
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 60
ENDRIN
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1991
This is a companion volume to Environmental Health Criteria 130:
Endrin
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Endrin : health and safety guide.
(Health and safety guide ; no. 60)
1. Endrin - standards I. Series
ISBN 92 4 151060 9 (NLM Classification: WA 240)
ISSN 0259-7268
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Exposure
2.2. Uptake, metabolism, and excretion
2.3. Effects on organisms in the environment
2.4. Effects on experimental animals and in vitro test systems
2.5. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Symptoms of poisoning
4.1.2. Medical treatment
4.1.3. Health surveillance advice
4.2. Safety in use
4.3. Explosion and fire hazards
4.3.1. Explosion hazard
4.3.2. Fire hazard
4.4. Storage
4.5. Transport
4.6. Spillage and disposal
4.6.1. Spillage
4.6.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluations by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Labelling, packaging, and transport
6.5. Waste disposal
6.6. Other measures
BIBLIOGRAPHY
ANNEX
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: endrin
Molecular formula: C12H8Cl6O
Chemical structure:
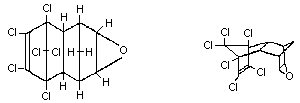 Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157, ENT 17251
OMS 197, and Mendrin
CAS chemical name: (1a,2,2a,3,6,6a,7,7a)-3,4,5,6,9,9-
hexachloro-1a,2,2a,3,6,6a,7,7a-
octahydro-2,7:3,6-dimethanonaphth[2,3-b]
oxirene (9CI-CAS).
Former CAS chemical name: 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,6,7,8,8a-octahydro-1,4-endo,endo-
5,8-dimethanonaphthalene)
IUPAC chemical name: (IR,4S,4aS,5S,6S,7R,8R,8aR)-
1,2,3,4,10.10-h exachloro-
1,4,4a,5,6,7,8,8a-octahydro-6,7-epoxy-
1,4:5,8-dimethano-naphthalene
CAS registry number: 72-20-8
RTECS registry number: IO1575000
Technical product
Trade name: Endrin
Purity: Not less than 92%. Impurities include
dieldrin (0.42%), aldrin (0.03%),
isodrin (0.73%), endrin half-cage ketone
(1.57%), endrin aldehyde (0.05%), and
heptachloro-norbornene (0.09%).
1.2 Physical and Chemical Properties
Endrin is a crystalline solid with a mild odour. Technical endrin is
stable when stored at ambient temperatures. It is stable in
formulations containing alkaline agents, emulsifiers, wetting agents,
and solvents. It decomposes with concentrated mineral acids, acid
catalysts, acid oxidizing agents, and active metals. When heated
above 200°C, endrin forms a less toxic and less insecticidally active
compound, delta-ketoendrin.
Some physical properties of endrin are given in Table 1.
Table 1. Physical properties of endrin
Melting point 226-230°C (above 200°C decomposition)
Flash-point None
Explosion limits Stable
Vapour pressure 2.7 x 10-7mmHg at 25°C
(3.6 x 10-5Pa at 25°C)
Relative molecular mass 380.9
Density 1.64 g/ml at 20°C
Solubility in water Practically insoluble
Solubility in organic Sparingly soluble in alcohols, petroleum
solvents hydrocarbons, moderately soluble in
aliphatic hydrocarbons, and quite soluble
in solvents, such as acetone, benzene,
carbon tetrachloride, and xylene
Partition coefficient
log P octanol/water 5.34
Conversion factors (20°C):
1 ppm = 16 mg/m3;
1 mg/m3 = 0.063 ppm.
1.3 Analytical Methods
Analytical methods for the determination of endrin are mainly based on
gas-liquid chromatography with electron-capture detection.
1.4 Production and Uses
Endrin has been manufactured since 1950, and was used throughout the
world up to the early 1970s. No actual production figures are
available, but the use of endrin has declined since the early 1970s,
because of severe restrictions on use, or banning, in several
countries.
It is a contact and stomach poison, used as a foliar insecticide,
which acts against a wide range of pests, particularly Lepidoptera.
It can be used at 0.2-0.5 kg a.i./ha on cotton, maize, sugar cane,
upland rice, and many other crops.
Endrin formulations include emulsifiable concentrates (ECs) at
190-200 g a.i./litre, wettable powders (WPs) at 500 g a.i./kg,
granules at 10-50 g a.i./kg, field strength dusts (FSDs), and pastes.
2. SUMMARY AND EVALUATION
2.1 Exposure
Endrin is an organochlorine insecticide that has been used since the
1950s to control a wide range of agricultural pests, mainly on cotton,
but also on rice, sugar cane, maize, and other crops. It is also used
as a rodenticide and avicide. Commercially, it is available in the
form of a dust, granules, paste, and as an emulsifiable concentrate
(EC).
Endrin enters the air mainly through volatilization and aerial drift.
In general, volatilization takes place after application to soils and
crops, and depends on many factors, such as the organic matter and
moisture content of the soil, humidity, air flow, and the surface area
of plants.
The most important route of contamination of surface water is run-off
from soil.
Contamination from precipitation, in the form of snow or rain, is
negligible. Local contamination of the environment may occur from
industrial effluents and careless application practices.
The main source of endrin in the soil is its direct application to
soil and crops. In soil, endrin can be retained, transported, or
degraded, depending on a number of factors. The highest retention
occurs in soils with a high organic matter content. The persistence
of endrin is highly dependent on local conditions. Its half-life in
soil can range up to 12 years.
Volatilization and photodecomposition are primary factors in the
disappearance of endrin from soil surfaces. Under the influence of
sunlight (UV radiation), the isomer delta-ketoendrin is formed. In
intense summer sunlight, about 50% of the endrin is isomerized to this
ketoendrin in 7 days. Microbial transformation (fungi and bacteria)
takes place, especially under anaerobic conditions, yielding the same
product.
Aquatic invertebrates and fish take up endrin rapidly from water.
Bioconcentration factors ranging between 14 and 10 000 have been
recorded after continuous exposure. Exposed fish transferred to
uncontaminated water lose endrin rapidly. Soil invertebrates may also
take up endrin readily.
The occasional presence of low levels of endrin in air and in surface
water or drinking-water (in agricultural areas) is of little
significance from the point of view of public health. The only
exposure that may be of relevance is the dietary intake. In general,
the reported intake levels are far below the ADI of 0.0002 mg/kg body
weight, established in 1970 (FAO/WHO, 1971).
2.2 Uptake, Metabolism, and Excretion
Unlike dieldrin, its stereoisomer endrin is rapidly metabolized by
animals; accumulation in the fat of animals is very low compared with
that of other compounds of similar chemical structure. In rats, it is
eliminated mainly in the faeces as endrin, anti-12-hydroxyendrin, and
a hydroxylated endrin derivative; a third metabolite, 12-ketoendrin,
accumulates in the tissues.
Both uptake and excretion after oral administration are rapid in rats.
The biological half-life is 1-6 days, depending on the dose level. A
steady state, when the excreted amount equals the daily intake, is
reached after 6 days. Excretion of both endrin and its metabolites
via the bile is much more rapid in male rats than in females,
resulting in lower accumulation in adipose tissue in males.
In rats, endrin and its metabolites are mainly eliminated via the
faeces in the first 24 h (70-75%). In rabbits, 50% of the metabolites
are excreted in the urine, compared with only 2% in rats. Only
unchanged endrin is found in the faeces of rabbits.
When cows were administered 0.1 mg endrin/kg diet for 21 days, up to
65% was excreted as metabolites in the urine, 20% was found in the
faeces, partly as unchanged endrin, and 3% was excreted in the milk,
also mainly as endrin. Residue levels of 0.003-0.006 mg/litre in
milk, 0.001-0.002 mg/ kg in meat, and 0.02-0.1 mg/kg in fat were
found.
Depending on dose levels, laying hens fed endrin showed residues of up
to 0.1 mg/kg in meat and 1 mg/kg in fat; eggs (yolk) contained
0.2-0.3 mg/kg and liver and kidneys each contained 0.2-0.5 mg/kg. The
residues found were mainly unchanged endrin, except in the liver and
kidneys. About 50% of the administered endrin was excreted in the
faeces, mainly in the form of metabolites.
It is clear that in the rat, rabbit, cow, hen, and man, the major
biotransformation metabolites of endrin are anti-12-hydroxyendrin, and
its sulfate and glucuronide conjugates. Four other metabolites are
present only in minor quantities. In body tissues and milk, mainly
unchanged endrin is found.
After application of endrin to plants, unchanged endrin and
transformation products including delta-ketoendrin and a very
hydrophilic compound were identified.
2.3 Effects on Organisms in the Environment
The effects of endrin on soil bacteria and fungi are minimal. Dose
levels of between 10 and 1000 mg/kg soil did not have any effects on
the decomposition of organic matter, denitrification, or the
generation of methane. Endrin is very toxic for fish, aquatic
invertebrates, and phytoplankton; the 96-h LC50 values are mostly
below 1.0 g/litre. In a life-cycle test, a lowest-observed-effect
level (LOEL) for the mysid shrimp (Mysidopsis bahia) was established
of 30 ng/litre.
The reported acute toxicity tests on aquatic organisms have been
conducted in aquaria without sediment. The presence of sediment would
be expected to attenuate the toxicity of endrin. Heavily contaminated
sediment had little effect on species living in open water, suggesting
low bioavailability of sediment-bound endrin. No tests have been
conducted on sediment-living aquatic animals.
The LD50 values for terrestrial mammals and birds are of the order
of 1.0-10.0 mg/kg body weight. Endrin fed to Mallard ducks at doses
of up to 3.0 mg/kg body weight, for 12 weeks, did not produce any
effects on egg production, fertility, or hatchability.
Resistance to endrin toxicity has been reported in several animal
groups including: aquatic invertebrates, fish, and small mammals.
Exposure to several different organochlorine pesticides led to the
selection of strains resistant to endrin.
Fish-kills occurring in agricultural (run-off) and industrial
(discharge) areas, and population decline in brown pelicans
(Louisiana, USA) and sandwich terns (the Netherlands) have been
attributed to a combination of endrin and other halogenated chemicals.
2.4 Effects on Experimental Animals and In vitro Test Systems
Endrin is a highly toxic pesticide. The oral LD50 values for
technical endrin in laboratory animals are in the range of 3-43 mg/kg
body weight. Dermal LD50 values for the rat range from 5 to 20 mg/kg
body weight. No substantial differences were found in the acute oral
and dermal toxicities of technical and formulated EC and wettable
powder (WP) products. Signs of intoxication are of a neurotoxic
nature.
