
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
DIELDRIN
IDENTITY
Synonyms
HEOD, Octalox (R)
Explanation
Dieldrin is a technical product containing 85 per cent of the chemical
known as HEOD of which the composition is as follows:
Chemical name
1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-exo-
1,4-endo-5,8-dimethanonaphthalene.
Formula
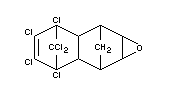 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-dieldrin was applied to growing cultures of Aspergilllus
and Penicillium species, no metabolites could be detected in the
culture medium or in the mycelium. Mosquito larvae (Aedes aegypti)
cultivated in an aqueous medium to which 14C.-dieldrin was added,
converted this compound to hydrophilic metabolites (Ludwig et al.,
1966).
A greater toxicity was found in rats kept on a low protein diet than
in rats on a high protein diet (Lee et al., 1964).
After stimulation of rat liver microsomal enzymes with phenobarbital,
the dieldrin content in fat was decreased in comparison with
non-stimulated rats (Cueto & Hayes, 1965).
In experiments with 14C-dieldrin it was demonstrated that dieldrin
in the blood is carried mainly in the erythrocyte contents and plasma,
not in the erythrocyte stroma, leucocytes or platelets. Haemoglobin is
largely but not entirely responsible for the intraerythrocytic
binding. In the plasma, dieldrin binds with soluble proteins (Moss &
Hathway, 1964).
In studies on the urine of individuals exposed to dieldrin, 2 neutral
polar metabolites of dieldrin were found by paper and column
chromatography. No dieldrin could be detected with this method, but
dieldrin or a material having the same retention time was found by gas
chromatography (Cueto Hayes, 1962).
In dogs fed 3 ppm of dieldrin 45.5 ppm were found in the fat, and in
dogs fed 1 ppm, 3.4 ppm were found in the fat. In other organs,
concentrations ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were
found (Borgmann et al., 1952).
14C-dieldrin is preferentially excreted in the bile with its
metabolites. Dieldrin and a hydrophilic metabolite were found in the
bile fifteen minutes after an intravenous injection (Mörsdorf et al.,
1963).
Studies with 36Cl-dieldrin have shown that the initial distribution
is general, but within a few hours of injection it concentrates more
in the fat. Excretion of 36Cl averages about 5 per cent per day and
is markedly increased by a restriction of diet, which reduces the body
fat. Excretion from the cannulated bile duct accelerates as the rat
loses weight, exceeding 10 per cent per day after a few days. Only 3
per cent of the 36Cl is excreted as dieldrin unless the bile is
cannulated, when up to 10 per cent may be excreted. The remainder of
36Cl, is found in the metabolites and these are excreted to 90 per
cent in the faeces and 10 per cent in the urine. The most important
metabolite, containing about 60 per cent of the total 36Cl, is
excreted in the bile (Heath & Vandekar, 1964).
Rats were fed 1 or 25 ppm dieldrin for 120 days. Two metabolites were
found in the urine, one of which was much more abundant in males than
in females. In the same study, small amounts of aldrin were found in
the urine of rats fed dieldrin (Datta et al., 1965).
Experiments with rats and rabbits showed that 14C-dieldrin given
intravenously was converted in 24 hours mainly into hydrophilic
metabolites. After 48 hours the presence of unconverted dieldrin and
the hydrophilic metabolites could be demonstrated in most organs and
tissues. A higher percentage of hydrophilic metabolites were found in
the kidney and liver. It was found that within one hour after
intravenous injection of 14C-dieldrin in rats, radioactive products
appeared in the bile. In 4 hours 13 per cent of the radioactivity was
excreted in the bile. Most of the radioactivity was found in
hydrophilic metabolites. After oral administration of 14C-dieldrin to
rabbits, six metabolites were isolated from the urine, all more
hydrophilic than the original compound. The main metabolite (86 per
cent of the total radioactivity in the urine) could be identified as
one of the two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The
oral LD50 of this compound in mice is 1250 mg/kg body-weight, and
the intravenous LD50 is 51 mg/kg body-weight (Ludwig et al., 1966).
After the feeding of dieldrin to animals it is stored in the adipose
tissues. Although small amounts of dieldrin are found in liver, kidney
and muscle tissues, the greatest amount of storage is in the fat,
where dieldrin is stored unchanged (Bann et al., 1956; Butcher et al.,
1957; Heath & Vandekar, 1964; Ivey et al., 1961; Lehmann, 1956; Street
et al., 1957). It is lost slowly from the body fat (Butcher et al.,
1957; Heath & Vandekar, 1964). It is stored in human fat in
significant amounts (Hunter et al., 1963).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 38 Borgmann at al., 1952
Rat (new-born) Intragastric 168 Lu et al., 1965
Rat (pre-weaning) Oral 25 Lu et al., 1965
Rat Oral 37-87 Borgmann et al., 1952
Gaines, 1960
Heath & Vandekar, 1964
Lehmann, 1951
Lu at al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann at al., 1952
Rabbit Oral 45-50 Borgmann et al., 1952
Dog Oral 56-80 Borgmann at al., 1952
Sheep Oral 50-75 Borgmann at al., 1952
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment groups of 10 male and 10 female rats were given, 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm of
technical dieldrin. The rats were killed after 2, 4, 6 and 8 months.
Food intake and growth were normal. No change in liver weight was
found. Cytoplasmic alteration of the liver cells was found at both
concentrations in males and females (Ortega et al., 1957).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and 10
male) were given dieldrin orally at dosage levels of 0.625, 1.25, 2.5,
5 and 10 mg/kg body-weight per day. Survival rates were affected at
all levels. At 2.5 mg/kg and above, all animals died (Borgmann et al.,
1952).
Dog. Dogs (1-4 animals per group) were given diets containing 1, 3,
10, 25 and 50 ppm dieldrin, six days per week. The animals on 25 and
50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm survived
for 9 months and on 1 and 3 ppm for 15 months. In the groups on 1 and
3 ppm the livers were significantly larger than those of the controls.
Histological changes were noted in brain, liver and kidney. Groups of
4 dogs (2 male and 2 female) were given 1 and 3 ppm of dieldrin in
their diet for 68 weeks. The concentration of 3 ppm increased the
liver/body-weight ratio and produced renal damage in 1 female. With 1
ppm of dieldrin, livers were enlarged but no histopathological changes
were found (Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of 0.2,
0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313 days. The
highest dosage killed all the dogs. Two of the 4 dogs died when given
0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day of
recrystallized dieldrin per kg of body-weight for one year; 3 of them
produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, with equal
numbers of each sex, were fed a diet containing 10 ppm of dieldrin for
their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in the
diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially
reduced the number of pregnancies but this effect tended to disappear
with continued feeding of the diet. All doses increased the mortality
among the suckling young. The effect on survival during suckling was
severe at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female) were
given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of 50
ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin in
the diet at a concentration of 75 ppm for 440 days. Twenty animals of
each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good condition
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight, ratio was markedly increased in the rats
killed during exposure, but was found to be normal in later
sacrifices. "Lesions of the hepatic parenchyma that have been
considered typical of exposure to the organochlorine insecticides in
rats" were observed only, in healthy animals killed during the
treatment. Rats dying spontaneously or killed after withdrawal of the
insecticide did not show such changes (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin in
their diets for a period of 40 months including 4 gestation periods.
At 25 ppm lambs died shortly after birth. Liver function as well as
other physiological tests on the ewes did not show any changes related
to the treatment with dieldrin (Shell, 1963).
Observations on man
In one study, a total of 13 men was divided into 4 groups receiving 0,
11, 57 and 211 µg dieldrin/man/day. No effects on body-weight, blood
cell counts, haemoglobin, total plasma protein, blood urea and serum
alkaline phosphatase, cholinesterase and transaminases were recorded
during the first 35 weeks. At the highest dose level, the average
concentration of dieldrin in the fat was 2.26 ppm. Ratios of fat
concentration at 35 weeks to fat concentration at 0 week ranged from
0.8-1.6 for men exposed to no dieldrin to 2.1-5.2 for those exposed to
11 µg/day; 2.5-6.0 for those exposed to 57 µg/day and 8.1-14.5 for
those exposed to 211 µg/day (Shell, 1966).
Several reports are now available on the storage of dieldrin in human
fat in the general population for several countries (Dale & Quinby,
1963; Dale et al., 1965; Egan et al., 1965; Hoffman et al., 1964;
Hunter et al., 1963; Robinson et al., 1965; Zavon, 1965). The average
concentration ranged between 0.03 and 0.30 ppm. It appears that in
those countries in which a higher DDT storage was recorded, this was
associated with a low dieldrin storage and vice-versa. In the United
States of America the amount of dieldrin stored in the fat increased
from 1950 to 1958 and then remained constant (USFDA Advisory
Committee, 1965). In the United Kingdom, from the above-mentioned
references, it appears that comparable figures were obtained in 1961
and 1965.
In one study, dieldrin was found in human milk at an average
concentration of 0.006 ppm (Egan et al., 1965).
Comments
The primary site of action of dieldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage.
In one long-term rat-feeding experiment, there was a general increase
in tumour production in the experimental animals at the lower dosage
levels as compared to the controls, but the liver was not particularly
affected. Liver tumours, however, were significantly increased at a
dose level of 10 ppm in one strain of mice susceptible to the
development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog. 1 ppm equivalent to 0.025 mg/kg/day produced liver changes.
Rat. 0.5 ppm in the diet equivalent to 0.025 mg/kg/day produced
minimal liver changes.
Estimate of acceptable dally intake for man
0-0.0001 mg/kg/day*
* Sum of aldrin and dieldrin by weight.
Further work required
Elucidation of the significance of the finding that dieldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Aldrin and dieldrin are used for soil treatment against various soil
insects (1.5-5 kg/ha), for seed treatment on grains, sugar beets,
beans (0.5-2.5 g/kg seed), leeks and onions (37-50 g/kg seed for
bulb- or root-dip), and for foliar application on various agricultural
crops, fruits, nursery stocks and ornamental plants.
The use pattern has recently changed considerably due to restrictions
in many countries. In the United States of America and Canada, aldrin
and dieldrin may not be used on any crop that is likely to be fed to
animals; the use in soil for sugar beets has been withdrawn, whereas
the use in soil where other root crops are grown in rotation is under
review. In Canada, the registration of aldrin and dieldrin in
fertilizer mixtures has been withdrawn. Soil and seed treatment is
recommended only for use in grain production. In Britain, the use for
treatment of cereal seeds sown in the spring and the use in fertilizer
mixtures has been withdrawn. In Germany, the prohibition of uses on
agricultural crops is being considered. In Austria, uses for
vegetables are forbidden. In the Netherlands, Belgium and Luxembourg,
aldrin and dieldrin may not be used on carrots and carrots may not be
grown in treated soil in the year of application. Furthermore, to
prevent edible crops obtaining too high residues, the soil used in
rotation for growing food crops, such as lettuce under glass, may not
be treated. In Belgium, seed treatment of grain-seed and pulses is
forbidden in order to avoid poisoning of seed-eating birds, and
subsequently of predatory birds and mammals.
(b) Post-harvest treatments
The use of dieldrin in food storage practice for treatment of empty
warehouses, etc., is decreasing, and is prohibited in many countries
(e.g. the Netherlands, Belgium). In Britain, there is a limited use
under strict precautions. Aldrin and dieldrin are not used for direct
treatment of stored commodities or for treatment of containers, etc.,
which may come into contact with foodstuffs.
(c) Other uses
The use of aldrin and dieldrin in sheep dips or cattle sprays is no
longer allowed in many countries (e.g. the United States of America,
Canada, Britain, Australia, New Zealand, the Netherlands and Belgium).
Dieldrin is used in many sub-tropical and tropical regions for the
control of mosquito larvae, adult mosquitos, chiggers, fleas, ticks,
reduviid bugs, tsetse-flies and other disease vectors. These
applications usually are carried out under supervision of trained
personnel of public health agencies or pest control operators.
