 62: 30-32.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
DUNKELBERG, H. & HARTMETZ, G. (1977) [Recording the air
pollution by ethylene oxide in the region of clinical
sterilization installations.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
164: 271-278 (in German).
EDELHAUSER, H.F., ANTOINE, M.E., PEDERSON, H.J., & HIDDEMAN,
J.W., & HARRIS, R.G. (1983) Intraocular safety evaluation of
ethylene oxide and sterilant residues. J. Toxicol.-Cut. ocular
Toxicol., 2: 7-39.
EHRENBERG, L. & HALLSTROM, T. (1967) In: Kalling, L.O., ed.
Haematologic studies on persons occupationally exposed to
ethylene oxide, Vienna, International Atomic Agency, pp.
327-334 (Report No. SM92-26).
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1956)
Chemically induced mutation and sterility in barley. Acta
chem. Scand., 10: 492-494.
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1959) The
mutagenic effects of ionizing radiations and reactive ethylene
derivatives in barley. Hereditas, 45: 351-368.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
tissue doses in the mouse from air contaminiated with ethylene
oxide. Mutat. Res., 24: 83-103.
EMBREE, J.W. & HINE, C.H. (1975) Mutagenicity of ethylene
oxide. Toxicol. appl. Pharmacol., 33: 172-173.
EMBREE, J.W., LYON, J.P., & HINE, C.H. (1977) The mutagenic
potential of ethylene oxide using the dominant-lethal assay in
rats. Toxicol. appl. Pharmacol., 40: 261-267.
FOMENKO, V.N. & STREKALOVA, E.Y. (1973) [The mutagenic
effect of some industrial toxins as a function of
concentration and exposure time.] Toksikol. Nov. Prom. Him.
Veshchestr., 7: 51-57 (in Russian).
FAHMY, G.G. & FAHMY, M.J. (1970) Gene elimination in
carcinogenesis: reinterpretation of the somatic mutation
theory. Cancer Res., 30: 195-205.
FINELLI, P., MORGAN, T.F., YAAR, I., & GRANGER, C.V. (1983)
Ethylene oxide-induced polyneuropathy: a clinical and
electrophysiologic study. Arch. Neurol., 40: 419-421.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione.
J. Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Controlling exposure to alkene oxides.
Chem. Eng. Prog., 79: 39-43.
GARRY, V.F., HOZIER, J., JACOBS, D., WADE, R.L., & GRAY, D.G.
(1979) Ethylene oxide: evidence of human chromosomal effects.
Environ. Mutagen., 1: 375-382.
GARRY, V.F., OPP, C.W., WIENCKE, J.K., & LAKATUA, D. (1982)
Ethylene oxide induced sister chromatid exchange in human
lymphocytes using a membrane dosimetry system. Pharmacology,
25: 214-221.
GENEROSO, W.M., CAIN, K.T., KRISHNA, M., SHEN, C.W., & GRYDER,
R.M. (1980) Heritable translocation and dominant-lethal
mutation induction with ethylene oxide in mice. Mutat. Res.,
73: 133-142.
GENEROSO, W.M., CUMMING, R.B., BANDY, J.A., & CAIN, K.T.
(1983) Increased dominant-lethal effects due to prolonged
exposure of mice to inhaled ethylene oxide. Mutat. Res., 119:
377-379.
GERHARDT, U. & LADD EFFIO, J.C. (1983) [Ethylene oxide
residues in spices.] Fleisch wirtsch., 63: 606-608 (in German).
GESSNER, P.K., PARKE, D.V., & WILLIAMS, R.T. (1961) The
metabolism of 14C-labelled ethylene glycol. Biochem. J., 79:
482-489.
GILDING, D.K., REED, A.M., & BASKETT, S.A. (1980) Ethylene
oxide sterilization: effect of polymer structure and
sterilization conditions on residue levels. Biomaterials, 1:
145-148.
GILLESPIE, E.H., JACKSON, J.M., & OWEN, G.R. (1979) Ethylene
oxide sterilization: is it safe? J. clin. Pathol., 32:
1184-1187.
GLASER, Z.R. (1979) Ethylene oxide: toxicology review and
field study results of hospital use. J. environ. Pathol.
Toxicol., 2: 173-208.
GORDON, H.T. & THORNBURG, W.W. (1959) Hydroxyethyl
derivatives in prunes fumigated with 14C-ethylene oxide.
J. agric. food Chem., 7: 196-200.
GREAVES WALKER, W.G. & GREESON, C.E. (1932) The toxicity of
ethylene oxide. J. Hyg., 32: 409-416.
GROSS, J.A., HAAS, M.L., & SWIFT, T.R. (1979) Ethylene oxide
neurotoxicity: report of four cases and review of the
literature. Neurology, 29: 978-983.
GRUNOW, W. & ALTMANN, H.-J. (1982) Toxicokinetics of
chloroethanol in the rat after single oral administration.
Arch. Toxicol., 49: 275-284.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Report No. PB
83-258038).
HANSEN, J.P., ALLEN, J., BROCK, K., FALCONER, J., HELMS, M.J.,
SHAVER, G.C., & STROHM, B. (1984) Normal sister chromatid
exchange levels in hospital sterilization employees exposed to
ethylene oxide. J. occup. Med., 26: 29-32.
HANIFIN, J.M. (1971) Ethylene oxide dermatitis. J. Am. Med.
Assoc., 217: 213.
HARTMAN, P.A. & BOWMAN, P.B. (1977) Simple GLC determination
of ethylene oxide and its reaction products in drugs and
formulations. J. Pharm. Sci., 66: 789-792.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEMMINKI, K., MUTANEN, P., SALONIEMI, I., NIEMI, M-L., &
VAINIO, H. (1982) Spontaneous abortions in hospital staff
engaged in sterilizing instruments with chemical agents.
Br. med. J., 285: 1461-1463.
HEMMINKI, K., MUTANEN, P., & NIEMI, M.-L. (1983) Spontaneous
abortions in hospital sterilizing staff. Br. med. J., 286:
1976-1977.
HENNE, W., DIETRICH, W., PELGER, M., & SENGBUSCH, G., VON
(1984) Residual ethylene oxide in hollow-fibre dialyzers.
Artif. Organs, 8: 306-309.
HINE, C.H. & ROWE, V.K. (1981) Epoxy compounds. In: Patty,
S.A., ed. Industrial hygiene and toxicology, 3rd revised ed.,
New York, Interscience Publishers, Vol. IIa, p. 2141.
HIROSE, T., GOLDSTEIN, R., & BAILEY, C. (1963) Hemolysis of
blood due to exposure to different types of plastic tubing and
the influence of ethylene oxide sterilization. J. thorac.
cardiovasc. Surg., 45: 245-251.
HOGSTEDT, C., MALMQVIST, N., & WADMAN, B. (1979a) Leukemia
in workers exposed to ethylene oxide. J. Am. Med. Assoc., 241:
1132-1133.
HOGSTEDT, C., ROHLEN, O., BERNDTSSON, B.S., AXELSON, O., &
EHRENBERG, L. (1979b) A cohort study of mortality and cancer
incidence in ethylene oxide production workers. Br. J. ind.
Med., 26: 276-280.
HOGSTEDT, B., GULLBERG, B., HEDNER, K., KOLNIG, A-M.,
MITELMAN, F., SKERFVING, S., & WIDEGREN, B. (1983)
Chromosome aberrations and micronuclei in bone marrow cells
and peripheral blood lymphocytes in humans exposed to ethylene
oxide. Hereditas, 98: 105-113.
HOGSTEDT, C., ARINGER, L., & GUSTAVSSON, A. (1984) [Ethylene
oxide and cancerreview of the literature and follow-up of 2
studies.] Arbete och Hälsa, 49: : 1-32 (in Swedish).
HOLLEY, H.S. & GILDEA, J.E. (1971) Vocal cord paralysis
after tracheal intubation. J. Am. Med. Assoc., 215: 281-284.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of ethylene oxide determined
on experimental animals. Arch. ind. Health, 13: 217-227.
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationship for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1985) Alkyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans No. 36).
IPPEN, H. VON & MATTHIES, V. (1970) [Protracted chemical
burns.] Berufsdermatosen, 18: 144-165 (in German).
IRPTC (1984) Data profile on ethylene oxide, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide vapors.
Arch. ind. Health, 13: 237-244.
JANA, M.K. & ROY, K. (1975) Effectiveness and efficiency of
ethyl methane-sulphonate and ethylene oxide for the induction
of mutations in mice. Mutat. Res., 28: 211-215.
JAY, W.M., SWIFT, T.R., & HULL, D.S. (1982) Possible
relationship of ethylene oxide exposure to cataract formation.
Am. J. Ophthalmol., 93: 727-732.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction
of carcinogenicity evaluated by 143 agents tested. Mutat.
Res., 75: 191-202.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rat.
Biochem. Pharmacol., 16: 185-199.
JONES, A.R. & WELLS, G. (1981) The comparative metabolism of
2-bromoethanol and ethylene oxide in the rat. Xenobiotica, 11:
763-770.
JORDY, A. (1983) [The course of the concentrations of
ethylene oxide reaction products in synthetic materials
following gas sterilization.] Hyg. Med., 8: 17-19 (in German).
JOSHI, S.B., DODGE, M.C., & BUFALINI, J.J. (1982)
Reactivities of selected organic compounds and contamination
effects. Atmos. Environ., 16: 1301-1310.
JOYNER, R.E. (1964) Chronic toxicity of ethylene oxide.
Arch. environ. Health, 8: 700-710.
KAUHANEN, K. (1978) Ethylene oxide. Rebuttable presumption
against registration maximum residue limits and daily levels
of exposure. Fed. Reg., 43:: 3804.
KLIGERMAN, A.D., EREXSON, G.L., PHELPS, M.E., & WILMER, J.L.
(1983) Sister-chromatid exchange induction in peripheral
blood lymphocytes of rats exposed to ethylene oxide by
inhalation. Mutat. Res., 120: 37-44.
KOLMARK, H.G. & KILBEY, B.J. (1968) Kinetic studies of
mutation induction by epoxides in Neurospora crassa. Molec.
Gen. Genet., 101: 89-98.
KOLMARK, H.G. & WESTERGAARD, M. (1953) Further studies on
chemically-induced reversions at the adenine locus of
Neurospora. Hereditas, 39: 209-224.
KORPELA, D.B., MCJILTON, C.E., & HAWKINSON, T.E. (1983)
Ethylene oxide dispersion from gas sterilizers. Am. Ind. Hyg.
Assoc. J., 44: 589-591.
KROLLER, E. VON (1966) [Investigations into the fumigation
of foodstuffs by ethylene oxide and into the determination of
its residues.] Dtsch. Lebensm. Rundsch., 62: 227-234 (in
German).
KUZUHARA, S., KANAZAWA, I., NAKANISHI, T., & EGASHIRA, T.
(1983) Ethylene oxide polyneuropathy. Neurology, 33: 377-380.
LABORDE, J.B. & KIMMEL, C.A. (1980) The teratogenicity of
ethylene oxide administered intraveneously to mice. Toxicol.
appl. Pharmacol., 56: 16-22.
LAHAYE, D., ASSCHE, F., VAN, & THEUNISSEN, A. [Ethylene oxide
levels in the sterilization units of hospitals.] Tijdschr.
Soc. Gezondheidsz., 62: 707-713 (in Dutch).
LAMY, F., LACHAPELLE, J.-M., & BRAEKEL, G. VAN (1974)
Dermites professionnelles a l'oxyde d'éthylène chez des sujets
travaillant en zone stérile. Arch. Mal. prof. Méd. Trav.
Sécur. soc., 35: 719-724.
LAURENT, C., FREDERIC, J., & LEONARD, A.Y. (1984) Sister
chromatid exchange frequency in workers exposed to high levels
of ethylene oxide, in a hospital sterilization service.
Int. Arch. occup. environ. Health, 54: 33-43.
LEBREC, D., MASQUET, C., & RUEFF, B. (1977) Collapsus après
exploration endovasculaire avec des cathéters stérilisés à
l'oxyde d'éthylène. Nouv. Presse Med., 6: 2991.
LIPTON, B., GUTIERREZ, R., BLAUGRUND, S., LITWAK, R.S., &
RENDELL-BAKER, L. (1971) Irridiated PVC plastic and gas
sterilization in the production of tracheal stenosis following
tracheostomy. Anest. Analg., 50: 578-586.
LYARSKII, P.P., YURCHENKO, V.V., ZHURKOV, V.S., & GLEIBERMAN,
S.E. (1983) [Mutagenic hazards of parenteral administration
of ethylene oxide to mammals.] Gig. i Sanit., 1: 23-26 (in
Russian).
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76: 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.H., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76: 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MACKEY, J. (1968) Mutagenesis in Vulgare wheat. Hereditas,
59: 505-517.
MCCHESNEY, E.W., GOLBERG, L., PAREKH, C.K., RUSSELL, J.C., &
MIN, B.H. (1971) Reappraisal of the toxicology of ethylene
glycol. II. Metabolism studies in laboratory animals. Food
cosmet. Toxicol., 9: 21-30.
MCDONALD, T.O., KASTEN, K., HERVEY, R., GREGG, S., BORGMANN,
A.R., & MURCHISON, T. (1973) Acute ocular toxicity of
ethylene oxide, ethylene glycol, and ethylene chlorohydrin.
Bull. Parenter. Drug Assoc., 27: 153-164.
MCGUNNIGLE, R.G., RENNER, J.A., ROMANO, S.J., & ABODEELY,
R.A. (1975) Residual ethylene oxide: levels in medical grade
tubing and effects on an in vitro biological system.
J. Biomed. Mater. Res., 9: 273-383.
MANTZ, J.M., TEMPE, J.D., JAEGER, A., & VIDAL, S. (1972)
Stenoses trachéales et stérilisation des canules de
trachéotomie par l'oxyde d'éthylène. Sem. Hôp. Paris, 48:
3367-3370.
MARTIS, L., KROES, R., DARBY, T.D., & WOODS, E.F. (1982)
Disposition kinetics of ethylene oxide, ethylene glycol, and
2-chloroethanol in the dog. J. Toxicol. environ. Health, 10:
847-856.
MARX, G.F., STEEN, S.N., SCHAPIRA, M., ERLANGER, H.L., ARKINS,
R.E., JADWAT, C.M., & KEPES, E. (1969) Hazards associated
with ethylene oxide sterilization. NY State J. Med., 69:
1319-1320.
METZ, E. VON (1939) [Poisoning by ethylene oxide (Cartox or
T-gas).] Samml. Vergiftungsfällen, 10: 37-38 (in German).
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on
Schizosaccharomyces pombe: the influence "in vitro" of
mouse-liver metabolizing system. Mutat. Res., 102: 425-437.
MORGAN, R.W., CLAXTON, K.W., DIVINE, B.J., KAPLAN, S.D., &
HARRIS, V.B. (1981) Mortality among ethylene oxide workers.
J. occup. Med., 23: 767-770.
MORPURGO, G. (1963) Induction of mitotic crossing-over in
Aspergillus nidulans by bifunctional alkylating agents.
Genetics, 48: 1259-1263.
MOUILLESEAUX, A., LAURENT, A.-M., FABRE, M., JOUAN, M., &
FESTY, B. (1983) Teneurs atmosphériques en oxyde d'éthylène
décelées dans l'environnement professionnel d'instalations de
stérilisation ou de désinfection. Arch. Mal. Prof., 44: 1-14.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., & EHRENBERG, L.
(1968) Note on the chromosome breaking activity of ethylene
oxide and ethyleneimine. Hereditas, 60: 267-269.
NAKAO, Y. & AUERBACH, C. (1961) Test of a possible
correlation between cross-linking and chromosome breaking
abilities of chemical mutagens. Z. Vererbungsl., 92: 457-461.
O'LEARY, R.K., WATKINS, W.D., & GUESS, W.L. (1969)
Comparative chemical and toxicological evaluation of residual
ethylene oxide in sterilized plastics. J. Pharm. Sci., 58:
1007-1010.
OSER, B.L. & HALL, L.A. (1956) The effect of ethylene oxide
treatment on the nutritive value of certain foods. Food
Technol., 10: 175-178.
OSTERMAN-GOLKAR, S (1983) Tissue doses in man: implications
in risk assessment. In: Hayes, A.W., ed. Developments in the
Science and Practice of Toxicology. Proceedings of the 3rd
International Congress on Toxicology, San Diego, California.
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor.
Mutat. Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog. Mutag.,
3: 395-405.
PERO, E.W., WIDEGREN, B., HOGSTEDT, B., & MITELMAN, F.
(1981) In vivo and in vitro ethylene oxide exposure of human
lymphocytes assessed by chemical stimulation of unscheduled
DNA synthesis. Mutat. Res., 83: 271-289.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food cosmet. Toxicol., 18: 115-118.
PFEILSTICKER, K. & SIDDIQUI, I.R. (1976) [Isolation of the
derivatives from cocoa-powder fumigated by ethylene oxide
1,2-14C and their structure suggested on the basis of I.R. and
mass-spectrometry.] Z. Lebensm. Unters. Forsch., 160: 19-27
(in German).
PFEILSTICKER, K., FABRICIUS, G., & TIMME, G. (1975)
[Simultaneous gas chromatographic determination of ethylene
oxide, ethylene chlorohydrin, and ethylene glycol in grain.]
Z. Lebensm. Unters. Forsch., 158: 21-25 (in German).
POIRIER, V. & PAPADOPOULO, D. (1982) Chromosomal aberrations
induced by ethylene oxide in a human amniotic cell line in
vitro. Mutat. Res., 104: 255-260.
POOTHULLIL, J., SHIMIZU, A., DAY, R.P., & DOLOVICH, J.
(1975) Anaphylaxis from the product(s) of ethylene oxide gas.
Ann. intern. Med., 82: 58-60.
QUAZI, A.H. & KETCHAM, N.H. (1977) A new method for
monitoring personal exposure to ethylene oxide in the
occupational environment. Am. Ind. Hyg. Assoc. J., 38: 635-647.
RADDING, S.B., LIU, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances, (EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide.
J. Assoc. Offic. Agric. Chem., 51: 709-715.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs.
Bull. environ. Contam. Toxicol., 27: 426-431.
RANNUG, U., GOTHE, R., & WACHTMEISTER, C.A. (1976) The
mutagenicity of chloroethylene oxide, chloroacetaldehyde,
2-chloroethanol, and chloroacetic acid, conceivable metabolites
of vinyl chloride. Chem.-Biol Interact., 12: 251-263.
RILLAER, W.G., VAN & BEERNAERT, H. (1982) Determination of
residual ethylene chlorohydrin (ECH) in fumigated foodstuffs
by glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 175: 175-178.
ROMANO, S.J. & RENNER, J.A. (1979) Analysis of ethylene
oxide-worker exposure. Am. Ind. Hyg. Assoc. J., 40: 742-745.
ROMANO, S.J., RENNER, J.A., & LEITNER, P.M. (1973) Gas
chromatographic determination of residual ethylene oxide by
head space analysis. Anal. Chem., 45: 2327-2330.
ROYCE, A. & MOORE, W.K.S. (1955) Occupational dermatitis
caused by ethylene oxide. Br. J. ind. Med., 12: 169-171.
SARTO, F., COMINATO, I., PINTON, A.M., BROVEDANI, P.G.,
FACCIOLI, C.M., BIANCHI, V., & LEVIS, A.G. (1984)
Cytogenetic damage in workers exposed to ethylene oxide.
Mutat. Res., 138: 185-195.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SEGERBACK, D. (1983) Alkylation of DNA and haemoglobin in
the mouse following exposure to ethene and ethene oxide.
Chem.-Biol. Interact., 45: 139-151.
SEXTON, R.J. & HENSON, E.V. (1949) Dermatological injuries
by ethylene oxide. J. ind. Hyg. Toxicol., 31: 297-300.
SEXTON, R.J. & HENSON, E.V. (1950) Experimental ethylene
oxide human skin injuries. J. ind. Hyg. Toxicol., 32: 549-564.
SHULOVSKA, K., LINDGREN, D., ERIKSSON, G., & EHRENBERG, L.
(1969) The mutagenic effect of low concentrations of ethylene
oxide in air. Hereditas, 62: 264-266.
SHUPACK, J.L., ANDERSEN, S.R., & ROMANO, S.J. (1981) Human
skin reactions to ethylene oxide. J. Lab. clin. Med., 98:
723-729.
SITTERT, N.J., VAN, JONG, G., DE, CLARE, M.G., DAVIES, R.,
DEAN, B.J., WREN, L.J., & WRIGHT, A.S. (1985) Cytogenetic,
immunological, and haematological effects in workers in an
ethylene oxide-manufacturing plant. Br. J. ind. Med., 42:
19-26.
SMITH, H.H. & LOTFY, T.A. (1954) Comparative effects of
certain chemicals on tradescantia chromosomes as observed at
pollen tube mitosis. Am. J. Bot., 41: 489-593.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg. Toxicol.,
23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1981)
Ethylene oxide: two-year inhalation study on rats, Pittsburgh,
Pennsylvania, Bushy Run Research Center (Final Report No.
44-20).
SNELLINGS, W.M., ZELENAK, J.P., & WEIL, C.S. (1982a) Effects
on reproduction in Fischer 344 rats exposed to ethylene oxide
by inhalation for one generation. Toxicol. appl. Pharmacol.,
63: 382-388.
SNELLINGS, W.M., MARONPOT, R.R., ZELENAK, J.P., & LAFFOON,
C.P. (1982b) Teratology study on Fischer 344 rats exposed to
ethylene oxide by inhalation. Toxicol. appl. Pharmacol., 64:
476-481.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984a) A
subchronic inhalation study on the toxicologic potential of
ethylene oxide in B6C3F1 mice. Toxicol. appl. Pharmacol., 76:
510-518.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984b) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPASOVSKI, M., HRISTEVA, V., PERNOV, K., KIRKOV, V.,
DRJANOVSKA, T., PANOVA, Z., BOBEV, G., GINCHEVA, N., &
IVANOVA, S. (1980) [Health status of the workers from the
production of ethylene and ethylene oxide.] Khig. Zdraveopaz.,
23: 41-47 (in Russian).
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropathological
evaluation of monkeys exposed to ethylene and propylene oxide,
Kansas City, Missouri, Midwest Research Institute (Prepared for
NIOSH) (PB 83-134817).
STANLEY, P., BERTRANOU, E., FOREST, F., & LANGEVIN, L.
(1971) Toxicity of ethylene oxide sterilization of polyvinyl
chloride in open-heart surgery. J. thorac. cardiovasc. Surg.,
61: 309-314.
STAR, E.G. (1980a) [Mutagenic and cytotoxic effect of
ethylene oxide on human cell cultures.] Zbl. Bakt. Hyg. (I. Abt.
Orig. B), 170: 548-556 (in German).
STAR, E.G. (1980b) [Absorption and desorption of ethylene
oxide in anaesthesia supplies.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
170: 557-569 (in German).
STAR, E.G. (1980c) [Ethylene oxide residues and aeration
time after use of modern heated aerators.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 171: 18-24 (in German).
STAR, E.G. (1980d) [Gamma-rays and ethylene oxide
sterilization.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 171: 33-41
(in German).
STAR, E.G., CASELITZ, J., & LONING, T. (1980) [The effect of
ethylene oxide and chemical desinfectant residues upon the
larynx- and tracheal mucosa of rabbits.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 170: 539-547 (in German).
STARK, W.J., ROSENBLUM, P., MAUMENEE, A.E., & COWEN, C.L.
(1980) Postoperative inflammatory reactions to intraocular
lenses sterilized with ethylene oxide. Opthalmology, 87:
385-389.
STIJVE, T., KALSBACH, R., & EYRING, G. (1976) Determination
and occurrence of ethylene chlorohydrin residues in foodstuffs
fumigated with ethylene oxide. Mitt. Geb. Lebensm. Unters.,
67: 403-428.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STOLLEY, P.D., SOPER, K.A., GALLOWAY, S.M., NICHOLS, W.W.,
NORMAN, S.A., & WOLMAN, S.R. (1984) Sister-chromatid
exchanges in association with occupational exposure to
ethylene oxide. Mutat. Res., 129: 89-102.
STREKALOVA, E.Y., CHIRKOVA, Y.M., & GOLUBOVICH, Y.Y. (1975)
[Mutagenic action of ethylene oxide on sex and somatic cells
in male white rats.] Toksikol. Nov. Prom. Him. Veshchestr., 6:
11-16 (in Russian).
TAN, E.-L., CUMMING, R.B., & HSIE, A.W. (1981) Mutagenicity
and cytotoxicity of ethylene oxide in the CHO/HGPRT system.
Environ. Mutagen., 3: 683-686.
TANOOKA, H. (1979) Application of Bacillus subtilis spores
in the detection of gas mutagens: a case of ethylene oxide.
Mutat. Res., 64: 433-435.
THIESS, A.M. (1963) [Observation on the adverse health
effects of ethylene oxide.] Archiv. Toxikol., 20: 127-140 (in
German).
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkene
oxides (ethylene oxide/propylene oxide) and derivatives.
J. occup. Med., 23: 343-347.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & STOCKER, W.G.
(1981b) Mortality study on employees exposed to alkene oxides
(ethylene oxide/propylene oxide) and their derivatives. In:
International Symposium on Prevention of Occupational Cancer,
Helsinki, pp. 249-259.
TROISI, F.M. (1965) [Aphonia of late onset due to
occupational exposure to ethylene oxide.] Med. Lav., 56:
373-377 (in Italian).
UKITA, T., OKUYAMA, H., & HAYATSU, H. (1963) Modifications
of nucleosides and nucleotides. I. Reaction of ethylene oxide
with uridine and uridylic acid. Chem. Pharm. Bull. (Tokyo),
11: 1399-1404.
US DEPARTMENT OF LABOR (1984) Occupational exposure to
ethylene oxide: final standard. Fed. Reg., 49: 25734-25809.
US EPA (1985) Health assessment document for ethylene oxide,
Washington DC, US Environmental Protection Agency (EPA
600/8-84/009F).
USITC (1971) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
USITC (1981) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
VAN DUUREN, B.L., ORRIS, L., & NELSON, N. (1965)
Carcinogenicity of epoxides, lactones, and peroxy compounds.
Part II. J. Natl Cancer Inst., 35: 707-717.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. I. Toxicity profiles, Boca Raton,
Florida, CRC Press Inc., pp. 55-56.
VIRTANEN, P.O.I. (1963) Kinetics of the reactions of
ethylene oxide with nucleophiles. Am. Acad. Sci. Fenn. Ser. A.
II, 124: 1-89.
WAITE, C.P., PATTY, F.A., & YANT, W.P. (1930) Acute response
of guinea-pigs to vapours of some new commercial organic
compounds. IV. Ethylene oxide. Pub. Health Rep., 45: 1832-1843.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WESLEY, F., ROURKE, B., & DARBISHIRE, O. (1965) The
formation of persistent toxic chlorohydrins in foodstuffs by
fumigation with ethylene oxide and with propylene oxide.
J. food Sci., 30: 1037-1042.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WINDMUELLER, H.G. & KAPLAN, N.O. (1961) The preparation and
properties of N-hydroxyethyl derivatives of adenosine,
adenosine tri-phosphate, and nicotinamide adenine
dinucleotide. J. Biol. Chem., 236: 2716-2726.
WINDMUELLER, H.G., ACKERMAN, C.J., BAKERMAN, H., & MICKELSEN,
O. (1959) Reaction of ethylene oxide with nicotinamide and
nicotinic acid. J. Biol. Chem., 234: 889-894.
WOLFS, P., DUTRIEUX, M., SCAILTEUR, V., HAXHE, J.-J., ZUMOFEN,
M., & LAUWERIJS, R. (1983) Surveillance des travailleurs
exposés a l'oxyde d'éthylène dans une enterprise de
distribution de gaz stérilisants et dans des unités de
stérilisation de matériel médical. Arch. Mal. Prof., 44:
321-328.
WOODARD, G. & WOODARD, M. (1971) Toxicity of residuals from
ethylene oxide gas sterilization. In: Proceedings of the 1971
HIA Technical Symposium, Washington DC, Health Industries
Association.
YAGER, J.W. & BENZ, R.D. (1982) Sister-chromatid exchanges
induced in rabbit lymphocytes by ethylene oxide after
inhalation exposure. Environ. Mutagen., 4: 121-134.
YAGER, J.W., HINES, C.J., & SPEAR, R.C. (1983) Exposure to
ethylene oxide at work increases sister-chromatid exchanges in
human peripheral lymphocytes. Science, 219: 1221-1223.
YAKUBOVA, Z.N., SHAMOVA, H.A., MUFTAKNOVA, F.A., & SHILOVA,
L.F. (1976) [Gynaecological disorders in workers engaged in
ethylene oxide production.] Kazan. Med. Zh., 57: 558-560 (in
Russian).
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
When a single dose of 2 mg labelled ethylene oxide in
propanediol/kg body weight was applied intraperitoneally to rats,
43% of the administered radioactivity was excreted in the urine
within 50 h (41% within 24 h) of exposure, 9% as S-(2-
hydroxyethyl)cysteine and 33% as N-acetyl- S-(2-hydroxyethyl)
cysteine, both products of glutathione conjugation. Via the lungs,
1.5% was excreted as carbon dioxide and 1% as unmetabolized
ethylene oxide (Jones & Wells, 1981). The involvement of
glutathione-epoxide- S-transferase (EC 4.4.1.7) has not been
investigated further. In vitro glutathione conjugation of the
homologue propylene oxide was shown to proceed only in the presence
of an enzyme (Fjellstedt et al., 1973). In rabbits, no effect was
found on liver- and blood-glutathione levels, after 12 weeks of
exposure to concentrations of ethylene oxide at 18, 90, or 450
mg/m3, for 5 days per week, 6 h per day (Yager & Benz, 1982).
As ethylene oxide can react with chloride ions, and this
reaction is acid catalysed, 2-chloroethanol might be expected to be
a metabolite, especially after oral administration. However,
neither 2-chloroethanol, nor its metabolites (Johnson, 1967; Grunow
& Altman, 1982) have been found in the plasma, tissues, or urine of
species exposed to ethylene oxide.
Ehrenberg et al. (1974) found that an average of 74% of
labelled ethylene oxide, inhaled by mice, was excreted in the urine
within 24 h in the form of unidentified metabolites, and only 4%
within the next 24 h. Thus, on the basis of this and previously-
presented excretion data, excretion of metabolites of ethylene
oxide mainly takes place via the urine, within 24 h following
exposure.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
A summary of the acute toxicity of ethylene oxide for aquatic
organisms is presented in Table 3. Data on the most likely
reaction products, 1,2-ethanediol and 2-chloroethanol, are
included.
LC50s of ethylene oxide for aquatic species have been reported
to range from 90 mg/litre (goldfish, 24-h exposure) to 745 mg/litre
(brine shrimp, 48-h exposure). Microorganisms in activated sludge
showed 50% inhibition at concentrations between 10 and 100
mg/litre. Hydrolysis to 1,2-ethanediol results in detoxification.
The toxicity of 2-chloroethanol for aquatic organisms resembles
that of ethylene oxide, though 2-chloroethanol seems to be more
toxic for Daphnia magna. Nevertheless, under environmental
conditions, the conversion of ethylene oxide to 2-chloroethanol or
1,2-ethanediol will be slow.
In a study on the albino guppy, Poecilia reticulata, desorption
of ethylene oxide or its reaction products from non-aerated plastic
materials into water can lead to behavioural disturbances in the
fish almost immediately, and to death within about one h. No such
toxicity was found when the materials were aerated for 24 h
(O'Leary et al., 1969).
Ethylene oxide is very soluble in aqueous media and evaporates
from water to a significant degree. The log n-octanol water-
partition coefficient was reported to be -0.30 (Radding et al.,
1977). Thus, ethylene oxide will not bioaccumulate.
Table 3. Acute aquatic toxicitya
-----------------------------------------------------------------------------------------------------------
Organism Description T pH Dissolved Parameter Test Concentration Reference
(°C) oxygen substance (mg/litre)
-----------------------------------------------------------------------------------------------------------
Micro- activated 22 50% growth ethylene oxide 10 - 100 Conway et al.
organisms sludge inhibition 1,2-ethanediol > 10 000 (1983)b
Crustacea water flea 17 7.0 minimal 48-h LC50 ethylene oxide 212 Conway et al.
(Daphnia magna) aeration 1,2-ethanediol > 10 000 (1983)c
2-chloroethanol 100
Fish fathead minnow 22 7.0 no 96-h LC50 ethylene oxide 84 Conway et al.
(Pimephales aeration 1,2-ethanediol > 10 000 (1983)c
promelas) 2-chloroethanol 90
Fish goldfish 20 6-8 no 24-h LC50 ethylene oxide 90 Bridié et al.
(Carassius aeration 1,2-ethanediol > 5000 (1979a)d
auratus) (> 4
mg/litre)
Fish brine shrimp 24 7.0 minimal 48-h LC50 ethylene oxide 745 Conway et al.
(Artemia aeration 1,2-ethanediol > 20 000 (1983)e
salina) 2-chloroethanol 680
-----------------------------------------------------------------------------------------------------------
a All tests were static. Water analysis for the substance under test was reported by all authors.
b Incubation on a shaker for 16 h.
c Medium was fresh water, reconstituted using dechlorinated, carbon-treated tap water; 10 fish per
concentration.
d Medium was local tap water; 6 fish per concentration.
e Medium was sea water, reconstituted using dechlorinated, carbon-treated tap water, 30 - 50 shrimps per
concentration.
7. EFFECTS ON ANIMALS
7.1. Acute Exposures
7.1.1. Oral, intravenous, and inhalation studies
The LD50s for ethylene oxide, administered orally and dissolved
in water, were 330 mg/kg body weight for male rats and 280 and
365 mg/kg body weight for female and male mice, respectively (Smyth
et al., 1941; Woodard & Woodard, 1971). After inhalation, the 4-h
LC50s were 1500 and 1730 mg/m3 for mouse and dog, respectively, and
2630 mg/m3 for rat (Jacobson et al., 1956). 1,2-Ethanediol, a
metabolite, is less toxic: LD50s for rat were above 10 000 mg/kg
body weight, after oral administration, and 5210 mg/kg body weight,
after intravenous administration (Woodard & Woodard, 1971).
The slope of the dose-response curve in relation to the
mortality rate for ethylene oxide was steep. After oral
administration to rats, the difference between 0.1% mortality
(325 mg/kg) and 99.9% mortality (975 mg/kg) was approximately
650 mg/kg body weight (Smyth et al., 1941). After inhalation for
4 h, this difference was approximately 3000 mg/m3, in mice, and
approximately 5000 mg/m3 in rats. No deaths occurred in dogs at
1280 mg/m3 (Jacobson et al., 1956). While no guinea-pigs died
after inhalation of 450 mg ethylene oxide/m3 air for 8 h, the
majority did so at 2400 mg/m3 (Waite et al., 1930). In the above
mortality studies, the lungs and nervous system were the main
targets in rodents and dogs. In dynamic inhalation exposure
studies on guinea-pigs (Waite et al., 1930), rats, mice, and dogs
(Jacobson et al., 1956), nasal irritation was the first clinical
effect, as evidenced by scratching the nose, nasal discharge,
lachrymation, and salivation. Respiratory problems occurred
ranging from gasping to laboured breathing. Dogs exhibited
laboured breathing, vomited, and suffered convulsions. Guinea-
pigs, exposed to a concentration of 13 000 mg ethylene oxide/m3,
for 2.5 h, were found lying on their sides, unable to stand, and
quiet. Gross pathological changes in animals that did not survive
included moderate congestion in the lungs of dogs, minor patchy
oedema in the lungs of rats, and congestion with oedema in the
lungs of guinea-pigs. In rats, moderate congestion with petecchial
haemorrhage of the trachea was also observed. Lobular pneumonia
and hyperaemia of the liver and kidneys were observed in guinea-
pigs. Parenchymatous changes in the kidney of guinea-pigs were
seen at 2300 mg/m3.
Ataxia, prostration, laboured breathing, and occasional tonic
convulsions were effects shown by rats and mice at lethal oral or
intravenous doses of ethylene oxide (Woodard & Woodard, 1971).
Vomiting was the only effect shown by dogs that had received 25 or
75 mg ethylene oxide/kg body weight intravenously (Martis et al.,
1982).
In order to investigate the effects of residues of ethylene
oxide or reaction products in sterilized medical equipment, rabbits
were exposed for 2 h via polyvinyl chloride endotracheal tubes
containing 0, 80, or 600 mg ethylene oxide/kg material. There were
no deaths, but rabbits receiving the tubes with the highest
residues showed increased incidences of hyperaemia, oedema,
leukocyte infiltration, and epithelial erosion of the larynx and
trachea (Star et al., 1980). Two groups of nine dogs each were
exposed through extracorporeal perfusion for 40 min via a polyvinyl
chloride oxygenator containing 12 g ethylene oxide/kg material.
Nine dogs with an interrupted pulmonary circulation died from shock
and pulmonary oedema. Six out of 9 dogs with a preserved pulmonary
circulation died from pulmonary distress. A group of 9 control
dogs was treated in the same manner using a steam-sterilized
oxygenator. Only 1 control dog died (Stanley et al., 1971).
7.1.2. Acute effects on eyes and skin
As noted above, ethylene oxide is an irritating agent for
several different species. A maximum non-damaging concentration of
0.1% ethylene oxide in balanced physiological salt solution
(prepared daily and kept at 0 °C) was established after
instillation of 0.05 ml solution, every 10 min for 6 h, into the
conjunctival sac of rabbits. The concentrations above 1% caused
reversible changes in conjunctiva such as hyperaemia and swelling,
and irreversible opacity, both in the cornea and in the lens.
Possible reaction products, 2-chloroethanol and 1,2-ethanediol,
were less irritating to the eye (McDonald et al., 1973). The
results of in vitro tests with isolated rabbit cornea were in
agreement with the results of these studies. In the in vitro
tests, the endothelia were perfused for 1 - 3 h with a balanced
salt solution containing 250 mg ethylene oxide, 2250 mg
2-chloroethanol, or 5000 mg 1,2-ethanediol/litre. No effects were
observed on corneal thickness and cellular ultrastructure
(Edelhauser et al., 1983).
Skin irritation with hyperaemia, oedema, and scar formation was
observed from 6 min after application of pads of cotton, moistened
with solutions of 100 or 500 g ethylene oxide/litre water, on the
shaved skin of rabbits, under a plastic cover. The intensity of
the response was reported to be roughly proportional to the length
of exposure time (1 - 60 min) and the concentration (Hollingsworth
et al., 1956).
According to Hine & Rowe (1981), liquid ethylene oxide is
apparently without adverse effects on rabbit and human skin, on
single mild exposures, if the material evaporates rapidly. If
large amounts of material are involved, evaporation may cause
sufficient cooling to cause a lesion similar to frost-bite.
7.2. Short-Term Studies
7.2.1. Inhalation exposure
Groups of 10 - 20 Wistar rats per sex, 8 guinea-pigs per sex,
1 - 2 rabbits per sex, and 1 - 2 female rhesus monkeys were each
exposed to concentrations of ethylene oxide at levels of 0, 90,
200, 370, 640, or 1510 mg/m3, for 7 h per day, and 5 days per week.
The female monkeys were not tested at 90 mg/m3, and an additional 3
male monkeys were tested at 640 mg/m3. The test period varied with
the species tested, and the severity of exposure, i.e.,
approximately 26 weeks at 90 mg/m3, 25 - 32 weeks at 200 and
370 mg/m3, 7 - 25 weeks at 640 mg/m3, and 10 days at 1510 mg/m3.
Guinea-pigs, rabbits, and monkeys tolerated 90 and 200 mg/m3, and
rats tolerated exposure to 90 mg/m3 without adverse effects on
general appearance, behaviour, mortality rate, growth, body and
organ weight, gross- and histopathology. Rats showed elevated
mortality rates from 370 mg/m3, rabbits from 640 mg/m3, and all
exposed animals died at 1510 mg/m3. Secondary respiratory
infection caused the deaths of an appreciable number of rats and
mice in these studies.
Surviving rats showed increased relative lung weights after
26 - 27 weeks at 200 and 370 mg/m3. At 370 mg/m3, haemorrhages,
hyperaemia, emphysema, and local alveolar collapse were observed in
these lungs. Lungs of male rabbits also showed hyperaemia and
slight oedema at 370 mg/m3. Even more severe lung injury was seen
in rats at 640 mg/m3 and the higher exposure. Gross respiratory
tract irritation was apparent in all species at 1510 mg/m3.
Delayed reversible effects were observed on the peripheral
nervous system. Monkeys and rabbits exhibited paralysis of the
hind legs at 370 mg/m3 and, together with rats, at 640 mg/m3. This
was accompanied by atrophy of the muscles of the hind legs, except
in rabbits at 370 mg/m3. The effects on the peripheral nervous
system were investigated further in monkeys, and loss of both
sensory and motor function was noted at levels of 370 and
640 mg/m3.
Significant increases in body weight were also observed in
rats, at levels of 200 mg/m3 or more. Rats showed slight but
significant increases in the relative weights of kidney and liver
at 370 mg/m3 (Hollingsworth et al. 1956).
The findings of Jacobson et al. (1956) are in agreement with
these results. Groups, comprising 20 male rats and 30 female mice
each, were exposed to concentrations of ethylene oxide at levels of
0, 180, or 730 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted 26 weeks at 180 mg/m3 and 6 weeks at 730 mg/m3.
Additional groups of 15 rats and mice at the higher, and 60 rats
and mice at the lower, exposure level were used for interim gross
pathology. No clear toxic effects were reported at 180 mg/m3. No
pathological changes were observed except for marked haemosiderosis
in the spleen of a few rats at 730 mg/m3. The highest exposure
(730 mg/m3) resulted in death for both species without clinical
signs in mice. Effects on the respiratory and nervous system were
shown by rats as laboured breathing, reddish nasal discharge,
diarrhoea, tendency towards a side position, and dragging of the
hind-quarters. Rats also lost weight, which was regained by
survivors.
More recently, groups of 30 B6C3F1 mice of each sex were
exposed to concentrations of ethylene oxide (purity 99.9%) at 0,
18, 86, 187, or 425 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted for 10 weeks for males and 11 weeks for females.
No effects were observed in relation to survival, body weight,
clinical signs, white blood cell count, serum clinical chemistry,
urinalysis, and histopathology. At the highest exposure level,
changes at terminal sacrifice included an increased relative liver
weight in female mice, and a decreased testicular weight in males.
A decreased relative spleen weight was observed at 187 and
425 mg/m3 in both sexes. In addition, the red blood cell count,
the packed cell volume, and the haemoglobin concentrations were
decreased at 425 mg/m3. Screening of neuromuscular function in
groups of 5 female mice, in week 6, and 5 mice of both sexes, in
week 10 or 11, revealed altered reflex responses at 425 mg/m3 and a
dose-related trend in alterations of locomotor function from
86 mg/m3 upwards (Snellings et al., 1984a).
Groups of 3 male beagle dogs each were exposed to
concentrations of ethylene oxide (purity 99.7%) of 180 and
530 mg/m3, for 1 - 3 days. No effects were observed on mortality
rate, body weight, electrocardiogram, blood-calcium and -urea,
icteric index, and rectal temperature. Anaemia was noted at both
exposure levels. Effects on the respiratory and nervous systems
were shown at 530 mg/m3, such as hyperaemia and local alveolar
collapse in lungs, vomiting, and occasional slight tremors and
transient weakness in the hind legs. Muscular atrophy was also
observed (Jacobson et al., 1956). No haematological changes were
noted when groups each comprising 3 male New Zealand rabbits were
exposed repeatedly for 12 weeks to 0, 18, 90, or 450 mg/m3 (Yager &
Benz, 1982). The white cell count was depressed in Fischer rats
exposed in groups of 3 or 4, for 3 days, 6 h per day, to 90, 270,
or 810 mg/m3. There was a poor correlation with exposure level
(Kligerman et al., 1983).
