
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
ENDRIN
IDENTITY
Chemical name
1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-
octahydro-1,4-endo-endo-5,8-dimethanonaphthalene
Synonyms
Mendrin (R), Compound 269 (R)
Structural formula (see also Figure 1)
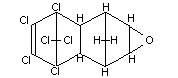 Other relevant chemical properties
Endrin (molecular weight 380.93) is a cream to light tan coloured
flowable powder which melts at 200+°C with decomposition. It has a
vapour pressure of 2 × 10-7mm Hg (Torr) at 77°F (25°C). It is stable
in the presence of ordinary alkaline reagents but tends to rearrange
to less insecticidally active substances in the presence of acids,
certain metal salts and catalytically active carriers. Endrin is
moderately soluble in benzene and acetone; sparingly soluble in
alcohols, paraffins, and xylene; insoluble in water.
Purity
Endrin, technical, 95 percent minimum
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The toxicology of this compound was evaluated by the WHO Expert
Committee on Pesticide Residues at the Joint FAO/WHO Meetings in 1963
and 1965 (FAO/WHO, 1964, 1965). Since that time considerable new
information has become available, and a completely revised monograph
has been produced.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
From early studies it was thought that, like other chlorinated
hydrocarbons, endrin, when fed to animals, was partly stored unchanged
in the tissues particularly in the body fat (Kiigemagi et al., 1958;
Street et al., 1957; Terriere et al., 1958, 1959 and Treon et al.,
1955). When fed at high levels it had been reported to be excreted in
milk and eggs (Ely et al., 1957; Street et al., 1957 and Terriere et
al., 1958). The ratio of the level in fatty tissue to the dietary
level has been estimated at 0.5-2, depending upon the dietary level
(Kiigemagi et al., 1958; Terriere et al., 1958 and Treon et al.,
1955).
Unlike the situation with its stereoisomer dieldrin, the extent of
storage of endrin is relatively small and the compound is eliminated
more quickly, due probably to its rapid biliary excretion (Cole et
al., 1970). Levels of the 9-keto metabolite of endrin in four human
fat samples were all less than 0.0004 ppm (Richardson, 1970).
A male rat was fed a dietary level of 30 ppm of 14C-labelled endrin
for eight days. About 60-70 percent excretion was noted from the first
day, and after three days the faeces contained more than 80 percent of
the administered radioactivity. On day 9, 84 percent had been
excreted, and there appeared to be a level of saturation after 6-7
days of feeding. The faeces contained about 75-80 percent
metabolities, of which there were at least two different compounds.
The fatty tissue stored 3-4 ppm of endrin, giving a storage ratio of
about 10. Compared to 84 percent excretion in the faeces, only about
0.5 percent was found in the urine (Ludwig, 1965, 1966).
The rapid rate of metabolism and excretion of endrin compared to that
of other chlorinated hydrocarbon insecticides has been confirmed by a
study on rats with and without bile fistula and on the isolated
perfused rat liver (Cole, et al., 1970; Altmeier, et al., 1969). In
rats on daily oral administration of 32 µg/kg, the storage reached a
state of equilibrium after 5-6 days. The half life in male rats was
three days and in female rats four days under these conditions (Klein
and Drefahl, 1970).
Biotransformation
The information available on the metabolism of endrin up to 1967 has
been reviewed (Soto and Deichmann, 1967; Brooks, 1969). The following
experimental data summarizes the pertinent information leading to the
current knowledge on the metabolism of endrin (see figure 1).
In rats, Klein et al. (1968a) and Richardson et al. (1970) found that
endrin is rapidly metabolized and excreted, principally in the faeces.
The faeces contain two metabolites as well as endrin itself. Baldwin
et al. (1970) have now found that the major faecal metabolite is a
secondary alcohol formed by substituting a hydroxyl group for one of
the hydrogens of the methano-bridge of endrin (II). The other faecal
Other relevant chemical properties
Endrin (molecular weight 380.93) is a cream to light tan coloured
flowable powder which melts at 200+°C with decomposition. It has a
vapour pressure of 2 × 10-7mm Hg (Torr) at 77°F (25°C). It is stable
in the presence of ordinary alkaline reagents but tends to rearrange
to less insecticidally active substances in the presence of acids,
certain metal salts and catalytically active carriers. Endrin is
moderately soluble in benzene and acetone; sparingly soluble in
alcohols, paraffins, and xylene; insoluble in water.
Purity
Endrin, technical, 95 percent minimum
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The toxicology of this compound was evaluated by the WHO Expert
Committee on Pesticide Residues at the Joint FAO/WHO Meetings in 1963
and 1965 (FAO/WHO, 1964, 1965). Since that time considerable new
information has become available, and a completely revised monograph
has been produced.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
From early studies it was thought that, like other chlorinated
hydrocarbons, endrin, when fed to animals, was partly stored unchanged
in the tissues particularly in the body fat (Kiigemagi et al., 1958;
Street et al., 1957; Terriere et al., 1958, 1959 and Treon et al.,
1955). When fed at high levels it had been reported to be excreted in
milk and eggs (Ely et al., 1957; Street et al., 1957 and Terriere et
al., 1958). The ratio of the level in fatty tissue to the dietary
level has been estimated at 0.5-2, depending upon the dietary level
(Kiigemagi et al., 1958; Terriere et al., 1958 and Treon et al.,
1955).
Unlike the situation with its stereoisomer dieldrin, the extent of
storage of endrin is relatively small and the compound is eliminated
more quickly, due probably to its rapid biliary excretion (Cole et
al., 1970). Levels of the 9-keto metabolite of endrin in four human
fat samples were all less than 0.0004 ppm (Richardson, 1970).
A male rat was fed a dietary level of 30 ppm of 14C-labelled endrin
for eight days. About 60-70 percent excretion was noted from the first
day, and after three days the faeces contained more than 80 percent of
the administered radioactivity. On day 9, 84 percent had been
excreted, and there appeared to be a level of saturation after 6-7
days of feeding. The faeces contained about 75-80 percent
metabolities, of which there were at least two different compounds.
The fatty tissue stored 3-4 ppm of endrin, giving a storage ratio of
about 10. Compared to 84 percent excretion in the faeces, only about
0.5 percent was found in the urine (Ludwig, 1965, 1966).
The rapid rate of metabolism and excretion of endrin compared to that
of other chlorinated hydrocarbon insecticides has been confirmed by a
study on rats with and without bile fistula and on the isolated
perfused rat liver (Cole, et al., 1970; Altmeier, et al., 1969). In
rats on daily oral administration of 32 µg/kg, the storage reached a
state of equilibrium after 5-6 days. The half life in male rats was
three days and in female rats four days under these conditions (Klein
and Drefahl, 1970).
Biotransformation
The information available on the metabolism of endrin up to 1967 has
been reviewed (Soto and Deichmann, 1967; Brooks, 1969). The following
experimental data summarizes the pertinent information leading to the
current knowledge on the metabolism of endrin (see figure 1).
In rats, Klein et al. (1968a) and Richardson et al. (1970) found that
endrin is rapidly metabolized and excreted, principally in the faeces.
The faeces contain two metabolites as well as endrin itself. Baldwin
et al. (1970) have now found that the major faecal metabolite is a
secondary alcohol formed by substituting a hydroxyl group for one of
the hydrogens of the methano-bridge of endrin (II). The other faecal
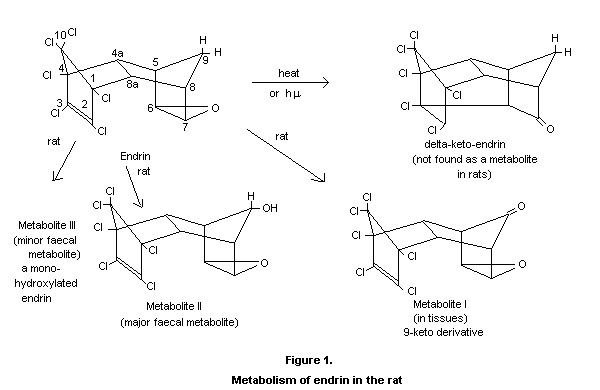 metabolite is also an alcohol. Three days after a single oral dose of
14C-labelled endrin, approximately half of the 14C radioactivity
remained in the bodies of the rats. This material was principally one
metabolite which was identified as 9-keto endrin (I), an oxidation
product of the secondary alcohol found in faeces.
When 14C-labelled endrin was applied orally to rabbits at 0.5 mg/kg
body-weight and at three and four day intervals, four metabolites were
isolated from the urine which appear to have the following chemical
natures: A (40% of excreted radioactivity) is a conjugate compound of
a hydroxy derivative of endrin; B (12%) is a monohydroxy derivative of
an unbridged endrin isomeric ketone; C (40%) is the 4a-hydroxyendrin;
D (8%) has a molecular weight of 420 and the C-C double bond intact in
the chlorinated ring. None of these compounds is the delta-keto endrin
(Korte and Porter, 1970).
Effects on enzymes and other biochemical parameters
In monkeys which had received exposure to an unspecified quantity of
endrin, there were significant changes in the enzymes serum
glutamic-oxaloacetic transaminase and serum glutamic-pyruvic
transaminase (Barth, 1967).
Elevation of serum alkaline phosphatase has been observed in rats fed
25 ppm and possibly 5 or 1 ppm of endrin for 16 weeks (Nelson et al.,
1956) but not in dogs fed 4 ppm of endrin for two years (Jolley et
al., 1969) nor in human subjects occupationally exposed to unspecified
levels of endrin (Shell, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
See under "Long-term studies" (Witherup et al., 1970).
Rat
Commencing at weaning, varying numbers of rats of mixed sex were fed
dietary levels of 0, 2, 6 or 12 ppm of endrin throughout their
lifetime. No primary malignant hepatic tumours were found in any
animals upon histological examination. Two benign hepatic tumours
(haemangiomas) were found in one male control rat and the other in a
female fed 6 ppm of endrin. Tumour incidence in other tissues was also
not significantly different between the control and experimental
animals (Diechmann et al., 1970).
Special studies on reproduction
Chicken egg
Hen eggs were injected with 0.5 or 5 mg per egg. The hatching rate was
40 or 20 percent, respectively (Dunachie and Fletcher, 1966).
When endrin was injected into the yolk of fertile eggs incubated for
seven days in amounts of 0.2 and 2.0 mg/egg, the hatchability was 40
and 6.9 percent, respectively (Smith et al., 1970).
Quail
No eggs were produced from quail which received 1 ppm of endrin in
their diet either as winter maintenance or during the reproductive
period (DeWitt, 1965).
Pheasant
In pheasants there was reduced egg production when fed 10 ppm of
endrin but not at 2 ppm or less. Survival of the chicks to two or six
weeks was also markedly reduced at 10 ppm, but not at lower doses
(DeWitt, 1965).
Mouse
Groups of male and female mice were fed endrin at dietary levels of 0
or 5 ppm for 30 days. Test and control mice were then randomly paired
and continued on the test diet for a further 90 days, there being a
total of 101 pairs in the group fed endrin. The first litters were
significantly smaller than the control group. The time taken to
produce the first litter was not significantly different between the
two groups (Good and Ware, 1969).
Deer mouse
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus manicalatus) of varying ages were fed dietary levels of
0, 1, 2, 4 or 7 ppm of endrin over intermittent periods between which
times the animals were either fed a normal diet or were subjected to
48 hours starvation. The animals were sacrificed by exposing them to
cold stress at -16°C and the time of death recorded. During feeding,
parental mortality increased in proportion to the level of endrin.
Young animals were more susceptible than old. Starvation increased
mortality in all test groups but not in the controls; this effect was
more evident with increasing dose levels. Litter production frequency
and mean litter size before and during experimental feeding were
similar. However, post-natal mortality prior to weaning increased in
the young from parents fed 4 or 7 ppm. Endrin adversely affected the
survival time during cold stress in the females but not in the males
(Morris, 1968).
Rat
In a later study from the same laboratory, groups of ten male and 20
female rats were fed dietary levels of 0, 0.1, 1.0 and 2.0 ppm of
endrin over a period of three generations. The F0 generation was
mated after 79 days on the test diets, and the males were rotated as
in the previous experiment.
The young from the first litter were discarded at weaning and the
parents mated again after ten days to form the F1b generation. Young
from this generation were mated when 100 days old, and this protocol
was followed for three generations, using the second litter in all
cases. The size of the litter in the F3 generation from the 2.0 ppm
group was significantly larger than that from the controls. Mortality
was high in the controls, which resulted in a greater percentage
survival in the F3a litters in the 0.1 ppm group and in all F3b
litters in the test groups. The weights of weanlings were comparable
to the controls except in the F3a litters from the 0.1 ppm, which
were significantly less due probably to the large litter sizes in that
group. Examination of the F3b weanlings revealed no differences in
organ to body-weight ratios. It was stated that there were no
histological abnormalities, but details of the pathology were not
available. Fertility, gestation, viability and lactation indices did
not indicate that endrin affected any of these parameters (Hine et
al., 1968).
Special studies on the photoisomerization product of endrin
When endrin is irradiated with short wavelength ultraviolet light, the
delta-keto compound is formed in 37 percent yield as well as an
aldehyde in 9 percent yield. Under the influence of sunlight, only the
ketone is formed. The ketone is about a quarter as toxic to rate as
endrin and like endrin is more toxic to the male than to the female
(Soto and Diechmann, 1970) (see also "Fate of residues").
Acute toxicity
The major clinical manifestation of endrin intoxication in man
involves convulsions of several minutes duration which may be isolated
and followed by semiconciousness for 15-30 minutes, with complete
recovery after 2-4 weeks. More serious symptoms are continuous
convulsions, high fever and decerebrate rigidity prior to death. Mild
symptoms of poisoning include dizziness, weakness of the legs,
abdominal discomfort and nausea. Temporary deafness and insomnia may
also occur. It has been estimated, based upon reports of outbreaks of
poisoning, that 0.2-0.25 mg/kg body-weight will produce a single
convulsion in man, and that repeated fits will result from 1 mg/kg
(Hayes, 1963).
LD50
Animal mg/kg body-weight References
(oral)
Rat, adult (F) 7 Treon et al., 1955
Rat, young (F) 17 Treon at al., 1955
Rat, adult (F) 40-43 Speck and Maaske, 1958
Rat, young (M) 29 Treon at al., 1955
Rabbit (F) 7-10 Treon at al., 1955
Guinea-pig 16-36 Treon at al., 1955
Monkey 3 Treon at al., 1955
Monkey 12 Barth, 1967
The acute toxicity of endrin appears to be influenced by the diet.
Three groups each comprising about 100 male rats were fed for 28 days
either a normal diet, a normal protein diet containing protein only as
casein or a low protein diet. The acute toxicity to endrin was then
determined by a single oral administration of the pesticide. The LD50
values were 27, 17 and 7 for the animals fed the respective diets,
indicating an approximately four-fold increase in toxicity between the
normal and low-protein diet as well as an effect due to the type of
protein fed (Boyd and Stefec, 1969).
Short-term studies
Quail
Groups each of 40 quail, starting when one day old, were fed dietary
levels of 0, 0.5, 1, 5, 10, 20 or 50 ppm in their diet. Survival was
adversely affected in all the test groups, and there were no survivors
beyond two weeks in the birds fed 10 ppm or more. Food consumption was
abnormally low. Symptoms involved lack of muscular coordination,
tremors, bedraggled appearance and rigidity with occasional convulsive
movements (De Witt, 1956).
Chicken
Groups each of 20 seven-day old chicks were unaffected by a diet
containing 0, 1.5 or 3 ppm of endrin. When the concentration was
increased to 6 or 12 ppm, the birds became highly excitable, failed to
gain as much weight as the controls and the survival rates over a 12
week period were 85 and 5 percent, respectively, compared to 100 per
cent in the controls (Sherman and Rosenberg, 1954).
Pheasant
Day-old pheasants, in groups of 40, did not survive beyond eight days
when fed dietary levels of 5 or 20 ppm. Reduced food consumption
occurred, and the symptoms were the same as those seen in quail (De
Witt, 1956).
Rat
Groups comprising five male and five female rats were fed dietary
levels of 0, 1, 5, 25, 50 or 100 ppm of endrin for up to 16 weeks. All
of the group fed 100 ppm died within the first two weeks, and only two
rats fed 50 ppm and three fed 25 ppm survived. Three males fed 5 ppm
also died; the other animals were continued on the test diet for the
full 16 weeks. Weight loss was roughly dose related but was evident in
all test groups, as was hypersensitivity to tactile stimuli. There was
an initial drop in serum alkaline phosphatase during the first three
to eight weeks' feeding, which was then followed by an increase at all
dose levels. At the end of the 16 weeks, the phosphatase level was
elevated above the controls in all the test groups, the levels being
highest in the groups fed 25 and 50 ppm (Nelson et al., 1956).
However, other statisticians have considered that the elevation of
serum alkaline phosphatase was not significant in the groups fed 1 and
5 ppm of endrin (Williams, 1966).
Dog
In a series of experiments, dogs were fed diets containing from 1 to
50 ppm endrin along with control groups. Two of four animals fed a
diet containing 8 ppm and the one fed 5 ppm died. The two surviving
dogs on 8 ppm were kept on the diet for about six months and then
sacrificed; increased organ to body-weight ratios for the liver,
kidney and brain were found, and histopathological examination showed
degeneration of kidney tissue. Three of four dogs on 4 ppm of endrin
survived, and there were no symptoms in dogs fed 1 or 3 ppm (Treon et
al., 1955).
In an experiment of about 19 months' duration, groups comprising two
male and two female dogs were placed on diets containing 0, 1 or 3 ppm
of endrin. All dogs on 3 ppm had increased organ to body-weight ratios
for the kidney and heart. Some female dogs fed 1 or 3 ppm of endrin
had a renal abnormality characterized by a slight tubular vacuolation;
this change was also observed in the female control dog. Male dogs in
both control and test groups had normal viscera (Treon et al., 1955).
Groups comprising seven male and seven female dogs were fed dietary
levels of 0, 0.1, 0.5, 1.0, 2.0 or 4.0 ppm of dieldrin for two years.
Scheduled autopsies were performed on two dogs of each sex from the 0,
1.0 and 4.0 ppm groups at six and 12 months. There were no deaths due
to the treatment nor were there any changes in body-weight increase or
food consumption in any group. The only clinical abnormalities were in
one female and two male dogs fed 4.0 ppm and one female fed 2.0 ppm
that showed evidence of, or were observed having, convulsions; the
earliest incidence in a male dog after five months on 4.0 ppm. The
only changes in organ weights were occasional slight increases in
liver or liver to body-weight ratios in the dogs fed 2.0 and 4.0 ppm.
After two years, pathological examination showed slight vacuolation of
hepatic cells in the females and diffuse pigmentation in one male and
all females. At 4.0 ppm, vacuolar degeneration and diffuse brown
pigment in the hepatic cells was evident in all dogs, without any sex
differentiation. In two only of the dogs, which had convulsions,
autopsies revealed some pathological changes in the brain. All other
organs in the dogs fed 2.0 or 4.0 ppm and all organs in the dogs fed
1.0 ppm or less showed no morphological changes which were considered
to be attributable to feeding endrin. There were no significant
changes in the blood picture or in the chemical or physical
characteristics of the urine attributable to endrin. After two years,
levels of liver enzymes, prothrombin time bromsulphthalein clearance,
serum protein electrophoresis, glucose, urea nitrogen, cholesterol,
calcium, inorganic phosphorus, total bilirubin or uric acid showed no
changes attributable to endrin feeding (Jolley et al., 1969).
Cattle and sheep
Cattle and sheep were not affected by 5 ppm of endrin in their diet
for 112 days (Radeleff, 1956).
Long-term studies
Mouse
A total of 1600 mice in equal numbers of each sex, consisting of one
inbred and one hybrid strain, were divided into four groups, two of
which were fed a control diet and the other two fed 0.3 or 3.0 ppm of
endrin. Feeding the test diet was started at five weeks of age and
continued through out their normal lifespan, or until sacrifice.
Because of an early high incidence of fibroadenomas occurring in both
control and test groups in the hybrid strain, all the females of that
strain were sacrificed after 72 weeks for pathological examination. A
few of the mice, fed 3.0 ppm only, displayed convulsions in the early
stages of feeding but recovered and survived. Mortality was not
adversely affected by endrin, nor was body-weight or food intake. No
haematological abnormalities were evident except in two males in the
hybrid group fed 0.3 ppm, which had severe leukaemia. In either sex,
the total number of neoplasms was not influenced by the endrin content
of the diet, except in the case of hepatomas in the females of the
hybrid strain, which were significantly higher than the controls in
the mice of the group fed 3.0 ppm, and sacrificed between weeks 53 and
60 of the feeding period. Because of a relatively high incidence of
hepatomas in one group of controls of this strain, the increase at the
3.0 ppm level was considered not due to endrin. It was noted that in
no animals of either sex were there any metastases of the hepatomas
into the lungs (Witherup et al., 1970).
Rat
In a two year experiment, groups each of 20 male and 20 female rats
were given diets containing 0, 1, 5, 25, 50 and 100 ppm of endrin.
Concentrations of 50 and 100 ppm were lethal within a few weeks. The
concentration of 25 ppm increased the mortality rate of the females.
Non-survivors at the three higher levels exhibited diffuse
degeneration of the brain, liver, kidneys and adrenal glands. The
survivors in the two higher levels showed degenerative changes in the
liver only, while those fed at the lower levels had normal viscera.
The level of 5 ppm caused an increase in liver to body-weight ratio in
males and an increase in kidney to body-weight ratio in females. There
was no effect at the 1 ppm level (Treon et al., 1955).
OBSERVATIONS IN MAN
A total of 874 persons were hospitalized, and there were 26 deaths in
several outbreaks of poisoning in Saudi Arabia in 1967 due to
consumption of bread containing endrin. Approximate average levels in
the bread in various outbreaks were 48, 1500 or 400 ppm, corresponding
to a percentage of fatalities of 1.4, 9.5 and 0.4, respectively, among
those poisoned. Signs and symptoms were typical of central nervous
system stimulation and all survivors rapidly returned to normal
(Weeks, 1967).
Three persons in Egypt experienced convulsions after eating bread
containing 126 to 176 ppm of diedrin. There were no deaths (Coble et
al., 1967).
In an incident in the UK, 59 people became ill from ingesting bread
which contained about 150 ppm of endrin, but there were no deaths
(Davies and Lewis, 1956). The maximum amount of endrin consumed has
been estimated to have been 1 mg/kg body-weight (Zavon, 1961).
Studies in human subjects experiencing intoxication from endrin (Coble
et al., 1967, Weeks, 1967) and from occupational workers (Hayes and
Curley, 1968, Jager, 1970) have demonstrated that endrin rapidly
disappears from the blood in cases of acute intoxication and cannot be
detected in the fat or blood of people exposed to endrin unless
symptoms of intoxication are evident.
Endrin has not been reported to be found from studies involving levels
of organochlorine pesticides in human body fat in India, UK or USA
using analytical methods sensitive to <0.03 ppm (Dale et al., 1965;
Hayes et al., 1965, Robinson et al., 1965 and Zavon et al., 1965).
Levels of the 9-keto metabolite of endrin in four human fat samples
were all less than 0.0004 ppm (Richardson, 1970).
Among workers in a plant manufacturing endrin and a number of other
pesticides, no detectable amounts of endrin were found in samples of
plasma, fat or urine. Exposure was for an average time of 2,106 hours.
Based upon the limit of detection, the levels of endrin, were <0.0030
ppm in plasma, <0.03 ppm in fat and <0.0016 ppm in urine. Endrin
has, however, been detected in the serum and urine of people who
received amounts sufficient to produce intoxication (Hayes and Curley,
1968).
Serum alkaline phosphatase was determined in 30 workers who had been
exposed to endrin for periods from six weeks to eight years. There was
no difference in the levels found in the exposed group and those found
in a group comprising nine unexposed individuals, nor was there any
relationship detected between the phosphatase levels and the duration
of exposure of the workers (Shell, 1965).
In workers exposed to endrin during periods up to eight years, no
significant changes in the level of serum alkaline phosphatase were
observed during a 13-month observation period (Van Dijk, 1968).
It is estimated that the blood level of endrin below which no signs or
symptoms of intoxication occurs is in the range of 0.05 - 0.100 µg/ml.
Measurable blood levels (detection level 0.005 µg/ml) occur only after
gross over-exposure. The half-life of endrin appears to be
approximately 24 hours. Medical control of a group of workers exposed
over periods up to 13 years has failed to show any effects of
long-term exposure. The blood picture, results of urinalysis,
activities of serum glutamic-oxaloacetic and glutamic-pyruvic
transaminases, alkaline phosphatase and lactic dehydrogenase remained
all within normal limits. Occasional electroencephalographic changes
returned to normal. Absenteeism due to disease or accidents was
comparable to that of a control group. Because of the short half-life
of endrin, it is (unlike dieldrin) impossible to calculate the average
level of exposure of the workers to endrin (Jager, 1970).
A decrease in the blood levels of pp'-DDE and an increased excretion
of 6-ß-hydrocortisol in relation to 17-hydroxy-corticosteroids was
present in the workers employed in manufacturing and who were exposed
to endrin and its intermediates. The compound responsible is not known
and further studies are in progress (Jager, 1970).
COMMENTS
The primary site of action of endrin is the central nervous system.
This fact is evidenced by convulsions which result from acute
poisoning and from administration of repeated relatively high doses.
Unlike dieldrin, endrin is rapidly metabolized by animals; the storage
in the fat of animals is very low compared with other compounds of
similar chemical structure. In rats, it is excreted mainly in the
faeces as endrin, 4a-hydroxyendrin, and an unidentified endrin
alcohol; a third metabolite, 9-keto-endrin, is stored in the tissues.
In rabbit, the main route of excretion is the urine, in which four
metabolites were demonstrated, one of which is the 4a-hydroxyendrin.
The plant metabolite of endrin, delta-keto endrin, is rapidly
metabolized by animals. Three metabolites were found in rabbit urine
after oral administration of delta-keto endrin. It is understood that
delta-keto endrin is unlikely to be formed under conditions of good
agricultural practice, and that the compound is less toxic to mammals
than endrin.
A long-term study in mice failed to produce conclusive results
relative to the carcinogenic potential of endrin. Endrin is more
acutely toxic to animals fed a low protein diet. Reproduction studies
with endrin in several species revealed no influence of endrin on
maturation, but foetal and postnatal mortality were increased. There
is no evidence of teratogenic activity. The two-year studies in the
dog and rat are used as a basis for determining a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1 ppm in the diet, equivalent to 0.05 mg/kg body-weight/day
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
The most important of all endrin uses, worldwide, and accounting for
some 80% of all endrin applied is as a spray for the control of
insects of cotton. It is also used extensively for the control of
insect pests in rice, to some extent in sugar cane and to a limited
extent in grain crops and sugar beets. In Australia, endrin is used
for insect control on tobacco and crucifers.
Post-harvest treatments
Endrin is accepted and occasionally used in some countries for rodent
control in orchards, where it is sprayed onto the ground under the
trees either in autumn or springtime. Rates vary from 2.4 kg/ha for
deciduous fruits in the United States to 0.02 or 0.03% solutions,
often in combination with mineral oil, in Australia.
Seed treatments
Endrin is used as a seed treatment on cotton at 2 oz /100 lb seed in
the United States and on beans at 1 oz /30 lb seed in Australia.
TABLE I
Summary of Registered Uses
Rate of
Crop application No. of Pre-harvest
(kg/ha) applications interval (days)
Cotton 0.25-0.50 1-12 none
Rice 0.2-0.50 1-4 30
Sugar cane 0.25 (only 1-8 45
granular)
Wheat 0.25-0.50 1 45
Barley " " "
Oats " " "
Sorghum " " "
Tobacco 0.25-0.5 - -
Crucifers 0.04% soln. - -
Potatoes 0.8 (USA) - -
0.03% soln.
(Australia)
Sugar beets 0.4 1-2 60
Other uses
Endrin has a minor use in the United States as a bait formulation for
control of cutworms in corn and potatoes. It is applied to the soil
surface at 0.8 kg/ha, and preharvest intervals are observed.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cotton seed and its products
From the view point of residues of endrin arising in food products,
cotton seed and its products are of primary interest; the refined
cottonseed oil is used for cooking or margarine manufacture, while the
extracted cake is used as cattle feed. Therefore residue levels in
cottonseed oil products which may reach the food consumer are
determined not so much by levels in products harvested in the field,
but to an appreciable extent by mill processes.
The effect of extraction processes on endrin residues was studied by
Smith et al. (1968) using crude cottonseed oil and soy bean oil
fortified with 1 ppm (mg/g) endrin. They found that alkali washing and
bleaching did not have a marked effect but that deodorization by
vacuum steam stripping effectively reduced endrin levels to below the
limits of detection (0.03 ppm). These authors also quote unpublished
work by Evans of the USDA using radio-labelled endrin. At initial
levels of up to 3.5 ppm in the crude oil, 96 percent of the added
radioactivity was lost during the process of steam stripping. The
effectiveness of the deodorization step in removing endrin residues
from vegetable oils was further demonstrated by Barrantine and Cain
(1970) in studies of commercial scale operations. Endrin residues are
summarized in Tables II and III. (Shell Development Co. Reports,
RES-64-3. RES-64-9; Shell Chemical Co., New York PRL-67-24; Velsicol
Corp., unpublished data).
