
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
DIELDRIN
Explanation
Since the previous comprehensive evaluation (FAO/WHO, 1968) additional
information has become available and is summarized and discussed in
the following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Because the conversion of aldrin to dieldrin appears to be the primary
metabolic step in all mammalian species studied, the data summarized
in this section can be considered to be applicable to aldrin also.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
The concentration of dieldrin in blood and liver of rats fed 50 ppm
increased for nine days and then remained fairly constant during the
remaining six months; after 16 days the dieldrin level in the fat also
reached a relatively constant value. The mean ratios after equilibrium
was reached were approximately 1 : 30 : 500 for blood, liver and fat
respectively (Diechmann et al., 1968).
It was found that highest concentrations of dieldrin in rats fed 10
ppm were found in the fat followed by the liver, brain and blood in
that order. The half-lives of dieldrin in the fat and brain were
estimated to be ten and three days respectively. (Robinson et al.,
1969).
In the rat reproduction study described below, the average
concentration of dieldrin in body fat was 18 times that in the diet,
and in the maternal milk it was 17 times greater. The maximum
excretory rate was estimated at 0.42 mg/kg body-weight per day and the
maximum excretion in milk was 1-4 mg per lactation period (Harr et
al., 1970).
In the two-year feeding study in rats and dogs, it was found that
equilibrium in tissue concentrations in rats was attained after six
months of feeding, the changes that occurred in the subsequent 18
months being very small. A similar situation was evident with dogs,
although an unexplained significant increase in the blood level of
dieldrin occurred during the last six months of the study (i.e. after
18 months' feeding). In female rats the tissue concentration of
dieldrin was 2-10 times that of males fed the same dietary
concentration; however, a similar sex difference was not noted with
dogs (Walker at al., 1969a).
The ratio of the concentration of dieldrin in human fat to that in
blood, once equilibrium was established, was about 140, a ratio which
is similar to that found with rats and dogs. After termination of
feeding dieldrin, the estimated biological half-life was 369 days.
Relationships between the intake levels of dieldrin and the levels in
blood and fat were developed from these studies and have been applied
to humans exposed to dieldrin (Hunter et al., 1969). See also
"Observations in Man" (Jager, 1970).
Of 43 workers who were removed from exposure to dieldrin, it was
calculated that the half-life of dieldrin in these subjects was seven
months. The mean dieldrin concentration in blood during the last half
year of exposure was 0.1 ppm which corresponded to an average daily
oral intake of 1.03 mg (about 0.17 mg/kg body-weight) (Jager, 1970).
When dairy cattle were fed 0.1 mg/kg body-weight per day of dieldrin
for four months, there was a sharp decline in the dieldrin content of
the milk after two weeks cessation, and a further less marked decline
after 11 weeks. (Braund et al., 1969).
Male rats, with or without bile fistulas were given intravenous doses
of 0.25 mg/kg body-weight of 14C-labelled dieldrin. In the intact
animals the mean total recovery of radioactivity in the faeces, urine
and carcasses was 96.9 percent of the original dose. Over 90 percent
occurred in the faeces. In the animals with bile fistulas the mean
total recovery was 86.9 percent of the original dose and over 90
percent occurred in the bile. A comparison was made with the
stereoisomer of dieldrin, endrin, which had more rapid faecal
elimination or biliary excretion. The liver has been identified as the
reason for the difference by in vitro studies using perfused liver.
The results may explain the greater storage of dieldrin in the body
than endrin (Cole et al., 1970).
The concentration of dieldrin in the fat of pregnant women was 0.08
ppm versus 0.17 ppm in the non-pregnant, a fact which suggests a more
rapid metabolism of this insecticide during pregnancy. Dieldrin was
also present in the maternal and umbilical cord blood and the foetal
blood concentration was very close to that in the maternal blood.
(Polishuk et al., 1970).
Biotransformation
Information available on the metabolism of dieldrin up to 1967 has
recently been reviewed (Brooks, 1969).
The mammalian conversion of aldrin to dieldrin has been known since it
was first discovered in the mouse (Winteringham and Barnes, 1955).
This reaction has been demonstrated in rabbit-liver microsomes and
requires the presence of NADPH, the activity being destroyed by heat
(Nakatsugawa et al., 1965).
The enzyme involved is aldrin epoxidase, the activity of which appears
to vary greatly, depending upon the preparation and the temperature of
storage (Chan and Terriere, 1969).
The information currently available on the various pathways of
metabolism of dieldrin in mammals is based on in vivo and in vitro
studies in several species, including the mouse, rat, rabbit, monkey,
sheep and man (Baldwin and Robinson, 1970; Baldwin et al., 1970;
Damico et al., 1968; Datta et al., 1965; Feil et al., 1970; Hedde et
al., 1970; Klein et al., 1968; Korte and Arent, 1965; Matthews and
Matsumura, 1969; Richardson et al., 1968; Robinson, 1970a, 1970b).
Although there appears to be some sex and species differences in
metabolism, the overall picture is summarized in Figure 1.
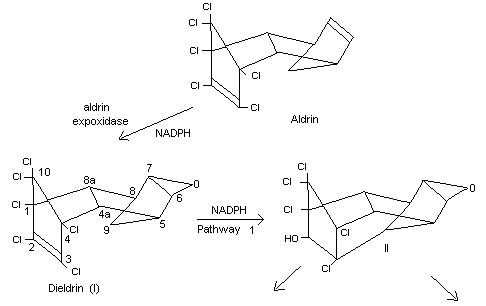
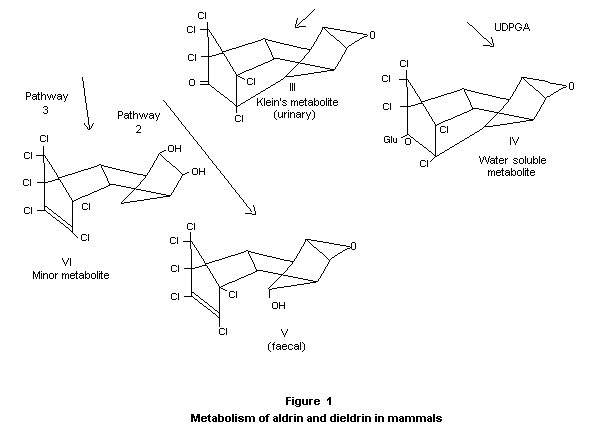 In a comparative study on the metabolism of dieldrin in mice and rats
administered 14C-labelled dieldrin, ten times more radioactivity
appeared in the faeces than in the urine of both species. More
unchanged dieldrin occurred in the rat urine than in mouse urine, and
Klein's metabolite (III) was found only in rat urine. Other minor
differences in urinary metabolites occurred between the two species,
and in the mouse they were largely unidentified (Baldwin and Robinson,
1970).
Some information on dieldrin metabolism has recently become available
both in humans and in non-human primates. Neither unchanged dieldrin
nor the urinary metabolites found in rats were identified in workers
occupationally exposed to dieldrin or in monkeys fed dieldrin. The
faeces contained the majority of the metabolites of dieldrin (Robinson
1970a, 1970b).
Effect on enzymes and other chemical parameters
A study of the ultrastructural and biochemical effects of dieldrin and
phenobarbitone upon the liver of rats, dogs and mice has been reported
(Donninger et al. 1967; Donninger, 1969 and Wright et al. 1968), and
the comparative effects have been reviewed (Potter et al. 1970).
Rats were fed dieldrin at a dose of 8 mg/kg and phenobarbitone at 140
mg/kg. Dogs were given 2.0 mg/kg of dieldrin and 20 mg/kg of
phenobarbitone. Mice received doses of dieldrin ranging from 0.16 to
7.5 mg/kg and doses of phenobarbitone between 1.2 and 120 mg/kg. In
addition, the effect of the administration of 80 mg/kg of the
carcinogen 4-amino-2,3-dimethylazobenzene (butter yellow) was studied
in mice. Increase in the activity of microsomal enzymes was observed
in all three species following the administration of dieldrin or
phenobarbitone, while no increase was seen after butter yellow
administration in mice. A marked increase in the liver smooth
endoplasmic reticulum (SER) occurred in rats and dogs. Considerably
less increase in SER was observed in mice. A further difference was
that in the mouse the relative increase of DNA indicated that increase
of liver-weight in the mouse was the result of new cell formation,
while in the rat and dog there was cellular hypertrophy. No effect
upon the ultrastructure of the mouse liver was observed following the
administration of butter yellow. A progressive decrease in the
activity of glucose-6-phosphatase was observed in the mouse liver
following administration of butter yellow, while the activity of this
enzyme was not changed following administration of dieldrin or
phenobarbitone (Potter et al. 1970).
Dieldrin was fed to male Rhesus monkeys at levels of 0.1, 0.5, 1.0,
1.75 and 5.0 ppm (0.0002-0.07 mg/kg) in the diet for approximately six
years. No significant liver changes were observed at dietary levels
below 1.0 ppm. A dose-related increase in microsomal P450 was found at
the higher levels. Increase of microsomal enzyme activity was observed
at the two highest levels, but no changes in sub-cellular structure
were found. (Wright et al., 1969, Zavon, 1970).
A no-effect level for enzyme induction was established in rats at
between 1 and 5 ppm of dieldrin (Gillett et al., 1968). Kinoshita et
al. (1970) also established a diet level of approximately 1 ppm as the
no-effect level. These workers observed the greatest amount of
increase after one to three weeks of feeding with dieldrin.
After an extensive study of workers in a manufacturing plant, it was
concluded that no measurable microsomal enzyme induction occurs in man
after long exposure to approximately 0.010 mg/kg/day. No evidence of
enzyme stimulation was observed in a smaller group of workers
absorbing higher amounts of dieldrin, up to 0.020 mg/kg/day (Jager,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of equal numbers (125-300) of male and female mice (CFI strain)
were fed dietary levels of 0, 0.1, 1.0 or 10 ppm of dieldrin for
periods of up to 132 weeks. In addition, a positive control group of
50 mice received 600 ppm of the carcinogen,
4-amino-2,3-dimethylazobenzene (butter yellow). Feeding was started at
four weeks of age. The presence of liver tumours was detected
initially by palpation, which was started on all animals after 16
weeks of feeding, and the animals were sacrificed when the tumours
became large enough to endanger health. No effect on health or
behaviour was evident in the first nine months of feeding, and no
liver tumours were detectable before 37 weeks at any dose level of
dieldrin. The morbidity of the mice receiving 10 ppm of dieldrin
increased after nine months, and at 15 months 50 percent of both sexes
had either died or been sacrificed because of tumour size. The control
group normally died after 20-24 months, and the life-span of the 0.1
and 1 ppm group wan the same; these groups displayed no palpable
masses. All the positive control group died after 14 months, although
feeding of butter yellow had been terminated after six months when the
first hepatoma appeared. Liver tumours increased in all test groups
but not in other tissues. Tumours were of two types: type (a)
consisted of solid cords of closely packed parenchymal cells with
morphology and staining characteristics similar to the rest of the
parenchyma. Little mitotic activity occurred, and growth appeared to
be by expansion. These growths were classified as benign tumours. In
the second type, type (b) tumours, a much more abnormal structure was
present with areas of cells proliferating in confluent sheets and
often with foci of necrosis. There were areas of papilliform or
adenoid formation of liver cells with wide and irregular vascular
channels within the growth. The mitotic activity was often increased
and multinucleate forms were seen. On purely morphological criteria,
the authors classified these tumours as hepatocarcinomas. They were
very uncommon in the control group of mice. In a few cases these type
(b) tumours had emboli of cells in the lungs. Dieldrin increased the
incidence of both types of tumour (see Table I) but did not produce
the fibrosis and bile duct proliferation sometimes observed in the
mice given butter yellow. In order to determine if tumour-formation
was reversible, certain mice from the group fed 10 ppm of dieldrin
were transferred to a normal diet after 0, 2, 4, 8, 16, 32 or 64 weeks
and sacrificed after 104 weeks. Incidence of tumours indicated that
removal of dieldrin from the diet did not cause the tumours to regress
or disappear. (Dieldrin also produced liver enlargement and
cytoplasmic changes which were reversible). In companion studies,
sterilizing the diet or the bedding did not influence the incidence of
tumours in mice fed dieldrin (Walker et al., 1970a).
In connection with the increase in liver tumours arising in
susceptible species of mice it is noted that the incidence of
tumour-formation in C3H mice was also affected by variations in the
diet (Tannenbaum and Silverstone, 1949a, 1949b) and in microbiological
status (Roe and Grant, 1970).
Rat
In groups of 25 male and 25 female rats fed 0, 0.1, 1.0 or 10 ppm of
dieldrin for up to two years, the only pathological findings
attributable to dieldrin were the existence of liver parenchymal cell
changes in one male and four females fed 10 ppm. In one female,
microscopic intrahepatic nodules were evident, and in addition nodules
were observed after two years in two females fed 10 ppm and in one
female in the control group (Walker et al., 1969a).
Weanling rats were fed, for a lifetime, diets supplemented with
aldrin, 0, 20, 30 or 50 ppm; dieldrin, 20, 30 or 50 ppm; or endrin, 2,
6 or 12 ppm. Benign and malignant tumours were observed in 23 tissues
or organs - in 199 of 800 experimental rats and in 79 of 163 controls,
examined histologically. The highest number of tumours in all groups
occurred in mammary and lymphatic tissues. When compared to the
controls, male and female rats fed aldrin, 20, 30 and 50 ppm, and
dieldrin, 20, 30 and 50 ppm, showed a dose-related significant
decrease in the incidence of all tumours, particularly those of the
mammary and lymphatic tissues. In male rats fed aldrin and dieldrin 50
ppm, the tumour incidence was reduced from 46 (in 75 control rats) to
13 (in 45 rats fed aldrin 50 ppm) and to one benign tumour (a skin
papilloma) in 44 rats fed dieldrin. In the females fed aldrin and
dieldrin 50 ppm, the total number of tumours was 23 in 41 and 16 in
31, respectively, while the female control rats showed 104 tumours in
88 rats. In all the 963 rats examined, no primary malignant hepatic
tumours were found, only two benign hepatic hemangiomas, one in male
control and one in a female fed endrin 6 ppm (Diechmann et al., 1970).
Special studies on reproduction
Bird embryos
Exposure of chick, quail and pheasant embryos to aldrin produced an
oestrogen-like effect in the male genital tract, leading to the
persistence of the Mullerian ducts and a retardation of testicular
development (Lutz-Ostertag and Lutz, 1969).
TABLE I
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Dieldrin
Males
0 288 10 4 20 0.7 (1)*
(16-25)
0.1 124 22 4 26 0.8 (1)
(18-35)
1.0 111 23 8 31 0.9 (1)
(23-41)
10.0 176 37 57 94 0.6 (1)
(89-97)
Dieldrin
Females
0 297 13 - 13 -
(9-17)
0.1 90 23 4 27 -
(18-38)
1.0 87 31 6 37 1.1 (1)
(26-48)
10.0 148 37 55 92 4.1 (5)
(86-96)
Butter yellow
600 ppm
Males** 23 13 4 17 -
(5-38)
TABLE I (cont'd)
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Females 21 43 38 81 9.5 (2)
(58-94)
* Figures in parentheses are the actual number of mice showing this
lesion.
** The group of male mice fed 600 ppm of butter yellow had a 61 percent
incidence of haemangiosarcomas and anaplastic sarcomas.
Wildlife
Studies in the sparrow hawk have provided evidence that the decline in
this species may be due to a failure in reproduction stemming from
increased shell breakage (Lehner and Egbert, 1969; Porter and
Wiemeyer, 1969), possibly caused by dieldrin.
Quail
When groups of four male and six female quail were fed diets
containing 0, 10, 20, 30 or 40 ppm of dieldrin for periods up to
eighteen weeks, survival and reproduction were markedly affected at
the 20, 30 and 40 ppm levels. At 10 ppm there was only a marginal
effect upon survival, and reproduction was not greatly affected.
Although egg production was reduced in the higher levels, even birds
fed 40 ppm laid fertile eggs which had residues of over 50 ppm of
dieldrin (Walker et al., 1969b).
Mouse
Groups of male and female mice were fed dietary levels 0 or 5 ppm of
dieldrin for 30 days. Test and control mice were then randomly paired
and were continued on the same diet for a further 90 days, there being
a total of 101 pairs in the group fed dieldrin. Mortality in the test
group was comparable to the controls, but the test group produced
significantly smaller litters. There was, however, no difference in
the time taken to produce the litters (Good and Ware, 1969).
Rat
Rats were weaned at 28 days of age and then were started on diets
containing dieldrin in ten two-fold levels from 0.01 to 40 ppm. The
animals were sacrificed at ten equal logarithmic intervals up to 750
days of age. In the females receiving diets containing 0.08 to 0.16
ppm of dieldrin, the conception rate, rate of survival of young, and
the number weaned were normal. At higher levels all these parameters
decreased so that at levels greater than 2.5 ppm few young survived,
and at 20 ppm none survived. The maximum dietary exposure which did
not interfere with reproduction was 0.24 ppm (Harr et al., 1970).
Sheep
36 ewes were fed diets containing 0, 1.0, 5.0 and 25.0 ppm of dieldrin
over a period of 40 months, including two gestation periods. No
teratogenic effects were observed and reproductive performance was
normal. However, at the 25 ppm level the lambs died shortly after
birth (Harris and Greenwood, 1963).
Special studies on the photoisomerization product of dieldrin
Of various compounds that can arise due to the influence of sunlight
on aldrin and dieldrin, an identified product, referred to as
"photodieldrin" is the only product of any established importance; the
corresponding "photoaldrin" is of negligible practical significance.
Through the work of Robinson et al. (1965, 1966a), Parsons and Moore
(1966), and Rosen et al. (1966), it has been established that
"photodieldrin" has the cage structure A shown in Figure 2.
Rats ware fed diets containing 10 or 30 ppm of the photoisomer for
thirteen weeks. The animals were sacrificed at the end of the period,
and brain, liver and fat were analysed. The presence of the unchanged
photoisomer as well as the pentochloroketone (Klein's metabolite, B,
found in dieldrin metabolism) was demonstrated (Baldwin and Robinson,
1969a, 1969b).
Exposure of dieldrin to ultraviolet light produced the
hexachlorocyclo-isomer VII. This compound was produced in 7 percent
yield after two months or in 25 percent yield after 12 months exposure
to sunlight. The compound was found to be twice as toxic as dieldrin
to the housefly and mosquito (Rosen et al., 1966; Robinson et al.,
1966b).
The photoisomerization product of dieldrin (photodieldrin) has been
determined in a number of vertebrate species. Using oral or
intragastric injection, either alone or in dimethylsulfoxide solution,
it was found to be substantially more toxic than dieldrin in most
species. Convulsions similar to those seen from dieldrin occurred
prior to death. Table II summarizes the acute toxicity compared to
dieldrin (Brown et al., 1967).
In a comparative study on the metabolism of dieldrin in mice and rats
administered 14C-labelled dieldrin, ten times more radioactivity
appeared in the faeces than in the urine of both species. More
unchanged dieldrin occurred in the rat urine than in mouse urine, and
Klein's metabolite (III) was found only in rat urine. Other minor
differences in urinary metabolites occurred between the two species,
and in the mouse they were largely unidentified (Baldwin and Robinson,
1970).
Some information on dieldrin metabolism has recently become available
both in humans and in non-human primates. Neither unchanged dieldrin
nor the urinary metabolites found in rats were identified in workers
occupationally exposed to dieldrin or in monkeys fed dieldrin. The
faeces contained the majority of the metabolites of dieldrin (Robinson
1970a, 1970b).
Effect on enzymes and other chemical parameters
A study of the ultrastructural and biochemical effects of dieldrin and
phenobarbitone upon the liver of rats, dogs and mice has been reported
(Donninger et al. 1967; Donninger, 1969 and Wright et al. 1968), and
the comparative effects have been reviewed (Potter et al. 1970).
Rats were fed dieldrin at a dose of 8 mg/kg and phenobarbitone at 140
mg/kg. Dogs were given 2.0 mg/kg of dieldrin and 20 mg/kg of
phenobarbitone. Mice received doses of dieldrin ranging from 0.16 to
7.5 mg/kg and doses of phenobarbitone between 1.2 and 120 mg/kg. In
addition, the effect of the administration of 80 mg/kg of the
carcinogen 4-amino-2,3-dimethylazobenzene (butter yellow) was studied
in mice. Increase in the activity of microsomal enzymes was observed
in all three species following the administration of dieldrin or
phenobarbitone, while no increase was seen after butter yellow
administration in mice. A marked increase in the liver smooth
endoplasmic reticulum (SER) occurred in rats and dogs. Considerably
less increase in SER was observed in mice. A further difference was
that in the mouse the relative increase of DNA indicated that increase
of liver-weight in the mouse was the result of new cell formation,
while in the rat and dog there was cellular hypertrophy. No effect
upon the ultrastructure of the mouse liver was observed following the
administration of butter yellow. A progressive decrease in the
activity of glucose-6-phosphatase was observed in the mouse liver
following administration of butter yellow, while the activity of this
enzyme was not changed following administration of dieldrin or
phenobarbitone (Potter et al. 1970).
Dieldrin was fed to male Rhesus monkeys at levels of 0.1, 0.5, 1.0,
1.75 and 5.0 ppm (0.0002-0.07 mg/kg) in the diet for approximately six
years. No significant liver changes were observed at dietary levels
below 1.0 ppm. A dose-related increase in microsomal P450 was found at
the higher levels. Increase of microsomal enzyme activity was observed
at the two highest levels, but no changes in sub-cellular structure
were found. (Wright et al., 1969, Zavon, 1970).
A no-effect level for enzyme induction was established in rats at
between 1 and 5 ppm of dieldrin (Gillett et al., 1968). Kinoshita et
al. (1970) also established a diet level of approximately 1 ppm as the
no-effect level. These workers observed the greatest amount of
increase after one to three weeks of feeding with dieldrin.
After an extensive study of workers in a manufacturing plant, it was
concluded that no measurable microsomal enzyme induction occurs in man
after long exposure to approximately 0.010 mg/kg/day. No evidence of
enzyme stimulation was observed in a smaller group of workers
absorbing higher amounts of dieldrin, up to 0.020 mg/kg/day (Jager,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of equal numbers (125-300) of male and female mice (CFI strain)
were fed dietary levels of 0, 0.1, 1.0 or 10 ppm of dieldrin for
periods of up to 132 weeks. In addition, a positive control group of
50 mice received 600 ppm of the carcinogen,
4-amino-2,3-dimethylazobenzene (butter yellow). Feeding was started at
four weeks of age. The presence of liver tumours was detected
initially by palpation, which was started on all animals after 16
weeks of feeding, and the animals were sacrificed when the tumours
became large enough to endanger health. No effect on health or
behaviour was evident in the first nine months of feeding, and no
liver tumours were detectable before 37 weeks at any dose level of
dieldrin. The morbidity of the mice receiving 10 ppm of dieldrin
increased after nine months, and at 15 months 50 percent of both sexes
had either died or been sacrificed because of tumour size. The control
group normally died after 20-24 months, and the life-span of the 0.1
and 1 ppm group wan the same; these groups displayed no palpable
masses. All the positive control group died after 14 months, although
feeding of butter yellow had been terminated after six months when the
first hepatoma appeared. Liver tumours increased in all test groups
but not in other tissues. Tumours were of two types: type (a)
consisted of solid cords of closely packed parenchymal cells with
morphology and staining characteristics similar to the rest of the
parenchyma. Little mitotic activity occurred, and growth appeared to
be by expansion. These growths were classified as benign tumours. In
the second type, type (b) tumours, a much more abnormal structure was
present with areas of cells proliferating in confluent sheets and
often with foci of necrosis. There were areas of papilliform or
adenoid formation of liver cells with wide and irregular vascular
channels within the growth. The mitotic activity was often increased
and multinucleate forms were seen. On purely morphological criteria,
the authors classified these tumours as hepatocarcinomas. They were
very uncommon in the control group of mice. In a few cases these type
(b) tumours had emboli of cells in the lungs. Dieldrin increased the
incidence of both types of tumour (see Table I) but did not produce
the fibrosis and bile duct proliferation sometimes observed in the
mice given butter yellow. In order to determine if tumour-formation
was reversible, certain mice from the group fed 10 ppm of dieldrin
were transferred to a normal diet after 0, 2, 4, 8, 16, 32 or 64 weeks
and sacrificed after 104 weeks. Incidence of tumours indicated that
removal of dieldrin from the diet did not cause the tumours to regress
or disappear. (Dieldrin also produced liver enlargement and
cytoplasmic changes which were reversible). In companion studies,
sterilizing the diet or the bedding did not influence the incidence of
tumours in mice fed dieldrin (Walker et al., 1970a).
In connection with the increase in liver tumours arising in
susceptible species of mice it is noted that the incidence of
tumour-formation in C3H mice was also affected by variations in the
diet (Tannenbaum and Silverstone, 1949a, 1949b) and in microbiological
status (Roe and Grant, 1970).
Rat
In groups of 25 male and 25 female rats fed 0, 0.1, 1.0 or 10 ppm of
dieldrin for up to two years, the only pathological findings
attributable to dieldrin were the existence of liver parenchymal cell
changes in one male and four females fed 10 ppm. In one female,
microscopic intrahepatic nodules were evident, and in addition nodules
were observed after two years in two females fed 10 ppm and in one
female in the control group (Walker et al., 1969a).
Weanling rats were fed, for a lifetime, diets supplemented with
aldrin, 0, 20, 30 or 50 ppm; dieldrin, 20, 30 or 50 ppm; or endrin, 2,
6 or 12 ppm. Benign and malignant tumours were observed in 23 tissues
or organs - in 199 of 800 experimental rats and in 79 of 163 controls,
examined histologically. The highest number of tumours in all groups
occurred in mammary and lymphatic tissues. When compared to the
controls, male and female rats fed aldrin, 20, 30 and 50 ppm, and
dieldrin, 20, 30 and 50 ppm, showed a dose-related significant
decrease in the incidence of all tumours, particularly those of the
mammary and lymphatic tissues. In male rats fed aldrin and dieldrin 50
ppm, the tumour incidence was reduced from 46 (in 75 control rats) to
13 (in 45 rats fed aldrin 50 ppm) and to one benign tumour (a skin
papilloma) in 44 rats fed dieldrin. In the females fed aldrin and
dieldrin 50 ppm, the total number of tumours was 23 in 41 and 16 in
31, respectively, while the female control rats showed 104 tumours in
88 rats. In all the 963 rats examined, no primary malignant hepatic
tumours were found, only two benign hepatic hemangiomas, one in male
control and one in a female fed endrin 6 ppm (Diechmann et al., 1970).
Special studies on reproduction
Bird embryos
Exposure of chick, quail and pheasant embryos to aldrin produced an
oestrogen-like effect in the male genital tract, leading to the
persistence of the Mullerian ducts and a retardation of testicular
development (Lutz-Ostertag and Lutz, 1969).
TABLE I
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Dieldrin
Males
0 288 10 4 20 0.7 (1)*
(16-25)
0.1 124 22 4 26 0.8 (1)
(18-35)
1.0 111 23 8 31 0.9 (1)
(23-41)
10.0 176 37 57 94 0.6 (1)
(89-97)
Dieldrin
Females
0 297 13 - 13 -
(9-17)
0.1 90 23 4 27 -
(18-38)
1.0 87 31 6 37 1.1 (1)
(26-48)
10.0 148 37 55 92 4.1 (5)
(86-96)
Butter yellow
600 ppm
Males** 23 13 4 17 -
(5-38)
TABLE I (cont'd)
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Females 21 43 38 81 9.5 (2)
(58-94)
* Figures in parentheses are the actual number of mice showing this
lesion.
** The group of male mice fed 600 ppm of butter yellow had a 61 percent
incidence of haemangiosarcomas and anaplastic sarcomas.
Wildlife
Studies in the sparrow hawk have provided evidence that the decline in
this species may be due to a failure in reproduction stemming from
increased shell breakage (Lehner and Egbert, 1969; Porter and
Wiemeyer, 1969), possibly caused by dieldrin.
Quail
When groups of four male and six female quail were fed diets
containing 0, 10, 20, 30 or 40 ppm of dieldrin for periods up to
eighteen weeks, survival and reproduction were markedly affected at
the 20, 30 and 40 ppm levels. At 10 ppm there was only a marginal
effect upon survival, and reproduction was not greatly affected.
Although egg production was reduced in the higher levels, even birds
fed 40 ppm laid fertile eggs which had residues of over 50 ppm of
dieldrin (Walker et al., 1969b).
Mouse
Groups of male and female mice were fed dietary levels 0 or 5 ppm of
dieldrin for 30 days. Test and control mice were then randomly paired
and were continued on the same diet for a further 90 days, there being
a total of 101 pairs in the group fed dieldrin. Mortality in the test
group was comparable to the controls, but the test group produced
significantly smaller litters. There was, however, no difference in
the time taken to produce the litters (Good and Ware, 1969).
Rat
Rats were weaned at 28 days of age and then were started on diets
containing dieldrin in ten two-fold levels from 0.01 to 40 ppm. The
animals were sacrificed at ten equal logarithmic intervals up to 750
days of age. In the females receiving diets containing 0.08 to 0.16
ppm of dieldrin, the conception rate, rate of survival of young, and
the number weaned were normal. At higher levels all these parameters
decreased so that at levels greater than 2.5 ppm few young survived,
and at 20 ppm none survived. The maximum dietary exposure which did
not interfere with reproduction was 0.24 ppm (Harr et al., 1970).
Sheep
36 ewes were fed diets containing 0, 1.0, 5.0 and 25.0 ppm of dieldrin
over a period of 40 months, including two gestation periods. No
teratogenic effects were observed and reproductive performance was
normal. However, at the 25 ppm level the lambs died shortly after
birth (Harris and Greenwood, 1963).
Special studies on the photoisomerization product of dieldrin
Of various compounds that can arise due to the influence of sunlight
on aldrin and dieldrin, an identified product, referred to as
"photodieldrin" is the only product of any established importance; the
corresponding "photoaldrin" is of negligible practical significance.
Through the work of Robinson et al. (1965, 1966a), Parsons and Moore
(1966), and Rosen et al. (1966), it has been established that
"photodieldrin" has the cage structure A shown in Figure 2.
Rats ware fed diets containing 10 or 30 ppm of the photoisomer for
thirteen weeks. The animals were sacrificed at the end of the period,
and brain, liver and fat were analysed. The presence of the unchanged
photoisomer as well as the pentochloroketone (Klein's metabolite, B,
found in dieldrin metabolism) was demonstrated (Baldwin and Robinson,
1969a, 1969b).
Exposure of dieldrin to ultraviolet light produced the
hexachlorocyclo-isomer VII. This compound was produced in 7 percent
yield after two months or in 25 percent yield after 12 months exposure
to sunlight. The compound was found to be twice as toxic as dieldrin
to the housefly and mosquito (Rosen et al., 1966; Robinson et al.,
1966b).
The photoisomerization product of dieldrin (photodieldrin) has been
determined in a number of vertebrate species. Using oral or
intragastric injection, either alone or in dimethylsulfoxide solution,
it was found to be substantially more toxic than dieldrin in most
species. Convulsions similar to those seen from dieldrin occurred
prior to death. Table II summarizes the acute toxicity compared to
dieldrin (Brown et al., 1967).
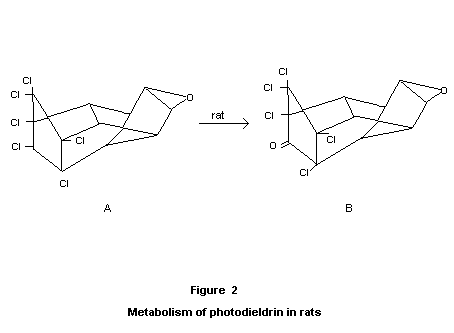 TABLE II
Toxicity of the photoisomerization product of dieldrin
compared with dieldrin
Species Approximate LD50, mg/kg body-weight
Photoisomerization
product of dieldrin Dieldrin
Pigeon 90 250
Chicken 80 48
Mouse 7 77
Rat 10 47
Guinea-pig 3 24
Dog (M) 140 120
Dog (F) 100 90
Dieldrin or its photoisomerization product was fed to five male and
five female mice at dietary levels of 1, 3 and 10 ppm for the
photoisomerization product and 3, 10 and 3O ppm for dieldrin for one
month. The mice exposed to the photoisomerization product were
unaffected by 1 ppm, there was one male and one female death at 3 ppm
and all died at 10 ppm. The mice fed dieldrin were unaffected by 3 or
10 ppm, but three males and two females died at 30 ppm. Autopsies of
the mice revealed no abnormalities (Brown et al., 1967).
A similar experiment with rats produced no deaths from 10 ppm of the
photoisomerization product; a higher level was not tested. Autopsies
revealed no abnormalities. Levels were measured in the tissues; the
biological half-life of the photoisomerization product was 1.7 for the
males and 2.6 for the females, compared to 10.0 and 12.7 respectively
for dieldrin (Brown et al., 1967).
Subacute levels (5 mg per day) of 14C-labelled dieldrin or
photodieldrin were administered to young adult rats of both sexes for
a period of twelve weeks. Photodieldrin was given both orally and
intraperitoneally; dieldrin was given by stomach tube only. About 60
percent of the activity of 14C-photodieldrin was excreted in the
urine and faeces of male rats and 47 percent in those of female rats,
compared to 60 and 37 percent respectively with 14C-dieldrin. Females
retained three-ten times more 14C activity in their tissues than did
male rats after photodieldrin administration. A similar sex difference
in tissue levels was found after administration of dieldrin and
photodieldrin in female rats and of dieldrin in male rats. Extremely
high levels of 14C activity were detected in the kidneys of male, but
not female, rats receiving 14C-photodieldrin (Dailey et al., 1970).
Storage of the photodieldrin was mainly in the fat and up to 10 ppm it
was 2-15 times higher in females than in males. A metabolite of
dieldrin was detected in the kidneys and urine. The concentration of
this metabolite in the kidneys was about ten times that of the
photodieldrin. No toxicologic effects were detected at 1 ppm (Walker
et al., 1970b).
Similar results were reported by Walton et al. (1970) who also found
slight stimulation of liver microsomal enzymes.
Photodieldrin was fed to groups of 12 male and 12 female rats at
levels of 0.1, 1.0, 10 and 30 ppm in the diet for 13 weeks. Tremors
were observed in rats in the 10 and 30 ppm groups. Reduced food intake
and depressed growth also occurred at these levels. Two of the 10 ppm
and six of the 30 ppm females died. Liver/body-weight ratios were
increased in the 30 ppm groups and kidney weight was increased in the
10 and 30 ppm males. Histologic liver changes were observed in the 10
and 30 ppm groups (Walker at al., 1970b).
Short-term studies
Studies on the effect of dieldrin on the liver microsomal enzymes have
been reviewed under "Biochemical aspects". The following additional
studies have become available.
Dog
Three groups each of six dogs of mixed sex were fed 0, 0.2 or 2 mg/kg
body-weight of dieldrin by capsule for five days a week. The group
given the low dose was started at 1.0 mg/kg for five days, then
reduced to 0.2 mg/kg until day 62 and from then on given 2 mg/kg until
signs of intoxication were evident. A direct relationship was found
between the severity of symptoms and the blood concentration of
dieldrin. As the blood level rose from 0.37 to about 0.8 ppm, changes
progressed from a reduced food intake and growth reduction to the
onset of muscular spasm and finally convulsions. A direct relationship
was also found between blood and fat levels of dieldrin (Keane and
Zavon, 1969).
In an extension of the above-mentioned study, three groups each of six
dogs of mixed sex were given a similar dose regimen of dieldrin. It
was found that the period between dieldrin exposure and the onset of
toxic signs was dependent upon the obesity of the animal; the greater
the amount of body fat the longer it took for toxicity to develop.
Since food refusal preceded symptoms of intoxication, forced feeding
was able to suppress toxicity by preventing fat mobilization and the
associated release of toxic concentrations of dieldrin into the blood
stream, so avoiding the death of some animals (Keane et al., 1969).
Groups of five male and five female dogs were given by capsule daily
oral doses of 0, 0.005 or 0.05 mg/kg body-weight of dieldrin for
periods up to two years. Health, behaviour and body-weight were
unaffected by giving dieldrin, and the electroencephalograph readings
showed no abnormalities. Urine and blood findings were normal except
for an increase in plasma alkaline phosphatase after 30 weeks and a
reduction in serum protein at the 0.05 mg/kg dose. Bromosulphthalein
clearance was normal throughout the exposure period. Liver-weight and
liver to body-weight ratios were increased in the females fed 0.05
mg/kg, and decreased heart-weights, but not heart to body-weight
ratios, were found in all males in the test groups and decreased
spleen-weights at 0.05 mg/kg in the males. No abnormal gross or
histopathological findings associated with administration of dieldrin
were evident (Walker et al., 1969a).
Long-term studies
Rat
Dieldrin was fed to groups comprising 25 male and 25 female rats (45
each for the controls) at dietary levels of 0, 0.1, 1.0 or 10 ppm for
periods up to two years. In addition, a separate group comprising 15
animals of each sex were sacrificed after six, 12 or 18 months
feeding. Body-weight and food intake were unaffected by dieldrin, but
at 10 ppm all the animals became irritable after eight or 13 weeks in
the males and females respectively. Occasional convulsions occurred in
this group during handling. No adverse effect on mortality was
observed. There were no changes in haematology or serum enzyme levels
attributable to dieldrin. Liver weights were normal for the first 18
months of feeding, but after two years, increased liver weights and
liver to body-weight ratios were evident in the groups fed 1 and 10
ppm (Walker et al., 1969a).
OBSERVATIONS IN MAN
In a continuation of the study described in the previous monograph
addendum on dieldrin (FAO/WHO 1968), the 12 male subjects were
continued on the dose regimen of 0, 0.01, 0.05 or 0.21 mg of dieldrin
for a total of two years and were further followed during an
eight-month post-exposure period. All twelve men remained in excellent
health, and there were no changes in body-weights, fatness or
leanness, or other clinical abnormalities throughout the entire
exposure and post-exposure periods. No departures from normal or
pre-exposure values in the biochemical and haemotological parameters
investigated were observed, although there was a slight trend towards
a fall in plasma alkaline phosphatase activity in some men fed the
higher dose levels. This drop was of doubtful significance, and the
enzyme level returned to normal after two months post-exposure. The
concentration of dieldrin in the blood did not significantly change in
the groups given 0.05 mg/day, but at 0.21 mg/day there was a slight
increase during months 18 to 21, but little over months 21 to 24.
This increase was small compared to the first 18 months when the blood
level tripled. Increase in dieldrin in adipose tissue was evident at
all levels after administration for 92 days (Hunter et al., 1969).
Extensive studies of workers exposed to dieldrin in a manufacturing
plant have been reported. The average blood concentration of a group
of 35 workers with an exposure of over ten years was 0.035 mg/ml,
calculated to be equivalent to a dietary intake of 407 mg/man/day.
Intoxications had occurred in some of these individuals prior to 1964.
No definite deleterious effects were found in this high exposure
group, including some individuals who had suffered intoxications.
Clinical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein and serum protein spectrum,
determined every three months remained within normal limits. In an
extreme exposure group with blood levels above 0.2 ppm, alkaline
phosphatase values, although within normal limits, were higher than in
the control group.
