
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
DIELDRIN
Chemical name
1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-
endo-1,4-exo-5,8,-dimethanonaphthalene or 1,2,3,4,10,10-hexachloro-
6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-exo-1,4-endo-5,8,-
dimethanophthalene.
Synonyms
HEOD, Octalox, Alvit, Quintox.
Empirical formula
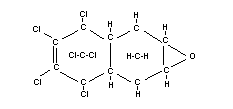
C12H8OCl6
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Dieldrin labelled with 14C is preferentially excreted together
with its metabolites in the bile. Fifteen minutes after an intravenous
injection, radioactive dieldrin and a hydrophilic metabolite were
found in the bile (Mörsdorf, et al. 1963).
Studies with 36Cl-dieldrin have shown that the initial
distribution is general, but within a few hours of injection it
concentrates more in the fat. Excretion of 36Cl averages about 5% per
day and is markedly increased by a restriction of diet, which reduces
the body fat. From the canulated bile duct, excretion accelerates as
the rat loses weight exceeding 10% per day after a few days. Only 3%
of the 36Cl is excreted as dieldrin unless the bile is canulated,
when up to 10% may be excreted. The remainder of 36Cl is found in the
metabolites and these are excreted to 90% in the faeces and 10% in the
urine. The most important metabolite containing about 60% of the total
36Cl is excreted in the bile (Heath & Vandekar, 1964).
In experiments 14C dieldrin it was demonstrated that in the
blood dieldrin is located mainly in the erythrocytes and plasma.
Haemoglobin is largely but not entirely responsible for the binding to
erythrocytes. In the plasma, dieldrin binds with soluble proteins
(Moss & Hathway, 1964).
After the feeding of dieldrin to animals it is stored in the
adipose tissues. Although small amounts of dieldrin are found in
liver, kidney and muscle tissues, the greatest amount of storage is in
the fat where dieldrin is stored unchanged, (Bann et al., 1956;
Butcher et al., 1957; Heath & Vandekar, 1964; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957). It is lost slowly from the body
fat (Butcher et al., 1957; Heath & Vandekar, 1964). It is stored in
human fat in significant amounts (Hunter et al., 1963).
In studies on the urine of individuals exposed to dieldrin, on
paper and column chromatography 2 neutral polar metabolites of
dieldrin were found. No dieldrin could be detected with this method,
but dieldrin or a material having the same retention time was found by
gas chromatography (Cueto & Hayes, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 38 Borgmann et al., 1952
Council of Europe, 1962
Rat Oral 37-87 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Heath & Vandekar, 1964
Lehman, 1951
Lu, et al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 45-50 Borgmann et al., 1952
Council of Europe, 1962
Dog Oral 56-80 Borgmann et al., 1952
Council of Europe, 1962
Sheep Oral 50-75 Borgmann et al., 1952
Council of Europe, 1962
In rats the LD50 for a day-old animal is 168 mg/kg body-weight,
the same as in adults (Lu et al., 1965).
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment, groups of 10 male and 10 female rats were given 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm
of technical dieldrin. The rats were killed after 2, 4, 6 and 8
months. Food intake and growth were normal. No significant change in
liver weight was found. Cytoplasmic alteration of the liver cells was
found at both concentrations in males and females (Ortega et al.,
1957).