Short-term oral toxicity studies were carried out on mice, rats,
rabbits, dogs, and domestic animals. In mice and rats, the maximum
tolerated doses for 6 weeks were 5 and 15 mg/kg diet (equivalent to
0.7 mg/kg body weight), respectivley. Rats survived a 16-week
exposure to a level of 1 mg/kg diet (equivalent to 0.05 mg/kg body
weight). Rabbits, administered repeated doses of 1 mg endrin/kg body
weight, died. In studies on dogs, a dietary level of 1mg/kg diet
(approx. 0.025 mg/kg body weight), given over 2 years, did not induce
any effects.
At low doses, the neurologically based sign of intoxication is an
inhibition of the GABA-ergic function. As is the case with other
chlorinated hydrocarbon insecticides, endrin also affects the liver.
Stimulation of enzyme systems involved in the metabolism of other
chemicals was evident as shown by, for instance, a decreased
hexobarbital sleeping time.
Doses of 75-150 mg endrin/kg, applied dermally as the dry powder for
2 h daily, caused convulsions and death in the rabbit, but did not
result in skin irritation. The production of systemic toxicity
without irritation at the site of contact is noteworthy.
Long-term toxicity/carcinogenicity studies were carried out on the
mouse and rat. No carcinogenic effects were found, but it should be
mentioned that there were shortcomings in the studies, e.g., poor
survival of the animals. In a 2-year study on the rat, the
no-observed-effect level was 1 mg/kg diet (ca 0.05 mg/kg body weight).
Tumour-promoting effects were not observed when endrin was tested in
combination with subminimal quantities of animal carcinogens. Endrin
was not found to be genotoxic in several mutagenicity studies. The
WHO Task Group on Environmental Health Criteria for Endrin concluded
that the data are insufficient to indicate that endrin is a
carcinogenic hazard for human beings.
Endrin was found not to be teratogenic in mice, rats, and hamsters,
even at dose levels causing maternal or fetotoxicity. NOELs of
0.5 mg/kg body weight in mice and rats and 0.75 mg/kg body weight in
hamsters were demonstrated. Endrin, at a dose of 2 mg/kg diet (ca
0.1 mg/kg body weight), did not induce reproductive effects over 3
generations in the rat.
A number of metabolites have acute toxicities that are similar to, or
higher than, that of endrin. The transformation product
delta-ketoendrin is less toxic. 12-Ketoendrin is considered to be the
most toxic metabolite of endrin in mammals, with an oral LD50 in the
rat of 0.8-1.1 mg/kg body weight.
2.5 Effects on Human Beings
Several episodes of fatal and non-fatal accidental and suicidal
poisoning have occurred. Cases of acute non-fatal intoxication, due
to accidental overexposure, have been observed in workers in an
endrin-manufacturing plant. The oral dose causing death was estimated
to be approximately 10 mg/kg body weight. The single oral dose
causing convulsions was estimated to be 0.25-1.0 mg/kg body weight.
The primary site of action of endrin is the central nervous system.
Exposure of humans to a toxic dose may lead to signs and symptoms of
intoxication, such as excitability and convulsions, within a few
hours, and death may occur within 2-12 h following exposure, if
appropriate treatment is not administered immediately. In cases of
non-fatal poisoning, recovery is rapid and complete.
Endrin does not accumulate in the human body to any significant
extent. Medical supervision of occupationally exposed workers
(duration of exposure ranging from 4 to 27 years) showed that
long-term adverse effects were not present (observation period for 232
workers ranged from 4 to 29 years). The only effect observed in the
workers was indirect evidence of a reversible stimulation of drug
metabolizing enzymes.
Endrin was not detected in a large number of samples of adipose
tissue, blood, and breast milk analysed in many countries. The Task
Group attributed this to the minor exposure of the general population
to endrin and its rapid metabolism.
Endrin could be detected in the blood (up to 450 µg/litre) and tissues
(adipose tissue, 89.5 mg/kg) in cases of fatal accidental poisonings.
No endrin was found in workers under normal circumstances. The
threshold level of endrin in the blood, below which no signs or
symptoms of intoxication occur, has been estimated to be in the range
of 50-100 µg/litre. The half-life of endrin in the blood may be of
the order of 24 h.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Endrin is an insecticide of high acute toxicity. Overexposure through
careless handling during manufacture or use, or from contaminated
food, may cause severe poisoning.
Exposure of the general population to endrin arises mainly through
residues in food. The reported intakes have generally been far below
the Acceptable Daily Intake established by FAO/WHO. Such exposure
should not constitute a health hazard for the general population.
When good work practices, hygiene measures, and safety precautions are
enforced, endrin is unlikely to present a hazard for those
occupationally exposed.
It is clear that the high toxicity of endrin can cause acute
environmental problems when there are uncontrolled discharges during
its manufacture, formulation, or use. Effects on wildlife from
agricultural use are less clear, though fish and fish-eating birds are
at risk from surface run-off.
Declines in the populations of some bird species have been associated
with high residues of various organochlorine pesticides in the tissues
of adults and in the eggs. While endrin has been found in some of
these species, it is very difficult to separate the effects of the
different organochlorines present.
3.2 Recommendations
Endrin should not be used, unless it is indispensable or less toxic
alternatives are not available.
For the health and welfare of workers and the general population, the
handling and application of endrin should only be entrusted to
competently supervised and well-trained operators, who will ensure
adequate safety precautions and apply endrin according to good
agricultural practice.
The manufacture, formulation, agricultural use, and disposal of endrin
should be carefully managed to minimize contamination of the
environment, particularly surface waters.
Periodic health evaluations should be carried out in those regularly
exposed to endrin.
Epidemiological studies of exposed populations of workers should be
continued.
In countries where endrin is still used, food should be monitored for
endrin residues.
If the use of endrin continues, more information is required on the
presence, ultimate fate, and toxicity of 12-ketoendrin and
delta-ketoendrin.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Endrin is an organochlorine insecticide. It is highly toxic (oral rat
LD50 approximately 7 mg/kg) and can be hazardous for human beings,
if incorrectly or carelessly handled. It is therefore essential that
the correct precautions should be observed during its handling and
use.
The human health hazards of endrin exposure, preventive and protective
measures, and first aid are listed in Table 2.
4.1.1 Symptoms of poisoning
Endrin is readily absorbed and toxic by mouth, by skin contact, and by
inhalation. It acts as a stimulant of the central nervous system. An
oral dose of 0.25 mg/kg body weight has been reported to cause
convulsions in human beings.
Symptoms may appear between 20 min and 12 h following accidental
ingestion or gross overexposure, and may include headache, dizziness,
nausea, vomiting, weakness in the legs, and convulsions, sometimes
leading to death.
Organochlorine compounds can cause respiratory depression. They may
also sensitize the heart to endogenous catecholamines, leading to
cardiac arrhythmias and, in severe exposure cases, to ventricular
fibrillation and cardiac arrest.
Respiratory depression may lead to metabolic acidosis, and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended if the symptoms are severe.
4.1.2 Medical treatment
Treatment of endrin poisoning requires immediate action; it is largely
symptomatic and supportive and directed against convulsions and
hypoxia.
Endrin is quickly eliminated from the blood and can only be detected
for 1 or 2 days following massive overexposures. Signs and symptoms
of poisoning occur only at concentrations in whole blood of more than
50 µg endrin/litre.
If endrin is swallowed, the stomach should be emptied as soon as
possible, by careful gastric lavage (with a cuffed endotracheal tube
already in place), avoiding aspiration into the lungs. In a rural
situation, where this is not feasible, vomiting should be induced
immediately, if the victim is conscious. This should be followed by
intragastric administration of 50 g of activated charcoal and 30 g
TABLE 2. HUMAN HEALTH HAZARDS, PREVENTIVE AND PROTECTIVE MEASURES, AND FIRST AID
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: may cause poisoning in Avoid contact with skin; After contact with skin, wash immediately
contact with skin wear suitable, impervious, protective with plenty of water and soap; remove
clothing and gloves all contaminated clothing immediately
and launder separately
EYES: may cause irritation to Avoid contact with eyes; wear In case of contact with eyes, rinse
eyes eye protection immediately with plenty of water and seek
medical advice
INHALATION: dusts and mist may Do not breathe dusts or spray;
cause poisoning by inhalation wear appropriate dust mask or
respirator
INGESTION: unlikely occupational Do not eat, drink, or smoke during
hazard work; wash hands before eating,
drinking, or smoking
Accidental or intentional ingestion If swallowed, seek medical advice immediately
may cause poisoning and show container or label; keep at rest and
ensure a clear airway; if gastric lavage is
not possible in a rural situation, induce
vomiting (only if victim is conscious)
magnesium or sodium sulfate in a 30% aqueous solution. Oily
purgatives are contraindicated. No fats, oils, or milk should be
given.
If convulsions occur, anticonvulsants should be given immediately,
e.g., 10 mg of diazepam, slowly, intravenously (children 1-5 mg),
repeated as necessary; or thiopental sodium or hexobarbital sodium
slowly, intravenously, in a dose of 10 mg/kg, with a maximum total
dose of up to 750 mg for an adult, or 5 ml of paraldehyde by
intramuscular injection. These short-acting anticonvulsants should
always be followed by phenobarbital given orally at 3 mg/kg (up to
200 mg for an adult), or phenobarbital sodium given intramuscularly at
3 mg/kg (also up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline, should
never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
Some guidelines on the management of major status epilepticus are
provided in Annex 1.
4.1.3 Health surveillance advice
A complete medical history and physical examination of regularly
exposed workers should be made at least annually, and a pre-employment
examination is recommended. Special attention should be paid to liver
function and signs and symptoms of stimulation of the central nervous
system (see 4.1.1).
4.2 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
apron and boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective gloves, cotton overalls,
rubber boots, and an appropriate
dust-mask or respirator. Follow
the advice relating to personal
hygiene.
Application in the field
Aerial application: Ensure that flag-men (markers) do
not stand in the spray-path of the
aircraft; do not spray over
residences occupied by human beings
or over surface waters, and avoid
spraying over ditches, canals,
rivers, streams, ponds, or lakes.
Ground spraying: Wear suitable protective clothing
(i.e., cap or hat, cotton overalls
or long-sleeved cotton shirt and
long trousers, boots or shoes);
when spraying tall crops or when
there is a risk of accidental
contamination by the spray, an
impermeable hood and jacket should
be worn; always avoid exposure to
the spray mist; do not spray into
the wind.
After application: Take off heavily splashed or
contaminated clothing; wash hands
and exposed skin before eating,
drinking, or smoking; wash
overalls, boots, hat, and other
protective clothing thoroughly,
especially the inside of gloves;
keep application equipment in good
condition, and free from leaks and
external contamination; keep
contents tightly closed in original
labelled container, when not fully
used; do not re-use empty
containers for any other purpose;
keep containers in a safe place
away from food, children, and
animals; empty containers must be
washed out and disposed of, as
advised in section 4.6.2.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazard
The explosion hazard depends on the solvent used in the formulation
and on the characteristics of the dust.