The possible neurotoxic effects of ethylene oxide noted in the
above studies were investigated further in groups each comprising
12 male cynomolgus monkeys. These animals were exposed to 0, 90,
or 180 mg ethylene oxide/m3 (purity 99.7%), for 7 h per day, 5 days
per week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related lesions found were in the medulla oblongata of the brain.
Axonal dystrophy was found in the nucleus gracilis, primarily in
the exposed groups. Demyelination of the terminal axons of the
fasciculus gracilis occurred in one monkey at each exposure level,
but not in the controls (Sprinz et al., 1982). Paralysis of the
hind limbs was observed in monkeys repeatedly exposed for up to
32 weeks to 370 mg/m3, for 7 h per day, 5 days per week
(Hollingsworth et al., 1956).
7.2.2. Oral exposure
Groups of 5 Wistar rats each received, by gavage, 22 doses of
3, 10, or 30 mg ethylene oxide/kg body weight in 30 days and 15
doses of 100 mg/kg in 21 days. The vehicle was olive oil. There
were 10 vehicle controls.
No effects on mortality rate, growth, haematology, blood urea-
nitrogen, organ weights, gross- and histopathology were reported at
the 3 lower dose levels. At 100 mg/kg, there was marked loss in
body weight, gastric irritation, and slight (not further specified)
liver damage (Hollingsworth et al., 1956).
7.3. Long-Term Inhalation Studies
In a combined toxicity-carcinogenicity study, groups of 120
male and 120 female Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 18 mg/m3 (10 ppm), 58 mg/m3
(32 ppm), and 173 mg/m3 (96 ppm), for 6 h per day, 5 days per week,
over 25 months. Two control groups each comprising 120 male and
120 female rats were used. There was an exposure-free period of 2
weeks in month 15, because of infection with sialoacryoadenitis
virus. Interim sacrfices occurred at 6, 12, and 18 months.
The mortality rates of male and female rats increased
significantly from the 22nd or 23rd month, at the highest exposure,
with a trend towards an increase at a level of 58 mg/m3. Body
weights in both sexes were depressed at 173 mg/m3, from the end of
the first week onwards, until the end of the study. At 58 mg/m3,
the body weights of female rats were decreased between week 10 and
80. In females, the relative liver weights were increased in the
18th month at 173 mg/m3. This effect on the liver could not be
related to increases in the activities of serum alkaline
phosphatase (EC 3.1.3.1), aspartate aminotransferase (EC 2.6.1.1),
or alanine aminotransferase (EC 2.6.1.2), found mainly at the 2
highest exposures during interim sacrifices. Relative spleen
weights were increased in rats that developed leukaemia (section
7.4.1). Haematological changes were found in rats at all doses,
but mainly at the end of the study in animals exposed to 173 mg/m3;
these included an elevated leukocyte count in both sexes, and a
depressed red blood cell count and haemoglobin value in females.
Some of these rats had leukaemia.
Non-neoplastic histopathological changes observed included an
elevated frequency of focal fatty metamorphosis of the adrenal
cortices in both sexes and bone marrow hyperplasia in females at
173 mg/m3. Although no effect was observed on the hind-quarter
lift reflex, examined monthly, mild skeletal muscular atrophy was
observed, after 2 years of exposure to 173 mg/m3. Observations on
general health and ophthalmology did not reveal anything abnormal.
Neoplastic changes are reported in section 7.4.1 (Snellings et al.,
1981, 1984b).
In another toxicity-carcinogenicity study (Lynch et al.,
1984a), groups of 80 male Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 92 mg/m3 (51 ppm) and 182 mg/m3
(101 ppm), for 7 h per day, 5 days per week, over 2 years. The
control group also comprised 80 rats. There was an exposure-free
period of 2 weeks in month 16 because of a pulmonary infection,
which contributed to the mortality rate.
The mortality rate increased at both exposure levels, the
increase being significant at 182 mg/m3. Only 19% of the rats
survived 2 years of exposure at 182 mg/m3 compared with 49% in the
unexposed group. Body weights were reduced from the 3rd or 4th
month onwards. The relative weights of adrenals and brain were
increased at both exposure levels. The relative weights of lung
and kidney were increased at 92 mg/m3. Serum aspartate
aminotransferase activity was increased in rats exposed to 92 and
182 mg/m3 (section 7.4.1). No other changes were found in
haematology or clinical chemistry.
Non-neoplastic histopathological changes included an elevated
incidence of vacuolization and hyperplasia or hypertrophy in the
adrenals at both exposure levels, and of atrophy and degeneration
of skeletal muscle fibres at 182 mg/m3. There were also increased
incidences of inflammatory lesions of the lungs, nasal cavities,
trachea, and internal ear at both exposure levels. Eye cataracts
developed in 9 out of 78 rats at 182 mg/m3, 3 out of 79 in the
92 mg/m3 group, and 2 out of 77 in the controls.
7.4. Carcinogenicity
7.4.1. Inhalation exposure
In the studies by Snellings et al. (1981, 1984b) (section 7.3)
(Table 4), several neoplasms were induced by ethylene oxide. A
dose-related increased incidence of mononuclear cell leukaemia was
found in both sexes, significant at the 2 highest exposures in
females, from the 18th or 19th month onwards. Trend test revealed
a treatment-related response in both sexes. In males, an increased
incidence of peritoneal mesotheliomas originating from the
testicular mesothelium, occurred at 58 and 173 mg/m3 from the 23rd
month onwards, and an increased incidence of subcutaneous fibroma
was seen in male rats exposed to 173 mg/m3 that had survived for 24
months. Trend analysis showed that there was a treatment-related
increase in peritoneal mesothelioma. There was no increased
incidence of pituitary tumours, but they appeared earlier in the
173 mg/m3 group.
Following the report by Lynch et al. (1984a) of an increased
incidence of brain tumours in Fischer 344 rats exposed to ethylene
oxide (see below), the brain tissue from this study was re-examined
both macro- and microscopically, and a dose-related incidence of
primary brain tumours was observed at 58 and 173 mg/m3 that
appeared to be treatment related in the trend test, but was not
statistically significant. The tumours were mainly diagnosed as
gliomas and malignant reticular tumours. The percentage of rats
with multiple neoplasms was greater than in controls at all
exposure levels in females and at 173 mg/m3 in males. At 58 and
173 mg/m3, the percentage of female rats with at least one
malignancy was increased. The authors considered that a
contribution of the viral outbreak to the toxicity of ethylene
oxide was unlikely (Snellings et al., 1981, 1984b).
Lynch et al. (1984a) (section 7.3, Table 4) also found an
increased incidence of mononuclear cell leukaemia, which was
significant at the lower exposure level. The absence of a dose-
relationship was attributed to the increased mortality rate at 182
mg/m3. Dose-related increased incidences of peritoneal
mesotheliomas, originating from the testicular mesothelium, and of
mixed-cell gliomas in the brain, were found. The increases in both
tumours were significant at 182 mg/m3 (Lynch et al., 1984a).
Table 4. Tumours induced by ethylene oxide in Fischer 344 rats
------------------------------------------------------------------------------
Concen- Leukaemia Meso- Pituitary Subcutaneous Braina
tration (mononuclear) thelioma adenoma fibromaa
(mg/m3)
------------------------------------------------------------------------------
M F M M F M M F
Snellings et al. (1981, 1984b)
173 26(119)b 28(113) 22(119) 27(117) 32(117) 11(30) 3(30) 2(26)
58 25(81) 24(79) 7(91) 16(79) 38(90) 1(39) 1(39) 2(48)
18 21(79) 14(77) 3(89) 27(80) 39(90) 3(51) 0(51) 0
0 20(116) 9(118) 2(114) 28(117) 38(119) 1(48) 1(48) 0
0 18(118) 13(117) 2(116) 22(117) 38(116) 2(49) 0(49) 0
Lynch et al. (1984a)c
182 30(76) NA 21(79) 21(67) NA NR 5(79) NA
92 38(79) NA 9(79) 20(66) NA NR 2(77) NA
0 24(77) NA 3(78) 48(73) NA NR 0(76) NA
------------------------------------------------------------------------------
a Only animals that survived for 24 months were included, because these
tumours appeared after the 18-month interim sacrifice.
b Numbers in brackets refer to the number of rats examined. They include
interim and final kills.
c Only male rats were used.
NA = Not applicable.
NR = Not reported.
7.4.2. Oral exposure
Groups of 50 female Sprague Dawley rats received, in salad oil,
7.5 or 30.0 mg ethylene oxide/kg body weight, by gavage, in the
empty stomach, twice a week, for 110 weeks. There were 50 vehicle
controls, 50 untreated controls, and 50 positive controls. The
rats were observed for their life span. No statistical analysis
was reported. The mean survival period was over 100 weeks for all
groups. The mortality rate increased at 30.0 mg/kg body weight,
from week 100 onward. Elevated incidences of tumours were only
observed in the forestomach, the first tumour appearing in week 79.
The incidences of squamous cell carcinomas were 0/50, 8/50, and
29/50 at 0, 7.5, and 30 mg/kg body weight, respectively. At
30 mg/kg body weight, invasive growth and metastases were observed
in 10 rats. At 30 mg/kg body weight, 2 fibrosarcomas were also
noted. At both doses, the incidences of hyperplasia,
hyperkeratosis, papillomas, and/or carcinomas were increased in the
forestomach (Dunkelberg, 1982).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice were injected once a week with a
tricaprylin solution containing 0.1, 0.3, or 1.0 mg ethylene oxide
per animal, for 106 weeks. There were 200 vehicle controls and 200
untreated controls. From week 35 to week 85, the mortality rate
increased by a maximum of 10% at a dose of 1.0 mg per mouse. The
mean length of survival in this group was 75 weeks. An elevated
incidence of tumours was only observed at the injection site, the
first tumour appearing in week 79. There was a dose-related
increased incidence of sarcomas, mainly fibrosarcomas, which was
significant at 0.3 and 1.0 mg per mouse. The tumour incidence was
11% at the highest dose compared with 2% in vehicle controls
(Dunkelberg, 1981).
7.4.4. Dermal exposure
Each of a group of 30 female Swiss Millerton mice received, for
their lifetime, approximately 100 mg of a 10% solution of ethylene
oxide (purity 99.7%) in acetone, brushed on the clipped dorsal
uncovered skin, 3 times a week. A group of 60 mice did not receive
any treatment, and a group of 60 mice received the vehicle only.
No skin tumours were found, nor was there any sign of skin
irritation. The median length of survival was 493 days for treated
mice and 445 days for controls (Van Duuren et al., 1965). It is
assumed that ethylene oxide, applied in this manner, evaporated
rapidly from the skin.
7.5. Mutagenicity and Related End-Points
Almost all the reports available demonstrate the mutagenic
action of ethylene oxide. A summary of mutagenicity tests with
positive results is presented in Table 5.
Ethylene oxide is an alkylating agent (section 5.2). It has
induced gene mutations in all plants, bacteria, fungi, insects, and
mammalian cells investigated in vitro, with and without metabolic
activation. Chromosome damage and sister chromatid exchanges were
observed in plants, insects, and mammalian somatic cells exposed in
vivo and in vitro. Fomenko & Strekalova (1973) and Strekalova et
al. (1975) reported an increased incidence of chromosomal
aberrations in the bone-marrow cells of rats exposed by inhalation
to concentrations of ethylene oxide vapour at 3.6 and 112 mg/m3.
Unscheduled DNA synthesis, induced by the N-acetoxy-2-acetylamino-
fluorene, was inhibited by ethylene oxide in human lymphocytes in
vitro (Pero et al., 1981). The positive results in the
micronucleus tests are in agreement with those from a distribution
study showing that ethylene oxide, or its metabolites, was retained
in the bone marrow of mice (Appelgren et al., 1977). An increased
incidence of micronuclei was observed after one intraperitoneal
dose of 100 mg/kg body weight in mice (Lyarskii et al., 1983) and
after 4 h of vapour exposure of rats to a concentration of 90 mg/m3
(Embree & Hine, 1975).
Table 5. Mutagenic tests for ethylene oxide with positive resultsa
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Gene mutations
Forward mutations plant barley Ehrenberg et al. (1956,
1959)
Forward mutations barley Shulovsk et al. (1969)
Forward mutations rice Jana & Roy (1975)
Forward mutations pea Blixt et al. (1963)
Reverse mutations bacterium Escherichia coli Hussain & Osterman-Golkar
SD-4 (1984)
Reverse mutations Salmonella typhimurium Rannug et al. (1976);
(base-pair TA1535, TA100 Kauhanen (1978)b;
substitutions) Pfeiffer & Dunkelberg
(1980)
Reverse mutations Bacillus subtilis Tanooka (1979)
(spores) HA101, TKJ5211,
TKJ8201
Reverse mutations fungus Neurospora crassa Kolmark & Westergaard
(macroconidia) (1953); Kolmark & Kilby
(1968)
Forward mutations Aspergillus nidulans Morpurgo (1963)
Forward mutations Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked insect Drosophila melanogaster Bird (1952); Nakao &
recessive lethals Auerbach (1961)
Forward mutations mammal Chinese hamster Tan et al. (1981)b;
on specific locus ( in vitro) ovary cells Zamora et al. (1983)
Chromosome damage
breaks, erosions, plant Tradescantia paludosa Smith & Lofty (1954)
contractions (pollen)
translocations barley Ehrenberg et al. (1956,
1959)
breaks barley Moutschen-Dahmen et al.
(1968)
breaks wheat (hexaploid) MacKey (1968)
translocations insect Drosophila melanogaster Nakao & Auerbach (1961)
small deletions insect Drosophila melanogaster Fahmy & Fahmy (1970)
(min)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Chromosome damage (contd.)
breaks, human anion cells Poirier & Papadopoulo
gaps, exchanges, ( in vitro) (1982)
complexes
sister chromatide human lymphocytes Star (1980a);
exchanges ( in vitro) fibroblasts Garry et al. (1982)
rat lymphocytes Kligerman et al. (1983)
(inhalation)
rabbit lymphocytes Yager & Benz (1982)
(inhalation)
sister chromatide monkey lymphocytes Lynch et al. (1984b)d
exchanges, (inhalation)
breaks, acentric
fragments,
dicentrics,
triradials,
quadriradials,
complex
rearrangements
chromosomal rat bone marrow cells Strekalova et al. (1975)
aberrations (inhalation)
breaks, gaps, rat bone marrow cells Embree & Hine (1975)
rearrangement, (inhalation)
exchanges,
ring formations
micronuclei mouse (ip) polychromatic Conan et al. (1979);
erythrocytes Jenssen & Ramel (1980);
Lyarskii et al. (1983)
micronuclei mouse (iv) polychromatic Appelgren et al. (1978)
erythrocytes
heritable mouse (ip) germ cells Generoso et al. (1980)
translocations
dominant lethals mouse germ cells Cumming & Michaud (1979)
(inhalation)
mouse (ip) Generoso et al. (1980);
Lyarskii et al. (1983);
mouse Generoso et al. (1983)
(inhalation)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
dominant lethals rat Embree et al. (1977)
(contd.) (inhalation)
DNA repair
unscheduled mouse germ cells Cumming & Michaud (1979)
DNA synthesis (inhalation)
unscheduled human lymphocytes Pero et al. (1981)
DNA synthesis ( in vitro)
----------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b A similar effect after metabolic activation by rat liver microsomal fraction.
c A slight reduction in mutagenicity after metabolic activation by mouse liver
microsomal fraction.
d 2-year exposure groups of 12 male monkeys to 0, 90, and 180 mg/m3 for 7 h per day and
5 days per week.
Dose-related damage to germ cells was established in the mid
and late spermatid stages in the dominant lethal assay. One oral
dose of 100 mg/kg body weight in mice generated inconsistent
results (Appelgren et al., 1977), but 150 mg/kg proved positive
(Generoso et al., 1980). After short-term repeated exposures,
dominant lethals were induced in mice at intraperitoneal doses from
40 mg/kg body weight, given over a period of 3 months, 5 times per
week (Lyarskii et al., 1983) and at vapour exposures from
460 mg/m3, for 6 h/day, 5 days per week, over 11 weeks (Generoso et
al., 1983). Heritable translocations were induced in the germ
cells of mice after repeated intraperitoneal exposure, at doses of
30 mg/kg body weight or more, for 5 days/week, over a 5-week period
(Generoso et al., 1980). Recent studies have investigated the
dose-response of inhaled ethylene oxide and have compared effects
of different dose rates (contributions of different concentrations
and durations of exposure, maintaining total exposure (C x t)
constant) on the dominant-lethal response in male mice. In the
dose-reponse study, male mice were exposed by inhalation to
ethylene oxide at concentrations of 540, 720, or 900 mg/m3 (300,
400, or 500 ppm), respectively. Exposures were for 6 h/day, for 4
consecutive days. A dose-related increase in dominant-lethal
mutations was observed; however, the dose-response curve was
nonlinear, i.e., increasing embryonic mortality occurred with
increasing mg/m3 x h. In the dose-rate study, mice had a total
exposure of 3240 mg/m3 (1800 ppm) x h/day for 4 consecutive days,
delivered either at 540 mg/m3 (300 ppm) in 6 h, 1080 mg/m3 in 3 h,
or 2160 mg/m3 (1200 ppm) in 1.5 h. The highest airborne
concentration resulted in the greatest embryonic mortality, 64%,
versus 32 and 11% for the intermediate and lowest airborne
concentrations, respectively (Generoso et al., 1985). According to
an abstract, DNA repair was induced in the germ cells of mice
exposed to 540 mg/m3, for 8 h. The repair seemed inhibited at
higher exposures (Cumming & Michaud, 1979). No details were
available.
Negative results were observed on a few occasions only. In one
study, with vapour-exposed rats, chromosome aberrations or slowing
of mitotic activity and cell cycle kinetics were not observed in
lymphocytes at levels at which sister chromatid exchanges occurred
(Kligerman et al., 1983). In a dominant-lethal assay with mice,
relatively high intravenous doses of ethylene oxide (up to
100 mg/kg body weight) did not cause any treatment-related effects
(Appelgren et al., 1977). An intraperitoneal dose of 10 mg/kg body
weight to mice was reported to give a slight increase in the number
of polychromatic erythrocytes with micronuclei, but the statistical
analysis was not adequate (Conan et al., 1979).
7.6. Effects on Reproduction
Rats and guinea-pigs were exposed to vapour concentrations of
370 and 640 mg ethylene oxide/m3, for 7 h per day, 5 days per week,
for up to 32 weeks. Among other effects (section 7.2.1),
degeneration of testes tubules was observed at the higher exposure
level in guinea-pigs, while at 370 mg/m3, there was a decrease in
the relative weights of testes in rats and guinea-pigs, which was
not statistically significant (Hollingsworth et al., 1956).
Significantly-decreased absolute testicular weights were observed
in mice exposed to ethylene oxide at a concentration of 425 mg/m3,
for 6 h/day, 5 days per week, over 10 - 11 weeks (Snellings et al.,
1984a). However, the testicular effects may have been secondary to
toxic effects (e.g., growth inhibition). Male and female Fischer
344 rats exposed repeatedly to concentrations of ethylene oxide of
up to 182 mg/m3, for 6 h/day, 5 days per week, over 25 months, did
not show any histopathological effects on the reproductive tissues
(Snellings et al., 1981).
When groups of 12 male Cynomolgus monkeys were exposed to
concentrations of ethylene oxide at 90 or 180 mg/m3, for 7 h/day,
5 days per week, over 2 years, spermatogenic functions were found
to differ from those of controls. At both exposure levels, sperm
motility and sperm count were decreased and the sperm drive range
was increased, but there was no increase in effect with increase in
dose. The incidence of abnormal sperm heads did not change (Lynch
et al., 1984c).
Groups, each comprising 30 male and 30 female Fischer 344 rats,
were exposed to concentrations of ethylene oxide (purity 99.9%) of
18, 58, or 173 mg/m3, for 6 h/day, 5 days per week, over 12 weeks.
Two control groups of 30 rats per sex each were exposed to air
only. After mating, females were further exposed for 7 days/week,
up to 3 weeks after delivery, with the exception of the first 5
days of lactation. Effects on the reproductive performance were
detected. The number of pups per litter was decreased at 173
mg/m3, as well as the number of implantation sites per female, and
the number of fetuses born per implantation site. The number of
females with a gestation period longer than 22 days was also
increased at this concentration, but no effects were noted on the
average length of the gestation period. Neither parents nor pups
showed signs of toxicity from ethylene oxide. The percentages of
pregnant females and fertile males were not affected (Snellings et
al., 1982a).
7.7. Teratogenicity
Groups of 22 female Fischer 344 rats were exposed to
concentrations of ethylene oxide of 18, 58, or 173 mg/m3, for
6 h/day, on days 6 - 15 of gestation. Two control groups
comprising 22 rats each were exposed to air only. The numbers of
pregnant dams ranged from 17 to 22. Maternal behaviour was normal,
and there were no deaths. The only effect on the fetuses was a 5 -
8% decrease in weight at 180 mg/m3 (Snellings et al., 1982b).
Groups of 32 - 45 female Sprague Dawley rats were exposed to
concentrations of ethylene oxide (purity 99.7%) of 0 or 270 mg/m3,
for 7 h/day, on days 7 - 16 of gestation (Group 1) or on days 1 -
16 of gestation (Group 2) or during 3 weeks before mating (5 days
per week), and on days 1 - 16 of gestation (Group 3). No dams
died, but body weights were decreased in Group 3. In all exposed
groups, the relative and absolute weights of kidney and spleen were
increased. The results of histopathological examination did not
show any abnormalities. There was a significant increase in
resorptions per litter and per implantation site in Group 3, with
no significant effects on the number of implants, live fetuses, and
pregnancies. In all exposed groups, weights and lengths of the
fetuses were decreased. Reduced ossification of sternebrae and
primary skull was observed (Hackett et al., 1982).
New Zealand rabbits were similarly exposed to a concentration
of ethylene oxide of 270 mg/m3 from days 1 - 19 or from days 7 - 19
of gestation. There was no evidence of toxicity in the mothers,
embryos, or fetuses, or of developmental defects (Hackett et al.,
1982).
Groups of 24 - 37 female CD-1 mice each received,
intraveneously, doses of 0, 75, or 150 mg ethylene oxide (purity
not stated)/kg body weight in an aqueous dextrose solution on days
4 - 6, 6 - 8, 8 - 10, or 10 - 12 of pregnancy. Dams exposed on
days 6 - 8 of pregnancy did not show toxic signs. In the other
groups, at the highest dose, toxic signs such as weakness, laboured
respiration, and tremor were observed with a mortality rate of 19 -
48%. In the group without signs of maternal toxicity, fetotoxicity
was observed at 150 mg/kg, as shown by a 20% decrease in mean fetal
weight. Fetal malformations were shown in 19.3% of fetuses in
exposed litters compared with 2% in control groups. These
malformations were mainly fused cervical arches. In addition,
fused thoracic arches, scrambled and fused sternebrae, and fused,
branched, or missing thoracic ribs were observed (Laborde & Kimmel,
1980).
8. EFFECTS ON MAN
8.1. Exposure of the Skin and Eyes
Undiluted ethylene oxide, applied to the skin of volunteers,
evaporated rapidly without leaving any mark or irritation. A
15-min exposure to cotton wool soaked in undiluted ethylene oxide
also did not produce any effects (Greaves Walker & Greeson, 1932).
However, with exposure to larger quantities, there may be
sufficient cooling to produce a lesion similar to frost-bite (Hine
& Rowe, 1981). Skin injury following exposure to aqueous solutions
of ethylene oxide is characterized by the appearance of oedema and
erythema, 1 - 5 h after exposure, followed by the formation of
vesicles. On healing, incrustation, often with itching and
desquamation, is observed. The magnitude of the skin injury seems
to depend on the length of contact and the concentration, a 50%
aqueous solution (500 g/litre) being most hazardous. More
concentrated solutions were less harmful. The lowest concentration
tested, a 1% solution, produced a mild reaction after a 50-min
exposure. Such effects have been observed in a number of accidents
(Sexton & Henson, 1949, 1950; Joyner, 1964; Ippen & Mathies, 1970).
Vapour exposure was found to produce the above dermal effects,
mainly on the humid parts of the skin (Ippen & Mathies, 1970). The
effects were also found, to different extents, after exposure via
ethylene oxide-sterilized materials such as face masks, gloves, and
surgical gowns (Royce & Moor, 1955; Marx et al., 1969; Hanifin,
1971; Biro et al., 1974; Lamy et al., 1974). Patch tests on
volunteers with various sterilized materials containing residues of
ethylene oxide and its reaction products, showed erythema, without
oedema, after 4 - 8 h of contact, from a residue level of 890 mg
ethylene oxide/kg up to 2890 mg/kg of a polyvinyl chloride block.
Most skin types tolerated residues of ethylene oxide of up to
2270 mg/kg polyvinyl chloride film, 2800 mg/kg brown-milled rubber,
and 5100 mg/kg non-woven fabric (Shupack et al., 1981).
Accidental skin exposure to a 1% aqueous solution, from the
waist down, was also reported to result in effects on the nervous
system, such as nausea and repeated vomiting (Sexton & Henson,
1949).
Accidental exposure of the eyes to the vapour of ethylene oxide
can lead to conjunctivitis (Thiess, 1963; Joyner, 1964). Exposure
of 12 men via a leaking sterilizer resulted in neurological
disorders (section 8.3) in 4 of the men, 3 of whom had eye
cataracts; one of the latter also showed an increase in corneal
thickness. Two additional men showed only an increase in corneal
thickness (Gross et al., 1979; Jay et al., 1982). In one case of
accidental exposure of the eyes to pure ethylene oxide, only slight
irritation of the conjunctiva was seen (Thiess, 1963).
The implantation of artificial lenses, sterilized with ethylene
oxide, in 103 eyes was compared with the implantation of lenses,
sterilized with sodium hydroxide (200 control eyes), in a
retrospective study. The follow-up period was 10 months for the
exposed patients. Post-operative inflammatory complications
occurred in 30% of the eyes exposed to residues of ethylene oxide
or its reaction products compared with 9% of the control eyes.
Cystoid macular oedema with reduction in visual acuity developed in
16% of the exposed eyes and in 7% of the control eyes (Stark et
al., 1980).
8.2. Sensitization
No dermal sensitization was observed in a total of 47 workers
frequently exposed to ethylene oxide (Royce & Moor, 1955; Thiess,
1963). In another study using patch tests, one of 12 volunteers
showed a recurrent reaction, 3 weeks after the trial. When
challenged afterwards with a 2 mm-thick patch of polyvinyl chloride
containing 100 mg ethylene oxide/kg, a mild reaction was observed,
which reappeared after 3 weeks (Shupack et al., 1981) (section
8.1). When the skin of 8 workers was exposed repeatedly to aqueous
solutions of ethylene oxide, all sites of previous contact, with
and without a primary reaction, flared up in 3 of them showing
pruritus, erythema, and slight oedema, 5 - 9 days after the last
exposure (Sexton & Henson, 1950). Another case of an apparently
allergic reaction was reported. The patient concerned was exposed
to ethylene oxide via a sterilized face mask (Alomar et al., 1981).
A case of anaphylaxis has been reported in a patient receiving
haemodialysis treatment with equipment that had been sterilized
with ethylene oxide (Poothullil et al., 1975). A cause-effect
relationship with ethylene oxide exposure was demonstrated by
haptan specificity (Dolovich & Bell, 1978).
8.3. Accidental Inhalation Exposure
Respiratory tract irritation was reported as hoarseness
(Thiess, 1963) and cough (Metz, 1939) in 5 cases of acute
accidental exposure to ethylene oxide vapour.
Acute effects on the nervous system in nearly all inhalation
cases were marked by nausea, recurrent vomiting, and headache.
Less frequently reported effects included decreased consciousness
(one case of coma), excitement, sleeplessness, muscular weakness,
diarrhoea, and abdominal discomfort (Blackwood & Erskine, 1938;
Metz, 1939; Thiess, 1963; Capellini & Ghezzi, 1965).
Because of a leaking sterilizer, 4 young men were exposed
intermittently, for 2 - 8 weeks, to ethylene oxide at levels of
approximately 1000 mg/m3. Three of the men developed a reversible
peripheral neuropathy showing abnormal nerve conduction and, in 2
cases, headache, weakness and decreased reflexes in the
extremities, incoordination, and a wide-based gait. The fourth man
developed a reversible acute encephalopathy with headache, nausea,
vomiting, lethargy, recurrent motor seizures, agitation, and a
diffusely slow electroencephalogram (Gross et al., 1979).
Following this, 6 more cases were reported concerning sterilizer
operators, suffering from reversible peripheral neuropathy
following ethylene oxide exposure for 0.5 - 1.5 years. Finelli et
al. (1983) described 3 persons showing subacute polyneuropathy with
bilateral foot-drop, slowing of nerve conduction velocity, and
denervation potential on electromyography as the main findings.
All 3 persons had noticed the smell of ethylene oxide regularly at
work, while 2 persons experienced eye irritation. Polyneuropathy
was also reported in 3 sterilizer operators by Kuzuhara et al.
(1983). Two of these cases were described in detail. Sural nerve
biopsies revealed axonal degeneration with mild changes in the
myeline sheath. Unmyelinated fibres were also involved. Muscle
biopsies showed typical denervation atrophy.
8.4. Other Accidental Exposures
Severe respiratory problems due to inflammatory reactions in
the trachea and larynx were reported in 17 hospital patients who
had received endotracheal intubation. The tubes had been
sterilized with ethylene oxide (Marx et al., 1969; Holley & Gildea,
1971; Lipton et al., 1971; Mantz et al., 1972).
Reversible vocal paralysis was reported to be associated with
ethylene oxide exposure in 5 cases: one woman had been exposed to
vapour (Troisi, 1965), and the other 4 patients were exposed via
sterilized endotracheal tubes (Holley & Gildea, 1971). The vocal
cords showed no or only slight damage. In one of these patients,
who died from a cause unrelated to the intubation, myelin
degeneration of parts of the nervus vagus was noted at autopsy. It
was suggested, therefore, that the paralysis was of neural origin.
Four cases of shock, 1 - 10 h after endovascular examination,
were associated with the catheters used, which contained residues
of ethylene oxide. The presence of bacterial toxins was considered
unlikely. One patient died as a result of renal insufficiency
(Lebrec et al., 1977). Cases of cardiovascular collapse in
children, 3 of which were fatal, were considered by the authors to
be the result of residues of ethylene oxide in a heart-lung machine
on the basis of subsequent studies on dogs (Stanley et al., 1971)
(section 7.1.1). Among others, Hirose et al. (1963) and Clarke et
al. (1966) measured haemolysis due to residues in ethylene oxide-
sterilized plastic tubes in vitro.
8.5. Occupational Inhalation Exposure
The health status of 37 male operators from an ethylene oxide-
producing plant in the USA during the period 1953 - 62 was compared
with that of age-matched operators from other production units.
The average employment period was 11 years for exposed workers and
12 years for controls. The usual average exposure level was
between 9 and 18 mg/m3, with occasional peaks up to 230 mg/m3 for
one particular job (collecting a sample of the product). According
to the medical records, the health of the exposed workers was
somewhat better than that of the controls. A physical examination
and extensive clinical tests did not reveal any exposure-related
effects with the exception of a slightly increased white blood cell
count (Joyner, 1964).
Chromosomal damage was found in a group of 12 workers from a
hospital sterilization facility in the USA (section 8.6). The
maximum exposure concentration measured during sterilization was
65 mg/m3. Another group of 12 persons, who worked in the adjacent
operating room area, volunteered as representatives of an unexposed
or accidentally exposed group. To insure adequate control
throughout the study, unexposed laboratory staff members served as
a third group. Frequently-reported subjective complaints indicated
irritation of the mouth, throat, and eyes, and effects on the
nervous system, such as headache, nausea, speech difficulty, memory
loss, dizziness, and incoordination (Garry et al., 1979).
In Belgium, a group of 18 workers, using or distributing the
sterilant ethylene oxide, was compared with a well-matched control
group by means of a questionnaire, and by analyses for urinary
retinol-binding protein and albumin, beta-microglobulin, and
chromosomal damage in lymphocytes. The overall mean exposure level
was 7.6 mg/m3, and the time-weighted average exposure, over a
working day, ranged between 0.2 and 95 mg/m3. A significant
increase in the incidence of sleeplessness and leg cramps was
recorded, but not irritation or allergy. These studies did not
reveal any abnormalities with the exception of an increase in
sister chromatid exchanges in lymphocytes (Wolfs et al., 1983;
Laurent et al., 1984; section 8.7).
In a plant in Bulgaria, 196 workers engaged in the production
of ethene and ethylene oxide were examined. About 73% of all
concentrations of ethylene oxide measured were 1 mg/m3 or less,
while 27% were between 1 mg/m3 and 3.5 mg/m3. Significant
increases were found in deviations of the autonomous nervous system
and in neurosis-like manifestations, especially in female workers
(Spasovski et al., 1980). Because of a mixed exposure, it is
difficult to evaluate the findings.
Haematological changes were reported in a group of 27 workers
in an ethylene oxide manufacturing and processing plant, in Sweden,
in 1967. The exposure period varied from 2 to 20 years, the
average length being 15 years. Controls worked with ethylene oxide
in other departments, where no leakages were possible. No exposure
data were reported. When 2 cases of anaemia were excluded, there
was still a significantly-decreased haemoglobin value in exposed
workers. There was a 30% increase in the number of lymphocytes,
and one case of chronic lymphatic leukaemia was noted (Ehrenberg &
Hällström, 1967).
In the Federal Republic of Germany, 279 employees from 8 plants
in which alkene oxides were produced or processed, were examined
for morbidity during 1978. They were employed for an average of
10.8 years. Of these workers, 21 had been involved in accidents
with ethylene oxide. Taking into account age and length of
exposure, they were compared with groups of industrial and clerical
workers within the same company. The exposure concentrations were
not reported. The workers were also exposed to many other
chemicals, some of which may be carcinogenic for man.
No abnormalities were found that could be related to exposure
to ethylene oxide or propylene oxide. The investigators related
increases in haemoglobin and mean erythrocyte volume to smoking
habits. Slight lymphocytosis was found to be unrelated to exposure
time, though there was a distinct age influence (Stocker & Thiess,
1979).
8.6. Mortality Studies
Two studies were conducted in Sweden to investigate the
possible neoplastic effects from occupational exposure to ethylene
oxide (Hogstedt et al., 1979a,b, 1984). The first study originally
included 58 male and 172 female workers in a small factory,
sterilizing hospital equipment with a 1:1 mixture of ethylene oxide
and methyl formate, over a period from 1968 to 1977 (Hogstedt et
al., 1979a).
Two cases of leukaemia (one was diagnosed as chronic myeloid
leukaemia and the other as acute myelogenous leukaemia) occurred
among 68 women who were exposed to vapours from sterilized boxes
stored for weekly periods in a factory storage hall where about 30
persons were exposed at any one time. A third case was the local
male manager who developed primary macroglobulinaemia (Morbus
Waldenström; this case was later diagnosed as a non-Hodgkin
lymphoma), 9 years after the installation of the sterilization
equipment; his exposure was estimated to be about 3 h per week in
the storage hall. He is also reported to have had some exposure to
benzene in the past. The concentration of ethylene oxide in the
hall was in the range of 3.6 - 128 mg/m3, and the 8-h time-weighted
average in the breathing zone was calculated to be between 36 ±
18 mg/m3. The other workers had occasional exposure to ethylene
oxide, and 7 operators had relatively high exposure (amount
unspecified) during the sterilization process.
In a follow-up study (Hogstedt et al., 1984), a further case of
leukaemia was found in a woman who had been exposed to ethylene
oxide in the storage hall between 1969 - 72. In this study, the
cohort consisted of 203 workers who had been employed for more than
one year at the plant.
Altogether, 4 deaths from malignancies of the lymphoreticular
system were reported from this factory. The expected number was
0.3.
A second study to investigate the carcinogenic effects of
ethylene oxide was conducted on 241 Swedish male workers in an
ethylene oxide-producing plant (Hogstedt et al., 1979b). These
men were examined medically in 1960. Twenty-three deaths
occurred during the 16-year observation period dating from
1961 - 77 (13.5 expected). The excess mortality was due to cancer
and cardiovascular disease. Three cases of stomach cancer (0.4
expected) and 2 cases of leukaemia (one chronic myeloid and once
acute myeloid leukaemia) (0.14 expected) accounted for the excess
mortality from cancer. No increase in mortality was observed among
86 maintenance workers exposed intermittently to ethylene oxide
among 66 unexposed controls. Average exposure levels during
1941 - 47 were estimated to be below 25 mg/m3 and, during the 1950s
up to 1963, these levels were 10 - 50 mg/m3, but peak exposures
above the odour threshold (about 1000 mg/m3) were known to occur.
The ethylene oxide was manufactured by the chlorohydrin process
so that significant exposure to other chemicals such as 1,2-
dichloroethane, ethylene, ethylene-chlorohydrin, and bis(2-
chloroethyl)ether might have occurred.
This investigation was followed up by a study that extended the
period of observation up to 1982. Seven more deaths had occurred
among the workers exposed to ethylene oxide for the whole of the
working day against 6.6 expected. Three of these were due to
cancer (1.6 expected). Two of these 3 cases were cancer of the
stomach (0.2 expected) and one an oesophageal cancer (0.04
expected). In the period from 1961 - 82, 6 deaths due to stomach
or oesophageal cancer had occurred in workers exposed to ethylene
oxide for the whole of the working day (0.7 expected). Alimentary
tract cancer was observed in 2 maintenance workers (0.8 expected)
and in 1 unexposed worker (0.8 expected). One new case of chronic
myeloid leukaemia was reported during this follow-up period.
During the 20-year period of observation, a total of 17 cases of
cancer were notified to the Cancer Registry against 7.9 expected
(Hogstedt et al., 1984).
The Task Group evaluated these data and concluded that the
evidence was adequate to consider the mixtures of compounds to
which these workers were exposed as carcinogenic for human beings,
but inadequate to label ethylene oxide as a proved human
carcinogen. This conclusion was in agreement with that arrived at
by an International Agency for Research on Cancer Working Group
(IARC, 1985).
A similar study in the USA concerned 767 male workers at an
ethylene oxide producing plant. They were employed for at least
5 years between January 1955 and December 1977 and "potentially
exposed". Concentrations of ethylene oxide were reported to be
below 18 mg/m3, but no further details concerning exposure levels
were reported. Exposure to other chemicals was not reported.
Control data came from national statistics and was adjusted for
sex, age, and calendar time. There were 46 deaths against an
expected 80; there were 11 malignant neoplasms against 15.2
expected. No statistically-significant excess deaths could be
found due to any cause. There were no deaths due to leukaemia, 3
deaths from pancreatic cancer (0.8 expected), 1 death from bladder
cancer (0.3 expected), 2 deaths from brain cancer (0.7 expected),
and 2 deaths from Hodgkin's disease (0.4 expected) (Morgan et al.,
1981).
In the Federal Republic of Germany, 602 workers were
investigated for mortality experience during the period 1928 - 80.
The workers had been employed for at least 6 months in 8 plants
producing or processing ethylene oxide and propylene oxide. A
subcohort of 351 workers was observed for more than 10 years.
Control data came from a styrene plant and from national
statistics. Since 1978, exposure to ethylene oxide had normally
remained below 9 mg/m3. In the past, occasional excursions above
90 mg/m3 (50 ppm) had been reported. On one occasion during plant
breakdown, 3420 mg/m3 (1900 ppm) was measured. No statements were
offered concerning human exposure or the use of personal protective
equipment. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. There were 14 deaths from
cancer compared with 16.6 expected. No statistically-significant
excess deaths could be found due to any cause in the cohort. In
the subcohort of 351 workers, there was a significant increase in
mortality rate due to kidney disease (3 against 0.4 expected).
There was 1 death from gall bladder cancer, 1 death from urinary
bladder cancer, 1 death from brain cancer, and 1 death from myeloid
leukaemia. Two stomach tumours were observed compared with 1.8
expected (Thiess et al., 1981a,b).
8.7. Mutagenicity and Related End-Points
An increase in chromosomal aberrations was found in the
lymphocytes of 3 groups of workers sterilizing medical equipment in
hospitals or factories (Abrahams, 1980; Pero et al., 1981; Högstedt
et al., 1983). A 50% increase in aberration rate was found in 28
workers exposed to 8-h time-weighted average concentrations of
ethylene oxide below 1.8 mg/m3 in the 2.5 years before the study.
Before this period, higher exposures were reported to have
occurred. The workers had been exposed for 0.5 - 8 years. The
mean number of micronuclei in the bone marrow cells of 18 of these
workers was 3 times higher than in the controls (Högstedt et al.,
1983). Pero et al. (1981) found that, while workers exposed to
concentrations of ethylene oxide between 0.9 and 1.8 mg/m3, for
40 h per week, did not show an increased aberration rate; others,
exposed to bursts of ethylene oxide at concentrations between 9 and
18 mg/m3, for 8 h per week, did. The Task Group noted that the
sterilization procedure involved the use of a 50:50 mixture of
ethylene oxide and methyl formate. At the same time, DNA repair,
induced in vitro by the mutagen N-acetoxy-2-acetylamino-fluorene,
was reversibly inhibited in the low exposure group compared with an
unexposed control group, but not affected in the other group. DNA
repair inhibition was positively correlated with duration of
exposure (Pero et al., 1981). In 43 male workers from the cohort
of 602 workers (section 8.5) (Thiess et al., 1981b), an increase in
chromosomal aberration rate was found that was significant for the
workers exposed for more than 20 years, but not for those
accidentally exposed or exposed for average periods of 12 and
17 years (Thiess et al., 1981a).
In another ethylene oxide manufacturing plant, no chromosomal
aberrations were detected in the lymphocytes of 36 male workers.
These men had been exposed for 1 - 14 years to average
concentrations of up to 0.28 mg/m3 (van Sittert et al., 1985).
The sister chromatid exchange rate in lymphocytes was not
increased in groups of 28 and 14 sterilization workers exposed to
8-h time-weighted averages, below 1.8 mg/m3 for 2.5 years before
the study (Högstedt et al., 1983) and below 8 mg/m3 (Hansen et al.,
1984), respectively. In the second study, peaks up to 1430 mg/m3
were also measured. Increases in sister chromatid exchange rate
were found in 4 other studies on sterilization workers (Garry et
al., 1979; Abrahams, 1980; Yager et al., 1983; Laurent et al.,
1984). In one of these studies, in which 75 workers were exposed
to levels generally below an 8-h time-weighted average of 90 mg/m3,
this increase was found in cells together with quadriradial
aberrations (Abrahams, 1980). In the other studies, groups were
small, exposure conditions often unclear, and the number of
metaphases scored, in some cases, limited. Yager et al. (1983)
only found an increased sister chromatid exchange rate at
relatively high calculated integrated doses of more than 100 mg per
person. The length of exposure to ethylene oxide averaged 3.6 min
per day; these tasks were performed between 6 and 120 times during
the 6-month study period. Laurent et al. (1984), studied sister
chromatid exchange rates in 2 groups of ethylene oxide-exposed
workers (estimated ethylene oxide dose in past 2 years, 530 -
715 mg and 1185 - 5800 mg, respectively) as well as unexposed
controls. Although the sister chromatid exchange rates in the
high-exposure group did not differ significantly from the rates in
the low-exposure groups (not adjusted for smoking), the difference
among non-smokers between the exposed (n = 20) and the controls
(n = 15) was significant.