Using as criteria of possible microsomal enzyme stimulation the blood
levels of pp'-DDE and the urinary ratio of 6-B-hydroxycortisol and
17-hydroxycortico steroids, no differences between exposed and
unexposed workers were found. The dieldrin workers were exposed to an
average daily intake of 593 mg/man. One group of ten workers with the
highest exposure had an average equivalent oral intake of 1224
mg/man/day.
Comparisons of work records between exposed and non-exposed groups did
not reveal any differences as to frequency or severity of diseases or
accidents or disability (Jager et al., 1970). See also "Biochemical
studies".
On the basis of fat samples only, Crabtree (1969) found in Australia a
mean fat concentration of 0.3 ppm equivalent to an intake of 16
mg/man/day. Bick (1967) reported lower figures while Wasserman (1968)
reported higher ones. These last figures may be due to analytical
errors (Lofroth, 1970).
Wasserman et al. (1969) reported very low levels of dieldrin in fat
tissues from South Africa. Jansen (1969), however, analyzing part of
the same samples found a higher mean of 0.159 ppm, equivalent to an
average absorption of 8.6 mg/man/day.
COMMENT
The increased risk of development of liver tumours in mice exposed to
dieldrin as compared with untreated control mice was regarded as the
main matter for concern. This increased risk was seen even at an
exposure level of 0.1 ppm in the diet. It was generally agreed that
the tumours were neoplasms and that those seen in treated mice were
similar in nature to those seen in untreated mice except insofar as
the tumours in dieldrin-treated mice tended to show the same
cytoplasmic changes as other liver parenchymal cells in the same
animals.
There was no evidence that tumours regressed or disappeared after
cessation of exposure to dieldrin, although the cytoplasmic changes
did regress under such circumstances. It is difficult to establish
unequivocal criteria for distinguishing between benign and malignant
neoplasms in mouse-liver. The incidence of mice showing tumour emboli
or metastases in the lungs was not significantly increased at any
levels of exposure to dieldrin up to 10 ppm for 132 weeks.
It was agreed that the effect of dieldrin in increasing the risk of
tumour development in mice could not at the present time be regarded
as sufficient evidence to categorize dieldrin as a carcinogen. Other
work showed that nonspecific factors such as calorie intake, hormonal
status and micro biological status may profoundly influence the risk
of liver tumour development in mice, some strains of which are in any
case peculiarly susceptible to each tumours. Also it was noted that
long term studies in rats and prolonged studies in dogs and monkeys
had revealed no evidence of hepatocarcinogenicity. It seems likely
that the effect of dieldrin in mice is probably a species-specific
phenomena.
Further information is urgently needed concerning the aetiology and
pathogenesis of liver tumours in mice. A comparison of the effect of
dieldrin in mice of high and low liver tumour susceptibility, with
parallel metabolic studies, might provide a basis for relating
observations of tumourogenicity in mice to other species, including
man. There are at present no experimental data referable to
teratogenicity.
Continued surveillance of persons occupationally exposed to dieldrin
for chronic toxic effects and manifestation of possible
carcinogenicity is recommended. In this connection, the extensive
surveillance of occupationally exposed workers, which is currently
being made, was noted.
Information on the varying patterns of metabolism in the mouse, sheep,
rat, monkey and man are as yet too incomplete to indicate whether the
possible carcinogenic potential in the mouse is likely to apply to
man.
For these reasons, it was felt that no change in the acceptable daily
intake established at the 1966 Joint Meeting could be made at this
time.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE IN MAN
0-0.0001 mg/kg body-weight, the sum of aldrin and dieldrin by weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Seed treatments
The use of aldrin and dieldrin as a rice seed treatment is now
considered important only in Brazil and to a lesser degree in Japan,
Costa Rica and El Salvador. The choice between aldrin and dieldrin is
primarily a question of agronomic factors, local preference and
economics prevailing. Treatment rates vary, but a rate of 2 oz /
bushel (0.2 percent active material) is standard. In Brazil, recent
figures show that out of 4,600,000 hectares planted, 3,680,000
hectares (80 percent) were treated; in Japan, out of 3,280,000
hectares planted, 169,000 hectares (0.5 percent) were treated; in
Costa Rica, out of 43,000 hectares planted, 21,500 hectares (50
percent) were treated and in El Salvador out of 31,000 hectares
planted, 6,200 hectares (20 percent) were treated.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In maize
The treatment of soils prior to planting maize represents the most
important of all uses for aldrin. Studies in the United States in
which aldrin was applied to soil at rates of 1, 2, 3, 4 and 6 lb /acre
and in which pre-harvest intervals ranged from 75-195 days resulted in
aldrin residues in maize grain ranging from <0.001-<0.01 ppm and
dieldrin residues from 0.002-0.02 ppm. Control values were equal to or
greater than treated values in virtually all cases. In the same
experiments, the corresponding residues of aldrin plus dieldrin in
green fodder were less than 0.034 ppm; in maize stover most levels
were less than 0.01 ppm and only in two subsamples did levels exceed
0.03 ppm. With rare exceptions, the totals for aldrin and dieldrin
fell well below 0.03 ppm (Shell Development Co., Reports RES 63-122,
64-135, 64-102, 63-111, 64-168, 64-166 Shell Chemical Co., Reports PRL
67-81, 67-62, 65-6, 67-92; Mourbry and Myrdal, pre-print
communication). Similar experiments in Germany, France, Spain,
Portugal, Australia, Mexico, Venezuela and Trinidad at application
rates of 1-5 kg/ha gave residues of aldrin plus dieldrin of <0.01 ppm
in maize grain; a second crop from treatment two years in succession
gave the same result. Residues in whole maize plants (green fodder) in
France, Spain, Australia and Trinidad were 0.01-ppm or less except for
one year samples from Trinidad which were 0.15 ppm. This appears to be
exceptional, since samples in the succeeding year were <0.01 ppm.
Limited data from France on residues in maize leaves ranged from
0.01-0.07 ppm in dry leaf. Based on this data, it is estimated that
whole stover would have residues less than 0.03 ppm. (Shell Research
Ltd., Reports WKGR 0114.70, 0053.68, ibid - unreported data). In the
U.S.A. extensive data have been obtained for maize grown in a series
of rotations typically employed in U.S. farm practice. Treatments were
between 1´-2´ lb/acre - occasionally 5 lb/acre - and covered 35 sites
and included two-eleven years. In no cases were residues detected in
grain (0.01 ppm dieldrin). In stover, 80 percent of the results were
below 0.01 ppm, and the upper limit was 0.02 ppm. No residues were
detected in green corn (Shell Chemical Co., Report PRL 66-73).
In potatoes
The treatment of potato soils at recommended rates (2-4 kg/ha) with
aldrin shortly before planting gave rise to residues in the whole
washed tubers of between <0.01 ppm and 0.1 ppm of aldrin plus
dieldrin. Typically, levels were 0.04-0.06 ppm. Levels in peeled
tubers were generally 1/3-´ of these values (Shell International
Chemical Co. Monograph, November 1968). More recent data developed by
Shell in summarized in Table III; data from Scandinavia is given in
Table IV.
Residues in potatoes resulting from two or three soil applications of
aldrin in consecutive years are summarized in Table V.
A trial in Portugal compared residues resulting from broadcast
applications with row applications made with aldrinised fertilizer,
all at 3 kg/ha. Results are shown in Table VI (Shell Research Ltd.,
Tech. Serv. Report WKTR.0025/69).
Potatoes were planted in plots in tropical countries from which maize,
grown on soil treated at 2-4 kg/ha just prior to sowing, had been
harvested. Aldrin + dieldrin residues in the tubers were from <0.01
to 0.07 ppm (mean--0.03 ppm) and in peeled tubers from <0.01 to 0.03
ppm (mean--0.01 ppm), as shown in Table VI (Shell Research Ltd.,
Report WKGR. 0114.70).
Residue data for potatoes grown in soils in temperate countries to
which aldrin treatment had been given one or two years before planting
are given in Table VII.
Analytical data for 256 samples of peeled potatoes and potato peel
obtained by the U.K. Ministry of Agriculture (Lee, 1968) from potatoes
grown commercially on treated (three or four lb./acre) land shows
residues of aldrin plus dieldrin in peeled tubers ranging from
0.0-0.14 ppm (mean--0.01 ppm). Calculated residues in whole tubers
based on 15 percent peel ranged from 0.0 to 0.23 ppm (mean--ca. 0.02
ppm).
In sugar beets, juice and pulp
Residues of aldrin plus dieldrin in sugar beets grown in soils treated
once with 2 kg/ha in the same year as cropping ranged from <0.01 to
0.03 ppm; 4 kg/ha resulted in residues ranging from <0.01 to 0.05 ppm
(Legar, 1966; van Steyvoort et al., 1968; Shell Research Ltd., Reports
WKTR.0053.68, WKGR.0123.69, WKGR.0114.70; Shell Chemical Co., Report
PRL-68-148). In contradiction to all these findings, Walker et al.,
(1965) found that one application of 5 lb./acre gave combined residues
in the whole fresh beet of 0.19 ppm and Smith et al., (1966) found
residues of 0.93 ppm resulted from 1 lb./acre of aldrin as a row
application at the time of sowing. However, 80 percent of this latter
residue occurred as aldrin, strongly indicating sample contamination.
Residues of dieldrin in sugar beet tops from beets grown in soils
treated once with aldrin at 1-6 kg/ha in various countries in Europe
and in Turkey ranged from <0.001 to 0.01 ppm (van Steyvoort et al.,
1968; Shell Research Ltd., Reports WKTR. 0053.68, WKGR.0123.69,
.0114.70). Residues of aldrin plus dieldrin in roots and tops of sugar
beets grown in soils treated two and sometimes three years in
succession are summarized in Table VIII.
Table III
Residues in potatoes arising from soil applications of aldrin
Application Range of Values, ppm No. of Results Arithmetic Mean
Rate Whole Peeled Whole Pooled Whole Peeled
kg/ha Tubers Tubers Tubers Tubers Tubers Tubers
2 <0.01-0.13 <0.01-0.05 24 10 0.06 0.02
3 0.01-0.16 - 18 - 0.08 -
4 0.03-0.24 0.01-0.07 10 10 0.11 0.04
2-4 <0.01-0.24 - 52 - 0.08 -
5 0.02-0.22 - 11 - 0.11 -
Shell Chemical Co., Reports PRL 68-147, PCY-64-10; Shell Research Ltd.,
Reports WKTR. 0025/69, Tech. Memo 137/65, Tech. Serv. Note 141/66,
WKTR. 0114. 70; Winnett and Reed, 1968.
Table IV
Aldrin and Dieldrin Residues in Potatoes Arising from single soil
applications in Scandinavia
Application Residues, ppm in
Rate, potato tubers
Country kg/ha Aldrin Dieldrin Reference
Sweden(1964) 2.5 <0.01 0.02
Sweden(1965) 2.5 <0.01-0.01 <0.01-0.02 Renvall and
Akerblom, (1968)
Sweden(1966) 2.5 <0.01 0.01
2.5 <0.01 0.05
2.5 0.01 0.02
2.5 0.02 0.13
2.5 0.03 0.12
Denmark 2.5 not found 0.05 Bro-Rasmussen
5.0* not found 0.02-0.05 and Voldum-
10.0* 0.02 0.03-0.10 Clausen, (1966)
* High rates
Table V
Aldrin plus dieldrin residnes in potatoes grown in plots which received
a number of annual treatments with aIdrin
Range of values
Application Aldrin + Dieldrin, Arithmetic
kg/ha ppm No. of results Mean, ppm
Whole Peeled Whole Peeled Whole Peeled
2 0.01-0.11 00.01-0-05 11 8 0.05 0.02
4 0.03-0.40 <0.01-0.01 8 8 0.14 0.03
Shell Research Ltd., Reports WKTR-0007.69, 0028.68, Tech. Serv. Note
141/66, Report WKGR.0114.70, 0153/69, Tech. Serv. Note WKTR. 0100.68.
Table VI
Comparison of residues in potato tubers from broadcast and
row treatments
Range of Results,
Type of Aldrin + Dieldrin, No. of Arithmetic
Application ppm Values Mean, ppm
Row treatment 0.09-0.27 4 0.15
Broadcast 0.04-0.07 3 0.05
(fertilized
mixture)
Broadcast (E.C.) 0.05-0.16 4 0.08
Table VII
Residues In potato tubers grown-in soils treated one or two years
prior to planting
Interval
Treatment Between Range of
Rate, Treatment and Results, No. of Arithmetic
kg/ha Planting, ppm Results Mean, ppm
years
2 1 <0.01-0.05 14 0.02
4 1 0.01-0.05 9 0.03
2-5 1 <0.01-0.11 29 0.04
2-5 2 0.01-0.02 12 0.01
Shell Research Ltd., Tech. Serv. Note 141/66, Report WKGR.0114.70,
Tech. Serv. Report WKTR 0007.69, .0028.68, Report WKGR 0153/69,
Tech. Serv. Note WKTR.0100.68, Winnett and Reed; 1968.
Residues of dieldrin in roots and tops of beets grown on plots treated
the previous year at 2 or 4 kg/ha were all <0.01 ppm (28 samples)
except for two root samples of 0.02 and 0.03 ppm; no aldrin was
detected (Elgar, 1966; Shell Research Ltd., Report WKGR.0114.70).
Recent studies by Onsager at al., (1970) show that aldrin plus
dieldrin residues in mature sugar beets are proportional to the
residues in the soil at the time of planting as illustrated in Table
IX.
Studies in Belgium (van Steyvoort et al., 1968) and in France (Shell
Research Ltd., Report WKTR.0053.68) indicate that no residues could be
found in purified juices from sugar beets grown in soil treated with
up to 6 kg/ha of aldrin. However, data from refineries in the U.S.A.
(Walker at al., 1965) show combined residues of 0.02 ppm (expressed as
ppm of pure sucrose) in clarified juice from beets with abnormally
high residue levels. Had these juices been processed, most of the
residue would have been found in the molasses rather than in the
sugar.
Data was obtained on dieldrin residues in dried pulp from beets grown
in soil which had received aldrin treatment for two years preceding
planting. A residue of 0.03 ppm in the fresh beet resulted in values
of 0.13-0.14 ppm in pulp dried at 105°C and 0.05-0.12 in pulp dried at
150°C; 0.07 ppm in fresh beet gave values of 0.28 ppm in pulp dried at
105°C and 0.34 ppm in pulp dried at 150°C (Shell Research Ltd.,
unreported data). The ratio of residues in dried pulp to fresh beet
was 3-4 which is lower than the figure of 10.6 reported by Walker et
al. (1965). The significance of pulp residues in terms of cattle feed
is questionable, however, due to the low amount of pulp (10-20
percent) in the total diet. Onsager et al. (1970) calculated that a
residue of 0.25 ppm in 4.5-8 lb of beet pulp fed daily to a 1200-lb
animal amounts to ingestion of 0.2-1.6 ppm of insecticide.
In crops derived from seed dressings
Both aldrin and dieldrin are extensively used as seed dressings on a
wide variety of crops; maize, rice, cereals, cotton and pulse crops
are the most important. A large amount of data collected primarily by
Shell shows that residues in the crop derived from dressed seed are
extremely small and usually below the limits of detection (about 0.005
ppm), except for occasional residues of 0.01-0.02 ppm in pulse crops.
In all cases where residues were much above the detection limit, there
are strong indications that samples were contaminated. Either residues
were high on control as well as treated samples, or else appreciable
proportions of aldrin were found in the total residue.
In vegetables
Residue data obtained up through 1968 is summarized in Table X (Shell
International Chemical Co., Monograph, Residues in Vegetables,
November 1968).
Table VIII
Residues in sugar beets grown in soils treated on more than one occasion
Rate of
Application, Range of Residues Arithmetic
kg/ha/ Part of Aldrin + Dieldrin, No. of Mean,
year Crop ppm Samples ppm
2 Root <0.01-0.04 8 0.01
4 Root <0.01-0.07 5 0.04
2 Tops <0.01-0.02 5 <0.01
4 Tops <0.01-0.02 5 <0.01
Table IX
Average residues of aldrin plus dieldrin in soil and sugar beet roots
1965-1968
Average residue, ppm
Aldrin Soil
dosage, sampled on Roots sampled on
lb ai/acre 4/10/65 10/5/65 10/25/66 10/17/67
0.625 0.05 <0.01 <0.01 <0.01
1.25 0.21 0.04 <0.01 <0.01
2.5 0.38 0.06 0.01 0.01
5.0 0.73 0.13 0.02 0.01
7.5 1.84 0.37 0.03 0.03
10.0 2.84 0.96 0.05 0.04
Table X
Residues from seed or soil treatment with aldrin and dieldrin at
application rates within the recommended range (typically 2-3 kg/ha).
Crop or Residues, ppm Location and
Crop Group Min Max Est. Median No. of Expts.
Carrots 0.02 0.51 0.20 USA:3, Switzerland:5,
Denmark:2, Holland, 3,
UK:3, Austria:2
Cruciferous 0.02 0.08 0.04 USA:20, Norway:1,
roots (1) Austria:2
Beetroot 0.01 0.22 0.10 USA:15
Cole crops (2) 0.01 0.02 0.015 USA:21
Onions ND ND ND UK:3, USA:1
Peas ND ND ND USA:1
Lettuce 0.10 0.16 - USA:2
Beans ND ND ND USA:3
Tomatoes 0.10 0.10 0.10 USA:3
Celery 0.02 ND ND UK:3
Cucurbits (3) 0.01 0.07 0.03 USA:10
(1) Including radish, turnip, rutabaga and swede.
(2) Including cabbage broccoli, brussels sprouts and cauliflower.
(3) Including cucumber, pumpkin and melon.
More recent data on beetroot, cauliflower, carrots, white cabbage,
lettuce, tomatoes, turnips, and sweet potatoes are consistent with the
findings in Table X and in general give slightly lower residue figures
(Bro-Rasmussen and Voldum-Clausen, 1966; Shell Research Ltd., Reports
WKTR. 0050.68, 0053.68; Shell Chemical Co., Reports PRL-68-112,
-68-210).
In cereal grain
Aldrin treatments of seed or soil at recommended rates have rarely
produced residues of aldrin plus dieldrin in grain of wheat, oats or
barley above 0.01 ppm. Foliar applications of aldrin or dieldrin to
the growing crop do not give measurable residues in grain or straw if
pre-harvest intervals of one month or more are observed (Shell
International Chemical Co., Monograph, Residues in Small Grains,
1968).
Recent data for wheat and barley grain from soils receiving a variety
of aldrin application regimes at rates of 2 or 4 kg/ha show no
detectable residues (<0.01 ppm) of either aldrin or dieldrin.
Residues in wheat straw ranged from <0.01-0.16 ppm and in barley
straw from <0.01-0.20 ppm. Straw residues are of limited significance
since wheat straw is not an appreciable component in the diet of dairy
cattle and barley straw is used as a feed primarily for beef cattle in
winter only (Shell Research Ltd., Reports WKGR.0114.70, WKTR-0053.68).
In citrus and sugar-cane
Soil applications of aldrin at 2.0-5.0 kg/ha in citrus groves resulted
in dieldrin residues of 0.01-0.02 ppm in whole oranges, 0.02 ppm in
chopped peel and 0.03-0.04 ppm in dried pulp; controls contained up to
0.01 ppm. Dieldrin residues were not found in whole grapefruit or
grapefruit juice, but peel contained 0.006-0.007 ppm and dried pulp
contained 0.01 ppm; residues were also found in control samples (Shell
Chemical Co., Report PRL-67-30).
No residues were found in any of six samples of sugar-cane grown in
soil treated with two applications of aldrin at 2 kg/ha (Shell
Chemical Co., Report PRL-68-51). Soil treatments with aldrin of 5 or
10 lb ai/acre resulted in apparent aldrin residues of 0.004 ppm in two
out of three foliage samples from the 10 lb/acre treatment and in
apparent dieldrin residues ranging from 0.008-0.015 ppm in 8 out of 16
samples of foliage and stalks. No residues were found in the juice at
any treatment rate, and one sample of bagasse (out of four) had 0.009
ppm dieldrin (Shell Chemical Co., Report PRL-68-236).
In milk and meat (from cattle feed)
Williams et al. (1964) fed 16 milking cows for 35 days with a feed
containing various amounts of dieldrin. Similar experiments were
carried out by Gannon et al. (1959a, 1959b) for 12 weeks and by Shell
(1970) for 21 days using dieldrin C-14. The results of these
investigations are summarized in Table XI.
After the feeding of dieldrin was stopped, dieldrin concentrations in
the milk began to fall with a half-life of 10-15 days in the case of
Williams' work and substantially longer in the case of Gannon's work.
The lack of consistency may be due to some cows coming to the end of
their lactation period, thus reducing the milk production per day.
Gannon et al. (1959a) also studied residues in body tissues of milk
cows and steers fed diets containing various amounts of dieldrin. His
results are shown in Table XII.
In poultry and eggs
The residues of dieldrin that could arise in poultry products from the
use of rice bran in the diet (typically 20 percent of the total diet)
are summarized in Table XIII.
Thus with residues of dieldrin in rice bran of 0.05 ppm, residues of
dieldrin in poultry products could be 0.012 ppm in eggs, 0.20 ppm in
fat and 0.02 ppm in poultry meat. Residues of up to 0.03 ppm dieldrin
are commonly found in rice bran.
FATE OF RESIDUES
General comments
The occurrence and toxicity of the photoisomer of dieldrin,
photodieldrin, has been reviewed in the section on biological studies.
Photodieldrin residues may be of significance only after foliar
application of dieldrin. No detectable residues were found in human
fat in the U.S.A. where the ratio of dieldrin to photodieldrin
residues was greater than 200 to 1 (Richardson, 1966). Photodieldrin
was also absent in human fat from U.K. and Holland (Richardson, 1968)
and milk (Robinson et al., 1966).
In animals
The fate of residues of dieldrin in animals has been covered under the
biochemical section of this monograph.
In plants
Extensive experimental work was carried out by Shell to determine
residues of photoaldrin and photodieldrin in crops grown on
aldrin-treated soil, grown from aldrin or dieldrin-treated seed, grown
in soils containing old residues or receiving foliar applications of
dieldrin, and general monitoring investigations. Their results
indicate that none of the soil or seed dressing uses of aldrin or
dieldrin lead to detectable residues of the photoisomers. Foliar
applications led to photodieldrin residues of 0.03 ppm in coffee beans
treated at 1-1/3 g of dieldrin/ tree, 0.02 ppm in leaves of forage
beets treated at 4.3 oz/acre, 0.31-0.89 ppm in wheat straw treated at
8 oz /acre, <0.01-0.04 ppm in apples and 0.01-0.06 ppm in apple
pomace treated at ´ lb/acre, 3.6 ppm in pasture grass one and a half
months after treatment with 1 oz/acre, and 1.1 ppm in fat of sheep
grazing for four months on the treated pasture grass. None of the
photoisomer was detected in any samples of vegetable oils and fats
monitored in the U.K., but some samples of food and feed components
contained traces.
Table XI
Dieldrin in milk from animals fed with feed containing
different amounts and for various lengths of time
Dieldrin in Butterfat Ratio,
feed, ppm, Milk level, ppm, equivalent, ppm, ppm B.F.
dry weight at time indicated at time indicated ppm Feed Reference
35 days 35 day
0 0.004 0.11 - Williams
0.09 0.021 0.53 5.9 et al.
0.23 0.058 1.52 6.6 (1964)
0.50 0.11 2.89 5.9
4 weeks 12 weeks 4 weeks 12 weeks
0 <0.01 0.01 - - - Gannon
0.1 <0.01 0.02 - 0.5 5.0 et al.
0.25 0.02 0.06 0.5 1.5 6.0 (1959b)
0.75 0.07 0.11 1.75 2.8 3.1
2.25 0.16 0.28 4.0 7.0 3.0
15-21 days 15-21 days
0.102 0.018 0.41 4.0 Shell
0.120 0.017 0.35 2.9 Development
0.111 0.015 0.38 3.5 Co., Report
M-32-70
Table XII
Dieldrin residues in body tissues of cows and steers after feeding at
various levels of dieldrin for twelve weeks
Milk cows Steers
Dieldrin in Feed, ppm Dieldrin in Feed, ppm
Body Part 0.25 2.25 0.1 0.25 0.75 2.25
Dieldrin in Tissues, ppm
Brain <0.1 0.1 - - - -
Heart 0.2 0.6 - - - -
Liver 0.2 0.7 - 0.1 - 0.7
Kidney <0.1 <0.1 - <0.1 - 0.2
Steak 0.1 1.3 - <0.1 - 1.0
Roast 0.2 1.2 - - - -
Renal Fat 0.9 6.2 - 0.9 - 9.7
Body Fat 0.9 4.8 0.4 0.8 3.5 8.7
Heart Fat - 7.0 - - - -
Udder Fat - 5.6 - - - -
The average ratio of dieldrin levels in fat to levels in feed was 2.75
in milk cows and 3.95 in steers.
Table XIII
Relation between dieldrin residues in poultry feed
and poultry products (eggs, meat, fat of meat)
Feed Range of Ratios,
Poultry Weeks Concentration Poultry Feed/
Product fed Range (ppm) Poultry Product Reference
Eggs 12 0.1-0.75 0.8-1.6 Gannon et al., 1959a
Eggs 16 0.006-5.0 1.0-3.0* Greaves et al., 1969a
Eggs 14 0.05-0.45 1.1-1.2 Cummings et al., 1966
Fat(abdominal) 2 0.1-0.15 20-29 Liska et al., 1967
Fat 12 0.1-0.75 41-48 Gannon et al., 1959a
Fat(abdominal) 14 0.05-0.45 8.8-9.7 Cummings et al., 1967
Meat 2 0.1-0.15 2-4 Liska et al., 1967
Meat 12 0.1-0.75 <0.2 Gannon et al., 1959a
Meat 14 0.05-0.45 0.1 Cummings et al., 1967
* The figure of 3 regarded as inconsistent with other data.
Lichtenstein et al. (1970) reported 0.015 ppm photodieldrin in soils
which had been treated annually with 5 lb/acre of aldrin for five
years and then not treated for five years. Potatoes and carrots grown
in these soils contained 0.0006 ppm and 0.002 ppm, respectively.
After foliar application of photodieldrin C-14 to white cabbage,
residues disappeared more slowly than in corresponding tests with
aldrin, dieldrin, isodrin or endrin. Four weeks after application, as
much as 75 percent of the applied radioactivity was present as
residues. Fifteen to 33 percent of metabolites were found mainly in
leaves. Metabolic fractions consisted of a very hydrophilic main
product and at least two less hydrophilic by-products (Korte and
Porter, 1970).
Four weeks after application of ca. 75 ppm of aldrin C-14 on the upper
side of cabbage leaves, only 17 percent of the applied radioactivity
was recovered in plants and soil. Hydrophilic metabolites made up 76
percent of the recovered activity; besides aldrin, five other
compounds were found, one of which appeared to be dieldrin. In similar
experiments with dieldrin C-14 on cabbage, 40 percent of the
radioactivity is recovered after four weeks and 34 percent of the
recovered activity is due to at least two hydrophilic metabolites
(Korte, 1970).
In soils
Maize grown in California in soil treated with 3 kg/ha of aldrin C-14
contained no detectable C-14 in the grain or cobs. The husks of the
ears contained 0.004 ppm aldrin, 0.004 ppm dieldrin and 0.032 ppm
metabolites. The leaves contained 0.35 ppm C-14 equivalents of which
0.02 ppm was aldrin and 0.05 ppm was dieldrin (Korte and Porter,
1970).
Evidence of residues in food in commerce
Belgium
Thirty local and sixteen imported samples of butter were examined
(Dejaukheere and Kips, 1968). In the local samples, residues of
dieldrin ranged from 0.01 to 0.24 ppm (mean 0.12 ppm), and in the
imported samples the range was 0.01 to 0.25 ppm.
Denmark
Samples of milk butterfat (44) contained mean levels of dieldrin of
0.03 ppm (<0.007-0.11) (Bro-Rasmussen et al., 1968). Samples of
butter examined over a three year period contained dieldrin residues
as follows:
1964 1965 1966
No. of samples 118 137 126
Range (ppm) 0.01-0.20 0.01-0.18 0.01-0.17
Mean (ppm) 0.05 0.04 0.03
The authors suggested from their data that 60-100 percent of these
residues originated from dieldrin residues in imported cattle feed.
France
Mestres, et al., (1967) in France found no residue of aldrin or
dieldrin in 44 samples of French butter or in four samples of Swiss
butter at a detection limit of 0.02 ppm.
West Germany
Of 65 samples of carrots taken in West Germany (Maier-Bode, 1967) 43
contained less than 0.01 ppm aldrin plus dieldrin. Of the remainder,
64 percent contained residues of 0.03 ppm or less, and only one sample
exceeded 0.1 ppm (0.17 ppm).
Norway
Twenty butter samples out of a total of 54 contained dieldrin residues
in the range 0.01-0.1 ppm (Sakshaug, 1968). The rest did not contain
residues, above the limit of detection.
U.K.
Routine analysis of food-stuffs by public analysts in the U.K. (Rymer
and Hammence, 1967) demonstrated that of 776 fruit, 461 vegetable and
237 meat and dairy produce samples, only three fruit samples and three
meat and dairy produce samples contained aldrin above the limits of
detection (maximum levels of 0.06 and 0.18 ppm respectively) and two
fruit, one vegetable and thirteen produce samples contained dieldrin
above the limits of detection (maximum 0.15 and 0.27 ppm).
A more recent examination of 388 fruit, 285 vegetable and 217 meat and
dairy produce samples (Dickes and Nicholas, 1969) revealed one
vegetable sample containing aldrin (0.09 ppm) and sixteen vegetable
samples containing dieldrin, although all were below 0.01 ppm.
Findlay and Hamilton (1968) examined 163 samples of eggs and 114
samples of poultry meat, liver and fat from nine sources within the
U.K. No egg samples exceeded 0.06 ppm of dieldrin and only one poultry
meat sample exceeded 0.1 ppm (0.13 ppm).
Table XIV
Dieldrin residues in dairy and meat products produced in U.K., 1965-1968
Butter Milk Mutton Fat Beef Fat
Year Mean Mean Mean Mean
Samples (ppm) Samples (ppm) Samples (ppm) Samples (ppm)
1964 127 0.84
1965 18 0.03 85 0.0025 107 1.15 59 0.06
1966 13 0.02 75 0.0025 101 0.44 63 0.04
1967 22 0.03 77 0.002 76 0.24 36 0.03
1968 16 0.03 76 0.001 77 0.21 34 0.03
A most comprehensive examination of home produced and imported meat
and dairy products has been carried out over a number of years by the
Laboratory of the Government Chemist (1966, 1967, 1968, 1969). In the
four years 1965-1968, 935 samples of home produced foods and 803
samples of foods imported from ten countries were analyzed. Table XIV
summarizes their findings for home-produced dairy and meat products.
Residues in food at time of consumption
A study of data accumulated over four years by the U.S. Food and Drug
Administration on pesticide residues in total diet samples was made by
Duggan and Lipscomb (1969).
Residues of dieldrin were found in from 15.6-21.3 percent of
composites analyzed over this period and aldrin residues were found in
from 3.3-5.6 percent. Measurable dieldrin residues were present in six
of the twelve food groups examined, and traces were found in another
four. Measurable aldrin residues were found in only one group. The
highest residues of dieldrin (max. 0.20 ppm) were found in samples of
meat, fish and poultry (Group 2). In the dairy products group (Group
1) the greatest level was 0.08 ppm and residues did not exceed 0.03
ppm in any other group. The maximum aldrin level was 0.07 ppm in the
first group (Group 9). No tendency to increase or decrease was
observed over this period. From the residue information and a
knowledge of the diet, the daily intake of each pesticide was
calculated. Since the diet was representative of that consumed by a
sixteen-nineteen year old American male, the intake of the average
population will be about half the calculated figure. The total daily
intake for aldrin and dieldrin over the four year period is given in
the following table (from Duggan and Lipscomb, 1969):
Daily Intake (mg/kg body-weight)
1965 1966 1967 1968
Aldrin 0.00001 0.00004 0.00001 0.00001
Dieldrin 0.00008 0.00009 0.00005 0.00005
Total 0.00009 0.00013 0.00006 0.00006
An examination of residues in groups of foodstuffs making up a
complete diet was undertaken by Abbott et al., (1969), at the
instigation of the U.K. Ministry of Agriculture, Fisheries and Food,
for one year from October 1966-October 1967. Of the foods analyzed,
the highest dieldrin residues were in fat (mean 0.024 ppm, range
0.009-0.075 ppm), followed by meat (mean 0.009 ppm, range 0.001-0.060
ppm). Because of the larger consumption of meat, this group provides
the largest intake of dieldrin 2.2 mg/person/day. The mean daily
intake in the diet calculated from the residues found was 0.00009
mg/kg body-weight. This figure confirms the decrease shown in the
second survey by McGill and Robinson (1968) compared with the first,
and may be attributed to the voluntary withdrawal of some previously
approved dieldrin uses in the U.K. over the period 1965-1967.
In 1965 the Association of Public Analysts prepared a survey to
determine the extent of contamination of foodstuffs by pesticides.
Data for the first year of this work has so far been reported
(Association of Public Analysts, 1969). Of the 2352 samples analyzed,
only ten contained residues of aldrin (two only above 0.1 ppm) and 76
contained dieldrin (again only two above 0.1 ppm). Dieldrin was most
commonly found in meat and dairy products. In no foodstuffs did the
mean level of dieldrin residues exceed 0.015 ppm, a figure that is
based on the assumption that all samples in which residues were not
detected (limit 0.002 ppm in solid foods and 0.0002 ppm in liquid milk
and infant foods) did in fact contain residues at half the detection
limit. Using the same assumption, the mean daily intake in the diet
was calculated to be 0.0001 mg/kg body-weight. A survey of residues in
welfare foods (dried full cream milk, cod-liver oil and concentrated
orange juice) by Ruzicka et al., (1967) revealed residues of up to
0.017 ppm dieldrin (mean 0.011 ppm) in dried milk and demonstrated a
15 percent loss in residue during the drying period. Cod-liver oil
contained 0.15-0.20 ppm (mean 0.16 ppm) and concentrated orange juice
contained up to 0.007 ppm (mean 0.002 ppm).
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967a) apply to the
determination of aldrin and dieldrin. Burke (1969) has studied the
application of the official AOAC multiresidue method to photodieldrin.
No methods of analysis have been well worked out for the more
hydrophilic metabolites of dieldrin such as monohydroxydieldrin,
dihydro-aldrin-diol (trans), dieldrin-ketone, dieldrin-ketone
photo-isomer, or Klein's metabolite. Although photoaldrin can be
determined by GLC techniques, the rapid conversion of aldrin to
dieldrin makes it unlikely that photoaldrin will be a significant
residue in crops or soils.
CONSIDERATION OF SPECIFIC POINTS RAISED BY THE CODEX COMMITTEE ON
PESTICIDE RESIDUES
The meeting considered three questions arising from the Draft Report
of the Fifth Session of the Codex Committee in 1970 as follows:
Further data on the use pattern of aldrin and dieldrin on rice are
provided in this monograph, as are data on residues resulting from the
use in animal feed.
The question of the fate of the residues on rice during processing was
not considered because the available data show that residues are so
small that any remaining following processing would be negligible.
No firm data were received on residues in fruit juices resulting from
the use of rice husks as clarifying agents. However, if it is assumed
that 10 percent rice husks are used for clarification, and if they
should contain up to 0.02 ppm of aldrin plus dieldrin, and if it is
further assumed that this is extracted by the fruit juices (unlikely
due to the lipophylic nature of residues), then it would be highly
improbable that residues in excess of 0.002 ppm in the juice would
occur.
APPRAISAL
The data provided on residues of aldrin plus dieldrin in maize grain
resulting from planting maize in soils treated at recommended rates
indicate that levels of 0.02 ppm or less could be expected and that
maize grain is within the recommended practical residue limit of 0.02
ppm on raw cereals listed in the 1969 Report of the Joint Meeting.
The data on residues of aldrin plus dieldrin in whole washed potato
tubers arising from soil applications of aldrin at recommended rates
reveals the possibility of exceeding the recommended tolerance of 0.1
ppm, especially when the potatoes are grown in plots receiving a
number of annual applications at the highest rate. An increased
tolerance of 0.2 ppm is recommended for potatoes.
Residues of aldrin plus dieldrin in mature sugar beets are
proportional to the residues in soil at planting. For example, an
aldrin dosage of 2.5 lb/acre gives after six months a soil residue of
0.11 ppm and a sugar beet root residue, grown in the same year, of
0.06 ppm. This would be expected to result in about 0.24 ppm of
dieldrin residue in the pulp dried at 105°C. This amount of residue
under normal feeding practices would not lead to excessive residues in
whole milk or meat (fat basis). Therefore, the recommended practical
residue limits appear to be adequate for residues from this source.
Residues in the crop derived from dressed seed (maize, rice, cereals,
cotton and pulse crops) are extremely small and usually below the
limits of detection, thus justifying the reduction of the tolerance in
rough rice to 0.02 ppm.
Residues in vegetables from seed or soil treatment are dependent on
the type of crop. Residues at or in excess of tolerance could arise in
carrots and lettuce planted in soil previously treated for other
crops. Data were received which justify a revision of the recommended
tolerance and the establishment of a practical residue limit of 0.2
ppm for lettuce and carrots.
Soil applications of aldrin of 2-5 kg/ha in citrus groves resulted in
dieldrin residues of 0.01-0.02 ppm in whole oranges and no detectable
residues in grapefruit. The presently recommended tolerance of 0.05
ppm appears to be adequate.
The importance of photodieldrin as a plant metabolite of aldrin or
dieldrin appears to be well established; however, its significance as
a terminal residue will depend on the results of experiments in
progress on rates of storage and excretion in animals.
A consideration of the new data supplied on residues in milk, milk
products and meat resulting from supervised trials and surveys of food
in commerce does not indicate a need for revision of the previously
recommended practical residue limits for these items at this time. The
previous practical residue limit of 0.125 ppm for milk products was
changed to 0.15 ppm to allow for the limitations and variability of
residue analytical procedures.
A suitable regulatory method of analysis exists for photodieldrin
(official AOAC multiresidue method) but no methods of analysis have
been worked out for the more hydrophilic metabolites of dieldrin.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The previously recommended tolerances and practical residue limits (no
longer temporary) are amended as follows:
ppm
Fruit (other than citrus), asparagus,
broccoli, brussels sprouts, cabbage,
cauliflower, cucumber, eggplant,
horse-radish, onions, parsnips, peppers,
pimentoes, radishes and radish tops 0.1
Citrus 0.05
Rice (rough) 0.02
Potatoes 0.2
PRACTICAL RESIDUE LIMITS
ppm
Carrots, lettuce, fat of meat 0.2
Raw cereals (other than rice) 0.02
Milk and milk products (fat basis) 0.15
Eggs (shell-free) 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further information relevant to the assessment of the significance
of the changes that occur in the livers of mice exposed to dieldrin. A
comparison of the response to dieldrin of high and low spontaneous
liver-tumour mouse strains in respect of liver tumour incidence and
the metabolism of dieldrin might be helpful in this respect.
2. The continued collection of follow-up information on persons
exposed occupationally in the manufacture of dieldrin.
3. Completion of the studies being conducted on the comparative
metabolism of dieldrin in man, monkey, rat and mouse.
4. Experimental studies to determine whether dieldrin might have
teratogenic potential.
5. A continuing study of the possible occurrence of residues of
photodieldrin in food surveys and total diet studies.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J.Sci.Fd.
