
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
ALDRIN
IDENTITY
Synonyms
HHDN, Octalene(R)
Explanation
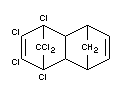
Aldrin is a technical product containing at least 95 per cent of HHDN
of which the composition is as follows:
1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-endo-1,4-exo-5,8-
dimethanonaphthalene;
1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-exo-1,4-endo-5,8-
dimethanonaphthalene.
Formula
 DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-aldrin was applied to growing cultures of Aspergillus and
Penicillium species, dieldrin and more hydrophilic metabolites were
found in the culture medium and in the mycelium. Mosquito larvae
(Aedes aegypti) cultivated in an aqueous medium to which
14C-aldrin is added, can convert this compound to hydrophilic
metabolites (Ludwig et al., 1966).
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1966; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
but this ratio decreases rapidly to less than one with an intake of 50
ppm (Lehman, 1956).
The aldrin content of the blood plasma of men occupationally exposed
for 259-6044 hours over a period of eight years ranged between 0.0007
and 0.0023 ppm and was directly proportional to the duration of
exposure. Aldrin added to human serum could be only partially
recovered, probably because of in vitro interaction or binding with
some constituent of serum such as lipoproteins. The plasma of an adult
male about 18 hours after ingestion of aldrin and 10 hours after the
last convulsion contained 0.036 ppm aldrin and 0.279 dieldrin. Twenty
days later these concentrations were 0.0018 and 0.090 ppm respectively
(Dale et al., 1966).
Aldrin is largely and readily converted in the animal body, especially
in the liver, to dieldrin (Bann et al., 1956; Ivey et al., 1961; Treon
& Cleveland, 1955). The rate of change has not been fully established,
and is independent of the site of entrance into the body. The dieldrin
is stored without further change and may be recovered as such from
animal products and tissues, including the eggs of fowls and the milk
of dairy cows within 24 hours after ingestion (Bann et al., 1956).
Fifteen minutes after an intravenous injection of 14C-aldrin, aldrin
and its metabolites were found in the bile (Mörsdorf et al., 1963).
In vitro epoxidation by rat liver microsomes was found to be about
10 times greater in males than in females (Wong & Terriere, 1965).
Rats were fed 1 and 25 ppm of aldrin for 120 days; two metabolites
were found in the urine, one of which was much more abundant in males
than in females (Datta et al., 1965).
Experiments with rats showed that 14C-aldrin given intravenously was
converted in 24 hours mainly into hydrophilic metabolites. After 48
hours the presence of these metabolites could be demonstrated in most
organs and tissues. In the urine of rabbits given 14C-aldrin
intravenously, the main metabolite could be identified as one of the
two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The oral
LD50 of this compound in mice is 1250 mg/kg bodyweight, and the
intravenous LD50 is 51 mg/kg body-weight. It was found that after
intravenous injection of labelled aldrin in rats, radioactive products
could be demonstrated in the bile within one hour. In 4 hours, 16.2
per cent of the radioactivity was excreted in the bile. Most of the
radioactivity was in the form of hydrophilic products. Perfusion tests
on rat livers showed conversion of aldrin into dieldrin. From these
results it was concluded that the conversion of the insecticide does
in fact take place in the animal organism (Ludwig et al., 1966).
Studies in rats with 14C-aldrin have shown that with daily
administration of a constant dose of aldrin (4.3 µg per animal,
calculated to be equivalent to 0.2 ppm in the diet) a saturation
equilibrium is reached after a certain time (in male rats after about
50 days; whereas in females this saturation equilibrium is reached
after as long as 200 days). After discontinuation of the daily oral
administration, the compound had a biological half-life period of
10-11 days in male rats and 100 days in female rats. In faeces and
urine, 70 and 95 per cent respectively of the radioactivity was in the
form of hydrophilic metabolites. The conversion and excretion rate in
female rats was lower at the same dose levels than in the male rats.
The radioactivity found in the tissues of female animals was about
double that found in male rats. Remarkable differences in the
concentration of metabolites in lung, liver, spleen and kidney were
found; in the female rats, the content of hydrophilic metabolites was
lower and the dieldrin content higher than in the males (Ludwig et
al., 1966).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Mouse Oral 44 Borgmann et al., 1952
Rat, male Oral 38-54 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Barnes, J. M., 1957
Guinea-pig Oral 33 Borgmann et al., 1952
Rabbit Oral 50-80 Borgmann et al., 1952
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Man A 25-year-old man intentionally ingested a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria
and albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level (Borgmann et al., 1952).
In a feeding study lasting from 6 to 7 months, dosage levels of 5, 10
and 25 ppm aldrin were used with groups of 5 females. No enlargement
of the liver or other gross change was noted. Histological data are
not described. In a 9-month feeding experiment, with 20 female rats
per group, the dosage levels were 5, 15, 25 and 45 ppm of aldrin.