The cottonseed cake left after the extraction of oil commonly contains
1%-5% oil. These cottonseed cakes are widely used as an important
ingredient of cattle feed concentrates and could contribute up to 20%
of the daily diet of a dairy cow. The relationship between cattle feed
and milk residues was studied by Williams and Mills (1964), Kiigemagi
et al., (1958), and Ely et al., (1957). Consideration of their
combined results indicates that at feed levels in the region of
0.25-2.0 ppm endrin in total feed, milk residues will eventually reach
0.04 times the concentration in the total feed, if feeding is
continued until a plateau is reached. Both Williams and Kiigemagi
found that when endrin was withdrawn from the feed, levels in the milk
fell fairly quickly. At 0.25 and 0.75 ppm in feed, levels fell to
0.002 ppm in six weeks after endrin was withdrawn (Kiigemagi, 1958).
At the 2 ppm feed level, residues fell from 0.06 to 0.02 ppm in the
same period. In Williams' experiments residue from the highest feed
level (0.50 ppm in whole dry feed) fell to 0.002 ppm in about 15 days.
In rice, rice bran and rice straw
The results of experiments in a number of rice growing regions are
summarized in Table IV.
TABLE II
Residues of endrin in whole cotton seed, U.S.A.
Dosage/ Harvest Residue ppm
No.of applic. interval delta-
Location applics. (lb/acre) (days) endrin keto
Oklahoma control - - <0.004 N.D.
2 0.5 58 <0.004 to
0.013 N.D.
2 1.0 58 <0.004 N.D.
Louisiana 4-5 2.67(total) - 0.02 to <0.02
0.03
Texas not stated 0.5 (total) - <0.04 <0.04
1.0 (total) - 0.06 to <0.04
0.10
2.0 (total) - <0.04 <0.04
TABLE III
Endrin residues in crude and alkali-extract
Cottonseed oil obtained from endrin treated cotton in Texas, U.S.A.
Residues in oil - ppm
Dosage rate Post harvest Crude Alkali extracted
lb/acre total interval-days Endrin delta-Keto Endrin delta-Keto
Control - <0.01 <0.03 <0.01 <0.03
0.5 not stated 0.05 <0.03 0.05 <0.03
1.0 not stated 0.43* <0.03 0.39* <0.03
1.0 not stated 0.05 <0.03 0.05 <0.03
2.0 not stated 0.04 <0.03 0.01 <0.03
Control - <0.01 - <0.01 -
0.4, ten times 5 0.05 - <0.01 -
10 <0.01 - <0.01 -
20 <0.01 - <0.01 -
30 <0.01 - <0.01 -
0.8, ten times 5 0.02 - <0.01 -
10 0.03 - <0.01 -
20 0.08 - <0.01 -
30 0.01 - <0.01 -
Control - <0.02-<0.03 - - -
0.4 8 0.02 - - -
13 <0.02 - - -
28 <0.02-<0.07 - - -
52 0.04 - - -
0.8 8 0.07 - - -
13 "neg" - - -
28 <0.02 - - -
42 <0.02-0.02 - - -
* These figures are regarded as exceptional in light of all other available data.
Although neither rice bran or straw is reported to be an appreciable
item of international trade, residues may be of importance insofar as
they may persist in animal products. A summary of data provided by the
references in Table IV shows bran residues for 11 sites to range from
<0.01 to 2.30 ppm, with a mean of 0.35 ppm. The data are heavily
weighted by one figure from India; without this figure, the range is
<0.01 to 0.80 ppm, with a mean of 0.15 ppm. Rice straw residues from
the same sources (25 samples) ranged from 0.04 to 2.90 ppm, with a
mean of 1.02 ppm. Residues of delta-keto endrin were found in bran and
straw containing high residues of endrin but seldom exceeded 10% of
the total residue and never exceeded 20%.
Rice bran is used mainly as a component of locally produced poultry
feed. The significance of endrin residues in poultry feed in giving
rise to residues in meat and eggs may be judged from data in the
published literature.
Cummings et al., (1967) fed diets containing between 0.05 and 0.45 ppm
endrin to White Leghorns for 14 weeks and found residues ranging from
<0.01 to 0.06 ppm endrin in breast meat and from 0.25 to 3.5 ppm
endrin in depot fat. Similar diets gave endrin residues of 0.03 to
0.32 ppm in eggs as estimated from graphs (Cummings et al., 1966).
Terriere et al. (1959) fed diets containing 0.10 to 0.75 ppm endrin
for 6 weeks and found <0.1 to 0.2 ppm endrin in breast meat, <0.1 to
0.3 ppm in leg meat, 0.6 to 3.6 ppm in depot fat and <0.1 to 0.3 ppm
in eggs. A feed level of 2.25 ppm for 6 weeks gave 17.0 ppm in depot
fat.
In sugar cane
In experiments extending from 1957 to 1965, Shell Development Co.
applied endrin to sugar cane in Louisiana and Florida. When 0.3 lb
ai/acre of 2 percent granular was applied at 3-6 applications and
postharvest intervals ranging from 12-120 days were observed, average
endrin residues ranging from <0.01 to 0.05 ppm were obtained.
Keto-endrin residues were determined in four samples and did not
exceed <0.02 on the average. Residues in control samples ranged from
<0.01 to 0.04 ppm. No endrin was detected in molasses or refined
sugar prepared from the treated cane. One group of samples treated
with 0.5 lb ai/acre of E. C. had <0.1 ppm endrin (sensitivity limit
of the method). When 2 percent granular was applied four times at 1.0
lb ai/acre and postharvest intervals of 7-45 days were observed, the
endrin residues ranged from 0.04 to 0.08 ppm. The same formulation
applied four times at 0.22 lb ai/acre and eight times at 0.23 to 0.29
lb ai/acre resulted, after postharvest intervals of 71 and 64 days,
respectively, in endrin residues of <0.10 ppm. (Shell Development Co.
Reports RES-61-53, RES-63-159 DLI-136 (1963), DLI-157 (1964),
RES-64-1, DLI-160 (1965), RES-58-48A, RES-57-44.
In grains
Data obtained on a variety of small grains in the U.S.A. are
summarized in Table V.
In India, endrin (2% granular) was applied to sorghum at 0.4 kg ai/ha
for 4 to 6 applications. Samples taken from 42 to 75 days after last
treatment had residues in grain ranging from <0.01 to 0.02 ppm endrin
and in straw ranging from 0.14 to 0.70 ppm endrin. Control samples had
<0.01 to 0.02 ppm in grain and 0.03 to 0.31 ppm in straw (Shell
Research Ltd., Report WKGR 0071/70).
In the U.S.A., endrin E.C. was applied to sorghum once at either 0.25
or 0.50 lb ai/acre. A pre-harvest interval of 111 days resulted in
endrin residues of <0.02 ppm in grain, <0.02 ppm in straw, and
<0.02 ppm in silage (73 days PHI). A pre-harvest interval of 63 days
resulted in endrin residues of <0.02 ppm in grain, 0.03 and 0.09 ppm
in straw, and <0.02 and 0.15 ppm in silage (39 days PHI); the latter
value is suspect, according to the analysts (Shell Chemical Co.,
Report PRL-66-113). A pre-harvest interval of 29 days resulted in
residues of <0.05 and 0.05 ppm in grain (Velsicol Corp., Report
TSR-2575).
Single applications of endrin E.C. to sweet corn (maize) in the U.S.A.
at 0.25 or 0.50 lb ai/acre, 37 days before harvest, resulted in
residues of <0.02 ppm of endrin in grain and husk. Some controls gave
an apparent residue of 0.02 to 0.03 ppm (Shell Chemical Co., Report
PRL 66-67g).
In apples
Experiments conducted by Shell in the U.S.A. in which endrin E.C. or
W.P. was applied at 1.5 to 4.0 lb ai/acre to the soil of orchards gave
no detectable residues in whole apples at harvest time; detection
limits were 0.01-0.002 ppm for the methods employed (Shell Development
Co., Report RES-61-60, Shell Chemical Co., Report PRL-69-119, Ibid,
PRL-69-95). In recent studies by Horsfall et al. (1970), picked fruit
residues ranged from <0.002-0.003 ppm. In fallen fruit, residues were
sometimes higher, ranging up to 0.023 ppm.
FATE OF RESIDUES
General comments
Endrin undergoes rearrangement to delta-keto endrin in sunlight, in
the presence of strong acids, and by thermal treatment. Endrin also
undergoes thermal and photo degradation to yield small amounts of the
aldehyde SD 7442. Delta-keto endrin can undergo a rearrangement to the
"bird cage alcohol" when treated with very dry Florisil adsorbent
(Korte and Porter, 1970).
TABLE IV
Endrin residues in polished and unpolished rice
Applic. rate Harvest Residues, ppm
kg am/ha × interval, Polished Brown
Country Formulation no. of applic. days rice rice Reference
India 2% gran. 0.2, 0.4, 0.6, 49-79 <0.01 <0.01-0.01 Shell Res.Ltd.Report, WKTR 0023/69
2.0 × 4-7
control - - <0.01 <0.01
Venezuela E.C. 0.29 × 1 95 0.02 0.03 Ibid,WKGR 0046/70
Thailand control - - <0.01 <0.01 Ibid,WKGR 0087/70
2% gran. 0.2, 0.4, 0.4 56-83 <0.01 <0.01
20% E.C. 0.16 × 3 46-72 <0.01 <0.01-0.23*
Philippines control - - <0.01-0.03 <0.01-0.03 Ibid,WKGR 0078/70
2% gran. 0.2, 0.4, 0.4 44-60 <0.01-0.02 <0.01-0.05
20% E.C. 0.16 × 3 33-49 <0.01-0.03 <0.01-0.03
Indonesia control - - - <0.01 Ibid,WKTR 0078/68
20% E.C. 0.2-0.62 21 - 0.03-0.05
× 7 or × 8
Thailand 20% gran. 0.4 X3, X4 not <0.02 - Ibid,BEGR.0045/70
0.8 × 3 stated (Endrin and Keto -
Endrin Keto Endrin Keto
India control - - <0.02 <0.02 0.09 <0.02 Ibid
2% gran. 0.4 - 0.02 <0.02 0.23 0.03
0.8 45 0.04 <0.02 0.47 0.04
* One exceptional result - reason not identified
TABLE V
Endrin residues in small grains - U.S.A.
Applic. rate, Postharvest Endrin residues,
lb ai/acre interval, ppm
Crop × no. of applic. days Grain Straw Reference
Wheat 0.25 × 1 26 0.03 - Shell Dev. Co.
0.50 × 1 26 0.06 - Report RES-
Oats 0.25 × 1 26 0.15 - 62-124 (1963)
0.50 × 1 26 0.50 -
Wheat 0.25 × 1 62 <0.01 0.27 Shell Chem.Co.
0.25 × 1 96 <0.01 0.02 Report PRL-
0.25 × 2 62 <0.01 0.37 65-41 (1966)
Barley 0.50 × 1 60 <0.05 0.07
0.50 × 1 32 <0.05 0.09-0.38
Wheat 0.25 × 1 >150 0.01 - Velsicol Corp.
0.50 × 1 >150 0.03 - Report TSR-
Barley 0.25 × 1 >150 <0.01 - 2578 (1966)
0.50 × 1 >150 <0.01 -
In animals
Homogenates from cow or pig liver upon addition of NADH2 converted 38
ug of endrin, after 72 hours incubation, to metabolites which were
identical to those found from living organisms (Korte, 1967; Klein et
al., 1968a). See "Biochemical aspects".
In plants
14C labelled endrin was applied at either 1.04 or 2.08 mg/plant to
the leaves of tobacco under conditions of free or restricted aeration.
Six weeks after treatment, 32-47% of the applied radioactivity was
found on and in the tobacco plants, the lowest residues being in the
plants grown under free aeration. (Korte and Porter, 1970).
Shortly after the first buds emerged, the leaves of each of 11 cotton
plants were treated with 4.2 mg 14C-labelled endrin; treatment was
repeated after two and six weeks (total ca. 120 ppm). Twelve weeks
after the last application, quantitative measurement of the residues
revealed only very low concentrations in the cotton seed (0.033 ppm),
somewhat more in the fibers (0.36 ppm), and about 80% in and on the
leaves. Two thirds of the applied radioactivity was lost to the
atmosphere during the test. Besides endrin, there were two groups of
degradation products in the cotton plants, one group slightly more
hydrophilic than endrin and one very hydrophilic. One component of the
less hydrophilic group was isolated and gave mass and IR spectra
identical with that of delta-keto endrin (Korte and Porter, 1970).
When 14C-labelled endrin was applied at 50 ug/plant to young cabbage
plants, 66 percent of the activity had evaporated after two weeks, 70
percent after three weeks, and 75 percent after four weeks. Activity
not evaporated was found in the plants as endrin and hydrophilic
metabolites. After administration of 500 ug/plant, the concentration
of activity decreased from leaves to stalks to roots to soil, while
the ratio of metabolites to endrin increases in the same order. The
metabolite fraction consisted of two compounds, a very hydrophilic
main metabolite and delta-keto endrin (Weisgerber et al., 1968).
Similar results were obtained with carrots (Klein et al., 1968b).
Foliar application of 14C-labelled delta-keto endrin to white cabbage
has shown that it is more persistent than endrin but metabolizes more
rapidly (about 15 percent compared with about 10 percent for endrin).
The converted keto-endrin consisted mainly of a very hydrophilic
metabolite, the concentration of which is highest in leaves and stalks
(Korte and Porter, 1970).
Residues in soybean plants grown in soil treated with 14C-labelled
endrin were mainly delta-keto endrin, and endrin alcohol in addition
to endrin aldehyde were believed present (Nash and Beall, private
communication).
In soil
Matsumura et al., (in press); incubated 14C-labelled endrin with 150
cultures of microorganisms isolated from soils and found 25 active in
degrading endrin, all of which had previously been found to degrade
dieldrin. Seven different metabolites of endrin were found, with three
major and four minor ones; tentative structures have been assigned
(Korte and Porter, 1970).
In storage and processing
The effects of processing on the residues of endrin in dairy products
was studied by Langlois et al., (1965). Butter, ice cream, cheese,
condensed milk and dry whole milk were manufactured from milk
contaminated with endrin either by direct addition or through feeding
to cows. In general, they found that the residues concentration in the
fat remained fairly constant during processing except for drastic heat
treatment to produce dry whole milk which caused major reductions
(>50 percent).
When hens fed on a diet containing endrin were cooked in water at
190-200°F for three hours, the residues were reduced up to 90 percent
(Liska et al., 1967). Autoclaving the carcass at 15 psi for three
hours removed essentially all residues. Metabolites of endrin ware not
determined.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of endrin were monitored in cottonseed oil produced by mills
in Venezuela, Brazil, India and the United States. In Venezuela,
weekly samples were taken for nine weeks, and endrin residues ranged
from <0.02 to 0.05 ppm for crude oil, <0.02 to 0.02 ppm for
decolorized oil and <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR. 0119.70, 1970). In Brazil, seven samples were taken over
thirteen weeks, and endrin residues ranged from <0.01 to 0.01 ppm for
crude oil and <0.01 to <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR.0120.70, 1970). In India, only crude oil samples were
available, and these contained from 0.04 to 0.06 ppm endrin (Shell
Research Ltd., Report WKGR 0091.70). In the United States, ten samples
of refined oil from California were analyzed and found to contain
<0.03 to 0.03 ppm endrin and <0.02 ppm delta-keto endrin (Shell
Development Co., RES-63-155).
In addition to oil, cottonseed cakes have also been studied for endrin
residues, since these are used widely as an important ingredient of
cattle feed concentrates. Figures for cattle cake samples
corresponding to the samples of cottonseed oils given in the preceding
paragraph are as follows: Venezuela, <0.01 to 0.02 ppm; Brazil <0.01
to 0.08 ppm; India, <0.01 ppm. In the United States, data obtained on
cakes and meals corresponding to the samples listed in Tables II and
III ranged from <0.01 ppm to <0.04 ppm; most residues were below the
detection limit of <0.01 ppm.
Total diet studies conducted in the U.K.and the U.S.A. indicate that
endrin residues are unlikely to arise in human diets as a result of
the use of endrin in cotton (Abbott et al., 1969; Assoc. of Public
Analysts, 1969; Duggan, 1968; USDA, 1968). In the U.K., no endrin
residues were found in vegetable oils, fats, milk, milk based infant
foods, beef, beef sausages or meats. In the U.S.A., no endrin residues
were found in finished or crude cotton-seed oil, milk, milk products
or manufactured milk products.
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967) apply to the
determination of endrin. The compound can be determined in fatty and
nonfatty foods by the multiresidue method and gas chromatography
(Pesticide Analytical Manual., Vol. I and II, U.S. FDA). Endrin in
eluted from the Florisil column in the 15% ethyl ether/petroleum ether
fraction. Additional cleanup by MgO-Celite column and alkaline
hydrolysis in required, and recovery is usually in the range of
80-100%. When a gas chromatograph containing 10% DC 200 on Gas-Chrom Q
column at 200°C with 120 ml/min N2flow and an electron capture
detector is used, the following retention times relative to aldrin are
obtained: endrin - 2.05, endrin aldehyde - 2.30, endrin alcohol
- 2.50, delta-keto endrin -3.50. The sensitivity of the method is
good; 2 ng of endrin gives a “ full scale response on a 10-9amp full
scale recorder. A procedure for the determination of delta-keto endrin
in mammalian samples has been developed (Zavon et al., 1965), and a
general method is available (Shell Development Co., Anal. Method MMS
53/64). Residue methods have not yet been developed for monohydroxy
endrin, 9-keto endrin, endrin, endrin aldehyde or the "bird cage
alcohol" isomer of endrin.
An infrared method for endrin has been described (Gershman, 1961). An
absorbance peak at 10.2 microns is used for calculations. The method
requires a 1 kg ample for 0.25 ppm.
NATIONAL TOLERANCES
Country Commodity Tolerance ppm
Australia vegetables including 0.1
beans, crucifers,
potatoes, tomatoes
United States broccoli, brussels 0
sprouts, cabbage,
cauliflower, cucumbers, extended1
cottonseed, eggplant,
peppers, potatoes,
United States
(cont'd) sugar beets and sugar extended1
beet tops, summer squash,
tomatoes
1 The word "extended" appears where action is being taken to acquire
data to petition for a tolerance.
Australia has not set a tolerance for endrin in cottonseed, however,
an action level has been set for endrin residues in animal feeds at
0.03 ppm.
APPRAISAL
Endrin is widely used to control insect pests on cotton and to lesser
extent on rice. It is used to a limited extent as an insecticide on
small grains, vegetables and sugar cane and is occasionally used for
mouse control in orchards. The ways in which endrin is applied vary
widely, as do the numbers of applications.
The most important consideration regarding endrin residues in food
arise from its use on cotton; cottonseed oil is used for cooking or
margarine manufacture, and the extracted cottonseed cake is used as
cattle feed. Controlled feeding experiments with cattle indicate that
whole milk residues would reach a plateau of 0.04 times the residue in
feed. Once ingestion stops, excretion in milk soon ceases (two to six
weeks). Cottonseed cake for feed should have less than 0.1 ppm of
endrin residues if the recommended practical residue limits for whole
milk and milk products are not to be exceeded.
Rice bran is used as a component of poultry feed; feeding studies on
hens indicate that endrin residues in depot fat would reach about five
times the level in feed; for eggs the ratio would be about 0.7 times.
Calculations indicate that it is unlikely that significant endrin
residues would be found in cooked poultry or eggs. The rice bran used
as a constituent of poultry feeds should not contain residues greater
than 1 ppm if the recommended practical residue limits for poultry and
eggs are not to be exceeded.
In the absence of experimental residue data at the recommended
preharvest interval of 45 days or longer, the meeting did not
recommend a tolerance for oats.
Under field conditions, endrin is rapidly loot from plants through
evaporation - 60-80 percent over six to 18 weeks, depending on the
crop.
Endrin is metabolized by plants to delta-keto endrin, a rearrangement
product, and to very hydrophilic metabolites; traces of the aldehyde
(SD 7442) and alcohol have been reported. The parent compound appears
to be the major residue at all times.
The widespread use of endrin on cotton does not appear to give rise to
measurable residues in the human diet. Dietary studies in the United
States have revealed no endrin residues in finished cottonseed oils,
milk, milk based infant foods, milk products, beef or meats and trace
amounts in margarine and fats. Exceptionally, one diet composite
sample in one sampling location in the United States contained 0.20
ppm of endrin in oils, fats and shortening.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The following tolerance and practical residue limits are to apply to
raw agricultural products moving in commerce unless otherwise
indicated. All figures include the sum of endrin and delta-keto
endrin.
TOLERANCES
ppm
Cottonseed, cottonseed oil (crude) 0.1
Cottonseed oil (finished), maize
(sweet), wheat, barley, sorghum,
rice (brown or polished), apples 0.02
PRACTICAL RESIDUES LIMITS
Milk and milk products (fat basis) 0.02
Fat of poultry 1
Eggs (shell-free) 0.2
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate long-term study in a strain of mice of known tumour
susceptibility.
REFERENCES
Abbot, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric. 20; 245
Altmeier, G., Klein, W. and Korte, F. (1969) Tetrahedron Letters,
49: 4269-4271
Association of American Pesticide Control Officials, Inc. (1966)
Pesticide Chemicals Official Compendium, 1966 Ed., 475-477
Association of Public Analysts. (1967) Joint Survey of Pesticide
Residues in Foodstuffs sold in England and Wales
Baldwin, M.K., Robinson, J. and Parke D.V. (1970) The metabolism of
endrin in the rat. Unpublished report from the Tunstall Laboratory,
Shell Research Ltd., Sittingbourne and the University of Surrey
Barrentine, B.F., and Cain, J.B. (1970) Unpublished work cited in
Comptes Rendus, 25th Conference of IUPAC, Commission VI.5.1, Appendix
VII
Barth, R.A.J. (1967) Pesticide toxicity in primates. Doctoral thesis,
Tulane University, Louisiana p. 110-111
Boyd, E.M. and Stefec, J. (1969) Dietary protein and pesticide
toxicity: with particular reference to endrin. Canad. med. Ass. J.,
101: 335-339
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27:81-138
Coble, Y., Hildebrandt, P., Davis, J., Raasch, F. and Curley, A.
(1967) Acute endrin poisoning. J. Amer. med. Ass. 202: 489-493
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Comptes Rendus. (1970) Fate of organochlorine pesticides in vegetable
oil processing. 25th Conference of IUPAC, Commission VI.5.1, Terminal
Pesticide Residues, Appendix VII. p. 187-189
Cummings, J.G., Zee, K.T., Turner, V., Quinn, F..and Cook R.E. (1966)
Residues in eggs from low level feeding of five chlorinated
hydrocarbon insecticides to hens. J. Assoc. Off. Anal. Chem. 49:
354-364
Cummings, J.G., Eidelman, M., Turner, V., Reed, D., Zee K.T. and Cook,
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydro-carbon insecticides to hens. J. Assoc. Off. Anal.
Chem. 50: 418-425
Dale, W.E., Copeland, M.F. and Hayes, W.J. Jr. (1965) Chlorinated
insecticides in the body fat of people of India. Bull. Wld Hlth Org.,
33: 471-477
Davies, G.M. and Lewis, I. (1956) Outbreak of food poisoning from
bread made of chemically contaminated flour. Brit. med. J., (2):
393-398
Dewitt, J.B. (1956) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J. Agr. Fd. Chem., 10: 863-866
Diechmann, W.B., MacDonald, W.E., Radomski, J. Blum, E.B. Bevilacqua,
M. and Keplinger, M. (1970) The tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Industr. Med., 39: 314
Duggan, R.E. (1968) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pesticides Monotoring
Journal 1 (4): 2-7
Dunachie F.F. and Fletcher W.W. (1966) Effect of some insecticides on
the hatching rate of hen's eggs. Nature, 212: 1062-1063
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. econ. Entomol., 50: 348-349
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. Econ. Ent. 50: 348-349
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23(1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FDA, (1967) Pesticide analytical manual, HEW, FDA, Vol. I and 11
Frear, D.E.H. (1955) Endrin in pesticide index, Third Edition, D. van
Nostrand Co. Inc., New York. p. 68-69
Gershman, L.L. (1961) Infra-red determination of endrin residues. J.
Assoc. Off. Anal. Chem. 44: 212-214
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Hayes, W.J., Jr., (1963) Endrin in Clinical handbook on economic
poisons, revised edition. pp. 68.70. Publ. Hlth. Ser. Publn No. 476.
US Govnmt Printing Off. Washington D.C.
Hayes, W.J., Jr., Dale, W.E. and Baise, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people of New Orleans. Life Sci.,
4: 1611-1615
Hayes, W.J. and Curley, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environm. Hlth, 16: 155-162
Hine, C.H., Eisenlord, G., Loquvam, G.S. and Leung, T. (1968) Results
of reproduction studies on rats fed diets containing endrin over three
generations. Unpublished report prepared by the Hine Laboratories,
Inc., San Francisco, submitted by Shell International Research
Horsfall, F. Jr., Webb, R.E., Price, N.O. and Young, R.W. (1970)
Residues in apples subsequent to ground sprays of endrin. J. Agr. Food
Chem. 18: 221-223
IUPAC, (1967) Proceedings of the Commission on Terminal Residues,
Vienna, August 1967. Appendixes VI and VII
Jager, K.W. (1970) Aldrin, dieldrin and telodrin. An epidemiological
and toxicological study of long-term exposure. Doctoral thesis,
Leiden, 1970
Jolley, W.P., Stemmer, K.L., Grande, F., Richmond, J. and Pfitzer,
E.A. (1969) The effects exerted upon beagle dogs during a period of
two years by the introduction of 1, 2, 3, 4, 10, 10-hexachloro-6,
7-epoxy-1, 4, 4a, 5, 6, 7, 8, 8a-octahydro-1, 4-endo, endo-5,
8-dimethanonaphthalene into their daily diets. Unpublished report from
the Kettering Laboratory University of Cincinnati submitted by Shell
International Research
Kiigemagi, U., Sprowls, R.G. and Terriere, L.C. (1958) Endrin content
of milk and body tissues of dairy cows receiving endrin daily in their
diet. J. Agr. Fd. Chem., 6: 518-521
Klein, W., Müller, W. and Korte, F. (1968a) Insektizide im
Stoffwechsel, XVI. Ausscheidung, Verteilung und Stoffwechsel von
Endrin (14C) in Ratten. Liebigs Ann. Chem. 713: 180-185
Klein, W. et al., (1968b) Qualitas Plant. Mater. Vegetabiles 15,
225-238
Klein, W. and Drefahl, B. (1970) "Jahresbericht 1969" Institut für
ökologische Chemie der Gesellschaft für Strahlenforschung mbH,
München, Schloss Birlinghoven, April 1970
Korte, F. (1967) Metabolism of chlorinated insecticides. Unpublished
report from the Radiochemical Laboratories, Bonn University
Korte, F. and Porter, P.E. (1970) Minutes of the fifth meeting of the
IUPAC Commission on terminal pesticide residues, September, 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Langlois, B.E., Liska, B.J. and Hill, D.L. (1965) The effects of
processing and storage of dairy products on chlorinated insecticide
residues. J. Milk and Food Technol. 28: 9-11
Liska, B.J., Stemp, A.R. and. Stadelman, W.J. (1967) Food Technol.
21: 435
Ludwig, G. (1965) Excretion, metabolism and storage of endrin 14C
after oral administration to a rat. Minutes of the fifth meeting on
the fate of organic compounds in the living organism. 20-21 October
1965, Birlinghoven Laboratory
Ludwig, G. (1966) Investigations with 14C-labelled insecticides. XX
Excretion, metabolism and storage of endrin - 14C after oral
administration to a rat. Birlinghoven Biomonthly Res. Summary
(January), p. 13-14
Morris, R.D. (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse Peromyscus maniculatus. Canad. J.
Zool., 46: 951-958
Nash, R.G. and Beall, M.L. Jr. (1970) Extraction and identification of
endrin and heptachlor degradation products. Submitted to J. Assoc.
Off. Anal. Chem., October 1970
Nelson, S.C., Bahler, T.L., Hartwell, W.V., Greenwood, D.A. and
Harris, L.E. (1968) Serum alkaline phosphatase levels, weight changes
and mortality ratios of rats fed endrin. J. Agr. Pd. Chem., 4:
696-700
Radeleff, R.D. (1956) Hazards to livestock of insecticides used in
mosquito control. Mosquito News, 16(2): 79-80
Richardson, A. (1970) Unpublished untitled letter from Tunstall
Laboratories to Shell International Research
Richardson, A. Robinson, J. and Baldwin, M.K. (1970) Metabolism of
endrin in the rat. Chem. Industr., 502-503
Robinson, J., Richardson, A., Hunter, C.G., Crabtree, A.N. and Rees,
H.J. (1965) Organo-chlorine insecticide content of human adipose
tissue in south-eastern England. Brit. J. industr. Med., 22: 220-229
Shell. (1965) Alkaline phosphatase in endrin-exposed workers.
