
FAO Meeting Report No. PL/1965/10/2
WHO/Food Add/28.65
EVALUATION OF THE HAZARDS TO CONSUMERS RESULTING FROM THE USE OF
FUMIGANTS IN THE PROTECTION OF FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met 15-22 March
19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65.
ETHYLENE OXIDE
Compound
Ethylene oxide
Chemical name
Ethylene oxide
Synonyms
Oxirane, 1,2-epoxyethane
Empirical formula
C2H4O
Structural formula
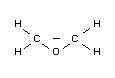 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: 10.7°C
Odour: irritating, mustard-like
Lowest concentration in air which is detectable by odour: hard to
detect in low concentrations
Flash point: < -18°C (open cup)
Flammability limits in air: 3-80% by volume
Solubility:
Water: soluble in all proportions
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 0.887
Specific, gravity (gas). 1.52
Uses
Ethylene oxide is used as an insecticide and sterilant for a wide
variety of foodstuffs. It is used mainly in vacuum fumigation mixed
with different proportions of carbon dioxide or non-flammable
halogenated hydrocarbons, such as dichloro-difluoromethane, to provide
non-explosive conditions. For insect control in foodstuffs fumigated
under vacuum a usual application rate is 100 g/m3 for three hours at
temperatures between 20°C and 25°C; for sterilizing foodstuffs 500
g/m3 for six hours at similar temperatures.
Residues
Residues of gaseous ethylene oxide as high as 68 ppm have been
reported in wheat fumigated by ethylene oxide at atmospheric pressure
and under vacuum in commercial treatments or in experiments simulating
practical conditions (Lubatti, 1944; El Nahal, 1954). Residues in raw
copra fumigated at atmospheric pressure did not exceed 4.5 ppm
(Benedict, 1957).
Effect of fumigant on treated crop
The chemical reactions of ethylene oxide with the ingredients of
certain foodstuffs have to be considered with the foodstuff as a
whole.
When proteins are exposed to ethylene oxide, the -COOH, -NH2,
-C6H4OH and -SH end groups react with the fumigant to give the
corresponding hydroxy-ethyl compounds (Bruhin et al., 1961a).
Fumigation of prunes with ethylene oxide-C14 revealed that the
major fraction of the fumigant is bound to prune cellulose as
hydroxyethyl cellulose. The only toxic products derived from ethylene
oxide were ethylene glycol and diethylene glycol in amounts of 0.002%,
dry weight (Gordon et al., 1959).
BIOLOGICAL DATA
Biochemical aspects
No information available
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat oral 330 Bruhin et al., 1961b
Guinea-pig " 270 "
Ethylene oxide has a moderate toxicity for rats. At a single oral
does of 0.1 g/kg all animals survived. Doses of 0.2 g/kg killed all
animals (Hollingsworth et al., 1956).
Short-term studies
Mice. Germ-free inbred albino mice were accidentally placed,
for 150 days, on ground corn-cob bedding treated with ethylene oxide
by the manufacturer. All males died with massive hemorrhages in the
thoracic and abdominal cavities and other sites, failure of the blood
to clot and jaundice (Reyniers et al., 1964). These findings agree
with the results in other similar experiments (Allen et al., 1962).
Rat. Feeding experiments with rats given fumigated diets
resulted in loss of body-weight of the animals and in early death,
probably due to thiamine destruction. Gain in weight started after
oral thiamine supplementation (Hawk and Mickelsen, 1955). In another
experiment young rats fed fumigated diets showed slight growth
retardation in the first week; in the second week weight gain was
normal (Oser and Hall, 1956).
Four groups of five young female rats were given 0.1 mg/kg (15
doses in 21 days), 0.03, 0.01 and 0.003 mg/kg (22 doses in 30 days)
in cold olive oil. There was no mortality due to the experimental
material. Doses of 0.1 mg/kg caused a marked loss of body-weight,
gastric irritation and a slight liver damage. The groups doses with
0.03, 0.01, and 0.003 mg/kg showed no adverse effects attributable
to ethylene oxide as judged by growth, haemotology, blood urea,
N-determinations, organ-weights and gross and microscopic examination
of the tissues (Hollingsworth et al., 1956).
