
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 22
CYPERMETHRIN
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA
This is a companion volume to Environmental Health Criteria 82:
Cypermethrin
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
ISBN 92 4 154344 2
ISSN 0259 - 7268
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure
2.2. Effects on animals and human health
2.3. Evaluation of effects on the environment
3. CONCLUSIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first
aid
4.1.1. Advice to physicians
4.1.2. Health surveillance advice
4.2. Explosion and fire hazards
4.3. Storage
4.4. Transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is an International Chemical Safety Card
which should be readily available, and should be clearly explained, to
all who could come into contact with the chemical. The section on
regulatory information has been extracted from the legal file of the
International Register of Potentially Toxic Chemicals (IRPTC) and from
other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: Cypermethrin
Chemical structure:
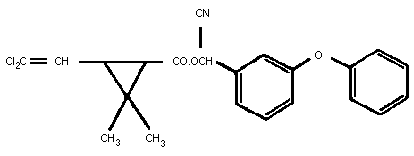 Molecular formula: C22H19O3NCl2
Chemical name: (IUPAC) (RS9-alpha-cyano-3-phenoxybenzyl (1RS)
cis-trans-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropane carboxylate
Chemical name: (CAS) (RS)-cyano(3-phenoxyphenyl)methyl
(1RS)- cis-trans-3-(2,2-
dichloroethenyl)-2,2-dimethyl-
cyclopropane carboxylate
CAS registry number: 52315-07-8 (formerly 69865-47-0)
RTECS registry number: GZ1250000
Synonyms: NRDC 149, WL43467, PP 383, CG-A 55186
Common trade names: Ammo, Avicade, Barricade, CCN 52,
Cymbush, Folcord, Imperator, Kafil
super, Polytrin, Ripcord, Stockade.
Relative molecular mass: 416.3
Cypermethrin is the ISO name for the pure racemic compound, consisting
of 8 stereo isomers. The technical products commonly available
contain more than 90% cypermethrin and the ratio of cis to trans
isomers varies from 50:50 to 40:60.
1.2 Physical and Chemical Properties
Technical cypermethrin varies from a viscous, yellow liquid to a
semi-solid crystalline mass at ambient temperatures.
Some physical and chemical properties are listed in the International
Chemical Safety Card on pages 20-23.
Cypermethrin is highly stable to light and at temperatures below
220°C. It is resistant to acidic rather than alkaline media with an
optimum stability at pH 4. Cypermethrin hydrolyses under alkaline
conditions in a similar way to simple aliphatic esters. Dilute
aqueous solutions are subject to photolysis, which occurs at a
moderate rate.
1.3 Analytical Methods
The most widely adopted procedures for the determination of
cypermethrin residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with an
organic solvent, clean-up of the extract, as necessary, by
solvent-solvent partition and adsorption-column chromatography,
followed by determination of the residue using gas chromatography
with electron capture detector (GC/ECD). The identity of residues can
be confirmed by GC with mass selective detection (GC-MSD) or by
thin-layer chromatography (TLC) followed by GC/ECD.
1.4 Production and Uses
Cypermethrin was first synthesized in 1974 and first marketed in 1977
as a highly active synthetic pyrethroid insecticide effective against
a wide range of pests in agriculture, public health, and animal
husbandry. In agriculture, its main use is against foliage pests and
certain surface soil pests, such as cutworms, but, because of its
physical and chemical properties, it is not recommended against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was used
on cotton; in 1982, world production was 340 tonnes of the active
material. It is used primarily in the form of an emulsifiable
concentrate, but ultra-low-volume concentrates, wettable powders, and
joint formulations with other pesticides are available.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
The levels of cypermethrin residues in food commodities after good
agricultural practice are generally low. The residue levels will be
further reduced during food processing. In food of animal origin,
residues may range between 0.01 and 0.2 mg/kg product. Residue levels
found in non-food commodities are generally higher, up to 20 mg/kg
product. Total-diet studies are not available, but the available
residue information indicates that the oral intake by the general
population is well below the ADI, and expected to be negligible.
2.2 Effects on Animals and Human Health
The absorption and elimination of cypermethrin was rapid in the
different mammalian species tested. The major metabolic reaction is
cleavage of the ester bond followed by hydroxylation and conjugation
of the cyclopropane and phenoxybenzyl moieties. The highest levels of
cypermethrin are found in body fat, which is consistent with the
lipophilic nature of the compound. The half-life in the fat of rats
is about 12-9 days for the cis-isomer and 3-4 days for the
trans-isomer.
The acute toxicity of cypermethrin for mammals is of a moderate order.
The oral LD50 for the rat ranged from 200-4000 mg/kg body weight.
Short-term and long-term toxicity studies on rats, mice, and dogs have
shown effects on growth, the liver and kidneys, the nervous system,
and the blood. A no-observed-adverse-effect level of 7.5 mg/kg body
weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic in mice or rats fed diets containing
the compound over a 2-year period. Cypermethrin was not teratogenic
in either rats at 70 mg/kg body weight or rabbits at 30 mg/kg body
weight. No effects on reproductive performance were seen in a
3-generation reproduction study on rats administered 10 mg
cypermethrin/kg diet. In a variety of mutagenicity studies,
cypermethrin was shown to be mainly without mutagenic activity.
