
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
ALDRIN
Chemical name
1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-endo-1,4-exo-
5,8-dimethanonaphthalene;
1,2,3,4,10,10-hexachloro-1-4-4a,5,8,8a-hexahydro-exo-1,4,endo-5,8-
dimethanonaphthalene.
Synonyms
HHDN, Octalene
Empirical formula
C12H8Cl6
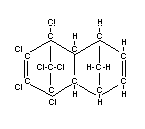
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1956; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
(Lehman, 1956), but this storage ratio decreases rapidly to less than
one with an intake of 50 ppm.
Aldrin is largely and readily converted in the animal body,
especially in the liver, to dieldrin (Bann et al., 1956; Ivey et al.,
1961; Treon & Cleveland, 1955). The rate of change has not been fully
established, and is independent of the site of entrance into the body.
The dieldrin is stored without further change and may be recovered as
such from animal products and tissues, including the eggs of fowls and
the milk of dairy cows within 24 hours after ingestion (Bann et al.,
1956). Fifteen minutes after an intravenous injection of 14C aldrin,
aldrin and its metabolites were found in the bile (Morsdorf et al.,
1963).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse Oral 44 Borgmann et al., 1952
Council of Europe, 1962
Rat, male Oral 38-54 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Council of Europe, 1962
Guinea-pig Oral 33 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 50-80 Borgmann et al., 1952
Council of Europe, 1962
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Council of Europe, 1962
Man. A 23-year-old man intentionally drank a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria and
albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level.
In a feeding study lasting from 6 to 7 months, dosage levels of
5, 10 and 25 ppm aldrin were used with groups of 5 females. No
enlargement of the liver or other gross change was noted. Histological
data are not described. In a 9-month feeding experiment, with 20
female rats per group, the dosage levels were 5, 15, 25 and 45 ppm of
aldrin. There was an increase in the liver body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 25 female rats were fed diets 5, 10 and 20 ppm of
recrystallized aldrin for 64 weeks. The group on 20 ppm showed an
increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
Groups of 5 animals of each were given 2.5, 5, 25, 75 or 300 ppm
of either purified or technical aldrin in the diet for 26 weeks. Two
rats of each group were killed before the end of the treatment, and
the last three were killed before the thirty-seventh week. All the
animals receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate
was good. Liver/body-weight ratio was increased in males at 25 ppm and
in both sexes at 75 and 300 ppm. Swelling of centrolobular liver cells
with peripheral distribution of the cytoplasmic granules were often
seen. At 2.5 and 5 ppm these changes were seen with the same frequency
as in the controls. They were markedly obvious at 25 ppm and over, but
regressed after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following
concentrations of 5 ppm of aldrin in the diet (Dewitt, 1955).
Dog. When dogs were fed, for 5 or 6 days per week, diets
containing 10 to 30 ppm of aldrin, death occurred after periods of
feeding ranging from a few days to about 7 months. Three groups of
suckling puppies (11 days old), 3 each group comprising 2 males and 1
female were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5
days per week. All the animals died within 38 days. A 2-month-old male
and a female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6
and 2.0 mg of recrystallized aldrin per kg of body-weight daily for
one year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of aldrin
for their life-span (maximum 2 years). The aldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. In a 2-year feeding experiment, groups of 20 rats (10 male
and 10 female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 50, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male
and 40 female) was given 2.5, 12.5 and 25 ppm of recrystallized
aldrin. There was a questionable increase in mortality rate at the 25
ppm level in females. Significant increase in the liver/body-weight
ratio occurred in males at all levels and at 12.5 and 25 ppm in
females. Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male
and 12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25
ppm for three generations: at 12.5 and 25 ppm the number of
pregnancies was reduced. The incorporation of aldrin into the diets of
lactating females has a "slight to moderate" effect on mortality among
the offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments on the experimental studies reported
The primary mode of action of aldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and,
in this respect, young dogs are more susceptible than rats.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased on a dosage of 10 ppm in one strain of mice susceptible to
the development of these tumours.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorigenic properties of aldrin could be related to a
general type of effect.
EVALUATION
Level causing no significant toxicological effect
In the rat and the dog a no-effect level has not been
demonstrated.
Acceptable daily intake for man
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of aldrin for man is kept
at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Kettering Lab., University of
Cincinnati Report, March.
Council of Europe (1962) Agricultural pesticides, Strasbourg
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol. 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxic., 2, 551
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 11, 378
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Morsdorf, K. et al. (1963) Med. Exp., 8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C. et al. (1957) Proc. West. Sec. Amer. Soc. Anim.