There was an increase in the liver/body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 5 animals of each sex were given 2.5, 5, 25, 75 or 300 ppm
of purified or technical aldrin in the diet for 26 weeks. Two rats of
each group were killed before the end of the treatment, and the last
three were killed before the thirty-seventh week. All the animals
receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate was
good. Liver/body-weight ratio in males at 25 ppm and in both sexes at
75 and 300 ppm. Swelling of centrolobular liver cells with peripheral
distribution of the cytoplasmic granules were often seen. At 2.5 and 5
ppm these changes were seen with the same frequency as in the
controls. They were markedly obvious at 25 ppm and over, but regressed
after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following concentrations of
5 ppm in the diet (Dewitt, 1955).
Dog When dogs were fed, for 5 or 6 days per week, diets containing
10 to 30 ppm of aldrin, death occurred after periods of feeding
ranging from a few days to about 7 months, Three groups of suckling
puppies (11 days old), 3 each group comprising 2 males and 1 female
were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5 days per
week. All the animals died within 38 days. A 2-month-old male and a
female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6 and
2.0 mg of recrystallized aldrin per kg of body-weight daily for one
year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, equally
divided by sex, were fed a diet containing 10 ppm of aldrin for their
life-span (maximum 2 years). The aldrin shortened their average
life-span by 2 months, as compared with an equal number of controls,
and significantly increased the incidence of hepatic tumours (Davis &
Fitzhugh, 1962).
Rat. Groups of 25 female rats were fed diets containing 5, 10 and 20
ppm of recrystallized aldrin for 64 weeks. The group on 20 ppm showed
an increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
In a 2-year feeding experiment, groups of 20 rats (10 male and 10
female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 10, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male and
40 female) was given 2.5, 12.5 and 25 ppm of recrystallized aldrin.
There was a questionable increase in mortality rate at the 25 ppm
level in females. Significant increase in the liver/body-weight ratio
occurred in males at all levels and at 12.5 and 25 ppm in females.
Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male and
12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25 ppm for
three generations; at 12.5 and 25 ppm the number of pregnancies was
reduced. The incorporation of aldrin into the diets of lactating
females has a "slight to moderate" effect on mortality among the
offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments
The primary site of action of aldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage and, in this respect, young
dogs are more susceptible than older dogs.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels an compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased at a dose level of 10 ppm in one strain of mice susceptible
to the development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog: Questionable effects were seen at 1 ppm in the diet, equivalent
to 0.025 mg/kg.
Rat: Minimal changes were produced at 0.5 ppm in the diet,
equivalent to 0.025 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0001 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that aldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
See the monograph on Dieldrin.
* Sum of aldrin and dieldrin by weight.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. P. (1956) J. Agr.
Food Chem. 4, 937
Barnes, J. M. (1957) Unpublished report.
Borgmann, A. R., Kitselman, C. H., Dahm, P. A. & Pankaskie, J. E.
(1952) Unpublished report from Kettering Laboratory, University of
Cincinnati
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life Sciences, 5,
47
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol., 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxicol., 9, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961), J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 41,
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plant Protection Conference, Budapest, Hungary
Mörsdorf K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. Exp.,
8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West. Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F., Dutra, F. R., Shaffer, F. R., Cleveland, F. P., Wagner,
W. & Gahegan, R. (1951) Unpublished report from Kettering Laboratory,
University of Cincinnati
Treon, J. F. (1955) Unpublished report from Kettering Laboratory,
University of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-aldrin was applied to growing cultures of Aspergillus and
Penicillium species, dieldrin and more hydrophilic metabolites were
found in the culture medium and in the mycelium. Mosquito larvae
(Aedes aegypti) cultivated in an aqueous medium to which
14C-aldrin is added, can convert this compound to hydrophilic
metabolites (Ludwig et al., 1966).
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1966; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
but this ratio decreases rapidly to less than one with an intake of 50
ppm (Lehman, 1956).
The aldrin content of the blood plasma of men occupationally exposed
for 259-6044 hours over a period of eight years ranged between 0.0007
and 0.0023 ppm and was directly proportional to the duration of
exposure. Aldrin added to human serum could be only partially
recovered, probably because of in vitro interaction or binding with
some constituent of serum such as lipoproteins. The plasma of an adult
male about 18 hours after ingestion of aldrin and 10 hours after the
last convulsion contained 0.036 ppm aldrin and 0.279 dieldrin. Twenty
days later these concentrations were 0.0018 and 0.090 ppm respectively
(Dale et al., 1966).