4.3.2 Fire hazard
Liquid formulations containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, endrin will
decompose, emitting toxic fumes. Fire-fighters should be equipped
with self-contained breathing apparatus, eye protection, and full
protective clothing.
The use of water spray should be confined to the cooling of unaffected
containers, thus avoiding the accumulation of polluted run-off from
the site.
4.4 Storage
Products should be stored in locked buildings, preferably buildings
dedicated to insecticides, and in compliance with label
recommendations. They should be segregated from incompatible
chemicals.
Keep the products out of reach of children and unauthorized personnel.
Do not store near foodstuffs or animal feed.
4.5 Transport
Comply with any national or local requirements regarding movement of
hazardous goods or wastes. Do not transport in the same compartment
as foodstuffs or animal feed. Before dispatch, check that containers
are sound and labels undamaged.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used (section
4.2). Empty any product remaining in damaged/leaking containers into
a clean empty drum, which should then be tightly closed and suitably
labelled.
Prevent liquid from spreading or contaminating other cargo and
vegetation, and avoid pollution of surface waters and ground water by
using the most suitable available material, e.g., earth or sand.
After emptying, leaking containers should be rinsed with at least
1 litre of water per 20-litre drum. Swirl around to rinse the walls
of the container, empty, and add the rinsings to the sawdust or earth.
Puncture or crush the container to prevent re-use.
As soon as possible after the spillage, and before re-use, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place in a closeable container for later transfer to a safe place for
disposal.
4.6.2 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organochlorine waste disposal, with
effluent gas scrubbing. If this is not possible, bury in an approved
dump or landfill, where there is no risk of contamination of surface
or ground water. Comply with any local requirements regarding the
disposal of toxic wastes. Puncture and/or crush all containers to
prevent re-use.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Endrin is highly toxic for all animal species, especially fish and
other aquatic organisms. It is readily bioaccumulated in fish, but
disappears rapidly when exposure is discontinued. It does not persist
for long periods in the water, but may persist in sediments.
Discharges from the manufacture, formulation, or use of endrin, and
any spillage or unused product, must be prevented from polluting the
environment and spreading to vegetation or waterways, and must be
treated and disposed of properly (section 4.6.2).
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative, but not an exhaustive, overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluations by International Bodies
The International Agency for Research on Cancer (IARC) reviewed endrin
in 1974 and 1987 and concluded that there was inadequate evidence for
the carcinogenicity of endrin in experimental animals and data in
humans were inadequate. Endrin was classified in Group 3: not
classifiable as to carcinogenicity to humans.
WHO classifies technical endrin as highly hazardous in normal use
(WHO, 1990). A data sheet on endrin was issued in 1978 (WHO/FAO,
1978).
Endrin was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1963, 1965, and 1970. In 1970, the JMPR
established an Acceptable Daily Intake (ADI) for man of 0-0.0002 mg/kg
body weight.
The maximum residue limits (MRL) established for endrin by the Joint
FAO/WHO Codex Alimentarious Commission 1986 are shown in Table 3.
6.2 Exposure Limit Values
Some exposure limit values are shown in the table on pages 28 and 29.
Table 3. Maximum residue limits for endrin
Commodity MRLa
in mg/kg product
Apples 0.02b
Barley 0.02b
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02b
Eggs 0.2
(on a shell-free basis)
Meat 0.1c
(in the carcass fat)
Milk 0.0008c
Poultry 1
(in the carcass fat)
Rice, husked or polished 0.02bb
Sorghum 0.02b
Sweet corn 0.02b
Wheat 0.02b
a Definition of residue: Sum of endrin and delta-ketoendrin.
b Level at, or about, the limit of determination.
c ERL:extraneous residue limit.
6.3 Specific Restrictions
The use of endrin is prohibited (with minor exceptions) in several
countries, including, Australia, the countries of the European
Community, Hungary, India, Japan, and Sweden. In the USSR, endrin is
prohibited for use in agriculture.
In some other countries, endrin is registered only for certain uses,
e.g., in Argentina, Brazil, and the USA.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transport of Dangerous
Goods classifies endrin in:
Hazard Class 6.1: poisonous substance;
Packing Group I: substances and preparations presenting a
very severe risk of poisoning, when the
content of the active ingredient is
60-100%;
Packing Group II: substances and preparations presenting a
serious risk of poisoning, when the
content of active ingredient is 6-60%;
Packing Group III: substance presenting a relatively low
risk of poisoning in transport, when the
content of active ingredient is 1-6%
(solid) or 0.5-6% (liquid).
As endrin may be carried in solution in flammable solvents, a
"Flammable liquid" subsidiary risk label (red) is also required when
the flash point of the solution is below or equal to 61°C (closed
cup); the flammable risk takes precedence when the flash-point is
below or equal to 23°C (closed cup); the solution is then classified
in Class 3, with a Class 6.1 subsidiary risk.
For the purposes of international transport, the types of labelling
shown below (page 30) are required by:
* the United Nations Committee of Experts on the Transport of
Dangerous Goods;
* the International Maritime Dangerous Goods (IMDG) Code;
* the ICAO Technical Instructions for the Safe Transport of
Dangerous Goods by Air;
* the European Agreement concerning the International Carriage of
Dangerous Goods by Road (ADR);
* the Regulations concerning the International Carriage of
Dangerous Goods by Rail (RID).
TABLE 4. EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Germany, Maximum worksite concentration (MAK) 1985
Federal - time weighted average (TWA) 0.1 mg/m3a
Republic of - short term exposure limit (STEL) 1.0 mg/m3
(30 min; 1 × /shift)
United Kingdom Recommended limit (RECL) 1985
- time-weighted average (TWA) 0.1 mg/m3a
- short term exposure level (STEL) 0.3 mg/m3
(10-min TWA)
USA - OSHA Permissible Exposure Limit (PEL) 1989
- time-weighted average (TWA) 0.1 mg/m3a
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.0002 mg/kg 1970
body weight
Residue FAO/WHO Maximum residue limit 0.0008-1mg/kg 1986
(for specified products)
WATER Ambient Mexico Maximum permissible concentration
(for drinking-water purification) 0.001 mg/litre 1973
(coastal) 0.0002 mg/litre 1973
(estuarine) 0.002 mg/litre 1973
USA Maximum permissible concentration 1981
(bottled water for human consumption) 0.0002 mg/litre
a Skin absorption.
Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157, ENT 17251
OMS 197, and Mendrin
CAS chemical name: (1a,2,2a,3,6,6a,7,7a)-3,4,5,6,9,9-
hexachloro-1a,2,2a,3,6,6a,7,7a-
octahydro-2,7:3,6-dimethanonaphth[2,3-b]
oxirene (9CI-CAS).
Former CAS chemical name: 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,6,7,8,8a-octahydro-1,4-endo,endo-
5,8-dimethanonaphthalene)
IUPAC chemical name: (IR,4S,4aS,5S,6S,7R,8R,8aR)-
1,2,3,4,10.10-h exachloro-
1,4,4a,5,6,7,8,8a-octahydro-6,7-epoxy-
1,4:5,8-dimethano-naphthalene
CAS registry number: 72-20-8
RTECS registry number: IO1575000
Technical product
Trade name: Endrin
Purity: Not less than 92%. Impurities include
dieldrin (0.42%), aldrin (0.03%),
isodrin (0.73%), endrin half-cage ketone
(1.57%), endrin aldehyde (0.05%), and
heptachloro-norbornene (0.09%).
1.2 Physical and Chemical Properties
Endrin is a crystalline solid with a mild odour. Technical endrin is
stable when stored at ambient temperatures. It is stable in
formulations containing alkaline agents, emulsifiers, wetting agents,
and solvents. It decomposes with concentrated mineral acids, acid
catalysts, acid oxidizing agents, and active metals. When heated
above 200°C, endrin forms a less toxic and less insecticidally active
compound, delta-ketoendrin.
Some physical properties of endrin are given in Table 1.
Table 1. Physical properties of endrin
Melting point 226-230°C (above 200°C decomposition)
Flash-point None
Explosion limits Stable
Vapour pressure 2.7 x 10-7mmHg at 25°C
(3.6 x 10-5Pa at 25°C)
Relative molecular mass 380.9
Density 1.64 g/ml at 20°C
Solubility in water Practically insoluble
Solubility in organic Sparingly soluble in alcohols, petroleum
solvents hydrocarbons, moderately soluble in
aliphatic hydrocarbons, and quite soluble
in solvents, such as acetone, benzene,
carbon tetrachloride, and xylene
Partition coefficient
log P octanol/water 5.34
Conversion factors (20°C):
1 ppm = 16 mg/m3;
1 mg/m3 = 0.063 ppm.
1.3 Analytical Methods
Analytical methods for the determination of endrin are mainly based on
gas-liquid chromatography with electron-capture detection.
1.4 Production and Uses
Endrin has been manufactured since 1950, and was used throughout the
world up to the early 1970s. No actual production figures are
available, but the use of endrin has declined since the early 1970s,
because of severe restrictions on use, or banning, in several
countries.
It is a contact and stomach poison, used as a foliar insecticide,
which acts against a wide range of pests, particularly Lepidoptera.
It can be used at 0.2-0.5 kg a.i./ha on cotton, maize, sugar cane,
upland rice, and many other crops.
Endrin formulations include emulsifiable concentrates (ECs) at
190-200 g a.i./litre, wettable powders (WPs) at 500 g a.i./kg,
granules at 10-50 g a.i./kg, field strength dusts (FSDs), and pastes.
2. SUMMARY AND EVALUATION
2.1 Exposure
Endrin is an organochlorine insecticide that has been used since the
1950s to control a wide range of agricultural pests, mainly on cotton,
but also on rice, sugar cane, maize, and other crops. It is also used
as a rodenticide and avicide. Commercially, it is available in the
form of a dust, granules, paste, and as an emulsifiable concentrate
(EC).
Endrin enters the air mainly through volatilization and aerial drift.
In general, volatilization takes place after application to soils and
crops, and depends on many factors, such as the organic matter and
moisture content of the soil, humidity, air flow, and the surface area
of plants.
The most important route of contamination of surface water is run-off
from soil.
Contamination from precipitation, in the form of snow or rain, is
negligible. Local contamination of the environment may occur from
industrial effluents and careless application practices.
The main source of endrin in the soil is its direct application to
soil and crops. In soil, endrin can be retained, transported, or
degraded, depending on a number of factors. The highest retention
occurs in soils with a high organic matter content. The persistence
of endrin is highly dependent on local conditions. Its half-life in
soil can range up to 12 years.
Volatilization and photodecomposition are primary factors in the
disappearance of endrin from soil surfaces. Under the influence of
sunlight (UV radiation), the isomer delta-ketoendrin is formed. In
intense summer sunlight, about 50% of the endrin is isomerized to this
ketoendrin in 7 days. Microbial transformation (fungi and bacteria)
takes place, especially under anaerobic conditions, yielding the same
product.