Prod.,44 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F. et al. (1951) Unpublished report from Kettering Lab.,
University of Cincinnati
Treon, J. F. et al. (1955) Kettering Lab., University of Cincinnati
Report, February
BIOLOGICAL DATA
Biochemical aspects
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1956; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
(Lehman, 1956), but this storage ratio decreases rapidly to less than
one with an intake of 50 ppm.
Aldrin is largely and readily converted in the animal body,
especially in the liver, to dieldrin (Bann et al., 1956; Ivey et al.,
1961; Treon & Cleveland, 1955). The rate of change has not been fully
established, and is independent of the site of entrance into the body.
The dieldrin is stored without further change and may be recovered as
such from animal products and tissues, including the eggs of fowls and
the milk of dairy cows within 24 hours after ingestion (Bann et al.,
1956). Fifteen minutes after an intravenous injection of 14C aldrin,
aldrin and its metabolites were found in the bile (Morsdorf et al.,
1963).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse Oral 44 Borgmann et al., 1952
Council of Europe, 1962
Rat, male Oral 38-54 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Council of Europe, 1962
Guinea-pig Oral 33 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 50-80 Borgmann et al., 1952
Council of Europe, 1962
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Council of Europe, 1962
Man. A 23-year-old man intentionally drank a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria and
albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level.
In a feeding study lasting from 6 to 7 months, dosage levels of
5, 10 and 25 ppm aldrin were used with groups of 5 females. No
enlargement of the liver or other gross change was noted. Histological
data are not described. In a 9-month feeding experiment, with 20
female rats per group, the dosage levels were 5, 15, 25 and 45 ppm of
aldrin. There was an increase in the liver body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 25 female rats were fed diets 5, 10 and 20 ppm of
recrystallized aldrin for 64 weeks. The group on 20 ppm showed an
increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
Groups of 5 animals of each were given 2.5, 5, 25, 75 or 300 ppm
of either purified or technical aldrin in the diet for 26 weeks. Two
rats of each group were killed before the end of the treatment, and
the last three were killed before the thirty-seventh week. All the
animals receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate
was good. Liver/body-weight ratio was increased in males at 25 ppm and
in both sexes at 75 and 300 ppm. Swelling of centrolobular liver cells
with peripheral distribution of the cytoplasmic granules were often
seen. At 2.5 and 5 ppm these changes were seen with the same frequency
as in the controls. They were markedly obvious at 25 ppm and over, but
regressed after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following
concentrations of 5 ppm of aldrin in the diet (Dewitt, 1955).
Dog. When dogs were fed, for 5 or 6 days per week, diets
containing 10 to 30 ppm of aldrin, death occurred after periods of
feeding ranging from a few days to about 7 months. Three groups of
suckling puppies (11 days old), 3 each group comprising 2 males and 1
female were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5
days per week. All the animals died within 38 days. A 2-month-old male
and a female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6
and 2.0 mg of recrystallized aldrin per kg of body-weight daily for
one year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of aldrin
for their life-span (maximum 2 years). The aldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. In a 2-year feeding experiment, groups of 20 rats (10 male
and 10 female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 50, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male
and 40 female) was given 2.5, 12.5 and 25 ppm of recrystallized
aldrin. There was a questionable increase in mortality rate at the 25
ppm level in females. Significant increase in the liver/body-weight
ratio occurred in males at all levels and at 12.5 and 25 ppm in
females. Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male
and 12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25
ppm for three generations: at 12.5 and 25 ppm the number of
pregnancies was reduced. The incorporation of aldrin into the diets of
lactating females has a "slight to moderate" effect on mortality among
the offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments on the experimental studies reported
The primary mode of action of aldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and,
in this respect, young dogs are more susceptible than rats.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased on a dosage of 10 ppm in one strain of mice susceptible to
the development of these tumours.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorigenic properties of aldrin could be related to a
general type of effect.
EVALUATION
Level causing no significant toxicological effect
In the rat and the dog a no-effect level has not been
demonstrated.
Acceptable daily intake for man
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of aldrin for man is kept
at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Kettering Lab., University of
Cincinnati Report, March.
Council of Europe (1962) Agricultural pesticides, Strasbourg
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol. 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxic., 2, 551
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 11, 378
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Morsdorf, K. et al. (1963) Med. Exp., 8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C. et al. (1957) Proc. West. Sec. Amer. Soc. Anim.
