
ETHYL VANILLIN
First draft prepared by
Dr Preben Olsen
Institute of Toxicology, National Food Agency
Ministry of Health
Soborg, Denmark
Explanation
Biological data
Biochemical aspeects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Long-term toxicity/carcinogenicity studies
Reproductive toxicity studies
Special studies on genotoxicity
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Ethyl vanillin was first evaluated at the eleventh meeting of the
Committee (Annex 1, reference 14), when an ADI of 0-10 mg/kg bw was
allocated on the basis of a long-term study in rats. At that time, the
Committee noted that few metabolism studies had been carried out on
ethyl vanillin and concluded that further studies of that type were
desirable. Ethyl vanillin was re-evaluated at the thirty-fifth meeting
of the Committee (Annex 1, reference 88) on the basis of the partial
application of the procedure for setting priorities for the safety
review of food flavouring ingredients (Annex 1, reference 83). At that
time, the Committee noted that none of the previously evaluated
long-term toxicity or carcinogenicity studies met modern standards in
that fewer animals per group had been used than would be the present
norm, and it therefore reduced the ADI to 0-5 mg/kg bw and made it
temporary. A consolidated monograph was prepared (Annex 1, reference
89). The Committee requested submission of the results of adequate
short-term toxicity and metabolism studies in rats for evaluation in
1992. At the thirty-ninth meeting (Annex 1, reference 101) the
Committee was informed that the studies requested had been initiated,
and that preliminary results did not indicate any cause for concern.
On the basis of this information, the Committee extended the
previously allocated temporary ADI of 0-5 mg/kg bw, pending the
submission of the final results of the ongoing short-term toxicity
and metabolism studies in rats for evaluation by 1994.
At the present meeting, the Committee reviewed the studies
requested. Relevant information from the previous monographs and
information received since the previous evaluation are summarized and
discussed in the following monograph addendum.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Early reports indicated that ethyl vanillin was probably
metabolized to glucuroethyl vanillin and ethyl vanillic acid, of which
some was conjugated with glucuronic and sulfuric acids (Williams,
1959).
Ethyl 14C-vanillin was administered to male and female
Sprague-Dawley CD rats by gavage in polyethylene glycol solution at
single doses of 50, 100, or 200 mg/kg bw. Ethyl vanillin was rapidly
absorbed and peak plasma radioactivity occurred within 2 h after
dosing at all dose levels, falling rapidly to undetectable levels
within 96 h. Plasma radioactivity tended to be higher in female than
male rats and it was postulated that this might reflect a lower
metabolic capacity of female rats.
Urinary excretion of radioactivity was rapid and more than 94%
of the dose was excreted by this route within 24 h. Only 1-5% of the
dose was excreted in faeces. After 5 days, more than 99% of the
administered dose was excreted. No radioactivity was detected in
expired air, indicating that the aromatic ring was in a metabolically
stable position (Hawkins et al., 1992).
2.1.2 Biotransformation
Ethyl 14C-vanillin was administered to male and female Sprague
Dawley CD rats at single oral doses of 50, 100, or 200 mg/kg bw. Rapid
metabolism occurred and the principal metabolite at all dose levels
was ethyl vanillic acid.
Analysis of urine after hydrolysis with glucuronidase and/or
sulfatase indicated that the major metabolites were glucuronide or
sulfate conjugates of ethyl vanillic acid (56-62%), ethyl vanillyl
alcohol (15-20%), and ethyl vanillin (7-12%). A minor proportion of
the dose (2-8%) was excreted as the glycine conjugate of vanillic acid
(ethyl vanilloyl glycine) (Hawkins et al., 1992). The major
metabolic pathways of ethyl vanillin in rats are shown in Figure 1.
Ethyl vanillic acid was also the major metabolite after dietary
administration of ethyl vanillin to rats at doses of 500, 1000 or
2000 mg/kg bw (Hooks et al., 1992a).
During urinary organic acid profiling in human subjects, several
patients excreted high concentrations of ethyl vanillic acid
(3-ethoxy-4-hydroxybenzoic acid) and traces of 3-ethoxy-4-hydroxy-
mandelic acid.
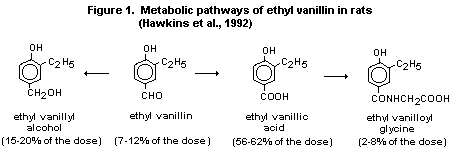 Ethyl vanillic acid was identified by GC/MS in the urine
of a 9-year old female patient who had received liquid dietary
supplementation flavoured with vanilla. Other patients excreting this
acid were also known to have consumed foodstuffs flavoured with ethyl
vanillin. Eight different urine samples containing more than 50 mg
ethyl vanillic acid/g creatinine were also found to contain small
amounts of vanillylmandelic acid. Unchanged ethyl vanillin was not
detected in any of the urine samples.
A healthy adult male volunteer drank a 235 ml aliquot of a liquid
dietary supplement containing an unknown quantity of ethyl vanillin. A
concentration of 13 mg ethyl vanillic acid/g creatinine was found in a
12-hour urine sample. The compound was not present in urine collected
before exposure (Mamer et al., 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ethyl vanillin are
summarized in Table 1.
The lowest lethal dermal dose in rats was reported to be
1800 mg/kg bw (RTECS, 1990).
When groups of 6 rabbits were given ethyl vanillin by gavage, a
dose of 150 mg/kg bw caused no adverse effects. At 2500 mg/kg bw, only
a transient increase in respiration rate was observed. The minimum
oral lethal dose was reported to be 3000 mg/kg bw (Deichmann &
Kitzmuller, 1940).
Table 1. Acute toxicity studies with ethyl vanillin
Species Route LD50 Reference
mg/kg bw
Mouse i.p. 7501 Caujolle & Meynier, 1954a
Rat oral 1590 Sporn, 1960
oral > 2000 Jenner et al., 1964
s.c. 1800 Deichmann & Kitzmuller, 1940
Guinea-pig i.p. 1140 Caujolle & Meynier, 1954b
Dog i.v. 760 Caujolle et al., 1953
1 Maximum non-lethal dose, 450 mg/kg bw; Lethal dose: 950 mg/kg bw
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 300 mg ethyl vanillin/kg bw were administered to rats by
gavage twice weekly for 14 weeks without any adverse affects. In
another experiment, groups of 16 rats were fed ethyl vanillin at a
dose of 20 mg/kg bw/day for 18 weeks without adverse effect. However,
64 mg/kg bw/day for 10 weeks reduced growth rate and caused
myocardial, renal, hepatic, lung, spleen and stomach injuries (nature
not specified) (Deichmann & Kitzmuller, 1940).
Sixteen rats were given 30 mg ethyl vanillin weekly for 7 weeks
without adverse effect on growth, food intake or protein utilization
(Spore, 1960).
Groups of 5 male rats were fed 0, 2%, or 5% ethyl vanillin in the
diet for 1 year without any adverse effects (Hagan et al., 1967).
Groups of CD Sprague-Dawley BR rats (20/sex/group) were fed ethyl
vanillin of > 99.9% purity (nature of diet e.g., semi-synthetic/chow
diet, not specified) at dose levels of 0, 500, 1000 or 2000 mg/kg
bw/day for 13 weeks. The study was designed in accordance with
toxicological principles for the safety assessment of food additives
established by the US FDA (FDA, 1982). The diet was prepared weekly
and showed stability for up to 18 days at room temperature. The
achieved mean dose over the 13-week period was within 1.5% of the
nominal value. Food consumption and body weight were recorded weekly.
Ophthalmoscopy was done before treatment and at termination of the
study. Detailed haematological and clinical chemical examinations were
carried out at week 6 and 13. At termination, all animals were
necropsied and organ weights recorded (adrenals, brain, heart,
kidneys, liver, lungs, ovaries, pituitary gland, prostate, spleen,
testes, thyroids gland, uterus). A complete histological examination
was performed on rats in the control and top-dose groups (adrenals,
alimentary tract, aorta, brain, eyes, femur, Harderian gland, heart,
kidneys, larynx and pharynx, liver, lungs, cervical and mesenteric
lymph nodes, mammary gland, ovaries, pancreas, pituitary gland,
prostate, salivary gland, sciatic nerve, seminal vesicles, skeletal
muscle, skin, spleen, sternum, testes, thymus, thyroid gland, tongue,
trachea, urinary bladder, uterus, vagina). The examination was
extended to the low and intermediate dosage groups where
treatment-related effects were suspected.
No clinical signs or treatment-related deaths of toxicological
significance were observed in treated animals during the study. Food
intake was statistically significantly reduced in females at the
highest dose group at week 1, and in treated male groups at weeks 1-4;
thereafter there were no significant differences in food intake
between controls and treated animals. Water intake, measured
accurately during week 12 of treatment, did not differ notably from
controls. Body-weight gain in males and females in the high-dose group
was significantly reduced compared to control throughout the study;
significant lower body-weight gain was also apparent in males of the
intermediate- and low-dose groups during the first 4 weeks of
treatment. The authors considered these differences from control not
to be treatment-related since the differences were not dosage-related
in magnitude, and because of intra-group variability noted in feeding
patterns of all groups of male rats. Impaired food efficiency was
noted for both male and female rats at the highest dose level.
There were no treatment-related differences from control in
haematological parameters at week 6 or at termination. Clinical
biochemical analyses showed statistically significant higher values in
the high-dose group compared to control for ALAT, ALP, cholesterol and
total plasma protein. Cholesterol levels were significantly increased
in males at the intermediate-dose group at week 6 only. The authors
considered the alteration of the clinical biochemical parameters
secondary to the hepatic changes seen histologically. Other sporadic
differences from control values were generally within normal ranges
for the strain and were not considered of toxicological significance.
At autopsy, enlarged cervical lymph nodes were noted in males at
the intermediate-dose group, and in both sexes at the highest dose
group. In addition, there was a reduction in adipose tissue in rats of
both sexes at the highest dose group. Absolute liver weights were
similar to controls but relative liver weights were increased in the
intermediate- and high-dose animals. Absolute and relative spleen
weights were increased in the intermediate- and high-dose groups.
Although relative spleen weights were increased in the low-dose males,
the absolute organ weights were unaffected, and in the absence of
histopathological changes this observation was considered by the
authors to be of no toxicological significance.