The mechanism of the action of cypermethrin on the nervous system has
been extensively studied. From these studies and the available
occupational studies, it seems that the skin sensation seen in workers
handling cypermethrin generally lasts only a few hours and does not
persist for more than one day after exposure. Other neurological
signs have not been observed. These skin sensations may be considered
as an early warning that exposure has occurred and that work practice
should be reviewed. Cypermethrin may cause eye irritation and may be
a sensitizer for certain persons.
No cases of accidental poisoning have been reported as a result of
occupational exposure.
2.3 Evaluation of Effects on the Environment
When cypermethrin is applied to crops, residues may occur in soils and
surface waters, but biological degradation is fairly rapid and
residues do not accumulate in the environment. Photodegradation is
unlikely to play an important role. The main route of degradation is
cleavage of the ester linkage to give two main degradation products
containing the cyclopropane, and the phenoxybenzyl moieties. The
half-life in the soil is determined by many factors, but is in the
range of 2-4 weeks. Breakdown products in plants are bound as
glucosides. Because of its rather fast breakdown forming less toxic
breakdown products, and the low dose rates used in good agricultural
practice, it is unlikely that cypermethrin will reach significant
levels in the environment.
Cypermethrin at high dose levels may exert transient effects on the
soil microflora. Earthworms and other soil organisms are generally
resistant to cypermethrin. Because of strong adsorption to soil, only
low levels of cypermethrin may leak into surface water. These may
have transient effects, mainly on surface-breathing insects.
Cypermethrin is very toxic for fish and aquatic invertebrates. The
presence of suspended solids decreases the toxicity by a factor of at
least 2, because of the adsorption of cypermethrin on the solids.
Accumulation studies have shown that cypermethrin is rapidly taken up
by fish (accumulation factor approximately 1000). The half-life of
residues in the rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it is concluded that, under practical
conditions, residues in fish will not reach measurable levels.
The toxicity of cypermethrin for birds is low. However, bees appeared
to be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect on them.
3. CONCLUSIONS
General population exposure: Under recommended conditions of use the
exposure of the general population to cypermethrin is negligible and
is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practices should be reviewed.
Environment: With recommended application rates, it is unlikely that
cypermethrin or its degradation products will attain levels of
environmental significance. The fact that cypermethrin is highly
toxic for fish and honey bees is only likely to cause a problem in the
case of spillage or overspraying.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Cypermethrin is a synthetic pyrethroid insecticide. Only one case of
poisoning has been described in the general population and none during
occupational exposure. The results of experimental animal studies
suggest that, following massive overexposure or accidental ingestion,
neurological signs and symptoms, e.g., ataxia, convulsions, could
occur.
The human health hazards associated with certain types of exposure to
cypermethrin, together with preventive and protective measures and
first aid recommendations, are listed on the International Chemical
Safety Card on pages 20-23.
4.1.1 Advice to Physicians
No specific antidote is known. If indicated, empty stomach. Treat
symptomatically. The main hazard with liquid formulations is
aspiration of the solvent into the lungs, resulting in chemical
pneumonitis.
4.1.2 Health Surveillance Advice
Occurrence of "facial sensations" is an indication of skin exposure.
Under these circumstances, work practices should be reviewed.
4.2 Explosion and Fire Hazards
Some solvents in pyrethroid formulations are highly flammable. Use
dry powder, carbon dioxide, alcohol-resistant foam, sand, or earth for
dealing with fires. Do not use water. Cool nearby drums with water
spray.
If pyrethroid products are involved in a major fire or in a fire
involving other products, advise the fire service that protective
clothing and breathing apparatus should be worn. Also, warn the
authorities that pyrethroids are highly toxic for fish, and that the
use of water should be confined to the cooling of unaffected stock,
thus avoiding the accumulation of polluted run-off from the site.
4.3 Storage
Store technical material and formulations away from heat, under lock
and key, and out of the reach of children, animals, and unauthorized
personnel. Store in an area designated for insecticide storage,
preferably without drains.
Store away from other chemicals, foodstuffs, and animal feed.
4.4 Transport
Pyrethroids are classified as "harmful" or "low hazard" for transport
purposes. Formulations based on flammable solvents may be subject to
local transport controls. Ensure that containers are sound and that
labels are securely fixed and undamaged before dispatch. Comply with
local transport regulations.
Do not load together with food and animal feed.
Accident procedures:
(a) Avoid exposure - if possible by the use of appropriate protective
clothing and masks. Keep spectators away from leaking or spilled
product and prevent smoking or the use of naked flames in the
immediate vicinity.
(b) Extinguish fires with dry powder, carbon dioxide,
alcohol-resistant foam, sand, or earth.
(c) Prevent liquid from spreading to other cargo, vegetation, or
waterways by containing it with the most readily available
barrier material, e.g., earth or sand.