Aldrin is largely and readily converted in the animal body, especially
in the liver, to dieldrin (Bann et al., 1956; Ivey et al., 1961; Treon
& Cleveland, 1955). The rate of change has not been fully established,
and is independent of the site of entrance into the body. The dieldrin
is stored without further change and may be recovered as such from
animal products and tissues, including the eggs of fowls and the milk
of dairy cows within 24 hours after ingestion (Bann et al., 1956).
Fifteen minutes after an intravenous injection of 14C-aldrin, aldrin
and its metabolites were found in the bile (Mörsdorf et al., 1963).
In vitro epoxidation by rat liver microsomes was found to be about
10 times greater in males than in females (Wong & Terriere, 1965).
Rats were fed 1 and 25 ppm of aldrin for 120 days; two metabolites
were found in the urine, one of which was much more abundant in males
than in females (Datta et al., 1965).
Experiments with rats showed that 14C-aldrin given intravenously was
converted in 24 hours mainly into hydrophilic metabolites. After 48
hours the presence of these metabolites could be demonstrated in most
organs and tissues. In the urine of rabbits given 14C-aldrin
intravenously, the main metabolite could be identified as one of the
two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The oral
LD50 of this compound in mice is 1250 mg/kg bodyweight, and the
intravenous LD50 is 51 mg/kg body-weight. It was found that after
intravenous injection of labelled aldrin in rats, radioactive products
could be demonstrated in the bile within one hour. In 4 hours, 16.2
per cent of the radioactivity was excreted in the bile. Most of the
radioactivity was in the form of hydrophilic products. Perfusion tests
on rat livers showed conversion of aldrin into dieldrin. From these
results it was concluded that the conversion of the insecticide does
in fact take place in the animal organism (Ludwig et al., 1966).
Studies in rats with 14C-aldrin have shown that with daily
administration of a constant dose of aldrin (4.3 µg per animal,
calculated to be equivalent to 0.2 ppm in the diet) a saturation
equilibrium is reached after a certain time (in male rats after about
50 days; whereas in females this saturation equilibrium is reached
after as long as 200 days). After discontinuation of the daily oral
administration, the compound had a biological half-life period of
10-11 days in male rats and 100 days in female rats. In faeces and
urine, 70 and 95 per cent respectively of the radioactivity was in the
form of hydrophilic metabolites. The conversion and excretion rate in
female rats was lower at the same dose levels than in the male rats.
The radioactivity found in the tissues of female animals was about
double that found in male rats. Remarkable differences in the
concentration of metabolites in lung, liver, spleen and kidney were
found; in the female rats, the content of hydrophilic metabolites was
lower and the dieldrin content higher than in the males (Ludwig et
al., 1966).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Mouse Oral 44 Borgmann et al., 1952
Rat, male Oral 38-54 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Barnes, J. M., 1957
Guinea-pig Oral 33 Borgmann et al., 1952
Rabbit Oral 50-80 Borgmann et al., 1952
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Man A 25-year-old man intentionally ingested a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria
and albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level (Borgmann et al., 1952).
In a feeding study lasting from 6 to 7 months, dosage levels of 5, 10
and 25 ppm aldrin were used with groups of 5 females. No enlargement
of the liver or other gross change was noted. Histological data are
not described. In a 9-month feeding experiment, with 20 female rats
per group, the dosage levels were 5, 15, 25 and 45 ppm of aldrin.
There was an increase in the liver/body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 5 animals of each sex were given 2.5, 5, 25, 75 or 300 ppm
of purified or technical aldrin in the diet for 26 weeks. Two rats of
each group were killed before the end of the treatment, and the last
three were killed before the thirty-seventh week. All the animals
receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate was
good. Liver/body-weight ratio in males at 25 ppm and in both sexes at
75 and 300 ppm. Swelling of centrolobular liver cells with peripheral
distribution of the cytoplasmic granules were often seen. At 2.5 and 5
ppm these changes were seen with the same frequency as in the
controls. They were markedly obvious at 25 ppm and over, but regressed
after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following concentrations of
5 ppm in the diet (Dewitt, 1955).
Dog When dogs were fed, for 5 or 6 days per week, diets containing
10 to 30 ppm of aldrin, death occurred after periods of feeding
ranging from a few days to about 7 months, Three groups of suckling
puppies (11 days old), 3 each group comprising 2 males and 1 female
were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5 days per
week. All the animals died within 38 days. A 2-month-old male and a
female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6 and
2.0 mg of recrystallized aldrin per kg of body-weight daily for one
year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, equally
divided by sex, were fed a diet containing 10 ppm of aldrin for their
life-span (maximum 2 years). The aldrin shortened their average
life-span by 2 months, as compared with an equal number of controls,
and significantly increased the incidence of hepatic tumours (Davis &
Fitzhugh, 1962).