Dog. Dogs (1-4 animals per group) were given diets containing
1, 3, 10, 25 and 50 ppm dieldrin on six days per week. The animals on
25 and 50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm
survived for 9 months and on 1 and 3 ppm for 15 months. In the groups
on 1 and 3 ppm the livers were significantly larger than those of the
controls. Histological changes were noted in brains, liver and kidneys
(Treon & Cleveland, 1955). Groups of 4 dogs (2 male and 2 female) were
given 1 and 3 ppm of dieldrin in their diet for 68 weeks. The
concentration of 3 ppm increased the liver/body-weight ratio and
produced renal damage in 1 female. With 1 ppm of dieldrin, livers were
enlarged but no histopathological changes were found. In the dogs fed
3 ppm of dieldrin, 45.5 ppm were found in the fat, and in the dogs fed
1 ppm, 3.4 ppm were found in the fat. In other organs, concentrations
ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were found (Borgmann
et al., 1952; Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of
0.2, 0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313
days. The highest dosage killed all the dogs. Two of the 4 dogs died
when given 0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day
of recrystallized dieldrin per kg of body-weight for one year; 3 of
them produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and
10 male) were given dieldrin orally at dosage levels of 0.625, 1.25,
2.5, 5 and 10 mg/kg body-weight per day. Survival rates were affected
at all levels. At 2.5 mg/kg and above, all animals died (Borgmann et
al., 1952).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of dieldrin
for their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in
the diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially reduced
the number of pregnancies but this effect tended to disappear with
continued feeding of the diet. All doses increased the mortality among
the suckling young. The effect on survival during suckling was severe
at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female)
were given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of
50 ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin
in the diet at a concentration of 75 ppm for 440 days. Twenty animals
of each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good conditions
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight ratio was markedly increased in the rats
killed during exposure, but it returned to normal after the end of it.
"Lesions of the hepatic parenchyma that have been considered typical
of exposure to the organochlorine insecticides in rats" were observed
only in healthy animals killed during the treatment. Rats dying
spontaneously or killed after withdrawal of the insecticide did not
show such type of change (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin
in their diets for a period of 40 months including 4 gestation
periods. At 25 ppm lambs died shortly after birth. Liver function as
well as other physiological tests on the ewes did not show any changes
related to the treatment with dieldrin (Shell, 1963).
Comments on the experimental studies reported
The primary mode of action of dieldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and in
this respect young dogs are more susceptible than rats.
In one long-term rat feeding experiment, there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected.
Liver tumours, however, were significantly increased on a dosage
of 10 ppm in one strain of mice susceptible to the development of
these tumours. Some evidence has been presented that the same effect
can occur in another strain of mice, but this requires more
information.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorgenic properties of dieldrin could be related to a
"general" type of effect.
EVALUATION
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of dieldrin for man is
kept at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Unpublished report, July
Butcher, J. E. et al. (1957) Research report, Agricultural
Experimental Station, Utah State Agricultural College
Council of Europe (1962) Agricultural pesticides, Strasbourg
Cueto, C. & Hayes, W. J., jr (1962) J. Agr. Food. Chem., 10, 366
Davis. K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. pharmacol.,
4, 187
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Data submitted
by Shell Company
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 3 (In press)
Mörsdorf, K. et al. (1963) Med. exp., 8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J. & Burham, W. F. (1957) Arch. Path., 64,
614
Shell Chemical Company (1963) Unpublished report
Street, J. C. et al. (1957) Proc. West Sec. Amer. Soc. Anim. Prod.,
46 (1)
Treon, J. F. & Cleveland. F. P. (1955) J. Agr. Food Chem., 3, 402
BIOLOGICAL DATA
Biochemical aspects
Dieldrin labelled with 14C is preferentially excreted together
with its metabolites in the bile. Fifteen minutes after an intravenous
injection, radioactive dieldrin and a hydrophilic metabolite were
found in the bile (Mörsdorf, et al. 1963).
Studies with 36Cl-dieldrin have shown that the initial
distribution is general, but within a few hours of injection it
concentrates more in the fat. Excretion of 36Cl averages about 5% per
day and is markedly increased by a restriction of diet, which reduces
the body fat. From the canulated bile duct, excretion accelerates as
the rat loses weight exceeding 10% per day after a few days. Only 3%
of the 36Cl is excreted as dieldrin unless the bile is canulated,
when up to 10% may be excreted. The remainder of 36Cl is found in the
metabolites and these are excreted to 90% in the faeces and 10% in the
urine. The most important metabolite containing about 60% of the total
36Cl is excreted in the bile (Heath & Vandekar, 1964).
