
WHO FOOD ADDITIVES SERIES: 52
First draft prepared by
Dr I. C. Munro
CANTOX Health Sciences International, Mississauga, Ontario, Canada
and
Dr A. Mattia
Division of Biotechnology and GRAS Notice Review, Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, United States Food & Drug Administration, Maryland, USA
|
Proposed modification to the current Procedure for the Safety Evaluation of Flavouring Agents |
For nearly 40 years, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has played a leading role in the development of principles and procedures for the safety evaluation of flavouring agents. In 1996, the Committee began an ongoing programme to conduct safety evaluations and establish specifications for individual flavouring agents. On the basis of this experience, the Committee recognizes that the safety evaluation of flavouring agents presents unique challenges, principally owing to the fact that there are more than 2000 such agents used in commerce, the vast majority of which are added at extremely low levels in food. In view of the fact that the majority of individual flavouring agents occur naturally in food, the Committee has concluded that to evaluate each of these substances by traditional toxicological testing is simply not warranted in most cases (WHO, 1987).
In 1987, WHO classified flavouring agents into four groups:
Since 1996, the Committee has evaluated the safety of approximately 1150 individual flavouring agents using the Procedure for the Safety Evaluation of Flavouring Agents (Annex 1, references 116 and 117 ). The Committe organized these agents into a number of well-defined chemical groups (i.e. congeneric groups) in order to effectively evaluate such a large number of substances. The evaluations conducted to date have demonstrated that flavouring agents within a congeneric group have a similar biochemical fate and toxicological potential. Most individual flavouring agents have been shown to be efficiently detoxicated to yield innocuous metabolites. Knowledge of their metabolic fate, coupled with low intake levels that are typically below thresholds of toxicological concern, has formed the basis for the majority of the Committee’s evaluations of individual flavouring agents. In instances where the metabolic fate of an individual flavouring agent was not well known or readily predictable, or where intake exceeded the threshold of toxicological concern for the relevant structural class, the Committee has relied on data on the toxicity of the agent itself or of a structurally related substance in order to perform a rigorous safety evaluation. The results of these evaluations comprise an extensive database, which has been published since 1996 in the WHO Food Additive Series as a series of monographs on congeneric groups.
A key development in the process by which the Committee evaluates the safety of flavouring agents has been the establishment of specifications, consistent with JECFA practice, for all flavouring agents. The criteria underpinning these specifications require that the chemical assay for individual flavouring agents be specified. The Committee has adopted the criterion of a minimum assay of 95% purity of the named flavouring agent. In some cases, flavouring agents have been reported to be of less than 95% purity. In such instances, the Committee has required the identification of the secondary components such that at least 95% of the chemical composition of the named flavouring agent can be accounted for. Key features of secondary components are that many are themselves flavouring agents that are likely have been evaluated previously by the Committee, as a member of a different congeneric group, or they are structurally related substances that belong to the same congeneric group as the named flavouring agent.
In order to provide for a rigorous safety evaluation when there are many secondary components, the Committee has collected and evaluated data on each of these components. This process has been facilitated by the fact that, since 1996, the Committee has reviewed and published monographs on most of the chemical groups of flavouring agents. Using these monographs on congeneric groups, the Committee now has the capacity to efficiently evaluate additional flavouring agents and numerous secondary components. An obvious extension of this process is that the Committee can begin to evaluate mixtures of flavouring agents, as found in natural flavouring complexes.
Natural flavouring complexes fall into the following categories:
These categories fall into groups (b), (c), and (d) listed above (WHO, 1987). The agents that comprise these categories range from those that consist almost entirely of a single chemical entity, such as bitter almond oil (benzaldehyde), to those with a highly complex composition (e.g. rosemary oil). In some instances, dozens of constituents are essential to the technical flavour characteristics of the natural flavouring complex. Although the chemical composition of these complexes is variable, their constituents can be assigned to relevant congeneric groups.
Table 1 gives examples of natural flavouring complexes and individual flavouring agents and provides an indication of the number of these substances that are used in commerce in the United States. Tables 2 and 3 list the constituents of two natural flavouring complexes, bois de rose oil and lemongrass oil, organized by congeneric group. While each of these complexes has numerous constituents, these constituents fit readily into congeneric groups, most of which have already been evaluated by the Committee.
