
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
METHOXYCHLOR
Chemical name
1,1,1-trichloro-2,2-di-(p-methoxyphenyl) ethane;
2,2-di-4-anisyl, 1,1,1,trichloroethane,
di-(p-methoxyphenyl)-trichloromethyl methane;
2,2-di(p-methoxyphenyl)-1,1,1,-trichloroethane.
Synonyms
DMDT; dimethoxy-DT; marlate.
Empirical formula
C16H15O2Cl3
Structural formula
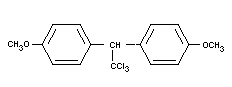 BIOLOGICAL DATA
Biochemical aspects
Methoxychlor is not excreted intact, and appears to undergo
metabolize yielding hydroxyphenyl derivatives (von Oettingen &
Sharpless, 1946).
Methoxychlor is very rapidly detoxicated in the liver, yielding
a metabolite which is excreted into the intestine and removed from the
body in the faeces. This rapid detoxication, together with fairly slow
gastro-intestinal absorption, explains the low mammalian oral toxicity
and low tissue storage of methoxychlor (Weikel, 1957).
Some tissue and fat storage takes place and reaches a maximum
in 4 weeks; the stored material is mobilized in 2-4 weeks after
exposure ceases (Kunze et al., 1950; Metcalf, 1955).
Methoxychlor showed very little tendency to be excreted in the
milk even when dietary concentrations up to 7000 ppm were given to
dairy cows. At 800 ppm and 7000 ppm the amounts found in the milk were
0.13 ppm and 2.14 ppm respectively at 16 weeks (Gannon et al., 1959).
The rate and completeness of methoxychlor metabolism may account for
the low storage and accumulation.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1850 Domenjoz, 1946
Rat Oral 5000-7000 Hodge et al., 1950
Lehman, 1951
Smith et al., 1946
Sheep Oral >2000 Negherbon, 1959
Welch, 1948
Steer Oral >500 Negherbon, 1959
Welch, 1948
Short-term studies
Rat. Groups of weaned rats, each of 10 males and 10 females per
group, were fed for 45 days on a ration containing 100, 1000 and 30
000 ppm methoxychlor. At 100 ppm there was no effect on growth; at
1000 ppm growth was slightly retarded; at 30 000 ppm very little
growth occurred. At 10 000 ppm, rats in paired-feeding tests over 30
days showed marked growth reduction attributed to a reduced food
intake.
There were no deaths in the 100 ppm and 1000 ppm groups; 8 of 10
rats died in each of the male and female groups receiving 30 000 ppm.
The blood picture was normal. At autopsy there was no significant
difference in the organ-weights of the rats on 100 ppm and 1000 ppm.
The 30 000 ppm group showed uniformly smaller organ-weights than the
controls. In the case of the testes, the decrease in weight was very
marked. There was no evidence of histopathological change in the
organs examined, except in the testes, which showed apparent
suppression of spermatogenesis beyond the spermatogonial phase; the
spermatogonia and Sertoli cells were relatively normal; the primary
spermatocytes were variable in number, usually with evidence of
necrosis. The more mature germ cells were absent (Hodge et al., 1950).
In paired-feeding experiments in which 10 000 ppm of
methoxychlor were added to the diet of weanling male rats, a marked
reduction in the weight of the testes, seminal vesicles and prostate
was found. These effects could be mediated through an oestrogenic
action inhibiting the production of anterior pituitary gonadotrophins
with consequent deficiencies in the development of the male
reproductive system. Cystic tubular nephropathy was also observed
(Tullner & Edgcomb, 1962).
Rabbit. Daily oral doses of 200 mg/kg body-weight killed the
rabbits after 4 to 15 days. The only symptoms noted were diarrhoea and
anorexia (Smith et al., 1946; Von Oettingen, 1955). Dermal application
of 2 or 3 ml of a 30% solution (in dimethyl phthalate) 5 days a week
for 13 weeks was toxic; growth was depressed and paralysis of the
forelegs occurred in some cases. Histopathological examination showed
some fatty degeneration of the liver and lesions of the central
nervous system. Applications of 1 ml or less had no effect (Haag et
al., 1950).
Dog. Groups, each of 2 dogs, were maintained for one year on
doses of 20, 100 and 300 mg/kg body-weight per day. There were no
deaths; the blood picture and organ-weights were normal; there were no
histopathological changes (Hodge et al., 1952).