Prod.,44 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F. et al. (1951) Unpublished report from Kettering Lab.,
University of Cincinnati
Treon, J. F. et al. (1955) Kettering Lab., University of Cincinnati
Report, February
 BIOLOGICAL DATA
Biochemical aspects
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1956; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
(Lehman, 1956), but this storage ratio decreases rapidly to less than
one with an intake of 50 ppm.
Aldrin is largely and readily converted in the animal body,
especially in the liver, to dieldrin (Bann et al., 1956; Ivey et al.,
1961; Treon & Cleveland, 1955). The rate of change has not been fully
established, and is independent of the site of entrance into the body.
The dieldrin is stored without further change and may be recovered as
such from animal products and tissues, including the eggs of fowls and
the milk of dairy cows within 24 hours after ingestion (Bann et al.,
1956). Fifteen minutes after an intravenous injection of 14C aldrin,
aldrin and its metabolites were found in the bile (Morsdorf et al.,
1963).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse Oral 44 Borgmann et al., 1952
Council of Europe, 1962
Rat, male Oral 38-54 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Council of Europe, 1962
Guinea-pig Oral 33 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 50-80 Borgmann et al., 1952
Council of Europe, 1962
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Council of Europe, 1962
Man. A 23-year-old man intentionally drank a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria and
albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level.
In a feeding study lasting from 6 to 7 months, dosage levels of
5, 10 and 25 ppm aldrin were used with groups of 5 females. No
enlargement of the liver or other gross change was noted. Histological
data are not described. In a 9-month feeding experiment, with 20
female rats per group, the dosage levels were 5, 15, 25 and 45 ppm of
aldrin. There was an increase in the liver body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 25 female rats were fed diets 5, 10 and 20 ppm of
recrystallized aldrin for 64 weeks. The group on 20 ppm showed an
increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
Groups of 5 animals of each were given 2.5, 5, 25, 75 or 300 ppm
of either purified or technical aldrin in the diet for 26 weeks. Two
rats of each group were killed before the end of the treatment, and
the last three were killed before the thirty-seventh week. All the
animals receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate
was good. Liver/body-weight ratio was increased in males at 25 ppm and
in both sexes at 75 and 300 ppm. Swelling of centrolobular liver cells
with peripheral distribution of the cytoplasmic granules were often
seen. At 2.5 and 5 ppm these changes were seen with the same frequency
as in the controls. They were markedly obvious at 25 ppm and over, but
regressed after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following
concentrations of 5 ppm of aldrin in the diet (Dewitt, 1955).
Dog. When dogs were fed, for 5 or 6 days per week, diets
containing 10 to 30 ppm of aldrin, death occurred after periods of
feeding ranging from a few days to about 7 months. Three groups of
suckling puppies (11 days old), 3 each group comprising 2 males and 1
female were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5
days per week. All the animals died within 38 days. A 2-month-old male
and a female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6
and 2.0 mg of recrystallized aldrin per kg of body-weight daily for
one year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of aldrin
for their life-span (maximum 2 years). The aldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. In a 2-year feeding experiment, groups of 20 rats (10 male
and 10 female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 50, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male
and 40 female) was given 2.5, 12.5 and 25 ppm of recrystallized
aldrin. There was a questionable increase in mortality rate at the 25
ppm level in females. Significant increase in the liver/body-weight
ratio occurred in males at all levels and at 12.5 and 25 ppm in
females. Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male
and 12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25
ppm for three generations: at 12.5 and 25 ppm the number of
pregnancies was reduced. The incorporation of aldrin into the diets of
lactating females has a "slight to moderate" effect on mortality among
the offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments on the experimental studies reported
The primary mode of action of aldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and,
in this respect, young dogs are more susceptible than rats.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased on a dosage of 10 ppm in one strain of mice susceptible to
the development of these tumours.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorigenic properties of aldrin could be related to a
general type of effect.
EVALUATION
Level causing no significant toxicological effect
In the rat and the dog a no-effect level has not been
demonstrated.
Acceptable daily intake for man
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of aldrin for man is kept
at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Kettering Lab., University of
Cincinnati Report, March.
Council of Europe (1962) Agricultural pesticides, Strasbourg
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol. 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxic., 2, 551
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 11, 378
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Morsdorf, K. et al. (1963) Med. Exp., 8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C. et al. (1957) Proc. West. Sec. Amer. Soc. Anim.
