
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
CYHALOTHRIN
IDENTITY
Common Name
Cyhalothrin
Chemical Names
(RS)-alpha-cyano-3-phenoxybenzyl Z-(1RS,3RS)-3-(2-chloro-
3,3,3-trifluoropropenyl)-2,2-dimethylcyclopropanecarboxylate
(IUPAC)
cyano-(3-phenoxyphenyl)methyl (Z)-cis-3-(2-chloro-3,3,3-
trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylate (CAS)
Cyhalothrin is a mixture in equal amounts of the four
Z-cis-isomers, which exist in two enantiomeric pairs:
(R)-alpha-cyano (R)-cis-Z-cyclopropanecarboxylate and
(S)-alpha-cyano (S)-cis-Z-cyclopropanecarboxylate, coded
R157836
(S)-alpha-cyano (R)-cis-Z-cyclopropanecarboxylate and
(R)-alpha-cyano (S)-cis-Z-cyclopropanecarboxylate, coded
R119321
Synonyms
PP563, ICI 146,814, 'GRENADE', 'LIBREKTO'
Empirical Formula
C23H19ClF3NO3
Structural Formula
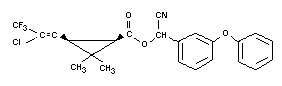 Other Information on Identity and Properties
Molecular weight 449.9
Technical material Contains at least 90 percent cyhalothrin (the
four Z-cis-isomers). Small amounts of
E-cis and Z/E-trans isomers are
present.
Physical form Technical material is a viscous, odourless,
yellow-brown liquid.
Vapour pressure 1 × 10-9 kPa at 20°C
Density 1.25 g/cm3 at 25°C
Solubility Insoluble in water; soluble in a range of
organic solvents, e.g. aliphatic and aromatic
hydrocarbons, ethers, ketones, esters and
alcohols
Formulations 5, 10 and 20 percent emulsifiable
available concentrates (E.C.); 5 percent wettable
commercially powder (W.P.)
TOXICOLOGY
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The absorption, distribution and excretion of Cyhalothrin was
studied in Alderley Park Wistar SPF albino rats. Two different
14C-labelled cis preparations, 14C-cyano-3-phenoxybenzyl-(14CHCN)
and 14C-cyclopropylcyhalothrin, each 99 percent radiochemically pure,
were administered orally (see Figure 1).
Other Information on Identity and Properties
Molecular weight 449.9
Technical material Contains at least 90 percent cyhalothrin (the
four Z-cis-isomers). Small amounts of
E-cis and Z/E-trans isomers are
present.
Physical form Technical material is a viscous, odourless,
yellow-brown liquid.
Vapour pressure 1 × 10-9 kPa at 20°C
Density 1.25 g/cm3 at 25°C
Solubility Insoluble in water; soluble in a range of
organic solvents, e.g. aliphatic and aromatic
hydrocarbons, ethers, ketones, esters and
alcohols
Formulations 5, 10 and 20 percent emulsifiable
available concentrates (E.C.); 5 percent wettable
commercially powder (W.P.)
TOXICOLOGY
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The absorption, distribution and excretion of Cyhalothrin was
studied in Alderley Park Wistar SPF albino rats. Two different
14C-labelled cis preparations, 14C-cyano-3-phenoxybenzyl-(14CHCN)
and 14C-cyclopropylcyhalothrin, each 99 percent radiochemically pure,
were administered orally (see Figure 1).
 Groups of six male and six female rats received a single dose of
1 mg/kg or 25 mg/kg of the test compound in maize oil and the
following parameters were determined: Cyhalothrin concentration in
whole blood (determined by GLC); total radioactivity in whole blood,
plasma, urine and faeces. Groups of four male and four female rats
with exteriorized bile duct cannulae were similarly dosed to enable
determination of total radioactivity excreted in bile. The expired air
from two male and two female rats of each group was monitored for
14CO2 for the first 48 h.
Absorption of radioactivity was variable but accounted for about
half of the administered dose in each case. Absorbed radioactivity was
fairly rapidly eliminated in urine and faeces. Twenty four hours after
administration, the faeces of rats dosed with 1 mg/kg Cyhalothrin
contained significant amounts (30-70 percent) of administered
radioactivity, as did the urine (2-38 percent). After seven days,
total excretion was high (85-104 percent). At 25 mg/kg, the pattern of
elimination of radioactivity was similar: after 24 h the faeces
contained significant radioactivity (6-58 percent) as did the urine
(10-27 percent). After seven days, total elimination was again high
(faeces (35-64 percent); urine, including cage washings (30-51
percent). 14CO2 was not detected in expired air.
After seven days, the rat carcases contained about 2-3 percent of
the radioactivity administered at either dose, which was present
mainly in adipose tissue, and was higher at 25 mg/kg than at 1 mg/kg.
In order to study the relative proportions excreted in the urine
and the faeces, 14C-CHCN-labelled Cyhalothrin was administered
subcutaneously (1 mg/kg) to five male and six female rats. After 24 h,
faeces contained only a relatively small amount of administered
radioactivity (0.2-3.2 percent) and the urine somewhat more (0.5-8.5
percent). After seven days, faecal elimination (1-10.6 percent) was
significantly less than urinary excretion (0.7-32.3 percent),
including cage washings. Total elimination was less than with oral
administration.
Biliary excretion of orally administered 14C-CHCN-Cyhalothrin
was studied in rats with externalized biliary duct cannulae. At
1 mg/kg, biliary excretion of radioactivity after 12 h was low
(0.8-1.85 percent). Total biliary excretion after four days was
significant (4.2-12.9 percent).
Studies with groups of six male and six female rats showed that
after a single oral dose of 25 mg/kg, the blood concentration of
14C-CHCN-Cyhalothrin peaked at 5.9-6.2 µg/ml about seven h after oral
administration. After 48 h, the blood concentration had fallen to
0.5-0.8 µg/ml. At 1 mg/kg, the corresponding peak concentration was
0.5-8.7 µg/ml, falling to 0.006-0.12 µg/ml at 48 h. Subcutaneous
administration of 1 mg/kg produced a peak blood concentration of
0.009-0.033 µg/ml at 12-24 h, which fell to 0.006-0.012 µg/ml after
48 h.
Comparison of gas chromatographic analysis of blood of rats,
analysed two h after oral dosing with cyhalothrin at 25 mg/kg, showed
that unmetabolized cyhalothrin accounted for only about 4.6-5.5
percent of total radioactivity.
Additional studies were conducted with the alternatively labelled
14C-cyclopropyl Cyhalothrin in groups of six male and six female
rats. When orally administered at 1 and 25 mg/kg, most radioactivity
was excreted in urine and faeces after 24 h (urine: 0.3-10.7 percent);
(faeces: 36-61 percent) and after seven days (urine, including cage
washings: 13-32 percent); (faeces: 2-68 percent). The whole blood
concentration of total radioactivity peaked (0.30-0.35 ug/ml) 4 h
after oral administration of a single dose of 1 mg/kg, the half life
being about 10-12 h. Carcases contained low residual levels
(1-3 percent) of radioactivity after seven days. 14CO2 was not
detected in expired air (Harrison & Case, 1981).
The bio-accumulation of Cyhalothrin in the rat was studied
further. A solution of 14C-cyclopropyl cyhalothrin in maize oil was
administered orally to 20 groups of three male Alpk/AP rats. Dosing
volumes were adjusted for body weight so that daily doses of 1 mg/kg
were given for up to four months. A group of 20 control rats received
similar volumes of the maize oil vehicle.
Groups of three test and one control animal were sacrificed 24 h
after receiving the last dose. Some animals were retained after dosing
was complete to permit estimation of the dissipation of Cyhalothrin
residues from the tissues.
Blood levels of radioactivity rose slowly over the duration of
the dosing period to a plateau at about 0.2 µg Cyhalothrin
equivalents/ml after 77 daily doses. Blood radioactivity had
dissipated 36 days after cessation of exposure. Levels of
radioactivity in fat rose progressively to about 9 ug equivalents/g at
100 days. On cessation of exposure, the level of radioactivity in
adipose tissue declined slowly with apparent first order kinetics with
a half-life of about 30 days. The levels of radioactivity in kidney
and liver plateaued at about 1.5 and 2.5 µg equivalents Cyhalothrin/g,
respectively, after about 80 days. These levels fell rapidly on
cessation of exposure but levels of radioactivity in the liver
remained detectable throughout the study, appearing to parallel the
rate of dissipation from the adipose tissue. Analysis of the fat
showed the presence of Cyhalothrin and that the ratio of the
Cyhalothrin enantiomers was essentially unchanged from that of the
material given initially (Prout, 1984).
Biotransformation
Chromatographic analysis of radioactive material excreted by rats
treated with 14C-CHCN Cyhalothrin at 25 mg/kg showed that extensive
metabolism to polar metabolites occurred. Unmetabolized Cyhalothrin
was not excreted in the urine, which contained at least two
metabolites unaffected by treatment with ß-glucuronidase or aryl
sulphatase. The bile contained at least three metabolites, which
differed from those in the urine and faeces. The faeces contained
unchanged Cyhalothrin (approximately 80 percent) plus small amounts of
three polar metabolites.
After dosing with 14C-cyclopropyl Cyhalothrin at 25 mg/kg,
unmetabolized Cyhalothrin was not detected in the urine, which
contained at least three metabolites with the 14C-cyclopropyl moeity
(Harrison & Case, 1981).
Further studies were undertaken to characterize the major urinary
metabolites of cyhalothrin in rats. Six male and six females received
the 14C-CHCN compound orally for a period of eight days at
12.5 mg/kg. Urine and faeces were collected daily up to three days
after the last dose. The urine contained about 64 percent of the
administered radioactivity.
Similarly, pooled urine samples from rats that had received 14
daily doses of 14C-cyclopropyl Cyhalothrin at 1 mg/kg contained about
50 percent of the total administered radioactivity.
The above samples were subject to thin layer chromatography and
high performance liquid chromatography and the metabolites identified
by mass spectrometry and nuclear magnetic resonance spectroscopy,
after appropriate hydrolysis with aryl sulphatase and ß-glucuronidase.
The identity of these metabolites is given in Figure 2 (Harrison &
Case, 1983).
TOXICOLOGICAL STUDIES
Special Studies on Cell Transformation
A cell transformation test, using BHK clone 13 Hamster fibroblast
cells, failed to show a carcinogenic potential for Cyhalothrin, either
with or without metabolic activation (see Table 1. See also under
Long-Term Studies).
Special Studies on Mutagenicity
Cyhalothrin was without mutagenic activity in a number of
mutagenicity assays (see Table 2).
Special Study on Reproduction
In a three-generation reproduction study, groups of 30 male and
30 female Alpk/AP (Wistar-derived) weanling rats received technical
Cyhalothrin (89.2 percent pure) in the diet at concentrations of 0,
10, 30 or 100 ppm for 12 weeks before mating and then continuously. A
similar dosing regime was maintained throughout two successive
generations resulting from the matings of the F1 parents selected
from the F1b generation and F2 parents selected from the F2b
generation.
Groups of six male and six female rats received a single dose of
1 mg/kg or 25 mg/kg of the test compound in maize oil and the
following parameters were determined: Cyhalothrin concentration in
whole blood (determined by GLC); total radioactivity in whole blood,
plasma, urine and faeces. Groups of four male and four female rats
with exteriorized bile duct cannulae were similarly dosed to enable
determination of total radioactivity excreted in bile. The expired air
from two male and two female rats of each group was monitored for
14CO2 for the first 48 h.
Absorption of radioactivity was variable but accounted for about
half of the administered dose in each case. Absorbed radioactivity was
fairly rapidly eliminated in urine and faeces. Twenty four hours after
administration, the faeces of rats dosed with 1 mg/kg Cyhalothrin
contained significant amounts (30-70 percent) of administered
radioactivity, as did the urine (2-38 percent). After seven days,
total excretion was high (85-104 percent). At 25 mg/kg, the pattern of
elimination of radioactivity was similar: after 24 h the faeces
contained significant radioactivity (6-58 percent) as did the urine
(10-27 percent). After seven days, total elimination was again high
(faeces (35-64 percent); urine, including cage washings (30-51
percent). 14CO2 was not detected in expired air.
After seven days, the rat carcases contained about 2-3 percent of
the radioactivity administered at either dose, which was present
mainly in adipose tissue, and was higher at 25 mg/kg than at 1 mg/kg.
In order to study the relative proportions excreted in the urine
and the faeces, 14C-CHCN-labelled Cyhalothrin was administered
subcutaneously (1 mg/kg) to five male and six female rats. After 24 h,
faeces contained only a relatively small amount of administered
radioactivity (0.2-3.2 percent) and the urine somewhat more (0.5-8.5
percent). After seven days, faecal elimination (1-10.6 percent) was
significantly less than urinary excretion (0.7-32.3 percent),
including cage washings. Total elimination was less than with oral
administration.
Biliary excretion of orally administered 14C-CHCN-Cyhalothrin
was studied in rats with externalized biliary duct cannulae. At
1 mg/kg, biliary excretion of radioactivity after 12 h was low
(0.8-1.85 percent). Total biliary excretion after four days was
significant (4.2-12.9 percent).
Studies with groups of six male and six female rats showed that
after a single oral dose of 25 mg/kg, the blood concentration of
14C-CHCN-Cyhalothrin peaked at 5.9-6.2 µg/ml about seven h after oral
administration. After 48 h, the blood concentration had fallen to
0.5-0.8 µg/ml. At 1 mg/kg, the corresponding peak concentration was
0.5-8.7 µg/ml, falling to 0.006-0.12 µg/ml at 48 h. Subcutaneous
administration of 1 mg/kg produced a peak blood concentration of
0.009-0.033 µg/ml at 12-24 h, which fell to 0.006-0.012 µg/ml after
48 h.
Comparison of gas chromatographic analysis of blood of rats,
analysed two h after oral dosing with cyhalothrin at 25 mg/kg, showed
that unmetabolized cyhalothrin accounted for only about 4.6-5.5
percent of total radioactivity.
Additional studies were conducted with the alternatively labelled
14C-cyclopropyl Cyhalothrin in groups of six male and six female
rats. When orally administered at 1 and 25 mg/kg, most radioactivity
was excreted in urine and faeces after 24 h (urine: 0.3-10.7 percent);
(faeces: 36-61 percent) and after seven days (urine, including cage
washings: 13-32 percent); (faeces: 2-68 percent). The whole blood
concentration of total radioactivity peaked (0.30-0.35 ug/ml) 4 h
after oral administration of a single dose of 1 mg/kg, the half life
being about 10-12 h. Carcases contained low residual levels
(1-3 percent) of radioactivity after seven days. 14CO2 was not
detected in expired air (Harrison & Case, 1981).
The bio-accumulation of Cyhalothrin in the rat was studied
further. A solution of 14C-cyclopropyl cyhalothrin in maize oil was
administered orally to 20 groups of three male Alpk/AP rats. Dosing
volumes were adjusted for body weight so that daily doses of 1 mg/kg
were given for up to four months. A group of 20 control rats received
similar volumes of the maize oil vehicle.
Groups of three test and one control animal were sacrificed 24 h
after receiving the last dose. Some animals were retained after dosing
was complete to permit estimation of the dissipation of Cyhalothrin
residues from the tissues.