Long-term studies
Rat. Groups each of 25 male and 25 female rats were fed for 2
years on diets containing 25, 200 and 1600 ppm of methoxychlor. There
was no effect on growth at doses of 25 ppm and 200 ppm in the diet,
but there was moderate reduction in growth at 1600 ppm. There was no
decrease in life-span; organ-weights and blood picture were
essentially normal and histopathological examination revealed no
significant changes (Hodge et al., 1952).
Comments on the experimental studies reported
From the studies reported the rat appears more sensitive than
the dog. Experiments with the rat covered the life-span and may be
used to estimate the acceptable daily intake for man.
EVALUATION
Level causing no toxicological effect in the rat
The maximum no-effect level in rat was 200 ppm in the diet,
equivalent to 10 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
0-0.10 mg/kg body-weight
Further work desirable
Additional biochemical studies. Long-term toxicity studies in
another species than the rat. Reproduction studies.
REFERENCES
Domenjoz, R. (1946) Arch. int. Pharmacodyn., 73, 128
Gannon, N., Link, R. P. & Decker, G. C. (1959) J. Agric. Food Chem.,
7, 829
Haag, H. B., Finnegan, J. K., Larson, P. S., Riese, W. & Dreyfuss, M.
L. (1950) Arch. int. Pharmacodyn, 83 (4), 491
Hodge, H. C., Elliott, A. M., Thomas, J. F., Blanchet, H. J., Wilt, W.
G. & Mason, K. E. (1950) J. Phamacol. exp. Ther., 99, 140
Hodge, H. C., Maynard, E. A. & Blanchet, H. J. jr (1952) J.
Pharmacol. exp. Ther., 104, 60
Kunze, F. M., Laug, E. P. & Prickett, C. S. (1950) Proc Soc. exp.
Biol. (N.Y.), 75, 415
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3., Saunders,
Philadelphia
Smith, M. I., Bauer, H., Stohlman, E. F. & Lillie, R. D. (1946) J.
Pharmacol, exp. Ther., 88, 359
Tullner, W. W. & Edgcomb, J. H. (1962) J. Pharmacol. exp. Ther.,
138 (1), 126
Von Oettingen, W. F. & Sharpless, N. (1946) J. Pharmacol. exp.
Ther., 88, 400
Von Oettingen, W. F. (1955) The halogenated hydrocarbons, toxicity
and potential dangers, United States Department of Health.
Education and Welfare. Public Health Service Bull. No. 414
Weikel, J. H. (1957) Arch. int. Pharmacodyn., 110 (4), 423
Welch, H. L. (1948) J. econ. Ent., 41, 36
BIOLOGICAL DATA
Biochemical aspects
Methoxychlor is not excreted intact, and appears to undergo
metabolize yielding hydroxyphenyl derivatives (von Oettingen &
Sharpless, 1946).
Methoxychlor is very rapidly detoxicated in the liver, yielding
a metabolite which is excreted into the intestine and removed from the
body in the faeces. This rapid detoxication, together with fairly slow
gastro-intestinal absorption, explains the low mammalian oral toxicity
and low tissue storage of methoxychlor (Weikel, 1957).
Some tissue and fat storage takes place and reaches a maximum
in 4 weeks; the stored material is mobilized in 2-4 weeks after
exposure ceases (Kunze et al., 1950; Metcalf, 1955).
Methoxychlor showed very little tendency to be excreted in the
milk even when dietary concentrations up to 7000 ppm were given to
dairy cows. At 800 ppm and 7000 ppm the amounts found in the milk were
0.13 ppm and 2.14 ppm respectively at 16 weeks (Gannon et al., 1959).
The rate and completeness of methoxychlor metabolism may account for
the low storage and accumulation.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1850 Domenjoz, 1946
Rat Oral 5000-7000 Hodge et al., 1950
Lehman, 1951
Smith et al., 1946
Sheep Oral >2000 Negherbon, 1959
Welch, 1948
Steer Oral >500 Negherbon, 1959
Welch, 1948
Short-term studies
Rat. Groups of weaned rats, each of 10 males and 10 females per
group, were fed for 45 days on a ration containing 100, 1000 and 30
000 ppm methoxychlor. At 100 ppm there was no effect on growth; at
1000 ppm growth was slightly retarded; at 30 000 ppm very little
growth occurred. At 10 000 ppm, rats in paired-feeding tests over 30
days showed marked growth reduction attributed to a reduced food
intake.