Table 1. Examples of individual flavouring agents and natural flavouring complexes
|
Type of flavouring agents (number of agents of this type) |
Examples (No.) |
Chemical assay |
|
Individual flavouring agents with minimum assay value of >95% (1400) |
Cinnamyl alcohol (No. 647) |
Minimum assay of >95% (JECFA) |
|
Individual flavouring agents with minimum assay value of <95% (230) |
(E,R)-3,7-Dimethyl-1,5,7-octatrien-3-ol (No. 1154) |
Minimum assay of 93%; 3-5% linalool, and lesser quantities of linalool oxide and nerol oxide |
|
Natural flavouring complexes Essential oils (190) |
Wintergreen oil (Group (b))a |
FCC chemical assay: not less than 98% and not more than 100.5% methyl salicylate |
|
Lemongrass oil (Group (c))a |
FCC chemical assay: not less than 75%, by volume, of aldehyde as citral |
|
Lemon oil, distilled (Group (d))a |
FCC chemical assay: between 1% and 3.5% aldehydes, calculated as citral |
|
Extracts (100) |
Vanilla extract (Group (c))a |
— |
|
Oleoresins (30) |
Black pepper oleoresin (Group (c))a |
FCC chemical assay: piperine: not less than 36%; volatile oil content: between 15 ml and 35 ml/100 g. |
a Group as classified by WHO (1987) (see Introduction)
FCC, Food Chemical Codex
This monograph proposes that the existing Procedure for the Safety Evaluation of Flavouring Agents (Annex 1, references 116 and 117), as refined by the Committee (Annex 1, references 122, 131), be further modified to accommodate the safety evaluation of natural flavouring complexes. After consideration of an approach to determining specifications that could be used to specify a chemical assay for a natural flavouring complex, details of the suggested modifications to the existing Procedure in order to provide for the evaluation of natural flavouring complexes are outlined herein.
The Committee has always insisted upon specifications that define the substance being considered and, as previously noted, has typically required that a chemical assay for individual flavouring agents be at least 95%. In cases in which the named substance could not practically be freed from secondary components, the Committee has evaluated each secondary component individually. The emphasis throughout has been on assuring the identity and safety of the substance being evaluated.
In dealing with natural flavouring complexes, it may be useful to invoke compendia of specifications published by organizations that have previously considered these agents. The Food Chemicals Codex is one such organization, recognized by regulation in the United States and elsewhere. Food Chemicals Codex specifications typically list physiochemical properties, contaminants (including heavy metals and polynuclear aromatics) and microbiological parameters, to ensure that food ingredients, including flavouring agents, are safe for human consumption. For many natural flavouring complexes, Food Chemicals Codex also specifies a minimum chemical assay for key chemical constituents. For example, the chemical assay for caraway oil specifies that it should contain not less than 50%, by volume, of ketones calculated as carvone. The assay for peppermint oil is not less than 5% esters, calculated as menthyl acetate, and not less than 50% menthol. Clearly, flexibility is maintained in a chemical assay specified by Food Chemicals Codex in that a minimum level is specified for a chemical group that may be measured or calculated as a key chemical constituent in the natural flavouring complex. This approach could be used effectively to specify a chemical assay for natural flavouring complexes evaluated by the Committee.
To perform an evaluation, the Committee would organize the constituents of the natural flavouring complex into congeneric groups (e.g. phenols, tertiary alcohols, etc.) and perform a series of evaluations for the congeneric group. Within key congeneric groups (e.g. alicyclic secondary alcohols and ketones) selected constituents (e.g. menthol) will be associated with the technical flavouring characteristics of the natural flavouring complex (e.g. peppermint oil). In a manner entirely consistent with Food Chemicals Codex, the evaluation of congeneric groups within the complex could be linked by chemical assay to constituents that are fundamental to the flavouring characteristics of the natural flavouring complex.
An essential general principle is that the specification should be no more complex than is essential to assure the most critical aspects of safety, identity, and technical function. Determination of these key characteristics requires the use of methods shown to be reliable through collaborative studies. Existing relevant specifications from Food Chemicals Codex for bois de rose oil and lemongrass oil are provided in Tables 4 and 5 (Food Chemicals Codex, 1996).
The principles and procedures used by the Committee to estimate intake in the safety evaluation of individual flavouring agents are equally valid and appropriate for natural flavouring complexes, provided that the intended conditions of use are similar to those for individual flavouring agents. One obvious difference between individual flavouring agents and natural flavouring complexes is that some of the latter have much broader patterns of use in the food supply and much higher volumes of disappearance into the marketplace than do individual agents. Natural flavouring complexes such as vanilla extract and lemon oil are used in a wide variety of food categories that have high rates of consumption from products such as baked goods, beverages, soft and hard candy, and dairy products. Annual volumes of production for twelve natural flavouring complexes in use in the United States exceed 1 000 000 kg.