Aldrin and dieldrin are used in various countries for termite control
in basements of buildings during construction and for spot treatment
against other domestic pests. In the Netherlands and Belgium, the use
for household purposes is no longer allowed. Dieldrin as a 0.05 per
cent solution is used for wood preservation to prevent attack by
wood-boring insects. In many countries, it is also used for
mothproofing of wool at a dosage of about 0.05 per cent dieldrin on
the weight of wool during the dyeing operation.
(d) Tolerances
The many countries and the great variety of crops in question make it
impossible to present a complete survey of all tolerances, established
or considered. The following table, therefore, is only intended to
serve as a rough guide to current levels. (The figures usually refer
to totals of aldrin plus dieldrin).
Residues resulting from supervised trials
(a) In crops grown in treated soils
Various factors influence the residues remaining in soil and in or on
the crop at harvest. Retention in soil has been greatest when the
organic matter has been high (Bowman, Schechter & Carter, 1965). Low
moisture content (Harris, 1964; Lichtenstein & Schulz, 1961) and dense
coverage of crops (Harris & Lichtenstein, 1961; Lichtenstein et al.,
1962) have also been found to favour retention. Water leaching through
the soil does not appear to play an important role in the decrease of
the residue (Lichtenstein, 1958).
After two yearly applications to soil of 2 kg/ha of aldrin and
dieldrin, Lichtenstein & Schulz (1965) found residues in carrots and
radishes in excess of 0.1 ppm, whereas in potatoes 0.1 ppm was
reached. Continued soil treatment has eventually led to residues in
potatoes, turnips, beet roots and chicory roots in excess of
tolerances (0.1-0.55 ppm) in the Netherlands (unpublished information
from Netherlands Government).
Country Food Tolerance
(parts per million)
United States of America fruits, vegetables 0.1-0.25
potatoes 0.1
animal feed 0
Canada fruits, vegetables 0.1-0.25
potatoes 0.1
Netherlands fruits, vegetables 0.1
Sweden fruits, vegetables 0.1
potatoes
Switzerland potatoes 0.1
Germany (Federal) all edible crops Limit of sensitivity of
analytical method
(under consideration)
Germany (DDR) all edible crops 0
(under consideration)
Translocation of aldrin and dieldrin from the soil to the aerial parts
of the crop has been demonstrated by several investigators, although
in the case of cereal crops, Morley & Chiba (1965) and Saha & McDonald
(1966) found no detectable amounts in the grains, residues were found
in the leaves.
(b) After direct application to growing crops
In several experiments carried out in the United States of America on
various fruits and vegetables at practical dosage levels, the residues
in the edible part of the plant varied from <0.01-0.2 ppm when the
recommended safety interval was observed (unpublished information from
Shell Company).
(c) In meat and meat products
Various workers have measured residues in the bodies of cattle dipped
in or sprayed with dieldrin, or receiving the insecticides in their
diet. For example, Ivey, Claborn & Mann (1961) measured residues in
various body tissues of animals which had received aldrin. The
residues have been highest in the fat; Egan (1965), for example, found
up to about 9 ppm in the fat of sheep slaughtered 4 1/2 weeks after an
experimental dipping.
Residues in raw food moving in commerce
In 1965, analyses were carried out in the Netherlands on glasshouse
lettuce grown in soil treated with aldrin (2.5 kg/ha) against
cutworms. A total of 105 samples from lettuces going on to the market
was examined, Seventy-eight per cent of the examinations showed a
residue of less than the tolerance (0.1 ppm), 11 samples contained
0.1-0.2 ppm, 7 samples contained 0.2-0.3 ppm, 3 samples contained
0.3-0.4 ppm, and 2 samples contained 0.4-0.55 ppm (unpublished
communication).
Residues at time of consumption
Although losses or residues are likely to occur from some foods
through mechanical means (cleaning, trimming, etc.), substantial
losses during cooking are not to be expected. In recent total diet
studies in the United States of America, where, during the months of
June, August, October and December 1965, and February and April 1966,
a total of 317 samples of total diet was analysed for the presence of
aldrin and dieldrin residues, aldrin was found in 13 samples at levels
varying between traces to 0.07 ppm; whereas in 76 samples, dieldrin
was found at levels varying between traces to 0.20 ppm (unpublished
communication from the United States Food and Drug Administration).
Analyses carried out in Britain during the years 1962-1965 showed
dieldrin residues in home-produced butter of 0.03-0.07 ppm, and in
home-produced milk of 0.002-0.003 ppm (Lewis, 1963, 1964, 1965).
Studies have been made in various countries of the dieldrin content of
human fat. In measurements undertaken between 1962 and 1965 in
Britain, the average residues were of the order of 0.25 ppm (Egan et
al., 1965). In a similar investigation carried out in the Netherlands
since 1963, the average residue in 15 samples of human fat was 0.15
ppm, with a range of 0.06 to 30 ppm (direct communication). However,
although it is likely that these residues mainly arise from food, the
extent to which other routes have been responsible, such as the
inhalation of contaminated atmosphere (Abbott et al., 1965, 1966) or
the wearing of treated clothing, has not been clearly demonstrated.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of aldrin and dieldrin, together with
residues of a number of other compounds. An example is the AOAC system
(1966) in which acetonitrile partition and Florisil column clean-up
are employed, and the residues are identified and measured by gas
chromatography coupled with thin layer or paper chromatography.
Alternative clean-up systems, e.g., that of de Faubert Maunder et al.
(1965) using dimethylformamide, and other methods of confirmation of
identity, using infra-red spectrophotometry, are also available. The
methods are sensitive to about 0.002 ppm of aldrin or dieldrin in milk
and 0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATION FOR TOLERANCE
In view of the exceptionally low acceptable daily intake of aldrin and
dieldrin, and the occurrence of unintentional residues, the meeting
came to the conclusion that no allowance could be made for a finite
tolerance figure as a result of agricultural use.
Total diet studies have revealed the presence of dieldrin in the human
food as consumed. Furthermore, aldrin and dieldrin have been shown to
persist for long periods in the soil after soil treatment, and to
occur quite widely in human fat, in aquatic and terrestrial wild life,
and in the abiotic environment. Consequently, it is recommended that
the use of these compounds should be reduced, and, as far as possible,
restricted to those usages which cannot result in residues in food or
in the biotic and abiotic environment.
Although various agricultural uses of aldrin and dieldrin have already
been restricted or prohibited in many countries, some foods moving in
international trade may continue to contain residues (e.g., resulting
from earlier soil treatments). It is, therefore, suggested that a
"practical residue limit" be established for such foods on the
following basis: vegetables, 0.05 ppm; fat in products of animal
origin (excluding milk), 0.2 ppm, or in whole milk, 0.003 ppm. The
above figures should be kept under review.
Further Information
Further information is required on the results of total diet studies
carried out in different countries.
More information is needed on the residence occurring in crops grown
in soils which were treated in previous years and on the possible
occurrence of residues in cereal crops grown in treated soil or from
treated seeds under conditions of extensive monoculture (i.e., no
other crops grown in rotation) such as prevail in certain countries.
Attention is also needed to residues present in products used for
animal feed which are moving in international trade.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. F. (1956) J. Agr.
Food Chem., 4, 937
Borgmann, A. R., Kitselman, C. H., Dahm, P. A., & Pankaskie, J. E.
(1952) Unpublished report
Butcher, J. E., Street. J. C., Shupe, J. L. & Biddulph, C. (1957)
Unpublished report, Agricultural Experimental Station, Utah State
Agricultural College
Cueto, C., jr & Hayes, W. J., jr (1962) J. Agr. Food Chem., 10, .366
Cueto, C., jr & Hayes, W. J., jr (1965) Toxicol. Appl. Pharmacol.,
7, 481
Dale, W. E. & Quinby, G. E. (1963) Science, 142, 593
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol.,
4, 187
Egan, H., Goulding, R., Roburn, J. & Tatton, J. O'G. (1965) Brit.
med. J., 2, 66
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Unpublished
report submitted by Shell Company
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lee, M., Harris, K. & Trowbridge, H. (1964) J. Nutr., 84, 136
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 9, 591
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plan Protection Conference, Budapest
Mörsdorf, K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. exp.,
8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J., jr. & Durham, W.F. (1957) Arch. Path.,
64, 614
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Shell Chemical Company (1963) Unpublished report
Shell Chemical Company (1966) Unpublished report
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
United States Food and Drug Administration (1965) Unpublished report
of Advisory Committee
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1965)
Organochlorine pesticides in the atmospheric environment Nature 208:
1317
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1966)
Organochlorine pesticides in the atmosphere. Nature, 211 : 259
A.O.A.C., (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49 : 222-30
Bowman, M. C., M. S. Schechter and R. L. Carter, (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types.
J. Agr. and Food Chem. 13 (4): 360-365
Burrage R. H. and J. G. Saha. (1966) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin and heptachlor,
In press. Can. J. Plant Sci.
Egan, H., R. Goulding, J. Roburn, J. O'G. Tatton. (1965)
Organochlorine pesticide residues in human fat and human milk. Brit.
Med. J., 2 : 66
Egan, H., (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Fd. Agric., 16;
489-498
de Faubert Maunder, M. J., H. Egan, E. W. Godly, E. W. Hammond, J.
Roburn and J. Thomson. (1965) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. 89:
168-174
Harris, C. R. (1964) Influence or soil moisture of the toxicity of
insecticidal in a mineral soils to insects, J. Econ. Ent., 57 :
946-950
Harris, C. R, and E. P. Lichtenstein. (1961) Factors affecting the
volatization of insecticidal residues from soils. J. Econ. Ent., 54
: 1038-1045
Ivey, M. C., H. K. Claborn and H. D. Mann. (1961) Aldrin and dieldrin
content of body tissues of live stock receiving aldrin in their diet
J. Agric. Fd. Chem., 9 : 374-6
Lewis, D. T. (1963, 1964, & 1965) Report of the Government Chemist.
H.M.S.O. London
Lichtenstein, E. P. (1958) Movement of Insecticides in soil. J. Econ,
Ent., 51 : 380-383
Lichtenstein, E. P. (1962) Vertical distribution and persistence of
insecticidal residues in soil as influenced by mode of application and
cover crop. J. Econ. Ent., 55 : 215-219
Lichtenstein, E. P. and K. R. Schulz. (1965) Residues of aldrin and
dieldrin and their translocation into various crops, J. Agr. Food
Chem., 13 : 57-63
Morley, H. V., and M. Chiba. (1965) Dieldrin uptake from soil by wheat
plants. Can. J. Plant Sci., 45 : 209-210
Saha, J. G. and H. McDonald. (1966) Insecticide Residues in wheat
grown in soil treated with aldrin and endrin. (For publication in J.
Agr. and Food Chem.)
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-dieldrin was applied to growing cultures of Aspergilllus
and Penicillium species, no metabolites could be detected in the
culture medium or in the mycelium. Mosquito larvae (Aedes aegypti)
cultivated in an aqueous medium to which 14C.-dieldrin was added,
converted this compound to hydrophilic metabolites (Ludwig et al.,
1966).
A greater toxicity was found in rats kept on a low protein diet than
in rats on a high protein diet (Lee et al., 1964).
After stimulation of rat liver microsomal enzymes with phenobarbital,
the dieldrin content in fat was decreased in comparison with
non-stimulated rats (Cueto & Hayes, 1965).
In experiments with 14C-dieldrin it was demonstrated that dieldrin
in the blood is carried mainly in the erythrocyte contents and plasma,
not in the erythrocyte stroma, leucocytes or platelets. Haemoglobin is
largely but not entirely responsible for the intraerythrocytic
binding. In the plasma, dieldrin binds with soluble proteins (Moss &
Hathway, 1964).
In studies on the urine of individuals exposed to dieldrin, 2 neutral
polar metabolites of dieldrin were found by paper and column
chromatography. No dieldrin could be detected with this method, but
dieldrin or a material having the same retention time was found by gas
chromatography (Cueto Hayes, 1962).
In dogs fed 3 ppm of dieldrin 45.5 ppm were found in the fat, and in
dogs fed 1 ppm, 3.4 ppm were found in the fat. In other organs,
concentrations ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were
found (Borgmann et al., 1952).