In a study on 41 sterilization workers in 8 hospitals in Italy,
increases in both sister chromatid exchanges and in chromosomal
aberrations were detected in lymphocytes; these effects persisted
for months after exposure was reduced or interrupted. The workers
were exposed to average 8-h time-weighted averages of either
0.63 mg/m3 or 19.3 mg/m3 (section 4.3). A statistically-
significant correlation was found between sister chromatid exchange
frequency and the level of ethylene oxide, as well as a multiple
correlation between sister chromatid exchange frequency and
ethylene oxide exposure, smoking, and age (Sarto et al., 1984).
Similarly, in the USA, the sister chromatid exchange frequencies in
the lymphocytes of 61 sterilization workers involved in sterilizing
health-care products, were monitored over a period of 2 years and
compared with those of 82 unexposed controls. During the study
period, 8-h time-weighted-average exposures were reported to be
less than 1.8 mg/m3. Prior to the start of the study, 8-h time-
weighted averages between 0.9 and 36 mg/m3 were measured. Results
were adjusted for smoking habits, sex, and age. Workers exposed to
low levels of ethylene oxide such as those at a worksite with 8-h
time-weighted-average ethylene oxide levels below 1.8 mg/m3 prior
to and during the study, did not show increased frequencies of
sister chromatid exchange. Workers who had been exposed to levels
of 5 - 36 mg/m3, prior to the study, showed an increased frequency
of sister chromatid exchange that persisted for at least 24 months
after cessation of exposure (Stolley et al., 1984).
8.8. Effects on Reproduction
In a study from the USSR (Yakubova et al., 1976), the course of
pregnancy and birth was followed in 57 operators, 38 laboratory
workers, and 65 adminstrative staff in an ethylene oxide-producing
plant, the majority of the women being between 20 and 29 years of
age. A group of 50 pregnant women working outside the plant served
as additional controls. It was estimated that the operators were
exposed to ethylene oxide concentrations of 0.2 - 0.3 mg/m3 for 80%
of the working time and 1.0 mg/m3 for the remaining 20%, while the
laboratory workers were exposed only to the lower concentrations.
Pregnancy toxaemia in the latter half of pregnancy and other
complications were higher in the operators (14.7%) and laboratory
workers (9.9%) than in the administrative staff (4.6%) and outside
controls (8%). On the other hand, the primiparae among the
operators lost less blood perinatally than those among the other
groups. Spontaneous abortion occurred in 6 out of 57 (10.5%)
operators, 3 out of 38 (7.9%) laboratory workers, and in 5 out of
65 (7.7%) administrative staff. The operators were subjected to
the additional stress of high levels of noise and vibration and
wide variations in atmospheric temperatures.
Findings in this study do not indicate any unequivocal adverse
effect of ethylene oxide exposure at these concentrations on the
outcome of pregnancy.
An increase in spontaneous abortions was also found in a study
on Finnish hospital sterilizing staff in 1980, using questionnaires
and hospital discharge records. The sterilizing agents were
ethylene oxide, glutaraldehyde, and formaldehyde. Controls were
nursing auxiliaries, from various hospitals, who did not work in
sterilization, anaesthetization, or X-ray recording departments.
Results were adjusted for age, parity, decade of the reported
pregnancy, coffee and alcohol consumption, and smoking habits.
The rate of spontaneous abortions in the sterilization staff
members as a whole (9.7% in 1443 pregnancies) was similar to the
rate in the controls (10.5% in 1179 pregnancies). A significant
increase, however, was observed when the adjusted spontaneous
abortion rate in the sterilization staff who were exposed during
pregnancy (15.1% in 545 pregnancies) was compared with the rate in
the staff members who were not exposed during pregnancy (4.6% in
605 pregnancies). On the basis of a separate study, the time-
weighted average exposure concentration was estimated to be in the
range of 0.18 - 0.90 mg/m3 (0.1 - 0.5 ppm), with peak concentrations
up to 450 mg/m3 (250 ppm). It was considered by the authors that
exposure to ethylene oxide accounted for most of the excess
spontaneous abortions (Hemminki et al., 1982).
In a new analysis of the data, controls were chosen from the
same hospitals, and only pregnancies that started during hospital
employment were analysed in all groups. The spontaneous abortion
rate was still highest for the pregnancies during which exposure to
ethylene oxide took place (20.4%), and the difference compared with
controls (11.3%) was significant. The abortion rate of the group
exposed to glutaraldehyde alone (16.6%) was also significantly
elevated (Hemminki et al., 1983).
Despite any methodological shortcomings of this reproductive
study, such as (a) the statement by Hemminki in 1983 that there was
limited exposure data, which prevented a comparison between
abortion rates and defined exposure levels; and (b) the fact that
the ethylene oxide-exposed and unexposed cohorts were not balanced
with regard to the incidence of prior abortions, there is a
suggestion of an association between ethylene oxide exposure and
adverse pregnancy outcome.
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
Human exposure is mainly through inhalation of the vapour.
Residues in medical equipment that has been sterilized with
ethylene oxide and not sufficiently aerated can migrate into
tissues and blood, producing primarily local effects (section 8.4).
Oral ingestion of ethylene oxide residues in most fumigated or
sterilized foodstuffs is unlikely, as they disappear rapidly
through evaporation or reaction with food constituents. A major
conversion product in foodstuffs is 2-chloroethanol, which is more
persistent than ethylene oxide (section 4.2.1).
Most ethylene oxide is used in the chemical plant in which it
is produced. Because of the explosion hazard, ethylene oxide is
stored and handled in chemical process plants in closed, automated
systems. This equipment is often located outdoors, and, except
during maintenance, workers have a minimal chance of exposure. Air
samples collected in processing areas of chemical production plants
have shown that ethylene oxide vapour concentrations are generally
less than 4 mg/m3 with occasional high peak exposures (section
4.3). Occupational exposure to ethylene oxide tends to be much
higher in health instrument manufacture and in hospitals than in
the chemical processing industries. Ethylene oxide concentrations
near malfunctioning or improperly designed equipment may reach
hundreds of mg/m3 of air for brief periods. However, 8-h time-
weighted average breathing-zone air concentrations in hospitals are
generally less than 36 mg/m3. It should be emphasized that the
exposure of hospital workers to ethylene oxide tends to be of a
short-term and intermittent nature with the likelihood of exposure
to short-term (5 - 120 min) concentrations of about 100 mg/m3 and
to peak concentrations of up to 1800 mg/m3 following the opening of
sterilization chambers (section 4.3).
Ethylene oxide has a high solubility in water but will
evaporate to a great extent. Degradation of ethylene oxide in
neutral water is slow, even in the presence of aerobic
microorganisms. Because of the low log n-octanol water-partition
coefficient, it is unlikely that ethylene oxide and its conversion
products (such as 1,2-ethanediol) will bioaccumulate. The toxicity
of ethylene oxide for aquatic organisms is low (all available LC50s
are above approximately 90 mg/litre) (Table 3). The probable
effects of ethylene oxide on the aquatic environment are,
therefore, considered negligible (sections 3.2, 6). There are no
data concerning the toxicity of ethylene oxide for terrestrial
organisms.
No ambient air monitoring data are available from which the
effects of ethylene oxide on the health of man and the environment
can be assessed. However, the risk for health from exposure to
ethylene oxide in the ambient air, apart from point source
emissions and accidental spillage, is likely to be negligible.
Inhaled ethylene oxide is readily absorbed into the blood,
distributed throughout the body, and rapidly metabolized. The
half-life in the tissues of man and rodents is approximately
10 min; clearance from the blood of dogs occurred with a half-
life of 33 min (section 5.2). Marked nausea and profuse vomiting
following dermal exposure of man to aqueous solutions of ethylene
oxide suggest that absorption can occur through the skin (section
5.1).
Case reports indicate that headache, nausea, vomiting,
dyspnoea, and respiratory tract irritation are common effects of
acute inhalation exposure to ethylene oxide (section 8.3). Case
reports and the results of animal studies indicate that
sensorimotor neuropathies may follow repeated exposure to
concentrations of ethylene oxide recognizable by its odour
(approximately 900 mg/m3 or more) (sections 2.2, 7.2.1, 8.3).
Dermatological effects in man following skin contact with
ethylene oxide include erythema, oedema, and vesiculation, in that
order. The severity of the skin injury is related to concentration
(a 50% (500 g/litre) solution being most hazardous) and duration of
contact. Liquid ethylene oxide, as it vaporizes, can result in a
freeze burn. On repeated exposure, ethylene oxide may cause
delayed allergic contact dermatitis (sections 8.1, 8.2). Ethylene
oxide and its conversion products are irritating to the eyes and
can produce corneal injury. Cataracts have occurred following
repeated exposure to concentrations of the vapour recognizable by
its odour (approximately 900 mg/m3 or more) (Table 1, sections 8.1,
8.3).
Ethylene oxide directly alkylates proteins and DNA and is
mutagenic in microorganisms, plants, insects, mammalian cells in
vitro, and mammals in vivo, including both gene mutations and
chromosomal abnormalities (section 7.5). In man, ethylene oxide
induces chromosomal aberrations and sister chromatid exchanges in
lymphocytes at air concentrations found at the workplace (section
8.7). Tissue distribution studies provide evidence that ethylene
oxide reaches the gonads, supporting the findings of heritable
mutations in insects and rodents (sections 5.2, 7.5). Ethylene
oxide may, therefore, be considered a potential human mutagen for
both somatic and germ cells.
The potential of ethylene oxide to cause teratogenic or adverse
reproductive effects has been examined in 4 animal species (mouse,
rat, rabbit, and monkey) by 2 routes of administration. Results
from these studies showed that ethylene oxide is toxic to
reproductive function in both males (reduced sperm number and sperm
motility, and an increased time to traverse a linear path) and
females (depression of fetal weight gain, fetal death, and fetal
malformation). The levels needed to produce these fetal effects
approach or equal the dose needed to produce maternal toxicity
(section 7.6). The results of animal studies suggest possible
reproductive impairment in human males but are inadequate for
assessing the fetal risk. Data on reproductive effects in human
beings are insufficient; one study, however, suggests an increase
in spontaneous abortion rate in women occupationally exposed to
ethylene oxide (section 8.8). However, the reported time-weighted
average air concentrations may not reflect the exposure levels that
induced the effect.
It has been clearly demonstrated in experimental animal studies
that ethylene oxide is carcinogenic via different routes of
exposure (intragastric, subcutaneous injection, and inhalation).
In 2 inhalation studies, confirmatory data demonstrated dose-
related increases in the incidences of leukaemia, peritoneal
mesothelioma, and cerebral glioma (section 7.4). Although the
evidence for the carcinogenicity of ethylene oxide in man is
inadequate, epidemiological studies indicate that exposure to
ethylene oxide (in mixtures with other chemicals) increases the
risk of malignancies (section 8.6).
Taking into account available data concerning the alkylating
nature of ethylene oxide, the demonstration of DNA adducts, the
overwhelming positive in vivo responses in mutagenic and
clastogenic assays, the reproducible positive carcinogenic findings
in animals, and the epidemiological findings suggesting an increase
in the incidence of human cancer, ethylene oxide should be
considered as a probable human carcinogen, and its levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. The study indicating that exposure to ethylene oxide may be
associated with spontaneous abortion needs to be
corroborated and the implication explored further.
2. The possible effects of ethylene oxide on the reproductive
function of man should be studied.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to ethylene oxide in combination
with other chemicals strongly suggest that additional
epidemiological studies should be carried out on
populations whose exposure has been primarily to ethylene
oxide, including adequate quantification of past exposure.
4. The temporal relationships of air concentrations of
ethylene oxide and duration of exposure must be examined to
determine which of these two factors has the greater impact
on health.
5. Development of methods and research should be conducted to
assess the endogenous occurrence of hydroxyethylation and
the exogenous (environmental) contribution of ethylene
oxide and its precursors to the formation of macromolecular
adducts as markers of internal dose.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of ethylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
ethylene oxide to experimental animals; there is limited
evidence for the carcinogenicity to humans of exposures to
ethylene oxide in combination with other chemicals; there is
inadequate evidence for the carcinogenicity to humans of
exposures to ethylene oxide alone. Taken together, the data
indicate that ethylene oxide is probably carcinogenic to
humans."
REFERENCES
ABRAHAMS, R.H. (1980) Recent studies with workers exposed to
ethylene oxide. In: Jorkasky, J.F., ed. Safe use of ethylene
oxide. Proceedings of the Educational Seminar, Washington DC,
Health Industries Manufacturers Association, pp. 27-38,
211-220 (HIMA Report No. 80-4)
ADLER, N. (1965) Residual ethylene oxide and ethylene glycol
in ethylene oxide sterilized pharmaceuticals. J. Pharm. Sci.,
54: 735-742.
ALOMAR, A., CAMARASA, J.M.G., NOGUERA, J., ASPINOLEA, F.
(1981) Ethylene oxide dermatitis. Contact dermatit., 7:
205-207.
ALTMAN, P.L. & DITTMER, D.S. (1974) Biological data book,
Bethesda, Maryland, Federation of American Societies for
Experimental Biology, Vol. 3.
APPELGREN, L.-E., ENEROTH, G., & GRANT, C. (1977) Studies on
ethylene oxide: whole-body autoradiography and dominant lethal
test in mice. In: Clinical toxicology. Proceedings of the
Meeting held at Edinburgh, June 1976, European Society of
Toxicology, Amsterdam, Excerpta Medica, Vol. 18, pp. 315-317.
APPELGREN, L.-E., ENEROTH, G., GRANT, C., LANDSTROM, L.-E., &
TENGHAGEN, K. (1978) Testing of ethylene oxide for
mutagenicity using the micronucleus test in mice and rats.
Acta pharmacol. toxicol., 43: 69-71.
BINDER, H. & LINDNER, W. (1972) [Determination of ethylene
oxide in the smoke of definitely unfumigated cigarettes.]
Fachliche Mitteilungen der Austria Tabakwerke A.G., 13:
215-220 (in German).
BIRD, M.J. (1952) Chemical production of mutations in
Drosophila: comparison of techniques. J. Genet., 50: 480-485.
BIRO, L., FISHER, A.A., & PRICE, E. (1974) Ethylene oxide
burns. Arch. Dermatol., 110: 924-925.
BLACKWOOD, J.D. & ERSKINE, E.B. (1938) Carboxide poisoning.
US Navy med. Bull., 36: 44-45.
BLIXT, S., EHRENBERGH, L., & GELIN, O. (1963) Studies of
induced mutations in peas. VII. Mutation spectrum and mutation
rate of different mutagenic agents. Agric. Hort. Genet., 21:
178-216.
BOGYO, S., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: epoxides, Syracuse, New York,
Center for Chemical Hazard Assessment, Syracuse Research
Corporation (Report prepared for US EPA) (Report No. EPA
560/11-80-005, PB 80-183197).
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
BROOKES, P. & LAWLEY, P.D. (1961) The alkylation of
guanosine and guanylic acid. J. Chem. Soc., pp. 3923-3928.
BUQUET, A. & MANCHON, P. (1970) Recherche et dosage des
résidus et dérivés dans un pain conservé a l'aide d'oxyde
d'éthylène. Chim. analytique, 52: 978-983.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CAPELLINI, A. & GHEZZI, I. (1965) [Two cases of acute
ethylene oxide poisoning.] Med. Lav., 56: 822-827 (in Italian).
CHAIGNEAU, M. & MURAZ, B. (1981) Action de l'oxyde
d'éthylène sur le tabac ( Nicotiana tabacum L.): absorption et
combinaisons. Ann. Pharm. Fr., 39: 305-311.
CLARKE, C.P., DAVIDSON, W.L., & JOHNSTON, J.B. (1966)
Haemolysis of blood following exposure to an Austrialian
manufactured plastic tubing sterilized by means of ethylene
oxide gas. Aust. NZ J. Surg., 36: 53-55.
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, Vol. 2a: toxicology, 3rd revised ed.,
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
p. 2166.
CONAN, L., FOUCAULT, B., SIOU, G., CHAIGNEAU, M., LE MOAN, G.,
& DOINEL, A. (1979) Contribution à la recherche d'une action
mutagène des résidus d'oxyde d'éthylène, d'éthylène glycol et
de chloro-2-éthanol dans le matériel plastique stérilisé par
l'oxyde d'éthylène. Ann. Fals. exp. Chim., 72: 141-151.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
CUMMING, R.B. & MICHAUD, T.A. (1979) Mutagenic effects of
inhaled ethylene oxide in male mice. Environ. Mutagen., 1:
166-167.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No.
600/3-80-084, PB 80-221948).
DAUVOIS, C., CHAIGNEAU, M., & LE MOAN, G. (1982)
Sterilisation de pansements par l'oxyde d'éthylène. I.
Physisorption. Ann. Pharm. Fr., 40: 125-132.
DOLOVICH, J. & BELL, B. (1978) Allergy to a product(s) of
ethylene oxide gas. Demonstration of IgE and IgG antibodies
and hapten specificity. J. Allergy clin. Immunol., 62: 30-32.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
DUNKELBERG, H. & HARTMETZ, G. (1977) [Recording the air
pollution by ethylene oxide in the region of clinical
sterilization installations.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
164: 271-278 (in German).
EDELHAUSER, H.F., ANTOINE, M.E., PEDERSON, H.J., & HIDDEMAN,
J.W., & HARRIS, R.G. (1983) Intraocular safety evaluation of
ethylene oxide and sterilant residues. J. Toxicol.-Cut. ocular
Toxicol., 2: 7-39.
EHRENBERG, L. & HALLSTROM, T. (1967) In: Kalling, L.O., ed.
Haematologic studies on persons occupationally exposed to
ethylene oxide, Vienna, International Atomic Agency, pp.
327-334 (Report No. SM92-26).
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1956)
Chemically induced mutation and sterility in barley. Acta
chem. Scand., 10: 492-494.
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1959) The
mutagenic effects of ionizing radiations and reactive ethylene
derivatives in barley. Hereditas, 45: 351-368.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
tissue doses in the mouse from air contaminiated with ethylene
oxide. Mutat. Res., 24: 83-103.
EMBREE, J.W. & HINE, C.H. (1975) Mutagenicity of ethylene
oxide. Toxicol. appl. Pharmacol., 33: 172-173.
EMBREE, J.W., LYON, J.P., & HINE, C.H. (1977) The mutagenic
potential of ethylene oxide using the dominant-lethal assay in
rats. Toxicol. appl. Pharmacol., 40: 261-267.
FOMENKO, V.N. & STREKALOVA, E.Y. (1973) [The mutagenic
effect of some industrial toxins as a function of
concentration and exposure time.] Toksikol. Nov. Prom. Him.
Veshchestr., 7: 51-57 (in Russian).
FAHMY, G.G. & FAHMY, M.J. (1970) Gene elimination in
carcinogenesis: reinterpretation of the somatic mutation
theory. Cancer Res., 30: 195-205.
FINELLI, P., MORGAN, T.F., YAAR, I., & GRANGER, C.V. (1983)
Ethylene oxide-induced polyneuropathy: a clinical and
electrophysiologic study. Arch. Neurol., 40: 419-421.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione.
J. Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Controlling exposure to alkene oxides.
Chem. Eng. Prog., 79: 39-43.
GARRY, V.F., HOZIER, J., JACOBS, D., WADE, R.L., & GRAY, D.G.
(1979) Ethylene oxide: evidence of human chromosomal effects.
Environ. Mutagen., 1: 375-382.
GARRY, V.F., OPP, C.W., WIENCKE, J.K., & LAKATUA, D. (1982)
Ethylene oxide induced sister chromatid exchange in human
lymphocytes using a membrane dosimetry system. Pharmacology,
25: 214-221.
GENEROSO, W.M., CAIN, K.T., KRISHNA, M., SHEN, C.W., & GRYDER,
R.M. (1980) Heritable translocation and dominant-lethal
mutation induction with ethylene oxide in mice. Mutat. Res.,
73: 133-142.
GENEROSO, W.M., CUMMING, R.B., BANDY, J.A., & CAIN, K.T.
(1983) Increased dominant-lethal effects due to prolonged
exposure of mice to inhaled ethylene oxide. Mutat. Res., 119:
377-379.
GERHARDT, U. & LADD EFFIO, J.C. (1983) [Ethylene oxide
residues in spices.] Fleisch wirtsch., 63: 606-608 (in German).
GESSNER, P.K., PARKE, D.V., & WILLIAMS, R.T. (1961) The
metabolism of 14C-labelled ethylene glycol. Biochem. J., 79:
482-489.
GILDING, D.K., REED, A.M., & BASKETT, S.A. (1980) Ethylene
oxide sterilization: effect of polymer structure and
sterilization conditions on residue levels. Biomaterials, 1:
145-148.
GILLESPIE, E.H., JACKSON, J.M., & OWEN, G.R. (1979) Ethylene
oxide sterilization: is it safe? J. clin. Pathol., 32:
1184-1187.
GLASER, Z.R. (1979) Ethylene oxide: toxicology review and
field study results of hospital use. J. environ. Pathol.
Toxicol., 2: 173-208.
GORDON, H.T. & THORNBURG, W.W. (1959) Hydroxyethyl
derivatives in prunes fumigated with 14C-ethylene oxide.
J. agric. food Chem., 7: 196-200.
GREAVES WALKER, W.G. & GREESON, C.E. (1932) The toxicity of
ethylene oxide. J. Hyg., 32: 409-416.
GROSS, J.A., HAAS, M.L., & SWIFT, T.R. (1979) Ethylene oxide
neurotoxicity: report of four cases and review of the
literature. Neurology, 29: 978-983.
GRUNOW, W. & ALTMANN, H.-J. (1982) Toxicokinetics of
chloroethanol in the rat after single oral administration.
Arch. Toxicol., 49: 275-284.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Report No. PB
83-258038).
HANSEN, J.P., ALLEN, J., BROCK, K., FALCONER, J., HELMS, M.J.,
SHAVER, G.C., & STROHM, B. (1984) Normal sister chromatid
exchange levels in hospital sterilization employees exposed to
ethylene oxide. J. occup. Med., 26: 29-32.
HANIFIN, J.M. (1971) Ethylene oxide dermatitis. J. Am. Med.
Assoc., 217: 213.
HARTMAN, P.A. & BOWMAN, P.B. (1977) Simple GLC determination
of ethylene oxide and its reaction products in drugs and
formulations. J. Pharm. Sci., 66: 789-792.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEMMINKI, K., MUTANEN, P., SALONIEMI, I., NIEMI, M-L., &
VAINIO, H. (1982) Spontaneous abortions in hospital staff
engaged in sterilizing instruments with chemical agents.
Br. med. J., 285: 1461-1463.
HEMMINKI, K., MUTANEN, P., & NIEMI, M.-L. (1983) Spontaneous
abortions in hospital sterilizing staff. Br. med. J., 286:
1976-1977.
HENNE, W., DIETRICH, W., PELGER, M., & SENGBUSCH, G., VON
(1984) Residual ethylene oxide in hollow-fibre dialyzers.
Artif. Organs, 8: 306-309.
HINE, C.H. & ROWE, V.K. (1981) Epoxy compounds. In: Patty,
S.A., ed. Industrial hygiene and toxicology, 3rd revised ed.,
New York, Interscience Publishers, Vol. IIa, p. 2141.
HIROSE, T., GOLDSTEIN, R., & BAILEY, C. (1963) Hemolysis of
blood due to exposure to different types of plastic tubing and
the influence of ethylene oxide sterilization. J. thorac.
cardiovasc. Surg., 45: 245-251.
HOGSTEDT, C., MALMQVIST, N., & WADMAN, B. (1979a) Leukemia
in workers exposed to ethylene oxide. J. Am. Med. Assoc., 241:
1132-1133.
HOGSTEDT, C., ROHLEN, O., BERNDTSSON, B.S., AXELSON, O., &
EHRENBERG, L. (1979b) A cohort study of mortality and cancer
incidence in ethylene oxide production workers. Br. J. ind.
Med., 26: 276-280.
HOGSTEDT, B., GULLBERG, B., HEDNER, K., KOLNIG, A-M.,
MITELMAN, F., SKERFVING, S., & WIDEGREN, B. (1983)
Chromosome aberrations and micronuclei in bone marrow cells
and peripheral blood lymphocytes in humans exposed to ethylene
oxide. Hereditas, 98: 105-113.
HOGSTEDT, C., ARINGER, L., & GUSTAVSSON, A. (1984) [Ethylene
oxide and cancerreview of the literature and follow-up of 2
studies.] Arbete och Hälsa, 49: : 1-32 (in Swedish).
HOLLEY, H.S. & GILDEA, J.E. (1971) Vocal cord paralysis
after tracheal intubation. J. Am. Med. Assoc., 215: 281-284.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of ethylene oxide determined
on experimental animals. Arch. ind. Health, 13: 217-227.
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationship for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1985) Alkyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans No. 36).
IPPEN, H. VON & MATTHIES, V. (1970) [Protracted chemical
burns.] Berufsdermatosen, 18: 144-165 (in German).
IRPTC (1984) Data profile on ethylene oxide, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide vapors.
Arch. ind. Health, 13: 237-244.
JANA, M.K. & ROY, K. (1975) Effectiveness and efficiency of
ethyl methane-sulphonate and ethylene oxide for the induction
of mutations in mice. Mutat. Res., 28: 211-215.
JAY, W.M., SWIFT, T.R., & HULL, D.S. (1982) Possible
relationship of ethylene oxide exposure to cataract formation.
Am. J. Ophthalmol., 93: 727-732.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction
of carcinogenicity evaluated by 143 agents tested. Mutat.
Res., 75: 191-202.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rat.
Biochem. Pharmacol., 16: 185-199.
JONES, A.R. & WELLS, G. (1981) The comparative metabolism of
2-bromoethanol and ethylene oxide in the rat. Xenobiotica, 11:
763-770.
JORDY, A. (1983) [The course of the concentrations of
ethylene oxide reaction products in synthetic materials
following gas sterilization.] Hyg. Med., 8: 17-19 (in German).
JOSHI, S.B., DODGE, M.C., & BUFALINI, J.J. (1982)
Reactivities of selected organic compounds and contamination
effects. Atmos. Environ., 16: 1301-1310.
JOYNER, R.E. (1964) Chronic toxicity of ethylene oxide.
Arch. environ. Health, 8: 700-710.
KAUHANEN, K. (1978) Ethylene oxide. Rebuttable presumption
against registration maximum residue limits and daily levels
of exposure. Fed. Reg., 43:: 3804.
KLIGERMAN, A.D., EREXSON, G.L., PHELPS, M.E., & WILMER, J.L.
(1983) Sister-chromatid exchange induction in peripheral
blood lymphocytes of rats exposed to ethylene oxide by
inhalation. Mutat. Res., 120: 37-44.
KOLMARK, H.G. & KILBEY, B.J. (1968) Kinetic studies of
mutation induction by epoxides in Neurospora crassa. Molec.
Gen. Genet., 101: 89-98.
KOLMARK, H.G. & WESTERGAARD, M. (1953) Further studies on
chemically-induced reversions at the adenine locus of
Neurospora. Hereditas, 39: 209-224.
KORPELA, D.B., MCJILTON, C.E., & HAWKINSON, T.E. (1983)
Ethylene oxide dispersion from gas sterilizers. Am. Ind. Hyg.
Assoc. J., 44: 589-591.
KROLLER, E. VON (1966) [Investigations into the fumigation
of foodstuffs by ethylene oxide and into the determination of
its residues.] Dtsch. Lebensm. Rundsch., 62: 227-234 (in
German).
KUZUHARA, S., KANAZAWA, I., NAKANISHI, T., & EGASHIRA, T.
(1983) Ethylene oxide polyneuropathy. Neurology, 33: 377-380.
LABORDE, J.B. & KIMMEL, C.A. (1980) The teratogenicity of
ethylene oxide administered intraveneously to mice. Toxicol.
appl. Pharmacol., 56: 16-22.
LAHAYE, D., ASSCHE, F., VAN, & THEUNISSEN, A. [Ethylene oxide
levels in the sterilization units of hospitals.] Tijdschr.
Soc. Gezondheidsz., 62: 707-713 (in Dutch).
LAMY, F., LACHAPELLE, J.-M., & BRAEKEL, G. VAN (1974)
Dermites professionnelles a l'oxyde d'éthylène chez des sujets
travaillant en zone stérile. Arch. Mal. prof. Méd. Trav.
Sécur. soc., 35: 719-724.
LAURENT, C., FREDERIC, J., & LEONARD, A.Y. (1984) Sister
chromatid exchange frequency in workers exposed to high levels
of ethylene oxide, in a hospital sterilization service.
Int. Arch. occup. environ. Health, 54: 33-43.
LEBREC, D., MASQUET, C., & RUEFF, B. (1977) Collapsus après
exploration endovasculaire avec des cathéters stérilisés à
l'oxyde d'éthylène. Nouv. Presse Med., 6: 2991.
LIPTON, B., GUTIERREZ, R., BLAUGRUND, S., LITWAK, R.S., &
RENDELL-BAKER, L. (1971) Irridiated PVC plastic and gas
sterilization in the production of tracheal stenosis following
tracheostomy. Anest. Analg., 50: 578-586.
LYARSKII, P.P., YURCHENKO, V.V., ZHURKOV, V.S., & GLEIBERMAN,
S.E. (1983) [Mutagenic hazards of parenteral administration
of ethylene oxide to mammals.] Gig. i Sanit., 1: 23-26 (in
Russian).
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76: 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.H., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76: 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MACKEY, J. (1968) Mutagenesis in Vulgare wheat. Hereditas,
59: 505-517.
MCCHESNEY, E.W., GOLBERG, L., PAREKH, C.K., RUSSELL, J.C., &
MIN, B.H. (1971) Reappraisal of the toxicology of ethylene
glycol. II. Metabolism studies in laboratory animals. Food
cosmet. Toxicol., 9: 21-30.
MCDONALD, T.O., KASTEN, K., HERVEY, R., GREGG, S., BORGMANN,
A.R., & MURCHISON, T. (1973) Acute ocular toxicity of
ethylene oxide, ethylene glycol, and ethylene chlorohydrin.
Bull. Parenter. Drug Assoc., 27: 153-164.
MCGUNNIGLE, R.G., RENNER, J.A., ROMANO, S.J., & ABODEELY,
R.A. (1975) Residual ethylene oxide: levels in medical grade
tubing and effects on an in vitro biological system.
J. Biomed. Mater. Res., 9: 273-383.
MANTZ, J.M., TEMPE, J.D., JAEGER, A., & VIDAL, S. (1972)
Stenoses trachéales et stérilisation des canules de
trachéotomie par l'oxyde d'éthylène. Sem. Hôp. Paris, 48:
3367-3370.
MARTIS, L., KROES, R., DARBY, T.D., & WOODS, E.F. (1982)
Disposition kinetics of ethylene oxide, ethylene glycol, and
2-chloroethanol in the dog. J. Toxicol. environ. Health, 10:
847-856.
MARX, G.F., STEEN, S.N., SCHAPIRA, M., ERLANGER, H.L., ARKINS,
R.E., JADWAT, C.M., & KEPES, E. (1969) Hazards associated
with ethylene oxide sterilization. NY State J. Med., 69:
1319-1320.
METZ, E. VON (1939) [Poisoning by ethylene oxide (Cartox or
T-gas).] Samml. Vergiftungsfällen, 10: 37-38 (in German).
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on
Schizosaccharomyces pombe: the influence "in vitro" of
mouse-liver metabolizing system. Mutat. Res., 102: 425-437.
MORGAN, R.W., CLAXTON, K.W., DIVINE, B.J., KAPLAN, S.D., &
HARRIS, V.B. (1981) Mortality among ethylene oxide workers.
J. occup. Med., 23: 767-770.
MORPURGO, G. (1963) Induction of mitotic crossing-over in
Aspergillus nidulans by bifunctional alkylating agents.
Genetics, 48: 1259-1263.
MOUILLESEAUX, A., LAURENT, A.-M., FABRE, M., JOUAN, M., &
FESTY, B. (1983) Teneurs atmosphériques en oxyde d'éthylène
décelées dans l'environnement professionnel d'instalations de
stérilisation ou de désinfection. Arch. Mal. Prof., 44: 1-14.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., & EHRENBERG, L.
(1968) Note on the chromosome breaking activity of ethylene
oxide and ethyleneimine. Hereditas, 60: 267-269.
NAKAO, Y. & AUERBACH, C. (1961) Test of a possible
correlation between cross-linking and chromosome breaking
abilities of chemical mutagens. Z. Vererbungsl., 92: 457-461.
O'LEARY, R.K., WATKINS, W.D., & GUESS, W.L. (1969)
Comparative chemical and toxicological evaluation of residual
ethylene oxide in sterilized plastics. J. Pharm. Sci., 58:
1007-1010.
OSER, B.L. & HALL, L.A. (1956) The effect of ethylene oxide
treatment on the nutritive value of certain foods. Food
Technol., 10: 175-178.
OSTERMAN-GOLKAR, S (1983) Tissue doses in man: implications
in risk assessment. In: Hayes, A.W., ed. Developments in the
Science and Practice of Toxicology. Proceedings of the 3rd
International Congress on Toxicology, San Diego, California.
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor.
Mutat. Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog. Mutag.,
3: 395-405.
PERO, E.W., WIDEGREN, B., HOGSTEDT, B., & MITELMAN, F.
(1981) In vivo and in vitro ethylene oxide exposure of human
lymphocytes assessed by chemical stimulation of unscheduled
DNA synthesis. Mutat. Res., 83: 271-289.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food cosmet. Toxicol., 18: 115-118.
PFEILSTICKER, K. & SIDDIQUI, I.R. (1976) [Isolation of the
derivatives from cocoa-powder fumigated by ethylene oxide
1,2-14C and their structure suggested on the basis of I.R. and
mass-spectrometry.] Z. Lebensm. Unters. Forsch., 160: 19-27
(in German).
PFEILSTICKER, K., FABRICIUS, G., & TIMME, G. (1975)
[Simultaneous gas chromatographic determination of ethylene
oxide, ethylene chlorohydrin, and ethylene glycol in grain.]
Z. Lebensm. Unters. Forsch., 158: 21-25 (in German).
POIRIER, V. & PAPADOPOULO, D. (1982) Chromosomal aberrations
induced by ethylene oxide in a human amniotic cell line in
vitro. Mutat. Res., 104: 255-260.
POOTHULLIL, J., SHIMIZU, A., DAY, R.P., & DOLOVICH, J.
(1975) Anaphylaxis from the product(s) of ethylene oxide gas.
Ann. intern. Med., 82: 58-60.
QUAZI, A.H. & KETCHAM, N.H. (1977) A new method for
monitoring personal exposure to ethylene oxide in the
occupational environment. Am. Ind. Hyg. Assoc. J., 38: 635-647.
RADDING, S.B., LIU, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances, (EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide.
J. Assoc. Offic. Agric. Chem., 51: 709-715.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs.
Bull. environ. Contam. Toxicol., 27: 426-431.
RANNUG, U., GOTHE, R., & WACHTMEISTER, C.A. (1976) The
mutagenicity of chloroethylene oxide, chloroacetaldehyde,
2-chloroethanol, and chloroacetic acid, conceivable metabolites
of vinyl chloride. Chem.-Biol Interact., 12: 251-263.
RILLAER, W.G., VAN & BEERNAERT, H. (1982) Determination of
residual ethylene chlorohydrin (ECH) in fumigated foodstuffs
by glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 175: 175-178.
ROMANO, S.J. & RENNER, J.A. (1979) Analysis of ethylene
oxide-worker exposure. Am. Ind. Hyg. Assoc. J., 40: 742-745.
ROMANO, S.J., RENNER, J.A., & LEITNER, P.M. (1973) Gas
chromatographic determination of residual ethylene oxide by
head space analysis. Anal. Chem., 45: 2327-2330.
ROYCE, A. & MOORE, W.K.S. (1955) Occupational dermatitis
caused by ethylene oxide. Br. J. ind. Med., 12: 169-171.
SARTO, F., COMINATO, I., PINTON, A.M., BROVEDANI, P.G.,
FACCIOLI, C.M., BIANCHI, V., & LEVIS, A.G. (1984)
Cytogenetic damage in workers exposed to ethylene oxide.
Mutat. Res., 138: 185-195.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SEGERBACK, D. (1983) Alkylation of DNA and haemoglobin in
the mouse following exposure to ethene and ethene oxide.
Chem.-Biol. Interact., 45: 139-151.
SEXTON, R.J. & HENSON, E.V. (1949) Dermatological injuries
by ethylene oxide. J. ind. Hyg. Toxicol., 31: 297-300.
SEXTON, R.J. & HENSON, E.V. (1950) Experimental ethylene
oxide human skin injuries. J. ind. Hyg. Toxicol., 32: 549-564.
SHULOVSKA, K., LINDGREN, D., ERIKSSON, G., & EHRENBERG, L.
(1969) The mutagenic effect of low concentrations of ethylene
oxide in air. Hereditas, 62: 264-266.
SHUPACK, J.L., ANDERSEN, S.R., & ROMANO, S.J. (1981) Human
skin reactions to ethylene oxide. J. Lab. clin. Med., 98:
723-729.
SITTERT, N.J., VAN, JONG, G., DE, CLARE, M.G., DAVIES, R.,
DEAN, B.J., WREN, L.J., & WRIGHT, A.S. (1985) Cytogenetic,
immunological, and haematological effects in workers in an
ethylene oxide-manufacturing plant. Br. J. ind. Med., 42:
19-26.
SMITH, H.H. & LOTFY, T.A. (1954) Comparative effects of
certain chemicals on tradescantia chromosomes as observed at
pollen tube mitosis. Am. J. Bot., 41: 489-593.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg. Toxicol.,
23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1981)
Ethylene oxide: two-year inhalation study on rats, Pittsburgh,
Pennsylvania, Bushy Run Research Center (Final Report No.
44-20).
SNELLINGS, W.M., ZELENAK, J.P., & WEIL, C.S. (1982a) Effects
on reproduction in Fischer 344 rats exposed to ethylene oxide
by inhalation for one generation. Toxicol. appl. Pharmacol.,
63: 382-388.
SNELLINGS, W.M., MARONPOT, R.R., ZELENAK, J.P., & LAFFOON,
C.P. (1982b) Teratology study on Fischer 344 rats exposed to
ethylene oxide by inhalation. Toxicol. appl. Pharmacol., 64:
476-481.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984a) A
subchronic inhalation study on the toxicologic potential of
ethylene oxide in B6C3F1 mice. Toxicol. appl. Pharmacol., 76:
510-518.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984b) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPASOVSKI, M., HRISTEVA, V., PERNOV, K., KIRKOV, V.,
DRJANOVSKA, T., PANOVA, Z., BOBEV, G., GINCHEVA, N., &
IVANOVA, S. (1980) [Health status of the workers from the
production of ethylene and ethylene oxide.] Khig. Zdraveopaz.,
23: 41-47 (in Russian).
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropathological
evaluation of monkeys exposed to ethylene and propylene oxide,
Kansas City, Missouri, Midwest Research Institute (Prepared for
NIOSH) (PB 83-134817).
STANLEY, P., BERTRANOU, E., FOREST, F., & LANGEVIN, L.
(1971) Toxicity of ethylene oxide sterilization of polyvinyl
chloride in open-heart surgery. J. thorac. cardiovasc. Surg.,
61: 309-314.
STAR, E.G. (1980a) [Mutagenic and cytotoxic effect of
ethylene oxide on human cell cultures.] Zbl. Bakt. Hyg. (I. Abt.
Orig. B), 170: 548-556 (in German).
STAR, E.G. (1980b) [Absorption and desorption of ethylene
oxide in anaesthesia supplies.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
170: 557-569 (in German).
STAR, E.G. (1980c) [Ethylene oxide residues and aeration
time after use of modern heated aerators.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 171: 18-24 (in German).
STAR, E.G. (1980d) [Gamma-rays and ethylene oxide
sterilization.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 171: 33-41
(in German).
STAR, E.G., CASELITZ, J., & LONING, T. (1980) [The effect of
ethylene oxide and chemical desinfectant residues upon the
larynx- and tracheal mucosa of rabbits.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 170: 539-547 (in German).
STARK, W.J., ROSENBLUM, P., MAUMENEE, A.E., & COWEN, C.L.
(1980) Postoperative inflammatory reactions to intraocular
lenses sterilized with ethylene oxide. Opthalmology, 87:
385-389.
STIJVE, T., KALSBACH, R., & EYRING, G. (1976) Determination
and occurrence of ethylene chlorohydrin residues in foodstuffs
fumigated with ethylene oxide. Mitt. Geb. Lebensm. Unters.,
67: 403-428.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STOLLEY, P.D., SOPER, K.A., GALLOWAY, S.M., NICHOLS, W.W.,
NORMAN, S.A., & WOLMAN, S.R. (1984) Sister-chromatid
exchanges in association with occupational exposure to
ethylene oxide. Mutat. Res., 129: 89-102.
STREKALOVA, E.Y., CHIRKOVA, Y.M., & GOLUBOVICH, Y.Y. (1975)
[Mutagenic action of ethylene oxide on sex and somatic cells
in male white rats.] Toksikol. Nov. Prom. Him. Veshchestr., 6:
11-16 (in Russian).
TAN, E.-L., CUMMING, R.B., & HSIE, A.W. (1981) Mutagenicity
and cytotoxicity of ethylene oxide in the CHO/HGPRT system.
Environ. Mutagen., 3: 683-686.
TANOOKA, H. (1979) Application of Bacillus subtilis spores
in the detection of gas mutagens: a case of ethylene oxide.
Mutat. Res., 64: 433-435.
THIESS, A.M. (1963) [Observation on the adverse health
effects of ethylene oxide.] Archiv. Toxikol., 20: 127-140 (in
German).
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkene
oxides (ethylene oxide/propylene oxide) and derivatives.
J. occup. Med., 23: 343-347.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & STOCKER, W.G.
(1981b) Mortality study on employees exposed to alkene oxides
(ethylene oxide/propylene oxide) and their derivatives. In:
International Symposium on Prevention of Occupational Cancer,
Helsinki, pp. 249-259.
TROISI, F.M. (1965) [Aphonia of late onset due to
occupational exposure to ethylene oxide.] Med. Lav., 56:
373-377 (in Italian).
UKITA, T., OKUYAMA, H., & HAYATSU, H. (1963) Modifications
of nucleosides and nucleotides. I. Reaction of ethylene oxide
with uridine and uridylic acid. Chem. Pharm. Bull. (Tokyo),
11: 1399-1404.
US DEPARTMENT OF LABOR (1984) Occupational exposure to
ethylene oxide: final standard. Fed. Reg., 49: 25734-25809.
US EPA (1985) Health assessment document for ethylene oxide,
Washington DC, US Environmental Protection Agency (EPA
600/8-84/009F).
USITC (1971) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
USITC (1981) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
VAN DUUREN, B.L., ORRIS, L., & NELSON, N. (1965)
Carcinogenicity of epoxides, lactones, and peroxy compounds.
Part II. J. Natl Cancer Inst., 35: 707-717.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. I. Toxicity profiles, Boca Raton,
Florida, CRC Press Inc., pp. 55-56.
VIRTANEN, P.O.I. (1963) Kinetics of the reactions of
ethylene oxide with nucleophiles. Am. Acad. Sci. Fenn. Ser. A.
II, 124: 1-89.
WAITE, C.P., PATTY, F.A., & YANT, W.P. (1930) Acute response
of guinea-pigs to vapours of some new commercial organic
compounds. IV. Ethylene oxide. Pub. Health Rep., 45: 1832-1843.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WESLEY, F., ROURKE, B., & DARBISHIRE, O. (1965) The
formation of persistent toxic chlorohydrins in foodstuffs by
fumigation with ethylene oxide and with propylene oxide.