There were no deaths in the 100 ppm and 1000 ppm groups; 8 of 10
rats died in each of the male and female groups receiving 30 000 ppm.
The blood picture was normal. At autopsy there was no significant
difference in the organ-weights of the rats on 100 ppm and 1000 ppm.
The 30 000 ppm group showed uniformly smaller organ-weights than the
controls. In the case of the testes, the decrease in weight was very
marked. There was no evidence of histopathological change in the
organs examined, except in the testes, which showed apparent
suppression of spermatogenesis beyond the spermatogonial phase; the
spermatogonia and Sertoli cells were relatively normal; the primary
spermatocytes were variable in number, usually with evidence of
necrosis. The more mature germ cells were absent (Hodge et al., 1950).
In paired-feeding experiments in which 10 000 ppm of
methoxychlor were added to the diet of weanling male rats, a marked
reduction in the weight of the testes, seminal vesicles and prostate
was found. These effects could be mediated through an oestrogenic
action inhibiting the production of anterior pituitary gonadotrophins
with consequent deficiencies in the development of the male
reproductive system. Cystic tubular nephropathy was also observed
(Tullner & Edgcomb, 1962).
Rabbit. Daily oral doses of 200 mg/kg body-weight killed the
rabbits after 4 to 15 days. The only symptoms noted were diarrhoea and
anorexia (Smith et al., 1946; Von Oettingen, 1955). Dermal application
of 2 or 3 ml of a 30% solution (in dimethyl phthalate) 5 days a week
for 13 weeks was toxic; growth was depressed and paralysis of the
forelegs occurred in some cases. Histopathological examination showed
some fatty degeneration of the liver and lesions of the central
nervous system. Applications of 1 ml or less had no effect (Haag et
al., 1950).
Dog. Groups, each of 2 dogs, were maintained for one year on
doses of 20, 100 and 300 mg/kg body-weight per day. There were no
deaths; the blood picture and organ-weights were normal; there were no
histopathological changes (Hodge et al., 1952).
Long-term studies
Rat. Groups each of 25 male and 25 female rats were fed for 2
years on diets containing 25, 200 and 1600 ppm of methoxychlor. There
was no effect on growth at doses of 25 ppm and 200 ppm in the diet,
but there was moderate reduction in growth at 1600 ppm. There was no
decrease in life-span; organ-weights and blood picture were
essentially normal and histopathological examination revealed no
significant changes (Hodge et al., 1952).
Comments on the experimental studies reported
From the studies reported the rat appears more sensitive than
the dog. Experiments with the rat covered the life-span and may be
used to estimate the acceptable daily intake for man.
EVALUATION
Level causing no toxicological effect in the rat
The maximum no-effect level in rat was 200 ppm in the diet,
equivalent to 10 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
0-0.10 mg/kg body-weight
Further work desirable
Additional biochemical studies. Long-term toxicity studies in
another species than the rat. Reproduction studies.
REFERENCES
Domenjoz, R. (1946) Arch. int. Pharmacodyn., 73, 128
Gannon, N., Link, R. P. & Decker, G. C. (1959) J. Agric. Food Chem.,
7, 829
Haag, H. B., Finnegan, J. K., Larson, P. S., Riese, W. & Dreyfuss, M.
L. (1950) Arch. int. Pharmacodyn, 83 (4), 491
Hodge, H. C., Elliott, A. M., Thomas, J. F., Blanchet, H. J., Wilt, W.
G. & Mason, K. E. (1950) J. Phamacol. exp. Ther., 99, 140
Hodge, H. C., Maynard, E. A. & Blanchet, H. J. jr (1952) J.
Pharmacol. exp. Ther., 104, 60
Kunze, F. M., Laug, E. P. & Prickett, C. S. (1950) Proc Soc. exp.
Biol. (N.Y.), 75, 415
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3., Saunders,
Philadelphia
Smith, M. I., Bauer, H., Stohlman, E. F. & Lillie, R. D. (1946) J.
Pharmacol, exp. Ther., 88, 359
Tullner, W. W. & Edgcomb, J. H. (1962) J. Pharmacol. exp. Ther.,
138 (1), 126
Von Oettingen, W. F. & Sharpless, N. (1946) J. Pharmacol. exp.
Ther., 88, 400
Von Oettingen, W. F. (1955) The halogenated hydrocarbons, toxicity
and potential dangers, United States Department of Health.