Aquatic invertebrates and fish take up endrin rapidly from water.
Bioconcentration factors ranging between 14 and 10 000 have been
recorded after continuous exposure. Exposed fish transferred to
uncontaminated water lose endrin rapidly. Soil invertebrates may also
take up endrin readily.
The occasional presence of low levels of endrin in air and in surface
water or drinking-water (in agricultural areas) is of little
significance from the point of view of public health. The only
exposure that may be of relevance is the dietary intake. In general,
the reported intake levels are far below the ADI of 0.0002 mg/kg body
weight, established in 1970 (FAO/WHO, 1971).
2.2 Uptake, Metabolism, and Excretion
Unlike dieldrin, its stereoisomer endrin is rapidly metabolized by
animals; accumulation in the fat of animals is very low compared with
that of other compounds of similar chemical structure. In rats, it is
eliminated mainly in the faeces as endrin, anti-12-hydroxyendrin, and
a hydroxylated endrin derivative; a third metabolite, 12-ketoendrin,
accumulates in the tissues.
Both uptake and excretion after oral administration are rapid in rats.
The biological half-life is 1-6 days, depending on the dose level. A
steady state, when the excreted amount equals the daily intake, is
reached after 6 days. Excretion of both endrin and its metabolites
via the bile is much more rapid in male rats than in females,
resulting in lower accumulation in adipose tissue in males.
In rats, endrin and its metabolites are mainly eliminated via the
faeces in the first 24 h (70-75%). In rabbits, 50% of the metabolites
are excreted in the urine, compared with only 2% in rats. Only
unchanged endrin is found in the faeces of rabbits.
When cows were administered 0.1 mg endrin/kg diet for 21 days, up to
65% was excreted as metabolites in the urine, 20% was found in the
faeces, partly as unchanged endrin, and 3% was excreted in the milk,
also mainly as endrin. Residue levels of 0.003-0.006 mg/litre in
milk, 0.001-0.002 mg/ kg in meat, and 0.02-0.1 mg/kg in fat were
found.
Depending on dose levels, laying hens fed endrin showed residues of up
to 0.1 mg/kg in meat and 1 mg/kg in fat; eggs (yolk) contained
0.2-0.3 mg/kg and liver and kidneys each contained 0.2-0.5 mg/kg. The
residues found were mainly unchanged endrin, except in the liver and
kidneys. About 50% of the administered endrin was excreted in the
faeces, mainly in the form of metabolites.
It is clear that in the rat, rabbit, cow, hen, and man, the major
biotransformation metabolites of endrin are anti-12-hydroxyendrin, and
its sulfate and glucuronide conjugates. Four other metabolites are
present only in minor quantities. In body tissues and milk, mainly
unchanged endrin is found.
After application of endrin to plants, unchanged endrin and
transformation products including delta-ketoendrin and a very
hydrophilic compound were identified.
2.3 Effects on Organisms in the Environment
The effects of endrin on soil bacteria and fungi are minimal. Dose
levels of between 10 and 1000 mg/kg soil did not have any effects on
the decomposition of organic matter, denitrification, or the
generation of methane. Endrin is very toxic for fish, aquatic
invertebrates, and phytoplankton; the 96-h LC50 values are mostly
below 1.0 g/litre. In a life-cycle test, a lowest-observed-effect
level (LOEL) for the mysid shrimp (Mysidopsis bahia) was established
of 30 ng/litre.
The reported acute toxicity tests on aquatic organisms have been
conducted in aquaria without sediment. The presence of sediment would
be expected to attenuate the toxicity of endrin. Heavily contaminated
sediment had little effect on species living in open water, suggesting
low bioavailability of sediment-bound endrin. No tests have been
conducted on sediment-living aquatic animals.
The LD50 values for terrestrial mammals and birds are of the order
of 1.0-10.0 mg/kg body weight. Endrin fed to Mallard ducks at doses
of up to 3.0 mg/kg body weight, for 12 weeks, did not produce any
effects on egg production, fertility, or hatchability.
Resistance to endrin toxicity has been reported in several animal
groups including: aquatic invertebrates, fish, and small mammals.
Exposure to several different organochlorine pesticides led to the
selection of strains resistant to endrin.
Fish-kills occurring in agricultural (run-off) and industrial
(discharge) areas, and population decline in brown pelicans
(Louisiana, USA) and sandwich terns (the Netherlands) have been
attributed to a combination of endrin and other halogenated chemicals.
2.4 Effects on Experimental Animals and In vitro Test Systems
Endrin is a highly toxic pesticide. The oral LD50 values for
technical endrin in laboratory animals are in the range of 3-43 mg/kg
body weight. Dermal LD50 values for the rat range from 5 to 20 mg/kg
body weight. No substantial differences were found in the acute oral
and dermal toxicities of technical and formulated EC and wettable
powder (WP) products. Signs of intoxication are of a neurotoxic
nature.
Short-term oral toxicity studies were carried out on mice, rats,
rabbits, dogs, and domestic animals. In mice and rats, the maximum
tolerated doses for 6 weeks were 5 and 15 mg/kg diet (equivalent to
0.7 mg/kg body weight), respectivley. Rats survived a 16-week
exposure to a level of 1 mg/kg diet (equivalent to 0.05 mg/kg body
weight). Rabbits, administered repeated doses of 1 mg endrin/kg body
weight, died. In studies on dogs, a dietary level of 1mg/kg diet
(approx. 0.025 mg/kg body weight), given over 2 years, did not induce
any effects.
At low doses, the neurologically based sign of intoxication is an
inhibition of the GABA-ergic function. As is the case with other
chlorinated hydrocarbon insecticides, endrin also affects the liver.
Stimulation of enzyme systems involved in the metabolism of other
chemicals was evident as shown by, for instance, a decreased
hexobarbital sleeping time.
Doses of 75-150 mg endrin/kg, applied dermally as the dry powder for
2 h daily, caused convulsions and death in the rabbit, but did not
result in skin irritation. The production of systemic toxicity
without irritation at the site of contact is noteworthy.
Long-term toxicity/carcinogenicity studies were carried out on the
mouse and rat. No carcinogenic effects were found, but it should be
mentioned that there were shortcomings in the studies, e.g., poor
survival of the animals. In a 2-year study on the rat, the
no-observed-effect level was 1 mg/kg diet (ca 0.05 mg/kg body weight).
Tumour-promoting effects were not observed when endrin was tested in
combination with subminimal quantities of animal carcinogens. Endrin
was not found to be genotoxic in several mutagenicity studies. The
WHO Task Group on Environmental Health Criteria for Endrin concluded
that the data are insufficient to indicate that endrin is a
carcinogenic hazard for human beings.
Endrin was found not to be teratogenic in mice, rats, and hamsters,
even at dose levels causing maternal or fetotoxicity. NOELs of
0.5 mg/kg body weight in mice and rats and 0.75 mg/kg body weight in
hamsters were demonstrated. Endrin, at a dose of 2 mg/kg diet (ca
0.1 mg/kg body weight), did not induce reproductive effects over 3
generations in the rat.
A number of metabolites have acute toxicities that are similar to, or
higher than, that of endrin. The transformation product
delta-ketoendrin is less toxic. 12-Ketoendrin is considered to be the
most toxic metabolite of endrin in mammals, with an oral LD50 in the
rat of 0.8-1.1 mg/kg body weight.
2.5 Effects on Human Beings
Several episodes of fatal and non-fatal accidental and suicidal
poisoning have occurred. Cases of acute non-fatal intoxication, due
to accidental overexposure, have been observed in workers in an
endrin-manufacturing plant. The oral dose causing death was estimated
to be approximately 10 mg/kg body weight. The single oral dose
causing convulsions was estimated to be 0.25-1.0 mg/kg body weight.
The primary site of action of endrin is the central nervous system.
Exposure of humans to a toxic dose may lead to signs and symptoms of
intoxication, such as excitability and convulsions, within a few
hours, and death may occur within 2-12 h following exposure, if
appropriate treatment is not administered immediately. In cases of
non-fatal poisoning, recovery is rapid and complete.
Endrin does not accumulate in the human body to any significant
extent. Medical supervision of occupationally exposed workers
(duration of exposure ranging from 4 to 27 years) showed that
long-term adverse effects were not present (observation period for 232
workers ranged from 4 to 29 years). The only effect observed in the
workers was indirect evidence of a reversible stimulation of drug
metabolizing enzymes.
Endrin was not detected in a large number of samples of adipose
tissue, blood, and breast milk analysed in many countries. The Task
Group attributed this to the minor exposure of the general population
to endrin and its rapid metabolism.
Endrin could be detected in the blood (up to 450 µg/litre) and tissues
(adipose tissue, 89.5 mg/kg) in cases of fatal accidental poisonings.
No endrin was found in workers under normal circumstances. The
threshold level of endrin in the blood, below which no signs or
symptoms of intoxication occur, has been estimated to be in the range
of 50-100 µg/litre. The half-life of endrin in the blood may be of
the order of 24 h.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
Endrin is an insecticide of high acute toxicity. Overexposure through
careless handling during manufacture or use, or from contaminated
food, may cause severe poisoning.
Exposure of the general population to endrin arises mainly through
residues in food. The reported intakes have generally been far below
the Acceptable Daily Intake established by FAO/WHO. Such exposure
should not constitute a health hazard for the general population.
When good work practices, hygiene measures, and safety precautions are
enforced, endrin is unlikely to present a hazard for those
occupationally exposed.
It is clear that the high toxicity of endrin can cause acute
environmental problems when there are uncontrolled discharges during
its manufacture, formulation, or use. Effects on wildlife from
agricultural use are less clear, though fish and fish-eating birds are
at risk from surface run-off.
Declines in the populations of some bird species have been associated
with high residues of various organochlorine pesticides in the tissues
of adults and in the eggs. While endrin has been found in some of
these species, it is very difficult to separate the effects of the
different organochlorines present.
3.2 Recommendations
Endrin should not be used, unless it is indispensable or less toxic
alternatives are not available.
For the health and welfare of workers and the general population, the
handling and application of endrin should only be entrusted to
competently supervised and well-trained operators, who will ensure
adequate safety precautions and apply endrin according to good
agricultural practice.
The manufacture, formulation, agricultural use, and disposal of endrin
should be carefully managed to minimize contamination of the
environment, particularly surface waters.
Periodic health evaluations should be carried out in those regularly
exposed to endrin.
Epidemiological studies of exposed populations of workers should be
continued.
In countries where endrin is still used, food should be monitored for
endrin residues.
If the use of endrin continues, more information is required on the
presence, ultimate fate, and toxicity of 12-ketoendrin and
delta-ketoendrin.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Endrin is an organochlorine insecticide. It is highly toxic (oral rat
LD50 approximately 7 mg/kg) and can be hazardous for human beings,
if incorrectly or carelessly handled. It is therefore essential that
the correct precautions should be observed during its handling and
use.