(d) Absorb spilled liquid and cover contaminated areas with earth,
lime, sand, or other absorbent material. Sweep up and place in a
secure container for subsequent safe disposal.
4.5 Spillage and Disposal
4.5.1 Spillage
Avoid exposure, if possible by the use of appropriate protective
clothing and masks.
Empty any product remaining in damaged or leaking containers into a
clean empty drum and label.
Absorb spillage with lime, damp sawdust, sand, or earth and dispose of
safely (see below). If spillage is large, contain it by building a
barrier of earth or sandbags.
Decontaminate empty, damaged, or leaking containers with a 10% sodium
carbonate solution added at the rate of at least 1 litre per 20-litre
drum. Puncture containers to prevent reuse.
4.5.2 Disposal
Waste containing cypermethrin should be burnt in a proper high
temperature incinerator with effluent scrubbing. Where no incinerator
is available, contaminated absorbents or surplus products should be
decomposed by hydrolysis at pH 12 or above. Contact with a suitable
hydrolysing agent is required to ensure degradation of the active
ingredient to a safe level.
(a) For emulsifiable material: 5% sodium hydroxide (caustic soda)
solution or saturated (7-10%) sodium carbonate (washing soda)
solution can be used.
(b) For non-emulsifiable material: a 1:1 mixture (by volume) can be
used of either of the above solutions and a water/oil soluble
solvent, such as denatured alcohol, monoethylene glycol, hexylene
glycol, or isopropanol.
The material should be covered with hydrolysing agent and put aside to
stand for 7 days. Before disposal of the resultant waste, the
material must be analysed to ensure that the active ingredient has
been degraded to a safe level.
Never pour untreated waste or surplus products into public sewers or
where there is any danger of run-off or seepage into streams,
water-courses, open waterways, ditches, fields with drainage systems,
or the catchment areas of boreholes, wells, springs, or ponds.
Puncture empty containers in order to avoid reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
With recommended applications rates, it is unlikely that cypermethrin
and its degradation products will reach levels of environmental
significance. Cypermethrin is very toxic for fish and honey bees,
but, because of the very low exposure levels that normally occur, this
may only cause a problem in the case of spillage or overspraying.
Avoid spraying over bodies of water. Do not contaminate ponds,
waterways, or ditches with the product or with used containers.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, cypermethrin. It should be displayed at, or near,
entrances to areas where there is potential exposure to cypermethrin,
and on processing equipment and containers. The card should be
translated into the appropriate language(s). All persons potentially
exposed to the chemical should also have the instructions on the
chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
CYPERMETHRIN
CAS chemical name: (RS)-cyano(3-phenoxyphenyl)methyl(1RS)- cis-trans-
3-(2,2-dichloro-ethenyl)-2.2-dimethyl-cyclopropane carboxylate
CAS registry no. 52315-07-8; RTECS registry no. GZ1250000
C22H19O3NCl2
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 416.3 Technical cypermethrin is a yellowish-brown liquid to
semi-solid, with a mild chemical odour. It is stable
Melting point up to 80°C (depending on to light and more stable in an acidic than in an
purity and cis or trans form) alkaline medium. It decomposes on heating above 220°C.
Readily absorbed via ingestion and inhalation and to a
Water solubility 0.01 mg/litre (20°C) lesser extent via the skin. It is a highly active synthetic
pyrethroid used as a stomach and contact insecticide.
Solubility in organic solvents The technical product is a mixture of eight steroisomers
(at 20°C)
xylene >450 g/litre
hexane 103 g/litre
Log n-octanol/water partition
coefficient 6.3
Density 1.12 (20°C)
Vapour pressure 1.4 x 10-9 mmHg (20°C)
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: tingling or burning sensation, Decrease exposure by using proper Remove contaminated clothing, wash skin
or numbness application technique, proper skin with water and soap
protection, face shield; wear protective
clothing when handling the concentrate
EYES: splashing may cause severe Wear face shield or goggles Flush immediately with clean water for at
irritation least 15 minutes
INHALATION: irritant to Avoid inhalation of fine dust and Fresh air
respiratory system mist
INGESTION: unlikely occupational Do not eat, drink, or smoke during
hazard work, wash hands
Accidental or deliberate ingestion Do not induce vomiting Obtain medical attention immediately; if
could lead to neurological signs and breathing has stopped, apply artificial
symptoms, such as ataxia and respiration
convulsions; main hazard of ingested
liquid formulations is aspiration Do not induce vomiting
into lungs
ENVIRONMENT: very toxic for Avoid the spraying of water;
fish and honey bees do not contaminate ponds,
waterways, or ditches with the
product or used containers
Spillage Storage Fire and Explosion
Absorb spillage with lime, damp Store in locked, well ventilated Some liquid formulations may be highly
sawdust, sand or earth; sweep up storeroom, away from feed flammable; use dry powder, carbon
and place in closed container and and foodstuffs, children and dioxide, or alcohol-resistant foam; cool
dispose of safely; avoid unauthorized personnel nearby drums with water spray
contamination of personnel, ponds,
and waterways
Waste disposal
Burn in high-temperature National Occupational
incinerator with effluent Exposure Limit:
scrubbing; alternatively, treat
with 5% caustic soda as a National Poison Control Centre:
hydrolysing agent; comply with
local regulations Local trade names:
Molecular formula: C22H19O3NCl2
Chemical name: (IUPAC) (RS9-alpha-cyano-3-phenoxybenzyl (1RS)
cis-trans-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropane carboxylate
Chemical name: (CAS) (RS)-cyano(3-phenoxyphenyl)methyl
(1RS)- cis-trans-3-(2,2-
dichloroethenyl)-2,2-dimethyl-
cyclopropane carboxylate
CAS registry number: 52315-07-8 (formerly 69865-47-0)
RTECS registry number: GZ1250000
Synonyms: NRDC 149, WL43467, PP 383, CG-A 55186
Common trade names: Ammo, Avicade, Barricade, CCN 52,
Cymbush, Folcord, Imperator, Kafil
super, Polytrin, Ripcord, Stockade.