Blood levels of radioactivity rose slowly over the duration of
the dosing period to a plateau at about 0.2 µg Cyhalothrin
equivalents/ml after 77 daily doses. Blood radioactivity had
dissipated 36 days after cessation of exposure. Levels of
radioactivity in fat rose progressively to about 9 ug equivalents/g at
100 days. On cessation of exposure, the level of radioactivity in
adipose tissue declined slowly with apparent first order kinetics with
a half-life of about 30 days. The levels of radioactivity in kidney
and liver plateaued at about 1.5 and 2.5 µg equivalents Cyhalothrin/g,
respectively, after about 80 days. These levels fell rapidly on
cessation of exposure but levels of radioactivity in the liver
remained detectable throughout the study, appearing to parallel the
rate of dissipation from the adipose tissue. Analysis of the fat
showed the presence of Cyhalothrin and that the ratio of the
Cyhalothrin enantiomers was essentially unchanged from that of the
material given initially (Prout, 1984).
Biotransformation
Chromatographic analysis of radioactive material excreted by rats
treated with 14C-CHCN Cyhalothrin at 25 mg/kg showed that extensive
metabolism to polar metabolites occurred. Unmetabolized Cyhalothrin
was not excreted in the urine, which contained at least two
metabolites unaffected by treatment with ß-glucuronidase or aryl
sulphatase. The bile contained at least three metabolites, which
differed from those in the urine and faeces. The faeces contained
unchanged Cyhalothrin (approximately 80 percent) plus small amounts of
three polar metabolites.
After dosing with 14C-cyclopropyl Cyhalothrin at 25 mg/kg,
unmetabolized Cyhalothrin was not detected in the urine, which
contained at least three metabolites with the 14C-cyclopropyl moeity
(Harrison & Case, 1981).
Further studies were undertaken to characterize the major urinary
metabolites of cyhalothrin in rats. Six male and six females received
the 14C-CHCN compound orally for a period of eight days at
12.5 mg/kg. Urine and faeces were collected daily up to three days
after the last dose. The urine contained about 64 percent of the
administered radioactivity.
Similarly, pooled urine samples from rats that had received 14
daily doses of 14C-cyclopropyl Cyhalothrin at 1 mg/kg contained about
50 percent of the total administered radioactivity.
The above samples were subject to thin layer chromatography and
high performance liquid chromatography and the metabolites identified
by mass spectrometry and nuclear magnetic resonance spectroscopy,
after appropriate hydrolysis with aryl sulphatase and ß-glucuronidase.
The identity of these metabolites is given in Figure 2 (Harrison &
Case, 1983).
TOXICOLOGICAL STUDIES
Special Studies on Cell Transformation
A cell transformation test, using BHK clone 13 Hamster fibroblast
cells, failed to show a carcinogenic potential for Cyhalothrin, either
with or without metabolic activation (see Table 1. See also under
Long-Term Studies).
Special Studies on Mutagenicity
Cyhalothrin was without mutagenic activity in a number of
mutagenicity assays (see Table 2).
Special Study on Reproduction
In a three-generation reproduction study, groups of 30 male and
30 female Alpk/AP (Wistar-derived) weanling rats received technical
Cyhalothrin (89.2 percent pure) in the diet at concentrations of 0,
10, 30 or 100 ppm for 12 weeks before mating and then continuously. A
similar dosing regime was maintained throughout two successive
generations resulting from the matings of the F1 parents selected
from the F1b generation and F2 parents selected from the F2b
generation.
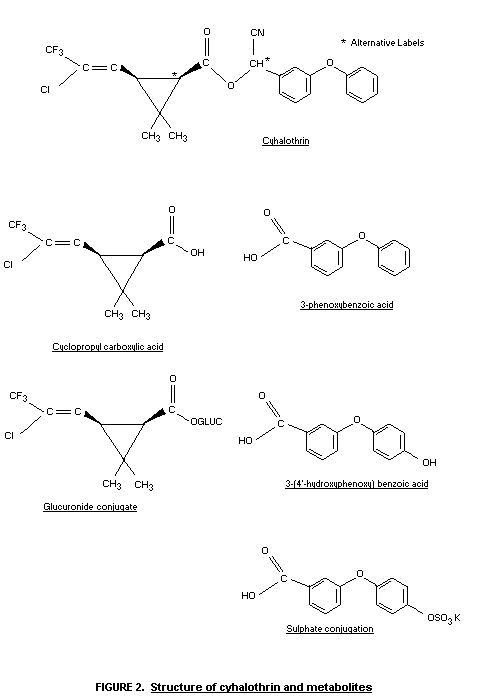 TABLE 1. Special Study of Cyhalothrin on Cell Transformation
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Cell BHK 21 C13 Without metabolic Unspecified Negative Richold et al., 1981
transformation activation
Hamster Cells: 50, 250, 500
0 ] 750, 1000 µg/ml
0.625 ]
1.25 ] x 105
2.5 ] cells/plate
5 ]
With S-9 Mix
1000, 2000, 3000 Negative
4000, 5000 µg/ml
4-nitroquinoline-N-oxide ] Unspecified Positive
p-dimethylaminobenzene as ]
positive controls Positive
TABLE 2. Special Studies of Cyhalothrin on Mutagenicity
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Ames' Test S. typhimurium 4, 20, 100, 500, 90.2% w/w ] Truman, 1981
(both with TA 1535 2500 µg/plate ]
and without TA 1537 (Cis:trans ]
metabolic TA 1538 97.1:2.9) ] Negative
activation) TA 98 ]
TA 100 ]
In Vivo Rat 0,1.5, 7.5 or 15 mg/kg 89.2% w/w No evidence Anderson et
cytogenetics orally in maize oil, once of mutagenic al., 1981-
and also four consecutive or clastogenic
daily doses. Ethyl potential due to
methanesulphonate at cyhalothrin.
200 mg/kg. Positive response
with ethyl
methanesulphonate
Dominant lethal Mouse 0, 1,5 and 10 mg/kg orally 89.25% w/w No dominant Irvine, 1981
in maize oil daily for five lethality due
days. Cyclophosphamide at to cyhalothrin.
200 mg/kg i.p. daily for Positive response
five days. with cyclophosphamide.
Bodyweight gains were reduced at 100 ppm in all generations.
There were no treatment-related effects on food consumption, fertility
rate, duration of pre-coital period and length of gestation. The F2a
and F3b litter sizes were slightly smaller at 100 ppm. The numbers of
liveborn pups per litter and survival rates up to day 22 were not
affected by treatment.
At autopsy, no treatment-related effects were found, either on
pathological or histopathological examination. Based on the findings
of reduced bodyweight gain, the no-effect level established by this
study is 30 ppm (Milburn et al., 1984).
Special Studies on Teratogenicity
Rat
Groups of 24 mated female Charles River CD rats received
technical Cyhalothrin (89.25 percent pure) in maize oil by gavage at
0, 5, 10, 15 mg/kg on days 6-15 (inclusive) of gestation and were
maintained without treatment until sacrificed at day 20.
Treatment at the highest dose (15 mg/kg) was associated with
weight loss and with uncoordinated limb movements in 2/24 animals. The
overall body weight gain over the initial seven days was significantly
reduced in this treatment group. The rate of food consumption for the
other two treatment groups was slightly lower than for the control
group during treatment. Cyhalothrin had no effect on the incidence
of pregnancy, which was complete in all treatment groups. Pre-
implantation losses were unaffected by treatment. Post-implantation
losses, determined from the number of implantations and the number of
live foetuses, were marginally, but not significantly, increased over
the control values but not at the higher rates of treatment (i.e., 10
and 15 mg/kg). The overall rate of implantations was unaffected by
treatment. The mean litter weights, mean foetal weights and mean
crown-rump lengths were also not affected by treatment.
Foetal abnormalities, considered as random, occurred only within
one of the 12 litters of the 10 mg/kg treatment group. These
abnormalities were bilateral agenesis of kidney and ureters in four
foetuses and kidney hypoplasia in the fifth of the 17 foetuses. Three
of the affected foetuses also had malformations of the thoracic and
lumbar vertebrae and fusions of the sternebrae and metacarpals. The
results of this study indicate that daily treatment up to 15 mg/kg
Cyhalothrin, which produces maternal toxicity in the rat, was without
any teratogenic effect (Killick, 1981a).
Rabbit
Groups of about 20 mated female New Zealand White rabbits
received a solution of Cyhalothrin (89.25 percent purity) in maize oil
by gavage at 0, 3, 10 and 30 mg/kg daily from days 6-18 of gestation,
inclusively. Survivors were sacrificed on gestation day 28.
Treatment with 30 mg/kg Cyhalothrin initially caused loss of body
weight and subsequent depression of body weight gain during the
treatment. Food intake was correspondingly reduced at this dose.
Although the mean weights of the gravid uteri were not affected by
treatment, the residual body weights of all Cyhalothrin-treated
rabbits were less than control values, although the differences were
not statistically significant.
Pre-implantation losses were slightly higher in the control
groups and the number of corpora lutea and implantations were not
affected by treatment with Cyhalothrin. Post-implantation losses were
slightly higher in the treated groups owing to a slight increase in
intra-uterine deaths, which was not dose-related. The mean number of
foetuses/litter was also reduced, but not significantly, by treatment.
The sex ratios were unchanged. The mean foetal weights were also
slightly, but not significantly, reduced at 30 mg/kg and similarly
slightly increased at 3 mg/kg. The type and incidence of foetal
abnormalities found was not related to treatment and did not differ
significantly from controls (Killick, 1981b).
The results of this study indicate no teratogenic potential for
Cyhalothrin, although treatment at 30 mg/kg daily caused maternal
toxicity.
Acute Toxicity
Signs of Cyhalothrin toxicity included: ungroomed appearance,
subdued behaviour, piloerection, ptosis, salivation, chromodacyrrhoea,
scouring, incontinence and staining of ventral surface, hypothermia,
hypersensitivity to noise, altered gait and unnatural posture (upward
curvature of spine). LD50 levels are reported in six species in Table
3.
Death usually occurred within two days of Cyhalothrin
administration and surviving animals appeared normal within nine days.
Short-Term Studies
Rat
In a 28-day feeding study, groups of 16 male and 16 female
Alpk/AP (Wistar-derived) rats were fed Cyhalothrin (89.0 percent pure;
100 percent cis isomer) at 0, 20, 100 and 250 ppm in the diet for 28
days. Because the experimental design was modified, groups of eight
male and eight female rats were similarly fed 500 and 750 ppm
cis-Cyhalothrin. A comparative group of eight male and eight females
also received 500 and 750 ppm of a 50:50 mixture of the geometrical
isomers of the chemical (84 percent pure) in the diet-for the same
period. Dose-related signs of toxicity (high stepping gait, hunching,
ataxia, hyperactivity, hypersensitivity to external stimuli,
piloerection, poor grooming and salivation) were observed in all
cis-Cyhalothrin groups treated above 100 ppm; one of the 100 ppm
treated males exhibited transient high stepping gait and
hypersensitivity to external stimuli at 20 and 250 ppm. Three of the
males and five of the females fed 750 ppm Cyhalothrin died during
treatment. The cis:trans Cyhalothrin mixture produced similar, but
less, toxicity.
Body weight gains were more depressed above 250 ppm by
cis-Cyhalothrin treatment than by the cis:trans mixture. Food
consumption was decreased above these doses but food utilization was
reduced only in females at and above 500 ppm. A dose-related decrease
of plasma triglyceride concentrations occurred at and above 500 ppm
for males, but only at 750 ppm for cis-Cyhalothrin treated females.
Urinalysis and haematological parameters were not affected by
treatment.
At necropsy, slight liver weight gains were found in the 250 ppm
and 500 ppm Cyhalothrin treatment groups and in the 750 ppm group
treated with the cis:trans mixture. Spleen, adrenal, lung, brain and
thymus weights were decreased at the highest male treatment groups,
consistent with the observed growth retardation. Testes weights were
increased in these same groups. Above 250 ppm there was a dose-related
decrease in the weight of the hearts of male rats treated with either
pyrethroid mixture.
Histopathological examination of males and females from the group
treated with 750 ppm Cyhalothrin showed thymic atrophy, adrenal
enlargement with vacuolation and differential staining of zona
fasciculata cells. The testes showed incomplete spermatogenesis and
loss of seminal vescicle secretion. However, the numbers of animals
autopsied was very small and other treatment groups were not
autopsied.
There was a dose-related increase in hepatic amino-pyrine
demethylase levels of male and female rats treated with
cis-Cyhalothrin and the cis:trans mixture. Electron microscopy showed
proliferation of hepatic smooth endoplasmic reticulum of all male rats
treated with 20, 100 and 250 ppm cis-Cyhalothrin; this effect was also
observed in females treated at 250 ppm. Higher treatment groups were
not examined (Tinston et al., 1984).
In a study to investigate the reversibility of
Cyhalothrin-induced liver changes in male Wistar rats, cyhalothrin
(89.2 percent pure) was fed at 250 ppm in the diet to a group of 32
male rats. A similar number of control rats received the diet alone.
After 28 days, eight rats from each group were sacrificed and examined
for hepatotoxicity. The remaining rats were then maintained on a
control diet until similarly sacrificed after 7, 14 or 28 days.
The bodyweight gain of the rats fed 250 ppm Cyhalothrin was
depressed during feeding and remained depressed until the day of
sacrifice.
TABLE 3. Acute Toxicity of Cyhalothrin in Animals
Animal Route LD50 References
(mg/kg bw)
Mouse M oral 36.7 Nixon & Jackson, 1981
F oral 62.3 ibid
Rat M oral 243 ibid
F oral 144 ibid
M dermal 1000-2500 ibid
F dermal 200-2500 ibid
M intraperitoneal 250-750 ibid
Guinea pig M oral 5000 ibid
Chicken F oral 10 000 Roberts et al., 1982
Rabbit F oral 1000 Jones, 1980
M dermal 2500 Nixon & Jackson, 1981
F dermal 2500 ibid
There was a tendency for a slight reduction of the liver weights
of the treated rats, but this was only statistically significant in
those rats (8/32) allowed a 14-day recovery period. Electron
microscopy showed significant proliferation of smooth endoplasmic
reticulum in only five rats; such proliferation was no longer apparent
in animals allowed a seven-day recovery. Hepatic aminopyrine-N-
demethylase activity was also elevated after 28 days feeding at
250 ppm but this too had reversed seven days after cessation of
exposure (Lindsay et al., 1982).
Groups of 20 male and 20 female Wistar rats were fed Cyhalothrin
(89.25 percent pure) at dietary concentrations of 0, 10, 50 and
250 ppm for 90 days. Only male rats fed 250 ppm Cyhalothrin exhibited
a significantly reduced bodyweight gain. Food consumption of male rats
was reduced at 50 and 250 ppm but, despite significant food wastage by
the 50 and 250 ppm male groups, overall food utilization (g food/g
bodyweight gain) were unaffected in all groups. Although haemoglobin
and haematocrit were not affected by treatment, there was a tendency
for slight reduction of mean erythrocyte volume in Cyhalothrin-treated
groups, with compensatory changes in other haematological parameters.
After four weeks, the mean erythrocyte volume of only female rats
treated at 250 ppm was slightly reduced. However, after 13 weeks,
there was a slight reduction of mean erythrocyte volume in all treated
males and in female rats fed 50 and 250 ppm. Corresponding slight
reduction of erythrocyte haemoglobin levels occurred in males fed
250 ppm and 10 ppm, but not 50 ppm.
Mean erythrocyte haemoglobin concentrations were reduced only in
female rats at 250 ppm. At the same time, male rats fed 50 and 250 ppm
Cyhalothrin exhibited decreased plasma triglyceride levels and a
dose-related increase in hepatic aminopyrine-N-demethylase activity.