Table 2. Constituents of bois de rose oil, organized by congeneric groupa
|
Congeneric group (date of JECFA review) |
DT class |
FEMA No. |
CAS No. |
Constituent |
|
3 |
I |
2303 |
141-27-5 |
|
|
3 |
I |
2507 |
106-24-1 |
|
|
3 |
I |
2509 |
105-87-3 |
|
|
3 |
I |
2303 |
106-26-3 |
|
|
3 |
I |
2770 |
106-25-2 |
|
|
3 (1997, 2003) |
I |
|
|
alpha,beta-Unsaturated aliphatic primary alcohol/ aldehyde/ acid/acetal/ ester |
|
6 |
I |
|
29171-20-8 |
|
|
6 |
I |
|
20053-88-7 |
|
|
6 |
I |
|
|
|
|
6 |
I |
2635 |
78-70-6 |
|
|
6 |
I |
2772 |
7212-44-4 |
|
|
6 |
I |
|
5986-38-9 |
|
|
6 |
I |
|
|
|
|
6 |
I |
2248 |
562-74-3 |
|
|
6 |
I |
3045 |
98-55-5 |
|
|
6 |
I |
|
|
|
|
6 (1998) |
I |
|
|
Aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohol and related ester |
|
16 |
II |
2465 |
470-82-6 |
|
|
16 |
II |
3746 |
5989-33-3 |
|
|
16 |
II |
3746 |
56752-50-2 |
|
|
16 |
II |
3746 |
68892-15-9 |
|
|
16 |
II |
3735 |
7392-19-0 |
|
|
16 (2003) |
II |
|
|
|
|
31 |
I |
|
29350-73-0 |
|
|
31 |
I |
|
483-76-1 |
|
|
31 |
I |
|
39029-41-9 |
|
|
31 |
I |
2229 |
79-92-5 |
|
|
31 |
I |
2252 |
87-44-5 |
|
|
31 |
I |
|
3856-25-5 |
|
|
31 |
I |
|
|
|
|
31 |
I |
2356 |
99-87-6 |
|
|
31 |
I |
|
33880-83-0 |
|
|
31 |
I |
|
|
|
|
31 |
I |
2633 |
138-86-3 |
|
|
31 |
I |
2762 |
123-35-3 |
|
|
31 |
I |
3539 |
3338-55-4 |
|
|
31 |
I |
3539 |
3779-61-1 |
|
|
31 |
I |
|
555-10-2 |
|
|
31 |
I |
2902 |
127-91-3 |
|
|
31 |
I |
2903 |
80-56-8 |
|
|
31 |
I |
|
17066-67-0 |
|
|
31 |
I |
|
473-13-2 |
|
|
31 |
I |
|
|
|
|
31 |
I |
3046 |
586-62-9 |
|
|
31 (2004) |
I |
|
|
|
|
|
|
|
|
FEMA, Flavour and Extract Manufacturers Association; CAS, Chemical abstracts service |
|
|
Notes: |
|
|
The composition data cited above are derived from industry and from published literature. The data are representative of the NFC in commerce (1, 2 and, in some cases, 4) and various analytical data for studies on the impact of various factors (geography, maturity, storage, etc.) on the composition of the NFC (mainly 3). Typically, four types of analyses are available: |
|
|
1. |
Target analyses from industry: routine quality control analysis for key constituents responsible for technical flavour function |
|
2. |
Complete analyses from industry: complete analysis for all constituents in the NFC intended for commerce |
|
3. |
Target analyses from the literature: published limited analysis for constituents in the NFC on the basis of the objective |
|
4. |
Complete analyses from the literature: published complete analysis for all constituents in the NFC |
|
a |
For bois de rose oil, constituents identified in complete analyses account for >95% |
|
b |
Only data available from complete analyses are used in the safety evaluation procedure for the NFC |
|
c |
All references for data from industry (Ind. 1-8): Industry (1999-2002) Private communication to the Flavour and Extract Manufacturers Association, Washington DC |
|
d |
References for data from the published literature: |
|
1. |
Formacek, K. & Kubeczka, K.H. (1982) The chemical composition of commercial bois-de-rose oil. In: Essential oils analysis by capillary chromatography and carbon-13 NMR spectroscopy. New York: John Wiley and Sons |
|
2. |
Buccallato, F. (1988) Bois-de-rose oil: A glimpse into the past. Perfumer Flavorist, 13, 35-36 |
|
3. |
Lawrence, B. (1984) Bois-de-rose oil. Perfumer Flavorist 24, 63 |
Table 3. Constituents of lemongrass oil organized by congeneric groupa
|
Congeneric group (Date of JECFA review) |
DT class |
FEMA No. |
CAS No. |
Constituent |
|
1 |
I |
2362 |
112-31-2 |
|
|
1 |
I |
2792 |
124-13-0 |
|
|
1 (1996, 1997) |
I |