Relative molecular mass: 416.3
Cypermethrin is the ISO name for the pure racemic compound, consisting
of 8 stereo isomers. The technical products commonly available
contain more than 90% cypermethrin and the ratio of cis to trans
isomers varies from 50:50 to 40:60.
1.2 Physical and Chemical Properties
Technical cypermethrin varies from a viscous, yellow liquid to a
semi-solid crystalline mass at ambient temperatures.
Some physical and chemical properties are listed in the International
Chemical Safety Card on pages 20-23.
Cypermethrin is highly stable to light and at temperatures below
220°C. It is resistant to acidic rather than alkaline media with an
optimum stability at pH 4. Cypermethrin hydrolyses under alkaline
conditions in a similar way to simple aliphatic esters. Dilute
aqueous solutions are subject to photolysis, which occurs at a
moderate rate.
1.3 Analytical Methods
The most widely adopted procedures for the determination of
cypermethrin residues in crops, soil, animal tissues and products, and
environmental samples are based on extraction of the residue with an
organic solvent, clean-up of the extract, as necessary, by
solvent-solvent partition and adsorption-column chromatography,
followed by determination of the residue using gas chromatography
with electron capture detector (GC/ECD). The identity of residues can
be confirmed by GC with mass selective detection (GC-MSD) or by
thin-layer chromatography (TLC) followed by GC/ECD.
1.4 Production and Uses
Cypermethrin was first synthesized in 1974 and first marketed in 1977
as a highly active synthetic pyrethroid insecticide effective against
a wide range of pests in agriculture, public health, and animal
husbandry. In agriculture, its main use is against foliage pests and
certain surface soil pests, such as cutworms, but, because of its
physical and chemical properties, it is not recommended against
soil-borne pests below the surface.
In 1980, 92.5% of all the cypermethrin produced in the world was used
on cotton; in 1982, world production was 340 tonnes of the active
material. It is used primarily in the form of an emulsifiable
concentrate, but ultra-low-volume concentrates, wettable powders, and
joint formulations with other pesticides are available.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
The levels of cypermethrin residues in food commodities after good
agricultural practice are generally low. The residue levels will be
further reduced during food processing. In food of animal origin,
residues may range between 0.01 and 0.2 mg/kg product. Residue levels
found in non-food commodities are generally higher, up to 20 mg/kg
product. Total-diet studies are not available, but the available
residue information indicates that the oral intake by the general
population is well below the ADI, and expected to be negligible.
2.2 Effects on Animals and Human Health
The absorption and elimination of cypermethrin was rapid in the
different mammalian species tested. The major metabolic reaction is
cleavage of the ester bond followed by hydroxylation and conjugation
of the cyclopropane and phenoxybenzyl moieties. The highest levels of
cypermethrin are found in body fat, which is consistent with the
lipophilic nature of the compound. The half-life in the fat of rats
is about 12-9 days for the cis-isomer and 3-4 days for the
trans-isomer.
The acute toxicity of cypermethrin for mammals is of a moderate order.
The oral LD50 for the rat ranged from 200-4000 mg/kg body weight.
Short-term and long-term toxicity studies on rats, mice, and dogs have
shown effects on growth, the liver and kidneys, the nervous system,
and the blood. A no-observed-adverse-effect level of 7.5 mg/kg body
weight has been adopted by the Task Group.
Cypermethrin was not carcinogenic in mice or rats fed diets containing
the compound over a 2-year period. Cypermethrin was not teratogenic
in either rats at 70 mg/kg body weight or rabbits at 30 mg/kg body
weight. No effects on reproductive performance were seen in a
3-generation reproduction study on rats administered 10 mg
cypermethrin/kg diet. In a variety of mutagenicity studies,
cypermethrin was shown to be mainly without mutagenic activity.
The mechanism of the action of cypermethrin on the nervous system has
been extensively studied. From these studies and the available
occupational studies, it seems that the skin sensation seen in workers
handling cypermethrin generally lasts only a few hours and does not
persist for more than one day after exposure. Other neurological
signs have not been observed. These skin sensations may be considered
as an early warning that exposure has occurred and that work practice
should be reviewed. Cypermethrin may cause eye irritation and may be
a sensitizer for certain persons.