J. food Sci., 30: 1037-1042.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WINDMUELLER, H.G. & KAPLAN, N.O. (1961) The preparation and
properties of N-hydroxyethyl derivatives of adenosine,
adenosine tri-phosphate, and nicotinamide adenine
dinucleotide. J. Biol. Chem., 236: 2716-2726.
WINDMUELLER, H.G., ACKERMAN, C.J., BAKERMAN, H., & MICKELSEN,
O. (1959) Reaction of ethylene oxide with nicotinamide and
nicotinic acid. J. Biol. Chem., 234: 889-894.
WOLFS, P., DUTRIEUX, M., SCAILTEUR, V., HAXHE, J.-J., ZUMOFEN,
M., & LAUWERIJS, R. (1983) Surveillance des travailleurs
exposés a l'oxyde d'éthylène dans une enterprise de
distribution de gaz stérilisants et dans des unités de
stérilisation de matériel médical. Arch. Mal. Prof., 44:
321-328.
WOODARD, G. & WOODARD, M. (1971) Toxicity of residuals from
ethylene oxide gas sterilization. In: Proceedings of the 1971
HIA Technical Symposium, Washington DC, Health Industries
Association.
YAGER, J.W. & BENZ, R.D. (1982) Sister-chromatid exchanges
induced in rabbit lymphocytes by ethylene oxide after
inhalation exposure. Environ. Mutagen., 4: 121-134.
YAGER, J.W., HINES, C.J., & SPEAR, R.C. (1983) Exposure to
ethylene oxide at work increases sister-chromatid exchanges in
human peripheral lymphocytes. Science, 219: 1221-1223.
YAKUBOVA, Z.N., SHAMOVA, H.A., MUFTAKNOVA, F.A., & SHILOVA,
L.F. (1976) [Gynaecological disorders in workers engaged in
ethylene oxide production.] Kazan. Med. Zh., 57: 558-560 (in
Russian).
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
 When a single dose of 2 mg labelled ethylene oxide in
propanediol/kg body weight was applied intraperitoneally to rats,
43% of the administered radioactivity was excreted in the urine
within 50 h (41% within 24 h) of exposure, 9% as S-(2-
hydroxyethyl)cysteine and 33% as N-acetyl- S-(2-hydroxyethyl)
cysteine, both products of glutathione conjugation. Via the lungs,
1.5% was excreted as carbon dioxide and 1% as unmetabolized
ethylene oxide (Jones & Wells, 1981). The involvement of
glutathione-epoxide- S-transferase (EC 4.4.1.7) has not been
investigated further. In vitro glutathione conjugation of the
homologue propylene oxide was shown to proceed only in the presence
of an enzyme (Fjellstedt et al., 1973). In rabbits, no effect was
found on liver- and blood-glutathione levels, after 12 weeks of
exposure to concentrations of ethylene oxide at 18, 90, or 450
mg/m3, for 5 days per week, 6 h per day (Yager & Benz, 1982).
As ethylene oxide can react with chloride ions, and this
reaction is acid catalysed, 2-chloroethanol might be expected to be
a metabolite, especially after oral administration. However,
neither 2-chloroethanol, nor its metabolites (Johnson, 1967; Grunow
& Altman, 1982) have been found in the plasma, tissues, or urine of
species exposed to ethylene oxide.
Ehrenberg et al. (1974) found that an average of 74% of
labelled ethylene oxide, inhaled by mice, was excreted in the urine
within 24 h in the form of unidentified metabolites, and only 4%
within the next 24 h. Thus, on the basis of this and previously-
presented excretion data, excretion of metabolites of ethylene
oxide mainly takes place via the urine, within 24 h following
exposure.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
A summary of the acute toxicity of ethylene oxide for aquatic
organisms is presented in Table 3. Data on the most likely
reaction products, 1,2-ethanediol and 2-chloroethanol, are
included.
LC50s of ethylene oxide for aquatic species have been reported
to range from 90 mg/litre (goldfish, 24-h exposure) to 745 mg/litre
(brine shrimp, 48-h exposure). Microorganisms in activated sludge
showed 50% inhibition at concentrations between 10 and 100
mg/litre. Hydrolysis to 1,2-ethanediol results in detoxification.
The toxicity of 2-chloroethanol for aquatic organisms resembles
that of ethylene oxide, though 2-chloroethanol seems to be more
toxic for Daphnia magna. Nevertheless, under environmental
conditions, the conversion of ethylene oxide to 2-chloroethanol or
1,2-ethanediol will be slow.
In a study on the albino guppy, Poecilia reticulata, desorption
of ethylene oxide or its reaction products from non-aerated plastic
materials into water can lead to behavioural disturbances in the
fish almost immediately, and to death within about one h. No such
toxicity was found when the materials were aerated for 24 h
(O'Leary et al., 1969).
Ethylene oxide is very soluble in aqueous media and evaporates
from water to a significant degree. The log n-octanol water-
partition coefficient was reported to be -0.30 (Radding et al.,
1977). Thus, ethylene oxide will not bioaccumulate.
Table 3. Acute aquatic toxicitya
-----------------------------------------------------------------------------------------------------------
Organism Description T pH Dissolved Parameter Test Concentration Reference
(°C) oxygen substance (mg/litre)
-----------------------------------------------------------------------------------------------------------
Micro- activated 22 50% growth ethylene oxide 10 - 100 Conway et al.
organisms sludge inhibition 1,2-ethanediol > 10 000 (1983)b
Crustacea water flea 17 7.0 minimal 48-h LC50 ethylene oxide 212 Conway et al.
(Daphnia magna) aeration 1,2-ethanediol > 10 000 (1983)c
2-chloroethanol 100
Fish fathead minnow 22 7.0 no 96-h LC50 ethylene oxide 84 Conway et al.
(Pimephales aeration 1,2-ethanediol > 10 000 (1983)c
promelas) 2-chloroethanol 90
Fish goldfish 20 6-8 no 24-h LC50 ethylene oxide 90 Bridié et al.
(Carassius aeration 1,2-ethanediol > 5000 (1979a)d
auratus) (> 4
mg/litre)
Fish brine shrimp 24 7.0 minimal 48-h LC50 ethylene oxide 745 Conway et al.
(Artemia aeration 1,2-ethanediol > 20 000 (1983)e
salina) 2-chloroethanol 680
-----------------------------------------------------------------------------------------------------------
a All tests were static. Water analysis for the substance under test was reported by all authors.
b Incubation on a shaker for 16 h.
c Medium was fresh water, reconstituted using dechlorinated, carbon-treated tap water; 10 fish per
concentration.
d Medium was local tap water; 6 fish per concentration.
e Medium was sea water, reconstituted using dechlorinated, carbon-treated tap water, 30 - 50 shrimps per
concentration.
7. EFFECTS ON ANIMALS
7.1. Acute Exposures
7.1.1. Oral, intravenous, and inhalation studies
The LD50s for ethylene oxide, administered orally and dissolved
in water, were 330 mg/kg body weight for male rats and 280 and
365 mg/kg body weight for female and male mice, respectively (Smyth
et al., 1941; Woodard & Woodard, 1971). After inhalation, the 4-h
LC50s were 1500 and 1730 mg/m3 for mouse and dog, respectively, and
2630 mg/m3 for rat (Jacobson et al., 1956). 1,2-Ethanediol, a
metabolite, is less toxic: LD50s for rat were above 10 000 mg/kg
body weight, after oral administration, and 5210 mg/kg body weight,
after intravenous administration (Woodard & Woodard, 1971).
The slope of the dose-response curve in relation to the
mortality rate for ethylene oxide was steep. After oral
administration to rats, the difference between 0.1% mortality
(325 mg/kg) and 99.9% mortality (975 mg/kg) was approximately
650 mg/kg body weight (Smyth et al., 1941). After inhalation for
4 h, this difference was approximately 3000 mg/m3, in mice, and
approximately 5000 mg/m3 in rats. No deaths occurred in dogs at
1280 mg/m3 (Jacobson et al., 1956). While no guinea-pigs died
after inhalation of 450 mg ethylene oxide/m3 air for 8 h, the
majority did so at 2400 mg/m3 (Waite et al., 1930). In the above
mortality studies, the lungs and nervous system were the main
targets in rodents and dogs. In dynamic inhalation exposure
studies on guinea-pigs (Waite et al., 1930), rats, mice, and dogs
(Jacobson et al., 1956), nasal irritation was the first clinical
effect, as evidenced by scratching the nose, nasal discharge,
lachrymation, and salivation. Respiratory problems occurred
ranging from gasping to laboured breathing. Dogs exhibited
laboured breathing, vomited, and suffered convulsions. Guinea-
pigs, exposed to a concentration of 13 000 mg ethylene oxide/m3,
for 2.5 h, were found lying on their sides, unable to stand, and
quiet. Gross pathological changes in animals that did not survive
included moderate congestion in the lungs of dogs, minor patchy
oedema in the lungs of rats, and congestion with oedema in the
lungs of guinea-pigs. In rats, moderate congestion with petecchial
haemorrhage of the trachea was also observed. Lobular pneumonia
and hyperaemia of the liver and kidneys were observed in guinea-
pigs. Parenchymatous changes in the kidney of guinea-pigs were
seen at 2300 mg/m3.
Ataxia, prostration, laboured breathing, and occasional tonic
convulsions were effects shown by rats and mice at lethal oral or
intravenous doses of ethylene oxide (Woodard & Woodard, 1971).
Vomiting was the only effect shown by dogs that had received 25 or
75 mg ethylene oxide/kg body weight intravenously (Martis et al.,
1982).
In order to investigate the effects of residues of ethylene
oxide or reaction products in sterilized medical equipment, rabbits
were exposed for 2 h via polyvinyl chloride endotracheal tubes
containing 0, 80, or 600 mg ethylene oxide/kg material. There were
no deaths, but rabbits receiving the tubes with the highest
residues showed increased incidences of hyperaemia, oedema,
leukocyte infiltration, and epithelial erosion of the larynx and
trachea (Star et al., 1980). Two groups of nine dogs each were
exposed through extracorporeal perfusion for 40 min via a polyvinyl
chloride oxygenator containing 12 g ethylene oxide/kg material.
Nine dogs with an interrupted pulmonary circulation died from shock
and pulmonary oedema. Six out of 9 dogs with a preserved pulmonary
circulation died from pulmonary distress. A group of 9 control
dogs was treated in the same manner using a steam-sterilized
oxygenator. Only 1 control dog died (Stanley et al., 1971).
7.1.2. Acute effects on eyes and skin
As noted above, ethylene oxide is an irritating agent for
several different species. A maximum non-damaging concentration of
0.1% ethylene oxide in balanced physiological salt solution
(prepared daily and kept at 0 °C) was established after
instillation of 0.05 ml solution, every 10 min for 6 h, into the
conjunctival sac of rabbits. The concentrations above 1% caused
reversible changes in conjunctiva such as hyperaemia and swelling,
and irreversible opacity, both in the cornea and in the lens.
Possible reaction products, 2-chloroethanol and 1,2-ethanediol,
were less irritating to the eye (McDonald et al., 1973). The
results of in vitro tests with isolated rabbit cornea were in
agreement with the results of these studies. In the in vitro
tests, the endothelia were perfused for 1 - 3 h with a balanced
salt solution containing 250 mg ethylene oxide, 2250 mg
2-chloroethanol, or 5000 mg 1,2-ethanediol/litre. No effects were
observed on corneal thickness and cellular ultrastructure
(Edelhauser et al., 1983).
Skin irritation with hyperaemia, oedema, and scar formation was
observed from 6 min after application of pads of cotton, moistened
with solutions of 100 or 500 g ethylene oxide/litre water, on the
shaved skin of rabbits, under a plastic cover. The intensity of
the response was reported to be roughly proportional to the length
of exposure time (1 - 60 min) and the concentration (Hollingsworth
et al., 1956).
According to Hine & Rowe (1981), liquid ethylene oxide is
apparently without adverse effects on rabbit and human skin, on
single mild exposures, if the material evaporates rapidly. If
large amounts of material are involved, evaporation may cause
sufficient cooling to cause a lesion similar to frost-bite.
7.2. Short-Term Studies
7.2.1. Inhalation exposure
Groups of 10 - 20 Wistar rats per sex, 8 guinea-pigs per sex,
1 - 2 rabbits per sex, and 1 - 2 female rhesus monkeys were each
exposed to concentrations of ethylene oxide at levels of 0, 90,
200, 370, 640, or 1510 mg/m3, for 7 h per day, and 5 days per week.
The female monkeys were not tested at 90 mg/m3, and an additional 3
male monkeys were tested at 640 mg/m3. The test period varied with
the species tested, and the severity of exposure, i.e.,
approximately 26 weeks at 90 mg/m3, 25 - 32 weeks at 200 and
370 mg/m3, 7 - 25 weeks at 640 mg/m3, and 10 days at 1510 mg/m3.
Guinea-pigs, rabbits, and monkeys tolerated 90 and 200 mg/m3, and
rats tolerated exposure to 90 mg/m3 without adverse effects on
general appearance, behaviour, mortality rate, growth, body and
organ weight, gross- and histopathology. Rats showed elevated
mortality rates from 370 mg/m3, rabbits from 640 mg/m3, and all
exposed animals died at 1510 mg/m3. Secondary respiratory
infection caused the deaths of an appreciable number of rats and
mice in these studies.
Surviving rats showed increased relative lung weights after
26 - 27 weeks at 200 and 370 mg/m3. At 370 mg/m3, haemorrhages,
hyperaemia, emphysema, and local alveolar collapse were observed in
these lungs. Lungs of male rabbits also showed hyperaemia and
slight oedema at 370 mg/m3. Even more severe lung injury was seen
in rats at 640 mg/m3 and the higher exposure. Gross respiratory
tract irritation was apparent in all species at 1510 mg/m3.
Delayed reversible effects were observed on the peripheral
nervous system. Monkeys and rabbits exhibited paralysis of the
hind legs at 370 mg/m3 and, together with rats, at 640 mg/m3. This
was accompanied by atrophy of the muscles of the hind legs, except
in rabbits at 370 mg/m3. The effects on the peripheral nervous
system were investigated further in monkeys, and loss of both
sensory and motor function was noted at levels of 370 and
640 mg/m3.
Significant increases in body weight were also observed in
rats, at levels of 200 mg/m3 or more. Rats showed slight but
significant increases in the relative weights of kidney and liver
at 370 mg/m3 (Hollingsworth et al. 1956).
The findings of Jacobson et al. (1956) are in agreement with
these results. Groups, comprising 20 male rats and 30 female mice
each, were exposed to concentrations of ethylene oxide at levels of
0, 180, or 730 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted 26 weeks at 180 mg/m3 and 6 weeks at 730 mg/m3.
Additional groups of 15 rats and mice at the higher, and 60 rats
and mice at the lower, exposure level were used for interim gross
pathology. No clear toxic effects were reported at 180 mg/m3. No
pathological changes were observed except for marked haemosiderosis
in the spleen of a few rats at 730 mg/m3. The highest exposure
(730 mg/m3) resulted in death for both species without clinical
signs in mice. Effects on the respiratory and nervous system were
shown by rats as laboured breathing, reddish nasal discharge,
diarrhoea, tendency towards a side position, and dragging of the
hind-quarters. Rats also lost weight, which was regained by
survivors.
More recently, groups of 30 B6C3F1 mice of each sex were
exposed to concentrations of ethylene oxide (purity 99.9%) at 0,
18, 86, 187, or 425 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted for 10 weeks for males and 11 weeks for females.
No effects were observed in relation to survival, body weight,
clinical signs, white blood cell count, serum clinical chemistry,
urinalysis, and histopathology. At the highest exposure level,
changes at terminal sacrifice included an increased relative liver
weight in female mice, and a decreased testicular weight in males.
A decreased relative spleen weight was observed at 187 and
425 mg/m3 in both sexes. In addition, the red blood cell count,
the packed cell volume, and the haemoglobin concentrations were
decreased at 425 mg/m3. Screening of neuromuscular function in
groups of 5 female mice, in week 6, and 5 mice of both sexes, in
week 10 or 11, revealed altered reflex responses at 425 mg/m3 and a
dose-related trend in alterations of locomotor function from
86 mg/m3 upwards (Snellings et al., 1984a).
Groups of 3 male beagle dogs each were exposed to
concentrations of ethylene oxide (purity 99.7%) of 180 and
530 mg/m3, for 1 - 3 days. No effects were observed on mortality
rate, body weight, electrocardiogram, blood-calcium and -urea,
icteric index, and rectal temperature. Anaemia was noted at both
exposure levels. Effects on the respiratory and nervous systems
were shown at 530 mg/m3, such as hyperaemia and local alveolar
collapse in lungs, vomiting, and occasional slight tremors and
transient weakness in the hind legs. Muscular atrophy was also
observed (Jacobson et al., 1956). No haematological changes were
noted when groups each comprising 3 male New Zealand rabbits were
exposed repeatedly for 12 weeks to 0, 18, 90, or 450 mg/m3 (Yager &
Benz, 1982). The white cell count was depressed in Fischer rats
exposed in groups of 3 or 4, for 3 days, 6 h per day, to 90, 270,
or 810 mg/m3. There was a poor correlation with exposure level
(Kligerman et al., 1983).
The possible neurotoxic effects of ethylene oxide noted in the
above studies were investigated further in groups each comprising
12 male cynomolgus monkeys. These animals were exposed to 0, 90,
or 180 mg ethylene oxide/m3 (purity 99.7%), for 7 h per day, 5 days
per week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related lesions found were in the medulla oblongata of the brain.
Axonal dystrophy was found in the nucleus gracilis, primarily in
the exposed groups. Demyelination of the terminal axons of the
fasciculus gracilis occurred in one monkey at each exposure level,
but not in the controls (Sprinz et al., 1982). Paralysis of the
hind limbs was observed in monkeys repeatedly exposed for up to
32 weeks to 370 mg/m3, for 7 h per day, 5 days per week
(Hollingsworth et al., 1956).
7.2.2. Oral exposure
Groups of 5 Wistar rats each received, by gavage, 22 doses of
3, 10, or 30 mg ethylene oxide/kg body weight in 30 days and 15
doses of 100 mg/kg in 21 days. The vehicle was olive oil. There
were 10 vehicle controls.
No effects on mortality rate, growth, haematology, blood urea-
nitrogen, organ weights, gross- and histopathology were reported at
the 3 lower dose levels. At 100 mg/kg, there was marked loss in
body weight, gastric irritation, and slight (not further specified)
liver damage (Hollingsworth et al., 1956).
7.3. Long-Term Inhalation Studies
In a combined toxicity-carcinogenicity study, groups of 120
male and 120 female Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 18 mg/m3 (10 ppm), 58 mg/m3
(32 ppm), and 173 mg/m3 (96 ppm), for 6 h per day, 5 days per week,
over 25 months. Two control groups each comprising 120 male and
120 female rats were used. There was an exposure-free period of 2
weeks in month 15, because of infection with sialoacryoadenitis
virus. Interim sacrfices occurred at 6, 12, and 18 months.
The mortality rates of male and female rats increased
significantly from the 22nd or 23rd month, at the highest exposure,
with a trend towards an increase at a level of 58 mg/m3. Body
weights in both sexes were depressed at 173 mg/m3, from the end of
the first week onwards, until the end of the study. At 58 mg/m3,
the body weights of female rats were decreased between week 10 and
80. In females, the relative liver weights were increased in the
18th month at 173 mg/m3. This effect on the liver could not be
related to increases in the activities of serum alkaline
phosphatase (EC 3.1.3.1), aspartate aminotransferase (EC 2.6.1.1),
or alanine aminotransferase (EC 2.6.1.2), found mainly at the 2
highest exposures during interim sacrifices. Relative spleen
weights were increased in rats that developed leukaemia (section
7.4.1). Haematological changes were found in rats at all doses,
but mainly at the end of the study in animals exposed to 173 mg/m3;
these included an elevated leukocyte count in both sexes, and a
depressed red blood cell count and haemoglobin value in females.
Some of these rats had leukaemia.
Non-neoplastic histopathological changes observed included an
elevated frequency of focal fatty metamorphosis of the adrenal
cortices in both sexes and bone marrow hyperplasia in females at
173 mg/m3. Although no effect was observed on the hind-quarter
lift reflex, examined monthly, mild skeletal muscular atrophy was
observed, after 2 years of exposure to 173 mg/m3. Observations on
general health and ophthalmology did not reveal anything abnormal.
Neoplastic changes are reported in section 7.4.1 (Snellings et al.,
1981, 1984b).
In another toxicity-carcinogenicity study (Lynch et al.,
1984a), groups of 80 male Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 92 mg/m3 (51 ppm) and 182 mg/m3
(101 ppm), for 7 h per day, 5 days per week, over 2 years. The
control group also comprised 80 rats. There was an exposure-free
period of 2 weeks in month 16 because of a pulmonary infection,
which contributed to the mortality rate.
The mortality rate increased at both exposure levels, the
increase being significant at 182 mg/m3. Only 19% of the rats
survived 2 years of exposure at 182 mg/m3 compared with 49% in the
unexposed group. Body weights were reduced from the 3rd or 4th
month onwards. The relative weights of adrenals and brain were
increased at both exposure levels. The relative weights of lung
and kidney were increased at 92 mg/m3. Serum aspartate
aminotransferase activity was increased in rats exposed to 92 and
182 mg/m3 (section 7.4.1). No other changes were found in
haematology or clinical chemistry.
Non-neoplastic histopathological changes included an elevated
incidence of vacuolization and hyperplasia or hypertrophy in the
adrenals at both exposure levels, and of atrophy and degeneration
of skeletal muscle fibres at 182 mg/m3. There were also increased
incidences of inflammatory lesions of the lungs, nasal cavities,
trachea, and internal ear at both exposure levels. Eye cataracts
developed in 9 out of 78 rats at 182 mg/m3, 3 out of 79 in the
92 mg/m3 group, and 2 out of 77 in the controls.
7.4. Carcinogenicity
7.4.1. Inhalation exposure
In the studies by Snellings et al. (1981, 1984b) (section 7.3)
(Table 4), several neoplasms were induced by ethylene oxide. A
dose-related increased incidence of mononuclear cell leukaemia was
found in both sexes, significant at the 2 highest exposures in
females, from the 18th or 19th month onwards. Trend test revealed
a treatment-related response in both sexes. In males, an increased
incidence of peritoneal mesotheliomas originating from the
testicular mesothelium, occurred at 58 and 173 mg/m3 from the 23rd
month onwards, and an increased incidence of subcutaneous fibroma
was seen in male rats exposed to 173 mg/m3 that had survived for 24
months. Trend analysis showed that there was a treatment-related
increase in peritoneal mesothelioma. There was no increased
incidence of pituitary tumours, but they appeared earlier in the
173 mg/m3 group.
Following the report by Lynch et al. (1984a) of an increased
incidence of brain tumours in Fischer 344 rats exposed to ethylene
oxide (see below), the brain tissue from this study was re-examined
both macro- and microscopically, and a dose-related incidence of
primary brain tumours was observed at 58 and 173 mg/m3 that
appeared to be treatment related in the trend test, but was not
statistically significant. The tumours were mainly diagnosed as
gliomas and malignant reticular tumours. The percentage of rats
with multiple neoplasms was greater than in controls at all
exposure levels in females and at 173 mg/m3 in males. At 58 and
173 mg/m3, the percentage of female rats with at least one
malignancy was increased. The authors considered that a
contribution of the viral outbreak to the toxicity of ethylene
oxide was unlikely (Snellings et al., 1981, 1984b).
Lynch et al. (1984a) (section 7.3, Table 4) also found an
increased incidence of mononuclear cell leukaemia, which was
significant at the lower exposure level. The absence of a dose-
relationship was attributed to the increased mortality rate at 182
mg/m3. Dose-related increased incidences of peritoneal
mesotheliomas, originating from the testicular mesothelium, and of
mixed-cell gliomas in the brain, were found. The increases in both
tumours were significant at 182 mg/m3 (Lynch et al., 1984a).
Table 4. Tumours induced by ethylene oxide in Fischer 344 rats
------------------------------------------------------------------------------
Concen- Leukaemia Meso- Pituitary Subcutaneous Braina
tration (mononuclear) thelioma adenoma fibromaa
(mg/m3)
------------------------------------------------------------------------------
M F M M F M M F
Snellings et al. (1981, 1984b)
173 26(119)b 28(113) 22(119) 27(117) 32(117) 11(30) 3(30) 2(26)
58 25(81) 24(79) 7(91) 16(79) 38(90) 1(39) 1(39) 2(48)
18 21(79) 14(77) 3(89) 27(80) 39(90) 3(51) 0(51) 0
0 20(116) 9(118) 2(114) 28(117) 38(119) 1(48) 1(48) 0
0 18(118) 13(117) 2(116) 22(117) 38(116) 2(49) 0(49) 0
Lynch et al. (1984a)c
182 30(76) NA 21(79) 21(67) NA NR 5(79) NA
92 38(79) NA 9(79) 20(66) NA NR 2(77) NA
0 24(77) NA 3(78) 48(73) NA NR 0(76) NA
------------------------------------------------------------------------------
a Only animals that survived for 24 months were included, because these
tumours appeared after the 18-month interim sacrifice.
b Numbers in brackets refer to the number of rats examined. They include
interim and final kills.
c Only male rats were used.
NA = Not applicable.
NR = Not reported.
7.4.2. Oral exposure
Groups of 50 female Sprague Dawley rats received, in salad oil,
7.5 or 30.0 mg ethylene oxide/kg body weight, by gavage, in the
empty stomach, twice a week, for 110 weeks. There were 50 vehicle
controls, 50 untreated controls, and 50 positive controls. The
rats were observed for their life span. No statistical analysis
was reported. The mean survival period was over 100 weeks for all
groups. The mortality rate increased at 30.0 mg/kg body weight,
from week 100 onward. Elevated incidences of tumours were only
observed in the forestomach, the first tumour appearing in week 79.
The incidences of squamous cell carcinomas were 0/50, 8/50, and
29/50 at 0, 7.5, and 30 mg/kg body weight, respectively. At
30 mg/kg body weight, invasive growth and metastases were observed
in 10 rats. At 30 mg/kg body weight, 2 fibrosarcomas were also
noted. At both doses, the incidences of hyperplasia,
hyperkeratosis, papillomas, and/or carcinomas were increased in the
forestomach (Dunkelberg, 1982).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice were injected once a week with a
tricaprylin solution containing 0.1, 0.3, or 1.0 mg ethylene oxide
per animal, for 106 weeks. There were 200 vehicle controls and 200
untreated controls. From week 35 to week 85, the mortality rate
increased by a maximum of 10% at a dose of 1.0 mg per mouse. The
mean length of survival in this group was 75 weeks. An elevated
incidence of tumours was only observed at the injection site, the
first tumour appearing in week 79. There was a dose-related
increased incidence of sarcomas, mainly fibrosarcomas, which was
significant at 0.3 and 1.0 mg per mouse. The tumour incidence was
11% at the highest dose compared with 2% in vehicle controls
(Dunkelberg, 1981).
7.4.4. Dermal exposure
Each of a group of 30 female Swiss Millerton mice received, for
their lifetime, approximately 100 mg of a 10% solution of ethylene
oxide (purity 99.7%) in acetone, brushed on the clipped dorsal
uncovered skin, 3 times a week. A group of 60 mice did not receive
any treatment, and a group of 60 mice received the vehicle only.
No skin tumours were found, nor was there any sign of skin
irritation. The median length of survival was 493 days for treated
mice and 445 days for controls (Van Duuren et al., 1965). It is
assumed that ethylene oxide, applied in this manner, evaporated
rapidly from the skin.
7.5. Mutagenicity and Related End-Points
Almost all the reports available demonstrate the mutagenic
action of ethylene oxide. A summary of mutagenicity tests with
positive results is presented in Table 5.
Ethylene oxide is an alkylating agent (section 5.2). It has
induced gene mutations in all plants, bacteria, fungi, insects, and
mammalian cells investigated in vitro, with and without metabolic
activation. Chromosome damage and sister chromatid exchanges were
observed in plants, insects, and mammalian somatic cells exposed in
vivo and in vitro. Fomenko & Strekalova (1973) and Strekalova et
al. (1975) reported an increased incidence of chromosomal
aberrations in the bone-marrow cells of rats exposed by inhalation
to concentrations of ethylene oxide vapour at 3.6 and 112 mg/m3.
Unscheduled DNA synthesis, induced by the N-acetoxy-2-acetylamino-
fluorene, was inhibited by ethylene oxide in human lymphocytes in
vitro (Pero et al., 1981). The positive results in the
micronucleus tests are in agreement with those from a distribution
study showing that ethylene oxide, or its metabolites, was retained
in the bone marrow of mice (Appelgren et al., 1977). An increased
incidence of micronuclei was observed after one intraperitoneal
dose of 100 mg/kg body weight in mice (Lyarskii et al., 1983) and
after 4 h of vapour exposure of rats to a concentration of 90 mg/m3
(Embree & Hine, 1975).
Table 5. Mutagenic tests for ethylene oxide with positive resultsa
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Gene mutations
Forward mutations plant barley Ehrenberg et al. (1956,
1959)
Forward mutations barley Shulovsk et al. (1969)
Forward mutations rice Jana & Roy (1975)
Forward mutations pea Blixt et al. (1963)
Reverse mutations bacterium Escherichia coli Hussain & Osterman-Golkar
SD-4 (1984)
Reverse mutations Salmonella typhimurium Rannug et al. (1976);
(base-pair TA1535, TA100 Kauhanen (1978)b;
substitutions) Pfeiffer & Dunkelberg
(1980)
Reverse mutations Bacillus subtilis Tanooka (1979)
(spores) HA101, TKJ5211,
TKJ8201
Reverse mutations fungus Neurospora crassa Kolmark & Westergaard
(macroconidia) (1953); Kolmark & Kilby
(1968)
Forward mutations Aspergillus nidulans Morpurgo (1963)
Forward mutations Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked insect Drosophila melanogaster Bird (1952); Nakao &
recessive lethals Auerbach (1961)
Forward mutations mammal Chinese hamster Tan et al. (1981)b;
on specific locus ( in vitro) ovary cells Zamora et al. (1983)
Chromosome damage
breaks, erosions, plant Tradescantia paludosa Smith & Lofty (1954)
contractions (pollen)
translocations barley Ehrenberg et al. (1956,
1959)
breaks barley Moutschen-Dahmen et al.
(1968)
breaks wheat (hexaploid) MacKey (1968)
translocations insect Drosophila melanogaster Nakao & Auerbach (1961)
small deletions insect Drosophila melanogaster Fahmy & Fahmy (1970)
(min)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Chromosome damage (contd.)
breaks, human anion cells Poirier & Papadopoulo
gaps, exchanges, ( in vitro) (1982)
complexes
sister chromatide human lymphocytes Star (1980a);
exchanges ( in vitro) fibroblasts Garry et al. (1982)
rat lymphocytes Kligerman et al. (1983)
(inhalation)
rabbit lymphocytes Yager & Benz (1982)
(inhalation)
sister chromatide monkey lymphocytes Lynch et al. (1984b)d
exchanges, (inhalation)
breaks, acentric
fragments,
dicentrics,
triradials,
quadriradials,
complex
rearrangements
chromosomal rat bone marrow cells Strekalova et al. (1975)
aberrations (inhalation)
breaks, gaps, rat bone marrow cells Embree & Hine (1975)
rearrangement, (inhalation)
exchanges,
ring formations
micronuclei mouse (ip) polychromatic Conan et al. (1979);
erythrocytes Jenssen & Ramel (1980);
Lyarskii et al. (1983)
micronuclei mouse (iv) polychromatic Appelgren et al. (1978)
erythrocytes
heritable mouse (ip) germ cells Generoso et al. (1980)
translocations
dominant lethals mouse germ cells Cumming & Michaud (1979)
(inhalation)
mouse (ip) Generoso et al. (1980);
Lyarskii et al. (1983);
mouse Generoso et al. (1983)
(inhalation)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
dominant lethals rat Embree et al. (1977)
(contd.) (inhalation)
DNA repair
unscheduled mouse germ cells Cumming & Michaud (1979)
DNA synthesis (inhalation)
unscheduled human lymphocytes Pero et al. (1981)
DNA synthesis ( in vitro)
----------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b A similar effect after metabolic activation by rat liver microsomal fraction.
c A slight reduction in mutagenicity after metabolic activation by mouse liver
microsomal fraction.
d 2-year exposure groups of 12 male monkeys to 0, 90, and 180 mg/m3 for 7 h per day and
5 days per week.
Dose-related damage to germ cells was established in the mid
and late spermatid stages in the dominant lethal assay. One oral
dose of 100 mg/kg body weight in mice generated inconsistent
results (Appelgren et al., 1977), but 150 mg/kg proved positive
(Generoso et al., 1980). After short-term repeated exposures,
dominant lethals were induced in mice at intraperitoneal doses from
40 mg/kg body weight, given over a period of 3 months, 5 times per
week (Lyarskii et al., 1983) and at vapour exposures from
460 mg/m3, for 6 h/day, 5 days per week, over 11 weeks (Generoso et
al., 1983). Heritable translocations were induced in the germ
cells of mice after repeated intraperitoneal exposure, at doses of
30 mg/kg body weight or more, for 5 days/week, over a 5-week period
(Generoso et al., 1980). Recent studies have investigated the
dose-response of inhaled ethylene oxide and have compared effects
of different dose rates (contributions of different concentrations
and durations of exposure, maintaining total exposure (C x t)
constant) on the dominant-lethal response in male mice. In the
dose-reponse study, male mice were exposed by inhalation to
ethylene oxide at concentrations of 540, 720, or 900 mg/m3 (300,
400, or 500 ppm), respectively. Exposures were for 6 h/day, for 4
consecutive days. A dose-related increase in dominant-lethal
mutations was observed; however, the dose-response curve was
nonlinear, i.e., increasing embryonic mortality occurred with
increasing mg/m3 x h. In the dose-rate study, mice had a total
exposure of 3240 mg/m3 (1800 ppm) x h/day for 4 consecutive days,
delivered either at 540 mg/m3 (300 ppm) in 6 h, 1080 mg/m3 in 3 h,
or 2160 mg/m3 (1200 ppm) in 1.5 h. The highest airborne
concentration resulted in the greatest embryonic mortality, 64%,
versus 32 and 11% for the intermediate and lowest airborne
concentrations, respectively (Generoso et al., 1985). According to
an abstract, DNA repair was induced in the germ cells of mice
exposed to 540 mg/m3, for 8 h. The repair seemed inhibited at
higher exposures (Cumming & Michaud, 1979). No details were
available.
Negative results were observed on a few occasions only. In one
study, with vapour-exposed rats, chromosome aberrations or slowing
of mitotic activity and cell cycle kinetics were not observed in
lymphocytes at levels at which sister chromatid exchanges occurred
(Kligerman et al., 1983). In a dominant-lethal assay with mice,
relatively high intravenous doses of ethylene oxide (up to
100 mg/kg body weight) did not cause any treatment-related effects
(Appelgren et al., 1977). An intraperitoneal dose of 10 mg/kg body
weight to mice was reported to give a slight increase in the number
of polychromatic erythrocytes with micronuclei, but the statistical
analysis was not adequate (Conan et al., 1979).
7.6. Effects on Reproduction
Rats and guinea-pigs were exposed to vapour concentrations of
370 and 640 mg ethylene oxide/m3, for 7 h per day, 5 days per week,
for up to 32 weeks. Among other effects (section 7.2.1),
degeneration of testes tubules was observed at the higher exposure
level in guinea-pigs, while at 370 mg/m3, there was a decrease in
the relative weights of testes in rats and guinea-pigs, which was
not statistically significant (Hollingsworth et al., 1956).
Significantly-decreased absolute testicular weights were observed
in mice exposed to ethylene oxide at a concentration of 425 mg/m3,
for 6 h/day, 5 days per week, over 10 - 11 weeks (Snellings et al.,
1984a). However, the testicular effects may have been secondary to
toxic effects (e.g., growth inhibition). Male and female Fischer
344 rats exposed repeatedly to concentrations of ethylene oxide of
up to 182 mg/m3, for 6 h/day, 5 days per week, over 25 months, did
not show any histopathological effects on the reproductive tissues
(Snellings et al., 1981).
When groups of 12 male Cynomolgus monkeys were exposed to
concentrations of ethylene oxide at 90 or 180 mg/m3, for 7 h/day,
5 days per week, over 2 years, spermatogenic functions were found
to differ from those of controls. At both exposure levels, sperm
motility and sperm count were decreased and the sperm drive range
was increased, but there was no increase in effect with increase in
dose. The incidence of abnormal sperm heads did not change (Lynch
et al., 1984c).
Groups, each comprising 30 male and 30 female Fischer 344 rats,
were exposed to concentrations of ethylene oxide (purity 99.9%) of
18, 58, or 173 mg/m3, for 6 h/day, 5 days per week, over 12 weeks.
Two control groups of 30 rats per sex each were exposed to air
only. After mating, females were further exposed for 7 days/week,
up to 3 weeks after delivery, with the exception of the first 5
days of lactation. Effects on the reproductive performance were
detected. The number of pups per litter was decreased at 173
mg/m3, as well as the number of implantation sites per female, and
the number of fetuses born per implantation site. The number of
females with a gestation period longer than 22 days was also
increased at this concentration, but no effects were noted on the
average length of the gestation period. Neither parents nor pups
showed signs of toxicity from ethylene oxide. The percentages of
pregnant females and fertile males were not affected (Snellings et
al., 1982a).
7.7. Teratogenicity
Groups of 22 female Fischer 344 rats were exposed to
concentrations of ethylene oxide of 18, 58, or 173 mg/m3, for
6 h/day, on days 6 - 15 of gestation. Two control groups
comprising 22 rats each were exposed to air only. The numbers of
pregnant dams ranged from 17 to 22. Maternal behaviour was normal,
and there were no deaths. The only effect on the fetuses was a 5 -
8% decrease in weight at 180 mg/m3 (Snellings et al., 1982b).
Groups of 32 - 45 female Sprague Dawley rats were exposed to
concentrations of ethylene oxide (purity 99.7%) of 0 or 270 mg/m3,
for 7 h/day, on days 7 - 16 of gestation (Group 1) or on days 1 -
16 of gestation (Group 2) or during 3 weeks before mating (5 days
per week), and on days 1 - 16 of gestation (Group 3). No dams
died, but body weights were decreased in Group 3. In all exposed
groups, the relative and absolute weights of kidney and spleen were
increased. The results of histopathological examination did not
show any abnormalities. There was a significant increase in
resorptions per litter and per implantation site in Group 3, with
no significant effects on the number of implants, live fetuses, and
pregnancies. In all exposed groups, weights and lengths of the
fetuses were decreased. Reduced ossification of sternebrae and
primary skull was observed (Hackett et al., 1982).
New Zealand rabbits were similarly exposed to a concentration
of ethylene oxide of 270 mg/m3 from days 1 - 19 or from days 7 - 19
of gestation. There was no evidence of toxicity in the mothers,
embryos, or fetuses, or of developmental defects (Hackett et al.,
1982).
Groups of 24 - 37 female CD-1 mice each received,
intraveneously, doses of 0, 75, or 150 mg ethylene oxide (purity
not stated)/kg body weight in an aqueous dextrose solution on days
4 - 6, 6 - 8, 8 - 10, or 10 - 12 of pregnancy. Dams exposed on
days 6 - 8 of pregnancy did not show toxic signs. In the other
groups, at the highest dose, toxic signs such as weakness, laboured
respiration, and tremor were observed with a mortality rate of 19 -
48%. In the group without signs of maternal toxicity, fetotoxicity
was observed at 150 mg/kg, as shown by a 20% decrease in mean fetal
weight. Fetal malformations were shown in 19.3% of fetuses in
exposed litters compared with 2% in control groups. These
malformations were mainly fused cervical arches. In addition,
fused thoracic arches, scrambled and fused sternebrae, and fused,
branched, or missing thoracic ribs were observed (Laborde & Kimmel,
1980).
8. EFFECTS ON MAN
8.1. Exposure of the Skin and Eyes
Undiluted ethylene oxide, applied to the skin of volunteers,
evaporated rapidly without leaving any mark or irritation. A
15-min exposure to cotton wool soaked in undiluted ethylene oxide
also did not produce any effects (Greaves Walker & Greeson, 1932).
However, with exposure to larger quantities, there may be
sufficient cooling to produce a lesion similar to frost-bite (Hine
& Rowe, 1981). Skin injury following exposure to aqueous solutions
of ethylene oxide is characterized by the appearance of oedema and
erythema, 1 - 5 h after exposure, followed by the formation of
vesicles. On healing, incrustation, often with itching and
desquamation, is observed. The magnitude of the skin injury seems
to depend on the length of contact and the concentration, a 50%
aqueous solution (500 g/litre) being most hazardous. More
concentrated solutions were less harmful. The lowest concentration
tested, a 1% solution, produced a mild reaction after a 50-min
exposure. Such effects have been observed in a number of accidents
(Sexton & Henson, 1949, 1950; Joyner, 1964; Ippen & Mathies, 1970).
Vapour exposure was found to produce the above dermal effects,
mainly on the humid parts of the skin (Ippen & Mathies, 1970). The
effects were also found, to different extents, after exposure via
ethylene oxide-sterilized materials such as face masks, gloves, and
surgical gowns (Royce & Moor, 1955; Marx et al., 1969; Hanifin,
1971; Biro et al., 1974; Lamy et al., 1974). Patch tests on
volunteers with various sterilized materials containing residues of
ethylene oxide and its reaction products, showed erythema, without
oedema, after 4 - 8 h of contact, from a residue level of 890 mg
ethylene oxide/kg up to 2890 mg/kg of a polyvinyl chloride block.
Most skin types tolerated residues of ethylene oxide of up to
2270 mg/kg polyvinyl chloride film, 2800 mg/kg brown-milled rubber,
and 5100 mg/kg non-woven fabric (Shupack et al., 1981).
Accidental skin exposure to a 1% aqueous solution, from the
waist down, was also reported to result in effects on the nervous
system, such as nausea and repeated vomiting (Sexton & Henson,
1949).
Accidental exposure of the eyes to the vapour of ethylene oxide
can lead to conjunctivitis (Thiess, 1963; Joyner, 1964). Exposure
of 12 men via a leaking sterilizer resulted in neurological
disorders (section 8.3) in 4 of the men, 3 of whom had eye
cataracts; one of the latter also showed an increase in corneal
thickness. Two additional men showed only an increase in corneal
thickness (Gross et al., 1979; Jay et al., 1982). In one case of
accidental exposure of the eyes to pure ethylene oxide, only slight
irritation of the conjunctiva was seen (Thiess, 1963).
The implantation of artificial lenses, sterilized with ethylene
oxide, in 103 eyes was compared with the implantation of lenses,
sterilized with sodium hydroxide (200 control eyes), in a
retrospective study. The follow-up period was 10 months for the
exposed patients. Post-operative inflammatory complications
occurred in 30% of the eyes exposed to residues of ethylene oxide
or its reaction products compared with 9% of the control eyes.
Cystoid macular oedema with reduction in visual acuity developed in
16% of the exposed eyes and in 7% of the control eyes (Stark et
al., 1980).