Man. Thirty-seven industrial workers, engaged in manufacturing
ethylene oxide, showed no ill effects at 5-10 ppm levels over an
average service of 10 years with an exposure of eight hours daily
(Joyner, 1964).
Thiess (1963) described the symptoms of ethylene oxide poisoning
derived from 41 cases as follows: after a short time of exposure
periodical vomiting; irritation of the respiratory passages leading to
emphysema, bronchitis and pulmonary oedema. Skin application caused
blisters.
Long-term studies
No information available.
Comments on experimental studies reported
The feeding studies are not satisfactory because of the small
number of animals and because only rats have been used.
Evaluation
Since no long-term experiments are available, no acceptable daily
intake for ethylene oxide can be estimated.
Further work required
The reaction of ethylene oxide with food should be further
investigated to determine the identity of the reaction products. The
nutritional and toxicological aspects of any changes that are detected
should be evaluated. Long-term experiments with the unchanged fumigant
should be carried out in two species.
REFERENCES
Allen, R. C., Meier, H. & Hoag, W. G. (1962) Nature (Lond.), 193,
387
Benedict, J. H. (1957) J. Amer. Oil Chem. Soc., 34, 450
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961a)
Schweiz. med. Wschr., 91, 607
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961b)
Schweiz. med. Wschr., 91, 635
El Nahal, A. K. N. (1954) J. Sci. Food Agric., 5, 205
Gordon, H. T., Thornburg, W. W. & Werum, L. N. (1959) J. Agric. Food
Chem., 7, 196
Hawk, E. A. & Mickelsen, O. (1955) Science, 121, 442
Hollingsworth, R. L., Rowe, V. K., Oyen, F., McCollister, D. D. &
Spencer, H. C., (1956) Arch. industr. Hlth, 13, 217
Joyner, R. E. (1964) Arch. environm. Hlth, 8, 700
Lubatti, O. F, (1944) J. Soc. Chem. Ind. (Lond.), 63, 353
Lubatti, O. F. & Harrison (1944) J. Soc. Chem. Ind. (Lond.), 63,
353
Oser, B. L. & Hall, L. A. (1956) Food Techn., 10, 175
Reyniers, J. A., Sachsteder, M. R. & Ashburn, L. L. (1964) J. nat.
Cancer Inst., 32, 1045
Thiess, A. M. (1963) Arch. Toxikol., 20, 127
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: 10.7°C
Odour: irritating, mustard-like
Lowest concentration in air which is detectable by odour: hard to
detect in low concentrations
Flash point: < -18°C (open cup)
Flammability limits in air: 3-80% by volume
Solubility:
Water: soluble in all proportions
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 0.887
Specific, gravity (gas). 1.52
Uses
Ethylene oxide is used as an insecticide and sterilant for a wide
variety of foodstuffs. It is used mainly in vacuum fumigation mixed
with different proportions of carbon dioxide or non-flammable
halogenated hydrocarbons, such as dichloro-difluoromethane, to provide
non-explosive conditions. For insect control in foodstuffs fumigated
under vacuum a usual application rate is 100 g/m3 for three hours at
temperatures between 20°C and 25°C; for sterilizing foodstuffs 500
g/m3 for six hours at similar temperatures.
Residues
Residues of gaseous ethylene oxide as high as 68 ppm have been
reported in wheat fumigated by ethylene oxide at atmospheric pressure
and under vacuum in commercial treatments or in experiments simulating
practical conditions (Lubatti, 1944; El Nahal, 1954). Residues in raw
copra fumigated at atmospheric pressure did not exceed 4.5 ppm
(Benedict, 1957).
Effect of fumigant on treated crop
The chemical reactions of ethylene oxide with the ingredients of
certain foodstuffs have to be considered with the foodstuff as a
whole.
When proteins are exposed to ethylene oxide, the -COOH, -NH2,
-C6H4OH and -SH end groups react with the fumigant to give the
corresponding hydroxy-ethyl compounds (Bruhin et al., 1961a).