No cases of accidental poisoning have been reported as a result of
occupational exposure.
2.3 Evaluation of Effects on the Environment
When cypermethrin is applied to crops, residues may occur in soils and
surface waters, but biological degradation is fairly rapid and
residues do not accumulate in the environment. Photodegradation is
unlikely to play an important role. The main route of degradation is
cleavage of the ester linkage to give two main degradation products
containing the cyclopropane, and the phenoxybenzyl moieties. The
half-life in the soil is determined by many factors, but is in the
range of 2-4 weeks. Breakdown products in plants are bound as
glucosides. Because of its rather fast breakdown forming less toxic
breakdown products, and the low dose rates used in good agricultural
practice, it is unlikely that cypermethrin will reach significant
levels in the environment.
Cypermethrin at high dose levels may exert transient effects on the
soil microflora. Earthworms and other soil organisms are generally
resistant to cypermethrin. Because of strong adsorption to soil, only
low levels of cypermethrin may leak into surface water. These may
have transient effects, mainly on surface-breathing insects.
Cypermethrin is very toxic for fish and aquatic invertebrates. The
presence of suspended solids decreases the toxicity by a factor of at
least 2, because of the adsorption of cypermethrin on the solids.
Accumulation studies have shown that cypermethrin is rapidly taken up
by fish (accumulation factor approximately 1000). The half-life of
residues in the rainbow trout was 8 days. In view of the low
concentrations of cypermethrin that are likely to arise in water
bodies and their rapid decline, it is concluded that, under practical
conditions, residues in fish will not reach measurable levels.
The toxicity of cypermethrin for birds is low. However, bees appeared
to be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect on them.
3. CONCLUSIONS
General population exposure: Under recommended conditions of use the
exposure of the general population to cypermethrin is negligible and
is unlikely to present a hazard.
Occupational exposure: With reasonable work practices, hygiene
measures, and safety precautions, the use of cypermethrin is unlikely
to present a hazard to those occupationally exposed to it. The
occurrence of "facial sensations" is an indication of exposure. Under
these circumstances work practices should be reviewed.
Environment: With recommended application rates, it is unlikely that
cypermethrin or its degradation products will attain levels of
environmental significance. The fact that cypermethrin is highly
toxic for fish and honey bees is only likely to cause a problem in the
case of spillage or overspraying.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Cypermethrin is a synthetic pyrethroid insecticide. Only one case of
poisoning has been described in the general population and none during
occupational exposure. The results of experimental animal studies
suggest that, following massive overexposure or accidental ingestion,
neurological signs and symptoms, e.g., ataxia, convulsions, could
occur.
The human health hazards associated with certain types of exposure to
cypermethrin, together with preventive and protective measures and
first aid recommendations, are listed on the International Chemical
Safety Card on pages 20-23.
4.1.1 Advice to Physicians
No specific antidote is known. If indicated, empty stomach. Treat
symptomatically. The main hazard with liquid formulations is
aspiration of the solvent into the lungs, resulting in chemical
pneumonitis.
4.1.2 Health Surveillance Advice
Occurrence of "facial sensations" is an indication of skin exposure.
Under these circumstances, work practices should be reviewed.
4.2 Explosion and Fire Hazards
Some solvents in pyrethroid formulations are highly flammable. Use
dry powder, carbon dioxide, alcohol-resistant foam, sand, or earth for
dealing with fires. Do not use water. Cool nearby drums with water
spray.
If pyrethroid products are involved in a major fire or in a fire
involving other products, advise the fire service that protective
clothing and breathing apparatus should be worn. Also, warn the
authorities that pyrethroids are highly toxic for fish, and that the
use of water should be confined to the cooling of unaffected stock,
thus avoiding the accumulation of polluted run-off from the site.
4.3 Storage
Store technical material and formulations away from heat, under lock
and key, and out of the reach of children, animals, and unauthorized
personnel. Store in an area designated for insecticide storage,
preferably without drains.
Store away from other chemicals, foodstuffs, and animal feed.
4.4 Transport
Pyrethroids are classified as "harmful" or "low hazard" for transport
purposes. Formulations based on flammable solvents may be subject to
local transport controls. Ensure that containers are sound and that
labels are securely fixed and undamaged before dispatch. Comply with
local transport regulations.
Do not load together with food and animal feed.
Accident procedures:
(a) Avoid exposure - if possible by the use of appropriate protective
clothing and masks. Keep spectators away from leaking or spilled
product and prevent smoking or the use of naked flames in the
immediate vicinity.
(b) Extinguish fires with dry powder, carbon dioxide,
alcohol-resistant foam, sand, or earth.
(c) Prevent liquid from spreading to other cargo, vegetation, or
waterways by containing it with the most readily available
barrier material, e.g., earth or sand.
(d) Absorb spilled liquid and cover contaminated areas with earth,
lime, sand, or other absorbent material. Sweep up and place in a
secure container for subsequent safe disposal.