In experiments 14C dieldrin it was demonstrated that in the
blood dieldrin is located mainly in the erythrocytes and plasma.
Haemoglobin is largely but not entirely responsible for the binding to
erythrocytes. In the plasma, dieldrin binds with soluble proteins
(Moss & Hathway, 1964).
After the feeding of dieldrin to animals it is stored in the
adipose tissues. Although small amounts of dieldrin are found in
liver, kidney and muscle tissues, the greatest amount of storage is in
the fat where dieldrin is stored unchanged, (Bann et al., 1956;
Butcher et al., 1957; Heath & Vandekar, 1964; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957). It is lost slowly from the body
fat (Butcher et al., 1957; Heath & Vandekar, 1964). It is stored in
human fat in significant amounts (Hunter et al., 1963).
In studies on the urine of individuals exposed to dieldrin, on
paper and column chromatography 2 neutral polar metabolites of
dieldrin were found. No dieldrin could be detected with this method,
but dieldrin or a material having the same retention time was found by
gas chromatography (Cueto & Hayes, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 38 Borgmann et al., 1952
Council of Europe, 1962
Rat Oral 37-87 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Heath & Vandekar, 1964
Lehman, 1951
Lu, et al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 45-50 Borgmann et al., 1952
Council of Europe, 1962
Dog Oral 56-80 Borgmann et al., 1952
Council of Europe, 1962
Sheep Oral 50-75 Borgmann et al., 1952
Council of Europe, 1962
In rats the LD50 for a day-old animal is 168 mg/kg body-weight,
the same as in adults (Lu et al., 1965).
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment, groups of 10 male and 10 female rats were given 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm
of technical dieldrin. The rats were killed after 2, 4, 6 and 8
months. Food intake and growth were normal. No significant change in
liver weight was found. Cytoplasmic alteration of the liver cells was
found at both concentrations in males and females (Ortega et al.,
1957).
Dog. Dogs (1-4 animals per group) were given diets containing
1, 3, 10, 25 and 50 ppm dieldrin on six days per week. The animals on
25 and 50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm
survived for 9 months and on 1 and 3 ppm for 15 months. In the groups
on 1 and 3 ppm the livers were significantly larger than those of the
controls. Histological changes were noted in brains, liver and kidneys
(Treon & Cleveland, 1955). Groups of 4 dogs (2 male and 2 female) were
given 1 and 3 ppm of dieldrin in their diet for 68 weeks. The
concentration of 3 ppm increased the liver/body-weight ratio and
produced renal damage in 1 female. With 1 ppm of dieldrin, livers were
enlarged but no histopathological changes were found. In the dogs fed
3 ppm of dieldrin, 45.5 ppm were found in the fat, and in the dogs fed
1 ppm, 3.4 ppm were found in the fat. In other organs, concentrations
ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were found (Borgmann
et al., 1952; Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of
0.2, 0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313
days. The highest dosage killed all the dogs. Two of the 4 dogs died
when given 0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day
of recrystallized dieldrin per kg of body-weight for one year; 3 of
them produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and
10 male) were given dieldrin orally at dosage levels of 0.625, 1.25,
2.5, 5 and 10 mg/kg body-weight per day. Survival rates were affected
at all levels. At 2.5 mg/kg and above, all animals died (Borgmann et
al., 1952).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of dieldrin
for their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in
the diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially reduced
the number of pregnancies but this effect tended to disappear with
continued feeding of the diet. All doses increased the mortality among
the suckling young. The effect on survival during suckling was severe
at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female)
were given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of
50 ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin
in the diet at a concentration of 75 ppm for 440 days. Twenty animals
of each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good conditions
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight ratio was markedly increased in the rats
killed during exposure, but it returned to normal after the end of it.