14C-dieldrin is preferentially excreted in the bile with its
metabolites. Dieldrin and a hydrophilic metabolite were found in the
bile fifteen minutes after an intravenous injection (Mörsdorf et al.,
1963).
Studies with 36Cl-dieldrin have shown that the initial distribution
is general, but within a few hours of injection it concentrates more
in the fat. Excretion of 36Cl averages about 5 per cent per day and
is markedly increased by a restriction of diet, which reduces the body
fat. Excretion from the cannulated bile duct accelerates as the rat
loses weight, exceeding 10 per cent per day after a few days. Only 3
per cent of the 36Cl is excreted as dieldrin unless the bile is
cannulated, when up to 10 per cent may be excreted. The remainder of
36Cl, is found in the metabolites and these are excreted to 90 per
cent in the faeces and 10 per cent in the urine. The most important
metabolite, containing about 60 per cent of the total 36Cl, is
excreted in the bile (Heath & Vandekar, 1964).
Rats were fed 1 or 25 ppm dieldrin for 120 days. Two metabolites were
found in the urine, one of which was much more abundant in males than
in females. In the same study, small amounts of aldrin were found in
the urine of rats fed dieldrin (Datta et al., 1965).
Experiments with rats and rabbits showed that 14C-dieldrin given
intravenously was converted in 24 hours mainly into hydrophilic
metabolites. After 48 hours the presence of unconverted dieldrin and
the hydrophilic metabolites could be demonstrated in most organs and
tissues. A higher percentage of hydrophilic metabolites were found in
the kidney and liver. It was found that within one hour after
intravenous injection of 14C-dieldrin in rats, radioactive products
appeared in the bile. In 4 hours 13 per cent of the radioactivity was
excreted in the bile. Most of the radioactivity was found in
hydrophilic metabolites. After oral administration of 14C-dieldrin to
rabbits, six metabolites were isolated from the urine, all more
hydrophilic than the original compound. The main metabolite (86 per
cent of the total radioactivity in the urine) could be identified as
one of the two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The
oral LD50 of this compound in mice is 1250 mg/kg body-weight, and
the intravenous LD50 is 51 mg/kg body-weight (Ludwig et al., 1966).
After the feeding of dieldrin to animals it is stored in the adipose
tissues. Although small amounts of dieldrin are found in liver, kidney
and muscle tissues, the greatest amount of storage is in the fat,
where dieldrin is stored unchanged (Bann et al., 1956; Butcher et al.,
1957; Heath & Vandekar, 1964; Ivey et al., 1961; Lehmann, 1956; Street
et al., 1957). It is lost slowly from the body fat (Butcher et al.,
1957; Heath & Vandekar, 1964). It is stored in human fat in
significant amounts (Hunter et al., 1963).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 38 Borgmann at al., 1952
Rat (new-born) Intragastric 168 Lu et al., 1965
Rat (pre-weaning) Oral 25 Lu et al., 1965
Rat Oral 37-87 Borgmann et al., 1952
Gaines, 1960
Heath & Vandekar, 1964
Lehmann, 1951
Lu at al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann at al., 1952
Rabbit Oral 45-50 Borgmann et al., 1952
Dog Oral 56-80 Borgmann at al., 1952
Sheep Oral 50-75 Borgmann at al., 1952
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment groups of 10 male and 10 female rats were given, 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm of
technical dieldrin. The rats were killed after 2, 4, 6 and 8 months.
Food intake and growth were normal. No change in liver weight was
found. Cytoplasmic alteration of the liver cells was found at both
concentrations in males and females (Ortega et al., 1957).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and 10
male) were given dieldrin orally at dosage levels of 0.625, 1.25, 2.5,
5 and 10 mg/kg body-weight per day. Survival rates were affected at
all levels. At 2.5 mg/kg and above, all animals died (Borgmann et al.,
1952).
Dog. Dogs (1-4 animals per group) were given diets containing 1, 3,
10, 25 and 50 ppm dieldrin, six days per week. The animals on 25 and
50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm survived
for 9 months and on 1 and 3 ppm for 15 months. In the groups on 1 and
3 ppm the livers were significantly larger than those of the controls.
Histological changes were noted in brain, liver and kidney. Groups of
4 dogs (2 male and 2 female) were given 1 and 3 ppm of dieldrin in
their diet for 68 weeks. The concentration of 3 ppm increased the
liver/body-weight ratio and produced renal damage in 1 female. With 1
ppm of dieldrin, livers were enlarged but no histopathological changes
were found (Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of 0.2,
0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313 days. The
highest dosage killed all the dogs. Two of the 4 dogs died when given
0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day of
recrystallized dieldrin per kg of body-weight for one year; 3 of them
produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, with equal
numbers of each sex, were fed a diet containing 10 ppm of dieldrin for
their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in the
diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially
reduced the number of pregnancies but this effect tended to disappear
with continued feeding of the diet. All doses increased the mortality
among the suckling young. The effect on survival during suckling was
severe at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female) were
given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of 50
ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin in
the diet at a concentration of 75 ppm for 440 days. Twenty animals of
each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good condition
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight, ratio was markedly increased in the rats
killed during exposure, but was found to be normal in later
sacrifices. "Lesions of the hepatic parenchyma that have been
considered typical of exposure to the organochlorine insecticides in
rats" were observed only, in healthy animals killed during the
treatment. Rats dying spontaneously or killed after withdrawal of the
insecticide did not show such changes (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin in
their diets for a period of 40 months including 4 gestation periods.
At 25 ppm lambs died shortly after birth. Liver function as well as
other physiological tests on the ewes did not show any changes related
to the treatment with dieldrin (Shell, 1963).
Observations on man
In one study, a total of 13 men was divided into 4 groups receiving 0,
11, 57 and 211 µg dieldrin/man/day. No effects on body-weight, blood
cell counts, haemoglobin, total plasma protein, blood urea and serum
alkaline phosphatase, cholinesterase and transaminases were recorded
during the first 35 weeks. At the highest dose level, the average
concentration of dieldrin in the fat was 2.26 ppm. Ratios of fat
concentration at 35 weeks to fat concentration at 0 week ranged from
0.8-1.6 for men exposed to no dieldrin to 2.1-5.2 for those exposed to
11 µg/day; 2.5-6.0 for those exposed to 57 µg/day and 8.1-14.5 for
those exposed to 211 µg/day (Shell, 1966).
Several reports are now available on the storage of dieldrin in human
fat in the general population for several countries (Dale & Quinby,
1963; Dale et al., 1965; Egan et al., 1965; Hoffman et al., 1964;
Hunter et al., 1963; Robinson et al., 1965; Zavon, 1965). The average
concentration ranged between 0.03 and 0.30 ppm. It appears that in
those countries in which a higher DDT storage was recorded, this was
associated with a low dieldrin storage and vice-versa. In the United
States of America the amount of dieldrin stored in the fat increased
from 1950 to 1958 and then remained constant (USFDA Advisory
Committee, 1965). In the United Kingdom, from the above-mentioned
references, it appears that comparable figures were obtained in 1961
and 1965.
In one study, dieldrin was found in human milk at an average
concentration of 0.006 ppm (Egan et al., 1965).
Comments
The primary site of action of dieldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage.
In one long-term rat-feeding experiment, there was a general increase
in tumour production in the experimental animals at the lower dosage
levels as compared to the controls, but the liver was not particularly
affected. Liver tumours, however, were significantly increased at a
dose level of 10 ppm in one strain of mice susceptible to the
development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog. 1 ppm equivalent to 0.025 mg/kg/day produced liver changes.
Rat. 0.5 ppm in the diet equivalent to 0.025 mg/kg/day produced
minimal liver changes.
Estimate of acceptable dally intake for man
0-0.0001 mg/kg/day*
* Sum of aldrin and dieldrin by weight.
Further work required
Elucidation of the significance of the finding that dieldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Aldrin and dieldrin are used for soil treatment against various soil
insects (1.5-5 kg/ha), for seed treatment on grains, sugar beets,
beans (0.5-2.5 g/kg seed), leeks and onions (37-50 g/kg seed for
bulb- or root-dip), and for foliar application on various agricultural
crops, fruits, nursery stocks and ornamental plants.
The use pattern has recently changed considerably due to restrictions
in many countries. In the United States of America and Canada, aldrin
and dieldrin may not be used on any crop that is likely to be fed to
animals; the use in soil for sugar beets has been withdrawn, whereas
the use in soil where other root crops are grown in rotation is under
review. In Canada, the registration of aldrin and dieldrin in
fertilizer mixtures has been withdrawn. Soil and seed treatment is
recommended only for use in grain production. In Britain, the use for
treatment of cereal seeds sown in the spring and the use in fertilizer
mixtures has been withdrawn. In Germany, the prohibition of uses on
agricultural crops is being considered. In Austria, uses for
vegetables are forbidden. In the Netherlands, Belgium and Luxembourg,
aldrin and dieldrin may not be used on carrots and carrots may not be
grown in treated soil in the year of application. Furthermore, to
prevent edible crops obtaining too high residues, the soil used in
rotation for growing food crops, such as lettuce under glass, may not
be treated. In Belgium, seed treatment of grain-seed and pulses is
forbidden in order to avoid poisoning of seed-eating birds, and
subsequently of predatory birds and mammals.
(b) Post-harvest treatments
The use of dieldrin in food storage practice for treatment of empty
warehouses, etc., is decreasing, and is prohibited in many countries
(e.g. the Netherlands, Belgium). In Britain, there is a limited use
under strict precautions. Aldrin and dieldrin are not used for direct
treatment of stored commodities or for treatment of containers, etc.,
which may come into contact with foodstuffs.
(c) Other uses
The use of aldrin and dieldrin in sheep dips or cattle sprays is no
longer allowed in many countries (e.g. the United States of America,
Canada, Britain, Australia, New Zealand, the Netherlands and Belgium).
Dieldrin is used in many sub-tropical and tropical regions for the
control of mosquito larvae, adult mosquitos, chiggers, fleas, ticks,
reduviid bugs, tsetse-flies and other disease vectors. These
applications usually are carried out under supervision of trained
personnel of public health agencies or pest control operators.
Aldrin and dieldrin are used in various countries for termite control
in basements of buildings during construction and for spot treatment
against other domestic pests. In the Netherlands and Belgium, the use
for household purposes is no longer allowed. Dieldrin as a 0.05 per
cent solution is used for wood preservation to prevent attack by
wood-boring insects. In many countries, it is also used for
mothproofing of wool at a dosage of about 0.05 per cent dieldrin on
the weight of wool during the dyeing operation.
(d) Tolerances
The many countries and the great variety of crops in question make it
impossible to present a complete survey of all tolerances, established
or considered. The following table, therefore, is only intended to
serve as a rough guide to current levels. (The figures usually refer
to totals of aldrin plus dieldrin).
Residues resulting from supervised trials
(a) In crops grown in treated soils
Various factors influence the residues remaining in soil and in or on
the crop at harvest. Retention in soil has been greatest when the
organic matter has been high (Bowman, Schechter & Carter, 1965). Low
moisture content (Harris, 1964; Lichtenstein & Schulz, 1961) and dense
coverage of crops (Harris & Lichtenstein, 1961; Lichtenstein et al.,
1962) have also been found to favour retention. Water leaching through
the soil does not appear to play an important role in the decrease of
the residue (Lichtenstein, 1958).
After two yearly applications to soil of 2 kg/ha of aldrin and
dieldrin, Lichtenstein & Schulz (1965) found residues in carrots and
radishes in excess of 0.1 ppm, whereas in potatoes 0.1 ppm was
reached. Continued soil treatment has eventually led to residues in
potatoes, turnips, beet roots and chicory roots in excess of
tolerances (0.1-0.55 ppm) in the Netherlands (unpublished information
from Netherlands Government).
Country Food Tolerance
(parts per million)
United States of America fruits, vegetables 0.1-0.25
potatoes 0.1
animal feed 0
Canada fruits, vegetables 0.1-0.25
potatoes 0.1
Netherlands fruits, vegetables 0.1
Sweden fruits, vegetables 0.1
potatoes
Switzerland potatoes 0.1
Germany (Federal) all edible crops Limit of sensitivity of
analytical method
(under consideration)
Germany (DDR) all edible crops 0
(under consideration)
Translocation of aldrin and dieldrin from the soil to the aerial parts
of the crop has been demonstrated by several investigators, although
in the case of cereal crops, Morley & Chiba (1965) and Saha & McDonald
(1966) found no detectable amounts in the grains, residues were found
in the leaves.