Histological examination revealed a dose-related increased
incidence of hepatic peribiliary inflammatory change in both males and
females of the intermediate- and high-dose groups, and minor bile duct
hyperplasia affecting 1/20 intermediate- and 4/20 high-dose males.
There were no changes observed in the liver parenchyma and no
degenerative or inflammatory changes of the bile duct epithelium.
Increased white pulp cellularity and prominence of germinal centres in
the spleen, and increased prominence of germinal centres and lymphoid
proliferation in cervical lymph nodes were seen in the intermediate-
and high-dose groups. The authors considered the findings of the
lymphoid tissue to be associated reactive changes to the hepatic
peribiliary inflammatory observations.
The authors concluded that no treatment-related changes were
observed at 500 mg/kg bw/day which was considered to be the NOEL in
this study (Hooks et al., 1992b).
2.2.2.2 Rabbits
Single rabbits were given ethyl vanillin orally in 10% aqueous
glycerine at doses of 15 mg/kg bw/day for 13 or 26 days; 32 mg/kg
bw/day for 15 days; 41 mg/kg bw/day for 26 days; or 49 mg/kg bw/day
for 43 days. At the highest close level, anaemia, diarrhoea and lack
of weight gain were observed but no toxic signs were reported at any
of the lower doses (Deichmann & Kitzmuller, 1940).
Subcutaneous injection of ethyl vanillin to rabbits at doses of
148-154 mg/kg bw/day for 6 days did not elicit any observed adverse
effects. Similarly, oral intubation of ethyl vanillin in a milk
vehicle at a dose of 240 mg/kg bw during 25 days (observation period
56 days), or during 54 days (observation period 126 days) did not
produce any observed effects (the parameters observed were not
specified in any of these studies) (Deichmann & Kitzmuller, 1940).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
The maximum tolerated dose for ethyl vanillin in strain A mice
when administered i.p. 3 times/week for 2 weeks was reported to be
75 mg/kg bw. Administration of ethyl vanillin i.p. at doses of 15 or
75 mg/kg bw, 3 times/week for 8 weeks resulted in mortalities of 8/20
and 10/20 animals, respectively. Control animals receiving i.p.
injections of the vehicle tricaprylin, had survival rates of 77/80
males and 77/80 females. In the control group, 28% of males and 23% of
females developed lung tumours whereas in the treated groups only one
animal, in the higher dose group, exhibited a single lung nodule. It
was concluded that ethyl vanillin did not potentiate the pulmonary
tumour response in strain A mice (Stoner et al., 1973).
2.2.3.2 Rats
Groups of Osborne-Mendel rats (12/sex/group) were fed diets
containing 0, 0.5, 1 or 2% ethyl vanillin for 2 years, and 2% or 5%
for 1 year. Haematological examinations (RBC, WBC, haemoglobin and
haematocrit) were performed at 3, 6, 12 and 22 months and at
termination in the 2-year study. All animals were necropsied and
liver, kidney, spleen, heart and testes weights recorded. Histological
examinations were performed on these organs and remaining thoracic and
abdominal viscera, bone and bone marrow, and muscle. No adverse
effects on growth, haematology, organ weights or histology of major
tissues were reported (Hagan et al., 1967).
2.2.4 Reproductive toxicity studies
No reproductive toxicity or teratogenicity studies have been
reported on ethyl vanillin.
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with ethyl vanillin are
summarized in Table 2.
From the SCE studies with human lymphocytes the authors concluded
that benzaldehyde derivatives, including ethyl vanillin, were probably
direct acting SCE inducers and the aldehyde moiety was of primary
importance (Jansson et al., 1988). This contrasts with the negative
effect in CHO cells (Sasaki et al., 1987).
In a study on the anti-mutagenic potential of flavourings, ethyl
vanillin was reported to show marked anti-mutagenic activity against
mutagenicity induced by 4-nitroquinoline 1-oxide, furylfuramide,
captan or methylglyoxal in Escherichia coli WP2s but was ineffective
against mutations induced by Trp-P-2 or IQ in Salmonella typhimurium
TA98. It was proposed that the anti-mutagenic activity was due to
enhancement of an error-free recombinant repair system (Ohta et al.,
1986; Watanabe et al., 1988).
2.3 Observations in humans
In a 24-hour closed patch test in 25 subjects, ethyl vanillin
tested at 2% in petrolatum produced a mild irritation. No
sensitization reactions occurred when ethyl vanillin was used at 2% in
petrolatum in a maximization test on 25 volunteers (Kligman, 1970).
People previously sensitized to balsam of Peru, benzoin, rosin,
benzoic acid, orange peel, cinnamon and cloves have been reported to
cross-react with hydroxybenzaldehydes such as vanillin or ethyl
vanillin. A patient with contact dermatitis showed strong reactions
to balsam of Peru, cassia oil and ethyl vanillin, it was not known
whether the dermatitis was a response to occupational exposure to
ethyl vanillin in a candy factory or to rubber (Rudzki & Grzwa, 1976).
Table 2. Results of genotoxicity assays on ethyl vanillin
Test system Test object Concentration of Results Reference
ethyl vanillin
Micronucleus Mouse 2 × 0-1000 Negative Wild et al., 1983
test mg/kg bw
Ames test1 Salmonella 0-10 mg/plate Negative Ishidate et al.,
typhimurium 1984
TA92, TA94,
TA98, TA100
TA1535,
TA1537
Ames test2 S. typhimurium 0-10 mg/plate Negative Mortelmans et al.,
TA98, TA100 1986
TA1535,
TA1537
Ames test1 S. typhimurium 0-3.6 mg/plate Negative Wild et al., 1983
TA98, TA100
TA1535,
TA1537
TA1538
Chromosomal Chinese hamster 0-0.25 mg/ml Negative3 Ishidate et al.,
aberrations ovary (CHO) 1984
cells in vitro
Sister chromatid Chinese 0-100 M Negative4 Sasaki et al., 1987
exchange (SCE) hamsterd ovary
cells in vitro
Sister chromatid Human 0-2 M Positive Jansson et al.,
exchange (SCE) lymphocytes in 1988
vitro
Table 2. Results of genotoxicity assays on ethyl vanillin (cont'd).
Test system Test object Concentration of Results Reference
ethyl vanillin
Heritable Drosophila 50 mM Negative Wild et al., 1983
mutations melanogaster
1 with or without metabolic activation using rat liver S9 fractions
2 with or without metabolic activation using rat or hamster liver S9 fractions
3 ethyl vanillin did not induce chromosomal aberrations but did cause an increase
in polyploid cells, however the significance of this was unclear and similar
polyploidy was induced by riboflavin
4 ethyl vanillin did not induce sister chromatid exchanges in cultured CHO cells
in vitro but was reported to enhance the ability of mitomycin C to cause sister
chromatid exchanges.
3. COMMENTS
The metabolism studies indicated that ethyl vanillin was rapidly
absorbed, metabolized and excreted in the rat. The principal
metabolite identified was ethyl vanillic acid (3-ethoxy-4-hydroxy-
benzoic acid). This compound, which is not a normal constituent of
human urine, has also been identified in the urine of humans known to
have ingested vanilla-flavoured foodstuffs.
In the recent 13-week toxicity study in which rats were fed ethyl
vanillin at 500, 1000 or 2000 mg/kg bw/day, treated males showed a
transient reduction in body-weight gain compared with controls during
the first 4 weeks of treatment. Since this effect was only transient
and associated with reduced food intake, probably due to impaired
palatability, the Committee concluded that the NOEL was 500 mg/kg
bw/day.
The Committee considered ethyl vanillin not to be genotoxic on
the basis of negative results in a large number of studies, although
one assay for sister chromatid exchange was positive.
4. EVALUATION
The Committee concluded that, in the light of the information
showing daily intakes to be in the range of 0.06-7 mg/person/day, the
safety evaluation could be based on the principles applicable to
materials occurring in foods in small amounts. In view of the limited
toxicological information available, the Committee withdrew the
previous temporary ADI and allocated an ADI of 0-3 mg/kg bw for ethyl
vanillin, based on a NOEL of 500 mg/kg bw/day in the 13-week toxicity
study in rats and a safety factor of 200.
5. REFERENCES
CAUJOLLE, F. & MEYNIER, D. (1954a). Toxicity of vanillin, o-vanillin
and ethyl vanillin and of the corresponding meta aldehydes.
Ann. Pharm. Fr., 12: 42-49.
CAUJOLLE, F. & MEYNIER, D. (1954b). The comparative toxicity of
orthovanillin and ethyl orthovanillin. Compt. Rend., 238: 2576-2578.
CAUJOLLE, F., MEYNIER, D. & MOSCARELLA, C. (1953). The comparative
toxicity of ethyl vanillin and 4-hydroxy-5-ethoxyisophthalic
dialdehyde. Compt. Rend., 237: 765-766.
DEICHMANN, W. & KITZMULLER, K.V. (1940). Toxicity of vanillin and
ethyl vanillin for rabbits and rats. J. Am. Pharm. Assoc.,
29: 425-428.
FDA, (1982). Toxicological Principles for the Safety Assessment of
Direct Food Additives and Colour Additives used in Food. Food and Drug
Administration, USA.
HAGAN, E.C, HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES, W.I.,
TAYLOR, J.M., LONG, EL., NELSON, A.A. & BROUWER, J.B. (1967). Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5: 141-157.
HAWKINS, D.R., MIDGLEY, I., PROCTOR, P., PARIKH, R., BENNETT, S.,
HUCKSTEP, M.R. & CHENG, K. (1992). Biokinetics and metabolism of ethyl
14C-vanillin following oral (gavage) administration to rats.
Unpublished Report No. HRC/EVT 5/921134 of Huntingdon Research Centre
Ltd. Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., GOPINATH, C., ANDERSON, A.,
DAWE, I.S., BRODIE, R. R. & CHASSEAUD, L F. (1992a). Ethyl vanillin
palatability/toxicity by dietary administration to rats for 2 weeks.
Unpublished report No. EVT 1/911052 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC). Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., CROOK, D., GIBSON, W.A., GREGSON
R.L., GOPINATH, C., ANDERSON, A. & DAWE, S.I. (1992b). Ethyl vanillin
toxicity to rats by repeated dietary administration for 13 weeks.