Straight-chain primary aliphatic alcohol/aldehyde/acid/acetal/ester |
||
|
3 |
I |
2303 |
141-27-5 |
|
|
3 |
I |
2307 |
106-24-1 |
|
|
3 |
I |
2509 |
105-87-3 |
|
|
3 |
I |
106-29-6 |
||
|
3 |
I |
105-86-2 |
||
|
3 |
I |
2303 |
106-26-3 |
|
|
3 |
I |
2770 |
106-25-2 |
|
|
3 |
I |
2773 |
141-12-8 |
|
|
3 (1997, 2003) |
I |
alpha,beta-Unsaturated aliphatic primary alcohol/aldehyde/acid/acetal/ester |
||
|
4 |
I |
2307 |
||
|
4 |
I |
2309 |
106-22-9 |
|
|
4 |
I |
2311 |
150-84-5 |
|
|
4 |
I |
106-72-9 |
||
|
4 |
I |
|||
|
4 |
I |
141-27-5 |
||
|
4 |
I |
|||
|
4 |
I |
50705-16-3 |
||
|
4 |
I |
112-54-9 |
||
|
4 |
I |
2401 |
68820-34-8 |
|
|
4 |
I |
55050-40-3 |
||
|
4 |
I |
55722-59-3 |
||
|
4 (1998) |
I |
|||
|
5 |
II |
409-02-9 |
||
|
5 |
II |
4485-09-0 |
||
|
5 |
I |
3388 |
593-08-8 |
|
|
5 |
I |
3093 |
112-12-9 |
|
|
5 (1998, 2002) |
II |
|||
|
6 |
I |
|||
|
6 |
I |
|||
|
6 |
I |
639-99-6 |
||
|
6 |
I |
473-16-5 |
||
|
6 |
I |
2635 |
78-70-6 |
|
|
6 |
I |
2772 |
40716-66-3 |
|
|
6 |
I |
2248 |
562-74-3 |
|
|
6 |
I |
3045 |
98-55-5 |
|
|
6 (1998) |
I |
|||
|
8 |
I |
2125 |
507-70-0 |
|
|
8 |
II |
2159 |
76-49-3 |
|
|
8 |
II |
2230 |
76-22-2 |
|
|
8 |
I |
2247 |
1197-06-4 |
|
|
8 |
I |
6753-98-6 |
||
|
8 |
II |
89-81-6 |
||
|
8 |
II |
546-80-5 |
||
|
8 |
II |
473-67-6 |
||
|
8 (2004) |
II |
Secondary alicyclic/saturated/unsaturated/ alcohol/ketone/ketal/ester |
||
|
31 |
I |
3331 |
495-62-5 |
|
|
31 |
I |
483-76-1 |
||
|
31 |
I |
39029-41-9 |
||
|
31 |
I |
2229 |
79-92-5 |
|
|
31 |
I |
2252 |
87-44-5 |
|
|
31 |
I |
3856-25-5u |
||
|
31 |
I |
|||
|
31 |
I |
2356 |
99-87-6 |
|
|
31 |
I |
515-13-9 |
||
|
31 |
I |
3839 |
18794-84-8 |
|
|
31 |
I |
23986-74-5 |
||
|
31 |
I |
1195-32-0 |
||
|
31 |
I |
2633 |
138-86-3 |
|
|
31 |
I |
2762 |
||
|
31 |
I |
3539 |
3779-61-1 |
|
|
31 |
I |
3539 |
3779-61-1 |
|
|
31 |
I |
2856 |
555-10-2 |
|
|
31 |
I |
2903 |
80-56-8 |
|
|
31 |
I |
3046 |
586-62-9 |
|
|
31 |
I |
|||
|
31 |
I |
508-32-7 |
||
|
31 |
I |
|||
|
31 |
I |
|||
|
31 (2004) |
I |
|||
|
32 |
III |
1139-30-6 |
||
|
32 |
III |
|||
|
32 |
III |
|||
|
32 |
III |
|||
|
32 |
III |
|||
|
32 (2005) |
III |
|||
Notes to Table 3
|
FEMA, Flavour and Extract Manufacturers Association; CAS, Chemical abstracts service |
|
|
Notes: |
|
|
The composition data cited above are derived from industry and from published literature. The data are representative of the NFC in commerce (1, 2 and, in some cases, 4) and various analytical data for studies on the impact of various factors (geography, maturity, storage, etc.) on the composition of the NFC (mainly 3). Typically, four types of analyses are available: |
|
|
1. |
Target analyses from industry: routine quality control analysis for key constituents responsible for technical flavour function; |
|
2. |
Complete analyses from industry: complete analysis for all constituents in the NFC intended for commerce; |
|
3. |
Target analyses from the literature: published limited analysis for constituents in the NFC on the basis of the objective; |
|
4. |
Complete analyses from the literature: published complete analysis for all constituents in the NFC. |
|
a |
For lemongrass oil, constituents identified in complete analyses account for >95% |
|
b |
Only data available from complete analyses are used in the safety evaluation procedure for the NFC (shaded columns) |
|
c |
References for data from industry (Ind. 1–4): Industry (1976–2003) Private communication to the Flavour and Extract Manufacturers Association, Washington D.C. |
|
d |
References for data from published literature: |
|
1. |