(b) After direct application to growing crops
In several experiments carried out in the United States of America on
various fruits and vegetables at practical dosage levels, the residues
in the edible part of the plant varied from <0.01-0.2 ppm when the
recommended safety interval was observed (unpublished information from
Shell Company).
(c) In meat and meat products
Various workers have measured residues in the bodies of cattle dipped
in or sprayed with dieldrin, or receiving the insecticides in their
diet. For example, Ivey, Claborn & Mann (1961) measured residues in
various body tissues of animals which had received aldrin. The
residues have been highest in the fat; Egan (1965), for example, found
up to about 9 ppm in the fat of sheep slaughtered 4 1/2 weeks after an
experimental dipping.
Residues in raw food moving in commerce
In 1965, analyses were carried out in the Netherlands on glasshouse
lettuce grown in soil treated with aldrin (2.5 kg/ha) against
cutworms. A total of 105 samples from lettuces going on to the market
was examined, Seventy-eight per cent of the examinations showed a
residue of less than the tolerance (0.1 ppm), 11 samples contained
0.1-0.2 ppm, 7 samples contained 0.2-0.3 ppm, 3 samples contained
0.3-0.4 ppm, and 2 samples contained 0.4-0.55 ppm (unpublished
communication).
Residues at time of consumption
Although losses or residues are likely to occur from some foods
through mechanical means (cleaning, trimming, etc.), substantial
losses during cooking are not to be expected. In recent total diet
studies in the United States of America, where, during the months of
June, August, October and December 1965, and February and April 1966,
a total of 317 samples of total diet was analysed for the presence of
aldrin and dieldrin residues, aldrin was found in 13 samples at levels
varying between traces to 0.07 ppm; whereas in 76 samples, dieldrin
was found at levels varying between traces to 0.20 ppm (unpublished
communication from the United States Food and Drug Administration).
Analyses carried out in Britain during the years 1962-1965 showed
dieldrin residues in home-produced butter of 0.03-0.07 ppm, and in
home-produced milk of 0.002-0.003 ppm (Lewis, 1963, 1964, 1965).
Studies have been made in various countries of the dieldrin content of
human fat. In measurements undertaken between 1962 and 1965 in
Britain, the average residues were of the order of 0.25 ppm (Egan et
al., 1965). In a similar investigation carried out in the Netherlands
since 1963, the average residue in 15 samples of human fat was 0.15
ppm, with a range of 0.06 to 30 ppm (direct communication). However,
although it is likely that these residues mainly arise from food, the
extent to which other routes have been responsible, such as the
inhalation of contaminated atmosphere (Abbott et al., 1965, 1966) or
the wearing of treated clothing, has not been clearly demonstrated.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of aldrin and dieldrin, together with
residues of a number of other compounds. An example is the AOAC system
(1966) in which acetonitrile partition and Florisil column clean-up
are employed, and the residues are identified and measured by gas
chromatography coupled with thin layer or paper chromatography.
Alternative clean-up systems, e.g., that of de Faubert Maunder et al.
(1965) using dimethylformamide, and other methods of confirmation of
identity, using infra-red spectrophotometry, are also available. The
methods are sensitive to about 0.002 ppm of aldrin or dieldrin in milk
and 0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATION FOR TOLERANCE
In view of the exceptionally low acceptable daily intake of aldrin and
dieldrin, and the occurrence of unintentional residues, the meeting
came to the conclusion that no allowance could be made for a finite
tolerance figure as a result of agricultural use.
Total diet studies have revealed the presence of dieldrin in the human
food as consumed. Furthermore, aldrin and dieldrin have been shown to
persist for long periods in the soil after soil treatment, and to
occur quite widely in human fat, in aquatic and terrestrial wild life,
and in the abiotic environment. Consequently, it is recommended that
the use of these compounds should be reduced, and, as far as possible,
restricted to those usages which cannot result in residues in food or
in the biotic and abiotic environment.
Although various agricultural uses of aldrin and dieldrin have already
been restricted or prohibited in many countries, some foods moving in
international trade may continue to contain residues (e.g., resulting
from earlier soil treatments). It is, therefore, suggested that a
"practical residue limit" be established for such foods on the
following basis: vegetables, 0.05 ppm; fat in products of animal
origin (excluding milk), 0.2 ppm, or in whole milk, 0.003 ppm. The
above figures should be kept under review.
Further Information
Further information is required on the results of total diet studies
carried out in different countries.
More information is needed on the residence occurring in crops grown
in soils which were treated in previous years and on the possible
occurrence of residues in cereal crops grown in treated soil or from
treated seeds under conditions of extensive monoculture (i.e., no
other crops grown in rotation) such as prevail in certain countries.
Attention is also needed to residues present in products used for
animal feed which are moving in international trade.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. F. (1956) J. Agr.
Food Chem., 4, 937
Borgmann, A. R., Kitselman, C. H., Dahm, P. A., & Pankaskie, J. E.
(1952) Unpublished report
Butcher, J. E., Street. J. C., Shupe, J. L. & Biddulph, C. (1957)
Unpublished report, Agricultural Experimental Station, Utah State
Agricultural College
Cueto, C., jr & Hayes, W. J., jr (1962) J. Agr. Food Chem., 10, .366
Cueto, C., jr & Hayes, W. J., jr (1965) Toxicol. Appl. Pharmacol.,
7, 481
Dale, W. E. & Quinby, G. E. (1963) Science, 142, 593
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol.,
4, 187
Egan, H., Goulding, R., Roburn, J. & Tatton, J. O'G. (1965) Brit.
med. J., 2, 66
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Unpublished
report submitted by Shell Company
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lee, M., Harris, K. & Trowbridge, H. (1964) J. Nutr., 84, 136
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 9, 591
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plan Protection Conference, Budapest
Mörsdorf, K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. exp.,
8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J., jr. & Durham, W.F. (1957) Arch. Path.,
64, 614
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Shell Chemical Company (1963) Unpublished report
Shell Chemical Company (1966) Unpublished report
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
United States Food and Drug Administration (1965) Unpublished report
of Advisory Committee
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1965)
Organochlorine pesticides in the atmospheric environment Nature 208:
1317
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1966)
Organochlorine pesticides in the atmosphere. Nature, 211 : 259
A.O.A.C., (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49 : 222-30
Bowman, M. C., M. S. Schechter and R. L. Carter, (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types.
J. Agr. and Food Chem. 13 (4): 360-365
Burrage R. H. and J. G. Saha. (1966) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin and heptachlor,
In press. Can. J. Plant Sci.
Egan, H., R. Goulding, J. Roburn, J. O'G. Tatton. (1965)
Organochlorine pesticide residues in human fat and human milk. Brit.
Med. J., 2 : 66
Egan, H., (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Fd. Agric., 16;
489-498
de Faubert Maunder, M. J., H. Egan, E. W. Godly, E. W. Hammond, J.
Roburn and J. Thomson. (1965) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. 89:
168-174
Harris, C. R. (1964) Influence or soil moisture of the toxicity of
insecticidal in a mineral soils to insects, J. Econ. Ent., 57 :
946-950
Harris, C. R, and E. P. Lichtenstein. (1961) Factors affecting the
volatization of insecticidal residues from soils. J. Econ. Ent., 54
: 1038-1045
Ivey, M. C., H. K. Claborn and H. D. Mann. (1961) Aldrin and dieldrin
content of body tissues of live stock receiving aldrin in their diet
J. Agric. Fd. Chem., 9 : 374-6
Lewis, D. T. (1963, 1964, & 1965) Report of the Government Chemist.
H.M.S.O. London
Lichtenstein, E. P. (1958) Movement of Insecticides in soil. J. Econ,
Ent., 51 : 380-383
Lichtenstein, E. P. (1962) Vertical distribution and persistence of
insecticidal residues in soil as influenced by mode of application and
cover crop. J. Econ. Ent., 55 : 215-219
Lichtenstein, E. P. and K. R. Schulz. (1965) Residues of aldrin and
dieldrin and their translocation into various crops, J. Agr. Food
Chem., 13 : 57-63
Morley, H. V., and M. Chiba. (1965) Dieldrin uptake from soil by wheat
plants. Can. J. Plant Sci., 45 : 209-210
Saha, J. G. and H. McDonald. (1966) Insecticide Residues in wheat
grown in soil treated with aldrin and endrin. (For publication in J.
Agr. and Food Chem.)
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada.
 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-dieldrin was applied to growing cultures of Aspergilllus
and Penicillium species, no metabolites could be detected in the
culture medium or in the mycelium. Mosquito larvae (Aedes aegypti)
cultivated in an aqueous medium to which 14C.-dieldrin was added,
converted this compound to hydrophilic metabolites (Ludwig et al.,
1966).
A greater toxicity was found in rats kept on a low protein diet than
in rats on a high protein diet (Lee et al., 1964).
After stimulation of rat liver microsomal enzymes with phenobarbital,
the dieldrin content in fat was decreased in comparison with
non-stimulated rats (Cueto & Hayes, 1965).
In experiments with 14C-dieldrin it was demonstrated that dieldrin
in the blood is carried mainly in the erythrocyte contents and plasma,
not in the erythrocyte stroma, leucocytes or platelets. Haemoglobin is
largely but not entirely responsible for the intraerythrocytic
binding. In the plasma, dieldrin binds with soluble proteins (Moss &
Hathway, 1964).
In studies on the urine of individuals exposed to dieldrin, 2 neutral
polar metabolites of dieldrin were found by paper and column
chromatography. No dieldrin could be detected with this method, but
dieldrin or a material having the same retention time was found by gas
chromatography (Cueto Hayes, 1962).
In dogs fed 3 ppm of dieldrin 45.5 ppm were found in the fat, and in
dogs fed 1 ppm, 3.4 ppm were found in the fat. In other organs,
concentrations ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were
found (Borgmann et al., 1952).
14C-dieldrin is preferentially excreted in the bile with its
metabolites. Dieldrin and a hydrophilic metabolite were found in the
bile fifteen minutes after an intravenous injection (Mörsdorf et al.,
1963).
Studies with 36Cl-dieldrin have shown that the initial distribution
is general, but within a few hours of injection it concentrates more
in the fat. Excretion of 36Cl averages about 5 per cent per day and
is markedly increased by a restriction of diet, which reduces the body
fat. Excretion from the cannulated bile duct accelerates as the rat
loses weight, exceeding 10 per cent per day after a few days. Only 3
per cent of the 36Cl is excreted as dieldrin unless the bile is
cannulated, when up to 10 per cent may be excreted. The remainder of
36Cl, is found in the metabolites and these are excreted to 90 per
cent in the faeces and 10 per cent in the urine. The most important
metabolite, containing about 60 per cent of the total 36Cl, is
excreted in the bile (Heath & Vandekar, 1964).
Rats were fed 1 or 25 ppm dieldrin for 120 days. Two metabolites were
found in the urine, one of which was much more abundant in males than
in females. In the same study, small amounts of aldrin were found in
the urine of rats fed dieldrin (Datta et al., 1965).
Experiments with rats and rabbits showed that 14C-dieldrin given
intravenously was converted in 24 hours mainly into hydrophilic
metabolites. After 48 hours the presence of unconverted dieldrin and
the hydrophilic metabolites could be demonstrated in most organs and
tissues. A higher percentage of hydrophilic metabolites were found in
the kidney and liver. It was found that within one hour after
intravenous injection of 14C-dieldrin in rats, radioactive products
appeared in the bile. In 4 hours 13 per cent of the radioactivity was
excreted in the bile. Most of the radioactivity was found in
hydrophilic metabolites. After oral administration of 14C-dieldrin to
rabbits, six metabolites were isolated from the urine, all more
hydrophilic than the original compound. The main metabolite (86 per
cent of the total radioactivity in the urine) could be identified as
one of the two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The
oral LD50 of this compound in mice is 1250 mg/kg body-weight, and
the intravenous LD50 is 51 mg/kg body-weight (Ludwig et al., 1966).