Unpublished report No EVT 2/920067 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
ISHIDATE, M., SOFUNI, T., YOSHIKAWA, K., HAYASHI, M., NOHMI, T.,
SAWADA, M. & MATSUOKA, A. (1984). Primary mutagenicity screening of
food additives currently used in Japan. Food Chem Toxicol.,
22: 623-636.
JANSSON, T., CURVALL, M., HEDIN, A. & ENZELL, C.R. (1988). In vitro
studies of the biological effects of cigarette smoke condensate III.
Induction of SCE by some phenolic and related constituents derived
from cigarette smoke. A study of structure-activity relationships.
Mutation Res., 206: 17-24.
JENNER, P.M., HAGEN, E.C., TAYLOR, J.M., COOK, E.L & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
KLIGMAN, A.M. (1970). Unpublished report to RIFM cited in
Opdyke, D.L.(1975). Monographs on fragrance raw materials.
Food Cosmet. Toxicol., 13:91-112.
MAMER, O.A., MONTGOMERY, J.A., DECKELBAUM, R.J. & GRANOT, E. (1985).
Identification of urinary 3-ethoxy-4-hydroxybenzoic and 3-ethoxy-4-
hydroxymandelic acids after dietary intake of ethyl vanillin.
Biomed. Mass. Spectrom. 12: 163-169.
MORTELMANS, K, HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986). Salmonella mutagenicity tests II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8, Suppl. 7: 1-119.
OHTA, T., WATANABE, M., WATANABE, K., SHIRASU, Y. & KADA, T. (1986).
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24: 51-54.
RTECS (1990). Registry of Toxic Effects of Chemical Substances
computer database.
RUDZKI, E. & GRYZWA, Z. (1976). Immediate reactions to balsam of Peru,
cassia oil and ethyl vanillin. Contact Dermatitis, 2: 360-361.
SASAKI, Y.F., IMANISHI, H., OHIA, T. & SHIRASU, Y. (1987). Effects of
antimutagenic flavourings on SCEs induced by chemical mutagens in
cultured Chinese hamster cells, Mutat. Res., 189: 313-318.
SPORN, A. (1960). Toxicity of aromatic ethyl vanillin. Igiena
(Bucharest), 9: 365.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G B. (1973). Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumour response
in strain A mice. Cancer Res., 33: 3069-3085.
WATANABE, K., OHTA, T. & SHIASU, Y. (1988). Antimutagenic effects of
benzaldehyde and its derivatives on mutagenesis induced by
4-nitroquinoline 1-oxide in Escherichia coli. Agric. Biol. Chem.,
52: 1041-1046.
WILD, D., KING, M-T., GOCKE, E. & ECKHARDT, K. (1983). Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, BASC and micronucleus tests. Food Chem.
Toxic., 21: 707-719.
WILLIAMS, R.T. (1959). Detoxication Mechanisms, 2nd Edition, Chapman &
Hall: London.
Ethyl vanillic acid was identified by GC/MS in the urine
of a 9-year old female patient who had received liquid dietary
supplementation flavoured with vanilla. Other patients excreting this
acid were also known to have consumed foodstuffs flavoured with ethyl
vanillin. Eight different urine samples containing more than 50 mg
ethyl vanillic acid/g creatinine were also found to contain small
amounts of vanillylmandelic acid. Unchanged ethyl vanillin was not
detected in any of the urine samples.
A healthy adult male volunteer drank a 235 ml aliquot of a liquid
dietary supplement containing an unknown quantity of ethyl vanillin. A
concentration of 13 mg ethyl vanillic acid/g creatinine was found in a
12-hour urine sample. The compound was not present in urine collected
before exposure (Mamer et al., 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ethyl vanillin are
summarized in Table 1.
The lowest lethal dermal dose in rats was reported to be
1800 mg/kg bw (RTECS, 1990).
When groups of 6 rabbits were given ethyl vanillin by gavage, a
dose of 150 mg/kg bw caused no adverse effects. At 2500 mg/kg bw, only
a transient increase in respiration rate was observed. The minimum
oral lethal dose was reported to be 3000 mg/kg bw (Deichmann &
Kitzmuller, 1940).
Table 1. Acute toxicity studies with ethyl vanillin
Species Route LD50 Reference
mg/kg bw
Mouse i.p. 7501 Caujolle & Meynier, 1954a
Rat oral 1590 Sporn, 1960
oral > 2000 Jenner et al., 1964
s.c. 1800 Deichmann & Kitzmuller, 1940
Guinea-pig i.p. 1140 Caujolle & Meynier, 1954b
Dog i.v. 760 Caujolle et al., 1953
1 Maximum non-lethal dose, 450 mg/kg bw; Lethal dose: 950 mg/kg bw
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 300 mg ethyl vanillin/kg bw were administered to rats by
gavage twice weekly for 14 weeks without any adverse affects. In
another experiment, groups of 16 rats were fed ethyl vanillin at a
dose of 20 mg/kg bw/day for 18 weeks without adverse effect. However,
64 mg/kg bw/day for 10 weeks reduced growth rate and caused
myocardial, renal, hepatic, lung, spleen and stomach injuries (nature
not specified) (Deichmann & Kitzmuller, 1940).
Sixteen rats were given 30 mg ethyl vanillin weekly for 7 weeks
without adverse effect on growth, food intake or protein utilization
(Spore, 1960).
Groups of 5 male rats were fed 0, 2%, or 5% ethyl vanillin in the
diet for 1 year without any adverse effects (Hagan et al., 1967).
Groups of CD Sprague-Dawley BR rats (20/sex/group) were fed ethyl
vanillin of > 99.9% purity (nature of diet e.g., semi-synthetic/chow
diet, not specified) at dose levels of 0, 500, 1000 or 2000 mg/kg
bw/day for 13 weeks. The study was designed in accordance with
toxicological principles for the safety assessment of food additives
established by the US FDA (FDA, 1982). The diet was prepared weekly
and showed stability for up to 18 days at room temperature. The
achieved mean dose over the 13-week period was within 1.5% of the
nominal value. Food consumption and body weight were recorded weekly.
Ophthalmoscopy was done before treatment and at termination of the
study. Detailed haematological and clinical chemical examinations were
carried out at week 6 and 13. At termination, all animals were
necropsied and organ weights recorded (adrenals, brain, heart,
kidneys, liver, lungs, ovaries, pituitary gland, prostate, spleen,
testes, thyroids gland, uterus). A complete histological examination
was performed on rats in the control and top-dose groups (adrenals,
alimentary tract, aorta, brain, eyes, femur, Harderian gland, heart,
kidneys, larynx and pharynx, liver, lungs, cervical and mesenteric
lymph nodes, mammary gland, ovaries, pancreas, pituitary gland,
prostate, salivary gland, sciatic nerve, seminal vesicles, skeletal
muscle, skin, spleen, sternum, testes, thymus, thyroid gland, tongue,
trachea, urinary bladder, uterus, vagina). The examination was
extended to the low and intermediate dosage groups where
treatment-related effects were suspected.
No clinical signs or treatment-related deaths of toxicological
significance were observed in treated animals during the study. Food
intake was statistically significantly reduced in females at the
highest dose group at week 1, and in treated male groups at weeks 1-4;
thereafter there were no significant differences in food intake
between controls and treated animals. Water intake, measured
accurately during week 12 of treatment, did not differ notably from
controls. Body-weight gain in males and females in the high-dose group
was significantly reduced compared to control throughout the study;
significant lower body-weight gain was also apparent in males of the
intermediate- and low-dose groups during the first 4 weeks of
treatment. The authors considered these differences from control not
to be treatment-related since the differences were not dosage-related
in magnitude, and because of intra-group variability noted in feeding
patterns of all groups of male rats. Impaired food efficiency was
noted for both male and female rats at the highest dose level.
There were no treatment-related differences from control in
haematological parameters at week 6 or at termination. Clinical
biochemical analyses showed statistically significant higher values in
the high-dose group compared to control for ALAT, ALP, cholesterol and
total plasma protein. Cholesterol levels were significantly increased
in males at the intermediate-dose group at week 6 only. The authors
considered the alteration of the clinical biochemical parameters
secondary to the hepatic changes seen histologically. Other sporadic
differences from control values were generally within normal ranges
for the strain and were not considered of toxicological significance.
At autopsy, enlarged cervical lymph nodes were noted in males at
the intermediate-dose group, and in both sexes at the highest dose
group. In addition, there was a reduction in adipose tissue in rats of
both sexes at the highest dose group. Absolute liver weights were
similar to controls but relative liver weights were increased in the
intermediate- and high-dose animals. Absolute and relative spleen
weights were increased in the intermediate- and high-dose groups.
Although relative spleen weights were increased in the low-dose males,
the absolute organ weights were unaffected, and in the absence of
histopathological changes this observation was considered by the
authors to be of no toxicological significance.
Histological examination revealed a dose-related increased
incidence of hepatic peribiliary inflammatory change in both males and
females of the intermediate- and high-dose groups, and minor bile duct
hyperplasia affecting 1/20 intermediate- and 4/20 high-dose males.
There were no changes observed in the liver parenchyma and no
degenerative or inflammatory changes of the bile duct epithelium.
Increased white pulp cellularity and prominence of germinal centres in
the spleen, and increased prominence of germinal centres and lymphoid
proliferation in cervical lymph nodes were seen in the intermediate-
and high-dose groups. The authors considered the findings of the
lymphoid tissue to be associated reactive changes to the hepatic
peribiliary inflammatory observations.
The authors concluded that no treatment-related changes were
observed at 500 mg/kg bw/day which was considered to be the NOEL in
this study (Hooks et al., 1992b).
2.2.2.2 Rabbits
Single rabbits were given ethyl vanillin orally in 10% aqueous
glycerine at doses of 15 mg/kg bw/day for 13 or 26 days; 32 mg/kg
bw/day for 15 days; 41 mg/kg bw/day for 26 days; or 49 mg/kg bw/day
for 43 days. At the highest close level, anaemia, diarrhoea and lack
of weight gain were observed but no toxic signs were reported at any
of the lower doses (Deichmann & Kitzmuller, 1940).