"Lesions of the hepatic parenchyma that have been considered typical
of exposure to the organochlorine insecticides in rats" were observed
only in healthy animals killed during the treatment. Rats dying
spontaneously or killed after withdrawal of the insecticide did not
show such type of change (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin
in their diets for a period of 40 months including 4 gestation
periods. At 25 ppm lambs died shortly after birth. Liver function as
well as other physiological tests on the ewes did not show any changes
related to the treatment with dieldrin (Shell, 1963).
Comments on the experimental studies reported
The primary mode of action of dieldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and in
this respect young dogs are more susceptible than rats.
In one long-term rat feeding experiment, there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected.
Liver tumours, however, were significantly increased on a dosage
of 10 ppm in one strain of mice susceptible to the development of
these tumours. Some evidence has been presented that the same effect
can occur in another strain of mice, but this requires more
information.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorgenic properties of dieldrin could be related to a
"general" type of effect.
EVALUATION
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of dieldrin for man is
kept at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Unpublished report, July
Butcher, J. E. et al. (1957) Research report, Agricultural
Experimental Station, Utah State Agricultural College
Council of Europe (1962) Agricultural pesticides, Strasbourg
Cueto, C. & Hayes, W. J., jr (1962) J. Agr. Food. Chem., 10, 366
Davis. K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. pharmacol.,
4, 187
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Data submitted
by Shell Company
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 3 (In press)
Mörsdorf, K. et al. (1963) Med. exp., 8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J. & Burham, W. F. (1957) Arch. Path., 64,
614
Shell Chemical Company (1963) Unpublished report
Street, J. C. et al. (1957) Proc. West Sec. Amer. Soc. Anim. Prod.,
46 (1)
Treon, J. F. & Cleveland. F. P. (1955) J. Agr. Food Chem., 3, 402
 BIOLOGICAL DATA
Biochemical aspects
Dieldrin labelled with 14C is preferentially excreted together
with its metabolites in the bile. Fifteen minutes after an intravenous
injection, radioactive dieldrin and a hydrophilic metabolite were
found in the bile (Mörsdorf, et al. 1963).
Studies with 36Cl-dieldrin have shown that the initial
distribution is general, but within a few hours of injection it
concentrates more in the fat. Excretion of 36Cl averages about 5% per
day and is markedly increased by a restriction of diet, which reduces
the body fat. From the canulated bile duct, excretion accelerates as
the rat loses weight exceeding 10% per day after a few days. Only 3%
of the 36Cl is excreted as dieldrin unless the bile is canulated,
when up to 10% may be excreted. The remainder of 36Cl is found in the
metabolites and these are excreted to 90% in the faeces and 10% in the
urine. The most important metabolite containing about 60% of the total
36Cl is excreted in the bile (Heath & Vandekar, 1964).
In experiments 14C dieldrin it was demonstrated that in the
blood dieldrin is located mainly in the erythrocytes and plasma.
Haemoglobin is largely but not entirely responsible for the binding to
erythrocytes. In the plasma, dieldrin binds with soluble proteins
(Moss & Hathway, 1964).
After the feeding of dieldrin to animals it is stored in the
adipose tissues. Although small amounts of dieldrin are found in
liver, kidney and muscle tissues, the greatest amount of storage is in
the fat where dieldrin is stored unchanged, (Bann et al., 1956;
Butcher et al., 1957; Heath & Vandekar, 1964; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957). It is lost slowly from the body
fat (Butcher et al., 1957; Heath & Vandekar, 1964). It is stored in
human fat in significant amounts (Hunter et al., 1963).
In studies on the urine of individuals exposed to dieldrin, on
paper and column chromatography 2 neutral polar metabolites of
dieldrin were found. No dieldrin could be detected with this method,
but dieldrin or a material having the same retention time was found by
gas chromatography (Cueto & Hayes, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 38 Borgmann et al., 1952
Council of Europe, 1962
Rat Oral 37-87 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Heath & Vandekar, 1964
Lehman, 1951
Lu, et al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 45-50 Borgmann et al., 1952
Council of Europe, 1962
Dog Oral 56-80 Borgmann et al., 1952
Council of Europe, 1962
Sheep Oral 50-75 Borgmann et al., 1952
Council of Europe, 1962
In rats the LD50 for a day-old animal is 168 mg/kg body-weight,
the same as in adults (Lu et al., 1965).