Prod.,44 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F. et al. (1951) Unpublished report from Kettering Lab.,
University of Cincinnati
Treon, J. F. et al. (1955) Kettering Lab., University of Cincinnati
Report, February
BIOLOGICAL DATA
Biochemical aspects
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1956; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
(Lehman, 1956), but this storage ratio decreases rapidly to less than
one with an intake of 50 ppm.
Aldrin is largely and readily converted in the animal body,
especially in the liver, to dieldrin (Bann et al., 1956; Ivey et al.,
1961; Treon & Cleveland, 1955). The rate of change has not been fully
established, and is independent of the site of entrance into the body.
The dieldrin is stored without further change and may be recovered as
such from animal products and tissues, including the eggs of fowls and
the milk of dairy cows within 24 hours after ingestion (Bann et al.,
1956). Fifteen minutes after an intravenous injection of 14C aldrin,
aldrin and its metabolites were found in the bile (Morsdorf et al.,
1963).
Acute toxicity
Animal Route LD50 mg/kg Reference
body-weight
Mouse Oral 44 Borgmann et al., 1952
Council of Europe, 1962
Rat, male Oral 38-54 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Council of Europe, 1962
Guinea-pig Oral 33 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 50-80 Borgmann et al., 1952
Council of Europe, 1962
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Council of Europe, 1962
Man. A 23-year-old man intentionally drank a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria and
albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level.
In a feeding study lasting from 6 to 7 months, dosage levels of
5, 10 and 25 ppm aldrin were used with groups of 5 females. No
enlargement of the liver or other gross change was noted. Histological
data are not described. In a 9-month feeding experiment, with 20
female rats per group, the dosage levels were 5, 15, 25 and 45 ppm of
aldrin. There was an increase in the liver body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 25 female rats were fed diets 5, 10 and 20 ppm of
recrystallized aldrin for 64 weeks. The group on 20 ppm showed an
increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
Groups of 5 animals of each were given 2.5, 5, 25, 75 or 300 ppm
of either purified or technical aldrin in the diet for 26 weeks. Two
rats of each group were killed before the end of the treatment, and
the last three were killed before the thirty-seventh week. All the
animals receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate
was good. Liver/body-weight ratio was increased in males at 25 ppm and
in both sexes at 75 and 300 ppm. Swelling of centrolobular liver cells
with peripheral distribution of the cytoplasmic granules were often
seen. At 2.5 and 5 ppm these changes were seen with the same frequency
as in the controls. They were markedly obvious at 25 ppm and over, but
regressed after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following
concentrations of 5 ppm of aldrin in the diet (Dewitt, 1955).
Dog. When dogs were fed, for 5 or 6 days per week, diets
containing 10 to 30 ppm of aldrin, death occurred after periods of
feeding ranging from a few days to about 7 months. Three groups of
suckling puppies (11 days old), 3 each group comprising 2 males and 1
female were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5
days per week. All the animals died within 38 days. A 2-month-old male
and a female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6
and 2.0 mg of recrystallized aldrin per kg of body-weight daily for
one year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of aldrin
for their life-span (maximum 2 years). The aldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. In a 2-year feeding experiment, groups of 20 rats (10 male
and 10 female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 50, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male
and 40 female) was given 2.5, 12.5 and 25 ppm of recrystallized
aldrin. There was a questionable increase in mortality rate at the 25
ppm level in females. Significant increase in the liver/body-weight
ratio occurred in males at all levels and at 12.5 and 25 ppm in
females. Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male
and 12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25
ppm for three generations: at 12.5 and 25 ppm the number of
pregnancies was reduced. The incorporation of aldrin into the diets of
lactating females has a "slight to moderate" effect on mortality among
the offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments on the experimental studies reported
The primary mode of action of aldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and,
in this respect, young dogs are more susceptible than rats.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased on a dosage of 10 ppm in one strain of mice susceptible to
the development of these tumours.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorigenic properties of aldrin could be related to a
general type of effect.
EVALUATION
Level causing no significant toxicological effect
In the rat and the dog a no-effect level has not been
demonstrated.
Acceptable daily intake for man
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of aldrin for man is kept
at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Kettering Lab., University of
Cincinnati Report, March.
Council of Europe (1962) Agricultural pesticides, Strasbourg
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol. 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxic., 2, 551
Gaines, Th. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 11, 378
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Morsdorf, K. et al. (1963) Med. Exp., 8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C. et al. (1957) Proc. West. Sec. Amer. Soc. Anim.
Prod.,44 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F. et al. (1951) Unpublished report from Kettering Lab.,
University of Cincinnati
Treon, J. F. et al. (1955) Kettering Lab., University of Cincinnati
Report, February