Subcutaneous injection of ethyl vanillin to rabbits at doses of
148-154 mg/kg bw/day for 6 days did not elicit any observed adverse
effects. Similarly, oral intubation of ethyl vanillin in a milk
vehicle at a dose of 240 mg/kg bw during 25 days (observation period
56 days), or during 54 days (observation period 126 days) did not
produce any observed effects (the parameters observed were not
specified in any of these studies) (Deichmann & Kitzmuller, 1940).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
The maximum tolerated dose for ethyl vanillin in strain A mice
when administered i.p. 3 times/week for 2 weeks was reported to be
75 mg/kg bw. Administration of ethyl vanillin i.p. at doses of 15 or
75 mg/kg bw, 3 times/week for 8 weeks resulted in mortalities of 8/20
and 10/20 animals, respectively. Control animals receiving i.p.
injections of the vehicle tricaprylin, had survival rates of 77/80
males and 77/80 females. In the control group, 28% of males and 23% of
females developed lung tumours whereas in the treated groups only one
animal, in the higher dose group, exhibited a single lung nodule. It
was concluded that ethyl vanillin did not potentiate the pulmonary
tumour response in strain A mice (Stoner et al., 1973).
2.2.3.2 Rats
Groups of Osborne-Mendel rats (12/sex/group) were fed diets
containing 0, 0.5, 1 or 2% ethyl vanillin for 2 years, and 2% or 5%
for 1 year. Haematological examinations (RBC, WBC, haemoglobin and
haematocrit) were performed at 3, 6, 12 and 22 months and at
termination in the 2-year study. All animals were necropsied and
liver, kidney, spleen, heart and testes weights recorded. Histological
examinations were performed on these organs and remaining thoracic and
abdominal viscera, bone and bone marrow, and muscle. No adverse
effects on growth, haematology, organ weights or histology of major
tissues were reported (Hagan et al., 1967).
2.2.4 Reproductive toxicity studies
No reproductive toxicity or teratogenicity studies have been
reported on ethyl vanillin.
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with ethyl vanillin are
summarized in Table 2.
From the SCE studies with human lymphocytes the authors concluded
that benzaldehyde derivatives, including ethyl vanillin, were probably
direct acting SCE inducers and the aldehyde moiety was of primary
importance (Jansson et al., 1988). This contrasts with the negative
effect in CHO cells (Sasaki et al., 1987).
In a study on the anti-mutagenic potential of flavourings, ethyl
vanillin was reported to show marked anti-mutagenic activity against
mutagenicity induced by 4-nitroquinoline 1-oxide, furylfuramide,
captan or methylglyoxal in Escherichia coli WP2s but was ineffective
against mutations induced by Trp-P-2 or IQ in Salmonella typhimurium
TA98. It was proposed that the anti-mutagenic activity was due to
enhancement of an error-free recombinant repair system (Ohta et al.,
1986; Watanabe et al., 1988).
2.3 Observations in humans
In a 24-hour closed patch test in 25 subjects, ethyl vanillin
tested at 2% in petrolatum produced a mild irritation. No
sensitization reactions occurred when ethyl vanillin was used at 2% in
petrolatum in a maximization test on 25 volunteers (Kligman, 1970).
People previously sensitized to balsam of Peru, benzoin, rosin,
benzoic acid, orange peel, cinnamon and cloves have been reported to
cross-react with hydroxybenzaldehydes such as vanillin or ethyl
vanillin. A patient with contact dermatitis showed strong reactions
to balsam of Peru, cassia oil and ethyl vanillin, it was not known
whether the dermatitis was a response to occupational exposure to
ethyl vanillin in a candy factory or to rubber (Rudzki & Grzwa, 1976).
Table 2. Results of genotoxicity assays on ethyl vanillin
Test system Test object Concentration of Results Reference
ethyl vanillin
Micronucleus Mouse 2 × 0-1000 Negative Wild et al., 1983
test mg/kg bw
Ames test1 Salmonella 0-10 mg/plate Negative Ishidate et al.,
typhimurium 1984
TA92, TA94,
TA98, TA100
TA1535,
TA1537
Ames test2 S. typhimurium 0-10 mg/plate Negative Mortelmans et al.,
TA98, TA100 1986
TA1535,
TA1537
Ames test1 S. typhimurium 0-3.6 mg/plate Negative Wild et al., 1983
TA98, TA100
TA1535,
TA1537
TA1538
Chromosomal Chinese hamster 0-0.25 mg/ml Negative3 Ishidate et al.,
aberrations ovary (CHO) 1984
cells in vitro
Sister chromatid Chinese 0-100 M Negative4 Sasaki et al., 1987
exchange (SCE) hamsterd ovary
cells in vitro
Sister chromatid Human 0-2 M Positive Jansson et al.,
exchange (SCE) lymphocytes in 1988
vitro
Table 2. Results of genotoxicity assays on ethyl vanillin (cont'd).
Test system Test object Concentration of Results Reference
ethyl vanillin
Heritable Drosophila 50 mM Negative Wild et al., 1983
mutations melanogaster
1 with or without metabolic activation using rat liver S9 fractions
2 with or without metabolic activation using rat or hamster liver S9 fractions
3 ethyl vanillin did not induce chromosomal aberrations but did cause an increase
in polyploid cells, however the significance of this was unclear and similar
polyploidy was induced by riboflavin
4 ethyl vanillin did not induce sister chromatid exchanges in cultured CHO cells
in vitro but was reported to enhance the ability of mitomycin C to cause sister
chromatid exchanges.
3. COMMENTS
The metabolism studies indicated that ethyl vanillin was rapidly
absorbed, metabolized and excreted in the rat. The principal
metabolite identified was ethyl vanillic acid (3-ethoxy-4-hydroxy-
benzoic acid). This compound, which is not a normal constituent of
human urine, has also been identified in the urine of humans known to
have ingested vanilla-flavoured foodstuffs.
In the recent 13-week toxicity study in which rats were fed ethyl
vanillin at 500, 1000 or 2000 mg/kg bw/day, treated males showed a
transient reduction in body-weight gain compared with controls during
the first 4 weeks of treatment. Since this effect was only transient
and associated with reduced food intake, probably due to impaired
palatability, the Committee concluded that the NOEL was 500 mg/kg
bw/day.
The Committee considered ethyl vanillin not to be genotoxic on
the basis of negative results in a large number of studies, although
one assay for sister chromatid exchange was positive.
4. EVALUATION
The Committee concluded that, in the light of the information
showing daily intakes to be in the range of 0.06-7 mg/person/day, the
safety evaluation could be based on the principles applicable to
materials occurring in foods in small amounts. In view of the limited
toxicological information available, the Committee withdrew the
previous temporary ADI and allocated an ADI of 0-3 mg/kg bw for ethyl
vanillin, based on a NOEL of 500 mg/kg bw/day in the 13-week toxicity
study in rats and a safety factor of 200.
5. REFERENCES
CAUJOLLE, F. & MEYNIER, D. (1954a). Toxicity of vanillin, o-vanillin
and ethyl vanillin and of the corresponding meta aldehydes.
Ann. Pharm. Fr., 12: 42-49.
CAUJOLLE, F. & MEYNIER, D. (1954b). The comparative toxicity of
orthovanillin and ethyl orthovanillin. Compt. Rend., 238: 2576-2578.
CAUJOLLE, F., MEYNIER, D. & MOSCARELLA, C. (1953). The comparative
toxicity of ethyl vanillin and 4-hydroxy-5-ethoxyisophthalic
dialdehyde. Compt. Rend., 237: 765-766.
DEICHMANN, W. & KITZMULLER, K.V. (1940). Toxicity of vanillin and
ethyl vanillin for rabbits and rats. J. Am. Pharm. Assoc.,
29: 425-428.
FDA, (1982). Toxicological Principles for the Safety Assessment of
Direct Food Additives and Colour Additives used in Food. Food and Drug
Administration, USA.
HAGAN, E.C, HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES, W.I.,
TAYLOR, J.M., LONG, EL., NELSON, A.A. & BROUWER, J.B. (1967). Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5: 141-157.
HAWKINS, D.R., MIDGLEY, I., PROCTOR, P., PARIKH, R., BENNETT, S.,
HUCKSTEP, M.R. & CHENG, K. (1992). Biokinetics and metabolism of ethyl
14C-vanillin following oral (gavage) administration to rats.
Unpublished Report No. HRC/EVT 5/921134 of Huntingdon Research Centre
Ltd. Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., GOPINATH, C., ANDERSON, A.,
DAWE, I.S., BRODIE, R. R. & CHASSEAUD, L F. (1992a). Ethyl vanillin
palatability/toxicity by dietary administration to rats for 2 weeks.
Unpublished report No. EVT 1/911052 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC). Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., CROOK, D., GIBSON, W.A., GREGSON
R.L., GOPINATH, C., ANDERSON, A. & DAWE, S.I. (1992b). Ethyl vanillin
toxicity to rats by repeated dietary administration for 13 weeks.
Unpublished report No EVT 2/920067 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
ISHIDATE, M., SOFUNI, T., YOSHIKAWA, K., HAYASHI, M., NOHMI, T.,
SAWADA, M. & MATSUOKA, A. (1984). Primary mutagenicity screening of
food additives currently used in Japan. Food Chem Toxicol.,
22: 623-636.
JANSSON, T., CURVALL, M., HEDIN, A. & ENZELL, C.R. (1988). In vitro
studies of the biological effects of cigarette smoke condensate III.
Induction of SCE by some phenolic and related constituents derived
from cigarette smoke. A study of structure-activity relationships.
Mutation Res., 206: 17-24.
JENNER, P.M., HAGEN, E.C., TAYLOR, J.M., COOK, E.L & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
KLIGMAN, A.M. (1970). Unpublished report to RIFM cited in
Opdyke, D.L.(1975). Monographs on fragrance raw materials.
Food Cosmet. Toxicol., 13:91-112.
MAMER, O.A., MONTGOMERY, J.A., DECKELBAUM, R.J. & GRANOT, E. (1985).
Identification of urinary 3-ethoxy-4-hydroxybenzoic and 3-ethoxy-4-
hydroxymandelic acids after dietary intake of ethyl vanillin.