Chowdhury, J.U. et. al. (1998) Studies on the essential oil bearing plants of Bangladesh. Part IV. Composition of the leaf oils of three Cymbopogon species: C. flexuosus Wats. J. Essent. Oil Res., 10: 301–306. |
|
2. |
Chalchat, J.C. et. al. (1997) Correlation between chemical composition and antimicrobial activity. VI. Activity of some African essential oils. J. Essent. Oil Res. 9, 67–75. |
Table 4. Food Chemicals Codex specifications for bois de rose oil
|
NFC cluster |
Bois de rose |
|
Common name |
Rosewood |
|
Botanical family |
Lauraceae |
|
Genus and species |
Aniba rosaeodora Ducke |
|
Synonyms |
Aniba rosaeodora var. amazonica |
|
Geographical source |
Wood is collected in principally in Brazil and, to a lesser extent, in Peru |
|
Description of botanical source |
Tropical, medium-sized, wild-growing evergreen |
|
Degree of maturity |
Mature trunk wood |
|
FEMA No(s). |
2156 |
|
FDA citation(s) |
182.20 |
|
COE No(s). |
Not applicable |
|
Other information |
Use in formulations has become less attractive as environmental concerns have grown over the destructive nature of rosewood oil production in Brazil. |
|
Plant parts used |
Trunk wood |
|
Derivatives used (e.g. absolute, oil, extract, etc.) |
Essential oil (FEMA2156) |
|
Yield, %, based on original botanical |
Yields of oil vary according to wood feedstock quality and moisture content. Typical yields of oil are approximately 1% (w/w). |
|
Method of isolation |
Steam distillation of water soaked comminuted trunk wood. Distillation is carried out in mild or galvanized steel vessels that may vary in size from 200 to 1000 kg capacity of chips. Steam generation is by boiler fueled with spent chips. No further processing of the oil is carried out either by the primary distiller or by the end-user. |
|
Solvents used |
Not applicable |
|
Appearance |
Colourless or pale yellow liquid with a refreshing, sweet woody, linalool-like, somewhat floral-spicy odour. |
|
Angular rotation |
FCC: -4 to+6° |
|
Heavy metals Specific gravity |
FCC: passes test (as lead, Pb) |
|
Refractive index |
FCC: 1.462-1.470 at 20 °C |
|
Distillation range Solubility in alcohol |
FCC: Not less than 70% distills between 195 and 205°C |
|
Solubility in water |
Very soluble |
|
Solubility in other solvents |
FCC: Soluble in mineral oil, most fixed oils and propylene glycol Slightly soluble in glycerin |
|
Moisture |
Not applicable |
|
Major components assay (if applicable) |
FCC: Total alcohols 82-92%, calculated as linalool |
|
Recommended JECFA specifications for chemical assay |
Not less than 80% and not more than 95% tertiary terpenoid alcohols and related esters, calculated as linalool (decision-tree class I). |
COE, Council of Europe; FCC, Food chemicals Codex; FEMA, Flavour and Extracts Manufacturers Association; ISO, International Organization for Standardization
Table 5. Food Chemicals Codex specifications for lemongrass oil
|
NFC cluster |
Lemongrass |
|
Common name |
Lemongrass |
|
Botanical family |
Gramineae |
|
Genus and species |
Cymbopogon flexuosus (Nees.) Stapf ("East Indian type") |
|
Synonyms |
Citral terpenes |
|
Geographical source |
India, West Africa, Central America, West Indies, South America |
|
Description of botanical source |
Cultivated herbaceous grass |
|
Degree of maturity |
Mature fresh or partly dried leaves |
|
FEMA No(s). |
2624 |
|
FDA citations(s). |
182.20 |
|
COE No(s). |
CE38 |
|
Other information: |
Lemongrass oil has one of the largest annual volumes of production of all essential oils in the world. Most of the volume produced is used for the production of citral. |
|
Plant parts used |
Leaves |
|
Derivatives used (e.g. absolute, oil, extract, etc.) |