The human health hazards of endrin exposure, preventive and protective
measures, and first aid are listed in Table 2.
4.1.1 Symptoms of poisoning
Endrin is readily absorbed and toxic by mouth, by skin contact, and by
inhalation. It acts as a stimulant of the central nervous system. An
oral dose of 0.25 mg/kg body weight has been reported to cause
convulsions in human beings.
Symptoms may appear between 20 min and 12 h following accidental
ingestion or gross overexposure, and may include headache, dizziness,
nausea, vomiting, weakness in the legs, and convulsions, sometimes
leading to death.
Organochlorine compounds can cause respiratory depression. They may
also sensitize the heart to endogenous catecholamines, leading to
cardiac arrhythmias and, in severe exposure cases, to ventricular
fibrillation and cardiac arrest.
Respiratory depression may lead to metabolic acidosis, and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended if the symptoms are severe.
4.1.2 Medical treatment
Treatment of endrin poisoning requires immediate action; it is largely
symptomatic and supportive and directed against convulsions and
hypoxia.
Endrin is quickly eliminated from the blood and can only be detected
for 1 or 2 days following massive overexposures. Signs and symptoms
of poisoning occur only at concentrations in whole blood of more than
50 µg endrin/litre.
If endrin is swallowed, the stomach should be emptied as soon as
possible, by careful gastric lavage (with a cuffed endotracheal tube
already in place), avoiding aspiration into the lungs. In a rural
situation, where this is not feasible, vomiting should be induced
immediately, if the victim is conscious. This should be followed by
intragastric administration of 50 g of activated charcoal and 30 g
TABLE 2. HUMAN HEALTH HAZARDS, PREVENTIVE AND PROTECTIVE MEASURES, AND FIRST AID
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: may cause poisoning in Avoid contact with skin; After contact with skin, wash immediately
contact with skin wear suitable, impervious, protective with plenty of water and soap; remove
clothing and gloves all contaminated clothing immediately
and launder separately
EYES: may cause irritation to Avoid contact with eyes; wear In case of contact with eyes, rinse
eyes eye protection immediately with plenty of water and seek
medical advice
INHALATION: dusts and mist may Do not breathe dusts or spray;
cause poisoning by inhalation wear appropriate dust mask or
respirator
INGESTION: unlikely occupational Do not eat, drink, or smoke during
hazard work; wash hands before eating,
drinking, or smoking
Accidental or intentional ingestion If swallowed, seek medical advice immediately
may cause poisoning and show container or label; keep at rest and
ensure a clear airway; if gastric lavage is
not possible in a rural situation, induce
vomiting (only if victim is conscious)
magnesium or sodium sulfate in a 30% aqueous solution. Oily
purgatives are contraindicated. No fats, oils, or milk should be
given.
If convulsions occur, anticonvulsants should be given immediately,
e.g., 10 mg of diazepam, slowly, intravenously (children 1-5 mg),
repeated as necessary; or thiopental sodium or hexobarbital sodium
slowly, intravenously, in a dose of 10 mg/kg, with a maximum total
dose of up to 750 mg for an adult, or 5 ml of paraldehyde by
intramuscular injection. These short-acting anticonvulsants should
always be followed by phenobarbital given orally at 3 mg/kg (up to
200 mg for an adult), or phenobarbital sodium given intramuscularly at
3 mg/kg (also up to 200 mg for an adult).
Morphine and its derivatives, adrenaline and noradrenaline, should
never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
Some guidelines on the management of major status epilepticus are
provided in Annex 1.
4.1.3 Health surveillance advice
A complete medical history and physical examination of regularly
exposed workers should be made at least annually, and a pre-employment
examination is recommended. Special attention should be paid to liver
function and signs and symptoms of stimulation of the central nervous
system (see 4.1.1).
4.2 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
apron and boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective gloves, cotton overalls,
rubber boots, and an appropriate
dust-mask or respirator. Follow
the advice relating to personal
hygiene.
Application in the field
Aerial application: Ensure that flag-men (markers) do
not stand in the spray-path of the
aircraft; do not spray over
residences occupied by human beings
or over surface waters, and avoid
spraying over ditches, canals,
rivers, streams, ponds, or lakes.
Ground spraying: Wear suitable protective clothing
(i.e., cap or hat, cotton overalls
or long-sleeved cotton shirt and
long trousers, boots or shoes);
when spraying tall crops or when
there is a risk of accidental
contamination by the spray, an
impermeable hood and jacket should
be worn; always avoid exposure to
the spray mist; do not spray into
the wind.
After application: Take off heavily splashed or
contaminated clothing; wash hands
and exposed skin before eating,
drinking, or smoking; wash
overalls, boots, hat, and other
protective clothing thoroughly,
especially the inside of gloves;
keep application equipment in good
condition, and free from leaks and
external contamination; keep
contents tightly closed in original
labelled container, when not fully
used; do not re-use empty
containers for any other purpose;
keep containers in a safe place
away from food, children, and
animals; empty containers must be
washed out and disposed of, as
advised in section 4.6.2.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazard
The explosion hazard depends on the solvent used in the formulation
and on the characteristics of the dust.
4.3.2 Fire hazard
Liquid formulations containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, endrin will
decompose, emitting toxic fumes. Fire-fighters should be equipped
with self-contained breathing apparatus, eye protection, and full
protective clothing.
The use of water spray should be confined to the cooling of unaffected
containers, thus avoiding the accumulation of polluted run-off from
the site.
4.4 Storage
Products should be stored in locked buildings, preferably buildings
dedicated to insecticides, and in compliance with label
recommendations. They should be segregated from incompatible
chemicals.
Keep the products out of reach of children and unauthorized personnel.
Do not store near foodstuffs or animal feed.
4.5 Transport
Comply with any national or local requirements regarding movement of
hazardous goods or wastes. Do not transport in the same compartment
as foodstuffs or animal feed. Before dispatch, check that containers
are sound and labels undamaged.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken as
required, and appropriate personal protection should be used (section
4.2). Empty any product remaining in damaged/leaking containers into
a clean empty drum, which should then be tightly closed and suitably
labelled.
Prevent liquid from spreading or contaminating other cargo and
vegetation, and avoid pollution of surface waters and ground water by
using the most suitable available material, e.g., earth or sand.
After emptying, leaking containers should be rinsed with at least
1 litre of water per 20-litre drum. Swirl around to rinse the walls
of the container, empty, and add the rinsings to the sawdust or earth.
Puncture or crush the container to prevent re-use.
As soon as possible after the spillage, and before re-use, cover all
contaminated areas with damp sawdust, sand, or earth. Sweep up and
place in a closeable container for later transfer to a safe place for
disposal.
4.6.2 Disposal
Any surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Waste material should be burned in
a proper incinerator designed for organochlorine waste disposal, with
effluent gas scrubbing. If this is not possible, bury in an approved
dump or landfill, where there is no risk of contamination of surface
or ground water. Comply with any local requirements regarding the
disposal of toxic wastes. Puncture and/or crush all containers to
prevent re-use.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Endrin is highly toxic for all animal species, especially fish and
other aquatic organisms. It is readily bioaccumulated in fish, but
disappears rapidly when exposure is discontinued. It does not persist
for long periods in the water, but may persist in sediments.
Discharges from the manufacture, formulation, or use of endrin, and
any spillage or unused product, must be prevented from polluting the
environment and spreading to vegetation or waterways, and must be
treated and disposed of properly (section 4.6.2).
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. Its intention is to give the
reader a representative, but not an exhaustive, overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluations by International Bodies
The International Agency for Research on Cancer (IARC) reviewed endrin
in 1974 and 1987 and concluded that there was inadequate evidence for
the carcinogenicity of endrin in experimental animals and data in
humans were inadequate. Endrin was classified in Group 3: not
classifiable as to carcinogenicity to humans.
WHO classifies technical endrin as highly hazardous in normal use
(WHO, 1990). A data sheet on endrin was issued in 1978 (WHO/FAO,
1978).
Endrin was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1963, 1965, and 1970. In 1970, the JMPR
established an Acceptable Daily Intake (ADI) for man of 0-0.0002 mg/kg
body weight.
The maximum residue limits (MRL) established for endrin by the Joint
FAO/WHO Codex Alimentarious Commission 1986 are shown in Table 3.
6.2 Exposure Limit Values
Some exposure limit values are shown in the table on pages 28 and 29.
Table 3. Maximum residue limits for endrin
Commodity MRLa
in mg/kg product
Apples 0.02b
Barley 0.02b
Cottonseed 0.1
Cottonseed oil (crude) 0.1
Cottonseed oil (edible) 0.02b
Eggs 0.2
(on a shell-free basis)
Meat 0.1c
(in the carcass fat)
Milk 0.0008c
Poultry 1
(in the carcass fat)
Rice, husked or polished 0.02bb
Sorghum 0.02b
Sweet corn 0.02b
Wheat 0.02b
a Definition of residue: Sum of endrin and delta-ketoendrin.
b Level at, or about, the limit of determination.
c ERL:extraneous residue limit.
6.3 Specific Restrictions
The use of endrin is prohibited (with minor exceptions) in several
countries, including, Australia, the countries of the European
Community, Hungary, India, Japan, and Sweden. In the USSR, endrin is
prohibited for use in agriculture.
In some other countries, endrin is registered only for certain uses,
e.g., in Argentina, Brazil, and the USA.
6.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transport of Dangerous
Goods classifies endrin in:
Hazard Class 6.1: poisonous substance;
Packing Group I: substances and preparations presenting a
very severe risk of poisoning, when the
content of the active ingredient is
60-100%;
Packing Group II: substances and preparations presenting a
serious risk of poisoning, when the
content of active ingredient is 6-60%;
Packing Group III: substance presenting a relatively low
risk of poisoning in transport, when the
content of active ingredient is 1-6%
(solid) or 0.5-6% (liquid).
As endrin may be carried in solution in flammable solvents, a
"Flammable liquid" subsidiary risk label (red) is also required when
the flash point of the solution is below or equal to 61°C (closed
cup); the flammable risk takes precedence when the flash-point is
below or equal to 23°C (closed cup); the solution is then classified
in Class 3, with a Class 6.1 subsidiary risk.
For the purposes of international transport, the types of labelling
shown below (page 30) are required by:
* the United Nations Committee of Experts on the Transport of
Dangerous Goods;
* the International Maritime Dangerous Goods (IMDG) Code;
* the ICAO Technical Instructions for the Safe Transport of
Dangerous Goods by Air;
* the European Agreement concerning the International Carriage of
Dangerous Goods by Road (ADR);
* the Regulations concerning the International Carriage of
Dangerous Goods by Rail (RID).