The latter was also elevated in female rats fed 250 ppm Cyhalothrin.
Small increases in urinary glucose excretions occurred in males fed 50
and 250 ppm Cyhalothrin after 13 weeks.
No treatment-related changes in organ weights and no macroscopic
pathological changes were found at autopsy. Electron microscopy showed
mild proliferation of smooth endoplasmic reticulum in three males of
each of the 50 ppm and 250 ppm groups; however, the group mean values
did not differ from control values.
In view of the above mentioned haematological effects, a
no-effect level was not established in this study (Lindsay et al.,
1981).
Dog
Groups of six male and six female purebred beagles received
Cyhalothrin orally in encapsulated maize oil solution at 0, 1.0, 2.5
and 10.0 mg/kg daily for 26 weeks.
Vomiting occurred in some dogs of the highest treatment group,
especially in the initial weeks of the study. Neurotoxicity,
manifested as unsteady gait and/or muscular tremors, excessive
salivation and head shaking, occurred in some dogs only at 10 mg/kg
and mostly in the second week of treatment; one dog of the group was
severely affected throughout the study.
Bodyweights were not affected by treatment, but food consumption
was reduced in the highest treatment group. An increase in water
consumption occurred at 10 mg/kg during the initial half of the study.
Ophthalmoscopy, performed before dosing and at 6, 12 and 24 weeks,
revealed no treatment-related effects. Haematological and biochemical
parameters and urinalysis were not affected by treatment. Bone
marrows, examined on the day before autopsy, were normal in all
groups. There were no treatment-related findings at autopsy.
The results of this study indicate 2.5 mg/kg/day as the no-effect
level of Cyhalothrin in the beagle (Chesterman et al., 1981).
Long-Term Studies
Mouse
In a combined chronic toxicity/carcinogenicity study, groups of
64 male and 64 female CD-1 mice were fed Cyhalothrin of unspecified
purity in their diet at 0, 20, 100 and 500 ppm. Twelve mice/sex group
were sacrificed at 52 weeks and the remainder at 104 weeks. Overall
survival was good and there were no dose-related effects on mortality.
High-dose animals and mid-dose males showed an increased incidence of
piloerection and hunched posture. Bodyweight gain was decreased for
males receiving 500 ppm. These animals exhibited postural hunching,
poor condition and aggressive behaviour; the latter was also observed
at 100 ppm.
Packed red cell volumes were reduced in males fed 100 and 500 ppm
and the mean corpuscular haemoglobin concentrations were increased and
the mean cell volume correspondingly reduced. Small changes in the red
cell indices were also observed at 49 weeks in males receiving
Cyhalothrin and at 101 weeks for females, but these were not
associated with changes in PCV, haemoglobin level or red cell count.
The incidence of animals showing elevated serum glutamic-oxaloacetic
and glutamic-pyruvate transaminase levels was somewhat increased in
mid- and high-dose groups at 104 weeks.
Widespread, severe amyloidosis was the most common finding at
autopsy, but controls and all treated groups were affected to a
similar degree. The incidence of mammary adenocarcinomas was increased
in female mice compared with concurrent controls, i.e. control 1/52,
low dose 0/52, mid-dose 7/52, high dose 6/52. However, historical data
show that the background incidence of this neoplasm in untreated
female CD-1 mice is up to 11.7 percent. The observed increase in
tumours was not proportional to dose. No mammary tumours were reported
in male mice and the incidence of all other tumours was unremarkable.
The results of this study indicate 20 ppm as the no-effect level
of dietary Cyhalothrin in the mouse (Colley et al., 1984).
Rat
In a combined chronic toxicity/oncogenicity study, groups of 72
male and 72 female Alpk/AP rats were fed diets containing 0, 10, 50 or
250 ppm Cyhalothrin (89.2 percent pure) for 104 weeks. Ten
rats/sex/group were sacrificed at 52 weeks.
Overall survival was good and no dose-related effects on
mortality were observed. There were no clinical signs of toxicity.
Bodyweight gains were reduced in high-dose groups of both sexes. No
toxicologically significant, treatment-related effects were reported
on haematology, clinical chemistry or urinalysis. Ophthalmoscopy was
unremarkable. Neurological effects were not observed.
At autopsy, no treatment-related macroscopic or histopathological
changes were found. A significant number of treated rats showed
oro-nasal fistulae attributable to the presence of fibrous particles
in the diet. Associated secondary metaplasia of the epithelium of the
nasal cavity with chronic suppurative rhinitis was not uncommon.
Marked rhinitis was a significant cause of death or removal from the
study.
The toxicological no-effect level from this study is 50 ppm
Cyhalothrin (Piggot et al., 1984).
COMMENTS
Cyhalothrin is a new synthetic pyrethroid; it is a mixture of
equal amounts of four Z-cis diastereoisomers.
Cyhalothrin, administered orally to rats, is only partly absorbed
and is readily excreted, with low levels remaining in the tissues. It
is extensively metabolized in the rat to 3-phenoxybenzoic acid,
substituted cyclopropanecarboxylic acid and other polar metabolites.
No mutagenicity was observed in a number of in vitro and in
vivo studies, and a cell transformation test was negative.
A three-generation study in rats showed decreased weight gains in
both females and males at 100 ppm; the no-effect level was 30 ppm
(1.5 mg/kg/day). A study in rats showed no teratogenicity at doses up
to 15 mg/kg/day, which produced maternal toxicity. Similarly, a rabbit
study showed no teratogenicity at doses up to 30 mg/kg/day, which also
caused maternal toxicity.
A 28-day feeding study in rats showed thymic atrophy, adrenal
enlargement with vacuolation and incomplete spermatogenesis at
750 ppm. A 90-day feeding study in rats showed decreased erythrocyte
haemoglobin at 10 and 250 ppm, but not at 50 ppm, and a no-effect
level was not established. A six-month study in dogs showed symptoms
of neurotoxicity at 10 mg/kg/day and the no-effect level was
2.5 mg/kg/day.
A two-year chronic toxicity/carcinogenicity study in mice showed
adverse clinical signs at doses exceeding 20 ppm, which is taken as
the no-effect level in the mouse. A chronic toxicity/carcinogenicity
study in rats showed decreased bodyweight gains at 250 ppm, but no
increased tumourigenicity, and the no-effect level was 50 ppm. A full
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 20 ppm in diet, equal to 2.0 mg/kg bw/day
Rat: 30 ppm in diet, equal to 1.5 mg/kg bw/day
Dog: 2.5 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OF INFORMATION
Desirable
Observations in humans
REFERENCES
Anderson, D.A., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B.
1981 & Godley, M.J. Cyhalothrin: A cytogenetic study in the rat.
ICI Central Toxicology Report No. CTL/P/664 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
1981 Gopinath, C. & Prentice, D.E. Cyhalothrin: Oral toxicity
study in beagle dogs. Huntingdon Research Centre Report No.
ICI/326/8162 submitted by Imperial Chemical Industries PLC
to WHO. (Unpublished)
Colley, J., Dawe, S., Heywood, R., Almond, R., Gibson, W.A., Gregson,
1984 R. & Gopinath, C. Cyhalothrin: Potential tumorigenic and
toxic effects in prolonged dietary administration to mice.
Huntingdon Research Centre Report No. ICI 395/83668
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The disposition and
1981 metabolism of 14C-ICI 146814 in rats. ICI Pharmaceuticals
Division Report No. 8/HA/003990 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The metabolism and
1983 disposition of ICI 146814 in the rat: Part IV. ICI
Pharmaceuticals Division Report No. 6/HC/005683 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Irvine, L.F.H. Cyhalothrin: Oral (gavage) dominant lethal study in the
1981 male mouse. Hazleton Laboratories Europe Ltd. Report No.
2647-72/213 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Jones, J.R. Cyhalothrin: Acute oral median lethal dose (LD50) in the
1980 female rabbit. Hazleton Laboratories Europe Ltd. Report No.
2364-72/214 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the rat.
1981a Hazleton Laboratories Europe Ltd. Report No. 2661-72/208
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the New
1981b Zealand white rabbit. Hazleton Laboratories Europe Ltd.
Report No. 2700-72/211 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
1981 I., Robinson, M. & Stonard, M. Cyhalothrin: 90-day feeding
study in rats. ICI Central Toxicology Laboratory Report No.
CTL/P/629 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Lindsay, S., Doe, J.E., Godley, M.J., Hall, M., Pratt, I., Robinson,
1982 M. & Stonard, M.D. Cyhalothrin-induced liver changes:
Reversibility study in male rats. ICI Central Toxicology
Laboratory Report No. CTL/P/668 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Milburn, G.M., Banham, P., Godley, M.J., Piggott, G. & Robinson, M.
1984 Cyhalothrin: Three-generation reproduction study in the rat.
ICI Central Toxicology Laboratory Report No. CTL/P/906
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Nixon, J. & Jackson, S.J. Cyhalothrin acute toxicity. ICI Central
1981 Toxicology Laboratory Report No. CTL/T/1555 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Piggott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
1984 Robinson, M., Taylor, K. & Tinston, D.J. Cyhalothrin: Two-
year feeding study in rats. ICI Central Toxicology
Laboratory Report No. CTL/P/980 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Prout, M.S. Cyhalothrin: Bioaccumulation in the rat. ICI Central
1984 Toxicology Laboratory Report No. CTL/P/1014 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wight, D.G.D.
1982 The acute oral toxicity (LD50) and neurotoxic effects of
Cyhalothrin to the domestic hen. Huntingdon Research Centre
Report No. ICI/374 NT/81742 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. Cell
1981 transformation test for potential carcinogenicity of
Y00102/010/005 (Cyhalothrin [PP563]). Huntingdon Research
Centre Report No. ICI 391/(B)/80948 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Tinston, D.J., Banham, P.B., Chart, I.S., Gore, C.W., Pratt, I.,
1984 Scales, M.D.C. & Weight, T.M. PP563: 28-day feeding study in
rats. ICI Central Toxicology Laboratory Report No.
CTL/P/1056 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Truman, R.W. Cyhalothrin: Results from the Salmonella reverse mutation
1981 assay. ICI Central Toxicology Laboratory Report No.
CTL/P/665 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
TABLE 1. Special Study of Cyhalothrin on Cell Transformation
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Cell BHK 21 C13 Without metabolic Unspecified Negative Richold et al., 1981
transformation activation
Hamster Cells: 50, 250, 500
0 ] 750, 1000 µg/ml
0.625 ]
1.25 ] x 105
2.5 ] cells/plate
5 ]
With S-9 Mix
1000, 2000, 3000 Negative
4000, 5000 µg/ml
4-nitroquinoline-N-oxide ] Unspecified Positive
p-dimethylaminobenzene as ]
positive controls Positive
TABLE 2. Special Studies of Cyhalothrin on Mutagenicity
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Ames' Test S. typhimurium 4, 20, 100, 500, 90.2% w/w ] Truman, 1981
(both with TA 1535 2500 µg/plate ]
and without TA 1537 (Cis:trans ]
metabolic TA 1538 97.1:2.9) ] Negative
activation) TA 98 ]
TA 100 ]
In Vivo Rat 0,1.5, 7.5 or 15 mg/kg 89.2% w/w No evidence Anderson et
cytogenetics orally in maize oil, once of mutagenic al., 1981-
and also four consecutive or clastogenic
daily doses. Ethyl potential due to
methanesulphonate at cyhalothrin.
200 mg/kg. Positive response
with ethyl
methanesulphonate
Dominant lethal Mouse 0, 1,5 and 10 mg/kg orally 89.25% w/w No dominant Irvine, 1981
in maize oil daily for five lethality due
days. Cyclophosphamide at to cyhalothrin.
200 mg/kg i.p. daily for Positive response
five days. with cyclophosphamide.
Bodyweight gains were reduced at 100 ppm in all generations.
There were no treatment-related effects on food consumption, fertility
rate, duration of pre-coital period and length of gestation. The F2a
and F3b litter sizes were slightly smaller at 100 ppm. The numbers of
liveborn pups per litter and survival rates up to day 22 were not
affected by treatment.
At autopsy, no treatment-related effects were found, either on
pathological or histopathological examination. Based on the findings
of reduced bodyweight gain, the no-effect level established by this
study is 30 ppm (Milburn et al., 1984).
Special Studies on Teratogenicity
Rat
Groups of 24 mated female Charles River CD rats received
technical Cyhalothrin (89.25 percent pure) in maize oil by gavage at
0, 5, 10, 15 mg/kg on days 6-15 (inclusive) of gestation and were
maintained without treatment until sacrificed at day 20.
Treatment at the highest dose (15 mg/kg) was associated with
weight loss and with uncoordinated limb movements in 2/24 animals. The
overall body weight gain over the initial seven days was significantly
reduced in this treatment group. The rate of food consumption for the
other two treatment groups was slightly lower than for the control
group during treatment. Cyhalothrin had no effect on the incidence
of pregnancy, which was complete in all treatment groups. Pre-
implantation losses were unaffected by treatment. Post-implantation
losses, determined from the number of implantations and the number of
live foetuses, were marginally, but not significantly, increased over
the control values but not at the higher rates of treatment (i.e., 10
and 15 mg/kg). The overall rate of implantations was unaffected by
treatment. The mean litter weights, mean foetal weights and mean
crown-rump lengths were also not affected by treatment.
Foetal abnormalities, considered as random, occurred only within
one of the 12 litters of the 10 mg/kg treatment group. These
abnormalities were bilateral agenesis of kidney and ureters in four
foetuses and kidney hypoplasia in the fifth of the 17 foetuses. Three
of the affected foetuses also had malformations of the thoracic and
lumbar vertebrae and fusions of the sternebrae and metacarpals. The
results of this study indicate that daily treatment up to 15 mg/kg
Cyhalothrin, which produces maternal toxicity in the rat, was without
any teratogenic effect (Killick, 1981a).
Rabbit
Groups of about 20 mated female New Zealand White rabbits
received a solution of Cyhalothrin (89.25 percent purity) in maize oil
by gavage at 0, 3, 10 and 30 mg/kg daily from days 6-18 of gestation,
inclusively. Survivors were sacrificed on gestation day 28.
Treatment with 30 mg/kg Cyhalothrin initially caused loss of body
weight and subsequent depression of body weight gain during the
treatment. Food intake was correspondingly reduced at this dose.
Although the mean weights of the gravid uteri were not affected by
treatment, the residual body weights of all Cyhalothrin-treated
rabbits were less than control values, although the differences were
not statistically significant.
Pre-implantation losses were slightly higher in the control
groups and the number of corpora lutea and implantations were not
affected by treatment with Cyhalothrin. Post-implantation losses were
slightly higher in the treated groups owing to a slight increase in
intra-uterine deaths, which was not dose-related. The mean number of
foetuses/litter was also reduced, but not significantly, by treatment.
The sex ratios were unchanged. The mean foetal weights were also
slightly, but not significantly, reduced at 30 mg/kg and similarly
slightly increased at 3 mg/kg. The type and incidence of foetal
abnormalities found was not related to treatment and did not differ
significantly from controls (Killick, 1981b).
The results of this study indicate no teratogenic potential for
Cyhalothrin, although treatment at 30 mg/kg daily caused maternal
toxicity.
Acute Toxicity
Signs of Cyhalothrin toxicity included: ungroomed appearance,
subdued behaviour, piloerection, ptosis, salivation, chromodacyrrhoea,
scouring, incontinence and staining of ventral surface, hypothermia,
hypersensitivity to noise, altered gait and unnatural posture (upward
curvature of spine). LD50 levels are reported in six species in Table
3.