Fumigation of prunes with ethylene oxide-C14 revealed that the
major fraction of the fumigant is bound to prune cellulose as
hydroxyethyl cellulose. The only toxic products derived from ethylene
oxide were ethylene glycol and diethylene glycol in amounts of 0.002%,
dry weight (Gordon et al., 1959).
BIOLOGICAL DATA
Biochemical aspects
No information available
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat oral 330 Bruhin et al., 1961b
Guinea-pig " 270 "
Ethylene oxide has a moderate toxicity for rats. At a single oral
does of 0.1 g/kg all animals survived. Doses of 0.2 g/kg killed all
animals (Hollingsworth et al., 1956).
Short-term studies
Mice. Germ-free inbred albino mice were accidentally placed,
for 150 days, on ground corn-cob bedding treated with ethylene oxide
by the manufacturer. All males died with massive hemorrhages in the
thoracic and abdominal cavities and other sites, failure of the blood
to clot and jaundice (Reyniers et al., 1964). These findings agree
with the results in other similar experiments (Allen et al., 1962).
Rat. Feeding experiments with rats given fumigated diets
resulted in loss of body-weight of the animals and in early death,
probably due to thiamine destruction. Gain in weight started after
oral thiamine supplementation (Hawk and Mickelsen, 1955). In another
experiment young rats fed fumigated diets showed slight growth
retardation in the first week; in the second week weight gain was
normal (Oser and Hall, 1956).
Four groups of five young female rats were given 0.1 mg/kg (15
doses in 21 days), 0.03, 0.01 and 0.003 mg/kg (22 doses in 30 days)
in cold olive oil. There was no mortality due to the experimental
material. Doses of 0.1 mg/kg caused a marked loss of body-weight,
gastric irritation and a slight liver damage. The groups doses with
0.03, 0.01, and 0.003 mg/kg showed no adverse effects attributable
to ethylene oxide as judged by growth, haemotology, blood urea,
N-determinations, organ-weights and gross and microscopic examination
of the tissues (Hollingsworth et al., 1956).
Man. Thirty-seven industrial workers, engaged in manufacturing
ethylene oxide, showed no ill effects at 5-10 ppm levels over an
average service of 10 years with an exposure of eight hours daily
(Joyner, 1964).
Thiess (1963) described the symptoms of ethylene oxide poisoning
derived from 41 cases as follows: after a short time of exposure
periodical vomiting; irritation of the respiratory passages leading to
emphysema, bronchitis and pulmonary oedema. Skin application caused
blisters.
Long-term studies
No information available.
Comments on experimental studies reported
The feeding studies are not satisfactory because of the small
number of animals and because only rats have been used.
Evaluation
Since no long-term experiments are available, no acceptable daily
intake for ethylene oxide can be estimated.
Further work required
The reaction of ethylene oxide with food should be further
investigated to determine the identity of the reaction products. The
nutritional and toxicological aspects of any changes that are detected
should be evaluated. Long-term experiments with the unchanged fumigant
should be carried out in two species.
REFERENCES
Allen, R. C., Meier, H. & Hoag, W. G. (1962) Nature (Lond.), 193,
387
Benedict, J. H. (1957) J. Amer. Oil Chem. Soc., 34, 450
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961a)
Schweiz. med. Wschr., 91, 607
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961b)
Schweiz. med. Wschr., 91, 635
El Nahal, A. K. N. (1954) J. Sci. Food Agric., 5, 205
Gordon, H. T., Thornburg, W. W. & Werum, L. N. (1959) J. Agric. Food
Chem., 7, 196
Hawk, E. A. & Mickelsen, O. (1955) Science, 121, 442
Hollingsworth, R. L., Rowe, V. K., Oyen, F., McCollister, D. D. &
Spencer, H. C., (1956) Arch. industr. Hlth, 13, 217
Joyner, R. E. (1964) Arch. environm. Hlth, 8, 700
Lubatti, O. F, (1944) J. Soc. Chem. Ind. (Lond.), 63, 353
Lubatti, O. F. & Harrison (1944) J. Soc. Chem. Ind. (Lond.), 63,
353
Oser, B. L. & Hall, L. A. (1956) Food Techn., 10, 175
Reyniers, J. A., Sachsteder, M. R. & Ashburn, L. L. (1964) J. nat.