TABLE 4. EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Workplace Germany, Maximum worksite concentration (MAK) 1985
Federal - time weighted average (TWA) 0.1 mg/m3a
Republic of - short term exposure limit (STEL) 1.0 mg/m3
(30 min; 1 × /shift)
United Kingdom Recommended limit (RECL) 1985
- time-weighted average (TWA) 0.1 mg/m3a
- short term exposure level (STEL) 0.3 mg/m3
(10-min TWA)
USA - OSHA Permissible Exposure Limit (PEL) 1989
- time-weighted average (TWA) 0.1 mg/m3a
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.0002 mg/kg 1970
body weight
Residue FAO/WHO Maximum residue limit 0.0008-1mg/kg 1986
(for specified products)
WATER Ambient Mexico Maximum permissible concentration
(for drinking-water purification) 0.001 mg/litre 1973
(coastal) 0.0002 mg/litre 1973
(estuarine) 0.002 mg/litre 1973
USA Maximum permissible concentration 1981
(bottled water for human consumption) 0.0002 mg/litre
a Skin absorption.
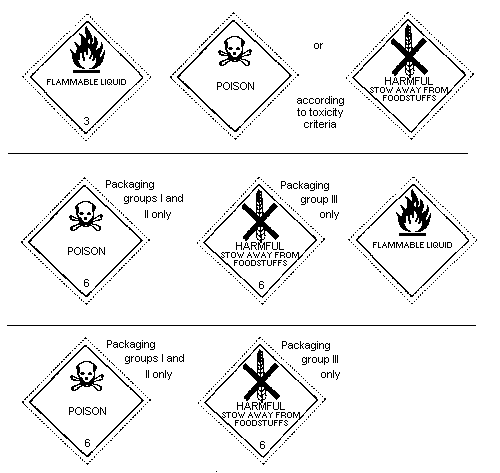 Note: The text on the label is optional, and is not required by
RID/ADR. The class number at the bottom of the main hazard
is not required by RID/ADR, but is not optional for the
other modes.
Endrin has been identified as a severe marine pollutant in the
International Maritime Dangerous Goods (IMDG) Code, therefore a
"Marine pollutant" mark is required for the transport by sea of all
concentrations greater than or equal to 1%.
Note: The text on the label is optional, and is not required by
RID/ADR. The class number at the bottom of the main hazard
is not required by RID/ADR, but is not optional for the
other modes.
Endrin has been identified as a severe marine pollutant in the
International Maritime Dangerous Goods (IMDG) Code, therefore a
"Marine pollutant" mark is required for the transport by sea of all
concentrations greater than or equal to 1%.
 The FAO specifications for plant protection products containing endrin
specify the composition and purity of the technical product and its
formulations. They also advise on methods for checking this. The
endrin content should be stated and may not differ by more than 4%
from this for the technical product (and up to 10% for some
formulations). Technical endrin should contain a minimum of 92%w/w
active material.
The European Economic Community legislation on the labelling of
pesticide preparations classified endrin in Class I/a for the purpose
of determining the label for preparations containing endrin and other
active ingredients.
The European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
The FAO specifications for plant protection products containing endrin
specify the composition and purity of the technical product and its
formulations. They also advise on methods for checking this. The
endrin content should be stated and may not differ by more than 4%
from this for the technical product (and up to 10% for some
formulations). Technical endrin should contain a minimum of 92%w/w
active material.
The European Economic Community legislation on the labelling of
pesticide preparations classified endrin in Class I/a for the purpose
of determining the label for preparations containing endrin and other
active ingredients.
The European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
 The label must read:
Very toxic by inhalation, in contact with skin and if swallowed;
keep locked up; keep away from food, drink and animal feeding
stuffs; after contact with skin, wash immediately with plenty of
water; in case of accident or if you feel unwell, seek medical
advice (show the label where possible).
6.5 Waste Disposal
In the USA, any non-domestic waste containing endrin and its
metabolites must be treated as a hazardous waste. Specific
instructions are given for notification and incineration.
Owners/operators of vessels or onshore or offshore facilities must
notify the US Government (National Response Center) of any release of
endrin in or on navigable waters, adjoining shorelines, in the
contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land, or ground water) in an amount equal
to, or greater than, one pound (0.454 kg).
An owner or operator of a hazardous waste incinerator must achieve
99.99% destruction and removal efficiency for this substance.
6.6 Other Measures
Aquatic environment
The European Economic Community legislation has established limit
values for the discharge of, and quality objectives for, aldrin,
dieldrin, endrin, and isodrin in the aquatic environment.
The limit values for emission standards are:
(a) Plants producing aldrin and/or dieldrin and/or endrin, including
formulation of these substances on the same site, must:
* on a monthly average value, not exceed 3 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 2 g/litre of water discharged (to be complied with as from
1 January 1989).
* on a daily average value, not exceed 15 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 10 g/litre of water discharged (to be complied with as from
1 January 1989).
(b) For inland surface waters, estuary waters, internal coastal waters
other than estuary waters, territorial waters, for the compounds
aldrin, dieldrin, endrin, and isodrin together:
* 30 ng/litre (to be complied with as from 1 January 1989); and
10 ng/litre for aldrin, 10 ng/litre for dieldrin, 5 ng/litre for
endrin, and 5 ng/litre for isodrin (to be complied with as from
1 January 1994).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice. Rome, Food and
Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues., 3rd ed. Rome, Codex Committee on Pesticide
Residues.
FAO/WHO (1986) Codex maximum limits for pesticide residues. CAC/Vol.
XIII. 2nd ed., Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
IRPTC (1986) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. United Nations Environment
Programme, Industry and Environment Office, Paris, 80 pp..
UNITED NATIONS (1984) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by governments, 1st ed. revised, United
Nations, New York.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 vol., Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-1991. Geneva, World Health
Organization (unpublished document WHO/PCS/90.1).
WHO (in preparation) Environmental Health Criteria 130: Endrin.
Geneva, World Health Organization.
WHO/FAO (1978) Data sheets on pesticides: Endrin. Geneva, World
Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX. MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
(A) Initial management
1. Assess the patient, verify the diagnosis, remove false teeth and
place in the lateral semi-prone position, establish an airway.
2. Diazepam, iv (see Note 1), (10 mg in 2 ml), 0.15-0.25 mg/kg,
usually 10 mg (2 ml) bolus followed immediately by a further 10 mg
(2 ml) over 1-2 minutes. This may be repeated according to response.
3. Take blood for determination of anticonvulsant drug levels, blood
alcohol level, and blood sugar (5 ml of blood in a sugar tube), also
blood for determination of calcium (5 ml in a plain tube), and a drop
of blood to determine blood glucose.
4. If this shows a low blood glucose level: administer glucose 50%,
iv, 25ml, preferably by catheter, and not into a small distal vein.
If alcohol is likely to be a factor: administer thiamine, iv, 100 mg.
5. Phenytoin, iv, 250 mg in 5 ml, 10-15 mg/kg, no faster than 50 mg
(1 ml) per minute, by infusion pump or slow iv injection (see Note 2).
(B) If fits continue, transfer to an intensive care unit, and consult
an anaesthetist
6. Chlormethiazole, iv (8 mg/ml). A loading dose of up to 800 mg
(100 ml) over 10 minutes (10 ml/min); maintain with 0.5-1 ml/min
(4-8 mg).
7. Thiopental, iv, 5 mg/kg loading dose, then 1-3 mg/kg per hour, to
a maximum blood thiopental level of 100 mg/litre.
8. If this fails - consult a neurologist.
a Adapted from a guideline prepared by Guy's Hospital, London.
NOTES
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there is
concurrent use of other CNS depressant drugs, especially
phenobarbital.
Diazepam must not be given:
* intramuscularly;
* added to an intravenous infusion;
* with phenobarbital unless artificial ventilation is available.
Rectal diazepam (using a rectal administration set), 5 or 10 mg in
2.5 ml, may be used for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
* intramuscularly;
* by central line;
* into a dextrose infusion;
* with any other drug.
Intravenous phenytoin should be monitored with continous ECG
recording. If this is not available, it may be safer to use a diluted
solution of 250 mg (5 ml) in 250 ml of normal saline, no faster than
50 mg/min. The diluted solution should be used immediately, provided
there is no evidence of precipitation (this use of phenytoin is not
licensed).
OPTIONS
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate, deep, intramuscular injection,
or 10ml diluted into 100 ml of normal saline given intravenously over
10-15 minutes.
Note: paraldehyde should only be used with glass syringes.
2. Phenobarbital (200 mg/ml). Should not be given intravenously,
except where artificial ventilation is available, and not at all if
the patient normally takes phenobarbital. The maximum rate of
infusion is 100 mg/min, to a maximum dose of 15 mg/kg.
3. Lignocaine, iv, 100 mg, by slow intravenous injection, followed by
50-100 mg of lignocaine in 250 ml of 5% dextrose at 1-2 mg/min.
Note: It is essential that this treatment is given with ECG
monitoring.
4. Diazepam, iv (10 mg in 2 ml), 40 mg in 500 ml of 5% dextrose, at a
maximum infusion rate of 100 mg/h.
5. Sodium valproate, iv (400 mg in 4 ml), 400-800 mg, iv, over
3-5 minutes (up to 10 mg/kg), followed by intravenous infusion, to a
maximum of 2.5 g/day (unlicensed).
Paediatric Doses
For children, dosing should be adapted as follows:
Diazepam 0.2-0.3 mg/kg intravenous.
Phenytoin 10-20 mg/kg intravenous.
Chlormethiazole 5-10 mg/kg per hour, which is equivalent to
0.6-1.25 ml/kg per hour.
The label must read:
Very toxic by inhalation, in contact with skin and if swallowed;
keep locked up; keep away from food, drink and animal feeding
stuffs; after contact with skin, wash immediately with plenty of
water; in case of accident or if you feel unwell, seek medical
advice (show the label where possible).
6.5 Waste Disposal
In the USA, any non-domestic waste containing endrin and its
metabolites must be treated as a hazardous waste. Specific
instructions are given for notification and incineration.
Owners/operators of vessels or onshore or offshore facilities must
notify the US Government (National Response Center) of any release of
endrin in or on navigable waters, adjoining shorelines, in the
contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land, or ground water) in an amount equal
to, or greater than, one pound (0.454 kg).
An owner or operator of a hazardous waste incinerator must achieve
99.99% destruction and removal efficiency for this substance.
6.6 Other Measures
Aquatic environment
The European Economic Community legislation has established limit
values for the discharge of, and quality objectives for, aldrin,
dieldrin, endrin, and isodrin in the aquatic environment.
