
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 45
Prepared by the
Fifty-fourth meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
CYHALOTHRIN
First draft prepared by
D.W. Renshaw
Joint Department of Health Ministry of Agriculture, Food and Fisheries
Food Standards and Safety Group, Department of Health, London, United
Kingdom
Explanation
Biological data
Biochemical aspects
Pharmacodynamics
Absorption, distribution, excretion, and
biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration studies
Developmental toxicity
Special studies
Neurotoxicity
Neurobehavioural effects
Immunotoxicity
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Cyhalothrin is a type II pyrethroid insecticide and acaricide.
Technical-grade cyhalothrin (about 98% pure), which was the material
used in most of the toxicological studies, consists mainly of four of
the possible 16 isomers. These four isomers comprise two pairs of
enantiomers, A and B, in a ratio of 60:40. Within each pair, the
enantiomers are present in equal amounts.
Cyhalothrin is used predominantly on cattle and sheep, and to a
lesser extent on pigs and goats, for the control of a broad range of
ectoparasites, including flies, lice, and ticks. Cyhalothrin is
applied topically as a pour-on formulation to cattle at doses of up to
60 ml (1.2 g) for ticks and 10 ml (0.2 g) for lice or flies, and to
sheep and pigs at a dose of 5 ml (0.1 g) for all applications.
Cyhalothrin is also available as a 20% (w/v) emulsifiable concentrate
for use as a spray or a dip, prepared by dilution to 0.002-0.2%. It is
applied at a dose of 0.1-4 L per animal, depending on the size of
animal and the pest for which it is applied. In general, the more
dilute the spray or dip, the greater the volume applied. A related
product, lambda-cyhalothrin, contains only the B pair of enantiomers.
The Committee has not previously evaluated cyhalothrin. It had
however been evaluated toxicologically by the 1984 Joint FAO/WHO
Meeting on Pesticide Residues, when an ADI of 0-0.02 mg/kg bw was
established (JMPR, 1985). It had also been reviewed in an
Environmental Health Criteria monograph (WHO, 1990).
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
Type II pyrethroids are characterized by the presence of an
alpha-cyano group. Their main action is to alter the sodium channels
in the nerve membrane reversibly, causing persistent prolongation of
the transient increase in the permeability of the neuronal membrane to
sodium during excitation (Vijverberg et al., 1982; Narahashi, 1985;
Aldridge, 1990; Coats, 1990; Vijverberg & van den Bercken, 1990). Type
II pyrethroids depress resting chloride conductance, thereby
amplifying any effects of sodium or calcium (Ray, 1991).
Cyhalothrin and permethrin have been shown in vitro to be
potent inhibitors of mitochondrial complex I but not of complex II,
III, IV, or V. In isolated mitochondria from rat liver,
concentration-dependent inhibition of glutamate and succinate stage 3
respiration was seen (Gassner et al., 1997).
It has been postulated that type II pyrethroids exert some of
their effects by binding to the gamma-aminobutyric acid (GABA)
receptor, but they inhibit this receptor much less than the cyclodiene
insecticides (Bloomquist et al., 1986).
Type II pyrethroids act primarily on the central nervous system,
whereas type I pyrethroids act mainly on peripheral nerves (Barnes &
Verschoyle, 1974; Lawrence & Casida, 1982).
2.1.2 Absorption, distribution, excretion, and biotransformation
The studies on cyhalothrin summarized in this section date from
the early 1980s, and many were not conducted in accordance with the
requirements of good laboratory practice (GLP). They were, however,
well conducted by the standards of the time and were monitored by a
quality assurance team. In the studies of Harrison, the batch of
cyhalothrin used was > 99% pure cis-Z-cyhalothrin, but individual
isomers were not measured in either the test material or tissues or
body fluids.
Rats
Groups of six male and six female Alderley Park Wistar (Alpk/Ap)
specific pathogen-free (SPF) rats were given a single dose of 1 or 25
mg/kg bw of 14C-cyhalothrin in corn oil by oral gavage. The
investigation was conducted with cyhalothrin labelled either at the
3-phenoxybenzyl side-chain (i.e. the carbon to which the cyanide
moiety is attached: 14CHCN) or at the 1 position of the cyclopropyl
moiety. Separate experiments were performed in rats with bile duct
cannulae. Another group of male and female rats were given a
subcuta-neous dose of 1 mg/kg bw of 14CHCN-labelled cyhalothrin.
Approximately 30-40% of the orally administered 14CHCN-labelled
material was recovered in urine, most of the remainder appearing in
the faeces. The rate of excretion was unaffected by the dose. Peak
blood concentrations (approximately 0.6 and 6 µg/kg of cyhalothrin
equivalents at 1 and 25 mg/kg bw, respectively) were reached 7 h after
dosing. By 48 h, the concentrations had depleted to less than 10% of
the peak value. In bile duct-cannulated rats, biliary excretion
accounted for 4.8% of the total excretion in males and 8.9% in
females, but the amounts excreted in both urine and bile were doubled
when the cyhalothrin was co-administered with bile. Most of the
administered material was recovered within 24 h, and only 2-3% of the
administered radiolabel remained in the carcass after 7 days. The
radiolabel was distributed mainly to fat. Table 1 shows the
concentrations in adipose tissues and liver; very little
(approximately 2-7 µg/kg as equivalents) was found in bone, muscle,
gonads, kidney, lungs, and heart. Elimination of radiolabel was slower
after subcutaneous administration of 14CHCN-labelled cyhalothrin
than after oral dosing, and 58% of the radiolabel was still present in
the carcass 7 days after dosing. With cyclopropyl-labelled
cyhalothrin, smaller proportions of the administered radiolabel were
recovered in the urine (18-30%), and the rate of excretion was slower
than with 14CHCN-cyhalothrin. Peak blood concentrations were
achieved at 4 h, but these had declined to 10% of the peak values by
48 h. Only 1-3% of the administered dose remained in the carcass after
7 days.
Analysis by thin-layer chromatography (TLC) showed that most of
the faecal radiolabel consisted of unchanged cyhalothrin (probably
unabsorbed material). In contrast, no unchanged material was detected
in urine or bile. Most of the radiolabel in urine (98%) was in the
form of highly polar compounds. The patterns of metabolites derived
from the two 14C-labelled forms of cyhalothrin were totally
different, suggesting that the metabolism involves initial cleavage of
the ester bond. Of the urinary material derived from
cyclopropyl-labelled cyhalothrin, 60% could be hydrolysed by
glucuronidase. Two major urinary metabolites were derived from
14CHCN-labelled cyhalothrin, representing 75% and 17% of the
administered dose. Both were polar and resistant to glucuronidase.
Three biliary metabolites were derived from 14CHCN-labelled
cyhalothrin, two of which (representing 12% and 67% of the dose) were
different from the urinary metabolites (Harrison, 1981, 1984a).
Table 1. Residues of cyhalothrin equivalents in selected tissues 7 days after
dosing
Tissue Tissue concentration (µg/kg as equivalents)
1 mg/kg bw 25 mg/kg bw
Males Females Males Females
14CHCN-labelled material
Brown fat 65 93 870 2 000
White fat 340 330 6400 12 000
Liver 320 540 320 540
14C-Cyclopropyl-labelled material
Brown fat 40 54 1600 2 200
White fat 160 300 6900 12 000
Liver 21 20 930 740
In a separate study, groups of six male and six female Alpk/Ap
rats were given daily doses of 1 mg/kg bw of 14CHCN- or
14C-cyclopropyl-cyhalothrin by oral gavage in corn oil for 14 days.
Groups of animals were killed 2, 5, and 7 days after the final dose
and the radiolabel was measured in various tissues, urine, and faeces.
Up to 50% of each radiolabelled material was excreted in the urine and
the remainder in the faeces. Excretion was rapid, over 90% of the
daily dose being excreted within 24 h and over 90% of the cumulative
dose being excreted within 7 days of the end of dosing. Most of the
residue was located in fat, the concentrations in other tissues
ranging from 20 to 460 µg/kg (as equivalents) 2 days after the end of
dosing. The residue in fat depleted slowly, with an estimated
half-time of 23 days. The depletion of residues from brown and white
fat is shown in Table 2. The half-time in white fat was estimated to
be 30 days. The concentrations of residue found at 2, 5, and 7 days in
the animals given 14CHCN-cyhalothrin were almost identical to those
in the animals given 14C-cyclopropyl-cyhalothrin. Analysis by
high-performance liquid chromatography (HPLC) showed that most of the
residue in white fat (79%) was in the form of unchanged cyhalothrin
(Harrison, 1984b).
Table 2. Depletion of residues from adipose tissues
Tissue Tissue concentration of radiolabel (µg/kg)
2 days 5 days 7 days
CHCN Cyclopropyl CHCN Cyclopropyl CHCN Cyclopropyl
Brown fat 1900 2300 1100 1000 820 500
White fat 4200 3600 4400 3500 3300 3400
The biotransformation of cyhalothrin was examined in groups of
six male and six female Alpk/Ap rats that were given either daily oral
doses of 12.5 mg/kg bw of 14CHCN-cyhalothrin for 8 days or daily
oral doses of 1 mg/kg bw of 14C-cyclopropyl-cyhalothrin for 14 days.
The major urinary metabolites were analysed by TLC, HPLC, mass
spectrometry, and nuclear magnetic resonance spectroscopy. Cyhalothrin
was initially cleaved at the ester bond, breaking the pyrethroid
structure and therefore presumably detoxifying it. This cleavage
produced a cyclopropyl carboxylic acid and the phenoxybenzyl cyano
alcohol, which was subsequently metabolized to
3-(4'-hydroxyphenoxy)benzoic acid. Cyclopropyl carboxylic acid was
excreted in the urine, largely as the glucuronic acid conjugate,
whereas 3-(4'-hydroxyphenoxy)benzoic acid was excreted mainly (~75%)
as the sulfate conjugate. Small amounts of free
3-(4'-hydroxyphenoxy)benzoic acid, 3-phenoxybenzoic acid, and
cyclopropyl carboxylic acid were also found in the urine. It was
concluded that the metabolism of cyhalothrin in rats proceeded along
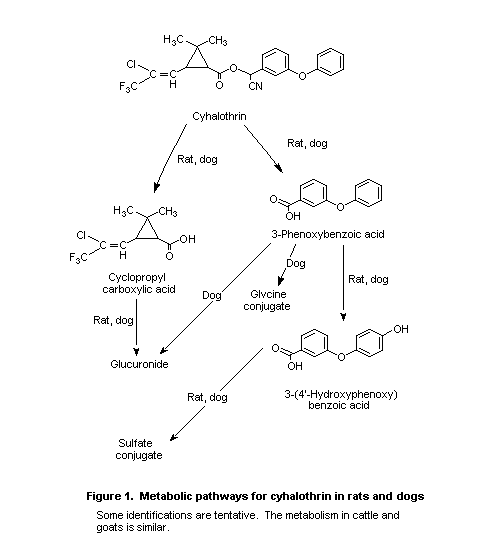
routes similar to those of other type II pyrethroids (Figure 1)
(Harrison, 1983).
Dogs
Groups of three male and three female beagle dogs were given a
single oral dose of 1 or 10 mg/kg bw of either 14CHCN- or
14C-cyclopropyl-cyhalothrin dissolved in corn oil and administered
in a gelatin capsule. Other groups of dogs were given a single
intravenous injection of 0.1 mg/kg bw (in ethanol:0.9% saline in a
3.2:1.2 ratio) of each material. Blood samples and excreta were
collected over 7 days after dosing and were analysed for radiolabel by
liquid scintillation counting. Metabolites were measured by TLC, and
the identities of selected metabolites were confirmed by mass
spectrometry.
 Excretion was initially rapid, most of the administered doses
being eliminated within the first 48 h after dosing, but excretion was
still incomplete at 7 days. After 14CHCN-cyhalothrin was given
orally, approximately 30% of the administered dose was found in urine
at 7 days and about 50% in faeces. When 14C-cyclopropyl-cyhalothrin
was given, the proportion of residue in urine was slightly lower (19%)
and the amount in faeces higher (68%). The proportions of residue in
urine and in faeces were approximately equal when the two
radiolabelled forms of cyhalothrin were given intravenously. A large
proportion (46-87%) of the faecal residue was in the form of unchanged
cyhalothrin after oral dosing, but only small amounts (1.4-1.5% of
faecal radiolabel) were found in faeces after intravenous injection,
suggesting that the oral dose of cyhalothrin was only partially
absorbed from the gut lumen, leaving unabsorbed cyhalothrin in the
faeces.
Metabolism occurred initially by cleavage of the ester bond to
give a phenoxybenzyl moiety and a cyclopropane acid moiety. Further
metabolism of the phenoxybenzyl moiety produced N-(3-phenoxybenzoyl)
glycine, 3-(4'-hydroxyphenoxy)benzoic acid and its sulfate conjugate,
3-phenoxybenzoyl glucuronide, small amounts of various other
conjugates of these compounds, and a little free phenoxybenzoic acid.
The cyclopropane acid moiety was extensively metabolized to at least
11 further metabolites, including 45% as the glucuronide of the
cyclopropane acid and up to 23% as the free cyclopropane acid (i.e.
3-( Z-2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclo-propane
carboxylic acid; see Figure 1 (Harrison, 1984c).
Humans
In a study of three teams of seven pesticide applicators in
Pakistan, the men were monitored over 15 days while they were using a
pesticide formulation which contained 10% lambda-cyhalothrin and for a
further 4 days after they had finished spraying. The men wore typical
work clothing with no personal protective equipment while spraying
dwellings to kill mosquitoes as part of a malaria control programme.
All of the workers underwent four medical examinations, including
blood sampling, before the study, in the middle of the first week, in
the middle of the second week, and 4 days after the last spraying, and
were questioned about adverse effects. Urine samples were collected
daily. Fifteen men who lived in the treated dwellings were monitored
for exposure by collection and analysis of 24-h urine samples for 3
consecutive days after the spraying of their houses. The samples of
urine and serum were treated enzymically to deconjugate the
metabolites and were then analysed by gas chromatography with mass
spectroscopy. The cyhalothrin metabolites measured were
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)-benzoic acid. The limit of determination for all
three metabolites was 0.01 µg/ml. These metabolites are products of
cleavage of the ester bond in cyhalothrin, and their presence
indicates that the initial metabolism of cyhalothrin is similar in
humans to that observed in other species.
The average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). 3-(2-Chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid was usually the most abundant
metabolite in urine. No metabolites were detected in serum samples.
Most of the inhabitants of treated houses had no detectable residues
in their urine, although they were measurable in 12 of 44 samples, and
the measured concentrations were at or close to the limit of
determination. Most values were not reported, but the highest
concentration of urinary metabolites was 0.1 mg/person per day
(Chester et al., 1992). The signs of toxicity found are reported
below.
2.2 Toxicological studies
Most of the toxicological studies of cyhalothrin were performed
during 1980-84, and some were therefore conducted before GLP was
widely introduced. The chemical identity of the cyhalothrin used in
each study is not clearly stated, and, although the purity was
reported in most cases, the isomers and the isomer ratios were not
mentioned in most of the reports. The manufacturer has claimed that
the analysis given in appendices to the report of the inclined plane
test (see below) is typical of technical-grade cyhalothrin. The
manufacturer also claimed that measurements made over the last
15 years have shown little variation in the isomer ratio. Two
measurements from 1985 gave about 56% for the A isomeric pair and 41%
for the B isomeric pair, leaving only about 2% for other isomers and
impurities (Woodward, 2000).
2.2.1 Acute toxicity
Acute poisoning of rats with type II pyrethroids such as
cyhalothrin is typically characterized by progressive development of
'nosing' and exaggerated jaw opening, increasing extensor tone in the
hind limbs causing a rolling gait, choreoathetosis (sinuous writhing
spasms), salivation, incoordination progressing to coarse tremor,
tonic seizures, apnoea, and death (Barnes & Verschoyle, 1974; Ray,
1991). This has been called the choreoathetosis/salivation syndrome or
CS syndrome. In dogs, similar symptoms are seen, but salivation, upper
airway hypersecretion, and gastrointestinal symptoms are more
prominent than in rats (Ray, 1991).
As in poisoning with type I pyrethroids, the plasma noradrenaline
concentration is increased by type II pyrethroids, but they also
increase plasma adrenaline and blood glucose concentrations. Type II
pyrethroids increase cardiac contractility both directly by action on
cardiac muscle and via circulating catecholamines. They also cause
repetitive firing and potentiate contraction in skeletal muscle. The
type II pyrethroids do not produce the repetitive activity in sensory
nerves that is seen after exposure to type I pyrethroids (Ray, 1991).
Table 3. LD50 values for cyhalothrin
Species Sex Route Purity Vehicle LD50 Reference
(%) (mg/kg bw)
Rat M Oral 90/94 cis Corn oil 240 Nixon & Jackson (1981)
Rat M Oral 92.9 Corn oil 170 Pritchard (1984)
Rat M Oral 90 Oilve oil 51 Ebino et al. (1984a)
Rat F Oral 90/94 cis Corn oil 140 Nixon & Jackson (1981)
Rat F Oral 92.9 Corn oil 110 Pritchard (1984)
Rat F Oral 90 Olive oil 65 Ebino et al. (1984a)
Mouse M Oral 90/94 cis Corn oil 37 Nixon & Jackson (1981)
Mouse F Oral 90/94 cis Corn oil 62 Nixon & Jackson (1981)
Guinea-pig M Oral 90/94 cis Corn oil > 5000 Nixon & Jackson (1981)
Rabbit F Oral 89.2 Corn oil > 1000 Jones (1980)
Chicken F Oral 93.4 Corn oil > 10 000 Roberts et al. (1982)
Rat M Dermal 90 Undiluted 3600 Ebino et al. (1984d)
Rat F Dermal 90 Undiluted 3700 Ebino et al. (1984d)
Rabbit M Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rabbit F Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rat M Intraperitoneal 90/94 cis Corn oil 250-750 Nixon & Jackson (1981)
Rat M Intraperitoneal 90 Oilve oil 500 Ebino et al. (1984c)
Rat F Intraperitoneal 90 Oilve oil 420 Ebino et al. (1984c)
Rat M Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
Rat F Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
The acute toxicity of cyhalothrin was investigated in a series of
studies that complied with GLP (Nixon & Jackson, 1981; Roberts et al.,
1982; Pritchard, 1984) and also in studies that, although not GLP
compliant, were accompanied by a suitable quality assurance statement
(Jones, 1980; Ebino et al., 1984a-e). The LD50 values estimated from
these studies are listed in Table 3. The LD50 of a wettable
formulation containing 5% cyhalothrin was 1200 mg/kg bw in male rats
and 900 mg/kg bw in females when it was administered orally in olive
oil (Ebino et al., 1981b).
The signs of toxicity seen in all species and with all routes of
administration were similar and included subdued behaviour, ungroomed
appearance, piloerection, salivation, incontinence, and unsteady gait.
A further account of the signs of acute toxicity in rats receiving
various oral doses of cyhalothrin is given in the preliminary report
of the inclined plane test (Denton, 1988), which is described below.
2.2.2 Short-term studies of toxicity
Mice
In a range-finding study for an assay of dominant lethal mutation
that complied with GLP, groups of 10 male CD-1 mice were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 5, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 5 days. At
the two higher doses, signs of severe toxicity (ataxia and
convulsions) were observed. One animal at 40 mg/kg bw per day and
eight at 80 mg/kg bw per day died. Less severe clinical signs were
seen at 20 mg/kg bw per day, including body-weight loss, ataxia, and a
rough coat. Only rough coats were seen at 5 mg/kg bw per day (Irvine,
1981a).
In a range-finding study for a long-term study of toxicity and
carcinogenicity, groups of 12 male and 12 female CD-1 mice were fed
diets containing technical-grade cyhalothrin (purity unspecified) at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of diet. Over the
course of the 4-week study, these concentrations gave mean doses of
cyhalothrin of 0, 0.65, 3.3, 14, 64, and 310 mg/kg bw per day for
males and 0, 0.80, 4.2, 15, 78, and 290 mg/kg bw per day for females.
No certificate of compliance with GLP was available for this study,
but quality assurance systems were in place.
Deaths were seen only at the highest dietary concentration, at
which six males and three females died prematurely. Severe signs of
toxicity were also seen at this dose, including abnormal gait
('walking on toes'), hunched posture, decreased body weight (Student's
t test, p < 0.01), and increased respiratory rate. Piloerection
was observed in several animals in each groups given cyhalothrin at
> 25 mg/kg and in one control male and one male at 5 mg/kg of diet.
Haematological changes were seen in males (statistically significant
in Williams' test: p < 0.05) but not in females. Animals at 2000
mg/kg of diet had a decreased total leukocyte count accompanied by a
lower lymphocyte count and a higher neutrophil count. Those at 100 and
500 mg/kg of diet also had marginally lowered lymphocyte counts.
Macroscopic examinations post mortem revealed no adverse
effects other than altered organ weights (statistically significant in
both Student's t test and Williams' test: p < 0.05). The weight
of the kidney relative to body weight was slightly elevated in males
given 100, 500, or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The relative liver weights were also slightly increased in
males given 25 or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The heart weights were marginally decreased in females given
100 or 2000 mg/kg of diet. The activity of aminopyrine- N-demethylase
in liver was increased in males given 2000 mg/kg of diet and in
females given 100, 500, or 2000 mg/kg of diet (Student's t test:
p < 0.05). Histopathological examination showed minimal
centrilobular hepatocyte enlargement in two females at 2000 mg/kg of
diet. Atrophy of the red pulp of the spleen was seen in two of the
three females at this dose that died. The Committee considered that
the effect may have been indicative of immunological changes. The NOEL
was 5 mg/kg of diet, equal to 0.65 mg/kg bw per day, on the basis of
piloerection at concentrations > 25 mg/kg of diet (Colley et al.,
1981).
When liver samples from this study were examined by electron
microscopy, minimal amounts of smooth endoplasmic reticulum were
observed in occasional hepatocytes from some mice from each group,
including controls. Most hepatocytes contained no observable smooth
endoplasmic reticulum. The incidence of mice with hepatocytes
containing smooth endoplasmic reticulum was highest in the group given
cyhalothrin at 2000 mg/kg of diet, consistent with the finding of
other hepatic effects in this group (Prentice et al., 1981).
Rats
In a range-finding study for an investigation of developmental
toxicity, groups of 10 female Charles River CD rats were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 10
consecutive days. After treatment, the animals were observed for a
7-day recovery period. No certificate of compliance with GLP was
supplied, but suitable quality assurance records had been kept.
All of the animals given 80 mg/kg bw per day, nine of those given
40 mg/kg bw per day, and two given 20 mg/kg bw per day had to be
killed for humane reasons after the first dose, as this caused severe
toxicity (ataxia and/or collapse). Body-weight loss and reduced food
intake were seen in the rats given 20 mg/kg bw per day throughout the
treatment period. Body-weight loss was also seen in the rats given 10
mg/kg bw per day during the first 5 days of the study. Food intake was
increased in the groups at 10 and 20 mg/kg bw per day during the
recovery period. Macroscopic examination of the animals at necropsy
revealed no treatment-related changes. No NOEL could be identified in
this study (Killick, 1980).
In a 28-day range-finding study, groups of 10 male and 10 female
Charles River CD rats were fed diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 5, 10, 20,
or 250 mg/kg of diet, equivalent to 0, 0.5, 1, 2, and 25 mg/kg bw per
day. The study did not comply with GLP. There were no
treatment-related clinical signs and no deaths. Food intake, food
efficiency, and body-weight gain were decreased only at the highest
dose (Student's t test: p < 0.05). Gross examination post
mortem revealed no treatment-related effects, and organ weights were
unaffected by treatment. Electron microscopy of liver samples showed a
moderate increase in the amount of smooth endoplasmic reticulum in
hepatocytes of animals at the highest dose, with a decrease in the
amount of glycogen. An increase in hepatocyte size was noted in the
centrilobular and mid-zonal regions of the livers of males at this
dose, which also had increased hepatic activity of
aminopyrine- N-demethylase (Student's t test: p < 0.001). The
NOEL was 20 mg/kg of diet, equivalent to 2 mg/kg bw per day (Colley et
al., 1980).
In a study that complied with GLP, groups of eight male and eight
female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%) at a concentration of 0,
1, 5, 10, 20, or 250 mg/kg of diet, equivalent to 0, 0.1, 0.5, 1, 2,
and 25 mg/kg bw per day. Full post-mortem examinations were not
performed, but samples of liver were taken for analysis of
aminopyrine- N-demethylase activity and electron microscopy. No
treatment-related clinical signs were reported. The mean body weight
of males given 250 mg/kg of diet was decreased when compared with that
of concurrent controls during weeks 1 and 2 of the study (two-sided
t test: p < 0.05). Body-weight gain was depressed during week 1
for males given 250 mg/kg of diet and for females given 10, 20, or 250
mg/kg of diet (two-sided t test: p < 0.05). The absolute weight
of the liver was reduced in males given 250 mg/kg of diet and in
females given 20 mg/kg of diet (two-sided t test: p < 0.05), but
the relative weight of the liver was increased in males at 250 mg/kg
of diet. Both males and females at this dose had increased hepatic
activity of aminopyrine- N-demethylase and mild proliferation of
smooth endoplasmic reticulum in hepatocytes. The NOEL was 5 mg/kg of
diet, equivalent to 0.5 mg/kg bw per day, on the basis of depression
of body-weight gain, whereas that for hepatic changes was 20 mg/kg of
diet, equivalent to 2 mg/kg bw per day (Moyes et al., 1984).
In a 28-day range-finding study, of which full details were not
available, a dietary concentration of cyhalothrin of 750 mg/kg
(equivalent to 75 mg/kg bw per day) increased the mortality rate.
'Characteristic signs of pyrethroid neurotoxicity' were seen at
dietary concentrations of 250 and 750 mg/kg. Treatment-related effects
on body weights, food consumption, organ weights, and hepatic activity
of aminopyrine- N-demethylase were seen at 250 mg/kg of diet. Hepatic
smooth endoplasmic reticulum proliferation occurred in males fed 20
and 250 mg/kg of diet. No NOEL could be identified (Lindsey et al.,
1981).
In a 90-day study that complied with GLP, groups of 20 male and
20 female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%; with a total pyrethroid
content of 92.2% of which 96.8% was cyhalothrin) at a concentration of
0, 10, 50, or 250 mg/kg of diet, providing mean doses of 0, 0.56, 2.6,
and 36 mg/kg bw per day for males and 0, 0.57, 3.2, and 15 mg/kg bw
per day for females. Blood samples were collected before treatment,
during week 4 of treatment, and at the end of the study. Urine samples
were collected before treatment and in weeks 4 and 13. Ophthalmoscopic
examinations were performed during the week before termination. Full
post-mortem examinations were performed, and the major organs and any
grossly abnormal tissues were examined histologically. Liver samples
were taken for analysis of aminopyrine- N-demethylase activity and
examination by electron microscopy.
Treatment with doses < 250 mg/kg of diet had no effect on the
mortality rate. The body-weight gain of males at the highest dose was
consistently reduced (two-sided t test: p < 0.01) throughout the
study. Food consumption was slightly reduced in males at 50 and 250
mg/kg of diet, and this effect was occasionally statistically
significant (two-sided t test: p < 0.05). There were no clearly
treatment-related haematological effects, although there were small,
significant, but not dose-related changes in some red blood cell
parameters (mean corpuscular volume, packed cell volume, haemoglobin,
mean corpuscular haemoglobin). Plasma alanine and aspartate
aminotransferase activities and cholesterol concentration were raised
at 4 weeks in males given 10 or 50 mg/kg of diet, and those at the
lower dose also had a raised plasma urea concentration (two-sided
t test: p < 0.05). At 13 weeks, however, the plasma concentration
of urea in males at 50 mg/kg of diet was decreased (two-sided
t test: p < 0.05). The observed effects on haematological
parameters, plasma enzyme activity, and concentrations of cholesterol
and urea were considered to be unrelated to treatment as there were no
clear dose-response relationships. At 13 weeks, however, the plasma
triglyceride concentration was reduced and there was a dose-related
increase in urinary glucose concentration in males at 50 and 250 mg/kg
of diet (two-sided t test: p < 0.05). Ophthalmoscopy revealed no
treatment-related effects. The results of gross examinations were not
reported, although the organ weights were recorded. The absolute
weight of the liver was increased in males at 250 mg/kg of diet, and
the absolute weight of the lung was decreased in animals given 250
mg/kg of diet, but the relative weights of these organs were
unaffected. No treatment-related changes were revealed by optical
microscopy of organs. Electron microscopy of the liver revealed mild
proliferation of smooth endoplasmic reticulum in the hepatocytes of
three males given 50 mg/kg of diet and three males given 250 mg/kg of
diet. There was a dose-related increased in hepatic
aminopyrine- N-demethylase activity in males given 50 or 250 mg/kg of
diet and in females given 250 mg/kg of diet (two-sided t test:
p < 0.05). The NOEL was 10 mg/kg of diet, equivalent to 0.56 mg/kg
bw per day, on the basis of hepatic changes in males at higher doses
(Lindsey et al., 1981).
Rabbits
In a range-finding study for an investigation of developmental
toxicity, groups of six mated female New Zealand white rabbits were
given cyhalothrin (purity, 89.25%) at a daily dose of 0, 20, 30, or 40
mg/kg bw by oral gavage in corn oil on days 6-18 of gestation. The
animals were killed on day 18 of gestation.
There were no unscheduled deaths and no consistent clinical signs
of toxicity. Body-weight gain was depressed only at 30 mg/kg bw per
day, although animals at 40 mg/kg bw per day showed reduced food
intake. Not all of the animals became pregnant, but in all groups,
including controls, few of the does had live fetuses. There was no
dose-response relationship for this effect. Furthermore, not all of
the pregnant does at 20 and 40 mg/kg bw per day produced live
offspring. Consequently, there were insufficient numbers of litters to
allow any meaningful inter-group comparison of litter parameters,
although no adverse effects were apparent from the limited data
available. No treatment-related macroscopic changes were seen post
mortem. The apparent NOEL in this limited study was 20 mg/kg bw per
day on the basis of reduced body-weight gain (Killick, 1981a).
Dogs
In a preliminary study, groups of one male and one female beagles
were given gelatin capsules containing cyhalothrin (purity
unspecified) in corn oil at a dose of 0, 2.5, 10, or 30 mg/kg bw per
day for 4 weeks. After 10 days, treatment with the high dose was
stopped, and the animals were allowed to recover for 4 days; treatment
was then resumed at a lower dose of 20 mg/kg bw per day for 4 weeks.
Blood was taken weekly. Urine samples were collected before dosing and
during week 4. Ophthalmoscopic examinations were performed on each dog
before dosing and during week 4. No certificate of compliance with GLP
was supplied, but suitable quality assurance records were kept.
None of the dogs died prematurely. Muscular trembling was seen in
all groups, including controls, but the degree of trembling was more
marked in animals at the two higher doses. Unsteady gait and
body-weight loss were observed in dogs given 30 mg/kg bw per day, but
this effect was not seen after the dose was reduced to 20 mg/kg bw per
day. Body weight was unaffected by doses up to 10 mg/kg bw per day,
and only slight fluctuations occurred at 20 mg/kg bw per day. Vomiting
was seen occasionally at doses > 10 mg/kg bw per day. Dogs at the
high dose (30 or 20 mg/kg bw per day) had loose stools and increased
serum alanine and aspartate aminotransferase activities. No
haematological, ophthalmological, or urinary changes were observed,
and no gross pathological or organ weight changes were noted post
mortem. Histopathological examination revealed no treatment-related
effects. No NOEL could be identified in this study owing to the
finding of an increased frequency of liquid faeces at all doses. The
LOEL was 2.5 mg/kg bw per day (Chesterman et al., 1983).
In a 26-week study that complied with GLP, groups of six male and
six female beagles were given cyhalothrin (purity unspecified)
dissolved in corn oil inside a gelatin capsule at a dose of 0, 1.0,
2.5, or 10 mg/kg bw per day. The dogs were examined
ophthalmoscopically before dosing and during weeks 6, 12, and 24 of
treatment. Blood and urine samples were taken every 4 weeks. The
functional integrity of the nervous system was assessed clinically
before treatment and during week 6. At the end of the treatment
period, full necropsies were performed on all dogs, and a large
selection of organs and tissues was examined histologically.
No deaths occurred during the study. A dose-related increase in
the incidence of liquid faeces affected all treated groups. At 10
mg/kg bw per day, the clinical signs seen included vomiting,
salivation, incoordination, unsteadiness, collapse, muscular spasms,
and convulsions. Body weight was unaffected by treatment.
Ophthalmoscopic and neurological examinations revealed no adverse
effects, and no consistent haematological, serum biochemical, or
urinary effects were seen. No treatment-related gross effects on organ
weights or appearance were noted post mortem, and histopathological
examination revealed no treatment-related effects. No NOEL could be
identified in this study owing to the finding of an increased
frequency of liquid faeces at all doses. The LOEL was 1.0 mg/kg bw per
day (Chesterman et al., 1981).
In a 52-week study, groups of six dogs of each sex were given
lambda-cyhalothrin at a dose of 0, 0.1, 0.5, or 3.5 mg/kg bw per day
orally in corn oil, but there was lack of agreement between summaries
of the study about whether the animals were treated by gelatin capsule
(European Medicines Evaluation Agency, 1999) or by gavage (WHO, 1990).
The report was not available to the Committee. Clinical signs of
neurological effects (muscular trembling, unsteadiness, and vomiting)
were seen in all dogs at the highest dose, but the signs were not
accompanied by histological changes in the nervous system. The
European Medicines Evaluation Agency (1999) reported a dose-related
increased incidence of liquid faeces at all doses, but WHO (1990)
reported that this effect was seen only at 0.5 and 3.5 mg/kg bw per
day and that the increased incidence was only slight at 0.5 mg/kg bw
per day. The incidence of liquid faeces was considered not to be
toxicologically significant. The NOEL was 0.5 mg/kg bw per day on the
basis of neurological signs seen at 3.5 mg/kg bw per day. It is
unclear how this dose relates to a dose of cyhalothrin (WHO, 1990;
European Medicines Evaluation Agency, 1999).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study that complied with GLP, groups of 52 male and 52
female CD-1 mice were given diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 20, 100, or
500 mg/kg for 104 weeks, equal to 0, 1.8, 9.2, and 53 mg/kg bw per day
for males and 0, 1.8, 11, and 51 mg/kg bw per day for females.
Satellite groups of 12 mice of each sex were given the same diets for
52 weeks and then killed.
The signs of toxicity observed in animals given high doses were
piloerection and hunched posture in mice of each sex given 500 mg/kg
of diet and in males given 100 mg/kg of diet. Males at 500 mg/kg of
diet also showed emaciation, pallor, hyperactivity, and increased
fighting activity. The mortality rate was unaffected by treatment.
Decreased body-weight gain was seen in males at 500 mg/kg of diet
throughout the study (two-sided Student t test: p < 0.05),
particularly during the first 13 weeks ( p < 0.001). Reduced feed
use efficiency was seen in the same group over the first 24 weeks of
the study (not calculated for the rest of the study). Water
consumption was also slightly increased in males at 500 mg/kg of diet
(two-sided Student t test: p < 0.05).
The results of haematological tests were unremarkable.
Statistically significant changes in various parameters were seen at
various times, but the effects were not consistent or dose-related.
Urinary analysis revealed no significant differences from control
values. The serum glucose concentration was slightly reduced
(two-sided Student t test: p < 0.05) in animals given 500 mg/kg
of diet. During week 100, serum alanine and aspartate aminotransferase
activities were increased in a dose-related manner, with occasional
significance ( p < 0.05) for both activities in mice of each sex at
100 and 500 mg/kg of diet and for alanine aminotransferase activity in
females at 20 mg/kg of diet.
Gross examination post mortem revealed increased incidences
(not dose-related) of thickening of the forestomach in treated
females. The ovarian weights were significantly (two-sided Student
t test: p < 0.05) decreased at all doses in animals in the main
group but not in the satellite groups. The effect was not
dose-related. Other organ weights were unaffected. Histopathological
examination revealed a variety of non-neoplastic lesions, but none was
considered to be due to treatment with cyhalothrin.
A non-dose-related increase in the incidence of mammary
adenocarcino-mas was seen in females in the main group, the incidences
being 1/52 in controls, 0/52 at 20 mg/kg of diet, 7/52 at 100 mg/kg of
diet, and 6/52 at 500 mg/kg of diet. (The detailed statistical
analysis of these results was given in an addendum to the report that
was not available to the Committee.) The incidence of mammary
adenocinoma in control females in 17 studies performed by the same
laboratory between 1980 and 1982 ranged from 2% to 12%. No
preneoplastic changes were found in the mammary glands of mice in the
main or satellite groups. A re-evaluation of mammary tissues from all
animals confirmed the original diagnoses of mammary neoplasia. As the
highest incidence of mammary adenocarcinoma in any group was only
slightly above the range of historical controls and as there was no
dose-response relationship, it was concluded that treatment with
cyhalothrin was unlikely to have caused these tumours. The NOEL was 20
mg/kg of diet, equal to 1.8 mg/kg bw per day, on the basis of clinical
signs of toxicity (piloerection and hunched posture) in males at
higher doses (Colley et al., 1984).
Rats
In a study that complied with GLP, groups of 72 male and 72
female Alpk/AP SPF rats were fed diets containing technical-grade
cyhalothrin (purity, 89.25%; containing 92.2% pyrethroids of which
96.8% was cyhalothrin) at a concentration of 0, 10, 50, or 250 mg/kg
for 104 weeks, providing mean doses of 0, 0.47, 2.3, and 12 mg/kg bw
per day for males and 0, 0.55, 2.7, and 14 mg/kg bw per day for
females. Satellite groups of 10 rats of each sex were given the same
diets but were killed after only 52 weeks. Blood was taken for
haematological measurements from 10 rats of each sex in each group at
4 and 13 weeks and thereafter at 13-week intervals. Blood was taken
for biochemical analyses and urine for urinary analyses from 12 rats
of each sex in each group at the same intervals. In weeks 52 and 103,
ophthalmoscopy was performed on 20 males and 20 females from each
group.
No clinical signs were seen that could be attributed to
treatment. The mortality rate was unaffected. Body-weight gain was
suppressed in both males and females given 250 mg/kg of diet
(two-sided Student t test: p < 0.05), and the males in this group
had increased food use efficiency. No consistent, dose-related
haematological effects were seen, although changes in various
parameters occasionally achieved statistical significance (two-sided
Student t test: p < 0.05). The volume of urine produced by rats
fed 250 mg/kg of diet tended to be decreased, but otherwise there was
no effect on urinary parameters. Ophthalmoscopy revealed no
treatment-related effects. Blood biochemistry showed intermittent
effects in rats at 250 mg/kg of diet, consisting of reduced plasma
concentrations of glucose and triglycerides, reduced plasma alkaline
phosphatase activity, and increased plasma urea concentration
(two-sided Student t test: p < 0.05). The reduction in plasma
triglycerides was the most marked finding, especially in females.
Long, pointed fibres, thought to originate from the diet, were
found embedded in the tissues of the palate, nasal turbinates, roof of
the nasal cavity, and peridontal space. The presence of the fibres
suggested that the lesions were caused by penetration of the tissue by
these fibres rather than by cyhalothrin. No treatment-related gross
lesions were detected post mortem, but several rats in all groups,
including controls, had rhinitis and oral lesions (oral granuloma
formation and erosion of the palate) in response to the presence of
the fibres in the diet. At the interim kill, the weight of the liver
(relative to body weight) was increased in rats of each sex given 250
mg/kg of diet, but this effect was not seen in rats killed at 104
weeks. The weight of the adrenals (relative to body weight) was
increased in females at 50 and 250 mg/kg of diet killed at 104 weeks.
Histopathological examination revealed no treatment-related effect on
the incidence or severity of any neoplastic or non-neoplastic lesions.
The NOEL was 50 mg/kg of diet, equal to 2.3 mg/kg bw per day (Pigott
et al., 1984).
2.2.4 Genotoxicity
Cyhalothrin was tested in assays for gene mutation in bacteria,
cytogenicity in rats in vivo, dominant lethal mutation in mice, and
cell transformation. The results of all the assays were negative. The
assays did not comply with GLP, but they appeared to be of good
quality and suitable quality assurance records had been maintained.
The studies are summarized in Table 4. The material tested by Trueman
(1981) was analysed by reversed-phase HPLC and found to have a
cis:trans ratio of 97.1:2.9.
lambda-Cyhalothrin was reported to be non-genotoxic when tested
for reverse mutation in bacteria, gene mutation in a mouse lymphoma
cell line, cytogenicity in vitro, unscheduled DNA damage in
vitro, and micronucleus formation in mice (WHO, 1990). Full reports
of these assays were not available, but the available details are
given in Table 5. lambda-Cyhalothrin produced micronuclei in an assay
in fish (Campana et al., 1999), but the relevance to human health of a
positive result in this unvalidated assay is not known.
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Mice
Only a brief account of a three-generation study of the effects
of cyhalothrin on reproduction in mice has been reported. It is
unclear whether the study was performed in accordance with the
principles of GLP. Groups of albino Swiss mice were given oral doses
of '0 (blank control), 2.5 ppm and 5.0 ppm cyhalothrin/kg body weight
daily' (the meaning of the term 'ppm cyhalothrin/kg body weight' is
unclear). The F0 generation consisted of groups of four males and
eight females. No further details of the protocol were available. No
signs of toxicity or behavioural changes were observed in the parents
or offspring for up to three generations. Maternal body weights were
not affected, although F2 generation dams at the low dose had
increased food intake. The weights of pups of F2 dams treated at the
high dose were reported to be significantly different from those of
control pups. Male and female fertility indices, pup live birth and
survival indices, litter size, and sex ratio were unaffected. The
meaning of the results was unclear. No NOEL could be identified
(Deshmukh, 1992).