Biomed. Mass. Spectrom. 12: 163-169.
MORTELMANS, K, HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986). Salmonella mutagenicity tests II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8, Suppl. 7: 1-119.
OHTA, T., WATANABE, M., WATANABE, K., SHIRASU, Y. & KADA, T. (1986).
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24: 51-54.
RTECS (1990). Registry of Toxic Effects of Chemical Substances
computer database.
RUDZKI, E. & GRYZWA, Z. (1976). Immediate reactions to balsam of Peru,
cassia oil and ethyl vanillin. Contact Dermatitis, 2: 360-361.
SASAKI, Y.F., IMANISHI, H., OHIA, T. & SHIRASU, Y. (1987). Effects of
antimutagenic flavourings on SCEs induced by chemical mutagens in
cultured Chinese hamster cells, Mutat. Res., 189: 313-318.
SPORN, A. (1960). Toxicity of aromatic ethyl vanillin. Igiena
(Bucharest), 9: 365.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G B. (1973). Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumour response
in strain A mice. Cancer Res., 33: 3069-3085.
WATANABE, K., OHTA, T. & SHIASU, Y. (1988). Antimutagenic effects of
benzaldehyde and its derivatives on mutagenesis induced by
4-nitroquinoline 1-oxide in Escherichia coli. Agric. Biol. Chem.,
52: 1041-1046.
WILD, D., KING, M-T., GOCKE, E. & ECKHARDT, K. (1983). Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, BASC and micronucleus tests. Food Chem.
Toxic., 21: 707-719.
WILLIAMS, R.T. (1959). Detoxication Mechanisms, 2nd Edition, Chapman &
Hall: London.
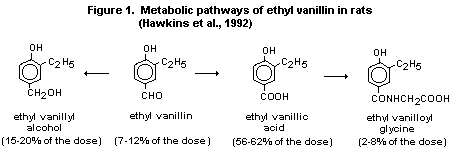 Ethyl vanillic acid was identified by GC/MS in the urine
of a 9-year old female patient who had received liquid dietary
supplementation flavoured with vanilla. Other patients excreting this
acid were also known to have consumed foodstuffs flavoured with ethyl
vanillin. Eight different urine samples containing more than 50 mg
ethyl vanillic acid/g creatinine were also found to contain small
amounts of vanillylmandelic acid. Unchanged ethyl vanillin was not
detected in any of the urine samples.
A healthy adult male volunteer drank a 235 ml aliquot of a liquid
dietary supplement containing an unknown quantity of ethyl vanillin. A
concentration of 13 mg ethyl vanillic acid/g creatinine was found in a
12-hour urine sample. The compound was not present in urine collected
before exposure (Mamer et al., 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ethyl vanillin are
summarized in Table 1.
The lowest lethal dermal dose in rats was reported to be
1800 mg/kg bw (RTECS, 1990).
When groups of 6 rabbits were given ethyl vanillin by gavage, a
dose of 150 mg/kg bw caused no adverse effects. At 2500 mg/kg bw, only
a transient increase in respiration rate was observed. The minimum
oral lethal dose was reported to be 3000 mg/kg bw (Deichmann &
Kitzmuller, 1940).
Table 1. Acute toxicity studies with ethyl vanillin
Species Route LD50 Reference
mg/kg bw
Mouse i.p. 7501 Caujolle & Meynier, 1954a
Rat oral 1590 Sporn, 1960
oral > 2000 Jenner et al., 1964
s.c. 1800 Deichmann & Kitzmuller, 1940
Guinea-pig i.p. 1140 Caujolle & Meynier, 1954b
Dog i.v. 760 Caujolle et al., 1953
1 Maximum non-lethal dose, 450 mg/kg bw; Lethal dose: 950 mg/kg bw
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 300 mg ethyl vanillin/kg bw were administered to rats by
gavage twice weekly for 14 weeks without any adverse affects. In
another experiment, groups of 16 rats were fed ethyl vanillin at a
dose of 20 mg/kg bw/day for 18 weeks without adverse effect. However,
64 mg/kg bw/day for 10 weeks reduced growth rate and caused
myocardial, renal, hepatic, lung, spleen and stomach injuries (nature
not specified) (Deichmann & Kitzmuller, 1940).
Sixteen rats were given 30 mg ethyl vanillin weekly for 7 weeks
without adverse effect on growth, food intake or protein utilization
(Spore, 1960).
Groups of 5 male rats were fed 0, 2%, or 5% ethyl vanillin in the
diet for 1 year without any adverse effects (Hagan et al., 1967).
Groups of CD Sprague-Dawley BR rats (20/sex/group) were fed ethyl
vanillin of > 99.9% purity (nature of diet e.g., semi-synthetic/chow
diet, not specified) at dose levels of 0, 500, 1000 or 2000 mg/kg
bw/day for 13 weeks. The study was designed in accordance with
toxicological principles for the safety assessment of food additives
established by the US FDA (FDA, 1982). The diet was prepared weekly
and showed stability for up to 18 days at room temperature. The
achieved mean dose over the 13-week period was within 1.5% of the
nominal value. Food consumption and body weight were recorded weekly.
Ophthalmoscopy was done before treatment and at termination of the
study. Detailed haematological and clinical chemical examinations were
carried out at week 6 and 13. At termination, all animals were
necropsied and organ weights recorded (adrenals, brain, heart,
kidneys, liver, lungs, ovaries, pituitary gland, prostate, spleen,
testes, thyroids gland, uterus). A complete histological examination
was performed on rats in the control and top-dose groups (adrenals,
alimentary tract, aorta, brain, eyes, femur, Harderian gland, heart,
kidneys, larynx and pharynx, liver, lungs, cervical and mesenteric
lymph nodes, mammary gland, ovaries, pancreas, pituitary gland,
prostate, salivary gland, sciatic nerve, seminal vesicles, skeletal
muscle, skin, spleen, sternum, testes, thymus, thyroid gland, tongue,
trachea, urinary bladder, uterus, vagina). The examination was
extended to the low and intermediate dosage groups where
treatment-related effects were suspected.
No clinical signs or treatment-related deaths of toxicological
significance were observed in treated animals during the study. Food
intake was statistically significantly reduced in females at the
highest dose group at week 1, and in treated male groups at weeks 1-4;
thereafter there were no significant differences in food intake
between controls and treated animals. Water intake, measured
accurately during week 12 of treatment, did not differ notably from
controls. Body-weight gain in males and females in the high-dose group
was significantly reduced compared to control throughout the study;
significant lower body-weight gain was also apparent in males of the
intermediate- and low-dose groups during the first 4 weeks of
treatment. The authors considered these differences from control not
to be treatment-related since the differences were not dosage-related
in magnitude, and because of intra-group variability noted in feeding
patterns of all groups of male rats. Impaired food efficiency was
noted for both male and female rats at the highest dose level.
There were no treatment-related differences from control in
haematological parameters at week 6 or at termination. Clinical
biochemical analyses showed statistically significant higher values in
the high-dose group compared to control for ALAT, ALP, cholesterol and
total plasma protein. Cholesterol levels were significantly increased
in males at the intermediate-dose group at week 6 only. The authors
considered the alteration of the clinical biochemical parameters
secondary to the hepatic changes seen histologically. Other sporadic
differences from control values were generally within normal ranges
for the strain and were not considered of toxicological significance.
At autopsy, enlarged cervical lymph nodes were noted in males at
the intermediate-dose group, and in both sexes at the highest dose
group. In addition, there was a reduction in adipose tissue in rats of
both sexes at the highest dose group. Absolute liver weights were
similar to controls but relative liver weights were increased in the
intermediate- and high-dose animals. Absolute and relative spleen
weights were increased in the intermediate- and high-dose groups.
Although relative spleen weights were increased in the low-dose males,
the absolute organ weights were unaffected, and in the absence of
histopathological changes this observation was considered by the
authors to be of no toxicological significance.
Histological examination revealed a dose-related increased
incidence of hepatic peribiliary inflammatory change in both males and
females of the intermediate- and high-dose groups, and minor bile duct
hyperplasia affecting 1/20 intermediate- and 4/20 high-dose males.
There were no changes observed in the liver parenchyma and no
degenerative or inflammatory changes of the bile duct epithelium.
Increased white pulp cellularity and prominence of germinal centres in
the spleen, and increased prominence of germinal centres and lymphoid
proliferation in cervical lymph nodes were seen in the intermediate-
and high-dose groups. The authors considered the findings of the
lymphoid tissue to be associated reactive changes to the hepatic
peribiliary inflammatory observations.
The authors concluded that no treatment-related changes were
observed at 500 mg/kg bw/day which was considered to be the NOEL in
this study (Hooks et al., 1992b).
2.2.2.2 Rabbits
Single rabbits were given ethyl vanillin orally in 10% aqueous
glycerine at doses of 15 mg/kg bw/day for 13 or 26 days; 32 mg/kg
bw/day for 15 days; 41 mg/kg bw/day for 26 days; or 49 mg/kg bw/day
for 43 days. At the highest close level, anaemia, diarrhoea and lack
of weight gain were observed but no toxic signs were reported at any
of the lower doses (Deichmann & Kitzmuller, 1940).
Subcutaneous injection of ethyl vanillin to rabbits at doses of
148-154 mg/kg bw/day for 6 days did not elicit any observed adverse
effects. Similarly, oral intubation of ethyl vanillin in a milk
vehicle at a dose of 240 mg/kg bw during 25 days (observation period
56 days), or during 54 days (observation period 126 days) did not
produce any observed effects (the parameters observed were not
specified in any of these studies) (Deichmann & Kitzmuller, 1940).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
The maximum tolerated dose for ethyl vanillin in strain A mice
when administered i.p. 3 times/week for 2 weeks was reported to be
75 mg/kg bw. Administration of ethyl vanillin i.p. at doses of 15 or
75 mg/kg bw, 3 times/week for 8 weeks resulted in mortalities of 8/20
and 10/20 animals, respectively. Control animals receiving i.p.
injections of the vehicle tricaprylin, had survival rates of 77/80
males and 77/80 females. In the control group, 28% of males and 23% of
females developed lung tumours whereas in the treated groups only one
animal, in the higher dose group, exhibited a single lung nodule. It
was concluded that ethyl vanillin did not potentiate the pulmonary
tumour response in strain A mice (Stoner et al., 1973).