8.2. Sensitization
No dermal sensitization was observed in a total of 47 workers
frequently exposed to ethylene oxide (Royce & Moor, 1955; Thiess,
1963). In another study using patch tests, one of 12 volunteers
showed a recurrent reaction, 3 weeks after the trial. When
challenged afterwards with a 2 mm-thick patch of polyvinyl chloride
containing 100 mg ethylene oxide/kg, a mild reaction was observed,
which reappeared after 3 weeks (Shupack et al., 1981) (section
8.1). When the skin of 8 workers was exposed repeatedly to aqueous
solutions of ethylene oxide, all sites of previous contact, with
and without a primary reaction, flared up in 3 of them showing
pruritus, erythema, and slight oedema, 5 - 9 days after the last
exposure (Sexton & Henson, 1950). Another case of an apparently
allergic reaction was reported. The patient concerned was exposed
to ethylene oxide via a sterilized face mask (Alomar et al., 1981).
A case of anaphylaxis has been reported in a patient receiving
haemodialysis treatment with equipment that had been sterilized
with ethylene oxide (Poothullil et al., 1975). A cause-effect
relationship with ethylene oxide exposure was demonstrated by
haptan specificity (Dolovich & Bell, 1978).
8.3. Accidental Inhalation Exposure
Respiratory tract irritation was reported as hoarseness
(Thiess, 1963) and cough (Metz, 1939) in 5 cases of acute
accidental exposure to ethylene oxide vapour.
Acute effects on the nervous system in nearly all inhalation
cases were marked by nausea, recurrent vomiting, and headache.
Less frequently reported effects included decreased consciousness
(one case of coma), excitement, sleeplessness, muscular weakness,
diarrhoea, and abdominal discomfort (Blackwood & Erskine, 1938;
Metz, 1939; Thiess, 1963; Capellini & Ghezzi, 1965).
Because of a leaking sterilizer, 4 young men were exposed
intermittently, for 2 - 8 weeks, to ethylene oxide at levels of
approximately 1000 mg/m3. Three of the men developed a reversible
peripheral neuropathy showing abnormal nerve conduction and, in 2
cases, headache, weakness and decreased reflexes in the
extremities, incoordination, and a wide-based gait. The fourth man
developed a reversible acute encephalopathy with headache, nausea,
vomiting, lethargy, recurrent motor seizures, agitation, and a
diffusely slow electroencephalogram (Gross et al., 1979).
Following this, 6 more cases were reported concerning sterilizer
operators, suffering from reversible peripheral neuropathy
following ethylene oxide exposure for 0.5 - 1.5 years. Finelli et
al. (1983) described 3 persons showing subacute polyneuropathy with
bilateral foot-drop, slowing of nerve conduction velocity, and
denervation potential on electromyography as the main findings.
All 3 persons had noticed the smell of ethylene oxide regularly at
work, while 2 persons experienced eye irritation. Polyneuropathy
was also reported in 3 sterilizer operators by Kuzuhara et al.
(1983). Two of these cases were described in detail. Sural nerve
biopsies revealed axonal degeneration with mild changes in the
myeline sheath. Unmyelinated fibres were also involved. Muscle
biopsies showed typical denervation atrophy.
8.4. Other Accidental Exposures
Severe respiratory problems due to inflammatory reactions in
the trachea and larynx were reported in 17 hospital patients who
had received endotracheal intubation. The tubes had been
sterilized with ethylene oxide (Marx et al., 1969; Holley & Gildea,
1971; Lipton et al., 1971; Mantz et al., 1972).
Reversible vocal paralysis was reported to be associated with
ethylene oxide exposure in 5 cases: one woman had been exposed to
vapour (Troisi, 1965), and the other 4 patients were exposed via
sterilized endotracheal tubes (Holley & Gildea, 1971). The vocal
cords showed no or only slight damage. In one of these patients,
who died from a cause unrelated to the intubation, myelin
degeneration of parts of the nervus vagus was noted at autopsy. It
was suggested, therefore, that the paralysis was of neural origin.
Four cases of shock, 1 - 10 h after endovascular examination,
were associated with the catheters used, which contained residues
of ethylene oxide. The presence of bacterial toxins was considered
unlikely. One patient died as a result of renal insufficiency
(Lebrec et al., 1977). Cases of cardiovascular collapse in
children, 3 of which were fatal, were considered by the authors to
be the result of residues of ethylene oxide in a heart-lung machine
on the basis of subsequent studies on dogs (Stanley et al., 1971)
(section 7.1.1). Among others, Hirose et al. (1963) and Clarke et
al. (1966) measured haemolysis due to residues in ethylene oxide-
sterilized plastic tubes in vitro.
8.5. Occupational Inhalation Exposure
The health status of 37 male operators from an ethylene oxide-
producing plant in the USA during the period 1953 - 62 was compared
with that of age-matched operators from other production units.
The average employment period was 11 years for exposed workers and
12 years for controls. The usual average exposure level was
between 9 and 18 mg/m3, with occasional peaks up to 230 mg/m3 for
one particular job (collecting a sample of the product). According
to the medical records, the health of the exposed workers was
somewhat better than that of the controls. A physical examination
and extensive clinical tests did not reveal any exposure-related
effects with the exception of a slightly increased white blood cell
count (Joyner, 1964).
Chromosomal damage was found in a group of 12 workers from a
hospital sterilization facility in the USA (section 8.6). The
maximum exposure concentration measured during sterilization was
65 mg/m3. Another group of 12 persons, who worked in the adjacent
operating room area, volunteered as representatives of an unexposed
or accidentally exposed group. To insure adequate control
throughout the study, unexposed laboratory staff members served as
a third group. Frequently-reported subjective complaints indicated
irritation of the mouth, throat, and eyes, and effects on the
nervous system, such as headache, nausea, speech difficulty, memory
loss, dizziness, and incoordination (Garry et al., 1979).
In Belgium, a group of 18 workers, using or distributing the
sterilant ethylene oxide, was compared with a well-matched control
group by means of a questionnaire, and by analyses for urinary
retinol-binding protein and albumin, beta-microglobulin, and
chromosomal damage in lymphocytes. The overall mean exposure level
was 7.6 mg/m3, and the time-weighted average exposure, over a
working day, ranged between 0.2 and 95 mg/m3. A significant
increase in the incidence of sleeplessness and leg cramps was
recorded, but not irritation or allergy. These studies did not
reveal any abnormalities with the exception of an increase in
sister chromatid exchanges in lymphocytes (Wolfs et al., 1983;
Laurent et al., 1984; section 8.7).
In a plant in Bulgaria, 196 workers engaged in the production
of ethene and ethylene oxide were examined. About 73% of all
concentrations of ethylene oxide measured were 1 mg/m3 or less,
while 27% were between 1 mg/m3 and 3.5 mg/m3. Significant
increases were found in deviations of the autonomous nervous system
and in neurosis-like manifestations, especially in female workers
(Spasovski et al., 1980). Because of a mixed exposure, it is
difficult to evaluate the findings.
Haematological changes were reported in a group of 27 workers
in an ethylene oxide manufacturing and processing plant, in Sweden,
in 1967. The exposure period varied from 2 to 20 years, the
average length being 15 years. Controls worked with ethylene oxide
in other departments, where no leakages were possible. No exposure
data were reported. When 2 cases of anaemia were excluded, there
was still a significantly-decreased haemoglobin value in exposed
workers. There was a 30% increase in the number of lymphocytes,
and one case of chronic lymphatic leukaemia was noted (Ehrenberg &
Hällström, 1967).
In the Federal Republic of Germany, 279 employees from 8 plants
in which alkene oxides were produced or processed, were examined
for morbidity during 1978. They were employed for an average of
10.8 years. Of these workers, 21 had been involved in accidents
with ethylene oxide. Taking into account age and length of
exposure, they were compared with groups of industrial and clerical
workers within the same company. The exposure concentrations were
not reported. The workers were also exposed to many other
chemicals, some of which may be carcinogenic for man.
No abnormalities were found that could be related to exposure
to ethylene oxide or propylene oxide. The investigators related
increases in haemoglobin and mean erythrocyte volume to smoking
habits. Slight lymphocytosis was found to be unrelated to exposure
time, though there was a distinct age influence (Stocker & Thiess,
1979).
8.6. Mortality Studies
Two studies were conducted in Sweden to investigate the
possible neoplastic effects from occupational exposure to ethylene
oxide (Hogstedt et al., 1979a,b, 1984). The first study originally
included 58 male and 172 female workers in a small factory,
sterilizing hospital equipment with a 1:1 mixture of ethylene oxide
and methyl formate, over a period from 1968 to 1977 (Hogstedt et
al., 1979a).
Two cases of leukaemia (one was diagnosed as chronic myeloid
leukaemia and the other as acute myelogenous leukaemia) occurred
among 68 women who were exposed to vapours from sterilized boxes
stored for weekly periods in a factory storage hall where about 30
persons were exposed at any one time. A third case was the local
male manager who developed primary macroglobulinaemia (Morbus
Waldenström; this case was later diagnosed as a non-Hodgkin
lymphoma), 9 years after the installation of the sterilization
equipment; his exposure was estimated to be about 3 h per week in
the storage hall. He is also reported to have had some exposure to
benzene in the past. The concentration of ethylene oxide in the
hall was in the range of 3.6 - 128 mg/m3, and the 8-h time-weighted
average in the breathing zone was calculated to be between 36 ±
18 mg/m3. The other workers had occasional exposure to ethylene
oxide, and 7 operators had relatively high exposure (amount
unspecified) during the sterilization process.
In a follow-up study (Hogstedt et al., 1984), a further case of
leukaemia was found in a woman who had been exposed to ethylene
oxide in the storage hall between 1969 - 72. In this study, the
cohort consisted of 203 workers who had been employed for more than
one year at the plant.
Altogether, 4 deaths from malignancies of the lymphoreticular
system were reported from this factory. The expected number was
0.3.
A second study to investigate the carcinogenic effects of
ethylene oxide was conducted on 241 Swedish male workers in an
ethylene oxide-producing plant (Hogstedt et al., 1979b). These
men were examined medically in 1960. Twenty-three deaths
occurred during the 16-year observation period dating from
1961 - 77 (13.5 expected). The excess mortality was due to cancer
and cardiovascular disease. Three cases of stomach cancer (0.4
expected) and 2 cases of leukaemia (one chronic myeloid and once
acute myeloid leukaemia) (0.14 expected) accounted for the excess
mortality from cancer. No increase in mortality was observed among
86 maintenance workers exposed intermittently to ethylene oxide
among 66 unexposed controls. Average exposure levels during
1941 - 47 were estimated to be below 25 mg/m3 and, during the 1950s
up to 1963, these levels were 10 - 50 mg/m3, but peak exposures
above the odour threshold (about 1000 mg/m3) were known to occur.
The ethylene oxide was manufactured by the chlorohydrin process
so that significant exposure to other chemicals such as 1,2-
dichloroethane, ethylene, ethylene-chlorohydrin, and bis(2-
chloroethyl)ether might have occurred.
This investigation was followed up by a study that extended the
period of observation up to 1982. Seven more deaths had occurred
among the workers exposed to ethylene oxide for the whole of the
working day against 6.6 expected. Three of these were due to
cancer (1.6 expected). Two of these 3 cases were cancer of the
stomach (0.2 expected) and one an oesophageal cancer (0.04
expected). In the period from 1961 - 82, 6 deaths due to stomach
or oesophageal cancer had occurred in workers exposed to ethylene
oxide for the whole of the working day (0.7 expected). Alimentary
tract cancer was observed in 2 maintenance workers (0.8 expected)
and in 1 unexposed worker (0.8 expected). One new case of chronic
myeloid leukaemia was reported during this follow-up period.
During the 20-year period of observation, a total of 17 cases of
cancer were notified to the Cancer Registry against 7.9 expected
(Hogstedt et al., 1984).
The Task Group evaluated these data and concluded that the
evidence was adequate to consider the mixtures of compounds to
which these workers were exposed as carcinogenic for human beings,
but inadequate to label ethylene oxide as a proved human
carcinogen. This conclusion was in agreement with that arrived at
by an International Agency for Research on Cancer Working Group
(IARC, 1985).
A similar study in the USA concerned 767 male workers at an
ethylene oxide producing plant. They were employed for at least
5 years between January 1955 and December 1977 and "potentially
exposed". Concentrations of ethylene oxide were reported to be
below 18 mg/m3, but no further details concerning exposure levels
were reported. Exposure to other chemicals was not reported.
Control data came from national statistics and was adjusted for
sex, age, and calendar time. There were 46 deaths against an
expected 80; there were 11 malignant neoplasms against 15.2
expected. No statistically-significant excess deaths could be
found due to any cause. There were no deaths due to leukaemia, 3
deaths from pancreatic cancer (0.8 expected), 1 death from bladder
cancer (0.3 expected), 2 deaths from brain cancer (0.7 expected),
and 2 deaths from Hodgkin's disease (0.4 expected) (Morgan et al.,
1981).
In the Federal Republic of Germany, 602 workers were
investigated for mortality experience during the period 1928 - 80.
The workers had been employed for at least 6 months in 8 plants
producing or processing ethylene oxide and propylene oxide. A
subcohort of 351 workers was observed for more than 10 years.
Control data came from a styrene plant and from national
statistics. Since 1978, exposure to ethylene oxide had normally
remained below 9 mg/m3. In the past, occasional excursions above
90 mg/m3 (50 ppm) had been reported. On one occasion during plant
breakdown, 3420 mg/m3 (1900 ppm) was measured. No statements were
offered concerning human exposure or the use of personal protective
equipment. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. There were 14 deaths from
cancer compared with 16.6 expected. No statistically-significant
excess deaths could be found due to any cause in the cohort. In
the subcohort of 351 workers, there was a significant increase in
mortality rate due to kidney disease (3 against 0.4 expected).
There was 1 death from gall bladder cancer, 1 death from urinary
bladder cancer, 1 death from brain cancer, and 1 death from myeloid
leukaemia. Two stomach tumours were observed compared with 1.8
expected (Thiess et al., 1981a,b).
8.7. Mutagenicity and Related End-Points
An increase in chromosomal aberrations was found in the
lymphocytes of 3 groups of workers sterilizing medical equipment in
hospitals or factories (Abrahams, 1980; Pero et al., 1981; Högstedt
et al., 1983). A 50% increase in aberration rate was found in 28
workers exposed to 8-h time-weighted average concentrations of
ethylene oxide below 1.8 mg/m3 in the 2.5 years before the study.
Before this period, higher exposures were reported to have
occurred. The workers had been exposed for 0.5 - 8 years. The
mean number of micronuclei in the bone marrow cells of 18 of these
workers was 3 times higher than in the controls (Högstedt et al.,
1983). Pero et al. (1981) found that, while workers exposed to
concentrations of ethylene oxide between 0.9 and 1.8 mg/m3, for
40 h per week, did not show an increased aberration rate; others,
exposed to bursts of ethylene oxide at concentrations between 9 and
18 mg/m3, for 8 h per week, did. The Task Group noted that the
sterilization procedure involved the use of a 50:50 mixture of
ethylene oxide and methyl formate. At the same time, DNA repair,
induced in vitro by the mutagen N-acetoxy-2-acetylamino-fluorene,
was reversibly inhibited in the low exposure group compared with an
unexposed control group, but not affected in the other group. DNA
repair inhibition was positively correlated with duration of
exposure (Pero et al., 1981). In 43 male workers from the cohort
of 602 workers (section 8.5) (Thiess et al., 1981b), an increase in
chromosomal aberration rate was found that was significant for the
workers exposed for more than 20 years, but not for those
accidentally exposed or exposed for average periods of 12 and
17 years (Thiess et al., 1981a).
In another ethylene oxide manufacturing plant, no chromosomal
aberrations were detected in the lymphocytes of 36 male workers.
These men had been exposed for 1 - 14 years to average
concentrations of up to 0.28 mg/m3 (van Sittert et al., 1985).
The sister chromatid exchange rate in lymphocytes was not
increased in groups of 28 and 14 sterilization workers exposed to
8-h time-weighted averages, below 1.8 mg/m3 for 2.5 years before
the study (Högstedt et al., 1983) and below 8 mg/m3 (Hansen et al.,
1984), respectively. In the second study, peaks up to 1430 mg/m3
were also measured. Increases in sister chromatid exchange rate
were found in 4 other studies on sterilization workers (Garry et
al., 1979; Abrahams, 1980; Yager et al., 1983; Laurent et al.,
1984). In one of these studies, in which 75 workers were exposed
to levels generally below an 8-h time-weighted average of 90 mg/m3,
this increase was found in cells together with quadriradial
aberrations (Abrahams, 1980). In the other studies, groups were
small, exposure conditions often unclear, and the number of
metaphases scored, in some cases, limited. Yager et al. (1983)
only found an increased sister chromatid exchange rate at
relatively high calculated integrated doses of more than 100 mg per
person. The length of exposure to ethylene oxide averaged 3.6 min
per day; these tasks were performed between 6 and 120 times during
the 6-month study period. Laurent et al. (1984), studied sister
chromatid exchange rates in 2 groups of ethylene oxide-exposed
workers (estimated ethylene oxide dose in past 2 years, 530 -
715 mg and 1185 - 5800 mg, respectively) as well as unexposed
controls. Although the sister chromatid exchange rates in the
high-exposure group did not differ significantly from the rates in
the low-exposure groups (not adjusted for smoking), the difference
among non-smokers between the exposed (n = 20) and the controls
(n = 15) was significant.
In a study on 41 sterilization workers in 8 hospitals in Italy,
increases in both sister chromatid exchanges and in chromosomal
aberrations were detected in lymphocytes; these effects persisted
for months after exposure was reduced or interrupted. The workers
were exposed to average 8-h time-weighted averages of either
0.63 mg/m3 or 19.3 mg/m3 (section 4.3). A statistically-
significant correlation was found between sister chromatid exchange
frequency and the level of ethylene oxide, as well as a multiple
correlation between sister chromatid exchange frequency and
ethylene oxide exposure, smoking, and age (Sarto et al., 1984).
Similarly, in the USA, the sister chromatid exchange frequencies in
the lymphocytes of 61 sterilization workers involved in sterilizing
health-care products, were monitored over a period of 2 years and
compared with those of 82 unexposed controls. During the study
period, 8-h time-weighted-average exposures were reported to be
less than 1.8 mg/m3. Prior to the start of the study, 8-h time-
weighted averages between 0.9 and 36 mg/m3 were measured. Results
were adjusted for smoking habits, sex, and age. Workers exposed to
low levels of ethylene oxide such as those at a worksite with 8-h
time-weighted-average ethylene oxide levels below 1.8 mg/m3 prior
to and during the study, did not show increased frequencies of
sister chromatid exchange. Workers who had been exposed to levels
of 5 - 36 mg/m3, prior to the study, showed an increased frequency
of sister chromatid exchange that persisted for at least 24 months
after cessation of exposure (Stolley et al., 1984).
8.8. Effects on Reproduction
In a study from the USSR (Yakubova et al., 1976), the course of
pregnancy and birth was followed in 57 operators, 38 laboratory
workers, and 65 adminstrative staff in an ethylene oxide-producing
plant, the majority of the women being between 20 and 29 years of
age. A group of 50 pregnant women working outside the plant served
as additional controls. It was estimated that the operators were
exposed to ethylene oxide concentrations of 0.2 - 0.3 mg/m3 for 80%
of the working time and 1.0 mg/m3 for the remaining 20%, while the
laboratory workers were exposed only to the lower concentrations.
Pregnancy toxaemia in the latter half of pregnancy and other
complications were higher in the operators (14.7%) and laboratory
workers (9.9%) than in the administrative staff (4.6%) and outside
controls (8%). On the other hand, the primiparae among the
operators lost less blood perinatally than those among the other
groups. Spontaneous abortion occurred in 6 out of 57 (10.5%)
operators, 3 out of 38 (7.9%) laboratory workers, and in 5 out of
65 (7.7%) administrative staff. The operators were subjected to
the additional stress of high levels of noise and vibration and
wide variations in atmospheric temperatures.
Findings in this study do not indicate any unequivocal adverse
effect of ethylene oxide exposure at these concentrations on the
outcome of pregnancy.
An increase in spontaneous abortions was also found in a study
on Finnish hospital sterilizing staff in 1980, using questionnaires
and hospital discharge records. The sterilizing agents were
ethylene oxide, glutaraldehyde, and formaldehyde. Controls were
nursing auxiliaries, from various hospitals, who did not work in
sterilization, anaesthetization, or X-ray recording departments.
Results were adjusted for age, parity, decade of the reported
pregnancy, coffee and alcohol consumption, and smoking habits.
The rate of spontaneous abortions in the sterilization staff
members as a whole (9.7% in 1443 pregnancies) was similar to the
rate in the controls (10.5% in 1179 pregnancies). A significant
increase, however, was observed when the adjusted spontaneous
abortion rate in the sterilization staff who were exposed during
pregnancy (15.1% in 545 pregnancies) was compared with the rate in
the staff members who were not exposed during pregnancy (4.6% in
605 pregnancies). On the basis of a separate study, the time-
weighted average exposure concentration was estimated to be in the
range of 0.18 - 0.90 mg/m3 (0.1 - 0.5 ppm), with peak concentrations
up to 450 mg/m3 (250 ppm). It was considered by the authors that
exposure to ethylene oxide accounted for most of the excess
spontaneous abortions (Hemminki et al., 1982).
In a new analysis of the data, controls were chosen from the
same hospitals, and only pregnancies that started during hospital
employment were analysed in all groups. The spontaneous abortion
rate was still highest for the pregnancies during which exposure to
ethylene oxide took place (20.4%), and the difference compared with
controls (11.3%) was significant. The abortion rate of the group
exposed to glutaraldehyde alone (16.6%) was also significantly
elevated (Hemminki et al., 1983).
Despite any methodological shortcomings of this reproductive
study, such as (a) the statement by Hemminki in 1983 that there was
limited exposure data, which prevented a comparison between
abortion rates and defined exposure levels; and (b) the fact that
the ethylene oxide-exposed and unexposed cohorts were not balanced
with regard to the incidence of prior abortions, there is a
suggestion of an association between ethylene oxide exposure and
adverse pregnancy outcome.
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
Human exposure is mainly through inhalation of the vapour.
Residues in medical equipment that has been sterilized with
ethylene oxide and not sufficiently aerated can migrate into
tissues and blood, producing primarily local effects (section 8.4).
Oral ingestion of ethylene oxide residues in most fumigated or
sterilized foodstuffs is unlikely, as they disappear rapidly
through evaporation or reaction with food constituents. A major
conversion product in foodstuffs is 2-chloroethanol, which is more
persistent than ethylene oxide (section 4.2.1).
Most ethylene oxide is used in the chemical plant in which it
is produced. Because of the explosion hazard, ethylene oxide is
stored and handled in chemical process plants in closed, automated
systems. This equipment is often located outdoors, and, except
during maintenance, workers have a minimal chance of exposure. Air
samples collected in processing areas of chemical production plants
have shown that ethylene oxide vapour concentrations are generally
less than 4 mg/m3 with occasional high peak exposures (section
4.3). Occupational exposure to ethylene oxide tends to be much
higher in health instrument manufacture and in hospitals than in
the chemical processing industries. Ethylene oxide concentrations
near malfunctioning or improperly designed equipment may reach
hundreds of mg/m3 of air for brief periods. However, 8-h time-
weighted average breathing-zone air concentrations in hospitals are
generally less than 36 mg/m3. It should be emphasized that the
exposure of hospital workers to ethylene oxide tends to be of a
short-term and intermittent nature with the likelihood of exposure
to short-term (5 - 120 min) concentrations of about 100 mg/m3 and
to peak concentrations of up to 1800 mg/m3 following the opening of
sterilization chambers (section 4.3).
Ethylene oxide has a high solubility in water but will
evaporate to a great extent. Degradation of ethylene oxide in
neutral water is slow, even in the presence of aerobic
microorganisms. Because of the low log n-octanol water-partition
coefficient, it is unlikely that ethylene oxide and its conversion
products (such as 1,2-ethanediol) will bioaccumulate. The toxicity
of ethylene oxide for aquatic organisms is low (all available LC50s
are above approximately 90 mg/litre) (Table 3). The probable
effects of ethylene oxide on the aquatic environment are,
therefore, considered negligible (sections 3.2, 6). There are no
data concerning the toxicity of ethylene oxide for terrestrial
organisms.
No ambient air monitoring data are available from which the
effects of ethylene oxide on the health of man and the environment
can be assessed. However, the risk for health from exposure to
ethylene oxide in the ambient air, apart from point source
emissions and accidental spillage, is likely to be negligible.
Inhaled ethylene oxide is readily absorbed into the blood,
distributed throughout the body, and rapidly metabolized. The
half-life in the tissues of man and rodents is approximately
10 min; clearance from the blood of dogs occurred with a half-
life of 33 min (section 5.2). Marked nausea and profuse vomiting
following dermal exposure of man to aqueous solutions of ethylene
oxide suggest that absorption can occur through the skin (section
5.1).
Case reports indicate that headache, nausea, vomiting,
dyspnoea, and respiratory tract irritation are common effects of
acute inhalation exposure to ethylene oxide (section 8.3). Case
reports and the results of animal studies indicate that
sensorimotor neuropathies may follow repeated exposure to
concentrations of ethylene oxide recognizable by its odour
(approximately 900 mg/m3 or more) (sections 2.2, 7.2.1, 8.3).
Dermatological effects in man following skin contact with
ethylene oxide include erythema, oedema, and vesiculation, in that
order. The severity of the skin injury is related to concentration
(a 50% (500 g/litre) solution being most hazardous) and duration of
contact. Liquid ethylene oxide, as it vaporizes, can result in a
freeze burn. On repeated exposure, ethylene oxide may cause
delayed allergic contact dermatitis (sections 8.1, 8.2). Ethylene
oxide and its conversion products are irritating to the eyes and
can produce corneal injury. Cataracts have occurred following
repeated exposure to concentrations of the vapour recognizable by
its odour (approximately 900 mg/m3 or more) (Table 1, sections 8.1,
8.3).
Ethylene oxide directly alkylates proteins and DNA and is
mutagenic in microorganisms, plants, insects, mammalian cells in
vitro, and mammals in vivo, including both gene mutations and
chromosomal abnormalities (section 7.5). In man, ethylene oxide
induces chromosomal aberrations and sister chromatid exchanges in
lymphocytes at air concentrations found at the workplace (section
8.7). Tissue distribution studies provide evidence that ethylene
oxide reaches the gonads, supporting the findings of heritable
mutations in insects and rodents (sections 5.2, 7.5). Ethylene
oxide may, therefore, be considered a potential human mutagen for
both somatic and germ cells.
The potential of ethylene oxide to cause teratogenic or adverse
reproductive effects has been examined in 4 animal species (mouse,
rat, rabbit, and monkey) by 2 routes of administration. Results
from these studies showed that ethylene oxide is toxic to
reproductive function in both males (reduced sperm number and sperm
motility, and an increased time to traverse a linear path) and
females (depression of fetal weight gain, fetal death, and fetal
malformation). The levels needed to produce these fetal effects
approach or equal the dose needed to produce maternal toxicity
(section 7.6). The results of animal studies suggest possible
reproductive impairment in human males but are inadequate for
assessing the fetal risk. Data on reproductive effects in human
beings are insufficient; one study, however, suggests an increase
in spontaneous abortion rate in women occupationally exposed to
ethylene oxide (section 8.8). However, the reported time-weighted
average air concentrations may not reflect the exposure levels that
induced the effect.
It has been clearly demonstrated in experimental animal studies
that ethylene oxide is carcinogenic via different routes of
exposure (intragastric, subcutaneous injection, and inhalation).
In 2 inhalation studies, confirmatory data demonstrated dose-
related increases in the incidences of leukaemia, peritoneal
mesothelioma, and cerebral glioma (section 7.4). Although the
evidence for the carcinogenicity of ethylene oxide in man is
inadequate, epidemiological studies indicate that exposure to
ethylene oxide (in mixtures with other chemicals) increases the
risk of malignancies (section 8.6).
Taking into account available data concerning the alkylating
nature of ethylene oxide, the demonstration of DNA adducts, the
overwhelming positive in vivo responses in mutagenic and
clastogenic assays, the reproducible positive carcinogenic findings
in animals, and the epidemiological findings suggesting an increase
in the incidence of human cancer, ethylene oxide should be
considered as a probable human carcinogen, and its levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. The study indicating that exposure to ethylene oxide may be
associated with spontaneous abortion needs to be
corroborated and the implication explored further.
2. The possible effects of ethylene oxide on the reproductive
function of man should be studied.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to ethylene oxide in combination
with other chemicals strongly suggest that additional
epidemiological studies should be carried out on
populations whose exposure has been primarily to ethylene
oxide, including adequate quantification of past exposure.
4. The temporal relationships of air concentrations of
ethylene oxide and duration of exposure must be examined to
determine which of these two factors has the greater impact
on health.
5. Development of methods and research should be conducted to
assess the endogenous occurrence of hydroxyethylation and
the exogenous (environmental) contribution of ethylene
oxide and its precursors to the formation of macromolecular
adducts as markers of internal dose.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of ethylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
ethylene oxide to experimental animals; there is limited
evidence for the carcinogenicity to humans of exposures to
ethylene oxide in combination with other chemicals; there is
inadequate evidence for the carcinogenicity to humans of
exposures to ethylene oxide alone. Taken together, the data
indicate that ethylene oxide is probably carcinogenic to
humans."
REFERENCES
ABRAHAMS, R.H. (1980) Recent studies with workers exposed to
ethylene oxide. In: Jorkasky, J.F., ed. Safe use of ethylene
oxide. Proceedings of the Educational Seminar, Washington DC,
Health Industries Manufacturers Association, pp. 27-38,
211-220 (HIMA Report No. 80-4)
ADLER, N. (1965) Residual ethylene oxide and ethylene glycol
in ethylene oxide sterilized pharmaceuticals. J. Pharm. Sci.,
54: 735-742.
ALOMAR, A., CAMARASA, J.M.G., NOGUERA, J., ASPINOLEA, F.
(1981) Ethylene oxide dermatitis. Contact dermatit., 7:
205-207.
ALTMAN, P.L. & DITTMER, D.S. (1974) Biological data book,
Bethesda, Maryland, Federation of American Societies for
Experimental Biology, Vol. 3.
APPELGREN, L.-E., ENEROTH, G., & GRANT, C. (1977) Studies on
ethylene oxide: whole-body autoradiography and dominant lethal
test in mice. In: Clinical toxicology. Proceedings of the
Meeting held at Edinburgh, June 1976, European Society of
Toxicology, Amsterdam, Excerpta Medica, Vol. 18, pp. 315-317.
APPELGREN, L.-E., ENEROTH, G., GRANT, C., LANDSTROM, L.-E., &
TENGHAGEN, K. (1978) Testing of ethylene oxide for
mutagenicity using the micronucleus test in mice and rats.
Acta pharmacol. toxicol., 43: 69-71.
BINDER, H. & LINDNER, W. (1972) [Determination of ethylene
oxide in the smoke of definitely unfumigated cigarettes.]
Fachliche Mitteilungen der Austria Tabakwerke A.G., 13:
215-220 (in German).
BIRD, M.J. (1952) Chemical production of mutations in
Drosophila: comparison of techniques. J. Genet., 50: 480-485.
BIRO, L., FISHER, A.A., & PRICE, E. (1974) Ethylene oxide
burns. Arch. Dermatol., 110: 924-925.
BLACKWOOD, J.D. & ERSKINE, E.B. (1938) Carboxide poisoning.
US Navy med. Bull., 36: 44-45.
BLIXT, S., EHRENBERGH, L., & GELIN, O. (1963) Studies of
induced mutations in peas. VII. Mutation spectrum and mutation
rate of different mutagenic agents. Agric. Hort. Genet., 21:
178-216.
BOGYO, S., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: epoxides, Syracuse, New York,
Center for Chemical Hazard Assessment, Syracuse Research
Corporation (Report prepared for US EPA) (Report No. EPA
560/11-80-005, PB 80-183197).
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
BROOKES, P. & LAWLEY, P.D. (1961) The alkylation of
guanosine and guanylic acid. J. Chem. Soc., pp. 3923-3928.
BUQUET, A. & MANCHON, P. (1970) Recherche et dosage des
résidus et dérivés dans un pain conservé a l'aide d'oxyde
d'éthylène. Chim. analytique, 52: 978-983.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CAPELLINI, A. & GHEZZI, I. (1965) [Two cases of acute
ethylene oxide poisoning.] Med. Lav., 56: 822-827 (in Italian).
CHAIGNEAU, M. & MURAZ, B. (1981) Action de l'oxyde
d'éthylène sur le tabac ( Nicotiana tabacum L.): absorption et
combinaisons. Ann. Pharm. Fr., 39: 305-311.
CLARKE, C.P., DAVIDSON, W.L., & JOHNSTON, J.B. (1966)
Haemolysis of blood following exposure to an Austrialian
manufactured plastic tubing sterilized by means of ethylene
oxide gas. Aust. NZ J. Surg., 36: 53-55.
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, Vol. 2a: toxicology, 3rd revised ed.,
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
p. 2166.
CONAN, L., FOUCAULT, B., SIOU, G., CHAIGNEAU, M., LE MOAN, G.,
& DOINEL, A. (1979) Contribution à la recherche d'une action
mutagène des résidus d'oxyde d'éthylène, d'éthylène glycol et
de chloro-2-éthanol dans le matériel plastique stérilisé par
l'oxyde d'éthylène. Ann. Fals. exp. Chim., 72: 141-151.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
CUMMING, R.B. & MICHAUD, T.A. (1979) Mutagenic effects of
inhaled ethylene oxide in male mice. Environ. Mutagen., 1:
166-167.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No.
600/3-80-084, PB 80-221948).
DAUVOIS, C., CHAIGNEAU, M., & LE MOAN, G. (1982)
Sterilisation de pansements par l'oxyde d'éthylène. I.
Physisorption. Ann. Pharm. Fr., 40: 125-132.
DOLOVICH, J. & BELL, B. (1978) Allergy to a product(s) of
ethylene oxide gas. Demonstration of IgE and IgG antibodies
and hapten specificity. J. Allergy clin. Immunol., 62: 30-32.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
DUNKELBERG, H. & HARTMETZ, G. (1977) [Recording the air
pollution by ethylene oxide in the region of clinical
sterilization installations.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
164: 271-278 (in German).
EDELHAUSER, H.F., ANTOINE, M.E., PEDERSON, H.J., & HIDDEMAN,
J.W., & HARRIS, R.G. (1983) Intraocular safety evaluation of
ethylene oxide and sterilant residues. J. Toxicol.-Cut. ocular
Toxicol., 2: 7-39.
EHRENBERG, L. & HALLSTROM, T. (1967) In: Kalling, L.O., ed.
Haematologic studies on persons occupationally exposed to
ethylene oxide, Vienna, International Atomic Agency, pp.
327-334 (Report No. SM92-26).
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1956)
Chemically induced mutation and sterility in barley. Acta
chem. Scand., 10: 492-494.
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1959) The
mutagenic effects of ionizing radiations and reactive ethylene
derivatives in barley. Hereditas, 45: 351-368.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
tissue doses in the mouse from air contaminiated with ethylene
oxide. Mutat. Res., 24: 83-103.
EMBREE, J.W. & HINE, C.H. (1975) Mutagenicity of ethylene
oxide. Toxicol. appl. Pharmacol., 33: 172-173.
EMBREE, J.W., LYON, J.P., & HINE, C.H. (1977) The mutagenic
potential of ethylene oxide using the dominant-lethal assay in
rats. Toxicol. appl. Pharmacol., 40: 261-267.
FOMENKO, V.N. & STREKALOVA, E.Y. (1973) [The mutagenic
effect of some industrial toxins as a function of
concentration and exposure time.] Toksikol. Nov. Prom. Him.
Veshchestr., 7: 51-57 (in Russian).
FAHMY, G.G. & FAHMY, M.J. (1970) Gene elimination in
carcinogenesis: reinterpretation of the somatic mutation
theory. Cancer Res., 30: 195-205.
FINELLI, P., MORGAN, T.F., YAAR, I., & GRANGER, C.V. (1983)
Ethylene oxide-induced polyneuropathy: a clinical and
electrophysiologic study. Arch. Neurol., 40: 419-421.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione.
J. Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Controlling exposure to alkene oxides.
Chem. Eng. Prog., 79: 39-43.
GARRY, V.F., HOZIER, J., JACOBS, D., WADE, R.L., & GRAY, D.G.
(1979) Ethylene oxide: evidence of human chromosomal effects.
Environ. Mutagen., 1: 375-382.
GARRY, V.F., OPP, C.W., WIENCKE, J.K., & LAKATUA, D. (1982)
Ethylene oxide induced sister chromatid exchange in human
lymphocytes using a membrane dosimetry system. Pharmacology,
25: 214-221.
GENEROSO, W.M., CAIN, K.T., KRISHNA, M., SHEN, C.W., & GRYDER,
R.M. (1980) Heritable translocation and dominant-lethal
mutation induction with ethylene oxide in mice. Mutat. Res.,
73: 133-142.
GENEROSO, W.M., CUMMING, R.B., BANDY, J.A., & CAIN, K.T.
(1983) Increased dominant-lethal effects due to prolonged
exposure of mice to inhaled ethylene oxide. Mutat. Res., 119:
377-379.
GERHARDT, U. & LADD EFFIO, J.C. (1983) [Ethylene oxide
residues in spices.] Fleisch wirtsch., 63: 606-608 (in German).
GESSNER, P.K., PARKE, D.V., & WILLIAMS, R.T. (1961) The
metabolism of 14C-labelled ethylene glycol. Biochem. J., 79:
482-489.
GILDING, D.K., REED, A.M., & BASKETT, S.A. (1980) Ethylene
oxide sterilization: effect of polymer structure and
sterilization conditions on residue levels. Biomaterials, 1:
145-148.
GILLESPIE, E.H., JACKSON, J.M., & OWEN, G.R. (1979) Ethylene
oxide sterilization: is it safe? J. clin. Pathol., 32:
1184-1187.
GLASER, Z.R. (1979) Ethylene oxide: toxicology review and
field study results of hospital use. J. environ. Pathol.
Toxicol., 2: 173-208.
GORDON, H.T. & THORNBURG, W.W. (1959) Hydroxyethyl
derivatives in prunes fumigated with 14C-ethylene oxide.
J. agric. food Chem., 7: 196-200.
GREAVES WALKER, W.G. & GREESON, C.E. (1932) The toxicity of
ethylene oxide. J. Hyg., 32: 409-416.
GROSS, J.A., HAAS, M.L., & SWIFT, T.R. (1979) Ethylene oxide
neurotoxicity: report of four cases and review of the
literature. Neurology, 29: 978-983.
GRUNOW, W. & ALTMANN, H.-J. (1982) Toxicokinetics of
chloroethanol in the rat after single oral administration.
Arch. Toxicol., 49: 275-284.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Report No. PB
83-258038).
HANSEN, J.P., ALLEN, J., BROCK, K., FALCONER, J., HELMS, M.J.,
SHAVER, G.C., & STROHM, B. (1984) Normal sister chromatid
exchange levels in hospital sterilization employees exposed to
ethylene oxide. J. occup. Med., 26: 29-32.
HANIFIN, J.M. (1971) Ethylene oxide dermatitis. J. Am. Med.
Assoc., 217: 213.
HARTMAN, P.A. & BOWMAN, P.B. (1977) Simple GLC determination
of ethylene oxide and its reaction products in drugs and
formulations. J. Pharm. Sci., 66: 789-792.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEMMINKI, K., MUTANEN, P., SALONIEMI, I., NIEMI, M-L., &
VAINIO, H. (1982) Spontaneous abortions in hospital staff
engaged in sterilizing instruments with chemical agents.
Br. med. J., 285: 1461-1463.
HEMMINKI, K., MUTANEN, P., & NIEMI, M.-L. (1983) Spontaneous
abortions in hospital sterilizing staff. Br. med. J., 286:
1976-1977.
HENNE, W., DIETRICH, W., PELGER, M., & SENGBUSCH, G., VON
(1984) Residual ethylene oxide in hollow-fibre dialyzers.
Artif. Organs, 8: 306-309.
HINE, C.H. & ROWE, V.K. (1981) Epoxy compounds. In: Patty,
S.A., ed. Industrial hygiene and toxicology, 3rd revised ed.,
New York, Interscience Publishers, Vol. IIa, p. 2141.
HIROSE, T., GOLDSTEIN, R., & BAILEY, C. (1963) Hemolysis of
blood due to exposure to different types of plastic tubing and
the influence of ethylene oxide sterilization. J. thorac.
cardiovasc. Surg., 45: 245-251.
HOGSTEDT, C., MALMQVIST, N., & WADMAN, B. (1979a) Leukemia
in workers exposed to ethylene oxide. J. Am. Med. Assoc., 241:
1132-1133.
HOGSTEDT, C., ROHLEN, O., BERNDTSSON, B.S., AXELSON, O., &
EHRENBERG, L. (1979b) A cohort study of mortality and cancer
incidence in ethylene oxide production workers. Br. J. ind.
Med., 26: 276-280.
HOGSTEDT, B., GULLBERG, B., HEDNER, K., KOLNIG, A-M.,
MITELMAN, F., SKERFVING, S., & WIDEGREN, B. (1983)
Chromosome aberrations and micronuclei in bone marrow cells
and peripheral blood lymphocytes in humans exposed to ethylene
oxide. Hereditas, 98: 105-113.
HOGSTEDT, C., ARINGER, L., & GUSTAVSSON, A. (1984) [Ethylene
oxide and cancerreview of the literature and follow-up of 2
studies.] Arbete och Hälsa, 49: : 1-32 (in Swedish).
HOLLEY, H.S. & GILDEA, J.E. (1971) Vocal cord paralysis
after tracheal intubation. J. Am. Med. Assoc., 215: 281-284.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of ethylene oxide determined
on experimental animals. Arch. ind. Health, 13: 217-227.
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationship for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1985) Alkyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans No. 36).
IPPEN, H. VON & MATTHIES, V. (1970) [Protracted chemical
burns.] Berufsdermatosen, 18: 144-165 (in German).
IRPTC (1984) Data profile on ethylene oxide, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide vapors.
Arch. ind. Health, 13: 237-244.
JANA, M.K. & ROY, K. (1975) Effectiveness and efficiency of
ethyl methane-sulphonate and ethylene oxide for the induction
of mutations in mice. Mutat. Res., 28: 211-215.
JAY, W.M., SWIFT, T.R., & HULL, D.S. (1982) Possible
relationship of ethylene oxide exposure to cataract formation.
Am. J. Ophthalmol., 93: 727-732.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction
of carcinogenicity evaluated by 143 agents tested. Mutat.
Res., 75: 191-202.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rat.
Biochem. Pharmacol., 16: 185-199.
JONES, A.R. & WELLS, G. (1981) The comparative metabolism of
2-bromoethanol and ethylene oxide in the rat. Xenobiotica, 11:
763-770.
JORDY, A. (1983) [The course of the concentrations of
ethylene oxide reaction products in synthetic materials
following gas sterilization.] Hyg. Med., 8: 17-19 (in German).
JOSHI, S.B., DODGE, M.C., & BUFALINI, J.J. (1982)
Reactivities of selected organic compounds and contamination
effects. Atmos. Environ., 16: 1301-1310.
JOYNER, R.E. (1964) Chronic toxicity of ethylene oxide.
Arch. environ. Health, 8: 700-710.
KAUHANEN, K. (1978) Ethylene oxide. Rebuttable presumption
against registration maximum residue limits and daily levels
of exposure. Fed. Reg., 43:: 3804.
KLIGERMAN, A.D., EREXSON, G.L., PHELPS, M.E., & WILMER, J.L.
(1983) Sister-chromatid exchange induction in peripheral
blood lymphocytes of rats exposed to ethylene oxide by
inhalation. Mutat. Res., 120: 37-44.