4.5 Spillage and Disposal
4.5.1 Spillage
Avoid exposure, if possible by the use of appropriate protective
clothing and masks.
Empty any product remaining in damaged or leaking containers into a
clean empty drum and label.
Absorb spillage with lime, damp sawdust, sand, or earth and dispose of
safely (see below). If spillage is large, contain it by building a
barrier of earth or sandbags.
Decontaminate empty, damaged, or leaking containers with a 10% sodium
carbonate solution added at the rate of at least 1 litre per 20-litre
drum. Puncture containers to prevent reuse.
4.5.2 Disposal
Waste containing cypermethrin should be burnt in a proper high
temperature incinerator with effluent scrubbing. Where no incinerator
is available, contaminated absorbents or surplus products should be
decomposed by hydrolysis at pH 12 or above. Contact with a suitable
hydrolysing agent is required to ensure degradation of the active
ingredient to a safe level.
(a) For emulsifiable material: 5% sodium hydroxide (caustic soda)
solution or saturated (7-10%) sodium carbonate (washing soda)
solution can be used.
(b) For non-emulsifiable material: a 1:1 mixture (by volume) can be
used of either of the above solutions and a water/oil soluble
solvent, such as denatured alcohol, monoethylene glycol, hexylene
glycol, or isopropanol.
The material should be covered with hydrolysing agent and put aside to
stand for 7 days. Before disposal of the resultant waste, the
material must be analysed to ensure that the active ingredient has
been degraded to a safe level.
Never pour untreated waste or surplus products into public sewers or
where there is any danger of run-off or seepage into streams,
water-courses, open waterways, ditches, fields with drainage systems,
or the catchment areas of boreholes, wells, springs, or ponds.
Puncture empty containers in order to avoid reuse.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
With recommended applications rates, it is unlikely that cypermethrin
and its degradation products will reach levels of environmental
significance. Cypermethrin is very toxic for fish and honey bees,
but, because of the very low exposure levels that normally occur, this
may only cause a problem in the case of spillage or overspraying.
Avoid spraying over bodies of water. Do not contaminate ponds,
waterways, or ditches with the product or with used containers.
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers concerned
with, and users of, cypermethrin. It should be displayed at, or near,
entrances to areas where there is potential exposure to cypermethrin,
and on processing equipment and containers. The card should be
translated into the appropriate language(s). All persons potentially
exposed to the chemical should also have the instructions on the
chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of the
National Poison Control Centre, and for local trade names.
CYPERMETHRIN
CAS chemical name: (RS)-cyano(3-phenoxyphenyl)methyl(1RS)- cis-trans-
3-(2,2-dichloro-ethenyl)-2.2-dimethyl-cyclopropane carboxylate
CAS registry no. 52315-07-8; RTECS registry no. GZ1250000
C22H19O3NCl2
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 416.3 Technical cypermethrin is a yellowish-brown liquid to
semi-solid, with a mild chemical odour. It is stable
Melting point up to 80°C (depending on to light and more stable in an acidic than in an
purity and cis or trans form) alkaline medium. It decomposes on heating above 220°C.
Readily absorbed via ingestion and inhalation and to a
Water solubility 0.01 mg/litre (20°C) lesser extent via the skin. It is a highly active synthetic
pyrethroid used as a stomach and contact insecticide.
Solubility in organic solvents The technical product is a mixture of eight steroisomers
(at 20°C)
xylene >450 g/litre
hexane 103 g/litre
Log n-octanol/water partition
coefficient 6.3
Density 1.12 (20°C)
Vapour pressure 1.4 x 10-9 mmHg (20°C)
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: tingling or burning sensation, Decrease exposure by using proper Remove contaminated clothing, wash skin
or numbness application technique, proper skin with water and soap
protection, face shield; wear protective
clothing when handling the concentrate
EYES: splashing may cause severe Wear face shield or goggles Flush immediately with clean water for at
irritation least 15 minutes
INHALATION: irritant to Avoid inhalation of fine dust and Fresh air
respiratory system mist
INGESTION: unlikely occupational Do not eat, drink, or smoke during
hazard work, wash hands
Accidental or deliberate ingestion Do not induce vomiting Obtain medical attention immediately; if
could lead to neurological signs and breathing has stopped, apply artificial
symptoms, such as ataxia and respiration
convulsions; main hazard of ingested
liquid formulations is aspiration Do not induce vomiting
into lungs
ENVIRONMENT: very toxic for Avoid the spraying of water;
fish and honey bees do not contaminate ponds,
waterways, or ditches with the
product or used containers
Spillage Storage Fire and Explosion
Absorb spillage with lime, damp Store in locked, well ventilated Some liquid formulations may be highly
sawdust, sand or earth; sweep up storeroom, away from feed flammable; use dry powder, carbon
and place in closed container and and foodstuffs, children and dioxide, or alcohol-resistant foam; cool
dispose of safely; avoid unauthorized personnel nearby drums with water spray
contamination of personnel, ponds,
and waterways
Waste disposal
Burn in high-temperature National Occupational
incinerator with effluent Exposure Limit:
scrubbing; alternatively, treat
with 5% caustic soda as a National Poison Control Centre:
hydrolysing agent; comply with
local regulations Local trade names:

 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Cypermethrin has been discussed several times by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR). In 1981, the JMPR established
an acceptable daily intake (ADI) for cypermethrin of 0-0.05 mg/kg body
weight.