The limit values for emission standards are:
(a) Plants producing aldrin and/or dieldrin and/or endrin, including
formulation of these substances on the same site, must:
* on a monthly average value, not exceed 3 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 2 g/litre of water discharged (to be complied with as from
1 January 1989).
* on a daily average value, not exceed 15 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 10 g/litre of water discharged (to be complied with as from
1 January 1989).
(b) For inland surface waters, estuary waters, internal coastal waters
other than estuary waters, territorial waters, for the compounds
aldrin, dieldrin, endrin, and isodrin together:
* 30 ng/litre (to be complied with as from 1 January 1989); and
10 ng/litre for aldrin, 10 ng/litre for dieldrin, 5 ng/litre for
endrin, and 5 ng/litre for isodrin (to be complied with as from
1 January 1994).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice. Rome, Food and
Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues., 3rd ed. Rome, Codex Committee on Pesticide
Residues.
FAO/WHO (1986) Codex maximum limits for pesticide residues. CAC/Vol.
XIII. 2nd ed., Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
IRPTC (1986) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. United Nations Environment
Programme, Industry and Environment Office, Paris, 80 pp..
UNITED NATIONS (1984) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by governments, 1st ed. revised, United
Nations, New York.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 vol., Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-1991. Geneva, World Health
Organization (unpublished document WHO/PCS/90.1).
WHO (in preparation) Environmental Health Criteria 130: Endrin.
Geneva, World Health Organization.
WHO/FAO (1978) Data sheets on pesticides: Endrin. Geneva, World
Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX. MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
(A) Initial management
1. Assess the patient, verify the diagnosis, remove false teeth and
place in the lateral semi-prone position, establish an airway.
2. Diazepam, iv (see Note 1), (10 mg in 2 ml), 0.15-0.25 mg/kg,
usually 10 mg (2 ml) bolus followed immediately by a further 10 mg
(2 ml) over 1-2 minutes. This may be repeated according to response.
3. Take blood for determination of anticonvulsant drug levels, blood
alcohol level, and blood sugar (5 ml of blood in a sugar tube), also
blood for determination of calcium (5 ml in a plain tube), and a drop
of blood to determine blood glucose.
4. If this shows a low blood glucose level: administer glucose 50%,
iv, 25ml, preferably by catheter, and not into a small distal vein.
If alcohol is likely to be a factor: administer thiamine, iv, 100 mg.
5. Phenytoin, iv, 250 mg in 5 ml, 10-15 mg/kg, no faster than 50 mg
(1 ml) per minute, by infusion pump or slow iv injection (see Note 2).
(B) If fits continue, transfer to an intensive care unit, and consult
an anaesthetist
6. Chlormethiazole, iv (8 mg/ml). A loading dose of up to 800 mg
(100 ml) over 10 minutes (10 ml/min); maintain with 0.5-1 ml/min
(4-8 mg).
7. Thiopental, iv, 5 mg/kg loading dose, then 1-3 mg/kg per hour, to
a maximum blood thiopental level of 100 mg/litre.
8. If this fails - consult a neurologist.
a Adapted from a guideline prepared by Guy's Hospital, London.
NOTES
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there is
concurrent use of other CNS depressant drugs, especially
phenobarbital.
Diazepam must not be given:
* intramuscularly;
* added to an intravenous infusion;
* with phenobarbital unless artificial ventilation is available.
Rectal diazepam (using a rectal administration set), 5 or 10 mg in
2.5 ml, may be used for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
* intramuscularly;
* by central line;
* into a dextrose infusion;
* with any other drug.
Intravenous phenytoin should be monitored with continous ECG
recording. If this is not available, it may be safer to use a diluted
solution of 250 mg (5 ml) in 250 ml of normal saline, no faster than
50 mg/min. The diluted solution should be used immediately, provided
there is no evidence of precipitation (this use of phenytoin is not
licensed).
OPTIONS
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate, deep, intramuscular injection,
or 10ml diluted into 100 ml of normal saline given intravenously over
10-15 minutes.
Note: paraldehyde should only be used with glass syringes.
2. Phenobarbital (200 mg/ml). Should not be given intravenously,
except where artificial ventilation is available, and not at all if
the patient normally takes phenobarbital. The maximum rate of
infusion is 100 mg/min, to a maximum dose of 15 mg/kg.
3. Lignocaine, iv, 100 mg, by slow intravenous injection, followed by
50-100 mg of lignocaine in 250 ml of 5% dextrose at 1-2 mg/min.
Note: It is essential that this treatment is given with ECG
monitoring.
4. Diazepam, iv (10 mg in 2 ml), 40 mg in 500 ml of 5% dextrose, at a
maximum infusion rate of 100 mg/h.
5. Sodium valproate, iv (400 mg in 4 ml), 400-800 mg, iv, over
3-5 minutes (up to 10 mg/kg), followed by intravenous infusion, to a
maximum of 2.5 g/day (unlicensed).
Paediatric Doses
For children, dosing should be adapted as follows:
Diazepam 0.2-0.3 mg/kg intravenous.
Phenytoin 10-20 mg/kg intravenous.
Chlormethiazole 5-10 mg/kg per hour, which is equivalent to
0.6-1.25 ml/kg per hour.
See Also:
Toxicological Abbreviations
Endrin (EHC 130, 1992)
Endrin (ICSC)
Endrin (FAO Meeting Report PL/1965/10/1)
Endrin (AGP:1970/M/12/1)
Endrin (WHO Pesticide Residues Series 4)
Endrin (WHO Pesticide Residues Series 5)
Endrin (IARC Summary & Evaluation, Volume 5, 1974)
 Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157, ENT 17251
OMS 197, and Mendrin
CAS chemical name: (1a,2,2a,3,6,6a,7,7a)-3,4,5,6,9,9-
hexachloro-1a,2,2a,3,6,6a,7,7a-
octahydro-2,7:3,6-dimethanonaphth[2,3-b]
oxirene (9CI-CAS).
Former CAS chemical name: 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,6,7,8,8a-octahydro-1,4-endo,endo-
5,8-dimethanonaphthalene)
IUPAC chemical name: (IR,4S,4aS,5S,6S,7R,8R,8aR)-
1,2,3,4,10.10-h exachloro-
1,4,4a,5,6,7,8,8a-octahydro-6,7-epoxy-
1,4:5,8-dimethano-naphthalene
CAS registry number:
Synonyms: Endrex, Experimental Insecticide 269,
Hexadrin, Nendrin, NCI-COO157, ENT 17251
OMS 197, and Mendrin
CAS chemical name: (1a,2,2a,3,6,6a,7,7a)-3,4,5,6,9,9-
hexachloro-1a,2,2a,3,6,6a,7,7a-
octahydro-2,7:3,6-dimethanonaphth[2,3-b]
oxirene (9CI-CAS).
Former CAS chemical name: 1,2,3,4,10,10-hexachloro-6,7-epoxy-
1,4,4a,6,7,8,8a-octahydro-1,4-endo,endo-
5,8-dimethanonaphthalene)
IUPAC chemical name: (IR,4S,4aS,5S,6S,7R,8R,8aR)-
1,2,3,4,10.10-h exachloro-
1,4,4a,5,6,7,8,8a-octahydro-6,7-epoxy-
1,4:5,8-dimethano-naphthalene
CAS registry number:  Note: The text on the label is optional, and is not required by
RID/ADR. The class number at the bottom of the main hazard
is not required by RID/ADR, but is not optional for the
other modes.
Endrin has been identified as a severe marine pollutant in the
International Maritime Dangerous Goods (IMDG) Code, therefore a
"Marine pollutant" mark is required for the transport by sea of all
concentrations greater than or equal to 1%.
Note: The text on the label is optional, and is not required by
RID/ADR. The class number at the bottom of the main hazard
is not required by RID/ADR, but is not optional for the
other modes.
Endrin has been identified as a severe marine pollutant in the
International Maritime Dangerous Goods (IMDG) Code, therefore a
"Marine pollutant" mark is required for the transport by sea of all
concentrations greater than or equal to 1%.
 The FAO specifications for plant protection products containing endrin
specify the composition and purity of the technical product and its
formulations. They also advise on methods for checking this. The
endrin content should be stated and may not differ by more than 4%
from this for the technical product (and up to 10% for some
formulations). Technical endrin should contain a minimum of 92%w/w
active material.
The European Economic Community legislation on the labelling of
pesticide preparations classified endrin in Class I/a for the purpose
of determining the label for preparations containing endrin and other
active ingredients.
The European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
The FAO specifications for plant protection products containing endrin
specify the composition and purity of the technical product and its
formulations. They also advise on methods for checking this. The
endrin content should be stated and may not differ by more than 4%
from this for the technical product (and up to 10% for some
formulations). Technical endrin should contain a minimum of 92%w/w
active material.
The European Economic Community legislation on the labelling of
pesticide preparations classified endrin in Class I/a for the purpose
of determining the label for preparations containing endrin and other
active ingredients.
The European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
 The label must read:
Very toxic by inhalation, in contact with skin and if swallowed;
keep locked up; keep away from food, drink and animal feeding
stuffs; after contact with skin, wash immediately with plenty of
water; in case of accident or if you feel unwell, seek medical
advice (show the label where possible).
6.5 Waste Disposal
In the USA, any non-domestic waste containing endrin and its
metabolites must be treated as a hazardous waste. Specific
instructions are given for notification and incineration.
Owners/operators of vessels or onshore or offshore facilities must
notify the US Government (National Response Center) of any release of
endrin in or on navigable waters, adjoining shorelines, in the
contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land, or ground water) in an amount equal
to, or greater than, one pound (0.454 kg).
An owner or operator of a hazardous waste incinerator must achieve
99.99% destruction and removal efficiency for this substance.
6.6 Other Measures
Aquatic environment
The European Economic Community legislation has established limit
values for the discharge of, and quality objectives for, aldrin,
dieldrin, endrin, and isodrin in the aquatic environment.
The limit values for emission standards are:
(a) Plants producing aldrin and/or dieldrin and/or endrin, including
formulation of these substances on the same site, must:
* on a monthly average value, not exceed 3 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 2 g/litre of water discharged (to be complied with as from
1 January 1989).
* on a daily average value, not exceed 15 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 10 g/litre of water discharged (to be complied with as from
1 January 1989).
(b) For inland surface waters, estuary waters, internal coastal waters
other than estuary waters, territorial waters, for the compounds
aldrin, dieldrin, endrin, and isodrin together:
* 30 ng/litre (to be complied with as from 1 January 1989); and
10 ng/litre for aldrin, 10 ng/litre for dieldrin, 5 ng/litre for
endrin, and 5 ng/litre for isodrin (to be complied with as from
1 January 1994).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice. Rome, Food and
Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues., 3rd ed. Rome, Codex Committee on Pesticide
Residues.
FAO/WHO (1986) Codex maximum limits for pesticide residues. CAC/Vol.
XIII. 2nd ed., Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
IRPTC (1986) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. United Nations Environment
Programme, Industry and Environment Office, Paris, 80 pp..