KOLMARK, H.G. & KILBEY, B.J. (1968) Kinetic studies of
mutation induction by epoxides in Neurospora crassa. Molec.
Gen. Genet., 101: 89-98.
KOLMARK, H.G. & WESTERGAARD, M. (1953) Further studies on
chemically-induced reversions at the adenine locus of
Neurospora. Hereditas, 39: 209-224.
KORPELA, D.B., MCJILTON, C.E., & HAWKINSON, T.E. (1983)
Ethylene oxide dispersion from gas sterilizers. Am. Ind. Hyg.
Assoc. J., 44: 589-591.
KROLLER, E. VON (1966) [Investigations into the fumigation
of foodstuffs by ethylene oxide and into the determination of
its residues.] Dtsch. Lebensm. Rundsch., 62: 227-234 (in
German).
KUZUHARA, S., KANAZAWA, I., NAKANISHI, T., & EGASHIRA, T.
(1983) Ethylene oxide polyneuropathy. Neurology, 33: 377-380.
LABORDE, J.B. & KIMMEL, C.A. (1980) The teratogenicity of
ethylene oxide administered intraveneously to mice. Toxicol.
appl. Pharmacol., 56: 16-22.
LAHAYE, D., ASSCHE, F., VAN, & THEUNISSEN, A. [Ethylene oxide
levels in the sterilization units of hospitals.] Tijdschr.
Soc. Gezondheidsz., 62: 707-713 (in Dutch).
LAMY, F., LACHAPELLE, J.-M., & BRAEKEL, G. VAN (1974)
Dermites professionnelles a l'oxyde d'éthylène chez des sujets
travaillant en zone stérile. Arch. Mal. prof. Méd. Trav.
Sécur. soc., 35: 719-724.
LAURENT, C., FREDERIC, J., & LEONARD, A.Y. (1984) Sister
chromatid exchange frequency in workers exposed to high levels
of ethylene oxide, in a hospital sterilization service.
Int. Arch. occup. environ. Health, 54: 33-43.
LEBREC, D., MASQUET, C., & RUEFF, B. (1977) Collapsus après
exploration endovasculaire avec des cathéters stérilisés à
l'oxyde d'éthylène. Nouv. Presse Med., 6: 2991.
LIPTON, B., GUTIERREZ, R., BLAUGRUND, S., LITWAK, R.S., &
RENDELL-BAKER, L. (1971) Irridiated PVC plastic and gas
sterilization in the production of tracheal stenosis following
tracheostomy. Anest. Analg., 50: 578-586.
LYARSKII, P.P., YURCHENKO, V.V., ZHURKOV, V.S., & GLEIBERMAN,
S.E. (1983) [Mutagenic hazards of parenteral administration
of ethylene oxide to mammals.] Gig. i Sanit., 1: 23-26 (in
Russian).
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76: 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.H., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76: 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MACKEY, J. (1968) Mutagenesis in Vulgare wheat. Hereditas,
59: 505-517.
MCCHESNEY, E.W., GOLBERG, L., PAREKH, C.K., RUSSELL, J.C., &
MIN, B.H. (1971) Reappraisal of the toxicology of ethylene
glycol. II. Metabolism studies in laboratory animals. Food
cosmet. Toxicol., 9: 21-30.
MCDONALD, T.O., KASTEN, K., HERVEY, R., GREGG, S., BORGMANN,
A.R., & MURCHISON, T. (1973) Acute ocular toxicity of
ethylene oxide, ethylene glycol, and ethylene chlorohydrin.
Bull. Parenter. Drug Assoc., 27: 153-164.
MCGUNNIGLE, R.G., RENNER, J.A., ROMANO, S.J., & ABODEELY,
R.A. (1975) Residual ethylene oxide: levels in medical grade
tubing and effects on an in vitro biological system.
J. Biomed. Mater. Res., 9: 273-383.
MANTZ, J.M., TEMPE, J.D., JAEGER, A., & VIDAL, S. (1972)
Stenoses trachéales et stérilisation des canules de
trachéotomie par l'oxyde d'éthylène. Sem. Hôp. Paris, 48:
3367-3370.
MARTIS, L., KROES, R., DARBY, T.D., & WOODS, E.F. (1982)
Disposition kinetics of ethylene oxide, ethylene glycol, and
2-chloroethanol in the dog. J. Toxicol. environ. Health, 10:
847-856.
MARX, G.F., STEEN, S.N., SCHAPIRA, M., ERLANGER, H.L., ARKINS,
R.E., JADWAT, C.M., & KEPES, E. (1969) Hazards associated
with ethylene oxide sterilization. NY State J. Med., 69:
1319-1320.
METZ, E. VON (1939) [Poisoning by ethylene oxide (Cartox or
T-gas).] Samml. Vergiftungsfällen, 10: 37-38 (in German).
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on
Schizosaccharomyces pombe: the influence "in vitro" of
mouse-liver metabolizing system. Mutat. Res., 102: 425-437.
MORGAN, R.W., CLAXTON, K.W., DIVINE, B.J., KAPLAN, S.D., &
HARRIS, V.B. (1981) Mortality among ethylene oxide workers.
J. occup. Med., 23: 767-770.
MORPURGO, G. (1963) Induction of mitotic crossing-over in
Aspergillus nidulans by bifunctional alkylating agents.
Genetics, 48: 1259-1263.
MOUILLESEAUX, A., LAURENT, A.-M., FABRE, M., JOUAN, M., &
FESTY, B. (1983) Teneurs atmosphériques en oxyde d'éthylène
décelées dans l'environnement professionnel d'instalations de
stérilisation ou de désinfection. Arch. Mal. Prof., 44: 1-14.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., & EHRENBERG, L.
(1968) Note on the chromosome breaking activity of ethylene
oxide and ethyleneimine. Hereditas, 60: 267-269.
NAKAO, Y. & AUERBACH, C. (1961) Test of a possible
correlation between cross-linking and chromosome breaking
abilities of chemical mutagens. Z. Vererbungsl., 92: 457-461.
O'LEARY, R.K., WATKINS, W.D., & GUESS, W.L. (1969)
Comparative chemical and toxicological evaluation of residual
ethylene oxide in sterilized plastics. J. Pharm. Sci., 58:
1007-1010.
OSER, B.L. & HALL, L.A. (1956) The effect of ethylene oxide
treatment on the nutritive value of certain foods. Food
Technol., 10: 175-178.
OSTERMAN-GOLKAR, S (1983) Tissue doses in man: implications
in risk assessment. In: Hayes, A.W., ed. Developments in the
Science and Practice of Toxicology. Proceedings of the 3rd
International Congress on Toxicology, San Diego, California.
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor.
Mutat. Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog. Mutag.,
3: 395-405.
PERO, E.W., WIDEGREN, B., HOGSTEDT, B., & MITELMAN, F.
(1981) In vivo and in vitro ethylene oxide exposure of human
lymphocytes assessed by chemical stimulation of unscheduled
DNA synthesis. Mutat. Res., 83: 271-289.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food cosmet. Toxicol., 18: 115-118.
PFEILSTICKER, K. & SIDDIQUI, I.R. (1976) [Isolation of the
derivatives from cocoa-powder fumigated by ethylene oxide
1,2-14C and their structure suggested on the basis of I.R. and
mass-spectrometry.] Z. Lebensm. Unters. Forsch., 160: 19-27
(in German).
PFEILSTICKER, K., FABRICIUS, G., & TIMME, G. (1975)
[Simultaneous gas chromatographic determination of ethylene
oxide, ethylene chlorohydrin, and ethylene glycol in grain.]
Z. Lebensm. Unters. Forsch., 158: 21-25 (in German).
POIRIER, V. & PAPADOPOULO, D. (1982) Chromosomal aberrations
induced by ethylene oxide in a human amniotic cell line in
vitro. Mutat. Res., 104: 255-260.
POOTHULLIL, J., SHIMIZU, A., DAY, R.P., & DOLOVICH, J.
(1975) Anaphylaxis from the product(s) of ethylene oxide gas.
Ann. intern. Med., 82: 58-60.
QUAZI, A.H. & KETCHAM, N.H. (1977) A new method for
monitoring personal exposure to ethylene oxide in the
occupational environment. Am. Ind. Hyg. Assoc. J., 38: 635-647.
RADDING, S.B., LIU, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances, (EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide.
J. Assoc. Offic. Agric. Chem., 51: 709-715.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs.
Bull. environ. Contam. Toxicol., 27: 426-431.
RANNUG, U., GOTHE, R., & WACHTMEISTER, C.A. (1976) The
mutagenicity of chloroethylene oxide, chloroacetaldehyde,
2-chloroethanol, and chloroacetic acid, conceivable metabolites
of vinyl chloride. Chem.-Biol Interact., 12: 251-263.
RILLAER, W.G., VAN & BEERNAERT, H. (1982) Determination of
residual ethylene chlorohydrin (ECH) in fumigated foodstuffs
by glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 175: 175-178.
ROMANO, S.J. & RENNER, J.A. (1979) Analysis of ethylene
oxide-worker exposure. Am. Ind. Hyg. Assoc. J., 40: 742-745.
ROMANO, S.J., RENNER, J.A., & LEITNER, P.M. (1973) Gas
chromatographic determination of residual ethylene oxide by
head space analysis. Anal. Chem., 45: 2327-2330.
ROYCE, A. & MOORE, W.K.S. (1955) Occupational dermatitis
caused by ethylene oxide. Br. J. ind. Med., 12: 169-171.
SARTO, F., COMINATO, I., PINTON, A.M., BROVEDANI, P.G.,
FACCIOLI, C.M., BIANCHI, V., & LEVIS, A.G. (1984)
Cytogenetic damage in workers exposed to ethylene oxide.
Mutat. Res., 138: 185-195.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SEGERBACK, D. (1983) Alkylation of DNA and haemoglobin in
the mouse following exposure to ethene and ethene oxide.
Chem.-Biol. Interact., 45: 139-151.
SEXTON, R.J. & HENSON, E.V. (1949) Dermatological injuries
by ethylene oxide. J. ind. Hyg. Toxicol., 31: 297-300.
SEXTON, R.J. & HENSON, E.V. (1950) Experimental ethylene
oxide human skin injuries. J. ind. Hyg. Toxicol., 32: 549-564.
SHULOVSKA, K., LINDGREN, D., ERIKSSON, G., & EHRENBERG, L.
(1969) The mutagenic effect of low concentrations of ethylene
oxide in air. Hereditas, 62: 264-266.
SHUPACK, J.L., ANDERSEN, S.R., & ROMANO, S.J. (1981) Human
skin reactions to ethylene oxide. J. Lab. clin. Med., 98:
723-729.
SITTERT, N.J., VAN, JONG, G., DE, CLARE, M.G., DAVIES, R.,
DEAN, B.J., WREN, L.J., & WRIGHT, A.S. (1985) Cytogenetic,
immunological, and haematological effects in workers in an
ethylene oxide-manufacturing plant. Br. J. ind. Med., 42:
19-26.
SMITH, H.H. & LOTFY, T.A. (1954) Comparative effects of
certain chemicals on tradescantia chromosomes as observed at
pollen tube mitosis. Am. J. Bot., 41: 489-593.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg. Toxicol.,
23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1981)
Ethylene oxide: two-year inhalation study on rats, Pittsburgh,
Pennsylvania, Bushy Run Research Center (Final Report No.
44-20).
SNELLINGS, W.M., ZELENAK, J.P., & WEIL, C.S. (1982a) Effects
on reproduction in Fischer 344 rats exposed to ethylene oxide
by inhalation for one generation. Toxicol. appl. Pharmacol.,
63: 382-388.
SNELLINGS, W.M., MARONPOT, R.R., ZELENAK, J.P., & LAFFOON,
C.P. (1982b) Teratology study on Fischer 344 rats exposed to
ethylene oxide by inhalation. Toxicol. appl. Pharmacol., 64:
476-481.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984a) A
subchronic inhalation study on the toxicologic potential of
ethylene oxide in B6C3F1 mice. Toxicol. appl. Pharmacol., 76:
510-518.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984b) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPASOVSKI, M., HRISTEVA, V., PERNOV, K., KIRKOV, V.,
DRJANOVSKA, T., PANOVA, Z., BOBEV, G., GINCHEVA, N., &
IVANOVA, S. (1980) [Health status of the workers from the
production of ethylene and ethylene oxide.] Khig. Zdraveopaz.,
23: 41-47 (in Russian).
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropathological
evaluation of monkeys exposed to ethylene and propylene oxide,
Kansas City, Missouri, Midwest Research Institute (Prepared for
NIOSH) (PB 83-134817).
STANLEY, P., BERTRANOU, E., FOREST, F., & LANGEVIN, L.
(1971) Toxicity of ethylene oxide sterilization of polyvinyl
chloride in open-heart surgery. J. thorac. cardiovasc. Surg.,
61: 309-314.
STAR, E.G. (1980a) [Mutagenic and cytotoxic effect of
ethylene oxide on human cell cultures.] Zbl. Bakt. Hyg. (I. Abt.
Orig. B), 170: 548-556 (in German).
STAR, E.G. (1980b) [Absorption and desorption of ethylene
oxide in anaesthesia supplies.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
170: 557-569 (in German).
STAR, E.G. (1980c) [Ethylene oxide residues and aeration
time after use of modern heated aerators.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 171: 18-24 (in German).
STAR, E.G. (1980d) [Gamma-rays and ethylene oxide
sterilization.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 171: 33-41
(in German).
STAR, E.G., CASELITZ, J., & LONING, T. (1980) [The effect of
ethylene oxide and chemical desinfectant residues upon the
larynx- and tracheal mucosa of rabbits.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 170: 539-547 (in German).
STARK, W.J., ROSENBLUM, P., MAUMENEE, A.E., & COWEN, C.L.
(1980) Postoperative inflammatory reactions to intraocular
lenses sterilized with ethylene oxide. Opthalmology, 87:
385-389.
STIJVE, T., KALSBACH, R., & EYRING, G. (1976) Determination
and occurrence of ethylene chlorohydrin residues in foodstuffs
fumigated with ethylene oxide. Mitt. Geb. Lebensm. Unters.,
67: 403-428.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STOLLEY, P.D., SOPER, K.A., GALLOWAY, S.M., NICHOLS, W.W.,
NORMAN, S.A., & WOLMAN, S.R. (1984) Sister-chromatid
exchanges in association with occupational exposure to
ethylene oxide. Mutat. Res., 129: 89-102.
STREKALOVA, E.Y., CHIRKOVA, Y.M., & GOLUBOVICH, Y.Y. (1975)
[Mutagenic action of ethylene oxide on sex and somatic cells
in male white rats.] Toksikol. Nov. Prom. Him. Veshchestr., 6:
11-16 (in Russian).
TAN, E.-L., CUMMING, R.B., & HSIE, A.W. (1981) Mutagenicity
and cytotoxicity of ethylene oxide in the CHO/HGPRT system.
Environ. Mutagen., 3: 683-686.
TANOOKA, H. (1979) Application of Bacillus subtilis spores
in the detection of gas mutagens: a case of ethylene oxide.
Mutat. Res., 64: 433-435.
THIESS, A.M. (1963) [Observation on the adverse health
effects of ethylene oxide.] Archiv. Toxikol., 20: 127-140 (in
German).
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkene
oxides (ethylene oxide/propylene oxide) and derivatives.
J. occup. Med., 23: 343-347.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & STOCKER, W.G.
(1981b) Mortality study on employees exposed to alkene oxides
(ethylene oxide/propylene oxide) and their derivatives. In:
International Symposium on Prevention of Occupational Cancer,
Helsinki, pp. 249-259.
TROISI, F.M. (1965) [Aphonia of late onset due to
occupational exposure to ethylene oxide.] Med. Lav., 56:
373-377 (in Italian).
UKITA, T., OKUYAMA, H., & HAYATSU, H. (1963) Modifications
of nucleosides and nucleotides. I. Reaction of ethylene oxide
with uridine and uridylic acid. Chem. Pharm. Bull. (Tokyo),
11: 1399-1404.
US DEPARTMENT OF LABOR (1984) Occupational exposure to
ethylene oxide: final standard. Fed. Reg., 49: 25734-25809.
US EPA (1985) Health assessment document for ethylene oxide,
Washington DC, US Environmental Protection Agency (EPA
600/8-84/009F).
USITC (1971) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
USITC (1981) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
VAN DUUREN, B.L., ORRIS, L., & NELSON, N. (1965)
Carcinogenicity of epoxides, lactones, and peroxy compounds.
Part II. J. Natl Cancer Inst., 35: 707-717.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. I. Toxicity profiles, Boca Raton,
Florida, CRC Press Inc., pp. 55-56.
VIRTANEN, P.O.I. (1963) Kinetics of the reactions of
ethylene oxide with nucleophiles. Am. Acad. Sci. Fenn. Ser. A.
II, 124: 1-89.
WAITE, C.P., PATTY, F.A., & YANT, W.P. (1930) Acute response
of guinea-pigs to vapours of some new commercial organic
compounds. IV. Ethylene oxide. Pub. Health Rep., 45: 1832-1843.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WESLEY, F., ROURKE, B., & DARBISHIRE, O. (1965) The
formation of persistent toxic chlorohydrins in foodstuffs by
fumigation with ethylene oxide and with propylene oxide.
J. food Sci., 30: 1037-1042.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WINDMUELLER, H.G. & KAPLAN, N.O. (1961) The preparation and
properties of N-hydroxyethyl derivatives of adenosine,
adenosine tri-phosphate, and nicotinamide adenine
dinucleotide. J. Biol. Chem., 236: 2716-2726.
WINDMUELLER, H.G., ACKERMAN, C.J., BAKERMAN, H., & MICKELSEN,
O. (1959) Reaction of ethylene oxide with nicotinamide and
nicotinic acid. J. Biol. Chem., 234: 889-894.
WOLFS, P., DUTRIEUX, M., SCAILTEUR, V., HAXHE, J.-J., ZUMOFEN,
M., & LAUWERIJS, R. (1983) Surveillance des travailleurs
exposés a l'oxyde d'éthylène dans une enterprise de
distribution de gaz stérilisants et dans des unités de
stérilisation de matériel médical. Arch. Mal. Prof., 44:
321-328.
WOODARD, G. & WOODARD, M. (1971) Toxicity of residuals from
ethylene oxide gas sterilization. In: Proceedings of the 1971
HIA Technical Symposium, Washington DC, Health Industries
Association.
YAGER, J.W. & BENZ, R.D. (1982) Sister-chromatid exchanges
induced in rabbit lymphocytes by ethylene oxide after
inhalation exposure. Environ. Mutagen., 4: 121-134.
YAGER, J.W., HINES, C.J., & SPEAR, R.C. (1983) Exposure to
ethylene oxide at work increases sister-chromatid exchanges in
human peripheral lymphocytes. Science, 219: 1221-1223.
YAKUBOVA, Z.N., SHAMOVA, H.A., MUFTAKNOVA, F.A., & SHILOVA,
L.F. (1976) [Gynaecological disorders in workers engaged in
ethylene oxide production.] Kazan. Med. Zh., 57: 558-560 (in
Russian).
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
When a single dose of 2 mg labelled ethylene oxide in
propanediol/kg body weight was applied intraperitoneally to rats,
43% of the administered radioactivity was excreted in the urine
within 50 h (41% within 24 h) of exposure, 9% as S-(2-
hydroxyethyl)cysteine and 33% as N-acetyl- S-(2-hydroxyethyl)
cysteine, both products of glutathione conjugation. Via the lungs,
1.5% was excreted as carbon dioxide and 1% as unmetabolized
ethylene oxide (Jones & Wells, 1981). The involvement of
glutathione-epoxide- S-transferase (EC 4.4.1.7) has not been
investigated further. In vitro glutathione conjugation of the
homologue propylene oxide was shown to proceed only in the presence
of an enzyme (Fjellstedt et al., 1973). In rabbits, no effect was
found on liver- and blood-glutathione levels, after 12 weeks of
exposure to concentrations of ethylene oxide at 18, 90, or 450
mg/m3, for 5 days per week, 6 h per day (Yager & Benz, 1982).
As ethylene oxide can react with chloride ions, and this
reaction is acid catalysed, 2-chloroethanol might be expected to be
a metabolite, especially after oral administration. However,
neither 2-chloroethanol, nor its metabolites (Johnson, 1967; Grunow
& Altman, 1982) have been found in the plasma, tissues, or urine of
species exposed to ethylene oxide.
Ehrenberg et al. (1974) found that an average of 74% of
labelled ethylene oxide, inhaled by mice, was excreted in the urine
within 24 h in the form of unidentified metabolites, and only 4%
within the next 24 h. Thus, on the basis of this and previously-
presented excretion data, excretion of metabolites of ethylene
oxide mainly takes place via the urine, within 24 h following
exposure.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
A summary of the acute toxicity of ethylene oxide for aquatic
organisms is presented in Table 3. Data on the most likely
reaction products, 1,2-ethanediol and 2-chloroethanol, are
included.
LC50s of ethylene oxide for aquatic species have been reported
to range from 90 mg/litre (goldfish, 24-h exposure) to 745 mg/litre
(brine shrimp, 48-h exposure). Microorganisms in activated sludge
showed 50% inhibition at concentrations between 10 and 100
mg/litre. Hydrolysis to 1,2-ethanediol results in detoxification.
The toxicity of 2-chloroethanol for aquatic organisms resembles
that of ethylene oxide, though 2-chloroethanol seems to be more
toxic for Daphnia magna. Nevertheless, under environmental
conditions, the conversion of ethylene oxide to 2-chloroethanol or
1,2-ethanediol will be slow.
In a study on the albino guppy, Poecilia reticulata, desorption
of ethylene oxide or its reaction products from non-aerated plastic
materials into water can lead to behavioural disturbances in the
fish almost immediately, and to death within about one h. No such
toxicity was found when the materials were aerated for 24 h
(O'Leary et al., 1969).
Ethylene oxide is very soluble in aqueous media and evaporates
from water to a significant degree. The log n-octanol water-
partition coefficient was reported to be -0.30 (Radding et al.,
1977). Thus, ethylene oxide will not bioaccumulate.
Table 3. Acute aquatic toxicitya
-----------------------------------------------------------------------------------------------------------
Organism Description T pH Dissolved Parameter Test Concentration Reference
(°C) oxygen substance (mg/litre)
-----------------------------------------------------------------------------------------------------------
Micro- activated 22 50% growth ethylene oxide 10 - 100 Conway et al.
organisms sludge inhibition 1,2-ethanediol > 10 000 (1983)b
Crustacea water flea 17 7.0 minimal 48-h LC50 ethylene oxide 212 Conway et al.
(Daphnia magna) aeration 1,2-ethanediol > 10 000 (1983)c
2-chloroethanol 100
Fish fathead minnow 22 7.0 no 96-h LC50 ethylene oxide 84 Conway et al.
(Pimephales aeration 1,2-ethanediol > 10 000 (1983)c
promelas) 2-chloroethanol 90
Fish goldfish 20 6-8 no 24-h LC50 ethylene oxide 90 Bridié et al.
(Carassius aeration 1,2-ethanediol > 5000 (1979a)d
auratus) (> 4
mg/litre)
Fish brine shrimp 24 7.0 minimal 48-h LC50 ethylene oxide 745 Conway et al.
(Artemia aeration 1,2-ethanediol > 20 000 (1983)e
salina) 2-chloroethanol 680
-----------------------------------------------------------------------------------------------------------
a All tests were static. Water analysis for the substance under test was reported by all authors.
b Incubation on a shaker for 16 h.
c Medium was fresh water, reconstituted using dechlorinated, carbon-treated tap water; 10 fish per
concentration.
d Medium was local tap water; 6 fish per concentration.
e Medium was sea water, reconstituted using dechlorinated, carbon-treated tap water, 30 - 50 shrimps per
concentration.
7. EFFECTS ON ANIMALS
7.1. Acute Exposures
7.1.1. Oral, intravenous, and inhalation studies
The LD50s for ethylene oxide, administered orally and dissolved
in water, were 330 mg/kg body weight for male rats and 280 and
365 mg/kg body weight for female and male mice, respectively (Smyth
et al., 1941; Woodard & Woodard, 1971). After inhalation, the 4-h
LC50s were 1500 and 1730 mg/m3 for mouse and dog, respectively, and
2630 mg/m3 for rat (Jacobson et al., 1956). 1,2-Ethanediol, a
metabolite, is less toxic: LD50s for rat were above 10 000 mg/kg
body weight, after oral administration, and 5210 mg/kg body weight,
after intravenous administration (Woodard & Woodard, 1971).
The slope of the dose-response curve in relation to the
mortality rate for ethylene oxide was steep. After oral
administration to rats, the difference between 0.1% mortality
(325 mg/kg) and 99.9% mortality (975 mg/kg) was approximately
650 mg/kg body weight (Smyth et al., 1941). After inhalation for
4 h, this difference was approximately 3000 mg/m3, in mice, and
approximately 5000 mg/m3 in rats. No deaths occurred in dogs at
1280 mg/m3 (Jacobson et al., 1956). While no guinea-pigs died
after inhalation of 450 mg ethylene oxide/m3 air for 8 h, the
majority did so at 2400 mg/m3 (Waite et al., 1930). In the above
mortality studies, the lungs and nervous system were the main
targets in rodents and dogs. In dynamic inhalation exposure
studies on guinea-pigs (Waite et al., 1930), rats, mice, and dogs
(Jacobson et al., 1956), nasal irritation was the first clinical
effect, as evidenced by scratching the nose, nasal discharge,
lachrymation, and salivation. Respiratory problems occurred
ranging from gasping to laboured breathing. Dogs exhibited
laboured breathing, vomited, and suffered convulsions. Guinea-
pigs, exposed to a concentration of 13 000 mg ethylene oxide/m3,
for 2.5 h, were found lying on their sides, unable to stand, and
quiet. Gross pathological changes in animals that did not survive
included moderate congestion in the lungs of dogs, minor patchy
oedema in the lungs of rats, and congestion with oedema in the
lungs of guinea-pigs. In rats, moderate congestion with petecchial
haemorrhage of the trachea was also observed. Lobular pneumonia
and hyperaemia of the liver and kidneys were observed in guinea-
pigs. Parenchymatous changes in the kidney of guinea-pigs were
seen at 2300 mg/m3.
Ataxia, prostration, laboured breathing, and occasional tonic
convulsions were effects shown by rats and mice at lethal oral or
intravenous doses of ethylene oxide (Woodard & Woodard, 1971).
Vomiting was the only effect shown by dogs that had received 25 or
75 mg ethylene oxide/kg body weight intravenously (Martis et al.,
1982).
In order to investigate the effects of residues of ethylene
oxide or reaction products in sterilized medical equipment, rabbits
were exposed for 2 h via polyvinyl chloride endotracheal tubes
containing 0, 80, or 600 mg ethylene oxide/kg material. There were
no deaths, but rabbits receiving the tubes with the highest
residues showed increased incidences of hyperaemia, oedema,
leukocyte infiltration, and epithelial erosion of the larynx and
trachea (Star et al., 1980). Two groups of nine dogs each were
exposed through extracorporeal perfusion for 40 min via a polyvinyl
chloride oxygenator containing 12 g ethylene oxide/kg material.
Nine dogs with an interrupted pulmonary circulation died from shock
and pulmonary oedema. Six out of 9 dogs with a preserved pulmonary
circulation died from pulmonary distress. A group of 9 control
dogs was treated in the same manner using a steam-sterilized
oxygenator. Only 1 control dog died (Stanley et al., 1971).
7.1.2. Acute effects on eyes and skin
As noted above, ethylene oxide is an irritating agent for
several different species. A maximum non-damaging concentration of
0.1% ethylene oxide in balanced physiological salt solution
(prepared daily and kept at 0 °C) was established after
instillation of 0.05 ml solution, every 10 min for 6 h, into the
conjunctival sac of rabbits. The concentrations above 1% caused
reversible changes in conjunctiva such as hyperaemia and swelling,
and irreversible opacity, both in the cornea and in the lens.
Possible reaction products, 2-chloroethanol and 1,2-ethanediol,
were less irritating to the eye (McDonald et al., 1973). The
results of in vitro tests with isolated rabbit cornea were in
agreement with the results of these studies. In the in vitro
tests, the endothelia were perfused for 1 - 3 h with a balanced
salt solution containing 250 mg ethylene oxide, 2250 mg
2-chloroethanol, or 5000 mg 1,2-ethanediol/litre. No effects were
observed on corneal thickness and cellular ultrastructure
(Edelhauser et al., 1983).
Skin irritation with hyperaemia, oedema, and scar formation was
observed from 6 min after application of pads of cotton, moistened
with solutions of 100 or 500 g ethylene oxide/litre water, on the
shaved skin of rabbits, under a plastic cover. The intensity of
the response was reported to be roughly proportional to the length
of exposure time (1 - 60 min) and the concentration (Hollingsworth
et al., 1956).
According to Hine & Rowe (1981), liquid ethylene oxide is
apparently without adverse effects on rabbit and human skin, on
single mild exposures, if the material evaporates rapidly. If
large amounts of material are involved, evaporation may cause
sufficient cooling to cause a lesion similar to frost-bite.
7.2. Short-Term Studies
7.2.1. Inhalation exposure
Groups of 10 - 20 Wistar rats per sex, 8 guinea-pigs per sex,
1 - 2 rabbits per sex, and 1 - 2 female rhesus monkeys were each
exposed to concentrations of ethylene oxide at levels of 0, 90,
200, 370, 640, or 1510 mg/m3, for 7 h per day, and 5 days per week.
The female monkeys were not tested at 90 mg/m3, and an additional 3
male monkeys were tested at 640 mg/m3. The test period varied with
the species tested, and the severity of exposure, i.e.,
approximately 26 weeks at 90 mg/m3, 25 - 32 weeks at 200 and
370 mg/m3, 7 - 25 weeks at 640 mg/m3, and 10 days at 1510 mg/m3.
Guinea-pigs, rabbits, and monkeys tolerated 90 and 200 mg/m3, and
rats tolerated exposure to 90 mg/m3 without adverse effects on
general appearance, behaviour, mortality rate, growth, body and
organ weight, gross- and histopathology. Rats showed elevated
mortality rates from 370 mg/m3, rabbits from 640 mg/m3, and all
exposed animals died at 1510 mg/m3. Secondary respiratory
infection caused the deaths of an appreciable number of rats and
mice in these studies.
Surviving rats showed increased relative lung weights after
26 - 27 weeks at 200 and 370 mg/m3. At 370 mg/m3, haemorrhages,
hyperaemia, emphysema, and local alveolar collapse were observed in
these lungs. Lungs of male rabbits also showed hyperaemia and
slight oedema at 370 mg/m3. Even more severe lung injury was seen
in rats at 640 mg/m3 and the higher exposure. Gross respiratory
tract irritation was apparent in all species at 1510 mg/m3.
Delayed reversible effects were observed on the peripheral
nervous system. Monkeys and rabbits exhibited paralysis of the
hind legs at 370 mg/m3 and, together with rats, at 640 mg/m3. This
was accompanied by atrophy of the muscles of the hind legs, except
in rabbits at 370 mg/m3. The effects on the peripheral nervous
system were investigated further in monkeys, and loss of both
sensory and motor function was noted at levels of 370 and
640 mg/m3.
Significant increases in body weight were also observed in
rats, at levels of 200 mg/m3 or more. Rats showed slight but
significant increases in the relative weights of kidney and liver
at 370 mg/m3 (Hollingsworth et al. 1956).
The findings of Jacobson et al. (1956) are in agreement with
these results. Groups, comprising 20 male rats and 30 female mice
each, were exposed to concentrations of ethylene oxide at levels of
0, 180, or 730 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted 26 weeks at 180 mg/m3 and 6 weeks at 730 mg/m3.
Additional groups of 15 rats and mice at the higher, and 60 rats
and mice at the lower, exposure level were used for interim gross
pathology. No clear toxic effects were reported at 180 mg/m3. No
pathological changes were observed except for marked haemosiderosis
in the spleen of a few rats at 730 mg/m3. The highest exposure
(730 mg/m3) resulted in death for both species without clinical
signs in mice. Effects on the respiratory and nervous system were
shown by rats as laboured breathing, reddish nasal discharge,
diarrhoea, tendency towards a side position, and dragging of the
hind-quarters. Rats also lost weight, which was regained by
survivors.
More recently, groups of 30 B6C3F1 mice of each sex were
exposed to concentrations of ethylene oxide (purity 99.9%) at 0,
18, 86, 187, or 425 mg/m3, for 6 h/day, and 5 days per week. The
exposures lasted for 10 weeks for males and 11 weeks for females.
No effects were observed in relation to survival, body weight,
clinical signs, white blood cell count, serum clinical chemistry,
urinalysis, and histopathology. At the highest exposure level,
changes at terminal sacrifice included an increased relative liver
weight in female mice, and a decreased testicular weight in males.
A decreased relative spleen weight was observed at 187 and
425 mg/m3 in both sexes. In addition, the red blood cell count,
the packed cell volume, and the haemoglobin concentrations were
decreased at 425 mg/m3. Screening of neuromuscular function in
groups of 5 female mice, in week 6, and 5 mice of both sexes, in
week 10 or 11, revealed altered reflex responses at 425 mg/m3 and a
dose-related trend in alterations of locomotor function from
86 mg/m3 upwards (Snellings et al., 1984a).
Groups of 3 male beagle dogs each were exposed to
concentrations of ethylene oxide (purity 99.7%) of 180 and
530 mg/m3, for 1 - 3 days. No effects were observed on mortality
rate, body weight, electrocardiogram, blood-calcium and -urea,
icteric index, and rectal temperature. Anaemia was noted at both
exposure levels. Effects on the respiratory and nervous systems
were shown at 530 mg/m3, such as hyperaemia and local alveolar
collapse in lungs, vomiting, and occasional slight tremors and
transient weakness in the hind legs. Muscular atrophy was also
observed (Jacobson et al., 1956). No haematological changes were
noted when groups each comprising 3 male New Zealand rabbits were
exposed repeatedly for 12 weeks to 0, 18, 90, or 450 mg/m3 (Yager &
Benz, 1982). The white cell count was depressed in Fischer rats
exposed in groups of 3 or 4, for 3 days, 6 h per day, to 90, 270,
or 810 mg/m3. There was a poor correlation with exposure level
(Kligerman et al., 1983).
The possible neurotoxic effects of ethylene oxide noted in the
above studies were investigated further in groups each comprising
12 male cynomolgus monkeys. These animals were exposed to 0, 90,
or 180 mg ethylene oxide/m3 (purity 99.7%), for 7 h per day, 5 days
per week, for 2 years. In 2 monkeys per group, brain, ulnar and
sciatic nerves, and spinal cord were examined histologically after
exposure. No clinical signs were reported. The only treatment-
related lesions found were in the medulla oblongata of the brain.
Axonal dystrophy was found in the nucleus gracilis, primarily in
the exposed groups. Demyelination of the terminal axons of the
fasciculus gracilis occurred in one monkey at each exposure level,
but not in the controls (Sprinz et al., 1982). Paralysis of the
hind limbs was observed in monkeys repeatedly exposed for up to
32 weeks to 370 mg/m3, for 7 h per day, 5 days per week
(Hollingsworth et al., 1956).
7.2.2. Oral exposure
Groups of 5 Wistar rats each received, by gavage, 22 doses of
3, 10, or 30 mg ethylene oxide/kg body weight in 30 days and 15
doses of 100 mg/kg in 21 days. The vehicle was olive oil. There
were 10 vehicle controls.
No effects on mortality rate, growth, haematology, blood urea-
nitrogen, organ weights, gross- and histopathology were reported at
the 3 lower dose levels. At 100 mg/kg, there was marked loss in
body weight, gastric irritation, and slight (not further specified)
liver damage (Hollingsworth et al., 1956).
7.3. Long-Term Inhalation Studies
In a combined toxicity-carcinogenicity study, groups of 120
male and 120 female Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 18 mg/m3 (10 ppm), 58 mg/m3
(32 ppm), and 173 mg/m3 (96 ppm), for 6 h per day, 5 days per week,
over 25 months. Two control groups each comprising 120 male and
120 female rats were used. There was an exposure-free period of 2
weeks in month 15, because of infection with sialoacryoadenitis
virus. Interim sacrfices occurred at 6, 12, and 18 months.
The mortality rates of male and female rats increased
significantly from the 22nd or 23rd month, at the highest exposure,
with a trend towards an increase at a level of 58 mg/m3. Body
weights in both sexes were depressed at 173 mg/m3, from the end of
the first week onwards, until the end of the study. At 58 mg/m3,
the body weights of female rats were decreased between week 10 and
80. In females, the relative liver weights were increased in the
18th month at 173 mg/m3. This effect on the liver could not be
related to increases in the activities of serum alkaline
phosphatase (EC 3.1.3.1), aspartate aminotransferase (EC 2.6.1.1),
or alanine aminotransferase (EC 2.6.1.2), found mainly at the 2
highest exposures during interim sacrifices. Relative spleen
weights were increased in rats that developed leukaemia (section
7.4.1). Haematological changes were found in rats at all doses,
but mainly at the end of the study in animals exposed to 173 mg/m3;
these included an elevated leukocyte count in both sexes, and a
depressed red blood cell count and haemoglobin value in females.
Some of these rats had leukaemia.
Non-neoplastic histopathological changes observed included an
elevated frequency of focal fatty metamorphosis of the adrenal
cortices in both sexes and bone marrow hyperplasia in females at
173 mg/m3. Although no effect was observed on the hind-quarter
lift reflex, examined monthly, mild skeletal muscular atrophy was
observed, after 2 years of exposure to 173 mg/m3. Observations on
general health and ophthalmology did not reveal anything abnormal.
Neoplastic changes are reported in section 7.4.1 (Snellings et al.,
1981, 1984b).
In another toxicity-carcinogenicity study (Lynch et al.,
1984a), groups of 80 male Fischer 344 rats were exposed to actual
concentrations of ethylene oxide of 92 mg/m3 (51 ppm) and 182 mg/m3
(101 ppm), for 7 h per day, 5 days per week, over 2 years. The
control group also comprised 80 rats. There was an exposure-free
period of 2 weeks in month 16 because of a pulmonary infection,
which contributed to the mortality rate.
The mortality rate increased at both exposure levels, the
increase being significant at 182 mg/m3. Only 19% of the rats
survived 2 years of exposure at 182 mg/m3 compared with 49% in the
unexposed group. Body weights were reduced from the 3rd or 4th
month onwards. The relative weights of adrenals and brain were
increased at both exposure levels. The relative weights of lung
and kidney were increased at 92 mg/m3. Serum aspartate
aminotransferase activity was increased in rats exposed to 92 and
182 mg/m3 (section 7.4.1). No other changes were found in
haematology or clinical chemistry.
Non-neoplastic histopathological changes included an elevated
incidence of vacuolization and hyperplasia or hypertrophy in the
adrenals at both exposure levels, and of atrophy and degeneration
of skeletal muscle fibres at 182 mg/m3. There were also increased
incidences of inflammatory lesions of the lungs, nasal cavities,
trachea, and internal ear at both exposure levels. Eye cataracts
developed in 9 out of 78 rats at 182 mg/m3, 3 out of 79 in the
92 mg/m3 group, and 2 out of 77 in the controls.
7.4. Carcinogenicity
7.4.1. Inhalation exposure
In the studies by Snellings et al. (1981, 1984b) (section 7.3)
(Table 4), several neoplasms were induced by ethylene oxide. A
dose-related increased incidence of mononuclear cell leukaemia was
found in both sexes, significant at the 2 highest exposures in
females, from the 18th or 19th month onwards. Trend test revealed
a treatment-related response in both sexes. In males, an increased
incidence of peritoneal mesotheliomas originating from the
testicular mesothelium, occurred at 58 and 173 mg/m3 from the 23rd
month onwards, and an increased incidence of subcutaneous fibroma
was seen in male rats exposed to 173 mg/m3 that had survived for 24
months. Trend analysis showed that there was a treatment-related
increase in peritoneal mesothelioma. There was no increased
incidence of pituitary tumours, but they appeared earlier in the
173 mg/m3 group.
Following the report by Lynch et al. (1984a) of an increased
incidence of brain tumours in Fischer 344 rats exposed to ethylene
oxide (see below), the brain tissue from this study was re-examined
both macro- and microscopically, and a dose-related incidence of
primary brain tumours was observed at 58 and 173 mg/m3 that
appeared to be treatment related in the trend test, but was not
statistically significant. The tumours were mainly diagnosed as
gliomas and malignant reticular tumours. The percentage of rats
with multiple neoplasms was greater than in controls at all
exposure levels in females and at 173 mg/m3 in males. At 58 and
173 mg/m3, the percentage of female rats with at least one
malignancy was increased. The authors considered that a
contribution of the viral outbreak to the toxicity of ethylene
oxide was unlikely (Snellings et al., 1981, 1984b).
Lynch et al. (1984a) (section 7.3, Table 4) also found an
increased incidence of mononuclear cell leukaemia, which was
significant at the lower exposure level. The absence of a dose-
relationship was attributed to the increased mortality rate at 182
mg/m3. Dose-related increased incidences of peritoneal
mesotheliomas, originating from the testicular mesothelium, and of
mixed-cell gliomas in the brain, were found. The increases in both
tumours were significant at 182 mg/m3 (Lynch et al., 1984a).
Table 4. Tumours induced by ethylene oxide in Fischer 344 rats
------------------------------------------------------------------------------
Concen- Leukaemia Meso- Pituitary Subcutaneous Braina
tration (mononuclear) thelioma adenoma fibromaa
(mg/m3)
------------------------------------------------------------------------------
M F M M F M M F
Snellings et al. (1981, 1984b)
173 26(119)b 28(113) 22(119) 27(117) 32(117) 11(30) 3(30) 2(26)
58 25(81) 24(79) 7(91) 16(79) 38(90) 1(39) 1(39) 2(48)
18 21(79) 14(77) 3(89) 27(80) 39(90) 3(51) 0(51) 0
0 20(116) 9(118) 2(114) 28(117) 38(119) 1(48) 1(48) 0
0 18(118) 13(117) 2(116) 22(117) 38(116) 2(49) 0(49) 0
Lynch et al. (1984a)c
182 30(76) NA 21(79) 21(67) NA NR 5(79) NA
92 38(79) NA 9(79) 20(66) NA NR 2(77) NA
0 24(77) NA 3(78) 48(73) NA NR 0(76) NA
------------------------------------------------------------------------------
a Only animals that survived for 24 months were included, because these
tumours appeared after the 18-month interim sacrifice.
b Numbers in brackets refer to the number of rats examined. They include
interim and final kills.
c Only male rats were used.
NA = Not applicable.
NR = Not reported.
7.4.2. Oral exposure
Groups of 50 female Sprague Dawley rats received, in salad oil,
7.5 or 30.0 mg ethylene oxide/kg body weight, by gavage, in the
empty stomach, twice a week, for 110 weeks. There were 50 vehicle
controls, 50 untreated controls, and 50 positive controls. The
rats were observed for their life span. No statistical analysis
was reported. The mean survival period was over 100 weeks for all
groups. The mortality rate increased at 30.0 mg/kg body weight,
from week 100 onward. Elevated incidences of tumours were only
observed in the forestomach, the first tumour appearing in week 79.
The incidences of squamous cell carcinomas were 0/50, 8/50, and
29/50 at 0, 7.5, and 30 mg/kg body weight, respectively. At
30 mg/kg body weight, invasive growth and metastases were observed
in 10 rats. At 30 mg/kg body weight, 2 fibrosarcomas were also
noted. At both doses, the incidences of hyperplasia,
hyperkeratosis, papillomas, and/or carcinomas were increased in the
forestomach (Dunkelberg, 1982).