Rat. Groups of 25 female rats were fed diets containing 5, 10 and 20
ppm of recrystallized aldrin for 64 weeks. The group on 20 ppm showed
an increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
In a 2-year feeding experiment, groups of 20 rats (10 male and 10
female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 10, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male and
40 female) was given 2.5, 12.5 and 25 ppm of recrystallized aldrin.
There was a questionable increase in mortality rate at the 25 ppm
level in females. Significant increase in the liver/body-weight ratio
occurred in males at all levels and at 12.5 and 25 ppm in females.
Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male and
12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25 ppm for
three generations; at 12.5 and 25 ppm the number of pregnancies was
reduced. The incorporation of aldrin into the diets of lactating
females has a "slight to moderate" effect on mortality among the
offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments
The primary site of action of aldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage and, in this respect, young
dogs are more susceptible than older dogs.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels an compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased at a dose level of 10 ppm in one strain of mice susceptible
to the development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog: Questionable effects were seen at 1 ppm in the diet, equivalent
to 0.025 mg/kg.
Rat: Minimal changes were produced at 0.5 ppm in the diet,
equivalent to 0.025 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0001 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that aldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
See the monograph on Dieldrin.
* Sum of aldrin and dieldrin by weight.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. P. (1956) J. Agr.
Food Chem. 4, 937
Barnes, J. M. (1957) Unpublished report.
Borgmann, A. R., Kitselman, C. H., Dahm, P. A. & Pankaskie, J. E.
(1952) Unpublished report from Kettering Laboratory, University of
Cincinnati
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life Sciences, 5,
47
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol., 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxicol., 9, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961), J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 41,
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plant Protection Conference, Budapest, Hungary
Mörsdorf K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. Exp.,
8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West. Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F., Dutra, F. R., Shaffer, F. R., Cleveland, F. P., Wagner,
W. & Gahegan, R. (1951) Unpublished report from Kettering Laboratory,
University of Cincinnati
Treon, J. F. (1955) Unpublished report from Kettering Laboratory,
University of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
 DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-aldrin was applied to growing cultures of Aspergillus and
Penicillium species, dieldrin and more hydrophilic metabolites were
found in the culture medium and in the mycelium. Mosquito larvae
(Aedes aegypti) cultivated in an aqueous medium to which
14C-aldrin is added, can convert this compound to hydrophilic
metabolites (Ludwig et al., 1966).
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1966; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
but this ratio decreases rapidly to less than one with an intake of 50
ppm (Lehman, 1956).
The aldrin content of the blood plasma of men occupationally exposed
for 259-6044 hours over a period of eight years ranged between 0.0007
and 0.0023 ppm and was directly proportional to the duration of
exposure. Aldrin added to human serum could be only partially
recovered, probably because of in vitro interaction or binding with
some constituent of serum such as lipoproteins. The plasma of an adult
male about 18 hours after ingestion of aldrin and 10 hours after the
last convulsion contained 0.036 ppm aldrin and 0.279 dieldrin. Twenty
days later these concentrations were 0.0018 and 0.090 ppm respectively
(Dale et al., 1966).
Aldrin is largely and readily converted in the animal body, especially
in the liver, to dieldrin (Bann et al., 1956; Ivey et al., 1961; Treon
& Cleveland, 1955). The rate of change has not been fully established,
and is independent of the site of entrance into the body. The dieldrin
is stored without further change and may be recovered as such from
animal products and tissues, including the eggs of fowls and the milk
of dairy cows within 24 hours after ingestion (Bann et al., 1956).
Fifteen minutes after an intravenous injection of 14C-aldrin, aldrin
and its metabolites were found in the bile (Mörsdorf et al., 1963).
In vitro epoxidation by rat liver microsomes was found to be about
10 times greater in males than in females (Wong & Terriere, 1965).
Rats were fed 1 and 25 ppm of aldrin for 120 days; two metabolites
were found in the urine, one of which was much more abundant in males
than in females (Datta et al., 1965).
Experiments with rats showed that 14C-aldrin given intravenously was
converted in 24 hours mainly into hydrophilic metabolites. After 48
hours the presence of these metabolites could be demonstrated in most
organs and tissues. In the urine of rabbits given 14C-aldrin
intravenously, the main metabolite could be identified as one of the
two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The oral
LD50 of this compound in mice is 1250 mg/kg bodyweight, and the
intravenous LD50 is 51 mg/kg body-weight. It was found that after
intravenous injection of labelled aldrin in rats, radioactive products
could be demonstrated in the bile within one hour. In 4 hours, 16.2
per cent of the radioactivity was excreted in the bile. Most of the
radioactivity was in the form of hydrophilic products. Perfusion tests
on rat livers showed conversion of aldrin into dieldrin. From these
results it was concluded that the conversion of the insecticide does
in fact take place in the animal organism (Ludwig et al., 1966).