Table 4. Assays for genotoxicity with cyhalothrin
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse S. typhimurium 0.16-2500 µg/ 90.2 Negativea Trueman (1981)
mutation TA1535, TA1537, plate ± S9
TA1538, TA98,
TA100
Cell Syrian hamster 50-1000 µg/ml NR Negativeb Richold et al.
transformation - S9 (1981)
1000-5000 µg/ml
+ S9
In vivo
Cytogenicity Bone marrow, Single oral dose 89.2 Negativec Anderson et al.
Wistar-derived of 1.5, 7.5,15 mg/ (1983)
male rats kg bw in corn oil
5 daily oral doses
of 1.5, 7.5, 15 mg/
kg bw per day
Dominant Male CD1 mice 1, 5, 10 mg/kg bw/ 89.2 Negatived Irvine (1981b)
lethal day for 5 days by
mutation oral gavage
in corn oil
a 2-Aminoanthracene, 9-aminoacridine, 4-nitroquinoline- N-oxide, and
N-methyl- N'-nitro- N-nitrosoguanidine used as positive controls
b 4-Nitroquinoline- N-oxide and para-dimethylaminoazobenzene (butter yellow) used
as positive controls
c Ethyl methanesulphonate used as a positive control
d Cyclophosphamide used as a positive control
Table 5. Assays for genotoxicity with lambda-cyhalothrin
End-point Test object Concentration Results Reference
In vitro
Reverse S. typhimurium < 5000 µg/plate Negative IPCS (1990)
mutation (strains not ± S9
specified)
Gene mutation L51787 mouse 125-4000 µg/ml Negative IPCS (1990)
lymphoma cell ± S9
line
Unscheduled Cultured human NR Negative IPCS (1990)
DNA synthesis HeLa cells
Cytogenicity Human lymphocytes NR Negative IPCS (1990)
In vivo
Micronucleus Erythrocytes of 0.001-0.05 µg/L Positive Campana et al.
formation Cheirodon interruptus (1999)
interruptus
Micronucleus Mice < 35 mg/kg bw Negative IPCS (1990)
formation (route not
specified)a
a Cyclophosphamide used as a positive control
Rats
Throughout a GLP-compliant three-generation (two litters per
generation) study, groups of SPF Alpk/AP Wistar-derived rats were
given diets containing technical-grade cyhalothrin (purity, 89.25%;
containing 92.2% pyrethroids of which 96.8% was cyhalothrin) at a
concentration of 0, 10, 30, or 100 mg/kg, providing mean doses of 0,
0.6, 1.7, and 5.6 mg/kg bw per day for males and 0, 0.7, 1.9, and 6.1
mg/kg bw per day for females. The F0 generation consisted of groups
of 15 male and 30 female rats. After 12 weeks on the test diets, these
animals were mated within their groups to produce a first litter
(F1a generation). Later, they were mated again to produce a second
litter (F1b generation). Breeding was repeated with F1 parents (15
males and 30 females per group) selected from the F1b animals and
F2 parents (15 males and 30 females per group) selected from the
F2b animals. At day 36 post partum, all pups not selected as the
next parental generation were killed and examined externally.
Approximately half of these were examined by autopsy, with
histopathological examination of any grossly abnormal tissues. A
fuller post-mortem examination with histopathology of all major organs
was performed on five males and five females from each group of the
F1b and F2b generations and 10 males and 10 females from each of
the F3b groups.
No clinical signs of toxicity were found. Minor reductions in
body-weight gain were seen in the premating period of the F0
generation of animals of each sex at 100 mg/kg of diet and
occasionally in females given 30 mg/kg of diet. At other times and in
other generations, occasional reductions in body-weight gain were seen
in rats given cyhalothrin at 30 or 100 mg/kg of diet. Male fertility
was unaffected by treatment. The fertility of F1 females at 30 mg/kg
of diet was statistically significantly (one-sided t test:
p < 0.05) reduced but remained within the range of control values.
At 100 mg/kg, the litter size of F2 and F3 pups was reduced, the
effect being statistically significant (one-sided t test:
p < 0.05) for the F2a and F3b litters. Small but statistically
significant decreases (one-sided t test: p < 0.05) were seen in
the percentages of live pups born to animals at 10 mg/kg in the F1b
generation and to dams at 30 and 100 mg/kg of diet in the F3b
generation. Post-mortem and histopathological examinations revealed no
adverse effects that could be attributed to the treatment. The
occasional effects seen at 10 and 30 mg/kg of diet were considered to
be incidental, and only the effects at 100 mg/kg of diet were regarded
as treatment-related. The NOEL was 30 mg/kg of diet, equal to 1.7
mg/kg bw per day, on the basis of reduced parental body-weight gain
and reduced litter size (Milburn et al., 1984).
(b) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley-derived rats (CD rats from
Charles River's SPF colony) were given technical-grade cyhalothrin
(purity, 89.25%; containing 92.2% pyrethroids of which 96.8% was
cyhalothrin) at a dose of 0, 5, 10, or 15 mg/kg bw per day by oral
gavage in corn oil on days 6-15 of gestation. The dams were killed on
day 20, and the contents of their uteri were examined. No certificate
of compliance with GLP was supplied, but suitable quality assurance
records had been kept.
Body-weight loss, reduced food intake, and signs of neurotoxicity
(loss of limb coordination in 2 out of 24 rats) were observed in dams
at the highest dose. The numbers of pregnant animals, pre-implantation
and post-implantation losses, mean litter weights, and mean weights
and lengths of fetuses were unaffected by treatment. Abnormalities
were seen in one litter of a dam at 10 mg/kg bw per day, in which 5 of
17 fetuses had major defects: four had bilateral agenesis of the
kidneys, and three had skeletal malformations of the vertebral
centrae, sternebrae, and/or metacarpals. The effects were considered
to be incidental and unlikely to be due to treatment. The NOEL was 10
mg/kg bw per day on the basis of maternal toxicity (Killick, 1981b).
Six pregnant Wistar-derived rats were given cyhalothrin (purity,
95.8%) by dermal application throughout the 21 days of gestation at a
daily dose of 1 ml of 0.02% (w/v; equal to approximately 0.8 mg/kg bw
per day if completely absorbed) in an aqueous vehicle applied to a
shaved area of the back. Controls were given the vehicle alone. After
parturition, six pups were left with each dam. At 90 days of age, 12
males in the treated group were tested for mating behaviour with
ovariectomized females that were showing estrus induced by
subcutaneous injections of 17ß-estradiol and progesterone. No
certificate of compliance with GLP was supplied for this study.
Treated male pups showed a delay in testicular descent, but the age of
vaginal opening was not affected. The mating behaviour of the males
was not affected by treatment. No NOEL could be identified (da Silva
Gomes et al., 1991a).
In another study which did not appear to conform to GLP, 12
pregnant Wistar-derived rats were given cyhalothrin (purity
unspecified) by dermal application throughout the 21 days of gestation
at a daily dose of 1 ml of 0.018% (w/v; equal to approximately 0.72
mg/kg bw per day if completely absorbed) in an aqueous vehicle. At
weaning (21 days) and at 90 days of age, the pups were tested for
locomotor activity in an open field. At 90 days, training began for
inhibitory avoidance behaviour. Adult males were also tested for
motivational response in a 'hole board' test.
The opening of ears and eyes, the development of fur, and the
descent of testes were all delayed in treated rats, but the time of
vaginal opening was not affected. The body weights of the pups of
cyhalothrin-treated dams were statistically significantly greater
(Student t test: p < 0.05) than those of controls at days 2, 7,
and 14 of age but not at day 21. Locomotor frequency was increased at
21 days and decreased at 90 days, but the effects were not
statistically significant in the Student t test. No effect was seen
on inhibitory avoidance behaviour. The results of the hole board test
showed a decreased motivational response in cyhalothrin-exposed rats.
No NOEL could be identified (da Silva Gomes et al., 1991b).
Rabbits
Groups of 20 pregnant New Zealand white rabbits were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) on days 6-18 of gestation
at a dose of 0, 3, 10, or 30 mg/kg bw per day by oral gavage in corn
oil. The animals were killed and examined on day 28 of gestation. No
certificate of compliance with GLP was supplied, but suitable quality
assurance records had been kept.
The highest dose was toxic to the dams, as indicated by an
initial body-weight loss followed by a decreased rate of body-weight
gain. No treatment-related deaths, clinical signs, or gross
pathological changes were seen in any group of does. There was no
effect on the incidence of pregnancy or on gravid uterine weight. The
mean weight of the fetuses of dams at 30 mg/kg bw per day was slightly
decreased, but the effect was not statistically significant (Wilcoxon
test). Mean fetal crown-rump length, sex ratio, and the numbers of
corpora lutea, intrauterine deaths, and implantations were unchanged
by treatment. There was no increased incidence of any type of fetal
abnormality in any treated group. The NOEL for maternal toxicity was
10 mg/kg bw per day (Killick, 1981c).
2.2.6 Special studies
(a) Neurotoxicity
Rats
Groups of rats were given single oral or intraperitoneal doses of
25-200 mg/kg bw 'cyhalothrin', referred to as 'PP 321, trade mark
Karate', which are terms associated with lambda-cyhalothrin rather
than cyhalothrin. Its purity was not reported. It was suspended as an
emulsion in 'solvents and surface active agents'. After treatment, the
behaviour of the animals in the open field was monitored, and their
electroencephalograms were measured while they were moving freely. No
further details of the protocol were reported. Treated animals were
found to have limited mobility as a consequence of discoordination of
the limbs. The reported signs of toxicity were respiratory
difficulties, tremor, salivation, piloerection, increased reactivity
to noise and during handling, with vocalization. Analysis of the
electroencephalograms showed 'changes in amplitudes (depressions in
majority of cases), in frequency composition (relative increases of
beta activity) and in repetition of cycles of high and low
amplitudes'. The results for individual groups were not presented. No
NOEL could be identified (Zufan et al., 1989).
Hens
In a study that complied with GLP, groups of 10 hens were given
cyhalothrin (purity, 93.4%) as a single dose of 2500, 5000, or 10 000
(two groups) mg/kg bw by oral gavage in corn oil. Negative controls
were given corn oil alone; positive controls received 500 mg/kg bw of
tri- ortho-cresyl phosphate. The hens were observed for 21 days after
dosing. The only hens that died during this period were two given
10 000 mg/kg bw, one in the negative and one in the positive control
group. The mean body weights of hens in all groups given cyhalothrin
and the positive controls were reduced. Clinical signs of toxicity
(ataxia) were seen only in the positive controls. The only
treatment-related gross change seen post mortem was muscular atrophy
in two of the positive control birds. Histopathological examination of
the cervical cranial, cervical caudal, thoracic, and lumbar spinal
cord and the proximal and distal sciatic nerve showed morphological
evidence of axonal damage at all levels of the spinal cord in all the
positive control birds. There were no consistent or dose-related
changes in the nerves of any of the groups given cyhalothrin. The NOEL
was 10 000 mg/kg bw, the highest dose tested (Roberts et al., 1982).
(b) Neurobehavioural effects
Inclined plane test
In a preliminary GLP-compliant study, cyhalothrin was
administered by oral gavage in corn oil to groups of Crl:CD.BR strain
rats of each sex. Initially, single doses of 50, 100, or 200 mg/kg bw
were given to groups of two males and doses of 25, 40, or 75 mg/kg bw
to groups of two females. Later, groups of three rats of each sex were
given a single dose of 15, 30, or 60 mg/kg bw. Controls received corn
oil only. The cyhalothrin contained A and B isomer pairs in a 60:40
ratio and was 95.8% pure, with a total pyrethroid content of 96.4%.
The other isomers measured were cis B' (0.2%), trans D and cis
A' (0.4%), and trans C (< 0.1%). The contaminants included
phenoxybenzalde-hyde (0.2%) and
(Z)3-(2-chloro-3,3,3-trifluoro-1-enyl)-2,2-dimethylcyclopro-pane
(0.1%). One hour before dosing and again at 30 min, 2 h, 7 h, and 24 h
after dosing, each rat was placed on a smooth plane with an angle of
inclination that was gradually increased until the rat could no longer
maintain its position. For each rat, three measurements were made at
each time. In the inclined plane test, any decrease in the angle at
which the rat loses its position (mean slip angle) is regarded as a
reflection of subtle changes in neuromuscular function.
Clinical signs of toxicity were seen at all doses tested. At 15
mg/kg bw, the only clinical sign was soft faeces in females. At 25-40
mg/kg bw, ungroomed appearance, soft faeces, and piloerection were
seen. At 50-75 mg/kg bw, there were marked clinical signs, including
ataxia, writhing, emprosthotonos, high stepping or splayed gait,
hunched posture, stained snout, salivation, and wasted appearance. At
100 mg/kg bw, the signs also included vocalization and closure of the
eyelids. Rats given 60 or 200 mg/kg bw were killed on humane grounds
after the inclined plane measurements had been made. Only one rat (a
female at 60 mg/kg bw) died during the study, but all rats given 60 or
200 mg/kg bw were killed prematurely on humane grounds. Body-weight
gains were decreased at doses > 50 mg/kg bw. Necropsy of the
animals showed no consistent gross pathological changes. The result of
the inclined plane test was uninterpretable, as the clinical signs
interfered with the conduct of the study: convulsing rats were unable
to stay on the plane, and rats with soiled fur adhered to it (Denton,
1988).
Acute startle response
A preliminary study was performed in two phases with groups of
three male Wistar-derived Alpk:ApfSD rats. The purity of the
cyhalothrin tested was 97.2%. In phase 1, rats were given a single
oral dose of 0, 25, 50, or 75 mg/kg bw of cyhalothrin in corn oil, and
auditory habituation tests were performed 1, 2, and 3 h after dosing.
Clinical signs of toxicity were seen on day 2 after dosing (the time
of dosing being the start of day 1) but not on day 1 or day 3, in the
group given 75 mg/kg bw. The signs included ataxia, subdued behaviour,
body-weight loss, and stained fur. Rats at this dose had a
statistically nonsignificant increase in mean response amplitude
(two-sided Student t test) but a significant ( p < 0.05) increase
in the time to maximum amplitude 3 h after dosing.
In phase 2, rats were given a single oral dose of 0, 75, or 100
mg/kg bw of cyhalothrin in corn oil, and auditory habituation tests
were performed on separate groups of animals 3, 5, or 7 h after
dosing. The signs of toxicity were similar to those seen in phase 1.
The clearest results in the auditory startle habituation test were
obtained at 7 h: with both doses tested, the mean response amplitude
was consistently statistically significantly ( p < 0.05) reduced and
the time to maximum amplitude was reduced (Brammer, 1998).
In the main study, groups of 10 male and 10 female Wistar-derived
Alpk:ApfSD rats were given a single dose of 0, 5, 15, or 75 mg/kg bw
of cyhalothrin (purity, 97.2%) by oral gavage in corn oil. An auditory
startle habituation test was performed 7 h after dosing on day 1 and
on day 8 with an automated recording apparatus. The animals were
killed after 8 days but were not examined post mortem. The study was
performed in accordance with GLP.
Body-weight gain was reduced in males at the highest dose and in
females at the two lower doses. Transient clinical signs of toxicity
were seen at the highest dose. Those seen at day 2 included subdued
behaviour, ataxia, high-stepping gait, salivation, and tip-toe gait,
and 'some of these signs' were also seen 7 h after dosing on day 1.
There were no signs of toxicity from day 3 until the end of the study.
In the startle response test, there was no effect on time to
maximum amplitude in either sex on day 1 or day 8. A reduction in the
mean response amplitude was seen, however, on day 1 in animals of each
sex at the highest dose. Statistically significant (two-sided Student
t test: p < 0.05), but not dose-related, reductions in mean
response amplitude were also seen on day 1 in females at all doses. No
effects were seen on day 8.
The authors reported that the NOEL for this study was 15 mg/kg
bw, as they dismissed the effects on response amplitude because of the
absence of a clear dose-response relationship. The Committee disagreed
with this conclusion. It has been reported that type II pyrethroids
generally decrease rather than increase the startle response to sound,
although this is a complex response and at low doses some type II
pyrethroids give an increased startle response (Hijzen & Slangen,
1988; Ray, 1991). Thus, although there was a NOEL for a reduced
response, lower doses may have caused an increased response, which
would indicate a lower NOEL. The Committee also noted that the startle
response was not evaluated at the most sensitive time, i.e. the time
of peak expression of acute toxicity as shown by clinical signs. No
NOEL could be identified (Brammer, 1999).
Inhibitory avoidance test
Newborn Wistar rat pups were exposed to cyhalothrin (purity
unspecified) in their mother's milk from birth until weaning at 21
days of age, and at 97, 104, and 111 days of age they were tested in
inhibitory avoidance tests. Cyhalothrin was given to nursing dams in
their drinking-water as a 200-mg/L solution in 400 mg/L sucrose. This
would be expected to provide a dose of cyhalothrin of approximately 20
mg/kg bw per day to the dams (assuming that 200-g rats drink about 20
ml/day). The intake of the pups was not measured, but the treated
groups consisted of 20 pups and the control group of 15. Control dams
were given sucrose solution at 400 mg/L. Drink was available
ad libidum to eight cyhalothrin-exposed dams and six control dams.
No clinical signs of toxicity were reported in either the dams or
the pups. The body weights at weaning and at 90 days of age were not
affected. The behaviour of the dams was unaffected by treatment
throughout the 21 days of lactation. The motor activity of the pups at
weaning was unaffected by the treatment. At 90 days, eight pups
exposed to cyhalothrin and 11 control pups were chosen (all the males)
for training and testing in a shuttle box to evaluate inhibitory
avoidance behaviour. Each rat was trained once by placing it in a
well-lit box and then opening a door to a dark compartment and giving
the rat an electric shock to the foot when it entered the dark
compartment. The test is a measure of the time taken by the rats to
cross again from the well-lit area to the dark compartment. Each rat
was tested once at 97, 104, and 111 days of age, receiving no electric
shock. At all times, there was a reduction in the learning avoidance
latency of the treated group, but the effect was statistically
significant (Mann-Whitney U-test: p < 0.05) only at 97 and 104 days
of age and not at 111 days. No NOEL could be identified (Moniz et al.,
1990).
In a separate study, described above, rats born to dams treated
dermally with an aqueous solution of cyhalothrin were tested for
behavioural changes. Inhibitory avoidance behaviour was unaffected by
the treatment, and there was no consistent effect on locomotor
frequency. The animals did, however, show a decreased motivational
response when they were tested as adults in a hole board test. No NOEL
could be identified for this study because of the production of subtle
but persistent neurobehavioural effects at the only dose tested. It
was also difficult to estimate the amount of cyhalothrin received by
the offspring (da Silva Gomes et al., 1991b).
2.2.7 Immunotoxicity
Some pyrethroids have been reported to be potent allergens that
may cause allergic rhinitis, asthma, or allergic alveolitis (Marrs,
1993). Cyhalothrin has not been specifically tested for immunotoxicity
but in a study of toxicity in mice given repeated doses, decreased
total leukocyte counts, decreased lymphocyte counts, and increased
neutrophil counts were observed in male but not female mice that had
consumed feed containing cyhalothrin for 28 days (Colley et al.,
1981). In the same study, atrophy of the red pulp of the spleen
occurred in some females given the high dose. Toxicological studies in
other species did not show alterations in total or differential
leukocyte counts and showed no histopathological changes in most
organs potentially involved in immunity (thymus, spleen, lymph nodes,
bone marrow), as described above.
A Magnusson and Kligman maximization test and a Buehler test both
showed that cyhalothrin is a skin sensitizer in guinea-pigs (European
Medicines Evaluation Agency, 1999; WHO, 1990). A Magnusson and Kligman
maximization test with lambda-cyhalothrin, however, showed no skin
sensitization in guinea-pigs (WHO, 1990).
2.3 Observations in humans
Cases of severe poisoning with pyrethroids are rare, but some
cases, for example in China, have been reported, including a few
deaths. Absorbed pyrethroids are rapidly detoxified by esterases, so
that any systemic effects would be of short duration (Marrs, 1993).
Topical application of pyrethrins or pyrethroids to the skin
produces local effects unrelated to their systemic action. The
synthetic pyrethroids have a characteristic irritating effect that is
not associated with inflammation and appears to be unique to
pyrethroids. The initial lesions are tenacious, painful pruritus
(pricking sensation) followed by a local burning sensation with
blotchy erythema that lasts about 2 days after cessation of exposure;
when exposure is intense, it is associated with numbness. Later,
desquamation may occur on the contaminated area of skin. There appear
to be no lasting ill effects. The condition is produced by all classes
of pyrethroids although most readily by type II pyrethroids such as
cyhalothrin. Repeated exposure to systemically toxic doses of
pyrethroids can produce a different effect: peripheral nerve damage
can occur subsequent to severe motor symptoms (Aldridge, 1990; Ray,
1991)
No clinical or haematological effects were observed in six
volunteers given a single dose of 5 mg of lambda-cyhalothrin in corn
oil (equal to 0.05-0.07 mg/kg bw) (European Medicines Evaluation
Agency, 1999). The route of administration was not reported, but it
seems likely to have been oral.
In the study of Pakistani pesticide workers described in section
2.2, the average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). Transient signs of toxicity, lasting up to 24
h, were reported by the workers, which included skin paraesthesia,
feeling hot, feeling cold, numbness, irritation of the skin, red eyes,
coughing, and sneezing. Medical examination revealed one case of face
rash that lasted 2 days (Chester et al., 1992).
In a study carried out in a village in the United Republic of
Tanzania, a lambda-cyhalothrin-based insecticide was sprayed inside
houses and shelters at a coverage of approximately 25 mg/m2. The
insecticide was supplied as a water-dispersible powder in a soluble
sachet. Every day for 6 days, 12 spraymen and 3 squad leaders were
interviewed about symptoms. Each sprayman used up to 62 g of
lambda-cyhalothrin over 2.7-5.1 h each day. The spraymen wore personal
protective equipment (rubber boots and gloves, cotton overalls, caps,
and gauze nose-mouth masks) which left much of the face exposed. One
sprayman also used a face shield for 3 days.
All the spraymen complained at least once of symptoms related to
exposure to lambda-cyhalothrin. The commonest symptoms were itching
and burning of the face and nose and throat irritation, frequently
accompanied by sneezing or coughing. Facial symptoms occurred only on
unprotected areas, and the worker who wore a face shield was free of
facial symptoms. All the symptoms had disappeared by the morning after
the spraying. The number of subjects affected and the duration of
facial symptoms were proportional to the amount of compound sprayed.
These parameters were not affected by use of lambda-cyhalothrin in the
previous 6 months. A sample of occupants was interviewed 1 and 5-6
days after their houses had been sprayed. One woman who entered her
house 30 min after the end of spraying complained of periorbicular
itching, but this lasted only a few minutes. The other inhabitants of
sprayed houses reported no other insecticide-related adverse effects.
Furthermore, a squad leader who entered almost every house a few
minutes after spraying reported no symptoms (Moretto, 1991)
As part of a field trial conducted in South India,
electrophysiological tests were conducted on 15 spraymen aged 19-48
years, before and after exposure to lambda-cyhalothrin (formulation
and purity unspecified). The tests performed on the subjects comprised
conduction of the right median, common peroneal, and facial motor
nerves; conduction of the right median and sural sensory nerves; blink
response with stimulation of the right supra-orbital nerve and
recording of R1 and R2 responses from the right orbicularis oculi
muscle with a pair of surface electrodes; concentric needle
electromyography of the tibialis anterior; repetitive stimulation of
the right median nerve at the wrist at 3 and 20 Hz and recording of
the responses from the abductor pollicis brevis; and multi-modality
visual, brainstem, auditory and somatosensory evoked potentials. The
evoked potentials were measured in only six of the subjects, but the
other measurements were made in all 15 subjects.
Clinical observation revealed no changes, and facial nerve
conduction, blink response, responses to repetitive stimulation, and
visual, auditory, and somatosensory evoked potentials were all normal.
Six of the 15 subjects had mild changes in peripheral nerve conduction
parameters (paired t test: p < 0.05), but comparison of the mean
values for the various nerve conduction parameters before and after
exposure showed no significant difference except for prolongation of
distal motor latency of the median nerve. Studies of nerve conduction
12-16 months later in three subjects who had shown abnormalities
immediately after exposure showed normal rates. The authors concluded
that occupational exposure to lambda-cyhalothrin can produce
transient, subclinical electrophysiological changes in the nerves of
the upper limbs (Arunodaya et al., 1997).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity,
neurotoxicity, and neurobehavioural effects of cyhalothrin and
observations of effects in humans exposed to this compound. Although
some of the studies were not fully compliant with codes of GLP, all of
the pivotal studies were carried out according to appropriate
standards for study protocol and conduct.
Oral doses of cyhalothrin were readily but incompletely absorbed
in the species studied (rats and dogs), and the subsequent metabolism
was similar, involving initial cleavage of the molecule at the ester
bond, presumably resulting in detoxification. The metabolites were
rapidly excreted, some as conjugates, whereas small amounts of
unchanged cyhalothrin persisted as residues in fatty tissues. Similar
results were seen in food-producing animals.
Serum and urine from exposed workers contained the metabolites
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)benzoic acid. These metabolites are products of
cleavage of the cyhalothrin molecule at the ester bond, and their
presence suggests that the initial metabolism of this compound in
humans is similar to that in the animal species that have been
investigated.
No toxicological studies on metabolites of cyhalothrin were
available, but the Committee considered it likely that the metabolites
would be less toxic than cyhalothrin, as none contains an intact
pyrethroid structure.
The acute toxicity of cyhalothrin is characterized by effects on
the nervous system, principally the central nervous system. The signs
of toxicity are typical of type II pyrethroids and comprise writhing,
salivation, exaggerated jaw opening, increasing extensor tone in the
hind legs causing a rolling gait, poor coordination progressing to
coarse tremor, tonic seizures, and apnoea. The LD50 values after
oral administration depended on the vehicle used but varied from 37 to
62 mg/kg bw in mice and from 51 to 240 mg/kg bw in rats. Higher values
were found in other species or after administration by other routes.
In mice that received a dose of 0, 5, 10, 20, 40, or 80 mg/kg bw
per day orally for 5 days, ataxia and convulsions were seen in those
given the two higher doses. The dose of 20 mg/kg bw per day caused
body-weight loss, ataxia, and rough coat. At 5 mg/kg bw per day, the
only adverse effect was a rough coat.
In a 28-day study in mice, cyhalothrin was added to the diet at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of feed. Atrophy of
the red pulp of the spleen and an increased mortality rate were seen
at the highest dose. Adaptive liver changes, including enlarged liver,
centrilobular hepatocellular hypertrophy, increased activity of
aminopyrine- N-demethylase, and proliferation of smooth endoplasmic
reticulum were seen at concentrations of 100 mg/kg of feed and above;
lowered lymphocyte counts were also seen at these doses. At 25 mg/kg
of feed, the only effect seen was piloerection. The NOEL was 5 mg/kg
of feed, equal to 0.65 mg/kg bw per day, on the basis of piloerection
at higher doses.
The main effects in five 10-90-day studies of toxicity in rats
were adaptive changes in the liver similar to those seen in mice. In a
28-day range-finding study in which rats were fed diets containing
cyhalothrin at a concentration of 0, 5, 10, 20, or 250 mg/kg of feed,
only the highest concentration caused adverse effects. These effects
were characterized by hepatocellular hypertrophy, increased activity
of aminopyrine- N-demethylase in the liver, and proliferation of
hepatic smooth endoplasmic reticulum. The NOEL was 20 mg/kg of feed,
equivalent to 2 mg/kg bw per day. In a 28-day study in rats given 0,
1, 5, 10, 20, or 250 mg/kg of feed, the activity of hepatic
aminopyrine- N-demethylase was increased and proliferation of hepatic
smooth endoplasmic reticulum was seen at the highest dose only. No
such effect was seen in the liver at doses equivalent to 2 mg/kg bw
per day or less. Females given 10 mg/kg of feed or more had decreased
body-weight gain. The NOEL was 5 mg/kg of feed, equivalent to 0.5
mg/kg bw per day, but this observation was not in line with the
findings of other short-term studies of toxicity in rats. A third
28-day study in rats was inadequately reported but showed that
administration of cyhalothrin at 20 mg/kg of feed (equivalent to 2
mg/kg bw per day) caused proliferation of hepatic smooth endoplasmic
reticulum; a NOEL was not identified in this study.
A 90-day study was performed in which rats given cyhalothrin at
0, 10, 50, or 250 mg/kg of feed. Males given the two higher doses
showed increased activity of hepatic aminopyrine- N-demethylase and
proliferation of hepatic smooth endoplasmic reticulum. Females at the
highest dose showed increased activity of hepatic
aminopyrine- N-demethylase activity. The NOEL was 10 mg/kg of feed,
equal to 0.56 mg/kg bw per day.
Groups of dogs received cyhalothrin at a dose of 0, 2.5, 10, or
30 mg/kg bw per day for 4 weeks or a dose of 0, 1.0, 2.5, or 10 mg/kg
bw per day for 26 weeks. In the 4-week study, the two higher doses
caused an increased incidence of vomiting, and the highest dose caused
body-weight loss, unsteady gait, and increased serum alanine and
aspartate aminotransferase activities. Muscular trembling was seen in
dogs in all groups, including the controls, in the 4-week study. In
the 26-week study, doses of 10 mg/kg bw per day or more caused
clinical signs including vomiting, salivation, lack of coordination,
unsteadiness, collapse, muscular spasms, and convulsions. As treatment
at all doses in both studies increased the prevalence of liquid
faeces, a NOEL was not identified. The Committee considered it
possible that the liquid faeces were a consequence of the neurological
effects of cyhalothrin. The LOEL was 1.0 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in mice,
cyhalothrin was given in the diet at a concentration of 0, 20, 100, or
500 mg/kg of feed for 104 weeks. An increased incidence of mammary
adenocarcinoma was seen in females at the two higher doses. The
highest incidence (14%) was only slightly greater then the upper limit
of the range in historical controls (2-12%). The Committee could not
exclude the possibility that the adenocarcinomas seen in the groups
given 100 or 500 mg/kg of feed were caused by cyhalothrin. Clinical
signs of toxicity (piloerection and hunched posture) and increased
serum activities of aspartate and alanine aminotransferases were seen
at these doses. The NOEL for these effects was 20 mg/kg of feed, equal
to 1.9 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in rats,
cyhalothrin was given in the diet at a concentration of 0, 10, 50, or
250 mg/kg of feed for 104 weeks. There was no treatment-related
increase in the incidence of any type of tumour. Adverse effects found
at the highest dose included decreased body weight, altered blood
biochemistry, and an increased weight of the liver relative to that of
the body in animals of each sex and of the adrenals in females only.
The NOEL was 50 mg/kg of feed, equal to 2.3 mg/kg bw per day.
Cyhalothrin was not genotoxic in a range of studies, including a
test for reverse mutation in Salmonella, a test for cytogenetic
effects in the bone marrow of rats treated in vivo, a test for
dominant lethal mutation in mice, and an assay of cell transformation
in vitro. The Committee concluded that cyhalothrin is not genotoxic.
Furthermore, the Committee considered it likely that the mammary
adenocarcinomas found in mice in the long-term study were due to a
non-genotoxic mechanism.
A three-generation study of reproductive toxicity was performed
in rats given cyhalothrin in the diet at a concentration of 0, 10, 30,
or 100 mg/kg of feed. Adverse effects, including reduced parental
body-weight gain and reduced litter size, were found only at the
highest dose. The NOEL for these effects was 30 mg/kg of feed, equal
to 1.7 mg/kg bw per day.
In a study of developmental toxicity, rats received a dose of 0,
5, 10, or 15 mg/kg bw per day by oral gavage on days 6-15 of
gestation. Maternal toxicity, characterized by body-weight loss and
poor coordination, was seen at the highest dose. Embryotoxicity also
occurred at this dose. Abnormalities were seen in 5 of 17 fetuses in
one litter from a dam at 10 mg/kg bw per day, but the effect was
considered not to be due to treatment with cyhalothrin as no
fetotoxicity was seen at the higher dose. The NOEL for maternal
toxicity was 10 mg/kg bw per day, and that for developmental toxicity
was 15 mg/kg bw per day, the highest dose tested.
Two studies of developmental toxicity in rats treated by dermal
administration provided some evidence that cyhalothrin can delay fetal
development at doses lower than those given orally. However, the
Committee considered that oral administration is a more relevant route
and that the NOEL in the study in which this route was used was the
appropriate one for evaluating developmental toxicity in rats.
Rabbits studied for developmental toxicity were given a dose of
0, 3, 10, or 30 mg/kg bw per day by oral gavage. The highest dose
caused initial body-weight loss in the does, which was followed by
reduced body-weight gain. There was no significant effect on
development at any dose. The NOEL for maternal toxicity was 10 mg/kg
bw per day, and the NOEL for developmental toxicity was 30 mg/kg bw
per day, the highest dose tested.
Single oral doses of up to 10 000 mg/kg bw did not induce
clinical or histopathological signs of neurotoxicity in hens.
Various studies of neurobehavioural effects have been performed
in rats, including a range-finding test of performance on an inclined
plane, a test for acute auditory startle response, and tests of
inhibitory avoidance. In the inclined plane test, rats were given a
single oral dose of 0, 15, 25, 30, 40, 50, 60, 75, 100, or 200 mg/kg
bw. All of these doses caused soft faeces in at least some of the
rats, and doses of 25 mg/kg bw or more caused clinical signs of
neurotoxicity. The results were, however, variable, and no conclusion
could be reached about neurotoxicity.
In the test for acute auditory startle response in which an oral
dose of 0, 5, 15, or 75 mg/kg bw was given, the highest dose resulted
in reduced body-weight gain, transient clinical signs, and a reduced
mean response amplitude in animals of each sex. Statistically
significant but not dose-related reductions in mean response amplitude
were also seen at 5 and 15 mg/kg bw in females only. The Committee was
aware that a biphasic auditory startle response has been reported with
some other type II pyrethroids, with a reduced response at high doses
and an increased response at lower doses. A NOEL for the startle
response was not identified in this study.
In the inhibitory avoidance tests, rats were exposed either
in utero (dams were given 200 mg/l in drinking-water) or during
lactation (dams were given 1 ml of a 0.018% solution dermally). The
doses received by the animals could not be estimated reliably. The
rats exposed in utero showed a decreased motivational response when
tested as adults, but no effects were seen on inhibitory avoidance
behaviour or locomotor frequency. Animals exposed during lactation had
a shorter latency in learning avoidance behaviour. There was no NOEL
in either study, as adverse effects were seen at the only dose tested
in each study and the doses received by the animals were not known.
4. EVALUATION
Observations and case reports in humans provided little
information relevant to the establishment of an ADI for cyhalothrin.
In most instances, no information on doses was available, the route of
exposure was dermal, and some studies were of exposure to
lambda-cyhalothrin rather than cyhalothrin.
The Committee established a temporary ADI of 0-0.002 mg/kg bw for
cyhalothrin by applying a 500-fold safety factor to the LOEL of 1.0
mg/kg bw per day for induction of liquid faeces in dogs in the 26-week
study. The Committee considered it possible that the liquid faeces
were a consequence of the neurological effects of cyhalothrin. The
Committee applied the 500-fold safety factor to account for the
absence of a NOEL for liquid faeces in dogs and because of the absence
of a NOEL for neurobehavioural effects. The ADI was made temporary
because cyhalothrin belongs to a class of substances that are
characterized by their toxicity to the central nervous system, and
therefore neurobehavioural effects may be the most sensitive indicator
of the toxicity of this compound. There was an adequate margin of
safety between the ADI and the NOELs identified in the studies on
cyhalothrin, including the NOEL of 0.65 mg/kg bw per day for
piloerection in the 28-day study in mice.
The results of studies appropriate for establishing a NOEL for
neurobehavioural effects in laboratory animals are required for
evaluation in 2002.
The Committee noted that the NOEL for toxicological effects other
than the neurotoxicity related to the pyrethroid structure was 2.3
mg/kg bw per day for adaptive changes in the livers of rats in the
long-term study. This suggests that the toxicity of the metabolites
(none of which has a pyrethroid structure) is no greater than 50% of
that of the parent drug.
5. REFERENCES
Aldridge, W.N. (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit. Rev. Toxicol., 21,
89-103.
Anderson, D., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B. &
Godley, M.J. (1983) Cyhalothrin: A cytogenetic study in the rat.
Unpublished study, report No. CTL/P/664, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Arunodaya, G.R., Kumar, K.R. & Swamy, H.S. (1997) Electrophysiologic
study of spraymen exposed to insecticide lambdacyhalothrin.
Neurol. India, 45, 189-192.
Barnes, J.M. & Verschoyle, R.D. (1974) Toxicity of new pyrethroid
insecticides. Nature, 248, 711.
Bicknell, C.E., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Milk
residues of cyhalothrin following a 10 mL application of a 2%
pour-on. Unpublished study, report No. CIBH 89-1, Coopers Animal
Health, Berkhamsted, United Kingdom. Submitted by Schering-Plough
Animal Health, Harefield, United Kingdom.
Bloomquist, J.R., Adams, P.M. & Soderlund, D.M. (1986) Inhibition of
gamma-amino butyric acid-stimulated chloride flux in mouse brain
vesicles by polycycloalkane and pyrethroid insecticides.
Neurotoxicology, 7, 11-20.
Brammer, A. (1998) Cyhalothrin: Preliminary acute startle response
study in rats. Unpublished study, report No. CTL/L/8516, Zeneca
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Brammer, A. (1999) Cyhalothrin: Acute startle response study in rats.
Unpublished study, report No. CTL/P/6162, Zeneca Central
Toxicology Laboratory, Macclesfield, Cheshire, United Kingdom.
Campana, M.A., Panzeri, A.M., Moreno, V.J. & Dulout, F.N. (1999)
Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using
the micronucleus test in erythrocytes of the fish Cheirodon
interruptus interruptus. Mutat. Res., 438, 155-161.
Chester, G., Sabapathy, N.N. & Woollon, B.H. (1992) Exposure and
health assessment during application of lambda-cyhalothrin for
malaria vector control in Pakistan. Bull. World Health Organ.,
70, 615-619.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
Gopinath, C. & Prentice, D. (1981) Cyhalothrin. Oral toxicity
study in beagle dogs (Final report: repeated daily dosing for 26
weeks). Unpublished study, report No. ICI 326/8162, Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, United Kingdom.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Prentice, D.,
Buckley, P. & Offer, J. (1983) PP 563. Preliminary oral toxicity
study in beagle dogs. Unpublished study, report No. ICI 325/8074,
Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom.
Coats, J.R. (1990) Mechanisms of toxic action and structure
relationships for organochloride and synthetic pyrethroid
insecticides. Environ. Health Perspectives, 87, 255-262.
Colley, J.C., Loane, J.A., Heywood, R., Gibson, W.A., Chasseaud, L.F.,
Edmundson, N., Lewis, D. & Woodhouse, R.N. (1980) PP563. 28-day
dose range finding study in rats. Unpublished study, report No.
ICI/321/8041, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Down, W.H., Woodhouse, R.N.,
Gibson, W.A., Gopinath, C., Zubaidy, A.J. & Prentice, D.E. (1981)
Cyhalothrin. 4-week dose range finding study on mice. Unpublished
study, report No. ICI/379/80910, Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Almond, R., Gibson, W.A.,
Gregson, R. & Gopinath, C. (1984) Cyhalothrin. Potential
tumorigenic and toxic effects in prolonged dietary administration
to mice (Final report). Unpublished study, report No.
ICI/395/83668, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Denton, S. (1988) Cyhalothrin: Preliminary neurotoxicity study in the
rat following a single oral dose. Unpublished study, report No.
S8104-001R, Covance, Harrogate, United Kingdom.
Deshmukh, P.B. (1992) Three-generation reproductive studies of a
synthetic pyrethroid, cyhalothrin. Toxicol. Lett., 64/65,
770-781.
Dow, P.R. & Parker, R.C. (1989) Attempted identification of 3 major
liver metabolites following dermal application of C-14 alcohol
lambda-cyhalothrin to lactating cows. Unpublished study, report
No. CIBH 89-2, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Dow, P.R., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989)
Metabolism of C-14 alcohol labelled lambda-cyhalothrin in
lactating cows following dermal application. Unpublished study,
report No. CIBH 88-4, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Ebino, K., Suzuki, H. & Harada, T. (1984a) PP-563: Acute oral toxicity
in rats. Unpublished study, report No. 4/HD/006931, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984b) PP-563 WP: Acute oral
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984c) PP-563: Acute
intraperitoneal toxicity in rats. Unpublished study, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984d) PP-563: Acute dermal
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984e) PP-563: Acute subcutaneous
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
European Medicines Evaluation Agency (1999) Cyhalothrin. Summary
report of the Committee for Veterinary Medicinal Products,
EMEA/MRL/699/99. Internet:
http://web.is.eudra.org/vetdocs/vets/mrl.htm.
Gassner, B., Wuthrich, A., Scholtysik, G. & Solioz, M. (1997) The
pyrethroids permethrin and cyhalothrin are potent inhibitors of
the mitochondrial complex I. J. Pharmacol. Exp. Ther., 281,
855-860.