Agric., 20: 245-249
Association of Public Analysts, London. (1969) Joint survey of
pesticide residues in foodstuffs sold in England and Wales, August
1966-July 1967
Baldwin, M.K. and Robinson, J. (1969a) The identification of a
metabolite of the photo-isomerization product of HEOD (dieldrin) found
in rat tissues. Unpublished report from the Tunstall Laboratory, Shell
Research Ltd., Sittingbourne
Baldwin, M.K. and Robinson, J. (1969b) Metabolism in the rat of the
photo-isomerization product of dieldrin. Nature, 224: 283-284
Baldwin, M.K. and Robinson, J. (1970) Comparison of the metabolism of
HEOD in the CFI mouse with that in the CFE rat - preliminary results.
Unpublished report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Baldwin, M.K., Robinson, J. and Carrington, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Industr., 595-597
Bick, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Austr., 54 (1): 1127-1130
Braund, D.G., Brown, L.D., Huber, J.J., Leeling, N.C. and Zabik, M.J.
(1969) Excretion and storage of dieldrin in dairy cows fed
thryoprotein and different levels of energy. J. Dairy Sci., 52:
172-182
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27: 81-138
Bro-Rasmussen, F., and Voldum-Clausen, K. (1966) Tids. Planteovl.,
20: 232
Bro-Rasmussen, F., Dalgaard-Mikkelsen, S., Jakobsen T., Koch, S.O.,
Rodin, F., Uhl, E. and Voldum-Clausen, K. (1968) Examinations of
Danish milk and butter for contaminating organochlorine insecticides.
Res. Rev., 23: 55-69
Brown, V.K., Robinson, J. and Richardson, A. (1967) Preliminary
studies on the acute and sub-acute toxicities of a photoisomerization
products of HEOD (dieldrin). Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Chan, T. and Terriere, L.C. (1969) Aldrin epoxidase activity of rat
liver and rat liver microsomes under various conditions of storage.
Biochem. Pharmacol., 18: 1061-1070
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Crabtree, A.N. (1969) The concentrations of organochlorine
insecticides in human fat and blood from Australia. Shell Research
Limited, Tunstall Report No. TL GR. 0059.69
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Ass. Off. Anal. Chem., 49: 354-364
Cummings, J.G., Gidelman M., Turner V., Reed D., Zee K.T. and Cook
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydrocarbon insecticides to hens. J. Ass. Off. Anal.
Chem., 50: 418-425
Dailey, R.E., Walton, M.S., Beck, V., Leavens, C.L. and Klein, A.K.
(1970) Excretion, distribution and tissue storage of a 14C-labelled
photoconversion product of 14C-dieldrin. J. Agr. Fd. Chem., 18:
443-445
Damico, J.N., Chen, J.T., Costello, C.E. and Haenni, E.O. (1968)
Structure of Klein's metabolites of aldrin and dieldrin. J. Ass. Off.
agr. Chem., 51: 48-55
Datta, P.R., Lang, E.P., Watts, J.O., Klein, A.K. and Nelson, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature, 208: 289-290
Dejaukheere, W. and Kips, R.N. (1968) Meded. Rijksfac.
Landbouwwenteusch (Ghent), 33: 1283
Dickes, G.J. and Nicholas, P.V.J. (1969) Assoc. Pub. Analysts, 7: 14
Diechmann, W.B., Dressler, I., Keplinger, M. and MacDonald, W.E.
(1968) Retention of dieldrin in blood, liver and fat of rats fed
dieldrin for six months. Industr. Med. Surg., 37: 837-839
Diechmann, W.B., MacDonald, W.E., Radomski, J., Blum, E.B.,
Bevilacqua, M., and Keplinger, M. (1970) The tumorigenicity of aldrin,
dieldrin and endrin in the albino rat. Industr. Med., 39: 314
Donninger, C., Potter, D., Potter, S.A. and Wright, A.S. (1967) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to rats. Unpublished report from Tunstall Laboratory,
Shell Research Ltd., Sittingbourne
Donninger, C. (1969) Liver cell changes following the oral
administration of dieldrin to mice. Unpublished report from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest.
Monit. J., 2(4): 153-162
Elgar, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Fd. Agr., 17: 541-545
FAO/WHO (1967) Evaluations of some pesticide residues in food,
FAO,PL:CP/15; WHO/Food Add./67.32 p. 3-4.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Feil, V.J., Hedda, R.D., Zayliskie, R.G. and Zachrison, C.N. (1970)
Dieldrin-14C metabolism in sheep. Identification of
trans-6-7-dihydroxyaldrin and 9-(synepoxy)
hydroxy-1,2,3,4,10,10-hexachloro-6,7-
epoxy-1,4,4a,5,6,7,8,8a-,octahydro-1,4-endo-5,8-
exodimethanonophthalene. J. Agr. Fd. Chem., 18: 120-24
Findlay, E. and Hamilton, G.A. (1968) Pesticide residues in foodstuffs
in Great Britain. VIII Organochlorine pesticide residues in eggs and
poultry. J. Sci. Fd. Agric., 19: 609-611
Gannon, N., Link, R.P. and Decker, G.C. (1959a) Pesticide residues in
meat. Storage of dieldrin in tissues of steers, hogs, lambs and
poultry fed dieldrin in their diets. J. Agr. Fd. Chem., 7: 826-828
Gannon, N., Link, R.P. and Decker, G.C. (1959b) Pesticide residues in
meat and milk. Storage of dieldrin in tissues and its excretion in
milk of dairy cows fed dieldrin in their diets. J. Agr. Fd. Chem.,
7: 824-826
Gillett, J.W. and Chan, I.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Food Chem., 16: 590-593
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Graves, J.B., Bonner, F.L., McKnight W.F., Watts, E.B. and Epps, E.A.
(1969) Residues in eggs, preening glands, liver and muscle from
feeding dieldrin-contaminated rice bran to hens and its effect on egg
production, egg hatch and chick survival. Bull. Env. Contam. Tox.,
4: 375-383
Harr, J.R., Claeys, R.R., Bone, J.F. and McCorcle, J.W. (1970)
Dieldrin toxicosis: rat reproduction. Amer. J. vet. Res., 31:
181-189
Harris, L.E. and Greenwood, D.A. (1963) Effect of residues of newer
insecticides on health. Unpublished progress report to Shell Chemical
Company, letter signed by Joseph Street, dated 8 March 1963
Hedde, R.D., Davison, K.C. and Robbins, J.D. (1970) Dieldrin 14-C
metabolism in sheep. Distribution and isolation of urinary
metabolites. J. Agr. Fd. Chem., 18: 116-119
Hunter, C.G., Robinson, J. and Roberts, M. Pharmacodynamics of
dieldrin (HEOD). Ingestion by human subjects for 18 to 24 months and
postexposure for eight months. Arch. environm. Hlth., 18: 12-21
Jager, K.W. (1970) "Aldrin, dieldrin, endrin and telodrin". An
epidemiological and toxicological study of long-term occupational
exposure. Thesis, 234 p., Elsevier, Amsterdam
Jansen, J.D. (1969) Letter to A.M. Coetzee dated 25 July 1969
Keane, W.T. and Zavon, M.R. (1969) Validity of a critical blood level
for prevention of dieldrin intoxication. Arch. environm. Hlth., 19:
36-44
Kinoshita, P.K. and Kempf, C.K. (1970) Quantitative measurements of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Tox. Appl. Pharmacol., 17: 288
Klein, A.K., Link J.D. and Ives, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed aldrin
and dieldrin. J. Ass. Off. agr. Chem., 51: 895-898
Korte, F. and Arent, H. (1965) Metabolism of insecticides. IX (1)
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin - 14C. Life Sci., 4:
2017-2026
Korte, F. (1970) Terminal residues of cyclodiene insecticides. Comptes
Rendus, XXV Conference, IUPAC, Cortina d'Ampezzo, Italy, July 1969.
Appendix II: p. 172-180
Korte, F. and Porter, P.E (1970) Minutes of the Fifth Meeting of the
IUPAC Terminal Pesticide Residues, September 1970, Erbach, West
Germany. Appendixes 4 and 4a
Lee, D.F. (1968) Pesticide residues in foodstuffs in Great Britain.
IX. Aldrin, dieldrin and other organochlorine pesticides in potatoes
and carrots. J. Sci. Fd. Agric., 19: 701-705
Lehner, P.N. and Egbert, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature, 224: 1218-1219
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, et al. (1967) Food Technol. 21: 435-438
Lofroth, G. (1970) Exposure of the general population to dieldrin.
Letter to editor and reply Robinson J. and Roberts M. Fd. Cosmet.
Toxicol., 8: 341-342
Lutz-Ostertag, Y. and Lutz, H. (1969) Embryologie expérimentale. Note
préliminaire sur les effets "oestrogènes" de l'"Aldrine" sur le
tractus urogénital de l'embryon d'Oiseau. C.R. Acad. Sci. (Paris) Ser.
D., 269: 484-486
Maier-Bode, H. (1967) The aldrin and dieldrin contents of German
edible carrots. Bull. environ. Contam. Toxicol., 2(1): 10-11
Matthews, H.B. and Matsumura, F. (1969) Metabolic fate of dieldrin in
the rat. J. Agr. Fd. Chem., 17: 845-852
McGill, A.E.J. and Robinson, J. (1968) Organochlorine insecticide
residues in complete prepared meals: a twelve-month survey in
southeast England. Fd. Cosmet. Toxicol., 6: 45-57
Mestres, R., Barthes, F. and Priu, M. (1967) DDT, aldrin and dieldrin
residue determinations in milk and butter. Bull. Environ. Contam.
Toxicol., 2(1): 25-33
Moubry, R.J. and Myrdal, G.E. (1970) private communication, Wisconsin
Department of Agriculture
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugarbeets. J. Econ. Entomol.,
63: 1143-1146
Parsons, A.M. and Moore, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem. Soc.
(C), 2026-2031
Polishuk, Z.W., Wasserman, M., Wasserman, D., Groner, Y., Luzarovici,
S. and Tomatis, L. (1970) Arch. environm. Hlth., 20: 215-217
Porter, R.D. and Wiemeyer, S.N. (1969) Dieldrin and DDT: Effects on
sparrow hawk eggshells and reproduction. Science, 165: 199-200
Potter, D., Wooder, M.F., Wright, A.S., Greenland, R.D. and
Zavon, M.R. (1970) The effects of ingestion of foreign compounds on
the sub-cellular structure and function of mammalian liver cells.
Paper given by A.S. Wright at Sittingbourne, 14 May 1970
Renvall, S. and Akerblom, M. (1968) Rester au organiska
klorpesticider, sörskilt aldrin och dieldrin, 2 nagra svenska
kalvöxter ochpotatis. Var. Föda, 20: 65-72
Reports of the Government Chemist, H.M.S.O., London (1965-1969)
Richardson, A. (1966) Ultra-violet conversion product of HEOD: Its
concentration in various samples received from the USA. Shell Research
Tunstall Report No. IRR TL/35/66
Richardson, A. (1968) Residues of dieldrin and its ultraviolet
decomposition product in selected samples. Shell Research Tunstall
Report No. TLGR 0014.63
Richardson, A., Baldwin, M. and Robinson, J. (1968) Identification of
metabolites of dieldrin (HEOD) in the faeces and urine of rats. J.
Sci. Fd. Agr., 19: 524-529
Robinson, et al. (1965-66) Shell Research Ltd., Tunstall, Report
PRRTL/12/65 and TU/23/66
Robinson, J., Richardson, A. and Bush, B. (1966b) A photoisomerization
product of dieldrin. Bull. environm. Contam. Toxicol., 1: 127-133
Robinson, J., Roberts, M., Baldwin, M. and Walker, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Fd. Cosmet. Toxicol.,
7: 317-332
Robinson, J. (1970a) Metabolites of dieldrin in the excreta of Rhesus
monkeys. Unpublished information submitted in a letter to H.G.S. van
Raalte
Robinson, J. (1970b) Metabolites of dieldrin in the excreta of man.
Unpublished information submitted in a letter to H.G.S. van Raalte
Roe, F.J.C. and Grant, G.A. (1970) Inhibition by germ-free status of
development of liver and lung tumours in mice exposed neonatally to
7,12-dimethyl-benz(a) anthracene: implications in relation to tests
for carcinogenicity. Int. J. Cancer, 6: 133-144
Rosen, J.D., Sutherland, D.J. and Lipton, G.R. (1966) The
photochemical isomerization of dieldrin and endrin and effects on
toxicity. Bull. environm. Contam. Toxicol., 1: 133-140
Ruzica, J.H.A., Simmond, J.H. and Tatton, J. O'G. (1967) Pesticide
residues in foodstuffs in Great Britain. IV. Organochlorine pesticide
residues in welfare foods. J. Sci. Fd. Agric., 18: 579-582
Rymer, T.E. and Hammence, J.H. (1967) J. Ass. Pub. Analysts 5: 24
Sakshaug, J. (1968) Residues of organochlorine insecticide in
Norwegian butter samples 1966 (in Norwegian). J. Nord. Vet.-Med.,
20: 696-701
Shell International Chemical Co. (1968) Residues in potatoes resulting
from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in vegetables
resulting from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in small grains
resulting from applications of aldrin, Monograph, November
Smith, Klosterman and Dogger. (1966) Ann. Proc. N. Dakota Acad. Sci.,
20: 96
Tannenbaum, A. and Silverstone, H. (1949a) The genesis and growth of
tumours. IV. Effects of varying the proportion of protein (casein) in
the diet. Cancer Res., 9: 162-173
Tannenbaum, A. and Silverstone, H. (1949b) The influences of the
degree of calorie restriction in the formation of skin tumours and
hepatomas in mice. Cancer Res., 9: 724-727
van Steyvoort, L. et al. (1968) Meded. Rijksfac. Landbouwwettensch XX,
the International Symposium, Ghent, May
Walker, K. et al. (1965) The fate of aldrin, dieldrin and endrin
residues daring the processing of sugar beets. United States
Department of Agriculture, ARS. Publication, ARS-33-107, p. 8
Walker, A.I.T., Stevenson, D.E., Robinson, J., Thorpe, E. and Roberts,
M. (1969a) The toxicology and pharmacodynamics of dieldrin (HEOD):
two-year oral exposures in rats and dogs. Toxicol. appl. Pharmacol.,
15: 345-373
Walker, A.I.T., Neill, C.H., Stevenson, D.E. and Robinson, J. (1969b)
The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix coturnix
japonica). Toxicol. appl. Pharmacol., 15: 69-73
Walker, A.I.T., Thorpe, E. and Stevenson D.E. (1970a) The toxicology
of dieldrin (HEOD): long-term oral toxicity experiments in mice.
Unpublished report from the Tunstall Laboratory, TU/13/70, Shell
Research Ltd., Sittingbourne. Proposed for publication in Fd. Cosmet.
Toxicol.
Walker, A.I.T., Thorpe, E. and Baldwin, M.K. (1970b) Toxicity studies
on the ultra-violet conversion product of dieldrin (UVCPD): thirteen
weeks oral experiment with rats. Shell Research Tunstall Report No.
TLGR. 0053.69
Walton, M.S., Beck, V. and Baron, R.L. (1970) Subacute toxicity of
photodieldrin 3,4,5,6,6,7-hexachloro-12-oxahexacyclo-tridecane, a
photodecomposition product of dieldrin. Tox. appl. Pharmacol., 17:
278.
Wasserman, M., Curnow, D.H., Forte, P.N. and Gurner Y. (1969) Storage
of organochlorine pesticides in the body fat of people in Western
Australia. Industr. Med. Surg., 37: 295-300
Wasserman, M., Coetzee, A.M., Wasserman, D. and Lazarovici, S. (1969)
Present state of the storage of the organochlorine insecticides in the
general population of South Africa. Paper given at the IUPAC
conference, Johannesburg
Williams, S., Mills, P.A. and McDowell, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J. Ass. Off. Anal. Chem., 47: 1124-1128
Winnett, G. and Reed, J.P. (1968) Aldrin, dieldrin, endrin and
chlordane persistence - a three-year study. Pest. Mon. J., 2(3):
133-136
Winteringham, F.P.W. and Barnes, J.M. (1955) Comparative response of
insects and mammals to certain halogenated hydrocarbons used as
insecticides. Physiol. Rev., 35: 701-739
Wright, A.S., Potter, D., Wooder, M.F., Donninger, C., Le Pelley,
J.R., Potter, S.A., Ferrigan, L.W., Sykes, R., Dean, B., Pickering,
R.G., Walker, A.I.T., Crabtree, A.N. and Richardson, A. (1968) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to female dogs. Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Wright, A.S., Greenland R.D., Zavon, M.R. and Donninger, C. (1969)
Liver cell changes following the oral administration of dieldrin to
Rhesus monkeys for 5.5-6 years. Unpublished report from the Tunstall
Laboratories, Shell Research Ltd., Sittingbourne
Zavon, M.R. (1970) The effect of long-continued ingestion of dieldrin
on Rhesus monkeys. A six-year study. Unpublished report from the
Kettering Laboratory, University of Cincinnati, submitted by Shell
International Research.
TABLE II
Toxicity of the photoisomerization product of dieldrin
compared with dieldrin
Species Approximate LD50, mg/kg body-weight
Photoisomerization
product of dieldrin Dieldrin
Pigeon 90 250
Chicken 80 48
Mouse 7 77
Rat 10 47
Guinea-pig 3 24
Dog (M) 140 120
Dog (F) 100 90
Dieldrin or its photoisomerization product was fed to five male and
five female mice at dietary levels of 1, 3 and 10 ppm for the
photoisomerization product and 3, 10 and 3O ppm for dieldrin for one
month. The mice exposed to the photoisomerization product were
unaffected by 1 ppm, there was one male and one female death at 3 ppm
and all died at 10 ppm. The mice fed dieldrin were unaffected by 3 or
10 ppm, but three males and two females died at 30 ppm. Autopsies of
the mice revealed no abnormalities (Brown et al., 1967).
A similar experiment with rats produced no deaths from 10 ppm of the
photoisomerization product; a higher level was not tested. Autopsies
revealed no abnormalities. Levels were measured in the tissues; the
biological half-life of the photoisomerization product was 1.7 for the
males and 2.6 for the females, compared to 10.0 and 12.7 respectively
for dieldrin (Brown et al., 1967).
Subacute levels (5 mg per day) of 14C-labelled dieldrin or
photodieldrin were administered to young adult rats of both sexes for
a period of twelve weeks. Photodieldrin was given both orally and
intraperitoneally; dieldrin was given by stomach tube only. About 60
percent of the activity of 14C-photodieldrin was excreted in the
urine and faeces of male rats and 47 percent in those of female rats,
compared to 60 and 37 percent respectively with 14C-dieldrin. Females
retained three-ten times more 14C activity in their tissues than did
male rats after photodieldrin administration. A similar sex difference
in tissue levels was found after administration of dieldrin and
photodieldrin in female rats and of dieldrin in male rats. Extremely
high levels of 14C activity were detected in the kidneys of male, but
not female, rats receiving 14C-photodieldrin (Dailey et al., 1970).
Storage of the photodieldrin was mainly in the fat and up to 10 ppm it
was 2-15 times higher in females than in males. A metabolite of
dieldrin was detected in the kidneys and urine. The concentration of
this metabolite in the kidneys was about ten times that of the
photodieldrin. No toxicologic effects were detected at 1 ppm (Walker
et al., 1970b).
Similar results were reported by Walton et al. (1970) who also found
slight stimulation of liver microsomal enzymes.
Photodieldrin was fed to groups of 12 male and 12 female rats at
levels of 0.1, 1.0, 10 and 30 ppm in the diet for 13 weeks. Tremors
were observed in rats in the 10 and 30 ppm groups. Reduced food intake
and depressed growth also occurred at these levels. Two of the 10 ppm
and six of the 30 ppm females died. Liver/body-weight ratios were
increased in the 30 ppm groups and kidney weight was increased in the
10 and 30 ppm males. Histologic liver changes were observed in the 10
and 30 ppm groups (Walker at al., 1970b).
Short-term studies
Studies on the effect of dieldrin on the liver microsomal enzymes have
been reviewed under "Biochemical aspects". The following additional
studies have become available.
Dog
Three groups each of six dogs of mixed sex were fed 0, 0.2 or 2 mg/kg
body-weight of dieldrin by capsule for five days a week. The group
given the low dose was started at 1.0 mg/kg for five days, then
reduced to 0.2 mg/kg until day 62 and from then on given 2 mg/kg until
signs of intoxication were evident. A direct relationship was found
between the severity of symptoms and the blood concentration of
dieldrin. As the blood level rose from 0.37 to about 0.8 ppm, changes
progressed from a reduced food intake and growth reduction to the
onset of muscular spasm and finally convulsions. A direct relationship
was also found between blood and fat levels of dieldrin (Keane and
Zavon, 1969).
In an extension of the above-mentioned study, three groups each of six
dogs of mixed sex were given a similar dose regimen of dieldrin. It
was found that the period between dieldrin exposure and the onset of
toxic signs was dependent upon the obesity of the animal; the greater
the amount of body fat the longer it took for toxicity to develop.
Since food refusal preceded symptoms of intoxication, forced feeding
was able to suppress toxicity by preventing fat mobilization and the
associated release of toxic concentrations of dieldrin into the blood
stream, so avoiding the death of some animals (Keane et al., 1969).
Groups of five male and five female dogs were given by capsule daily
oral doses of 0, 0.005 or 0.05 mg/kg body-weight of dieldrin for
periods up to two years. Health, behaviour and body-weight were
unaffected by giving dieldrin, and the electroencephalograph readings
showed no abnormalities. Urine and blood findings were normal except
for an increase in plasma alkaline phosphatase after 30 weeks and a
reduction in serum protein at the 0.05 mg/kg dose. Bromosulphthalein
clearance was normal throughout the exposure period. Liver-weight and
liver to body-weight ratios were increased in the females fed 0.05
mg/kg, and decreased heart-weights, but not heart to body-weight
ratios, were found in all males in the test groups and decreased
spleen-weights at 0.05 mg/kg in the males. No abnormal gross or
histopathological findings associated with administration of dieldrin
were evident (Walker et al., 1969a).
Long-term studies
Rat
Dieldrin was fed to groups comprising 25 male and 25 female rats (45
each for the controls) at dietary levels of 0, 0.1, 1.0 or 10 ppm for
periods up to two years. In addition, a separate group comprising 15
animals of each sex were sacrificed after six, 12 or 18 months
feeding. Body-weight and food intake were unaffected by dieldrin, but
at 10 ppm all the animals became irritable after eight or 13 weeks in
the males and females respectively. Occasional convulsions occurred in
this group during handling. No adverse effect on mortality was
observed. There were no changes in haematology or serum enzyme levels
attributable to dieldrin. Liver weights were normal for the first 18
months of feeding, but after two years, increased liver weights and
liver to body-weight ratios were evident in the groups fed 1 and 10
ppm (Walker et al., 1969a).
OBSERVATIONS IN MAN
In a continuation of the study described in the previous monograph
addendum on dieldrin (FAO/WHO 1968), the 12 male subjects were
continued on the dose regimen of 0, 0.01, 0.05 or 0.21 mg of dieldrin
for a total of two years and were further followed during an
eight-month post-exposure period. All twelve men remained in excellent
health, and there were no changes in body-weights, fatness or
leanness, or other clinical abnormalities throughout the entire
exposure and post-exposure periods. No departures from normal or
pre-exposure values in the biochemical and haemotological parameters
investigated were observed, although there was a slight trend towards
a fall in plasma alkaline phosphatase activity in some men fed the
higher dose levels. This drop was of doubtful significance, and the
enzyme level returned to normal after two months post-exposure. The
concentration of dieldrin in the blood did not significantly change in
the groups given 0.05 mg/day, but at 0.21 mg/day there was a slight
increase during months 18 to 21, but little over months 21 to 24.
This increase was small compared to the first 18 months when the blood
level tripled. Increase in dieldrin in adipose tissue was evident at
all levels after administration for 92 days (Hunter et al., 1969).
Extensive studies of workers exposed to dieldrin in a manufacturing
plant have been reported. The average blood concentration of a group
of 35 workers with an exposure of over ten years was 0.035 mg/ml,
calculated to be equivalent to a dietary intake of 407 mg/man/day.
Intoxications had occurred in some of these individuals prior to 1964.
No definite deleterious effects were found in this high exposure
group, including some individuals who had suffered intoxications.
Clinical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein and serum protein spectrum,
determined every three months remained within normal limits. In an
extreme exposure group with blood levels above 0.2 ppm, alkaline
phosphatase values, although within normal limits, were higher than in
the control group.
Using as criteria of possible microsomal enzyme stimulation the blood
levels of pp'-DDE and the urinary ratio of 6-B-hydroxycortisol and
17-hydroxycortico steroids, no differences between exposed and
unexposed workers were found. The dieldrin workers were exposed to an
average daily intake of 593 mg/man. One group of ten workers with the
highest exposure had an average equivalent oral intake of 1224
mg/man/day.
Comparisons of work records between exposed and non-exposed groups did
not reveal any differences as to frequency or severity of diseases or
accidents or disability (Jager et al., 1970). See also "Biochemical
studies".
On the basis of fat samples only, Crabtree (1969) found in Australia a
mean fat concentration of 0.3 ppm equivalent to an intake of 16
mg/man/day. Bick (1967) reported lower figures while Wasserman (1968)
reported higher ones. These last figures may be due to analytical
errors (Lofroth, 1970).
Wasserman et al. (1969) reported very low levels of dieldrin in fat
tissues from South Africa. Jansen (1969), however, analyzing part of
the same samples found a higher mean of 0.159 ppm, equivalent to an
average absorption of 8.6 mg/man/day.
COMMENT
The increased risk of development of liver tumours in mice exposed to
dieldrin as compared with untreated control mice was regarded as the
main matter for concern. This increased risk was seen even at an
exposure level of 0.1 ppm in the diet. It was generally agreed that
the tumours were neoplasms and that those seen in treated mice were
similar in nature to those seen in untreated mice except insofar as
the tumours in dieldrin-treated mice tended to show the same
cytoplasmic changes as other liver parenchymal cells in the same
animals.
There was no evidence that tumours regressed or disappeared after
cessation of exposure to dieldrin, although the cytoplasmic changes
did regress under such circumstances. It is difficult to establish
unequivocal criteria for distinguishing between benign and malignant
neoplasms in mouse-liver. The incidence of mice showing tumour emboli
or metastases in the lungs was not significantly increased at any
levels of exposure to dieldrin up to 10 ppm for 132 weeks.
It was agreed that the effect of dieldrin in increasing the risk of
tumour development in mice could not at the present time be regarded
as sufficient evidence to categorize dieldrin as a carcinogen. Other
work showed that nonspecific factors such as calorie intake, hormonal
status and micro biological status may profoundly influence the risk
of liver tumour development in mice, some strains of which are in any
case peculiarly susceptible to each tumours. Also it was noted that
long term studies in rats and prolonged studies in dogs and monkeys
had revealed no evidence of hepatocarcinogenicity. It seems likely
that the effect of dieldrin in mice is probably a species-specific
phenomena.
Further information is urgently needed concerning the aetiology and
pathogenesis of liver tumours in mice. A comparison of the effect of
dieldrin in mice of high and low liver tumour susceptibility, with
parallel metabolic studies, might provide a basis for relating
observations of tumourogenicity in mice to other species, including
man. There are at present no experimental data referable to
teratogenicity.
Continued surveillance of persons occupationally exposed to dieldrin
for chronic toxic effects and manifestation of possible
carcinogenicity is recommended. In this connection, the extensive
surveillance of occupationally exposed workers, which is currently
being made, was noted.
Information on the varying patterns of metabolism in the mouse, sheep,
rat, monkey and man are as yet too incomplete to indicate whether the
possible carcinogenic potential in the mouse is likely to apply to
man.
For these reasons, it was felt that no change in the acceptable daily
intake established at the 1966 Joint Meeting could be made at this
time.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE IN MAN
0-0.0001 mg/kg body-weight, the sum of aldrin and dieldrin by weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Seed treatments
The use of aldrin and dieldrin as a rice seed treatment is now
considered important only in Brazil and to a lesser degree in Japan,
Costa Rica and El Salvador. The choice between aldrin and dieldrin is
primarily a question of agronomic factors, local preference and
economics prevailing. Treatment rates vary, but a rate of 2 oz /
bushel (0.2 percent active material) is standard. In Brazil, recent
figures show that out of 4,600,000 hectares planted, 3,680,000
hectares (80 percent) were treated; in Japan, out of 3,280,000
hectares planted, 169,000 hectares (0.5 percent) were treated; in
Costa Rica, out of 43,000 hectares planted, 21,500 hectares (50
percent) were treated and in El Salvador out of 31,000 hectares
planted, 6,200 hectares (20 percent) were treated.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In maize
The treatment of soils prior to planting maize represents the most
important of all uses for aldrin. Studies in the United States in
which aldrin was applied to soil at rates of 1, 2, 3, 4 and 6 lb /acre
and in which pre-harvest intervals ranged from 75-195 days resulted in
aldrin residues in maize grain ranging from <0.001-<0.01 ppm and
dieldrin residues from 0.002-0.02 ppm. Control values were equal to or
greater than treated values in virtually all cases. In the same
experiments, the corresponding residues of aldrin plus dieldrin in
green fodder were less than 0.034 ppm; in maize stover most levels
were less than 0.01 ppm and only in two subsamples did levels exceed
0.03 ppm. With rare exceptions, the totals for aldrin and dieldrin
fell well below 0.03 ppm (Shell Development Co., Reports RES 63-122,
64-135, 64-102, 63-111, 64-168, 64-166 Shell Chemical Co., Reports PRL
67-81, 67-62, 65-6, 67-92; Mourbry and Myrdal, pre-print
communication). Similar experiments in Germany, France, Spain,
Portugal, Australia, Mexico, Venezuela and Trinidad at application
rates of 1-5 kg/ha gave residues of aldrin plus dieldrin of <0.01 ppm
in maize grain; a second crop from treatment two years in succession
gave the same result. Residues in whole maize plants (green fodder) in
France, Spain, Australia and Trinidad were 0.01-ppm or less except for
one year samples from Trinidad which were 0.15 ppm. This appears to be
exceptional, since samples in the succeeding year were <0.01 ppm.
Limited data from France on residues in maize leaves ranged from
0.01-0.07 ppm in dry leaf. Based on this data, it is estimated that
whole stover would have residues less than 0.03 ppm. (Shell Research
Ltd., Reports WKGR 0114.70, 0053.68, ibid - unreported data). In the
U.S.A. extensive data have been obtained for maize grown in a series
of rotations typically employed in U.S. farm practice. Treatments were
between 1´-2´ lb/acre - occasionally 5 lb/acre - and covered 35 sites
and included two-eleven years. In no cases were residues detected in
grain (0.01 ppm dieldrin). In stover, 80 percent of the results were
below 0.01 ppm, and the upper limit was 0.02 ppm. No residues were
detected in green corn (Shell Chemical Co., Report PRL 66-73).
In potatoes
The treatment of potato soils at recommended rates (2-4 kg/ha) with
aldrin shortly before planting gave rise to residues in the whole
washed tubers of between <0.01 ppm and 0.1 ppm of aldrin plus
dieldrin. Typically, levels were 0.04-0.06 ppm. Levels in peeled
tubers were generally 1/3-´ of these values (Shell International
Chemical Co. Monograph, November 1968). More recent data developed by
Shell in summarized in Table III; data from Scandinavia is given in
Table IV.
Residues in potatoes resulting from two or three soil applications of
aldrin in consecutive years are summarized in Table V.
A trial in Portugal compared residues resulting from broadcast
applications with row applications made with aldrinised fertilizer,
all at 3 kg/ha. Results are shown in Table VI (Shell Research Ltd.,
Tech. Serv. Report WKTR.0025/69).
Potatoes were planted in plots in tropical countries from which maize,
grown on soil treated at 2-4 kg/ha just prior to sowing, had been
harvested. Aldrin + dieldrin residues in the tubers were from <0.01
to 0.07 ppm (mean--0.03 ppm) and in peeled tubers from <0.01 to 0.03
ppm (mean--0.01 ppm), as shown in Table VI (Shell Research Ltd.,
Report WKGR. 0114.70).
Residue data for potatoes grown in soils in temperate countries to
which aldrin treatment had been given one or two years before planting
are given in Table VII.
Analytical data for 256 samples of peeled potatoes and potato peel
obtained by the U.K. Ministry of Agriculture (Lee, 1968) from potatoes
grown commercially on treated (three or four lb./acre) land shows
residues of aldrin plus dieldrin in peeled tubers ranging from
0.0-0.14 ppm (mean--0.01 ppm). Calculated residues in whole tubers
based on 15 percent peel ranged from 0.0 to 0.23 ppm (mean--ca. 0.02
ppm).
In sugar beets, juice and pulp
Residues of aldrin plus dieldrin in sugar beets grown in soils treated
once with 2 kg/ha in the same year as cropping ranged from <0.01 to
0.03 ppm; 4 kg/ha resulted in residues ranging from <0.01 to 0.05 ppm
(Legar, 1966; van Steyvoort et al., 1968; Shell Research Ltd., Reports
WKTR.0053.68, WKGR.0123.69, WKGR.0114.70; Shell Chemical Co., Report
PRL-68-148). In contradiction to all these findings, Walker et al.,
(1965) found that one application of 5 lb./acre gave combined residues
in the whole fresh beet of 0.19 ppm and Smith et al., (1966) found
residues of 0.93 ppm resulted from 1 lb./acre of aldrin as a row
application at the time of sowing. However, 80 percent of this latter
residue occurred as aldrin, strongly indicating sample contamination.
Residues of dieldrin in sugar beet tops from beets grown in soils
treated once with aldrin at 1-6 kg/ha in various countries in Europe
and in Turkey ranged from <0.001 to 0.01 ppm (van Steyvoort et al.,
1968; Shell Research Ltd., Reports WKTR. 0053.68, WKGR.0123.69,
.0114.70). Residues of aldrin plus dieldrin in roots and tops of sugar
beets grown in soils treated two and sometimes three years in
succession are summarized in Table VIII.
Table III
Residues in potatoes arising from soil applications of aldrin
Application Range of Values, ppm No. of Results Arithmetic Mean
Rate Whole Peeled Whole Pooled Whole Peeled
kg/ha Tubers Tubers Tubers Tubers Tubers Tubers
2 <0.01-0.13 <0.01-0.05 24 10 0.06 0.02
3 0.01-0.16 - 18 - 0.08 -
4 0.03-0.24 0.01-0.07 10 10 0.11 0.04
2-4 <0.01-0.24 - 52 - 0.08 -
5 0.02-0.22 - 11 - 0.11 -
Shell Chemical Co., Reports PRL 68-147, PCY-64-10; Shell Research Ltd.,
Reports WKTR. 0025/69, Tech. Memo 137/65, Tech. Serv. Note 141/66,
WKTR. 0114. 70; Winnett and Reed, 1968.
Table IV
Aldrin and Dieldrin Residues in Potatoes Arising from single soil
applications in Scandinavia
Application Residues, ppm in
Rate, potato tubers
Country kg/ha Aldrin Dieldrin Reference
Sweden(1964) 2.5 <0.01 0.02
Sweden(1965) 2.5 <0.01-0.01 <0.01-0.02 Renvall and
Akerblom, (1968)
Sweden(1966) 2.5 <0.01 0.01
2.5 <0.01 0.05
2.5 0.01 0.02
2.5 0.02 0.13
2.5 0.03 0.12
Denmark 2.5 not found 0.05 Bro-Rasmussen
5.0* not found 0.02-0.05 and Voldum-
10.0* 0.02 0.03-0.10 Clausen, (1966)
* High rates
Table V
Aldrin plus dieldrin residnes in potatoes grown in plots which received
a number of annual treatments with aIdrin
Range of values
Application Aldrin + Dieldrin, Arithmetic
kg/ha ppm No. of results Mean, ppm
Whole Peeled Whole Peeled Whole Peeled
2 0.01-0.11 00.01-0-05 11 8 0.05 0.02
4 0.03-0.40 <0.01-0.01 8 8 0.14 0.03
Shell Research Ltd., Reports WKTR-0007.69, 0028.68, Tech. Serv. Note
141/66, Report WKGR.0114.70, 0153/69, Tech. Serv. Note WKTR. 0100.68.
Table VI
Comparison of residues in potato tubers from broadcast and
row treatments
Range of Results,
Type of Aldrin + Dieldrin, No. of Arithmetic
Application ppm Values Mean, ppm
Row treatment 0.09-0.27 4 0.15
Broadcast 0.04-0.07 3 0.05
(fertilized
mixture)
Broadcast (E.C.) 0.05-0.16 4 0.08
Table VII
Residues In potato tubers grown-in soils treated one or two years
prior to planting
Interval
Treatment Between Range of
Rate, Treatment and Results, No. of Arithmetic
kg/ha Planting, ppm Results Mean, ppm
years
2 1 <0.01-0.05 14 0.02
4 1 0.01-0.05 9 0.03
2-5 1 <0.01-0.11 29 0.04
2-5 2 0.01-0.02 12 0.01
Shell Research Ltd., Tech. Serv. Note 141/66, Report WKGR.0114.70,
Tech. Serv. Report WKTR 0007.69, .0028.68, Report WKGR 0153/69,
Tech. Serv. Note WKTR.0100.68, Winnett and Reed; 1968.
Residues of dieldrin in roots and tops of beets grown on plots treated
the previous year at 2 or 4 kg/ha were all <0.01 ppm (28 samples)
except for two root samples of 0.02 and 0.03 ppm; no aldrin was
detected (Elgar, 1966; Shell Research Ltd., Report WKGR.0114.70).
Recent studies by Onsager at al., (1970) show that aldrin plus
dieldrin residues in mature sugar beets are proportional to the
residues in the soil at the time of planting as illustrated in Table
IX.
Studies in Belgium (van Steyvoort et al., 1968) and in France (Shell
Research Ltd., Report WKTR.0053.68) indicate that no residues could be
found in purified juices from sugar beets grown in soil treated with
up to 6 kg/ha of aldrin. However, data from refineries in the U.S.A.
(Walker at al., 1965) show combined residues of 0.02 ppm (expressed as
ppm of pure sucrose) in clarified juice from beets with abnormally
high residue levels. Had these juices been processed, most of the
residue would have been found in the molasses rather than in the
sugar.
Data was obtained on dieldrin residues in dried pulp from beets grown
in soil which had received aldrin treatment for two years preceding
planting. A residue of 0.03 ppm in the fresh beet resulted in values
of 0.13-0.14 ppm in pulp dried at 105°C and 0.05-0.12 in pulp dried at
150°C; 0.07 ppm in fresh beet gave values of 0.28 ppm in pulp dried at
105°C and 0.34 ppm in pulp dried at 150°C (Shell Research Ltd.,
unreported data). The ratio of residues in dried pulp to fresh beet
was 3-4 which is lower than the figure of 10.6 reported by Walker et
al. (1965). The significance of pulp residues in terms of cattle feed
is questionable, however, due to the low amount of pulp (10-20
percent) in the total diet. Onsager et al. (1970) calculated that a
residue of 0.25 ppm in 4.5-8 lb of beet pulp fed daily to a 1200-lb
animal amounts to ingestion of 0.2-1.6 ppm of insecticide.