2.2.3.2 Rats
Groups of Osborne-Mendel rats (12/sex/group) were fed diets
containing 0, 0.5, 1 or 2% ethyl vanillin for 2 years, and 2% or 5%
for 1 year. Haematological examinations (RBC, WBC, haemoglobin and
haematocrit) were performed at 3, 6, 12 and 22 months and at
termination in the 2-year study. All animals were necropsied and
liver, kidney, spleen, heart and testes weights recorded. Histological
examinations were performed on these organs and remaining thoracic and
abdominal viscera, bone and bone marrow, and muscle. No adverse
effects on growth, haematology, organ weights or histology of major
tissues were reported (Hagan et al., 1967).
2.2.4 Reproductive toxicity studies
No reproductive toxicity or teratogenicity studies have been
reported on ethyl vanillin.
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with ethyl vanillin are
summarized in Table 2.
From the SCE studies with human lymphocytes the authors concluded
that benzaldehyde derivatives, including ethyl vanillin, were probably
direct acting SCE inducers and the aldehyde moiety was of primary
importance (Jansson et al., 1988). This contrasts with the negative
effect in CHO cells (Sasaki et al., 1987).
In a study on the anti-mutagenic potential of flavourings, ethyl
vanillin was reported to show marked anti-mutagenic activity against
mutagenicity induced by 4-nitroquinoline 1-oxide, furylfuramide,
captan or methylglyoxal in Escherichia coli WP2s but was ineffective
against mutations induced by Trp-P-2 or IQ in Salmonella typhimurium
TA98. It was proposed that the anti-mutagenic activity was due to
enhancement of an error-free recombinant repair system (Ohta et al.,
1986; Watanabe et al., 1988).
2.3 Observations in humans
In a 24-hour closed patch test in 25 subjects, ethyl vanillin
tested at 2% in petrolatum produced a mild irritation. No
sensitization reactions occurred when ethyl vanillin was used at 2% in
petrolatum in a maximization test on 25 volunteers (Kligman, 1970).
People previously sensitized to balsam of Peru, benzoin, rosin,
benzoic acid, orange peel, cinnamon and cloves have been reported to
cross-react with hydroxybenzaldehydes such as vanillin or ethyl
vanillin. A patient with contact dermatitis showed strong reactions
to balsam of Peru, cassia oil and ethyl vanillin, it was not known
whether the dermatitis was a response to occupational exposure to
ethyl vanillin in a candy factory or to rubber (Rudzki & Grzwa, 1976).
Table 2. Results of genotoxicity assays on ethyl vanillin
Test system Test object Concentration of Results Reference
ethyl vanillin
Micronucleus Mouse 2 × 0-1000 Negative Wild et al., 1983
test mg/kg bw
Ames test1 Salmonella 0-10 mg/plate Negative Ishidate et al.,
typhimurium 1984
TA92, TA94,
TA98, TA100
TA1535,
TA1537
Ames test2 S. typhimurium 0-10 mg/plate Negative Mortelmans et al.,
TA98, TA100 1986
TA1535,
TA1537
Ames test1 S. typhimurium 0-3.6 mg/plate Negative Wild et al., 1983
TA98, TA100
TA1535,
TA1537
TA1538
Chromosomal Chinese hamster 0-0.25 mg/ml Negative3 Ishidate et al.,
aberrations ovary (CHO) 1984
cells in vitro
Sister chromatid Chinese 0-100 M Negative4 Sasaki et al., 1987
exchange (SCE) hamsterd ovary
cells in vitro
Sister chromatid Human 0-2 M Positive Jansson et al.,
exchange (SCE) lymphocytes in 1988
vitro
Table 2. Results of genotoxicity assays on ethyl vanillin (cont'd).
Test system Test object Concentration of Results Reference
ethyl vanillin
Heritable Drosophila 50 mM Negative Wild et al., 1983
mutations melanogaster
1 with or without metabolic activation using rat liver S9 fractions
2 with or without metabolic activation using rat or hamster liver S9 fractions
3 ethyl vanillin did not induce chromosomal aberrations but did cause an increase
in polyploid cells, however the significance of this was unclear and similar
polyploidy was induced by riboflavin
4 ethyl vanillin did not induce sister chromatid exchanges in cultured CHO cells
in vitro but was reported to enhance the ability of mitomycin C to cause sister
chromatid exchanges.
3. COMMENTS
The metabolism studies indicated that ethyl vanillin was rapidly
absorbed, metabolized and excreted in the rat. The principal
metabolite identified was ethyl vanillic acid (3-ethoxy-4-hydroxy-
benzoic acid). This compound, which is not a normal constituent of
human urine, has also been identified in the urine of humans known to
have ingested vanilla-flavoured foodstuffs.
In the recent 13-week toxicity study in which rats were fed ethyl
vanillin at 500, 1000 or 2000 mg/kg bw/day, treated males showed a
transient reduction in body-weight gain compared with controls during
the first 4 weeks of treatment. Since this effect was only transient
and associated with reduced food intake, probably due to impaired
palatability, the Committee concluded that the NOEL was 500 mg/kg
bw/day.
The Committee considered ethyl vanillin not to be genotoxic on
the basis of negative results in a large number of studies, although
one assay for sister chromatid exchange was positive.
4. EVALUATION
The Committee concluded that, in the light of the information
showing daily intakes to be in the range of 0.06-7 mg/person/day, the
safety evaluation could be based on the principles applicable to
materials occurring in foods in small amounts. In view of the limited
toxicological information available, the Committee withdrew the
previous temporary ADI and allocated an ADI of 0-3 mg/kg bw for ethyl
vanillin, based on a NOEL of 500 mg/kg bw/day in the 13-week toxicity
study in rats and a safety factor of 200.
5. REFERENCES
CAUJOLLE, F. & MEYNIER, D. (1954a). Toxicity of vanillin, o-vanillin
and ethyl vanillin and of the corresponding meta aldehydes.
Ann. Pharm. Fr., 12: 42-49.
CAUJOLLE, F. & MEYNIER, D. (1954b). The comparative toxicity of
orthovanillin and ethyl orthovanillin. Compt. Rend., 238: 2576-2578.
CAUJOLLE, F., MEYNIER, D. & MOSCARELLA, C. (1953). The comparative
toxicity of ethyl vanillin and 4-hydroxy-5-ethoxyisophthalic
dialdehyde. Compt. Rend., 237: 765-766.
DEICHMANN, W. & KITZMULLER, K.V. (1940). Toxicity of vanillin and
ethyl vanillin for rabbits and rats. J. Am. Pharm. Assoc.,
29: 425-428.
FDA, (1982). Toxicological Principles for the Safety Assessment of
Direct Food Additives and Colour Additives used in Food. Food and Drug
Administration, USA.
HAGAN, E.C, HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES, W.I.,
TAYLOR, J.M., LONG, EL., NELSON, A.A. & BROUWER, J.B. (1967). Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5: 141-157.
HAWKINS, D.R., MIDGLEY, I., PROCTOR, P., PARIKH, R., BENNETT, S.,
HUCKSTEP, M.R. & CHENG, K. (1992). Biokinetics and metabolism of ethyl
14C-vanillin following oral (gavage) administration to rats.
Unpublished Report No. HRC/EVT 5/921134 of Huntingdon Research Centre
Ltd. Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., GOPINATH, C., ANDERSON, A.,
DAWE, I.S., BRODIE, R. R. & CHASSEAUD, L F. (1992a). Ethyl vanillin
palatability/toxicity by dietary administration to rats for 2 weeks.
Unpublished report No. EVT 1/911052 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC). Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., CROOK, D., GIBSON, W.A., GREGSON
R.L., GOPINATH, C., ANDERSON, A. & DAWE, S.I. (1992b). Ethyl vanillin
toxicity to rats by repeated dietary administration for 13 weeks.
Unpublished report No EVT 2/920067 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
ISHIDATE, M., SOFUNI, T., YOSHIKAWA, K., HAYASHI, M., NOHMI, T.,
SAWADA, M. & MATSUOKA, A. (1984). Primary mutagenicity screening of
food additives currently used in Japan. Food Chem Toxicol.,
22: 623-636.
JANSSON, T., CURVALL, M., HEDIN, A. & ENZELL, C.R. (1988). In vitro
studies of the biological effects of cigarette smoke condensate III.
Induction of SCE by some phenolic and related constituents derived
from cigarette smoke. A study of structure-activity relationships.
Mutation Res., 206: 17-24.
JENNER, P.M., HAGEN, E.C., TAYLOR, J.M., COOK, E.L & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
KLIGMAN, A.M. (1970). Unpublished report to RIFM cited in
Opdyke, D.L.(1975). Monographs on fragrance raw materials.
Food Cosmet. Toxicol., 13:91-112.
MAMER, O.A., MONTGOMERY, J.A., DECKELBAUM, R.J. & GRANOT, E. (1985).
Identification of urinary 3-ethoxy-4-hydroxybenzoic and 3-ethoxy-4-
hydroxymandelic acids after dietary intake of ethyl vanillin.
Biomed. Mass. Spectrom. 12: 163-169.
MORTELMANS, K, HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986). Salmonella mutagenicity tests II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8, Suppl. 7: 1-119.
OHTA, T., WATANABE, M., WATANABE, K., SHIRASU, Y. & KADA, T. (1986).
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24: 51-54.
RTECS (1990). Registry of Toxic Effects of Chemical Substances
computer database.
RUDZKI, E. & GRYZWA, Z. (1976). Immediate reactions to balsam of Peru,
cassia oil and ethyl vanillin. Contact Dermatitis, 2: 360-361.
SASAKI, Y.F., IMANISHI, H., OHIA, T. & SHIRASU, Y. (1987). Effects of
antimutagenic flavourings on SCEs induced by chemical mutagens in
cultured Chinese hamster cells, Mutat. Res., 189: 313-318.
SPORN, A. (1960). Toxicity of aromatic ethyl vanillin. Igiena
(Bucharest), 9: 365.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G B. (1973). Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumour response
in strain A mice. Cancer Res., 33: 3069-3085.