Harrison, M.P. (1981) Cyhalothrin: The disposition and metabolism of
14C-ICI 146,814 in rats. Unpublished study, report No. 146814
KMR 002/01, Imperial Chemical Industries plc, Macclesfield,
Cheshire, United Kingdom.
Harrison, M.P. (1983) Cyhalothrin:The metabolism and disposition of
14C-ICI 146,814 in the rat; Part IV: Isolation and
identification of the major urinary metabolites derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 following oral
administration. Unpublished study, report No. YIBH 85-C9,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984a) Cyhalothrin: The metabolism and disposition of
14C-ICI 146,814 in rats; Part II, tissue residues derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 after a single
oral dose of 1 or 25 mg/kg bw. Unpublished study, report No. KMR
002/02, Imperial Chemical Industries plc, Macclesfield, Cheshire,
United Kingdom.
Harrison, M.P. (1984b) Cyhalothrin (ICI 146,814): The metabolism and
disposition of ICI 146,814 in rats; Part III, Studies to
determine radioactive residues in rats following 14 days repeated
administration. Unpublished study, report No. KMR 002/03,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984c) Cyhalothrin (ICI 146,814): The disposition and
metabolism of ICI 146,814 in the dog. Unpublished study, report
No. 146814KMD 005, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Harrison, M.P. (1985) Cyhalothrin (ICI 146,814): The metabolism,
excretion and residues in the cow after 7 days oral
administration with two [14C]-labelled forms of ICI 146,814 at
1 mg/kg/day. Unpublished study, report No. YIBH 85-C11, Imperial
Chemical Industries plc, Macclesfield, Cheshire, United Kingdom.
Hijzen, T.H. & Slangen, J.L. (1988) Effects of type I and type II
pyrethroids on the startle response in rats. Toxicol. Lett.,
40, 141-152.
Irvine, L.F.H. (1981a) Cyhalothrin: Oral (gavage) range finding study
in the male mouse. Unpublished study, report No. 2647-72/212,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Irvine, L.F.H. (1981b) Cyhalothrin: Oral (gavage) dominant lethal
study in the male mouse. Unpublished study, report No.
2647-72/213, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
JMPR (Joint FAO/WHO Meeting on Pesticide Residues) (1985) Pesticide
residues in food - 1984. Report of the Joint Meeting on
Pesticide Residues. FAO Plant Production and Protection Paper
62, Rome: Food and Agriculture Organization of the United
Nations.
Jones, J.R. (1980) Cyhalothrin: Acute oral median lethal dose (LD50)
in the female rabbit. Unpublished study, report No. 2364-72/214,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1980) Cyhalothrin: Oral (gavage) maximum tolerated dose
study in the non-pregnant rat. Unpublished study, report No.
2537-72/206, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981a) Cyhalothrin: Oral (gavage) dose ranging study in
the pregnant New Zealand white rabbit. Unpublished study, report
No. 2603-72/210, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in
the rat. Unpublished study, report No. 2661-72, Hazleton
Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1981c) Cyhalothrin: Oral (gavage) teratology study in
the New Zealand white rabbit. Unpublished study, report No.
270072/211, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Metabolism of C-14
alcohol labelled lambdacyhalothrin in lactating cows following
dermal application. Unpublished study, report No. CIBH 88-2,
Coopers Animal Health, Berkhamsted, United Kingdom. Submitted by
Schering-Plough Animal Health, Harefield, United Kingdom.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationships. Pest. Biochem.
Physiol., 18, 9-14.
Leahey, J.P., French, D.A. & Heath, J. (1985) PP321: Metabolism in a
goat. Unpublished study, report No. RJ 0435B, Imperial Chemical
Industries, Plant Protection Division, Jealott's Hill Research
Station, Bracknell, Berkshire, United Kingdom.
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
I., Robinson, M. & Stonard, M. (1981) Cyhalothrin: 90-day feeding
study in rats. Unpublished report No. CTL/P/629, Imperial
Chemical Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Marrs, T.C. (1993) Toxicology of pesticides. In: Ballantyne, B.,
Marrs, T.C. & Turner, P., eds, General and Applied Toxicology,
Basingstoke:MacMillan, Vol. 2, Ch. 62, p. 1334.
Milburn, G.M., Banham, P., Godley, M.J., Pigott, G. & Robinson, M.
(1984) Cyhalothrin: Three-generation reproduction study in the
rat. Unpublished study, report No. CTL/P/906, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Moniz, A.C., Bernadi, M.M., Souza-Spinosa, H.S. & Palermo-Neto, J.
(1990) Effects of exposure to a pyrethroid insecticide during
lactation on the behaviour of infant and adult rats. Braz. J.
Med. Biol. Res., 23, 45-48.
Moretto, A. (1991) Indoor spraying with the pyrethroid insecticide
lambda-cyhalothrin: Effects on spraymen and inhabitants of
sprayed houses. Bull. World Health Organ., 69, 591-594.
Moyes, A., Godley, M.J., Hall, M., Pratt, I., Stonard, M.D. & Tinston,
D.J. (1984) Cyhalothrin: 28-day feeding study in the rat (second
study). Unpublished report No. CTL/P/1013, Imperial Chemical
Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Narahashi, T. (1985) Nerve membrane ionic channels as the primary
target of pyrethroids. Neurotoxicology, 2, 3-22.
Nixon, J. & Jackson, S.J. (1981) Cyhalothrin: Acute toxicity.
Unpublished study, report No. CTL/T/555, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Pigott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
Robinson, M., Taylor, K. & Tinston, D.J. (1984) Cyhalothrin. Two
year feeding study in rats. Unpublished report, report No.
CTL/1023, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Prentice, D.E., Edmondson, N.A. & Lewis, D.J. (1981) 28 day dose range
finding study of cyhalothrin in dietary administration to mice.
Electron microscopy of livers. Unpublished report No.
5/HA/003712, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Pritchard, V.K. (1984) Cyhalothrin: Acute oral toxicity. Unpublished
study, report No. CTL/P/1023, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Ray, D. (1991) Chapter 13. In: Hayes, W.J., Jr & Laws, E.R., Jr, eds,
Handbook of Pesticide Toxicology, London: Academic Press, Vol.
2, pp. 585-636.
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. (1981) Cell
transformation test for potential carcinogenicity of
Y00102/010/005 (cyhalothrin (PP563)). Unpublished study, report
Number CTL/1023, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wright, D.G.D.
(1982) The acute oral toxicity (LD50) and neurotoxic effects of
cyhalothrin to the domestic hen. Unpublished study, report Number
ICI/374 NT/81742, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991a)
Effects of prenatal pyrethroid exposure on the sexual development
of rats. Vet. Hum. Toxicol., 33, 427-428.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991b)
Pyrethroid insecticides and pregnancy: Effect on physical and
behavioural development in rats. Vet. Hum. Toxicol., 33,
315-317.
Tomlin, C., ed. (1994) The Pesticide Manual, 10th Ed., Cambridge:
Crop Protection Publications / Royal Society of Chemistry.
Trueman, R.W. (1981) Cyhalothrin: Results from the Salmonella
reverse mutation assay. Unpublished study, report No. CTL/P/665,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Vijverberg, H.P.M. & van den Bercken, J. (1990) Neurotoxicological
effects and mode of action of pyrethroid insecticides. Crit.
Rev. Toxicol., 21, 105-126.
Vijverberg, H.P.M., van den Zalm, J.M. & van den Bercken, J. (1982)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature, 295, 601-603.
WHO (1990) Cyhalothrin. Environmental Health Criteria 99, Geneva:
World Health Organization.
Woodward, K. (2000) Personal communication from Kevin Woodward of
Schering-Plough Animal Health, Uxbridge, United Kingdom, to Mr
Derek Renshaw of the Department of Health. E-mail message dated
14 January 2000.
Zufan, L., Kubat, J., Formanek, J., Fuchs, A., Tobiskova, G., Zajicek,
P., Vodickova, J., Rehak, P. & Dvorak, J. (1989) Behavioural and
EEG changes induced by the pyrethroid insecticide cyhalothrin.
Int. J. Psychophysiol., 7, 447-448.
Excretion was initially rapid, most of the administered doses
being eliminated within the first 48 h after dosing, but excretion was
still incomplete at 7 days. After 14CHCN-cyhalothrin was given
orally, approximately 30% of the administered dose was found in urine
at 7 days and about 50% in faeces. When 14C-cyclopropyl-cyhalothrin
was given, the proportion of residue in urine was slightly lower (19%)
and the amount in faeces higher (68%). The proportions of residue in
urine and in faeces were approximately equal when the two
radiolabelled forms of cyhalothrin were given intravenously. A large
proportion (46-87%) of the faecal residue was in the form of unchanged
cyhalothrin after oral dosing, but only small amounts (1.4-1.5% of
faecal radiolabel) were found in faeces after intravenous injection,
suggesting that the oral dose of cyhalothrin was only partially
absorbed from the gut lumen, leaving unabsorbed cyhalothrin in the
faeces.
Metabolism occurred initially by cleavage of the ester bond to
give a phenoxybenzyl moiety and a cyclopropane acid moiety. Further
metabolism of the phenoxybenzyl moiety produced N-(3-phenoxybenzoyl)
glycine, 3-(4'-hydroxyphenoxy)benzoic acid and its sulfate conjugate,
3-phenoxybenzoyl glucuronide, small amounts of various other
conjugates of these compounds, and a little free phenoxybenzoic acid.
The cyclopropane acid moiety was extensively metabolized to at least
11 further metabolites, including 45% as the glucuronide of the
cyclopropane acid and up to 23% as the free cyclopropane acid (i.e.
3-( Z-2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclo-propane
carboxylic acid; see Figure 1 (Harrison, 1984c).
Humans
In a study of three teams of seven pesticide applicators in
Pakistan, the men were monitored over 15 days while they were using a
pesticide formulation which contained 10% lambda-cyhalothrin and for a
further 4 days after they had finished spraying. The men wore typical
work clothing with no personal protective equipment while spraying
dwellings to kill mosquitoes as part of a malaria control programme.
All of the workers underwent four medical examinations, including
blood sampling, before the study, in the middle of the first week, in
the middle of the second week, and 4 days after the last spraying, and
were questioned about adverse effects. Urine samples were collected
daily. Fifteen men who lived in the treated dwellings were monitored
for exposure by collection and analysis of 24-h urine samples for 3
consecutive days after the spraying of their houses. The samples of
urine and serum were treated enzymically to deconjugate the
metabolites and were then analysed by gas chromatography with mass
spectroscopy. The cyhalothrin metabolites measured were
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)-benzoic acid. The limit of determination for all
three metabolites was 0.01 µg/ml. These metabolites are products of
cleavage of the ester bond in cyhalothrin, and their presence
indicates that the initial metabolism of cyhalothrin is similar in
humans to that observed in other species.
The average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). 3-(2-Chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid was usually the most abundant
metabolite in urine. No metabolites were detected in serum samples.
Most of the inhabitants of treated houses had no detectable residues
in their urine, although they were measurable in 12 of 44 samples, and
the measured concentrations were at or close to the limit of
determination. Most values were not reported, but the highest
concentration of urinary metabolites was 0.1 mg/person per day
(Chester et al., 1992). The signs of toxicity found are reported
below.
2.2 Toxicological studies
Most of the toxicological studies of cyhalothrin were performed
during 1980-84, and some were therefore conducted before GLP was
widely introduced. The chemical identity of the cyhalothrin used in
each study is not clearly stated, and, although the purity was
reported in most cases, the isomers and the isomer ratios were not
mentioned in most of the reports. The manufacturer has claimed that
the analysis given in appendices to the report of the inclined plane
test (see below) is typical of technical-grade cyhalothrin. The
manufacturer also claimed that measurements made over the last
15 years have shown little variation in the isomer ratio. Two
measurements from 1985 gave about 56% for the A isomeric pair and 41%
for the B isomeric pair, leaving only about 2% for other isomers and
impurities (Woodward, 2000).
2.2.1 Acute toxicity
Acute poisoning of rats with type II pyrethroids such as
cyhalothrin is typically characterized by progressive development of
'nosing' and exaggerated jaw opening, increasing extensor tone in the
hind limbs causing a rolling gait, choreoathetosis (sinuous writhing
spasms), salivation, incoordination progressing to coarse tremor,
tonic seizures, apnoea, and death (Barnes & Verschoyle, 1974; Ray,
1991). This has been called the choreoathetosis/salivation syndrome or
CS syndrome. In dogs, similar symptoms are seen, but salivation, upper
airway hypersecretion, and gastrointestinal symptoms are more
prominent than in rats (Ray, 1991).
As in poisoning with type I pyrethroids, the plasma noradrenaline
concentration is increased by type II pyrethroids, but they also
increase plasma adrenaline and blood glucose concentrations. Type II
pyrethroids increase cardiac contractility both directly by action on
cardiac muscle and via circulating catecholamines. They also cause
repetitive firing and potentiate contraction in skeletal muscle. The
type II pyrethroids do not produce the repetitive activity in sensory
nerves that is seen after exposure to type I pyrethroids (Ray, 1991).
Table 3. LD50 values for cyhalothrin
Species Sex Route Purity Vehicle LD50 Reference
(%) (mg/kg bw)
Rat M Oral 90/94 cis Corn oil 240 Nixon & Jackson (1981)
Rat M Oral 92.9 Corn oil 170 Pritchard (1984)
Rat M Oral 90 Oilve oil 51 Ebino et al. (1984a)
Rat F Oral 90/94 cis Corn oil 140 Nixon & Jackson (1981)
Rat F Oral 92.9 Corn oil 110 Pritchard (1984)
Rat F Oral 90 Olive oil 65 Ebino et al. (1984a)
Mouse M Oral 90/94 cis Corn oil 37 Nixon & Jackson (1981)
Mouse F Oral 90/94 cis Corn oil 62 Nixon & Jackson (1981)
Guinea-pig M Oral 90/94 cis Corn oil > 5000 Nixon & Jackson (1981)
Rabbit F Oral 89.2 Corn oil > 1000 Jones (1980)
Chicken F Oral 93.4 Corn oil > 10 000 Roberts et al. (1982)
Rat M Dermal 90 Undiluted 3600 Ebino et al. (1984d)
Rat F Dermal 90 Undiluted 3700 Ebino et al. (1984d)
Rabbit M Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rabbit F Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rat M Intraperitoneal 90/94 cis Corn oil 250-750 Nixon & Jackson (1981)
Rat M Intraperitoneal 90 Oilve oil 500 Ebino et al. (1984c)
Rat F Intraperitoneal 90 Oilve oil 420 Ebino et al. (1984c)
Rat M Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
Rat F Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
The acute toxicity of cyhalothrin was investigated in a series of
studies that complied with GLP (Nixon & Jackson, 1981; Roberts et al.,
1982; Pritchard, 1984) and also in studies that, although not GLP
compliant, were accompanied by a suitable quality assurance statement
(Jones, 1980; Ebino et al., 1984a-e). The LD50 values estimated from
these studies are listed in Table 3. The LD50 of a wettable
formulation containing 5% cyhalothrin was 1200 mg/kg bw in male rats
and 900 mg/kg bw in females when it was administered orally in olive
oil (Ebino et al., 1981b).
The signs of toxicity seen in all species and with all routes of
administration were similar and included subdued behaviour, ungroomed
appearance, piloerection, salivation, incontinence, and unsteady gait.
A further account of the signs of acute toxicity in rats receiving
various oral doses of cyhalothrin is given in the preliminary report
of the inclined plane test (Denton, 1988), which is described below.
2.2.2 Short-term studies of toxicity
Mice
In a range-finding study for an assay of dominant lethal mutation
that complied with GLP, groups of 10 male CD-1 mice were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 5, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 5 days. At
the two higher doses, signs of severe toxicity (ataxia and
convulsions) were observed. One animal at 40 mg/kg bw per day and
eight at 80 mg/kg bw per day died. Less severe clinical signs were
seen at 20 mg/kg bw per day, including body-weight loss, ataxia, and a
rough coat. Only rough coats were seen at 5 mg/kg bw per day (Irvine,
1981a).
In a range-finding study for a long-term study of toxicity and
carcinogenicity, groups of 12 male and 12 female CD-1 mice were fed
diets containing technical-grade cyhalothrin (purity unspecified) at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of diet. Over the
course of the 4-week study, these concentrations gave mean doses of
cyhalothrin of 0, 0.65, 3.3, 14, 64, and 310 mg/kg bw per day for
males and 0, 0.80, 4.2, 15, 78, and 290 mg/kg bw per day for females.
No certificate of compliance with GLP was available for this study,
but quality assurance systems were in place.
Deaths were seen only at the highest dietary concentration, at
which six males and three females died prematurely. Severe signs of
toxicity were also seen at this dose, including abnormal gait
('walking on toes'), hunched posture, decreased body weight (Student's
t test, p < 0.01), and increased respiratory rate. Piloerection
was observed in several animals in each groups given cyhalothrin at
> 25 mg/kg and in one control male and one male at 5 mg/kg of diet.
Haematological changes were seen in males (statistically significant
in Williams' test: p < 0.05) but not in females. Animals at 2000
mg/kg of diet had a decreased total leukocyte count accompanied by a
lower lymphocyte count and a higher neutrophil count. Those at 100 and
500 mg/kg of diet also had marginally lowered lymphocyte counts.
Macroscopic examinations post mortem revealed no adverse
effects other than altered organ weights (statistically significant in
both Student's t test and Williams' test: p < 0.05). The weight
of the kidney relative to body weight was slightly elevated in males
given 100, 500, or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The relative liver weights were also slightly increased in
males given 25 or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The heart weights were marginally decreased in females given
100 or 2000 mg/kg of diet. The activity of aminopyrine- N-demethylase
in liver was increased in males given 2000 mg/kg of diet and in
females given 100, 500, or 2000 mg/kg of diet (Student's t test:
p < 0.05). Histopathological examination showed minimal
centrilobular hepatocyte enlargement in two females at 2000 mg/kg of
diet. Atrophy of the red pulp of the spleen was seen in two of the
three females at this dose that died. The Committee considered that
the effect may have been indicative of immunological changes. The NOEL
was 5 mg/kg of diet, equal to 0.65 mg/kg bw per day, on the basis of
piloerection at concentrations > 25 mg/kg of diet (Colley et al.,
1981).
When liver samples from this study were examined by electron
microscopy, minimal amounts of smooth endoplasmic reticulum were
observed in occasional hepatocytes from some mice from each group,
including controls. Most hepatocytes contained no observable smooth
endoplasmic reticulum. The incidence of mice with hepatocytes
containing smooth endoplasmic reticulum was highest in the group given
cyhalothrin at 2000 mg/kg of diet, consistent with the finding of
other hepatic effects in this group (Prentice et al., 1981).
Rats
In a range-finding study for an investigation of developmental
toxicity, groups of 10 female Charles River CD rats were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 10
consecutive days. After treatment, the animals were observed for a
7-day recovery period. No certificate of compliance with GLP was
supplied, but suitable quality assurance records had been kept.
All of the animals given 80 mg/kg bw per day, nine of those given
40 mg/kg bw per day, and two given 20 mg/kg bw per day had to be
killed for humane reasons after the first dose, as this caused severe
toxicity (ataxia and/or collapse). Body-weight loss and reduced food
intake were seen in the rats given 20 mg/kg bw per day throughout the
treatment period. Body-weight loss was also seen in the rats given 10
mg/kg bw per day during the first 5 days of the study. Food intake was
increased in the groups at 10 and 20 mg/kg bw per day during the
recovery period. Macroscopic examination of the animals at necropsy
revealed no treatment-related changes. No NOEL could be identified in
this study (Killick, 1980).
In a 28-day range-finding study, groups of 10 male and 10 female
Charles River CD rats were fed diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 5, 10, 20,
or 250 mg/kg of diet, equivalent to 0, 0.5, 1, 2, and 25 mg/kg bw per
day. The study did not comply with GLP. There were no
treatment-related clinical signs and no deaths. Food intake, food
efficiency, and body-weight gain were decreased only at the highest
dose (Student's t test: p < 0.05). Gross examination post
mortem revealed no treatment-related effects, and organ weights were
unaffected by treatment. Electron microscopy of liver samples showed a
moderate increase in the amount of smooth endoplasmic reticulum in
hepatocytes of animals at the highest dose, with a decrease in the
amount of glycogen. An increase in hepatocyte size was noted in the
centrilobular and mid-zonal regions of the livers of males at this
dose, which also had increased hepatic activity of
aminopyrine- N-demethylase (Student's t test: p < 0.001). The
NOEL was 20 mg/kg of diet, equivalent to 2 mg/kg bw per day (Colley et
al., 1980).
In a study that complied with GLP, groups of eight male and eight
female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%) at a concentration of 0,
1, 5, 10, 20, or 250 mg/kg of diet, equivalent to 0, 0.1, 0.5, 1, 2,
and 25 mg/kg bw per day. Full post-mortem examinations were not
performed, but samples of liver were taken for analysis of
aminopyrine- N-demethylase activity and electron microscopy. No
treatment-related clinical signs were reported. The mean body weight
of males given 250 mg/kg of diet was decreased when compared with that
of concurrent controls during weeks 1 and 2 of the study (two-sided
t test: p < 0.05). Body-weight gain was depressed during week 1
for males given 250 mg/kg of diet and for females given 10, 20, or 250
mg/kg of diet (two-sided t test: p < 0.05). The absolute weight
of the liver was reduced in males given 250 mg/kg of diet and in
females given 20 mg/kg of diet (two-sided t test: p < 0.05), but
the relative weight of the liver was increased in males at 250 mg/kg
of diet. Both males and females at this dose had increased hepatic
activity of aminopyrine- N-demethylase and mild proliferation of
smooth endoplasmic reticulum in hepatocytes. The NOEL was 5 mg/kg of
diet, equivalent to 0.5 mg/kg bw per day, on the basis of depression
of body-weight gain, whereas that for hepatic changes was 20 mg/kg of
diet, equivalent to 2 mg/kg bw per day (Moyes et al., 1984).
In a 28-day range-finding study, of which full details were not
available, a dietary concentration of cyhalothrin of 750 mg/kg
(equivalent to 75 mg/kg bw per day) increased the mortality rate.
'Characteristic signs of pyrethroid neurotoxicity' were seen at
dietary concentrations of 250 and 750 mg/kg. Treatment-related effects
on body weights, food consumption, organ weights, and hepatic activity
of aminopyrine- N-demethylase were seen at 250 mg/kg of diet. Hepatic
smooth endoplasmic reticulum proliferation occurred in males fed 20
and 250 mg/kg of diet. No NOEL could be identified (Lindsey et al.,
1981).
In a 90-day study that complied with GLP, groups of 20 male and
20 female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%; with a total pyrethroid
content of 92.2% of which 96.8% was cyhalothrin) at a concentration of
0, 10, 50, or 250 mg/kg of diet, providing mean doses of 0, 0.56, 2.6,
and 36 mg/kg bw per day for males and 0, 0.57, 3.2, and 15 mg/kg bw
per day for females. Blood samples were collected before treatment,
during week 4 of treatment, and at the end of the study. Urine samples
were collected before treatment and in weeks 4 and 13. Ophthalmoscopic
examinations were performed during the week before termination. Full
post-mortem examinations were performed, and the major organs and any
grossly abnormal tissues were examined histologically. Liver samples
were taken for analysis of aminopyrine- N-demethylase activity and
examination by electron microscopy.
Treatment with doses < 250 mg/kg of diet had no effect on the
mortality rate. The body-weight gain of males at the highest dose was
consistently reduced (two-sided t test: p < 0.01) throughout the
study. Food consumption was slightly reduced in males at 50 and 250
mg/kg of diet, and this effect was occasionally statistically
significant (two-sided t test: p < 0.05). There were no clearly
treatment-related haematological effects, although there were small,
significant, but not dose-related changes in some red blood cell
parameters (mean corpuscular volume, packed cell volume, haemoglobin,
mean corpuscular haemoglobin). Plasma alanine and aspartate
aminotransferase activities and cholesterol concentration were raised
at 4 weeks in males given 10 or 50 mg/kg of diet, and those at the
lower dose also had a raised plasma urea concentration (two-sided
t test: p < 0.05). At 13 weeks, however, the plasma concentration
of urea in males at 50 mg/kg of diet was decreased (two-sided
t test: p < 0.05). The observed effects on haematological
parameters, plasma enzyme activity, and concentrations of cholesterol
and urea were considered to be unrelated to treatment as there were no
clear dose-response relationships. At 13 weeks, however, the plasma
triglyceride concentration was reduced and there was a dose-related
increase in urinary glucose concentration in males at 50 and 250 mg/kg
of diet (two-sided t test: p < 0.05). Ophthalmoscopy revealed no
treatment-related effects. The results of gross examinations were not
reported, although the organ weights were recorded. The absolute
weight of the liver was increased in males at 250 mg/kg of diet, and
the absolute weight of the lung was decreased in animals given 250
mg/kg of diet, but the relative weights of these organs were
unaffected. No treatment-related changes were revealed by optical
microscopy of organs. Electron microscopy of the liver revealed mild
proliferation of smooth endoplasmic reticulum in the hepatocytes of
three males given 50 mg/kg of diet and three males given 250 mg/kg of
diet. There was a dose-related increased in hepatic
aminopyrine- N-demethylase activity in males given 50 or 250 mg/kg of
diet and in females given 250 mg/kg of diet (two-sided t test:
p < 0.05). The NOEL was 10 mg/kg of diet, equivalent to 0.56 mg/kg
bw per day, on the basis of hepatic changes in males at higher doses
(Lindsey et al., 1981).
Rabbits
In a range-finding study for an investigation of developmental
toxicity, groups of six mated female New Zealand white rabbits were
given cyhalothrin (purity, 89.25%) at a daily dose of 0, 20, 30, or 40
mg/kg bw by oral gavage in corn oil on days 6-18 of gestation. The
animals were killed on day 18 of gestation.
There were no unscheduled deaths and no consistent clinical signs
of toxicity. Body-weight gain was depressed only at 30 mg/kg bw per
day, although animals at 40 mg/kg bw per day showed reduced food
intake. Not all of the animals became pregnant, but in all groups,
including controls, few of the does had live fetuses. There was no
dose-response relationship for this effect. Furthermore, not all of
the pregnant does at 20 and 40 mg/kg bw per day produced live
offspring. Consequently, there were insufficient numbers of litters to
allow any meaningful inter-group comparison of litter parameters,
although no adverse effects were apparent from the limited data
available. No treatment-related macroscopic changes were seen post
mortem. The apparent NOEL in this limited study was 20 mg/kg bw per
day on the basis of reduced body-weight gain (Killick, 1981a).
Dogs
In a preliminary study, groups of one male and one female beagles
were given gelatin capsules containing cyhalothrin (purity
unspecified) in corn oil at a dose of 0, 2.5, 10, or 30 mg/kg bw per
day for 4 weeks. After 10 days, treatment with the high dose was
stopped, and the animals were allowed to recover for 4 days; treatment
was then resumed at a lower dose of 20 mg/kg bw per day for 4 weeks.
Blood was taken weekly. Urine samples were collected before dosing and
during week 4. Ophthalmoscopic examinations were performed on each dog
before dosing and during week 4. No certificate of compliance with GLP
was supplied, but suitable quality assurance records were kept.
None of the dogs died prematurely. Muscular trembling was seen in
all groups, including controls, but the degree of trembling was more
marked in animals at the two higher doses. Unsteady gait and
body-weight loss were observed in dogs given 30 mg/kg bw per day, but
this effect was not seen after the dose was reduced to 20 mg/kg bw per
day. Body weight was unaffected by doses up to 10 mg/kg bw per day,
and only slight fluctuations occurred at 20 mg/kg bw per day. Vomiting
was seen occasionally at doses > 10 mg/kg bw per day. Dogs at the
high dose (30 or 20 mg/kg bw per day) had loose stools and increased
serum alanine and aspartate aminotransferase activities. No
haematological, ophthalmological, or urinary changes were observed,
and no gross pathological or organ weight changes were noted post
mortem. Histopathological examination revealed no treatment-related
effects. No NOEL could be identified in this study owing to the
finding of an increased frequency of liquid faeces at all doses. The
LOEL was 2.5 mg/kg bw per day (Chesterman et al., 1983).
In a 26-week study that complied with GLP, groups of six male and
six female beagles were given cyhalothrin (purity unspecified)
dissolved in corn oil inside a gelatin capsule at a dose of 0, 1.0,
2.5, or 10 mg/kg bw per day. The dogs were examined
ophthalmoscopically before dosing and during weeks 6, 12, and 24 of
treatment. Blood and urine samples were taken every 4 weeks. The
functional integrity of the nervous system was assessed clinically
before treatment and during week 6. At the end of the treatment
period, full necropsies were performed on all dogs, and a large
selection of organs and tissues was examined histologically.
No deaths occurred during the study. A dose-related increase in
the incidence of liquid faeces affected all treated groups. At 10
mg/kg bw per day, the clinical signs seen included vomiting,
salivation, incoordination, unsteadiness, collapse, muscular spasms,
and convulsions. Body weight was unaffected by treatment.
Ophthalmoscopic and neurological examinations revealed no adverse
effects, and no consistent haematological, serum biochemical, or
urinary effects were seen. No treatment-related gross effects on organ
weights or appearance were noted post mortem, and histopathological
examination revealed no treatment-related effects. No NOEL could be
identified in this study owing to the finding of an increased
frequency of liquid faeces at all doses. The LOEL was 1.0 mg/kg bw per
day (Chesterman et al., 1981).
In a 52-week study, groups of six dogs of each sex were given
lambda-cyhalothrin at a dose of 0, 0.1, 0.5, or 3.5 mg/kg bw per day
orally in corn oil, but there was lack of agreement between summaries
of the study about whether the animals were treated by gelatin capsule
(European Medicines Evaluation Agency, 1999) or by gavage (WHO, 1990).
The report was not available to the Committee. Clinical signs of
neurological effects (muscular trembling, unsteadiness, and vomiting)
were seen in all dogs at the highest dose, but the signs were not
accompanied by histological changes in the nervous system. The
European Medicines Evaluation Agency (1999) reported a dose-related
increased incidence of liquid faeces at all doses, but WHO (1990)
reported that this effect was seen only at 0.5 and 3.5 mg/kg bw per
day and that the increased incidence was only slight at 0.5 mg/kg bw
per day. The incidence of liquid faeces was considered not to be
toxicologically significant. The NOEL was 0.5 mg/kg bw per day on the
basis of neurological signs seen at 3.5 mg/kg bw per day. It is
unclear how this dose relates to a dose of cyhalothrin (WHO, 1990;
European Medicines Evaluation Agency, 1999).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study that complied with GLP, groups of 52 male and 52
female CD-1 mice were given diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 20, 100, or
500 mg/kg for 104 weeks, equal to 0, 1.8, 9.2, and 53 mg/kg bw per day
for males and 0, 1.8, 11, and 51 mg/kg bw per day for females.
Satellite groups of 12 mice of each sex were given the same diets for
52 weeks and then killed.
The signs of toxicity observed in animals given high doses were
piloerection and hunched posture in mice of each sex given 500 mg/kg
of diet and in males given 100 mg/kg of diet. Males at 500 mg/kg of
diet also showed emaciation, pallor, hyperactivity, and increased
fighting activity. The mortality rate was unaffected by treatment.
Decreased body-weight gain was seen in males at 500 mg/kg of diet
throughout the study (two-sided Student t test: p < 0.05),
particularly during the first 13 weeks ( p < 0.001). Reduced feed
use efficiency was seen in the same group over the first 24 weeks of
the study (not calculated for the rest of the study). Water
consumption was also slightly increased in males at 500 mg/kg of diet
(two-sided Student t test: p < 0.05).
The results of haematological tests were unremarkable.
Statistically significant changes in various parameters were seen at
various times, but the effects were not consistent or dose-related.
Urinary analysis revealed no significant differences from control
values. The serum glucose concentration was slightly reduced
(two-sided Student t test: p < 0.05) in animals given 500 mg/kg
of diet. During week 100, serum alanine and aspartate aminotransferase
activities were increased in a dose-related manner, with occasional
significance ( p < 0.05) for both activities in mice of each sex at
100 and 500 mg/kg of diet and for alanine aminotransferase activity in
females at 20 mg/kg of diet.
Gross examination post mortem revealed increased incidences
(not dose-related) of thickening of the forestomach in treated
females. The ovarian weights were significantly (two-sided Student
t test: p < 0.05) decreased at all doses in animals in the main
group but not in the satellite groups. The effect was not
dose-related. Other organ weights were unaffected. Histopathological
examination revealed a variety of non-neoplastic lesions, but none was
considered to be due to treatment with cyhalothrin.
A non-dose-related increase in the incidence of mammary
adenocarcino-mas was seen in females in the main group, the incidences
being 1/52 in controls, 0/52 at 20 mg/kg of diet, 7/52 at 100 mg/kg of
diet, and 6/52 at 500 mg/kg of diet. (The detailed statistical
analysis of these results was given in an addendum to the report that
was not available to the Committee.) The incidence of mammary
adenocinoma in control females in 17 studies performed by the same
laboratory between 1980 and 1982 ranged from 2% to 12%. No
preneoplastic changes were found in the mammary glands of mice in the
main or satellite groups. A re-evaluation of mammary tissues from all
animals confirmed the original diagnoses of mammary neoplasia. As the
highest incidence of mammary adenocarcinoma in any group was only
slightly above the range of historical controls and as there was no
dose-response relationship, it was concluded that treatment with
cyhalothrin was unlikely to have caused these tumours. The NOEL was 20
mg/kg of diet, equal to 1.8 mg/kg bw per day, on the basis of clinical
signs of toxicity (piloerection and hunched posture) in males at
higher doses (Colley et al., 1984).
Rats
In a study that complied with GLP, groups of 72 male and 72
female Alpk/AP SPF rats were fed diets containing technical-grade
cyhalothrin (purity, 89.25%; containing 92.2% pyrethroids of which
96.8% was cyhalothrin) at a concentration of 0, 10, 50, or 250 mg/kg
for 104 weeks, providing mean doses of 0, 0.47, 2.3, and 12 mg/kg bw
per day for males and 0, 0.55, 2.7, and 14 mg/kg bw per day for
females. Satellite groups of 10 rats of each sex were given the same
diets but were killed after only 52 weeks. Blood was taken for
haematological measurements from 10 rats of each sex in each group at
4 and 13 weeks and thereafter at 13-week intervals. Blood was taken
for biochemical analyses and urine for urinary analyses from 12 rats
of each sex in each group at the same intervals. In weeks 52 and 103,
ophthalmoscopy was performed on 20 males and 20 females from each
group.
No clinical signs were seen that could be attributed to
treatment. The mortality rate was unaffected. Body-weight gain was
suppressed in both males and females given 250 mg/kg of diet
(two-sided Student t test: p < 0.05), and the males in this group
had increased food use efficiency. No consistent, dose-related
haematological effects were seen, although changes in various
parameters occasionally achieved statistical significance (two-sided
Student t test: p < 0.05). The volume of urine produced by rats
fed 250 mg/kg of diet tended to be decreased, but otherwise there was
no effect on urinary parameters. Ophthalmoscopy revealed no
treatment-related effects. Blood biochemistry showed intermittent
effects in rats at 250 mg/kg of diet, consisting of reduced plasma
concentrations of glucose and triglycerides, reduced plasma alkaline
phosphatase activity, and increased plasma urea concentration
(two-sided Student t test: p < 0.05). The reduction in plasma
triglycerides was the most marked finding, especially in females.
Long, pointed fibres, thought to originate from the diet, were
found embedded in the tissues of the palate, nasal turbinates, roof of
the nasal cavity, and peridontal space. The presence of the fibres
suggested that the lesions were caused by penetration of the tissue by
these fibres rather than by cyhalothrin. No treatment-related gross
lesions were detected post mortem, but several rats in all groups,
including controls, had rhinitis and oral lesions (oral granuloma
formation and erosion of the palate) in response to the presence of
the fibres in the diet. At the interim kill, the weight of the liver
(relative to body weight) was increased in rats of each sex given 250
mg/kg of diet, but this effect was not seen in rats killed at 104
weeks. The weight of the adrenals (relative to body weight) was
increased in females at 50 and 250 mg/kg of diet killed at 104 weeks.
Histopathological examination revealed no treatment-related effect on
the incidence or severity of any neoplastic or non-neoplastic lesions.
The NOEL was 50 mg/kg of diet, equal to 2.3 mg/kg bw per day (Pigott
et al., 1984).
2.2.4 Genotoxicity
Cyhalothrin was tested in assays for gene mutation in bacteria,
cytogenicity in rats in vivo, dominant lethal mutation in mice, and
cell transformation. The results of all the assays were negative. The
assays did not comply with GLP, but they appeared to be of good
quality and suitable quality assurance records had been maintained.
The studies are summarized in Table 4. The material tested by Trueman
(1981) was analysed by reversed-phase HPLC and found to have a
cis:trans ratio of 97.1:2.9.
lambda-Cyhalothrin was reported to be non-genotoxic when tested
for reverse mutation in bacteria, gene mutation in a mouse lymphoma
cell line, cytogenicity in vitro, unscheduled DNA damage in
vitro, and micronucleus formation in mice (WHO, 1990). Full reports
of these assays were not available, but the available details are
given in Table 5. lambda-Cyhalothrin produced micronuclei in an assay
in fish (Campana et al., 1999), but the relevance to human health of a
positive result in this unvalidated assay is not known.
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Mice
Only a brief account of a three-generation study of the effects
of cyhalothrin on reproduction in mice has been reported. It is
unclear whether the study was performed in accordance with the
principles of GLP. Groups of albino Swiss mice were given oral doses
of '0 (blank control), 2.5 ppm and 5.0 ppm cyhalothrin/kg body weight
daily' (the meaning of the term 'ppm cyhalothrin/kg body weight' is
unclear). The F0 generation consisted of groups of four males and
eight females. No further details of the protocol were available. No
signs of toxicity or behavioural changes were observed in the parents
or offspring for up to three generations. Maternal body weights were
not affected, although F2 generation dams at the low dose had
increased food intake. The weights of pups of F2 dams treated at the
high dose were reported to be significantly different from those of
control pups. Male and female fertility indices, pup live birth and
survival indices, litter size, and sex ratio were unaffected. The
meaning of the results was unclear. No NOEL could be identified
(Deshmukh, 1992).
Table 4. Assays for genotoxicity with cyhalothrin
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse S. typhimurium 0.16-2500 µg/ 90.2 Negativea Trueman (1981)
mutation TA1535, TA1537, plate ± S9
TA1538, TA98,
TA100
Cell Syrian hamster 50-1000 µg/ml NR Negativeb Richold et al.
transformation - S9 (1981)
1000-5000 µg/ml
+ S9
In vivo
Cytogenicity Bone marrow, Single oral dose 89.2 Negativec Anderson et al.
Wistar-derived of 1.5, 7.5,15 mg/ (1983)
male rats kg bw in corn oil
5 daily oral doses
of 1.5, 7.5, 15 mg/
kg bw per day
Dominant Male CD1 mice 1, 5, 10 mg/kg bw/ 89.2 Negatived Irvine (1981b)
lethal day for 5 days by
mutation oral gavage
in corn oil
a 2-Aminoanthracene, 9-aminoacridine, 4-nitroquinoline- N-oxide, and
N-methyl- N'-nitro- N-nitrosoguanidine used as positive controls
b 4-Nitroquinoline- N-oxide and para-dimethylaminoazobenzene (butter yellow) used
as positive controls
c Ethyl methanesulphonate used as a positive control
d Cyclophosphamide used as a positive control
Table 5. Assays for genotoxicity with lambda-cyhalothrin
End-point Test object Concentration Results Reference
In vitro
Reverse S. typhimurium < 5000 µg/plate Negative IPCS (1990)
mutation (strains not ± S9
specified)
Gene mutation L51787 mouse 125-4000 µg/ml Negative IPCS (1990)
lymphoma cell ± S9
line
Unscheduled Cultured human NR Negative IPCS (1990)
DNA synthesis HeLa cells
Cytogenicity Human lymphocytes NR Negative IPCS (1990)
In vivo
Micronucleus Erythrocytes of 0.001-0.05 µg/L Positive Campana et al.
formation Cheirodon interruptus (1999)
interruptus
Micronucleus Mice < 35 mg/kg bw Negative IPCS (1990)
formation (route not
specified)a
a Cyclophosphamide used as a positive control
Rats
Throughout a GLP-compliant three-generation (two litters per
generation) study, groups of SPF Alpk/AP Wistar-derived rats were
given diets containing technical-grade cyhalothrin (purity, 89.25%;
containing 92.2% pyrethroids of which 96.8% was cyhalothrin) at a
concentration of 0, 10, 30, or 100 mg/kg, providing mean doses of 0,
0.6, 1.7, and 5.6 mg/kg bw per day for males and 0, 0.7, 1.9, and 6.1
mg/kg bw per day for females. The F0 generation consisted of groups
of 15 male and 30 female rats. After 12 weeks on the test diets, these
animals were mated within their groups to produce a first litter
(F1a generation). Later, they were mated again to produce a second
litter (F1b generation). Breeding was repeated with F1 parents (15
males and 30 females per group) selected from the F1b animals and
F2 parents (15 males and 30 females per group) selected from the
F2b animals. At day 36 post partum, all pups not selected as the
next parental generation were killed and examined externally.
Approximately half of these were examined by autopsy, with
histopathological examination of any grossly abnormal tissues. A
fuller post-mortem examination with histopathology of all major organs
was performed on five males and five females from each group of the
F1b and F2b generations and 10 males and 10 females from each of
the F3b groups.