Studies in rats with 14C-aldrin have shown that with daily
administration of a constant dose of aldrin (4.3 µg per animal,
calculated to be equivalent to 0.2 ppm in the diet) a saturation
equilibrium is reached after a certain time (in male rats after about
50 days; whereas in females this saturation equilibrium is reached
after as long as 200 days). After discontinuation of the daily oral
administration, the compound had a biological half-life period of
10-11 days in male rats and 100 days in female rats. In faeces and
urine, 70 and 95 per cent respectively of the radioactivity was in the
form of hydrophilic metabolites. The conversion and excretion rate in
female rats was lower at the same dose levels than in the male rats.
The radioactivity found in the tissues of female animals was about
double that found in male rats. Remarkable differences in the
concentration of metabolites in lung, liver, spleen and kidney were
found; in the female rats, the content of hydrophilic metabolites was
lower and the dieldrin content higher than in the males (Ludwig et
al., 1966).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Mouse Oral 44 Borgmann et al., 1952
Rat, male Oral 38-54 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Barnes, J. M., 1957
Guinea-pig Oral 33 Borgmann et al., 1952
Rabbit Oral 50-80 Borgmann et al., 1952
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Man A 25-year-old man intentionally ingested a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria
and albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level (Borgmann et al., 1952).
In a feeding study lasting from 6 to 7 months, dosage levels of 5, 10
and 25 ppm aldrin were used with groups of 5 females. No enlargement
of the liver or other gross change was noted. Histological data are
not described. In a 9-month feeding experiment, with 20 female rats
per group, the dosage levels were 5, 15, 25 and 45 ppm of aldrin.
There was an increase in the liver/body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 5 animals of each sex were given 2.5, 5, 25, 75 or 300 ppm
of purified or technical aldrin in the diet for 26 weeks. Two rats of
each group were killed before the end of the treatment, and the last
three were killed before the thirty-seventh week. All the animals
receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate was
good. Liver/body-weight ratio in males at 25 ppm and in both sexes at
75 and 300 ppm. Swelling of centrolobular liver cells with peripheral
distribution of the cytoplasmic granules were often seen. At 2.5 and 5
ppm these changes were seen with the same frequency as in the
controls. They were markedly obvious at 25 ppm and over, but regressed
after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following concentrations of
5 ppm in the diet (Dewitt, 1955).
Dog When dogs were fed, for 5 or 6 days per week, diets containing
10 to 30 ppm of aldrin, death occurred after periods of feeding
ranging from a few days to about 7 months, Three groups of suckling
puppies (11 days old), 3 each group comprising 2 males and 1 female
were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5 days per
week. All the animals died within 38 days. A 2-month-old male and a
female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6 and
2.0 mg of recrystallized aldrin per kg of body-weight daily for one
year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, equally
divided by sex, were fed a diet containing 10 ppm of aldrin for their
life-span (maximum 2 years). The aldrin shortened their average
life-span by 2 months, as compared with an equal number of controls,
and significantly increased the incidence of hepatic tumours (Davis &
Fitzhugh, 1962).
Rat. Groups of 25 female rats were fed diets containing 5, 10 and 20
ppm of recrystallized aldrin for 64 weeks. The group on 20 ppm showed
an increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
In a 2-year feeding experiment, groups of 20 rats (10 male and 10
female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 10, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male and
40 female) was given 2.5, 12.5 and 25 ppm of recrystallized aldrin.
There was a questionable increase in mortality rate at the 25 ppm
level in females. Significant increase in the liver/body-weight ratio
occurred in males at all levels and at 12.5 and 25 ppm in females.
Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male and
12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25 ppm for
three generations; at 12.5 and 25 ppm the number of pregnancies was
reduced. The incorporation of aldrin into the diets of lactating
females has a "slight to moderate" effect on mortality among the
offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments
The primary site of action of aldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage and, in this respect, young
dogs are more susceptible than older dogs.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels an compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased at a dose level of 10 ppm in one strain of mice susceptible
to the development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog: Questionable effects were seen at 1 ppm in the diet, equivalent
to 0.025 mg/kg.
Rat: Minimal changes were produced at 0.5 ppm in the diet,
equivalent to 0.025 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0001 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that aldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
See the monograph on Dieldrin.
* Sum of aldrin and dieldrin by weight.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. P. (1956) J. Agr.
Food Chem. 4, 937
Barnes, J. M. (1957) Unpublished report.
Borgmann, A. R., Kitselman, C. H., Dahm, P. A. & Pankaskie, J. E.