Cancer Inst., 32, 1045
Thiess, A. M. (1963) Arch. Toxikol., 20, 127
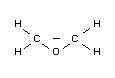 Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: 10.7°C
Odour: irritating, mustard-like
Lowest concentration in air which is detectable by odour: hard to
detect in low concentrations
Flash point: < -18°C (open cup)
Flammability limits in air: 3-80% by volume
Solubility:
Water: soluble in all proportions
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 0.887
Specific, gravity (gas). 1.52
Uses
Ethylene oxide is used as an insecticide and sterilant for a wide
variety of foodstuffs. It is used mainly in vacuum fumigation mixed
with different proportions of carbon dioxide or non-flammable
halogenated hydrocarbons, such as dichloro-difluoromethane, to provide
non-explosive conditions. For insect control in foodstuffs fumigated
under vacuum a usual application rate is 100 g/m3 for three hours at
temperatures between 20°C and 25°C; for sterilizing foodstuffs 500
g/m3 for six hours at similar temperatures.
Residues
Residues of gaseous ethylene oxide as high as 68 ppm have been
reported in wheat fumigated by ethylene oxide at atmospheric pressure
and under vacuum in commercial treatments or in experiments simulating
practical conditions (Lubatti, 1944; El Nahal, 1954). Residues in raw
copra fumigated at atmospheric pressure did not exceed 4.5 ppm
(Benedict, 1957).
Effect of fumigant on treated crop
The chemical reactions of ethylene oxide with the ingredients of
certain foodstuffs have to be considered with the foodstuff as a
whole.
When proteins are exposed to ethylene oxide, the -COOH, -NH2,
-C6H4OH and -SH end groups react with the fumigant to give the
corresponding hydroxy-ethyl compounds (Bruhin et al., 1961a).
Fumigation of prunes with ethylene oxide-C14 revealed that the
major fraction of the fumigant is bound to prune cellulose as
hydroxyethyl cellulose. The only toxic products derived from ethylene
oxide were ethylene glycol and diethylene glycol in amounts of 0.002%,
dry weight (Gordon et al., 1959).
BIOLOGICAL DATA
Biochemical aspects
No information available
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat oral 330 Bruhin et al., 1961b
Guinea-pig " 270 "
Ethylene oxide has a moderate toxicity for rats. At a single oral
does of 0.1 g/kg all animals survived. Doses of 0.2 g/kg killed all
animals (Hollingsworth et al., 1956).
Short-term studies
Mice. Germ-free inbred albino mice were accidentally placed,
for 150 days, on ground corn-cob bedding treated with ethylene oxide
by the manufacturer. All males died with massive hemorrhages in the
thoracic and abdominal cavities and other sites, failure of the blood
to clot and jaundice (Reyniers et al., 1964). These findings agree
with the results in other similar experiments (Allen et al., 1962).
Rat. Feeding experiments with rats given fumigated diets
resulted in loss of body-weight of the animals and in early death,
probably due to thiamine destruction. Gain in weight started after
oral thiamine supplementation (Hawk and Mickelsen, 1955). In another
experiment young rats fed fumigated diets showed slight growth
retardation in the first week; in the second week weight gain was
normal (Oser and Hall, 1956).
Four groups of five young female rats were given 0.1 mg/kg (15
doses in 21 days), 0.03, 0.01 and 0.003 mg/kg (22 doses in 30 days)
in cold olive oil. There was no mortality due to the experimental
material. Doses of 0.1 mg/kg caused a marked loss of body-weight,
gastric irritation and a slight liver damage. The groups doses with
0.03, 0.01, and 0.003 mg/kg showed no adverse effects attributable
to ethylene oxide as judged by growth, haemotology, blood urea,
N-determinations, organ-weights and gross and microscopic examination
of the tissues (Hollingsworth et al., 1956).
Man. Thirty-seven industrial workers, engaged in manufacturing
ethylene oxide, showed no ill effects at 5-10 ppm levels over an
average service of 10 years with an exposure of eight hours daily
(Joyner, 1964).
Thiess (1963) described the symptoms of ethylene oxide poisoning
derived from 41 cases as follows: after a short time of exposure
periodical vomiting; irritation of the respiratory passages leading to
emphysema, bronchitis and pulmonary oedema. Skin application caused
blisters.