No clinical signs of toxicity were found. Minor reductions in
body-weight gain were seen in the premating period of the F0
generation of animals of each sex at 100 mg/kg of diet and
occasionally in females given 30 mg/kg of diet. At other times and in
other generations, occasional reductions in body-weight gain were seen
in rats given cyhalothrin at 30 or 100 mg/kg of diet. Male fertility
was unaffected by treatment. The fertility of F1 females at 30 mg/kg
of diet was statistically significantly (one-sided t test:
p < 0.05) reduced but remained within the range of control values.
At 100 mg/kg, the litter size of F2 and F3 pups was reduced, the
effect being statistically significant (one-sided t test:
p < 0.05) for the F2a and F3b litters. Small but statistically
significant decreases (one-sided t test: p < 0.05) were seen in
the percentages of live pups born to animals at 10 mg/kg in the F1b
generation and to dams at 30 and 100 mg/kg of diet in the F3b
generation. Post-mortem and histopathological examinations revealed no
adverse effects that could be attributed to the treatment. The
occasional effects seen at 10 and 30 mg/kg of diet were considered to
be incidental, and only the effects at 100 mg/kg of diet were regarded
as treatment-related. The NOEL was 30 mg/kg of diet, equal to 1.7
mg/kg bw per day, on the basis of reduced parental body-weight gain
and reduced litter size (Milburn et al., 1984).
(b) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley-derived rats (CD rats from
Charles River's SPF colony) were given technical-grade cyhalothrin
(purity, 89.25%; containing 92.2% pyrethroids of which 96.8% was
cyhalothrin) at a dose of 0, 5, 10, or 15 mg/kg bw per day by oral
gavage in corn oil on days 6-15 of gestation. The dams were killed on
day 20, and the contents of their uteri were examined. No certificate
of compliance with GLP was supplied, but suitable quality assurance
records had been kept.
Body-weight loss, reduced food intake, and signs of neurotoxicity
(loss of limb coordination in 2 out of 24 rats) were observed in dams
at the highest dose. The numbers of pregnant animals, pre-implantation
and post-implantation losses, mean litter weights, and mean weights
and lengths of fetuses were unaffected by treatment. Abnormalities
were seen in one litter of a dam at 10 mg/kg bw per day, in which 5 of
17 fetuses had major defects: four had bilateral agenesis of the
kidneys, and three had skeletal malformations of the vertebral
centrae, sternebrae, and/or metacarpals. The effects were considered
to be incidental and unlikely to be due to treatment. The NOEL was 10
mg/kg bw per day on the basis of maternal toxicity (Killick, 1981b).
Six pregnant Wistar-derived rats were given cyhalothrin (purity,
95.8%) by dermal application throughout the 21 days of gestation at a
daily dose of 1 ml of 0.02% (w/v; equal to approximately 0.8 mg/kg bw
per day if completely absorbed) in an aqueous vehicle applied to a
shaved area of the back. Controls were given the vehicle alone. After
parturition, six pups were left with each dam. At 90 days of age, 12
males in the treated group were tested for mating behaviour with
ovariectomized females that were showing estrus induced by
subcutaneous injections of 17ß-estradiol and progesterone. No
certificate of compliance with GLP was supplied for this study.
Treated male pups showed a delay in testicular descent, but the age of
vaginal opening was not affected. The mating behaviour of the males
was not affected by treatment. No NOEL could be identified (da Silva
Gomes et al., 1991a).
In another study which did not appear to conform to GLP, 12
pregnant Wistar-derived rats were given cyhalothrin (purity
unspecified) by dermal application throughout the 21 days of gestation
at a daily dose of 1 ml of 0.018% (w/v; equal to approximately 0.72
mg/kg bw per day if completely absorbed) in an aqueous vehicle. At
weaning (21 days) and at 90 days of age, the pups were tested for
locomotor activity in an open field. At 90 days, training began for
inhibitory avoidance behaviour. Adult males were also tested for
motivational response in a 'hole board' test.
The opening of ears and eyes, the development of fur, and the
descent of testes were all delayed in treated rats, but the time of
vaginal opening was not affected. The body weights of the pups of
cyhalothrin-treated dams were statistically significantly greater
(Student t test: p < 0.05) than those of controls at days 2, 7,
and 14 of age but not at day 21. Locomotor frequency was increased at
21 days and decreased at 90 days, but the effects were not
statistically significant in the Student t test. No effect was seen
on inhibitory avoidance behaviour. The results of the hole board test
showed a decreased motivational response in cyhalothrin-exposed rats.
No NOEL could be identified (da Silva Gomes et al., 1991b).
Rabbits
Groups of 20 pregnant New Zealand white rabbits were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) on days 6-18 of gestation
at a dose of 0, 3, 10, or 30 mg/kg bw per day by oral gavage in corn
oil. The animals were killed and examined on day 28 of gestation. No
certificate of compliance with GLP was supplied, but suitable quality
assurance records had been kept.
The highest dose was toxic to the dams, as indicated by an
initial body-weight loss followed by a decreased rate of body-weight
gain. No treatment-related deaths, clinical signs, or gross
pathological changes were seen in any group of does. There was no
effect on the incidence of pregnancy or on gravid uterine weight. The
mean weight of the fetuses of dams at 30 mg/kg bw per day was slightly
decreased, but the effect was not statistically significant (Wilcoxon
test). Mean fetal crown-rump length, sex ratio, and the numbers of
corpora lutea, intrauterine deaths, and implantations were unchanged
by treatment. There was no increased incidence of any type of fetal
abnormality in any treated group. The NOEL for maternal toxicity was
10 mg/kg bw per day (Killick, 1981c).
2.2.6 Special studies
(a) Neurotoxicity
Rats
Groups of rats were given single oral or intraperitoneal doses of
25-200 mg/kg bw 'cyhalothrin', referred to as 'PP 321, trade mark
Karate', which are terms associated with lambda-cyhalothrin rather
than cyhalothrin. Its purity was not reported. It was suspended as an
emulsion in 'solvents and surface active agents'. After treatment, the
behaviour of the animals in the open field was monitored, and their
electroencephalograms were measured while they were moving freely. No
further details of the protocol were reported. Treated animals were
found to have limited mobility as a consequence of discoordination of
the limbs. The reported signs of toxicity were respiratory
difficulties, tremor, salivation, piloerection, increased reactivity
to noise and during handling, with vocalization. Analysis of the
electroencephalograms showed 'changes in amplitudes (depressions in
majority of cases), in frequency composition (relative increases of
beta activity) and in repetition of cycles of high and low
amplitudes'. The results for individual groups were not presented. No
NOEL could be identified (Zufan et al., 1989).
Hens
In a study that complied with GLP, groups of 10 hens were given
cyhalothrin (purity, 93.4%) as a single dose of 2500, 5000, or 10 000
(two groups) mg/kg bw by oral gavage in corn oil. Negative controls
were given corn oil alone; positive controls received 500 mg/kg bw of
tri- ortho-cresyl phosphate. The hens were observed for 21 days after
dosing. The only hens that died during this period were two given
10 000 mg/kg bw, one in the negative and one in the positive control
group. The mean body weights of hens in all groups given cyhalothrin
and the positive controls were reduced. Clinical signs of toxicity
(ataxia) were seen only in the positive controls. The only
treatment-related gross change seen post mortem was muscular atrophy
in two of the positive control birds. Histopathological examination of
the cervical cranial, cervical caudal, thoracic, and lumbar spinal
cord and the proximal and distal sciatic nerve showed morphological
evidence of axonal damage at all levels of the spinal cord in all the
positive control birds. There were no consistent or dose-related
changes in the nerves of any of the groups given cyhalothrin. The NOEL
was 10 000 mg/kg bw, the highest dose tested (Roberts et al., 1982).
(b) Neurobehavioural effects
Inclined plane test
In a preliminary GLP-compliant study, cyhalothrin was
administered by oral gavage in corn oil to groups of Crl:CD.BR strain
rats of each sex. Initially, single doses of 50, 100, or 200 mg/kg bw
were given to groups of two males and doses of 25, 40, or 75 mg/kg bw
to groups of two females. Later, groups of three rats of each sex were
given a single dose of 15, 30, or 60 mg/kg bw. Controls received corn
oil only. The cyhalothrin contained A and B isomer pairs in a 60:40
ratio and was 95.8% pure, with a total pyrethroid content of 96.4%.
The other isomers measured were cis B' (0.2%), trans D and cis
A' (0.4%), and trans C (< 0.1%). The contaminants included
phenoxybenzalde-hyde (0.2%) and
(Z)3-(2-chloro-3,3,3-trifluoro-1-enyl)-2,2-dimethylcyclopro-pane
(0.1%). One hour before dosing and again at 30 min, 2 h, 7 h, and 24 h
after dosing, each rat was placed on a smooth plane with an angle of
inclination that was gradually increased until the rat could no longer
maintain its position. For each rat, three measurements were made at
each time. In the inclined plane test, any decrease in the angle at
which the rat loses its position (mean slip angle) is regarded as a
reflection of subtle changes in neuromuscular function.
Clinical signs of toxicity were seen at all doses tested. At 15
mg/kg bw, the only clinical sign was soft faeces in females. At 25-40
mg/kg bw, ungroomed appearance, soft faeces, and piloerection were
seen. At 50-75 mg/kg bw, there were marked clinical signs, including
ataxia, writhing, emprosthotonos, high stepping or splayed gait,
hunched posture, stained snout, salivation, and wasted appearance. At
100 mg/kg bw, the signs also included vocalization and closure of the
eyelids. Rats given 60 or 200 mg/kg bw were killed on humane grounds
after the inclined plane measurements had been made. Only one rat (a
female at 60 mg/kg bw) died during the study, but all rats given 60 or
200 mg/kg bw were killed prematurely on humane grounds. Body-weight
gains were decreased at doses > 50 mg/kg bw. Necropsy of the
animals showed no consistent gross pathological changes. The result of
the inclined plane test was uninterpretable, as the clinical signs
interfered with the conduct of the study: convulsing rats were unable
to stay on the plane, and rats with soiled fur adhered to it (Denton,
1988).
Acute startle response
A preliminary study was performed in two phases with groups of
three male Wistar-derived Alpk:ApfSD rats. The purity of the
cyhalothrin tested was 97.2%. In phase 1, rats were given a single
oral dose of 0, 25, 50, or 75 mg/kg bw of cyhalothrin in corn oil, and
auditory habituation tests were performed 1, 2, and 3 h after dosing.
Clinical signs of toxicity were seen on day 2 after dosing (the time
of dosing being the start of day 1) but not on day 1 or day 3, in the
group given 75 mg/kg bw. The signs included ataxia, subdued behaviour,
body-weight loss, and stained fur. Rats at this dose had a
statistically nonsignificant increase in mean response amplitude
(two-sided Student t test) but a significant ( p < 0.05) increase
in the time to maximum amplitude 3 h after dosing.
In phase 2, rats were given a single oral dose of 0, 75, or 100
mg/kg bw of cyhalothrin in corn oil, and auditory habituation tests
were performed on separate groups of animals 3, 5, or 7 h after
dosing. The signs of toxicity were similar to those seen in phase 1.
The clearest results in the auditory startle habituation test were
obtained at 7 h: with both doses tested, the mean response amplitude
was consistently statistically significantly ( p < 0.05) reduced and
the time to maximum amplitude was reduced (Brammer, 1998).
In the main study, groups of 10 male and 10 female Wistar-derived
Alpk:ApfSD rats were given a single dose of 0, 5, 15, or 75 mg/kg bw
of cyhalothrin (purity, 97.2%) by oral gavage in corn oil. An auditory
startle habituation test was performed 7 h after dosing on day 1 and
on day 8 with an automated recording apparatus. The animals were
killed after 8 days but were not examined post mortem. The study was
performed in accordance with GLP.
Body-weight gain was reduced in males at the highest dose and in
females at the two lower doses. Transient clinical signs of toxicity
were seen at the highest dose. Those seen at day 2 included subdued
behaviour, ataxia, high-stepping gait, salivation, and tip-toe gait,
and 'some of these signs' were also seen 7 h after dosing on day 1.
There were no signs of toxicity from day 3 until the end of the study.
In the startle response test, there was no effect on time to
maximum amplitude in either sex on day 1 or day 8. A reduction in the
mean response amplitude was seen, however, on day 1 in animals of each
sex at the highest dose. Statistically significant (two-sided Student
t test: p < 0.05), but not dose-related, reductions in mean
response amplitude were also seen on day 1 in females at all doses. No
effects were seen on day 8.
The authors reported that the NOEL for this study was 15 mg/kg
bw, as they dismissed the effects on response amplitude because of the
absence of a clear dose-response relationship. The Committee disagreed
with this conclusion. It has been reported that type II pyrethroids
generally decrease rather than increase the startle response to sound,
although this is a complex response and at low doses some type II
pyrethroids give an increased startle response (Hijzen & Slangen,
1988; Ray, 1991). Thus, although there was a NOEL for a reduced
response, lower doses may have caused an increased response, which
would indicate a lower NOEL. The Committee also noted that the startle
response was not evaluated at the most sensitive time, i.e. the time
of peak expression of acute toxicity as shown by clinical signs. No
NOEL could be identified (Brammer, 1999).
Inhibitory avoidance test
Newborn Wistar rat pups were exposed to cyhalothrin (purity
unspecified) in their mother's milk from birth until weaning at 21
days of age, and at 97, 104, and 111 days of age they were tested in
inhibitory avoidance tests. Cyhalothrin was given to nursing dams in
their drinking-water as a 200-mg/L solution in 400 mg/L sucrose. This
would be expected to provide a dose of cyhalothrin of approximately 20
mg/kg bw per day to the dams (assuming that 200-g rats drink about 20
ml/day). The intake of the pups was not measured, but the treated
groups consisted of 20 pups and the control group of 15. Control dams
were given sucrose solution at 400 mg/L. Drink was available
ad libidum to eight cyhalothrin-exposed dams and six control dams.
No clinical signs of toxicity were reported in either the dams or
the pups. The body weights at weaning and at 90 days of age were not
affected. The behaviour of the dams was unaffected by treatment
throughout the 21 days of lactation. The motor activity of the pups at
weaning was unaffected by the treatment. At 90 days, eight pups
exposed to cyhalothrin and 11 control pups were chosen (all the males)
for training and testing in a shuttle box to evaluate inhibitory
avoidance behaviour. Each rat was trained once by placing it in a
well-lit box and then opening a door to a dark compartment and giving
the rat an electric shock to the foot when it entered the dark
compartment. The test is a measure of the time taken by the rats to
cross again from the well-lit area to the dark compartment. Each rat
was tested once at 97, 104, and 111 days of age, receiving no electric
shock. At all times, there was a reduction in the learning avoidance
latency of the treated group, but the effect was statistically
significant (Mann-Whitney U-test: p < 0.05) only at 97 and 104 days
of age and not at 111 days. No NOEL could be identified (Moniz et al.,
1990).
In a separate study, described above, rats born to dams treated
dermally with an aqueous solution of cyhalothrin were tested for
behavioural changes. Inhibitory avoidance behaviour was unaffected by
the treatment, and there was no consistent effect on locomotor
frequency. The animals did, however, show a decreased motivational
response when they were tested as adults in a hole board test. No NOEL
could be identified for this study because of the production of subtle
but persistent neurobehavioural effects at the only dose tested. It
was also difficult to estimate the amount of cyhalothrin received by
the offspring (da Silva Gomes et al., 1991b).
2.2.7 Immunotoxicity
Some pyrethroids have been reported to be potent allergens that
may cause allergic rhinitis, asthma, or allergic alveolitis (Marrs,
1993). Cyhalothrin has not been specifically tested for immunotoxicity
but in a study of toxicity in mice given repeated doses, decreased
total leukocyte counts, decreased lymphocyte counts, and increased
neutrophil counts were observed in male but not female mice that had
consumed feed containing cyhalothrin for 28 days (Colley et al.,
1981). In the same study, atrophy of the red pulp of the spleen
occurred in some females given the high dose. Toxicological studies in
other species did not show alterations in total or differential
leukocyte counts and showed no histopathological changes in most
organs potentially involved in immunity (thymus, spleen, lymph nodes,
bone marrow), as described above.
A Magnusson and Kligman maximization test and a Buehler test both
showed that cyhalothrin is a skin sensitizer in guinea-pigs (European
Medicines Evaluation Agency, 1999; WHO, 1990). A Magnusson and Kligman
maximization test with lambda-cyhalothrin, however, showed no skin
sensitization in guinea-pigs (WHO, 1990).
2.3 Observations in humans
Cases of severe poisoning with pyrethroids are rare, but some
cases, for example in China, have been reported, including a few
deaths. Absorbed pyrethroids are rapidly detoxified by esterases, so
that any systemic effects would be of short duration (Marrs, 1993).
Topical application of pyrethrins or pyrethroids to the skin
produces local effects unrelated to their systemic action. The
synthetic pyrethroids have a characteristic irritating effect that is
not associated with inflammation and appears to be unique to
pyrethroids. The initial lesions are tenacious, painful pruritus
(pricking sensation) followed by a local burning sensation with
blotchy erythema that lasts about 2 days after cessation of exposure;
when exposure is intense, it is associated with numbness. Later,
desquamation may occur on the contaminated area of skin. There appear
to be no lasting ill effects. The condition is produced by all classes
of pyrethroids although most readily by type II pyrethroids such as
cyhalothrin. Repeated exposure to systemically toxic doses of
pyrethroids can produce a different effect: peripheral nerve damage
can occur subsequent to severe motor symptoms (Aldridge, 1990; Ray,
1991)
No clinical or haematological effects were observed in six
volunteers given a single dose of 5 mg of lambda-cyhalothrin in corn
oil (equal to 0.05-0.07 mg/kg bw) (European Medicines Evaluation
Agency, 1999). The route of administration was not reported, but it
seems likely to have been oral.
In the study of Pakistani pesticide workers described in section
2.2, the average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). Transient signs of toxicity, lasting up to 24
h, were reported by the workers, which included skin paraesthesia,
feeling hot, feeling cold, numbness, irritation of the skin, red eyes,
coughing, and sneezing. Medical examination revealed one case of face
rash that lasted 2 days (Chester et al., 1992).
In a study carried out in a village in the United Republic of
Tanzania, a lambda-cyhalothrin-based insecticide was sprayed inside
houses and shelters at a coverage of approximately 25 mg/m2. The
insecticide was supplied as a water-dispersible powder in a soluble
sachet. Every day for 6 days, 12 spraymen and 3 squad leaders were
interviewed about symptoms. Each sprayman used up to 62 g of
lambda-cyhalothrin over 2.7-5.1 h each day. The spraymen wore personal
protective equipment (rubber boots and gloves, cotton overalls, caps,
and gauze nose-mouth masks) which left much of the face exposed. One
sprayman also used a face shield for 3 days.
All the spraymen complained at least once of symptoms related to
exposure to lambda-cyhalothrin. The commonest symptoms were itching
and burning of the face and nose and throat irritation, frequently
accompanied by sneezing or coughing. Facial symptoms occurred only on
unprotected areas, and the worker who wore a face shield was free of
facial symptoms. All the symptoms had disappeared by the morning after
the spraying. The number of subjects affected and the duration of
facial symptoms were proportional to the amount of compound sprayed.
These parameters were not affected by use of lambda-cyhalothrin in the
previous 6 months. A sample of occupants was interviewed 1 and 5-6
days after their houses had been sprayed. One woman who entered her
house 30 min after the end of spraying complained of periorbicular
itching, but this lasted only a few minutes. The other inhabitants of
sprayed houses reported no other insecticide-related adverse effects.
Furthermore, a squad leader who entered almost every house a few
minutes after spraying reported no symptoms (Moretto, 1991)
As part of a field trial conducted in South India,
electrophysiological tests were conducted on 15 spraymen aged 19-48
years, before and after exposure to lambda-cyhalothrin (formulation
and purity unspecified). The tests performed on the subjects comprised
conduction of the right median, common peroneal, and facial motor
nerves; conduction of the right median and sural sensory nerves; blink
response with stimulation of the right supra-orbital nerve and
recording of R1 and R2 responses from the right orbicularis oculi
muscle with a pair of surface electrodes; concentric needle
electromyography of the tibialis anterior; repetitive stimulation of
the right median nerve at the wrist at 3 and 20 Hz and recording of
the responses from the abductor pollicis brevis; and multi-modality
visual, brainstem, auditory and somatosensory evoked potentials. The
evoked potentials were measured in only six of the subjects, but the
other measurements were made in all 15 subjects.
Clinical observation revealed no changes, and facial nerve
conduction, blink response, responses to repetitive stimulation, and
visual, auditory, and somatosensory evoked potentials were all normal.
Six of the 15 subjects had mild changes in peripheral nerve conduction
parameters (paired t test: p < 0.05), but comparison of the mean
values for the various nerve conduction parameters before and after
exposure showed no significant difference except for prolongation of
distal motor latency of the median nerve. Studies of nerve conduction
12-16 months later in three subjects who had shown abnormalities
immediately after exposure showed normal rates. The authors concluded
that occupational exposure to lambda-cyhalothrin can produce
transient, subclinical electrophysiological changes in the nerves of
the upper limbs (Arunodaya et al., 1997).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity,
neurotoxicity, and neurobehavioural effects of cyhalothrin and
observations of effects in humans exposed to this compound. Although
some of the studies were not fully compliant with codes of GLP, all of
the pivotal studies were carried out according to appropriate
standards for study protocol and conduct.
Oral doses of cyhalothrin were readily but incompletely absorbed
in the species studied (rats and dogs), and the subsequent metabolism
was similar, involving initial cleavage of the molecule at the ester
bond, presumably resulting in detoxification. The metabolites were
rapidly excreted, some as conjugates, whereas small amounts of
unchanged cyhalothrin persisted as residues in fatty tissues. Similar
results were seen in food-producing animals.
Serum and urine from exposed workers contained the metabolites
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)benzoic acid. These metabolites are products of
cleavage of the cyhalothrin molecule at the ester bond, and their
presence suggests that the initial metabolism of this compound in
humans is similar to that in the animal species that have been
investigated.
No toxicological studies on metabolites of cyhalothrin were
available, but the Committee considered it likely that the metabolites
would be less toxic than cyhalothrin, as none contains an intact
pyrethroid structure.
The acute toxicity of cyhalothrin is characterized by effects on
the nervous system, principally the central nervous system. The signs
of toxicity are typical of type II pyrethroids and comprise writhing,
salivation, exaggerated jaw opening, increasing extensor tone in the
hind legs causing a rolling gait, poor coordination progressing to
coarse tremor, tonic seizures, and apnoea. The LD50 values after
oral administration depended on the vehicle used but varied from 37 to
62 mg/kg bw in mice and from 51 to 240 mg/kg bw in rats. Higher values
were found in other species or after administration by other routes.
In mice that received a dose of 0, 5, 10, 20, 40, or 80 mg/kg bw
per day orally for 5 days, ataxia and convulsions were seen in those
given the two higher doses. The dose of 20 mg/kg bw per day caused
body-weight loss, ataxia, and rough coat. At 5 mg/kg bw per day, the
only adverse effect was a rough coat.
In a 28-day study in mice, cyhalothrin was added to the diet at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of feed. Atrophy of
the red pulp of the spleen and an increased mortality rate were seen
at the highest dose. Adaptive liver changes, including enlarged liver,
centrilobular hepatocellular hypertrophy, increased activity of
aminopyrine- N-demethylase, and proliferation of smooth endoplasmic
reticulum were seen at concentrations of 100 mg/kg of feed and above;
lowered lymphocyte counts were also seen at these doses. At 25 mg/kg
of feed, the only effect seen was piloerection. The NOEL was 5 mg/kg
of feed, equal to 0.65 mg/kg bw per day, on the basis of piloerection
at higher doses.
The main effects in five 10-90-day studies of toxicity in rats
were adaptive changes in the liver similar to those seen in mice. In a
28-day range-finding study in which rats were fed diets containing
cyhalothrin at a concentration of 0, 5, 10, 20, or 250 mg/kg of feed,
only the highest concentration caused adverse effects. These effects
were characterized by hepatocellular hypertrophy, increased activity
of aminopyrine- N-demethylase in the liver, and proliferation of
hepatic smooth endoplasmic reticulum. The NOEL was 20 mg/kg of feed,
equivalent to 2 mg/kg bw per day. In a 28-day study in rats given 0,
1, 5, 10, 20, or 250 mg/kg of feed, the activity of hepatic
aminopyrine- N-demethylase was increased and proliferation of hepatic
smooth endoplasmic reticulum was seen at the highest dose only. No
such effect was seen in the liver at doses equivalent to 2 mg/kg bw
per day or less. Females given 10 mg/kg of feed or more had decreased
body-weight gain. The NOEL was 5 mg/kg of feed, equivalent to 0.5
mg/kg bw per day, but this observation was not in line with the
findings of other short-term studies of toxicity in rats. A third
28-day study in rats was inadequately reported but showed that
administration of cyhalothrin at 20 mg/kg of feed (equivalent to 2
mg/kg bw per day) caused proliferation of hepatic smooth endoplasmic
reticulum; a NOEL was not identified in this study.
A 90-day study was performed in which rats given cyhalothrin at
0, 10, 50, or 250 mg/kg of feed. Males given the two higher doses
showed increased activity of hepatic aminopyrine- N-demethylase and
proliferation of hepatic smooth endoplasmic reticulum. Females at the
highest dose showed increased activity of hepatic
aminopyrine- N-demethylase activity. The NOEL was 10 mg/kg of feed,
equal to 0.56 mg/kg bw per day.
Groups of dogs received cyhalothrin at a dose of 0, 2.5, 10, or
30 mg/kg bw per day for 4 weeks or a dose of 0, 1.0, 2.5, or 10 mg/kg
bw per day for 26 weeks. In the 4-week study, the two higher doses
caused an increased incidence of vomiting, and the highest dose caused
body-weight loss, unsteady gait, and increased serum alanine and
aspartate aminotransferase activities. Muscular trembling was seen in
dogs in all groups, including the controls, in the 4-week study. In
the 26-week study, doses of 10 mg/kg bw per day or more caused
clinical signs including vomiting, salivation, lack of coordination,
unsteadiness, collapse, muscular spasms, and convulsions. As treatment
at all doses in both studies increased the prevalence of liquid
faeces, a NOEL was not identified. The Committee considered it
possible that the liquid faeces were a consequence of the neurological
effects of cyhalothrin. The LOEL was 1.0 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in mice,
cyhalothrin was given in the diet at a concentration of 0, 20, 100, or
500 mg/kg of feed for 104 weeks. An increased incidence of mammary
adenocarcinoma was seen in females at the two higher doses. The
highest incidence (14%) was only slightly greater then the upper limit
of the range in historical controls (2-12%). The Committee could not
exclude the possibility that the adenocarcinomas seen in the groups
given 100 or 500 mg/kg of feed were caused by cyhalothrin. Clinical
signs of toxicity (piloerection and hunched posture) and increased
serum activities of aspartate and alanine aminotransferases were seen
at these doses. The NOEL for these effects was 20 mg/kg of feed, equal
to 1.9 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in rats,
cyhalothrin was given in the diet at a concentration of 0, 10, 50, or
250 mg/kg of feed for 104 weeks. There was no treatment-related
increase in the incidence of any type of tumour. Adverse effects found
at the highest dose included decreased body weight, altered blood
biochemistry, and an increased weight of the liver relative to that of
the body in animals of each sex and of the adrenals in females only.
The NOEL was 50 mg/kg of feed, equal to 2.3 mg/kg bw per day.
Cyhalothrin was not genotoxic in a range of studies, including a
test for reverse mutation in Salmonella, a test for cytogenetic
effects in the bone marrow of rats treated in vivo, a test for
dominant lethal mutation in mice, and an assay of cell transformation
in vitro. The Committee concluded that cyhalothrin is not genotoxic.
Furthermore, the Committee considered it likely that the mammary
adenocarcinomas found in mice in the long-term study were due to a
non-genotoxic mechanism.
A three-generation study of reproductive toxicity was performed
in rats given cyhalothrin in the diet at a concentration of 0, 10, 30,
or 100 mg/kg of feed. Adverse effects, including reduced parental
body-weight gain and reduced litter size, were found only at the
highest dose. The NOEL for these effects was 30 mg/kg of feed, equal
to 1.7 mg/kg bw per day.
In a study of developmental toxicity, rats received a dose of 0,
5, 10, or 15 mg/kg bw per day by oral gavage on days 6-15 of
gestation. Maternal toxicity, characterized by body-weight loss and
poor coordination, was seen at the highest dose. Embryotoxicity also
occurred at this dose. Abnormalities were seen in 5 of 17 fetuses in
one litter from a dam at 10 mg/kg bw per day, but the effect was
considered not to be due to treatment with cyhalothrin as no
fetotoxicity was seen at the higher dose. The NOEL for maternal
toxicity was 10 mg/kg bw per day, and that for developmental toxicity
was 15 mg/kg bw per day, the highest dose tested.
Two studies of developmental toxicity in rats treated by dermal
administration provided some evidence that cyhalothrin can delay fetal
development at doses lower than those given orally. However, the
Committee considered that oral administration is a more relevant route
and that the NOEL in the study in which this route was used was the
appropriate one for evaluating developmental toxicity in rats.
Rabbits studied for developmental toxicity were given a dose of
0, 3, 10, or 30 mg/kg bw per day by oral gavage. The highest dose
caused initial body-weight loss in the does, which was followed by
reduced body-weight gain. There was no significant effect on
development at any dose. The NOEL for maternal toxicity was 10 mg/kg
bw per day, and the NOEL for developmental toxicity was 30 mg/kg bw
per day, the highest dose tested.
Single oral doses of up to 10 000 mg/kg bw did not induce
clinical or histopathological signs of neurotoxicity in hens.
Various studies of neurobehavioural effects have been performed
in rats, including a range-finding test of performance on an inclined
plane, a test for acute auditory startle response, and tests of
inhibitory avoidance. In the inclined plane test, rats were given a
single oral dose of 0, 15, 25, 30, 40, 50, 60, 75, 100, or 200 mg/kg
bw. All of these doses caused soft faeces in at least some of the
rats, and doses of 25 mg/kg bw or more caused clinical signs of
neurotoxicity. The results were, however, variable, and no conclusion
could be reached about neurotoxicity.
In the test for acute auditory startle response in which an oral
dose of 0, 5, 15, or 75 mg/kg bw was given, the highest dose resulted
in reduced body-weight gain, transient clinical signs, and a reduced
mean response amplitude in animals of each sex. Statistically
significant but not dose-related reductions in mean response amplitude
were also seen at 5 and 15 mg/kg bw in females only. The Committee was
aware that a biphasic auditory startle response has been reported with
some other type II pyrethroids, with a reduced response at high doses
and an increased response at lower doses. A NOEL for the startle
response was not identified in this study.
In the inhibitory avoidance tests, rats were exposed either
in utero (dams were given 200 mg/l in drinking-water) or during
lactation (dams were given 1 ml of a 0.018% solution dermally). The
doses received by the animals could not be estimated reliably. The
rats exposed in utero showed a decreased motivational response when
tested as adults, but no effects were seen on inhibitory avoidance
behaviour or locomotor frequency. Animals exposed during lactation had
a shorter latency in learning avoidance behaviour. There was no NOEL
in either study, as adverse effects were seen at the only dose tested
in each study and the doses received by the animals were not known.
4. EVALUATION
Observations and case reports in humans provided little
information relevant to the establishment of an ADI for cyhalothrin.
In most instances, no information on doses was available, the route of
exposure was dermal, and some studies were of exposure to
lambda-cyhalothrin rather than cyhalothrin.
The Committee established a temporary ADI of 0-0.002 mg/kg bw for
cyhalothrin by applying a 500-fold safety factor to the LOEL of 1.0
mg/kg bw per day for induction of liquid faeces in dogs in the 26-week
study. The Committee considered it possible that the liquid faeces
were a consequence of the neurological effects of cyhalothrin. The
Committee applied the 500-fold safety factor to account for the
absence of a NOEL for liquid faeces in dogs and because of the absence
of a NOEL for neurobehavioural effects. The ADI was made temporary
because cyhalothrin belongs to a class of substances that are
characterized by their toxicity to the central nervous system, and
therefore neurobehavioural effects may be the most sensitive indicator
of the toxicity of this compound. There was an adequate margin of
safety between the ADI and the NOELs identified in the studies on
cyhalothrin, including the NOEL of 0.65 mg/kg bw per day for
piloerection in the 28-day study in mice.
The results of studies appropriate for establishing a NOEL for
neurobehavioural effects in laboratory animals are required for
evaluation in 2002.
The Committee noted that the NOEL for toxicological effects other
than the neurotoxicity related to the pyrethroid structure was 2.3
mg/kg bw per day for adaptive changes in the livers of rats in the
long-term study. This suggests that the toxicity of the metabolites
(none of which has a pyrethroid structure) is no greater than 50% of
that of the parent drug.
5. REFERENCES
Aldridge, W.N. (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit. Rev. Toxicol., 21,
89-103.
Anderson, D., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B. &
Godley, M.J. (1983) Cyhalothrin: A cytogenetic study in the rat.
Unpublished study, report No. CTL/P/664, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Arunodaya, G.R., Kumar, K.R. & Swamy, H.S. (1997) Electrophysiologic
study of spraymen exposed to insecticide lambdacyhalothrin.
Neurol. India, 45, 189-192.
Barnes, J.M. & Verschoyle, R.D. (1974) Toxicity of new pyrethroid
insecticides. Nature, 248, 711.
Bicknell, C.E., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Milk
residues of cyhalothrin following a 10 mL application of a 2%
pour-on. Unpublished study, report No. CIBH 89-1, Coopers Animal
Health, Berkhamsted, United Kingdom. Submitted by Schering-Plough
Animal Health, Harefield, United Kingdom.
Bloomquist, J.R., Adams, P.M. & Soderlund, D.M. (1986) Inhibition of
gamma-amino butyric acid-stimulated chloride flux in mouse brain
vesicles by polycycloalkane and pyrethroid insecticides.
Neurotoxicology, 7, 11-20.
Brammer, A. (1998) Cyhalothrin: Preliminary acute startle response
study in rats. Unpublished study, report No. CTL/L/8516, Zeneca
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Brammer, A. (1999) Cyhalothrin: Acute startle response study in rats.
Unpublished study, report No. CTL/P/6162, Zeneca Central
Toxicology Laboratory, Macclesfield, Cheshire, United Kingdom.
Campana, M.A., Panzeri, A.M., Moreno, V.J. & Dulout, F.N. (1999)
Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using
the micronucleus test in erythrocytes of the fish Cheirodon
interruptus interruptus. Mutat. Res., 438, 155-161.
Chester, G., Sabapathy, N.N. & Woollon, B.H. (1992) Exposure and
health assessment during application of lambda-cyhalothrin for
malaria vector control in Pakistan. Bull. World Health Organ.,
70, 615-619.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
Gopinath, C. & Prentice, D. (1981) Cyhalothrin. Oral toxicity
study in beagle dogs (Final report: repeated daily dosing for 26
weeks). Unpublished study, report No. ICI 326/8162, Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, United Kingdom.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Prentice, D.,
Buckley, P. & Offer, J. (1983) PP 563. Preliminary oral toxicity
study in beagle dogs. Unpublished study, report No. ICI 325/8074,
Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom.
Coats, J.R. (1990) Mechanisms of toxic action and structure
relationships for organochloride and synthetic pyrethroid
insecticides. Environ. Health Perspectives, 87, 255-262.
Colley, J.C., Loane, J.A., Heywood, R., Gibson, W.A., Chasseaud, L.F.,
Edmundson, N., Lewis, D. & Woodhouse, R.N. (1980) PP563. 28-day
dose range finding study in rats. Unpublished study, report No.
ICI/321/8041, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Down, W.H., Woodhouse, R.N.,
Gibson, W.A., Gopinath, C., Zubaidy, A.J. & Prentice, D.E. (1981)
Cyhalothrin. 4-week dose range finding study on mice. Unpublished
study, report No. ICI/379/80910, Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Almond, R., Gibson, W.A.,
Gregson, R. & Gopinath, C. (1984) Cyhalothrin. Potential
tumorigenic and toxic effects in prolonged dietary administration
to mice (Final report). Unpublished study, report No.
ICI/395/83668, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Denton, S. (1988) Cyhalothrin: Preliminary neurotoxicity study in the
rat following a single oral dose. Unpublished study, report No.
S8104-001R, Covance, Harrogate, United Kingdom.
Deshmukh, P.B. (1992) Three-generation reproductive studies of a
synthetic pyrethroid, cyhalothrin. Toxicol. Lett., 64/65,
770-781.
Dow, P.R. & Parker, R.C. (1989) Attempted identification of 3 major
liver metabolites following dermal application of C-14 alcohol
lambda-cyhalothrin to lactating cows. Unpublished study, report
No. CIBH 89-2, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Dow, P.R., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989)
Metabolism of C-14 alcohol labelled lambda-cyhalothrin in
lactating cows following dermal application. Unpublished study,
report No. CIBH 88-4, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Ebino, K., Suzuki, H. & Harada, T. (1984a) PP-563: Acute oral toxicity
in rats. Unpublished study, report No. 4/HD/006931, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984b) PP-563 WP: Acute oral
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984c) PP-563: Acute
intraperitoneal toxicity in rats. Unpublished study, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984d) PP-563: Acute dermal
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984e) PP-563: Acute subcutaneous
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
European Medicines Evaluation Agency (1999) Cyhalothrin. Summary
report of the Committee for Veterinary Medicinal Products,
EMEA/MRL/699/99. Internet:
http://web.is.eudra.org/vetdocs/vets/mrl.htm.
Gassner, B., Wuthrich, A., Scholtysik, G. & Solioz, M. (1997) The
pyrethroids permethrin and cyhalothrin are potent inhibitors of
the mitochondrial complex I. J. Pharmacol. Exp. Ther., 281,
855-860.
Harrison, M.P. (1981) Cyhalothrin: The disposition and metabolism of
14C-ICI 146,814 in rats. Unpublished study, report No. 146814
KMR 002/01, Imperial Chemical Industries plc, Macclesfield,
Cheshire, United Kingdom.
Harrison, M.P. (1983) Cyhalothrin:The metabolism and disposition of
14C-ICI 146,814 in the rat; Part IV: Isolation and
identification of the major urinary metabolites derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 following oral
administration. Unpublished study, report No. YIBH 85-C9,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984a) Cyhalothrin: The metabolism and disposition of
14C-ICI 146,814 in rats; Part II, tissue residues derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 after a single
oral dose of 1 or 25 mg/kg bw. Unpublished study, report No. KMR
002/02, Imperial Chemical Industries plc, Macclesfield, Cheshire,
United Kingdom.
Harrison, M.P. (1984b) Cyhalothrin (ICI 146,814): The metabolism and
disposition of ICI 146,814 in rats; Part III, Studies to
determine radioactive residues in rats following 14 days repeated
administration. Unpublished study, report No. KMR 002/03,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984c) Cyhalothrin (ICI 146,814): The disposition and
metabolism of ICI 146,814 in the dog. Unpublished study, report
No. 146814KMD 005, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Harrison, M.P. (1985) Cyhalothrin (ICI 146,814): The metabolism,
excretion and residues in the cow after 7 days oral
administration with two [14C]-labelled forms of ICI 146,814 at
1 mg/kg/day. Unpublished study, report No. YIBH 85-C11, Imperial
Chemical Industries plc, Macclesfield, Cheshire, United Kingdom.
Hijzen, T.H. & Slangen, J.L. (1988) Effects of type I and type II
pyrethroids on the startle response in rats. Toxicol. Lett.,
40, 141-152.
Irvine, L.F.H. (1981a) Cyhalothrin: Oral (gavage) range finding study
in the male mouse. Unpublished study, report No. 2647-72/212,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Irvine, L.F.H. (1981b) Cyhalothrin: Oral (gavage) dominant lethal
study in the male mouse. Unpublished study, report No.
2647-72/213, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
JMPR (Joint FAO/WHO Meeting on Pesticide Residues) (1985) Pesticide
residues in food - 1984. Report of the Joint Meeting on
Pesticide Residues. FAO Plant Production and Protection Paper
62, Rome: Food and Agriculture Organization of the United
Nations.
Jones, J.R. (1980) Cyhalothrin: Acute oral median lethal dose (LD50)
in the female rabbit. Unpublished study, report No. 2364-72/214,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1980) Cyhalothrin: Oral (gavage) maximum tolerated dose
study in the non-pregnant rat. Unpublished study, report No.
2537-72/206, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981a) Cyhalothrin: Oral (gavage) dose ranging study in
the pregnant New Zealand white rabbit. Unpublished study, report
No. 2603-72/210, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in
the rat. Unpublished study, report No. 2661-72, Hazleton
Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1981c) Cyhalothrin: Oral (gavage) teratology study in
the New Zealand white rabbit. Unpublished study, report No.
270072/211, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Metabolism of C-14
alcohol labelled lambdacyhalothrin in lactating cows following
dermal application. Unpublished study, report No. CIBH 88-2,
Coopers Animal Health, Berkhamsted, United Kingdom. Submitted by
Schering-Plough Animal Health, Harefield, United Kingdom.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationships. Pest. Biochem.
Physiol., 18, 9-14.
Leahey, J.P., French, D.A. & Heath, J. (1985) PP321: Metabolism in a
goat. Unpublished study, report No. RJ 0435B, Imperial Chemical
Industries, Plant Protection Division, Jealott's Hill Research
Station, Bracknell, Berkshire, United Kingdom.