Death usually occurred within two days of Cyhalothrin
administration and surviving animals appeared normal within nine days.
Short-Term Studies
Rat
In a 28-day feeding study, groups of 16 male and 16 female
Alpk/AP (Wistar-derived) rats were fed Cyhalothrin (89.0 percent pure;
100 percent cis isomer) at 0, 20, 100 and 250 ppm in the diet for 28
days. Because the experimental design was modified, groups of eight
male and eight female rats were similarly fed 500 and 750 ppm
cis-Cyhalothrin. A comparative group of eight male and eight females
also received 500 and 750 ppm of a 50:50 mixture of the geometrical
isomers of the chemical (84 percent pure) in the diet-for the same
period. Dose-related signs of toxicity (high stepping gait, hunching,
ataxia, hyperactivity, hypersensitivity to external stimuli,
piloerection, poor grooming and salivation) were observed in all
cis-Cyhalothrin groups treated above 100 ppm; one of the 100 ppm
treated males exhibited transient high stepping gait and
hypersensitivity to external stimuli at 20 and 250 ppm. Three of the
males and five of the females fed 750 ppm Cyhalothrin died during
treatment. The cis:trans Cyhalothrin mixture produced similar, but
less, toxicity.
Body weight gains were more depressed above 250 ppm by
cis-Cyhalothrin treatment than by the cis:trans mixture. Food
consumption was decreased above these doses but food utilization was
reduced only in females at and above 500 ppm. A dose-related decrease
of plasma triglyceride concentrations occurred at and above 500 ppm
for males, but only at 750 ppm for cis-Cyhalothrin treated females.
Urinalysis and haematological parameters were not affected by
treatment.
At necropsy, slight liver weight gains were found in the 250 ppm
and 500 ppm Cyhalothrin treatment groups and in the 750 ppm group
treated with the cis:trans mixture. Spleen, adrenal, lung, brain and
thymus weights were decreased at the highest male treatment groups,
consistent with the observed growth retardation. Testes weights were
increased in these same groups. Above 250 ppm there was a dose-related
decrease in the weight of the hearts of male rats treated with either
pyrethroid mixture.
Histopathological examination of males and females from the group
treated with 750 ppm Cyhalothrin showed thymic atrophy, adrenal
enlargement with vacuolation and differential staining of zona
fasciculata cells. The testes showed incomplete spermatogenesis and
loss of seminal vescicle secretion. However, the numbers of animals
autopsied was very small and other treatment groups were not
autopsied.
There was a dose-related increase in hepatic amino-pyrine
demethylase levels of male and female rats treated with
cis-Cyhalothrin and the cis:trans mixture. Electron microscopy showed
proliferation of hepatic smooth endoplasmic reticulum of all male rats
treated with 20, 100 and 250 ppm cis-Cyhalothrin; this effect was also
observed in females treated at 250 ppm. Higher treatment groups were
not examined (Tinston et al., 1984).
In a study to investigate the reversibility of
Cyhalothrin-induced liver changes in male Wistar rats, cyhalothrin
(89.2 percent pure) was fed at 250 ppm in the diet to a group of 32
male rats. A similar number of control rats received the diet alone.
After 28 days, eight rats from each group were sacrificed and examined
for hepatotoxicity. The remaining rats were then maintained on a
control diet until similarly sacrificed after 7, 14 or 28 days.
The bodyweight gain of the rats fed 250 ppm Cyhalothrin was
depressed during feeding and remained depressed until the day of
sacrifice.
TABLE 3. Acute Toxicity of Cyhalothrin in Animals
Animal Route LD50 References
(mg/kg bw)
Mouse M oral 36.7 Nixon & Jackson, 1981
F oral 62.3 ibid
Rat M oral 243 ibid
F oral 144 ibid
M dermal 1000-2500 ibid
F dermal 200-2500 ibid
M intraperitoneal 250-750 ibid
Guinea pig M oral 5000 ibid
Chicken F oral 10 000 Roberts et al., 1982
Rabbit F oral 1000 Jones, 1980
M dermal 2500 Nixon & Jackson, 1981
F dermal 2500 ibid
There was a tendency for a slight reduction of the liver weights
of the treated rats, but this was only statistically significant in
those rats (8/32) allowed a 14-day recovery period. Electron
microscopy showed significant proliferation of smooth endoplasmic
reticulum in only five rats; such proliferation was no longer apparent
in animals allowed a seven-day recovery. Hepatic aminopyrine-N-
demethylase activity was also elevated after 28 days feeding at
250 ppm but this too had reversed seven days after cessation of
exposure (Lindsay et al., 1982).
Groups of 20 male and 20 female Wistar rats were fed Cyhalothrin
(89.25 percent pure) at dietary concentrations of 0, 10, 50 and
250 ppm for 90 days. Only male rats fed 250 ppm Cyhalothrin exhibited
a significantly reduced bodyweight gain. Food consumption of male rats
was reduced at 50 and 250 ppm but, despite significant food wastage by
the 50 and 250 ppm male groups, overall food utilization (g food/g
bodyweight gain) were unaffected in all groups. Although haemoglobin
and haematocrit were not affected by treatment, there was a tendency
for slight reduction of mean erythrocyte volume in Cyhalothrin-treated
groups, with compensatory changes in other haematological parameters.
After four weeks, the mean erythrocyte volume of only female rats
treated at 250 ppm was slightly reduced. However, after 13 weeks,
there was a slight reduction of mean erythrocyte volume in all treated
males and in female rats fed 50 and 250 ppm. Corresponding slight
reduction of erythrocyte haemoglobin levels occurred in males fed
250 ppm and 10 ppm, but not 50 ppm.
Mean erythrocyte haemoglobin concentrations were reduced only in
female rats at 250 ppm. At the same time, male rats fed 50 and 250 ppm
Cyhalothrin exhibited decreased plasma triglyceride levels and a
dose-related increase in hepatic aminopyrine-N-demethylase activity.
The latter was also elevated in female rats fed 250 ppm Cyhalothrin.
Small increases in urinary glucose excretions occurred in males fed 50
and 250 ppm Cyhalothrin after 13 weeks.
No treatment-related changes in organ weights and no macroscopic
pathological changes were found at autopsy. Electron microscopy showed
mild proliferation of smooth endoplasmic reticulum in three males of
each of the 50 ppm and 250 ppm groups; however, the group mean values
did not differ from control values.
In view of the above mentioned haematological effects, a
no-effect level was not established in this study (Lindsay et al.,
1981).
Dog
Groups of six male and six female purebred beagles received
Cyhalothrin orally in encapsulated maize oil solution at 0, 1.0, 2.5
and 10.0 mg/kg daily for 26 weeks.
Vomiting occurred in some dogs of the highest treatment group,
especially in the initial weeks of the study. Neurotoxicity,
manifested as unsteady gait and/or muscular tremors, excessive
salivation and head shaking, occurred in some dogs only at 10 mg/kg
and mostly in the second week of treatment; one dog of the group was
severely affected throughout the study.
Bodyweights were not affected by treatment, but food consumption
was reduced in the highest treatment group. An increase in water
consumption occurred at 10 mg/kg during the initial half of the study.
Ophthalmoscopy, performed before dosing and at 6, 12 and 24 weeks,
revealed no treatment-related effects. Haematological and biochemical
parameters and urinalysis were not affected by treatment. Bone
marrows, examined on the day before autopsy, were normal in all
groups. There were no treatment-related findings at autopsy.
The results of this study indicate 2.5 mg/kg/day as the no-effect
level of Cyhalothrin in the beagle (Chesterman et al., 1981).
Long-Term Studies
Mouse
In a combined chronic toxicity/carcinogenicity study, groups of
64 male and 64 female CD-1 mice were fed Cyhalothrin of unspecified
purity in their diet at 0, 20, 100 and 500 ppm. Twelve mice/sex group
were sacrificed at 52 weeks and the remainder at 104 weeks. Overall
survival was good and there were no dose-related effects on mortality.
High-dose animals and mid-dose males showed an increased incidence of
piloerection and hunched posture. Bodyweight gain was decreased for
males receiving 500 ppm. These animals exhibited postural hunching,
poor condition and aggressive behaviour; the latter was also observed
at 100 ppm.
Packed red cell volumes were reduced in males fed 100 and 500 ppm
and the mean corpuscular haemoglobin concentrations were increased and
the mean cell volume correspondingly reduced. Small changes in the red
cell indices were also observed at 49 weeks in males receiving
Cyhalothrin and at 101 weeks for females, but these were not
associated with changes in PCV, haemoglobin level or red cell count.
The incidence of animals showing elevated serum glutamic-oxaloacetic
and glutamic-pyruvate transaminase levels was somewhat increased in
mid- and high-dose groups at 104 weeks.
Widespread, severe amyloidosis was the most common finding at
autopsy, but controls and all treated groups were affected to a
similar degree. The incidence of mammary adenocarcinomas was increased
in female mice compared with concurrent controls, i.e. control 1/52,
low dose 0/52, mid-dose 7/52, high dose 6/52. However, historical data
show that the background incidence of this neoplasm in untreated
female CD-1 mice is up to 11.7 percent. The observed increase in
tumours was not proportional to dose. No mammary tumours were reported
in male mice and the incidence of all other tumours was unremarkable.
The results of this study indicate 20 ppm as the no-effect level
of dietary Cyhalothrin in the mouse (Colley et al., 1984).
Rat
In a combined chronic toxicity/oncogenicity study, groups of 72
male and 72 female Alpk/AP rats were fed diets containing 0, 10, 50 or
250 ppm Cyhalothrin (89.2 percent pure) for 104 weeks. Ten
rats/sex/group were sacrificed at 52 weeks.
Overall survival was good and no dose-related effects on
mortality were observed. There were no clinical signs of toxicity.
Bodyweight gains were reduced in high-dose groups of both sexes. No
toxicologically significant, treatment-related effects were reported
on haematology, clinical chemistry or urinalysis. Ophthalmoscopy was
unremarkable. Neurological effects were not observed.
At autopsy, no treatment-related macroscopic or histopathological
changes were found. A significant number of treated rats showed
oro-nasal fistulae attributable to the presence of fibrous particles
in the diet. Associated secondary metaplasia of the epithelium of the
nasal cavity with chronic suppurative rhinitis was not uncommon.
Marked rhinitis was a significant cause of death or removal from the
study.
The toxicological no-effect level from this study is 50 ppm
Cyhalothrin (Piggot et al., 1984).
COMMENTS
Cyhalothrin is a new synthetic pyrethroid; it is a mixture of
equal amounts of four Z-cis diastereoisomers.
Cyhalothrin, administered orally to rats, is only partly absorbed
and is readily excreted, with low levels remaining in the tissues. It
is extensively metabolized in the rat to 3-phenoxybenzoic acid,
substituted cyclopropanecarboxylic acid and other polar metabolites.
No mutagenicity was observed in a number of in vitro and in
vivo studies, and a cell transformation test was negative.
A three-generation study in rats showed decreased weight gains in
both females and males at 100 ppm; the no-effect level was 30 ppm
(1.5 mg/kg/day). A study in rats showed no teratogenicity at doses up
to 15 mg/kg/day, which produced maternal toxicity. Similarly, a rabbit
study showed no teratogenicity at doses up to 30 mg/kg/day, which also
caused maternal toxicity.
A 28-day feeding study in rats showed thymic atrophy, adrenal
enlargement with vacuolation and incomplete spermatogenesis at
750 ppm. A 90-day feeding study in rats showed decreased erythrocyte
haemoglobin at 10 and 250 ppm, but not at 50 ppm, and a no-effect
level was not established. A six-month study in dogs showed symptoms
of neurotoxicity at 10 mg/kg/day and the no-effect level was
2.5 mg/kg/day.
A two-year chronic toxicity/carcinogenicity study in mice showed
adverse clinical signs at doses exceeding 20 ppm, which is taken as
the no-effect level in the mouse. A chronic toxicity/carcinogenicity
study in rats showed decreased bodyweight gains at 250 ppm, but no
increased tumourigenicity, and the no-effect level was 50 ppm. A full
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 20 ppm in diet, equal to 2.0 mg/kg bw/day
Rat: 30 ppm in diet, equal to 1.5 mg/kg bw/day
Dog: 2.5 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OF INFORMATION
Desirable
Observations in humans
REFERENCES
Anderson, D.A., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B.
1981 & Godley, M.J. Cyhalothrin: A cytogenetic study in the rat.
ICI Central Toxicology Report No. CTL/P/664 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
1981 Gopinath, C. & Prentice, D.E. Cyhalothrin: Oral toxicity
study in beagle dogs. Huntingdon Research Centre Report No.
ICI/326/8162 submitted by Imperial Chemical Industries PLC
to WHO. (Unpublished)
Colley, J., Dawe, S., Heywood, R., Almond, R., Gibson, W.A., Gregson,
1984 R. & Gopinath, C. Cyhalothrin: Potential tumorigenic and
toxic effects in prolonged dietary administration to mice.
Huntingdon Research Centre Report No. ICI 395/83668
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The disposition and
1981 metabolism of 14C-ICI 146814 in rats. ICI Pharmaceuticals
Division Report No. 8/HA/003990 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The metabolism and
1983 disposition of ICI 146814 in the rat: Part IV. ICI
Pharmaceuticals Division Report No. 6/HC/005683 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Irvine, L.F.H. Cyhalothrin: Oral (gavage) dominant lethal study in the
1981 male mouse. Hazleton Laboratories Europe Ltd. Report No.
2647-72/213 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Jones, J.R. Cyhalothrin: Acute oral median lethal dose (LD50) in the
1980 female rabbit. Hazleton Laboratories Europe Ltd. Report No.
2364-72/214 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the rat.
1981a Hazleton Laboratories Europe Ltd. Report No. 2661-72/208
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the New
1981b Zealand white rabbit. Hazleton Laboratories Europe Ltd.
Report No. 2700-72/211 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
1981 I., Robinson, M. & Stonard, M. Cyhalothrin: 90-day feeding
study in rats. ICI Central Toxicology Laboratory Report No.
CTL/P/629 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Lindsay, S., Doe, J.E., Godley, M.J., Hall, M., Pratt, I., Robinson,
1982 M. & Stonard, M.D. Cyhalothrin-induced liver changes:
Reversibility study in male rats. ICI Central Toxicology
Laboratory Report No. CTL/P/668 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Milburn, G.M., Banham, P., Godley, M.J., Piggott, G. & Robinson, M.
1984 Cyhalothrin: Three-generation reproduction study in the rat.
ICI Central Toxicology Laboratory Report No. CTL/P/906
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Nixon, J. & Jackson, S.J. Cyhalothrin acute toxicity. ICI Central
1981 Toxicology Laboratory Report No. CTL/T/1555 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Piggott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
1984 Robinson, M., Taylor, K. & Tinston, D.J. Cyhalothrin: Two-
year feeding study in rats. ICI Central Toxicology
Laboratory Report No. CTL/P/980 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Prout, M.S. Cyhalothrin: Bioaccumulation in the rat. ICI Central
1984 Toxicology Laboratory Report No. CTL/P/1014 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wight, D.G.D.
1982 The acute oral toxicity (LD50) and neurotoxic effects of
Cyhalothrin to the domestic hen. Huntingdon Research Centre
Report No. ICI/374 NT/81742 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. Cell
1981 transformation test for potential carcinogenicity of
Y00102/010/005 (Cyhalothrin [PP563]). Huntingdon Research
Centre Report No. ICI 391/(B)/80948 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Tinston, D.J., Banham, P.B., Chart, I.S., Gore, C.W., Pratt, I.,
1984 Scales, M.D.C. & Weight, T.M. PP563: 28-day feeding study in
rats. ICI Central Toxicology Laboratory Report No.