Long-term studies
No information available.
Comments on experimental studies reported
The feeding studies are not satisfactory because of the small
number of animals and because only rats have been used.
Evaluation
Since no long-term experiments are available, no acceptable daily
intake for ethylene oxide can be estimated.
Further work required
The reaction of ethylene oxide with food should be further
investigated to determine the identity of the reaction products. The
nutritional and toxicological aspects of any changes that are detected
should be evaluated. Long-term experiments with the unchanged fumigant
should be carried out in two species.
REFERENCES
Allen, R. C., Meier, H. & Hoag, W. G. (1962) Nature (Lond.), 193,
387
Benedict, J. H. (1957) J. Amer. Oil Chem. Soc., 34, 450
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961a)
Schweiz. med. Wschr., 91, 607
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961b)
Schweiz. med. Wschr., 91, 635
El Nahal, A. K. N. (1954) J. Sci. Food Agric., 5, 205
Gordon, H. T., Thornburg, W. W. & Werum, L. N. (1959) J. Agric. Food
Chem., 7, 196
Hawk, E. A. & Mickelsen, O. (1955) Science, 121, 442
Hollingsworth, R. L., Rowe, V. K., Oyen, F., McCollister, D. D. &
Spencer, H. C., (1956) Arch. industr. Hlth, 13, 217
Joyner, R. E. (1964) Arch. environm. Hlth, 8, 700
Lubatti, O. F, (1944) J. Soc. Chem. Ind. (Lond.), 63, 353
Lubatti, O. F. & Harrison (1944) J. Soc. Chem. Ind. (Lond.), 63,
353
Oser, B. L. & Hall, L. A. (1956) Food Techn., 10, 175
Reyniers, J. A., Sachsteder, M. R. & Ashburn, L. L. (1964) J. nat.
Cancer Inst., 32, 1045
Thiess, A. M. (1963) Arch. Toxikol., 20, 127
Relevant physical and chemical properties
Physical state (atmospheric pressure, 20°C): gas
Boiling-point: 10.7°C
Odour: irritating, mustard-like
Lowest concentration in air which is detectable by odour: hard to
detect in low concentrations
Flash point: < -18°C (open cup)
Flammability limits in air: 3-80% by volume
Solubility:
Water: soluble in all proportions
Organic solvents: infinitely soluble in alcohol and ether
Specific gravity (liquid): 0.887
Specific, gravity (gas). 1.52
Uses
Ethylene oxide is used as an insecticide and sterilant for a wide
variety of foodstuffs. It is used mainly in vacuum fumigation mixed
with different proportions of carbon dioxide or non-flammable
halogenated hydrocarbons, such as dichloro-difluoromethane, to provide
non-explosive conditions. For insect control in foodstuffs fumigated
under vacuum a usual application rate is 100 g/m3 for three hours at
temperatures between 20°C and 25°C; for sterilizing foodstuffs 500
g/m3 for six hours at similar temperatures.
Residues
Residues of gaseous ethylene oxide as high as 68 ppm have been
reported in wheat fumigated by ethylene oxide at atmospheric pressure
and under vacuum in commercial treatments or in experiments simulating
practical conditions (Lubatti, 1944; El Nahal, 1954). Residues in raw
copra fumigated at atmospheric pressure did not exceed 4.5 ppm
(Benedict, 1957).
Effect of fumigant on treated crop
The chemical reactions of ethylene oxide with the ingredients of
certain foodstuffs have to be considered with the foodstuff as a
whole.
When proteins are exposed to ethylene oxide, the -COOH, -NH2,
-C6H4OH and -SH end groups react with the fumigant to give the
corresponding hydroxy-ethyl compounds (Bruhin et al., 1961a).
Fumigation of prunes with ethylene oxide-C14 revealed that the
major fraction of the fumigant is bound to prune cellulose as
hydroxyethyl cellulose. The only toxic products derived from ethylene
oxide were ethylene glycol and diethylene glycol in amounts of 0.002%,
dry weight (Gordon et al., 1959).