WHO has classified cypermethrin as "irritant to eyes and sensitizer of
skin" in the list of "technical products unlikely to present an acute
hazard in normal use" (WHO, 1986). WHO has also issued a data sheet
on cypermethrin (WHO/FAO, 1975-85).
7.2 Exposure Limit Values
Some exposure limit values are given in the table on page 25.
7.3 Specific Restrictions
Cypermethrin is officially approved for use as a pesticide in many
countries, in each of which specific uses are defined, as well as
limitations and precautions. Its toxicity classification is also
determined according to the country or region concerned.
a The regulations and guidelines of all countries are subject to
change and should always be verified with appropriate regulatory
authorities before application.
EXPOSURE LIMIT VALUES
Medium Country/ Exposure limit description Value Effective
organization date
FOOD FAO/WHO Acceptable daily intake (ADI) 0.05 mg/kg 1985
body weight
FAO/WHO Maximum residue limits (MRL) 0.02-2.0 mg/kg 1982
(in specified products)
Brazil Acceptable limit 0.01-0.5 mg/kg 1985
Sweden Maximum tolerable concentration 2 mg/kg 1985
(provisional limit)
USA Acceptable residue limit 0.05-0.5 mg/kg 1985
(in specified products)
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pyrethroids in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low risk
of poisoning in transport.
The following label should be used:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Cypermethrin has been discussed several times by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR). In 1981, the JMPR established
an acceptable daily intake (ADI) for cypermethrin of 0-0.05 mg/kg body
weight.
WHO has classified cypermethrin as "irritant to eyes and sensitizer of
skin" in the list of "technical products unlikely to present an acute
hazard in normal use" (WHO, 1986). WHO has also issued a data sheet
on cypermethrin (WHO/FAO, 1975-85).
7.2 Exposure Limit Values
Some exposure limit values are given in the table on page 25.
7.3 Specific Restrictions
Cypermethrin is officially approved for use as a pesticide in many
countries, in each of which specific uses are defined, as well as
limitations and precautions. Its toxicity classification is also
determined according to the country or region concerned.
a The regulations and guidelines of all countries are subject to
change and should always be verified with appropriate regulatory
authorities before application.
EXPOSURE LIMIT VALUES
Medium Country/ Exposure limit description Value Effective
organization date
FOOD FAO/WHO Acceptable daily intake (ADI) 0.05 mg/kg 1985
body weight
FAO/WHO Maximum residue limits (MRL) 0.02-2.0 mg/kg 1982
(in specified products)
Brazil Acceptable limit 0.01-0.5 mg/kg 1985
Sweden Maximum tolerable concentration 2 mg/kg 1985
(provisional limit)
USA Acceptable residue limit 0.05-0.5 mg/kg 1985
(in specified products)
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pyrethroids in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low risk
of poisoning in transport.
The following label should be used:
 The European Community legislation requires labelling as a dangerous
substance using the symbol:
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 The label must read:
Harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuff.
7.5 Waste Disposal
In the USA, permits are required for discharge of pyrethroids from any
point source into national waters. This requirement contains detailed
instructions.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of
pesticide poisoning. Brussels, Groupement International des
Associations Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
WHO (1989) EHC No. 82: Cypermethrin. Geneva, World Health
Organization.
WHO (1986) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87. Geneva, World
Health Organization (Unpublished WHO document VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. (Unpublished WHO
documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuff.
7.5 Waste Disposal
In the USA, permits are required for discharge of pyrethroids from any
point source into national waters. This requirement contains detailed
instructions.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of
pesticide poisoning. Brussels, Groupement International des
Associations Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
WHO (1989) EHC No. 82: Cypermethrin. Geneva, World Health
Organization.
WHO (1986) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87. Geneva, World
Health Organization (Unpublished WHO document VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. (Unpublished WHO
documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
See Also:
Toxicological Abbreviations
Cypermethrin (EHC 82, 1989)
Cypermethrin (ICSC)
CYPERMETHRIN (JECFA Evaluation)
Cypermethrin (PIM 163)
Cypermethrin (Pesticide residues in food: 1981 evaluations)
Cypermethrin (Pesticide residues in food: 1982 evaluations)
Cypermethrin (Pesticide residues in food: 1983 evaluations)
Cypermethrin (Pesticide residues in food: 1984 evaluations)
 Molecular formula: C22H19O3NCl2
Chemical name: (IUPAC) (RS9-alpha-cyano-3-phenoxybenzyl (1RS)
cis-trans-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropane carboxylate
Chemical name: (CAS) (RS)-cyano(3-phenoxyphenyl)methyl
(1RS)- cis-trans-3-(2,2-
dichloroethenyl)-2,2-dimethyl-
cyclopropane carboxylate
CAS registry number:
Molecular formula: C22H19O3NCl2
Chemical name: (IUPAC) (RS9-alpha-cyano-3-phenoxybenzyl (1RS)
cis-trans-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropane carboxylate
Chemical name: (CAS) (RS)-cyano(3-phenoxyphenyl)methyl
(1RS)- cis-trans-3-(2,2-
dichloroethenyl)-2,2-dimethyl-
cyclopropane carboxylate
CAS registry number: 
 7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Cypermethrin has been discussed several times by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR). In 1981, the JMPR established
an acceptable daily intake (ADI) for cypermethrin of 0-0.05 mg/kg body
weight.