7.4.3. Subcutaneous exposure
Groups of 100 female NMRI mice were injected once a week with a
tricaprylin solution containing 0.1, 0.3, or 1.0 mg ethylene oxide
per animal, for 106 weeks. There were 200 vehicle controls and 200
untreated controls. From week 35 to week 85, the mortality rate
increased by a maximum of 10% at a dose of 1.0 mg per mouse. The
mean length of survival in this group was 75 weeks. An elevated
incidence of tumours was only observed at the injection site, the
first tumour appearing in week 79. There was a dose-related
increased incidence of sarcomas, mainly fibrosarcomas, which was
significant at 0.3 and 1.0 mg per mouse. The tumour incidence was
11% at the highest dose compared with 2% in vehicle controls
(Dunkelberg, 1981).
7.4.4. Dermal exposure
Each of a group of 30 female Swiss Millerton mice received, for
their lifetime, approximately 100 mg of a 10% solution of ethylene
oxide (purity 99.7%) in acetone, brushed on the clipped dorsal
uncovered skin, 3 times a week. A group of 60 mice did not receive
any treatment, and a group of 60 mice received the vehicle only.
No skin tumours were found, nor was there any sign of skin
irritation. The median length of survival was 493 days for treated
mice and 445 days for controls (Van Duuren et al., 1965). It is
assumed that ethylene oxide, applied in this manner, evaporated
rapidly from the skin.
7.5. Mutagenicity and Related End-Points
Almost all the reports available demonstrate the mutagenic
action of ethylene oxide. A summary of mutagenicity tests with
positive results is presented in Table 5.
Ethylene oxide is an alkylating agent (section 5.2). It has
induced gene mutations in all plants, bacteria, fungi, insects, and
mammalian cells investigated in vitro, with and without metabolic
activation. Chromosome damage and sister chromatid exchanges were
observed in plants, insects, and mammalian somatic cells exposed in
vivo and in vitro. Fomenko & Strekalova (1973) and Strekalova et
al. (1975) reported an increased incidence of chromosomal
aberrations in the bone-marrow cells of rats exposed by inhalation
to concentrations of ethylene oxide vapour at 3.6 and 112 mg/m3.
Unscheduled DNA synthesis, induced by the N-acetoxy-2-acetylamino-
fluorene, was inhibited by ethylene oxide in human lymphocytes in
vitro (Pero et al., 1981). The positive results in the
micronucleus tests are in agreement with those from a distribution
study showing that ethylene oxide, or its metabolites, was retained
in the bone marrow of mice (Appelgren et al., 1977). An increased
incidence of micronuclei was observed after one intraperitoneal
dose of 100 mg/kg body weight in mice (Lyarskii et al., 1983) and
after 4 h of vapour exposure of rats to a concentration of 90 mg/m3
(Embree & Hine, 1975).
Table 5. Mutagenic tests for ethylene oxide with positive resultsa
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Gene mutations
Forward mutations plant barley Ehrenberg et al. (1956,
1959)
Forward mutations barley Shulovsk et al. (1969)
Forward mutations rice Jana & Roy (1975)
Forward mutations pea Blixt et al. (1963)
Reverse mutations bacterium Escherichia coli Hussain & Osterman-Golkar
SD-4 (1984)
Reverse mutations Salmonella typhimurium Rannug et al. (1976);
(base-pair TA1535, TA100 Kauhanen (1978)b;
substitutions) Pfeiffer & Dunkelberg
(1980)
Reverse mutations Bacillus subtilis Tanooka (1979)
(spores) HA101, TKJ5211,
TKJ8201
Reverse mutations fungus Neurospora crassa Kolmark & Westergaard
(macroconidia) (1953); Kolmark & Kilby
(1968)
Forward mutations Aspergillus nidulans Morpurgo (1963)
Forward mutations Schizosaccharomyces Migliore et al. (1982)c
pombe Pl
Sex-linked insect Drosophila melanogaster Bird (1952); Nakao &
recessive lethals Auerbach (1961)
Forward mutations mammal Chinese hamster Tan et al. (1981)b;
on specific locus ( in vitro) ovary cells Zamora et al. (1983)
Chromosome damage
breaks, erosions, plant Tradescantia paludosa Smith & Lofty (1954)
contractions (pollen)
translocations barley Ehrenberg et al. (1956,
1959)
breaks barley Moutschen-Dahmen et al.
(1968)
breaks wheat (hexaploid) MacKey (1968)
translocations insect Drosophila melanogaster Nakao & Auerbach (1961)
small deletions insect Drosophila melanogaster Fahmy & Fahmy (1970)
(min)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
Chromosome damage (contd.)
breaks, human anion cells Poirier & Papadopoulo
gaps, exchanges, ( in vitro) (1982)
complexes
sister chromatide human lymphocytes Star (1980a);
exchanges ( in vitro) fibroblasts Garry et al. (1982)
rat lymphocytes Kligerman et al. (1983)
(inhalation)
rabbit lymphocytes Yager & Benz (1982)
(inhalation)
sister chromatide monkey lymphocytes Lynch et al. (1984b)d
exchanges, (inhalation)
breaks, acentric
fragments,
dicentrics,
triradials,
quadriradials,
complex
rearrangements
chromosomal rat bone marrow cells Strekalova et al. (1975)
aberrations (inhalation)
breaks, gaps, rat bone marrow cells Embree & Hine (1975)
rearrangement, (inhalation)
exchanges,
ring formations
micronuclei mouse (ip) polychromatic Conan et al. (1979);
erythrocytes Jenssen & Ramel (1980);
Lyarskii et al. (1983)
micronuclei mouse (iv) polychromatic Appelgren et al. (1978)
erythrocytes
heritable mouse (ip) germ cells Generoso et al. (1980)
translocations
dominant lethals mouse germ cells Cumming & Michaud (1979)
(inhalation)
mouse (ip) Generoso et al. (1980);
Lyarskii et al. (1983);
mouse Generoso et al. (1983)
(inhalation)
----------------------------------------------------------------------------------------
Table 5. (contd.)
----------------------------------------------------------------------------------------
Test description System description Reference
Organism Strain/cell type
----------------------------------------------------------------------------------------
dominant lethals rat Embree et al. (1977)
(contd.) (inhalation)
DNA repair
unscheduled mouse germ cells Cumming & Michaud (1979)
DNA synthesis (inhalation)
unscheduled human lymphocytes Pero et al. (1981)
DNA synthesis ( in vitro)
----------------------------------------------------------------------------------------
a For details of these studies, see text and data profile (IRPTC, 1984).
b A similar effect after metabolic activation by rat liver microsomal fraction.
c A slight reduction in mutagenicity after metabolic activation by mouse liver
microsomal fraction.
d 2-year exposure groups of 12 male monkeys to 0, 90, and 180 mg/m3 for 7 h per day and
5 days per week.
Dose-related damage to germ cells was established in the mid
and late spermatid stages in the dominant lethal assay. One oral
dose of 100 mg/kg body weight in mice generated inconsistent
results (Appelgren et al., 1977), but 150 mg/kg proved positive
(Generoso et al., 1980). After short-term repeated exposures,
dominant lethals were induced in mice at intraperitoneal doses from
40 mg/kg body weight, given over a period of 3 months, 5 times per
week (Lyarskii et al., 1983) and at vapour exposures from
460 mg/m3, for 6 h/day, 5 days per week, over 11 weeks (Generoso et
al., 1983). Heritable translocations were induced in the germ
cells of mice after repeated intraperitoneal exposure, at doses of
30 mg/kg body weight or more, for 5 days/week, over a 5-week period
(Generoso et al., 1980). Recent studies have investigated the
dose-response of inhaled ethylene oxide and have compared effects
of different dose rates (contributions of different concentrations
and durations of exposure, maintaining total exposure (C x t)
constant) on the dominant-lethal response in male mice. In the
dose-reponse study, male mice were exposed by inhalation to
ethylene oxide at concentrations of 540, 720, or 900 mg/m3 (300,
400, or 500 ppm), respectively. Exposures were for 6 h/day, for 4
consecutive days. A dose-related increase in dominant-lethal
mutations was observed; however, the dose-response curve was
nonlinear, i.e., increasing embryonic mortality occurred with
increasing mg/m3 x h. In the dose-rate study, mice had a total
exposure of 3240 mg/m3 (1800 ppm) x h/day for 4 consecutive days,
delivered either at 540 mg/m3 (300 ppm) in 6 h, 1080 mg/m3 in 3 h,
or 2160 mg/m3 (1200 ppm) in 1.5 h. The highest airborne
concentration resulted in the greatest embryonic mortality, 64%,
versus 32 and 11% for the intermediate and lowest airborne
concentrations, respectively (Generoso et al., 1985). According to
an abstract, DNA repair was induced in the germ cells of mice
exposed to 540 mg/m3, for 8 h. The repair seemed inhibited at
higher exposures (Cumming & Michaud, 1979). No details were
available.
Negative results were observed on a few occasions only. In one
study, with vapour-exposed rats, chromosome aberrations or slowing
of mitotic activity and cell cycle kinetics were not observed in
lymphocytes at levels at which sister chromatid exchanges occurred
(Kligerman et al., 1983). In a dominant-lethal assay with mice,
relatively high intravenous doses of ethylene oxide (up to
100 mg/kg body weight) did not cause any treatment-related effects
(Appelgren et al., 1977). An intraperitoneal dose of 10 mg/kg body
weight to mice was reported to give a slight increase in the number
of polychromatic erythrocytes with micronuclei, but the statistical
analysis was not adequate (Conan et al., 1979).
7.6. Effects on Reproduction
Rats and guinea-pigs were exposed to vapour concentrations of
370 and 640 mg ethylene oxide/m3, for 7 h per day, 5 days per week,
for up to 32 weeks. Among other effects (section 7.2.1),
degeneration of testes tubules was observed at the higher exposure
level in guinea-pigs, while at 370 mg/m3, there was a decrease in
the relative weights of testes in rats and guinea-pigs, which was
not statistically significant (Hollingsworth et al., 1956).
Significantly-decreased absolute testicular weights were observed
in mice exposed to ethylene oxide at a concentration of 425 mg/m3,
for 6 h/day, 5 days per week, over 10 - 11 weeks (Snellings et al.,
1984a). However, the testicular effects may have been secondary to
toxic effects (e.g., growth inhibition). Male and female Fischer
344 rats exposed repeatedly to concentrations of ethylene oxide of
up to 182 mg/m3, for 6 h/day, 5 days per week, over 25 months, did
not show any histopathological effects on the reproductive tissues
(Snellings et al., 1981).
When groups of 12 male Cynomolgus monkeys were exposed to
concentrations of ethylene oxide at 90 or 180 mg/m3, for 7 h/day,
5 days per week, over 2 years, spermatogenic functions were found
to differ from those of controls. At both exposure levels, sperm
motility and sperm count were decreased and the sperm drive range
was increased, but there was no increase in effect with increase in
dose. The incidence of abnormal sperm heads did not change (Lynch
et al., 1984c).
Groups, each comprising 30 male and 30 female Fischer 344 rats,
were exposed to concentrations of ethylene oxide (purity 99.9%) of
18, 58, or 173 mg/m3, for 6 h/day, 5 days per week, over 12 weeks.
Two control groups of 30 rats per sex each were exposed to air
only. After mating, females were further exposed for 7 days/week,
up to 3 weeks after delivery, with the exception of the first 5
days of lactation. Effects on the reproductive performance were
detected. The number of pups per litter was decreased at 173
mg/m3, as well as the number of implantation sites per female, and
the number of fetuses born per implantation site. The number of
females with a gestation period longer than 22 days was also
increased at this concentration, but no effects were noted on the
average length of the gestation period. Neither parents nor pups
showed signs of toxicity from ethylene oxide. The percentages of
pregnant females and fertile males were not affected (Snellings et
al., 1982a).
7.7. Teratogenicity
Groups of 22 female Fischer 344 rats were exposed to
concentrations of ethylene oxide of 18, 58, or 173 mg/m3, for
6 h/day, on days 6 - 15 of gestation. Two control groups
comprising 22 rats each were exposed to air only. The numbers of
pregnant dams ranged from 17 to 22. Maternal behaviour was normal,
and there were no deaths. The only effect on the fetuses was a 5 -
8% decrease in weight at 180 mg/m3 (Snellings et al., 1982b).
Groups of 32 - 45 female Sprague Dawley rats were exposed to
concentrations of ethylene oxide (purity 99.7%) of 0 or 270 mg/m3,
for 7 h/day, on days 7 - 16 of gestation (Group 1) or on days 1 -
16 of gestation (Group 2) or during 3 weeks before mating (5 days
per week), and on days 1 - 16 of gestation (Group 3). No dams
died, but body weights were decreased in Group 3. In all exposed
groups, the relative and absolute weights of kidney and spleen were
increased. The results of histopathological examination did not
show any abnormalities. There was a significant increase in
resorptions per litter and per implantation site in Group 3, with
no significant effects on the number of implants, live fetuses, and
pregnancies. In all exposed groups, weights and lengths of the
fetuses were decreased. Reduced ossification of sternebrae and
primary skull was observed (Hackett et al., 1982).
New Zealand rabbits were similarly exposed to a concentration
of ethylene oxide of 270 mg/m3 from days 1 - 19 or from days 7 - 19
of gestation. There was no evidence of toxicity in the mothers,
embryos, or fetuses, or of developmental defects (Hackett et al.,
1982).
Groups of 24 - 37 female CD-1 mice each received,
intraveneously, doses of 0, 75, or 150 mg ethylene oxide (purity
not stated)/kg body weight in an aqueous dextrose solution on days
4 - 6, 6 - 8, 8 - 10, or 10 - 12 of pregnancy. Dams exposed on
days 6 - 8 of pregnancy did not show toxic signs. In the other
groups, at the highest dose, toxic signs such as weakness, laboured
respiration, and tremor were observed with a mortality rate of 19 -
48%. In the group without signs of maternal toxicity, fetotoxicity
was observed at 150 mg/kg, as shown by a 20% decrease in mean fetal
weight. Fetal malformations were shown in 19.3% of fetuses in
exposed litters compared with 2% in control groups. These
malformations were mainly fused cervical arches. In addition,
fused thoracic arches, scrambled and fused sternebrae, and fused,
branched, or missing thoracic ribs were observed (Laborde & Kimmel,
1980).
8. EFFECTS ON MAN
8.1. Exposure of the Skin and Eyes
Undiluted ethylene oxide, applied to the skin of volunteers,
evaporated rapidly without leaving any mark or irritation. A
15-min exposure to cotton wool soaked in undiluted ethylene oxide
also did not produce any effects (Greaves Walker & Greeson, 1932).
However, with exposure to larger quantities, there may be
sufficient cooling to produce a lesion similar to frost-bite (Hine
& Rowe, 1981). Skin injury following exposure to aqueous solutions
of ethylene oxide is characterized by the appearance of oedema and
erythema, 1 - 5 h after exposure, followed by the formation of
vesicles. On healing, incrustation, often with itching and
desquamation, is observed. The magnitude of the skin injury seems
to depend on the length of contact and the concentration, a 50%
aqueous solution (500 g/litre) being most hazardous. More
concentrated solutions were less harmful. The lowest concentration
tested, a 1% solution, produced a mild reaction after a 50-min
exposure. Such effects have been observed in a number of accidents
(Sexton & Henson, 1949, 1950; Joyner, 1964; Ippen & Mathies, 1970).
Vapour exposure was found to produce the above dermal effects,
mainly on the humid parts of the skin (Ippen & Mathies, 1970). The
effects were also found, to different extents, after exposure via
ethylene oxide-sterilized materials such as face masks, gloves, and
surgical gowns (Royce & Moor, 1955; Marx et al., 1969; Hanifin,
1971; Biro et al., 1974; Lamy et al., 1974). Patch tests on
volunteers with various sterilized materials containing residues of
ethylene oxide and its reaction products, showed erythema, without
oedema, after 4 - 8 h of contact, from a residue level of 890 mg
ethylene oxide/kg up to 2890 mg/kg of a polyvinyl chloride block.
Most skin types tolerated residues of ethylene oxide of up to
2270 mg/kg polyvinyl chloride film, 2800 mg/kg brown-milled rubber,
and 5100 mg/kg non-woven fabric (Shupack et al., 1981).
Accidental skin exposure to a 1% aqueous solution, from the
waist down, was also reported to result in effects on the nervous
system, such as nausea and repeated vomiting (Sexton & Henson,
1949).
Accidental exposure of the eyes to the vapour of ethylene oxide
can lead to conjunctivitis (Thiess, 1963; Joyner, 1964). Exposure
of 12 men via a leaking sterilizer resulted in neurological
disorders (section 8.3) in 4 of the men, 3 of whom had eye
cataracts; one of the latter also showed an increase in corneal
thickness. Two additional men showed only an increase in corneal
thickness (Gross et al., 1979; Jay et al., 1982). In one case of
accidental exposure of the eyes to pure ethylene oxide, only slight
irritation of the conjunctiva was seen (Thiess, 1963).
The implantation of artificial lenses, sterilized with ethylene
oxide, in 103 eyes was compared with the implantation of lenses,
sterilized with sodium hydroxide (200 control eyes), in a
retrospective study. The follow-up period was 10 months for the
exposed patients. Post-operative inflammatory complications
occurred in 30% of the eyes exposed to residues of ethylene oxide
or its reaction products compared with 9% of the control eyes.
Cystoid macular oedema with reduction in visual acuity developed in
16% of the exposed eyes and in 7% of the control eyes (Stark et
al., 1980).
8.2. Sensitization
No dermal sensitization was observed in a total of 47 workers
frequently exposed to ethylene oxide (Royce & Moor, 1955; Thiess,
1963). In another study using patch tests, one of 12 volunteers
showed a recurrent reaction, 3 weeks after the trial. When
challenged afterwards with a 2 mm-thick patch of polyvinyl chloride
containing 100 mg ethylene oxide/kg, a mild reaction was observed,
which reappeared after 3 weeks (Shupack et al., 1981) (section
8.1). When the skin of 8 workers was exposed repeatedly to aqueous
solutions of ethylene oxide, all sites of previous contact, with
and without a primary reaction, flared up in 3 of them showing
pruritus, erythema, and slight oedema, 5 - 9 days after the last
exposure (Sexton & Henson, 1950). Another case of an apparently
allergic reaction was reported. The patient concerned was exposed
to ethylene oxide via a sterilized face mask (Alomar et al., 1981).
A case of anaphylaxis has been reported in a patient receiving
haemodialysis treatment with equipment that had been sterilized
with ethylene oxide (Poothullil et al., 1975). A cause-effect
relationship with ethylene oxide exposure was demonstrated by
haptan specificity (Dolovich & Bell, 1978).
8.3. Accidental Inhalation Exposure
Respiratory tract irritation was reported as hoarseness
(Thiess, 1963) and cough (Metz, 1939) in 5 cases of acute
accidental exposure to ethylene oxide vapour.
Acute effects on the nervous system in nearly all inhalation
cases were marked by nausea, recurrent vomiting, and headache.
Less frequently reported effects included decreased consciousness
(one case of coma), excitement, sleeplessness, muscular weakness,
diarrhoea, and abdominal discomfort (Blackwood & Erskine, 1938;
Metz, 1939; Thiess, 1963; Capellini & Ghezzi, 1965).
Because of a leaking sterilizer, 4 young men were exposed
intermittently, for 2 - 8 weeks, to ethylene oxide at levels of
approximately 1000 mg/m3. Three of the men developed a reversible
peripheral neuropathy showing abnormal nerve conduction and, in 2
cases, headache, weakness and decreased reflexes in the
extremities, incoordination, and a wide-based gait. The fourth man
developed a reversible acute encephalopathy with headache, nausea,
vomiting, lethargy, recurrent motor seizures, agitation, and a
diffusely slow electroencephalogram (Gross et al., 1979).
Following this, 6 more cases were reported concerning sterilizer
operators, suffering from reversible peripheral neuropathy
following ethylene oxide exposure for 0.5 - 1.5 years. Finelli et
al. (1983) described 3 persons showing subacute polyneuropathy with
bilateral foot-drop, slowing of nerve conduction velocity, and
denervation potential on electromyography as the main findings.
All 3 persons had noticed the smell of ethylene oxide regularly at
work, while 2 persons experienced eye irritation. Polyneuropathy
was also reported in 3 sterilizer operators by Kuzuhara et al.
(1983). Two of these cases were described in detail. Sural nerve
biopsies revealed axonal degeneration with mild changes in the
myeline sheath. Unmyelinated fibres were also involved. Muscle
biopsies showed typical denervation atrophy.
8.4. Other Accidental Exposures
Severe respiratory problems due to inflammatory reactions in
the trachea and larynx were reported in 17 hospital patients who
had received endotracheal intubation. The tubes had been
sterilized with ethylene oxide (Marx et al., 1969; Holley & Gildea,
1971; Lipton et al., 1971; Mantz et al., 1972).
Reversible vocal paralysis was reported to be associated with
ethylene oxide exposure in 5 cases: one woman had been exposed to
vapour (Troisi, 1965), and the other 4 patients were exposed via
sterilized endotracheal tubes (Holley & Gildea, 1971). The vocal
cords showed no or only slight damage. In one of these patients,
who died from a cause unrelated to the intubation, myelin
degeneration of parts of the nervus vagus was noted at autopsy. It
was suggested, therefore, that the paralysis was of neural origin.
Four cases of shock, 1 - 10 h after endovascular examination,
were associated with the catheters used, which contained residues
of ethylene oxide. The presence of bacterial toxins was considered
unlikely. One patient died as a result of renal insufficiency
(Lebrec et al., 1977). Cases of cardiovascular collapse in
children, 3 of which were fatal, were considered by the authors to
be the result of residues of ethylene oxide in a heart-lung machine
on the basis of subsequent studies on dogs (Stanley et al., 1971)
(section 7.1.1). Among others, Hirose et al. (1963) and Clarke et
al. (1966) measured haemolysis due to residues in ethylene oxide-
sterilized plastic tubes in vitro.
8.5. Occupational Inhalation Exposure
The health status of 37 male operators from an ethylene oxide-
producing plant in the USA during the period 1953 - 62 was compared
with that of age-matched operators from other production units.
The average employment period was 11 years for exposed workers and
12 years for controls. The usual average exposure level was
between 9 and 18 mg/m3, with occasional peaks up to 230 mg/m3 for
one particular job (collecting a sample of the product). According
to the medical records, the health of the exposed workers was
somewhat better than that of the controls. A physical examination
and extensive clinical tests did not reveal any exposure-related
effects with the exception of a slightly increased white blood cell
count (Joyner, 1964).
Chromosomal damage was found in a group of 12 workers from a
hospital sterilization facility in the USA (section 8.6). The
maximum exposure concentration measured during sterilization was
65 mg/m3. Another group of 12 persons, who worked in the adjacent
operating room area, volunteered as representatives of an unexposed
or accidentally exposed group. To insure adequate control
throughout the study, unexposed laboratory staff members served as
a third group. Frequently-reported subjective complaints indicated
irritation of the mouth, throat, and eyes, and effects on the
nervous system, such as headache, nausea, speech difficulty, memory
loss, dizziness, and incoordination (Garry et al., 1979).
In Belgium, a group of 18 workers, using or distributing the
sterilant ethylene oxide, was compared with a well-matched control
group by means of a questionnaire, and by analyses for urinary
retinol-binding protein and albumin, beta-microglobulin, and
chromosomal damage in lymphocytes. The overall mean exposure level
was 7.6 mg/m3, and the time-weighted average exposure, over a
working day, ranged between 0.2 and 95 mg/m3. A significant
increase in the incidence of sleeplessness and leg cramps was
recorded, but not irritation or allergy. These studies did not
reveal any abnormalities with the exception of an increase in
sister chromatid exchanges in lymphocytes (Wolfs et al., 1983;
Laurent et al., 1984; section 8.7).
In a plant in Bulgaria, 196 workers engaged in the production
of ethene and ethylene oxide were examined. About 73% of all
concentrations of ethylene oxide measured were 1 mg/m3 or less,
while 27% were between 1 mg/m3 and 3.5 mg/m3. Significant
increases were found in deviations of the autonomous nervous system
and in neurosis-like manifestations, especially in female workers
(Spasovski et al., 1980). Because of a mixed exposure, it is
difficult to evaluate the findings.
Haematological changes were reported in a group of 27 workers
in an ethylene oxide manufacturing and processing plant, in Sweden,
in 1967. The exposure period varied from 2 to 20 years, the
average length being 15 years. Controls worked with ethylene oxide
in other departments, where no leakages were possible. No exposure
data were reported. When 2 cases of anaemia were excluded, there
was still a significantly-decreased haemoglobin value in exposed
workers. There was a 30% increase in the number of lymphocytes,
and one case of chronic lymphatic leukaemia was noted (Ehrenberg &
Hällström, 1967).
In the Federal Republic of Germany, 279 employees from 8 plants
in which alkene oxides were produced or processed, were examined
for morbidity during 1978. They were employed for an average of
10.8 years. Of these workers, 21 had been involved in accidents
with ethylene oxide. Taking into account age and length of
exposure, they were compared with groups of industrial and clerical
workers within the same company. The exposure concentrations were
not reported. The workers were also exposed to many other
chemicals, some of which may be carcinogenic for man.
No abnormalities were found that could be related to exposure
to ethylene oxide or propylene oxide. The investigators related
increases in haemoglobin and mean erythrocyte volume to smoking
habits. Slight lymphocytosis was found to be unrelated to exposure
time, though there was a distinct age influence (Stocker & Thiess,
1979).
8.6. Mortality Studies
Two studies were conducted in Sweden to investigate the
possible neoplastic effects from occupational exposure to ethylene
oxide (Hogstedt et al., 1979a,b, 1984). The first study originally
included 58 male and 172 female workers in a small factory,
sterilizing hospital equipment with a 1:1 mixture of ethylene oxide
and methyl formate, over a period from 1968 to 1977 (Hogstedt et
al., 1979a).
Two cases of leukaemia (one was diagnosed as chronic myeloid
leukaemia and the other as acute myelogenous leukaemia) occurred
among 68 women who were exposed to vapours from sterilized boxes
stored for weekly periods in a factory storage hall where about 30
persons were exposed at any one time. A third case was the local
male manager who developed primary macroglobulinaemia (Morbus
Waldenström; this case was later diagnosed as a non-Hodgkin
lymphoma), 9 years after the installation of the sterilization
equipment; his exposure was estimated to be about 3 h per week in
the storage hall. He is also reported to have had some exposure to
benzene in the past. The concentration of ethylene oxide in the
hall was in the range of 3.6 - 128 mg/m3, and the 8-h time-weighted
average in the breathing zone was calculated to be between 36 ±
18 mg/m3. The other workers had occasional exposure to ethylene
oxide, and 7 operators had relatively high exposure (amount
unspecified) during the sterilization process.
In a follow-up study (Hogstedt et al., 1984), a further case of
leukaemia was found in a woman who had been exposed to ethylene
oxide in the storage hall between 1969 - 72. In this study, the
cohort consisted of 203 workers who had been employed for more than
one year at the plant.
Altogether, 4 deaths from malignancies of the lymphoreticular
system were reported from this factory. The expected number was
0.3.
A second study to investigate the carcinogenic effects of
ethylene oxide was conducted on 241 Swedish male workers in an
ethylene oxide-producing plant (Hogstedt et al., 1979b). These
men were examined medically in 1960. Twenty-three deaths
occurred during the 16-year observation period dating from
1961 - 77 (13.5 expected). The excess mortality was due to cancer
and cardiovascular disease. Three cases of stomach cancer (0.4
expected) and 2 cases of leukaemia (one chronic myeloid and once
acute myeloid leukaemia) (0.14 expected) accounted for the excess
mortality from cancer. No increase in mortality was observed among
86 maintenance workers exposed intermittently to ethylene oxide
among 66 unexposed controls. Average exposure levels during
1941 - 47 were estimated to be below 25 mg/m3 and, during the 1950s
up to 1963, these levels were 10 - 50 mg/m3, but peak exposures
above the odour threshold (about 1000 mg/m3) were known to occur.
The ethylene oxide was manufactured by the chlorohydrin process
so that significant exposure to other chemicals such as 1,2-
dichloroethane, ethylene, ethylene-chlorohydrin, and bis(2-
chloroethyl)ether might have occurred.
This investigation was followed up by a study that extended the
period of observation up to 1982. Seven more deaths had occurred
among the workers exposed to ethylene oxide for the whole of the
working day against 6.6 expected. Three of these were due to
cancer (1.6 expected). Two of these 3 cases were cancer of the
stomach (0.2 expected) and one an oesophageal cancer (0.04
expected). In the period from 1961 - 82, 6 deaths due to stomach
or oesophageal cancer had occurred in workers exposed to ethylene
oxide for the whole of the working day (0.7 expected). Alimentary
tract cancer was observed in 2 maintenance workers (0.8 expected)
and in 1 unexposed worker (0.8 expected). One new case of chronic
myeloid leukaemia was reported during this follow-up period.
During the 20-year period of observation, a total of 17 cases of
cancer were notified to the Cancer Registry against 7.9 expected
(Hogstedt et al., 1984).
The Task Group evaluated these data and concluded that the
evidence was adequate to consider the mixtures of compounds to
which these workers were exposed as carcinogenic for human beings,
but inadequate to label ethylene oxide as a proved human
carcinogen. This conclusion was in agreement with that arrived at
by an International Agency for Research on Cancer Working Group
(IARC, 1985).
A similar study in the USA concerned 767 male workers at an
ethylene oxide producing plant. They were employed for at least
5 years between January 1955 and December 1977 and "potentially
exposed". Concentrations of ethylene oxide were reported to be
below 18 mg/m3, but no further details concerning exposure levels
were reported. Exposure to other chemicals was not reported.
Control data came from national statistics and was adjusted for
sex, age, and calendar time. There were 46 deaths against an
expected 80; there were 11 malignant neoplasms against 15.2
expected. No statistically-significant excess deaths could be
found due to any cause. There were no deaths due to leukaemia, 3
deaths from pancreatic cancer (0.8 expected), 1 death from bladder
cancer (0.3 expected), 2 deaths from brain cancer (0.7 expected),
and 2 deaths from Hodgkin's disease (0.4 expected) (Morgan et al.,
1981).
In the Federal Republic of Germany, 602 workers were
investigated for mortality experience during the period 1928 - 80.
The workers had been employed for at least 6 months in 8 plants
producing or processing ethylene oxide and propylene oxide. A
subcohort of 351 workers was observed for more than 10 years.
Control data came from a styrene plant and from national
statistics. Since 1978, exposure to ethylene oxide had normally
remained below 9 mg/m3. In the past, occasional excursions above
90 mg/m3 (50 ppm) had been reported. On one occasion during plant
breakdown, 3420 mg/m3 (1900 ppm) was measured. No statements were
offered concerning human exposure or the use of personal protective
equipment. The workers were also exposed to many other chemicals,
some of which might be carcinogenic for human beings. There were
56 deaths compared with 76.6 expected. There were 14 deaths from
cancer compared with 16.6 expected. No statistically-significant
excess deaths could be found due to any cause in the cohort. In
the subcohort of 351 workers, there was a significant increase in
mortality rate due to kidney disease (3 against 0.4 expected).
There was 1 death from gall bladder cancer, 1 death from urinary
bladder cancer, 1 death from brain cancer, and 1 death from myeloid
leukaemia. Two stomach tumours were observed compared with 1.8
expected (Thiess et al., 1981a,b).
8.7. Mutagenicity and Related End-Points
An increase in chromosomal aberrations was found in the
lymphocytes of 3 groups of workers sterilizing medical equipment in
hospitals or factories (Abrahams, 1980; Pero et al., 1981; Högstedt
et al., 1983). A 50% increase in aberration rate was found in 28
workers exposed to 8-h time-weighted average concentrations of
ethylene oxide below 1.8 mg/m3 in the 2.5 years before the study.
Before this period, higher exposures were reported to have
occurred. The workers had been exposed for 0.5 - 8 years. The
mean number of micronuclei in the bone marrow cells of 18 of these
workers was 3 times higher than in the controls (Högstedt et al.,
1983). Pero et al. (1981) found that, while workers exposed to
concentrations of ethylene oxide between 0.9 and 1.8 mg/m3, for
40 h per week, did not show an increased aberration rate; others,
exposed to bursts of ethylene oxide at concentrations between 9 and
18 mg/m3, for 8 h per week, did. The Task Group noted that the
sterilization procedure involved the use of a 50:50 mixture of
ethylene oxide and methyl formate. At the same time, DNA repair,
induced in vitro by the mutagen N-acetoxy-2-acetylamino-fluorene,
was reversibly inhibited in the low exposure group compared with an
unexposed control group, but not affected in the other group. DNA
repair inhibition was positively correlated with duration of
exposure (Pero et al., 1981). In 43 male workers from the cohort
of 602 workers (section 8.5) (Thiess et al., 1981b), an increase in
chromosomal aberration rate was found that was significant for the
workers exposed for more than 20 years, but not for those
accidentally exposed or exposed for average periods of 12 and
17 years (Thiess et al., 1981a).
In another ethylene oxide manufacturing plant, no chromosomal
aberrations were detected in the lymphocytes of 36 male workers.
These men had been exposed for 1 - 14 years to average
concentrations of up to 0.28 mg/m3 (van Sittert et al., 1985).
The sister chromatid exchange rate in lymphocytes was not
increased in groups of 28 and 14 sterilization workers exposed to
8-h time-weighted averages, below 1.8 mg/m3 for 2.5 years before
the study (Högstedt et al., 1983) and below 8 mg/m3 (Hansen et al.,
1984), respectively. In the second study, peaks up to 1430 mg/m3
were also measured. Increases in sister chromatid exchange rate
were found in 4 other studies on sterilization workers (Garry et
al., 1979; Abrahams, 1980; Yager et al., 1983; Laurent et al.,
1984). In one of these studies, in which 75 workers were exposed
to levels generally below an 8-h time-weighted average of 90 mg/m3,
this increase was found in cells together with quadriradial
aberrations (Abrahams, 1980). In the other studies, groups were
small, exposure conditions often unclear, and the number of
metaphases scored, in some cases, limited. Yager et al. (1983)
only found an increased sister chromatid exchange rate at
relatively high calculated integrated doses of more than 100 mg per
person. The length of exposure to ethylene oxide averaged 3.6 min
per day; these tasks were performed between 6 and 120 times during
the 6-month study period. Laurent et al. (1984), studied sister
chromatid exchange rates in 2 groups of ethylene oxide-exposed
workers (estimated ethylene oxide dose in past 2 years, 530 -
715 mg and 1185 - 5800 mg, respectively) as well as unexposed
controls. Although the sister chromatid exchange rates in the
high-exposure group did not differ significantly from the rates in
the low-exposure groups (not adjusted for smoking), the difference
among non-smokers between the exposed (n = 20) and the controls
(n = 15) was significant.
In a study on 41 sterilization workers in 8 hospitals in Italy,
increases in both sister chromatid exchanges and in chromosomal
aberrations were detected in lymphocytes; these effects persisted
for months after exposure was reduced or interrupted. The workers
were exposed to average 8-h time-weighted averages of either
0.63 mg/m3 or 19.3 mg/m3 (section 4.3). A statistically-
significant correlation was found between sister chromatid exchange
frequency and the level of ethylene oxide, as well as a multiple
correlation between sister chromatid exchange frequency and
ethylene oxide exposure, smoking, and age (Sarto et al., 1984).
Similarly, in the USA, the sister chromatid exchange frequencies in
the lymphocytes of 61 sterilization workers involved in sterilizing
health-care products, were monitored over a period of 2 years and
compared with those of 82 unexposed controls. During the study
period, 8-h time-weighted-average exposures were reported to be
less than 1.8 mg/m3. Prior to the start of the study, 8-h time-
weighted averages between 0.9 and 36 mg/m3 were measured. Results
were adjusted for smoking habits, sex, and age. Workers exposed to
low levels of ethylene oxide such as those at a worksite with 8-h
time-weighted-average ethylene oxide levels below 1.8 mg/m3 prior
to and during the study, did not show increased frequencies of
sister chromatid exchange. Workers who had been exposed to levels
of 5 - 36 mg/m3, prior to the study, showed an increased frequency
of sister chromatid exchange that persisted for at least 24 months
after cessation of exposure (Stolley et al., 1984).
8.8. Effects on Reproduction
In a study from the USSR (Yakubova et al., 1976), the course of
pregnancy and birth was followed in 57 operators, 38 laboratory
workers, and 65 adminstrative staff in an ethylene oxide-producing
plant, the majority of the women being between 20 and 29 years of
age. A group of 50 pregnant women working outside the plant served
as additional controls. It was estimated that the operators were
exposed to ethylene oxide concentrations of 0.2 - 0.3 mg/m3 for 80%
of the working time and 1.0 mg/m3 for the remaining 20%, while the
laboratory workers were exposed only to the lower concentrations.
Pregnancy toxaemia in the latter half of pregnancy and other
complications were higher in the operators (14.7%) and laboratory
workers (9.9%) than in the administrative staff (4.6%) and outside
controls (8%). On the other hand, the primiparae among the
operators lost less blood perinatally than those among the other
groups. Spontaneous abortion occurred in 6 out of 57 (10.5%)
operators, 3 out of 38 (7.9%) laboratory workers, and in 5 out of
65 (7.7%) administrative staff. The operators were subjected to
the additional stress of high levels of noise and vibration and
wide variations in atmospheric temperatures.
Findings in this study do not indicate any unequivocal adverse
effect of ethylene oxide exposure at these concentrations on the
outcome of pregnancy.
An increase in spontaneous abortions was also found in a study
on Finnish hospital sterilizing staff in 1980, using questionnaires
and hospital discharge records. The sterilizing agents were
ethylene oxide, glutaraldehyde, and formaldehyde. Controls were
nursing auxiliaries, from various hospitals, who did not work in
sterilization, anaesthetization, or X-ray recording departments.
Results were adjusted for age, parity, decade of the reported
pregnancy, coffee and alcohol consumption, and smoking habits.
The rate of spontaneous abortions in the sterilization staff
members as a whole (9.7% in 1443 pregnancies) was similar to the
rate in the controls (10.5% in 1179 pregnancies). A significant
increase, however, was observed when the adjusted spontaneous
abortion rate in the sterilization staff who were exposed during
pregnancy (15.1% in 545 pregnancies) was compared with the rate in
the staff members who were not exposed during pregnancy (4.6% in
605 pregnancies). On the basis of a separate study, the time-
weighted average exposure concentration was estimated to be in the
range of 0.18 - 0.90 mg/m3 (0.1 - 0.5 ppm), with peak concentrations
up to 450 mg/m3 (250 ppm). It was considered by the authors that
exposure to ethylene oxide accounted for most of the excess
spontaneous abortions (Hemminki et al., 1982).
In a new analysis of the data, controls were chosen from the
same hospitals, and only pregnancies that started during hospital
employment were analysed in all groups. The spontaneous abortion
rate was still highest for the pregnancies during which exposure to
ethylene oxide took place (20.4%), and the difference compared with
controls (11.3%) was significant. The abortion rate of the group
exposed to glutaraldehyde alone (16.6%) was also significantly
elevated (Hemminki et al., 1983).
Despite any methodological shortcomings of this reproductive
study, such as (a) the statement by Hemminki in 1983 that there was
limited exposure data, which prevented a comparison between
abortion rates and defined exposure levels; and (b) the fact that
the ethylene oxide-exposed and unexposed cohorts were not balanced
with regard to the incidence of prior abortions, there is a
suggestion of an association between ethylene oxide exposure and
adverse pregnancy outcome.
9. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
Human exposure is mainly through inhalation of the vapour.
Residues in medical equipment that has been sterilized with
ethylene oxide and not sufficiently aerated can migrate into
tissues and blood, producing primarily local effects (section 8.4).
Oral ingestion of ethylene oxide residues in most fumigated or
sterilized foodstuffs is unlikely, as they disappear rapidly
through evaporation or reaction with food constituents. A major
conversion product in foodstuffs is 2-chloroethanol, which is more
persistent than ethylene oxide (section 4.2.1).
Most ethylene oxide is used in the chemical plant in which it
is produced. Because of the explosion hazard, ethylene oxide is
stored and handled in chemical process plants in closed, automated
systems. This equipment is often located outdoors, and, except
during maintenance, workers have a minimal chance of exposure. Air
samples collected in processing areas of chemical production plants
have shown that ethylene oxide vapour concentrations are generally
less than 4 mg/m3 with occasional high peak exposures (section
4.3). Occupational exposure to ethylene oxide tends to be much
higher in health instrument manufacture and in hospitals than in
the chemical processing industries. Ethylene oxide concentrations
near malfunctioning or improperly designed equipment may reach
hundreds of mg/m3 of air for brief periods. However, 8-h time-
weighted average breathing-zone air concentrations in hospitals are
generally less than 36 mg/m3. It should be emphasized that the
exposure of hospital workers to ethylene oxide tends to be of a
short-term and intermittent nature with the likelihood of exposure
to short-term (5 - 120 min) concentrations of about 100 mg/m3 and
to peak concentrations of up to 1800 mg/m3 following the opening of
sterilization chambers (section 4.3).
Ethylene oxide has a high solubility in water but will
evaporate to a great extent. Degradation of ethylene oxide in
neutral water is slow, even in the presence of aerobic
microorganisms. Because of the low log n-octanol water-partition
coefficient, it is unlikely that ethylene oxide and its conversion
products (such as 1,2-ethanediol) will bioaccumulate. The toxicity
of ethylene oxide for aquatic organisms is low (all available LC50s
are above approximately 90 mg/litre) (Table 3). The probable
effects of ethylene oxide on the aquatic environment are,
therefore, considered negligible (sections 3.2, 6). There are no
data concerning the toxicity of ethylene oxide for terrestrial
organisms.
No ambient air monitoring data are available from which the
effects of ethylene oxide on the health of man and the environment
can be assessed. However, the risk for health from exposure to
ethylene oxide in the ambient air, apart from point source
emissions and accidental spillage, is likely to be negligible.
Inhaled ethylene oxide is readily absorbed into the blood,
distributed throughout the body, and rapidly metabolized. The
half-life in the tissues of man and rodents is approximately
10 min; clearance from the blood of dogs occurred with a half-
life of 33 min (section 5.2). Marked nausea and profuse vomiting
following dermal exposure of man to aqueous solutions of ethylene
oxide suggest that absorption can occur through the skin (section
5.1).
Case reports indicate that headache, nausea, vomiting,
dyspnoea, and respiratory tract irritation are common effects of
acute inhalation exposure to ethylene oxide (section 8.3). Case
reports and the results of animal studies indicate that
sensorimotor neuropathies may follow repeated exposure to
concentrations of ethylene oxide recognizable by its odour
(approximately 900 mg/m3 or more) (sections 2.2, 7.2.1, 8.3).
Dermatological effects in man following skin contact with
ethylene oxide include erythema, oedema, and vesiculation, in that
order. The severity of the skin injury is related to concentration
(a 50% (500 g/litre) solution being most hazardous) and duration of
contact. Liquid ethylene oxide, as it vaporizes, can result in a
freeze burn. On repeated exposure, ethylene oxide may cause
delayed allergic contact dermatitis (sections 8.1, 8.2). Ethylene
oxide and its conversion products are irritating to the eyes and
can produce corneal injury. Cataracts have occurred following
repeated exposure to concentrations of the vapour recognizable by
its odour (approximately 900 mg/m3 or more) (Table 1, sections 8.1,
8.3).