BIOLOGICAL DATA
Biochemical aspects
No information available
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Rat oral 330 Bruhin et al., 1961b
Guinea-pig " 270 "
Ethylene oxide has a moderate toxicity for rats. At a single oral
does of 0.1 g/kg all animals survived. Doses of 0.2 g/kg killed all
animals (Hollingsworth et al., 1956).
Short-term studies
Mice. Germ-free inbred albino mice were accidentally placed,
for 150 days, on ground corn-cob bedding treated with ethylene oxide
by the manufacturer. All males died with massive hemorrhages in the
thoracic and abdominal cavities and other sites, failure of the blood
to clot and jaundice (Reyniers et al., 1964). These findings agree
with the results in other similar experiments (Allen et al., 1962).
Rat. Feeding experiments with rats given fumigated diets
resulted in loss of body-weight of the animals and in early death,
probably due to thiamine destruction. Gain in weight started after
oral thiamine supplementation (Hawk and Mickelsen, 1955). In another
experiment young rats fed fumigated diets showed slight growth
retardation in the first week; in the second week weight gain was
normal (Oser and Hall, 1956).
Four groups of five young female rats were given 0.1 mg/kg (15
doses in 21 days), 0.03, 0.01 and 0.003 mg/kg (22 doses in 30 days)
in cold olive oil. There was no mortality due to the experimental
material. Doses of 0.1 mg/kg caused a marked loss of body-weight,
gastric irritation and a slight liver damage. The groups doses with
0.03, 0.01, and 0.003 mg/kg showed no adverse effects attributable
to ethylene oxide as judged by growth, haemotology, blood urea,
N-determinations, organ-weights and gross and microscopic examination
of the tissues (Hollingsworth et al., 1956).
Man. Thirty-seven industrial workers, engaged in manufacturing
ethylene oxide, showed no ill effects at 5-10 ppm levels over an
average service of 10 years with an exposure of eight hours daily
(Joyner, 1964).
Thiess (1963) described the symptoms of ethylene oxide poisoning
derived from 41 cases as follows: after a short time of exposure
periodical vomiting; irritation of the respiratory passages leading to
emphysema, bronchitis and pulmonary oedema. Skin application caused
blisters.
Long-term studies
No information available.
Comments on experimental studies reported
The feeding studies are not satisfactory because of the small
number of animals and because only rats have been used.
Evaluation
Since no long-term experiments are available, no acceptable daily
intake for ethylene oxide can be estimated.
Further work required
The reaction of ethylene oxide with food should be further
investigated to determine the identity of the reaction products. The
nutritional and toxicological aspects of any changes that are detected
should be evaluated. Long-term experiments with the unchanged fumigant
should be carried out in two species.
REFERENCES
Allen, R. C., Meier, H. & Hoag, W. G. (1962) Nature (Lond.), 193,
387
Benedict, J. H. (1957) J. Amer. Oil Chem. Soc., 34, 450
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961a)
Schweiz. med. Wschr., 91, 607
Bruhin, H., Bühlmann, X., Vischer, W. A. & Lammers, T. (1961b)
Schweiz. med. Wschr., 91, 635
El Nahal, A. K. N. (1954) J. Sci. Food Agric., 5, 205
Gordon, H. T., Thornburg, W. W. & Werum, L. N. (1959) J. Agric. Food
Chem., 7, 196
Hawk, E. A. & Mickelsen, O. (1955) Science, 121, 442
Hollingsworth, R. L., Rowe, V. K., Oyen, F., McCollister, D. D. &
Spencer, H. C., (1956) Arch. industr. Hlth, 13, 217
Joyner, R. E. (1964) Arch. environm. Hlth, 8, 700
Lubatti, O. F, (1944) J. Soc. Chem. Ind. (Lond.), 63, 353
Lubatti, O. F. & Harrison (1944) J. Soc. Chem. Ind. (Lond.), 63,
353
Oser, B. L. & Hall, L. A. (1956) Food Techn., 10, 175
Reyniers, J. A., Sachsteder, M. R. & Ashburn, L. L. (1964) J. nat.
Cancer Inst., 32, 1045
Thiess, A. M. (1963) Arch. Toxikol., 20, 127