CTL/P/1056 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Truman, R.W. Cyhalothrin: Results from the Salmonella reverse mutation
1981 assay. ICI Central Toxicology Laboratory Report No.
CTL/P/665 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
 Other Information on Identity and Properties
Molecular weight 449.9
Technical material Contains at least 90 percent cyhalothrin (the
four Z-cis-isomers). Small amounts of
E-cis and Z/E-trans isomers are
present.
Physical form Technical material is a viscous, odourless,
yellow-brown liquid.
Vapour pressure 1 × 10-9 kPa at 20°C
Density 1.25 g/cm3 at 25°C
Solubility Insoluble in water; soluble in a range of
organic solvents, e.g. aliphatic and aromatic
hydrocarbons, ethers, ketones, esters and
alcohols
Formulations 5, 10 and 20 percent emulsifiable
available concentrates (E.C.); 5 percent wettable
commercially powder (W.P.)
TOXICOLOGY
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The absorption, distribution and excretion of Cyhalothrin was
studied in Alderley Park Wistar SPF albino rats. Two different
14C-labelled cis preparations, 14C-cyano-3-phenoxybenzyl-(14CHCN)
and 14C-cyclopropylcyhalothrin, each 99 percent radiochemically pure,
were administered orally (see Figure 1).
Other Information on Identity and Properties
Molecular weight 449.9
Technical material Contains at least 90 percent cyhalothrin (the
four Z-cis-isomers). Small amounts of
E-cis and Z/E-trans isomers are
present.
Physical form Technical material is a viscous, odourless,
yellow-brown liquid.
Vapour pressure 1 × 10-9 kPa at 20°C
Density 1.25 g/cm3 at 25°C
Solubility Insoluble in water; soluble in a range of
organic solvents, e.g. aliphatic and aromatic
hydrocarbons, ethers, ketones, esters and
alcohols
Formulations 5, 10 and 20 percent emulsifiable
available concentrates (E.C.); 5 percent wettable
commercially powder (W.P.)
TOXICOLOGY
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
The absorption, distribution and excretion of Cyhalothrin was
studied in Alderley Park Wistar SPF albino rats. Two different
14C-labelled cis preparations, 14C-cyano-3-phenoxybenzyl-(14CHCN)
and 14C-cyclopropylcyhalothrin, each 99 percent radiochemically pure,
were administered orally (see Figure 1).
 Groups of six male and six female rats received a single dose of
1 mg/kg or 25 mg/kg of the test compound in maize oil and the
following parameters were determined: Cyhalothrin concentration in
whole blood (determined by GLC); total radioactivity in whole blood,
plasma, urine and faeces. Groups of four male and four female rats
with exteriorized bile duct cannulae were similarly dosed to enable
determination of total radioactivity excreted in bile. The expired air
from two male and two female rats of each group was monitored for
14CO2 for the first 48 h.
Absorption of radioactivity was variable but accounted for about
half of the administered dose in each case. Absorbed radioactivity was
fairly rapidly eliminated in urine and faeces. Twenty four hours after
administration, the faeces of rats dosed with 1 mg/kg Cyhalothrin
contained significant amounts (30-70 percent) of administered
radioactivity, as did the urine (2-38 percent). After seven days,
total excretion was high (85-104 percent). At 25 mg/kg, the pattern of
elimination of radioactivity was similar: after 24 h the faeces
contained significant radioactivity (6-58 percent) as did the urine
(10-27 percent). After seven days, total elimination was again high
(faeces (35-64 percent); urine, including cage washings (30-51
percent). 14CO2 was not detected in expired air.
After seven days, the rat carcases contained about 2-3 percent of
the radioactivity administered at either dose, which was present
mainly in adipose tissue, and was higher at 25 mg/kg than at 1 mg/kg.
In order to study the relative proportions excreted in the urine
and the faeces, 14C-CHCN-labelled Cyhalothrin was administered
subcutaneously (1 mg/kg) to five male and six female rats. After 24 h,
faeces contained only a relatively small amount of administered
radioactivity (0.2-3.2 percent) and the urine somewhat more (0.5-8.5
percent). After seven days, faecal elimination (1-10.6 percent) was
significantly less than urinary excretion (0.7-32.3 percent),
including cage washings. Total elimination was less than with oral
administration.
Biliary excretion of orally administered 14C-CHCN-Cyhalothrin
was studied in rats with externalized biliary duct cannulae. At
1 mg/kg, biliary excretion of radioactivity after 12 h was low
(0.8-1.85 percent). Total biliary excretion after four days was
significant (4.2-12.9 percent).
Studies with groups of six male and six female rats showed that
after a single oral dose of 25 mg/kg, the blood concentration of
14C-CHCN-Cyhalothrin peaked at 5.9-6.2 µg/ml about seven h after oral
administration. After 48 h, the blood concentration had fallen to
0.5-0.8 µg/ml. At 1 mg/kg, the corresponding peak concentration was
0.5-8.7 µg/ml, falling to 0.006-0.12 µg/ml at 48 h. Subcutaneous
administration of 1 mg/kg produced a peak blood concentration of
0.009-0.033 µg/ml at 12-24 h, which fell to 0.006-0.012 µg/ml after
48 h.
Comparison of gas chromatographic analysis of blood of rats,
analysed two h after oral dosing with cyhalothrin at 25 mg/kg, showed
that unmetabolized cyhalothrin accounted for only about 4.6-5.5
percent of total radioactivity.
Additional studies were conducted with the alternatively labelled
14C-cyclopropyl Cyhalothrin in groups of six male and six female
rats. When orally administered at 1 and 25 mg/kg, most radioactivity
was excreted in urine and faeces after 24 h (urine: 0.3-10.7 percent);
(faeces: 36-61 percent) and after seven days (urine, including cage
washings: 13-32 percent); (faeces: 2-68 percent). The whole blood
concentration of total radioactivity peaked (0.30-0.35 ug/ml) 4 h
after oral administration of a single dose of 1 mg/kg, the half life
being about 10-12 h. Carcases contained low residual levels
(1-3 percent) of radioactivity after seven days. 14CO2 was not
detected in expired air (Harrison & Case, 1981).
The bio-accumulation of Cyhalothrin in the rat was studied
further. A solution of 14C-cyclopropyl cyhalothrin in maize oil was
administered orally to 20 groups of three male Alpk/AP rats. Dosing
volumes were adjusted for body weight so that daily doses of 1 mg/kg
were given for up to four months. A group of 20 control rats received
similar volumes of the maize oil vehicle.
Groups of three test and one control animal were sacrificed 24 h
after receiving the last dose. Some animals were retained after dosing
was complete to permit estimation of the dissipation of Cyhalothrin
residues from the tissues.
Blood levels of radioactivity rose slowly over the duration of
the dosing period to a plateau at about 0.2 µg Cyhalothrin
equivalents/ml after 77 daily doses. Blood radioactivity had
dissipated 36 days after cessation of exposure. Levels of
radioactivity in fat rose progressively to about 9 ug equivalents/g at
100 days. On cessation of exposure, the level of radioactivity in
adipose tissue declined slowly with apparent first order kinetics with
a half-life of about 30 days. The levels of radioactivity in kidney
and liver plateaued at about 1.5 and 2.5 µg equivalents Cyhalothrin/g,
respectively, after about 80 days. These levels fell rapidly on
cessation of exposure but levels of radioactivity in the liver
remained detectable throughout the study, appearing to parallel the
rate of dissipation from the adipose tissue. Analysis of the fat
showed the presence of Cyhalothrin and that the ratio of the
Cyhalothrin enantiomers was essentially unchanged from that of the
material given initially (Prout, 1984).
Biotransformation
Chromatographic analysis of radioactive material excreted by rats
treated with 14C-CHCN Cyhalothrin at 25 mg/kg showed that extensive
metabolism to polar metabolites occurred. Unmetabolized Cyhalothrin
was not excreted in the urine, which contained at least two
metabolites unaffected by treatment with ß-glucuronidase or aryl
sulphatase. The bile contained at least three metabolites, which
differed from those in the urine and faeces. The faeces contained
unchanged Cyhalothrin (approximately 80 percent) plus small amounts of
three polar metabolites.
After dosing with 14C-cyclopropyl Cyhalothrin at 25 mg/kg,
unmetabolized Cyhalothrin was not detected in the urine, which
contained at least three metabolites with the 14C-cyclopropyl moeity
(Harrison & Case, 1981).
Further studies were undertaken to characterize the major urinary
metabolites of cyhalothrin in rats. Six male and six females received
the 14C-CHCN compound orally for a period of eight days at
12.5 mg/kg. Urine and faeces were collected daily up to three days
after the last dose. The urine contained about 64 percent of the
administered radioactivity.
Similarly, pooled urine samples from rats that had received 14
daily doses of 14C-cyclopropyl Cyhalothrin at 1 mg/kg contained about
50 percent of the total administered radioactivity.
The above samples were subject to thin layer chromatography and
high performance liquid chromatography and the metabolites identified
by mass spectrometry and nuclear magnetic resonance spectroscopy,
after appropriate hydrolysis with aryl sulphatase and ß-glucuronidase.
The identity of these metabolites is given in Figure 2 (Harrison &
Case, 1983).
TOXICOLOGICAL STUDIES
Special Studies on Cell Transformation
A cell transformation test, using BHK clone 13 Hamster fibroblast
cells, failed to show a carcinogenic potential for Cyhalothrin, either
with or without metabolic activation (see Table 1. See also under
Long-Term Studies).
Special Studies on Mutagenicity
Cyhalothrin was without mutagenic activity in a number of
mutagenicity assays (see Table 2).
Special Study on Reproduction
In a three-generation reproduction study, groups of 30 male and
30 female Alpk/AP (Wistar-derived) weanling rats received technical
Cyhalothrin (89.2 percent pure) in the diet at concentrations of 0,
10, 30 or 100 ppm for 12 weeks before mating and then continuously. A
similar dosing regime was maintained throughout two successive
generations resulting from the matings of the F1 parents selected
from the F1b generation and F2 parents selected from the F2b
generation.
Groups of six male and six female rats received a single dose of
1 mg/kg or 25 mg/kg of the test compound in maize oil and the
following parameters were determined: Cyhalothrin concentration in
whole blood (determined by GLC); total radioactivity in whole blood,
plasma, urine and faeces. Groups of four male and four female rats
with exteriorized bile duct cannulae were similarly dosed to enable
determination of total radioactivity excreted in bile. The expired air
from two male and two female rats of each group was monitored for
14CO2 for the first 48 h.
Absorption of radioactivity was variable but accounted for about
half of the administered dose in each case. Absorbed radioactivity was
fairly rapidly eliminated in urine and faeces. Twenty four hours after
administration, the faeces of rats dosed with 1 mg/kg Cyhalothrin
contained significant amounts (30-70 percent) of administered
radioactivity, as did the urine (2-38 percent). After seven days,
total excretion was high (85-104 percent). At 25 mg/kg, the pattern of
elimination of radioactivity was similar: after 24 h the faeces
contained significant radioactivity (6-58 percent) as did the urine
(10-27 percent). After seven days, total elimination was again high
(faeces (35-64 percent); urine, including cage washings (30-51
percent). 14CO2 was not detected in expired air.
After seven days, the rat carcases contained about 2-3 percent of
the radioactivity administered at either dose, which was present
mainly in adipose tissue, and was higher at 25 mg/kg than at 1 mg/kg.
In order to study the relative proportions excreted in the urine
and the faeces, 14C-CHCN-labelled Cyhalothrin was administered
subcutaneously (1 mg/kg) to five male and six female rats. After 24 h,
faeces contained only a relatively small amount of administered
radioactivity (0.2-3.2 percent) and the urine somewhat more (0.5-8.5
percent). After seven days, faecal elimination (1-10.6 percent) was
significantly less than urinary excretion (0.7-32.3 percent),
including cage washings. Total elimination was less than with oral
administration.
Biliary excretion of orally administered 14C-CHCN-Cyhalothrin
was studied in rats with externalized biliary duct cannulae. At
1 mg/kg, biliary excretion of radioactivity after 12 h was low
(0.8-1.85 percent). Total biliary excretion after four days was
significant (4.2-12.9 percent).
Studies with groups of six male and six female rats showed that
after a single oral dose of 25 mg/kg, the blood concentration of
14C-CHCN-Cyhalothrin peaked at 5.9-6.2 µg/ml about seven h after oral
administration. After 48 h, the blood concentration had fallen to
0.5-0.8 µg/ml. At 1 mg/kg, the corresponding peak concentration was
0.5-8.7 µg/ml, falling to 0.006-0.12 µg/ml at 48 h. Subcutaneous
administration of 1 mg/kg produced a peak blood concentration of
0.009-0.033 µg/ml at 12-24 h, which fell to 0.006-0.012 µg/ml after
48 h.
Comparison of gas chromatographic analysis of blood of rats,
analysed two h after oral dosing with cyhalothrin at 25 mg/kg, showed
that unmetabolized cyhalothrin accounted for only about 4.6-5.5
percent of total radioactivity.
Additional studies were conducted with the alternatively labelled
14C-cyclopropyl Cyhalothrin in groups of six male and six female
rats. When orally administered at 1 and 25 mg/kg, most radioactivity
was excreted in urine and faeces after 24 h (urine: 0.3-10.7 percent);
(faeces: 36-61 percent) and after seven days (urine, including cage
washings: 13-32 percent); (faeces: 2-68 percent). The whole blood
concentration of total radioactivity peaked (0.30-0.35 ug/ml) 4 h
after oral administration of a single dose of 1 mg/kg, the half life
being about 10-12 h. Carcases contained low residual levels
(1-3 percent) of radioactivity after seven days. 14CO2 was not
detected in expired air (Harrison & Case, 1981).
The bio-accumulation of Cyhalothrin in the rat was studied
further. A solution of 14C-cyclopropyl cyhalothrin in maize oil was
administered orally to 20 groups of three male Alpk/AP rats. Dosing
volumes were adjusted for body weight so that daily doses of 1 mg/kg
were given for up to four months. A group of 20 control rats received
similar volumes of the maize oil vehicle.
Groups of three test and one control animal were sacrificed 24 h
after receiving the last dose. Some animals were retained after dosing
was complete to permit estimation of the dissipation of Cyhalothrin
residues from the tissues.
Blood levels of radioactivity rose slowly over the duration of
the dosing period to a plateau at about 0.2 µg Cyhalothrin
equivalents/ml after 77 daily doses. Blood radioactivity had
dissipated 36 days after cessation of exposure. Levels of
radioactivity in fat rose progressively to about 9 ug equivalents/g at
100 days. On cessation of exposure, the level of radioactivity in
adipose tissue declined slowly with apparent first order kinetics with
a half-life of about 30 days. The levels of radioactivity in kidney
and liver plateaued at about 1.5 and 2.5 µg equivalents Cyhalothrin/g,
respectively, after about 80 days. These levels fell rapidly on
cessation of exposure but levels of radioactivity in the liver
remained detectable throughout the study, appearing to parallel the
rate of dissipation from the adipose tissue. Analysis of the fat
showed the presence of Cyhalothrin and that the ratio of the
Cyhalothrin enantiomers was essentially unchanged from that of the
material given initially (Prout, 1984).