WATANABE, K., OHTA, T. & SHIASU, Y. (1988). Antimutagenic effects of
benzaldehyde and its derivatives on mutagenesis induced by
4-nitroquinoline 1-oxide in Escherichia coli. Agric. Biol. Chem.,
52: 1041-1046.
WILD, D., KING, M-T., GOCKE, E. & ECKHARDT, K. (1983). Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, BASC and micronucleus tests. Food Chem.
Toxic., 21: 707-719.
WILLIAMS, R.T. (1959). Detoxication Mechanisms, 2nd Edition, Chapman &
Hall: London.
Ethyl vanillic acid was identified by GC/MS in the urine
of a 9-year old female patient who had received liquid dietary
supplementation flavoured with vanilla. Other patients excreting this
acid were also known to have consumed foodstuffs flavoured with ethyl
vanillin. Eight different urine samples containing more than 50 mg
ethyl vanillic acid/g creatinine were also found to contain small
amounts of vanillylmandelic acid. Unchanged ethyl vanillin was not
detected in any of the urine samples.
A healthy adult male volunteer drank a 235 ml aliquot of a liquid
dietary supplement containing an unknown quantity of ethyl vanillin. A
concentration of 13 mg ethyl vanillic acid/g creatinine was found in a
12-hour urine sample. The compound was not present in urine collected
before exposure (Mamer et al., 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with ethyl vanillin are
summarized in Table 1.
The lowest lethal dermal dose in rats was reported to be
1800 mg/kg bw (RTECS, 1990).
When groups of 6 rabbits were given ethyl vanillin by gavage, a
dose of 150 mg/kg bw caused no adverse effects. At 2500 mg/kg bw, only
a transient increase in respiration rate was observed. The minimum
oral lethal dose was reported to be 3000 mg/kg bw (Deichmann &
Kitzmuller, 1940).
Table 1. Acute toxicity studies with ethyl vanillin
Species Route LD50 Reference
mg/kg bw
Mouse i.p. 7501 Caujolle & Meynier, 1954a
Rat oral 1590 Sporn, 1960
oral > 2000 Jenner et al., 1964
s.c. 1800 Deichmann & Kitzmuller, 1940
Guinea-pig i.p. 1140 Caujolle & Meynier, 1954b
Dog i.v. 760 Caujolle et al., 1953
1 Maximum non-lethal dose, 450 mg/kg bw; Lethal dose: 950 mg/kg bw
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Doses of 300 mg ethyl vanillin/kg bw were administered to rats by
gavage twice weekly for 14 weeks without any adverse affects. In
another experiment, groups of 16 rats were fed ethyl vanillin at a
dose of 20 mg/kg bw/day for 18 weeks without adverse effect. However,
64 mg/kg bw/day for 10 weeks reduced growth rate and caused
myocardial, renal, hepatic, lung, spleen and stomach injuries (nature
not specified) (Deichmann & Kitzmuller, 1940).
Sixteen rats were given 30 mg ethyl vanillin weekly for 7 weeks
without adverse effect on growth, food intake or protein utilization
(Spore, 1960).
Groups of 5 male rats were fed 0, 2%, or 5% ethyl vanillin in the
diet for 1 year without any adverse effects (Hagan et al., 1967).
Groups of CD Sprague-Dawley BR rats (20/sex/group) were fed ethyl
vanillin of > 99.9% purity (nature of diet e.g., semi-synthetic/chow
diet, not specified) at dose levels of 0, 500, 1000 or 2000 mg/kg
bw/day for 13 weeks. The study was designed in accordance with
toxicological principles for the safety assessment of food additives
established by the US FDA (FDA, 1982). The diet was prepared weekly
and showed stability for up to 18 days at room temperature. The
achieved mean dose over the 13-week period was within 1.5% of the
nominal value. Food consumption and body weight were recorded weekly.
Ophthalmoscopy was done before treatment and at termination of the
study. Detailed haematological and clinical chemical examinations were
carried out at week 6 and 13. At termination, all animals were
necropsied and organ weights recorded (adrenals, brain, heart,
kidneys, liver, lungs, ovaries, pituitary gland, prostate, spleen,
testes, thyroids gland, uterus). A complete histological examination
was performed on rats in the control and top-dose groups (adrenals,
alimentary tract, aorta, brain, eyes, femur, Harderian gland, heart,
kidneys, larynx and pharynx, liver, lungs, cervical and mesenteric
lymph nodes, mammary gland, ovaries, pancreas, pituitary gland,
prostate, salivary gland, sciatic nerve, seminal vesicles, skeletal
muscle, skin, spleen, sternum, testes, thymus, thyroid gland, tongue,
trachea, urinary bladder, uterus, vagina). The examination was
extended to the low and intermediate dosage groups where
treatment-related effects were suspected.
No clinical signs or treatment-related deaths of toxicological
significance were observed in treated animals during the study. Food
intake was statistically significantly reduced in females at the
highest dose group at week 1, and in treated male groups at weeks 1-4;
thereafter there were no significant differences in food intake
between controls and treated animals. Water intake, measured
accurately during week 12 of treatment, did not differ notably from
controls. Body-weight gain in males and females in the high-dose group
was significantly reduced compared to control throughout the study;
significant lower body-weight gain was also apparent in males of the
intermediate- and low-dose groups during the first 4 weeks of
treatment. The authors considered these differences from control not
to be treatment-related since the differences were not dosage-related
in magnitude, and because of intra-group variability noted in feeding
patterns of all groups of male rats. Impaired food efficiency was
noted for both male and female rats at the highest dose level.
There were no treatment-related differences from control in
haematological parameters at week 6 or at termination. Clinical
biochemical analyses showed statistically significant higher values in
the high-dose group compared to control for ALAT, ALP, cholesterol and
total plasma protein. Cholesterol levels were significantly increased
in males at the intermediate-dose group at week 6 only. The authors
considered the alteration of the clinical biochemical parameters
secondary to the hepatic changes seen histologically. Other sporadic
differences from control values were generally within normal ranges
for the strain and were not considered of toxicological significance.
At autopsy, enlarged cervical lymph nodes were noted in males at
the intermediate-dose group, and in both sexes at the highest dose
group. In addition, there was a reduction in adipose tissue in rats of
both sexes at the highest dose group. Absolute liver weights were
similar to controls but relative liver weights were increased in the
intermediate- and high-dose animals. Absolute and relative spleen
weights were increased in the intermediate- and high-dose groups.
Although relative spleen weights were increased in the low-dose males,
the absolute organ weights were unaffected, and in the absence of
histopathological changes this observation was considered by the
authors to be of no toxicological significance.
Histological examination revealed a dose-related increased
incidence of hepatic peribiliary inflammatory change in both males and
females of the intermediate- and high-dose groups, and minor bile duct
hyperplasia affecting 1/20 intermediate- and 4/20 high-dose males.
There were no changes observed in the liver parenchyma and no
degenerative or inflammatory changes of the bile duct epithelium.
Increased white pulp cellularity and prominence of germinal centres in
the spleen, and increased prominence of germinal centres and lymphoid
proliferation in cervical lymph nodes were seen in the intermediate-
and high-dose groups. The authors considered the findings of the
lymphoid tissue to be associated reactive changes to the hepatic
peribiliary inflammatory observations.
The authors concluded that no treatment-related changes were
observed at 500 mg/kg bw/day which was considered to be the NOEL in
this study (Hooks et al., 1992b).
2.2.2.2 Rabbits
Single rabbits were given ethyl vanillin orally in 10% aqueous
glycerine at doses of 15 mg/kg bw/day for 13 or 26 days; 32 mg/kg
bw/day for 15 days; 41 mg/kg bw/day for 26 days; or 49 mg/kg bw/day
for 43 days. At the highest close level, anaemia, diarrhoea and lack
of weight gain were observed but no toxic signs were reported at any
of the lower doses (Deichmann & Kitzmuller, 1940).
Subcutaneous injection of ethyl vanillin to rabbits at doses of
148-154 mg/kg bw/day for 6 days did not elicit any observed adverse
effects. Similarly, oral intubation of ethyl vanillin in a milk
vehicle at a dose of 240 mg/kg bw during 25 days (observation period
56 days), or during 54 days (observation period 126 days) did not
produce any observed effects (the parameters observed were not
specified in any of these studies) (Deichmann & Kitzmuller, 1940).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Mice
The maximum tolerated dose for ethyl vanillin in strain A mice
when administered i.p. 3 times/week for 2 weeks was reported to be
75 mg/kg bw. Administration of ethyl vanillin i.p. at doses of 15 or
75 mg/kg bw, 3 times/week for 8 weeks resulted in mortalities of 8/20
and 10/20 animals, respectively. Control animals receiving i.p.
injections of the vehicle tricaprylin, had survival rates of 77/80
males and 77/80 females. In the control group, 28% of males and 23% of
females developed lung tumours whereas in the treated groups only one
animal, in the higher dose group, exhibited a single lung nodule. It
was concluded that ethyl vanillin did not potentiate the pulmonary
tumour response in strain A mice (Stoner et al., 1973).
2.2.3.2 Rats
Groups of Osborne-Mendel rats (12/sex/group) were fed diets
containing 0, 0.5, 1 or 2% ethyl vanillin for 2 years, and 2% or 5%
for 1 year. Haematological examinations (RBC, WBC, haemoglobin and
haematocrit) were performed at 3, 6, 12 and 22 months and at
termination in the 2-year study. All animals were necropsied and
liver, kidney, spleen, heart and testes weights recorded. Histological
examinations were performed on these organs and remaining thoracic and
abdominal viscera, bone and bone marrow, and muscle. No adverse
effects on growth, haematology, organ weights or histology of major
tissues were reported (Hagan et al., 1967).
2.2.4 Reproductive toxicity studies
No reproductive toxicity or teratogenicity studies have been
reported on ethyl vanillin.
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with ethyl vanillin are
summarized in Table 2.
From the SCE studies with human lymphocytes the authors concluded
that benzaldehyde derivatives, including ethyl vanillin, were probably
direct acting SCE inducers and the aldehyde moiety was of primary
importance (Jansson et al., 1988). This contrasts with the negative
effect in CHO cells (Sasaki et al., 1987).