Oil |
|
Yield, %, based on original botanical |
0.2-0.4% |
|
Method of isolation |
Steam distillation |
|
Solvents class used |
No solvents |
|
Cymbopogon flexuosus |
|
|
Appearance |
ISO: Pale yellow to yellowish brown, mobile liquid with odour resembling that of citral |
|
Optical rotation |
ISO: -3 to+1° at 20 °C |
|
Heavy metals |
FCC: passes test |
|
Specific gravity |
ISO: 0.885-0.905 at 20/20 °C |
|
Refractive index |
ISO: 1.4830-1.4890 at 20 °C |
|
Distillation range |
Not applicable |
|
Solubility in alcohol |
ISO: Miscible 1:3 in 70% (v/v) ethanol at 20 °C |
|
Solubility in water |
Not available |
|
Solubility in other solvents |
Not available |
|
Moisture |
Not available |
|
Major components assay (if applicable) |
ISO: Carbonyl content (as citral) 73% min |
|
Other |
ISO: Residue from vacuum distillation: 10% (m/m) max. |
|
Cymbopogon citrates |
|
|
Appearance |
ISO: Pale yellow to orange-yellow, mobile liquid with characteristic strong note of citral. |
|
Optical rotation |
ISO: -3 to +1 at 20°C |
|
Heavy metals |
FCC: passes test |
|
Specific gravity |
ISO: 0.872-0.897 at 20/20°C |
|
Refractive index |
ISO: 1.4830-1.4890 at 20°C |
|
Distillation range |
Not applicable |
|
Solubility in alcohol |
ISO: Soluble in 70% (v/v) ethanol at 20°C |
|
Solubility in water |
Not available |
|
Solubility in other solvents |
Not available |
|
Moisture |
Not available |
|
Major components assay (if applicable) |
ISO: Carbonyl content, as citral: 75% min. |
|
Other |
FCC: Steam-volatile oil 93.0% min. |
|
Recommended JECFA specifications for chemical assay |
Not less than 70% and not more than 90% primary terpenoid alcohols, aldehydes, carboxylic acids, and related esters, calculated as citral (decision-tree class I). |
COE, Council of Europe; FCC, Food Chemical Codex; FDA, Food and Drug Administration; ISO, International Organization for Standardization
As has been common practice by the Committee since 1996, intake of individual flavouring agents is based on a conservative estimate, with the assumption that only 10% of the population consumes all of the substance as a flavouring agent, Clearly the use of natural flavouring complexes, such as vanilla extract and lemon oil, that are used at high volume is not limited to 10% of the population. It is recommended that in the case of natural flavouring complexes that are used at high volume (e.g. >100 000 kg/year) that the Committee use the actual percentage of users as estimated from databases on food intake. This would provide more realistic estimates of daily intake.
The data on poundage and intake for bois de rose oil and lemongrass oil are show in Table 6. For these natural flavouring complexes, it is valid to estimate daily intake using the principles and procedures that are used for individual flavouring agents.
Exposure to constituents of the natural flavouring complex in the various con-generic groups does not solely occur via intake of the complex. These constituents, mainly terpenes, are also present as common components of many traditional foods. In fact, the majority of terpenes that are constituents of a natural flavouring complex are also consumed in the normal diet. For example, exposure to linalool, limonene, linalyl acetate and other terpenes in bois de rose oil also occurs by daily consumption of spices (e.g. coriander), fruits (e.g. oranges), wine, and certain vegetables. Exposure to the constituents found in bois de rose oil occurs mainly from consumption of a normal diet. This is to be expected, given the ubiquitous presence of simple monoterpenes in all plants.