(1952) Unpublished report from Kettering Laboratory, University of
Cincinnati
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life Sciences, 5,
47
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol., 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxicol., 9, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961), J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 41,
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plant Protection Conference, Budapest, Hungary
Mörsdorf K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. Exp.,
8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West. Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F., Dutra, F. R., Shaffer, F. R., Cleveland, F. P., Wagner,
W. & Gahegan, R. (1951) Unpublished report from Kettering Laboratory,
University of Cincinnati
Treon, J. F. (1955) Unpublished report from Kettering Laboratory,
University of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-aldrin was applied to growing cultures of Aspergillus and
Penicillium species, dieldrin and more hydrophilic metabolites were
found in the culture medium and in the mycelium. Mosquito larvae
(Aedes aegypti) cultivated in an aqueous medium to which
14C-aldrin is added, can convert this compound to hydrophilic
metabolites (Ludwig et al., 1966).
Following the feeding of aldrin to animals it is stored in the
tissues, especially in the fat (Bann et al., 1966; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957; Treon & Cleveland, 1955). At low
levels of intake (1 ppm) the storage ratio is large (about 60 times)
but this ratio decreases rapidly to less than one with an intake of 50
ppm (Lehman, 1956).
The aldrin content of the blood plasma of men occupationally exposed
for 259-6044 hours over a period of eight years ranged between 0.0007
and 0.0023 ppm and was directly proportional to the duration of
exposure. Aldrin added to human serum could be only partially
recovered, probably because of in vitro interaction or binding with
some constituent of serum such as lipoproteins. The plasma of an adult
male about 18 hours after ingestion of aldrin and 10 hours after the
last convulsion contained 0.036 ppm aldrin and 0.279 dieldrin. Twenty
days later these concentrations were 0.0018 and 0.090 ppm respectively
(Dale et al., 1966).
Aldrin is largely and readily converted in the animal body, especially
in the liver, to dieldrin (Bann et al., 1956; Ivey et al., 1961; Treon
& Cleveland, 1955). The rate of change has not been fully established,
and is independent of the site of entrance into the body. The dieldrin
is stored without further change and may be recovered as such from
animal products and tissues, including the eggs of fowls and the milk
of dairy cows within 24 hours after ingestion (Bann et al., 1956).
Fifteen minutes after an intravenous injection of 14C-aldrin, aldrin
and its metabolites were found in the bile (Mörsdorf et al., 1963).
In vitro epoxidation by rat liver microsomes was found to be about
10 times greater in males than in females (Wong & Terriere, 1965).
Rats were fed 1 and 25 ppm of aldrin for 120 days; two metabolites
were found in the urine, one of which was much more abundant in males
than in females (Datta et al., 1965).
Experiments with rats showed that 14C-aldrin given intravenously was
converted in 24 hours mainly into hydrophilic metabolites. After 48
hours the presence of these metabolites could be demonstrated in most
organs and tissues. In the urine of rabbits given 14C-aldrin
intravenously, the main metabolite could be identified as one of the
two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The oral
LD50 of this compound in mice is 1250 mg/kg bodyweight, and the
intravenous LD50 is 51 mg/kg body-weight. It was found that after
intravenous injection of labelled aldrin in rats, radioactive products
could be demonstrated in the bile within one hour. In 4 hours, 16.2
per cent of the radioactivity was excreted in the bile. Most of the
radioactivity was in the form of hydrophilic products. Perfusion tests
on rat livers showed conversion of aldrin into dieldrin. From these
results it was concluded that the conversion of the insecticide does
in fact take place in the animal organism (Ludwig et al., 1966).
Studies in rats with 14C-aldrin have shown that with daily
administration of a constant dose of aldrin (4.3 µg per animal,
calculated to be equivalent to 0.2 ppm in the diet) a saturation
equilibrium is reached after a certain time (in male rats after about
50 days; whereas in females this saturation equilibrium is reached
after as long as 200 days). After discontinuation of the daily oral
administration, the compound had a biological half-life period of
10-11 days in male rats and 100 days in female rats. In faeces and
urine, 70 and 95 per cent respectively of the radioactivity was in the
form of hydrophilic metabolites. The conversion and excretion rate in
female rats was lower at the same dose levels than in the male rats.
The radioactivity found in the tissues of female animals was about
double that found in male rats. Remarkable differences in the
concentration of metabolites in lung, liver, spleen and kidney were
found; in the female rats, the content of hydrophilic metabolites was
lower and the dieldrin content higher than in the males (Ludwig et
al., 1966).