Education and Welfare. Public Health Service Bull. No. 414
Weikel, J. H. (1957) Arch. int. Pharmacodyn., 110 (4), 423
Welch, H. L. (1948) J. econ. Ent., 41, 36
 BIOLOGICAL DATA
Biochemical aspects
Methoxychlor is not excreted intact, and appears to undergo
metabolize yielding hydroxyphenyl derivatives (von Oettingen &
Sharpless, 1946).
Methoxychlor is very rapidly detoxicated in the liver, yielding
a metabolite which is excreted into the intestine and removed from the
body in the faeces. This rapid detoxication, together with fairly slow
gastro-intestinal absorption, explains the low mammalian oral toxicity
and low tissue storage of methoxychlor (Weikel, 1957).
Some tissue and fat storage takes place and reaches a maximum
in 4 weeks; the stored material is mobilized in 2-4 weeks after
exposure ceases (Kunze et al., 1950; Metcalf, 1955).
Methoxychlor showed very little tendency to be excreted in the
milk even when dietary concentrations up to 7000 ppm were given to
dairy cows. At 800 ppm and 7000 ppm the amounts found in the milk were
0.13 ppm and 2.14 ppm respectively at 16 weeks (Gannon et al., 1959).
The rate and completeness of methoxychlor metabolism may account for
the low storage and accumulation.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1850 Domenjoz, 1946
Rat Oral 5000-7000 Hodge et al., 1950
Lehman, 1951
Smith et al., 1946
Sheep Oral >2000 Negherbon, 1959
Welch, 1948
Steer Oral >500 Negherbon, 1959
Welch, 1948
Short-term studies
Rat. Groups of weaned rats, each of 10 males and 10 females per
group, were fed for 45 days on a ration containing 100, 1000 and 30
000 ppm methoxychlor. At 100 ppm there was no effect on growth; at
1000 ppm growth was slightly retarded; at 30 000 ppm very little
growth occurred. At 10 000 ppm, rats in paired-feeding tests over 30
days showed marked growth reduction attributed to a reduced food
intake.
There were no deaths in the 100 ppm and 1000 ppm groups; 8 of 10
rats died in each of the male and female groups receiving 30 000 ppm.
The blood picture was normal. At autopsy there was no significant
difference in the organ-weights of the rats on 100 ppm and 1000 ppm.
The 30 000 ppm group showed uniformly smaller organ-weights than the
controls. In the case of the testes, the decrease in weight was very
marked. There was no evidence of histopathological change in the
organs examined, except in the testes, which showed apparent
suppression of spermatogenesis beyond the spermatogonial phase; the
spermatogonia and Sertoli cells were relatively normal; the primary
spermatocytes were variable in number, usually with evidence of
necrosis. The more mature germ cells were absent (Hodge et al., 1950).
In paired-feeding experiments in which 10 000 ppm of
methoxychlor were added to the diet of weanling male rats, a marked
reduction in the weight of the testes, seminal vesicles and prostate
was found. These effects could be mediated through an oestrogenic
action inhibiting the production of anterior pituitary gonadotrophins
with consequent deficiencies in the development of the male
reproductive system. Cystic tubular nephropathy was also observed
(Tullner & Edgcomb, 1962).
Rabbit. Daily oral doses of 200 mg/kg body-weight killed the
rabbits after 4 to 15 days. The only symptoms noted were diarrhoea and
anorexia (Smith et al., 1946; Von Oettingen, 1955). Dermal application
of 2 or 3 ml of a 30% solution (in dimethyl phthalate) 5 days a week
for 13 weeks was toxic; growth was depressed and paralysis of the
forelegs occurred in some cases. Histopathological examination showed
some fatty degeneration of the liver and lesions of the central
nervous system. Applications of 1 ml or less had no effect (Haag et
al., 1950).
Dog. Groups, each of 2 dogs, were maintained for one year on
doses of 20, 100 and 300 mg/kg body-weight per day. There were no
deaths; the blood picture and organ-weights were normal; there were no
histopathological changes (Hodge et al., 1952).
Long-term studies
Rat. Groups each of 25 male and 25 female rats were fed for 2
years on diets containing 25, 200 and 1600 ppm of methoxychlor. There
was no effect on growth at doses of 25 ppm and 200 ppm in the diet,
but there was moderate reduction in growth at 1600 ppm. There was no
decrease in life-span; organ-weights and blood picture were
essentially normal and histopathological examination revealed no
significant changes (Hodge et al., 1952).