In crops derived from seed dressings
Both aldrin and dieldrin are extensively used as seed dressings on a
wide variety of crops; maize, rice, cereals, cotton and pulse crops
are the most important. A large amount of data collected primarily by
Shell shows that residues in the crop derived from dressed seed are
extremely small and usually below the limits of detection (about 0.005
ppm), except for occasional residues of 0.01-0.02 ppm in pulse crops.
In all cases where residues were much above the detection limit, there
are strong indications that samples were contaminated. Either residues
were high on control as well as treated samples, or else appreciable
proportions of aldrin were found in the total residue.
In vegetables
Residue data obtained up through 1968 is summarized in Table X (Shell
International Chemical Co., Monograph, Residues in Vegetables,
November 1968).
Table VIII
Residues in sugar beets grown in soils treated on more than one occasion
Rate of
Application, Range of Residues Arithmetic
kg/ha/ Part of Aldrin + Dieldrin, No. of Mean,
year Crop ppm Samples ppm
2 Root <0.01-0.04 8 0.01
4 Root <0.01-0.07 5 0.04
2 Tops <0.01-0.02 5 <0.01
4 Tops <0.01-0.02 5 <0.01
Table IX
Average residues of aldrin plus dieldrin in soil and sugar beet roots
1965-1968
Average residue, ppm
Aldrin Soil
dosage, sampled on Roots sampled on
lb ai/acre 4/10/65 10/5/65 10/25/66 10/17/67
0.625 0.05 <0.01 <0.01 <0.01
1.25 0.21 0.04 <0.01 <0.01
2.5 0.38 0.06 0.01 0.01
5.0 0.73 0.13 0.02 0.01
7.5 1.84 0.37 0.03 0.03
10.0 2.84 0.96 0.05 0.04
Table X
Residues from seed or soil treatment with aldrin and dieldrin at
application rates within the recommended range (typically 2-3 kg/ha).
Crop or Residues, ppm Location and
Crop Group Min Max Est. Median No. of Expts.
Carrots 0.02 0.51 0.20 USA:3, Switzerland:5,
Denmark:2, Holland, 3,
UK:3, Austria:2
Cruciferous 0.02 0.08 0.04 USA:20, Norway:1,
roots (1) Austria:2
Beetroot 0.01 0.22 0.10 USA:15
Cole crops (2) 0.01 0.02 0.015 USA:21
Onions ND ND ND UK:3, USA:1
Peas ND ND ND USA:1
Lettuce 0.10 0.16 - USA:2
Beans ND ND ND USA:3
Tomatoes 0.10 0.10 0.10 USA:3
Celery 0.02 ND ND UK:3
Cucurbits (3) 0.01 0.07 0.03 USA:10
(1) Including radish, turnip, rutabaga and swede.
(2) Including cabbage broccoli, brussels sprouts and cauliflower.
(3) Including cucumber, pumpkin and melon.
More recent data on beetroot, cauliflower, carrots, white cabbage,
lettuce, tomatoes, turnips, and sweet potatoes are consistent with the
findings in Table X and in general give slightly lower residue figures
(Bro-Rasmussen and Voldum-Clausen, 1966; Shell Research Ltd., Reports
WKTR. 0050.68, 0053.68; Shell Chemical Co., Reports PRL-68-112,
-68-210).
In cereal grain
Aldrin treatments of seed or soil at recommended rates have rarely
produced residues of aldrin plus dieldrin in grain of wheat, oats or
barley above 0.01 ppm. Foliar applications of aldrin or dieldrin to
the growing crop do not give measurable residues in grain or straw if
pre-harvest intervals of one month or more are observed (Shell
International Chemical Co., Monograph, Residues in Small Grains,
1968).
Recent data for wheat and barley grain from soils receiving a variety
of aldrin application regimes at rates of 2 or 4 kg/ha show no
detectable residues (<0.01 ppm) of either aldrin or dieldrin.
Residues in wheat straw ranged from <0.01-0.16 ppm and in barley
straw from <0.01-0.20 ppm. Straw residues are of limited significance
since wheat straw is not an appreciable component in the diet of dairy
cattle and barley straw is used as a feed primarily for beef cattle in
winter only (Shell Research Ltd., Reports WKGR.0114.70, WKTR-0053.68).
In citrus and sugar-cane
Soil applications of aldrin at 2.0-5.0 kg/ha in citrus groves resulted
in dieldrin residues of 0.01-0.02 ppm in whole oranges, 0.02 ppm in
chopped peel and 0.03-0.04 ppm in dried pulp; controls contained up to
0.01 ppm. Dieldrin residues were not found in whole grapefruit or
grapefruit juice, but peel contained 0.006-0.007 ppm and dried pulp
contained 0.01 ppm; residues were also found in control samples (Shell
Chemical Co., Report PRL-67-30).
No residues were found in any of six samples of sugar-cane grown in
soil treated with two applications of aldrin at 2 kg/ha (Shell
Chemical Co., Report PRL-68-51). Soil treatments with aldrin of 5 or
10 lb ai/acre resulted in apparent aldrin residues of 0.004 ppm in two
out of three foliage samples from the 10 lb/acre treatment and in
apparent dieldrin residues ranging from 0.008-0.015 ppm in 8 out of 16
samples of foliage and stalks. No residues were found in the juice at
any treatment rate, and one sample of bagasse (out of four) had 0.009
ppm dieldrin (Shell Chemical Co., Report PRL-68-236).
In milk and meat (from cattle feed)
Williams et al. (1964) fed 16 milking cows for 35 days with a feed
containing various amounts of dieldrin. Similar experiments were
carried out by Gannon et al. (1959a, 1959b) for 12 weeks and by Shell
(1970) for 21 days using dieldrin C-14. The results of these
investigations are summarized in Table XI.
After the feeding of dieldrin was stopped, dieldrin concentrations in
the milk began to fall with a half-life of 10-15 days in the case of
Williams' work and substantially longer in the case of Gannon's work.
The lack of consistency may be due to some cows coming to the end of
their lactation period, thus reducing the milk production per day.
Gannon et al. (1959a) also studied residues in body tissues of milk
cows and steers fed diets containing various amounts of dieldrin. His
results are shown in Table XII.
In poultry and eggs
The residues of dieldrin that could arise in poultry products from the
use of rice bran in the diet (typically 20 percent of the total diet)
are summarized in Table XIII.
Thus with residues of dieldrin in rice bran of 0.05 ppm, residues of
dieldrin in poultry products could be 0.012 ppm in eggs, 0.20 ppm in
fat and 0.02 ppm in poultry meat. Residues of up to 0.03 ppm dieldrin
are commonly found in rice bran.
FATE OF RESIDUES
General comments
The occurrence and toxicity of the photoisomer of dieldrin,
photodieldrin, has been reviewed in the section on biological studies.
Photodieldrin residues may be of significance only after foliar
application of dieldrin. No detectable residues were found in human
fat in the U.S.A. where the ratio of dieldrin to photodieldrin
residues was greater than 200 to 1 (Richardson, 1966). Photodieldrin
was also absent in human fat from U.K. and Holland (Richardson, 1968)
and milk (Robinson et al., 1966).
In animals
The fate of residues of dieldrin in animals has been covered under the
biochemical section of this monograph.
In plants
Extensive experimental work was carried out by Shell to determine
residues of photoaldrin and photodieldrin in crops grown on
aldrin-treated soil, grown from aldrin or dieldrin-treated seed, grown
in soils containing old residues or receiving foliar applications of
dieldrin, and general monitoring investigations. Their results
indicate that none of the soil or seed dressing uses of aldrin or
dieldrin lead to detectable residues of the photoisomers. Foliar
applications led to photodieldrin residues of 0.03 ppm in coffee beans
treated at 1-1/3 g of dieldrin/ tree, 0.02 ppm in leaves of forage
beets treated at 4.3 oz/acre, 0.31-0.89 ppm in wheat straw treated at
8 oz /acre, <0.01-0.04 ppm in apples and 0.01-0.06 ppm in apple
pomace treated at ´ lb/acre, 3.6 ppm in pasture grass one and a half
months after treatment with 1 oz/acre, and 1.1 ppm in fat of sheep
grazing for four months on the treated pasture grass. None of the
photoisomer was detected in any samples of vegetable oils and fats
monitored in the U.K., but some samples of food and feed components
contained traces.
Table XI
Dieldrin in milk from animals fed with feed containing
different amounts and for various lengths of time
Dieldrin in Butterfat Ratio,
feed, ppm, Milk level, ppm, equivalent, ppm, ppm B.F.
dry weight at time indicated at time indicated ppm Feed Reference
35 days 35 day
0 0.004 0.11 - Williams
0.09 0.021 0.53 5.9 et al.
0.23 0.058 1.52 6.6 (1964)
0.50 0.11 2.89 5.9
4 weeks 12 weeks 4 weeks 12 weeks
0 <0.01 0.01 - - - Gannon
0.1 <0.01 0.02 - 0.5 5.0 et al.
0.25 0.02 0.06 0.5 1.5 6.0 (1959b)
0.75 0.07 0.11 1.75 2.8 3.1
2.25 0.16 0.28 4.0 7.0 3.0
15-21 days 15-21 days
0.102 0.018 0.41 4.0 Shell
0.120 0.017 0.35 2.9 Development
0.111 0.015 0.38 3.5 Co., Report
M-32-70
Table XII
Dieldrin residues in body tissues of cows and steers after feeding at
various levels of dieldrin for twelve weeks
Milk cows Steers
Dieldrin in Feed, ppm Dieldrin in Feed, ppm
Body Part 0.25 2.25 0.1 0.25 0.75 2.25
Dieldrin in Tissues, ppm
Brain <0.1 0.1 - - - -
Heart 0.2 0.6 - - - -
Liver 0.2 0.7 - 0.1 - 0.7
Kidney <0.1 <0.1 - <0.1 - 0.2
Steak 0.1 1.3 - <0.1 - 1.0
Roast 0.2 1.2 - - - -
Renal Fat 0.9 6.2 - 0.9 - 9.7
Body Fat 0.9 4.8 0.4 0.8 3.5 8.7
Heart Fat - 7.0 - - - -
Udder Fat - 5.6 - - - -
The average ratio of dieldrin levels in fat to levels in feed was 2.75
in milk cows and 3.95 in steers.
Table XIII
Relation between dieldrin residues in poultry feed
and poultry products (eggs, meat, fat of meat)
Feed Range of Ratios,
Poultry Weeks Concentration Poultry Feed/
Product fed Range (ppm) Poultry Product Reference
Eggs 12 0.1-0.75 0.8-1.6 Gannon et al., 1959a
Eggs 16 0.006-5.0 1.0-3.0* Greaves et al., 1969a
Eggs 14 0.05-0.45 1.1-1.2 Cummings et al., 1966
Fat(abdominal) 2 0.1-0.15 20-29 Liska et al., 1967
Fat 12 0.1-0.75 41-48 Gannon et al., 1959a
Fat(abdominal) 14 0.05-0.45 8.8-9.7 Cummings et al., 1967
Meat 2 0.1-0.15 2-4 Liska et al., 1967
Meat 12 0.1-0.75 <0.2 Gannon et al., 1959a
Meat 14 0.05-0.45 0.1 Cummings et al., 1967
* The figure of 3 regarded as inconsistent with other data.
Lichtenstein et al. (1970) reported 0.015 ppm photodieldrin in soils
which had been treated annually with 5 lb/acre of aldrin for five
years and then not treated for five years. Potatoes and carrots grown
in these soils contained 0.0006 ppm and 0.002 ppm, respectively.
After foliar application of photodieldrin C-14 to white cabbage,
residues disappeared more slowly than in corresponding tests with
aldrin, dieldrin, isodrin or endrin. Four weeks after application, as
much as 75 percent of the applied radioactivity was present as
residues. Fifteen to 33 percent of metabolites were found mainly in
leaves. Metabolic fractions consisted of a very hydrophilic main
product and at least two less hydrophilic by-products (Korte and
Porter, 1970).
Four weeks after application of ca. 75 ppm of aldrin C-14 on the upper
side of cabbage leaves, only 17 percent of the applied radioactivity
was recovered in plants and soil. Hydrophilic metabolites made up 76
percent of the recovered activity; besides aldrin, five other
compounds were found, one of which appeared to be dieldrin. In similar
experiments with dieldrin C-14 on cabbage, 40 percent of the
radioactivity is recovered after four weeks and 34 percent of the
recovered activity is due to at least two hydrophilic metabolites
(Korte, 1970).
In soils
Maize grown in California in soil treated with 3 kg/ha of aldrin C-14
contained no detectable C-14 in the grain or cobs. The husks of the
ears contained 0.004 ppm aldrin, 0.004 ppm dieldrin and 0.032 ppm
metabolites. The leaves contained 0.35 ppm C-14 equivalents of which
0.02 ppm was aldrin and 0.05 ppm was dieldrin (Korte and Porter,
1970).
Evidence of residues in food in commerce
Belgium
Thirty local and sixteen imported samples of butter were examined
(Dejaukheere and Kips, 1968). In the local samples, residues of
dieldrin ranged from 0.01 to 0.24 ppm (mean 0.12 ppm), and in the
imported samples the range was 0.01 to 0.25 ppm.
Denmark
Samples of milk butterfat (44) contained mean levels of dieldrin of
0.03 ppm (<0.007-0.11) (Bro-Rasmussen et al., 1968). Samples of
butter examined over a three year period contained dieldrin residues
as follows:
1964 1965 1966
No. of samples 118 137 126
Range (ppm) 0.01-0.20 0.01-0.18 0.01-0.17
Mean (ppm) 0.05 0.04 0.03
The authors suggested from their data that 60-100 percent of these
residues originated from dieldrin residues in imported cattle feed.
France
Mestres, et al., (1967) in France found no residue of aldrin or
dieldrin in 44 samples of French butter or in four samples of Swiss
butter at a detection limit of 0.02 ppm.
West Germany
Of 65 samples of carrots taken in West Germany (Maier-Bode, 1967) 43
contained less than 0.01 ppm aldrin plus dieldrin. Of the remainder,
64 percent contained residues of 0.03 ppm or less, and only one sample
exceeded 0.1 ppm (0.17 ppm).
Norway
Twenty butter samples out of a total of 54 contained dieldrin residues
in the range 0.01-0.1 ppm (Sakshaug, 1968). The rest did not contain
residues, above the limit of detection.
U.K.
Routine analysis of food-stuffs by public analysts in the U.K. (Rymer
and Hammence, 1967) demonstrated that of 776 fruit, 461 vegetable and
237 meat and dairy produce samples, only three fruit samples and three
meat and dairy produce samples contained aldrin above the limits of
detection (maximum levels of 0.06 and 0.18 ppm respectively) and two
fruit, one vegetable and thirteen produce samples contained dieldrin
above the limits of detection (maximum 0.15 and 0.27 ppm).
A more recent examination of 388 fruit, 285 vegetable and 217 meat and
dairy produce samples (Dickes and Nicholas, 1969) revealed one
vegetable sample containing aldrin (0.09 ppm) and sixteen vegetable
samples containing dieldrin, although all were below 0.01 ppm.
Findlay and Hamilton (1968) examined 163 samples of eggs and 114
samples of poultry meat, liver and fat from nine sources within the
U.K. No egg samples exceeded 0.06 ppm of dieldrin and only one poultry
meat sample exceeded 0.1 ppm (0.13 ppm).
Table XIV
Dieldrin residues in dairy and meat products produced in U.K., 1965-1968
Butter Milk Mutton Fat Beef Fat
Year Mean Mean Mean Mean
Samples (ppm) Samples (ppm) Samples (ppm) Samples (ppm)
1964 127 0.84
1965 18 0.03 85 0.0025 107 1.15 59 0.06
1966 13 0.02 75 0.0025 101 0.44 63 0.04
1967 22 0.03 77 0.002 76 0.24 36 0.03
1968 16 0.03 76 0.001 77 0.21 34 0.03
A most comprehensive examination of home produced and imported meat
and dairy products has been carried out over a number of years by the
Laboratory of the Government Chemist (1966, 1967, 1968, 1969). In the
four years 1965-1968, 935 samples of home produced foods and 803
samples of foods imported from ten countries were analyzed. Table XIV
summarizes their findings for home-produced dairy and meat products.
Residues in food at time of consumption
A study of data accumulated over four years by the U.S. Food and Drug
Administration on pesticide residues in total diet samples was made by
Duggan and Lipscomb (1969).
Residues of dieldrin were found in from 15.6-21.3 percent of
composites analyzed over this period and aldrin residues were found in
from 3.3-5.6 percent. Measurable dieldrin residues were present in six
of the twelve food groups examined, and traces were found in another
four. Measurable aldrin residues were found in only one group. The
highest residues of dieldrin (max. 0.20 ppm) were found in samples of
meat, fish and poultry (Group 2). In the dairy products group (Group
1) the greatest level was 0.08 ppm and residues did not exceed 0.03
ppm in any other group. The maximum aldrin level was 0.07 ppm in the
first group (Group 9). No tendency to increase or decrease was
observed over this period. From the residue information and a
knowledge of the diet, the daily intake of each pesticide was
calculated. Since the diet was representative of that consumed by a
sixteen-nineteen year old American male, the intake of the average
population will be about half the calculated figure. The total daily
intake for aldrin and dieldrin over the four year period is given in
the following table (from Duggan and Lipscomb, 1969):
Daily Intake (mg/kg body-weight)
1965 1966 1967 1968
Aldrin 0.00001 0.00004 0.00001 0.00001
Dieldrin 0.00008 0.00009 0.00005 0.00005
Total 0.00009 0.00013 0.00006 0.00006
An examination of residues in groups of foodstuffs making up a
complete diet was undertaken by Abbott et al., (1969), at the
instigation of the U.K. Ministry of Agriculture, Fisheries and Food,
for one year from October 1966-October 1967. Of the foods analyzed,
the highest dieldrin residues were in fat (mean 0.024 ppm, range
0.009-0.075 ppm), followed by meat (mean 0.009 ppm, range 0.001-0.060
ppm). Because of the larger consumption of meat, this group provides
the largest intake of dieldrin 2.2 mg/person/day. The mean daily
intake in the diet calculated from the residues found was 0.00009
mg/kg body-weight. This figure confirms the decrease shown in the
second survey by McGill and Robinson (1968) compared with the first,
and may be attributed to the voluntary withdrawal of some previously
approved dieldrin uses in the U.K. over the period 1965-1967.
In 1965 the Association of Public Analysts prepared a survey to
determine the extent of contamination of foodstuffs by pesticides.
Data for the first year of this work has so far been reported
(Association of Public Analysts, 1969). Of the 2352 samples analyzed,
only ten contained residues of aldrin (two only above 0.1 ppm) and 76
contained dieldrin (again only two above 0.1 ppm). Dieldrin was most
commonly found in meat and dairy products. In no foodstuffs did the
mean level of dieldrin residues exceed 0.015 ppm, a figure that is
based on the assumption that all samples in which residues were not
detected (limit 0.002 ppm in solid foods and 0.0002 ppm in liquid milk
and infant foods) did in fact contain residues at half the detection
limit. Using the same assumption, the mean daily intake in the diet
was calculated to be 0.0001 mg/kg body-weight. A survey of residues in
welfare foods (dried full cream milk, cod-liver oil and concentrated
orange juice) by Ruzicka et al., (1967) revealed residues of up to
0.017 ppm dieldrin (mean 0.011 ppm) in dried milk and demonstrated a
15 percent loss in residue during the drying period. Cod-liver oil
contained 0.15-0.20 ppm (mean 0.16 ppm) and concentrated orange juice
contained up to 0.007 ppm (mean 0.002 ppm).
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967a) apply to the
determination of aldrin and dieldrin. Burke (1969) has studied the
application of the official AOAC multiresidue method to photodieldrin.
No methods of analysis have been well worked out for the more
hydrophilic metabolites of dieldrin such as monohydroxydieldrin,
dihydro-aldrin-diol (trans), dieldrin-ketone, dieldrin-ketone
photo-isomer, or Klein's metabolite. Although photoaldrin can be
determined by GLC techniques, the rapid conversion of aldrin to
dieldrin makes it unlikely that photoaldrin will be a significant
residue in crops or soils.
CONSIDERATION OF SPECIFIC POINTS RAISED BY THE CODEX COMMITTEE ON
PESTICIDE RESIDUES
The meeting considered three questions arising from the Draft Report
of the Fifth Session of the Codex Committee in 1970 as follows:
Further data on the use pattern of aldrin and dieldrin on rice are
provided in this monograph, as are data on residues resulting from the
use in animal feed.
The question of the fate of the residues on rice during processing was
not considered because the available data show that residues are so
small that any remaining following processing would be negligible.
No firm data were received on residues in fruit juices resulting from
the use of rice husks as clarifying agents. However, if it is assumed
that 10 percent rice husks are used for clarification, and if they
should contain up to 0.02 ppm of aldrin plus dieldrin, and if it is
further assumed that this is extracted by the fruit juices (unlikely
due to the lipophylic nature of residues), then it would be highly
improbable that residues in excess of 0.002 ppm in the juice would
occur.
APPRAISAL
The data provided on residues of aldrin plus dieldrin in maize grain
resulting from planting maize in soils treated at recommended rates
indicate that levels of 0.02 ppm or less could be expected and that
maize grain is within the recommended practical residue limit of 0.02
ppm on raw cereals listed in the 1969 Report of the Joint Meeting.
The data on residues of aldrin plus dieldrin in whole washed potato
tubers arising from soil applications of aldrin at recommended rates
reveals the possibility of exceeding the recommended tolerance of 0.1
ppm, especially when the potatoes are grown in plots receiving a
number of annual applications at the highest rate. An increased
tolerance of 0.2 ppm is recommended for potatoes.
Residues of aldrin plus dieldrin in mature sugar beets are
proportional to the residues in soil at planting. For example, an
aldrin dosage of 2.5 lb/acre gives after six months a soil residue of
0.11 ppm and a sugar beet root residue, grown in the same year, of
0.06 ppm. This would be expected to result in about 0.24 ppm of
dieldrin residue in the pulp dried at 105°C. This amount of residue
under normal feeding practices would not lead to excessive residues in
whole milk or meat (fat basis). Therefore, the recommended practical
residue limits appear to be adequate for residues from this source.
Residues in the crop derived from dressed seed (maize, rice, cereals,
cotton and pulse crops) are extremely small and usually below the
limits of detection, thus justifying the reduction of the tolerance in
rough rice to 0.02 ppm.
Residues in vegetables from seed or soil treatment are dependent on
the type of crop. Residues at or in excess of tolerance could arise in
carrots and lettuce planted in soil previously treated for other
crops. Data were received which justify a revision of the recommended
tolerance and the establishment of a practical residue limit of 0.2
ppm for lettuce and carrots.
Soil applications of aldrin of 2-5 kg/ha in citrus groves resulted in
dieldrin residues of 0.01-0.02 ppm in whole oranges and no detectable
residues in grapefruit. The presently recommended tolerance of 0.05
ppm appears to be adequate.
The importance of photodieldrin as a plant metabolite of aldrin or
dieldrin appears to be well established; however, its significance as
a terminal residue will depend on the results of experiments in
progress on rates of storage and excretion in animals.
A consideration of the new data supplied on residues in milk, milk
products and meat resulting from supervised trials and surveys of food
in commerce does not indicate a need for revision of the previously
recommended practical residue limits for these items at this time. The
previous practical residue limit of 0.125 ppm for milk products was
changed to 0.15 ppm to allow for the limitations and variability of
residue analytical procedures.
A suitable regulatory method of analysis exists for photodieldrin
(official AOAC multiresidue method) but no methods of analysis have
been worked out for the more hydrophilic metabolites of dieldrin.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The previously recommended tolerances and practical residue limits (no
longer temporary) are amended as follows:
ppm
Fruit (other than citrus), asparagus,
broccoli, brussels sprouts, cabbage,
cauliflower, cucumber, eggplant,
horse-radish, onions, parsnips, peppers,
pimentoes, radishes and radish tops 0.1
Citrus 0.05
Rice (rough) 0.02
Potatoes 0.2
PRACTICAL RESIDUE LIMITS
ppm
Carrots, lettuce, fat of meat 0.2
Raw cereals (other than rice) 0.02
Milk and milk products (fat basis) 0.15
Eggs (shell-free) 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further information relevant to the assessment of the significance
of the changes that occur in the livers of mice exposed to dieldrin. A
comparison of the response to dieldrin of high and low spontaneous
liver-tumour mouse strains in respect of liver tumour incidence and
the metabolism of dieldrin might be helpful in this respect.
2. The continued collection of follow-up information on persons
exposed occupationally in the manufacture of dieldrin.
3. Completion of the studies being conducted on the comparative
metabolism of dieldrin in man, monkey, rat and mouse.
4. Experimental studies to determine whether dieldrin might have
teratogenic potential.
5. A continuing study of the possible occurrence of residues of
photodieldrin in food surveys and total diet studies.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J.Sci.Fd.
Agric., 20: 245-249
Association of Public Analysts, London. (1969) Joint survey of
pesticide residues in foodstuffs sold in England and Wales, August
1966-July 1967
Baldwin, M.K. and Robinson, J. (1969a) The identification of a
metabolite of the photo-isomerization product of HEOD (dieldrin) found
in rat tissues. Unpublished report from the Tunstall Laboratory, Shell
Research Ltd., Sittingbourne
Baldwin, M.K. and Robinson, J. (1969b) Metabolism in the rat of the
photo-isomerization product of dieldrin. Nature, 224: 283-284
Baldwin, M.K. and Robinson, J. (1970) Comparison of the metabolism of
HEOD in the CFI mouse with that in the CFE rat - preliminary results.
Unpublished report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Baldwin, M.K., Robinson, J. and Carrington, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Industr., 595-597
Bick, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Austr., 54 (1): 1127-1130
Braund, D.G., Brown, L.D., Huber, J.J., Leeling, N.C. and Zabik, M.J.
(1969) Excretion and storage of dieldrin in dairy cows fed
thryoprotein and different levels of energy. J. Dairy Sci., 52:
172-182
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27: 81-138
Bro-Rasmussen, F., and Voldum-Clausen, K. (1966) Tids. Planteovl.,
20: 232
Bro-Rasmussen, F., Dalgaard-Mikkelsen, S., Jakobsen T., Koch, S.O.,
Rodin, F., Uhl, E. and Voldum-Clausen, K. (1968) Examinations of
Danish milk and butter for contaminating organochlorine insecticides.
Res. Rev., 23: 55-69
Brown, V.K., Robinson, J. and Richardson, A. (1967) Preliminary
studies on the acute and sub-acute toxicities of a photoisomerization
products of HEOD (dieldrin). Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Chan, T. and Terriere, L.C. (1969) Aldrin epoxidase activity of rat
liver and rat liver microsomes under various conditions of storage.
Biochem. Pharmacol., 18: 1061-1070
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Crabtree, A.N. (1969) The concentrations of organochlorine
insecticides in human fat and blood from Australia. Shell Research
Limited, Tunstall Report No. TL GR. 0059.69
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Ass. Off. Anal. Chem., 49: 354-364
Cummings, J.G., Gidelman M., Turner V., Reed D., Zee K.T. and Cook
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydrocarbon insecticides to hens. J. Ass. Off. Anal.
Chem., 50: 418-425
Dailey, R.E., Walton, M.S., Beck, V., Leavens, C.L. and Klein, A.K.
(1970) Excretion, distribution and tissue storage of a 14C-labelled
photoconversion product of 14C-dieldrin. J. Agr. Fd. Chem., 18:
443-445
Damico, J.N., Chen, J.T., Costello, C.E. and Haenni, E.O. (1968)
Structure of Klein's metabolites of aldrin and dieldrin. J. Ass. Off.
agr. Chem., 51: 48-55
Datta, P.R., Lang, E.P., Watts, J.O., Klein, A.K. and Nelson, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature, 208: 289-290
Dejaukheere, W. and Kips, R.N. (1968) Meded. Rijksfac.
Landbouwwenteusch (Ghent), 33: 1283
Dickes, G.J. and Nicholas, P.V.J. (1969) Assoc. Pub. Analysts, 7: 14
Diechmann, W.B., Dressler, I., Keplinger, M. and MacDonald, W.E.
(1968) Retention of dieldrin in blood, liver and fat of rats fed
dieldrin for six months. Industr. Med. Surg., 37: 837-839
Diechmann, W.B., MacDonald, W.E., Radomski, J., Blum, E.B.,
Bevilacqua, M., and Keplinger, M. (1970) The tumorigenicity of aldrin,
dieldrin and endrin in the albino rat. Industr. Med., 39: 314
Donninger, C., Potter, D., Potter, S.A. and Wright, A.S. (1967) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to rats. Unpublished report from Tunstall Laboratory,
Shell Research Ltd., Sittingbourne
Donninger, C. (1969) Liver cell changes following the oral
administration of dieldrin to mice. Unpublished report from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest.
Monit. J., 2(4): 153-162
Elgar, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Fd. Agr., 17: 541-545
FAO/WHO (1967) Evaluations of some pesticide residues in food,
FAO,PL:CP/15; WHO/Food Add./67.32 p. 3-4.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Feil, V.J., Hedda, R.D., Zayliskie, R.G. and Zachrison, C.N. (1970)
Dieldrin-14C metabolism in sheep. Identification of
trans-6-7-dihydroxyaldrin and 9-(synepoxy)
hydroxy-1,2,3,4,10,10-hexachloro-6,7-
epoxy-1,4,4a,5,6,7,8,8a-,octahydro-1,4-endo-5,8-
exodimethanonophthalene. J. Agr. Fd. Chem., 18: 120-24
Findlay, E. and Hamilton, G.A. (1968) Pesticide residues in foodstuffs
in Great Britain. VIII Organochlorine pesticide residues in eggs and
poultry. J. Sci. Fd. Agric., 19: 609-611
Gannon, N., Link, R.P. and Decker, G.C. (1959a) Pesticide residues in
meat. Storage of dieldrin in tissues of steers, hogs, lambs and
poultry fed dieldrin in their diets. J. Agr. Fd. Chem., 7: 826-828
Gannon, N., Link, R.P. and Decker, G.C. (1959b) Pesticide residues in
meat and milk. Storage of dieldrin in tissues and its excretion in
milk of dairy cows fed dieldrin in their diets. J. Agr. Fd. Chem.,
7: 824-826
Gillett, J.W. and Chan, I.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Food Chem., 16: 590-593
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Graves, J.B., Bonner, F.L., McKnight W.F., Watts, E.B. and Epps, E.A.
(1969) Residues in eggs, preening glands, liver and muscle from
feeding dieldrin-contaminated rice bran to hens and its effect on egg
production, egg hatch and chick survival. Bull. Env. Contam. Tox.,
4: 375-383
Harr, J.R., Claeys, R.R., Bone, J.F. and McCorcle, J.W. (1970)
Dieldrin toxicosis: rat reproduction. Amer. J. vet. Res., 31:
181-189
Harris, L.E. and Greenwood, D.A. (1963) Effect of residues of newer
insecticides on health. Unpublished progress report to Shell Chemical
Company, letter signed by Joseph Street, dated 8 March 1963
Hedde, R.D., Davison, K.C. and Robbins, J.D. (1970) Dieldrin 14-C
metabolism in sheep. Distribution and isolation of urinary
metabolites. J. Agr. Fd. Chem., 18: 116-119
Hunter, C.G., Robinson, J. and Roberts, M. Pharmacodynamics of
dieldrin (HEOD). Ingestion by human subjects for 18 to 24 months and
postexposure for eight months. Arch. environm. Hlth., 18: 12-21
Jager, K.W. (1970) "Aldrin, dieldrin, endrin and telodrin". An
epidemiological and toxicological study of long-term occupational
exposure. Thesis, 234 p., Elsevier, Amsterdam
Jansen, J.D. (1969) Letter to A.M. Coetzee dated 25 July 1969
Keane, W.T. and Zavon, M.R. (1969) Validity of a critical blood level
for prevention of dieldrin intoxication. Arch. environm. Hlth., 19:
36-44
Kinoshita, P.K. and Kempf, C.K. (1970) Quantitative measurements of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Tox. Appl. Pharmacol., 17: 288
Klein, A.K., Link J.D. and Ives, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed aldrin
and dieldrin. J. Ass. Off. agr. Chem., 51: 895-898
Korte, F. and Arent, H. (1965) Metabolism of insecticides. IX (1)
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin - 14C. Life Sci., 4:
2017-2026
Korte, F. (1970) Terminal residues of cyclodiene insecticides. Comptes
Rendus, XXV Conference, IUPAC, Cortina d'Ampezzo, Italy, July 1969.
Appendix II: p. 172-180
Korte, F. and Porter, P.E (1970) Minutes of the Fifth Meeting of the
IUPAC Terminal Pesticide Residues, September 1970, Erbach, West
Germany. Appendixes 4 and 4a
Lee, D.F. (1968) Pesticide residues in foodstuffs in Great Britain.
IX. Aldrin, dieldrin and other organochlorine pesticides in potatoes
and carrots. J. Sci. Fd. Agric., 19: 701-705
Lehner, P.N. and Egbert, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature, 224: 1218-1219
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, et al. (1967) Food Technol. 21: 435-438
Lofroth, G. (1970) Exposure of the general population to dieldrin.
Letter to editor and reply Robinson J. and Roberts M. Fd. Cosmet.
Toxicol., 8: 341-342
Lutz-Ostertag, Y. and Lutz, H. (1969) Embryologie expérimentale. Note
préliminaire sur les effets "oestrogènes" de l'"Aldrine" sur le
tractus urogénital de l'embryon d'Oiseau. C.R. Acad. Sci. (Paris) Ser.
D., 269: 484-486
Maier-Bode, H. (1967) The aldrin and dieldrin contents of German
edible carrots. Bull. environ. Contam. Toxicol., 2(1): 10-11
Matthews, H.B. and Matsumura, F. (1969) Metabolic fate of dieldrin in
the rat. J. Agr. Fd. Chem., 17: 845-852
McGill, A.E.J. and Robinson, J. (1968) Organochlorine insecticide
residues in complete prepared meals: a twelve-month survey in
southeast England. Fd. Cosmet. Toxicol., 6: 45-57
Mestres, R., Barthes, F. and Priu, M. (1967) DDT, aldrin and dieldrin
residue determinations in milk and butter. Bull. Environ. Contam.
Toxicol., 2(1): 25-33
Moubry, R.J. and Myrdal, G.E. (1970) private communication, Wisconsin
Department of Agriculture
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugarbeets. J. Econ. Entomol.,
63: 1143-1146
Parsons, A.M. and Moore, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem. Soc.
(C), 2026-2031
Polishuk, Z.W., Wasserman, M., Wasserman, D., Groner, Y., Luzarovici,
S. and Tomatis, L. (1970) Arch. environm. Hlth., 20: 215-217
Porter, R.D. and Wiemeyer, S.N. (1969) Dieldrin and DDT: Effects on
sparrow hawk eggshells and reproduction. Science, 165: 199-200
Potter, D., Wooder, M.F., Wright, A.S., Greenland, R.D. and
Zavon, M.R. (1970) The effects of ingestion of foreign compounds on
the sub-cellular structure and function of mammalian liver cells.
Paper given by A.S. Wright at Sittingbourne, 14 May 1970
Renvall, S. and Akerblom, M. (1968) Rester au organiska
klorpesticider, sörskilt aldrin och dieldrin, 2 nagra svenska
kalvöxter ochpotatis. Var. Föda, 20: 65-72
Reports of the Government Chemist, H.M.S.O., London (1965-1969)
Richardson, A. (1966) Ultra-violet conversion product of HEOD: Its
concentration in various samples received from the USA. Shell Research
Tunstall Report No. IRR TL/35/66
Richardson, A. (1968) Residues of dieldrin and its ultraviolet
decomposition product in selected samples. Shell Research Tunstall
Report No. TLGR 0014.63
Richardson, A., Baldwin, M. and Robinson, J. (1968) Identification of
metabolites of dieldrin (HEOD) in the faeces and urine of rats. J.
Sci. Fd. Agr., 19: 524-529
Robinson, et al. (1965-66) Shell Research Ltd., Tunstall, Report
PRRTL/12/65 and TU/23/66
Robinson, J., Richardson, A. and Bush, B. (1966b) A photoisomerization
product of dieldrin. Bull. environm. Contam. Toxicol., 1: 127-133
Robinson, J., Roberts, M., Baldwin, M. and Walker, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Fd. Cosmet. Toxicol.,
7: 317-332
Robinson, J. (1970a) Metabolites of dieldrin in the excreta of Rhesus
monkeys. Unpublished information submitted in a letter to H.G.S. van
Raalte
Robinson, J. (1970b) Metabolites of dieldrin in the excreta of man.
Unpublished information submitted in a letter to H.G.S. van Raalte
Roe, F.J.C. and Grant, G.A. (1970) Inhibition by germ-free status of
development of liver and lung tumours in mice exposed neonatally to
7,12-dimethyl-benz(a) anthracene: implications in relation to tests
for carcinogenicity. Int. J. Cancer, 6: 133-144
Rosen, J.D., Sutherland, D.J. and Lipton, G.R. (1966) The
photochemical isomerization of dieldrin and endrin and effects on
toxicity. Bull. environm. Contam. Toxicol., 1: 133-140
Ruzica, J.H.A., Simmond, J.H. and Tatton, J. O'G. (1967) Pesticide
residues in foodstuffs in Great Britain. IV. Organochlorine pesticide
residues in welfare foods. J. Sci. Fd. Agric., 18: 579-582
Rymer, T.E. and Hammence, J.H. (1967) J. Ass. Pub. Analysts 5: 24
Sakshaug, J. (1968) Residues of organochlorine insecticide in
Norwegian butter samples 1966 (in Norwegian). J. Nord. Vet.-Med.,
20: 696-701
Shell International Chemical Co. (1968) Residues in potatoes resulting
from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in vegetables
resulting from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in small grains
resulting from applications of aldrin, Monograph, November
Smith, Klosterman and Dogger. (1966) Ann. Proc. N. Dakota Acad. Sci.,
20: 96
Tannenbaum, A. and Silverstone, H. (1949a) The genesis and growth of
tumours. IV. Effects of varying the proportion of protein (casein) in
the diet. Cancer Res., 9: 162-173
Tannenbaum, A. and Silverstone, H. (1949b) The influences of the
degree of calorie restriction in the formation of skin tumours and
hepatomas in mice. Cancer Res., 9: 724-727
van Steyvoort, L. et al. (1968) Meded. Rijksfac. Landbouwwettensch XX,
the International Symposium, Ghent, May
Walker, K. et al. (1965) The fate of aldrin, dieldrin and endrin
residues daring the processing of sugar beets. United States
Department of Agriculture, ARS. Publication, ARS-33-107, p. 8
Walker, A.I.T., Stevenson, D.E., Robinson, J., Thorpe, E. and Roberts,
M. (1969a) The toxicology and pharmacodynamics of dieldrin (HEOD):
two-year oral exposures in rats and dogs. Toxicol. appl. Pharmacol.,
15: 345-373
Walker, A.I.T., Neill, C.H., Stevenson, D.E. and Robinson, J. (1969b)
The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix coturnix
japonica). Toxicol. appl. Pharmacol., 15: 69-73
Walker, A.I.T., Thorpe, E. and Stevenson D.E. (1970a) The toxicology
of dieldrin (HEOD): long-term oral toxicity experiments in mice.
Unpublished report from the Tunstall Laboratory, TU/13/70, Shell
Research Ltd., Sittingbourne. Proposed for publication in Fd. Cosmet.
Toxicol.