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment, groups of 10 male and 10 female rats were given 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm
of technical dieldrin. The rats were killed after 2, 4, 6 and 8
months. Food intake and growth were normal. No significant change in
liver weight was found. Cytoplasmic alteration of the liver cells was
found at both concentrations in males and females (Ortega et al.,
1957).
Dog. Dogs (1-4 animals per group) were given diets containing
1, 3, 10, 25 and 50 ppm dieldrin on six days per week. The animals on
25 and 50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm
survived for 9 months and on 1 and 3 ppm for 15 months. In the groups
on 1 and 3 ppm the livers were significantly larger than those of the
controls. Histological changes were noted in brains, liver and kidneys
(Treon & Cleveland, 1955). Groups of 4 dogs (2 male and 2 female) were
given 1 and 3 ppm of dieldrin in their diet for 68 weeks. The
concentration of 3 ppm increased the liver/body-weight ratio and
produced renal damage in 1 female. With 1 ppm of dieldrin, livers were
enlarged but no histopathological changes were found. In the dogs fed
3 ppm of dieldrin, 45.5 ppm were found in the fat, and in the dogs fed
1 ppm, 3.4 ppm were found in the fat. In other organs, concentrations
ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were found (Borgmann
et al., 1952; Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of
0.2, 0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313
days. The highest dosage killed all the dogs. Two of the 4 dogs died
when given 0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day
of recrystallized dieldrin per kg of body-weight for one year; 3 of
them produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and
10 male) were given dieldrin orally at dosage levels of 0.625, 1.25,
2.5, 5 and 10 mg/kg body-weight per day. Survival rates were affected
at all levels. At 2.5 mg/kg and above, all animals died (Borgmann et
al., 1952).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of dieldrin
for their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in
the diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially reduced
the number of pregnancies but this effect tended to disappear with
continued feeding of the diet. All doses increased the mortality among
the suckling young. The effect on survival during suckling was severe
at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female)
were given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of
50 ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin
in the diet at a concentration of 75 ppm for 440 days. Twenty animals
of each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good conditions
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight ratio was markedly increased in the rats
killed during exposure, but it returned to normal after the end of it.
"Lesions of the hepatic parenchyma that have been considered typical
of exposure to the organochlorine insecticides in rats" were observed
only in healthy animals killed during the treatment. Rats dying
spontaneously or killed after withdrawal of the insecticide did not
show such type of change (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin
in their diets for a period of 40 months including 4 gestation
periods. At 25 ppm lambs died shortly after birth. Liver function as
well as other physiological tests on the ewes did not show any changes
related to the treatment with dieldrin (Shell, 1963).
Comments on the experimental studies reported
The primary mode of action of dieldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and in
this respect young dogs are more susceptible than rats.
In one long-term rat feeding experiment, there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected.
Liver tumours, however, were significantly increased on a dosage
of 10 ppm in one strain of mice susceptible to the development of
these tumours. Some evidence has been presented that the same effect
can occur in another strain of mice, but this requires more
information.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorgenic properties of dieldrin could be related to a
"general" type of effect.
EVALUATION
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of dieldrin for man is
kept at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Unpublished report, July
Butcher, J. E. et al. (1957) Research report, Agricultural
Experimental Station, Utah State Agricultural College
Council of Europe (1962) Agricultural pesticides, Strasbourg
Cueto, C. & Hayes, W. J., jr (1962) J. Agr. Food. Chem., 10, 366
Davis. K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. pharmacol.,
4, 187
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Data submitted
by Shell Company
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 3 (In press)
Mörsdorf, K. et al. (1963) Med. exp., 8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J. & Burham, W. F. (1957) Arch. Path., 64,
614
Shell Chemical Company (1963) Unpublished report
Street, J. C. et al. (1957) Proc. West Sec. Amer. Soc. Anim. Prod.,
46 (1)
Treon, J. F. & Cleveland. F. P. (1955) J. Agr. Food Chem., 3, 402
BIOLOGICAL DATA
Biochemical aspects
Dieldrin labelled with 14C is preferentially excreted together
with its metabolites in the bile. Fifteen minutes after an intravenous
injection, radioactive dieldrin and a hydrophilic metabolite were
found in the bile (Mörsdorf, et al. 1963).