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
I., Robinson, M. & Stonard, M. (1981) Cyhalothrin: 90-day feeding
study in rats. Unpublished report No. CTL/P/629, Imperial
Chemical Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Marrs, T.C. (1993) Toxicology of pesticides. In: Ballantyne, B.,
Marrs, T.C. & Turner, P., eds, General and Applied Toxicology,
Basingstoke:MacMillan, Vol. 2, Ch. 62, p. 1334.
Milburn, G.M., Banham, P., Godley, M.J., Pigott, G. & Robinson, M.
(1984) Cyhalothrin: Three-generation reproduction study in the
rat. Unpublished study, report No. CTL/P/906, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Moniz, A.C., Bernadi, M.M., Souza-Spinosa, H.S. & Palermo-Neto, J.
(1990) Effects of exposure to a pyrethroid insecticide during
lactation on the behaviour of infant and adult rats. Braz. J.
Med. Biol. Res., 23, 45-48.
Moretto, A. (1991) Indoor spraying with the pyrethroid insecticide
lambda-cyhalothrin: Effects on spraymen and inhabitants of
sprayed houses. Bull. World Health Organ., 69, 591-594.
Moyes, A., Godley, M.J., Hall, M., Pratt, I., Stonard, M.D. & Tinston,
D.J. (1984) Cyhalothrin: 28-day feeding study in the rat (second
study). Unpublished report No. CTL/P/1013, Imperial Chemical
Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Narahashi, T. (1985) Nerve membrane ionic channels as the primary
target of pyrethroids. Neurotoxicology, 2, 3-22.
Nixon, J. & Jackson, S.J. (1981) Cyhalothrin: Acute toxicity.
Unpublished study, report No. CTL/T/555, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Pigott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
Robinson, M., Taylor, K. & Tinston, D.J. (1984) Cyhalothrin. Two
year feeding study in rats. Unpublished report, report No.
CTL/1023, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Prentice, D.E., Edmondson, N.A. & Lewis, D.J. (1981) 28 day dose range
finding study of cyhalothrin in dietary administration to mice.
Electron microscopy of livers. Unpublished report No.
5/HA/003712, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Pritchard, V.K. (1984) Cyhalothrin: Acute oral toxicity. Unpublished
study, report No. CTL/P/1023, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Ray, D. (1991) Chapter 13. In: Hayes, W.J., Jr & Laws, E.R., Jr, eds,
Handbook of Pesticide Toxicology, London: Academic Press, Vol.
2, pp. 585-636.
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. (1981) Cell
transformation test for potential carcinogenicity of
Y00102/010/005 (cyhalothrin (PP563)). Unpublished study, report
Number CTL/1023, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wright, D.G.D.
(1982) The acute oral toxicity (LD50) and neurotoxic effects of
cyhalothrin to the domestic hen. Unpublished study, report Number
ICI/374 NT/81742, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991a)
Effects of prenatal pyrethroid exposure on the sexual development
of rats. Vet. Hum. Toxicol., 33, 427-428.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991b)
Pyrethroid insecticides and pregnancy: Effect on physical and
behavioural development in rats. Vet. Hum. Toxicol., 33,
315-317.
Tomlin, C., ed. (1994) The Pesticide Manual, 10th Ed., Cambridge:
Crop Protection Publications / Royal Society of Chemistry.
Trueman, R.W. (1981) Cyhalothrin: Results from the Salmonella
reverse mutation assay. Unpublished study, report No. CTL/P/665,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Vijverberg, H.P.M. & van den Bercken, J. (1990) Neurotoxicological
effects and mode of action of pyrethroid insecticides. Crit.
Rev. Toxicol., 21, 105-126.
Vijverberg, H.P.M., van den Zalm, J.M. & van den Bercken, J. (1982)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature, 295, 601-603.
WHO (1990) Cyhalothrin. Environmental Health Criteria 99, Geneva:
World Health Organization.
Woodward, K. (2000) Personal communication from Kevin Woodward of
Schering-Plough Animal Health, Uxbridge, United Kingdom, to Mr
Derek Renshaw of the Department of Health. E-mail message dated
14 January 2000.
Zufan, L., Kubat, J., Formanek, J., Fuchs, A., Tobiskova, G., Zajicek,
P., Vodickova, J., Rehak, P. & Dvorak, J. (1989) Behavioural and
EEG changes induced by the pyrethroid insecticide cyhalothrin.
Int. J. Psychophysiol., 7, 447-448.
 Excretion was initially rapid, most of the administered doses
being eliminated within the first 48 h after dosing, but excretion was
still incomplete at 7 days. After 14CHCN-cyhalothrin was given
orally, approximately 30% of the administered dose was found in urine
at 7 days and about 50% in faeces. When 14C-cyclopropyl-cyhalothrin
was given, the proportion of residue in urine was slightly lower (19%)
and the amount in faeces higher (68%). The proportions of residue in
urine and in faeces were approximately equal when the two
radiolabelled forms of cyhalothrin were given intravenously. A large
proportion (46-87%) of the faecal residue was in the form of unchanged
cyhalothrin after oral dosing, but only small amounts (1.4-1.5% of
faecal radiolabel) were found in faeces after intravenous injection,
suggesting that the oral dose of cyhalothrin was only partially
absorbed from the gut lumen, leaving unabsorbed cyhalothrin in the
faeces.
Metabolism occurred initially by cleavage of the ester bond to
give a phenoxybenzyl moiety and a cyclopropane acid moiety. Further
metabolism of the phenoxybenzyl moiety produced N-(3-phenoxybenzoyl)
glycine, 3-(4'-hydroxyphenoxy)benzoic acid and its sulfate conjugate,
3-phenoxybenzoyl glucuronide, small amounts of various other
conjugates of these compounds, and a little free phenoxybenzoic acid.
The cyclopropane acid moiety was extensively metabolized to at least
11 further metabolites, including 45% as the glucuronide of the
cyclopropane acid and up to 23% as the free cyclopropane acid (i.e.
3-( Z-2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclo-propane
carboxylic acid; see Figure 1 (Harrison, 1984c).
Humans
In a study of three teams of seven pesticide applicators in
Pakistan, the men were monitored over 15 days while they were using a
pesticide formulation which contained 10% lambda-cyhalothrin and for a
further 4 days after they had finished spraying. The men wore typical
work clothing with no personal protective equipment while spraying
dwellings to kill mosquitoes as part of a malaria control programme.
All of the workers underwent four medical examinations, including
blood sampling, before the study, in the middle of the first week, in
the middle of the second week, and 4 days after the last spraying, and
were questioned about adverse effects. Urine samples were collected
daily. Fifteen men who lived in the treated dwellings were monitored
for exposure by collection and analysis of 24-h urine samples for 3
consecutive days after the spraying of their houses. The samples of
urine and serum were treated enzymically to deconjugate the
metabolites and were then analysed by gas chromatography with mass
spectroscopy. The cyhalothrin metabolites measured were
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)-benzoic acid. The limit of determination for all
three metabolites was 0.01 µg/ml. These metabolites are products of
cleavage of the ester bond in cyhalothrin, and their presence
indicates that the initial metabolism of cyhalothrin is similar in
humans to that observed in other species.
The average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). 3-(2-Chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid was usually the most abundant
metabolite in urine. No metabolites were detected in serum samples.
Most of the inhabitants of treated houses had no detectable residues
in their urine, although they were measurable in 12 of 44 samples, and
the measured concentrations were at or close to the limit of
determination. Most values were not reported, but the highest
concentration of urinary metabolites was 0.1 mg/person per day
(Chester et al., 1992). The signs of toxicity found are reported
below.
2.2 Toxicological studies
Most of the toxicological studies of cyhalothrin were performed
during 1980-84, and some were therefore conducted before GLP was
widely introduced. The chemical identity of the cyhalothrin used in
each study is not clearly stated, and, although the purity was
reported in most cases, the isomers and the isomer ratios were not
mentioned in most of the reports. The manufacturer has claimed that
the analysis given in appendices to the report of the inclined plane
test (see below) is typical of technical-grade cyhalothrin. The
manufacturer also claimed that measurements made over the last
15 years have shown little variation in the isomer ratio. Two
measurements from 1985 gave about 56% for the A isomeric pair and 41%
for the B isomeric pair, leaving only about 2% for other isomers and
impurities (Woodward, 2000).
2.2.1 Acute toxicity
Acute poisoning of rats with type II pyrethroids such as
cyhalothrin is typically characterized by progressive development of
'nosing' and exaggerated jaw opening, increasing extensor tone in the
hind limbs causing a rolling gait, choreoathetosis (sinuous writhing
spasms), salivation, incoordination progressing to coarse tremor,
tonic seizures, apnoea, and death (Barnes & Verschoyle, 1974; Ray,
1991). This has been called the choreoathetosis/salivation syndrome or
CS syndrome. In dogs, similar symptoms are seen, but salivation, upper
airway hypersecretion, and gastrointestinal symptoms are more
prominent than in rats (Ray, 1991).
As in poisoning with type I pyrethroids, the plasma noradrenaline
concentration is increased by type II pyrethroids, but they also
increase plasma adrenaline and blood glucose concentrations. Type II
pyrethroids increase cardiac contractility both directly by action on
cardiac muscle and via circulating catecholamines. They also cause
repetitive firing and potentiate contraction in skeletal muscle. The
type II pyrethroids do not produce the repetitive activity in sensory
nerves that is seen after exposure to type I pyrethroids (Ray, 1991).
Table 3. LD50 values for cyhalothrin
Species Sex Route Purity Vehicle LD50 Reference
(%) (mg/kg bw)
Rat M Oral 90/94 cis Corn oil 240 Nixon & Jackson (1981)
Rat M Oral 92.9 Corn oil 170 Pritchard (1984)
Rat M Oral 90 Oilve oil 51 Ebino et al. (1984a)
Rat F Oral 90/94 cis Corn oil 140 Nixon & Jackson (1981)
Rat F Oral 92.9 Corn oil 110 Pritchard (1984)
Rat F Oral 90 Olive oil 65 Ebino et al. (1984a)
Mouse M Oral 90/94 cis Corn oil 37 Nixon & Jackson (1981)
Mouse F Oral 90/94 cis Corn oil 62 Nixon & Jackson (1981)
Guinea-pig M Oral 90/94 cis Corn oil > 5000 Nixon & Jackson (1981)
Rabbit F Oral 89.2 Corn oil > 1000 Jones (1980)
Chicken F Oral 93.4 Corn oil > 10 000 Roberts et al. (1982)
Rat M Dermal 90 Undiluted 3600 Ebino et al. (1984d)
Rat F Dermal 90 Undiluted 3700 Ebino et al. (1984d)
Rabbit M Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rabbit F Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rat M Intraperitoneal 90/94 cis Corn oil 250-750 Nixon & Jackson (1981)
Rat M Intraperitoneal 90 Oilve oil 500 Ebino et al. (1984c)
Rat F Intraperitoneal 90 Oilve oil 420 Ebino et al. (1984c)
Rat M Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
Rat F Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
The acute toxicity of cyhalothrin was investigated in a series of
studies that complied with GLP (Nixon & Jackson, 1981; Roberts et al.,
1982; Pritchard, 1984) and also in studies that, although not GLP
compliant, were accompanied by a suitable quality assurance statement
(Jones, 1980; Ebino et al., 1984a-e). The LD50 values estimated from
these studies are listed in Table 3. The LD50 of a wettable
formulation containing 5% cyhalothrin was 1200 mg/kg bw in male rats
and 900 mg/kg bw in females when it was administered orally in olive
oil (Ebino et al., 1981b).
The signs of toxicity seen in all species and with all routes of
administration were similar and included subdued behaviour, ungroomed
appearance, piloerection, salivation, incontinence, and unsteady gait.
A further account of the signs of acute toxicity in rats receiving
various oral doses of cyhalothrin is given in the preliminary report
of the inclined plane test (Denton, 1988), which is described below.
2.2.2 Short-term studies of toxicity
Mice
In a range-finding study for an assay of dominant lethal mutation
that complied with GLP, groups of 10 male CD-1 mice were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 5, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 5 days. At
the two higher doses, signs of severe toxicity (ataxia and
convulsions) were observed. One animal at 40 mg/kg bw per day and
eight at 80 mg/kg bw per day died. Less severe clinical signs were
seen at 20 mg/kg bw per day, including body-weight loss, ataxia, and a
rough coat. Only rough coats were seen at 5 mg/kg bw per day (Irvine,
1981a).
In a range-finding study for a long-term study of toxicity and
carcinogenicity, groups of 12 male and 12 female CD-1 mice were fed
diets containing technical-grade cyhalothrin (purity unspecified) at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of diet. Over the
course of the 4-week study, these concentrations gave mean doses of
cyhalothrin of 0, 0.65, 3.3, 14, 64, and 310 mg/kg bw per day for
males and 0, 0.80, 4.2, 15, 78, and 290 mg/kg bw per day for females.
No certificate of compliance with GLP was available for this study,
but quality assurance systems were in place.
Deaths were seen only at the highest dietary concentration, at
which six males and three females died prematurely. Severe signs of
toxicity were also seen at this dose, including abnormal gait
('walking on toes'), hunched posture, decreased body weight (Student's
t test, p < 0.01), and increased respiratory rate. Piloerection
was observed in several animals in each groups given cyhalothrin at
> 25 mg/kg and in one control male and one male at 5 mg/kg of diet.
Haematological changes were seen in males (statistically significant
in Williams' test: p < 0.05) but not in females. Animals at 2000
mg/kg of diet had a decreased total leukocyte count accompanied by a
lower lymphocyte count and a higher neutrophil count. Those at 100 and
500 mg/kg of diet also had marginally lowered lymphocyte counts.
Macroscopic examinations post mortem revealed no adverse
effects other than altered organ weights (statistically significant in
both Student's t test and Williams' test: p < 0.05). The weight
of the kidney relative to body weight was slightly elevated in males
given 100, 500, or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The relative liver weights were also slightly increased in
males given 25 or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The heart weights were marginally decreased in females given
100 or 2000 mg/kg of diet. The activity of aminopyrine- N-demethylase
in liver was increased in males given 2000 mg/kg of diet and in
females given 100, 500, or 2000 mg/kg of diet (Student's t test:
p < 0.05). Histopathological examination showed minimal
centrilobular hepatocyte enlargement in two females at 2000 mg/kg of
diet. Atrophy of the red pulp of the spleen was seen in two of the
three females at this dose that died. The Committee considered that
the effect may have been indicative of immunological changes. The NOEL
was 5 mg/kg of diet, equal to 0.65 mg/kg bw per day, on the basis of
piloerection at concentrations > 25 mg/kg of diet (Colley et al.,
1981).
When liver samples from this study were examined by electron
microscopy, minimal amounts of smooth endoplasmic reticulum were
observed in occasional hepatocytes from some mice from each group,
including controls. Most hepatocytes contained no observable smooth
endoplasmic reticulum. The incidence of mice with hepatocytes
containing smooth endoplasmic reticulum was highest in the group given
cyhalothrin at 2000 mg/kg of diet, consistent with the finding of
other hepatic effects in this group (Prentice et al., 1981).
Rats
In a range-finding study for an investigation of developmental
toxicity, groups of 10 female Charles River CD rats were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 10
consecutive days. After treatment, the animals were observed for a
7-day recovery period. No certificate of compliance with GLP was
supplied, but suitable quality assurance records had been kept.
All of the animals given 80 mg/kg bw per day, nine of those given
40 mg/kg bw per day, and two given 20 mg/kg bw per day had to be
killed for humane reasons after the first dose, as this caused severe
toxicity (ataxia and/or collapse). Body-weight loss and reduced food
intake were seen in the rats given 20 mg/kg bw per day throughout the
treatment period. Body-weight loss was also seen in the rats given 10
mg/kg bw per day during the first 5 days of the study. Food intake was
increased in the groups at 10 and 20 mg/kg bw per day during the
recovery period. Macroscopic examination of the animals at necropsy
revealed no treatment-related changes. No NOEL could be identified in
this study (Killick, 1980).
In a 28-day range-finding study, groups of 10 male and 10 female
Charles River CD rats were fed diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 5, 10, 20,
or 250 mg/kg of diet, equivalent to 0, 0.5, 1, 2, and 25 mg/kg bw per
day. The study did not comply with GLP. There were no
treatment-related clinical signs and no deaths. Food intake, food
efficiency, and body-weight gain were decreased only at the highest
dose (Student's t test: p < 0.05). Gross examination post
mortem revealed no treatment-related effects, and organ weights were
unaffected by treatment. Electron microscopy of liver samples showed a
moderate increase in the amount of smooth endoplasmic reticulum in
hepatocytes of animals at the highest dose, with a decrease in the
amount of glycogen. An increase in hepatocyte size was noted in the
centrilobular and mid-zonal regions of the livers of males at this
dose, which also had increased hepatic activity of
aminopyrine- N-demethylase (Student's t test: p < 0.001). The
NOEL was 20 mg/kg of diet, equivalent to 2 mg/kg bw per day (Colley et
al., 1980).
In a study that complied with GLP, groups of eight male and eight
female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%) at a concentration of 0,
1, 5, 10, 20, or 250 mg/kg of diet, equivalent to 0, 0.1, 0.5, 1, 2,
and 25 mg/kg bw per day. Full post-mortem examinations were not
performed, but samples of liver were taken for analysis of
aminopyrine- N-demethylase activity and electron microscopy. No
treatment-related clinical signs were reported. The mean body weight
of males given 250 mg/kg of diet was decreased when compared with that
of concurrent controls during weeks 1 and 2 of the study (two-sided
t test: p < 0.05). Body-weight gain was depressed during week 1
for males given 250 mg/kg of diet and for females given 10, 20, or 250
mg/kg of diet (two-sided t test: p < 0.05). The absolute weight
of the liver was reduced in males given 250 mg/kg of diet and in
females given 20 mg/kg of diet (two-sided t test: p < 0.05), but
the relative weight of the liver was increased in males at 250 mg/kg
of diet. Both males and females at this dose had increased hepatic
activity of aminopyrine- N-demethylase and mild proliferation of
smooth endoplasmic reticulum in hepatocytes. The NOEL was 5 mg/kg of
diet, equivalent to 0.5 mg/kg bw per day, on the basis of depression
of body-weight gain, whereas that for hepatic changes was 20 mg/kg of
diet, equivalent to 2 mg/kg bw per day (Moyes et al., 1984).
In a 28-day range-finding study, of which full details were not
available, a dietary concentration of cyhalothrin of 750 mg/kg
(equivalent to 75 mg/kg bw per day) increased the mortality rate.
'Characteristic signs of pyrethroid neurotoxicity' were seen at
dietary concentrations of 250 and 750 mg/kg. Treatment-related effects
on body weights, food consumption, organ weights, and hepatic activity
of aminopyrine- N-demethylase were seen at 250 mg/kg of diet. Hepatic
smooth endoplasmic reticulum proliferation occurred in males fed 20
and 250 mg/kg of diet. No NOEL could be identified (Lindsey et al.,
1981).
In a 90-day study that complied with GLP, groups of 20 male and
20 female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%; with a total pyrethroid
content of 92.2% of which 96.8% was cyhalothrin) at a concentration of
0, 10, 50, or 250 mg/kg of diet, providing mean doses of 0, 0.56, 2.6,
and 36 mg/kg bw per day for males and 0, 0.57, 3.2, and 15 mg/kg bw
per day for females. Blood samples were collected before treatment,
during week 4 of treatment, and at the end of the study. Urine samples
were collected before treatment and in weeks 4 and 13. Ophthalmoscopic
examinations were performed during the week before termination. Full
post-mortem examinations were performed, and the major organs and any
grossly abnormal tissues were examined histologically. Liver samples
were taken for analysis of aminopyrine- N-demethylase activity and
examination by electron microscopy.
Treatment with doses < 250 mg/kg of diet had no effect on the
mortality rate. The body-weight gain of males at the highest dose was
consistently reduced (two-sided t test: p < 0.01) throughout the
study. Food consumption was slightly reduced in males at 50 and 250
mg/kg of diet, and this effect was occasionally statistically
significant (two-sided t test: p < 0.05). There were no clearly
treatment-related haematological effects, although there were small,
significant, but not dose-related changes in some red blood cell
parameters (mean corpuscular volume, packed cell volume, haemoglobin,
mean corpuscular haemoglobin). Plasma alanine and aspartate
aminotransferase activities and cholesterol concentration were raised
at 4 weeks in males given 10 or 50 mg/kg of diet, and those at the
lower dose also had a raised plasma urea concentration (two-sided
t test: p < 0.05). At 13 weeks, however, the plasma concentration
of urea in males at 50 mg/kg of diet was decreased (two-sided
t test: p < 0.05). The observed effects on haematological
parameters, plasma enzyme activity, and concentrations of cholesterol
and urea were considered to be unrelated to treatment as there were no
clear dose-response relationships. At 13 weeks, however, the plasma
triglyceride concentration was reduced and there was a dose-related
increase in urinary glucose concentration in males at 50 and 250 mg/kg
of diet (two-sided t test: p < 0.05). Ophthalmoscopy revealed no
treatment-related effects. The results of gross examinations were not
reported, although the organ weights were recorded. The absolute
weight of the liver was increased in males at 250 mg/kg of diet, and
the absolute weight of the lung was decreased in animals given 250
mg/kg of diet, but the relative weights of these organs were
unaffected. No treatment-related changes were revealed by optical
microscopy of organs. Electron microscopy of the liver revealed mild
proliferation of smooth endoplasmic reticulum in the hepatocytes of
three males given 50 mg/kg of diet and three males given 250 mg/kg of
diet. There was a dose-related increased in hepatic
aminopyrine- N-demethylase activity in males given 50 or 250 mg/kg of
diet and in females given 250 mg/kg of diet (two-sided t test:
p < 0.05). The NOEL was 10 mg/kg of diet, equivalent to 0.56 mg/kg
bw per day, on the basis of hepatic changes in males at higher doses
(Lindsey et al., 1981).
Rabbits
In a range-finding study for an investigation of developmental
toxicity, groups of six mated female New Zealand white rabbits were
given cyhalothrin (purity, 89.25%) at a daily dose of 0, 20, 30, or 40
mg/kg bw by oral gavage in corn oil on days 6-18 of gestation. The
animals were killed on day 18 of gestation.
There were no unscheduled deaths and no consistent clinical signs
of toxicity. Body-weight gain was depressed only at 30 mg/kg bw per
day, although animals at 40 mg/kg bw per day showed reduced food
intake. Not all of the animals became pregnant, but in all groups,
including controls, few of the does had live fetuses. There was no
dose-response relationship for this effect. Furthermore, not all of
the pregnant does at 20 and 40 mg/kg bw per day produced live
offspring. Consequently, there were insufficient numbers of litters to
allow any meaningful inter-group comparison of litter parameters,
although no adverse effects were apparent from the limited data
available. No treatment-related macroscopic changes were seen post
mortem. The apparent NOEL in this limited study was 20 mg/kg bw per
day on the basis of reduced body-weight gain (Killick, 1981a).
Dogs
In a preliminary study, groups of one male and one female beagles
were given gelatin capsules containing cyhalothrin (purity
unspecified) in corn oil at a dose of 0, 2.5, 10, or 30 mg/kg bw per
day for 4 weeks. After 10 days, treatment with the high dose was
stopped, and the animals were allowed to recover for 4 days; treatment
was then resumed at a lower dose of 20 mg/kg bw per day for 4 weeks.
Blood was taken weekly. Urine samples were collected before dosing and
during week 4. Ophthalmoscopic examinations were performed on each dog
before dosing and during week 4. No certificate of compliance with GLP
was supplied, but suitable quality assurance records were kept.
None of the dogs died prematurely. Muscular trembling was seen in
all groups, including controls, but the degree of trembling was more
marked in animals at the two higher doses. Unsteady gait and
body-weight loss were observed in dogs given 30 mg/kg bw per day, but
this effect was not seen after the dose was reduced to 20 mg/kg bw per
day. Body weight was unaffected by doses up to 10 mg/kg bw per day,
and only slight fluctuations occurred at 20 mg/kg bw per day. Vomiting
was seen occasionally at doses > 10 mg/kg bw per day. Dogs at the
high dose (30 or 20 mg/kg bw per day) had loose stools and increased
serum alanine and aspartate aminotransferase activities. No
haematological, ophthalmological, or urinary changes were observed,
and no gross pathological or organ weight changes were noted post
mortem. Histopathological examination revealed no treatment-related
effects. No NOEL could be identified in this study owing to the
finding of an increased frequency of liquid faeces at all doses. The
LOEL was 2.5 mg/kg bw per day (Chesterman et al., 1983).
In a 26-week study that complied with GLP, groups of six male and
six female beagles were given cyhalothrin (purity unspecified)
dissolved in corn oil inside a gelatin capsule at a dose of 0, 1.0,
2.5, or 10 mg/kg bw per day. The dogs were examined
ophthalmoscopically before dosing and during weeks 6, 12, and 24 of
treatment. Blood and urine samples were taken every 4 weeks. The
functional integrity of the nervous system was assessed clinically
before treatment and during week 6. At the end of the treatment
period, full necropsies were performed on all dogs, and a large
selection of organs and tissues was examined histologically.
No deaths occurred during the study. A dose-related increase in
the incidence of liquid faeces affected all treated groups. At 10
mg/kg bw per day, the clinical signs seen included vomiting,
salivation, incoordination, unsteadiness, collapse, muscular spasms,
and convulsions. Body weight was unaffected by treatment.
Ophthalmoscopic and neurological examinations revealed no adverse
effects, and no consistent haematological, serum biochemical, or
urinary effects were seen. No treatment-related gross effects on organ
weights or appearance were noted post mortem, and histopathological
examination revealed no treatment-related effects. No NOEL could be
identified in this study owing to the finding of an increased
frequency of liquid faeces at all doses. The LOEL was 1.0 mg/kg bw per
day (Chesterman et al., 1981).
In a 52-week study, groups of six dogs of each sex were given
lambda-cyhalothrin at a dose of 0, 0.1, 0.5, or 3.5 mg/kg bw per day
orally in corn oil, but there was lack of agreement between summaries
of the study about whether the animals were treated by gelatin capsule
(European Medicines Evaluation Agency, 1999) or by gavage (WHO, 1990).
The report was not available to the Committee. Clinical signs of
neurological effects (muscular trembling, unsteadiness, and vomiting)
were seen in all dogs at the highest dose, but the signs were not
accompanied by histological changes in the nervous system. The
European Medicines Evaluation Agency (1999) reported a dose-related
increased incidence of liquid faeces at all doses, but WHO (1990)
reported that this effect was seen only at 0.5 and 3.5 mg/kg bw per
day and that the increased incidence was only slight at 0.5 mg/kg bw
per day. The incidence of liquid faeces was considered not to be
toxicologically significant. The NOEL was 0.5 mg/kg bw per day on the
basis of neurological signs seen at 3.5 mg/kg bw per day. It is
unclear how this dose relates to a dose of cyhalothrin (WHO, 1990;
European Medicines Evaluation Agency, 1999).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study that complied with GLP, groups of 52 male and 52
female CD-1 mice were given diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 20, 100, or
500 mg/kg for 104 weeks, equal to 0, 1.8, 9.2, and 53 mg/kg bw per day
for males and 0, 1.8, 11, and 51 mg/kg bw per day for females.
Satellite groups of 12 mice of each sex were given the same diets for
52 weeks and then killed.
The signs of toxicity observed in animals given high doses were
piloerection and hunched posture in mice of each sex given 500 mg/kg
of diet and in males given 100 mg/kg of diet. Males at 500 mg/kg of
diet also showed emaciation, pallor, hyperactivity, and increased
fighting activity. The mortality rate was unaffected by treatment.
Decreased body-weight gain was seen in males at 500 mg/kg of diet
throughout the study (two-sided Student t test: p < 0.05),
particularly during the first 13 weeks ( p < 0.001). Reduced feed
use efficiency was seen in the same group over the first 24 weeks of
the study (not calculated for the rest of the study). Water
consumption was also slightly increased in males at 500 mg/kg of diet
(two-sided Student t test: p < 0.05).
The results of haematological tests were unremarkable.
Statistically significant changes in various parameters were seen at
various times, but the effects were not consistent or dose-related.
Urinary analysis revealed no significant differences from control
values. The serum glucose concentration was slightly reduced
(two-sided Student t test: p < 0.05) in animals given 500 mg/kg
of diet. During week 100, serum alanine and aspartate aminotransferase
activities were increased in a dose-related manner, with occasional
significance ( p < 0.05) for both activities in mice of each sex at
100 and 500 mg/kg of diet and for alanine aminotransferase activity in
females at 20 mg/kg of diet.
Gross examination post mortem revealed increased incidences
(not dose-related) of thickening of the forestomach in treated
females. The ovarian weights were significantly (two-sided Student
t test: p < 0.05) decreased at all doses in animals in the main
group but not in the satellite groups. The effect was not
dose-related. Other organ weights were unaffected. Histopathological
examination revealed a variety of non-neoplastic lesions, but none was
considered to be due to treatment with cyhalothrin.
A non-dose-related increase in the incidence of mammary
adenocarcino-mas was seen in females in the main group, the incidences
being 1/52 in controls, 0/52 at 20 mg/kg of diet, 7/52 at 100 mg/kg of
diet, and 6/52 at 500 mg/kg of diet. (The detailed statistical
analysis of these results was given in an addendum to the report that
was not available to the Committee.) The incidence of mammary
adenocinoma in control females in 17 studies performed by the same
laboratory between 1980 and 1982 ranged from 2% to 12%. No
preneoplastic changes were found in the mammary glands of mice in the
main or satellite groups. A re-evaluation of mammary tissues from all
animals confirmed the original diagnoses of mammary neoplasia. As the
highest incidence of mammary adenocarcinoma in any group was only
slightly above the range of historical controls and as there was no
dose-response relationship, it was concluded that treatment with
cyhalothrin was unlikely to have caused these tumours. The NOEL was 20
mg/kg of diet, equal to 1.8 mg/kg bw per day, on the basis of clinical
signs of toxicity (piloerection and hunched posture) in males at
higher doses (Colley et al., 1984).
Rats
In a study that complied with GLP, groups of 72 male and 72
female Alpk/AP SPF rats were fed diets containing technical-grade
cyhalothrin (purity, 89.25%; containing 92.2% pyrethroids of which
96.8% was cyhalothrin) at a concentration of 0, 10, 50, or 250 mg/kg
for 104 weeks, providing mean doses of 0, 0.47, 2.3, and 12 mg/kg bw
per day for males and 0, 0.55, 2.7, and 14 mg/kg bw per day for
females. Satellite groups of 10 rats of each sex were given the same
diets but were killed after only 52 weeks. Blood was taken for
haematological measurements from 10 rats of each sex in each group at
4 and 13 weeks and thereafter at 13-week intervals. Blood was taken
for biochemical analyses and urine for urinary analyses from 12 rats
of each sex in each group at the same intervals. In weeks 52 and 103,
ophthalmoscopy was performed on 20 males and 20 females from each
group.
No clinical signs were seen that could be attributed to
treatment. The mortality rate was unaffected. Body-weight gain was
suppressed in both males and females given 250 mg/kg of diet
(two-sided Student t test: p < 0.05), and the males in this group
had increased food use efficiency. No consistent, dose-related
haematological effects were seen, although changes in various
parameters occasionally achieved statistical significance (two-sided
Student t test: p < 0.05). The volume of urine produced by rats
fed 250 mg/kg of diet tended to be decreased, but otherwise there was
no effect on urinary parameters. Ophthalmoscopy revealed no
treatment-related effects. Blood biochemistry showed intermittent
effects in rats at 250 mg/kg of diet, consisting of reduced plasma
concentrations of glucose and triglycerides, reduced plasma alkaline
phosphatase activity, and increased plasma urea concentration
(two-sided Student t test: p < 0.05). The reduction in plasma
triglycerides was the most marked finding, especially in females.
Long, pointed fibres, thought to originate from the diet, were
found embedded in the tissues of the palate, nasal turbinates, roof of
the nasal cavity, and peridontal space. The presence of the fibres
suggested that the lesions were caused by penetration of the tissue by
these fibres rather than by cyhalothrin. No treatment-related gross
lesions were detected post mortem, but several rats in all groups,
including controls, had rhinitis and oral lesions (oral granuloma
formation and erosion of the palate) in response to the presence of
the fibres in the diet. At the interim kill, the weight of the liver
(relative to body weight) was increased in rats of each sex given 250
mg/kg of diet, but this effect was not seen in rats killed at 104
weeks. The weight of the adrenals (relative to body weight) was
increased in females at 50 and 250 mg/kg of diet killed at 104 weeks.
Histopathological examination revealed no treatment-related effect on
the incidence or severity of any neoplastic or non-neoplastic lesions.
The NOEL was 50 mg/kg of diet, equal to 2.3 mg/kg bw per day (Pigott
et al., 1984).
2.2.4 Genotoxicity
Cyhalothrin was tested in assays for gene mutation in bacteria,
cytogenicity in rats in vivo, dominant lethal mutation in mice, and
cell transformation. The results of all the assays were negative. The
assays did not comply with GLP, but they appeared to be of good
quality and suitable quality assurance records had been maintained.
The studies are summarized in Table 4. The material tested by Trueman
(1981) was analysed by reversed-phase HPLC and found to have a
cis:trans ratio of 97.1:2.9.
lambda-Cyhalothrin was reported to be non-genotoxic when tested
for reverse mutation in bacteria, gene mutation in a mouse lymphoma
cell line, cytogenicity in vitro, unscheduled DNA damage in
vitro, and micronucleus formation in mice (WHO, 1990). Full reports
of these assays were not available, but the available details are
given in Table 5. lambda-Cyhalothrin produced micronuclei in an assay
in fish (Campana et al., 1999), but the relevance to human health of a
positive result in this unvalidated assay is not known.
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Mice
Only a brief account of a three-generation study of the effects
of cyhalothrin on reproduction in mice has been reported. It is
unclear whether the study was performed in accordance with the
principles of GLP. Groups of albino Swiss mice were given oral doses
of '0 (blank control), 2.5 ppm and 5.0 ppm cyhalothrin/kg body weight
daily' (the meaning of the term 'ppm cyhalothrin/kg body weight' is
unclear). The F0 generation consisted of groups of four males and
eight females. No further details of the protocol were available. No
signs of toxicity or behavioural changes were observed in the parents
or offspring for up to three generations. Maternal body weights were
not affected, although F2 generation dams at the low dose had
increased food intake. The weights of pups of F2 dams treated at the
high dose were reported to be significantly different from those of
control pups. Male and female fertility indices, pup live birth and
survival indices, litter size, and sex ratio were unaffected. The
meaning of the results was unclear. No NOEL could be identified
(Deshmukh, 1992).
Table 4. Assays for genotoxicity with cyhalothrin
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse S. typhimurium 0.16-2500 µg/ 90.2 Negativea Trueman (1981)
mutation TA1535, TA1537, plate ± S9
TA1538, TA98,
TA100
Cell Syrian hamster 50-1000 µg/ml NR Negativeb Richold et al.
transformation - S9 (1981)
1000-5000 µg/ml
+ S9
In vivo
Cytogenicity Bone marrow, Single oral dose 89.2 Negativec Anderson et al.
Wistar-derived of 1.5, 7.5,15 mg/ (1983)
male rats kg bw in corn oil
5 daily oral doses
of 1.5, 7.5, 15 mg/
kg bw per day
Dominant Male CD1 mice 1, 5, 10 mg/kg bw/ 89.2 Negatived Irvine (1981b)
lethal day for 5 days by
mutation oral gavage
in corn oil
a 2-Aminoanthracene, 9-aminoacridine, 4-nitroquinoline- N-oxide, and
N-methyl- N'-nitro- N-nitrosoguanidine used as positive controls
b 4-Nitroquinoline- N-oxide and para-dimethylaminoazobenzene (butter yellow) used
as positive controls
c Ethyl methanesulphonate used as a positive control
d Cyclophosphamide used as a positive control
Table 5. Assays for genotoxicity with lambda-cyhalothrin
End-point Test object Concentration Results Reference
In vitro
Reverse S. typhimurium < 5000 µg/plate Negative IPCS (1990)
mutation (strains not ± S9
specified)
Gene mutation L51787 mouse 125-4000 µg/ml Negative IPCS (1990)
lymphoma cell ± S9
line
Unscheduled Cultured human NR Negative IPCS (1990)
DNA synthesis HeLa cells
Cytogenicity Human lymphocytes NR Negative IPCS (1990)
In vivo
Micronucleus Erythrocytes of 0.001-0.05 µg/L Positive Campana et al.
formation Cheirodon interruptus (1999)
interruptus
Micronucleus Mice < 35 mg/kg bw Negative IPCS (1990)
formation (route not
specified)a
a Cyclophosphamide used as a positive control
Rats
Throughout a GLP-compliant three-generation (two litters per
generation) study, groups of SPF Alpk/AP Wistar-derived rats were
given diets containing technical-grade cyhalothrin (purity, 89.25%;
containing 92.2% pyrethroids of which 96.8% was cyhalothrin) at a
concentration of 0, 10, 30, or 100 mg/kg, providing mean doses of 0,
0.6, 1.7, and 5.6 mg/kg bw per day for males and 0, 0.7, 1.9, and 6.1
mg/kg bw per day for females. The F0 generation consisted of groups
of 15 male and 30 female rats. After 12 weeks on the test diets, these
animals were mated within their groups to produce a first litter
(F1a generation). Later, they were mated again to produce a second
litter (F1b generation). Breeding was repeated with F1 parents (15
males and 30 females per group) selected from the F1b animals and
F2 parents (15 males and 30 females per group) selected from the
F2b animals. At day 36 post partum, all pups not selected as the
next parental generation were killed and examined externally.
Approximately half of these were examined by autopsy, with
histopathological examination of any grossly abnormal tissues. A
fuller post-mortem examination with histopathology of all major organs
was performed on five males and five females from each group of the
F1b and F2b generations and 10 males and 10 females from each of
the F3b groups.
No clinical signs of toxicity were found. Minor reductions in
body-weight gain were seen in the premating period of the F0
generation of animals of each sex at 100 mg/kg of diet and
occasionally in females given 30 mg/kg of diet. At other times and in
other generations, occasional reductions in body-weight gain were seen
in rats given cyhalothrin at 30 or 100 mg/kg of diet. Male fertility
was unaffected by treatment. The fertility of F1 females at 30 mg/kg
of diet was statistically significantly (one-sided t test:
p < 0.05) reduced but remained within the range of control values.
At 100 mg/kg, the litter size of F2 and F3 pups was reduced, the
effect being statistically significant (one-sided t test:
p < 0.05) for the F2a and F3b litters. Small but statistically
significant decreases (one-sided t test: p < 0.05) were seen in
the percentages of live pups born to animals at 10 mg/kg in the F1b
generation and to dams at 30 and 100 mg/kg of diet in the F3b
generation. Post-mortem and histopathological examinations revealed no
adverse effects that could be attributed to the treatment. The
occasional effects seen at 10 and 30 mg/kg of diet were considered to
be incidental, and only the effects at 100 mg/kg of diet were regarded
as treatment-related. The NOEL was 30 mg/kg of diet, equal to 1.7
mg/kg bw per day, on the basis of reduced parental body-weight gain
and reduced litter size (Milburn et al., 1984).
(b) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley-derived rats (CD rats from
Charles River's SPF colony) were given technical-grade cyhalothrin
(purity, 89.25%; containing 92.2% pyrethroids of which 96.8% was
cyhalothrin) at a dose of 0, 5, 10, or 15 mg/kg bw per day by oral
gavage in corn oil on days 6-15 of gestation. The dams were killed on
day 20, and the contents of their uteri were examined. No certificate
of compliance with GLP was supplied, but suitable quality assurance
records had been kept.
Body-weight loss, reduced food intake, and signs of neurotoxicity
(loss of limb coordination in 2 out of 24 rats) were observed in dams
at the highest dose. The numbers of pregnant animals, pre-implantation
and post-implantation losses, mean litter weights, and mean weights
and lengths of fetuses were unaffected by treatment. Abnormalities
were seen in one litter of a dam at 10 mg/kg bw per day, in which 5 of
17 fetuses had major defects: four had bilateral agenesis of the
kidneys, and three had skeletal malformations of the vertebral
centrae, sternebrae, and/or metacarpals. The effects were considered
to be incidental and unlikely to be due to treatment. The NOEL was 10
mg/kg bw per day on the basis of maternal toxicity (Killick, 1981b).
Six pregnant Wistar-derived rats were given cyhalothrin (purity,
95.8%) by dermal application throughout the 21 days of gestation at a
daily dose of 1 ml of 0.02% (w/v; equal to approximately 0.8 mg/kg bw
per day if completely absorbed) in an aqueous vehicle applied to a
shaved area of the back. Controls were given the vehicle alone. After
parturition, six pups were left with each dam. At 90 days of age, 12
males in the treated group were tested for mating behaviour with
ovariectomized females that were showing estrus induced by
subcutaneous injections of 17ß-estradiol and progesterone. No
certificate of compliance with GLP was supplied for this study.
Treated male pups showed a delay in testicular descent, but the age of
vaginal opening was not affected. The mating behaviour of the males
was not affected by treatment. No NOEL could be identified (da Silva
Gomes et al., 1991a).
In another study which did not appear to conform to GLP, 12
pregnant Wistar-derived rats were given cyhalothrin (purity
unspecified) by dermal application throughout the 21 days of gestation
at a daily dose of 1 ml of 0.018% (w/v; equal to approximately 0.72
mg/kg bw per day if completely absorbed) in an aqueous vehicle. At
weaning (21 days) and at 90 days of age, the pups were tested for
locomotor activity in an open field. At 90 days, training began for
inhibitory avoidance behaviour. Adult males were also tested for
motivational response in a 'hole board' test.