After the feeding of dieldrin to animals it is stored in the adipose
tissues. Although small amounts of dieldrin are found in liver, kidney
and muscle tissues, the greatest amount of storage is in the fat,
where dieldrin is stored unchanged (Bann et al., 1956; Butcher et al.,
1957; Heath & Vandekar, 1964; Ivey et al., 1961; Lehmann, 1956; Street
et al., 1957). It is lost slowly from the body fat (Butcher et al.,
1957; Heath & Vandekar, 1964). It is stored in human fat in
significant amounts (Hunter et al., 1963).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 38 Borgmann at al., 1952
Rat (new-born) Intragastric 168 Lu et al., 1965
Rat (pre-weaning) Oral 25 Lu et al., 1965
Rat Oral 37-87 Borgmann et al., 1952
Gaines, 1960
Heath & Vandekar, 1964
Lehmann, 1951
Lu at al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann at al., 1952
Rabbit Oral 45-50 Borgmann et al., 1952
Dog Oral 56-80 Borgmann at al., 1952
Sheep Oral 50-75 Borgmann at al., 1952
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment groups of 10 male and 10 female rats were given, 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm of
technical dieldrin. The rats were killed after 2, 4, 6 and 8 months.
Food intake and growth were normal. No change in liver weight was
found. Cytoplasmic alteration of the liver cells was found at both
concentrations in males and females (Ortega et al., 1957).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and 10
male) were given dieldrin orally at dosage levels of 0.625, 1.25, 2.5,
5 and 10 mg/kg body-weight per day. Survival rates were affected at
all levels. At 2.5 mg/kg and above, all animals died (Borgmann et al.,
1952).
Dog. Dogs (1-4 animals per group) were given diets containing 1, 3,
10, 25 and 50 ppm dieldrin, six days per week. The animals on 25 and
50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm survived
for 9 months and on 1 and 3 ppm for 15 months. In the groups on 1 and
3 ppm the livers were significantly larger than those of the controls.
Histological changes were noted in brain, liver and kidney. Groups of
4 dogs (2 male and 2 female) were given 1 and 3 ppm of dieldrin in
their diet for 68 weeks. The concentration of 3 ppm increased the
liver/body-weight ratio and produced renal damage in 1 female. With 1
ppm of dieldrin, livers were enlarged but no histopathological changes
were found (Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of 0.2,
0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313 days. The
highest dosage killed all the dogs. Two of the 4 dogs died when given
0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day of
recrystallized dieldrin per kg of body-weight for one year; 3 of them
produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, with equal
numbers of each sex, were fed a diet containing 10 ppm of dieldrin for
their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in the
diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially
reduced the number of pregnancies but this effect tended to disappear
with continued feeding of the diet. All doses increased the mortality
among the suckling young. The effect on survival during suckling was
severe at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female) were
given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of 50
ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin in
the diet at a concentration of 75 ppm for 440 days. Twenty animals of
each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good condition
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight, ratio was markedly increased in the rats
killed during exposure, but was found to be normal in later
sacrifices. "Lesions of the hepatic parenchyma that have been
considered typical of exposure to the organochlorine insecticides in
rats" were observed only, in healthy animals killed during the
treatment. Rats dying spontaneously or killed after withdrawal of the
insecticide did not show such changes (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin in
their diets for a period of 40 months including 4 gestation periods.
At 25 ppm lambs died shortly after birth. Liver function as well as
other physiological tests on the ewes did not show any changes related
to the treatment with dieldrin (Shell, 1963).
Observations on man
In one study, a total of 13 men was divided into 4 groups receiving 0,
11, 57 and 211 µg dieldrin/man/day. No effects on body-weight, blood
cell counts, haemoglobin, total plasma protein, blood urea and serum
alkaline phosphatase, cholinesterase and transaminases were recorded
during the first 35 weeks. At the highest dose level, the average
concentration of dieldrin in the fat was 2.26 ppm. Ratios of fat
concentration at 35 weeks to fat concentration at 0 week ranged from
0.8-1.6 for men exposed to no dieldrin to 2.1-5.2 for those exposed to
11 µg/day; 2.5-6.0 for those exposed to 57 µg/day and 8.1-14.5 for
those exposed to 211 µg/day (Shell, 1966).
Several reports are now available on the storage of dieldrin in human
fat in the general population for several countries (Dale & Quinby,
1963; Dale et al., 1965; Egan et al., 1965; Hoffman et al., 1964;
Hunter et al., 1963; Robinson et al., 1965; Zavon, 1965). The average
concentration ranged between 0.03 and 0.30 ppm. It appears that in
those countries in which a higher DDT storage was recorded, this was
associated with a low dieldrin storage and vice-versa. In the United
States of America the amount of dieldrin stored in the fat increased
from 1950 to 1958 and then remained constant (USFDA Advisory
Committee, 1965). In the United Kingdom, from the above-mentioned
references, it appears that comparable figures were obtained in 1961
and 1965.
In one study, dieldrin was found in human milk at an average
concentration of 0.006 ppm (Egan et al., 1965).
Comments
The primary site of action of dieldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage.
In one long-term rat-feeding experiment, there was a general increase
in tumour production in the experimental animals at the lower dosage
levels as compared to the controls, but the liver was not particularly
affected. Liver tumours, however, were significantly increased at a
dose level of 10 ppm in one strain of mice susceptible to the
development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog. 1 ppm equivalent to 0.025 mg/kg/day produced liver changes.
Rat. 0.5 ppm in the diet equivalent to 0.025 mg/kg/day produced
minimal liver changes.
Estimate of acceptable dally intake for man
0-0.0001 mg/kg/day*
* Sum of aldrin and dieldrin by weight.
Further work required
Elucidation of the significance of the finding that dieldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Aldrin and dieldrin are used for soil treatment against various soil
insects (1.5-5 kg/ha), for seed treatment on grains, sugar beets,
beans (0.5-2.5 g/kg seed), leeks and onions (37-50 g/kg seed for
bulb- or root-dip), and for foliar application on various agricultural
crops, fruits, nursery stocks and ornamental plants.
The use pattern has recently changed considerably due to restrictions
in many countries. In the United States of America and Canada, aldrin
and dieldrin may not be used on any crop that is likely to be fed to
animals; the use in soil for sugar beets has been withdrawn, whereas
the use in soil where other root crops are grown in rotation is under
review. In Canada, the registration of aldrin and dieldrin in
fertilizer mixtures has been withdrawn. Soil and seed treatment is
recommended only for use in grain production. In Britain, the use for
treatment of cereal seeds sown in the spring and the use in fertilizer
mixtures has been withdrawn. In Germany, the prohibition of uses on
agricultural crops is being considered. In Austria, uses for
vegetables are forbidden. In the Netherlands, Belgium and Luxembourg,
aldrin and dieldrin may not be used on carrots and carrots may not be
grown in treated soil in the year of application. Furthermore, to
prevent edible crops obtaining too high residues, the soil used in
rotation for growing food crops, such as lettuce under glass, may not
be treated. In Belgium, seed treatment of grain-seed and pulses is
forbidden in order to avoid poisoning of seed-eating birds, and
subsequently of predatory birds and mammals.
(b) Post-harvest treatments
The use of dieldrin in food storage practice for treatment of empty
warehouses, etc., is decreasing, and is prohibited in many countries
(e.g. the Netherlands, Belgium). In Britain, there is a limited use
under strict precautions. Aldrin and dieldrin are not used for direct
treatment of stored commodities or for treatment of containers, etc.,
which may come into contact with foodstuffs.
(c) Other uses
The use of aldrin and dieldrin in sheep dips or cattle sprays is no
longer allowed in many countries (e.g. the United States of America,
Canada, Britain, Australia, New Zealand, the Netherlands and Belgium).
Dieldrin is used in many sub-tropical and tropical regions for the
control of mosquito larvae, adult mosquitos, chiggers, fleas, ticks,
reduviid bugs, tsetse-flies and other disease vectors. These
applications usually are carried out under supervision of trained
personnel of public health agencies or pest control operators.
Aldrin and dieldrin are used in various countries for termite control
in basements of buildings during construction and for spot treatment
against other domestic pests. In the Netherlands and Belgium, the use
for household purposes is no longer allowed. Dieldrin as a 0.05 per
cent solution is used for wood preservation to prevent attack by
wood-boring insects. In many countries, it is also used for
mothproofing of wool at a dosage of about 0.05 per cent dieldrin on
the weight of wool during the dyeing operation.
(d) Tolerances
The many countries and the great variety of crops in question make it
impossible to present a complete survey of all tolerances, established
or considered. The following table, therefore, is only intended to
serve as a rough guide to current levels. (The figures usually refer
to totals of aldrin plus dieldrin).
Residues resulting from supervised trials
(a) In crops grown in treated soils
Various factors influence the residues remaining in soil and in or on
the crop at harvest. Retention in soil has been greatest when the
organic matter has been high (Bowman, Schechter & Carter, 1965). Low
moisture content (Harris, 1964; Lichtenstein & Schulz, 1961) and dense
coverage of crops (Harris & Lichtenstein, 1961; Lichtenstein et al.,
1962) have also been found to favour retention. Water leaching through
the soil does not appear to play an important role in the decrease of
the residue (Lichtenstein, 1958).
After two yearly applications to soil of 2 kg/ha of aldrin and
dieldrin, Lichtenstein & Schulz (1965) found residues in carrots and
radishes in excess of 0.1 ppm, whereas in potatoes 0.1 ppm was
reached. Continued soil treatment has eventually led to residues in
potatoes, turnips, beet roots and chicory roots in excess of
tolerances (0.1-0.55 ppm) in the Netherlands (unpublished information
from Netherlands Government).
Country Food Tolerance
(parts per million)
United States of America fruits, vegetables 0.1-0.25
potatoes 0.1
animal feed 0
Canada fruits, vegetables 0.1-0.25
potatoes 0.1
Netherlands fruits, vegetables 0.1
Sweden fruits, vegetables 0.1
potatoes
Switzerland potatoes 0.1
Germany (Federal) all edible crops Limit of sensitivity of
analytical method
(under consideration)
Germany (DDR) all edible crops 0
(under consideration)
Translocation of aldrin and dieldrin from the soil to the aerial parts
of the crop has been demonstrated by several investigators, although
in the case of cereal crops, Morley & Chiba (1965) and Saha & McDonald
(1966) found no detectable amounts in the grains, residues were found
in the leaves.
(b) After direct application to growing crops
In several experiments carried out in the United States of America on
various fruits and vegetables at practical dosage levels, the residues
in the edible part of the plant varied from <0.01-0.2 ppm when the
recommended safety interval was observed (unpublished information from
Shell Company).
(c) In meat and meat products
Various workers have measured residues in the bodies of cattle dipped
in or sprayed with dieldrin, or receiving the insecticides in their
diet. For example, Ivey, Claborn & Mann (1961) measured residues in
various body tissues of animals which had received aldrin. The
residues have been highest in the fat; Egan (1965), for example, found
up to about 9 ppm in the fat of sheep slaughtered 4 1/2 weeks after an
experimental dipping.
Residues in raw food moving in commerce
In 1965, analyses were carried out in the Netherlands on glasshouse
lettuce grown in soil treated with aldrin (2.5 kg/ha) against
cutworms. A total of 105 samples from lettuces going on to the market
was examined, Seventy-eight per cent of the examinations showed a
residue of less than the tolerance (0.1 ppm), 11 samples contained
0.1-0.2 ppm, 7 samples contained 0.2-0.3 ppm, 3 samples contained
0.3-0.4 ppm, and 2 samples contained 0.4-0.55 ppm (unpublished
communication).
Residues at time of consumption
Although losses or residues are likely to occur from some foods
through mechanical means (cleaning, trimming, etc.), substantial
losses during cooking are not to be expected. In recent total diet
studies in the United States of America, where, during the months of
June, August, October and December 1965, and February and April 1966,
a total of 317 samples of total diet was analysed for the presence of
aldrin and dieldrin residues, aldrin was found in 13 samples at levels
varying between traces to 0.07 ppm; whereas in 76 samples, dieldrin
was found at levels varying between traces to 0.20 ppm (unpublished
communication from the United States Food and Drug Administration).