UNITED NATIONS (1984) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by governments, 1st ed. revised, United
Nations, New York.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 vol., Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-1991. Geneva, World Health
Organization (unpublished document WHO/PCS/90.1).
WHO (in preparation) Environmental Health Criteria 130: Endrin.
Geneva, World Health Organization.
WHO/FAO (1978) Data sheets on pesticides: Endrin. Geneva, World
Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX. MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
(A) Initial management
1. Assess the patient, verify the diagnosis, remove false teeth and
place in the lateral semi-prone position, establish an airway.
2. Diazepam, iv (see Note 1), (10 mg in 2 ml), 0.15-0.25 mg/kg,
usually 10 mg (2 ml) bolus followed immediately by a further 10 mg
(2 ml) over 1-2 minutes. This may be repeated according to response.
3. Take blood for determination of anticonvulsant drug levels, blood
alcohol level, and blood sugar (5 ml of blood in a sugar tube), also
blood for determination of calcium (5 ml in a plain tube), and a drop
of blood to determine blood glucose.
4. If this shows a low blood glucose level: administer glucose 50%,
iv, 25ml, preferably by catheter, and not into a small distal vein.
If alcohol is likely to be a factor: administer thiamine, iv, 100 mg.
5. Phenytoin, iv, 250 mg in 5 ml, 10-15 mg/kg, no faster than 50 mg
(1 ml) per minute, by infusion pump or slow iv injection (see Note 2).
(B) If fits continue, transfer to an intensive care unit, and consult
an anaesthetist
6. Chlormethiazole, iv (8 mg/ml). A loading dose of up to 800 mg
(100 ml) over 10 minutes (10 ml/min); maintain with 0.5-1 ml/min
(4-8 mg).
7. Thiopental, iv, 5 mg/kg loading dose, then 1-3 mg/kg per hour, to
a maximum blood thiopental level of 100 mg/litre.
8. If this fails - consult a neurologist.
a Adapted from a guideline prepared by Guy's Hospital, London.
NOTES
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there is
concurrent use of other CNS depressant drugs, especially
phenobarbital.
Diazepam must not be given:
* intramuscularly;
* added to an intravenous infusion;
* with phenobarbital unless artificial ventilation is available.
Rectal diazepam (using a rectal administration set), 5 or 10 mg in
2.5 ml, may be used for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
* intramuscularly;
* by central line;
* into a dextrose infusion;
* with any other drug.
Intravenous phenytoin should be monitored with continous ECG
recording. If this is not available, it may be safer to use a diluted
solution of 250 mg (5 ml) in 250 ml of normal saline, no faster than
50 mg/min. The diluted solution should be used immediately, provided
there is no evidence of precipitation (this use of phenytoin is not
licensed).
OPTIONS
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate, deep, intramuscular injection,
or 10ml diluted into 100 ml of normal saline given intravenously over
10-15 minutes.
Note: paraldehyde should only be used with glass syringes.
2. Phenobarbital (200 mg/ml). Should not be given intravenously,
except where artificial ventilation is available, and not at all if
the patient normally takes phenobarbital. The maximum rate of
infusion is 100 mg/min, to a maximum dose of 15 mg/kg.
3. Lignocaine, iv, 100 mg, by slow intravenous injection, followed by
50-100 mg of lignocaine in 250 ml of 5% dextrose at 1-2 mg/min.
Note: It is essential that this treatment is given with ECG
monitoring.
4. Diazepam, iv (10 mg in 2 ml), 40 mg in 500 ml of 5% dextrose, at a
maximum infusion rate of 100 mg/h.
5. Sodium valproate, iv (400 mg in 4 ml), 400-800 mg, iv, over
3-5 minutes (up to 10 mg/kg), followed by intravenous infusion, to a
maximum of 2.5 g/day (unlicensed).
Paediatric Doses
For children, dosing should be adapted as follows:
Diazepam 0.2-0.3 mg/kg intravenous.
Phenytoin 10-20 mg/kg intravenous.
Chlormethiazole 5-10 mg/kg per hour, which is equivalent to
0.6-1.25 ml/kg per hour.
The label must read:
Very toxic by inhalation, in contact with skin and if swallowed;
keep locked up; keep away from food, drink and animal feeding
stuffs; after contact with skin, wash immediately with plenty of
water; in case of accident or if you feel unwell, seek medical
advice (show the label where possible).
6.5 Waste Disposal
In the USA, any non-domestic waste containing endrin and its
metabolites must be treated as a hazardous waste. Specific
instructions are given for notification and incineration.
Owners/operators of vessels or onshore or offshore facilities must
notify the US Government (National Response Center) of any release of
endrin in or on navigable waters, adjoining shorelines, in the
contiguous zone or beyond the contiguous zone or to any other
environmental media (air, land, or ground water) in an amount equal
to, or greater than, one pound (0.454 kg).
An owner or operator of a hazardous waste incinerator must achieve
99.99% destruction and removal efficiency for this substance.
6.6 Other Measures
Aquatic environment
The European Economic Community legislation has established limit
values for the discharge of, and quality objectives for, aldrin,
dieldrin, endrin, and isodrin in the aquatic environment.
The limit values for emission standards are:
(a) Plants producing aldrin and/or dieldrin and/or endrin, including
formulation of these substances on the same site, must:
* on a monthly average value, not exceed 3 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 2 g/litre of water discharged (to be complied with as from
1 January 1989).
* on a daily average value, not exceed 15 g in effluent per tonne
of production capacity (g/tonne) or a concentration in effluent
of 10 g/litre of water discharged (to be complied with as from
1 January 1989).
(b) For inland surface waters, estuary waters, internal coastal waters
other than estuary waters, territorial waters, for the compounds
aldrin, dieldrin, endrin, and isodrin together:
* 30 ng/litre (to be complied with as from 1 January 1989); and
10 ng/litre for aldrin, 10 ng/litre for dieldrin, 5 ng/litre for
endrin, and 5 ng/litre for isodrin (to be complied with as from
1 January 1994).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice. Rome, Food and
Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues., 3rd ed. Rome, Codex Committee on Pesticide
Residues.
FAO/WHO (1986) Codex maximum limits for pesticide residues. CAC/Vol.
XIII. 2nd ed., Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964-present) Evaluation of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
IRPTC (1986) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished WHO document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. United Nations Environment
Programme, Industry and Environment Office, Paris, 80 pp..
UNITED NATIONS (1984) Consolidated list of products whose
consumption and/or sale have been banned, withdrawn, severely
restricted or not approved by governments, 1st ed. revised, United
Nations, New York.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 vol., Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-1991. Geneva, World Health
Organization (unpublished document WHO/PCS/90.1).
WHO (in preparation) Environmental Health Criteria 130: Endrin.
Geneva, World Health Organization.
WHO/FAO (1978) Data sheets on pesticides: Endrin. Geneva, World
Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.,
Lavenham, Lavenham Press Limited, British Crop Protection Council.
ANNEX. MANAGEMENT OF MAJOR STATUS EPILEPTICUS IN ADULTSa
(A) Initial management
1. Assess the patient, verify the diagnosis, remove false teeth and
place in the lateral semi-prone position, establish an airway.
2. Diazepam, iv (see Note 1), (10 mg in 2 ml), 0.15-0.25 mg/kg,
usually 10 mg (2 ml) bolus followed immediately by a further 10 mg
(2 ml) over 1-2 minutes. This may be repeated according to response.
3. Take blood for determination of anticonvulsant drug levels, blood
alcohol level, and blood sugar (5 ml of blood in a sugar tube), also
blood for determination of calcium (5 ml in a plain tube), and a drop
of blood to determine blood glucose.
4. If this shows a low blood glucose level: administer glucose 50%,
iv, 25ml, preferably by catheter, and not into a small distal vein.
If alcohol is likely to be a factor: administer thiamine, iv, 100 mg.
5. Phenytoin, iv, 250 mg in 5 ml, 10-15 mg/kg, no faster than 50 mg
(1 ml) per minute, by infusion pump or slow iv injection (see Note 2).
(B) If fits continue, transfer to an intensive care unit, and consult
an anaesthetist
6. Chlormethiazole, iv (8 mg/ml). A loading dose of up to 800 mg
(100 ml) over 10 minutes (10 ml/min); maintain with 0.5-1 ml/min
(4-8 mg).
7. Thiopental, iv, 5 mg/kg loading dose, then 1-3 mg/kg per hour, to
a maximum blood thiopental level of 100 mg/litre.
8. If this fails - consult a neurologist.
a Adapted from a guideline prepared by Guy's Hospital, London.
NOTES
1. Diazepam: A bolus injection of 10 mg may cause respiratory
depression and hypotension, which may be pronounced if there is
concurrent use of other CNS depressant drugs, especially
phenobarbital.
Diazepam must not be given:
* intramuscularly;
* added to an intravenous infusion;
* with phenobarbital unless artificial ventilation is available.
Rectal diazepam (using a rectal administration set), 5 or 10 mg in
2.5 ml, may be used for the immediate treatment of epilepsy instead of
intravenous diazepam.
2. Phenytoin must not be given:
* intramuscularly;
* by central line;
* into a dextrose infusion;
* with any other drug.
Intravenous phenytoin should be monitored with continous ECG
recording. If this is not available, it may be safer to use a diluted
solution of 250 mg (5 ml) in 250 ml of normal saline, no faster than
50 mg/min. The diluted solution should be used immediately, provided
there is no evidence of precipitation (this use of phenytoin is not
licensed).
OPTIONS
The following drugs may also be used:
1. Paraldehyde: 2 x 5 ml by separate, deep, intramuscular injection,
or 10ml diluted into 100 ml of normal saline given intravenously over
10-15 minutes.
Note: paraldehyde should only be used with glass syringes.
2. Phenobarbital (200 mg/ml). Should not be given intravenously,
except where artificial ventilation is available, and not at all if
the patient normally takes phenobarbital. The maximum rate of
infusion is 100 mg/min, to a maximum dose of 15 mg/kg.
3. Lignocaine, iv, 100 mg, by slow intravenous injection, followed by
50-100 mg of lignocaine in 250 ml of 5% dextrose at 1-2 mg/min.
Note: It is essential that this treatment is given with ECG
monitoring.
4. Diazepam, iv (10 mg in 2 ml), 40 mg in 500 ml of 5% dextrose, at a
maximum infusion rate of 100 mg/h.
5. Sodium valproate, iv (400 mg in 4 ml), 400-800 mg, iv, over
3-5 minutes (up to 10 mg/kg), followed by intravenous infusion, to a
maximum of 2.5 g/day (unlicensed).
Paediatric Doses
For children, dosing should be adapted as follows:
Diazepam 0.2-0.3 mg/kg intravenous.
Phenytoin 10-20 mg/kg intravenous.
Chlormethiazole 5-10 mg/kg per hour, which is equivalent to
0.6-1.25 ml/kg per hour.