Biotransformation
Chromatographic analysis of radioactive material excreted by rats
treated with 14C-CHCN Cyhalothrin at 25 mg/kg showed that extensive
metabolism to polar metabolites occurred. Unmetabolized Cyhalothrin
was not excreted in the urine, which contained at least two
metabolites unaffected by treatment with ß-glucuronidase or aryl
sulphatase. The bile contained at least three metabolites, which
differed from those in the urine and faeces. The faeces contained
unchanged Cyhalothrin (approximately 80 percent) plus small amounts of
three polar metabolites.
After dosing with 14C-cyclopropyl Cyhalothrin at 25 mg/kg,
unmetabolized Cyhalothrin was not detected in the urine, which
contained at least three metabolites with the 14C-cyclopropyl moeity
(Harrison & Case, 1981).
Further studies were undertaken to characterize the major urinary
metabolites of cyhalothrin in rats. Six male and six females received
the 14C-CHCN compound orally for a period of eight days at
12.5 mg/kg. Urine and faeces were collected daily up to three days
after the last dose. The urine contained about 64 percent of the
administered radioactivity.
Similarly, pooled urine samples from rats that had received 14
daily doses of 14C-cyclopropyl Cyhalothrin at 1 mg/kg contained about
50 percent of the total administered radioactivity.
The above samples were subject to thin layer chromatography and
high performance liquid chromatography and the metabolites identified
by mass spectrometry and nuclear magnetic resonance spectroscopy,
after appropriate hydrolysis with aryl sulphatase and ß-glucuronidase.
The identity of these metabolites is given in Figure 2 (Harrison &
Case, 1983).
TOXICOLOGICAL STUDIES
Special Studies on Cell Transformation
A cell transformation test, using BHK clone 13 Hamster fibroblast
cells, failed to show a carcinogenic potential for Cyhalothrin, either
with or without metabolic activation (see Table 1. See also under
Long-Term Studies).
Special Studies on Mutagenicity
Cyhalothrin was without mutagenic activity in a number of
mutagenicity assays (see Table 2).
Special Study on Reproduction
In a three-generation reproduction study, groups of 30 male and
30 female Alpk/AP (Wistar-derived) weanling rats received technical
Cyhalothrin (89.2 percent pure) in the diet at concentrations of 0,
10, 30 or 100 ppm for 12 weeks before mating and then continuously. A
similar dosing regime was maintained throughout two successive
generations resulting from the matings of the F1 parents selected
from the F1b generation and F2 parents selected from the F2b
generation.
 TABLE 1. Special Study of Cyhalothrin on Cell Transformation
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Cell BHK 21 C13 Without metabolic Unspecified Negative Richold et al., 1981
transformation activation
Hamster Cells: 50, 250, 500
0 ] 750, 1000 µg/ml
0.625 ]
1.25 ] x 105
2.5 ] cells/plate
5 ]
With S-9 Mix
1000, 2000, 3000 Negative
4000, 5000 µg/ml
4-nitroquinoline-N-oxide ] Unspecified Positive
p-dimethylaminobenzene as ]
positive controls Positive
TABLE 2. Special Studies of Cyhalothrin on Mutagenicity
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Ames' Test S. typhimurium 4, 20, 100, 500, 90.2% w/w ] Truman, 1981
(both with TA 1535 2500 µg/plate ]
and without TA 1537 (Cis:trans ]
metabolic TA 1538 97.1:2.9) ] Negative
activation) TA 98 ]
TA 100 ]
In Vivo Rat 0,1.5, 7.5 or 15 mg/kg 89.2% w/w No evidence Anderson et
cytogenetics orally in maize oil, once of mutagenic al., 1981-
and also four consecutive or clastogenic
daily doses. Ethyl potential due to
methanesulphonate at cyhalothrin.
200 mg/kg. Positive response
with ethyl
methanesulphonate
Dominant lethal Mouse 0, 1,5 and 10 mg/kg orally 89.25% w/w No dominant Irvine, 1981
in maize oil daily for five lethality due
days. Cyclophosphamide at to cyhalothrin.
200 mg/kg i.p. daily for Positive response
five days. with cyclophosphamide.
Bodyweight gains were reduced at 100 ppm in all generations.
There were no treatment-related effects on food consumption, fertility
rate, duration of pre-coital period and length of gestation. The F2a
and F3b litter sizes were slightly smaller at 100 ppm. The numbers of
liveborn pups per litter and survival rates up to day 22 were not
affected by treatment.
At autopsy, no treatment-related effects were found, either on
pathological or histopathological examination. Based on the findings
of reduced bodyweight gain, the no-effect level established by this
study is 30 ppm (Milburn et al., 1984).
Special Studies on Teratogenicity
Rat
Groups of 24 mated female Charles River CD rats received
technical Cyhalothrin (89.25 percent pure) in maize oil by gavage at
0, 5, 10, 15 mg/kg on days 6-15 (inclusive) of gestation and were
maintained without treatment until sacrificed at day 20.
Treatment at the highest dose (15 mg/kg) was associated with
weight loss and with uncoordinated limb movements in 2/24 animals. The
overall body weight gain over the initial seven days was significantly
reduced in this treatment group. The rate of food consumption for the
other two treatment groups was slightly lower than for the control
group during treatment. Cyhalothrin had no effect on the incidence
of pregnancy, which was complete in all treatment groups. Pre-
implantation losses were unaffected by treatment. Post-implantation
losses, determined from the number of implantations and the number of
live foetuses, were marginally, but not significantly, increased over
the control values but not at the higher rates of treatment (i.e., 10
and 15 mg/kg). The overall rate of implantations was unaffected by
treatment. The mean litter weights, mean foetal weights and mean
crown-rump lengths were also not affected by treatment.
Foetal abnormalities, considered as random, occurred only within
one of the 12 litters of the 10 mg/kg treatment group. These
abnormalities were bilateral agenesis of kidney and ureters in four
foetuses and kidney hypoplasia in the fifth of the 17 foetuses. Three
of the affected foetuses also had malformations of the thoracic and
lumbar vertebrae and fusions of the sternebrae and metacarpals. The
results of this study indicate that daily treatment up to 15 mg/kg
Cyhalothrin, which produces maternal toxicity in the rat, was without
any teratogenic effect (Killick, 1981a).
Rabbit
Groups of about 20 mated female New Zealand White rabbits
received a solution of Cyhalothrin (89.25 percent purity) in maize oil
by gavage at 0, 3, 10 and 30 mg/kg daily from days 6-18 of gestation,
inclusively. Survivors were sacrificed on gestation day 28.
Treatment with 30 mg/kg Cyhalothrin initially caused loss of body
weight and subsequent depression of body weight gain during the
treatment. Food intake was correspondingly reduced at this dose.
Although the mean weights of the gravid uteri were not affected by
treatment, the residual body weights of all Cyhalothrin-treated
rabbits were less than control values, although the differences were
not statistically significant.
Pre-implantation losses were slightly higher in the control
groups and the number of corpora lutea and implantations were not
affected by treatment with Cyhalothrin. Post-implantation losses were
slightly higher in the treated groups owing to a slight increase in
intra-uterine deaths, which was not dose-related. The mean number of
foetuses/litter was also reduced, but not significantly, by treatment.
The sex ratios were unchanged. The mean foetal weights were also
slightly, but not significantly, reduced at 30 mg/kg and similarly
slightly increased at 3 mg/kg. The type and incidence of foetal
abnormalities found was not related to treatment and did not differ
significantly from controls (Killick, 1981b).
The results of this study indicate no teratogenic potential for
Cyhalothrin, although treatment at 30 mg/kg daily caused maternal
toxicity.
Acute Toxicity
Signs of Cyhalothrin toxicity included: ungroomed appearance,
subdued behaviour, piloerection, ptosis, salivation, chromodacyrrhoea,
scouring, incontinence and staining of ventral surface, hypothermia,
hypersensitivity to noise, altered gait and unnatural posture (upward
curvature of spine). LD50 levels are reported in six species in Table
3.
Death usually occurred within two days of Cyhalothrin
administration and surviving animals appeared normal within nine days.
Short-Term Studies
Rat
In a 28-day feeding study, groups of 16 male and 16 female
Alpk/AP (Wistar-derived) rats were fed Cyhalothrin (89.0 percent pure;
100 percent cis isomer) at 0, 20, 100 and 250 ppm in the diet for 28
days. Because the experimental design was modified, groups of eight
male and eight female rats were similarly fed 500 and 750 ppm
cis-Cyhalothrin. A comparative group of eight male and eight females
also received 500 and 750 ppm of a 50:50 mixture of the geometrical
isomers of the chemical (84 percent pure) in the diet-for the same
period. Dose-related signs of toxicity (high stepping gait, hunching,
ataxia, hyperactivity, hypersensitivity to external stimuli,
piloerection, poor grooming and salivation) were observed in all
cis-Cyhalothrin groups treated above 100 ppm; one of the 100 ppm
treated males exhibited transient high stepping gait and
hypersensitivity to external stimuli at 20 and 250 ppm. Three of the
males and five of the females fed 750 ppm Cyhalothrin died during
treatment. The cis:trans Cyhalothrin mixture produced similar, but
less, toxicity.
Body weight gains were more depressed above 250 ppm by
cis-Cyhalothrin treatment than by the cis:trans mixture. Food
consumption was decreased above these doses but food utilization was
reduced only in females at and above 500 ppm. A dose-related decrease
of plasma triglyceride concentrations occurred at and above 500 ppm
for males, but only at 750 ppm for cis-Cyhalothrin treated females.
Urinalysis and haematological parameters were not affected by
treatment.
At necropsy, slight liver weight gains were found in the 250 ppm
and 500 ppm Cyhalothrin treatment groups and in the 750 ppm group
treated with the cis:trans mixture. Spleen, adrenal, lung, brain and
thymus weights were decreased at the highest male treatment groups,
consistent with the observed growth retardation. Testes weights were
increased in these same groups. Above 250 ppm there was a dose-related
decrease in the weight of the hearts of male rats treated with either
pyrethroid mixture.
Histopathological examination of males and females from the group
treated with 750 ppm Cyhalothrin showed thymic atrophy, adrenal
enlargement with vacuolation and differential staining of zona
fasciculata cells. The testes showed incomplete spermatogenesis and
loss of seminal vescicle secretion. However, the numbers of animals
autopsied was very small and other treatment groups were not
autopsied.
There was a dose-related increase in hepatic amino-pyrine
demethylase levels of male and female rats treated with
cis-Cyhalothrin and the cis:trans mixture. Electron microscopy showed
proliferation of hepatic smooth endoplasmic reticulum of all male rats
treated with 20, 100 and 250 ppm cis-Cyhalothrin; this effect was also
observed in females treated at 250 ppm. Higher treatment groups were
not examined (Tinston et al., 1984).
In a study to investigate the reversibility of
Cyhalothrin-induced liver changes in male Wistar rats, cyhalothrin
(89.2 percent pure) was fed at 250 ppm in the diet to a group of 32
male rats. A similar number of control rats received the diet alone.
After 28 days, eight rats from each group were sacrificed and examined
for hepatotoxicity. The remaining rats were then maintained on a
control diet until similarly sacrificed after 7, 14 or 28 days.
The bodyweight gain of the rats fed 250 ppm Cyhalothrin was
depressed during feeding and remained depressed until the day of
sacrifice.
TABLE 3. Acute Toxicity of Cyhalothrin in Animals
Animal Route LD50 References
(mg/kg bw)
Mouse M oral 36.7 Nixon & Jackson, 1981
F oral 62.3 ibid
Rat M oral 243 ibid
F oral 144 ibid
M dermal 1000-2500 ibid
F dermal 200-2500 ibid
M intraperitoneal 250-750 ibid
Guinea pig M oral 5000 ibid
Chicken F oral 10 000 Roberts et al., 1982
Rabbit F oral 1000 Jones, 1980
M dermal 2500 Nixon & Jackson, 1981
F dermal 2500 ibid
There was a tendency for a slight reduction of the liver weights
of the treated rats, but this was only statistically significant in
those rats (8/32) allowed a 14-day recovery period. Electron
microscopy showed significant proliferation of smooth endoplasmic
reticulum in only five rats; such proliferation was no longer apparent
in animals allowed a seven-day recovery. Hepatic aminopyrine-N-
demethylase activity was also elevated after 28 days feeding at
250 ppm but this too had reversed seven days after cessation of
exposure (Lindsay et al., 1982).
Groups of 20 male and 20 female Wistar rats were fed Cyhalothrin
(89.25 percent pure) at dietary concentrations of 0, 10, 50 and
250 ppm for 90 days. Only male rats fed 250 ppm Cyhalothrin exhibited
a significantly reduced bodyweight gain. Food consumption of male rats
was reduced at 50 and 250 ppm but, despite significant food wastage by
the 50 and 250 ppm male groups, overall food utilization (g food/g
bodyweight gain) were unaffected in all groups. Although haemoglobin
and haematocrit were not affected by treatment, there was a tendency
for slight reduction of mean erythrocyte volume in Cyhalothrin-treated
groups, with compensatory changes in other haematological parameters.
After four weeks, the mean erythrocyte volume of only female rats
treated at 250 ppm was slightly reduced. However, after 13 weeks,
there was a slight reduction of mean erythrocyte volume in all treated
males and in female rats fed 50 and 250 ppm. Corresponding slight
reduction of erythrocyte haemoglobin levels occurred in males fed
250 ppm and 10 ppm, but not 50 ppm.
Mean erythrocyte haemoglobin concentrations were reduced only in
female rats at 250 ppm. At the same time, male rats fed 50 and 250 ppm
Cyhalothrin exhibited decreased plasma triglyceride levels and a
dose-related increase in hepatic aminopyrine-N-demethylase activity.
The latter was also elevated in female rats fed 250 ppm Cyhalothrin.
Small increases in urinary glucose excretions occurred in males fed 50
and 250 ppm Cyhalothrin after 13 weeks.
No treatment-related changes in organ weights and no macroscopic
pathological changes were found at autopsy. Electron microscopy showed
mild proliferation of smooth endoplasmic reticulum in three males of
each of the 50 ppm and 250 ppm groups; however, the group mean values
did not differ from control values.
In view of the above mentioned haematological effects, a
no-effect level was not established in this study (Lindsay et al.,
1981).
Dog
Groups of six male and six female purebred beagles received
Cyhalothrin orally in encapsulated maize oil solution at 0, 1.0, 2.5
and 10.0 mg/kg daily for 26 weeks.
Vomiting occurred in some dogs of the highest treatment group,
especially in the initial weeks of the study. Neurotoxicity,
manifested as unsteady gait and/or muscular tremors, excessive
salivation and head shaking, occurred in some dogs only at 10 mg/kg
and mostly in the second week of treatment; one dog of the group was
severely affected throughout the study.
Bodyweights were not affected by treatment, but food consumption
was reduced in the highest treatment group. An increase in water
consumption occurred at 10 mg/kg during the initial half of the study.
Ophthalmoscopy, performed before dosing and at 6, 12 and 24 weeks,
revealed no treatment-related effects. Haematological and biochemical
parameters and urinalysis were not affected by treatment. Bone
marrows, examined on the day before autopsy, were normal in all
groups. There were no treatment-related findings at autopsy.
The results of this study indicate 2.5 mg/kg/day as the no-effect
level of Cyhalothrin in the beagle (Chesterman et al., 1981).