Studies with 36Cl-dieldrin have shown that the initial
distribution is general, but within a few hours of injection it
concentrates more in the fat. Excretion of 36Cl averages about 5% per
day and is markedly increased by a restriction of diet, which reduces
the body fat. From the canulated bile duct, excretion accelerates as
the rat loses weight exceeding 10% per day after a few days. Only 3%
of the 36Cl is excreted as dieldrin unless the bile is canulated,
when up to 10% may be excreted. The remainder of 36Cl is found in the
metabolites and these are excreted to 90% in the faeces and 10% in the
urine. The most important metabolite containing about 60% of the total
36Cl is excreted in the bile (Heath & Vandekar, 1964).
In experiments 14C dieldrin it was demonstrated that in the
blood dieldrin is located mainly in the erythrocytes and plasma.
Haemoglobin is largely but not entirely responsible for the binding to
erythrocytes. In the plasma, dieldrin binds with soluble proteins
(Moss & Hathway, 1964).
After the feeding of dieldrin to animals it is stored in the
adipose tissues. Although small amounts of dieldrin are found in
liver, kidney and muscle tissues, the greatest amount of storage is in
the fat where dieldrin is stored unchanged, (Bann et al., 1956;
Butcher et al., 1957; Heath & Vandekar, 1964; Ivey et al., 1961;
Lehman, 1956; Street et al., 1957). It is lost slowly from the body
fat (Butcher et al., 1957; Heath & Vandekar, 1964). It is stored in
human fat in significant amounts (Hunter et al., 1963).
In studies on the urine of individuals exposed to dieldrin, on
paper and column chromatography 2 neutral polar metabolites of
dieldrin were found. No dieldrin could be detected with this method,
but dieldrin or a material having the same retention time was found by
gas chromatography (Cueto & Hayes, 1962).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 38 Borgmann et al., 1952
Council of Europe, 1962
Rat Oral 37-87 Borgmann et al., 1952
Council of Europe, 1962
Gaines, 1960
Heath & Vandekar, 1964
Lehman, 1951
Lu, et al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann et al., 1952
Council of Europe, 1962
Rabbit Oral 45-50 Borgmann et al., 1952
Council of Europe, 1962
Dog Oral 56-80 Borgmann et al., 1952
Council of Europe, 1962
Sheep Oral 50-75 Borgmann et al., 1952
Council of Europe, 1962
In rats the LD50 for a day-old animal is 168 mg/kg body-weight,
the same as in adults (Lu et al., 1965).
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment, groups of 10 male and 10 female rats were given 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm
of technical dieldrin. The rats were killed after 2, 4, 6 and 8
months. Food intake and growth were normal. No significant change in
liver weight was found. Cytoplasmic alteration of the liver cells was
found at both concentrations in males and females (Ortega et al.,
1957).