Comments on the experimental studies reported
From the studies reported the rat appears more sensitive than
the dog. Experiments with the rat covered the life-span and may be
used to estimate the acceptable daily intake for man.
EVALUATION
Level causing no toxicological effect in the rat
The maximum no-effect level in rat was 200 ppm in the diet,
equivalent to 10 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
0-0.10 mg/kg body-weight
Further work desirable
Additional biochemical studies. Long-term toxicity studies in
another species than the rat. Reproduction studies.
REFERENCES
Domenjoz, R. (1946) Arch. int. Pharmacodyn., 73, 128
Gannon, N., Link, R. P. & Decker, G. C. (1959) J. Agric. Food Chem.,
7, 829
Haag, H. B., Finnegan, J. K., Larson, P. S., Riese, W. & Dreyfuss, M.
L. (1950) Arch. int. Pharmacodyn, 83 (4), 491
Hodge, H. C., Elliott, A. M., Thomas, J. F., Blanchet, H. J., Wilt, W.
G. & Mason, K. E. (1950) J. Phamacol. exp. Ther., 99, 140
Hodge, H. C., Maynard, E. A. & Blanchet, H. J. jr (1952) J.
Pharmacol. exp. Ther., 104, 60
Kunze, F. M., Laug, E. P. & Prickett, C. S. (1950) Proc Soc. exp.
Biol. (N.Y.), 75, 415
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3., Saunders,
Philadelphia
Smith, M. I., Bauer, H., Stohlman, E. F. & Lillie, R. D. (1946) J.
Pharmacol, exp. Ther., 88, 359
Tullner, W. W. & Edgcomb, J. H. (1962) J. Pharmacol. exp. Ther.,
138 (1), 126
Von Oettingen, W. F. & Sharpless, N. (1946) J. Pharmacol. exp.
Ther., 88, 400
Von Oettingen, W. F. (1955) The halogenated hydrocarbons, toxicity
and potential dangers, United States Department of Health.
Education and Welfare. Public Health Service Bull. No. 414
Weikel, J. H. (1957) Arch. int. Pharmacodyn., 110 (4), 423
Welch, H. L. (1948) J. econ. Ent., 41, 36
BIOLOGICAL DATA
Biochemical aspects
Methoxychlor is not excreted intact, and appears to undergo
metabolize yielding hydroxyphenyl derivatives (von Oettingen &
Sharpless, 1946).
Methoxychlor is very rapidly detoxicated in the liver, yielding
a metabolite which is excreted into the intestine and removed from the
body in the faeces. This rapid detoxication, together with fairly slow
gastro-intestinal absorption, explains the low mammalian oral toxicity
and low tissue storage of methoxychlor (Weikel, 1957).
Some tissue and fat storage takes place and reaches a maximum
in 4 weeks; the stored material is mobilized in 2-4 weeks after
exposure ceases (Kunze et al., 1950; Metcalf, 1955).
Methoxychlor showed very little tendency to be excreted in the
milk even when dietary concentrations up to 7000 ppm were given to
dairy cows. At 800 ppm and 7000 ppm the amounts found in the milk were
0.13 ppm and 2.14 ppm respectively at 16 weeks (Gannon et al., 1959).
The rate and completeness of methoxychlor metabolism may account for
the low storage and accumulation.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1850 Domenjoz, 1946
Rat Oral 5000-7000 Hodge et al., 1950
Lehman, 1951
Smith et al., 1946
Sheep Oral >2000 Negherbon, 1959
Welch, 1948
Steer Oral >500 Negherbon, 1959
Welch, 1948
Short-term studies
Rat. Groups of weaned rats, each of 10 males and 10 females per
group, were fed for 45 days on a ration containing 100, 1000 and 30
000 ppm methoxychlor. At 100 ppm there was no effect on growth; at
1000 ppm growth was slightly retarded; at 30 000 ppm very little
growth occurred. At 10 000 ppm, rats in paired-feeding tests over 30
days showed marked growth reduction attributed to a reduced food
intake.
There were no deaths in the 100 ppm and 1000 ppm groups; 8 of 10
rats died in each of the male and female groups receiving 30 000 ppm.
The blood picture was normal. At autopsy there was no significant
difference in the organ-weights of the rats on 100 ppm and 1000 ppm.