Unpublished report prepared by the Industrial Medical Department,
Shell Nederland Chemische Fabrieken N.V. Rotterdam
Shell Chemical Co., Reports. (1965-69) PRL-65-41, PRL-66-67g,
PRL-66-113, PRL-67-24, PRL-69-28, PRL-69-95, PRL-69-119
Shell Development Co., Reports. (1961-65) RES-61-60, RES-62-124,
RES-63-155, RES-64-39 RES-64-9
Shell Research Ltd., Report (1968-70) WKTR 0078/68, 0023/69, WKGR
0046/70, 0072/70, 0078/70, 0087/70, 0071.70, 0091.70, 0119.70, 0120.70
Sherman, M. and Rosenberg, M.M. (1954) Sub-chronic toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 47: 1082-1083
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chem. Soc., 45:
866-869
Smith, S.I., Weber, C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocarbon insecticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Soto, A.R. and Diechmann, W.B. (1967) Major metabolism and acute
toxicity of aldrin, dieldrin and endrin. Environm. Res., 1: 307-322
Speck, L.B. and Maaske, C.A. (1958) The effects of chronic and acute
exposure of rats to endrin. A.M.A. Arch. industr. Hlth, 18: 268-272
Street, J.C., Butcher, J.E., Raleigh, R.J. and Clanton, D.C. (1957)
Tissue storage and transferral to the lamb of aldrin, dieldrin and
endrin when fed to bred ewes. Proc. West. Sec. Amer. Soc. Anim. Prod.,
(Pullman, Washington), 46: 1-6
Terriere, L.C., Kiigemagi, U. and England, D.C. (1958) Endrin content
of body tissues of steers, lambs and hogs receiving endrin in their
daily diet. J. Agr. Fd. Chem., 6: 516-518
Terriere, L.C., Arscott, G.H. and Kiigemagi, U. (1959) The endrin
content of eggs and body tissue of poultry receiving endrin in their
daily diet. J. Agr. Fd Chem., 7: 502-504
Treon, J.F., Clevelend, F.P. and Cappel, J. (1955) Toxicity of endrin
for laboratory animals. J. Agr. Fd. Chem., 3: 842-848
USDA, Agriculture handbook No. 331. (1968) Suggested guide for the use
of insecticides to control insects affecting crops, livestock,
households, stored products, forests and forest products
USDA, Residues in food and feed. (1968) Pesticide residue levels in
foods in the United States from 1 July 1963 to 30 June 1967.
Pesticides Monitoring Journal, 2(1): 2-46
USDA, Summary of registered agricultural pesticide chemical uses.
(1969) Vol. III, Insecticides, repellents, acaricides. Endrin,
III-E-2.1, 2.2, 2.3
Van Dijk, M.C. (1968) Chlorinated insecticides and the serum alkaline
phosphatase. Proc. Sixth Shell Industrial Doctors Meeting, 28-30 May
1968, Amsterdam
Velsicol Corp., (1968-70) Reports TSR 2578, TSR 2575 (Unpublished -
1970)
Weeks, D.E. Endrin food-poisoning. A report of four outbreaks caused
by two separate shipments of endrin-contaminated flour. Bull. Wld Hlth
Org., 37: 499-512
Weisgerber, I., Klein, W., Djirsarai, A., and Korte, F. (1968) Ann.
Chem. 713: 175-179
Williams, S., and Mills, P.A. and McDowell, R.E. (1964) Residues in
milk of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Anal. Chem., 47:
1124-1128
Williams, D.A. (1966) Alkaline phosphatase activity following exposure
to endrin, comments on the paper by Nelson et al. Unpublished report
from the Statistics Unit, Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Witherup, S., Stemmer, K.L., Taylor, P., Bietsch, P. and Pfitzer, E.A.
The incidence of neoplasms in two strains of mice sustained on diets
containing endrin. Unpublished report from the Kettering Laboratory,
University of Cincinnati submitted by Shell International Research
Zavon, M.R. (1961) The toxicology and pharmacology of endrin.
Unpublished report from the Kettering Laboratory, University of
Cincinnati
Zavon, M.R., Hine, C.H. and Parker, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Amer. med. Ass., 193: 837-839
metabolite is also an alcohol. Three days after a single oral dose of
14C-labelled endrin, approximately half of the 14C radioactivity
remained in the bodies of the rats. This material was principally one
metabolite which was identified as 9-keto endrin (I), an oxidation
product of the secondary alcohol found in faeces.
When 14C-labelled endrin was applied orally to rabbits at 0.5 mg/kg
body-weight and at three and four day intervals, four metabolites were
isolated from the urine which appear to have the following chemical
natures: A (40% of excreted radioactivity) is a conjugate compound of
a hydroxy derivative of endrin; B (12%) is a monohydroxy derivative of
an unbridged endrin isomeric ketone; C (40%) is the 4a-hydroxyendrin;
D (8%) has a molecular weight of 420 and the C-C double bond intact in
the chlorinated ring. None of these compounds is the delta-keto endrin
(Korte and Porter, 1970).
Effects on enzymes and other biochemical parameters
In monkeys which had received exposure to an unspecified quantity of
endrin, there were significant changes in the enzymes serum
glutamic-oxaloacetic transaminase and serum glutamic-pyruvic
transaminase (Barth, 1967).
Elevation of serum alkaline phosphatase has been observed in rats fed
25 ppm and possibly 5 or 1 ppm of endrin for 16 weeks (Nelson et al.,
1956) but not in dogs fed 4 ppm of endrin for two years (Jolley et
al., 1969) nor in human subjects occupationally exposed to unspecified
levels of endrin (Shell, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
See under "Long-term studies" (Witherup et al., 1970).
Rat
Commencing at weaning, varying numbers of rats of mixed sex were fed
dietary levels of 0, 2, 6 or 12 ppm of endrin throughout their
lifetime. No primary malignant hepatic tumours were found in any
animals upon histological examination. Two benign hepatic tumours
(haemangiomas) were found in one male control rat and the other in a
female fed 6 ppm of endrin. Tumour incidence in other tissues was also
not significantly different between the control and experimental
animals (Diechmann et al., 1970).
Special studies on reproduction
Chicken egg
Hen eggs were injected with 0.5 or 5 mg per egg. The hatching rate was
40 or 20 percent, respectively (Dunachie and Fletcher, 1966).
When endrin was injected into the yolk of fertile eggs incubated for
seven days in amounts of 0.2 and 2.0 mg/egg, the hatchability was 40
and 6.9 percent, respectively (Smith et al., 1970).
Quail
No eggs were produced from quail which received 1 ppm of endrin in
their diet either as winter maintenance or during the reproductive
period (DeWitt, 1965).
Pheasant
In pheasants there was reduced egg production when fed 10 ppm of
endrin but not at 2 ppm or less. Survival of the chicks to two or six
weeks was also markedly reduced at 10 ppm, but not at lower doses
(DeWitt, 1965).
Mouse
Groups of male and female mice were fed endrin at dietary levels of 0
or 5 ppm for 30 days. Test and control mice were then randomly paired
and continued on the test diet for a further 90 days, there being a
total of 101 pairs in the group fed endrin. The first litters were
significantly smaller than the control group. The time taken to
produce the first litter was not significantly different between the
two groups (Good and Ware, 1969).
Deer mouse
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus manicalatus) of varying ages were fed dietary levels of
0, 1, 2, 4 or 7 ppm of endrin over intermittent periods between which
times the animals were either fed a normal diet or were subjected to
48 hours starvation. The animals were sacrificed by exposing them to
cold stress at -16°C and the time of death recorded. During feeding,
parental mortality increased in proportion to the level of endrin.
Young animals were more susceptible than old. Starvation increased
mortality in all test groups but not in the controls; this effect was
more evident with increasing dose levels. Litter production frequency
and mean litter size before and during experimental feeding were
similar. However, post-natal mortality prior to weaning increased in
the young from parents fed 4 or 7 ppm. Endrin adversely affected the
survival time during cold stress in the females but not in the males
(Morris, 1968).
Rat
In a later study from the same laboratory, groups of ten male and 20
female rats were fed dietary levels of 0, 0.1, 1.0 and 2.0 ppm of
endrin over a period of three generations. The F0 generation was
mated after 79 days on the test diets, and the males were rotated as
in the previous experiment.
The young from the first litter were discarded at weaning and the
parents mated again after ten days to form the F1b generation. Young
from this generation were mated when 100 days old, and this protocol
was followed for three generations, using the second litter in all
cases. The size of the litter in the F3 generation from the 2.0 ppm
group was significantly larger than that from the controls. Mortality
was high in the controls, which resulted in a greater percentage
survival in the F3a litters in the 0.1 ppm group and in all F3b
litters in the test groups. The weights of weanlings were comparable
to the controls except in the F3a litters from the 0.1 ppm, which
were significantly less due probably to the large litter sizes in that
group. Examination of the F3b weanlings revealed no differences in
organ to body-weight ratios. It was stated that there were no
histological abnormalities, but details of the pathology were not
available. Fertility, gestation, viability and lactation indices did
not indicate that endrin affected any of these parameters (Hine et
al., 1968).
Special studies on the photoisomerization product of endrin
When endrin is irradiated with short wavelength ultraviolet light, the
delta-keto compound is formed in 37 percent yield as well as an
aldehyde in 9 percent yield. Under the influence of sunlight, only the
ketone is formed. The ketone is about a quarter as toxic to rate as
endrin and like endrin is more toxic to the male than to the female
(Soto and Diechmann, 1970) (see also "Fate of residues").
Acute toxicity
The major clinical manifestation of endrin intoxication in man
involves convulsions of several minutes duration which may be isolated
and followed by semiconciousness for 15-30 minutes, with complete
recovery after 2-4 weeks. More serious symptoms are continuous
convulsions, high fever and decerebrate rigidity prior to death. Mild
symptoms of poisoning include dizziness, weakness of the legs,
abdominal discomfort and nausea. Temporary deafness and insomnia may
also occur. It has been estimated, based upon reports of outbreaks of
poisoning, that 0.2-0.25 mg/kg body-weight will produce a single
convulsion in man, and that repeated fits will result from 1 mg/kg
(Hayes, 1963).
LD50
Animal mg/kg body-weight References
(oral)
Rat, adult (F) 7 Treon et al., 1955
Rat, young (F) 17 Treon at al., 1955
Rat, adult (F) 40-43 Speck and Maaske, 1958
Rat, young (M) 29 Treon at al., 1955
Rabbit (F) 7-10 Treon at al., 1955
Guinea-pig 16-36 Treon at al., 1955
Monkey 3 Treon at al., 1955
Monkey 12 Barth, 1967
The acute toxicity of endrin appears to be influenced by the diet.
Three groups each comprising about 100 male rats were fed for 28 days
either a normal diet, a normal protein diet containing protein only as
casein or a low protein diet. The acute toxicity to endrin was then
determined by a single oral administration of the pesticide. The LD50
values were 27, 17 and 7 for the animals fed the respective diets,
indicating an approximately four-fold increase in toxicity between the
normal and low-protein diet as well as an effect due to the type of
protein fed (Boyd and Stefec, 1969).
Short-term studies
Quail
Groups each of 40 quail, starting when one day old, were fed dietary
levels of 0, 0.5, 1, 5, 10, 20 or 50 ppm in their diet. Survival was
adversely affected in all the test groups, and there were no survivors
beyond two weeks in the birds fed 10 ppm or more. Food consumption was
abnormally low. Symptoms involved lack of muscular coordination,
tremors, bedraggled appearance and rigidity with occasional convulsive
movements (De Witt, 1956).
Chicken
Groups each of 20 seven-day old chicks were unaffected by a diet
containing 0, 1.5 or 3 ppm of endrin. When the concentration was
increased to 6 or 12 ppm, the birds became highly excitable, failed to
gain as much weight as the controls and the survival rates over a 12
week period were 85 and 5 percent, respectively, compared to 100 per
cent in the controls (Sherman and Rosenberg, 1954).
Pheasant
Day-old pheasants, in groups of 40, did not survive beyond eight days
when fed dietary levels of 5 or 20 ppm. Reduced food consumption
occurred, and the symptoms were the same as those seen in quail (De
Witt, 1956).
Rat
Groups comprising five male and five female rats were fed dietary
levels of 0, 1, 5, 25, 50 or 100 ppm of endrin for up to 16 weeks. All
of the group fed 100 ppm died within the first two weeks, and only two
rats fed 50 ppm and three fed 25 ppm survived. Three males fed 5 ppm
also died; the other animals were continued on the test diet for the
full 16 weeks. Weight loss was roughly dose related but was evident in
all test groups, as was hypersensitivity to tactile stimuli. There was
an initial drop in serum alkaline phosphatase during the first three
to eight weeks' feeding, which was then followed by an increase at all
dose levels. At the end of the 16 weeks, the phosphatase level was
elevated above the controls in all the test groups, the levels being
highest in the groups fed 25 and 50 ppm (Nelson et al., 1956).
However, other statisticians have considered that the elevation of
serum alkaline phosphatase was not significant in the groups fed 1 and
5 ppm of endrin (Williams, 1966).
Dog
In a series of experiments, dogs were fed diets containing from 1 to
50 ppm endrin along with control groups. Two of four animals fed a
diet containing 8 ppm and the one fed 5 ppm died. The two surviving
dogs on 8 ppm were kept on the diet for about six months and then
sacrificed; increased organ to body-weight ratios for the liver,
kidney and brain were found, and histopathological examination showed
degeneration of kidney tissue. Three of four dogs on 4 ppm of endrin
survived, and there were no symptoms in dogs fed 1 or 3 ppm (Treon et
al., 1955).
In an experiment of about 19 months' duration, groups comprising two
male and two female dogs were placed on diets containing 0, 1 or 3 ppm
of endrin. All dogs on 3 ppm had increased organ to body-weight ratios
for the kidney and heart. Some female dogs fed 1 or 3 ppm of endrin
had a renal abnormality characterized by a slight tubular vacuolation;
this change was also observed in the female control dog. Male dogs in
both control and test groups had normal viscera (Treon et al., 1955).
Groups comprising seven male and seven female dogs were fed dietary
levels of 0, 0.1, 0.5, 1.0, 2.0 or 4.0 ppm of dieldrin for two years.
Scheduled autopsies were performed on two dogs of each sex from the 0,
1.0 and 4.0 ppm groups at six and 12 months. There were no deaths due
to the treatment nor were there any changes in body-weight increase or
food consumption in any group. The only clinical abnormalities were in
one female and two male dogs fed 4.0 ppm and one female fed 2.0 ppm
that showed evidence of, or were observed having, convulsions; the
earliest incidence in a male dog after five months on 4.0 ppm. The
only changes in organ weights were occasional slight increases in
liver or liver to body-weight ratios in the dogs fed 2.0 and 4.0 ppm.
After two years, pathological examination showed slight vacuolation of
hepatic cells in the females and diffuse pigmentation in one male and
all females. At 4.0 ppm, vacuolar degeneration and diffuse brown
pigment in the hepatic cells was evident in all dogs, without any sex
differentiation. In two only of the dogs, which had convulsions,
autopsies revealed some pathological changes in the brain. All other
organs in the dogs fed 2.0 or 4.0 ppm and all organs in the dogs fed
1.0 ppm or less showed no morphological changes which were considered
to be attributable to feeding endrin. There were no significant
changes in the blood picture or in the chemical or physical
characteristics of the urine attributable to endrin. After two years,
levels of liver enzymes, prothrombin time bromsulphthalein clearance,
serum protein electrophoresis, glucose, urea nitrogen, cholesterol,
calcium, inorganic phosphorus, total bilirubin or uric acid showed no
changes attributable to endrin feeding (Jolley et al., 1969).
Cattle and sheep
Cattle and sheep were not affected by 5 ppm of endrin in their diet
for 112 days (Radeleff, 1956).
Long-term studies
Mouse
A total of 1600 mice in equal numbers of each sex, consisting of one
inbred and one hybrid strain, were divided into four groups, two of
which were fed a control diet and the other two fed 0.3 or 3.0 ppm of
endrin. Feeding the test diet was started at five weeks of age and
continued through out their normal lifespan, or until sacrifice.
Because of an early high incidence of fibroadenomas occurring in both
control and test groups in the hybrid strain, all the females of that
strain were sacrificed after 72 weeks for pathological examination. A
few of the mice, fed 3.0 ppm only, displayed convulsions in the early
stages of feeding but recovered and survived. Mortality was not
adversely affected by endrin, nor was body-weight or food intake. No
haematological abnormalities were evident except in two males in the
hybrid group fed 0.3 ppm, which had severe leukaemia. In either sex,
the total number of neoplasms was not influenced by the endrin content
of the diet, except in the case of hepatomas in the females of the
hybrid strain, which were significantly higher than the controls in
the mice of the group fed 3.0 ppm, and sacrificed between weeks 53 and
60 of the feeding period. Because of a relatively high incidence of
hepatomas in one group of controls of this strain, the increase at the
3.0 ppm level was considered not due to endrin. It was noted that in
no animals of either sex were there any metastases of the hepatomas
into the lungs (Witherup et al., 1970).
Rat
In a two year experiment, groups each of 20 male and 20 female rats
were given diets containing 0, 1, 5, 25, 50 and 100 ppm of endrin.
Concentrations of 50 and 100 ppm were lethal within a few weeks. The
concentration of 25 ppm increased the mortality rate of the females.
Non-survivors at the three higher levels exhibited diffuse
degeneration of the brain, liver, kidneys and adrenal glands. The
survivors in the two higher levels showed degenerative changes in the
liver only, while those fed at the lower levels had normal viscera.
The level of 5 ppm caused an increase in liver to body-weight ratio in
males and an increase in kidney to body-weight ratio in females. There
was no effect at the 1 ppm level (Treon et al., 1955).
OBSERVATIONS IN MAN
A total of 874 persons were hospitalized, and there were 26 deaths in
several outbreaks of poisoning in Saudi Arabia in 1967 due to
consumption of bread containing endrin. Approximate average levels in
the bread in various outbreaks were 48, 1500 or 400 ppm, corresponding
to a percentage of fatalities of 1.4, 9.5 and 0.4, respectively, among
those poisoned. Signs and symptoms were typical of central nervous
system stimulation and all survivors rapidly returned to normal
(Weeks, 1967).
Three persons in Egypt experienced convulsions after eating bread
containing 126 to 176 ppm of diedrin. There were no deaths (Coble et
al., 1967).
In an incident in the UK, 59 people became ill from ingesting bread
which contained about 150 ppm of endrin, but there were no deaths
(Davies and Lewis, 1956). The maximum amount of endrin consumed has
been estimated to have been 1 mg/kg body-weight (Zavon, 1961).
Studies in human subjects experiencing intoxication from endrin (Coble
et al., 1967, Weeks, 1967) and from occupational workers (Hayes and
Curley, 1968, Jager, 1970) have demonstrated that endrin rapidly
disappears from the blood in cases of acute intoxication and cannot be
detected in the fat or blood of people exposed to endrin unless
symptoms of intoxication are evident.
Endrin has not been reported to be found from studies involving levels
of organochlorine pesticides in human body fat in India, UK or USA
using analytical methods sensitive to <0.03 ppm (Dale et al., 1965;
Hayes et al., 1965, Robinson et al., 1965 and Zavon et al., 1965).
Levels of the 9-keto metabolite of endrin in four human fat samples
were all less than 0.0004 ppm (Richardson, 1970).
Among workers in a plant manufacturing endrin and a number of other
pesticides, no detectable amounts of endrin were found in samples of
plasma, fat or urine. Exposure was for an average time of 2,106 hours.
Based upon the limit of detection, the levels of endrin, were <0.0030
ppm in plasma, <0.03 ppm in fat and <0.0016 ppm in urine. Endrin
has, however, been detected in the serum and urine of people who
received amounts sufficient to produce intoxication (Hayes and Curley,
1968).
Serum alkaline phosphatase was determined in 30 workers who had been
exposed to endrin for periods from six weeks to eight years. There was
no difference in the levels found in the exposed group and those found
in a group comprising nine unexposed individuals, nor was there any
relationship detected between the phosphatase levels and the duration
of exposure of the workers (Shell, 1965).
In workers exposed to endrin during periods up to eight years, no
significant changes in the level of serum alkaline phosphatase were
observed during a 13-month observation period (Van Dijk, 1968).
It is estimated that the blood level of endrin below which no signs or
symptoms of intoxication occurs is in the range of 0.05 - 0.100 µg/ml.
Measurable blood levels (detection level 0.005 µg/ml) occur only after
gross over-exposure. The half-life of endrin appears to be
approximately 24 hours. Medical control of a group of workers exposed
over periods up to 13 years has failed to show any effects of
long-term exposure. The blood picture, results of urinalysis,
activities of serum glutamic-oxaloacetic and glutamic-pyruvic
transaminases, alkaline phosphatase and lactic dehydrogenase remained
all within normal limits. Occasional electroencephalographic changes
returned to normal. Absenteeism due to disease or accidents was
comparable to that of a control group. Because of the short half-life
of endrin, it is (unlike dieldrin) impossible to calculate the average
level of exposure of the workers to endrin (Jager, 1970).
A decrease in the blood levels of pp'-DDE and an increased excretion
of 6-ß-hydrocortisol in relation to 17-hydroxy-corticosteroids was
present in the workers employed in manufacturing and who were exposed
to endrin and its intermediates. The compound responsible is not known
and further studies are in progress (Jager, 1970).
COMMENTS
The primary site of action of endrin is the central nervous system.
This fact is evidenced by convulsions which result from acute
poisoning and from administration of repeated relatively high doses.
Unlike dieldrin, endrin is rapidly metabolized by animals; the storage
in the fat of animals is very low compared with other compounds of
similar chemical structure. In rats, it is excreted mainly in the
faeces as endrin, 4a-hydroxyendrin, and an unidentified endrin
alcohol; a third metabolite, 9-keto-endrin, is stored in the tissues.
In rabbit, the main route of excretion is the urine, in which four
metabolites were demonstrated, one of which is the 4a-hydroxyendrin.
The plant metabolite of endrin, delta-keto endrin, is rapidly
metabolized by animals. Three metabolites were found in rabbit urine
after oral administration of delta-keto endrin. It is understood that
delta-keto endrin is unlikely to be formed under conditions of good
agricultural practice, and that the compound is less toxic to mammals
than endrin.
A long-term study in mice failed to produce conclusive results
relative to the carcinogenic potential of endrin. Endrin is more
acutely toxic to animals fed a low protein diet. Reproduction studies
with endrin in several species revealed no influence of endrin on
maturation, but foetal and postnatal mortality were increased. There
is no evidence of teratogenic activity. The two-year studies in the
dog and rat are used as a basis for determining a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1 ppm in the diet, equivalent to 0.05 mg/kg body-weight/day
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
The most important of all endrin uses, worldwide, and accounting for
some 80% of all endrin applied is as a spray for the control of
insects of cotton. It is also used extensively for the control of
insect pests in rice, to some extent in sugar cane and to a limited
extent in grain crops and sugar beets. In Australia, endrin is used
for insect control on tobacco and crucifers.
Post-harvest treatments
Endrin is accepted and occasionally used in some countries for rodent
control in orchards, where it is sprayed onto the ground under the
trees either in autumn or springtime. Rates vary from 2.4 kg/ha for
deciduous fruits in the United States to 0.02 or 0.03% solutions,
often in combination with mineral oil, in Australia.
Seed treatments
Endrin is used as a seed treatment on cotton at 2 oz /100 lb seed in
the United States and on beans at 1 oz /30 lb seed in Australia.
TABLE I
Summary of Registered Uses
Rate of
Crop application No. of Pre-harvest
(kg/ha) applications interval (days)
Cotton 0.25-0.50 1-12 none
Rice 0.2-0.50 1-4 30
Sugar cane 0.25 (only 1-8 45
granular)
Wheat 0.25-0.50 1 45
Barley " " "
Oats " " "
Sorghum " " "
Tobacco 0.25-0.5 - -
Crucifers 0.04% soln. - -
Potatoes 0.8 (USA) - -
0.03% soln.
(Australia)
Sugar beets 0.4 1-2 60
Other uses
Endrin has a minor use in the United States as a bait formulation for
control of cutworms in corn and potatoes. It is applied to the soil
surface at 0.8 kg/ha, and preharvest intervals are observed.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cotton seed and its products
From the view point of residues of endrin arising in food products,
cotton seed and its products are of primary interest; the refined
cottonseed oil is used for cooking or margarine manufacture, while the
extracted cake is used as cattle feed. Therefore residue levels in
cottonseed oil products which may reach the food consumer are
determined not so much by levels in products harvested in the field,
but to an appreciable extent by mill processes.
The effect of extraction processes on endrin residues was studied by
Smith et al. (1968) using crude cottonseed oil and soy bean oil
fortified with 1 ppm (mg/g) endrin. They found that alkali washing and
bleaching did not have a marked effect but that deodorization by
vacuum steam stripping effectively reduced endrin levels to below the
limits of detection (0.03 ppm). These authors also quote unpublished
work by Evans of the USDA using radio-labelled endrin. At initial
levels of up to 3.5 ppm in the crude oil, 96 percent of the added
radioactivity was lost during the process of steam stripping. The
effectiveness of the deodorization step in removing endrin residues
from vegetable oils was further demonstrated by Barrantine and Cain
(1970) in studies of commercial scale operations. Endrin residues are
summarized in Tables II and III. (Shell Development Co. Reports,
RES-64-3. RES-64-9; Shell Chemical Co., New York PRL-67-24; Velsicol
Corp., unpublished data).
The cottonseed cake left after the extraction of oil commonly contains
1%-5% oil. These cottonseed cakes are widely used as an important
ingredient of cattle feed concentrates and could contribute up to 20%
of the daily diet of a dairy cow. The relationship between cattle feed
and milk residues was studied by Williams and Mills (1964), Kiigemagi
et al., (1958), and Ely et al., (1957). Consideration of their
combined results indicates that at feed levels in the region of
0.25-2.0 ppm endrin in total feed, milk residues will eventually reach
0.04 times the concentration in the total feed, if feeding is
continued until a plateau is reached. Both Williams and Kiigemagi
found that when endrin was withdrawn from the feed, levels in the milk
fell fairly quickly. At 0.25 and 0.75 ppm in feed, levels fell to
0.002 ppm in six weeks after endrin was withdrawn (Kiigemagi, 1958).
At the 2 ppm feed level, residues fell from 0.06 to 0.02 ppm in the
same period. In Williams' experiments residue from the highest feed
level (0.50 ppm in whole dry feed) fell to 0.002 ppm in about 15 days.
In rice, rice bran and rice straw
The results of experiments in a number of rice growing regions are
summarized in Table IV.
TABLE II
Residues of endrin in whole cotton seed, U.S.A.
Dosage/ Harvest Residue ppm
No.of applic. interval delta-
Location applics. (lb/acre) (days) endrin keto
Oklahoma control - - <0.004 N.D.
2 0.5 58 <0.004 to
0.013 N.D.
2 1.0 58 <0.004 N.D.
Louisiana 4-5 2.67(total) - 0.02 to <0.02
0.03
Texas not stated 0.5 (total) - <0.04 <0.04
1.0 (total) - 0.06 to <0.04
0.10
2.0 (total) - <0.04 <0.04
TABLE III
Endrin residues in crude and alkali-extract
Cottonseed oil obtained from endrin treated cotton in Texas, U.S.A.
Residues in oil - ppm
Dosage rate Post harvest Crude Alkali extracted
lb/acre total interval-days Endrin delta-Keto Endrin delta-Keto
Control - <0.01 <0.03 <0.01 <0.03
0.5 not stated 0.05 <0.03 0.05 <0.03
1.0 not stated 0.43* <0.03 0.39* <0.03
1.0 not stated 0.05 <0.03 0.05 <0.03
2.0 not stated 0.04 <0.03 0.01 <0.03
Control - <0.01 - <0.01 -
0.4, ten times 5 0.05 - <0.01 -
10 <0.01 - <0.01 -
20 <0.01 - <0.01 -
30 <0.01 - <0.01 -
0.8, ten times 5 0.02 - <0.01 -
10 0.03 - <0.01 -
20 0.08 - <0.01 -
30 0.01 - <0.01 -
Control - <0.02-<0.03 - - -
0.4 8 0.02 - - -
13 <0.02 - - -
28 <0.02-<0.07 - - -
52 0.04 - - -
0.8 8 0.07 - - -
13 "neg" - - -
28 <0.02 - - -
42 <0.02-0.02 - - -
* These figures are regarded as exceptional in light of all other available data.
Although neither rice bran or straw is reported to be an appreciable
item of international trade, residues may be of importance insofar as
they may persist in animal products. A summary of data provided by the
references in Table IV shows bran residues for 11 sites to range from
<0.01 to 2.30 ppm, with a mean of 0.35 ppm. The data are heavily
weighted by one figure from India; without this figure, the range is
<0.01 to 0.80 ppm, with a mean of 0.15 ppm. Rice straw residues from
the same sources (25 samples) ranged from 0.04 to 2.90 ppm, with a
mean of 1.02 ppm. Residues of delta-keto endrin were found in bran and
straw containing high residues of endrin but seldom exceeded 10% of
the total residue and never exceeded 20%.
Rice bran is used mainly as a component of locally produced poultry
feed. The significance of endrin residues in poultry feed in giving
rise to residues in meat and eggs may be judged from data in the
published literature.