Dog. Dogs (1-4 animals per group) were given diets containing
1, 3, 10, 25 and 50 ppm dieldrin on six days per week. The animals on
25 and 50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm
survived for 9 months and on 1 and 3 ppm for 15 months. In the groups
on 1 and 3 ppm the livers were significantly larger than those of the
controls. Histological changes were noted in brains, liver and kidneys
(Treon & Cleveland, 1955). Groups of 4 dogs (2 male and 2 female) were
given 1 and 3 ppm of dieldrin in their diet for 68 weeks. The
concentration of 3 ppm increased the liver/body-weight ratio and
produced renal damage in 1 female. With 1 ppm of dieldrin, livers were
enlarged but no histopathological changes were found. In the dogs fed
3 ppm of dieldrin, 45.5 ppm were found in the fat, and in the dogs fed
1 ppm, 3.4 ppm were found in the fat. In other organs, concentrations
ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were found (Borgmann
et al., 1952; Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of
0.2, 0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313
days. The highest dosage killed all the dogs. Two of the 4 dogs died
when given 0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day
of recrystallized dieldrin per kg of body-weight for one year; 3 of
them produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and
10 male) were given dieldrin orally at dosage levels of 0.625, 1.25,
2.5, 5 and 10 mg/kg body-weight per day. Survival rates were affected
at all levels. At 2.5 mg/kg and above, all animals died (Borgmann et
al., 1952).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice,
equally divided by sex, were fed a diet containing 10 ppm of dieldrin
for their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in
the diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially reduced
the number of pregnancies but this effect tended to disappear with
continued feeding of the diet. All doses increased the mortality among
the suckling young. The effect on survival during suckling was severe
at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female)
were given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of
50 ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin
in the diet at a concentration of 75 ppm for 440 days. Twenty animals
of each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good conditions
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight ratio was markedly increased in the rats
killed during exposure, but it returned to normal after the end of it.
"Lesions of the hepatic parenchyma that have been considered typical
of exposure to the organochlorine insecticides in rats" were observed
only in healthy animals killed during the treatment. Rats dying
spontaneously or killed after withdrawal of the insecticide did not
show such type of change (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin
in their diets for a period of 40 months including 4 gestation
periods. At 25 ppm lambs died shortly after birth. Liver function as
well as other physiological tests on the ewes did not show any changes
related to the treatment with dieldrin (Shell, 1963).
Comments on the experimental studies reported
The primary mode of action of dieldrin is on the central nervous
system. This is the mechanism of death in acute poisoning. Symptoms of
central nervous system stimulation are also seen after repeated high
doses. Repeated doses at lower levels give rise to liver damage and in
this respect young dogs are more susceptible than rats.
In one long-term rat feeding experiment, there was a general
increase in tumour production in the experimental animals at the lower
dosage levels as compared to the controls, but the liver was not
particularly affected.
Liver tumours, however, were significantly increased on a dosage
of 10 ppm in one strain of mice susceptible to the development of
these tumours. Some evidence has been presented that the same effect
can occur in another strain of mice, but this requires more
information.
In rats, it has been suggested (Fitzhugh et al., 1964) that the
apparent tumorgenic properties of dieldrin could be related to a
"general" type of effect.
EVALUATION
From the data presented, an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming, every
effort should be made to see that the intake of dieldrin for man is
kept at the lowest possible level.
Further work required
Additional long-term toxicity studies in other species than the
rat, including further reproduction studies. Determination of a
no-effect level in more than one species.
REFERENCES
Bann, J. M. et al. (1956) J. Agr. Food Chem., 4, 937
Borgmann, A. R. et al. (1952) Unpublished report, July
Butcher, J. E. et al. (1957) Research report, Agricultural
Experimental Station, Utah State Agricultural College
Council of Europe (1962) Agricultural pesticides, Strasbourg
Cueto, C. & Hayes, W. J., jr (1962) J. Agr. Food. Chem., 10, 366
Davis. K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. pharmacol.,
4, 187
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Data submitted
by Shell Company
Ivey, M. C. et al. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 3 (In press)
Mörsdorf, K. et al. (1963) Med. exp., 8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J. & Burham, W. F. (1957) Arch. Path., 64,
614
Shell Chemical Company (1963) Unpublished report
Street, J. C. et al. (1957) Proc. West Sec. Amer. Soc. Anim. Prod.,
46 (1)
Treon, J. F. & Cleveland. F. P. (1955) J. Agr. Food Chem., 3, 402