Table 6. Poundage and intake data for bois de rose oil and lemongrass oil
|
Bois de rose oil |
Lemongrass oil |
|
USA annual poundage (1995) |
2902 kg |
1470 kg |
|
Per capita intake, USA |
38 mg/person per day |
19 mg/person |
|
"Eaters only", per capita intake, USA × 10 |
380 mg/person per day |
194 mg/person |
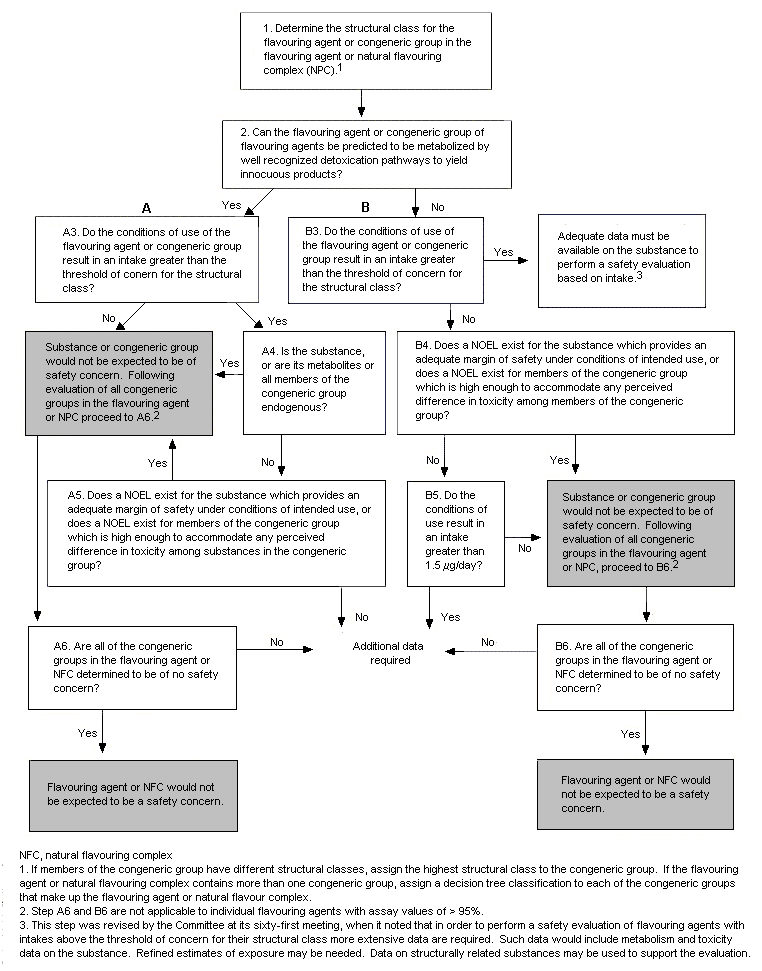
It is recommended that the current Procedure for the Safety Evaluation of Flavouring Agents be expanded to include the option to evaluate both individual flavouring agents and natural flavouring complexes. Like individual flavouring agents, flavouring agents present in a natural flavouring complex will be assigned to the appropriate congeneric groups. Subsequently, each congeneric group will be evaluated according to the current steps in the Procedure (see Figure 1). Once all congeneric groups contained within the natural flavouring complex have been evaluated, a conclusion can be made concerning the safety of the combination of congeneric groups that constitute the complex. This modification to the existing Procedure to allow the inclusion of assessments for congeneric groups will permit the evaluation of all types of flavouring agents, including relatively pure individual flavouring agents (chemical assay >95%), agents containing appreciable amounts of secondary components minimum chemical assay, <95%), and natural flavouring complexes containing different congeneric groups of individual flavouring agents. The proposed modification to the Procedure constitutes an efficient use of resources because it builds upon the evaluations previously conducted and relies on the extensive series of monographs on well-defined congeneric groups of flavouring agents already published by the Committee.

Figure 1. Procedure for the Safety Evaluation of Flavouring Agents and Natural Flavouring Complexes
As a test, two natural flavouring complexes, bois de rose oil and lemongrass oil, were evaluated using the modified Procedure. The results of these evaluations (shown in Table 7 and Table 8, respectively) indicate that at current levels of intake these natural flavouring complexes would not be expected to present a safety concern.