Long-Term Studies
Mouse
In a combined chronic toxicity/carcinogenicity study, groups of
64 male and 64 female CD-1 mice were fed Cyhalothrin of unspecified
purity in their diet at 0, 20, 100 and 500 ppm. Twelve mice/sex group
were sacrificed at 52 weeks and the remainder at 104 weeks. Overall
survival was good and there were no dose-related effects on mortality.
High-dose animals and mid-dose males showed an increased incidence of
piloerection and hunched posture. Bodyweight gain was decreased for
males receiving 500 ppm. These animals exhibited postural hunching,
poor condition and aggressive behaviour; the latter was also observed
at 100 ppm.
Packed red cell volumes were reduced in males fed 100 and 500 ppm
and the mean corpuscular haemoglobin concentrations were increased and
the mean cell volume correspondingly reduced. Small changes in the red
cell indices were also observed at 49 weeks in males receiving
Cyhalothrin and at 101 weeks for females, but these were not
associated with changes in PCV, haemoglobin level or red cell count.
The incidence of animals showing elevated serum glutamic-oxaloacetic
and glutamic-pyruvate transaminase levels was somewhat increased in
mid- and high-dose groups at 104 weeks.
Widespread, severe amyloidosis was the most common finding at
autopsy, but controls and all treated groups were affected to a
similar degree. The incidence of mammary adenocarcinomas was increased
in female mice compared with concurrent controls, i.e. control 1/52,
low dose 0/52, mid-dose 7/52, high dose 6/52. However, historical data
show that the background incidence of this neoplasm in untreated
female CD-1 mice is up to 11.7 percent. The observed increase in
tumours was not proportional to dose. No mammary tumours were reported
in male mice and the incidence of all other tumours was unremarkable.
The results of this study indicate 20 ppm as the no-effect level
of dietary Cyhalothrin in the mouse (Colley et al., 1984).
Rat
In a combined chronic toxicity/oncogenicity study, groups of 72
male and 72 female Alpk/AP rats were fed diets containing 0, 10, 50 or
250 ppm Cyhalothrin (89.2 percent pure) for 104 weeks. Ten
rats/sex/group were sacrificed at 52 weeks.
Overall survival was good and no dose-related effects on
mortality were observed. There were no clinical signs of toxicity.
Bodyweight gains were reduced in high-dose groups of both sexes. No
toxicologically significant, treatment-related effects were reported
on haematology, clinical chemistry or urinalysis. Ophthalmoscopy was
unremarkable. Neurological effects were not observed.
At autopsy, no treatment-related macroscopic or histopathological
changes were found. A significant number of treated rats showed
oro-nasal fistulae attributable to the presence of fibrous particles
in the diet. Associated secondary metaplasia of the epithelium of the
nasal cavity with chronic suppurative rhinitis was not uncommon.
Marked rhinitis was a significant cause of death or removal from the
study.
The toxicological no-effect level from this study is 50 ppm
Cyhalothrin (Piggot et al., 1984).
COMMENTS
Cyhalothrin is a new synthetic pyrethroid; it is a mixture of
equal amounts of four Z-cis diastereoisomers.
Cyhalothrin, administered orally to rats, is only partly absorbed
and is readily excreted, with low levels remaining in the tissues. It
is extensively metabolized in the rat to 3-phenoxybenzoic acid,
substituted cyclopropanecarboxylic acid and other polar metabolites.
No mutagenicity was observed in a number of in vitro and in
vivo studies, and a cell transformation test was negative.
A three-generation study in rats showed decreased weight gains in
both females and males at 100 ppm; the no-effect level was 30 ppm
(1.5 mg/kg/day). A study in rats showed no teratogenicity at doses up
to 15 mg/kg/day, which produced maternal toxicity. Similarly, a rabbit
study showed no teratogenicity at doses up to 30 mg/kg/day, which also
caused maternal toxicity.
A 28-day feeding study in rats showed thymic atrophy, adrenal
enlargement with vacuolation and incomplete spermatogenesis at
750 ppm. A 90-day feeding study in rats showed decreased erythrocyte
haemoglobin at 10 and 250 ppm, but not at 50 ppm, and a no-effect
level was not established. A six-month study in dogs showed symptoms
of neurotoxicity at 10 mg/kg/day and the no-effect level was
2.5 mg/kg/day.
A two-year chronic toxicity/carcinogenicity study in mice showed
adverse clinical signs at doses exceeding 20 ppm, which is taken as
the no-effect level in the mouse. A chronic toxicity/carcinogenicity
study in rats showed decreased bodyweight gains at 250 ppm, but no
increased tumourigenicity, and the no-effect level was 50 ppm. A full
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 20 ppm in diet, equal to 2.0 mg/kg bw/day
Rat: 30 ppm in diet, equal to 1.5 mg/kg bw/day
Dog: 2.5 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OF INFORMATION
Desirable
Observations in humans
REFERENCES
Anderson, D.A., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B.
1981 & Godley, M.J. Cyhalothrin: A cytogenetic study in the rat.
ICI Central Toxicology Report No. CTL/P/664 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
1981 Gopinath, C. & Prentice, D.E. Cyhalothrin: Oral toxicity
study in beagle dogs. Huntingdon Research Centre Report No.
ICI/326/8162 submitted by Imperial Chemical Industries PLC
to WHO. (Unpublished)
Colley, J., Dawe, S., Heywood, R., Almond, R., Gibson, W.A., Gregson,
1984 R. & Gopinath, C. Cyhalothrin: Potential tumorigenic and
toxic effects in prolonged dietary administration to mice.
Huntingdon Research Centre Report No. ICI 395/83668
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The disposition and
1981 metabolism of 14C-ICI 146814 in rats. ICI Pharmaceuticals
Division Report No. 8/HA/003990 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The metabolism and
1983 disposition of ICI 146814 in the rat: Part IV. ICI
Pharmaceuticals Division Report No. 6/HC/005683 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Irvine, L.F.H. Cyhalothrin: Oral (gavage) dominant lethal study in the
1981 male mouse. Hazleton Laboratories Europe Ltd. Report No.
2647-72/213 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Jones, J.R. Cyhalothrin: Acute oral median lethal dose (LD50) in the
1980 female rabbit. Hazleton Laboratories Europe Ltd. Report No.
2364-72/214 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the rat.
1981a Hazleton Laboratories Europe Ltd. Report No. 2661-72/208
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the New
1981b Zealand white rabbit. Hazleton Laboratories Europe Ltd.
Report No. 2700-72/211 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
1981 I., Robinson, M. & Stonard, M. Cyhalothrin: 90-day feeding
study in rats. ICI Central Toxicology Laboratory Report No.
CTL/P/629 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Lindsay, S., Doe, J.E., Godley, M.J., Hall, M., Pratt, I., Robinson,
1982 M. & Stonard, M.D. Cyhalothrin-induced liver changes:
Reversibility study in male rats. ICI Central Toxicology
Laboratory Report No. CTL/P/668 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Milburn, G.M., Banham, P., Godley, M.J., Piggott, G. & Robinson, M.
1984 Cyhalothrin: Three-generation reproduction study in the rat.
ICI Central Toxicology Laboratory Report No. CTL/P/906
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Nixon, J. & Jackson, S.J. Cyhalothrin acute toxicity. ICI Central
1981 Toxicology Laboratory Report No. CTL/T/1555 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Piggott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
1984 Robinson, M., Taylor, K. & Tinston, D.J. Cyhalothrin: Two-
year feeding study in rats. ICI Central Toxicology
Laboratory Report No. CTL/P/980 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Prout, M.S. Cyhalothrin: Bioaccumulation in the rat. ICI Central
1984 Toxicology Laboratory Report No. CTL/P/1014 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wight, D.G.D.
1982 The acute oral toxicity (LD50) and neurotoxic effects of
Cyhalothrin to the domestic hen. Huntingdon Research Centre
Report No. ICI/374 NT/81742 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. Cell
1981 transformation test for potential carcinogenicity of
Y00102/010/005 (Cyhalothrin [PP563]). Huntingdon Research
Centre Report No. ICI 391/(B)/80948 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Tinston, D.J., Banham, P.B., Chart, I.S., Gore, C.W., Pratt, I.,
1984 Scales, M.D.C. & Weight, T.M. PP563: 28-day feeding study in
rats. ICI Central Toxicology Laboratory Report No.
CTL/P/1056 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Truman, R.W. Cyhalothrin: Results from the Salmonella reverse mutation
1981 assay. ICI Central Toxicology Laboratory Report No.
CTL/P/665 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
TABLE 1. Special Study of Cyhalothrin on Cell Transformation
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Cell BHK 21 C13 Without metabolic Unspecified Negative Richold et al., 1981
transformation activation
Hamster Cells: 50, 250, 500
0 ] 750, 1000 µg/ml
0.625 ]
1.25 ] x 105
2.5 ] cells/plate
5 ]
With S-9 Mix
1000, 2000, 3000 Negative
4000, 5000 µg/ml
4-nitroquinoline-N-oxide ] Unspecified Positive
p-dimethylaminobenzene as ]
positive controls Positive
TABLE 2. Special Studies of Cyhalothrin on Mutagenicity
Test system Test organism Concentrations of Purity Results Reference
Cyhalothrin used
Ames' Test S. typhimurium 4, 20, 100, 500, 90.2% w/w ] Truman, 1981
(both with TA 1535 2500 µg/plate ]
and without TA 1537 (Cis:trans ]
metabolic TA 1538 97.1:2.9) ] Negative
activation) TA 98 ]
TA 100 ]
In Vivo Rat 0,1.5, 7.5 or 15 mg/kg 89.2% w/w No evidence Anderson et
cytogenetics orally in maize oil, once of mutagenic al., 1981-
and also four consecutive or clastogenic
daily doses. Ethyl potential due to
methanesulphonate at cyhalothrin.
200 mg/kg. Positive response
with ethyl
methanesulphonate
Dominant lethal Mouse 0, 1,5 and 10 mg/kg orally 89.25% w/w No dominant Irvine, 1981
in maize oil daily for five lethality due
days. Cyclophosphamide at to cyhalothrin.
200 mg/kg i.p. daily for Positive response
five days. with cyclophosphamide.
Bodyweight gains were reduced at 100 ppm in all generations.
There were no treatment-related effects on food consumption, fertility
rate, duration of pre-coital period and length of gestation. The F2a
and F3b litter sizes were slightly smaller at 100 ppm. The numbers of
liveborn pups per litter and survival rates up to day 22 were not
affected by treatment.
At autopsy, no treatment-related effects were found, either on
pathological or histopathological examination. Based on the findings
of reduced bodyweight gain, the no-effect level established by this
study is 30 ppm (Milburn et al., 1984).
Special Studies on Teratogenicity
Rat
Groups of 24 mated female Charles River CD rats received
technical Cyhalothrin (89.25 percent pure) in maize oil by gavage at
0, 5, 10, 15 mg/kg on days 6-15 (inclusive) of gestation and were
maintained without treatment until sacrificed at day 20.
Treatment at the highest dose (15 mg/kg) was associated with
weight loss and with uncoordinated limb movements in 2/24 animals. The
overall body weight gain over the initial seven days was significantly
reduced in this treatment group. The rate of food consumption for the
other two treatment groups was slightly lower than for the control
group during treatment. Cyhalothrin had no effect on the incidence
of pregnancy, which was complete in all treatment groups. Pre-
implantation losses were unaffected by treatment. Post-implantation
losses, determined from the number of implantations and the number of
live foetuses, were marginally, but not significantly, increased over
the control values but not at the higher rates of treatment (i.e., 10
and 15 mg/kg). The overall rate of implantations was unaffected by
treatment. The mean litter weights, mean foetal weights and mean
crown-rump lengths were also not affected by treatment.
Foetal abnormalities, considered as random, occurred only within
one of the 12 litters of the 10 mg/kg treatment group. These
abnormalities were bilateral agenesis of kidney and ureters in four
foetuses and kidney hypoplasia in the fifth of the 17 foetuses. Three
of the affected foetuses also had malformations of the thoracic and
lumbar vertebrae and fusions of the sternebrae and metacarpals. The
results of this study indicate that daily treatment up to 15 mg/kg
Cyhalothrin, which produces maternal toxicity in the rat, was without
any teratogenic effect (Killick, 1981a).
Rabbit
Groups of about 20 mated female New Zealand White rabbits
received a solution of Cyhalothrin (89.25 percent purity) in maize oil
by gavage at 0, 3, 10 and 30 mg/kg daily from days 6-18 of gestation,
inclusively. Survivors were sacrificed on gestation day 28.
Treatment with 30 mg/kg Cyhalothrin initially caused loss of body
weight and subsequent depression of body weight gain during the
treatment. Food intake was correspondingly reduced at this dose.
Although the mean weights of the gravid uteri were not affected by
treatment, the residual body weights of all Cyhalothrin-treated
rabbits were less than control values, although the differences were
not statistically significant.
Pre-implantation losses were slightly higher in the control
groups and the number of corpora lutea and implantations were not
affected by treatment with Cyhalothrin. Post-implantation losses were
slightly higher in the treated groups owing to a slight increase in
intra-uterine deaths, which was not dose-related. The mean number of
foetuses/litter was also reduced, but not significantly, by treatment.
The sex ratios were unchanged. The mean foetal weights were also
slightly, but not significantly, reduced at 30 mg/kg and similarly
slightly increased at 3 mg/kg. The type and incidence of foetal
abnormalities found was not related to treatment and did not differ
significantly from controls (Killick, 1981b).
The results of this study indicate no teratogenic potential for
Cyhalothrin, although treatment at 30 mg/kg daily caused maternal
toxicity.
Acute Toxicity
Signs of Cyhalothrin toxicity included: ungroomed appearance,
subdued behaviour, piloerection, ptosis, salivation, chromodacyrrhoea,
scouring, incontinence and staining of ventral surface, hypothermia,
hypersensitivity to noise, altered gait and unnatural posture (upward
curvature of spine). LD50 levels are reported in six species in Table
3.
Death usually occurred within two days of Cyhalothrin
administration and surviving animals appeared normal within nine days.
Short-Term Studies
Rat
In a 28-day feeding study, groups of 16 male and 16 female
Alpk/AP (Wistar-derived) rats were fed Cyhalothrin (89.0 percent pure;
100 percent cis isomer) at 0, 20, 100 and 250 ppm in the diet for 28
days. Because the experimental design was modified, groups of eight
male and eight female rats were similarly fed 500 and 750 ppm
cis-Cyhalothrin. A comparative group of eight male and eight females
also received 500 and 750 ppm of a 50:50 mixture of the geometrical
isomers of the chemical (84 percent pure) in the diet-for the same
period. Dose-related signs of toxicity (high stepping gait, hunching,
ataxia, hyperactivity, hypersensitivity to external stimuli,
piloerection, poor grooming and salivation) were observed in all
cis-Cyhalothrin groups treated above 100 ppm; one of the 100 ppm
treated males exhibited transient high stepping gait and
hypersensitivity to external stimuli at 20 and 250 ppm. Three of the
males and five of the females fed 750 ppm Cyhalothrin died during
treatment. The cis:trans Cyhalothrin mixture produced similar, but
less, toxicity.
Body weight gains were more depressed above 250 ppm by
cis-Cyhalothrin treatment than by the cis:trans mixture. Food
consumption was decreased above these doses but food utilization was
reduced only in females at and above 500 ppm. A dose-related decrease
of plasma triglyceride concentrations occurred at and above 500 ppm
for males, but only at 750 ppm for cis-Cyhalothrin treated females.