Analyses carried out in Britain during the years 1962-1965 showed
dieldrin residues in home-produced butter of 0.03-0.07 ppm, and in
home-produced milk of 0.002-0.003 ppm (Lewis, 1963, 1964, 1965).
Studies have been made in various countries of the dieldrin content of
human fat. In measurements undertaken between 1962 and 1965 in
Britain, the average residues were of the order of 0.25 ppm (Egan et
al., 1965). In a similar investigation carried out in the Netherlands
since 1963, the average residue in 15 samples of human fat was 0.15
ppm, with a range of 0.06 to 30 ppm (direct communication). However,
although it is likely that these residues mainly arise from food, the
extent to which other routes have been responsible, such as the
inhalation of contaminated atmosphere (Abbott et al., 1965, 1966) or
the wearing of treated clothing, has not been clearly demonstrated.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of aldrin and dieldrin, together with
residues of a number of other compounds. An example is the AOAC system
(1966) in which acetonitrile partition and Florisil column clean-up
are employed, and the residues are identified and measured by gas
chromatography coupled with thin layer or paper chromatography.
Alternative clean-up systems, e.g., that of de Faubert Maunder et al.
(1965) using dimethylformamide, and other methods of confirmation of
identity, using infra-red spectrophotometry, are also available. The
methods are sensitive to about 0.002 ppm of aldrin or dieldrin in milk
and 0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATION FOR TOLERANCE
In view of the exceptionally low acceptable daily intake of aldrin and
dieldrin, and the occurrence of unintentional residues, the meeting
came to the conclusion that no allowance could be made for a finite
tolerance figure as a result of agricultural use.
Total diet studies have revealed the presence of dieldrin in the human
food as consumed. Furthermore, aldrin and dieldrin have been shown to
persist for long periods in the soil after soil treatment, and to
occur quite widely in human fat, in aquatic and terrestrial wild life,
and in the abiotic environment. Consequently, it is recommended that
the use of these compounds should be reduced, and, as far as possible,
restricted to those usages which cannot result in residues in food or
in the biotic and abiotic environment.
Although various agricultural uses of aldrin and dieldrin have already
been restricted or prohibited in many countries, some foods moving in
international trade may continue to contain residues (e.g., resulting
from earlier soil treatments). It is, therefore, suggested that a
"practical residue limit" be established for such foods on the
following basis: vegetables, 0.05 ppm; fat in products of animal
origin (excluding milk), 0.2 ppm, or in whole milk, 0.003 ppm. The
above figures should be kept under review.
Further Information
Further information is required on the results of total diet studies
carried out in different countries.
More information is needed on the residence occurring in crops grown
in soils which were treated in previous years and on the possible
occurrence of residues in cereal crops grown in treated soil or from
treated seeds under conditions of extensive monoculture (i.e., no
other crops grown in rotation) such as prevail in certain countries.
Attention is also needed to residues present in products used for
animal feed which are moving in international trade.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. F. (1956) J. Agr.
Food Chem., 4, 937
Borgmann, A. R., Kitselman, C. H., Dahm, P. A., & Pankaskie, J. E.
(1952) Unpublished report
Butcher, J. E., Street. J. C., Shupe, J. L. & Biddulph, C. (1957)
Unpublished report, Agricultural Experimental Station, Utah State
Agricultural College
Cueto, C., jr & Hayes, W. J., jr (1962) J. Agr. Food Chem., 10, .366
Cueto, C., jr & Hayes, W. J., jr (1965) Toxicol. Appl. Pharmacol.,
7, 481
Dale, W. E. & Quinby, G. E. (1963) Science, 142, 593
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol.,
4, 187
Egan, H., Goulding, R., Roburn, J. & Tatton, J. O'G. (1965) Brit.
med. J., 2, 66
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Unpublished
report submitted by Shell Company
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lee, M., Harris, K. & Trowbridge, H. (1964) J. Nutr., 84, 136
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 9, 591
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plan Protection Conference, Budapest
Mörsdorf, K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. exp.,
8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J., jr. & Durham, W.F. (1957) Arch. Path.,
64, 614
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Shell Chemical Company (1963) Unpublished report
Shell Chemical Company (1966) Unpublished report
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
United States Food and Drug Administration (1965) Unpublished report
of Advisory Committee
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1965)
Organochlorine pesticides in the atmospheric environment Nature 208:
1317
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1966)
Organochlorine pesticides in the atmosphere. Nature, 211 : 259
A.O.A.C., (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49 : 222-30
Bowman, M. C., M. S. Schechter and R. L. Carter, (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types.
J. Agr. and Food Chem. 13 (4): 360-365
Burrage R. H. and J. G. Saha. (1966) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin and heptachlor,
In press. Can. J. Plant Sci.
Egan, H., R. Goulding, J. Roburn, J. O'G. Tatton. (1965)
Organochlorine pesticide residues in human fat and human milk. Brit.
Med. J., 2 : 66
Egan, H., (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Fd. Agric., 16;
489-498
de Faubert Maunder, M. J., H. Egan, E. W. Godly, E. W. Hammond, J.
Roburn and J. Thomson. (1965) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. 89:
168-174
Harris, C. R. (1964) Influence or soil moisture of the toxicity of
insecticidal in a mineral soils to insects, J. Econ. Ent., 57 :
946-950
Harris, C. R, and E. P. Lichtenstein. (1961) Factors affecting the
volatization of insecticidal residues from soils. J. Econ. Ent., 54
: 1038-1045
Ivey, M. C., H. K. Claborn and H. D. Mann. (1961) Aldrin and dieldrin
content of body tissues of live stock receiving aldrin in their diet
J. Agric. Fd. Chem., 9 : 374-6
Lewis, D. T. (1963, 1964, & 1965) Report of the Government Chemist.
H.M.S.O. London
Lichtenstein, E. P. (1958) Movement of Insecticides in soil. J. Econ,
Ent., 51 : 380-383
Lichtenstein, E. P. (1962) Vertical distribution and persistence of
insecticidal residues in soil as influenced by mode of application and
cover crop. J. Econ. Ent., 55 : 215-219
Lichtenstein, E. P. and K. R. Schulz. (1965) Residues of aldrin and
dieldrin and their translocation into various crops, J. Agr. Food
Chem., 13 : 57-63
Morley, H. V., and M. Chiba. (1965) Dieldrin uptake from soil by wheat
plants. Can. J. Plant Sci., 45 : 209-210
Saha, J. G. and H. McDonald. (1966) Insecticide Residues in wheat
grown in soil treated with aldrin and endrin. (For publication in J.
Agr. and Food Chem.)
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada.
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
When 14C-dieldrin was applied to growing cultures of Aspergilllus
and Penicillium species, no metabolites could be detected in the
culture medium or in the mycelium. Mosquito larvae (Aedes aegypti)
cultivated in an aqueous medium to which 14C.-dieldrin was added,
converted this compound to hydrophilic metabolites (Ludwig et al.,
1966).
A greater toxicity was found in rats kept on a low protein diet than
in rats on a high protein diet (Lee et al., 1964).
After stimulation of rat liver microsomal enzymes with phenobarbital,
the dieldrin content in fat was decreased in comparison with
non-stimulated rats (Cueto & Hayes, 1965).
In experiments with 14C-dieldrin it was demonstrated that dieldrin
in the blood is carried mainly in the erythrocyte contents and plasma,
not in the erythrocyte stroma, leucocytes or platelets. Haemoglobin is
largely but not entirely responsible for the intraerythrocytic
binding. In the plasma, dieldrin binds with soluble proteins (Moss &
Hathway, 1964).
In studies on the urine of individuals exposed to dieldrin, 2 neutral
polar metabolites of dieldrin were found by paper and column
chromatography. No dieldrin could be detected with this method, but
dieldrin or a material having the same retention time was found by gas
chromatography (Cueto Hayes, 1962).
In dogs fed 3 ppm of dieldrin 45.5 ppm were found in the fat, and in
dogs fed 1 ppm, 3.4 ppm were found in the fat. In other organs,
concentrations ranging from 2.9 ppm (liver) to 0.05 ppm (brain) were
found (Borgmann et al., 1952).
14C-dieldrin is preferentially excreted in the bile with its
metabolites. Dieldrin and a hydrophilic metabolite were found in the
bile fifteen minutes after an intravenous injection (Mörsdorf et al.,
1963).
Studies with 36Cl-dieldrin have shown that the initial distribution
is general, but within a few hours of injection it concentrates more
in the fat. Excretion of 36Cl averages about 5 per cent per day and
is markedly increased by a restriction of diet, which reduces the body
fat. Excretion from the cannulated bile duct accelerates as the rat
loses weight, exceeding 10 per cent per day after a few days. Only 3
per cent of the 36Cl is excreted as dieldrin unless the bile is
cannulated, when up to 10 per cent may be excreted. The remainder of
36Cl, is found in the metabolites and these are excreted to 90 per
cent in the faeces and 10 per cent in the urine. The most important
metabolite, containing about 60 per cent of the total 36Cl, is
excreted in the bile (Heath & Vandekar, 1964).
Rats were fed 1 or 25 ppm dieldrin for 120 days. Two metabolites were
found in the urine, one of which was much more abundant in males than
in females. In the same study, small amounts of aldrin were found in
the urine of rats fed dieldrin (Datta et al., 1965).
Experiments with rats and rabbits showed that 14C-dieldrin given
intravenously was converted in 24 hours mainly into hydrophilic
metabolites. After 48 hours the presence of unconverted dieldrin and
the hydrophilic metabolites could be demonstrated in most organs and
tissues. A higher percentage of hydrophilic metabolites were found in
the kidney and liver. It was found that within one hour after
intravenous injection of 14C-dieldrin in rats, radioactive products
appeared in the bile. In 4 hours 13 per cent of the radioactivity was
excreted in the bile. Most of the radioactivity was found in
hydrophilic metabolites. After oral administration of 14C-dieldrin to
rabbits, six metabolites were isolated from the urine, all more
hydrophilic than the original compound. The main metabolite (86 per
cent of the total radioactivity in the urine) could be identified as
one of the two enantiomorphs of 6,7-trans-dihydroxy-dihydroaldrin. The
oral LD50 of this compound in mice is 1250 mg/kg body-weight, and
the intravenous LD50 is 51 mg/kg body-weight (Ludwig et al., 1966).
After the feeding of dieldrin to animals it is stored in the adipose
tissues. Although small amounts of dieldrin are found in liver, kidney
and muscle tissues, the greatest amount of storage is in the fat,
where dieldrin is stored unchanged (Bann et al., 1956; Butcher et al.,
1957; Heath & Vandekar, 1964; Ivey et al., 1961; Lehmann, 1956; Street
et al., 1957). It is lost slowly from the body fat (Butcher et al.,
1957; Heath & Vandekar, 1964). It is stored in human fat in
significant amounts (Hunter et al., 1963).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
Mouse Oral 38 Borgmann at al., 1952
Rat (new-born) Intragastric 168 Lu et al., 1965
Rat (pre-weaning) Oral 25 Lu et al., 1965
Rat Oral 37-87 Borgmann et al., 1952
Gaines, 1960
Heath & Vandekar, 1964
Lehmann, 1951
Lu at al., 1965
Treon & Cleveland, 1955
Guinea-pig Oral 49 Borgmann at al., 1952
Rabbit Oral 45-50 Borgmann et al., 1952
Dog Oral 56-80 Borgmann at al., 1952
Sheep Oral 50-75 Borgmann at al., 1952
Short-term studies
Rat. In a 90-day feeding study, groups of 12 rats (6 male and 6
female) were fed diets containing 25, 50 and 125 ppm of dieldrin; an
increased mortality rate was observed at 125 ppm. In another
experiment groups of 10 male and 10 female rats were given, 2, 5, 10,
50, 100 and 150 ppm dieldrin. All rats on 150 ppm died. Histological
liver changes were observed in rats on 10 ppm and above (Borgmann et
al., 1952).