Cummings et al., (1967) fed diets containing between 0.05 and 0.45 ppm
endrin to White Leghorns for 14 weeks and found residues ranging from
<0.01 to 0.06 ppm endrin in breast meat and from 0.25 to 3.5 ppm
endrin in depot fat. Similar diets gave endrin residues of 0.03 to
0.32 ppm in eggs as estimated from graphs (Cummings et al., 1966).
Terriere et al. (1959) fed diets containing 0.10 to 0.75 ppm endrin
for 6 weeks and found <0.1 to 0.2 ppm endrin in breast meat, <0.1 to
0.3 ppm in leg meat, 0.6 to 3.6 ppm in depot fat and <0.1 to 0.3 ppm
in eggs. A feed level of 2.25 ppm for 6 weeks gave 17.0 ppm in depot
fat.
In sugar cane
In experiments extending from 1957 to 1965, Shell Development Co.
applied endrin to sugar cane in Louisiana and Florida. When 0.3 lb
ai/acre of 2 percent granular was applied at 3-6 applications and
postharvest intervals ranging from 12-120 days were observed, average
endrin residues ranging from <0.01 to 0.05 ppm were obtained.
Keto-endrin residues were determined in four samples and did not
exceed <0.02 on the average. Residues in control samples ranged from
<0.01 to 0.04 ppm. No endrin was detected in molasses or refined
sugar prepared from the treated cane. One group of samples treated
with 0.5 lb ai/acre of E. C. had <0.1 ppm endrin (sensitivity limit
of the method). When 2 percent granular was applied four times at 1.0
lb ai/acre and postharvest intervals of 7-45 days were observed, the
endrin residues ranged from 0.04 to 0.08 ppm. The same formulation
applied four times at 0.22 lb ai/acre and eight times at 0.23 to 0.29
lb ai/acre resulted, after postharvest intervals of 71 and 64 days,
respectively, in endrin residues of <0.10 ppm. (Shell Development Co.
Reports RES-61-53, RES-63-159 DLI-136 (1963), DLI-157 (1964),
RES-64-1, DLI-160 (1965), RES-58-48A, RES-57-44.
In grains
Data obtained on a variety of small grains in the U.S.A. are
summarized in Table V.
In India, endrin (2% granular) was applied to sorghum at 0.4 kg ai/ha
for 4 to 6 applications. Samples taken from 42 to 75 days after last
treatment had residues in grain ranging from <0.01 to 0.02 ppm endrin
and in straw ranging from 0.14 to 0.70 ppm endrin. Control samples had
<0.01 to 0.02 ppm in grain and 0.03 to 0.31 ppm in straw (Shell
Research Ltd., Report WKGR 0071/70).
In the U.S.A., endrin E.C. was applied to sorghum once at either 0.25
or 0.50 lb ai/acre. A pre-harvest interval of 111 days resulted in
endrin residues of <0.02 ppm in grain, <0.02 ppm in straw, and
<0.02 ppm in silage (73 days PHI). A pre-harvest interval of 63 days
resulted in endrin residues of <0.02 ppm in grain, 0.03 and 0.09 ppm
in straw, and <0.02 and 0.15 ppm in silage (39 days PHI); the latter
value is suspect, according to the analysts (Shell Chemical Co.,
Report PRL-66-113). A pre-harvest interval of 29 days resulted in
residues of <0.05 and 0.05 ppm in grain (Velsicol Corp., Report
TSR-2575).
Single applications of endrin E.C. to sweet corn (maize) in the U.S.A.
at 0.25 or 0.50 lb ai/acre, 37 days before harvest, resulted in
residues of <0.02 ppm of endrin in grain and husk. Some controls gave
an apparent residue of 0.02 to 0.03 ppm (Shell Chemical Co., Report
PRL 66-67g).
In apples
Experiments conducted by Shell in the U.S.A. in which endrin E.C. or
W.P. was applied at 1.5 to 4.0 lb ai/acre to the soil of orchards gave
no detectable residues in whole apples at harvest time; detection
limits were 0.01-0.002 ppm for the methods employed (Shell Development
Co., Report RES-61-60, Shell Chemical Co., Report PRL-69-119, Ibid,
PRL-69-95). In recent studies by Horsfall et al. (1970), picked fruit
residues ranged from <0.002-0.003 ppm. In fallen fruit, residues were
sometimes higher, ranging up to 0.023 ppm.
FATE OF RESIDUES
General comments
Endrin undergoes rearrangement to delta-keto endrin in sunlight, in
the presence of strong acids, and by thermal treatment. Endrin also
undergoes thermal and photo degradation to yield small amounts of the
aldehyde SD 7442. Delta-keto endrin can undergo a rearrangement to the
"bird cage alcohol" when treated with very dry Florisil adsorbent
(Korte and Porter, 1970).
TABLE IV
Endrin residues in polished and unpolished rice
Applic. rate Harvest Residues, ppm
kg am/ha × interval, Polished Brown
Country Formulation no. of applic. days rice rice Reference
India 2% gran. 0.2, 0.4, 0.6, 49-79 <0.01 <0.01-0.01 Shell Res.Ltd.Report, WKTR 0023/69
2.0 × 4-7
control - - <0.01 <0.01
Venezuela E.C. 0.29 × 1 95 0.02 0.03 Ibid,WKGR 0046/70
Thailand control - - <0.01 <0.01 Ibid,WKGR 0087/70
2% gran. 0.2, 0.4, 0.4 56-83 <0.01 <0.01
20% E.C. 0.16 × 3 46-72 <0.01 <0.01-0.23*
Philippines control - - <0.01-0.03 <0.01-0.03 Ibid,WKGR 0078/70
2% gran. 0.2, 0.4, 0.4 44-60 <0.01-0.02 <0.01-0.05
20% E.C. 0.16 × 3 33-49 <0.01-0.03 <0.01-0.03
Indonesia control - - - <0.01 Ibid,WKTR 0078/68
20% E.C. 0.2-0.62 21 - 0.03-0.05
× 7 or × 8
Thailand 20% gran. 0.4 X3, X4 not <0.02 - Ibid,BEGR.0045/70
0.8 × 3 stated (Endrin and Keto -
Endrin Keto Endrin Keto
India control - - <0.02 <0.02 0.09 <0.02 Ibid
2% gran. 0.4 - 0.02 <0.02 0.23 0.03
0.8 45 0.04 <0.02 0.47 0.04
* One exceptional result - reason not identified
TABLE V
Endrin residues in small grains - U.S.A.
Applic. rate, Postharvest Endrin residues,
lb ai/acre interval, ppm
Crop × no. of applic. days Grain Straw Reference
Wheat 0.25 × 1 26 0.03 - Shell Dev. Co.
0.50 × 1 26 0.06 - Report RES-
Oats 0.25 × 1 26 0.15 - 62-124 (1963)
0.50 × 1 26 0.50 -
Wheat 0.25 × 1 62 <0.01 0.27 Shell Chem.Co.
0.25 × 1 96 <0.01 0.02 Report PRL-
0.25 × 2 62 <0.01 0.37 65-41 (1966)
Barley 0.50 × 1 60 <0.05 0.07
0.50 × 1 32 <0.05 0.09-0.38
Wheat 0.25 × 1 >150 0.01 - Velsicol Corp.
0.50 × 1 >150 0.03 - Report TSR-
Barley 0.25 × 1 >150 <0.01 - 2578 (1966)
0.50 × 1 >150 <0.01 -
In animals
Homogenates from cow or pig liver upon addition of NADH2 converted 38
ug of endrin, after 72 hours incubation, to metabolites which were
identical to those found from living organisms (Korte, 1967; Klein et
al., 1968a). See "Biochemical aspects".
In plants
14C labelled endrin was applied at either 1.04 or 2.08 mg/plant to
the leaves of tobacco under conditions of free or restricted aeration.
Six weeks after treatment, 32-47% of the applied radioactivity was
found on and in the tobacco plants, the lowest residues being in the
plants grown under free aeration. (Korte and Porter, 1970).
Shortly after the first buds emerged, the leaves of each of 11 cotton
plants were treated with 4.2 mg 14C-labelled endrin; treatment was
repeated after two and six weeks (total ca. 120 ppm). Twelve weeks
after the last application, quantitative measurement of the residues
revealed only very low concentrations in the cotton seed (0.033 ppm),
somewhat more in the fibers (0.36 ppm), and about 80% in and on the
leaves. Two thirds of the applied radioactivity was lost to the
atmosphere during the test. Besides endrin, there were two groups of
degradation products in the cotton plants, one group slightly more
hydrophilic than endrin and one very hydrophilic. One component of the
less hydrophilic group was isolated and gave mass and IR spectra
identical with that of delta-keto endrin (Korte and Porter, 1970).
When 14C-labelled endrin was applied at 50 ug/plant to young cabbage
plants, 66 percent of the activity had evaporated after two weeks, 70
percent after three weeks, and 75 percent after four weeks. Activity
not evaporated was found in the plants as endrin and hydrophilic
metabolites. After administration of 500 ug/plant, the concentration
of activity decreased from leaves to stalks to roots to soil, while
the ratio of metabolites to endrin increases in the same order. The
metabolite fraction consisted of two compounds, a very hydrophilic
main metabolite and delta-keto endrin (Weisgerber et al., 1968).
Similar results were obtained with carrots (Klein et al., 1968b).
Foliar application of 14C-labelled delta-keto endrin to white cabbage
has shown that it is more persistent than endrin but metabolizes more
rapidly (about 15 percent compared with about 10 percent for endrin).
The converted keto-endrin consisted mainly of a very hydrophilic
metabolite, the concentration of which is highest in leaves and stalks
(Korte and Porter, 1970).
Residues in soybean plants grown in soil treated with 14C-labelled
endrin were mainly delta-keto endrin, and endrin alcohol in addition
to endrin aldehyde were believed present (Nash and Beall, private
communication).
In soil
Matsumura et al., (in press); incubated 14C-labelled endrin with 150
cultures of microorganisms isolated from soils and found 25 active in
degrading endrin, all of which had previously been found to degrade
dieldrin. Seven different metabolites of endrin were found, with three
major and four minor ones; tentative structures have been assigned
(Korte and Porter, 1970).
In storage and processing
The effects of processing on the residues of endrin in dairy products
was studied by Langlois et al., (1965). Butter, ice cream, cheese,
condensed milk and dry whole milk were manufactured from milk
contaminated with endrin either by direct addition or through feeding
to cows. In general, they found that the residues concentration in the
fat remained fairly constant during processing except for drastic heat
treatment to produce dry whole milk which caused major reductions
(>50 percent).
When hens fed on a diet containing endrin were cooked in water at
190-200°F for three hours, the residues were reduced up to 90 percent
(Liska et al., 1967). Autoclaving the carcass at 15 psi for three
hours removed essentially all residues. Metabolites of endrin ware not
determined.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of endrin were monitored in cottonseed oil produced by mills
in Venezuela, Brazil, India and the United States. In Venezuela,
weekly samples were taken for nine weeks, and endrin residues ranged
from <0.02 to 0.05 ppm for crude oil, <0.02 to 0.02 ppm for
decolorized oil and <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR. 0119.70, 1970). In Brazil, seven samples were taken over
thirteen weeks, and endrin residues ranged from <0.01 to 0.01 ppm for
crude oil and <0.01 to <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR.0120.70, 1970). In India, only crude oil samples were
available, and these contained from 0.04 to 0.06 ppm endrin (Shell
Research Ltd., Report WKGR 0091.70). In the United States, ten samples
of refined oil from California were analyzed and found to contain
<0.03 to 0.03 ppm endrin and <0.02 ppm delta-keto endrin (Shell
Development Co., RES-63-155).
In addition to oil, cottonseed cakes have also been studied for endrin
residues, since these are used widely as an important ingredient of
cattle feed concentrates. Figures for cattle cake samples
corresponding to the samples of cottonseed oils given in the preceding
paragraph are as follows: Venezuela, <0.01 to 0.02 ppm; Brazil <0.01
to 0.08 ppm; India, <0.01 ppm. In the United States, data obtained on
cakes and meals corresponding to the samples listed in Tables II and
III ranged from <0.01 ppm to <0.04 ppm; most residues were below the
detection limit of <0.01 ppm.
Total diet studies conducted in the U.K.and the U.S.A. indicate that
endrin residues are unlikely to arise in human diets as a result of
the use of endrin in cotton (Abbott et al., 1969; Assoc. of Public
Analysts, 1969; Duggan, 1968; USDA, 1968). In the U.K., no endrin
residues were found in vegetable oils, fats, milk, milk based infant
foods, beef, beef sausages or meats. In the U.S.A., no endrin residues
were found in finished or crude cotton-seed oil, milk, milk products
or manufactured milk products.
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967) apply to the
determination of endrin. The compound can be determined in fatty and
nonfatty foods by the multiresidue method and gas chromatography
(Pesticide Analytical Manual., Vol. I and II, U.S. FDA). Endrin in
eluted from the Florisil column in the 15% ethyl ether/petroleum ether
fraction. Additional cleanup by MgO-Celite column and alkaline
hydrolysis in required, and recovery is usually in the range of
80-100%. When a gas chromatograph containing 10% DC 200 on Gas-Chrom Q
column at 200°C with 120 ml/min N2flow and an electron capture
detector is used, the following retention times relative to aldrin are
obtained: endrin - 2.05, endrin aldehyde - 2.30, endrin alcohol
- 2.50, delta-keto endrin -3.50. The sensitivity of the method is
good; 2 ng of endrin gives a “ full scale response on a 10-9amp full
scale recorder. A procedure for the determination of delta-keto endrin
in mammalian samples has been developed (Zavon et al., 1965), and a
general method is available (Shell Development Co., Anal. Method MMS
53/64). Residue methods have not yet been developed for monohydroxy
endrin, 9-keto endrin, endrin, endrin aldehyde or the "bird cage
alcohol" isomer of endrin.
An infrared method for endrin has been described (Gershman, 1961). An
absorbance peak at 10.2 microns is used for calculations. The method
requires a 1 kg ample for 0.25 ppm.
NATIONAL TOLERANCES
Country Commodity Tolerance ppm
Australia vegetables including 0.1
beans, crucifers,
potatoes, tomatoes
United States broccoli, brussels 0
sprouts, cabbage,
cauliflower, cucumbers, extended1
cottonseed, eggplant,
peppers, potatoes,
United States
(cont'd) sugar beets and sugar extended1
beet tops, summer squash,
tomatoes
1 The word "extended" appears where action is being taken to acquire
data to petition for a tolerance.
Australia has not set a tolerance for endrin in cottonseed, however,
an action level has been set for endrin residues in animal feeds at
0.03 ppm.
APPRAISAL
Endrin is widely used to control insect pests on cotton and to lesser
extent on rice. It is used to a limited extent as an insecticide on
small grains, vegetables and sugar cane and is occasionally used for
mouse control in orchards. The ways in which endrin is applied vary
widely, as do the numbers of applications.
The most important consideration regarding endrin residues in food
arise from its use on cotton; cottonseed oil is used for cooking or
margarine manufacture, and the extracted cottonseed cake is used as
cattle feed. Controlled feeding experiments with cattle indicate that
whole milk residues would reach a plateau of 0.04 times the residue in
feed. Once ingestion stops, excretion in milk soon ceases (two to six
weeks). Cottonseed cake for feed should have less than 0.1 ppm of
endrin residues if the recommended practical residue limits for whole
milk and milk products are not to be exceeded.
Rice bran is used as a component of poultry feed; feeding studies on
hens indicate that endrin residues in depot fat would reach about five
times the level in feed; for eggs the ratio would be about 0.7 times.
Calculations indicate that it is unlikely that significant endrin
residues would be found in cooked poultry or eggs. The rice bran used
as a constituent of poultry feeds should not contain residues greater
than 1 ppm if the recommended practical residue limits for poultry and
eggs are not to be exceeded.
In the absence of experimental residue data at the recommended
preharvest interval of 45 days or longer, the meeting did not
recommend a tolerance for oats.
Under field conditions, endrin is rapidly loot from plants through
evaporation - 60-80 percent over six to 18 weeks, depending on the
crop.
Endrin is metabolized by plants to delta-keto endrin, a rearrangement
product, and to very hydrophilic metabolites; traces of the aldehyde
(SD 7442) and alcohol have been reported. The parent compound appears
to be the major residue at all times.
The widespread use of endrin on cotton does not appear to give rise to
measurable residues in the human diet. Dietary studies in the United
States have revealed no endrin residues in finished cottonseed oils,
milk, milk based infant foods, milk products, beef or meats and trace
amounts in margarine and fats. Exceptionally, one diet composite
sample in one sampling location in the United States contained 0.20
ppm of endrin in oils, fats and shortening.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The following tolerance and practical residue limits are to apply to
raw agricultural products moving in commerce unless otherwise
indicated. All figures include the sum of endrin and delta-keto
endrin.
TOLERANCES
ppm
Cottonseed, cottonseed oil (crude) 0.1
Cottonseed oil (finished), maize
(sweet), wheat, barley, sorghum,
rice (brown or polished), apples 0.02
PRACTICAL RESIDUES LIMITS
Milk and milk products (fat basis) 0.02
Fat of poultry 1
Eggs (shell-free) 0.2
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate long-term study in a strain of mice of known tumour
susceptibility.
REFERENCES
Abbot, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric. 20; 245
Altmeier, G., Klein, W. and Korte, F. (1969) Tetrahedron Letters,
49: 4269-4271
Association of American Pesticide Control Officials, Inc. (1966)
Pesticide Chemicals Official Compendium, 1966 Ed., 475-477
Association of Public Analysts. (1967) Joint Survey of Pesticide
Residues in Foodstuffs sold in England and Wales
Baldwin, M.K., Robinson, J. and Parke D.V. (1970) The metabolism of
endrin in the rat. Unpublished report from the Tunstall Laboratory,
Shell Research Ltd., Sittingbourne and the University of Surrey
Barrentine, B.F., and Cain, J.B. (1970) Unpublished work cited in
Comptes Rendus, 25th Conference of IUPAC, Commission VI.5.1, Appendix
VII
Barth, R.A.J. (1967) Pesticide toxicity in primates. Doctoral thesis,
Tulane University, Louisiana p. 110-111
Boyd, E.M. and Stefec, J. (1969) Dietary protein and pesticide
toxicity: with particular reference to endrin. Canad. med. Ass. J.,
101: 335-339
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27:81-138
Coble, Y., Hildebrandt, P., Davis, J., Raasch, F. and Curley, A.
(1967) Acute endrin poisoning. J. Amer. med. Ass. 202: 489-493
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Comptes Rendus. (1970) Fate of organochlorine pesticides in vegetable
oil processing. 25th Conference of IUPAC, Commission VI.5.1, Terminal
Pesticide Residues, Appendix VII. p. 187-189
Cummings, J.G., Zee, K.T., Turner, V., Quinn, F..and Cook R.E. (1966)
Residues in eggs from low level feeding of five chlorinated
hydrocarbon insecticides to hens. J. Assoc. Off. Anal. Chem. 49:
354-364
Cummings, J.G., Eidelman, M., Turner, V., Reed, D., Zee K.T. and Cook,
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydro-carbon insecticides to hens. J. Assoc. Off. Anal.
Chem. 50: 418-425
Dale, W.E., Copeland, M.F. and Hayes, W.J. Jr. (1965) Chlorinated
insecticides in the body fat of people of India. Bull. Wld Hlth Org.,
33: 471-477
Davies, G.M. and Lewis, I. (1956) Outbreak of food poisoning from
bread made of chemically contaminated flour. Brit. med. J., (2):
393-398
Dewitt, J.B. (1956) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J. Agr. Fd. Chem., 10: 863-866
Diechmann, W.B., MacDonald, W.E., Radomski, J. Blum, E.B. Bevilacqua,
M. and Keplinger, M. (1970) The tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Industr. Med., 39: 314
Duggan, R.E. (1968) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pesticides Monotoring
Journal 1 (4): 2-7
Dunachie F.F. and Fletcher W.W. (1966) Effect of some insecticides on
the hatching rate of hen's eggs. Nature, 212: 1062-1063
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. econ. Entomol., 50: 348-349
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. Econ. Ent. 50: 348-349
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23(1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FDA, (1967) Pesticide analytical manual, HEW, FDA, Vol. I and 11
Frear, D.E.H. (1955) Endrin in pesticide index, Third Edition, D. van
Nostrand Co. Inc., New York. p. 68-69
Gershman, L.L. (1961) Infra-red determination of endrin residues. J.
Assoc. Off. Anal. Chem. 44: 212-214
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Hayes, W.J., Jr., (1963) Endrin in Clinical handbook on economic
poisons, revised edition. pp. 68.70. Publ. Hlth. Ser. Publn No. 476.
US Govnmt Printing Off. Washington D.C.
Hayes, W.J., Jr., Dale, W.E. and Baise, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people of New Orleans. Life Sci.,
4: 1611-1615
Hayes, W.J. and Curley, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environm. Hlth, 16: 155-162
Hine, C.H., Eisenlord, G., Loquvam, G.S. and Leung, T. (1968) Results
of reproduction studies on rats fed diets containing endrin over three
generations. Unpublished report prepared by the Hine Laboratories,
Inc., San Francisco, submitted by Shell International Research
Horsfall, F. Jr., Webb, R.E., Price, N.O. and Young, R.W. (1970)
Residues in apples subsequent to ground sprays of endrin. J. Agr. Food
Chem. 18: 221-223
IUPAC, (1967) Proceedings of the Commission on Terminal Residues,
Vienna, August 1967. Appendixes VI and VII
Jager, K.W. (1970) Aldrin, dieldrin and telodrin. An epidemiological
and toxicological study of long-term exposure. Doctoral thesis,
Leiden, 1970
Jolley, W.P., Stemmer, K.L., Grande, F., Richmond, J. and Pfitzer,
E.A. (1969) The effects exerted upon beagle dogs during a period of
two years by the introduction of 1, 2, 3, 4, 10, 10-hexachloro-6,
7-epoxy-1, 4, 4a, 5, 6, 7, 8, 8a-octahydro-1, 4-endo, endo-5,
8-dimethanonaphthalene into their daily diets. Unpublished report from
the Kettering Laboratory University of Cincinnati submitted by Shell
International Research
Kiigemagi, U., Sprowls, R.G. and Terriere, L.C. (1958) Endrin content
of milk and body tissues of dairy cows receiving endrin daily in their
diet. J. Agr. Fd. Chem., 6: 518-521
Klein, W., Müller, W. and Korte, F. (1968a) Insektizide im
Stoffwechsel, XVI. Ausscheidung, Verteilung und Stoffwechsel von
Endrin (14C) in Ratten. Liebigs Ann. Chem. 713: 180-185
Klein, W. et al., (1968b) Qualitas Plant. Mater. Vegetabiles 15,
225-238
Klein, W. and Drefahl, B. (1970) "Jahresbericht 1969" Institut für
ökologische Chemie der Gesellschaft für Strahlenforschung mbH,
München, Schloss Birlinghoven, April 1970
Korte, F. (1967) Metabolism of chlorinated insecticides. Unpublished
report from the Radiochemical Laboratories, Bonn University
Korte, F. and Porter, P.E. (1970) Minutes of the fifth meeting of the
IUPAC Commission on terminal pesticide residues, September, 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Langlois, B.E., Liska, B.J. and Hill, D.L. (1965) The effects of
processing and storage of dairy products on chlorinated insecticide
residues. J. Milk and Food Technol. 28: 9-11
Liska, B.J., Stemp, A.R. and. Stadelman, W.J. (1967) Food Technol.
21: 435
Ludwig, G. (1965) Excretion, metabolism and storage of endrin 14C
after oral administration to a rat. Minutes of the fifth meeting on
the fate of organic compounds in the living organism. 20-21 October
1965, Birlinghoven Laboratory
Ludwig, G. (1966) Investigations with 14C-labelled insecticides. XX
Excretion, metabolism and storage of endrin - 14C after oral
administration to a rat. Birlinghoven Biomonthly Res. Summary
(January), p. 13-14
Morris, R.D. (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse Peromyscus maniculatus. Canad. J.
Zool., 46: 951-958
Nash, R.G. and Beall, M.L. Jr. (1970) Extraction and identification of
endrin and heptachlor degradation products. Submitted to J. Assoc.
Off. Anal. Chem., October 1970
Nelson, S.C., Bahler, T.L., Hartwell, W.V., Greenwood, D.A. and
Harris, L.E. (1968) Serum alkaline phosphatase levels, weight changes
and mortality ratios of rats fed endrin. J. Agr. Pd. Chem., 4:
696-700
Radeleff, R.D. (1956) Hazards to livestock of insecticides used in
mosquito control. Mosquito News, 16(2): 79-80
Richardson, A. (1970) Unpublished untitled letter from Tunstall
Laboratories to Shell International Research
Richardson, A. Robinson, J. and Baldwin, M.K. (1970) Metabolism of
endrin in the rat. Chem. Industr., 502-503
Robinson, J., Richardson, A., Hunter, C.G., Crabtree, A.N. and Rees,
H.J. (1965) Organo-chlorine insecticide content of human adipose
tissue in south-eastern England. Brit. J. industr. Med., 22: 220-229
Shell. (1965) Alkaline phosphatase in endrin-exposed workers.
Unpublished report prepared by the Industrial Medical Department,
Shell Nederland Chemische Fabrieken N.V. Rotterdam
Shell Chemical Co., Reports. (1965-69) PRL-65-41, PRL-66-67g,
PRL-66-113, PRL-67-24, PRL-69-28, PRL-69-95, PRL-69-119
Shell Development Co., Reports. (1961-65) RES-61-60, RES-62-124,
RES-63-155, RES-64-39 RES-64-9
Shell Research Ltd., Report (1968-70) WKTR 0078/68, 0023/69, WKGR
0046/70, 0072/70, 0078/70, 0087/70, 0071.70, 0091.70, 0119.70, 0120.70
Sherman, M. and Rosenberg, M.M. (1954) Sub-chronic toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 47: 1082-1083
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chem. Soc., 45:
866-869
Smith, S.I., Weber, C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocarbon insecticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Soto, A.R. and Diechmann, W.B. (1967) Major metabolism and acute
toxicity of aldrin, dieldrin and endrin. Environm. Res., 1: 307-322
Speck, L.B. and Maaske, C.A. (1958) The effects of chronic and acute
exposure of rats to endrin. A.M.A. Arch. industr. Hlth, 18: 268-272
Street, J.C., Butcher, J.E., Raleigh, R.J. and Clanton, D.C. (1957)
Tissue storage and transferral to the lamb of aldrin, dieldrin and
endrin when fed to bred ewes. Proc. West. Sec. Amer. Soc. Anim. Prod.,
(Pullman, Washington), 46: 1-6
Terriere, L.C., Kiigemagi, U. and England, D.C. (1958) Endrin content
of body tissues of steers, lambs and hogs receiving endrin in their
daily diet. J. Agr. Fd. Chem., 6: 516-518
Terriere, L.C., Arscott, G.H. and Kiigemagi, U. (1959) The endrin
content of eggs and body tissue of poultry receiving endrin in their
daily diet. J. Agr. Fd Chem., 7: 502-504
Treon, J.F., Clevelend, F.P. and Cappel, J. (1955) Toxicity of endrin
for laboratory animals. J. Agr. Fd. Chem., 3: 842-848
USDA, Agriculture handbook No. 331. (1968) Suggested guide for the use
of insecticides to control insects affecting crops, livestock,
households, stored products, forests and forest products
USDA, Residues in food and feed. (1968) Pesticide residue levels in
foods in the United States from 1 July 1963 to 30 June 1967.
Pesticides Monitoring Journal, 2(1): 2-46
USDA, Summary of registered agricultural pesticide chemical uses.
(1969) Vol. III, Insecticides, repellents, acaricides. Endrin,
III-E-2.1, 2.2, 2.3
Van Dijk, M.C. (1968) Chlorinated insecticides and the serum alkaline
phosphatase. Proc. Sixth Shell Industrial Doctors Meeting, 28-30 May
1968, Amsterdam
Velsicol Corp., (1968-70) Reports TSR 2578, TSR 2575 (Unpublished -
1970)
Weeks, D.E. Endrin food-poisoning. A report of four outbreaks caused
by two separate shipments of endrin-contaminated flour. Bull. Wld Hlth
Org., 37: 499-512
Weisgerber, I., Klein, W., Djirsarai, A., and Korte, F. (1968) Ann.
Chem. 713: 175-179
Williams, S., and Mills, P.A. and McDowell, R.E. (1964) Residues in
milk of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Anal. Chem., 47:
1124-1128
Williams, D.A. (1966) Alkaline phosphatase activity following exposure
to endrin, comments on the paper by Nelson et al. Unpublished report
from the Statistics Unit, Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Witherup, S., Stemmer, K.L., Taylor, P., Bietsch, P. and Pfitzer, E.A.
The incidence of neoplasms in two strains of mice sustained on diets
containing endrin. Unpublished report from the Kettering Laboratory,
University of Cincinnati submitted by Shell International Research
Zavon, M.R. (1961) The toxicology and pharmacology of endrin.