In a study on the anti-mutagenic potential of flavourings, ethyl
vanillin was reported to show marked anti-mutagenic activity against
mutagenicity induced by 4-nitroquinoline 1-oxide, furylfuramide,
captan or methylglyoxal in Escherichia coli WP2s but was ineffective
against mutations induced by Trp-P-2 or IQ in Salmonella typhimurium
TA98. It was proposed that the anti-mutagenic activity was due to
enhancement of an error-free recombinant repair system (Ohta et al.,
1986; Watanabe et al., 1988).
2.3 Observations in humans
In a 24-hour closed patch test in 25 subjects, ethyl vanillin
tested at 2% in petrolatum produced a mild irritation. No
sensitization reactions occurred when ethyl vanillin was used at 2% in
petrolatum in a maximization test on 25 volunteers (Kligman, 1970).
People previously sensitized to balsam of Peru, benzoin, rosin,
benzoic acid, orange peel, cinnamon and cloves have been reported to
cross-react with hydroxybenzaldehydes such as vanillin or ethyl
vanillin. A patient with contact dermatitis showed strong reactions
to balsam of Peru, cassia oil and ethyl vanillin, it was not known
whether the dermatitis was a response to occupational exposure to
ethyl vanillin in a candy factory or to rubber (Rudzki & Grzwa, 1976).
Table 2. Results of genotoxicity assays on ethyl vanillin
Test system Test object Concentration of Results Reference
ethyl vanillin
Micronucleus Mouse 2 × 0-1000 Negative Wild et al., 1983
test mg/kg bw
Ames test1 Salmonella 0-10 mg/plate Negative Ishidate et al.,
typhimurium 1984
TA92, TA94,
TA98, TA100
TA1535,
TA1537
Ames test2 S. typhimurium 0-10 mg/plate Negative Mortelmans et al.,
TA98, TA100 1986
TA1535,
TA1537
Ames test1 S. typhimurium 0-3.6 mg/plate Negative Wild et al., 1983
TA98, TA100
TA1535,
TA1537
TA1538
Chromosomal Chinese hamster 0-0.25 mg/ml Negative3 Ishidate et al.,
aberrations ovary (CHO) 1984
cells in vitro
Sister chromatid Chinese 0-100 M Negative4 Sasaki et al., 1987
exchange (SCE) hamsterd ovary
cells in vitro
Sister chromatid Human 0-2 M Positive Jansson et al.,
exchange (SCE) lymphocytes in 1988
vitro
Table 2. Results of genotoxicity assays on ethyl vanillin (cont'd).
Test system Test object Concentration of Results Reference
ethyl vanillin
Heritable Drosophila 50 mM Negative Wild et al., 1983
mutations melanogaster
1 with or without metabolic activation using rat liver S9 fractions
2 with or without metabolic activation using rat or hamster liver S9 fractions
3 ethyl vanillin did not induce chromosomal aberrations but did cause an increase
in polyploid cells, however the significance of this was unclear and similar
polyploidy was induced by riboflavin
4 ethyl vanillin did not induce sister chromatid exchanges in cultured CHO cells
in vitro but was reported to enhance the ability of mitomycin C to cause sister
chromatid exchanges.
3. COMMENTS
The metabolism studies indicated that ethyl vanillin was rapidly
absorbed, metabolized and excreted in the rat. The principal
metabolite identified was ethyl vanillic acid (3-ethoxy-4-hydroxy-
benzoic acid). This compound, which is not a normal constituent of
human urine, has also been identified in the urine of humans known to
have ingested vanilla-flavoured foodstuffs.
In the recent 13-week toxicity study in which rats were fed ethyl
vanillin at 500, 1000 or 2000 mg/kg bw/day, treated males showed a
transient reduction in body-weight gain compared with controls during
the first 4 weeks of treatment. Since this effect was only transient
and associated with reduced food intake, probably due to impaired
palatability, the Committee concluded that the NOEL was 500 mg/kg
bw/day.
The Committee considered ethyl vanillin not to be genotoxic on
the basis of negative results in a large number of studies, although
one assay for sister chromatid exchange was positive.
4. EVALUATION
The Committee concluded that, in the light of the information
showing daily intakes to be in the range of 0.06-7 mg/person/day, the
safety evaluation could be based on the principles applicable to
materials occurring in foods in small amounts. In view of the limited
toxicological information available, the Committee withdrew the
previous temporary ADI and allocated an ADI of 0-3 mg/kg bw for ethyl
vanillin, based on a NOEL of 500 mg/kg bw/day in the 13-week toxicity
study in rats and a safety factor of 200.
5. REFERENCES
CAUJOLLE, F. & MEYNIER, D. (1954a). Toxicity of vanillin, o-vanillin
and ethyl vanillin and of the corresponding meta aldehydes.
Ann. Pharm. Fr., 12: 42-49.
CAUJOLLE, F. & MEYNIER, D. (1954b). The comparative toxicity of
orthovanillin and ethyl orthovanillin. Compt. Rend., 238: 2576-2578.
CAUJOLLE, F., MEYNIER, D. & MOSCARELLA, C. (1953). The comparative
toxicity of ethyl vanillin and 4-hydroxy-5-ethoxyisophthalic
dialdehyde. Compt. Rend., 237: 765-766.
DEICHMANN, W. & KITZMULLER, K.V. (1940). Toxicity of vanillin and
ethyl vanillin for rabbits and rats. J. Am. Pharm. Assoc.,
29: 425-428.
FDA, (1982). Toxicological Principles for the Safety Assessment of
Direct Food Additives and Colour Additives used in Food. Food and Drug
Administration, USA.
HAGAN, E.C, HANSEN, W.H., FITZHUGH, O.G., JENNER, P.M., JONES, W.I.,
TAYLOR, J.M., LONG, EL., NELSON, A.A. & BROUWER, J.B. (1967). Food
flavourings and compounds of related structure. II. Subacute and
chronic toxicity. Food Cosmet. Toxicol., 5: 141-157.
HAWKINS, D.R., MIDGLEY, I., PROCTOR, P., PARIKH, R., BENNETT, S.,
HUCKSTEP, M.R. & CHENG, K. (1992). Biokinetics and metabolism of ethyl
14C-vanillin following oral (gavage) administration to rats.
Unpublished Report No. HRC/EVT 5/921134 of Huntingdon Research Centre
Ltd. Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., GOPINATH, C., ANDERSON, A.,
DAWE, I.S., BRODIE, R. R. & CHASSEAUD, L F. (1992a). Ethyl vanillin
palatability/toxicity by dietary administration to rats for 2 weeks.
Unpublished report No. EVT 1/911052 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC). Atlanta, Georgia, USA.
HOOKS, W.N., KIRK, S.J., SMITH, H.L., CROOK, D., GIBSON, W.A., GREGSON
R.L., GOPINATH, C., ANDERSON, A. & DAWE, S.I. (1992b). Ethyl vanillin
toxicity to rats by repeated dietary administration for 13 weeks.
Unpublished report No EVT 2/920067 of Huntingdon Research Centre Ltd.
Submitted to WHO by Ethyl Vanillin Task Force, International Food
Additives Council (IFAC), Atlanta, Georgia, USA.
ISHIDATE, M., SOFUNI, T., YOSHIKAWA, K., HAYASHI, M., NOHMI, T.,
SAWADA, M. & MATSUOKA, A. (1984). Primary mutagenicity screening of
food additives currently used in Japan. Food Chem Toxicol.,
22: 623-636.
JANSSON, T., CURVALL, M., HEDIN, A. & ENZELL, C.R. (1988). In vitro
studies of the biological effects of cigarette smoke condensate III.
Induction of SCE by some phenolic and related constituents derived
from cigarette smoke. A study of structure-activity relationships.
Mutation Res., 206: 17-24.
JENNER, P.M., HAGEN, E.C., TAYLOR, J.M., COOK, E.L & FITZHUGH, O.G.
(1964). Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
KLIGMAN, A.M. (1970). Unpublished report to RIFM cited in
Opdyke, D.L.(1975). Monographs on fragrance raw materials.
Food Cosmet. Toxicol., 13:91-112.
MAMER, O.A., MONTGOMERY, J.A., DECKELBAUM, R.J. & GRANOT, E. (1985).
Identification of urinary 3-ethoxy-4-hydroxybenzoic and 3-ethoxy-4-
hydroxymandelic acids after dietary intake of ethyl vanillin.
Biomed. Mass. Spectrom. 12: 163-169.
MORTELMANS, K, HAWORTH, S., LAWLOR, T., SPECK, W., TAINER, B. &
ZEIGER, E. (1986). Salmonella mutagenicity tests II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8, Suppl. 7: 1-119.
OHTA, T., WATANABE, M., WATANABE, K., SHIRASU, Y. & KADA, T. (1986).
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24: 51-54.
RTECS (1990). Registry of Toxic Effects of Chemical Substances
computer database.
RUDZKI, E. & GRYZWA, Z. (1976). Immediate reactions to balsam of Peru,
cassia oil and ethyl vanillin. Contact Dermatitis, 2: 360-361.
SASAKI, Y.F., IMANISHI, H., OHIA, T. & SHIRASU, Y. (1987). Effects of
antimutagenic flavourings on SCEs induced by chemical mutagens in
cultured Chinese hamster cells, Mutat. Res., 189: 313-318.
SPORN, A. (1960). Toxicity of aromatic ethyl vanillin. Igiena
(Bucharest), 9: 365.
STONER, G.D., SHIMKIN, M.B., KNIAZEFF, A.J., WEISBURGER, J.H.,
WEISBURGER, E.K. & GORI, G B. (1973). Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumour response
in strain A mice. Cancer Res., 33: 3069-3085.
WATANABE, K., OHTA, T. & SHIASU, Y. (1988). Antimutagenic effects of
benzaldehyde and its derivatives on mutagenesis induced by
4-nitroquinoline 1-oxide in Escherichia coli. Agric. Biol. Chem.,
52: 1041-1046.
WILD, D., KING, M-T., GOCKE, E. & ECKHARDT, K. (1983). Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, BASC and micronucleus tests. Food Chem.
Toxic., 21: 707-719.
WILLIAMS, R.T. (1959). Detoxication Mechanisms, 2nd Edition, Chapman &
Hall: London.