The opening of ears and eyes, the development of fur, and the
descent of testes were all delayed in treated rats, but the time of
vaginal opening was not affected. The body weights of the pups of
cyhalothrin-treated dams were statistically significantly greater
(Student t test: p < 0.05) than those of controls at days 2, 7,
and 14 of age but not at day 21. Locomotor frequency was increased at
21 days and decreased at 90 days, but the effects were not
statistically significant in the Student t test. No effect was seen
on inhibitory avoidance behaviour. The results of the hole board test
showed a decreased motivational response in cyhalothrin-exposed rats.
No NOEL could be identified (da Silva Gomes et al., 1991b).
Rabbits
Groups of 20 pregnant New Zealand white rabbits were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) on days 6-18 of gestation
at a dose of 0, 3, 10, or 30 mg/kg bw per day by oral gavage in corn
oil. The animals were killed and examined on day 28 of gestation. No
certificate of compliance with GLP was supplied, but suitable quality
assurance records had been kept.
The highest dose was toxic to the dams, as indicated by an
initial body-weight loss followed by a decreased rate of body-weight
gain. No treatment-related deaths, clinical signs, or gross
pathological changes were seen in any group of does. There was no
effect on the incidence of pregnancy or on gravid uterine weight. The
mean weight of the fetuses of dams at 30 mg/kg bw per day was slightly
decreased, but the effect was not statistically significant (Wilcoxon
test). Mean fetal crown-rump length, sex ratio, and the numbers of
corpora lutea, intrauterine deaths, and implantations were unchanged
by treatment. There was no increased incidence of any type of fetal
abnormality in any treated group. The NOEL for maternal toxicity was
10 mg/kg bw per day (Killick, 1981c).
2.2.6 Special studies
(a) Neurotoxicity
Rats
Groups of rats were given single oral or intraperitoneal doses of
25-200 mg/kg bw 'cyhalothrin', referred to as 'PP 321, trade mark
Karate', which are terms associated with lambda-cyhalothrin rather
than cyhalothrin. Its purity was not reported. It was suspended as an
emulsion in 'solvents and surface active agents'. After treatment, the
behaviour of the animals in the open field was monitored, and their
electroencephalograms were measured while they were moving freely. No
further details of the protocol were reported. Treated animals were
found to have limited mobility as a consequence of discoordination of
the limbs. The reported signs of toxicity were respiratory
difficulties, tremor, salivation, piloerection, increased reactivity
to noise and during handling, with vocalization. Analysis of the
electroencephalograms showed 'changes in amplitudes (depressions in
majority of cases), in frequency composition (relative increases of
beta activity) and in repetition of cycles of high and low
amplitudes'. The results for individual groups were not presented. No
NOEL could be identified (Zufan et al., 1989).
Hens
In a study that complied with GLP, groups of 10 hens were given
cyhalothrin (purity, 93.4%) as a single dose of 2500, 5000, or 10 000
(two groups) mg/kg bw by oral gavage in corn oil. Negative controls
were given corn oil alone; positive controls received 500 mg/kg bw of
tri- ortho-cresyl phosphate. The hens were observed for 21 days after
dosing. The only hens that died during this period were two given
10 000 mg/kg bw, one in the negative and one in the positive control
group. The mean body weights of hens in all groups given cyhalothrin
and the positive controls were reduced. Clinical signs of toxicity
(ataxia) were seen only in the positive controls. The only
treatment-related gross change seen post mortem was muscular atrophy
in two of the positive control birds. Histopathological examination of
the cervical cranial, cervical caudal, thoracic, and lumbar spinal
cord and the proximal and distal sciatic nerve showed morphological
evidence of axonal damage at all levels of the spinal cord in all the
positive control birds. There were no consistent or dose-related
changes in the nerves of any of the groups given cyhalothrin. The NOEL
was 10 000 mg/kg bw, the highest dose tested (Roberts et al., 1982).
(b) Neurobehavioural effects
Inclined plane test
In a preliminary GLP-compliant study, cyhalothrin was
administered by oral gavage in corn oil to groups of Crl:CD.BR strain
rats of each sex. Initially, single doses of 50, 100, or 200 mg/kg bw
were given to groups of two males and doses of 25, 40, or 75 mg/kg bw
to groups of two females. Later, groups of three rats of each sex were
given a single dose of 15, 30, or 60 mg/kg bw. Controls received corn
oil only. The cyhalothrin contained A and B isomer pairs in a 60:40
ratio and was 95.8% pure, with a total pyrethroid content of 96.4%.
The other isomers measured were cis B' (0.2%), trans D and cis
A' (0.4%), and trans C (< 0.1%). The contaminants included
phenoxybenzalde-hyde (0.2%) and
(Z)3-(2-chloro-3,3,3-trifluoro-1-enyl)-2,2-dimethylcyclopro-pane
(0.1%). One hour before dosing and again at 30 min, 2 h, 7 h, and 24 h
after dosing, each rat was placed on a smooth plane with an angle of
inclination that was gradually increased until the rat could no longer
maintain its position. For each rat, three measurements were made at
each time. In the inclined plane test, any decrease in the angle at
which the rat loses its position (mean slip angle) is regarded as a
reflection of subtle changes in neuromuscular function.
Clinical signs of toxicity were seen at all doses tested. At 15
mg/kg bw, the only clinical sign was soft faeces in females. At 25-40
mg/kg bw, ungroomed appearance, soft faeces, and piloerection were
seen. At 50-75 mg/kg bw, there were marked clinical signs, including
ataxia, writhing, emprosthotonos, high stepping or splayed gait,
hunched posture, stained snout, salivation, and wasted appearance. At
100 mg/kg bw, the signs also included vocalization and closure of the
eyelids. Rats given 60 or 200 mg/kg bw were killed on humane grounds
after the inclined plane measurements had been made. Only one rat (a
female at 60 mg/kg bw) died during the study, but all rats given 60 or
200 mg/kg bw were killed prematurely on humane grounds. Body-weight
gains were decreased at doses > 50 mg/kg bw. Necropsy of the
animals showed no consistent gross pathological changes. The result of
the inclined plane test was uninterpretable, as the clinical signs
interfered with the conduct of the study: convulsing rats were unable
to stay on the plane, and rats with soiled fur adhered to it (Denton,
1988).
Acute startle response
A preliminary study was performed in two phases with groups of
three male Wistar-derived Alpk:ApfSD rats. The purity of the
cyhalothrin tested was 97.2%. In phase 1, rats were given a single
oral dose of 0, 25, 50, or 75 mg/kg bw of cyhalothrin in corn oil, and
auditory habituation tests were performed 1, 2, and 3 h after dosing.
Clinical signs of toxicity were seen on day 2 after dosing (the time
of dosing being the start of day 1) but not on day 1 or day 3, in the
group given 75 mg/kg bw. The signs included ataxia, subdued behaviour,
body-weight loss, and stained fur. Rats at this dose had a
statistically nonsignificant increase in mean response amplitude
(two-sided Student t test) but a significant ( p < 0.05) increase
in the time to maximum amplitude 3 h after dosing.
In phase 2, rats were given a single oral dose of 0, 75, or 100
mg/kg bw of cyhalothrin in corn oil, and auditory habituation tests
were performed on separate groups of animals 3, 5, or 7 h after
dosing. The signs of toxicity were similar to those seen in phase 1.
The clearest results in the auditory startle habituation test were
obtained at 7 h: with both doses tested, the mean response amplitude
was consistently statistically significantly ( p < 0.05) reduced and
the time to maximum amplitude was reduced (Brammer, 1998).
In the main study, groups of 10 male and 10 female Wistar-derived
Alpk:ApfSD rats were given a single dose of 0, 5, 15, or 75 mg/kg bw
of cyhalothrin (purity, 97.2%) by oral gavage in corn oil. An auditory
startle habituation test was performed 7 h after dosing on day 1 and
on day 8 with an automated recording apparatus. The animals were
killed after 8 days but were not examined post mortem. The study was
performed in accordance with GLP.
Body-weight gain was reduced in males at the highest dose and in
females at the two lower doses. Transient clinical signs of toxicity
were seen at the highest dose. Those seen at day 2 included subdued
behaviour, ataxia, high-stepping gait, salivation, and tip-toe gait,
and 'some of these signs' were also seen 7 h after dosing on day 1.
There were no signs of toxicity from day 3 until the end of the study.
In the startle response test, there was no effect on time to
maximum amplitude in either sex on day 1 or day 8. A reduction in the
mean response amplitude was seen, however, on day 1 in animals of each
sex at the highest dose. Statistically significant (two-sided Student
t test: p < 0.05), but not dose-related, reductions in mean
response amplitude were also seen on day 1 in females at all doses. No
effects were seen on day 8.
The authors reported that the NOEL for this study was 15 mg/kg
bw, as they dismissed the effects on response amplitude because of the
absence of a clear dose-response relationship. The Committee disagreed
with this conclusion. It has been reported that type II pyrethroids
generally decrease rather than increase the startle response to sound,
although this is a complex response and at low doses some type II
pyrethroids give an increased startle response (Hijzen & Slangen,
1988; Ray, 1991). Thus, although there was a NOEL for a reduced
response, lower doses may have caused an increased response, which
would indicate a lower NOEL. The Committee also noted that the startle
response was not evaluated at the most sensitive time, i.e. the time
of peak expression of acute toxicity as shown by clinical signs. No
NOEL could be identified (Brammer, 1999).
Inhibitory avoidance test
Newborn Wistar rat pups were exposed to cyhalothrin (purity
unspecified) in their mother's milk from birth until weaning at 21
days of age, and at 97, 104, and 111 days of age they were tested in
inhibitory avoidance tests. Cyhalothrin was given to nursing dams in
their drinking-water as a 200-mg/L solution in 400 mg/L sucrose. This
would be expected to provide a dose of cyhalothrin of approximately 20
mg/kg bw per day to the dams (assuming that 200-g rats drink about 20
ml/day). The intake of the pups was not measured, but the treated
groups consisted of 20 pups and the control group of 15. Control dams
were given sucrose solution at 400 mg/L. Drink was available
ad libidum to eight cyhalothrin-exposed dams and six control dams.
No clinical signs of toxicity were reported in either the dams or
the pups. The body weights at weaning and at 90 days of age were not
affected. The behaviour of the dams was unaffected by treatment
throughout the 21 days of lactation. The motor activity of the pups at
weaning was unaffected by the treatment. At 90 days, eight pups
exposed to cyhalothrin and 11 control pups were chosen (all the males)
for training and testing in a shuttle box to evaluate inhibitory
avoidance behaviour. Each rat was trained once by placing it in a
well-lit box and then opening a door to a dark compartment and giving
the rat an electric shock to the foot when it entered the dark
compartment. The test is a measure of the time taken by the rats to
cross again from the well-lit area to the dark compartment. Each rat
was tested once at 97, 104, and 111 days of age, receiving no electric
shock. At all times, there was a reduction in the learning avoidance
latency of the treated group, but the effect was statistically
significant (Mann-Whitney U-test: p < 0.05) only at 97 and 104 days
of age and not at 111 days. No NOEL could be identified (Moniz et al.,
1990).
In a separate study, described above, rats born to dams treated
dermally with an aqueous solution of cyhalothrin were tested for
behavioural changes. Inhibitory avoidance behaviour was unaffected by
the treatment, and there was no consistent effect on locomotor
frequency. The animals did, however, show a decreased motivational
response when they were tested as adults in a hole board test. No NOEL
could be identified for this study because of the production of subtle
but persistent neurobehavioural effects at the only dose tested. It
was also difficult to estimate the amount of cyhalothrin received by
the offspring (da Silva Gomes et al., 1991b).
2.2.7 Immunotoxicity
Some pyrethroids have been reported to be potent allergens that
may cause allergic rhinitis, asthma, or allergic alveolitis (Marrs,
1993). Cyhalothrin has not been specifically tested for immunotoxicity
but in a study of toxicity in mice given repeated doses, decreased
total leukocyte counts, decreased lymphocyte counts, and increased
neutrophil counts were observed in male but not female mice that had
consumed feed containing cyhalothrin for 28 days (Colley et al.,
1981). In the same study, atrophy of the red pulp of the spleen
occurred in some females given the high dose. Toxicological studies in
other species did not show alterations in total or differential
leukocyte counts and showed no histopathological changes in most
organs potentially involved in immunity (thymus, spleen, lymph nodes,
bone marrow), as described above.
A Magnusson and Kligman maximization test and a Buehler test both
showed that cyhalothrin is a skin sensitizer in guinea-pigs (European
Medicines Evaluation Agency, 1999; WHO, 1990). A Magnusson and Kligman
maximization test with lambda-cyhalothrin, however, showed no skin
sensitization in guinea-pigs (WHO, 1990).
2.3 Observations in humans
Cases of severe poisoning with pyrethroids are rare, but some
cases, for example in China, have been reported, including a few
deaths. Absorbed pyrethroids are rapidly detoxified by esterases, so
that any systemic effects would be of short duration (Marrs, 1993).
Topical application of pyrethrins or pyrethroids to the skin
produces local effects unrelated to their systemic action. The
synthetic pyrethroids have a characteristic irritating effect that is
not associated with inflammation and appears to be unique to
pyrethroids. The initial lesions are tenacious, painful pruritus
(pricking sensation) followed by a local burning sensation with
blotchy erythema that lasts about 2 days after cessation of exposure;
when exposure is intense, it is associated with numbness. Later,
desquamation may occur on the contaminated area of skin. There appear
to be no lasting ill effects. The condition is produced by all classes
of pyrethroids although most readily by type II pyrethroids such as
cyhalothrin. Repeated exposure to systemically toxic doses of
pyrethroids can produce a different effect: peripheral nerve damage
can occur subsequent to severe motor symptoms (Aldridge, 1990; Ray,
1991)
No clinical or haematological effects were observed in six
volunteers given a single dose of 5 mg of lambda-cyhalothrin in corn
oil (equal to 0.05-0.07 mg/kg bw) (European Medicines Evaluation
Agency, 1999). The route of administration was not reported, but it
seems likely to have been oral.
In the study of Pakistani pesticide workers described in section
2.2, the average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). Transient signs of toxicity, lasting up to 24
h, were reported by the workers, which included skin paraesthesia,
feeling hot, feeling cold, numbness, irritation of the skin, red eyes,
coughing, and sneezing. Medical examination revealed one case of face
rash that lasted 2 days (Chester et al., 1992).
In a study carried out in a village in the United Republic of
Tanzania, a lambda-cyhalothrin-based insecticide was sprayed inside
houses and shelters at a coverage of approximately 25 mg/m2. The
insecticide was supplied as a water-dispersible powder in a soluble
sachet. Every day for 6 days, 12 spraymen and 3 squad leaders were
interviewed about symptoms. Each sprayman used up to 62 g of
lambda-cyhalothrin over 2.7-5.1 h each day. The spraymen wore personal
protective equipment (rubber boots and gloves, cotton overalls, caps,
and gauze nose-mouth masks) which left much of the face exposed. One
sprayman also used a face shield for 3 days.
All the spraymen complained at least once of symptoms related to
exposure to lambda-cyhalothrin. The commonest symptoms were itching
and burning of the face and nose and throat irritation, frequently
accompanied by sneezing or coughing. Facial symptoms occurred only on
unprotected areas, and the worker who wore a face shield was free of
facial symptoms. All the symptoms had disappeared by the morning after
the spraying. The number of subjects affected and the duration of
facial symptoms were proportional to the amount of compound sprayed.
These parameters were not affected by use of lambda-cyhalothrin in the
previous 6 months. A sample of occupants was interviewed 1 and 5-6
days after their houses had been sprayed. One woman who entered her
house 30 min after the end of spraying complained of periorbicular
itching, but this lasted only a few minutes. The other inhabitants of
sprayed houses reported no other insecticide-related adverse effects.
Furthermore, a squad leader who entered almost every house a few
minutes after spraying reported no symptoms (Moretto, 1991)
As part of a field trial conducted in South India,
electrophysiological tests were conducted on 15 spraymen aged 19-48
years, before and after exposure to lambda-cyhalothrin (formulation
and purity unspecified). The tests performed on the subjects comprised
conduction of the right median, common peroneal, and facial motor
nerves; conduction of the right median and sural sensory nerves; blink
response with stimulation of the right supra-orbital nerve and
recording of R1 and R2 responses from the right orbicularis oculi
muscle with a pair of surface electrodes; concentric needle
electromyography of the tibialis anterior; repetitive stimulation of
the right median nerve at the wrist at 3 and 20 Hz and recording of
the responses from the abductor pollicis brevis; and multi-modality
visual, brainstem, auditory and somatosensory evoked potentials. The
evoked potentials were measured in only six of the subjects, but the
other measurements were made in all 15 subjects.
Clinical observation revealed no changes, and facial nerve
conduction, blink response, responses to repetitive stimulation, and
visual, auditory, and somatosensory evoked potentials were all normal.
Six of the 15 subjects had mild changes in peripheral nerve conduction
parameters (paired t test: p < 0.05), but comparison of the mean
values for the various nerve conduction parameters before and after
exposure showed no significant difference except for prolongation of
distal motor latency of the median nerve. Studies of nerve conduction
12-16 months later in three subjects who had shown abnormalities
immediately after exposure showed normal rates. The authors concluded
that occupational exposure to lambda-cyhalothrin can produce
transient, subclinical electrophysiological changes in the nerves of
the upper limbs (Arunodaya et al., 1997).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity,
neurotoxicity, and neurobehavioural effects of cyhalothrin and
observations of effects in humans exposed to this compound. Although
some of the studies were not fully compliant with codes of GLP, all of
the pivotal studies were carried out according to appropriate
standards for study protocol and conduct.
Oral doses of cyhalothrin were readily but incompletely absorbed
in the species studied (rats and dogs), and the subsequent metabolism
was similar, involving initial cleavage of the molecule at the ester
bond, presumably resulting in detoxification. The metabolites were
rapidly excreted, some as conjugates, whereas small amounts of
unchanged cyhalothrin persisted as residues in fatty tissues. Similar
results were seen in food-producing animals.
Serum and urine from exposed workers contained the metabolites
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)benzoic acid. These metabolites are products of
cleavage of the cyhalothrin molecule at the ester bond, and their
presence suggests that the initial metabolism of this compound in
humans is similar to that in the animal species that have been
investigated.
No toxicological studies on metabolites of cyhalothrin were
available, but the Committee considered it likely that the metabolites
would be less toxic than cyhalothrin, as none contains an intact
pyrethroid structure.
The acute toxicity of cyhalothrin is characterized by effects on
the nervous system, principally the central nervous system. The signs
of toxicity are typical of type II pyrethroids and comprise writhing,
salivation, exaggerated jaw opening, increasing extensor tone in the
hind legs causing a rolling gait, poor coordination progressing to
coarse tremor, tonic seizures, and apnoea. The LD50 values after
oral administration depended on the vehicle used but varied from 37 to
62 mg/kg bw in mice and from 51 to 240 mg/kg bw in rats. Higher values
were found in other species or after administration by other routes.
In mice that received a dose of 0, 5, 10, 20, 40, or 80 mg/kg bw
per day orally for 5 days, ataxia and convulsions were seen in those
given the two higher doses. The dose of 20 mg/kg bw per day caused
body-weight loss, ataxia, and rough coat. At 5 mg/kg bw per day, the
only adverse effect was a rough coat.
In a 28-day study in mice, cyhalothrin was added to the diet at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of feed. Atrophy of
the red pulp of the spleen and an increased mortality rate were seen
at the highest dose. Adaptive liver changes, including enlarged liver,
centrilobular hepatocellular hypertrophy, increased activity of
aminopyrine- N-demethylase, and proliferation of smooth endoplasmic
reticulum were seen at concentrations of 100 mg/kg of feed and above;
lowered lymphocyte counts were also seen at these doses. At 25 mg/kg
of feed, the only effect seen was piloerection. The NOEL was 5 mg/kg
of feed, equal to 0.65 mg/kg bw per day, on the basis of piloerection
at higher doses.
The main effects in five 10-90-day studies of toxicity in rats
were adaptive changes in the liver similar to those seen in mice. In a
28-day range-finding study in which rats were fed diets containing
cyhalothrin at a concentration of 0, 5, 10, 20, or 250 mg/kg of feed,
only the highest concentration caused adverse effects. These effects
were characterized by hepatocellular hypertrophy, increased activity
of aminopyrine- N-demethylase in the liver, and proliferation of
hepatic smooth endoplasmic reticulum. The NOEL was 20 mg/kg of feed,
equivalent to 2 mg/kg bw per day. In a 28-day study in rats given 0,
1, 5, 10, 20, or 250 mg/kg of feed, the activity of hepatic
aminopyrine- N-demethylase was increased and proliferation of hepatic
smooth endoplasmic reticulum was seen at the highest dose only. No
such effect was seen in the liver at doses equivalent to 2 mg/kg bw
per day or less. Females given 10 mg/kg of feed or more had decreased
body-weight gain. The NOEL was 5 mg/kg of feed, equivalent to 0.5
mg/kg bw per day, but this observation was not in line with the
findings of other short-term studies of toxicity in rats. A third
28-day study in rats was inadequately reported but showed that
administration of cyhalothrin at 20 mg/kg of feed (equivalent to 2
mg/kg bw per day) caused proliferation of hepatic smooth endoplasmic
reticulum; a NOEL was not identified in this study.
A 90-day study was performed in which rats given cyhalothrin at
0, 10, 50, or 250 mg/kg of feed. Males given the two higher doses
showed increased activity of hepatic aminopyrine- N-demethylase and
proliferation of hepatic smooth endoplasmic reticulum. Females at the
highest dose showed increased activity of hepatic
aminopyrine- N-demethylase activity. The NOEL was 10 mg/kg of feed,
equal to 0.56 mg/kg bw per day.
Groups of dogs received cyhalothrin at a dose of 0, 2.5, 10, or
30 mg/kg bw per day for 4 weeks or a dose of 0, 1.0, 2.5, or 10 mg/kg
bw per day for 26 weeks. In the 4-week study, the two higher doses
caused an increased incidence of vomiting, and the highest dose caused
body-weight loss, unsteady gait, and increased serum alanine and
aspartate aminotransferase activities. Muscular trembling was seen in
dogs in all groups, including the controls, in the 4-week study. In
the 26-week study, doses of 10 mg/kg bw per day or more caused
clinical signs including vomiting, salivation, lack of coordination,
unsteadiness, collapse, muscular spasms, and convulsions. As treatment
at all doses in both studies increased the prevalence of liquid
faeces, a NOEL was not identified. The Committee considered it
possible that the liquid faeces were a consequence of the neurological
effects of cyhalothrin. The LOEL was 1.0 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in mice,
cyhalothrin was given in the diet at a concentration of 0, 20, 100, or
500 mg/kg of feed for 104 weeks. An increased incidence of mammary
adenocarcinoma was seen in females at the two higher doses. The
highest incidence (14%) was only slightly greater then the upper limit
of the range in historical controls (2-12%). The Committee could not
exclude the possibility that the adenocarcinomas seen in the groups
given 100 or 500 mg/kg of feed were caused by cyhalothrin. Clinical
signs of toxicity (piloerection and hunched posture) and increased
serum activities of aspartate and alanine aminotransferases were seen
at these doses. The NOEL for these effects was 20 mg/kg of feed, equal
to 1.9 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in rats,
cyhalothrin was given in the diet at a concentration of 0, 10, 50, or
250 mg/kg of feed for 104 weeks. There was no treatment-related
increase in the incidence of any type of tumour. Adverse effects found
at the highest dose included decreased body weight, altered blood
biochemistry, and an increased weight of the liver relative to that of
the body in animals of each sex and of the adrenals in females only.
The NOEL was 50 mg/kg of feed, equal to 2.3 mg/kg bw per day.
Cyhalothrin was not genotoxic in a range of studies, including a
test for reverse mutation in Salmonella, a test for cytogenetic
effects in the bone marrow of rats treated in vivo, a test for
dominant lethal mutation in mice, and an assay of cell transformation
in vitro. The Committee concluded that cyhalothrin is not genotoxic.
Furthermore, the Committee considered it likely that the mammary
adenocarcinomas found in mice in the long-term study were due to a
non-genotoxic mechanism.
A three-generation study of reproductive toxicity was performed
in rats given cyhalothrin in the diet at a concentration of 0, 10, 30,
or 100 mg/kg of feed. Adverse effects, including reduced parental
body-weight gain and reduced litter size, were found only at the
highest dose. The NOEL for these effects was 30 mg/kg of feed, equal
to 1.7 mg/kg bw per day.
In a study of developmental toxicity, rats received a dose of 0,
5, 10, or 15 mg/kg bw per day by oral gavage on days 6-15 of
gestation. Maternal toxicity, characterized by body-weight loss and
poor coordination, was seen at the highest dose. Embryotoxicity also
occurred at this dose. Abnormalities were seen in 5 of 17 fetuses in
one litter from a dam at 10 mg/kg bw per day, but the effect was
considered not to be due to treatment with cyhalothrin as no
fetotoxicity was seen at the higher dose. The NOEL for maternal
toxicity was 10 mg/kg bw per day, and that for developmental toxicity
was 15 mg/kg bw per day, the highest dose tested.
Two studies of developmental toxicity in rats treated by dermal
administration provided some evidence that cyhalothrin can delay fetal
development at doses lower than those given orally. However, the
Committee considered that oral administration is a more relevant route
and that the NOEL in the study in which this route was used was the
appropriate one for evaluating developmental toxicity in rats.
Rabbits studied for developmental toxicity were given a dose of
0, 3, 10, or 30 mg/kg bw per day by oral gavage. The highest dose
caused initial body-weight loss in the does, which was followed by
reduced body-weight gain. There was no significant effect on
development at any dose. The NOEL for maternal toxicity was 10 mg/kg
bw per day, and the NOEL for developmental toxicity was 30 mg/kg bw
per day, the highest dose tested.
Single oral doses of up to 10 000 mg/kg bw did not induce
clinical or histopathological signs of neurotoxicity in hens.
Various studies of neurobehavioural effects have been performed
in rats, including a range-finding test of performance on an inclined
plane, a test for acute auditory startle response, and tests of
inhibitory avoidance. In the inclined plane test, rats were given a
single oral dose of 0, 15, 25, 30, 40, 50, 60, 75, 100, or 200 mg/kg
bw. All of these doses caused soft faeces in at least some of the
rats, and doses of 25 mg/kg bw or more caused clinical signs of
neurotoxicity. The results were, however, variable, and no conclusion
could be reached about neurotoxicity.
In the test for acute auditory startle response in which an oral
dose of 0, 5, 15, or 75 mg/kg bw was given, the highest dose resulted
in reduced body-weight gain, transient clinical signs, and a reduced
mean response amplitude in animals of each sex. Statistically
significant but not dose-related reductions in mean response amplitude
were also seen at 5 and 15 mg/kg bw in females only. The Committee was
aware that a biphasic auditory startle response has been reported with
some other type II pyrethroids, with a reduced response at high doses
and an increased response at lower doses. A NOEL for the startle
response was not identified in this study.
In the inhibitory avoidance tests, rats were exposed either
in utero (dams were given 200 mg/l in drinking-water) or during
lactation (dams were given 1 ml of a 0.018% solution dermally). The
doses received by the animals could not be estimated reliably. The
rats exposed in utero showed a decreased motivational response when
tested as adults, but no effects were seen on inhibitory avoidance
behaviour or locomotor frequency. Animals exposed during lactation had
a shorter latency in learning avoidance behaviour. There was no NOEL
in either study, as adverse effects were seen at the only dose tested
in each study and the doses received by the animals were not known.
4. EVALUATION
Observations and case reports in humans provided little
information relevant to the establishment of an ADI for cyhalothrin.
In most instances, no information on doses was available, the route of
exposure was dermal, and some studies were of exposure to
lambda-cyhalothrin rather than cyhalothrin.
The Committee established a temporary ADI of 0-0.002 mg/kg bw for
cyhalothrin by applying a 500-fold safety factor to the LOEL of 1.0
mg/kg bw per day for induction of liquid faeces in dogs in the 26-week
study. The Committee considered it possible that the liquid faeces
were a consequence of the neurological effects of cyhalothrin. The
Committee applied the 500-fold safety factor to account for the
absence of a NOEL for liquid faeces in dogs and because of the absence
of a NOEL for neurobehavioural effects. The ADI was made temporary
because cyhalothrin belongs to a class of substances that are
characterized by their toxicity to the central nervous system, and
therefore neurobehavioural effects may be the most sensitive indicator
of the toxicity of this compound. There was an adequate margin of
safety between the ADI and the NOELs identified in the studies on
cyhalothrin, including the NOEL of 0.65 mg/kg bw per day for
piloerection in the 28-day study in mice.
The results of studies appropriate for establishing a NOEL for
neurobehavioural effects in laboratory animals are required for
evaluation in 2002.
The Committee noted that the NOEL for toxicological effects other
than the neurotoxicity related to the pyrethroid structure was 2.3
mg/kg bw per day for adaptive changes in the livers of rats in the
long-term study. This suggests that the toxicity of the metabolites
(none of which has a pyrethroid structure) is no greater than 50% of
that of the parent drug.
5. REFERENCES
Aldridge, W.N. (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit. Rev. Toxicol., 21,
89-103.
Anderson, D., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B. &
Godley, M.J. (1983) Cyhalothrin: A cytogenetic study in the rat.
Unpublished study, report No. CTL/P/664, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Arunodaya, G.R., Kumar, K.R. & Swamy, H.S. (1997) Electrophysiologic
study of spraymen exposed to insecticide lambdacyhalothrin.
Neurol. India, 45, 189-192.
Barnes, J.M. & Verschoyle, R.D. (1974) Toxicity of new pyrethroid
insecticides. Nature, 248, 711.
Bicknell, C.E., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Milk
residues of cyhalothrin following a 10 mL application of a 2%
pour-on. Unpublished study, report No. CIBH 89-1, Coopers Animal
Health, Berkhamsted, United Kingdom. Submitted by Schering-Plough
Animal Health, Harefield, United Kingdom.
Bloomquist, J.R., Adams, P.M. & Soderlund, D.M. (1986) Inhibition of
gamma-amino butyric acid-stimulated chloride flux in mouse brain
vesicles by polycycloalkane and pyrethroid insecticides.
Neurotoxicology, 7, 11-20.
Brammer, A. (1998) Cyhalothrin: Preliminary acute startle response
study in rats. Unpublished study, report No. CTL/L/8516, Zeneca
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Brammer, A. (1999) Cyhalothrin: Acute startle response study in rats.
Unpublished study, report No. CTL/P/6162, Zeneca Central
Toxicology Laboratory, Macclesfield, Cheshire, United Kingdom.
Campana, M.A., Panzeri, A.M., Moreno, V.J. & Dulout, F.N. (1999)
Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using
the micronucleus test in erythrocytes of the fish Cheirodon
interruptus interruptus. Mutat. Res., 438, 155-161.
Chester, G., Sabapathy, N.N. & Woollon, B.H. (1992) Exposure and
health assessment during application of lambda-cyhalothrin for
malaria vector control in Pakistan. Bull. World Health Organ.,
70, 615-619.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
Gopinath, C. & Prentice, D. (1981) Cyhalothrin. Oral toxicity
study in beagle dogs (Final report: repeated daily dosing for 26
weeks). Unpublished study, report No. ICI 326/8162, Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, United Kingdom.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Prentice, D.,
Buckley, P. & Offer, J. (1983) PP 563. Preliminary oral toxicity
study in beagle dogs. Unpublished study, report No. ICI 325/8074,
Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom.
Coats, J.R. (1990) Mechanisms of toxic action and structure
relationships for organochloride and synthetic pyrethroid
insecticides. Environ. Health Perspectives, 87, 255-262.
Colley, J.C., Loane, J.A., Heywood, R., Gibson, W.A., Chasseaud, L.F.,
Edmundson, N., Lewis, D. & Woodhouse, R.N. (1980) PP563. 28-day
dose range finding study in rats. Unpublished study, report No.
ICI/321/8041, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Down, W.H., Woodhouse, R.N.,
Gibson, W.A., Gopinath, C., Zubaidy, A.J. & Prentice, D.E. (1981)
Cyhalothrin. 4-week dose range finding study on mice. Unpublished
study, report No. ICI/379/80910, Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Almond, R., Gibson, W.A.,
Gregson, R. & Gopinath, C. (1984) Cyhalothrin. Potential
tumorigenic and toxic effects in prolonged dietary administration
to mice (Final report). Unpublished study, report No.
ICI/395/83668, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Denton, S. (1988) Cyhalothrin: Preliminary neurotoxicity study in the
rat following a single oral dose. Unpublished study, report No.
S8104-001R, Covance, Harrogate, United Kingdom.
Deshmukh, P.B. (1992) Three-generation reproductive studies of a
synthetic pyrethroid, cyhalothrin. Toxicol. Lett., 64/65,
770-781.
Dow, P.R. & Parker, R.C. (1989) Attempted identification of 3 major
liver metabolites following dermal application of C-14 alcohol
lambda-cyhalothrin to lactating cows. Unpublished study, report
No. CIBH 89-2, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Dow, P.R., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989)
Metabolism of C-14 alcohol labelled lambda-cyhalothrin in
lactating cows following dermal application. Unpublished study,
report No. CIBH 88-4, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Ebino, K., Suzuki, H. & Harada, T. (1984a) PP-563: Acute oral toxicity
in rats. Unpublished study, report No. 4/HD/006931, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984b) PP-563 WP: Acute oral
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984c) PP-563: Acute
intraperitoneal toxicity in rats. Unpublished study, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984d) PP-563: Acute dermal
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984e) PP-563: Acute subcutaneous
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
European Medicines Evaluation Agency (1999) Cyhalothrin. Summary
report of the Committee for Veterinary Medicinal Products,
EMEA/MRL/699/99. Internet:
http://web.is.eudra.org/vetdocs/vets/mrl.htm.
Gassner, B., Wuthrich, A., Scholtysik, G. & Solioz, M. (1997) The
pyrethroids permethrin and cyhalothrin are potent inhibitors of
the mitochondrial complex I. J. Pharmacol. Exp. Ther., 281,
855-860.
Harrison, M.P. (1981) Cyhalothrin: The disposition and metabolism of
14C-ICI 146,814 in rats. Unpublished study, report No. 146814
KMR 002/01, Imperial Chemical Industries plc, Macclesfield,
Cheshire, United Kingdom.
Harrison, M.P. (1983) Cyhalothrin:The metabolism and disposition of
14C-ICI 146,814 in the rat; Part IV: Isolation and
identification of the major urinary metabolites derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 following oral
administration. Unpublished study, report No. YIBH 85-C9,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984a) Cyhalothrin: The metabolism and disposition of
14C-ICI 146,814 in rats; Part II, tissue residues derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 after a single
oral dose of 1 or 25 mg/kg bw. Unpublished study, report No. KMR
002/02, Imperial Chemical Industries plc, Macclesfield, Cheshire,
United Kingdom.
Harrison, M.P. (1984b) Cyhalothrin (ICI 146,814): The metabolism and
disposition of ICI 146,814 in rats; Part III, Studies to
determine radioactive residues in rats following 14 days repeated
administration. Unpublished study, report No. KMR 002/03,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984c) Cyhalothrin (ICI 146,814): The disposition and
metabolism of ICI 146,814 in the dog. Unpublished study, report
No. 146814KMD 005, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Harrison, M.P. (1985) Cyhalothrin (ICI 146,814): The metabolism,
excretion and residues in the cow after 7 days oral
administration with two [14C]-labelled forms of ICI 146,814 at
1 mg/kg/day. Unpublished study, report No. YIBH 85-C11, Imperial
Chemical Industries plc, Macclesfield, Cheshire, United Kingdom.
Hijzen, T.H. & Slangen, J.L. (1988) Effects of type I and type II
pyrethroids on the startle response in rats. Toxicol. Lett.,
40, 141-152.
Irvine, L.F.H. (1981a) Cyhalothrin: Oral (gavage) range finding study
in the male mouse. Unpublished study, report No. 2647-72/212,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Irvine, L.F.H. (1981b) Cyhalothrin: Oral (gavage) dominant lethal
study in the male mouse. Unpublished study, report No.
2647-72/213, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
JMPR (Joint FAO/WHO Meeting on Pesticide Residues) (1985) Pesticide
residues in food - 1984. Report of the Joint Meeting on
Pesticide Residues. FAO Plant Production and Protection Paper
62, Rome: Food and Agriculture Organization of the United
Nations.
Jones, J.R. (1980) Cyhalothrin: Acute oral median lethal dose (LD50)
in the female rabbit. Unpublished study, report No. 2364-72/214,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1980) Cyhalothrin: Oral (gavage) maximum tolerated dose
study in the non-pregnant rat. Unpublished study, report No.
2537-72/206, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981a) Cyhalothrin: Oral (gavage) dose ranging study in
the pregnant New Zealand white rabbit. Unpublished study, report
No. 2603-72/210, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in
the rat. Unpublished study, report No. 2661-72, Hazleton
Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1981c) Cyhalothrin: Oral (gavage) teratology study in
the New Zealand white rabbit. Unpublished study, report No.
270072/211, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Metabolism of C-14
alcohol labelled lambdacyhalothrin in lactating cows following
dermal application. Unpublished study, report No. CIBH 88-2,
Coopers Animal Health, Berkhamsted, United Kingdom. Submitted by
Schering-Plough Animal Health, Harefield, United Kingdom.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationships. Pest. Biochem.
Physiol., 18, 9-14.
Leahey, J.P., French, D.A. & Heath, J. (1985) PP321: Metabolism in a
goat. Unpublished study, report No. RJ 0435B, Imperial Chemical
Industries, Plant Protection Division, Jealott's Hill Research
Station, Bracknell, Berkshire, United Kingdom.
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
I., Robinson, M. & Stonard, M. (1981) Cyhalothrin: 90-day feeding
study in rats. Unpublished report No. CTL/P/629, Imperial
Chemical Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Marrs, T.C. (1993) Toxicology of pesticides. In: Ballantyne, B.,
Marrs, T.C. & Turner, P., eds, General and Applied Toxicology,
Basingstoke:MacMillan, Vol. 2, Ch. 62, p. 1334.
Milburn, G.M., Banham, P., Godley, M.J., Pigott, G. & Robinson, M.
(1984) Cyhalothrin: Three-generation reproduction study in the
rat. Unpublished study, report No. CTL/P/906, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Moniz, A.C., Bernadi, M.M., Souza-Spinosa, H.S. & Palermo-Neto, J.
(1990) Effects of exposure to a pyrethroid insecticide during
lactation on the behaviour of infant and adult rats. Braz. J.
Med. Biol. Res., 23, 45-48.
Moretto, A. (1991) Indoor spraying with the pyrethroid insecticide
lambda-cyhalothrin: Effects on spraymen and inhabitants of
sprayed houses. Bull. World Health Organ., 69, 591-594.
Moyes, A., Godley, M.J., Hall, M., Pratt, I., Stonard, M.D. & Tinston,
D.J. (1984) Cyhalothrin: 28-day feeding study in the rat (second
study). Unpublished report No. CTL/P/1013, Imperial Chemical
Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Narahashi, T. (1985) Nerve membrane ionic channels as the primary
target of pyrethroids. Neurotoxicology, 2, 3-22.
Nixon, J. & Jackson, S.J. (1981) Cyhalothrin: Acute toxicity.
Unpublished study, report No. CTL/T/555, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Pigott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
Robinson, M., Taylor, K. & Tinston, D.J. (1984) Cyhalothrin. Two
year feeding study in rats. Unpublished report, report No.
CTL/1023, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Prentice, D.E., Edmondson, N.A. & Lewis, D.J. (1981) 28 day dose range
finding study of cyhalothrin in dietary administration to mice.
Electron microscopy of livers. Unpublished report No.
5/HA/003712, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Pritchard, V.K. (1984) Cyhalothrin: Acute oral toxicity. Unpublished
study, report No. CTL/P/1023, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Ray, D. (1991) Chapter 13. In: Hayes, W.J., Jr & Laws, E.R., Jr, eds,
Handbook of Pesticide Toxicology, London: Academic Press, Vol.
2, pp. 585-636.
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. (1981) Cell
transformation test for potential carcinogenicity of
Y00102/010/005 (cyhalothrin (PP563)). Unpublished study, report
Number CTL/1023, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wright, D.G.D.
(1982) The acute oral toxicity (LD50) and neurotoxic effects of
cyhalothrin to the domestic hen. Unpublished study, report Number
ICI/374 NT/81742, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991a)
Effects of prenatal pyrethroid exposure on the sexual development
of rats. Vet. Hum. Toxicol., 33, 427-428.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991b)
Pyrethroid insecticides and pregnancy: Effect on physical and
behavioural development in rats. Vet. Hum. Toxicol., 33,
315-317.
Tomlin, C., ed. (1994) The Pesticide Manual, 10th Ed., Cambridge:
Crop Protection Publications / Royal Society of Chemistry.
Trueman, R.W. (1981) Cyhalothrin: Results from the Salmonella
reverse mutation assay. Unpublished study, report No. CTL/P/665,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Vijverberg, H.P.M. & van den Bercken, J. (1990) Neurotoxicological
effects and mode of action of pyrethroid insecticides. Crit.
Rev. Toxicol., 21, 105-126.
Vijverberg, H.P.M., van den Zalm, J.M. & van den Bercken, J. (1982)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature, 295, 601-603.