Unpublished report from the Kettering Laboratory, University of
Cincinnati
Zavon, M.R., Hine, C.H. and Parker, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Amer. med. Ass., 193: 837-839
 Other relevant chemical properties
Endrin (molecular weight 380.93) is a cream to light tan coloured
flowable powder which melts at 200+°C with decomposition. It has a
vapour pressure of 2 × 10-7mm Hg (Torr) at 77°F (25°C). It is stable
in the presence of ordinary alkaline reagents but tends to rearrange
to less insecticidally active substances in the presence of acids,
certain metal salts and catalytically active carriers. Endrin is
moderately soluble in benzene and acetone; sparingly soluble in
alcohols, paraffins, and xylene; insoluble in water.
Purity
Endrin, technical, 95 percent minimum
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The toxicology of this compound was evaluated by the WHO Expert
Committee on Pesticide Residues at the Joint FAO/WHO Meetings in 1963
and 1965 (FAO/WHO, 1964, 1965). Since that time considerable new
information has become available, and a completely revised monograph
has been produced.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
From early studies it was thought that, like other chlorinated
hydrocarbons, endrin, when fed to animals, was partly stored unchanged
in the tissues particularly in the body fat (Kiigemagi et al., 1958;
Street et al., 1957; Terriere et al., 1958, 1959 and Treon et al.,
1955). When fed at high levels it had been reported to be excreted in
milk and eggs (Ely et al., 1957; Street et al., 1957 and Terriere et
al., 1958). The ratio of the level in fatty tissue to the dietary
level has been estimated at 0.5-2, depending upon the dietary level
(Kiigemagi et al., 1958; Terriere et al., 1958 and Treon et al.,
1955).
Unlike the situation with its stereoisomer dieldrin, the extent of
storage of endrin is relatively small and the compound is eliminated
more quickly, due probably to its rapid biliary excretion (Cole et
al., 1970). Levels of the 9-keto metabolite of endrin in four human
fat samples were all less than 0.0004 ppm (Richardson, 1970).
A male rat was fed a dietary level of 30 ppm of 14C-labelled endrin
for eight days. About 60-70 percent excretion was noted from the first
day, and after three days the faeces contained more than 80 percent of
the administered radioactivity. On day 9, 84 percent had been
excreted, and there appeared to be a level of saturation after 6-7
days of feeding. The faeces contained about 75-80 percent
metabolities, of which there were at least two different compounds.
The fatty tissue stored 3-4 ppm of endrin, giving a storage ratio of
about 10. Compared to 84 percent excretion in the faeces, only about
0.5 percent was found in the urine (Ludwig, 1965, 1966).
The rapid rate of metabolism and excretion of endrin compared to that
of other chlorinated hydrocarbon insecticides has been confirmed by a
study on rats with and without bile fistula and on the isolated
perfused rat liver (Cole, et al., 1970; Altmeier, et al., 1969). In
rats on daily oral administration of 32 µg/kg, the storage reached a
state of equilibrium after 5-6 days. The half life in male rats was
three days and in female rats four days under these conditions (Klein
and Drefahl, 1970).
Biotransformation
The information available on the metabolism of endrin up to 1967 has
been reviewed (Soto and Deichmann, 1967; Brooks, 1969). The following
experimental data summarizes the pertinent information leading to the
current knowledge on the metabolism of endrin (see figure 1).
In rats, Klein et al. (1968a) and Richardson et al. (1970) found that
endrin is rapidly metabolized and excreted, principally in the faeces.
The faeces contain two metabolites as well as endrin itself. Baldwin
et al. (1970) have now found that the major faecal metabolite is a
secondary alcohol formed by substituting a hydroxyl group for one of
the hydrogens of the methano-bridge of endrin (II). The other faecal
Other relevant chemical properties
Endrin (molecular weight 380.93) is a cream to light tan coloured
flowable powder which melts at 200+°C with decomposition. It has a
vapour pressure of 2 × 10-7mm Hg (Torr) at 77°F (25°C). It is stable
in the presence of ordinary alkaline reagents but tends to rearrange
to less insecticidally active substances in the presence of acids,
certain metal salts and catalytically active carriers. Endrin is
moderately soluble in benzene and acetone; sparingly soluble in
alcohols, paraffins, and xylene; insoluble in water.
Purity
Endrin, technical, 95 percent minimum
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The toxicology of this compound was evaluated by the WHO Expert
Committee on Pesticide Residues at the Joint FAO/WHO Meetings in 1963
and 1965 (FAO/WHO, 1964, 1965). Since that time considerable new
information has become available, and a completely revised monograph
has been produced.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
From early studies it was thought that, like other chlorinated
hydrocarbons, endrin, when fed to animals, was partly stored unchanged
in the tissues particularly in the body fat (Kiigemagi et al., 1958;
Street et al., 1957; Terriere et al., 1958, 1959 and Treon et al.,
1955). When fed at high levels it had been reported to be excreted in
milk and eggs (Ely et al., 1957; Street et al., 1957 and Terriere et
al., 1958). The ratio of the level in fatty tissue to the dietary
level has been estimated at 0.5-2, depending upon the dietary level
(Kiigemagi et al., 1958; Terriere et al., 1958 and Treon et al.,
1955).
Unlike the situation with its stereoisomer dieldrin, the extent of
storage of endrin is relatively small and the compound is eliminated
more quickly, due probably to its rapid biliary excretion (Cole et
al., 1970). Levels of the 9-keto metabolite of endrin in four human
fat samples were all less than 0.0004 ppm (Richardson, 1970).
A male rat was fed a dietary level of 30 ppm of 14C-labelled endrin
for eight days. About 60-70 percent excretion was noted from the first
day, and after three days the faeces contained more than 80 percent of
the administered radioactivity. On day 9, 84 percent had been
excreted, and there appeared to be a level of saturation after 6-7
days of feeding. The faeces contained about 75-80 percent
metabolities, of which there were at least two different compounds.
The fatty tissue stored 3-4 ppm of endrin, giving a storage ratio of
about 10. Compared to 84 percent excretion in the faeces, only about
0.5 percent was found in the urine (Ludwig, 1965, 1966).
The rapid rate of metabolism and excretion of endrin compared to that
of other chlorinated hydrocarbon insecticides has been confirmed by a
study on rats with and without bile fistula and on the isolated
perfused rat liver (Cole, et al., 1970; Altmeier, et al., 1969). In
rats on daily oral administration of 32 µg/kg, the storage reached a
state of equilibrium after 5-6 days. The half life in male rats was
three days and in female rats four days under these conditions (Klein
and Drefahl, 1970).
Biotransformation
The information available on the metabolism of endrin up to 1967 has
been reviewed (Soto and Deichmann, 1967; Brooks, 1969). The following
experimental data summarizes the pertinent information leading to the
current knowledge on the metabolism of endrin (see figure 1).
In rats, Klein et al. (1968a) and Richardson et al. (1970) found that
endrin is rapidly metabolized and excreted, principally in the faeces.
The faeces contain two metabolites as well as endrin itself. Baldwin
et al. (1970) have now found that the major faecal metabolite is a
secondary alcohol formed by substituting a hydroxyl group for one of
the hydrogens of the methano-bridge of endrin (II). The other faecal
 metabolite is also an alcohol. Three days after a single oral dose of
14C-labelled endrin, approximately half of the 14C radioactivity
remained in the bodies of the rats. This material was principally one
metabolite which was identified as 9-keto endrin (I), an oxidation
product of the secondary alcohol found in faeces.
When 14C-labelled endrin was applied orally to rabbits at 0.5 mg/kg
body-weight and at three and four day intervals, four metabolites were
isolated from the urine which appear to have the following chemical
natures: A (40% of excreted radioactivity) is a conjugate compound of
a hydroxy derivative of endrin; B (12%) is a monohydroxy derivative of
an unbridged endrin isomeric ketone; C (40%) is the 4a-hydroxyendrin;
D (8%) has a molecular weight of 420 and the C-C double bond intact in
the chlorinated ring. None of these compounds is the delta-keto endrin
(Korte and Porter, 1970).
Effects on enzymes and other biochemical parameters
In monkeys which had received exposure to an unspecified quantity of
endrin, there were significant changes in the enzymes serum
glutamic-oxaloacetic transaminase and serum glutamic-pyruvic
transaminase (Barth, 1967).
Elevation of serum alkaline phosphatase has been observed in rats fed
25 ppm and possibly 5 or 1 ppm of endrin for 16 weeks (Nelson et al.,
1956) but not in dogs fed 4 ppm of endrin for two years (Jolley et
al., 1969) nor in human subjects occupationally exposed to unspecified
levels of endrin (Shell, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
See under "Long-term studies" (Witherup et al., 1970).
Rat
Commencing at weaning, varying numbers of rats of mixed sex were fed
dietary levels of 0, 2, 6 or 12 ppm of endrin throughout their
lifetime. No primary malignant hepatic tumours were found in any
animals upon histological examination. Two benign hepatic tumours
(haemangiomas) were found in one male control rat and the other in a
female fed 6 ppm of endrin. Tumour incidence in other tissues was also
not significantly different between the control and experimental
animals (Diechmann et al., 1970).
Special studies on reproduction
Chicken egg
Hen eggs were injected with 0.5 or 5 mg per egg. The hatching rate was
40 or 20 percent, respectively (Dunachie and Fletcher, 1966).
When endrin was injected into the yolk of fertile eggs incubated for
seven days in amounts of 0.2 and 2.0 mg/egg, the hatchability was 40
and 6.9 percent, respectively (Smith et al., 1970).
Quail
No eggs were produced from quail which received 1 ppm of endrin in
their diet either as winter maintenance or during the reproductive
period (DeWitt, 1965).
Pheasant
In pheasants there was reduced egg production when fed 10 ppm of
endrin but not at 2 ppm or less. Survival of the chicks to two or six
weeks was also markedly reduced at 10 ppm, but not at lower doses
(DeWitt, 1965).
Mouse
Groups of male and female mice were fed endrin at dietary levels of 0
or 5 ppm for 30 days. Test and control mice were then randomly paired
and continued on the test diet for a further 90 days, there being a
total of 101 pairs in the group fed endrin. The first litters were
significantly smaller than the control group. The time taken to
produce the first litter was not significantly different between the
two groups (Good and Ware, 1969).
Deer mouse
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus manicalatus) of varying ages were fed dietary levels of
0, 1, 2, 4 or 7 ppm of endrin over intermittent periods between which
times the animals were either fed a normal diet or were subjected to
48 hours starvation. The animals were sacrificed by exposing them to
cold stress at -16°C and the time of death recorded. During feeding,
parental mortality increased in proportion to the level of endrin.
Young animals were more susceptible than old. Starvation increased
mortality in all test groups but not in the controls; this effect was
more evident with increasing dose levels. Litter production frequency
and mean litter size before and during experimental feeding were
similar. However, post-natal mortality prior to weaning increased in
the young from parents fed 4 or 7 ppm. Endrin adversely affected the
survival time during cold stress in the females but not in the males
(Morris, 1968).
Rat
In a later study from the same laboratory, groups of ten male and 20
female rats were fed dietary levels of 0, 0.1, 1.0 and 2.0 ppm of
endrin over a period of three generations. The F0 generation was
mated after 79 days on the test diets, and the males were rotated as
in the previous experiment.
The young from the first litter were discarded at weaning and the
parents mated again after ten days to form the F1b generation. Young
from this generation were mated when 100 days old, and this protocol
was followed for three generations, using the second litter in all
cases. The size of the litter in the F3 generation from the 2.0 ppm
group was significantly larger than that from the controls. Mortality
was high in the controls, which resulted in a greater percentage
survival in the F3a litters in the 0.1 ppm group and in all F3b
litters in the test groups. The weights of weanlings were comparable
to the controls except in the F3a litters from the 0.1 ppm, which
were significantly less due probably to the large litter sizes in that
group. Examination of the F3b weanlings revealed no differences in
organ to body-weight ratios. It was stated that there were no
histological abnormalities, but details of the pathology were not
available. Fertility, gestation, viability and lactation indices did
not indicate that endrin affected any of these parameters (Hine et
al., 1968).
Special studies on the photoisomerization product of endrin
When endrin is irradiated with short wavelength ultraviolet light, the
delta-keto compound is formed in 37 percent yield as well as an
aldehyde in 9 percent yield. Under the influence of sunlight, only the
ketone is formed. The ketone is about a quarter as toxic to rate as
endrin and like endrin is more toxic to the male than to the female
(Soto and Diechmann, 1970) (see also "Fate of residues").
Acute toxicity
The major clinical manifestation of endrin intoxication in man
involves convulsions of several minutes duration which may be isolated
and followed by semiconciousness for 15-30 minutes, with complete
recovery after 2-4 weeks. More serious symptoms are continuous
convulsions, high fever and decerebrate rigidity prior to death. Mild
symptoms of poisoning include dizziness, weakness of the legs,
abdominal discomfort and nausea. Temporary deafness and insomnia may
also occur. It has been estimated, based upon reports of outbreaks of
poisoning, that 0.2-0.25 mg/kg body-weight will produce a single
convulsion in man, and that repeated fits will result from 1 mg/kg
(Hayes, 1963).
LD50
Animal mg/kg body-weight References
(oral)
Rat, adult (F) 7 Treon et al., 1955
Rat, young (F) 17 Treon at al., 1955
Rat, adult (F) 40-43 Speck and Maaske, 1958
Rat, young (M) 29 Treon at al., 1955
Rabbit (F) 7-10 Treon at al., 1955
Guinea-pig 16-36 Treon at al., 1955
Monkey 3 Treon at al., 1955
Monkey 12 Barth, 1967
The acute toxicity of endrin appears to be influenced by the diet.
Three groups each comprising about 100 male rats were fed for 28 days
either a normal diet, a normal protein diet containing protein only as
casein or a low protein diet. The acute toxicity to endrin was then
determined by a single oral administration of the pesticide. The LD50
values were 27, 17 and 7 for the animals fed the respective diets,
indicating an approximately four-fold increase in toxicity between the
normal and low-protein diet as well as an effect due to the type of
protein fed (Boyd and Stefec, 1969).
Short-term studies
Quail
Groups each of 40 quail, starting when one day old, were fed dietary
levels of 0, 0.5, 1, 5, 10, 20 or 50 ppm in their diet. Survival was
adversely affected in all the test groups, and there were no survivors
beyond two weeks in the birds fed 10 ppm or more. Food consumption was
abnormally low. Symptoms involved lack of muscular coordination,
tremors, bedraggled appearance and rigidity with occasional convulsive
movements (De Witt, 1956).
Chicken
Groups each of 20 seven-day old chicks were unaffected by a diet
containing 0, 1.5 or 3 ppm of endrin. When the concentration was
increased to 6 or 12 ppm, the birds became highly excitable, failed to
gain as much weight as the controls and the survival rates over a 12
week period were 85 and 5 percent, respectively, compared to 100 per
cent in the controls (Sherman and Rosenberg, 1954).
Pheasant
Day-old pheasants, in groups of 40, did not survive beyond eight days
when fed dietary levels of 5 or 20 ppm. Reduced food consumption
occurred, and the symptoms were the same as those seen in quail (De
Witt, 1956).
Rat
Groups comprising five male and five female rats were fed dietary
levels of 0, 1, 5, 25, 50 or 100 ppm of endrin for up to 16 weeks. All
of the group fed 100 ppm died within the first two weeks, and only two
rats fed 50 ppm and three fed 25 ppm survived. Three males fed 5 ppm
also died; the other animals were continued on the test diet for the
full 16 weeks. Weight loss was roughly dose related but was evident in
all test groups, as was hypersensitivity to tactile stimuli. There was
an initial drop in serum alkaline phosphatase during the first three
to eight weeks' feeding, which was then followed by an increase at all
dose levels. At the end of the 16 weeks, the phosphatase level was
elevated above the controls in all the test groups, the levels being
highest in the groups fed 25 and 50 ppm (Nelson et al., 1956).
However, other statisticians have considered that the elevation of
serum alkaline phosphatase was not significant in the groups fed 1 and
5 ppm of endrin (Williams, 1966).
Dog
In a series of experiments, dogs were fed diets containing from 1 to
50 ppm endrin along with control groups. Two of four animals fed a
diet containing 8 ppm and the one fed 5 ppm died. The two surviving
dogs on 8 ppm were kept on the diet for about six months and then
sacrificed; increased organ to body-weight ratios for the liver,
kidney and brain were found, and histopathological examination showed
degeneration of kidney tissue. Three of four dogs on 4 ppm of endrin
survived, and there were no symptoms in dogs fed 1 or 3 ppm (Treon et
al., 1955).
In an experiment of about 19 months' duration, groups comprising two
male and two female dogs were placed on diets containing 0, 1 or 3 ppm
of endrin. All dogs on 3 ppm had increased organ to body-weight ratios
for the kidney and heart. Some female dogs fed 1 or 3 ppm of endrin
had a renal abnormality characterized by a slight tubular vacuolation;
this change was also observed in the female control dog. Male dogs in
both control and test groups had normal viscera (Treon et al., 1955).
Groups comprising seven male and seven female dogs were fed dietary
levels of 0, 0.1, 0.5, 1.0, 2.0 or 4.0 ppm of dieldrin for two years.
Scheduled autopsies were performed on two dogs of each sex from the 0,
1.0 and 4.0 ppm groups at six and 12 months. There were no deaths due
to the treatment nor were there any changes in body-weight increase or
food consumption in any group. The only clinical abnormalities were in
one female and two male dogs fed 4.0 ppm and one female fed 2.0 ppm
that showed evidence of, or were observed having, convulsions; the
earliest incidence in a male dog after five months on 4.0 ppm. The
only changes in organ weights were occasional slight increases in
liver or liver to body-weight ratios in the dogs fed 2.0 and 4.0 ppm.
After two years, pathological examination showed slight vacuolation of
hepatic cells in the females and diffuse pigmentation in one male and
all females. At 4.0 ppm, vacuolar degeneration and diffuse brown
pigment in the hepatic cells was evident in all dogs, without any sex
differentiation. In two only of the dogs, which had convulsions,
autopsies revealed some pathological changes in the brain. All other
organs in the dogs fed 2.0 or 4.0 ppm and all organs in the dogs fed
1.0 ppm or less showed no morphological changes which were considered
to be attributable to feeding endrin. There were no significant
changes in the blood picture or in the chemical or physical
characteristics of the urine attributable to endrin. After two years,
levels of liver enzymes, prothrombin time bromsulphthalein clearance,
serum protein electrophoresis, glucose, urea nitrogen, cholesterol,
calcium, inorganic phosphorus, total bilirubin or uric acid showed no
changes attributable to endrin feeding (Jolley et al., 1969).
Cattle and sheep
Cattle and sheep were not affected by 5 ppm of endrin in their diet
for 112 days (Radeleff, 1956).
Long-term studies
Mouse
A total of 1600 mice in equal numbers of each sex, consisting of one
inbred and one hybrid strain, were divided into four groups, two of
which were fed a control diet and the other two fed 0.3 or 3.0 ppm of
endrin. Feeding the test diet was started at five weeks of age and
continued through out their normal lifespan, or until sacrifice.
Because of an early high incidence of fibroadenomas occurring in both
control and test groups in the hybrid strain, all the females of that
strain were sacrificed after 72 weeks for pathological examination. A
few of the mice, fed 3.0 ppm only, displayed convulsions in the early
stages of feeding but recovered and survived. Mortality was not
adversely affected by endrin, nor was body-weight or food intake. No
haematological abnormalities were evident except in two males in the
hybrid group fed 0.3 ppm, which had severe leukaemia. In either sex,
the total number of neoplasms was not influenced by the endrin content
of the diet, except in the case of hepatomas in the females of the
hybrid strain, which were significantly higher than the controls in
the mice of the group fed 3.0 ppm, and sacrificed between weeks 53 and
60 of the feeding period. Because of a relatively high incidence of
hepatomas in one group of controls of this strain, the increase at the
3.0 ppm level was considered not due to endrin. It was noted that in
no animals of either sex were there any metastases of the hepatomas
into the lungs (Witherup et al., 1970).
Rat
In a two year experiment, groups each of 20 male and 20 female rats
were given diets containing 0, 1, 5, 25, 50 and 100 ppm of endrin.
Concentrations of 50 and 100 ppm were lethal within a few weeks. The
concentration of 25 ppm increased the mortality rate of the females.
Non-survivors at the three higher levels exhibited diffuse
degeneration of the brain, liver, kidneys and adrenal glands. The
survivors in the two higher levels showed degenerative changes in the
liver only, while those fed at the lower levels had normal viscera.
The level of 5 ppm caused an increase in liver to body-weight ratio in
males and an increase in kidney to body-weight ratio in females. There
was no effect at the 1 ppm level (Treon et al., 1955).
OBSERVATIONS IN MAN
A total of 874 persons were hospitalized, and there were 26 deaths in
several outbreaks of poisoning in Saudi Arabia in 1967 due to
consumption of bread containing endrin. Approximate average levels in
the bread in various outbreaks were 48, 1500 or 400 ppm, corresponding
to a percentage of fatalities of 1.4, 9.5 and 0.4, respectively, among
those poisoned. Signs and symptoms were typical of central nervous
system stimulation and all survivors rapidly returned to normal
(Weeks, 1967).
Three persons in Egypt experienced convulsions after eating bread
containing 126 to 176 ppm of diedrin. There were no deaths (Coble et
al., 1967).
In an incident in the UK, 59 people became ill from ingesting bread
which contained about 150 ppm of endrin, but there were no deaths
(Davies and Lewis, 1956). The maximum amount of endrin consumed has
been estimated to have been 1 mg/kg body-weight (Zavon, 1961).
Studies in human subjects experiencing intoxication from endrin (Coble
et al., 1967, Weeks, 1967) and from occupational workers (Hayes and
Curley, 1968, Jager, 1970) have demonstrated that endrin rapidly
disappears from the blood in cases of acute intoxication and cannot be
detected in the fat or blood of people exposed to endrin unless
symptoms of intoxication are evident.
Endrin has not been reported to be found from studies involving levels
of organochlorine pesticides in human body fat in India, UK or USA
using analytical methods sensitive to <0.03 ppm (Dale et al., 1965;
Hayes et al., 1965, Robinson et al., 1965 and Zavon et al., 1965).
Levels of the 9-keto metabolite of endrin in four human fat samples
were all less than 0.0004 ppm (Richardson, 1970).
Among workers in a plant manufacturing endrin and a number of other
pesticides, no detectable amounts of endrin were found in samples of
plasma, fat or urine. Exposure was for an average time of 2,106 hours.
Based upon the limit of detection, the levels of endrin, were <0.0030
ppm in plasma, <0.03 ppm in fat and <0.0016 ppm in urine. Endrin
has, however, been detected in the serum and urine of people who
received amounts sufficient to produce intoxication (Hayes and Curley,
1968).
Serum alkaline phosphatase was determined in 30 workers who had been
exposed to endrin for periods from six weeks to eight years. There was
no difference in the levels found in the exposed group and those found
in a group comprising nine unexposed individuals, nor was there any
relationship detected between the phosphatase levels and the duration
of exposure of the workers (Shell, 1965).
In workers exposed to endrin during periods up to eight years, no
significant changes in the level of serum alkaline phosphatase were
observed during a 13-month observation period (Van Dijk, 1968).
It is estimated that the blood level of endrin below which no signs or
symptoms of intoxication occurs is in the range of 0.05 - 0.100 µg/ml.
Measurable blood levels (detection level 0.005 µg/ml) occur only after
gross over-exposure. The half-life of endrin appears to be
approximately 24 hours. Medical control of a group of workers exposed
over periods up to 13 years has failed to show any effects of
long-term exposure. The blood picture, results of urinalysis,
activities of serum glutamic-oxaloacetic and glutamic-pyruvic
transaminases, alkaline phosphatase and lactic dehydrogenase remained
all within normal limits. Occasional electroencephalographic changes
returned to normal. Absenteeism due to disease or accidents was
comparable to that of a control group. Because of the short half-life
of endrin, it is (unlike dieldrin) impossible to calculate the average
level of exposure of the workers to endrin (Jager, 1970).
A decrease in the blood levels of pp'-DDE and an increased excretion
of 6-ß-hydrocortisol in relation to 17-hydroxy-corticosteroids was
present in the workers employed in manufacturing and who were exposed
to endrin and its intermediates. The compound responsible is not known
and further studies are in progress (Jager, 1970).
COMMENTS
The primary site of action of endrin is the central nervous system.
This fact is evidenced by convulsions which result from acute
poisoning and from administration of repeated relatively high doses.
Unlike dieldrin, endrin is rapidly metabolized by animals; the storage
in the fat of animals is very low compared with other compounds of
similar chemical structure. In rats, it is excreted mainly in the
faeces as endrin, 4a-hydroxyendrin, and an unidentified endrin
alcohol; a third metabolite, 9-keto-endrin, is stored in the tissues.
In rabbit, the main route of excretion is the urine, in which four
metabolites were demonstrated, one of which is the 4a-hydroxyendrin.
The plant metabolite of endrin, delta-keto endrin, is rapidly
metabolized by animals. Three metabolites were found in rabbit urine
after oral administration of delta-keto endrin. It is understood that
delta-keto endrin is unlikely to be formed under conditions of good
agricultural practice, and that the compound is less toxic to mammals
than endrin.
A long-term study in mice failed to produce conclusive results
relative to the carcinogenic potential of endrin. Endrin is more
acutely toxic to animals fed a low protein diet. Reproduction studies
with endrin in several species revealed no influence of endrin on
maturation, but foetal and postnatal mortality were increased. There
is no evidence of teratogenic activity. The two-year studies in the
dog and rat are used as a basis for determining a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1 ppm in the diet, equivalent to 0.05 mg/kg body-weight/day
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
The most important of all endrin uses, worldwide, and accounting for
some 80% of all endrin applied is as a spray for the control of
insects of cotton. It is also used extensively for the control of
insect pests in rice, to some extent in sugar cane and to a limited
extent in grain crops and sugar beets. In Australia, endrin is used
for insect control on tobacco and crucifers.
Post-harvest treatments
Endrin is accepted and occasionally used in some countries for rodent
control in orchards, where it is sprayed onto the ground under the
trees either in autumn or springtime. Rates vary from 2.4 kg/ha for
deciduous fruits in the United States to 0.02 or 0.03% solutions,
often in combination with mineral oil, in Australia.
Seed treatments
Endrin is used as a seed treatment on cotton at 2 oz /100 lb seed in
the United States and on beans at 1 oz /30 lb seed in Australia.
TABLE I
Summary of Registered Uses
Rate of
Crop application No. of Pre-harvest
(kg/ha) applications interval (days)
Cotton 0.25-0.50 1-12 none
Rice 0.2-0.50 1-4 30
Sugar cane 0.25 (only 1-8 45
granular)
Wheat 0.25-0.50 1 45
Barley " " "
Oats " " "
Sorghum " " "
Tobacco 0.25-0.5 - -
Crucifers 0.04% soln. - -
Potatoes 0.8 (USA) - -
0.03% soln.
(Australia)
Sugar beets 0.4 1-2 60
Other uses
Endrin has a minor use in the United States as a bait formulation for
control of cutworms in corn and potatoes. It is applied to the soil
surface at 0.8 kg/ha, and preharvest intervals are observed.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cotton seed and its products
From the view point of residues of endrin arising in food products,
cotton seed and its products are of primary interest; the refined
cottonseed oil is used for cooking or margarine manufacture, while the
extracted cake is used as cattle feed. Therefore residue levels in
cottonseed oil products which may reach the food consumer are
determined not so much by levels in products harvested in the field,
but to an appreciable extent by mill processes.
The effect of extraction processes on endrin residues was studied by
Smith et al. (1968) using crude cottonseed oil and soy bean oil
fortified with 1 ppm (mg/g) endrin. They found that alkali washing and
bleaching did not have a marked effect but that deodorization by
vacuum steam stripping effectively reduced endrin levels to below the
limits of detection (0.03 ppm). These authors also quote unpublished
work by Evans of the USDA using radio-labelled endrin. At initial
levels of up to 3.5 ppm in the crude oil, 96 percent of the added
radioactivity was lost during the process of steam stripping. The
effectiveness of the deodorization step in removing endrin residues
from vegetable oils was further demonstrated by Barrantine and Cain
(1970) in studies of commercial scale operations. Endrin residues are
summarized in Tables II and III. (Shell Development Co. Reports,
RES-64-3. RES-64-9; Shell Chemical Co., New York PRL-67-24; Velsicol
Corp., unpublished data).
The cottonseed cake left after the extraction of oil commonly contains
1%-5% oil. These cottonseed cakes are widely used as an important
ingredient of cattle feed concentrates and could contribute up to 20%
of the daily diet of a dairy cow. The relationship between cattle feed
and milk residues was studied by Williams and Mills (1964), Kiigemagi
et al., (1958), and Ely et al., (1957). Consideration of their
combined results indicates that at feed levels in the region of
0.25-2.0 ppm endrin in total feed, milk residues will eventually reach
0.04 times the concentration in the total feed, if feeding is
continued until a plateau is reached. Both Williams and Kiigemagi
found that when endrin was withdrawn from the feed, levels in the milk
fell fairly quickly. At 0.25 and 0.75 ppm in feed, levels fell to
0.002 ppm in six weeks after endrin was withdrawn (Kiigemagi, 1958).
At the 2 ppm feed level, residues fell from 0.06 to 0.02 ppm in the
same period. In Williams' experiments residue from the highest feed
level (0.50 ppm in whole dry feed) fell to 0.002 ppm in about 15 days.