Acute toxicity
Animal Route LD50 Reference
mg/kg body-weight
Mouse Oral 44 Borgmann et al., 1952
Rat, male Oral 38-54 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Oral 46-67 Borgmann et al., 1952
Gaines, 1960
Lehman, 1951
Treon & Cleveland, 1955
Rat, female Intravenous 18 Barnes, J. M., 1957
Guinea-pig Oral 33 Borgmann et al., 1952
Rabbit Oral 50-80 Borgmann et al., 1952
Treon & Cleveland, 1955
Dog Oral 65-95 Borgmann et al., 1952
Man A 25-year-old man intentionally ingested a quantity of aldrin
equivalent to 25.6 mg per kg of body-weight. The following symptoms
were noticed: generalized convulsions, E.E.G. changes, haematuria
and albuminuria. Recovery was complete (Spiotta, 1951).
Short-term studies
Rat. Groups of 12 rats (6 male and 6 female) were fed diets
containing 0.5, 2.5, 75 and 150 ppm aldrin for 90 days. The liver
weight was increased at the two higher dosages. The mortality rate was
increased at the 150 ppm level (Borgmann et al., 1952).
In a feeding study lasting from 6 to 7 months, dosage levels of 5, 10
and 25 ppm aldrin were used with groups of 5 females. No enlargement
of the liver or other gross change was noted. Histological data are
not described. In a 9-month feeding experiment, with 20 female rats
per group, the dosage levels were 5, 15, 25 and 45 ppm of aldrin.
There was an increase in the liver/body-weight ratio at 45 ppm
(Borgmann et al., 1952).
Groups of 5 animals of each sex were given 2.5, 5, 25, 75 or 300 ppm
of purified or technical aldrin in the diet for 26 weeks. Two rats of
each group were killed before the end of the treatment, and the last
three were killed before the thirty-seventh week. All the animals
receiving 300 ppm died in 2 weeks. At 75 ppm the survival rate was
good. Liver/body-weight ratio in males at 25 ppm and in both sexes at
75 and 300 ppm. Swelling of centrolobular liver cells with peripheral
distribution of the cytoplasmic granules were often seen. At 2.5 and 5
ppm these changes were seen with the same frequency as in the
controls. They were markedly obvious at 25 ppm and over, but regressed
after the end of the treatment (Treon et al., 1951).
Quail and pheasants. These animals died following concentrations of
5 ppm in the diet (Dewitt, 1955).
Dog When dogs were fed, for 5 or 6 days per week, diets containing
10 to 30 ppm of aldrin, death occurred after periods of feeding
ranging from a few days to about 7 months, Three groups of suckling
puppies (11 days old), 3 each group comprising 2 males and 1 female
were given 1.5, 3.0 and 4.5 mg/kg per day respectively, on 5 days per
week. All the animals died within 38 days. A 2-month-old male and a
female survived about 6 to 7 months when given 0.9 to 1.8 mg/kg
body-weight per day for 6 days per week (Treon & Cleveland, 1955).
When 3 groups of 3 dogs (both sexes) were given orally 0.2, 0.6 and
2.0 mg of recrystallized aldrin per kg of body-weight daily for one
year, 5 of them produced litters but the pups died early, probably
because of high quantities of aldrin or dieldrin in the milk of the
dams. Histological liver changes were found in the dogs (Kitselman,
1953).
Groups of 4 dogs (2 male and 2 female) were given 1 and 3 ppm of
aldrin in their diet for 68 weeks. Liver damage occurred in 3 animals
on the 3 ppm dosage level. There were significant increases in
liver/body-weight ratios in the dogs on 3 ppm of aldrin. Kidney damage
occurred in the female at the 1 ppm dosage level. An average
concentration of aldrin of 0.3 ppm remained in the adipose tissue in
the animals fed 3 ppm and 0.18 ppm remained at 1 ppm. Dieldrin
occurred at a concentration of 25.4 ppm in the fat of a dog fed 1 ppm
of aldrin (Treon & Cleveland, 1955; Treon et al., 1955).
A group of 12 dogs was given aldrin orally for 2 years at the
following daily doses - 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2
and 5 mg/kg (2 dogs each). The animals at 5 mg/kg and one of those
given 2 mg/kg died within 24 days. The other animal at 2 mg/kg and the
2 given 1 mg/kg died in 1 year. All the others survived until the end
of the experiment but for a dog at 0.5 mg/kg which died in a few days.
Fatty changes in the liver and kidney, associated with "mild bone
marrow changes" were observed at the highest doses. At 0.5 mg/kg one
animal showed convulsions. No effects were seen at 0.2 mg/kg (Fitzhugh
et al., 1964).
Sheep and cattle. Heifers given 0.5-1 mg/kg/day for 64 days and
cattle given 1.9 mg/kg/day for 10 days were not affected, whereas
sheep given 6 mg/kg/day died within 28 days (Kitselman et al., 1950).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, equally
divided by sex, were fed a diet containing 10 ppm of aldrin for their
life-span (maximum 2 years). The aldrin shortened their average
life-span by 2 months, as compared with an equal number of controls,
and significantly increased the incidence of hepatic tumours (Davis &
Fitzhugh, 1962).