WHO (1990) Cyhalothrin. Environmental Health Criteria 99, Geneva:
World Health Organization.
Woodward, K. (2000) Personal communication from Kevin Woodward of
Schering-Plough Animal Health, Uxbridge, United Kingdom, to Mr
Derek Renshaw of the Department of Health. E-mail message dated
14 January 2000.
Zufan, L., Kubat, J., Formanek, J., Fuchs, A., Tobiskova, G., Zajicek,
P., Vodickova, J., Rehak, P. & Dvorak, J. (1989) Behavioural and
EEG changes induced by the pyrethroid insecticide cyhalothrin.
Int. J. Psychophysiol., 7, 447-448.
Excretion was initially rapid, most of the administered doses
being eliminated within the first 48 h after dosing, but excretion was
still incomplete at 7 days. After 14CHCN-cyhalothrin was given
orally, approximately 30% of the administered dose was found in urine
at 7 days and about 50% in faeces. When 14C-cyclopropyl-cyhalothrin
was given, the proportion of residue in urine was slightly lower (19%)
and the amount in faeces higher (68%). The proportions of residue in
urine and in faeces were approximately equal when the two
radiolabelled forms of cyhalothrin were given intravenously. A large
proportion (46-87%) of the faecal residue was in the form of unchanged
cyhalothrin after oral dosing, but only small amounts (1.4-1.5% of
faecal radiolabel) were found in faeces after intravenous injection,
suggesting that the oral dose of cyhalothrin was only partially
absorbed from the gut lumen, leaving unabsorbed cyhalothrin in the
faeces.
Metabolism occurred initially by cleavage of the ester bond to
give a phenoxybenzyl moiety and a cyclopropane acid moiety. Further
metabolism of the phenoxybenzyl moiety produced N-(3-phenoxybenzoyl)
glycine, 3-(4'-hydroxyphenoxy)benzoic acid and its sulfate conjugate,
3-phenoxybenzoyl glucuronide, small amounts of various other
conjugates of these compounds, and a little free phenoxybenzoic acid.
The cyclopropane acid moiety was extensively metabolized to at least
11 further metabolites, including 45% as the glucuronide of the
cyclopropane acid and up to 23% as the free cyclopropane acid (i.e.
3-( Z-2-chloro-3,3,3-trifluoropropenyl)-2,2-dimethylcyclo-propane
carboxylic acid; see Figure 1 (Harrison, 1984c).
Humans
In a study of three teams of seven pesticide applicators in
Pakistan, the men were monitored over 15 days while they were using a
pesticide formulation which contained 10% lambda-cyhalothrin and for a
further 4 days after they had finished spraying. The men wore typical
work clothing with no personal protective equipment while spraying
dwellings to kill mosquitoes as part of a malaria control programme.
All of the workers underwent four medical examinations, including
blood sampling, before the study, in the middle of the first week, in
the middle of the second week, and 4 days after the last spraying, and
were questioned about adverse effects. Urine samples were collected
daily. Fifteen men who lived in the treated dwellings were monitored
for exposure by collection and analysis of 24-h urine samples for 3
consecutive days after the spraying of their houses. The samples of
urine and serum were treated enzymically to deconjugate the
metabolites and were then analysed by gas chromatography with mass
spectroscopy. The cyhalothrin metabolites measured were
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)-benzoic acid. The limit of determination for all
three metabolites was 0.01 µg/ml. These metabolites are products of
cleavage of the ester bond in cyhalothrin, and their presence
indicates that the initial metabolism of cyhalothrin is similar in
humans to that observed in other species.
The average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). 3-(2-Chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid was usually the most abundant
metabolite in urine. No metabolites were detected in serum samples.
Most of the inhabitants of treated houses had no detectable residues
in their urine, although they were measurable in 12 of 44 samples, and
the measured concentrations were at or close to the limit of
determination. Most values were not reported, but the highest
concentration of urinary metabolites was 0.1 mg/person per day
(Chester et al., 1992). The signs of toxicity found are reported
below.
2.2 Toxicological studies
Most of the toxicological studies of cyhalothrin were performed
during 1980-84, and some were therefore conducted before GLP was
widely introduced. The chemical identity of the cyhalothrin used in
each study is not clearly stated, and, although the purity was
reported in most cases, the isomers and the isomer ratios were not
mentioned in most of the reports. The manufacturer has claimed that
the analysis given in appendices to the report of the inclined plane
test (see below) is typical of technical-grade cyhalothrin. The
manufacturer also claimed that measurements made over the last
15 years have shown little variation in the isomer ratio. Two
measurements from 1985 gave about 56% for the A isomeric pair and 41%
for the B isomeric pair, leaving only about 2% for other isomers and
impurities (Woodward, 2000).
2.2.1 Acute toxicity
Acute poisoning of rats with type II pyrethroids such as
cyhalothrin is typically characterized by progressive development of
'nosing' and exaggerated jaw opening, increasing extensor tone in the
hind limbs causing a rolling gait, choreoathetosis (sinuous writhing
spasms), salivation, incoordination progressing to coarse tremor,
tonic seizures, apnoea, and death (Barnes & Verschoyle, 1974; Ray,
1991). This has been called the choreoathetosis/salivation syndrome or
CS syndrome. In dogs, similar symptoms are seen, but salivation, upper
airway hypersecretion, and gastrointestinal symptoms are more
prominent than in rats (Ray, 1991).
As in poisoning with type I pyrethroids, the plasma noradrenaline
concentration is increased by type II pyrethroids, but they also
increase plasma adrenaline and blood glucose concentrations. Type II
pyrethroids increase cardiac contractility both directly by action on
cardiac muscle and via circulating catecholamines. They also cause
repetitive firing and potentiate contraction in skeletal muscle. The
type II pyrethroids do not produce the repetitive activity in sensory
nerves that is seen after exposure to type I pyrethroids (Ray, 1991).
Table 3. LD50 values for cyhalothrin
Species Sex Route Purity Vehicle LD50 Reference
(%) (mg/kg bw)
Rat M Oral 90/94 cis Corn oil 240 Nixon & Jackson (1981)
Rat M Oral 92.9 Corn oil 170 Pritchard (1984)
Rat M Oral 90 Oilve oil 51 Ebino et al. (1984a)
Rat F Oral 90/94 cis Corn oil 140 Nixon & Jackson (1981)
Rat F Oral 92.9 Corn oil 110 Pritchard (1984)
Rat F Oral 90 Olive oil 65 Ebino et al. (1984a)
Mouse M Oral 90/94 cis Corn oil 37 Nixon & Jackson (1981)
Mouse F Oral 90/94 cis Corn oil 62 Nixon & Jackson (1981)
Guinea-pig M Oral 90/94 cis Corn oil > 5000 Nixon & Jackson (1981)
Rabbit F Oral 89.2 Corn oil > 1000 Jones (1980)
Chicken F Oral 93.4 Corn oil > 10 000 Roberts et al. (1982)
Rat M Dermal 90 Undiluted 3600 Ebino et al. (1984d)
Rat F Dermal 90 Undiluted 3700 Ebino et al. (1984d)
Rabbit M Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rabbit F Dermal 90/94 cis Propylene > 2 ml/kg Nixon & Jackson (1981)
glycol
Rat M Intraperitoneal 90/94 cis Corn oil 250-750 Nixon & Jackson (1981)
Rat M Intraperitoneal 90 Oilve oil 500 Ebino et al. (1984c)
Rat F Intraperitoneal 90 Oilve oil 420 Ebino et al. (1984c)
Rat M Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
Rat F Subcutaneous 90 Oilve oil > 5000 Ebino et al. (1984e)
The acute toxicity of cyhalothrin was investigated in a series of
studies that complied with GLP (Nixon & Jackson, 1981; Roberts et al.,
1982; Pritchard, 1984) and also in studies that, although not GLP
compliant, were accompanied by a suitable quality assurance statement
(Jones, 1980; Ebino et al., 1984a-e). The LD50 values estimated from
these studies are listed in Table 3. The LD50 of a wettable
formulation containing 5% cyhalothrin was 1200 mg/kg bw in male rats
and 900 mg/kg bw in females when it was administered orally in olive
oil (Ebino et al., 1981b).
The signs of toxicity seen in all species and with all routes of
administration were similar and included subdued behaviour, ungroomed
appearance, piloerection, salivation, incontinence, and unsteady gait.
A further account of the signs of acute toxicity in rats receiving
various oral doses of cyhalothrin is given in the preliminary report
of the inclined plane test (Denton, 1988), which is described below.
2.2.2 Short-term studies of toxicity
Mice
In a range-finding study for an assay of dominant lethal mutation
that complied with GLP, groups of 10 male CD-1 mice were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 5, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 5 days. At
the two higher doses, signs of severe toxicity (ataxia and
convulsions) were observed. One animal at 40 mg/kg bw per day and
eight at 80 mg/kg bw per day died. Less severe clinical signs were
seen at 20 mg/kg bw per day, including body-weight loss, ataxia, and a
rough coat. Only rough coats were seen at 5 mg/kg bw per day (Irvine,
1981a).
In a range-finding study for a long-term study of toxicity and
carcinogenicity, groups of 12 male and 12 female CD-1 mice were fed
diets containing technical-grade cyhalothrin (purity unspecified) at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of diet. Over the
course of the 4-week study, these concentrations gave mean doses of
cyhalothrin of 0, 0.65, 3.3, 14, 64, and 310 mg/kg bw per day for
males and 0, 0.80, 4.2, 15, 78, and 290 mg/kg bw per day for females.
No certificate of compliance with GLP was available for this study,
but quality assurance systems were in place.
Deaths were seen only at the highest dietary concentration, at
which six males and three females died prematurely. Severe signs of
toxicity were also seen at this dose, including abnormal gait
('walking on toes'), hunched posture, decreased body weight (Student's
t test, p < 0.01), and increased respiratory rate. Piloerection
was observed in several animals in each groups given cyhalothrin at
> 25 mg/kg and in one control male and one male at 5 mg/kg of diet.
Haematological changes were seen in males (statistically significant
in Williams' test: p < 0.05) but not in females. Animals at 2000
mg/kg of diet had a decreased total leukocyte count accompanied by a
lower lymphocyte count and a higher neutrophil count. Those at 100 and
500 mg/kg of diet also had marginally lowered lymphocyte counts.
Macroscopic examinations post mortem revealed no adverse
effects other than altered organ weights (statistically significant in
both Student's t test and Williams' test: p < 0.05). The weight
of the kidney relative to body weight was slightly elevated in males
given 100, 500, or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The relative liver weights were also slightly increased in
males given 25 or 2000 mg/kg of diet and in females given 2000 mg/kg
of diet. The heart weights were marginally decreased in females given
100 or 2000 mg/kg of diet. The activity of aminopyrine- N-demethylase
in liver was increased in males given 2000 mg/kg of diet and in
females given 100, 500, or 2000 mg/kg of diet (Student's t test:
p < 0.05). Histopathological examination showed minimal
centrilobular hepatocyte enlargement in two females at 2000 mg/kg of
diet. Atrophy of the red pulp of the spleen was seen in two of the
three females at this dose that died. The Committee considered that
the effect may have been indicative of immunological changes. The NOEL
was 5 mg/kg of diet, equal to 0.65 mg/kg bw per day, on the basis of
piloerection at concentrations > 25 mg/kg of diet (Colley et al.,
1981).
When liver samples from this study were examined by electron
microscopy, minimal amounts of smooth endoplasmic reticulum were
observed in occasional hepatocytes from some mice from each group,
including controls. Most hepatocytes contained no observable smooth
endoplasmic reticulum. The incidence of mice with hepatocytes
containing smooth endoplasmic reticulum was highest in the group given
cyhalothrin at 2000 mg/kg of diet, consistent with the finding of
other hepatic effects in this group (Prentice et al., 1981).
Rats
In a range-finding study for an investigation of developmental
toxicity, groups of 10 female Charles River CD rats were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) at a dose of 0, 10, 20,
40, or 80 mg/kg bw per day by oral gavage in corn oil for 10
consecutive days. After treatment, the animals were observed for a
7-day recovery period. No certificate of compliance with GLP was
supplied, but suitable quality assurance records had been kept.
All of the animals given 80 mg/kg bw per day, nine of those given
40 mg/kg bw per day, and two given 20 mg/kg bw per day had to be
killed for humane reasons after the first dose, as this caused severe
toxicity (ataxia and/or collapse). Body-weight loss and reduced food
intake were seen in the rats given 20 mg/kg bw per day throughout the
treatment period. Body-weight loss was also seen in the rats given 10
mg/kg bw per day during the first 5 days of the study. Food intake was
increased in the groups at 10 and 20 mg/kg bw per day during the
recovery period. Macroscopic examination of the animals at necropsy
revealed no treatment-related changes. No NOEL could be identified in
this study (Killick, 1980).
In a 28-day range-finding study, groups of 10 male and 10 female
Charles River CD rats were fed diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 5, 10, 20,
or 250 mg/kg of diet, equivalent to 0, 0.5, 1, 2, and 25 mg/kg bw per
day. The study did not comply with GLP. There were no
treatment-related clinical signs and no deaths. Food intake, food
efficiency, and body-weight gain were decreased only at the highest
dose (Student's t test: p < 0.05). Gross examination post
mortem revealed no treatment-related effects, and organ weights were
unaffected by treatment. Electron microscopy of liver samples showed a
moderate increase in the amount of smooth endoplasmic reticulum in
hepatocytes of animals at the highest dose, with a decrease in the
amount of glycogen. An increase in hepatocyte size was noted in the
centrilobular and mid-zonal regions of the livers of males at this
dose, which also had increased hepatic activity of
aminopyrine- N-demethylase (Student's t test: p < 0.001). The
NOEL was 20 mg/kg of diet, equivalent to 2 mg/kg bw per day (Colley et
al., 1980).
In a study that complied with GLP, groups of eight male and eight
female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%) at a concentration of 0,
1, 5, 10, 20, or 250 mg/kg of diet, equivalent to 0, 0.1, 0.5, 1, 2,
and 25 mg/kg bw per day. Full post-mortem examinations were not
performed, but samples of liver were taken for analysis of
aminopyrine- N-demethylase activity and electron microscopy. No
treatment-related clinical signs were reported. The mean body weight
of males given 250 mg/kg of diet was decreased when compared with that
of concurrent controls during weeks 1 and 2 of the study (two-sided
t test: p < 0.05). Body-weight gain was depressed during week 1
for males given 250 mg/kg of diet and for females given 10, 20, or 250
mg/kg of diet (two-sided t test: p < 0.05). The absolute weight
of the liver was reduced in males given 250 mg/kg of diet and in
females given 20 mg/kg of diet (two-sided t test: p < 0.05), but
the relative weight of the liver was increased in males at 250 mg/kg
of diet. Both males and females at this dose had increased hepatic
activity of aminopyrine- N-demethylase and mild proliferation of
smooth endoplasmic reticulum in hepatocytes. The NOEL was 5 mg/kg of
diet, equivalent to 0.5 mg/kg bw per day, on the basis of depression
of body-weight gain, whereas that for hepatic changes was 20 mg/kg of
diet, equivalent to 2 mg/kg bw per day (Moyes et al., 1984).
In a 28-day range-finding study, of which full details were not
available, a dietary concentration of cyhalothrin of 750 mg/kg
(equivalent to 75 mg/kg bw per day) increased the mortality rate.
'Characteristic signs of pyrethroid neurotoxicity' were seen at
dietary concentrations of 250 and 750 mg/kg. Treatment-related effects
on body weights, food consumption, organ weights, and hepatic activity
of aminopyrine- N-demethylase were seen at 250 mg/kg of diet. Hepatic
smooth endoplasmic reticulum proliferation occurred in males fed 20
and 250 mg/kg of diet. No NOEL could be identified (Lindsey et al.,
1981).
In a 90-day study that complied with GLP, groups of 20 male and
20 female Wistar-derived Alderley Park rats were fed diets containing
technical-grade cyhalothrin (purity, 89.2%; with a total pyrethroid
content of 92.2% of which 96.8% was cyhalothrin) at a concentration of
0, 10, 50, or 250 mg/kg of diet, providing mean doses of 0, 0.56, 2.6,
and 36 mg/kg bw per day for males and 0, 0.57, 3.2, and 15 mg/kg bw
per day for females. Blood samples were collected before treatment,
during week 4 of treatment, and at the end of the study. Urine samples
were collected before treatment and in weeks 4 and 13. Ophthalmoscopic
examinations were performed during the week before termination. Full
post-mortem examinations were performed, and the major organs and any
grossly abnormal tissues were examined histologically. Liver samples
were taken for analysis of aminopyrine- N-demethylase activity and
examination by electron microscopy.
Treatment with doses < 250 mg/kg of diet had no effect on the
mortality rate. The body-weight gain of males at the highest dose was
consistently reduced (two-sided t test: p < 0.01) throughout the
study. Food consumption was slightly reduced in males at 50 and 250
mg/kg of diet, and this effect was occasionally statistically
significant (two-sided t test: p < 0.05). There were no clearly
treatment-related haematological effects, although there were small,
significant, but not dose-related changes in some red blood cell
parameters (mean corpuscular volume, packed cell volume, haemoglobin,
mean corpuscular haemoglobin). Plasma alanine and aspartate
aminotransferase activities and cholesterol concentration were raised
at 4 weeks in males given 10 or 50 mg/kg of diet, and those at the
lower dose also had a raised plasma urea concentration (two-sided
t test: p < 0.05). At 13 weeks, however, the plasma concentration
of urea in males at 50 mg/kg of diet was decreased (two-sided
t test: p < 0.05). The observed effects on haematological
parameters, plasma enzyme activity, and concentrations of cholesterol
and urea were considered to be unrelated to treatment as there were no
clear dose-response relationships. At 13 weeks, however, the plasma
triglyceride concentration was reduced and there was a dose-related
increase in urinary glucose concentration in males at 50 and 250 mg/kg
of diet (two-sided t test: p < 0.05). Ophthalmoscopy revealed no
treatment-related effects. The results of gross examinations were not
reported, although the organ weights were recorded. The absolute
weight of the liver was increased in males at 250 mg/kg of diet, and
the absolute weight of the lung was decreased in animals given 250
mg/kg of diet, but the relative weights of these organs were
unaffected. No treatment-related changes were revealed by optical
microscopy of organs. Electron microscopy of the liver revealed mild
proliferation of smooth endoplasmic reticulum in the hepatocytes of
three males given 50 mg/kg of diet and three males given 250 mg/kg of
diet. There was a dose-related increased in hepatic
aminopyrine- N-demethylase activity in males given 50 or 250 mg/kg of
diet and in females given 250 mg/kg of diet (two-sided t test:
p < 0.05). The NOEL was 10 mg/kg of diet, equivalent to 0.56 mg/kg
bw per day, on the basis of hepatic changes in males at higher doses
(Lindsey et al., 1981).
Rabbits
In a range-finding study for an investigation of developmental
toxicity, groups of six mated female New Zealand white rabbits were
given cyhalothrin (purity, 89.25%) at a daily dose of 0, 20, 30, or 40
mg/kg bw by oral gavage in corn oil on days 6-18 of gestation. The
animals were killed on day 18 of gestation.
There were no unscheduled deaths and no consistent clinical signs
of toxicity. Body-weight gain was depressed only at 30 mg/kg bw per
day, although animals at 40 mg/kg bw per day showed reduced food
intake. Not all of the animals became pregnant, but in all groups,
including controls, few of the does had live fetuses. There was no
dose-response relationship for this effect. Furthermore, not all of
the pregnant does at 20 and 40 mg/kg bw per day produced live
offspring. Consequently, there were insufficient numbers of litters to
allow any meaningful inter-group comparison of litter parameters,
although no adverse effects were apparent from the limited data
available. No treatment-related macroscopic changes were seen post
mortem. The apparent NOEL in this limited study was 20 mg/kg bw per
day on the basis of reduced body-weight gain (Killick, 1981a).
Dogs
In a preliminary study, groups of one male and one female beagles
were given gelatin capsules containing cyhalothrin (purity
unspecified) in corn oil at a dose of 0, 2.5, 10, or 30 mg/kg bw per
day for 4 weeks. After 10 days, treatment with the high dose was
stopped, and the animals were allowed to recover for 4 days; treatment
was then resumed at a lower dose of 20 mg/kg bw per day for 4 weeks.
Blood was taken weekly. Urine samples were collected before dosing and
during week 4. Ophthalmoscopic examinations were performed on each dog
before dosing and during week 4. No certificate of compliance with GLP
was supplied, but suitable quality assurance records were kept.
None of the dogs died prematurely. Muscular trembling was seen in
all groups, including controls, but the degree of trembling was more
marked in animals at the two higher doses. Unsteady gait and
body-weight loss were observed in dogs given 30 mg/kg bw per day, but
this effect was not seen after the dose was reduced to 20 mg/kg bw per
day. Body weight was unaffected by doses up to 10 mg/kg bw per day,
and only slight fluctuations occurred at 20 mg/kg bw per day. Vomiting
was seen occasionally at doses > 10 mg/kg bw per day. Dogs at the
high dose (30 or 20 mg/kg bw per day) had loose stools and increased
serum alanine and aspartate aminotransferase activities. No
haematological, ophthalmological, or urinary changes were observed,
and no gross pathological or organ weight changes were noted post
mortem. Histopathological examination revealed no treatment-related
effects. No NOEL could be identified in this study owing to the
finding of an increased frequency of liquid faeces at all doses. The
LOEL was 2.5 mg/kg bw per day (Chesterman et al., 1983).
In a 26-week study that complied with GLP, groups of six male and
six female beagles were given cyhalothrin (purity unspecified)
dissolved in corn oil inside a gelatin capsule at a dose of 0, 1.0,
2.5, or 10 mg/kg bw per day. The dogs were examined
ophthalmoscopically before dosing and during weeks 6, 12, and 24 of
treatment. Blood and urine samples were taken every 4 weeks. The
functional integrity of the nervous system was assessed clinically
before treatment and during week 6. At the end of the treatment
period, full necropsies were performed on all dogs, and a large
selection of organs and tissues was examined histologically.
No deaths occurred during the study. A dose-related increase in
the incidence of liquid faeces affected all treated groups. At 10
mg/kg bw per day, the clinical signs seen included vomiting,
salivation, incoordination, unsteadiness, collapse, muscular spasms,
and convulsions. Body weight was unaffected by treatment.
Ophthalmoscopic and neurological examinations revealed no adverse
effects, and no consistent haematological, serum biochemical, or
urinary effects were seen. No treatment-related gross effects on organ
weights or appearance were noted post mortem, and histopathological
examination revealed no treatment-related effects. No NOEL could be
identified in this study owing to the finding of an increased
frequency of liquid faeces at all doses. The LOEL was 1.0 mg/kg bw per
day (Chesterman et al., 1981).
In a 52-week study, groups of six dogs of each sex were given
lambda-cyhalothrin at a dose of 0, 0.1, 0.5, or 3.5 mg/kg bw per day
orally in corn oil, but there was lack of agreement between summaries
of the study about whether the animals were treated by gelatin capsule
(European Medicines Evaluation Agency, 1999) or by gavage (WHO, 1990).
The report was not available to the Committee. Clinical signs of
neurological effects (muscular trembling, unsteadiness, and vomiting)
were seen in all dogs at the highest dose, but the signs were not
accompanied by histological changes in the nervous system. The
European Medicines Evaluation Agency (1999) reported a dose-related
increased incidence of liquid faeces at all doses, but WHO (1990)
reported that this effect was seen only at 0.5 and 3.5 mg/kg bw per
day and that the increased incidence was only slight at 0.5 mg/kg bw
per day. The incidence of liquid faeces was considered not to be
toxicologically significant. The NOEL was 0.5 mg/kg bw per day on the
basis of neurological signs seen at 3.5 mg/kg bw per day. It is
unclear how this dose relates to a dose of cyhalothrin (WHO, 1990;
European Medicines Evaluation Agency, 1999).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In a study that complied with GLP, groups of 52 male and 52
female CD-1 mice were given diets containing technical-grade
cyhalothrin (purity unspecified) at a concentration of 0, 20, 100, or
500 mg/kg for 104 weeks, equal to 0, 1.8, 9.2, and 53 mg/kg bw per day
for males and 0, 1.8, 11, and 51 mg/kg bw per day for females.
Satellite groups of 12 mice of each sex were given the same diets for
52 weeks and then killed.
The signs of toxicity observed in animals given high doses were
piloerection and hunched posture in mice of each sex given 500 mg/kg
of diet and in males given 100 mg/kg of diet. Males at 500 mg/kg of
diet also showed emaciation, pallor, hyperactivity, and increased
fighting activity. The mortality rate was unaffected by treatment.
Decreased body-weight gain was seen in males at 500 mg/kg of diet
throughout the study (two-sided Student t test: p < 0.05),
particularly during the first 13 weeks ( p < 0.001). Reduced feed
use efficiency was seen in the same group over the first 24 weeks of
the study (not calculated for the rest of the study). Water
consumption was also slightly increased in males at 500 mg/kg of diet
(two-sided Student t test: p < 0.05).
The results of haematological tests were unremarkable.
Statistically significant changes in various parameters were seen at
various times, but the effects were not consistent or dose-related.
Urinary analysis revealed no significant differences from control
values. The serum glucose concentration was slightly reduced
(two-sided Student t test: p < 0.05) in animals given 500 mg/kg
of diet. During week 100, serum alanine and aspartate aminotransferase
activities were increased in a dose-related manner, with occasional
significance ( p < 0.05) for both activities in mice of each sex at
100 and 500 mg/kg of diet and for alanine aminotransferase activity in
females at 20 mg/kg of diet.
Gross examination post mortem revealed increased incidences
(not dose-related) of thickening of the forestomach in treated
females. The ovarian weights were significantly (two-sided Student
t test: p < 0.05) decreased at all doses in animals in the main
group but not in the satellite groups. The effect was not
dose-related. Other organ weights were unaffected. Histopathological
examination revealed a variety of non-neoplastic lesions, but none was
considered to be due to treatment with cyhalothrin.
A non-dose-related increase in the incidence of mammary
adenocarcino-mas was seen in females in the main group, the incidences
being 1/52 in controls, 0/52 at 20 mg/kg of diet, 7/52 at 100 mg/kg of
diet, and 6/52 at 500 mg/kg of diet. (The detailed statistical
analysis of these results was given in an addendum to the report that
was not available to the Committee.) The incidence of mammary
adenocinoma in control females in 17 studies performed by the same
laboratory between 1980 and 1982 ranged from 2% to 12%. No
preneoplastic changes were found in the mammary glands of mice in the
main or satellite groups. A re-evaluation of mammary tissues from all
animals confirmed the original diagnoses of mammary neoplasia. As the
highest incidence of mammary adenocarcinoma in any group was only
slightly above the range of historical controls and as there was no
dose-response relationship, it was concluded that treatment with
cyhalothrin was unlikely to have caused these tumours. The NOEL was 20
mg/kg of diet, equal to 1.8 mg/kg bw per day, on the basis of clinical
signs of toxicity (piloerection and hunched posture) in males at
higher doses (Colley et al., 1984).
Rats
In a study that complied with GLP, groups of 72 male and 72
female Alpk/AP SPF rats were fed diets containing technical-grade
cyhalothrin (purity, 89.25%; containing 92.2% pyrethroids of which
96.8% was cyhalothrin) at a concentration of 0, 10, 50, or 250 mg/kg
for 104 weeks, providing mean doses of 0, 0.47, 2.3, and 12 mg/kg bw
per day for males and 0, 0.55, 2.7, and 14 mg/kg bw per day for
females. Satellite groups of 10 rats of each sex were given the same
diets but were killed after only 52 weeks. Blood was taken for
haematological measurements from 10 rats of each sex in each group at
4 and 13 weeks and thereafter at 13-week intervals. Blood was taken
for biochemical analyses and urine for urinary analyses from 12 rats
of each sex in each group at the same intervals. In weeks 52 and 103,
ophthalmoscopy was performed on 20 males and 20 females from each
group.
No clinical signs were seen that could be attributed to
treatment. The mortality rate was unaffected. Body-weight gain was
suppressed in both males and females given 250 mg/kg of diet
(two-sided Student t test: p < 0.05), and the males in this group
had increased food use efficiency. No consistent, dose-related
haematological effects were seen, although changes in various
parameters occasionally achieved statistical significance (two-sided
Student t test: p < 0.05). The volume of urine produced by rats
fed 250 mg/kg of diet tended to be decreased, but otherwise there was
no effect on urinary parameters. Ophthalmoscopy revealed no
treatment-related effects. Blood biochemistry showed intermittent
effects in rats at 250 mg/kg of diet, consisting of reduced plasma
concentrations of glucose and triglycerides, reduced plasma alkaline
phosphatase activity, and increased plasma urea concentration
(two-sided Student t test: p < 0.05). The reduction in plasma
triglycerides was the most marked finding, especially in females.
Long, pointed fibres, thought to originate from the diet, were
found embedded in the tissues of the palate, nasal turbinates, roof of
the nasal cavity, and peridontal space. The presence of the fibres
suggested that the lesions were caused by penetration of the tissue by
these fibres rather than by cyhalothrin. No treatment-related gross
lesions were detected post mortem, but several rats in all groups,
including controls, had rhinitis and oral lesions (oral granuloma
formation and erosion of the palate) in response to the presence of
the fibres in the diet. At the interim kill, the weight of the liver
(relative to body weight) was increased in rats of each sex given 250
mg/kg of diet, but this effect was not seen in rats killed at 104
weeks. The weight of the adrenals (relative to body weight) was
increased in females at 50 and 250 mg/kg of diet killed at 104 weeks.
Histopathological examination revealed no treatment-related effect on
the incidence or severity of any neoplastic or non-neoplastic lesions.
The NOEL was 50 mg/kg of diet, equal to 2.3 mg/kg bw per day (Pigott
et al., 1984).
2.2.4 Genotoxicity
Cyhalothrin was tested in assays for gene mutation in bacteria,
cytogenicity in rats in vivo, dominant lethal mutation in mice, and
cell transformation. The results of all the assays were negative. The
assays did not comply with GLP, but they appeared to be of good
quality and suitable quality assurance records had been maintained.
The studies are summarized in Table 4. The material tested by Trueman
(1981) was analysed by reversed-phase HPLC and found to have a
cis:trans ratio of 97.1:2.9.
lambda-Cyhalothrin was reported to be non-genotoxic when tested
for reverse mutation in bacteria, gene mutation in a mouse lymphoma
cell line, cytogenicity in vitro, unscheduled DNA damage in
vitro, and micronucleus formation in mice (WHO, 1990). Full reports
of these assays were not available, but the available details are
given in Table 5. lambda-Cyhalothrin produced micronuclei in an assay
in fish (Campana et al., 1999), but the relevance to human health of a
positive result in this unvalidated assay is not known.
2.2.5 Reproductive toxicity
(a) Multigeneration studies
Mice
Only a brief account of a three-generation study of the effects
of cyhalothrin on reproduction in mice has been reported. It is
unclear whether the study was performed in accordance with the
principles of GLP. Groups of albino Swiss mice were given oral doses
of '0 (blank control), 2.5 ppm and 5.0 ppm cyhalothrin/kg body weight
daily' (the meaning of the term 'ppm cyhalothrin/kg body weight' is
unclear). The F0 generation consisted of groups of four males and
eight females. No further details of the protocol were available. No
signs of toxicity or behavioural changes were observed in the parents
or offspring for up to three generations. Maternal body weights were
not affected, although F2 generation dams at the low dose had
increased food intake. The weights of pups of F2 dams treated at the
high dose were reported to be significantly different from those of
control pups. Male and female fertility indices, pup live birth and
survival indices, litter size, and sex ratio were unaffected. The
meaning of the results was unclear. No NOEL could be identified
(Deshmukh, 1992).
Table 4. Assays for genotoxicity with cyhalothrin
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse S. typhimurium 0.16-2500 µg/ 90.2 Negativea Trueman (1981)
mutation TA1535, TA1537, plate ± S9
TA1538, TA98,
TA100
Cell Syrian hamster 50-1000 µg/ml NR Negativeb Richold et al.
transformation - S9 (1981)
1000-5000 µg/ml
+ S9
In vivo
Cytogenicity Bone marrow, Single oral dose 89.2 Negativec Anderson et al.
Wistar-derived of 1.5, 7.5,15 mg/ (1983)
male rats kg bw in corn oil
5 daily oral doses
of 1.5, 7.5, 15 mg/
kg bw per day
Dominant Male CD1 mice 1, 5, 10 mg/kg bw/ 89.2 Negatived Irvine (1981b)
lethal day for 5 days by
mutation oral gavage
in corn oil
a 2-Aminoanthracene, 9-aminoacridine, 4-nitroquinoline- N-oxide, and
N-methyl- N'-nitro- N-nitrosoguanidine used as positive controls
b 4-Nitroquinoline- N-oxide and para-dimethylaminoazobenzene (butter yellow) used
as positive controls
c Ethyl methanesulphonate used as a positive control
d Cyclophosphamide used as a positive control
Table 5. Assays for genotoxicity with lambda-cyhalothrin
End-point Test object Concentration Results Reference
In vitro
Reverse S. typhimurium < 5000 µg/plate Negative IPCS (1990)
mutation (strains not ± S9
specified)
Gene mutation L51787 mouse 125-4000 µg/ml Negative IPCS (1990)
lymphoma cell ± S9
line
Unscheduled Cultured human NR Negative IPCS (1990)
DNA synthesis HeLa cells
Cytogenicity Human lymphocytes NR Negative IPCS (1990)
In vivo
Micronucleus Erythrocytes of 0.001-0.05 µg/L Positive Campana et al.
formation Cheirodon interruptus (1999)
interruptus
Micronucleus Mice < 35 mg/kg bw Negative IPCS (1990)
formation (route not
specified)a
a Cyclophosphamide used as a positive control
Rats
Throughout a GLP-compliant three-generation (two litters per
generation) study, groups of SPF Alpk/AP Wistar-derived rats were
given diets containing technical-grade cyhalothrin (purity, 89.25%;
containing 92.2% pyrethroids of which 96.8% was cyhalothrin) at a
concentration of 0, 10, 30, or 100 mg/kg, providing mean doses of 0,
0.6, 1.7, and 5.6 mg/kg bw per day for males and 0, 0.7, 1.9, and 6.1
mg/kg bw per day for females. The F0 generation consisted of groups
of 15 male and 30 female rats. After 12 weeks on the test diets, these
animals were mated within their groups to produce a first litter
(F1a generation). Later, they were mated again to produce a second
litter (F1b generation). Breeding was repeated with F1 parents (15
males and 30 females per group) selected from the F1b animals and
F2 parents (15 males and 30 females per group) selected from the
F2b animals. At day 36 post partum, all pups not selected as the
next parental generation were killed and examined externally.
Approximately half of these were examined by autopsy, with
histopathological examination of any grossly abnormal tissues. A
fuller post-mortem examination with histopathology of all major organs
was performed on five males and five females from each group of the
F1b and F2b generations and 10 males and 10 females from each of
the F3b groups.
No clinical signs of toxicity were found. Minor reductions in
body-weight gain were seen in the premating period of the F0
generation of animals of each sex at 100 mg/kg of diet and
occasionally in females given 30 mg/kg of diet. At other times and in
other generations, occasional reductions in body-weight gain were seen
in rats given cyhalothrin at 30 or 100 mg/kg of diet. Male fertility
was unaffected by treatment. The fertility of F1 females at 30 mg/kg
of diet was statistically significantly (one-sided t test:
p < 0.05) reduced but remained within the range of control values.
At 100 mg/kg, the litter size of F2 and F3 pups was reduced, the
effect being statistically significant (one-sided t test:
p < 0.05) for the F2a and F3b litters. Small but statistically
significant decreases (one-sided t test: p < 0.05) were seen in
the percentages of live pups born to animals at 10 mg/kg in the F1b
generation and to dams at 30 and 100 mg/kg of diet in the F3b
generation. Post-mortem and histopathological examinations revealed no
adverse effects that could be attributed to the treatment. The
occasional effects seen at 10 and 30 mg/kg of diet were considered to
be incidental, and only the effects at 100 mg/kg of diet were regarded
as treatment-related. The NOEL was 30 mg/kg of diet, equal to 1.7
mg/kg bw per day, on the basis of reduced parental body-weight gain
and reduced litter size (Milburn et al., 1984).
(b) Developmental toxicity
Rats
Groups of 24 pregnant Sprague-Dawley-derived rats (CD rats from
Charles River's SPF colony) were given technical-grade cyhalothrin
(purity, 89.25%; containing 92.2% pyrethroids of which 96.8% was
cyhalothrin) at a dose of 0, 5, 10, or 15 mg/kg bw per day by oral
gavage in corn oil on days 6-15 of gestation. The dams were killed on
day 20, and the contents of their uteri were examined. No certificate
of compliance with GLP was supplied, but suitable quality assurance
records had been kept.
Body-weight loss, reduced food intake, and signs of neurotoxicity
(loss of limb coordination in 2 out of 24 rats) were observed in dams
at the highest dose. The numbers of pregnant animals, pre-implantation
and post-implantation losses, mean litter weights, and mean weights
and lengths of fetuses were unaffected by treatment. Abnormalities
were seen in one litter of a dam at 10 mg/kg bw per day, in which 5 of
17 fetuses had major defects: four had bilateral agenesis of the
kidneys, and three had skeletal malformations of the vertebral
centrae, sternebrae, and/or metacarpals. The effects were considered
to be incidental and unlikely to be due to treatment. The NOEL was 10
mg/kg bw per day on the basis of maternal toxicity (Killick, 1981b).
Six pregnant Wistar-derived rats were given cyhalothrin (purity,
95.8%) by dermal application throughout the 21 days of gestation at a
daily dose of 1 ml of 0.02% (w/v; equal to approximately 0.8 mg/kg bw
per day if completely absorbed) in an aqueous vehicle applied to a
shaved area of the back. Controls were given the vehicle alone. After
parturition, six pups were left with each dam. At 90 days of age, 12
males in the treated group were tested for mating behaviour with
ovariectomized females that were showing estrus induced by
subcutaneous injections of 17ß-estradiol and progesterone. No
certificate of compliance with GLP was supplied for this study.
Treated male pups showed a delay in testicular descent, but the age of
vaginal opening was not affected. The mating behaviour of the males
was not affected by treatment. No NOEL could be identified (da Silva
Gomes et al., 1991a).
In another study which did not appear to conform to GLP, 12
pregnant Wistar-derived rats were given cyhalothrin (purity
unspecified) by dermal application throughout the 21 days of gestation
at a daily dose of 1 ml of 0.018% (w/v; equal to approximately 0.72
mg/kg bw per day if completely absorbed) in an aqueous vehicle. At
weaning (21 days) and at 90 days of age, the pups were tested for
locomotor activity in an open field. At 90 days, training began for
inhibitory avoidance behaviour. Adult males were also tested for
motivational response in a 'hole board' test.
The opening of ears and eyes, the development of fur, and the
descent of testes were all delayed in treated rats, but the time of
vaginal opening was not affected. The body weights of the pups of
cyhalothrin-treated dams were statistically significantly greater
(Student t test: p < 0.05) than those of controls at days 2, 7,
and 14 of age but not at day 21. Locomotor frequency was increased at
21 days and decreased at 90 days, but the effects were not
statistically significant in the Student t test. No effect was seen
on inhibitory avoidance behaviour. The results of the hole board test
showed a decreased motivational response in cyhalothrin-exposed rats.
No NOEL could be identified (da Silva Gomes et al., 1991b).
Rabbits
Groups of 20 pregnant New Zealand white rabbits were given
technical-grade cyhalothrin (purity, 89.25%; containing 92.2%
pyrethroids of which 96.8% was cyhalothrin) on days 6-18 of gestation
at a dose of 0, 3, 10, or 30 mg/kg bw per day by oral gavage in corn
oil. The animals were killed and examined on day 28 of gestation. No
certificate of compliance with GLP was supplied, but suitable quality
assurance records had been kept.
The highest dose was toxic to the dams, as indicated by an
initial body-weight loss followed by a decreased rate of body-weight
gain. No treatment-related deaths, clinical signs, or gross
pathological changes were seen in any group of does. There was no
effect on the incidence of pregnancy or on gravid uterine weight. The
mean weight of the fetuses of dams at 30 mg/kg bw per day was slightly
decreased, but the effect was not statistically significant (Wilcoxon
test). Mean fetal crown-rump length, sex ratio, and the numbers of
corpora lutea, intrauterine deaths, and implantations were unchanged
by treatment. There was no increased incidence of any type of fetal
abnormality in any treated group. The NOEL for maternal toxicity was
10 mg/kg bw per day (Killick, 1981c).
2.2.6 Special studies
(a) Neurotoxicity
Rats
Groups of rats were given single oral or intraperitoneal doses of
25-200 mg/kg bw 'cyhalothrin', referred to as 'PP 321, trade mark
Karate', which are terms associated with lambda-cyhalothrin rather
than cyhalothrin. Its purity was not reported. It was suspended as an
emulsion in 'solvents and surface active agents'. After treatment, the
behaviour of the animals in the open field was monitored, and their
electroencephalograms were measured while they were moving freely. No
further details of the protocol were reported. Treated animals were
found to have limited mobility as a consequence of discoordination of
the limbs. The reported signs of toxicity were respiratory
difficulties, tremor, salivation, piloerection, increased reactivity
to noise and during handling, with vocalization. Analysis of the
electroencephalograms showed 'changes in amplitudes (depressions in
majority of cases), in frequency composition (relative increases of
beta activity) and in repetition of cycles of high and low
amplitudes'. The results for individual groups were not presented. No
NOEL could be identified (Zufan et al., 1989).