Walker, A.I.T., Thorpe, E. and Baldwin, M.K. (1970b) Toxicity studies
on the ultra-violet conversion product of dieldrin (UVCPD): thirteen
weeks oral experiment with rats. Shell Research Tunstall Report No.
TLGR. 0053.69
Walton, M.S., Beck, V. and Baron, R.L. (1970) Subacute toxicity of
photodieldrin 3,4,5,6,6,7-hexachloro-12-oxahexacyclo-tridecane, a
photodecomposition product of dieldrin. Tox. appl. Pharmacol., 17:
278.
Wasserman, M., Curnow, D.H., Forte, P.N. and Gurner Y. (1969) Storage
of organochlorine pesticides in the body fat of people in Western
Australia. Industr. Med. Surg., 37: 295-300
Wasserman, M., Coetzee, A.M., Wasserman, D. and Lazarovici, S. (1969)
Present state of the storage of the organochlorine insecticides in the
general population of South Africa. Paper given at the IUPAC
conference, Johannesburg
Williams, S., Mills, P.A. and McDowell, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J. Ass. Off. Anal. Chem., 47: 1124-1128
Winnett, G. and Reed, J.P. (1968) Aldrin, dieldrin, endrin and
chlordane persistence - a three-year study. Pest. Mon. J., 2(3):
133-136
Winteringham, F.P.W. and Barnes, J.M. (1955) Comparative response of
insects and mammals to certain halogenated hydrocarbons used as
insecticides. Physiol. Rev., 35: 701-739
Wright, A.S., Potter, D., Wooder, M.F., Donninger, C., Le Pelley,
J.R., Potter, S.A., Ferrigan, L.W., Sykes, R., Dean, B., Pickering,
R.G., Walker, A.I.T., Crabtree, A.N. and Richardson, A. (1968) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to female dogs. Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Wright, A.S., Greenland R.D., Zavon, M.R. and Donninger, C. (1969)
Liver cell changes following the oral administration of dieldrin to
Rhesus monkeys for 5.5-6 years. Unpublished report from the Tunstall
Laboratories, Shell Research Ltd., Sittingbourne
Zavon, M.R. (1970) The effect of long-continued ingestion of dieldrin
on Rhesus monkeys. A six-year study. Unpublished report from the
Kettering Laboratory, University of Cincinnati, submitted by Shell
International Research.

 In a comparative study on the metabolism of dieldrin in mice and rats
administered 14C-labelled dieldrin, ten times more radioactivity
appeared in the faeces than in the urine of both species. More
unchanged dieldrin occurred in the rat urine than in mouse urine, and
Klein's metabolite (III) was found only in rat urine. Other minor
differences in urinary metabolites occurred between the two species,
and in the mouse they were largely unidentified (Baldwin and Robinson,
1970).
Some information on dieldrin metabolism has recently become available
both in humans and in non-human primates. Neither unchanged dieldrin
nor the urinary metabolites found in rats were identified in workers
occupationally exposed to dieldrin or in monkeys fed dieldrin. The
faeces contained the majority of the metabolites of dieldrin (Robinson
1970a, 1970b).
Effect on enzymes and other chemical parameters
A study of the ultrastructural and biochemical effects of dieldrin and
phenobarbitone upon the liver of rats, dogs and mice has been reported
(Donninger et al. 1967; Donninger, 1969 and Wright et al. 1968), and
the comparative effects have been reviewed (Potter et al. 1970).
Rats were fed dieldrin at a dose of 8 mg/kg and phenobarbitone at 140
mg/kg. Dogs were given 2.0 mg/kg of dieldrin and 20 mg/kg of
phenobarbitone. Mice received doses of dieldrin ranging from 0.16 to
7.5 mg/kg and doses of phenobarbitone between 1.2 and 120 mg/kg. In
addition, the effect of the administration of 80 mg/kg of the
carcinogen 4-amino-2,3-dimethylazobenzene (butter yellow) was studied
in mice. Increase in the activity of microsomal enzymes was observed
in all three species following the administration of dieldrin or
phenobarbitone, while no increase was seen after butter yellow
administration in mice. A marked increase in the liver smooth
endoplasmic reticulum (SER) occurred in rats and dogs. Considerably
less increase in SER was observed in mice. A further difference was
that in the mouse the relative increase of DNA indicated that increase
of liver-weight in the mouse was the result of new cell formation,
while in the rat and dog there was cellular hypertrophy. No effect
upon the ultrastructure of the mouse liver was observed following the
administration of butter yellow. A progressive decrease in the
activity of glucose-6-phosphatase was observed in the mouse liver
following administration of butter yellow, while the activity of this
enzyme was not changed following administration of dieldrin or
phenobarbitone (Potter et al. 1970).
Dieldrin was fed to male Rhesus monkeys at levels of 0.1, 0.5, 1.0,
1.75 and 5.0 ppm (0.0002-0.07 mg/kg) in the diet for approximately six
years. No significant liver changes were observed at dietary levels
below 1.0 ppm. A dose-related increase in microsomal P450 was found at
the higher levels. Increase of microsomal enzyme activity was observed
at the two highest levels, but no changes in sub-cellular structure
were found. (Wright et al., 1969, Zavon, 1970).
A no-effect level for enzyme induction was established in rats at
between 1 and 5 ppm of dieldrin (Gillett et al., 1968). Kinoshita et
al. (1970) also established a diet level of approximately 1 ppm as the
no-effect level. These workers observed the greatest amount of
increase after one to three weeks of feeding with dieldrin.
After an extensive study of workers in a manufacturing plant, it was
concluded that no measurable microsomal enzyme induction occurs in man
after long exposure to approximately 0.010 mg/kg/day. No evidence of
enzyme stimulation was observed in a smaller group of workers
absorbing higher amounts of dieldrin, up to 0.020 mg/kg/day (Jager,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of equal numbers (125-300) of male and female mice (CFI strain)
were fed dietary levels of 0, 0.1, 1.0 or 10 ppm of dieldrin for
periods of up to 132 weeks. In addition, a positive control group of
50 mice received 600 ppm of the carcinogen,
4-amino-2,3-dimethylazobenzene (butter yellow). Feeding was started at
four weeks of age. The presence of liver tumours was detected
initially by palpation, which was started on all animals after 16
weeks of feeding, and the animals were sacrificed when the tumours
became large enough to endanger health. No effect on health or
behaviour was evident in the first nine months of feeding, and no
liver tumours were detectable before 37 weeks at any dose level of
dieldrin. The morbidity of the mice receiving 10 ppm of dieldrin
increased after nine months, and at 15 months 50 percent of both sexes
had either died or been sacrificed because of tumour size. The control
group normally died after 20-24 months, and the life-span of the 0.1
and 1 ppm group wan the same; these groups displayed no palpable
masses. All the positive control group died after 14 months, although
feeding of butter yellow had been terminated after six months when the
first hepatoma appeared. Liver tumours increased in all test groups
but not in other tissues. Tumours were of two types: type (a)
consisted of solid cords of closely packed parenchymal cells with
morphology and staining characteristics similar to the rest of the
parenchyma. Little mitotic activity occurred, and growth appeared to
be by expansion. These growths were classified as benign tumours. In
the second type, type (b) tumours, a much more abnormal structure was
present with areas of cells proliferating in confluent sheets and
often with foci of necrosis. There were areas of papilliform or
adenoid formation of liver cells with wide and irregular vascular
channels within the growth. The mitotic activity was often increased
and multinucleate forms were seen. On purely morphological criteria,
the authors classified these tumours as hepatocarcinomas. They were
very uncommon in the control group of mice. In a few cases these type
(b) tumours had emboli of cells in the lungs. Dieldrin increased the
incidence of both types of tumour (see Table I) but did not produce
the fibrosis and bile duct proliferation sometimes observed in the
mice given butter yellow. In order to determine if tumour-formation
was reversible, certain mice from the group fed 10 ppm of dieldrin
were transferred to a normal diet after 0, 2, 4, 8, 16, 32 or 64 weeks
and sacrificed after 104 weeks. Incidence of tumours indicated that
removal of dieldrin from the diet did not cause the tumours to regress
or disappear. (Dieldrin also produced liver enlargement and
cytoplasmic changes which were reversible). In companion studies,
sterilizing the diet or the bedding did not influence the incidence of
tumours in mice fed dieldrin (Walker et al., 1970a).
In connection with the increase in liver tumours arising in
susceptible species of mice it is noted that the incidence of
tumour-formation in C3H mice was also affected by variations in the
diet (Tannenbaum and Silverstone, 1949a, 1949b) and in microbiological
status (Roe and Grant, 1970).
Rat
In groups of 25 male and 25 female rats fed 0, 0.1, 1.0 or 10 ppm of
dieldrin for up to two years, the only pathological findings
attributable to dieldrin were the existence of liver parenchymal cell
changes in one male and four females fed 10 ppm. In one female,
microscopic intrahepatic nodules were evident, and in addition nodules
were observed after two years in two females fed 10 ppm and in one
female in the control group (Walker et al., 1969a).
Weanling rats were fed, for a lifetime, diets supplemented with
aldrin, 0, 20, 30 or 50 ppm; dieldrin, 20, 30 or 50 ppm; or endrin, 2,
6 or 12 ppm. Benign and malignant tumours were observed in 23 tissues
or organs - in 199 of 800 experimental rats and in 79 of 163 controls,
examined histologically. The highest number of tumours in all groups
occurred in mammary and lymphatic tissues. When compared to the
controls, male and female rats fed aldrin, 20, 30 and 50 ppm, and
dieldrin, 20, 30 and 50 ppm, showed a dose-related significant
decrease in the incidence of all tumours, particularly those of the
mammary and lymphatic tissues. In male rats fed aldrin and dieldrin 50
ppm, the tumour incidence was reduced from 46 (in 75 control rats) to
13 (in 45 rats fed aldrin 50 ppm) and to one benign tumour (a skin
papilloma) in 44 rats fed dieldrin. In the females fed aldrin and
dieldrin 50 ppm, the total number of tumours was 23 in 41 and 16 in
31, respectively, while the female control rats showed 104 tumours in
88 rats. In all the 963 rats examined, no primary malignant hepatic
tumours were found, only two benign hepatic hemangiomas, one in male
control and one in a female fed endrin 6 ppm (Diechmann et al., 1970).
Special studies on reproduction
Bird embryos
Exposure of chick, quail and pheasant embryos to aldrin produced an
oestrogen-like effect in the male genital tract, leading to the
persistence of the Mullerian ducts and a retardation of testicular
development (Lutz-Ostertag and Lutz, 1969).
TABLE I
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Dieldrin
Males
0 288 10 4 20 0.7 (1)*
(16-25)
0.1 124 22 4 26 0.8 (1)
(18-35)
1.0 111 23 8 31 0.9 (1)
(23-41)
10.0 176 37 57 94 0.6 (1)
(89-97)
Dieldrin
Females
0 297 13 - 13 -
(9-17)
0.1 90 23 4 27 -
(18-38)
1.0 87 31 6 37 1.1 (1)
(26-48)
10.0 148 37 55 92 4.1 (5)
(86-96)
Butter yellow
600 ppm
Males** 23 13 4 17 -
(5-38)
TABLE I (cont'd)
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Females 21 43 38 81 9.5 (2)
(58-94)
* Figures in parentheses are the actual number of mice showing this
lesion.
** The group of male mice fed 600 ppm of butter yellow had a 61 percent
incidence of haemangiosarcomas and anaplastic sarcomas.
Wildlife
Studies in the sparrow hawk have provided evidence that the decline in
this species may be due to a failure in reproduction stemming from
increased shell breakage (Lehner and Egbert, 1969; Porter and
Wiemeyer, 1969), possibly caused by dieldrin.
Quail
When groups of four male and six female quail were fed diets
containing 0, 10, 20, 30 or 40 ppm of dieldrin for periods up to
eighteen weeks, survival and reproduction were markedly affected at
the 20, 30 and 40 ppm levels. At 10 ppm there was only a marginal
effect upon survival, and reproduction was not greatly affected.
Although egg production was reduced in the higher levels, even birds
fed 40 ppm laid fertile eggs which had residues of over 50 ppm of
dieldrin (Walker et al., 1969b).
Mouse
Groups of male and female mice were fed dietary levels 0 or 5 ppm of
dieldrin for 30 days. Test and control mice were then randomly paired
and were continued on the same diet for a further 90 days, there being
a total of 101 pairs in the group fed dieldrin. Mortality in the test
group was comparable to the controls, but the test group produced
significantly smaller litters. There was, however, no difference in
the time taken to produce the litters (Good and Ware, 1969).
Rat
Rats were weaned at 28 days of age and then were started on diets
containing dieldrin in ten two-fold levels from 0.01 to 40 ppm. The
animals were sacrificed at ten equal logarithmic intervals up to 750
days of age. In the females receiving diets containing 0.08 to 0.16
ppm of dieldrin, the conception rate, rate of survival of young, and
the number weaned were normal. At higher levels all these parameters
decreased so that at levels greater than 2.5 ppm few young survived,
and at 20 ppm none survived. The maximum dietary exposure which did
not interfere with reproduction was 0.24 ppm (Harr et al., 1970).
Sheep
36 ewes were fed diets containing 0, 1.0, 5.0 and 25.0 ppm of dieldrin
over a period of 40 months, including two gestation periods. No
teratogenic effects were observed and reproductive performance was
normal. However, at the 25 ppm level the lambs died shortly after
birth (Harris and Greenwood, 1963).
Special studies on the photoisomerization product of dieldrin
Of various compounds that can arise due to the influence of sunlight
on aldrin and dieldrin, an identified product, referred to as
"photodieldrin" is the only product of any established importance; the
corresponding "photoaldrin" is of negligible practical significance.
Through the work of Robinson et al. (1965, 1966a), Parsons and Moore
(1966), and Rosen et al. (1966), it has been established that
"photodieldrin" has the cage structure A shown in Figure 2.
Rats ware fed diets containing 10 or 30 ppm of the photoisomer for
thirteen weeks. The animals were sacrificed at the end of the period,
and brain, liver and fat were analysed. The presence of the unchanged
photoisomer as well as the pentochloroketone (Klein's metabolite, B,
found in dieldrin metabolism) was demonstrated (Baldwin and Robinson,
1969a, 1969b).
Exposure of dieldrin to ultraviolet light produced the
hexachlorocyclo-isomer VII. This compound was produced in 7 percent
yield after two months or in 25 percent yield after 12 months exposure
to sunlight. The compound was found to be twice as toxic as dieldrin
to the housefly and mosquito (Rosen et al., 1966; Robinson et al.,
1966b).
The photoisomerization product of dieldrin (photodieldrin) has been
determined in a number of vertebrate species. Using oral or
intragastric injection, either alone or in dimethylsulfoxide solution,
it was found to be substantially more toxic than dieldrin in most
species. Convulsions similar to those seen from dieldrin occurred
prior to death. Table II summarizes the acute toxicity compared to
dieldrin (Brown et al., 1967).
In a comparative study on the metabolism of dieldrin in mice and rats
administered 14C-labelled dieldrin, ten times more radioactivity
appeared in the faeces than in the urine of both species. More
unchanged dieldrin occurred in the rat urine than in mouse urine, and
Klein's metabolite (III) was found only in rat urine. Other minor
differences in urinary metabolites occurred between the two species,
and in the mouse they were largely unidentified (Baldwin and Robinson,
1970).
Some information on dieldrin metabolism has recently become available
both in humans and in non-human primates. Neither unchanged dieldrin
nor the urinary metabolites found in rats were identified in workers
occupationally exposed to dieldrin or in monkeys fed dieldrin. The
faeces contained the majority of the metabolites of dieldrin (Robinson
1970a, 1970b).
Effect on enzymes and other chemical parameters
A study of the ultrastructural and biochemical effects of dieldrin and
phenobarbitone upon the liver of rats, dogs and mice has been reported
(Donninger et al. 1967; Donninger, 1969 and Wright et al. 1968), and
the comparative effects have been reviewed (Potter et al. 1970).
Rats were fed dieldrin at a dose of 8 mg/kg and phenobarbitone at 140
mg/kg. Dogs were given 2.0 mg/kg of dieldrin and 20 mg/kg of
phenobarbitone. Mice received doses of dieldrin ranging from 0.16 to
7.5 mg/kg and doses of phenobarbitone between 1.2 and 120 mg/kg. In
addition, the effect of the administration of 80 mg/kg of the
carcinogen 4-amino-2,3-dimethylazobenzene (butter yellow) was studied
in mice. Increase in the activity of microsomal enzymes was observed
in all three species following the administration of dieldrin or
phenobarbitone, while no increase was seen after butter yellow
administration in mice. A marked increase in the liver smooth
endoplasmic reticulum (SER) occurred in rats and dogs. Considerably
less increase in SER was observed in mice. A further difference was
that in the mouse the relative increase of DNA indicated that increase
of liver-weight in the mouse was the result of new cell formation,
while in the rat and dog there was cellular hypertrophy. No effect
upon the ultrastructure of the mouse liver was observed following the
administration of butter yellow. A progressive decrease in the
activity of glucose-6-phosphatase was observed in the mouse liver
following administration of butter yellow, while the activity of this
enzyme was not changed following administration of dieldrin or
phenobarbitone (Potter et al. 1970).
Dieldrin was fed to male Rhesus monkeys at levels of 0.1, 0.5, 1.0,
1.75 and 5.0 ppm (0.0002-0.07 mg/kg) in the diet for approximately six
years. No significant liver changes were observed at dietary levels
below 1.0 ppm. A dose-related increase in microsomal P450 was found at
the higher levels. Increase of microsomal enzyme activity was observed
at the two highest levels, but no changes in sub-cellular structure
were found. (Wright et al., 1969, Zavon, 1970).
A no-effect level for enzyme induction was established in rats at
between 1 and 5 ppm of dieldrin (Gillett et al., 1968). Kinoshita et
al. (1970) also established a diet level of approximately 1 ppm as the
no-effect level. These workers observed the greatest amount of
increase after one to three weeks of feeding with dieldrin.
After an extensive study of workers in a manufacturing plant, it was
concluded that no measurable microsomal enzyme induction occurs in man
after long exposure to approximately 0.010 mg/kg/day. No evidence of
enzyme stimulation was observed in a smaller group of workers
absorbing higher amounts of dieldrin, up to 0.020 mg/kg/day (Jager,
1970).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
Groups of equal numbers (125-300) of male and female mice (CFI strain)
were fed dietary levels of 0, 0.1, 1.0 or 10 ppm of dieldrin for
periods of up to 132 weeks. In addition, a positive control group of
50 mice received 600 ppm of the carcinogen,
4-amino-2,3-dimethylazobenzene (butter yellow). Feeding was started at
four weeks of age. The presence of liver tumours was detected
initially by palpation, which was started on all animals after 16
weeks of feeding, and the animals were sacrificed when the tumours
became large enough to endanger health. No effect on health or
behaviour was evident in the first nine months of feeding, and no
liver tumours were detectable before 37 weeks at any dose level of
dieldrin. The morbidity of the mice receiving 10 ppm of dieldrin
increased after nine months, and at 15 months 50 percent of both sexes
had either died or been sacrificed because of tumour size. The control
group normally died after 20-24 months, and the life-span of the 0.1
and 1 ppm group wan the same; these groups displayed no palpable
masses. All the positive control group died after 14 months, although
feeding of butter yellow had been terminated after six months when the
first hepatoma appeared. Liver tumours increased in all test groups
but not in other tissues. Tumours were of two types: type (a)
consisted of solid cords of closely packed parenchymal cells with
morphology and staining characteristics similar to the rest of the
parenchyma. Little mitotic activity occurred, and growth appeared to
be by expansion. These growths were classified as benign tumours. In
the second type, type (b) tumours, a much more abnormal structure was
present with areas of cells proliferating in confluent sheets and
often with foci of necrosis. There were areas of papilliform or
adenoid formation of liver cells with wide and irregular vascular
channels within the growth. The mitotic activity was often increased
and multinucleate forms were seen. On purely morphological criteria,
the authors classified these tumours as hepatocarcinomas. They were
very uncommon in the control group of mice. In a few cases these type
(b) tumours had emboli of cells in the lungs. Dieldrin increased the
incidence of both types of tumour (see Table I) but did not produce
the fibrosis and bile duct proliferation sometimes observed in the
mice given butter yellow. In order to determine if tumour-formation
was reversible, certain mice from the group fed 10 ppm of dieldrin
were transferred to a normal diet after 0, 2, 4, 8, 16, 32 or 64 weeks
and sacrificed after 104 weeks. Incidence of tumours indicated that
removal of dieldrin from the diet did not cause the tumours to regress
or disappear. (Dieldrin also produced liver enlargement and
cytoplasmic changes which were reversible). In companion studies,
sterilizing the diet or the bedding did not influence the incidence of
tumours in mice fed dieldrin (Walker et al., 1970a).
In connection with the increase in liver tumours arising in
susceptible species of mice it is noted that the incidence of
tumour-formation in C3H mice was also affected by variations in the
diet (Tannenbaum and Silverstone, 1949a, 1949b) and in microbiological
status (Roe and Grant, 1970).
Rat
In groups of 25 male and 25 female rats fed 0, 0.1, 1.0 or 10 ppm of
dieldrin for up to two years, the only pathological findings
attributable to dieldrin were the existence of liver parenchymal cell
changes in one male and four females fed 10 ppm. In one female,
microscopic intrahepatic nodules were evident, and in addition nodules
were observed after two years in two females fed 10 ppm and in one
female in the control group (Walker et al., 1969a).
Weanling rats were fed, for a lifetime, diets supplemented with
aldrin, 0, 20, 30 or 50 ppm; dieldrin, 20, 30 or 50 ppm; or endrin, 2,
6 or 12 ppm. Benign and malignant tumours were observed in 23 tissues
or organs - in 199 of 800 experimental rats and in 79 of 163 controls,
examined histologically. The highest number of tumours in all groups
occurred in mammary and lymphatic tissues. When compared to the
controls, male and female rats fed aldrin, 20, 30 and 50 ppm, and
dieldrin, 20, 30 and 50 ppm, showed a dose-related significant
decrease in the incidence of all tumours, particularly those of the
mammary and lymphatic tissues. In male rats fed aldrin and dieldrin 50
ppm, the tumour incidence was reduced from 46 (in 75 control rats) to
13 (in 45 rats fed aldrin 50 ppm) and to one benign tumour (a skin
papilloma) in 44 rats fed dieldrin. In the females fed aldrin and
dieldrin 50 ppm, the total number of tumours was 23 in 41 and 16 in
31, respectively, while the female control rats showed 104 tumours in
88 rats. In all the 963 rats examined, no primary malignant hepatic
tumours were found, only two benign hepatic hemangiomas, one in male
control and one in a female fed endrin 6 ppm (Diechmann et al., 1970).
Special studies on reproduction
Bird embryos
Exposure of chick, quail and pheasant embryos to aldrin produced an
oestrogen-like effect in the male genital tract, leading to the
persistence of the Mullerian ducts and a retardation of testicular
development (Lutz-Ostertag and Lutz, 1969).
TABLE I
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Dieldrin
Males
0 288 10 4 20 0.7 (1)*
(16-25)
0.1 124 22 4 26 0.8 (1)
(18-35)
1.0 111 23 8 31 0.9 (1)
(23-41)
10.0 176 37 57 94 0.6 (1)
(89-97)
Dieldrin
Females
0 297 13 - 13 -
(9-17)
0.1 90 23 4 27 -
(18-38)
1.0 87 31 6 37 1.1 (1)
(26-48)
10.0 148 37 55 92 4.1 (5)
(86-96)
Butter yellow
600 ppm
Males** 23 13 4 17 -
(5-38)
TABLE I (cont'd)
The percentage incidence of liver tumours in mice fed dieldrin for
132 weeks
Diet Number % Liver tumours (type) % Secondary
concn of tumour deposits
(ppm) animals %(a) %(b) %(a+b) in lung
Females 21 43 38 81 9.5 (2)
(58-94)
* Figures in parentheses are the actual number of mice showing this
lesion.
** The group of male mice fed 600 ppm of butter yellow had a 61 percent
incidence of haemangiosarcomas and anaplastic sarcomas.
Wildlife
Studies in the sparrow hawk have provided evidence that the decline in
this species may be due to a failure in reproduction stemming from
increased shell breakage (Lehner and Egbert, 1969; Porter and
Wiemeyer, 1969), possibly caused by dieldrin.
Quail
When groups of four male and six female quail were fed diets
containing 0, 10, 20, 30 or 40 ppm of dieldrin for periods up to
eighteen weeks, survival and reproduction were markedly affected at
the 20, 30 and 40 ppm levels. At 10 ppm there was only a marginal
effect upon survival, and reproduction was not greatly affected.
Although egg production was reduced in the higher levels, even birds
fed 40 ppm laid fertile eggs which had residues of over 50 ppm of
dieldrin (Walker et al., 1969b).
Mouse
Groups of male and female mice were fed dietary levels 0 or 5 ppm of
dieldrin for 30 days. Test and control mice were then randomly paired
and were continued on the same diet for a further 90 days, there being
a total of 101 pairs in the group fed dieldrin. Mortality in the test
group was comparable to the controls, but the test group produced
significantly smaller litters. There was, however, no difference in
the time taken to produce the litters (Good and Ware, 1969).
Rat
Rats were weaned at 28 days of age and then were started on diets
containing dieldrin in ten two-fold levels from 0.01 to 40 ppm. The
animals were sacrificed at ten equal logarithmic intervals up to 750
days of age. In the females receiving diets containing 0.08 to 0.16
ppm of dieldrin, the conception rate, rate of survival of young, and
the number weaned were normal. At higher levels all these parameters
decreased so that at levels greater than 2.5 ppm few young survived,
and at 20 ppm none survived. The maximum dietary exposure which did
not interfere with reproduction was 0.24 ppm (Harr et al., 1970).
Sheep
36 ewes were fed diets containing 0, 1.0, 5.0 and 25.0 ppm of dieldrin
over a period of 40 months, including two gestation periods. No
teratogenic effects were observed and reproductive performance was
normal. However, at the 25 ppm level the lambs died shortly after
birth (Harris and Greenwood, 1963).
Special studies on the photoisomerization product of dieldrin
Of various compounds that can arise due to the influence of sunlight
on aldrin and dieldrin, an identified product, referred to as
"photodieldrin" is the only product of any established importance; the
corresponding "photoaldrin" is of negligible practical significance.
Through the work of Robinson et al. (1965, 1966a), Parsons and Moore
(1966), and Rosen et al. (1966), it has been established that
"photodieldrin" has the cage structure A shown in Figure 2.
Rats ware fed diets containing 10 or 30 ppm of the photoisomer for
thirteen weeks. The animals were sacrificed at the end of the period,
and brain, liver and fat were analysed. The presence of the unchanged
photoisomer as well as the pentochloroketone (Klein's metabolite, B,
found in dieldrin metabolism) was demonstrated (Baldwin and Robinson,
1969a, 1969b).
Exposure of dieldrin to ultraviolet light produced the
hexachlorocyclo-isomer VII. This compound was produced in 7 percent
yield after two months or in 25 percent yield after 12 months exposure
to sunlight. The compound was found to be twice as toxic as dieldrin
to the housefly and mosquito (Rosen et al., 1966; Robinson et al.,
1966b).
The photoisomerization product of dieldrin (photodieldrin) has been
determined in a number of vertebrate species. Using oral or
intragastric injection, either alone or in dimethylsulfoxide solution,
it was found to be substantially more toxic than dieldrin in most
species. Convulsions similar to those seen from dieldrin occurred
prior to death. Table II summarizes the acute toxicity compared to
dieldrin (Brown et al., 1967).
 TABLE II
Toxicity of the photoisomerization product of dieldrin
compared with dieldrin
Species Approximate LD50, mg/kg body-weight
Photoisomerization
product of dieldrin Dieldrin
Pigeon 90 250
Chicken 80 48
Mouse 7 77
Rat 10 47
Guinea-pig 3 24
Dog (M) 140 120
Dog (F) 100 90
Dieldrin or its photoisomerization product was fed to five male and
five female mice at dietary levels of 1, 3 and 10 ppm for the
photoisomerization product and 3, 10 and 3O ppm for dieldrin for one
month. The mice exposed to the photoisomerization product were
unaffected by 1 ppm, there was one male and one female death at 3 ppm
and all died at 10 ppm. The mice fed dieldrin were unaffected by 3 or
10 ppm, but three males and two females died at 30 ppm. Autopsies of
the mice revealed no abnormalities (Brown et al., 1967).
A similar experiment with rats produced no deaths from 10 ppm of the
photoisomerization product; a higher level was not tested. Autopsies
revealed no abnormalities. Levels were measured in the tissues; the
biological half-life of the photoisomerization product was 1.7 for the
males and 2.6 for the females, compared to 10.0 and 12.7 respectively
for dieldrin (Brown et al., 1967).
Subacute levels (5 mg per day) of 14C-labelled dieldrin or
photodieldrin were administered to young adult rats of both sexes for
a period of twelve weeks. Photodieldrin was given both orally and
intraperitoneally; dieldrin was given by stomach tube only. About 60
percent of the activity of 14C-photodieldrin was excreted in the
urine and faeces of male rats and 47 percent in those of female rats,
compared to 60 and 37 percent respectively with 14C-dieldrin. Females
retained three-ten times more 14C activity in their tissues than did
male rats after photodieldrin administration. A similar sex difference
in tissue levels was found after administration of dieldrin and
photodieldrin in female rats and of dieldrin in male rats. Extremely
high levels of 14C activity were detected in the kidneys of male, but
not female, rats receiving 14C-photodieldrin (Dailey et al., 1970).
Storage of the photodieldrin was mainly in the fat and up to 10 ppm it
was 2-15 times higher in females than in males. A metabolite of
dieldrin was detected in the kidneys and urine. The concentration of
this metabolite in the kidneys was about ten times that of the
photodieldrin. No toxicologic effects were detected at 1 ppm (Walker
et al., 1970b).
Similar results were reported by Walton et al. (1970) who also found
slight stimulation of liver microsomal enzymes.
Photodieldrin was fed to groups of 12 male and 12 female rats at
levels of 0.1, 1.0, 10 and 30 ppm in the diet for 13 weeks. Tremors
were observed in rats in the 10 and 30 ppm groups. Reduced food intake
and depressed growth also occurred at these levels. Two of the 10 ppm
and six of the 30 ppm females died. Liver/body-weight ratios were
increased in the 30 ppm groups and kidney weight was increased in the
10 and 30 ppm males. Histologic liver changes were observed in the 10
and 30 ppm groups (Walker at al., 1970b).
Short-term studies
Studies on the effect of dieldrin on the liver microsomal enzymes have
been reviewed under "Biochemical aspects". The following additional
studies have become available.
Dog
Three groups each of six dogs of mixed sex were fed 0, 0.2 or 2 mg/kg
body-weight of dieldrin by capsule for five days a week. The group
given the low dose was started at 1.0 mg/kg for five days, then
reduced to 0.2 mg/kg until day 62 and from then on given 2 mg/kg until
signs of intoxication were evident. A direct relationship was found
between the severity of symptoms and the blood concentration of
dieldrin. As the blood level rose from 0.37 to about 0.8 ppm, changes
progressed from a reduced food intake and growth reduction to the
onset of muscular spasm and finally convulsions. A direct relationship
was also found between blood and fat levels of dieldrin (Keane and
Zavon, 1969).
In an extension of the above-mentioned study, three groups each of six
dogs of mixed sex were given a similar dose regimen of dieldrin. It
was found that the period between dieldrin exposure and the onset of
toxic signs was dependent upon the obesity of the animal; the greater
the amount of body fat the longer it took for toxicity to develop.
Since food refusal preceded symptoms of intoxication, forced feeding
was able to suppress toxicity by preventing fat mobilization and the
associated release of toxic concentrations of dieldrin into the blood
stream, so avoiding the death of some animals (Keane et al., 1969).
Groups of five male and five female dogs were given by capsule daily
oral doses of 0, 0.005 or 0.05 mg/kg body-weight of dieldrin for
periods up to two years. Health, behaviour and body-weight were
unaffected by giving dieldrin, and the electroencephalograph readings
showed no abnormalities. Urine and blood findings were normal except
for an increase in plasma alkaline phosphatase after 30 weeks and a
reduction in serum protein at the 0.05 mg/kg dose. Bromosulphthalein
clearance was normal throughout the exposure period. Liver-weight and
liver to body-weight ratios were increased in the females fed 0.05
mg/kg, and decreased heart-weights, but not heart to body-weight
ratios, were found in all males in the test groups and decreased
spleen-weights at 0.05 mg/kg in the males. No abnormal gross or
histopathological findings associated with administration of dieldrin
were evident (Walker et al., 1969a).
Long-term studies
Rat
Dieldrin was fed to groups comprising 25 male and 25 female rats (45
each for the controls) at dietary levels of 0, 0.1, 1.0 or 10 ppm for
periods up to two years. In addition, a separate group comprising 15
animals of each sex were sacrificed after six, 12 or 18 months
feeding. Body-weight and food intake were unaffected by dieldrin, but
at 10 ppm all the animals became irritable after eight or 13 weeks in
the males and females respectively. Occasional convulsions occurred in
this group during handling. No adverse effect on mortality was
observed. There were no changes in haematology or serum enzyme levels
attributable to dieldrin. Liver weights were normal for the first 18
months of feeding, but after two years, increased liver weights and
liver to body-weight ratios were evident in the groups fed 1 and 10
ppm (Walker et al., 1969a).
OBSERVATIONS IN MAN
In a continuation of the study described in the previous monograph
addendum on dieldrin (FAO/WHO 1968), the 12 male subjects were
continued on the dose regimen of 0, 0.01, 0.05 or 0.21 mg of dieldrin
for a total of two years and were further followed during an
eight-month post-exposure period. All twelve men remained in excellent
health, and there were no changes in body-weights, fatness or
leanness, or other clinical abnormalities throughout the entire
exposure and post-exposure periods. No departures from normal or
pre-exposure values in the biochemical and haemotological parameters
investigated were observed, although there was a slight trend towards
a fall in plasma alkaline phosphatase activity in some men fed the
higher dose levels. This drop was of doubtful significance, and the
enzyme level returned to normal after two months post-exposure. The
concentration of dieldrin in the blood did not significantly change in
the groups given 0.05 mg/day, but at 0.21 mg/day there was a slight
increase during months 18 to 21, but little over months 21 to 24.
This increase was small compared to the first 18 months when the blood
level tripled. Increase in dieldrin in adipose tissue was evident at
all levels after administration for 92 days (Hunter et al., 1969).
Extensive studies of workers exposed to dieldrin in a manufacturing
plant have been reported. The average blood concentration of a group
of 35 workers with an exposure of over ten years was 0.035 mg/ml,
calculated to be equivalent to a dietary intake of 407 mg/man/day.
Intoxications had occurred in some of these individuals prior to 1964.
No definite deleterious effects were found in this high exposure
group, including some individuals who had suffered intoxications.
Clinical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein and serum protein spectrum,
determined every three months remained within normal limits. In an
extreme exposure group with blood levels above 0.2 ppm, alkaline
phosphatase values, although within normal limits, were higher than in
the control group.
Using as criteria of possible microsomal enzyme stimulation the blood
levels of pp'-DDE and the urinary ratio of 6-B-hydroxycortisol and
17-hydroxycortico steroids, no differences between exposed and
unexposed workers were found. The dieldrin workers were exposed to an
average daily intake of 593 mg/man. One group of ten workers with the
highest exposure had an average equivalent oral intake of 1224
mg/man/day.
Comparisons of work records between exposed and non-exposed groups did
not reveal any differences as to frequency or severity of diseases or
accidents or disability (Jager et al., 1970). See also "Biochemical
studies".
On the basis of fat samples only, Crabtree (1969) found in Australia a
mean fat concentration of 0.3 ppm equivalent to an intake of 16
mg/man/day. Bick (1967) reported lower figures while Wasserman (1968)
reported higher ones. These last figures may be due to analytical
errors (Lofroth, 1970).
Wasserman et al. (1969) reported very low levels of dieldrin in fat
tissues from South Africa. Jansen (1969), however, analyzing part of
the same samples found a higher mean of 0.159 ppm, equivalent to an
average absorption of 8.6 mg/man/day.
COMMENT
The increased risk of development of liver tumours in mice exposed to
dieldrin as compared with untreated control mice was regarded as the
main matter for concern. This increased risk was seen even at an
exposure level of 0.1 ppm in the diet. It was generally agreed that
the tumours were neoplasms and that those seen in treated mice were
similar in nature to those seen in untreated mice except insofar as
the tumours in dieldrin-treated mice tended to show the same
cytoplasmic changes as other liver parenchymal cells in the same
animals.
There was no evidence that tumours regressed or disappeared after
cessation of exposure to dieldrin, although the cytoplasmic changes
did regress under such circumstances. It is difficult to establish
unequivocal criteria for distinguishing between benign and malignant
neoplasms in mouse-liver. The incidence of mice showing tumour emboli
or metastases in the lungs was not significantly increased at any
levels of exposure to dieldrin up to 10 ppm for 132 weeks.
It was agreed that the effect of dieldrin in increasing the risk of
tumour development in mice could not at the present time be regarded
as sufficient evidence to categorize dieldrin as a carcinogen. Other
work showed that nonspecific factors such as calorie intake, hormonal
status and micro biological status may profoundly influence the risk
of liver tumour development in mice, some strains of which are in any
case peculiarly susceptible to each tumours. Also it was noted that
long term studies in rats and prolonged studies in dogs and monkeys
had revealed no evidence of hepatocarcinogenicity. It seems likely
that the effect of dieldrin in mice is probably a species-specific
phenomena.
Further information is urgently needed concerning the aetiology and
pathogenesis of liver tumours in mice. A comparison of the effect of
dieldrin in mice of high and low liver tumour susceptibility, with
parallel metabolic studies, might provide a basis for relating
observations of tumourogenicity in mice to other species, including
man. There are at present no experimental data referable to
teratogenicity.
Continued surveillance of persons occupationally exposed to dieldrin
for chronic toxic effects and manifestation of possible
carcinogenicity is recommended. In this connection, the extensive
surveillance of occupationally exposed workers, which is currently
being made, was noted.
Information on the varying patterns of metabolism in the mouse, sheep,
rat, monkey and man are as yet too incomplete to indicate whether the
possible carcinogenic potential in the mouse is likely to apply to
man.