In rice, rice bran and rice straw
The results of experiments in a number of rice growing regions are
summarized in Table IV.
TABLE II
Residues of endrin in whole cotton seed, U.S.A.
Dosage/ Harvest Residue ppm
No.of applic. interval delta-
Location applics. (lb/acre) (days) endrin keto
Oklahoma control - - <0.004 N.D.
2 0.5 58 <0.004 to
0.013 N.D.
2 1.0 58 <0.004 N.D.
Louisiana 4-5 2.67(total) - 0.02 to <0.02
0.03
Texas not stated 0.5 (total) - <0.04 <0.04
1.0 (total) - 0.06 to <0.04
0.10
2.0 (total) - <0.04 <0.04
TABLE III
Endrin residues in crude and alkali-extract
Cottonseed oil obtained from endrin treated cotton in Texas, U.S.A.
Residues in oil - ppm
Dosage rate Post harvest Crude Alkali extracted
lb/acre total interval-days Endrin delta-Keto Endrin delta-Keto
Control - <0.01 <0.03 <0.01 <0.03
0.5 not stated 0.05 <0.03 0.05 <0.03
1.0 not stated 0.43* <0.03 0.39* <0.03
1.0 not stated 0.05 <0.03 0.05 <0.03
2.0 not stated 0.04 <0.03 0.01 <0.03
Control - <0.01 - <0.01 -
0.4, ten times 5 0.05 - <0.01 -
10 <0.01 - <0.01 -
20 <0.01 - <0.01 -
30 <0.01 - <0.01 -
0.8, ten times 5 0.02 - <0.01 -
10 0.03 - <0.01 -
20 0.08 - <0.01 -
30 0.01 - <0.01 -
Control - <0.02-<0.03 - - -
0.4 8 0.02 - - -
13 <0.02 - - -
28 <0.02-<0.07 - - -
52 0.04 - - -
0.8 8 0.07 - - -
13 "neg" - - -
28 <0.02 - - -
42 <0.02-0.02 - - -
* These figures are regarded as exceptional in light of all other available data.
Although neither rice bran or straw is reported to be an appreciable
item of international trade, residues may be of importance insofar as
they may persist in animal products. A summary of data provided by the
references in Table IV shows bran residues for 11 sites to range from
<0.01 to 2.30 ppm, with a mean of 0.35 ppm. The data are heavily
weighted by one figure from India; without this figure, the range is
<0.01 to 0.80 ppm, with a mean of 0.15 ppm. Rice straw residues from
the same sources (25 samples) ranged from 0.04 to 2.90 ppm, with a
mean of 1.02 ppm. Residues of delta-keto endrin were found in bran and
straw containing high residues of endrin but seldom exceeded 10% of
the total residue and never exceeded 20%.
Rice bran is used mainly as a component of locally produced poultry
feed. The significance of endrin residues in poultry feed in giving
rise to residues in meat and eggs may be judged from data in the
published literature.
Cummings et al., (1967) fed diets containing between 0.05 and 0.45 ppm
endrin to White Leghorns for 14 weeks and found residues ranging from
<0.01 to 0.06 ppm endrin in breast meat and from 0.25 to 3.5 ppm
endrin in depot fat. Similar diets gave endrin residues of 0.03 to
0.32 ppm in eggs as estimated from graphs (Cummings et al., 1966).
Terriere et al. (1959) fed diets containing 0.10 to 0.75 ppm endrin
for 6 weeks and found <0.1 to 0.2 ppm endrin in breast meat, <0.1 to
0.3 ppm in leg meat, 0.6 to 3.6 ppm in depot fat and <0.1 to 0.3 ppm
in eggs. A feed level of 2.25 ppm for 6 weeks gave 17.0 ppm in depot
fat.
In sugar cane
In experiments extending from 1957 to 1965, Shell Development Co.
applied endrin to sugar cane in Louisiana and Florida. When 0.3 lb
ai/acre of 2 percent granular was applied at 3-6 applications and
postharvest intervals ranging from 12-120 days were observed, average
endrin residues ranging from <0.01 to 0.05 ppm were obtained.
Keto-endrin residues were determined in four samples and did not
exceed <0.02 on the average. Residues in control samples ranged from
<0.01 to 0.04 ppm. No endrin was detected in molasses or refined
sugar prepared from the treated cane. One group of samples treated
with 0.5 lb ai/acre of E. C. had <0.1 ppm endrin (sensitivity limit
of the method). When 2 percent granular was applied four times at 1.0
lb ai/acre and postharvest intervals of 7-45 days were observed, the
endrin residues ranged from 0.04 to 0.08 ppm. The same formulation
applied four times at 0.22 lb ai/acre and eight times at 0.23 to 0.29
lb ai/acre resulted, after postharvest intervals of 71 and 64 days,
respectively, in endrin residues of <0.10 ppm. (Shell Development Co.
Reports RES-61-53, RES-63-159 DLI-136 (1963), DLI-157 (1964),
RES-64-1, DLI-160 (1965), RES-58-48A, RES-57-44.
In grains
Data obtained on a variety of small grains in the U.S.A. are
summarized in Table V.
In India, endrin (2% granular) was applied to sorghum at 0.4 kg ai/ha
for 4 to 6 applications. Samples taken from 42 to 75 days after last
treatment had residues in grain ranging from <0.01 to 0.02 ppm endrin
and in straw ranging from 0.14 to 0.70 ppm endrin. Control samples had
<0.01 to 0.02 ppm in grain and 0.03 to 0.31 ppm in straw (Shell
Research Ltd., Report WKGR 0071/70).
In the U.S.A., endrin E.C. was applied to sorghum once at either 0.25
or 0.50 lb ai/acre. A pre-harvest interval of 111 days resulted in
endrin residues of <0.02 ppm in grain, <0.02 ppm in straw, and
<0.02 ppm in silage (73 days PHI). A pre-harvest interval of 63 days
resulted in endrin residues of <0.02 ppm in grain, 0.03 and 0.09 ppm
in straw, and <0.02 and 0.15 ppm in silage (39 days PHI); the latter
value is suspect, according to the analysts (Shell Chemical Co.,
Report PRL-66-113). A pre-harvest interval of 29 days resulted in
residues of <0.05 and 0.05 ppm in grain (Velsicol Corp., Report
TSR-2575).
Single applications of endrin E.C. to sweet corn (maize) in the U.S.A.
at 0.25 or 0.50 lb ai/acre, 37 days before harvest, resulted in
residues of <0.02 ppm of endrin in grain and husk. Some controls gave
an apparent residue of 0.02 to 0.03 ppm (Shell Chemical Co., Report
PRL 66-67g).
In apples
Experiments conducted by Shell in the U.S.A. in which endrin E.C. or
W.P. was applied at 1.5 to 4.0 lb ai/acre to the soil of orchards gave
no detectable residues in whole apples at harvest time; detection
limits were 0.01-0.002 ppm for the methods employed (Shell Development
Co., Report RES-61-60, Shell Chemical Co., Report PRL-69-119, Ibid,
PRL-69-95). In recent studies by Horsfall et al. (1970), picked fruit
residues ranged from <0.002-0.003 ppm. In fallen fruit, residues were
sometimes higher, ranging up to 0.023 ppm.
FATE OF RESIDUES
General comments
Endrin undergoes rearrangement to delta-keto endrin in sunlight, in
the presence of strong acids, and by thermal treatment. Endrin also
undergoes thermal and photo degradation to yield small amounts of the
aldehyde SD 7442. Delta-keto endrin can undergo a rearrangement to the
"bird cage alcohol" when treated with very dry Florisil adsorbent
(Korte and Porter, 1970).
TABLE IV
Endrin residues in polished and unpolished rice
Applic. rate Harvest Residues, ppm
kg am/ha × interval, Polished Brown
Country Formulation no. of applic. days rice rice Reference
India 2% gran. 0.2, 0.4, 0.6, 49-79 <0.01 <0.01-0.01 Shell Res.Ltd.Report, WKTR 0023/69
2.0 × 4-7
control - - <0.01 <0.01
Venezuela E.C. 0.29 × 1 95 0.02 0.03 Ibid,WKGR 0046/70
Thailand control - - <0.01 <0.01 Ibid,WKGR 0087/70
2% gran. 0.2, 0.4, 0.4 56-83 <0.01 <0.01
20% E.C. 0.16 × 3 46-72 <0.01 <0.01-0.23*
Philippines control - - <0.01-0.03 <0.01-0.03 Ibid,WKGR 0078/70
2% gran. 0.2, 0.4, 0.4 44-60 <0.01-0.02 <0.01-0.05
20% E.C. 0.16 × 3 33-49 <0.01-0.03 <0.01-0.03
Indonesia control - - - <0.01 Ibid,WKTR 0078/68
20% E.C. 0.2-0.62 21 - 0.03-0.05
× 7 or × 8
Thailand 20% gran. 0.4 X3, X4 not <0.02 - Ibid,BEGR.0045/70
0.8 × 3 stated (Endrin and Keto -
Endrin Keto Endrin Keto
India control - - <0.02 <0.02 0.09 <0.02 Ibid
2% gran. 0.4 - 0.02 <0.02 0.23 0.03
0.8 45 0.04 <0.02 0.47 0.04
* One exceptional result - reason not identified
TABLE V
Endrin residues in small grains - U.S.A.
Applic. rate, Postharvest Endrin residues,
lb ai/acre interval, ppm
Crop × no. of applic. days Grain Straw Reference
Wheat 0.25 × 1 26 0.03 - Shell Dev. Co.
0.50 × 1 26 0.06 - Report RES-
Oats 0.25 × 1 26 0.15 - 62-124 (1963)
0.50 × 1 26 0.50 -
Wheat 0.25 × 1 62 <0.01 0.27 Shell Chem.Co.
0.25 × 1 96 <0.01 0.02 Report PRL-
0.25 × 2 62 <0.01 0.37 65-41 (1966)
Barley 0.50 × 1 60 <0.05 0.07
0.50 × 1 32 <0.05 0.09-0.38
Wheat 0.25 × 1 >150 0.01 - Velsicol Corp.
0.50 × 1 >150 0.03 - Report TSR-
Barley 0.25 × 1 >150 <0.01 - 2578 (1966)
0.50 × 1 >150 <0.01 -
In animals
Homogenates from cow or pig liver upon addition of NADH2 converted 38
ug of endrin, after 72 hours incubation, to metabolites which were
identical to those found from living organisms (Korte, 1967; Klein et
al., 1968a). See "Biochemical aspects".
In plants
14C labelled endrin was applied at either 1.04 or 2.08 mg/plant to
the leaves of tobacco under conditions of free or restricted aeration.
Six weeks after treatment, 32-47% of the applied radioactivity was
found on and in the tobacco plants, the lowest residues being in the
plants grown under free aeration. (Korte and Porter, 1970).
Shortly after the first buds emerged, the leaves of each of 11 cotton
plants were treated with 4.2 mg 14C-labelled endrin; treatment was
repeated after two and six weeks (total ca. 120 ppm). Twelve weeks
after the last application, quantitative measurement of the residues
revealed only very low concentrations in the cotton seed (0.033 ppm),
somewhat more in the fibers (0.36 ppm), and about 80% in and on the
leaves. Two thirds of the applied radioactivity was lost to the
atmosphere during the test. Besides endrin, there were two groups of
degradation products in the cotton plants, one group slightly more
hydrophilic than endrin and one very hydrophilic. One component of the
less hydrophilic group was isolated and gave mass and IR spectra
identical with that of delta-keto endrin (Korte and Porter, 1970).
When 14C-labelled endrin was applied at 50 ug/plant to young cabbage
plants, 66 percent of the activity had evaporated after two weeks, 70
percent after three weeks, and 75 percent after four weeks. Activity
not evaporated was found in the plants as endrin and hydrophilic
metabolites. After administration of 500 ug/plant, the concentration
of activity decreased from leaves to stalks to roots to soil, while
the ratio of metabolites to endrin increases in the same order. The
metabolite fraction consisted of two compounds, a very hydrophilic
main metabolite and delta-keto endrin (Weisgerber et al., 1968).
Similar results were obtained with carrots (Klein et al., 1968b).
Foliar application of 14C-labelled delta-keto endrin to white cabbage
has shown that it is more persistent than endrin but metabolizes more
rapidly (about 15 percent compared with about 10 percent for endrin).
The converted keto-endrin consisted mainly of a very hydrophilic
metabolite, the concentration of which is highest in leaves and stalks
(Korte and Porter, 1970).
Residues in soybean plants grown in soil treated with 14C-labelled
endrin were mainly delta-keto endrin, and endrin alcohol in addition
to endrin aldehyde were believed present (Nash and Beall, private
communication).
In soil
Matsumura et al., (in press); incubated 14C-labelled endrin with 150
cultures of microorganisms isolated from soils and found 25 active in
degrading endrin, all of which had previously been found to degrade
dieldrin. Seven different metabolites of endrin were found, with three
major and four minor ones; tentative structures have been assigned
(Korte and Porter, 1970).
In storage and processing
The effects of processing on the residues of endrin in dairy products
was studied by Langlois et al., (1965). Butter, ice cream, cheese,
condensed milk and dry whole milk were manufactured from milk
contaminated with endrin either by direct addition or through feeding
to cows. In general, they found that the residues concentration in the
fat remained fairly constant during processing except for drastic heat
treatment to produce dry whole milk which caused major reductions
(>50 percent).
When hens fed on a diet containing endrin were cooked in water at
190-200°F for three hours, the residues were reduced up to 90 percent
(Liska et al., 1967). Autoclaving the carcass at 15 psi for three
hours removed essentially all residues. Metabolites of endrin ware not
determined.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of endrin were monitored in cottonseed oil produced by mills
in Venezuela, Brazil, India and the United States. In Venezuela,
weekly samples were taken for nine weeks, and endrin residues ranged
from <0.02 to 0.05 ppm for crude oil, <0.02 to 0.02 ppm for
decolorized oil and <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR. 0119.70, 1970). In Brazil, seven samples were taken over
thirteen weeks, and endrin residues ranged from <0.01 to 0.01 ppm for
crude oil and <0.01 to <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR.0120.70, 1970). In India, only crude oil samples were
available, and these contained from 0.04 to 0.06 ppm endrin (Shell
Research Ltd., Report WKGR 0091.70). In the United States, ten samples
of refined oil from California were analyzed and found to contain
<0.03 to 0.03 ppm endrin and <0.02 ppm delta-keto endrin (Shell
Development Co., RES-63-155).
In addition to oil, cottonseed cakes have also been studied for endrin
residues, since these are used widely as an important ingredient of
cattle feed concentrates. Figures for cattle cake samples
corresponding to the samples of cottonseed oils given in the preceding
paragraph are as follows: Venezuela, <0.01 to 0.02 ppm; Brazil <0.01
to 0.08 ppm; India, <0.01 ppm. In the United States, data obtained on
cakes and meals corresponding to the samples listed in Tables II and
III ranged from <0.01 ppm to <0.04 ppm; most residues were below the
detection limit of <0.01 ppm.
Total diet studies conducted in the U.K.and the U.S.A. indicate that
endrin residues are unlikely to arise in human diets as a result of
the use of endrin in cotton (Abbott et al., 1969; Assoc. of Public
Analysts, 1969; Duggan, 1968; USDA, 1968). In the U.K., no endrin
residues were found in vegetable oils, fats, milk, milk based infant
foods, beef, beef sausages or meats. In the U.S.A., no endrin residues
were found in finished or crude cotton-seed oil, milk, milk products
or manufactured milk products.
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967) apply to the
determination of endrin. The compound can be determined in fatty and
nonfatty foods by the multiresidue method and gas chromatography
(Pesticide Analytical Manual., Vol. I and II, U.S. FDA). Endrin in
eluted from the Florisil column in the 15% ethyl ether/petroleum ether
fraction. Additional cleanup by MgO-Celite column and alkaline
hydrolysis in required, and recovery is usually in the range of
80-100%. When a gas chromatograph containing 10% DC 200 on Gas-Chrom Q
column at 200°C with 120 ml/min N2flow and an electron capture
detector is used, the following retention times relative to aldrin are
obtained: endrin - 2.05, endrin aldehyde - 2.30, endrin alcohol
- 2.50, delta-keto endrin -3.50. The sensitivity of the method is
good; 2 ng of endrin gives a “ full scale response on a 10-9amp full
scale recorder. A procedure for the determination of delta-keto endrin
in mammalian samples has been developed (Zavon et al., 1965), and a
general method is available (Shell Development Co., Anal. Method MMS
53/64). Residue methods have not yet been developed for monohydroxy
endrin, 9-keto endrin, endrin, endrin aldehyde or the "bird cage
alcohol" isomer of endrin.
An infrared method for endrin has been described (Gershman, 1961). An
absorbance peak at 10.2 microns is used for calculations. The method
requires a 1 kg ample for 0.25 ppm.
NATIONAL TOLERANCES
Country Commodity Tolerance ppm
Australia vegetables including 0.1
beans, crucifers,
potatoes, tomatoes
United States broccoli, brussels 0
sprouts, cabbage,
cauliflower, cucumbers, extended1
cottonseed, eggplant,
peppers, potatoes,
United States
(cont'd) sugar beets and sugar extended1
beet tops, summer squash,
tomatoes
1 The word "extended" appears where action is being taken to acquire
data to petition for a tolerance.
Australia has not set a tolerance for endrin in cottonseed, however,
an action level has been set for endrin residues in animal feeds at
0.03 ppm.
APPRAISAL
Endrin is widely used to control insect pests on cotton and to lesser
extent on rice. It is used to a limited extent as an insecticide on
small grains, vegetables and sugar cane and is occasionally used for
mouse control in orchards. The ways in which endrin is applied vary
widely, as do the numbers of applications.
The most important consideration regarding endrin residues in food
arise from its use on cotton; cottonseed oil is used for cooking or
margarine manufacture, and the extracted cottonseed cake is used as
cattle feed. Controlled feeding experiments with cattle indicate that
whole milk residues would reach a plateau of 0.04 times the residue in
feed. Once ingestion stops, excretion in milk soon ceases (two to six
weeks). Cottonseed cake for feed should have less than 0.1 ppm of
endrin residues if the recommended practical residue limits for whole
milk and milk products are not to be exceeded.
Rice bran is used as a component of poultry feed; feeding studies on
hens indicate that endrin residues in depot fat would reach about five
times the level in feed; for eggs the ratio would be about 0.7 times.
Calculations indicate that it is unlikely that significant endrin
residues would be found in cooked poultry or eggs. The rice bran used
as a constituent of poultry feeds should not contain residues greater
than 1 ppm if the recommended practical residue limits for poultry and
eggs are not to be exceeded.
In the absence of experimental residue data at the recommended
preharvest interval of 45 days or longer, the meeting did not
recommend a tolerance for oats.
Under field conditions, endrin is rapidly loot from plants through
evaporation - 60-80 percent over six to 18 weeks, depending on the
crop.
Endrin is metabolized by plants to delta-keto endrin, a rearrangement
product, and to very hydrophilic metabolites; traces of the aldehyde
(SD 7442) and alcohol have been reported. The parent compound appears
to be the major residue at all times.
The widespread use of endrin on cotton does not appear to give rise to
measurable residues in the human diet. Dietary studies in the United
States have revealed no endrin residues in finished cottonseed oils,
milk, milk based infant foods, milk products, beef or meats and trace
amounts in margarine and fats. Exceptionally, one diet composite
sample in one sampling location in the United States contained 0.20
ppm of endrin in oils, fats and shortening.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The following tolerance and practical residue limits are to apply to
raw agricultural products moving in commerce unless otherwise
indicated. All figures include the sum of endrin and delta-keto
endrin.
TOLERANCES
ppm
Cottonseed, cottonseed oil (crude) 0.1
Cottonseed oil (finished), maize
(sweet), wheat, barley, sorghum,
rice (brown or polished), apples 0.02
PRACTICAL RESIDUES LIMITS
Milk and milk products (fat basis) 0.02
Fat of poultry 1
Eggs (shell-free) 0.2
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate long-term study in a strain of mice of known tumour
susceptibility.
REFERENCES
Abbot, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric. 20; 245
Altmeier, G., Klein, W. and Korte, F. (1969) Tetrahedron Letters,
49: 4269-4271
Association of American Pesticide Control Officials, Inc. (1966)
Pesticide Chemicals Official Compendium, 1966 Ed., 475-477
Association of Public Analysts. (1967) Joint Survey of Pesticide
Residues in Foodstuffs sold in England and Wales
Baldwin, M.K., Robinson, J. and Parke D.V. (1970) The metabolism of
endrin in the rat. Unpublished report from the Tunstall Laboratory,
Shell Research Ltd., Sittingbourne and the University of Surrey
Barrentine, B.F., and Cain, J.B. (1970) Unpublished work cited in
Comptes Rendus, 25th Conference of IUPAC, Commission VI.5.1, Appendix
VII
Barth, R.A.J. (1967) Pesticide toxicity in primates. Doctoral thesis,
Tulane University, Louisiana p. 110-111
Boyd, E.M. and Stefec, J. (1969) Dietary protein and pesticide
toxicity: with particular reference to endrin. Canad. med. Ass. J.,
101: 335-339
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27:81-138
Coble, Y., Hildebrandt, P., Davis, J., Raasch, F. and Curley, A.
(1967) Acute endrin poisoning. J. Amer. med. Ass. 202: 489-493
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Comptes Rendus. (1970) Fate of organochlorine pesticides in vegetable
oil processing. 25th Conference of IUPAC, Commission VI.5.1, Terminal
Pesticide Residues, Appendix VII. p. 187-189
Cummings, J.G., Zee, K.T., Turner, V., Quinn, F..and Cook R.E. (1966)
Residues in eggs from low level feeding of five chlorinated
hydrocarbon insecticides to hens. J. Assoc. Off. Anal. Chem. 49:
354-364
Cummings, J.G., Eidelman, M., Turner, V., Reed, D., Zee K.T. and Cook,
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydro-carbon insecticides to hens. J. Assoc. Off. Anal.
Chem. 50: 418-425
Dale, W.E., Copeland, M.F. and Hayes, W.J. Jr. (1965) Chlorinated
insecticides in the body fat of people of India. Bull. Wld Hlth Org.,
33: 471-477
Davies, G.M. and Lewis, I. (1956) Outbreak of food poisoning from
bread made of chemically contaminated flour. Brit. med. J., (2):
393-398
Dewitt, J.B. (1956) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J. Agr. Fd. Chem., 10: 863-866
Diechmann, W.B., MacDonald, W.E., Radomski, J. Blum, E.B. Bevilacqua,
M. and Keplinger, M. (1970) The tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Industr. Med., 39: 314
Duggan, R.E. (1968) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pesticides Monotoring
Journal 1 (4): 2-7
Dunachie F.F. and Fletcher W.W. (1966) Effect of some insecticides on
the hatching rate of hen's eggs. Nature, 212: 1062-1063
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. econ. Entomol., 50: 348-349
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. Econ. Ent. 50: 348-349
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23(1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FDA, (1967) Pesticide analytical manual, HEW, FDA, Vol. I and 11
Frear, D.E.H. (1955) Endrin in pesticide index, Third Edition, D. van
Nostrand Co. Inc., New York. p. 68-69
Gershman, L.L. (1961) Infra-red determination of endrin residues. J.
Assoc. Off. Anal. Chem. 44: 212-214
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Hayes, W.J., Jr., (1963) Endrin in Clinical handbook on economic
poisons, revised edition. pp. 68.70. Publ. Hlth. Ser. Publn No. 476.
US Govnmt Printing Off. Washington D.C.
Hayes, W.J., Jr., Dale, W.E. and Baise, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people of New Orleans. Life Sci.,
4: 1611-1615
Hayes, W.J. and Curley, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environm. Hlth, 16: 155-162
Hine, C.H., Eisenlord, G., Loquvam, G.S. and Leung, T. (1968) Results
of reproduction studies on rats fed diets containing endrin over three
generations. Unpublished report prepared by the Hine Laboratories,
Inc., San Francisco, submitted by Shell International Research
Horsfall, F. Jr., Webb, R.E., Price, N.O. and Young, R.W. (1970)
Residues in apples subsequent to ground sprays of endrin. J. Agr. Food
Chem. 18: 221-223
IUPAC, (1967) Proceedings of the Commission on Terminal Residues,
Vienna, August 1967. Appendixes VI and VII
Jager, K.W. (1970) Aldrin, dieldrin and telodrin. An epidemiological
and toxicological study of long-term exposure. Doctoral thesis,
Leiden, 1970
Jolley, W.P., Stemmer, K.L., Grande, F., Richmond, J. and Pfitzer,
E.A. (1969) The effects exerted upon beagle dogs during a period of
two years by the introduction of 1, 2, 3, 4, 10, 10-hexachloro-6,
7-epoxy-1, 4, 4a, 5, 6, 7, 8, 8a-octahydro-1, 4-endo, endo-5,
8-dimethanonaphthalene into their daily diets. Unpublished report from
the Kettering Laboratory University of Cincinnati submitted by Shell
International Research
Kiigemagi, U., Sprowls, R.G. and Terriere, L.C. (1958) Endrin content
of milk and body tissues of dairy cows receiving endrin daily in their
diet. J. Agr. Fd. Chem., 6: 518-521
Klein, W., Müller, W. and Korte, F. (1968a) Insektizide im
Stoffwechsel, XVI. Ausscheidung, Verteilung und Stoffwechsel von
Endrin (14C) in Ratten. Liebigs Ann. Chem. 713: 180-185
Klein, W. et al., (1968b) Qualitas Plant. Mater. Vegetabiles 15,
225-238
Klein, W. and Drefahl, B. (1970) "Jahresbericht 1969" Institut für
ökologische Chemie der Gesellschaft für Strahlenforschung mbH,
München, Schloss Birlinghoven, April 1970
Korte, F. (1967) Metabolism of chlorinated insecticides. Unpublished
report from the Radiochemical Laboratories, Bonn University
Korte, F. and Porter, P.E. (1970) Minutes of the fifth meeting of the
IUPAC Commission on terminal pesticide residues, September, 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Langlois, B.E., Liska, B.J. and Hill, D.L. (1965) The effects of
processing and storage of dairy products on chlorinated insecticide
residues. J. Milk and Food Technol. 28: 9-11
Liska, B.J., Stemp, A.R. and. Stadelman, W.J. (1967) Food Technol.
21: 435
Ludwig, G. (1965) Excretion, metabolism and storage of endrin 14C
after oral administration to a rat. Minutes of the fifth meeting on
the fate of organic compounds in the living organism. 20-21 October
1965, Birlinghoven Laboratory
Ludwig, G. (1966) Investigations with 14C-labelled insecticides. XX
Excretion, metabolism and storage of endrin - 14C after oral
administration to a rat. Birlinghoven Biomonthly Res. Summary
(January), p. 13-14
Morris, R.D. (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse Peromyscus maniculatus. Canad. J.
Zool., 46: 951-958
Nash, R.G. and Beall, M.L. Jr. (1970) Extraction and identification of
endrin and heptachlor degradation products. Submitted to J. Assoc.
Off. Anal. Chem., October 1970
Nelson, S.C., Bahler, T.L., Hartwell, W.V., Greenwood, D.A. and
Harris, L.E. (1968) Serum alkaline phosphatase levels, weight changes
and mortality ratios of rats fed endrin. J. Agr. Pd. Chem., 4:
696-700
Radeleff, R.D. (1956) Hazards to livestock of insecticides used in
mosquito control. Mosquito News, 16(2): 79-80
Richardson, A. (1970) Unpublished untitled letter from Tunstall
Laboratories to Shell International Research
Richardson, A. Robinson, J. and Baldwin, M.K. (1970) Metabolism of
endrin in the rat. Chem. Industr., 502-503
Robinson, J., Richardson, A., Hunter, C.G., Crabtree, A.N. and Rees,
H.J. (1965) Organo-chlorine insecticide content of human adipose
tissue in south-eastern England. Brit. J. industr. Med., 22: 220-229
Shell. (1965) Alkaline phosphatase in endrin-exposed workers.
Unpublished report prepared by the Industrial Medical Department,
Shell Nederland Chemische Fabrieken N.V. Rotterdam
Shell Chemical Co., Reports. (1965-69) PRL-65-41, PRL-66-67g,
PRL-66-113, PRL-67-24, PRL-69-28, PRL-69-95, PRL-69-119
Shell Development Co., Reports. (1961-65) RES-61-60, RES-62-124,
RES-63-155, RES-64-39 RES-64-9
Shell Research Ltd., Report (1968-70) WKTR 0078/68, 0023/69, WKGR
0046/70, 0072/70, 0078/70, 0087/70, 0071.70, 0091.70, 0119.70, 0120.70
Sherman, M. and Rosenberg, M.M. (1954) Sub-chronic toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 47: 1082-1083
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chem. Soc., 45:
866-869
Smith, S.I., Weber, C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocarbon insecticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Soto, A.R. and Diechmann, W.B. (1967) Major metabolism and acute
toxicity of aldrin, dieldrin and endrin. Environm. Res., 1: 307-322
Speck, L.B. and Maaske, C.A. (1958) The effects of chronic and acute
exposure of rats to endrin. A.M.A. Arch. industr. Hlth, 18: 268-272
Street, J.C., Butcher, J.E., Raleigh, R.J. and Clanton, D.C. (1957)
Tissue storage and transferral to the lamb of aldrin, dieldrin and
endrin when fed to bred ewes. Proc. West. Sec. Amer. Soc. Anim. Prod.,
(Pullman, Washington), 46: 1-6
Terriere, L.C., Kiigemagi, U. and England, D.C. (1958) Endrin content
of body tissues of steers, lambs and hogs receiving endrin in their
daily diet. J. Agr. Fd. Chem., 6: 516-518
Terriere, L.C., Arscott, G.H. and Kiigemagi, U. (1959) The endrin
content of eggs and body tissue of poultry receiving endrin in their
daily diet. J. Agr. Fd Chem., 7: 502-504
Treon, J.F., Clevelend, F.P. and Cappel, J. (1955) Toxicity of endrin
for laboratory animals. J. Agr. Fd. Chem., 3: 842-848
USDA, Agriculture handbook No. 331. (1968) Suggested guide for the use
of insecticides to control insects affecting crops, livestock,
households, stored products, forests and forest products
USDA, Residues in food and feed. (1968) Pesticide residue levels in
foods in the United States from 1 July 1963 to 30 June 1967.