Urinalysis and haematological parameters were not affected by
treatment.
At necropsy, slight liver weight gains were found in the 250 ppm
and 500 ppm Cyhalothrin treatment groups and in the 750 ppm group
treated with the cis:trans mixture. Spleen, adrenal, lung, brain and
thymus weights were decreased at the highest male treatment groups,
consistent with the observed growth retardation. Testes weights were
increased in these same groups. Above 250 ppm there was a dose-related
decrease in the weight of the hearts of male rats treated with either
pyrethroid mixture.
Histopathological examination of males and females from the group
treated with 750 ppm Cyhalothrin showed thymic atrophy, adrenal
enlargement with vacuolation and differential staining of zona
fasciculata cells. The testes showed incomplete spermatogenesis and
loss of seminal vescicle secretion. However, the numbers of animals
autopsied was very small and other treatment groups were not
autopsied.
There was a dose-related increase in hepatic amino-pyrine
demethylase levels of male and female rats treated with
cis-Cyhalothrin and the cis:trans mixture. Electron microscopy showed
proliferation of hepatic smooth endoplasmic reticulum of all male rats
treated with 20, 100 and 250 ppm cis-Cyhalothrin; this effect was also
observed in females treated at 250 ppm. Higher treatment groups were
not examined (Tinston et al., 1984).
In a study to investigate the reversibility of
Cyhalothrin-induced liver changes in male Wistar rats, cyhalothrin
(89.2 percent pure) was fed at 250 ppm in the diet to a group of 32
male rats. A similar number of control rats received the diet alone.
After 28 days, eight rats from each group were sacrificed and examined
for hepatotoxicity. The remaining rats were then maintained on a
control diet until similarly sacrificed after 7, 14 or 28 days.
The bodyweight gain of the rats fed 250 ppm Cyhalothrin was
depressed during feeding and remained depressed until the day of
sacrifice.
TABLE 3. Acute Toxicity of Cyhalothrin in Animals
Animal Route LD50 References
(mg/kg bw)
Mouse M oral 36.7 Nixon & Jackson, 1981
F oral 62.3 ibid
Rat M oral 243 ibid
F oral 144 ibid
M dermal 1000-2500 ibid
F dermal 200-2500 ibid
M intraperitoneal 250-750 ibid
Guinea pig M oral 5000 ibid
Chicken F oral 10 000 Roberts et al., 1982
Rabbit F oral 1000 Jones, 1980
M dermal 2500 Nixon & Jackson, 1981
F dermal 2500 ibid
There was a tendency for a slight reduction of the liver weights
of the treated rats, but this was only statistically significant in
those rats (8/32) allowed a 14-day recovery period. Electron
microscopy showed significant proliferation of smooth endoplasmic
reticulum in only five rats; such proliferation was no longer apparent
in animals allowed a seven-day recovery. Hepatic aminopyrine-N-
demethylase activity was also elevated after 28 days feeding at
250 ppm but this too had reversed seven days after cessation of
exposure (Lindsay et al., 1982).
Groups of 20 male and 20 female Wistar rats were fed Cyhalothrin
(89.25 percent pure) at dietary concentrations of 0, 10, 50 and
250 ppm for 90 days. Only male rats fed 250 ppm Cyhalothrin exhibited
a significantly reduced bodyweight gain. Food consumption of male rats
was reduced at 50 and 250 ppm but, despite significant food wastage by
the 50 and 250 ppm male groups, overall food utilization (g food/g
bodyweight gain) were unaffected in all groups. Although haemoglobin
and haematocrit were not affected by treatment, there was a tendency
for slight reduction of mean erythrocyte volume in Cyhalothrin-treated
groups, with compensatory changes in other haematological parameters.
After four weeks, the mean erythrocyte volume of only female rats
treated at 250 ppm was slightly reduced. However, after 13 weeks,
there was a slight reduction of mean erythrocyte volume in all treated
males and in female rats fed 50 and 250 ppm. Corresponding slight
reduction of erythrocyte haemoglobin levels occurred in males fed
250 ppm and 10 ppm, but not 50 ppm.
Mean erythrocyte haemoglobin concentrations were reduced only in
female rats at 250 ppm. At the same time, male rats fed 50 and 250 ppm
Cyhalothrin exhibited decreased plasma triglyceride levels and a
dose-related increase in hepatic aminopyrine-N-demethylase activity.
The latter was also elevated in female rats fed 250 ppm Cyhalothrin.
Small increases in urinary glucose excretions occurred in males fed 50
and 250 ppm Cyhalothrin after 13 weeks.
No treatment-related changes in organ weights and no macroscopic
pathological changes were found at autopsy. Electron microscopy showed
mild proliferation of smooth endoplasmic reticulum in three males of
each of the 50 ppm and 250 ppm groups; however, the group mean values
did not differ from control values.
In view of the above mentioned haematological effects, a
no-effect level was not established in this study (Lindsay et al.,
1981).
Dog
Groups of six male and six female purebred beagles received
Cyhalothrin orally in encapsulated maize oil solution at 0, 1.0, 2.5
and 10.0 mg/kg daily for 26 weeks.
Vomiting occurred in some dogs of the highest treatment group,
especially in the initial weeks of the study. Neurotoxicity,
manifested as unsteady gait and/or muscular tremors, excessive
salivation and head shaking, occurred in some dogs only at 10 mg/kg
and mostly in the second week of treatment; one dog of the group was
severely affected throughout the study.
Bodyweights were not affected by treatment, but food consumption
was reduced in the highest treatment group. An increase in water
consumption occurred at 10 mg/kg during the initial half of the study.
Ophthalmoscopy, performed before dosing and at 6, 12 and 24 weeks,
revealed no treatment-related effects. Haematological and biochemical
parameters and urinalysis were not affected by treatment. Bone
marrows, examined on the day before autopsy, were normal in all
groups. There were no treatment-related findings at autopsy.
The results of this study indicate 2.5 mg/kg/day as the no-effect
level of Cyhalothrin in the beagle (Chesterman et al., 1981).
Long-Term Studies
Mouse
In a combined chronic toxicity/carcinogenicity study, groups of
64 male and 64 female CD-1 mice were fed Cyhalothrin of unspecified
purity in their diet at 0, 20, 100 and 500 ppm. Twelve mice/sex group
were sacrificed at 52 weeks and the remainder at 104 weeks. Overall
survival was good and there were no dose-related effects on mortality.
High-dose animals and mid-dose males showed an increased incidence of
piloerection and hunched posture. Bodyweight gain was decreased for
males receiving 500 ppm. These animals exhibited postural hunching,
poor condition and aggressive behaviour; the latter was also observed
at 100 ppm.
Packed red cell volumes were reduced in males fed 100 and 500 ppm
and the mean corpuscular haemoglobin concentrations were increased and
the mean cell volume correspondingly reduced. Small changes in the red
cell indices were also observed at 49 weeks in males receiving
Cyhalothrin and at 101 weeks for females, but these were not
associated with changes in PCV, haemoglobin level or red cell count.
The incidence of animals showing elevated serum glutamic-oxaloacetic
and glutamic-pyruvate transaminase levels was somewhat increased in
mid- and high-dose groups at 104 weeks.
Widespread, severe amyloidosis was the most common finding at
autopsy, but controls and all treated groups were affected to a
similar degree. The incidence of mammary adenocarcinomas was increased
in female mice compared with concurrent controls, i.e. control 1/52,
low dose 0/52, mid-dose 7/52, high dose 6/52. However, historical data
show that the background incidence of this neoplasm in untreated
female CD-1 mice is up to 11.7 percent. The observed increase in
tumours was not proportional to dose. No mammary tumours were reported
in male mice and the incidence of all other tumours was unremarkable.
The results of this study indicate 20 ppm as the no-effect level
of dietary Cyhalothrin in the mouse (Colley et al., 1984).
Rat
In a combined chronic toxicity/oncogenicity study, groups of 72
male and 72 female Alpk/AP rats were fed diets containing 0, 10, 50 or
250 ppm Cyhalothrin (89.2 percent pure) for 104 weeks. Ten
rats/sex/group were sacrificed at 52 weeks.
Overall survival was good and no dose-related effects on
mortality were observed. There were no clinical signs of toxicity.
Bodyweight gains were reduced in high-dose groups of both sexes. No
toxicologically significant, treatment-related effects were reported
on haematology, clinical chemistry or urinalysis. Ophthalmoscopy was
unremarkable. Neurological effects were not observed.
At autopsy, no treatment-related macroscopic or histopathological
changes were found. A significant number of treated rats showed
oro-nasal fistulae attributable to the presence of fibrous particles
in the diet. Associated secondary metaplasia of the epithelium of the
nasal cavity with chronic suppurative rhinitis was not uncommon.
Marked rhinitis was a significant cause of death or removal from the
study.
The toxicological no-effect level from this study is 50 ppm
Cyhalothrin (Piggot et al., 1984).
COMMENTS
Cyhalothrin is a new synthetic pyrethroid; it is a mixture of
equal amounts of four Z-cis diastereoisomers.
Cyhalothrin, administered orally to rats, is only partly absorbed
and is readily excreted, with low levels remaining in the tissues. It
is extensively metabolized in the rat to 3-phenoxybenzoic acid,
substituted cyclopropanecarboxylic acid and other polar metabolites.
No mutagenicity was observed in a number of in vitro and in
vivo studies, and a cell transformation test was negative.
A three-generation study in rats showed decreased weight gains in
both females and males at 100 ppm; the no-effect level was 30 ppm
(1.5 mg/kg/day). A study in rats showed no teratogenicity at doses up
to 15 mg/kg/day, which produced maternal toxicity. Similarly, a rabbit
study showed no teratogenicity at doses up to 30 mg/kg/day, which also
caused maternal toxicity.
A 28-day feeding study in rats showed thymic atrophy, adrenal
enlargement with vacuolation and incomplete spermatogenesis at
750 ppm. A 90-day feeding study in rats showed decreased erythrocyte
haemoglobin at 10 and 250 ppm, but not at 50 ppm, and a no-effect
level was not established. A six-month study in dogs showed symptoms
of neurotoxicity at 10 mg/kg/day and the no-effect level was
2.5 mg/kg/day.
A two-year chronic toxicity/carcinogenicity study in mice showed
adverse clinical signs at doses exceeding 20 ppm, which is taken as
the no-effect level in the mouse. A chronic toxicity/carcinogenicity
study in rats showed decreased bodyweight gains at 250 ppm, but no
increased tumourigenicity, and the no-effect level was 50 ppm. A full
ADI was allocated.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 20 ppm in diet, equal to 2.0 mg/kg bw/day
Rat: 30 ppm in diet, equal to 1.5 mg/kg bw/day
Dog: 2.5 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OF INFORMATION
Desirable
Observations in humans
REFERENCES
Anderson, D.A., Richardson, C.R., Hulme, A., Morris, J., Banham, P.B.
1981 & Godley, M.J. Cyhalothrin: A cytogenetic study in the rat.
ICI Central Toxicology Report No. CTL/P/664 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Chesterman, H., Heywood, R., Allen, T.R., Street, A.E., Kelly, D.F.,
1981 Gopinath, C. & Prentice, D.E. Cyhalothrin: Oral toxicity
study in beagle dogs. Huntingdon Research Centre Report No.
ICI/326/8162 submitted by Imperial Chemical Industries PLC
to WHO. (Unpublished)
Colley, J., Dawe, S., Heywood, R., Almond, R., Gibson, W.A., Gregson,
1984 R. & Gopinath, C. Cyhalothrin: Potential tumorigenic and
toxic effects in prolonged dietary administration to mice.
Huntingdon Research Centre Report No. ICI 395/83668
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The disposition and
1981 metabolism of 14C-ICI 146814 in rats. ICI Pharmaceuticals
Division Report No. 8/HA/003990 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Harrison, M.P. & Case, D.E. Cyhalothrin: The metabolism and
1983 disposition of ICI 146814 in the rat: Part IV. ICI
Pharmaceuticals Division Report No. 6/HC/005683 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Irvine, L.F.H. Cyhalothrin: Oral (gavage) dominant lethal study in the
1981 male mouse. Hazleton Laboratories Europe Ltd. Report No.
2647-72/213 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Jones, J.R. Cyhalothrin: Acute oral median lethal dose (LD50) in the
1980 female rabbit. Hazleton Laboratories Europe Ltd. Report No.
2364-72/214 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the rat.
1981a Hazleton Laboratories Europe Ltd. Report No. 2661-72/208
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Killick, M.E. Cyhalothrin: Oral (gavage) teratology study in the New
1981b Zealand white rabbit. Hazleton Laboratories Europe Ltd.
Report No. 2700-72/211 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Lindsay, S., Chart, I.S., Godley, M.J., Gore, C.W., Hall, M., Pratt,
1981 I., Robinson, M. & Stonard, M. Cyhalothrin: 90-day feeding
study in rats. ICI Central Toxicology Laboratory Report No.
CTL/P/629 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Lindsay, S., Doe, J.E., Godley, M.J., Hall, M., Pratt, I., Robinson,
1982 M. & Stonard, M.D. Cyhalothrin-induced liver changes:
Reversibility study in male rats. ICI Central Toxicology
Laboratory Report No. CTL/P/668 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Milburn, G.M., Banham, P., Godley, M.J., Piggott, G. & Robinson, M.
1984 Cyhalothrin: Three-generation reproduction study in the rat.
ICI Central Toxicology Laboratory Report No. CTL/P/906
submitted by Imperial Chemical Industries PLC to WHO.
(Unpublished)
Nixon, J. & Jackson, S.J. Cyhalothrin acute toxicity. ICI Central
1981 Toxicology Laboratory Report No. CTL/T/1555 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Piggott, G.H., Chart, I.S., Godley, M.J., Gore, C.W., Hollis, K.J.,
1984 Robinson, M., Taylor, K. & Tinston, D.J. Cyhalothrin: Two-
year feeding study in rats. ICI Central Toxicology
Laboratory Report No. CTL/P/980 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Prout, M.S. Cyhalothrin: Bioaccumulation in the rat. ICI Central
1984 Toxicology Laboratory Report No. CTL/P/1014 submitted by
Imperial Chemical Industries PLC to WHO. (Unpublished)
Roberts, N.L., Fairley, C., Hakin, B., Prentice, D.E. & Wight, D.G.D.
1982 The acute oral toxicity (LD50) and neurotoxic effects of
Cyhalothrin to the domestic hen. Huntingdon Research Centre
Report No. ICI/374 NT/81742 submitted by Imperial Chemical
Industries PLC to WHO. (Unpublished)
Richold, M., Allen, J.A., Williams, A. & Ransome, S.J. Cell
1981 transformation test for potential carcinogenicity of
Y00102/010/005 (Cyhalothrin [PP563]). Huntingdon Research
Centre Report No. ICI 391/(B)/80948 submitted by Imperial
Chemical Industries PLC to WHO. (Unpublished)
Tinston, D.J., Banham, P.B., Chart, I.S., Gore, C.W., Pratt, I.,
1984 Scales, M.D.C. & Weight, T.M. PP563: 28-day feeding study in
rats. ICI Central Toxicology Laboratory Report No.
CTL/P/1056 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
Truman, R.W. Cyhalothrin: Results from the Salmonella reverse mutation
1981 assay. ICI Central Toxicology Laboratory Report No.
CTL/P/665 submitted by Imperial Chemical Industries PLC to
WHO. (Unpublished)