For these reasons, it was felt that no change in the acceptable daily
intake established at the 1966 Joint Meeting could be made at this
time.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE IN MAN
0-0.0001 mg/kg body-weight, the sum of aldrin and dieldrin by weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Seed treatments
The use of aldrin and dieldrin as a rice seed treatment is now
considered important only in Brazil and to a lesser degree in Japan,
Costa Rica and El Salvador. The choice between aldrin and dieldrin is
primarily a question of agronomic factors, local preference and
economics prevailing. Treatment rates vary, but a rate of 2 oz /
bushel (0.2 percent active material) is standard. In Brazil, recent
figures show that out of 4,600,000 hectares planted, 3,680,000
hectares (80 percent) were treated; in Japan, out of 3,280,000
hectares planted, 169,000 hectares (0.5 percent) were treated; in
Costa Rica, out of 43,000 hectares planted, 21,500 hectares (50
percent) were treated and in El Salvador out of 31,000 hectares
planted, 6,200 hectares (20 percent) were treated.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In maize
The treatment of soils prior to planting maize represents the most
important of all uses for aldrin. Studies in the United States in
which aldrin was applied to soil at rates of 1, 2, 3, 4 and 6 lb /acre
and in which pre-harvest intervals ranged from 75-195 days resulted in
aldrin residues in maize grain ranging from <0.001-<0.01 ppm and
dieldrin residues from 0.002-0.02 ppm. Control values were equal to or
greater than treated values in virtually all cases. In the same
experiments, the corresponding residues of aldrin plus dieldrin in
green fodder were less than 0.034 ppm; in maize stover most levels
were less than 0.01 ppm and only in two subsamples did levels exceed
0.03 ppm. With rare exceptions, the totals for aldrin and dieldrin
fell well below 0.03 ppm (Shell Development Co., Reports RES 63-122,
64-135, 64-102, 63-111, 64-168, 64-166 Shell Chemical Co., Reports PRL
67-81, 67-62, 65-6, 67-92; Mourbry and Myrdal, pre-print
communication). Similar experiments in Germany, France, Spain,
Portugal, Australia, Mexico, Venezuela and Trinidad at application
rates of 1-5 kg/ha gave residues of aldrin plus dieldrin of <0.01 ppm
in maize grain; a second crop from treatment two years in succession
gave the same result. Residues in whole maize plants (green fodder) in
France, Spain, Australia and Trinidad were 0.01-ppm or less except for
one year samples from Trinidad which were 0.15 ppm. This appears to be
exceptional, since samples in the succeeding year were <0.01 ppm.
Limited data from France on residues in maize leaves ranged from
0.01-0.07 ppm in dry leaf. Based on this data, it is estimated that
whole stover would have residues less than 0.03 ppm. (Shell Research
Ltd., Reports WKGR 0114.70, 0053.68, ibid - unreported data). In the
U.S.A. extensive data have been obtained for maize grown in a series
of rotations typically employed in U.S. farm practice. Treatments were
between 1´-2´ lb/acre - occasionally 5 lb/acre - and covered 35 sites
and included two-eleven years. In no cases were residues detected in
grain (0.01 ppm dieldrin). In stover, 80 percent of the results were
below 0.01 ppm, and the upper limit was 0.02 ppm. No residues were
detected in green corn (Shell Chemical Co., Report PRL 66-73).
In potatoes
The treatment of potato soils at recommended rates (2-4 kg/ha) with
aldrin shortly before planting gave rise to residues in the whole
washed tubers of between <0.01 ppm and 0.1 ppm of aldrin plus
dieldrin. Typically, levels were 0.04-0.06 ppm. Levels in peeled
tubers were generally 1/3-´ of these values (Shell International
Chemical Co. Monograph, November 1968). More recent data developed by
Shell in summarized in Table III; data from Scandinavia is given in
Table IV.
Residues in potatoes resulting from two or three soil applications of
aldrin in consecutive years are summarized in Table V.
A trial in Portugal compared residues resulting from broadcast
applications with row applications made with aldrinised fertilizer,
all at 3 kg/ha. Results are shown in Table VI (Shell Research Ltd.,
Tech. Serv. Report WKTR.0025/69).
Potatoes were planted in plots in tropical countries from which maize,
grown on soil treated at 2-4 kg/ha just prior to sowing, had been
harvested. Aldrin + dieldrin residues in the tubers were from <0.01
to 0.07 ppm (mean--0.03 ppm) and in peeled tubers from <0.01 to 0.03
ppm (mean--0.01 ppm), as shown in Table VI (Shell Research Ltd.,
Report WKGR. 0114.70).
Residue data for potatoes grown in soils in temperate countries to
which aldrin treatment had been given one or two years before planting
are given in Table VII.
Analytical data for 256 samples of peeled potatoes and potato peel
obtained by the U.K. Ministry of Agriculture (Lee, 1968) from potatoes
grown commercially on treated (three or four lb./acre) land shows
residues of aldrin plus dieldrin in peeled tubers ranging from
0.0-0.14 ppm (mean--0.01 ppm). Calculated residues in whole tubers
based on 15 percent peel ranged from 0.0 to 0.23 ppm (mean--ca. 0.02
ppm).
In sugar beets, juice and pulp
Residues of aldrin plus dieldrin in sugar beets grown in soils treated
once with 2 kg/ha in the same year as cropping ranged from <0.01 to
0.03 ppm; 4 kg/ha resulted in residues ranging from <0.01 to 0.05 ppm
(Legar, 1966; van Steyvoort et al., 1968; Shell Research Ltd., Reports
WKTR.0053.68, WKGR.0123.69, WKGR.0114.70; Shell Chemical Co., Report
PRL-68-148). In contradiction to all these findings, Walker et al.,
(1965) found that one application of 5 lb./acre gave combined residues
in the whole fresh beet of 0.19 ppm and Smith et al., (1966) found
residues of 0.93 ppm resulted from 1 lb./acre of aldrin as a row
application at the time of sowing. However, 80 percent of this latter
residue occurred as aldrin, strongly indicating sample contamination.
Residues of dieldrin in sugar beet tops from beets grown in soils
treated once with aldrin at 1-6 kg/ha in various countries in Europe
and in Turkey ranged from <0.001 to 0.01 ppm (van Steyvoort et al.,
1968; Shell Research Ltd., Reports WKTR. 0053.68, WKGR.0123.69,
.0114.70). Residues of aldrin plus dieldrin in roots and tops of sugar
beets grown in soils treated two and sometimes three years in
succession are summarized in Table VIII.
Table III
Residues in potatoes arising from soil applications of aldrin
Application Range of Values, ppm No. of Results Arithmetic Mean
Rate Whole Peeled Whole Pooled Whole Peeled
kg/ha Tubers Tubers Tubers Tubers Tubers Tubers
2 <0.01-0.13 <0.01-0.05 24 10 0.06 0.02
3 0.01-0.16 - 18 - 0.08 -
4 0.03-0.24 0.01-0.07 10 10 0.11 0.04
2-4 <0.01-0.24 - 52 - 0.08 -
5 0.02-0.22 - 11 - 0.11 -
Shell Chemical Co., Reports PRL 68-147, PCY-64-10; Shell Research Ltd.,
Reports WKTR. 0025/69, Tech. Memo 137/65, Tech. Serv. Note 141/66,
WKTR. 0114. 70; Winnett and Reed, 1968.
Table IV
Aldrin and Dieldrin Residues in Potatoes Arising from single soil
applications in Scandinavia
Application Residues, ppm in
Rate, potato tubers
Country kg/ha Aldrin Dieldrin Reference
Sweden(1964) 2.5 <0.01 0.02
Sweden(1965) 2.5 <0.01-0.01 <0.01-0.02 Renvall and
Akerblom, (1968)
Sweden(1966) 2.5 <0.01 0.01
2.5 <0.01 0.05
2.5 0.01 0.02
2.5 0.02 0.13
2.5 0.03 0.12
Denmark 2.5 not found 0.05 Bro-Rasmussen
5.0* not found 0.02-0.05 and Voldum-
10.0* 0.02 0.03-0.10 Clausen, (1966)
* High rates
Table V
Aldrin plus dieldrin residnes in potatoes grown in plots which received
a number of annual treatments with aIdrin
Range of values
Application Aldrin + Dieldrin, Arithmetic
kg/ha ppm No. of results Mean, ppm
Whole Peeled Whole Peeled Whole Peeled
2 0.01-0.11 00.01-0-05 11 8 0.05 0.02
4 0.03-0.40 <0.01-0.01 8 8 0.14 0.03
Shell Research Ltd., Reports WKTR-0007.69, 0028.68, Tech. Serv. Note
141/66, Report WKGR.0114.70, 0153/69, Tech. Serv. Note WKTR. 0100.68.
Table VI
Comparison of residues in potato tubers from broadcast and
row treatments
Range of Results,
Type of Aldrin + Dieldrin, No. of Arithmetic
Application ppm Values Mean, ppm
Row treatment 0.09-0.27 4 0.15
Broadcast 0.04-0.07 3 0.05
(fertilized
mixture)
Broadcast (E.C.) 0.05-0.16 4 0.08
Table VII
Residues In potato tubers grown-in soils treated one or two years
prior to planting
Interval
Treatment Between Range of
Rate, Treatment and Results, No. of Arithmetic
kg/ha Planting, ppm Results Mean, ppm
years
2 1 <0.01-0.05 14 0.02
4 1 0.01-0.05 9 0.03
2-5 1 <0.01-0.11 29 0.04
2-5 2 0.01-0.02 12 0.01
Shell Research Ltd., Tech. Serv. Note 141/66, Report WKGR.0114.70,
Tech. Serv. Report WKTR 0007.69, .0028.68, Report WKGR 0153/69,
Tech. Serv. Note WKTR.0100.68, Winnett and Reed; 1968.
Residues of dieldrin in roots and tops of beets grown on plots treated
the previous year at 2 or 4 kg/ha were all <0.01 ppm (28 samples)
except for two root samples of 0.02 and 0.03 ppm; no aldrin was
detected (Elgar, 1966; Shell Research Ltd., Report WKGR.0114.70).
Recent studies by Onsager at al., (1970) show that aldrin plus
dieldrin residues in mature sugar beets are proportional to the
residues in the soil at the time of planting as illustrated in Table
IX.
Studies in Belgium (van Steyvoort et al., 1968) and in France (Shell
Research Ltd., Report WKTR.0053.68) indicate that no residues could be
found in purified juices from sugar beets grown in soil treated with
up to 6 kg/ha of aldrin. However, data from refineries in the U.S.A.
(Walker at al., 1965) show combined residues of 0.02 ppm (expressed as
ppm of pure sucrose) in clarified juice from beets with abnormally
high residue levels. Had these juices been processed, most of the
residue would have been found in the molasses rather than in the
sugar.
Data was obtained on dieldrin residues in dried pulp from beets grown
in soil which had received aldrin treatment for two years preceding
planting. A residue of 0.03 ppm in the fresh beet resulted in values
of 0.13-0.14 ppm in pulp dried at 105°C and 0.05-0.12 in pulp dried at
150°C; 0.07 ppm in fresh beet gave values of 0.28 ppm in pulp dried at
105°C and 0.34 ppm in pulp dried at 150°C (Shell Research Ltd.,
unreported data). The ratio of residues in dried pulp to fresh beet
was 3-4 which is lower than the figure of 10.6 reported by Walker et
al. (1965). The significance of pulp residues in terms of cattle feed
is questionable, however, due to the low amount of pulp (10-20
percent) in the total diet. Onsager et al. (1970) calculated that a
residue of 0.25 ppm in 4.5-8 lb of beet pulp fed daily to a 1200-lb
animal amounts to ingestion of 0.2-1.6 ppm of insecticide.
In crops derived from seed dressings
Both aldrin and dieldrin are extensively used as seed dressings on a
wide variety of crops; maize, rice, cereals, cotton and pulse crops
are the most important. A large amount of data collected primarily by
Shell shows that residues in the crop derived from dressed seed are
extremely small and usually below the limits of detection (about 0.005
ppm), except for occasional residues of 0.01-0.02 ppm in pulse crops.
In all cases where residues were much above the detection limit, there
are strong indications that samples were contaminated. Either residues
were high on control as well as treated samples, or else appreciable
proportions of aldrin were found in the total residue.
In vegetables
Residue data obtained up through 1968 is summarized in Table X (Shell
International Chemical Co., Monograph, Residues in Vegetables,
November 1968).
Table VIII
Residues in sugar beets grown in soils treated on more than one occasion
Rate of
Application, Range of Residues Arithmetic
kg/ha/ Part of Aldrin + Dieldrin, No. of Mean,
year Crop ppm Samples ppm
2 Root <0.01-0.04 8 0.01
4 Root <0.01-0.07 5 0.04
2 Tops <0.01-0.02 5 <0.01
4 Tops <0.01-0.02 5 <0.01
Table IX
Average residues of aldrin plus dieldrin in soil and sugar beet roots
1965-1968
Average residue, ppm
Aldrin Soil
dosage, sampled on Roots sampled on
lb ai/acre 4/10/65 10/5/65 10/25/66 10/17/67
0.625 0.05 <0.01 <0.01 <0.01
1.25 0.21 0.04 <0.01 <0.01
2.5 0.38 0.06 0.01 0.01
5.0 0.73 0.13 0.02 0.01
7.5 1.84 0.37 0.03 0.03
10.0 2.84 0.96 0.05 0.04
Table X
Residues from seed or soil treatment with aldrin and dieldrin at
application rates within the recommended range (typically 2-3 kg/ha).
Crop or Residues, ppm Location and
Crop Group Min Max Est. Median No. of Expts.
Carrots 0.02 0.51 0.20 USA:3, Switzerland:5,
Denmark:2, Holland, 3,
UK:3, Austria:2
Cruciferous 0.02 0.08 0.04 USA:20, Norway:1,
roots (1) Austria:2
Beetroot 0.01 0.22 0.10 USA:15
Cole crops (2) 0.01 0.02 0.015 USA:21
Onions ND ND ND UK:3, USA:1
Peas ND ND ND USA:1
Lettuce 0.10 0.16 - USA:2
Beans ND ND ND USA:3
Tomatoes 0.10 0.10 0.10 USA:3
Celery 0.02 ND ND UK:3
Cucurbits (3) 0.01 0.07 0.03 USA:10
(1) Including radish, turnip, rutabaga and swede.
(2) Including cabbage broccoli, brussels sprouts and cauliflower.
(3) Including cucumber, pumpkin and melon.
More recent data on beetroot, cauliflower, carrots, white cabbage,
lettuce, tomatoes, turnips, and sweet potatoes are consistent with the
findings in Table X and in general give slightly lower residue figures
(Bro-Rasmussen and Voldum-Clausen, 1966; Shell Research Ltd., Reports
WKTR. 0050.68, 0053.68; Shell Chemical Co., Reports PRL-68-112,
-68-210).
In cereal grain
Aldrin treatments of seed or soil at recommended rates have rarely
produced residues of aldrin plus dieldrin in grain of wheat, oats or
barley above 0.01 ppm. Foliar applications of aldrin or dieldrin to
the growing crop do not give measurable residues in grain or straw if
pre-harvest intervals of one month or more are observed (Shell
International Chemical Co., Monograph, Residues in Small Grains,
1968).
Recent data for wheat and barley grain from soils receiving a variety
of aldrin application regimes at rates of 2 or 4 kg/ha show no
detectable residues (<0.01 ppm) of either aldrin or dieldrin.
Residues in wheat straw ranged from <0.01-0.16 ppm and in barley
straw from <0.01-0.20 ppm. Straw residues are of limited significance
since wheat straw is not an appreciable component in the diet of dairy
cattle and barley straw is used as a feed primarily for beef cattle in
winter only (Shell Research Ltd., Reports WKGR.0114.70, WKTR-0053.68).
In citrus and sugar-cane
Soil applications of aldrin at 2.0-5.0 kg/ha in citrus groves resulted
in dieldrin residues of 0.01-0.02 ppm in whole oranges, 0.02 ppm in
chopped peel and 0.03-0.04 ppm in dried pulp; controls contained up to
0.01 ppm. Dieldrin residues were not found in whole grapefruit or
grapefruit juice, but peel contained 0.006-0.007 ppm and dried pulp
contained 0.01 ppm; residues were also found in control samples (Shell
Chemical Co., Report PRL-67-30).
No residues were found in any of six samples of sugar-cane grown in
soil treated with two applications of aldrin at 2 kg/ha (Shell
Chemical Co., Report PRL-68-51). Soil treatments with aldrin of 5 or
10 lb ai/acre resulted in apparent aldrin residues of 0.004 ppm in two
out of three foliage samples from the 10 lb/acre treatment and in
apparent dieldrin residues ranging from 0.008-0.015 ppm in 8 out of 16
samples of foliage and stalks. No residues were found in the juice at
any treatment rate, and one sample of bagasse (out of four) had 0.009
ppm dieldrin (Shell Chemical Co., Report PRL-68-236).
In milk and meat (from cattle feed)
Williams et al. (1964) fed 16 milking cows for 35 days with a feed
containing various amounts of dieldrin. Similar experiments were
carried out by Gannon et al. (1959a, 1959b) for 12 weeks and by Shell
(1970) for 21 days using dieldrin C-14. The results of these
investigations are summarized in Table XI.
After the feeding of dieldrin was stopped, dieldrin concentrations in
the milk began to fall with a half-life of 10-15 days in the case of
Williams' work and substantially longer in the case of Gannon's work.
The lack of consistency may be due to some cows coming to the end of
their lactation period, thus reducing the milk production per day.
Gannon et al. (1959a) also studied residues in body tissues of milk
cows and steers fed diets containing various amounts of dieldrin. His
results are shown in Table XII.
In poultry and eggs
The residues of dieldrin that could arise in poultry products from the
use of rice bran in the diet (typically 20 percent of the total diet)
are summarized in Table XIII.
Thus with residues of dieldrin in rice bran of 0.05 ppm, residues of
dieldrin in poultry products could be 0.012 ppm in eggs, 0.20 ppm in
fat and 0.02 ppm in poultry meat. Residues of up to 0.03 ppm dieldrin
are commonly found in rice bran.
FATE OF RESIDUES
General comments
The occurrence and toxicity of the photoisomer of dieldrin,
photodieldrin, has been reviewed in the section on biological studies.
Photodieldrin residues may be of significance only after foliar
application of dieldrin. No detectable residues were found in human
fat in the U.S.A. where the ratio of dieldrin to photodieldrin
residues was greater than 200 to 1 (Richardson, 1966). Photodieldrin
was also absent in human fat from U.K. and Holland (Richardson, 1968)
and milk (Robinson et al., 1966).
In animals
The fate of residues of dieldrin in animals has been covered under the
biochemical section of this monograph.
In plants
Extensive experimental work was carried out by Shell to determine
residues of photoaldrin and photodieldrin in crops grown on
aldrin-treated soil, grown from aldrin or dieldrin-treated seed, grown
in soils containing old residues or receiving foliar applications of
dieldrin, and general monitoring investigations. Their results
indicate that none of the soil or seed dressing uses of aldrin or
dieldrin lead to detectable residues of the photoisomers. Foliar
applications led to photodieldrin residues of 0.03 ppm in coffee beans
treated at 1-1/3 g of dieldrin/ tree, 0.02 ppm in leaves of forage
beets treated at 4.3 oz/acre, 0.31-0.89 ppm in wheat straw treated at
8 oz /acre, <0.01-0.04 ppm in apples and 0.01-0.06 ppm in apple
pomace treated at ´ lb/acre, 3.6 ppm in pasture grass one and a half
months after treatment with 1 oz/acre, and 1.1 ppm in fat of sheep
grazing for four months on the treated pasture grass. None of the
photoisomer was detected in any samples of vegetable oils and fats
monitored in the U.K., but some samples of food and feed components
contained traces.
Table XI
Dieldrin in milk from animals fed with feed containing
different amounts and for various lengths of time
Dieldrin in Butterfat Ratio,
feed, ppm, Milk level, ppm, equivalent, ppm, ppm B.F.
dry weight at time indicated at time indicated ppm Feed Reference
35 days 35 day
0 0.004 0.11 - Williams
0.09 0.021 0.53 5.9 et al.
0.23 0.058 1.52 6.6 (1964)
0.50 0.11 2.89 5.9
4 weeks 12 weeks 4 weeks 12 weeks
0 <0.01 0.01 - - - Gannon
0.1 <0.01 0.02 - 0.5 5.0 et al.
0.25 0.02 0.06 0.5 1.5 6.0 (1959b)
0.75 0.07 0.11 1.75 2.8 3.1
2.25 0.16 0.28 4.0 7.0 3.0
15-21 days 15-21 days
0.102 0.018 0.41 4.0 Shell
0.120 0.017 0.35 2.9 Development
0.111 0.015 0.38 3.5 Co., Report
M-32-70
Table XII
Dieldrin residues in body tissues of cows and steers after feeding at
various levels of dieldrin for twelve weeks
Milk cows Steers
Dieldrin in Feed, ppm Dieldrin in Feed, ppm
Body Part 0.25 2.25 0.1 0.25 0.75 2.25
Dieldrin in Tissues, ppm
Brain <0.1 0.1 - - - -
Heart 0.2 0.6 - - - -
Liver 0.2 0.7 - 0.1 - 0.7
Kidney <0.1 <0.1 - <0.1 - 0.2
Steak 0.1 1.3 - <0.1 - 1.0
Roast 0.2 1.2 - - - -
Renal Fat 0.9 6.2 - 0.9 - 9.7
Body Fat 0.9 4.8 0.4 0.8 3.5 8.7
Heart Fat - 7.0 - - - -
Udder Fat - 5.6 - - - -
The average ratio of dieldrin levels in fat to levels in feed was 2.75
in milk cows and 3.95 in steers.
Table XIII
Relation between dieldrin residues in poultry feed
and poultry products (eggs, meat, fat of meat)
Feed Range of Ratios,
Poultry Weeks Concentration Poultry Feed/
Product fed Range (ppm) Poultry Product Reference
Eggs 12 0.1-0.75 0.8-1.6 Gannon et al., 1959a
Eggs 16 0.006-5.0 1.0-3.0* Greaves et al., 1969a
Eggs 14 0.05-0.45 1.1-1.2 Cummings et al., 1966
Fat(abdominal) 2 0.1-0.15 20-29 Liska et al., 1967
Fat 12 0.1-0.75 41-48 Gannon et al., 1959a
Fat(abdominal) 14 0.05-0.45 8.8-9.7 Cummings et al., 1967
Meat 2 0.1-0.15 2-4 Liska et al., 1967
Meat 12 0.1-0.75 <0.2 Gannon et al., 1959a
Meat 14 0.05-0.45 0.1 Cummings et al., 1967
* The figure of 3 regarded as inconsistent with other data.
Lichtenstein et al. (1970) reported 0.015 ppm photodieldrin in soils
which had been treated annually with 5 lb/acre of aldrin for five
years and then not treated for five years. Potatoes and carrots grown
in these soils contained 0.0006 ppm and 0.002 ppm, respectively.
After foliar application of photodieldrin C-14 to white cabbage,
residues disappeared more slowly than in corresponding tests with
aldrin, dieldrin, isodrin or endrin. Four weeks after application, as
much as 75 percent of the applied radioactivity was present as
residues. Fifteen to 33 percent of metabolites were found mainly in
leaves. Metabolic fractions consisted of a very hydrophilic main
product and at least two less hydrophilic by-products (Korte and
Porter, 1970).
Four weeks after application of ca. 75 ppm of aldrin C-14 on the upper
side of cabbage leaves, only 17 percent of the applied radioactivity
was recovered in plants and soil. Hydrophilic metabolites made up 76
percent of the recovered activity; besides aldrin, five other
compounds were found, one of which appeared to be dieldrin. In similar
experiments with dieldrin C-14 on cabbage, 40 percent of the
radioactivity is recovered after four weeks and 34 percent of the
recovered activity is due to at least two hydrophilic metabolites
(Korte, 1970).
In soils
Maize grown in California in soil treated with 3 kg/ha of aldrin C-14
contained no detectable C-14 in the grain or cobs. The husks of the
ears contained 0.004 ppm aldrin, 0.004 ppm dieldrin and 0.032 ppm
metabolites. The leaves contained 0.35 ppm C-14 equivalents of which
0.02 ppm was aldrin and 0.05 ppm was dieldrin (Korte and Porter,
1970).
Evidence of residues in food in commerce
Belgium
Thirty local and sixteen imported samples of butter were examined
(Dejaukheere and Kips, 1968). In the local samples, residues of
dieldrin ranged from 0.01 to 0.24 ppm (mean 0.12 ppm), and in the
imported samples the range was 0.01 to 0.25 ppm.
Denmark
Samples of milk butterfat (44) contained mean levels of dieldrin of
0.03 ppm (<0.007-0.11) (Bro-Rasmussen et al., 1968). Samples of
butter examined over a three year period contained dieldrin residues
as follows:
1964 1965 1966
No. of samples 118 137 126
Range (ppm) 0.01-0.20 0.01-0.18 0.01-0.17
Mean (ppm) 0.05 0.04 0.03
The authors suggested from their data that 60-100 percent of these
residues originated from dieldrin residues in imported cattle feed.
France
Mestres, et al., (1967) in France found no residue of aldrin or
dieldrin in 44 samples of French butter or in four samples of Swiss
butter at a detection limit of 0.02 ppm.
West Germany
Of 65 samples of carrots taken in West Germany (Maier-Bode, 1967) 43
contained less than 0.01 ppm aldrin plus dieldrin. Of the remainder,
64 percent contained residues of 0.03 ppm or less, and only one sample
exceeded 0.1 ppm (0.17 ppm).
Norway
Twenty butter samples out of a total of 54 contained dieldrin residues
in the range 0.01-0.1 ppm (Sakshaug, 1968). The rest did not contain
residues, above the limit of detection.
U.K.
Routine analysis of food-stuffs by public analysts in the U.K. (Rymer
and Hammence, 1967) demonstrated that of 776 fruit, 461 vegetable and
237 meat and dairy produce samples, only three fruit samples and three
meat and dairy produce samples contained aldrin above the limits of
detection (maximum levels of 0.06 and 0.18 ppm respectively) and two
fruit, one vegetable and thirteen produce samples contained dieldrin
above the limits of detection (maximum 0.15 and 0.27 ppm).
A more recent examination of 388 fruit, 285 vegetable and 217 meat and
dairy produce samples (Dickes and Nicholas, 1969) revealed one
vegetable sample containing aldrin (0.09 ppm) and sixteen vegetable
samples containing dieldrin, although all were below 0.01 ppm.
Findlay and Hamilton (1968) examined 163 samples of eggs and 114
samples of poultry meat, liver and fat from nine sources within the
U.K. No egg samples exceeded 0.06 ppm of dieldrin and only one poultry
meat sample exceeded 0.1 ppm (0.13 ppm).
Table XIV
Dieldrin residues in dairy and meat products produced in U.K., 1965-1968
Butter Milk Mutton Fat Beef Fat
Year Mean Mean Mean Mean
Samples (ppm) Samples (ppm) Samples (ppm) Samples (ppm)
1964 127 0.84
1965 18 0.03 85 0.0025 107 1.15 59 0.06
1966 13 0.02 75 0.0025 101 0.44 63 0.04
1967 22 0.03 77 0.002 76 0.24 36 0.03
1968 16 0.03 76 0.001 77 0.21 34 0.03
A most comprehensive examination of home produced and imported meat
and dairy products has been carried out over a number of years by the
Laboratory of the Government Chemist (1966, 1967, 1968, 1969). In the
four years 1965-1968, 935 samples of home produced foods and 803
samples of foods imported from ten countries were analyzed. Table XIV
summarizes their findings for home-produced dairy and meat products.
Residues in food at time of consumption
A study of data accumulated over four years by the U.S. Food and Drug
Administration on pesticide residues in total diet samples was made by
Duggan and Lipscomb (1969).
Residues of dieldrin were found in from 15.6-21.3 percent of
composites analyzed over this period and aldrin residues were found in
from 3.3-5.6 percent. Measurable dieldrin residues were present in six
of the twelve food groups examined, and traces were found in another
four. Measurable aldrin residues were found in only one group. The
highest residues of dieldrin (max. 0.20 ppm) were found in samples of
meat, fish and poultry (Group 2). In the dairy products group (Group
1) the greatest level was 0.08 ppm and residues did not exceed 0.03
ppm in any other group. The maximum aldrin level was 0.07 ppm in the
first group (Group 9). No tendency to increase or decrease was
observed over this period. From the residue information and a
knowledge of the diet, the daily intake of each pesticide was
calculated. Since the diet was representative of that consumed by a
sixteen-nineteen year old American male, the intake of the average
population will be about half the calculated figure. The total daily
intake for aldrin and dieldrin over the four year period is given in
the following table (from Duggan and Lipscomb, 1969):
Daily Intake (mg/kg body-weight)
1965 1966 1967 1968
Aldrin 0.00001 0.00004 0.00001 0.00001
Dieldrin 0.00008 0.00009 0.00005 0.00005
Total 0.00009 0.00013 0.00006 0.00006
An examination of residues in groups of foodstuffs making up a
complete diet was undertaken by Abbott et al., (1969), at the
instigation of the U.K. Ministry of Agriculture, Fisheries and Food,
for one year from October 1966-October 1967. Of the foods analyzed,
the highest dieldrin residues were in fat (mean 0.024 ppm, range
0.009-0.075 ppm), followed by meat (mean 0.009 ppm, range 0.001-0.060
ppm). Because of the larger consumption of meat, this group provides
the largest intake of dieldrin 2.2 mg/person/day. The mean daily
intake in the diet calculated from the residues found was 0.00009
mg/kg body-weight. This figure confirms the decrease shown in the
second survey by McGill and Robinson (1968) compared with the first,
and may be attributed to the voluntary withdrawal of some previously
approved dieldrin uses in the U.K. over the period 1965-1967.
In 1965 the Association of Public Analysts prepared a survey to
determine the extent of contamination of foodstuffs by pesticides.
Data for the first year of this work has so far been reported
(Association of Public Analysts, 1969). Of the 2352 samples analyzed,
only ten contained residues of aldrin (two only above 0.1 ppm) and 76
contained dieldrin (again only two above 0.1 ppm). Dieldrin was most
commonly found in meat and dairy products. In no foodstuffs did the
mean level of dieldrin residues exceed 0.015 ppm, a figure that is
based on the assumption that all samples in which residues were not
detected (limit 0.002 ppm in solid foods and 0.0002 ppm in liquid milk
and infant foods) did in fact contain residues at half the detection
limit. Using the same assumption, the mean daily intake in the diet
was calculated to be 0.0001 mg/kg body-weight. A survey of residues in
welfare foods (dried full cream milk, cod-liver oil and concentrated
orange juice) by Ruzicka et al., (1967) revealed residues of up to
0.017 ppm dieldrin (mean 0.011 ppm) in dried milk and demonstrated a
15 percent loss in residue during the drying period. Cod-liver oil
contained 0.15-0.20 ppm (mean 0.16 ppm) and concentrated orange juice
contained up to 0.007 ppm (mean 0.002 ppm).
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967a) apply to the
determination of aldrin and dieldrin. Burke (1969) has studied the
application of the official AOAC multiresidue method to photodieldrin.
No methods of analysis have been well worked out for the more
hydrophilic metabolites of dieldrin such as monohydroxydieldrin,
dihydro-aldrin-diol (trans), dieldrin-ketone, dieldrin-ketone
photo-isomer, or Klein's metabolite. Although photoaldrin can be
determined by GLC techniques, the rapid conversion of aldrin to
dieldrin makes it unlikely that photoaldrin will be a significant
residue in crops or soils.
CONSIDERATION OF SPECIFIC POINTS RAISED BY THE CODEX COMMITTEE ON
PESTICIDE RESIDUES
The meeting considered three questions arising from the Draft Report
of the Fifth Session of the Codex Committee in 1970 as follows:
Further data on the use pattern of aldrin and dieldrin on rice are
provided in this monograph, as are data on residues resulting from the
use in animal feed.
The question of the fate of the residues on rice during processing was
not considered because the available data show that residues are so
small that any remaining following processing would be negligible.
No firm data were received on residues in fruit juices resulting from
the use of rice husks as clarifying agents. However, if it is assumed
that 10 percent rice husks are used for clarification, and if they
should contain up to 0.02 ppm of aldrin plus dieldrin, and if it is
further assumed that this is extracted by the fruit juices (unlikely
due to the lipophylic nature of residues), then it would be highly
improbable that residues in excess of 0.002 ppm in the juice would
occur.
APPRAISAL
The data provided on residues of aldrin plus dieldrin in maize grain
resulting from planting maize in soils treated at recommended rates
indicate that levels of 0.02 ppm or less could be expected and that
maize grain is within the recommended practical residue limit of 0.02
ppm on raw cereals listed in the 1969 Report of the Joint Meeting.
The data on residues of aldrin plus dieldrin in whole washed potato
tubers arising from soil applications of aldrin at recommended rates
reveals the possibility of exceeding the recommended tolerance of 0.1
ppm, especially when the potatoes are grown in plots receiving a
number of annual applications at the highest rate. An increased
tolerance of 0.2 ppm is recommended for potatoes.
Residues of aldrin plus dieldrin in mature sugar beets are
proportional to the residues in soil at planting. For example, an
aldrin dosage of 2.5 lb/acre gives after six months a soil residue of
0.11 ppm and a sugar beet root residue, grown in the same year, of
0.06 ppm. This would be expected to result in about 0.24 ppm of
dieldrin residue in the pulp dried at 105°C. This amount of residue
under normal feeding practices would not lead to excessive residues in
whole milk or meat (fat basis). Therefore, the recommended practical
residue limits appear to be adequate for residues from this source.
Residues in the crop derived from dressed seed (maize, rice, cereals,
cotton and pulse crops) are extremely small and usually below the
limits of detection, thus justifying the reduction of the tolerance in
rough rice to 0.02 ppm.
Residues in vegetables from seed or soil treatment are dependent on
the type of crop. Residues at or in excess of tolerance could arise in
carrots and lettuce planted in soil previously treated for other
crops. Data were received which justify a revision of the recommended
tolerance and the establishment of a practical residue limit of 0.2
ppm for lettuce and carrots.
Soil applications of aldrin of 2-5 kg/ha in citrus groves resulted in
dieldrin residues of 0.01-0.02 ppm in whole oranges and no detectable
residues in grapefruit. The presently recommended tolerance of 0.05
ppm appears to be adequate.
The importance of photodieldrin as a plant metabolite of aldrin or
dieldrin appears to be well established; however, its significance as
a terminal residue will depend on the results of experiments in
progress on rates of storage and excretion in animals.
A consideration of the new data supplied on residues in milk, milk
products and meat resulting from supervised trials and surveys of food
in commerce does not indicate a need for revision of the previously
recommended practical residue limits for these items at this time. The
previous practical residue limit of 0.125 ppm for milk products was
changed to 0.15 ppm to allow for the limitations and variability of
residue analytical procedures.
A suitable regulatory method of analysis exists for photodieldrin
(official AOAC multiresidue method) but no methods of analysis have
been worked out for the more hydrophilic metabolites of dieldrin.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The previously recommended tolerances and practical residue limits (no
longer temporary) are amended as follows:
ppm
Fruit (other than citrus), asparagus,
broccoli, brussels sprouts, cabbage,
cauliflower, cucumber, eggplant,
horse-radish, onions, parsnips, peppers,
pimentoes, radishes and radish tops 0.1
Citrus 0.05
Rice (rough) 0.02
Potatoes 0.2
PRACTICAL RESIDUE LIMITS
ppm
Carrots, lettuce, fat of meat 0.2
Raw cereals (other than rice) 0.02
Milk and milk products (fat basis) 0.15
Eggs (shell-free) 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further information relevant to the assessment of the significance
of the changes that occur in the livers of mice exposed to dieldrin. A
comparison of the response to dieldrin of high and low spontaneous
liver-tumour mouse strains in respect of liver tumour incidence and
the metabolism of dieldrin might be helpful in this respect.
2. The continued collection of follow-up information on persons
exposed occupationally in the manufacture of dieldrin.
3. Completion of the studies being conducted on the comparative
metabolism of dieldrin in man, monkey, rat and mouse.
4. Experimental studies to determine whether dieldrin might have
teratogenic potential.
5. A continuing study of the possible occurrence of residues of
photodieldrin in food surveys and total diet studies.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J.Sci.Fd.
Agric., 20: 245-249
Association of Public Analysts, London. (1969) Joint survey of
pesticide residues in foodstuffs sold in England and Wales, August
1966-July 1967
Baldwin, M.K. and Robinson, J. (1969a) The identification of a
metabolite of the photo-isomerization product of HEOD (dieldrin) found
in rat tissues. Unpublished report from the Tunstall Laboratory, Shell
Research Ltd., Sittingbourne
Baldwin, M.K. and Robinson, J. (1969b) Metabolism in the rat of the
photo-isomerization product of dieldrin. Nature, 224: 283-284
Baldwin, M.K. and Robinson, J. (1970) Comparison of the metabolism of
HEOD in the CFI mouse with that in the CFE rat - preliminary results.
Unpublished report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Baldwin, M.K., Robinson, J. and Carrington, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Industr., 595-597
Bick, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Austr., 54 (1): 1127-1130
Braund, D.G., Brown, L.D., Huber, J.J., Leeling, N.C. and Zabik, M.J.
(1969) Excretion and storage of dieldrin in dairy cows fed
thryoprotein and different levels of energy. J. Dairy Sci., 52:
172-182
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27: 81-138
Bro-Rasmussen, F., and Voldum-Clausen, K. (1966) Tids. Planteovl.,
20: 232
Bro-Rasmussen, F., Dalgaard-Mikkelsen, S., Jakobsen T., Koch, S.O.,
Rodin, F., Uhl, E. and Voldum-Clausen, K. (1968) Examinations of
Danish milk and butter for contaminating organochlorine insecticides.
Res. Rev., 23: 55-69
Brown, V.K., Robinson, J. and Richardson, A. (1967) Preliminary
studies on the acute and sub-acute toxicities of a photoisomerization
products of HEOD (dieldrin). Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Chan, T. and Terriere, L.C. (1969) Aldrin epoxidase activity of rat
liver and rat liver microsomes under various conditions of storage.
Biochem. Pharmacol., 18: 1061-1070
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Crabtree, A.N. (1969) The concentrations of organochlorine
insecticides in human fat and blood from Australia. Shell Research
Limited, Tunstall Report No. TL GR. 0059.69
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Ass. Off. Anal. Chem., 49: 354-364
Cummings, J.G., Gidelman M., Turner V., Reed D., Zee K.T. and Cook
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydrocarbon insecticides to hens. J. Ass. Off. Anal.
Chem., 50: 418-425
Dailey, R.E., Walton, M.S., Beck, V., Leavens, C.L. and Klein, A.K.
(1970) Excretion, distribution and tissue storage of a 14C-labelled
photoconversion product of 14C-dieldrin. J. Agr. Fd. Chem., 18:
443-445
Damico, J.N., Chen, J.T., Costello, C.E. and Haenni, E.O. (1968)
Structure of Klein's metabolites of aldrin and dieldrin. J. Ass. Off.
agr. Chem., 51: 48-55
Datta, P.R., Lang, E.P., Watts, J.O., Klein, A.K. and Nelson, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature, 208: 289-290
Dejaukheere, W. and Kips, R.N. (1968) Meded. Rijksfac.
Landbouwwenteusch (Ghent), 33: 1283
Dickes, G.J. and Nicholas, P.V.J. (1969) Assoc. Pub. Analysts, 7: 14
Diechmann, W.B., Dressler, I., Keplinger, M. and MacDonald, W.E.
(1968) Retention of dieldrin in blood, liver and fat of rats fed
dieldrin for six months. Industr. Med. Surg., 37: 837-839
Diechmann, W.B., MacDonald, W.E., Radomski, J., Blum, E.B.,
Bevilacqua, M., and Keplinger, M. (1970) The tumorigenicity of aldrin,
dieldrin and endrin in the albino rat. Industr. Med., 39: 314
Donninger, C., Potter, D., Potter, S.A. and Wright, A.S. (1967) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to rats. Unpublished report from Tunstall Laboratory,
Shell Research Ltd., Sittingbourne
Donninger, C. (1969) Liver cell changes following the oral
administration of dieldrin to mice. Unpublished report from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest.