Pesticides Monitoring Journal, 2(1): 2-46
USDA, Summary of registered agricultural pesticide chemical uses.
(1969) Vol. III, Insecticides, repellents, acaricides. Endrin,
III-E-2.1, 2.2, 2.3
Van Dijk, M.C. (1968) Chlorinated insecticides and the serum alkaline
phosphatase. Proc. Sixth Shell Industrial Doctors Meeting, 28-30 May
1968, Amsterdam
Velsicol Corp., (1968-70) Reports TSR 2578, TSR 2575 (Unpublished -
1970)
Weeks, D.E. Endrin food-poisoning. A report of four outbreaks caused
by two separate shipments of endrin-contaminated flour. Bull. Wld Hlth
Org., 37: 499-512
Weisgerber, I., Klein, W., Djirsarai, A., and Korte, F. (1968) Ann.
Chem. 713: 175-179
Williams, S., and Mills, P.A. and McDowell, R.E. (1964) Residues in
milk of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Anal. Chem., 47:
1124-1128
Williams, D.A. (1966) Alkaline phosphatase activity following exposure
to endrin, comments on the paper by Nelson et al. Unpublished report
from the Statistics Unit, Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Witherup, S., Stemmer, K.L., Taylor, P., Bietsch, P. and Pfitzer, E.A.
The incidence of neoplasms in two strains of mice sustained on diets
containing endrin. Unpublished report from the Kettering Laboratory,
University of Cincinnati submitted by Shell International Research
Zavon, M.R. (1961) The toxicology and pharmacology of endrin.
Unpublished report from the Kettering Laboratory, University of
Cincinnati
Zavon, M.R., Hine, C.H. and Parker, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Amer. med. Ass., 193: 837-839
metabolite is also an alcohol. Three days after a single oral dose of
14C-labelled endrin, approximately half of the 14C radioactivity
remained in the bodies of the rats. This material was principally one
metabolite which was identified as 9-keto endrin (I), an oxidation
product of the secondary alcohol found in faeces.
When 14C-labelled endrin was applied orally to rabbits at 0.5 mg/kg
body-weight and at three and four day intervals, four metabolites were
isolated from the urine which appear to have the following chemical
natures: A (40% of excreted radioactivity) is a conjugate compound of
a hydroxy derivative of endrin; B (12%) is a monohydroxy derivative of
an unbridged endrin isomeric ketone; C (40%) is the 4a-hydroxyendrin;
D (8%) has a molecular weight of 420 and the C-C double bond intact in
the chlorinated ring. None of these compounds is the delta-keto endrin
(Korte and Porter, 1970).
Effects on enzymes and other biochemical parameters
In monkeys which had received exposure to an unspecified quantity of
endrin, there were significant changes in the enzymes serum
glutamic-oxaloacetic transaminase and serum glutamic-pyruvic
transaminase (Barth, 1967).
Elevation of serum alkaline phosphatase has been observed in rats fed
25 ppm and possibly 5 or 1 ppm of endrin for 16 weeks (Nelson et al.,
1956) but not in dogs fed 4 ppm of endrin for two years (Jolley et
al., 1969) nor in human subjects occupationally exposed to unspecified
levels of endrin (Shell, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
See under "Long-term studies" (Witherup et al., 1970).
Rat
Commencing at weaning, varying numbers of rats of mixed sex were fed
dietary levels of 0, 2, 6 or 12 ppm of endrin throughout their
lifetime. No primary malignant hepatic tumours were found in any
animals upon histological examination. Two benign hepatic tumours
(haemangiomas) were found in one male control rat and the other in a
female fed 6 ppm of endrin. Tumour incidence in other tissues was also
not significantly different between the control and experimental
animals (Diechmann et al., 1970).
Special studies on reproduction
Chicken egg
Hen eggs were injected with 0.5 or 5 mg per egg. The hatching rate was
40 or 20 percent, respectively (Dunachie and Fletcher, 1966).
When endrin was injected into the yolk of fertile eggs incubated for
seven days in amounts of 0.2 and 2.0 mg/egg, the hatchability was 40
and 6.9 percent, respectively (Smith et al., 1970).
Quail
No eggs were produced from quail which received 1 ppm of endrin in
their diet either as winter maintenance or during the reproductive
period (DeWitt, 1965).
Pheasant
In pheasants there was reduced egg production when fed 10 ppm of
endrin but not at 2 ppm or less. Survival of the chicks to two or six
weeks was also markedly reduced at 10 ppm, but not at lower doses
(DeWitt, 1965).
Mouse
Groups of male and female mice were fed endrin at dietary levels of 0
or 5 ppm for 30 days. Test and control mice were then randomly paired
and continued on the test diet for a further 90 days, there being a
total of 101 pairs in the group fed endrin. The first litters were
significantly smaller than the control group. The time taken to
produce the first litter was not significantly different between the
two groups (Good and Ware, 1969).
Deer mouse
Five groups each of 13-14 pairs of Saskatchewan deer mice
(Peromyscus manicalatus) of varying ages were fed dietary levels of
0, 1, 2, 4 or 7 ppm of endrin over intermittent periods between which
times the animals were either fed a normal diet or were subjected to
48 hours starvation. The animals were sacrificed by exposing them to
cold stress at -16°C and the time of death recorded. During feeding,
parental mortality increased in proportion to the level of endrin.
Young animals were more susceptible than old. Starvation increased
mortality in all test groups but not in the controls; this effect was
more evident with increasing dose levels. Litter production frequency
and mean litter size before and during experimental feeding were
similar. However, post-natal mortality prior to weaning increased in
the young from parents fed 4 or 7 ppm. Endrin adversely affected the
survival time during cold stress in the females but not in the males
(Morris, 1968).
Rat
In a later study from the same laboratory, groups of ten male and 20
female rats were fed dietary levels of 0, 0.1, 1.0 and 2.0 ppm of
endrin over a period of three generations. The F0 generation was
mated after 79 days on the test diets, and the males were rotated as
in the previous experiment.
The young from the first litter were discarded at weaning and the
parents mated again after ten days to form the F1b generation. Young
from this generation were mated when 100 days old, and this protocol
was followed for three generations, using the second litter in all
cases. The size of the litter in the F3 generation from the 2.0 ppm
group was significantly larger than that from the controls. Mortality
was high in the controls, which resulted in a greater percentage
survival in the F3a litters in the 0.1 ppm group and in all F3b
litters in the test groups. The weights of weanlings were comparable
to the controls except in the F3a litters from the 0.1 ppm, which
were significantly less due probably to the large litter sizes in that
group. Examination of the F3b weanlings revealed no differences in
organ to body-weight ratios. It was stated that there were no
histological abnormalities, but details of the pathology were not
available. Fertility, gestation, viability and lactation indices did
not indicate that endrin affected any of these parameters (Hine et
al., 1968).
Special studies on the photoisomerization product of endrin
When endrin is irradiated with short wavelength ultraviolet light, the
delta-keto compound is formed in 37 percent yield as well as an
aldehyde in 9 percent yield. Under the influence of sunlight, only the
ketone is formed. The ketone is about a quarter as toxic to rate as
endrin and like endrin is more toxic to the male than to the female
(Soto and Diechmann, 1970) (see also "Fate of residues").
Acute toxicity
The major clinical manifestation of endrin intoxication in man
involves convulsions of several minutes duration which may be isolated
and followed by semiconciousness for 15-30 minutes, with complete
recovery after 2-4 weeks. More serious symptoms are continuous
convulsions, high fever and decerebrate rigidity prior to death. Mild
symptoms of poisoning include dizziness, weakness of the legs,
abdominal discomfort and nausea. Temporary deafness and insomnia may
also occur. It has been estimated, based upon reports of outbreaks of
poisoning, that 0.2-0.25 mg/kg body-weight will produce a single
convulsion in man, and that repeated fits will result from 1 mg/kg
(Hayes, 1963).
LD50
Animal mg/kg body-weight References
(oral)
Rat, adult (F) 7 Treon et al., 1955
Rat, young (F) 17 Treon at al., 1955
Rat, adult (F) 40-43 Speck and Maaske, 1958
Rat, young (M) 29 Treon at al., 1955
Rabbit (F) 7-10 Treon at al., 1955
Guinea-pig 16-36 Treon at al., 1955
Monkey 3 Treon at al., 1955
Monkey 12 Barth, 1967
The acute toxicity of endrin appears to be influenced by the diet.
Three groups each comprising about 100 male rats were fed for 28 days
either a normal diet, a normal protein diet containing protein only as
casein or a low protein diet. The acute toxicity to endrin was then
determined by a single oral administration of the pesticide. The LD50
values were 27, 17 and 7 for the animals fed the respective diets,
indicating an approximately four-fold increase in toxicity between the
normal and low-protein diet as well as an effect due to the type of
protein fed (Boyd and Stefec, 1969).
Short-term studies
Quail
Groups each of 40 quail, starting when one day old, were fed dietary
levels of 0, 0.5, 1, 5, 10, 20 or 50 ppm in their diet. Survival was
adversely affected in all the test groups, and there were no survivors
beyond two weeks in the birds fed 10 ppm or more. Food consumption was
abnormally low. Symptoms involved lack of muscular coordination,
tremors, bedraggled appearance and rigidity with occasional convulsive
movements (De Witt, 1956).
Chicken
Groups each of 20 seven-day old chicks were unaffected by a diet
containing 0, 1.5 or 3 ppm of endrin. When the concentration was
increased to 6 or 12 ppm, the birds became highly excitable, failed to
gain as much weight as the controls and the survival rates over a 12
week period were 85 and 5 percent, respectively, compared to 100 per
cent in the controls (Sherman and Rosenberg, 1954).
Pheasant
Day-old pheasants, in groups of 40, did not survive beyond eight days
when fed dietary levels of 5 or 20 ppm. Reduced food consumption
occurred, and the symptoms were the same as those seen in quail (De
Witt, 1956).
Rat
Groups comprising five male and five female rats were fed dietary
levels of 0, 1, 5, 25, 50 or 100 ppm of endrin for up to 16 weeks. All
of the group fed 100 ppm died within the first two weeks, and only two
rats fed 50 ppm and three fed 25 ppm survived. Three males fed 5 ppm
also died; the other animals were continued on the test diet for the
full 16 weeks. Weight loss was roughly dose related but was evident in
all test groups, as was hypersensitivity to tactile stimuli. There was
an initial drop in serum alkaline phosphatase during the first three
to eight weeks' feeding, which was then followed by an increase at all
dose levels. At the end of the 16 weeks, the phosphatase level was
elevated above the controls in all the test groups, the levels being
highest in the groups fed 25 and 50 ppm (Nelson et al., 1956).
However, other statisticians have considered that the elevation of
serum alkaline phosphatase was not significant in the groups fed 1 and
5 ppm of endrin (Williams, 1966).
Dog
In a series of experiments, dogs were fed diets containing from 1 to
50 ppm endrin along with control groups. Two of four animals fed a
diet containing 8 ppm and the one fed 5 ppm died. The two surviving
dogs on 8 ppm were kept on the diet for about six months and then
sacrificed; increased organ to body-weight ratios for the liver,
kidney and brain were found, and histopathological examination showed
degeneration of kidney tissue. Three of four dogs on 4 ppm of endrin
survived, and there were no symptoms in dogs fed 1 or 3 ppm (Treon et
al., 1955).
In an experiment of about 19 months' duration, groups comprising two
male and two female dogs were placed on diets containing 0, 1 or 3 ppm
of endrin. All dogs on 3 ppm had increased organ to body-weight ratios
for the kidney and heart. Some female dogs fed 1 or 3 ppm of endrin
had a renal abnormality characterized by a slight tubular vacuolation;
this change was also observed in the female control dog. Male dogs in
both control and test groups had normal viscera (Treon et al., 1955).
Groups comprising seven male and seven female dogs were fed dietary
levels of 0, 0.1, 0.5, 1.0, 2.0 or 4.0 ppm of dieldrin for two years.
Scheduled autopsies were performed on two dogs of each sex from the 0,
1.0 and 4.0 ppm groups at six and 12 months. There were no deaths due
to the treatment nor were there any changes in body-weight increase or
food consumption in any group. The only clinical abnormalities were in
one female and two male dogs fed 4.0 ppm and one female fed 2.0 ppm
that showed evidence of, or were observed having, convulsions; the
earliest incidence in a male dog after five months on 4.0 ppm. The
only changes in organ weights were occasional slight increases in
liver or liver to body-weight ratios in the dogs fed 2.0 and 4.0 ppm.
After two years, pathological examination showed slight vacuolation of
hepatic cells in the females and diffuse pigmentation in one male and
all females. At 4.0 ppm, vacuolar degeneration and diffuse brown
pigment in the hepatic cells was evident in all dogs, without any sex
differentiation. In two only of the dogs, which had convulsions,
autopsies revealed some pathological changes in the brain. All other
organs in the dogs fed 2.0 or 4.0 ppm and all organs in the dogs fed
1.0 ppm or less showed no morphological changes which were considered
to be attributable to feeding endrin. There were no significant
changes in the blood picture or in the chemical or physical
characteristics of the urine attributable to endrin. After two years,
levels of liver enzymes, prothrombin time bromsulphthalein clearance,
serum protein electrophoresis, glucose, urea nitrogen, cholesterol,
calcium, inorganic phosphorus, total bilirubin or uric acid showed no
changes attributable to endrin feeding (Jolley et al., 1969).
Cattle and sheep
Cattle and sheep were not affected by 5 ppm of endrin in their diet
for 112 days (Radeleff, 1956).
Long-term studies
Mouse
A total of 1600 mice in equal numbers of each sex, consisting of one
inbred and one hybrid strain, were divided into four groups, two of
which were fed a control diet and the other two fed 0.3 or 3.0 ppm of
endrin. Feeding the test diet was started at five weeks of age and
continued through out their normal lifespan, or until sacrifice.
Because of an early high incidence of fibroadenomas occurring in both
control and test groups in the hybrid strain, all the females of that
strain were sacrificed after 72 weeks for pathological examination. A
few of the mice, fed 3.0 ppm only, displayed convulsions in the early
stages of feeding but recovered and survived. Mortality was not
adversely affected by endrin, nor was body-weight or food intake. No
haematological abnormalities were evident except in two males in the
hybrid group fed 0.3 ppm, which had severe leukaemia. In either sex,
the total number of neoplasms was not influenced by the endrin content
of the diet, except in the case of hepatomas in the females of the
hybrid strain, which were significantly higher than the controls in
the mice of the group fed 3.0 ppm, and sacrificed between weeks 53 and
60 of the feeding period. Because of a relatively high incidence of
hepatomas in one group of controls of this strain, the increase at the
3.0 ppm level was considered not due to endrin. It was noted that in
no animals of either sex were there any metastases of the hepatomas
into the lungs (Witherup et al., 1970).
Rat
In a two year experiment, groups each of 20 male and 20 female rats
were given diets containing 0, 1, 5, 25, 50 and 100 ppm of endrin.
Concentrations of 50 and 100 ppm were lethal within a few weeks. The
concentration of 25 ppm increased the mortality rate of the females.
Non-survivors at the three higher levels exhibited diffuse
degeneration of the brain, liver, kidneys and adrenal glands. The
survivors in the two higher levels showed degenerative changes in the
liver only, while those fed at the lower levels had normal viscera.
The level of 5 ppm caused an increase in liver to body-weight ratio in
males and an increase in kidney to body-weight ratio in females. There
was no effect at the 1 ppm level (Treon et al., 1955).
OBSERVATIONS IN MAN
A total of 874 persons were hospitalized, and there were 26 deaths in
several outbreaks of poisoning in Saudi Arabia in 1967 due to
consumption of bread containing endrin. Approximate average levels in
the bread in various outbreaks were 48, 1500 or 400 ppm, corresponding
to a percentage of fatalities of 1.4, 9.5 and 0.4, respectively, among
those poisoned. Signs and symptoms were typical of central nervous
system stimulation and all survivors rapidly returned to normal
(Weeks, 1967).
Three persons in Egypt experienced convulsions after eating bread
containing 126 to 176 ppm of diedrin. There were no deaths (Coble et
al., 1967).
In an incident in the UK, 59 people became ill from ingesting bread
which contained about 150 ppm of endrin, but there were no deaths
(Davies and Lewis, 1956). The maximum amount of endrin consumed has
been estimated to have been 1 mg/kg body-weight (Zavon, 1961).
Studies in human subjects experiencing intoxication from endrin (Coble
et al., 1967, Weeks, 1967) and from occupational workers (Hayes and
Curley, 1968, Jager, 1970) have demonstrated that endrin rapidly
disappears from the blood in cases of acute intoxication and cannot be
detected in the fat or blood of people exposed to endrin unless
symptoms of intoxication are evident.
Endrin has not been reported to be found from studies involving levels
of organochlorine pesticides in human body fat in India, UK or USA
using analytical methods sensitive to <0.03 ppm (Dale et al., 1965;
Hayes et al., 1965, Robinson et al., 1965 and Zavon et al., 1965).
Levels of the 9-keto metabolite of endrin in four human fat samples
were all less than 0.0004 ppm (Richardson, 1970).
Among workers in a plant manufacturing endrin and a number of other
pesticides, no detectable amounts of endrin were found in samples of
plasma, fat or urine. Exposure was for an average time of 2,106 hours.
Based upon the limit of detection, the levels of endrin, were <0.0030
ppm in plasma, <0.03 ppm in fat and <0.0016 ppm in urine. Endrin
has, however, been detected in the serum and urine of people who
received amounts sufficient to produce intoxication (Hayes and Curley,
1968).
Serum alkaline phosphatase was determined in 30 workers who had been
exposed to endrin for periods from six weeks to eight years. There was
no difference in the levels found in the exposed group and those found
in a group comprising nine unexposed individuals, nor was there any
relationship detected between the phosphatase levels and the duration
of exposure of the workers (Shell, 1965).
In workers exposed to endrin during periods up to eight years, no
significant changes in the level of serum alkaline phosphatase were
observed during a 13-month observation period (Van Dijk, 1968).
It is estimated that the blood level of endrin below which no signs or
symptoms of intoxication occurs is in the range of 0.05 - 0.100 µg/ml.
Measurable blood levels (detection level 0.005 µg/ml) occur only after
gross over-exposure. The half-life of endrin appears to be
approximately 24 hours. Medical control of a group of workers exposed
over periods up to 13 years has failed to show any effects of
long-term exposure. The blood picture, results of urinalysis,
activities of serum glutamic-oxaloacetic and glutamic-pyruvic
transaminases, alkaline phosphatase and lactic dehydrogenase remained
all within normal limits. Occasional electroencephalographic changes
returned to normal. Absenteeism due to disease or accidents was
comparable to that of a control group. Because of the short half-life
of endrin, it is (unlike dieldrin) impossible to calculate the average
level of exposure of the workers to endrin (Jager, 1970).
A decrease in the blood levels of pp'-DDE and an increased excretion
of 6-ß-hydrocortisol in relation to 17-hydroxy-corticosteroids was
present in the workers employed in manufacturing and who were exposed
to endrin and its intermediates. The compound responsible is not known
and further studies are in progress (Jager, 1970).
COMMENTS
The primary site of action of endrin is the central nervous system.
This fact is evidenced by convulsions which result from acute
poisoning and from administration of repeated relatively high doses.
Unlike dieldrin, endrin is rapidly metabolized by animals; the storage
in the fat of animals is very low compared with other compounds of
similar chemical structure. In rats, it is excreted mainly in the
faeces as endrin, 4a-hydroxyendrin, and an unidentified endrin
alcohol; a third metabolite, 9-keto-endrin, is stored in the tissues.
In rabbit, the main route of excretion is the urine, in which four
metabolites were demonstrated, one of which is the 4a-hydroxyendrin.
The plant metabolite of endrin, delta-keto endrin, is rapidly
metabolized by animals. Three metabolites were found in rabbit urine
after oral administration of delta-keto endrin. It is understood that
delta-keto endrin is unlikely to be formed under conditions of good
agricultural practice, and that the compound is less toxic to mammals
than endrin.
A long-term study in mice failed to produce conclusive results
relative to the carcinogenic potential of endrin. Endrin is more
acutely toxic to animals fed a low protein diet. Reproduction studies
with endrin in several species revealed no influence of endrin on
maturation, but foetal and postnatal mortality were increased. There
is no evidence of teratogenic activity. The two-year studies in the
dog and rat are used as a basis for determining a no-effect level.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 1 ppm in the diet, equivalent to 0.05 mg/kg body-weight/day
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0002 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
The most important of all endrin uses, worldwide, and accounting for
some 80% of all endrin applied is as a spray for the control of
insects of cotton. It is also used extensively for the control of
insect pests in rice, to some extent in sugar cane and to a limited
extent in grain crops and sugar beets. In Australia, endrin is used
for insect control on tobacco and crucifers.
Post-harvest treatments
Endrin is accepted and occasionally used in some countries for rodent
control in orchards, where it is sprayed onto the ground under the
trees either in autumn or springtime. Rates vary from 2.4 kg/ha for
deciduous fruits in the United States to 0.02 or 0.03% solutions,
often in combination with mineral oil, in Australia.
Seed treatments
Endrin is used as a seed treatment on cotton at 2 oz /100 lb seed in
the United States and on beans at 1 oz /30 lb seed in Australia.
TABLE I
Summary of Registered Uses
Rate of
Crop application No. of Pre-harvest
(kg/ha) applications interval (days)
Cotton 0.25-0.50 1-12 none
Rice 0.2-0.50 1-4 30
Sugar cane 0.25 (only 1-8 45
granular)
Wheat 0.25-0.50 1 45
Barley " " "
Oats " " "
Sorghum " " "
Tobacco 0.25-0.5 - -
Crucifers 0.04% soln. - -
Potatoes 0.8 (USA) - -
0.03% soln.
(Australia)
Sugar beets 0.4 1-2 60
Other uses
Endrin has a minor use in the United States as a bait formulation for
control of cutworms in corn and potatoes. It is applied to the soil
surface at 0.8 kg/ha, and preharvest intervals are observed.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In cotton seed and its products
From the view point of residues of endrin arising in food products,
cotton seed and its products are of primary interest; the refined
cottonseed oil is used for cooking or margarine manufacture, while the
extracted cake is used as cattle feed. Therefore residue levels in
cottonseed oil products which may reach the food consumer are
determined not so much by levels in products harvested in the field,
but to an appreciable extent by mill processes.
The effect of extraction processes on endrin residues was studied by
Smith et al. (1968) using crude cottonseed oil and soy bean oil
fortified with 1 ppm (mg/g) endrin. They found that alkali washing and
bleaching did not have a marked effect but that deodorization by
vacuum steam stripping effectively reduced endrin levels to below the
limits of detection (0.03 ppm). These authors also quote unpublished
work by Evans of the USDA using radio-labelled endrin. At initial
levels of up to 3.5 ppm in the crude oil, 96 percent of the added
radioactivity was lost during the process of steam stripping. The
effectiveness of the deodorization step in removing endrin residues
from vegetable oils was further demonstrated by Barrantine and Cain
(1970) in studies of commercial scale operations. Endrin residues are
summarized in Tables II and III. (Shell Development Co. Reports,
RES-64-3. RES-64-9; Shell Chemical Co., New York PRL-67-24; Velsicol
Corp., unpublished data).
The cottonseed cake left after the extraction of oil commonly contains
1%-5% oil. These cottonseed cakes are widely used as an important
ingredient of cattle feed concentrates and could contribute up to 20%
of the daily diet of a dairy cow. The relationship between cattle feed
and milk residues was studied by Williams and Mills (1964), Kiigemagi
et al., (1958), and Ely et al., (1957). Consideration of their
combined results indicates that at feed levels in the region of
0.25-2.0 ppm endrin in total feed, milk residues will eventually reach
0.04 times the concentration in the total feed, if feeding is
continued until a plateau is reached. Both Williams and Kiigemagi
found that when endrin was withdrawn from the feed, levels in the milk
fell fairly quickly. At 0.25 and 0.75 ppm in feed, levels fell to
0.002 ppm in six weeks after endrin was withdrawn (Kiigemagi, 1958).
At the 2 ppm feed level, residues fell from 0.06 to 0.02 ppm in the
same period. In Williams' experiments residue from the highest feed
level (0.50 ppm in whole dry feed) fell to 0.002 ppm in about 15 days.
In rice, rice bran and rice straw
The results of experiments in a number of rice growing regions are
summarized in Table IV.
TABLE II
Residues of endrin in whole cotton seed, U.S.A.
Dosage/ Harvest Residue ppm
No.of applic. interval delta-
Location applics. (lb/acre) (days) endrin keto
Oklahoma control - - <0.004 N.D.
2 0.5 58 <0.004 to
0.013 N.D.
2 1.0 58 <0.004 N.D.
Louisiana 4-5 2.67(total) - 0.02 to <0.02
0.03
Texas not stated 0.5 (total) - <0.04 <0.04
1.0 (total) - 0.06 to <0.04
0.10
2.0 (total) - <0.04 <0.04
TABLE III
Endrin residues in crude and alkali-extract
Cottonseed oil obtained from endrin treated cotton in Texas, U.S.A.
Residues in oil - ppm
Dosage rate Post harvest Crude Alkali extracted
lb/acre total interval-days Endrin delta-Keto Endrin delta-Keto
Control - <0.01 <0.03 <0.01 <0.03
0.5 not stated 0.05 <0.03 0.05 <0.03
1.0 not stated 0.43* <0.03 0.39* <0.03
1.0 not stated 0.05 <0.03 0.05 <0.03
2.0 not stated 0.04 <0.03 0.01 <0.03
Control - <0.01 - <0.01 -
0.4, ten times 5 0.05 - <0.01 -
10 <0.01 - <0.01 -
20 <0.01 - <0.01 -
30 <0.01 - <0.01 -
0.8, ten times 5 0.02 - <0.01 -
10 0.03 - <0.01 -
20 0.08 - <0.01 -
30 0.01 - <0.01 -
Control - <0.02-<0.03 - - -
0.4 8 0.02 - - -
13 <0.02 - - -
28 <0.02-<0.07 - - -
52 0.04 - - -
0.8 8 0.07 - - -
13 "neg" - - -
28 <0.02 - - -
42 <0.02-0.02 - - -
* These figures are regarded as exceptional in light of all other available data.
Although neither rice bran or straw is reported to be an appreciable
item of international trade, residues may be of importance insofar as
they may persist in animal products. A summary of data provided by the
references in Table IV shows bran residues for 11 sites to range from
<0.01 to 2.30 ppm, with a mean of 0.35 ppm. The data are heavily
weighted by one figure from India; without this figure, the range is
<0.01 to 0.80 ppm, with a mean of 0.15 ppm. Rice straw residues from
the same sources (25 samples) ranged from 0.04 to 2.90 ppm, with a
mean of 1.02 ppm. Residues of delta-keto endrin were found in bran and
straw containing high residues of endrin but seldom exceeded 10% of
the total residue and never exceeded 20%.
Rice bran is used mainly as a component of locally produced poultry
feed. The significance of endrin residues in poultry feed in giving
rise to residues in meat and eggs may be judged from data in the
published literature.
Cummings et al., (1967) fed diets containing between 0.05 and 0.45 ppm
endrin to White Leghorns for 14 weeks and found residues ranging from
<0.01 to 0.06 ppm endrin in breast meat and from 0.25 to 3.5 ppm
endrin in depot fat. Similar diets gave endrin residues of 0.03 to
0.32 ppm in eggs as estimated from graphs (Cummings et al., 1966).
Terriere et al. (1959) fed diets containing 0.10 to 0.75 ppm endrin
for 6 weeks and found <0.1 to 0.2 ppm endrin in breast meat, <0.1 to
0.3 ppm in leg meat, 0.6 to 3.6 ppm in depot fat and <0.1 to 0.3 ppm
in eggs. A feed level of 2.25 ppm for 6 weeks gave 17.0 ppm in depot
fat.
In sugar cane
In experiments extending from 1957 to 1965, Shell Development Co.
applied endrin to sugar cane in Louisiana and Florida. When 0.3 lb
ai/acre of 2 percent granular was applied at 3-6 applications and
postharvest intervals ranging from 12-120 days were observed, average
endrin residues ranging from <0.01 to 0.05 ppm were obtained.