Groups of 12 rats, 6 females and 6 males, were fed 2.5 and 25 ppm of
technical dieldrin. The rats were killed after 2, 4, 6 and 8 months.
Food intake and growth were normal. No change in liver weight was
found. Cytoplasmic alteration of the liver cells was found at both
concentrations in males and females (Ortega et al., 1957).
Rabbit. In a 90-day study, groups of 20 rabbits (10 female and 10
male) were given dieldrin orally at dosage levels of 0.625, 1.25, 2.5,
5 and 10 mg/kg body-weight per day. Survival rates were affected at
all levels. At 2.5 mg/kg and above, all animals died (Borgmann et al.,
1952).
Dog. Dogs (1-4 animals per group) were given diets containing 1, 3,
10, 25 and 50 ppm dieldrin, six days per week. The animals on 25 and
50 ppm died after 5 and 33 days respectively. Dogs on 10 ppm survived
for 9 months and on 1 and 3 ppm for 15 months. In the groups on 1 and
3 ppm the livers were significantly larger than those of the controls.
Histological changes were noted in brain, liver and kidney. Groups of
4 dogs (2 male and 2 female) were given 1 and 3 ppm of dieldrin in
their diet for 68 weeks. The concentration of 3 ppm increased the
liver/body-weight ratio and produced renal damage in 1 female. With 1
ppm of dieldrin, livers were enlarged but no histopathological changes
were found (Treon & Cleveland, 1955).
Dogs (both sexes) in groups of 3 or 4 were given dosage levels of 0.2,
0.6 and 2.0 mg/kg per day by mouth for a maximum time of 313 days. The
highest dosage killed all the dogs. Two of the 4 dogs died when given
0.6 mg/kg body-weight per day (Borgmann et al., 1952).
Three groups of 3 dogs were given orally 0.2 and 0.6 mg per day of
recrystallized dieldrin per kg of body-weight for one year; 3 of them
produced litters but none of the pups of the group given 0.6 mg
survived, probably because of high quantities of dieldrin in the milk
of the dams. Histological changes were found in the liver and/or
kidneys of adult dogs (Kitselman, 1953).
A group of 14 dogs was given dieldrin orally for 25 months at the
following daily doses: 0.2 mg/kg (2 dogs), 0.5 mg/kg (4 dogs), 1, 2, 5
and 10 mg/kg (2 dogs in each group). All the dogs given 2, 5 or 10
mg/kg died in 35 days. All those given 1 mg/kg or 0.5 mg/kg (with one
exception) died in one year. Convulsions and fatty liver changes were
frequently seen. At 0.2 mg/kg no effects were observed (Fitzhugh et
al., 1964).
Long-term studies
Mouse. Groups of approximately 200 young C3HeB/Fe mice, with equal
numbers of each sex, were fed a diet containing 10 ppm of dieldrin for
their life-span (maximum 2 years). The dieldrin shortened their
average life-span by 2 months, as compared with an equal number of
controls, and significantly increased the incidence of hepatic tumours
(Davis & Fitzhugh, 1962).
Rat. Groups of 40 rats (20 male and 20 female) were fed diets
containing 2.5, 12.5 and 25 ppm dieldrin for 2 years. The
liver/body-weight ratio increased and characteristic histological
liver damage was seen at all dosages (Treon & Cleveland, 1955).
With groups of 16 female rats each, dieldrin was incorporated in the
diet for three generations at 2.5, 12.5 and 25 ppm. Two litters of
offspring were taken from each generation of these groups. The
presence of dieldrin in the diet at 2.5 and 12.5 ppm initially
reduced the number of pregnancies but this effect tended to disappear
with continued feeding of the diet. All doses increased the mortality
among the suckling young. The effect on survival during suckling was
severe at 12.5 and 25 ppm dieldrin (Treon & Cleveland, 1955).
In a 2-year experiment, groups of 24 rats (12 male and 12 female) were
given 0.5, 2, 10, 50, 100 and 150 ppm dieldrin. Concentrations of 50
ppm and above increased the mortality rate in a dose-response
relationship. The liver/body-weight ratio increased and characteristic
histological lesions occurred in the liver at all levels; these were
minimal at 0.5 ppm but increased in severity with increasing dose.
There was an increase in the number of tumours in the experimental
groups, especially at the lower levels of feeding, in contrast to the
control group (Fitzhugh et al., 1964).
In other experiments, 40 males and 40 females were given dieldrin in
the diet at a concentration of 75 ppm for 440 days. Twenty animals of
each sex were used as controls. All the females and 22 males of the
experimental group, and 5 control males died spontaneously before the
end of the treatment. Seven males were killed in good condition
between 300 and 440 days and the last 11 males were killed after 440
days. The liver/body-weight, ratio was markedly increased in the rats
killed during exposure, but was found to be normal in later
sacrifices. "Lesions of the hepatic parenchyma that have been
considered typical of exposure to the organochlorine insecticides in
rats" were observed only, in healthy animals killed during the
treatment. Rats dying spontaneously or killed after withdrawal of the
insecticide did not show such changes (Hunter et al., 1964).
Ewes. Thirty-six ewes were given 0, 1, 5 or 25 ppm of dieldrin in
their diets for a period of 40 months including 4 gestation periods.
At 25 ppm lambs died shortly after birth. Liver function as well as
other physiological tests on the ewes did not show any changes related
to the treatment with dieldrin (Shell, 1963).
Observations on man
In one study, a total of 13 men was divided into 4 groups receiving 0,
11, 57 and 211 µg dieldrin/man/day. No effects on body-weight, blood
cell counts, haemoglobin, total plasma protein, blood urea and serum
alkaline phosphatase, cholinesterase and transaminases were recorded
during the first 35 weeks. At the highest dose level, the average
concentration of dieldrin in the fat was 2.26 ppm. Ratios of fat
concentration at 35 weeks to fat concentration at 0 week ranged from
0.8-1.6 for men exposed to no dieldrin to 2.1-5.2 for those exposed to
11 µg/day; 2.5-6.0 for those exposed to 57 µg/day and 8.1-14.5 for
those exposed to 211 µg/day (Shell, 1966).
Several reports are now available on the storage of dieldrin in human
fat in the general population for several countries (Dale & Quinby,
1963; Dale et al., 1965; Egan et al., 1965; Hoffman et al., 1964;
Hunter et al., 1963; Robinson et al., 1965; Zavon, 1965). The average
concentration ranged between 0.03 and 0.30 ppm. It appears that in
those countries in which a higher DDT storage was recorded, this was
associated with a low dieldrin storage and vice-versa. In the United
States of America the amount of dieldrin stored in the fat increased
from 1950 to 1958 and then remained constant (USFDA Advisory
Committee, 1965). In the United Kingdom, from the above-mentioned
references, it appears that comparable figures were obtained in 1961
and 1965.
In one study, dieldrin was found in human milk at an average
concentration of 0.006 ppm (Egan et al., 1965).
Comments
The primary site of action of dieldrin is the central nervous system.
CNS stimulation is the cause of death in acute poisoning. Signs of CNS
stimulation are also seen after repeated high doses. Repeated doses at
lower levels give rise to liver damage.
In one long-term rat-feeding experiment, there was a general increase
in tumour production in the experimental animals at the lower dosage
levels as compared to the controls, but the liver was not particularly
affected. Liver tumours, however, were significantly increased at a
dose level of 10 ppm in one strain of mice susceptible to the
development of these tumours.
TOXICOLOGICAL EVALUATION
Levels causing no toxicological effect
Dog. 1 ppm equivalent to 0.025 mg/kg/day produced liver changes.
Rat. 0.5 ppm in the diet equivalent to 0.025 mg/kg/day produced
minimal liver changes.
Estimate of acceptable dally intake for man
0-0.0001 mg/kg/day*
* Sum of aldrin and dieldrin by weight.
Further work required
Elucidation of the significance of the finding that dieldrin is one of
the compounds which affect liver cellular metabolism (p. 3).
Development of methods of toxicological investigation aimed at
defining and clarifying the various biological changes seen in the
reported studies of this compound, with a view to removing doubts
which may remain as to its safety in use.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Aldrin and dieldrin are used for soil treatment against various soil
insects (1.5-5 kg/ha), for seed treatment on grains, sugar beets,
beans (0.5-2.5 g/kg seed), leeks and onions (37-50 g/kg seed for
bulb- or root-dip), and for foliar application on various agricultural
crops, fruits, nursery stocks and ornamental plants.
The use pattern has recently changed considerably due to restrictions
in many countries. In the United States of America and Canada, aldrin
and dieldrin may not be used on any crop that is likely to be fed to
animals; the use in soil for sugar beets has been withdrawn, whereas
the use in soil where other root crops are grown in rotation is under
review. In Canada, the registration of aldrin and dieldrin in
fertilizer mixtures has been withdrawn. Soil and seed treatment is
recommended only for use in grain production. In Britain, the use for
treatment of cereal seeds sown in the spring and the use in fertilizer
mixtures has been withdrawn. In Germany, the prohibition of uses on
agricultural crops is being considered. In Austria, uses for
vegetables are forbidden. In the Netherlands, Belgium and Luxembourg,
aldrin and dieldrin may not be used on carrots and carrots may not be
grown in treated soil in the year of application. Furthermore, to
prevent edible crops obtaining too high residues, the soil used in
rotation for growing food crops, such as lettuce under glass, may not
be treated. In Belgium, seed treatment of grain-seed and pulses is
forbidden in order to avoid poisoning of seed-eating birds, and
subsequently of predatory birds and mammals.
(b) Post-harvest treatments
The use of dieldrin in food storage practice for treatment of empty
warehouses, etc., is decreasing, and is prohibited in many countries
(e.g. the Netherlands, Belgium). In Britain, there is a limited use
under strict precautions. Aldrin and dieldrin are not used for direct
treatment of stored commodities or for treatment of containers, etc.,
which may come into contact with foodstuffs.
(c) Other uses
The use of aldrin and dieldrin in sheep dips or cattle sprays is no
longer allowed in many countries (e.g. the United States of America,
Canada, Britain, Australia, New Zealand, the Netherlands and Belgium).
Dieldrin is used in many sub-tropical and tropical regions for the
control of mosquito larvae, adult mosquitos, chiggers, fleas, ticks,
reduviid bugs, tsetse-flies and other disease vectors. These
applications usually are carried out under supervision of trained
personnel of public health agencies or pest control operators.
Aldrin and dieldrin are used in various countries for termite control
in basements of buildings during construction and for spot treatment
against other domestic pests. In the Netherlands and Belgium, the use
for household purposes is no longer allowed. Dieldrin as a 0.05 per
cent solution is used for wood preservation to prevent attack by
wood-boring insects. In many countries, it is also used for
mothproofing of wool at a dosage of about 0.05 per cent dieldrin on
the weight of wool during the dyeing operation.
(d) Tolerances
The many countries and the great variety of crops in question make it
impossible to present a complete survey of all tolerances, established
or considered. The following table, therefore, is only intended to
serve as a rough guide to current levels. (The figures usually refer
to totals of aldrin plus dieldrin).
Residues resulting from supervised trials
(a) In crops grown in treated soils
Various factors influence the residues remaining in soil and in or on
the crop at harvest. Retention in soil has been greatest when the
organic matter has been high (Bowman, Schechter & Carter, 1965). Low
moisture content (Harris, 1964; Lichtenstein & Schulz, 1961) and dense
coverage of crops (Harris & Lichtenstein, 1961; Lichtenstein et al.,
1962) have also been found to favour retention. Water leaching through
the soil does not appear to play an important role in the decrease of
the residue (Lichtenstein, 1958).
After two yearly applications to soil of 2 kg/ha of aldrin and
dieldrin, Lichtenstein & Schulz (1965) found residues in carrots and
radishes in excess of 0.1 ppm, whereas in potatoes 0.1 ppm was
reached. Continued soil treatment has eventually led to residues in
potatoes, turnips, beet roots and chicory roots in excess of
tolerances (0.1-0.55 ppm) in the Netherlands (unpublished information
from Netherlands Government).