Monit. J., 2(4): 153-162
Elgar, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Fd. Agr., 17: 541-545
FAO/WHO (1967) Evaluations of some pesticide residues in food,
FAO,PL:CP/15; WHO/Food Add./67.32 p. 3-4.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Feil, V.J., Hedda, R.D., Zayliskie, R.G. and Zachrison, C.N. (1970)
Dieldrin-14C metabolism in sheep. Identification of
trans-6-7-dihydroxyaldrin and 9-(synepoxy)
hydroxy-1,2,3,4,10,10-hexachloro-6,7-
epoxy-1,4,4a,5,6,7,8,8a-,octahydro-1,4-endo-5,8-
exodimethanonophthalene. J. Agr. Fd. Chem., 18: 120-24
Findlay, E. and Hamilton, G.A. (1968) Pesticide residues in foodstuffs
in Great Britain. VIII Organochlorine pesticide residues in eggs and
poultry. J. Sci. Fd. Agric., 19: 609-611
Gannon, N., Link, R.P. and Decker, G.C. (1959a) Pesticide residues in
meat. Storage of dieldrin in tissues of steers, hogs, lambs and
poultry fed dieldrin in their diets. J. Agr. Fd. Chem., 7: 826-828
Gannon, N., Link, R.P. and Decker, G.C. (1959b) Pesticide residues in
meat and milk. Storage of dieldrin in tissues and its excretion in
milk of dairy cows fed dieldrin in their diets. J. Agr. Fd. Chem.,
7: 824-826
Gillett, J.W. and Chan, I.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Food Chem., 16: 590-593
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Graves, J.B., Bonner, F.L., McKnight W.F., Watts, E.B. and Epps, E.A.
(1969) Residues in eggs, preening glands, liver and muscle from
feeding dieldrin-contaminated rice bran to hens and its effect on egg
production, egg hatch and chick survival. Bull. Env. Contam. Tox.,
4: 375-383
Harr, J.R., Claeys, R.R., Bone, J.F. and McCorcle, J.W. (1970)
Dieldrin toxicosis: rat reproduction. Amer. J. vet. Res., 31:
181-189
Harris, L.E. and Greenwood, D.A. (1963) Effect of residues of newer
insecticides on health. Unpublished progress report to Shell Chemical
Company, letter signed by Joseph Street, dated 8 March 1963
Hedde, R.D., Davison, K.C. and Robbins, J.D. (1970) Dieldrin 14-C
metabolism in sheep. Distribution and isolation of urinary
metabolites. J. Agr. Fd. Chem., 18: 116-119
Hunter, C.G., Robinson, J. and Roberts, M. Pharmacodynamics of
dieldrin (HEOD). Ingestion by human subjects for 18 to 24 months and
postexposure for eight months. Arch. environm. Hlth., 18: 12-21
Jager, K.W. (1970) "Aldrin, dieldrin, endrin and telodrin". An
epidemiological and toxicological study of long-term occupational
exposure. Thesis, 234 p., Elsevier, Amsterdam
Jansen, J.D. (1969) Letter to A.M. Coetzee dated 25 July 1969
Keane, W.T. and Zavon, M.R. (1969) Validity of a critical blood level
for prevention of dieldrin intoxication. Arch. environm. Hlth., 19:
36-44
Kinoshita, P.K. and Kempf, C.K. (1970) Quantitative measurements of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Tox. Appl. Pharmacol., 17: 288
Klein, A.K., Link J.D. and Ives, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed aldrin
and dieldrin. J. Ass. Off. agr. Chem., 51: 895-898
Korte, F. and Arent, H. (1965) Metabolism of insecticides. IX (1)
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin - 14C. Life Sci., 4:
2017-2026
Korte, F. (1970) Terminal residues of cyclodiene insecticides. Comptes
Rendus, XXV Conference, IUPAC, Cortina d'Ampezzo, Italy, July 1969.
Appendix II: p. 172-180
Korte, F. and Porter, P.E (1970) Minutes of the Fifth Meeting of the
IUPAC Terminal Pesticide Residues, September 1970, Erbach, West
Germany. Appendixes 4 and 4a
Lee, D.F. (1968) Pesticide residues in foodstuffs in Great Britain.
IX. Aldrin, dieldrin and other organochlorine pesticides in potatoes
and carrots. J. Sci. Fd. Agric., 19: 701-705
Lehner, P.N. and Egbert, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature, 224: 1218-1219
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, et al. (1967) Food Technol. 21: 435-438
Lofroth, G. (1970) Exposure of the general population to dieldrin.
Letter to editor and reply Robinson J. and Roberts M. Fd. Cosmet.
Toxicol., 8: 341-342
Lutz-Ostertag, Y. and Lutz, H. (1969) Embryologie expérimentale. Note
préliminaire sur les effets "oestrogènes" de l'"Aldrine" sur le
tractus urogénital de l'embryon d'Oiseau. C.R. Acad. Sci. (Paris) Ser.
D., 269: 484-486
Maier-Bode, H. (1967) The aldrin and dieldrin contents of German
edible carrots. Bull. environ. Contam. Toxicol., 2(1): 10-11
Matthews, H.B. and Matsumura, F. (1969) Metabolic fate of dieldrin in
the rat. J. Agr. Fd. Chem., 17: 845-852
McGill, A.E.J. and Robinson, J. (1968) Organochlorine insecticide
residues in complete prepared meals: a twelve-month survey in
southeast England. Fd. Cosmet. Toxicol., 6: 45-57
Mestres, R., Barthes, F. and Priu, M. (1967) DDT, aldrin and dieldrin
residue determinations in milk and butter. Bull. Environ. Contam.
Toxicol., 2(1): 25-33
Moubry, R.J. and Myrdal, G.E. (1970) private communication, Wisconsin
Department of Agriculture
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugarbeets. J. Econ. Entomol.,
63: 1143-1146
Parsons, A.M. and Moore, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem. Soc.
(C), 2026-2031
Polishuk, Z.W., Wasserman, M., Wasserman, D., Groner, Y., Luzarovici,
S. and Tomatis, L. (1970) Arch. environm. Hlth., 20: 215-217
Porter, R.D. and Wiemeyer, S.N. (1969) Dieldrin and DDT: Effects on
sparrow hawk eggshells and reproduction. Science, 165: 199-200
Potter, D., Wooder, M.F., Wright, A.S., Greenland, R.D. and
Zavon, M.R. (1970) The effects of ingestion of foreign compounds on
the sub-cellular structure and function of mammalian liver cells.
Paper given by A.S. Wright at Sittingbourne, 14 May 1970
Renvall, S. and Akerblom, M. (1968) Rester au organiska
klorpesticider, sörskilt aldrin och dieldrin, 2 nagra svenska
kalvöxter ochpotatis. Var. Föda, 20: 65-72
Reports of the Government Chemist, H.M.S.O., London (1965-1969)
Richardson, A. (1966) Ultra-violet conversion product of HEOD: Its
concentration in various samples received from the USA. Shell Research
Tunstall Report No. IRR TL/35/66
Richardson, A. (1968) Residues of dieldrin and its ultraviolet
decomposition product in selected samples. Shell Research Tunstall
Report No. TLGR 0014.63
Richardson, A., Baldwin, M. and Robinson, J. (1968) Identification of
metabolites of dieldrin (HEOD) in the faeces and urine of rats. J.
Sci. Fd. Agr., 19: 524-529
Robinson, et al. (1965-66) Shell Research Ltd., Tunstall, Report
PRRTL/12/65 and TU/23/66
Robinson, J., Richardson, A. and Bush, B. (1966b) A photoisomerization
product of dieldrin. Bull. environm. Contam. Toxicol., 1: 127-133
Robinson, J., Roberts, M., Baldwin, M. and Walker, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Fd. Cosmet. Toxicol.,
7: 317-332
Robinson, J. (1970a) Metabolites of dieldrin in the excreta of Rhesus
monkeys. Unpublished information submitted in a letter to H.G.S. van
Raalte
Robinson, J. (1970b) Metabolites of dieldrin in the excreta of man.
Unpublished information submitted in a letter to H.G.S. van Raalte
Roe, F.J.C. and Grant, G.A. (1970) Inhibition by germ-free status of
development of liver and lung tumours in mice exposed neonatally to
7,12-dimethyl-benz(a) anthracene: implications in relation to tests
for carcinogenicity. Int. J. Cancer, 6: 133-144
Rosen, J.D., Sutherland, D.J. and Lipton, G.R. (1966) The
photochemical isomerization of dieldrin and endrin and effects on
toxicity. Bull. environm. Contam. Toxicol., 1: 133-140
Ruzica, J.H.A., Simmond, J.H. and Tatton, J. O'G. (1967) Pesticide
residues in foodstuffs in Great Britain. IV. Organochlorine pesticide
residues in welfare foods. J. Sci. Fd. Agric., 18: 579-582
Rymer, T.E. and Hammence, J.H. (1967) J. Ass. Pub. Analysts 5: 24
Sakshaug, J. (1968) Residues of organochlorine insecticide in
Norwegian butter samples 1966 (in Norwegian). J. Nord. Vet.-Med.,
20: 696-701
Shell International Chemical Co. (1968) Residues in potatoes resulting
from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in vegetables
resulting from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in small grains
resulting from applications of aldrin, Monograph, November
Smith, Klosterman and Dogger. (1966) Ann. Proc. N. Dakota Acad. Sci.,
20: 96
Tannenbaum, A. and Silverstone, H. (1949a) The genesis and growth of
tumours. IV. Effects of varying the proportion of protein (casein) in
the diet. Cancer Res., 9: 162-173
Tannenbaum, A. and Silverstone, H. (1949b) The influences of the
degree of calorie restriction in the formation of skin tumours and
hepatomas in mice. Cancer Res., 9: 724-727
van Steyvoort, L. et al. (1968) Meded. Rijksfac. Landbouwwettensch XX,
the International Symposium, Ghent, May
Walker, K. et al. (1965) The fate of aldrin, dieldrin and endrin
residues daring the processing of sugar beets. United States
Department of Agriculture, ARS. Publication, ARS-33-107, p. 8
Walker, A.I.T., Stevenson, D.E., Robinson, J., Thorpe, E. and Roberts,
M. (1969a) The toxicology and pharmacodynamics of dieldrin (HEOD):
two-year oral exposures in rats and dogs. Toxicol. appl. Pharmacol.,
15: 345-373
Walker, A.I.T., Neill, C.H., Stevenson, D.E. and Robinson, J. (1969b)
The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix coturnix
japonica). Toxicol. appl. Pharmacol., 15: 69-73
Walker, A.I.T., Thorpe, E. and Stevenson D.E. (1970a) The toxicology
of dieldrin (HEOD): long-term oral toxicity experiments in mice.
Unpublished report from the Tunstall Laboratory, TU/13/70, Shell
Research Ltd., Sittingbourne. Proposed for publication in Fd. Cosmet.
Toxicol.
Walker, A.I.T., Thorpe, E. and Baldwin, M.K. (1970b) Toxicity studies
on the ultra-violet conversion product of dieldrin (UVCPD): thirteen
weeks oral experiment with rats. Shell Research Tunstall Report No.
TLGR. 0053.69
Walton, M.S., Beck, V. and Baron, R.L. (1970) Subacute toxicity of
photodieldrin 3,4,5,6,6,7-hexachloro-12-oxahexacyclo-tridecane, a
photodecomposition product of dieldrin. Tox. appl. Pharmacol., 17:
278.
Wasserman, M., Curnow, D.H., Forte, P.N. and Gurner Y. (1969) Storage
of organochlorine pesticides in the body fat of people in Western
Australia. Industr. Med. Surg., 37: 295-300
Wasserman, M., Coetzee, A.M., Wasserman, D. and Lazarovici, S. (1969)
Present state of the storage of the organochlorine insecticides in the
general population of South Africa. Paper given at the IUPAC
conference, Johannesburg
Williams, S., Mills, P.A. and McDowell, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J. Ass. Off. Anal. Chem., 47: 1124-1128
Winnett, G. and Reed, J.P. (1968) Aldrin, dieldrin, endrin and
chlordane persistence - a three-year study. Pest. Mon. J., 2(3):
133-136
Winteringham, F.P.W. and Barnes, J.M. (1955) Comparative response of
insects and mammals to certain halogenated hydrocarbons used as
insecticides. Physiol. Rev., 35: 701-739
Wright, A.S., Potter, D., Wooder, M.F., Donninger, C., Le Pelley,
J.R., Potter, S.A., Ferrigan, L.W., Sykes, R., Dean, B., Pickering,
R.G., Walker, A.I.T., Crabtree, A.N. and Richardson, A. (1968) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to female dogs. Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Wright, A.S., Greenland R.D., Zavon, M.R. and Donninger, C. (1969)
Liver cell changes following the oral administration of dieldrin to
Rhesus monkeys for 5.5-6 years. Unpublished report from the Tunstall
Laboratories, Shell Research Ltd., Sittingbourne
Zavon, M.R. (1970) The effect of long-continued ingestion of dieldrin
on Rhesus monkeys. A six-year study. Unpublished report from the
Kettering Laboratory, University of Cincinnati, submitted by Shell
International Research.
TABLE II
Toxicity of the photoisomerization product of dieldrin
compared with dieldrin
Species Approximate LD50, mg/kg body-weight
Photoisomerization
product of dieldrin Dieldrin
Pigeon 90 250
Chicken 80 48
Mouse 7 77
Rat 10 47
Guinea-pig 3 24
Dog (M) 140 120
Dog (F) 100 90
Dieldrin or its photoisomerization product was fed to five male and
five female mice at dietary levels of 1, 3 and 10 ppm for the
photoisomerization product and 3, 10 and 3O ppm for dieldrin for one
month. The mice exposed to the photoisomerization product were
unaffected by 1 ppm, there was one male and one female death at 3 ppm
and all died at 10 ppm. The mice fed dieldrin were unaffected by 3 or
10 ppm, but three males and two females died at 30 ppm. Autopsies of
the mice revealed no abnormalities (Brown et al., 1967).
A similar experiment with rats produced no deaths from 10 ppm of the
photoisomerization product; a higher level was not tested. Autopsies
revealed no abnormalities. Levels were measured in the tissues; the
biological half-life of the photoisomerization product was 1.7 for the
males and 2.6 for the females, compared to 10.0 and 12.7 respectively
for dieldrin (Brown et al., 1967).
Subacute levels (5 mg per day) of 14C-labelled dieldrin or
photodieldrin were administered to young adult rats of both sexes for
a period of twelve weeks. Photodieldrin was given both orally and
intraperitoneally; dieldrin was given by stomach tube only. About 60
percent of the activity of 14C-photodieldrin was excreted in the
urine and faeces of male rats and 47 percent in those of female rats,
compared to 60 and 37 percent respectively with 14C-dieldrin. Females
retained three-ten times more 14C activity in their tissues than did
male rats after photodieldrin administration. A similar sex difference
in tissue levels was found after administration of dieldrin and
photodieldrin in female rats and of dieldrin in male rats. Extremely
high levels of 14C activity were detected in the kidneys of male, but
not female, rats receiving 14C-photodieldrin (Dailey et al., 1970).
Storage of the photodieldrin was mainly in the fat and up to 10 ppm it
was 2-15 times higher in females than in males. A metabolite of
dieldrin was detected in the kidneys and urine. The concentration of
this metabolite in the kidneys was about ten times that of the
photodieldrin. No toxicologic effects were detected at 1 ppm (Walker
et al., 1970b).
Similar results were reported by Walton et al. (1970) who also found
slight stimulation of liver microsomal enzymes.
Photodieldrin was fed to groups of 12 male and 12 female rats at
levels of 0.1, 1.0, 10 and 30 ppm in the diet for 13 weeks. Tremors
were observed in rats in the 10 and 30 ppm groups. Reduced food intake
and depressed growth also occurred at these levels. Two of the 10 ppm
and six of the 30 ppm females died. Liver/body-weight ratios were
increased in the 30 ppm groups and kidney weight was increased in the
10 and 30 ppm males. Histologic liver changes were observed in the 10
and 30 ppm groups (Walker at al., 1970b).
Short-term studies
Studies on the effect of dieldrin on the liver microsomal enzymes have
been reviewed under "Biochemical aspects". The following additional
studies have become available.
Dog
Three groups each of six dogs of mixed sex were fed 0, 0.2 or 2 mg/kg
body-weight of dieldrin by capsule for five days a week. The group
given the low dose was started at 1.0 mg/kg for five days, then
reduced to 0.2 mg/kg until day 62 and from then on given 2 mg/kg until
signs of intoxication were evident. A direct relationship was found
between the severity of symptoms and the blood concentration of
dieldrin. As the blood level rose from 0.37 to about 0.8 ppm, changes
progressed from a reduced food intake and growth reduction to the
onset of muscular spasm and finally convulsions. A direct relationship
was also found between blood and fat levels of dieldrin (Keane and
Zavon, 1969).
In an extension of the above-mentioned study, three groups each of six
dogs of mixed sex were given a similar dose regimen of dieldrin. It
was found that the period between dieldrin exposure and the onset of
toxic signs was dependent upon the obesity of the animal; the greater
the amount of body fat the longer it took for toxicity to develop.
Since food refusal preceded symptoms of intoxication, forced feeding
was able to suppress toxicity by preventing fat mobilization and the
associated release of toxic concentrations of dieldrin into the blood
stream, so avoiding the death of some animals (Keane et al., 1969).
Groups of five male and five female dogs were given by capsule daily
oral doses of 0, 0.005 or 0.05 mg/kg body-weight of dieldrin for
periods up to two years. Health, behaviour and body-weight were
unaffected by giving dieldrin, and the electroencephalograph readings
showed no abnormalities. Urine and blood findings were normal except
for an increase in plasma alkaline phosphatase after 30 weeks and a
reduction in serum protein at the 0.05 mg/kg dose. Bromosulphthalein
clearance was normal throughout the exposure period. Liver-weight and
liver to body-weight ratios were increased in the females fed 0.05
mg/kg, and decreased heart-weights, but not heart to body-weight
ratios, were found in all males in the test groups and decreased
spleen-weights at 0.05 mg/kg in the males. No abnormal gross or
histopathological findings associated with administration of dieldrin
were evident (Walker et al., 1969a).
Long-term studies
Rat
Dieldrin was fed to groups comprising 25 male and 25 female rats (45
each for the controls) at dietary levels of 0, 0.1, 1.0 or 10 ppm for
periods up to two years. In addition, a separate group comprising 15
animals of each sex were sacrificed after six, 12 or 18 months
feeding. Body-weight and food intake were unaffected by dieldrin, but
at 10 ppm all the animals became irritable after eight or 13 weeks in
the males and females respectively. Occasional convulsions occurred in
this group during handling. No adverse effect on mortality was
observed. There were no changes in haematology or serum enzyme levels
attributable to dieldrin. Liver weights were normal for the first 18
months of feeding, but after two years, increased liver weights and
liver to body-weight ratios were evident in the groups fed 1 and 10
ppm (Walker et al., 1969a).
OBSERVATIONS IN MAN
In a continuation of the study described in the previous monograph
addendum on dieldrin (FAO/WHO 1968), the 12 male subjects were
continued on the dose regimen of 0, 0.01, 0.05 or 0.21 mg of dieldrin
for a total of two years and were further followed during an
eight-month post-exposure period. All twelve men remained in excellent
health, and there were no changes in body-weights, fatness or
leanness, or other clinical abnormalities throughout the entire
exposure and post-exposure periods. No departures from normal or
pre-exposure values in the biochemical and haemotological parameters
investigated were observed, although there was a slight trend towards
a fall in plasma alkaline phosphatase activity in some men fed the
higher dose levels. This drop was of doubtful significance, and the
enzyme level returned to normal after two months post-exposure. The
concentration of dieldrin in the blood did not significantly change in
the groups given 0.05 mg/day, but at 0.21 mg/day there was a slight
increase during months 18 to 21, but little over months 21 to 24.
This increase was small compared to the first 18 months when the blood
level tripled. Increase in dieldrin in adipose tissue was evident at
all levels after administration for 92 days (Hunter et al., 1969).
Extensive studies of workers exposed to dieldrin in a manufacturing
plant have been reported. The average blood concentration of a group
of 35 workers with an exposure of over ten years was 0.035 mg/ml,
calculated to be equivalent to a dietary intake of 407 mg/man/day.
Intoxications had occurred in some of these individuals prior to 1964.
No definite deleterious effects were found in this high exposure
group, including some individuals who had suffered intoxications.
Clinical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein and serum protein spectrum,
determined every three months remained within normal limits. In an
extreme exposure group with blood levels above 0.2 ppm, alkaline
phosphatase values, although within normal limits, were higher than in
the control group.
Using as criteria of possible microsomal enzyme stimulation the blood
levels of pp'-DDE and the urinary ratio of 6-B-hydroxycortisol and
17-hydroxycortico steroids, no differences between exposed and
unexposed workers were found. The dieldrin workers were exposed to an
average daily intake of 593 mg/man. One group of ten workers with the
highest exposure had an average equivalent oral intake of 1224
mg/man/day.
Comparisons of work records between exposed and non-exposed groups did
not reveal any differences as to frequency or severity of diseases or
accidents or disability (Jager et al., 1970). See also "Biochemical
studies".
On the basis of fat samples only, Crabtree (1969) found in Australia a
mean fat concentration of 0.3 ppm equivalent to an intake of 16
mg/man/day. Bick (1967) reported lower figures while Wasserman (1968)
reported higher ones. These last figures may be due to analytical
errors (Lofroth, 1970).
Wasserman et al. (1969) reported very low levels of dieldrin in fat
tissues from South Africa. Jansen (1969), however, analyzing part of
the same samples found a higher mean of 0.159 ppm, equivalent to an
average absorption of 8.6 mg/man/day.
COMMENT
The increased risk of development of liver tumours in mice exposed to
dieldrin as compared with untreated control mice was regarded as the
main matter for concern. This increased risk was seen even at an
exposure level of 0.1 ppm in the diet. It was generally agreed that
the tumours were neoplasms and that those seen in treated mice were
similar in nature to those seen in untreated mice except insofar as
the tumours in dieldrin-treated mice tended to show the same
cytoplasmic changes as other liver parenchymal cells in the same
animals.
There was no evidence that tumours regressed or disappeared after
cessation of exposure to dieldrin, although the cytoplasmic changes
did regress under such circumstances. It is difficult to establish
unequivocal criteria for distinguishing between benign and malignant
neoplasms in mouse-liver. The incidence of mice showing tumour emboli
or metastases in the lungs was not significantly increased at any
levels of exposure to dieldrin up to 10 ppm for 132 weeks.
It was agreed that the effect of dieldrin in increasing the risk of
tumour development in mice could not at the present time be regarded
as sufficient evidence to categorize dieldrin as a carcinogen. Other
work showed that nonspecific factors such as calorie intake, hormonal
status and micro biological status may profoundly influence the risk
of liver tumour development in mice, some strains of which are in any
case peculiarly susceptible to each tumours. Also it was noted that
long term studies in rats and prolonged studies in dogs and monkeys
had revealed no evidence of hepatocarcinogenicity. It seems likely
that the effect of dieldrin in mice is probably a species-specific
phenomena.
Further information is urgently needed concerning the aetiology and
pathogenesis of liver tumours in mice. A comparison of the effect of
dieldrin in mice of high and low liver tumour susceptibility, with
parallel metabolic studies, might provide a basis for relating
observations of tumourogenicity in mice to other species, including
man. There are at present no experimental data referable to
teratogenicity.
Continued surveillance of persons occupationally exposed to dieldrin
for chronic toxic effects and manifestation of possible
carcinogenicity is recommended. In this connection, the extensive
surveillance of occupationally exposed workers, which is currently
being made, was noted.
Information on the varying patterns of metabolism in the mouse, sheep,
rat, monkey and man are as yet too incomplete to indicate whether the
possible carcinogenic potential in the mouse is likely to apply to
man.
For these reasons, it was felt that no change in the acceptable daily
intake established at the 1966 Joint Meeting could be made at this
time.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
Dog: 1 ppm in the diet, equivalent to 0.025 mg/kg body-weight/day.
ESTIMATE OF ACCEPTABLE DAILY INTAKE IN MAN
0-0.0001 mg/kg body-weight, the sum of aldrin and dieldrin by weight.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Seed treatments
The use of aldrin and dieldrin as a rice seed treatment is now
considered important only in Brazil and to a lesser degree in Japan,
Costa Rica and El Salvador. The choice between aldrin and dieldrin is
primarily a question of agronomic factors, local preference and
economics prevailing. Treatment rates vary, but a rate of 2 oz /
bushel (0.2 percent active material) is standard. In Brazil, recent
figures show that out of 4,600,000 hectares planted, 3,680,000
hectares (80 percent) were treated; in Japan, out of 3,280,000
hectares planted, 169,000 hectares (0.5 percent) were treated; in
Costa Rica, out of 43,000 hectares planted, 21,500 hectares (50
percent) were treated and in El Salvador out of 31,000 hectares
planted, 6,200 hectares (20 percent) were treated.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In maize
The treatment of soils prior to planting maize represents the most
important of all uses for aldrin. Studies in the United States in
which aldrin was applied to soil at rates of 1, 2, 3, 4 and 6 lb /acre
and in which pre-harvest intervals ranged from 75-195 days resulted in
aldrin residues in maize grain ranging from <0.001-<0.01 ppm and
dieldrin residues from 0.002-0.02 ppm. Control values were equal to or
greater than treated values in virtually all cases. In the same
experiments, the corresponding residues of aldrin plus dieldrin in
green fodder were less than 0.034 ppm; in maize stover most levels
were less than 0.01 ppm and only in two subsamples did levels exceed
0.03 ppm. With rare exceptions, the totals for aldrin and dieldrin
fell well below 0.03 ppm (Shell Development Co., Reports RES 63-122,
64-135, 64-102, 63-111, 64-168, 64-166 Shell Chemical Co., Reports PRL
67-81, 67-62, 65-6, 67-92; Mourbry and Myrdal, pre-print
communication). Similar experiments in Germany, France, Spain,
Portugal, Australia, Mexico, Venezuela and Trinidad at application
rates of 1-5 kg/ha gave residues of aldrin plus dieldrin of <0.01 ppm
in maize grain; a second crop from treatment two years in succession
gave the same result. Residues in whole maize plants (green fodder) in
France, Spain, Australia and Trinidad were 0.01-ppm or less except for
one year samples from Trinidad which were 0.15 ppm. This appears to be
exceptional, since samples in the succeeding year were <0.01 ppm.
Limited data from France on residues in maize leaves ranged from
0.01-0.07 ppm in dry leaf. Based on this data, it is estimated that
whole stover would have residues less than 0.03 ppm. (Shell Research
Ltd., Reports WKGR 0114.70, 0053.68, ibid - unreported data). In the
U.S.A. extensive data have been obtained for maize grown in a series
of rotations typically employed in U.S. farm practice. Treatments were
between 1´-2´ lb/acre - occasionally 5 lb/acre - and covered 35 sites
and included two-eleven years. In no cases were residues detected in
grain (0.01 ppm dieldrin). In stover, 80 percent of the results were
below 0.01 ppm, and the upper limit was 0.02 ppm. No residues were
detected in green corn (Shell Chemical Co., Report PRL 66-73).
In potatoes
The treatment of potato soils at recommended rates (2-4 kg/ha) with
aldrin shortly before planting gave rise to residues in the whole
washed tubers of between <0.01 ppm and 0.1 ppm of aldrin plus
dieldrin. Typically, levels were 0.04-0.06 ppm. Levels in peeled
tubers were generally 1/3-´ of these values (Shell International
Chemical Co. Monograph, November 1968). More recent data developed by
Shell in summarized in Table III; data from Scandinavia is given in
Table IV.
Residues in potatoes resulting from two or three soil applications of
aldrin in consecutive years are summarized in Table V.
A trial in Portugal compared residues resulting from broadcast
applications with row applications made with aldrinised fertilizer,
all at 3 kg/ha. Results are shown in Table VI (Shell Research Ltd.,
Tech. Serv. Report WKTR.0025/69).
Potatoes were planted in plots in tropical countries from which maize,
grown on soil treated at 2-4 kg/ha just prior to sowing, had been
harvested. Aldrin + dieldrin residues in the tubers were from <0.01
to 0.07 ppm (mean--0.03 ppm) and in peeled tubers from <0.01 to 0.03
ppm (mean--0.01 ppm), as shown in Table VI (Shell Research Ltd.,
Report WKGR. 0114.70).
Residue data for potatoes grown in soils in temperate countries to
which aldrin treatment had been given one or two years before planting
are given in Table VII.
Analytical data for 256 samples of peeled potatoes and potato peel
obtained by the U.K. Ministry of Agriculture (Lee, 1968) from potatoes
grown commercially on treated (three or four lb./acre) land shows
residues of aldrin plus dieldrin in peeled tubers ranging from
0.0-0.14 ppm (mean--0.01 ppm). Calculated residues in whole tubers
based on 15 percent peel ranged from 0.0 to 0.23 ppm (mean--ca. 0.02
ppm).
In sugar beets, juice and pulp
Residues of aldrin plus dieldrin in sugar beets grown in soils treated
once with 2 kg/ha in the same year as cropping ranged from <0.01 to
0.03 ppm; 4 kg/ha resulted in residues ranging from <0.01 to 0.05 ppm
(Legar, 1966; van Steyvoort et al., 1968; Shell Research Ltd., Reports
WKTR.0053.68, WKGR.0123.69, WKGR.0114.70; Shell Chemical Co., Report
PRL-68-148). In contradiction to all these findings, Walker et al.,
(1965) found that one application of 5 lb./acre gave combined residues
in the whole fresh beet of 0.19 ppm and Smith et al., (1966) found
residues of 0.93 ppm resulted from 1 lb./acre of aldrin as a row
application at the time of sowing. However, 80 percent of this latter
residue occurred as aldrin, strongly indicating sample contamination.
Residues of dieldrin in sugar beet tops from beets grown in soils
treated once with aldrin at 1-6 kg/ha in various countries in Europe
and in Turkey ranged from <0.001 to 0.01 ppm (van Steyvoort et al.,
1968; Shell Research Ltd., Reports WKTR. 0053.68, WKGR.0123.69,
.0114.70). Residues of aldrin plus dieldrin in roots and tops of sugar
beets grown in soils treated two and sometimes three years in
succession are summarized in Table VIII.
Table III
Residues in potatoes arising from soil applications of aldrin
Application Range of Values, ppm No. of Results Arithmetic Mean
Rate Whole Peeled Whole Pooled Whole Peeled
kg/ha Tubers Tubers Tubers Tubers Tubers Tubers
2 <0.01-0.13 <0.01-0.05 24 10 0.06 0.02
3 0.01-0.16 - 18 - 0.08 -
4 0.03-0.24 0.01-0.07 10 10 0.11 0.04
2-4 <0.01-0.24 - 52 - 0.08 -
5 0.02-0.22 - 11 - 0.11 -
Shell Chemical Co., Reports PRL 68-147, PCY-64-10; Shell Research Ltd.,
Reports WKTR. 0025/69, Tech. Memo 137/65, Tech. Serv. Note 141/66,
WKTR. 0114. 70; Winnett and Reed, 1968.
Table IV
Aldrin and Dieldrin Residues in Potatoes Arising from single soil
applications in Scandinavia
Application Residues, ppm in
Rate, potato tubers
Country kg/ha Aldrin Dieldrin Reference
Sweden(1964) 2.5 <0.01 0.02
Sweden(1965) 2.5 <0.01-0.01 <0.01-0.02 Renvall and
Akerblom, (1968)
Sweden(1966) 2.5 <0.01 0.01
2.5 <0.01 0.05
2.5 0.01 0.02
2.5 0.02 0.13
2.5 0.03 0.12
Denmark 2.5 not found 0.05 Bro-Rasmussen
5.0* not found 0.02-0.05 and Voldum-
10.0* 0.02 0.03-0.10 Clausen, (1966)
* High rates
Table V
Aldrin plus dieldrin residnes in potatoes grown in plots which received
a number of annual treatments with aIdrin
Range of values
Application Aldrin + Dieldrin, Arithmetic
kg/ha ppm No. of results Mean, ppm
Whole Peeled Whole Peeled Whole Peeled
2 0.01-0.11 00.01-0-05 11 8 0.05 0.02
4 0.03-0.40 <0.01-0.01 8 8 0.14 0.03
Shell Research Ltd., Reports WKTR-0007.69, 0028.68, Tech. Serv. Note
141/66, Report WKGR.0114.70, 0153/69, Tech. Serv. Note WKTR. 0100.68.
Table VI
Comparison of residues in potato tubers from broadcast and
row treatments
Range of Results,
Type of Aldrin + Dieldrin, No. of Arithmetic
Application ppm Values Mean, ppm
Row treatment 0.09-0.27 4 0.15
Broadcast 0.04-0.07 3 0.05
(fertilized
mixture)
Broadcast (E.C.) 0.05-0.16 4 0.08
Table VII
Residues In potato tubers grown-in soils treated one or two years
prior to planting
Interval
Treatment Between Range of
Rate, Treatment and Results, No. of Arithmetic
kg/ha Planting, ppm Results Mean, ppm
years
2 1 <0.01-0.05 14 0.02
4 1 0.01-0.05 9 0.03
2-5 1 <0.01-0.11 29 0.04
2-5 2 0.01-0.02 12 0.01
Shell Research Ltd., Tech. Serv. Note 141/66, Report WKGR.0114.70,
Tech. Serv. Report WKTR 0007.69, .0028.68, Report WKGR 0153/69,
Tech. Serv. Note WKTR.0100.68, Winnett and Reed; 1968.
Residues of dieldrin in roots and tops of beets grown on plots treated
the previous year at 2 or 4 kg/ha were all <0.01 ppm (28 samples)
except for two root samples of 0.02 and 0.03 ppm; no aldrin was
detected (Elgar, 1966; Shell Research Ltd., Report WKGR.0114.70).
Recent studies by Onsager at al., (1970) show that aldrin plus
dieldrin residues in mature sugar beets are proportional to the
residues in the soil at the time of planting as illustrated in Table
IX.
Studies in Belgium (van Steyvoort et al., 1968) and in France (Shell
Research Ltd., Report WKTR.0053.68) indicate that no residues could be
found in purified juices from sugar beets grown in soil treated with
up to 6 kg/ha of aldrin. However, data from refineries in the U.S.A.
(Walker at al., 1965) show combined residues of 0.02 ppm (expressed as
ppm of pure sucrose) in clarified juice from beets with abnormally
high residue levels. Had these juices been processed, most of the
residue would have been found in the molasses rather than in the
sugar.
Data was obtained on dieldrin residues in dried pulp from beets grown
in soil which had received aldrin treatment for two years preceding
planting. A residue of 0.03 ppm in the fresh beet resulted in values
of 0.13-0.14 ppm in pulp dried at 105°C and 0.05-0.12 in pulp dried at
150°C; 0.07 ppm in fresh beet gave values of 0.28 ppm in pulp dried at
105°C and 0.34 ppm in pulp dried at 150°C (Shell Research Ltd.,
unreported data). The ratio of residues in dried pulp to fresh beet
was 3-4 which is lower than the figure of 10.6 reported by Walker et
al. (1965). The significance of pulp residues in terms of cattle feed
is questionable, however, due to the low amount of pulp (10-20
percent) in the total diet. Onsager et al. (1970) calculated that a
residue of 0.25 ppm in 4.5-8 lb of beet pulp fed daily to a 1200-lb
animal amounts to ingestion of 0.2-1.6 ppm of insecticide.
In crops derived from seed dressings
Both aldrin and dieldrin are extensively used as seed dressings on a
wide variety of crops; maize, rice, cereals, cotton and pulse crops
are the most important. A large amount of data collected primarily by
Shell shows that residues in the crop derived from dressed seed are
extremely small and usually below the limits of detection (about 0.005
ppm), except for occasional residues of 0.01-0.02 ppm in pulse crops.
In all cases where residues were much above the detection limit, there
are strong indications that samples were contaminated. Either residues
were high on control as well as treated samples, or else appreciable
proportions of aldrin were found in the total residue.
In vegetables
Residue data obtained up through 1968 is summarized in Table X (Shell
International Chemical Co., Monograph, Residues in Vegetables,
November 1968).
Table VIII
Residues in sugar beets grown in soils treated on more than one occasion
Rate of
Application, Range of Residues Arithmetic
kg/ha/ Part of Aldrin + Dieldrin, No. of Mean,
year Crop ppm Samples ppm
2 Root <0.01-0.04 8 0.01
4 Root <0.01-0.07 5 0.04
2 Tops <0.01-0.02 5 <0.01
4 Tops <0.01-0.02 5 <0.01
Table IX
Average residues of aldrin plus dieldrin in soil and sugar beet roots
1965-1968
Average residue, ppm
Aldrin Soil
dosage, sampled on Roots sampled on
lb ai/acre 4/10/65 10/5/65 10/25/66 10/17/67
0.625 0.05 <0.01 <0.01 <0.01
1.25 0.21 0.04 <0.01 <0.01
2.5 0.38 0.06 0.01 0.01
5.0 0.73 0.13 0.02 0.01
7.5 1.84 0.37 0.03 0.03
10.0 2.84 0.96 0.05 0.04
Table X
Residues from seed or soil treatment with aldrin and dieldrin at
application rates within the recommended range (typically 2-3 kg/ha).
Crop or Residues, ppm Location and
Crop Group Min Max Est. Median No. of Expts.
Carrots 0.02 0.51 0.20 USA:3, Switzerland:5,
Denmark:2, Holland, 3,
UK:3, Austria:2
Cruciferous 0.02 0.08 0.04 USA:20, Norway:1,
roots (1) Austria:2
Beetroot 0.01 0.22 0.10 USA:15
Cole crops (2) 0.01 0.02 0.015 USA:21
Onions ND ND ND UK:3, USA:1
Peas ND ND ND USA:1
Lettuce 0.10 0.16 - USA:2
Beans ND ND ND USA:3
Tomatoes 0.10 0.10 0.10 USA:3
Celery 0.02 ND ND UK:3
Cucurbits (3) 0.01 0.07 0.03 USA:10
(1) Including radish, turnip, rutabaga and swede.
(2) Including cabbage broccoli, brussels sprouts and cauliflower.
(3) Including cucumber, pumpkin and melon.
More recent data on beetroot, cauliflower, carrots, white cabbage,
lettuce, tomatoes, turnips, and sweet potatoes are consistent with the
findings in Table X and in general give slightly lower residue figures
(Bro-Rasmussen and Voldum-Clausen, 1966; Shell Research Ltd., Reports
WKTR. 0050.68, 0053.68; Shell Chemical Co., Reports PRL-68-112,
-68-210).
In cereal grain
Aldrin treatments of seed or soil at recommended rates have rarely
produced residues of aldrin plus dieldrin in grain of wheat, oats or
barley above 0.01 ppm. Foliar applications of aldrin or dieldrin to
the growing crop do not give measurable residues in grain or straw if
pre-harvest intervals of one month or more are observed (Shell
International Chemical Co., Monograph, Residues in Small Grains,
1968).
Recent data for wheat and barley grain from soils receiving a variety
of aldrin application regimes at rates of 2 or 4 kg/ha show no
detectable residues (<0.01 ppm) of either aldrin or dieldrin.
Residues in wheat straw ranged from <0.01-0.16 ppm and in barley
straw from <0.01-0.20 ppm. Straw residues are of limited significance
since wheat straw is not an appreciable component in the diet of dairy
cattle and barley straw is used as a feed primarily for beef cattle in
winter only (Shell Research Ltd., Reports WKGR.0114.70, WKTR-0053.68).