Keto-endrin residues were determined in four samples and did not
exceed <0.02 on the average. Residues in control samples ranged from
<0.01 to 0.04 ppm. No endrin was detected in molasses or refined
sugar prepared from the treated cane. One group of samples treated
with 0.5 lb ai/acre of E. C. had <0.1 ppm endrin (sensitivity limit
of the method). When 2 percent granular was applied four times at 1.0
lb ai/acre and postharvest intervals of 7-45 days were observed, the
endrin residues ranged from 0.04 to 0.08 ppm. The same formulation
applied four times at 0.22 lb ai/acre and eight times at 0.23 to 0.29
lb ai/acre resulted, after postharvest intervals of 71 and 64 days,
respectively, in endrin residues of <0.10 ppm. (Shell Development Co.
Reports RES-61-53, RES-63-159 DLI-136 (1963), DLI-157 (1964),
RES-64-1, DLI-160 (1965), RES-58-48A, RES-57-44.
In grains
Data obtained on a variety of small grains in the U.S.A. are
summarized in Table V.
In India, endrin (2% granular) was applied to sorghum at 0.4 kg ai/ha
for 4 to 6 applications. Samples taken from 42 to 75 days after last
treatment had residues in grain ranging from <0.01 to 0.02 ppm endrin
and in straw ranging from 0.14 to 0.70 ppm endrin. Control samples had
<0.01 to 0.02 ppm in grain and 0.03 to 0.31 ppm in straw (Shell
Research Ltd., Report WKGR 0071/70).
In the U.S.A., endrin E.C. was applied to sorghum once at either 0.25
or 0.50 lb ai/acre. A pre-harvest interval of 111 days resulted in
endrin residues of <0.02 ppm in grain, <0.02 ppm in straw, and
<0.02 ppm in silage (73 days PHI). A pre-harvest interval of 63 days
resulted in endrin residues of <0.02 ppm in grain, 0.03 and 0.09 ppm
in straw, and <0.02 and 0.15 ppm in silage (39 days PHI); the latter
value is suspect, according to the analysts (Shell Chemical Co.,
Report PRL-66-113). A pre-harvest interval of 29 days resulted in
residues of <0.05 and 0.05 ppm in grain (Velsicol Corp., Report
TSR-2575).
Single applications of endrin E.C. to sweet corn (maize) in the U.S.A.
at 0.25 or 0.50 lb ai/acre, 37 days before harvest, resulted in
residues of <0.02 ppm of endrin in grain and husk. Some controls gave
an apparent residue of 0.02 to 0.03 ppm (Shell Chemical Co., Report
PRL 66-67g).
In apples
Experiments conducted by Shell in the U.S.A. in which endrin E.C. or
W.P. was applied at 1.5 to 4.0 lb ai/acre to the soil of orchards gave
no detectable residues in whole apples at harvest time; detection
limits were 0.01-0.002 ppm for the methods employed (Shell Development
Co., Report RES-61-60, Shell Chemical Co., Report PRL-69-119, Ibid,
PRL-69-95). In recent studies by Horsfall et al. (1970), picked fruit
residues ranged from <0.002-0.003 ppm. In fallen fruit, residues were
sometimes higher, ranging up to 0.023 ppm.
FATE OF RESIDUES
General comments
Endrin undergoes rearrangement to delta-keto endrin in sunlight, in
the presence of strong acids, and by thermal treatment. Endrin also
undergoes thermal and photo degradation to yield small amounts of the
aldehyde SD 7442. Delta-keto endrin can undergo a rearrangement to the
"bird cage alcohol" when treated with very dry Florisil adsorbent
(Korte and Porter, 1970).
TABLE IV
Endrin residues in polished and unpolished rice
Applic. rate Harvest Residues, ppm
kg am/ha × interval, Polished Brown
Country Formulation no. of applic. days rice rice Reference
India 2% gran. 0.2, 0.4, 0.6, 49-79 <0.01 <0.01-0.01 Shell Res.Ltd.Report, WKTR 0023/69
2.0 × 4-7
control - - <0.01 <0.01
Venezuela E.C. 0.29 × 1 95 0.02 0.03 Ibid,WKGR 0046/70
Thailand control - - <0.01 <0.01 Ibid,WKGR 0087/70
2% gran. 0.2, 0.4, 0.4 56-83 <0.01 <0.01
20% E.C. 0.16 × 3 46-72 <0.01 <0.01-0.23*
Philippines control - - <0.01-0.03 <0.01-0.03 Ibid,WKGR 0078/70
2% gran. 0.2, 0.4, 0.4 44-60 <0.01-0.02 <0.01-0.05
20% E.C. 0.16 × 3 33-49 <0.01-0.03 <0.01-0.03
Indonesia control - - - <0.01 Ibid,WKTR 0078/68
20% E.C. 0.2-0.62 21 - 0.03-0.05
× 7 or × 8
Thailand 20% gran. 0.4 X3, X4 not <0.02 - Ibid,BEGR.0045/70
0.8 × 3 stated (Endrin and Keto -
Endrin Keto Endrin Keto
India control - - <0.02 <0.02 0.09 <0.02 Ibid
2% gran. 0.4 - 0.02 <0.02 0.23 0.03
0.8 45 0.04 <0.02 0.47 0.04
* One exceptional result - reason not identified
TABLE V
Endrin residues in small grains - U.S.A.
Applic. rate, Postharvest Endrin residues,
lb ai/acre interval, ppm
Crop × no. of applic. days Grain Straw Reference
Wheat 0.25 × 1 26 0.03 - Shell Dev. Co.
0.50 × 1 26 0.06 - Report RES-
Oats 0.25 × 1 26 0.15 - 62-124 (1963)
0.50 × 1 26 0.50 -
Wheat 0.25 × 1 62 <0.01 0.27 Shell Chem.Co.
0.25 × 1 96 <0.01 0.02 Report PRL-
0.25 × 2 62 <0.01 0.37 65-41 (1966)
Barley 0.50 × 1 60 <0.05 0.07
0.50 × 1 32 <0.05 0.09-0.38
Wheat 0.25 × 1 >150 0.01 - Velsicol Corp.
0.50 × 1 >150 0.03 - Report TSR-
Barley 0.25 × 1 >150 <0.01 - 2578 (1966)
0.50 × 1 >150 <0.01 -
In animals
Homogenates from cow or pig liver upon addition of NADH2 converted 38
ug of endrin, after 72 hours incubation, to metabolites which were
identical to those found from living organisms (Korte, 1967; Klein et
al., 1968a). See "Biochemical aspects".
In plants
14C labelled endrin was applied at either 1.04 or 2.08 mg/plant to
the leaves of tobacco under conditions of free or restricted aeration.
Six weeks after treatment, 32-47% of the applied radioactivity was
found on and in the tobacco plants, the lowest residues being in the
plants grown under free aeration. (Korte and Porter, 1970).
Shortly after the first buds emerged, the leaves of each of 11 cotton
plants were treated with 4.2 mg 14C-labelled endrin; treatment was
repeated after two and six weeks (total ca. 120 ppm). Twelve weeks
after the last application, quantitative measurement of the residues
revealed only very low concentrations in the cotton seed (0.033 ppm),
somewhat more in the fibers (0.36 ppm), and about 80% in and on the
leaves. Two thirds of the applied radioactivity was lost to the
atmosphere during the test. Besides endrin, there were two groups of
degradation products in the cotton plants, one group slightly more
hydrophilic than endrin and one very hydrophilic. One component of the
less hydrophilic group was isolated and gave mass and IR spectra
identical with that of delta-keto endrin (Korte and Porter, 1970).
When 14C-labelled endrin was applied at 50 ug/plant to young cabbage
plants, 66 percent of the activity had evaporated after two weeks, 70
percent after three weeks, and 75 percent after four weeks. Activity
not evaporated was found in the plants as endrin and hydrophilic
metabolites. After administration of 500 ug/plant, the concentration
of activity decreased from leaves to stalks to roots to soil, while
the ratio of metabolites to endrin increases in the same order. The
metabolite fraction consisted of two compounds, a very hydrophilic
main metabolite and delta-keto endrin (Weisgerber et al., 1968).
Similar results were obtained with carrots (Klein et al., 1968b).
Foliar application of 14C-labelled delta-keto endrin to white cabbage
has shown that it is more persistent than endrin but metabolizes more
rapidly (about 15 percent compared with about 10 percent for endrin).
The converted keto-endrin consisted mainly of a very hydrophilic
metabolite, the concentration of which is highest in leaves and stalks
(Korte and Porter, 1970).
Residues in soybean plants grown in soil treated with 14C-labelled
endrin were mainly delta-keto endrin, and endrin alcohol in addition
to endrin aldehyde were believed present (Nash and Beall, private
communication).
In soil
Matsumura et al., (in press); incubated 14C-labelled endrin with 150
cultures of microorganisms isolated from soils and found 25 active in
degrading endrin, all of which had previously been found to degrade
dieldrin. Seven different metabolites of endrin were found, with three
major and four minor ones; tentative structures have been assigned
(Korte and Porter, 1970).
In storage and processing
The effects of processing on the residues of endrin in dairy products
was studied by Langlois et al., (1965). Butter, ice cream, cheese,
condensed milk and dry whole milk were manufactured from milk
contaminated with endrin either by direct addition or through feeding
to cows. In general, they found that the residues concentration in the
fat remained fairly constant during processing except for drastic heat
treatment to produce dry whole milk which caused major reductions
(>50 percent).
When hens fed on a diet containing endrin were cooked in water at
190-200°F for three hours, the residues were reduced up to 90 percent
(Liska et al., 1967). Autoclaving the carcass at 15 psi for three
hours removed essentially all residues. Metabolites of endrin ware not
determined.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Residues of endrin were monitored in cottonseed oil produced by mills
in Venezuela, Brazil, India and the United States. In Venezuela,
weekly samples were taken for nine weeks, and endrin residues ranged
from <0.02 to 0.05 ppm for crude oil, <0.02 to 0.02 ppm for
decolorized oil and <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR. 0119.70, 1970). In Brazil, seven samples were taken over
thirteen weeks, and endrin residues ranged from <0.01 to 0.01 ppm for
crude oil and <0.01 to <0.02 ppm for deodorized oil (Shell Research
Ltd., WKGR.0120.70, 1970). In India, only crude oil samples were
available, and these contained from 0.04 to 0.06 ppm endrin (Shell
Research Ltd., Report WKGR 0091.70). In the United States, ten samples
of refined oil from California were analyzed and found to contain
<0.03 to 0.03 ppm endrin and <0.02 ppm delta-keto endrin (Shell
Development Co., RES-63-155).
In addition to oil, cottonseed cakes have also been studied for endrin
residues, since these are used widely as an important ingredient of
cattle feed concentrates. Figures for cattle cake samples
corresponding to the samples of cottonseed oils given in the preceding
paragraph are as follows: Venezuela, <0.01 to 0.02 ppm; Brazil <0.01
to 0.08 ppm; India, <0.01 ppm. In the United States, data obtained on
cakes and meals corresponding to the samples listed in Tables II and
III ranged from <0.01 ppm to <0.04 ppm; most residues were below the
detection limit of <0.01 ppm.
Total diet studies conducted in the U.K.and the U.S.A. indicate that
endrin residues are unlikely to arise in human diets as a result of
the use of endrin in cotton (Abbott et al., 1969; Assoc. of Public
Analysts, 1969; Duggan, 1968; USDA, 1968). In the U.K., no endrin
residues were found in vegetable oils, fats, milk, milk based infant
foods, beef, beef sausages or meats. In the U.S.A., no endrin residues
were found in finished or crude cotton-seed oil, milk, milk products
or manufactured milk products.
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967) apply to the
determination of endrin. The compound can be determined in fatty and
nonfatty foods by the multiresidue method and gas chromatography
(Pesticide Analytical Manual., Vol. I and II, U.S. FDA). Endrin in
eluted from the Florisil column in the 15% ethyl ether/petroleum ether
fraction. Additional cleanup by MgO-Celite column and alkaline
hydrolysis in required, and recovery is usually in the range of
80-100%. When a gas chromatograph containing 10% DC 200 on Gas-Chrom Q
column at 200°C with 120 ml/min N2flow and an electron capture
detector is used, the following retention times relative to aldrin are
obtained: endrin - 2.05, endrin aldehyde - 2.30, endrin alcohol
- 2.50, delta-keto endrin -3.50. The sensitivity of the method is
good; 2 ng of endrin gives a “ full scale response on a 10-9amp full
scale recorder. A procedure for the determination of delta-keto endrin
in mammalian samples has been developed (Zavon et al., 1965), and a
general method is available (Shell Development Co., Anal. Method MMS
53/64). Residue methods have not yet been developed for monohydroxy
endrin, 9-keto endrin, endrin, endrin aldehyde or the "bird cage
alcohol" isomer of endrin.
An infrared method for endrin has been described (Gershman, 1961). An
absorbance peak at 10.2 microns is used for calculations. The method
requires a 1 kg ample for 0.25 ppm.
NATIONAL TOLERANCES
Country Commodity Tolerance ppm
Australia vegetables including 0.1
beans, crucifers,
potatoes, tomatoes
United States broccoli, brussels 0
sprouts, cabbage,
cauliflower, cucumbers, extended1
cottonseed, eggplant,
peppers, potatoes,
United States
(cont'd) sugar beets and sugar extended1
beet tops, summer squash,
tomatoes
1 The word "extended" appears where action is being taken to acquire
data to petition for a tolerance.
Australia has not set a tolerance for endrin in cottonseed, however,
an action level has been set for endrin residues in animal feeds at
0.03 ppm.
APPRAISAL
Endrin is widely used to control insect pests on cotton and to lesser
extent on rice. It is used to a limited extent as an insecticide on
small grains, vegetables and sugar cane and is occasionally used for
mouse control in orchards. The ways in which endrin is applied vary
widely, as do the numbers of applications.
The most important consideration regarding endrin residues in food
arise from its use on cotton; cottonseed oil is used for cooking or
margarine manufacture, and the extracted cottonseed cake is used as
cattle feed. Controlled feeding experiments with cattle indicate that
whole milk residues would reach a plateau of 0.04 times the residue in
feed. Once ingestion stops, excretion in milk soon ceases (two to six
weeks). Cottonseed cake for feed should have less than 0.1 ppm of
endrin residues if the recommended practical residue limits for whole
milk and milk products are not to be exceeded.
Rice bran is used as a component of poultry feed; feeding studies on
hens indicate that endrin residues in depot fat would reach about five
times the level in feed; for eggs the ratio would be about 0.7 times.
Calculations indicate that it is unlikely that significant endrin
residues would be found in cooked poultry or eggs. The rice bran used
as a constituent of poultry feeds should not contain residues greater
than 1 ppm if the recommended practical residue limits for poultry and
eggs are not to be exceeded.
In the absence of experimental residue data at the recommended
preharvest interval of 45 days or longer, the meeting did not
recommend a tolerance for oats.
Under field conditions, endrin is rapidly loot from plants through
evaporation - 60-80 percent over six to 18 weeks, depending on the
crop.
Endrin is metabolized by plants to delta-keto endrin, a rearrangement
product, and to very hydrophilic metabolites; traces of the aldehyde
(SD 7442) and alcohol have been reported. The parent compound appears
to be the major residue at all times.
The widespread use of endrin on cotton does not appear to give rise to
measurable residues in the human diet. Dietary studies in the United
States have revealed no endrin residues in finished cottonseed oils,
milk, milk based infant foods, milk products, beef or meats and trace
amounts in margarine and fats. Exceptionally, one diet composite
sample in one sampling location in the United States contained 0.20
ppm of endrin in oils, fats and shortening.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The following tolerance and practical residue limits are to apply to
raw agricultural products moving in commerce unless otherwise
indicated. All figures include the sum of endrin and delta-keto
endrin.
TOLERANCES
ppm
Cottonseed, cottonseed oil (crude) 0.1
Cottonseed oil (finished), maize
(sweet), wheat, barley, sorghum,
rice (brown or polished), apples 0.02
PRACTICAL RESIDUES LIMITS
Milk and milk products (fat basis) 0.02
Fat of poultry 1
Eggs (shell-free) 0.2
FURTHER WORK OR INFORMATION
DESIRABLE
An adequate long-term study in a strain of mice of known tumour
susceptibility.
REFERENCES
Abbot, D.C., Holmes, D.C. and Tatton, J. O'G. (1969) J. Sci. Fd.
Agric. 20; 245
Altmeier, G., Klein, W. and Korte, F. (1969) Tetrahedron Letters,
49: 4269-4271
Association of American Pesticide Control Officials, Inc. (1966)
Pesticide Chemicals Official Compendium, 1966 Ed., 475-477
Association of Public Analysts. (1967) Joint Survey of Pesticide
Residues in Foodstuffs sold in England and Wales
Baldwin, M.K., Robinson, J. and Parke D.V. (1970) The metabolism of
endrin in the rat. Unpublished report from the Tunstall Laboratory,
Shell Research Ltd., Sittingbourne and the University of Surrey
Barrentine, B.F., and Cain, J.B. (1970) Unpublished work cited in
Comptes Rendus, 25th Conference of IUPAC, Commission VI.5.1, Appendix
VII
Barth, R.A.J. (1967) Pesticide toxicity in primates. Doctoral thesis,
Tulane University, Louisiana p. 110-111
Boyd, E.M. and Stefec, J. (1969) Dietary protein and pesticide
toxicity: with particular reference to endrin. Canad. med. Ass. J.,
101: 335-339
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27:81-138
Coble, Y., Hildebrandt, P., Davis, J., Raasch, F. and Curley, A.
(1967) Acute endrin poisoning. J. Amer. med. Ass. 202: 489-493
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Comptes Rendus. (1970) Fate of organochlorine pesticides in vegetable
oil processing. 25th Conference of IUPAC, Commission VI.5.1, Terminal
Pesticide Residues, Appendix VII. p. 187-189
Cummings, J.G., Zee, K.T., Turner, V., Quinn, F..and Cook R.E. (1966)
Residues in eggs from low level feeding of five chlorinated
hydrocarbon insecticides to hens. J. Assoc. Off. Anal. Chem. 49:
354-364
Cummings, J.G., Eidelman, M., Turner, V., Reed, D., Zee K.T. and Cook,
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydro-carbon insecticides to hens. J. Assoc. Off. Anal.
Chem. 50: 418-425
Dale, W.E., Copeland, M.F. and Hayes, W.J. Jr. (1965) Chlorinated
insecticides in the body fat of people of India. Bull. Wld Hlth Org.,
33: 471-477
Davies, G.M. and Lewis, I. (1956) Outbreak of food poisoning from
bread made of chemically contaminated flour. Brit. med. J., (2):
393-398
Dewitt, J.B. (1956) Chronic toxicity to quail and pheasants of some
chlorinated insecticides. J. Agr. Fd. Chem., 10: 863-866
Diechmann, W.B., MacDonald, W.E., Radomski, J. Blum, E.B. Bevilacqua,
M. and Keplinger, M. (1970) The tumorigenicity of aldrin, dieldrin and
endrin in the albino rat. Industr. Med., 39: 314
Duggan, R.E. (1968) Residues in food and feed. Pesticide residues in
vegetable oil seeds, oils and by-products. Pesticides Monotoring
Journal 1 (4): 2-7
Dunachie F.F. and Fletcher W.W. (1966) Effect of some insecticides on
the hatching rate of hen's eggs. Nature, 212: 1062-1063
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. econ. Entomol., 50: 348-349
Ely, R.E., Moore, L.A., Carter, R.H. and App, B.A. (1957) Excretion of
endrin in the milk of cows fed endrin-sprayed alfalfa and technical
endrin. J. Econ. Ent. 50: 348-349
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1963/13; WHO/Food Add./23(1964)
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FDA, (1967) Pesticide analytical manual, HEW, FDA, Vol. I and 11
Frear, D.E.H. (1955) Endrin in pesticide index, Third Edition, D. van
Nostrand Co. Inc., New York. p. 68-69
Gershman, L.L. (1961) Infra-red determination of endrin residues. J.
Assoc. Off. Anal. Chem. 44: 212-214
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Hayes, W.J., Jr., (1963) Endrin in Clinical handbook on economic
poisons, revised edition. pp. 68.70. Publ. Hlth. Ser. Publn No. 476.
US Govnmt Printing Off. Washington D.C.
Hayes, W.J., Jr., Dale, W.E. and Baise, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people of New Orleans. Life Sci.,
4: 1611-1615
Hayes, W.J. and Curley, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environm. Hlth, 16: 155-162
Hine, C.H., Eisenlord, G., Loquvam, G.S. and Leung, T. (1968) Results
of reproduction studies on rats fed diets containing endrin over three
generations. Unpublished report prepared by the Hine Laboratories,
Inc., San Francisco, submitted by Shell International Research
Horsfall, F. Jr., Webb, R.E., Price, N.O. and Young, R.W. (1970)
Residues in apples subsequent to ground sprays of endrin. J. Agr. Food
Chem. 18: 221-223
IUPAC, (1967) Proceedings of the Commission on Terminal Residues,
Vienna, August 1967. Appendixes VI and VII
Jager, K.W. (1970) Aldrin, dieldrin and telodrin. An epidemiological
and toxicological study of long-term exposure. Doctoral thesis,
Leiden, 1970
Jolley, W.P., Stemmer, K.L., Grande, F., Richmond, J. and Pfitzer,
E.A. (1969) The effects exerted upon beagle dogs during a period of
two years by the introduction of 1, 2, 3, 4, 10, 10-hexachloro-6,
7-epoxy-1, 4, 4a, 5, 6, 7, 8, 8a-octahydro-1, 4-endo, endo-5,
8-dimethanonaphthalene into their daily diets. Unpublished report from
the Kettering Laboratory University of Cincinnati submitted by Shell
International Research
Kiigemagi, U., Sprowls, R.G. and Terriere, L.C. (1958) Endrin content
of milk and body tissues of dairy cows receiving endrin daily in their
diet. J. Agr. Fd. Chem., 6: 518-521
Klein, W., Müller, W. and Korte, F. (1968a) Insektizide im
Stoffwechsel, XVI. Ausscheidung, Verteilung und Stoffwechsel von
Endrin (14C) in Ratten. Liebigs Ann. Chem. 713: 180-185
Klein, W. et al., (1968b) Qualitas Plant. Mater. Vegetabiles 15,
225-238
Klein, W. and Drefahl, B. (1970) "Jahresbericht 1969" Institut für
ökologische Chemie der Gesellschaft für Strahlenforschung mbH,
München, Schloss Birlinghoven, April 1970
Korte, F. (1967) Metabolism of chlorinated insecticides. Unpublished
report from the Radiochemical Laboratories, Bonn University
Korte, F. and Porter, P.E. (1970) Minutes of the fifth meeting of the
IUPAC Commission on terminal pesticide residues, September, 1970,
Erbach, Federal Republic of Germany, Appendixes 4 and 4a
Langlois, B.E., Liska, B.J. and Hill, D.L. (1965) The effects of
processing and storage of dairy products on chlorinated insecticide
residues. J. Milk and Food Technol. 28: 9-11
Liska, B.J., Stemp, A.R. and. Stadelman, W.J. (1967) Food Technol.
21: 435
Ludwig, G. (1965) Excretion, metabolism and storage of endrin 14C
after oral administration to a rat. Minutes of the fifth meeting on
the fate of organic compounds in the living organism. 20-21 October
1965, Birlinghoven Laboratory
Ludwig, G. (1966) Investigations with 14C-labelled insecticides. XX
Excretion, metabolism and storage of endrin - 14C after oral
administration to a rat. Birlinghoven Biomonthly Res. Summary
(January), p. 13-14
Morris, R.D. (1968) Effects of endrin feeding on survival and
reproduction in the deer mouse Peromyscus maniculatus. Canad. J.
Zool., 46: 951-958
Nash, R.G. and Beall, M.L. Jr. (1970) Extraction and identification of
endrin and heptachlor degradation products. Submitted to J. Assoc.
Off. Anal. Chem., October 1970
Nelson, S.C., Bahler, T.L., Hartwell, W.V., Greenwood, D.A. and
Harris, L.E. (1968) Serum alkaline phosphatase levels, weight changes
and mortality ratios of rats fed endrin. J. Agr. Pd. Chem., 4:
696-700
Radeleff, R.D. (1956) Hazards to livestock of insecticides used in
mosquito control. Mosquito News, 16(2): 79-80
Richardson, A. (1970) Unpublished untitled letter from Tunstall
Laboratories to Shell International Research
Richardson, A. Robinson, J. and Baldwin, M.K. (1970) Metabolism of
endrin in the rat. Chem. Industr., 502-503
Robinson, J., Richardson, A., Hunter, C.G., Crabtree, A.N. and Rees,
H.J. (1965) Organo-chlorine insecticide content of human adipose
tissue in south-eastern England. Brit. J. industr. Med., 22: 220-229
Shell. (1965) Alkaline phosphatase in endrin-exposed workers.
Unpublished report prepared by the Industrial Medical Department,
Shell Nederland Chemische Fabrieken N.V. Rotterdam
Shell Chemical Co., Reports. (1965-69) PRL-65-41, PRL-66-67g,
PRL-66-113, PRL-67-24, PRL-69-28, PRL-69-95, PRL-69-119
Shell Development Co., Reports. (1961-65) RES-61-60, RES-62-124,
RES-63-155, RES-64-39 RES-64-9
Shell Research Ltd., Report (1968-70) WKTR 0078/68, 0023/69, WKGR
0046/70, 0072/70, 0078/70, 0087/70, 0071.70, 0091.70, 0119.70, 0120.70
Sherman, M. and Rosenberg, M.M. (1954) Sub-chronic toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 47: 1082-1083
Smith, K.J., Polen, P.B., De Vries, D.M. and Coon, F.B. (1968) Removal
of chlorinated pesticides from crude vegetable oils by simulated
commercial processing procedures. J. Amer. Oil Chem. Soc., 45:
866-869
Smith, S.I., Weber, C.W. and Reid, B.L. (1970) The effect of injection
of chlorinated hydrocarbon insecticides on hatchability of eggs.
Toxicol. appl. Pharmacol, 16: 179-185
Soto, A.R. and Diechmann, W.B. (1967) Major metabolism and acute
toxicity of aldrin, dieldrin and endrin. Environm. Res., 1: 307-322
Speck, L.B. and Maaske, C.A. (1958) The effects of chronic and acute
exposure of rats to endrin. A.M.A. Arch. industr. Hlth, 18: 268-272
Street, J.C., Butcher, J.E., Raleigh, R.J. and Clanton, D.C. (1957)
Tissue storage and transferral to the lamb of aldrin, dieldrin and
endrin when fed to bred ewes. Proc. West. Sec. Amer. Soc. Anim. Prod.,
(Pullman, Washington), 46: 1-6
Terriere, L.C., Kiigemagi, U. and England, D.C. (1958) Endrin content
of body tissues of steers, lambs and hogs receiving endrin in their
daily diet. J. Agr. Fd. Chem., 6: 516-518
Terriere, L.C., Arscott, G.H. and Kiigemagi, U. (1959) The endrin
content of eggs and body tissue of poultry receiving endrin in their
daily diet. J. Agr. Fd Chem., 7: 502-504
Treon, J.F., Clevelend, F.P. and Cappel, J. (1955) Toxicity of endrin
for laboratory animals. J. Agr. Fd. Chem., 3: 842-848
USDA, Agriculture handbook No. 331. (1968) Suggested guide for the use
of insecticides to control insects affecting crops, livestock,
households, stored products, forests and forest products
USDA, Residues in food and feed. (1968) Pesticide residue levels in
foods in the United States from 1 July 1963 to 30 June 1967.
Pesticides Monitoring Journal, 2(1): 2-46
USDA, Summary of registered agricultural pesticide chemical uses.
(1969) Vol. III, Insecticides, repellents, acaricides. Endrin,
III-E-2.1, 2.2, 2.3
Van Dijk, M.C. (1968) Chlorinated insecticides and the serum alkaline
phosphatase. Proc. Sixth Shell Industrial Doctors Meeting, 28-30 May
1968, Amsterdam
Velsicol Corp., (1968-70) Reports TSR 2578, TSR 2575 (Unpublished -
1970)
Weeks, D.E. Endrin food-poisoning. A report of four outbreaks caused
by two separate shipments of endrin-contaminated flour. Bull. Wld Hlth
Org., 37: 499-512
Weisgerber, I., Klein, W., Djirsarai, A., and Korte, F. (1968) Ann.
Chem. 713: 175-179
Williams, S., and Mills, P.A. and McDowell, R.E. (1964) Residues in
milk of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Anal. Chem., 47:
1124-1128
Williams, D.A. (1966) Alkaline phosphatase activity following exposure
to endrin, comments on the paper by Nelson et al. Unpublished report
from the Statistics Unit, Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Witherup, S., Stemmer, K.L., Taylor, P., Bietsch, P. and Pfitzer, E.A.
The incidence of neoplasms in two strains of mice sustained on diets
containing endrin. Unpublished report from the Kettering Laboratory,
University of Cincinnati submitted by Shell International Research
Zavon, M.R. (1961) The toxicology and pharmacology of endrin.
Unpublished report from the Kettering Laboratory, University of
Cincinnati
Zavon, M.R., Hine, C.H. and Parker, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Amer. med. Ass., 193: 837-839