Table 7. Safety evaluation of bois de rose oila,b
|
Flavouring agent or congeneric group of NFC |
Congeneric group number |
|
Structural class I |
|
|
Aliphatic, alicyclic, and aromatic saturated and unsaturated tertiary alcohols and esters |
6 |
|
31 |
|
|
3 |
|
|
Structural class II |
|
|
16 |
|
NFC, natural flavouring complex; NR, not required for evaluation because consumption of the substance was determined to be of no safety concern at step A3 of the decision-tree |
|
|
a |
If members of the congeneric group have different structural classes, assign the highest structural class to the congeneric group. If the flavouring agent or natural flavouring complex contains more than one congeneric group, assign a decision-tree classification to each of the congeneric groups that make up the flavouring agent or natural flavour complex |
|
b |
Step 2: All of the congeneric groups in this NFC are expected to be metabolized to innocuous products. The evaluation of these congeneric groups therefore proceeded via the A-side of the decision-tree |
|
c |
The thresholds for human intake for structural classes I and II are 1800 and 540 µg/day, respectively. Daily intake for each congeneric group was calculated by taking the highest analytical value (%) from the complete analyses and multiplying by the daily per capita intake ("eaters only") of the NFC. The daily per capita intake ("eaters only") is 380 µg/person per day from use of bois de rose oil as a flavouring agent in the USA |
|
Notes: |
|
|
1. |
To be evaluated by the Committee in 2004: aliphatic and aromatic hydrocarbons (Group 2) |
|
2. |
To be evaluated by the Committee in 2004: a,b-unsaturated aldehydes (Group 3) |
|
3. |
To be evaluated by the Committee in 2004: monocyclic and bicyclic secondary alcohols and ketones (Group 4) |
|
4. |
Evaluated by the Committee in 2003 (WHO Food Additives Series 52, in preparation): aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters |
|
5. |
Evaluated by the Committee in 2003 (WHO Food Additives Series 52, in preparation): aliphatic and aromatic ethers |
Table 8. Safety evaluation of lemongrass oila,b
|
|
Congeneric group number |
|
Structural class I |
|
|
3 |
|
|
31 |
|
|
Unsaturated straight and branched chain ali primary alcohols, aldehydes, acids, acetals and esters |
4 |
|
Straight chain aliphatic primary alcohols, aldehydes, acids, acetals and esters |
1 |
|
Structural class II |
|
|
Aliphatic, alicyclic secondary alcohols, ketones, ketals and esters |
5 |
|
Aliphatic, alicyclic and aromatic tertiary alcohols and esters |
6 |
|
Secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters |
8 |
|
Structural class III |
|
|
32 |
Notes to Table 8
|
NR, not required for evaluation because consumption of the substance was determined to be of no safety concern at step A3 or B3 of the decision-tree; NFC, natural flavouin complex |
|
|
a |
If members of the congeneric group have different structural classes, assign the highest structural class to the congeneric group. If the flavouring agent or natural flavouring complex contains more than one congeneric group, assign a decision-tree classification to each of the congeneric groups that make up the flavouring agent or natural flavour complex |
|
b |
Step 2: Seven congeneric groups in this natural flavouring complex are expected to be metabolized to innocuous products. The evaluation of these congeneric groups therefore proceeded via the A-side of the decision-tree. Congeneric group 32, epoxides, is in structural class III; and limited metabolic data exists for this congeneric group. The evaluation of this congeneric group therefore proceeded via the B-side of the decision-tree |
|
c |
The thresholds for human intake for structural classes I, II and III are 1800, 540 and 90 µg/day, respectively. All intake values are expressed in mg/person per day. Daily intake for each congeneric group was calculated by taking the highest analytical value (%) from the complete analyses and multiplying by the daily per capita intake ("eaters only") of the natural flavouring complex. The daily per capita intake ("eaters only") is 194 µg/person per day from use of lemongrass oil as a flavouring agent in the USA |
|
Notes: |
|
|
1. |
To be evaluated by the Committee in 2004: aliphatic and aromatic hydrocarbons (Group 2) |
|
2. |
To be evaluated by the Committee in 2004: a,b-unsaturated aldehydes (Group 3) |
|
3. |
To be evaluated by the Committee in 2004: monocyclic and bicyclic secondary alcohols and ketones (Group 4) |
|
4. |
Evaluated by the Committee in 2003 (WHO Food Additives Series 52, in preparation): aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters |
|
5. |
To be evaluated by the Committee in 2005 |
Procedures that allow the Committee to evaluate individual flavouring agents as well as natural flavouring complexes are already in place. The examples provided indicate that the modified Procedure for the Safety Evaluation of Flavouring Agents also works for natural flavouring complexes. It is recommended that the Committee should evaluate several natural flavouring complexes using the modified Procedure at a future meeting.
Food Chemicals Codex (1996) Food Chemicals Codex, 4th Ed., Washington: National Academy Press.
WHO (1987) Principles for the Safety Assessment of Food Additives and Contaminants in Food (Environmental Health Criteria, No. 70), Geneva.
Acknowledgement
The authors offer special thanks to Dr Timothy Adams and Ms Christie Gavin of the Flavor and Extract Manufacturers of the United States for providing analytical data on flavour composition and for many useful ideas in the formulation of the modified Procedure for the Safety Evaluation Of Flavouring Agents.
See Also:
Toxicological Abbreviations