In citrus and sugar-cane
Soil applications of aldrin at 2.0-5.0 kg/ha in citrus groves resulted
in dieldrin residues of 0.01-0.02 ppm in whole oranges, 0.02 ppm in
chopped peel and 0.03-0.04 ppm in dried pulp; controls contained up to
0.01 ppm. Dieldrin residues were not found in whole grapefruit or
grapefruit juice, but peel contained 0.006-0.007 ppm and dried pulp
contained 0.01 ppm; residues were also found in control samples (Shell
Chemical Co., Report PRL-67-30).
No residues were found in any of six samples of sugar-cane grown in
soil treated with two applications of aldrin at 2 kg/ha (Shell
Chemical Co., Report PRL-68-51). Soil treatments with aldrin of 5 or
10 lb ai/acre resulted in apparent aldrin residues of 0.004 ppm in two
out of three foliage samples from the 10 lb/acre treatment and in
apparent dieldrin residues ranging from 0.008-0.015 ppm in 8 out of 16
samples of foliage and stalks. No residues were found in the juice at
any treatment rate, and one sample of bagasse (out of four) had 0.009
ppm dieldrin (Shell Chemical Co., Report PRL-68-236).
In milk and meat (from cattle feed)
Williams et al. (1964) fed 16 milking cows for 35 days with a feed
containing various amounts of dieldrin. Similar experiments were
carried out by Gannon et al. (1959a, 1959b) for 12 weeks and by Shell
(1970) for 21 days using dieldrin C-14. The results of these
investigations are summarized in Table XI.
After the feeding of dieldrin was stopped, dieldrin concentrations in
the milk began to fall with a half-life of 10-15 days in the case of
Williams' work and substantially longer in the case of Gannon's work.
The lack of consistency may be due to some cows coming to the end of
their lactation period, thus reducing the milk production per day.
Gannon et al. (1959a) also studied residues in body tissues of milk
cows and steers fed diets containing various amounts of dieldrin. His
results are shown in Table XII.
In poultry and eggs
The residues of dieldrin that could arise in poultry products from the
use of rice bran in the diet (typically 20 percent of the total diet)
are summarized in Table XIII.
Thus with residues of dieldrin in rice bran of 0.05 ppm, residues of
dieldrin in poultry products could be 0.012 ppm in eggs, 0.20 ppm in
fat and 0.02 ppm in poultry meat. Residues of up to 0.03 ppm dieldrin
are commonly found in rice bran.
FATE OF RESIDUES
General comments
The occurrence and toxicity of the photoisomer of dieldrin,
photodieldrin, has been reviewed in the section on biological studies.
Photodieldrin residues may be of significance only after foliar
application of dieldrin. No detectable residues were found in human
fat in the U.S.A. where the ratio of dieldrin to photodieldrin
residues was greater than 200 to 1 (Richardson, 1966). Photodieldrin
was also absent in human fat from U.K. and Holland (Richardson, 1968)
and milk (Robinson et al., 1966).
In animals
The fate of residues of dieldrin in animals has been covered under the
biochemical section of this monograph.
In plants
Extensive experimental work was carried out by Shell to determine
residues of photoaldrin and photodieldrin in crops grown on
aldrin-treated soil, grown from aldrin or dieldrin-treated seed, grown
in soils containing old residues or receiving foliar applications of
dieldrin, and general monitoring investigations. Their results
indicate that none of the soil or seed dressing uses of aldrin or
dieldrin lead to detectable residues of the photoisomers. Foliar
applications led to photodieldrin residues of 0.03 ppm in coffee beans
treated at 1-1/3 g of dieldrin/ tree, 0.02 ppm in leaves of forage
beets treated at 4.3 oz/acre, 0.31-0.89 ppm in wheat straw treated at
8 oz /acre, <0.01-0.04 ppm in apples and 0.01-0.06 ppm in apple
pomace treated at ´ lb/acre, 3.6 ppm in pasture grass one and a half
months after treatment with 1 oz/acre, and 1.1 ppm in fat of sheep
grazing for four months on the treated pasture grass. None of the
photoisomer was detected in any samples of vegetable oils and fats
monitored in the U.K., but some samples of food and feed components
contained traces.
Table XI
Dieldrin in milk from animals fed with feed containing
different amounts and for various lengths of time
Dieldrin in Butterfat Ratio,
feed, ppm, Milk level, ppm, equivalent, ppm, ppm B.F.
dry weight at time indicated at time indicated ppm Feed Reference
35 days 35 day
0 0.004 0.11 - Williams
0.09 0.021 0.53 5.9 et al.
0.23 0.058 1.52 6.6 (1964)
0.50 0.11 2.89 5.9
4 weeks 12 weeks 4 weeks 12 weeks
0 <0.01 0.01 - - - Gannon
0.1 <0.01 0.02 - 0.5 5.0 et al.
0.25 0.02 0.06 0.5 1.5 6.0 (1959b)
0.75 0.07 0.11 1.75 2.8 3.1
2.25 0.16 0.28 4.0 7.0 3.0
15-21 days 15-21 days
0.102 0.018 0.41 4.0 Shell
0.120 0.017 0.35 2.9 Development
0.111 0.015 0.38 3.5 Co., Report
M-32-70
Table XII
Dieldrin residues in body tissues of cows and steers after feeding at
various levels of dieldrin for twelve weeks
Milk cows Steers
Dieldrin in Feed, ppm Dieldrin in Feed, ppm
Body Part 0.25 2.25 0.1 0.25 0.75 2.25
Dieldrin in Tissues, ppm
Brain <0.1 0.1 - - - -
Heart 0.2 0.6 - - - -
Liver 0.2 0.7 - 0.1 - 0.7
Kidney <0.1 <0.1 - <0.1 - 0.2
Steak 0.1 1.3 - <0.1 - 1.0
Roast 0.2 1.2 - - - -
Renal Fat 0.9 6.2 - 0.9 - 9.7
Body Fat 0.9 4.8 0.4 0.8 3.5 8.7
Heart Fat - 7.0 - - - -
Udder Fat - 5.6 - - - -
The average ratio of dieldrin levels in fat to levels in feed was 2.75
in milk cows and 3.95 in steers.
Table XIII
Relation between dieldrin residues in poultry feed
and poultry products (eggs, meat, fat of meat)
Feed Range of Ratios,
Poultry Weeks Concentration Poultry Feed/
Product fed Range (ppm) Poultry Product Reference
Eggs 12 0.1-0.75 0.8-1.6 Gannon et al., 1959a
Eggs 16 0.006-5.0 1.0-3.0* Greaves et al., 1969a
Eggs 14 0.05-0.45 1.1-1.2 Cummings et al., 1966
Fat(abdominal) 2 0.1-0.15 20-29 Liska et al., 1967
Fat 12 0.1-0.75 41-48 Gannon et al., 1959a
Fat(abdominal) 14 0.05-0.45 8.8-9.7 Cummings et al., 1967
Meat 2 0.1-0.15 2-4 Liska et al., 1967
Meat 12 0.1-0.75 <0.2 Gannon et al., 1959a
Meat 14 0.05-0.45 0.1 Cummings et al., 1967
* The figure of 3 regarded as inconsistent with other data.
Lichtenstein et al. (1970) reported 0.015 ppm photodieldrin in soils
which had been treated annually with 5 lb/acre of aldrin for five
years and then not treated for five years. Potatoes and carrots grown
in these soils contained 0.0006 ppm and 0.002 ppm, respectively.
After foliar application of photodieldrin C-14 to white cabbage,
residues disappeared more slowly than in corresponding tests with
aldrin, dieldrin, isodrin or endrin. Four weeks after application, as
much as 75 percent of the applied radioactivity was present as
residues. Fifteen to 33 percent of metabolites were found mainly in
leaves. Metabolic fractions consisted of a very hydrophilic main
product and at least two less hydrophilic by-products (Korte and
Porter, 1970).
Four weeks after application of ca. 75 ppm of aldrin C-14 on the upper
side of cabbage leaves, only 17 percent of the applied radioactivity
was recovered in plants and soil. Hydrophilic metabolites made up 76
percent of the recovered activity; besides aldrin, five other
compounds were found, one of which appeared to be dieldrin. In similar
experiments with dieldrin C-14 on cabbage, 40 percent of the
radioactivity is recovered after four weeks and 34 percent of the
recovered activity is due to at least two hydrophilic metabolites
(Korte, 1970).
In soils
Maize grown in California in soil treated with 3 kg/ha of aldrin C-14
contained no detectable C-14 in the grain or cobs. The husks of the
ears contained 0.004 ppm aldrin, 0.004 ppm dieldrin and 0.032 ppm
metabolites. The leaves contained 0.35 ppm C-14 equivalents of which
0.02 ppm was aldrin and 0.05 ppm was dieldrin (Korte and Porter,
1970).
Evidence of residues in food in commerce
Belgium
Thirty local and sixteen imported samples of butter were examined
(Dejaukheere and Kips, 1968). In the local samples, residues of
dieldrin ranged from 0.01 to 0.24 ppm (mean 0.12 ppm), and in the
imported samples the range was 0.01 to 0.25 ppm.
Denmark
Samples of milk butterfat (44) contained mean levels of dieldrin of
0.03 ppm (<0.007-0.11) (Bro-Rasmussen et al., 1968). Samples of
butter examined over a three year period contained dieldrin residues
as follows:
1964 1965 1966
No. of samples 118 137 126
Range (ppm) 0.01-0.20 0.01-0.18 0.01-0.17
Mean (ppm) 0.05 0.04 0.03
The authors suggested from their data that 60-100 percent of these
residues originated from dieldrin residues in imported cattle feed.
France
Mestres, et al., (1967) in France found no residue of aldrin or
dieldrin in 44 samples of French butter or in four samples of Swiss
butter at a detection limit of 0.02 ppm.
West Germany
Of 65 samples of carrots taken in West Germany (Maier-Bode, 1967) 43
contained less than 0.01 ppm aldrin plus dieldrin. Of the remainder,
64 percent contained residues of 0.03 ppm or less, and only one sample
exceeded 0.1 ppm (0.17 ppm).
Norway
Twenty butter samples out of a total of 54 contained dieldrin residues
in the range 0.01-0.1 ppm (Sakshaug, 1968). The rest did not contain
residues, above the limit of detection.
U.K.
Routine analysis of food-stuffs by public analysts in the U.K. (Rymer
and Hammence, 1967) demonstrated that of 776 fruit, 461 vegetable and
237 meat and dairy produce samples, only three fruit samples and three
meat and dairy produce samples contained aldrin above the limits of
detection (maximum levels of 0.06 and 0.18 ppm respectively) and two
fruit, one vegetable and thirteen produce samples contained dieldrin
above the limits of detection (maximum 0.15 and 0.27 ppm).
A more recent examination of 388 fruit, 285 vegetable and 217 meat and
dairy produce samples (Dickes and Nicholas, 1969) revealed one
vegetable sample containing aldrin (0.09 ppm) and sixteen vegetable
samples containing dieldrin, although all were below 0.01 ppm.
Findlay and Hamilton (1968) examined 163 samples of eggs and 114
samples of poultry meat, liver and fat from nine sources within the
U.K. No egg samples exceeded 0.06 ppm of dieldrin and only one poultry
meat sample exceeded 0.1 ppm (0.13 ppm).
Table XIV
Dieldrin residues in dairy and meat products produced in U.K., 1965-1968
Butter Milk Mutton Fat Beef Fat
Year Mean Mean Mean Mean
Samples (ppm) Samples (ppm) Samples (ppm) Samples (ppm)
1964 127 0.84
1965 18 0.03 85 0.0025 107 1.15 59 0.06
1966 13 0.02 75 0.0025 101 0.44 63 0.04
1967 22 0.03 77 0.002 76 0.24 36 0.03
1968 16 0.03 76 0.001 77 0.21 34 0.03
A most comprehensive examination of home produced and imported meat
and dairy products has been carried out over a number of years by the
Laboratory of the Government Chemist (1966, 1967, 1968, 1969). In the
four years 1965-1968, 935 samples of home produced foods and 803
samples of foods imported from ten countries were analyzed. Table XIV
summarizes their findings for home-produced dairy and meat products.
Residues in food at time of consumption
A study of data accumulated over four years by the U.S. Food and Drug
Administration on pesticide residues in total diet samples was made by
Duggan and Lipscomb (1969).
Residues of dieldrin were found in from 15.6-21.3 percent of
composites analyzed over this period and aldrin residues were found in
from 3.3-5.6 percent. Measurable dieldrin residues were present in six
of the twelve food groups examined, and traces were found in another
four. Measurable aldrin residues were found in only one group. The
highest residues of dieldrin (max. 0.20 ppm) were found in samples of
meat, fish and poultry (Group 2). In the dairy products group (Group
1) the greatest level was 0.08 ppm and residues did not exceed 0.03
ppm in any other group. The maximum aldrin level was 0.07 ppm in the
first group (Group 9). No tendency to increase or decrease was
observed over this period. From the residue information and a
knowledge of the diet, the daily intake of each pesticide was
calculated. Since the diet was representative of that consumed by a
sixteen-nineteen year old American male, the intake of the average
population will be about half the calculated figure. The total daily
intake for aldrin and dieldrin over the four year period is given in
the following table (from Duggan and Lipscomb, 1969):
Daily Intake (mg/kg body-weight)
1965 1966 1967 1968
Aldrin 0.00001 0.00004 0.00001 0.00001
Dieldrin 0.00008 0.00009 0.00005 0.00005
Total 0.00009 0.00013 0.00006 0.00006
An examination of residues in groups of foodstuffs making up a
complete diet was undertaken by Abbott et al., (1969), at the
instigation of the U.K. Ministry of Agriculture, Fisheries and Food,
for one year from October 1966-October 1967. Of the foods analyzed,
the highest dieldrin residues were in fat (mean 0.024 ppm, range
0.009-0.075 ppm), followed by meat (mean 0.009 ppm, range 0.001-0.060
ppm). Because of the larger consumption of meat, this group provides
the largest intake of dieldrin 2.2 mg/person/day. The mean daily
intake in the diet calculated from the residues found was 0.00009
mg/kg body-weight. This figure confirms the decrease shown in the
second survey by McGill and Robinson (1968) compared with the first,
and may be attributed to the voluntary withdrawal of some previously
approved dieldrin uses in the U.K. over the period 1965-1967.
In 1965 the Association of Public Analysts prepared a survey to
determine the extent of contamination of foodstuffs by pesticides.
Data for the first year of this work has so far been reported
(Association of Public Analysts, 1969). Of the 2352 samples analyzed,
only ten contained residues of aldrin (two only above 0.1 ppm) and 76
contained dieldrin (again only two above 0.1 ppm). Dieldrin was most
commonly found in meat and dairy products. In no foodstuffs did the
mean level of dieldrin residues exceed 0.015 ppm, a figure that is
based on the assumption that all samples in which residues were not
detected (limit 0.002 ppm in solid foods and 0.0002 ppm in liquid milk
and infant foods) did in fact contain residues at half the detection
limit. Using the same assumption, the mean daily intake in the diet
was calculated to be 0.0001 mg/kg body-weight. A survey of residues in
welfare foods (dried full cream milk, cod-liver oil and concentrated
orange juice) by Ruzicka et al., (1967) revealed residues of up to
0.017 ppm dieldrin (mean 0.011 ppm) in dried milk and demonstrated a
15 percent loss in residue during the drying period. Cod-liver oil
contained 0.15-0.20 ppm (mean 0.16 ppm) and concentrated orange juice
contained up to 0.007 ppm (mean 0.002 ppm).
METHODS OF RESIDUE ANALYSIS
The remarks on methods for residues of organochlorine pesticides and
multidetection systems of analysis (FAO/WHO, 1967a) apply to the
determination of aldrin and dieldrin. Burke (1969) has studied the
application of the official AOAC multiresidue method to photodieldrin.
No methods of analysis have been well worked out for the more
hydrophilic metabolites of dieldrin such as monohydroxydieldrin,
dihydro-aldrin-diol (trans), dieldrin-ketone, dieldrin-ketone
photo-isomer, or Klein's metabolite. Although photoaldrin can be
determined by GLC techniques, the rapid conversion of aldrin to
dieldrin makes it unlikely that photoaldrin will be a significant
residue in crops or soils.
CONSIDERATION OF SPECIFIC POINTS RAISED BY THE CODEX COMMITTEE ON
PESTICIDE RESIDUES
The meeting considered three questions arising from the Draft Report
of the Fifth Session of the Codex Committee in 1970 as follows:
Further data on the use pattern of aldrin and dieldrin on rice are
provided in this monograph, as are data on residues resulting from the
use in animal feed.
The question of the fate of the residues on rice during processing was
not considered because the available data show that residues are so
small that any remaining following processing would be negligible.
No firm data were received on residues in fruit juices resulting from
the use of rice husks as clarifying agents. However, if it is assumed
that 10 percent rice husks are used for clarification, and if they
should contain up to 0.02 ppm of aldrin plus dieldrin, and if it is
further assumed that this is extracted by the fruit juices (unlikely
due to the lipophylic nature of residues), then it would be highly
improbable that residues in excess of 0.002 ppm in the juice would
occur.
APPRAISAL
The data provided on residues of aldrin plus dieldrin in maize grain
resulting from planting maize in soils treated at recommended rates
indicate that levels of 0.02 ppm or less could be expected and that
maize grain is within the recommended practical residue limit of 0.02
ppm on raw cereals listed in the 1969 Report of the Joint Meeting.
The data on residues of aldrin plus dieldrin in whole washed potato
tubers arising from soil applications of aldrin at recommended rates
reveals the possibility of exceeding the recommended tolerance of 0.1
ppm, especially when the potatoes are grown in plots receiving a
number of annual applications at the highest rate. An increased
tolerance of 0.2 ppm is recommended for potatoes.
Residues of aldrin plus dieldrin in mature sugar beets are
proportional to the residues in soil at planting. For example, an
aldrin dosage of 2.5 lb/acre gives after six months a soil residue of
0.11 ppm and a sugar beet root residue, grown in the same year, of
0.06 ppm. This would be expected to result in about 0.24 ppm of
dieldrin residue in the pulp dried at 105°C. This amount of residue
under normal feeding practices would not lead to excessive residues in
whole milk or meat (fat basis). Therefore, the recommended practical
residue limits appear to be adequate for residues from this source.
Residues in the crop derived from dressed seed (maize, rice, cereals,
cotton and pulse crops) are extremely small and usually below the
limits of detection, thus justifying the reduction of the tolerance in
rough rice to 0.02 ppm.
Residues in vegetables from seed or soil treatment are dependent on
the type of crop. Residues at or in excess of tolerance could arise in
carrots and lettuce planted in soil previously treated for other
crops. Data were received which justify a revision of the recommended
tolerance and the establishment of a practical residue limit of 0.2
ppm for lettuce and carrots.
Soil applications of aldrin of 2-5 kg/ha in citrus groves resulted in
dieldrin residues of 0.01-0.02 ppm in whole oranges and no detectable
residues in grapefruit. The presently recommended tolerance of 0.05
ppm appears to be adequate.
The importance of photodieldrin as a plant metabolite of aldrin or
dieldrin appears to be well established; however, its significance as
a terminal residue will depend on the results of experiments in
progress on rates of storage and excretion in animals.
A consideration of the new data supplied on residues in milk, milk
products and meat resulting from supervised trials and surveys of food
in commerce does not indicate a need for revision of the previously
recommended practical residue limits for these items at this time. The
previous practical residue limit of 0.125 ppm for milk products was
changed to 0.15 ppm to allow for the limitations and variability of
residue analytical procedures.
A suitable regulatory method of analysis exists for photodieldrin
(official AOAC multiresidue method) but no methods of analysis have
been worked out for the more hydrophilic metabolites of dieldrin.
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
The previously recommended tolerances and practical residue limits (no
longer temporary) are amended as follows:
ppm
Fruit (other than citrus), asparagus,
broccoli, brussels sprouts, cabbage,
cauliflower, cucumber, eggplant,
horse-radish, onions, parsnips, peppers,
pimentoes, radishes and radish tops 0.1
Citrus 0.05
Rice (rough) 0.02
Potatoes 0.2
PRACTICAL RESIDUE LIMITS
ppm
Carrots, lettuce, fat of meat 0.2
Raw cereals (other than rice) 0.02
Milk and milk products (fat basis) 0.15
Eggs (shell-free) 0.1
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further information relevant to the assessment of the significance
of the changes that occur in the livers of mice exposed to dieldrin. A
comparison of the response to dieldrin of high and low spontaneous
liver-tumour mouse strains in respect of liver tumour incidence and
the metabolism of dieldrin might be helpful in this respect.
2. The continued collection of follow-up information on persons
exposed occupationally in the manufacture of dieldrin.
3. Completion of the studies being conducted on the comparative
metabolism of dieldrin in man, monkey, rat and mouse.
4. Experimental studies to determine whether dieldrin might have
teratogenic potential.
5. A continuing study of the possible occurrence of residues of
photodieldrin in food surveys and total diet studies.
REFERENCES
Abbott, D.C., Holmes, D.C. and Tatton, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-1967. J.Sci.Fd.
Agric., 20: 245-249
Association of Public Analysts, London. (1969) Joint survey of
pesticide residues in foodstuffs sold in England and Wales, August
1966-July 1967
Baldwin, M.K. and Robinson, J. (1969a) The identification of a
metabolite of the photo-isomerization product of HEOD (dieldrin) found
in rat tissues. Unpublished report from the Tunstall Laboratory, Shell
Research Ltd., Sittingbourne
Baldwin, M.K. and Robinson, J. (1969b) Metabolism in the rat of the
photo-isomerization product of dieldrin. Nature, 224: 283-284
Baldwin, M.K. and Robinson, J. (1970) Comparison of the metabolism of
HEOD in the CFI mouse with that in the CFE rat - preliminary results.
Unpublished report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Baldwin, M.K., Robinson, J. and Carrington, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Industr., 595-597
Bick, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Austr., 54 (1): 1127-1130
Braund, D.G., Brown, L.D., Huber, J.J., Leeling, N.C. and Zabik, M.J.
(1969) Excretion and storage of dieldrin in dairy cows fed
thryoprotein and different levels of energy. J. Dairy Sci., 52:
172-182
Brooks, G.T. (1969) The metabolism of diene-organochlorine
(cyclodiene) insecticides. Res. Rev., 27: 81-138
Bro-Rasmussen, F., and Voldum-Clausen, K. (1966) Tids. Planteovl.,
20: 232
Bro-Rasmussen, F., Dalgaard-Mikkelsen, S., Jakobsen T., Koch, S.O.,
Rodin, F., Uhl, E. and Voldum-Clausen, K. (1968) Examinations of
Danish milk and butter for contaminating organochlorine insecticides.
Res. Rev., 23: 55-69
Brown, V.K., Robinson, J. and Richardson, A. (1967) Preliminary
studies on the acute and sub-acute toxicities of a photoisomerization
products of HEOD (dieldrin). Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Chan, T. and Terriere, L.C. (1969) Aldrin epoxidase activity of rat
liver and rat liver microsomes under various conditions of storage.
Biochem. Pharmacol., 18: 1061-1070
Cole, J.F., Klevay, L.M. and Zavon, M.R. (1970) Endrin and dieldrin: a
comparison of hepatic excretion in the rat. Toxicol. appl. Pharmacol.,
16: 547-555
Crabtree, A.N. (1969) The concentrations of organochlorine
insecticides in human fat and blood from Australia. Shell Research
Limited, Tunstall Report No. TL GR. 0059.69
Cummings, J.G., Zee, K.T., Turner, V. and Quinn, F. (1966) Residues in
eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Ass. Off. Anal. Chem., 49: 354-364
Cummings, J.G., Gidelman M., Turner V., Reed D., Zee K.T. and Cook
R.E. (1967) Residues in poultry tissues from low level feeding of five
chlorinated hydrocarbon insecticides to hens. J. Ass. Off. Anal.
Chem., 50: 418-425
Dailey, R.E., Walton, M.S., Beck, V., Leavens, C.L. and Klein, A.K.
(1970) Excretion, distribution and tissue storage of a 14C-labelled
photoconversion product of 14C-dieldrin. J. Agr. Fd. Chem., 18:
443-445
Damico, J.N., Chen, J.T., Costello, C.E. and Haenni, E.O. (1968)
Structure of Klein's metabolites of aldrin and dieldrin. J. Ass. Off.
agr. Chem., 51: 48-55
Datta, P.R., Lang, E.P., Watts, J.O., Klein, A.K. and Nelson, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature, 208: 289-290
Dejaukheere, W. and Kips, R.N. (1968) Meded. Rijksfac.
Landbouwwenteusch (Ghent), 33: 1283
Dickes, G.J. and Nicholas, P.V.J. (1969) Assoc. Pub. Analysts, 7: 14
Diechmann, W.B., Dressler, I., Keplinger, M. and MacDonald, W.E.
(1968) Retention of dieldrin in blood, liver and fat of rats fed
dieldrin for six months. Industr. Med. Surg., 37: 837-839
Diechmann, W.B., MacDonald, W.E., Radomski, J., Blum, E.B.,
Bevilacqua, M., and Keplinger, M. (1970) The tumorigenicity of aldrin,
dieldrin and endrin in the albino rat. Industr. Med., 39: 314
Donninger, C., Potter, D., Potter, S.A. and Wright, A.S. (1967) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to rats. Unpublished report from Tunstall Laboratory,
Shell Research Ltd., Sittingbourne
Donninger, C. (1969) Liver cell changes following the oral
administration of dieldrin to mice. Unpublished report from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Duggan, R.E. and Lipscomb, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States (II), June 1966-April 1968. Pest.
Monit. J., 2(4): 153-162
Elgar, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Fd. Agr., 17: 541-545
FAO/WHO (1967) Evaluations of some pesticide residues in food,
FAO,PL:CP/15; WHO/Food Add./67.32 p. 3-4.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30
Feil, V.J., Hedda, R.D., Zayliskie, R.G. and Zachrison, C.N. (1970)
Dieldrin-14C metabolism in sheep. Identification of
trans-6-7-dihydroxyaldrin and 9-(synepoxy)
hydroxy-1,2,3,4,10,10-hexachloro-6,7-
epoxy-1,4,4a,5,6,7,8,8a-,octahydro-1,4-endo-5,8-
exodimethanonophthalene. J. Agr. Fd. Chem., 18: 120-24
Findlay, E. and Hamilton, G.A. (1968) Pesticide residues in foodstuffs
in Great Britain. VIII Organochlorine pesticide residues in eggs and
poultry. J. Sci. Fd. Agric., 19: 609-611
Gannon, N., Link, R.P. and Decker, G.C. (1959a) Pesticide residues in
meat. Storage of dieldrin in tissues of steers, hogs, lambs and
poultry fed dieldrin in their diets. J. Agr. Fd. Chem., 7: 826-828
Gannon, N., Link, R.P. and Decker, G.C. (1959b) Pesticide residues in
meat and milk. Storage of dieldrin in tissues and its excretion in
milk of dairy cows fed dieldrin in their diets. J. Agr. Fd. Chem.,
7: 824-826
Gillett, J.W. and Chan, I.M. (1968) Cyclodiene insecticides as
inducers, substrates and inhibitors of microsomal epoxidation. J. Agr.
Food Chem., 16: 590-593
Good, E.E. and Ware, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV Endrin and dieldrin. Toxicol.
appl. Pharmacol., 14: 201-203
Graves, J.B., Bonner, F.L., McKnight W.F., Watts, E.B. and Epps, E.A.
(1969) Residues in eggs, preening glands, liver and muscle from
feeding dieldrin-contaminated rice bran to hens and its effect on egg
production, egg hatch and chick survival. Bull. Env. Contam. Tox.,
4: 375-383
Harr, J.R., Claeys, R.R., Bone, J.F. and McCorcle, J.W. (1970)
Dieldrin toxicosis: rat reproduction. Amer. J. vet. Res., 31:
181-189
Harris, L.E. and Greenwood, D.A. (1963) Effect of residues of newer
insecticides on health. Unpublished progress report to Shell Chemical
Company, letter signed by Joseph Street, dated 8 March 1963
Hedde, R.D., Davison, K.C. and Robbins, J.D. (1970) Dieldrin 14-C
metabolism in sheep. Distribution and isolation of urinary
metabolites. J. Agr. Fd. Chem., 18: 116-119
Hunter, C.G., Robinson, J. and Roberts, M. Pharmacodynamics of
dieldrin (HEOD). Ingestion by human subjects for 18 to 24 months and
postexposure for eight months. Arch. environm. Hlth., 18: 12-21
Jager, K.W. (1970) "Aldrin, dieldrin, endrin and telodrin". An
epidemiological and toxicological study of long-term occupational
exposure. Thesis, 234 p., Elsevier, Amsterdam
Jansen, J.D. (1969) Letter to A.M. Coetzee dated 25 July 1969
Keane, W.T. and Zavon, M.R. (1969) Validity of a critical blood level
for prevention of dieldrin intoxication. Arch. environm. Hlth., 19:
36-44
Kinoshita, P.K. and Kempf, C.K. (1970) Quantitative measurements of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Tox. Appl. Pharmacol., 17: 288
Klein, A.K., Link J.D. and Ives, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed aldrin
and dieldrin. J. Ass. Off. agr. Chem., 51: 895-898
Korte, F. and Arent, H. (1965) Metabolism of insecticides. IX (1)
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin - 14C. Life Sci., 4:
2017-2026
Korte, F. (1970) Terminal residues of cyclodiene insecticides. Comptes
Rendus, XXV Conference, IUPAC, Cortina d'Ampezzo, Italy, July 1969.
Appendix II: p. 172-180
Korte, F. and Porter, P.E (1970) Minutes of the Fifth Meeting of the
IUPAC Terminal Pesticide Residues, September 1970, Erbach, West
Germany. Appendixes 4 and 4a
Lee, D.F. (1968) Pesticide residues in foodstuffs in Great Britain.
IX. Aldrin, dieldrin and other organochlorine pesticides in potatoes
and carrots. J. Sci. Fd. Agric., 19: 701-705
Lehner, P.N. and Egbert, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature, 224: 1218-1219
Lichtenstein, E.P., Schulz, K.R., Fuhremann, T.W. and Liang T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. Agr. Fd. Chem., 18:
100-106
Liska, et al. (1967) Food Technol. 21: 435-438
Lofroth, G. (1970) Exposure of the general population to dieldrin.
Letter to editor and reply Robinson J. and Roberts M. Fd. Cosmet.
Toxicol., 8: 341-342
Lutz-Ostertag, Y. and Lutz, H. (1969) Embryologie expérimentale. Note
préliminaire sur les effets "oestrogènes" de l'"Aldrine" sur le
tractus urogénital de l'embryon d'Oiseau. C.R. Acad. Sci. (Paris) Ser.
D., 269: 484-486
Maier-Bode, H. (1967) The aldrin and dieldrin contents of German
edible carrots. Bull. environ. Contam. Toxicol., 2(1): 10-11
Matthews, H.B. and Matsumura, F. (1969) Metabolic fate of dieldrin in
the rat. J. Agr. Fd. Chem., 17: 845-852
McGill, A.E.J. and Robinson, J. (1968) Organochlorine insecticide
residues in complete prepared meals: a twelve-month survey in
southeast England. Fd. Cosmet. Toxicol., 6: 45-57
Mestres, R., Barthes, F. and Priu, M. (1967) DDT, aldrin and dieldrin
residue determinations in milk and butter. Bull. Environ. Contam.
Toxicol., 2(1): 25-33
Moubry, R.J. and Myrdal, G.E. (1970) private communication, Wisconsin
Department of Agriculture
Nakatsugawa, T., Ishida, M. and Dahm, P.A. (1965) Microsomal
epoxidation of cyclodiene insecticides. Biochem. Pharmacol., 14:
1853-1865
Onsager, J.A., Rusk, H.W. and Butler, L.I. (1970) Residues of aldrin,
dieldrin, chlordane and DDT in soil and sugarbeets. J. Econ. Entomol.,
63: 1143-1146
Parsons, A.M. and Moore, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem. Soc.
(C), 2026-2031
Polishuk, Z.W., Wasserman, M., Wasserman, D., Groner, Y., Luzarovici,
S. and Tomatis, L. (1970) Arch. environm. Hlth., 20: 215-217
Porter, R.D. and Wiemeyer, S.N. (1969) Dieldrin and DDT: Effects on
sparrow hawk eggshells and reproduction. Science, 165: 199-200
Potter, D., Wooder, M.F., Wright, A.S., Greenland, R.D. and
Zavon, M.R. (1970) The effects of ingestion of foreign compounds on
the sub-cellular structure and function of mammalian liver cells.
Paper given by A.S. Wright at Sittingbourne, 14 May 1970
Renvall, S. and Akerblom, M. (1968) Rester au organiska
klorpesticider, sörskilt aldrin och dieldrin, 2 nagra svenska
kalvöxter ochpotatis. Var. Föda, 20: 65-72
Reports of the Government Chemist, H.M.S.O., London (1965-1969)
Richardson, A. (1966) Ultra-violet conversion product of HEOD: Its
concentration in various samples received from the USA. Shell Research
Tunstall Report No. IRR TL/35/66
Richardson, A. (1968) Residues of dieldrin and its ultraviolet
decomposition product in selected samples. Shell Research Tunstall
Report No. TLGR 0014.63
Richardson, A., Baldwin, M. and Robinson, J. (1968) Identification of
metabolites of dieldrin (HEOD) in the faeces and urine of rats. J.
Sci. Fd. Agr., 19: 524-529
Robinson, et al. (1965-66) Shell Research Ltd., Tunstall, Report
PRRTL/12/65 and TU/23/66
Robinson, J., Richardson, A. and Bush, B. (1966b) A photoisomerization
product of dieldrin. Bull. environm. Contam. Toxicol., 1: 127-133
Robinson, J., Roberts, M., Baldwin, M. and Walker, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Fd. Cosmet. Toxicol.,
7: 317-332
Robinson, J. (1970a) Metabolites of dieldrin in the excreta of Rhesus
monkeys. Unpublished information submitted in a letter to H.G.S. van
Raalte
Robinson, J. (1970b) Metabolites of dieldrin in the excreta of man.
Unpublished information submitted in a letter to H.G.S. van Raalte
Roe, F.J.C. and Grant, G.A. (1970) Inhibition by germ-free status of
development of liver and lung tumours in mice exposed neonatally to
7,12-dimethyl-benz(a) anthracene: implications in relation to tests
for carcinogenicity. Int. J. Cancer, 6: 133-144
Rosen, J.D., Sutherland, D.J. and Lipton, G.R. (1966) The
photochemical isomerization of dieldrin and endrin and effects on
toxicity. Bull. environm. Contam. Toxicol., 1: 133-140
Ruzica, J.H.A., Simmond, J.H. and Tatton, J. O'G. (1967) Pesticide
residues in foodstuffs in Great Britain. IV. Organochlorine pesticide
residues in welfare foods. J. Sci. Fd. Agric., 18: 579-582
Rymer, T.E. and Hammence, J.H. (1967) J. Ass. Pub. Analysts 5: 24
Sakshaug, J. (1968) Residues of organochlorine insecticide in
Norwegian butter samples 1966 (in Norwegian). J. Nord. Vet.-Med.,
20: 696-701
Shell International Chemical Co. (1968) Residues in potatoes resulting
from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in vegetables
resulting from soil applications of aldrin, Monograph, November
Shell International Chemical Co. (1968) Residues in small grains
resulting from applications of aldrin, Monograph, November
Smith, Klosterman and Dogger. (1966) Ann. Proc. N. Dakota Acad. Sci.,
20: 96
Tannenbaum, A. and Silverstone, H. (1949a) The genesis and growth of
tumours. IV. Effects of varying the proportion of protein (casein) in
the diet. Cancer Res., 9: 162-173
Tannenbaum, A. and Silverstone, H. (1949b) The influences of the
degree of calorie restriction in the formation of skin tumours and
hepatomas in mice. Cancer Res., 9: 724-727
van Steyvoort, L. et al. (1968) Meded. Rijksfac. Landbouwwettensch XX,
the International Symposium, Ghent, May
Walker, K. et al. (1965) The fate of aldrin, dieldrin and endrin
residues daring the processing of sugar beets. United States
Department of Agriculture, ARS. Publication, ARS-33-107, p. 8
Walker, A.I.T., Stevenson, D.E., Robinson, J., Thorpe, E. and Roberts,
M. (1969a) The toxicology and pharmacodynamics of dieldrin (HEOD):
two-year oral exposures in rats and dogs. Toxicol. appl. Pharmacol.,
15: 345-373
Walker, A.I.T., Neill, C.H., Stevenson, D.E. and Robinson, J. (1969b)
The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix coturnix
japonica). Toxicol. appl. Pharmacol., 15: 69-73
Walker, A.I.T., Thorpe, E. and Stevenson D.E. (1970a) The toxicology
of dieldrin (HEOD): long-term oral toxicity experiments in mice.
Unpublished report from the Tunstall Laboratory, TU/13/70, Shell
Research Ltd., Sittingbourne. Proposed for publication in Fd. Cosmet.
Toxicol.
Walker, A.I.T., Thorpe, E. and Baldwin, M.K. (1970b) Toxicity studies
on the ultra-violet conversion product of dieldrin (UVCPD): thirteen
weeks oral experiment with rats. Shell Research Tunstall Report No.
TLGR. 0053.69
Walton, M.S., Beck, V. and Baron, R.L. (1970) Subacute toxicity of
photodieldrin 3,4,5,6,6,7-hexachloro-12-oxahexacyclo-tridecane, a
photodecomposition product of dieldrin. Tox. appl. Pharmacol., 17:
278.
Wasserman, M., Curnow, D.H., Forte, P.N. and Gurner Y. (1969) Storage
of organochlorine pesticides in the body fat of people in Western
Australia. Industr. Med. Surg., 37: 295-300
Wasserman, M., Coetzee, A.M., Wasserman, D. and Lazarovici, S. (1969)
Present state of the storage of the organochlorine insecticides in the
general population of South Africa. Paper given at the IUPAC
conference, Johannesburg
Williams, S., Mills, P.A. and McDowell, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five chlorinated
hydrocarbon pesticides. J. Ass. Off. Anal. Chem., 47: 1124-1128
Winnett, G. and Reed, J.P. (1968) Aldrin, dieldrin, endrin and
chlordane persistence - a three-year study. Pest. Mon. J., 2(3):
133-136
Winteringham, F.P.W. and Barnes, J.M. (1955) Comparative response of
insects and mammals to certain halogenated hydrocarbons used as
insecticides. Physiol. Rev., 35: 701-739
Wright, A.S., Potter, D., Wooder, M.F., Donninger, C., Le Pelley,
J.R., Potter, S.A., Ferrigan, L.W., Sykes, R., Dean, B., Pickering,
R.G., Walker, A.I.T., Crabtree, A.N. and Richardson, A. (1968) Liver
cell changes induced by the oral administration of dieldrin and
phenobarbitone to female dogs. Unpublished report from the Tunstall
Laboratory, Shell Research Ltd., Sittingbourne
Wright, A.S., Greenland R.D., Zavon, M.R. and Donninger, C. (1969)
Liver cell changes following the oral administration of dieldrin to
Rhesus monkeys for 5.5-6 years. Unpublished report from the Tunstall
Laboratories, Shell Research Ltd., Sittingbourne
Zavon, M.R. (1970) The effect of long-continued ingestion of dieldrin
on Rhesus monkeys. A six-year study. Unpublished report from the
Kettering Laboratory, University of Cincinnati, submitted by Shell
International Research.