Rat. Groups of 25 female rats were fed diets containing 5, 10 and 20
ppm of recrystallized aldrin for 64 weeks. The group on 20 ppm showed
an increase in weight over the controls which was correlated with an
increased food intake. At the 10 ppm and 20 ppm levels the oestrus
cycle was disturbed (Ball et al., 1953).
In a 2-year feeding experiment, groups of 20 rats (10 male and 10
female) were given 5, 10, 50, 100 and 150 ppm of aldrin. The
concentrations of 100 and 150 ppm increased the mortality rate and
those of 10, 100 and 150 ppm produced microscopic changes in the
liver. A single rat on 10 ppm of aldrin had specific liver changes;
the rats on 5 ppm of aldrin had no noticeable liver changes. Aldrin
was stored in the tissues at all dosage levels (Borgmann et al.,
1952).
In a second 2-year feeding experiment a group of 80 rats (40 male and
40 female) was given 2.5, 12.5 and 25 ppm of recrystallized aldrin.
There was a questionable increase in mortality rate at the 25 ppm
level in females. Significant increase in the liver/body-weight ratio
occurred in males at all levels and at 12.5 and 25 ppm in females.
Histological liver changes characteristic of organic chlorine
compounds occurred at all dosage levels of aldrin (Treon & Cleveland,
1955).
In a third 2-year feeding experiment, groups of 24 rats (12 male and
12 female) were given 0.5, 2, 10, 50, 100 and 150 ppm of aldrin.
Concentrations of 50 ppm and above in the diet increased the mortality
rate in a dose-response relationship. Liver/body-weight ratio
increased at all levels of feeding. Characteristic microscopic lesions
occurred in the liver at all levels; these were minimal at 0.5 ppm but
increased in severity with dosage. There was an increase in tumour
incidence among treated animals at all feeding levels and particularly
at lower levels, but no single type of tumour predominated (Fitzhugh &
Nelson, 1963).
Aldrin was fed to groups of 16 female rats at 2.5, 12.5 and 25 ppm for
three generations; at 12.5 and 25 ppm the number of pregnancies was
reduced. The incorporation of aldrin into the diets of lactating
females has a "slight to moderate" effect on mortality among the
offspring at 2.5 ppm. It was severe at higher doses (Treon &
Cleveland, 1955).
Comments
The primary site of action of aldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage and, in this respect, young
dogs are more susceptible than older dogs.
In one long-term feeding experiment in rats there was a general
increase in tumour production in the experimental animals at the lower
dosage levels an compared to the controls, but the liver was not
particularly affected. Liver tumours were, however, significantly
increased at a dose level of 10 ppm in one strain of mice susceptible
to the development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog: Questionable effects were seen at 1 ppm in the diet, equivalent
to 0.025 mg/kg.
Rat: Minimal changes were produced at 0.5 ppm in the diet,
equivalent to 0.025 mg/kg/day.
Estimate of acceptable daily intake for man
0-0.0001 mg/kg body-weight*
Further work required
Elucidation of the significance of the finding that aldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
See the monograph on Dieldrin.
* Sum of aldrin and dieldrin by weight.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Ball, W. L., Kay, K. & Sinclair, J. W. (1953) Arch. industr. Hyg.,
7, 292
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. P. (1956) J. Agr.
Food Chem. 4, 937
Barnes, J. M. (1957) Unpublished report.
Borgmann, A. R., Kitselman, C. H., Dahm, P. A. & Pankaskie, J. E.
(1952) Unpublished report from Kettering Laboratory, University of
Cincinnati
Dale, W. E., Curley, A. & Cueto, C., jr (1966) Life Sciences, 5,
47
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol., 4,
187
Dewitt, J. B. (1955) J. Agr. Food Chem., 3, 672
Fitzhugh, O. G. & Nelson, A. A. (1963) Unpublished data from the
United States Food and Drug Administration
Fitzhugh, O. G., Nelson, A. A. & Quaife, M. L. (1964) Food Cosmet.
Toxicol., 9, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961), J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Kitselman, C. H., Dahm, P. A. & Borgmann, A. R. (1950) Amer. J. vet.
Res., 41,
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plant Protection Conference, Budapest, Hungary
Mörsdorf K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. Exp.,
8, 90
Spiotta, E. J. (1951) Arch. industr. Hyg., 4, 560
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West. Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Treon, J. F., Dutra, F. R., Shaffer, F. R., Cleveland, F. P., Wagner,
W. & Gahegan, R. (1951) Unpublished report from Kettering Laboratory,
University of Cincinnati
Treon, J. F. (1955) Unpublished report from Kettering Laboratory,
University of Cincinnati
Wong, D. T. & Terriere, L. C. (1965) Biochem. Pharmacol., 14, 375