Country Food Tolerance
(parts per million)
United States of America fruits, vegetables 0.1-0.25
potatoes 0.1
animal feed 0
Canada fruits, vegetables 0.1-0.25
potatoes 0.1
Netherlands fruits, vegetables 0.1
Sweden fruits, vegetables 0.1
potatoes
Switzerland potatoes 0.1
Germany (Federal) all edible crops Limit of sensitivity of
analytical method
(under consideration)
Germany (DDR) all edible crops 0
(under consideration)
Translocation of aldrin and dieldrin from the soil to the aerial parts
of the crop has been demonstrated by several investigators, although
in the case of cereal crops, Morley & Chiba (1965) and Saha & McDonald
(1966) found no detectable amounts in the grains, residues were found
in the leaves.
(b) After direct application to growing crops
In several experiments carried out in the United States of America on
various fruits and vegetables at practical dosage levels, the residues
in the edible part of the plant varied from <0.01-0.2 ppm when the
recommended safety interval was observed (unpublished information from
Shell Company).
(c) In meat and meat products
Various workers have measured residues in the bodies of cattle dipped
in or sprayed with dieldrin, or receiving the insecticides in their
diet. For example, Ivey, Claborn & Mann (1961) measured residues in
various body tissues of animals which had received aldrin. The
residues have been highest in the fat; Egan (1965), for example, found
up to about 9 ppm in the fat of sheep slaughtered 4 1/2 weeks after an
experimental dipping.
Residues in raw food moving in commerce
In 1965, analyses were carried out in the Netherlands on glasshouse
lettuce grown in soil treated with aldrin (2.5 kg/ha) against
cutworms. A total of 105 samples from lettuces going on to the market
was examined, Seventy-eight per cent of the examinations showed a
residue of less than the tolerance (0.1 ppm), 11 samples contained
0.1-0.2 ppm, 7 samples contained 0.2-0.3 ppm, 3 samples contained
0.3-0.4 ppm, and 2 samples contained 0.4-0.55 ppm (unpublished
communication).
Residues at time of consumption
Although losses or residues are likely to occur from some foods
through mechanical means (cleaning, trimming, etc.), substantial
losses during cooking are not to be expected. In recent total diet
studies in the United States of America, where, during the months of
June, August, October and December 1965, and February and April 1966,
a total of 317 samples of total diet was analysed for the presence of
aldrin and dieldrin residues, aldrin was found in 13 samples at levels
varying between traces to 0.07 ppm; whereas in 76 samples, dieldrin
was found at levels varying between traces to 0.20 ppm (unpublished
communication from the United States Food and Drug Administration).
Analyses carried out in Britain during the years 1962-1965 showed
dieldrin residues in home-produced butter of 0.03-0.07 ppm, and in
home-produced milk of 0.002-0.003 ppm (Lewis, 1963, 1964, 1965).
Studies have been made in various countries of the dieldrin content of
human fat. In measurements undertaken between 1962 and 1965 in
Britain, the average residues were of the order of 0.25 ppm (Egan et
al., 1965). In a similar investigation carried out in the Netherlands
since 1963, the average residue in 15 samples of human fat was 0.15
ppm, with a range of 0.06 to 30 ppm (direct communication). However,
although it is likely that these residues mainly arise from food, the
extent to which other routes have been responsible, such as the
inhalation of contaminated atmosphere (Abbott et al., 1965, 1966) or
the wearing of treated clothing, has not been clearly demonstrated.
Methods of residue analysis
A number of multidetection systems are available for the detection and
determination of residues of aldrin and dieldrin, together with
residues of a number of other compounds. An example is the AOAC system
(1966) in which acetonitrile partition and Florisil column clean-up
are employed, and the residues are identified and measured by gas
chromatography coupled with thin layer or paper chromatography.
Alternative clean-up systems, e.g., that of de Faubert Maunder et al.
(1965) using dimethylformamide, and other methods of confirmation of
identity, using infra-red spectrophotometry, are also available. The
methods are sensitive to about 0.002 ppm of aldrin or dieldrin in milk
and 0.02 ppm in most other foods, though under favourable conditions
greater sensitivity can, if appropriate, be obtained.
RECOMMENDATION FOR TOLERANCE
In view of the exceptionally low acceptable daily intake of aldrin and
dieldrin, and the occurrence of unintentional residues, the meeting
came to the conclusion that no allowance could be made for a finite
tolerance figure as a result of agricultural use.
Total diet studies have revealed the presence of dieldrin in the human
food as consumed. Furthermore, aldrin and dieldrin have been shown to
persist for long periods in the soil after soil treatment, and to
occur quite widely in human fat, in aquatic and terrestrial wild life,
and in the abiotic environment. Consequently, it is recommended that
the use of these compounds should be reduced, and, as far as possible,
restricted to those usages which cannot result in residues in food or
in the biotic and abiotic environment.
Although various agricultural uses of aldrin and dieldrin have already
been restricted or prohibited in many countries, some foods moving in
international trade may continue to contain residues (e.g., resulting
from earlier soil treatments). It is, therefore, suggested that a
"practical residue limit" be established for such foods on the
following basis: vegetables, 0.05 ppm; fat in products of animal
origin (excluding milk), 0.2 ppm, or in whole milk, 0.003 ppm. The
above figures should be kept under review.
Further Information
Further information is required on the results of total diet studies
carried out in different countries.
More information is needed on the residence occurring in crops grown
in soils which were treated in previous years and on the possible
occurrence of residues in cereal crops grown in treated soil or from
treated seeds under conditions of extensive monoculture (i.e., no
other crops grown in rotation) such as prevail in certain countries.
Attention is also needed to residues present in products used for
animal feed which are moving in international trade.
REFERENCES PERTINENT TO BIOLOGICAL DATA
Bann, J. M., DeCino, T. J., Earle, N. W. & Sun, Y. F. (1956) J. Agr.
Food Chem., 4, 937
Borgmann, A. R., Kitselman, C. H., Dahm, P. A., & Pankaskie, J. E.
(1952) Unpublished report
Butcher, J. E., Street. J. C., Shupe, J. L. & Biddulph, C. (1957)
Unpublished report, Agricultural Experimental Station, Utah State
Agricultural College
Cueto, C., jr & Hayes, W. J., jr (1962) J. Agr. Food Chem., 10, .366
Cueto, C., jr & Hayes, W. J., jr (1965) Toxicol. Appl. Pharmacol.,
7, 481
Dale, W. E. & Quinby, G. E. (1963) Science, 142, 593
Dale, W. E., Copeland, M. F. & Hayes, W. J., jr (1965) Bull. Wld
Hlth Org., 33, 471
Datta, P. R., Laug, E. P., Watts, J. O., Klein, A. K. & Nelson, M. J.
(1965) Nature, 208, 289
Davis, K. J. & Fitzhugh, O. G. (1962) Toxicol. Appl. Pharmacol.,
4, 187
Egan, H., Goulding, R., Roburn, J. & Tatton, J. O'G. (1965) Brit.
med. J., 2, 66
Fitzhugh, O. G., Nelson. A. A. & Quaife, M. L. (1964) Food &
Cosmetic Toxicol., 2, 551
Gaines, T. B. (1960) Toxicol. Appl. Pharmacol., 2, 88
Heath, D. F. & Vandekar, M. (1964) Brit. J. industr. Med., 21, 269
Hoffmann, W. S., Fishbein, W. I. & Andelman, M. B. (1964) Arch.
environ. Hlth, 9, 387
Hunter, C. G., Robinson, J. & Richardson, A. (1963) Brit. med. J.,
3, 221
Hunter, C. G., Stevenson, D. E. & Ferrigan, L. W. (1964) Unpublished
report submitted by Shell Company
Ivey, M. C., Claborn, H. V., Mann, H. D., Radeleff, R. D. & Woodard,
G. T. (1961) J. Agr. Food Chem., 9, 374
Kitselman, C. H. (1953) J. Amer. vet. med. Ass., 123, 28
Lee, M., Harris, K. & Trowbridge, H. (1964) J. Nutr., 84, 136
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Lehman, A. J. (1956) Quart. Bull. Assoc. Food and Drug Officials
U.S., 20, 95
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetic
Toxicol., 9, 591
Ludwig, G., Arent, H., Kochen, W., Poonawalla, N., Rechmeier, G.,
Stiasni, M., Vogel, J. & Korte, F. (1966) Paper presented at the
Scientific Plan Protection Conference, Budapest
Mörsdorf, K., Ludwig, G., Vogel, J. & Korte, F. (1963) Med. exp.,
8, 90
Moss, J. A. & Hathway, D. E. (1964) Biochem. J., 91, 384
Ortega, P., Hayes, W. J., jr. & Durham, W.F. (1957) Arch. Path.,
64, 614
Robinson, J., Richardson, A., Hunter, C. G., Crabtree, A. N. & Rees,
H. J. (1965) Brit. J. industr. Med., 22, 220
Shell Chemical Company (1963) Unpublished report
Shell Chemical Company (1966) Unpublished report
Street, J. C., Butcher, J. E., Raleigh, R. J. & Clanton, D. C. (1957)
Proc. West Sec. Amer. Soc. Anim. Prod., 46 (1)
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
United States Food and Drug Administration (1965) Unpublished report
of Advisory Committee
Zavon, M. R., Hine, C. H. & Parker, K. D. (1965) J. Amer. med. Ass.,
193, 181
REFERENCES PERTINENT TO AGRICULTURAL DATA
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1965)
Organochlorine pesticides in the atmospheric environment Nature 208:
1317
Abbott, D. C., R. B. Harrison, J. O'G. Tatton, J. Thomson. (1966)
Organochlorine pesticides in the atmosphere. Nature, 211 : 259
A.O.A.C., (1966) Changes in Methods of Analysis. J. Assoc. Offic.
Analytical Chem., 49 : 222-30
Bowman, M. C., M. S. Schechter and R. L. Carter, (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types.
J. Agr. and Food Chem. 13 (4): 360-365
Burrage R. H. and J. G. Saha. (1966) Insecticide residues in spring
wheat plants field-grown from seed treated with aldrin and heptachlor,
In press. Can. J. Plant Sci.
Egan, H., R. Goulding, J. Roburn, J. O'G. Tatton. (1965)
Organochlorine pesticide residues in human fat and human milk. Brit.
Med. J., 2 : 66
Egan, H., (1965) Chlorinated pesticide residues in lamb and mutton fat
following dipping and other treatment. J. Sci. Fd. Agric., 16;
489-498
de Faubert Maunder, M. J., H. Egan, E. W. Godly, E. W. Hammond, J.
Roburn and J. Thomson. (1965) Clean-up of animal fats and dairy
products for the analysis of chlorinated pesticide residues. 89:
168-174
Harris, C. R. (1964) Influence or soil moisture of the toxicity of
insecticidal in a mineral soils to insects, J. Econ. Ent., 57 :
946-950
Harris, C. R, and E. P. Lichtenstein. (1961) Factors affecting the
volatization of insecticidal residues from soils. J. Econ. Ent., 54
: 1038-1045
Ivey, M. C., H. K. Claborn and H. D. Mann. (1961) Aldrin and dieldrin
content of body tissues of live stock receiving aldrin in their diet
J. Agric. Fd. Chem., 9 : 374-6
Lewis, D. T. (1963, 1964, & 1965) Report of the Government Chemist.
H.M.S.O. London
Lichtenstein, E. P. (1958) Movement of Insecticides in soil. J. Econ,
Ent., 51 : 380-383
Lichtenstein, E. P. (1962) Vertical distribution and persistence of
insecticidal residues in soil as influenced by mode of application and
cover crop. J. Econ. Ent., 55 : 215-219
Lichtenstein, E. P. and K. R. Schulz. (1965) Residues of aldrin and
dieldrin and their translocation into various crops, J. Agr. Food
Chem., 13 : 57-63
Morley, H. V., and M. Chiba. (1965) Dieldrin uptake from soil by wheat
plants. Can. J. Plant Sci., 45 : 209-210
Saha, J. G. and H. McDonald. (1966) Insecticide Residues in wheat
grown in soil treated with aldrin and endrin. (For publication in J.
Agr. and Food Chem.)
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada.