The 30 000 ppm group showed uniformly smaller organ-weights than the
controls. In the case of the testes, the decrease in weight was very
marked. There was no evidence of histopathological change in the
organs examined, except in the testes, which showed apparent
suppression of spermatogenesis beyond the spermatogonial phase; the
spermatogonia and Sertoli cells were relatively normal; the primary
spermatocytes were variable in number, usually with evidence of
necrosis. The more mature germ cells were absent (Hodge et al., 1950).
In paired-feeding experiments in which 10 000 ppm of
methoxychlor were added to the diet of weanling male rats, a marked
reduction in the weight of the testes, seminal vesicles and prostate
was found. These effects could be mediated through an oestrogenic
action inhibiting the production of anterior pituitary gonadotrophins
with consequent deficiencies in the development of the male
reproductive system. Cystic tubular nephropathy was also observed
(Tullner & Edgcomb, 1962).
Rabbit. Daily oral doses of 200 mg/kg body-weight killed the
rabbits after 4 to 15 days. The only symptoms noted were diarrhoea and
anorexia (Smith et al., 1946; Von Oettingen, 1955). Dermal application
of 2 or 3 ml of a 30% solution (in dimethyl phthalate) 5 days a week
for 13 weeks was toxic; growth was depressed and paralysis of the
forelegs occurred in some cases. Histopathological examination showed
some fatty degeneration of the liver and lesions of the central
nervous system. Applications of 1 ml or less had no effect (Haag et
al., 1950).
Dog. Groups, each of 2 dogs, were maintained for one year on
doses of 20, 100 and 300 mg/kg body-weight per day. There were no
deaths; the blood picture and organ-weights were normal; there were no
histopathological changes (Hodge et al., 1952).
Long-term studies
Rat. Groups each of 25 male and 25 female rats were fed for 2
years on diets containing 25, 200 and 1600 ppm of methoxychlor. There
was no effect on growth at doses of 25 ppm and 200 ppm in the diet,
but there was moderate reduction in growth at 1600 ppm. There was no
decrease in life-span; organ-weights and blood picture were
essentially normal and histopathological examination revealed no
significant changes (Hodge et al., 1952).
Comments on the experimental studies reported
From the studies reported the rat appears more sensitive than
the dog. Experiments with the rat covered the life-span and may be
used to estimate the acceptable daily intake for man.
EVALUATION
Level causing no toxicological effect in the rat
The maximum no-effect level in rat was 200 ppm in the diet,
equivalent to 10 mg/kg body-weight per day.
Estimate of acceptable daily intakes for man
0-0.10 mg/kg body-weight
Further work desirable
Additional biochemical studies. Long-term toxicity studies in
another species than the rat. Reproduction studies.
REFERENCES
Domenjoz, R. (1946) Arch. int. Pharmacodyn., 73, 128
Gannon, N., Link, R. P. & Decker, G. C. (1959) J. Agric. Food Chem.,
7, 829
Haag, H. B., Finnegan, J. K., Larson, P. S., Riese, W. & Dreyfuss, M.
L. (1950) Arch. int. Pharmacodyn, 83 (4), 491
Hodge, H. C., Elliott, A. M., Thomas, J. F., Blanchet, H. J., Wilt, W.
G. & Mason, K. E. (1950) J. Phamacol. exp. Ther., 99, 140
Hodge, H. C., Maynard, E. A. & Blanchet, H. J. jr (1952) J.
Pharmacol. exp. Ther., 104, 60
Kunze, F. M., Laug, E. P. & Prickett, C. S. (1950) Proc Soc. exp.
Biol. (N.Y.), 75, 415
Lehman, A. J. (1951) Quart. Bull. Assoc. Food and Drug Officials
U.S., 15, 122
Metcalf, R. L. (1955) Organic insecticides, Interscience, New York
Negherbon, W. O. (1959) Handbook of Toxicology, vol. 3., Saunders,
Philadelphia
Smith, M. I., Bauer, H., Stohlman, E. F. & Lillie, R. D. (1946) J.
Pharmacol, exp. Ther., 88, 359
Tullner, W. W. & Edgcomb, J. H. (1962) J. Pharmacol. exp. Ther.,
138 (1), 126
Von Oettingen, W. F. & Sharpless, N. (1946) J. Pharmacol. exp.
Ther., 88, 400
Von Oettingen, W. F. (1955) The halogenated hydrocarbons, toxicity
and potential dangers, United States Department of Health.
Education and Welfare. Public Health Service Bull. No. 414
Weikel, J. H. (1957) Arch. int. Pharmacodyn., 110 (4), 423
Welch, H. L. (1948) J. econ. Ent., 41, 36