WHO has classified cypermethrin as "irritant to eyes and sensitizer of
skin" in the list of "technical products unlikely to present an acute
hazard in normal use" (WHO, 1986). WHO has also issued a data sheet
on cypermethrin (WHO/FAO, 1975-85).
7.2 Exposure Limit Values
Some exposure limit values are given in the table on page 25.
7.3 Specific Restrictions
Cypermethrin is officially approved for use as a pesticide in many
countries, in each of which specific uses are defined, as well as
limitations and precautions. Its toxicity classification is also
determined according to the country or region concerned.
a The regulations and guidelines of all countries are subject to
change and should always be verified with appropriate regulatory
authorities before application.
EXPOSURE LIMIT VALUES
Medium Country/ Exposure limit description Value Effective
organization date
FOOD FAO/WHO Acceptable daily intake (ADI) 0.05 mg/kg 1985
body weight
FAO/WHO Maximum residue limits (MRL) 0.02-2.0 mg/kg 1982
(in specified products)
Brazil Acceptable limit 0.01-0.5 mg/kg 1985
Sweden Maximum tolerable concentration 2 mg/kg 1985
(provisional limit)
USA Acceptable residue limit 0.05-0.5 mg/kg 1985
(in specified products)
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pyrethroids in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low risk
of poisoning in transport.
The following label should be used:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this paragraph has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. Its intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
Cypermethrin has been discussed several times by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR). In 1981, the JMPR established
an acceptable daily intake (ADI) for cypermethrin of 0-0.05 mg/kg body
weight.
WHO has classified cypermethrin as "irritant to eyes and sensitizer of
skin" in the list of "technical products unlikely to present an acute
hazard in normal use" (WHO, 1986). WHO has also issued a data sheet
on cypermethrin (WHO/FAO, 1975-85).
7.2 Exposure Limit Values
Some exposure limit values are given in the table on page 25.
7.3 Specific Restrictions
Cypermethrin is officially approved for use as a pesticide in many
countries, in each of which specific uses are defined, as well as
limitations and precautions. Its toxicity classification is also
determined according to the country or region concerned.
a The regulations and guidelines of all countries are subject to
change and should always be verified with appropriate regulatory
authorities before application.
EXPOSURE LIMIT VALUES
Medium Country/ Exposure limit description Value Effective
organization date
FOOD FAO/WHO Acceptable daily intake (ADI) 0.05 mg/kg 1985
body weight
FAO/WHO Maximum residue limits (MRL) 0.02-2.0 mg/kg 1982
(in specified products)
Brazil Acceptable limit 0.01-0.5 mg/kg 1985
Sweden Maximum tolerable concentration 2 mg/kg 1985
(provisional limit)
USA Acceptable residue limit 0.05-0.5 mg/kg 1985
(in specified products)
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies pyrethroids in:
- Hazard Class 6.1: poisonous substance
- Packing Group III: a substance presenting a relatively low risk
of poisoning in transport.
The following label should be used:
 The European Community legislation requires labelling as a dangerous
substance using the symbol:
The European Community legislation requires labelling as a dangerous
substance using the symbol:
 The label must read:
Harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuff.
7.5 Waste Disposal
In the USA, permits are required for discharge of pyrethroids from any
point source into national waters. This requirement contains detailed
instructions.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of
pesticide poisoning. Brussels, Groupement International des
Associations Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
WHO (1989) EHC No. 82: Cypermethrin. Geneva, World Health
Organization.
WHO (1986) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87. Geneva, World
Health Organization (Unpublished WHO document VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. (Unpublished WHO
documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Harmful by inhalation, in contact with skin and if swallowed;
keep out of reach of children; keep away from food, drink, and
animal feeding stuff.
7.5 Waste Disposal
In the USA, permits are required for discharge of pyrethroids from any
point source into national waters. This requirement contains detailed
instructions.
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and
use of pesticides. Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed., Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of
pesticide poisoning. Brussels, Groupement International des
Associations Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyons, International Agency
for Research on Cancer.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(Unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vols., Washington DC, US Department of Health and Human
Services, US Departmnent of Labor (Publication No. DHSS(NIOSH)
01-123).
WHO (1989) EHC No. 82: Cypermethrin. Geneva, World Health
Organization.
WHO (1986) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87. Geneva, World
Health Organization (Unpublished WHO document VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. (Unpublished WHO
documents).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual. 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