Hens
In a study that complied with GLP, groups of 10 hens were given
cyhalothrin (purity, 93.4%) as a single dose of 2500, 5000, or 10 000
(two groups) mg/kg bw by oral gavage in corn oil. Negative controls
were given corn oil alone; positive controls received 500 mg/kg bw of
tri- ortho-cresyl phosphate. The hens were observed for 21 days after
dosing. The only hens that died during this period were two given
10 000 mg/kg bw, one in the negative and one in the positive control
group. The mean body weights of hens in all groups given cyhalothrin
and the positive controls were reduced. Clinical signs of toxicity
(ataxia) were seen only in the positive controls. The only
treatment-related gross change seen post mortem was muscular atrophy
in two of the positive control birds. Histopathological examination of
the cervical cranial, cervical caudal, thoracic, and lumbar spinal
cord and the proximal and distal sciatic nerve showed morphological
evidence of axonal damage at all levels of the spinal cord in all the
positive control birds. There were no consistent or dose-related
changes in the nerves of any of the groups given cyhalothrin. The NOEL
was 10 000 mg/kg bw, the highest dose tested (Roberts et al., 1982).
(b) Neurobehavioural effects
Inclined plane test
In a preliminary GLP-compliant study, cyhalothrin was
administered by oral gavage in corn oil to groups of Crl:CD.BR strain
rats of each sex. Initially, single doses of 50, 100, or 200 mg/kg bw
were given to groups of two males and doses of 25, 40, or 75 mg/kg bw
to groups of two females. Later, groups of three rats of each sex were
given a single dose of 15, 30, or 60 mg/kg bw. Controls received corn
oil only. The cyhalothrin contained A and B isomer pairs in a 60:40
ratio and was 95.8% pure, with a total pyrethroid content of 96.4%.
The other isomers measured were cis B' (0.2%), trans D and cis
A' (0.4%), and trans C (< 0.1%). The contaminants included
phenoxybenzalde-hyde (0.2%) and
(Z)3-(2-chloro-3,3,3-trifluoro-1-enyl)-2,2-dimethylcyclopro-pane
(0.1%). One hour before dosing and again at 30 min, 2 h, 7 h, and 24 h
after dosing, each rat was placed on a smooth plane with an angle of
inclination that was gradually increased until the rat could no longer
maintain its position. For each rat, three measurements were made at
each time. In the inclined plane test, any decrease in the angle at
which the rat loses its position (mean slip angle) is regarded as a
reflection of subtle changes in neuromuscular function.
Clinical signs of toxicity were seen at all doses tested. At 15
mg/kg bw, the only clinical sign was soft faeces in females. At 25-40
mg/kg bw, ungroomed appearance, soft faeces, and piloerection were
seen. At 50-75 mg/kg bw, there were marked clinical signs, including
ataxia, writhing, emprosthotonos, high stepping or splayed gait,
hunched posture, stained snout, salivation, and wasted appearance. At
100 mg/kg bw, the signs also included vocalization and closure of the
eyelids. Rats given 60 or 200 mg/kg bw were killed on humane grounds
after the inclined plane measurements had been made. Only one rat (a
female at 60 mg/kg bw) died during the study, but all rats given 60 or
200 mg/kg bw were killed prematurely on humane grounds. Body-weight
gains were decreased at doses > 50 mg/kg bw. Necropsy of the
animals showed no consistent gross pathological changes. The result of
the inclined plane test was uninterpretable, as the clinical signs
interfered with the conduct of the study: convulsing rats were unable
to stay on the plane, and rats with soiled fur adhered to it (Denton,
1988).
Acute startle response
A preliminary study was performed in two phases with groups of
three male Wistar-derived Alpk:ApfSD rats. The purity of the
cyhalothrin tested was 97.2%. In phase 1, rats were given a single
oral dose of 0, 25, 50, or 75 mg/kg bw of cyhalothrin in corn oil, and
auditory habituation tests were performed 1, 2, and 3 h after dosing.
Clinical signs of toxicity were seen on day 2 after dosing (the time
of dosing being the start of day 1) but not on day 1 or day 3, in the
group given 75 mg/kg bw. The signs included ataxia, subdued behaviour,
body-weight loss, and stained fur. Rats at this dose had a
statistically nonsignificant increase in mean response amplitude
(two-sided Student t test) but a significant ( p < 0.05) increase
in the time to maximum amplitude 3 h after dosing.
In phase 2, rats were given a single oral dose of 0, 75, or 100
mg/kg bw of cyhalothrin in corn oil, and auditory habituation tests
were performed on separate groups of animals 3, 5, or 7 h after
dosing. The signs of toxicity were similar to those seen in phase 1.
The clearest results in the auditory startle habituation test were
obtained at 7 h: with both doses tested, the mean response amplitude
was consistently statistically significantly ( p < 0.05) reduced and
the time to maximum amplitude was reduced (Brammer, 1998).
In the main study, groups of 10 male and 10 female Wistar-derived
Alpk:ApfSD rats were given a single dose of 0, 5, 15, or 75 mg/kg bw
of cyhalothrin (purity, 97.2%) by oral gavage in corn oil. An auditory
startle habituation test was performed 7 h after dosing on day 1 and
on day 8 with an automated recording apparatus. The animals were
killed after 8 days but were not examined post mortem. The study was
performed in accordance with GLP.
Body-weight gain was reduced in males at the highest dose and in
females at the two lower doses. Transient clinical signs of toxicity
were seen at the highest dose. Those seen at day 2 included subdued
behaviour, ataxia, high-stepping gait, salivation, and tip-toe gait,
and 'some of these signs' were also seen 7 h after dosing on day 1.
There were no signs of toxicity from day 3 until the end of the study.
In the startle response test, there was no effect on time to
maximum amplitude in either sex on day 1 or day 8. A reduction in the
mean response amplitude was seen, however, on day 1 in animals of each
sex at the highest dose. Statistically significant (two-sided Student
t test: p < 0.05), but not dose-related, reductions in mean
response amplitude were also seen on day 1 in females at all doses. No
effects were seen on day 8.
The authors reported that the NOEL for this study was 15 mg/kg
bw, as they dismissed the effects on response amplitude because of the
absence of a clear dose-response relationship. The Committee disagreed
with this conclusion. It has been reported that type II pyrethroids
generally decrease rather than increase the startle response to sound,
although this is a complex response and at low doses some type II
pyrethroids give an increased startle response (Hijzen & Slangen,
1988; Ray, 1991). Thus, although there was a NOEL for a reduced
response, lower doses may have caused an increased response, which
would indicate a lower NOEL. The Committee also noted that the startle
response was not evaluated at the most sensitive time, i.e. the time
of peak expression of acute toxicity as shown by clinical signs. No
NOEL could be identified (Brammer, 1999).
Inhibitory avoidance test
Newborn Wistar rat pups were exposed to cyhalothrin (purity
unspecified) in their mother's milk from birth until weaning at 21
days of age, and at 97, 104, and 111 days of age they were tested in
inhibitory avoidance tests. Cyhalothrin was given to nursing dams in
their drinking-water as a 200-mg/L solution in 400 mg/L sucrose. This
would be expected to provide a dose of cyhalothrin of approximately 20
mg/kg bw per day to the dams (assuming that 200-g rats drink about 20
ml/day). The intake of the pups was not measured, but the treated
groups consisted of 20 pups and the control group of 15. Control dams
were given sucrose solution at 400 mg/L. Drink was available
ad libidum to eight cyhalothrin-exposed dams and six control dams.
No clinical signs of toxicity were reported in either the dams or
the pups. The body weights at weaning and at 90 days of age were not
affected. The behaviour of the dams was unaffected by treatment
throughout the 21 days of lactation. The motor activity of the pups at
weaning was unaffected by the treatment. At 90 days, eight pups
exposed to cyhalothrin and 11 control pups were chosen (all the males)
for training and testing in a shuttle box to evaluate inhibitory
avoidance behaviour. Each rat was trained once by placing it in a
well-lit box and then opening a door to a dark compartment and giving
the rat an electric shock to the foot when it entered the dark
compartment. The test is a measure of the time taken by the rats to
cross again from the well-lit area to the dark compartment. Each rat
was tested once at 97, 104, and 111 days of age, receiving no electric
shock. At all times, there was a reduction in the learning avoidance
latency of the treated group, but the effect was statistically
significant (Mann-Whitney U-test: p < 0.05) only at 97 and 104 days
of age and not at 111 days. No NOEL could be identified (Moniz et al.,
1990).
In a separate study, described above, rats born to dams treated
dermally with an aqueous solution of cyhalothrin were tested for
behavioural changes. Inhibitory avoidance behaviour was unaffected by
the treatment, and there was no consistent effect on locomotor
frequency. The animals did, however, show a decreased motivational
response when they were tested as adults in a hole board test. No NOEL
could be identified for this study because of the production of subtle
but persistent neurobehavioural effects at the only dose tested. It
was also difficult to estimate the amount of cyhalothrin received by
the offspring (da Silva Gomes et al., 1991b).
2.2.7 Immunotoxicity
Some pyrethroids have been reported to be potent allergens that
may cause allergic rhinitis, asthma, or allergic alveolitis (Marrs,
1993). Cyhalothrin has not been specifically tested for immunotoxicity
but in a study of toxicity in mice given repeated doses, decreased
total leukocyte counts, decreased lymphocyte counts, and increased
neutrophil counts were observed in male but not female mice that had
consumed feed containing cyhalothrin for 28 days (Colley et al.,
1981). In the same study, atrophy of the red pulp of the spleen
occurred in some females given the high dose. Toxicological studies in
other species did not show alterations in total or differential
leukocyte counts and showed no histopathological changes in most
organs potentially involved in immunity (thymus, spleen, lymph nodes,
bone marrow), as described above.
A Magnusson and Kligman maximization test and a Buehler test both
showed that cyhalothrin is a skin sensitizer in guinea-pigs (European
Medicines Evaluation Agency, 1999; WHO, 1990). A Magnusson and Kligman
maximization test with lambda-cyhalothrin, however, showed no skin
sensitization in guinea-pigs (WHO, 1990).
2.3 Observations in humans
Cases of severe poisoning with pyrethroids are rare, but some
cases, for example in China, have been reported, including a few
deaths. Absorbed pyrethroids are rapidly detoxified by esterases, so
that any systemic effects would be of short duration (Marrs, 1993).
Topical application of pyrethrins or pyrethroids to the skin
produces local effects unrelated to their systemic action. The
synthetic pyrethroids have a characteristic irritating effect that is
not associated with inflammation and appears to be unique to
pyrethroids. The initial lesions are tenacious, painful pruritus
(pricking sensation) followed by a local burning sensation with
blotchy erythema that lasts about 2 days after cessation of exposure;
when exposure is intense, it is associated with numbness. Later,
desquamation may occur on the contaminated area of skin. There appear
to be no lasting ill effects. The condition is produced by all classes
of pyrethroids although most readily by type II pyrethroids such as
cyhalothrin. Repeated exposure to systemically toxic doses of
pyrethroids can produce a different effect: peripheral nerve damage
can occur subsequent to severe motor symptoms (Aldridge, 1990; Ray,
1991)
No clinical or haematological effects were observed in six
volunteers given a single dose of 5 mg of lambda-cyhalothrin in corn
oil (equal to 0.05-0.07 mg/kg bw) (European Medicines Evaluation
Agency, 1999). The route of administration was not reported, but it
seems likely to have been oral.
In the study of Pakistani pesticide workers described in section
2.2, the average exposure of the workers to lambda-cyhalothrin was
estimated to be 54 µg/person per day (extrapolated from measured
metabolites in urine). Transient signs of toxicity, lasting up to 24
h, were reported by the workers, which included skin paraesthesia,
feeling hot, feeling cold, numbness, irritation of the skin, red eyes,
coughing, and sneezing. Medical examination revealed one case of face
rash that lasted 2 days (Chester et al., 1992).
In a study carried out in a village in the United Republic of
Tanzania, a lambda-cyhalothrin-based insecticide was sprayed inside
houses and shelters at a coverage of approximately 25 mg/m2. The
insecticide was supplied as a water-dispersible powder in a soluble
sachet. Every day for 6 days, 12 spraymen and 3 squad leaders were
interviewed about symptoms. Each sprayman used up to 62 g of
lambda-cyhalothrin over 2.7-5.1 h each day. The spraymen wore personal
protective equipment (rubber boots and gloves, cotton overalls, caps,
and gauze nose-mouth masks) which left much of the face exposed. One
sprayman also used a face shield for 3 days.
All the spraymen complained at least once of symptoms related to
exposure to lambda-cyhalothrin. The commonest symptoms were itching
and burning of the face and nose and throat irritation, frequently
accompanied by sneezing or coughing. Facial symptoms occurred only on
unprotected areas, and the worker who wore a face shield was free of
facial symptoms. All the symptoms had disappeared by the morning after
the spraying. The number of subjects affected and the duration of
facial symptoms were proportional to the amount of compound sprayed.
These parameters were not affected by use of lambda-cyhalothrin in the
previous 6 months. A sample of occupants was interviewed 1 and 5-6
days after their houses had been sprayed. One woman who entered her
house 30 min after the end of spraying complained of periorbicular
itching, but this lasted only a few minutes. The other inhabitants of
sprayed houses reported no other insecticide-related adverse effects.
Furthermore, a squad leader who entered almost every house a few
minutes after spraying reported no symptoms (Moretto, 1991)
As part of a field trial conducted in South India,
electrophysiological tests were conducted on 15 spraymen aged 19-48
years, before and after exposure to lambda-cyhalothrin (formulation
and purity unspecified). The tests performed on the subjects comprised
conduction of the right median, common peroneal, and facial motor
nerves; conduction of the right median and sural sensory nerves; blink
response with stimulation of the right supra-orbital nerve and
recording of R1 and R2 responses from the right orbicularis oculi
muscle with a pair of surface electrodes; concentric needle
electromyography of the tibialis anterior; repetitive stimulation of
the right median nerve at the wrist at 3 and 20 Hz and recording of
the responses from the abductor pollicis brevis; and multi-modality
visual, brainstem, auditory and somatosensory evoked potentials. The
evoked potentials were measured in only six of the subjects, but the
other measurements were made in all 15 subjects.
Clinical observation revealed no changes, and facial nerve
conduction, blink response, responses to repetitive stimulation, and
visual, auditory, and somatosensory evoked potentials were all normal.
Six of the 15 subjects had mild changes in peripheral nerve conduction
parameters (paired t test: p < 0.05), but comparison of the mean
values for the various nerve conduction parameters before and after
exposure showed no significant difference except for prolongation of
distal motor latency of the median nerve. Studies of nerve conduction
12-16 months later in three subjects who had shown abnormalities
immediately after exposure showed normal rates. The authors concluded
that occupational exposure to lambda-cyhalothrin can produce
transient, subclinical electrophysiological changes in the nerves of
the upper limbs (Arunodaya et al., 1997).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity,
neurotoxicity, and neurobehavioural effects of cyhalothrin and
observations of effects in humans exposed to this compound. Although
some of the studies were not fully compliant with codes of GLP, all of
the pivotal studies were carried out according to appropriate
standards for study protocol and conduct.
Oral doses of cyhalothrin were readily but incompletely absorbed
in the species studied (rats and dogs), and the subsequent metabolism
was similar, involving initial cleavage of the molecule at the ester
bond, presumably resulting in detoxification. The metabolites were
rapidly excreted, some as conjugates, whereas small amounts of
unchanged cyhalothrin persisted as residues in fatty tissues. Similar
results were seen in food-producing animals.
Serum and urine from exposed workers contained the metabolites
3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-
dimethylcyclopropanecarboxylic acid, 3-phenoxybenzoic acid, and
3-(4'-hydroxyphenoxy)benzoic acid. These metabolites are products of
cleavage of the cyhalothrin molecule at the ester bond, and their
presence suggests that the initial metabolism of this compound in
humans is similar to that in the animal species that have been
investigated.
No toxicological studies on metabolites of cyhalothrin were
available, but the Committee considered it likely that the metabolites
would be less toxic than cyhalothrin, as none contains an intact
pyrethroid structure.
The acute toxicity of cyhalothrin is characterized by effects on
the nervous system, principally the central nervous system. The signs
of toxicity are typical of type II pyrethroids and comprise writhing,
salivation, exaggerated jaw opening, increasing extensor tone in the
hind legs causing a rolling gait, poor coordination progressing to
coarse tremor, tonic seizures, and apnoea. The LD50 values after
oral administration depended on the vehicle used but varied from 37 to
62 mg/kg bw in mice and from 51 to 240 mg/kg bw in rats. Higher values
were found in other species or after administration by other routes.
In mice that received a dose of 0, 5, 10, 20, 40, or 80 mg/kg bw
per day orally for 5 days, ataxia and convulsions were seen in those
given the two higher doses. The dose of 20 mg/kg bw per day caused
body-weight loss, ataxia, and rough coat. At 5 mg/kg bw per day, the
only adverse effect was a rough coat.
In a 28-day study in mice, cyhalothrin was added to the diet at a
concentration of 0, 5, 25, 100, 500, or 2000 mg/kg of feed. Atrophy of
the red pulp of the spleen and an increased mortality rate were seen
at the highest dose. Adaptive liver changes, including enlarged liver,
centrilobular hepatocellular hypertrophy, increased activity of
aminopyrine- N-demethylase, and proliferation of smooth endoplasmic
reticulum were seen at concentrations of 100 mg/kg of feed and above;
lowered lymphocyte counts were also seen at these doses. At 25 mg/kg
of feed, the only effect seen was piloerection. The NOEL was 5 mg/kg
of feed, equal to 0.65 mg/kg bw per day, on the basis of piloerection
at higher doses.
The main effects in five 10-90-day studies of toxicity in rats
were adaptive changes in the liver similar to those seen in mice. In a
28-day range-finding study in which rats were fed diets containing
cyhalothrin at a concentration of 0, 5, 10, 20, or 250 mg/kg of feed,
only the highest concentration caused adverse effects. These effects
were characterized by hepatocellular hypertrophy, increased activity
of aminopyrine- N-demethylase in the liver, and proliferation of
hepatic smooth endoplasmic reticulum. The NOEL was 20 mg/kg of feed,
equivalent to 2 mg/kg bw per day. In a 28-day study in rats given 0,
1, 5, 10, 20, or 250 mg/kg of feed, the activity of hepatic
aminopyrine- N-demethylase was increased and proliferation of hepatic
smooth endoplasmic reticulum was seen at the highest dose only. No
such effect was seen in the liver at doses equivalent to 2 mg/kg bw
per day or less. Females given 10 mg/kg of feed or more had decreased
body-weight gain. The NOEL was 5 mg/kg of feed, equivalent to 0.5
mg/kg bw per day, but this observation was not in line with the
findings of other short-term studies of toxicity in rats. A third
28-day study in rats was inadequately reported but showed that
administration of cyhalothrin at 20 mg/kg of feed (equivalent to 2
mg/kg bw per day) caused proliferation of hepatic smooth endoplasmic
reticulum; a NOEL was not identified in this study.
A 90-day study was performed in which rats given cyhalothrin at
0, 10, 50, or 250 mg/kg of feed. Males given the two higher doses
showed increased activity of hepatic aminopyrine- N-demethylase and
proliferation of hepatic smooth endoplasmic reticulum. Females at the
highest dose showed increased activity of hepatic
aminopyrine- N-demethylase activity. The NOEL was 10 mg/kg of feed,
equal to 0.56 mg/kg bw per day.
Groups of dogs received cyhalothrin at a dose of 0, 2.5, 10, or
30 mg/kg bw per day for 4 weeks or a dose of 0, 1.0, 2.5, or 10 mg/kg
bw per day for 26 weeks. In the 4-week study, the two higher doses
caused an increased incidence of vomiting, and the highest dose caused
body-weight loss, unsteady gait, and increased serum alanine and
aspartate aminotransferase activities. Muscular trembling was seen in
dogs in all groups, including the controls, in the 4-week study. In
the 26-week study, doses of 10 mg/kg bw per day or more caused
clinical signs including vomiting, salivation, lack of coordination,
unsteadiness, collapse, muscular spasms, and convulsions. As treatment
at all doses in both studies increased the prevalence of liquid
faeces, a NOEL was not identified. The Committee considered it
possible that the liquid faeces were a consequence of the neurological
effects of cyhalothrin. The LOEL was 1.0 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in mice,
cyhalothrin was given in the diet at a concentration of 0, 20, 100, or
500 mg/kg of feed for 104 weeks. An increased incidence of mammary
adenocarcinoma was seen in females at the two higher doses. The
highest incidence (14%) was only slightly greater then the upper limit
of the range in historical controls (2-12%). The Committee could not
exclude the possibility that the adenocarcinomas seen in the groups
given 100 or 500 mg/kg of feed were caused by cyhalothrin. Clinical
signs of toxicity (piloerection and hunched posture) and increased
serum activities of aspartate and alanine aminotransferases were seen
at these doses. The NOEL for these effects was 20 mg/kg of feed, equal
to 1.9 mg/kg bw per day.
In a long-term study of toxicity and carcinogenicity in rats,
cyhalothrin was given in the diet at a concentration of 0, 10, 50, or
250 mg/kg of feed for 104 weeks. There was no treatment-related
increase in the incidence of any type of tumour. Adverse effects found
at the highest dose included decreased body weight, altered blood
biochemistry, and an increased weight of the liver relative to that of
the body in animals of each sex and of the adrenals in females only.
The NOEL was 50 mg/kg of feed, equal to 2.3 mg/kg bw per day.
Cyhalothrin was not genotoxic in a range of studies, including a
test for reverse mutation in Salmonella, a test for cytogenetic
effects in the bone marrow of rats treated in vivo, a test for
dominant lethal mutation in mice, and an assay of cell transformation
in vitro. The Committee concluded that cyhalothrin is not genotoxic.
Furthermore, the Committee considered it likely that the mammary
adenocarcinomas found in mice in the long-term study were due to a
non-genotoxic mechanism.
A three-generation study of reproductive toxicity was performed
in rats given cyhalothrin in the diet at a concentration of 0, 10, 30,
or 100 mg/kg of feed. Adverse effects, including reduced parental
body-weight gain and reduced litter size, were found only at the
highest dose. The NOEL for these effects was 30 mg/kg of feed, equal
to 1.7 mg/kg bw per day.
In a study of developmental toxicity, rats received a dose of 0,
5, 10, or 15 mg/kg bw per day by oral gavage on days 6-15 of
gestation. Maternal toxicity, characterized by body-weight loss and
poor coordination, was seen at the highest dose. Embryotoxicity also
occurred at this dose. Abnormalities were seen in 5 of 17 fetuses in
one litter from a dam at 10 mg/kg bw per day, but the effect was
considered not to be due to treatment with cyhalothrin as no
fetotoxicity was seen at the higher dose. The NOEL for maternal
toxicity was 10 mg/kg bw per day, and that for developmental toxicity
was 15 mg/kg bw per day, the highest dose tested.
Two studies of developmental toxicity in rats treated by dermal
administration provided some evidence that cyhalothrin can delay fetal
development at doses lower than those given orally. However, the
Committee considered that oral administration is a more relevant route
and that the NOEL in the study in which this route was used was the
appropriate one for evaluating developmental toxicity in rats.
Rabbits studied for developmental toxicity were given a dose of
0, 3, 10, or 30 mg/kg bw per day by oral gavage. The highest dose
caused initial body-weight loss in the does, which was followed by
reduced body-weight gain. There was no significant effect on
development at any dose. The NOEL for maternal toxicity was 10 mg/kg
bw per day, and the NOEL for developmental toxicity was 30 mg/kg bw
per day, the highest dose tested.
Single oral doses of up to 10 000 mg/kg bw did not induce
clinical or histopathological signs of neurotoxicity in hens.
Various studies of neurobehavioural effects have been performed
in rats, including a range-finding test of performance on an inclined
plane, a test for acute auditory startle response, and tests of
inhibitory avoidance. In the inclined plane test, rats were given a
single oral dose of 0, 15, 25, 30, 40, 50, 60, 75, 100, or 200 mg/kg
bw. All of these doses caused soft faeces in at least some of the
rats, and doses of 25 mg/kg bw or more caused clinical signs of
neurotoxicity. The results were, however, variable, and no conclusion
could be reached about neurotoxicity.
In the test for acute auditory startle response in which an oral
dose of 0, 5, 15, or 75 mg/kg bw was given, the highest dose resulted
in reduced body-weight gain, transient clinical signs, and a reduced
mean response amplitude in animals of each sex. Statistically
significant but not dose-related reductions in mean response amplitude
were also seen at 5 and 15 mg/kg bw in females only. The Committee was
aware that a biphasic auditory startle response has been reported with
some other type II pyrethroids, with a reduced response at high doses
and an increased response at lower doses. A NOEL for the startle
response was not identified in this study.
In the inhibitory avoidance tests, rats were exposed either
in utero (dams were given 200 mg/l in drinking-water) or during
lactation (dams were given 1 ml of a 0.018% solution dermally). The
doses received by the animals could not be estimated reliably. The
rats exposed in utero showed a decreased motivational response when
tested as adults, but no effects were seen on inhibitory avoidance
behaviour or locomotor frequency. Animals exposed during lactation had
a shorter latency in learning avoidance behaviour. There was no NOEL
in either study, as adverse effects were seen at the only dose tested
in each study and the doses received by the animals were not known.
4. EVALUATION
Observations and case reports in humans provided little
information relevant to the establishment of an ADI for cyhalothrin.
In most instances, no information on doses was available, the route of
exposure was dermal, and some studies were of exposure to
lambda-cyhalothrin rather than cyhalothrin.
The Committee established a temporary ADI of 0-0.002 mg/kg bw for
cyhalothrin by applying a 500-fold safety factor to the LOEL of 1.0
mg/kg bw per day for induction of liquid faeces in dogs in the 26-week
study. The Committee considered it possible that the liquid faeces
were a consequence of the neurological effects of cyhalothrin. The
Committee applied the 500-fold safety factor to account for the
absence of a NOEL for liquid faeces in dogs and because of the absence
of a NOEL for neurobehavioural effects. The ADI was made temporary
because cyhalothrin belongs to a class of substances that are
characterized by their toxicity to the central nervous system, and
therefore neurobehavioural effects may be the most sensitive indicator
of the toxicity of this compound. There was an adequate margin of
safety between the ADI and the NOELs identified in the studies on
cyhalothrin, including the NOEL of 0.65 mg/kg bw per day for
piloerection in the 28-day study in mice.
The results of studies appropriate for establishing a NOEL for
neurobehavioural effects in laboratory animals are required for
evaluation in 2002.
The Committee noted that the NOEL for toxicological effects other
than the neurotoxicity related to the pyrethroid structure was 2.3
mg/kg bw per day for adaptive changes in the livers of rats in the
long-term study. This suggests that the toxicity of the metabolites
(none of which has a pyrethroid structure) is no greater than 50% of
that of the parent drug.
5. REFERENCES
Aldridge, W.N. (1990) An assessment of the toxicological properties of
pyrethroids and their neurotoxicity. Crit. Rev. Toxicol., 21,
89-103.
Anderson, D., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B. &
Godley, M.J. (1983) Cyhalothrin: A cytogenetic study in the rat.
Unpublished study, report No. CTL/P/664, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Arunodaya, G.R., Kumar, K.R. & Swamy, H.S. (1997) Electrophysiologic
study of spraymen exposed to insecticide lambdacyhalothrin.
Neurol. India, 45, 189-192.
Barnes, J.M. & Verschoyle, R.D. (1974) Toxicity of new pyrethroid
insecticides. Nature, 248, 711.
Bicknell, C.E., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Milk
residues of cyhalothrin following a 10 mL application of a 2%
pour-on. Unpublished study, report No. CIBH 89-1, Coopers Animal
Health, Berkhamsted, United Kingdom. Submitted by Schering-Plough
Animal Health, Harefield, United Kingdom.
Bloomquist, J.R., Adams, P.M. & Soderlund, D.M. (1986) Inhibition of
gamma-amino butyric acid-stimulated chloride flux in mouse brain
vesicles by polycycloalkane and pyrethroid insecticides.
Neurotoxicology, 7, 11-20.
Brammer, A. (1998) Cyhalothrin: Preliminary acute startle response
study in rats. Unpublished study, report No. CTL/L/8516, Zeneca
Central Toxicology Laboratory, Macclesfield, United Kingdom.
Brammer, A. (1999) Cyhalothrin: Acute startle response study in rats.
Unpublished study, report No. CTL/P/6162, Zeneca Central
Toxicology Laboratory, Macclesfield, Cheshire, United Kingdom.
Campana, M.A., Panzeri, A.M., Moreno, V.J. & Dulout, F.N. (1999)
Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using
the micronucleus test in erythrocytes of the fish Cheirodon
interruptus interruptus. Mutat. Res., 438, 155-161.
Chester, G., Sabapathy, N.N. & Woollon, B.H. (1992) Exposure and
health assessment during application of lambda-cyhalothrin for
malaria vector control in Pakistan. Bull. World Health Organ.,
70, 615-619.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
Gopinath, C. & Prentice, D. (1981) Cyhalothrin. Oral toxicity
study in beagle dogs (Final report: repeated daily dosing for 26
weeks). Unpublished study, report No. ICI 326/8162, Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, United Kingdom.
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Prentice, D.,
Buckley, P. & Offer, J. (1983) PP 563. Preliminary oral toxicity
study in beagle dogs. Unpublished study, report No. ICI 325/8074,
Huntingdon Research Centre plc, Huntingdon, Cambridgeshire,
United Kingdom.
Coats, J.R. (1990) Mechanisms of toxic action and structure
relationships for organochloride and synthetic pyrethroid
insecticides. Environ. Health Perspectives, 87, 255-262.
Colley, J.C., Loane, J.A., Heywood, R., Gibson, W.A., Chasseaud, L.F.,
Edmundson, N., Lewis, D. & Woodhouse, R.N. (1980) PP563. 28-day
dose range finding study in rats. Unpublished study, report No.
ICI/321/8041, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Down, W.H., Woodhouse, R.N.,
Gibson, W.A., Gopinath, C., Zubaidy, A.J. & Prentice, D.E. (1981)
Cyhalothrin. 4-week dose range finding study on mice. Unpublished
study, report No. ICI/379/80910, Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom.
Colley, J.C., Dawe, S., Heywood, R., Almond, R., Gibson, W.A.,
Gregson, R. & Gopinath, C. (1984) Cyhalothrin. Potential
tumorigenic and toxic effects in prolonged dietary administration
to mice (Final report). Unpublished study, report No.
ICI/395/83668, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Denton, S. (1988) Cyhalothrin: Preliminary neurotoxicity study in the
rat following a single oral dose. Unpublished study, report No.
S8104-001R, Covance, Harrogate, United Kingdom.
Deshmukh, P.B. (1992) Three-generation reproductive studies of a
synthetic pyrethroid, cyhalothrin. Toxicol. Lett., 64/65,
770-781.
Dow, P.R. & Parker, R.C. (1989) Attempted identification of 3 major
liver metabolites following dermal application of C-14 alcohol
lambda-cyhalothrin to lactating cows. Unpublished study, report
No. CIBH 89-2, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Dow, P.R., Knight, P.J., Parker, R.C. & Woollon, R.M. (1989)
Metabolism of C-14 alcohol labelled lambda-cyhalothrin in
lactating cows following dermal application. Unpublished study,
report No. CIBH 88-4, Coopers Animal Health, Berkhamsted, United
Kingdom. Submitted to WHO by Schering-Plough Animal Health,
Harefield, United Kingdom.
Ebino, K., Suzuki, H. & Harada, T. (1984a) PP-563: Acute oral toxicity
in rats. Unpublished study, report No. 4/HD/006931, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984b) PP-563 WP: Acute oral
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984c) PP-563: Acute
intraperitoneal toxicity in rats. Unpublished study, Institute of
Environmental Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984d) PP-563: Acute dermal
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
Ebino, K., Suzuki, H. & Harada, T. (1984e) PP-563: Acute subcutaneous
toxicity in rats. Unpublished study, Institute of Environmental
Toxicology, Tokyo, Japan.
European Medicines Evaluation Agency (1999) Cyhalothrin. Summary
report of the Committee for Veterinary Medicinal Products,
EMEA/MRL/699/99. Internet:
http://web.is.eudra.org/vetdocs/vets/mrl.htm.
Gassner, B., Wuthrich, A., Scholtysik, G. & Solioz, M. (1997) The
pyrethroids permethrin and cyhalothrin are potent inhibitors of
the mitochondrial complex I. J. Pharmacol. Exp. Ther., 281,
855-860.
Harrison, M.P. (1981) Cyhalothrin: The disposition and metabolism of
14C-ICI 146,814 in rats. Unpublished study, report No. 146814
KMR 002/01, Imperial Chemical Industries plc, Macclesfield,
Cheshire, United Kingdom.
Harrison, M.P. (1983) Cyhalothrin:The metabolism and disposition of
14C-ICI 146,814 in the rat; Part IV: Isolation and
identification of the major urinary metabolites derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 following oral
administration. Unpublished study, report No. YIBH 85-C9,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984a) Cyhalothrin: The metabolism and disposition of
14C-ICI 146,814 in rats; Part II, tissue residues derived from
(14C-benzyl) or (14C-cyclopropyl)-ICI 146,814 after a single
oral dose of 1 or 25 mg/kg bw. Unpublished study, report No. KMR
002/02, Imperial Chemical Industries plc, Macclesfield, Cheshire,
United Kingdom.
Harrison, M.P. (1984b) Cyhalothrin (ICI 146,814): The metabolism and
disposition of ICI 146,814 in rats; Part III, Studies to
determine radioactive residues in rats following 14 days repeated
administration. Unpublished study, report No. KMR 002/03,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Harrison, M.P. (1984c) Cyhalothrin (ICI 146,814): The disposition and
metabolism of ICI 146,814 in the dog. Unpublished study, report
No. 146814KMD 005, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Harrison, M.P. (1985) Cyhalothrin (ICI 146,814): The metabolism,
excretion and residues in the cow after 7 days oral
administration with two [14C]-labelled forms of ICI 146,814 at
1 mg/kg/day. Unpublished study, report No. YIBH 85-C11, Imperial
Chemical Industries plc, Macclesfield, Cheshire, United Kingdom.
Hijzen, T.H. & Slangen, J.L. (1988) Effects of type I and type II
pyrethroids on the startle response in rats. Toxicol. Lett.,
40, 141-152.
Irvine, L.F.H. (1981a) Cyhalothrin: Oral (gavage) range finding study
in the male mouse. Unpublished study, report No. 2647-72/212,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Irvine, L.F.H. (1981b) Cyhalothrin: Oral (gavage) dominant lethal
study in the male mouse. Unpublished study, report No.
2647-72/213, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
JMPR (Joint FAO/WHO Meeting on Pesticide Residues) (1985) Pesticide
residues in food - 1984. Report of the Joint Meeting on
Pesticide Residues. FAO Plant Production and Protection Paper
62, Rome: Food and Agriculture Organization of the United
Nations.
Jones, J.R. (1980) Cyhalothrin: Acute oral median lethal dose (LD50)
in the female rabbit. Unpublished study, report No. 2364-72/214,
Hazleton Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1980) Cyhalothrin: Oral (gavage) maximum tolerated dose
study in the non-pregnant rat. Unpublished study, report No.
2537-72/206, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981a) Cyhalothrin: Oral (gavage) dose ranging study in
the pregnant New Zealand white rabbit. Unpublished study, report
No. 2603-72/210, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Killick, M.E. (1981b) Cyhalothrin: Oral (gavage) teratology study in
the rat. Unpublished study, report No. 2661-72, Hazleton
Laboratories Europe, Harrogate, United Kingdom.
Killick, M.E. (1981c) Cyhalothrin: Oral (gavage) teratology study in
the New Zealand white rabbit. Unpublished study, report No.
270072/211, Hazleton Laboratories Europe, Harrogate, United
Kingdom.
Knight, P.J., Parker, R.C. & Woollon, R.M. (1989) Metabolism of C-14
alcohol labelled lambdacyhalothrin in lactating cows following
dermal application. Unpublished study, report No. CIBH 88-2,
Coopers Animal Health, Berkhamsted, United Kingdom. Submitted by
Schering-Plough Animal Health, Harefield, United Kingdom.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationships. Pest. Biochem.
Physiol., 18, 9-14.
Leahey, J.P., French, D.A. & Heath, J. (1985) PP321: Metabolism in a
goat. Unpublished study, report No. RJ 0435B, Imperial Chemical
Industries, Plant Protection Division, Jealott's Hill Research
Station, Bracknell, Berkshire, United Kingdom.
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
I., Robinson, M. & Stonard, M. (1981) Cyhalothrin: 90-day feeding
study in rats. Unpublished report No. CTL/P/629, Imperial
Chemical Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Marrs, T.C. (1993) Toxicology of pesticides. In: Ballantyne, B.,
Marrs, T.C. & Turner, P., eds, General and Applied Toxicology,
Basingstoke:MacMillan, Vol. 2, Ch. 62, p. 1334.
Milburn, G.M., Banham, P., Godley, M.J., Pigott, G. & Robinson, M.
(1984) Cyhalothrin: Three-generation reproduction study in the
rat. Unpublished study, report No. CTL/P/906, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Moniz, A.C., Bernadi, M.M., Souza-Spinosa, H.S. & Palermo-Neto, J.
(1990) Effects of exposure to a pyrethroid insecticide during
lactation on the behaviour of infant and adult rats. Braz. J.
Med. Biol. Res., 23, 45-48.
Moretto, A. (1991) Indoor spraying with the pyrethroid insecticide
lambda-cyhalothrin: Effects on spraymen and inhabitants of
sprayed houses. Bull. World Health Organ., 69, 591-594.
Moyes, A., Godley, M.J., Hall, M., Pratt, I., Stonard, M.D. & Tinston,
D.J. (1984) Cyhalothrin: 28-day feeding study in the rat (second
study). Unpublished report No. CTL/P/1013, Imperial Chemical
Industries Ltd, Macclesfield, Cheshire, United Kingdom.
Narahashi, T. (1985) Nerve membrane ionic channels as the primary
target of pyrethroids. Neurotoxicology, 2, 3-22.
Nixon, J. & Jackson, S.J. (1981) Cyhalothrin: Acute toxicity.
Unpublished study, report No. CTL/T/555, Imperial Chemical
Industries plc, Macclesfield, Cheshire, United Kingdom.
Pigott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
Robinson, M., Taylor, K. & Tinston, D.J. (1984) Cyhalothrin. Two
year feeding study in rats. Unpublished report, report No.
CTL/1023, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Prentice, D.E., Edmondson, N.A. & Lewis, D.J. (1981) 28 day dose range
finding study of cyhalothrin in dietary administration to mice.
Electron microscopy of livers. Unpublished report No.
5/HA/003712, Imperial Chemical Industries Ltd, Central Toxicology
Laboratories Ltd, Macclesfield, Cheshire, United Kingdom.
Pritchard, V.K. (1984) Cyhalothrin: Acute oral toxicity. Unpublished
study, report No. CTL/P/1023, Imperial Chemical Industries plc,
Macclesfield, Cheshire, United Kingdom.
Ray, D. (1991) Chapter 13. In: Hayes, W.J., Jr & Laws, E.R., Jr, eds,
Handbook of Pesticide Toxicology, London: Academic Press, Vol.
2, pp. 585-636.
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. (1981) Cell
transformation test for potential carcinogenicity of
Y00102/010/005 (cyhalothrin (PP563)). Unpublished study, report
Number CTL/1023, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wright, D.G.D.
(1982) The acute oral toxicity (LD50) and neurotoxic effects of
cyhalothrin to the domestic hen. Unpublished study, report Number
ICI/374 NT/81742, Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991a)
Effects of prenatal pyrethroid exposure on the sexual development
of rats. Vet. Hum. Toxicol., 33, 427-428.
da Silva Gomes, M., Bernadi, M.M. & de Souza Spinosa, H. (1991b)
Pyrethroid insecticides and pregnancy: Effect on physical and
behavioural development in rats. Vet. Hum. Toxicol., 33,
315-317.
Tomlin, C., ed. (1994) The Pesticide Manual, 10th Ed., Cambridge:
Crop Protection Publications / Royal Society of Chemistry.
Trueman, R.W. (1981) Cyhalothrin: Results from the Salmonella
reverse mutation assay. Unpublished study, report No. CTL/P/665,
Imperial Chemical Industries plc, Macclesfield, Cheshire, United
Kingdom.
Vijverberg, H.P.M. & van den Bercken, J. (1990) Neurotoxicological
effects and mode of action of pyrethroid insecticides. Crit.
Rev. Toxicol., 21, 105-126.
Vijverberg, H.P.M., van den Zalm, J.M. & van den Bercken, J. (1982)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature, 295, 601-603.
WHO (1990) Cyhalothrin. Environmental Health Criteria 99, Geneva:
World Health Organization.
Woodward, K. (2000) Personal communication from Kevin Woodward of
Schering-Plough Animal Health, Uxbridge, United Kingdom, to Mr
Derek Renshaw of the Department of Health. E-mail message dated
14 January 2000.
Zufan, L., Kubat, J., Formanek, J., Fuchs, A., Tobiskova, G., Zajicek,
P., Vodickova, J., Rehak, P. & Dvorak, J. (1989) Behavioural and
EEG changes induced by the pyrethroid insecticide cyhalothrin.
Int. J. Psychophysiol., 7, 447-448.