Ethylene oxide directly alkylates proteins and DNA and is
mutagenic in microorganisms, plants, insects, mammalian cells in
vitro, and mammals in vivo, including both gene mutations and
chromosomal abnormalities (section 7.5). In man, ethylene oxide
induces chromosomal aberrations and sister chromatid exchanges in
lymphocytes at air concentrations found at the workplace (section
8.7). Tissue distribution studies provide evidence that ethylene
oxide reaches the gonads, supporting the findings of heritable
mutations in insects and rodents (sections 5.2, 7.5). Ethylene
oxide may, therefore, be considered a potential human mutagen for
both somatic and germ cells.
The potential of ethylene oxide to cause teratogenic or adverse
reproductive effects has been examined in 4 animal species (mouse,
rat, rabbit, and monkey) by 2 routes of administration. Results
from these studies showed that ethylene oxide is toxic to
reproductive function in both males (reduced sperm number and sperm
motility, and an increased time to traverse a linear path) and
females (depression of fetal weight gain, fetal death, and fetal
malformation). The levels needed to produce these fetal effects
approach or equal the dose needed to produce maternal toxicity
(section 7.6). The results of animal studies suggest possible
reproductive impairment in human males but are inadequate for
assessing the fetal risk. Data on reproductive effects in human
beings are insufficient; one study, however, suggests an increase
in spontaneous abortion rate in women occupationally exposed to
ethylene oxide (section 8.8). However, the reported time-weighted
average air concentrations may not reflect the exposure levels that
induced the effect.
It has been clearly demonstrated in experimental animal studies
that ethylene oxide is carcinogenic via different routes of
exposure (intragastric, subcutaneous injection, and inhalation).
In 2 inhalation studies, confirmatory data demonstrated dose-
related increases in the incidences of leukaemia, peritoneal
mesothelioma, and cerebral glioma (section 7.4). Although the
evidence for the carcinogenicity of ethylene oxide in man is
inadequate, epidemiological studies indicate that exposure to
ethylene oxide (in mixtures with other chemicals) increases the
risk of malignancies (section 8.6).
Taking into account available data concerning the alkylating
nature of ethylene oxide, the demonstration of DNA adducts, the
overwhelming positive in vivo responses in mutagenic and
clastogenic assays, the reproducible positive carcinogenic findings
in animals, and the epidemiological findings suggesting an increase
in the incidence of human cancer, ethylene oxide should be
considered as a probable human carcinogen, and its levels in the
environment should be kept as low as feasible.
10. RECOMMENDATIONS FOR FURTHER RESEARCH
1. The study indicating that exposure to ethylene oxide may be
associated with spontaneous abortion needs to be
corroborated and the implication explored further.
2. The possible effects of ethylene oxide on the reproductive
function of man should be studied.
3. The epidemiological studies indicating an increased risk of
cancer in workers exposed to ethylene oxide in combination
with other chemicals strongly suggest that additional
epidemiological studies should be carried out on
populations whose exposure has been primarily to ethylene
oxide, including adequate quantification of past exposure.
4. The temporal relationships of air concentrations of
ethylene oxide and duration of exposure must be examined to
determine which of these two factors has the greater impact
on health.
5. Development of methods and research should be conducted to
assess the endogenous occurrence of hydroxyethylation and
the exogenous (environmental) contribution of ethylene
oxide and its precursors to the formation of macromolecular
adducts as markers of internal dose.
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
An International Agency for Research on Cancer Working Group
(IARC, 1985) evaluated the carcinogenicity of ethylene oxide and
concluded that:
"There is sufficient evidence for the carcinogenicity of
ethylene oxide to experimental animals; there is limited
evidence for the carcinogenicity to humans of exposures to
ethylene oxide in combination with other chemicals; there is
inadequate evidence for the carcinogenicity to humans of
exposures to ethylene oxide alone. Taken together, the data
indicate that ethylene oxide is probably carcinogenic to
humans."
REFERENCES
ABRAHAMS, R.H. (1980) Recent studies with workers exposed to
ethylene oxide. In: Jorkasky, J.F., ed. Safe use of ethylene
oxide. Proceedings of the Educational Seminar, Washington DC,
Health Industries Manufacturers Association, pp. 27-38,
211-220 (HIMA Report No. 80-4)
ADLER, N. (1965) Residual ethylene oxide and ethylene glycol
in ethylene oxide sterilized pharmaceuticals. J. Pharm. Sci.,
54: 735-742.
ALOMAR, A., CAMARASA, J.M.G., NOGUERA, J., ASPINOLEA, F.
(1981) Ethylene oxide dermatitis. Contact dermatit., 7:
205-207.
ALTMAN, P.L. & DITTMER, D.S. (1974) Biological data book,
Bethesda, Maryland, Federation of American Societies for
Experimental Biology, Vol. 3.
APPELGREN, L.-E., ENEROTH, G., & GRANT, C. (1977) Studies on
ethylene oxide: whole-body autoradiography and dominant lethal
test in mice. In: Clinical toxicology. Proceedings of the
Meeting held at Edinburgh, June 1976, European Society of
Toxicology, Amsterdam, Excerpta Medica, Vol. 18, pp. 315-317.
APPELGREN, L.-E., ENEROTH, G., GRANT, C., LANDSTROM, L.-E., &
TENGHAGEN, K. (1978) Testing of ethylene oxide for
mutagenicity using the micronucleus test in mice and rats.
Acta pharmacol. toxicol., 43: 69-71.
BINDER, H. & LINDNER, W. (1972) [Determination of ethylene
oxide in the smoke of definitely unfumigated cigarettes.]
Fachliche Mitteilungen der Austria Tabakwerke A.G., 13:
215-220 (in German).
BIRD, M.J. (1952) Chemical production of mutations in
Drosophila: comparison of techniques. J. Genet., 50: 480-485.
BIRO, L., FISHER, A.A., & PRICE, E. (1974) Ethylene oxide
burns. Arch. Dermatol., 110: 924-925.
BLACKWOOD, J.D. & ERSKINE, E.B. (1938) Carboxide poisoning.
US Navy med. Bull., 36: 44-45.
BLIXT, S., EHRENBERGH, L., & GELIN, O. (1963) Studies of
induced mutations in peas. VII. Mutation spectrum and mutation
rate of different mutagenic agents. Agric. Hort. Genet., 21:
178-216.
BOGYO, S., LANDE, S.S., MEYLAND, W.M., HOWARD, P.H., &
SANTODONATO, J. (1980) Investigation of selected potential
environmental contaminants: epoxides, Syracuse, New York,
Center for Chemical Hazard Assessment, Syracuse Research
Corporation (Report prepared for US EPA) (Report No. EPA
560/11-80-005, PB 80-183197).
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) The acute
toxicity of some petrochemicals to goldfish. Water Res., 13:
623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and
COD of some petrochemicals. Water Res., 13: 627-630.
BROOKES, P. & LAWLEY, P.D. (1961) The alkylation of
guanosine and guanylic acid. J. Chem. Soc., pp. 3923-3928.
BUQUET, A. & MANCHON, P. (1970) Recherche et dosage des
résidus et dérivés dans un pain conservé a l'aide d'oxyde
d'éthylène. Chim. analytique, 52: 978-983.
CALLEMAN, C.J., EHRENBERG, L., JANSSON, B., OSTERMAN-GOLKAR,
S., SEGERBACK, D., SVENSSON, K., & WACHTMEISTER, C.A. (1978)
Monitoring and risk assessment by means of alkyl groups in
haemoglobin in persons occupationally exposed to ethylene
oxide. J. environ. Pathol. Toxicol., 2: 247-442.
CAPELLINI, A. & GHEZZI, I. (1965) [Two cases of acute
ethylene oxide poisoning.] Med. Lav., 56: 822-827 (in Italian).
CHAIGNEAU, M. & MURAZ, B. (1981) Action de l'oxyde
d'éthylène sur le tabac ( Nicotiana tabacum L.): absorption et
combinaisons. Ann. Pharm. Fr., 39: 305-311.
CLARKE, C.P., DAVIDSON, W.L., & JOHNSTON, J.B. (1966)
Haemolysis of blood following exposure to an Austrialian
manufactured plastic tubing sterilized by means of ethylene
oxide gas. Aust. NZ J. Surg., 36: 53-55.
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, Vol. 2a: toxicology, 3rd revised ed.,
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
p. 2166.
CONAN, L., FOUCAULT, B., SIOU, G., CHAIGNEAU, M., LE MOAN, G.,
& DOINEL, A. (1979) Contribution à la recherche d'une action
mutagène des résidus d'oxyde d'éthylène, d'éthylène glycol et
de chloro-2-éthanol dans le matériel plastique stérilisé par
l'oxyde d'éthylène. Ann. Fals. exp. Chim., 72: 141-151.
CONWAY, R.A., WAGGY, G.T., SPIEGEL, M.H., & BERGLUND, R.L.
(1983) Environmental fate and effects of ethylene oxide.
Environ. Sci. Technol., 17: 107-112.
CUMMING, R.B. & MICHAUD, T.A. (1979) Mutagenic effects of
inhaled ethylene oxide in male mice. Environ. Mutagen., 1:
166-167.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
US Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No.
600/3-80-084, PB 80-221948).
DAUVOIS, C., CHAIGNEAU, M., & LE MOAN, G. (1982)
Sterilisation de pansements par l'oxyde d'éthylène. I.
Physisorption. Ann. Pharm. Fr., 40: 125-132.
DOLOVICH, J. & BELL, B. (1978) Allergy to a product(s) of
ethylene oxide gas. Demonstration of IgE and IgG antibodies
and hapten specificity. J. Allergy clin. Immunol., 62: 30-32.
DUNKELBERG, H. (1981) [Carcinogenic activity of ethylene
oxide and its reaction products 2-chloroethanol,
2-bromoethanol, ethylene glycol, and diethylene glycol. I.
Carcinogenicity of ethylene oxide in comparison with
1,2-propylene oxide after subcutaneous administration in
mice.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 174: 383-404 (in
German).
DUNKELBERG, H. (1982) Carcinogenicity of ethylene oxide and
1,2-propylene oxide upon intragastric administration to rats.
Br. J. Cancer, 46: 924-933.
DUNKELBERG, H. & HARTMETZ, G. (1977) [Recording the air
pollution by ethylene oxide in the region of clinical
sterilization installations.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
164: 271-278 (in German).
EDELHAUSER, H.F., ANTOINE, M.E., PEDERSON, H.J., & HIDDEMAN,
J.W., & HARRIS, R.G. (1983) Intraocular safety evaluation of
ethylene oxide and sterilant residues. J. Toxicol.-Cut. ocular
Toxicol., 2: 7-39.
EHRENBERG, L. & HALLSTROM, T. (1967) In: Kalling, L.O., ed.
Haematologic studies on persons occupationally exposed to
ethylene oxide, Vienna, International Atomic Agency, pp.
327-334 (Report No. SM92-26).
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1956)
Chemically induced mutation and sterility in barley. Acta
chem. Scand., 10: 492-494.
EHRENBERG, L., GUSTAFSSON, A., LUNDQVIST, U. (1959) The
mutagenic effects of ionizing radiations and reactive ethylene
derivatives in barley. Hereditas, 45: 351-368.
EHRENBERG, L., HIESCHE, K.D., OSTERMAN-GOLKAR, S., & WENNBERG,
I. (1974) Evaluation of genetic risks of alkylating agents:
tissue doses in the mouse from air contaminiated with ethylene
oxide. Mutat. Res., 24: 83-103.
EMBREE, J.W. & HINE, C.H. (1975) Mutagenicity of ethylene
oxide. Toxicol. appl. Pharmacol., 33: 172-173.
EMBREE, J.W., LYON, J.P., & HINE, C.H. (1977) The mutagenic
potential of ethylene oxide using the dominant-lethal assay in
rats. Toxicol. appl. Pharmacol., 40: 261-267.
FOMENKO, V.N. & STREKALOVA, E.Y. (1973) [The mutagenic
effect of some industrial toxins as a function of
concentration and exposure time.] Toksikol. Nov. Prom. Him.
Veshchestr., 7: 51-57 (in Russian).
FAHMY, G.G. & FAHMY, M.J. (1970) Gene elimination in
carcinogenesis: reinterpretation of the somatic mutation
theory. Cancer Res., 30: 195-205.
FINELLI, P., MORGAN, T.F., YAAR, I., & GRANGER, C.V. (1983)
Ethylene oxide-induced polyneuropathy: a clinical and
electrophysiologic study. Arch. Neurol., 40: 419-421.
FJELLSTEDT, T.A., ALLEN, R.H., DUNCAN, B.K., & JAKOBY, W.B.
(1973) Enzymatic conjugation of epoxides with glutathione.
J. Biol. Chem., 248: 3702-3707.
FLORES, G.H. (1983) Controlling exposure to alkene oxides.
Chem. Eng. Prog., 79: 39-43.
GARRY, V.F., HOZIER, J., JACOBS, D., WADE, R.L., & GRAY, D.G.
(1979) Ethylene oxide: evidence of human chromosomal effects.
Environ. Mutagen., 1: 375-382.
GARRY, V.F., OPP, C.W., WIENCKE, J.K., & LAKATUA, D. (1982)
Ethylene oxide induced sister chromatid exchange in human
lymphocytes using a membrane dosimetry system. Pharmacology,
25: 214-221.
GENEROSO, W.M., CAIN, K.T., KRISHNA, M., SHEN, C.W., & GRYDER,
R.M. (1980) Heritable translocation and dominant-lethal
mutation induction with ethylene oxide in mice. Mutat. Res.,
73: 133-142.
GENEROSO, W.M., CUMMING, R.B., BANDY, J.A., & CAIN, K.T.
(1983) Increased dominant-lethal effects due to prolonged
exposure of mice to inhaled ethylene oxide. Mutat. Res., 119:
377-379.
GERHARDT, U. & LADD EFFIO, J.C. (1983) [Ethylene oxide
residues in spices.] Fleisch wirtsch., 63: 606-608 (in German).
GESSNER, P.K., PARKE, D.V., & WILLIAMS, R.T. (1961) The
metabolism of 14C-labelled ethylene glycol. Biochem. J., 79:
482-489.
GILDING, D.K., REED, A.M., & BASKETT, S.A. (1980) Ethylene
oxide sterilization: effect of polymer structure and
sterilization conditions on residue levels. Biomaterials, 1:
145-148.
GILLESPIE, E.H., JACKSON, J.M., & OWEN, G.R. (1979) Ethylene
oxide sterilization: is it safe? J. clin. Pathol., 32:
1184-1187.
GLASER, Z.R. (1979) Ethylene oxide: toxicology review and
field study results of hospital use. J. environ. Pathol.
Toxicol., 2: 173-208.
GORDON, H.T. & THORNBURG, W.W. (1959) Hydroxyethyl
derivatives in prunes fumigated with 14C-ethylene oxide.
J. agric. food Chem., 7: 196-200.
GREAVES WALKER, W.G. & GREESON, C.E. (1932) The toxicity of
ethylene oxide. J. Hyg., 32: 409-416.
GROSS, J.A., HAAS, M.L., & SWIFT, T.R. (1979) Ethylene oxide
neurotoxicity: report of four cases and review of the
literature. Neurology, 29: 978-983.
GRUNOW, W. & ALTMANN, H.-J. (1982) Toxicokinetics of
chloroethanol in the rat after single oral administration.
Arch. Toxicol., 49: 275-284.
HACKETT, P.L., BROWN, M.G., BUSCHBOM, R.L., CLARK, M.L.,
MILLER, R.A., MUSIC, R.L., ROWE, S.E., SCHIRMER, R.E., &
SIKOV, M.R. (1982) Teratogenic study of ethylene and
propylene oxide and N-butyl acetate, Richland, Washington,
Batelle Pacific Northwest Laboratories (Report No. PB
83-258038).
HANSEN, J.P., ALLEN, J., BROCK, K., FALCONER, J., HELMS, M.J.,
SHAVER, G.C., & STROHM, B. (1984) Normal sister chromatid
exchange levels in hospital sterilization employees exposed to
ethylene oxide. J. occup. Med., 26: 29-32.
HANIFIN, J.M. (1971) Ethylene oxide dermatitis. J. Am. Med.
Assoc., 217: 213.
HARTMAN, P.A. & BOWMAN, P.B. (1977) Simple GLC determination
of ethylene oxide and its reaction products in drugs and
formulations. J. Pharm. Sci., 66: 789-792.
HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEMMINKI, K., MUTANEN, P., SALONIEMI, I., NIEMI, M-L., &
VAINIO, H. (1982) Spontaneous abortions in hospital staff
engaged in sterilizing instruments with chemical agents.
Br. med. J., 285: 1461-1463.
HEMMINKI, K., MUTANEN, P., & NIEMI, M.-L. (1983) Spontaneous
abortions in hospital sterilizing staff. Br. med. J., 286:
1976-1977.
HENNE, W., DIETRICH, W., PELGER, M., & SENGBUSCH, G., VON
(1984) Residual ethylene oxide in hollow-fibre dialyzers.
Artif. Organs, 8: 306-309.
HINE, C.H. & ROWE, V.K. (1981) Epoxy compounds. In: Patty,
S.A., ed. Industrial hygiene and toxicology, 3rd revised ed.,
New York, Interscience Publishers, Vol. IIa, p. 2141.
HIROSE, T., GOLDSTEIN, R., & BAILEY, C. (1963) Hemolysis of
blood due to exposure to different types of plastic tubing and
the influence of ethylene oxide sterilization. J. thorac.
cardiovasc. Surg., 45: 245-251.
HOGSTEDT, C., MALMQVIST, N., & WADMAN, B. (1979a) Leukemia
in workers exposed to ethylene oxide. J. Am. Med. Assoc., 241:
1132-1133.
HOGSTEDT, C., ROHLEN, O., BERNDTSSON, B.S., AXELSON, O., &
EHRENBERG, L. (1979b) A cohort study of mortality and cancer
incidence in ethylene oxide production workers. Br. J. ind.
Med., 26: 276-280.
HOGSTEDT, B., GULLBERG, B., HEDNER, K., KOLNIG, A-M.,
MITELMAN, F., SKERFVING, S., & WIDEGREN, B. (1983)
Chromosome aberrations and micronuclei in bone marrow cells
and peripheral blood lymphocytes in humans exposed to ethylene
oxide. Hereditas, 98: 105-113.
HOGSTEDT, C., ARINGER, L., & GUSTAVSSON, A. (1984) [Ethylene
oxide and cancerreview of the literature and follow-up of 2
studies.] Arbete och Hälsa, 49: : 1-32 (in Swedish).
HOLLEY, H.S. & GILDEA, J.E. (1971) Vocal cord paralysis
after tracheal intubation. J. Am. Med. Assoc., 215: 281-284.
HOLLINGSWORTH, R.L., ROWE, V.K., OYEN, F., MCCOLLISTER, D.D.,
& SPENCER, H.C. (1956) Toxicity of ethylene oxide determined
on experimental animals. Arch. ind. Health, 13: 217-227.
HUSSAIN, S. & OSTERMAN-GOLKAR, S. (1984) Dose-response
relationship for mutations induced in E. coli by some model
compounds. Hereditas, 101: 57-68.
IARC (1985) Alkyl compounds, aldehydes, epoxides, and
peroxides, Lyons, International Agency for Research on Cancer
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans No. 36).
IPPEN, H. VON & MATTHIES, V. (1970) [Protracted chemical
burns.] Berufsdermatosen, 18: 144-165 (in German).
IRPTC (1984) Data profile on ethylene oxide, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBSON, K.H., HACKLEY, E.B., & FEINSILVER, L. (1956) The
toxicity of inhaled ethylene oxide and propylene oxide vapors.
Arch. ind. Health, 13: 237-244.
JANA, M.K. & ROY, K. (1975) Effectiveness and efficiency of
ethyl methane-sulphonate and ethylene oxide for the induction
of mutations in mice. Mutat. Res., 28: 211-215.
JAY, W.M., SWIFT, T.R., & HULL, D.S. (1982) Possible
relationship of ethylene oxide exposure to cataract formation.
Am. J. Ophthalmol., 93: 727-732.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction
of carcinogenicity evaluated by 143 agents tested. Mutat.
Res., 75: 191-202.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rat.
Biochem. Pharmacol., 16: 185-199.
JONES, A.R. & WELLS, G. (1981) The comparative metabolism of
2-bromoethanol and ethylene oxide in the rat. Xenobiotica, 11:
763-770.
JORDY, A. (1983) [The course of the concentrations of
ethylene oxide reaction products in synthetic materials
following gas sterilization.] Hyg. Med., 8: 17-19 (in German).
JOSHI, S.B., DODGE, M.C., & BUFALINI, J.J. (1982)
Reactivities of selected organic compounds and contamination
effects. Atmos. Environ., 16: 1301-1310.
JOYNER, R.E. (1964) Chronic toxicity of ethylene oxide.
Arch. environ. Health, 8: 700-710.
KAUHANEN, K. (1978) Ethylene oxide. Rebuttable presumption
against registration maximum residue limits and daily levels
of exposure. Fed. Reg., 43:: 3804.
KLIGERMAN, A.D., EREXSON, G.L., PHELPS, M.E., & WILMER, J.L.
(1983) Sister-chromatid exchange induction in peripheral
blood lymphocytes of rats exposed to ethylene oxide by
inhalation. Mutat. Res., 120: 37-44.
KOLMARK, H.G. & KILBEY, B.J. (1968) Kinetic studies of
mutation induction by epoxides in Neurospora crassa. Molec.
Gen. Genet., 101: 89-98.
KOLMARK, H.G. & WESTERGAARD, M. (1953) Further studies on
chemically-induced reversions at the adenine locus of
Neurospora. Hereditas, 39: 209-224.
KORPELA, D.B., MCJILTON, C.E., & HAWKINSON, T.E. (1983)
Ethylene oxide dispersion from gas sterilizers. Am. Ind. Hyg.
Assoc. J., 44: 589-591.
KROLLER, E. VON (1966) [Investigations into the fumigation
of foodstuffs by ethylene oxide and into the determination of
its residues.] Dtsch. Lebensm. Rundsch., 62: 227-234 (in
German).
KUZUHARA, S., KANAZAWA, I., NAKANISHI, T., & EGASHIRA, T.
(1983) Ethylene oxide polyneuropathy. Neurology, 33: 377-380.
LABORDE, J.B. & KIMMEL, C.A. (1980) The teratogenicity of
ethylene oxide administered intraveneously to mice. Toxicol.
appl. Pharmacol., 56: 16-22.
LAHAYE, D., ASSCHE, F., VAN, & THEUNISSEN, A. [Ethylene oxide
levels in the sterilization units of hospitals.] Tijdschr.
Soc. Gezondheidsz., 62: 707-713 (in Dutch).
LAMY, F., LACHAPELLE, J.-M., & BRAEKEL, G. VAN (1974)
Dermites professionnelles a l'oxyde d'éthylène chez des sujets
travaillant en zone stérile. Arch. Mal. prof. Méd. Trav.
Sécur. soc., 35: 719-724.
LAURENT, C., FREDERIC, J., & LEONARD, A.Y. (1984) Sister
chromatid exchange frequency in workers exposed to high levels
of ethylene oxide, in a hospital sterilization service.
Int. Arch. occup. environ. Health, 54: 33-43.
LEBREC, D., MASQUET, C., & RUEFF, B. (1977) Collapsus après
exploration endovasculaire avec des cathéters stérilisés à
l'oxyde d'éthylène. Nouv. Presse Med., 6: 2991.
LIPTON, B., GUTIERREZ, R., BLAUGRUND, S., LITWAK, R.S., &
RENDELL-BAKER, L. (1971) Irridiated PVC plastic and gas
sterilization in the production of tracheal stenosis following
tracheostomy. Anest. Analg., 50: 578-586.
LYARSKII, P.P., YURCHENKO, V.V., ZHURKOV, V.S., & GLEIBERMAN,
S.E. (1983) [Mutagenic hazards of parenteral administration
of ethylene oxide to mammals.] Gig. i Sanit., 1: 23-26 (in
Russian).
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GROTH,
D.H., KHAN, A., ACKERMAN, L.J., & COCKRELL, B.Y. (1984a)
Carcinogenic and toxicologic effects of inhaled ethylene oxide
and propylene oxide in F344 rats. Toxicol. appl. Pharmacol.,
76: 69-84.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., BURG, J.R., GULATI,
D.H., KAUR, P., & SABHARWAL, P.S. (1984b) Sister-chromatid
exchanges and chromosome aberrations in lymphocytes from
monkeys exposed to ethylene oxide and propylene oxide by
inhalation. Toxicol. appl. Pharmacol., 76: 85-95.
LYNCH, D.W., LEWIS, T.R., MOORMAN, W.J., LAL, J.B., BURG,
J.R., GULATI, D.K., ZAVOS, P.M., & SABHARWAL, P.S. (1984c)
Toxic and mutagenic effects of inhaled ethylene oxide and
propylene oxide on spermatogenic functions in monkeys.
Toxicologist, 3: 60.
MACKEY, J. (1968) Mutagenesis in Vulgare wheat. Hereditas,
59: 505-517.
MCCHESNEY, E.W., GOLBERG, L., PAREKH, C.K., RUSSELL, J.C., &
MIN, B.H. (1971) Reappraisal of the toxicology of ethylene
glycol. II. Metabolism studies in laboratory animals. Food
cosmet. Toxicol., 9: 21-30.
MCDONALD, T.O., KASTEN, K., HERVEY, R., GREGG, S., BORGMANN,
A.R., & MURCHISON, T. (1973) Acute ocular toxicity of
ethylene oxide, ethylene glycol, and ethylene chlorohydrin.
Bull. Parenter. Drug Assoc., 27: 153-164.
MCGUNNIGLE, R.G., RENNER, J.A., ROMANO, S.J., & ABODEELY,
R.A. (1975) Residual ethylene oxide: levels in medical grade
tubing and effects on an in vitro biological system.
J. Biomed. Mater. Res., 9: 273-383.
MANTZ, J.M., TEMPE, J.D., JAEGER, A., & VIDAL, S. (1972)
Stenoses trachéales et stérilisation des canules de
trachéotomie par l'oxyde d'éthylène. Sem. Hôp. Paris, 48:
3367-3370.
MARTIS, L., KROES, R., DARBY, T.D., & WOODS, E.F. (1982)
Disposition kinetics of ethylene oxide, ethylene glycol, and
2-chloroethanol in the dog. J. Toxicol. environ. Health, 10:
847-856.
MARX, G.F., STEEN, S.N., SCHAPIRA, M., ERLANGER, H.L., ARKINS,
R.E., JADWAT, C.M., & KEPES, E. (1969) Hazards associated
with ethylene oxide sterilization. NY State J. Med., 69:
1319-1320.
METZ, E. VON (1939) [Poisoning by ethylene oxide (Cartox or
T-gas).] Samml. Vergiftungsfällen, 10: 37-38 (in German).
MIGLIORE, L., ROSSI, A.M., & LOPRIENO, N. (1982) Mutagenic
action of structurally-related alkene oxides on
Schizosaccharomyces pombe: the influence "in vitro" of
mouse-liver metabolizing system. Mutat. Res., 102: 425-437.
MORGAN, R.W., CLAXTON, K.W., DIVINE, B.J., KAPLAN, S.D., &
HARRIS, V.B. (1981) Mortality among ethylene oxide workers.
J. occup. Med., 23: 767-770.
MORPURGO, G. (1963) Induction of mitotic crossing-over in
Aspergillus nidulans by bifunctional alkylating agents.
Genetics, 48: 1259-1263.
MOUILLESEAUX, A., LAURENT, A.-M., FABRE, M., JOUAN, M., &
FESTY, B. (1983) Teneurs atmosphériques en oxyde d'éthylène
décelées dans l'environnement professionnel d'instalations de
stérilisation ou de désinfection. Arch. Mal. Prof., 44: 1-14.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., & EHRENBERG, L.
(1968) Note on the chromosome breaking activity of ethylene
oxide and ethyleneimine. Hereditas, 60: 267-269.
NAKAO, Y. & AUERBACH, C. (1961) Test of a possible
correlation between cross-linking and chromosome breaking
abilities of chemical mutagens. Z. Vererbungsl., 92: 457-461.
O'LEARY, R.K., WATKINS, W.D., & GUESS, W.L. (1969)
Comparative chemical and toxicological evaluation of residual
ethylene oxide in sterilized plastics. J. Pharm. Sci., 58:
1007-1010.
OSER, B.L. & HALL, L.A. (1956) The effect of ethylene oxide
treatment on the nutritive value of certain foods. Food
Technol., 10: 175-178.
OSTERMAN-GOLKAR, S (1983) Tissue doses in man: implications
in risk assessment. In: Hayes, A.W., ed. Developments in the
Science and Practice of Toxicology. Proceedings of the 3rd
International Congress on Toxicology, San Diego, California.
OSTERMAN-GOLKAR, S., EHRENBERG, L., SEGERBACK, D., &
HALLSTROM, I. (1976) Evaluation of genetic risks of
alkylating agents. II. Haemoglobin as a dose monitor.
Mutat. Res., 34: 1-10.
OSTERMAN-GOLKAR, S., FARMER, P.B., SEGERBACK, D., BAILEY, E.,
CALLEMAN, C.J., SVENSSON, K., & EHRENBERG, L. (1983)
Dosimetry of ethylene oxide in the rat by quantitation of
alkylated histidine in haemoglobin. Teratog. Carcinog. Mutag.,
3: 395-405.
PERO, E.W., WIDEGREN, B., HOGSTEDT, B., & MITELMAN, F.
(1981) In vivo and in vitro ethylene oxide exposure of human
lymphocytes assessed by chemical stimulation of unscheduled
DNA synthesis. Mutat. Res., 83: 271-289.
PFEIFFER, E.H. & DUNKELBERG, H. (1980) Mutagenicity of
ethylene oxide and propylene oxide and of the glycols and
halohydrins formed from them during the fumigation of
foodstuffs. Food cosmet. Toxicol., 18: 115-118.
PFEILSTICKER, K. & SIDDIQUI, I.R. (1976) [Isolation of the
derivatives from cocoa-powder fumigated by ethylene oxide
1,2-14C and their structure suggested on the basis of I.R. and
mass-spectrometry.] Z. Lebensm. Unters. Forsch., 160: 19-27
(in German).
PFEILSTICKER, K., FABRICIUS, G., & TIMME, G. (1975)
[Simultaneous gas chromatographic determination of ethylene
oxide, ethylene chlorohydrin, and ethylene glycol in grain.]
Z. Lebensm. Unters. Forsch., 158: 21-25 (in German).
POIRIER, V. & PAPADOPOULO, D. (1982) Chromosomal aberrations
induced by ethylene oxide in a human amniotic cell line in
vitro. Mutat. Res., 104: 255-260.
POOTHULLIL, J., SHIMIZU, A., DAY, R.P., & DOLOVICH, J.
(1975) Anaphylaxis from the product(s) of ethylene oxide gas.
Ann. intern. Med., 82: 58-60.
QUAZI, A.H. & KETCHAM, N.H. (1977) A new method for
monitoring personal exposure to ethylene oxide in the
occupational environment. Am. Ind. Hyg. Assoc. J., 38: 635-647.
RADDING, S.B., LIU, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances, (EPA 560/5-77-003, PB 267121).
RAGELIS, E.P., FISHER, B.S., KLIMECK, B.A., & JOHNSON, C.
(1968) Isolation and determination of chlorohydrins in foods
fumigated with ethylene oxide or with propylene oxide.
J. Assoc. Offic. Agric. Chem., 51: 709-715.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs.
Bull. environ. Contam. Toxicol., 27: 426-431.
RANNUG, U., GOTHE, R., & WACHTMEISTER, C.A. (1976) The
mutagenicity of chloroethylene oxide, chloroacetaldehyde,
2-chloroethanol, and chloroacetic acid, conceivable metabolites
of vinyl chloride. Chem.-Biol Interact., 12: 251-263.
RILLAER, W.G., VAN & BEERNAERT, H. (1982) Determination of
residual ethylene chlorohydrin (ECH) in fumigated foodstuffs
by glass capillary gas chromatography. Z. Lebensm. Unters.
Forsch., 175: 175-178.
ROMANO, S.J. & RENNER, J.A. (1979) Analysis of ethylene
oxide-worker exposure. Am. Ind. Hyg. Assoc. J., 40: 742-745.
ROMANO, S.J., RENNER, J.A., & LEITNER, P.M. (1973) Gas
chromatographic determination of residual ethylene oxide by
head space analysis. Anal. Chem., 45: 2327-2330.
ROYCE, A. & MOORE, W.K.S. (1955) Occupational dermatitis
caused by ethylene oxide. Br. J. ind. Med., 12: 169-171.
SARTO, F., COMINATO, I., PINTON, A.M., BROVEDANI, P.G.,
FACCIOLI, C.M., BIANCHI, V., & LEVIS, A.G. (1984)
Cytogenetic damage in workers exposed to ethylene oxide.
Mutat. Res., 138: 185-195.
SCUDAMORE, K.A. & HEUSER, S.G. (1971) Ethylene oxide and its
persistent reaction products in wheat flour and other
commodities: residues from fumigation or sterilization, and
effects of processing. Pestic. Sci., 2: 80-91.
SEGERBACK, D. (1983) Alkylation of DNA and haemoglobin in
the mouse following exposure to ethene and ethene oxide.
Chem.-Biol. Interact., 45: 139-151.
SEXTON, R.J. & HENSON, E.V. (1949) Dermatological injuries
by ethylene oxide. J. ind. Hyg. Toxicol., 31: 297-300.
SEXTON, R.J. & HENSON, E.V. (1950) Experimental ethylene
oxide human skin injuries. J. ind. Hyg. Toxicol., 32: 549-564.
SHULOVSKA, K., LINDGREN, D., ERIKSSON, G., & EHRENBERG, L.
(1969) The mutagenic effect of low concentrations of ethylene
oxide in air. Hereditas, 62: 264-266.
SHUPACK, J.L., ANDERSEN, S.R., & ROMANO, S.J. (1981) Human
skin reactions to ethylene oxide. J. Lab. clin. Med., 98:
723-729.
SITTERT, N.J., VAN, JONG, G., DE, CLARE, M.G., DAVIES, R.,
DEAN, B.J., WREN, L.J., & WRIGHT, A.S. (1985) Cytogenetic,
immunological, and haematological effects in workers in an
ethylene oxide-manufacturing plant. Br. J. ind. Med., 42:
19-26.
SMITH, H.H. & LOTFY, T.A. (1954) Comparative effects of
certain chemicals on tradescantia chromosomes as observed at
pollen tube mitosis. Am. J. Bot., 41: 489-593.
SMYTH, H.F., SEATON, J., & FISHER, L. (1941) The single dose
toxicity of some glycols and derivatives. J. ind. Hyg. Toxicol.,
23: 259-268.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1981)
Ethylene oxide: two-year inhalation study on rats, Pittsburgh,
Pennsylvania, Bushy Run Research Center (Final Report No.
44-20).
SNELLINGS, W.M., ZELENAK, J.P., & WEIL, C.S. (1982a) Effects
on reproduction in Fischer 344 rats exposed to ethylene oxide
by inhalation for one generation. Toxicol. appl. Pharmacol.,
63: 382-388.
SNELLINGS, W.M., MARONPOT, R.R., ZELENAK, J.P., & LAFFOON,
C.P. (1982b) Teratology study on Fischer 344 rats exposed to
ethylene oxide by inhalation. Toxicol. appl. Pharmacol., 64:
476-481.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984a) A
subchronic inhalation study on the toxicologic potential of
ethylene oxide in B6C3F1 mice. Toxicol. appl. Pharmacol., 76:
510-518.
SNELLINGS, W.M., WEIL, C.S., & MARONPOT, R.R. (1984b) A
two-year inhalation study of the carcinogenic potential of
ethylene oxide in Fischer 344 rats. Toxicol. appl. Pharmacol.,
75: 105-117.
SPASOVSKI, M., HRISTEVA, V., PERNOV, K., KIRKOV, V.,
DRJANOVSKA, T., PANOVA, Z., BOBEV, G., GINCHEVA, N., &
IVANOVA, S. (1980) [Health status of the workers from the
production of ethylene and ethylene oxide.] Khig. Zdraveopaz.,
23: 41-47 (in Russian).
SPRINZ, H., MATZKE, H., & CARTER, J. (1982) Neuropathological
evaluation of monkeys exposed to ethylene and propylene oxide,
Kansas City, Missouri, Midwest Research Institute (Prepared for
NIOSH) (PB 83-134817).
STANLEY, P., BERTRANOU, E., FOREST, F., & LANGEVIN, L.
(1971) Toxicity of ethylene oxide sterilization of polyvinyl
chloride in open-heart surgery. J. thorac. cardiovasc. Surg.,
61: 309-314.
STAR, E.G. (1980a) [Mutagenic and cytotoxic effect of
ethylene oxide on human cell cultures.] Zbl. Bakt. Hyg. (I. Abt.
Orig. B), 170: 548-556 (in German).
STAR, E.G. (1980b) [Absorption and desorption of ethylene
oxide in anaesthesia supplies.] Zbl. Bakt. Hyg. (I. Abt. Orig. B),
170: 557-569 (in German).
STAR, E.G. (1980c) [Ethylene oxide residues and aeration
time after use of modern heated aerators.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 171: 18-24 (in German).
STAR, E.G. (1980d) [Gamma-rays and ethylene oxide
sterilization.] Zbl. Bakt. Hyg. (I. Abt. Orig. B), 171: 33-41
(in German).
STAR, E.G., CASELITZ, J., & LONING, T. (1980) [The effect of
ethylene oxide and chemical desinfectant residues upon the
larynx- and tracheal mucosa of rabbits.] Zbl. Bakt. Hyg.
(I. Abt. Orig. B), 170: 539-547 (in German).
STARK, W.J., ROSENBLUM, P., MAUMENEE, A.E., & COWEN, C.L.
(1980) Postoperative inflammatory reactions to intraocular
lenses sterilized with ethylene oxide. Opthalmology, 87:
385-389.
STIJVE, T., KALSBACH, R., & EYRING, G. (1976) Determination
and occurrence of ethylene chlorohydrin residues in foodstuffs
fumigated with ethylene oxide. Mitt. Geb. Lebensm. Unters.,
67: 403-428.
STOCKER, W.G. & THIESS, A.M. (1979) Morbidity study on
workers exposed to ethylene oxide/propylene oxide. In: The 7th
Medichem Congress, Gera, 11-15 September, 1979.
STOLLEY, P.D., SOPER, K.A., GALLOWAY, S.M., NICHOLS, W.W.,
NORMAN, S.A., & WOLMAN, S.R. (1984) Sister-chromatid
exchanges in association with occupational exposure to
ethylene oxide. Mutat. Res., 129: 89-102.
STREKALOVA, E.Y., CHIRKOVA, Y.M., & GOLUBOVICH, Y.Y. (1975)
[Mutagenic action of ethylene oxide on sex and somatic cells
in male white rats.] Toksikol. Nov. Prom. Him. Veshchestr., 6:
11-16 (in Russian).
TAN, E.-L., CUMMING, R.B., & HSIE, A.W. (1981) Mutagenicity
and cytotoxicity of ethylene oxide in the CHO/HGPRT system.
Environ. Mutagen., 3: 683-686.
TANOOKA, H. (1979) Application of Bacillus subtilis spores
in the detection of gas mutagens: a case of ethylene oxide.
Mutat. Res., 64: 433-435.
THIESS, A.M. (1963) [Observation on the adverse health
effects of ethylene oxide.] Archiv. Toxikol., 20: 127-140 (in
German).
THIESS, A.M., SCHWEGLER, H., FLEIG, I., & STOCKER, W.G.
(1981a) Mutagenicity study of workers exposed to alkene
oxides (ethylene oxide/propylene oxide) and derivatives.
J. occup. Med., 23: 343-347.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & STOCKER, W.G.
(1981b) Mortality study on employees exposed to alkene oxides
(ethylene oxide/propylene oxide) and their derivatives. In:
International Symposium on Prevention of Occupational Cancer,
Helsinki, pp. 249-259.
TROISI, F.M. (1965) [Aphonia of late onset due to
occupational exposure to ethylene oxide.] Med. Lav., 56:
373-377 (in Italian).
UKITA, T., OKUYAMA, H., & HAYATSU, H. (1963) Modifications
of nucleosides and nucleotides. I. Reaction of ethylene oxide
with uridine and uridylic acid. Chem. Pharm. Bull. (Tokyo),
11: 1399-1404.
US DEPARTMENT OF LABOR (1984) Occupational exposure to
ethylene oxide: final standard. Fed. Reg., 49: 25734-25809.
US EPA (1985) Health assessment document for ethylene oxide,
Washington DC, US Environmental Protection Agency (EPA
600/8-84/009F).
USITC (1971) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
USITC (1981) Synthetic organic chemicals. United States
production and sales, Washington DC, United States
International Trade Commission.
VAN DUUREN, B.L., ORRIS, L., & NELSON, N. (1965)
Carcinogenicity of epoxides, lactones, and peroxy compounds.
Part II. J. Natl Cancer Inst., 35: 707-717.
VETTORAZZI, G. (1979) International regulatory aspects for
pesticide chemicals. I. Toxicity profiles, Boca Raton,
Florida, CRC Press Inc., pp. 55-56.
VIRTANEN, P.O.I. (1963) Kinetics of the reactions of
ethylene oxide with nucleophiles. Am. Acad. Sci. Fenn. Ser. A.
II, 124: 1-89.
WAITE, C.P., PATTY, F.A., & YANT, W.P. (1930) Acute response
of guinea-pigs to vapours of some new commercial organic
compounds. IV. Ethylene oxide. Pub. Health Rep., 45: 1832-1843.
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. Eng. News, May 7: 8-10.
WESLEY, F., ROURKE, B., & DARBISHIRE, O. (1965) The
formation of persistent toxic chlorohydrins in foodstuffs by
fumigation with ethylene oxide and with propylene oxide.
J. food Sci., 30: 1037-1042.
WHO (1978) Environmental health problems associated with the
manufacture and uses of synthetic organic chemicals, Geneva,
World Health Organization (Report No. HCS/78.2).
WINDMUELLER, H.G. & KAPLAN, N.O. (1961) The preparation and
properties of N-hydroxyethyl derivatives of adenosine,
adenosine tri-phosphate, and nicotinamide adenine
dinucleotide. J. Biol. Chem., 236: 2716-2726.
WINDMUELLER, H.G., ACKERMAN, C.J., BAKERMAN, H., & MICKELSEN,
O. (1959) Reaction of ethylene oxide with nicotinamide and
nicotinic acid. J. Biol. Chem., 234: 889-894.
WOLFS, P., DUTRIEUX, M., SCAILTEUR, V., HAXHE, J.-J., ZUMOFEN,
M., & LAUWERIJS, R. (1983) Surveillance des travailleurs
exposés a l'oxyde d'éthylène dans une enterprise de
distribution de gaz stérilisants et dans des unités de
stérilisation de matériel médical. Arch. Mal. Prof., 44:
321-328.
WOODARD, G. & WOODARD, M. (1971) Toxicity of residuals from
ethylene oxide gas sterilization. In: Proceedings of the 1971
HIA Technical Symposium, Washington DC, Health Industries
Association.
YAGER, J.W. & BENZ, R.D. (1982) Sister-chromatid exchanges
induced in rabbit lymphocytes by ethylene oxide after
inhalation exposure. Environ. Mutagen., 4: 121-134.
YAGER, J.W., HINES, C.J., & SPEAR, R.C. (1983) Exposure to
ethylene oxide at work increases sister-chromatid exchanges in
human peripheral lymphocytes. Science, 219: 1221-1223.
YAKUBOVA, Z.N., SHAMOVA, H.A., MUFTAKNOVA, F.A., & SHILOVA,
L.F. (1976) [Gynaecological disorders in workers engaged in
ethylene oxide production.] Kazan. Med. Zh., 57: 558-560 (in
Russian).
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
